
Saadeldeen majeed
Professor of cardiology and internal medicine
Power point 1
4
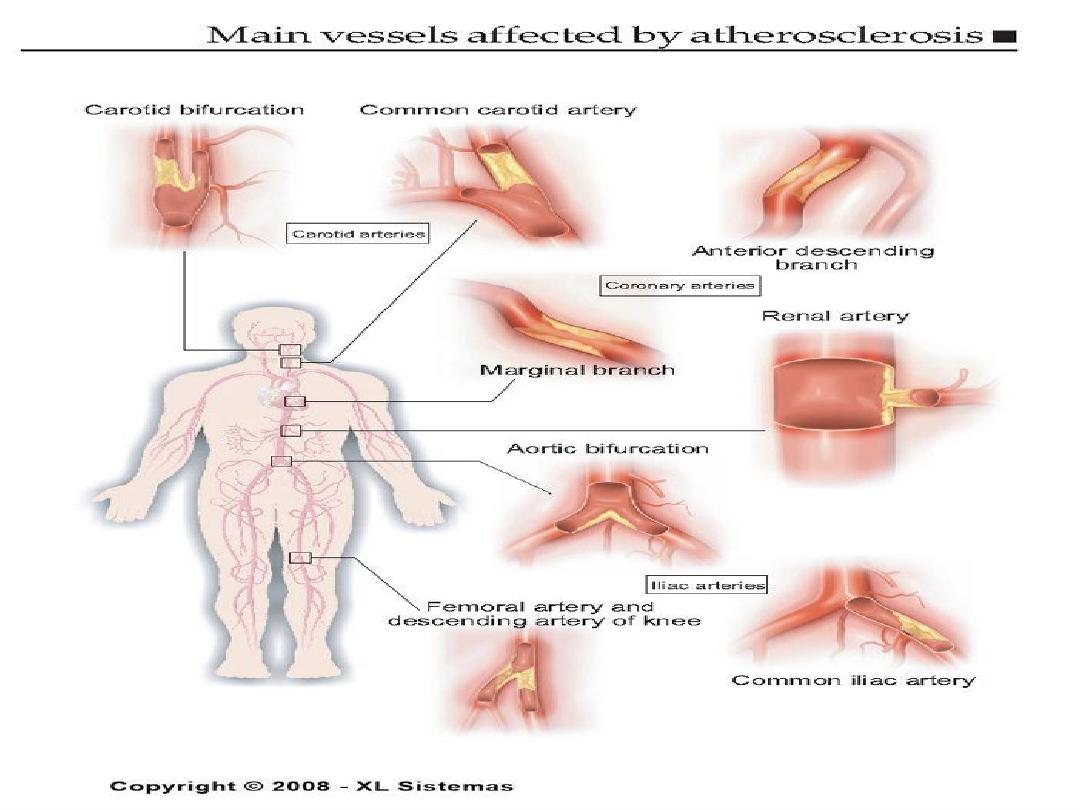
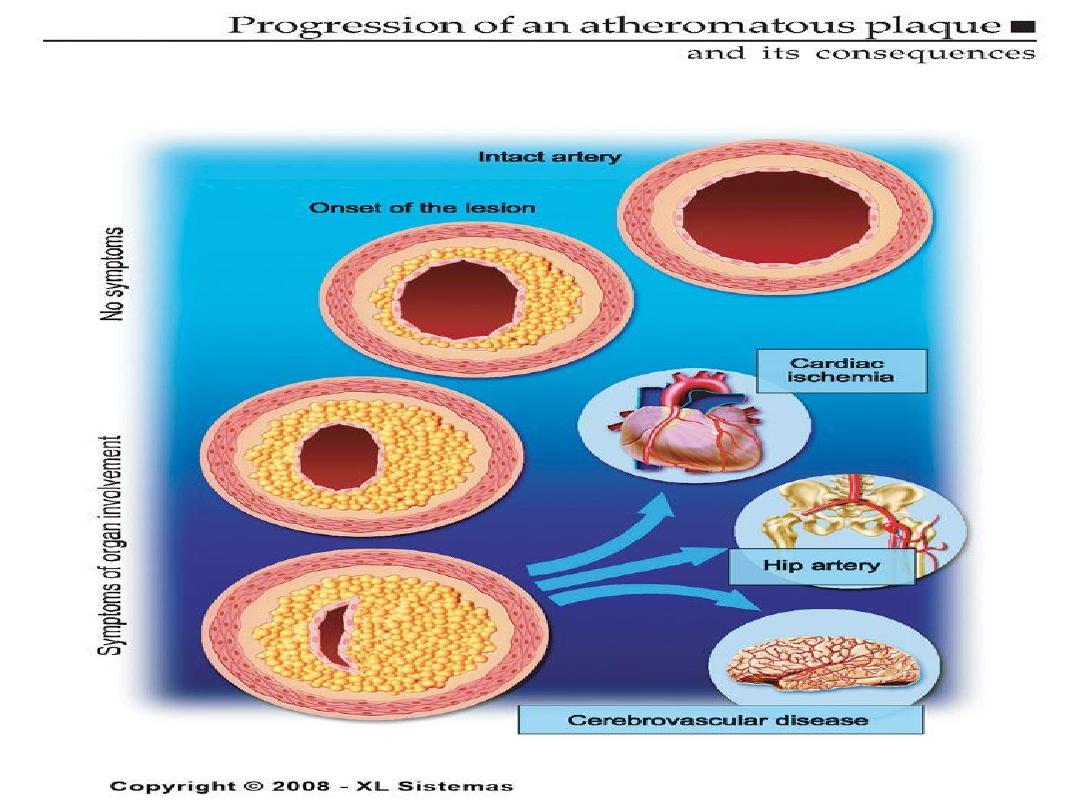
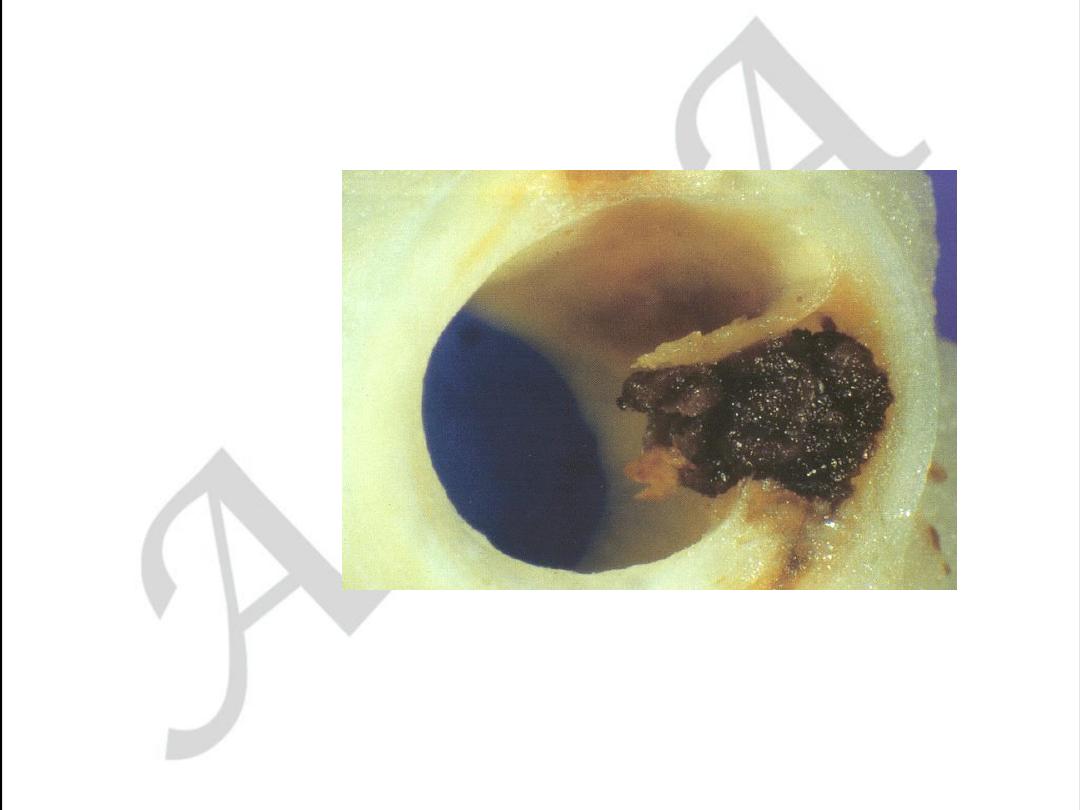
Aortic form of atherosclerosis
Various forms of aorta lesion
What Does It Look Like?
•
The coronary
artery is narrowed
reducing the flow
of oxygen to the
heart.
•
It is easier for
plaque to get
inside a narrower
artery.
6
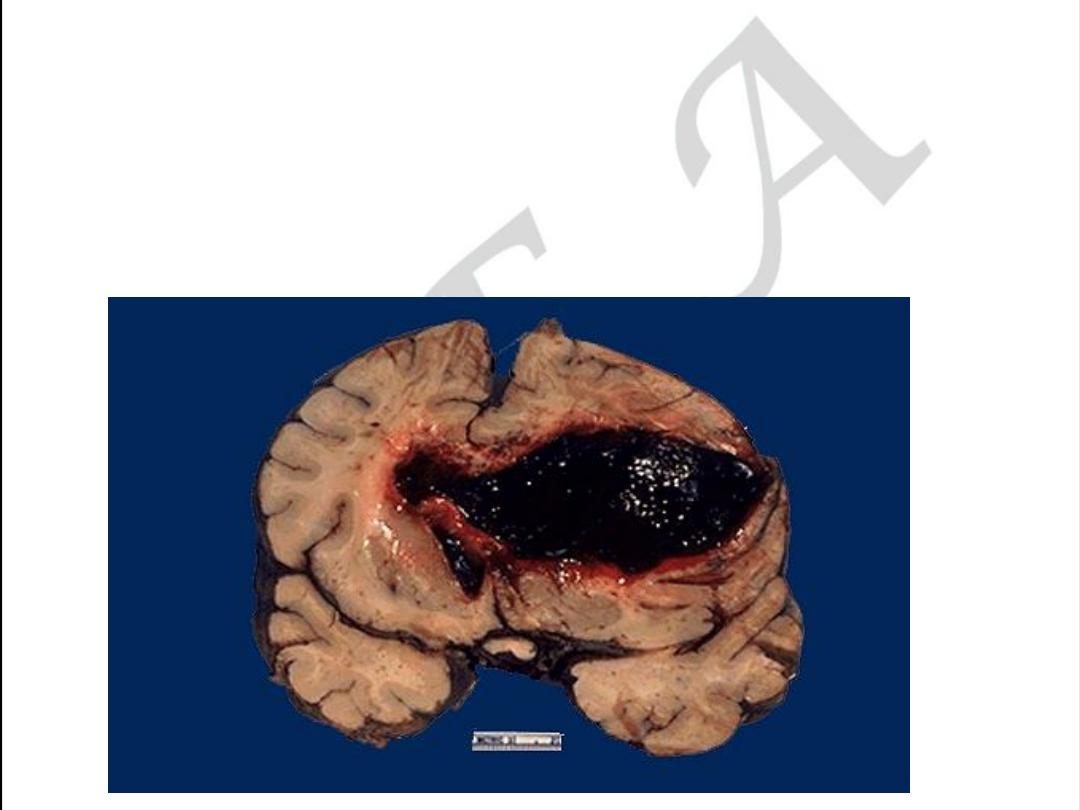
CEREBRAL FORM OF ATHEROSCLEROSIS
Acute form may be as Hemorrhage within
The brain due to rupture
Of atherosclerotic aneurism
7
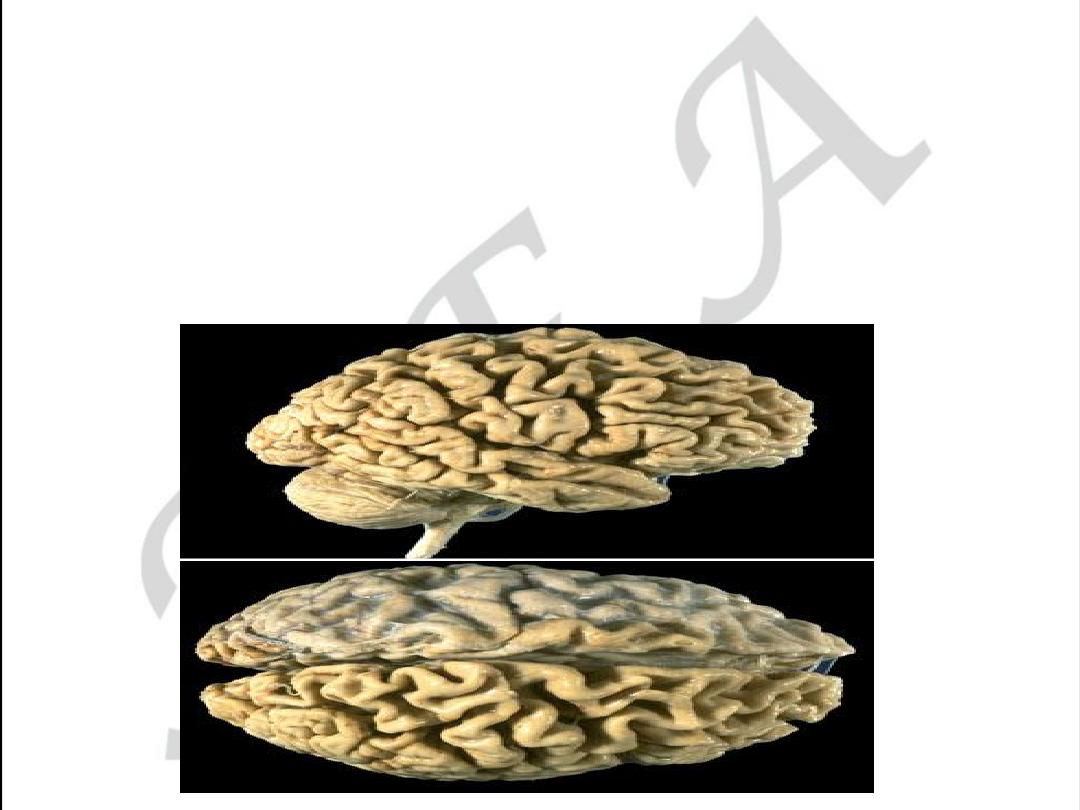
CEREBRAL FORM OF ATHEROSCLEROSIS
• Chronic form may be as encephalopathy
With cerebral atrophy (decreasing memory)
9
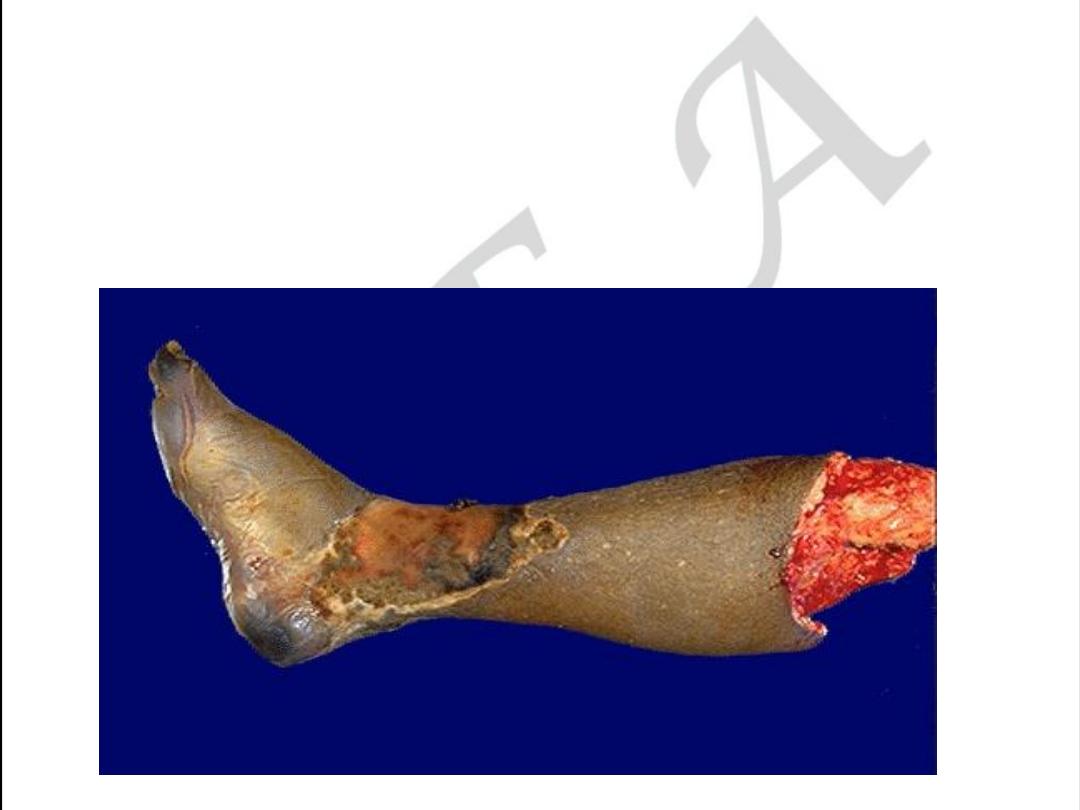
Extremity form of atherosclerosis
• Acute form may be as gangrenous necrosis.
10
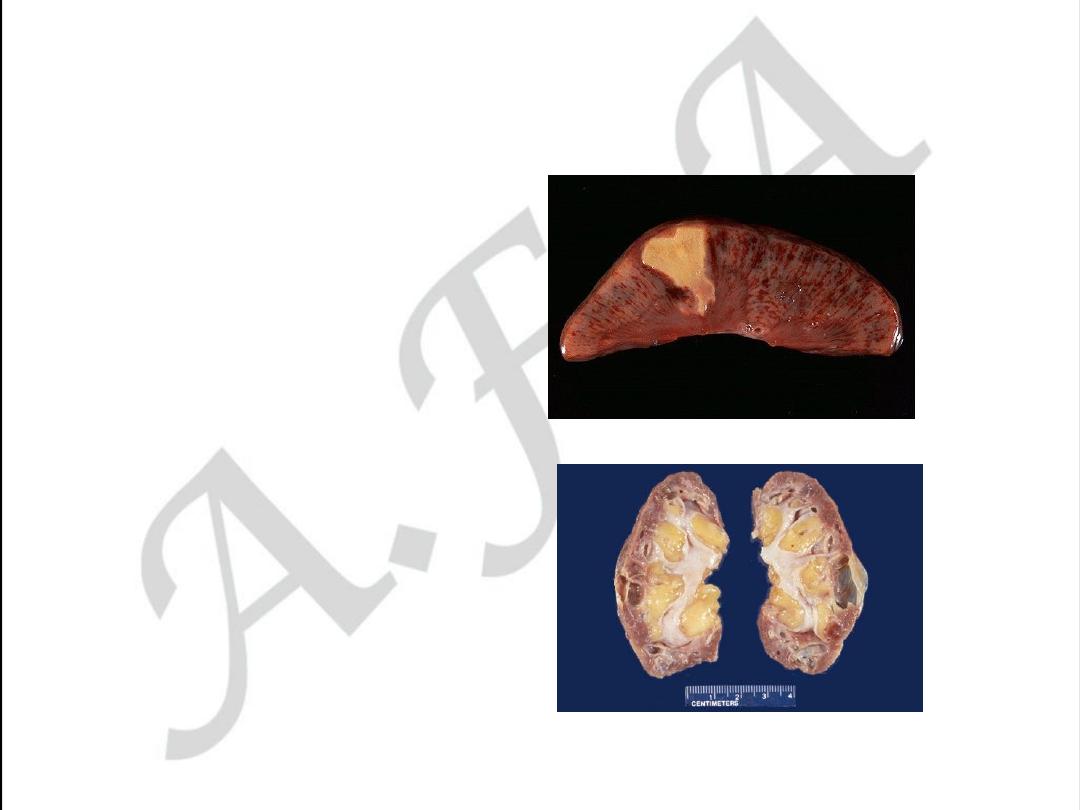
RENAL FORM OF ATHEROSCLEROSIS
• Acute form may be as
infarction
• Chronic form is called
Atherosclerotic
Nephrosclerosis or
Primary contracted
kidney
11
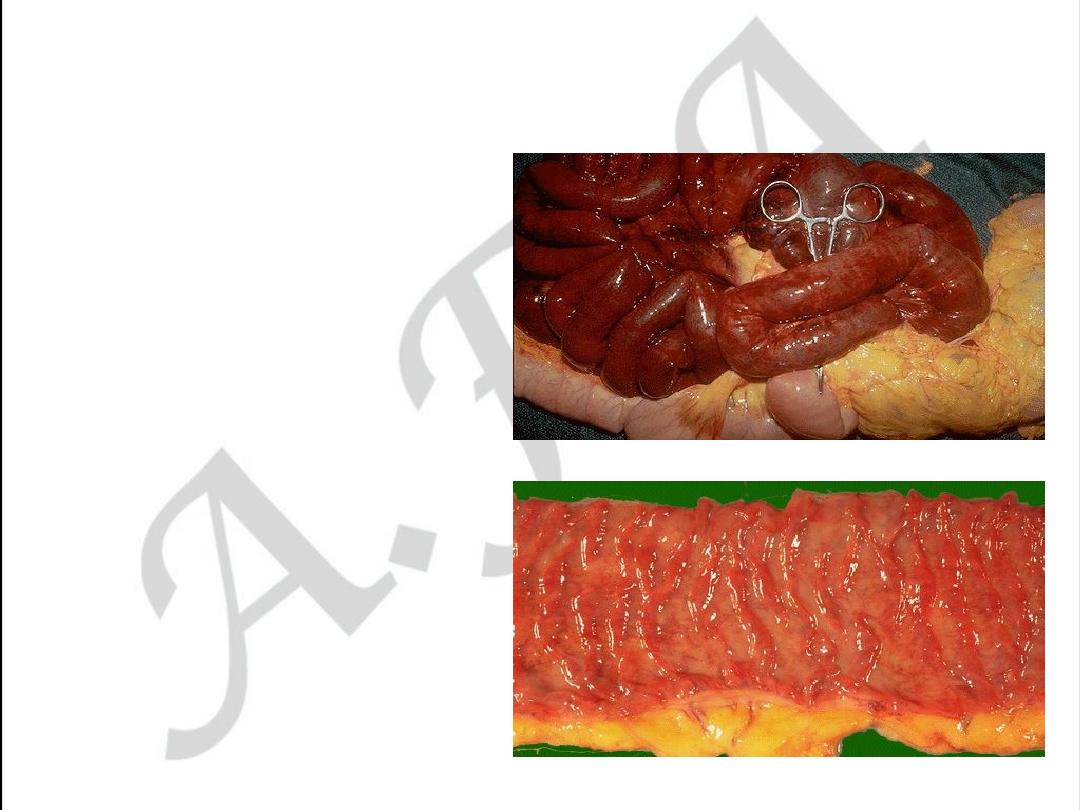
Intestinal form of atherosclerosis
• Acute form may be as
gangrenous necrosis of
the intestine
• Chronic form may be as
ischemic enterocolitis

