
Dr.Asaad 2016
Human Immunodeficiency Virus Disease
EPIDEMIOLOGY AND BIOLOGY OF HIV
The acquired immunodeficiency syndrome (AIDS) was first recognized in 1981. It is caused
by the human immunodeficiency virus (HIV-1). HIV-2 causes a similar illness to HIV-1 but
is less aggressive and restricted mainly to western Africa. The viruses almost certainly
originated from closely related African primate viruses, simian immunodeficiency viruses
(SIVs). Sequence analysis has led to the estimate that HIV-1 was introduced into humans in
the early 1930s.
Since 1981 AIDS has grown to be the second leading cause of disease burden world-wide
and the leading cause of death in Africa, where it accounts for over 20% of deaths. Highly
active retroviral therapy (HAART) with three or more drugs has improved life expectancy to
near normal in the majority of patients receiving it, with an 80% reduction of mortality since
its introduction. Immune deficiency is a consequence of continuous high-level HIV
replication leading to virus and immune-mediated destruction of the key immune effector
cell, the CD4 lymphocyte.
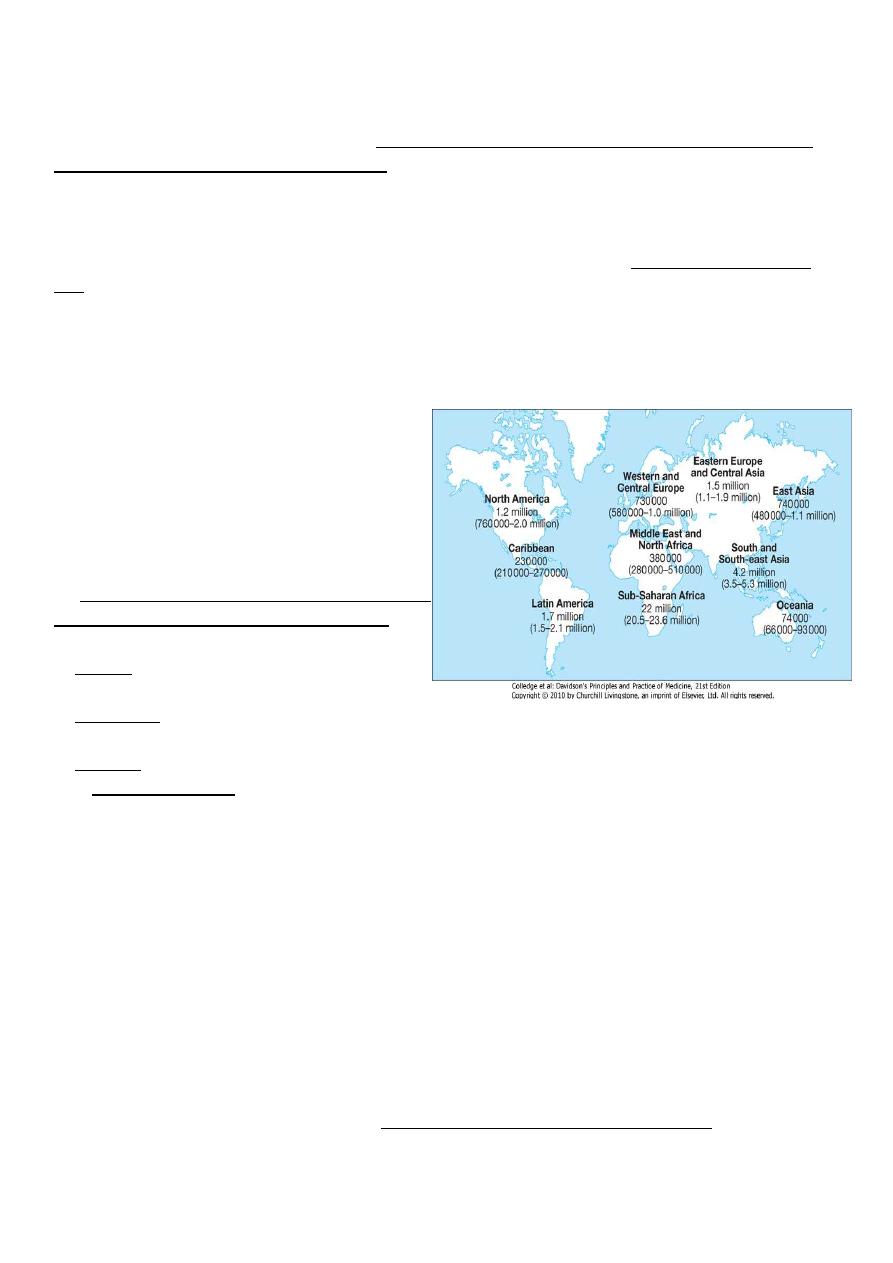
In 2012, the World Health Organization (WHO) estimated that there were 35.3 million
people living with HIV/AIDS, 2.5 million new infections and 2.1 million deaths. The
cumulative death toll since the epidemic began is over 36 million(2012) in 2013 1.4 million
death, the vast majority of cases occurring in sub-Saharan Africa where over 11.4 million
children are now orphaned.
