1
Fifth stage
Medicine
Lec-10
د . منوع
1/1/2014
Cancer Chemotherapy
OUTLINE
Introduction
Cell cycle
Definition of terms
Commonly used cytotoxic drugs
Routes of administration
Pre-chemotherapy assessment
Response & Resistance to Chemo.
Complications & Management
Introduction
• Cancer chemotherapy is a branch of cancer treatment that involve the use of
chemical agents to destroy cancer cells.
• Other modalities of cancer Rx include surgery, radiation, immune modulation, &
marrow transplants.
• The aim of cancer chemotherapy is to cure where possible and palliate where cure is
impossible.
• The effective use of cancer chemotherapy requires an understanding of the principles
of tumor biology, cellular kinetics, pharmacology, and drug resistance.
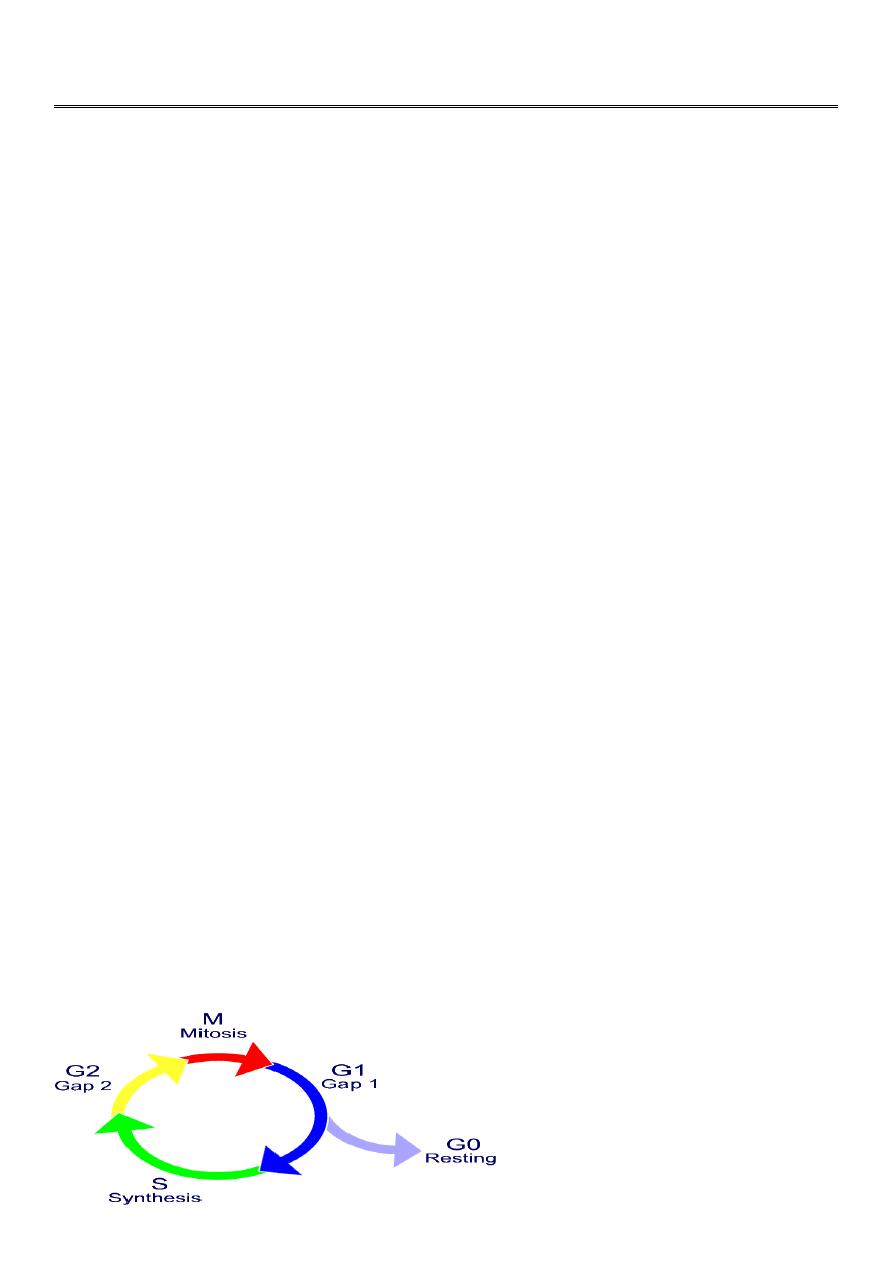
Cell Cycle
A constantly proliferating cell (normal or malignant) repeats a cycle of events that
eventually end with cell division

2
• The progression of a cell through this cycle is promoted by cycline dependent kinases
which when complexed with cyclines drives the cell through cell cycle.
, the cell cycle consists of: G
1
= growth and preparation of the chromosomes for replication;
S = synthesis of DNA and duplication of the
G
2
= preparation for
M =
Definition of terms
• Induction: chemotherapy given with the intent of inducing complete remission when
initiating a curative regimen. usually applied to hematologic malignancies.
• Consolidation: Repetition of the induction regimen in a patient who has achieved a
complete remission after induction, with the intent of increasing cure rate or prolonging
remission.
• Intensification: Chemotherapy after complete remission with higher doses of the same
agents used for induction or with different agents at high doses with the intent of
increasing cure rate or remission duration.
• Maintenance: Long-term, low-dose, single or combination chemotherapy in a patient
who has achieved a complete remission, with the intent of delaying the regrowth of
residual tumor cells.
• Adjuvant: A short course of high-dose, usually combination chemotherapy in a patient
with no evidence of residual cancer after surgery or radiotherapy, given with the intent
of destroying a low number of residual tumor cells.
• Neoadjuvant: Adjuvant chemotherapy given in the preoperative or perioperative period.
• Palliative: Chemotherapy given to control symptoms or prolong life in a patient in whom
cure is unlikely.
• Salvage: A potentially curative, high-dose, usually combination, regimen given in a
patient who has failed or recurred following a different curative regimen.
