Chapter 5 – Cardiovascular disease
105
“” Presenting problems in cardiovascular disease “”
(Printed by Mostafa Hatim)
- Chest pain - Breathlessness (dyspnoea) - Acute circulatory failure (cardiogenic shock) -
Heart failure - Hypertension - Syncope & presyncope - Palpitation - Cardiac arrest and
sudden cardiac death - Abnormal heart sounds and murmurs
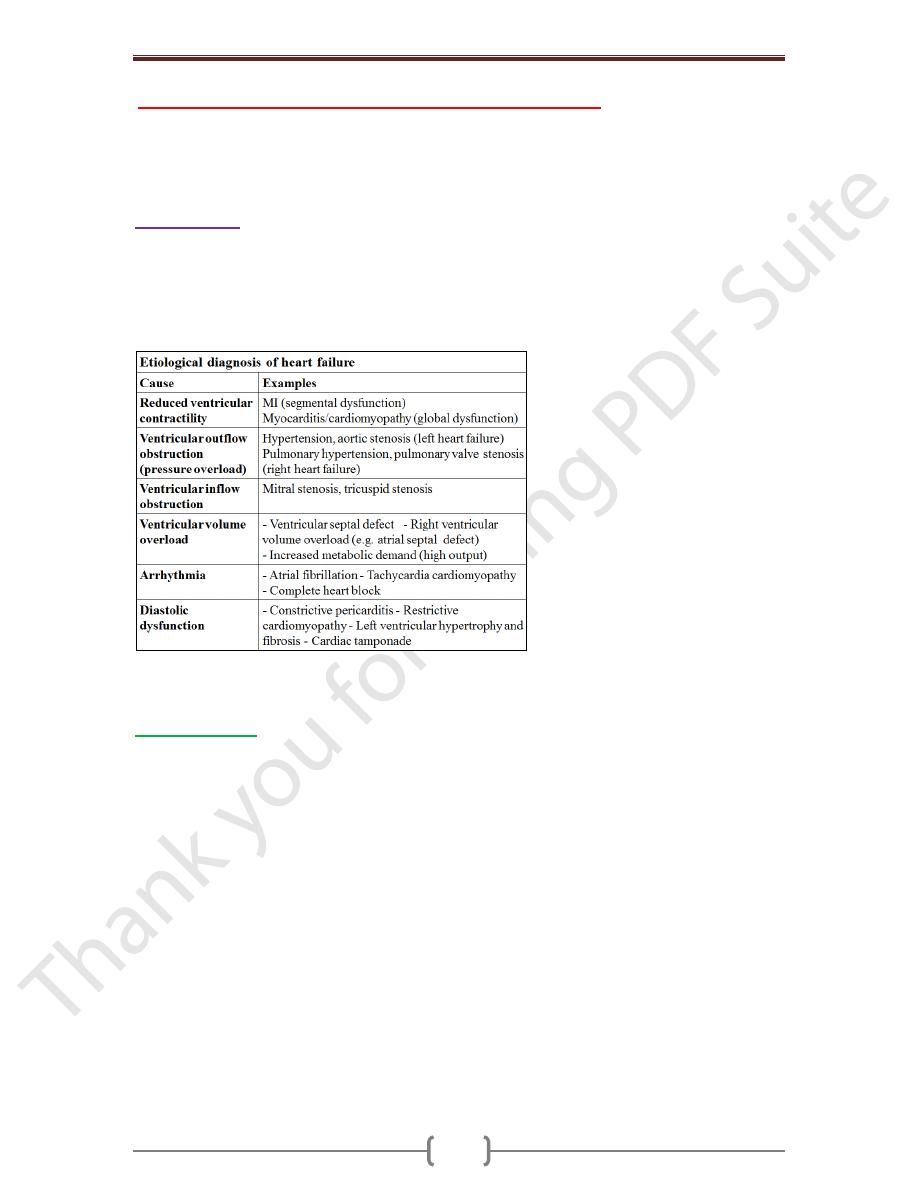
Heart failure
Describes the clinical syndrome that develops when the heart cannot maintain an
adequate cardiac output, or can do so only at the expense of an elevated filling pressure.