No cure no vaccin
MODES OF TRANSMISSION
HIV is present in blood, semen and other
body fluids such as breast milk and saliva.
Exposure to infected fluid leads to a risk of
contracting infection, which is dependent on
the integrity of the exposed site, the type and
volume of body fluid, and the viral load.
The modes of spread are :
1. Sexual (man to man, heterosexual and
oral).
2. Parenteral (blood or blood product
recipients, injection drug-users and those experiencing occupational injury) and
3. Vertical.
The transmission risk after exposure is over 90% for blood or blood products, 15-40% for
the vertical route, 0.5-1.0% for injection drug use, 0.2-0.5% for genital mucous membrane
spread and under 0.1% for non-genital mucous membrane spread. After >25 years of
scrutiny, there is no evidence that HIV is transmitted by casual contact or that the virus can
be spread by insects, such as by a mosquito bite.
World-wide, the major route of transmission (> 75%) is heterosexual. About 5-10% of new
HIV infections are in children and more than 90% of these are infected during pregnancy,
birth or breastfeeding. The rate of mother-to-child transmission is higher in developing
countries (25-44%) than in industrialised nations (13-25%); postnatal transmission via breast
milk may account for some of this increased risk.
VIROLOGY AND IMMUNOLOGY
HIV is a single-stranded RNA retrovirus from the Lentivirus family. After mucosal exposure,
HIV is transported to the lymph nodes via dendritic, CD4 or Langerhans cells, where
infection becomes established. Dendritic cells express various receptor that facilitate capture
and transport of HIV-1. Free or cell-associated virus is then disseminated widely through the
blood with seeding of 'sanctuary' sites (e.g. central nervous system) and latent CD4 cell
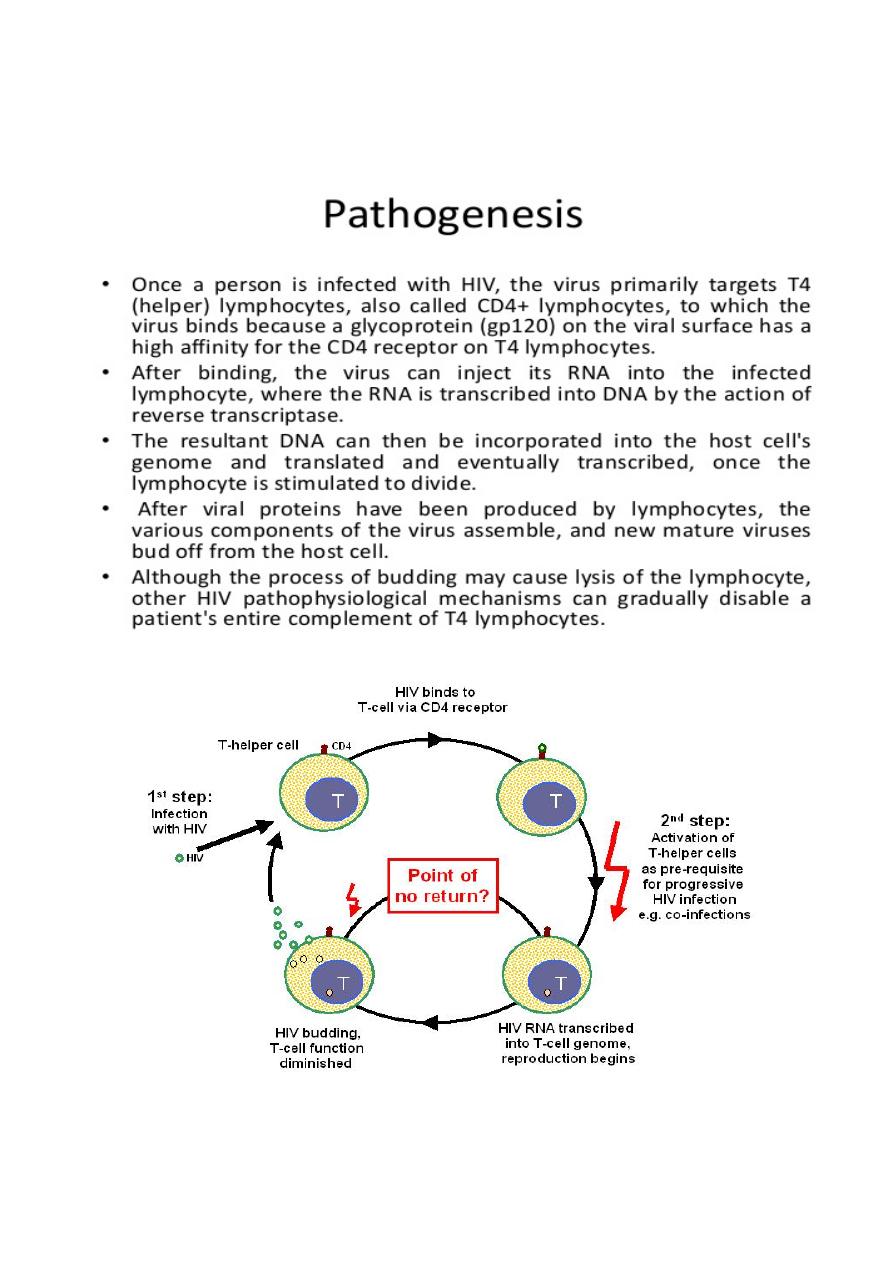
reservoirs. With time, there is gradual attrition of the CD4 cell population, resulting in
increasing impairment of cell-mediated immunity and susceptibility to opportunistic
infections.
As CD4 cells are pivotal in orchestrating the immune response, any depletion in numbers
renders the body susceptible to opportunistic infections and oncogenic virus-related tumours.
The predominant opportunist infections seen in HIV disease are intracellular parasites(e.g.
Mycobacterium tuberculosis) or pathogens susceptible to cell-mediated rather than
antibody-mediated immune responses. The reduction in the number of CD4 cells circulating
in peripheral blood is tightly correlated with the amount of plasma viral load. Both are
monitored closely in patients and are used as measures of disease progression.
