
بورد عراقي
(
دكتوراه
)
في الطب الباطني
بورد عربي
(
دكتوراه
)
في الطب الباطني
بورد عراقي
(
دكتوراه
)
تخصص دقيق في أمراض
وقسطرة القلب والشرايين

DEFINITION
Heart failure (HF) is a clinical syndrome that
occurs in patients who, because of an inherited
or acquired abnormality of cardiac structure
and/or function, develop a constellation of
clinical symptoms (dyspnea and fatigue) and
signs (edema and rales) that lead to frequent
hospitalizations, a poor quality of life, and a
shortened life expectancy.

EPIDEMIOLOGY
The overall prevalence of HF in the adult population in developed countries
is 2%. rising with age, and affects 6–10% of people over age 65.
Although the relative incidence of HF is lower in women than in men, women
constitute at least one-half the cases of HF because of their longer life
expectancy.
The overall prevalence of HF is thought to be increasing, in part because
current therapies for cardiac disorders, such as myocardial infarction (MI),
valvular heart disease, and arrhythmias, are allowing patients to survive
longer.
Although HF once was thought to arise primarily in the setting of a
depressed left ventricular (LV) ejection fraction (EF), epidemiologic studies
have shown that approximately one-half of patients who develop HF have a
normal or preserved EF (EF 40–50%).
Accordingly, HF patients are now broadly categorized into one of two groups:
(1) HF with a depressed EF (commonly referred to as systolic failure) or (2)
HF with a preserved EF (commonly referred to as diastolic failure).

ETIOLOGY
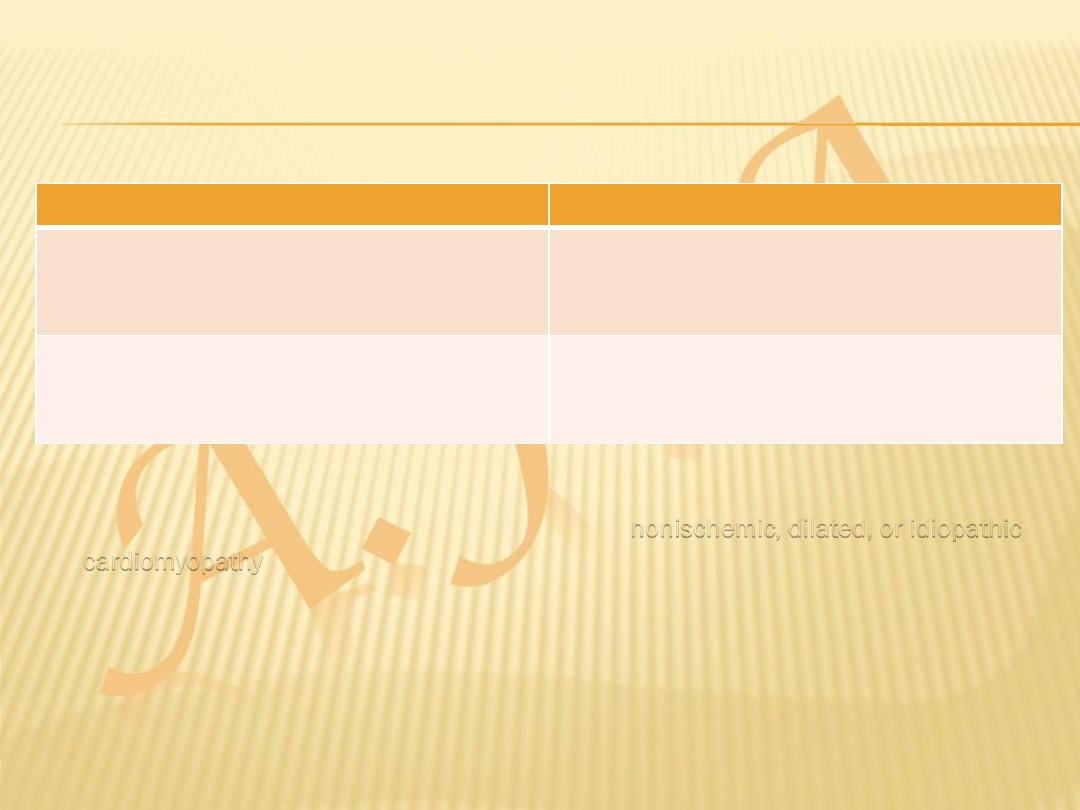
Depressed Ejection Fraction (<40%)
Preserved Ejection Fraction (>40–50%)
Coronary artery disease
• Myocardial infarction
• Myocardial ischemia
Pathologic hypertrophy
• Primary (hypertrophic cardiomyopathies)
• Secondary (hypertension)
Chronic pressure overload
• Hypertension
• Obstructive valvular disease
Aging
Chronic volume overload
• Regurgitant valvular disease
• Intracardiac shunting (L to R)
• Extrracardiac shunting
Restrictive cardiomyopathy
• Infiltrative disorders (amyloidosis,
sarcoidosis)
• Storage diseases (hemochromatosis)
• Fibrosis
• Endomyocardial disorders
Nonischemic dilated cardiomyopathy
• Familial or genetic disorders
• Toxic or drug-induced damage
• Metabolic disorder
• Viral or other infectious agents
Disorders of rate and rhythm
• Chronic bradyarrhythmias
• Chronic tachyarrhythmias
Pulmonary Heart Disease
High-Output States
Cor pulmonale
Metabolic disorders
• Thyrotoxicosis
• Nutritional disorders (beriberi)
Pulmonary vascular disorders
Excessive blood-flow requirements
• Systemic arteriovenous shunting
• Chronic anemia
• In 20–30% of the cases of HF with a depressed EF, the exact etiologic basis is not
known. These patients are referred to as having
nonischemic, dilated, or idiopathic
cardiomyopathy
if the cause is unknown

