
23
Lec.6 General Aspects of Minerals + Iron
Definition
Are inorganic elemental atoms that are essential nutrients.
They are not changed by digestion or metabolism.
Functions of Minerals
1) Participate with enzymes in metabolic processes (cofactors) Fe, Cu, Zn.
2) Structural functions (Ca, P in bone; S in keratin).
3) Acid-base and water balance (Na, K, Cl).
4) Nerve & muscle function (Ca, Na, K).
5) Blood clotting: Ca.
6) Unique functions (Fe, Co).
7) Synthesis of Hormones: thyroid hormones (iodine).
ls
Classification of minera
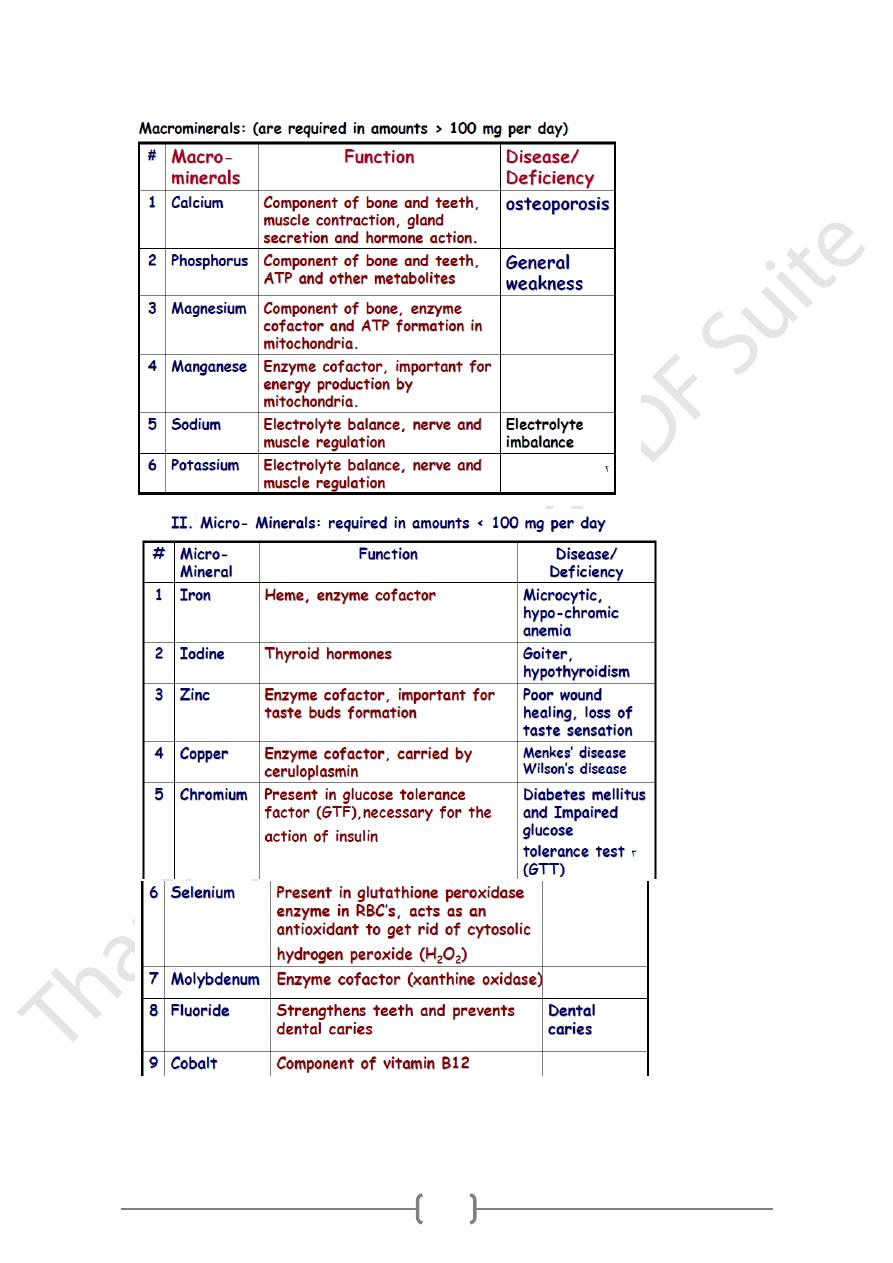
A. Macro or Major minerals
1) Present in body tissues at concentrations more than 50 mg/kg.
