4
• These vitamins are released, absorbed & transported with the fat of the diet.
• They are not readily excreted in the urine & significant quantities are stored in the
liver &adipose tissue.
• Consumption of lipid soluble vitamins in excess of the recommended dietary
allowances can lead to accumulation of toxic quantities of these vitamins.
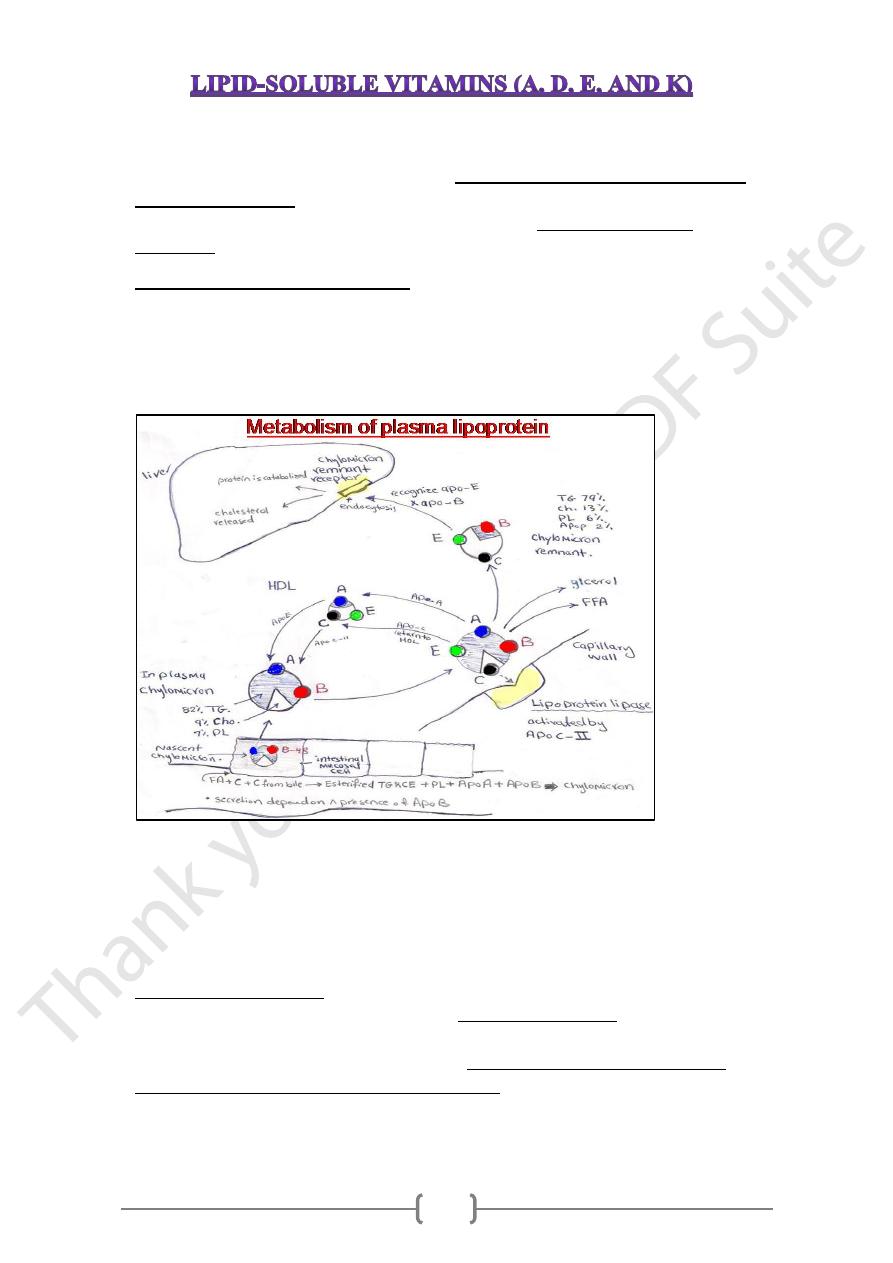
Absorption of lipid soluble vitamins
These vitamins solubilized within bile salt emulsification & mixed micelle formation
before being transported into the intestinal mucosal cells (enterocytes), then moved
across the mucosal membrane & packaged into chylomicrons which secreted into the
intracellular spaces and enter the lymphatic system & pass into systemic circulation.