NATURAL HISTORY AND
CLASSIFICATION OF HIV
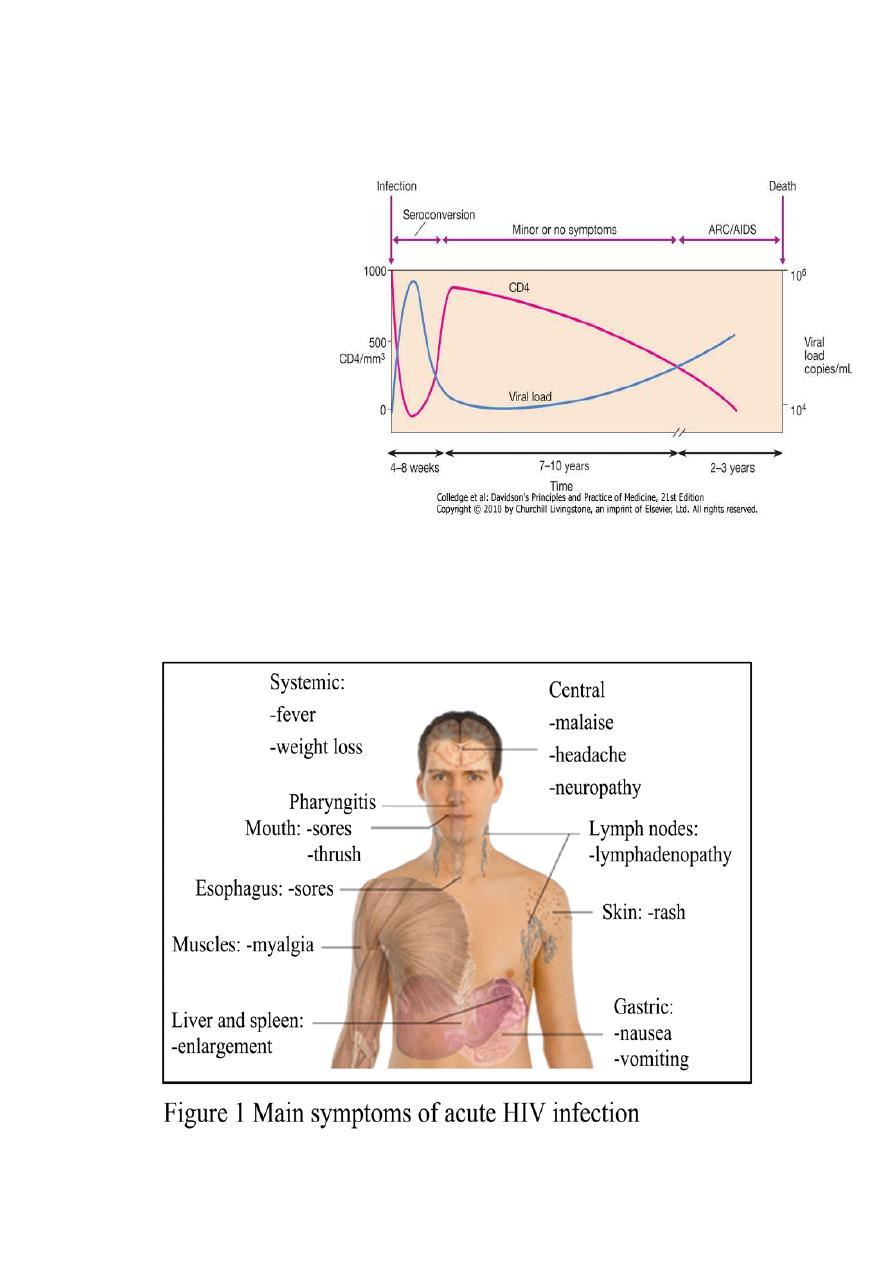
Primary infection is symptomatic
in 70-80% of cases and usually
occurs 2-6 weeks after exposure:
CLINICAL FEATURES OF
PRIMARY INFECTION
Fever with rash
Pharyngitis with cervical
lymphadenopathy
Myalgia/arthralgia
Headache
Mucosal ulceration
Rarely, presentation may be neurological (aseptic meningitis, encephalitis, myelitis,
polyneuritis).

Asymptomatic infection
Asymptomatic infection follows primary infection and lasts for a variable period, during
which the infected individual remains well with no evidence of disease except for the
possible presence of persistent generalised lymphadenopathy (PGL, defined as enlarged
glands at ≥ 2 extra-inguinal sites).
Mildly symptomatic disease
Mildly symptomatic disease then develops in the majority, indicating some impairment of
the cellular immune system. These diseases correspond to AIDS-related complex (ARC)
conditions but by definition are not AIDS-defining. The median interval from infection to the
development of symptoms is around 7-10 years.