In mild to moderate forms of heart failure, cardiac output is adequate at rest & only
becomes inadequate when the metabolic demand increases during exercise or some other
form of stress. Almost all forms of heart disease can lead to heart failure.
HF is a common problem. It is a killing disease, 50% of patients with severe HF will die
within 6 months, 50% of patients with moderate HF will die in 2 years.
Pathophysiology
Cardiac output is a function of the preload (the volume and pressure of blood in the
ventricle at the end of diastole), the afterload (the volume and pressure of blood in the
ventricle during systole) and myocardial contractility; this is the basis of Starling’s Law.
In patients without valvular disease, the primary abnormality is impairment of ventricular
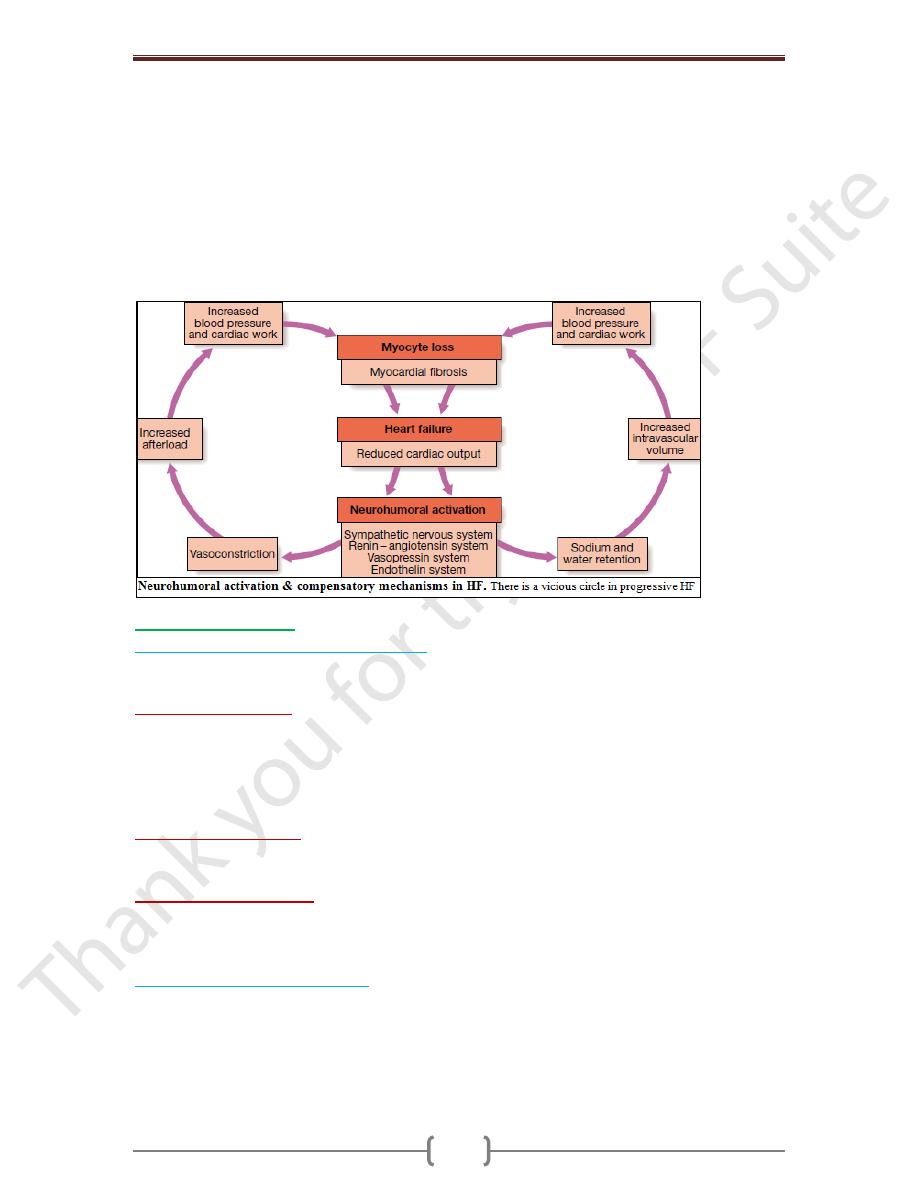
function leading to a fall in cardiac output. This activates counter-regulatory
neurohumoral mechanisms that in normal physiological circumstances would support
cardiac function, but in the setting of impaired ventricular function can lead to a
deleterious increase in both afterload and preload. A vicious circle may be established
because any additional fall in cardiac output will cause further neurohumoral activation
and increasing peripheral vascular resistance.