• The overall dietary absorption approximately 70–90%, with less efficient absorption
at very high doses (generally, the rate of absorption decreases as the amount taken
increases).
• It has been reported that only about 50% of a dose of vitamin D is absorbed ,
considering that sufficient amounts of vitamin D can be produced daily by exposure
to sunlight .
• Endogenous & exogenous
• The absorbed vitamins is stored in the body, primarily in the liver .
• Measurement of plasma concentration of lipid soluble vitamins is the most convenient
& widely used assessment of vit.A status,but it does not ideal indicator because it
does not decline until liver stores become depleted

5
vitamin D
Transport of
The actual site of transfer of vitamin D from the chylomicrons to its specific
(synthesized in liver) &circulate in
Binding protein
-
D
vit.
plasma carrier protein is
in pregnancy & with estrogen
increased
great excess; its concentrations are
therapy & decreased in nephrotic syndrome.
Transport of vit.A
serum
Retinol secreted from liver in blood & transported in blood by specific
cytosolic retinal binding proteins.
and
binding protein
-
retinal
Transport of vitamin E
• Following delivery to the liver, tocopherol is repackaged with very low-density
lipoproteins (VLDL-c) and secreted into plasma.
• During the conversion of VLDL-c to low-density lipoproteins (LDL-c) in the
circulation, a portion of tocopherol is transferred to LDL- c.
• Exchange of tocopherol also occurs between LDLs and high-density lipoproteins
(HDL-c).
Sources
1) Retinoids (retinol, retinal, and retinoic acid) found only in foods of animal origin;
Retinol is the principal vitamin A, can oxidized reversibly to retinal (share all the
biological activity of retinol), further oxidized to retinoic acid (which shows some of
biological activity)
2) Carotenoids (provitamin A), found in plants, β-Carotene oxidized by oxidative
cleavage, to yield two molecules of retinal by enzyme (carotenoid dioxygenase),
which is found in the intestine, liver, and kidney
.
Storage
which
retinol palmitate
in the liver as
stored primarily
The absorbed vitamin A is
1 year's.
comprise approximately

6
Metabolic roles of vitamin A
Vitamin A has two metabolic:
1. Prosthetic group of the visual pigments. 2. Nuclear modulator of gene expression
1) Role of vitamin A ( retinal) in vision
• In the pigment epithelium of the retina, all-trans-retinol
from circulation is isomerized to 11-cis-retinol and then
oxidized to 11-cis-retinal, which reacts with apoprotein
(opsin), forming the rhodopsin (visual pigment) found in
the retina.
• The adsorption of light by rhodopsin causes
isomerization of the retinal from 11-cis retinal to all-
trans retinal, and conformational changes in opsin,
results in the release of all-trans retinal from the opsin
and the initiation of a nerve impulse.
• The final step is release of all-trans-retinol and opsin.
2) Role of retinoic acid in the regulation of gene expression
& tissue differentiation in embryonic development
.
differentiation and turnover
Vitamin A control cell
All-trans retinoic acid and 9-cis-retinoic acid regulate
retinoic acid binds to nuclear
because
tissue differentiation
&
,
development
,
growth
receptors that bind to response elements of DNA and regulate the transcription of
specific genes.
Vitamin A deficiency:
• In children :
1) Leads to increased susceptibility to infectious diseases because vitamin A has an
important role in differentiation of immune system cells including modulating the
expression of cytokines.
2) The synthesis of RBP (retinol-binding protein) in response to infection is reduced
(it is a negative acute phase protein) and therefore there is further impairment of
immune responses.
• In adults :
Excessive alcohol consumption reduces liver reserves of vitamin A, as a result of
alcoholic liver damage and also by induction of cytochrome P450enzymes that
catalyze the oxidation of retinol to retinoic acid.
Toxic effect of excessive vitamin A intake.
