
stances, such as glucose and amino acids, are almost completely reabsorbed
. Some sub-
Second, unlike glomerular filtration, which is relatively nonselective (that is,
glomerular filtration are closely coordinated, so that large fluctuations in urinary
remained constant. In reality, however, changes in tubular reabsorption and
reabsorption, from 178.5 to 160.7 L/day, would increase urine volume from 1.5
large change in urinary excretion. For example, a 10 per cent decrease in tubular
ative to urinary excretion for many substances. This means that a small change
From Table 27–1, two things are immediately apparent. First, the processes of
glucose reabsorption is also 180 g/day.
Because virtually none of the filtered glucose is normally excreted, the rate of
1 g/L, or 180 g/day.
amount of glucose filtered each day is about 180 L/day
to plasma proteins. For example, if plasma glucose concentration is 1 g/L, the
This calculation assumes that the substance is freely filtered and not bound
Filtration
The rate at which each of these substances is filtered is calculated as
tered in the kidneys and reabsorbed at variable rates.
Table 27–1 shows the renal handling of several substances that are all freely fil-
Tubular Reabsorption Is Selective and Quantitatively Large
few other substances that appear in the urine.
tion accounts for significant amounts of potassium ions, hydrogen ions, and a
does secretion in determining the final urinary excretion rate. However, secre-
For many substances, reabsorption plays a much more important role than
Tubular secretion
Tubular reabsorption
reabsorption, and tubular secretion—as follows:
represent the sum of three basic renal processes—glomerular filtration, tubular
lumen. Eventually, the urine that is formed and all the substances in the urine
back into the blood, whereas others are secreted from the blood into the tubular
Along this course, some substances are selectively reabsorbed from the tubules
before it is excreted as urine.
, and, finally,
, the
, the
proximal tubule
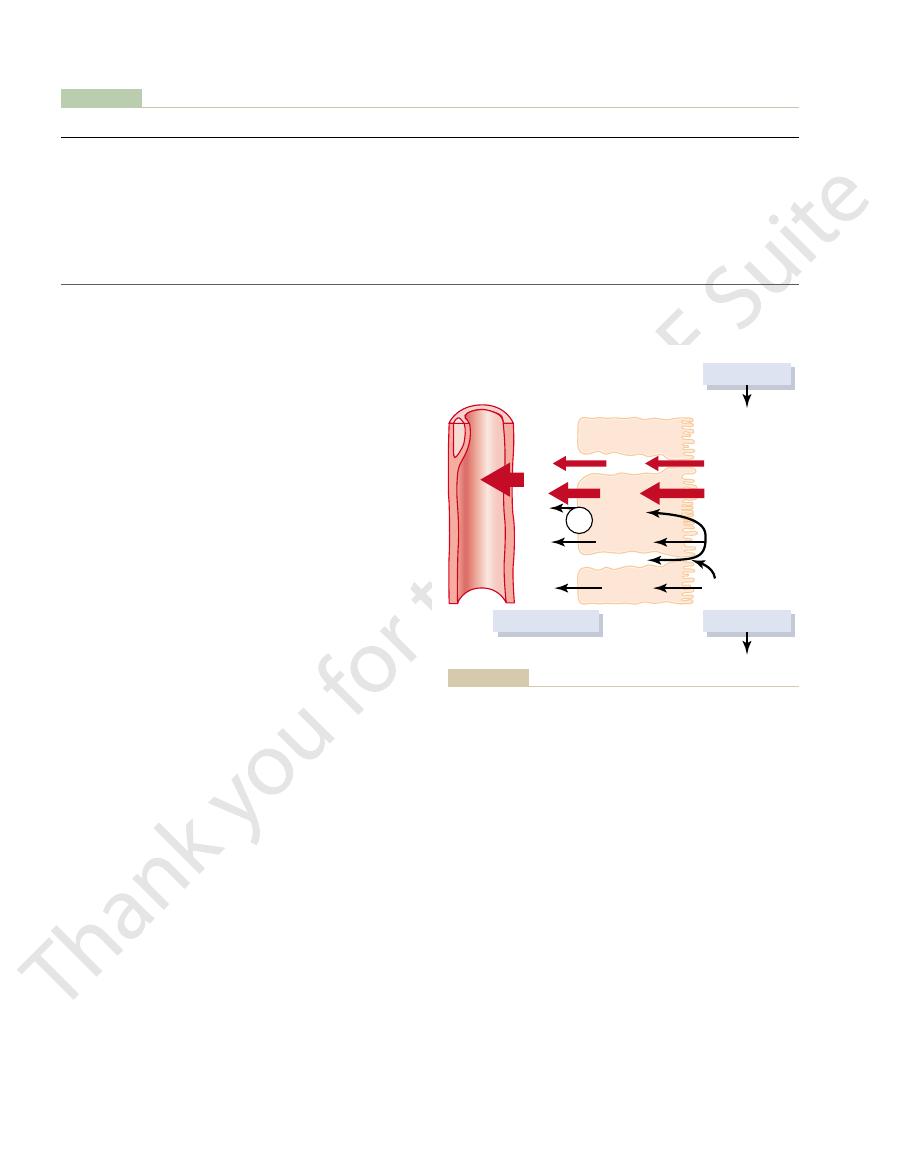
As the glomerular filtrate enters the renal tubules,
by the Renal Tubules
Reabsorption and Secretion
Glomerular Filtrate
II. Tubular Processing of the
Urine Formation by the Kidneys:
C
H
A
P
T
E
R
2
7
327
it flows sequentially through the successive parts of
the tubule—the
loop of Henle,
the distal tubule
collecting tubule
the collecting duct—
Urinary excretion
= Glomerular filtration -
+
= Glomerular filtration rate ¥ Plasma concentration
¥
glomerular filtration and tubular reabsorption are quantitatively very large rel-
in glomerular filtration or tubular reabsorption can potentially cause a relatively
to 19.3 L/day (almost a 13-fold increase) if the glomerular filtration rate (GFR)
excretion are avoided.
essentially all solutes in the plasma are filtered except the plasma proteins or
substances bound to them), tubular reabsorption is highly selective
the tubule, water is always reabsorbed by a passive
secondary active transport. Although solutes can be
. Reabsorp-
an energy source, such as that due to an ion gradient,
the renal tubule. Transport that is coupled
ATPase pump that functions throughout most parts of
. A good example of this is the sodium-potassium
triphosphate (ATP), is termed
an energy source, such as the hydrolysis of adenosine
from metabolism. Transport that is coupled directly to
Active Transport
and colloid osmotic forces. The peritubular capillaries
bulk flow
tubular epithelial cells into the interstitial fluid, water
). Then, after absorption across the
across other membranes of the body. For instance,
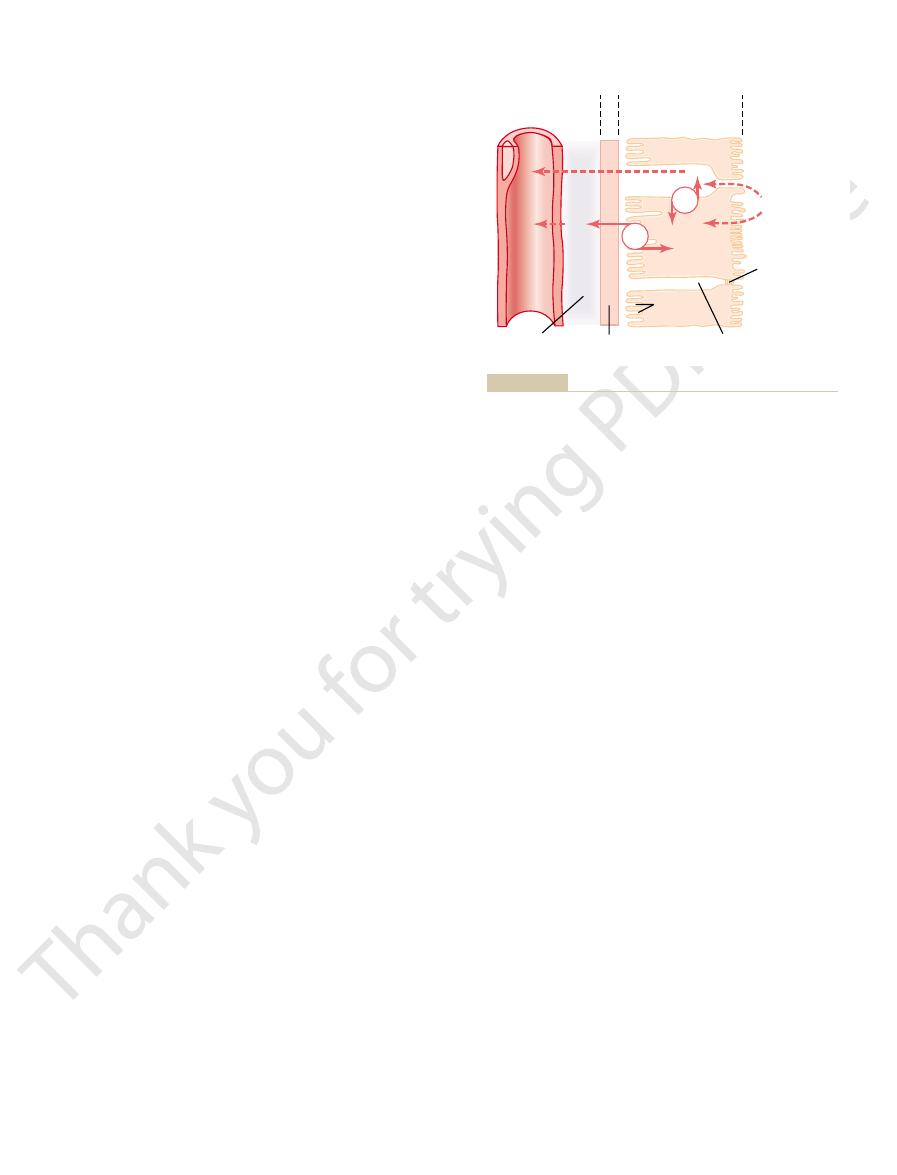
includes a series of transport steps. Reabsorption
(Figure 27–1). Thus, reabsorption of water and solutes
For a substance to be reabsorbed, it must first be trans-
Tubular Reabsorption
rates.
composition of body fluids. In this chapter, we discuss
excretion of solutes independently of one another, a
reabsorb different substances, the kidneys regulate the
Therefore, by controlling the rate at which they
tubules and excreted in relatively large amounts.
nine, conversely, are poorly reabsorbed from the
body. Certain waste products, such as urea and creati-
excretion are variable, depending on the needs of the
reabsorbed, but their rates of reabsorption and urinary
as sodium, chloride, and bicarbonate, are also highly
essentially zero. Many of the ions in the plasma, such
from the tubules, so that the urinary excretion rate is
328
Unit V
The Body Fluids and Kidneys
capability that is essential for precise control of the
the mechanisms that allow the kidneys to selectively
reabsorb or secrete different substances at variable
Includes Passive and
Active Mechanisms
ported (1) across the tubular epithelial membranes
into the renal interstitial fluid and then (2) through the
peritubular capillary membrane back into the blood
across the tubular epithelium into the interstitial fluid
includes active or passive transport by way of the same
basic mechanisms discussed in Chapter 4 for transport
water and solutes can be transported either through
the cell membranes themselves (transcellular route) or
through the junctional spaces between the cells (para-
cellular route
and solutes are transported the rest of the way through
the peritubular capillary walls into the blood by ultra-
filtration (
) that is mediated by hydrostatic
behave very much like the venous ends of most other
capillaries because there is a net reabsorptive force
that moves the fluid and solutes from the interstitium
into the blood.
Active transport can move a solute against an elec-
trochemical gradient and requires energy derived
primary active trans-
port
indirectly to
is referred to as secondary active transport
tion of glucose by the renal tubule is an example of
reabsorbed by active and/or passive mechanisms by
Table 27–1
Urea (g/day)
46.8
23.4
23.4
50
Potassium (mEq/day)
756
664
92
87.8
Chloride (mEq/day)
19,440
19,260
180
99.1
Sodium (mEq/day)
25,560
25,410
150
99.4
Bicarbonate (mEq/day)
4,320
4,318
2
Glucose (g/day)
180
180
0
100
Amount Filtered
Amount Reabsorbed
Amount Excreted
% of Filtered Load Reabsorbed
Filtration, Reabsorption, and Excretion Rates of Different Substances by the Kidneys
>99.9
Creatinine (g/day)
1.8
0
1.8
0
ATP
(diffusion)
FILTRATION
Transcellular
Tubular
Peritubular
capillary
cells
Lumen
Paracellular
path
path
Solutes
H
2
O
EXCRETION
REABSORPTION
Bulk
flow
Blood
Active
Passive
Osmosis
through the cells and between the tubular cells by osmosis. Trans-
the cells (paracellular route) by diffusion. Water is transported
cellular route) by passive diffusion or active transport, or between
back into the blood. Solutes are transported through the cells (trans-
across the tubular epithelial cells, through the renal interstitium, and
Figure 27–1
Reabsorption of filtered water and solutes from the tubular lumen
port of water and solutes from the interstitial fluid into the peri-
tubular capillaries occurs by ultrafiltration (bulk flow).
membrane. As one of the substances (for instance,
In secondary active transport, two or more substances
Secondary Active Reabsorption Through the Tubular Membrane.
osmotic pressure gradients.
peritubular capillaries by ultrafiltration, a passive
3. Sodium, water, and other substances are
by the sodium-potassium ATPase pump.
2. Sodium is transported across the basolateral
basolateral side of the membrane.
by the sodium-potassium ATPase pump on the
1. Sodium diffuses across the luminal membrane
Thus, the net reabsorption of sodium ions from the
as discussed later.
of other substances, such as glucose and amino acids,
the membrane into the cell. These sodium carrier pro-
facilitated diffusion
cell, providing
the surface area about 20-fold. There are also sodium
the cell. In the proximal tubule, there is an extensive
In certain parts of the nephron, there are additional
potassium ATPase occurs in most parts of the tubule.
high (140 mEq/L). (2) The negative,
into the cell, for two reasons: (1) There is a concen-
membrane of the cell, from the tubular lumen
70 millivolts within the cell. This pumping of
cell. The operation of this ion pump maintains low
cell into the interstitium. At the same time, potassium
ATPase system that hydrolyzes ATP and uses the
proximal tubular membrane, as shown in Figure 27–2.
calcium ATPase
, and
potassium ATPase
hydrogen ATPase
potassium ATPase
moves solutes across the cell membranes. The primary
way of membrane-bound ATPase; the ATPase is also
active transport comes from the hydrolysis of ATP by
The energy for this
against an electrochemical gradient.
primary active transport is that it can move solutes
Linked to Hydrolysis of ATP
Primary Active Transport Through the Tubular Membrane Is
between the cells.
ride ions, are carried with the reabsorbed fluid
the water, especially potassium, magnesium, and chlo-
paracellular pathway, and substances dissolved in
proximal tubule, water is also reabsorbed across the
pathway. In some nephron segments, especially the
moves through both routes, although most of the
. Sodium is a substance that
paracellular pathway
pathway
epithelial cells of the tubule. Solutes can be reabsorbed
. Lateral intercellular
Renal tubular cells, like other epithelial cells, are
Solutes Can Be Transported Through Epithelial Cells or Between
, which
Urine Formation by the Kidneys: II. Tubular Processing of the Glomerular Filtrate
Chapter 27
329
(nonactive) physical mechanism called osmosis
means water diffusion from a region of low solute con-
centration (high water concentration) to one of high
solute concentration (low water concentration).