Routes of Administration
• Intravenous
• Oral
• Intrathecal / Intraventricular – used in meningeal metastasis.
• Intrapericardial – used in malignant pericardial effusion.
• Intraperitoneal – used in ovarian cancer, colorectal cancer, mesothelioma.
• Intra-arterial – used in Liver cancer

3
chemotherapy dosage
• Based on Body Surface Area( BSA) or weight
• BSA gives more accurate measure of fluid and tissue proportions
• Use normogram or BSA conversion calculator
• BSA (m²) = √ ht(cm) × w(kg)
3600
Pre-Chemotherapy Assessment
• Detailed Hx
-
Age
-
Occupation
-
Duration of symptoms
-
Weight loss
-
PMHx
Physical Examination
-
Lymphadenopathy
-
Organomegaly
-
States of the heart & lungs
-
Bone involvement
-
Ht, Wt, BSA
Performance status
Popular instrument used in oncology include
• Karnofsky performance index :
Normal – 100%
Death – 0%
• Eastern Cooperative Oncology Grp ECOG :
0 – Asymptomatic
5 - Death

4
Investigations
• Hematologic – FBC, ESR, BM biopsy
• Biochemical – LFT, RFT
• Imaging – CXR, USS, Bone scan, CT, MRI
• Tumour markers –
gene products expressed in some cancers & may be used as diagnostic & monitoring tools.
E.g. a-feto protein, PSA
Response to Chemotherapy
• Complete response (CR)
Disappearance of all known disease, confirmed at ≥ 4 weeks
• Partial response (PR)
≥ 50% decrease from baseline, confirmed at ≥ 4weeks
• Progressive disease (PD)
≥ 25% increase in one or more lesions or appearance of new lesions
• Stable disease (SD)
Neither PR nor PD criteria met (no change)
Resistance to Chemotherapy
• Primary Resistance
When the cancer does not respond to standard chemotherapy from the very first exposure.
• Acquired Resistance
When the tumour initially responds to chemotherapy then becomes resistant.
• Cancer cells may mutate & develop pathways that are independent of those blocked by
cytotoxic drugs.
• Gene amplification may lead to overproduction of proteins that are blocked by
anticancer drugs.
• Cancer cells may develop mechanism that inactivate anticancer drugs.
• They may learn to repair the DNA & protein damages induced by anticancer drugs.
• Resistant clones of cancer cells may develop
5
Complications of Chemotherapy
• Skin
-
Alopecia
-
Darkening of the skin & Nails
-
Noticeable with 5FU
• Hematological
- Anaemia, Leucopaenia, Thrombocytopenia
- Almost all cytotoxic drugs
• Gastrointestinal
-
Nausea, Vomiting, Mucositis, GIT infection
-
Almost all drugs
• Endocrine
-
Infertility, Amenorrhea, Irregular menses
-
Almost all drugs
• Neurological
-
Peripheral neuropathy
-
Loss of interest
-
Confusion
-
Common with plant alkaloids
Others
• Ototoxicity – Platinum compounds
• Second tumors – Alkylating agents
• Cardiac toxicity – Anthracyclines
• Pulmonary toxicity – Bleomycin
• Bladder toxicity – Cyclophosphamide
• Nephrotoxicity – Platinum compound
• Tumour lysis syndrome
6
Prevention & Management of Complications
• Nausea & Vomiting
-
5-HT3 receptor antagonist
-
Ondasetron, Ganisetron
• Secondary tumors
- Careful follow-up
• Anaemia
-
Erythropoietin
-
Blood transfusion
• Immunosupression:
-
Colony stimulating factor
-
BM transplant
-
Broad-spectrum antibiotics
• Supportive Rx
-
Leucovorin (folinic acid) is added when a very high dose Antimetabolites are given
-
Colony stimulating factor & Erythropoietin can be used to supplement high dose
chemotherapy

7
Classification
Cell Cycle–Specific (CCS) Agents
Cell Cycle–Nonspecific (CCNS)
Agents
Antimetabolites (S phase)
Alkylating agents
Capecitabine
Altretamine
Cladribine
Bendamustine
Clofarabine
Busulfan
Cytarabine
(ara-C)
Carmustine
Fludarabine
Chlorambucil
5-Fluorouracil (5-FU)
Cyclophosphamide
Gemcitabine
Dacarbazine
6-Mercaptopurine (6-MP)
Lomustine
Methotrexate (MTX)
Mechlorethamine
6-Thioguanine
(
6-TG
)
Melphalan
Epipodophyllotoxin
(topoisomerase II
inhibitor) (G
1
–S phase)
Temozolomide
Thiotepa
Etoposide
Anthracyclines
Taxanes (M phase)
Daunorubicin
Albumin-bound paclitaxel
Doxorubicin
Docetaxel
Epirubicin
Paclitaxel
Idarubicin
Vinca alkaloids (M phase)
Mitoxantrone
Vinblastine
Antitumor antibiotics
Vincristine
Dactinomycin
Vinorelbine
Mitomycin
Antimicrotubule inhibitor (M phase)
Camptothecins (topoisomerase I
inhibitors)
Ixabepilone
Irinotecan
Antitumor antibiotics (G
2
–M phase)
Topotecan
Platinum analogs
Bleomycin
Carboplatin
Cisplatin
Alkylating agents
• Cyclophosphamide
• Cisplatin
• Procarbazine
• Busulfan

8
• Mechlorethamine
Mechanism Of Action (MOA)
• They are highly reactive drugs that restrict the action of biological molecules such as
proteins and DNA by binding to them
•
They add alkyl groups to the electro negative groups in cancer cells
Alkylating Agents- Used in wide variety of hematologic and solid tumors
• Thiotepa – ovarian cancer
• Busulfan – DOC in CML (***Important ADR-Pulmonary fibrosis).
• ***Nitrosoureas (Carmustine and lomustine ) - brain tumors
– Highly lipid soluble drugs hence reach high concentration in the brain and CSF.