There is only a limited capacity to metabolize vitamin A, and excessive intakes
so that unbound
lead to accumulation beyond the capacity of binding proteins,
vitamin A causes tissue damage.

7
Vitamin D is really a hormone (why)
Vitamin D is not consider as a vitamin since it can be synthesized in the skin, and
it is not required in the diet, only under
under most conditions its major source,
inadequate exposure to sunlight .
conditions of
Source of vitamin D
1) Exogenous (Diet):
Ergocalciferol (Vit. D2 ) found in plants.
Cholecalciferol (Vit. D3) found in animal tissues, milk & egg yolks.
2) Endogenous Vit. D precursor :
hich is synthesized in the liver (intermediate
w
Dehydrocholesterol (provitamin D3)
-
7
in the synthesis of cholesterol) & accumulates in the skin, undergoes a non-enzymatic
, which is
Cholecalciferol (vit .D3)
reaction on exposure to UV of sunlight, yielding
then absorbed into the blood stream.
.
So that Cholecalciferol synthesized in the body & taken from the diet
Activation
• Ergocalciferol-D2 & Cholcalciferol -D3 undergoes two successive hydroxylation
(Hydroxylase enz).
• The 1
st
occurs at C- 25 in liver &
• 2nd hydroxylation occurs at C-1, in the kidney, producing the active vit.D [1α-, 25-
dihydroxy D3&dihydroxyD2].
• When calcium levels are adequate, 2nd hydroxylation occurs at C-24, yielding the
inactive 24, 25(OH) D3 form.
• The activity of1α- hydroxylase and hence the production of active vit. D is
Stimulated by ;
Low plasma phosphate & An increase in plasma parathyroid hormone level.
inhibited by:
High plasma phosphate level & High level of free ionized calcium.
Role of vitamin D
1) Effect on the intestine:
.
absorption of Ca & phosphate
Active vit. D, stimulates the intestinal
Binding of (D) to its cytosolic receptor in intestinal cell, lead to formation of a
complex, which moves to the nucleus where it interacts with the DNA, leading to
.
binding protein
-
Ca
increase synthesis of a
increase Ca uptake by
2) Effect on bone resorption:
(D3) stimulates (increase) the mobilization of Ca & phosphate from bone by a
, the result is an increase in plasma Ca&
parathyroid hormone
process that requires
is an important reservoir of Ca that can be mobilized to
Thus, bone
phosphate .
maintain plasma levels.
3) Effect on kidney Active vit. D inhibits its own synthesis &stimulates its metabolism.

8
Vitamin D deficiency
• Results in inadequate intestinal absorption (site of absorption), and
renal reabsorption & (site of active hydroxylation) of calcium and
phosphate, thus serum calcium and phosphate levels fall and serum
alkaline phosphatase activity increases.
• In response to these low serum calcium levels, hyperparathyroidism
occurs. The result of increased levels of PTH, with 1α, 25(OH)2
D3 at the onset of the deficiency, lead to the demineralization of
bone , this will leads to
rickets in children and
Osteomalacia in adults this is aggravated by insufficient
sunlight and low calcium intake or absorption.
Vitamin D is toxic in excess
•
Some infants are sensitive to intakes of vitamin D as low as 50 µg
/d, resulting in an elevated plasma concentration of calcium.
This can lead to contraction of blood vessels, high blood pressure, and calcinosis (the
calcification of soft tissues).
•
Although excess dietary vitamin D is toxic, excessive exposure to sunlight does not
lead to vitamin D poisoning because there is a limited capacity to form the precursor
7-dehydrocholesterol and to take up cholecalciferol from the skin.
• Vitamin E is the term suggested for naturally occurring tocopherols &tocotrienols, all
derivatives qualitatively exhibiting the biological activity of α-tocopherol.
• 90% of vitamin E present in human tissues is in the form of the natural isomer,
α-tocopherol (most active form).
Dietary sources
• The dietary vitamin E are oils and fats, particularl sunflower oil, grains, and nuts.
• Meats, fruits and vegetables contribute little vitamin E.