Stimulation of the renin–angiotensin–aldosterone system leads to vasoconstriction, salt
and water retention, and sympathetic nervous system activation. This is mediated by
angiotensin II, a potent constrictor of arterioles in both the kidney and the systemic
circulation. Activation of the sympathetic nervous system may initially maintain cardiac
output through an increase in myocardial contractility, heart rate & peripheral
vasoconstriction. However, prolonged
sympathetic stimulation leads to cardiac myocyte apoptosis, hypertrophy & focal
myocardial necrosis. Salt & water retention is promoted by release of aldosterone,
Chapter 5 – Cardiovascular disease
106
endothelin-1 (a potent vasoconstrictor peptide with marked effects on the renal
vasculature) and, in severe heart failure, antidiuretic hormone (ADH). Natriuretic
peptides are released from the atria in response to atrial stretch, and act as physiological
antagonists to the fluid conserving effect of aldosterone.
After MI, cardiac contractility is impaired & neurohumoral activation causes hypertrophy
of non-infarcted segments, with thinning, dilatation and expansion of the infarcted
segment (remodeling). This leads to further deterioration in ventricular function and
worsening heart failure.
The onset of pulmonary & peripheral oedema is due to high atrial pressures compounded by
salt & water retention caused by impaired renal perfusion & secondary hyperaldosteronism.
Types of heart failure
Left, right and biventricular heart failure
The left side of the heart comprises the functional unit of the LA & LV, together with the
mitral & aortic valves; the right heart comprises the RA, RV, & tricuspid & pulmonary valves.
o
Left-sided heart failure.
There is a reduction in the left ventricular output and an
increase in the left atrial or pulmonary venous pressure. An acute increase in left atrial
pressure causes pulmonary congestion or pulmonary oedema; a more gradual increase in
left atrial pressure, as occurs with mitral stenosis, leads to reflex pulmonary
vasoconstriction, which protects the patient from pulmonary oedema at the cost of
increasing pulmonary hypertension.
o
Right-sided heart failure
. There is a reduction in right ventricular output for any given
right atrial pressure. Causes of isolated right HF include chronic lung disease (cor
pulmonale), multiple pulmonary emboli & pulmonary valvular stenosis.
o
Biventricular heart failure.
Failure of the left and right heart may develop because the
disease process, such as dilated cardiomyopathy or ischaemic heart disease, affects both
ventricles or because disease of the left heart leads to chronic elevation of the left atrial
pressure, pulmonary hypertension and right heart failure.
Diastolic and systolic dysfunction
Heart failure may develop as a result of impaired myocardial contraction (systolic
dysfunction) but can also be due to poor ventricular filling and high filling pressures
caused by abnormal ventricular relaxation (diastolic dysfunction). The latter is caused by
a stiff non-compliant ventricle and is commonly found in patients with left ventricular
hypertrophy. Systolic and diastolic dysfunction often coexist, particularly in patients with
coronary artery disease.
Chapter 5 – Cardiovascular disease
107
High-output failure
Conditions such as large arteriovenous shunt, beri-beri, severe anaemia or thyrotoxicosis
can occasionally cause heart failure due to an excessively high cardiac output.