Cells.
held together by tight junctions
spaces lie behind the tight junctions and separate the
or secreted across the cells by way of the transcellular
or between the cells by moving across the
tight junctions and intercellular spaces by way of
the
sodium is transported through the transcellular
. The special importance of
a component of the carrier mechanism that binds and
active transporters that are known include sodium-
,
, hydrogen-
.
A good example of a primary active transport
system is the reabsorption of sodium ions across the
On the basolateral sides of the tubular epithelial cell,
the cell membrane has an extensive sodium-potassium
released energy to transport sodium ions out of the
is transported from the interstitium to the inside of the
intracellular sodium and high intracellular potassium
concentrations and creates a net negative charge of
about
-
sodium out of the cell across the basolateral membrane
of the cell favors passive diffusion of sodium across the
luminal
tration gradient favoring sodium diffusion into the cell
because intracellular sodium concentration is low
(12 mEq/L) and tubular fluid sodium concentration is
-70-millivolt,
intracellular potential attracts the positive sodium ions
from the tubular lumen into the cell.
Active reabsorption of sodium by sodium-
provisions for moving large amounts of sodium into
brush border on the luminal side of the membrane
(the side that faces the tubular lumen) that multiplies
carrier proteins that bind sodium ions on the luminal
surface of the membrane and release them inside the
of sodium through
teins are also important for secondary active transport
tubular lumen back into the blood involves at least
three steps:
(also called the apical membrane) into the cell
down an electrochemical gradient established
membrane against an electrochemical gradient
reabsorbed from the interstitial fluid into the
process driven by the hydrostatic and colloid
interact with a specific membrane protein (a carrier
molecule) and are transported together across the
sodium) diffuses down its electrochemical gradient,
the energy released is used to drive another substance
ATP
ATP
Tubular
Tight junction
Tubular
Peritubular
capillary
epithelial cells
Basement
membrane
Intercellular space
Interstitial
fluid
lumen
(
-
3 mv)
(
-
70 mV)
Brush border
(luminal
membrane)
Basal
channels
Na
+
Na
+
Na
+
K
+
K
+
the tubular lumen into the cell through the brush border.
electrical potential. The low intracellular sodium concentration and
the interior of the cell across the basolateral membrane, creating a
epithelial cell. The sodium-potassium pump transports sodium from
Figure 27–2
Basic mechanism for active transport of sodium through the tubular
low intracellular sodium concentration and a negative intracellular
the negative electrical potential cause sodium ions to diffuse from
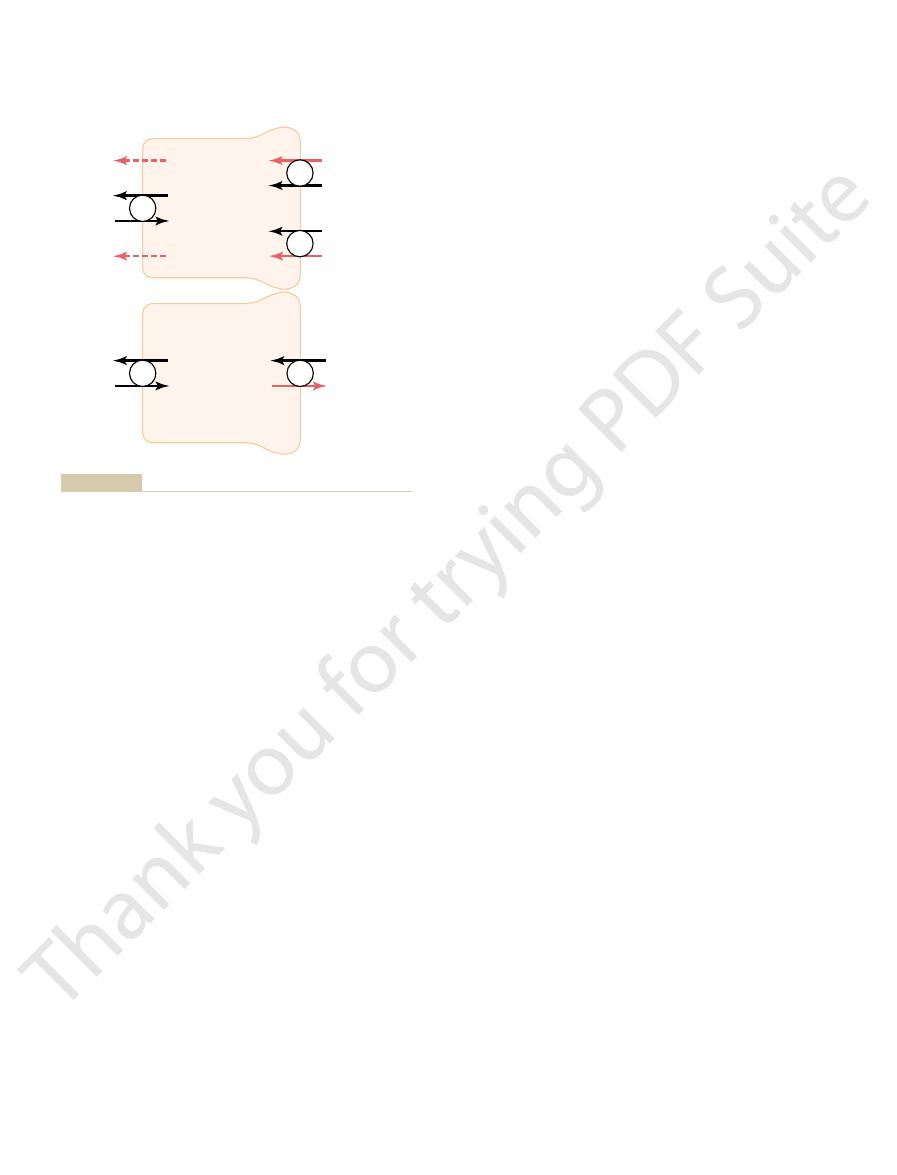
port process.
. This limit is due to saturation
the solute can be transported, often referred to as the
sorbed or secreted, there is a limit to the rate at which
For most substances that are actively reab-
Transport Maximum for Substances That Are Actively Reab-
Because pinocytosis requires energy, it is considered a
stituent amino acids, which are reabsorbed through the
inside the cell, the protein is digested into its con-
and a vesicle is formed containing the protein. Once
attaches to the brush border of the luminal membrane,
. In this process, the protein
proximal tubule, reabsorb large molecules such as
Some parts of the tubule, especially the
Pinocytosis—An Active Transport Mechanism for Reabsorption
in the opposite direction into the tubular lumen. The
interior of the cell, hydrogen ions are forced outward
of the luminal membrane. As sodium is carried to the
by sodium-hydrogen counter-transport. This transport
the proximal tubule. In this case, sodium entry into the
27–3, is the active secretion of hydrogen ions coupled
One example of counter-transport, shown in Figure
movement of one of the substances (for example,
transport, the energy liberated from the downhill
of the substance with sodium ions. In counter-
counter-transport
active transport. This often involves
Secondary Active Secretion into the Tubules.
capillaries.
occurs at the basolateral membrane, and passive
luminal membrane, but passive facilitated diffusion
reabsorption, secondary active transport occurs at the
the reabsorption process may be passive. For glucose
ary active transport, even though other steps in
to undergo “active” transport when at least one of the
gradient, but it is “secondary” to primary active trans-
is referred to as “secondary active transport” because
luminal membrane. Thus, this reabsorption of glucose
maintained, and it is this downhill diffusion of sodium
this pump, an electrochemical gradient for facilitated
basolateral membrane. Because of the activity of
primary active sodium-potassium ATPase pump in the
of glucose depends on energy expended by the
gradient does not directly use ATP, the reabsorption
tated diffusion, driven by the high glucose and amino
lumen. After entry into the cell, glucose and amino
or a glucose molecule at the same time. These trans-
both instances, a specific carrier protein in the brush
glucose and amino acids in the proximal tubule. In
Figure 27–3 shows secondary active transport of
energy phosphate sources. Rather, the direct source of
require energy directly from ATP or from other high-
gradient. Thus, secondary active transport does not
(for instance, glucose) against its electrochemical
330
Unit V
The Body Fluids and Kidneys
the energy is that liberated by the simultaneous facil-
itated diffusion of another transported substance
down its own electrochemical gradient.
border combines with a sodium ion and an amino acid
port mechanisms are so efficient that they remove vir-
tually all the glucose and amino acids from the tubular
acids exit across the basolateral membranes by facili-
acid concentrations in the cell.
Although transport of glucose against a chemical
diffusion of sodium across the luminal membrane is
to the interior of the cell that provides the energy for
the simultaneous uphill transport of glucose across the
glucose itself is reabsorbed uphill against a chemical
port of sodium.
Another important point is that a substance is said
steps in the reabsorption involves primary or second-
uptake by bulk flow occurs at the peritubular
Some sub-
stances are secreted into the tubules by secondary
sodium ions) enables uphill movement of a second
substance in the opposite direction.
to sodium reabsorption in the luminal membrane of
cell is coupled with hydrogen extrusion from the cell
is mediated by a specific protein in the brush border
basic principles of primary and secondary active trans-
port are discussed in additional detail in Chapter 4.
of Proteins.
proteins by pinocytosis
and this portion of the membrane then invaginates to
the interior of the cell until it is completely pinched off
basolateral membrane into the interstitial fluid.
form of active transport.
sorbed.
transport maximum
of the specific transport systems involved when the
amount of solute delivered to the tubule (referred to
as tubular load) exceeds the capacity of the carrier
proteins and specific enzymes involved in the trans-
Amino acids
Amino acids
ATP
ATP
Tubular
Tubular
cells
-
70 mV
Co-transport
Interstitial
fluid
lumen
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
K
+
K
+
-
70 mV
Counter-transport
Na
+
Na
+
K
+
K
+
Na
+
Na
+
H
+
H
+
Glucose
Glucose
membrane, provides the energy for transport of the hydrogen ions
movement of sodium ions into the cell, down an electrochemical gra-
counter-transport
facilitated diffusion through the basolateral membranes. The lower
through the apical side of the tubular epithelial cells, followed by
Mechanisms of secondary active transport. The upper cell shows the
Figure 27–3
co-transport of glucose and amino acids along with sodium ions
cell shows the
of hydrogen ions from the interior
of the cell across the apical membrane and into the tubular lumen;
dient established by the sodium-potassium pump on the basolateral
from inside the cell into the tubular lumen.
resulting in urinary glucose excretion. Some of the
glucose may rise to high levels, causing the filtered load
diabetes mellitus,
However, in uncontrolled
glucose in the urine, even after eating a meal.
The plasma glucose of a healthy person almost
sorb glucose.
nephrons have reached their maximal capacity to reab-
is normally about 375 mg/min, is reached when all
The overall transport maximum for the kidneys, which
glucose, and some of the nephrons excrete glucose
. One reason for the difference between
reached
for glucose.
This point is termed the
small amount of glucose begins to appear in the urine.
increasing the filtered load to about 250 mg/min, a
glucose in the urine. However, when the plasma con-
is at its normal level, 125 mg/min, there is no loss of
loss in the urine. Note that when the plasma glucose
transport maximum for glucose, and rate of glucose
centration of glucose, filtered load of glucose, tubular
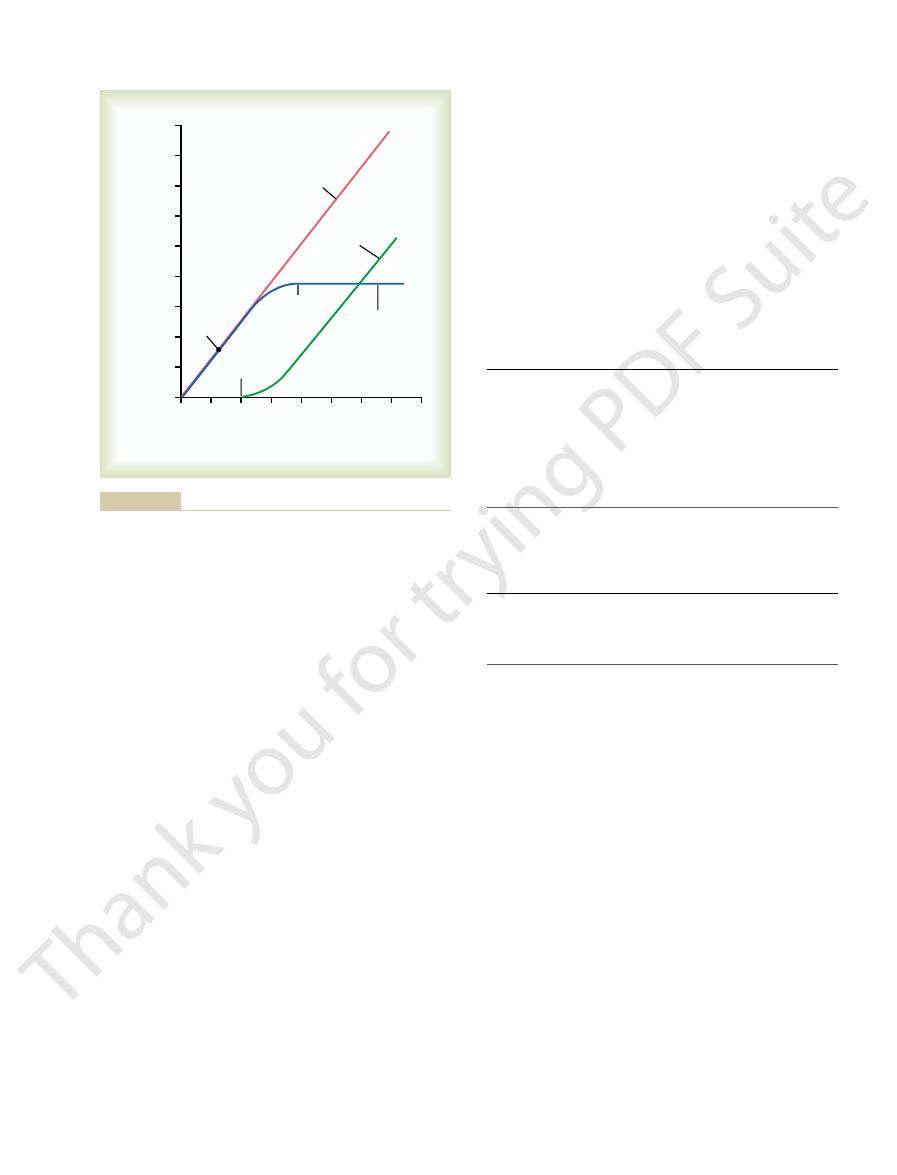
Figure 27–4 shows the relation between plasma con-
passes into the urine.
min, the excess glucose filtered is not reabsorbed and
1 mg/ml). With large
glucose averages about 375 mg/min, whereas the fil-
In the adult human, the transport maximum for
tion of glucose does occur.
ity of the tubules to reabsorb glucose, urinary excre-
However, when the filtered load exceeds the capabil-
tered glucose is reabsorbed in the proximal tubule.
is a good example. Normally, measurable glucose does
The glucose transport system in the proximal tubule
Urine Formation by the Kidneys: II. Tubular Processing of the Glomerular Filtrate
Chapter 27
331
not appear in the urine because essentially all the fil-
tered load of glucose is only about 125 mg/min (GFR
¥ plasma glucose = 125 ml/min ¥
increases in GFR and/or plasma glucose concentration
that increase the filtered load of glucose above 375 mg/
concentration is 100 mg/100 mL and the filtered load
centration of glucose rises above about 200 mg/100 ml,
threshold
Note
that this appearance of glucose in the urine (at the
threshold) occurs before the transport maximum is
threshold and transport maximum is that not all
nephrons have the same transport maximum for
before others have reached their transport maximum.
never becomes high enough to cause excretion of
plasma
of glucose to exceed the transport maximum and
important transport maximums for substances actively
reabsorbed by the tubules are as follows:
Lactate
75 mg/min
Urate
15 mg/min
Amino acids
1.5 mM/min
Sulfate
0.06 mM/min
Phosphate
0.10 mM/min
Glucose
375 mg/min
Substance
Transport Maximum
Transport Maximums for Substances That Are Actively
Plasma protein
30 mg/min
Secreted.