• Streptozocin – insulin-secreting islet cell carcinoma of the pancreas
• Mechlorethamine – Prodrug. Component of MOPP regimen for Hodgkin’s disease. It
is a highly irritant drug so care should be taken to avoid extravasation during IV
administration
• Chlorambucil (Leukeran): for CLL. Slow acting and least toxic nitrogen mustard.
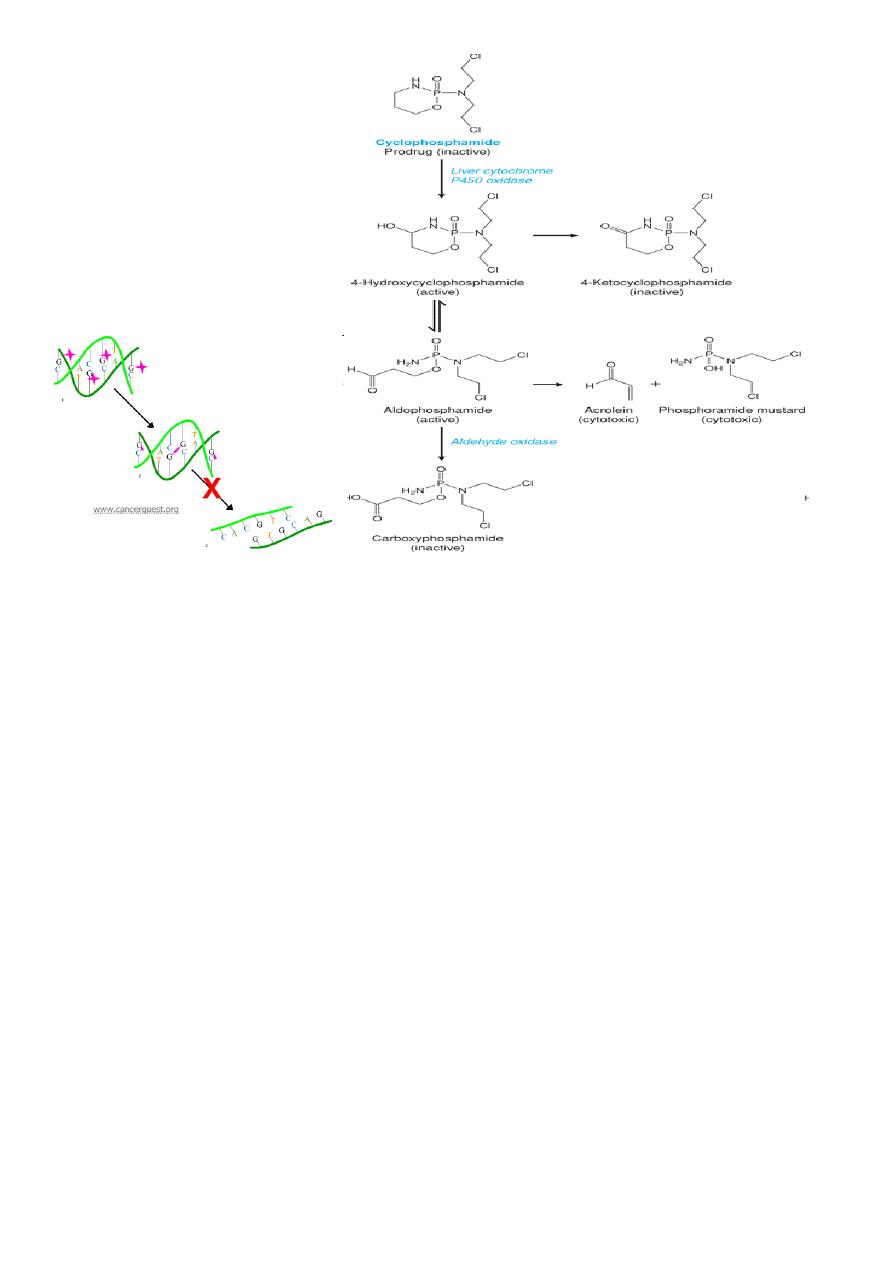
Cyclophosphamide
It is a prodrug and is activated by the P-450 enzymes to its active form phosphoramide
mustard
• The active drug alkylates nucleophilic groups on DNA bases
– Particularly at the N-7 position of guanine
• This leads to cross linking of bases, abnormal base pairing and DNA strand breakage
9
Mechanism of resistance
• *** The mechanisms mentioned below are common for all the alkylyting agents
• Increased DNA repair
• Decreased drug permeability
• Production of “trapping” agents (thiols)
Uses
• Non-Hodgkin’s lymphoma
• Breast Ca
• Ovarian Ca
• Neuroblastoma
Adverse effect (ADR)
• Acrolein is the metabolite
• Responsible for causing hemorrhagic cystitis
– Suprapubic pain
– Hematuria
– Cyctoscopic findings
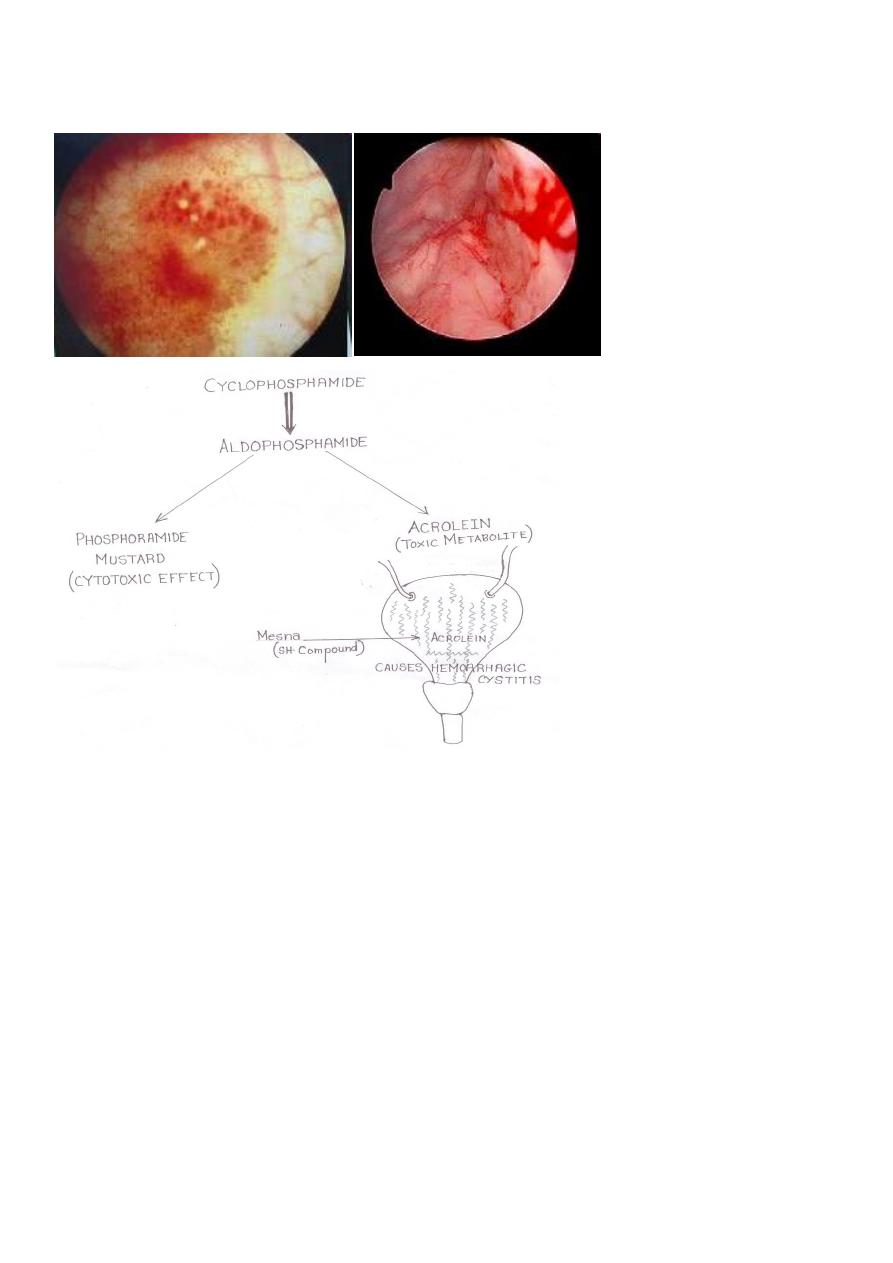
10
• ***This is prevented/treated by MESNA (mercaptoethanesulfonate)
• Rarely cyclophosphamide can cause SIADH and pulmonary toxicity
Cisplatin
• Platinum analog
• Same MOA as cyclophosphamide
• **Used in testicular carcinoma
• Also used for Ca of bladder, lung and ovary
• Carboplatin is new drug with better safety profile
ADR
• Nephrotoxicity (prevented by Amifostine***)
• ***Ototoxicity (acoustic nerve damage)
• Peripheral neuritis
• Severe nausea and vomiting

11
Procarbazine
• MOA: forms hydrogen peroxide, which generates free radicals that cause DNA damage
• Important component of regimens especially for Hodgkin’s lymphoma
ADR
• ***Disulfiram like reactions
Disulfiram produces a sensitivity to alcohol which results in a highly unpleasant reaction