13
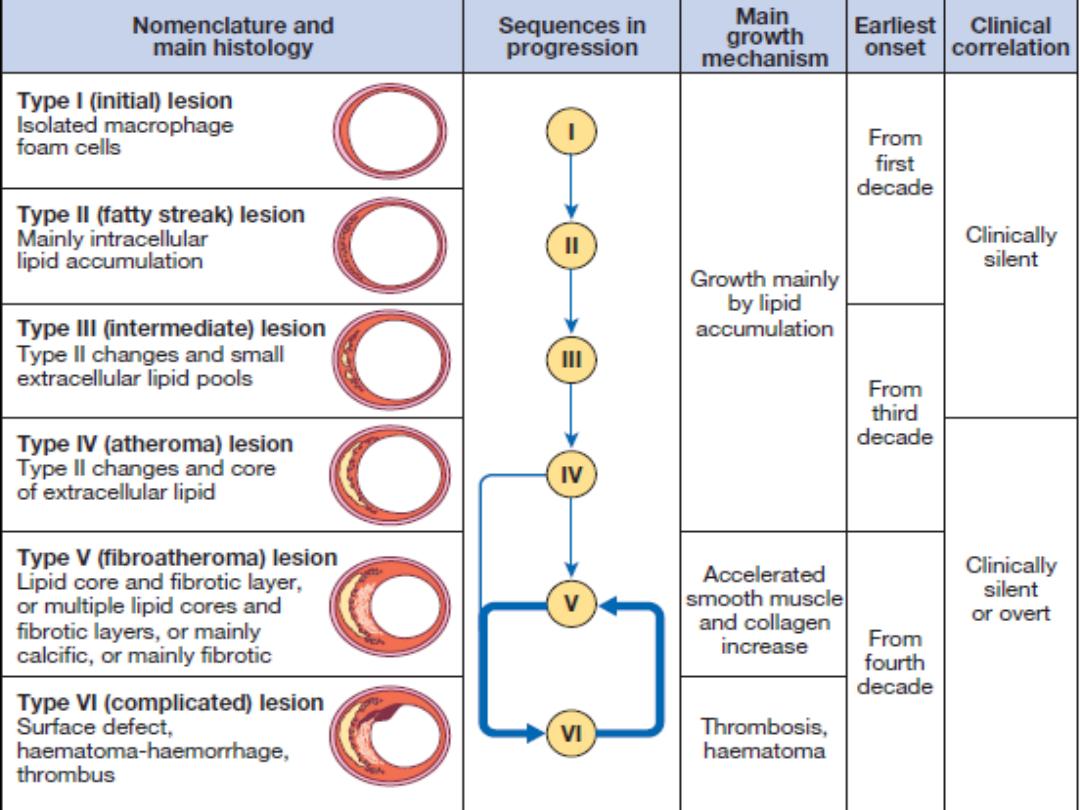
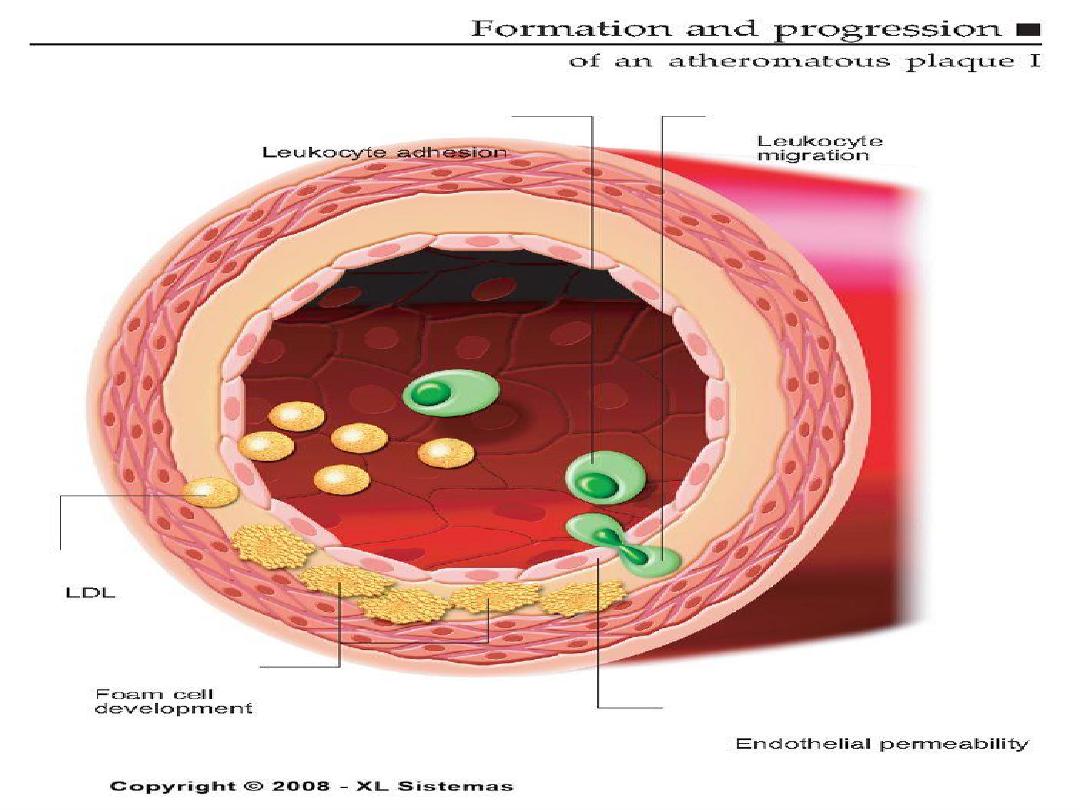
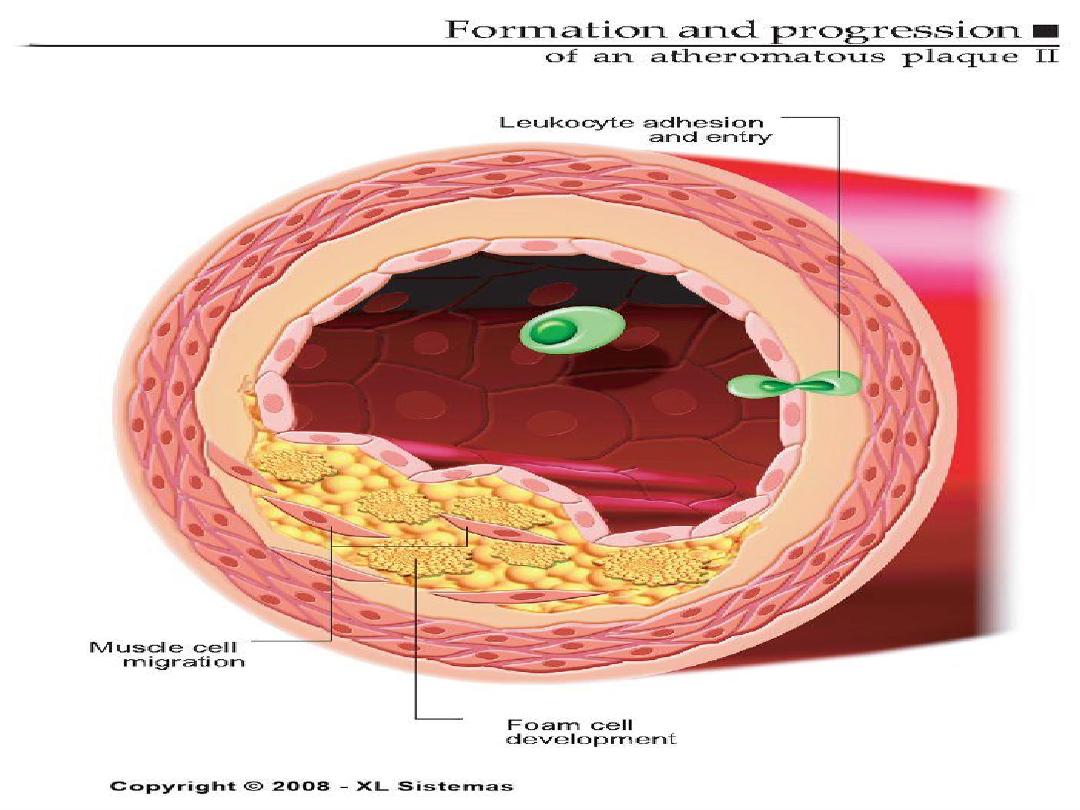
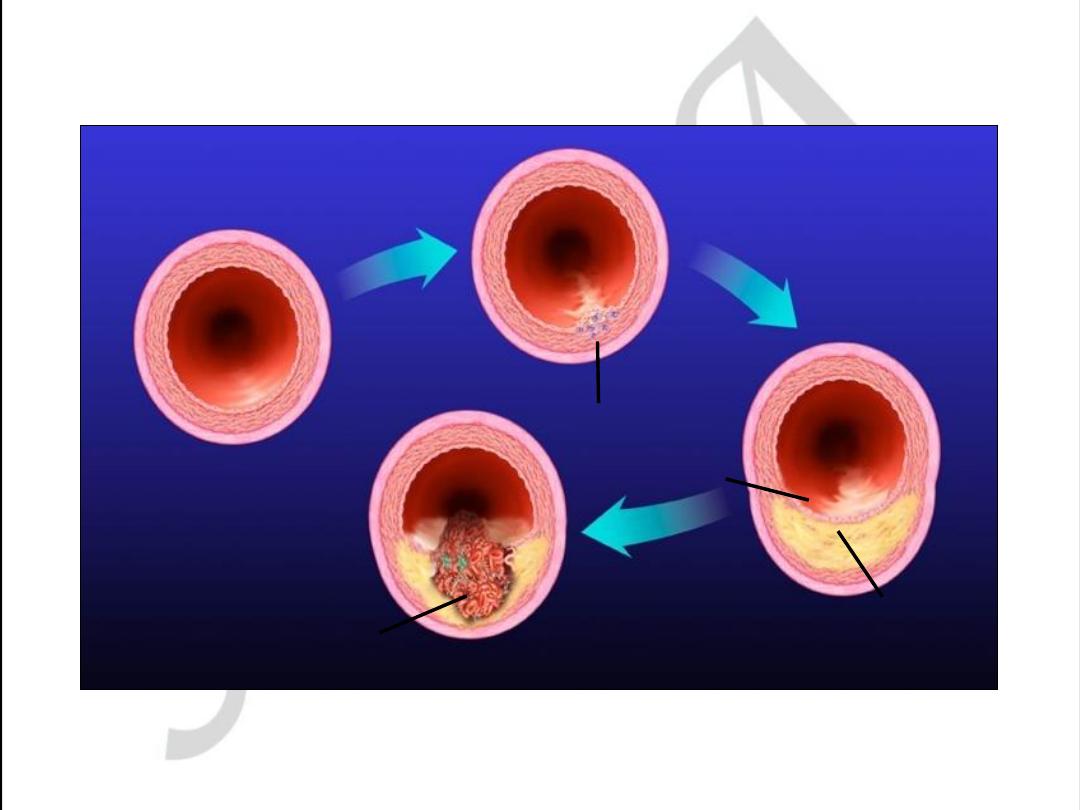
Pathogenesis of Atherosclerosis
• According to injury hypothesis
considers atherosclerosis to be a
chronic inflammatory response of the
arterial wall initiated by injury:
14
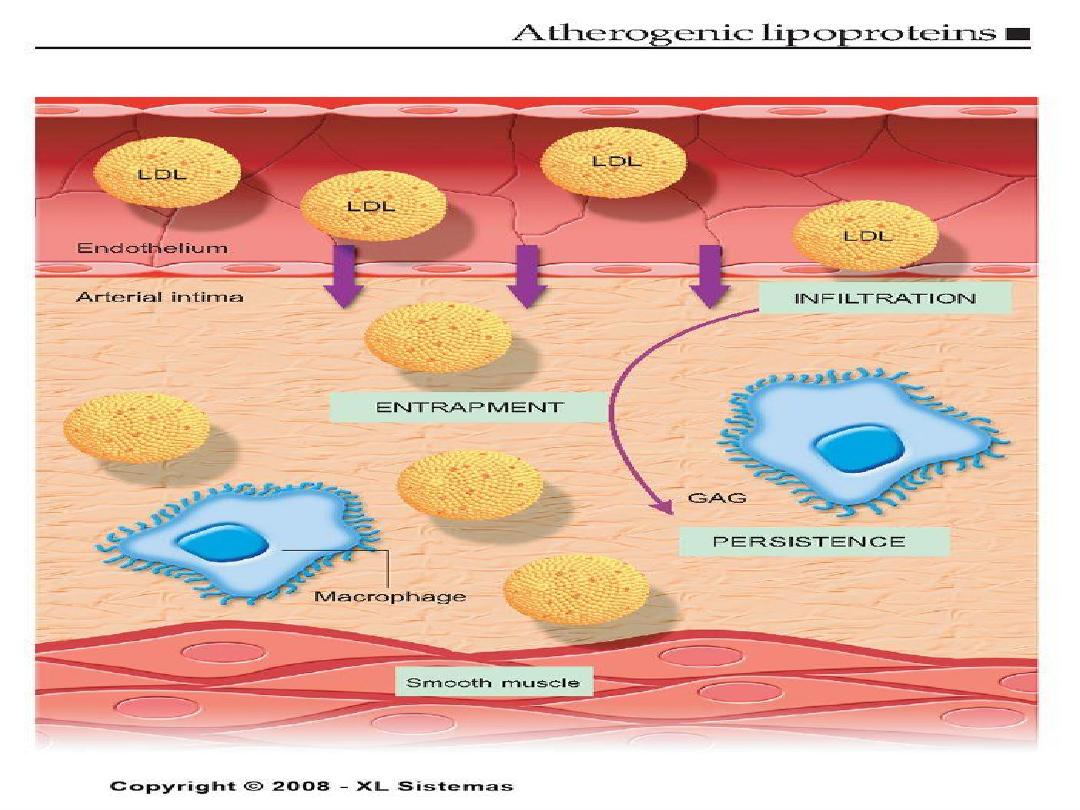
Pathogenesis of Atherosclerosis
1.
Chronic endothelial injury.
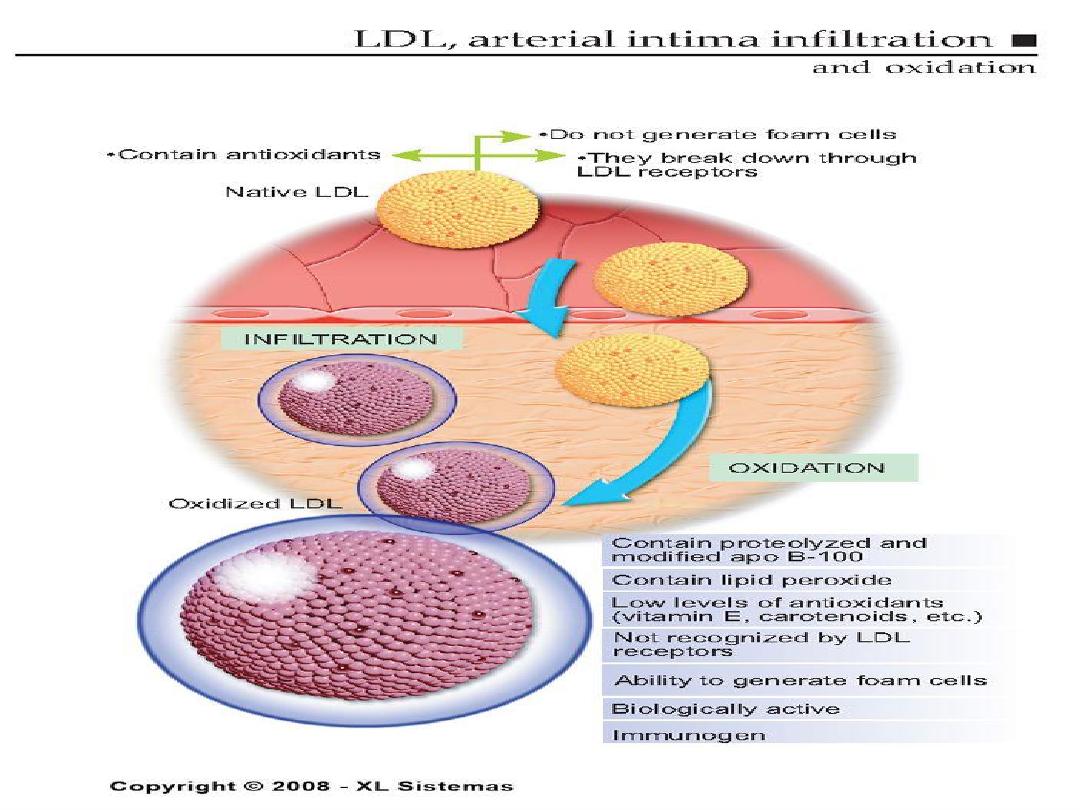
2 .Insudation of lipoproteins [LDL].
3. Modification of lipoproteins by
oxidation.
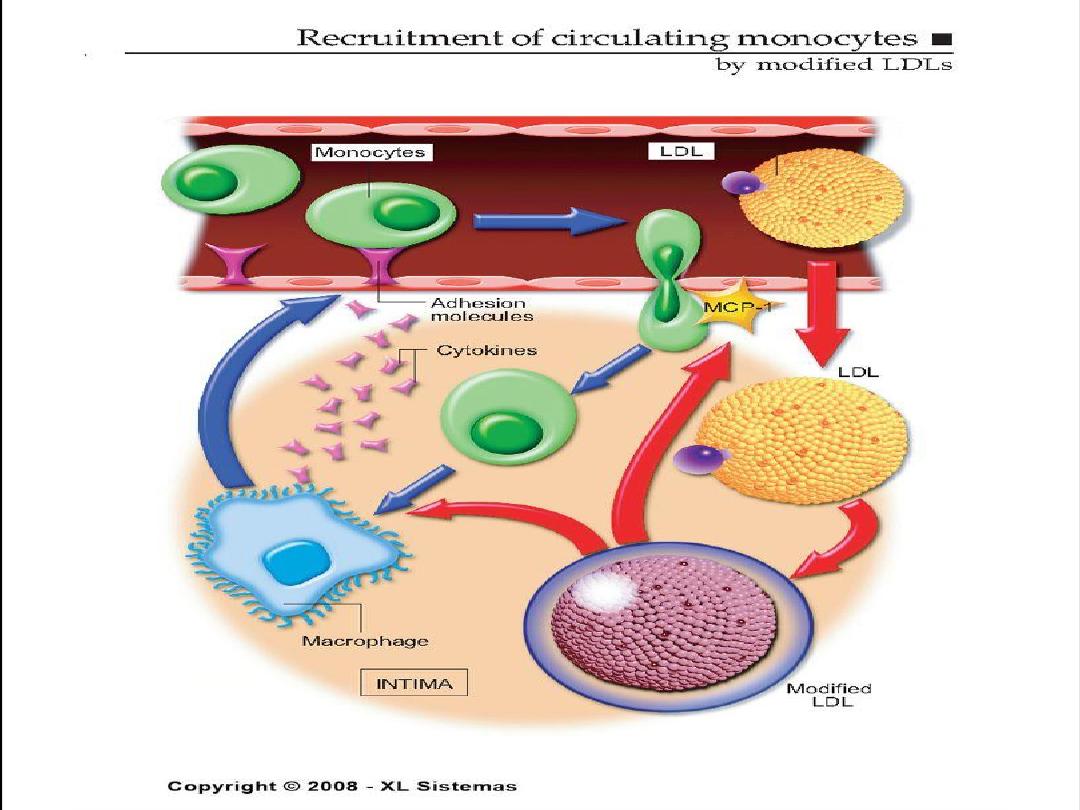
4. Adhesion of blood monocytes.
5. Adhesion of platelets.
15
Pathogenesis of Atherosclerosis
6. migration of smooth muscle cells
from the media into the intima.
7. proliferation of smooth muscle cells
in the intima.
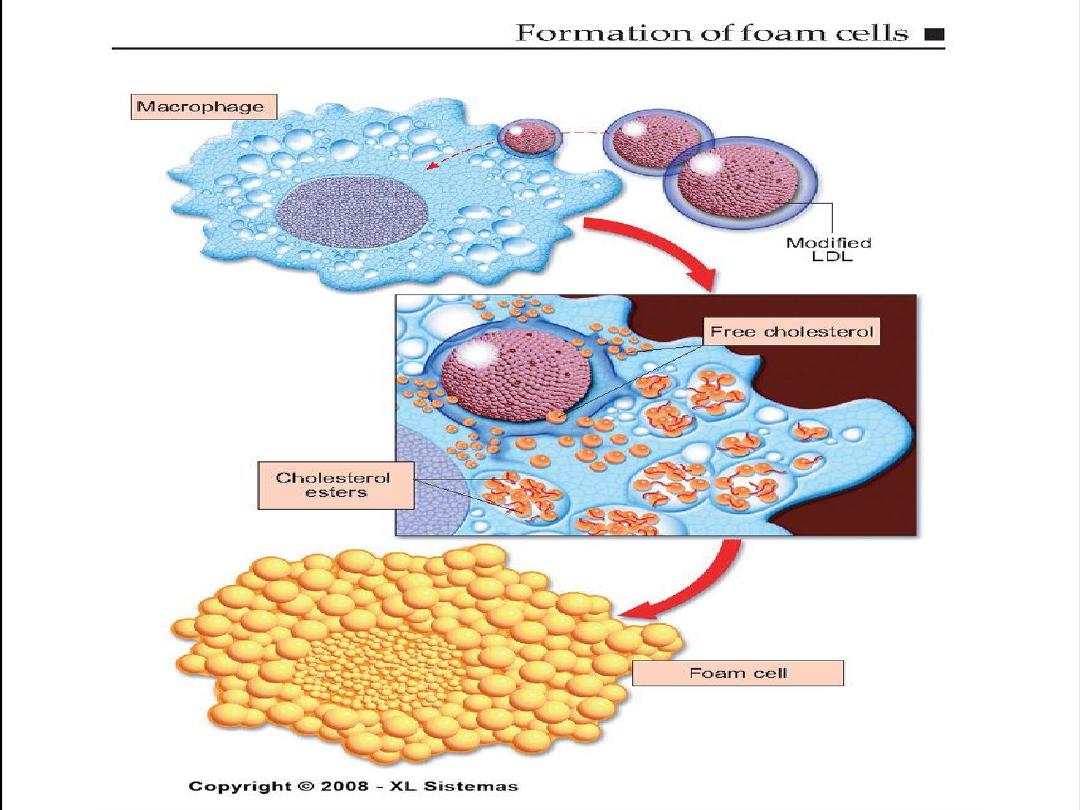
8. enhanced accumulation of intra and
extra cellular lipids.
16
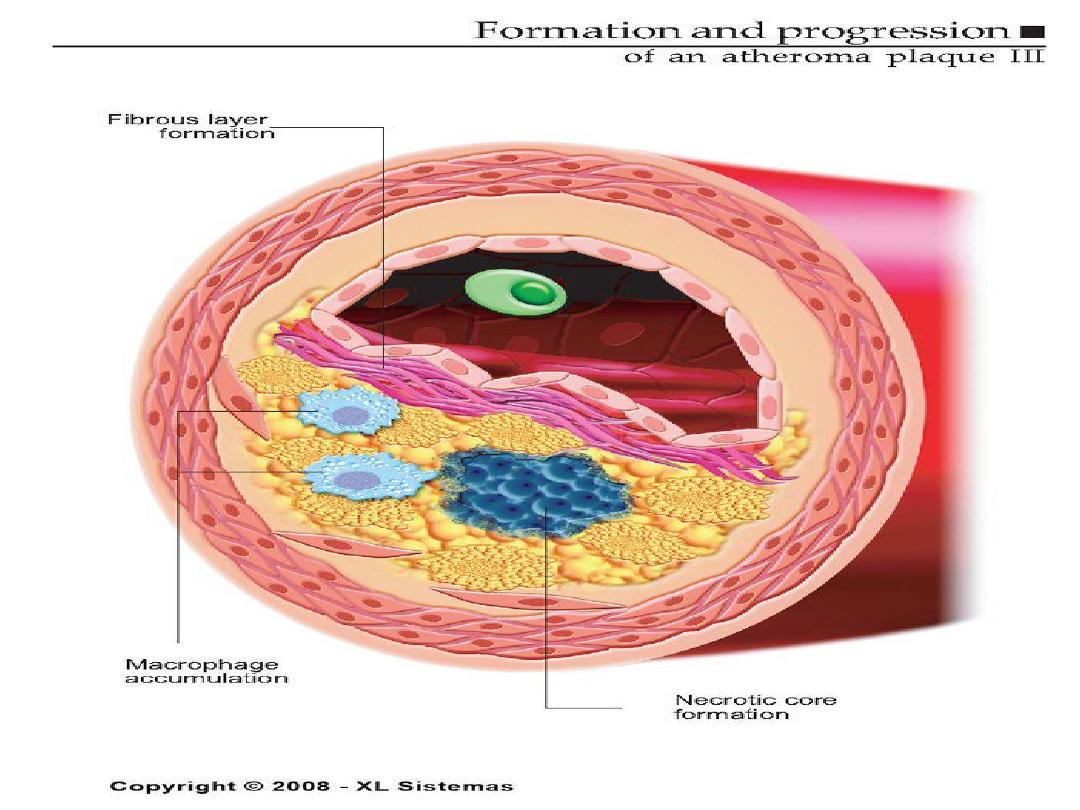
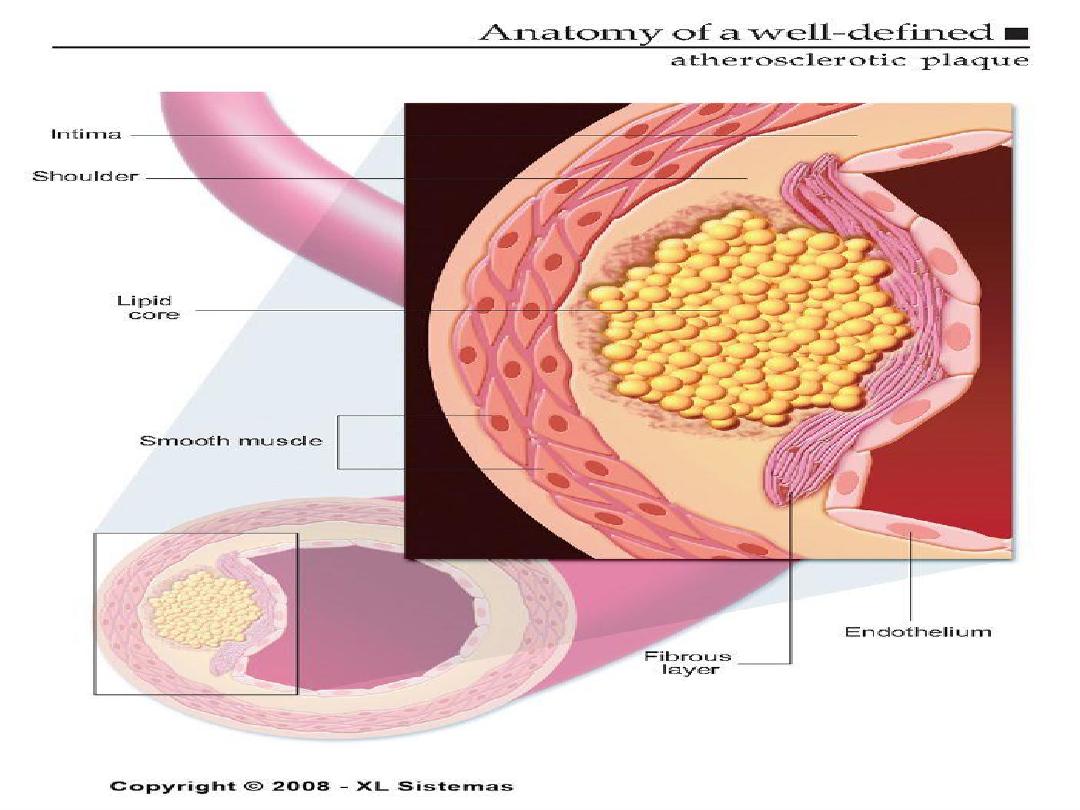
ATHEROSCLEROTIC PLAQUE
• The change of the large arterial intima is
called atherosclerotic plaque or
atheroma
•
atherosclerotic plaque is the intimal
thickening with lipid accumulation
• It consists of fibrous cap, necrotic core and
fibrous basis.
17
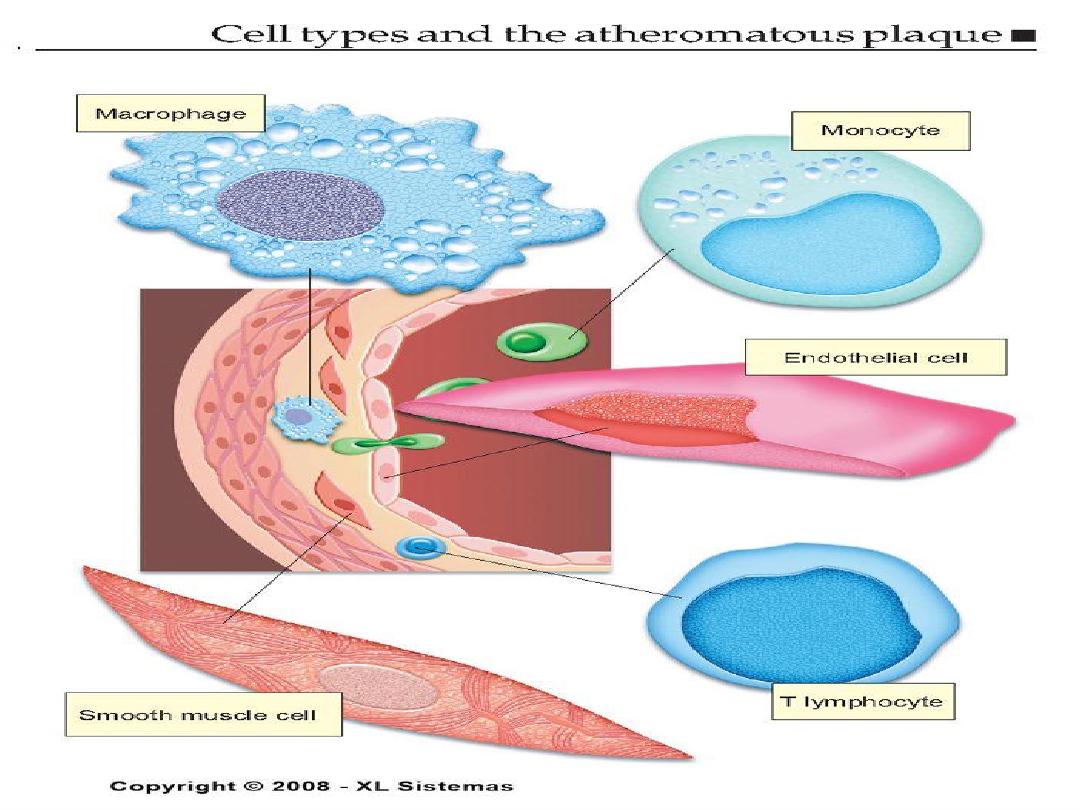
Atherosclerotic plaque
•
It has three principle components:
1- cells –smooth muscle cells, macrophages,
other leukocytes.
2 - Extra cellular matrix- collagen, elastic fibers,
and proteoglycans.
3 - Intra cellular and extra cellular lipids.
Normal coronary artery
Lumen has been distended at a pressure of 100mmHg
with 10% formal saline
used with permission from
M.J. Davies
Atlas of Coronary Artery Disease 1998
Lippincott-Raven Publishers
used with permission from
M.J. Davies
Atlas of Coronary Artery Disease 1998
Lippincott-Raven Publishers
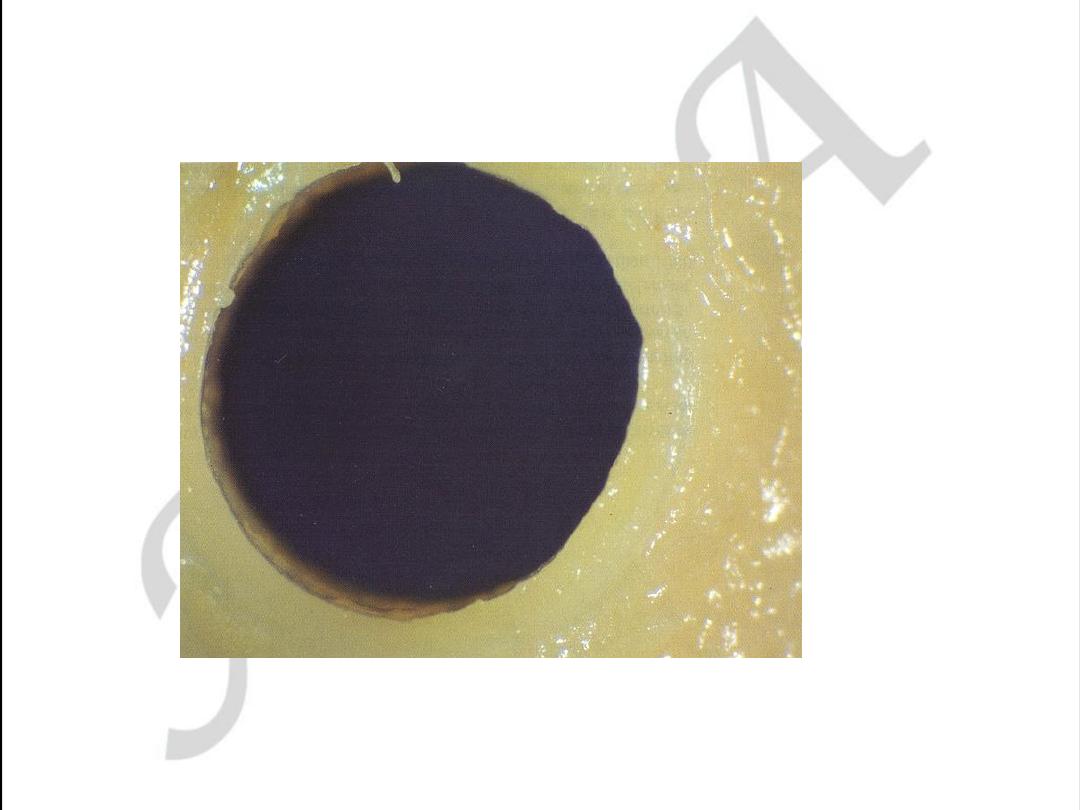
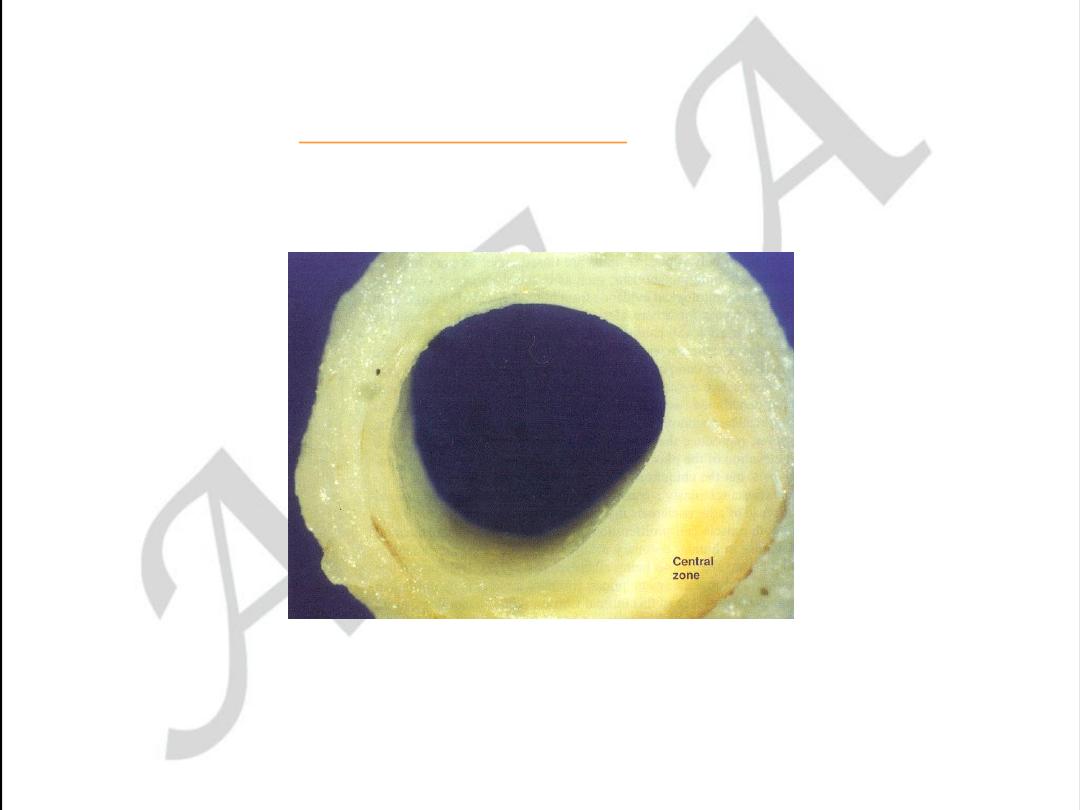
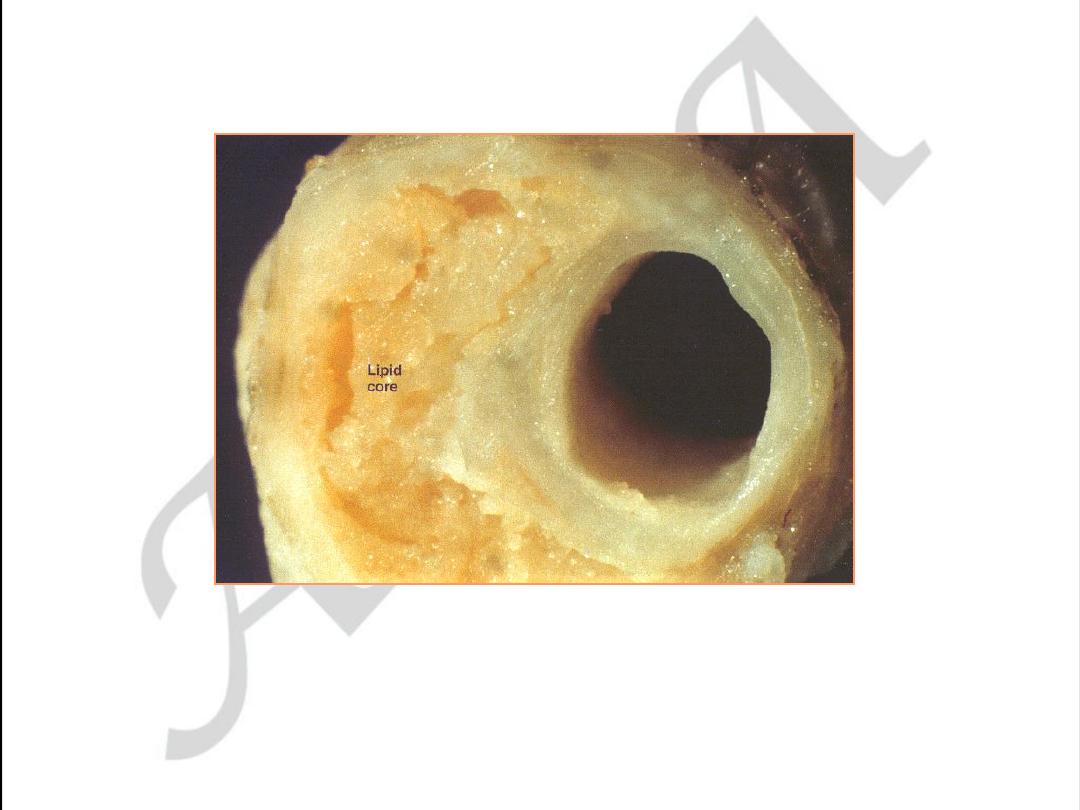
Early coronary atherosclerosis
Eccentric plaque with a central zone
containing yellow lipid
Stable angina. Eccentric coronary stenosis
used with permission from
M.J. Davies
Atlas of Coronary Artery Disease 1998
Lippincott-Raven Publishers
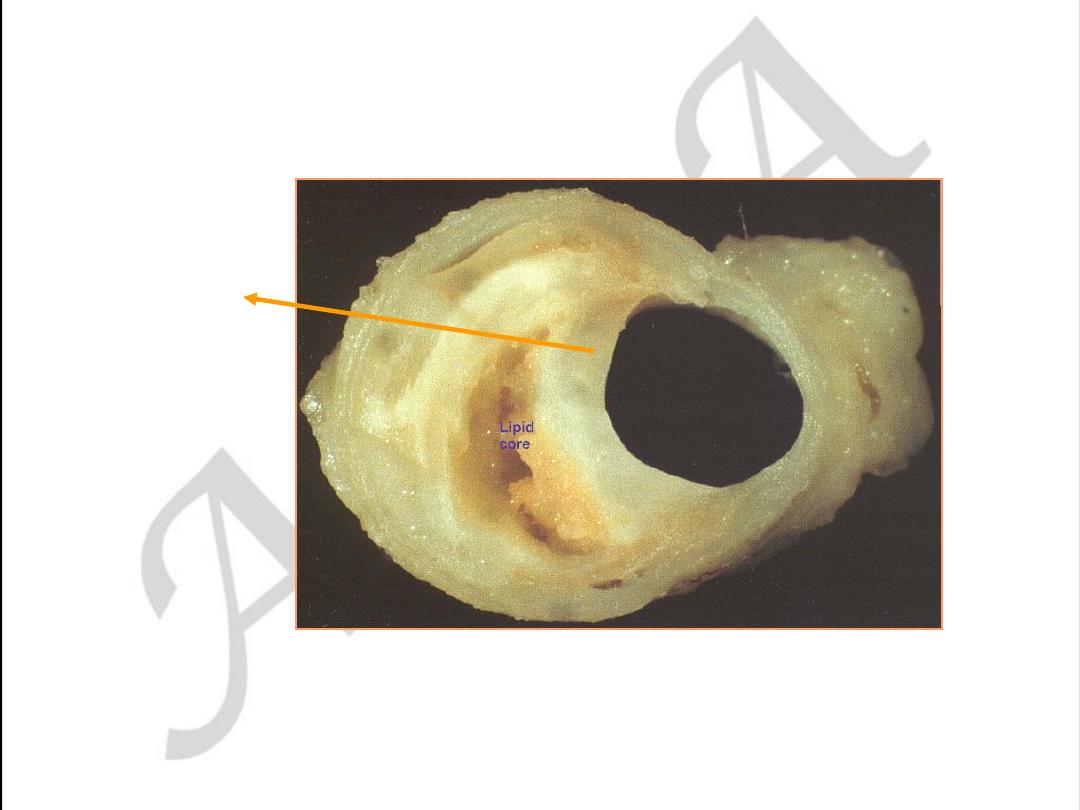
Stable angina. Eccentric coronary stenosis
used with permission from M.J. Davies
Atlas of Coronary Artery Disease 1998
Lippincott-Raven Publishers
thick cap
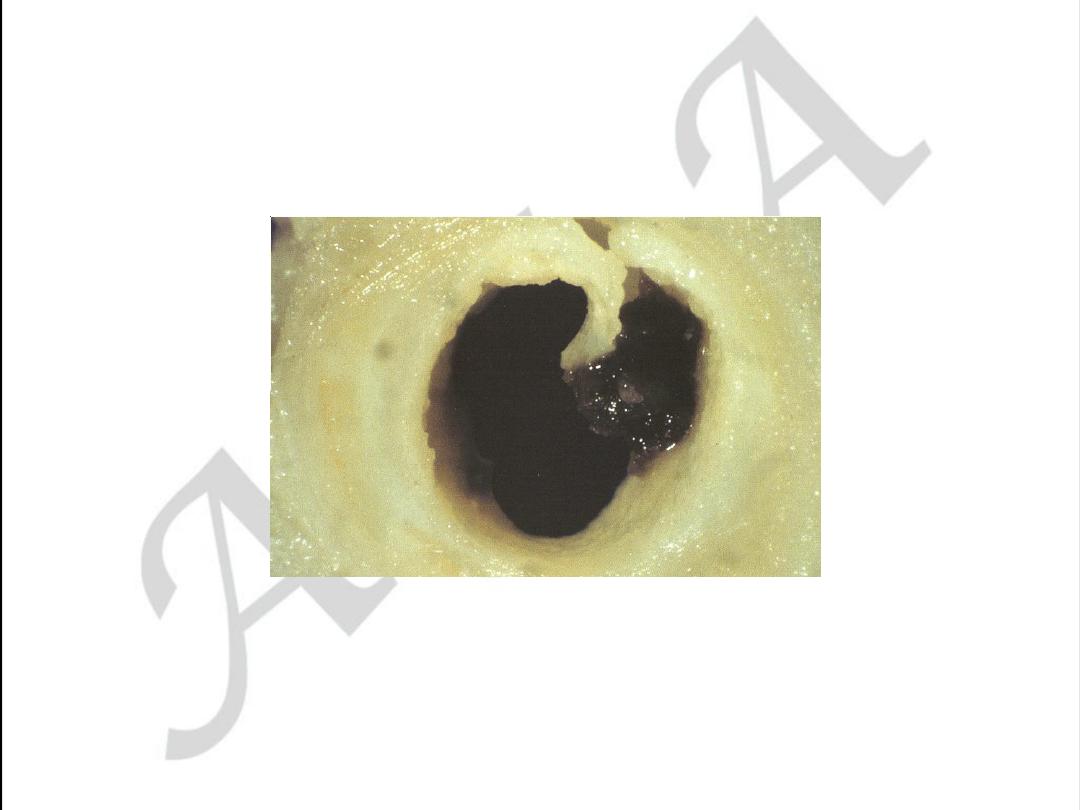
Unstable angina with
plaque disruption
used with permission from M.J. Davies
Atlas of Coronary Artery Disease 1998
Lippincott-Raven Publishers
Unstable angina with plaque disruption
used with permission from
M.J. Davies
Atlas of Coronary Artery Disease 1998
Lippincott-Raven Publishers
The plaque cap is torn,
projects into the
lumen, exposing a
mass of thrombus
filling the lipid core
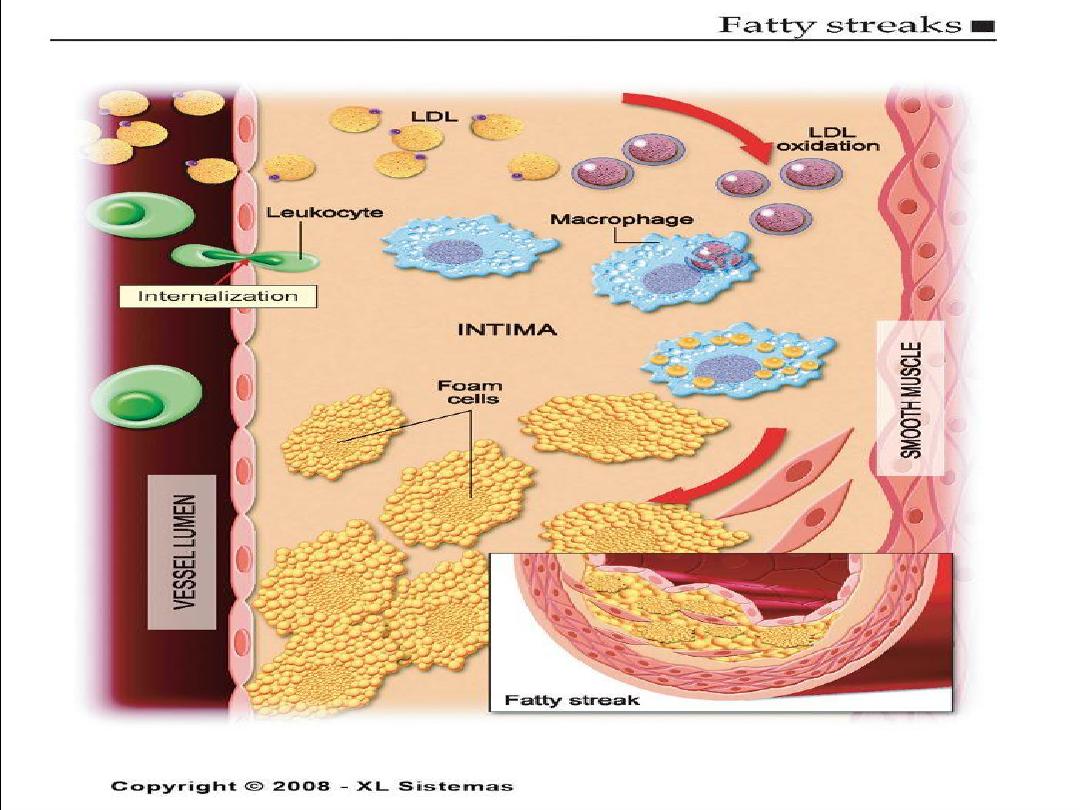
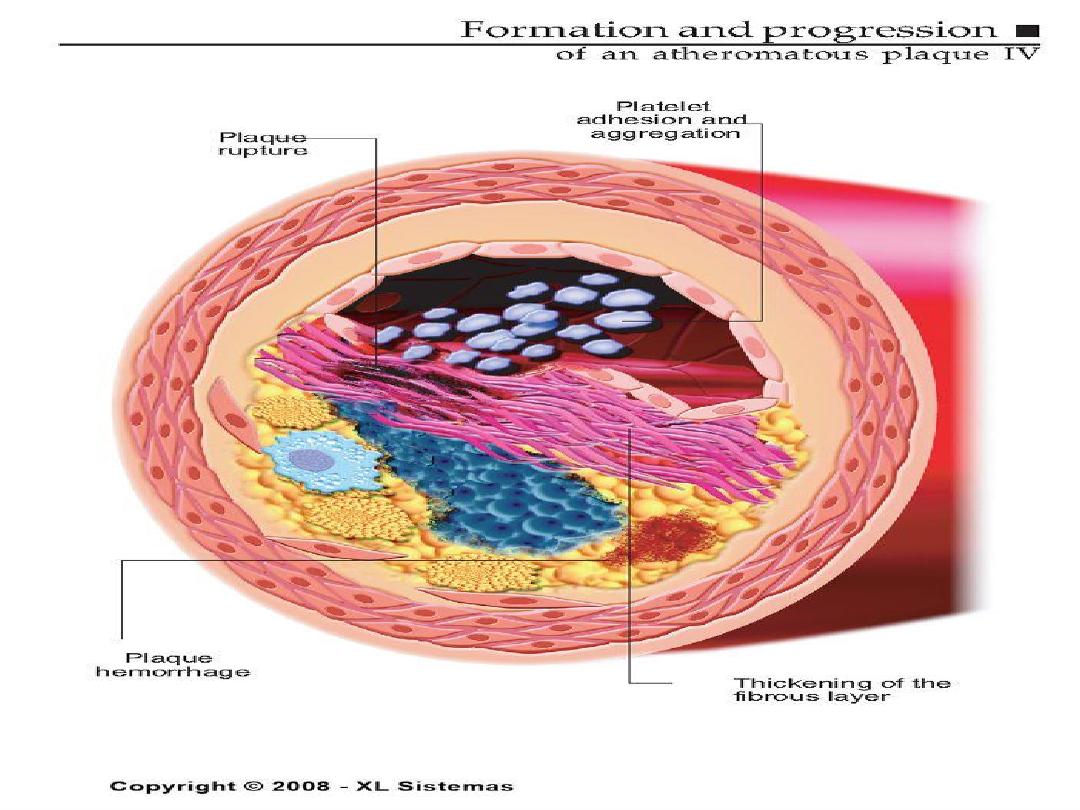
Development of Atherosclerotic Plaques
Normal
Fatty streak
Foam cells
Lipid-rich plaque
Lipid core
Fibrous cap
Thrombus
Ross R.
Nature.
1993;362:801-809.

