
19
Unit 2: Inflammation and Repair
20
Definition
Is a protective response intended to eliminate the initial
cause of cell injury as well as the necrotic cells and tissues
resulting from the original insult.
Acute inflammation
Causes (Stimuli for Acute Inflammation)
Infection
Trauma
Physical injury from thermal extremes or from ionizing
radiation
Chemical injury
Immunologic injury
Tissue death. Inflammatory changes occur in viable tissue
adjacent to necrotic areas
Processes
1) Recognition of the injurious agent,
2) Recruitment of leukocytes,
3) Removal of the agent,
4) Regulation (control) of the response, and
5) Resolution (repair).
Cardinal signs
Rubor (redness caused by dilation of vessels)
Dolor (pain due to increased pressure exerted by the
accumulation of interstitial fluid and to mediators such as
bradykinin)
Calor (heat caused by increased blood flow)
Tumor (swelling due to an extravascular accumulation of
fluid)
Functio laesa (loss of function)
Types
1- Acute
2- Chronic
Components of inflammation
A) Vascular changes.
B) Cellular events.
Vasoactive changes
These changes begin with a brief period of
vasoconstriction, followed shortly by dilation of
arterioles, capillaries, and postcapillary venules.
The resultant marked increase in blood flow to the
affected area is clinically manifest by redness and
increased warmth of the affected area.
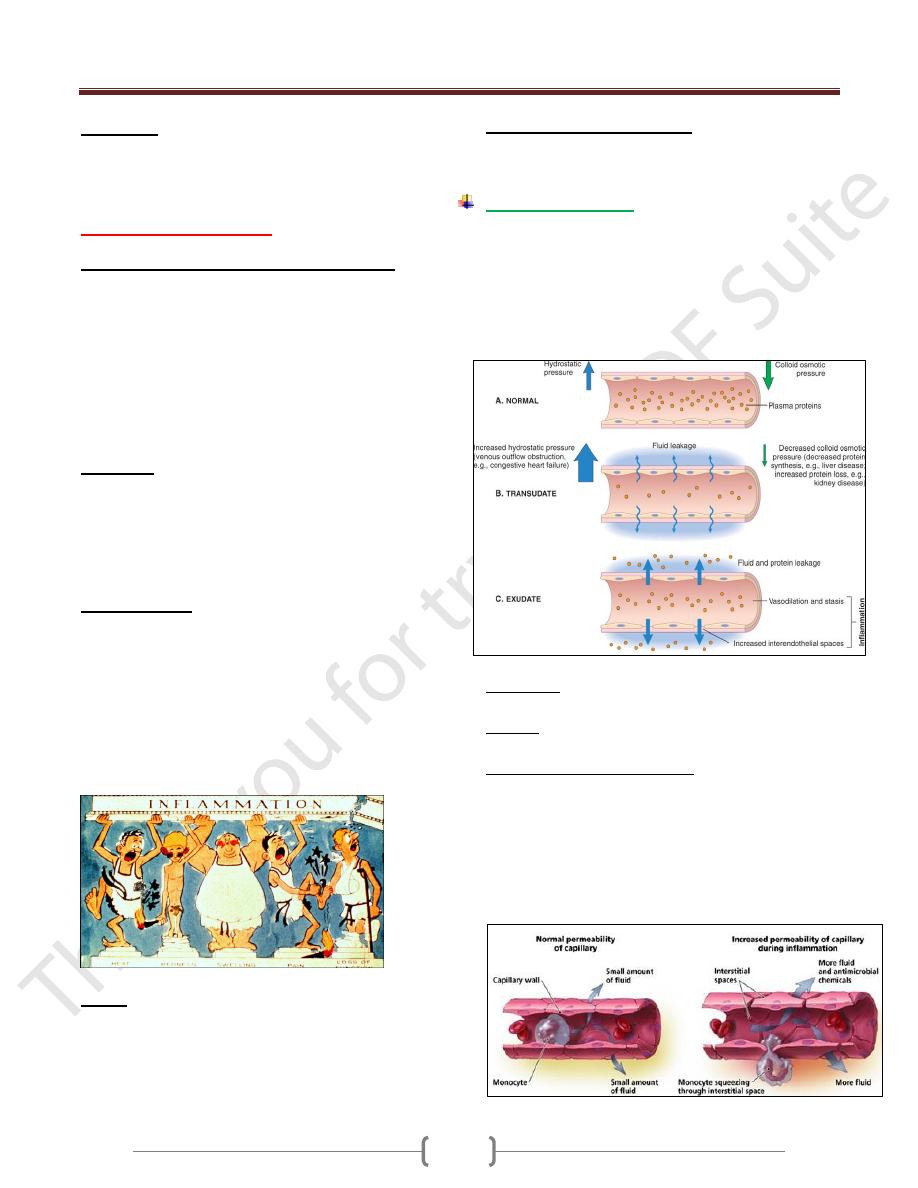
Transudate: is an ultrafiltrate of blood plasma and
contains little protein.