HIV SYMPTOMATIC DISEASES
Oral hairy leucoplakia
Recurrent oropharyngeal candidiasis
Recurrent vaginal candidiasis
Severe pelvic inflammatory disease
Bacillary angiomatosis
Cervical dysplasia
Idiopathic thrombocytopenic purpura
Weight loss
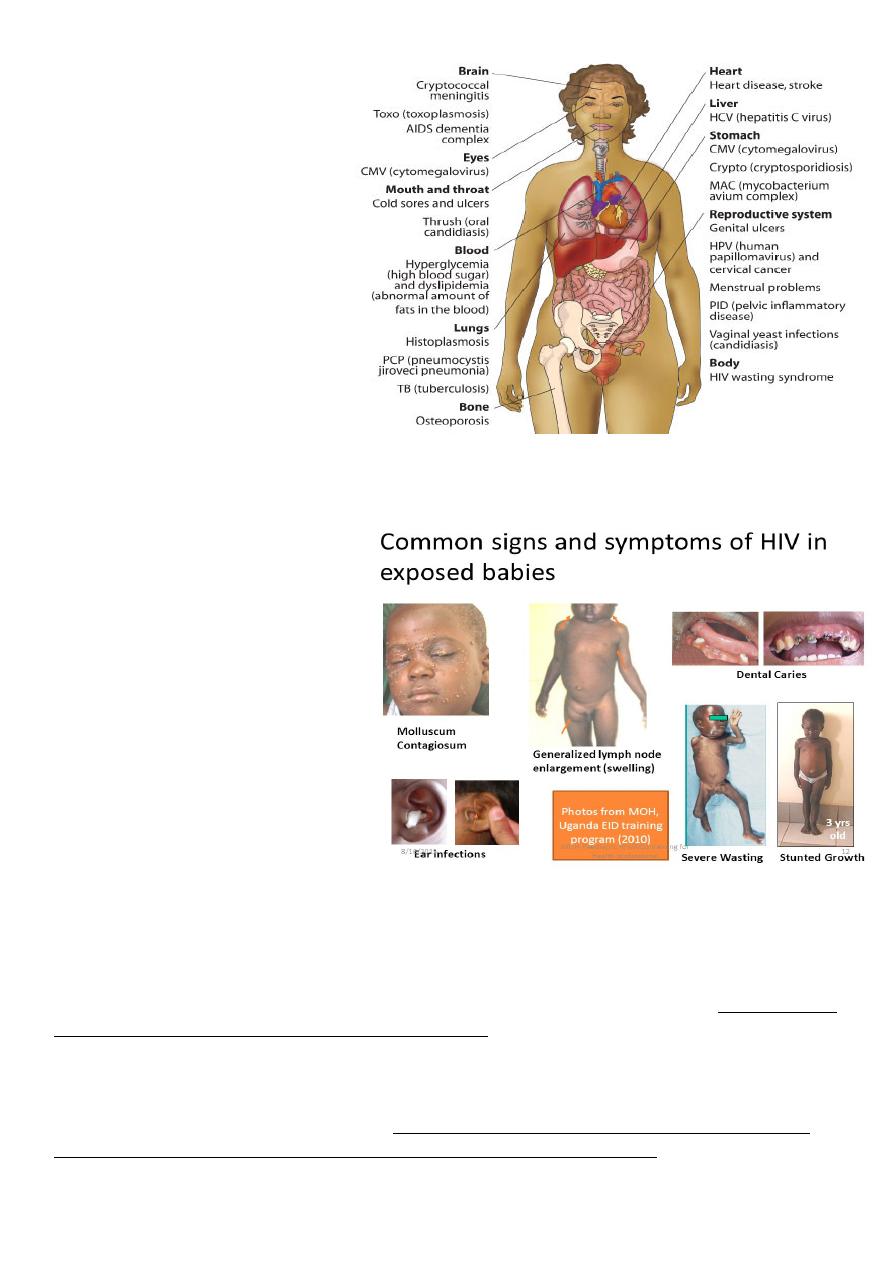
Chronic diarrhoea
Herpes zoster
Peripheral neuropathy
Low-grade fever/night sweats
Acquired immunodeficiency
syndrome (AIDS)
AIDS is defined by the
development of specified
opportunistic infections, tumours
etc.
