
1

Practical
Biochemistry
2

List of contents
Lab 1 - Sampling
Lab 2 - Calorimetric Analysis
Lab 3 - Estimation of Blood Glucose
Lab 4 - Measurement of enzyme activity
(Alkaline phosphatase)
Lab 5 - Estimation of Serum Calcium
Lab 6 - Estimation of Serum Phosphorous
Lab 7 - Uric acid
Lab 8 - Total cholesterol
Lab 9 - Measurement of enzyme activity (GPT)
Lab 10 - Estimation of glucose
Lab 11 - lipids
Lab 12 - lipoproteins
Lab 13 - Alkaline phosphatase & transaminase
Lab 14 - Liver Function Test
Lab 15 - Total protein
Lab 16 - General Urine Test
Lab 17 - Serum Creatinine
3

4
5

6

7

8
9

10
11
Lab 3 - Estimation of Blood Glucose
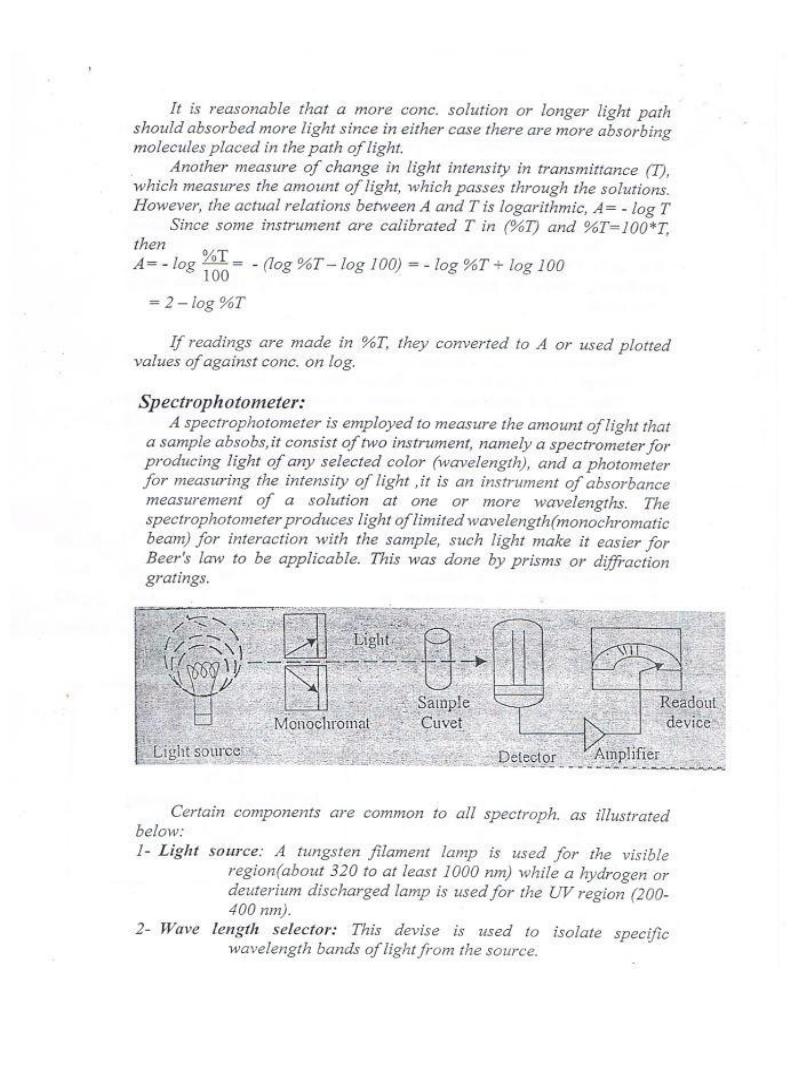
Principle
This method uses two coupled enzyme reactions. In this case,
the initial reaction is the specific one, and the indicator
reaction is nonspecific. The first reaction employs glucose
oxidase (GOD) to oxidized glucose to gluconate and hydrogen
peroxide. The hydrogen peroxide from the (GOD) reaction is
consumed by a peroxidase - dye indicator reaction in which
the oxidized dye is colored, allowing the reaction to be
monitored photometrically. The dyes employed as a final
oxygen acceptors can vary, the most widely used compound is
4-amino-antipyrine (PAP), this explained by the following
equation.
Procedure
Working reagent: prepared by dissolve reagents contained
glucose oxidase, peroxidase, 4-amino-antipyrine with reagents
contained buffer solution.
Samples: serum (not hemolyzed), plasma, spinal fluid.
Calculations:
( )
( )
Conc. of standard
Conc. of standard= 100 mg/dl
(A) Standard = 0.212
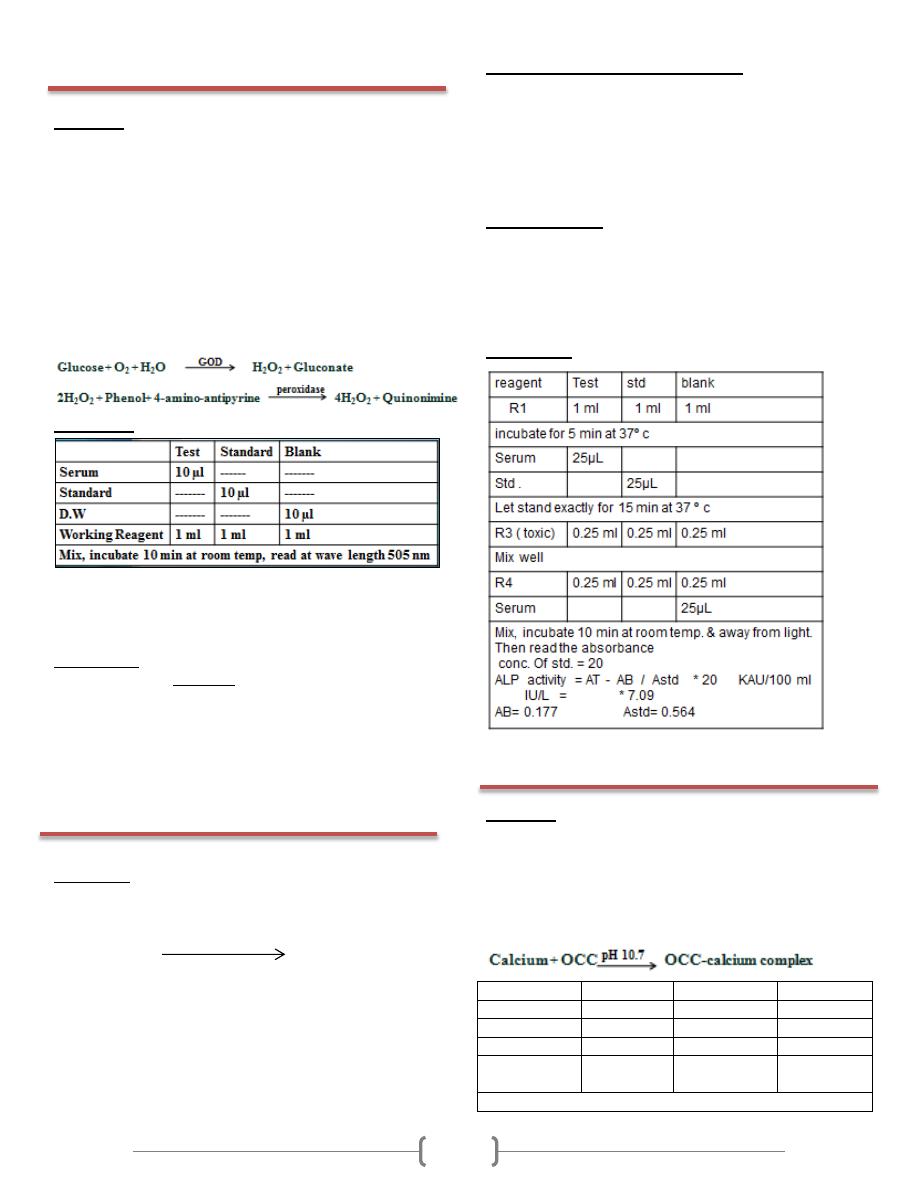
Lab 4 - Measurement of enzyme activity
(Alkaline phosphatase)
Principle:
Colorimetric determination of the ALP activity which reaction
scheme is as follows:
Phenyl phosphate alkalinephophatase phenol + phosphate
Free phenol librated by hydrolysis of the substrate reacts then
with 4- amino antipyrine in the presence of alkaline
potassium ferriccyanide to form a red-coloured complex
which absorbance measured at 510 nm is directly proportional
to the ALP activity in the specimen.
Sodium arsenate incorporated in the reagent abolishes further
enzyme activity and prevents the dilution of the colour
inherent in earlier methods.
Specimen collection and handling
Un hemolysed serum oe heparinized plasma, immediately
refrigerated:
ALP activity is stable in the specimen for:
2-3 days at 2-8º c
1 month at -25º c
Normal values:
adults = 3 – 13 kind & king units / 100ml
IU / L = 21 – 92
King Armstrong unit is 1 mg of product(phenol) liberated by
100ml of serum in 15 min under the conditions of the test.
Procedure:
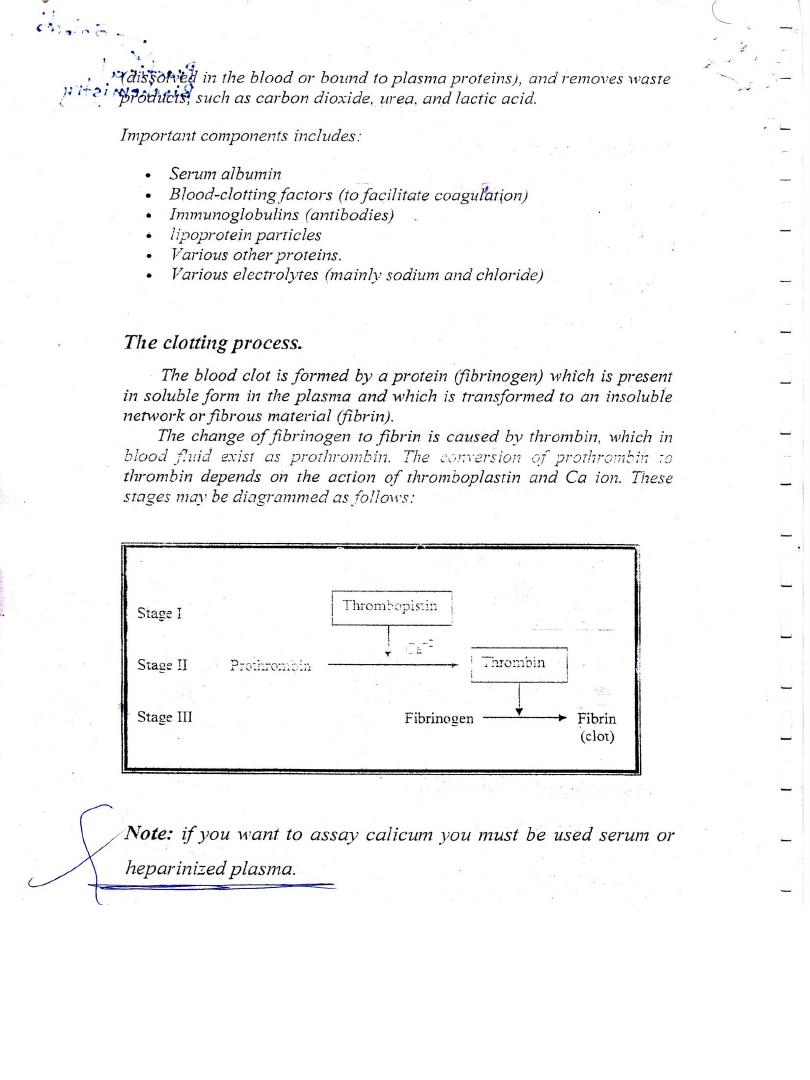
Lab 5 - Estimation of Serum Calcium
Principle
Tn the samplhe methods is based on the specific binding of
cresolftalein complexone (OCC), a metallochromic indicator,
and calcium at alkaine pH with the resulting shift in the
absorbtion wavelength of the complex. The intensity of the
cromophore formed is proportional to the concentration of
total calcium in the sample.
Test
Standard
Blank
Serum
10 µl
------
------
Standard
------
10 µl
------
D.W
------
------
10 µl
Working
Reagent
1 ml
1 ml
1 ml
Mix, incubate 2 min at room temp, read at wave length 570nm