Exudate: interstitial fluid with high concentration of
plasma protein.
Increased capillary permeability
This results in leakage of proteinaceous fluid, which
causes edema.
Causes
1) Endothelial changes that vary from contraction of
endothelial cells in postcapillary venules, with widening
of interendothelial gaps.
2) Endothelial damage involving arterioles, capillaries & venules
Unit 2: Inflammation and Repair
21
Cellular Events: Leukocyte Recruitment &
Activation (Cellular response of leukocytes)
1- Emigration 2- Chemotaxis.
3- Phagocytosis. 4- Intracellular microbial killing
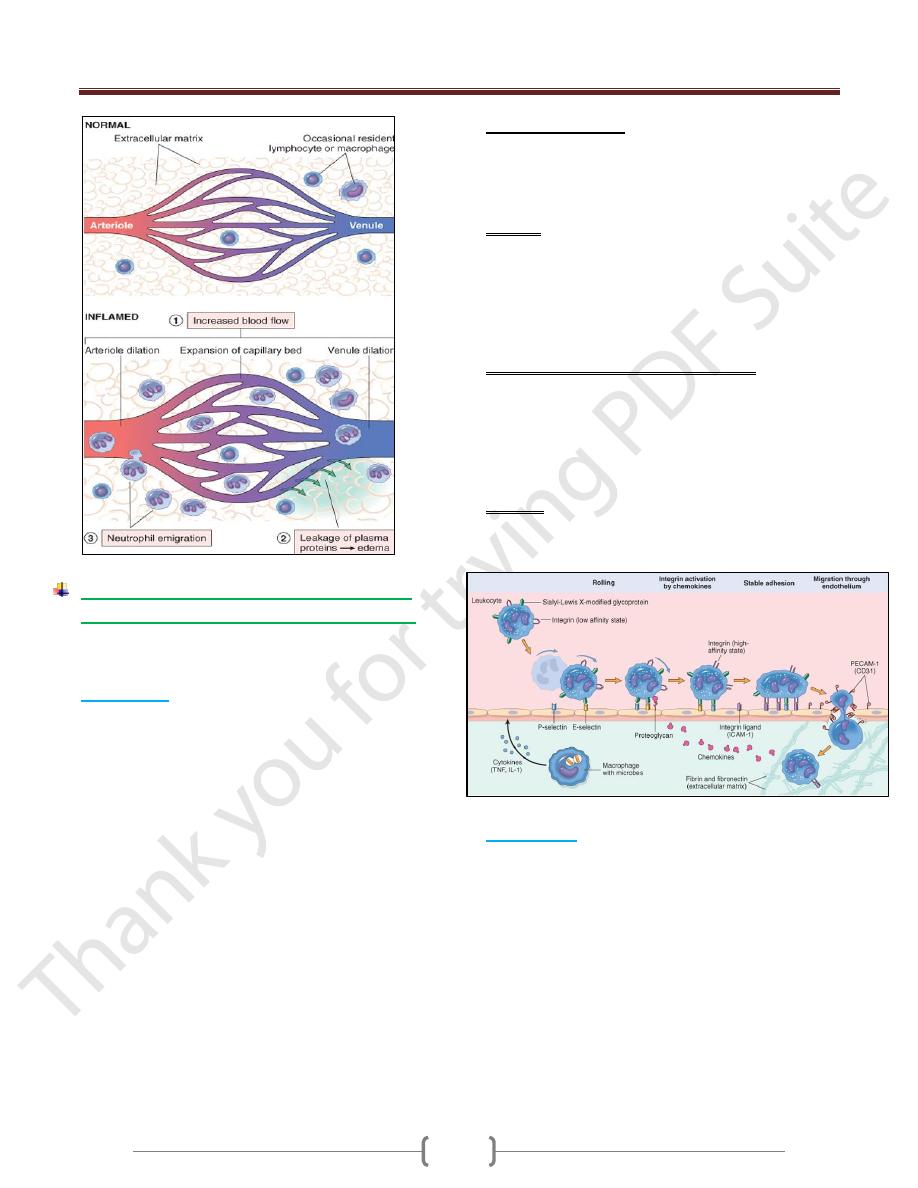
1) Emigration
Is the passage of inflammatory leukocytes between the
endothelial cells into the adjacent interstitial tissue.
Through many steps:
Margination occurs as leukocytes localize to the outer
margin of the blood flow adjacent to the vascular
endothelium.
Pavementing occurs as leukocytes line the endothelial
surface.
Rolling is mediated by the action of endothelial selectins
loosely binding to leukocytes, producing a characteristic
"rolling" movement of the leukocytes along the
endothelial surface.
Adhesion occurs as leukocytes adhere to the endothelial
surface and is mediated by the interaction of integrins on
leukocytes binding to immunoglobulin-family adhesion
proteins on endothelium.