AIDS-DEFINING DISEASES
Oesophageal candidiasis
Cryptococcal meningitis
Chronic cryptosporidial diarrhoea
CMV retinitis or colitis
Chronic mucocutaneous herpes simplex
Disseminated Mycobacterium avium intracellulare
Pulmonary or extrapulmonary
tuberculosis
Pneumocystis carinii pneumonia
Progressive multifocal
leucoencephalopathy
Recurrent non-typhi Salmonella
septicemia
Cerebral toxoplasmosis
Extrapulmonary coccidioidomycosis
Invasive cervical cancer
Extrapulmonary histoplasmosis
Kaposi's sarcoma
Non-Hodgkin lymphoma
Primary cerebral lymphoma
HIV-associated wasting
HIV-associated dementia
Diagnosis of HIV Infection
The CDC has recommended that screening for HIV infection be performed as a matter of
routine health care. The diagnosis of HIV infection depends upon the demonstration of
antibodies to HIV and/or the direct detection of HIV or one of its components. Antibodies to
HIV generally appear in the circulation 2–12 weeks following infection. The standard blood
screening test for HIV infection is the ELISA, also referred to as (EIA) an enzyme
immunoassay is an extremely good screening test with a sensitivity of >99.5%. Most
diagnostic laboratories use a commercial EIA kit that contains antigens from both HIV-1 and
HIV-2 and thus are able to detect either. EIA tests are generally scored as positive (highly
reactive), negative (nonreactive), or indeterminate) partially reactive.
)
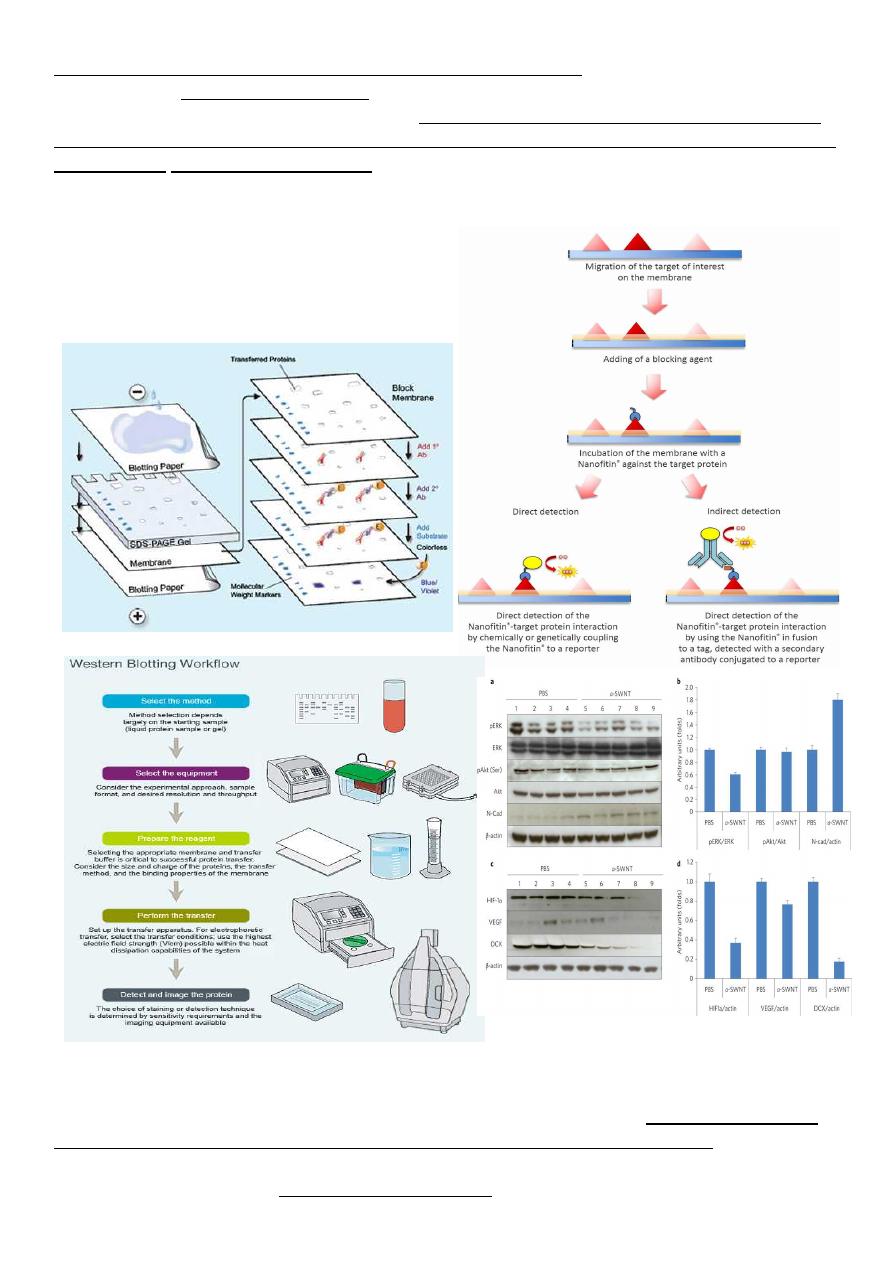
The most commonly used confirmatory test is the Western blot. This assay takes advantage
of the fact that multiple HIV antigens of different, well-characterized molecular weights
elicit the production of specific antibodies. These antigens can be separated on the basis of
molecular weight, and antibodies to each component can be detected as discrete bands on the
Western blot. A negative Western blot is one in which no bands are present at molecular
weights corresponding to HIV gene products. In a patient with a positive or indeterminate
EIA and a negative Western blot, one can conclude with certainty that the EIA reactivity was
a false positive. On the other hand, a Western
blot demonstrating antibodies to products of all
three of the major genes of of HIV (env, pol
&gag) is conclusive evidence of infection with
HIV.
CD4+ T Cell Counts
