and collecting ducts to water, as discussed in Chapter 27. This allows large
gland secretes more ADH, which increases the permeability of the distal tubules
solutes in the body fluids become too concentrated), the posterior pituitary
When osmolarity of the body fluids increases above normal (that is, the
, also called
pendently of the rate of solute excretion. A primary effector of this feedback is
There is a powerful feedback system for regulating plasma osmolarity and
Antidiuretic Hormone Controls Urine Concentration
independently of solute excretion is necessary for survival, especially when fluid
solutes such as sodium and potassium. This ability to regulate water excretion
important, the kidney can excrete a large volume of dilute urine or a small
kidney can excrete urine with a concentration of 1200 to 1400 mOsm/L. Equally
when there is a deficit of water and extracellular fluid osmolarity is high, the
only about one sixth the osmolarity of normal extracellular fluid. Conversely,
excrete urine with an osmolarity as low as 50 mOsm/L, a concentration that is
is excess water in the body and body fluid osmolarity is reduced, the kidney can
of solutes and water in the urine in response to various challenges. When there
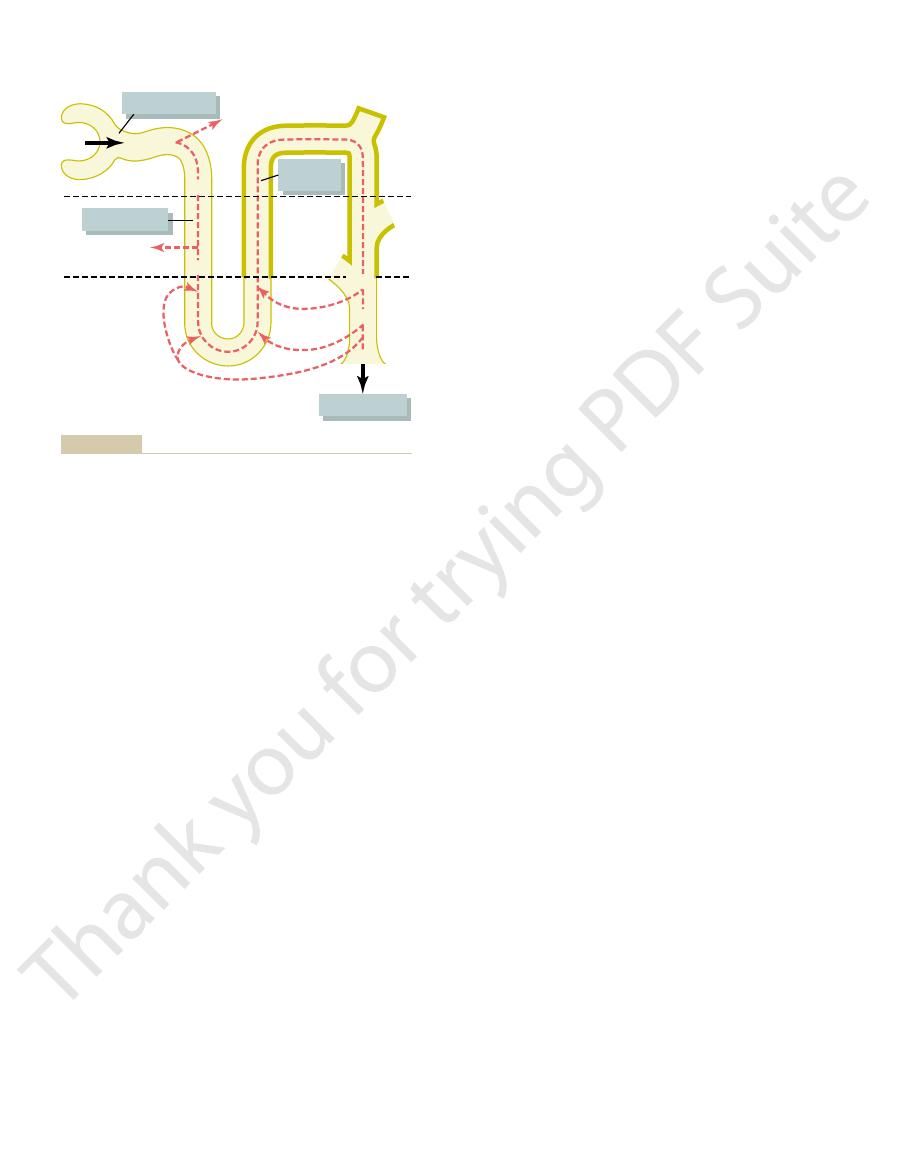
The normal kidney has tremendous capability to vary the relative proportions
a Dilute Urine
The Kidneys Excrete Excess Water by Forming
extracellular fluid volume, osmolarity, and sodium concentration.
