بسم ه الرحمن الرحيم
ACIDOSIS & ALKALOSIS
Compensatory changes

Respiratory Acidosis & Alkalosis
• A rise in arterial PCO
2
due to decreased
ventilation causes
respiratory acidosis
.
• The CO
2
that is retained is in equilibrium with
H
2
CO
3
, which in turn is in equilibrium with HCO
3
-
,
so that the plasma HCO
3
-
rises and
a new
equilibrium is reached at a lower pH
.
• This can be indicated graphically on a plot of
plasma HCO
3
-
concentration versus pH .
• Conversely, a decline in PCO
2
causes
respiratory
alkalosis.
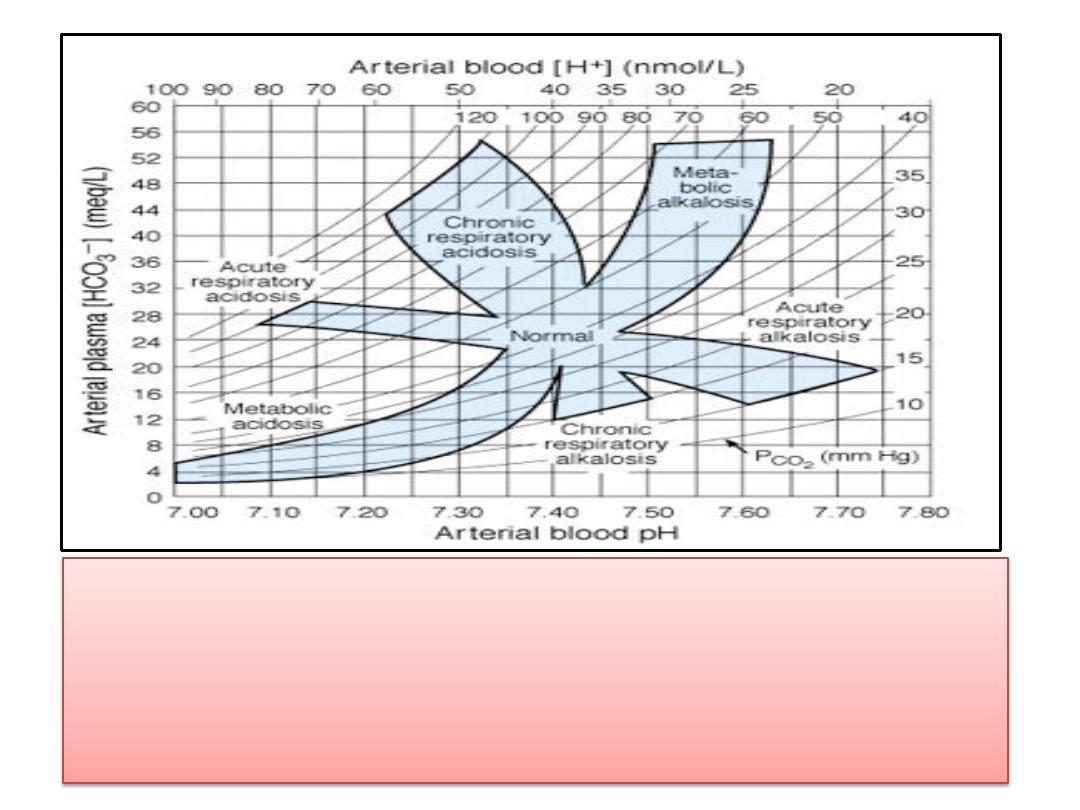
(1)Acid-base nomogram showing changes in the PCO
2
(curved
lines), plasma HCO
3
-
, and pH of arterial blood in respiratory
and metabolic acidosis. Note the shifts in HCO
3
-
and pH as
acute respiratory acidosis and alkalosis are compensated,
producing their chronic counterparts.

Respiratory Acidosis & Alkalosis
• The initial changes in Figure are those
that occur independently of any
compensatory mechanism; ie, they are
those of
uncompensated
respiratory
acidosis or alkalosis.
• In either situation, changes are
produced in the kidneys, which then tend
to
compensate
for the acidosis or
alkalosis, adjusting the pH toward
normal.

Renal Compensation
• HCO
3
-
reabsorption in the renal tubules depends not
only on the
filtered load of HCO
3
-
, which is the product
of the GFR and the plasma HCO
3
-
level, but also on the
rate of H
+
secretion
by the renal tubular cells, since
HCO
3
-
is reabsorbed by exchange for H
+
.
• The rate of H
+
secretion
—and hence the rate of HCO
3
-
reabsorption
—
is proportionate to the arterial PCO
2
,
probably because the
more CO
2
that is available to
form H
2
CO
3
in the cells, the
more H
+
can be secreted .
• In
respiratory acidosis
, renal tubular H
+
secretion is
therefore increased, removing H
+
from the body; and
even though the plasma HCO
3
-
is elevated, HCO
3
-
reabsorption is increased, further raising the plasma
HCO
3
-
.

Renal Compensation
• This renal compensation for respiratory
acidosis is shown graphically in the shift from
acute to chronic
respiratory acidosis in Figure
(2 ).
• Conversely, in respiratory alkalosis, the low
PCO
2
hinders renal H
+
secretion, HCO
3
-
reabsorption is depressed, and HCO
3
-
is
excreted, further reducing the already low
plasma HCO
3
-
and lowering the pH toward
normal .