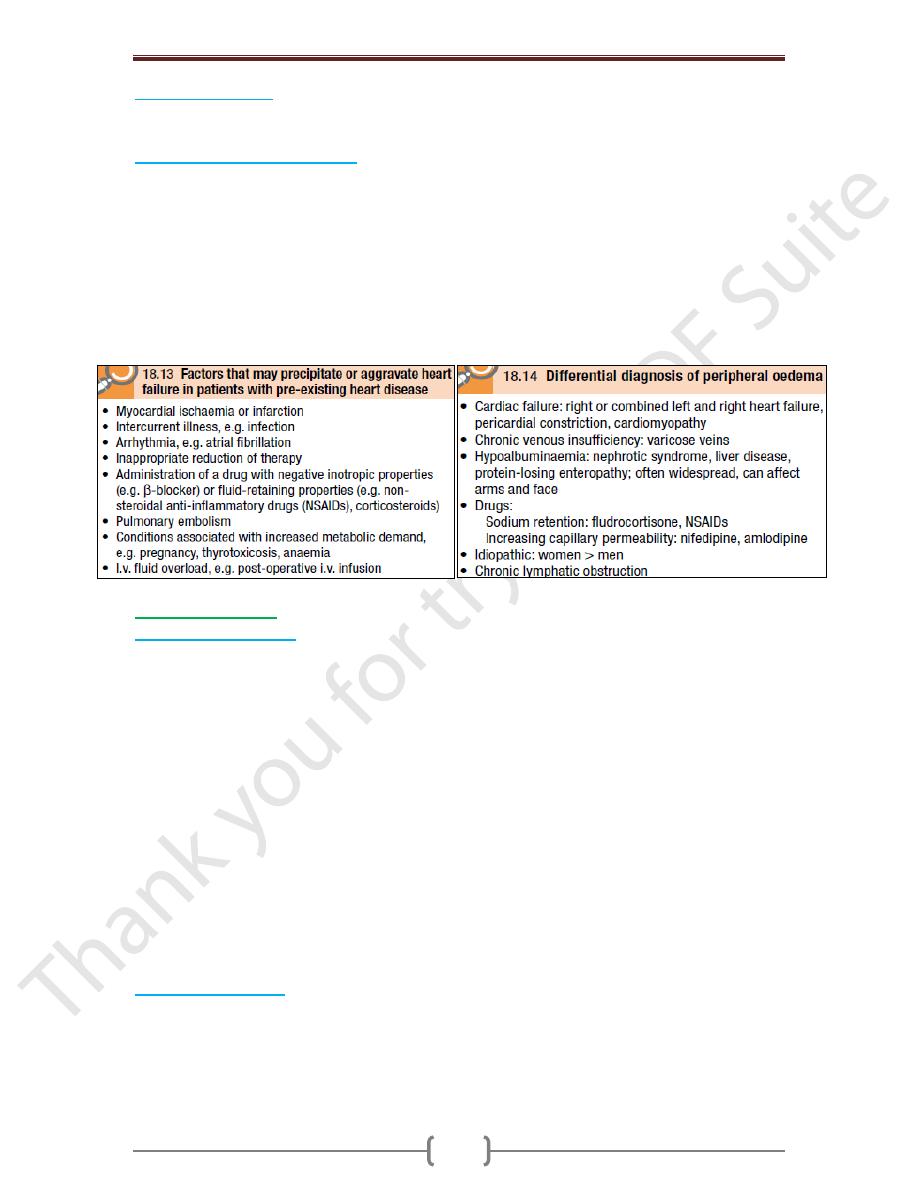
Acute and chronic heart failure
Heart failure may develop suddenly, as in MI, or gradually, as in progressive valvular
heart disease. When there is gradual impairment of cardiac function, a variety of
compensatory changes may take place. The term ‘compensated heart failure’ is
sometimes used to describe those with impaired cardiac function in whom adaptive
changes have prevented the development of overt heart failure. A minor event, such as an
intercurrent infection or development of atrial fibrillation, may precipitate overt or acute
heart failure. Acute left heart failure occurs either de novo or as an acute decompensated
episode on a background of chronic heart failure, so-called acute-on-chronic heart failure.