anisms that determine the intakes of water and salt, which also help to control
sodium concentration and osmolarity; and (4) the thirst and salt appetite mech-
urine; (3) the renal feedback mechanisms that control the extracellular fluid
kidneys to eliminate excess water by excreting a dilute urine; (2) the mecha-
In this chapter, we discuss specifically (1) the mechanisms that cause the
that determine thirst, and (2) renal excretion of water, which is controlled by
body water in turn is controlled by (1) fluid intake, which is regulated by factors
tion and osmolarity are regulated by the amount of extracellular water. The
large extent, extracellular fluid sodium concentra-
by the volume of the extracellular fluid. Thus, to a
other solutes. The total concentration of solutes in
For the cells of the body to function properly, they
C
H
A
P
T
E
R
2
8
348
Regulation of Extracellular Fluid
Osmolarity and Sodium
Concentration
must be bathed in extracellular fluid with a rela-
tively constant concentration of electrolytes and
the extracellular fluid—and therefore the osmolar-
ity—is determined by the amount of solute divided
multiple factors that influence glomerular filtration and tubular reabsorption.
nisms that cause the kidneys to conserve water by excreting a concentrated
volume of concentrated urine without major changes in rates of excretion of
intake is limited.
sodium concentration that operates by altering renal excretion of water inde-
antidiuretic hormone (ADH)
vasopressin.
amounts of water to be reabsorbed and decreases urine volume but does not
tubular segment is impermeable to water, even in
are avidly reabsorbed. However, this portion of the
in the thick segment, sodium, potassium, and chloride
In the ascending limb of the loop of Henle, especially
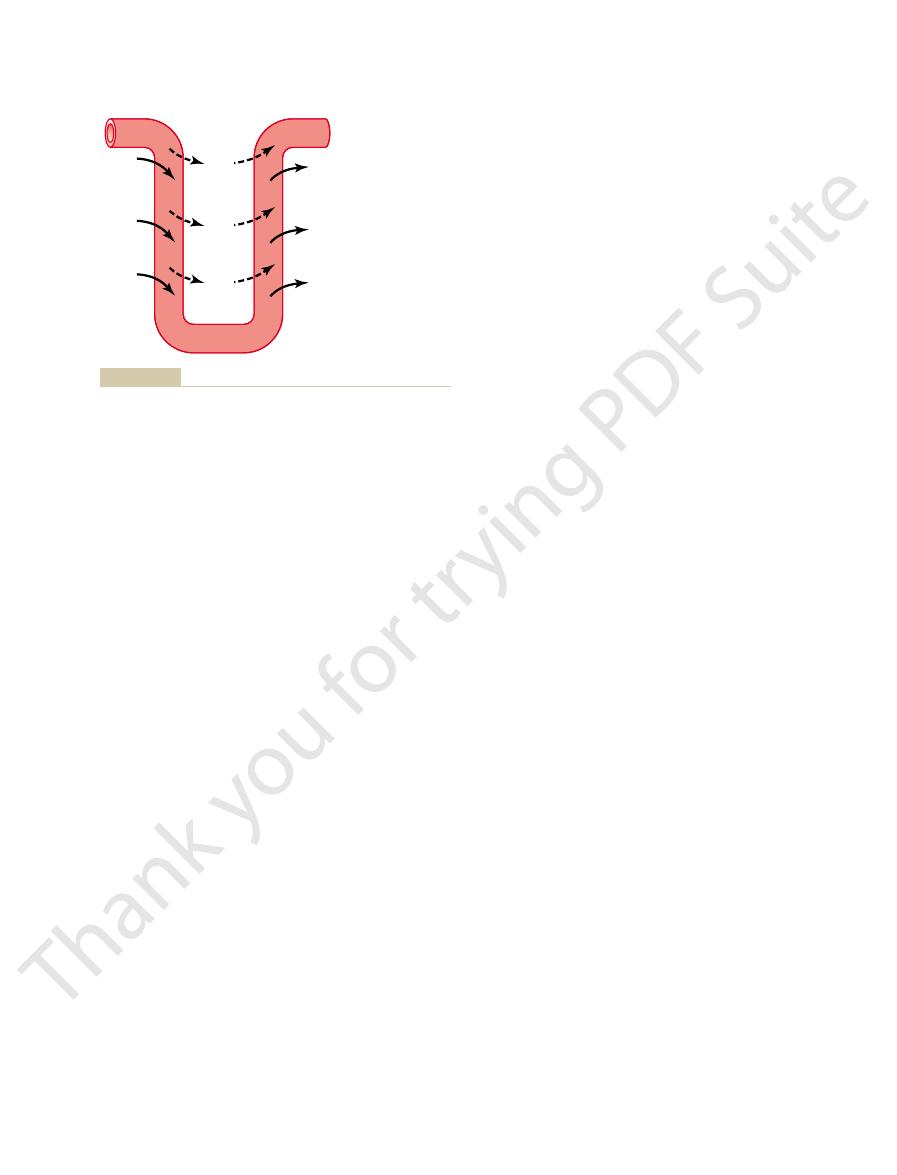
Tubular Fluid Becomes Dilute in the Ascending Loop of Henle.
fore, the tubular fluid becomes more concentrated as
osmolarity of the original glomerular filtrate. There-
the descending loop of Henle, water is reabsorbed by