12
Normal values: 8.4 -10.4 mg/dl
Calculations:
( )
( )
Conc. of standard= 10 mg/dl
(A) Standard = 0.630
Lab 6 - Estimation of Serum Phosphorous
Principle
Inorganic phosphorus reacts with molybdic acid forming a
phosphomolybdic complex. Its subsequent reduction in
alkaline medium originates a blue molybdenum colour.
The intensity of the color formed is proportional to the
inorganic phosphorus concentration in the sample
Sample:
Serum:
Free of hemolysis. Serum should be removed from the clot as
quickly as possible to avoid elevation of serum phosphorus
from hydrolysis or leakage of phosphate present in
erythrocytes.
Urine:
Collect the specimen into a bottle containing 10 mL of 10%
v/v hydrochloric acid (HCl) to avoid phosphate precipitations.
Normal values:
Children : 4.0 - 7.0 mg/dL
Adults : 2.5 - 5.0 mg/dL
Procedure
Calculations:
( )
( )
Conc. of standard= 5 mg/dl
(A) Standard = 0.401
Lab 7 - Uric acid
Principle:
Uric acid is oxidized by uricase to allantoin with the formation
of hydrogen peroxide. In the presence of peroxidase (POD), a
mixture of dichlorophenol sulphonate (DCPS) and 4-
aminoantipyrine (4-AA) is oxidized by hydrogen peroxide to
form quinoneimine dye proportional to the concentration of
uric acid in the sample.
Specimen collection and handling
Whenever possible medication should be suspended 12 hr.
before sample collection.
Hemolysis –free serum, EDTA or heparinized plasma & urine
Uric acid in plasma or serum is stable up to 5 days at 2-8ºC
and for 6 months at -20ºC.
Normal values:
Serum , plasma
Men = 3.5 – 7.2 mg / dl
women = 2.6 – 6.0 mg / dl
Procedure:
Mix, and let the tubes stand 10 min at room temperature
Read the absorbance (A) of the sample and & the standared at
520 nm
Calculation
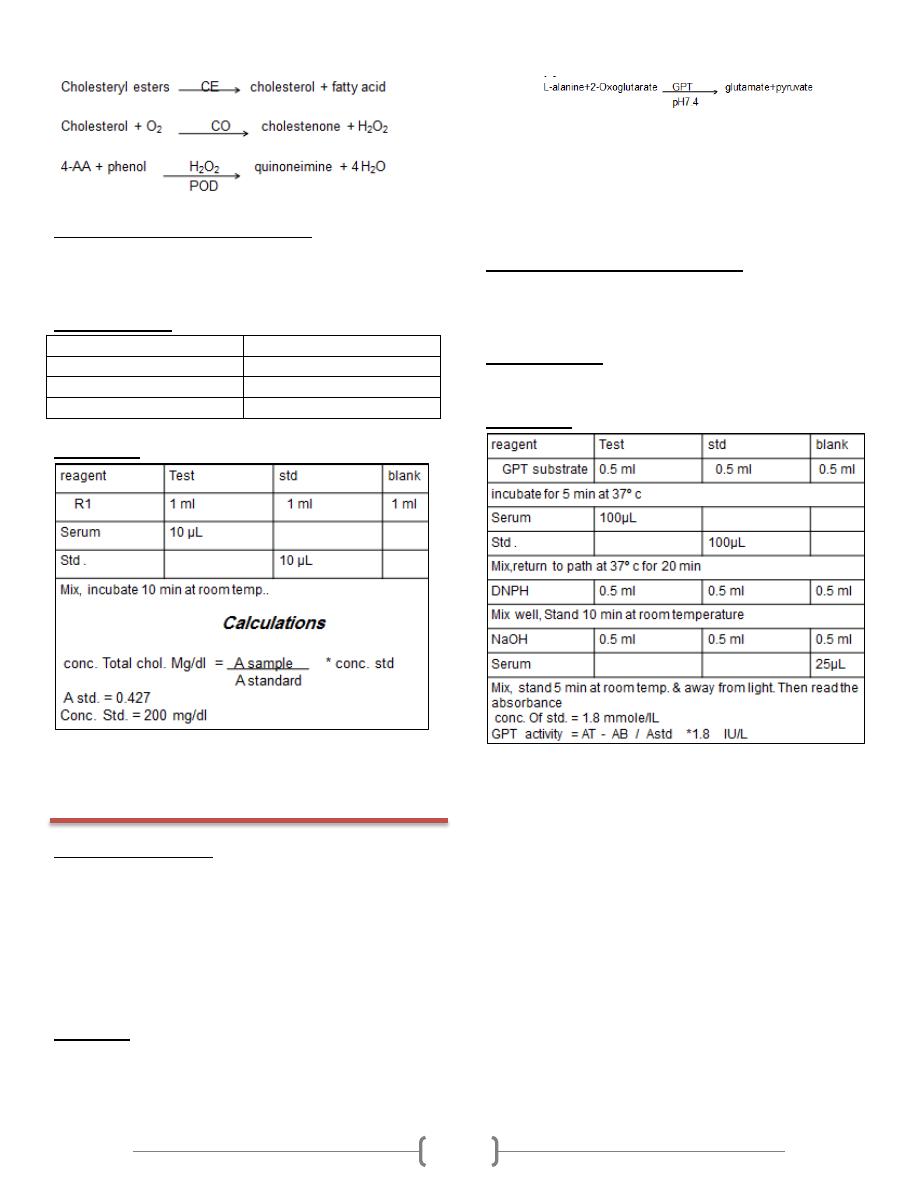
Lab 8 - Total cholesterol
Principle:
This method for the measurement of total cholesterol in serum
involves the use of three enzymes: cholesterol esterase (CE),
cholesterol oxidase (CO) and peroxidase (POD). In the
presence of the former the mixture of phenol and 4-
aminoantipyrine (4-AA) are condensed by hydrogen peroxide
to form quinoneimine dye proportional to the concentration of
cholesterol in the sample.
13
Specimen collection and handling
Un hemolysed serum or heparinized or EDTA plasma
Cholesterol in serum or plasma is stable up to 5 days at 2-
8ºC and for a few months at -20ºC.
Normal values:
Total cholesterol
Risk classification
˂200 mg / dl
Desirable
200 – 239 mg / dl
Borderline high
˃ 240 mg / dl
High
Procedure:
Lab 9 - Measurement of enzyme activity
(GPT)
Clinical significance
The group of enzymes called transaminase exist in tissues
of many organs. Necrotic activity in these organs causes a
release of abnormal quantitaties of enzyme into the blood.
The liver is specially rich in GPT. This enzyme
measurement is used primarily as a test for hepatitis. The
GPT value is useful in diagnosing infecttious hepatitis.
Neither test is specific.
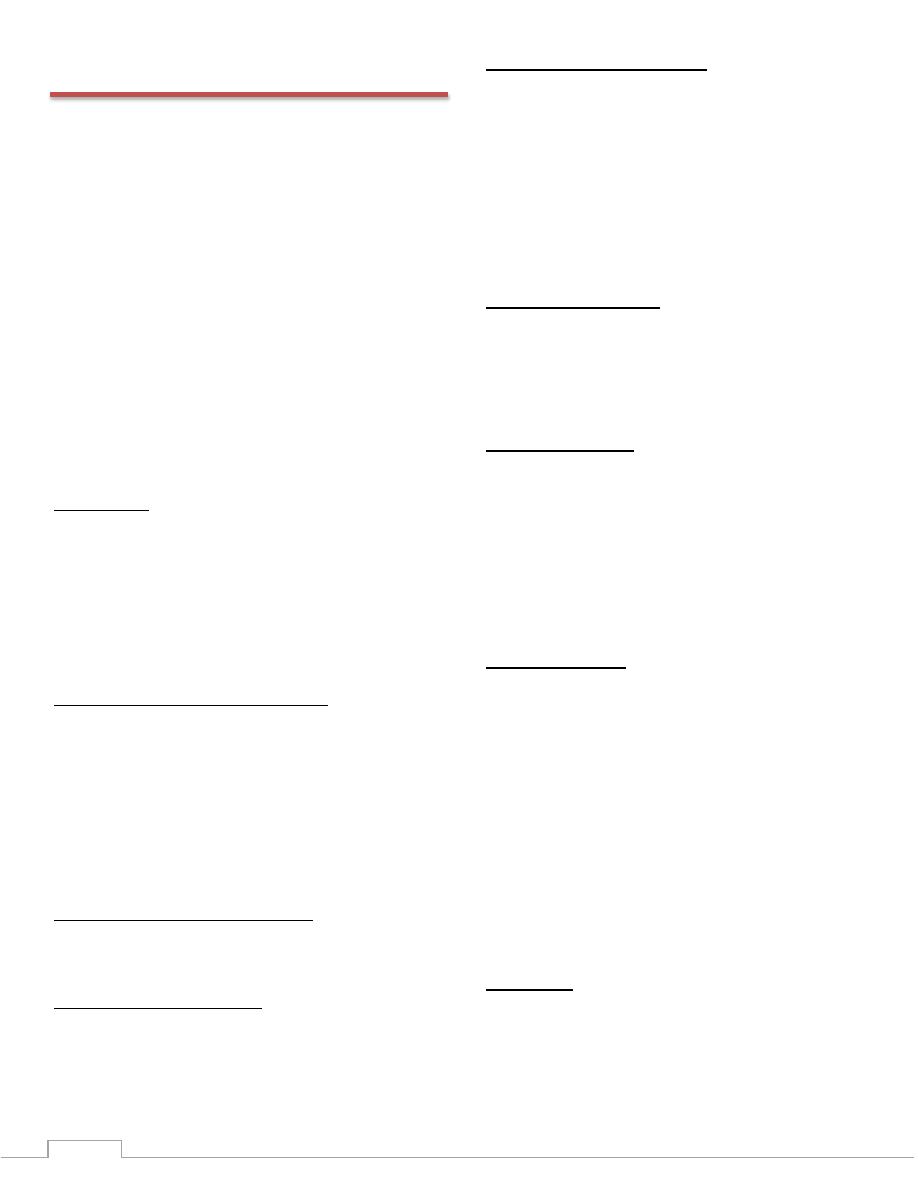
Principle:
Alanine aminotranferase (GPT) catalyzes the taransfer of
the amino group from alanine to oxoglutarate with the
formation of glutamate and pyruvate.
The transaminase activity is proportional to the amount of
oxalacetate or
pyruvate formed over adefinite period of time and is measured
by the reaction with 2,4-dinitrophenylhydrazine(DNPH) and
measurement of the color formed in an alkaline solution.
Specimen collection and handling
Serum free of hemolysis.
Transaminases are stable in serum 24 hr. at room
temperature and for 1 week at 2-8ºC.
Normal values:
GPT/ALT = 5-30 IU/L
Procedure:

14
Lab 10 - Estimation of glucose
• Glucose test measures the amount of a type of sugar, called
glucose. It is one of the simple sugars that are products of
carbohydrates digestion.
• Normally, your serum glucose levels increases slightly after
you eat; this increase causes your pancreas to release insulin
so that your blood glucose levels do not get too high. Glucose
levels that remain high over time can damage your eye,
kidneys, nerves, and blood vessels.
Specimens:
• Serum or Plasma: It is the best choice than whole blood,
because glucose in whole blood can undergo glycolysis; the
blood samples should be centrifuged and removed from cells
as soon as possible because blood cell will utilize glucose and
cause a reduction or a lowering in the estimated in blood
glucose.
• Note: collection of blood in plane tubes for serum chemistry
analysis permits the metabolism of glucose in the sample by
blood cells until separated by centrifugation(higher than
normal amount of WBC, and RBC counts can lead to
excessive glycolysis in sample with substantial reduction of
glucose level if sample is not process quickly. Therefore we
can used fluoride as inhibitors of glycolysis to preserve blood
that cannot be separated rapidly.
• Urine: in a normal person glucose is not detectable in urine. It
appears in urine only when its level in the serum exceeds 180
mg/dl. In this cases it will called glucosuria.
• Capillary blood glucose: the capillary glucose concentrations
are approximately 2-5% higher than venous blood.
Types of serum glucose tests
• Fasting serum glucose (FSG): measures the serum glucose
after you have stop eaten for at least 8 hours.
• 2Postprandial serum glucose: measures serum glucose
exactly 2 hours after you eats a meal.
• Oral glucose tolerance test (GTT).
Normal value:
When glucose level rises above the normal range, the
condition called hyperglycemia. When glucose levels fall
below the range, the condition called hypoglycemia.