PROGNOSIS
Despite many recent advances in the evaluation
and management of HF, the development of
symptomatic HF still carries a poor prognosis.
Although it is difficult to predict prognosis in an
individual, patients with symptoms at rest [New
York Heart Association (NYHA) class IV] have a
30–70% annual mortality rate, whereas patients
with symptoms with moderate activity (NYHA class
II) have an annual mortality rate of 5–10%.
Thus, functional status is an important predictor
of patient outcome

NEW YORK HEART ASSOCIATION
CLASSIFICATION

PATHOGENESIS
HF may be viewed as a progressive disorder that is
initiated after an
index event
either damages the heart
muscle, with a resultant loss of functioning cardiac
myocytes, or, alternatively, disrupts the ability of the
myocardium to generate force, thereby preventing the
heart from contracting normally.
This index event may have an
abrupt onset
, as in the
case of a myocardial infarction (MI); it may have a
gradual or insidious onset
, as in the case of
hemodynamic pressure or volume overloading; or it may
be hereditary, as in the case of many of the genetic
cardiomyopathies.
Regardless of the nature of the inciting event, the
feature that is common to each of these index events is
that they all in some manner produce
a decline in the
pumping capacity of the heart.
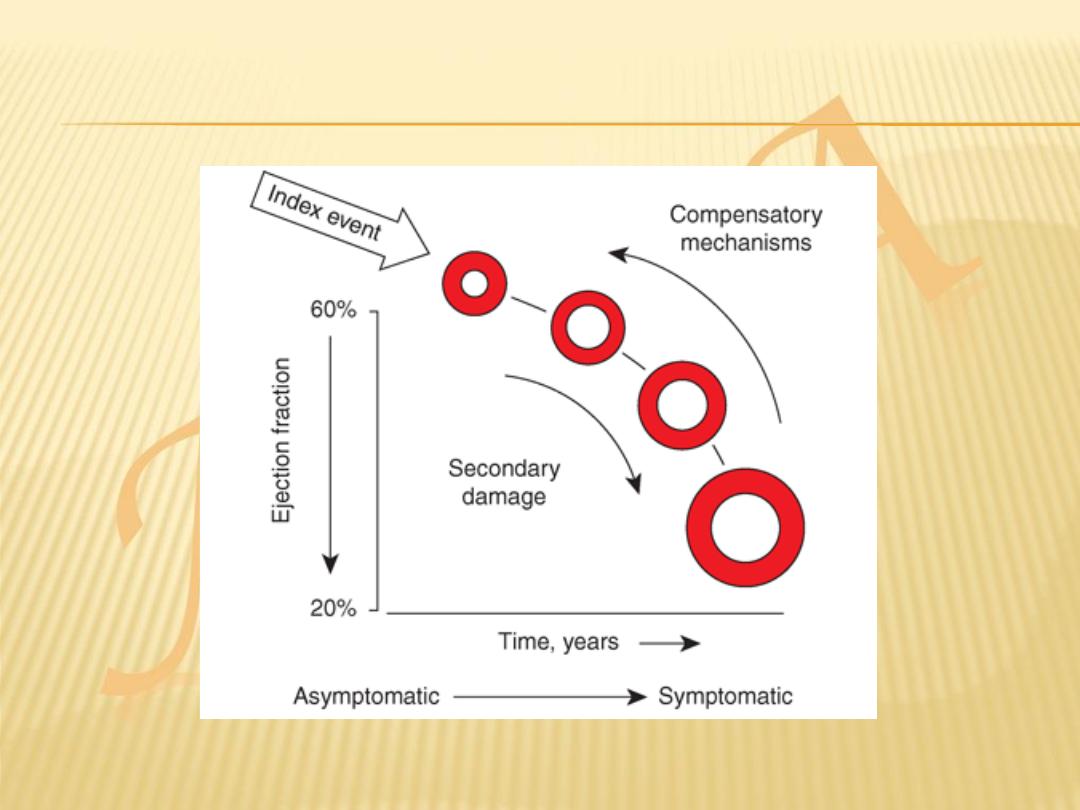

Although the precise reasons why patients with LV dysfunction may remain
asymptomatic is not certain, one potential explanation is that a number of
compensatory mechanisms
become activated in the presence of cardiac
injury and/or LV dysfunction allowing patients to sustain and modulate LV
function for a period of months to years.
The list of
compensatory mechanisms
that have been described thus far
include:
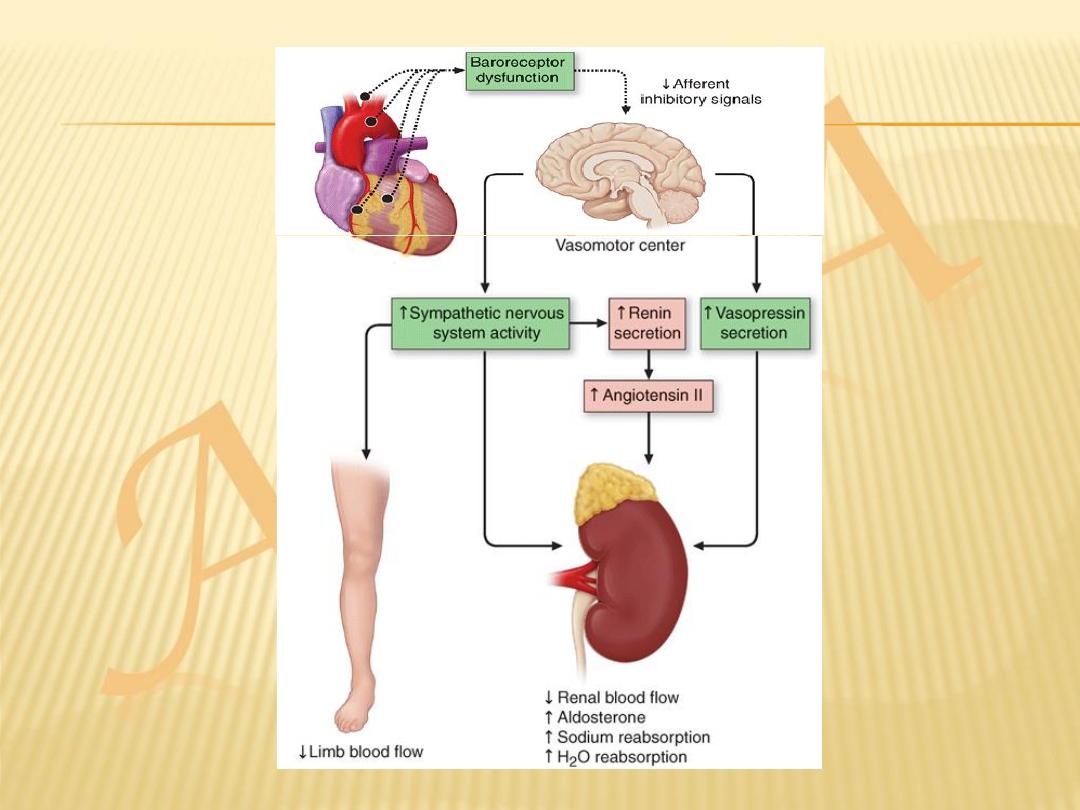
(1) activation of the renin-angiotensin-aldosterone (RAA) and adrenergic nervous
systems, which are responsible for maintaining cardiac output through
increased retention of salt and water
and (2) increased myocardial contractility.
In addition, there is activation of a family of countervailing vasodilatory
molecules, including the atrial and brain natriuretic peptides (ANP and
BNP), prostaglandins (PGE2 and PGI2), and nitric oxide (NO), that offsets
the excessive peripheral vascular vasoconstriction.