2) Required in amounts > 100 mg per day.
3) Ex: Calcium, Magnesium, Phosphorus, Sodium, Potassium, Sulfur.
2. Micro or Trace minerals
1) Present in body tissues at concentrations less than 50 mg/kg.
2) Required in amounts < 100 mg per day)
3) Ex: Iron, Iodine, Copper, Manganese, Chloride, Zinc, Fluoride, Tin, Arsenic,
Nickel…
Nutritionally Important Minerals
TRACE
MACRO
mg/Kg
ELEMENT
g/Kg
ELEMENT
20-50
Fe
15
Ca
10-50
Zn
10
P
1-5
Cu
0.4
Mg
1-4
Mo
1.6
Na
1-2
Se
2
K
0.3-0.6
I
1.1
Cl
o.2-0.5
Mn
1.5
S
24
25
Factors Affecting Requirements
1) Physiological state.
2) Tissue storage: Bone, Liver , Specific proteins to hold and transport.
3) Form of food: organic or inorganic.
Factors affecting absorption
1) Amount of mineral present in the food.
2) Gastrointestinal diseases.
3) Physiological state and Tissue storage.
4) substances in foods decrease absorption of minerals:
absorption of calcium in spinach.
, prevents
Oxalate
, a form of phosphorous in most plants makes
Phytate
phosphte poorly available.
5) Substances increase absorption of minerals: Vit C, increase iron absorption.
Deficiencies and Excesses
• Most minerals have an optimal range.
Ca = 8.5-10.5 mg/dl, Cu = 80-150 ug/dl, Mg = 1.8-2.4 mg/dl, Ph= 2.5-4.5 mg/dl, Fe
= 70- 180 ug/dl
• Below leads to deficiency symptoms.
• Above leads to toxicity symptoms.
• May take many months to develop.
• Time impacted by body stores.
• Physiological state.
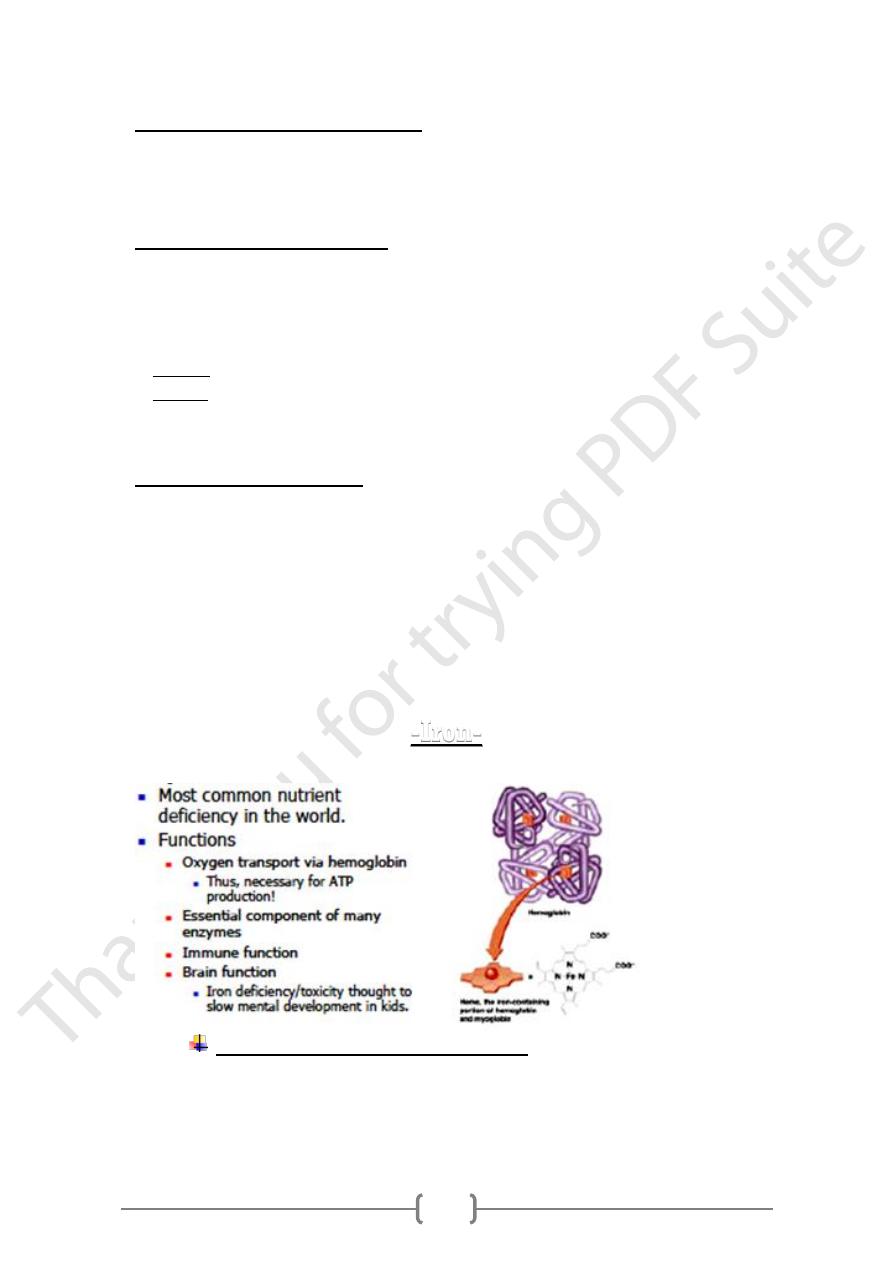
-Iron-
Iron body content and distribution
The body contains about 4-5 g of iron.