Transmigration is the movement of leukocytes across
the endothelium and is mediated by platelet endothelial
cell adhesion molecule-1 (PECAM-1) on both leukocytes
and endothelium
Adhesion molecules
Adhesion molecules play an important role in acute
inflammation.
They are of three families: selectins, immunoglobulin-
family adhesion proteins, and integrins.
Selectins
o These molecules are induced by the cytokines interleukin-
1 (IL-1) and tumor necrosis factor (TNF).
o L-selectins are expressed on neutrophils and bind to
endothelial mucin-like molecules such as GlyCam-1.
o E- & P-selectins are expressed on endothelial cells and
bind to sialyl-Lewis X on the surface of leukocytes.
Immunoglobulin-family adhesion proteins
o Intercellular adhesion molecules 1 and 2 (ICAM-1 and
ICAM-2) are expressed on endothelial cells and bind to
integrin molecules on leukocytes.
o Vascular cell adhesion molecules (VCAMs) similarly are
expressed on endothelial cells and bind to integrin
molecules on leukocytes.
Integrins.
Examples include leukocyte MAC-1, and VLA-4which
bind to endothelial immunoglobulin-family adhesion proteins
2) Chemotaxis
Process by which leukocytes are attracted to and move
toward an injury.
This process is mediated by diffusible chemical agents.
Movement of leukocytes occurs along a chemical gradient.
