
barrier against excessive loss by diffusion. When the cornified layer becomes
minimized by the cholesterol-filled cornified layer of the skin, which provides a
water loss by diffusion through the skin is about 300 to 400 ml/day. This loss is
and is present even in people who are born without sweat glands; the average
The insensible water loss through the skin occurs independently of sweating
tinually in all living humans.
because we are not consciously aware of it, even though it occurs con-
700 ml/day of water loss under normal conditions. This is termed
tory tract and diffusion through the skin, which together account for about
example, there is a continuous loss of water by evaporation from the respira-
Some of the water losses cannot be precisely regulated. For
Insensible Water Loss.
Daily Loss of Body Water
depending on climate, habits, and level of physical activity.
among different people and even within the same person on different days,
of about 2300 ml/day (Table 25–1). Intake of water, however, is highly variable
of carbohydrates, adding about 200 ml/day. This provides a total water intake
to the body fluids, and (2) it is synthesized in the body as a result of oxidation
of liquids or water in the food, which together normally add about 2100 ml/day
Water is added to the body by two major sources: (1) it is ingested in the form
Daily Intake of Water
body to prevent body fluid volumes from increasing or decreasing.
within the different compartments of the body. For example, there is a highly
The relative constancy of the body fluids is remarkable because there is con-
Fluid Intake and Output Are Balanced During
compartments.
balance, and control of fluid exchange between extracellular and intracellular
ulation of body fluid volume, constituents of the extracellular fluid, acid-base
chapters on the kidneys, we discuss the overall reg-
the body fluids. In this chapter and in the following
the control systems that maintain this constancy of
tial for homeostasis, as discussed in Chapter 1. Some
The maintenance of a relatively constant volume
The Body Fluid Compartments:
C
H
A
P
T
E
R
2
5
291
Extracellular and
Intracellular Fluids;
Interstitial Fluid and Edema
and a stable composition of the body fluids is essen-
of the most common and important problems in
clinical medicine arise because of abnormalities in
Steady-State Conditions
tinuous exchange of fluid and solutes with the external environment as well as
variable fluid intake that must be carefully matched by equal output from the
insensible
water loss
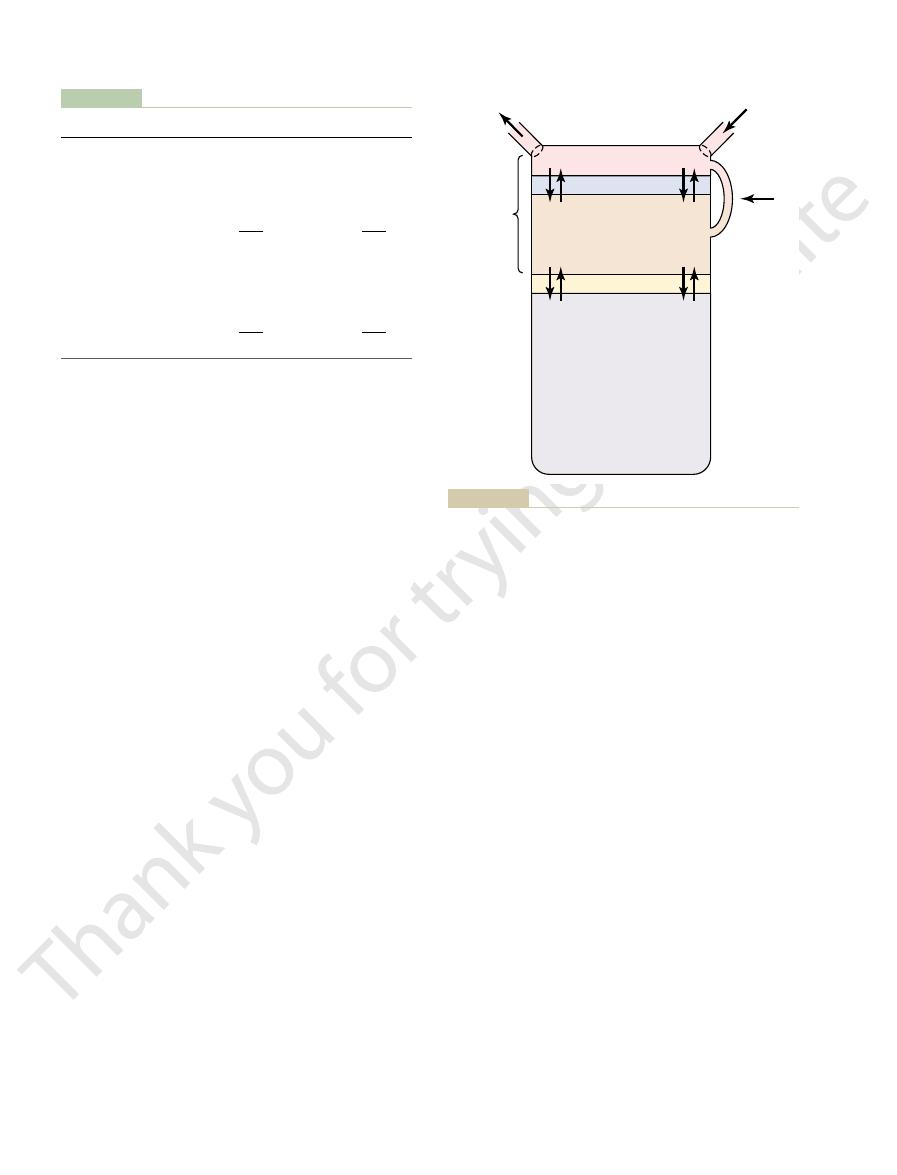
constitute about 1 to 2 liters.
or interstitial fluid. All the transcellular fluids together
of extracellular fluid, although in some cases, its com-
fluid; it is usually considered to be a specialized type
and intraocular spaces, as well as the cerebrospinal
includes fluid in the synovial, peritoneal, pericardial,
This compartment
There is another small compartment of fluid that is
(Figure 25–1). The extracellular fluid is
compartments: the
The total body fluid is distributed mainly between two
Body Fluid Compartments
these remarkable tasks.
disease states. In Chapters 26 through 30, we discuss
these substances, as well as compensating for excessive
may be as high as 300 to 500 mEq/day. The kidneys are
low as 20 mEq/day, whereas in others, sodium intake
potassium. In some people, sodium intake may be as
electrolytes of the body, such as sodium, chloride, and
This variability of intake is also true for most of the
dous amounts of water.
20 L/day in a person who has been drinking tremen-
as 0.5 L/day in a dehydrated person or as high as
substances. For example, urine volume can be as low
and output of most electrolytes in the body, is by con-
intake and output, as well as a balance between intake
of urine excretion. In fact, the most important means
There are multiple mechanisms that control the rate
the body occurs in the urine excreted by the kidneys.
The remaining water loss from
Water Loss by the Kidneys.
threatening if not corrected within a few days.
diarrhea. For this reason, severe diarrhea can be life
(100 ml/day) normally is lost in the feces. This can
Water Loss in Feces.
sionally increases to 1 to 2 L/hour. This would rapidly
or during heavy exercise, water loss in sweat occa-
normally is about 100 ml/day, but in very hot weather
and environmental temperature. The volume of sweat
ing is highly variable, depending on physical activity
The amount of water lost by sweat-
weather.
the lungs as the temperature decreases. This explains
nearly 0, causing an even greater loss of water from
weather, the atmospheric vapor pressure decreases to
lost through the lungs with respiration. In cold
air is usually less than 47 mm Hg, water is continuously
expelled. Because the vapor pressure of the inspired
to a vapor pressure of about 47 mm Hg, before it is
respiratory tract, it becomes saturated with moisture,
averages about 300 to 400 ml/day. As air enters the
balance fluid loss.
large amounts of fluid, usually intravenously, to
5 L/day. For this reason, burn victims must be given
evaporation can increase as much as 10-fold, to 3 to
denuded, as occurs with extensive burns, the rate of
292
Unit V
The Body Fluids and Kidneys
Insensible water loss through the respiratory tract
the dry feeling in the respiratory passages in cold
Fluid Loss in Sweat.
deplete the body fluids if intake were not also
increased by activating the thirst mechanism discussed
in Chapter 29.
Only a small amount of water
increase to several liters a day in people with severe
by which the body maintains a balance between water
trolling the rates at which the kidneys excrete these
faced with the task of adjusting the excretion rate of
water and electrolytes to match precisely the intake of
losses of fluids and electrolytes that occur in certain
the mechanisms that allow the kidneys to perform
extracellular fluid and the intracel-
lular fluid
divided into the interstitial fluid and the blood plasma.
referred to as transcellular fluid.
position may differ markedly from that of the plasma
Table 25–1
Fluids ingested
2100
?
Intake
Normal
Heavy Exercise
Daily Intake and Output of Water (ml/day)
Prolonged,
Feces
100
100
Sweat
100
5000
Insensible—lungs
350
650
Insensible—skin
350
350
Total intake
2300
?