when the patient under treatment ingests even small amounts of alcohol. Disulfiram blocks
the oxidation of alcohol at the acetaldehyde stage.
During alcohol metabolism after disulfiram intake, the concentration of acetaldehyde
occurring in the blood may be 5 to 10 times higher than that found during metabolism of
the same amount of alcohol alone.
Accumulation of acetaldehyde in the blood produces a complex of highly unpleasant
symptoms referred to as the disulfiram-alcohol reaction. This reaction, which is
proportional to the dosage of both disulfiram and alcohol, will persist as long as alcohol is
being metabolized. Disulfiram does not appear to influence the rate of alcohol elimination
from the body.
Disulfiram plus even small amounts of alcohol produces flushing, throbbing in head and
neck, throbbing headache, respiratory difficulty, nausea, copious vomiting, sweating, thirst,
chest pain, palpitation, dyspnea, hyperventilation, tachycardia, hypotension, syncope,
marked uneasiness, weakness, vertigo, blurred vision, and confusion.
In severe reactions, there may be respiratory depression, cardiovascular collapse,
arrhythmias, myocardial infarction, acute congestive heart failure, unconsciousness,
convulsions, and death.
The intensity of the reaction may vary with each individual but is generally proportional to
the amount of disulfiram and alcohol ingested.
In the sensitive individual, mild reactions may occur when the blood alcohol concentration
is increased to as little as 5 to 10 mg/100 mL. At a concentration of 50 mg/100 mL
symptoms are usually fully developed, and when the concentration reaches 125 to 150
mg/100 mL unconsciousness may occur.
12
Blood in the urine!!!
• A 30-year-old man is seen at the ER complaining about severe suprapubic pain, fever
and signs of passing blood in the urine. He is currently in treatment for non-Hodgkin’s
lymphoma (four drug regimen).