Libby P.
Circulation.
1995;91:2844-2850.
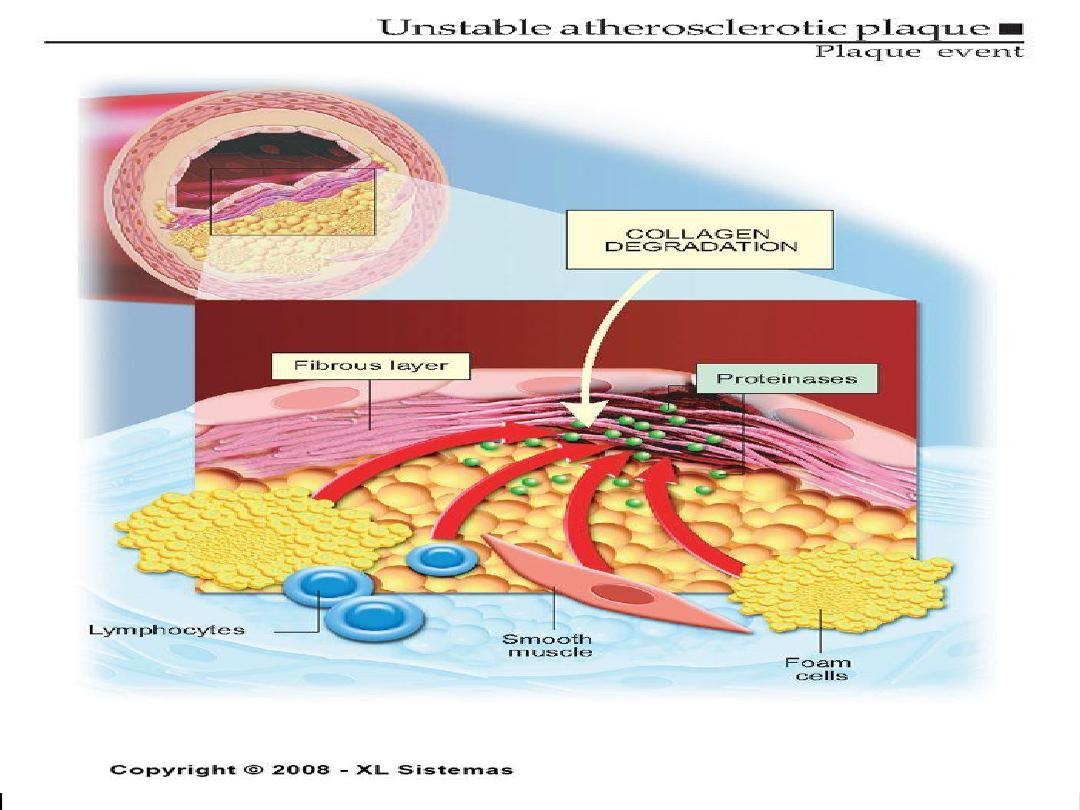
Vulnerable Plaque
• Thin fibrous cap
• Inflammatory cell infiltrates:
proteolytic activity
• Lipid-rich plaque
Lumen
Lipid
Core
Fibrous Cap
• Thick fibrous cap
• Smooth muscle cells:
more extracellular matrix
• Lipid-poor plaque
Stable Plaque
Lumen
Lipid
Core
Fibrous Cap
Vulnerable Versus Stable
Atherosclerotic Plaques