Substances that are actively secreted also
exhibit transport maximums as follows:
Creatinine
16 mg/min
Substance
Transport Maximum
the basolateral sodium-potassium ATPase pump is
proximal tubules, the maximum transport capacity of
maximum rate of active transport. For example, in the
sodium reabsorption in the proximal tubule. The
. An example is
Some actively transported substances also have char-
in turn depends on the tubular flow rate.
and the time that the substance is in the tubule, which
remains within the tubule. Transport of this type is
the permeability of the membrane for the substance,
diffusion of the substance across the membrane, (2)
factors, such as (1) the electrochemical gradient for
the tubular load increases.
The reason that actively trans-
Transport Maximum.
Substances That Are Actively Transported but Do Not Exhibit a
Para-aminohippuric acid
80 mg/min
ported solutes often exhibit a transport maximum is
that the transport carrier system becomes saturated as
Substances that are passively
reabsorbed do not demonstrate a transport maximum
because their rate of transport is determined by other
and (3) the time that the fluid containing the substance
referred to as gradient-time transport because the rate
of transport depends on the electrochemical gradient
acteristics of gradient-time transport
main reason that sodium transport in the proximal
tubule does not exhibit a transport maximum is that
other factors limit the reabsorption rate besides the
usually far greater than the actual rate of net sodium
Transport
0
800
Glucose filtered load, reabsorption
or excretion (mg/min)
Plasma glucose concentration
(mg/100 ml)
700
600
500
400
300
200
100
Filtered
load
Filtered
load
900
800
700
600
500
400
300
200
100
Normal
Threshold
maximum
Reabsorption
Excretion
0
begins to be excreted in the urine.
glucose can be reabsorbed from the tubules. The
in the urine. The
reabsorption by the renal tubules, and the rate of glucose excretion
Relations among the filtered load of glucose, the rate of glucose
Figure 27–4
transport maximum is the maximum rate at which
threshold for
glucose refers to the filtered load of glucose at which glucose first
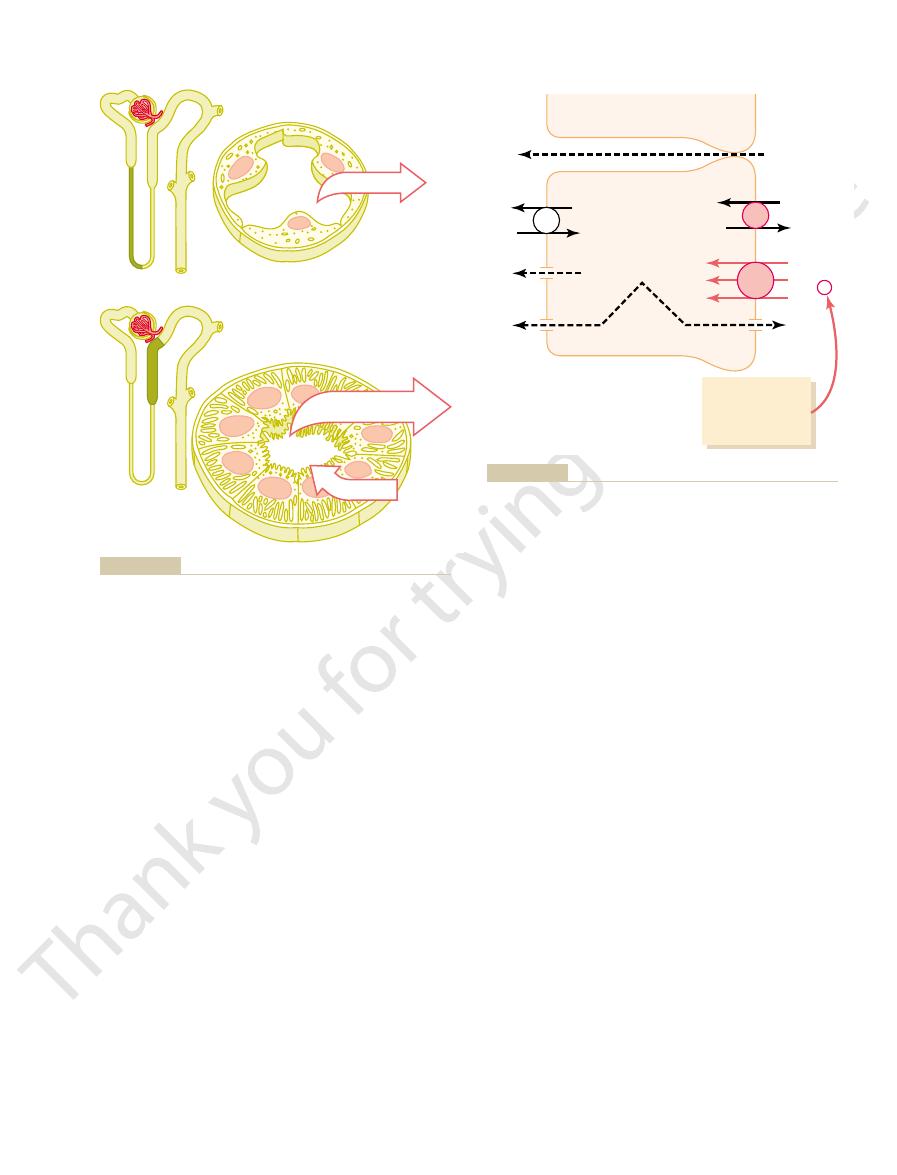
(Figure 27–5). Thus, the active reabsorption of sodium
reabsorbed from the tubule by osmosis, thereby con-
. Additional reab-
paracellular pathway
fluid. This causes chloride ions to diffuse
negatively charged, compared with the interstitial
tials. That is, transport of positively charged sodium
epithelial cell, negative ions such as chloride are trans-
When sodium is reabsorbed through the tubular
Reabsorption of Chloride, Urea, and
low, depending on the presence or absence of ADH.
lecting tubules, and collecting ducts—can be high or
the last parts of the tubules—the distal tubules, col-
despite a large osmotic gradient. Water permeability in
always low, so that almost no water is reabsorbed,
the ascending loop of Henle, water permeability is
and water is reabsorbed as rapidly as the solutes. In
proximal tubule, the water permeability is always high,
water, no matter how large the osmotic gradient. In the
Thus, water movement across the tubular epithe-
distal and collecting tubules, as discussed later.
membrane by osmosis. However, antidiuretic hormone
Therefore, water cannot move easily across the tubular
also have a greatly decreased membrane surface area.
meable to water and solutes, and the epithelial cells
lecting tubule, the tight junctions become far less per-
In the more distal parts of the nephron, beginning
of water and many other solutes.
coupled to sodium reabsorption, changes in sodium
reabsorption of water, organic solutes, and ions is
. And because the
osmosis, it can also carry with it some of the solutes, a
calcium, and magnesium.
most ions, such as sodium, chloride, potassium,
proximal tubules, which have a high permeability for
of water and small ions. This is especially true in the
name would imply, and they allow significant diffusion
The reason for this, as already discussed, is that the
epithelial cells as well as through the cells themselves.
tubular membrane.
proximal tubule, are highly permeable to water, and
tium. Some parts of the renal tubule, especially the
ported, from the tubular lumen to the renal intersti-
while increasing in the renal interstitium. This creates
either primary or secondary active transport, their
When solutes are transported out of the tubule by
to Sodium Reabsorption
by Osmosis Is Coupled Mainly
Passive Water Reabsorption
in response to certain hormones, such as
Furthermore, this transport maximum can be increased
similar to that for other actively transported substances.
much smaller amounts of sodium. In these segments,
In the more distal parts of the nephron, the epithe-
the proximal tubules.
slower the flow rate of tubular fluid, the greater the
mal tubules, the greater its reabsorption rate. Also, the
maximum transport characteristics. This means that
the peritubular capillaries. Therefore, sodium trans-
interstitial physical forces, which determine the rate of
leak occurs depends on several factors, including (1)
epithelial tight junctions. The rate at which this back-
reabsorption. One of the reasons for this is that a sig-
332
Unit V
The Body Fluids and Kidneys
nificant amount of sodium transported out of the
cell leaks back into the tubular lumen through the
the permeability of the tight junctions and (2) the
bulk flow reabsorption from the interstitial fluid into
port in the proximal tubules obeys mainly gradient-
time transport principles rather than tubular
the greater the concentration of sodium in the proxi-
percentage of sodium that can be reabsorbed from
lial cells have much tighter junctions and transport
sodium reabsorption exhibits a transport maximum
aldosterone.
concentrations tend to decrease inside the tubule
a concentration difference that causes osmosis of
water in the same direction that the solutes are trans-
water reabsorption occurs so rapidly that there is only
a small concentration gradient for solutes across the
A large part of the osmotic flow of water occurs
through the so-called tight junctions between the
junctions between the cells are not as tight as their
water and a smaller but significant permeability to
As water moves across the tight junctions by
process referred to as solvent drag
reabsorption significantly influence the reabsorption
in the loop of Henle and extending through the col-
(ADH) greatly increases the water permeability in the
lium can occur only if the membrane is permeable to
Other Solutes by Passive Diffusion
ported along with sodium because of electrical poten-
ions out of the lumen leaves the inside of the lumen
passively
through the
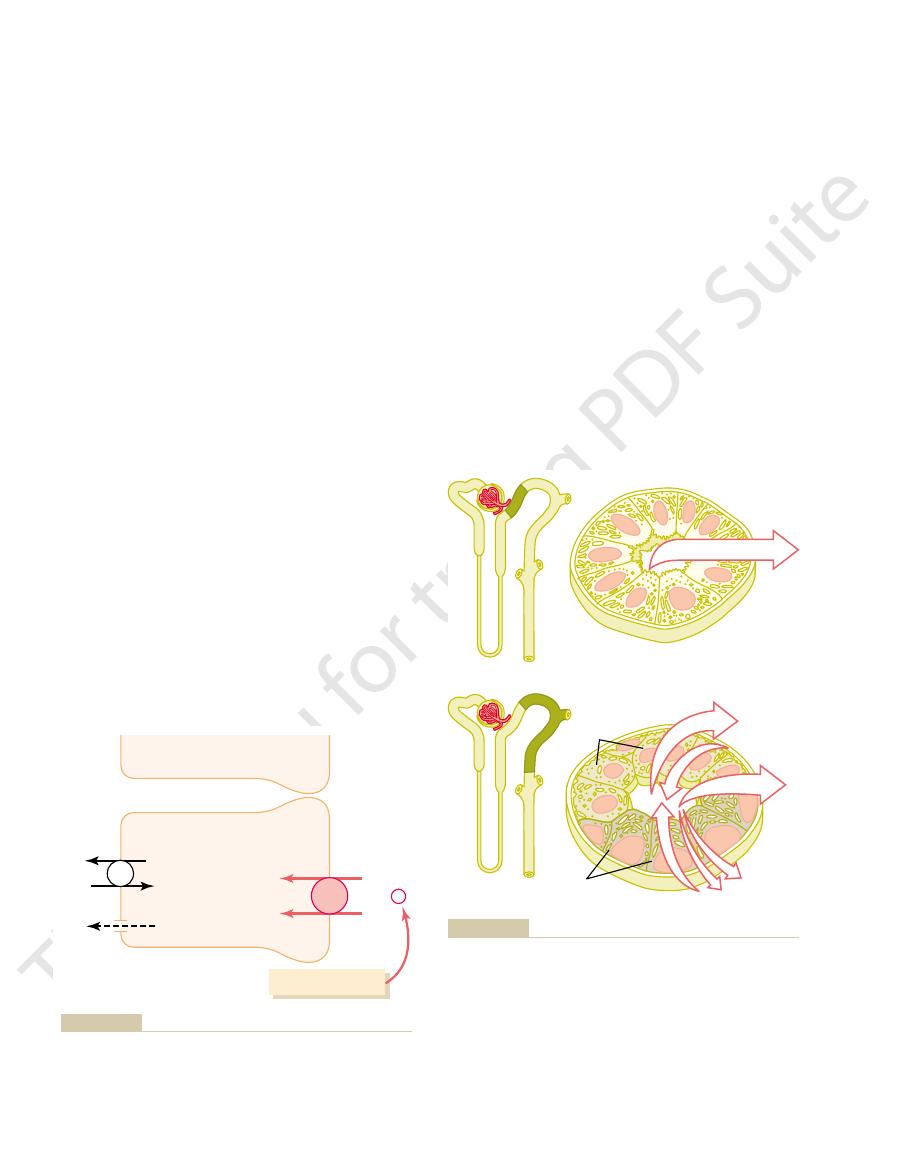
sorption of chloride ions occurs because of a chloride
concentration gradient that develops when water is
centrating the chloride ions in the tubular lumen
is closely coupled to the passive reabsorption of chlo-
ride by way of an electrical potential and a chloride
concentration gradient.
Passive Cl
-
reabsorption
Passive urea
reabsorption
Na
+
reabsorption
H
2
O reabsorption
Lumen
negative
potential
Lumen
negative
potential
Luminal Cl
-
concentration
Luminal Cl
-
concentration
Luminal
urea
concentration
Luminal
urea
concentration
Mechanisms by which water, chloride, and urea reabsorption are
Figure 27–5
coupled with sodium reabsorption.

