Lecture 3
Types of metals alloys
1
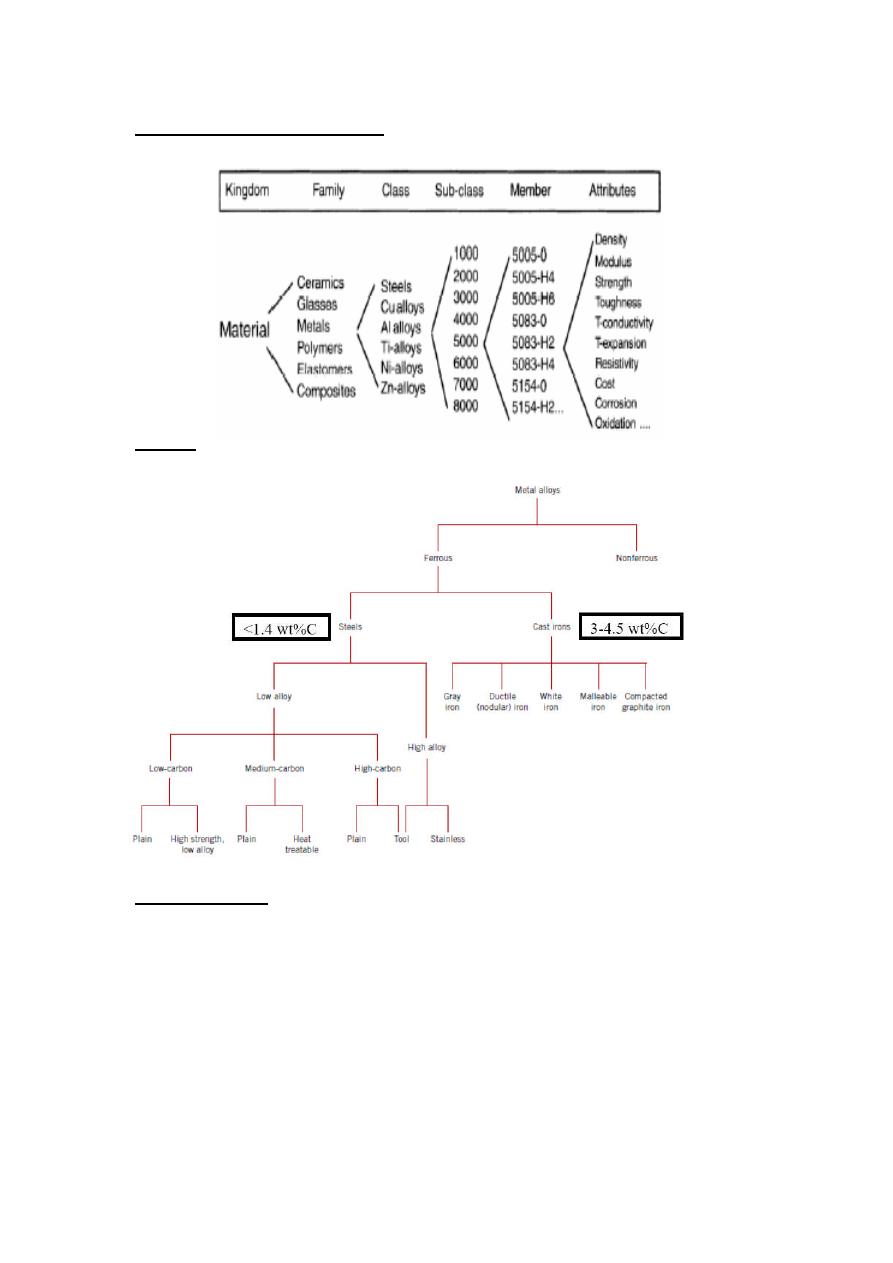
General Material Classifications
Metals
Ferrous Alloys
Those with iron as prime constituent (except for high alloy steel)
Important as engineering construction material (especially steel Fe-C) is
because:
They important – Abundant within the earth-crust – low cost
Easier to be produced
Good strength toughness and ductility
Versatile – wide range of mechanical and physical properties
Lecture 3
Types of metals alloys
2
.
