
1
2
List of contents
Topics
Directed by
Page number
1
Heart
د. مهند
(3
– 14)
Heart Failure
4 - 6
Pericardial disease
7
Valvular heart disease
8 - 10
Cardiomyopathies
11
Congenital heart disease
12 - 14
2
Oral cavity & GIT
د. سالم
(15
– 50)
Oral cavity & Oropharynx
16 - 21
Esophagus
22 - 27
Stomach
28 - 34
Small & Large Intestine
35 - 49
Appendix
50
3
Lymphoid System
(51
– 59)
Lymph Node
52 - 59
4
Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
(60
– 83)
The Liver
61 - 76
Disorders of gallbladder and extrahepatic biliary tract
77 - 80
Exocrine Pancreas
81 - 83
5
Kidney & Its Collecting System, and Lower Urinary Tract
(84 - 106)
Clinical Manifestations of Renal Diseases
86 – 91
Glomerular diseases
86 – 91
Tubulo-interstitial diseases
92 – 95
Diseases of renal blood vessels
96 - 97
Cystic diseases of the kidney
98
Urinary outflow obstruction
99 - 100
Tumors
101 - 102
Renal pelvis, ureter, urinary bladder & urethra
103 - 106
6
Male Genital System
(107
– 117)
The penis
108
Scrotum
109
Testis and Epididymis
110 – 113
The prostate
114 - 117
7
Female genital system & Breast
(118
– 144)
Vulva
119 - 120
Vagina
120
Cervix
121 – 123
Body of uterus
124 - 127
Fallopian tubes
128
Ovaries
129 – 132
Diseases of pregnancy
133 - 136
Breast
137 – 144
8
Endocrine system
(145
–166)
Pituitary
146 - 148
Thyroid
149 - 155
Parathyroid glands
156 – 157
Endocrine pancreas
158 - 160
Adrenal glands
161 - 165
Multiple Endocrine Neoplasia Syndromes (MEN)
166
9
Bones, Joints, and Soft Tissue Tumors
(167
–185)
Bones
168 - 178
Joints
179 - 181
Soft Tissue Tumors
182 – 185
3
Chapter contents (3 – 14)
Topics
Directed by
Page number
1
Heart Failure
د. مهند
(4 - 6)
Left-Sided Heart Failure
4
Right-Sided Heart Failure
5
2
Pericardial Disease
(7)
Pericarditis
7
Pericardial Effusion
7
3
Valvular Heart Disease
(8 - 10)
Rheumatic Valvular Disease
8
Infective Endocarditis
9
Noninfective Vegetations
10
4
Cardiomyopathies
(11)
Myocarditis
11
5
Congenital heart disease
(12 - 14)
Left-to-Right Shunts
12
Right-to-Left Shunts
13
Obstructive Lesions
13

Chapter 1 - Heart
4
“” Heart Failure “”
Left-Sided Heart Failure

Chapter 1 - Heart
5
Right-Sided Heart Failure

Chapter 1 - Heart
6

Chapter 1 - Heart
7
“” Pericardial disease “”
Pericarditis
Pericardial effusion

Chapter 1 - Heart
8
“” Valvular Heart Disease “”
Rheumatic Valvular Disease

Chapter 1 - Heart
9
Infective Endocarditis

Chapter 1 - Heart
10
Noninfected Vegetations
Nonbacterial Thrombotic Endocarditis

Chapter 1 - Heart
11
“” Cardiomyopathies “”
Myocarditis

Chapter 1 - Heart
12
“” Congenital Heart Disease “”
Left-to-Right Shunts
Atrial septal defect (ASD)
Ventricular septal defect

Chapter 1 - Heart
13
Patent ductus arteriosus
Right-to-Left Shunts
Teratology of fallot
Transposition of great arteries (TGA)
Obstructive Lesions
Aortic Coarctation

Chapter 1 - Heart
14

15
Chapter contents (15 – 50)
Topics
Directed by
Page number
1
Oral cavity & Oropharynx
.د
سالم
(16 - 21)
Gingivitis
16
Proliferative lesions
16
Inflammatory conditions
16
Infections
17
Oral manifestations of systemic diseases
17
Tumors & Precancerous lesions
18
Salivary Glands
19
2
Esophagus
(22 - 27)
Congenital anomalies
22
Lesions associated with motor dysfunction
22
Esophageal varices
23
Esophagitis
24
Tumors of the esophagus
26
3
Stomach
(28 - 34)
Congenital anomalies
28
Gastritis
28
Peptic ulcer
30
Acute gastric ulceration
32
Tumors of the stomach
32
4
Small & Large intestine
(35 - 49)
Congenital anomalies
35
Enterocolitis
35
Vascular diseases
44
Diverticular disease
45
Intestinal obstruction
45
Tumors of the small & large intestine
46
5
Appendix
(50)
Acute appendicitis
50
Tumors of the appendix
50
Mucocele and pseudomyxoma peritonei
50
Chapter 2 - Oral Cavity & Gastrintestinal Tract
16
“” The oral cavity & oropharynx “”
Many pathological processes can affect the constituents of the oral cavity. The more
important and frequent conditions will covered in this lecture. Diseases involving the
teeth and related structures will not be discussed.
Gingivitis
Gingivitis refers to "inflammation of soft tissues surrounding the teeth (gum)." It is
primarily due to lack of proper oral hygiene that leads to accumulation of dental plaques.
The latter consist of normal flora, proteins of saliva, and desquamated epithelial cells.
Plaques are converted to calculi through mineralization. Continuous and persistent
irritation by these calculi leads to chronic gingivitis. The condition manifests as redness,
edema, and bleeding. Eventually there is loss of soft tissue adaptation to the teeth (loose
tooth). (Fig. 5-1)
Proliferative lesions
The most common proliferative lesions of the oral cavity are
1. Irritation Fibroma and Ossifying fibroma
2. Ossifying fibroma
2. Pyogenic granuloma
3. Peripheral giant cell granuloma
Irritation fibroma
is a nodular mass of fibrous tissue that occurs mainly in the buccal
mucosa along the bite line & gingivo-dental margin. (Fig. 5-2)
Ossifying fibroma
is a common growth of the gingiva. Some may be due to maturation
of a long-standing pyogenic granuloma.
Pyogenic granuloma (granuloma pyogenicum)
is a highly vascular lesion that is
usually seen in the gingiva of children, young adults, and pregnant women (pregnancy
tumor). The lesion is typically ulcerated and bright red in color (due to rich vascularity)
(Fig. 5-3). Microscopically there is vascular proliferation similar to that of granulation
tissue. (Fig. 5-3) They lesion either regresses (particularly after pregnancy), or undergoes
fibrous maturation and may develop into ossifying fibroma.
Peripheral giant cell granuloma (giant cell epulis)
is a relatively common lesion that
characteristically protrudes from the gingiva at sites of chronic inflammation. It is so
named because microscopically there are aggregates of multinucleate giant cells separated
by a fibro-vascular stroma. (Fig. 5-4)
Inflammatory conditions
Inflammatory ulcerations
The most common inflammatory ulcerations of the oral cavity are
1)
Traumatic ulcers
, usually the result of trauma (e.g. fist fighting) or licking a jagged tooth.
2)
Aphthous ulcers
are extremely common, single or multiple, painful, recurrent,
superficial, ulcerations of the oral mucosa. The ulcer is covered by a thin yellow exudate
and rimmed by a narrow zone of erythema. (Fig. 5-5)
3)
Herpetic ulcers
(see under herpes simplex infection)
Glossitis
This is inflammation of the tongue, but sometimes it is also applied to the beefy-red
tongue that occurs in certain deficiency states. The latter is the result of atrophy of the
papillae and thinning of the mucosa, thus exposing the underlying vasculature. (Fig. 5-6)
However, in some instances, the atrophy leads to inflammation (and even shallow
ulcerations). Examples of such deficiency states that include:
1. B12 (pernicious anemia), 2. Riboflavin 3. Niacin 4. Pyridoxine 5.Iron-deficiency anemia
Plummer-Vinson syndrome
is a combination of
1. Iron-deficiency anemia 2. Glossitis 3. Esophageal dysphagia (due to esophageal webs)
Chapter 2 - Oral Cavity & Gastrintestinal Tract
17
Glossitis (with ulcerations) may also be seen with
* Jagged carious teeth * Ill-fitting dentures
* Exposure to hot fluids or corrosive chemicals
* Inhalation of burn fumes including excessive smoking
* Syphilis
Infections
1) Herpes simplex infections
Most of these are caused by herpes simplex virus (HSV) type 1 & sometimes 2. Primary
HSV infection typically occurs in children aged 2 to 4 years; is often asymptomatic, but
sometime presents as acute herpetic gingivostomatitis, characterized by vesicles and
ulcerations throughout the oral cavity. The great majority of affected adults harbor latent
HSV-1 (the virus migrates along the regional nerves and eventually becomes dormant in
the local ganglia e.g., the trigeminal). In some individuals, usually young adults, the virus
becomes reactivated to produce the common but usually mild cold sore. (Fig. 5-7)
Factors activating the virus include
1. Trauma 2. Allergies 3. Exposure to ultraviolet light (sunlight)
4. Upper respiratory tract infections 5. Pregnancy 6. Menstruation 7. Immunosuppression
The viral infection is associated with intracellular and intercellular edema, yielding clefts that
may become transformed into vesicles. The vesicles range from a few millimeters to large
ones that eventually rupture to yield extremely painful, red-rimmed, shallow ulcerations.
2) Other Viral Infections
These include
- Herpes zoster - EBV (infectious mononucleosis) - CMV - Enterovirus - Measles
3) Oral Candidiasis (thrush)
This is the most common fungal infection of the oral cavity. The thrush is a grayish white,
superficial, inflammatory psudomembrane composed of the fungus enmeshed in fibrino-
suppurative exudates. (Fig. 5-8) This can be readily scraped off to reveal an underlying
red inflammatory base. The fungus is a normal oral flora but causes troubles only
1) In the setting of immunosuppression (e.g. diabetes mellitus, organ or bone marrow
transplant recipients, neutropenia, cancer chemotherapy, or AIDS) or
2) When broad-spectrum antibiotics are taken; these eliminate or alter the normal
bacterial flora of the mouth.
3) In infants, where the condition is relatively common, presumably due to immaturity of
the immune system in them.
4) Deep Fungal Infections
Some fungal infections may extends deeply to involve the muscles & bones in relation to
oral cavity. These include, among others, histoplasmosis, blastomycosis, and
aspergillosis. The incidence of such infections has been increasing due to increasing
number of patients with AIDS, therapies for cancer, & organ transplantation
5)
Diphtheria
Characterized grossly by dirty white, fibrino-suppurative, tough, inflammatory
pseudomembrane over tonsils & posterior pharynx. (Fig. 5-9)
Oral manifestations of systemic disease
Many systemic diseases are associated with oral lesions & it is not uncommon for oral
lesions to be the first manifestation of some underlying systemic condition.
1)
Scarlet fever:
strawberry tongue: white coated tongue with hyperemic papillae projecting (Fig. 5-10)
2)
Measles:
Koplik spots: small whitish ulcerations (spots) on a reddened base, about Stensen
duct (Fig. 5-11)
3)
Diphtheria:
Dirty white, fibrinosuppurative, tough pseudomembrane over the tonsils and retropharynx
Chapter 2 - Oral Cavity & Gastrintestinal Tract
18
4)
AIDS
A. opportunistic oral infections: herpesvirus, Candida, other fungi
b. Kaposi sarcoma (Fig. 5-12)
c. hairy leukoplakia; Approximately 80% of patients with hairy leukoplakia have been
infected with HIV; the remainig 20% are seen in association with other
immunodeficiency states. The condition presents as a white fluffy ("hairy") patches that
are situated on the lateral border of the tongue. (Fig. 5-15) EBV is now accepted as the
cause of the condition. When hairy leukoplakia precedes HIV infection, manifestations of
AIDS generally appear within 2 or 3 years.
5)
AML
(especially monocytic leukemia): enlargement of the gingivae + periodontitis (Fig. 5-13)
6)
Melanotic pigmentation
(Fig. 5-14)
a. Addison disease
b. hemochromatosis
c. fibrous dysplasia of bone
d. Peutz-Jegher syndrome
7)
Pregnancy:
pyogenic granuloma ("pregnancy tumor")
Tumors and precancerous lesions
Many of the oral cavity tumors (e.g., papillomas, hemangiomas, lymphomas) are not
different from their homologous tumors elsewhere in the body. Here we will consider
only oral squamous cell carcinoma and its associated precancerous lesions.
Leukoplakia and Erythroplakia are considered premalignant lesions of squamous cell
carcinoma.
Leukoplakia
(Fig. 5-16) is a white patch that cannot be scraped off and cannot be attributed clinically
or microscopically to any other disease i.e. if a white lesion in the oral cavity can be given
a specific diagnosis it is not a leukoplakia. As such, white patches caused by entities such
as candidiasis are not leukoplakias. All leukoplakias must be considered precancerous
(have the potential to progress to squamous cell carcinoma) until proved otherwise
through histologic evaluation.
Erythroplakias
(Fig. 5-17) are red velvety patches that are much less common, yet much more serious
than leukoplakias. The incidence of dysplasia and thus the risk of complicating squamous
cell carcinoma is much more frequent in erythroplakia compared to leukoplakias.
Both leukoplakia and erythroplakia are usually found between ages of 40 and 70 years,
and are much more common in males than females. The use of tobacco (cigarettes, pipes,
cigars, and chewing tobacco) is the most common incriminated factor.
Squamous cell carcinoma
The vast majority (95%) of cancers of the head and neck are squamous cell carcinomas;
these arise most commonly in the oral cavity. The 5-year survival rate of early-stage oral
cancer is approximately 80%, but this drops to about 20% for late-stage disease. These
figures highlight the importance of early diagnosis & treatment, optimally of the
precancerous lesions.
The pathogenesis of squamous cell carcinoma is multifactorial.
1) Chronic smoking and alcohol consumption
2) Oncogenic variants of human papilloma virus (HPV). It is now known that at least
50% of oropharyngeal cancers, particularly those of the tonsils and the base of tongue,
harbor oncogenic variants of HPV.
3) Inheritance of genomic instability; a family history of head & neck cancer is a risk factor.
4) Exposure to actinic radiation (sunlight) & pipe smoking are known predisposing
factors for cancer of the lower lip.
Chapter 2 - Oral Cavity & Gastrintestinal Tract
19
Gross features (Fig. 5-18 A)
may arise anywhere in the oral cavity, but the favored locations are
1. The tongue 2. Floor of mouth 3. Lower lip 4. Soft palate 5. Gingiva
In the early stages, cancers of the oral cavity appear as roughened areas of the mucosa. As
the lesion enlarges, it typically appears as either an ulcer or a protruding mass (fungating).
Microscopic features (Fig. 5-18 B)
Early there is full-thickness dysplasia (carcinoma in situ) followed by invasion of the
underlying connective tissue stroma.
The grade varies from from well-differentiated keratinizing to poorly differentiated.
As a group, these tumors tend to infiltrate and extend locally before they eventually
metastasize to cervical lymph nodes and more remotely. The most common sites of
distant metastasis are mediastinal lymph nodes, lung, liver and bones
Salivary glands
There are three major salivary glands—parotid, submandibular, and sublingual.
Additionally, there are numerous minor salivary glands distributed throughout the mucosa
of the oral cavity.
Xerostomia
refers to dry mouth due to a lack of salivary secretion; the causes include
1. Sjögren syndrome: an autoimmune disorder, that is usually also accompanied by
involvement of the lacrimal glands that produces dry eyes (keratoconjunctivitis sicca).
2. Radiation therapy
Inflammation (sialadenitis)
Sialadenitis refers to inflammation of a salivary gland; it may be
1) Traumatic
2) Infectious: viral, bacterial
The most common form of viral sialadenitis is mumps, which usually affects the
parotids. The pancreas and testes may also be involved.
Bacterial sialadenitis is seen as a complication of
1. Stones obstructing ducts of a major salivary gland (Sialolithiasis), particularly the
submandibular. (Fig. 5-19)
2. Dehydration with decreased secretory function as is sometimes occurs in
a. patients on long-term phenothiazines that suppress salivary secretion.
b. elderly patients with a recent major thoracic or abdominal surgery.
Unilateral involvement of a single gland is the rule & the inflammation may be suppurative.
The inflammatory involvement causes painful enlargement and sometimes a purulent
ductal discharge.
3) Autoimmune
Sjögren syndrome causes an immunolgically mediated sialadenitis i.e. inflammatory
damage of the salivary tissues.
Mucocele
This is common salivary lesion results from interruption of salivary outflow due to
blockage or rupture of a salivary gland duct. This leads to seepage of saliva into the
surrounding tissues. The lower lip is the most common location due to exposure of this
site to trauma (fist fighting, falling etc.). It presents as fluctuant swelling.
Microscopically, there is accumulation of mucin with inflammatory cells. (Fig. 5-20)
Ranula is a mucocele of the sublingual gland; it may become extremely large.
Neoplasms of salivary glands
Neoplasms of the salivary glands (benign and malignant) are generally uncommon,
constituting less than 2% of human tumors. We will restrict our discussion on the more
common examples.
Chapter 2 - Oral Cavity & Gastrintestinal Tract
20
The relative frequency distributions of these tumors in relation to various salivary glands
are as follows
Salivary gland
Relative frequency of tumors (%)
Parotid gland
80%
Submandibular gland
10%
Minor salivary and sublingual glands
10%
The incidence of malignant tumors within the glands is, however, different from the
above
Salivary gland affected
Relative frequency of malignant tumors (%)
Sublingual tumors
80%
Minor salivary glands
50%
Submandibular glands
40%
Parotid glands
25%
These tumors usually occur in adults, with a slight female predominance. Excluded from
this rule is Warthin tumor, which occurs much more frequently in males than in females.
The benign tumors occur most often around the age of 50 to 60 years; the malignant ones
tend to appear in older age groups. Neoplasms of the parotid produce distinctive swellings
in front of, or below the ear. Clinically, there are no reliable criteria to differentiate benign
from the malignant tumors; therefore, pathological evaluation is necessary. (Fig. 5-21)
Pleomorphic Adenomas (Mixed Salivary Gland Tumors)
These benign tumors commonly occur within the parotid gland (constitute 60% of all
parotid tumors).
o Gross features (Fig. 5-22 A)
Most tumors are rounded, encapsulated masses.
The cut surface is gray-white with myxoid and light blue translucent areas of chondroid.
o Microscopic features (Fig. 5-22 B)
The main constituents are a mixture of ductal epithelial and myoepithelial cells, and it is
believed that all the other elements, including mesenchymal, are derived from the above
cells (hence the name adenoma).
The epithelial/myoepithelial components of the neoplasm are arranged as glands, strands,
or sheets. These various epithelial/myoepithelial elements are dispersed within a
background of loose myxoid tissue that may contain islands of cartilage-like islands and,
rarely bone. Sometimes, squamous differentiation is present.
In some instances, the tumor capsule is focally deficient allowing the tumor to extend as
tongue-like protrusions into the surrounding normal tissue.
Enucleation of the tumor is, therefore, not advisable because residual foci (the protrusions)
may be left behind and act as a potential source of multifocal recurrences. (Fig. 5-23)The
incidence of malignant transformation increases with the duration of the tumor.
Warthin Tumor
Is the second most common salivary gland neoplasm. It is benign, arises usually in the
parotid gland and occurs more commonly in males than in females. About 10% are
multifocal and 10% bilateral. Smokers have a higher risk than nonsmokers for developing
these tumors. Grossly, it is round to oval, encapsulated mass & on section display gray
tissue with narrow cystic or cleft-like spaces filled with secretion. Microscopically, these
spaces are lined by a double layer of neoplastic epithelial cells resting on a dense
lymphoid stroma, sometimes with germinal centers. This lympho-epithelial lining
frequently project into the spaces. The epithelial cells are oncoytes as evidenced by their
eosinophilic granular cytoplasm (stuffed with mitochondria). (Fig. 5-24)
Mucoepidermoid Carcinoma
As the name indicates, these neoplasms are composed of variable mixtures of mucus-
secreting cells (muco), and squamous cells (epidermoid). They are the most common form
of primary malignant tumor of the salivary glands. They occur mainly in the parotids.

Chapter 2 - Oral Cavity & Gastrintestinal Tract
21
Low-grade tumors may invade locally but do not metastasize. By contrast, high-grade
neoplasms metastasize to distant sites in 30% of cases. Grossly, mucoepidermoid
carcinomas are gray-white, infiltrative tumors that frequently show small, mucin-
containing cysts. Microscopically, there are cords, sheets, and cysts of squamous and
mucous-secreting cells. (Fig. 5-25)
Other Salivary Gland Tumors
Two less common neoplasms worth brief description:
o Adenoid cystic carcinoma: half of the cases are found in the minor salivary glands (in
particular the palate). Although slow growing, they have a tendency to invade perineural
spaces and to recur. Eventually, 50% or more disseminate widely to distant sites such as
bone, liver, and brain. Microscopically, they are composed of small cells having dark,
compact nuclei and scant cytoplasm. These cells tend to be disposed in sieve-like
(cribriform) patterns. The spaces between the tumor cells are often filled with a hyaline
material thought to represent excess basement membrane. (Fig. 5-26)
o Acinic cell tumor is composed of cells resembling the normal acinar cells (hence the
name). Most arise in the parotids; the small remainder arises in the submandibular glands.
On histologic examination, they reveal a variable architecture and cell morphology. Most
characteristically, the cells have clear cytoplasm & are disposed in sheets, microcysts,
glands, or papillae. About 10% to 15% of these neoplasms metastasize to lymph nodes.
(Fig. 5-27)
Chapter 2 - Oral Cavity & Gastrintestinal Tract
22
Esophagus
The main functions of the esophagus are to 1. Conduct food and fluids from the pharynx to
the stomach 2. Prevent reflux of gastric contents into the esophagus. These functions
require motor activity coordinated with swallowing, i.e. wave of peristalsis, associated
with relaxation of the LES in anticipation of the peristaltic wave. This is followed by
closure of the LES after the swallowing reflex. Maintenance of sphincter tone (positive
pressure relative to the rest of esophagus) is necessary to prevent reflux of gastric contents.
Congenital anomalies
Several congenital anomalies affect the esophagus including
The presence of
ectopic
gastric mucosa & pancreatic tissues within the esophageal wall,
congenital cysts
&
congenital herniation
of the esophageal wall into the thorax. The
latter is due to impaired formation of the diaphragm.
Atresia and fistulas
are uncommon but must be recognized & corrected early because
they cause immediate regurgitation, suffocation & aspiration pneumontis when feeding is
attempted. In atresia, a segment of the esophagus is represented by only a noncanalized
cord, with the upper pouch connected to the bronchus or the trachea and a lower pouch
leading to the stomach. (Fig. 5-28)
Webs, rings, and stenosis
(Fig. 5-29)
Mucosal webs
are shelf-like, eccentric protrusions of the mucosa into the esophageal
lumen. These are most common in the upper esophagus. The triad of upper esophageal
web, iron-deficiency anemia, and glossitis is referred to as Plummer-Vinson syndrome.
This condition is associated with an increased risk for postcricoid esophageal carcinoma.
Esophageal rings
unlike webs are concentric plates of tissue protruding into the lumen of
the distal esophagus. Esophageal webs and rings are encountered most frequently in
women over age 40. Episodic dysphagia is the main symptom.
Stenosis
consists of fibrous thickening of the esophageal wall. Although it may be
congenital, it is more frequently the result of severe esophageal injury with inflammatory
scarring, as from gastroesophageal reflux disease (GERD), radiation, scleroderma and
caustic injury. Stenosis usually manifests as progressive dysphagia, at first to solid foods
but eventually to fluids as well.
Lesions associated with motor dysfunction (fig. 5-30)
Coordinated motor activity is important for proper function of the esophagus. The major
entities that are caused by motor dysfunction of the esophagus are
1. Achalasia 2. Hiatal hernia 3. Diverticula 4. Mallory-Weiss tear
Achalasia
Achalasia means "failure to relax." It is characterized by three major abnormalities:
1. Aperistalsis (failure of peristalsis)
2. Increased resting tone of the LES
3. Icomplete relaxation of the LES in response to swallowing
In most instances, achalasia is an idiopathic disorder. In this condition there is progressive
dilation of the esophagus above the persistently contracted LES. The wall of the
esophagus may be of normal thickness, thicker than normal owing to hypertrophy of the
muscular wall, or markedly thinned by dilation (when dilatation overruns hypertrophy).
The mucosa just above the LES may show inflammation and ulceration. Young adults are
usually affected and present with progressive dysphagia. (Fig. 5-31)
Complications of achalasia are
1. Aspiration pneumonitis of undigested food 2. Monilial esophagitis
3. Esophageal squamous cell carcinoma (about 5% of patients)
4. Lower esophageal diverticula
Chapter 2 - Oral Cavity & Gastrintestinal Tract
23
Hiatal Hernia (Fig. 5-32)
Hiatal hernia is characterized by separation of the diaphragmatic crura leading to
widening of the space around the esophageal wall. Two types of hiatal hernia are
recognized: 1. The sliding type (95%)
2. The paraesophageal type
In the sliding hernia the stomach skates up through the patent hiatus above the diaphragm
creating a bell-shaped dilation. In paraesophageal hernias, a separate portion of the
stomach, usually along the greater curvature, enters the thorax through the widened
foramen. The cause of hiatal hernia is unknown. It is not clear whether it is a congenital
malformation or is acquired during life. Only about 10% of adults with a sliding hernia
suffer from heartburn or regurgitation of gastric juices into the mouth. These are due to
incompetence of the LES and are accentuated by positions favoring reflux (bending
forward, lying supine) and obesity.
Complications of hiatal hernias include
1. Ulceration, bleeding and perforation (both types)
2. Reflux esophagitis (frequent with sliding hernias)
3. Strangulation of paraesophageal hernias
Diverticula
(Fig. 5-33)
By definition a diverticulum is a "focal out pouching of the alimentary tract wall that
contains all or some of its constituents"; they are divided into
1. False diverticulum
is an out pouching of the mucosa and submucosa through weak
points in the muscular wall.
2. True diverticulum
consists of all the layers of the wall and is thought to be due to
motor dysfunction of the esophagus. They may develop in three regions of the esophagus
a. Zenker diverticulum, located immediately above the UES
b. Traction diverticulum near the midpoint of the esophagus
c. Epiphrenic diverticulum immediately above the LES.
Lacerations (Mallory-Weiss Syndrome)
These refer to longitudinal tears at the GEJ or gastric cardia and are the consequence of
severe retching or vomiting. They are encountered most commonly in alcoholics, since
they are susceptible to episodes of excessive vomiting, but have been reported in persons
with no history of vomiting or alcoholism. During episodes of prolonged vomiting, reflex
relaxation of LES fails to occur. The refluxing gastric contents suddenly overcome the
contracted musculature leading to forced, massive dilation of the lower esophagus with
tearing of the stretched wall.
Pathological features
The linear irregular lacerations, which are usually found astride the GEJ or in the gastric
cardia, are oriented along the axis of the esophageal lumen. The tears may involve only
the mucosa or may penetrate deeply to perforate the wall. (Fig. 5-34) Infection of the
mucosal defect may lead to inflammatory ulcer or to mediastinitis. Usually the bleeding is
not profuse and stops without surgical intervention. Healing is the usual outcome. Rarely
esophageal rupture occurs.
Esophageal Varices
Portal hypertension when sufficiently prolonged or severe induces the formation of
collateral bypass veins wherever the portal and caval venous systems communicate.
Esophageal varices refer to the prominent plexus of deep mucosal and submucosal venous
collaterals of the lower esophagus subsequent to the diversion of portal blood through
them through the coronary veins of the stomach. From the varices the blood is diverted
into the azygos veins, and eventually into the systemic veins. Varices develop in 90% of

Chapter 2 - Oral Cavity & Gastrintestinal Tract
24
cirrhotic patients. Worldwide, after alcoholic cirrhosis, hepatic schistosomiasis is the
second most common cause of variceal bleeding.
Pathological features (Fig. 5-35)
The increased pressure in the esophageal plexus produces dilated tortuous vessels that are
liable to rupture.
Varices appear as tortuous dilated veins lying primarily within the submucosa of the distal
esophagus and proximal stomach.
The net effect is irregular protrusion of the overlying mucosa into the lumen. The mucosa
is often eroded because of its exposed position.
Variceal rupture produces massive hemorrhage into the lumen. In this instance, the
overlying mucosa appears ulcerated and necrotic.
Rupture of esophageal varices usually produces massive hematemesis. Among patients
with advanced liver cirrhosis, such a rupture is responsible for 50% of the deaths. Some
patients die as a direct consequence of the hemorrhage (hypovolemic shock); others of
hepatic coma triggered by the hemorrhage.
Esophagitis
This term refers to inflammation of the esophageal mucosa. It may be caused by a variety
of physical, chemical, or biologic agents.
Reflux Esophagitis (Gastroesophageal Reflux Disease or GERD)
Is the most important cause of esophagitis & signifies esophagitis associated with reflux of
gastric contents into the lower esophagus. Many causative factors are involved, the most
important is decreased efficacy of esophageal antireflux mechanisms, particularly LES tone.
In most instances, no cause is identified. However, the following may be contributatory
a. Central nervous system depressants including alcohol
b. Smoking
c. Pregnancy
d. Nasogastric tube
e. Sliding hiatal hernia
f. hypothyroidism
g. Systemic sclerosis
Any one of the above mechanism may be the primary cause in an individual case, but
more than one is likely to be involved in most instances. The action of gastric juices is
vital to the development of esophageal mucosal injury.
Gross (endoscopic) features (Fig. 5-36 A)
These depend on the causative agent and on the duration and severity of the exposure.
Mild esophagitis may appear grossly as simple hyperemia. In contrast, the mucosa in
severe esophagitis shows confluent erosions or total ulceration into the submucosa.
Microscopic features (Fig. 5-36 B)
Three histologic features are characteristic:
1. Inflammatory cells including eosinophils within the squamous mucosa.
2. Basal cells hyperplasia
3. Extension of lamina propria papillae into the upper third of the mucosa.
The disease mostly affects those over the age of 40 years. The clinical manifestations
consist of dysphagia, heartburn, regurgitation of a sour fluid into the mouth, hematemesis,
or melena. Rarely, there are episodes of severe chest pain that may be mistaken for a
"heart attack."
The potential consequences of severe reflux esophagitis are
1. Bleeding
2. Ulceration
3. Stricture formation
4. Tendency to develop Barrett esophagus

Chapter 2 - Oral Cavity & Gastrintestinal Tract
25
Barrett Esophagus (BE)
o 10% of patients with long-standing GERD develop this complication. BE is the single
most important risk factor for esophageal adenocarcinoma. BE refers to columnar
metaplasia of the distal squamous mucosa; this occurs in response to prolonged injury
induced by refluxing gastric contents. Two criteria are required for the diagnosis of
Barrett esophagus:
1. Endoscopic evidence of columnar lining above the GEJ
2. Histologic confirmation of the above in biopsy specimens.
The pathogenesis of Barrett esophagus appears to be due to a change in the differentiation
program of stem cells of the esophageal mucosa. Since the most frequent metaplastic
change is the presence of columnar cells admixed with goblet cells, the term "intestinal
metaplasia" is used to describe the histological alteration.
o Gross features
Barrett esophagus is recognized as a red, velvety mucosa located between the smooth,
pale pink esophageal squamous mucosa and the light brown gastric mucosa.
It is displayed as tongues, patches or broad circumferential bands replacing the
squamocolumnar junction several centimeters. (Fig. 5-37 A)
o Microscopic features
the esophageal squamous epithelium is replaced by metaplastic columnar epithelium,
including interspersed goblet cells, & may show a villous pattern (as that of the small
intestine hence the term intestinal metaplasia). (Fig. 5-37 B)
Critical to the pathologic evaluation of patients with Barrett mucosa is the search for
dysplasia within the metaplastic epithelium. This dysplastic change is the presumed
precursor of malignancy (adenocarcinoma). Dysplasia is recognized by the presence of
cytologic and architectural abnormalities in the columnar epithelium, consisting of
enlarged, crowded, and stratified hyperchromatic nuclei with loss of intervening stroma
between adjacent glandular structures. Depending on the severity of the changes,
dysplasia is classified as low-grade or high-grade.
Approximately 50% of patients with high-grade dysplasia may already have adjacent
adenocarcinoma.
o Most patients with the first diagnosis of Barrett esophagus are between 40 and 60 years.
Barrett esophagus is clinically significant due to
1. The secondary complications of local peptic ulceration with bleeding and stricture.
2. The development of adenocarcinoma, which in patients with long segment disease
(over 3 cm of Barrett mucosa), occurs at a frequency that is 30- to 40 times greater than
that of the general population.
Other causes of esophagitis
In addition to GERD (which is, in fact, a chemical injury), esophageal inflammation may
have many origins. Examples include ingestion of mucosal irritants (such as alcohol,
corrosive acids or alkalis as in suicide attempts), cytotoxic anticancer therapy, bacteremia
or viremia (in immunosuppressed patients), fungal infection (in debilitated or
immunosuppressed patients or during broad-spectrum antimicrobial therapy; candidiasis
by far the most common), and uremia.
Chapter 2 - Oral Cavity & Gastrintestinal Tract
26
Tumors
Benign Tumors
Leiomyomas are the most common benign tumors of the esophagus. (Fig. 5-38)
Malignant Tumors
Carcinomas of the esophagus (5% of all cancers of the GIT) have, generally, a poor
prognosis because they are often discovered too late. Worldwide, squamous cell
carcinomas constitute 90% of esophageal cancers, followed by adenocarcinoma.
Other tumors are rare.
Squamous Cell Carcinoma (SCC)
o Most SCCs occur in adults over the age of 50. The disease is more common in males than
females. The regions with high incidence include Iran & China. Blacks throughout the
world are at higher risk than are whites.
o Etiology and Pathogenesis
Factors Associated with the Development of Squamous Cell Carcinoma of the Esophagus
are classified as
1. Dietary
Deficiency of vitamins (A, C, riboflavin, thiamine, and pyridoxine) & trace elements (zinc)
Fungal contamination of foodstuffs
High content of nitrites/nitrosamines
Betel chewing (betel: the leaf of a climbing evergreen shrub, of the pepper family, which
is chewed in the East with a little lime.)
2. Lifestyle
Burning-hot food
Alcohol consumption
Tobacco abuse
3. Esophageal Disorders
Long-standing esophagitis
Achalasia
Plummer-Vinson syndrome
4. Genetic Predisposition
Long-standing celiac disease - Racial disposition
The marked geographical variations in the incidence of the disease strongly implicate
dietary and environmental factors, with a contribution from genetic predisposition. The
majority of cancers in Europe and the United States are attributable to alcohol and
tobacco. Some alcoholic drinks contain significant amounts of such carcinogens as
polycyclic hydrocarbons, nitrosamines, and other mutagenic compounds. Nutritional
deficiencies associated with alcoholism may contribute to the process of carcinogenesis.
Human papillomavirus DNA is found frequently in esophageal squamous cell
carcinomas from high-incidence regions.
o Gross features (Fig. 5-39 A)
Like squamous cell carcinomas arising in other locations, those of the esophagus begin as
in situ lesions.
When they become overt, about 20% of these tumors are located in the upper third, 50%
in the middle third, and 30% in the lower third of the esophagus.
Early lesions appear as small, gray-white, plaque-like thickenings of the mucosa but with
progression, three gross patterns are encountered:
1. Fungating (polypoid) (60%) that protrudes into the lumen
2. Flat (diffusely infiltrative) (15%) that tends to spread within the wall of the esophagus,
causing thickening, rigidity, and narrowing of the lumen
3. Excavated (ulcerated) (25%) that digs deeply into surrounding structures and may
erode into the respiratory tree (with resultant fistula and pneumonia) or aorta (with
catastrophic bleeding) or may permeate the mediastinum and pericardium.

Chapter 2 - Oral Cavity & Gastrintestinal Tract
27
Local extension into adjacent mediastinal structures occurs early, possibly due to the
absence of serosa for most of the esophagus. Tumors located in the upper third of the
esophagus also metastasize to cervical lymph nodes; those in the middle third to the
mediastinal, paratracheal, and tracheobronchial lymph nodes; and those in the lower third
most often spread to the gastric and celiac groups of nodes.
o Microscopic features (Fig. 5-39 B)
Most squamous cell carcinomas are moderately to well-differentiated,
They are invasive tumors that have infiltrated through the wall or beyond.
The rich lymphatic network in the submucosa promotes extensive circumferential and
longitudinal spread.
o Esophageal carcinomas are usually quite large by the time of diagnosis, produces
dysphagia and obstruction gradually. Cachexia is frequent. Hemorrhage and sepsis may
accompany ulceration of the tumor.
The five-year survival rate in patients with superficial esophageal carcinoma is about
75%, compared to 25% in patients who undergo "curative" surgery for more advanced
disease. Local recurrence and distant metastasis following surgery are common. The
presence of lymph node metastases at the time of resection significantly reduces survival.
Adenocarcinoma
With increasing recognition of Barrett mucosa, most adenocarcinomas in the lower third
of the esophagus arise from the Barrett mucosa.
o Etiology and Pathogenesis
These focus on Barrett esophagus. The lifetime risk for cancer development from Barrett
esophagus is approximately 10%. Tobacco exposure and obesity are risk factors.
Helicobacter pylori infection may be a contributing factor.
o Gross features: (Fig. 5-40 A)
Adenocarcinomas arising in the setting of Barrett esophagus are usually located in the
distal esophagus and may invade the adjacent gastric cardia.
As is the case with squamous cell carcinomas, adenocarcinomas initially appear as flat
raised patches that may develop into large nodular fungating masses or may exhibit
diffusely infiltrative or deeply ulcerative features.
o Microscopic features (Fig. 5-40 B)
Most tumors are mucin-producing glandular tumors exhibiting intestinal-type features.
Multiple foci of dysplastic mucosa are frequently present adjacent to the tumor.
o Adenocarcinomas arising in Barrett esophagus chiefly occur in patients over the age of 40
years and similar to Barrett esophagus, it is more common in men than in women, and in
whites more than blacks (in contrast to squamous cell carcinomas). As in other forms of
esophageal carcinoma, patients usually present because of difficulty swallowing,
progressive weight loss, bleeding, and chest pain. The prognosis is as poor as that for
other forms of esophageal cancer, with under 20% overall five-year survival.
Identification and resection of early cancers with invasion limited to the mucosa or
submucosa improves five-year survival to over 80%. Regression or surgical removal of
Barrett esophagus has not yet been shown to eliminate the risk for adenocarcinoma.

Chapter 2 - Oral Cavity & Gastrintestinal Tract
28
Stomach
In developed countries, peptic ulcers occur in up to 10% of the general population.
Chronic infection of the gastric mucosa by the bacterium H. pylori is the most common
infection worldwide. Gastric cancer is still a significant cause of death, despite its
decreasing incidence.
Congenital anomalies
These include
Various
heterotopias
(normal tissues in abnormal locations), which are usually
asymptomatic, e.g. pancreatic heterotopia.
Congenital diaphgramatic hernia
, which is due to defective closure of the diaphragm;
this leads to herniation of abdominal contents into the thorax in utero.
Congenital hypertrophic pyloric stenosis
is encountered in male infants four times more
often than females. Persistent, projectile vomiting usually appears in the second or third
week of life. There is visible peristalsis and a firm, ovoid palpable mass in the region of
the pylorus resulting from hypertrophy and hyperplasia of the muscularis propria of the
pylorus. (Fig. 5-41)
Acquired pyloric stenosis in adults may complicate
1. Antral gastritis or peptic ulcers close to the pylorus.
2. Malignancy e.g. carcinomas of the pyloric region or adjacent panceas, or gastric lymphomas
3. Hypertrophic pyloric stenosis (rare) due to prolonged pyloric spasm
Gastritis
This is by definition, "inflammation of the gastric mucosa". It is a microscopic diagnosis.
The inflammation may be acute, with neutrophilic infiltration, or chronic, with
lymphocytes and/or plasma cells.
Acute gastritis
Is usually transient in nature. The inflammation may be accompanied by hemorrhage into
the mucosa (acute hemorrhagic gastritis) and, sometimes by sloughing (erosions) of the
superficial mucosa (acute erosive gastritis). (Fig. 5-42) The latter is a severe form of the
disease & an important cause of acute gastrointestinal bleeding. Although a large number
of cases have no obvious cause (idiopathic), acute gastritis is frequently associated with
1) Heavy use of nonsteroidal anti-inflammatory drugs (NSAIDs) particularly aspirin, cancer
chemotherapeutic drugs, or radiation
2) Excessive consumption of alcohol, heavy smoking, and ingestion of strong acids or alkali
as in suicidal attempts
3) Uremia
4) Severe stress (e.g., trauma, burns, surgery)
5) Mechanical trauma (e.g., nasogastric intubation)
6) Distal gastrectomy (reflux of duodenal contents).
Chronic Gastritis
Is defined as "chronic inflammation of the gastric mucosa that eventuates in mucosal
atrophy and intestinal metaplasia". The epithelial changes may progress to dysplasia,
which constitute a soil for the development of carcinoma.
Pathogenesis
The major etiologic associations of chronic gastritis are:
1. Chronic infection by H. pylori 2. autoimmune damage
3. Excessive alcohol consumption & heavy cigarette smoking
4. Post-antrectomy (due to reflux of bile-containing duodenal secretions)
5. Outlet obstruction, uremia, and other rare causes
Chapter 2 - Oral Cavity & Gastrintestinal Tract
29
o Helicobacter pylori Infection and Chronic Gastritis
Infection by H. pylori is the most important etiologic cause of chronic gastritis. Effective
treatment with antibiotics has revolutionized the management of chronic gastritis and
peptic ulcer disease. Those with H. pylori-associated chronic gastritis are at increased
risk for the development of
1. Peptic ulcer disease 2. Gastric carcinoma
3. Gastric lymphoma
H. pylori are curvilinear, gram-negative rods. They have adapted to survive within gastric
mucus, which is lethal to most bacteria. The specialized features that allow these
bacteria to flourish include:
1) Motility (via flagella), allowing them to swim through the viscous mucus
2) Urease production, which releases ammonia and CO
2
from endogenous urea, thereby
buffering the harmful gastric acid in the immediate vicinity of the bacteria
3) Expression of adhesion molecules, which enhances binding of the bacteria to adjacent
foveolar cells
The bacteria appear to cause gastritis by stimulating production of pro-inflammatory
cytokines as well as by directly damaging epithelial cells by the liberation of toxins &
degrading enzymes e.g. vacuolating toxin (VacA), urease, proteases and phospholipases.
After exposure to H. pylori, gastritis occurs in two patterns:
1) Antral-predominant gastritis with high acid production & elevated risk for duodenal ulcer
2) Pan gastritis with low acid secretion and higher risk for adenocarcinoma
IL-1β is a potent pro-inflammatory cytokine and a powerful gastric acid inhibitor. Patients
who have higher IL-1β production in response to the infection tend to develop pangastritis,
while patients who have lower IL-1β production exhibit antral-predominant gastritis.
A number of diagnostic tests have been developed for the detection of H. pylori. (Fig. 5-43)
1. Noninvasive tests including
a. Serologic test for antibodies b. Stool culture for bacterial detection
c. Urea breath test: based on the generation of ammonia by bacterial urease.
2. Invasive tests (through gastroscopy): detection of H. pylori in gastric biopsy tissue
samples including
a. visualization of the bacteria in histologic sections with special stains
b. bacterial culture of the biopsy c. bacterial DNA detection by the PCR
o Autoimmune gastritis
About 10% of chronic gastritis are autoimmune in nature. It results from the presence of
autoantibodies to components of parietal cells, including the acid-producing enzyme
H+/K+-ATPase, gastrin receptor, and intrinsic factor. Gland destruction and mucosal
atrophy lead to loss of acid production (hypo- or achlorhydria). (Fig. 5-44) In the most
severe cases, production of intrinsic factor is also impaired, leading to pernicious anemia.
Affected patients have a significant risk for developing gastric carcinoma and endocrine
tumors (carcinoid tumor).
Pathological features of chronic gastritis
Autoimmune gastritis is characterized by diffuse mucosal damage of the body-fundic
mucosa, with sparing of antral rgion (Corpus-predominant gastritis).
Environmental gastritis i.e. due to environmental etiologies (including H. pylori infection) tends
to affect antral mucosa (antral gastritis) or both antral and body-fundic mucosa (pangastritis).
o Gross (endoscopic) features
The mucosa of the affected regions is usually hyperemic & has coarser rugae than normal.
With long-standing disease, the mucosa may become thinned & flattened because of atrophy.
o Microscopic features (Fig. 5-45)
Irrespective of cause or location, the microscopic changes are similar:
The mucosa is infiltrated by lymphocytes & plasma cells.
Frequently the lymphocytes are disposed into aggregates i.e. follicles, some with germinal centers.
Neutrophils may or may not be present.

Chapter 2 - Oral Cavity & Gastrintestinal Tract
30
Several additional histologic features are characteristic; these include
* Intestinal metaplasia: the mucosa may become partially replaced by metaplastic
columnar cells and goblet cells of intestinal morphology; these may display flat or villous
arrangement. If the columnar cells are absorptive (with ciliated border) the metaplasia is
termed complete, otherwise it is incomplete.
* Atrophy as evidecnced by marked loss of the mucosal glands. Parietal cells, in
particular, may be absent in the autoimmune form.
* Dysplasia: with long-standing chronic gastritis, the epithelium develops dysplastic
changes. Dysplastic alterations may become so severe as to constitute in situ carcinoma.
The development of dysplasia is thought to be a precursor lesion of gastric cancer. It
occurs in both autoimmune and H. pylori- associated chronic gastritis.
* In those individuals infected by H. pylori, the organism lies in the superficial mucus
layer on the surface and within the gastric pits. They do not invade the mucosa. These
bacteria are most easily demonstrated with silver or Giemsa (special) stains.
Peptic ulcer disease
An ulcer is defined as "a breach in the mucosa of the alimentary tract that extends into the
submucosa or deeper." Although they may occur anywhere in the alimentary tract, they
are most common in the duodenum and stomach. Ulcers have to be distinguished from
erosions. The latter is limited to the mucosa and does not extend into the submucosa.
Peptic Ulcers are chronic, most often solitary lesions and usually small. They occur in
any portion of the GIT exposed to the aggressive action of acid-peptic juices. They are
located, in descending order of frequency in:
1. Duodenum (first portion) 2. Stomach, (usually antral, along the lesser curve)
3. Gastro-esophageal junction (complicating GERD or Barrett esophagus)
4. Margins of a gastro-jejunostomy
5. Multiple in the duodenum, stomach, and/or jejunum (in Zollinger-Ellison syndrome)
6. Within or adjacent to a Meckel diverticulum (containing ectopic gastric mucosa)
The male-to-female ratio for duodenal ulcers is 3:1, and for gastric ulcers 2:1. Women are
most often affected at or after menopause.
Pathogenesis of peptic ulcers
Peptic ulcers are produced by an imbalance between gastro-duodenal mucosal defenses
and the damaging forces, particularly of gastric acid and pepsin.
Hyperacidity is not necessary; only a minority of patients with duodenal ulcers has
hyperacidity, and it is even less common in those with gastric ulcers.
H. pylori infection is a major factor in the pathogenesis of peptic ulcer. It is present in
virtually all patients with duodenal ulcers and in about 70% of those with gastric ulcers;
that is why peptic ulcer disease is now considered infectious in nature. Antibiotic
treatment of the infection promotes healing of ulcers and prevents their recurrence. The
possible mechanisms by which this tiny organism impairs mucosal defenses include:
1) H. pylori induce intense inflammatory and immune responses. As a result there is
increased production of pro-inflammatory cytokines, most notably, IL-8, by the mucosal
epithelial cells. This recruits and activates neutrophils with their damaging properties.
2) Several bacterial products cause epithelial cell injury; this is mostly caused by a
vacuolating toxin called VacA. H. pylori also secrete urease, proteases and
phospholipases, which also cause direct epithelial damage.
3) H. pylori enhance gastric acid secretion and impair duodenal bicarbonate production, thus
reducing luminal pH in the duodenum with its damaging effects on the duodenal mucosa.
4) Thrombotic occlusion of surface capillaries is provoked by a bacterial platelet-activating
factor. Thus, an additional ischemic element may contribute to the mucosal damage.
Most persons (80-90%) infected with H. pylori do not develop peptic ulcers. Perhaps there are
unknown interactions between H. pylori & the mucosa that occur only in some individuals.
Chapter 2 - Oral Cavity & Gastrintestinal Tract
31
Other factors may act alone or in concert with H. pylori to encourage peptic ulceration:
1) Gastric hyperacidity: this when present, may be strongly ulcerogenic. The classic example
is Zollinger-Ellison syndrome, in which there are multiple peptic ulcerations in the
stomach, duodenum, and even jejunum. This is due to excess gastrin secretion by a
gastrinoma and, hence, excess gastric acid production.
2) Chronic use of NSAIDs: this suppresses mucosal prostaglandin synthesis; aspirin also is a
direct irritant.
3) Cigarette smoking: this impairs mucosal blood flow and healing of the ulcer.
4) Corticosteroids: these in high doses and with repeated use encourage ulcer formation.
5) Rapid gastric emptying: this is present inn some patients with duodenal ulcers; this
phenomeneon exposes the duodenal mucosa to an excessive acid load & hence ulcerations
6) Patients with the following diseases are more prone to develop duodenal ulcer exposes
a. alcoholic cirrhosis b. chronic obstructive pulmonary disease
c. chronic renal failure d. hyperparathyroidism.
Chronic renal failure and hyperparathyroidism are associated with hypercalcemia. The
latter stimulates gastrin production and therefore acid secretion.
7) Personality and psychological stress seems to be important contributing factors.
Gross features (Fig. 5-46)
The vast majority of peptic ulcers are located in the first portion of the duodenum or in the
stomach, in a ratio of about 4:1. Gastric and duodenal ulcers may coexist in up to 20% of
the cases. Gastric ulcers are predominantly located along the lesser curvature.
Although over 50% of peptic ulcers have a diameter less than 2 cm but about 10% are
greater than 4 cm. Ulcerated carcinomas (which tend to be large) may be less than 4 cm in
diameter and may be located anywhere in the stomach. Thus, size and location do not
differentiate a benign from a malignant ulcer.
The classic peptic ulcer is a round to oval with sharply demarcated crater. The margins are
usually level with the surrounding mucosa or only slightly elevated. Heaping-up of these
margins is rare in the benign ulcer but is characteristic of the malignant ones.
Peptic ulcers penetrate the wall to a variable extent. When the entire wall is penetrated,
the base of the ulcer may be formed by adherent pancreas, omental fat, or liver.
The base of a peptic ulcer is smooth and clean, owing to peptic digestion of any exudate
that may form. Sometimes, thrombosed or patent blood vessels (the source of life
threatening hemorrhage) are evident at the base of the ulcer.
Ulcer-related scarring may involve the entire thickness of the gastric wall; puckering of
the surrounding mucosa creates mucosal folds that radiate from the crater in spoke-like
fashion. This is different from malignant ulcers where there is flattening of the mucosal
folds (because of malignant infiltration) in the immediately surrounding of the ulcerative.
Microscopic features (Fig. 5-46 B)
In active ulcers four zones are recognized
1) The base and walls have a superficial thin layer of necrotic fibrinoid necrosis.
2) Beneath this layer is a zone of predominantly neutrophilic inflammatory
infiltrate.
3) Deeper still, there is granulation tissue infiltrated with inflammatory cells. This rests on
4) Fibrous or collagenous scar.
H. pylori-associated chronic gastritis is seen in up to 100% of patients with duodenal ulcers
and in 70% with gastric ulcers. With present-day therapies aimed at inhibition of acid
secretion (H
2
receptor antagonists and parietal cell H
+
/K
+
-ATPase pump inhibitors), and
eradication of H. pylori infection (with antibiotics), most ulcers heal within a few weeks.
The complications of peptic ulcer disease are
1) Bleeding is the most frequent complication (20%). It may be life-threatening; fatal in 25%
of the affected patients. It may be the first warning of an ulcer.
2) Perforation is much less frequent (5% of patients) but much more serious being fatal in
60% of patients.
Chapter 2 - Oral Cavity & Gastrintestinal Tract
32
3) Obstruction (from edema or scarring) occurs in 2%, most often due to pyloric channel ulcers
but may occur with duodenal ulcers. Total obstruction with intractable vomiting is rare.
4) Malignant transformation does not occur with duodenal ulcers and is extremely rare with
gastric ulcers. When it occurs, it is always possible that a seemingly benign gastric ulcer
was, from the outset an ulcerative gastric carcinoma.
Acute Gastric Ulceration
Focal, acutely developing gastric mucosal defects are a well-known complication of
1) Therapy with NSAIDs
2) Severe stress (stress ulcers) as in shock states, extensive burns & severe trauma; they
usually occur in proximal duodenum (Curling ulcers)
3) Sepsis
4) Rraised intracranial pressure or intracranial surgery; these are seen as gastric, duodenal,
and esophageal ulcers & are designated as Cushing ulcers, which carry a high incidence
of perforation.
Generally, acute ulcers are multiple lesions predominantly gastric but sometimes also
duodenal. They range in depth from mere shedding of the superficial epithelium
(erosions) to deeper lesions that involve the entire mucosal thickness and deeper
(ulceration). Acute ulcers are not precursors of chronic peptic ulcers.
Gross features (Fig. 5-47)
Acute ulcers are usually small (less than 1 cm) and circular.
The ulcer base is frequently stained a dark brown by the acid digestion of blood.
They differ from chronic peptic ulcers by the following
1. They are found anywhere in the stomach, and are often multiple
2. The margins and base of the ulcers are not indurated
3. The related mucosal folds (rugae) are normal (cf. chronic peptic ulcer, which show
convergence on the ulcer)
Microscopically
There is focal loss of the mucosa & at least part of the submucosa
Unlike chronic peptic ulcers, there is no chronic gastritis or scarring.
Healing with complete re-epithelialization occurs after the causative factor is removed.
Bleeding from superficial gastric erosions or ulcers sufficient to require transfusion develops
in up to 5% of these patients. If the underlying cause is corrected recovery is complete.
Tumors of the stomach
These can be classified as benign and malignant lesions.
Benign tumors
Gastric polyps
In the alimentary tract, the term polyp is applied to any nodule or mass
that projects above the level of the surrounding mucosa. They are uncommon and
classified as non-neoplastic or neoplastic.
Hyperplastic polyps
(The most frequent; 90%) are small, sessile and multiple in about 25% of cases. There is
hyperplasia of the surface epithelium and cystically dilated glandular tissue. (Fig. 5-48)
Adenomatous polyp (adenoma)
(10% of polypoid lesions) (Fig. 5-49):
They contain proliferative dysplastic epithelium and hence have malignant potential. They
are usually single, and may grow up to 4 cm in size before detection. Up to 40% of gastric
adenomas contain a focus of carcinoma; there may also be an adjacent carcinoma that is
why histologic examination of all gastric polyps is obligatory.
Other specific types of gastric polyps are relatively uncommon and include fundic gland
polyps, hamartomatous Peutz-Jeghers polyps, juvenile polyps, and inflammatory fibroid polyp

Chapter 2 - Oral Cavity & Gastrintestinal Tract
33
Cancers of the stomach
Carcinoma is the most important and the most common (90%) of malignant tumors of the
stomach. Next in order of frequency are lymphomas (5%); the rest of the tumors are even
rarer e.g. carcinoids, and gastrointestinal stromal tumors (GISTs), leiomyosarcoma, and
schwannoma.
Gastric Carcinoma
Is a quite common tumor in the world. There are, however, marked geographical variations
in its incidence; it is particularly high in countries such as Japan. It is more common in
lower socioeconomic groups. There has been a steady decline in both the incidence and the
mortality of gastric cancer. There are mainly 2 subtypes of carcinoma: intestinal & diffuse.
These sub-types appear to have different pathogenetic mechanisms of evolution.
o Pathogenesis
The major factors thought to affect the genesis of gastric cancer apply more to the
intestinal type, as the risk factors for diffuse gastric cancer are not well defined.
1) Helicobacter pylori Infection: this generally increases the risk five- fold. The bacterial
infection causes chronic gastritis, followed by atrophy, intestinal metaplasia, dysplasia,
and carcinoma. Long-standing mucosal inflammation is associated with damage of
epithelial cells, which leads to compensatory epithelial cell proliferation, and hence
increased risk of genomic mutation. Since most individuals infected with H. pylori do not
develop cancer, other factors must be involved in carcinogenesis.
2) Adenomatous polyps: 40% of adenomas harbor carcinmatous foci; also adjacent
carcinoma is found in relation to adenomatous polyps in 30% of the cases.
3) Environmental factors: when families migrate from high-risk to low-risk areas (or the
reverse), successive generations acquire the level of risk that prevails in the new
environments. The diet is suspected to be a primary factor. Consumption of preserved and
salted foods; water contamination with nitrates; and lack of fresh fruit and vegetables are
common in high-risk areas. The intake of green, leafy vegetables and citrus fruits, which
contain antioxidants such as vitamin C, vitamin E and beta-carotene, seems to play a
protective role.
4) Autoimmune gastritis, like H. pylori infection, increases the risk of gastric cancer.
o Gross features
The most common location of gastric carcinomas is the pyloric antrum (50%). A favored
location is the lesser curvature. Although less common, an ulcerative lesion on the greater
curvature is more likely to be malignant.
Depth of invasion is the most important determinant of prognosis. Early gastric carcinoma
is defined as "a lesion confined to the mucosa and submucosa." Advanced gastric
carcinoma is a neoplasm extending into the muscular wall.
The three macroscopic growth patterns of gastric carcinoma, which may be evident at
both the early and advanced stages, are: 1. Fungating (exophytic) 2. Flat or depressed 3.
(Fig. 5-50) Ulcerative (excavated). Fungating tumors are readily identified by radiography
and endoscopy in contrast to flat (depressed) malignancy. Ulcerative cancers may closely
mimic chronic peptic ulcers. In advanced cases, there are heaped-up, beaded margins and
necrotic bases. The neoplastic tissue extends into the surrounding mucosa and wall; this
leads to flattening of the mucosa surrounding the ulcer. (Fig. 5-51)
Uncommonly, a broad region of the gastric wall or the entire stomach is extensively
infiltrated by malignancy, creating a rigid, thickened "leather bottle," termed linitis
plastica. (Fig. 5-52)
o Microscopic features
There are two main microscopic type of gastric carcinoma; intestinal and diffuse.
The intestinal variant is composed of neoplastic glands with mucin in their lumina. The
diffuse variant is composed of mucus-containing cells, which do not form glands, but
infiltrate the mucosa and wall as scattered individual and small clusters of cells. In this
Chapter 2 - Oral Cavity & Gastrintestinal Tract
34
variant, mucin formation expands the malignant cells and pushes the nucleus to the
periphery, creating"signet ring" morphology. (Fig. 5-53)
Sometimes, there is excessive mucin production that generates large mucin lakes
(mucinous carcinoma).
Infiltrative tumors often evoke a strong desmoplastic reaction (fibrosis), in which the
scattered cells are embedded; the fibrosis creates local rigidity of the wall.
Whatever the microscopic type, all gastric carcinomas eventually penetrate the wall to
involve the serosa and spread to regional and more distant lymph nodes.
o For obscure reasons, gastric carcinomas frequently metastasize to the supraclavicular
(Virchow) node as the first clinical manifestation of an occult neoplasm. (Fig. 5-54) The
tumor can also metastasize to the periumbilical region to form a subcutaneous nodule.
This nodule is called a Sister Mary Joseph nodule, after the nun who noted this lesion as a
marker of metastatic carcinoma. (Fig. 5-55) Local extension of gastric carcinoma into the
duodenum, pancreas, and retroperitoneum is also characteristic. At the time of death,
widespread peritoneal seeding and metastases to the liver and lungs are common. A
notable site of visceral metastasis is to one or both ovaries. Although uncommon,
metastatic adenocarcinoma to the ovaries (from stomach, breast, pancreas, and even
gallbladder) is distinctive & designated Krukenberg tumor. (Fig. 5-56)
o Gastric carcinoma is an insidious disease that is generally asymptomatic until late in its course.
The symptoms include weight loss, abdominal pain, anorexia, vomiting, dysphagia,
anemia, and hemorrhage. In Japan, where mass endoscopy screening programs are
employed (because of the high incidence of the disease), early gastric cancer constitutes
about one third of all newly diagnosed gastric cancers. In Europe and the United States,
this figure is only 10% to 15%.
o Prognosis
This depends primarily on
1. The depth of invasion and 2. The extent of nodal and distant metastasis
The histologic type (intestinal or diffuse) has minimal independent prognostic
significance. The five-year survival rate of surgically treated early gastric cancer is 90%;
this drops to below 15% for advanced gastric cancer.
Gastric Lymphomas
Represent 5% of all gastric malignancies. However, the stomach is the most common site
for extra-nodal lymphoma (20%). Nearly all primary gastric lymphomas are B-cell type
and of mucosa-associated lymphoid tissue (MALT lymphomas). The majority of gastric
lymphomas (>80%) are associated with chronic gastritis and H. pylori infection. The role
of H. pylori infection as an important etiologic factor for gastric lymphoma is supported
by the elimination of about 50% of early gastric lymphomas with antibiotic treatment for
H. pylori. Generally, the prognosis of gastric lymphoma is better than carcinoma.
Gastrointestinal Stromal Tumors (GISTs)
These are thought to originate from the interstitial cells of Cajal (normally control
gastrointestinal peristalsis). 95% of GISTs stain with antibodies against c-KIT (CD117).
The tumor can protrude into the lumen or extrude on the serosal side of the gastric wall.
Microscopically, the tumor can exhibit spindle cells, plump "epithelioid" cells, or a
mixture of both. Most of the tumors are quite cellular but mitotic activity is variable.
Gastric Neuroendocrine Cell (Carcinoid) Tumors
Most gastric carcinoid tumors originate from the enterochromaffin-like cells (ECL) cells
in the oxyntic mucosa. The tumor can arise in the setting of chronic atrophic gastritis. The
underlying pathogenesis is probably related to the hypergastrinemia, resulting in ECL-cell
hyperplasia, a presumed pre-neoplastic condition. Gastric carcinoid tumors exhibit similar
histologic features to other carcinoid tumors. The clinical course is quite variable.