15
Lab 11 - lipids
Classification of lipids:
1. Triglyceride (natural fat)
2. Phospholipids: contain a nitrogenous base, a phosphoric
acid residue, one or more fatty acids and a complex alcohol
either glycerol or sphingosin like lecithin.
3. Steroid: like cholesterol is a typical steroid
4. Fatty acid
-The lipid transported by lipoproteins, namely triglycerides,
phospholipids, cholesterol and cholesteryl ester
Hyperlipidemia:
1. Hypercholesterolaemia
2. Hyper TG
Hypolipidaemia:
1. Hypocholesterolaemia
2. Hypo TG
Triglyceride
• Triglyceride contain three fatty acid molecules attached to
one molecule of glycerol by ester bond.
• normal value = 35 – 160 mg / dl
Sources of triglyceride:
• Plant sources : corn, sunflower seeds and safflower seed are
rich in poly unsaturated fatty acid and are oils.
• Animal sources: contain mostly saturated fatty acids and are
usually solid at room temp.
• TG are influenced by a number of hormones, such as
insulin, glucagon, pituitary growth hormone.
Hyper triglyceridemia:
• 1 primary: (shows creamy layer) ↑ VLDL
Can be a consequence of genetic abnormalities, called familial
hypertriglyceridemia.
• 2 secondary:
Such as hormonal abnormalities associated with the pancreas,
adrenal glands and pituitary or diabetes mellitus or nephrosis
or alcoholic occure in chronic renal disease patient or
oestrogen therapy (↑VLDL, ↑HDL).
• Treatment of hypertriglyceridemia consists of dietary
modifications. Fish oil or triglyceride lowering drugs
(primarily, fibric acid derivatives) in cases of sever
hypertriglyceridemia or when accompanied with low HDL
cholesterol.
Hypotriglyceridemia: (hypolipoproteinaemia)
• Hypo Abeta lipoprotein has intestinal malabsorption of lipid
and has defect in the synthesis of apo β, CM, VLDL, absent
from the plasma.
• Very low plasma chol & absent β- lipoprotein & chy, VLDL,
LDL.
Method for TG: GPO method in human serum
or plasma
Principle: Reaction scheme is as follow:
• The absorbance of the coloured complex (quinoneimine),
proportional to the amount of triglycerides in the specimen.
• POD peroxydase
• GPO glycerol 3 phosphate oxydase
• GK glycerol kinase
• PAP 4-amino antipyrine
• ATP adenosine triphosphate Na
Procedure:
16
Lab 12 - lipoproteins
•
Cholesterol and its esters, triglycerides and phospholipids are
all transported in plasma as lipoprotein particles. Five main
types of particle can be recognized. These vary in their
functions, in their lipid and protein composition, and in their
size and density, as well as in other properties. Fatty acids are
transported in plasma bound to albumin.
•
Lipoproteins are composed of both lipids and proteins, called
apolipoproteins.
•
The protein components of the lipoprotein, the
apolipoproteins, are a complex family of polypeptides,
separable into four main groups (apo A,B,C and E) and two
minor groups (apoD and apoF). Each group of apolipoproteins
serves a distinct functional role in plasma lipid metabolism.
•
The lipid/ protein complexes that together comprise the
plasma lipoproteins can be separated into five main classes,
defined according to their behavior in the ultracentrifuge. The
five classes are:
1) Chylomicrons: (large, lipid-rich transport vessels) these are
large particles which consist mainly of triglycerides. They
have the lowest density of the lipoprotein classes, and contain
very little protein. They are formed in the intestinal mucosa
and reach the systemic circulation via the thoracic duct.
•
Because the large size for chylomicrons. They reflect light and
account for the turbidity of postprandial plasma. Because they
are so light, they also readily float to the top of stored plasma
and form a creamy layer.
2) The very low density lipoproteins (VLDL): these are
moderately large particles in which triglycerides are again the
main lipid component. They are mainly formed in the liver,
but to a lesser extent by the intestinal mucosa, and are secreted
into plasma from these two sites.
•
Like Chylomicrons, they are also reflect light and account for
most of the turbidity observed in fasting hyperlipidemic
plasma specimens, although they do not form a creamy top
layer like Chylomicrons, because they are smaller and less
buoyant.
3) Intermediate density lipoproteins (IDL): these arise from
VLDL, by catabolism within the circulation, this results in the
removal of some triglyceride and apolipoprotein from VLDL,
leaving IDL or remnant particles.
4) Low density lipoproteins (LDL): rich in cholesterol, are the
nearly empty tankers that deliver cholesterol to peripheral
cells and liver after the triglycerides have been off-loaded
,LDL may be a better marker for coronary heart disease
risk.LDL formed from IDL by the removal of more
triglyceride and apolipoprotein.
5) High density liopoproteins (HDL): these are the smallest of
the lipoprotein particles and the most dense. They contain a
large amount of protein, and approximately equal amounts of
cholesterol and phospholipid, but very little triglyceride.HDL
are synthesized by both the liver and intestine, is the most
active form in removing excess cholesterol from peripheral
cells. The ability of HDL to remove cholesterol from cells,
called reverse cholesterol transport.
•
*serum lipoprotein concentrations differ between adult men
and women, primarily as a result of differences in sex
hormone level.
Hypolipoproteinemia:
exist in two forms
•
Hypoalphalipoproteinemia: indicates an isolated decrease in
circulating HDL (tangier disease)
•
Hypobetalipoproteinemia: is associated with isolated low
levels of LDL cholesterol & VLDL present in low conc. &
deficiency in CM.
Hyperlipoproteinemia:
• They are diseases associated with elevated lipoprotein levels,
can be subdivided into hyper cholesterolemia,
hypertriglyceridemia and combined hyperlipidemia.
• The investigation of hyperlipoproteinaemia, it is usual to
analyze plasma after 10-14 hr. fasting (and preferably a
normal diet for two weeks), and no alcohol for 24 hr.
• Plasma should be looked at after being allowed to stand at 4
o
c
for 18 hr. A creamy layer signifies Chylomicrons, diffuse
turbidity means endogenous hypertriglyceridaemia
Primary disorder:
• Type I (hyperchylomicronaemia ) ↑TG, slightly ↑Chol
• Which are rare, shows a slightly creamy plasma with a heavy
chylomicron band, high TG & slightly raised chol.
• Type II (hyper betalipoproteinaemia) ↑chol, normal TG
• Shows a clear plasma, a high chol and normal TG. This type
includes familial hypercholesterolaemia.
• Type III (hyper cholestrolaemia) ↑TG, ↑chol
• Which is rare, shows a turbid plasma, and raised cholesterol
and triglycerides.
• Type IV (endogenous hypertriglyceridaemia) ↑TG > chol
• Shows a turbid plasma with an increased TG raised more than
chol., defect in production or in catabolism of VLDL ,
↓LDL&HDL.
• Type V : increased Chylomicrons
Secondary:
• Hypercholesterolaemia: hypothyroidism, nephritic
syndrome, obstruction of bile duct, ↑plasma LDL, ↑VLDL
• Hypertriglyceraemia: due to diabetes mellitus or alcoholic
occure in chronic renal disease patient or oestrogen therapy
decrease HDL