1. What is your preliminary diagnosis?
2. If it is drug induced, who is “the culprit”?
3. What is the mechanism behind the latest complication?
4. How to treat the complication?
5. Which drug we covered until now has serious nephrotoxic effects?
6. How to prevent that particular drug induced renal damage?
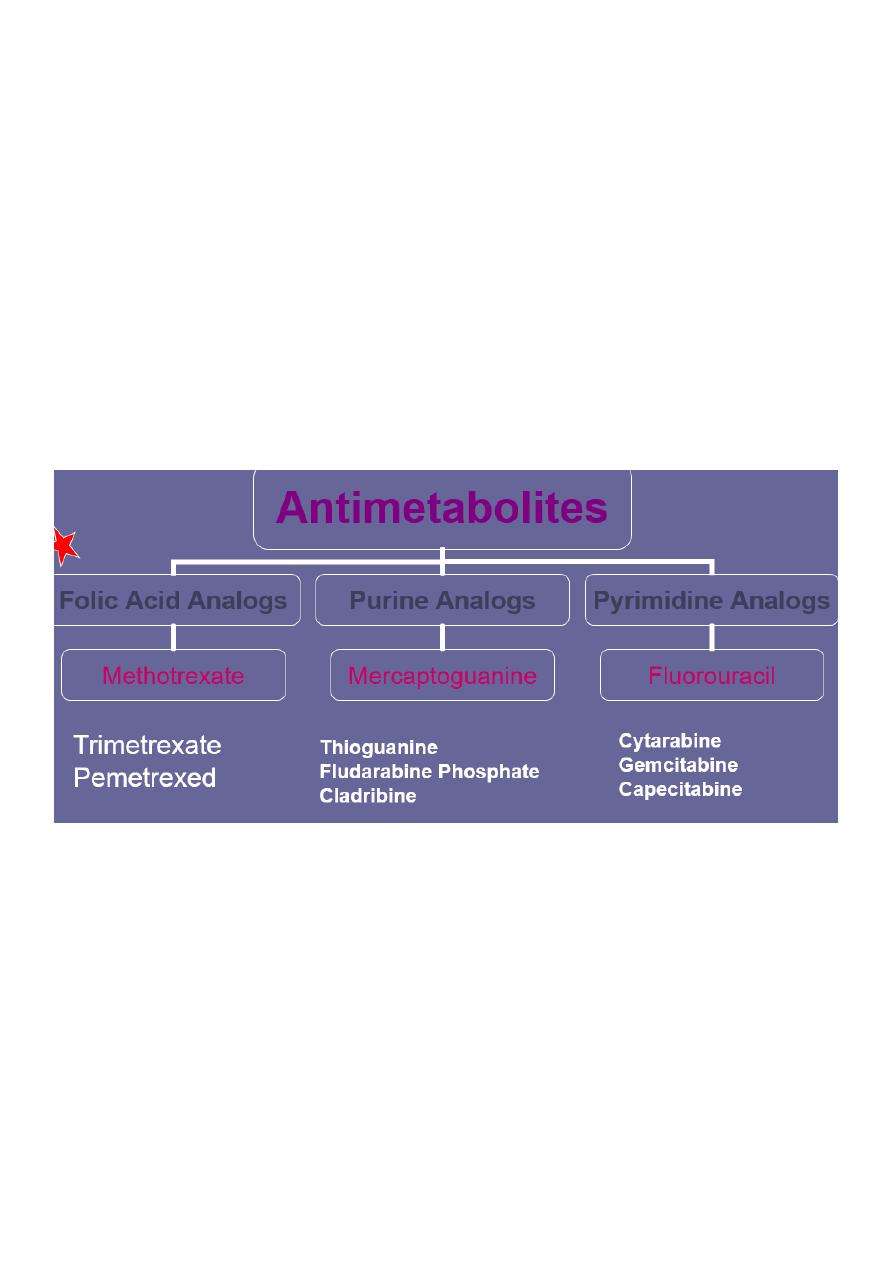
Antimetabolites
• They are structurally similar to endogenous compounds
• They act as antagonists of:
– Folic acid (methotrexate)
– Purines (Mercaptopurine and thioguanine)
– Pyrimidine (fluorouracil, cytarabine)
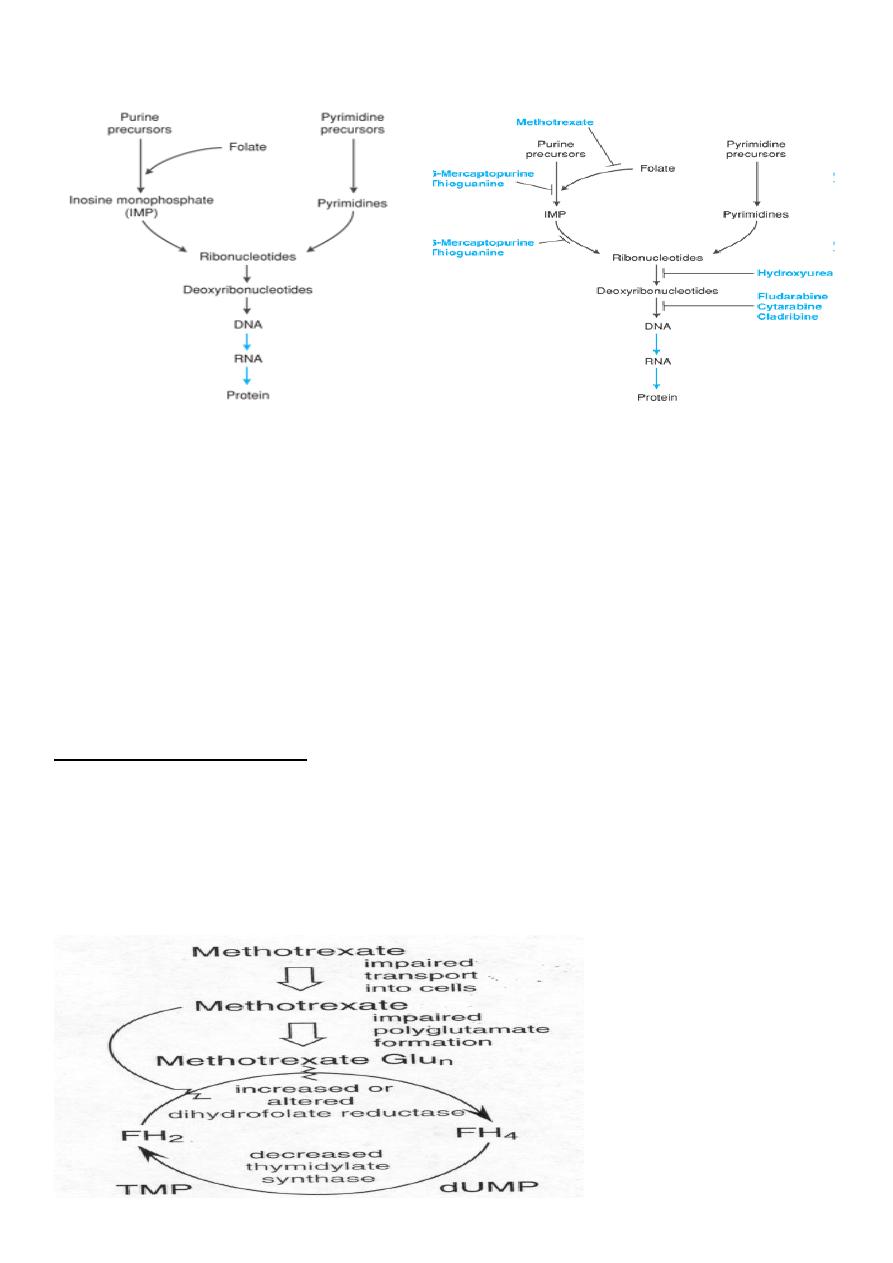
13
Antimetabolits: sites of drug action
Methotrexate (MTX)
• MTX is a folic acid analog that binds with high affinity to the active catalytic site of
dihydrofolate reductase (DHFR)
• Thus it interferes with the synthesis of tetrahydrofolate (THF)
• THF serves as the key one-carbon carrier for enzymatic processes involved in de novo
synthesis of thymidylate, purine nucleotides, and the amino acids serine and
methionine.