Thus, patients may remain asymptomatic or
minimally symptomatic for a period of years;
however, at some point patients become overtly
symptomatic, with a resultant striking increase in
morbidity and mortality rates.
the transition to symptomatic HF is accompanied
by increasing activation of neurohormonal,
adrenergic, and cytokine systems that lead to a
series of adaptive changes within the myocardium
collectively referred to as
LV remodeling
.

PATHOPHYSIOLOGICAL CHANGES IN
HEART FAILURE
Ventricular dilatation
Myocyte hypertrophy
Increased collagen synthesis
Altered myosin gene expression
Altered sarcoplasmic Ca2+-ATPase density
Increased ANP secretion
Salt and water retention
Sympathetic stimulation
Peripheral vasoconstriction

TYPES OF HEART FAILURE
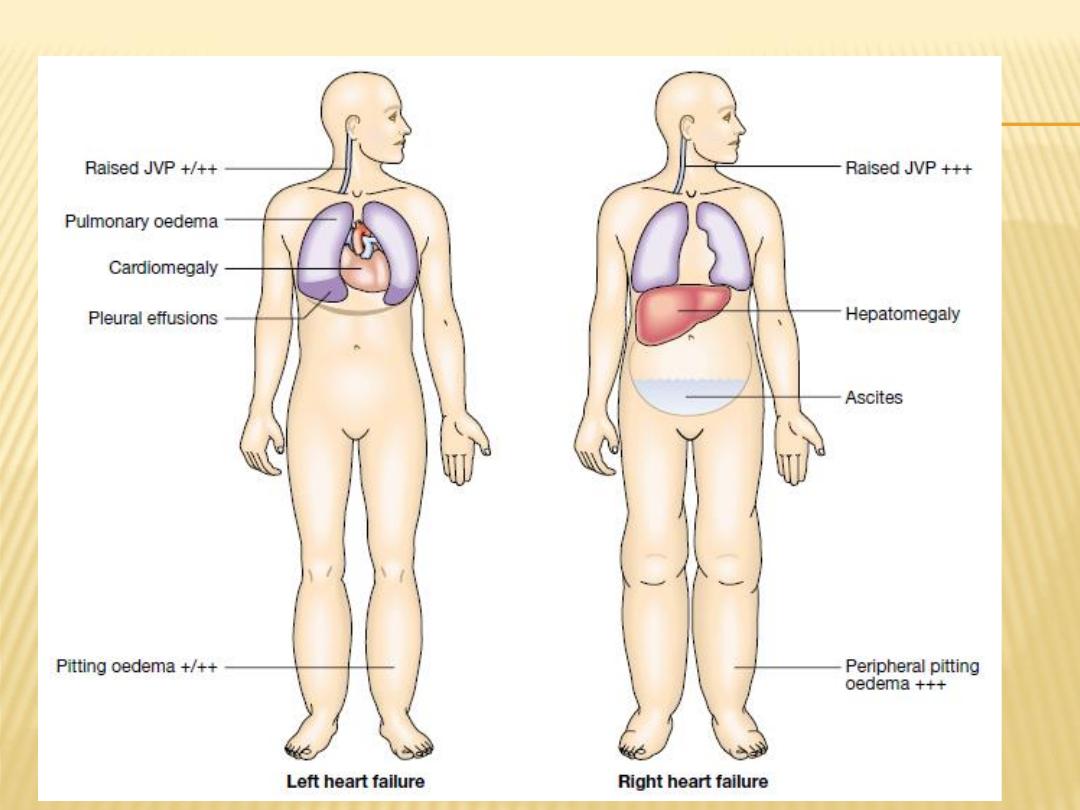
Left-sided heart failure
. There is a reduction in the
left ventricular output and an increase in the left
atrial or pulmonary venous pressure.
Right-sided heart failure
. There is a reduction in
right ventricular output for any given right atrial
pressure.
Causes of isolated right heart failure include chronic
lung disease (cor pulmonale), multiple pulmonary
emboli and pulmonary valvular stenosis.
Left, right and biventricular heart failure

Biventricular heart failure
. Failure of the left
and right heart may develop because
the disease process, such as dilated
cardiomyopathy or ischaemic heart disease, affects
both ventricles
or because disease of the left heart leads to
chronic elevation of the left atrial pressure,
pulmonary hypertension and right heart failure.