Chapter 2 - Oral Cavity & Gastrintestinal Tract
35
Small & large intestine
Several pathological conditions, such as infections, inflammatory diseases, motility
disorders, and tumors, affect both the small and large intestines simultaneously. These
two organs will therefore be considered together.
Congenital anomalies
Anomalies of the intestine are rarely encountered; these include
Duplication
of the small intestine or colon;
Malrotation
of the entire bowel;
Omphalocele
(birth of an infant with herniation of abdominal contents into a ventral
membranous sac related to umbilicus);
Heterotopia
of pancreatic tissue or gastric mucosa;
Atresia and stenosis
;
Imperforate anus
(due to failure of the cloacal diaphragm to rupture).
Meckel diverticulum
(Fig. 5-57)
Results from failure of involution of the vitelline duct, which embryologically connects
the lumen of developing gut to the yolk sac. The small pouch lies on the antimesenteric
side of the bowel, usually 30 cm proximal to the ileo-cecal valve. It consists of mucosa,
submucosa, and muscularis propria. The mucosal lining may be that of normal small
intestine, but heterotopic gastric mucosa or pancreatic tissue are frequently found. Meckel
diverticula are present in 2% of the normal population, but most remain asymptomatic.
When peptic ulceration occurs in the small intestinal mucosa adjacent to the heterotopic
gastric mucosa, intestinal bleeding or symptoms simulating those of an acute appendicitis
may result. Other complications include intussusception, incarceration, or perforation.
Congenital Aganglionic Megacolon (Hirschsprung Disease)
(Fig. 5-58)
This congenital disorder is characterized by the absence of ganglia of the submucosal and
myenteric neural plexuses, within a portion of the intestinal tract. The outcome is
contraction and functional obstruction of the aganglionic segment with secondary
proximal dilation. The rectum is always affected and most cases involve the rectum and
sigmoid colon only (short-segment disease). In some cases longer segments, and rarely
the entire colon may be aganglionic (long-segment disease). Proximal to the aganglionic
segment, the ganglionic colon undergoes progressive dilation and hypertrophy, sometimes
massively (megacolon). When distention overruns hypertrophy, the colonic wall becomes
markedly thinned and may rupture. Diagnosis of Hirschsprung is made histologically by
failure to detect ganglion cells in intestinal biopsy samples of the contracted (agnaglionic)
segment. The disease usually manifests itself in the immediate neonatal period by failure
to pass meconium, followed by obstructive constipation. Abdominal distention may
secondarily develop. The major threats to life are superimposed enterocolitis with fluid
and electrolyte disturbances and perforation with peritonitis.
Enterocolitis
These are divided into three etiological categories
I. Infectious (caused by microbiologic agents)
II. Malabsorption-associated
III. Idiopathic inflammatory bowel diseases
Diarrhea and Dysentery
Diarrhea is present when there is an increase in stool frequency, and/or stool fluidity.
This consists of daily stool production in excess of 250 gm, containing 70% to 95% water.
However, over 6 liters of fluid may be lost per day in severe cases of diarrhea (e.g.
cholera); this is the equivalent of the circulating blood volume.
Chapter 2 - Oral Cavity & Gastrintestinal Tract
36
Dysentery is characterized by low-volume, painful diarrhea with blood.
Malabsorption-associated diarrhea is the result of interference with absorption of
nutrients, which produces bulky stools containig excess fat (steatorrhea).
Infectious enterocolitis
This is the cause of more than 12,000 deaths per day among children in developing
countries & constituting 50% of all deaths before the age of 5 years worldwide. Acute, self-
limited infectious diarrhea is most frequently caused by enteric viruses (such as rotavirus
and adenoviruses). Bacterial infections, such as that caused by enterotoxigenic Escherichia
coli, are also common. In up to 50% of cases, the specific agent cannot be isolated.
Viral enterocolitis
The lesions caused by enteric viruses in the intestinal tract are similar. The small intestinal
mucosa shows partial villous atrophy (shortening of the villi) with infiltration of the
lamina propria by lymphocytes. However, in infants, rotavirus and adenoviruses can
produce total villous atrophy (flat mucosa), thus resembling celiac disease (see later).
Bacterial enterocolitis
o Salmonellosis and Typhoid Fever
Salmonellae are gram-negative bacteria that cause either a self-limited food-borne and
water-borne gastroenteritis (S. enteritidis, S. typhimurium, and others) or typhoid fever
(S. typhi) a life-threatening systemic illness. In typhoid fever, Peyer patches in the
terminal ileum become sharply prominent forming flat elevations. Shedding of the
mucosa and the lymphoid tissue creates oval ulcers with their long axes parallel to that of
the ileum. Microscopic examination reveals macrophages containing bacteria & red blood
cells. Intermingled with the phagocytes are lymphocytes and plasma cells; the infiltrate is
characteristically devoid of neutrophils.
o Campylobacter Enterocolitis
This flagellated, comma-shaped bacterium may involve the entire intestine from the
jejunum to the anus. The small intestine exhibits partial villous atrophy. In colonic
infection, the colonic mucosa appears friable and superficially ulcerated. Microscopically,
the formation of colonic crypt abscesses and mucosal ulceration may be confused with
those of ulcerative colitis.
o Cholera
Vibrio cholerae affect the proximal small intestine. The mucosa remains essentially
intact as the bacteria never invade the epithelium but remain within the lumen and secrete
an enterotoxin that stimulates excessive secretions from the enterocytes. The re-absorptive
function of the colon is not affected but overwhelmed, thus liters of dilute "rice water"
diarrhea containing flecks of mucus occurs. This causes dehydration and electrolyte
disturbances. Because overall absorption in the gut remains intact, oral electrolytes-
containing fluids can replace the massive sodium, chloride, bicarbonate, and fluid losses
and reduce the mortality rate from 50% to less than 1%.
o Antibiotic-Associated Colitis (Pseudomembranous Colitis) (Fig. 5-59)
This is an acute colitis characterized by formation of an adherent layer of pseudomembrane
overlying sites of mucosal injury. It is caused by toxins of Clostridium difficile, which is a
normal gut commensal. The disease mostly follows a course of broad-spectrum antibiotic
therapy. Clostridium difficile flourish following alteration of the normal intestinal flora.
Diagnosis is confirmed by the detection of the C. difficile cytotoxin in stool. Grossly, there
are plaque-like adhesions of fibrinopurulent-necrotic debris and mucus to damaged colonic
mucosa. Microscopically, the lesion is characteristic in that the superficially damaged
crypts are distended by a mucopurulent exudate, which erupts out of the crypt to form a
mushrooming cloud (akin to volcano eruption).
Chapter 2 - Oral Cavity & Gastrintestinal Tract
37
o Tuberculous enteritis
Intestinal tuberculosis contracted by the drinking of contaminated milk was common as a
primary focus of the disease. In developed countries today, intestinal tuberculosis is more
often a complication of advanced pulmonary tuberculosis i.e. secondary to the swallowing
of coughed-up infective sputum. Typically, the organisms are trapped in mucosal
lymphoid aggregations of the small and large bowel, which then undergo inflammatory
enlargement with ulceration of the overlying mucosa, particularly in the ileum (Fig. 5-60).
Microscopy shows typical caseating granulomas that coalesce to form larger ones.
General pathological features of bacterial enteric diseases
These are quite variable.
Dramatic, even lethal, diarrhea may occur without a significant pathologic lesion, as in cholera.
Characteristic histology may enable diagnosis with reasonable certainty, as with C.
difficile-induced pseudomembranous colitis, and caseating granulomas of TB.
Parasitic enterocolitis
Parasitic diseases collectively affect over one-half of the world's population on a chronic
or recurrent basis. The intestine can harbor as many as 20 species of parasites, including
roundworms Ascaris and Strongyloides, hookworms, pinworms, flatworms, tapeworms,
flukes, and protozoa.
o Ascaris lumbricoides
This is the most common nematode, infecting over a billion individuals worldwide. Adult
worm masses can physically obstruct the intestine or the biliary tree. Diagnosis is usually
made by detection of the eggs in the feces.
o Strongyloides
The eggs of Strongyloides can hatch within the intestine & larvae can penetrate the mucosa,
causing autoinfection. Hence, Strongyloides infection can persist in one individual for life.
Immunosuppressed individuals can have overwhelming autoinfection. Strongyloides incites
a strong tissue eosinophilic reaction associated with blood eosinophilia.
o Hookworm (Necator duodenale and Ancylostoma duodenale) infection
This affects an estimated 1 billion people worldwide and causes significant morbidity.
The worms attach to the mucosa of small intestine, suck blood, and reproduce. Long-term
infection causes iron deficiency anemia. Diagnosis can be made by detection of the eggs
in fecal smear.
o Enterobius vermicularis (pinworms)
These do not invade host tissue and live their entire life within the intestinal lumen. Adult
worms living in the intestine migrate to the anal orifice at night, where the female
deposits eggs on the perirectal mucosa. As the eggs are quite irritative, rectal and perineal
pruritus ensues. Diagnosis is easily made by applying cellophane tape to the perianal skin
and examining the tape for eggs under the microscope.
o
Amebiasis
Is caused by the protozoan Entamoeba histolytica. The parasite infects approximately 500
million persons in developing countries resulting in approximately 40 million cases of
dysentery and liver abscesses. The presence in stool of trophozoites containing ingested
RBCs is diagnostic. The patient may present with abdominal pain & bloody diarrhea.
Pathologic features (Fig. 5-61)
Amebiasis most frequently involves the cecum and ascending colon. In severe cases,
however, the entire colon is involved (pancolitis).
Amebae invade through the crypt epithelium and burrow into the mucosa and submucosa,
eliciting a neutrophilic reaction.
They are stopped by the muscularis propria thus forced to spread out laterally to create a
flask-shaped ulcer with a narrow neck and broad base.
The mucosa between ulcers is often normal.

Chapter 2 - Oral Cavity & Gastrintestinal Tract
38
o Giardiasis
Giardia lamblia is the most common pathogenic parasitic infection in humans. Infection
may cause acute or chronic diarrhea, steatorrhea, or constipation. Trophozoites multiply
in the intestinal lumen. The flagellated trophozoite has two nuclei & resides in the
duodenum, adhering to, but does not invade the intestinal epithelial cells. Tight contact
between the parasite and the epithelial cell is made by a sucker-like disc. It also contains
surface protein that resembles diarrhea-causing toxins secreted by certain snakes. The
physical presence of rapidly proliferating trophozoites as well as their toxic proteins that
damages the microvillus brush border are responsible for the associated malabsorption.
Pathological features (Fig. 5-62)
In stool smears, G. Iamblia trophozoites are pear shaped and binucleate.
Duodenal biopsy specimens are often packed with sickle or pear-shaped trophozoites,
which are tightly bound to the surface of the small intestinal villi.
As Giardia does not invade the mucosa, small intestinal morphology is often normal.
Diagnosis
This is readily made by
1) Examination of stool for cysts & trophozoites
2) Small intestinal biopsy or examination of a small intestinal aspirate that permit
identification of the trophzoite
Necrotizing enterocolitis (nec)
This acute, necrotizing inflammation of the small & large intestines may progress to
transmural necrosis of intestinal segments. It is the most common acquired gastrointestinal
emergency of neonates, particularly those who are premature or of low birth weight.
Collagenous and lymphocytic colitis
These distinctive disorders of the colon are characterized by chronic watery diarrhea (no
blood). Collagenous colitis is characterized by band-like collagen deposits directly under
the surface epithelium, whereas lymphocytic colitis is characterized by prominent
intraepithelial infiltrate of lymphocytes. Collagenous colitis occurs primarily in middle-
aged and older women; lymphocytic colitis affects males and females equally.
Radiographic studies are unremarkable, and endoscopy characteristically reveals normal
mucosa. The pathogenesis of these conditions remains unclear. While collagenous colitis
and lymphocytic colitis are uncommon, they must be considered in every adult patient
who presents with a noninflammatory, watery diarrhea.
Solitary rectal ulcer syndrome
This is an inflammatory condition of the rectum resulting from motor dysfunction of the
anorectal musculature, in particular impaired relaxation of the anorectal sling. The latter may
create sharp angulation of the anterior rectal wall. Abrasion of the overlying rectal mucosa
creates an oval ulcer & surrounding mucosal inflammation. Associated partial prolapse of
the rectal mucosa is common. Patients experience rectal bleeding & mucus discharge.
The malabsorption syndromes
Malabsorption is characterized by defective absorption of fats, fat-soluble and other
vitamins, proteins, carbohydrates, electrolytes and minerals, and water. The most common
clinical presentation is chronic diarrhea, and the hallmark of malabsorption is steatorrhea
(excessive fecal fat content). Although many causes of malabsorption can be established
clinically, small intestinal mucosal biopsy may be required to satisfactorily identify or
exclude celiac disease.
Major Malabsorption Syndromes
Clinically, the malabsorption syndromes resemble each other more than they differ. The
consequences of malabsorption affect many organ systems. The passage of abnormally

Chapter 2 - Oral Cavity & Gastrintestinal Tract
39
bulky, frothy, greasy, yellow, or gray stools (steatorrhea) is a prominent feature of
malabsorption; this is accompanied by weight loss, abdominal distention & muscle wasting.
The malabsorptive disorders most commonly encountered are
1) Celiac disease 2) Pancreatic insufficiency 3) Crohn disease
Pancreatic insufficiency
Primarily from chronic pancreatitis or cystic fibrosis, is a major cause of defective
intraluminal digestion that leads to diarrhea and steatorrhea.
Celiac Disease
Celiac disease (gluten-sensitive enteropathy, GSE) is a chronic disease, in which there is a
characteristic mucosal lesion of the small intestine and impaired nutrient absorption,
which improves on withdrawal of wheat gluten from the diet.
o Pathogenesis
The fundamental disorder in celiac disease is sensitivity to gluten component called
gliadin, which is a protein present in wheat and closely related grains (e.g. oat).
There is a T-cell mediated chronic inflammatory reaction, which develops as a
consequence of a loss of tolerance to gluten.
Interplay between genetic predisposing factors, the host immune response, and
environmental factors, is central to disease pathogenesis.
The small intestinal mucosa, when exposed to gluten, accumulates intraepithelial CD8+ T
cells and large numbers of lamina propria CD4+ T cells, which are sensitized to gliadin.
Gliadin is deamidated by the enzyme transglutaminase; the resultant peptides are recognized
by CD4+ T cells. This leads to secretion of interferon γ, which damages enterocytes.
o Pathological features (5-63)
By endoscopy, the duodenal mucosa appears flat (normally shows mucosal folds).
Biopsies demonstrate enteritis with partial or total loss of villi (partial villous atrophy or
completely flat mucosa respectively)
The surface epithelium shows degeneration, loss of the microvillus brush border, and an
increased number of intraepithelial lymphocytes.
The crypts exhibit increased mitotic activity and are hyperplastic, so that, despite villous
atrophy, the overall mucosal thickness remains the same.
The lamina propria has an overall increase in plasma cells and lymphocytes.
Although the above changes are characteristic of celiac disease, they can be mimicked by
other diseases, most notably tropical sprue.
Mucosal histology usually reverts to normal or near-normal following gluten exclusion
from the diet.
Dermatitis herpetiformis (DH) is a characteristic itchy skin-blistering disease can occur in
some patients with celiac disease.
Detection of serum anti-gliadin or "anti-endomysial" antibodies strongly favors the diagnosis
of celiac disease.
o Definitive diagnosis of celiac disease rests on
1. clinical documentation of malabsorption
2. demonstration of the intestinal lesions by small bowel biopsy and
3. Definite improvement in both symptoms and mucosal histology on gluten withdrawal
from the diet.
4. If there is doubt about the diagnosis, gluten challenge (reintroduction of gluten to the diet)
followed by rebiopsy has been advocated.
5. Serologic tests, mentioned above, are used for screening or treatment follow-up.
Most patients with celiac disease who adhere to a gluten-free diet remain well indefinitely
and ultimately die of unrelated causes. However, there is a long-term risk of malignant
disease, which includes small intestinal non-Hodgkin lymphoma (moderate risk), small
intestinal adenocarcinoma, and esophageal squamous cell carcinoma (50- to 100-fold
higher risk than the general population).
Chapter 2 - Oral Cavity & Gastrintestinal Tract
40
o Tropical Sprue (Postinfectious Sprue)
This condition is a celiac-like disease that occurs almost exclusively in people living in or
visiting the tropics. Malabsorption usually becomes apparent within days or a few weeks.
The condition improves on treatment with broad-spectrum antibiotics. This supports an
infectious etiology. Intestinal lymphoma does not appear to be associated with this
disorder (cf. celiac disease).
o Whipple Disease
Whipple disease is a rare systemic disease that principally affects the intestine, central
nervous system, and joints. It is caused by the bacterium Tropheryma whippelii. The
bacteria proliferate preferentially within macrophages. The hallmark of Whipple disease
is a small-intestinal mucosa laden with distended macrophages (stuffed with the bacteria).
Whipple disease is principally encountered in adults, with a strong male predominance. It
usually presents as malabsorption with diarrhea and weight loss. Response to antibiotic
therapy is usually rapid.
o Disaccharidase (Lactase) Deficiency
The disaccharidases, of which the most important is lactase, are located in the apical cell
membrane of the absorptive epithelial cells. Congenital lactase deficiency is a very rare
condition, but acquired lactase deficiency is common. Incomplete breakdown of the
disaccharide lactose into its monosaccharides glucose and galactose leads to osmotic
diarrhea from the unabsorbed lactose. Bacterial fermentation of the unabsorbed sugars
leads to increased hydrogen production, which is readily measured in exhaled air by gas
chromatography. Malabsorption is quickly corrected when exposure to milk and milk
products is terminated. In the adult, lactase insufficiency develops as an acquired disorder,
sometimes in association with viral and bacterial enteric infections. Neither light nor
electron microscopy has disclosed abnormalities of the mucosal cells of the bowel.
o Abetalipoproteinemia
This condition is a rare autosomal recessive inborn error of metabolism and characterized
by a defect in the synthesis and export of lipoproteins from intestinal mucosal cells. The
failure to absorb certain essential fatty acids leads to lipid membrane defects, evident in
the characteristic acanthocytic RBCs (burr cells) in blood films. The disease becomes
manifest in infancy and is dominated by failure to thrive, diarrhea, and steatorrhea.
Idiopathic inflammatory bowel disease (IBD)
The two disorders known as inflammatory bowel disease (IBD) are Crohn's disease (CD)
and ulcerative colitis (UC). These diseases have distinctly different clinical and
pathological features. Both CD and UC are chronic, relapsing inflammatory disorders of
obscure origin. CD is an autoimmune disease that may affect any portion of the
gastrointestinal tract from mouth to anus, but most often involves the distal small intestine
and colon. UC is a chronic inflammatory disease limited to the rectum and colon. Both
exhibit extra-intestinal inflammatory manifestations.
Etiology and Pathogenesis
In the normal GIT, the mucosal immune system is always ready to respond against
ingested pathogens but is unresponsive to normal intestinal microflora. In IBD, this state
of homeostasis is disrupted, leading to two key pathogenic abnormalities
1. Strong immune responses against normal microflora
2. Defects in epithelial barrier that cause microflora to reach the lymphoid tissue of the
intestine
The exact cause (s) leading to the above is still not established, hence the designation
idiopathic. It is postulated that IBD result from exaggerated local immune responses to
microflora in the gut, in genetically susceptible individuals.
Thus, the pathogenesis of IBD involves
1. Failure of immune regulation

Chapter 2 - Oral Cavity & Gastrintestinal Tract
41
2. Genetic susceptibility
3. Environmental triggers specifically microbial flora.
CD appears to be the result of a chronic delayed-type hypersensitivity reaction induced by
IFN-γ- producing T
H
1 cells. This is supported by the presence of granulomas in this
disease.
Experiments on animals suggest that UC is caused by excessive activation of T
H
2 cells.
Diagnosis of IBD
Since the exact etiology of IBD is not known, the diagnosis of IBD and the distinction
between CD and UC depend on clinical history, radiographic examination, laboratory
findings (serum ANCA is positive in 75% of UC Vs only 10% of CD.), and pathologic
examination of tissues involved. Pathologic appearances, both macroscopic and
microscopic, play a central role in establishing a definitive diagnosis.
Crohn disease (CD)
o This disease may involve any level of the alimentary tract. CD occurs at any age, but the
peak age of incidence is between 10 and 30 years. Smoking has been found to be a strong
risk factor.
o Pathological features
When fully developed, Crohn disease is characterized pathologically by
1. Sharply segmental and typically transmural involvement of the bowel by an
inflammatory process with mucosal damage
2. The presence of
- Small noncaseating granulomas
- Deep fissures that may eventuate in the formation of fistulae
In CD, there is involvement of the small intestine alone in about 40% of cases, of small
intestine and colon in 30%, and of the colon alone in about 30%. Other portions of the
GIT may also be uncommonly involved.
o Gross features (Fig. 5-64 A)
Segments of the small bowel involved by the disease show granular and dull gray serosa
(normally transparent and glistening).
Often the mesenteric fat wraps around the bowel (creeping fat)
The involved bowel wall is thick and rubbery (because of edema, inflammation, and
fibrosis). As a result, the lumen is narrowed.
A classic feature of CD is the sharp demarcation of diseased bowel segments from
adjacent uninvolved, essentially normal bowel (skip lesions).
Early disease shows small mucosal ulcers that coalesce to form long, serpentine linear
ulcers (i.e. long and twisted or sinuous).
As the intervening mucosa (between the ulcers) tends to be accentuated by inflammation
and edema, it acquires a cobblestone appearance. (Cobble-stone, is a rounded stone, esp.
of the size used for paving).
Narrow fissures develop between the mucosal folds, often penetrating deeply through the
bowel wall. Further extension of these fissures leads to fistulae or sinus tracts formation,
between the diseased intestinal segment and adherent structures (bowel loops, vagina,
urinary bladder, skin of the abdomen) or the sinuses may end blindly within the
abdominal cavity.
Free perforation or localized abscesses may develop.
o Microscopic features (Fig. 5- 64 B)
The characteristic histologic features of CD are:
1) Acute mucosal inflammation: there is neutrophilic infiltration of the surface & crypt
epithelium that eventually collects within the lumen of the crypts forming crypt abscesses.
2) Chronic mucosal damage: This is the hallmark of chronicity of CD (and UC). It
manifests as architectural distortion (in the small intestine as villus blunting; in the colon,

Chapter 2 - Oral Cavity & Gastrintestinal Tract
42
the crypts exhibit irregularity, and branching). Crypt destruction leads to progressive
mucosal atrophy.
3) Ulcerations are the usual outcome of severe active disease; these may be superficial, or
may penetrate deeply (as fissures) into underlying tissue layers.
4) Transmural chronic inflammation affecting all layers: chronic inflammatory cells
(lymphocytes & plasma cells) fill the affected mucosa and, to a lesser extent, all underlying
intestinal layers. Lymphoid aggregates are usually scattered throughout the bowel wall.
5) Noncaseating granulomas: in about 50% of the cases, noncaseating small granulomas
may be present in all tissue layers. Because they are not always present; the absence of
granulomas does not rule out the diagnosis of CD.
6) Other mural changes: in diseased segments, the muscularis mucosae usually exhibits
duplication & thickening. There is also fibrosis of the submucosa, muscularis propria, and
serosa that eventually leads to stricture formation.
7) Dysplastic changes of the mucosal epithelial cells are particularly important in persons
with long-standing chronic disease are. These may be focal or widespread, tend to
increase with time, and are thought to be related to increased risk of carcinoma,
particularly of the colon.
o Clinical Features
The disease usually begins with intermittent attacks of diarrhea, fever, and abdominal
pain, spaced by asymptomatic periods lasting for weeks to many months. In those with
colonic involvement, occult or overt fecal blood loss may lead to anemia. During this
lengthy, chronic disease, complications may arise from
1. Fibrosing strictures, particularly of the terminal ileum (intestinal obstruction)
2. Fistulas formed to other loops of bowel, urinary bladder, vagina, or perianal skin, or
into a peritoneum. In the latter focal abscesses may occur.
3. Extensive involvement of the small bowel, including the terminal ileum, may cause
a. marked loss of albumin (protein-losing enteropathy) b. generalized malabsorption
c. specific malabsorption of vitamin B
12
(pernicious anemia), or malabsorption of bile
salts, leading to steatorrhea.
Extraintestinal manifestations of this disease include
1. Arthritis & finger clubbing 2. Red nodules of the skin (Erythema nodosum)
3. Primary sclerosing cholangitis, (but the association is not as strong as in UC).
4. Renal disorders secondary to trapping of the ureters in the inflammatory process
sometimes develop and leading to hydronephrosis and pyelonephritis.
5. Systemic amyloidosis (rare late consequence).
6. An increased incidence of cancer of the GIT in patients with long-standing progressive
CD; however, the risk of cancer in CD is considerably less than in patients with chronic UC.
Ulcerative colitis
o In contradistinction to CD, ulcerative colitis is a chronic ulcero-inflammatory disease
limited to the colon and affecting only the mucosa and submucosa; it extends in a
continuous fashion proximally from the rectum. Well-formed granulomas are absent.
However, like CD, UC is a systemic disorder associated in some patients with arthritis,
uveitis, hepatic involvement (primary sclerosing cholangitis), and skin lesions. The onset
of disease peaks between ages 20 and 25 years. Nonsmoking is associated with UC; ex-
smokers are at higher risk for developing UC than never-smokers.
o Pathological features
Ulcerative colitis involves the rectum and extends proximally in a retrograde fashion to
involve the entire colon ("pancolitis") in the more severe cases. It is a disease of
continuity, and "skip" lesions are not found (cf. CD).
o Gross features (Fig. 5-65 A)
A key feature of UC is that the mucosal damage is continuous from the rectum and
extending proximally.

Chapter 2 - Oral Cavity & Gastrintestinal Tract
43
The mucosa may exhibit reddening and granularity with easy bleeding.
With fully developed severe, active inflammation, there may be extensive ulcerations of
the mucosa.
Isolated islands of regenerating mucosa bulge upward to create polypoid projections
(pseudopolyps).
With chronicity or healing of active disease, progressive mucosal atrophy occurs.
Thickening of the bowel wall does not occur in UC; the serosal surface is usually
completely normal (cf. CD).
Only in the most severe cases of ulcerative disease (UC, CD,& other severe inflammatory
diseases) does toxic damage to the muscularis propria and neural plexus lead to complete
shutdown of neuromuscular function. In this instance the colon progressively swells and
becomes gangrenous, a life-threatening condition called toxic megacolon.
o Microscopic features (Fig. 5-65 B)
The basic mucosal alterations in UC are similar to those of colonic CD, with
inflammation, chronic mucosal damage, and ulceration.
There is diffuse, predominantly chronic inflammatory infiltrate in the lamina propria.
Neutrophilic infiltration of the epithelial layer may produce crypt abscesses. The latter are
not specific for UC and may be observed in CD or any active inflammatory colitis.
Unlike CD, there are no granulomas.
Destruction of the mucosa leads to broad-based ulcerations that are superficial i.e.
extending at most into the submucosa.
Isolated islands of regenerating mucosa bulge upward to create pseudopolyps.
Features of chronic but healed (inactive) disease include submucosal fibrosis; mucosal
architectural distortion and atrophy.
Particularly significant is the spectrum of epithelial dysplasias, which are divided into
low-grade and high-grade depending on the severity. Invasive carcinoma is the ultimate
lesion arising from dysplasia.
To summarize UC differs pathologically from CD in the following
a. Well-formed granulomas are absent.
b. There are no skip lesions.
c. The mucosal ulcers rarely extend below the submucosa, and
d. There is surprisingly little fibrosis.
e. Mural thickening does not occur, and the serosal surface is usually completely normal.
f. There appears to be a higher risk of carcinoma development.
o Course & prognosis
Ulcerative colitis typically presents as a recurrent attacks of bloody mucoid diarrhea that
may persist for days, weeks, or months and then subside, only to recur after an
asymptomatic interval of months to years.
The outcome of UC depends on two factors:
1. The severity of active disease 2. The duration of the disease
The majority of the cases can be controlled medically; however, about 30% of patients
require colectomy due to uncontrollable active disease. On rare occasion, the disease runs
a fulminant course; unless medically or surgically controlled, this toxic form of the
disease can lead to death soon after onset.
The most serious long-term complication of UC is colonic carcinoma. There is a tendency
for dysplasia to arise in multiple sites. The associated carcinomas are often infiltrative
without obvious exophytic masses. Historically, the risk of cancer is highest in patients
with pancolitis of 10 or more years' duration. It is believed that with 10 years of disease
limited to the left colon the risk is minimal, and at 20 years the risk is on the order of 2%.
With pancolitis, the risk of carcinoma is 10% at 20 years and up to 25% by 30 years.
Overall, the annual incidence of colon cancer in persons with ulcerative colitis of more
than 10 years' duration is 1%.
Chapter 2 - Oral Cavity & Gastrintestinal Tract
44
Vascular disorders
Ischemic Bowel Disease
Ischemic lesions may be restricted to the small or large intestine, or may affect both,
depending on the particular vessel(s) affected. Acute occlusion of one of the three major
supply arteries of the intestines—celiac, superior mesenteric, and inferior mesenteric
arteries—may lead to infarction of several meters of intestine. However, gradual occlusion
of one vessel may be without effect, due to the rich anastomotic interconnections. Lesions
within the end arteries, which penetrate the gut wall, produce small, focal ischemic
lesions. Pathologically the outcome of ischemic injury is divided into
1. Transmural infarction, involving all layers (full thickness infarction)
2. Mural infarction involving the mucosa and submucosa.
3. Mucosal infarction extends no deeper than the muscularis mucosae.
Almost always, transmural infarction is caused by occlusion of major mesenteric blood
vessels, whereas mucosal or mural infarctions often result from hypoperfusion.
Mesenteric venous thrombosis is a less frequent cause of vascular insufficiency.
The predisposing conditions for ischemia are:
1. Arterial thrombosis complicating usually severe atherosclerosis.
2. Arterial embolism complicating cardiac vegetations and aortic atheroembolism.
3. Venous thrombosis complicating hypercoagulable states, oral contraceptives,
intraperitoneal sepsis, etc.
4. Nonocclusive ischemia complicating cardiac failure, shock, dehydration, and
vasoconstrictive drugs (e.g., digitalis, vasopressin, propranolol)
5. Miscellaneous such as radiation injury, volvulus, and internal or external herniae.
Pathological features (Fig. 5-66 A)
Transmural Infarction
Small intestinal infarction may follow sudden and total occlusion of mesenteric arterial
blood flow.
It more often involves a considerable length of the bowel.
The splenic flexure of the colon is also at greatest risk of ischemic injury because it is the
watershed between the distribution of the superior and inferior mesenteric arteries.
(Watershed area: a narrow zone between two areas that are supplied by two vessels).
Mesenteric venous occlusion, with subsequent propagation of thrombus may lead to
extensive infarction.
The infarction appears hemorrhagic (dusky red) because of blood re-flow into the
damaged area. The lumen of the affected segment commonly contains bloody mucus or
frank blood.
Within 1 to 4 days, intestinal bacteria produce gangrene & sometimes perforation of the bowel.
Microscopically, (Fig. 5-66 B)
The picture is that of coagulative necrosis with inflammatory cell infiltration.
Chronic Ischemia: Chronic vascular insufficiency, e.g. due to gradual atherosclerotic
narrowing of the relevant vessel to a region of intestine, produces mucosal inflammation &
ulceration. Submucosal chronic inflammation & fibrosis may also occur & lead to strictures.
Angiodysplasia: Is characterized by tortuous dilations of submucosal and mucosal blood
vessels are seen most often in the cecum or right colon, usually only after the age of 50
years. They are responsible for 20% of significant lower intestinal bleeding.
Hemorrhoids
(Fig. 5-67)
Are essentially varices of the anal and perianal venous plexuses. They are extremely
common affecting 5% of the general population. They develop secondary to persistently
elevated venous pressure within the hemorrhoidal plexus. The most frequent predisposing
influences are constipation with straining at stool and the venous stasis of pregnancy.
Except for pregnant women, they are rarely encountered in persons under the age of 30.
Much more rarely, but much more importantly, hemorrhoids may reflect collateral
Chapter 2 - Oral Cavity & Gastrintestinal Tract
45
anastomotic channels that develop because of portal hypertension (as in liver cirrhosis).
The varicosities may develop in the inferior hemorrhoidal plexus and thus are located
below the anorectal line (external hemorrhoids) or from dilation of the superior
hemorrhoidal plexus (internal hemorrhoids). Commonly, both plexuses are affected
(combined hemorrhoids). Microscopically these lesions consist of thin-walled, dilated,
submucosal vessels. Superficial ulceration, fissure formation, and infarction of the
hemorrhoids secondary to their strangulation may develop.
Diverticular disease
A diverticulum is a blind pouch related to the alimentary tract. It is lined by mucosa that
communicates with the lumen of the gut. Congenital diverticula is typified by Meckel
diverticulum. Virtually all other diverticula are acquired and either lack or have an
attenuated muscularis propria. The most common site of multiple diverticula is the left
side of the colon, with the majority in the sigmoid colon; this is termed diverticular
disease of the colon. It manifests mostly after the age of 30 years. They are much less
frequent in underdeveloped tropical countries than in developed countries.
Intestinal obstruction (fig. 5-68)
The small intestine is most often involved due to its narrow lumen. Tumors and
infarction, although the most serious, account for only up to 20% of small-bowel
obstructions. The remaining 80% are due to
1. Hernias
2. Intestinal adhesions
3. Intussusception
4. Volvulus
The clinical manifestations include abdominal pain and distention, vomiting, constipation,
and in complete obstruction failure to pass flatus.
Hernias (Fig. 5-69)
A weakness or defect in the wall of the peritoneal cavity may permit protrusion of a pouch-
like sac of peritoneum called hernial sac. The usual sites of such weakness are at the
1. Inguinal canal 2. Femoral canal 3. Umbilicus 4. In surgical scars
Hernias are of concern chiefly because segments of viscera frequently protrude and
become trapped in hernial sac. This is particularly true with inguinal hernias, since they
tend to have narrow orifices and large sacs. The most frequent contents of a hernial sac
are small-bowel loops, but portions of omentum or large bowel may also become trapped.
Pressure at the neck of the pouch may impair venous drainage of the trapped bowel
segment. The resultant stasis and edema increase the bulk of the herniated loop, leading to
permanent trapping (incarceration). With time, interference with arterial supply and
venous drainage (strangulation) leads to infarction of the trapped segment.
Adhesions (Fig. 5-70)
Surgical procedures, infection, and endometriosis often cause localized or more general
peritonitis. As the peritonitis heals, adhesions may also develop between bowel segments
and abdominal wall at the operative site. These fibrous bridges can create closed loops
through which other viscera may slide and eventually become trapped (internal hernias).
These internal hernias are liable for the same complications as external ones ( obstruction
and strangulation)
Intussusception (Fig. 5-71)
This occurs when one segment of the intestine, constricted by a wave of peristalsis,
suddenly becomes telescoped into the immediately distal segment of bowel. Once trapped,
the invaginated segment is propelled by peristalsis farther into the distal segment, pulling
its mesentery along with it. When encountered in infants and children, there is usually no
underlying anatomic lesion or defect in the bowl, but some cases of intussusception are
associated with rotavirus infection, suggesting that localized intestinal inflammation may
serve as a traction point for the intussusception. However, intussusception in adults
Chapter 2 - Oral Cavity & Gastrintestinal Tract
46
signifies an intraluminal mass or tumor as the point of traction. In both settings, intestinal
obstruction ensues, and trapping of mesenteric vessels leads to infarction.
Volvulus (Fig. 5-72)
This signifies complete twisting of a loop of bowel about its mesenteric base of
attachment. It produces intestinal obstruction and infarction. This lesion occurs most often
in large redundant loops of sigmoid, followed in frequency by the cecum& small intestine
Tumors of the small and large intestine
The large intestine is responsible for more primary neoplasms than any other organ in the
body. The vast majority are adenocarcinomas. The small intestine, despite its great length
(3/4 of the GIT), is an uncommon site for benign or malignant neoplasms.
Tumors of the small intestine
The most common benign tumors in the small intestine are adenomas (Fig. 5-73) and
mesenchymal tumors. Of malignant tumors adenocarcinomas and carcinoids have
roughly equal incidence, followed in order by lymphomas and sarcomas.
Tumors of the Colon and Rectum
Non-neoplastic and benign neoplastic lesions of the colo-rectum are collectively known as
polyps, which are common in the older adult population. Epithelial polyps that arise as the
result of proliferation and dysplasia are termed adenomatous polyps (adenomas). They are
precursors of carcinoma.
Non-Neoplastic Polyps include
1) Hyperplastic polyp 2) hamartomatous polyp 3) inflammatory polyp 4) lymphoid polyp
o Hyperplastic Polyps
These are the most common polyps of the colon and rectum. They are small (usually <5
mm in diameter) and appear as smooth protrusions of the mucosa. They are often multiple
and consists of well-formed glands and crypts lined by non-neoplastic epithelial cells.
o Hamartomatous Polyps
1) Juvenile polyps (Fig. 5-74) are essentially hamartomatous proliferations, mainly of the
lamina propria, enclosing widely spaced, dilated cystic glands. They occur most
frequently in children younger than 5 years old but also are found in adults of any age; in
the latter group they may be called retention polyps. The lesions are usually large in
children (1-3 cm in diameter) but smaller in adults; they are rounded, smooth, or slightly
lobulated and sometimes have a stalk as long as 2 cm. In general, they occur singly and in
the rectum, and have no malignant potential. Juvenile polyps may be the source of rectal
bleeding and in some cases become twisted on their stalks to undergo painful infarction.
2) Peutz-Jeghers polyps (Fig. 5-75) are also hamartomatous polyps that involve the
mucosal epithelium, lamina propria, and muscularis mucosae. They may occur
sporadically or in the setting Peutz-Jeghers syndrome (PJS). PJS is a rare autosomal
dominant syndrome characterized by
a) Multiple hamartomatous polyps scattered throughout the entire GIT
b) Melanotic mucosal & cutaneous pigmentation especially around the lips & in the oral mucosa.
Patients with this syndrome are at risk for intussusception, which is a common cause of mortality.
The polyps are present most frequently in the small intestine.
Adenomas (Adenomatous polyps)
Adenomas are intraepithelial neoplasms that range from small, often pedunculated lesions
to large neoplasms that are usually sessile. The prevalence of colonic adenomas increases
progressively with age. Males and females are affected equally. Adenomatous polyps are
divided into three subtypes on the basis of the epithelial architecture (Fig. 5-76):
1. Tubular adenomas: compose of tubular glands
Chapter 2 - Oral Cavity & Gastrintestinal Tract
47
2. Villous adenomas: composed of villous projections
3. Tubulovillous adenoma: composed of a mixture of the above two.
All adenomas by definition arise as the result of dysplastic epithelial proliferation. The
dysplasia ranges from low-grade to high-grade. There is strong evidence that adenomas are
precursors for invasive colorectal adenocarcinomas. The risk of cancer is high (approaching
40%) in villous adenomas more than 4 cm in diameter. Adenomas may be single or multiple,
may be asymptomatic, and many are discovered during evaluation of anemia (due to occult
bleeding) through endoscopy. Villous adenomas are often are discovered because of overt
rectal bleeding. The most distal villous adenomas may secrete sufficient amounts of mucoid
material rich in protein & potassium to produce hypoproteinemia or hypokalemia. The only
adequate treatment for adenomas is complete resection.
Familial polyposis syndromes
These are uncommon autosomal dominant disorders. Their importance lies in their
tendency for malignant transformation.
1. Peutz-Jeghers syndrome
2. Juvenile polyposis syndrome
3. Familial adenomatous polyposis (FAP)
Another hereditary condition in this context but is not associated with polyp formations is
the hereditary nonpolyposis colorectal cancer syndrome (Lynch syndrome).
o Familial Adenomatous Polyposis (FAP) Syndrome (Fig. 5-77)
This is caused by mutations of the adenomatous polyposis coli (APC) gene on
chromosome 5.
In the classic FAP syndrome, affected patients typically develop 500 to 2500 colonic
adenomas that carpet the mucosal surface; the presence of a minimum of 100 polyps is
necessary for a diagnosis. The lifetime risk of cancer development is 100%. Some
patients already have cancer of the colon or rectum at the time of diagnosis. Cancer-
prevention measures include early detection of the condition and prophylactic colectomy.
Colorectal carcinoma
o A widely accepted proposal of carcinogensis is the adenoma-carcinoma sequence (Fig.
5-78), i.e. most carcinomas arise from preexisting adenomas. This has been supported by
the following observations:
1) Populations that have a high prevalence of adenomas have a high prevalence of colorectal cancer.
2) The distribution of adenomas parallel that of colorectal cancer.
3) The peak incidence of adenomas precedes that of carcinoma by some years.
4) When invasive carcinoma is identified at an early stage, a related adenoma is often present
5) The risk of cancer is directly related to the number of adenomas, that is why carcinoma
complicates all those with FAP syndrome.
6) Removal of all adenomas that are suspicious reduces significantgly the incidence of
cancerinoma. 98% of all cancers in the large intestine are adenocarcinomas. They usually
arise in polyps and produce symptoms relatively early and at a stage generally curable by
surgical resection. Yet, it is responsible for 10% of all cancer-related deaths. The peak
incidence for colorectal carcinoma is between ages 60 and 79.When colorectal carcinoma
is found in a young person, pre-existing ulcerative colitis or one of the polyposis
syndromes must be suspected. Environmental factors, particularly dietary practices, are
implicated in the striking geographic variations in incidence. Japanese families that have
migrated from their low-risk areas to the United States (high-risk areas) have acquired,
over the course of 20 years, the rate prevailing in the new environment; mainly because
the immigrants adopted the common dietary practices of the U.S. population. The dietary
factors receiving the most attention as predisposing to a higher incidence of cancer are
1. Excess dietary caloric intake relative to requirements 2. Low content of unabsorbable
vegetable fibers & high content of refined carbohydrates 3. Intake of red meat

Chapter 2 - Oral Cavity & Gastrintestinal Tract
48
Several epidemiological studies suggest that the use of aspirin and other nonsteroidal anti-
inflammatory drugs exerts a protective effect against colon cancer.
o Gross features (Fig. 5-79 A)
The rectosigmoid colon is the most frequent location (60%), followed by
cecum/ascending colon (20%).
Tumors in the proximal colon tend to grow as polypoid, exophytic masses; obstruction is
uncommon. In the distal colon, they tend to be annular, encircling lesions that produce
napkin-ring constrictions. The lumen is markedly narrowed leading to obstruction with
secondary proximal distention.
Both forms (polypoid and annular) directly penetrate the bowel wall over the course of
time (probably years) and may appear as subserosal and serosal white, firm masses.
o Microscopic features (Fig. 5-79 B)
The features of right- and left-sided colonic adenocarcinomas are similar.
Differentiation (grade) may range from well-differentiated tumors to undifferentiated,
frankly anaplastic masses.
Invasive tumor provokes a strong desmoplastic (fibrotic) stromal response (responsible
for the characteristic firm, hard consistency of most carcinomas).
Carcinomas arising in the anal canal are mostly of squamous cell type.
o Clinical features
remain asymptomatic for years; symptoms develop insidiously and frequently have been
present for months, sometimes years, before diagnosis. Patients with cecal and right
colonic cancers are most often presented with iron-deficiency anemia (due to insidious
blood loss). Left-sided lesions come to attention by producing occult bleeding, changes in
bowel habit or intestinal obstrcution. Iron-deficiency anemia in an older male means GI
cancer until proved otherwise. In females the situation is less clear, since menstrual
losses, multiple pregnancies, or abnormal uterine bleeding may underlie such an anemia.
o Spread & metastsis
All colorectal tumors spread by direct extension into adjacent structures and by metastasis
through the lymphatics and blood vessels. The favored sites of metastatic spread are the
regional lymph nodes, liver, lungs, and bones. In general, the disease has spread beyond
the range of curative surgery in 25% of patients. The single most important prognostic
factor of colorectal carcinoma is the extent of the tumor at the time of diagnosis (stage).
Currently, the staging system most widely used is the tumor-nodes-metastasis (TNM).
(Fig. 5-80) The principal aim is to discover these neoplasms when curative resection is
possible. Indeed, each death from colonic cancer must be viewed as a preventable tragedy.
Carcinoid tumors
o The term carcinoid means carcinoma-like lesion because it shows a much more indolent
clinical course than genuine carcinoma. Carcinoid tumor is derived from resident
endocrine cells, with the gastrointestinal tract and lung as the predominant sites of
occurrence. They comprise less than 2% of colorectal malignancies but 50% of small
intestinal malignant tumors. These tumors may be confined to the mucosa and submucosa
or may be deeply invasive with metastatic spread to regional lymph nodes and the liver.
Appendiceal and rectal carcinoids almost never metastasize.
o Gross features (Fig. 5-81) The appendix is the most common site of gut carcinoid
tumors. In the appendix, they appear as rounded swellings of the tip. A characteristic
feature is a solid, yellow-tan appearance on transection.
o Microscopic features
The neoplastic cells may form islands, trabeculae, glands, or sheets. The tumor cells show
very little if any variation in cell and nuclear size, having a scant, pink granular cytoplasm
and a round to oval nucleus. Mitoses are infrequent or absent.
Electron microscopy: the cells contain dense-core secretory granules in the cytoplasm.
Most carcinoids contain endocrine markers.

Chapter 2 - Oral Cavity & Gastrintestinal Tract
49
o Gastrointestinal carcinoids only rarely produce local symptoms; many (especially rectal
and appendiceal) are asymptomatic and are found incidentally. However, the secretory
products of some carcinoids may produce a variety of syndromes such as Zollinger-
Ellison syndrome (excess production of gastrin), Cushing syndrome (excess production of
corticotrophin). Hyperinsulinism may also occur leading to hypoglycemia. Some
neoplasms are associated with the distinctive carcinoid syndrome, which occurs
especially with carcinoids associated with widespread metastases. Most manifestations
are thought to arise from excess elaboration of serotonin (5-hydroxytryptamine, 5-HT).
Elevated levels of 5-HT and its metabolite, 5-hydroxyindoleacetic acid (5-HIAA), are
present in the blood and urine of most patients with the classic syndrome. The syndrome
is characterized by
- Cyanosis of the face and anterior part of the chest
- Intermittent hypertension & palpitation
- Frequent watery stools
The tumor, sometimes, induces fibrosis of the right-sided cardiac valves that results in so-
called carcinoid heart disease.
Gastrointestinal lymphomas
Any segment of the gastrointestinal tract may be secondarily involved by systemic
dissemination of nodal-based non-Hodgkin lymphomas. However, up to 40% of
lymphomas arise in sites other than lymph nodes (extra-nodal lymphomas), and the gut is
the most common location.
Primary gastrointestinal lymphomas usually arise without an obvious predisposing factor
but they also occur more frequently in certain patient groups
1. Chronic gastritis caused by H. pylori 2. Chronic celiac disease
3. Natives of the Mediterranean region (Mediterranean lymphoma)
4. Congenital immunodeficiency states, infection with HIV or following organ
transplantation with immunosuppression
Most gut lymphomas are of B-cell type (over 95%) and are either low- or high-grade
tumors. Early discovery is the key to survival. The depth of local invasion, size of tumor
and its histologic grade as well as extension into adjacent viscera are important
determinants of prognosis.
Mesenchymal tumors
Mesenchymal tumors may occur anywhere in the alimentary tract and include lipomas,
leiomyosarcomas, gastrointestinal stromal tumors (GISTs) and Kaposi sarcomas.
Tumors of the anal canal
Pure squamous cell carcinomas of the anal canal are closely associated with chronic HPV
infection. The latter often causes precursor lesions such as condyloma acuminatum,
squamous epithelium dysplasia, and carcinoma in situ. Pure adenocarcinoma of the anal
canal is often the extension of rectal adenocarcinoma.
Chapter 2 - Oral Cavity & Gastrintestinal Tract
50
Appendix
Acute appendicitis
Is the most common acute abdominal surgical emergency. It is divided into
1) Acute appendicitis associated with obstruction (up to 80% of cases). The obstructing
agent is usually a fecalith and, less commonly, a gallstone, tumor, or ball of worms
(Entrobias vermicularis). Continued secretion of mucinous fluid in the obstructed portion
leads to a progressive increase in intraluminal pressure that eventuates in compression and
obstruction of the draining veins. The ischemic injury favors bacterial proliferation with
additional inflammatory edema& exudation, which further interfere with the blood supply
2) Acute appendicitis without obstruction, which is of unknown pathogenesis.
Gross features (Fig. 5-82 A)
The transmural inflammatory reaction converts the normal glistening serosa into a dull, red
membrane; this transformation signifies early acute appendicitis for the operating surgeon.
Later the prominent neutrophilic exudate leads to fibrino-purulent reaction over the serosa.
As the inflammatory process worsens, there is abscess formation within the wall, along
with ulcerations and foci of suppurative necrosis in the mucosa. This constitutes acute
suppurative appendicitis.
Further progression leads to large areas of hemorrhagic green ulceration of the mucosa &
green-black gangrenous necrosis through the wall, extending to the serosa, creating acute
gangrenous appendicitis, which is quickly followed by rupture and suppurative peritonitis.
Microscopy (Fig. 5-82 B)
The microscopic criterion for the diagnosis of acute appendicitis is neutrophilic
infiltration of the muscularis propria (transmural inflammation)
Usually, neutrophils and ulcerations are also present within the mucosa.
Tumors of the appendix
Carcinoid tumor
(see above)
Conventional adenomas or adenocarcinomas
of the appendix
These are rare and pathologically identical to those of the large intestine and may cause a
neoplastic enlargement of the appendix.
Mucocele and pseudomyxoma peritonei
Mucocele is the macroscopic description of a dilated appendix filled with mucin. The true
pathologic nature of mucocele runs the range from an innocuous obstructed appendix
containing inspissated mucin, to a mucin-secreting adenoma (mucinous cystadenoma) and
adenocarcinoma (mucinous cystadenocarcinoma). In the last instance, invasion through
the appendiceal wall with intraperitoneal seeding and spread of tumor may occur, this
may be associated with pseudomyxoma peritoneii. (Fig. 5-83) Mucoceles are generally
encountered as an incidental lesion. Mucinous cystadenomas and adenocarcinomas may
present with pain, attributable to distention of the viscus. Laparotomy for presumed acute
appendicitis is a typical diagnostic setting. For lesions confined to the resected specimen
(appendix or more radical excision), the outlook is excellent. Pseudomyxoma peritoneii
may be held in check for years by repeated debulking procedures but in most instances
eventually runs its inexorable fatal course.
51
Chapter contents (51 – 59)
Topics
Directed by
Page number
1
Lymph Node
د. سالم
(52
– 59)
Non-neoplastic conditions
52
Lymphoid neoplasms
53
Miscellaneous lymphoid neoplasms
59
Chapter 3 - lymphoid system
52
Lymph node pathology
Non-neoplastic conditions
Reactive Lymphadenitis
Any immune response against foreign antigens (infectious & noninfectious inflammatory
stimuli) is often associated with lymph node enlargement (lymphadenopathy). The
infections that cause lymphadenitis are numerous and varied and may be acute or chronic.
In most instances, the histological appearance of the nodes is entirely nonspecific i.e.
different etiologies are associated with similar microscopic changes.
Acute Nonspecific Lymphadenitis
Grossly, inflamed nodes are swollen & congested i.e. gray-red. Microscopically, there are
large germinal centers containing numerous mitotic figures. When the cause is a
pyogenic organism, a neutrophilic infiltrate is seen about the follicles and within the
lymphoid sinuses. With severe infections, the centers of follicles can undergo suppurative
necrosis. Clinically, affected nodes are tender, and become fluctuant when abscess is
formed. The overlying skin is frequently red, and penetration of the infection to the skin
can produce draining sinuses. With control of the infection, the lymph nodes can revert to
their normal appearance or, if damaged undergo fibrosis.
Chronic Nonspecific Lymphadenitis
Morphology: This condition can assume 1 of 3 patterns, depending on the causative agent:
1) Follicular Hyperplasia
Is associated with infections or inflammatory processes that activate B cells in the B-cell
areas i.e. the follicles, & thus create the follicular (or germinal center) reaction. The cells
in the reactive follicles include the activated B cells (centrocytes & centroblasts also
called follicular center cells), scattered phagocytic macrophages containing nuclear debris
(tingible body macrophages), and the not easily seen meshwork of follicular dendritic
cells (function in antigen display to the B cells). Causes of follicular hyperplasia include
rheumatoid arthritis, toxoplasmosis, and the early stages of HIV infection. This form of
lymphadenitis can be confused microscopically with follicular lymphomas (see later).
2) Paracortical Hyperplasia
The paracortex is the zone situated between the cortex and the medulla, which contains the
mobile pool of T lymphocytes responsible for cell-mediated immune responses.
Paracortical hyperplasia is characterized by reactive changes within the T-cell regions of
the lymph node, which is reflected microscopically as expanded zones between the cortical
follicles. On immune activation parafollicular T cells transform into large proliferating
immunoblasts that can efface the B-cell follicles. Paracortical hyperplasia is encountered in
1) Viral infections (such as EBV) 2) Following certain vaccinations (e.g., smallpox), &
2) In immune reactions induced by certain drugs (especially the antiepileptic drug phenytoin)
3) Sinus Histiocytosis
Is characterized by distention and prominence of the lymphatic sinusoids, owing to a
marked hypertrophy of lining endothelial cells and an infiltrate of macrophages. Sinus
histiocytosis is often encountered in lymph nodes draining cancers and may represent an
immune response to the tumor or its products.
Granulomatous lymphadenitis
There are a large number of diseases that can result in granuloma formations in lymph
nodes. However, the mere presence of granulomas in a lymph node biopsy will definitely
shorten the list of the differential diagnosis in a case of lymphadenopathy.