Metabolic Acidosis
• When acids stronger than HHb and the other buffer acids
are added to blood,
metabolic acidosis
is produced; and
when the free H
+
level falls as a result of addition of alkali
or removal of acid
, metabolic alkalosis
results.
• If, for example, H
2
SO
4
is added, the H
+
is buffered and the
Hb
-
, Prot
-
, and HCO
3
-
levels in plasma drop.
• The H
2
CO
3
formed is converted to H
2
O and CO
2
, and the
CO
2
is rapidly excreted via the lungs. This is the situation in
uncompensated
metabolic acidosis .
• Actually, the rise in
plasma H
+
stimulates respiration
, so
that the
PCO
2
, instead of rising or remaining constant
, is
reduced
.
• This
respiratory compensation
raises the pH even further.
• The
renal
compensatory
mechanisms then bring about the
excretion of the extra H
+
and return the buffer systems to
normal.
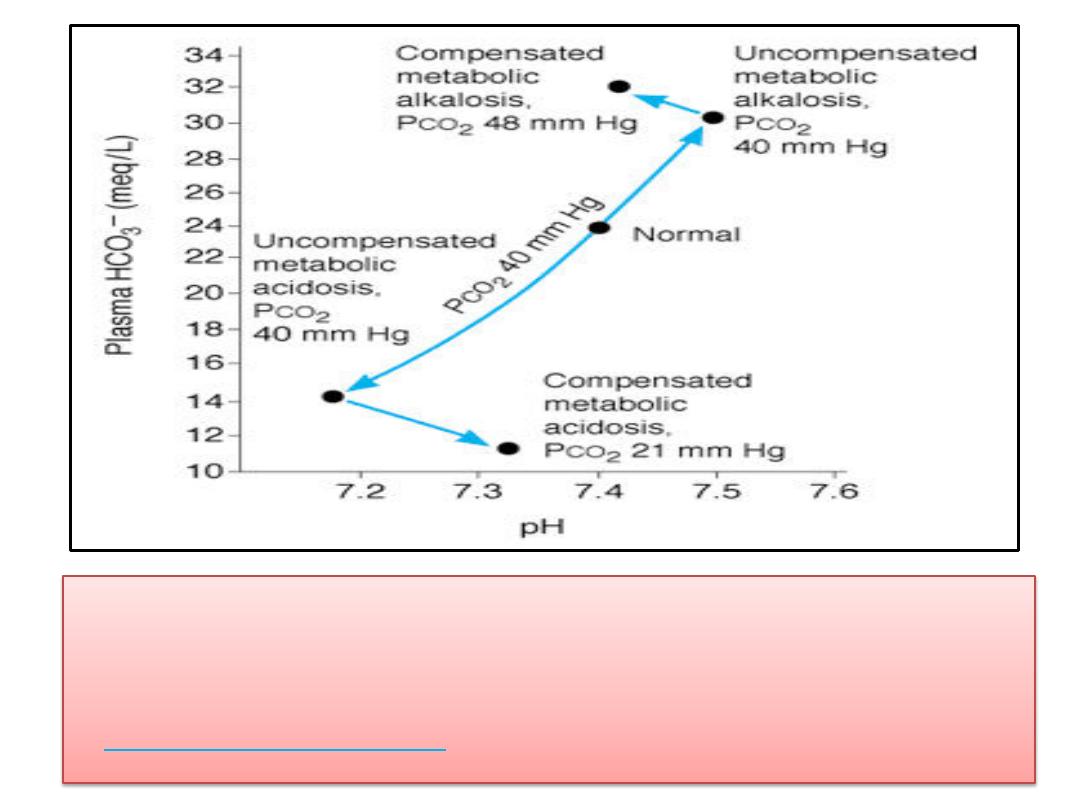
(2)Changes in true plasma pH, HCO
3
-
, and PCO
2
in
metabolic acidosis and alkalosis. (This is called a
Davenport diagram
and is based on Davenport HW:

Renal Compensation
• The renal tubular cells
secrete H
+
into the tubular fluid
in
exchange for Na
+
;
and for
each H
+
secreted,
one Na
+
and
one HCO
3
-
are added to the blood .
• The limiting urinary pH of 4.5
would be reached rapidly
and the total amount of H
+
secreted would be small if
there were no buffers in the urine that "
tied up
" H
+
.
• However,
secreted H
+
reacts with HCO
3
-
to form CO
2
and H
2
O (bicarbonate reabsorption);
with HPO
4
2-
to
form H
2
PO
4
-
; and
with NH
3
to form NH
4
+
.
• In this way large amounts of H
+
can be secreted,
permitting correspondingly large amounts of HCO
3
-
to
be returned to (in the case of bicarbonate
reabsorption) or added to the depleted body stores
and large numbers of the cations to be reabsorbed.

Renal Compensation
• It is only when the
acid load is very large
that
cations are lost with the anions, producing
diuresis and depletion of body cation stores.
• The overall reaction in blood when a strong
acid such as H
2
SO
4
is added is
NaHCO + H SO → Na SO +
2H2CO3

Renal Compensation
• For
each mole
of H
+
added,
1 mole
of NaHCO
3
is lost. The kidney in effect reverses the
reaction:
Na SO + H CO → NaHCO + H + SO
and the H
+
and SO
4
2-
are excreted.

Metabolic Alkalosis
• In metabolic alkalosis, the plasma HCO
3
-
level and pH rise .
• The respiratory compensation is a
decrease in ventilation
produced by the decline in H
+
concentration, and this
elevates
the PCO
2
.
• This brings the pH back toward normal while elevating the
plasma HCO
3
-
level still further.
• The
magnitude
of this compensation is
limited
by the carotid
and aortic
chemoreceptor
mechanisms, which drive the
respiratory center if there is any appreciable fall in the arterial
PO
2
.
• In metabolic alkalosis, more renal H
+
secretion is expended in
reabsorbing the increased filtered load of HCO
3
-
; and if the
HCO
3
-
level in plasma exceeds 26-28 meq/L, HCO
3
-
appears in
the urine.

Thank you