From metabolism
200
200
Output
Urine
1400
500
Total output
2300
6600
Plasma
3.0 L
Interstitial
fluid
11.0 L
INTAKE
Intracellular
fluid
28.0 L
Capillary membrane
Lymphatics
Extracellular
fluid (14.0 L)
Cell membrane
OUTPUT
•Kidneys
•Lungs
•Feces
•Sweat
•Skin
ments. The values shown are for an average 70-kilogram person.
compartments and the membranes that separate these compart-
Summary of body fluid regulation, including the major body fluid
Figure 25–1

purposes, however, the concentration of ions in the
repel the negatively charged anions. For practical
plasma proteins. Conversely, negatively charged ions
sodium and potassium ions, thus holding extra
charge and, therefore, tend to bind cations, such as
tial fluid. The plasma proteins have a net negative
the interstitial spaces in most tissues.
teins, only small amounts of proteins are leaked into
concentration of protein in the plasma; because the
ionic composition is similar. The most important dif-
only by highly permeable capillary membranes, their
Interstitial Fluid Is Similar
and in Table 25–2.
intracellular fluid are shown in Figures 25–2 and 25–3
fluid, including the plasma and interstitial fluid, and the
conditions, the hematocrit can rise to 0.65.
of red blood cells, resulting in
barely sufficient to sustain life. Conversely, there are
the hematocrit may fall as low as 0.10, a value that is
0.40, and in women, it is about 0.36. In severe
In men, the measured hematocrit is normally about
of the plasma remains entrapped among the cells, and
the red cells together; therefore, about 3 to 4 per cent
bottom of the tube. It is impossible to completely pack
tube” until the cells become tightly packed in the
as determined by centrifuging blood in a “hematocrit
the fraction of the blood composed of red blood cells,
The hematocrit is
Hematocrit (Packed Red Cell Volume).
other factors.
different people, depending on gender, weight, and
cells, but these percentages can vary considerably in
cent of body weight, or about 5 liters. About 60 per
The average blood volume of adults is about 7 per
diovascular dynamics.
chamber of its own, the circulatory system. The blood
blood cells). However, blood is considered to be a sep-
Blood Volume
about the same composition except for proteins, which
mixing, so that the plasma and interstitial fluids have
Therefore, the extracellular fluids are constantly
solutes in the extracellular fluid except the proteins.
branes. These pores are highly permeable to almost all
liters. The plasma is the noncellular part of the blood;
almost one fourth of the extracellular fluid, or about 3
extracellular fluid, and the
in a normal 70-kilogram adult. The two largest com-
about 20 per cent of the body weight, or about 14 liters
Together these fluids account for
Compartment
reason, the intracellular fluid of all the different
most primitive microorganisms to humans. For this
similar even in different animals, ranging from the
In fact, the composition of cell fluids is remarkably
these substances are similar from one cell to another.
of different constituents, but the concentrations of
The fluid of each cell contains its individual mixture
“average” person.
Thus, the intracellular fluid constitutes
that variations exist, depending on age, gender, and
“average” body fluid compartments, we should realize
their body weight. Therefore, when discussing the
women normally have more body fat than men, they
decreases the percentage of water in the body. Because
percentage of the body weight being fat, which
is fluid gradually decreases. This is due in part to the
grows older, the percentage of total body weight that
on age, gender, and degree of obesity. As a person
about 42 liters. This percentage can change, depending
body water is about 60 per cent of the body weight, or
In the average 70-kilogram adult human, the total
The Body Fluid Compartments: Extracellular and Intracellular Fluids; Interstitial Fluid and Edema
Chapter 25
293
fact that aging is usually associated with an increased
contain slightly less water than men in proportion to
percentage of body fat.
Intracellular Fluid Compartment
About 28 of the 42 liters of fluid in the body are inside
the 75 trillion cells and are collectively called the intra-
cellular fluid.
about 40 per cent of the total body weight in an
cells together is considered to be one large fluid
compartment.
Extracellular Fluid
All the fluids outside the cells are collectively called
the extracellular fluid.
partments of the extracellular fluid are the interstitial
fluid, which makes up more than three fourths of the
plasma, which makes up
it exchanges substances continuously with the intersti-
tial fluid through the pores of the capillary mem-
have a higher concentration in the plasma.
Blood contains both extracellular fluid (the fluid in
plasma) and intracellular fluid (the fluid in the red
arate fluid compartment because it is contained in a
volume is especially important in the control of car-
cent of the blood is plasma and 40 per cent is red blood
the true hematocrit is only about 96 per cent of the
measured hematocrit.
anemia,
some conditions in which there is excessive production
polycythemia. In these
Constituents of Extracellular
and Intracellular Fluids
Comparisons of the composition of the extracellular
Ionic Composition of Plasma and
Because the plasma and interstitial fluid are separated
ference between these two compartments is the higher
capillaries have a low permeability to the plasma pro-
Because of the Donnan effect, the concentration of
positively charged ions (cations) is slightly greater
(about 2 per cent) in the plasma than in the intersti-
amounts of these cations in the plasma along with the
(anions) tend to have a slightly higher concentration
in the interstitial fluid compared with the plasma,
because the negative charges of the plasma proteins

294
Unit V
The Body Fluids and Kidneys
Cations
Anions
––––
EXTRACELLULAR
mEq/L
INTRACELLULAR
150
150
100
100
50
50
0
Ca
++
Na
+
HCO
3
-
PO
4
and organic anions
Protein
Mg
++
K
+
Cl
-
free ions and complexed ions.
these two ions. The concentrations shown represent the total of
represent the sum of
Figure 25–2
Major cations and anions of the intracellular and extracellular
fluids. The concentrations of Ca
++
and Mg
++
Phospholipids – 280 mg/dl
Cholesterol – 150 mg/dl
Glucose – 100 mg/dl
Urea – 15 mg/dl
Lactic acid – 10 mg/dl
Uric acid – 3 mg/dl
Creatinine – 1.5 mg/dl
Bilirubin – 0.5 mg/dl
Bile salts – trace
Neutral fat – 125 mg/dl
Nonelectrolytes of the plasma.
Figure 25–3
Table 25–2
Others
4.8
3.9
10
Urea
4
4
4
Protein
1.2
0.2
4
Glucose
5.6
5.6
Lactate
1.2
1.2
1.5
Creatine
0.2
0.2
9
Amino acids
2
2
8
0.5
0.5
1
2
2
11
24
28.3
10
108
108
4
0.8
0.7
20
1.3
1.2
0
4.2
4.0
140
142
139
14
O)
Intracellular (mOsm/L H
O)
Interstitial (mOsm/L H
Osmolar Substances in Extracellular and Intracellular Fluids
Plasma (mOsm/L H
2
2
2
O)
Na
+
K
+
Ca
++
Mg
+
Cl
–
HCO
3
–
HPO
4
–
, H
2
PO
4
–
SO
4
–
Phosphocreatine
45
Carnosine
14
Adenosine triphosphate
5
Hexose monophosphate
3.7
Total mOsm/L
301.8
300.8
301.2
Corrected osmolar activity (mOsm/L)
282.0
281.0
281.0
C (mm Hg)
5443
5423
5423
Total osmotic pressure at 37
∞
after being injected into the blood, and the dilution
be used to measure total body water. These forms of
Measurement of Total Body Water.
Compartments
Determination of Volumes
ent body fluids.
is not metabolized or excreted. Several substances can
partment that is being measured, and (3) the indicator
partment, (2) the indicator disperses only in the com-
This method can be used to measure the volume of
ligram for each milliliter of fluid, the unknown volume
10 mg/ml of dye is dispersed into chamber B and
For example, if 1 milliliter of a solution containing
tion, one can calculate the unknown volume of
centration A). By simple rearrangement of the equa-
mass of the substance injected (Volume A
(Volume B
chemically, photoelectrically, or by other means. If
Then a sample of fluid containing the dispersed sub-
becomes mixed in equal concentrations in all areas.
syringe is injected into a chamber, and the substance
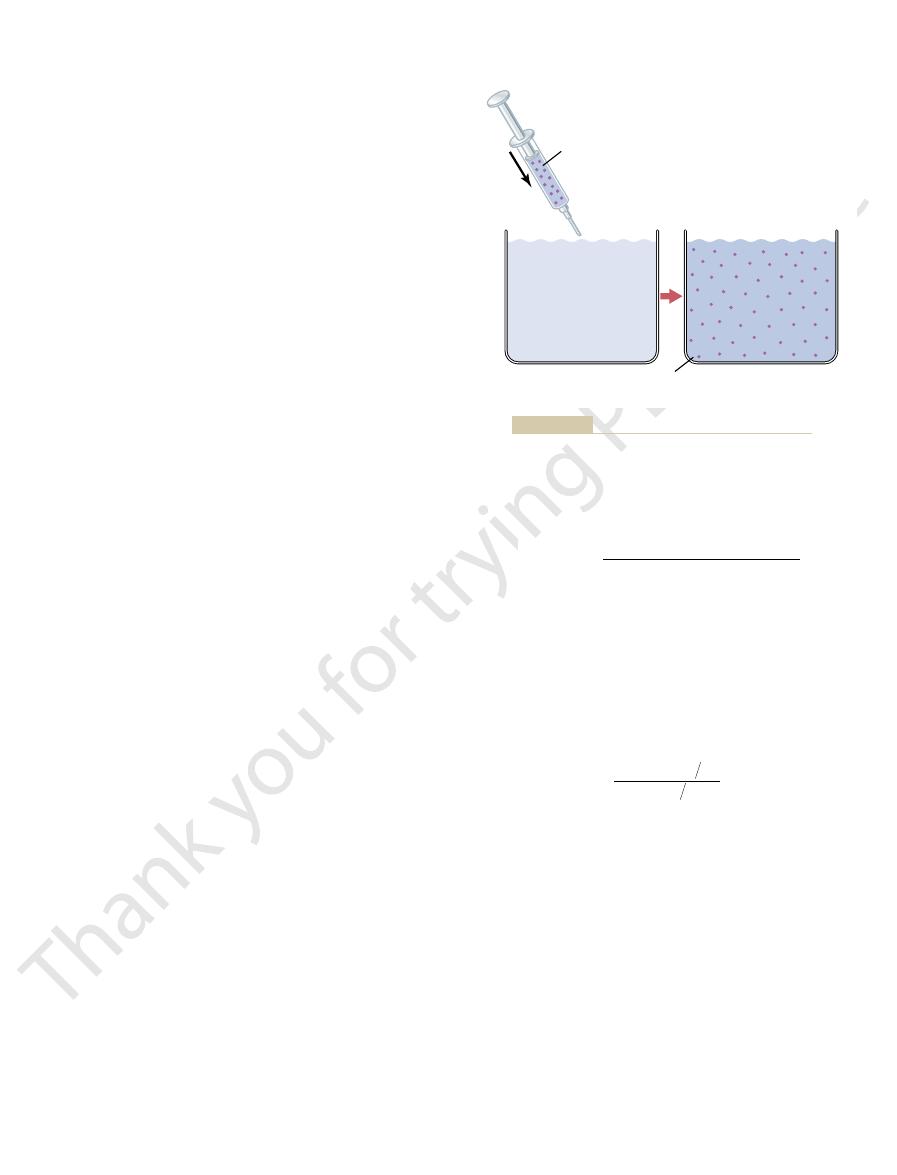
In the example shown in Figure 25–4, a small
based on the principle of conservation of mass. This
uring the volume of a fluid compartment, which is
25–4 shows this “indicator-dilution” method of meas-
extent to which the substance becomes diluted. Figure
out the compartment’s fluid, and then analyzing the
compartment, allowing it to disperse evenly through-
The volume of a fluid compartment in the body can be
Indicator-Dilution Principle
Fluid Compartments—The
Volumes in the Different Body
Measurement of Fluid
protein, almost four times as much as in the plasma.
extracellular fluid. Also, cells contain large amounts of
sulfate ions, all of which have low concentrations in the
chloride ions and almost no calcium ions. Instead, it
In contrast to the extracellular fluid, the intracellu-
to water but not to most of the electrolytes in the body.