Diastolic and systolic dysfunction
Heart failure may develop as a result of
impaired myocardial contraction (systolic
dysfunction)
but can also be due to poor ventricular filling and
high filling pressures caused by abnormal
ventricular relaxation (diastolic dysfunction).
The latter is caused by a stiff non-compliant ventricle
and is commonly found in patients with left ventricular
hypertrophy.
NB
: Systolic and diastolic dysfunction often coexist,
particularly in patients with coronary artery disease.

Heart failure may develop suddenly, as in MI, or
gradually, as in progressive valvular heart
disease.
When there is gradual impairment of cardiac
function, a variety of compensatory changes
may take place.

High-output failure
Conditions such as large arteriovenous shunt,
beri-beri , severe anaemia or thyrotoxicosis can
occasionally cause heart failure due to an
excessively high cardiac output.

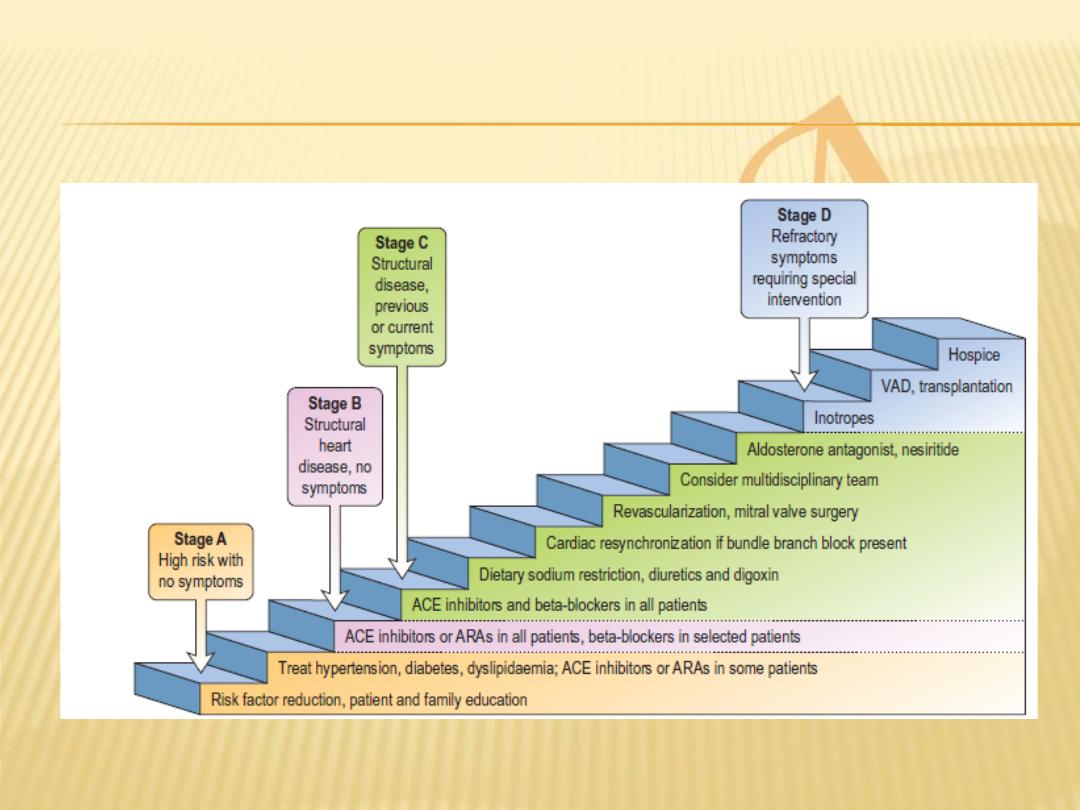
Stages of HF
Stage A
: patients at high risk for developing heart
failure but without structural disorders of the heart
Stage B
: patients with a structural disorder of the
heart but no symptoms of heart failure
Stage C
: patients with past or current symptoms of
heart failure associated with underlying structural
heart disease
Stage D
: patients with end-stage disease who
require specialized treatment strategies such as
mechanical circulatory support, continuous
inotropic infusions, cardiac transplantation, or
hospice care

Clinical Manifestations
Symptoms
The cardinal symptoms of HF are
fatigue
and
shortness of breath
.
Although fatigue traditionally has been ascribed to the
low cardiac output in HF, it is likely that skeletal-muscle
abnormalities and other noncardiac comorbidities (e.g.,
anemia) also contribute to this symptom.
Orthopnea
, which is defined as dyspnea occurring
in the recumbent position, is usually a later
manifestation of HF than is exertional dyspnea.
It results from redistribution of fluid from the
splanchnic circulation and lower extremities into the
central circulation during recumbency, with a resultant
increase in pulmonary capillary pressure.

Paroxysmal Nocturnal Dyspnea
(PND): This term
refers to acute episodes of severe shortness of
breath and coughing that generally occur at night
and awaken the patient from sleep, usually 1–3
hours after the patient retires. PND may be
manifest by coughing or wheezing, possibly
because of increased pressure in the bronchial
arteries leading to airway compression, along with
interstitial pulmonary edema that leads to
increased airway resistance
Cheyne-Stokes Respiration
( 40%)
Acute Pulmonary Edema

OTHER SYMPTOMS
Gastrointestinal symptoms
. Anorexia, nausea, and early
satiety associated with abdominal pain and fullness are
common complaints and may be related to edema of
the bowel wall and/or a congested liver.
Cerebral symptoms
such as confusion, disorientation,
and sleep and mood disturbances may be observed in
patients with severe HF, particularly elderly patients with
cerebral arteriosclerosis and reduced cerebral
perfusion.
Nocturia
is common in HF and may contribute to
insomnia.