• Triglycerides have been shown to enhance the absorption process,
• Whereas long-chain polyunsaturated fatty acids reduce the absorption of α-tocopherol
, this is may be due to an increase in oxidation of tocopherol during digestion.
Deficiency Symptoms
• Fat malabsorption reduces the body fat content of vitamin E and after a prolonged
period, vitamin E deficiency has been reported.
• Low vitamin E intake in pregnancy and newborn infants is associated with hemolytic
anemia and increased susceptibility to hemolytic stress ,this is reflects the shortened
life span of erythrocytes with fragile membranes &it doesn't respond to iron therapy.
9
• Premature &low birth weight infants are susceptible to development of vit. E
deficiency because placental transfer is poor & infants have limited adipose tissue.
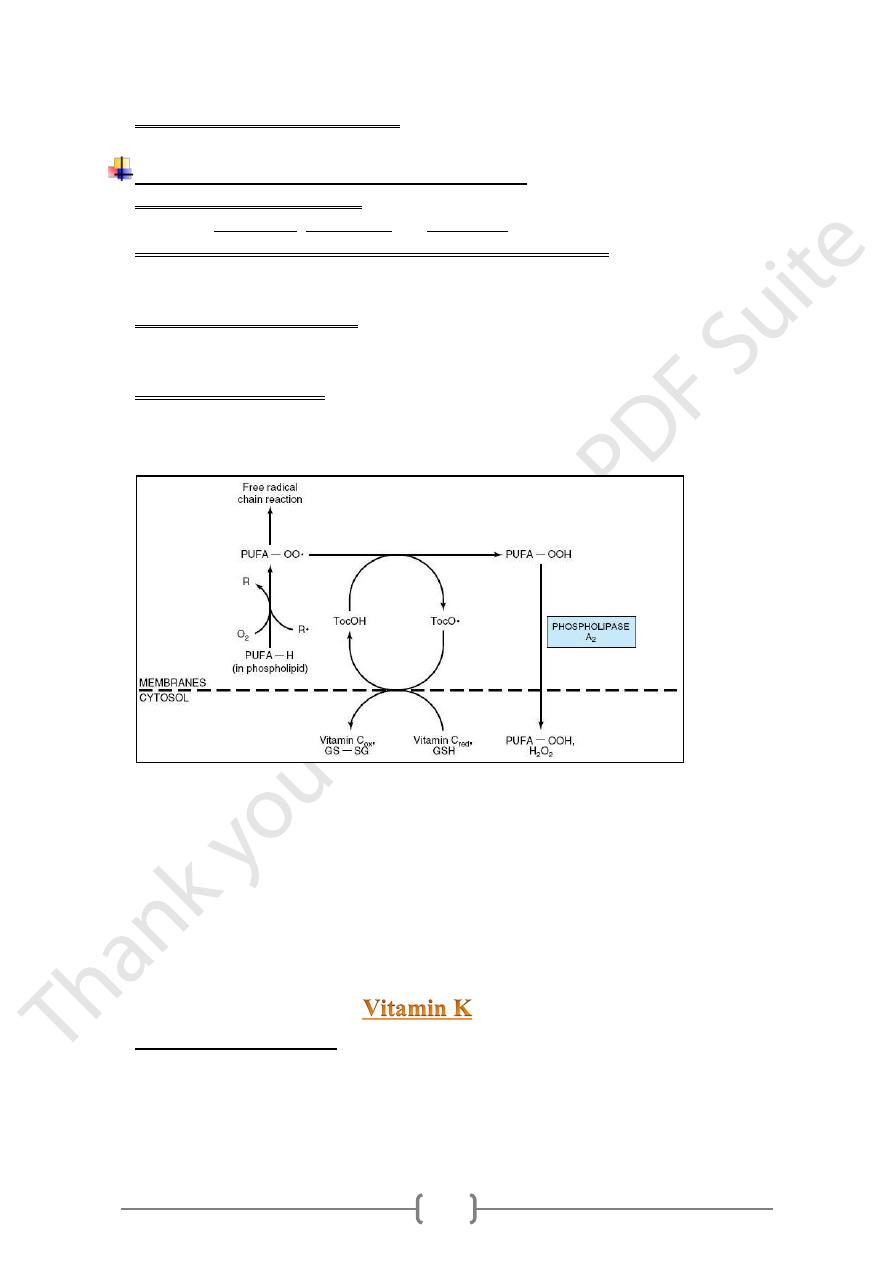
Role of vitamin E as free radicals scavenger
Vitamin E acts as antioxidant, because it is associated with all lipid-containing
structures: membranes, lipoproteins and fat deposit owing to its lipid solubility.