chloride (around 140 mEq/L) compared with the early
mainly with chloride ions. The second half of the prox-
to be reabsorbed. Instead, sodium is now reabsorbed
proximal tubule, little glucose and amino acids remain
acids, and other solutes. But in the second half of the
reabsorbed by co-transport along with glucose, amino
In the first half of the proximal tubule, sodium is
proximal tubular membrane.
chloride, and water throughout the proximal tubule,
Although the sodium-potassium ATPase pump pro-
, which then dissociates
to form H
in Chapter 30, the secretion of hydrogen ions into the
tubular lumen, especially hydrogen ions. As discussed
mechanisms, which reabsorb
counter-transport
such as amino acids and glucose. The remainder of the
The extensive membrane surface of the epithelial
other substances.
extensive labyrinth of intercellular and basal channels,
active transport processes. In addition, the proximal
acteristics, as shown in Figure 27–6. The proximal
The high capacity of the proximal tubule
Proximal Tubules Have a High Capacity for Active and Passive
different physiologic conditions, as discussed later.
These percentages can be increased or decreased in
tubule before the filtrate reaches the loops of Henle.
Normally, about 65 per cent of the filtered load of
Proximal Tubular Reabsorption
subsequent chapters, we discuss the reabsorption and
to the reabsorption of sodium, chloride, and water. In
most important are discussed, especially as they relate
perform their specific excretory functions. Only the
mind, we can now discuss the different characteristics
the tubular membrane. With these generalizations in
In the previous sections, we discussed the basic princi-
the Nephron
Along Different Parts of
Reabsorption and Secretion
the glomerulus is excreted in the urine.
sorbed, so that virtually all the creatinine filtered by
impermeant to the tubular membrane. Therefore,
Another waste product of metabolism, creatinine, is
passes into the urine, allowing the kidneys to excrete
sorbed from the tubules. The remainder of the urea
. Yet only about one half of the urea
duct, passive urea reabsorption is facilitated by specific
nephron, especially the inner medullary collecting
the tubule as readily as water. In some parts of the
sorption of urea. However, urea does not permeate
in the tubular lumen increases (see Figure 27–5). This
coupled to sodium reabsorption), urea concentration
but to a much lesser extent than chloride ions. As
Urea is also passively reabsorbed from the tubule,
the luminal membrane.
active transport. The most important of the secondary
Urine Formation by the Kidneys: II. Tubular Processing of the Glomerular Filtrate
Chapter 27
333
Chloride ions can also be reabsorbed by secondary
active transport processes for chloride reabsorption
involves co-transport of chloride with sodium across
water is reabsorbed from the tubules (by osmosis
creates a concentration gradient favoring the reab-
urea transporters
that is filtered by the glomerular capillaries is reab-
large amounts of this waste product of metabolism.
an even larger molecule than urea and is essentially
almost none of the creatinine that is filtered is reab-
ples by which water and solutes are transported across
of the individual tubular segments that enable them to
tubular transport functions that are quantitatively
secretion of other specific substances in different parts
of the tubular system.
sodium and water and a slightly lower percentage of
filtered chloride are reabsorbed by the proximal
Reabsorption.
for reabsorption results from its special cellular char-
tubule epithelial cells are highly metabolic and have
large numbers of mitochondria to support potent
tubular cells have an extensive brush border on the
luminal (apical) side of the membrane as well as an
all of which together provide an extensive membrane
surface area on the luminal and basolateral sides of the
epithelium for rapid transport of sodium ions and
brush border is also loaded with protein carrier mole-
cules that transport a large fraction of the sodium ions
across the luminal membrane linked by way of the co-
transport mechanism with multiple organic nutrients
sodium is transported from the tubular lumen into the
cell by
sodium while secreting other substances into the
tubular lumen is an important step in the removal of
bicarbonate ions from the tubule (by combining H
+
with the HCO
3
_
2
CO
3
into H
2
O and CO
2
).
vides the major force for reabsorption of sodium,
there are some differences in the mechanisms by
which sodium and chloride are transported through
the luminal side of the early and late portions of the
imal tubule has a relatively high concentration of
Proximal tubule
65%
Isosmotic
H
+
, organic acids, bases
Na
+
, Cl
-
, HCO
3
-
, K
+
,
H
2
O, glucose, amino acids
tubules also secrete organic acids, bases, and hydrogen ions into the
essentially all the filtered glucose and amino acids. The proximal
of the filtered sodium, chloride, bicarbonate, and potassium and
proximal tubule. The proximal tubules reabsorb about 65 per cent
Figure 27–6
Cellular ultrastructure and primary transport characteristics of the
tubular lumen.

The thin segment of the ascending limb has a much
also reabsorbed in the thick ascending loop of Henle.
ions, such as calcium, bicarbonate, and magnesium, are
thick ascending limb. Considerable amounts of other
are reabsorbed in the loop of Henle, mostly in the
the filtered loads of sodium, chloride, and potassium
and potassium (see Figure 27–8). About 25 per cent of
are capable of active reabsorption of sodium, chloride,
begins about halfway up the ascending limb, has thick
The thick segment of the loop of Henle, which
trating the urine.
water, a characteristic that is important for concen-
and the thick portions, is virtually impermeable to
ing limb. The ascending limb, including both the thin
Henle, and almost all of this occurs in the thin descend-
fusion of substances through its walls. About 20 per
most solutes, including urea and sodium. The function
The descending part of the thin segment is highly
levels of metabolic activity (Figure 27–8).
with no brush borders, few mitochondria, and minimal
their names imply, have thin epithelial membranes
The thin descending and thin ascending segments, as
thick ascending segment
, and the
, the
tinct segments: the
The loop of Henle consists of three functionally dis-
Loop of Henle
Solute and Water Transport in the
cussed later.
can be used to estimate the renal plasma flow, as dis-
the urine. For this reason, the rate of PAH clearance
can clear about 90 per cent of the PAH from the
PAH is secreted so rapidly that the average person
proximal tubule is para-aminohippuric acid (PAH).
and salicylates, the rapid clearance by the kidneys
blood. In the case of certain drugs, such as penicillin
In addition to the waste products of metabolism, the
the urine.
tubules, all combined, contribute to rapid excretion in
be rapidly removed from the body. The
. Many of these sub-
catecholamines
, and
oxalate
The proximal tubule is also an important site for secre-
Secretion of Organic Acids and Bases by the Proximal Tubule.
meability of this part of the nephron to water.
osmolarity, remains essentially the same all along the
tubule. The total solute concentration, as reflected by
nine, increase their concentration along the proximal
permeant and not actively reabsorbed, such as creati-
the proximal tubule. Other organic solutes that are less
much more avidly reabsorbed than water, so that their
such as glucose, amino acids, and bicarbonate, are
with sodium reabsorption. Certain organic solutes,
markedly along the proximal tubule, the
various solutes along the proximal tubule. Although
Figure
Concentrations of Solutes Along the Proximal Tubule.
the proximal tubule, the higher chloride concentration
higher concentration of chloride. In the second half of
proximal tubule, leaving behind a solution that has a
glucose, bicarbonate, and organic ions in the early
sodium is reabsorbed, it preferentially carries with it
proximal tubule (about 105 mEq/L) because when
334
Unit V
The Body Fluids and Kidneys
favors the diffusion of this ion from the tubule lumen
through the intercellular junctions into the renal inter-
stitial fluid.
27–7 summarizes the changes in concentrations of
the amount of sodium in the tubular fluid decreases
concentration
of sodium (and the total osmolarity) remains relatively
constant because water permeability of the proximal
tubules is so great that water reabsorption keeps pace
concentrations decrease markedly along the length of
proximal tubule because of the extremely high per-
tion of organic acids and bases such as bile salts,
, urate
stances are the end products of metabolism and must
secretion of
these substances into the proximal tubule plus filtra-
tion into the proximal tubule by the glomerular capil-
laries and the almost total lack of reabsorption by the
kidneys secrete many potentially harmful drugs or
toxins directly through the tubular cells into the
tubules and rapidly clear these substances from the
creates a problem in maintaining a therapeutically
effective drug concentration.
Another compound that is rapidly secreted by the
plasma flowing through the kidneys and excrete it in
thin descending segment
thin
ascending segment
.
permeable to water and moderately permeable to
of this nephron segment is mainly to allow simple dif-
cent of the filtered water is reabsorbed in the loop of
epithelial cells that have high metabolic activity and
lower reabsorptive capacity than the thick segment,
40
60
80
100
T
ubular fluid / plasma concentration
% Total proximal tubule length
20
0
Glucose
Amino acids
Osmolality
Urea
Creatinine
HCO
3
-
Cl
-
Na
+
5.0
2.0
1.0
0.5
0.05
0.2
0.1
0.01
sorbed to a lesser extent than water or is secreted into the tubules.
water, whereas values above 1.0 indicate that the substance is reab-
tubular fluid is the same as the concentration in the plasma. Values
of these substances in the plasma and in the glomerular filtrate. A
Figure 27–7
Changes in concentrations of different substances in tubular fluid
along the proximal convoluted tubule relative to the concentrations
value of 1.0 indicates that the concentration of the substance in the
below 1.0 indicate that the substance is reabsorbed more avidly than
water delivered to this segment remains in the tubule,
. Therefore, most of the
The thick segment of the ascending loop of Henle is
hydrogen counter-transport mechanism in its luminal
The thick ascending limb also has a sodium-
8 millivolts in the tubular lumen. This positive
sium ions into the lumen, creating a positive charge of
anions into the cell, there is a slight backleak of potas-
Although the 1-sodium, 2-chloride, 1-potassium co-
, in the thick
, and K
, Na
, Ca
cations, such as Mg
There is also significant paracellular reabsorption of
potassium co-transporter. These diuretics are dis-
which inhibit the action of the sodium 2-chloride,
, all of
, and
furosemide, ethacrynic acid
the site of action of the powerful “
The thick ascending limb of the loop of Henle is
(Figure 27–9). This co-transport protein carrier in the
2-chloride
the luminal membrane is mediated primarily by a
movement of sodium across
thick ascending loop
tion. The low intracellular sodium concentration in
capability of the sodium-potassium ATPase pump,
branes. As in the proximal tubule, the reabsorption of
ATPase pump in the epithelial cell basolateral mem-
nificant amounts of any of these solutes.
Urine Formation by the Kidneys: II. Tubular Processing of the Glomerular Filtrate
Chapter 27
335
and the thin descending limb does not reabsorb sig-
An important component of solute reabsorption in
the thick ascending limb is the sodium-potassium
other solutes in the thick segment of the ascending
loop of Henle is closely linked to the reabsorptive
which maintains a low intracellular sodium concentra-
turn provides a favorable gradient for movement
of sodium from the tubular fluid into the cell. In the
,
1-sodium,
,
1-potassium co-transporter
luminal membrane uses the potential energy released
by downhill diffusion of sodium into the cell to drive
the reabsorption of potassium into the cell against a
concentration gradient.
loop” diuretics
bumetanide
cussed in Chapter 31.
++
++
+
+
ascending limb owing to the slight positive charge of
the tubular lumen relative to the interstitial fluid.
transporter moves equal amounts of cations and
about
+
charge forces cations such as Mg
++
and Ca
++
to diffuse
from the tubular lumen through the paracellular space
and into the interstitial fluid.
cell membrane that mediates sodium reabsorption and
hydrogen secretion in this segment.
virtually impermeable to water
Thick ascending
loop of Henle
25%
Hypo-
osmotic
H
+
Na
+
, Cl
-
, K
+
,
Ca
++
, HCO
3
-
, Mg
++
Thin descending
loop of Henle
H
2
O
and magnesium. This segment also secretes hydrogen ions into the
and potassium, as well as large amounts of calcium, bicarbonate,
reabsorbs about 25 per cent of the filtered loads of sodium, chloride,
active reabsorption. The thick ascending limb of the loop of Henle
). The descending part of the thin segment
Figure 27–8
Cellular ultrastructure and transport characteristics of the thin
descending loop of Henle (top) and the thick ascending segment of
the loop of Henle (bottom
of the loop of Henle is highly permeable to water and moderately
permeable to most solutes but has few mitochondria and little or no
tubular lumen.
ATP
diffusion
Tubular
Tubular
Paracellular
Renal
interstitial
fluid
lumen
(
+
8 mV)
cells
Mg
++
, Ca
++
Mg
++
, Ca
++
Na
+
, K
+
Na
+
, K
+
Na
+
Na
+
Cl
-
Cl
-
K
+
K
+
K
+
K
+
Na
+
Na
+
H
+
H
+
Loop diuretics
• Furosemide
• Ethacrynic acid
• Bumetanide
-
-
Na
+
Na
+
2Cl
-
2Cl
-
K
+
K
+
from the lumen to the interstitial fluid via the paracellular pathway.
port. The positive charge (
transported into the tubular cell by sodium-hydrogen counter-trans-
down an electrochemical gradient into the cells. Sodium is also
the cells, using the potential energy released by diffusion of sodium
The 1-sodium, 2-chloride, 1-potassium co-transporter in the luminal
thick ascending loop of Henle. The sodium-potassium ATPase pump
Mechanisms of sodium, chloride, and potassium transport in the
Figure 27–9
in the basolateral cell membrane maintains a low intracellular
sodium concentration and a negative electrical potential in the cell.
membrane transports these three ions from the tubular lumen into
+8 mV) of the tubular lumen relative to
the interstitial fluid forces cations such as Mg
++
and Ca
++
to diffuse
sodium concentration inside the cell and, therefore,
membrane (Figure 27–12). This pump maintains a low
potassium ATPase pump in each cell’s basolateral
potassium ions into the lumen. The intercalated cells
(Figure 27–11). The principal cells reab-
two distinct cell types, the
characteristics. Anatomically, they are composed of
The second half of the distal tubule and the subse-
Collecting Tubule
Late Distal Tubule and Cortical
chloride co-transporter.
hypertension and heart failure, inhibit the sodium-
, which are widely used to treat disorders such as
nels in the basolateral membrane. The
brane (Figure 27–10). Chloride diffuses out of the cell
and the sodium-potassium ATPase pump transports
tubule. The sodium-chloride co-transporter moves
tually impermeable to water and urea. For this reason,
including sodium, potassium, and chloride, but is vir-
Henle. That is, it avidly reabsorbs most of the ions,
GFR and blood flow in this same nephron. The next
. The very first
The thick segment of the ascending limb of the loop
Distal Tubule
trate the urine under different conditions, as we
as it flows toward the distal tubule, a feature that is
despite reabsorption of large amounts of solute. The
336
Unit V
The Body Fluids and Kidneys
tubular fluid in the ascending limb becomes very dilute
important in allowing the kidneys to dilute or concen-
discuss much more fully in Chapter 28.
of Henle empties into the distal tubule
portion of the distal tubule forms part of the juxta-
glomerular complex that provides feedback control of
part of the distal tubule is highly convoluted and has
many of the same reabsorptive characteristics of the
thick segment of the ascending limb of the loop of
it is referred to as the diluting segment because it also
dilutes the tubular fluid.