Can be alloyed and heat treated to get desired mechanical properties
Alloying is combining or mixing other material like carbon or other
metals to iron
Heat treatment is a process of heating and cooling a metal to achieve
specific microstructure which in turns display specific mechanical
properties (e.g. Quenching austenite gives martensite witch be treated
to martensite which is can heat (tempered) produced tempered more
ductile
Nomenclature of Ferrous Alloys
Nomenclature AISI (American Iron and Steel Institute) & SAE (Society of
Automotive Engineers)
10xx Plain Carbon Steels
11xx Plain Carbon Steels (resulfurized for machinability)
15xx Mn (10 ~ 20%)
40xx Mo (0.20 ~ 0.30%)
43xx Ni (1.65 - 2.00%), Cr (0.4 - 0.90%), Mo (0.2 - 0.3%)
44xx Mo (0.5%)
where xx is wt% C x 100
example: 1060 steel – plain carbon steel with 0.60 wt% C
Stainless Steel -- >11% Cr – AISI number e.g. 409
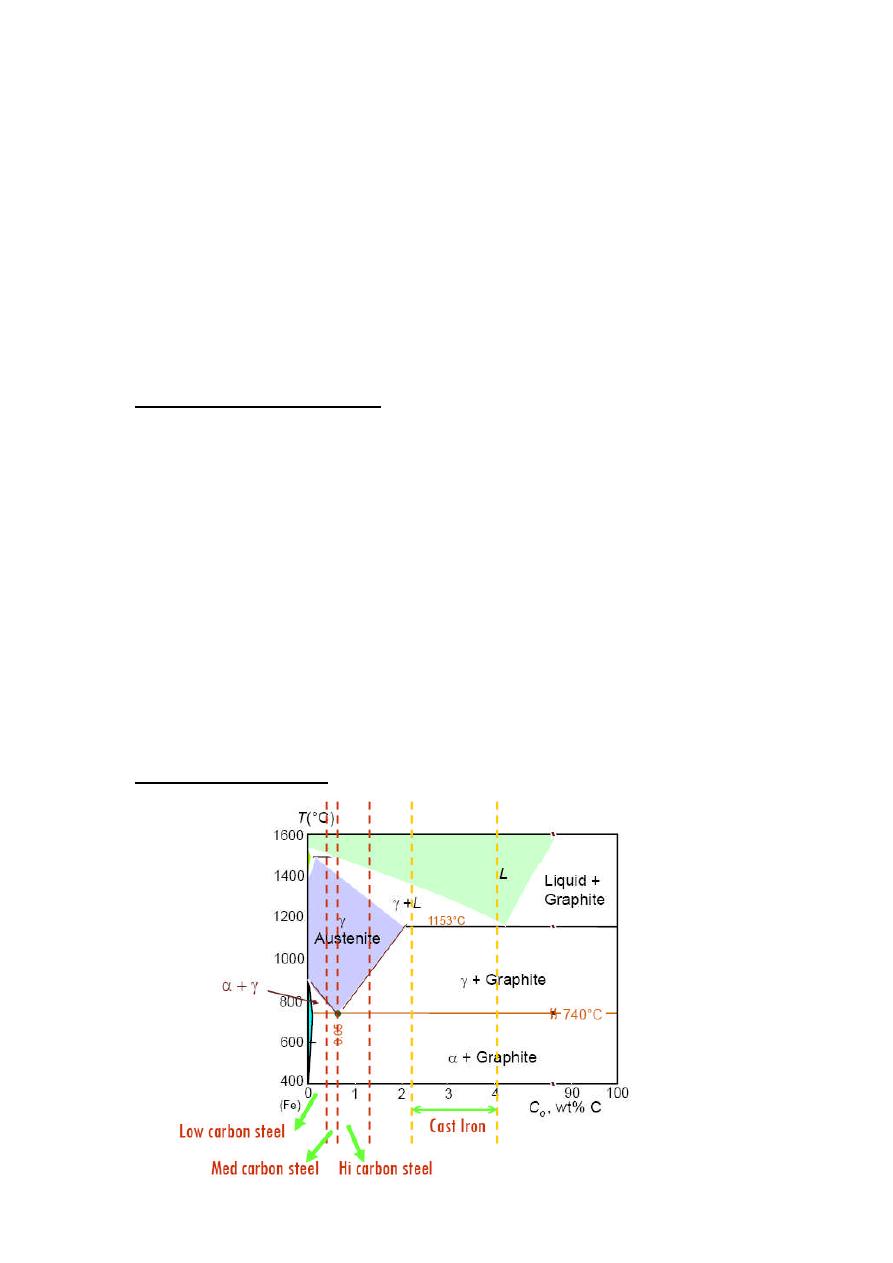
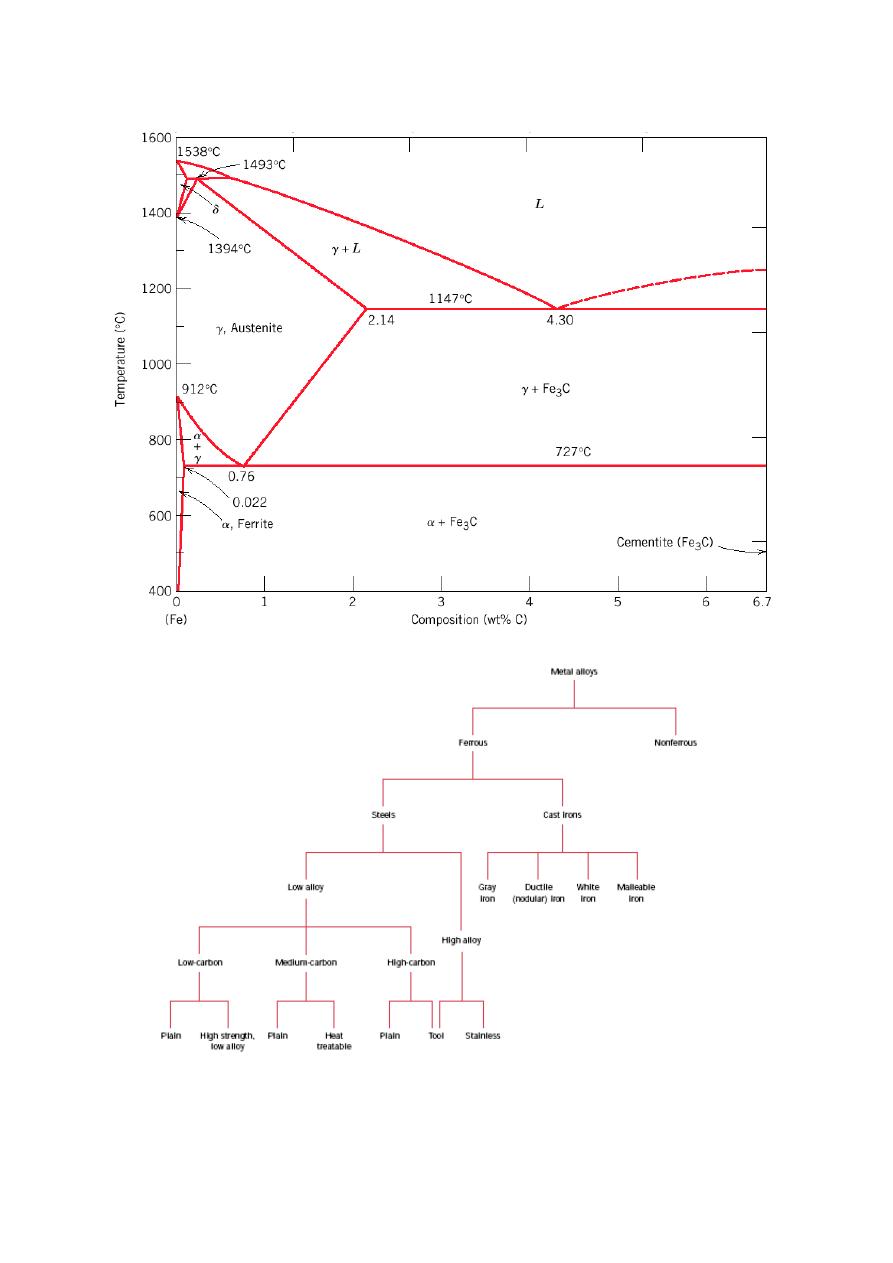
Iron-C phase Digram

Lecture 3
Types of metals alloys
3
Low Carbon steel
Alloys of low carbon steel being produced in the greatest quantity
<0.25wt %C
Consists of ferrites and pearlites and weak but can be treated to achieve high
strength
Machineable, weldable and cheaper to be produced
Types:
Plain low carbon steel
no alloying, variable tensile strength (TS) (415-550)MPa and Yield Strength
(YS) = 275MPa