Physical Examination
In mild or moderately severe HF
, the patient appears to be in no
distress at rest except for feeling uncomfortable when lying flat for
more than a few minutes.
In more severe HF
, the patient must sit upright, may have labored
breathing, and may not be able to finish a sentence because of
shortness of breath.
Systolic blood pressure
may be normal or high in early HF, but
generally is reduced in advanced HF because of severe LV
dysfunction.
The pulse pressure
may be diminished, reflecting a reduction in
stroke volume.
Sinus tachycardia
is a nonspecific sign caused by increased
adrenergic activity.
Peripheral vasoconstriction
leading to cool peripheral extremities and
cyanosis of the lips and nail beds is also caused by excessive
adrenergic activity.
General Appearance and Vital Signs

JUGULAR VEINS
Elevated

PULMONARY EXAMINATION
Pulmonary crackles (rales or crepitations)
result from the transudation of fluid from the
intravascular space into the alveoli.
In patients with pulmonary edema, rales may be
heard widely over both lung fields and may be
accompanied by expiratory wheezing (
cardiac
asthma
).
Pleural effusions(unilateral on the right or
bilateral)

CARDIAC EXAMINATION
Cardiomegaly
, the point of maximal impulse (PMI)
usually is displaced below the fifth intercostal space
and/or lateral to the midclavicular line, and the impulse
is palpable over two interspaces.
In some patients, a
third heart sound
(S
3
) is audible and
palpable at the apex.
Patients with enlarged or hypertrophied right ventricles
may have a sustained and prolonged
left parasternal
impulse
extending throughout systole.
A fourth heart sound
(S
4
) is not a specific indicator of HF
but is usually present in patients with diastolic
dysfunction.
The
murmurs of mitral and tricuspid regurgitation
are
frequently present in patients with advanced HF.

ABDOMEN AND EXTREMITIES
Hepatomegaly
is an important sign in patients with
HF. When it is present, the enlarged liver is
frequently tender and may pulsate during systole if
tricuspid regurgitation is present.
Ascites
, a late sign, occurs as a consequence of
increased pressure in the hepatic veins and the
veins draining the peritoneum.
Jaundice
, also a late finding in HF, results from
impairment of hepatic function secondary to
hepatic congestion and hepatocellular hypoxemia
and is associated with elevations of both direct
and indirect bilirubin.

Peripheral edema
is a cardinal manifestation of
HF, but it is nonspecific and usually is absent in
patients who have been treated adequately
with diuretics.
Long-standing edema may be associated with
indurated and pigmented skin.
Cardiac Cachexia

Diagnosis
The diagnosis of heart failure should not be
based on history and clinical findings; it
requires evidence of cardiac dysfunction with
appropriate investigation using objective
measures of left ventricular structure and
function (usually echocardiography).

INVESTIGATIONS IN HEART FAILURE
Blood tests
: complete blood count, a panel of
electrolytes, blood urea nitrogen, serum
creatinine, hepatic enzymes, and a urinalysis.
Selected patients should have assessment for
diabetes mellitus
(fasting serum glucose or oral
glucose tolerance test),
dyslipidemia
(fasting lipid
panel), and
thyroid abnormalities
(thyroid-
stimulating hormone level).

BIOMARKERS
Circulating levels of natriuretic peptides are useful
adjunctive tools in the diagnosis of patients with HF.
Both B-type natriuretic peptide (BNP) and N-terminal
pro-BNP, which are released from the failing heart, are
relatively sensitive markers for the presence of HF with
depressed EF;
they also are elevated in HF patients with a preserved EF,
albeit to a lesser degree.
However, it is important to recognize that natriuretic
peptide levels increase with
age
and
renal impairment
,
are more elevated in
women
, and can be elevated in
right HF
from any cause.
Levels can be falsely low in
obese patients
and may
normalize in some patients after appropriate treatment.

Chest X-ray.
Look for cardiomegaly, pulmonary
congestion with upper lobe diversion, fluid in
fissures, Kerley B lines, and pulmonary oedema.
Electrocardiogram
for ischaemia, chamber
enlargement or arrhythmia.
Echocardiography.
Cardiac chamber dimension,
systolic and diastolic function, regional wall motion
abnormalities, valvular heart disease,
cardiomyopathies.
Stress echocardiography.
Assessment of viability
in dysfunctional myocardium

Nuclear cardiology.
Radionucleotide angiography
(RNA) can quantify ventricular ejection fraction,
single photon-emission computed tomography (SPECT)
or
positron emission tomography (PET)
can
demonstrate myocardial ischaemia and viability in
dysfunctional myocardium.
CMR (cardiac MRI).
Assessment of viability in
dysfunctional myocardium with the use of
dobutamine for contractile reserve or with
gadolinium for delayed enhancement (‘infarct
imaging’).
Cardiac catheterization.
Diagnosis of ischaemic
heart failure (and suitability for revascularization),

Cardiac biopsy
.
Diagnosis of cardiomyopathies, e.g. amyloid,
follow-up of transplanted patients to assess rejection.
Cardiopulmonary exercise testing
. Peak oxygen
consumption (VO2) is predictive of hospital
admission and death in heart failure.
A 6-minute exercise walk is an alternative.
Ambulatory ECG monitoring (Holter).
In patients
with suspected arrhythmia.

Complications
In advanced heart failure, the following may occur:
Renal failure
is caused by poor renal perfusion
due to a low cardiac output and may be
exacerbated by diuretic therapy, angiotensin-
converting enzyme (ACE) inhibitors and
angiotensin receptor blockers.
Hypokalaemia
may be the result of treatment with
potassium-losing diuretics or hyperaldosteronism
caused by activation of the renin–angiotensin
system and impaired aldosterone metabolism due
to hepatic congestion.

Hyperkalaemia
may be due to the effects of drug
treatment, particularly the combination of ACE inhibitors
and spironolactone (which both promote potassium
retention), and renal dysfunction.
Hyponatraemia
is a feature of severe heart failure and
is a poor prognostic sign.
Impaired liver function
is caused by hepatic venous
congestion and poor arterial perfusion, which frequently
cause mild jaundice and abnormal liver function tests;
reduced synthesis of clotting factors can make
anticoagulant control difficult.