Chemotactic factors for neutrophils
1) Products from bacteria
2) Complement components, especially C5a
3) Arachidonic acid metabolites, especially leukotriene
B4 (LTB4)and kallikrein
Unit 2: Inflammation and Repair
22
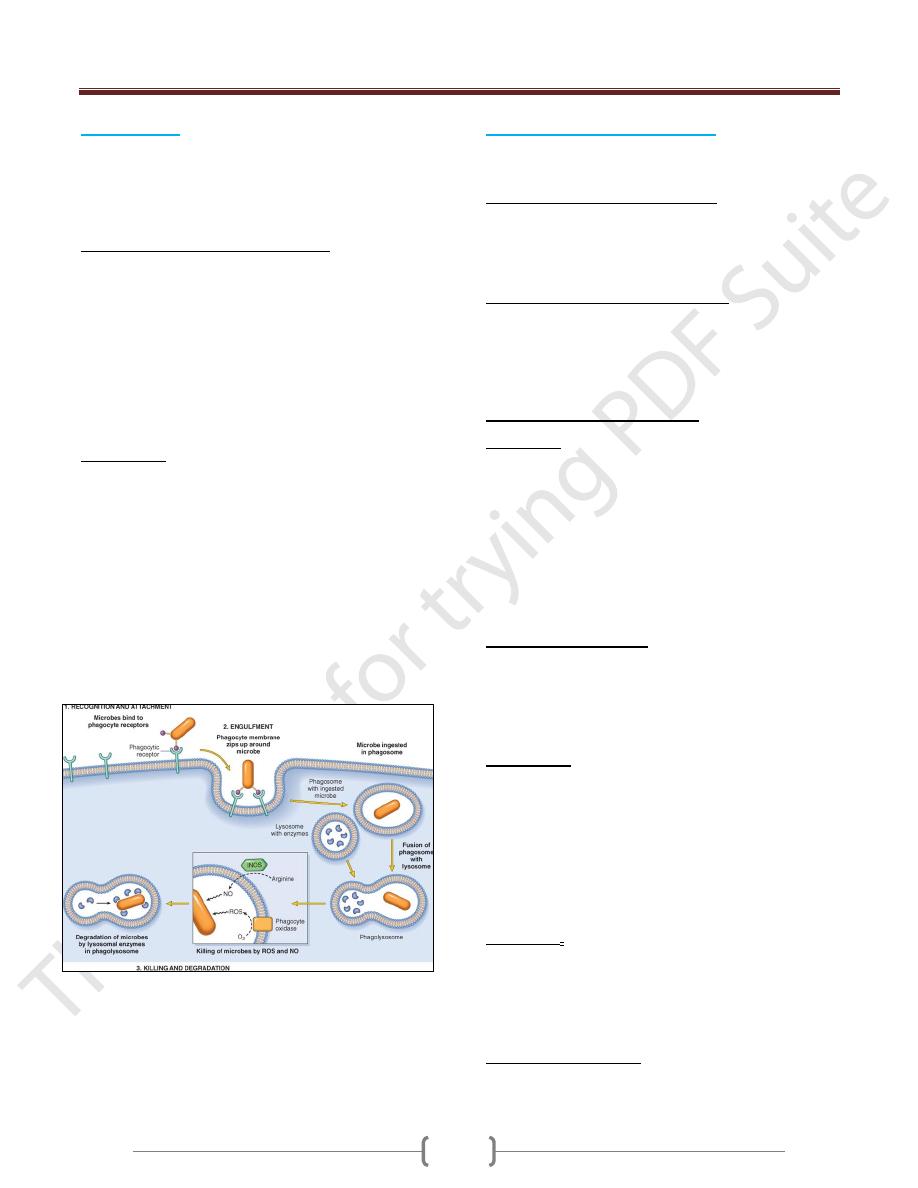
3) Phagocytosis
It is the ingestion of particulate material (e.g., tissue
debris, living or dead bacteria, other foreign cells) by
phagocytic cells. Neutrophils and monocytes-
macrophages are the most important phagocytic cells.
Anatomic changes during phagocytosis
Phagocytosis is characterized morphologically by
internalization of the attached opsonized particle by
pseudopodial extensions from the surface of the
leukocyte, which enclose the foreign particle, forming an
internalized vesicle, the phagosome.
Phagosomes fuse with cytoplasmic lysosomes and form
phagolysosomes.
Phagolysosome formation is associated with leukocytic
degranulation.
Opsonization
This process facilitates phagocytosis. It is the coating of
particulate material by substances referred to as opsonins,
which immobilize the particles on the surface of the
phagocyte.
The most important opsonins are immunoglobulin G
(IgG) subtypes and C3b, a complement component.
Fragments opsonized by IgG are bound to phagocytic
cells by cell-surface receptors for the Fc portion of the
IgG molecule.
Fragments opsonized by C3b bind to cellular receptors for
C3b.
