
Pleural Diseases
PLEURISY
Pleurisy is not a diagnosis but a term used to describe pleuritic pain
resulting from any one of a number of disease processes involving
the pleura. Pleurisy is a common feature of pulmonary infection
and infarction; it may also occur in malignancy.
Clinical features
Sharp pain that is aggravated by deep breathing or coughing is
characteristic.
On examination, rib movement is restricted and a pleural rub may
be present. Sometimes this is only audible in deep inspiration or
near the pericardium (pleuro-pericardial rub).
The other clinical features depend on the cause
•Loss of a pleural rub and diminution in the chest pain may
indicate either recovery or the development of a pleural effusion.
•Management
•The primary cause of pleurisy must be treated
PLEURAL EFFUSION
The accumulation of serous fluid within the pleural space is termed
pleural effusion.Accumulations of frank pus (empyema) or blood
(haemothorax).
In general, pleural fluid accumulates as a result of either increased
hydrostatic pressure or decreased osmotic pressure ('transudative
effusion' as seen in cardiac, liver or renal failure), or from increased
microvascular pressure due to disease of the pleural surface itself,
or injury in the adjacent lung ('exudative effusion').
Causes of pleural effusion
Common causes
1.Pneumonia ('para-pneumonic effusion')
2.Tuberculosis
3.Pulmonary infarction
4.Malignant disease

5.Cardiac failure
6.Subdiaphragmatic disorders (subphrenic abscess, pancreatitis
etc.)
Uncommon
causes
1.Hypoproteinemia(nephrotic syndrom,liver failure,malnutrition)
2.Connective tissue diseases (particularly systemic lupus
erythematosus and rheumatoid arthritis)
3.Acute rheumatic fever
4.Post-myocardial infarction syndrome
5.Meigs' syndrome (ovarian tumour plus pleural effusion)