17
Friedewald calculation:
• Total cholesterol= LDL+ HDL+ {VLDL or (TG/5)}
• LDL cholesterol= total cholesterol- HDL – Trig /5 in
mg/dl units
• If the quantities are expressed in mmole/L, It will be
TG/2.2
• This formula is invalid if the triglyceride conc. Exceeds
4.5 mmole/L or 400 mg/dl
Notes:
• Chylomicrons : consist TG, apoproteins A,B,C
• LDL: β-lipoproteins contains chol, apoB, phospholipid, little
TG
• VLDL: pre β-lipoprotein, contain endogenous TG, apoB,C
• HDL : α-lipoprotein, contain apoA&C, chol, phospholipid&
little TG
• Normal value HDL-cholesterol = 40-60 mg/dl
= 1- 1.5 mmole/L
LDL- cholesterol = 50-130 mg/dl
Method for estimation HDL-cholesterol:
(PTA)precipitant
• Principle:
This reagent is only for treatment of specimens before
determination of HDL-cholesterol with a reagent for total
cholesterol.
Low density lipoproteins (LDL), very low density (VLDL)
and Chylomicrons from specimens are precipitated by
phosphotungstic acid (PTA) and magnesium chloride. HDL-
cholesterol obtained in supernatant after centrifugation is then
measured with total cholesterol reagent.
Procedure:
Lab 13 - Alkaline phosphatase &
transaminase
Alkaline phosphatase
•
Phosphatase: They are enzymes catalyzes the splitting of
phosphoric group from mono phosphoric esters
•
Two types are commonly estimated in serum:
ALP (Alkaline Phosphatase) with maximum activity at pH 10
ACP (Acidic Phosphatase) with maximum activity
Alkaline phosphatase (sources):
Is present in most tissues, the richest sources being:
•
Osteoblast in bone
•
The bile canalculi in the liver
•
Small intestine epithelium
•
Proximal renal tubules (kidney)
•
Placental and in breast during lactation
ALP function
The precise biochemical role of alkaline phosphatase is not
known.
•
In many tissues it is attached to cell membranes,
suggesting an association between alkaline phosphatase
activity and membrane transport
•
Alp involved in the bone calcification
Clinical significance
:
•
Normal value in adult 3-13K.A.U/dl or 22-100 IU/L
•
ALP raised in pregnancy , during lactation , in growing
child and after male therefore the sample should be taken
at least after two hours after last male
Unit
•
King Armstrong Unit of ALP activiyy:
•
One unit being the mg of phenol liberated by 100ml of
serum in 15 min under specified conditions
•
IU is the amount of enzyme that will catalyze the reaction
of 1 μmol of substrate per minute under specified
conditions of temperature, pH, substrates, and activators.
ALP properties:
•
Optimum activity of it is exhibited at a pH 10 & temp 37
o
c
•
ALP requires Mg
+2
, Mn
+2
and Co
+2
for activation
•
ALP activity is inhibited by Ca
+2
, PO
4
-3
, C
2
O
4
-2
, EDTA &
CN
-
therefore they should be avoided during preparation of
plasma
•
The assays of ALP activity uses p-nitro phenyl phosphate
or phenyl phosphate or α- phosphate as a substrate
Clinical Significance:
•
Increased S.ALP:
Bone diseases:
18
o Paget's disease: increased 10-25 time than normal value
o Ricket's & osteomalacia : increased 2-4 times ,increased
osteoblasict activity
o Bone cancer with osteoblastic metastase
Liver disease:
Cholestasis has a dual effect since it causes increased
synthesis of hepatic alkaline phosphatase and solubilization
followed by regurgitation of the hepatic enzyme into plasma.
o obstructive jaundice
o biliary obstruction
•
Decreased S.ALP level:
Hypo phosphatasia
Transaminase (Aminotransferase) Enzymes
•
The function of these enzymes is a transfer of an amino group
from α-amino acid to α-keto acid in a reversible reaction with
pyridoxal-5'-phosphate as a coenzyme
•
The transamination reaction is important in the synthesis
°radation of a.a. The keto acids formed are ultimately
oxidized by TCA cycle to provide energy.
•
The clinical importance of measuring these enzymes is to
assess organ function and/or tissue damage, because
transaminase are normally intracellular enzyme with low level
found in plasma representing the release of cellular contents
during normal cell turn over, the presence of high plasma
enzyme level indicate damage to cell rich in these enzyme
A. AST (GOT):
•
Present in heart, liver, skeletal muscle and to a lesser extent
kidney, pancreas and RBCs.
•
N.R. is 5-30 U/L.
•
Diagnostic significance:
Acute MI: ↑ within 6-8 hours, peak at 12-24 hours and
return normal within 5 days.
Hepatocellular damage: ↑ in relation to the extent of tissue
damage;
Toxic liver injury by drugs or toxins, AST has an
important role in assessing alcoholic hepatitis.
Skeletal muscle disease: associated with an increase in CK
enzyme level
Hemolytic anemia: the enzyme is released from RBCs.
B. ALT (GPT):
•
Present mainly in liver and to a lesser extent in kidney,
skeletal muscle and pancreas.
•
N.R. is 6-35 U/L.
•
Acute hepatitis: it is more important than AST because it
appears earlier (may be before jaundice)
•
Liver cirrhosis.
Lab 14 – Liver Function Test
The liver is of vital importance in intermediary metabolism
and in the detoxification and elimination of toxic substances.
1) Test of liver- cells damage: GPT&GOT
2) Test for liver dys function
•
Test of conjucation capacity of the liver :total bilirubin
•
Test for the ability of liver to generate proteins: total
protein, alb&glod.
3) Test of cholestasis : ALP & 5 Nucleotidase
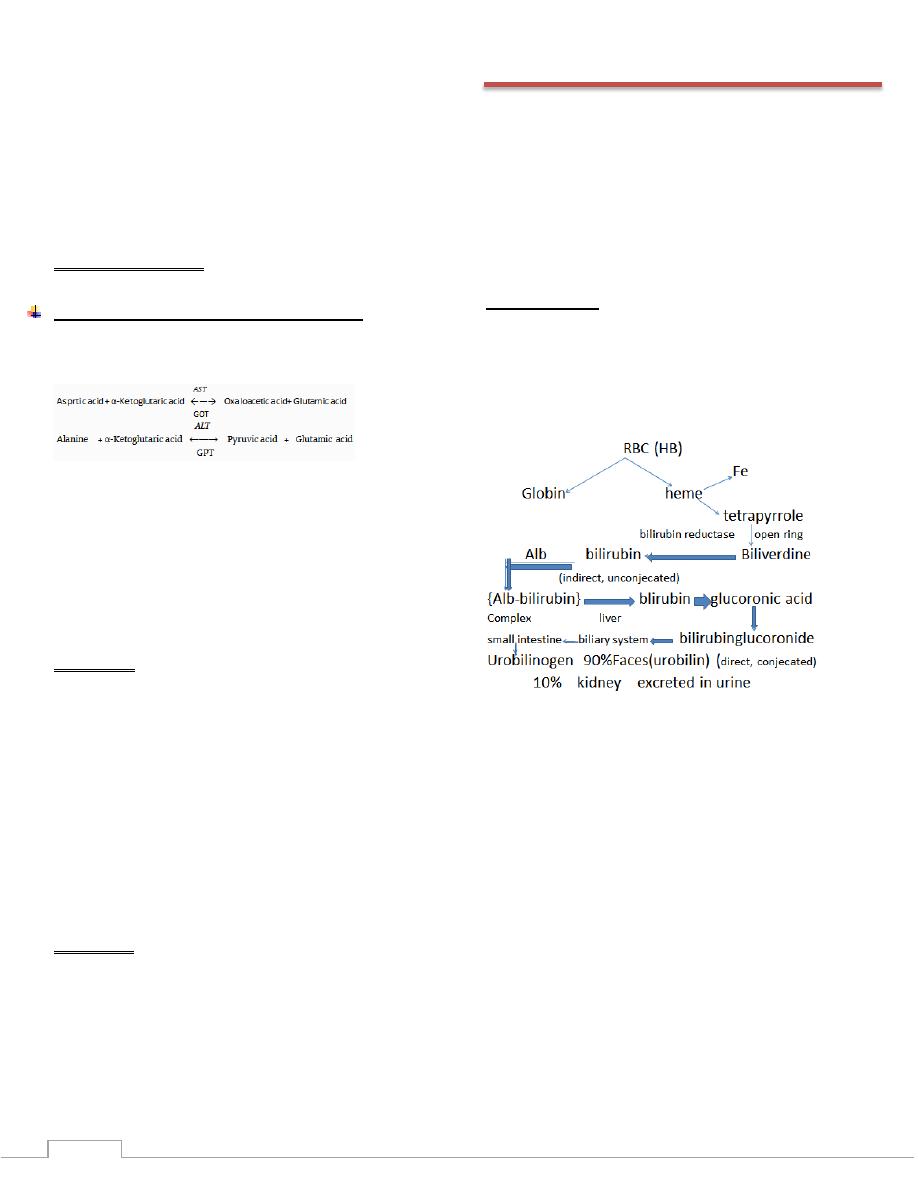
Serum bilirubin
•
Bilirubin is the yellow breakdown product of normal heme
catabolism. Heme is found in hemoglobin, a principal
compenent of red blood cells. Biliribun is excreted in bile and
urine, and elevated levels may indicate certain disease.
•
The iron in haem is reutilized but the tetrapyrrole ring is
degraded to bilirubin.
•
Unconjugated bilirubin is not water-soluble; it is transported
in the blood stream bound to albumin. In the liver it is taken
up by hepatocytes where it undergoes conjucation, principally
with glucuronic acid.
•
Conjucated bilirubin is water soluble and is secreted into the
biliary canaliculi, reaching the small intestine via the duct of
the biliary system.
•
In the gut, bilirubin is converted by bacterial action into
urobilinogen, a colourless compound. Some urobilinogen is
absorbed from the gut into the portal blood. Hepatic uptake of
this is incomplete; a small quantity reaches the systemic
circulation and is excreted in the urine. Most of the
urobilinogen in the gut is oxidized in the colon to a brown
pigment, stercobilin, which is excreted in the stool.
•
The bilirubin normally present in the plasma is mainly
unconjucated; since it is protein bound, it is not filtered by the
renal glomeruli and, in health; bilirubin is not detectable in the
urine.