osmolarity of about 300 mOsm/L.As fluid passes down
tubule fluid remains isosmotic to the plasma, with an
little change in osmolarity occurs; that is, the proximal
water are reabsorbed in equal proportions, so that
fluid flows through the proximal tubule, solutes and
Tubular Fluid Remains Isosmotic in the Proximal Tubule.
follows.
than water, as shown in Figure 28–2, but this occurs
to dilute the filtrate as it passes along the tubule. This
(300 mOsm/L). To excrete excess water, it is necessary
When the glomerular filtrate is initially formed,
does not excrete excess amounts of solutes.
water, the kidney rids the body of the excess water but
about 100 mOsm/L. Thus, after ingestion of excess
However, the total amount of solute excreted remains
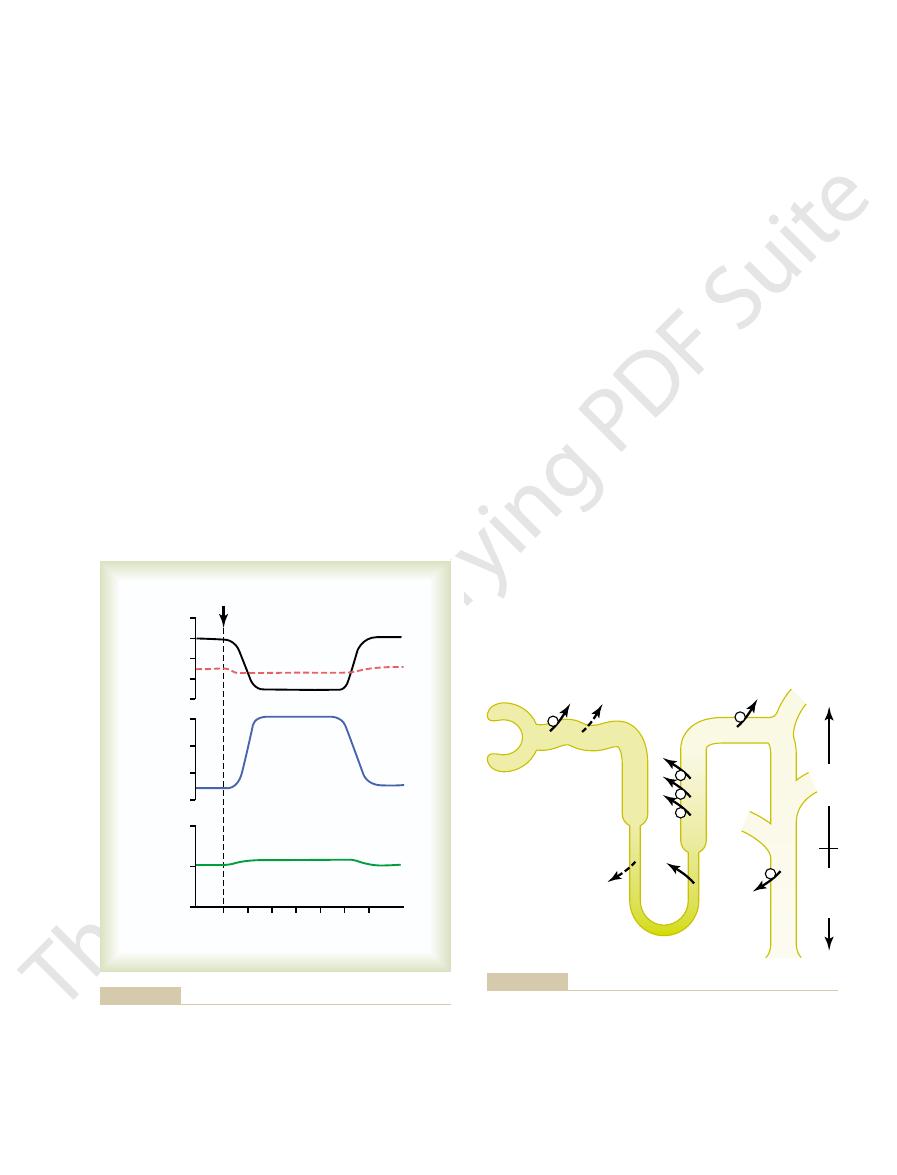
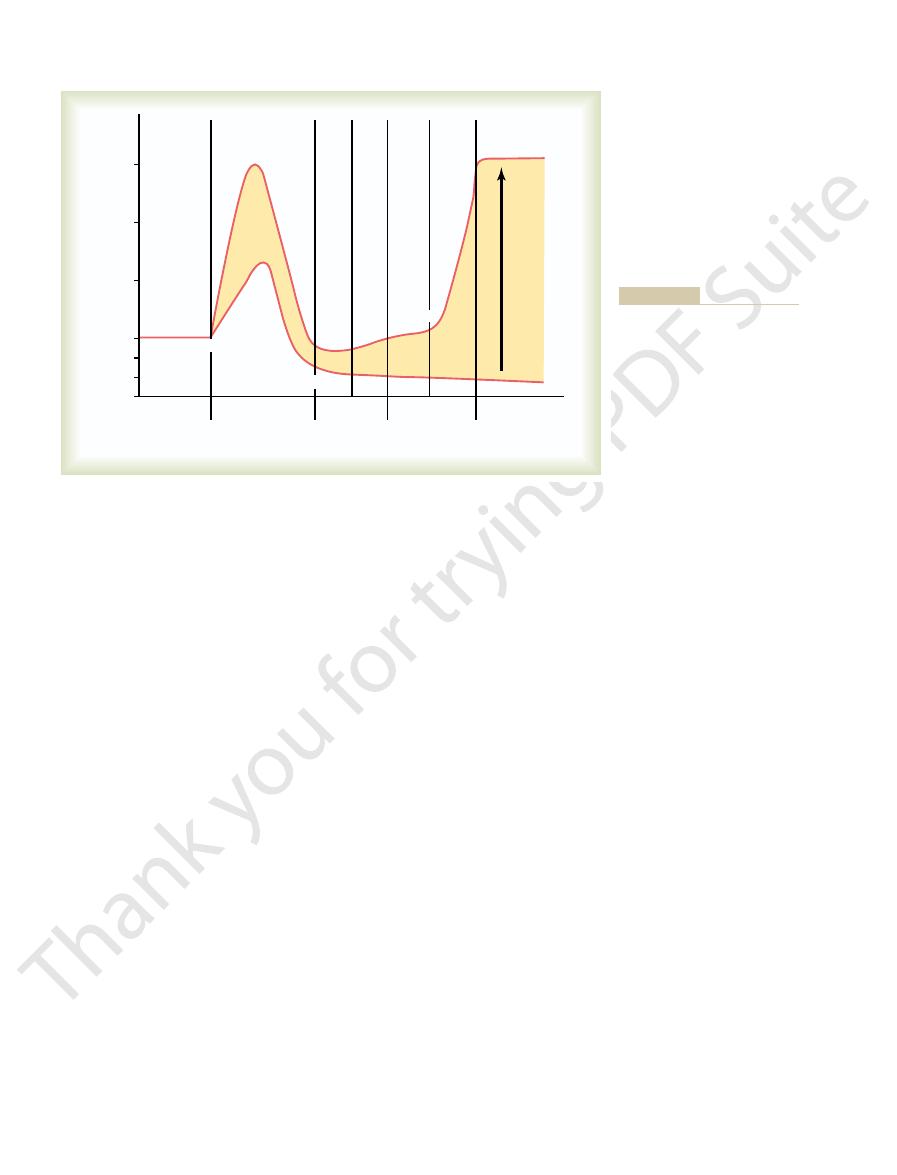
in a human after ingestion of 1 liter of water. Note that
Figure 28–1 shows the approximate renal responses
late distal tubule and the collecting ducts.