The intracellular fluid is separated from the extracel-
kidneys, as discussed later. This allows the cells to
regulated by various mechanisms, but especially by the
The composition of extracellular fluid is carefully
calcium, magnesium, phosphate, and organic acid ions.
bonate ions, but only small quantities of potassium,
and chloride ions, reasonably large amounts of bicar-
interstitial fluid, contains large amounts of sodium
the extracellular fluid, including the plasma and the
Referring again to Figure 25–2, one can see that
The Body Fluid Compartments: Extracellular and Intracellular Fluids; Interstitial Fluid and Edema
Chapter 25
295
interstitial fluid and in the plasma is considered to be
about equal.
remain continually bathed in a fluid that contains the
proper concentration of electrolytes and nutrients for
optimal cell function.
Important Constituents of the
Intracellular Fluid
lular fluid by a cell membrane that is highly permeable
lar fluid contains only small quantities of sodium and
contains large amounts of potassium and phosphate
ions plus moderate quantities of magnesium and
measured by placing an indicator substance in the
means that the total mass of a substance after disper-
sion in the fluid compartment will be the same as the
total mass injected into the compartment.
amount of dye or other substance contained in the
is allowed to disperse throughout the chamber until it
stance is removed and the concentration is analyzed
none of the substance leaks out of the compartment,
the total mass of substance in the compartment
¥ Concentration B) will equal the total
¥ Con-
chamber B as
Note that all one needs to know for this calculation
is (1) the total amount of substance injected into
the chamber (the numerator of the equation) and (2)
the concentration of the fluid in the chamber after the
substance has been dispersed (the denominator).
the final concentration in the chamber is 0.01 mil-
of the chamber can be calculated as follows:
virtually any compartment in the body as long as (1)
the indicator disperses evenly throughout the com-
be used to measure the volume of each of the differ-
of Specific Body Fluid
Radioactive water
(tritium,
3
H
2
O) or heavy water (deuterium,
2
H
2
O) can
water mix with the total body water within a few hours
0.01 mg ml
ml
10 mg ml
Volume B
ml
=
¥
=
1
1000
Volume A
Concentration A
Volume B
Concentration B
=
¥
Indicator Mass A = Volume A x Concentration A
Indicator Mass B = Volume B x Concentration B
Volume B = Indicator Mass B / Concentration B
Indicator Mass A = Indicator Mass B
Indicator-dilution method for measuring fluid volumes.
Figure 25–4

When a solute is added to pure water, this reduces
concentration to one that has a lower water concentra-
were presented in Chapter 4. Therefore, we review
The basic principles of osmosis and osmotic pressure
and Osmotic Pressure
between these two compartments.
In the next section, we discuss the interrelations
the cell membrane rapidly, so that the intracellular
sodium and chloride. Therefore, water moves across
acting across the cell membrane. The reason for this is
especially sodium, chloride, and other electrolytes
extracellular compartments, in contrast, is determined
The distribution of
forces across the capillary membranes.
in Chapter 16 and later in this chapter, the relative
cellular and extracellular compartments. As discussed
cells.
Cr), which binds tightly with the red blood
culated using the dilution principle. A substance fre-
can be measured, and the total blood volume can be cal-
circulation, the radioactivity of a mixed blood sample
labeled with radioactive material. After these mix in the
ocrit is 0.40, total blood volume would be calculated as
For example, if plasma volume is 3 liters and hemat-
posed of cells), using the following equation:
volume using the methods described earlier, blood
Measurement of Blood Volume.
Plasma volume
volume cannot be measured directly, but it can be cal-
Calculation of Interstitial Fluid Volume.
measure plasma volume.
), can be used to
T-1824
Also, dyes that avidly bind to the plasma proteins, such
cular system after injection. One of the most commonly
volume, a substance must be used that does not readily
To measure plasma
Measurement of Plasma Volume.
Extracellular volume
Total body water
volume cannot be measured directly. However, it can be
The intracellular
Calculation of Intracellular Volume.
uid volume.
inulin space,
Therefore, one frequently speaks of the
sodium, may diffuse into the cells in small amounts.
of these substances, however, such as radioactive
uid within 30 to 60 minutes. Some
blood, it usually disperses almost completely through-
When any one of these substances is injected into the
radioactive iothalamate, thiosulfate ion, and inulin.
They include radioactive sodium, radioactive chloride,
uid but do not readily permeate the cell membrane.
The volume of
Measurement of Extracellular Fluid Volume.
lular and extracellular compartments.
antipyrine,
3). Another substance that has been used to
(Table 25
296
Unit V
The Body Fluids and Kidneys
principle can be used to calculate total body water
–
measure total body water is
which is very
lipid soluble and can rapidly penetrate cell membranes
and distribute itself uniformly throughout the intracel-
extracellular fluid can be estimated using any of several
substances that disperse in the plasma and interstitial
fl
out the extracellular fl
sodium space or
the
instead of calling the measurement the
true extracellular fl
calculated as
Intracellular volume
=
–
penetrate capillary membranes but remains in the vas-
used substances for measuring plasma volume is serum
albumin labeled with radioactive iodine (
125
I-albumin).
as Evans blue dye (also called
Interstitial fluid
culated as
Interstitial fluid volume
= Extracellular fluid volume
–
If one measures plasma
volume can also be calculated if one knows the
hematocrit (the fraction of the total blood volume com-
Another way to measure blood volume is to inject
into the circulation red blood cells that have been
quently used to label the red blood cells is radioactive
chromium (
51
Regulation of Fluid Exchange
and Osmotic Equilibrium
Between Intracellular and
Extracellular Fluid
A frequent problem in treating seriously ill patients is
maintaining adequate fluids in one or both of the intra-
amounts of extracellular fluid distributed between the
plasma and interstitial spaces are determined mainly
by the balance of hydrostatic and colloid osmotic
fluid between intracellular and
mainly by the osmotic effect of the smaller solutes—
—
that the cell membranes are highly permeable to water
but relatively impermeable to even small ions such as
fluid remains isotonic with the extracellular fluid.
between intracellular and extracellular fluid volumes
and the osmotic factors that can cause shifts of fluid
Basic Principles of Osmosis
here only the most important aspects of these princi-
ples as they apply to volume regulation.
Osmosis is the net diffusion of water across a selec-
tively permeable membrane from a region of high water
tion.
3 liters
1
liters
-
=
0 4
5
.
1 Hematocrit
Total blood volume
Plasma volume
=
-
Table 25–3
uid
(Calculated as Extracellular
Cr-labeled red blood cells, or calculated
I-albumin, Evans blue dye (T-1824)
uid
(Calculated as Total body water
I-iothalamate, thiosulfate, inulin
O, antipyrine
Total body water
Volume
Indicators
Measurement of Body Fluid Volumes
3
H
2
O,
2
H
2
Extracellular fluid
22
Na,
125
Intracellular fl
–
Extracellular fluid volume)
Plasma volume
125
Blood volume
51
as Blood volume
= Plasma volume/
(1
- Hematocrit)
Interstitial fl
fluid
volume
- Plasma volume)
6th ed. Philadelphia: WB Saunders, 1997.
From Guyton AC, Hall JE: Human Physiology and Mechanisms of Disease,

For
between them. One can correct for these deviations
This calculation is only an approximation, because
19.3 mm Hg/mOsm/L, or 5944 mm Hg.
of this solution would therefore be 308 mOsm/L
tion is 308 mOsm/L. The potential osmotic pressure
or 0.308 osm/L. Therefore, the osmolarity of this solu-
2 osmoles, the osmolarity of the solution is 0.154
divided by 58.5 g/mol, or about 0.154 mol/L. Because
ride is 58.5 g/mol, the molarity of the solution is 9 g/L
9 g/L. Because the molecular weight of sodium chlo-
sodium chloride per 100 milliliters of solution, or
sodium chloride solution is calculated as follows: A
For example, the osmotic pressure of a 0.9 per cent
the cell membrane is impermeable to the solute.
potential osmotic pressure of a solution, assuming that
s law, one can calculate the
membrane, 19.3 mm Hg osmotic pressure is exerted.
is equal to 19.3 mm Hg. Thus, for each
1 mOsm/L,
of 1 osm/L. This means that for a concentration of
19,300 mm Hg for a solution having a concentration
kelvin), the value of
and T is normal body temperature (273
uids,
is expressed in millimeters of mercury (mm Hg), the
). If
liter, R is the ideal gas constant, and T is the absolute
CRT
law, osmotic pressure (
Expressed mathematically, according to van
measure of the concentration of solute particles.
of a solution is proportional to its osmolarity, a
ecule or a glucose molecule. Thus, the osmotic pressure
, and therefore
osmotically active particles, Na
One molecule of sodium chloride, however, has two
example, one molecule of albumin with a molecular
solute is a large molecule or a small molecule. For
that solution. This is true regardless of whether the
The
Relation Between Osmotic Pressure and Osmolarity.
tion. The higher the osmotic pressure of a solution,
Osmotic pressure, therefore, is an indirect measure-
osmotic pressure.
the osmosis. The precise amount of pressure required
based on osmolarities rather than osmolalities.
fore, most of the calculations used clinically and the
uid rather than in kilograms of water. There-
cases, it is easier to express body
mously because the differences are small. In most
uids, these two terms can be used almost synony-
The osmolal concentration of
Osmolality and Osmolarity.
osmole, is commonly used.