19
•
Hyperbilirubinaemia can be caused by increase production of
bilirubin, impaired metabolism, decrease excretion or a
combination of these.
Jaundace:
•
Increased total bilirubin (TBIL) causes
jaundice
,
•
Yellowish discoloration of skin, nail bed and sclera of the eye
caused by deposition of bilirubin secondary to increase
bilirubin level in blood.
•
Types of jaundice:
1) pre-hepatic ( hemolytic): Increased bilirubin production.
This can be due to a number of causes, including
hemolytic
anemias
•
The liver has a capacity to conjucate and excrete over
3000mg of bilirubin per day , whereas the normal
production is only 300mg/ day.
•
Massive lysis of RBC e.g.( sickle cell anemia, pyruvate
kinase deficiency , G
6
PD, malaria) may produce bilirubin
faster than it can conjucated which lead to increase level of
bilirubin excreted into the bile .
2) hepatocellular :
•
Damage to the liver cell, which are reflected as deficiencies in
bilrubin metabolism, e.g. patients with hepatitis or cirrhosis.
•
Unconjucated bilirubin level increase due to decrease
conjucation and regurgitation of conjucated bilirubin to the
blood because conjucated bilirubin not efficiently excreated
3) Post-hepatic: Obstruction of the bile ducts, reflected as
deficiencies in bilirubin excretion. (Obstruction can be located
either within the liver or in the
bile duct
).
•
Patient with obstructive jaundice experience
gastrointestinal pain, nausea with pale clay color stool.
•
Conjucated bilirubin regurgitates from the liver which
increase in its level.
Refrences range:
- serum/plasma [ for total bilirubin] < 1.5 mg/dl
- serum/plasma [ for direct bilirubin] < 0.2 mg/dl
- urine ( random) : negative.
Determination of bilirubin:
Principle:
Sulfanilic acid reacts with sodium nitrite to form diazotized
sulfanilic acid. In the presence of Dimethyl sulfoxide, total
bilirubin reacts with diazotized sulfanilic acid to form
azobilirubin. In the absence of Dimethyl sulfoxide only direct
bilirubin reacts with diazotized sulfanilic acid to form
azobilirubin.

20
Lab 15 - Total protein
A total serum protein test measure the total amount of protein
in the blood with exception of coagulation proteins.
Proteins are polymers of α – amino acids occurring in both
soluble and insoluble in the body, it is present in all body
fluids, the three common fluid that submitted for these
analyses are serum, urine, and cerebrospinal fluid (CSF).
Serum (as contrast with plasma) is deficient in those
coagulation proteins, which are consumed during the process
of blood coagulation. Therefore, serum protein will be
approximately 0.25 mg/dl lower than plasma protein, because
of the absence of fibrinogen.
In very general terms, variation of plasma proteins
concentrations can be due to changes in any of three factors:
The rate of proteins synthesis.
The rate of removal proteins.
The volume of distribution.
The two major causes for alterations of total serum proteins
are:
1) Change in the volume of plasma water
2) Change in the concentration of one or more of the specific
proteins in the plasma.
↓ In the volume plasma water (haemo concentration) occure in
the dehydration due to chronic vomiting, diarrhea, poly urea
(hyperproteinemia), malnutrition, malabsorption.
↑in the volume of plasma water ( haemo dilution) which
occurs in water intoxification & massive intra-venus infusion
(hypoproteinemia).
One or more of specific proteins, albumin is present in high
concentration ( any decrease in albumin→hypoalbuminamia
(hypoproteinemia) like polyurea (nephritic synd rome), burns
Function of proteins:
Nutritive
Control the body water distribution
Buffers
Transporting agents
In blood coagulation
Protective
Enzymes
Normal values may vary from lab to lab.
Total serum protein
Total protein: 5.5–9.0 (g/dl)
Albumin: 3.8–5 g/dl
Globulin: 2.0–3.5 g/dL
Clinical Significance:
Hyperproteinemia:
Dehydration.
Multiple myloma.
Cirrhoses of the liver.
Certain chronic disease. (in this case the immunoglobulins
are mostely increase).
Aretefacuial stasis during venepuncture.
Exercise.
Drugs like steroids& insulin
Hypoproteinemia:
Overhydration.
Kidney disease.
Sever malabsorption.
Sever burns, extensive bleeding
Sever protein deficiency. (starvation)
Increase requirement, as growth, pregnancy, and
hyperthyroidism.
Many proteins including albumin, fibrinogen & most
globuline are formed in the liver. The technical methods used
for separation S.protein types are:
Salt fraction
Electrophoresis
Method of serum total protein estimation:
Principle of Biuret method:
In the biuret reaction , a chelate is formed between the Cu
+2
ion
and the peptide bonds of the proteins in alkaline solution to
form a violet colored complex whose absorbance is measured
photometrically. The intensity of the color produced is
proportional to the concentration of protein in the sample.
Serum albumin:
Albumin is the major plasma protein
Its (carbohydrates- free) single poly peptide chain with mw.of
6500 dalton.
Albumin is synthesized in liver at a rate of 10-12 gm/day
Albumin ½ life 20 day