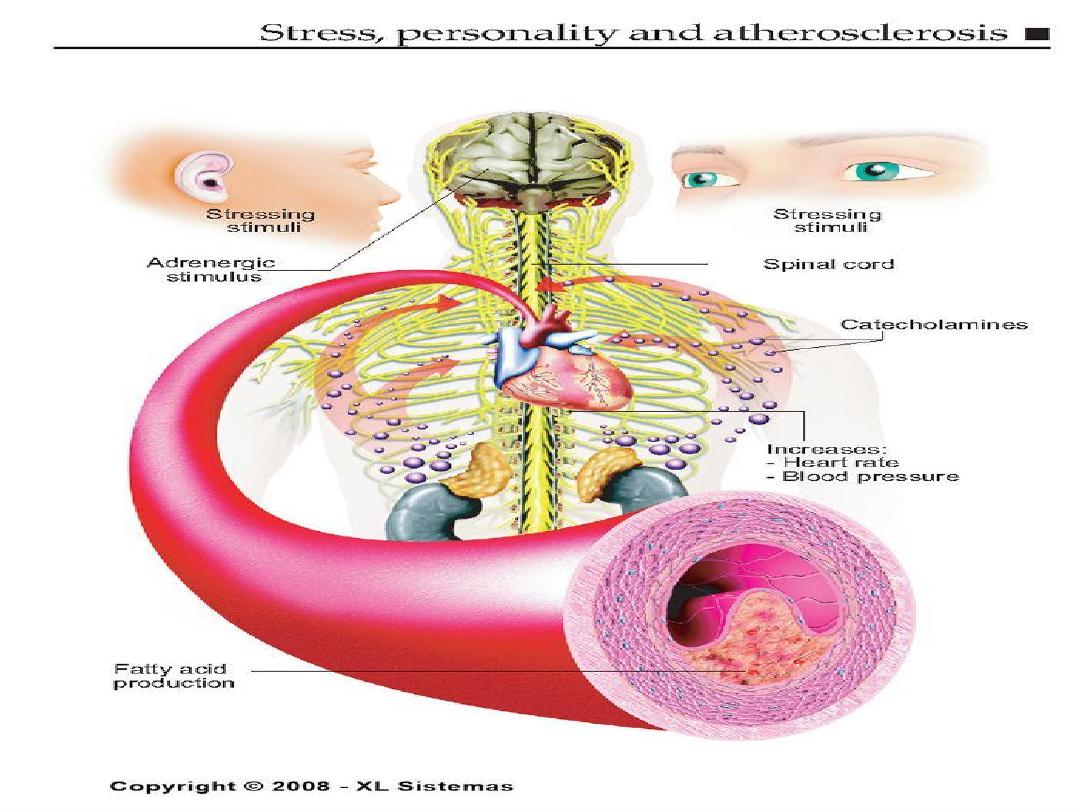
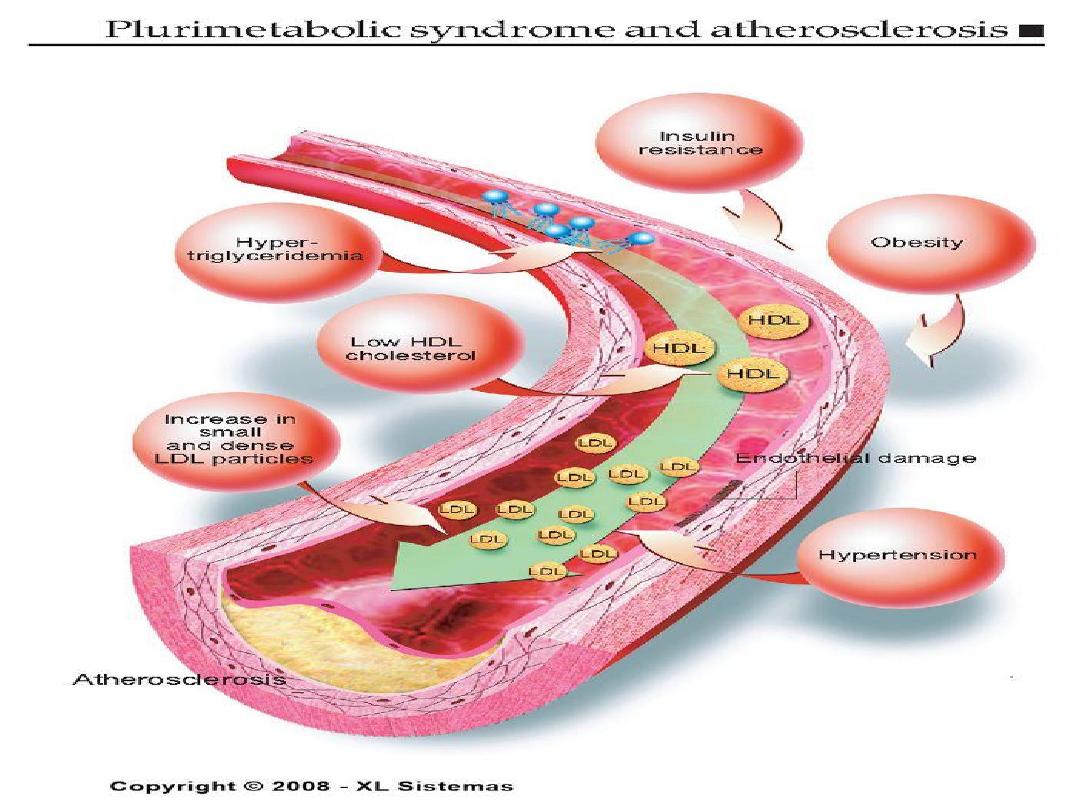
Major modifiable Risk Factors
• Cigarette smoking (passive smoking?)
• Elevated total or LDL-cholesterol
• Hypertension (BP
140/90 mmHg or on
antihypertensive medication)
.. Low HDL cholesterol (<40 mg/dL)
†
•
Obesity: Body Mass Index (BMI)
– Weight (kg)/height (m
2
)
– Weight (lb)/height (in
2
) x 703
• Obesity BMI >30 kg/m
2
with overweight defined as
25-<30 kg/m
2
• Abdominal obesity involves waist circumference >40
in. in men, >35 in. in women
• Physical inactivity: most experts recommend at least
30 minutes moderate activity at least 4-5 days/week
†
HDL cholesterol
60
mg/dL counts as a “negative” risk factor; its
presence removes one risk factor from the total count.

Nonmodifiable Risk Factors
• Age- Age (men
45 years; women
55 years)
the older you get, the greater the chance.
• Sex- males have a greater rate even after women
pass menopause.
• Race- minorities have a greater chance.
• Family history- if family members have had CHD,
there is a greater chance.
Family history of premature CHD
– CHD in male first degree relative <55 years
– CHD in female first degree relative <65 years
Unchangeable Risk Factors: Age, the older you get the greater the chance of heart disease.
Four out of five people who die of congestive heart disease are 65 years of age or older. Sex,
males have a greater rate of congestive heart disease. Race, minorities have a greater chance of
heart disease. African Americans have a greater chance of high blood pressure. The risk is also
higher in Mexican Americans, America Indians, native Hawaiians and Asian Americans. Also
included as unchangeable risk factors is your family history and your own personal medical
history.

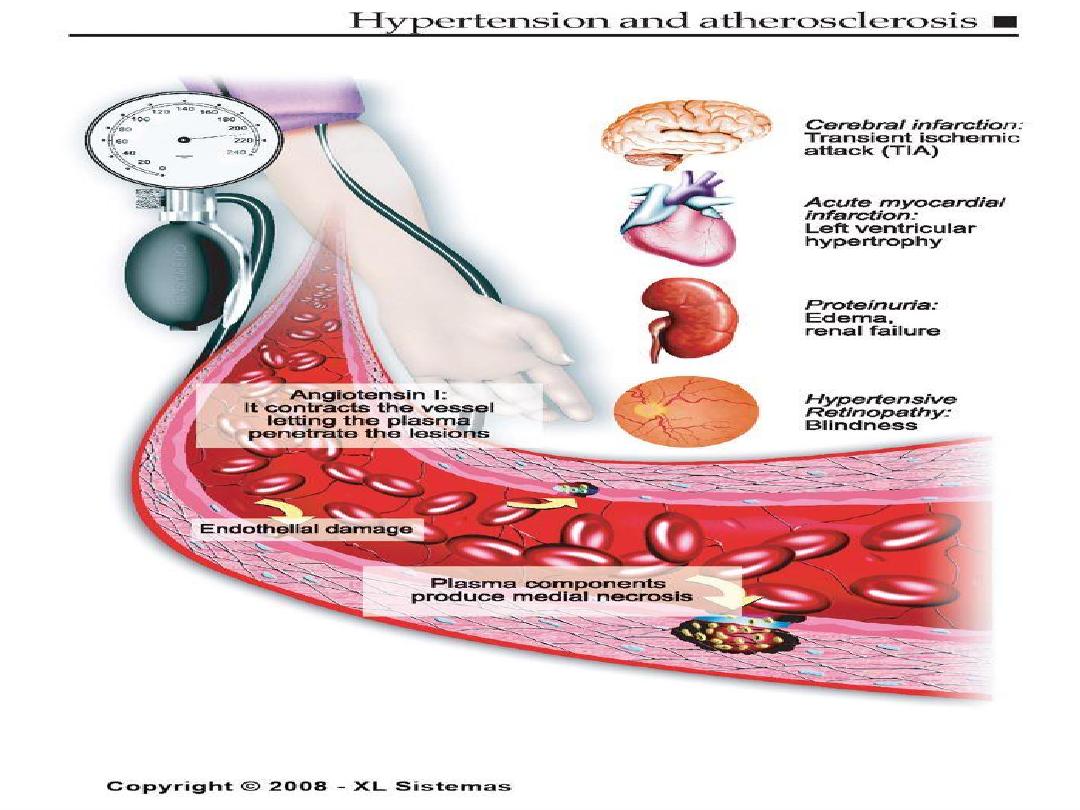
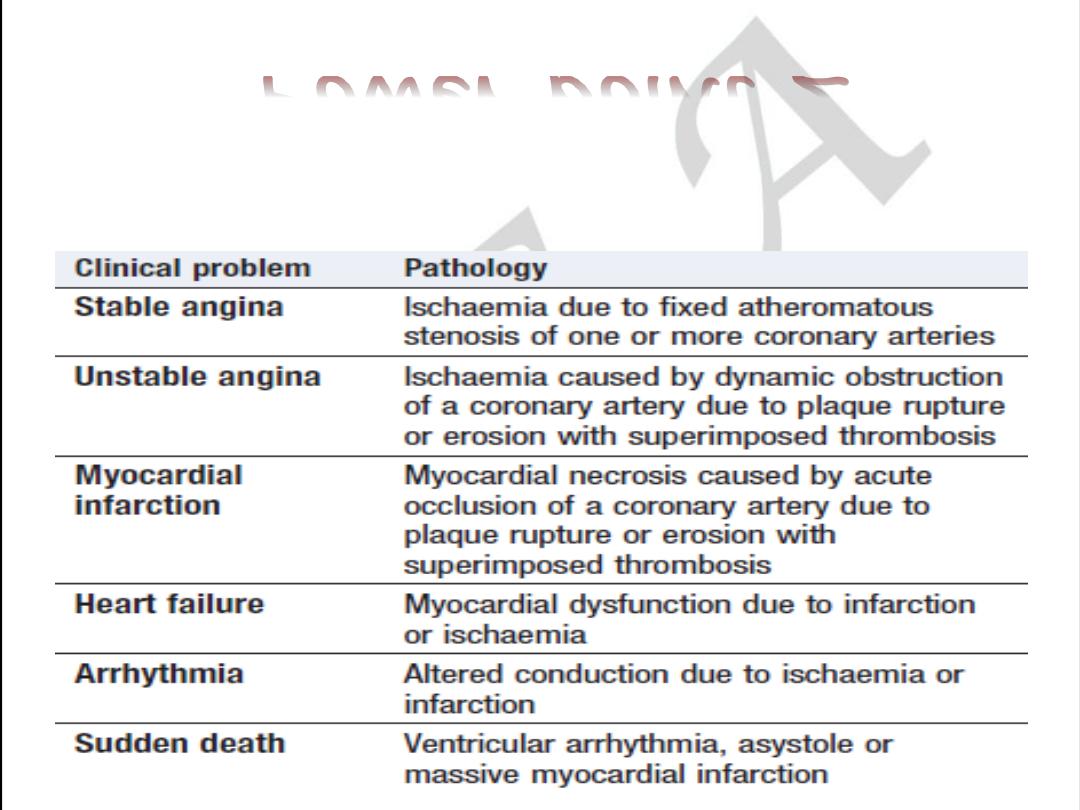
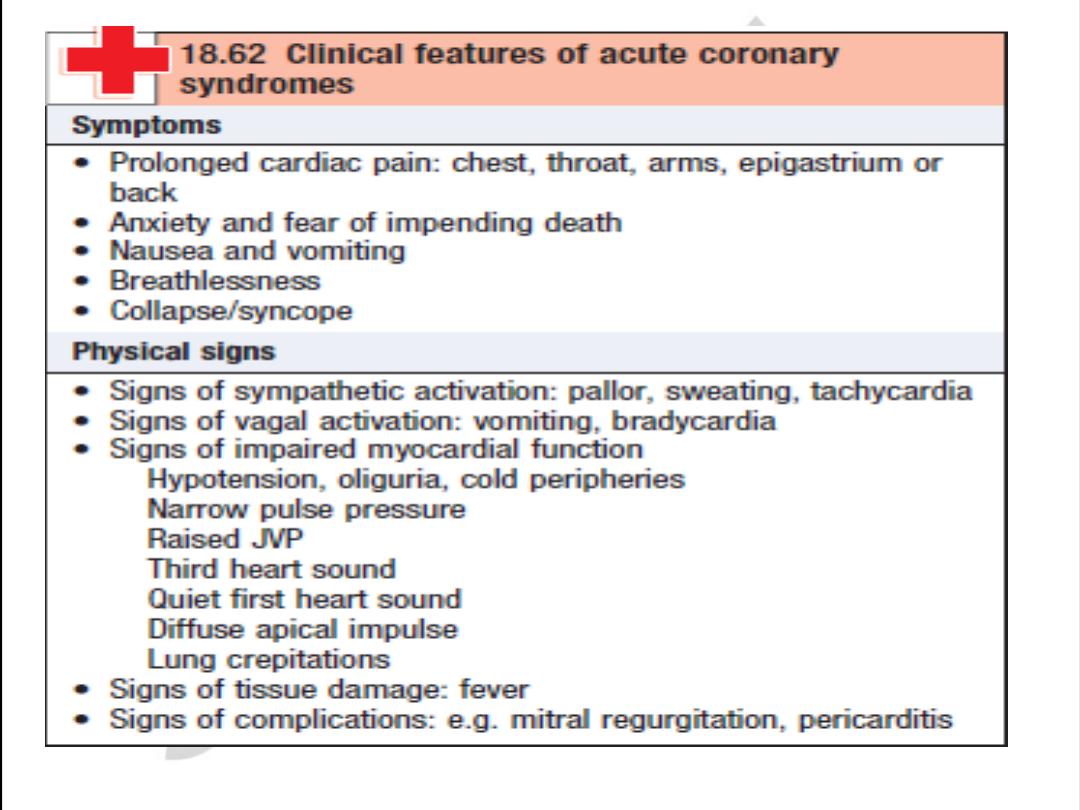
Clinical Manifestations
of Atherosclerosis
• Coronary heart disease
– Stable angina, acute myocardial infarction, sudden death,
unstable angina
• Cerebrovascular disease
– Stroke, TIAs
• Peripheral arterial disease
– Intermittent claudication, increased risk of death from heart
attack and stroke
American Heart Association, 2000.
41
FORMS OF ATHEROSCLEROSIS
• CEREBRAL ARTERIES INJURY
• CARDIAC ARTERIES INJURY
• RENAL ARTERIES INJURY
• AORTA INJURY
• INTESTINAL ARTERIES INJURY
• EXTREMITY ARTERIES INJURY
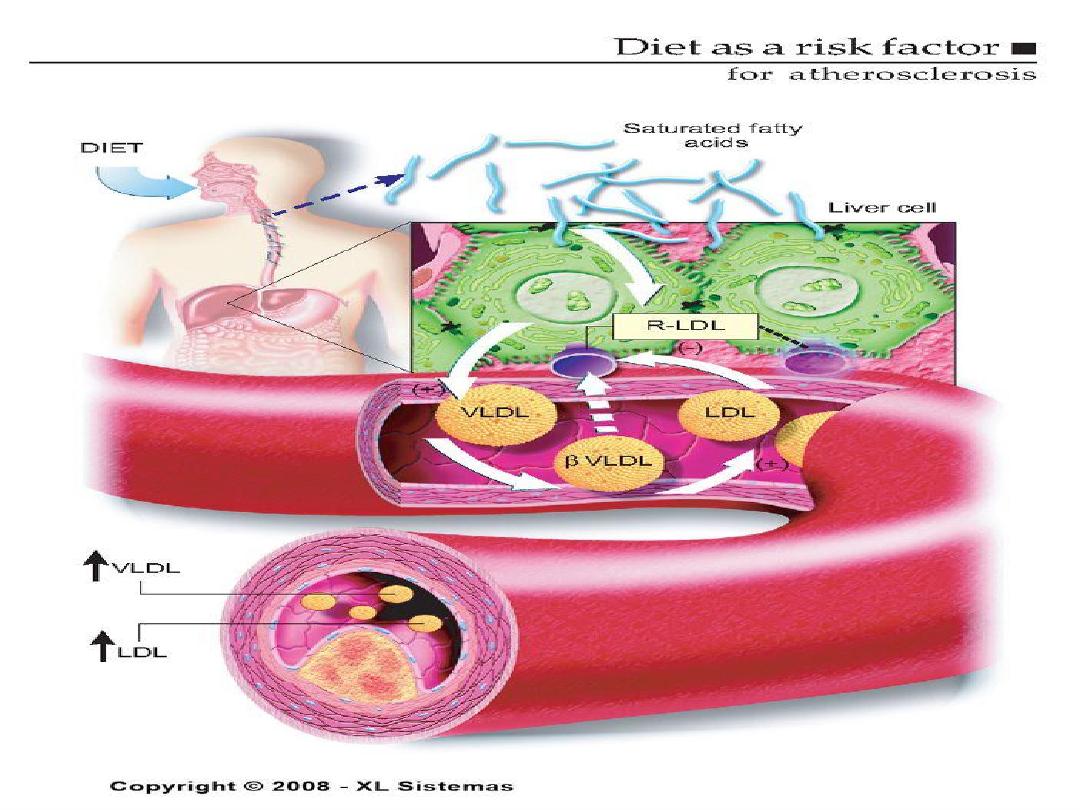


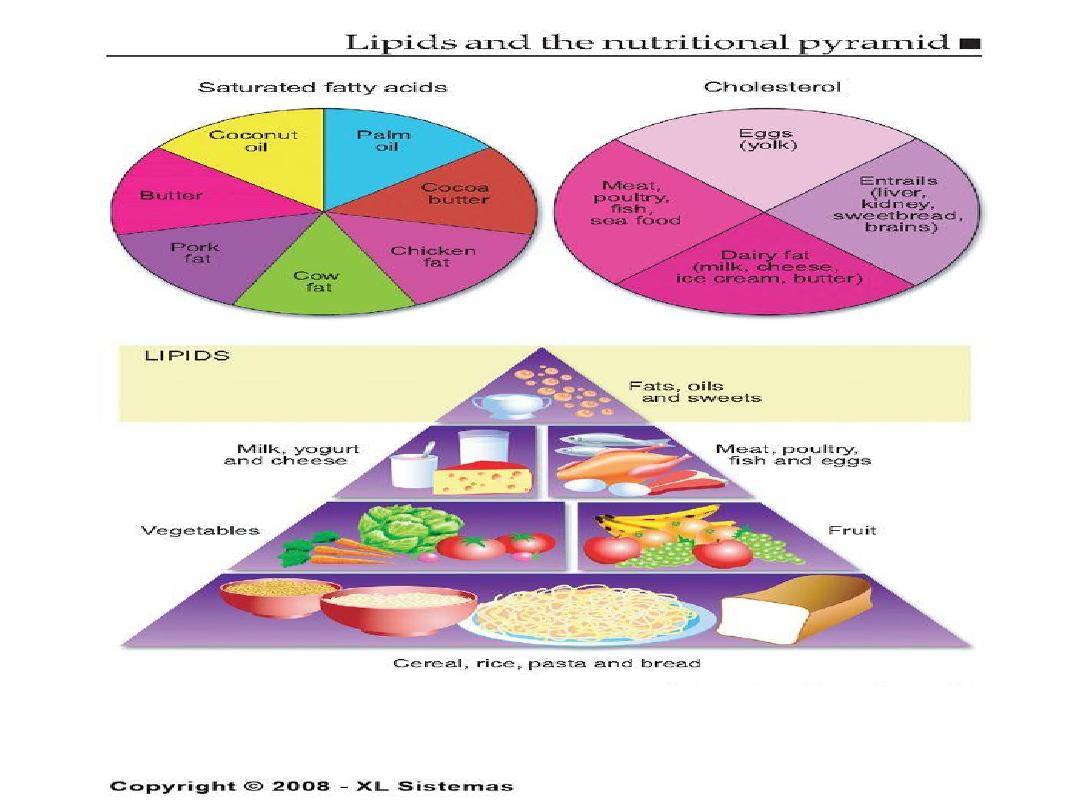
The Skinny on Fat
• Saturated fats- basically means the fat is saturated
with hydrogen, they are solid at room temperature.
Examples are lard and butter.
• Why are they bad for you? They increase levels of
LDL , decrease HDL and increase total cholesterol.
Saturated fats can cause an increase in cholesterol. What is
saturated fat? It is fat that is saturated with hydrogen and is a
solid at room temperature. Examples are lard and butter.
Saturated fats increase levels of LDL, decrease levels of HDL
and increases total cholesterol.
The American Heart
Association recommends you limit saturated fat intake to 7-
10% of your total calories.