Approximately 5 percent of the filtered load of
sodium chloride is reabsorbed in the early distal
sodium chloride from the tubular lumen into the cell,
sodium out of the cell across the basolateral mem-
into the renal interstitial fluid through chloride chan-
thiazide diuret-
ics
quent cortical collecting tubule have similar functional
principal cells and the inter-
calated cells
sorb sodium and water from the lumen and secrete
reabsorb potassium ions and secrete hydrogen ions
into the tubular lumen.
Principal Cells Reabsorb Sodium and Secrete Potassium.
Sodium reabsorption and potassium secretion by the
principal cells depend on the activity of a sodium-
favors sodium diffusion into the cell through special
ATP
Tubular
Tubular
Renal
interstitial
fluid
lumen
(
-
10mV)
cells
Na
+
Na
+
Cl
-
Cl
-
K
+
K
+
Thiazide diuretics:
Thiazide diuretics:
-
-
Na
+
Na
+
Cl
-
Cl
-
chloride diffuses into the interstitial fluid via chloride channels.
Sodium is pumped out of the cell by sodium-potassium ATPase and
the cell by a co-transporter that is inhibited by thiazide diuretics.
Mechanism of sodium chloride transport in the early distal tubule.
Figure 27–10
Sodium and chloride are transported from the tubular lumen into
Intercalated
cells
Early distal tubule
Late distal tubule
and collecting tubule
Principal
cells
Na
+
, Cl
-
, Ca
++
, Mg
++
Na
+
, Cl
-
(
+
ADH) H
2
O
HCO
3
-
H
+
K
+
K
+
ions into the lumen. The reabsorption of water from this tubular
potassium ions into the lumen. The intercalated cells reabsorb potas-
The principal cells reabsorb sodium from the lumen and secrete
two distinct cell types, the
and magnesium but is virtually impermeable to water and urea. The
ascending loop of Henle and reabsorbs sodium, chloride, calcium,
distal tubule and the late distal tubule and collecting tubule. The
Figure 27–11
Cellular ultrastructure and transport characteristics of the early
early distal tubule has many of the same characteristics as the thick
late distal tubules and cortical collecting tubules are composed of
principal cells and the intercalated cells.
sium and bicarbonate ions from the lumen and secrete hydrogen
segment is controlled by the concentration of antidiuretic hormone.
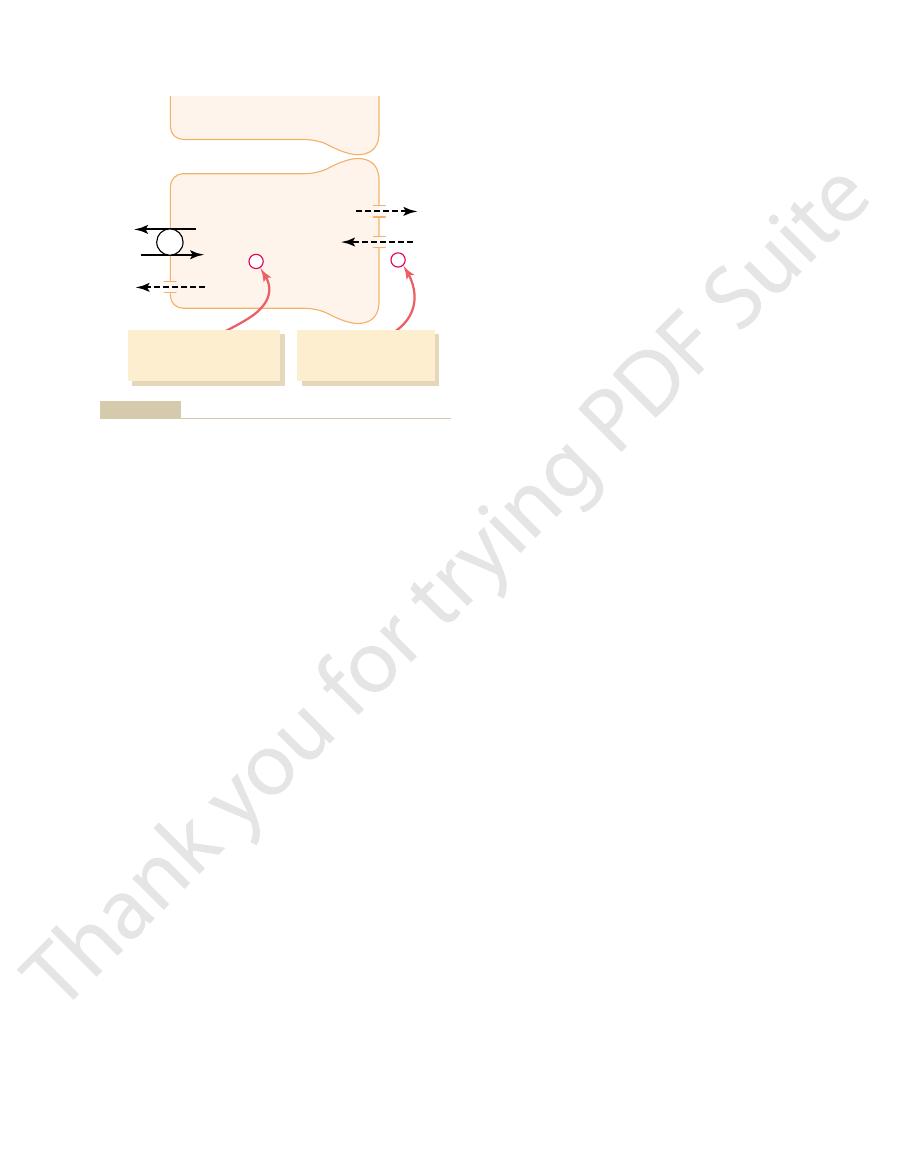
The epithelial cells of the collecting ducts are nearly
the final urine output of water and solutes.
fore, play an extremely important role in determining
are the final site for processing the urine and, there-
than 10 per cent of the filtered water and sodium, they
Medullary Collecting Duct
urine.
impermeable to water. This special characteristic
in the absence of ADH, they are virtually
tubular segments are permeable to water, but
. With high levels of ADH, these
, which is also called
4. The permeability of the late distal tubule and
in acid-base regulation of the body fluids.
tubule. Thus, the intercalated cells play a key role
concentration gradient, as much as 1000 to 1. This
hydrogen-ATPase mechanism. This process is
3. The
fluids.
tubular lumen, a process that is also controlled
time, these segments secrete potassium ions
hormones, especially aldosterone. At the same
collecting tubule segments reabsorb sodium ions,
2. Both the late distal tubule and the cortical
in the medullary collecting ducts.
collecting duct to be excreted in the urine,
thus, almost all the urea that enters these
almost completely impermeable to urea, similar
1. The tubular membranes of both segments are
The functional characteristics of the
ions.
The intercalated cells can also reabsorb potassium
across the basolateral membrane. A more detailed dis-
tubular lumen, and for each hydrogen ion secreted, a
ions. The hydrogen ions are then secreted into the
water and carbon dioxide to form carbonic acid, which
ATPase transport mechanism. Hydrogen is generated
Intercalated Cells Avidly Secrete Hydrogen and Reabsorb Bicar-
potassium-sparing diuretics.
tubular fluid. For this reason the sodium channel
the sodium-potassium ATPase pump. This, in turn,
sodium reabsorption and potassium secretion. Sodium
lactone, eplerenone, amiloride, and triamterene.
, including spirono-
potassium-sparing diuretics
The principal cells are the primary sites of action
(2) once in the cell, potassium diffuses down its con-
high intracellular potassium concentration, and then
sodium-potassium ATPase pump, which maintains a
steps: (1) Potassium enters the cell because of the
channels. The secretion of potassium by these cells
Urine Formation by the Kidneys: II. Tubular Processing of the Glomerular Filtrate
Chapter 27
337
from the blood into the tubular lumen involves two
centration gradient across the luminal membrane into
the tubular fluid.
of the
Aldosterone antagonists compete with aldosterone for
receptor sites in the principal cells and therefore
inhibit the stimulatory effects of aldosterone on
channel blockers directly inhibit the entry of sodium
into the sodium channels of the luminal membranes
and therefore reduce the amount of sodium that can
be transported across the basolateral membranes by
decreases transport of potassium into the cells and
ultimately reduces potassium secretion into the
blockers as well as the aldosterone antagonists
decrease urinary excretion of potassium and act as
bonate and Potassium Ions.
Hydrogen ion secretion by
the intercalated cells is mediated by a hydrogen-
in this cell by the action of carbonic anhydrase on
then dissociates into hydrogen ions and bicarbonate
bicarbonate ion becomes available for reabsorption
cussion of this mechanism is presented in Chapter 30.
late distal tubule
and cortical collecting tubule can be summarized as
follows:
to the diluting segment of the early distal tubule;
segments passes on through and into the
although some reabsorption of urea occurs
and the rate of reabsorption is controlled by
from the peritubular capillary blood into the
by aldosterone and by other factors such as
the concentration of potassium ions in the body
intercalated cells of these nephron segments
avidly secrete hydrogen ions by an active
different from the secondary active secretion of
hydrogen ions by the proximal tubule because it is
capable of secreting hydrogen ions against a large
is in contrast to the relatively small gradient (4- to
10-fold) for hydrogen ions that can be achieved
by secondary active secretion in the proximal
cortical collecting duct to water is controlled by
the concentration of ADH
vasopressin
provides an important mechanism for controlling
the degree of dilution or concentration of the
Although the medullary collecting ducts reabsorb less
cuboidal in shape with smooth surfaces and relatively
Triamterene
Amiloride
ATP
Tubular
Tubular
Renal
interstitial
fluid
lumen
(
-
50 mV)
cells
Na
+
Na
+
K
+
K
+
Na
+
Na
+
Cl
-
Cl
-
K
+
K
+
Na
+
channel blockers
•
•
-
-
Aldosterone antagonists
• Spironolactone
• Eplerenone
-
-
inhibit the entry of sodium into the sodium channels.
sorption and potassium secretion. Sodium channel blockers directly
cell by the sodium-potassium ATPase pump. Aldosterone antago-
tion in the late distal tubules and cortical collecting tubules. Sodium
Figure 27–12
Mechanism of sodium chloride reabsorption and potassium secre-
enters the cell through special channels and is transported out of the
nists compete with aldosterone for binding sites in the cell and
therefore inhibit the effects of aldosterone to stimulate sodium reab-
lost in the urine.
body needs to conserve, and almost none of them are
strongly reabsorbed; these are all substances that the
of the figure, such as glucose and amino acids, are all
versely, the substances represented toward the bottom
ing especially great quantities into the urine. Con-
even to secrete them into the tubules, thereby excret-
adapted to reabsorb them only slightly or not at all, or
needed by the body, and the kidneys have become
in the urine. In general, these substances are not
27–14, such as creatinine, become highly concentrated
The substances represented at the top of Figure
been reabsorbed than water.
than 1.0, this means that relatively more solute has
more water is reabsorbed than solute, or if there has
As the filtrate moves along the tubular system, the
is assumed to be constant, any change in the ratio
a substance. If plasma concentration of the substance
several substances in the different tubular segments.
Figure 27–14 shows the degree of concentration of
percentage of the solute is reabsorbed, the substance
the substance becomes more concentrated. If a greater
water. If a greater percentage of water is reabsorbed,
Whether a solute will become concentrated in the
Tubular Segments
Different Solutes in the Different
acid-base balance.
cortical collecting tubule. Thus, the medullary
concentration gradient, as also occurs in the
3. The medullary collecting duct is capable of
a concentrated urine.
into the medullary interstitium, helping to raise
Therefore, some of the tubular urea is reabsorbed
2. Unlike the cortical collecting tubule, the
solutes in the urine.
into the medullary interstitium, thereby reducing
high levels of ADH, water is avidly reabsorbed
to water is controlled by the level of ADH. With
1. The permeability of the medullary collecting duct
few mitochondria (Figure 27–13). Special characteris-
338
Unit V
The Body Fluids and Kidneys
tics of this tubular segment are as follows:
the urine volume and concentrating most of the
medullary collecting duct is permeable to urea.
the osmolality in this region of the kidneys and
contributing to the kidneys’ overall ability to form
secreting hydrogen ions against a large
collecting duct also plays a key role in regulating
Summary of Concentrations of
tubular fluid is determined by the relative degree of
reabsorption of that solute versus the reabsorption of
becomes more diluted.
All the values in this figure represent the tubular fluid
concentration divided by the plasma concentration of
of tubular fluid/plasma concentration rate reflects
changes in tubular fluid concentration.
concentration rises to progressively greater than 1.0 if
been a net secretion of the solute into the tubular fluid.
If the concentration ratio becomes progressively less
Medullary
collecting duct
Na
+
, Cl
-
Urea
HCO
3
-
(
+
ADH) H
2
O
H
+
centration of antidiuretic hormone.
urea, which is reabsorbed in these tubular segments. The reabsorp-
medullary collecting duct. The medullary collecting ducts actively
Figure 27–13
Cellular ultrastructure and transport characteristics of the
reabsorb sodium and secrete hydrogen ions and are permeable to
tion of water in medullary collecting ducts is controlled by the con-
PAH
PAH
T
ubular fluid/plasma concentration
Proximal
tubule
Loop of
Henle
Distal
tubule
Collecting
tubule
Cl
Cl
Cl
Cl
K
K
Na
Na
to 585
to 585
to 140
to 140
to 125
to 125
HCO
3
HCO
3
K
and Na
K
and Na
Cre
atinine
Cre
atinine
Glucose
Glucose
Protein
Protein
Amino acids
Amino acids
Inulin
Inulin
Urea
Urea
100.0
50.0
20.0
10.0
5.0
2.0
1.0
0.50
0.20
0.10
0.05
0.02
the tubules.
avidly than water, whereas values above 1.0 indicate that the sub-
Values below 1.0 indicate that the substance is reabsorbed more
substance in the plasma and in the glomerular filtrate. A value of 1.0
Figure 27–14
Changes in average concentrations of different substances at differ-
ent points in the tubular system relative to the concentration of that
indicates that the concentration of the substance in the tubular fluid
is the same as the concentration of that substance in the plasma.
stance is reabsorbed to a lesser extent than water or is secreted into

Figure 27–15 shows the approximate normal forces
), which opposes reabsorption.