21
Function of albumin:
Transport of large organic comp.( billirubin, thyroxine)
Maintance of plasma osmotic pressure
Source of endogenous amino acids
Function of globuline:
Defence function like fibrinogen or thrombin
Hypo albuminaemia( low serum albumin):
Decrease of alb. Synthesis as in liver disease, malnutrition
Loss of alb. From the body with urine (protein urea)
(nephritic syndrome)or skin ( burns)
Change in distribution between the intravascular & extra
vascular compartment as in shock
Hyper albuminaemia:(elevated level of serum alb.)
Is rare & may result from dehydration
Principle of serum albumin estimation (BCG):
The method is based on the specific binding of bromocresol
green(BCG), an anionic dye, and the protein at acid pH with
the resulting shift in the absorption wavelength of the
complex. The intensity of the color formed is proportional to
the concentration of alb. In the sample.
Procedure:
reagent
Test
Std
reagent
test
Std
R1
reagent
albumin
2 ml
2 ml
R1
reagent
total
protein
1 ml
1 ml
sample
10µL
sample
20µL
Mix & let stand 1 min at
room temp.
Conc. Of std = 5g/dl
Mix & incubate 10 min at
37C
Conc. Of std=7 g/dl
Lab 16 - General Urine Test
Tests on urine provide information and clues to many
diseases, and can also be
indications
of the condition of a
patient's health. A routine urine-screening test may be done to
help find the cause for a number of different symptoms.
The kidneys remove waste material, fluids, and other
substances from the blood for elimination through urine.
Therefore, urine can contain hundreds of different bodily
waste products.
Many factors such as diet,fluid intake, exercise, and kidney
function) affect the constituents of urine. Over 100 different
tests can be done on urine. However, a routine urinalysis
usually involves the following tests:
A. Physical charecterestics
B. Chemical analysis
C. Microscopic analysis
A. Physical characteristics:
Specimen Collection: early morning specimen is preferred
because it’s the more concentrated, the specimen should be
obtained by a clean midstream catch or catheterization, and
the urine should be freshly collected into a clean dry container
& must be analyzed within 1 hour of collection if at room
temp.urine sample could be randomly taken.
1) Color: Many factors affect the color of urine, including fluid
balance, diet, medications and disease. The intensity of the
color generally indicates the concentration of the urine,yellow
and amber are due to urochrome(from urobilinogen),yellow-
brown to green is a result bile pigment oxidation.
pale or colorless urine indicates that the urine is dilute, and
deep yellow urine indicates that it is concentrated. Reddish
brown urine comes from hemoglobin or RBC . certain
medications, eating blackberries or beets or may alter the urine
color.
2) Clarity
This test (also called opacity or turbidity) determines the
cloudiness of the urine. Urine is normally clear, but bacteria,
blood, crystals, or mucus can make urine appear cloudy.
3) Odor
Some diseases cause a change in the normal odor of urine. For
example, an infection with E. coli bacteria can cause a foul
odor while diabetes or starvation can cause a sweet, fruity
odor.
4) Specific gravity
Specific gravity measures the amount of substances dissolved
in the urine (density). it is the weight of 1 ml of urine in gram
divided by the weight of 1 ml of water. It also indicates how
well the kidneys are able to adjust the amount of water in
urine. The higher the specific gravity, the more the solid
material dissolved in the urine,normally between 1.005-1.030
,low SG is found in diabetes insipidus,glomerulonephritis.
high SG is found diabetes mellitus and dehydration.
5) PH :
Normally 4.5-8, The pH is a measure of how acidic or alkaline
(basic) the urine is a strongly acidic urine may occur in
systemic acidosis as in DM, alkaline urine is found in certain
UTI, Certain types of treatment adjust the pH of the urine. For
example, efforts are made to keep urine either acidic or
alkaline to prevent formation of certain types of kidney stones.
B. Chemical Analysis:
can be performed by reagent strips or dip sticks.which are
plastic strips coated with reagents band directed to different

22
analytes.when dipped into urine a color change signal
deviation from normality.
1) Protein: Normally there is very small amount of protein
(mainly albumin) in the urine(less than30 mg/day). Sometimes
a small amount of protein is released into the urine when a
person stands up ,Fever, exercise, normal pregnancy, and
some kidney disease) may also cause protein in the urine.
2) Glucose: Normally there is very little or no glucose in urine.
However, when the blood sugar level is very high, as in
uncontrolled diabetes, it spills over into the urine.
3) Ketones: When fat is broken down for energy, the body
produces ketones and releases them into the urine. Large
amounts of ketones in the urine may signal a dangerous
condition known as Diabetic Ketoacidosis, starvation, or
prolonged vomiting may also cause ketones in the urine.
4) Nitrite :positive in certain UTI(urinary tract infection).
5) 5.Leukocyte esterase:present in the leukocytes ,positivitity
indicate excessive WBC in the urine as in UTI
6) Bilirubin & urobilinogen: normally bilirubin is absent from
the urine and urobilinogen is present in very small amount,
change in both or one of them may be seen in liver diseases.
C.Microscopic analysis
In this test, urine is spun in a centrifuge so the solid materials
(sediment) settle out. The sediment is spread on a slide and
examined under a microscope. The materials that may be
found include:
Red blood cells: RBCgreater than 0-2/hpf is abnormal
Inflammation, disease, or injury to the kidneys, ureters,
bladder can cause blood in urine. exercise can also cause
blood in urine. RBC with White blood cells are often a sign of
infection.
white blood cells: WBC greater than 0-1 /hpf is abnormal.
White blood cells are often a sign of glomerulonephritis,UTI
or inflammation of any type.
Epithelial cells :large number are found in urinary
catherterization, bladder inflammation,cancer.
Casts. Some types of kidney disease can cause plugs of
material (called casts) to form in tiny tubes in the kidneys. The
casts can then get flushed out into the urine. Casts can be
made of different types of material, such as red or white blood
cells, waxy or fatty substances, or protein. The type of cast can
provide clues about the type of kidney disease that may be
present in the body.
Crystals. Healthy people often have only a few crystals in
their urine. However, a large number of crystals, , may
indicate kidney stones or a problem with (metabolism).
Bacteria, yeast cells, or parasites. Normally there are no
bacteria, yeast cells, or parasites in urine. The presence of
these indicates an infection.
Lab 17 - Serum Creatinine
Creatinine: It is a catabolic end product, an anhydride of
creatine (or phosphocreatine) produced by loss of water (or
phosphoric acid) from the molecule in an irreversible reaction.
Creatine synthesized in the liver & to some extent in the
pancreas from 3 amino acids- arginine, glycine & methionine.
Creatine Blood Muscle cells.
At rest
Creatine+ ATP Creatine-P + ADP
Muscle contraction High energy stored compound
Spontaneous Pi
spontaneous
Creatine Creatinine+ H
2
O
Urine Kidney Blood
There is some spontaneous conversion of Creatine to
Creatinine(2% day). Creatinine is a waste product of nitrogen
metabolism.
Clinical significance:
Normal range of
Serum Creatinine: 62-124 μmol / L (0.1-1.4 mg/dl)
Urinary Creatinine: 9-17 mmol / L (1-2 g/day)
Increase in serum Creatinine:
1. Reduction in kidney function from any cause (pre-renal,
renal, post-renal) However, with mild renal impairment, serum
Creatinine remains fairly within its normal level until the GFR
Is 50-60 % of its normal value, therefore for mild renal
impairment, calculation of Creatinine clearance is required.
2. Extensive muscle destruction,CPK also increase.
Note: In interpreting plasma [Creatinine] results,
the following factors should be borne in mind:
1) In ketosis, false high Creatinine, due to the reaction
between ketone bodies and picric acid (the color reagent
used in Creatinine estimation)
2) Values are lower in children than in adults, lower in
women than in men, and lower during pregnancy.
3)
4) Certain drugs (e.g. salicylates, cimetidine) increase plasma
[Creatinine] by inhibiting tubular secretion of Creatinine.