The Skinny on Fat
• What are monounsaturated fats?
• They are liquid at room temperature but start
to solidify in the refrigerator.
• Decrease total cholesterol and lower LDL
levels.
Monounsaturated fat includes canola, olive and peanut oils and
avocados.

The Skinny on Fat
• What are trans fatty acids? They are
unsaturated fats but they tend to raise total
and bad cholesterol.
• Where do you find them?
• In fast-food restaurants
• Commercial baked goods. Examples:
doughnuts, potato chips, cupcakes.
Trans fatty acids have hydrogen added to them to give them a
longer shelf life and they also tend to lower HDL levels.

What about Omega 3?
• Type of polyunsaturated fat.
• Consistently lowers serum triglycerides and may
also have an effect on lowering blood pressure.
• Found in oily fish such as salmon, tuna, and herring.
• Is available as a supplement.
The American Heart Association recommends eating fish two
times per week. Mention other fish that contain Omega 3
fatting acids. Omega 3 fatty acids are available as a
supplement but research is till being done to determine the
supplements’ effectiveness.

Physical Inactivity
• Increasing physical activity has been shown to
decrease blood pressure.
• Moderate to intense physical activity for 30-45
minutes on most days of the week is
recommended.
Exercise can help control blood cholesterol,
diabetes and obesity, as well as help lower
blood pressure.

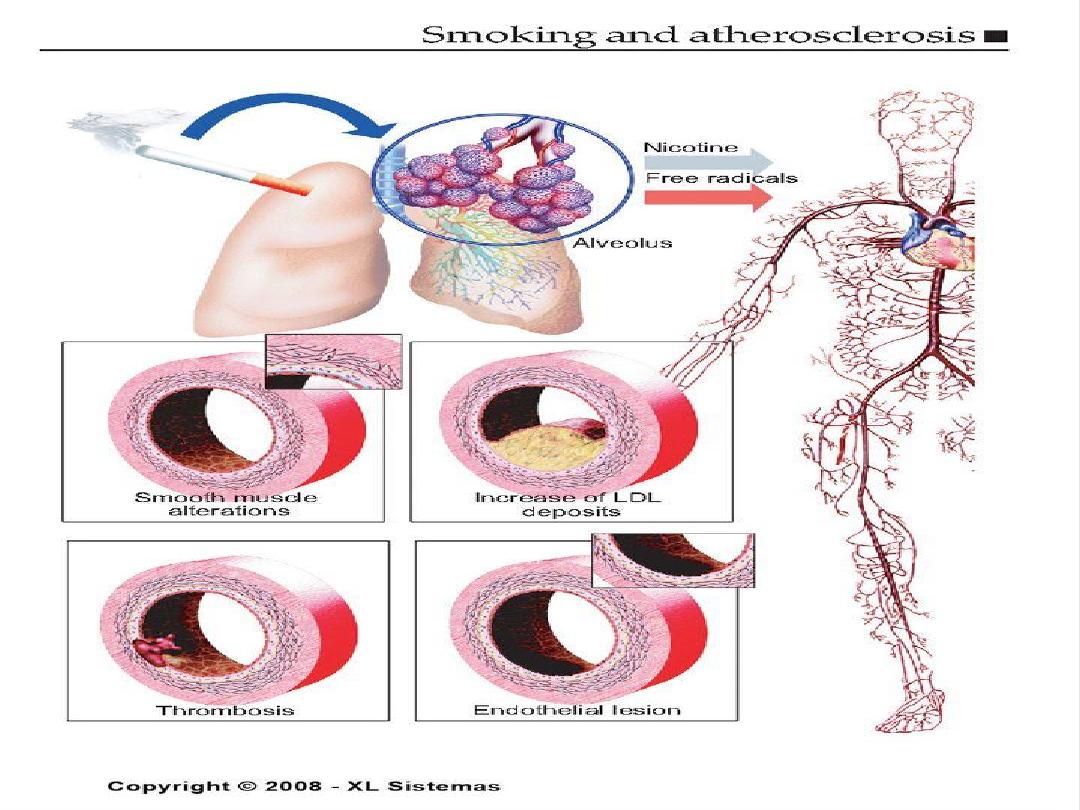
Cigarette Smoking
• Causes an increase in blood pressure
• Usually have lower levels of HDL
• Within 1 year of quitting, CHD risk decreases,
within 2 years it reaches the level of a
nonsmoker.

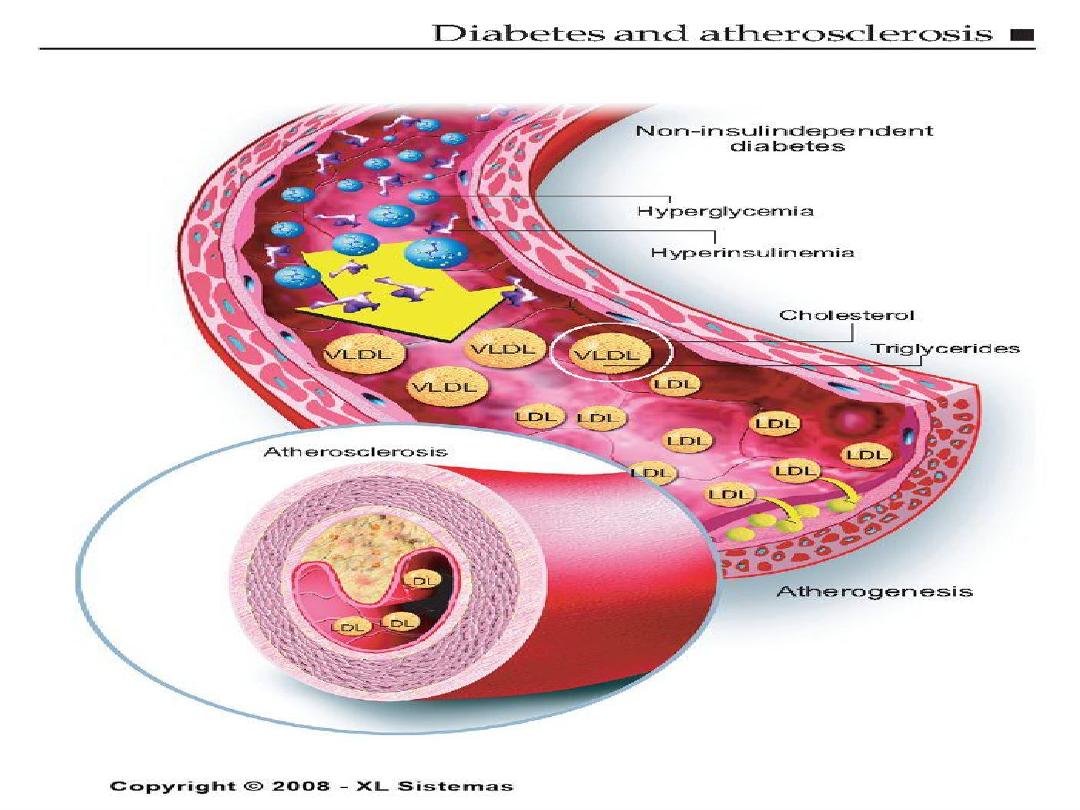
Diabetes Mellitus
• At any given cholesterol level, diabetic persons
have a 2 or 3 x higher risk of atherosclerosis!
• Insulin is required to maintain adequate levels
of lipoprotein lipase, an enzyme needed to
break down bad cholesterols.
About 2/3 of the people with diabetes die of some
type of heart or blood vessel disease.

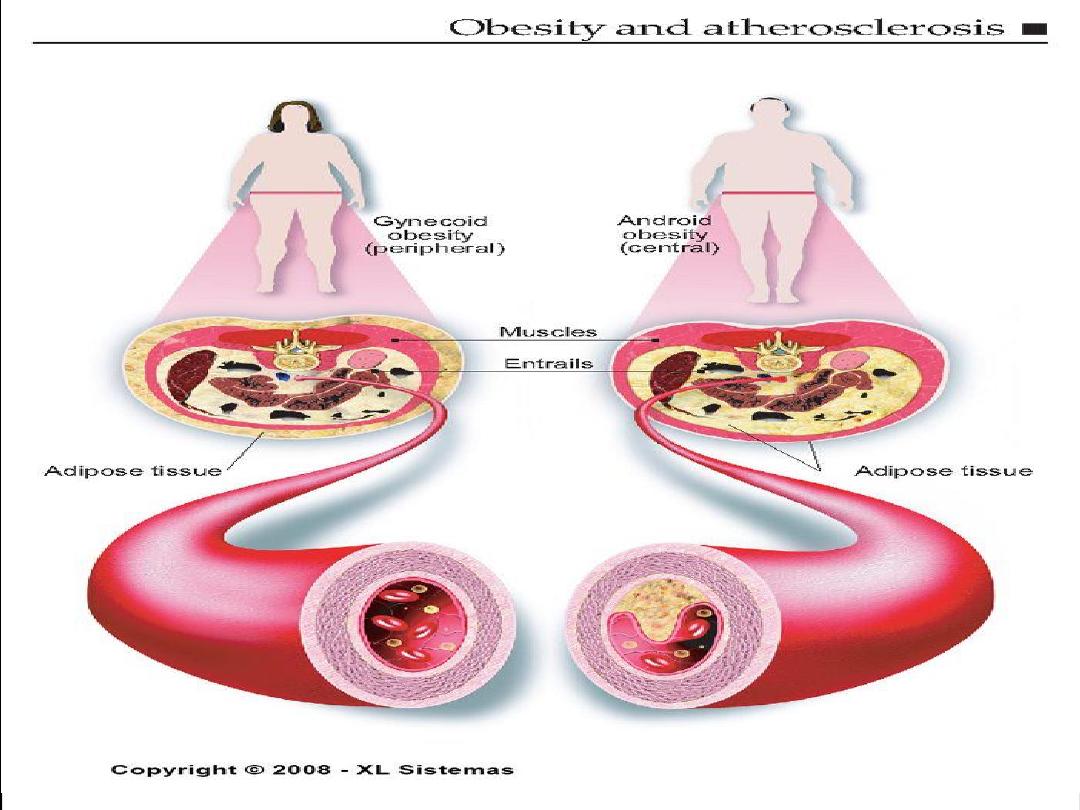
Obesity
• People who are obese have 2 to 6 times the
risk of developing hypertension.
• Location of the body fat is significant.
• Pears of apples?
People who are obese have 2 to 6 times the risk of
developing hypertension even if they have no other risk
factors.

Approaches to Primary and
Secondary Prevention
• Primary prevention involves prevention of
onset of disease in persons without symptoms.
• Primordial prevention involves the prevention
of risk factors causative o the disease, thereby
reducing the likelihood of development of the
disease.
• Secondary prevention refers to the prevention
of death or recurrence of disease in those who
are already symptomatic

Prevention of atherosclerosis
• Primary prevention:
• Population strategy.
• Targeted strategy.
• Secondary prevention

•
Get regular medical checkups.
•
Control your blood pressure.
•
Check your cholesterol.
•
Don’t smoke.
•
Exercise regularly.
•
Maintain a healthy weight.
•
Eat a heart-healthy diet.
•
Manage stress.

The End
Coronary artery disease
Clinical manifestation and pathology
Power point 2
Angina pectoris
Angina pectoris refers to the
PAIN
caused by
myocardial ischemia
.
Ischemia
is
usually
caused
by
mismatched
oxygen
demand
(tachycardia, anemia, aortic stenosis, left ventricular hypertrophy of
other etiologies)
and delivery in the setting of a hemodynamicaly
significant coronary stenosis due to atheroma
, but it may have other
causes such
as coronary artery spasm
(Prinzmetal’s variant angina).
In more unusual cases the etiology is not completely understood
—
e.g.,
syndrome
X
(chest
pain
with
normal
coronary
arteries).
Alternatively, these conditions may coexist and be exacerbated by
emotional stress.
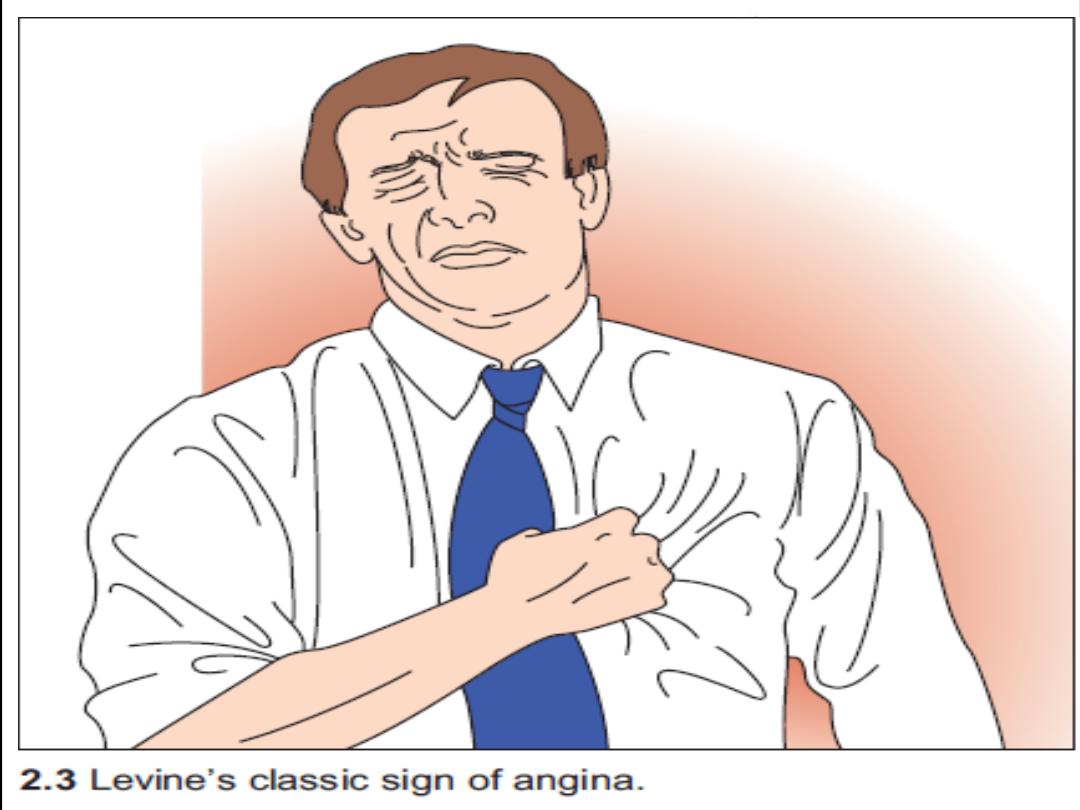
History and examination
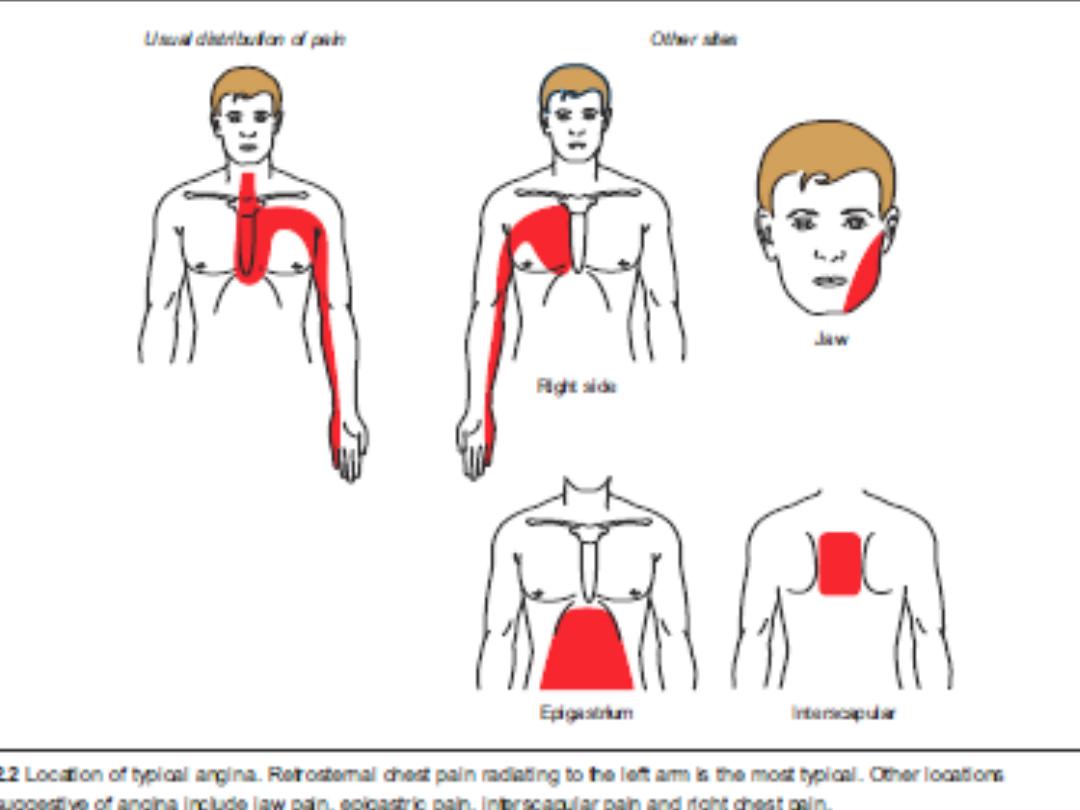
Angina pectoris is characterized by a deep and diffusely distributed
central chest
discomfort
. Certain features of pain are of discriminative value.
Patients will not be able to point to where the pain is coming from with
one finger but will use an open palm or fist over the center or left para-
sternal aspect of their chest ( Levin s sign ) .
• The pain is not sharp (some patients confuse “sharp” with “severe”).
• Pain lasts longer than a few seconds and rarely exceeds an hour
without varying in severity. Most episodes will last 1–5 minutes.
• The response to nitroglycerin, if any, will be almost immediate.
Generally, responses taking more than 5 minutes are unlikely to be
related to the drug.
Angina equivalent symptoms -Dyspnea, fatigue, nausea, and recurrent belching may
also represent underlying ischemia and can occur in the absence of the classical
central chest pain. The clue to underlying ischemic heart disease (IHD) lies in their
precipitation by exertion or emotional stress.
•
Chest wall tenderness suggests musculoskeletal pain and does not accompany
angina.


Classification of angina
Angina is often classified according to its temporal pattern and its relation to exertion because
this loosely reflects prognosis.
• Stable angina is characterized by pain occurring after a relatively constant level of exertion.
• Unstable angina is characterized by pain on minor exertion or at rest, which is either new onset
or a dramatic worsening of existing angina.
Also, it can present as pain on ever-diminishing levels of exertion, usually over a period of days.
The Canadian Cardiovascular Society (CCS) system
provides a quantitative means to
describe exertional capacity and is divided into four classes:
I. Minimal limitation of ordinary activity. Angina occurs with strenuous, rapid, or prolonged
exertion at work or recreation.
II. Slight limitation of ordinary activity; angina occurs on walking or climbing stairs rapidly;
walking in cold, in wind, or under emotional stress.
III. Marked limitation of ordinary physical activity; angina occurs on walking 50–100 m on level
ground or climbing 1 flight of stairs at a normal pace in normal conditions.
IV. Inability to perform any physical activity without discomfort; angina symptoms may be
present at rest.

Physical examination
• Measure the pulse rate.
This may be slowed by inferior ischemia due to
atrioventricular (AV) node ischemia. A resting tachycardia, if present, usually
represents activation of the sympathetic nervous system but may be due to
an arrhythmia precipitated by ischemia.
•
Blood pressure measurement
is essential to look for evidence of hypertension
(predisposing to atheroma) or hypotension (may reflect cardiac dysfunction or
overmedication).
• Precordial examination
should include palpation for left ventricular hypertrophy
(LVH), cardiac enlargement, or dyskinesis, and auscultation for added heart
sounds (heart failure or acute ischemia), aortic stenosis, or mitral
regurgitation (due to papillary muscle dysfunction).
• Examine for signs of heart failure
by listening for fine, late-inspiratory crackles
at the lung bases and looking for dependent pitting edema (typically bilateral
ankle ± leg edema, but sacral edema may be the only manifestation if the
patient has been recumbent for some time).
•
Look for evidence of peripheral vascular disease
by palpating for aortic
aneurysm; feeling the carotid and limb pulses; listening for carotid, renal, or
femoral artery bruits; and assessing tissue integrity and capillary refill of the
legs and feet.
• Examine for signs of hypercholesterolemia:
the eyes for xanthelasmata and
corneal arcus, and the skin and tendons (especially the Achilles) for
xanthomata.

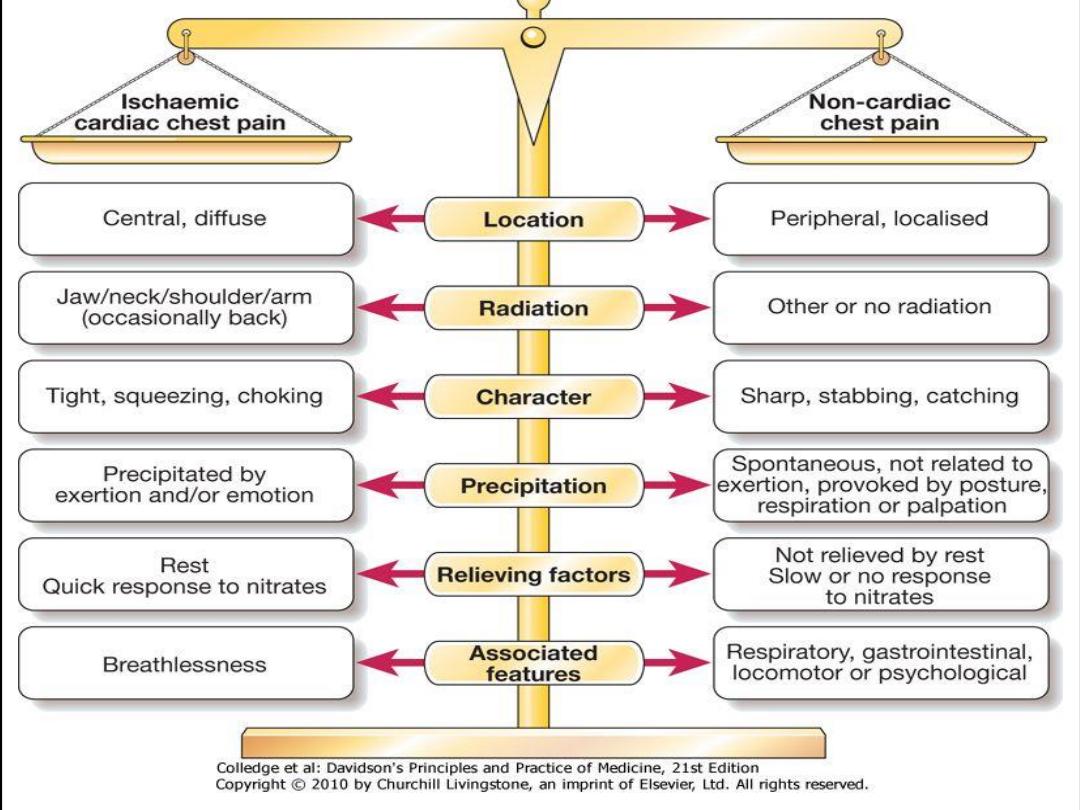
Differential diagnosis
The differential diagnosis of anginal chest pain is wide and includes
• Anxiety and hyperventilation
• Musculoskeletal chest wall pain
• Cervical or thoracic root pain
• Pneumothorax, pneumonia, or pulmonary embolus
• Esophageal problem (inflammation/spasm)
• Other upper gastrointestinal (GI) problem (gastritis, peptic ulcer,
pancreatitis, cholecystitis)
• Pericarditis
• Aortic dissection
• Mitral valve prolapse
• Coronary emboli (LV mural thrombus, atrial myxoma)