), which favors reabsorption; and
outside the capillaries, which favors reabsorption; (3)
]), which opposes reabsorption;
laries. These forces include (1) hydrostatic pressure
The net reabsorptive force represents the sum of the
laries. The normal rate of peritubular capillary reab-
of the solutes are normally reabsorbed. Fluid and elec-
tubules, more than 99 per cent of the water and most
Normal Values for Physical Forces and Reabsorption Rate.
the renal tubules.
and, ultimately, reabsorption of water and solutes from
glomerular capillaries. Changes in peritubular capil-
of reabsorption across the peritubular capillaries,
Interstitial Fluid Physical Forces
homeostasis.
changes in GFR.) Working together, the autoregula-
cially tubuloglomerular feedback, which help prevent
includes the renal autoregulatory mechanisms, espe-
output. (The first line of defense, discussed earlier,
segments when GFR increases. Glomerulotubular
The importance of glomerulotubular balance is that
pletely isolated proximal tubular segments.
ing renal interstitium, as discussed later. It is clear that
of Henle. The precise mechanisms responsible for this
occurs in other tubular segments, especially the loop
as the filtered load increases, even though the
cent of GFR). Thus, glomerulotubular balance refers
min to 150 ml/min, the absolute rate of proximal
. For example, if GFR is increased from 125 ml/
This phenomenon is referred to as
to Increased Tubular Load
Ability of the Tubules to Increase
control mechanisms.
independently of others, especially through hormonal
tion. An important feature of tubular reabsorption is
tion, just as there are for control of glomerular filtra-
tion, there are multiple nervous, hormonal, and local
Regulation of Tubular
than 99% has been reabsorbed.
125 (see Figure 27–14), indicating that only 1/125 of
At the end of the collecting ducts, the tubular
sorbed as the fluid passes through the proximal tubule.
sorbed from the tubules, a tubular fluid/plasma con-
glomerular filtrate. Since inulin is not secreted or reab-
rises to about 3.0 at the end of the proximal tubules,
water present in the tubular fluid. For example, the
tubule, therefore, reflect changes in the amount of
sorbed or secreted by the renal tubules. Changes in
a polysaccharide used to measure GFR, is not reab-
to Measure Water Reabsorption by the Renal Tubules.
Tubular Fluid /Plasma Inulin Concentration Ratio Can Be Used
Urine Formation by the Kidneys: II. Tubular Processing of the Glomerular Filtrate
Chapter 27
339
Inulin,
inulin concentration at different points along the renal
tubular fluid/plasma concentration ratio for inulin
indicating that inulin concentration in the tubular fluid
is 3 times greater than in the plasma and in the
centration ratio of 3.0 means that only one third of the
water that was filtered remains in the renal tubule and
that two thirds of the filtered water has been reab-
fluid/plasma inulin concentration ratio rises to about
the filtered water remains in the tubule and that more
Reabsorption
Because it is essential to maintain a precise balance
between tubular reabsorption and glomerular filtra-
control mechanisms that regulate tubular reabsorp-
that reabsorption of some solutes can be regulated
Glomerulotubular Balance—The
Reabsorption Rate in Response
One of the most basic mechanisms for controlling
tubular reabsorption is the intrinsic ability of the
tubules to increase their reabsorption rate in response
to increased tubular load (increased tubular inflow).
glomerulotubular
balance
tubular reabsorption also increases from about 81 ml/
min (65 per cent of GFR) to about 97.5 ml/min (65 per
to the fact that the total rate of reabsorption increases
percentage of GFR reabsorbed in the proximal
tubule remains relatively constant at about 65 per
cent.
Some degree of glomerulotubular balance also
are not fully understood but may be due partly to
changes in physical forces in the tubule and surround-
the mechanisms for glomerulotubular balance can
occur independently of hormones and can be demon-
strated in completely isolated kidneys or even in com-
it helps to prevent overloading of the distal tubular
balance acts as a second line of defense to buffer the
effects of spontaneous changes in GFR on urine
tory and glomerulotubular balance mechanisms
prevent large changes in fluid flow in the distal tubules
when the arterial pressure changes or when there
are other disturbances that would otherwise wreak
havoc with the maintenance of sodium and volume
Peritubular Capillary and Renal
Hydrostatic and colloid osmotic forces govern the rate
just as these physical forces control filtration in the
lary reabsorption can in turn influence the hydrostatic
and colloid osmotic pressures of the renal interstitium
As
the glomerular filtrate passes through the renal
trolytes are reabsorbed from the tubules into the renal
interstitium and from there into the peritubular capil-
sorption is about 124 ml/min.
Reabsorption across the peritubular capillaries can
be calculated as
Reabsorption
= K
f
¥ Net reabsorptive force
hydrostatic and colloid osmotic forces that either favor
or oppose reabsorption across the peritubular capil-
inside the peritubular capillaries (peritubular hydro-
static pressure [P
c
(2) hydrostatic pressure in the renal interstitium (P
if
)
colloid osmotic pressure of the peritubular capillary
plasma proteins (
p
c
(4) colloid osmotic pressure of the proteins in the renal
interstitium (
p
if
that favor and oppose peritubular reabsorption.
tubules.
tubules into the interstitium, especially in the proximal
proteins in the renal interstitium. These changes then
capillaries. This in turn raises renal interstitial fluid
colloid osmotic pressure, reduces the uptake of fluid
tive force across the peritubular capillary membranes,
the tubules. For example, a decrease in the reabsorp-
Ultimately, changes in peritubular capillary physical
rate.
conditions. Table 27–2 summarizes the factors that
tion. K
raise reabsorption, whereas
capillaries. Increases in K
cussed later.
plasma flow and increasing filtration fraction, as dis-
renal vasoconstrictors, such as angiotensin II, increase
increased GFR or decreased renal plasma flow. Some
tion is defined as the ratio of GFR/renal plasma flow,
lar capillary reabsorption rate. Because filtration frac-
the plasma that remains behind. Thus, increasing the
quently, the more concentrated the protein becomes in
plasma filtered through the glomerulus and, conse-
the filtration fraction, the greater the fraction of
reabsorption; and (2)
capillary colloid osmotic pressure, thereby increasing
is determined by
plasma in these capillaries; raising the colloid osmotic
The second major determinant of peritubular capil-
hydrostatic pressure.
hydrostatic pressure, it lowers peritubular capillary
reabsorption rate. Although constriction of the
blood vessels. (2) Increase in resistance of either the
decrease reabsorption rate. This effect is buffered to
. (1) Increases in arterial pressure tend to
sures of the peritubular capillaries. The
The two
Regulation of Peritubular Capillary Physical Forces.
normally is about 12.4 ml/min/mm Hg.
10 mm Hg, K
capillaries. Because the reabsorption rate is normally
The other factor that contributes to the high rate of
in the glomerular capillaries, but in the opposite
10 mm Hg. This is a high value, similar to that found
reabsorption. Therefore, subtracting the net hydro-
net colloid osmotic force of about 17 mm Hg, favoring
which opposes reabsorption, is 15 mm Hg, causing a
sure, which favors reabsorption, is about 32 mm Hg,
favor reabsorption. The plasma colloid osmotic pres-
which opposes fluid reabsorption. This is more than
lar capillary to the interstitial fluid of about 7 mm Hg,
hydrostatic pressure averages 6 mm Hg, there is a pos-
averages about 13 mm Hg and renal interstitial fluid
340
Unit V
The Body Fluids and Kidneys
Because the normal peritubular capillary pressure
itive hydrostatic pressure gradient from the peritubu-
counterbalanced by the colloid osmotic pressures that
and the colloid osmotic pressure of the interstitium,
static forces that oppose reabsorption (7 mm Hg) from
the net colloid osmotic forces that favor reabsorption
(17 mm Hg) gives a net reabsorptive force of about
direction.
fluid reabsorption in the peritubular capillaries is a
large filtration coefficient (K
f
) because of the high
hydraulic conductivity and large surface area of the
about 124 ml/min and net reabsorption pressure is
f
determinants of peritubular capillary reabsorption
that are directly influenced by renal hemodynamic
changes are the hydrostatic and colloid osmotic pres-
peritubular
capillary hydrostatic pressure is influenced by the arte-
rial pressure and resistances of the afferent and efferent
arterioles
raise peritubular capillary hydrostatic pressure and
some extent by autoregulatory mechanisms that main-
tain relatively constant renal blood flow as well as
relatively constant hydrostatic pressures in the renal
afferent or the efferent arterioles reduces peritubular
capillary hydrostatic pressure and tends to increase
efferent arterioles increases glomerular capillary
lary reabsorption is the colloid osmotic pressure of the
pressure increases peritubular capillary reabsorption.
The colloid osmotic pressure of peritubular capillaries
(1) the systemic plasma colloid
osmotic pressure; increasing the plasma protein con-
centration of systemic blood tends to raise peritubular
the filtration fraction; the higher
filtration fraction also tends to increase the peritubu-
increased filtration fraction can occur as a result of
peritubular capillary reabsorption by decreasing renal
Changes in the peritubular capillary K
f
can also
influence the reabsorption rate because K
f
is a
measure of the permeability and surface area of the
f
decreases in K
f
lower peritubular capillary reabsorp-
f
remains relatively constant in most physiologic
can influence the peritubular capillary reabsorption
Renal Interstitial Hydrostatic and Colloid Osmotic Pressures.
forces influence tubular reabsorption by changing the
physical forces in the renal interstitium surrounding
caused by either increased peritubular capillary hydro-
static pressure or decreased peritubular capillary
and solutes from the interstitium into the peritubular
hydrostatic pressure and decreases interstitial fluid
colloid osmotic pressure because of dilution of the
decrease the net reabsorption of fluid from the renal
Tubular
Tubular
Tubular
Tubular
ATP
Na
+
Na
+
cells
cells
lumen
lumen
Interstitial
fluid
Interstitial
fluid
Peritubular
capillary
Peritubular
capillary
H
2
O
Na
+
Na
+
10 mm Hg
Net reabsorption
pressure
10 mm Hg
Net reabsorption
pressure
P
c
13 mm Hg
P
c
13 mm Hg
π
c
32 mm Hg
π
c
32 mm Hg
π
if
15 mm Hg
π
if
15 mm Hg
P
if
6 mm Hg
P
if
6 mm Hg
H
2
O
Bulk
flow
Bulk
flow
, interstitial fluid colloid osmotic pressure.
, peritubular capillary colloid
, inter-
, peritubular capillary hydrostatic pressure; P
triphosphate; P
are transported across the renal tubular cells. ATP, adenosine
net reabsorptive pressure is normally about 10 mm Hg, causing fluid
cal values shown are estimates of the normal values for humans. The
mine fluid reabsorption by the peritubular capillaries. The numeri-
Figure 27–15
Summary of the hydrostatic and colloid osmotic forces that deter-
and solutes to be reabsorbed into the peritubular capillaries as they
c
if
stitial fluid hydrostatic pressure;
p
c
osmotic pressure;
p
if
kidney disease, increases in arterial pressure cause
GFR autoregulation is impaired, as often occurs in
increased arterial pressure on urine output. When
renal blood flow and GFR. The slight increase in GFR
pressure diuresis.
water, phenomena that are referred to as
Effect of Arterial Pressure on Urine
hemodynamic changes
Therefore, in general,
osmotic pressures of the renal interstitium, the uptake
Thus, through changes in the hydrostatic and colloid
sorption increases.
the tubular lumen is reduced, and net tubular reab-
stitium; therefore, backleak of water and solutes into
pressure. Both of these forces favor movement of fluid
The opposite is true when there is increased peri-
rate of net reabsorption (refer again to Figure 27–16).
backleak into the tubular lumen, thereby reducing the
tendency for greater amounts of solute and water to
tubular capillary reabsorption is reduced, there is
into the lumen of the tubule. However, when peri-
reabsorption, the net movement of water and solutes
With the normal high rate of peritubular capillary
can diffuse in both directions through these junctions.
actually leaky, so that considerable amounts of sodium
into the tubular lumen. The so-called tight junctions
once the water and solutes are in the interstitial spaces,
tubular lumen into the interstitium by osmosis. And
transport or passive diffusion, water is drawn from the
reabsorbed (Figure 27–16). Once the solutes enter the
The mechanisms by which changes in interstitial
Urine Formation by the Kidneys: II. Tubular Processing of the Glomerular Filtrate
Chapter 27
341
fluid hydrostatic and colloid osmotic pressures influ-
ence tubular reabsorption can be understood by exam-
ining the pathways through which solute and water are
intercellular channels or renal interstitium by active
they can either be swept up into the peritubular cap-
illaries or diffuse back through the epithelial junctions
between the epithelial cells of the proximal tubule are
is into the peritubular capillaries with little backleak
increased interstitial fluid hydrostatic pressure and a
tubular capillary reabsorption above the normal level.
An initial increase in reabsorption by the peritubular
capillaries tends to reduce interstitial fluid hydrostatic
pressure and raise interstitial fluid colloid osmotic
and solutes out of the tubular lumen and into the inter-
of water and solutes by the peritubular capillaries is
closely matched to the net reabsorption of water and
solutes from the tubular lumen into the interstitium.
forces that increase peritubular
capillary reabsorption also increase reabsorption from
the renal tubules. Conversely,
that inhibit peritubular capillary reabsorption also
inhibit tubular reabsorption of water and solutes.
Output—The Pressure-Natriuresis and
Pressure-Diuresis Mechanisms
Even small increases in arterial pressure often cause
marked increases in urinary excretion of sodium and
pressure
natriuresis and
Because of the
autoregulatory mechanisms described in Chapter 26,
increasing the arterial pressure between the limits of
75 and 160 mm Hg usually has only a small effect on
that does occur contributes in part to the effect of
much larger increases in GFR.
Factors That Can Influence Peritubular Capillary
Table 27–2
Reabsorption
≠ P
c
Æ Ø Reabsorption
•
Ø R
A
Æ ≠ P
c
•
Ø R
E
Æ ≠ P
c
•
≠ Arterial Pressure Æ ≠ P
c
≠ p
c
Æ ≠ Reabsorption
•
≠ p
A
Æ ≠ p
c
•
≠ FF Æ ≠ p
c
≠ K
f
Æ ≠ Reabsorption
, peritubular capillary filtration coefficient.
, arterial plasma colloid osmotic pressure; FF, filtration fraction;
, peritubular capillary colloid osmotic
ent arteriolar resistances, respectively;
, afferent and effer-
, peritubular capillary hydrostatic pressure; R
P
c
A
and R
E
p
c
pressure;
p
A
K
f
ATP
ATP
Tubular
Tubular
ATP
ATP
Decreased reabsorption
Decreased reabsorption
Normal
Normal
cells
cells
Lumen
Lumen
Backleak
Backleak
Interstitial
fluid
Interstitial
fluid
Peritubular
capillary
Peritubular
capillary
Net
reabsorption
Net
reabsorption
Increased
backleak
Increased
backleak
Decreased net
reabsorption
Decreased net
reabsorption
P
c
P
c
π
c
π
c
P
c
P
c
π
c
π
c
lial cells, especially in the proximal tubule.
sorption, in turn, decreases the net reabsorption of solutes and water
). Reduced peritubular capillary reab-
Figure 27–16
Proximal tubular and peritubular capillary reabsorption under
normal conditions (top) and during decreased peritubular capillary
reabsorption (bottom) caused by either increasing peritubular cap-
illary hydrostatic pressure (P
c
) or decreasing peritubular capillary
colloid osmotic pressure (
p
c
by increasing the amounts of solutes and water that leak back into
the tubular lumen through the tight junctions of the tubular epithe-

loss of salt and water from the body fluids. The
cellular fluid volume, such as during hemorrhage or
19, angiotensin II formation increases in circumstances
sodium-retaining hormone. As discussed in Chapter
Angiotensin II is perhaps the body’s most powerful
Angiotensin II Increases Sodium and Water Reabsorption.
fluids. Thus, aldosterone is even more important as a
minimal levels of aldosterone are present, the inabil-
and potassium depletion. Although day-to-day regula-
Conn’s syndrome
secretion, as occurs in patients with adrenal tumors
lation of potassium. Conversely, excess aldosterone
), there
Addison’s disease
In the absence of aldosterone, as occurs with adrenal
The cellular mechanisms of aldosterone action are
permeability of the luminal side of the membrane.
membrane. Aldosterone also increases the sodium
stimulating the sodium-potassium ATPase pump on
. The mechanism by which
secretion by the renal tubules.
glomerulosa cells of the adrenal cortex, is an impor-
Aldosterone, secreted by the zona
tubular actions in the next few paragraphs.