Thromboembolism
.
Deep vein thrombosis and pulmonary embolism may
occur due to the effects of a low cardiac output and
enforced immobility,
whereas systemic emboli may be related to
arrhythmias, atrial flutter or fibrillation, or intracardiac
thrombus complicating conditions such as mitral
stenosis, MI or left ventricular aneurysm.
Atrial and ventricular arrhythmias
are very
common and may be related to
electrolyte changes (e.g. hypokalaemia,
hypomagnesaemia),
the underlying structural heart disease,
and the pro-arrhythmic effects of increased circulating
catecholamines or drugs.

Management of HF with Depressed Ejection
Fraction (<40%)
Clinicians should aim to screen for and
treat comorbidities
such as
hypertension, CAD, diabetes mellitus, anemia, and sleep-disordered
breathing, as these conditions tend to exacerbate HF.
HF patients should be advised to
stop smoking
and to
limit alcohol
consumption
to two standard drinks per day in men or one per day in
women.
Patients suspected of having an alcohol-induced cardiomyopathy should be
urged to abstain from alcohol consumption indefinitely.
Extremes of temperature and heavy physical exertion
should be avoided.
Certain
drugs
are known to make HF worse and should be avoided .For
example, nonsteroidal anti-inflammatory drugs, including cyclooxygenase 2
inhibitors, are not recommended in patients with chronic HF because the
risk of renal failure and fluid retention is markedly increased in the
presence of reduced renal function or ACE inhibitor therapy.
Patients should
receive immunization
with influenza and pneumococcal
vaccines to prevent respiratory infections.
It is equally important to
educate
the patient and family about HF, the
importance of proper diet, and the importance of compliance with the
medical regimen.
General Measures

Diuretics
Many of the clinical manifestations of moderate to severe HF result
from excessive salt and water retention that leads to volume
expansion and congestive symptoms.
Diuretics are the only pharmacologic agents that can adequately
control fluid retention in advanced HF, and they should be used to
restore and maintain normal volume status in patients with
congestive symptoms (dyspnea, orthopnea, edema) or signs of
elevated filling pressures (rales, jugular venous distention, peripheral
edema).
Furosemide, torsemide, and bumetanide
act at the loop of Henle
(loop diuretics) by reversibly inhibiting the reabsorption of Na
+
, K
+
,
and Cl
–
in the thick ascending limb of Henle's loop;
thiazides and metolazone
reduce the reabsorption of Na
+
and Cl
–
in
the first half of the distal convoluted tubule;
and potassium-sparing diuretics
such as spironolactone act at the
level of the collecting duct.

Preventing Disease Progression
Drugs that interfere with excessive activation of
the RAA system and the adrenergic nervous
system can relieve the symptoms of HF with a
depressed EF by stabilizing and/or reversing
cardiac remodeling.
In this regard, ACE inhibitors and beta blockers
have emerged as the cornerstones of modern
therapy for HF with a depressed EF.

ACE INHIBITORS
There is overwhelming evidence that ACE inhibitors should
be used in symptomatic and asymptomatic patients with a
depressed EF (<40%).
ACE inhibitors interfere with the renin-angiotensin system by
inhibiting the enzyme that is responsible for the conversion
of angiotensin I to angiotensin II.
ACE inhibitors stabilize
LV remodeling, improve symptoms,
reduce hospitalization, and prolong life.
Because fluid retention can attenuate the effects of ACE
inhibitors, it is preferable to optimize the dose of diuretic
before starting the ACE inhibitor.
ACE inhibitors should be initiated in low doses, followed by
gradual increments if the lower doses have been well
tolerated.
Higher doses are more effective than lower doses in
preventing hospitalization.

Angiotensin Receptor Blockers
These drugs are well tolerated in patients who are intolerant of ACE
inhibitors because of
cough, skin rash, and angioedema.
ARBs should be used in symptomatic and asymptomatic patients
with an EF <40% who are ACE-intolerant for reasons other than
hyperkalemia or renal insufficiency .
Although ACE inhibitors and ARBs inhibit the renin-angiotensin
system, they do so by different mechanisms. Whereas ACE inhibitors
block the enzyme responsible for converting angiotensin I to
angiotensin II, ARBs block the effects of angiotensin II on the
angiotensin type 1 receptor.
When given in concert with beta blockers, ARBs reverse the process
of LV remodeling, improve patient symptoms, prevent hospitalization,
and prolong life.

B-Adrenergic Receptor Blockers
Beta-blocker therapy represents a major advance in the
treatment of patients with a depressed EF.
These drugs interfere with the harmful effects of
sustained activation of the adrenergic nervous system
by competitively antagonizing one or more adrenergic
receptors (a
1
, B
1
, and B
2
).
When given in concert with ACE inhibitors, beta blockers
reverse the process of LV remodeling, improve patient
symptoms, prevent hospitalization, and prolong life.
Therefore, beta blockers are indicated for patients with
symptomatic or asymptomatic HF and a depressed EF
<40%.

Analogous to the use of ACE inhibitors, beta
blockers should be initiated in low doses , followed
by gradual increments in the dose if lower doses
have been well tolerated.
However, unlike ACE inhibitors, which may be
titrated upward relatively rapidly, the titration of
beta blockers should proceed no more rapidly than
at 2-week intervals, because the initiation and/or
increased dosing of these agents may lead to
worsening fluid retention consequent to the
withdrawal of adrenergic support to the heart and
the circulation.

ALDOSTERONE ANTAGONISTS
Although classified as potassium-sparing diuretics,
drugs that block the effects of aldosterone
(spironolactone or eplerenone) have beneficial effects
that are independent of the effects of these agents on
sodium balance.
Although ACE inhibition may transiently decrease
aldosterone secretion, with chronic therapy there is a
rapid return of aldosterone to levels similar to those
before ACE inhibition.
Accordingly, the administration of an aldosterone
antagonist is recommended for patients with NYHA
class II-IV HF who have a depressed EF (<35%) and are
receiving standard therapy, including diuretics, ACE
inhibitors, and beta blockers.