Vitamin E act as scavenger for reactive oxygen &free radicals Superoxide radicals
& nitric oxide, are the most significant reactive oxygen species generated in systems
living in aerobic environments.
Tocopherols and tocotrienols inhibit lipid peroxidation because they scavenge lipid
peroxyl radicals faster than the radical can react with adjacent fatty acid side chains or
membrane proteins.
The resultant tocopheryl radicals may then react with further peroxyl radicals to
produce tocopherones (nonradicals), and the chain reaction terminated by: The action
of antioxidant (AH)
R• (or ROO•) +AH → RH (or ROOH) +A•
-R•, free radical;
-PUFA-OO•, peroxyl free radical of polyunsaturated fatty acid in membrane
phospholipid;
-PUFA-OOH, hydroperoxy polyunsaturated fatty acid in membrane phospholipid
released as hydroperoxy free fatty acid into cytosol by the action of phospholipase
A2;
-PUFA-OH, hydroxy polyunsaturated fatty acid;
-TocOH, vitamin E (α-tocopherol); TocO•, free radical of α-tocopherol;
• Exists in several vitamers:
1- Vitamin K1 phylloquinone: In plants
2- Vitamin K2 menaquinones: produced in human by intestinal bacterial flora
3- Vitamin K3 menadione: Synthetic analogues of the vitamin K, metabolized to
phylloquinone.
01
• Food content
Green vegetables, fruits, milk products, vegetable oil, egg yolks &liver
Tissue deposition and storage.
• Vitamin K circulates as phylloquinone and its hepatic stores are in the form of
menaquinones.
After an overnight fast, about half the plasma vitamin K is present in chylomicron
remnants.
• After a dose of radioactive phylloquinone, it's rapidly accumulated in the liver, and
then lost from the body within 1.5 days.
• This suggests that there is rapid turnover and little storage of vitamin K & there is a
considerable enterohepatic recirculation of the conjugates excreted in the bile.
Biochemical function
• Vitamin K acts as a cofactor to vitamin K dependent carboxylase [enzyme
necessary for conversion of specific glutamyl residues to γ-carboxyglutamyl
residues]
• this carboxylation increase the affinity of these proteins for Ca++, the
antihemorrhagic function of vit.K depend upon the formation γ- carboxyglutamyl
residues of proteins factor II , factor VII, factor IX, factor X .
• The
-carboxyglutamate residues of factor II (prothrombin) are good chelators of Ca
+
• The (prothrombin - Ca++) complex is then able to bind phospholipids essential for
blood clotting on the surface of platelets.
Deficiency
• The requirement for vit. K is extremely low (why), and there is little possibility of a
dietary deficiency ( high levels of vitamin K found in most diets ) had prevented an
accurate assessment of the requirement.
• The classical method used to define an inadequate intake of vitamin K was measure
the plasma concentration of the vitamin K–dependent clotting factors.
• Deficiency of vitamin K is rare but may develop in those with.
1) Liver disease & fat malabsorption states (bile duct obstruction, chronic pancreatitis)
2) In the newborn ,especially at risk of hemorrhagic disease because;
a. Poor placental transfer of vitamin K to the fetus, therefore, immediately after
birth the circulating concentration decreases.
b. The gut of the newborn is sterile, so that the intestinal microflora does not
provide a source of vitamin K for several days after birth.
c. Hepatic immaturity leading to inadequate synthesis of coagulation proteins.
d. Low vitamin K content of early breast milk.
Upper levels of intake
Even large intakes of phylloquinone have nontoxic effects, although they may be
Why
dangerous in patients receiving anticoagulant therapy.