The CD4+ T cell count is the laboratory test generally accepted as the best indicator of the
immediate state of immunologic competence of the patient with HIV infection. This
measurement has been shown to correlate very well with the level of immunologic
competence. Patients with CD4+ T cell counts <200 are at high risk of disease from P.
jiroveci while patients with CD4+ T cell counts <50 are at high risk of disease from CMV,
mycobacteria of M.avium complex &/0r T.gondi. Patients with HIV infection should have
CD4+ T cell measurements performed at the time of diagnosis and every 3–6 months
thereafter. More frequent measurements should be made if a declining trend is noted.
Treatment slow the course and lead to near normal life expectancy, reduce the risk of death
and complication
Without treat. Avarage survival time from infection is 9-11 year
MANAGEMENT OF HIV
Management of HIV involves both treatment of the virus and prevention of opportunistic
infections. The aims of HIV treatment are to:
1*reduce the viral load to an undetectable level (< 50 copies/ml) for as long as possible
2*improve the CD4 count (above 200 cells/mm significant HIV-related events rarely occur).
3*increase the quantity and improve the quality of life without unacceptable drug-related
side-effects or lifestyle alteration.
4*reduce transmission (mother-to-child and person-to-person).
DRUGS
Nucleoside reverse transcriptase inhibitors (NRTIs)
Zalcitabine (ddc)
Didanosine (ddI)
Lamivudine (3TC)
Zidovudine (ZDV)
Stavudine (d4T)
Abacavir
Emitricitabine (FTC)
Non-nucleoside reverse transcriptase inhibitors (NNRTIs)
Nevirapine
Efavirenz
Delavirdine
Protease inhibitors (PIs)
Indinavir
Ritonavir
Nelfinavir
Lopinavir
Atazanavir
PREVENTION MEASURES FOR HIV TRANSMISSION:
Parenteral
1. Blood product transmission: donor questionnaire, routine screening of donated blood,
blood substitute use.
2. Injection drug use: education, needle/syringe exchange, sharing and support for
methadone maintenance programmes.
Perinatal
1. Routine antenatal HIV antibody testing.
2.Preconception family planning if HIV- seropositive.
3. Measures to reduce vertical transmission.

Occupational
1. Education/training: universal precautions, needle stick avoidance.
2. Post-exposure prophylaxis.
Formatted by Mohammed Musa