• 65% of this is in Heamoglobin (Hb).
• 25% in iron stores: Liver, spleen and bone marrow.

26
• 5-10% is contained in heam proteins: (myoglobin, and the cytochromes).
• 1% is present free in the plasma.
Iron balance
• Is regulated by alteration in intestinal absorption.
• There is only a limited capacity to increase or decrease the rate of loss of iron.
• The amount that is absorbed daily is only 1-3 mg.
• The exact amount depending on:
• Age, physiological state and blood loss.
• Most of the needs of iron are met by:
• Reutilization of iron from heam protein degradations.
The internal iron cycle
Thus there is
Means the reutilization of iron released by heam protein digestion.
an internal iron cycle which means that: once iron enters human body, it cycles in
a closed system with only a little iron entering the body daily to replace the small
amount that is lost.
Iron pools and iron binding proteins in the bod
y
1) Heamoglobin (O
2
transporter) is by far the largest pool of body iron.
2) Iron storage pool: ferritin & Hemosiderin.
A. Ferritin: is a water soluble protein.
• Ferritin contains up to 20% of the total body iron.
• Is present in plasma, liver, and bone marrow. Each of which contain about 1/3 of
total iron stores.
• Iron is released from ferritin by the action of the enzyme ferritin reductase. then
become available for heam synthesis anywhere.
B. Hemosiderin:
• Formed from denaturation of ferritin but only after abnormal conditions such as:
repeated blood transfusion. & high iron IV or oral doses.
• Is a water insoluble protein found mainly in the spleen, bone marrow and kupffer
cells of the liver but affects all the cells of the body
• Hemosiderin iron is also available for heam synthesis but is mobilized much more
slowly than that of ferritin.

27
3) Myoglobin:
• O2 storage protein of skeletal and cardiac muscles.
• Contains about 3 to 4% of total body iron.
• Found in muscle cells.
• Heme group + protein subunit.
• Releases oxygen to cells when needed for: ATP production & Muscle contraction.
4) Cytochromes:
• They are:
1. Heme-containing complexes. play an important role in: cellular respiration.
2. Are: a mixture of 3 haemochromogens called: cytochromes A,B, C.
3. Function in electron transport chain.
4. Allow conversion of ADP to ATP.
• In Cytochromes Iron acts as cofactor in
Electron Transport Chain.
Citric Acid Cycle.
Gluconeogensis.
• Cytochrome p-450: A protein similar to Hb, present in the microsomes:
1. Catalyzing the metabolism of:steroid hormones and fatty acids.
2. Acts as a cofactor for antioxidant enzymes to Protect DNA, cell membranes,
proteins.
• Cytochromeoxidase: Is an enzyme of importance in biological oxidation.
Functioning in the transfer of: Electrons from Cytochromes to Oxygen. Thus
activating O2 union with H2 to form water.
5) Transferrine:
1. is a protein specific for iron transport in the circulation after its absorption from
GIT.
2. Transferrine has specific binding sites for iron, that is 30% saturated in normal
situations.
3. its main action is to transport iron from intestine to different iron stores.