Chapter 3 - lymphoid system
53
The causes of granulomatous lymphadenitis include
1) Infections 2) Foreign body reactions 3) Malignancy
Among the infectious causes are
1) Tuberculosis 2) Atypical mycobacteriosis
3) Sarcoidosis (although of still of unknown etiology; evidences suggest an infectious cause).
4) Fungal infections 5) Toxoplasmosis 6) Syphilis & Leprosy
7) Mesenteric lymphadenitis 8) Cat-scratch disease 9) Brucellosis
Malignancy related granulomatous lymphadenitis occurs whether the neoplasm is
primary (Hodgkin & nonHodgkin lymphomas), or secondary (metastatic carcinoma). It
occurs whether the node is involved by the malignancy or not.
Sometimes the appearance of the granulomas is such that a specific diagnosis can be
strongly suggested e.g. presence of caseation necrosis. In most cases, however, a
combination of clinical, morphologic, & bacteriologic data (sometimes with the help of
more sophisticated techniques) is necessary to determine the etiology of the granulomas.
Lymphoid neoplasms
Encompass a group of entities that vary widely in their clinical presentation and behavior.
These include leukemias, lymphomas, and plasma cell dyscrasias. All these have the
potential to spread to lymph nodes and various tissues throughout the body, especially the
liver, spleen, and bone marrow. In some cases lymphomas spill over into the peripheral
blood, creating a leukemia-like picture. Conversely, leukemias of lymphoid cells,
originating in the bone marrow, can infiltrate lymph nodes and other tissues, creating the
histologic picture of lymphoma. Because of the overlap in clinical presentations, the
diagnosis and prognosis of various lymphoid neoplasms can only be established
through the microscopic appearance and molecular characteristics of the tumor cells.
Two groups of lymphomas are recognized:
1. Hodgkin lymphoma and (HL) 2. Non-Hodgkin lymphomas (NHL)
According to the most recent issue of the Iraqi Cancer Registry (2002), malignant
lymphoma represents 9% of all cancers in Iraq.
The behavior and treatment of Hodgkin lymphoma differ from those of most NHLs,
thus making the distinction between the two of practical importance.
The WHO has established a widely accepted classification that depends on a combination
of clinical, microscopic, immunophenotypic, and genotypic features (e.g., karyotype,
presence of viral genomes, etc.)). B- and T-cell tumors are composed of cells derived
from specific stages of their normal differentiation pathways. The diagnosis and
classification of these tumors relies heavily on tests (e.g. immunohistochemistry) that
detect specific antigens (e.g., B-cell, T-cell markers). Many such markers are identified
according to their cluster of differentiation (CD) number. All lymphomas are derived
from a single transformed cell and thus are by definition monoclonal. During the
differentiation of precursor B and T cells there is a somatic rearrangement of their antigen
receptor genes. This process ensures that each lymphocyte makes a single, unique antigen
receptor. This rearrangement occurs before malignant transformation, thus the daughter
cells derived from a given malignant ancestor share the same antigen receptor gene
configuration and thus synthesize identical antigen receptor proteins (either
immunoglobulins or T-cell receptors). For this reason, analysis of antigen receptor genes
and their protein products is frequently used to differentiate monoclonal neoplasms
from polyclonal, reactive processes. It has been shown that NHLs is often widely
disseminated at the time of diagnosis, even when the disease appears clinically localized,

Chapter 3 - lymphoid system
54
that is why only systemic therapies are curative. In contrast, Hodgkin lymphoma often
presents at a single site and spreads in a predictable fashion to neighboring lymph node
groups. For this reason, early in the disease, local therapy may be effective.
The WHO classification embraces all lymphoid neoplasms, including leukemias and
multiple myeloma & separates them on the basis of origin into three major categories:
A. Tumors of B cells B. Tumors of T cells & NK cells C. Hodgkin lymphoma
Some neoplasms are common and collectively make up the vast majority of lymphoid
neoplasms (more than 90%), these include
1. Precursor B- & T-cell lymphoblastic leukemia/lymphoma
2. Small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL)
3. Follicular lymphoma 4. Diffuse large B-cell lymphomas 5. Burkitt lymphoma
6. Multiple myeloma and related plasma cell dyscrasias
7. Mantle cell lymphoma 8. Hodgkin lymphoma
Precursor B- and T-Cell Lymphoblastic Leukemia/Lymphoma
These are aggressive tumors, composed of immature lymphocytes (lymphoblasts), which
occur predominantly in children and young adults. They are microscopically
indistinguishable. Both pre-B- and pre-T-lymphoblastic lymphomas usually take on the
clinical appearance of an acute lymphoblastic leukemia (ALL) at some time during
their course. As a group, ALLs constitute 80% of childhood leukemia, peaking in
incidence at age of 4 years, with most of the cases being of pre-B-cell origin. The pre-T-
cell tumors, which initially present as thymic tumors, are most common in adolescent
males of between 15 and 20 years of age.
Small Lymphocytic Lymphoma/Chronic Lymphocytic Leukemia (Cll/Sll)
These two disorders differ only in the extent of peripheral blood involvement. If the
peripheral blood lymphocytosis exceeds 4000 cells/mm
3
, the patient is diagnosed
with chronic lymphocytic leukemia (CLL); if not, a diagnosis of small lymphocytic
lymphoma (SLL) is made.
Microscopic features
There is diffuse effacement of the lymph node architecture by lymphoid cells.
The predominant cells are compact, monomorphic, small, resting lymphocytes with dark-
staining round nuclei & scanty cytoplasm.
There are, in addition, foci of mitotically active larger cells; these aggregated to form
proliferation centers. The latter are pathognomonic for CLL/SLL. (Fig. 4-3)
CLL/SLL is a neoplasm of mature B cells expressing pan-B-cell markers such as CD19,
CD20, and surface immunoglobulin heavy and light chains.
In addition to the lymph nodes, the bone marrow, spleen, and liver are involved in almost
all cases; this is associated with peripheral absolute lymphocytosis. Generalized
lymphadenopathy & hepatosplenomegaly are present in 50% of cases. The median
survival is about 5 years. CLL/SLL tends to transform to more aggressive lymphoid
malignancies. Once transformation occurs, the median survival drops to less than one year.
Follicular Lymphomas
Are common adult NHLs in the United States, but (Like CLL/SLL), they occur much less
frequently in Asian populations.
Microscopic features (Fig. 4-4)
o The native lymph node architecture is effaced by nodular (follicular) aggregates of
proliferating lymphoid cells
Chapter 3 - lymphoid system
55
o Most commonly, the predominant neoplastic cells are "centrocyte-like" cells; these are
slightly larger than resting lymphocytes & have "cleaved" nuclei due to prominent
indentations and linear infoldings of the nuclear membrane.
o The small, cleaved cells are mixed with variable numbers of larger "centroblast-like"
cells that have vesicular chromatin, several nucleoli, and moderate amounts of cytoplasm.
In most tumors, centroblast-like cells are a minor component of the overall cellularity.
These tumors express the pan-B-cell markers (CD19 & CD20)
The majority of tumors have a characteristic t(14;18) translocation. This translocation
fuses the BCL2 gene to the IgH locus on chromosome 14 and leads to the inappropriate
expression of BCL2 protein, which functions to prevent apoptosis.
Follicular lymphoma usually presents as frequently generalized painless
lymphadenopathy. The bone marrow almost always involved at the time of diagnosis. The
median survival is 8 years, but this lymphoma is not easily curable. In about 40% of
patients, it progresses to a diffuse large B-cell lymphoma, which is an ominous change.
Diffuse Large B-Cell Lymphoma (DLBCL)
As a group, this is the most important type of NHL lymphoma in adults (50% of
adult NHL).
Microscopic features (Fig. 4-5)
o The nuclei of the neoplastic B cells are large (3-4 times the size of resting lymphocytes).
o There are two morphologic variants of DLBCLs
A. Centroblastic: the cells have round, or cleaved nuclear contours & several distinct
nucleoli, and moderate amounts of pale cytoplasm.
B. Immunoblastic: the cells have a large round or multilobulated nucleus, one or two
centrally placed prominent nucleoli, and abundant cytoplasm.
o The cells express pan-B-cell Ags (CD19 &20). Many also express surface IgM &/or IgG.
o Approximately 30% of tumors have a t(14;18) translocation involving the BCL2 gene.
Such tumors may represent "transformed" follicular lymphomas.
Several distinctive clinicopathologic subtypes are included in the general category of
diffuse large B-cell lymphoma.
1) Diffuse large B-cell lymphomas complicating AIDS & iatrogenic immunosuppression
(e.g., in transplant patients). EBV is implicated in the pathogenesis of this type.
2) Primary effusion lymphomas within the pleura, pericardium, or peritoneum. Human
herpesvirus type 8 (HHV-8), the agent of Kaposi sarcoma, is associated with this rare
subtype. Patients are usually immunosuppressed.
3) Mediastinal large B-cell lymphoma usually presents in young females and shows a
predilection for spread to abdominal viscera and the central nervous system.
Although the median age at presentation is about 60 years, DLBCLs can arise at any age;
they constitute 15% of childhood lymphomas. Patients typically present with a rapidly
enlarging mass at one or several sites. Extranodal presentations are common, with the
GIT and the brain among the more frequent sites. DLBCLs are aggressive tumors that are
rapidly fatal if untreated.
Burkitt Lymphoma
Is endemic in some parts of Africa and sporadic elsewhere. Both the African and
nonendemic forms are identical, although there are clinical and virologic differences. In
endemic areas, tumor cells in almost all patients carry EBV genome. In such endemic
areas concomitant (endemic) malaria (or other infections) impairs immune competence,
allowing sustained B-cell proliferation. Lymphoma cells emerge only when additional
mutations, such as the t(8;14) translocation. This involves MYC proto-oncogene on

Chapter 3 - lymphoid system
56
chromosome 8. The latter becomes activated to MYC oncogene as a result of fusion with
the IgH gene on chromosomes 14. The net result is the dysregulation and overexpression
of the MYC protein. In nonendemic areas, 80% of tumors do not harbor the EBV
genome, but all tumors possess the specific t(8 ; 14) translocation. This suggests that
although non-African Burkitt lymphomas are triggered by mechanisms other than EBV,
they develop cancer by similar pathways.
Microscopic features (Fig. 4-6)
The tumor cells are uniform and intermediate in size with round nuclei containing 2 to 5
prominent nucleoli. The nuclear size approximates that of benign macrophages within the
tumor. There is a moderate amount of basophilic cytoplasm, which on cytological smears
often contains small lipid vacuoles.
A high mitotic rate is very characteristic of this tumor, as is cell death. The latter
accounts for the presence of numerous tissue macrophages containing ingested nuclear
debris. Because these benign macrophages with their pale cytoplasm are scattered within
a blue background (of lymphoma cells) a "starry sky" pattern is thus created.
Both the endemic and nonendemic forms affect mainly children and young adults. In both
forms, the disease usually arises at extranodal sites. In African patients, involvement of
the maxilla or mandible is the common mode of presentation, whereas abdominal tumors
involving the bowel, retroperitoneum, and ovaries are more common in nonendemic
areas. Burkitt lymphoma is a high-grade tumor that is among the fastest growing human
neoplasms; however, with very aggressive chemo -therapy regimens, the majority of
patients can be cured.
Mantle Cell Lymphomas
are composed of B cells that resemble cells in the mantle zone of normal lymphoid
follicles. They constitute approximately 4% of all NHLs and occur mainly in older males.
They involve lymph nodes in a diffuse or vaguely nodular pattern. The tumor cells are
usually slightly larger than normal lymphocytes and have an irregular nucleus. Less
commonly, the cells are larger. The bone marrow is involved in the majority of cases, and
about 20% of patients have peripheral blood involvement. One unexplained but
characteristic tendency is the frequent involvement of the GIT.
Multiple Myeloma and Related Disorders (The plasma cell dyscrasias)
Hodgkin Lymphoma (HL)
Encompasses a distinctive group of neoplasms that arise almost invariably in a single
lymph node or chain of lymph nodes and spread characteristically in a stepwise fashion to
the anatomically contiguous nodes. Despite the fact that HL is a totally separate entity
from NHL, molecular studies have shown that it is a tumor of B-cell origin. HL accounts
for 30% of all lymphomas
Classification
HL has been reclassified by the rather recently into nodular lymphocyte predominant &
classic HLs. Classical Hodgkin's lymphoma are subclassified into 4 pathologic subtypes
based upon Reed-Sternberg cell morphology and the composition of the reactive cell
infiltrate seen in the lymph node biopsy specimen.
I. Nodular lymphocyte predominant HL in which the neoplastic RS cells are B-cells
reacting with CD20& 79a. Most of the background small lymphocytes are also B-cells.
II. Classical HL: nearly all RS cells are also derived from B-cells but show different
markers from the above, namely CD15 & CD30. The background lymphocytes are predo-
minantly of T-cells except in the lymphocyte-rich variant, where they are mainly B-cells.
Chapter 3 - lymphoid system
57
Under this heading are the following subtypes 1) Nodular sclerosis 2) Mixed cellularity
3) Lymphocyte rich (rare) 4) lymphocyte depletion (rare)
Microscopic features
The characteristic microscopic feature of HL is the Reed-Sternberg (RS) cell. This is a
large cell (15-45 μm in diameter) with an enlarged bi- or multilobated nucleus, prominent
nucleoli, and abundant, usually slightly eosinophilic cytoplasm. Particularly
characteristic are cells with two mirror-image nuclei or nuclear lobes, each containing a
large acidophilic nucleolus surrounded by a distinctive clear zone; together they impart
an owl-eye appearance. The nuclear membrane is thick. (Fig. 4-7)
Nodular Sclerosis Hodgkin Lymphoma
Is the most common form. It is equally frequent in men and women and has a striking
tendency to involve the lower cervical, supraclavicular, and mediastinal lymph nodes.
Most of the patients are adolescents or young adults, and the overall prognosis is
excellent. It is characterized microscopically by: (Fig. 4-8)
o The presence of a particular variant of the RS cell, the lacunar cell. This cell is large and
has a single multilobate nucleus with multiple small nucleoli and an abundant, pale-
staining cytoplasm. In formalin-fixed tissue, the cytoplasm often retracts, giving rise to
the appearance of cells lying in empty spaces, or lacunae.
o The presence of collagen bands that divide the lymphoid tissue into circumscribed
nodules. The cellular infiltrate may show varying proportions of lymphocytes,
eosinophils, histiocytes, and lacunar cells. Classic RS cells are infrequent.
o The immunophenotype of the lacunar variants is identical to that of classic RS cells i.e.
express CD15 and CD30.
Mixed-Cellularity Hodgkin Lymphoma (MCHL)
Is the most common form of Hodgkin lymphoma in patients older than the age of 50
years with a male predominance. Classic RS cells are plentiful within a distinctive mixed
cellular infiltrate, which includes small lymphocytes, eosinophils, plasma cells, and
benign histiocytes (Fig. 4-9). Compared with the other subtypes, more patients with
mixed cellularity have disseminated disease and systemic manifestations.
Lymphocyte-depleted Hodgkin disease (LDHL)
,
(< 1% of cases) is characterized by
the presence of large numbers of RS that are often bizarre morphologically. It is associated
with older age & HIV positive status. Patients usually present with advanced-stage disease
Lymphocyte-rich HL (LRHL),
(5%) of cases: in this type of Hodgkin disease, RS cells of the classic or lacunar type are
observed, with a background infiltrate of lymphocytes. It requires immunohistochemical
diagnosis. Clinically, the presentation & survival patterns are similar to those for MCHL.
Lymphocyte-predominance hodgkin lymphoma (LPHL)
Is a rare subgroup that often shows formation of poorly defined nodules, which contain a
large number of small mature lymphocytes admixed with a variable number of benign
histiocytes (Fig. 4-10). Classic RS cells are extremely difficult to find. Instead there is a
scattered of lymphohistiocytic (L&H) variant RS cells that have a delicate multilobed,
puffy nucleus, which has been likened in appearance to popcorn ("popcorn cell"). Unlike
RS cells, L&H cells are positive for B-cell antigens, such as CD19 & 20, & are negative
for CD15 & 30. A diagnosis of NLPHD needs to be supported by immunohistochemical
studies because it can appear similar to LRHD or even some non-HL. LPHL presents
mostly as isolated cervical or axillary lymphadenopathy and have an excellent prognosis.
The histologic diagnosis of Hodgkin lymphoma rests on the definitive identification
of RS cells or their variants in the appropriate background of reactive cells.
Chapter 3 - lymphoid system
58
Immunophenotyping plays an important adjunct role in helping to distinguish
Hodgkin lymphoma from reactive conditions and other forms of lymphoma.
In all forms involvement of the spleen, liver, bone marrow & other organs may appear in
due course. In advanced disease, the spleen and the liver can be enlarged by tumor.
Clinical Staging of Hodgkin (& Non-HL) (Ann Arbor Classification)
1) Stage I: involvement of a single lymph node region (I) or involvement of a single
extralymphatic organ or tissue (I
E
)
2) Stage II: involvement of 2 or more lymph node regions on the same side of the diaphragm
alone (II) or with involvement of limited contiguous extralymphatic organs or tissue (II
E
)
3) Stage III: involvement of lymph node regions on both sides of the diaphragm (III),
which may include the spleen (III
S
), limited contiguous extralymphatic organ or site
(III
E
), or both (III
ES
)
4) Stage IV: multiple or disseminated foci of involvement of one or more extralymphatic
organs or tissues with or without lymphatic involvement
All stages are further divided on the basis of the absence (A) or presence (B) of the
following systemic symptoms: significant fever, night sweats, unexplained loss of more
than 10% of normal body weight.
Etiology and Pathogenesis of HL
o L&H variants of RS cells found in nodular lymphocyte-predominance Hodgkin
lymphoma express B-cell markers (e.g. CD20). By contrast, the RS cells in all classic
subtypes do not express such markers. However, all show the same immunoglobulin gene
rearrangements and that the rearranged immunoglobulin genes have undergone somatic
hypermutation; this precludes expression of B cell markers. Thus, it is now generally
agreed that Hodgkin lymphoma, whether classic or not, is a neoplasm arising from
germinal center B cells. The events that transform these B cells and alter their appearance
and gene expression programs are still unknown.
o The EBV genome is present in the RS cells in up to 70% of cases of the mixed-cellularity
type and a smaller fraction of the nodular sclerosis type. Thus, EBV infection is likely to
be a contributing step to the development of Hodgkin lymphoma, particularly the mixed-
cellularity type.
o It has been found that the RS cells in classical forms of Hodgkin lymphoma, regardless of
their EBV status, contain high levels of activated NF-κB, a transcription factor that
normally stimulates B-cell proliferation and protects B cells from pro-apoptotic signals.
Several EBV proteins that are known to activate NF-κB are expressed in EBV-positive
RS cells. Thus, hyperactivation of NF-κB may be a central event in the genesis, growth,
and survival of RS cells.
o The characteristic non-neoplastic, inflammatory-cell infiltrate seems to result from a
number of cytokines secreted by RS cells, including IL-5 (which attracts & activates
eosinophils). Conversely, the responding inflammatory cells produce factors (such as
CD30 ligand) that can aid the growth & survival of RS cells.
Prognosis of HL
A definitive distinction from NHL can be made only by examination of a lymph node biopsy
specimen. With current modalities of therapy, however, the clinical stage is the most
important prognostic indicator. The 5-year survival rate of patients with stage I-A or II-A
disease is close to 100%. Even with advanced disease (stage IV-A or IV-B), the overall 5-
year disease-free survival rate is around 50%. However, therapeutic successes have also
brought problems. Long-term survivors of radiotherapy protocols are at much higher risk of
developing certain malignancies, including lung cancer, melanoma, and breast cancer.

Chapter 3 - lymphoid system
59
Miscellaneous lymphoid neoplasms
Extranodal Marginal Zone Lymphomas
Are low-grade mature B-cell tumors that arise mostly in mucosal-associated lymphoid
tissue (MALT), such as salivary glands, small and large bowel, and lungs. They tend to
occur in the setting of autoimmune disorders (such as Sjögren syndrome and Hashimoto
thyroiditis) or chronic infections with such organisms as Helicobacter pylori, suggesting
that sustained antigenic stimulation contributes to their development. In the case of H.
pylori-associated gastric MALT lymphoma, eradication of the organism with antibiotic
therapy often leads to regression of the tumor. When arising at other sites, MALT tumors
can often be cured by local excision or radiotherapy.
Mycosis Fungoides (MF) & Sézary Syndrome
(F 4-11)
These are composed of neoplastic CD4+ T cells that involve the skin, thus are examples
of cutaneous T-cell lymphomas. MF usually presents initially as reddish cutaneous rash
(erythrodermic phase) that progresses to plaque phase & then a tumor phase.
Microscopically, there is infiltration of the epidermis & upper dermis by neoplastic T
cells, with a marked infolding of the nuclear membrane (cerebriform nucleus).
Involvement of the epidermis is in the form of localized collections referred to as
Pautrier microabscess. With progressive disease, there is lymph & visceral
dissemination. Sézary syndrome is a clinical variant characterized by the presence of
1. Generalized exfoliative erythroderma
2. Tumor cells (Sézary cells) in the peripheral blood.
Patients with the initial erythrodermic-phase MF often survive for many years, whereas
survival is reduced to 2 years for patients with tumor-phase disease, visceral disease, or
Sézary syndrome.
Peripheral T-Cell Lymphomas
This is a heterogeneous group of tumors that together make up about 15% of adult NHLs. In
general, they present as disseminated disease, are aggressive & respond poorly to therapy.

60
Chapter contents (60 – 83)
Topics
Directed by
Page number
1
The Liver
د. سالم
(61
– 76)
Clinical syndromes
61
Inflammatory & Infectious disorders
64
Alcoholic liver disease
70
Drug-induced liver disease
71
Metabolic liver disease
71
Tumors & Hepatic nodules
74
2
Disorders of the gallbladder and the extrahepatic biliary tract
(77
– 80)
Gallbladder diseases
77
Disorders of the extrahepatic bile ducts
79
Tumors
80
3
Exocrine Pancreas
(81
– 83)
Congenital anomalies
81
Pancreatitis
81
Pancreatic neoplasms
83

Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
61
The Liver
The dominant primary diseases of the liver are 1. Viral hepatitis
2. Alcoholic liver disease (in the Western world; rare in Iraq) 3. Hepatocellular carcinoma
Clinical Syndromes
Hepatic failure
This is the gravest consequence of liver disease. It should be noted that 80% of hepatic
functional capacity must be damaged before failure ensues. In many cases
decompensation arises as a result of intercurrent diseases that place further burden on an
already sick liver; these include 1. Gastrointestinal bleeding 2. Systemic infection
3. Electrolyte disturbances 4. Severe stress such as major surgery or heart failure.
The morphologic alterations that cause liver failure fall into three categories:
1) Massive hepatic necrosis; most often drug-induced, as from paracetamol overdose,
halothane & antituberculous drugs (rifampin, isoniazid). Hepatitis A & hepatitis B
infection, and other causes (including unknown) account for about one-third of the
cases. Hepatitis C infection does not cause massive hepatic necrosis.
2) Chronic liver disease, which is the most common road to hepatic failure and is the
endpoint of persistent chronic hepatitis ending in cirrhosis.
3) Hepatic dysfunction without overt necrosis: hepatocytes may be viable but unable to
perform normal metabolic function, as with Reye syndrome, tetracycline toxicity, and
acute fatty liver of pregnancy.
Regardless of cause, the clinical signs of hepatic failure are much the same. Jaundice is
an almost always present. Hypoalbuminemia, which predisposes to peripheral edema, and
hyperammonemia, which may play a role in cerebral dysfunction, are extremely worrying
developments. Fetor hepaticus (a characteristic musty body odor) occurs occasionally.
Impaired estrogen metabolism and consequent hyperestrogenemia are thought to be the
causes of a) Palmar erythema & spider angiomas of the skin
b) Hypogonadism & gynecomastia in males
Hepatic failure is life-threatening because with severely impaired liver function, patients
are highly susceptible to failure of multiple organ systems. Thus, respiratory failure with
pneumonia and sepsis combine with renal failure to claim the lives of many patients. A
coagulopathy develops due to impaired hepatic synthesis of blood clotting factors. The
resultant bleeding tendency can lead to massive gastrointestinal bleeding as well as
petechiae elsewhere (see also esophageal varices). Intestinal absorption of blood places a
metabolic load on the liver, which worsens the extent of hepatic failure. The outlook of
full-blown hepatic failure is grave: A rapid downhill course is usual, & without liver
transplantation, death occurrs within weeks to a few months in about 80% of cases .
Two particular complications signal the gravest stages of hepatic failure
1) Hepatic encephalopathy, which is manifested disturbances of consciousness with
rigidity, hyperreflexia, and tremor. It is regarded as a disorder of neurotransmission in the
CNS and neuromuscular system and appears to be associated with elevated blood
ammonia levels, which impair neuronal function & promote generalized brain edema.
2) Hepatorenal syndrome refers to the appearance of renal failure in patients with severe
chronic liver disease, in whom there are no intrinsic morphologic or functional causes for
the renal failure. Na retention, impaired water excretion & decreased renal perfusion &
glomerular filtration rate are the main renal functional abnormalities. There is oliguria
associated with rising blood urea nitrogen & creatinine. The prognosis is poor, with a median
survival of only 2 weeks in the rapid-onset form and 6 months with the insidious-onset form.
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
62
Cirrhosis
Cirrhosis is the end-stage of chronic liver disease & is defined by 3 characteristics:
1) Bridging fibrous septae in the form of delicate or broad bands of fibrosis that link portal
tracts with one another and portal tracts with centrilobular veins.
2) Parenchymal nodules containing regenerating hepatocytes encircled by fibrosis, with
diameters varying from very small micronodules to large macronodules.
3) Disruption of the architecture of the entire liver
Classification of cirrhosis
The only satisfactory classification of cirrhosis is based on the underlying etiology. The
descriptive terms "micronodular" and "macronodular" should not be used as primary
classifications. Many forms of cirrhosis are initially micronodular (nodules < 3 mm), but
there is a tendency for nodules to increase in size; thus converting it to mixed (micro- &
macronodular) & eventually to macronodular form (nodules > 3mm).
The etiology of cirrhosis varies both geographically & socially. The following are
established causes of cirrhosis 1. Alcoholic liver disease (70% in Western countries)
2. Viral hepatitis (a very common cause in our country) 3. Biliary diseases
4. Primary hemochromatosis 5. Wilson disease
6. α1-Antitrypsin deficiency 7. Cryptogenic cirrhosis
Infrequent types of cirrhosis also include those cmplicating galactosemia & tyrosinosis in
infants & children, & drug-induced cirrhosis, as with α-methyldopa (aldomet). After all the
categories of cirrhosis of known causation have been excluded, a substantial number of cases
remain (15%) & is referred to as cryptogenic cirrhosis. It is possible that many of these
cases are due to undiagnosed nonalcoholic fatty liver disease. Once cirrhosis is established,
it is usually impossible to establish an etiologic diagnosis on morphologic grounds alone.
Pathogenesis of cirrhosis
The central pathogenetic processes in cirrhosis are progressive fibrosis and reorganization
of the vascular microarchitecture of the liver. In cirrhosis, types I and III collagen are
deposited in the lobule, creating delicate or broad septal tracts. New vascular channels in
the septae connect the vascular structures in the portal region (hepatic arteries and portal
veins) and terminal hepatic veins (centrilobulat & larger veins), shunting blood around
the parenchyma. Continued deposition of collagen in the space of Disse is accompanied
by the loss of fenestrations in the sinusoidal endothelial cells. As a result hepatocellular
secretion of proteins (e.g., albumin, clotting factors, and lipoproteins) is greatly impaired.
The major source of excess collagen in cirrhosis is the perisinusoidal stellate cells, which
lie in the space of Disse. Although normally functioning as vitamin A fat-storing cells,
during the development of cirrhosis they become activated. It is predominantly the
cytokines secreted by activated Kupffer cells and other inflammatory cells that stimulate
perisinusoidal stellate cells to divide & to produce large amounts of extracellular matrix.
Throughout the process of liver cell damage and fibrosis, remaining hepatocytes are
stimulated to regenerate and proliferate as spherical regenerative nodules within the
confines of the fibrous septae. The net outcome is a fibrotic, nodular liver in which
delivery of blood to hepatocytes is severely impaired, as is the ability of hepatocytes to
secrete substances into plasma. Disruption of the interface between the parenchyma and
portal tracts obliterates biliary channels as well. Thus, the cirrhotic patient may develop
jaundice and even hepatic failure, despite having a liver of normal mass.
In cirrhosis death is usually due to one or more of the following
1. Progressive liver failure 2. Portal hypertension related complications
3. The development of hepatocellular carcinoma.

Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
63
Portal hypertension
Increased resistance to portal blood flow may develop in a variety of circumstances,
which can be divided into prehepatic, intrahepatic, and posthepatic causes.
o Prehepatic conditions include
1. Portal vein thrombosis & narrowing
2. Massive splenomegaly through shunting excessive blood into the splenic vein.
o Posthepatic causes are 1. Severe right-sided heart failure 2. Constrictive pericarditis
3. Hepatic vein outflow obstruction.
o Intrahepatic causes:
1. Cirrhosis is the dominant cause accounting for most cases of portal hypertension.
2. Schistosomiasis 3. Massive fatty change
4. Diffuse fibrosing granulomatous disease such as sarcoidosis and miliary tuberculosis
5. Diseases affecting the portal microcirculation, exemplified by nodular regenerative hyperplasia
Portal hypertension in cirrhosis results from increased resistance to portal flow at the
level of the sinusoids, and compression of terminal hepatic veins by perivenular scarring
and expansile parenchymal nodules. Anastomoses between the arterial and portal systems
in the fibrous septa also contribute to portal hypertension by imposing arterial pressure on
the low-pressure hepatic venous system.
The 4 major consequences of portal hypertension in the setting of cirrhosis are
1. Ascites 2. The formation of portosystemic venous shunts leading to esophageal
varices & hemorrhoids 3. Congestive splenomegaly 4. Hepatic encephalopathy
o Ascites
Refers to the collection of excess fluid in the peritoneal cavity. It usually becomes
clinically detectable when at least 500 mL has accumulated, but many liters may collect
and cause massive abdominal distention. It is generally a serous fluid having less than 3
gm/dL of protein (largely albumin) as well as the same concentrations of solutes such as
glucose, sodium, and potassium as in the blood. Influx of neutrophils suggests secondary
infection, whereas red cells point to possible disseminated intra-abdominal cancer. With
long-standing ascites, seepage of peritoneal fluid through transdiaphragmatic lymphatics
may produce hydrothorax, more often on the right side.
The pathogenesis of ascites This is complex, involving the following mechanisms:
1) Sinusoidal hypertension, altering Starling's forces and driving fluid into the space of
Disse, which is then removed by hepatic lymphatics; this movement of fluid is also
promoted by hypoalbuminemia.
2) Percolation of hepatic lymph into the peritoneal cavity: normal thoracic duct lymph
flow approximates 800 to 1000 mL/day. With cirrhosis, hepatic lymphatic flow may
approach 20 L/day, exceeding thoracic duct capacity.
3) Intestinal fluid leakage: portal hypertension also causes increased perfusion pressure in
intestinal capillaries. This promotes movement of additional fluid out of intestinal
capillaries into the abdomen.
4) Renal retention of sodium and water due to secondary hyperaldosteronism
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
64
Inflammatory & infectious disorders
The liver is almost always involved in blood-borne infections such as bacterial (pyogenic
abscesses, miliary tuberculosis, salmonelloses), parasitic (malaria, amebiasis), fungal
(candidiasis), & viral (infectious mononucleosis, cytomegalovirus & herpes virus). Never
the less Viral hepatitis is the leading primary liver infection.
Viral hepatitis
Unless otherwise specified, the term viral hepatitis is reserved for “infection of the liver
caused by a group of hepatotropic viruses” i.e. having a particular affinity for the liver.
This group comprises
1. (HAV) 2.(HBV) 3. (HCV) 4. (HDV) 5.(HEV)
o Hepatitis A Virus (HAV)
Acute viral hepatitis A (infectious hepatitis) is a benign, self-limited disease with an
average incubation period of 4 weeks. HAV does not cause chronic hepatitis or a carrier
state and only rarely causes fulminant hepatitis. Nevertheless, most viral hepatitis
epidemics are attributed to HAV. In children, where most cases occur, the disease tends
to be mild or asymptomatic. HAV spreads by ingestion of contaminated water and foods.
The viremia is short-lived, thus, blood-borne transmission of occurs rarely; therefore,
donated blood is not screened for this virus.
HAV is a small, RNA virus. It reaches the liver from the intestinal tract after ingestion,
replicates in hepatocytes, and is shed in the bile and feces. It appears that the liver cell
injury is not directly related to the virus but results from T cell-mediated damage of
infected hepatocytes. Detection of anti-HAV IgM antibody is the best diagnostic marker
for the disease.
o Hepatitis B Virus (HBV)
This can produce
1. Acute viral hepatitis B with recovery & clearance of the virus
2. Chronic viral hepatitis B, which is either
a. Non-progressive or b. Progressive ending in cirrhosis
3. Fulminant hepatitis with massive liver necrosis 4. An asymptomatic carrier state.
Chronic viral hepatitis B is an important precursor of hepatocellular carcinoma. Liver
disease caused by HBV is a real worldwide problem, with an estimated carrier rate of
400 million. HBV remains in blood during the last stages of a long incubation period (4-
26 weeks) and during active episodes of both acute and chronic hepatitis. It is also
present in all physiologic and pathologic body fluids, with the exception of stool.
Whereas blood and body fluids are the primary vehicles of transmission, virus may also
be spread by contact with body secretions such as semen, saliva, sweat, tears, breast milk,
and pathologic effusions. In endemic regions, vertical transmission from mother to child
during birth constitutes the main mode of transmission. HBV infection in adults is mostly
cleared, but vertical transmission produces a high rate of chronic infection.
HBV is a DNA virus & its replication does not require integration of the virus in the host
DNA, however, integrated HBV is frequently found in cells. After exposure to the virus,
there is a long incubation period (average 16 weeks), which may be followed by acute
disease lasting weeks to months. The natural course of acute disease can be followed by
serum markers. HBsAg appears before the onset of symptoms, peaks during overt
disease, and then declines to undetectable levels in 3 to 6 months. Anti-HBs persists for
life, conferring protection; this is the basis for current vaccination policy using
noninfectious HBsAg. HBeAg appears in serum shortly after HBsAg to signify active
viral replication. Persistence of HBeAg is an important indicator of
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
65
1. Continued viral replication 2. Infectivity 3. Probable progression to chronic hepatitis
IgM anti-HBc (c for core Ag) is detectable with the onset of elevated serum
aminotransferase levels and thus indicative of hepatocyte destruction. Later this IgM is
replaced by IgG anti-HBc.
The host immune response to the virus is the main determinant of the outcome of the
infection. A strong response by virus-specific CD4+ & CD8+ interferon γ-producing cells
are associated with the resolution of acute infection. HBV, (like HAV) does not seem to
cause direct hepatocyte injury as many chronic carriers have virions in their hepatocytes
without any evidence of cell injury. Hepatocyte injury & damage seem to be mediated by
CD8+ cytotoxic T cells of the virus-infected hepatocytes.
o Hepatitis C Virus (HCV)
Is another major cause of liver disease. The worldwide carrier rate is estimated at 175
million persons. A decrease in the incidence has resulted from the marked reduction in
transfusion-associated hepatitis C (as a result of screening procedures). The major route of
transmission is through blood inoculation, with low rates of sexual and vertical
transmissions. HCV infection has a much higher rate (than HBV) of progression to chronic
liver disease and eventual cirrhosis. It is a single-stranded RNA virus. Based on the genetic
sequence, HCV is subclassified into six genotypes. An infected person may carry many
HCV variants. This variability seriously hinders efforts to develop an HCV vaccine. The
incubation period for hepatitis C has a mean of 6 to 12 weeks. The clinical course of acute
viral hepatitis C is usually asymptomatic and is easily missed. Strong immune responses
involving CD4+ and CD8+ cells are associated with self-limited HCV infections, but it is
not known why only a minority of individuals is capable of clearing HCV infection.
Persistent infection is the hallmark of HCV; in 80% of such cases it complicates subclinical
acute infection. Cirrhosis develops in 20% of such patients. Fulminant hepatitis is rare.
o Hepatitis D Virus (HDV) (Hepatitis delta virus)
Is a unique RNA virus in that it is replication defective, causing infection only when it is
encapsulated by HBsAg i.e. HDV is absolutely dependent on HBV co-infection for
multiplication. Delta hepatitis arises in two settings:
1. Acute coinfection after exposure to serum containing both HDV and HBV and
2. Superinfection of a chronic carrier of HBV with a new inoculum of HDV. In the first
case, most coinfected individuals can clear the viruses and recover completely. The
course is different in superinfected individuals in that most cases show acceleration of
hepatitis, progressing to more severe chronic hepatitis. Infection by HDV is worldwide,
with the highest prevalence rates (40%) in Africa & the Middle East. IgM anti-HDV AB
is the most reliable indicator of recent HDV exposure, but its appearance is transient.
o Hepatitis E Virus (HEV)
HEV hepatitis is a single-stranded RNA virus that is fecally transmitted. HEV is endemic
in India (where it was first documented). Epidemics have been reported from Asia and
Africa. HEV is not associated with chronic liver disease or persistent viremia. A
characteristic feature of the infection is the high mortality rate among pregnant women,
approaching 20%. A specific antigen (HEV Ag) can be identified in the cytoplasm of
hepatocytes during active infection. Virus can be detected in stools, and anti-HEV IgG
and IgM antibodies are detectable in serum.
Clinical features & Outcomes of viral hepatitis
A number of clinical syndromes may develop after exposure to hepatitis viruses:
1. Asymptomatic infection (serologic evidence only) 2. Acute hepatitis (anicteric or icteric)
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
66
3. Chronic hepatitis (with or without progression to cirrhosis)
4. Chronic carrier state (asymptomatic)
5. Fulminant hepatitis (submassive to massive hepatic necrosis with acute liver failure)
With rare exceptions, HAV, HCV, and HEV do not generate a carrier state, and HAV and
HEV infections do not progress to chronic hepatitis. Viral persistence and development
of chronic disease is much more common after HCV infection than HBV infection.
Because other infectious or noninfectious causes (such as drugs and toxins), can lead to
essentially identical syndromes, serologic studies are decisive for the diagnosis of viral
hepatitis and identification of virus types.
o Asymptomatic Infection:
The patients are identified only on the basis of minimally elevated serum
aminotransferases or the presence of antiviral antibodies.
o Acute viral hepatitis
Any one of the hepatotropic viruses can cause acute viral hepatitis. Acute infections are
easily detected for HBV infections but only rarely diagnosed for HCV. A hepatitis virus
etiology is suggested by elevated serum aminotransferase levels. As jaundice appears
(icteric phase), symptoms begin to fade away. To begin with there is predominantly
conjugated hyperbilirubinemia but later hepatocellular injury interferes with bilirubin
conjugation, thus, unconjugated hyperbilirubinemia can also occur. An icteric phase is
usual especially in adults infected with HAV, but is absent in about 50% of the cases
infected with HBV and in most cases of HCV infection. Within weeks, the jaundice and
most systemic symptoms clear as convalescence begins.
o Chronic Hepatitis
Is defined as “the presence of clinical, biochemical, or serologic evidence of continuing
hepatic disease for more than 6 months, with histological documentation of inflammation
and necrosis.” Although chronic hepatitis is mostly caused by hepatitis viruses, there
are other causes of this condition, these include
Drugs (isoniazide, α-methyldopa, methotrexate),
Auto-immune damage (autoimmune hepatitis)
Wilson disease, α
1
-antitrypsin deficiency, chronic alcoholism.
In chronic hepatitis, it is the etiology that determines the likelihood of progression. In
particular, HCV is notorious for causing a chronic hepatitis evolving to cirrhosis in a
significant percentage of patients. Chronic hepatitides are highly variable in their clinical
features. The most common signs are spider angiomas, palmar erythema, & mild tender
hepatomegaly. Laboratory studies may show prolongation of the prothrombin time,
hypergammaglobulinemia, hyperbilirubinemia, & mild elevations in alkaline phosphatase
levels. The course is again highly variable. Persons with hepatitis C may have indolent
disease without progression for years. Conversely, others have rapidly progressive
disease & develop cirrhosis within a few years. Causes of death in chronic hepatitis are
related to the complicating cirrhosis e.g. liver failure, hepatic encephalopathy, massive
hematemesis from esophageal varices, and hepatocellular carcinoma.
o The Carrier State
With hepatotropic viruses, carriers are those who harbor one of the viruses & may have
nonprogressive liver damage, but are essentially free of symptoms. They constitute
reservoirs of infection. HBV infection early in life, particularly through vertical
transmission during childbirth, produces a carrier state in 90% to 95% of the cases. In
contrast, less than 10% of HBV infections acquired in adulthood yield a carrier state.
Individuals with impaired immunity are particularly likely to become carriers. HCV can
induce a carrier state, which is estimated to affect 0.2% to0.6% of the general population
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
67
o Fulminant Hepatitis
A very small proportion of patients with acute viral hepatitis A, B, or E may develop acute
liver failure, resulting from massive hepatic necrosis. Cases with a more prolonged course
of several weeks or months are usually referred to as subacute hepatic necrosis; livers of
these individuals show both massive necrosis and regenerative hyperplasia. It should be
remembered that drugs & chemicals can also cause massive hepatic necrosis.
Pathological features of viral heaptitis
The morphologic changes in acute and chronic viral hepatitis are shared among the
hepatotropic viruses and can be mimicked by drug reactions.
o Acute viral hepatitis (Fig. 6-1)
The normal radial array of the lobules is lost. There is diffuse ballooning degeneration of
hepatocytes; the cells are swollen with clear, wispy cytoplasm.
Hepatocytes necrosis assume one of 3 morphologic types
1) Cytolysis i.e. dissolution of the hepatocytes. The necrotic cells vanish (cell dropped out).
This is detected indirectly as macrophage aggregation
2) Apoptosis i.e. hepatocytes shrink, become intensely eosinophilic, and have fragmented
nuclei. Apoptotic cells are phagocytosed within hours by macrophages & hence may be
difficult to find despite extensive apoptosis.
3) Confluent necrosis of hepatocytes, seen in severe cases & may lead to bridging necrosis
that extends through portal-portal, central-central, or portal-central areas. (f 6-2)
Hepatocyte regeneration as evidenced by irregularly thickened plates with occasional
rosettes & multinucleation.
Inflammation is usually a prominent feature of acute hepatitis. The portal tracts are
infiltrated predominantly by lymphocytes. The inflammatory infiltrate may spill over into
the parenchyma to cause necrosis of periportal hepatocytes (interface hepatitis) and may
also infiltrate the sinusoids.
Hypertrophy & hyperplasia of Kuppfer cells
Cholestasis may be present, both intracellular (brown pigmentation of hepatocytes) &
canalicular (bile plugs in canaliculi).
HBV infection, acute or chronic, may produce 2 distinctive features of the infected hepatocytes.
1) Ground-glass" hepatocytes: a finely granular, eosinophilic cytoplasm due to massive
quantities of HBsAg (as seen by electron microscopy).
2) Sanded nuclei, resulting from abundant intranuclear HBcAg.
o Chronic hepatitis (Fig. 6-3)
The changes are of variable severity, ranging from very mild to severe.
Hepatocyte necrosis may occur in all forms of chronic hepatitis.
The inflammatory component consists mainly of lymphocytes, macrophages, and
occasional plasma cells. In the mildest forms, significant inflammation is limited to portal
tracts. Lymphoid aggregates in the portal tract are often seen in HCV infection.
The liver architecture is usually well preserved. Continued periportal necrosis (interface
hepatitis) and bridging necrosis are forerunners of progressive liver damage.
The hallmark of serious liver damage is the deposition of fibrous tissue. At first, at the
portal tracts, but with time periportal fibrosis occurs. This is followed by bridging
fibrosis that links fibrous septa between lobules.
Continued loss of hepatocytes with fibrosis results in cirrhosis, with fibrous septa &
hepatocyte regenerative nodules. This pattern of cirrhosis is characterized by irregularly sized
nodules separated by variable but mostly broad bands of fibrosis. The nodules are typically
greater than 0.3 cm in diameter; accordingly, the cirrhosis is by definition macronodular
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
68
Autoimmune Hepatitis
Is microscopically indistinguishable from chronic viral hepatitis but is associated with a
set of immunologic abnormalities. This disease may run an indolent or severe course.
Salient features include: - Female predominance
- Absence of viral infection serologic markers
- Immunological abnormalities
a) Elevated serum IgG (>2.5 g/dl)
b) High titers of autoantibodies (80% of cases)
c) Presence of other autoimmune diseases (in 2/3 of the patients), including rheumatoid
arthritis, thyroiditis, Sjögren syndrome, and ulcerative colitis.
Pathogenesis: Most patients have a variety of auto-antibodies such as antinuclear, anti-
smooth muscle, liver kidney microsomal antibody, etc. The best characterized among
these antibodies are smooth muscle antibodies directed against cytoskeletal proteins that
include actin, and troponin, and liver kidney microsomal antibodies. The main effectors
of cell damage are believed to be CD4+ helper cells. Response to immunosuppressive
therapy is usually dramatic. The overall risk of cirrhosis, the main cause of death, is 5%.
Pyogenic liver abscesses
In developing countries most liver abscesses result from parasitic infections, such as
amebic, echinococcal, etc. In the Western world, bacterial abscesses are more common,
representing a complication of an infection elsewhere. Gram-negative bacteria such as E.
coli and Klebsiella sp. are the usual offenders. The organisms reach the liver through one
of the following pathways:
1. Ascending cholangitis 2. Vascular seeding, predominantly portal i.e. from the GIT
3. Direct invasion from a nearby focus 4. A penetrating injury.
Debilitating disease with immune deficiency is a common background e.g. extreme old
age, immunosuppression, or chemotherapy.
Gross features
Pyogenic abscesses may be solitary or multiple, ranging from very small to massive lesions.
Bacteremic spread through the arterial or portal system tends to produce multiple small
abscesses, whereas direct extension & trauma usually cause solitary large abscesses.
(F6-4 A)
Microscopic features
These are identical to pyogenic abscesses elsewhere.
Liver abscesses are associated with fever and right upper quadrant pain and tender
hepatomegaly. Jaundice is often the result of extrahepatic biliary obstruction. Surgical
drainage
is often necessary.
Amebic liver abscess
o Is usually single, mostly right sided, & close to liver dome, but tends to be multicentric in
immunocompromised patients. Adults are mostly affected but can develop in infants & children.
o Gross features (Fig. 6-4 B)
The necrotic center contains odorless, pasty, chocolate brown fluid.
o Microscopic:
Most of the lesion consists of necrotic debris. There few if any neutrophils.
This centre is surrounded by fibrin, macrophages, lymphocytes & a few fibroblasts with
clusters of amebic trophozoites (up to 60 microns with small eccentric nucleus and
cytoplasmic vacuoles that may contain red blood cells; resemble histiocytes).
o Complications
1. Bacterial superinfection
2. Extension or perforation into the following 1. Pleuro-pulmonary structures

Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
69
2. Subphrenic space 3. Peritoneal cavity, and pericardial sac, bile ducts
6. Kidney, mediastinum, chest wall, abdominal wall & flank.
o Diagnosis: serology is 90% sensitive
Hydatid disease of the liver
Three quarters of infected individuals develop one or more hepatic cysts, which grow
slowly. The typical hydatid cyst is spherical and may measure up to more than 30 cm in
diameter. The majority occur in the right lobe, but they may be multiple, involving all
lobes. A characteristic gross feature is the presence of the soft, whitish laminate
membrane. (Fig. 6-4 C) Histologic examination of the cyst wall shows an outer fibrous
layer; a midlle onionskin like laminated membrane, and an inner germinal layer.
Calcification in the latter layer signifies that the cyst is dead. The adjacent liver
parenchyma often shows pressure atrophy and a portal infiltrate in which eosinophils may
be prominent. The viable cyst is filled with colorless fluid, which contains daughter cysts
and brood capsules with scolices. Communication with the biliary tract & superimposed
infection are frequent. Rupture of the cysts into the peritoneal cavity may result in a fatal
anaphylactic reaction or in the formation of innumerable small granulomas grossly
resembling peritoneal tuberculosis. Identification of fragments of germinal membrane or
scolices in their center points to the diagnosis. Hepatic echinococcus cysts can also
rupture inside the gallbladder or through the diaphragm into the pleural space and lung.
The laboratory diagnosis can usually be made by hydatid serology and confirmed or
established by ultrasound or computed tomography.

Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
70
Alcoholic liver disease
Excessive ethanol consumption is a common cause of chronic liver disease in Western
countries and accounts for up to 50% of deaths due to cirrhosis. Chronic heavy drinkers
are predisposed to 3 distinctive forms of alcoholic liver disease; these may overlap.
Pathological features:
Hepatic Steatosis (Fatty Liver):
(almost all heavy drinkers)
Gross features: The liver is large (up to 4 or even 6 kg), soft, yellow, and greasy.
Microscopic features (Fig. 6-6 B)
Initially small lipid droplets accumulate in hepatocytes (microvesicular steatosis). Persistent
chronic intake of alcohol is associated with lipid accumulates in a large single vacuole that
compresses & displaces the nucleus to the periphery of hepatocyte (macrovesicular steatosis)
The affected hepatocytes are centrilobular, but in severe cases the entire lobule is affected.
With continued alcohol intake fibrous tissue develops around the central veins and
extends into the adjacent sinusoids. Until fibrosis appears, the fatty change is completely
reversible if there is abstention from further intake of alcohol
Alcoholic Hepatitis (30%)
Gross features: The liver is mottled red & yellow-green (bile stained). It may increase
in size. Visible nodules and fibrosis signify progression to cirrhosis.
Microscopic features (Fig. 6-7) Alcoholic hepatitis is characterized by
1) Ballooning degeneration and necrosis of hepatocytes
2) Mallory Bodies: these appear as pinkish, tangled filaments within the cytoplasm of
degenerating hepatocytes
3) Neutrophil Infiltrations that permeate the lobule & accumulate around degenerating hepatocytes.
4) Fibrosis is characteristically sinusoidal and perivenular
5) Cholestasis and hemosiderin deposition in hepatocytes and Kupffer cells
Alcoholic Cirrhosis (15%)
Is the final, irreversible form of alcoholic liver disease.
Gross features (Fig. 6-8)
Initially, the liver is yellow, fatty, enlarged, (usually over 2 kg), & finely (micro-) nodular
externally & on section. Over several years it becomes brown, shrunken (weighing < 1 kg)
with variably sized small & large nodules that create a "hobnail" appearance externally.
Microscopic features
Initially there are delicate fibrous septa that extend from central veins through the
sinusoids to the portal tracts and from portal tracts to portal tracts.
Regenerative activity of entrapped hepatocytes generates in the early stages uniform small
nodules (< 0.3 cm in diameter). This is by definition a micronodular cirrhosis. The nodules
eventually become larger & more prominent & are engulfed by ever wider bands of fibrous
tissue & the liver is converted into a mixed micronodular & macronodular cirrhosis.
Bile stasis often develops.
The induction of cytochrome P-450 by alcohol leads to enhanced transformation of other
drugs to toxic metabolites e.g. accelerated metabolism of paracetamol into highly toxic
metabolites that increase the risk of liver injury even with therapeutic doses of this
commonly used analgesic. Concurrent viral hepatitis, particularly hepatitis C is a major
accelerator of liver disease in alcoholics. In chronic alcoholics, alcohol may become a major
caloric source in the diet, displacing other nutrients and leading to malnutrition & vitamin
deficiencies (e.g., thiamine & vitamin B
12
). This is accentuated by impaired digestive
function, primarily related to chronic gastric & intestinal mucosal damage & pancreatitis.
With alcoholic cirrhosis, the immediate causes of death are 1. Hepatic failure 2.
Massive GI hemorrhage 3. Intercurrent infection 4. Hepatorenal syndrome and 5.
Hepatocellular carcinoma (5% of cases).
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
71
Drug-induced liver disease
The liver is a major drug metabolizing and detoxifying organ in the body, thus, it is
subjected to injury from a wide range of therapeutic and environmental chemicals.
Injury may result from
1. Direct toxicity 2. Hepatic conversion of a foreign chemical to an active toxin
3. Immune damage, usually initiated by the drug or its metabolites acting as a hapten to
convert a cellular protein into an immunogen.
Exposure to a toxin or therapeutic agent should always be included in the differential
diagnosis of any form of liver disease. Drug reactions may be classified as predictable
(intrinsic) reactions or unpredictable (idiosyncratic) ones. Predictable drug reactions are
dose dependent & thus may occur in anyone who accumulates a sufficient dose.
Unpredictable reactions are idiosyncratic & depend on the host's ability to mount an
immune response to the antigenic stimulus.
Drug-induced chronic hepatitis is clinically and histologically indistinguishable from
chronic viral hepatitis or autoimmune hepatitis, and hence serologic markers of viral
infection are critical for making the distinction. Examples of predictable drug reactions
are associated with paracetamol, tetracycline, antineoplastic drugs, carbon tetrachloride,
and alcohol. Examples of drugs that can cause idiosyncratic reactions include
chlorpromazine, halothane anesthetic, sulfonamides, α-methyldopa, and allopurinol.
Drugs that may cause acute liver failure due to massive hepatic necrosis include
1. Paracetamol (the most common cause)
2. Halothane, 3. AntiTB drugs (rifampin, isoniazide),
4. Industrial chemicals such as carbon tetrachloride 5. Mushroom poisoning.
Gross features of massive hepatic necrosis (F. 6-9 A)
The entire liver may be involved, (or only random areas are affected).
With such extensive loss of hepatic substance, the liver shrinks to 500 gm & becomes a
floppy, red or bile-stained, soft organ covered by a wrinkled, redundant capsule.
Microscopic features (Fig. 6-9 B)
Complete necrosis of hepatocytes in contiguous lobules leaves only a collapsed reticulin
framework and preserved portal tracts.
If the patient survives for more than a week, regeneration of surviving hepatocytes occurs.
With massive destruction of confluent lobules, regeneration is disorderly, yielding nodular
masses of liver cells surrounded by bands of scarring (macronodular cirrhosis).
Metabolic liver disease
Under this heading come the following entities 1) Nonalcoholic fatty liver disease
2) Hemochromatosis, 3) Wilson disease, and 4) α
1
-antitrypsin deficiency.
Nonalcoholic Fatty Liver Disease (NAFLD)
May present as steatosis (fatty liver), nonalcoholic steatohepatitis (NASH) or cirrhosis.
The latter is similar to alcoholic hepatitis. NAFLD is associated with
1. Insulin resistance including Type 2 diabetes, is the most common associated condition
2. Obesity
3. Dyslipidemia (hypertriglyceridemia, low HDLP cholesterol, high LDLP cholesterol).
The presence of type 2 diabetes and obesity are the best predictors of severe fibrosis and
disease progression. Insulin resistance results in the accumulation of triglycerides in
hepatocytes. Fat-laden hepatocytes are highly sensitive to lipid peroxidation products
generated by oxidative stress, which can damage mitochondrial and plasma membranes,

Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
72
causing apoptosis. Most persons with steatosis are asymptomatic; patients with NASH or
may also be asymptomatic, or present with symptoms of chronic liver disease. Liver
biopsy is required for diagnosis. Fortunately, the frequency of progression from steatosis
to NASH, and from NASH to cirrhosis seems to be low.
Hereditary hemochromatosis
The most common form of this genetic disease is an autosomal recessive variant of adult
onset caused by mutations in the HFE gene. Iron accumulates over the lifetime of the
affected individual from excessive intestinal absorption. Total iron accumulation may
exceed 50 gm (N: 2-6g), over one-third of which accumulates in the liver (N: 0.5 g).
Fully developed cases of hereditary hemochromatosis show
1. Cirrhosis (all patients) 2. Diabetes mellitus (75%) 3. Skin pigmentation (75%).
Iron accumulations from known sources of excess iron are called secondary iron
overload or (secondary hemochromatosis), the most important of these are
1. Multiple transfusions 2. Ineffective erythropoiesis (as in β-thalassemia and
sideroblastic anemia) 3. Increased iron intake (Bantu siderosis).
Chronic liver diseases can also cause iron accumulation in the liver e.g. alcoholic liver disease.
Pathogenesis In hereditary hemochromatosis there is a dysregulation of intestinal
absorption of dietary iron. This leads to net iron accumulation of up to 1.0 gm/year. It
appears that HFE gene regulates the levels of hepcidin, the iron hormone produced by the
liver. Hepicidin normally interferes with the flowing out of iron from the intestines and
macrophages into the plasma and inhibits iron absorption. As might be expected,
hepicidin levels are reduced in all currently known genetic forms of hemochromatosis.
Gross features (Fig. 6-10 A)
Hemosiderin deposition occurs in several organs & tissues e.g. the myocardium,
pituitary, adrenal, thyroid, parathyroid gland, joints, skin
The liver is typically chocolate brown in color. Fibrous septa develop slowly, leading
ultimately to micronodular cirrhosis in an intensely pigmented liver.
Microscopic features
The golden-yellow hemosiderin granules accumulate in the cytoplasm of periportal
hepatocytes; these stain blue with the Prussian blue stain (Fig. 6-10 B).
With increasing iron load, there is progressive deposition in the rest of the lobule, along
with bile duct epithelium and Kupffer cells. (In secondary iron overload, the iron is
mainly in Kupffer cells, not hepatocytes, at least initially)
Iron is a direct hepatotoxin, and inflammation is characteristically absent.
In normal individuals the iron content of unfixed liver tissue is less than 1000 μg/gm dry
weight. Adult patients with hereditary hemochromatosis exhibit over 10,000 μg/gm dry
weight of iron; hepatic iron concentrations in excess of 22,000 μg/gm dry weight are
associated with the development of fibrosis and cirrhosis.
Males predominate (ratio of 5 to 7:1), partly because physiologic iron loss (menstruation,
pregnancy) retards iron accumulation in women. In the most common forms, caused by
HFE mutations, symptoms usually first appear in the age range of 40 to 60 years. The
classic clinical triad of cirrhosis (with hepatomegaly), skin pigmentation & diabetes mellitu
may not develop until late in the course of the disease. Death may result from cirrhosis,
hepatocellular carcinoma, or cardiac involvement. Treatment of iron overload does not
remove the risk of hepatocellular carcinoma (200 times higher than in normal populations).
Wilson Disease (Hepatolenticular degeneration)
This autosomal recessive disorder of copper metabolism is characterized by the
accumulation of toxic levels of copper in many tissues and organs, principally the liver,

Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
73
brain, and eyes. The genetic defect responsible for Wilson disease is a mutation in
ATP7B, the defective function of which leads to failure on the part of hepatocytes to
excrete copper into bile, which is the primary route for copper elimination from the body.
The defect also inhibits secretion of ceruloplasmin from hepatocytes into the plasma.
Copper thus accumulates progressively in the liver, apparently causing toxic liver injury.
Usually by the age of 5 years, copper that is not ceruloplasmin bound spills over into the
circulation, causing hemolysis and pathologic changes at other sites, such as brain,
cornea, and kidneys. Concomitantly, urinary excretion of copper increases markedly. The
biochemical diagnosis of Wilson disease is based on a decrease in serum ceruloplasmin,
increase in hepatic copper content, and increase in urinary excretion of copper.
Pathological features
Fatty change of the liver may be mild to moderate, the hepatocytes also show vacuolated
nuclei (glycogen or water) and focal necrosis. An acute hepatitis can mimic acute
viral hepatitis, except for the fatty change.
A chronic hepatitis resembles chronic hepatitis due to other causes but may show such
distinguishing features as fatty change, vacuolated nuclei, and Mallory bodies.
With progression of chronic hepatitis, cirrhosis develop.
The demonstration of increased copper content of the hepatocytes by using special stains
is not an exclusive feature of Wilson disease. However, the demonstration of hepatic
copper content in excess of 250 μg/gm dry weight is most helpful for making the
diagnosis. In the brain, toxic injury affects the basal ganglia, particularly the putamen,
which shows atrophy & cavitation (hepatolenticular degeneration). Nearly all patients
with neurologic involvement develop eye lesions called Kayser-Fleischer rings (green to
brown deposits of copper in the Descemet’s membrane of the cornea). (Fig. 6-11)Wilson
disease rarely manifests before 6 years of age. The most common presentation is acute or
chronic liver disease. Neuropsychiatric manifestations, including frank psychosis, or a
Parkinson disease-like syndrome, are the initial features in most of the remaining cases.
Demonstration of Kayser-Fleischer rings or markedly elevated hepatic copper levels in a
person with a low serum ceruloplasmin value strongly favor the diagnosis.
α
1
-Antitrypsin (AAT) Deficiency
AAT deficiency is an autosomal recessive disorder characterized by abnormally low
serum levels of this protease inhibitor. The major function of AAT is the inhibition of
proteases, particularly neutrophil elastase released at sites of inflammation. AAT
deficiency leads to pulmonary emphysema because a relative lack of this protein permits
the unrestrained activity of destructive proteases. AAT is a plasma glycoprotein
synthesized predominantly by hepatocytes. The AAT gene is very polymorphic, and at
least 75 forms have been identified. However, homozygotes for the Z allele (PiZZ
genotype) have circulating AAT levels that are only 10% of normal levels. Because the
mutant protein cannot be secreted by the hepatocyte, it accumulates in the endoplasmic
reticulum. Curiously, all individuals with the PiZZ genotype accumulate AAT in the
liver, but only 8% to 20% develop significant liver damage.
Pathological features
Hepatocytes in AAT deficiency contain round to oval cytoplasmic globular inclusions of
retained AAT, which are strongly positive with PAS stain (Fig. 6-12).
Hepatic injury associated with PiZZ may range from marked cholestasis with hepatocyte
necrosis in newborns, to childhood cirrhosis, or to a chronic hepatitis or cirrhosis that
becomes apparent only late in life.
In older children, adolescents & adults, presenting symptoms may be related to chronic
hepatitis, cirrhosis, or pulmonary disease. Hepatocellular CA develops in 3% of PiZZ adults.
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
74
Tumors and hepatic nodules
The most common hepatic neoplasms are metastatic carcinomas, with colon, lung, and
breast heading the list as sites of the primary tumor.
Solitary or multiple benign hepatocellular nodules
May develop in the liver. These include
1) Focal nodular hyperplasia
Is localized, well-demarcated but poorly encapsulated
nodular regeneration. It consists of hyperplastic hepatocyte with a central fibrous scar.
(Fig. 6-18) The nodules appear in non-cirrhotic livers in response to local vascular injury
and may reach up to many centimeters in diameter. It is usually an incidental finding,
most commonly in women of reproductive age. In about 20% of cases focal nodular
hyperplasia coexists with hepatic cavernous hemangiomas
2) Macroregenerative nodules
Appear in cirrhotic livers (Fig. 6-19). They are larger
than surrounding cirrhotic nodules but do not display atypical features. They do not
seem to be precursors of malignant lesions
.
3) Dysplastic nodules
Are lesions larger than 1 mm in diameter that appear in cirrhotic
livers. Hepatocytes in dysplastic nodules & in smaller lesions called dysplastic foci are
highly proliferative & show atypical features such as crowding and pleomorphism. The
dysplastic features can be of low or high grade. High-grade dysplastic lesions are
considered to be precursors of hepatocelluar cancers.
Benign Tumors
Cavernous hemangiomas (Fig. 6-20)
Are most common benign lesions of the liver are. These well-circumscribed lesions
consist of vascular channels and intervening stroma. They appear as discrete red-blue,
soft nodules, usually less than 2 cm in diameter, often directly beneath the capsule. Their
chief clinical significance is the importance of not mistaking them for metastatic tumors;
blind percutaneous needle biopsy may cause severe intra-abdominal bleeding.
Hepatic (liver cell) Adenoma
Usually occurs in women of childbearing age who have used oral contraceptive steroids
& it may regress on discontinuance of hormone use. The tumor is yellow-tan, well-
demarcated nodules, up to 30 cm in diameter & is often located beneath the capsule (Fig.
6-21).
It is composed of sheets and cords of cells that may resemble normal hepatocytes
with prominent arteries & veins. Liver cell adenomas are significant for 3 reasons:
1. May be mistaken for HCC
2. May rupture, particularly during pregnancy causing
life-threatening intra-abdominal hemorrhage
3. With β-catenin mutations they carry a risk of cancerous transformation.
Malignant
Hepatocellular Carcinomas (HCC)
o The incidence (generally 5% of all cancers) varies widely in different areas of the world.
More than 85% of cases occur in countries with high rates of chronic HBV infection e.g.
Asian and African countries in which HBV is transmitted vertically, and thus the carrier
state starts in infancy. Moreover, many of these populations are exposed to aflatoxin,
which, combined with HBV infection, increases the risk of HCC development by more
than 200-fold over noninfected, nonexposed populations. The peak incidence of HCC in
these areas is between 20 and 40 years of age, and in almost 50% of cases, HCC may
appear in the absence of cirrhosis. In Western populations HCC is rare and seldom
present before age 60, and in 90% of cases tumors develop in cirrhotic livers. There is a
pronounced male preponderance of HCC throughout the world.
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
75
o Pathogenesis
Three major etiologic associations have been established:
1. Infection with HBV or HCV 2. Alcoholic cirrhosis 3. Aflatoxin exposure
Other assiciations include
4. Hemochromatosis 5. Hereditary tyrosinemia (40% of patients develop HCC)
Cirrhosis seems to be an important, but not essential contributor to the occurrence of
HCC. In most cases, HCC develops from high-grade dysplastic nodules.
Carcinogenesis is greatly enhanced in the presence of cell injury and replication, as
occurs in chronic viral hepatitis. HCV infection is the greatest risk factor; HCC in such
patients occurs almost exclusively in the setting of cirrhosis. In certain regions of the
world, such as China and South Africa, where HBV is endemic, there is also high
exposure to dietary aflatoxins derived from the fungus Aspergillus flavus. Aflatoxin can
bind covalently with cellular DNA and cause a mutation in p53. Neither HBV nor HCV
contains oncogenes. The carcinogenic capacity of these viruses probably relates to their
capacity to cause continuing cell death, chronic inflammation, and regeneration; these are
believed to be main contributors to DNA damage.
Autonomous hepatocyte replication can occur by over-expression of specific cellular
genes (such as β-catenin), mutation of the tumor suppressor gene p53, methylation
changes, and expression of growth factors.
o Gross features
There are three gross forms of HCC
1. Unifocal, usually a massive tumor (Fig. 6-22 A)
2. Multifocal i.e. made of variably sized nodules
3. Diffusely infiltrative i.e. permeating widely and sometimes involving the entire liver
Particularly in the latter two patterns, it may be difficult to distinguish regenerative nodules
of cirrhotic liver from nodules of neoplasm of similar size. The cancerous masses usually
are yellow-white, punctuated sometimes by bile staining & areas of hemorrhage or necrosis
All patterns of HCC have a strong propensity for invasion of vascular channels.
Extensive intrahepatic metastases ensue, and occasionally snakelike masses of tumor
invade the portal vein (with occlusion of the portal circulation) or inferior vena cava,
extending even into the right side of the heart.
o Microscopic features (Fig. 6-22 B)
HCCs range from well- to poorly- differentiated lesions. In well differentiated HCC the
neoplastic hepatocytes are arranged in broad trabeculae, which are separated by sinusoids.
Central necrosis in the broad trabeculae may produce a pseudoglandular pattern.
Poorly differentiated tumors are composed of large multinucleate anaplastic tumor giant cells.
In the better differentiated variants, globules of bile may be found within the cytoplasm
of cells and in pseudocanaliculi between cells.
Mallory bodies may be found within the cytoplasm of the neoplastic cells.
HCC displays scant connective tissue stroma (that is why it is soft in consistency)
Fibrolamellar carcinoma
o Is a distinctive clinicopathologic variant of HCC, in that
- It occurs in young male and female adults (20-40 years of age) with equal incidence
- It has no association with cirrhosis or other risk factors
- It usually appears grossly as a single large, hard "scirrhous" tumor
- Histologically it is composed of well-differentiated neoplastic hepatocytes growing in nests
or cords & separated by thick fibrous lamellae around groups of tumor cells. (F. 6-23)
o In patients with cirrhosis a rapid increase in liver size, sudden worsening of ascites, or the
appearance of bloody ascites, fever, and pain call attention to the development of HCC.
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
76
o Laboratory studies are helpful but not diagnostic. Half of the patients have elevated levels
of serum α-fetoprotein. However, this tumor "marker" lacks specificity, because moderate
elevations are also encountered in other conditions, such as cirrhosis, chronic hepatitis,
normal pregnancy, fetal death, fetal neural tube defects and gonadal germ cell tumors. Very
high levels (>1000 ng/mL), however, are rarely encountered except in HCC. This marker is
only rarely elevated to the levels considered diagnostic for HCC. The overall prognosis of
HCC is poor, but it is significantly better for individuals who have a single tumor less than
2 cm in diameter & good liver function. The median survival is 7 months.
o Metastatic tumors (Fig. 6-24)
The following malignant tumors frequently involve the liver by direct extension
1. Gallbladder 2. Extrahepatic bile ducts
3. Pancreas 4. Stomach
The following carcinomas metastasize to the liver with regularly
1) Large bowel 2) lung 3) breast 4) Pancreas 5) Kidney 6) Stomach
Sarcomas of soft tissues or internal organs and malignant melanomas also frequently
metastasize to this organ.
o Gross features
Most metastatic tumors in the liver form discrete masses that may locally elevate the capsule.
Central necrosis with umbilication occurs in the larger lesions
metastases are very rare in cirrhotic livers; whatever the reason for this may be
(nonreceptive soil for the metastatic growth or simply the fact that most cirrhotic patients
do not live long enough to develop them), the conclusion can be drawn that the large
majority of malignant tumors occurring in cirrhotic li vers are primary.
o The microscopic picture reflects the features of the primary cancer.

Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
77
Disorders of gallbladder and extrahepatic biliary tract
Gallbladder diseases
Cholelithiasis (Gallstones)
Gallstones trouble up to 20% of adult populations and are mainly of
two types
1. Cholesterol stones composed of crystalline cholesterol monohydrate (80%)
2. Pigment stones composed predominantly of bilirubin calcium salts (20%)
Pathogenesis and Risk Factors
o Bile is a major pathway for elimination of excess cholesterol from the body. Cholesterol
is rendered water soluble through mixing with bile salts and lecithins that are secreted
into bile. When cholesterol concentrations exceed the solubilizing capacity of bile
(supersaturation), cholesterol deposited as solid cholesterol crystals.
o Cholesterol gallstone formation involves four concurrently occurring steps:
1) Supersaturation of the bile with cholesterol
2) Establishment of a nidus by microprecipitates of calcium salts
3) Hypomobility of the gallbladder (stasis), which promotes nidus formation
4) Mucus hypersecretion to trap the crystals and thus enhancing their aggregation
o The presence of unconjugated bilirubin in the biliary tree increases the likelihood of
pigment stone formation. This occurs in hemolytic anemias and biliary tract infections.
The precipitates are insoluble calcium bilirubinate salts.
o The majority of individuals with gallstones (80%) have no identifying risk factors
o Contributory risk factors include
1) Age and gender: the incidence of gall stones increases with age in that only 5% of the
population younger than age 40 but 25% of those older than 80 years develop stones. The
prevalence in women is about twice as high as in men.
2) Ethnic and geographic: gallstones are more prevalent in Western industrialized societies
and uncommon in developing ones.
3) Heredity: family history imparts increased risk, as do a variety of inborn errors of
metabolism such as those associated with impaired bile salt synthesis and secretion.
4) Environment: estrogenic influences, including oral contraceptives & pregnancy, increase
hepatic cholesterol uptake & synthesis, leading to excess biliary secretion of cholesterol.
5) Obesity, rapid weight loss, and treatment with the hypocholesterolemic agent
clofibrate are strongly associated with increased biliary cholesterol secretion.
6) Gallbladder hypomotility predisposes to gallstones. It is associated with pregnancy,
rapid weight loss, and spinal cord injury. In most cases, however, the hypomotility is
present without obvious cause.
Pathologic features
o Pure cholesterol stones always formed within the gall bladder as pale to tan yellow, and
are ovoid and firm (Fig. 6-25). They may be single but most are often multiple. In the
latter instance, they assume a faceted surface from apposition to one another. Most
cholesterol stones are radiolucent, but 20%of them may have sufficient calcium
carbonate to render them radiopaque.
o Pigment stones may arise anywhere in the biliary tree (gall bladder, intra- or extra-
hepatic bile ducts) and are either black or brown. In general, black pigment stones are
found in sterile bile, while brown stones are found in infected bile. Black stones are
usually small, present in large numbers (Fig. 6-26), and crumble easily. Brown stones
tend to be single or few in number. Because of the incorporation of calcium carbonates
and phosphates, 50% to 75% of black stones are radiopaque. Brown stones, which
contain calcium soaps, are radiolucent.
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
78
Gallstones are asymptomatic in 75% of the cases. Pain is the principal symptom and it
tends to be severe, either constant or "colicky" from an obstructed gallbladder or when
small gallstones move down-stream and lodge in the biliary tree. Inflammation of the
gallbladder, in association with stones, also generates pain.
Complications of gall stones include
1. Empyema 2. Perforation 3. Fistulae 4. Cholongitis 5. Obstructive cholestasis
6. Pancreatitis. It is the very small stones that are dangerous; the larger the calculi,
the less likely they are to enter the cystic or common ducts to produce obstruction.
Occasionally a large stone may erode directly into an adjacent loop of small bowel,
generating intestinal obstruction ("gallstone ileus").
Cholecystitis
This may be acute, chronic, or acute superimposed on chronic & almost always occurs in
association with gallstones. Its epidemiologic distribution closely parallels that of gallstones
Acute cholecystitis (Fig. 6-27)
o Gross features
The gallbladder is usually enlarged, tense, and bright red or blotchy, violaceous to green-
black discoloration. The latter is due to subserosal hemorrhages.
The serosal covering is frequently covered by fibrin or suppurative exudate.
In 90% of cases stones are present, often obstructing the cystic duct.
The gallbladder lumen is filled with cloudy or turbid bile (contain fibrin, blood & frank pus).
When the exudate is pure pus, the condition is referred to as empyema of the gallbladder.
In mild cases the gallbladder wall is thickened, edematous, and hyperemic.
In more severe cases the gallbladder is transformed into a green-black necrotic organ,
termed gangrenous cholecystitis.
o Microscopical features
The inflammatory reactions consist of the usual patterns of acute inflammation (i.e.,
edema, neutrophilic infiltration, vascular congestion. It may be suppurative with frank
abscess formation, or eventuates in gangrenous necrosis.
o Types
Acute Calculous Cholecystitis refers to acute inflammation of a gallbladder that contains
stones and is precipitated by obstruction of the gallbladder neck or cystic duct. It is the
most common major complication of gallstones and the most common reason for
emergency cholecystectomy. Initially it is the result of chemical irritation and
inflammation of the gallbladder wall in the setting of obstruction to bile outflow.
Acute Non-Calculous Cholecystitis
Up to 10% of gallbladders removed for acute cholecystitis contain no gallstones. Most of
these cases occur in seriously ill patients e.g. after severe trauma such as a major surgery,
motor vehicle accidents, severe burns as well as sepsis. In such cases many events are
thought to contribute to this condition such dehydration, gallbladder stasis and sludging,
vascular compromise, and, ultimately, bacterial contamination.
Chronic Cholecystitis
may be the sequel to repeated bouts of acute cholecystitis, but in
most instances it develops de novo. Like acute cholecystitis it is almost always
associated with gallstones but these do not seem to have a direct role in the initiation of
inflammation. Rather, supersaturation of bile predisposes to both chronic inflammation
and, in most instances, stone formation. Microorganisms, usually E. coli and enterococci,
can be cultured from the bile in only about one-third of cases.
o Pathological features (Fig. 6-28)
The changes are extremely variable and sometimes minimal.
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
79
The mere presence of stones within the gallbladder, even in the absence of acute
inflammation, is often taken as sufficient justification for the diagnosis.
The gallbladder may be contracted, of normal size, or enlarged.
The submucosa and subserosa are often thickened from fibrosis.
In the absence of superimposed acute cholecystitis, mural lymphocytes are the only
feature of inflammation.
Disorders of extrahepatic bile ducts
Choledocholithiasis and Cholangitis
are frequently seen together.
Choledocholithiasis
is the presence of stones within the biliary tree. Almost all these
stones are derived from the gallbladder. Symptoms are absent in 10% of the cases, but
when occur they are due to biliary obstruction or its sequele such as pancreatitis,
cholangitis, hepatic abscess, secondary biliary cirrhosis, or acute calculous cholecystitis.
Cholangitis
refers to acute mostly bacterial inflammation of the wall of bile ducts. Most
cases are due to obstruction bile flow, mostly by choledocholithiasis. However, surgical
reconstruction of the biliary tree is also a recognized cause. Uncommon causes include
tumors, indwelling stents or catheters, acute pancreatitis, and benign strictures. Bacteria
most likely enter the biliary tract through the sphincter of Oddi, rather than by the
hematogenous route. Ascending cholangitis refers to the tendency of bacteria, once
within the biliary tree, to infect intrahepatic biliary ducts. The usual pathogens are E. coli,
Klebsiella, Clostridium, Bacteroides, etc. Charcot's triad (pain, jaundice and fever) is the
most common mode of presentation.
The most severe form of cholangitis is suppurative cholangitis, in which purulent bile
fills and distends bile ducts, with an attendant risk of liver abscess formation.
Secondary Biliary Cirrhosis
Prolonged obstruction of the extrahepatic biliary tree results in secondary biliary cirrhosis.
Causes include
1. Extrahepatic cholelithiasis (the most common cause)
2. Malignancies of the biliary tree and head of the pancreas
3. Strictures resulting from previous surgical procedures 4. Biliary atresia
The initial morphologic features of cholestasis are entirely reversible with correction of
the obstruction. However, secondary inflammation resulting from biliary obstruction
initiates periportal fibrogenesis, which eventually leads to scarring and nodule formation,
generating secondary biliary cirrhosis. Subtotal obstruction may promote ascending
cholangitis, which further contributes to the damage. Enteric organisms such as coliforms
and enterococci are common offenders.
Biliary Atresia
is a major cause of neonatal cholestasis (30%). Biliary atresia is defined
as a complete obstruction of bile flow caused by destruction or absence of all or part of
the extrahepatic bile ducts. It is the most frequent cause of death from liver disease in
early childhood. The salient features of biliary atresia include
1. Inflammatory fibrosing stricture of extrahepatic biliary tree (hepatic or common bile ducts)
2. Inflammatory destruction of the major intrahepatic bile ducts
3. Features of biliary obstruction on liver biopsy
4. Periportal fibrosis and cirrhosis within 3 to 6 months of birth
Laboratory findings do not distinguish between biliary atresia & intrahepatic cholestasis,
but a liver biopsy provides evidence of bile duct obstruction in 90% of cases of biliary
atresia. Without surgical intervention, death usually occurs within 2 years of birth.

Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
80
Tumors
Carcinoma of the Gallbladder
Is the most frequent malignant tumor of the biliary tract. It occurs most frequently in the
age group 60-70 years. The mean 5-year survival is 5% because it is rarely discovered at
a resectable stage. Gallstones are present in about 75% of the cases. Presumably,
gallbladders containing stones or infectious agents develop cancer as a result of recurrent
trauma and chronic inflammation. The presence of abnormal choledocho-pancreatic duct
junction is considered to be a risk factor.
Gross features
The cancer is either exophytic (fungating) or infiltrative growth.
The infiltrative pattern, which is the more common, usually appears as a poorly-defined
area of thickening and induration of part or whole of gall bladder wall.
The exophytic pattern grows into the lumen as cauliflower mass, but at the same time it
invades the underlying wall (Fig. 6-29).
Microscopic features
Well- to poorly-differentiated infiltrative adenocarcinomas that is sometimes papillary.
By the time gallbladder cancers are discovered, most have invaded the liver directly & many
have extended to the cystic duct & adjacent bile ducts & lymph nodes at the portahepatis.
Preoperative diagnosis of gall bladder carcinoma is seen in only a 20% of the cases. The
fortunate person develops early obstruction and acute cholecystitis before extension of
the tumor into adjacent structures or undergoes cholecystectomy for coexistent
symptomatic gallstones. Preoperative diagnosis rests largely on detection of gallstones
along with abnormalities in
the gallbladder wall documented by imaging studies.
Cholangiocarcinomas
Are adenocarcinomas arising from cholangiocytes (epithelial cells lining) in bile ducts
within and outside of the liver. Extrahepatic cholangiocarcinomas (2/3 of the cases) may
develop at the hilum (Klatskin tumors) or more distally in the biliary tree, down to the
peripancreatic portion of the distal common bile duct. They occur mostly in individuals
50 to 70 years of age. The prognosis of cholangiocarcinomas is poor because they are
generally asymptomatic until late, and most patients have unresectable tumors.
Risk factors include
1. Primary sclerosing cholangitis 2. Fibrocystic diseases of the biliary tree
3. Exposure to Thorotrast (which is no longer used in radiography of the biliary tree).
Pathological features (Fig. 6-30).
Due to early development of obstructive jaundice, these tumors are detected as small
firm, gray nodules within the bile duct wall. Alternatively, they are diffusely infiltrative
lesions that create thickening of the wall.
These adenocarcinomas are generally well-differentiated with an abundant fibrous stroma
Cholangiocarcinomas may spread to extrahepatic sites such as regional lymph nodes,
lungs, & bones. Mean survival
time is around 12 months.

Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
81
Exocrine Pancreas
Congenital anomalies
Pancreas divisum
is the most common clinically significant congenital anomaly
resulting from failure of fusion of pancreatic ducts. This leads to the main pancreatic duct
draining only a small portion of the head, while the bulk of the pancreas drains through a
minor duct. This creates a state of inadequate drainage for the bulky pancreatic secretions
thus predisposes to chronic pancreatitis.
Annular pancreas
results from abnormal pancreatic fusion due to failure of ventral bud
to rotate properly; head of pancreas encircles duodenum as a collar & may constrict
lumen completely. It can lead to duodenal obstruction.
Ectopic Pancreas
is uncommon; favored sites are the stomach and duodenum. The
ectopic tissue is small and submucosal in location. It can cause localized inflammation, or
mucosal bleeding.
Congenital cysts:
the kidney, liver & pancreas can all contain cysts (polycystic disease).
Pancreatitis
Acute Pancreatitis
Is relatively common in developed countries. Causes implicated include
1. Gallstones and excessive alcohol intake; these are the main offenders.
2. Non-gallstone obstruction of the pancreatic ducts e.g. by periampullary tumors
3. Medications as with thiazide & frusemide diuretics
4. Trauma, both blunt and iatrogenic during surgery or endoscopy
5. Others such as metabolic disorders, ischemia and infections as with mumps
In up to 20% of patients there is no identifiable cause (idiopathic pancreatitis).
Gross features (Fig. 6-31)
In milder forms there is edema & congestion of the organ with foci of fat necrosis. Fat
necrosis results from enzymatic destruction of fat cells; the released fatty acids combine
with calcium to form insoluble salts that precipitate locally and appear as yellow-white
chalky deposits within & outside the pancreas e.g. in the omentum and mesentery.
The peritoneal cavity contains a brown-tinged fluid with fat globules.
In the most severe forms there is extensive parenchymal necrosis accompanied by diffuse hemorrhage.
Microscopical features
These parallel the gross changes of edema, acute inflammation, and focal fat necrosis.
In more severe forms, necrosis involves all tissue constituents including islets of Langerhans
Vascular damage causes hemorrhage into the parenchyma of the pancreas.
Pathogenesis
The microscopic changes favor autodigestion of the pancreatic substance by activated
pancreatic enzymes. Trypsin seems to have a central role because it can activate other
enzymes (e.g. phospholipases and elastases) that can participate in the process of
autodigestion. Trypsin can also leads to activation of the kinin system & factor XII and
thus the clotting and complement systems.
Pancreatic duct obstruction causes an ↑ in intraductal pressure, thus allowing accumulation
of an enzyme-rich interstitial fluid that causes tissue injury. Edema further compromises
local blood flow, causing vascular insufficiency & ischemic injury to acinar cells.
The role of alcohol as a cause of pancreatitis is still unknown, proposed mechanisms
include contraction of the sphincter of Oddi and direct toxic effects on acinar cells.
Inherited mutations in genes important for normal pancreatic exocrine function are investigated.
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
82
The manifestations of severe acute pancreatitis are attributable to systemic release of
digestive enzymes and explosive activation of the inflammatory response. Patients show
increased vascular permeability, leukocytosis, DIC, ARDS (due to alveolar capillary
injury), and diffuse fat necrosis. Shock can occur rapidly due to electrolyte disturbances
and loss of blood volume. These catastrophic events can be complicated by endotoxemia
resulting from infection of the necrotic debris by gram-negative organisms as there is
break down of barriers between gastrointestinal, pancreas and blood stream.
Laboratory findings include markedly elevated serum amylase during the first 24 hours,
followed (within 3-4 days) by rising serum lipase levels. Hypocalcemia can result from
precipitation of calcium in the extensive areas of fat necrosis. The enlarged inflamed
pancreas can be visualized by CT or MRI. Although most individuals with acute
pancreatitis eventually recover, some die from shock; ARDS and acute renal failure. In
those who survive complications include pancreatic abscesses or pancreatic pseudocysts.
Pancreatic Pseudocyst is the most common cystic lesion of the pancreas and a common
complication of acute pancreatitis. Liquefied necrotic pancreatic tissues become
surrounded by fibrous tissue wall to form a cystic space, lacking an epithelial lining
("pseudo"). Drainage of pancreatic secretions into this space over months to years (from
damaged pancreatic ducts) can cause massive enlargement of the cyst (up to 30 cm in
diameter). They can become secondarily infected, and larger pseudocysts can compress
or even perforate into adjacent structures. It is commonly attached to the surface of the
gland and may involve peripancreatic tissues. (Fig. 6-32)
Chronic Pancreatitis
Is characterized by longstanding inflammation and fibrosis with destruction of the
exocrine pancreas; in the late stages, the islets are also lost.
Causes of chronic pancreatitis include
1) Chronic alcoholism (the most common cause).
2) Long-standing pancreatic duct obstruction (e.g., by pseudocysts, calculi, neoplasms)
3) Tropical pancreatitis: seen in Africa and Asia, and attributed to malnutrition
4) Hereditary pancreatitis due to mutations of genes, some encoding trypsin inhibitor.
5) Cystic fibrosis
In some cases there is no obvious cause (idiopathic); as with acute pancreatitis, a
growing number of these cases are associated with inherited mutations in genes
concerned with normal pancreatic exocrine function.
Gross features:
The gland is hard, sometimes with extremely dilated ducts & visible calcifications
Microscopic features (Fig. 6-33)
Parenchymal fibrosis, reduced number and size of acini & variable dilation of the ducts.
A chronic inflammatory infiltrate around remaining lobules and ducts.
Islets of Langerhans are relatively spared but eventually they disappear.
Pathogenesis
This is still not established with certainity. However, several hypotheses are proposed:
1) Ductal obstruction by concretions: alcohol increases the protein concentration of
pancreatic secretions; these can form ductal plugs.
2) Toxic: alcohol can exert a direct toxic effect on acinar cells leading to their destruction
3) Oxidative stress induced by alcohol generates free radicals in acinar cells, which lead to
fusion of lysosomes and zymogen granules with resulting acinar cell necrosis,
inflammation, and fibrosis.
4) Necrosis-fibrosis due to recurrent episodes of acute pancreatitis
Chapter 4 – Liver, Gallbladder, Biliary Tract & Exocrine Pancreas
83
Chronic pancreatitis can present with repeated bouts of jaundice, persistent or recurrent
abdominal and back pain. It may be entirely silent until pancreatic insufficiency and
diabetes develop.
Chronic pancreatitis may present as attacks of abdominal pain with some elevation of
serum amylase. Gallstone-induced obstruction may cause jaundice &/or elevation in
serum alkaline phosphatase. A helpful finding is visualization of calcifications within the
pancreas by CT or ultrasonography. Weight loss and hypoalbuminemia with edema from
malabsorption can also occur.
Pancreatic neoplasms
Cystic Neoplasms
Some of these are entirely benign (e.g., serous cystadenoma); others, such as mucinous
cystic neoplasms, can be benign but frequently have malignant potential.
Pancreatic Carcinoma
Has a very poor prognosis in that the 5-year survival rate is less than 5%.
Pathogenesis: like all cancers, it arises as a consequence of inherited and acquired
mutations in cancer-associated genes. There is a progressive accumulation of genetic
changes in pancreatic epithelium as it proceeds from non-neoplastic, to noninvasive
lesions in small ducts and ductules, to invasive carcinoma. Antecedent lesions are called
"pancreatic intraepithelial neoplasias" (PanINs). They are often found adjacent to
infiltrating carcinomas and share with the latter a number of the same genetic mutations.
The more common molecular alterations in pancreatic carcinogenesis affect K-RAS
(oncogene), and the tumor suppressor genes p16, SMAD4, and p53.
Carcinoma is primarily a disease of the elderly (60 and 80 years). Smoking has the
strongest environmental influence. Chronic pancreatitis and diabetes mellitus are also
associated with an increased risk. Familial clustering of pancreatic cancer has been
reported, and familial pancreatitis (related to mutations in a trypsinogen gene) is
associated with up to 80-fold increased risk.
Gross features (Fig. 6-34)
The head is most commonly involved (60%), whereas the tail is the least common site (5%)
The cancer is usually hard, gray-white, poorly defined mass.
Most carcinomas of the head obstruct the distal common bile duct leading to distention of
the biliary tree, and obstructive jaundice.
In marked contrast, carcinomas of the body and tail do not interrupt the biliary tract and
hence remain silent for some time. They may be quite large and widely disseminated by
the time of the diagnosis.
The regional lymph nodes & the liver are often involved by metastases as are the lungs & bones.
Microscopical features
Most carcinomas are ductal adenocarcinomas.
Two features are characteristic of pancreatic cancer:
1) It is highly invasive; even in the early stages, thus infiltrates peripancreatic tissues extensively
2) It elicits an intense fibroblastic (desmoplastic) response.
Perineural and lymphatic invasions are commonly seen.
Migratory thrombophlebitis (Trousseau syndrome) occurs in about 10% of patients.
Endoscopic ultrasonography and CT, are helpful in diagnosis and in performing guided
percutaneous needle biopsy.

84
Chapter contents (84 – 106)
Topics
Directed by
Page number
1
Clinical Manifestations of Renal Diseases
د. سالم
(85)
2
Glomerular diseases
(86
– 91)
Mechanisms (Pathogenesis) of Glomerular Injury and Disease
86
The nephrotic syndrome
88
The nephritic syndrome
90
Chronic glomerulonephritis
91
3
Tubulo-interstitial diseases
(92
– 95)
Tubulointerstitial nephritis (TIN)
92
Acute Tubular Necrosis (ATN)
94
4
Diseases of renal blood vessels
(96 - 97)
Benign Nephrosclerosis (BNS)
96
Malignant Nephrosclerosis
96
5
Cystic diseases of the kidney
(98)
Simple Cysts
98
Autosomal Dominant (Adult) Polycystic Kidney Disease (ADPKD)
98
Autosomal Recessive (Childhood) Polycystic Kidney Disease (ARPKD)
98
Medullary Cystic Disease (MCD)
98
6
Urinary outflow obstruction
(99 - 100)
Renal Stones (Urolithiasis)
99
Hydronephrosis
99
7
Tumors
(101 - 102)
Renal Cell Carcinoma (RCC)
101
Wilms Tumor (WT) (nephroblastoma)
102
8
Renal pelvis, ureter, urinary bladder & urethra
(103 - 106)
Ureters
103
Urinary bladder
103

Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
85
Introduction
Diseases of the kidney are divided into four categories depending on which component of
the kidney is primarily affected; these are
1. Glomerular 2. Tubular 3. Interstitial 4. Vascular
This division is useful because
a. The early manifestations of each group of diseases tend to be distinctive.
b. These groups differ in their pathogenesis, for e.g., glomerular diseases are often
immunologically mediated, whereas tubular and interstitial disorders are more likely to
be caused by toxic or infectious agents. However, it should be noted that
1. The interdependence of renal components translated into that damage to one
component is almost always affects secondarily the others.
2. All forms of chronic renal disease tend ultimately to damage all four components of
the kidney thus, eventuates in chronic renal failure (end-stage kidney disease).
Clinical Manifestations of Renal Diseases
These can be grouped into well-defined syndromes, each of which is distinctive to the
component that is primarily affected. At this point certain terms need to be clarified
Azotemia is an "elevation of blood urea, nitrogen and creatinine levels". It is largely
related to a decreased glomerular filtration rate (GFR). Azotemia is divided into
1. Prerenal azotemia, encountered with hypoperfusion of the kidneys, which decreases
GFR as in shock states
2. Renal, which is due to renal parenchymal damage
3. Postrenal that results from urine out flow obstruction below the level of the kidney.
Uremia signifies "azotemia associated with biochemical and systemic
clinicopathological alterations" such as metabolic and endocrine changes, uremic
gastroenteritis, peripheral neuropathy and fibrinous pericarditis.
The major renal syndromes are
Acute nephritic syndrome is characterized by acute onset of usually gross hematuria,
mild to moderate proteinuria, azotemia, edema, and hypertension; it is the classic
presentation of acute poststreptococcal glomerulonephritis.
The nephrotic syndrome is characterized by heavy proteinuria (excretion of >3.5 gm of
protein/day in adults), hypoalbuminemia, severe edema, hyperlipidemia, and lipiduria.
Asymptomatic (microscopic) hematuria or proteinuria, or a combination thereof, is
usually a manifestation of mild glomerular abnormalities.
Acute renal failure refers to recent onset of oliguria or anuria, with azotemia.
Chronic renal failure refers to prolonged symptoms and signs of uremia; it is the end
result of all chronic renal diseases.
Urinary tract infection (UTI) is characterized by bacteriuria and pyuria (bacteria and
leukocytes in the urine. The infection may be symptomatic or asymptomatic, and may
affect the kidney (pyelonephritis) or the bladder (cystitis) only.
Nephrolithiasis (renal stones) is manifested by renal colic, hematuria, and recurrent
stone formation.

Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
86
Glomerular diseases
The glomerulus consists of an anastomosing network of capillaries invested by two layers
of epithelium; visceral & parietal. The visceral epithelium (podocytes) is an intrinsic part
of the capillary wall, whereas the parietal epithelium lines Bowman space (urinary
space), the cavity in which plasma ultrafiltrate first collects. The glomerular capillary
wall is the filtration unit and consists of the following structures (Fig. 8-1):
1) A thin layer of fenestrated endothelial cells (each hole is about 100 nm in diameter).
2) Glomerular basement membrane
(GBM)
, ultrastructally made up of a thick, dense central
layer (lamina densa) & thinner, lucent peripheral layers, (lamina rara interna & externa).
3) The visceral epithelial cells (podocytes) that possess foot processes adherent to the lamina
rara externa of the basement membrane. Adjacent foot processes are separated by 25-nm-
wide filtration slits bridged by a thin slit diaphragm composed in largely of nephrin.
4) The entire glomerular tuft is supported by mesangial cells lying between the capillaries.
Basement membrane-like mechanical matrix forms a meshwork through which the
mesangial cells are scattered.
The glomerular basement membrane shows selective permeability, which is size-
dependent & charge-dependent. The major characteristics of glomerular filtration are
1. A high permeability to water and small solutes
2. Almost complete impermeability to molecules of the size & molecular charge of albumin.
3. More permeability to cations than anions.
The podocyte is decisive to the glomerular barrier function by providing a distal
resistance to the flow of water and a barrier to the filtration of proteins. It is also largely
responsible for synthesis of GBM components.
Mechanisms (Pathogenesis) of Glomerular Injury and Disease
Immune mechanisms (antibody-associated & cellular)
Underlie most primary and many secondary glomerular diseases.
Circulating Immune Complex-mediated Nephritis
(type III hypersensitivity reactions)
o With circulating immune complex-mediated disease, the glomerulus is an "innocent
bystander" because it is not responsible for their formation. The antigen in these
complexes may be
1. Endogenous, as in SLE or
2. Exogenous, as in bacterial (streptococcal), viral (hepatitis B), parasitic (Plasmodium
falciparum malaria), and spirochetal (Treponema pallidum) infections.
3. Unknown as often the case in membranous nephropathy.
o The antigen-antibody complexes are trapped in the glomeruli, where they produce injury,
mainly through the activation of complement and the recruitment of leukocytes. Electron
microscopy reveals the immune complexes as electron-dense deposits or clumps that are:
1. Mesangial 2. Subendothelial i.e. between the endothelial cells and the GBM
3. Subepithelial i.e. at the outer surface of the GBM and the podocytes
o The presence of immunoglobulins and complement in these deposits can be demonstrated
by immunofluorescence microscopy. When fluoresceinated anti-immunoglobulin or anti-
complement antibodies are used, the immune complexes are seen as granular deposits in
the glomerulus (Fig. 8-2).
Cell-Mediated Immune Glomerulonephritis:
T cell-mediated injury may account for some cases of glomerulonephritis (GN) in which
either there are no deposits of antibodies or immune complexes, or the deposits do not
correlate with the severity of damage.
Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
87
Mediators of Immune Injury:
o A major pathway of antibody-initiated injury is activation of the complement that leads to
the generation of chemotactic agents (mainly C5a) and thus recruitment of neutrophils
and monocytes. Neutrophils in turn release proteases, which cause GBM degradation;
2. Oxygen-derived free radicals, which cause cell damage; and
3. Arachidonic acid metabolites, which contribute to reduction in GFR.
o In some cases, however, complement-dependent (but not neutrophil-dependent) injury
occurs through the effect of the C5-C9 lytic component (membrane attack complex) of
complement, which causes 1. Epithelial cell detachment and
2. Stimulation of mesangial and epithelial cells to secrete various mediators of cell injury.
3. Up-regulation of (TGF-β) receptors on podocytes; TGF-β stimulates synthesis of
extracellular matrix, thus giving rise to altered GBM composition and thickening.
Glomerulonephritis Caused by In Situ Immune Complexes
Antibodies in this form of injury react directly with planted antigens in the glomerulus.
The best-characterized disease in this group is anti-GBM antibody GN, where
antibodies are directed against fixed antigens in the GBM. It results from the formation of
autoantibodies directed against the GBM. Deposition of these antibodies creates a linear
pattern of staining when visualized with immunofluorescence microscopy (Fig. 8-2); this
is in contrast to the granular pattern described for other forms of immune complex-
mediated nephritis. Sometimes, the anti-GBM antibodies cross-react with basement
membranes of lung alveoli, resulting in combined lung and kidney lesions (Goodpasture
syndrome). Planted antigens also include DNA, bacterial products, aggregated IgG,
which deposit in the mesangium because of their size. Most of these planted Ags induce a
granular pattern of Ig deposition as seen by immunofluorescence microscopy.
Other Mechanisms of Glomerular Injury
1) Podocyte injury
: this can be induced by antibodies to visceral epithelial cell antigens as
in some cases of focal and segmental glomerulosclerosis. Such injury is reflected by
effacement of podocyte's foot processes associated with proteinuria. In most forms loss of
normal slit diaphragms is a key feature in the development of proteinuria.
2) Nephron Loss:
any renal disease destroying sufficient nephrons to the extent of reducing
the GFR to 30%-50% of normal shows relentless progression to end-stage renal failure.
Such individuals develop proteinuria, and their kidneys show widespread
glomerulosclerosis. The latter is initiated by the adaptive hypertrophy of the remaining
unaffected glomeruli to maintain renal function. These adaptations ultimately lead to
further endothelial and epithelial cell injury, which is followed by capillary collapse and
obliteration, increased deposition of mesangial matrix, and eventually by segmental or
global sclerosis of glomeruli. This results in further reductions in the nephron mass and a
vicious cycle of continuing glomerulosclerosis.
Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
88
The nephrotic syndrome
This refers to a clinical complex that includes
1. Massive proteinuria i.e. daily urine protein loss of 3.5 gm or more in adults
2. Hypoalbuminemia i.e. plasma albumin levels less than 3 gm/dl
3. Generalized edema 4. Hyperlipidemia and lipiduria
The initial event is a derangement in the glomerular capillary walls that leads to increased
permeability to plasma proteins allowing their escape from the plasma into the glomerular
filtrate. With long-standing or extremely heavy proteinuria, serum albumin is decreased,
resulting in hypoalbuminemia. The generalized edema is, in turn, a consequence of the
drop in plasma colloid osmotic pressure as a result of hypoalbuminemia, and retention of
salt and water by the kidney. As fluid escapes from the vascular tree into the tissues, there
is a concomitant drop in plasma volume, with diminished glomerular filtration.
Compensatory secretion of aldosterone, along with the reduced GFR and reduction of
secretion of natriuretic peptides, promotes retention of salt and water by the kidneys, thus
further aggravating the edema. By repetition of this chain of events, generalized edema
(anasarca) may develop. It is possible that hypoalbuminemia triggers increased synthesis
of lipoproteins in the liver & thus hyperlipidemia. The lipiduria reflects the increased
permeability of the GBM to lipoproteins.
The relative frequencies of the several causes of the nephrotic syndrome vary according
to age. In children 1 to 7 years of age, the nephrotic syndrome is almost always caused by
a primary kidney disease, whereas among adults it is often due to renal manifestations of
a systemic disease. The most frequent systemic causes of the nephrotic syndrome in
adults are 1. Diabetes
2. Amyloidosis
3. SLE
The most important primary glomerular lesions that lead to the nephrotic syndrome are
1. Focal and segmental glomerulosclerosis (FSGS), which is more important in adults.
2. Minimal-change disease (MCD), which is more important in children
3. Membranous nephropathy 4. Membranoproliferative GN
Minimal-Change Disease (MCD) (Lipoid Nephrosis)
This is the most frequent cause of the nephrotic syndrome in children, mostly between
ages 1 and 7 years (65%). The glomeruli have a normal appearance under light
microscopy; however, electron microscopy shows diffuse effacement of podocyte foot
processes. This is presumably due to a T-cell derived factor that causes podocyte damage.
The cells of the proximal convoluted tubules are often heavily loaded with protein droplets
& lipids secondary to tubular reabsorption of the leaking lipoproteins. The renal function
is preserved in most individuals. The protein loss is confined to the smaller serum proteins
, chiefly albumin (selective proteinuria). The prognosis in children with this disorder is
generally good; more than 90% of cases respond to a short course of corticosteroid therapy
Focal and Segmental Glomerulosclerosis (FSGS)
Is characterized histologically by sclerosis (fibrosis) affecting some but not all glomeruli
(focal) & involving only segments of each affected glomerulus (segmental). FSGS is either
primary (idiopathic) or secondary. The former is a common cause of nephrotic syndrome in
adults (30%) & frequent cause in children (10%). The secondary form is seen with
1. HIV immunodeficiency or heroin abuse (HIV nephropathy, heroin nephropathy)
2. IgA nephropathy 3. Maladaptation after nephron loss (see above)
4. Mutations affecting cytoskeletal proteins of podocytes (e.g., nephrin) (inherited form)
Unlike MCD, there is a higher incidence of hematuria and hypertension; the proteinuria is
nonselective, and the response to corticosteroid therapy is poor. At least 50% of
individuals with FSGS develop end-stage renal failure within 10 years of diagnosis. The

Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
89
pathogenesis of primary FSGS is unknown. As with MCD, permeability-increasing factors
produced by lymphocytes have been proposed. The entrapment of plasma proteins and
lipids occurs in foci of injury where sclerosis develops. FSGS initially affects focally the
juxtamedullary glomeruli. With progression, eventually all levels of the cortex are
affected. The affected segment of the glomerulus shows increased mesangial matrix,
obliterated capillary lumens, and deposition of hyaline masses. (Fig. 8-3) In time,
progression of the disease leads to global sclerosis of the glomeruli with secondary tubular
atrophy & interstitial fibrosis. About 50% of individuals suffer renal failure after 10 years.
Membranous Nephropathy (Membranous Glomerulonephritis, MGN)
This is a slowly progressive disease, most common in adults 30 to 50 years of age. The
disease is idiopathic (primary) in about 85% of cases. In the remainder it may be
secondary to other disorders, including:
1. Infections (chronic hepatitis B, syphilis, schistosomiasis, malaria)
2. Malignancy, particularly lung and colonic carcinomas and melanoma
3. Autoimmune diseases e.g. SLE 4. Exposure to inorganic salts (gold, mercury)
5. Drugs (penicillamin, captoril, NSIAD)
MGN is a chronic immune complex nephritis. Most idiopathic forms are considered
autoimmune disease caused by antibodies to renal GBM autoantigen. There seems to be a
direct action of C5b-C9 (the membrane attack complex) on the podocyte and mesangial
cells, inducing them to liberate proteases and oxidants that can cause the damage. The
basic change microscopically is diffuse thickening of the GBM (Fig. 8-4). By electron
microscopy, this thickening is caused by subepithelial deposits that are separated from
each other by small, spike-like protrusions of GBM matrix ("spike and dome" pattern);
these can be highlighted by silver stains. With progression, these spikes close over the
deposits, to incorporate them into the GBM. Podocytes show effacement of foot
processes. Eventually the glomeruli become gradually sclerosed. Immunofluorescence
microscopy shows typical granular deposits of immunoglobulins and complement along
the GBM. The proteinuria is nonselective, involving globulins & albumin molecules.
Overall, only 40% suffer progressive disease terminating in renal failure after 2-20 years.
Membranoproliferative Glomerulonephritis (MPGN)
This is manifested histologically not only by GBM thickening but also by proliferation of
glomerular cells. It accounts for up to 10% of cases of primary nephrotic syndrome in
children and adults. Some individuals present only with hematuria or subnephrotic
proteinuria; others have a combined nephrotic-nephritic picture. Two major types of
MPGN (I and II) are recognized; type I is far more common (80% of cases). Different
pathogenic mechanisms are involved in the development of type I and type II disease.
Most cases of type I MPGN seem to be caused by circulating immune complexes, but the
inciting antigen is not known (primary); it also occurs as a secondary form in association
with hepatitis B and C antigenemia, SLE, & extra-renal infections. The fundamental
abnormality in type II appears to be excessive complement activation. By light
microscopy, both types of MPGN are similar. The glomeruli are large, with an accentuated
lobular appearance, and proliferation of mesangial and endothelial cells as well as
leukocytic infiltration (Fig. 8-5). The GBM is thickened, and the glomerular capillary wall
often shows a double contour, or "tram track," appearance, especially evident in silver or
periodic acid-Schiff (PAS) stains. This is caused by "splitting" of the GBM due to the
inclusion within it of processes of mesangial and inflammatory cells. Types I & II have
different ultrastructural & immunofluorescence microscopic features. The prognosis of
MPGN is generally poor in that 40% of the cases progressed to end-stage renal failure.
Type II MPGN (also called dense-deposit disease) has a worse prognosis.

Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
90
The nephritic syndrome
This is a clinical complex, usually of acute onset, characterized by
1. Hematuria
2. Oliguria with azotemia
3. Hypertension
The lesions that cause the nephritic syndrome have in common proliferation of the cells
within the glomeruli, accompanied by leukocytic infiltrate. This inflammatory reaction
injures the capillary walls, permitting escape of red cells into the urine, and induces
hemodynamic changes that lead to a reduction in the GFR. The reduced GFR is
manifested clinically by oliguria, fluid retention, and azotemia. Hypertension is the result
of both the fluid retention and excessive renin release. The acute nephritic syndrome may
be secondary to a systemic disorders such as SLE, or it may be the result of primary
glomerular disease e.g. acute postinfectious GN.
Acute postinfectious (Poststreptococcal) GN
Is typically associated with streptococcal infection, but other infectious agents may be
responsible. The latter include certain pneumococcal and staphylococcal infections, as
well as some common viral diseases such as mumps, measles, chickenpox, and hepatitis
B and C. The classic case of poststreptococcal GN develops in a child 1 to 4 weeks after
recovery from "nephritogenic" strains of β-hemolytic, group A streptococcal infection,
usually of the pharynx or skin. Immune complex deposition is involved in the
pathogenesis. The relevant antigens are probably streptococcal proteins. Serum
complement levels are low and serum anti-streptolysin O antibody titers are elevated.
Characteristically the histology shows a uniformly increased cellularity of the glomerular
tufts that affects nearly all glomeruli (Fig. 8-6). This increased cellularity is caused both
by proliferation and swelling of both endothelial and mesangial cells as well as by a
neutrophilic and monocytic infiltrate. Electron microscopy shows subepithelial "humps".
Immunofluorescence displays these immune complexes as scattered granular deposits of
IgG and complement within the capillary walls. Recovery occurs in most children.
Conversely up to 50% of adults develop end-stage renal disease.
IgA Nephropathy (Berger Disease)
Usually affects children and young adults as an episode of gross hematuria occurring
within 1 or 2 days of a nonspecific upper respiratory tract infection, to lasts several days
and then subsides, only to recur every few months. IgA nephropathy is one of the most
common causes of recurrent microscopic or gross hematuria and is the most common
glomerular disease revealed by renal biopsies worldwide. The pathogenic hallmark is the
deposition of IgA in the mesangium. IgA nephropathy may be viewed as a localized
(renal) variant of Henoch-Schönlein purpura, which is also characterized by IgA
deposition in the mesangium but it is a systemic syndrome characterized by purpuric
rash, abdominal pain and arthritis. Microscopically, the glomeruli may be normal or show
one of the following:
1. Focal proliferative GN
2. Diffuse mesangioproliferative GN
3. Crescentic GN (rare).
The characteristic immunofluorescence picture is of mesangial deposition of IgA.
Electron microscopy shows mesangial electron-dense deposits. Serum IgA is increased in
50% of patients due to its increased production in the marrow presumably in response to
respiratory or GIT exposure to viruses, bacteria, or food proteins. The deposition of IgA
and IgA-immune complexes in the mesangium activate the alternative complement
pathway and initiate glomerular injury. Slow progression to chronic renal failure occurs
in up to 50% of cases.
Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
91
Rapidly Progressive (Crescentic) Glomerulonephritis (RPGN; CrGN)
This is a clinical syndrome associated, irrespective of the etiology, with glomerular
crescents; these are produced by proliferation of the parietal epithelial cells associated
with infiltration by monocytes and macrophages (Fig. 8-8). CrGN is characterized
clinically by 1. Rapidly progressive loss of renal function 2. Nephritic syndrome
3. Severe oliguria (often) 4. Death from renal failure within weeks to months (if untreated).
A practical classification divides CrGN into 3 groups on the basis of immunologic findings.
In each group, the disease may be associated with a known disorder or it may be idiopathic.
1) Type I (Anti-GBM Antibody)
a. Idiopathic
b. Goodpasture syndrome
This group is characterized by linear IgG deposits along the GBM. In some of these
individuals the anti-GBM antibodies also bind to pulmonary alveolar capillary basement
membranes to produce the clinical picture of pulmonary hemorrhages associated with renal
failure (Goodpasture syndrome). In idiopathic cases the renal involvement occurs in the
absence of pulmonary disease. Anti-GBM Abs are present in the serum & are helpful in
diagnosis. Immunofluorescence is characteristic with strong linear staining of deposited
IgG & C3 along the GBM; these deposits are not visualized by electron microscopy.
2) Type II (Immune Complex)
a. Idiopathic b. Postinfectious/infection related
c. SLE d. Henoch-Schönlein purpura/IgA nephropathy
In all of these cases, immunofluorescence shows the characteristic granular pattern of
staining of the GBM and/or mesangium for immunoglobulin and/or complement. In
addition to the crescents, segments of glomeruli show evidence of the underlying GN.
3) Type III (Pauci-Immune) ANCA Associated
a. Idiopathic b. Wegener granulomatosis c. Microscopic angiitis
This group is defined by the lack morphologically of both anti-GBM antibodies and
immune complex deposition. Most of these individuals have antineutrophil cytoplasmic
antibodies (ANCA) in the serum. The latter have a role in some vasculitides. Therefore,
in some cases type there is a component of a systemic vasculitis. In the idiopathic form
the disease is limited to the kidney. The uninvolved segments of glomeruli appear
normal. Immunofluorescence studies for immunoglobulin and complement are negative
(cf. type I and II), and there are no ultrastructural deposits.
The onset of RPGN is much like that of the nephritic syndrom. Proteinuria sometimes
approaching nephrotic range may occur. The prognosis can be related to the number of
crescents; those with crescents in less than 80% of the glomeruli have a better prognosis
than those with higher percentages of crescents.
Chronic glomerulonephritis
Is one of the outcomes of the various glomerular diseases already discussed (Fig. 8-9). It
is an important cause of end-stage renal disease. Among all individuals who require
chronic hemodialysis or renal transplantation, 30% to 50% have the diagnosis of chronic
GN. By the time chronic GN is discovered, the glomerular changes are so advanced that
it is difficult to detect the nature of the original lesion. Classically, the kidneys are
symmetrically contracted with red-brown surface. Microscopically, the feature common
to all cases is advanced scarring of the glomeruli, sometimes to in the point of complete
sclerosis (Fig. 8-10). There is relentless progression to uremia and death. The rate of
progression is extremely variable. Renal dialysis and kidney transplantation alter this
course and allow long-term survival.

Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
92
Tubulo-interstitial diseases
Most forms of tubular injury also involve the interstitium, so the 2 are discussed together.
Under this heading are 2 categories 1) Inflammatory involvement of the tubules & interstitium
(tubulo-interstitial nephritis) 2) Ischemic or toxic tubular necrosis leading to acute renal failure
Tubulointerstitial nephritis (TIN)
Is a group of inflammatory diseases of the kidneys primarily involving the interstitium &
tubules. The glomeruli are spared or affected late in the course. TIN is subdivided into
1. Bacterial TIN, the renal pelvis is prominently involved (pyelonephritis)
2. Nonbacterial TIN ; these include tubular injury resulting from
a. drugs b. metabolic disorders (e.g. hypokalemia) c. physical injury (e.g. irradiation)
d. viral infections e. immune reactions
Pyelonephritis
This is divided into acute and chronic forms
Acute pyelonephritis
o Is a common bacterial suppurative inflammation of the kidney (nephritis) and renal pelvis
(pyelitis); hence the term pyelonephritis. The great majority of cases of pyelonephritis are
associated with lower UTI (cystitis, prostatitis, and urethritis). E. coli is by far the most
common offender. Other important organisms are Proteus, Klebsiella, Enterobacter, and
Pseudomonas; these are usually associated with recurrent infections, especially due to
urinary tract instrumentations (e.g. cystoscopy, catheterization) or congenital or acquired
anomalies of the lower urinary tract (e.g. vesicoureteral reflux or polycystic kidney
disease, presence of stones, prostatic hyperplasia in the elderly).
o Pathogenesis
Bacteria can reach the kidneys either through the bloodstream (hematogenous) or from
the lower urinary tract (ascending infection). The former is exemplified by acute pyelo-
nephritis complicating septicemia or infective endocarditis. Ascending infection from the
lower urinary tract is the most common & is an important route by which bacteria reach
the kidney. The evolution of acute pyelonephritis occurs through the following steps
1) Bacterial adhesion to the urethral urothelium is influenced by genetically determined
properties of both the urothelium and the offending bacteria.
2) Gaining access to the bladder is by growth expansion of the colonies and by moving
against urine flow; the latter is overcome by urethral instrumentation, including
catheterization and cystoscopy. In the absence of instrumentation, UTI most commonly
affects females because of factors that facilitate entry of bacteria to the bladder; these are
a. Close proximity of the urethra to the enteric bacteria-loaded rectum favoring colonization
b. Short urethra c. Trauma to the urethra during sexual intercourse (honeymoon cystitis)
3) Outflow obstruction: normally, bladder urine is sterile as a result of the antimicrobial
properties of the bladder mucosa and the flushing action of periodic voiding of urine.
With outflow obstruction or bladder dysfunction, these natural defenses are
overwhelmed, setting the stage for UTI. Obstruction at the level of the urinary bladder
(e.g. by prostatic hyperplasia) results in incomplete bladder emptying and hence
increased residual volume of urine (urine stasis). In the presence of stasis, bacteria
introduced into the bladder can multiply freely, without being flushed out or destroyed by
the bladder mucosa. From the contaminated bladder urine, the bacteria ascend along the
ureters to infect the renal pelvis and parenchyma. Thus, UTI is particularly frequent in
association with benign prostatic hyperplasia in male and uterine prolapse in females.
UTI is also frequent in diabetics because of the increased susceptibility to infection and
neurogenic bladder, which in turn predisposes to urine stasis.
Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
93
4) Vesicoureteral reflux (VUR): incompetence of the vesicoureteral orifice allows bacterial
ascent along the ureter & then into the pelvis. The normal ureteral insertion into the bladder
is a competent one-way valve that prevents retrograde flow of urine, especially during
micturition, when the intra-vesical pressure rises. An incompetent vesicoureteral orifice
allows the reflux of bladder urine into the ureters; this is termed vesicoureteral reflux
(VUR). Up to 40% of young children with UTI have this anomaly, which is usually a
congenital defect. VUR can also be acquired in individuals with a flaccid bladder resulting
from spinal cord injury & with neurogenic bladder dysfunction secondary to diabetes. The
effect of VUR is similar to that of an obstruction in that after voiding there is residual urine
in the UT, which favors bacterial growth. Furthermore, VUR affords a ready mechanism by
which the infected bladder urine can be pushed up to the renal pelvis & further into the
renal parenchyma through open ducts at the tips of the papillae (intrarenal reflux).
o Gross features of acute pyelonephritis
One or both kidneys may be involved. The affected kidney may be normal in size or enlarged
Characteristically, discrete, yellowish, raised abscesses are grossly apparent on the renal
surface. They are variably scattered & may coalesce to form a single large abscess.
When obstruction is prominent, the pus may fill the renal pelvis, calyces, and ureter,
producing pyonephrosis.
o Microscopic features
The characteristic feature of acute pyelonephritis is suppurative inflammation within the
renal parenchyma. Both the tubules & interstitium are involved. (Fig. 8-12 B).
Large numbers of intratubular neutrophils frequently extend into the collecting ducts,
giving rise to the characteristic white cell casts found in the urine.
Typically, the glomeruli are not affected.
o Papillary necrosis (necrotizing papillitis)
This is an infrequent form of pyelonephritis, which may be encountered
1. In diabetics 2. With significant urinary tract obstruction
3. Chronic interstitial nephritis associated with analgesic abuse
This lesion consists of ischemic and suppurative necrosis of the renal papillae (tips of the
renal pyramids). The pathognomonic gross feature is sharply defined gray-white to
yellow necrosis of the apical two-thirds of the pyramids. The involvement ranges from
one to all papillae. (Fig. 8-13) Microscopically, the papillae show characteristic
coagulative necrosis, with surrounding neutrophilic infiltrate.
Chronic Pyelonephritis (CPN) and Reflux Nephropathy
o For the pathological diagnosis of CPN two criteria must be met
1. Grossly visible scarring deformity of the pelvicalyceal system
2. Microscopic predominance of interstitial inflammation & fibrosis
o CPN is an important cause of chronic renal failure. It can be divided into two forms:
1. Chronic obstructive pyelonephritis: recurrent infections superimposed on obstructive
lesions lead to recurrent bouts of renal inflammation and scarring, which eventually cause
chronic pyelonephritis. The disease can be bilateral, as with congenital anomalies of the
urethra (posterior urethral valves), or unilateral, such as occurs with calculi and unilateral
obstructive lesions of the ureter.
2. Chronic reflux-associated pyelonephritis is the more common form and results from
superimposition of a UTI on congenital vesicoureteral reflux and intrarenal reflux. Reflux
may be unilateral or bilateral; thus, the resultant renal damage is either unilateral or bilateral.
o Gross features (Fig. 8-14 A).
One or both kidneys may be involved, either diffusely or in patches. Even when
involvement is bilateral, the kidneys are not equally damaged and therefore are not
Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
94
equally contracted. This uneven involvement is useful in differentiating CPN from the
more symmetrically contracted kidneys of benign nephrosclerosis and chronic GN.
The hallmark of CPN is scarring involving the pelvis &/or calyces leading to papillary
blunting and marked calyceal deformities
o Microscopic features (Fig. 8-14 B)
These are largely non-diagnostic since similar alterations may be seen with other tubulo-
interstitial disorders such as analgesic nephropathy.
The parenchyma shows the following features:
- Interstitial fibrosis with infiltration by lymphocytes, plasma cells & sometimes neutrophils
- Dilation or contraction of tubules, with atrophic lining epithelium. Many of the dilated
tubules contain pink to blue colloid-like casts; the overall appearance is reminiscent of
thyroid tissue, hence the descriptive term thyroidization.
Chronic inflammation with fibrosis involving the pelvi-calyceal mucosa and wall. This is
an important feature that is used in the differentiation from other conditions that give
otherwise similar parenchymal changes.
Vascular changes of benign arteriolosclerosis caused by the frequently associated hp.
Although glomeruli may be normal, some are sclerosed (glomerulosclerosis). Such
changes represent maladaptive changes secondary to nephron loss.
o Absence of significant bacteriuria should not rule out CPN. If the disease is bilateral and
progressive, tubular dysfunction occurs with loss of concentrating ability, manifested by
polyuria and nocturia. Some persons with CPN or reflux nephropathy ultimately develop
glomerular lesions of global sclerosis and secondary FSGS. These are associated with
proteinuria and eventually contribute to progressive chronic renal failure.
Drug-Induced Interstitial Nephritis (DIN)
Drugs are important causes of renal injury. There are two forms of DITN:
1) Acute DIN
: this is most frequently occurs with such drugs as methicillin, ampicillin,
rifampin, thiazide diuretics, NSAID, phenindione, and cimetidine. Most likely, the drugs
act as haptens that bind to a cytoplasmic or extracellular component of the secreting
tubular cells and become immunogenic. The resultant tubulointerstitial injury is either
caused by IgE (type I) or cell-mediated (type IV) immune reactions to tubular cells. The
interstitium shows infiltration by principally lymphocytes and macrophages but
eosinophils and neutrophils may be present (Fig. 8-15). With some drugs (e.g.,
methicillin, thiazides, rifampin), interstitial non-necrotizing granulomas with giant cells
may be seen. The glomeruli are normal. NSAID may cause membranous GN-like
reaction associated with nephrotic syndrome. It is important to recognize drug-induced
renal damage, because withdrawal of the offending drug is followed by recovery.
2) Analgesic Nephropathy
: with the intake of large quantities of analgesics, patients may
develop chronic interstitial nephritis, often associated with renal papillary necrosis. Most
people who develop this nephropathy consume mixtures containing some combination of
aspirin, paracetamol, caffeine, and codeine for long periods. Papillary necrosis is the initial
event, and the interstitial nephritis in the overlying renal parenchyma is a secondary
phenomenon. Cessation of analgesic intake may stabilize or even improve renal function.
A complication of analgesic abuse is the increased incidence of transitional-cell carcinoma
of the renal pelvis or bladder in persons who survive the renal failure.
Acute Tubular Necrosis (ATN)
This is a clinicopathologic entity characterized acute renal failure due to necrosis of
tubular epithelial cells. It is the most common cause of acute renal failure. ATN is a

Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
95
reversible renal lesion that arises in clinical settings that have in common a period of
inadequate blood flow to the peripheral organs, often in the setting of marked
hypotension and shock. The pattern of ATN associated with shock is called ischemic
ATN. Hemolytic crises including mismatched blood transfusions, and myoglobinuria,
also produce a picture resembling ischemic ATN.
A second pattern, called nephrotoxic ATN, is caused by a variety of poisons, including
heavy metals (e.g., mercury); organic solvents (e.g., carbon tetrachloride); and drugs such
as gentamicin and other antibiotics, and radiographic contrast agents.
Pathogenesis
The decisive events in both ischemic and nephrotoxic ATN are believed to be
1. Tubular injury and 2. Severe disturbances in blood flow to tubular cells.
Tubular epithelial cells are sensitive to both anoxia & toxins.
Toxic injury eventuates in decreased Na
+
reabsorption by proximal tubules and hence
increased sodium delivery to distal tubules. The latter, through a tubulo-glomerular
feedback system, contributes to vasoconstriction and thus ischemia.
The debris resulting from shedding of tubular cells results can block urine outflow, and
eventually increases intratubular pressure, thereby decreasing GFR.
Additionally, fluid from the damaged tubules could leak into the interstitium, resulting in
increased interstitial pressure and collapse of the tubules.
Ischemic tubular cells also express chemokines, cytokines, and adhesion molecules that
recruit and immobilize leukocytes that can participate in tissue injury.
Ischemic renal injury is also characterized by severe hemodynamic alterations that cause
reduced GFR. The major one is intrarenal vasoconstriction, which results in both reduced
glomerular plasma flow and reduced oxygen delivery to the functionally important
tubules in the outer medulla.
Vasoconstriction is mediated by sublethal endothelial injury, leading to increased release
of the endothelial vasoconstrictor endothelin and decreased production of vasodilatory
nitric oxide and prostaglandins.
Pathological features
Ischemic ATN is characterized by
o Necrosis of short segments mostly of the proximal tubule; thus necrosis may be missed in
biopsy samples.
o Frequently a variety of tubular injuries are noted in the epithelial cells of proximal
convoluted tubules e.g. brush borders attenuation, blebbing and sloughing, vacuolization
and detachment of tubular cells into the urine.
o Presence of proteinaceous casts in the distal tubules and collecting ducts. They consist of
Tamm-Horsfall protein (secreted normally by tubular epithelium) along with hemoglobin
and other plasma proteins.
o When crush injuries have produced ATN, the casts are composed of myoglobin.
o The interstitium usually shows generalized edema along with a mild inflammatory
infiltrate consisting of polymorphonuclear leukocytes, lymphocytes, and plasma cells.
Toxic ATN (Fig. 8-16)
o The microscopic picture is basically similar, with some differences. Necrosis is more
diffuse but again most prominent in the proximal tubule, and the tubular basement
membranes are generally spared.
o If the patient survives for a week, epithelial regeneration becomes apparent in the form of a
low cuboidal epithelial covering & mitotic activity in the persisting tubular epithelial cells.
o Except where the basement membrane is destroyed, regeneration is total and complete.
Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
96
Diseases of renal blood vessels
Changes affecting renal blood vessels are both frequent & important for the following reasons
1. The renal vasculature is secondarily involved in almost all diseases of the kidney.
2. Various forms of systemic arteritis also involve renal vessels & such involvement is
clinically important. 3. The kidney is intimately involved in the pathogenesis of both
essential & secondary hypertension.
Benign Nephrosclerosis (BNS)
Some degree of BNS is present in many of those older than 60 years of age. The
frequency and severity of the lesions are increased at any age when hypertension or
diabetes mellitus are present. It is not clear whether BNS is the cause of hypertension or
conversely, hypertension just accelerates an age-related vascular sclerosis. However,
many renal diseases cause hypertension, which in turn is associated with BNS. Thus, this
renal lesion is often seen superimposed on other primary kidney diseases.
Gross features
: (Fig. 8-17)
The kidneys are symmetrically shrunken, with diffuse fine granularity of the surface.
Microscopical features
The basic change is hyaline thickening of the walls of the small arteries and arterioles
(hyaline arteriolosclerosis). This appears as a homogeneous, pink, hyaline thickening that
reduces the lumen (Fig. 8-17).
The narrowing of the lumen results in markedly decreased blood flow through the
affected vessels and thus producing ischemic atrophy of all structures of the kidney. In
advanced cases the glomeruli become globally sclerosed. Diffuse tubular atrophy and
interstitial fibrosis are often present. BNS rarely causes severe damage to the kidney.
However, all persons with this lesion usually show some functional impairment.
Malignant Nephrosclerosis
Malignant hypertension occurs in only about 5% of hypertensive patients. It may occur
de novo, or suddenly complicates mild hypertension.
Pathogenesis
The following sequence of events is suggested.
1) Initially, there is renal arteriolar vascular damage, mostly from long-standing benign
hypertension. The result is increased permeability of the small vessels to fibrinogen and
other plasma proteins, endothelial cell injury, and platelet deposition.
2) This leads to occurrence of fibrinoid necrosis of arterioles & small arteries with thrombosis.
3) Platelet-derived and other growth factors cause intimal smooth muscle hyperplasia that
results in hyperplastic arteriolosclerosis (typical of malignant hypertension) associated
with further narrowing of the vascular lumina. The kidneys become markedly ischemic.
4) With severe involvement of the renal afferent arterioles, the renin-angiotensin system is
stimulated. A self-perpetuating cycle is thus created in which angiotensin II causes
intrarenal vasoconstriction, and the resultant renal ischemia stimulates renin secretion.
5) Aldosterone levels are elevated; the salt retention contributes to the elevation of blood pressure.
The consequences of the markedly elevated blood pressure on the blood vessels
throughout the body are known as malignant arteriolosclerosis, and the renal disorder is
referred to as malignant nephrosclerosis.
Gross feature
: The kidneys, which may be normal in size or slightly shrunken display
small, pinpoint petechial hemorrhages on the cortical surface due to rupture of arterioles or
glomerular capillaries. These give the kidney a peculiar, flea-bitten appearance (Fig. 8-18).

Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
97
Microscopic:
(Fig. 8-18)
There is fibrinoid necrosis of the arterioles. The vessel walls show a homogeneous,
granular eosinophilic appearance.
In the small arteries and large arterioles, proliferation of intimal smooth muscle cells
produces hyperplastic arteriolosclerosis in which the intimal smooth muscle cells show
concentric arrangement (onion-skin appearance). This lesion causes marked narrowing of
arterioles and small arteries, to the point of total obliteration.
Necrosis may also involve glomeruli, with microthrombi within the glomeruli & necrotic
arterioles.
The full-blown syndrome of malignant hypertension is characterized by diastolic
pressures greater than 120 mm Hg, papilledema, encephalopathy, cardiovascular
abnormalities, and renal failure. At the onset of rapidly rising blood pressure there is
marked proteinuria and microscopic, or sometimes macroscopic, hematuria followed
soon by renal failure. The syndrome is a true medical emergency. About 50% of patients
survive at least 5 years. Ninety percent of deaths are caused by uremia and the other 10%
by cerebral hemorrhage or cardiac failure.
Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
98
Cystic diseases of the kidney
These are a heterogeneous group comprising
a. hereditary
b. developmental but nonhereditary
c. acquired disorders.
They are important for several reasons: 1) They are practically common & often present
diagnostic problems. 2) Some are major causes of chronic renal failure (adult polycystic
disease). 3) They can occasionally be confused clinically with malignant tumors.
Simple Cysts
Are a common but have no clinical significance. They can be multiple or single,
commonly up to 5 cm in diameter. They are translucent; filled with clear fluid; lined by a
single layer of cuboidal or flattened epithelium.
Dialysis-associated acquired cysts
occur with prolonged dialysis in those with end-
stage renal disease. They may bleed, causing hematuria. Occasionally, renal adenomas or
carcinomas arise in the walls of these cysts.
Autosomal Dominant (Adult) Polycystic Kidney Disease (ADPKD)
Is characterized by multiple expanding cysts of both kidneys that ultimately destroy the
intervening parenchyma. ADPKD is responsible for 10% of all chronic renal failures. It
is caused by inheritance of one of two autosomal dominant genes of very high
penetrance. The kidneys may be very large (up to 4 kg for each), and thus are readily
palpable abdominally. Grossly the kidney is composed of a mass of cysts of varying
sizes (up to 4 cm). The cysts are filled with fluid (clear, turbid, or hemorrhagic) (Fig 8-
19). Microscopically, the cysts have often atrophic, lining. The pressure of the expanding
cysts leads to ischemic atrophy of the intervening renal substance. Evidence of
superimposed hypertension or infection is common. ADPKD in adults usually does not
produce symptoms until the fourth decade, by which time the kidneys are quite large.
Intermittent gross hematuria commonly occurs. The most important complications are
hypertension and urinary infection. Saccular aneurysms of the circle of Willis are present
in up to 30% of patients, and these individuals have a high incidence of subarachnoid
hemorrhage. Asymptomatic liver cysts occur in one-third of patients.
Autosomal Recessive (Childhood) Polycystic Kidney Disease (ARPKD)
Is a rare developmental anomaly that is genetically distinct from ADPKD. Perinatal,
neonatal, infantile, and juvenile subcategories have been defined, depending on time of
presentation and the presence of associated hepatic lesions. Both kidneys are invariably
involved with numerous small cysts that give them a sponge-like appearance (Fig. 8-20).
The cysts are lined by cuboidal cells. ARPKD is associated with multiple epithelium-
lined cysts in the liver. Young infants may die quickly from hepatic or renal failure.
Medullary Cystic Disease (MCD)
This is of two major types of
1)
Medullary sponge kidney
a relatively common and usually harmless condition and
2)
Nephronophthisis-medullary cystic disease complex
, which is associated with renal
dysfunction. On the basis of the time of onset they are divided into, infantile, juvenile
(the most common), adolescent, and adult types. They may be associated with other
extra-renal abnormalities including cerebellar malformations, and liver fibrosis.
Pathologic features of medullary cystic disease include small contracted kidneys with
numerous small cysts lined by flattened or cuboidal epithelium, typically at the cortico-
medullary junction (Fig. 14- ). Progression to end-stage renal disease occurs within a 5-
to 10-year period. The disease is difficult to diagnose, since the cysts may be too small to
be seen with imaging techniques and may not be apparent on renal biopsy if the cortico-
medullary junction is not well sampled.

Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
99
Urinary outflow obstruction
Renal Stones (Urolithiasis)
These are frequent (1% of all autopsies) & mostly form in the kidney. The majority
(80%) are composed of either calcium oxalate or calcium oxalate mixed with calcium
phosphate; 10% are of magnesium ammonium phosphate & the remainder 10% are either
uric acid or cystine stones. The most important cause is increased urine concentration of
the stone's constituents (supersaturation). Those who develop calcium stones have
1. Hypercalciuria not associated with hypercalcemia due to either
a. Absorption of calcium from the gut in excessive amounts (i.e. absorptive hypercalciuria)
b. Primary renal defect of calcium reabsorption (renal hypercalciuria).
Hypercalcemia is present in only 5% to 10% (due to hyperparathyroidism, vitamin D
intoxication, or sarcoidosis) and consequent hypercalciuria. In 20% of this subgroup, there
is excessive excretion of uric acid in the urine, which favors calcium stone formation; the
urates provide a nidus for calcium deposition. A high urine pH favors crystallization of
calcium phosphate and stone formation. Magnesium ammonium phosphate stones almost
always occur in persistently alkaline urine due to UTIs, particularly with urea-splitting
bacteria (as Proteus vulgaris and the Staphylococci). Gout and diseases involving rapid
cell turnover (as the leukemias), lead to high uric acid levels in the urine and uric acid
stones. About half of the individuals with uric acid stones, however, have no
hyperuricemia but unexplained tendency to excrete persistently acid urine (under pH 5.5),
which favors uric acid stone formation (cf. stones containing calcium phosphate).
Common sites of formation are renal pelvis and calyces as well as the bladder. Stone may
be small with smooth or jagged surface. Occasionally, progressive accumulation of salts
leads to the development of branching structures known as staghorn calculi, which create
a cast of the renal pelvis and calyceal system (Fig. 8-22). These massive stones are
usually composed of magnesium ammonium phosphate; these do not produce symptoms
or significant renal damage. Smaller stones may pass into the ureter, producing a typical
intense pain known as renal colic. Often at this time there is gross hematuria. The clinical
significance of stones lies in their capacity to obstruct urine flow or to produce sufficient
trauma to cause ulceration and bleeding. In either case, they predispose the sufferer to
bacterial infection.
Hydronephrosis
Refers to dilation of the renal pelvis and calyces, with accompanying atrophy of the renal
parenchyma, caused by obstruction to the outflow of urine. The obstruction may be
sudden or insidious, and it may occur at any level of the urinary tract, from the urethra to
the renal pelvis.
The most common causes are
1. Congenital: e.g. urethral atresia, ureteric or urethral valves, aberrant renal artery
2. Acquired:
a. Foreign bodies: calculi
b. Tumors of the prostate or bladder (benign or malignant)
c. Contiguous malignant disease: (retroperitoneal lymphoma, carcinoma of the cervix or uterus)
d. Inflammation: prostatitis, ureteritis, urethritis
e. Neurogenic: Spinal cord damage with paralysis of the bladder
f. Normal pregnancy: mild and reversible

Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
100
Bilateral hydronephrosis occurs only when the obstruction is below the level of the
ureters. If blockage is at the ureters or above, the lesion is unilateral. Sometimes
obstruction is complete, allowing no urine to pass; usually it is only partial.
Pathogenesis
Even with complete obstruction, glomerular filtration persists for some time, and the
filtrate subsequently diffuses back into the lymphatic and venous systems. Because of the
continued filtration, the affected calyces and pelvis become dilated, often markedly so.
The unusually high pressure thus generated in the renal pelvis, as well as that transmitted
back through the collecting ducts, causes compression of the renal blood vessels. Both
arterial insufficiency and venous stasis result. The most severe effects are seen in the
papillae, because they are subjected to the greatest increases in pressure. Accordingly, the
initial functional disturbances are largely tubular, manifested primarily by impaired
concentrating ability. Thereafter the glomerular filtration begins to diminish. Serious
irreversible damage occurs in about 3 weeks with complete obstruction, and in 3 months
with incomplete obstruction.
Gross features
The changes vary with the degree and speed of obstruction
- With subtotal or intermittent obstruction, the kidney is massively enlarged consisting
almost entirely of the greatly distended pelvicalyceal system. The renal parenchyma shows
compression atrophy, with obliteration of the papillae & flattening of the pyramids.
- With sudden complete obstruction, glomerular filtration is reduced relatively early and
as a consequence, renal function may cease while dilation is still slight.
Depending on the level of the obstruction, 1 or both ureters may also be dilated (hydroureter).
Microscpic features
Early lesions show tubular dilation, followed by atrophy and fibrous replacement with
relative sparing of the glomeruli. Eventually, in severe cases the glomeruli also become
atrophic and disappear, converting the entire kidney into a thin shell of fibrous tissue.
With sudden & complete obstruction, there may be coagulative necrosis of the renal papillae.
In uncomplicated cases the accompanying inflammatory reaction is minimal.
Complicating pyelonephritis, however, is common.
Course & prognosis
Bilateral complete obstruction produces anuria. When the obstruction is below the bladder,
there is bladder distention. Incomplete bilateral obstruction causes polyuria rather than
oliguria, as a result of defects in tubular concentrating mechanisms. Unilateral
hydronephrosis may remain completely silent. Removal of obstruction within a few weeks
usually permits full return of function; however, with time the changes become irreversible

Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
101
Tumors
The kidney is the site of benign and malignant tumors. In general, benign tumors (such as
small cortical adenomas or medullary fibromas) have no clinical significance. The most
common malignant tumors of the kidney in descending order of frequency are
1. Renal cell carcinoma 2. Nephroblastoma (Wilms tumor)
3. Transitional cell carcinoma of the calyces and pelvis
Renal Cell Carcinoma (RCC)
This is derived from the renal tubular epithelium and represents the most common
primary malignant tumor of the kidney (80%). The mea age of incidence is 50 to 70
years. Risk factors include smoking, hypertension, obesity, occupational exposure to
cadmium, and acquired polycystic disease complicating chronic dialysis (30-fold). RCC
are currently classified according to the molecular origins of these tumors.
The three most common forms are
1)
Clear Cell Carcinomas
(80% of RCCs): these are made up of cells with clear or
granular cytoplasm. The majority are sporadic, but may occur in familial forms or in
association with von Hippel-Lindau disease (VHLD). The latter is autosomal dominant
and characterized by predisposition to a variety of neoplasms including bilateral & often
multiple RCC of clear cell type. Patients with VHLD inherit a germ-line mutation of the
VHL gene on chromosome 3 and lose the second allele by somatic mutation. Thus, the
loss of the normal copies of both these tumor suppressor genes gives rise to clear cell
carcinoma. It has been also confirmed that homozygous loss of the VHL gene seems to be
the common underlying molecular abnormality in both sporadic and familial forms of
clear cell carcinomas.
2) Papillary Renal Cell Carcinomas
(10%). These tumors are frequently multifocal and
bilateral and like clear cell carcinomas, they occur in familial and sporadic forms.
However, they have no abnormality of chromosome 3. The cause is the MET proto-
oncogene. It is the increased dosage of the MET gene (due to duplications of
chromosome 7) seems to encourage neoplastic growth abnormal growth in the proximal
tubular epithelial cell precursors. In support of this, trisomy of chromosome 7 is seen
commonly in the familial cases. In the sporadic cases, although there is duplication of
chromosome 7, there is no mutation of the MET gene.
3) Chromophobe RCCs
: these are the least common; they arise from intercalated cells of
collecting ducts. Their name indicates that the tumor cells stain more darkly than cells in
clear cell carcinomas. These tumors are unique in having multiple losses of entire
chromosomes, including chromosomes 1 and 2. In general, they have a good prognosis.
Pathologic features
Clear cell carcinoma
o Gross features: (Fig. 8-24 A)
There is usually solitary spherical mass; large when symptomatic.
The cut surface is variegated; yellow-orange to gray-white with red areas of hemorrhage.
There are prominent areas of cystic degeneration. Although the margins of the tumor are
well defined, with enlargement extension may occur in two directions
1. Into the pelvicalyceal system & as far down as the ureter.
2. Into the renal vein, then the inferior vena cava & even into the right side of the heart.
o Microscopic features
The classic lipid- & glycogen-laden clear cells have well defined cell membranes. The
nuclei are usually small and round (Fig. 8-24 B).
Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
102
These cells are mixed to varying extent with cells having granular pink cytoplasm.
Some tumors exhibit marked degrees of anaplasia, with numerous mitotic figures and
markedly enlarged, hyperchromatic, pleomorphic nuclei (sarcomatoid RCC).
The stroma is usually scant but highly vascularized.
Papillary renal cell carcinomas
Exhibit papilla formation with fibrovascular cores.
They tend to be bilateral and multiple.
The cells can have clear or, more commonly, pink cytoplasm.
The classical triad of painless hematuria (the most frequent), a palpable abdominal mass,
and dull flank pain is characteristic. RCCs are well-known for their association with
several paraneoplastic syndromes. Polycythemia may occur; it results from excessive
secretion of erythropoietin by the tumor. Uncommonly, these tumors also produce a
variety of hormone-like substances, resulting in hypercalcemia, hypertension, Cushing
syndrome, etc. In many individuals the primary tumor remains silent and is discovered
only after its metastases have produced symptoms. Common locations for metastases are
the lungs and the bones. Forty percent of patients die of the disease.
Wilms Tumor (WT) (nephroblastoma)
This is not only the most common primary renal tumor of children (mostly between the
ages of 2 and 5 years) but also the third most common organ cancer in those younger than
10 years. WT may arise sporadically or be familial. Some congenital malformations are
associated with an increased risk of developing Wilms' tumor such as aniridia, mental
retardation, gonadal dysgenesis and renal abnormalities. These conditions are associated
with inactivation of the Wilms' tumor 1 (WT1) gene, located on chromosome11. This gene
is critical to normal renal and gonadal development. Patients with the Beckwith syndrome
(BWS); characterized by enlargement of individual body organs (e.g., tongue, kidneys, or
liver) or hemihypertrophy of the entire body segments. In addition to Wilms' tumors,
patients with
BWS
are also at increased risk for developing other cancers e.g.
hepatoblastoma
Gross features
WT tends to be large, well-circumscribed mass with soft, homogenous, tan to gray cut
section accentuated with occasional foci of hemorrhage, necrosis and cystic degeneration.
Microscopic features
In WT there are attempts to recapitulate different stages of nephrogenesis.
WT contain a variety of tissue components, but all derived from the mesoderm.
The classic triphasic combination of blastemal, stromal, and epithelial cell types is
observed in most lesions (Fig. 14-25 B).
- The blastemal component is represented by sheets of small blue cells
- The epithelial component (differentiation) is represented by abortive tubules or glomeruli.
- The stromal component is represented usually by fibroblastic cells or myxoid areas,
although skeletal muscle "differentiation" and other mesenchymal elements may be seen.
The presence of anaplasia correlates with underlying p53 mutations, and the emergence
of resistance to chemotherapy.
Clinically, there is a readily palpable abdominal mass, which may extend across the
midline and down into the pelvis. Fever and abdominal pain, with hematuria, are less
frequent. The prognosis of Wilms' tumor is generally very good, and excellent results are
obtained with a combination of nephrectomy and chemotherapy. WTs with diffuse
anaplasia, especially those with extra-renal spread, have the least favorable outcome.
Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
103
Renal pelvis, ureter, urinary bladder & urethra
Ureters
Congenital Anomalies
These are rare, and mostly of little clinical significance. However, some may contribute
to urine outflow obstruction. Incompetent ureterovesical junction predisposes to
pyelonephritis. The majority of double ureters are unilateral and of no clinical
significance. A congenital ureteropelvic junction obstruction is the most common cause
of hydronephrosis in infants and children. There is agenesis of the kidney on the opposite
side in a significant number of cases
.
Diverticula are saccular outpouchings acting as
pockets of stasis and secondary infections. Hydroureter with elongation and tortuosity
may be congenital leading to hydronephrosis if untreated.
Ureteritis may be one component of UTI. With long-standing chronic ureteritis, there
may be aggregation of lymphocytes in the subepithelial region causing fine granular
mucosal surface (ureteritis follicularis), or the mucosa may become sprinkled with tiny
cysts (ureteritis cystica). Identical changes are found in the bladder. The two most
common tumors and tumor-like lesions
are fibroepithelial polyps and leiomyomas.
Primary malignant tumors are similar to those arising in the renal pelvis, calyces, and
bladder; the majority are transitional cell carcinomas. They cause obstruction and are
most frequently during the sixth and seventh decades. They can be multiple and may
occur concurrently with similar tumors in the bladder or renal pelvis.
Obstructive lesions of the ureters
A great variety of pathologic lesions may obstruct the ureters & give rise to hydroureter,
hydronephrosis, and sometimes pyelonephritis. The more important causes include
1. impacted small stones 2. strictures (congenital or acquired),
3. primary carcinoma, 4. pregnancy,
5. retroperitoneal fibrosis & cancers of the rectum, bladder, prostate, ovaries, uterus & cervix.
Sclerosing Retroperitoneal Fibrosis
is a fibrous proliferative inflammatory process
encasing the retroperitoneal structures including the ureters and causing hydronephrosis.
Two thirds of the cases are idiopathic. The remaining cases are attributed to drugs (ergot
derivatives, β-adrenergic blockers), adjacent inflammatory conditions or malignant
disease. It may be associated with mediastinal fibrosis, sclerosing cholangitis, and Riedel
thyroiditis. This suggests that the disorder is systemic in distribution but preferentially
involves the retroperitoneum. An autoimmune reaction, sometimes triggered by drugs,
has been proposed.
Urinary bladder
Congenital anomalies
Diverticula
are pouch-like evaginations of the bladder wall that may be congenital, but
more commonly acquired due to persistent urethral obstruction (e.g. prostatic hyperplasia or
neoplasia). In both forms, there are frequently multiple sac-like pouches that range from
less than 1 cm to 10 cm in diameter. Most diverticula are small and asymptomatic, but may
be sites of urinary stasis that predispose to infection & the formation of bladder calculi.
Exstrophy
is a developmental failure in the anterior wall of the abdomen and the
bladder, so that the bladder communicates directly through a large defect with the surface
of the body. The exposed bladder mucosa may undergo colonic glandular metaplasia and
is subjected to infections. There is an increased risk of carcinoma.

Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
104
Vesicoureteral reflux
is the most common and serious anomaly that contributes to renal
infection and scarring.
Congenital fistulas
are abnormal connections between the bladder and the vagina,
rectum, or uterus.
Persistent urachus
refers to failure of the urachus to close in part or in whole. When it is
totally patent, a fistulous urinary tract is created that connects the bladder with the
umbilicus. Sometimes, only the central region of the urachus persists, giving rise to
urachal cysts. Carcinomas, mostly adenocarcinomas, may arise in such cysts.
Acute & chronic cystitis
Etiologic agents
o The common etiologic agents of bacterial cystitis are the E. coli, followed by Proteus, &
Klebsiella. Women are more likely to develop cystitis due to their shorter urethras.
Bacterial pyelonephritis is frequently preceded by cystitis, with retrograde spread of
microorganisms into the kidneys and their collecting systems. Tuberculous cystitis is
almost always a consequence of renal tuberculosis.
o Fungal cystitis is usually due to Candida albican. It is particularly seen in
immunosuppressed patients or those receiving long-term antibiotics.
o Schistosomal cystitis (Schistosoma haematobium) is common in certain Middle Eastern
countries, notably Egypt. Viruses (e.g., adenovirus), Chlamydia, and Mycoplasma may
also be causes of cystitis.
Predisposing factors of cystitis include
1. Urinary obstruction e.g. prostatic hyperplasia, bladder calculi,tumors
2. cystocele or diverticula 3. Diabetes mellitus
4. Instrumentation 5. Immune deficiency.
Hemorrhagic cystitis sometimes complicates cytotoxic antitumor drugs (e.g.
cyclophosphamide). Radiation cystitis is due to radiation of the bladder region. Most cases
of cystitis take the form of nonspecific acute or chronic inflammation of the bladder.
Gross features
o There is hyperemia of the mucosa.
o Hemorrhagic cystitis shows in addition a hemorrhagic component; this form is
sometimes follows radiation injury, antitumor chemotherapy, or adenovirus infection.
o Suppurative cystitis characterized by the accumulation of large amounts of suppurative exudate.
o Ulcerative cystitis refers to cystitis associated with ulceration of large areas of the
mucosa, or sometimes the entire bladder mucosa.
o Persistence of the infection leads to chronic cystitis, which shows red, friable, granular,
sometimes ulcerated mucosa. Chronicity is also associated with fibrous thickening and
inelasticity of the bladder wall.
Microscopic features
In acute cystitis there are the expected features of acute inflammation.
In chronic forms there is chronic inflammatory cells infiltration with fibrosis.
Variants of chronic cystitis include Follicular cystitis and Eosinophilic cystitis
Schistomal cystitis
: (Fig. 21-26)
Urogenital bilharziasis is caused by S. haematobium. Eggs are deposited in the superior
rectal vein. From there, they pass through anastomoses into the veins of the wall of
urinary bladder. There they cause granulomatous cystitis with eosinophilic infiltrate &
fibrosis. These granulomas are visible under endoscopy as minute granules referred to as
“sand grain” cystitis. The eggs eventually die in the tissue with regressive
calcification.The condition may be complicated by
Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
105
a. Extensive fibrosis that may impinge on the ureteric orifices with eventual hydronephrosis
b. Carcinoma of bladder that is frequently squamous in type; as this form of cystitis can
be associated with squamous metaplasia of the native transitional epithelium.
Special Forms of Cystitis
These are distinctive by either their morphologic appearance or their causation.
1) Interstitial Cystitis (Hunner Ulcer)
: a painful form of chronic cystitis occurring most
frequently in women. Cystoscopy shows fissures and punctate hemorrhages in the
mucosa, sometimes with chronic mucosal ulcers (Hunner ulcers). Infiltration by mast
cells is characteristic of this disease. The condition may be of autoimmune origin.
2) Malakoplakia
is characterized macroscopically by soft, yellow, slightly raised mucosal
plaques 3 to 4 cm in diameter and histologically by infiltration with large, foamy
macrophages with debris of bacterial origin (mostly E. coli) (Fig. 21-27). In addition,
laminated mineralized concretions (Michaelis-Gutmann bodies) are typically present.
Similar lesions have been described in other organs e.g. colon, lungs, bones. It occurs
with increased frequency in immuno-suppressed transplant recipients and as a result of
defects in phagocytic or degradative function of macrophages.
3) Polypoid Cystitis
is an inflammatory condition resulting from irritation to the bladder
mucosa mostly by indwelling catheters. The urothelium is thrown into broad, bulbous,
polypoid projections as a result of marked submucosal edema.
Metaplastic lesions
Cystitis Glandularis and Cystitis Cystica
: these terms refer to common lesions in
which nests of transitional epithelium (Brunn nests) grow downward into the lamina
propria and undergo transformation of their central epithelial cells into columnar
epithelium lining (cystitis glandularis) or cystic spaces lined by urothelium (cystitis
cystica). The two processes often coexist. In a variant of cystitis glandularis, goblet cells
are present (intestinal metaplasia).
Both variants are common microscopic incidental findings in relatively normal bladders
and are not associated with an increased risk of adenocarcinoma.
Two forms of metaplasia occur in response to injury
1. Squamous Metaplasia
2. Nephrogenic Metaplasia (Nephrogenic Adenoma): the urothelium may be focally
replaced by cuboidal epithelium, which can assume a papillary growth pattern with
subjacent tubular proliferation.
Tumors of the urinary bladder and collecting system (Renal Calyces, Renal
Pelvis, Ureter, and Urethra)
The entire urinary collecting system from renal pelvis to urethra is lined with transitional
epithelium, so its epithelial tumors assume similar morphologic patterns. Tumors in the
collecting system above the bladder are relatively uncommon; those in the bladder,
however, are a more frequent cause of death than are kidney tumors.
Gross features
Four morphologic patterns are recognized that range from small benign papillomas to
large invasive cancers. These tumors are classified into (Fig. 8-28)
1. Benign papilloma (rare) 2. Papillary urothelial neoplasms of low malignant potential
3. Urothelial carcinoma (low and high grade)
o Papillomas
are very rare, small (up to 1 cm) benign tumors with frondlike structures
having a delicate fibrovascular core covered by multilayered, well-differentiated
transitional epithelium. Such lesions are usually solitary. They rarely recur once removed.
Chapter 5 – Kidney & Its Collecting System, and Lower Urinary Tract
106
o Urothelial (transitional) cell carcinomas
range from papillary to flat, noninvasive to
invasive and low grade to high grade.
Low-grade carcinomas (Fig. 8-29) are always papillary and are rarely invasive, but they
may recur after removal.
High-grade carcinomas (Fig. 8-30) can be papillary or occasionally flat; they may cover
larger areas of the mucosal surface, invade deeper, and have a shaggier necrotic surface
than do low-grade tumors. Occasionally, these cancers show foci of squamous cell
differentiation, but only 5% of bladder cancers are true squamous cell carcinomas.
Carcinomas of grades II and III infiltrate surrounding structures, spread to regional nodes,
and, on occasion, metastasize widely.
Painless hematuria is the dominant
clinical presentation
of all these tumors. They affect
men 3 times more frequently than women & usually develop between the ages of 50-70 years.
Risk factors of bladder cancer are
1. Exposure to β-naphthylamine (50 times increased risk).
2. Cigarette smoking
3. Chronic cystitis
4. schistosomiasis of the bladder
5. Certain drugs (cyclophosphamide).
A wide variety of genetic abnormalities are seen in bladder cancers; of these, mutations
involving several genes on chromosome 9, p53, and FGFR3 are the most common.
The prognosis
of bladder tumors depends on their histologic grade and the depth of
invasion of the lesion; the latter is much more important. Except for benign papillomas,
all tend to recur after removal. Lesions that invade the ureteral or urethral orifices cause
urinary tract obstruction. Overall 5-year survival is 57%. With deep penetration of the
bladder wall the 5-year survival rate is less than 20%.
Papillary tumors occur much less frequently in the renal pelvis than in the bladder, they
nonetheless make up to 10% of primary renal tumors. (Fig. 8-31) Patients present with
painless hematuria, and may develop hydronephrosis. Infiltration of the walls of the
pelvis, calyces, and renal vein worsens the prognosis. Despite removal of the tumor by
nephrectomy, fewer than 50% of patients survive for 5 years.
Cancer of the ureter is fortunately the rarest of the tumors of the collecting system. The 5-
year survival rate is less than 10%.
107
Chapter contents (107 – 117)
Topics
Directed by
Page number
1
The penis
د. سالم
(108)
Malformations of the Penis
108
Inflammatory Lesions of the penis
108
Penile Neoplasms
108
2
Scrotum
(109)
3
Testis and Epididymis
(110
– 113)
Cryptorchidism
110
Inflammatory Lesions of the testis & epididymis
110
Testicular neoplasms
111
4
The prostate
(114 - 117)
Prostatitis
114
Benign prostatic hyperplasia (BPH) (Nodular hyperplasia of the prostate)
115
Carcinoma of the prostate
116
Chapter 6 - Male Genital System
108
The penis
Malformations of the Penis
Hypospadias
is the most common malformation (1 in 250 live male births). It refers to
the abnormal location of the distal urethral orifice along the ventral aspect of the penis.
The urethral orifice is sometimes constricted, resulting in urinary tract obstruction.
Epispadias
: the urethral orifice is situated on the dorsal aspect of the penis. Like
hypospadias, epispadias may produce lower urinary tract obstruction or incontinence.
Inflammatory Lesions of the penis
Balanitis
refer to local inflammation of the glans penis; it may be associated with
inflammation of the overlying prepuce. Most cases occur due to poor local hygiene in
uncircumcised males, with accumulations of smegma (desquamated epithelial cells,
sweat, and debris) that acts as a local irritant. The distal penis is typically red, swollen,
and tender; a purulent discharge may be present.
Phimosis
refers to the difficulty of retracting the prepuce over the glans. It may be
congenital but most cases are acquired from scarring of the prepuce secondary to
previous balanitis.
Genital candidiasis
is particularly common in patients with diabetes mellitus. Candidiasis
presents as a painful, intensely pruritic erosions involving the glans penis, scrotum, &
adjacent intertriginous areas. Scrapings or biopsy specimens of the lesions reveal
characteristic budding yeast forms & pseudohyphae within the superficial epidermis.
Penile Neoplasms
Squamous cell carcinomas
Are relatively uncommon. Most cases occur in uncircumcised patients older than 40 years
of age. Etiological factors include poor hygiene that expose the area to potential
carcinogens in smegma, smoking, and infection with human papillomavirus (HPV),
particularly types 16 & 18. These carcinomas are generally preceded by dysplastic changes
termed intraepithelial neoplasia that may culminate in carcinoma in situ.
Clinical variants of carcinoma in situ, all strongly associated with HPV infection, occur
on the penis
1) Bowen disease (Erythroplasia of Queyrat) a solitary, erythematous, plaque-like lesion
on the shaft of the penis that displays microscopically malignant cells throughout the
epidermis. It may progress to invasive carcinoma in one third of the cases. (Fig. 9-1)
2) Bowenoid papulosis presents as multiple reddish brown papules on the shaft, glans or
scrotum; it is and most often transient, with only rare progression to carcinoma. (Fig. 9-2)
Gross features of invasive Squamous cell carcinoma
This tumor appears as a gray, crusted, papular lesion, most commonly on the glans penis
or prepuce. In many cases, the carcinoma infiltrates the underlying connective tissue to
produce an indurated, ulcerated lesion with irregular margins (Fig. 9-3).
Microscopic features
SCC is usually a keratinizing with infiltrating margins.
Verrucous carcinoma is very well differentiated variant of squamous cell carcinoma
characterized by a papillary architecture & rounded, pushing margins.
Most cases of squamous cell carcinoma of the penis are indolent. Regional metastases are
present in the inguinal lymph nodes in approximately 25% of patients at the time of
diagnosis. Distant metastases are relatively uncommon. The overall 5-year survival rate
averages 70%.

Chapter 6 - Male Genital System
109
Scrotum
The skin of the scrotum may be affected by several inflammatory conditions, including
local fungal infections and systemic dermatoses.
Squamous cell carcinoma
, the most common of the generally rare scrotal tumors,
but represents the first observed human malignancy associated with environmental
influences i.e. a high incidence in chimney sweeps.
Hydrocele
is an accumulation of serous fluid within the tunica vaginalis & represents
the most common cause of scrotal enlargement. It may arise in response to neighboring
infections or tumors, or it may be idiopathic.
Hematoceles & chyloceles
, represent accumulations of blood or lymphatic fluid
within the tunica vaginalis respectively. In extreme cases of lymphatic obstruction,
caused, for example, by filariasis, the scrotum and the lower extremities may enlarge to
dreadful proportions, a condition termed elephantiasis.

Chapter 6 - Male Genital System
110
Testis and Epididymis
Cryptorchidism
This refers to failure of testicular descent into the scrotum leading to malposition of the
gonad anywhere along its migration pathway. Normally, the testes descend from the
coelomic cavity into the pelvis and then through the inguinal canals into the scrotum during
intrauterine life. The diagnosis of cryptorchidism is difficult before 1 year of age, because
complete testicular descent into the scrotum is not invariably present at birth. By 1 year of
age, cryptorchidism is present in 1% of the males and in 10% of these cases is bilateral.
Several influences may interfere with testicular descent including
1. Hormonal abnormalities 2. Intrinsic testicular abnormalities
3. Obstruction of the inguinal canal 4. Congenital syndromes.
In the vast majority of cases, however, the cause is unknown.
Complications of cryptorchidism include
1. Sterility in bilateral cases.
2. Atrophy of the cryptorchid testis and even of the contralateral descended gonad.
3. Malignancy of the cryptorchid testis (up to 5 times increased risk). An increased risk is
also noted in the contralateral, normally descended testis, suggesting that some intrinsic
abnormality, rather than simple failure of descent, may be responsible for the increased
cancer risk.
Surgical placement of the undescended testis into the scrotum (orchiopexy) before
puberty decreases the likelihood of testicular atrophy and reduces, but does not
eliminate, the risk of cancer and infertility. The cryptorchid testis may be of normal size
early in life, although some degree of atrophy is usually present by the time of puberty
with microscopic evidence of tubular atrophy, fibrosis & hyalinization. Loss of tubules is
usually accompanied by hyperplasia of Leydig cells. Foci of intratubular germ cell
neoplasia may be present in cryptorchid testes and may be the source of subsequent
cancers developing in these organs.
Other causes of testicular atrophy (apart from cryptorchidism) include (Fig. 9-4)
1. Chronic ischemia 2. Trauma 3. Radiation 4. Antineoplastic chemotherapy
5. Conditions associated with elevation in estrogen levels (e.g., cirrhosis)
Inflammatory Lesions of the testis & epididymis
Inflammatory lesions are more common in the epididymis than in the testis.
Causes include venereal diseases, nonspecific epididymitis & orchitis, mumps & tb.
Nonspecific epididymitis and orchitis
usually begin as a primary UTI with subsequent
climbing infection of the testis through the vas deferens or lymphatics of the spermatic
cord. The involved testis is typically swollen and tender and contains a predominantly
neutrophilic inflammatory infiltrate.
Orchitis complicates mumps infection
in roughly 20% of infected adult males but rarely
occurs in children. The affected testis is edematous& congested and contains a predominantly
lymphoplasmacytic inflammatory infiltrate. There may be an associated considerable loss of
seminiferous epithelium with resultant tubular atrophy, fibrosis & sterility.
Granulomatous orchitis
may be due to infections and autoimmune injury. Of these,
tuberculosis is the most common. Testicular tuberculosis generally begins as an
epididymitis, with secondary involvement of the testis. The histologic changes include
granulomatous inflammation and caseous necrosis, identical to that seen in active
tuberculosis in other sites.
Chapter 6 - Male Genital System
111
Testicular neoplasms
Testicular neoplasms are the most important cause of firm, painless enlargement of the
testis. The peak incidence is between the ages of 20 and 34 years. In adults, 95% of
testicular tumors arise from germ cells, and all are malignant. Neoplasms derived from
Sertoli or Leydig cells (sex cord/stromal tumors) are uncommon and, in contrast to
tumors of germ cell origin, usually pursue a benign clinical course.
The etiology of testicular neoplasms is not known.
The following are risk factors
1. Cryptorchidism: a history of this condition is present in 10% of testicular cancer.
2. Intersex syndromes, including androgen insensitivity syndrome & gonadal dysgenesis.
3. Genetic & ethnic influences as manifested by
a. Family hx: the risk of neoplasia is increased in siblings of males with testicular cancers
b. Cancer in one testis is associated with a markedly increased risk in the contralateral testis.
A wide range of abnormalities in testicular germ cell neoplasms have been detected, the
most common of which is an isochromosome of the short arm of chromosome 12.
However, the role of such aberrations remains unclear.
c. Whites are affected more commonly than blacks.
Classification and Histogenesis of Testicular tumors
The WHO classification is the most widely used. In this, germ cell tumors of the testis are
divided into two broad categories, based on whether they contain 1. A single histologic
pattern (60% of cases) or 2. Multiple histologic patterns (40% of cases).
Germ cell tumors of the testis arise from primitive cells that may either differentiate
along gonadal lines to produce seminomas or transform into a totipotential cell
population, giving rise to nonseminomatous germ cell tumors. Such totipotential cells
may remain largely undifferentiated to form embryonal carcinomas, may differentiate
along extra-embryonic lines to form yolk sac tumors & choriocarcinomas, or may
differentiate along somatic cell lines to produce teratomas. This proposed histogenesis is
supported by the high frequency of mixed histologic patterns among nonseminomatous
germ cell tumors. Most testicular tumors arise from in situ lesions designated
intratubular germ cell neoplasia. Such in situ lesions are seen in adjacent to a testicular
germ cell tumor in virtually all cases.
Seminomas
This is the most common testicular germ cell neoplasm, accounting for 50% of these
tumors. The peak incidence is between 34 to 45 years, which is about 1 decade later than
that of most other GCTs. Before puberty, seminoma is extremely rare; in fact, it does not
occur in the first decade of life especially in children younger than 5 years. Most patients
present with a typically painless testicular enlargement.
Gross features
They are soft, well-demarcated, usually homogeneous, gray-white or pale tumors that
bulge from the cut surface of the affected testis (Fig. 9-5 A).
The neoplasms are typically confined to the testis.
Large tumors may contain foci of coagulation necrosis, usually without hemorrhage.
Microscopically
o Seminomas are composed of large, uniform cells with distinct cell borders, clear,
glycogen-rich cytoplasm, and round nuclei with conspicuous nucleoli (Fig. 9-5 B).
o The cells are often disposed in small lobules with intervening fibrous septa.
o A lymphocytic infiltrate is usually present.
o A granulomatous inflammatory reaction may also be present.
Chapter 6 - Male Genital System
112
o In as many as 25% of cases, cells staining positively for human chorionic gonadotropin
(hCG) can be seen. Some of these hCG-expressing cells are morphologically similar to
syncytiotrophoblasts, and they are presumably the source of the elevated serum hCG
concentrations that may be encountered in some males with pure seminoma.
Spermatocytic seminoma
Is a much less common morphologic variant of seminoma that tend to occur in older
patients. It contains cells with round nuclei with spiral deposition of chromatin that are
reminiscent of secondary spermatocytes. The cytoplasm of tumor cells is eosinophilic and
does not contain glycogen .A high mitotic activity is often present . It typically occurs in
men older than 50 years of age (median, 55 years)
Embryonal carcinoma
Occurs most frequently between 25 and 35 years of age, which is 10 years earlier than the
age range for seminoma. It is rare after the age of 50 years and does not occur in infancy.
Most patients present with a painless unilateral enlarging testicular mass. Approximately
2 thirds of cases have retroperitoneal lymph node or distant metastases at the time of dx.
Gross features
Is an ill-defined, invasive tumor that contains foci of hemorrhage and necrosis.
The primary lesions may be small, even in patients with systemic metastases.
Larger lesions may invade the epididymis and spermatic cord.
Microscopic features (Fig. 9-7 B).
The constituent cells are large and primitive (undifferentiated) looking, with basophilic
cytoplasm, indistinct cell borders, and large nuclei with prominent nucleoli. Mitoses are
frequent including abnormal ones. The neoplastic cells are disposed in solid sheets that
may contain glandular structures and irregular papillae
In most cases, other germ cell tumors (e.g., yolk sac tumor, teratoma, choriocarcinoma) are
admixed with the embryonal carcinoma. In fact, pure embryonal carcinomas are quite rare.
Yolk sac tumors (YST) (endodermal sinus tumors)
These are the most common primary testicular neoplasm in children younger than 3 years
of age. In adults, yolk sac tumors are most often seen admixed with embryonal carcinoma.
Yolk sac tumors represent endodermal sinus differentiation of totipotential neoplastic cells.
Grossly, these tumors are often large and may be well demarcated.
Microscopy discloses low cuboidal to columnar epithelial cells forming microcysts,
sheets, glands, and papillae, often associated with eosinophilic hyaline globules. A
distinctive feature is the presence of structures resembling primitive glomeruli, the so-
called Schiller-Duvall bodies. α-fetoprotein (AFP) can be demonstrated within the
cytoplasm of the neoplastic cells by immunohistochemical techniques. (Fig. 9-8)
Choriocarcinomas
Represent differentiation of pluripotent neoplastic germ cells along trophoblastic lines.
Grossly, they are often small, nonpalpable lesions, even with extensive systemic
metastases. Microscopically, they are composed of sheets of small cuboidal cells
intermingled with large, eosinophilic syncytial cells containing multiple dark, pleomorphic
nuclei; these represent cytotrophoblastic and syncytiotrophoblastic differentiation,
respectively. The hormone hCG can be identified with immunohistochemical staining.
Teratomas
Represent differentiation of neoplastic germ cells along somatic cell lines.
Grossly There are firm masses that on cut surface often contain cysts and recognizable
areas of cartilage. (Fig. 9-10)
Chapter 6 - Male Genital System
113
Microscopically, three major variants of pure teratoma are recognized:
1. Mature teratomas contain fully differentiated tissues from one or more germ cell layers
(e.g., neural tissue, cartilage, adipose tissue, bone & epithelium) in a haphazard array.
2. Immature teratomas, in contrast, contain immature somatic elements reminiscent of
those in developing fetal tissue.
3. Teratomas with somatic-type malignancies are characterized by the development of
frank malignancy in preexisting teratomatous elements, usually in the form of a
squamous cell carcinoma or adenocarcinoma.
Pure teratomas in prepubertal males are usually benign. In adults, teratomas metastasize
in as many as one third of cases. As with other germ cell tumors, testicular teratomas in
adults often contain other malignant germ cell elements and therefore should be generally
regarded as malignant neoplasms.
Mixed germ cell tumors
Account for 40% of all testicular germ cell neoplasms. Combinations of any of the
described patterns may occur in mixed tumors, the most common of which is a
combination of teratoma, embryonal carcinoma & yolk sac tumors.
Clinically, testicular germ cell tumors are divided in to two broad categories:
1. Seminomas and
2. Non-seminomatous tumors
These two groups of tumors have somewhat distinctive clinical presentation and natural
history. Individuals with testicular germ cell neoplasms present most frequently with
painless testicular enlargement. However, some tumors, especially nonseminomatous
germ cell neoplasms, may have widespread metastases at diagnosis, in the absence of a
palpable testicular lesion. Seminomas often remain confined to the testis for prolonged
intervals and may reach considerable size before diagnosis. Metastases are most
commonly encountered in the iliac and para-aortic lymph nodes. Hematogenous
metastases occur later. In contrast, nonseminomatous germ cell neoplasms tend to
metastasize earlier, by both lymphatic and hematogenous routes. Hematogenous
metastases are most common in the liver and lungs.
Testicular germ cell neoplasms are staged as follows:
Stage I: Tumor confined to the testis Stage II: Regional lymph node metastases only
Stage III: Nonregional lymph node and/or distant organ metastases
Assay of tumor markers
Secreted by tumor cells is important in the clinical evaluation & staging of germ cell neoplasms.
1. hCG, produced by neoplastic syncytiotrophoblastic cells, is always elevated in patients
with choriocarcinoma. Other germ cell tumors, including seminoma, may also contain
syncytiotrophoblastic cells without cytotrophoblastic elements and hence up to 25% of
seminomas secrete hCG.
2. AFP is normally synthesized by the yolk sac and several other fetal tissues. Germ cell
tumors containing elements of yolk sac (endodermal sinus) often produce AFP; in
contrast to hCG, the presence of AFP is a reliable indicator of the presence of a
nonseminomatous component to the germ cell neoplasm, because yolk sac elements are
not found in pure seminomas.
Because mixed patterns are common, most nonseminomatous tumors have elevations of
both hCG and AFP. In addition to their role in the primary diagnosis and staging of
testicular germ cell tumors, serial determinations of hCG and AFP are useful for
monitoring patients for persistent or recurrent tumor after therapy.
Chapter 6 - Male Genital System
114
The prostate
Prostatitis
May be acute or chronic.
1)
Acute bacterial prostatitis
is caused by the same organisms associated with acute UTIs, particularly Escherichia
coli. Most patients with acute prostatitis also have concomitant acute urethritis and
cystitis. In these cases, organisms may reach the prostate by direct extension from the
urethra or urinary bladder. Alternatively prostatitis may complicate infections from
distant sites through the blood.
2) Chronic prostatitis
may follow episodes of acute prostatitis, or may develop insidiously, without previous
episodes of acute infection. In some cases of chronic prostatitis bacteria can be isolated
(chronic bacterial prostatitis). In other instances the presence of an increased number of
leukocytes in prostatic secretions confirms prostatic inflammation, but bacteriologic
findings are negative (chronic abacterial prostatitis), which account for most cases of
chronic prostatitis. Several nonbacterial agents implicated in the pathogenesis of
nongonococcal urethritis, including Chlamydia trachomatis.
Pathological features
o Acute prostatitis:
there is an acute, neutrophilic inflammatory infiltrate, congestion, and stromal edema.
Neutrophils are initially within the prostatic glands but as the infection progresses, they
destroy glandular epithelium and extends into the surrounding stroma, resulting in the
formation of microabscesses. Grossly visible abscesses can develop with extensive tissue
destruction, e.g. in diabetic patients.
o Chronic prostatitis:
evidence of tissue destruction and fibroblastic proliferation, along with the presence of
inflammatory cells, such as lymphocytes & neutrophils, is required for a histologic
diagnosis of chronic prostatitis.
3) Granulomatous prostatitis
is a variant of chronic prostatitis, it is not a single disease but instead a morphologic
reaction to a variety of insults. It may be seen
1. With systemic inflammatory diseases (disseminated TB, sacoidosis, fungal infections).
2. As a nonspecific reaction to inspissated prostatic secretions
3. After transurethral resection of prostatic tissue.
Microscopically, there are multinucleated giant cells and variable numbers of foamy
histiocytes, sometimes accompanied by eosinophils. Caseous necrosis is only seen in the
setting of tuberculous prostatitis.
Clinical features
Prostatitis is manifested as dysuria, frequency, lower back pain, and poorly localized
suprapubic or pelvic pain. The prostate may be enlarged and tender, particularly in acute
prostatitis with often fever& leukocytosis. Chronic prostatitis, even if asymptomatic, may
become a reservoir for organisms capable of causing UTIs. Chronic bacterial prostatitis,
therefore, is one of the most important causes of recurrent urinary tract infection in men.
Chapter 6 - Male Genital System
115
Benign prostatic hyperplasia (BPH) (Nodular hyperplasia of the prostate)
The normal prostate consists of glandular and stromal elements surrounding the urethra.
The prostatic parenchyma can be divided into the following biologically distinct zones:
1. Peripheral 2. Central 3. Transitional, and 4. Periurethral zones (Fig. 9-11).
The types of proliferative lesions are different in each zone. For example, most
hyperplastic lesions arise in the inner transitional and central zones of the prostate, while
most carcinomas arise in the peripheral zones.
BPH is an extremely common & affects a significant number of men by the age of 40,
and its frequency rises progressively with age, reaching 90% by the eighth decade. BPH
is characterized by proliferation of both stromal and epithelial elements, with resultant
enlargement of the gland and, in some cases, urinary obstruction. Androgens have a
central role in the pathogenesis of BPH. Dihydrotestosterone. (DHT) is derived from
testosterone through the action of 5α-reductase & appears to be major hormonal
stimulus for stromal and glandular proliferation in men with nodular hyperplasia. DHT
binds to nuclear androgen receptors and, in turn, stimulates synthesis of DNA, RNA,
growth factors, and other cytoplasmic proteins, leading to hyperplasia. This is the base
for the current use of 5α-reductase inhibitors in the treatment of symptomatic nodular
hyperplasia. Local, intra-prostatic concentrations of androgens and androgen receptors
contribute to the pathogenesis of this condition. Age-related increases in estrogen levels
that may increase the expression of DHT receptors on prostatic parenchymal cells,
thereby functioning in the pathogenesis of nodular hyperplasia.
Gross features BPH arises mostly in the inner, peri-urethral glands of the prostate,
particularly from those that lie above the verumontanum.
The affected prostate is enlarged. The cut surface contains many well-circumscribed
nodules (Fig. 9-12 A). The nodules may have a solid appearance or may contain cystic
spaces, the latter corresponding to dilated glandular elements seen in histologic sections.
The urethra is usually compressed by the hyperplastic nodules, often to a slitlike orifice.
In some cases, hyperplastic glandular and stromal elements lying just under the proximal
prostatic urethra may project into the bladder lumen as a pedunculated mass, resulting in
a ball-valve type of urethral obstruction.
Microscopic features
The hyperplastic nodules are composed of varying proportions of proliferating glandular
elements and fibromuscular stroma. The hyperplastic glands are lined by tall, columnar
epithelial cells & a peripheral layer of flattened basal cells; crowding of the proliferating
epithelium results in the formation of papillary projections in some glands (Fig. 9-12 B).
The glandular lumina often contain inspissated, proteinaceous secretory material, termed
corpora amylacea. The glands are surrounded by proliferating stromal elements.
Areas of infarction are frequent in advanced cases of BPH and are accompanied by foci
of squamous metaplasia in adjacent glands. (Fig. 9-13)
Clinical manifestations of BPH: Occur in only 10% of men with the disease. Because
nodular hyperplasia preferentially involves the inner portions of the prostate, its most
common manifestations are those of lower urinary tract obstruction. These include
difficulty in starting the stream of urine (hesitancy) and intermittent interruption of the
urinary stream while voiding. Some may develop complete urinary obstruction, with
resultant painful distention of the bladder and, if neglected, hydronephrosis. Symptoms of
obstruction are frequently accompanied by urinary urgency, frequency, and nocturia, all
indicative of bladder irritation. The combination of residual urine in the bladder and
chronic obstruction increases the risk of urinary tract infections.
Chapter 6 - Male Genital System
116
Carcinoma of the prostate
This is the most common visceral cancer in males and the second most common cause of
cancer-related deaths in men older than 50 years of age, after carcinoma of the lung. The
peak incidence is around the age of 70 years. Hidden (Latent) cancers of the prostate are
even more common than those that are clinically apparent, with an overall frequency of
more than 50% in men older than 80 years of age.
Hormones, genes, and environment are thought to be of pathogenetic importance:
A. Androgens; their role in prostatic CA is supported by the following observations
1. Cancer of the prostate does not develop in males castrated before puberty.
2. The growth of many prostatic carcinomas can be inhibited by orchiectomy or by the
administration of estrogens such as diethylstilbestrol.
B. Hereditary & Racial contributions are supported by
1. The increased risk of the disease among 1
st
-degree relatives of patients with prostate cancer.
2. Symptomatic carcinoma is more common and occurs at an earlier age in blacks &
Asians. Whether such racial differences occur as a consequence of genetic influences &/or
environmental factors remains unknown. However, the frequency of latent
(incidental) prostatic cancers is similar in all races, suggesting that race is more importantly
in the growth of established lesions than in the initial development of carcinoma.
In familial cases, several susceptibility loci on chromosome 1 have been identified.
C. Environmental influences is suggested by the increased frequency of prostatic
carcinoma in certain industrial settings and by significant geographic differences in the
incidence of the disease. Carcinoma of the prostate is particularly common in
Scandinavian countries and relatively uncommon in Japan and certain other Asian
countries. Males immigrating from low-risk to high-risk areas maintain a lower risk of
prostate cancer; the risk of disease is intermediate in subsequent generations, in keeping
with an environmental influence on the development of this disease. A diet high in animal
fat has been suggested as a risk factor.
Gross features (Fig. 9-14 A)
The majority of prostate cancers (80%) arise in the outer (peripheral) glands and hence
may be palpable as irregular hard nodules by rectal digital examination. Because of the
peripheral location, prostate cancer is less likely to cause urethral obstruction in its initial
stages than is nodular hyperplasia.
Early lesions typically appear as ill-defined masses just beneath the capsule of the prostate.
On cut surface, foci of carcinoma appear as firm, gray-white to yellow lesions that
infiltrate the adjacent native prostatic tissues with ill-defined margins.
Metastases to regional pelvic lymph nodes may occur early.
Locally advanced cancers often infiltrate the seminal vesicles and periurethral zones of
the prostate and may invade the adjacent soft tissues and the wall of the urinary bladder.
Denonvilliers fascia, the connective tissue layer separating the lower genitourinary
structures from the rectum, usually prevents growth of the tumor posteriorly. Invasion of
the rectum therefore is less common than is invasion of other contiguous structures.
Microscopic features (Fig. 9-14 B)
Most carcinomas are adenocarcinomas with variable degrees of differentiation.
The better differentiated lesions are composed of small glands that infiltrate the adjacent
stroma in an irregular, haphazard fashion.
In contrast to normal and hyperplastic prostate, the glands in carcinomas
o Lie "back to back" and appear to dissect sharply though the native stroma
o Are lined by a single layer of cuboidal cells with conspicuous nucleoli; the basal cell
layer seen in normal or hyperplastic glands is absent.
Chapter 6 - Male Genital System
117
With increasing degrees of anaplasia, irregular glandular, papillary or cribriform
epithelial structures, &, in extreme cases, sheets of poorly differentiated cells are present.
Glands adjacent to areas of invasive carcinoma of the prostate often contain foci of
epithelial atypia, or prostatic intraepithelial neoplasia (PIN).
Because of its frequent coexistence with infiltrating carcinoma, PIN has been suggested
as a probable precursor to carcinoma of the prostate. PIN has been subdivided into high-
grade and low-grade patterns, depending on the degree of atypia. Importantly, high-grade
PIN shares molecular changes with invasive carcinoma, lending support to the argument
that PIN is an intermediate between normal and frankly malignant tissue.
A number of histologic grading schemes have been proposed for carcinoma of the
prostate. They are based on features such as the degree of glandular differentiation, the
architecture of the neoplastic glands, nuclear anaplasia, and mitotic activity. A commonly
used method for grading is the Gleason system, which has proved to correlate reasonably
well with both the stage of prostatic carcinoma and its prognosis.
Clinically, 10% of carcinomas are localized and discovered unexpectedly during
histologic examination of tissues removed for nodular hyperplasia. Because most cancers
begin in the peripheral regions of the prostate, they may be discovered during digital
rectal examination. More extensive disease may produce local discomfort and evidence
of lower urinary tract obstruction. PR exam. in such cases reveals a hard, fixed prostate.
Aggressive carcinomas may first come to attention through the presence of metastases.
Bone metastases, particularly to the axial skeleton, are common and may cause either
osteolytic (destructive) or, more commonly, osteoblastic (bone-producing) lesions. The
presence of osteoblastic metastases in an older male is strongly suggestive of advanced
prostatic carcinoma.
Assay of serum levels of prostate-specific antigen (PSA) has gained widespread use in
the diagnosis of early carcinomas. PSA is proteolytic enzyme produced by both normal
and neoplastic prostatic epithelium. Traditionally, a serum PSA level of 4.0 ng/L has
been used as the upper limit of normal. Cancer cells produce more PSA, but any
condition that disrupts the normal architecture of the prostate, including adenocarcinoma,
BPH, and prostatitis, may also cause an elevation in serum levels of PSA. Moreover, in
some cases of cancer of the prostate, especially those confined to the prostate, serum PSA
is not elevated. Because of these problems, PSA is of limited value when used as an
isolated screening test for cancer of the prostate. However, it has a much more value
when it is used in conjunction with other procedures, such as digital rectal examination,
transrectal sonography, and needle biopsy. In contrast to its limitations as a diagnostic
screening test, serum PSA concentration is of great value in monitoring patients after
treatment for prostate cancer, with rising levels after therapy indicative of recurrence
and/or the development of metastases.
Staging of the extent of disease has an important role in the evaluation and treatment of
prostatic carcinoma. The anatomic extent of disease and the histologic grade influence
the therapy for prostate cancer and correlate well with prognosis. Carcinoma of the
prostate is treated with various combinations of surgery, radiation therapy, and hormonal
manipulations. Most prostate cancers are androgen sensitive and are inhibited to some
degree by surgical or pharmacologic castration, estrogens, and androgen receptor-
blocking agents. These all have been used to control the growth of disseminated lesions.
The prognosis for patients with limited-stage disease is favorable: more than 90% of
patients with stage T1 or T2 lesions (localized to the prostate) survive 10 years or longer.
The outlook for patients with disseminated disease remains poor, with 10-year survival
rates in this group ranging from 10% to 40%.
118
Chapter contents (118 – 144)
Topics
Directed by
Page number
1
Vulva
د. سالم
(119 - 120)
Vulvitis
119
Non-neoplastic epithelial disorders (NNED) (previously vulvar dystrophies)
119
Tumors
119
2
Vagina
(120)
- Congenital Anomalies - Vaginitis - Malignant Tumors
120
3
Cervix
(121
– 123)
Cervicitis
121
Cervical Tumors
121
4
Body of uterus
(124 - 127)
Endometritis
124
Adenomyosis
124
Endometriosis
124
Organic & Dysfunctional Uterine Bleeding
125
Endometrial Hyperplasia
125
Tumors of the Endometrium and Myometrium
126
5
Fallopian tubes
(128)
6
Ovaries
(129
– 132)
Follicle cyst and Luteal Cyst
129
Polycystic Ovaries (Stein-Leventhal syndrome)
129
Tumors of the ovary
129
7
Diseases of pregnancy
(133 - 136)
Placental Inflammations and Infections
133
Ectopic Pregnancy
133
Gestational Trophoblastic Tumors
133
Pre-eclampsia, Eclampsia (Toxemia Of Pregnancy)
136
8
Breast
(137
– 144)
Congenital anomalies
137
Galactocele
137
Fibrocystic changes (FCC)
137
Inflammations
139
Tumors of the breast
140
Lesions of the Male breast
144

Chapter 7 - Female genital system & Breast
119
Vulva
Vulvitis
The most important infectious agents are
1. Human papillomavirus (HPV), producing condylomata acuminata & vulvar
intraepithelial neoplasia
2. Herpes simplex genitalis (HSV 1 or 2), causing a vesicular eruption
3. Gonococci producing suppurative infection of the vulvovaginal glands
4. Syphilis causing primary chancre at the site of inoculation 5. Candida causing vulvitis.
Contact Dermatitis is one of the most common causes of vulvar pruritus. It presents as
erythematous weeping and crusting papules and plaques. Causes include urine, soaps,
detergents, deodorants, etc.
Non-neoplastic epithelial disorders (NNED) (previously vulvar dystrophies)
There are two forms of NNED 1. Lichen sclerosus and 2. Lichen simplex chronicus.
Both appear clinically as a white lesion i.e. leukoplakia. The latter, however, has many
other underlying conditions including, vitiligo, psoriasis, lichen planus, & carcinoma.
Only biopsy and microscopic examinations can differentiate among these lekoplakias.
Lichen Sclerosus is characterized by thinning of the epidermis, hydropic degeneration of
the basal cells, and dermal fibrosis. When the entire vulva is affected, the labia become
atrophic with constriction of the vaginal orifice. It is most common in postmenopausal
women. An autoimmune reaction is probably involved in its pathogenesis.
Lichen Simplex Chronicus is the end stage of many inflammatory dermatoses and is
marked by epidermal hyperplasia and significant surface hyperkeratosis.
Tumors
1) Condylomas and Low-Grade Vulvar Intraepithelial Neoplasia (VIN)
There are two biologic forms of anogenital warts (condylomas)
a. Condylomata lata: are manifestations of secondary syphilis & are flat & slightly elevated.
b. Condylomata accuminata: are viral (HPV) warts and appear as elevated warty or flat &
wrinkled localized lesions. They are often multiple, red-pink lesions that measure up to
several centimeters in diameter. Microscopically, there is acanthosis & hyper/parakeratosis,
but koilocytosis. The latter are squamous cells with perinuclear cytoplasmic vacuoles and
nuclear angulation. The koilocytes are characteristic of HPV infection. C. acuminata are
not precancerous but may coexist with foci of low-grade intraepithelial neoplasia in the vulva
(VIN 1) & cervix. Indeed, VIN I & condylomas are both related to HPV 6 & 11 infections.
2) High-Grade VIN and Carcinoma of the Vulva
Carcinoma of the vulva is used to be seen mostly in elderly women. However, there has
been an increase in the frequency of high grade VIN principally among younger women.
The vast majority of vulvar carcinomas are of squamous type. Two biologic forms of
vulvar carcinoma seem to exist
A. HPV-positive carcinoma (especially type 16) is seen in younger patients, particularly
cigarette smokers. Many cases show coexisting carcinoma in situ, or condylomata acuminata.
B. HPV-negative carcinoma seen in older women; frequently it is not associated with
VIN
.
VIN and early vulvar carcinomas appear as areas of leukoplakia due to epithelial
thickening. These areas eventually transform into exophytic or ulcerative cancers.
Microscopic features: HPV-positive neoplasms tend to be poorly differentiated squamous
cell carcinoma, whereas the HPV-negative lesions tend to be well-differentiated
keratinizing. Ultimately, direct invasion with involvement of regional nodes and more
distant spread occurs. Women with a tumor less than 2 cm in diameter have a much better
prognosis than those with larger lesions.
Chapter 7 - Female genital system & Breast
120
3) Paget Disease of the vulva
is a form of intraepithelial carcinoma. The majority of cases
have no underlying carcinoma (cf. mammary Paget disease); it is considered as
carcinoma of progenitor cells of the epidermis. The condition presents as a red, scaly,
crusted plaque. Microscopically, large epithelial cells infiltrate the epithelium, singly and
in groups, with abundant granular cytoplasm and occasional cytoplasmic mucin vacuoles
Vagina
Congenital Anomalies
Are uncommon and include total absence of the vagina, a septate or double vagina.
Vaginitis
Is a relatively common problem that produces a vaginal discharge (leukorrhea).
Many of the offenders are normal commensals that become pathogenic in conditions such as
diabetes, systemic antibiotic therapy, after abortion or pregnancy, or in AIDS. Gonorrheal
vaginitis may occur and be transmitted to the newborn of the infected mother. Candidal
vaginitis produces a curdy white discharge. Trichomonas vaginalis like candidiasis, is
frequent, but produces a watery, profuse, gray-green discharge in which parasites can be
identified microscopically. However, (like monilia) the infection can be asymptomatic.
Nonspecific atrophic vaginitis is a common cause of postmenopausal bleeding. It is
associated with thinning (atrophy) of the squamous vaginal mucosa. It is due to lack of
estrogenic support of the vaginal epithelium.
Malignant Tumors
1)
Squamous cell carcinoma
is very rare & usually occurs in elderly women, with risk factors similar to those for
cervical carcinoma. Vaginal intraepithelial neoplasia is a precursor lesion associated with
HPV infection.
2)
Clear cell adenocarcinoma
Usually affects adolescent and young females whose mothers took diethylstilbestrol
during pregnancy. The tumor may arise from the cervix rather than the vagina in a third
of the cases. Vaginal adenosis presents as red foci consisting of small glands lined by
columnar cells; this is a benign condition from which the clear cell adenocarcinoma is
thought to arise.
3)
Sarcoma botryoides (embryonal rhabdomyosarcoma)
,
Produces soft polypoid masses and is usually seen in infants and children younger 5 years
of age. (Fig. 10-5)
Chapter 7 - Female genital system & Breast
121
The cervix uteri
Cervicitis
Is a very common condition that is associated with a mucopurulent discharge. Cytologic
examination of the discharge reveals inflammatory cells admixed with cervical epithelial
cells, and possible microorganisms. It is often due to vaginal flora, streptococci,
staphylococci, and E. coli. Much more important are Chlamydia trachomatis, Ureaplasma,
Trich. vaginalis, Candida spp., Neisseria gonorrhoeae, herpes simplex II (genitalis), and
HPV. Many of these microorganisms are transmitted sexually, and so the cervicitis may
represent a sexually transmitted disease. Among these pathogens, C. trachomatis is by far
the most common sexually transmitted cervicitis. Herpetic infections of the cervix are
important because the infection may be transmitted to the infant during its passage through
the birth canal, sometimes resulting in a serious, sometimes fatal, systemic infection.
Pathologic features
Nonspecific cervicitis may be either acute or chronic. Excluding gonococcal infection,
the uncommon acute nonspecific cervicitis is limited to postpartum women and is usually
caused by staphylococci or streptococci. The chronic form is very common and usually
referred to as nonspecific cervicitis. Specific forms include herpesvirus ulcerative lesions
and changes caused by C. trachomatis. Chronic cervicitis consists of inflammation and
epithelial regeneration. These changes may occur in both squamous and columnar
mucosa. Eventually, the columnar epithelium undergoes squamous metaplasia.
Cervical Tumors
Despite dramatic improvements in early diagnosis and treatment, cervical carcinoma
continues to be one of the major causes of cancer-related deaths in women in the
developing world.
Cervical Intraepithelial Neoplasia (CIN)
The Pap smear, introduced 50 years ago by Papanicolaou, remains the most successful
cancer screening test ever developed. In populations that are screened regularly, cervical
cancer mortality is reduced by up to 99%. Nearly all invasive cervical squamous cell
carcinomas arise from precursor epithelial changes referred to as cervical intraepithelial
neoplasia (CIN). Detection of CIN by the Pap smear at an early stage permits curative
treatment. Cytological examination can detect CIN long before any abnormality can be
seen grossly. CIN begins as low-grade lesion that may progress to higher grade CIN, or it is
a highgrade lesions from the outset; this depends on the location of the HPV infection in the
transformation zone, the type of HPV infection (high versus low risk)& other host factors.
On the basis of histology, precancerous changes are graded as
CIN I: Mild dysplasia CIN II: Moderate dysplasia
CIN III: Severe dysplasia/carcinoma in situ
The current Bethesda system divides the precancerous lesions into only two groups:
1. Low-grade SIL (SIL for squamous intraepithelial lesions), equivalent to CIN I
2. High-grade SIL. Equivalent to CIN II & III
Progression from low- to high-grade SIL may or may not occur. The higher the grade of
CIN the greater the likelihood of progression to invasive carcinoma, this reaches to 70%
with CIN III.
Epidemiology and Pathogenesis
o The peak age of CIN incidence is about 30 yrs, whereas that of invasive carcinoma is about
45 yrs i.e. precancerous changes usually take many yrs to evolve into overt carcinomas.
Chapter 7 - Female genital system & Breast
122
o Important risk factors for the development of CIN and invasive carcinoma are:
1. Early age at first intercourse 2. Multiple sexual partners
3. Persistent infection by "high-risk" papilloma viruses 4. Low socio-economic status
o All of the above favor a sexually transmitted causative agent (HPV). Indeed, HPV can be
detected by molecular techniques in nearly all precancerous and cancerous lesions.
Specifically, high-risk HPV types including 16 & 18, account for the majority of cervical
carcinomas. By contrast, condylomas, which are benign lesions, are caused by low-risk
HPV types (i.e., 6 & 11). In these benign lesions the viral DNA does not integrate into the
host genome.
o By contrast, HPV types 16 & 18 usually integrate into the host genome with subsequent
inactivation of the tumor suppressor genes p53 and RB. The result is a transformed cell,
capable of autonomous growth and susceptible to the acquisition of further mutations
(cancer progression). The recently introduced HPV vaccine is very effective in preventing
HPV infections and hence cervical cancers.
o Although many women harbor these viruses, only a few develop cancer, suggesting other
pathogenetic influences play a role e.g. cigarette smoking and immunodeficiency states
such as AIDS.
Microscopic features
o CIN & Carcinoma in situ
CIN begins with CIN I. This lesion is characterized by koilocytotic changes mostly in the
superficial layers of the epithelium. Koilocytosis is composed of nuclear hyperchromasia
and angulation with perinuclear vacuolization produced by cytopathic effect of HPV. The
dysplastic epithelium is limited to the lower third of the mucosa.
In CIN II the dysplasia is more severe, involving the lower two-thirds of the mucosa. The
superficial layer in some cases shows the koilocytotic changes.
CIN III shows dysplastic changes that affect virtually all layers of the epithelium. Surface
cells and their koilocytotic changes are usually absent. (Figs. 10-6).
In time, dysplastic changes become more atypical and may extend into the endocervical
glands, but the alterations are confined to the epithelial layer and its glands. These
changes constitute carcinoma in situ.
The next stage is invasive canrcinoma.
The above progression sequences do not occur in all the cases.
Cervical cytology & cervical colposcopy remain the basis of cervical cancer prevention.
o Invasive Carcinoma of the Cervix
The most common cervical carcinomas are (in descending order)
1. Squamous cell carcinomas (75%)
2. Adenocarcinomas and adenosquamous carcinomas (20%)
3. Small-cell neuroendocrine carcinomas (<5%).
The squamous cell carcinomas are increasingly appearing in younger women, (peak
incidence at about 45 years); 10 to 15 years after detection of their precursors (CIN).
Invasive carcinomas of the cervix develop in the region of the transformation zone (the
squamo-columnar junction) and range from invisible microscopic foci of early stromal
invasion to grossly visible exophytic ulcerating masses or deeply infiltrative cancer that
encircle the os (Fig. 10-7 A).
Tumors encircling the cervix and penetrating into the underlying stroma produce a
"barrel cervix," which can be identified by direct palpation.
Extension into the parametrial soft tissues can fix the uterus to the pelvic structures.
Spread to pelvic lymph nodes is determined by tumor depth of invasion, ranging from
less than 1% for tumors under 3 mm in depth to over 10% once invasion exceeds 5 mm.