• Inhibition of these various metabolic processes thereby interferes with the formation
of DNA, RNA, and key cellular proteins.
Mechanism of Resistance
1. Decreased drug transport
2. Altered DHFR
3. Decreased polyglutamate formation
4. Increased levels of DHFR

14
• Most commonly used anticancer drug.
• Cell cycle specific (CCS) drug and acts during S phase of the cell cycle.
• Antineoplastic, immunosuppressant and antiinflammatory
• Used in RA, psoriasis
• Well absorbed orally; can also be given IM, IV or intrathecally**.
• It is bound to plasma proteins, does not cross the BBB and most of the drug is excreted
unchanged in urine.
• It is a weak acid and so is excreted better at high urine pH. Appropriate hydration and
alkalinizing the urine is important to prevent renal tox with MTX
ADR
• Bone marrow suppression (BMS)
• Mucositis
• Folic acid deficiency
• The toxic effects of MTX on normal cells is reduced by administering folinic acid
(leucovorin)
– This is called leucovorin rescue ****
– Higher the dose of MTX more the leucovorin you give**
Leucovorin Rescue
Mechanism of action of methotrexate and the effect of administration of leucovorin.
• FH2 = dihydrofolate
• FH4 = tetrahydrofolate
• dTMP = deoxythymidine monophosphate
•
dUMP = deoxyuridine mono
phosphate.
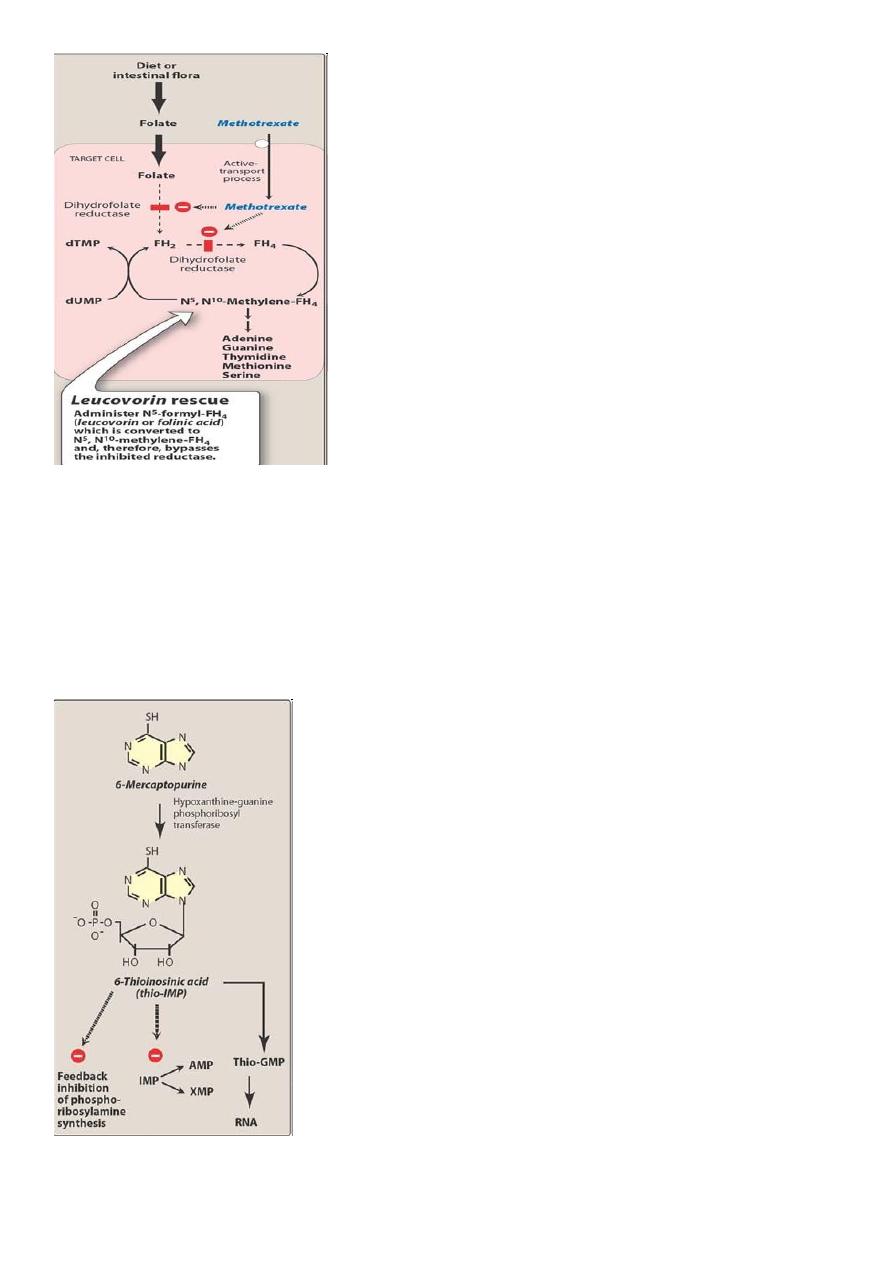
15
6-Mercaptopurine (6-MP) & Thioguanine
• Both 6-MP and Thioguanine are activated by hypoxanthine guanine phosphoribosyl
transferase(HGPRT) to toxic nucleotides that inhibit several enzymes involved in purine
metabolism
• ***Resistance is due to cancer cells having
d activity of HGPRT
• Cancer cells also
es alkaline phosphatase that inactivate toxic nucleotides

16
6-MP & Allopurinol
• 6-MP is metabolized in the liver by xanthine oxidase and the inactive metabolites are
excreted in the urine
• ***Allopurinol is used frequently to treat/prevent hyperuricemia caused by many
anticancer drugs.