Chapters 28 and 29, but we briefly review their renal
their effects on solute and water excretion. Some
their principal sites of action on the renal tubule, and
water. Table 27–3 summarizes some of the most impor-
major changes in excretion of other electrolytes.
when sodium intake is changed, the kidneys must
excretion of sodium and other electrolytes. Likewise,
potassium intake is increased, the kidneys must
independently of one another. For example, when
ent solutes and water at variable rates, sometimes
Hormonal Control of Tubular
increases sodium reabsorption. Therefore, decreased
stimulates aldosterone secretion,
which further
increases sodium reabsorption by the tubules; it also
reduced angiotensin II formation. Angiotensin II itself
arterial pressure rises.
leak of sodium into the tubular lumen, thereby reduc-
sure. As discussed earlier, an increase in the renal
vasa recta of the renal medulla, and a subsequent
tubular capillary hydrostatic pressure, especially in the
is reabsorbed by the tubules. The mechanisms respon-
342
Unit V
The Body Fluids and Kidneys
A second effect of increased renal arterial pressure
that raises urine output is that it decreases the per-
centage of the filtered load of sodium and water that
sible for this effect include a slight increase in peri-
increase in the renal interstitial fluid hydrostatic pres-
interstitial fluid hydrostatic pressure enhances back-
ing the net reabsorption of sodium and water and
further increasing the rate of urine output when renal
A third factor that contributes to the pressure-
natriuresis and pressure-diuresis mechanisms is
angiotensin II formation contributes to the decreased
tubular sodium reabsorption that occurs when arterial
pressure is increased.
Reabsorption
Precise regulation of body fluid volumes and solute
concentrations requires the kidneys to excrete differ-
excrete more potassium while maintaining normal
appropriately adjust urinary sodium excretion without
Several hormones in the body provide this specificity
of tubular reabsorption for different electrolytes and
tant hormones for regulating tubular reabsorption,
of these hormones are discussed in more detail in
Aldosterone Increases Sodium Reabsorption and Increases
Potassium Secretion.
tant regulator of sodium reabsorption and potassium
The primary site
of aldosterone action is on the principal cells of the
cortical collecting tubule
aldosterone increases sodium reabsorption while at
the same time increasing potassium secretion is by
the basolateral side of the cortical collecting tubule
discussed in Chapter 77.
destruction or malfunction (
is marked loss of sodium from the body and accumu-
(
) is associated with sodium retention
tion of sodium balance can be maintained as long as
ity to appropriately adjust aldosterone secretion
greatly impairs the regulation of renal potassium
excretion and potassium concentration of the body
regulator of potassium concentration than it is for
sodium concentration.
associated with low blood pressure and/or low extra-
increased formation of angiotensin II helps to return
blood pressure and extracellular volume toward
normal by increasing sodium and water reabsorption
from the renal tubules through three main effects:
1. Angiotensin II stimulates aldosterone secretion,
which in turn increases sodium reabsorption.
2. Angiotensin II constricts the efferent arterioles,
which has two effects on peritubular capillary
dynamics that raise sodium and water
Table 27–3
- - -
Parathyroid hormone
Proximal tubule, thick ascending loop of
Atrial natriuretic peptide
Distal tubule/collecting tubule and duct
Antidiuretic hormone
Distal tubule/collecting tubule and duct
tubule, collecting tubule
NaCl, H
Angiotensin II
Proximal tubule, thick ascending loop of Henle/distal
NaCl, H
Aldosterone
Collecting tubule and duct
Hormone
Site of Action
Effects
Hormones That Regulate Tubular Reabsorption
≠
2
O reabsorption,
≠ K
+
secretion
≠
2
O reabsorption,
≠ H
+
secretion
≠ H
2
O reabsorption
Ø NaCl reabsorption
Ø PO
4
reabsorption,
≠ Ca
++
reabsorption
Henle/distal tubule

of the plasma is “cleared” of the substance. Thus,
excreted into the urine each minute, then 1 ml/min
lowing example: If the plasma passing through the
To illustrate the clearance principle, consider the fol-
tion, and tubular secretion.
of the kidneys: glomerular filtration, tubular reabsorp-
later, can be used to quantify the rate at which blood
the excretory function of the kidneys and, as discussed
cleared of a substance. However,
. This concept is somewhat
that is completely cleared of the substance by the
various substances (Table 27–4). By definition, the
The rates at which different substances are “cleared”
formation, which adds to the overall effect to increase
renal tubule. And finally, sympathetic nervous system
loop of Henle, and perhaps in more distal parts of the
the proximal tubule, the thick ascending limb of the
the renal arterioles, thereby reducing GFR. Sympa-
Henle, as discussed in Chapter 29.
hormone also has other actions, including inhibition of
perhaps also in the loops of Henle. Parathyroid
tion of calcium, especially in the distal tubules and
calcium-regulating hormones in the body. Its principal
excretion, which helps to return blood volume back
especially in the collecting ducts. This decreased
sorption of sodium and water by the renal tubules,
Specific cells of the cardiac atria, when
Atrial Natriuretic Peptide Decreases Sodium and Water
reducing water permeability.
tled back to the cell cytoplasm, thereby removing the
of ADH decreases, the molecules of AQP-2 are shut-
ing AQP-2 gene transcription. When the concentration
AQP-2 protein in the renal tubular cells by stimulat-
increases in ADH levels also increase the formation of
are not believed to be regulated by ADH. Chronic
for water to rapidly exit the cells, although these
are other aquaporins, AQP-3 and AQP-4, in the baso-
permit rapid diffusion of water through the cells. There
water channels
membrane by exocytosis to form
ecules of AQP-2 cluster together and fuse with the cell
, to the luminal side of the cell membranes. The mol-
aquaporin-2 (AQP-
of an intracellular protein, called
protein kinases. This, in turn, stimulates the movement
increasing the formation of cyclic AMP and activating
tubules, collecting tubules, and collecting ducts,
degree of dilution or concentration of the urine, as dis-
the actions of ADH play a key role in controlling the
kidneys to excrete large amounts of dilute urine. Thus,
tubules and collecting ducts to water is low, causing the
In the absence of ADH, the permeability of the distal
lecting duct epithelia. This effect helps the body to
ability of the distal tubule, collecting tubule, and col-
renal action of ADH is to increase the water perme-
The most important
ADH Increases Water Reabsorption.
These multiple actions of angiotensin II cause
epithelial cell membrane in the tubules.
in the proximal tubule. Thus, angiotensin II
exchange in the luminal membrane, especially
the tubular epithelial cell basolateral membrane.
stimulate the sodium-potassium ATPase pump on
reabsorption in the proximal tubules
water.
the peritubular capillaries; this increases the
blood flow, raises filtration fraction in the
efferent arteriolar constriction, by reducing renal
especially from the proximal tubules. Second,
reduces peritubular capillary hydrostatic pressure,
reabsorption. First, efferent arteriolar constriction
Urine Formation by the Kidneys: II. Tubular Processing of the Glomerular Filtrate
Chapter 27
343
which increases net tubular reabsorption,
glomerulus and increases the concentration of
proteins and the colloid osmotic pressure in
reabsorptive force at the peritubular capillaries
and raises tubular reabsorption of sodium and
3. Angiotensin II directly stimulates sodium
, the loops of
Henle, the distal tubules, and the collecting tubules.
One of the direct effects of angiotensin II is to
A second effect is to stimulate sodium-hydrogen
stimulates sodium transport across both the
luminal and the basolateral surfaces of the
marked sodium retention by the kidneys when
angiotensin II levels are increased.
conserve water in circumstances such as dehydration.
cussed further in Chapters 28 and 75.
ADH binds to specific V
2
receptors in the late distal
2)
that
lateral side of the cell membrane that provide a path
water channels from the luminal membrane and
Reabsorption.
distended because of plasma volume expansion,
secrete a peptide called atrial natriuretic peptide.
Increased levels of this peptide in turn inhibit the reab-
sodium and water reabsorption increases urinary
toward normal.
Parathyroid Hormone Increases Calcium Reabsorption.
Parathyroid hormone is one of the most important
action in the kidneys is to increase tubular reabsorp-
phosphate reabsorption by the proximal tubule and
stimulation of magnesium reabsorption by the loop of
Sympathetic Nervous System
Activation Increases Sodium
Reabsorption
Activation of the sympathetic nervous system can
decrease sodium and water excretion by constricting
thetic activation also increases sodium reabsorption in
stimulation increases renin release and angiotensin II
tubular reabsorption and decrease renal excretion of
sodium.
Use of Clearance Methods
to Quantify Kidney Function
from the plasma provide a useful way of quantitating
the effectiveness with which the kidneys excrete
renal clearance of a substance is the volume of plasma
kidneys per unit time
abstract because there is no single volume of plasma
that is completely
renal clearance provides a useful way of quantifying
flows through the kidneys as well as the basic functions
kidneys contains 1 milligram of a substance in each mil-
liliter and if 1 milligram of this substance is also

, a polysac-
The GFR, therefore, can be calculated as the clearance
). Thus,
tubules, then the rate at which that substance is excreted
to Estimate GFR
Thus, renal clearance of a substance is calculated from
rate. Rearranging this equation, clearance can be
concentration of that substance, and V is the urine flow
plasma concentration of the substance, U
is the clearance rate of a substance s, P
in the urine per unit time. Stated mathematically,
344
Unit V
The Body Fluids and Kidneys
clearance refers to the volume of plasma that would be
necessary to supply the amount of substance excreted
C
s
¥ P
s
= U
s
¥ V,
where C
s
s
is the
s
is the urine
expressed as
the urinary excretion rate (U
s
¥ V) of that substance
divided by its plasma concentration.
Inulin Clearance Can Be Used
If a substance is freely filtered (filtered as freely as
water) and is not reabsorbed or secreted by the renal
in the urine (U
s
¥ V) is equal to the filtration rate of the
substance by the kidneys (GFR
¥ P
s
GFR
¥ P
s
= U
s
¥ V
of the substance as follows:
A substance that fits these criteria is inulin
charide molecule with a molecular weight of about
GFR
U
V
P
C
s
s
s
=
¥
=
C
U
V
P
s
s
s
=
¥
ltered. There is normally
tubules, so that the amount of creatinine excreted
However, creatinine clearance is not a perfect marker
used than inulin clearance for estimating GFR clinically.
sion into the patient, this method is much more widely
also be used to assess GFR. Because measurement of
ltration. Therefore, the clearance of creatinine can
creatinine.
determining GFR. Other substances that have been
ltered to deliver the inulin that appears in the urine.
tration, which yields a value of 125 ml/min. Thus, 125
urine. Then, inulin clearance is calculated as the urine
min. Therefore, 125 mg/min of inulin passes into the
concentration is 125 mg/ml, and urine
this example, the plasma concentration is 1 mg/ml, urine
17 shows the renal handling of inulin. In
Figure 27
5200. Inulin, which is not produced in the body, is found
in the roots of certain plants and must be administered
intravenously to a patient to measure GFR.
–
flow rate is 1 ml/
excretion rate of inulin divided by the plasma concen-
milliliters of plasma flowing through the kidneys must
be fi
Inulin is not the only substance that can be used for
used clinically to estimate GFR include radioactive
iothalamate and
Creatinine Clearance and Plasma
Creatinine Concentration Can Be
Used to Estimate GFR
Creatinine is a by-product of muscle metabolism and is
cleared from the body fluids almost entirely by glomeru-
lar fi
creatinine clearance does not require intravenous infu-
of GFR because a small amount of it is secreted by the
slightly exceeds the amount fi
a slight error in measuring plasma creatinine that
leads to an overestimate of the plasma creatinine
Table 27–4
Filtered load
mg/min, mmol/min, or mEq/min
Secretion rate
Secretion rate
Excretion rate
mg/min, mmol/min, or mEq/min
Filtered load
Reabsorption rate
Reabsorption rate
V
mg/min, mmol/min, or mEq/min
Excretion rate
Excretion rate
ow (ERPF)
ml/min
Term
Equation
Units
Use of Clearance to Quantify Kidney Function
Clearance rate (C
s
)
ml/min
Glomerular filtration rate (GFR)
Clearance ratio
None
Effective renal plasma fl
Renal plasma flow (RPF)
ml/min
Renal blood flow (RBF)
ml/min
= U
s
¥
=
-
= (GFR ¥ P
s
)
- (U
s
¥ V)
= Excretion rate -
, renal venous PAH concentration.