4. Estimation of transferrin in blood can give available information about the body
iron status.
6) 6- Gastroferrine:
Is a protein secreted by the stomach to enhance iron absorption.
Factors affecting daily iron absorption
1) The amount of iron present in the food.
2) the chemical status of the ingested iron.
3) The presence of : ferritin, apoferritin and gastroferrine in the mucosal cells & antrum.
4) The availability of acidic medium in the stomach.
5) Normal intestine and suitable alkaline medium.
6) The presence of other chemical in the food stuff.
28
7) Other factors also affecting Iron uptake are: increased erythropoiesis, hypoxia,
anemia, and depletion of Iron stores.
Factors increasing absorption:
Reducing agents (Eg: vitamin C, SH-containing proteins), gastric HCl, intestinal
bicarbonate & healthy GIT.
Factors decreasing absorption:
Oxalate, Phytate, Tannic acid, Carbonic acid,malabsorption & calcium ions.
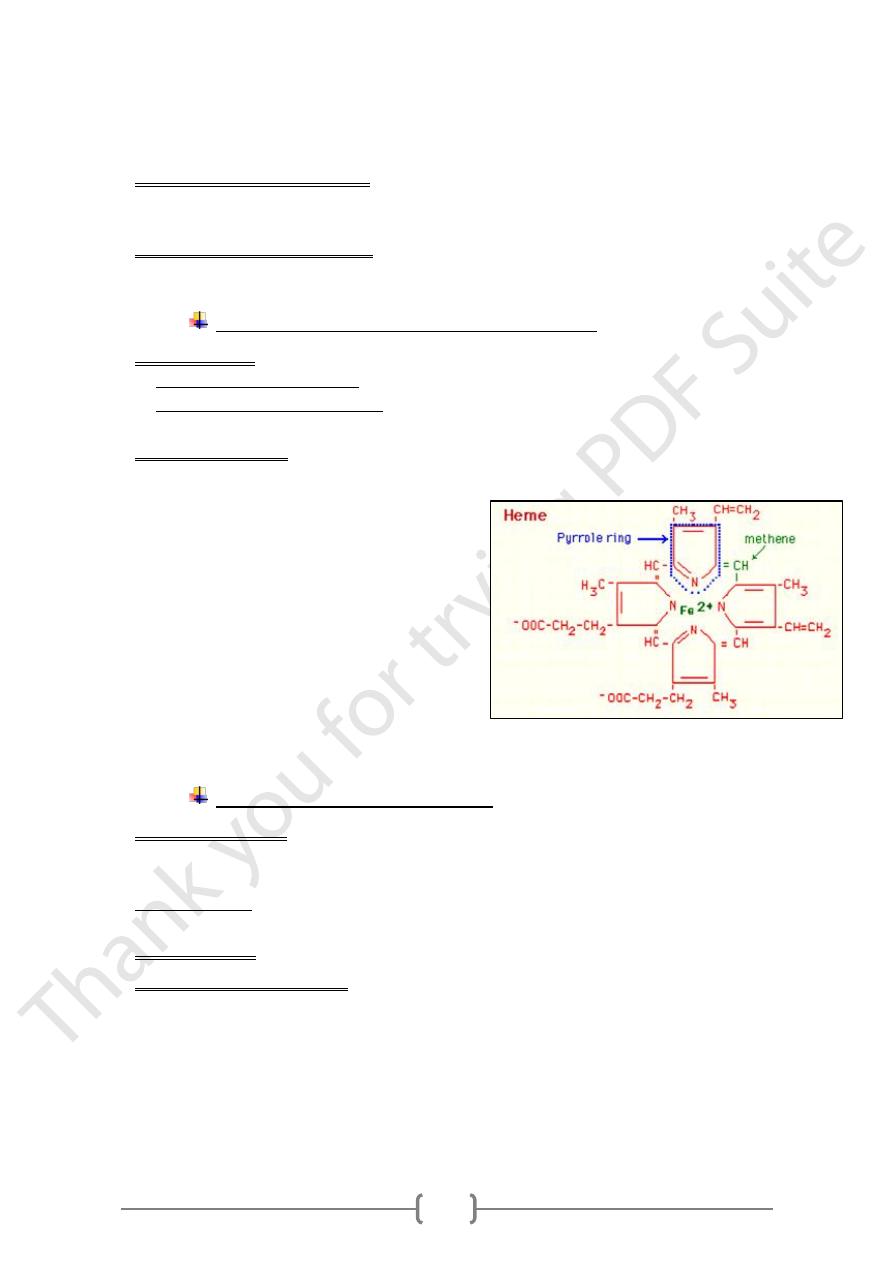
Mechanism of iron absorption and storage
A. In the stomach: ferric ions (+++) is reduced to ferrous ions (++)
• This reduction is favored by: low pH, Reducing agents, such as ascorbic acid
• Reduction is important because: ferrous ions (++) dissociate from Ligands more
easily than ferric ions (+++)
B. B- in the duodenum
• Hame is absorbed directly by the mucosal cells.