Chapter 7 - Female genital system & Breast
123
Three microscopic variants of cervical SCC squamous cell carcinoma exist, although
admixtures and intermediate forms occur: 1. Large cell nonkeratinizing 2. Keratinizing
3. Small cell; this should be distinguished from small cell neuroendocrine carcinoma
Distant metastases, including para-aortic nodal involvement, remote organ involvement, or
invasion of adjacent structures such as bladder or rectum, occur late in the course of disease.
With the exception of neuroendocrine tumors, which are uniformly aggressive, cervical
carcinomas are graded from 1 to 3 based on the degree of cellular differentiation and
staged from 1 to 4 depending on the extent of clinical spread.
Ideally cervical carcinomas should be diagnosed in the preinvasive phase; these appear as
white areas on colposcopic examination after application of dilute acetic acid (Schiller
test). More advanced cases of cervical cancer are invariably seen in women who either
have never had a Pap smear or have waited many years since the prior smear. Such
tumors call to attention by unexpected vaginal bleeding, leukorrhea, painful coitus
(dyspareunia), and dysuria. Mortality is most strongly related to the tumor stage. The 5-
year survival in stage 1 is 90% but this figure drops to 10% in stage 4.
Endocervical Polyp
This may protrude, sometimes, through the exocervix. The trend is to regard these polyps
as inflammatory rather than neoplastic. They are generally small, soft, and have smooth,
glistening surface and subjacent cystically dilated spaces filled with mucinous secretion.

Chapter 7 - Female genital system & Breast
124
Body of uterus
Endometritis
This may be associated with retained products of conception subsequent to miscarriage or
delivery, or a foreign body such as an intrauterine device. Retained tissues or foreign bodies
act as a nidus for infection, frequently by flora ascending from the vaginal or anal region.
Endometritis is either acute or chronic depending on whether there is a predominant
neutrophilic or lymphoplasmacytic response; however, components of both may be present
in otherwise normal endometrium. Generally the diagnosis of chronic endometritis requires
the presence of plasma cells. Acute endometritis is frequently due to N. gonorrhoeae or C.
trachomatis. Microscopically, neutrophilic infiltrate in the superficial endometrium and
glands coexists with a stromal lymphoplasmacytic infiltrate. All forms of endometritis may
present with menstrual abnormalities, infertility and ectopic pregnancy due to extension of
the damaging inflammation to the fallopian tubes. Occasionally tuberculosis may present as
granulomatous endometritis, frequently with tuberculous salpingitis and peritonitis.
Adenomyosis
This refers to the “invagination of the stratum basalis of endometrium down into the
myometrium.” Nests of endometrial stroma, glands, or both, are found well down in the
myometrium between the muscle bundles. The latter become hypertrophied. Accordingly,
the uterine wall often becomes thickened and the uterus is enlarged and globular. Because
these glands derive from the stratum basalis, they do not undergo cyclical bleeding.
Nevertheless, marked adenomyosis may produce menorrhagia, dysmenorrhea, and pelvic
pain before the onset of menstruation.
Endometriosis
This is characterized by “the presence of endometrial glands & stroma in a location outside
the endomyometrium.” It occurs in as many as 10% of women in their reproductive years
and in nearly half of women with infertility. It may present as a pelvic mass filled with
degenerating blood (chocolate cyst). It is frequently multifocal & may involve any tissue in
the pelvis (ovaries, pouch of Douglas, uterine ligaments, tubes & rectovaginal septum), less
frequently in more remote sites of the peritoneal cavity & about the umbilicus.
Three possibilities have been suugested to explain the origin of these lesions.
1. The regurgitation theory, currently the most accepted, proposes menstrual backflow
through the fallopian tubes with subsequent implantation.
2. The metaplastic theory proposes endometrial differentiation of coelomic epithelium.
3. The vascular or lymphatic dissemination theory has been raised to explain extrapelvic endometriosis.
Gross features
Endometriosis almost always contains functioning endometrium, which undergoes cyclic
bleeding. Because blood collects in these ectopic foci, they usually appear grossly as red-
blue to yellow-brown nodules.
They vary in size from microscopic up to 2 cm in diameter and lie on or just under the
affected serosal surface. Often individual lesions coalesce to form larger masses.
When the ovaries are involved, the lesions may form large, blood-filled cysts that are
transformed into so-called chocolate cysts as the blood ages.
Seepage and organization of the blood leads to widespread fibrosis, adherence of pelvic
structures, sealing of the tubal fimbriated ends, and distortion of the oviducts and ovaries.
Microscopic features
The histologic diagnosis depends on finding 2 of the following 3 features within the lesions:
1. Endometrial glands 2. Endometrial stroma, or 3. Hemosiderin pigment.
Chapter 7 - Female genital system & Breast
125
Extensive scarring of the oviducts & ovaries often causes sterility. Pain on defecation
reflects rectal wall involvement & dyspareunia (painful intercourse) & dysuria reflect
involvement of the uterine & bladder serosa, respectively. In almost all cases, there is severe
dysmenorrhea & pelvic pain as a result of intrapelvic bleeding & periuterine adhesions.
Organic & Dysfunctional Uterine Bleeding
Menorrhagia, metrorrhagia, & postmenopausal bleeding are common complaints.
Common organic causes include polyps, leiomyomas, endometrial carcinoma,
endometrial hyperplasia, and endometritis, as well as cervical and vaginal lesions
(polyps, cervicitis, or carcinoma).
Causes of Abnormal Uterine Bleeding by Age Group include
Prepuberty: precocious puberty (hypothalamic, pituitary, or ovarian origin)
Adolescence: anovulatory cycle
Reproductive age:
o complications of pregnancy (abortion, trophoblastic disease, ectopic pregnancy),
o Organic lesions (leiomyoma, adenomyosis, polyps, endometrial hyperplasia, carcinoma),
o anovulatory cycle,
○
ovulatory dysfunctional bleeding (e.g., inadequate luteal phase)
Perimenopause: anovulatory cycle, irregular shedding, Organic lesions (carcinoma,
hyperplasia, polyps)
Postmenopause: Organic lesions (carcinoma, hyperplasia, polyps), endometrial atrophy
Dysfunctional Uterine Bleeding (DUB)
Is an abnormal bleeding in the absence of a
well-defined organic lesion in the uterus. Examples of the causes of DUB include
Anovulatory cycles very common at both ends or reproductive life & have several causes:
1) Endocrine dysfunctions e.g. of the hypothalamic-pituitary axis, adrenal, or thyroid
2) Ovarian lesions producing an excess of estrogen
3) Malnutrition, obesity, or debilitating disease 4) Severe physical or emotional stress.
Regardless of the cause, an anovulatory cycle leads to an excess of estrogen relative to
progesterone; the endometrial glands may appear crowded with mild cystic changes with
a relative scant stroma. The poorly supported endometrium partially collapses, with
rupture of spiral arteries, accounting for the bleeding.
Inadequate luteal phase. The corpus luteum may fail to mature normally or may regress
prematurely, leading to a relative lack of progesterone. The endometrium under these
circumstances shows delay in the development of the secretory changes expected at the
date of biopsy.
Contraceptive-induced bleeding: older oral contraceptives containing synthetic
estrogens and progestin induced a variety of endometrial responses-for example, inactive,
nonsecretory glands. The pills in current use have corrected these abnormalities.
Endometrial Hyperplasia
This is an exaggerated endometrial proliferation induced by sufficiently prolonged excess
of estrogen relative to progestin. The severity of hyperplasia is classified according to
2 parameters: a. architectural crowding of the glands & b. cytologic atypia.
Accordingly there are three categories
1. Simple hyperplasia 2. Complex hyperplasia 3. Atypical hyperplasia
These three categories represent a spectrum based on the level and duration of the estrogen
excess. The risk of developing carcinoma depends on the severity of the hyperplastic
changes and associated cellular atypia. Simple hyperplasia carries a negligible risk, while
a woman with atypical hyperplasia has a 20% risk of developing endometrial carcinoma.
Chapter 7 - Female genital system & Breast
126
Thus When atypical hyperplasia is discovered, it must be carefully evaluated for the
presence of cancer and must be monitored by repeated endometrial biopsy.
Any estrogen excess may lead to hyperplasia.
Potential contributors include
1. Failure of ovulation, e.g. around the menopause
2. Prolonged administration of estrogenic steroids
3. Estrogen-producing ovarian lesions such as
a. polycystic ovaries (Stein-Leventhal syndrome)
b. cortical stromal hyperplasia c. granulosa-theca cell tumors of the ovary
4. Obesity, because adipose tissue processes steroid precursors into estrogens.
Gross features
In simple hyperplasia the endometrium is diffusely thickened
In complex & atypical hyperplasia there is usually focal thickening of the endometrium.
Microscopic features
Simple hyperplasia involves both the glands & stroma; the normal gland to stroma ratio is
maintained. The glands are proliferative and some are cystically dilated.
Complex hyperplasia: there is glandular crowding with little stroma separating the
proliferative glands. Typically, this is a focal process.
Simple and complex hyperplasias are further divided into atypical and non-atypical
Atypical hyperplasia: characterized by atypical nuclei of the proliferative glands as
evidenced by nuclear stratification, nuclear rounding and the presence of nucleoli
Tumors of the Endometrium and Myometrium
The most common neoplasms of the body of the uterus are
1. Endometrial polyps, 2. Smooth muscle tumors, and 3. Endometrial carcinomas.
All tend to produce bleeding from the uterus as the earliest manifestation.
Endometrial Polyps:
are usually sessile, hemispheric lesions up to 3 cm in diameter.
Larger polyps tend to be pedunculated & may project from the endometrial mucosa into
the uterine cavity & sometimes through the cervix into the vagina. Microscopically, they
are composed of basalis-type, often cystically dilated glands with a rather fibroblastic
stroma. Small muscular arteries are often prominent. The stromal cells have been found
to be monoclonal. This signifies that they are the neoplastic component. The clinical
significance of these polyps lies in the production of abnormal uterine bleeding and, more
important, the risk (however rare) of giving rise to a cancer.
Leiomyomas
are benign tumors that arise from the smooth muscle cells in the myometrium. Because of their
firmness they are also called fibroids. They are the most common benign tumor in females &
are found in 30% to 50% of women during reproductive life. Estrogens & possibly oral
contraceptives stimulate their growth; conversely, they shrink postmenopausally.
Gross features:
o They are sharply circumscribed, firm gray-white masses with a characteristic whorled cut surface.
o They may occur singly, but are often multiple tumors scattered within the uterus, ranging
in size from small seedlings to massive neoplasms that dwarf the size of the uterus.
o Some are embedded within the myometrium (intramural), whereas others may lie directly
beneath the endometrium (submucosal) or directly beneath the serosa (subserosal).
o Larger neoplasms may show foci of ischemic necrosis with areas of hemorrhage and
cystic softening (red degeneration)
o After menopause they may become densely collagenous and even calcified.

Chapter 7 - Female genital system & Breast
127
Microscopic features: There are whorling bundles of smooth muscle cells. Foci of fibrosis,
calcification, ischemic necrosis, cystic degeneration & hemorrhage may be present.
Leiomyomas of the uterus may be entirely asymptomatic and be discovered only on
routine pelvic examination or imaging studies. The most frequent manifestation, when
present, is menorrhagia. Large masses in the pelvic region may become palpable or may
produce a dragging sensation. Benign leiomyomas rarely transform into sarcomas.
Leiomyosarcomas
Typically arise de novo from the mesenchymal cells of the myometrium. They are almost
always solitary tumors, in contradistinction to the frequently multiple leiomyomas.
Gross features: The tumor is typically bulky. It infiltrates the uterine wall. Sometimes it
projects into the endometrial cavity. They are frequently soft, hemorrhagic, and necrotic.
Microscopic features
They show a wide range of differentiation, from those that closely resemble leiomyoma
to wildly anaplastic tumors. The diagnostic features of leiomyosarcoma include tumor
necrosis, cytologic atypia, and mitotic activity. Since increased mitotic activity alone is
sometimes seen in benign smooth muscle tumors in young women, an assessment of all
three features is necessary to make a diagnosis of malignancy. Recurrence after removal
is common with these cancers & many metastasize, typically to the lungs.
Endometrial Carcinoma (EMC)
After the dramatic drop in the incidence of cervical carcinoma, EMC is currently the most
frequent cancer occurring in the female genital tract.
Epidemiology and Pathogenesis
Appears most frequently around the age of 60 yrs.
There are 2 clinico-pathological settings in which EMC arise:
1. In perimenopausal women with estrogen excess; these are of endometrioid type
2. In older women with endometrial atrophy; these are of serous type.
Well-defined risk factors for endometrioid carcinoma include
a. Obesity: associated with increased synthesis of estrogens in fat depots b. Diabetes
c. Hypertension d. Infertility: women tend to be nulliparous, often with anovulatory cycles.
At least some of these risk factors point to increased estrogen stimulation & indeed it is
well recognized that prolonged estrogen replacement therapy and estrogen-secreting
ovarian tumors increase the risk of this form of cancer. Many of these risk factors are the
same as those for endometrial hyperplasia, and endometrial carcinoma frequently arises on
a background of endometrial hyperplasia. Mutations in DNA mismatch repair genes &
PTEN have been demonstrated. Serous carcinoma of the endometrium typically arises in a
background of atrophy. Nearly all cases have mutations in the p53 tumor suppressor gene.
Gross features: EMC may be exophytic (fungating, polypoid) or infiltrative.
Microscopic features
o Consists of malignant endometrial-like tubular glands of varying grades. Squamous
metaplasia is frequent. Sometimes, the tumor is adeno-squamous carcinoma.
o Tumors originate in the mucosa and may infiltrate the myometrium and enter vascular
spaces, with metastases to regional lymph nodes.
o Serous carcinoma forms small tufts & papillary arrangements rather than the glands seen in
endometrioid carcinoma & has much greater cytologic atypia. They are particularly aggressive.
Patients with EMC presents with leukorrhea and irregular bleeding. With progression, the
uterus may become palpable, and in time it becomes fixed to surrounding structures by
extension of the cancer beyond the uterus. EMCs are usually late-metastasizing
neoplasms, but dissemination eventually occurs, with involvement of regional nodes and
more distant sites. The prognosis depends heavily on the stage of the disease.

Chapter 7 - Female genital system & Breast
128
Fallopian tubes
Salpingitis
Is inflammations of the tube and is almost always microbial in origin. With the declining
incidence of gonorrhea, nongonococcal organisms, such as Chlamydia, Mycoplasma
hominis, coliforms, and (in the postpartum setting) streptococci and staphylococci, are
now the major offenders. Compared to gonococci, the nongonococcal infections differ in
that they are more invasive, penetrating the wall of the tubes and thus tend more often to
give rise to blood-borne infections and seeding of the meninges, joint spaces, and
sometimes the heart valves. Tuberculous salpingitis is far less common and is
encountered almost always in combination with tuberculosis of the endometrium.
All forms of salpingitis may produce pelvic masses when the tubes become distended
with either exudate or, later, burned-out inflammatory debris and secretions. Adherence
of the tube to the ovary and adjacent ligamentous tissues results in a tubo-ovarian
abscess, or when infection subsides, a tubo-ovarian complex. Even more serious is the
potential for adhesions that increases the risk of tubal ectopic pregnancy. Damage or
obstruction of the tubal lumina may produce permanent sterility.
Tubal pregnancy
(see later)
Primary adenocarcinomas
may be of papillary serous or endometrioid histology and frequently involve the
omentum and peritoneal cavity at presentation.
Chapter 7 - Female genital system & Breast
129
Ovaries
Follicle cyst and Luteal Cyst
Are common and usually harmless lesions originate in unruptured graafian follicles or in
follicles that have ruptured and immediately sealed. Such cysts are often multiple and
subserosal. Occasionally, they achieve diameters of 4 to 5 cm and may thus become
palpable and produce pelvic pain. They may also rupture, producing intraperitoneal
bleeding and acute abdominal symptoms.
Polycystic Ovaries (Stein-Leventhal syndrome)
Oligomenorrhea, hirsutism, infertility, and sometimes obesity may appear in young women
secondary to excessive production of androgens by multiple cystic follicles in the ovaries.
The ovaries are usually twice the normal size, gray-white with a smooth outer surface,&
studded with subcortical cysts 0.5 to 1.5 cm in diameter. Microscopically, there is a
thickened, fibrotic tunica with underlying follicular cysts. Stigmata of previous ovulation
are usually absent (corpora lutea or albicans). In most patients there are excessive
production of androgens, high concentrations of luteinizing hormone & low concentrations
of follicle-stimulating hormone. These changes inhibit ovulation. It is proposed that the
ovaries in this condition elaborate excess androgens and these, through the hypothalamus,
inhibit the secretion of follicle-stimulating hormone by the pituitary. The basis of excess
ovarian androgen secretion is not clear. The diagnosis of this syndrome can not be made on
morphological grounds alone; both clinical & endocrine data are also required.
Tumors of the ovary
Ovarian cancer is the 5
th
leading cause of cancer death in women. Ovary tumors are
diverse & this diversity is attributable to the three cell types that make up the normal ovary:
1. The surface (coelomic) covering epithelium
2. The germ cells 3. The sex cord/stromal cells.
Each of these cell types gives rise to a variety of tumors. Neoplasms of surface epithelial
constitute the great majority of primary ovarian tumors (70%) & their malignant forms
account 90% of ovarian cancers. Germ-cell & sex cord/stromal cell tumors constitute 20-30%
of ovarian tumors, but are collectively responsible for fewer than 10% of malignant tumors.
Pathogenesis
Nulliparity and family history are the two most important risk factors of epithelial ovarian
cancers. There is a higher incidence of carcinoma in unmarried women and married
women with low parity. Up to10% of ovarian cancers are familial; the majority of these
hereditary cancers seem to be caused by mutations in BRCA1 and BRCA2 genes, these
are also associated with hereditary breast cancer. Indeed, with mutations in these genes
there is increased risk for both ovarian and breast cancers. Mutations in BRCA genes are
also present in 10% of sporadic (nonfamilial) ovarian cancers. Other molecular changes
of ovarian neoplasms include HER2/NEU & K-RAS proteins over-expression and p53
mutation. The latter is present in about 50% of all ovarian cancers.
Surface epithelial tumors
These neoplasms are derived from the surface coelomic mesothelial covering of the
ovary. With repeated ovulation and scarring the surface epithelium is pulled into the
subjacent cortex, forming small epithelial cysts. These can undergo metaplasia with
subsequent neoplastic transformation into the various histological types of the epithelial
tumors. Benign lesions are usually cystic (cystadenoma) or can have an accompanying
Chapter 7 - Female genital system & Breast
130
stromal component (cystadenofibroma). Malignant tumors may also be cystic
(cystadenocarcinoma) or solid (carcinoma). The surface epithelial tumors also have an
intermediate, borderline category referred to as tumors of low malignant potential. These
seem to be low-grade cancers with limited invasive potential. Thus, they have a better
prognosis than the fully malignant ovarian carcinomas. Surface epithelial tumors are
divided into serous, mucinous, endometrioid & Brenner tumors.
A.
Serous Tumors
are the most frequent of the ovarian tumors. Combined, borderline & frankly malignant
serous carcinomas are the most common malignant ovarian tumors (60% of all ovarian
cancers). Benign lesions are usually encountered around 35 yrs of age & malignant ones
around 55.
Gross features
o Most serous tumors are large, spherical, and cystic. About 25% of the benign forms are
bilateral. These benign tumors display a smooth and glistening serosal covering.
o They are generally unilocular, but larger examples may be multilocular.
o The cystic spaces are usually filled with a clear serous fluid. Projecting into the cystic
cavities are papillary projections that become more marked in malignant tumors.
o In contrast to the benign examples, the surface of cystadenocarcinoma shows nodular or
warty irregularities that represent cancerous penetration of the serosa.
Microscopic features
o The benign tumors show a single layer of tall columnar epithelium that lines the cyst (s)
o Psammoma bodies (rounded laminated calcified structures) are common.
o In carcinoma, the lining cells display malignant features with invasion of the stroma.
Papillary formations are complex and multilayered.
o Borderline tumors show milder cytologic atypia and typically, no stromal invasion. These
tumors may seed the peritoneum by tumors implants that are typically also noninvasive.
In general, malignant serous tumors spread to regional lymph nodes, including para-
aortic lymph nodes, but distant lymphatic and hematogenous metastases are infrequent.
The prognosis for the clearly invasive serous cystadenocarcinoma is poor and depends
heavily on the stage of the disease at the time of diagnosis. But it is much better for the
borderline tumors even with the presence of peritoneal implants.
B. Mucinous Tumors
Differ from serous tumors essentially in the epithelium, which is of mucin-secreting cells
similar to those of the endocervical or intestinal mucosa. These tumors occur in women
in the same age range as those with serous tumors, but the majority are benign (80%),
only 10% are malignant (cystadenocarcinomas), and 10% are of low malignant potential.
Gross features
The incidence of bilateral ovarian involvement is much lower than for their serous
counterparts. Bilateral mucinous carcinomas of the ovary must be differentiated from
metastatic adenocarcinomas of the gastrointestinal tract, which may present as ovarian
masses (Krukemberg tumors).
Compared to their serous tumors, they show mucinous cystic contents and tend to be
larger and multilocular but papillary formations are less common.
Microscopic features
o Mucinous tumors are classified according to the type of the mucin-producing epithelial
cells into endocervical, intestinal and müllerian-types. The intestinal type is almost
always present in borderline mucinous tumors and mucinous carcinomas.
o Unlike in their serous counterparts, psammoma bodies are not found.
o Serosal penetration and solid areas point to malignancy.
Chapter 7 - Female genital system & Breast
131
Rupture of mucinous tumors may result in mucinous deposits in the peritoneum but
typically do not result in the malignant growth referred to as pseudomyxoma peritonei.
The vast majority if not all cases of the latter are caused by metastasis from the GIT,
primarily the appendix. Metastasis of mucinous tumor of the gastrointestinal tract to the
ovaries (Krukenberg tumor) may also mimic an ovarian primary tumor.
The prognosis of mucinous cystadenocarcinoma is somewhat better than that of the serous
counterpart, but the stage rather than histologic type is the major determinant of prognosis.
C.
Endometrioid Tumors
May be solid or cystic, but sometimes they develop as a mass projecting from the wall of
an endometriotic cyst. They are distinguished by the formation of tubular glands (similar
to those of the endometrium) within the linings of cystic spaces. Endometrioid tumors are
usually malignant. They are bilateral in about 30% of cases, and up to 30% of women
with these ovarian tumors have a concomitant endometrial carcinoma.
D.
Brenner Tumor
Is an uncommon, solid, usually unilateral ovarian tumor consisting of an abundant stroma
containing nests of transitional epithelium resembling that of the urinary tract. Although
most are benign, both malignant and borderline tumors have been described.
Germ cell tumors
1) Dysgerminoma
: these usually presents within 10 to 30 years of age. Their microscopic
picture is analogous to testicular seminoma. All are malignant but only 30% are
aggressive and disseminate. All are radiosensitive with 80% cure.
2) Yolk sac tumor & embryonal carcinoma
are similar to their testicular counterparts.
3) Choriocarcinoma
presents within the first three decades of life. They are pathologically
identical to placental choriocarcinoma.
4) Teratomas
Constitute up to 20% of ovarian tumors and arise in the first two decades of life; the
younger the person, the greater is the likelihood of malignancy. However, more than 90%
of these germ-cell neoplasms are benign mature cystic teratomas.
Benign (Mature) Cystic Teratomas
are characterized by differentiation of totipotential
germ cells into mature tissues representing all three germ cell layers: ectoderm,
endoderm, and mesoderm. Usually there is the formation of a cyst lined by recognizable
epidermis stuffed with adnexal appendages-hence the common designation dermoid
cysts. They rarely exceed 10 cm in diameter. On opening, they are often filled with
sebaceous secretion and matted hair. Sometimes there is a nodular projection from which
teeth protrude. Occasionally, foci of bone and cartilage, nests of bronchial or
gastrointestinal epithelium, and other recognizable lines of development are also present.
Thyroid tissues are present in 10% of the cases. These tumors are prone to undergo
torsion (10% of cases), producing an acute surgical emergency.
Immature Malignant Teratomas
differ from mature cystic teratoma by being often
bulky, predominantly solid or near-solid, and punctuated by areas of necrosis.
Microscopically, the distinguishing feature is a variety of immature tissues such as
cartilage, bone, muscle, nerve & other structures. Particularly worrying are foci of
neuroepithelial differentiation, because most of these are aggressive & metastasize widely.
Specialized Teratomas
: These include
1. Struma ovarii: composed entirely of mature thyroid tissue that may hyperfunction and
produce hyperthyroidism.
2. Ovarian carcinoid, which in rare instances has produced the carcinoid syndrome.
Chapter 7 - Female genital system & Breast
132
Sex cord tumors
these include
1) Granulosa-thecal cell tumors
:
mostly seen in postmenopausal but can occur at any age. They may be tiny or large, gray to
yellow, with cystic spaces. They are composed of mixture of granulosa cells (in cords,
sheets, or strands) and spindled, plump lipid-laden thecal cells. Granulosal elements may
recapitulate ovarian follicle as Call-Exner bodies (small round cavity containing
eosinophilic material and often shrunken nuclei). Nuclear folds or grooves (coffee-bean"
appearance) is an important diagnostic feature (Fig. 10-30) Hyperestrinism occurs in 75%
of cases; the effects depend on age: in children there is isosexual precocity; in adults &
elderly there is abnormal uterine bleeding due to estrogen-induced endometrial hyperplasia.
However, the tumor may be hormonally inactive or paradoxically androgenic The large
amounts of estrogen (from thecal elements) may encourage the development of endometrial
or breast carcinoma. Granulosa cell element may be malignant.
2) Thecoma-fibroma group of tumors
Occur at any age. Ovarian fibroma is solid gray and consists of spindle fibroblastic cells.
Ovarian thecoma is yellow in color because they are composed of lipid-laden, plump
thecal cells. Few tumors in this group elaborate estrogens. About 40%, for obscure
reasons, produce ascites and hydrothorax (Meigs syndrome). They are rarely malignant.
3) Sertoli-Leydig cell tumors
Occur at any age. They are usually small and recapitulate the development of testis with
tubules, or cords (Sertoli cells) and plump pink Leydig cells. Many of these tumors are
functional (masculinizing or defeminizing). They are rarely malignant.
Metastases to ovary
Are usually encountered in older ages. Mostly both ovaries are involved. Grossly there are
solid gray-white masses as large as 20cm in diameter. Microscopically, there are malignant
tumor cells arranged in cords or glands & dispersed through a usually prominent
fibroblastic background. Primaries include GIT, breast, and lung, & endometrium.
When
the infiltration is by mucin-containing signet ring cells the term Krukenberg tumor is
applied. This is usually bilateral and nearly always of metastatic origin.
Clinical Correlations of ovarian cancers:
All ovarian neoplasms produce no symptoms or signs until they are well advanced.
Indeed, about a third of all ovarian neoplasms are discovered incidentally on routine
gynecologic examination. The clinical presentation of all ovarian tumors is remarkably
similar, except for the functioning neoplasms that have hormonal effects. When they
become large enough they cause local pressure symptoms (e.g., pain, GI complaints, and
urinary frequency). Larger masses, notably the common epithelial tumors, may cause an
increase in abdominal girth. Smaller masses, particularly dermoid cysts, sometimes
become twisted on their pedicles (torsion), producing severe abdominal pain mimicking
an "acute abdomen." Fibromas and malignant serous tumors often cause ascites, the latter
resulting from metastatic seeding of the peritoneal cavity, so that tumor cells can be
identified in the ascitic fluid. Among the many markers that have been explored,
elevations of the protein CA-125 have been reported in 75% to 90% of women with
epithelial ovarian cancer. However, as with carcinoembryonic antigen (CEA) in colon
cancer, CA-125 measurements are of greatest value in monitoring response to therapy.

Chapter 7 - Female genital system & Breast
133
Diseases of pregnancy
Placental Inflammations and Infections
Infections reach the placenta either as an ascending infection through the birth canal (the
most common) or through hematogenous route (transplacental infection).
Ascending infections are mostly bacterial and are complicated by premature rupture of the
membranes that eventuates in premature birth. The chorioamnion shows neutrophilic
infiltration with edema and congestion (acute chorioamnionitis). Extension of the infection
may involve the umbilical cord and placental villi and cause acute vasculitis of the cord. In
hematogenous spread of bacteria & other organisms the villi are most often affected (villitis)
Ectopic Pregnancy
Refers to implantation of the fertilized ovum in any site other than the normal uterine
location. It occurs in 1% of pregnancies. In more than 90% it is a tubal pregnancy; other
sites include the ovaries & abdominal cavity. Any factor that retards passage of the ovum
along its course through the tube to the uterus predisposes to an ectopic pregnancy. In
about half of the cases, such obstruction is a complication of chronic salpingitis,
although intrauterine tumors & endometriosis may also impede passage of the ovum. In
approximately 50% of tubal pregnancies, no anatomic cause can be demonstrated.
Ectopic pregnancies are characterized by a normal early development of the embryo, with
the formation of placental tissue, the amniotic sac, and decidual changes. With tubal
pregnancies, however, the invading placenta eventually burrows through the wall,
causing intratubal hematoma (hematosalpinx), intraperitoneal hemorrhage, or both. The
tube is usually locally distended (up to 4 cm in diameter) by a contained mass of freshly
clotted blood in which may be seen bits of gray placental tissue and fetal parts. The
histologic diagnosis depends on the visualization of placental villi or, rarely, of the
embryo. Until rupture occurs, an ectopic pregnancy may be indistinguishable from a
normal one, with cessation of menstruation and elevation of serum and urinary placental
hormones. Under the influence of these hormones, the endometrium (in 50% of cases)
undergoes the characteristic hypersecretory and decidual changes. However, the absence
of elevated gonadotropin levels does not exclude this diagnosis, because poor attachment
with necrosis of the placenta is common. Rupture of an ectopic pregnancy may be
catastrophic, with the sudden onset of intense abdominal pain and signs of an acute
abdomen, often followed by shock. Prompt surgical intervention is necessary.
Gestational Trophoblastic Tumors
These are divided into four morphologic categories:
1. Hydatidiform mole a. Complete b. Incomplete
2. Invasive mole
3. Choriocarcinoma.
4. Placental site trophoblastic tumor
All the three produce human chorionic gonadotropin (hCG), which can be detected in the
circulating blood and urine, at much higher titers than those found during normal
pregnancy. The titers are progressively rising from hydatidiform mole to invasive mole to
choriocarcinoma. In addition to aiding diagnosis, the fall or rise in the level of the
hormone in the blood or urine can be used to monitor the effectiveness of treatment.
Clinicians therefore prefer to lump the three conditions under the heading of gestational
trophoblastic disease, because the response to therapy as judged by the hormone titers is
more important than any arbitrary anatomic segregation of one lesion from another.
Chapter 7 - Female genital system & Breast
134
1) Hydatidiform Mole: Complete and Partial
The typical hydatidiform mole is a large mass of swollen chorionic villi, appearing
grossly as grapelike structures. The swollen villi are covered by varying amounts of
sometimes highly atypical chorionic epithelium.
2 distinctive subtypes of moles have been characterized: complete and partial moles.
A. The complete hydatidiform mole does not permit embryogenesis and thus does not
contain fetal parts. All of the chorionic villi are abnormal, and the chorionic epithelial
cells are diploid (46, XX or, uncommonly, 46, XY).
B. The partial hydatidiform mole permits early embryogenesis and therefore contains
fetal parts, has some normal chorionic villi, and is almost always triploid (e.g., 69, XXY).
The two patterns result from abnormal fertilization; in both two spermatozoa fertlize an
ovum; in a complete mole an empty egg is fertilized, which yields a diploid karyotype
composed of entirely paternal genes, while in a partial mole a normal egg is fertilized,
resulting in a triploid karyotype with a preponderance of paternal genes.
For unknown reasons there is a much higher incidence of complete moles in Asian
countries. Moles are most common before the age of 20 years and after the age 40 years,
and a history of the condition increases the risk in subsequent pregnancies. Although
traditionally discovered around week 12 of pregnancy because of a gestation that was
"too large for dates," early monitoring of pregnancies by ultrasound has lowered the
gestational age of detection, leading to the more frequent diagnosis of "early complete
hydatidiform mole." In either instance, elevations of hCG in the maternal blood and
absence of fetal parts or fetal heart sounds are typical.
Gross features
In fully developed cases of complete moles the uterine cavity is filled with a delicate,
friable mass of thin-walled, translucent cystic structures.
Fetal parts are rarely seen in complete moles but are common in partial moles. In the
latter only some but not all of the villi are swollen.
Microscopic features
The complete mole
o There is hydropic swelling of all the chorionic villi, which are avascular.
o The central substance of the villi is a loose, edematous stroma.
o The chorionic epithelium almost always shows some degree of proliferation of both
cytotrophoblast and syncytiotrophoblast. The proliferation may be mild, but in many
cases there is striking circumferential hyperplasia.
Partial moles
o The villous edematous swelling involves only some of the villi and the trophoblastic
proliferation is focal and slight.
o The villi of partial moles have a characteristic irregular scalloped margin.
o In most cases of partial mole there is evidence of an embryo or fetus. This may be in the
form of fetal red blood cells in placental villi or, in some cases, a fully formed fetus that,
despite a triploid karyotype, is morphologically nearly normal in appearance.
Overall, 80% to 90% of moles do not recur after thorough curettage; 10% of complete
moles are invasive, but not more than 2% give rise to choriocarcinoma. Partial moles
rarely give rise to choriocarcinomas. With complete moles, monitoring the post-curettage
blood and urinary hCG concentrations, particularly the more definitive β-subunit of the
hormone, permits detection of incomplete removal or a more ominous complication and
leads to the institution of appropriate therapy, including in some cases chemotherapy,
which is almost always curative.

Chapter 7 - Female genital system & Breast
135
2) Invasive Mole
Is a complete mole that is more invasive locally but do not have the metastatic potential
of a choriocarcinoma. An invasive mole retains hydropic villi, which penetrate the uterine
wall deeply, possibly causing rupture and sometimes life-threatening hemorrhage. Local
spread to the broad ligament and vagina may also occur. Microscopically, the epithelium
of the villi is hyperplastic and atypical. Metastases do not occur but hydropic villi may
embolize to distant organs, such as lungs or brain. However, these emboli are not
metastatic & may regress spontaneously. Because of the greater depth of invasion into the
myometrium, an invasive mole is difficult to remove completely by curettage & therefore
serum hCG may remain elevated. In most cases cure is possible through chemotherapy.
3) Choriocarcinoma
Is a very aggressive malignant tumor arises either from gestational chorionic epithelium
or, less frequently, from totipotential cells within the gonads or elsewhere. They are much
more common in Asian and African countries, reaching a frequency of 1 in 2000
pregnancies. The risk is somewhat greater before age 20 and is significantly elevated after
age 40. Approximately 50% of choriocarcinomas complicate complete hyaditidiform
moles; about 25% arise after an abortion, and most of the remainder occur during what had
been a normal pregnancy. Most cases are discovered by the appearance of a bloody or
brownish discharge accompanied by a rising titer of hCG, particularly the β-subunit, in
blood and urine & the absence of marked uterine enlargement, such as would be anticipated
with a mole. In general, the titers are much higher than those associated with a mole.
Gross features
The tumor appears as very hemorrhagic, soft, nodular, necrotic masses within the uterus.
Sometimes the primary lesion may self-destruct, and only the metastases tell the story.
Very early, the tumor insinuates itself into the myometrium and into vessels.
Microscopic features
Cytotrophblastic cells tend to grow in clusters and sheets, separated by streaming masses
of syncytiotrophoblast, forming the characteristic dimorphic growth pattern of
mononucleate cytotrophoblast and syncytiotrophoblast.
Hemorrhage and necrosis are usually present. In contrast to the case with hydatidiform
moles & invasive moles, chorionic villi are not formed.
By the time most choriocarcinomas are discovered, there is usually widespread
dissemination via the blood, most often to the lungs, vagina, brain, liver, and kidneys.
Lymphatic invasion is uncommon. Despite the extreme aggressiveness of these
neoplasms, which made them nearly uniformly fatal in the past, present-day chemotherapy
has achieved nearly 100% cure. By contrast, there is relatively poor response to
chemotherapy in choriocarcinomas that arise in the gonads (ovary or testis). This striking
difference in prognosis may be related to the presence of paternal antigens on placental
choriocarcinomas but not on gonadal lesions. Conceivably, a maternal immune response
against the foreign (paternal) antigens helps by acting as an adjunct to chemotherapy.
4) Placental Site Trophoblastic Tumor
These uncommon tumors are diploid, often XX in karyotype, and are derived from the
placental site intermediate trophoblast. They typically arise a few months after a
pregnancy. Because intermediate trophoblasts do not produce hCG in large amounts,
hCG concentrations are elevated, but only slightly. More typically these tumors produce
human placental lactogen. These tumors are indolent and generally have a favorable
outcome if confined to the endomyometrium. However, they are not as sensitive to
chemotherapy as other trophoblastic tumors, and the prognosis is poor when spread has
occurred beyond the uterus.
Chapter 7 - Female genital system & Breast
136
Pre-eclampsia, Eclampsia (Toxemia Of Pregnancy)
The syndrome of hypertension, proteinuria and edema developing in the third trimester of
pregnancy, is referred to as preeclampsia. This occurs in 5% to 10% of pregnancies,
chiefly with first pregnancies in women older than age 35 yrs. In those severely affected,
convulsive seizures may appear, and the symptom complex is then termed eclampsia.
Full-blown eclampsia may lead to disseminated intravascular coagulation (DIC), with all
of its widespread ischemic organ injuries, and so eclampsia is potentially fatal.
Pathogenesis:
A basic underlying feature in all cases is inadequate maternal blood flow to the placenta
secondary to inadequate development of the spiral arteries of the uteroplacental bed. In the
third trimester of normal pregnancy, the musculo-elastic walls of the spiral arteries are
replaced by a fibrinous material, permitting them to dilate into wide vascular sinusoids. In
preeclampsia and eclampsia, the musculoelastic walls are retained & the channels remain
narrow. A decrease in vascular endothelial growth factor (VEGF) & an increase in
antiangiogenic factors have been found to predate the onset of preeclampsia. While the
exact basis of vascular abnormalities remains unknown, several consequences ensue:
1) Placental hypoperfusion with an increased predisposition to the development of
infarcts. 2) Reduced elaboration by the trophoblast of the vasodilators prostacyclin,
prostaglandin E
2
, and nitric oxide, which in normal pregnancies oppose the effects of
renin-angiotensin-hence the hypertension of preeclampsia and eclampsia
3) Production by the ischemic placenta of thromboplastic substances such as tissue factor
and thromboxane, which probably account for the development of DIC.
Pathologic features
A. Placental changes include:
1) Infarcts, which are a feature of normal pregnancy, are much more numerous.
However, they may be absent.
2) Retroplacental hemorrhages occur in 15% of patients.
3) Premature aging of the villi may occur as evidenced by villous edema,
hypovascularity, and increased production of syncytial epithelial knots.
4) Acute atherosis of spiral arterioles: prominent in well-advanced eclampsia &
characterized by thickening and fibrinoid necrosis of the vessel wall with focal
accumulations of lipid-containing macrophages. Necrosis of these cells releases lipid.
Such lesions accentuate the placental ischemia.
B. Multiorgan changes
May be present, reflecting the development of DIC. The kidneys are basically, shows
changes consist of fibrin thrombi within the glomerular capillaries, accompanied by
endothelial swelling. Focal glomerulitis may ensue. When numerous glomeruli are
affected, blood flow to the cortex is reduced, possibly resulting in renal cortical necrosis
that may be bilateral and fatal. Microvascular thrombi are also found in the brain,
pituitary, heart, and elsewhere. They have the potential of producing focal ischemic
lesions accompanied by microhemorrhages.
Preeclampsia appears insidiously in the 24th to 25th weeks of gestation, with the
development of edema, proteinuria, and rising blood pressure. Should the condition
evolve into eclampsia, renal function is impaired, the blood pressure mounts, and
convulsions may occur. Prompt therapy early in the course aborts the organ changes, with
clearance of all abnormalities promptly after delivery or cesarean section.
Chapter 7 - Female genital system & Breast
137
Breast
Fortunately, most lesions of female breast are innocent, but as is well known, breast
cancer was the foremost cause of cancer deaths in women in the United States until 1986,
when it was replaced by carcinoma of the lung.
Congenital anomalies
Supernumerary nipples or breasts
may be found along the embryonic ridge (milk line).
Congenital inversion of the nipple
is of significance because similar changes may be
produced by an underlying cancer.
Galactocele
is a cystic dilation of an obstructed duct that arises during lactation. Besides being
painful, the cysts may rupture to provoke a local inflammatory reaction, which may yield
a persistent focus of induration that may arouse suspicion of malignancy.
Fibrocystic changes (FCC)
are a group of alterations in the female breast ranging from harmless to those associated
with an increased risk of breast carcinoma. Some of these changes (stromal fibrosis and
microcysts or macrocysts) produce palpable lumps. FCC are in essence an exaggeration
and distortion of the cyclic breast changes that occur normally in the menstrual cycle.
Traditionally, these breast alterations have been called fibrocystic disease. However,
most of the changes included within this diagnosis have little clinical significance except
that they cause nodularity; only a small minority (epithelial hyperplasia) are clinically
important. Thus, the term FCC is preferred, since it does not mark the subject with a
disease. The lumps produced by the various patterns of fibrocystic change must be
distinguished from cancer, and the distinction between the unimportant variants and the
more significant ones can be made by examination of fine-needle aspiration material or
more definitively by biopsy and histologic evaluation.
The alterations encountered within the FCC are subdivided into
1. Nonproliferative (simple FCC) that include cysts &/or fibrosis (the most common)
2. Proliferative that include a range of harmless to atypical epithelial cell hyperplasias of
the ducts or ductules as well as sclerosing adenosis.
All the entities within FCC tend to arise during reproductive period of life but may persist
after menopause. The various changes, particularly the nonproliferative ones, are so
common, being found at autopsy in up to 80% of women, that they almost constitute
physiologic variants.
Cysts and Fibrosis:
Characterized by an increase in fibrous stroma associated with dilation of ducts and
formation of cysts of various sizes. Grossly, a single large cyst may form within one
breast, but the disorder is usually multifocal and often bilateral. The cysts are up to 5 cm
in diameter. Unopened, they are brown to blue (blue dome cysts) and are filled with
serous, turbid fluid. Microscopically, in smaller cysts, the epithelium is cuboidal to
columnar. In larger cysts it may be flattened. Frequently, cysts are lined by large cells
with abundant granular, eosinophilic cytoplasm, with small, round, deeply stained nuclei;
this is called apocrine metaplasia, which is virtually always benign.
Proliferative Changes
1) Epithelial Hyperplasia
comprises a range of proliferative lesions within the ductules, the
terminal ducts (ductal hyperplasia), and sometimes the lobules (lobular hyperplasia) of
Chapter 7 - Female genital system & Breast
138
the breast. Some of the epithelial hyperplasias are mild and carry little risk of carcinoma,
but others are the more florid and atypical that carry a significantly greater risk.
Microscopically, the ducts, ductules, or lobules may be filled with cuboidal cells, within
which small glands are disposed (fenestrations). The degree of hyperplasia can be mild,
moderate, or severe. In some instances the hyperplastic cells show complex architectural
patterns and approaching morphologically those of ductal carcinoma in situ, such
hyperplasia is called atypical. The line separating the epithelial hyperplasias without
atypia from atypical hyperplasia is difficult to define, just as it is difficult to clearly
distinguish between atypical hyperplasia and carcinoma in situ.
Atypical lobular hyperplasia describes hyperplasias that cytologically resemble lobular
carcinoma in situ, but the cells do not fill or distend more than 50% of the acini within a lobule.
Atypical lobular hyperplasia is associated with an increased risk of invasive carcinoma.
Epithelial hyperplasia per se does not often produce a clinically discrete breast mass.
2) Sclerosing Adenosis
: a significant variant of FCC because its clinical and morphologic
features may be deceptively similar to those of carcinoma. Grossly, the lesion has a hard,
rubbery consistency, & thus simulates that of breast cancer. Microscopically, there is
proliferation of lining epithelial cells and myoepithelial cells in small ducts and ductules,
yielding masses of distorted small glands within a fibrous stroma. Aggregated glands or
proliferating ductules may be virtually back to back (adenosis). Marked stromal fibrosis,
which may compress and distort the proliferating glandular structures, is always
associated with the adenosis; hence, the designation sclerosing adenosis. This overgrowth
of fibrous tissue may completely compress the lumina of the acini and ducts, so that they
appear as solid cords or trabeculae of cells. This pattern may then be difficult to
distinguish histologically from an invasive scirrhous carcinoma. The presence of double
layers of epithelium and the identification of myoepithelial elements are helpful in
suggesting a benign diagnosis. Sclerosing adenosis is associated with only a minimally
increased risk of progression to carcinoma.
Relationship of Fibrocystic Changes to Breast Carcinoma
This is a controversial issue. With respect to the relationship of the various patterns of
fibrocystic change to cancer, the statements below currently represent the most widely
accepted opinions:
1) Minimal or no increased risk of breast carcinoma:
- Fibrosis,
- Cystic changes (microscopic or macroscopic)
- Apocrine metaplasia
- Mild hyperplasia
- Fibroadenoma
2) Slightly increased risk (1.5-2 times):
- Moderate to florid hyperplasia (without atypia)
- Ductal papillomatosis
- Sclerosing adenosis.
3) Significantly increased risk (5 times):
- Atypical hyperplasia, ductular or lobular. Proliferative lesions may be multifocal, and
the risk of subsequent carcinoma extends to both breasts.
A family history of breast cancer may increase the risk in all categories (e.g., to about10-
fold with atypical hyperplasia).
Fortunately, most women who have lumps related to fibrocystic change can be reassured
that there is little or no increased predisposition to cancer.

Chapter 7 - Female genital system & Breast
139
Inflammations
Acute mastitis
develops when bacteria gain access to the breast tissue through the ducts; when there is
inspissation of secretions; through fissures in the nipples, which usually develop during
the early weeks of lactation; or from various forms of dermatitis involving the nipple.
Staphylococcal infections induce single or multiple abscesses accompanied by the typical
clinical acute inflammatory changes. They are usually small, but may be large.
Streptococcal infections generally spread throughout the entire breast, causing pain,
marked swelling, and breast tenderness.
Tuberculosis of the breast
Both clinical and radiological features of tuberculous mastitis are not diagnostic and
easily can be confused with either breast cancer or pyogenic breast abscess by clinicians .
The involvement is usually unilateral without pulmonary involvement. Multiple draining
sinuses may occur.
Although rare, this entity should be taken into consideration; remember the fact that the
prognosis for complete cure with appropriate antituberculous drug therapy is excellent.
Definitive diagnosis of the disease is based on identification of TB bacilli (ZN stain)
within typical histological features under microscopy or detection of the tubercle bacilli
with mycobacterial culture .For breast cancer patients with granulomatous axillary
lymphadenitis, PCR may be required to rule out TB in endemic regions.
Idiopathic Granulomatous mastitis (IGM)
This rare condition is seen mainly in young women, usually after pregnancy. Patients
present with firm tender mass. It may be complicated by overlying skin ulcerations &
multiple draining sinuses. The dx is by excluding other granulomatous conditions e.g.
TB, fungal infections, sarcoidosis, fat necrosis, foreign body reaction, etc. Its distinctive
microscopic feather is that the noncaseating granulomas are centered on the lobules.
Mammary duct ectasia (MDE)
is characterized by inspissation of breast secretions in the main excretory ducts. Ductal
dilation with subsequent rupture leads to nonbacterial chronic inflammation in the
surrounding periductal breast tissues. MDE is usually encountered in multiparous women
in their 40s and 50s. Patients present with a poorly defined palpable periareolar mass,
sometimes with skin retraction, often accompanied by thick, white nipple secretions.
Grossly, sectioning shows ropelike dilated ducts from which thick, cheesy secretions can
be extruded. Microscopically, the dilated ducts are filled by granular debris, sometimes
containing lipid-laden macrophages. The most distinguishing features are
1. The prominence of a lymphocytic and plasma cell infiltration
2. The occasional granulomas in the periductal stroma.
Mammary duct ectasia is important because of its clinical confusion with malignancy as
it leads to induration of the affected tissue and, more significantly, to retraction of the
skin or nipple.
Traumatic fat necrosis
is significant only because of its confusion clinically with carcinoma. Most women give a
history of antecedent trauma to the breast. The lesion consists of a central focus of
necrotic fat cells surrounded by neutrophils and lipid-laden macrophages.

Chapter 7 - Female genital system & Breast
140
Tumors of the breast
Fibroadenoma
is the most common benign neoplasm of the female breast. An increase in estrogen activity is
thought to contribute to its development & indeed similar lesions may appear with fibrocystic
changes (fibroadenomatoid changes). The peak incidence is in the 3
rd
decade of life.
Gross features
The tumor is usually solitary, well-defined, and freely movable.
It is variable size & may reach up to 10 cm in diameter. Larger tumors are referred to as
giant fibroadenoma. It is firm, with a uniform tan-white color on cut section.
Microscopically
o There is a loose fibroblastic stroma containing epithelium-lined duct-like spaces of
various forms and sizes.
o The duct-like spaces are lined with single or multiple layers of benign epithelial cells
having an intact basement membrane.
o In some lesions the ductal spaces are open, and fairly regular (pericanalicular
fibroadenoma), whereas in others they are compressed by the proliferation stroma and
thus appear as slits or irregular, star-shaped structures (intracanalicular fibroadenoma).
Cytogenetic studies reveal that the stromal cells are monoclonal and thus represent the
neoplastic element of these tumors. It is possible that the neoplastic stromal cells secrete
growth factors that induce proliferation of epithelial cells.
Phyllodes tumors
are much less common than fibroadenomas. They are thought to arise from the periductal
stroma. Most grow to large, possibly massive size; the patient typically has a history of a
rapidly growing palpable breast mass. Grossly, the tumors are lobulated and cystic and on
sectioning exhibit leaf-like clefts and slits. The latter feature is responsible for the naming
them as phyllodes tumors (Greek for "leaflike"). Microscopically, there is are expansion
and increased cellularity of the stromal component. The most ominous change is the
appearance of anaplasia and high mitotic activity, usually with invasion of adjacent
breast tissue (malignant phyllodes tumor). Most of these tumors remain localized and are
cured by excision; malignant lesions may recur, but they also tend to remain localized.
Only the most malignant, about 15% of cases, metastasize to distant sites.
Intraductal papilloma
is a neoplastic papillary growth within a duct. Most lesions are solitary, found within the
principal lactiferous ducts or sinuses, thus present clinically with a serous or bloody nipple
discharge and/or a small subareolar nodule and rarely, nipple retraction. Grossly, the
tumors are usually solitary and less than 1 cm in diameter. Microscopically, they consist
of delicate, branching papillary growths within a dilated duct (or cyst). Each papillary
projection has a connective tissue core covered by double layer of cells; outer cuboidal
epithelial cells that overlies myoepithelial cells. Some cases display multiple papillomas
(intraductal papillomatosis). The latter sometimes become malignant, whereas the solitary
papilloma is virtually benign. Papillary carcinoma is distinguished by
1. The absence of a myoepithelial component and
2. The epithelial cells show either severe cytologic atypia or monotonous ductal morphology.
Carcinoma (BRCA)
Breast carcinoma in the USA ranks second only to lung cancer as a cause of cancer death
in women; in our country it probably ranks first. Despite advances in diagnosis and
Chapter 7 - Female genital system & Breast
141
treatment, almost 25% of women who develop these neoplasms will die of the disease.
This has incite an intense study of the possible causes and origins of this form of cancer
and of ways to diagnose it early enough to permit cure. 75% of women with breast
cancer are older than age 50; only 5% are younger than the age of 40.
Epidemiology and Risk Factors
large number of risk factors have been identified that modify the likelihood of developing
BRCA. These RF are divided into well-established & less well-established groups.
1) Geographic Variations: the risk for BRCA is significantly higher in North America and
northern Europe than in Asia and Africa. For example, the incidence and mortality rates
are five times higher in the United States than in Japan. These differences seem to be
environmental rather than genetic in origin, because migrants from low-incidence to
high-incidence areas tend to acquire the rates of their adoptive countries, and vice versa.
Diet, reproductive patterns, and nursing habits are thought to be involved.
2) Age: BRCA is uncommon in women younger than age 30. Thereafter, the risk steadily
increases throughout life reaching a plateau after menopause.
3) Genetics and Family History: up to 10% of BRCA are related to specific inherited
mutations. Women are more likely to carry a BRCA susceptibility gene if they have
a. BRCA before menopause b. bilateral cancer
c. other associated cancers (e.g., ovarian cancer)
d. a significant family history (i.e., multiple relatives affected before menopause)
About 50% of women with hereditary BRCA have mutations in gene BRCA1, and an
additional 30% have mutations in BRCA2. Both BRCA 1 & 2 seem to be involved in DNA
repair and act as tumor suppressor genes. Cancer arises when both alleles are inactive
(defective); one due to a germ-line mutation and the second by a subsequent somatic
mutation. It is possible that other mechanisms, such as methylation of regulatory regions,
act to inactivate the genes in sporadic (nonhereditary) cancer.
4) Prolonged exposure to exogenous estrogens: short-term use of combined estrogen plus
progestin as hormonal replacement therapy in postmenopausal women is associated with
an increased risk of breast cancer. However, a large study concluded that birth control
pills do not increase the risk of breast cancer.
5) Ionizing radiation: e.g. to the chest increases the risk of breast cancer. Only women
irradiated before age 30, during breast development, seem to be affected. For example,
20% to 30% of women irradiated for Hodgkin lymphoma in their teens and 20s develop
breast cancer, but the risk for women treated later in life is not elevated.
6) Other less well-established risk factors, such as obesity, alcohol consumption, and a
diet high in fat, have been implicated in the development of breast cancer on the basis of
population studies. Obesity is a recognized risk factor in postmenopausal women.
Pathogenesis
:
The exact cause of breast cancer remains unknown. However, 3 sets of
influences seem to be important:
1) Genetic Changes
In addition to those producing the well-established familial BRCA, genetic changes have
also been implicated in the genesis of sporadic (nonfamilial) breast cancer. Mutations
affecting proto-oncogenes and tumor suppressor genes in breast epithelium contribute to
the malignant transformation process. Overexpression of the HER2/NEU proto-oncogene
has been found to be amplified in up to 30% of invasive breast cancers. This gene is a
member of the epidermal growth factor receptor family & its overexpression is associated
with a poor prognosis. Similarly, amplification of RAS and MYC genes has also been
reported in some human breast cancers. Mutations of the well-known tumor suppressor

Chapter 7 - Female genital system & Breast
142
genes RB and p53 may also be present. Multiple acquired genetic alterations seem to be
involved in the sequential transformation of a normal epithelial cell into a cancerous cell.
2) Hormonal Influences: Endogenous estrogen excess clearly has a significant role. This is
supported by the following observations
a. Many of risk factors mentioned (long duration of reproductive life, nulliparity & late age at
birth of 1
st
child) imply increased exposure to estrogen peaks during the menstrual cycle.
b. Functioning ovarian tumors that elaborate estrogens are associated with breast cancer in
postmenopausal women.
c. Estrogens stimulate the production of growth factors by normal breast epithelial cells and
by cancer cells. It seems that estrogen (and progesterone) receptors normally present in
breast epithelium, and often in breast cancer cells, may interact with growth promoters
(such as transforming growth factor α) produced by human breast cancer cells, to create
an autocrine mechanism of tumor development.
3) Environmental factors are suggested by the variable incidence of breast cancer in
genetically identical groups & the geographic differences in prevalence. Other important
environmental variables include irradiation and exogenous estrogens, described earlier.
Pathological features of BRCA
About 4% of women with breast cancer have bilateral primary tumors. The locations of the
tumors within the breast are: - Upper outer quadrant (50%) - Central sector (subareolar)
(20%) - Upper inner (10%) - Lower outer (10%) - Lower inner (10%)
Of these, invasive ductal carcinoma is the most common. Because it usually has an
abundant fibrous stroma, it is also referred to as scirrhous carcinoma. There are 2 types of
noninvasive breast carcinoma: ductal carcinoma in situ (DCIS) and lobular carcinoma in
situ (LCIS) both usually arise from the terminal duct lobular unit (TDLU). Both are
confined by a basement membrane & don’t invade into stroma or lymphovascular channels.
o DCIS: Has several of histologic appearances. Architectural patterns are often mixed and
include solid, comedo, cribriform, papillary, etc. - Nuclear appearance ranges from bland
and monotonous (low nuclear grade) to pleomorphic (high nuclear grade).
The comedo subtype is distinctive and is characterized by cells with high-grade nuclei
distending spaces with extensive central necrosis. The name derives from the toothpaste-
like necrotic tissue that can be extruded from transected ducts with gentle pressure.
DCIS only rarely presents as a palpable or radiologically detectable mass. If detection is
delayed, a palpable mass or nipple discharge may develop. The cells in the better
differentiated tumors express estrogen &, less often, progesterone receptors. The prognosis
for DCIS is excellent, with over 97% long-term survival after simple mastectomy.
o Paget disease of the nipple is caused by the extension of DCIS up to the lactiferous
ducts and into the contiguous skin of the nipple. The clinical appearance is usually of a
unilateral crusting exudate over the nipple and areolar skin. In about half of cases, an
underlying invasive carcinoma will also be present. Prognosis is based on the underlying
carcinoma and is not worsened by the presence of Paget disease.
o In LCIS the cells are small & monomorphic with bland, round nuclei & occur in loosely
cohesive clusters within distended lobular ductules & acini. Intracellular mucin vacuoles
(signet ring cells) are common. LCIS is virtually always an incidental finding,& , unlike
DCIS, it does not form masses. Approximately one-third of women with LCIS will
eventually develop invasive carcinoma. Unlike DCIS, subsequent invasive carcinomas
arise in either breast at significant frequency. Current treatment requires either close
clinical and radiologic follow-up of both breasts or bilateral prophylactic mastectomy.
o Invasive (Infiltrating) Carcinoma (IDC) is a term used for all carcinomas that cannot
be subclassified into one of the specialized types described below; it does not indicate

Chapter 7 - Female genital system & Breast
143
that this tumor specifically arises from the ductal system. Carcinomas of "no special
type" or "not otherwise specified"(NOS) are synonyms for ductal carcinomas. The
majority (75%) of BRCA fall into this group. This type of cancer is usually associated
with DCIS. Most ductal carcinomas produce a desmoplastic response, which replaces
normal breast fat and forms a hard, palpable mass. The microscopic appearance is quite
variable, ranging from tumors with well-developed tubule formation and low-grade
nuclei to tumors consisting of sheets of anaplastic cells. The tumor margins are usually
irregular. Advanced cancers may cause dimpling of the skin, retraction of the nipple, or
fixation to the chest wall. About two-thirds express estrogen or progestagen receptors,
and about one-third overexpress HER2/NEU.
o Inflammatory carcinoma is defined clinically by an enlarged, swollen, erythematous
breast, usually without a palpable mass. The underlying carcinoma is generally poorly
differentiated and diffusely invades the breast parenchyma. The blockage of numerous
dermal lymphatic spaces by carcinoma results in the clinical appearance. True
inflammation is minimal or absent. Most of these tumors have distant metastases, and the
prognosis is extremely poor.
o Invasive lobular carcinoma consists of cells morphologically identical to & is usually
associated with LCIS. The cells invade individually into stroma and are often aligned in
strands (Indian file). Occasionally they surround cancerous or normal-appearing acini or
ducts, creating a so-called bull's-eye pattern. Lobular carcinomas are also more frequently
multicentric and bilateral (10% to 20%). Almost all of these carcinomas express hormone
receptors, but HER2/NEU overexpression is very rare or absent. These tumors comprise
fewer than 20% of all breast carcinomas.
o Medullary carcinoma is a rare subtype, constituting 1% of cases. These cancers consist
of sheets of large anaplastic cells with pushing, well-circumscribed borders. There is also
a pronounced lymphoplasmacytic infiltrate. These carcinomas uniformly lack hormone
receptors and do not overexpress HER2/NEU.
o Colloid (mucinous) carcinoma is also a rare subtype. The tumor cells produce abundant
quantities of extracellular mucin that dissects into the surrounding stroma. Grossly the
tumors are usually soft and gelatinous.
o Tubular carcinoma rarely presents as palpable masses but account for 10% of invasive
carcinomas smaller than 1 cm found with mammographic screening. It consists of well-formed
tubules with low-grade nuclei. Lymph node metastases are rare & prognosis is excellent.
Features Common to All Invasive Cancers
:
in all forms of BRCA discussed previously,
progression of the disease leads to certain local morphologic features. These include a
tendency to become adherent to the pectoral muscles or deep fascia of the chest wall, with
consequent fixation of the lesion, as well as adherence to the overlying skin, with retraction or
dimpling of the skin or nipple. The latter is an important sign, because it may be the first
indication of a lesion, observed by the woman herself during self-examination. Involvement of
the lymphatic pathways may cause localized lymphedema. In these cases the skin becomes
thickened around exaggerated hair follicles, a change known as peau d'orange (orange peel).
Spread of Breast Cancer
Spread eventually occurs through lymphatic and hematogenous channels.
Lymph node metastases are present in about 40% of cancers presenting as palpable
masses but in fewer than 15% of cases found by mammography. Outer quadrant and
centrally located lesions typically spread first to the axillary nodes. Those in the inner
quadrants often involve the lymph node along the internal mammary arteries. The
supraclavicular nodes are sometimes the primary site of spread, but they may become
Chapter 7 - Female genital system & Breast
144
involved only after the axillary and internal mammary nodes are affected. More distant
dissemination eventually ensues, with metastatic involvement of almost any organ or
tissue in the body. Favored locations are the lungs, skeleton, liver, and adrenals and (less
commonly) the brain. However, no site is immune. Metastases may appear many years
after apparent therapeutic control of the primary lesion, sometimes 15 years later.
Clinical course
:
When BRCA is discovered by the woman or her physician, it is felt as a
deceptively discrete, solitary, painless, and movable mass. At this time, the carcinoma is
typically 2 to 3 cm in size & involvement of the regional lymph nodes (most often axillary)
is already present in about half of patients. With mammographic screening, carcinomas are
frequently detected before they become palpable. The average invasive carcinoma found by
screening is around 1 cm in size & only 15% of these have nodal metastases. In addition, in
many women DCIS is detected before the development of invasive carcinoma.
Prognosis
:
is influenced by the following (note that the 1
st
3 are components of tumor stage):
1) The size of the primary carcinoma. Invasive carcinomas smaller than 1 cm have an
excellent prognosis in the absence of lymph node metastases
2) Lymph node involvement and the number of lymph nodes involved by metastases. With no
axillary node involvement, the 5-year survival rate is close to 90%. The survival rate
decreases with each involved lymph node & is less than 50% with 16 or more involved nodes.
3) Distant metastases. At this stage the disease is rarely curable, although chemotherapy
may prolong survival. 4) The grade; Well-differentiated carcinomas have a significantly
better prognosis as compared with poorly differentiated carcinomas.
5) The histologic type; all specialized types of breast carcinoma (tubular, medullary, and
mucinous) have a somewhat better prognosis than carcinomas NOS.
6) Estrogen or progesterone receptors status: determining the presence or absence of these
receptos is to predict the response to therapy and thus indirectly the prognosis. The highest
rate of response is to anti-estrogen therapy (oophorectomy or tamoxifen) is seen in women
whose tumors have both estrogen and progesterone receptors. Lower rates of response are
seen if only one of the receptors is present. If both are absent, very few patients respond.
7) The proliferative rate of the cancer as measured by mitotic counts. Mitotic counts are
included as part of the grading system. High proliferative rates are associated with a poorer px.
8) Aneuploidy i.e. carcinomas with an abnormal DNA content; these have a slightly worse prognosis.
9) Overexpression of HER2/NEU is caused by amplification of the gene. Overexpression is
associated with a poorer prognosis. However, the importance of evaluating HER2/NEU is
to predict response to a monoclonal antibody ("Herceptin") to the gene product. This is
one of the first examples whereby an antitumor antibody therapy has been developed on
the basis of a specific gene abnormality present in the tumor.
Lesions of the Male breast
Gynecomastia
refers to enlargement of the male breast, which may occur in response to
absolute or relative estrogen excesses. The most important cause of such hyperestrinism
in the male is cirrhosis of the liver, with consequent inability of the liver to metabolize
estrogens. Other causes include Klinefelter syndrome, estrogen-secreting tumors,
estrogen therapy, and digitalis therapy. Grossly, a button-like, subareolar swelling
develops, usually in both breasts but occasionally in only one.
Carcinoma
is a rare, with a frequency ratio to breast cancer in the female of 1: 125. It
occurs in advanced age. Because of the scant amount of breast substance in the male, the
tumor rapidly infiltrates the overlying skin and underlying thoracic wall. Both
morphologically & biologically, these tumors resemble invasive carcinomas in the female.