(mOsm), which equals 1/1000
The term
uids.
In general, the osmole is too large a unit for express-
3 osm/L. Thus, the term osmole refers to the number
), will contain
ions, such as sodium sulfate (Na
centration of 2 osm/L. Likewise, a solution that con-
ride ionizing to give chloride and sodium ions, then a
two ions (giving two particles), such as sodium chlo-
centration of 1 osm/L. If a molecule dissociates into
) of solute particles. Therefore, a solution
osmoles.
particles, regardless of their exact composition. The
solute particles in the solution, a concentration term is
rate of osmosis.
membranes and into the cells. The rate of diffusion of
uid, water
becomes equal. Conversely, if a solute such as sodium
uid, water rapidly diffuses from the cells through the
region of higher solute concentration. Thus, if a solute
brane, water diffuses across the membrane toward the
selectively permeable), whenever there is a higher
to most solutes but highly permeable to water (i.e.,
the water concentration. Further, water diffuses from
higher the solute concentration in a solution, the lower
the concentration of water in the mixture. Thus, the
The Body Fluid Compartments: Extracellular and Intracellular Fluids; Interstitial Fluid and Edema
Chapter 25
297
a region of low solute concentration (high water con-
centration) to one with a high solute concentration
(low water concentration).
Because cell membranes are relatively impermeable
concentration of solute on one side of the cell mem-
such as sodium chloride is added to the extracellular
fl
cell membranes into the extracellular fluid until the
water concentration on both sides of the membrane
chloride is removed from the extracellular fl
diffuses from the extracellular fluid through the cell
water is called the
Relation Between Moles and Osmoles.
Because the water
concentration of a solution depends on the number of
needed to describe the total concentration of solute
total number of particles in a solution is measured in
One osmole (osm) is equal to 1 mole (mol)
(6.02
¥ 10
23
containing 1 mole of glucose in each liter has a con-
solution containing 1 mol/L will have an osmolar con-
tains 1 mole of a molecule that dissociates into three
2
SO
4
of osmotically active particles in a solution rather than
to the molar concentration.
ing osmotic activity of solutes in the body fl
milliosmole
a solution is called osmolality when the concentration
is expressed as osmoles per kilogram of water; it is
called osmolarity when it is expressed as osmoles per
liter of solution. In dilute solutions such as the body
fl
fluid quantities in
liters of fl
calculations expressed in the next several chapters are
Osmotic Pressure.
Osmosis of water molecules across a
selectively permeable membrane can be opposed by
applying a pressure in the direction opposite that of
to prevent the osmosis is called the
ment of the water and solute concentrations of a solu-
the lower the water concentration and the higher the
solute concentration of the solution.
osmotic pressure of a solution is directly proportional
to the concentration of osmotically active particles in
weight of 70,000 has the same osmotic effect as one
molecule of glucose with a molecular weight of 180.
+
and Cl
–
has twice the osmotic effect of either an albumin mol-
’t Hoff’s
p) can be calculated as
p =
where C is the concentration of solutes in osmoles per
temperature in degrees kelvin (273°
+ centigrade°
p
unit of pressure commonly used for biological fl
°
+ 37° =
310°
p calculates to be about
p
milliosmole concentration gradient across the cell
Calculation of the Osmolarity and Osmotic Pressure of a
Solution.
Using van’t Hoff’
0.9 per cent solution means that there is 0.9 gram of
each molecule of sodium chloride is equal to
¥ 2,
¥
sodium and chloride ions do not behave entirely inde-
pendently in solution because of interionic attraction
from the predictions of van’t Hoff’s law by using a
correction factor called the osmotic coefficient.
the same osmolarity. Solutions of sodium chloride with
it to swell; water will continue to diffuse into the cell,
282 mOsm/L), water will diffuse into the cell, causing
uids.
5 per cent glucose solution. These solutions are impor-
nor swells the cells. Examples of isotonic solutions
and the solutes cannot enter or leave the cell. Such a
solutes having an osmolarity of 282 mOsm/L, the cells
5. If a cell is placed in a solution of impermeant
uid on cell volume are shown in Figure
The effects of
can cause large changes in cell volume.
these forces, relatively small changes in the concen-
uids are not in osmotic equilibrium. As a result of
cell membrane is more than 5400 mm Hg. This demon-
uid is 282 mOsm/L, the
osmotic pressure is exerted across the cell membrane.
permeate the cell membrane), about 19.3 mm Hg
cussed earlier, for each milliosmole concentration
uid. As dis-
plasma, which is 19.3 times the corrected osmolarity of
membrane with pure water on the other side. Note
Table
Total Osmotic Pressure Exerted by the Body Fluids.
of the dissolved substance.
the next, and these two effects can cause, respectively,
uid.The reason for these corrections is that molecules
uid, and intracellular
of plasma, interstitial
bottom of Table 25
At the
illaries than in the surrounding interstitial spaces, as
by the osmotic effects of the plasma proteins, which
uids. The slight dif-
of the three compartments is about 300 mOsm/L, with
2, the total osmolarity of each
As shown in Table 25
other intracellular substances.
sium ions, and the remainder is divided among many
uid, almost half the osmolarity is due to potas-
due to sodium and chloride ions, whereas for intracel-
uid. Note that about 80 per cent of the
uid, and
ically active substances in plasma, interstitial
Turning back to Table 25
physiologic solutions.
286 mOsm/L. For practical reasons, the osmotic coef-
0.93, or about
Therefore, the actual osmolarity of a 0.9 per cent
sodium chloride, the osmotic coef
298
Unit V
The Body Fluids and Kidneys
ficient is about 0.93.
sodium chloride solution is 308
¥
ficients of different solutes are sometimes neglected in
determining the osmolarity and osmotic pressures of
Osmolarity of the Body Fluids.
–2,
note the approximate osmolarity of the various osmot-
fl
intracellular fl
total osmolarity of the interstitial fluid and plasma is
lular fl
–
the plasma being about 1 mOsm/L greater than that
of the interstitial and intracellular fl
ference between plasma and interstitial fluid is caused
maintain about 20 mm Hg greater pressure in the cap-
discussed in Chapter 16.
Corrected Osmolar Activity of the Body Fluids.
–2 are shown corrected osmolar
activities
fl
fl
and ions in solution exert interionic and intermolecu-
lar attraction or repulsion from one solute molecule to
a slight decrease or an increase in the osmotic “activ-
ity”
25–2 also shows the total osmotic pressure in millime-
ters of mercury that would be exerted by each of the
different fluids if it were placed on one side of the cell
that this total pressure averages about 5443 mm Hg for
282 mOsm/L for plasma.
Osmotic Equilibrium Is
Maintained Between
Intracellular and
Extracellular Fluids
Large osmotic pressures can develop across the cell
membrane with relatively small changes in the con-
centrations of solutes in the extracellular fl
gradient of an impermeant solute (one that will not
If the cell membrane is exposed to pure water and the
osmolarity of intracellular fl
potential osmotic pressure that can develop across the
strates the large force that can move water across the
cell membrane when the intracellular and extracellu-
lar fl
tration of impermeant solutes in the extracellular fluid
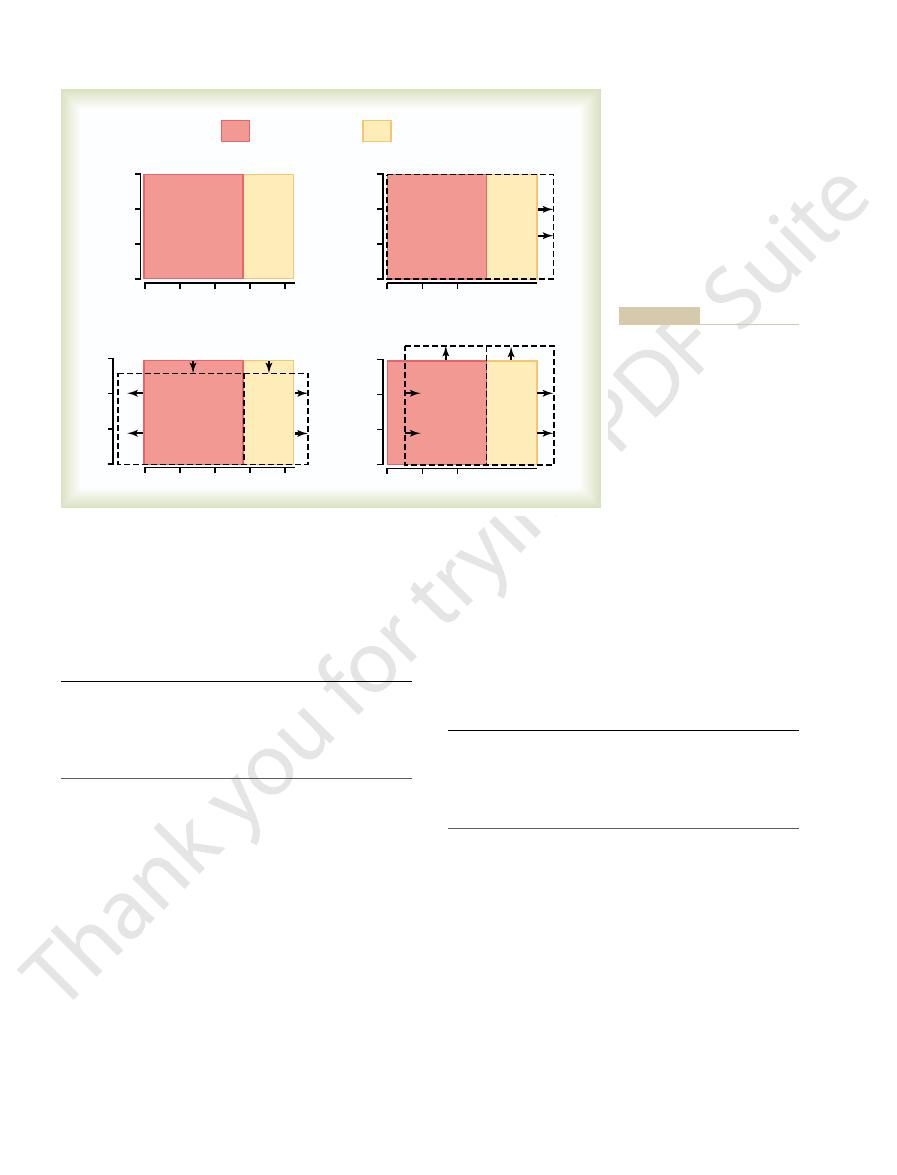
Isotonic, Hypotonic, and Hypertonic Fluids.