water in the distal parts of the nephron, including the
with a concentration as low as 50 mOsm/L. The kidney
kidney can excrete as much as 20 L/day of dilute urine,
When there is a large excess of water in the body, the
Dilute Urine
kidney excretes a dilute or a concentrated urine.
secretion determines, to a large extent, whether the
dilute urine to be excreted. Thus, the rate of ADH
lecting ducts to water, which causes large amounts of
ADH by the posterior pituitary decreases, thereby
cellular fluid osmolarity is reduced, the secretion of
When there is excess water in the body and extra-
solutes.
Chapter 28
Regulation of Extracellular Fluid Osmolarity and Sodium Concentration
349
markedly alter the rate of renal excretion of the
reducing the permeability of the distal tubule and col-
Renal Mechanisms for Excreting a
performs this impressive feat by continuing to reab-
sorb solutes while failing to reabsorb large amounts of
urine volume increases to about six times normal
within 45 minutes after the water has been drunk.
relatively constant because the urine formed becomes
very dilute and urine osmolarity decreases from 600 to
its osmolarity is about the same as that of plasma
is achieved by reabsorbing solutes to a greater extent
only in certain segments of the tubular system as
As
osmosis and the tubular fluid reaches equilibrium with
the surrounding interstitial fluid of the renal medulla,
which is very hypertonic—about two to four times the
it flows into the inner medulla.
Drink 1.0 L H
Time (minutes)
0
180
Urinary solute
excretion
(mOsm/min)
1.2
0.6
0
120
60
Urine flow rate
(ml/min)
6
4
2
0
Osmolarity
(mOsm/L)
800
Urine
osmolarity
Plasma
osmolarity
400
0
2
O
prevent plasma osmolarity from decreasing markedly during
remains relatively constant. These responses of the kidneys
urine; however, the total amount of solute excreted by the kidneys
larity decreases, causing the excretion of a large volume of dilute
that after water ingestion, urine volume increases and urine osmo-
Water diuresis in a human after ingestion of 1 liter of water. Note
Figure 28–1
excess water ingestion.
100
100
Medulla
Cortex
NaCl
NaCl
NaCl
NaCl
NaCl
300
300
400
400
600
400
600
H
2
O
H
2
O
600
300
70
50
(Numerical values are in milliosmoles per liter.)
reabsorption of solutes lead to a large volume of dilute urine.
levels are very low. The failure to reabsorb water and continued
sodium chloride and the failure to reabsorb water when ADH
tubules, the tubular fluid is further diluted by the reabsorption of
fluid becomes very dilute. In the distal tubules and collecting
are very low. Note that in the ascending loop of Henle, the tubular
Formation of a dilute urine when antidiuretic hormone (ADH) levels
Figure 28–2

occur in the presence of high levels of ADH.
, which provides the
avidly reabsorb water, and (2)
to water, thereby allowing these tubular segments to
, which increases the
high level of ADH
The basic requirements for forming a concentrated
Concentrated Urine—High ADH Levels
However, a shipwreck victim’s pet Australian hopping
that occurs in shipwreck victims who drink seawater.