23
5) Some endogenous substances (e.g. acetoacetate) and
exogenous substances (e.g. drugs)may affect the analytical
method.
Glomerular filtration rate
The Glomerular filtration rate (GFR) depends on:
1. The net pressure across the Glomerular membrane.
2. The physical nature of the membrane.
3. The surface area of the membrane, which reflects the
number of functioning glomeruli.
Measuring of GFR:
It can be determined by measuring the concentration in plasma
and urine of a substance which ideally fulfils the following
criteria:
1. It is readily filtered from the plasma at the glomerulus.
2. It is neither reabsorbed nor secreted by the tubules.
3. Its concentration in plasma remains constant throughout
the period of urine collection.
4. The measurement of its concentration in plasma and urine
is convenient and reliable.
Creatinine clearance Test:
The renal clearance tests are extremely useful in measuring the
actual capacity of the kidney eliminate certain substances
present in plasma.
The substances used to evaluate Glomerular filtration rate:
1. Should be excreted either completely or predominately by
glomeruli
2. Should not be absorbed or excreted by tubules
3. Should be present in blood in nearly constant level during
the time of urine collection
4. Should be analyzed both in blood & urine using relatively
simple technique.
So Creatinine is the best to fit these criteria:
Creatinine clearance test is the most sensitive measure of renal
function due to the fact that we relate the quantity of urine
Creatinine to its quantity in serum or plasma.
Advantages:
1. Actual measurement of the kidney capacity to eliminate
Creatinine.
2. Test of the glomerular filtration rate.
3. Creatinine not absorbed by tubules.
4. It is endogenous, not affected by protein diet.
5. Easy to measure.
Creatine clearance
U = urine Creatinine (μmol / L)
P = plasma or serum Creatinine (μmol / L)
V = volume of urine (ml)
T = time of urine collection (min)
Normal value for Creatinine clearance:
85-125 ml/min. ♂
75-115 ml/min.♀
Instruction & method for endogenous Creatinine
clearance test:
1. Hydrate the patient with at least 600 ml water.
2. Collect the urine for exactly 24 hrs.
3. Collect the blood sample at mid time of urine collection.
4. Calculate the volume of 24 hrs. urine collection.
5. Perform the assay of serum & urine Creatinine.
Other clearance tests:
1. Urea clearance test.
2. Inulin clearance test.
3. P-amino hippuric acid clearance test.
Estimating GFR:
In men:
Creatinine clearance ( ml /min)
(140- age[years]) * weight (kg)
72 * plasma creatinine (µmol / L)
In women:
(140- age[ year] ) * weight (kg)
*0.85
72 * plasma creatinine (µmol / L)
Plasma[creatinine]will be more precise than
creatinine clearance:
1. Plasma [Creatinine] remains fairly constant throughout adult
life, whereas Creatinine clearance declines with advancing
age.
2. Plasma [Creatinine] correlates as well (or as poorly)with GFR
as does creatinine clearance in patients with renal disease.
3. Measurement of plasma [Creatinine]is as effective in detecting
early renal disease as Creatinine clearance.
4. Plasma [Creatinine] measurements enable the progress of
renal disease to be followed with better precision than
Creatinine clearance.
Determination of serum Creatinine by jaff'e
reaction:
This method is based on the reaction of Creatinine with
alkaline picrate to give a red Picrate Creatinine complex.
Color reagent Picrate Creatinine
Procedure:
Procedure
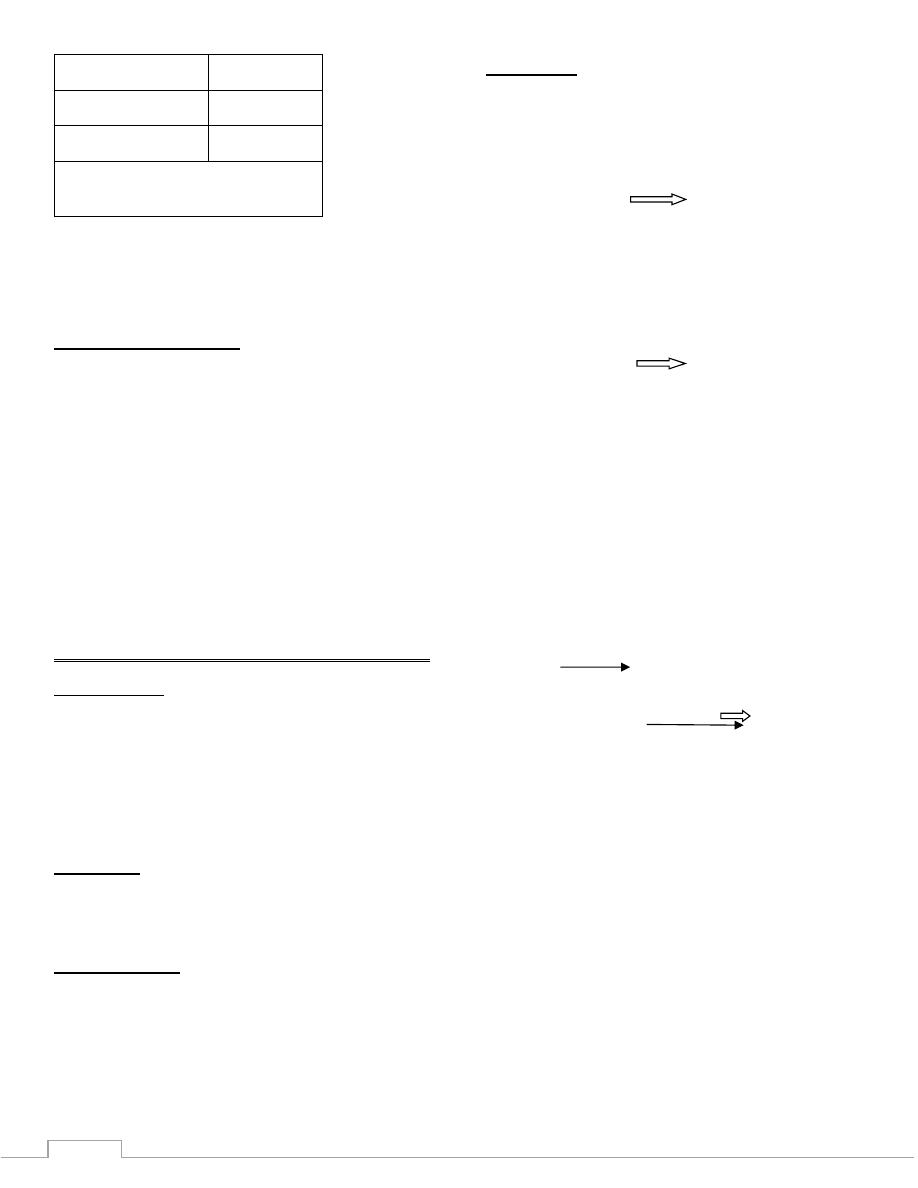
24
reagent
test
serum
1 ml
w.reagent
1 ml
Mix , and let stand 20 min at 25
o
c and
then read the absorbance
Conc. Creatinine= (A test / A std) *conc. Std
A std. = 0.604
Conc. Std= 2.04 mg/dl
Kidney function tests
Involves:
1. Blood urea or serum urea
2. Serum creatinine
3. Creatinine clearance test
4. General urine analysis
The major function of the kidney is to excrete waste products
of metabolism &plays an essential hemostasis role by
adjusting the body balance of water and solute, this depends
on :
a. Normal integrity of glomeruli & tubular cells.
b. Normal blood supply
c. Normal secretion & feed back control of hormones acting
on the kidney
In general the kidney function can be affected by 3 events:
1) Pre-renal events: causing decrease in kidney function due to
reduction of the blood supply low blood pressure
low renal flow.
This may occure in :
1. Sever vomiting due to pyloric stenosis or intestinal
obstruction.
2. Sever diarrhea (prolonged).
3. Sever poly urea as in diabetic coma.
4. Shock due to severe burn or heamorrage.
2) Renal events: affecting the glomerular filtration or tubular
function as in :
1. Acute glomerulo nephritis.
2. Chronic pylonephritis.
3. Hydro nephrosis
3) Post- renal events : affecting the urine out- flow from the
kidney, this cause retention of urine & so decrease the
effective filtration pressure at the glomeruli, this is due to:
1. enlargement of the prostate
2. Stones in the urinary tract.
3. Stricture in the urethra or ureter
4. Tumors in the bladder obstructing the urine out- flow
5. Bilharizia.
Blood urea:
Urea is the main product of protein metabolism, it is
synthesized in the liver from ammonia produced as a result of
the deomination of a.a.
N.R. =20 -45 mg/ dl
Increase in blood urea uremia.
This could occur from pre- renal – post causes of decreased
kidney function.
Other causes of increased blood urea are:
1. Increase in protein breakdown as in fever & other toxic
conditions.
2. Bleeding in the alimentary tract.
Decrease in blood urea is rare, but may occur in:
1. Pregnancy due to haemodilution
2. Low protein diet
3. Sever liver disease
Blood urea (oxime method):
Principle:
Urea is hydrolyzed by urease into ammonia and carbon
dioxide.the ammonia generated reacts with alkaline
hydrochlorite and sodium salicylate in presence of sodium
nitroprusside as coupling agent to yield agreen Crompphore.
The intensity of the color formed is proportional to the
concentration of urea in the sample
urease
Urea + H
2
O 2 NH
3
+ CO
2
Nitroprusside
NH
4
+
+ salycilate + NaCIO indophenols + Nacl
OH
-