Applications –– crankshafts, bolts, hammers, hand tools, gears, knives
High strength low allow (HSLA)
with alloying, higher TS and YS
Applications –– automobiles opines, nails, wire, pipe structural and sheet,
steel, railway cars
Medium Carbon Steel
0.25 –– 0.6 wt %C
Heat treated to achieve good mechanical properties
Used tampered condition (tampered martinsitic)
Strength - ductility combination can be tailored by heat treatment and alloying
(with Ni, Cr and Mo)
Applications: high strength structural components –– railway wheels, tracks,
crankshafts
High Carbon Steel
0.6 –– 1.4 %C wt
Hardest, strongest and least ductile carbon steel
Used in harden and tempered conditions
Can be alloyed with carbon and other metals to form vary hard and wear
resistance material (e.g C, Cr, Ni, W, Mo and V)
Applications: cutting tools, drills, embossing dies, saws, cutlery, paper cutters,
concrete drill, blacksmith tools.

Lecture 3
Types of metals alloys
4
Stainless--Steels
Highly resistance alloy to corrosion (rusting) in a variety of environment.
Mechanical integrity maintains
Consisting of iron-- carbon and > 11 wt% Cr. Ni, Mn may also be present
Example 0.08 C, 11.0 Cr, 1. Mn, 0.50 Ni, 0.75 Ti : S40900
Cast Irons
3.0-4.5 wt% C
Liquid at 1150-1300
o
C
Easily melted and amenable to casting
Gray Iron
graphite flakes
weak& brittle under tension
stronger under compression
excellent vibrational dampening