CARDIAC GLYCOSIDES
Digoxin is a cardiac glycoside that is indicated
in:
1.
Patients in atrial fibrillation with heart failure.
2.
It is used as add-on therapy in symptomatic
heart failure patients (in sinus rhythm) already
receiving ACEI and beta-blockers as it is
demonstrated that digoxin result in reduction
of hospital admissions in those patients .

VASODILATORS AND NITRATES
The combination of hydralazine and nitrates
reduces afterload and pre-load and is used in
patients intolerant of ACEI or ARB.
this combination improved survival in patients
with chronic heart failure.

INOTROPIC AND VASOPRESSOR AGENTS
Intravenous inotropes and vasopressor agents
are used in patients with chronic heart failure
who are not responding to oral medication.
Although they produce haemodynamic
improvements they have not been shown to
improve long-term mortality when compared
with placebo.

Anticoagulation and Antiplatelet Therapy
Treatment with warfarin [goal international normalized
ratio (INR) 2–3] is recommended for patients with:
1.
HF and chronic or paroxysmal atrial fibrillation
2.
or with a history of systemic or pulmonary emboli,
including stroke or transient ischemic attack.
3.
Patients with recent MI & documented LV thrombus
should be treated with warfarin (goal INR 2–3) for the initial
3 months after the MI unless there are contraindications to
its use.

Aspirin is recommended in HF patients with
ischemic heart disease for the prevention of MI
and death.
However, lower doses of aspirin (100mg) may
be preferable because of the concern of
worsening of HF at higher doses.

Management of Cardiac Arrhythmias
Atrial fibrillation occurs in 15–30% of patients with HF and is a
common cause of cardiac decompensation.
Most antiarrhythmic agents, with the exception of amiodarone and
dofetilide, have negative inotropic effects and are proarrhythmic.
Amiodarone is a class III antiarrhythmic that has few or no negative
inotropic and/or proarrhythmic effects and is effective against most
supraventricular arrhythmias.
Amiodarone is the preferred drug for restoring and maintaining sinus
rhythm, and it may improve the success of electrical cardioversion in
patients with HF.
Amiodarone increases the level of phenytoin and digoxin and
prolongs the INR in patients taking warfarin.
Therefore, it is often necessary to reduce the dose of these drugs by as
much as 50% when initiating therapy with amiodarone.
The risk of adverse events such as hyperthyroidism, hypothyroidism,
pulmonary fibrosis, and hepatitis is relatively low, particularly when
lower doses of amiodarone are used (100–200 mg/d).

Implantable cardiac defibrillators (ICDs)
are highly
effective in treating recurrences of sustained
ventricular tachycardia and/or ventricular
fibrillation in HF patients with recurrent
arrhythmias and/or cardiac syncope, and they may
be used as stand-alone therapy or in combination
with amiodarone and/or a beta blocker .
There is no role for treating ventricular arrhythmias
with an antiarrhythmic agent without an ICD.

NON-PHARMACOLOGICAL TREATMENT OF
HEART FAILURE
Coronary artery bypass surgery or
percutaneous coronary intervention may
improve function in areas of the myocardium
that are ‘hibernating’ because of inadequate
blood supply,
Revascularization

CARDIAC RESYNCHRONIZATION
Approximately one-third of patients with a depressed EF and symptomatic HF (NYHA
class III–IV) manifest a QRS duration >120 ms. (Bundle Branch Block, BBB)
This ECG finding of abnormal inter- or intraventricular conduction has been used to
identify patients with dyssynchronous ventricular contraction.
The mechanical consequences of ventricular dyssynchrony include suboptimal
ventricular filling, a reduction in LV contractility, prolonged duration (and therefore
greater severity) of mitral regurgitation, and paradoxical septal wall motion.
Biventricular pacing, also termed cardiac resynchronization therapy (CRT),
stimulates both ventricles nearly simultaneously, thereby improving the coordination
of ventricular contraction and reducing the severity of mitral regurgitation.
When CRT is added to optimal medical therapy in patients in sinus rhythm,
there is a
significant decrease in patient mortality rates and hospitalization and a reversal of
LV remodeling, as well as improved quality of life and exercise capacity.
Accordingly, CRT is recommended for patients in sinus rhythm with an
1.
EF <35%
2.
a QRS >150 ms (with LBBB configuration)
3.
those who remain symptomatic (NYHA II–IV) despite optimal medical therapy.

IMPLANTABLE CARDIAC DEFIBRILLATORS
The prophylactic implantation of ICDs in patients
with mild to moderate HF (NYHA class II–III) has
been shown to reduce the incidence of sudden
cardiac death in patients with ischemic or
nonischemic cardiomyopathy.
Accordingly, implantation of an ICD should be
considered for patients in NYHA class II–III HF with
a depressed EF of <35% who are already on
optimal background therapy, including an ACE
inhibitor (or ARB), a beta blocker, and an
aldosterone antagonist.

CARDIAC TRANSPLANTATION
Cardiac transplantation has become the treatment
of choice for younger patients with severe
intractable heart failure, whose life-expectancy is
less than 6 months.
With careful recipient selection, the expected 1-
year survival for patients following transplantation
is over 90%, and is 75% at 5 years.
Irrespective of survival, quality of life is
dramatically improved for the majority of patients.
The availability of heart transplantation is limited.