Investigations
Further risk stratification will add to the diagnostic certainty achieved by
history and examination.
Measure complete blood count (CBC), chemistries,
a full fasting lipid profile (total, LDL and HDL cholesterol and triglyceride
levels), and blood glucose.
Chest X-ray (CXR)
is not mandatory but should be performed if there is
suspicion of heart failure, aortic dissection, a pulmonary condition or an
abnormality of the bony structures of the chest wall.
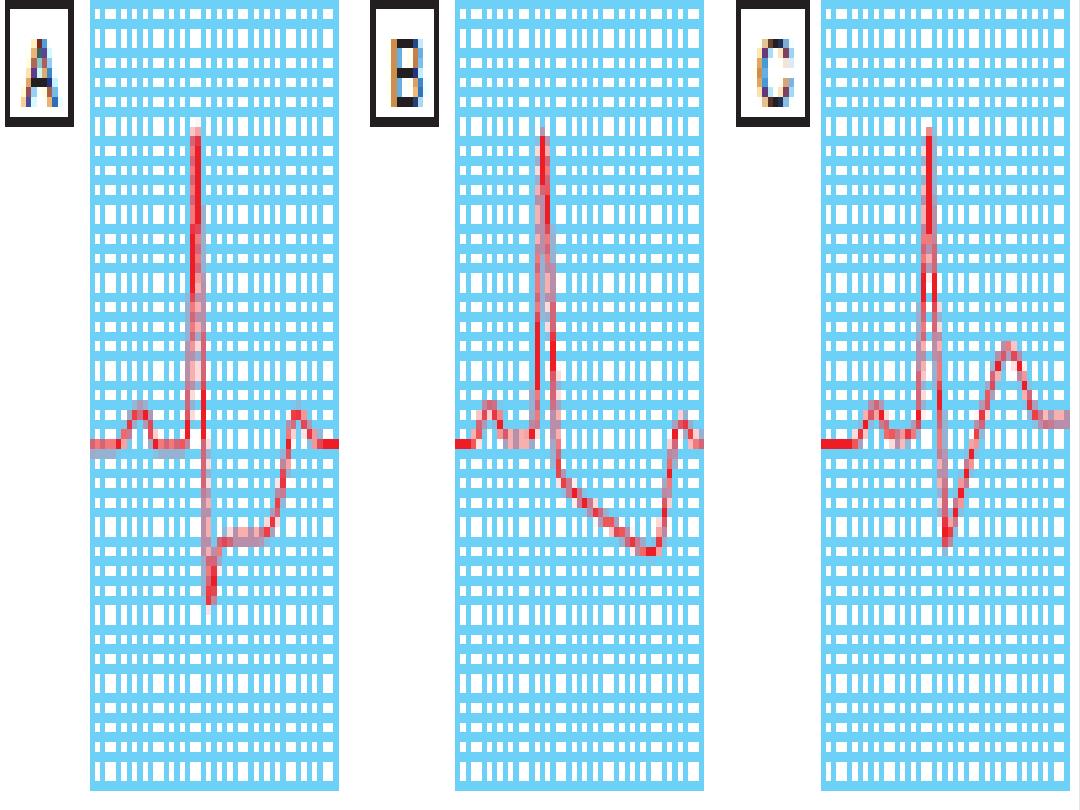
12 Lead ECG
A resting electrocardiogram (ECG) may not confirm the diagnosis but can point
toward ischemic heart disease. The presence of Q waves suggests previous
myocardial injury. The presence of ST depression and, to a lesser extent, T-
wave inversion during pain is a marker of ischemia and patients with these
signs should be further investigated. If ST-segment deviation is observed at
rest, an acute coronary syndrome must be excluded. A 12-lead ECG can
also help identify other causes of chest pain (LVH,arrhythmia, pericarditis).
Tests for inducible ischemia
Tests such as exercise ECG, stress echocardiogram (ECHO), or myocardial
perfusion scanning are useful adjuncts to confirm the diagnosis and aid
management.
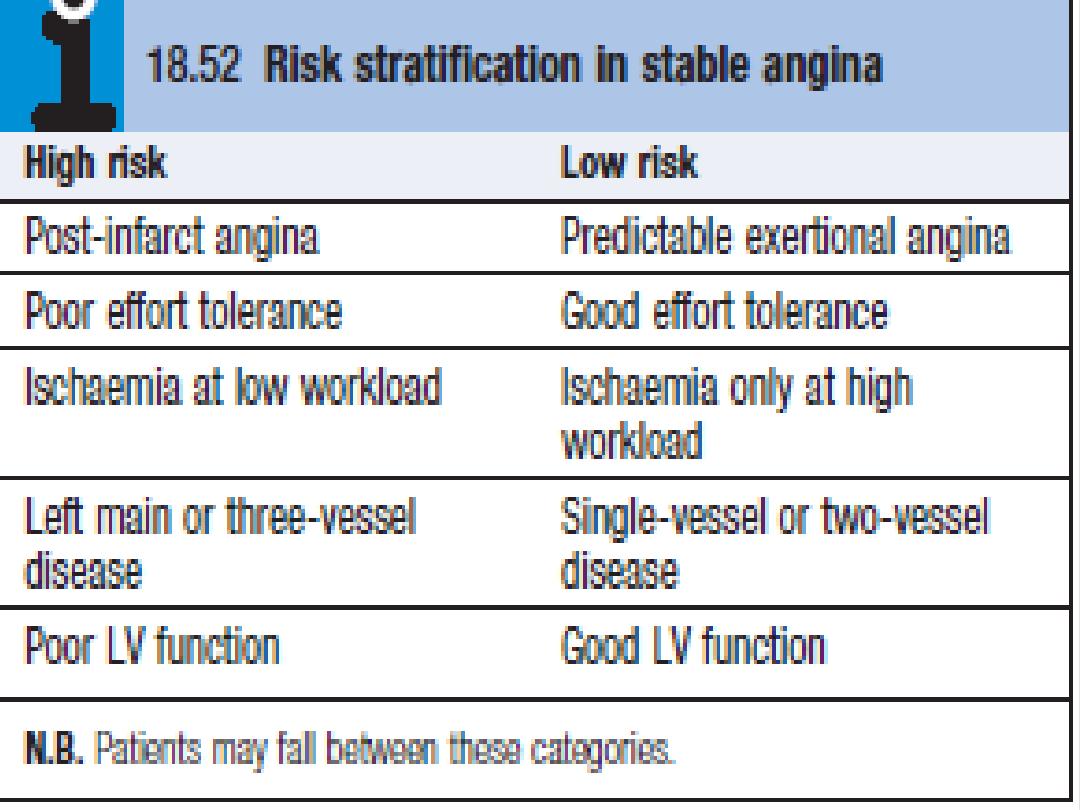



Management
Lifestyle
Smoking cessation is of paramount importance. Encourage daily aerobic exercise within limits of exercise capacity. Look
at the patient’s occupational needs and advise adjustment if symptom level is not compatible. Advise a healthy diet,
collaborating with dieticians if required.
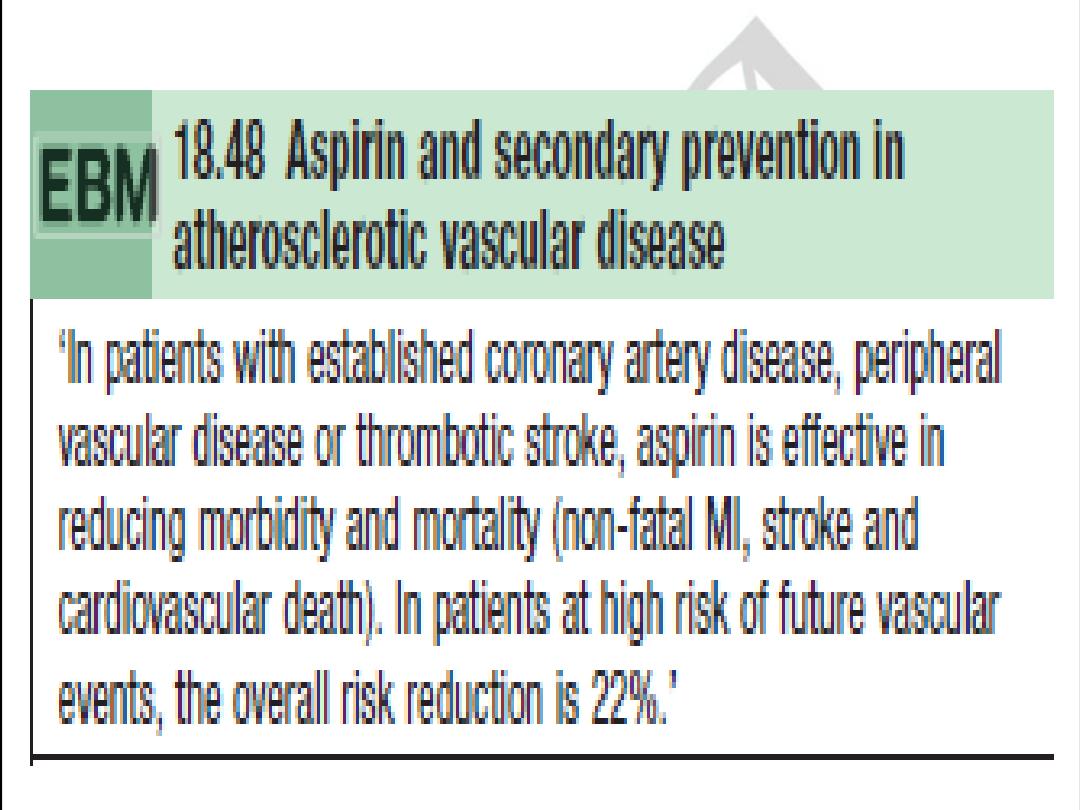
Aspirin
Provide aspirin in all cases unless there is active peptic ulcer disease, allergy (desensitizing may be required), or bleeding
diathesis. Those with past peptic ulcer disease may take a gastro protective agent such as an H2 antagonist or proton
pump inhibitor.
Anti-anginals
• B-Blockers:
First line (e.g., atenolol 25–100 mg qd or metoprolol 25–50 mg bid). Start on suspicion of ischemic heart
disease. Avoid only if contraindicated (asthma with confirmed B-agonist response
(mortality improved in patients with angina and concomitant COPD if they can tolerate bronchospasm), uncontrolled
severe LV dysfunction, bradycardia, coronary artery spasm).
• Calcium antagonists
(e.g., amlodipine or diltiazem): If B-blocker contraindicated or concern for vasospasm, calcium
antagonists become the drug of choice.
• Nitrates
(e.g., nitroglycerin): Used for control of breakthrough angina. Long-acting nitrates (e.g., isosorbide
mononitrate 60–120 mg qd) are a
useful addition to B-blockers for prevention of attacks.
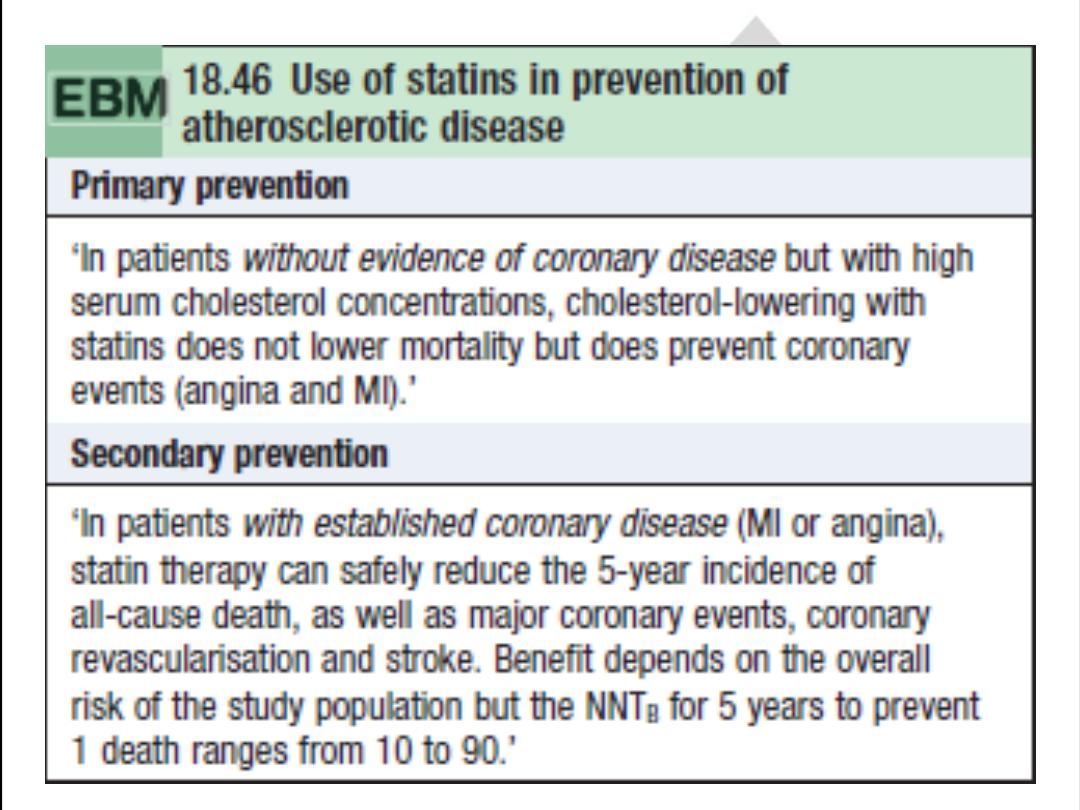
.Statins
Statins (HMG-CoA reductase inhibitors)
reduce mortality by approximately one-third in all risk groups. However, the
underlying risk of events must be taken into account when considering starting the drug, because absolute risk
reduction in young patients with low-risk IHD may be very small, with possible harm of myositis, hepatic failure, and
reduced compliance with other medications


The End

Coronary artery disease
Acute coronary syndromes
Power point 3

Definition
• Acute coronary syndrome (ACS) is a term used
to describe a constellation of symptoms
resulting from acute myocardial ischemia.
ACS includes the diagnosis of unstable angina
(UA), non-ST elevation myocardial infarction
(NSTEMI), and ST elevation myocardial
infarction (STEMI).
An ACS resulting in myocardial injury is termed
myocardial infarction (MI).

Acute coronary syndromes
The current nomenclature divides ACS into two major groups on the basis of
delivered treatment modalities :-
1- ST elevation myocardial infarction (STEMI)—
an ACS in which patients
present with chest discomfort and ST-segment elevation on ECG. This group
of patients must undergo reperfusion therapy on
presentation.
2-Non-ST elevation myocardial infarction (NSTEMI) and unstable angina
(UA)—ACS
in which patients present with ischemic chest discomfort
associated with transient or permanent non-ST-elevation ischemic ECG
changes. If there is biochemical evidence of myocardial injury, the condi-
tion is termed NSTEMI, and in the absence of biochemical myocardial
injury the condition is termed UA. This group of patients is not treated
with thrombolysis.
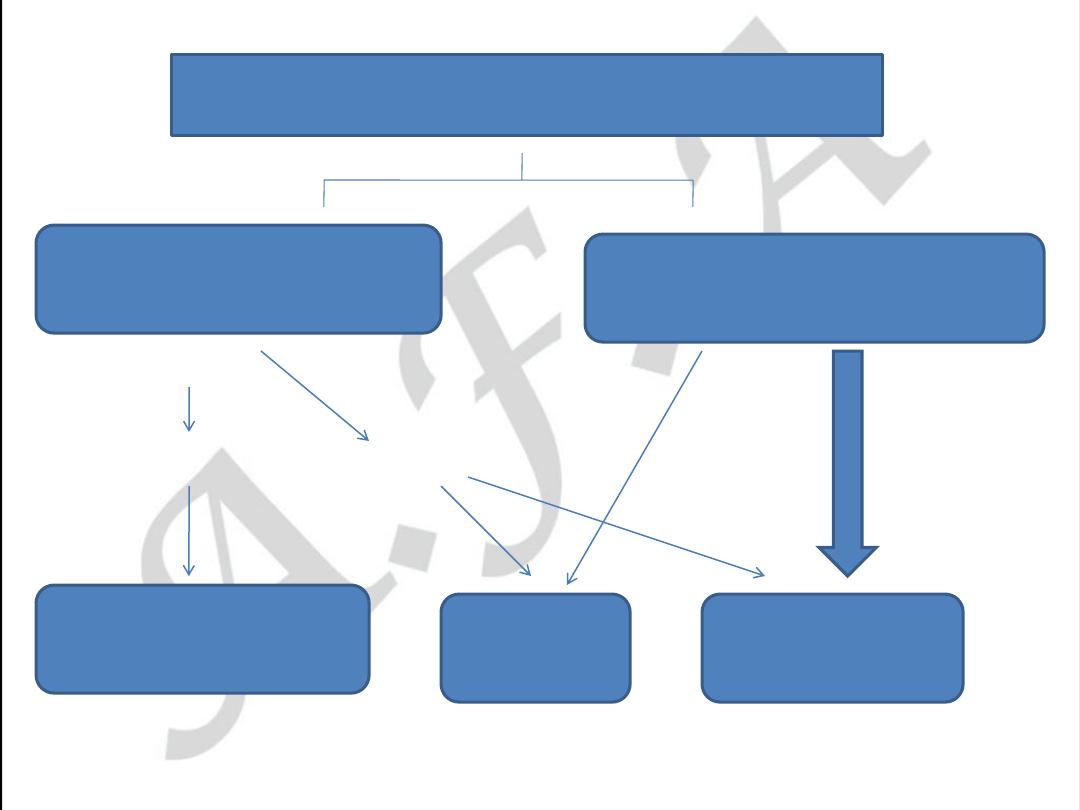
ACUTE CORONARY SYNDROMES
NO ST - elevation
ST - elevation
Unstable angina
NQ w- MI
Q w - MI
NO BIOMARKER RISE
NSTEMI

Conditions mimicking pain in ACS
• Pericarditis
• Dissecting aortic aneurysm
• Pulmonary embolism
• Esophageal reflux, spasm, or rupture
• Biliary tract disease
• Perforated peptic ulcer
• Pancreatitis

Initial management of ACS
• All patients with suspected ACS should have
continuous ECG monitoring and access to a
defibrillator.
• Rapid assessment and stabilization is
imperative.

management of ACS
• Rapid examination to exclude hypotension, note the
presence of murmurs, and identify and treat acute
pulmonary edema.
• Secure IV access.
• 12 Lead ECG should be obtained and reported within 10
minutes of presentation.
• Give the following:
• Oxygen (initially only 28% if history of COPD)
• Morphine 2.5–10 mg IV prn for pain relief
• Aspirin 325 mg po
• Nitroglycerin, unless hypotensive
• Heparin IV and/or Integrilin
• Consider addition of Plavix
• Take blood for the following:
• CBC/chemistries Supplement K+ to keep it at 4–5
mmol/L
• GlucoseMay be i acutely post-MI, even in nondiabetic
reflecting a stress-catecholamine response and may
resolve without treatment
• Biochemical markers of cardiac injury
• Lipid profileTotal cholesterol, LDL, HDL, triglycerides
• Serum cholesterol and HDL remain close to baseline for
24–48 hours but fall thereafter and take 8 weeks to
return to baseline.
• Portable CXR to assess cardiac size and pulmonary
edema and to
exclude mediastinal enlargement.
• General examination should include peripheral
pulses, fundoscopy, and
abdominal examination for organomegaly and
aortic aneurysm.

The End

Acute coronary syndrome
Non-ST elevation myocardial
infarction
(NSTEMI)/unstable angina (UA)
Power point 4
UA and NSTEMI
are closely related conditions with similar clinical
presentation, treatment, and pathogenesis but of varying severity. If there
is
biochemical evidence of myocardial damage, the condition is termed
NSTEMI; in the absence of damage it is termed UA.
Unlike patients with a STEMI, in whom diagnosis is generally made on
presentation in the emergency department, diagnosis of NSTEMI/UA may
not be definitive on presentation and evolves over the subsequent hours to
days. Therefore, management of patients with NSTEMI/UA is a progression
through a number of risk strati
fication processes dependent on history,
clinical features, and investigative results. These in turn determine
the
choice and timing of a number of medical and/or invasive treatment
strategies.

Clinical presentation
There are three distinct presentations:
• New-onset angina (in a patient without prior
angina)
• Rest angina (angina when patient is at rest; may
occur in a patient with prior stable, exertional
angina)
• Increasing angina (in a patient with previously
diagnosed angina for whom angina has become
more frequent or longer in duration or requires a
lower threshold to elicit)
NSTEMI/UA: diagnosis
Diagnosis in NSTEMI/UA is an
evolving process
and may not be
clear on presentation. A combination of history, serial changes
in ECG, and biochemical markers of myocardial injury (usually
over a 24- to 48-hourperiod) determine the diagnosis.
Serial ECGs :
Changes can be transient and/or fixed,
especially if a diagnosis of NSTEMI is made.
• ST-segment depression of 0.05 mV is highly specific of
myocardial ischemia (unless isolated in V1–V3, suggesting a
posterior STEMI).
• T-wave inversion is sensitive but nonspecific for acute ischemia
unless very deep ( 0.3 mV).
• Rarely, Q waves may evolve or there may be transient or new
LBBB.
Serial biochemical markers of cardiac injury
These are used to differentiate between NSTEMI and UA, as
well as to determine prognosis. Levels at 0, 6, and 12 hours
after
the last episode of pain. A positive biochemical marker
(CK, CK-MB, or
troponin) in the context of one or more of the
ECG changes listed above
is diagnostic of NSTEMI. If serial
markers over a 24-hour period from the
last episode of chest
pain remain negative, UA is diagnosed
.
Cardiac troponin T and I
Both of these are highly cardiac
speci
fic and sensitive, can detect “micro-
infarction
” in the presence of normal CK-MB, are not affected by skeletal
muscle injury, and convey prognostic information (worse prognosis if posi-
tive)
. Troponins can be raised in nonatherosclerotic myocardial damage
(cardiomyopathy, myocarditis, pericarditis chronic renal failure) and should
thus be interpreted
in the context of the clinical picture.
Both TnT and TnI rise within 3 hours of infarction. TnT may persist up
to 10
–14 days and TnI up to 7–10 days.
•
CK levels do not always reach the diagnostic twice upper-limit of
normal and generally have little value in diagnosis of NSTEMI.
•
CK-MB has low sensitivity and speci
ficity. CK-MB isoforms improve
sensitivity (CK-MB2>1 U/L or CK-MB2/CK-MB1 ratio >1.5), but isoform
assays are not widely available clinically.
•
Myoglobine is non
–cardiac specific, but levels can be detected as early
as 2 hours after onset of symptoms. A negative test is useful in ruling
out myocardial necrosis.

NSTEMI/UA: risk stratification
Early risk stratification
This should take place on presentation and forms part of the initial
assessment used to make a diagnosis. It involves a combination of clinical
features, ECG changes, and biochemical markers of cardiac injury.
Patients are divided into high risk and intermediate/low risk.
• High-risk patients should be admitted to the CCU, follow an early invasive
strategy, and be managed with a combination of :-
. ASA, clopidogrel, LMWH (or UFH), and/or gpIIb/IIIa antagonists
. Anti-ischemic therapy (first-line B-blocker, nitroglycerin)
. Early invasive strategy (inpatient catheterization and PCI within 48 hours
of admission)
• Intermediate- to low-risk patients should be admitted to a monitored
bed on a step-down unit and undergo a second inpatient risk stratification
once their symptoms have settled, to determine timing of invasive
investigations. Initial management should include :-
• ASA, clopidogrel, LMWH (or UFH)
• Anti-ischemic therapy (first-line B-blocker, nitroglycerin)
• Undergo a late risk stratification in 48–72 hours from admission

Late risk strati
fication
This involves a number of noninvasive tests to determine the optimal tim-
ing for invasive investigations in intermediate/low-risk patients. It is gener-
ally performed if there have been no further episodes of pain or ischemia
at 24
–48 hours after admission.
•
Intermediate/low-risk patients who develop recurrent pain and/or
ischemic ECG changes, heart failure, or hemodynamic instability (in
the absence of a noncardiac cause) should be managed as a high-risk
•
. High-risk patients from these assessments should also follow an early
invasive strategy and intermediate/low-risk patients, a more conservative
strategy.

NSTEMI/UA: medical management
1. Analgesia
Morphine 2.5–5 mg IV. Acts as anxiolytic,
reduces pain and systolic blood pressure
through venodilatation and reduction in
sympathetic arteriolar constriction. It can
result in hypotension (responsive to volume
therapy) and respiratory depression (reversal
with naloxone 400 µg to 2 mg IV).

2. Nitrates
•
Nitroglycerin infusion (50 mg in 50 mL normal saline at 1
–10 mL/hr) titrated to pain and keeping SBP >100 mmHg. T
3. B-Blockers
•
These should be started on presentation. Initially use a short-acting agent (e.g., metoprolol 12.5
–100 mg po bid),
which if tolerated, may be converted to a longer acting agent (e.g., atenolol 25
–1000 mg qd).
Rapid B-blockade may be achieved using short-acting IV agents such as metoprolol. Aim for HR of ~50
–60
beats/min.
4. Calcium antagonists
•
Diltiazem 60
–360 mg po, verapamil 40–120 mg po tid. These aim to reduce HR and BP and are a useful adjunct to
treatments 1
–3 above.
Amlodipine/felodipine 5
–10 mg po qd can be used with pulmonary edema and in poor LV function.
5. Statins (HMG-CoA reductase inhibitors)
High-dose statins (e.g., atorvastatin 80 mg qd) have been shown to
re
duce mortality and recurrent MI in the acute
setting. The role of stat
in
s in primary and secondary prevention of cardiovascular events is well
d
ocumented.
Antiplatelet therapy
All patients should be given aspirin and clopidogrel (unless
c
ontraindications)
—gp IIb/IIIa antagonists to high-risk
patients only.
Antithrombotic therapy
They should be used in conjunction with aspirin and clopidogrel in all
p
atients on presentation and continued
for 2
–5 days after the last episode
o
f pain and ischemic ECG changes.