• If Allopurinol is used with 6-MP then the dose of 6-MP is reduced by more than 75%
– Why??
Cytarabine (Ara-C)
• Cytarabine arabinoside is a pyrimidine antimetabolite
• The drug is activated by kinases
– This acts as an inhibitor of DNA polymerase
• ***of all antimetabolites, this is the most specific for S phase of tumor cell cycle
• It is an important component in acute leukaemia regimens
• ADR: at high doses cause neurotoxicity (cerebellar dysfunction and peripheral
neuritis)
• Hand-foot syndrome
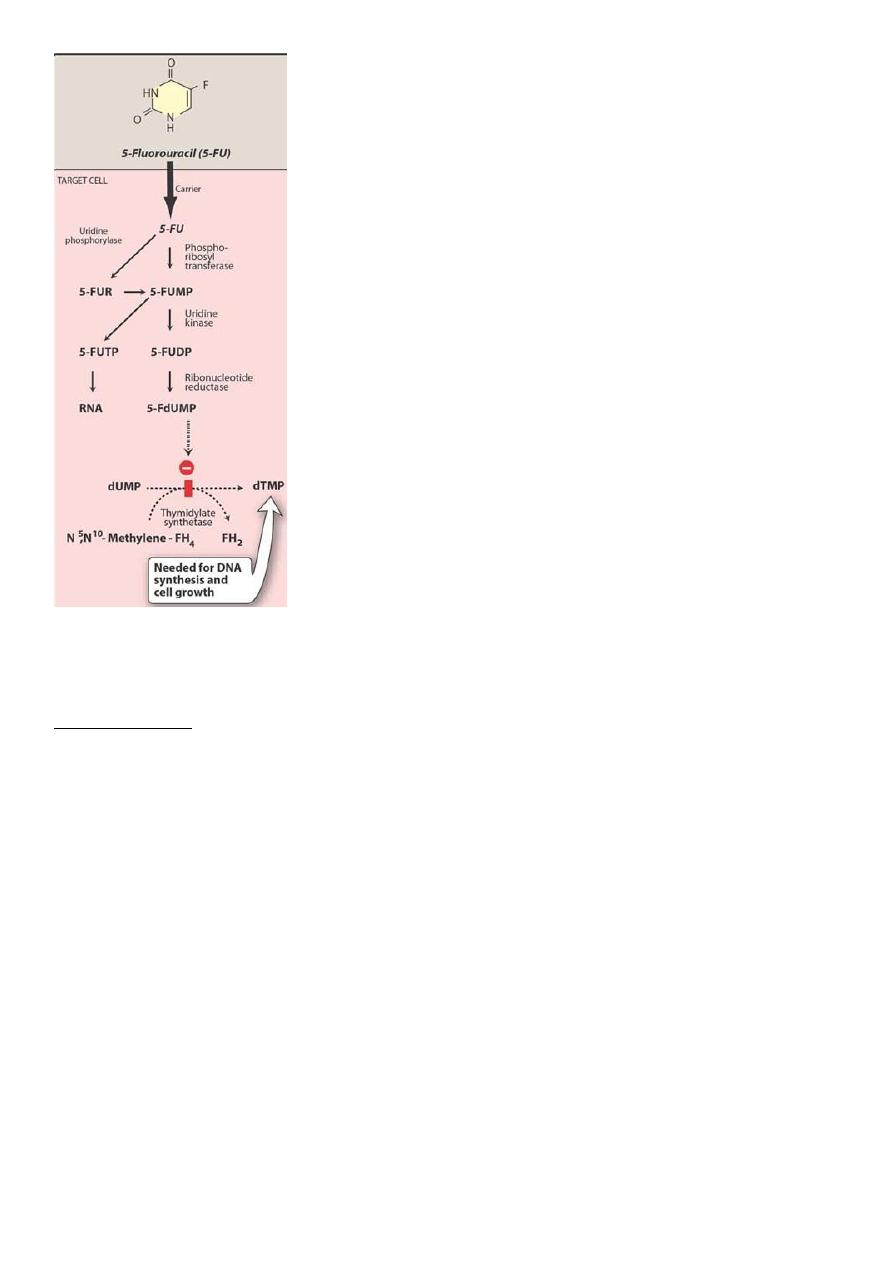
5-FU
Mechanism of the cytotoxic action of 5-FU
• 5-FU is converted to 5-FdUMP, which competes with deoxyuridine monophosphate
(dUMP) for the enzyme thymidylate synthetase.