6.Myxoedema
7.Uraemia
8.Asbestos-related benign pleural effusion
Clinical assessment:
Symptoms and signs of pleurisy often precede the development of
an effusion, especially in patients with underlying pneumonia,
pulmonary infarction or connective tissue disease. However, the
onset may be insidious. Breathlessness is the only symptom related
to the effusion and its severity depends on the size and rate of
accumulation.
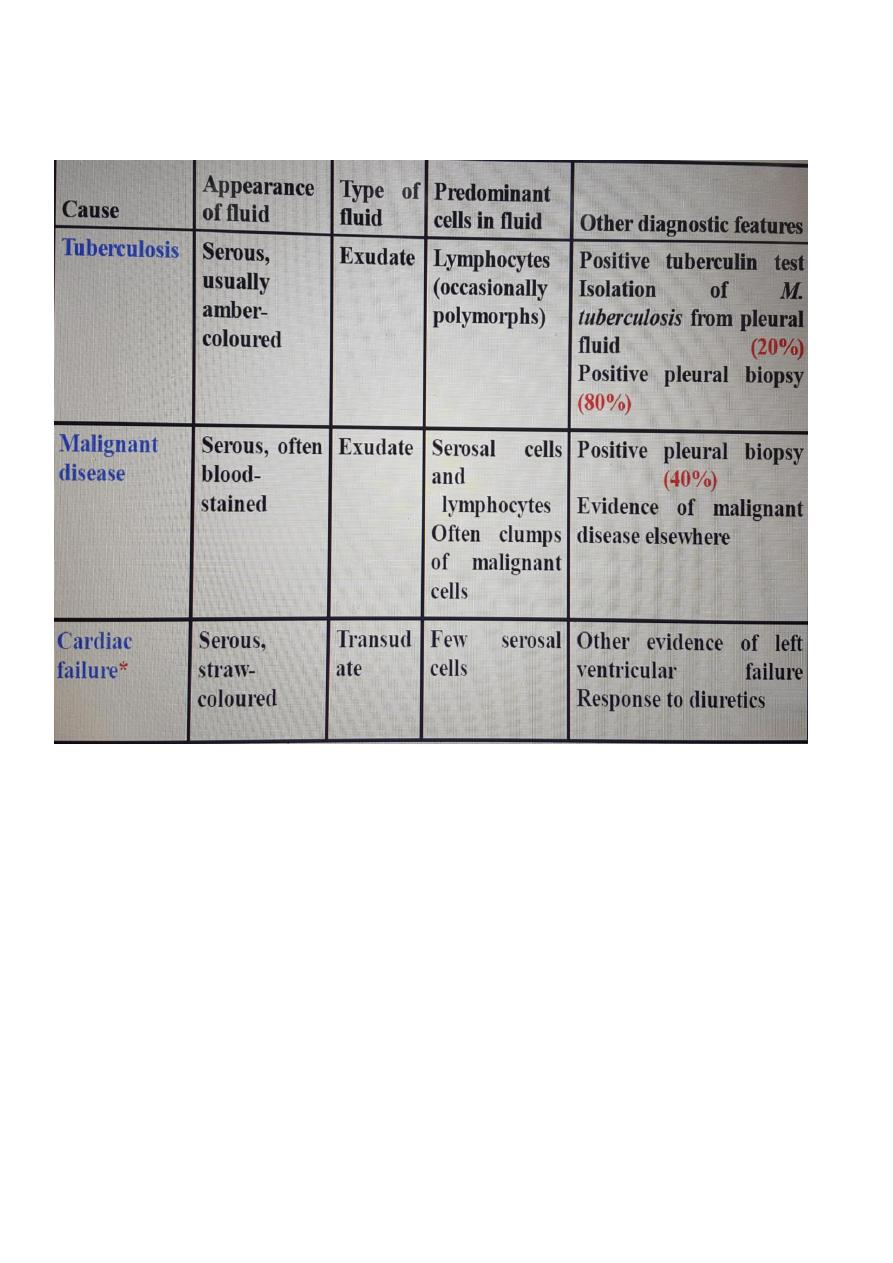
Pleural effusion: main causes and features:
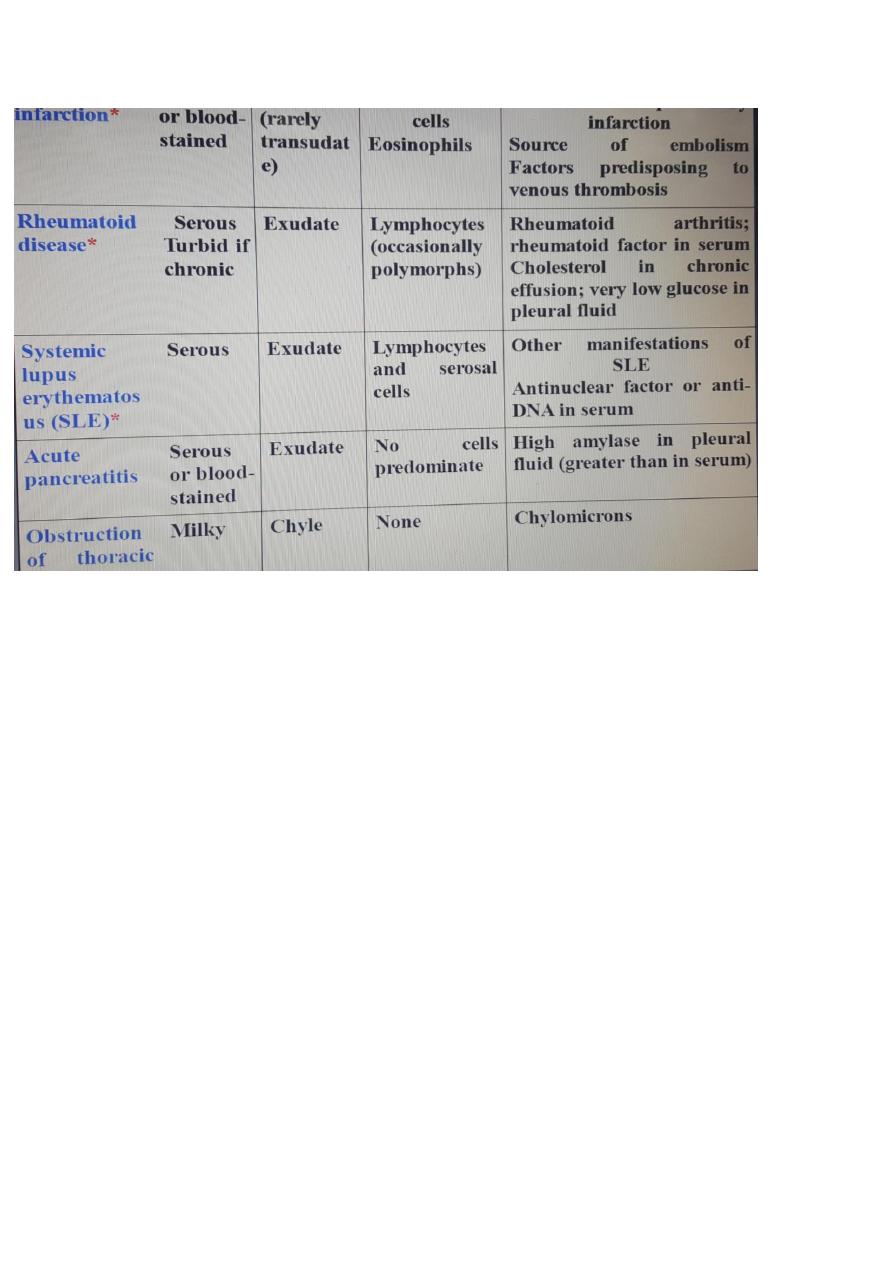
Investigations
Radiological investigations:
The classical appearance of pleural fluid on the erect PA chest film
is of a curved shadow at the lung base, blunting the costophrenic
angle and ascending towards the
axilla.
Fluid appears to track up the lateral chest wall.Around 200 ml of
fluid is required to be detectable on a PA chest X-ray, but smaller
effusions can be identified by ultrasound or CT
scanning.
Previous scarring or adhesions in the pleural space can cause
localised effusions.
•Pleural fluid localised below the lower lobe ('subpulmonary
effusion') simulates an elevated
hemidiaphragm.
•Fluid localised within an oblique fissure may produce a rounded
opacity simulating a tumour.
Ultrasonography:
CT scanning:displays pleural abnormalities more readily than
either plain radiography or ultrasound, and may distinguish
benign from malignant pleural disease.

Pleural aspiration and biopsy:Pleural fluid is an exudate if one or
more of the following criteria are met:
1.Pleural fluid protein:serum protein ratio >
0.5
2.Pleural fluid LDH: serum LDH ratio > 0.6 3.Pleural fluid LDH >
two
3.Pleural fluid LDH > two-thirds of the upper limit of normal serum
LDH
Management:
Therapeutic aspiration may be required to palliate breathlessness
but removing more than 1.5 litres in one episode is inadvisable as
there is a small risk of re-expansion pulmonary oedema.An effusion
should never be drained to dryness before establishing a diagnosis
as further biopsy may be precluded until further fluid
accumulates.
Treatment of the underlying cause-
for example, heart failure,
pneumonia, pulmonary embolism or subphrenic abscess-will often
be followed by resolution of the effusion.
EMPYEMA
This term describes the presence of pus in the pleural space.The pus
may be as thin as serous fluid or so thick that it is impossible to
aspirate even through a wide-bore needle. Microscopically,
neutrophil leucocytes are present in large numbers.The causative
organism may or may not be isolated from the pus.
An empyema may involve the whole pleural space or only part of it
('loculated' or 'encysted' empyema) and is almost invariably
unilateral
Aetiology:
Empyema is always secondary to infection in a neighbouring
structure, usually the lung. The principal infections liable to
produce empyema are the bacterial pneumonias and
TB.
Over 40% of patients with community-acquired pneumonia
develop an associated pleural effusion ('para-pneumonic' effusion)

and about 15% of these become secondarily
infected.
Other causes are infection of a haemothorax and rupture of a
subphrenic abscess through the diaphragm.
Pathology :
Both layers of pleura are covered with a thick, shaggy inflammatory
exudate.The pus in the pleural space is often under considerable
pressure and if the condition is not adequately treated pus may
rupture into a bronchus causing a bronchopleural fistula and
pyopneumothorax, or track through the chest wall with the
formation of a subcutaneous abscess or
sinus.