In HF, CO is about 5L/min & the heart can’t elevate the CO > 5L/min.
Clinical assessment
Acute left heart failure
o Acute de novo left ventricular failure presents with a sudden onset of dyspnoea at rest
that rapidly progresses to acute respiratory distress, orthopnoea & prostration. The
precipitant, such as acute MI, is often apparent from the hx.
o The patient appears agitated, pale and clammy. The peripheries are cool to the touch and
the pulse is rapid. Inappropriate bradycardia or excessive tachycardia should be identified
promptly, as this may be the precipitant for the acute episode of heart failure. The BP is
usually high because of sympathetic nervous system activation, but may be normal or low
if the patient is in cardiogenic shock.
o The jugular venous pressure (JVP) is usually elevated, particularly with associated fluid
overload or right HF. In acute de novo HF, there has been no time for ventricular
dilatation & the apex is not displaced. Auscultation occasionally identifies the murmur of
a catastrophic valvular or septal rupture, or reveals a triple ‘gallop’ rhythm. Crepitations
are heard at the lung bases, consistent with pulmonary oedema.
o Acute-on-chronic heart failure will have additional features of long-standing heart failure
(see below). Potential precipitants, such as an upper respiratory tract infection or
inappropriate cessation of diuretic medication, should be identified.
Chronic heart failure
o Patients with chronic HF commonly follow a relapsing and remitting course, with periods
of stability and episodes of decompensation leading to worsening symptoms that may
necessitate hospitalisation. The clinical picture depends on the nature of the underlying
heart disease, the type of heart failure that it has evoked, and the neurohumoral changes
that have developed.

Chapter 5 – Cardiovascular disease
108
o A low CO causes fatigue, listlessness & a poor effort tolerance; the peripheries are cold
& BP is low. To maintain perfusion of vital organs, blood flow is diverted away from
skeletal ms & this may contribute to fatigue & weakness. Poor renal perfusion leads to
oliguria & uraemia.
o Pulmonary oedema due to left heart failure presents as described above and with
inspiratory crepitations over the lung bases. In contrast, right heart failure produces a
high JVP with hepatic congestion and dependent peripheral oedema. In ambulant
patients, the oedema affects the ankles, whereas in bed-bound patients it collects around
the thighs and sacrum. Ascites or pleural effusion occurs in some cases. Heart failure is
not the only cause of oedema.
o Chronic heart failure is sometimes associated with marked weight loss (cardiac cachexia)
caused by a combination of anorexia and impaired absorption due to gastrointestinal
congestion, poor tissue perfusion due to a low cardiac output, and skeletal muscle atrophy
due to immobility.
Complications
In advanced heart failure, the following may occur:
Renal failure
is caused by poor renal perfusion due to a low cardiac output and may be
exacerbated by diuretic therapy, (ACE) inhibitors & angiotensin receptor blockers.
Hypokalaemia
may be the result of treatment with potassium-losing diuretics or
hyperaldosteronism caused by activation of the renin–angiotensin system and impaired
aldosterone metabolism due to hepatic congestion. Most of the body’s potassium is
intracellular and there may be substantial depletion of potassium stores, even when the
plasma potassium concentration is in the normal range.
Hyperkalaemia
may be due to the effects of drug treatment, particularly combination of ACE
inhibitors & spironolactone (which both promote potassium retention) & renal dysfunction.
Hyponatraemia
is a feature of severe heart failure and is a poor prognostic sign. It may
be caused by diuretic therapy, inappropriate water retention due to high ADH secretion,
or failure of the cell membrane ion pump.
Impaired liver function
caused by hepatic venous congestion and poor arterial
perfusion, which frequently cause mild jaundice and abnormal liver function tests;
reduced synthesis of clotting factors can make anticoagulant control difficult.
Thromboembolism.