Discharge and secondary prevention
•
Length of hospital stay will be determined by symptoms and the rate of
progression through the NSTEMI/UA pathway. Generally patients are
hospitalized for 3
–7 days.
•
Secondary prevention remains of paramount importance and is similar in
principle to that for STEMI patients.
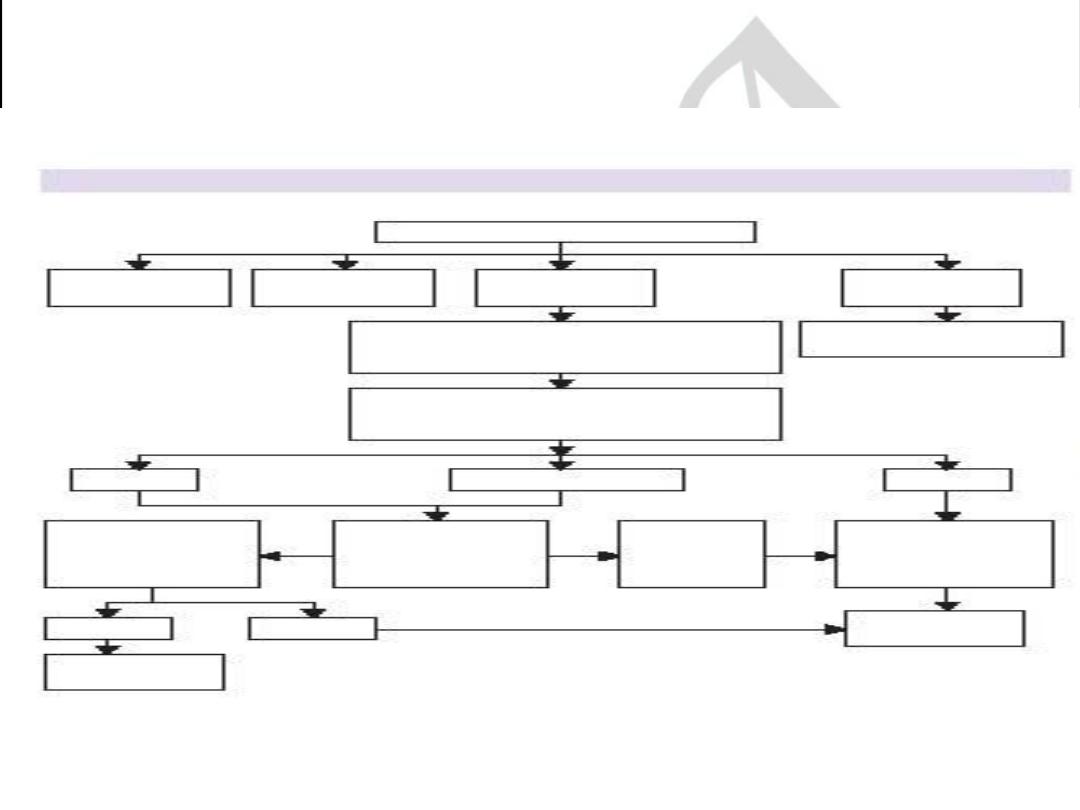
Symptoms suggestive of ACS
Noncardiac
d
iagnosis
Chronic
st
able angina
NSTEMI/UA
STEMI
Admit
,
A
SA, Clopidogrel, LMWH (UFH),
N
TG infusion, B-blocker, statin
Admit to CCU,Reperfusion ther
.
Early risk stratifiction in E.R
Low risk
Intermid. risk
High risk
No,
Late risk stratification
Admit, monitor.
Sympt.cont.?
Yes, treat as
High risk
Admit to CCU
Early invasive strategy
Low risk
High risk
O.P angiography
In patient angiog.

• Mention 3 characteristics of vulnerable
atheromatus plaque.

The End

Acute coronary syndromes
ST elevation myocardial infarction
(STEMI)
Power point 5

ST elevation myocardial infarction
(STEMI)
Patients with ACS who have ST-segment
elevation or new left bundle branch block
(LBBB) on their presenting ECG benefit
significantly from immediate reperfusion and
are treated as one group under the term ST-
elevation myocardial infarction (STEMI).
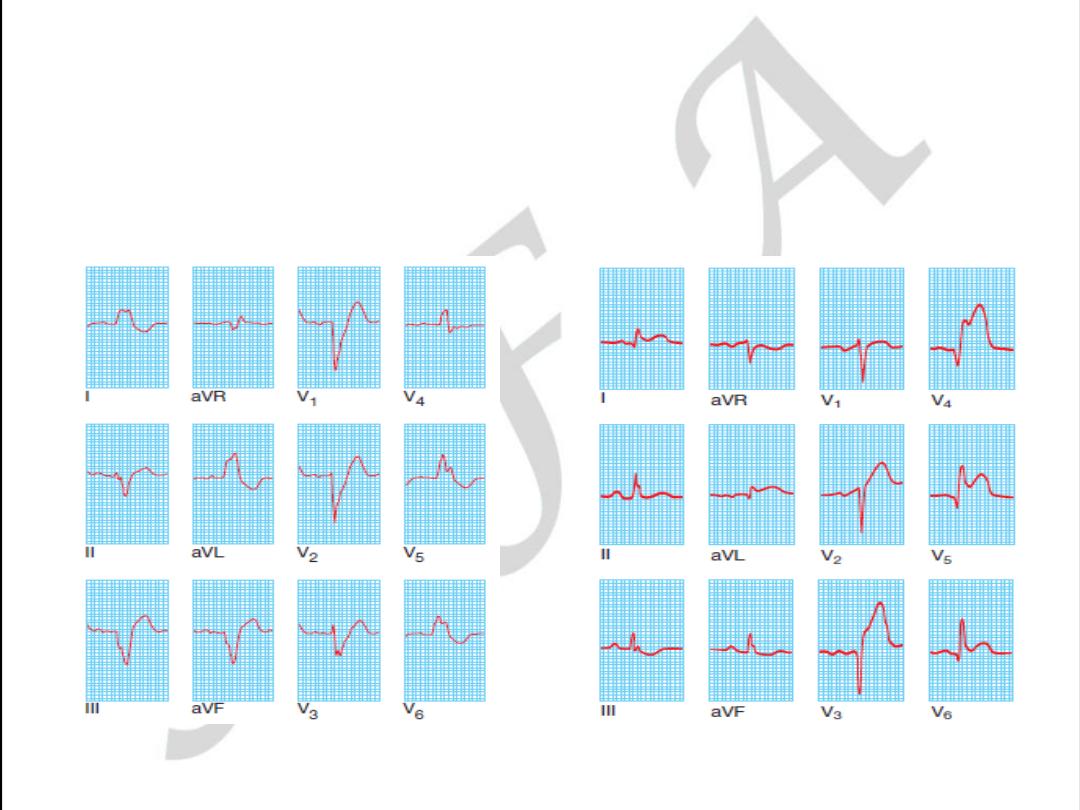
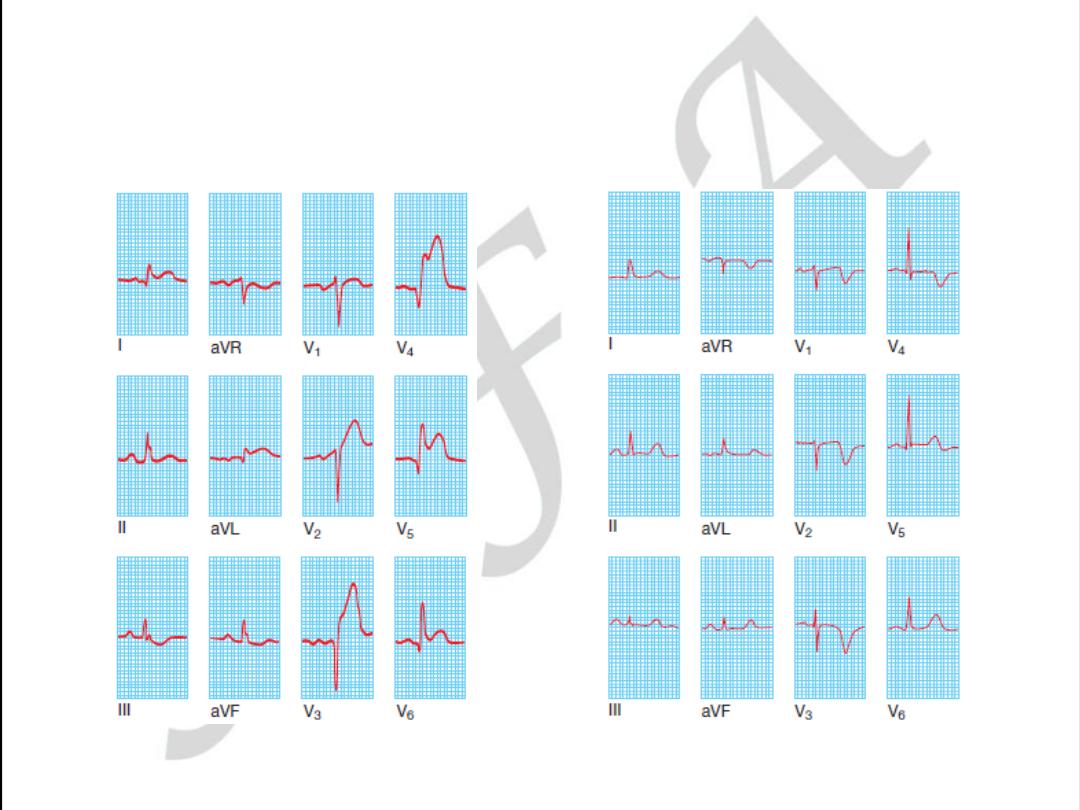
LEFT BANDLE BRANCH BLOCK
ANT.WALL STEMI
Presentation
• Chest pain is usually similar in nature to angina, but of greater severity
and longer duration and is
not
relieved by sublingual (SL) nitroglycerin.
Associated features are nausea and vomiting, sweating, breathlessness,
and extreme distress.
• The pain may be atypical (e.g., epigastric) or radiate to the back.
• Diabetics, elderly, women, or hypertensive patients may suffer painless
(“silent” infarcts) and/or atypical infarction. Presenting features
include breathlessness from acute pulmonary edema, syncope or coma
from arrhythmias, acute confusional states (mania/psychosis), diabetic
hyperglycemic crises, hypotension or cardiogenic shock, central nervous
system (CNS) manifestations resembling stroke secondary to sudden
reduction in cardiac output, and peripheral embolization.

STEMI: diagnosis
• STEMI diagnosis is based on a combination of
history, ECG, and biochemical markers of
cardiac injury.
• In practice, history and ECG changes are
diagnostic.
• Biochemical markers of cardiac injury usually
become available later and help reconfirm the
diagnosis and provide prognostic information
(magnitude of rise).

-- Aspects of Diagnosis of Myocardial Infarction by Different Techniques
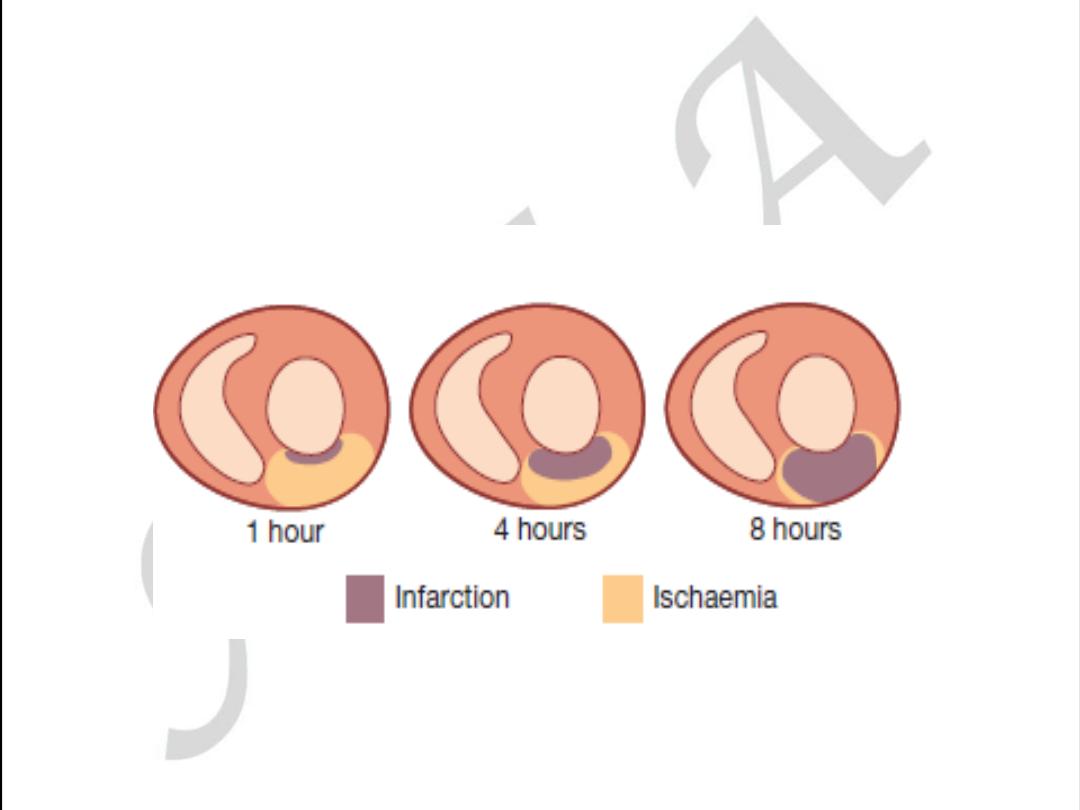
Pathology
Myocardial cell death
Biochemistry
Markers of myocardial cell death recovered from blood samples
Electrocardiography Evidence of myocardial ischemia (ST and T wave abnormalities)
Evidence of loss of electrically functioning cardiac tissue (Q
waves)
Imaging
Reduction or loss of tissue perfusion
Cardiac wall motion abnormalities

Revised Definition of Myocardial Infarction (MI)
Criteria for Acute, Evolving, or Recent MI
Either of the following criteria satisfies the diagnosis for acute, evolving, or recent MI:
1. Typical rise and/or fall of biochemical markers of myocardial necrosis with at
least one of the following:
a) Ischemic symptoms
b) Development of pathological Q waves in the ECG
c) ECG changes indicative of ischemia (ST segment elevation or
depression)
d) Imaging evidence of new loss of viable myocardium or new regional
wall motion abnormality
2. Pathological findings of an acute myocardial infarction
Criteria for Healing or Healed Myocardial Infarction
Any one of the following criteria satisfies the diagnosis for healing or healed myocardial
infarction:
1. Development of new pathological Q waves in serial ECGs. The patient may or
may not remember previous symptoms. Biochemical markers of myocardial
necrosis may have normalized depending on the length of time that has passed
since the infarction developed.
2. Pathological findings of a healed or healing infarction
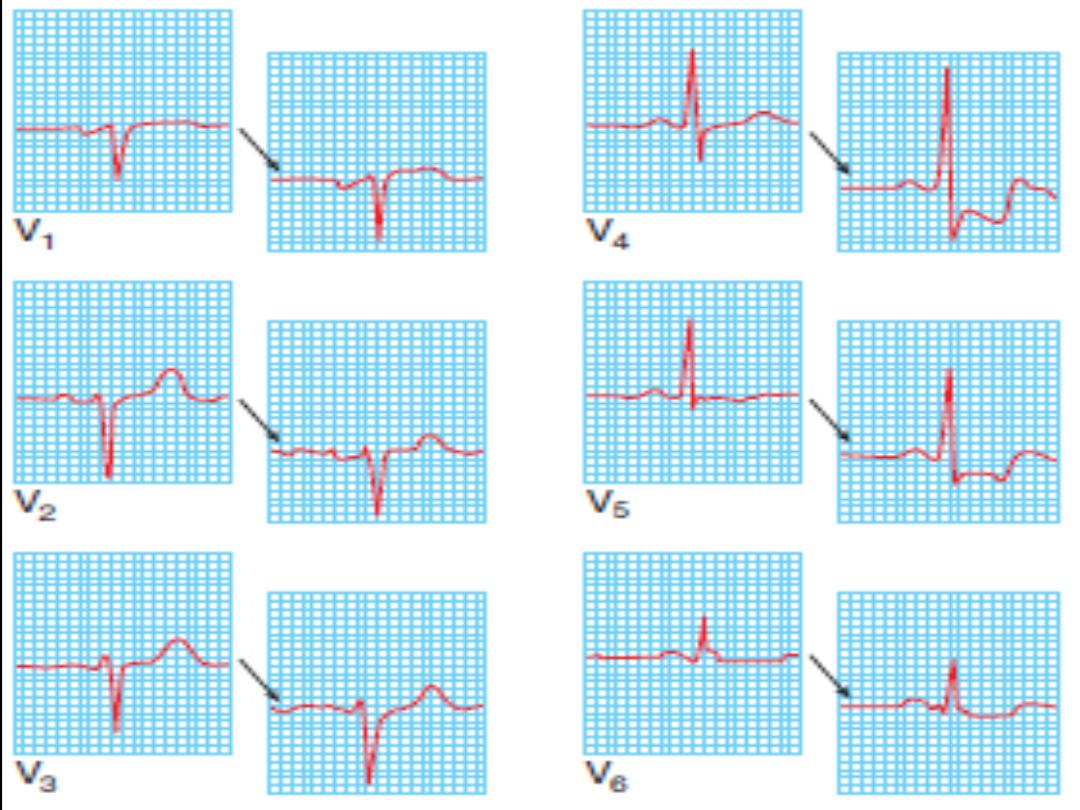
ECG changes
ST-segment elevation
occurs within minutes and may last for up to
2 weeks. ST elevation of 2 mm in adjacent chest leads and 1 mm in
adjacent limb leads is necessary to fulfill thrombolysis criteria. Persisting
ST elevation after 1 month suggests formation of LV aneurysm.
Pathological Q waves
indicate significant abnormal electrical
conduction but are not synonymous with irreversible myocardial
damage. In the context of a “transmural infarction” the Q waves may
take hours or days to develop and usually remain indefinitely. In the
standard leads, the Q wave should be 25% of the R wave, 0.04 s in
duration, with negative T waves. In the precordial leads, Q waves in V4
should be >0.4 mV (4 small sq) and in V6 >0.2 mV (2 small sq), in the
absence of LBBB (QRS width <0.1 s or 3 small sq).
ST-segment depression in a second territory
(in patients with
ST-segment elevation) is secondary to ischemia in a territory other
than the area of infarction (often indicative of multivessel disease) or
reciprocal electrical phenomena. Overall, it implies a poorer prognosis.
T-wave inversion
may be immediate or delayed and generally persists after
the ST elevation has resolved.
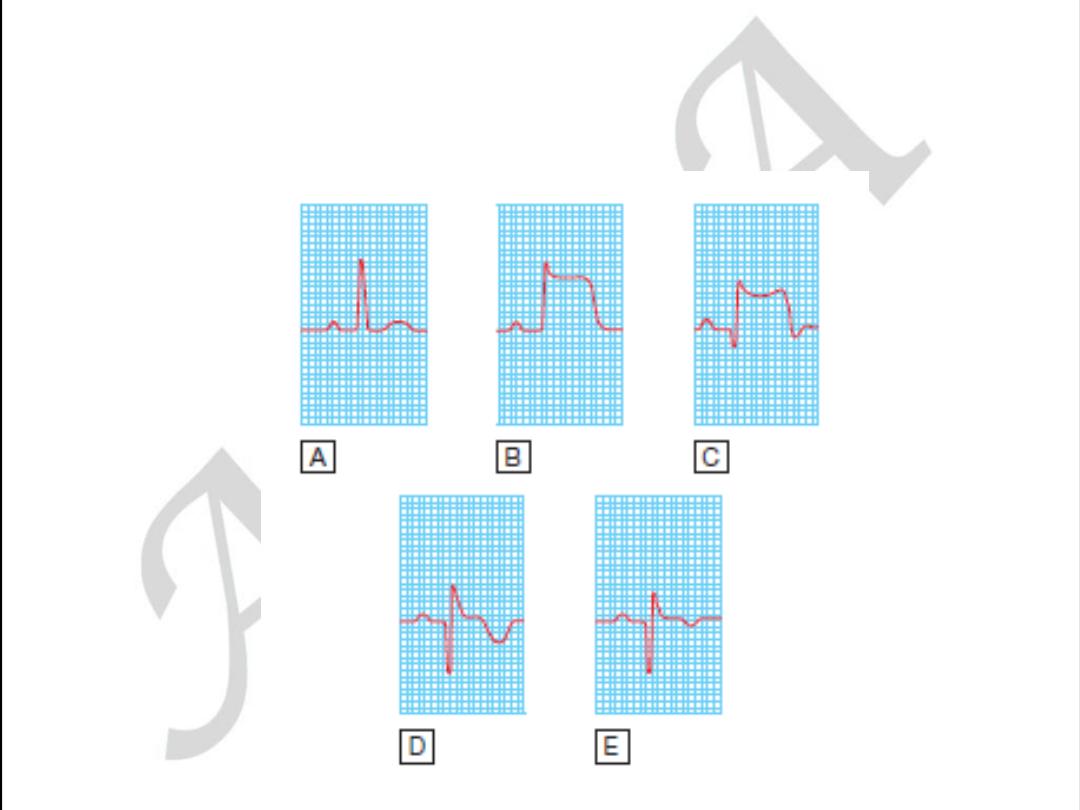
Anterior
ST elevation and/or Q waves in V1–V4/V5
Anterseptal
ST elevation and/or Q waves in V1–V3
Lateral
ST elevation and/or Q waves in V5–V6 and T-wave
inversion/ST elevation/Q waves in I and aVL
Inferoseptal
ST elevation and/or Q waves in II, III, aVF, and 5–V6
(sometimes I and aVL)
True posterior
Tall R waves in V1–V2 with ST depression in V1–V3. T
waves remain upright in V1–V2. This can be confirmed with an esophageal
lead if available (method similar to an NG tube). This usually occurs in
conjunction with an inferior or lateral infarct
RV infarction ST-segment elevation in the right precordial leads (V3R–
V4R). Usually found in conjunction with inferior infarction. This may only
be present in the early hours of infarction
Localization of infarcts from ECG
changes

Conditions that may mimic ECG
changes of a STEMI
• Left or right ventricular hypertrophy
• LBBB or left anterior fascicular block
• Wolff–Parkinson–White syndrome
• Pericarditis or myocarditis
• Cardiomyopathy (hypertrophic or dilated)
• Trauma to myocardium
• Cardiac tumors (primary and metastatic)
• Pulmonary embolus
• Pneumothorax
• Intracranial hemorrhage
• Hyperkalemia
• Cardiac sarcoid or amyloid
• Pancreatitis