wear resistant
used e.g. as base structure for machines and heavy equipment
Ductile Iron
add Mg or Ce
graphite in nodules not flakes
matrix often pearlite- better ductility
Applications: valves, pump bodies, gears, crankshafts
White Iron
< 1wt% Si so harder but brittle
more cementite
Typical applications: connecting rods, transmission gears, pipe fittings,
flanges
Malleable Iron
- heat treat at 800-900
o
C
- graphite in rosettes
- more ductile

Lecture 3
Types of metals alloys
5
Nonferrous alloys
Aluminum and its Alloys
Pure aluminum is a silvery-white metal with many desirable characteristics. It is light,
nontoxic (as the metal), nonmagnetic and nonsparking. It is easily formed, machined,
and cast. Pure aluminum is soft and lacks strength, but alloys with small amounts of
copper, magnesium, silicon, manganese, and other elements have very useful
properties. Aluminum is an abundant element in the earth's crust, but it is not found
free in nature. The Bayer process is used to refine aluminum from bauxite, an
aluminum ore. Because of aluminum's mechanical and physical properties, it is an
extremely convenient and widely used metal. Aluminium alloys can be classified as;
cast or wrought alloys, examples are; Al--Li, Al-Cu-Si etc
Properties
Very lightweight (about 1/3 the mass of an equivalent volume of steel or
copper) but with alloying can become very strong.