Deep vein thrombosis and pulmonary embolism may occur due to
the effects of a low CO & enforced immobility, whereas systemic emboli may be related
to arrhythmias, atrial flutter or fibrillation, or intracardiac
thrombus complicating conditions such as mitral stenosis, MI or left ventricular aneurysm.
Atrial and ventricular arrhythmias
are very common and may be related to
electrolyte changes (e.g. hypokalaemia, hypomagnesaemia), the underlying structural
heart disease, and the pro-arrhythmic effects of increased circulating catecholamines or
drugs. Sudden death occurs in up to 50% of patients with HF and is often due to a
ventricular arrhythmia. Frequent ventricular ectopic beats and runs of non-sustained
ventricular tachycardia are common findings in patients with heart failure and are
associated with an adverse prognosis.
Investigations
Serum urea & electrolytes, Hb, thyroid function, ECG & chest X-ray may help to establish
the nature & severity of the underlying heart disease & detect any complications.
Brain natriuretic peptide (BNP) is elevated in heart failure and is a marker of risk; it is
useful in the investigation of patients with breathlessness or peripheral oedema.
Chapter 5 – Cardiovascular disease
109
Echocardiography
is very useful and should be considered in all patients with heart
failure in order to:
o determine the aetiology
o detect hitherto unsuspected valvular heart disease, such as occult mitral stenosis, and
other conditions that may be amenable to specific remedies
o Identify patients who will benefit from long-term therapy with drugs, such as ACE
inhibitors (see below).
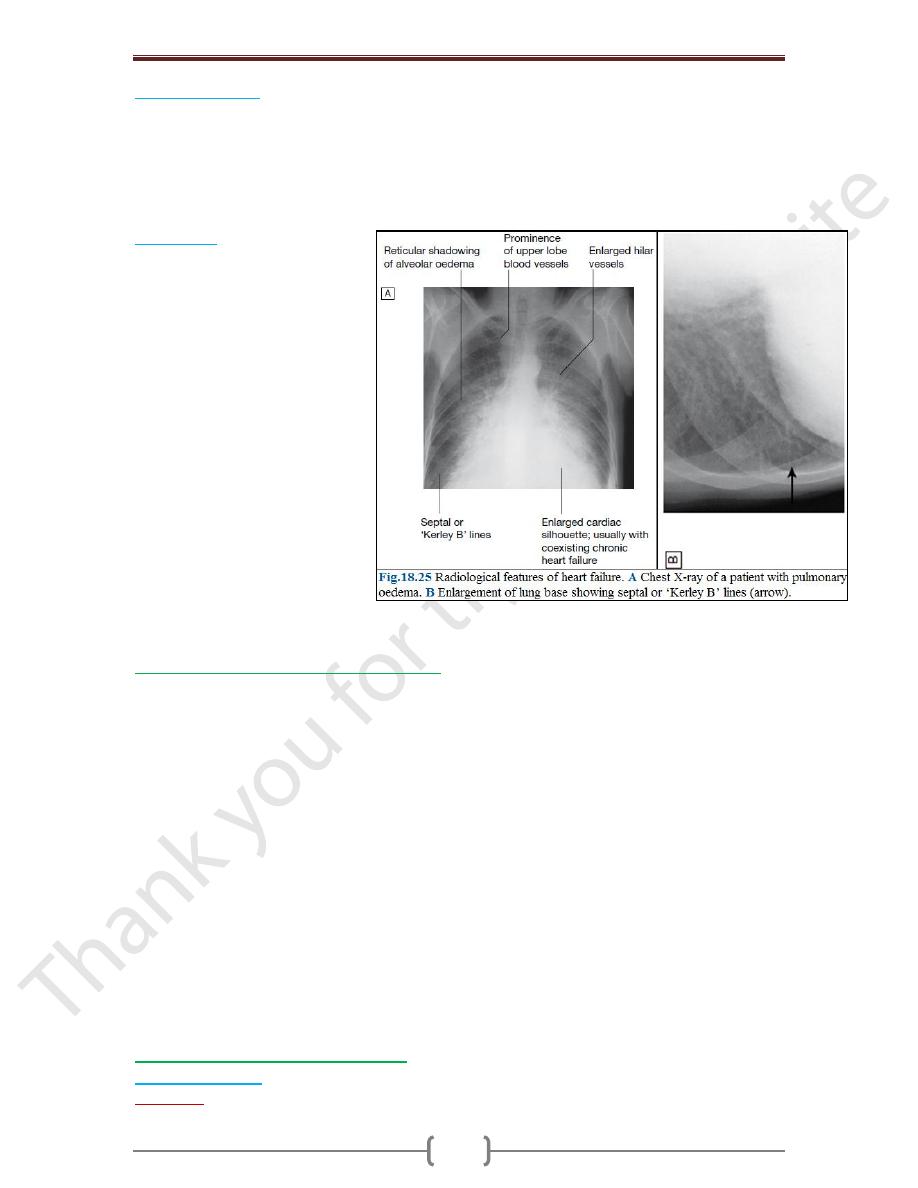
Chest X-ray
A rise in pulmonary venous
pressure from left-sided HF first
shows on the chest X-ray as an
abnormal distension of upper lobe
pulmonary veins (with the patient
in the erect position). The
vascularity of the lung fields
becomes more prominent, and the
right and left pulmonary arteries
dilate. Subsequently, interstitial
oedema causes thickened
interlobular septa and dilated
lymphatics. These are evident as
horizontal lines in the costophrenic
angles (septal or ‘Kerley B’ lines).
More advanced changes due to
alveolar oedema cause a hazy
opacification spreading from the
hilar regions & pleural effusions.
Management of acute pulmonary oedema
This is urgent:
o Sit the patient up in order to reduce pulmonary congestion.