4)
Intracellular microbial killing
Is mediated within phagocytic cells by oxygen-dependent
and oxygen-independent mechanisms.
Oxygen-dependent microbial killing
Is the most important intracellular microbicidal process
which mediated by formation of free radicals like
superoxide anion (O2·-), hydrogen peroxide (H2O2) and
hydroxyl radical (OH·).
Oxygen-independent microbial killing
This process is much less effective than oxygen-
dependent microbial killing.
This process is mediated by proteins, such as lysozyme,
lactoferrin, and cationic proteins, such as defensins
Types of inflammatory cells
1) Neutrophils are the most prominent inflammatory cells in
foci of acute inflammation during the first 24 hours.
Important causes of neutrophilia (increased neutrophils in
the peripheral blood) include bacterial infections and
other causes of acute inflammation, such as infarction.
The early release of neutrophils into the peripheral blood
in acute inflammation is from the bone marrow
postmitotic reserve pool. There is often an increase in the
proportion of less mature cells such as band neutrophils.
After 2–3 days, neutrophils are replaced mainly by
2) Monocytes-macrophages, which are capable of
engulfing larger particles, are longer-lived, and are
capable of dividing and proliferating within the inflamed
tissue. Important causes of monocytosis (i.e., increased
number of monocytes in the peripheral blood) include
tuberculosis, brucellosis, typhus, and salmonella infection.
3) Lymphocytes are the most prominent inflammatory cells
in many viral infections and, along with monocytes-
macrophages and plasma cells, are the most prominent
cells in chronic inflammation. Lymphocytosis (i.e., an
increased number of lymphocytes in the peripheral blood)
is most often caused by viral infections such as influenza,
mumps, rubella, and infectious mononucleosis and certain
bacterial infections such as whooping cough and
tuberculosis.
4) Eosinophils are the predominant inflammatory cells in
allergic reactions and parasitic infestations. The most
important causes of eosinophilia include allergies such as
asthma, hay fever, and hives and also parasitic infections.
Other causes include polyarteritis nodosa & Hodgkin
lymphoma.
5) Mast cells and basophils are sources of histamine.
Important causes of basophilia include chronic
myelogenous leukemia.