Biochemical markers of cardiac
injury
CK (creatine kinase)
• Levels
twice the upper limit of normal
are considered abnormal.
• Serum levels rise within 4–8 hours post-STEMI and fall to normal within 3–4
days. The peak level occurs at about 24 hours but may be earlier (12 hours)
and higher in patients who have had reperfusion (thrombolysis or
percutaneous coronary intervention [PCI]).
• False-positive rates of ~15% occur in patients with alcohol intoxication, muscle
disease or trauma, vigorous exercise, convulsions, IM injections,
hypothyroidism, pulmonary embolism (PE), and thoracic outlet
syndrome.
CK-MB isoenzyme
is more specific for myocardial disease. Levels may be
elevated despite a normal total CK. However, CK-MB is also present in
small quantities in other tissues (skeletal muscle, tongue, diaphragm,
uterus, and prostate) and trauma or surgery may lead to false-positive
results. If there is doubt about myocardial injury with CK-MB levels
obtained, a cardiac troponin must be measured.
Cardiac troponins ( Tn T ,Tn I
)
•
Both TnT and TnI are highly sensitive and speci
fic markers of cardiac injury.
•
Serum levels start to rise by 3 hours post-MI and elevation may persist up to 7
–14 days. This is
advantageous for diagnosis
of late MI.
•
In most STEMI cases, the diagnosis can be made using a combination of the clinical picture
and serial CK/CK-MB levels.
In the event of normal CK-MB levels and suspected noncardiac
sources of CK,
troponins can be used.
•
Troponins can also be elevated
in non ischemic
myocyte damage, such as myocarditis,
cardiomyopathy, and pericarditis
.
Other markers
There are multiple other markers, but with increasing clinical availability of Troponin,
measurements of these markers are not recommended. These include aspartamine
transferase (AST) (rise 18–36 hours post-MI) and lactate dehydrogenase (LDH) (rise 24–36
hours post-MI).
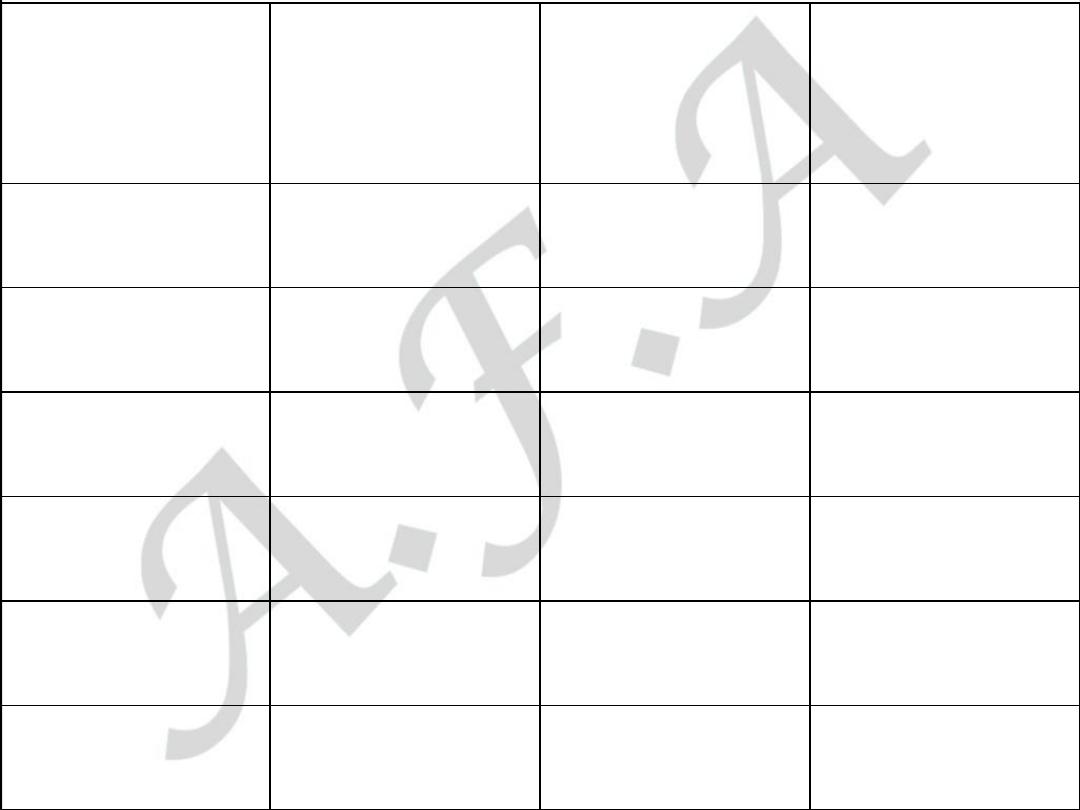
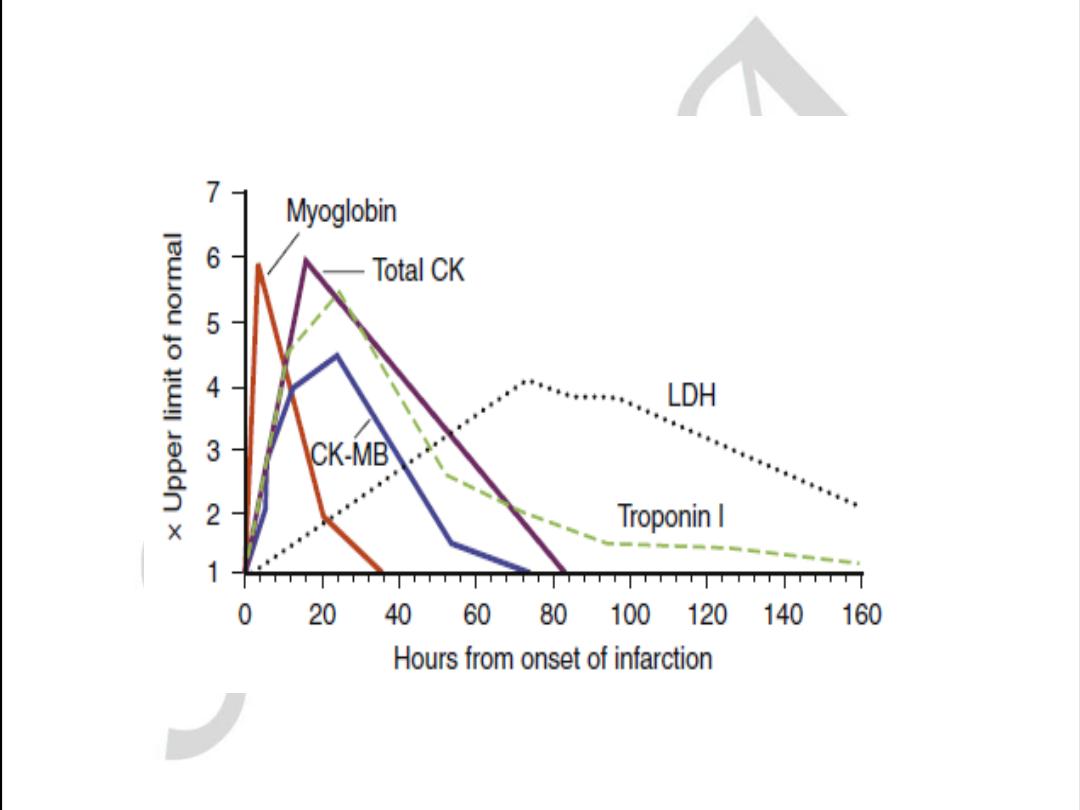
Biomarker
Begins to rise
Peak value
Returns to
normal
Myoglobin
1-2 hours
6-8 hours
1-2 days
CK-MB
2-6 hours
16-20 hours
1-2 days
CK
4-8 hours
16-24 hours
3-4 days
Trop T
4-6 hours
12-24 hours
7-10 days
AST
12-24 hours
36-48 hours
3-4 days
LDH
24-48 hours
72 hours
8-10 days

Factors associated with a poor
prognosis
• Age >70 years
• Previous MI or chronic stable angina
• Anterior MI or right ventricular infarction
• Left ventricular failure at presentation
• Hypotension (and sinus tachycardia) at
presentation
• Diabetes mellitus
• Mitral regurgitation (acute)
• Ventricular septal defect

Mention 3 differences between
unstable angina and myocardial
infarction.

Treatment of STEMI
All patients with STEMI should be admitted to an intensive care unit
(ICU), e.g., coronary ICU (CICU).
Stabilizing measures
are generally similar for all ACS patients.
• All patients with suspected STEMI should have continuous ECG
monitoring in an area with full resuscitation facilities.
• Patients should receive immediate aspirin 325 mg po (if no
contraindications), analgesia, and oxygen. Secure IV access.
• Conduct rapid examination to exclude hypotension, note the
presence of murmurs, and identify and treat acute pulmonary
edema.
Right ventricular failure (RVF) out of proportion to left ventricular
failure (LVF) suggests RV infarction.
Diagnosis
is normally made on presentation (history and ECG changes of ST –
elevation /new LBBBB )followed by
rapid stabilizing measures to
ensure institution
of
reperfusion therapy
without delay.
This is in contrast to NSTEMI/UA, where diagnosis may evolve over a period of
24–72 hours. (Reperfusion must not be delayed to wait for biochemical markers.)
Control of cardiac pain
•
Morphine
2.5
–10 mg IV is the drug of choice and may be repeated
to ensure adequate pain relief, unless there is evidence of emerging
toxicity (hypotension, respiratory depression). Nausea and vomiting
should be treated with metoclopramide (10 mg IV) or a phenothiazine.
•
Oxygen
should be administered at 2
–5 L/min for at least 2–3 hours.
Hypoxemia is frequently seen post-MI due to ventilation
–perfusion
abnormalities secondary to LVF. In patients with refractory pulmonary
edema, endotracheal intubation may be necessary. Beware of CO2
retention in patients with COPD.
•
Nitrates
may lessen pain and can be given (sublingual or IV) provided
that the patient is not hypotensive. These drugs should be used
cautiously in inferior STEMI, especially with right ventricular infarction,
as venodilation may decrease RV
filling and precipitate hypotension.
Nitrate therapy has no demonstrated effect on mortality (ISIS-4).
Correction of electrolytes
Both low potassium and low magnesium may be arrhythmogenic and must
be supplemented, especially in the context of arrhythmias.
Strategies to limit infarct size
B-Blockade
Early B-blockade has been shown to be beneficial by limiting infarct size,
reducing mortality, and decreasing early malignant arrhythmias.
All patients (including primary PCI and thrombolysis patients) should have
early B-blockade.
Patients with the following features may benefit most from B-blocker therapy:
• Hyperdynamic state (sinus tachycardia, hypertensive)
• Ongoing or recurrent pain or reinfarction
• Tachyarrhythmias such as AF
Use a short-acting agent IV initially (metoprolol 5 mg at a time repeated at
5-minute intervals to a maximum dose of 15 mg) under continuous ECG
and BP monitoring. Aim for a HR of 60 beats per minute (bpm) and SBP
100–110 mmHg. If hemodynamic stability continues 15–30 minutes after
the last IV dose, start metoprolol 50 mg po bid. Esmolol is an ultra-short
acting IV B-blocker, which may be tried if there is concern whether the
patient will tolerate B-blockers.
CONTRAINDICATIONS TO THE USE OF B- BLOCKERS
Absolute contraindications:
heart rate (HR) <50, systolic blood
pressure (SBP) <90 mmHg, moderate to severe heart failure, AV
conduction defect, severe airways disease.
Relative contraindications
: asthma, current use of calcium
channel blocker and/or B-blocker, severe peripheral vascular
disease with critical limb ischemia, large inferior MI involving the
right ventricle.

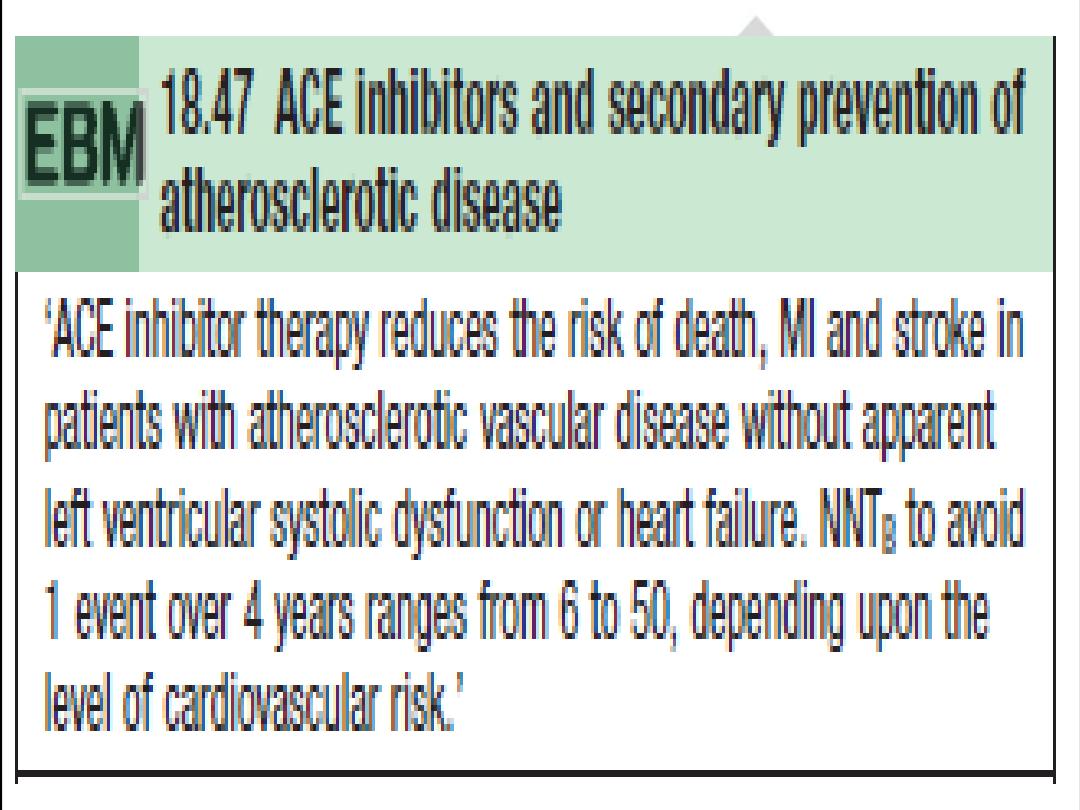
ACE inhibitors
After receiving aspirin, B-blockade (if appropriate), and reperfusion, all
patients with STEMI/LBBB infarction should receive an ACE inhibitor
within the
first 24 hours of presentation.
•
Patients with high risk or large infarcts, particularly with an
anterior STEMI, a previous MI, heart failure, and impaired LV
function on imaging
, will bene
fit most.
•
The effect of ACE inhibitors appears to be a class effect; therefore,
one may use the drug that the physician is familiar with.
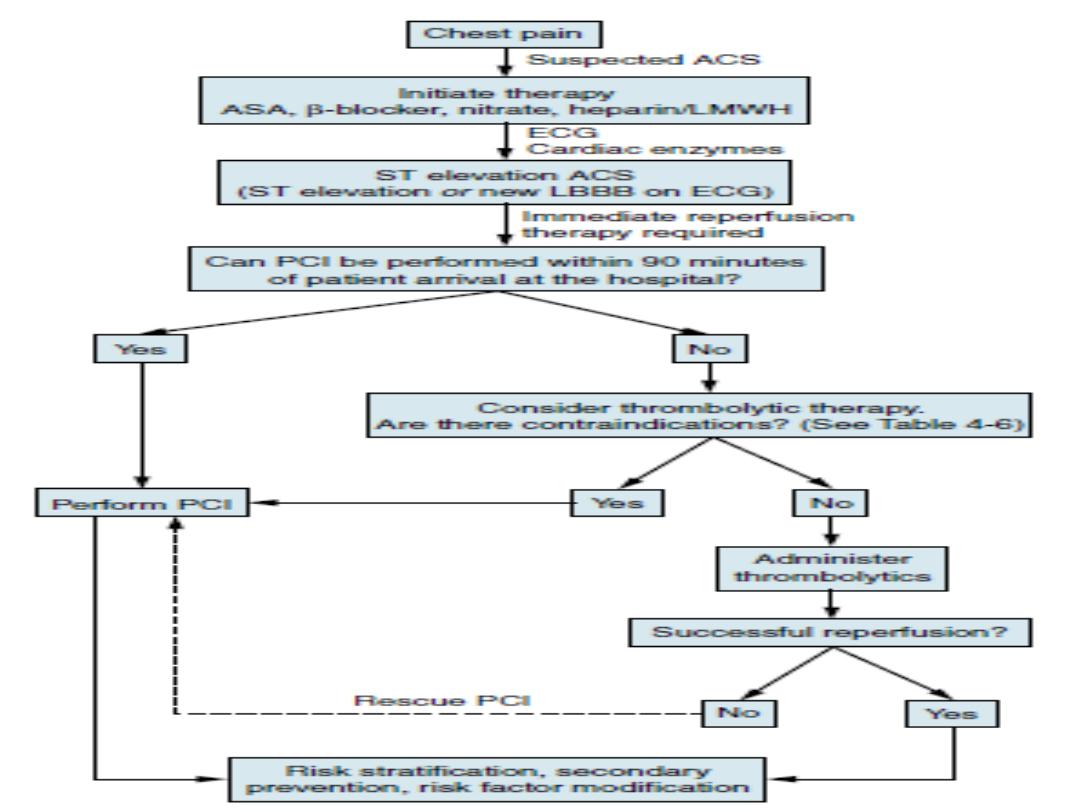
Assessment of Reperfusion Options for STEMI Patients
Step 1: Assess time and risk.
• Time since onset of symptoms
• Risk of STEMI
• Risk of fibrinolysis
• Time required for transport to a skilled PCI laboratory
Step 2: Determine if fibrinolysis or invasive strategy is preferred.
• If presentation is <3 hr and there is no delay to an invasive strateg y, there is no
preference for either strategy.
Fibrinolysis is generally preferred if:
• Early presentation (≤3 hr from symptom onset and delay to invasive strategy)
• Invasive strategy is not an option
• Catheterization laboratory occupied or not available
• Vascular access difficulties
• Lack of access to a skilled PCI laboratory
[*][†]
• Delay to invasive strategy
• Prolonged transport
• (Door-to-Balloon)–(Door-to-Needle) more than 1 hr
[‡][§]
• Medical contact-to-balloon or door-to-balloon more than 90 min
Reperfusion therapy (thrombolysis)
Indications for thrombolysis
•
Typical history of cardiac pain within previous 12 hours and ST elevation in two
contiguous ECG leads (>1 mm in limb leads or >2 mm in V1
–V6).
•
Cardiac pain with newly presumed LBBB on ECG.
•
If ECG is equivocal on arrival, repeat at 15- to 30-minute intervals to monitor
progression.
•
Thrombolysis
should not
be given if the ECG is normal or if there is isolated ST
depression (true posterior infarct must be excluded).
Timing of thrombolysis
•
Greatest bene
fit is achieved with early thrombolysis (especially if given within 1
1/2 hours(90min.s) of onset of
first pain.
•
Patients presenting between 12 and 24 hours from onset of pain
should undergo
thrombolysis only with persisting symptoms and ST-segment elevation.
•
Elderly patients (>65 years) presenting within the 12- to 24-hour time period with
symptoms are best managed by primary PCI, as thrombolysis has been
demonstrated to result in increased incidence
of cardiac rupture
.

Choice of thrombolytic agent
•
This is partly determined by each center
’s local thrombolysis strategy.
•
Allergic reactions and episodes of hypotension are greater with streptokinase (SK).
•
Bolus agents are easier and quicker to administer, with a decrease in drug errors in
comparison to
first-generation infusions.
•
Recombinant tissue-type plasminogen activator (rtPA) has a greater reperfusion
capacity and a marginally higher 30-day survival bene
fit than that of SK, but this
agent has been associated with an increased risk of
hemorrhage
.
•
More recent rtPA derivatives have demonstrated a higher 90-minute TIMI-III
(coronary reperfusion scale)
flow rate, but have shown similar 30-day mortality
bene
fits to those with rtPA.
•
An rtPA derivative (rather than SK) should be considered for any patient with any of
the following:
• Large anterior MI, especially if within 4 hours of onset
• Previous SK therapy or recent streptococcal infection (as this has been shown to
be a risk factor for allergic reactions to SK)
• Hypotension (systolic BP <100 mmHg)
• Low risk of stroke (age <55 years, systolic BP <144 mmHg)
• Reinfarction during hospitalization where immediate PCI facilities are not
available
Doses and administration of thrombolytic agents
Streptokinase (SK)
•
Give as 1.5 million units in 100 mL normal saline IV over 1 hour.
•
There is no indication for routine heparinization after SK; there is no clear mortality
bene
fit and there is a small increase in risk of hemorrhage.
Recombinant tissue-type plasminogen activator (rtPA, alteplase)
•
The GUSTO trial suggested that the
“front-loaded” or accelerated rtPA is the most
effective dosage regimen.
•
Give 15 mg bolus IV then 0.75 mg/kg over 30 minutes (not to exceed 50 mg), then
0.5 mg/kg over 60 minutes (not to exceed 35 mg).
•
This should be followed by IV heparin
.
Reteplase
•
Give two IV bolus doses of 10 units 10 minutes apart.
Tenectaplase
•
Give as injection over 10 seconds at 30
–50 mg according to body weight (500–600
µg/kg). Maximum dose is 50 mg.
APSAC (anistreplase)
•
Give as an IV bolus of 30 mg over 2
–5 minutes.

Complications of thrombolysis
• Bleeding is seen in up to 10% of patients. Most incidents are minor and at
sites of vascular puncture. Local pressure is generally sufficient to stop this
bleeding but occasionally transfusion may be required. In extreme cases,
SK may be reversed by tranexamic acid (10 mg/kg slow
IV infusion).
• Hypotension during the infusion is common with SK. Treatment consists of
placing the patient in a supine position and slowing/stopping infusion
until the blood pressure rises. Treatment with cautious (100–500 mL) fluid
challenges may be required, especially in inferior/ RV infarction.
Hypotension is not necessarily evidence of an allergic reaction and may
not warrant treatment as such.
• Allergic reactions are common with SK and include a low-grade fever, rash,
nausea, headaches, flushing, and, rarely (0.1%), anaphylaxis. Give
hydrocortisone 100 mg IV with chlorpheniramine 10 mg IV.
• Intracranial hemorrhage is seen in ~0.3% of patients treated with SK and in
~0.6% with rtPA.
• Reperfusion arrhythmias (most commonly a short, self-limiting run of
idioventricular rhythm) may occur.
• Systemic embolization may occur from lysis of thrombus within the left
atrium, LV, or aortic aneurysm.
Contraindications to thrombolysis
Absolute contraindications to thrombolysis
Active internal bleeding
Suspected aortic dissection
Recent head trauma and/or intracranial
neoplasm
Previous hemorrhagic stroke at any time
Previous ischemic stroke within the past
1 year
Previous allergic reaction to fibrinolytic
agents
Trauma and/or surgery within past 2
weeks at risk of bleeding
Relative contraindications to thrombolysis
Trauma and/or surgery more than 2
weeks previously
Severe uncontrolled hypertension (BP
>180/110)
Nonhemorrhagic stroke over 1 year ago
Known bleeding diathesis or current use
of anticoagulation within therapeutic
range (INR 2)
Prolonged (>10 minutes)
cardiopulmonary resuscitation
Prior exposure to SK (if planning to give
SK, especially previous 6–9 months)
Pregnancy or postpartum Lumbar
puncture within previous 1 month
Menstrual bleeding or lactation
History of chronic severe hypertension
Non compressible vascular punctures
(e.g., subclavian central venous lines)

Reperfusion by primary PCI
Primary PCI is the current gold-standard reperfusion
strategy for treatment of STEMI.
Indication for primary PCI
• All patients with chest pain and ST-segment elevation or
new LBBB fulfill primary PCI criteria (compare with
indications for thrombolysis).
• This includes a group of patients in whom ST-segment
elevation may not fulfill all criteria for thrombolysis.
• In general, patients in whom thrombolysis is
contraindicated should be managed by primary PCI.

Outcome in primary PCI
• A superior outcome in patients with STEMI who are treated with primary PCI in
comparison to outcomes with thrombolysis.
• There is a significant short-term, as well as long-term, reduction in mortality and
major adverse cardiac events (MACE) (death, nonfatal reinfarction, and nonfatal
stroke) in STEMI patients treated with primary PCI, with better LV function, a higher
vessel patency rate, and less recurrent myocardial ischemia.
• Interhospital transportation for primary PCI (community hospital to invasive center)
is safe, and primary PCI remains superior to thrombolysis despite the time delays
involved.
It is generally accepted that in the acute phase only the “culprit” lesion(s) and vessel(s)
will be treated. The pattern of disease in the remainder of the vessels will determine
whether further revascularization should be performed as an inpatient or an elective
case in the future.
• STEMI patients treated with uncomplicated primary PCI can often be discharged
safely within 72 hours of admission without the need for further risk stratification.
• Primary PCI is more cost-effective in the long term than thrombolysis with significant
savings from fewer days in the hospital, less need for readmission, and less heart
failure.
• Post-discharge care, secondary prevention, and rehabilitation remain identical to
that for other MI cases.

Rescue PCI
Rescue PCI may be performed as an adjunct to thrombolysis but should
be reserved
for patients who remain symptomatic post-thrombolysis
failure to reperfuse) or
develop cardiogenic shock. We recommend
that all patients who continue to have
post-thrombolysis symptoms
and/or ongoing ST-elevation with or without symptoms
be discussed
with the local invasive cardiac center for urgent catheterization and
revascularization.
Surgery for acute STEMI
CABG in the context of an acute STEMI is of value in the following
situations:
•
High-risk coronary anatomy on catheterization (left main stenosis, left anterior
descending [LAD] ostial disease).
•
Complicated STEMI (acute mitral regurgitation, or ventricular rupture)
•
STEMI patients (with or without successful thrombolysis) with additional coronary
lesions whose anatomy is best served by CABG on prior or subsequent
catheterization

STEMI: additional measures
Low-molecular-weight and unfractionated heparin
UFH
•
There is no indication for
“routine” IV heparin following SK.
•
IV heparin (4000 U/max IV bolus followed by 1000 U/hr max adjustedf or an aPTT ratio of 1.5
–2.0 times control)
should be used routinely following rtPA and its derivatives for 24
–48 hours.
LMWH
•
There is clinical trial data supporting the use of LMWH and thrombolysis (e.g., enoxaparin 30 mg IV bolus, then 1
mg/kg SC q12h).
•
As an alternative to UFH, LMWH can be used at a prophylactic dose to prevent thromboembolic events in high-risk
patients.
Clopidogrel
Calcium antagonists
•
This should be administered to all patients undergoing primary PCI (loading dose 300
–600 mg po followed by 75 mg
qd).
•
The length of therapy is determined by the type of stent used. Drug eluting stents require longer term use of
clopidogrel than bare-metal stents (current guidelines for ACS is 1 year of Plavix).
•
These are best avoided, especially in the presence of LV impairment.
•
Diltiazem and verapamil started after day 4
–5 in post-MI patients with normal LV function may have a small
bene
ficial effect.
•
Amlodipine is safe to use in patients with poor LV function post-MI.
•
Nifedipine has been shown to increase mortality and should be avoided.

The End