The only way in which an empyema can heal is by eradication of the
infection, obliteration of the empyema space and apposition of the
visceral and parietal pleural layers.
This cannot occur unless re-expansion of the compressed lung is
secured at an early stage by removal of all the pus from the pleural
space.
This cannot take place if:
1-the visceral pleura becomes grossly thickened and rigid due to
delayed treatment.
2-inadequate drainage of the infected pleural
fluid
3-the pleural layers are kept apart by air entering the pleura
through a bronchopleural
fistula
4-there is underlying disease in the lung, such as bronchiectasis,
bronchial carcinoma or pulmonary TB preventing re-expansion.In
all these circumstances an empyema tends to become chronic, and
healing is unlikely without surgical intervention.
Clinical features:
An empyema should be suspected in patients with pulmonary
infection if there is persistence or recurrence of pyrexia despite the
administration of a suitable antibiotic. Once an empyema has

developed, two separate groups of clinical features are
found.
1-
Systemic
features
Pyrexia, usually high and remittent Rigors, sweating, malaise and
weight loss Polymorphonuclear leucocytosis, high CRP
2-Local
features
Pleural pain; breathlessness; cough and sputum usually because of
underlying lung disease; copious purulent sputum if empyema
ruptures into a bronchus (bronchopleural fistula) Clinical signs of
fluid in the pleural space
Investigations
Radiological examinationThe appearances are often
indistinguishable from those of pleural effusion.
Ultrasoundshows the position of the fluid, the extent of pleural
thickening and whether fluid is in a single collection or
multiloculated by fibrin and debris. In addition to showing the
pleura,
CT canbe useful in assessing the underlying lung parenchyma and
patency of the major bronchi.
Aspiration of pusThis confirms the presence of an empyema.The
pus is frequently sterile when antibiotics have already been given;
the distinction between tuberculous and non-tuberculous disease
can be difficult and often requires pleural histology and culture.
Management
Treatment of non-tuberculous
empyema
When the patient is acutely ill and the pus is thin an intercostal
tube should be inserted under ultrasound or CT guidance into the
most dependent part of the empyema space and connected to a
water-seal drain system. If the initial aspirate reveals turbid fluid or
frank pus, or if loculations are seen on ultrasound, the tube should
be put on suction (5-10 cm H2O) and flushed regularly with 20 ml
normal saline.

Finally, an antibiotic directed against the organism causing the
empyema should be given for 2-4 weeks.
An empyema can often be aborted if these measures are started
early. If, however, the intercostal tube is not providing adequate
drainage, which can happen when the pus is thick or loculated,
surgical intervention is required. The empyema cavity is cleared of
pus and adhesions, and a wide-bore tube inserted to allow optimal
drainage. Surgical 'decortication' of the lung may also be required if
gross thickening of the visceral pleura has developed and is
preventing re-expansion of the lung.
•Treatment of tuberculous
empyema
•Antituberculosis chemotherapy must be started immediately and
the pus in the pleural space aspirated through a wide-bore needle
until it ceases to
reaccumulate.
•Intercostal tube drainage is often required. In many patients no
other treatment is necessary but surgery is occasionally required to
ablate a residual empyema space.
PNEUMOTHORAX
Classification of pneumothorax:
1-
Spontaneous
PrimaryWithout evidence of overt lung disease. Air escapes from
the lung into the pleural space through rupture of a small
subpleural emphysematous bulla or pleural bleb, or the pulmonary
end of a pleural adhesion It principally affects males aged 15-30 in
whom smoking, tall stature.
SecondaryUnderlying lung disease, most commonly COPD and TB;
also seen in asthma, lung abscess, pulmonary infarcts,
bronchogenic carcinoma, all forms of fibrotic and cystic lung
diseaseis most common in older patients, and is associated with the
highest mortality rates.

2-TraumaticIatrogenic (e.g. following thoracic surgery or biopsy) or
non-iatrogenic.
Clinical features:
The commonest symptoms are sudden-onset unilateral pleuritic
chest pain or breathlessness. In those with underlying chest disease,
breathlessness can be severe and may not resolve spontaneously. In
patients with a small pneumothorax the physical examination may
be normal.