different concentrations of impermeant solutes in the
extracellular fl
25–
will not shrink or swell because the water concentra-
tion in the intracellular and extracellular fluids is equal
solution is said to be isotonic because it neither shrinks
include a 0.9 per cent solution of sodium chloride or a
tant in clinical medicine because they can be infused
into the blood without the danger of upsetting osmotic
equilibrium between the intracellular and extracellu-
lar fl
If a cell is placed into a hypotonic solution that has
a lower concentration of impermeant solutes (less than
diluting the intracellular fluid while also concentrating
the extracellular fluid until both solutions have about
a concentration of less than 0.9 per cent are hypotonic
and cause cells to swell.
HYPOTONIC
Cell swells
200 mOsm/L
HYPERTONIC
Cell shrinks
360 mOsm/L
ISOTONIC
No change
280 mOsm/L
B
C
A
), hypertonic (
Effects of isotonic (
Figure 25–5
A
B), and hypotonic (C) solutions
on cell volume.

including the volume, concentration, and total
rst step is to calculate the initial conditions,
The
initial plasma osmolarity is 280 mOsm/L, what would
example, if 2 liters of a hypertonic 3.0 per cent sodium
uid volumes and osmolarities. For
We can calculate the sequential
ments have the same osmolarity (see Figure 25
uid, the osmolarity of the extracellular
compartments.
intracellular volume, and a rise in osmolarity in both
uid added), a decrease in
The net effect is an increase in extracellular volume
compartment, and
). Again, almost all the
compartment (see Figure 25
uid, the extracellular osmolarity increases and causes
chloride.
). The sodium and chloride largely remain in the
uid volume (Figure
occurs through the cell membranes. The only effect is
uid does not change; therefore, no osmosis
uid compartment, the osmolarity of the extra-
to the Extracellular Fluid
Effect of Adding Saline Solution
osmolarities.
With these basic principles in mind, we can analyze
therefore, the
change in one of the compartments.
therefore, the osmolarities of intracellular and
Water moves rapidly across cell membranes;
kidneys.
uid from the gastrointestinal tract, and loss of abnor-
of different types of solutions, loss of large amounts of
ingestion of water, dehydration, intravenous infusion
Fluids in Abnormal States
Volume and Osmolality of
in the body after drinking water.
osmotic equilibrium can occur. It usually takes about
same short period. The reason for this is that
minutes. This rapid movement of water across the cell
usually corrected within seconds or, at the most,
The transfer of
under steady-state conditions.
given enough time, the concentrations of these sub-
uids, but
such as urea, can cause transient shifts in
the cell membrane. Highly permeating substances,
uid, without regard for whether the solute permeates
respectively, compared with the normal extracellular
solutions that have a higher or lower osmolarity,
The terms
membrane.
isosmotic,
permeate the cell membrane. Solutions with an osmo-
of impermeant solutes. Some solutes, however, can
The tonicity of solutions depends on the concentration
whether solutions will cause a change in cell volume.
terms isotonic, hypotonic, and hypertonic refer to
The
solutions of greater than 0.9 per cent are hypertonic.
two concentrations become equal. Sodium chloride
uid. In this case, the cell will shrink until the
uid, con-
higher concentration of impermeant solutes, water will
The Body Fluid Compartments: Extracellular and Intracellular Fluids; Interstitial Fluid and Edema
Chapter 25
299
If a cell is placed in a hypertonic solution having a
flow out of the cell into the extracellular fl
centrating the intracellular fluid and diluting the extra-
cellular fl
Isosmotic, Hyperosmotic, and Hypo-osmotic Fluids.
larity the same as the cell are called
regard-
less of whether the solute can penetrate the cell
hyperosmotic and hypo-osmotic refer to
fl
fluid volume
between the intracellular and extracellular fl
stances eventually become equal in the two compart-
ments and have little effect on intracellular volume
Osmotic Equilibrium Between Intracellular and Extracellular
Fluids Is Rapidly Attained.
fluid across the
cell membrane occurs so rapidly that any differences
in osmolarities between these two compartments are
membrane does not mean that complete equilibrium
occurs between the intracellular and extracellular
compartments throughout the whole body within the
fluid
usually enters the body through the gut and must be
transported by the blood to all tissues before complete
30 minutes to achieve osmotic equilibrium everywhere
Extracellular and Intracellular
Some of the different factors that can cause extracel-
lular and intracellular volumes to change markedly are
fl
mal amounts of fluid by sweating or through the
One can calculate both the changes in intracellular
and extracellular fluid volumes and the types of
therapy that should be instituted if the following basic
principles are kept in mind:
1.
extracellular fluids remain almost exactly equal
to each other except for a few minutes after a
2. Cell membranes are almost completely
impermeable to many solutes;
number of osmoles in the extracellular or
intracellular fluid generally remains constant
unless solutes are added to or lost from the
extracellular compartment.
the effects of different abnormal fluid conditions on
extracellular and intracellular fluid volumes and
If an isotonic saline solution is added to the extracel-
lular fl
cellular fl
an increase in extracellular fl
25–6A
extracellular fluid because the cell membrane behaves
as though it were virtually impermeable to the sodium
If a hypertonic solution is added to the extracellular
fl
osmosis of water out of the cells into the extracellular
–6B
added sodium chloride remains in the extracellular
fluid diffuses from the cells into the
extracellular space to achieve osmotic equilibrium.
(greater than the volume of fl
If a hypotonic solution is added to the extracellular
fl
fluid decreases
and some of the extracellular water diffuses into the
cells until the intracellular and extracellular compart-
–6C).
Both the intracellular and the extracellular volumes
are increased by the addition of hypotonic fluid,
although the intracellular volume increases to a
greater extent.
Calculation of Fluid Shifts and Osmolarities After Infusion
of Hypertonic Saline.
effects of infusing different solutions on extracellular
and intracellular fl
chloride solution are infused into the extracellular
fluid compartment of a 70-kilogram patient whose
be the intracellular and extracellular fluid volumes and
osmolarities after osmotic equilibrium?
fi
weight, the following volumes and concentrations
milliosmoles in each compartment. Assuming that
300
Unit V
The Body Fluids and Kidneys
extracellular fluid volume is 20 per cent of body weight
and intracellular fluid volume is 40 per cent of body
can be calculated.
Step 1. Initial Conditions
uid
28
280
7,840
uid
14
280
3,920
(Liters)
(mOsm/L)
(mOsm)
Volume
Concentration
Total
Extracellular fl
Intracellular fl
Step 2. Instantaneous Effect of Adding 2 Liters of
centration of 373 mOsm/L. Thus, the following values
volume, the concentration can be calculated by divid-
solute, yielding a total of 5791 milliosmoles. Because
however, there
equilibrium. In the
tration or volume, and there would be no osmotic
uid along with 2 liters of volume. There
In Step 2, we calculate the instantaneous effect of
cally active particles per mole), the net effect of adding
chloride. Because 1 mole of sodium chloride is about
liters of solution, this would be 1.026 mole of sodium
mole of sodium chloride per liter of solution. For 2
about 58.5 g/mol, this means that there is about 0.513
3.0 g/100 ml, or 30 grams of sodium chloride per liter.
chloride. A 3.0 per cent solution means that there are
Next, we calculate the total milliosmoles added to
uid
42
280
11,760
Total body fl
the extracellular fluid in 2 liters of 3.0 per cent sodium
Because the molecular weight of sodium chloride is
equal to 2 osmoles (sodium chloride has two osmoti-
2 liters of this solution is to add 2051 milliosmoles of
sodium chloride to the extracellular fluid.
adding 2051 milliosmoles of sodium chloride to the
extracellular fl
would be no change in the intracellular fluid concen-
extracellular fluid,
would be an additional 2051 milliosmoles of total
the extracellular compartment now has 16 liters of
ing 5791 milliosmoles by 16 liters to yield a con-
would occur instantly after adding the solution.