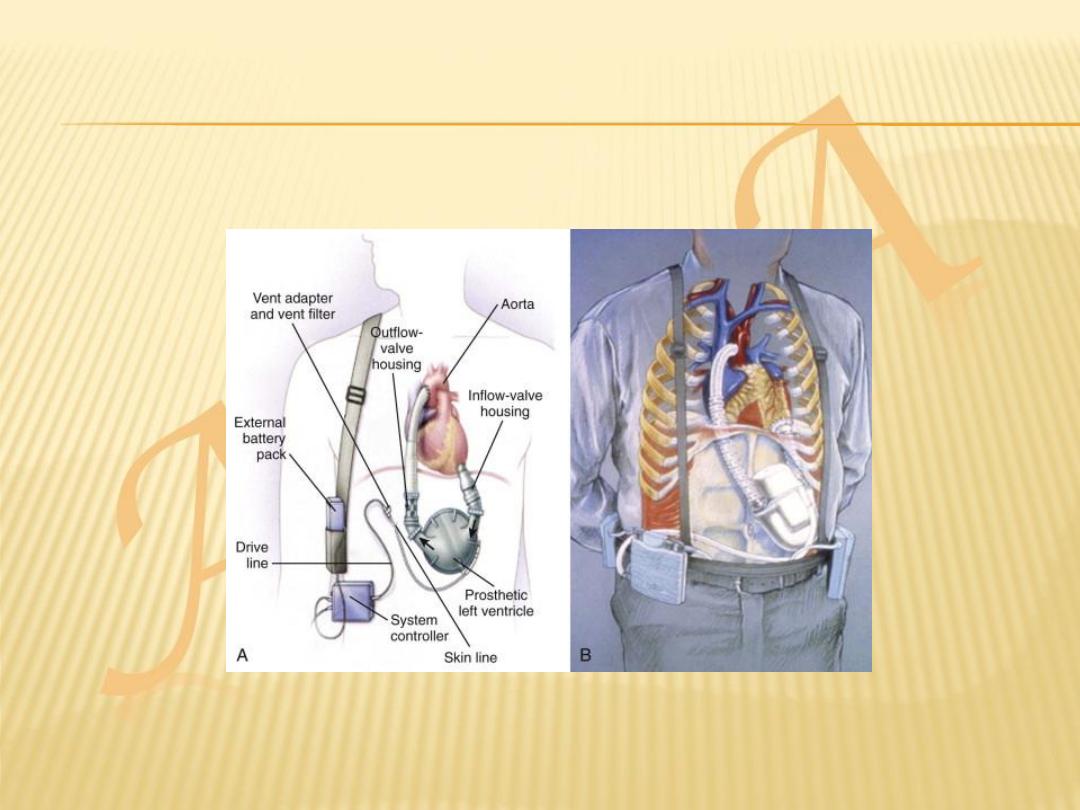
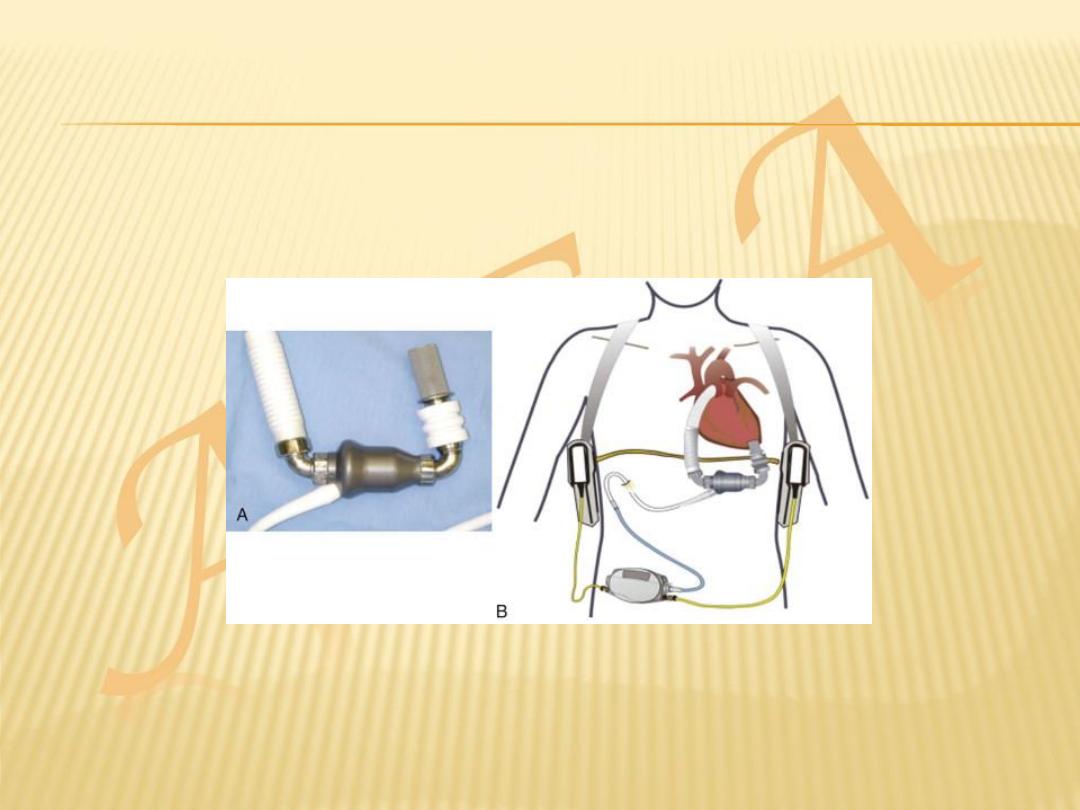
VENTRICULAR ASSIST DEVICES
Because of the limited supply of donor organs,
ventricular assist devices (VADs) have been
employed as:
a bridge to cardiac transplantation
potential long-term ‘destination’ therapy
short-term restoration therapy following a
potentially reversible insult such as viral
myocarditis.

MANAGEMENT OF HF WITH A PRESERVED
EJECTION FRACTION (>40–50%)
Despite the wealth of information with respect to the
evaluation and management of HF with a depressed EF,
there are no proven and/or approved pharmacologic or
device therapies for the management of patients with
HF and a preserved EF.
Therefore, it is recommended that :
1.
Control systolic and diastolic hypertension
2.
Control ventricular rate in patients with atrial fibrillation
3.
Diuretics to control pulmonary congestion and peripheral
edema

Thank you