• Then within the cells, the iron released
from heme by (hame oxidase) then reduced
to ferrous Ions (Fe++) ions
o Iron is bound and transported in the body via
transferrine and stored in ferritin molecules.
o Once iron is absorbed, there is no physiologic
mechanism for excretion of excess iron from
the body other than blood loss i.e. pregnancy,
menstruation or intestinal mucosal
desquamation.
Nutritional Iron Requirements
1) In newborn infants: there is more hemoglobin in their blood by:
A. High Hb concentration in the RBC.
B. high erythrocytes count of blood
and Iron accumulates:
erythropoiesis
exceeds
destruction
cell
:
Few weeks later
which will be reused in later months for synthesis of Hb.
2) During growth: the Requirements of Iron increase markedly.
3) In adult life: Iron turnover:
A. Hb is metabolized into heme + globin. Heme is then utilized into Iron and reused.
B. The Daily hemoglobin turnover is equivalent to 0.3 – 0.7 mg per dl of blood. Or
about 25 mg per day for an adult.
C. The iron produced by this process is bound to transferrin and stored or reutilized
again for the formation of new Hb in the RBC.
D. 1-3mg /d is only required to restore losses.
29
4) menarche:
• Females need iron 30 to 90 percent > than males.
• Until menopause 50% of the females iron is used in the replacement of Hb lost in
menses.
• The average menstrual loss is about 35ml of blood per cycle. Replacement of this
amount of blood alone requires 0.6 mg of iron per day.
• This is not so important since the average diet contains enough iron to meet these
requirements.
5) during pregnancy and lactation:
A. The requirements for iron are about 60% greater than the amount lost in
menstrual cycle during a similar period.
B. Mother food must therefore provide: large quantities of iron especially during the
last 3 months of gestation.
C. This may result in: anemia with Hb values between 11-12 g/dl. Which are
physiological during pregnancy.
Abnormal Iron Metabolism
A. Iron deficiency:
This is a common condition affecting mainly growing children, pregnant women
and lactating mothers.
The main causes of iron deficiency are:
1) Deficient intake: low iron in diet.
2) Decreased absorption.
3) Increased requirement for iron.
4) Iron loss from the body:
a. Abnormal menstrual cycle.
b. GIT bleeding: Esophageal Varices, Gastric and Duodenal Ulcers.
c. Urinary tract bleeding
Consequences of iron deficiency
1) Iron plasma falls.
2) Plasma ferritin falls.
3) Plasma transferrin and (TIBC) increase
4) Anemia becomes evident.
Iron deficiency anemia
o Causes: iron deficiency decreases the rate of both erythrocyte formation and
hemoglobin synthesis leading to what is called hypochromic microcytic anemia.
o Signs and symptoms:
1) Pallor of face and mucous membranes.
2) Excessive tiredness and fatigability.
3) Poor resistance to infections.