PAH
extraction ratio; V
, PAH
PAH
, renal arterial PAH concentration; E
PAH
ow rate; P, plasma concentration; PAH, para-aminohippuric acid; P
S, a substance; U, urine concentration; V, urine fl
1 Hematocrit
RBF
RPF
=
-
PAH
PAH
PAH
PAH
PAH
PAH
PAH
PAH
PAH
P
V
P
U
V P
RPF
C
E
U
V
P
V
PAH
PAH
=
=
¥
(
)
-
(
)
=
¥
-
ERPF
C
U
V
P
PAH
PAH
PAH
=
=
¥
Clearance ratio
C
C
s
inulin
=
GFR
U
V
P
inulin
inulin
=
¥
C
U
V
P
s
s
s
=
¥
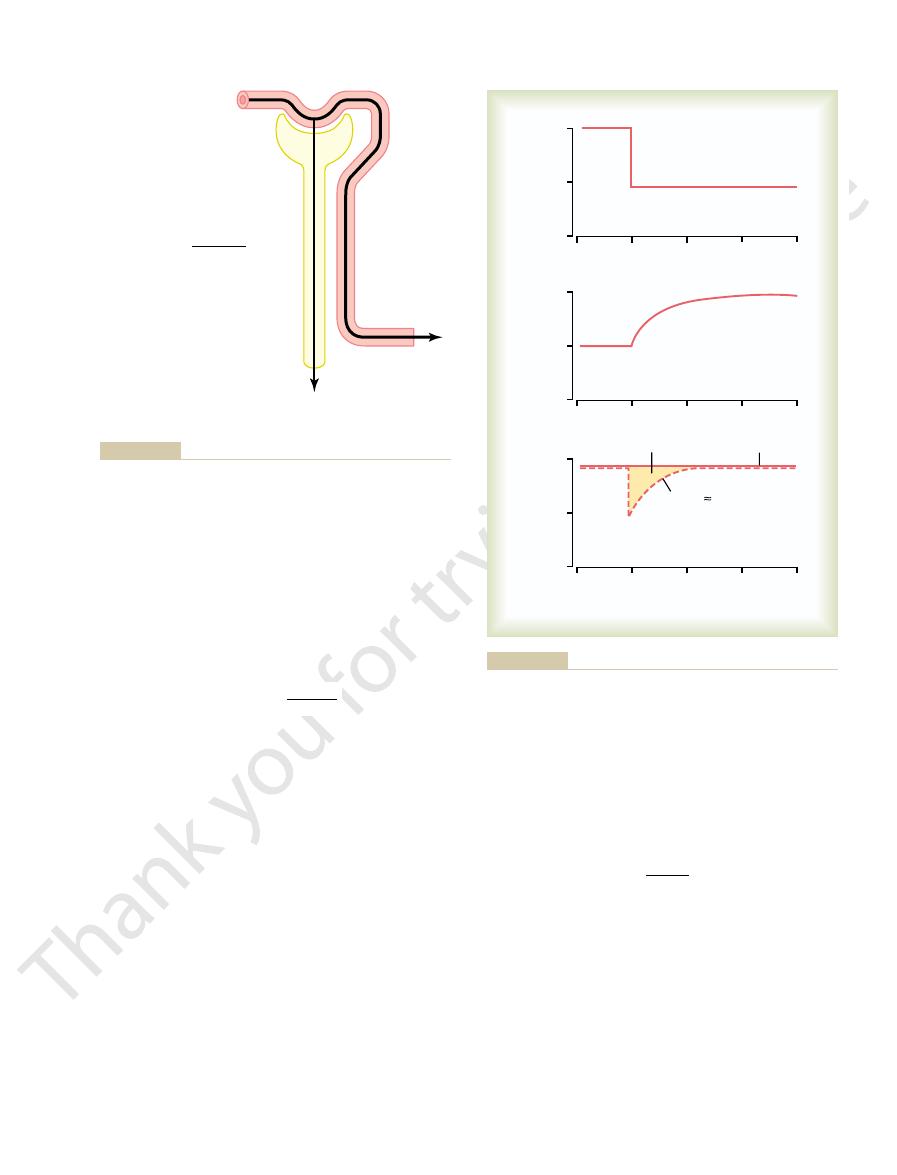
the plasma, the clearance rate of that substance is equal
Theoretically, if a substance is
PAH Clearance Can Be Used to
centration, as shown in Figure 27
GFR. However, this normal rate of creatinine excretion
rate of creatinine production, despite reductions in
state conditions, the creatinine excretion rate equals the
plasma creatinine to 8 times normal. Thus, under steady-
creatinine would increase to about 4 times normal, and
18. If GFR falls to one-fourth normal, plasma
Figure 27
increases to approximately twice normal, as shown in
is reestablished. This will occur when plasma creatinine
uids and raising plasma concentration. Plasma con-
nine, causing accumulation of creatinine in the body
If GFR suddenly decreases by 50%, the kidneys will
), which is inversely proportional to GFR:
in GFR, however, can be
changes
). An
In some cases, it may not be practical to collect urine
cancel each other. Therefore, creatinine clearance pro-
concentration, and fortuitously, these two errors tend to
Urine Formation by the Kidneys: II. Tubular Processing of the Glomerular Filtrate
Chapter 27
345
vides a reasonable estimate of GFR.
in a patient for measuring creatinine clearance (C
Cr
approximation of
obtained by simply measuring plasma creatinine con-
centration (P
Cr
transiently filter and excrete only half as much creati-
fl
centration of creatinine will continue to rise until the fil-
tered load of creatinine (P
Cr
¥ GFR) and creatinine
excretion (U
Cr
¥ V) return to normal and a balance
between creatinine production and creatinine excretion
–
a decrease of GFR to one-eighth normal would raise
occurs at the expense of elevated plasma creatinine con-
–19.
Estimate Renal Plasma Flow
completely cleared from
GFR
C
U
V
P
Cr
Cr
Cr
ª
=
¥
diseased kidneys, this extraction ratio may be reduced
and averages about 90 per cent in normal kidneys. In
extraction ratio of PAH
leaves the kidneys. The percentage of PAH removed
the percentage of PAH that is still in the blood when it
ow. To be more accurate, one can correct for
of PAH can be used as an approximation of renal
cent cleared from the plasma. Therefore, the clearance
kidneys. One substance, however, PAH, is about 90 per
20). There is
ltration (Figure 27
ow, a substance that is completely cleared
V). Thus, renal
ow. In other words, the
to the total renal plasma fl
amount of the substance delivered to the kidneys in
the blood (renal plasma flow
¥ P
s
) would be equal to
the amount excreted in the urine (U
s
¥
plasma flow (RPF) could be calculated as
Because the GFR is only about 20 per cent of the
total plasma fl
from the plasma must be excreted by tubular secretion
as well as glomerular fi
–
no known substance that is completely cleared by the
plasma fl
from the blood is known as the
RPF
U
V
P
C
s
s
s
=
¥
=
U
inulin
= 125 mg/ml
P
inulin
= 1 mg/ml
V = 1 ml/min
Amount filtered = Amount excreted
GFR x P
inulin
= U
inulin
x V
U
inulin
x V
P
inulin
GFR =
GFR = 125 ml/min
, urine inulin concentration; V, urine flow
illaries but is not reabsorbed by the renal tubules. P
clearance of inulin. Inulin is freely filtered by the glomerular cap-
Measurement of glomerular filtration rate (GFR) from the renal
Figure 27–17
inulin
, plasma
inulin concentration; U
inulin
rate.
Positive balance
Production
0
1
2
3
4
Creatinine production and
renal excretion (g/day)
Days
2
1
0
Excretion GFR x P
Creatinine
Serum creatinine
concentration (mg/dl)
2
1
0
GFR (ml/min)
100
50
0
, plasma creatinine concentration.
Creatinine
rate when the production rate of creatinine remains constant.
on serum creatinine concentration and on creatinine excretion
Effect of reducing glomerular filtration rate (GRF) by 50 per cent
Figure 27–18
P

0.45), or 1182 ml/min.
650 ml/min, the total blood
(the percentage of red blood cells in the blood). If the
arterial PAH concentration:
) concentrations, divided by the renal
PAH
venous PAH (V
PAH
ference between the renal arterial PAH (P
PAH
The extraction ratio (E
Clearance of PAH/Extraction ratio of PAH
Total renal plasma
585 ml/min by 0.9, yielding a value of 650 ml/min. Thus,
If the extraction ratio for PAH is 90 per cent, the
concentration (0.01 mg/ml). Thus, clearance of PAH cal-
1 ml/min) divided by the plasma PAH
can be calculated from the rate of urinary PAH excre-
ow rate is 1 ml/min. PAH clearance
mg/ml, and urine
tion of PAH is 0.01 mg/ml, urine concentration is 5.85
following example: Assume that the plasma concentra-
The calculation of RPF can be demonstrated by the
because of inability of damaged tubules to secrete PAH
346
Unit V
The Body Fluids and Kidneys
into the tubular fluid.
fl
tion (5.85 mg/ml
¥
culates to be 585 ml/min.
actual renal plasma flow can be calculated by dividing
total renal plasma flow can be calculated as
flow
=
) is calculated as the dif-
) and renal
One can calculate the total blood flow through the
kidneys from the total renal plasma flow and hematocrit
hematocrit is 0.45 and the total renal plasma flow is
flow through both kidneys
is 650/(1
-
PAH
PAH
E
P
V
P
PAH
PAH
=
-
of tubular reabsorption. Assume the following labora-
The following example demonstrates the calculation
ltered load, then the rate
renal tubules. Conversely, if the excretion rate of the
), then some of
stance by the renal tubules. For example, if the rate of
of a substance are known, one can calculate whether
Calculation of Tubular Reabsorption
ow is 650 ml/min and GFR is 125 ml/min, the
clearance) and the GFR (inulin clearance). If renal
ow (PAH
brane, one must
ltration fraction, which is the fraction
To calculate the
from GFR Divided by Renal
Filtration Fraction Is Calculated
Plasma Flow
fi
of plasma that filters through the glomerular mem-
first know the renal plasma fl
plasma fl
filtration fraction (FF) is calculated as
FF
= GFR/RPF = 125/650 = 0.19
or Secretion from Renal Clearances
If the rates of glomerular filtration and renal excretion
there is a net reabsorption or a net secretion of that sub-
excretion of the substance (U
s
¥ V) is less than the fil-
tered load of the substance (GFR
¥ P
s
the substance must have been reabsorbed from the
substance is greater than its fi
at which it appears in the urine represents the sum of
the rate of glomerular filtration plus tubular secretion.
tory values for a patient were obtained:
75
100
125
150
25
50
Plasma creatinine concentration
(mg/100 ml)
Glomerular filtration rate
(ml/min)
14
12
10
8
6
4
2
Normal
and plasma creatinine concentration under steady-state conditions.
Figure 27–19
Approximate relationship between glomerular filtration rate (GFR)
Decreasing GFR by 50 per cent will increase plasma creatinine to
twice normal if creatinine production by the body remains constant.
U
PAH
= 5.85 mg/ml
P
PAH
= 0.01 mg/ml
V = 1 ml/min
U
PAH
x V
P
PAH
Renal plasma flow
=
Renal venous
PAH =
0.001 mg/ml
ow rate.
PAH concentration; V, urine
, urine
PAH
, arterial plasma PAH concentration; U
PAH
the kidneys. P
for the percentage of PAH that is still in the blood when it leaves
). To be more accurate, one can correct
PAH
the clearance of PAH (C
the urine. Therefore, the renal plasma
renal artery is about equal to the amount of PAH excreted in
into the tubular lumen. The amount of PAH in the plasma of the
aminohippuric acid (PAH). PAH is freely
Figure 27–20
Measurement of renal plasma flow from the renal clearance of para-
filtered by the glomerular
capillaries and is also secreted from the peritubular capillary blood
flow can be calculated from
fl

be secreted by the nephron tubules. Listed below are
stance is greater than that of inulin, the substance must
nephron tubules; and (3) if the clearance rate of a sub-
ance, the substance must have been reabsorbed by the
ltered and not reabsorbed or secreted; (2) if the
rate of the substance equals that of inulin, the substance
ance of inulin, a measure of GFR: (1) if the clearance
The following generalizations can be made by
ltered load and urinary excretion, or
Therefore, tubular reabsorption of sodium is the differ-
Eq/min. Urinary
In this example, the
Urine Formation by the Kidneys: II. Tubular Processing of the Glomerular Filtrate
Chapter 27
347
Urine flow rate
= 1 ml/min
Urine concentration of sodium (U
Na
)
= 70 mEq/L
= 70 mEq/ml
Plasma sodium concentration
= 140 mEq/L
= 140 mEq/ml
GFR (inulin clearance)
= 100 ml/min
filtered sodium load is GFR
¥ P
Na
,
or 100 ml/min
¥ 140 mEq/ml = 14,000 m
sodium excretion (U
Na
¥ urine flow rate) is 70 mEq/min.
ence between the fi
14,000
mEq/min - 70 mEq/min = 13,930 mEq/min.
Comparisons of Inulin Clearance with Clearances of Different
Solutes.
comparing the clearance of a substance with the clear-
is only fi
clearance rate of a substance is less than inulin clear-
the approximate clearance rates for some of the sub-
stances normally handled by the kidneys:
Inulin
125.0
Phosphate
25.0
Potassium
12.0
Chloride
1.3
Sodium
0.9
Glucose
0
Substance
Clearance Rate (ml/min)
Physiol Renal Physiol 280:F10, 2001.
)-glucose cotransporters. Am J
Wright EM: Renal Na(
Physiol Renal Physiol 284:F871, 2003.
trolyte transport: acknowledging our uncertainty. Am J
Weinstein AM: Mathematical models of renal
J Physiol Renal Physiol 283:F221, 2002.
salt retention and salt wasting by the collecting duct. Am
Schafer JA: Abnormal regulation of ENaC: syndromes of
Rev 80:211, 2000.
Russell JM: Sodium-potassium-chloride cotransport. Physiol
Rev Physiol 64:877, 2002.
action between genetic and environmental factors. Annu
sodium channel and the control of sodium balance: inter-
Rossier BC, Praderv S, Schild L, Hummler E: Epithelial
80:277, 2000.
pathophysiology, and molecular anatomy. Physiol Rev
Reilly RF, Ellison DH: Mammalian distal tubule: physiology,
Press, 2000.
iology and Pathophysiology, 3rd ed. New York: Raven
duct. In Seldin DW, Giebisch G (eds): The Kidney
loop of Henle, distal convoluted tubule and collecting
Reeves WB, Andreoli TE: Sodium chloride transport in the
17:309, 1999.
glomerular function and damage in humans. J Hypertens
Rahn KH, Heidenreich S, Bruckner D: How to assess
by the cortical collecting duct. Kidney Int 57:1324, 2000.
Palmer LG, Frindt G: Aldosterone and potassium secretion
kidney: from molecules to medicine. Physiol Rev 82:205,
r J, Marples D, et al: Aquaporins in the
Nielsen S, Fr
Physiol Rev 82:735, 2002.
degenerin family of ion channels: A variety of functions
Kellenberger S, Schild L: Epithelial sodium channel/
and Pathophysiology, 3rd ed. New York: Raven Press,
In Seldin DW, Giebisch G (eds): The Kidney
Humphreys MH, Valentin J-P: Natriuretic hormonal agents.
and Pathophysiology, 3rd ed. New York: Raven Press,
In Seldin DW, Giebisch G (eds): The Kidney
system: renal mechanisms and circulatory homeostasis.
Hall JE, Brands MW: The renin-angiotensin-aldosterone
sure natriuresis. Curr Hypertens Rep 4:152, 2002.
Granger JP, Alexander BT, Llinas M: Mechanisms of pres-
kidney: Hormonal control. Physiol Rev 81:345, 2001.
raille E,
Doucet A:
Sodium-potassium-adenosine-
Physiol Sci 12:55, 1997.
channels: insights and outlooks. News
Benos DJ, Fuller CM, Shlyonsky VG, et al: Amiloride-
renal proximal tubule. Cell Biochem Biophys 36:147, 2002.
Aronson PS: Ion exchangers mediating NaCl transport in the
Creatinine
140.0
References
sensitive Na
+
Fé
triphosphatase–dependent sodium transport in the
—Physiology
2000.
—Physiology
2000.
for a shared structure.
økiæ
2002.
—Phys-
fluid and elec-
+