145
Chapter contents (145 –166)
Topics
Directed by
Page number
1
Pituitary
د. سالم
(146 - 148)
Hyperpituitarism
146
Hypopituitarism
147
Posterior Pituitary Syndromes
148
2
Thyroid
(149 - 155)
Hyperthyroidism (thyrotoxicosis)
149
Hypothyroidism
149
Thyroiditis
149
Graves disease
150
Diffuse and Multinodular Goiters
151
Neoplasms of the thyroid
152
3
Parathyroid glands
(156
– 157)
Hyperparathyroidism
156
Hypoparathyroidism
157
4
Endocrine pancreas
(158 - 160)
Diabetes mellitus
158
Pancreatic endocrine neoplasms (islet cell tumors)
160
5
Adrenal glands
(161 - 165)
Adrenal cortex
161
Adrenal medulla
164
6
Multiple Endocrine Neoplasia Syndromes (MEN)
(166)
Chapter 8 - Endocrine system
146
Pituitary
This is a small, bean-shaped structure that lies at the base of the brain within the confines of
the sella turcica. It is connected to the hypothalamus by a "stalk," composed of axons
extending from the hypothalamus. The pituitary is composed of two morphologically &
functionally distinct components: the anterior lobe (adenohypophysis) & the posterior lobe
(neurohypophysis). The adenohypophysis, in H&E stained sections, shows a colorful
collection of cells with basophilic, eosinophilic or poorly staining ("chromophobic")
cytoplasm. Immnohistochemistry (&sometimes electron microscopy) is usually employed in
typing the hormone (s) present in various pituitary lesions. The release of trophic hormones
is under the control of releasing or inhibiting factors produced in the hypothalamus.
Hyperpituitarism
Causes
A. Pituitary; usually anterior lobe
1. Adenoma (the most common cause) 2. Hyperplasia 3. Carcinoma
B. Extra-pituitary causes
1. Hormone producing extra-pituitary tumors (ectopic hormone production)
2. Certain hypothalamic disorders
Pituitary adenomas
Are classified according to the hormone(s) produced by the neoplastic cells; these are
detected by immunohistochemically-stained tissue sections. Pituitary adenomas can be
functional (associated with hormone excess with their associated clinical manifestations)
or silent. Adenomas are usually composed of a single cell type and produce a single
predominant hormone. However, some adenomas can secrete two hormones (e.g. growth
hormone and prolactin). Other adenomas are hormone negative. Adenomas are divided
on the basis of whether the size is less or exceeds 1 cm. (microadenomas and
macroademomas respectively). Macroadenomas can cause hypopituitarism by
compressing the adjacent non-neoplastic parenchyma.
Pathogenesis: Guanine nucleotide-binding protein (G-protein) mutations are the best
characterized molecular abnormalities. Such mutations eventuate in a persistent increase
in intracellular cAMP, which is a potent mitogenic stimulus promoting cellular
proliferation and hormone synthesis and secretion.
Gross features: Adenomas are usually soft & well-circumscribed. Larger lesions extend
superiorly through the sellar diaphragm compressing the optic chiasm and adjacent
structures. Invasive adenomas refer to nonencapsulated tumors that infiltrate adjacent
bone, dura, and even brain.
Microscopic features
o Adenomas are composed of monomorphic, polygonal cells displayed in sheets, cords, or
papillae. Their nuclei may be uniform or pleomorphic but the mitotic activity is scanty.
The cytoplasm of the constituent cells may be acidophilic, basophilic, or chromophobic.
o The connective tissue is scanty that is why many lesions are soft & even gelatinous in consistency.
The functional status of an adenoma cannot be predicted from its histologic appearance.
Pituitary adenomas are responsible for 10% of intracranial neoplasms. High-resolution
CT or MRI suggests that 20% of "normal" adult pituitary glands contain an incidental
usually silent adenoma measuring 3 mm or more in diameter. The peak incidence of
pituitary adenomas is from 30 to 50 years.
Prolactinomas
are the most common type of hyperfunctioning pituitary adenoma.
Hyperprolactinemia causes amenorrhea, galactorrhea, loss of libido, and infertility.

Chapter 8 - Endocrine system
147
Growth Hormone-Producing Adenomas
(somatotroph cell adenomas) are the second
most common type of functional pituitary adenoma. Because the clinical manifestations of
excessive growth hormone may be slight, the tumor may be quite large by the time they
come to clinical attention. If such tumors occur before closure of epiphyses (prepubertal
children), excessive levels of growth hormone result in gigantism. If elevated levels
persist, or present after closure of the epiphyses, individuals develop acromegaly.
Corticotroph Cell Adenomas
are mostly small (microadenomas) at the time of dx. They
may be clinically silent or cause hypercortisolism referred to as Cushing disease (originally
described by Dr. Harvey Cushing). Large, clinically aggressive corticotroph adenomas may
develop after surgical removal of the adrenal glands for treatment of Cushing syndrome.
This condition, known as Nelson syndrome, occurs in most cases because of a loss of the
inhibitory effect of adrenal corticosteroids on a preexisting corticotroph microadenoma.
Because the adrenals are absent in individuals with Nelson syndrome, hypercortisolism
does not develop. Instead, patients present with the mass effects of the pituitary tumor.
Because ACTH is synthesized as part of a larger prohormone that includes melanocyte-
stimulating hormone (MSH), there may also be hyperpigmentation.
Other Anterior Pituitary Neoplasms
Gonadotroph adenomas
(luteinizing hormone [LH]-producing and follicle-stimulating
hormone [FSH]-producing)
Thyrotroph
(thyroid-stimulating hormone [TSH]-producing) adenomas
Nonfunctioning pituitary adenomas
(hormone-negative (null cell) adenomas)
Nonfunctioning adenomas constitute approximately 25% of all pituitary tumors; they
typically present through their mass effects.
Ptuitary carcinomas
are exceedingly rare. In addition to local extension beyond the sella
turcica, these tumors virtually always have distant metastases.
Hypopituitarism
Caused by
1. Loss of the anterior pituitary parenchyma a. congenital b. acquired
2. Disorders of the hypothalamus e.g. tumors; these interfere with the delivery of
pituitary hormone-releasing factors from the hypothalamus.
Most cases of anterior pituitary hypofunction are caused by the following:
1) Nonfunctioning pituitary adenomas
2) Ischemic necrosis of the anterior pituitary
is an important cause of pituitary
insufficiency. This requires destruction of 75% of the anterior pituitary. Causes include
a. Sheehan syndrome, refers to postpartum necrosis of the anterior pituitary, and is the
most cause. During pregnancy the anterior pituitary enlarges considerably because of an
increase in the size and number of prolactin-secreting cells. However, this physiologic
enlargement of the gland is not accompanied by an increase in blood supply. The
enlarged gland is therefore vulnerable to ischemic injury, especially in women who
develop significant hemorrhage and hypotension during the peripartum period. The
posterior pituitary is usually not affected.
b. Disseminated intravascular coagulation c. Sickle cell anemia
d. Elevated intracranial pressure e. Traumatic injury f. Shock states
3. Iatrogenic i.e. surgical removal or radiation-induced destruction
4. Inflammatory lesions such as sarcoidosis or tuberculosis
5. Metastatic neoplasms involving the pituitary.
6. Mutations affecting the pituitary transcription factor Pit-1

Chapter 8 - Endocrine system
148
Children can develop growth failure (pituitary dwarfism) as a result of growth hormone
deficiency. Gonadotropin or gonadotropin-releasing hormone (GnRH) deficiency leads to
amenorrhea and infertility in women and decreased libido, impotence, and loss of pubic
& axillary hair in men. TSH & ACTH deficiencies result in symptoms of hypothyroidism
and hypoadrenalism. Prolactin deficiency results in failure of postpartum lactation.
Posterior Pituitary Syndromes
The posterior pituitary, or neurohypophysis, is composed of modified glial cells (termed
pituicytes) and axonal processes extending from nerve cell bodies in the hypothalamus.
The hypothalamic neurons produce two peptides: antidiuretic hormone (ADH) and
oxytocin that are
stored in axon terminals in the neurohypophysis. The clinically
important posterior pituitary syndromes involve ADH production and include
1. Diabetes insipidus and
2. Inappropriate secretion of (high levels of) ADH.
ADH is released into the general circulation in response to increased plasma oncotic
pressure & left atrial distention. It acts on the renal collecting tubules to increase the
resorption of free water. ADH deficiency causes diabetes insipidus, a condition
characterized by polyuria. Diabetes insipidus from ADH deficiency is designated as
central, to differentiate it from nephrogenic diabetes insipidus due to renal tubular
unresponsiveness to otherwise normal levels of circulating ADH. The clinical
manifestations of both diseases are similar and include the excretion of large volumes of
dilute urine with low specific gravity. Serum sodium and osmolality are increased as a
result of excessive renal loss of free water, resulting in thirst and polydipsia. In the
syndrome of inappropriate ADH secretion, ADH excess causes resorption of excessive
amounts of free water, with resultant hyponatremia. The most common causes of the
syndrome include the secretion of ectopic ADH by malignant neoplasms (particularly
small-cell carcinomas of the lung), and local injury to the hypothalamus and/or
neurohypophysis. The clinical manifestations are dominated by hyponatremia, cerebral
edema, and resultant neurologic dysfunction.
Chapter 8 - Endocrine system
149
Thyroid
The thyroid gland develops embryologically from the developing pharyngeal epithelium
that descends from the foramen cecum at the base of the tongue to its normal position in
the anterior neck. This pattern of descent explains the occasional presence of ectopic
thyroid tissue, most commonly located at the base of the tongue (lingual thyroid) or at
other sites abnormally high in the neck.
Hyperthyroidism (thyrotoxicosis)
Is “a hypermetabolic state caused by elevated circulating levels of free T
3
and T
4
”. This
may primary (Graves disease) or rarely, secondary (due to pituitary or hypothalamic
diseases). The diagnosis is based on clinical features and laboratory data. The
measurement of serum TSH concentration provides the most useful single screening test
for hyperthyroidism, because TSH levels are decreased in primary cases, even when the
disease is still be subclinical. In secondary cases TSH levels are either normal or raised.
A low TSH value is usually associated with increased levels of free T
4
. Occasionally,
hyperthyroidism results from increased levels of T
3
(T
3
toxicosis).
Hypothyroidism
Is caused by decreased production of adequate levels of thyroid hormones. It is
sometimes divided into primary and secondary categories, depending on whether the
condition arises from diseases affecting the thyroid or hypothalamic/pituitary disease.
The clinical manifestations of hypothyroidism include cretinism and myxedema.
Cretinism refers to hypothyroidism developing in infancy or early childhood that is
associated with impaired development of the skeletal and central nervous systems; these
lead to short stature & severe mental retardation. Hypothyroidism that develops in older
children and adults, results in myxedema, which manifests as mental sluggishness,
coarsening of facial features, enlargement of the tongue, and deepening of the voice. The
latter three are due to accumulation of mucopolysaccharides in the skin, subcutaneous
tissue, tongue and larynx. Pericardial effusions are common and in later stages the heart
is enlarged, and heart failure may occur. Laboratory evaluation has a vital role in the
diagnosis of suspected hypothyroidism because of the nonspecific nature of symptoms.
Measurement of the serum TSH is the most sensitive screening test for this disorder. The
serum TSH is increased in primary hypothyroidism. The TSH concentration is not
increased in hypothyroidism caused by hypothalamic or pituitary disease. Serum T
4
is
decreased in individuals with hypothyroidism of any origin.
Thyroiditis
The more common and clinically significant thyroidites are:
1. Hashimoto thyroiditis 2. Subacute granulomatous 3. Subacute lymphocytic
Hashimoto thyroiditis (Chronic Lymphocytic Thyroiditis)
Is the most common cause of hypothyroidism. It results from gradual autoimmune
destruction of the thyroid gland. There is striking female predominance (10: 1 to 20:1),
and is most prevalent around a mean age of 50 years.
Pathogenesis
o The dominant feature is progressive destruction of thyroid follicular epithelial cells with
gradual replacement by mononuclear cell infiltration and fibrosis.
o Sensitization of CD4+ T-helper cells to thyroid antigens seems to be the initiating event.
Chapter 8 - Endocrine system
150
o The reaction of CD4+ T cells with thyroid antigens produces interferon γ which promote
inflammation and activate macrophages. Injury to the thyroid results from the toxic
products of these inflammatory cells.
o CD8+ cytotoxic T cells also contribute to epithelial cells killing as are natural killer cells.
o There is a significant genetic component to disease pathogenesis. This is supported by
1. The increased frequency of the disease in first-degree relatives,
2. Unaffected family members often have circulating thyroid autoantibodies.
Gross features shows moderate, diffuse, and symmetric enlargement. The cut surface is
pale, gray-tan, firm, nodular and somewhat friable. Eventually there is thyroid atrophy
Microscopic features
o There is widespread, diffuse infiltration of the parenchyma by small lymphocytes, plasma
cells. The lymphocytes are also form follicles some with well-developed germinal centers
o The thyroid follicles are atrophic and lined by epithelial cells having abundant
eosinophilic, granular cytoplasm (Hürthle cells). This is a metaplastic response to the
ongoing injury; ultrastructurally the Hürthle cells are stuffed by numerous mitochondria.
o Interstitial connective tissue is increased and may be abundant.
Hashimoto thyroiditis presents as painless symmetrical goiter with clinical features of
hypothyroidism. This is associated with progressive fall of serum T
4
and T
3
levels &
elevated serum TSH levels. Patients often have other autoimmune diseases and are at
increased risk for
the development of B-cell non-Hodgkin lymphomas.
Subacute Granulomatous (de Quervain) Thyroiditis
Is much less common than
Hashimoto disease. Patients often have an upper respiratory infection just before the
onset of thyroiditis. Thus, a viral infection is probably the cause. Microscopically, there is
disruption of thyroid follicles, with extravasation of colloid. The extravasated colloid
provokes a granulomatous reaction, with giant cells. The condition is self-limited.
Subacute lymphocytic thyroiditis
May follow pregnancy (postpartum thyroiditis)
but mostly affects middle-aged women It is most likely autoimmune in etiology, because
circulating antithyroid antibodies are found in the majority of patients.. In a minority
there is progression to hypothyroidism. Microscopically, there is a lymphocytic
infiltration and hyperplastic germinal center within the thyroid parenchyma. There is no
significant destruction of the thyroid follicles (cf. Hashimoto thyroiditis).
Riedel thyroiditis
Is a rare disorder of unknown etiology, characterized by extensive
fibrosis involving the thyroid and the surrounding neck structures. The presence of a hard
and fixed thyroid mass may be confused clinically with thyroid cancer. It may be
associated with idiopathic fibrosis in other sites, such as the retroperitoneum. The presence
of circulating antithyroid antibodies in most patients suggests an autoimmune etiology.
Palpation thyroiditis
Is caused by vigorous clinical palpation of the thyroid gland that
results in disruption of thyroid follicles at multiple sites associated with chronic
inflammatory cells. It is usually an incidental finding in thyroids resected for other reasons.
Graves disease
Robert Graves in 1835 was the first to report the association of enlargement of the
thyroid gland with long continued palpitations in females. Graves disease is the most
common cause of hyperthyroidism
and characterized by the a triad of
1. Thyrotoxicosis, caused by a diffusely enlarged, hyperfunctioning thyroid
2. Exophthalmos, a form of infiltrative ophthalmopathy (40% of patients)
3. Pretibial myxedema (minority of cases)
The peak incidence is between 20 and 40 years, with women predominating (7:1).
Chapter 8 - Endocrine system
151
Pathogenesis
Graves disease is an autoimmune disorder in which autoantibodies to the TSH receptors
are central to disease pathogenesis; the most important of these is thyroid-stimulating
immunoglobulin (TSI). The latter is an IgG antibody that binds to the TSH receptors of
follicular cells with resultant increased release of thyroid hormones. This antibody is
present in most patients and is relatively specific for Graves disease. The initiation of the
autoimmune reaction in Graves disease seems to be a breakdown in helper T-cell
tolerance, resulting in the production of anti-TSH autoantibodies. Genetic susceptibility
to the disease is evidenced by an increased in the incidence of the disease among family
members of affected patients.
Autoimmune disorders of the thyroid thus span a spectrum in which Graves disease lies
at one extreme and Hashimoto disease occupies the other end. Sometimes
hyperthyroidism may supervene on preexisting Hashimoto thyroiditis (hashitoxicosis),
while at other times individuals with Graves disease may spontaneously develop thyroid
hypofunction. In both disorders the frequency of other autoimmune diseases, such as
SLE, pernicious anemia, & type 1 diabetes, is increased.
Gross features
The thyroid gland is diffusely but moderately enlarged. It is usually smooth, soft & hyperemic
Microscopic features
The follicular epithelial cells are tall, columnar, and more crowded than usual. This
crowding often results in the formation of small pseudopapillae, which project into the
follicular lumen
The colloid within the follicle lumens is pale, with scalloped margins.
Lymphoid infiltrates and mature plasma cells are present throughout the interstitium;
germinal centers are common.
Clinical features:
There are features of thyrotoxicosis, as well as signs that are associated exclusively with Graves
disease 1. Diffuse, smooth & symmetric enlargement of the thyroid, ophthalmopathy
2. Abnormal protrusion of the eyeball (exophthalmos).
3. Scaly thickening and induration of the skin (pretibial myxedema), most common in the
skin overlying the shins. Laboratory findings in Graves disease include elevated serum
free T
4
and T
3
and depressed serum TSH.
Diffuse and Multinodular Goiters
These reflect impaired synthesis of thyroid hormones, most often caused by dietary iodine
deficiency. Impairment of thyroid hormone synthesis leads to a compensatory rise in the
serum TSH, which, in turn, causes compensatory hypertrophy and hyperplasia of thyroid
follicular cells and, ultimately, gross enlargement of the thyroid gland. These changes are
able to overcome the hormone deficiency resulting in euthyroid metabolic state. If the
underlying disorder is sufficiently severe (e.g., a congenital biosynthetic defect), the
compensatory responses may be inadequate to overcome the impairment in hormone
synthesis, resulting in goitrous hypothyroidism. The degree of thyroid enlargement is
proportional to the level and duration of thyroid hormone deficiency.
Endemic goiter
Occurs in geographic areas (typically mountainous) where the soil, water & food supply
contain little iodine. The term endemic is used when goiters are present in more than 10%
of the population in a given region. With increasing availability of dietary iodine
supplementation, the frequency & severity of endemic goiter have declined significantly.
Chapter 8 - Endocrine system
152
Sporadic goiter is less common than endemic goiter. The condition is more common in
females than in males, with a peak incidence in puberty or young adult life, when there is
an increased physiologic demand for T
4
. Sporadic goiter may be caused by
1. Ingestion of substances that interfere with thyroid hormone synthesis, such as excessive
intake of calcium, cabbage, cauliflower, etc. 2. Hereditary enzymatic defects interfering
with thyroid hormone synthesis (dyshormonogenetic goiter).
In most cases, however, the cause of sporadic goiter is not apparent.
Pathological features
Initially there is diffuse, symmetric enlargement of the gland (diffuse goiter). The
follicles are lined by crowded columnar cells, which may pile up and form
pseudopapillae similar to those seen in Graves disease.
If dietary iodine subsequently increases, or if the demands for thyroid hormone decrease,
the stimulated follicular epithelium involutes to form an enlarged, colloid-rich gland
(simple colloid goiter). The cut surface of the thyroid in such cases is usually brown, glassy
& translucent. Microscopically, the follicular epithelium may be hyperplastic in the early
stages of disease or flattened & cuboidal during periods of involution. Colloid is abundant.
Recurrent episodes of hyperplasia and involution combine to produce a more irregular
enlargement of the thyroid (multinodular goiter). They may be nontoxic or may induce
thyrotoxicosis (toxic multinodular goiter). Grossly, Multinodular goiters are
multilobulated, asymmetrically enlarged glands, which may reach massive size. On the cut
surface, irregular nodules containing variable amounts of brown, gelatinous colloid are
present. Regressive changes are quite common, particularly in older lesions, and include
areas of fibrosis, hemorrhage, calcification, and cystic change. Microscopically, there are
colloid-rich follicles lined by flattened, inactive epithelium & areas of follicular epithelial
hypertrophy and hyperplasia, accompanied by the regressive changes mentioned above.
The dominant clinical features:
in addition to the obvious disfigurement induced by a large neck mass, goiters may also
cause airway obstruction, dysphagia, and compression of large vessels in the neck and
upper thorax. A hyperfunctioning ("toxic") nodule may develop within a long-standing
goiter, resulting in hyperthyroidism. This condition is not accompanied by the infiltrative
ophthalmopathy and dermopathy. Less commonly, there may be hypothyroidism.
Neoplasms of the thyroid
Fortunately, the overwhelming majority of solitary thyroid nodules are benign lesions.
Causes of a solitary thyroid nodule include
1. Non-neoplastic conditions
a. a dominant nodule of otherwise multinodular goiter
b. simple cysts c. foci of thyroiditis
2. Neoplastic conditions a. Follicular adenomas b. Carcinomas
It is the microscopic evaluation of a given thyroid nodule, done by fine-needle aspiration
biopsy and/or histologic study of surgically resected thyroid tissue, that provides the most
definitive information about its nature.
Adenomas
are derived from follicular epithelium. As in the case of all thyroid neoplasms, follicular
adenomas are usually solitary. Clinically and morphologically, they may be difficult to
distinguish, from hyperplastic nodules & from the less common follicular carcinomas.
Although the vast majority of adenomas are nonfunctional, a small proportion are "toxic
adenomas" and causes clinically apparent thyrotoxicosis.

Chapter 8 - Endocrine system
153
Pathogenesis
Somatic mutations most often in the TSH receptor itself, cause chronic overproduction of
cAMP, generating cells that acquire proliferative advantage. This results in clonal
expansion of epithelial cells that leads to the formation of follicular adenoma. About 20%
of follicular adenomas have point mutations in the RAS family of oncogenes, which have
been also identified in 50% of follicular carcinomas. This raises the possibility that some
adenomas may progress to carcinomas.
Gross features
The adenoma is solitary, spherical mass that compresses adjacent thyroid tissue.
It is well demarcated from the adjacent parenchyma by a well-defined capsule.
Microscopic features
o The uniform neoplastic cells are arranged in uniform follicles that contain colloid.
o The follicular growth pattern within the adenoma is usually quite distinct from the
adjacent non-neoplastic thyroid.
o The tumor is surrounded by a fibrous capsule
o The above 2 features help distinguish an adenoma from a nodule of a multinodular goiter,
in which nodular and uninvolved parenchyma demonstrate comparable growth patterns.
o Occasionally, the neoplastic cells acquire brightly eosinophilic granular cytoplasm
(Hürthle cell adenoma), which does not differ clinically and in behavior from those of a
conventional adenoma.
o The hallmark of all follicular adenomas is the presence of an intact well-formed capsule
encircling the tumor. Careful evaluation of the integrity of the capsule is critical in the
distinction of follicular adenomas from follicular carcinomas, which demonstrate
capsular and/or vascular invasion.
Because of the need for evaluating capsular integrity, the definitive diagnosis of thyroid
adenoma involves exclusion of follicular carcinoma, & this can only be made after
careful histologic examination of the resected specimen. Suspected adenomas of the
thyroid are therefore removed surgically to exclude malignancy.
Thyroid carcinomas
Most cases of thyroid carcinoma occur in adults, although papillary carcinomas, may
present in childhood. A female predominance has been noted in the early and middle
adult years; this is probably related to the expression of estrogen receptors on neoplastic
thyroid epithelium. In contrast, cases presenting in childhood and late adult life are
distributed equally among males and females.
The major subtypes of thyroid carcinoma & their relative frequencies are as follows:
Papillary carcinoma
80% Follicular carcinoma
15%
Medullary carcinoma
4% Anaplastic carcinomas
1%
Most thyroid carcinomas are derived from the follicular epithelium, except for medullary
carcinomas, which is derived from the parafollicular C cells.
Pathogenesis:
Both
1) Genetic influences
are implicated in both familial & sporadic forms of thyroid cancer.
o Papillary thyroid carcinomas: two major types of genetic alterations are involved in the
pathogenesis of papillary thyroid carcinomas.
a) Chromosomal rearrangements involving the tyrosine kinase receptor gene RET . Such
rearrangements result in the formation of fusion genes, known as ret/PTC (receptor of
tyrosine kinase/papillary thyroid carcinoma), b) Point mutations in the BRAF oncogene
Both these genetic changes independently activate the carcinogenic MAP kinase signaling pathway.
o Follicular thyroid carcinomas: approximately 50% of such tumors harbor mutations in
the RAS family of oncogenes.
Chapter 8 - Endocrine system
154
o Medullary carcinomas: familial medullary thyroid carcinomas occur in MEN 2, and are
associated with germ-line RET proto-oncogene mutations. RET mutations are also seen
in nonfamilial (sporadic) medullary thyroid cancers.
o Anaplastic carcinomas: inactivating point mutations in the p53 tumor suppressor gene
are common in anaplastic tumors.
2) Environmental factors
a) Exposure to ionizing radiation, particularly during the first 2 decades of life, is one of
the most important predisposing factor for the development of thyroid cancer. The
incidence of carcinoma of the thyroid is substantially higher among atomic bomb
survivors in Japan and in those exposed to ionizing radiation after the Chernobyl nuclear
plant disaster. The overwhelming majority of cancers arising in this setting are papillary
thyroid cancers, and most have RET gene rearrangements.
b) b) Long-standing multinodular goiter has been suggested as a predisposing factor since
areas with iodine deficiency-related endemic goiter have a higher prevalence of follicular CA.
Papillary Carcinoma
o This is the most common thyroid malignancy & although it can present in any age group,
the mean age at the time of initial diagnosis is 40 years.
o Gross features
The size of the primary tumor ranges from microscopic to huge. A very high proportion
of thyroid cancers measuring < 1 cm in diameter are of papillary type.
The tumor may be solitary or multifocal. It is either well circumscribed encapsulated or
ill-defined with infiltrative margins. Most cases are solid, whitish, firm, and clearly
invasive; sometimes papillary formations are evident.
o Microscopic features
The diagnosis is based on nuclear features even in the absence of a papillary architecture.
The nuclei of papillary carcinoma cells contain very finely dispersed chromatin, which
imparts an optically clear appearance referred to as "ground-glass" nuclei. In addition,
invaginations of the cytoplasm may give the appearance of intranuclear pseudo-
inclusions. Nuclear clefting is another feature of papillary carcinoma cells.
A papillary architecture is present in many cases, although some tumors are composed
predominantly or exclusively of follicles; these follicular variants still behave
biologically as papillary carcinomas if they have the nuclear features described above.
Concentrically calcified structures termed psammoma bodies are often present within tumor.
o Papillary carcinomas present most often as a painless mass in the neck, either within the
thyroid or as metastasis in a cervical lymph node. Metastases to adjacent cervical lymph
nodes occur in 50% of cases. The presence cervical nodal metastases does not seem to
influence the generally good prognosis of these cancers. In a minority of patients,
hematogenous metastases are present, most commonly to the lung. Papillary carcinomas
are indolent lesions, with 10-year survival rates in excess of 95%.
Follicular Carcinoma
o This is the second most common form of thyroid cancer. It shares with papillary
carcinoma the same predilection for females, but it occurs, on the average, in patients
who are a decade older. The incidence of follicular carcinoma is increased in areas of
dietary iodine deficiency. The high frequency of RAS mutations in follicular adenomas
and carcinomas suggests that they may be related tumors.
o Pathological features
Most follicular carcinomas are composed of fairly uniform cells forming small follicles,
reminiscent of normal thyroid. Similar to follicular adenomas, Hürthle cell variants of
follicular carcinomas may be seen.

Chapter 8 - Endocrine system
155
Follicular carcinomas may be frankly infiltrative or minimally invasive. The latter are
sharply demarcated lesions that may be impossible to distinguish from follicular
adenomas on gross examination. This distinction requires extensive histological sampling
of the tumor-capsule-thyroid interface, to exclude of confirm the presence of capsular
and/or vascular invasion. √ Follicular lesions in which the nuclear features are typical
of papillary carcinomas should be regarded as papillary cancers.
o Follicular carcinomas present most frequently as solitary "cold" thyroid nodules. These
neoplasms tend to metastasize through the bloodstream to the lungs, bone, and liver.
Regional nodal metastases are uncommon, in contrast to papillary carcinomas.
Medullary Carcinoma
o This is a neuroendocrine neoplasm derived from the parafollicular cells C cells. Like
normal C cells, medullary carcinomas secrete calcitonin, the measurement of which plays
an important role in the diagnosis and postoperative follow-up of patients. In some cases,
the tumor cells secrete other polypeptide hormones. Medullary carcinomas are either
sporadic (80% of cases) or familial (FMTC; 20%). The latter occur either in the setting of
MEN syndromes or without an associated MEN syndrome. Sporadic & FMTC without
MEN occur in adults, with a peak incidence around the age of 60 years. Cases associated
with MEN, in contrast, occur in younger patients and may even arise in children.
o Gross features
The tumor may arise as a solitary multiple nodules involving both lobes of the thyroid.
Multicentricity is particularly common in familial cases.
The tumor is unencapsulated, solid,, and yellowish tan in color. Larger lesions often
contain areas of necrosis and hemorrhage and may extend through outside the thyroid.
o Microscopic features
These tumors are composed of polygonal to spindle-shaped cells, which may form nests,
trabeculae, and even follicles.
Amyloid deposits, derived from altered calcitonin molecules, are present in the adjacent
stroma in many cases and are a distinctive feature of these tumors.
Calcitonin is readily demonstrable both within the cytoplasm of the tumor cells and in the
stromal amyloid by immunohistochemical methods.
Electron microscopy reveals variable numbers of intracytoplasmic membrane-bound
electron-dense granules.
One of the peculiar features of familial medullary carcinomas is the presence of
multicentric C-cell hyperplasia in the surrounding thyroid parenchyma. Foci of C-cell
hyperplasia are believed to represent the precursor lesions of medullary carcinomas.
o Sporadic cases of medullary carcinoma present most often as a mass in the neck,
sometimes associated with compression effects such as dysphagia or hoarseness. Notably,
hypocalcemia is not a feature, despite the presence of raised calcitonin levels. Screening
of relatives for elevated calcitonin levels or RET mutations permits early detection of
tumors in familial cases. Specific RET mutations have been found to correlate with an
aggressive behavior in medullary carcinomas.
Anaplastic (undifferentiated) Carcinoma
are among the most aggressive, uniformly fatal human neoplasms. The mean age of
occurrence is 65 years. It is speculated that anaplastic carcinoma develops by
"dedifferentiation" from more differentiated tumors (e.g. papillary carcinoma) as a result
of genetic changes, including loss of function of the p53 tumor suppressor gene.
The cancer presents as a bulky mass that grows rapidly beyond the thyroid capsule into
adjacent neck structures. Microscopically, the tumors are composed of highly anaplastic
large cells either pleomorphic giant cells &/or spindle cells (sarcomatoid).

Chapter 8 - Endocrine system
156
Parathyroid glands
Hyperparathyroidism
This is either primary or secondary, and, less commonly, tertiary. The primary form
represents an autonomous overproduction of PTH, while the latter two conditions occur
as secondary phenomena in individuals with chronic renal failure.
Primary hyperparathyroidism
Is an important cause of hypercalcemia. It is caused by parathyroid
1. Adenoma (80%) 2. Primary hyperplasia (15%) 3. Parathyroid carcinoma (5%).
Pathologic features
The changes seen in primary hyperparathyroidism include those
1. In the parathyroid glands 2. In other organs affected by elevated levels of calcium.
o Parathyroid changes: these take one of three possibilities
1) Adenoma this is a solitary, well-circumscribed, soft, brownish to yellow, encapsulated
nodule. By definition, parathyroid adenomas are almost invariably confined to single
glands, and the remaining glands are either of normal in size or shrunken due to feedback
inhibition by elevated serum calcium. Microscopically, parathyroid adenomas are
composed predominantly of chief cells. A rim of compressed, non-neoplastic parathyroid
tissue is often visible at the edge of the adenoma.
2) Hyperplasia, which affects typically all the glands. Microscopically, the most common
pattern seen is that of chief-cell hyperplasia. Less commonly, the constituent cells contain
abundant clear cytoplasm due to accumulation of glycogen, a condition designated as
water-clear cell hyperplasia.
3) Carcinoma, which is usually a hard tumor. Intra-operatively, a hard and adherent
parathyroid is often a hint to the possibility of carcinoma rather than adenoma. Like
adenoma, they typically a single-gland lesion and chief cells tend to predominate in most
cases. The only two valid criteria for malignancy are (1) invasion of surrounding tissues
and (2) metastatic dissemination.
o Changes in other organs; these are found in the skeleton and kidneys.
1) Skeletal changes include prominence of osteoclasts that erode bone matrix, particularly
in the metaphyses of long tubular bones. Bone resorption is accompanied by increased
osteoblastic activity and the formation of new bone trabeculae. In severe cases the cortex
is grossly thinned and the marrow contains increased amounts of fibrous tissue
accompanied by foci of hemorrhage and cyst formation (osteitis fibrosa cystica).
Aggregates of osteoclasts, reactive giant cells, and hemorrhagic debris occasionally form
masses that may be mistaken for neoplasms (brown tumors of hyperparathyroidism).
2) Nephrolithiasis & nephrocalcinosis: PTH-induced hypercalcemia favors the formation
of urinary tract stones (nephrolithiasis) as well as calcification of the renal interstitium
and tubules (nephrocalcinosis).
3) Metastatic calcification secondary to hypercalcemia may also be seen in the stomach,
lungs, myocardium, and
blood vessels.
Primary hyperparathyroidism is usually a disease of adults and is more common in
women than in men by a ratio of nearly 3:1. The most common lab. feature is an increase
in serum ionized calcium. Malignancy is the most common cause of clinically apparent
hypercalcemia in adults and primary hyperparathyroidism is the most common cause of
clinically silent hypercalcemia. In persons with hypercalcemia caused by parathyroid
hyperfunction, serum PTH is inappropriately elevated, whereas serum PTH is low to
undetectable in hypercalcemia caused by nonparathyroid diseases, including malignancy.

Chapter 8 - Endocrine system
157
Secondary hyperparathyroidism
is caused by any condition associated with chronic hypocalcemia, which leads to
compensatory overactivity of the parathyroids. Renal failure is by far the most common
cause. The parathyroid glands are hyperplastic. Bone changes and metastatic
calcification are similar to those seen in primary hyperparathyroidism.
The clinical manifestations of secondary hyperparathyroidism are usually dominated by
those related to chronic renal failure and elevated serum levels of PTH. In a minority of
patients, parathyroid activity may become autonomous and excessive, with resultant
hypercalcemia, a process sometimes termed Tertiary hyperparathyroidism.
Hypoparathyroidism
The major causes of hypoparathyroidism include
1. Iatrogenic i.e. unintentional removal of parathyroids during thyroidectomy.
2. Congenital absence of the parathyroid glands
3. Autoimmune hypoparathyroidism: a hereditary deficiency syndrome arising from
autoantibodies to multiple endocrine organs (parathyroid, thyroid, adrenals & pancreas).
The major clinical manifestations are referable to hypocalcemia and include increased
neuromuscular irritability, cardiac arrhythmias, and, on occasion, seizures.

Chapter 8 - Endocrine system
158
Endocrine pancreas
Diabetes mellitus
A group of metabolic disorders sharing the common underlying characteristic of hyperglycemia
Diabetes is an important disease because
1. It is common (affects 7% of the population).
2. It increases the risk of atherosclerotic coronary artery and cerebrovascular diseases.
3. It is a leading cause of a. Chronic renal failure b. Adult-onset blindness
c. Nontraumatic lower extremity amputations (due to gangrene)
Classification
Diabetes is divided into two broad classes:
1. Type 1 diabetes (10%): characterized by an absolute deficiency of insulin secretion
caused by pancreatic β-cell destruction, usually as a result of an autoimmune attack.
2. Type 2 diabetes (80%): caused by a combination of peripheral resistance to insulin
action and an inadequate secretion of insulin from the pancreatic β cells in response to
elevated blood glucose levels. The long-term complications in kidneys, eyes, nerves,
and blood vessels are the same in both types.
Pathogenesis
1) Type 1 diabetes is an autoimmune disease & as in all such diseases, genetic susceptibility
& environmental influences play important roles in the pathogenesis. The islet destruction
is caused primarily by T lymphocytes reacting against immunologic epitopes on the
insulin hormone located within β-cell; this results in a reduction of β-cell mass. The
reactive T cells include CD4+ T cells of the T
H
1 subset, which cause tissue injury by
activating macrophages & CD8+ cytotoxic T lymphocytes; these directly kill β cells &
also secrete cytokines that activate further macrophages. The islets show cellular necrosis
& lymphocytic infiltration (insulitis). Autoantibodies against a variety of β-cell antigens,
including insulin are also detected in the blood & may also contribute to islet damage.
2) Type 2 Diabetes Mellitus: the pathogenesis remains unsettled. Environmental
influences, such as inactive life style and dietary habits that eventuates in obesity, clearly
have a role. Nevertheless, genetic factors are even more important than in type 1diabetes.
Among first-degree relatives with type 2 diabetes the risk of developing the disease is
20% to 40%, as compared with 5% in the general population. The two metabolic defects
that characterize type 2 diabetes are 1) A decreased ability of peripheral tissues to
respond to insulin (insulin resistance) & 2) β-cell dysfunction manifested as inadequate
insulin secretion in the face of hyperglycemia. In most cases, insulin resistance is the
primary event & is followed by increasing degrees of β-cell dysfunction.
Morphology of Diabetes and Its Late Complications
The important morphologic changes are related to the many late systemic complications
of diabetes and thus are likely to be found in arteries (macrovascular disease), basement
membranes of small vessels (microangiopathy), kidneys (diabetic nephropathy), retina
(retinopathy) & nerves (neuropathy). These changes are seen in both type 1 & 2 diabetes.
The changes are divided into pancreatic & extrapancreatic
A. Pancreatic changes
Are inconstant and are more commonly associated with type 1 than with type 2 diabetes.
One or more of the following alterations may be present.
1. Reduction in the number and size of islets
2. Leukocytic infiltration of the islets (insulitis) principally byT lymphocytes.
3. Amyloid replacement of islets; which is seen in advanced stages.
Chapter 8 - Endocrine system
159
B. Extrapancreatic changes
1) Diabetic macrovascular disease is reflected as accelerated atherosclerosis affecting the
aorta and other large and medium-sized arteries including the coronaries. Myocardial
infarction is the most common cause of death in diabetics. Gangrene of the lower limbs
due to advanced vascular disease, is about 100 times more common in diabetics than in
the general population.
2) Hyaline arteriolosclerosis is the vascular lesion associated with hypertension. It is both
more prevalent and more severe in diabetics than in nondiabetics, but it is not specific for
diabetes and may be seen in elderly nondiabetics without hypertension.
3) Diabetic microangiopathy is one of the most consistent morphologic features of
diabetes, which reflected morphologically as diffuse thickening of basement membranes.
The thickening is most evident in the capillaries of the retina, renal glomeruli, and
peripheral nerves. The thickened capillary basement membranes are associated with
leakiness to plasma proteins. The microangiopathy underlies the development of diabetic
nephropathy, retinopathy, and some forms of neuropathy.
4) Diabetic Nephropathy: renal
failure is second only to myocardial infarction as a cause
of death from diabetes. Three lesions encountered are:
1. Glomerular lesions 2. Renal vascular lesions, principally arteriolosclerosis; and
3. Pyelonephritis, including necrotizing papillitis.
Glomerular lesions: these include
a. diffuse glomerular capillary basement membrane thickening
b. diffuse glomerular sclerosis: diffuse increase in mesangial matrix; always associated
with the above.
c. nodular glomerulosclerosis (Kimmelstiel-Wilson lesion) refers to a rounded deposits of
a laminated matrix situated in the periphery of the glomerulus. These nodules are PAS
positive. Nodular glomerulosclerosis is encountered in up to 30% of long-term diabetics
and is a major cause of morbidity and mortality. The change is pathogonomic of diabetes.
Both forms of glomerulosclerosis (diffuse and nodular) induce sufficient ischemia to
cause scarring of the kidneys, manifested by a finely granular cortical surface.
Pyelonephritis: both acute and chronic pyelonephritis are more common & more severe
in diabetics than in the general population. One special pattern of acute pyelonephritis,
necrotizing papillitis (or papillary necrosis), is also much more prevalent in diabetics.
5) Ocular Complications of Diabetes:
Visual impairment up to total blindness may occur in long-standing diabetes. The ocular
involvement may take the form of a. retinopathy b. cataract formation c. glaucoma
Retinopathy
is the most common of the above three. It consists of a group of changes that together are
considered diagnostic of the disease. The lesion in the retina takes two forms:
A) Nonproliferative retinopathy includes retinal hemorrhages, exudates,
microaneurysms, and most importantly, thickening of the retinal capillaries (micro-
angiopathy). Underlying all of these changes is thickening of the retinal capillaries
(microangiopathy), which leads to focal weakening of capillary structure.
B) Proliferative retinopathy is a process of neovascularization & fibrosis. This lesion leads
to serious consequences, including blindness, especially if it involves the macula. Vitreous
hemorrhages can result from rupture of newly formed capillaries; the resultant organization
of the hemorrhage can pull the retina off its foundation leading to retinal detachment.
6) Diabetic Neuropathy:
the most frequent is a peripheral, symmetric neuropathy of the lower extremities that
affects mainly the sensory function. Other forms produce disturbances in bowel and

Chapter 8 - Endocrine system
160
bladder function, sexual impotence, and diabetic mononeuropathy. The latter may
manifest as sudden footdrop, wristdrop, or isolated cranial nerve palsies. One of the
causes of the neurologic changes is again microangiopathy. The loss of pain sensation in
the lower limbs can result in the development of ulcers that heal poorly and are a major
cause of morbidity.
In both forms of long-standing diabetes, cardiovascular events such as myocardial
infarction, renal vascular insufficiency, and cerebrovascular accidents are the most
common causes of mortality. Diabetic nephropathy is a leading cause of end-stage renal
disease. By 20 years after diagnosis, more than 75% of type 1 diabetics and about 20% of
type 2 diabetics with overt renal disease will develop end-stage renal disease, requiring
dialysis or renal transplantation. Diabetics are plagued by an enhanced susceptibility to
infections of the skin, as well as to tuberculosis, pneumonia, and pyelonephritis. Such
infections cause the deaths of about 5% of diabetics.
Pancreatic endocrine neoplasms (islet cell tumors)
These are most common in adults, may be single or multiple, and benign or malignant.
The latter metastasize to lymph nodes and liver. They tend to be functional. Like other
endocrine neoplasms, it is difficult to predict their behavior purely on their light
microscopic criteria. In general, tumors less than 2 cm in size tend to behave in an
indolent manner. The vast majority of insulinomas (the most common subtype) are benign,
while the vast majority of other pancreatic endocrine neoplasms tend to be malignant.
Insulinomas (β-cell tumors)
are the most common of pancreatic endocrine neoplasms; they are generally benign but
may produce sufficient insulin to induce hypoglycemia.
Gross features
These are mostly solitary lesions, & usually small (<2 cm in diameter), They tend to be
encapsulated, pale to red-brown nodules located anywhere in the pancreas.
Microscopically,
They look like giant islets, with preservation of the regular cords of monotonous cells.
By immunocytochemistry, insulin can be localized in the tumor cells.
Under the electron microscope, neoplastic β cells, like their normal counterparts, display
distinctive round granules.
The critical laboratory findings are high circulating levels of insulin and a high
insulin-to-glucose ratio.
Gastrinomas
are associated with hypersecretion of gastrin. These tumors may be pancreatic, duodenal
or peripancreatic ("gastrinoma triangle"). Zollinger and Ellison were the first to report the
association of pancreatic islet cell lesions with hypersecretion of gastric acid and severe
peptic ulceration (Zollinger-Ellison syndrome). Over half of gastrinomas are locally
invasive or have already metastasized at the time of diagnosis. In 25% of the cases,
gastrinomas form a member of MEN-1 syndrome. In Zollinger-Ellison syndrome,
hypergastrinemia stimulates extreme gastric acid secretion, which in turn leads to
multiple duodenal and gastric ulcers. In addition, ulcers may also occur in the jejunum.
Other rare pancreatic endocrine neoplasms
α-Cell tumors (glucagonomas) characterized by extremely high plasma glucagon levels.
δ-Cell tumors (somatostatinomas) characterized by high plasma somatostatin.
VIPoma characterized by production of vasoactive intestinal peptide (VIP).

Chapter 8 - Endocrine system
161
Adrenal glands
Adrenal cortex
The adrenal cortex synthesizes three different types of steroids:
1. Glucocorticoids (principally cortisol), which are synthesized primarily in the zona fasciculata
2. Mineralocorticoids, the most important being aldosterone, which is generated in the
zona glomerulosa; and
3. Sex steroids (estrogens & androgens), which are produced largely in the zona reticularis.
Adrenocortical Hyperfunction (Hyperadrenalism)
Under this heading come 3 distinctive clinical syndromes: 1. Hypercortisolism (Cushing
syndrome) 2. Hyperaldosteronism 3. Adrenogenital syndrome (virilizing)
Hypercortisolism (Cushing Syndrome)
o Casuses
is caused by any condition that produces an elevation in glucocorticoid levels. The causes
of this syndrome are
A. Exogenous through administration of exogenous glucocorticoids; the most common cause.
B. Endogenous
1. Hypothalamic-pituitary diseases causing hypersecretion of ACTH (Cushing disease)
1) Hypothalamic-pituitary disease associated with oversecretion of ACTH (Cushing
disease) accounts for more than 50% of the endogenous hypercortisolism. The disorder
affects mainly women, and it occurs most frequently during the 20s and 30s. ACTH-
producing microadenoma (& rarely macroadenoma) is responsible for the vast majority
of such cases.
2) Adrenocortical hyperplasia or neoplasia: Alternatively, the anterior pituitary shows
areas of corticotroph cell hyperplasia. The latter may be primary, or arise secondarily
from excessive stimulation of ACTH release by the hypothalamus. The adrenal glands in
patients with Cushing disease show bilateral nodular cortical hyperplasia secondary to
overstimulation by elevated levels of ACTH. The cortical hyperplasia, in turn, is
responsible for the hypercortisolism.
3. Ectopic ACTH secretion by nonendocrine neoplasms (paraneoplastic)
Primary adrenal neoplasms, such as adrenal adenoma and carcinoma, and rarely by
primary bilateral adrenal cortical hyperplasia, are responsible for up to 20% of cases of
endogenous Cushing syndrome. This group is designated as ACTH-independent Cushing
syndrome (or adrenal Cushing syndrome) because the adrenals function autonomously.
Secretion of ectopic ACTH by nonendocrine tumors is commonly due to a small-cell
carcinoma of the lung.
o Pathological features
The main lesions of Cushing syndrome are found in the pituitary and adrenal glands.
The most common change in the pituitary, results from high levels of endogenous or
exogenous glucocorticoids, is termed Crooke hyaline change. In this condition, the
normal granular, basophilic cytoplasm of the ACTH-producing cells in the anterior
pituitary is replaced by homogeneous, lightly basophilic material. This is due to
accumulation of intermediate keratin filaments in the cytoplasm.
There is one of four changes in the adrenal glands, which depends on the cause.
1. Cortical atrophy
2. Diffuse hyperplasia
3. Nodular hyperplasia
4. Adenoma, rarely a carcinoma

Chapter 8 - Endocrine system
162
In patients in whom the syndrome results from exogenous glucocorticoids, suppression of
endogenous ACTH results in bilateral cortical atrophy, due to a lack of stimulation of
the cortex by ACTH. In cases of endogenous hypercortisolism, in contrast, the adrenals
either are hyperplastic or contain a cortical neoplasm. In Diffuse hyperplasia the adrenal
cortex is diffusely thickened and yellow, as a result of an increase in the size and number
of lipid-rich cells in the zonae fasciculata and reticularis. Alternatively, there is nodular
hyperplasia, which takes the form of bilateral, up to 2.0-cm, yellow nodules scattered
throughout the cortex. This macronodularity appears to be an extension of the diffuse
hyperplasia, because the cortex between the nodules exactly resembles that found in the
diffuse form of this condition.
Primary adrenocortical neoplasms causing Cushing syndrome may be benign or
malignant. The adrenocortical adenomas are yellow tumors surrounded by capsules, and
most weigh < 30 gm. Microscopically, they are composed of cells that are similar to
those encountered in the normal zona fasciculata. The carcinomas associated with
Cushing syndrome are unencapsulated masses frequently > 300 gm in weight, having all
of the anaplastic characteristics of cancer. With both functioning benign and malignant
tumors, the adjacent adrenal cortex and that of the contralateral adrenal gland are atrophic
because of suppression of endogenous ACTH by high cortisol levels.
Adrenal insufficiency
Adrenocortical hypofunction is either primary (adrenocrtical) or secondary (ACTH deficiency).
Primary insufficiency is divided into acute & chronic.
1) Acute Adrenocortical Insufficiency
Occurs most commonly in the following clinical settings
A) Massive adrenal hemorrhage may destroy the adrenal cortex sufficiently to cause
acute adrenocortical insufficiency. This condition may occur
1. in patients maintained on anticoagulant therapy
2. in postoperative patients who develop DIC 3. during pregnancy
4. in patients suffering from overwhelming sepsis (Waterhouse-Friderichsen syndrome)
Waterhouse-Friderichsen syndrome is a catastrophic syndrome classically associated
with Neisseria meningitidis septicemia but can also be caused by other organisms,
including Pseudomonas species, pneumococci & Haemophilus influenzae. The
pathogenesis of the syndrome remains unclear, but probably involves endotoxin-induced
vascular injury with associated DIC.
B) Sudden withdrawal of long-term corticosteroid therapy
C) Stress in those with chronic adrenal insufficiency
2) Chronic adrenocortical insufficiency (Addison disease)
Results from progressive destruction of the adrenal cortex. More than 90% of all cases
are attributable to one of 4 disorders:
In such primary diseases, there is hyperpigmentation of the skin & oral mucosa due to
high levels of MSH (associated with high levels of ACTH).
A) Autoimmune adrenalitis is due to autoimmune destruction of steroid-producing
cells. It is either isolated or associated other autoimmune diseases, such as Hashimoto
disease, pernicious anemia, etc.
B) Infections, particularly tuberculous and fungal
Tuberculous adrenalitis, which once was responsible for as many as 90% of cases of
Addison disease, has become less common with the advent of antituberculous therapy.
When present, tuberculous adrenalitis is usually associated with active infection
Chapter 8 - Endocrine system
163
elsewhere, particularly the lungs and genitourinary tract. Among fungi, disseminated
infections caused by Histoplasma capsulatum is the main cause.
C) AIDS patients are at risk for developing adrenal insufficiency from several infectious
(cytomegalovirus, Mycobacterium avium-intracellulare) and noninfectious (Kaposi
sarcoma) complications.
D) Metastatic neoplasms: the adrenals are a fairly common site for metastases in
persons with disseminated carcinomas. Although adrenal function is preserved in most
such patients, the metastatic growths sometimes destroy sufficient adrenal cortex to
produce a degree of adrenal insufficiency. Carcinomas of the lung and breast are the
major primary sources.
Secondary Adrenocortical Insufficiency
o Any disorder of the hypothalamus and pituitary, such as metastatic cancer, infection,
infarction, or irradiation, that reduces the output of ACTH leads to a syndrome of
hypoadrenalism having many similarities to Addison disease. In such secondary disease,
the hyperpigmentation of primary Addison disease is lacking because melanotropic
hormone levels are low. ACTH deficiency may occur alone, but in some instances, it is
only one part of panhypopituitarism, associated with multiple tropic hormone
deficiencies. Secondary adrenocortical insufficiency is characterized by low serum ACTH
and a prompt rise in plasma cortisol levels in response to ACTH administration.
o Pathological features of adrenocortical deficiency
The appearance of the adrenal glands varies with the cause of the insufficiency.
In secondary hypoadrenalism the adrenals are reduced to small, uniform, thin rim of
atrophic yellow cortex that surrounds a central, intact medulla. Histologically, there is
atrophy of cortical cells with loss of cytoplasmic lipid, particularly in the zonae
fasciculata and reticularis.
In primary autoimmune adrenalitis there is also atrophy of the cortex associated with a
variable lymphoid infiltrate that may extend into the subjacent medulla. The medulla is
otherwise nromal.
In tuberculosis or fungal diseases there is granulomatous inflammatory reaction.
Demonstration of the responsible organism may require the use of special stains.
With metastatic carcinoma, the adrenals are enlarged and their normal architecture is
obscured by the infiltrating neoplasm.
o At least 90% of the gland cortices have to be destroyed for the condition to be clinically apparent.
Adrenocortical tumors
Functional adenomas are commonly associated with hyperaldosteronism & with Cushing
syndrome, whereas a virilizing neoplasm is more likely to be a carcinoma. Determination
of of the functional status of a tumor is based on clinical evaluation & measurement of
the hormone or its metabolites. In other words, functional and nonfunctional
adrenocortical neoplasms cannot be distinguished on the basis of morphologic features.
Patholgical features
o Adrenocortical adenomas
Most cortical adenomas do not cause hyperfunction & are usually encountered as incidental
findings at the time of autopsy or during abdominal imaging for an unrelated cause.
They are generally small, 1 to 2 cm in diameter.
On cut surface, adenomas are usually yellow to yellow-brown due to presence of lipid
within the neoplastic cells
Microscopically, adenomas are composed of cells similar to those populating the normal
adrenal cortex. The nuclei tend to be small, although some degree of pleomorphism may
Chapter 8 - Endocrine system
164
be encountered even in benign lesions ("endocrine atypia"). The cytoplasm ranges from
eosinophilic to vacuolated, depending on their lipid content.
o Adrenocortical carcinomas
These are rare and may occur at any age, including in childhood.
Carcinomas are generally large, invasive lesions.
The cut surface is typically variegated and poorly demarcated with areas of necrosis,
hemorrhage, and cystic change.
Microscopically, they are composed of well-differentiated cells resembling those of
cortical adenomas or bizarre, pleomorphic cells, which may be difficult to distinguish
from those of an undifferentiated carcinoma metastatic to the adrenal.
o Adrenal cancers have a strong tendency to invade the adrenal vein, vena cava &
lymphatics. Metastases to regional and periaortic nodes are common, as are distant
hematogenous spread to the lungs & other viscera. The median survival is about 2 years.
Adrenal medulla
The adrenal medulla is populated by cells derived from the neural crest (chromaffin cells)
and their supporting (sustentacular) cells. The chromaffin cells are so named because they
stain brown-black after exposure to potassium dichromate. They secrete catecholamines in
response to signals from preganglionic nerve fibers in the sympathetic nervous system.
Similar collections of cells are distributed throughout the body in the extra-adrenal
paraganglion system. Tumors are the most important diseases of the adrenal medulla.
Pheochromocytoma
Pheochromocytomas are neoplasms composed of chromaffin cells, which as their normal
counterparts synthesize and release catecholamines. The "rule of 10s" is conviently
applied to this tumor: 10% of pheochromocytomas
1. Arise in association with one of several familial syndromes such as MEN syndromes,
type 1 neurofibromatosis, von Hippel-Lindau disease, and Sturge-Weber syndrome.
2. Are extra-adrenal, occurring in sites such as the organ of Zuckerkandl and the carotid
body, where they are usually called paragangliomas rather than pheochromocytomas.
3. Are bilateral; but in association with familial syndromes, this figure may rise to 50%.
4. Are malignant; frank malignancy, however, is more common in extra-adrenal tumors.
Gross features
o The size of these tumors is quite variable ranging from small to huge masses.
o Sectioning shows yellow-tan, well-defined tumor that compress the adjacent adrenal.
Large lesions display areas of hemorrhage, necrosis, and cystic degeneration.
o Incubation of the fresh tissue with potassium dichromate solutions converts the tumor a
dark brown color.
Microscopic features
o These tumors are composed of polygonal to spindle-shaped chromaffin cells and their
supporting sustentacular cells, arranged in well-defined small nests (Zellballen),"
rimmed by a rich vascular network. The cytoplasm is often finely granular
(catecholamine-containing granules). The nuclei are often quite pleomorphic.
o Both capsular and vascular invasion may be encountered in benign lesions, and the
presence of mitotic figures per se does not imply malignancy. Therefore, the definitive
diagnosis of malignancy in pheochromocytomas is based exclusively on the presence of
metastases. These may involve regional lymph nodes as well as more distant sites,
including liver, lung, and bone.
o Electron microscopy reveals variable numbers of membrane-bound, electron-dense
granules, representing catecholamines.