A larger pneumothorax (> 15% of the hemithorax) results in
decreased or absent breath sounds .
The combination of absent breath sounds and resonant percussion
note is diagnostic of pneumothorax.
If the communication between the airway and the pleura is small, it
can act as a one-way valve allowing air to enter the pleural space
during inspiration but not to escape on expiration.
Large amounts of trapped air accumulate in the pleural space and
the intrapleural pressure may rise to well above atmospheric levels
(a so-called 'tension' pneumothorax).
The pressure causes mediastinal displacement towards the opposite
side, with compression of the opposite normal lung and
impairment of systemic venous return, causing cardiovascular
compromise .
Clinically, the findings are rapidly progressive breathlessness
associated with a marked tachycardia, hypotension, cyanosis and
tracheal displacement away from the side of the silent
hemithorax.
Occasionally, 'tension' pneumothorax may occur without
mediastinal shift if malignant disease or scarring has splinted the
mediastinum. Where the communication between the lung and
pleural space seals off as the lung deflates and does not reopen, the
pneumothorax is referred to as 'closed' .
In such circumstances the mean pleural pressure remains negative,
spontaneous reabsorption of air and re-expansion of the lung occur
over a few days or weeks, and infection is
uncommon.
This contrasts with an 'open' pneumothorax, where the
communication fails to seal and air continues to transfer freely
between the lung and pleural space . An example of the latter is a
bronchopleural fistula which, if large, can also facilitate the

transmission of infection from the air passages into the pleural
space, leading to
empyema.
An open pneumothorax is commonly seen following rupture of an
emphysematous bulla, tuberculous cavity or lung abscess into the
pleural space.
Investigations
The chest X-ray shows the sharply defined edge of the deflated lung
with complete translucency (no lung markings) between this and
the chest wall . Care must be taken to differentiate between a large
pre-existing emphysematous bulla and a pneumothorax to avoid
misdirected attempts at aspiration. Where doubt
exists,CTis useful in distinguishing bullae from pleural air.
X-rays also show the extent of any mediastinal displacement and
give information regarding the presence or absence of pleural fluid
and underlying pulmonary disease.
Management
Primary pneumothorax where the lung edge is less than 2 cm from