3.0 Per Cent Sodium Chloride
uid
28
280
7,840
uid
16
373
5,971
(Liters)
(mOsm/L)
(mOsm)
Volume Concentration Total
Extracellular fl
Intracellular fl
the intracellular and extracellular compartments. The
out of the cells, we then calculate the volumes of
centration after osmotic equilibrium. Assuming that
yields a concentration of 313.9 mOsm/L. Therefore, all
13,811, by the total volume, which is now 44 liters. This
lated by dividing the total milliosmoles in the body,
after osmotic equilibrium develops. In this case, the
In the third step, we calculate the volumes and con-
uid
44
No equilibrium
13,811
Total body fl
centrations that would occur within a few minutes
concentrations in the intracellular and extracellular
fluid compartments would be equal and can be calcu-
the body fluid compartments will have this same con-
no solute or water has been lost from the body and
that there is no movement of sodium chloride into or
intracellular fluid volume is calculated by dividing the
total milliosmoles in the intracellular fluid (7840) by
Volume (liters)
Volume (liters)
30
40
C. Add Hypotonic NaCl
B. Add Hypertonic NaCl
Normal State
A. Add Isotonic NaCl
Osmolarity
20
10
Intracellular fluid
300
200
100
0
Extracellular fluid
on the ordinates.
these compartments are shown
are shown in the abscissa of each
extracellular fluid compartments
are shown by the shaded areas.
lines, and the shifts from normal
osmotic equilibrium. The normal
Effect of adding isotonic, hyper-
Figure 25–6
tonic, and hypotonic solutions
to the extracellular fluid after
state is indicated by the solid
The volumes of intracellular and
diagram, and the osmolarities of

Chloride After Osmotic Equilibrium
Step 3. Effect of Adding 2 Liters of 3.0 Per Cent Sodium
not move into the cells.
a volume of 19.02 liters. Again, these calculations are
(5971) by the concentration (313.9 mOsm/L), to yield
of 24.98 liters. Extracellular
the concentration (313.9 mOsm/L), to yield a volume
The Body Fluid Compartments: Extracellular and Intracellular Fluids; Interstitial Fluid and Edema
Chapter 25
301
fluid volume is calculated
by dividing the total milliosmoles in extracellular fluid
based on the assumption that the sodium chloride
added to the extracellular fluid remains there and does
uid
24.98
313.9
7,840
uid
19.02
313.9
5,971
(Liters)
(mOsm/L)
(mOsm)
Volume
Concentration
Total
Extracellular fl
Intracellular fl
, which causes the kidney
For example,
osmotic overhydration.
uid, a condition that is referred to as
water retention, which dilutes the sodium in the extra-
from decreased secretion of the hormone aldosterone,
, which results
Addison’s disease
hyponatremia. Finally,
Overuse of
uid volume. Conditions
4). A primary loss of sodium chloride usually
(Table 25
Water or Loss of Sodium
sodium concentration is elevated above normal, a
When plasma
of plasma osmolarity under many conditions. When
routinely measured, but because sodium and its associ-
plasma sodium concentration. Plasma osmolarity is not
The primary measurement that is readily available to
Hypernatremia
Hyponatremia and
Fluid Volume Regulation:
Clinical Abnormalities of
is the addition of only nutrients to the body.
the form of a very dilute urine. The net result, therefore,
uid is ingested. Ordinarily, the kidneys excrete this in
excess of water often remains, especially if additional
After the glucose or other nutrients are metabolized, an
uids.
isotonicity, or they are given slowly enough that they do
tions are administered, their concentrations of osmoti-
solutions are used to a lesser extent. When these solu-
are widely used, and amino acid and homogenized fat
take adequate amounts of nutrition. Glucose solutions
Solutions Administered
volume regulation. The reader should be familiar with
This method of calculating changes in intracellular
almost 3 liters.
Thus, one can see from this example that adding 2
uid
44.0
313.9
13,811
Total body fl
liters of a hypertonic sodium chloride solution causes
more than a 5-liter increase in extracellular fluid
volume while decreasing intracellular fluid volume by
and extracellular fluid volumes and osmolarities can
be applied to virtually any clinical problem of fluid
such calculations because an understanding of the
mathematical aspects of osmotic equilibrium between
intracellular and extracellular fluid compartments is
essential for understanding almost all fluid abnormal-
ities of the body and their treatment.
Glucose and Other
for Nutritive Purposes
Many types of solutions are administered intravenously
to provide nutrition to people who cannot otherwise
cally active substances are usually adjusted nearly to
not upset the osmotic equilibrium of the body fl
fl
the clinician for evaluating a patient’s fluid status is the
ated anions (mainly chloride) account for more than
90 per cent of the solute in the extracellular fluid,
plasma sodium concentration is a reasonable indicator
plasma sodium concentration is reduced more than a
few milliequivalents below normal (about 142 mEq/L),
a person is said to have hyponatremia.
person is said to have hypernatremia.
Causes of Hyponatremia: Excess
Decreased plasma sodium concentration can result
from loss of sodium chloride from the extracellular fluid
or addition of excess water to the extracellular fluid
–
results in hypo-osmotic dehydration and is associated
with decreased extracellular fl
that can cause hyponatremia owing to loss of sodium
chloride include diarrhea and vomiting.
diuretics that inhibit the ability of the kidneys to con-
serve sodium and certain types of sodium-wasting
kidney diseases can also cause modest degrees of
impairs the ability of the kidneys to reabsorb sodium
and can cause a modest degree of hyponatremia.
Hyponatremia can also be associated with excess
cellular fl
hypo-
excessive secretion
of antidiuretic hormone
Table 25–4
≠
≠
Ø
s disease; primary aldosteronism
Hyper-osmotic overhydration
Cushing
≠
Ø
Ø
Hyper-osmotic dehydration
Diabetes insipidus; excessive sweating
Ø
≠
≠
Hypo-osmotic overhydration
Excess ADH; bronchogenic tumor
Ø
Ø
≠
ciency; overuse of diuretics
Hypo-osmotic dehydration
Adrenal insuf
Abnormality
Cause
Concentration
Fluid Volume
Fluid Volume
Abnormalities of Body Fluid Volume Regulation: Hyponatremia and Hypernatremia
Plasma Na
+
Extracellular Intracellular
fi
’
ADH, antidiuretic hormone.

vessels are removed during radical mastectomy,
obstructed. For example, large numbers of lymph
. Blockage of the lymph
with infections of the lymph nodes, such as occurs with
ow can be especially severe
uid out of the capillaries.
uid, which draws
The rise in protein concentration raises the colloid
When lymphatic blockage occurs, edema can become
Lymphatic Blockage Causes Edema
Decreased plasma colloid osmotic pressure.
Increased capillary hydrostatic pressure.
changes can increase the capillary filtration rate:
that any one of the following
equation, one can see
uid colloid osmotic pressure. From this
capillary plasma colloid osmotic pressure, and
uid hydrostatic pressure,
is the capillary hydrostatic pressure, P
capillaries), P
Filtration
ltration discussed in Chapter 16. Mathematically, cap-
tion, it is useful to review the determinants of capillary
To understand the causes of excessive capillary
Factors That Can Increase Capillary Filtration
back into the blood. The most common clinical cause
uid from the interstitium
stitial spaces across the capillaries, and (2) failure of
are two general causes of extracellular edema: (1)
uid accumulation in the extracellular spaces. There
cells.
of the cell, with subsequent osmosis of water into the
cell membranes to increase their permeability, allow-
tissues. In
death of the tissue.
normal. When this occurs, it is usually a prelude to
ischemic leg, for example
water into the cells. Sometimes this can increase intra-
cell can no longer be pumped out of the cells, and the
ionic pumps become depressed. When this occurs,
tain normal tissue metabolism, the cell membrane
reduced. If the blood
is decreased, the delivery of oxygen and nutrients is
the cells. For example, when blood
of the tissues, and (2) lack of adequate nutrition to
lular swelling: (1) depression of the metabolic systems
Two conditions are especially prone to cause intracel-
uid compartment, but it can
body tissues. In most instances, edema occurs mainly
in the Tissues
loss or gain of water.
concentration and deciding on proper therapy, one
Thus, in analyzing abnormalities of plasma sodium
degree of hypernatremia and overhydration. The reason
kidneys as well. For example,
hyperosmotic overhydration
uid. This
during prolonged, heavy exercise.
that is less than water loss, as can occur with sweating
nephrogenic diabetes insipidus.
respond to antidiuretic hormone, also causing a type of
certain types of renal diseases, the kidneys cannot
uid. In
), causing dehydration and increased concen-
diuretic hormone, the kidneys excrete large amounts
kidneys to conserve water. As a result of lack of anti-
secrete antidiuretic hormone, which is needed for the
This condition can occur from an inability to
uid, this results in
uid. When there is primary loss of water from the
the sodium ions, or excess sodium in the extracellular
uid, which concentrates
causes increased osmolarity, can be due to either loss of
Increased plasma sodium concentration, which also
Causes of Hypernatremia: Water
tubules to reabsorb more water, can lead to hypona-
302
Unit V
The Body Fluids and Kidneys
tremia and overhydration.
Loss or Excess Sodium
water from the extracellular fl
fl
extracellular fl
hyperosmotic dehydra-
tion.
of dilute urine (a disorder referred to as diabetes
insipidus
tration of sodium chloride in the extracellular fl
A more common cause
of hypernatremia associated with decreased extracellu-
lar fluid volume is dehydration caused by water intake
Hypernatremia can also occur as a result of excessive
sodium chloride added to the extracellular fl
often results in
because
excess extracellular sodium chloride is usually associ-
ated with at least some degree of water retention by the
excessive secretion of the
sodium-retaining hormone aldosterone can cause a mild
that the hypernatremia is not more severe is that
increased aldosterone secretion causes the kidneys to
reabsorb greater amounts of water as well as sodium.
should first determine whether the abnormality is
caused by a primary loss or gain of sodium or a primary
Edema: Excess Fluid
Edema refers to the presence of excess fluid in the
in the extracellular fl
involve intracellular fluid as well.
Intracellular Edema
flow to a tissue
flow becomes too low to main-
sodium ions that normally leak into the interior of the
excess sodium ions inside the cells cause osmosis of
cellular volume of a tissue area—even of an entire
—to two to three times
Intracellular edema can also occur in inflamed
flammation usually has a direct effect on the
ing sodium and other ions to diffuse into the interior
Extracellular Edema
Extracellular fluid edema occurs when there is excess
fl
abnormal leakage of fluid from the plasma to the inter-
the lymphatics to return fl
of interstitial fluid accumulation is excessive capillary
fluid filtration.
filtra-
fi
illary filtration rate can be expressed as
= K
f
¥ (P
c
– P
if
–
p
c
+ p
if
),
where K
f
is the capillary filtration coefficient (the
product of the permeability and surface area of the
c
if
is the interstitial fl
p
c
is the
p
if
is the
interstitial fl
∑ Increased capillary filtration coefficient.