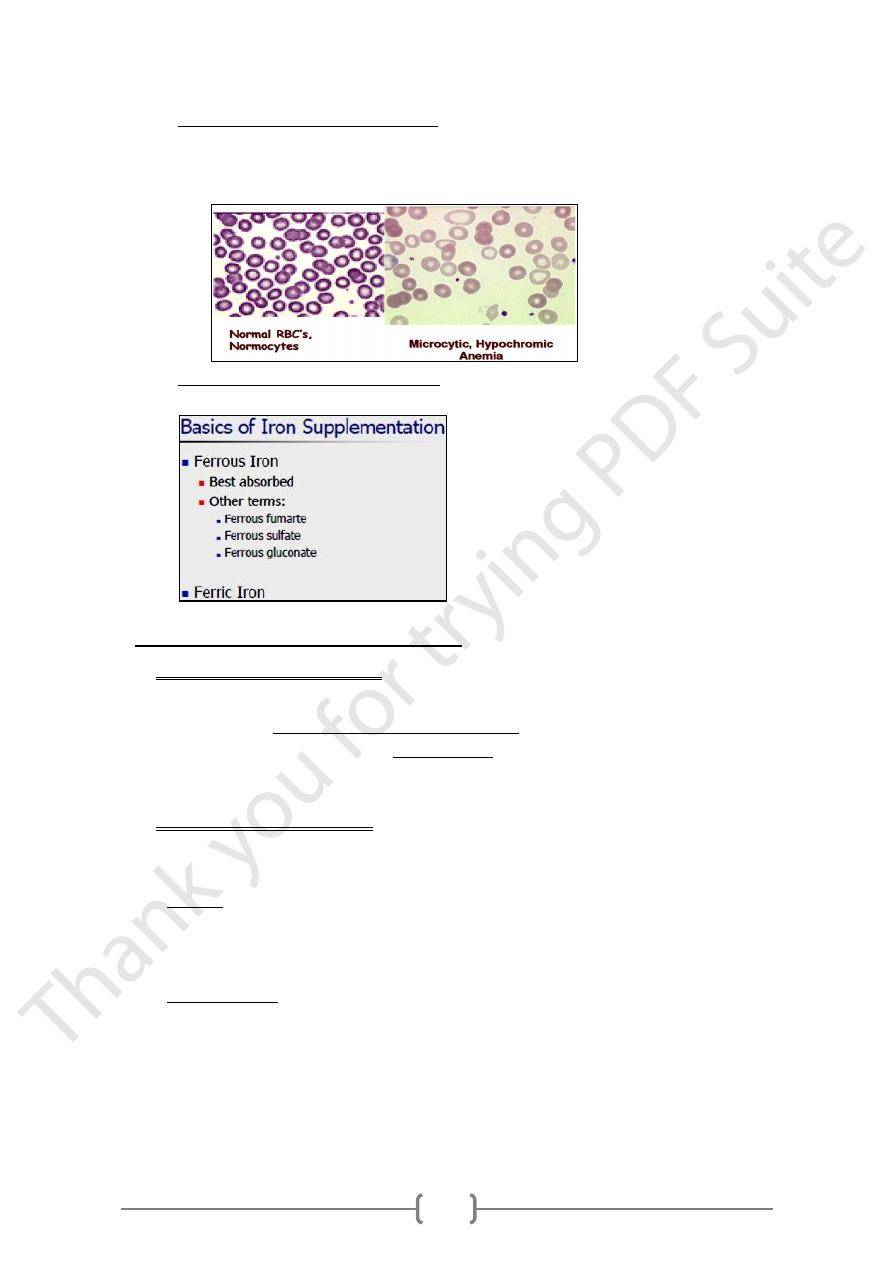
31
o Diagnosis of iron deficiency anemia
1) Signs and symptoms.
2) Blood film examination under microscope.
3) Measuring iron containing proteins.
o Treatment of iron deficiency anemia is by the supplementation of iron to the
patient.
B. Iron over load: (Haemochromatosis)
1) idiopathic haemochromatosis
• Is a rare hereditary disorder leads to an increased absorption of dietary iron due to
a defect in the mechanism controlling absorption from the intestine.
• Leads to Excessive deposits of Haemosiderin in several tissues with high plasma
ferritin and highly saturated transferrine levels.
• It is a slowly progressing and even affecting more than one member in the family.
2) acquired haemochromatosis
• This is much less common than iron deficiency, raised plasma ferritin often to
more than 1000 µg/L is characteristic of iron overload.
• Causes:
1. Increase intake and absorption of iron.
2. Treatment with iron preparation. Especially Parenteral administration of iron.
3. Repeated blood transfusion.
• Clinical picture : is due to deposition of iron in:
liver --------------- cirrhosis
pancreas --------- diabetes
skin --------------- bronze pigmentation
brain -------------- mental disturbance
gonads ----------- infertility

31
• treatment is by avoiding the causes and by removing excess iron from the body
by: phlebotomy and chelating agents (desferal).
Acute iron intoxication
o Is caused by accidental ingestion of large amounts of medicinal iron supplement
especially by children.
o It is the most common cause of childhood poisoning.
o Symptoms: Vomiting, diarrhea or constipation and black stool.
o Complications: Excess deposits in liver, heart, muscles and brain. Death if not
treated.