Unit 2: Inflammation and Repair
23
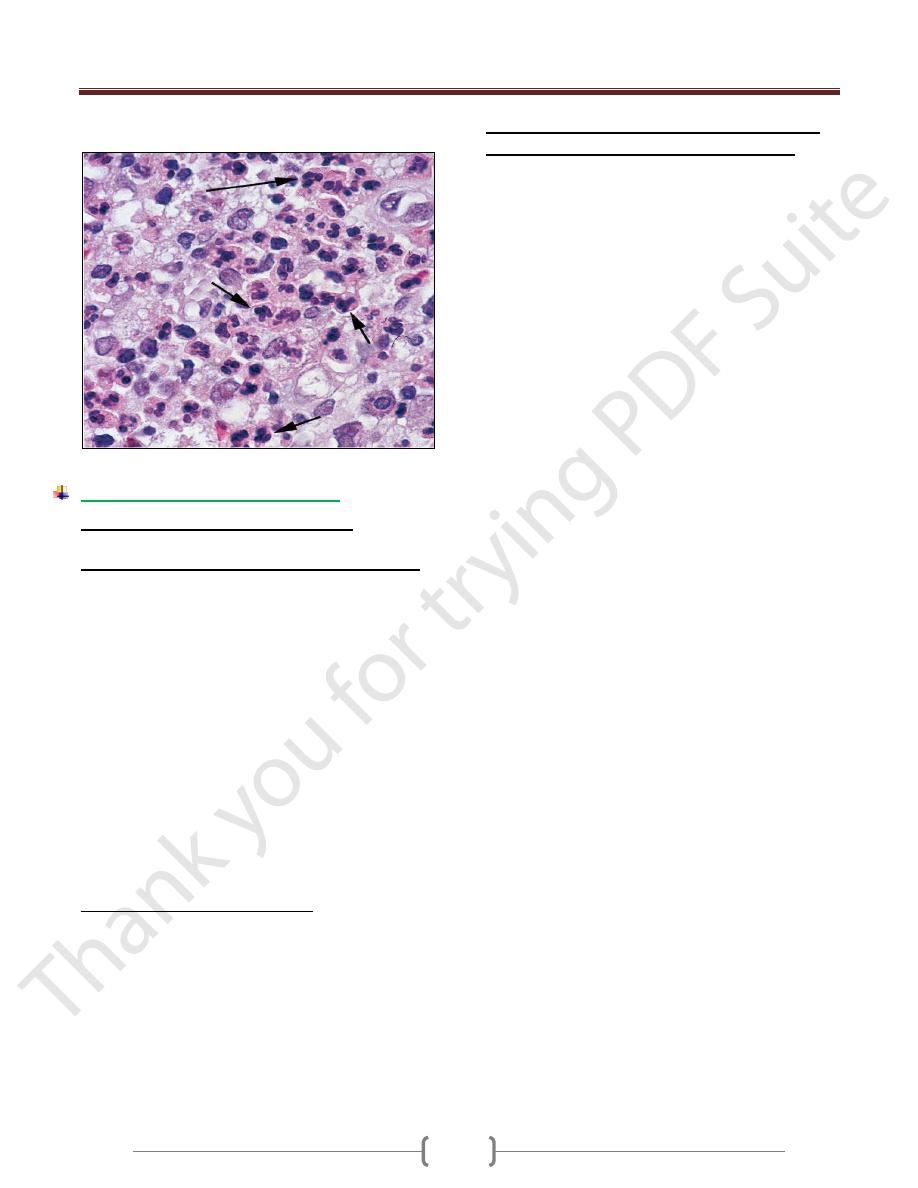
Neutrophils (polymorphonuclear leukocytes, PMNs) in tissue.
PMN infiltration typifies the early stages of acute inflammation.
Outcome of acute inflammation
1) Resolution of tissue structure and function often occurs
if the injurious agent is eliminated.
2) Tissue destruction and persistent acute inflammation
Abscess. This is a cavity filled with pus (neutrophils,
monocytes, and liquefied cellular debris).
It is often walled off by fibrous tissue and is relatively
inaccessible to the circulation.
It results from tissue destruction by lysosomal
products and other degradative enzymes.
It is usually caused by bacterial infections, often by
staphylococci.
Ulcer. This is the loss of surface epithelium.
This can be caused by acute inflammation of epithelial
surfaces (e.g., peptic ulcer, ulcers of the skin).
Fistula. This is an abnormal communication between two
organs or between an organ and a surface.
Scar. This is the final result of tissue destruction, with
resultant distortion of structure & in some cases, altered
function.
3) Conversion to chronic inflammation
This change is marked by the replacement of neutrophils &
monocytes with lymphocytes, plasma cells & macrophages.
It often includes proliferation of fibroblasts& new vessels,
with resultant scarring and distortion of architecture.
It is important to remember that there are both
good and bad aspects to acute inflammation
The Good.
Dilution of toxins
Entry of antibodies
Transport of drugs
Fibrin formation (traps microbes, serves as a matrix
for granulation tissue)
Delivery of nutrients & oxygen
Stimulation of immune response
The Bad.
Swelling (can occlude airways, raise intra-cranial pressure
and so on)
Inappropriate inflammatory response.
Digestion of normal tissues

Unit 2: Inflammation and Repair
24
Lecture 2 - chemical mediators and regulators of inflammation

Unit 2: Inflammation and Repair
25

Unit 2: Inflammation and Repair
26

Unit 2: Inflammation and Repair
27

Unit 2: Inflammation and Repair
28
Lecture 3 – Chronic Inflammation & Systemic effects of Inflammation
Chronic Inflammation

Unit 2: Inflammation and Repair
29

Unit 2: Inflammation and Repair
30

Unit 2: Inflammation and Repair
31

Unit 2: Inflammation and Repair
32
Systemic effects of Inflammation

Unit 2: Inflammation and Repair
33