∑
∑
especially severe because plasma proteins that leak
into the interstitium have no other way to be removed.
osmotic pressure of the interstitial fl
even more fl
Blockage of lymph fl
infection by filaria nematodes
vessels can occur in certain types of cancer or after
surgery in which lymph vessels are removed or
impairing removal of fluid from the breast and arm
areas and causing edema and swelling of the tissue

the plasma into the intra-abdominal areas. When this
cient plasma proteins,
among the liver parenchymal cells. One result is failure
a reduction in plasma protein concentration. Cirrhosis
tion in plasma protein concentration occurs. Serious
the ability of the body to synthesize proteins, a reduc-
proteins to pass into the urine. When this loss exceeds
nephrotic syndrome.
urine in certain kidney diseases, a condition referred to
colloid osmotic pressure to fall. This leads to increased
the edema, these children usually develop severe
uid edema in the entire body; along with
uid, also develop serious
acute glomerulonephritis, in which the renal glomeruli
in Chapter 19. As an example, children who develop
because of the increase in blood volume, as explained
but some remains in the blood. The main effects of this
water leaks from the blood into the interstitial spaces,
uid. Most of this salt and
water, large amounts of sodium chloride and water are
only small amounts enter the cells. Therefore, in kidney
blood remains in the extracellular compartment, and
As discussed earlier, most sodium chloride added to the
Edema Caused by Decreased Kidney Excretion of Salt and Water.
progress, causing death within a few hours.
serious and life-threatening pulmonary edema. When
ary capillary pressure, rise far above normal, causing
the pulmonary vascular pressures, including pulmon-
heart has been greatly weakened. Consequently, all
cant failure of the right side of the heart, blood is
by the kidneys. Thus, in untreated heart failure, all these
angiotensin II and increased secretion of aldosterone,
secretion of renin, causing increased formation of
Also, diminished blood
decreased excretion of salt and water by the kidneys,
In addition, the arterial pressure tends to fall, causing
capillary pressure, causing increased capillary
veins into the arteries; this raises venous pressure and
failure, the heart fails to pump blood normally from the
most common causes of edema is heart failure. In heart
Edema Caused by Heart Failure.
D. Congenital absence or abnormality of
C. Surgery
B. Infections (e.g.,
A. Cancer
IV. Blockage of lymph return
F. Burns
E. Prolonged ischemia
ciency, especially vitamin C
D. Vitamin de
C. Bacterial infections
B. Toxins
A. Immune reactions that cause release of
III. Increased capillary permeability
2. Serious protein or caloric malnutrition
1. Liver disease (e.g., cirrhosis)
C. Failure to produce proteins
2. Wounds
1. Burns
B. Loss of protein from denuded skin areas
A. Loss of proteins in urine (nephrotic
II. Decreased plasma proteins
3. Vasodilator drugs
ciency of sympathetic nervous system
2. Insuf
1. Excessive body heat
C. Decreased arteriolar resistance
(c) Failure of venous valves
(b) Immobilization of parts of the body
(a) Paralysis of muscles
3. Failure of venous pumps
2. Venous obstruction
1. Heart failure
B. High venous pressure and venous constriction
2. Mineralocorticoid excess
1. Acute or chronic kidney failure
A. Excessive kidney retention of salt and water
I. Increased capillary pressure
to the circulation. The following is a partial list of con-
usually temporary.
this type of surgery, so that the interstitial edema is
spaces. A few lymph vessels eventually regrow after
The Body Fluid Compartments: Extracellular and Intracellular Fluids; Interstitial Fluid and Edema
Chapter 25
303
Summary of Causes of
Extracellular Edema
A large number of conditions can cause fluid accumu-
lation in the interstitial spaces by the abnormal leaking
of fluid from the capillaries or by preventing the lym-
phatics from returning fluid from the interstitium back
ditions that can cause extracellular edema by these two
types of abnormalities:
fi
syndrome)
histamine and other immune products
fi
filaria nematodes)
lymphatic vessels
One of the most serious and
filtration.
which increases blood volume and further raises capil-
lary hydrostatic pressure to cause still more edema.
flow to the kidneys stimulates
both of which cause additional salt and water retention
factors acting together cause serious generalized extra-
cellular edema.
In patients with left-sided heart failure but without
signifi
pumped into the lungs normally by the right side of the
heart but cannot escape easily from the pulmonary
veins to the left side of the heart because this part of the
untreated, fluid accumulation in the lungs can rapidly
diseases that compromise urinary excretion of salt and
added to the extracellular fl
are to cause (1) widespread increases in interstitial fluid
volume (extracellular edema) and (2) hypertension
are injured by inflammation and therefore fail to filter
adequate amounts of fl
extracellular fl
hypertension.
Edema Caused by Decreased Plasma Proteins.
A reduction in
plasma concentration of proteins because of either
failure to produce normal amounts of proteins or
leakage of proteins from the plasma causes the plasma
capillary filtration throughout the body as well as extra-
cellular edema.
One of the most important causes of decreased
plasma protein concentration is loss of proteins in the
as
Multiple types of renal diseases
can damage the membranes of the renal glomeruli,
causing the membranes to become leaky to the plasma
proteins and often allowing large quantities of these
generalized edema occurs when the plasma protein con-
centration falls below 2.5 g/100 ml.
Cirrhosis of the liver is another condition that causes
means development of large amounts of fibrous tissue
of these cells to produce suffi
leading to decreased plasma colloid osmotic pressure
and the generalized edema that goes with this condition.
Another way that liver cirrhosis causes edema is that
the liver fibrosis sometimes compresses the abdominal
portal venous drainage vessels as they pass through the
liver before emptying back into the general circulation.
Blockage of this portal venous outflow raises capillary
hydrostatic pressure throughout the gastrointestinal
area and further increases filtration of fluid out of
negative pressure range.
words, the compliance of the tissues is very low in the
pressure or 10 to 20 mm Hg negative pressure. In other
not change greatly, regardless of whether the degree of
uid pressure range, the interstitial
an elastic resistance to compression. In the negative
negative values, the gel does not contract greatly
Also, when the interstitial
laments.
flowing
hundredths of a micrometer in diameter. The impor-
uid in the interstitium is in gel form. That is, the
uid pressure, virtually
Note in Figure 25
ance of the tissues.
itive tissue pressure range, this safety factor against
uid hydrostatic pressure. Thus, in the pos-
the compliance of the tissues increases markedly,
uid pressure rises above 0 mm Hg,
uid pressure of about 3 mm Hg.
Therefore, the safety factor against edema is a change
uid will begin to accumulate in the tissues.
3 mm Hg, the interstitial
tissues.
pressure, opposing further
hydrostatic pressure is in the negative pressure range,
ltration. Therefore, as long as the interstitial
uid hydrostatic pressure increases, this
ltration discussed previously. When
edema? To answer this question, recall the determi-
low.
volume per millimeter of mercury pressure change, is
of the tissues, de
pressure. Therefore, in the negative pressure range, the
uid pressure is in the negative range, small
animal studies. Note in Figure 25
volume, as extrapolated to the human being from
tissues helps hold the tissues together. Figure 25
3 mm Hg. This slight suction in the
the body is slightly less than atmospheric pressure,
In Chapter 16, we noted that interstitial
Pressure Range
ltration increases.
uid protein concentration, which reduces
to increase 10- to 50-fold, and (3) washdown of inter-
tive pressure range, (2) the ability of lymph
the interstitial spaces: (1) low compliance of the inter-
edema develops. The reason for this is that three major
ascites.
protein into the abdominal cavity, a condition referred
occurs, the combined effects of decreased plasma
304
Unit V
The Body Fluids and Kidneys
protein concentration and high portal capillary pres-
sures cause transudation of large amounts of fluid and
to as
Safety Factors That Normally
Prevent Edema
Even though many disturbances can cause edema,
usually the abnormality must be severe before serious
safety factors prevent excessive fluid accumulation in
stitium when interstitial fluid pressure is in the nega-
flow
stitial fl
interstitial fluid colloid osmotic pressure as capillary
fi
Safety Factor Caused by Low Compliance
of the Interstitium in the Negative
fluid hydro-
static pressure in most loose subcutaneous tissues of
averaging about –
–7
shows the approximate relations between different
levels of interstitial fluid pressure and interstitial fluid
–7 that as long as the
interstitial fl
changes in interstitial fluid volume are associated with
relatively large changes in interstitial fluid hydrostatic
compliance
fined as the change in
How does the low compliance of the tissues in the
negative pressure range act as a safety factor against
nants of capillary fi
interstitial fl
increased pressure tends to oppose further capillary
fi
fluid
small increases in interstitial fluid volume cause rela-
tively large increases in interstitial fluid hydrostatic
filtration of fluid into the
Because the normal interstitial fluid hydrostatic
pressure is –
fluid hydrostatic
pressure must increase by about 3 mm Hg before large
amounts of fl
of interstitial fl
Once interstitial fl
allowing large amounts of fluid to accumulate in the
tissues with relatively small additional increases in
interstitial fl
edema is lost because of the large increase in compli-
Importance of Interstitial Gel in Preventing Fluid Accumulation in
the Interstitium.
–7 that in normal
tissues with negative interstitial fl
all the fl
fluid is bound in a proteoglycan meshwork so that there
are virtually no “free” fluid spaces larger than a few
tance of the gel is that it prevents fluid from
easily through the tissues because of impediment from
the “brush pile” of trillions of proteoglycan fi
fluid pressure falls to very
because the meshwork of proteoglycan filaments offers
fl
fluid volume does
suction is only a few millimeters of mercury negative
Free fluid
Gel fluid
2
4
6
T
o
tal inte
Interstitial fluid volume (liters)
-
10
-
8
-
6
-
4
-
2
0
(Low compliance)
rstitial fluid
(High
compliance)
Normal
60
56
52
48
44
40
36
32
28
24
20
16
12
8
4
0
Interstitial free fluid pressure
(mm Hg)
Interstitial free fluid pressure
(mm Hg)
Taylor AE: Interstitial fluid pressure. Physiol Rev 51:527, 1971.)
sure becomes positive. (Modified from Guyton AC, Granger HJ,
cant amounts of free fluid occur only when the interstitial fluid pres-
stitial fluid volumes, including total volume, free fluid volume, and
Relation between interstitial fluid hydrostatic pressure and inter-
Figure 25–7
gel fluid volume, for loose tissues such as skin. Note that signifi-

ease. Therefore, each potential space is in reality a
uids, electrolytes, or even proteins, which
The surface membrane of a potential space
surfaces.
sliding, a viscous proteinaceous
and the surfaces slide over each other. To facilitate the
each other, with only a thin layer of
ing both the joint cavities and the bursae. Virtually all
cavity, peritoneal cavity, and synovial cavities, includ-
is to list some examples: pleural cavity, pericardial
before marked edema would occur.