o Give oxygen (high-flow, high-concentration). Noninvasive +ve pressure ventilation
(continuous +ve airways pressure (CPAP) of 5-10 mmHg) by a tight-fitting facemask
results in a more rapid improvement in the patient’s clinical state.
o Administer nitrates, such as i.v. glyceryl trinitrate 10–200 μg/min or buccal glyceryl
trinitrate 2–5 mg, titrated upwards every 10 minutes, until clinical improvement occurs or
systolic BP falls to < 110 mmHg.
o Administer a loop diuretic such as furosemide 50-100mg i.v.
The patient should initially be kept on strict bed rest with continuous monitoring of
cardiac rhythm, BP and pulse oximetry. IV opiates may be cautiously used when patients
are in extremis. They reduce sympathetically mediated peripheral vasoconstriction but
may cause respiratory depression & exacerbation of hypoxaemia & hypercapnia.
If these measures prove ineffective, inotropic agents may be required to augment cardiac
output, particularly in hypotensive patients. Insertion of an intra-aortic balloon pump can
be very beneficial in patients with acute cardiogenic pulmonary oedema, especially when
secondary to myocardial ischaemia.
Management of chronic heart failure
General measures
o Education:
Explanation of nature of disease, treatment and self-help strategies

Chapter 5 – Cardiovascular disease
110
o Diet:
Good general nutrition and weight reduction for the obese. Avoidance of high-salt
foods and added salt, especially for patients with severe congestive heart failure
o Alcohol:
Moderation or elimination of alcohol consumption. Alcohol-induced
cardiomyopathy requires abstinence
o Smoking:
Cessation
o Exercise:
Regular moderate aerobic exercise within limits of symptoms
o Vaccination:
Influenza and pneumococcal vaccination should be considered
In patients with coronary heart disease, secondary preventative measures, such as low-
dose aspirin and lipid-lowering therapy, are required. However, statins do not appear to
be effective in patients with severe heart failure.
Drug therapy
o Diuretic therapy
In HF, diuretics produce an increase in urinary sodium & water excretion, leading to a
reduction in blood & plasma volume. Diuretic therapy reduces preload and improves
pulmonary & systemic venous congestion. It may also reduce afterload and ventricular
volume, leading to a fall in wall tension & increased cardiac efficiency.