the chest wall and the patient is not breathless normally resolves
without intervention. In young patients presenting with a
moderate or large spontaneous primary pneumothorax,
percutaneous needle aspiration of air is a simple and well-tolerated
alternative to intercostal tube drainage, with a 60-80% chance of
avoiding the need for a chest drain .
In patients with underlying chronic lung disease, however, even a
small secondary pneumothorax may cause respiratory failure;
hence all such patients require intercostal tube drainage and
inpatient observation.
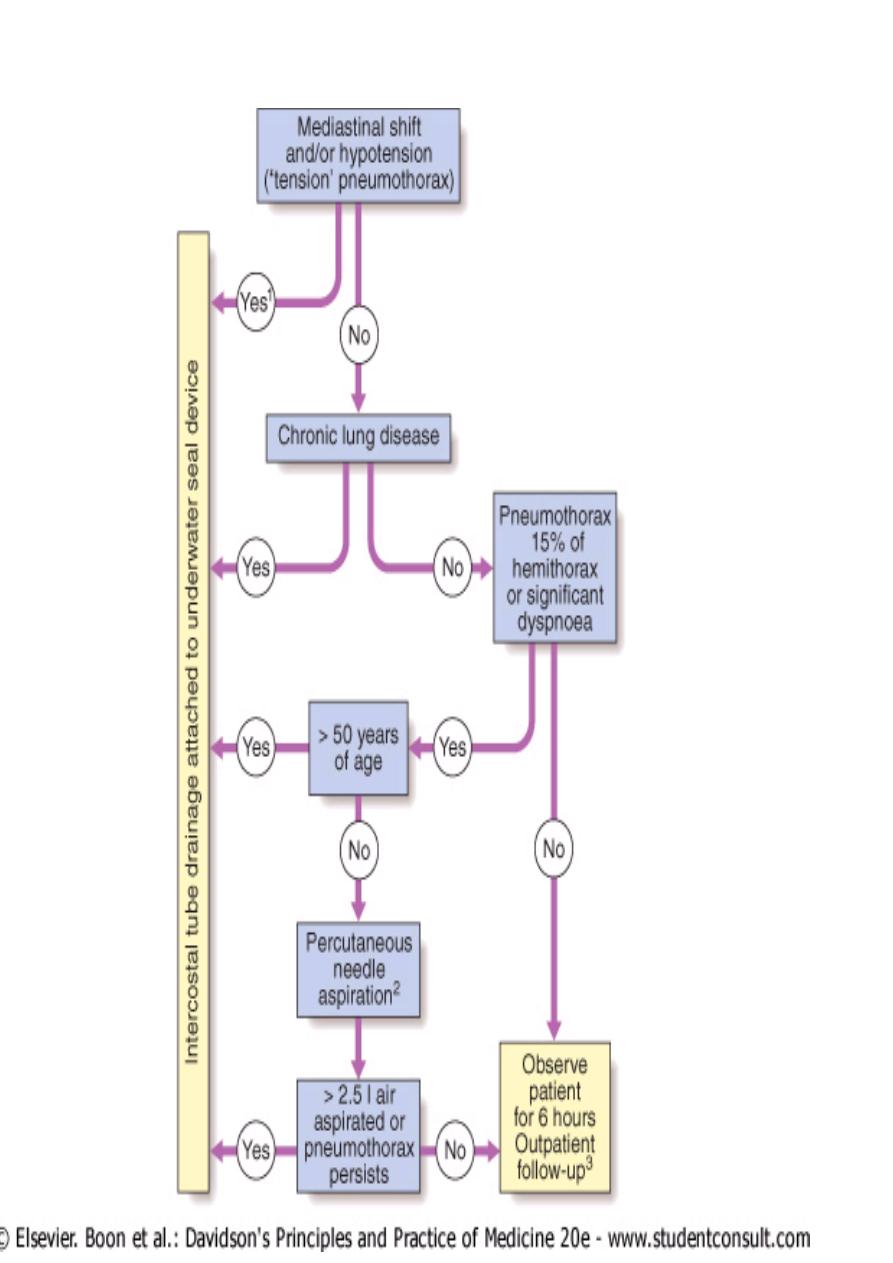

When needed, intercostal drains should be inserted in the 4th, 5th
or 6th intercostal space in the mid-axillary line following blunt
dissection through to the parietal
pleura.
The tube should be advanced in an apical direction, connected to
an underwater seal or one-way Heimlich valve, and secured firmly
to the chest wall.
Clamping of the drain is potentially dangerous and never
indicated.
The drain should be removed 24 hours after the lung has fully
reinflated and bubbling stopped.
Continued bubbling after 5-7 days is an indication for
surgery.
If bubbling in the underwater bottle stops prior to full reinflation,
the tube is either blocked, kinked or
displaced.
All patients should receive supplemental oxygen as this accelerates
the rate at which air is reabsorbed by the
pleura.
Patients with a closed pneumothorax should not fly as the trapped
gas expands at
altitude.
After complete resolution, there is no clear evidence on how long
patients should avoid flying, although guidelines suggest that a
wait of 1-2 weeks, with confirmation of full inflation prior to flight, is
prudent.
Patients should also be advised to stop smoking and be informed
about the risks of a recurrent
pneumothorax.
Recurrent spontaneous pneumothorax:
After primary
spontaneous pneumothorax, recurrence occurs within a year of
either aspiration or tube drainage in approximately 25% of
patients, and should prompt definitive treatment.
Surgical pleurodesis is recommended in all patients following
a
(1)second pneumothorax (even if
ipsilateral).
(2)first episode of secondary pneumothorax if low respiratory
reserve makes recurrence hazardous.

(3)Patients who plan to continue activities where pneumothorax
would be particularly dangerous (e.g. flying or diving) should also
undergo definitive treatment after the first episode of a primary
spontaneous pneumothorax.
Pleurodesis can be achieved by pleural abrasion or parietal
pleurectomy at thoracotomy or thoracoscopy.