17 mm Hg, or approximately double the normal value,
about 17 mm Hg. This means that the capillary pres-
Therefore, the total safety factor against edema is
from the interstitial spaces is about 7 mm Hg.
3. The safety factor caused by washdown of proteins
about 7 mm Hg.
2. The safety factor caused by increased lymph
in the negative pressure range is about 3 mm Hg.
1. The safety factor caused by low tissue compliance
Putting together all the safety factors against edema, we
about 7 mm Hg.
uid. The
the capillaries, decreasing the interstitial
increases.
with the lymph vessels. Therefore, the proteins are
are relatively impermeable to proteins, compared
capillaries; the reason for this is that the capillaries
ow is increased, because larger amounts of
ow. In most tissues, the
uid pressure increases,
interstitium, the interstitial
“Washdown” of the Interstitial Fluid Protein as
be about 7 mm Hg.
into the positive pressure range. The safety factor
tion, preventing the interstitial pressure from rising
uid begins to accumulate in the tissues. This allows
The lymphatics act as a safety factor against edema
and interstitial edema would occur.
blood, the plasma volume would be rapidly depleted,
the capillaries into the interstitium. Without this con-
Increased Lymph Flow as a Safety Factor
as they normally diffuse. Therefore, the usual diffusion
laments, different substances within the
flow
simply elevating the legs.
the legs, the edema
interstitium. Therefore, when severe edema occurs in
tium, as occurs in edema, this extra
body. When too much
laments, the simple act of a
owing too easily through the tissue spaces. If it were
The proteoglycan
tance from one another.
spacing between the cells, these nutrients, electrolytes,
through cell membranes; therefore, without adequate
the cells. Nutrients and ions do not diffuse readily
brils in the interstitial spaces, act as a
laments, along with much larger collagen
The
Cells and in Preventing Rapid Flow of Fluid in the Tissues.
spaces.
back from the surrounding tissues. This type of edema
uid out of the area. When the thumb is removed, a pit
spaces because it is not in gel form. When this occurs,
Therefore, the
uid hydrostatic pressure. Most
range, the tissues are compliant, allowing large amounts
in the tissues. In this pressure
the positive pressure range, there is a tremendous accu-
By contrast, when interstitial
The Body Fluid Compartments: Extracellular and Intracellular Fluids; Interstitial Fluid and Edema
Chapter 25
305
fluid pressure rises to
mulation of free fluid
of fluid to accumulate with relatively small additional
increases in interstitial fl
of the extra fluid that accumulates is “free fluid” because
it pushes the brush pile of proteoglycan filaments apart.
fluid can flow freely through the tissue
the edema is said to be pitting edema because one can
press the thumb against the tissue area and push the
fl
is left in the skin for a few seconds until the fluid flows
is distinguished from nonpitting edema, which occurs
when the tissue cells swell instead of the interstitium or
when the fluid in the interstitium becomes clotted with
fibrinogen so that it cannot move freely within the tissue
Importance of the Proteoglycan Filaments as a “Spacer” for the
proteoglycan fi
fi
“spacer” between
and cell waste products could not be rapidly exchanged
between the blood capillaries and cells located at a dis-
filaments also prevent fluid from
fl
not for the proteoglycan fi
person standing up would cause large amounts of inter-
stitial fluid to flow from the upper body to the lower
fluid accumulates in the intersti-
fluid creates large
channels that allow the fluid to flow readily through the
fluid often can be decreased by
Even though fluid does not
easily through the
tissues in the presence of the compacted proteoglycan
fi
fluid can
diffuse through the tissues at least 95 per cent as easily
of nutrients to the cells and the removal of waste prod-
ucts from the cells are not compromised by the proteo-
glycan filaments of the interstitium.
Against Edema
A major function of the lymphatic system is to return
to the circulation the fluid and proteins filtered from
tinuous return of the filtered proteins and fluid to the
because lymph flow can increase 10- to 50-fold when
fl
the lymphatics to carry away large amounts of fluid
and proteins in response to increased capillary filtra-
caused by increased lymph flow has been calculated to
a Safety Factor Against Edema
As increased amounts of fluid are filtered into the
fl
causing increased lymph fl
protein concentration of the interstitium decreases as
lymph fl
protein are carried away than can be filtered out of the
“washed out” of the interstitial fluid as lymph flow
Because the interstitial fluid colloid osmotic pres-
sure caused by the proteins tends to draw fluid out of
fluid proteins
lowers the net filtration force across the capillaries and
tends to prevent further accumulation of fl
safety factor from this effect has been calculated to be
Summary of Safety Factors That
Prevent Edema
find the following:
flow is
sure in a peripheral tissue could theoretically rise by
Fluids in the “Potential
Spaces” of the Body
Perhaps the best way to describe a “potential space”
these potential spaces have surfaces that almost touch
fluid in between,
fluid lubricates the
Fluid Is Exchanged Between the Capillaries and the Potential
Spaces.
usually does not offer significant resistance to the
passage of fl
all move back and forth between the space and the
interstitial fluid in the surrounding tissue with relative

6th ed. Philadelphia: WB Saunders, 2000.
formation. In Brenner BM, Rector FC (eds): The Kidney,
Winaver J, Abassi Z, Green J, Skorecki KL: Control of extra-
phedema. Ann N Y Acad Sci 979:94, 2002.
Saaristo A, Karkkainen MJ, Alitalo K: Insights into the
23:371, 2002.
children: hyponatremia and hypernatremia. Pediatr Rev
Moritz ML, Ayus JC: Disorders of water metabolism in
Lippincott Williams & Wilkins, 2000.
Physiology & Pathophysiology, 3rd ed. Philadelphia:
cular walls. In Seldin DW, Giebisch G (eds): The Kidney
Michel CC: Exchange of
Rev 82:569, 2002.
transport and the resolution of pulmonary edema. Physiol
Matthay MA, Folkesson HG, Clerici C: Lung epithelial
giogenesis. Physiol Rev 82:673, 2002.
Jussila L, Alitalo K: Vascular growth factors and lymphan-
Crit Care Clin 18:249, 2002.
and water balance: emphasis on integrative physiology.
Halperin ML, Bohn D: Clinical approach to disorders of salt
phia: WB Saunders, 1975.
II: Dynamics and Control of the Body Fluids. Philadel-
Guyton AC, Taylor AE, Granger HJ: Circulatory Physiology
sure. Physiol Rev 51:527, 1971.
Guyton AC, Granger HJ, Taylor AE: Interstitial
function: current perspectives. Ann N Y Acad Sci 979:178,
Gashev AA: Physiologic aspects of lymphatic contractile
Sci 17:223, 2002.
left atrial pressure: four hours and beyond. News Physiol
Drake RE, Doursout MF: Pulmonary edema and elevated
tremia. Am J Med Sci 326:25, 2003.
Decaux G, Soupart A: Treatment of symptomatic hypona-
Cho S, Atwood JE: Peripheral edema. Am J Med 113:580,
361:1967, 2003.
Basnyat B, Murdoch DR: High-altitude illness. Lancet
News Physiol Sci 9:214, 1994.
Aukland K: Why don
water transport in the brain. Nat Rev Neurosci 4:991, 2003.
Amiry-Moghaddam M, Ottersen OP: The molecular basis of
cardial cavity.
in the joint spaces, and
8 mm Hg in the pleural cavity,
pheric) in loose subcutaneous tissue. For instance, the
spaces as well. It is especially interesting that the
are discussed in detail in Chapter 38. These dynamics
The dynamics of
swelling in the cavity.
ties often blocks the lymph drainage, causing isolated
Also, injury or local infection in any one of the cavi-
cavity, pericardial cavity, and joint spaces, can become
The other potential spaces, such as the pleural
uid can accumulate.
In serious cases, 20 liters or
ascites.
uid, and in this instance, the
tial edema is caused.The abdominal cavity is especially
Thus, lymph blockage or any of the multiple
in the potential space as well, and this
cent to the potential space, edema
When edema occurs in the subcutaneous tissues adja-
vessels arise directly from the cavity itself.
the pleural cavity and peritoneal cavity, large lymph
connected with lymph vessels. In some cases, such as
body. The protein must be removed through lymphat-
leakage out of the capillaries, similar to the collection
Lymphatic Vessels Drain Protein from the Potential Spaces.
space.
large tissue space. Consequently,
306
Unit V
The Body Fluids and Kidneys
fluid in the capillar-
ies adjacent to the potential space diffuses not only
into the interstitial fluid but also into the potential
Proteins collect in the potential spaces because of
of protein in the interstitial spaces throughout the
ics or other channels and returned to the circulation.
Each potential space is either directly or indirectly
Edema Fluid in the Potential Spaces Is Called “Effusion.”
fluid usually collects
fluid is called
effusion.
abnormalities that can cause excessive capillary filtra-
tion can cause effusion in the same way that intersti-
prone to collect effusion fl
effusion is called
more of ascitic fl
seriously swollen when there is generalized edema.
fluid exchange in the pleural cavity
are mainly representative of all the other potential
normal fluid pressure in most or all of the potential
spaces in the nonedematous state is negative in the
same way that this pressure is negative (subatmos-
interstitial fluid hydrostatic pressure is normally about
–7 to –
–3 to –5 mm Hg
–5 to –6 mm Hg in the peri-
References
’t our feet swell in the upright position?
2002.
2002.
fluid pres-
fluid
fluid and solutes across microvas-
—
molecular pathogenesis and targeted treatment of lym-
cellular fluid volume and the pathophysiology of edema