In some patients with severe chronic heart failure, particularly in the presence of chronic
renal impairment, oedema may persist despite oral loop diuretics. In such patients an
intravenous infusion of furosemide 10 mg/hr may initiate a diuresis. Combining a loop
diuretic with a thiazide (e.g. bendroflumethiazide 5 mg daily) or a thiazide-like diuretic
(e.g. metolazone 5 mg daily) may prove effective, but this can cause an excessive
diuresis. Aldosterone receptor antagonists, such as spironolactone and eplerenone, are
potassium-sparing diuretics that are of particular benefit in patients with heart failure.
They may cause hyperkalaemia, particularly when used with an ACE inhibitor. They
improve long-term clinical outcome in patients with severe heart failure or heart failure
following acute MI.
o Vasodilator therapy
These drugs are valuable in chronic heart failure. Venodilators, such as nitrates, reduce
preload, & Arterial dilators, such as hydralazine, reduce afterload. Their use is limited by
pharmacological tolerance and hypotension.
o Angiotensin-converting enzyme (ACE) inhibition therapy
This interrupts the vicious circle of neurohumoral activation that is characteristic of
moderate and severe heart failure by preventing the conversion of angiotensin I to
angiotensin II, thereby preventing salt and water retention, peripheral arterial and venous
vasoconstriction, & activation of the sympathetic nervous system. These drugs also
prevent the undesirable activation of the renin–angiotensin system caused by diuretic
therapy. Whilst the major benefit of ACE inhibition in heart failure is a reduction in
afterload, it also reduces preload and causes a modest rise in the plasma potassium
concentrations. Treatment with a combination of a loop diuretic and an ACE inhibitor
therefore has many potential advantages.
In moderate and severe HF, ACE inhibitors can produce a substantial improvement in
effort tolerance and in mortality. They can also improve outcome and prevent the onset of
overt heart failure in patients with poor residual left ventricular function following MI.
They can cause symptomatic hypotension & impairment of renal function. Short-acting
ACE inhibitors can cause marked falls in BP, particularly in the elderly or when started in
the presence of hypotension, hypovolaemia or hyponatraemia.
o Angiotensin receptor blocker (ARB) therapy
These drugs act by blocking the action of angiotensin II on the heart, peripheral vasculature
& kidney. In HF, they produce beneficial haemodynamic changes that are similar to the

Chapter 5 – Cardiovascular disease
111
effects of ACE inhibitors but are generally better tolerated. They have comparable
effects on mortality & are a useful alternative for patients who cannot tolerate ACE
inhibitors. Unfortunately, they share all the more serious adverse effects of ACE inhibitors,
including renal dysfunction & hyperkalaemia. They may be considered in combination
with ACE inhibitors, especially in those with recurrent hospitalisations for HF.
o Beta-adrenoceptor blocker therapy
helps to counteract the deleterious effects of enhanced sympathetic stimulation & reduces
the risk of arrhythmias and sudden death. When initiated in standard doses, they may
precipitate acute-on-chronic HF, but when given in small incremental doses (e.g.
bisoprolol started at a dose of 1.25 mg daily, & increased gradually over a 12-week
period to a target maintenance dose of 10 mg daily), they can increase ejection fraction,
improve symptoms, reduce the frequency of hospitalization and reduce mortality in
patients with chronic HF. Beta-blockers are more effective at reducing mortality than
ACE inhibitors: relative risk reduction of 33% versus 20% respectively.
o Digoxin
can be used to provide rate control in patients with HF & atrial fibrillation. In patients
with severe HF (NYHA class III–IV), digoxin reduces the likelihood of hospitalisation
for heart failure, although it has no effect on long-term survival.
o Amiodarone
This is a potent anti-arrhythmic drug that has little negative inotropic effect and may be
valuable in patients with poor left ventricular function. It is only effective in the treatment
of symptomatic arrhythmias, and should not be used as a preventative agent in
asymptomatic patients.
Implantable cardiac defibrillators and resynchronisation therapy
Coronary revascularisation
Heart transplantation
Ventricular assist devices