• 5-FU = 5-fluorouracil
• 5-FUR = 5-fluorouridine
• 5-FUMP = 5-fluorouridine monophosphate
• 5-FUDP = 5-fluorouridine diphosphate
• 5-FUTP = 5-fluorouridine triphosphate
• dUMP = deoxyuridine monophosphate
• dTMP = deoxythymidine monophosphate
•
5-FdUMP = 5
-fluorodeoxyuridine monophosphate.
17
• 5-FU causes, “thymidineless death” of cells
• Resistance is due to
d activation of 5-FU and
d thymidylate synthase activity
Uses and ADR
• Metastatic carcinomas of the breast and the GI tract, hepatoma
• Carcinomas of the ovary, cervix, urinary bladder, prostate, pancreas, and
oropharyngeal areas
• Combined with levamisole for Rx of colon cancer
• ADR: nausea, mucositis, diarrhea, ***hand and foot syndrome, Alopecia,
hyperpigmentation, neurologic deficits, bone marrow depression
Hand –foot syndrome is a side effect of some chemotherapy drugs that results when a
small amount of drug leaks out of the blood vessels, damaging tissues. This tends to happen
in the hands and the feet because of the increased friction and heat that the extremities
are exposed to through daily activities. Symptoms can be prevented by avoiding friction and
heat. Treatment consists of reducing or stopping treatment with the drug that caused the
syndrome. prevent symptoms by avoiding friction or heat.
18
Symptoms of hand-foot syndrome include:
Tingling or burning
Redness
Flaking
Swelling
Small blisters
Small sores on the palms of the hands or soles of the feet
• A 50-year-old man is undergoing chemotherapy with a multi-drug regimen after
being diagnosed to have a malignant tumor. Soon after the first cycle of
chemotherapy, he develops symptoms of severe fatigue and pallor. His Hb is very low
and the peripheral smear shows presence of macrocytes
1. What is your prelim. Diagnosis?
2. Which of the drugs we discussed can be most likely responsible for this complication?
3. Which compound (and how) administered soon after chemotherapy could have
prevented the toxicity?
4. Which are the anticancer drugs which can cause tingling, feeling of warmth, redness,
flaking and blisters (what is this)?
19
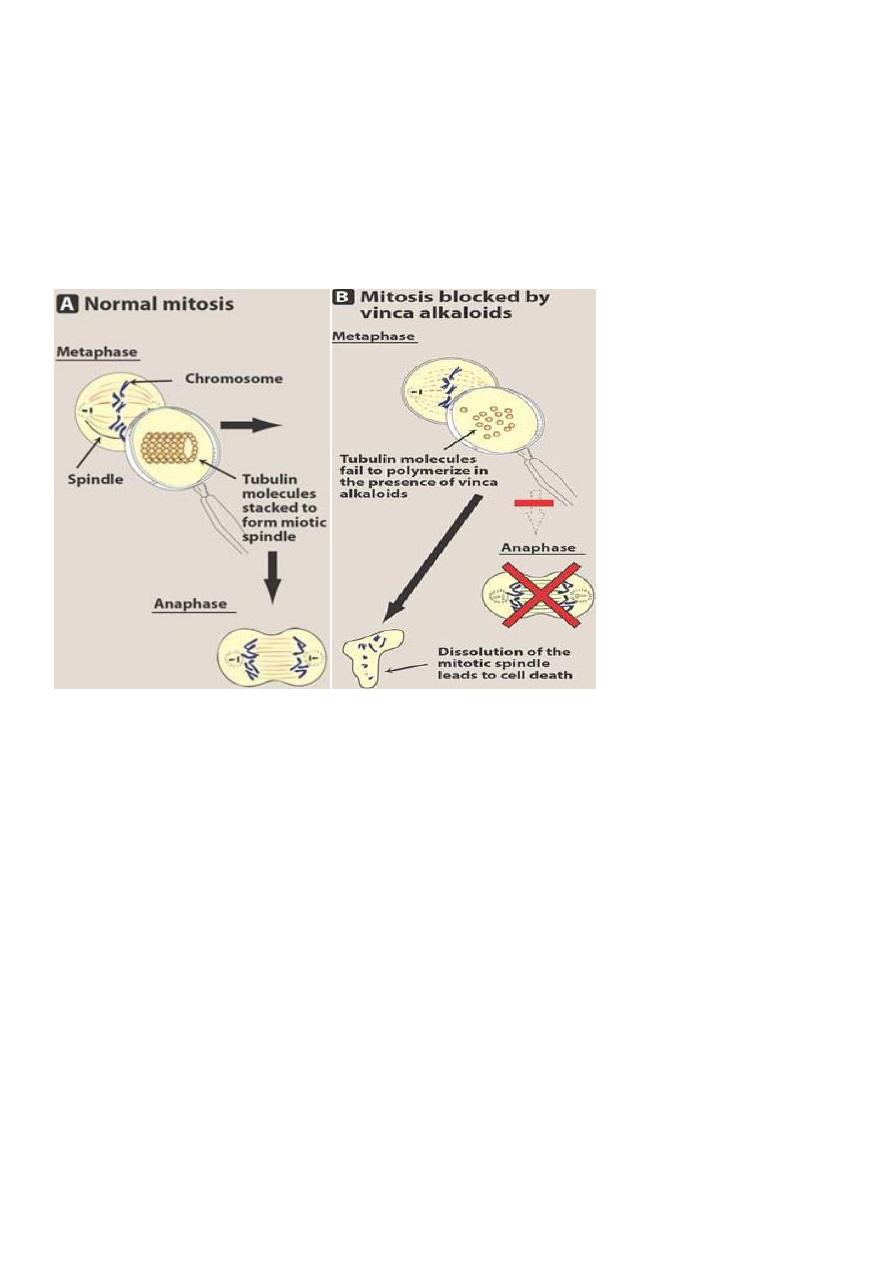
Vinka alkaloids (Vinblastine, vincristine)
• These drugs block the formation of mitotic spindle by preventing the assembly of tubulin
dimers into microtubules
• ***They act primarily on the M phase of cancer cell cycle
• Resistance is due to
d efflux of drugs from tumor cells