excellent thermal conductor
excellent electrical conductor (on a weight-for-mass basis, aluminium will
conduct more than twice as much electricity as copper)
highly reflective to radiant energy in the electromagnetic spectrum
highly corrosion resistant in air and water (including sea water)
highly workable and can be formed into almost any structural shape
non-magnetic
non-toxic
Lecture 3
Types of metals alloys
6
Applications
door and window frames
high tension power lines, wires, cables, bus bars, components for television,
radios, refrigerators and air-conditioners
beverage cans, bottle tops
propellers, airplane and vehicle body sheet, gear boxes, motor parts
Al-Cu food/ chemicals handing and storage equipments.
Al-Cu-Mn-Zn- Cooking utensils.
Al-Zn-Mg-Cu-Cr
Aircraft structural parts
Copper and its Alloys
Properties
Good thermal and electrical conductivity
Ease of forming, ease of joining, and color.
However, copper and its alloys have relatively low strength-to-weight ratios
Low strengths at elevated temperatures.
Copper is resistant to corrosion in most atmospheres including marine and
industrial environments. It is corroded by oxidizing acids, halogens, sulphides
and ammonia based solutions
Copper and its alloys -- the brasses and bronzes -- are available in rod, plate,
strip, sheet, tube shapes, forgings, wire, and castings.
Applications
Pure Cu
Electrical and thermal conductors (cast Cu), transistor components,
coaxial cables rectifiers, lead in wires (cold--worked Cu)
Cu- Be- Co
moulds for plastic parts, bearings, valves, gears(cast Cu)
Cu--30Zn & Cu --40Zn (cold --work brass)
fasteners, locks, heal exchange
components, large nuts and bolts, plumbing accessories, pints and rivets.
Cu--4Si
bearing, belts, marine fittings

Lecture 3
Types of metals alloys
7
Magnesium and its Alloys
Properties
Low density metal (1.7g/cm
3
)
limited cold working
Relatively soft low elastic modulus
Easily oxidized
Magnesium is expensive compared to Al
Mg is difficult to cast and it burn in air (handling must be with care and cannot
be used temperature at high)
Mg- Al- Zn alloys –– improve strength
Applications
Mg--alloys replace engineering plastics that have similar densities
Handheld devices (chain saw, power told etc)
Automobiles (steering wheels, tyre ream, seat, frames)
Laptop computers, cam coders, TV etc
Titanium and its Alloys
Properties
Low density metal (4.5 g/cm cm
3
)
High melting point = 1668
o
C
elastic modulus = 107MPa
Chemical reactivity with other material at elevated temperatures
Corrosion resistance
Ti—alloys ,very high elastic modulus ~1400MPa, easily forged and machined,
high ductility
Applications
•
Pure Ti - Jet engine cases and airframe skins, corrosion--resistance equipment
for marine's applications chemical processing, industries.
•
Ti--5Al--2.55Sn – Gas turbine engine casing
•
Ti--6Al-4V – High strength prosthetic implants, orthopedics, airframe
structured components
Lecture 3
Types of metals alloys
8
Nickel and nickel alloys
•
Nickel (Ni) has strength, toughness, and corrosion resistance to metals.
•
Used in stainless steels and nickel-base alloys.
•
Alloys are used for high temperature applications, such as jet-engine
components and rockets.
Superalloys
•
Superalloys are high-temperature alloys use in jet engines, gas turbines and
reciprocating engines.
Refractory metals
•
Refractory metals have a high melting point and retain their strength at
elevated temperatures.
•
Applications are electronics, nuclear power and chemical industries.
•
Molybdenum, columbium, tungsten, and tantalum are referred to as refractory
metal
Other nonferrous metals
1. Beryllium
2. Zirconium
3. Low-melting-point metals:
- Lead
- Zinc
- Tin
4. Precious metals:
- Gold
- Silver
- Platinum
Lecture 3
Types of metals alloys
9