Chapter 8 - Endocrine system
165
The clinical course is dominated by hypertension that may be episodic, which is
associated with tachycardia, palpitations, headache, sweating and tremor. Sudden cardiac
death may occur, probably secondary to catecholamine-induced myocardial irritability
and ventricular arrhythmias.
The laboratory dx is based on demonstration of increased urinary excretion of free
catecholamines & their metabolites, such as vanillylmandelic acid (VMA)& metanephrines.
Neuroblastoma and Related Neoplasms
Neuroblastoma is the second most common solid malignancy of childhood after brain
tumors, accounting for up to10% of all pediatric neoplasms. They are most common
during the first 5 years of life. Neuroblastomas may occur anywhere along the
sympathetic nervous system and occasionally within the brain. Most neuroblastomas are
sporadic. Spontaneous regression and spontaneous- or therapy-induced maturation are
their unique features.
Gross features
o The adrenal medulla is the commonest site of neuroblastomas. The remainder occur
along the sympathetic chain, mostly in the paravertebral region of the abdomen and
posterior mediastinum.
o They range in size from minute nodules to large masses weighing more than 1 kg.
o Some tumors are delineated by a fibrous pseudo-capsule, but others invade surrounding
structures, including the kidneys, renal vein, vena cava, and the aorta.
o Sectioning shows soft, gray-tan, brain-like tissue. Areas of necrosis, cystic softening, and
hemorrhage may be present in large tumors.
Microscopic features
o Neuroblastomas are composed of small, primitive-appearing neuroblasts with dark nuclei
& scant cytoplasm, growing in solid sheets.
o The background consists of light pinkish fibrillary material corresponding to neuritic
processes of the primitive cells.
o Typically, rosettes can be found in which the tumor cells are concentrically arranged
about a central space filled with the fibrillary neurites.
o Supporting features include include immunochemical detection of neuron-specific
enolase and ultrastructural demonstration of small, membrane-bound, cytoplasmic
catecholamine-containing secretory granules.
o Some neoplasms show signs of maturation, either spontaneous or therapy-induced.
Larger ganglion-like cells having more abundant cytoplasm with large vesicular nuclei
and prominent nucleoli may be found in tumors admixed with primitive neuroblasts
(ganglioneuroblastoma). Further maturation leads to tumors containing many mature
ganglion-like cells in the absence of residual neuroblasts (ganglioneuroma).
Many factors influence prognosis, but the most important are the stage of the tumor and
the age of the patient. Children below 1 year of age have a much more favorable outlook
than do older children at a comparable stage of disease. Miscroscopic features are also an
independent prognostic factor; evidence of gangliocytic differentiation is indicative of a
"favorable" histology. Amplification of the MYCN oncogene in neuroblastomas is a
molecular event that has profound impact on prognosis. The greater the number of
copies, the worse is the prognosis. MYCN amplification is currently the most important
genetic abnormality used in risk stratification of neuroblastic tumors.
Aout 90% of neuroblastomas produce catecholamines (as pheochromocytomas), which
are an important diagnostic feature (i.e., elevated blood levels of catecholamines and
elevated urine levels of catecholamine metabolites such as vanillylmandelic acid [VMA]
and homovanillic acid [HVA]).
Chapter 8 - Endocrine system
166
Multiple Endocrine Neoplasia Syndromes (MEN)
The MEN syndromes are a group of inherited diseases resulting in proliferative lesions
(hyperplasias, adenomas, and carcinomas) of multiple endocrine organs. Even in one
organ, the tumors are often multifocal. These tumors are usually more aggressive and
recur in a higher proportion of cases than similar but sporadic endocrine tumors.
1) Multiple Endocrine Neoplasia Type 1 (MEN1)
is inherited in an autosomal dominant pattern. The gene (MEN1) is a tumor suppressor
gene; thus, inactivation of both alleles of the gene is believed to be the basis of
tumorigenesis. Organs commonly involved include the parathyroid, pancreas, and
pituitary (the 3 Ps). Parathyroid hyperplasia is the most consistent feature of MEN-1 but
endocrine tumors of the pancreas are the leading cause of death because such tumors are
usually aggressive and present with metastatic disease. Zollinger-Ellison syndrome,
associated with gastrinomas, and hypoglycemia, related to insulinomas, are common
endocrine manifestations. Prolactin-secreting macroadenoma is the most frequent
pituitary tumor in MEN-1 patients.
2) Multiple Endocrine Neoplasia Type 2 (MEN2)
MEN type 2 is actually two distinct groups of disorders that are unified by the occurrence
of activating mutations of the RET protooncogene. Both are inherited in an autosomal
dominant pattern.
A. MEN 2A
Organs commonly involved include:
Medullary carcinoma of the thyroid develops in virtually all cases, and the tumors
usually occur in the first 2 decades of life. The tumors are commonly multifocal, and foci
of C-cell hyperplasia can be found in the adjacent thyroid. Adrenal pheochromocytomas
develop in 50% of patients; fortunately, no more than 10% are malignant. Parathyroid
gland hyperplasia with primary hyperparathyroidism occurs in a third of patients.
B. Multiple Endocrine Neoplasia, Type 2B
Organs commonly involved include the thyroid and adrenal medulla. The spectrum of
thyroid and adrenal medullary disease is similar to that in MEN-2A. However, unlike
MEN-2A, patients with MEN-2B:
1. Do not develop primary hyperparathyroidism
2. Develop extraendocrine manifestations: ganglioneuromas of mucosal sites
(gastrointestinal tract, lips, tongue) and marfanoid habitus

167
Chapter contents (167 –185)
Topics
Directed by
Page number
1
Bones
د. سالم
(168 - 178)
Congenital diseases of bone
168
Acquired diseases of bone development
168
Fractures
171
Osteonecrosis
172
Osteomyelitis
172
Bone tumors
173
2
Joints
(179 - 181)
Arthritis
179
Joint tumors and tumor-like lesions
181
3
Soft Tissue Tumors
(182
– 185)
Tumors of Adipose Tissue (Fatty Tumors)
182
Fibrous Tumors and Tumor-Like Lesions
182
Fibrohistiocytic Tumors
183
Skeletal Muscle Tumors
184
Smooth Muscle Tumors
184
Synovial Sarcoma
185
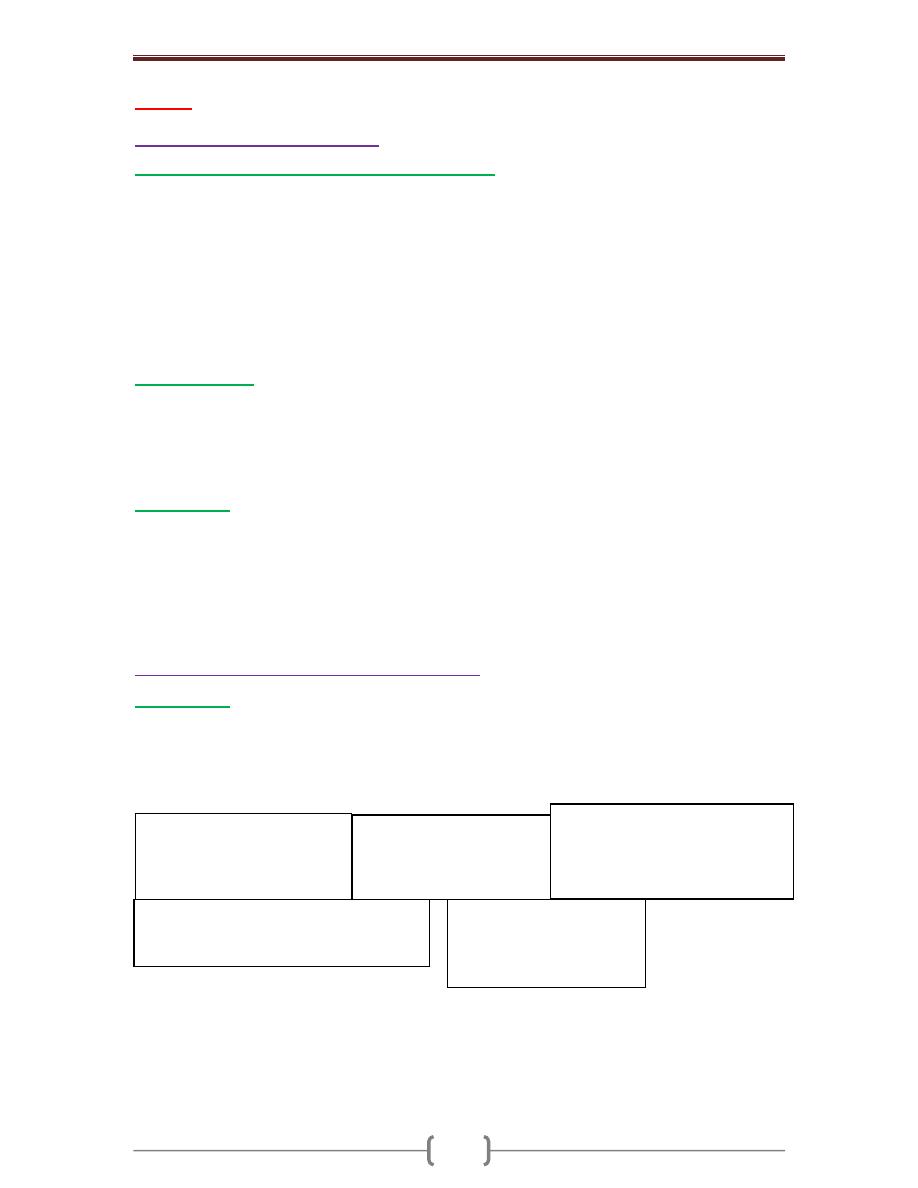
Chapter 9 - Bones, Joints, and Soft Tissue Tumors
168
Bones
Congenital diseases of bone
Osteogenesis Imperfecta (OI) (Brittle bone diseases)
is a group of hereditary disorders
caused by gene mutations that eventuate in defective synthesis of and thus premature
degradation of type I collagen. The fundamental abnormality in all forms of OI is too little
bone, resulting in extreme susceptibility to fractures. The bones show marked cortical
thinning and attenuation of trabeculae.
Extraskeletal manifestations also occur because type I collagen is a major component of
extracellular matrix in other parts of the body. The classic finding of blue sclerae (Fig. 12-1)
is attributable to decreased scleral collagen content; this causes a relative transparency that
allows the underlying choroid to be seen. Hearing loss can be related to conduction defects in
the middle and inner ear bones, and small misshapen teeth are a result of dentin deficiency.
Achrondroplasia
is a major cause of dwarfism. The underlying etiology is a point mutation
in the fibroblast growth factor receptor, which causes inhibition of chondrocyte proliferation,
which is associated with suppression of the normal epiphyseal growth plate expansion. Thus,
long bone growth is markedly shortened. The most conspicuous changes include
disproportionate shortening of the proximal extremities, bowing of the legs, and a lordotic
posture. Microscopically, the cartilage growth plates are disorganized & hypoplastic.
Osteopetrosis
is a group of rare genetic disorders characterized by reduced osteoclast-
mediated bone resorption and therefore defective bone remodelling. The bones are solid and
heavy with no medullary canal, long ends are bulbous, small neural foramina compress
nerves. The affected bone is grossly dense but fractures occur readily like a piece of chalk.
Patients frequently have cranial nerve compressions by the surrouding bone, and recurrent
infections. The latter is attributable to diminished hematopoiesis resulting from reduced
marrow space with impressive hepatosplenomegaly due to extramedullary hematopoiesis.
Acquired diseases of bone development
Osteoporosis
is characterized by increased porosity of the skeleton resulting from reduced bone mass. The
disorder may be localized to a certain bone (s), as in disuse osteoporosis of a limb, or generalized
involving the entire skeleton. Generalized osteoporosis may be primary, or secondary.
Primary generalized osteoporosis: - Postmenopausal - Senile
Secondary generalized osteoporosis
Senile and postmenopausal osteoporosis are the most common forms. In the fourth decade in
both sexes, bone resorption begins to overrun bone deposition. Such losses generally occur in
areas containing abundant cancelloues bone such as the vertebrae & femoral neck. The
postmenopausal state accelerates the rate of loss; that is why females are more susceptible to
osteoporosis and its complications.
B. Neoplasia
Multiple myeloma
Carcinomatosis
A. Endocrine disorders
Hyperparathyroidism
Hypo or hyperthyroidism
Others
C. Gastrointestinal disorders
Malnutrition & malabsorption
Vit D & C deficiency
Hepatic insufficiency
D. Drugs
Corticosteroids
●
Anticoagulants
Chemotherapy
●
Alcohol
E. Miscellaneous
osteogenesis imperfecta
immobilization
pulmonary disease

Chapter 9 - Bones, Joints, and Soft Tissue Tumors
169
Gross features
Because of bone loss, the bony trabeculae are thinner and more widely separated than usual.
This leads to obvious porosity of otherwise spongy cancellous bones. (Fig. 12-3).
Microscopic features
There is thinning of the trabeculae and widening of Haversian canals.
The mineral content of the thinned bone is normal, and thus there is no alteration in the ratio
of minerals to protein matrix.
Etiology & Pathogenesis
o Osteoporosis involves an imbalance of bone formation, bone resorption, & regulation of
osteoclast activation. It occurs when the balance tilts in favor of resorption.
o Osteoclasts (as macrophages) bear receptors (called RANK receptors) that when stimulated
activate the nuclear factor (NFκB) transcriptional pathway. RANK ligand synthesized by
bone stromal cells and osteoblasts activates RANK. RANK activation converts macrophages
into bone-crunching osteoclasts and is therefore a major stimulus for bone resorption.
o Osteoprotegerin (OPG) is a receptor secreted by osteoblasts and stromal cells, which can bind
RANK ligand and by doing so makes the ligand unavailable to activate RANK, thus limiting
osteoclast bone-resorbing activity.
o Dysregulation of RANK, RANK ligand, and OPG interactions seems to be a major
contributor in the pathogenesis of osteoporosis. Such dysregulation can occur for a variety of
reasons, including aging and estrogen deficiency (Fig. 12-4).
o Influence of age: with increasing age, osteoblasts synthetic activity of bone matrix
progressively diminished in the face of fully active osteoclasts.
o The hypoestrogenic effects: the decline in estrogen levels associated with menopause
correlates with an annual decline of as much as 2% of cortical bone and 9% of cancellous
bone. The hypoestrogenic effects are attributable in part to augmented cytokine production
(especially interleukin-1 and TNF). These translate into increased RANK-RANK ligand
activity and diminished OPG.
o Physical activity: reduced physical activity increases bone loss. This effect is obvious in an
immobilized limb, but also occurs diffusely with decreased physical activity in older individuals.
o Genetic factors: these influence vitamin D receptors efficiency, calcium uptake, or PTH
synthesis and responses.
o Calcium nutritional insufficiency: the majority of adolescent girls (but not boys) have
insufficient dietary intake of calcium. As a result, they do not achieve the maximal peak bone
mass, and are therefore likely to develop clinically significant osteoporosis at an earlier age.
o Secondary causes of osteoporosis: these include prolonged glucocorticoid therapy (increases
bone resorption and reduce bone synthesis.)
The clinical outcome of osteoporosis depends on which bones are involved. Thoracic & lumbar
vertebral fractures are extremely common, and produce loss of height & various deformities,
including kyphoscoliosis that can compromise respiratory function. Pulmonary embolism &
pneumonia are common complications of fractures of the femoral neck, pelvis, or spine.
Paget Disease (Osteitis Deformans)
This unique bone disease is characterized by repetitive episodes of exaggerated, regional
osteoclastic activity (osteolytic stage), followed by exuberant bone formation (mixed
osteoclastic-osteoblastic stage), and finally by exhaustion of cellular activity (osteosclerotic
stage). The net effect of this process is a gain in bone mass; however, the newly formed bone
is disordered and lacks strength. Paget disease usually does not occur until mid-adulthood but
becomes progressively more common thereafter. The pathognomonic histologic feature is a
mosaic pattern of lamellar bone (likened to a jigsaw puzzle) due to prominent cement lines
that haphazardly fuse units of lamellar bone. (Fig. 12-5) The axial skeleton and proximal

Chapter 9 - Bones, Joints, and Soft Tissue Tumors
170
femur are involved in the majority of cases. In patients with extensive disease,
hypervascularity of the marrow spaces can result in high-output congestive heart failure.
Cranial nerves impingement also occurs and can lead to head ache and auditory disturbances.
Rarely Paget disease is complicated by bone sarcoma (usually osteogenic).
Rickets and Osteomalacia
Rickets in growing children and osteomalacia in adults are skeletal diseases with worldwide
distribution. They may result from
1. Diets deficient in calcium and vitamin D 2. Limited exposure to sunlight (in heavily
veiled women, and inhabitants of northern climates with scant sunlight)
3. Renal disorders causing decreased synthesis of 1,25 (OH)
2
-D or phosphate depletion
4. Malabsorption disorders.
Although rickets and osteomalacia rarely occur outside high-risk groups, milder forms of
vitamin D deficiency (also called vitamin D insufficiency) leading to bone loss and hip
fractures are quite common in the elderly.
Whatever the basis, a deficiency of vitamin D tends to cause hypocalcemia. When
hypocalcemia occurs, PTH production is increased, that ultimately leads to restoration of the
serum level of calcium to near normal levels (through mobilization of Ca from bone &
decrease in its tubular reabsorption) with persistent hypophosphatemia (through increase renal
exretion of phosphate); so mineralization of bone is impaired or there is high bone turnover.
The basic derangement in both rickets and osteomalacia is an excess of unmineralized
matrix. This complicated in rickets by derangement of endochondral bone growth.
The following sequence ensues in rickets:
1. Overgrowth of epiphyseal cartilage with distorted, irregular masses of cartilage
2. Deposition of osteoid matrix on inadequately mineralized cartilage
3. Disruption of the orderly replacement of cartilage by osteoid matrix, with enlargement and
lateral expansion of the osteochondral junction
4. Microfractures and stresses of the inadequately mineralized, weak, poorly formed bone
5. Deformation of the skeleton due to the loss of structural rigidity of the developing bones
Gross features
o The gross skeletal changes depend on the severity of the disease; its duration, & the stresses
to which individual bones are subjected.
o During the nonambulatory stage of infancy, the head and chest sustain the greatest stresses. The
softened occipital bones may become flattened. An excess of osteoid produces frontal bossing.
Deformation of the chest results from overgrowth of cartilage or osteoid tissue at the
costochondral junction, producing the "rachitic rosary." The weakened metaphyseal areas of the
ribs are subject to the pull of the respiratory muscles and thus bend inward, creating anterior
protrusion of the sternum (pigeon breast deformity). The pelvis may become deformed.
o When an ambulating child develops rickets, deformities are likely to affect the spine, pelvis,
and long bones (e.g., tibia), causing, most notably, lumbar lordosis and bowing of the legs.
o In adults the lack of vitamin D deranges the normal bone remodeling that occurs throughout
life. The newly formed osteoid matrix laid down by osteoblasts is inadequately mineralized,
thus producing the excess of persistent osteoid that is characteristic of osteomalacia. Although
the contours of the bone are not affected, the bone is weak and vulnerable to gross fractures
or microfractures, which are most likely to affect vertebral bodies and femoral necks.
Microscopic features
The unmineralized osteoid can be visualized as a thickened layer of matrix (which stains pink
in hematoxylin and eosin preparations) arranged about the more basophilic, normally
mineralized trabeculae. (Fig. 12-7)
Chapter 9 - Bones, Joints, and Soft Tissue Tumors
171
Hyperparathyroidism
Abnormally high levels of parathyroid hormone (PTH) cause hypercalcemia. This can result
from either primary or secondary causes. Primary hyperparathyroidism is caused usually
by a parathyroid adenoma, which is associated with autonomous PTH secretion. Secondary
hyperparathyroidism, on the other hand, can occur in the setting of chronic renal failure. In
either situation, the presence of excessive amounts of this hormone leads to significant
skeletal changes related to a persistently exuberant osteoclast activity that is associated with
increased bone resorption and calcium mobilization. The entire skeleton is affected. PTH is
directly responsible for the bone changes seen in primary hyperparathyroidism, but in
secondary hyperparathyroidism additional influences also contribute. In chronic renal failure
there is inadequate 1,25-(OH)
2
-D synthesis that ultimately affects gastrointestinal calcium
absorption. The hyperphosphatemia of renal failure also suppresses renal α
1
-hydroxylase,
which further impair vitamin D synthesis; all these eventuate in hypocalcemia, which
stimulates excessive secretion of PTH by the parathyroid glands, & hence elevation in PTH
serum levels.
Gross features
There is increased osteoclastic activity, with bone resorption. Cortical and trabecular bone are
lost and replaced by loose connective tissue. (Fig. 12-8)
Bone resorption is especially pronounced in the subperiosteal regions and produces
characteristic radiographic changes, best seen along the radial aspect of the middle phalanges
of the second and third fingers.
o Microscopical features
o There is increased numbers of osteoclasts and accompanying erosion of bone surfaces.
o The marrow space contains increased amounts of loose fibrovascular tissue.
o Hemosiderin deposits are present, reflecting episodes of hemorrhage resulting from
microfractures of the weakened bone.
o In some instances, collections of osteoclasts, reactive giant cells, and hemorrhagic debris
form a distinct mass, termed "brown tumor of hyperparathyroidism". (Fig. 12-8 B) Cystic
change is common in such lesions (hence the name osteitis fibrosa cystica).
Patients with hyperparathyroidism have reduced bone mass, and hence are increasingly
susceptible to fractures and bone deformities.
Fractures
Fractures are classified into the following types
Complete or incomplete fractures: the fracture line either extends through the whole or part
of the thickness of the bone involved.
Closed when the overlying tissue is intact, or compound when the fracture extends into the
overlying skin
Comminuted when the bone is splintered into small fragments
Displaced, when the fractured bone ends are not aligned
Pathologic fracture refers to bone break that occurs at the site of previous disease (e.g., a
bone cyst, a malignant tumor, etc).
Stress fracture develops slowly over time as a collection of micro-fractures associated with
increased physical activity, especially with repetitive weight on bone (as in military boot camps).
The fracture mechanically ruptures associated blood vessels; the resulting blood hematoma
creates a fibrin mesh scaffold to recruit inflammatory cells, fibroblasts, and endothelium. This
soft tissue callus is able to hold the ends of the fractured bone in apposition, but it is non-
calcified and cannot support weight bearing. Bone progenitors in the medullary cavity deposit
Chapter 9 - Bones, Joints, and Soft Tissue Tumors
172
new foci of woven bone, and activated mesenchymal cells at the fracture site to differentiate
into cartilage-synthesizing chondroblasts. In uncomplicated fractures, this early repair process
peaks within 2-3 weeks. The newly formed cartilage acts as a nidus for endochondral
ossification. This connects the trabeculae in adjacent bone. With ossification, the fractured
ends are bridged by a bony callus. Although excess fibrous tissue, cartilage, and bone are
produced in the early callus, subsequent weight-bearing leads to resorption of the callus from
non-stressed sites; at the same time there is strengthening of regions that support greater load
stresses. This callus remodeling restores the original size and shape of the bone. (Fig. 12-9)
The healing of a fracture can be disrupted by many factors:
Displaced and comminuted fractures frequently result in some deformity; devitalized
fragments of splintered bone require resorption, which delays healing, enlarges the callus, and
requires long periods of remodeling that may never completely normalize.
Inadequate immobilization permits constant movement at the fracture site so that the normal
constituents of callus do not form. In this case, the healing site is composed mainly of fibrous
tissue and cartilage. This causes instability and resulting in delayed union or even nonunion.
Also too much movement along the fracture gap causes the central portion of the callus to
undergo cystic degeneration that may become lined by synovial-like cells, creating a false
joint, (pseudoarthrosis).
Infection (a risk in comminuted and open fractures) is a serious obstacle to fracture healing.
Inadequate levels of calcium or phosphorus, vitamin deficiencies, systemic infection,
diabetes, and vascular insufficiency all cause impairment of bone repair.
Osteonecrosis (avascular necrosis)
(Fig. 12-10)
Ischemic necrosis with resultant bone infarction occurs mostly due to fracture or after
corticosteroid use. Microscopically, dead bon trabevulae (characterized by empty lacunae) are
interspersed with areas of fat necrosis. The cortex is usually not affected because of collateral
blood supply; in subchondral infarcts, the overlying articular cartilage also remains viable
because the synovial fluid can provide nutritional support. With time, osteoclasts can resorb
many of the necrotic bony trabeculae; any dead bone fragments that remain act as scaffolds
for new bone formation, a process called creeping substitution. Symptoms depend on the size
and location of injury. Subchondral infarcts often collapse & can lead to severe osteoarthritis.
Osteomyelitis
This refers to inflammation of the bone and related marrow cavity almost always due to
infection. Osteomyelitis can be acute or a chronic. The most common etiologic agents are
pyogenic bacteria and Mycobacterium tuberculosis.
Pyogenic Osteomyelitis
The offending organisms reach the bone by one of three routes:
1. Hematogenous dissemination (most common)
2. Extension from a nearby infection (in adjacent joint or soft tissue)
3. Traumatic implantation of bacteria (as after compound fractures or orthopedic procedures).
Staphylococcus aureus is the most frequent cause. Mixed bacterial infections, including
anaerobes, are responsible for osteomyelitis complicating bone trauma. In as many as 50% of
cases, no organisms can be isolated.
Pathologic features (Fig. 12-11)
o The offending bacteria proliferate & induce an acute inflammatory reaction.
o Entrapped bone undergoes early necrosis; the dead bone is called sequestrum.
Chapter 9 - Bones, Joints, and Soft Tissue Tumors
173
o The inflammation with its bacteria can permeate the Haversian systems to reach the
periosteum. In children, the periosteum is loosely attached to the cortex; therefore, sizable
subperiosteal abscesses can form and extend for long distances along the bone surface.
o Lifting of the periosteum further impairs the blood supply to the affected region, and both
suppurative and ischemic injury can cause segmental bone necrosis.
o Rupture of the periosteum can lead to an abscess in the surrounding soft tissue and eventually
the formation of cutaneous draining sinus. Sometimes the sequestrum crumbles and passes
through the sinus tract.
o In infants (uncommonly in adults), epiphyseal infection can spread into the adjoining joint to
produce suppurative arthritis, sometimes with extensive destruction of the articular cartilage
and permanent disability.
o After the first week of infection chronic inflammatory cells become more numerous.
Leukocyte cytokine release stimulates osteoclastic bone resorption, fibrous tissue ingrowth,
and bone formation in the periphery, this occurs as a shell of living tissue (involucrum)
around a segment of dead bone. Viable organisms can persist in the sequestrum for years after
the original infection.
Chronicity may develop when there is delay in diagnosis, extensive bone necrosis, and
improper management. Complications of chronic osteomyelitis include
1. A source of acute exacerbations
2. Pathologic fracture
3. Secondary amyloidosis
4. Endocarditis
5. Development of squamous cell carcinoma in the sinus tract (rarely osteosarcoma).
Tuberculous Osteomyelitis
(Fig. 12-12)
Bone infection complicates up to 3% of those with pulmonary tuberculosis. Young adults or
children are usually affected. The organisms usually reach the bone hematogenously. The
long bones and vertebrae are favored sites. The lesions are often solitary (multifocal in AIDS
patients). The infection often spreads from the initial site of bacterial deposition (the
synovium of the vertebrae, hip, knee, ankle, elbow, wrist, etc) into the adjacent epiphysis,
where it causes typical granulomatous inflammation with caseous necrosis and extensive
bone destruction. Tuberculosis of the vertebral bodies (Pott disease), is an important form of
osteomyelitis. Infection at this site causes vertebral deformity and collapse, with secondary
neurologic deficits. Extension of the infection to the adjacent soft tissues with the
development of psoas muscle abscesses is fairly common in Pott disease. Advanced cases are
associated with cutaneous sinuses, which cause secondary bacterial infections. Diagnosis is
established by synovial fluid direct examination, culture or PCR.
Bone tumors
Primary bone tumors are classified according to their normal cell of origin or line of
differentiation. Among the benign mass lesions, osteochondroma and fibrous cortical defect
occur most frequently. Osteosarcoma is the most common primary bone cancer, followed by
chondrosarcoma and Ewing sarcoma. Benign tumors markedly outnumber their malignant
counterparts, particularly before age 40; bone tumors in the elderly are much more likely to
be malignant.
Most bone tumors develop during the first few decades of life and tend to originate in the
long bones of the extremities. Nevertheless, specific tumor types target certain age groups and
anatomic sites; such clinical information is often critical for the appropriate diagnosis. For
instance, most osteosarcomas occur during adolescence, with half arising around the knee,
Chapter 9 - Bones, Joints, and Soft Tissue Tumors
174
either in the distal femur or proximal tibia. In contrast, chondrosarcomas tend to develop
during mid- to late adulthood and involve the trunk, limb girdles, and proximal long bones.
Benign lesions are frequently asymptomatic and are detected as incidental findings. Others
produce pain or a slowly growing mass. Occasionally, a sudden pathologic fracture is the first
manifestation. Radiologic imaging is important in the evaluation of bone tumors; however,
biopsy and microscopic evaluations are necessary for the final diagnosis.
Bone-Forming Tumors
1)
Osteoma
is a benign lesion of bone that in many cases represent a developmental abnormaly or
reactive growth rather than true neoplasms. They are most common in the head, including the
paranasal sinuses. Microscopically, there is a mixture of woven and lamellar bone. They may
cause local mechanical problems (e.g., obstruction of a sinus cavity) & cosmetic deformities.
2)
Osteoid Osteoma and Osteoblastoma
are benign neoplasms with very similar histologic
features. Both lesions typically arise during the 2
nd
& 3
rd
decades. They are well-
circumscribed lesions, usually involving the cortex. The central area of the tumor, termed the
nidus, is characteristically radiolucent. Osteoid osteomas arise most often in the proximal
femur and tibia, and are by definition less than 2 cm, whereas osteoblastomas are larger.
Localized pain is an almost universal complaint with osteoid osteomas, and is usually relieved
by aspirin. Osteoblastomas arise most often in the vertebral column; they also cause pain,
which is not responsive to aspirin. Malignant transformation is rare unless the lesion is
treated with radiation.
Gross features (Fig. 12-13)
Both lesions are round-to-oval masses of hemorrhagic gritty tan tissue.
A rim of sclerotic bone is present at the edge of both types of tumors.
Microscopic features
There are interlacing trabeculae of woven bone surrounded by osteoblasts. The intervening
connective tissue is loose, vascular & contains variable numbers of giant cells.
3) Osteosarcoma
This is “a bone-producing malignant mesenchymal tumor.” Excluding myeloma and
lymphoma, osteosarcoma is the most common primary malignant tumor of bone (20%). The
peak age of incidence is 10-25 years with 75% of the affected patients are younger than age
20 years; there is a second peak that occurrs in the elderly, usually secondary to other
conditions, e.g. Paget disease, bone infarcts, and prior irradiation. Most tumors arise in the
metaphysis of the long bones of the extremities, with 60% occurring about the knee, 15%
around the hip, & 10% at the shoulder. The most common type of osteosarcoma is primary,
solitary, intramedullary, and poorly differentiated, producing a predominantly bony matrix.
Gross features (Fig. 12-14 A)
The tumor is gritty, gray-white, often with foci of hemorrhage and cystic degeneration.
It frequently destroys the surrounding cortex to extend into the soft tissue.
There is extensive spread within the medullary canal, with replacement of the marrow.
However, penetration of the epiphyseal plate or the joint space is infrequent.
Microscopic features
o Tumor cells are pleomorphic with large hyperchromatic nuclei; bizarre tumor giant cells are
common, as are mitoses.
o The direct production of mineralized or unmineralized bone (osteoid) by malignant cells is
essential for diagnosis of osteosarcoma. The neoplastic bone is typically fine, lace-like but
can also be deposited in broad sheets. (Fig. 12-14 B)
o Cartilage can be present in varying amounts. When malignant cartilage is abundant, the tumor
is called a chondroblastic osteosarcoma.

Chapter 9 - Bones, Joints, and Soft Tissue Tumors
175
Pathogenesis
o Several genetic mutations are closely associated with the development of osteosarcoma. In
particular, RB gene mutations that occur in both sporadic tumors, and in individuals with
hereditary retinoblastomas. In the latter there are germ-line mutations in the RB gene (inherited).
o Spontaneous osteosarcomas also frequently exhibit mutations in genes that regulate the cell
cycle including p53, cyclins, etc.
Osteosarcomas typically present as painful enlarging masses. Radiographs usually show a
large, destructive, mixed lytic and blastic mass with infiltrating margins. The tumor
frequently breaks the cortex and lifts the periosteum. The latter results in a reactive periosteal
bone formation; a triangular shadow on x-ray between the cortex and raised periosteum
(Codman triangle) is characteristic but not specific of osteosarcomas. Osteosarcomas
typically spread hematogenously; 10% to 20% of patients have demonstrable pulmonary
metastases at the time of diagnosis.
Cartilage-Forming Tumors
1)
Osteochondroma (Exostosis)
is a relatively common benign cartilage-capped outgrowth attached by a bony stalk to the
underlying skeleton. Solitary osteochondromas are usually first diagnosed in late adolescence
and early adulthood (male-to-female ratio of 3:1); multiple osteochondromas become
apparent during childhood, occurring as multiple hereditary exostosis, an autosomal dominant
disorder. Inactivation of both copies of the EXT gene (a tumor suppressor gne) in
chondrocytes is implicated in both sporadic and hereditary osteochondromas.
Osteochondromas develop only in bones of endochondral origin arising at the metaphysis
near the growth plate of long tubular bones, especially about the knee. They tend to stop
growing once the normal growth of the skeleton is completed. Occasionally they develop
from flat bones (pelvis, scapula, and ribs). Rarely, exostoses involve the short tubular bones
of hands and feet.
Pathological features (Fig. 12-15)
Osteochondromas vary from 1-20cm in size. The cap is benign hyaline cartilage.
Newly formed bone forms the inner portion of the head and stalk, with the stalk cortex
merging with the cortex of the host bone.
Osteochondromas are slow-growing masses that may be painful. Osteochondromas rarely
progress to chondrosarcoma or other sarcoma, although patients with the multiple hereditary
exostoses are at increased risk of malignant transformation.
2)
Chondroma
is a benign tumor of hyaline cartilage. When it arises within the medullary cavity, it is termed
enchondroma; when on the bone surface it is called juxtacortical chondroma.
Enchondromas are usually diagnosed in persons between ages 20 and 50 years; they are
typically solitary and located in the metaphyseal region of tubular bones, the favored sites
being the short tubular bones of the hands and feet. Ollier disease is characterized by
multiple chondromas preferentially involving one side of the body. Chondromas probably
develop from slowly proliferating rests of growth plate cartilage.
Pathological features (Fig. 12-16)
Enchondromas are gray-blue, translucent nodules usually smaller than 3 cm. Microscopically,
there is well-circumscribed hyaline matrix and cytologically benign chondrocytes.
Most enchondromas are detected as incidental findings; occasionally they are painful or cause
pathologic fractures. Solitary chondromas rarely undergo malignant transformation, but those
associated with enchondromatosis are at increased risk.

Chapter 9 - Bones, Joints, and Soft Tissue Tumors
176
3)
Chondrosarcomas
are malignant tumors of cartilage forming tissues. They are divided into conventional
chondrosarcomas and chondrosarcoma variants. Each of these categories comprises several
distinct types, some defined on microscopic grounds & others on the basis of location within
the affected bone, for e.g. they are divided into central (medullary), peripheral (cortical), and
juxtacortical (periosteal). The common denominator of chondrosarcoma is the production of a
cartilaginous matrix and the lack of direct bone formation by the tumor cells (cf
osteosarcoma). Chondrosarcomas occur roughly half as frequently as osteosarcomas; most
patients age 40 years or more, with men affected twice as frequently as women.
Pathological features (Fig. 12-17)
Conventional chondrosarcomas arise within the medullary cavity of the bone to form an
expansile glistening mass that often erodes the cortex. They exhibit malignant hyaline or
myxoid stroma. Spotty calcifications are typically present. The tumor grows with broad
pushing fronts into marrow spaces and the surrounding soft tissue. Tumor grade is determined
by cellularity, cytologic atypia, and mitotic activity. Low-grade tumors resemble normal
cartilage. Higher grade lesions contain pleomorphic chondrocytes with frequent mitotic
figures with multinucleate cells and lacunae containing two or more chondrocytes.
Dedifferentiated chondrosarcomas refers to the presence of a poorly differentiated
sarcomatous component at the periphery of an otherwise typical low-grade chondrosarcoma.
Other histologic variants include myxoid, clear-cell and mesenchymal chondrosarcomas.
Chondrosarcomas commonly arise in the pelvis, shoulder, and ribs. A slowly growing low-
grade tumor causes reactive thickening of the cortex, whereas a more aggressive high-grade
neoplasm destroys the cortex and forms a soft tissue mass. There is also a direct correlation
between grade and biologic behavior. Size is another prognostic feature, with tumors larger
than 10 cm being significantly more aggressive than smaller tumors. High-grade
Chondrosarcomas metastasize hematogenously, preferentially to the lungs and skeleton.
Fibrous and Fibro-Osseous Tumors
Fibrous tumors of bone are common and comprise several morphological variants.
1) Fibrous Cortical Defect and Nonossifying Fibroma
(Fig. 12-18)
Fibrous cortical defects occur in 30% to 50% of all children older than 2 years of age; they
are probably developmental rather than true neoplasms. The vast majority are smaller than 0.5
cm and arise in the metaphysis of the distal femur or proximal tibia; almost half are bilateral
or multiple. They may enlarge in size (5-6 cm) to form nonossifying fibromas. Both lesions
present as sharply demarcated radiolucencies surrounded by a thin zone of sclerosis.
Microscopically are cellular and composed of benign fibroblasts and macrophages, including
multinucleated forms. The fibroblasts classically exhibit a storiform pattern. Fibrous cortical
defects are asymptomatic and are usually only detected as incidental radiographic lesions.
Most undergo spontaneous differentiation into normal cortical bone. The few that enlarge into
nonossifying fibromas can present with pathologic fracture; in such cases biopsy is necessary
to rule out other tumors.
2) Fibrous Dysplasia
is a benign mass lesion in which all components of normal bone are present, but they fail to
differentiate into mature structures. Fibrous dysplasia occurs as one of three clinical patterns:
A. Involvement of a single bone (monostotic)
B. nvolvement of multiple bones (polyostotic)
C. Polyostotic disease, associated with café au lait skin pigmentations and endocrine
abnormalities, especially precocious puberty (Albright syndrome).
Chapter 9 - Bones, Joints, and Soft Tissue Tumors
177
Monostotic fibrous dysplasia accounts for 70% of cases. It usually begins in early
adolescence, and ceases with epiphyseal closure. It frequently involves ribs, femur, tibia &
jawbones. Lesions are asymptomatic and usually discovered incidentally. However, fibrous
dysplasia can cause marked enlargement and distortion of bone, so that if the face or skull is
involved, disfigurement can occur.
Polyostotic fibrous dysplasia without endocrine dysfunction accounts for the majority of the
remaining cases. It tends to involve the shoulder and pelvic girdles, resulting in severe
deformities and spontaneous fractures.
Albright syndrome accounts for 3% of all cases. The bone lesions are often unilateral, and the
skin pigmentation is usually limited to the same side of the body. The cutaneous macules are
classically large, dark to light brown (café au lait), and irregular.
Gross features (Fig. 12-19)
The lesion is well-circumscribed, intramedullary; large masses expand and distort the bone.
On section it is tan-white and gritty.
Microscopic features
There are curved trabeculae of woven bone (mimicking Chinese characters), without
osteoblastic rimming. The above are set within fibroblastic proliferation
Individuals with monostotic disease usually have minimal symptoms. By x-ray, lesions
exhibit a characteristic ground-glass appearance with well-defined margins. Polyostotic
involvement is frequently associated with progressive disease, and more severe skeletal
complications (e.g., fractures, long bone deformities, and craniofacial distortion). Rarely,
polyostotic disease can transform into osteosarcoma, especially following radiotherapy.
Miscellaneous Bone Tumors
1)
Ewing Sarcoma & Primitive Neuroectodermal Tumor (PNET)
are primary malignant small round-cell tumors of bone and soft tissue. They are viewed as
the same tumor because they share an identical chromosome translocation; they differ only in
degree of differentiation. PNETs demonstrate neural differentiation whereas Ewing sarcomas
are undifferentiated. After osteosarcomas, they are the second most common pediatric bone
sarcomas. Most patients are 10 to 15 years old. The common chromosomal abnormality is a
translocation that causes fusion of the EWS gene with a member of the ETS family of
transcription factors. The resulting hybrid protein functions as an active transcription factor to
stimulate cell proliferation. These translocations are of diagnostic importance since almost
all patients with Ewing tumor have t(11;22).
Pathological features (Fig. 12-20)
o Ewing sarcoma and PNETs arise in the medullary cavity but eventually invade the cortex and
periosteum to produce a soft tissue mass. The tumor is tan-white, frequently with foci of
hemorrhage and necrosis.
o Microscopic features
There are sheets of uniform small, round cells that are slightly larger than lymphocytes with
few mitoses and little intervening stroma. The cells have scant glycogen-rich cytoplasm.
The presence of Homer-Wright rosettes (tumor cells circled about a central fibrillary space)
indicates neural differentiation, and hence indicates by definition PNET.
Ewing sarcoma and PNETs typically present as painful enlarging masses in the diaphyses of
long tubular bones (especially the femur) and the pelvic flat bones. The tumor may be
confused with osteomyelitis because of its association with systemic signs & symptoms of
infection. X-rays show a destructive lytic tumor with infiltrative margins and extension into
surrounding soft tissues. There is a characteristic periosteal reaction depositing bone in an
onionskin fashion.

Chapter 9 - Bones, Joints, and Soft Tissue Tumors
178
2)
Giant-Cell Tumor of Bone (GCT)
(Fig. 12-21)
is dominated by multinucleated osteoclast-type giant cells, hence the synonym osteoclastoma.
GCT is benign but locally aggressive, usually arising in individuals in their 20s to 40s. Current
opinion suggests that the giant cell component is likely a reactive macrophage population and
the mononuclear cells are neoplastic. Tumors are large and red-brown with frequent cystic
degeneration. They are composed of uniform oval mononuclear cells with frequent mitoses,
with scattered osteoclast-type giant cells that may contain 30 or more nuclei.
The majority of GCTs arise in the epiphysis of long bones around the knee (distal femur and
proximal tibia). Radiographically, GCTs are large, purely lytic, and eccentric; the overlying
cortex is frequently destroyed, producing a bulging soft tissue mass with a thin shell of
reactive bone. Although GCTs are benign, roughly 50% recur after simple curettage; some
malignant examples (5%) metastasize to the lungs.
3)
Metastatic Tumors
(Fig. 12-22)
These are the most common malignant tumor of bone. Certain tumors exhibit a distinct
skeletal prediliction. In adults more than 75% of skeletal metastases originate from cancers
of the prostate, breast, kidney, and lung. In children, neuroblastoma, Wilms' tumor,
osteosarcoma, Ewing sarcoma, and rhabdomyosarcoma are the common sources of bony
metastases. Most metastases involve the axial skeleton (vertebral column, pelvis, ribs, skull,
sternum), proximal femur, and humerus. The radiologic appearance of metastases can be
purely osteolytic, purely osteoblastic, or mixed osteolytic-osteoblastic (majority of cases). In
lytic lesions (e.g., kidney& lung), the metastatic cells secrete substances such as
prostaglandins, interleukins, etc. that stimulate osteoclastic bone resorption; the tumor cells
themselves do not directly resorb bone. Similarly, metastases that elicit a blastic response
(e.g., prostate adenocarcinoma) do so by stimulating osteoblastic bone formation.
Chapter 9 - Bones, Joints, and Soft Tissue Tumors
179
Joints
Arthritis
Osteoarthritis (Degenerative joint disease)
is the most common joint disorder. It is a frequent consequence of aging and is an important
cause of physical disability in individuals over the age of 65. The fundamental feature of
osteoarthritis is degeneration of the articular cartilage.
In most cases, osteoarthritis appears insidiously with age and without apparent initiating
cause (primary osteoarthritis). When osteoarthritis manifests in youth, there is typically some
predisposing condition, such as previous traumatic injury, developmental deformity, or
marked obesity. In these osettings the disease is called secondary osteoarthritis. Gender has
some influence; knees and hands are more commonly affected in women, whereas hips are
more commonly affected in men.
Pathological features (Fig. 12-23)
o Early changes include proliferation with disorganization of the chondrocytes in the superficial
part of the articular cartilage; (this is accompanied by increasing water content of the matrix
thus decreasing concentration of the proteoglycans with subsequent reduction of elasticity).
o As the superficial part of the cartilage are degraded vertical and horizontal fibrillation and
cracking of the matrix occur.
o Eventually, full-thickness portions of the cartilage are lost, and the subchondral bone plate is
exposed. Friction smooths and polishes the exposed bone (eburnation).
o Small fractures displace pieces of cartilage and subchondral bone into the joint, forming loose
bodies (joint mice). The fracture gaps are filled with synovial fluid to form eventually fibrous
walled cysts.
o Mushroom-shaped osteophytes (bony outgrowths) develop at the margins of the articular surface.
o In severe disease, a fibrous synovial pannus covers the peripheral portions of the articular surface.
Pathogenesis
o Regardless of the inciting stimulus (wear & tear of aging, estrogens in females, and genetic
susceptibility), early osteoarthritis is marked by degenerating cartilage containing more water
and less proteoglycan. The collagen network is also diminished, presumably as a result of
decreased local synthesis and increased breakdown; chondrocyte apoptosis is increased.
o Overall, cartilage tensile strength and resilience are reduced. In response to these degenerative
changes, chondrocytes in the deeper layers proliferate and attempt to "repair" the damage by
synthesizing new collagen and proteoglycans. Although these reparative changes are initially
able to keep pace, matrix changes and chondrocyte loss eventually predominate.
Osteoarthritis predominantly affects patients beginning in their 50s and 60s. Characteristic
symptoms include deep, aching pain exacerbated by use, morning stiffness, and limited range
of movement. Osteophyte impingement on spinal foramina can cause nerve root compression
with radicular pain, muscle spasms, muscle atrophy, and neurologic deficits. Hips, knees,
lower lumbar and cervical vertebrae, proximal and distal interphalangeal joints of the fingers
are commonly involved. Heberden nodes in the fingers, representing prominent osteophytes
at the distal interphalangeal joints, are characteristic in women. With time, significant joint
deformity can occur, but unlike rheumatoid arthritis, fusion does not take place.
Gout
This is a disorder caused by the tissue accumulation of excessive amounts of uric acid, an end
product of purine metabolism. It is marked by recurrent episodes of acute arthritis, sometimes
accompanied by the formation of large crystalline aggregates called tophi & chronic joint
deformity. All of these are the result of precipitation of monosodium urate crystals from
Chapter 9 - Bones, Joints, and Soft Tissue Tumors
180
supersaturated body fluids. Not all individuals with hyperuricemia develop gout; this indicates
that influences besides hyperuricemia contribute to the pathogenesis. Gout is traditionally
divided into primary (90%) and secondary forms (10%). Primary gout designates cases in
whom the basic cause is unknown or when it is due to an inborn metabolic defect that causes
hyperuricemia. In secondary gout the cause of the hyperuricemia is known.
Pathologic features (Fig. 12-24)
The major morphologic manifestations of gout are
1. Acute arthritis 2. Chronic tophaceous arthritis
3. Tophi in various sites, and 4. Gouty nephropathy
o Acute arthritis: The synovium is edematous and congested,
There is an intense infiltration of the synovium & synovial fluid by neutrophils.
Long, slender, needle-shaped monosodium urate crystals are frequently found in the
cytoplasm of the neutrophils as well as in small clusters in the synovium.
o Chronic tophaceous arthritis:
This evolves from repetitive precipitation of urate crystals during acute attacks. The urates
can heavily encrust the articular surfaces and form visible deposits in the synovium.
The synovium becomes hyperplastic, fibrotic, and thickened by inflammatory cells, forming a
pannus that destroys the underlying cartilage, and leading to erosions of subjacent bone.
In severe cases, fibrous or bony ankylosis occurs, resulting in loss of joint function.
o Tophi
These are the pathognomonic hallmarks of gout.
Tophi can appear in the articular cartilage, periarticular ligaments, tendons, and soft tissues,
including the ear lobes. Superficial tophi can lead to large ulcerations of the overlying skin.
Microscopically, they are formed by large aggregations of urate crystals surrounded by an
intense inflammatory reaction of lymphocytes, macrophages, and foreign-body giant cells,
attempting to engulf the masses of crystals.
o Gouty nephropathy
This refers to the renal complications associated with urate deposition including medullary
tophi, intratubular precipitations and renal calculi. Secondary complications such as
pyelonephritis can occur, especially when there is urinary obstruction.
Pathogenesis
(Fig. 12-25)
o Although the cause of excessive uric acid biosynthesis in primary gout is unknown in most
cases, rare patients have identifiable enzymatic defects or deficiencies that are associated with
excess production of uric acid.
o In secondary gout, hyperuricemia can be caused by increased urate production (e.g., rapid cell
lysis during chemotherapy for lymphoma or leukemia) or decreased excretion (chronic renal
failure), or both. Reduced renal excretion may also be caused by drugs such as thiazide
diuretics, because of their effects on uric acid tubular transport.
o Whatever the cause, increased levels of uric acid in the blood and other body fluids (e.g.,
synovium) lead to the precipitation of monosodium urate crystals. The precipitated crystals
are chemotactic to neutrophils & macrophages through activation of complement components
C3a and C5a fragments. This leads to a local accumulation of neutrophils and macrophages in
the joints and synovial membranes to phagocytize the crystals. The activated neutrophils
liberate destructive lysosomal enzymes. Macrophages participate in joint injury by secreting a
variety of proinflammatory mediators such as IL-1, IL-6, and TNF. While intensifying the
inflammatory response, these cytokines can also directly activate synovial cells and cartilage
cells to release proteases (e.g., collagenases) that cause tissue injury.
o Repeated bouts of acute arthritis, however, can lead to the permanent damage seen in chronic
tophaceous arthritis.b
Chapter 9 - Bones, Joints, and Soft Tissue Tumors
181
Pseudogout (chondrocalcinosis) (Calcium pyrophosphate crystal deposition disease).
Pseudogout typically first occurs in the age 50 years or older. It involves enzymes that lead to
accumulation and eventual crystallization of pyrophosphate with calcium. The pathology in
pseudogout involves the recruitment and activation of inflammatory cells, and is reminiscent of
gout. The knees, followed by the wrists, elbows, shoulders, and ankles, are most commonly
affected. Approximately 50% of patients experience significant joint damage.
Infectious Arthritis
can cause rapid joint destruction and permanent deformities. Microorganisms can lodge in
joints during hematogenous dissemination, by direct inoculation or by contiguous spread
from osteomyelitis or a soft tissue abscess.
Suppurative Arthritis is a subtype of infectious arthritis in which the bacteria seed the joint
during episodes of bacteremia. Haemophilus influenzae predominates in children under age 2
years, S. aureus is the main causative agent in older children and adults, and gonococcus is
prevalent during late adolescence and young adulthood. There is sudden onset of pain,
redness, and swelling of the joint with fever, leukocytosis, and elevated ESR. In 90% of
nongonococcal suppurative arthritis, the infection involves only a single joint-usually the
knee. Joint aspiration is typically purulent, and allows identification of the causal agent.
Joint tumors and tumor-like lesions
Ganglion and Synovial Cysts
(Fig. 12-27)
A ganglion is a small (<1.5 cm) cyst located near a joint capsule or tendon sheath; the wrist is
an especially common site. It is firm to fluctuant pea-sized nodules. Because they arise by
cystic degeneration of connective tissue they are grossly translucent and microscopically lack
a true cell lining. They are usually asymptomatic.
Synovial cyst occurs due to herniation of synovium through a joint capsule or massive
enlargement of a bursa. (Fig. 12-28) A good example is the Baker cyst that occurs in the
popliteal fossa.
Pigmented Villonodular Tenosynovitis (PVNS) & Giant-Cell Tumor (GCT) of
Tendon Sheath
(Fig. 12-30)
These are closely related benign neoplasms of synovium. PVNS tends to involve joints
diffusely, whereas GCT usually occurs as a single tendon sheath nodule.
Grossly, both lesions are red-brown to orange-yellow. In PVNS the joint synovium is diffusely
converted into red-brown finger-like projections, and nodules. In contrast, GCT is well
circumscribed and contained. Microscopically, Tumor cells in both lesions resemble
synoviocytes. In PVNS they spread along the surface and infiltrate the subsynovial
compartment. In GCT the cells grow in a solid nodular aggregate. Other typical findings include
hemosiderin deposits, foamy macrophages, & multinucleated giant cells. PVNS usually affects
the knee (80% of cases). Patients typically complain of pain, locking, and recurrent swelling. In
contrast, GCT manifests as a solitary, slowly growing mass frequently involving wrist and
finger tendon sheaths. GCT is the most common soft tissue tumor of the hand.

Chapter 9 - Bones, Joints, and Soft Tissue Tumors
182
Soft Tissue Tumors
Tumors of Adipose Tissue (Fatty Tumors)
1)
Lipomas
are benign tumors of fat, and are the most common soft tissue tumors of adulthood. Most
lipomas are solitary lesions. Lipomas can be subclassified based on their histologic features
(e.g., conventional, myolipoma, spindle cell, myelolipoma, pleomorphic, angiolipoma),
and/or characteristic chromosomal rearrangements. Most lipomas are mobile, slowly
enlarging, painless masses. They are usually seen in adults age 40+; associated with obesity;
there are no gender differences. They are rare in children. Multiple lipomas are more common
in women. The condition is often familial, & may be associated with neurofibromatosis &
multiple endocrine neoplasia syndromes. Lipomas mostly involve the subcutaneous tissue of
the trunk, back, shoulder, neck, proximal extremities but are rare on hands, feet, face, &in
retroperitoneum
Pathological features (Fig. 12-35)
Conventional lipomas (the most common subtype) are soft, yellow, well-encapsulated
masses. They can vary considerably in size.
Microscopically, they consist of mature fat cells with no pleomorphism.
2)
Liposarcoma
is a malignant neoplasm of adipocytes. These tumors occur most commonly in the 40 to 60
years of age & mostly in the deep soft tissues (cf. lipoma) or in visceral sites. The prognosis
of liposarcomas is greatly influenced by the histologic subtype; well-differentiated and
myxoid variants tend to grow in a fairly indolent fashion and have a more favorable outlook
than do the more aggressive round cell and pleomorphic variants, which tend to recur after
excision and metastasize to lungs. A t(12;16) chromosomal translocation is associated with
myxoid liposarcomas; the rearrangement affects a transcription factor that plays a role in
normal adipocyte differentiation.
Pathological features (Fig. 12-36)
Usually they are relatively well-circumscribed large masses.
Several different histologic subtypes are recognized, including two low-grade variants, the
well-differentiated liposarcoma and the myxoid liposarcoma, the latter characterized by
abundant, mucoid extracellular matrix.
Some well-differentiated lesions can be difficult to distinguish histologically from lipomas,
whereas very poorly differentiated tumors can resemble various other high-grade malignancies.
In most cases, cells indicative of fatty differentiation are present. Such cells are known as
lipoblasts; they recapitulate fetal fat cells with cytoplasmic lipid vacuoles that scallop the nucleus.
Fibrous Tumors and Tumor-Like Lesions
Reactive Proliferations
1)
Nodular Fasciitis
is a self-limited, reactive fibroblastic proliferation that typically occurs in
adults on the volar aspect of the forearm. Patients characteristically present with a several-week
history of a solitary, rapidly growing mass. Preceding trauma is noted in up to 15% of cases.
Pathological features (Fig. 12-37)
Characteristically, the lesion is several centimeters in greatest dimension and nodular.
Mcroscopically, it is richly cellular and consists of plump, randomly arranged, immature-
appearing fibroblasts in an abundant myxoid stroma. The cells vary in size and shape (spindle
to stellate) and have conspicuous nucleoli and numerous mitoses.
Chapter 9 - Bones, Joints, and Soft Tissue Tumors
183
2)
Myositis Ossifican
is distinguished from other fibroblastic proliferations by the presence of
metaplastic bone. It usually develops in the proximal muscles of the extremities in athletic
adolescents and young adults after trauma. The involved area is initially swollen and painful,
eventually evolving into a painless, hard, well-demarcated mass. It is vital to distinguish the
lesion from extra-skeletal osteosarcoma.
3)
Fibromatoses
are a group of fibroblastic proliferations distinguished by their tendency to
grow in an infiltrative fashion and, in many cases, to recur after surgical removal. Although
some lesions are locally aggressive, they do not metastasize. The fibromatoses are divided
into two major clinicopathologic groups: superficial and deep.
The superficial fibromatoses arise in the superficial fascia and include such entities as
palmar fibromatosis (Dupuytren contracture) (Fig. 12-38) and penile fibromatosis (Peyronie
disease). Superficial lesions are genetically distinct from their deep-seated cousins and are
generally more innocuous; they also come to clinical attention earlier, because they cause
deformity of the involved structure. The deep fibromatoses include the so-called desmoid
tumors that arise in the abdominal wall & muscles of the trunk and extremities, and within
the abdominal cavity (mesentery and pelvic walls). Deep fibromatoses tend to grow in a
locally aggressive manner and recur after excision. These tumors are gray-white, firm to
rubbery, poorly demarcated, infiltrative masses 1 to 15 cm in greatest dimension.
Microscopically, they are composed of plump cells arranged in broad sweeping fascicles that
penetrate the adjacent tissue.
Fibrosarcoma
is a malignant neoplasm composed of fibroblasts. Most occur in adults, typically in the deep
tissues of the thigh and retroperitoneal area. As with other sarcomas, fibrosarcomas often
recur locally after excision (>50% of cases) and can metastasize hematogenously (>25% of
cases), usually to the lungs.
Pathological features (Fig. 12-39)
These unecapsulated, infiltrative sarcomas show frequently areas of hemorrhage and necrosis.
Microscopically, the low-grade tumors may closely resemble fibromatosis; less differentiated
examples show densely packed spindled cells growing in a herringbone fashion, there are
frequent mitoses, and necrosis.
Fibrohistiocytic Tumors
These are composed of a mixture of fibroblasts and phagocytic, lipid-laden cells resembling
activated macrophages. The neoplastic cells are most likely fibroblasts. Nevertheless, a
significant number of such tumors actually derive from other mesenchymal cell types. Thus,
the term fibrohistiocytic, especially in regard to the malignant variants, should be considered
descriptive and not necessarily referring to a specific cellular origin. These tumors are divided
into benign, of intermediate malignant potential, frankly malignant.
1)
Benign Fibrous Histiocytoma (Dermatofibroma)
are relatively common benign lesions in adults presenting as small (<1 cm) mobile nodules in
the dermis or subcutaneous tissue. Microscopically, the lesion consists of bland, interlacing
spindle cells admixed with foamy, lipid-rich histiocyte-like cells. The borders of the lesions
tend to be infiltrative. The pathogenesis of these lesions is uncertain.
2)
Malignant Fibrous Histiocytoma (MFH)
is a term rather loosely applied to a variety of soft tissue sarcomas characterized by
considerable cytologic pleomorphism, the presence of bizarre multinucleate cells, and
storiform architecture. (Fig. 12-40) Despite the name, the phenotype of many such tumors is
fibroblastic and not histiocytic. Nevertheless, it is also important to note that several tumors
Chapter 9 - Bones, Joints, and Soft Tissue Tumors
184
diagnosed as MFH actually exhibit markers for cells of other origin (e.g., smooth muscle
cells, adipocytes, skeletal muscle cells) and are therefore more appropriately classified as
leiomyosarcomas, liposarcomas, etc. MFH exhibiting fibroblastic differentiation are usually
large (5-20 cm), gray-white unencapsulated masses that often appear deceptively
circumscribed. They usually arise in the musculature of the proximal extremities or in the
retroperitoneum. Most of these tumors are extremely aggressive, recur unless widely excised,
and have a metastatic rate of 30% to 50%.
Skeletal Muscle Tumors
Rhabdomyosarcoma
Rhabdomyosarcoma is the most common soft tissue sarcoma of childhood and adolescence,
usually appearing before age 20. They occur most commonly in the head and neck or
genitourinary tract.
Chromosomal translocations are found in most cases; the more common t(2;13) translocation
fuses the PAX3 gene (controls skeletal muscle differentiation & development) on
chromosome 2 with the FKHR gene on chromosome 13. The hybrid PAX3-FKHR protein
probably involves dysregulation of muscle differentiation.
Gross features (Fig. 12-34)
Tumors arising near the mucosal surfaces of the bladder or vagina, can present as soft,
gelatinous, grapelike masses, designated sarcoma botryoides. In other cases they are
deceptively demarcated or infiltrative grayish-white to brownish masses.
Microscopical features
o Rhabdomyosarcoma is histologically subclassified into the embryonal, alveolar, and
pleomorphic variants.
o The rhabdomyoblast is the diagnostic cell in all types; it exhibits granular eosinophilic
cytoplasm, and may be round or elongated; the latter are known as tadpole or strap cells and
may contain cross-striations visible by light microscopy.
o The diagnosis of rhabdomyosarcoma is based on the demonstration of skeletal muscle
differentiation, either in the form of sarcomeres under the electron microscope or by
immunohistochemical demonstration of muscle-associated antigens such as desmin and
muscle-specific actin.
Rhabdomyosarcomas are aggressive neoplasms. Location and the histologic variant of the
tumor influence survival; embryonal, pleomorphic, and alveolar variants have progressively
worsening prognoses. The malignancy is curable in almost two-thirds of children, but adults
do much more poorly.
Smooth Muscle Tumors
1)
Leiomyomas
are common, well-circumscribed neoplasms that can arise from smooth muscle cells
anywhere in the body, but are encountered most commonly in the uterus.
2)
Leiomyosarcomas
(15% of soft tissue sarcomas).
They occur in adults, more commonly females. Deep soft tissues of the extremities and
retroperitoneum are common sites. Microscopically, they show spindle cells with cigar-
shaped nuclei arranged in interweaving fascicles. (Fig. 12-41)Superficial leiomyosarcomas
are usually small and have a good prognosis, whereas retroperitoneal tumors are large, cannot
be entirely excised, and cause death by both local extension and metastatic spread.

Chapter 9 - Bones, Joints, and Soft Tissue Tumors
185
Synovial Sarcoma
(10% of all soft tissue sarcomas)
The cell of origin is unclear and is most certainly not a synoviocyte. Reflecting a non-joint
origin, 90% of synovial sarcomas are not intra-articular but rather paraarticular. They are
typically seen in the 20-40 years of age. Most develop in deep soft tissues around the large
joints of the extremities, especially around the knee (Fig. 12-42). Most synovial sarcomas
show a characteristic t(X;18) translocation, which relates to prognosis. Microscopically,
synovial sarcomas may be biphasic or monophasic. Classic biphasic synovial sarcoma
exhibits differentiation of tumor cells into both epithelial-like cells and spindle cells. The
epithelial cells are cuboidal to columnar and form glands or grow in solid cords or aggregates.
The spindle cells are arranged in densely cellular fascicles that surround the epithelial cells.
Immunohistochemistry is helpful, because the tumor cells are positive for keratin and
epithelial membrane antigen. Common metastatic sites are lung, bone, and regional lymph
nodes. Only 10% to 30% live for more than 10 years.