of seawater drunk, explaining the rapid dehydration
ingested in addition to other solutes such as urea. This
drunk, 2 liters of urine volume would be required to rid
about 600 mOsm/L. Thus, for every liter of seawater
centrated. Therefore, the maximum concentration of
excrete other solutes, especially urea, which contribute
dehydration? The answer is that the kidney must also
or 1.0 liter. Why then does drinking seawater cause
urine concentrating ability is 1200 mOsm/L, the amount
sodium chloride intake of 1200 milliosmoles. If maximal
1200 mOsm/L. Drinking 1 liter of seawater with a con-
per cent, with an osmolarity between about 1000 and
if one attempts to drink seawater. Sodium chloride
The limited ability of the human kidney to con-
ratory tract, and gastrointestinal tract, when water is not
dehydration, along with water loss from the skin, respi-
This minimal loss of volume in the urine contributes to
, can be calculated as
must excrete about 600 milliosmoles of solute each day.
ions that are ingested. A normal 70-kilogram human
The maximal concentrating ability of the kidney dic-
Obligatory Urine Volume
beaver, have minimal urine concentrating ability; they
Animals adapted to aquatic environments, such as the
survive in the desert without drinking water; sufficient
high as 10,000 mOsm/L. This allows the mouse to
tralian hopping mouse, can concentrate urine to as
ity of plasma. Some desert animals, such as the Aus-
1200 to 1400 mOsm/L, four to five times the osmolar-
decreasing the volume of urine formed. The human
When there is a water deficit in the body, the kidney
tant when water is in short supply.
tain homeostasis, a function that is especially impor-
intake is required to match this loss, but the ability of
and the kidneys through the excretion of urine. Fluid
feces, the skin through evaporation and perspiration,
expired air, the gastrointestinal tract by way of the
routes, including the lungs by evaporation into the
mammals that live on land, including humans. Water is
The ability of the kidney to form a urine that is more
by Excreting a Concentrated
The Kidneys Conserve Water
distal tubule and collecting ducts, and a large volume
absence of ADH, the urine is further diluted in the late
always dilute, regardless of the level of ADH. In the
sorb water. In healthy kidneys, fluid leaving the
To summarize, the mechanism for forming a dilute
of dilute urine.
50 mOsm/L. The failure to reabsorb water and the con-
more dilute, decreasing its osmolarity to as low as
impermeable to water, and the additional reabsorption
the absence of ADH, this portion of the tubule is also
there is additional reabsorption of sodium chloride. In
tubule, cortical collecting duct, and collecting duct,
Tubular Fluid in Distal and Collecting Tubules Is Further Diluted
only about one third the osmolarity of plasma.
regardless of whether
ascending loop of Henle into the early distal tubule,
the presence of large amounts of ADH. Therefore, the
350
Unit V
The Body Fluids and Kidneys
tubular fluid becomes more dilute as it flows up the
with the osmolarity decreasing progressively to about
100 mOsm/L by the time the fluid enters the early
distal tubular segment. Thus,
ADH is present or absent, fluid leaving the early distal
tubular segment is hypo-osmotic, with an osmolarity of
in the Absence of ADH.
As the dilute fluid in the early
distal tubule passes into the late distal convoluted
of solutes causes the tubular fluid to become even
tinued reabsorption of solutes lead to a large volume
urine is to continue reabsorbing solutes from the distal
segments of the tubular system while failing to reab-
ascending loop of Henle and early distal tubule is
of dilute urine is excreted.
Urine
concentrated than plasma is essential for survival of
continuously lost from the body through various
the kidney to form a small volume of concentrated
urine minimizes the intake of fluid required to main-
forms a concentrated urine by continuing to excrete
solutes while increasing water reabsorption and
kidney can produce a maximal urine concentration of
water can be obtained through the food ingested and
water produced in the body by metabolism of the food.
can concentrate the urine to only about 500 mOsm/L.
tates how much urine volume must be excreted each
day to rid the body of waste products of metabolism and
If maximal urine concentrating ability is 1200 mOsm/L,
the minimal volume of urine that must be excreted,
called the obligatory urine volume
available to drink.
centrate the urine to a maximal concentration of
1200 mOsm/L explains why severe dehydration occurs
concentration in the oceans averages about 3.0 to 3.5
centration of 1200 mOsm/L would provide a total
of urine volume needed to excrete 1200 milliosmoles
would be 1200 milliosmoles divided by 1200 mOsm/L,
about 600 mOsm/L when the urine is maximally con-
sodium chloride that can be excreted by the kidneys is
the body of 1200 milliosmoles of sodium chloride
would result in a net fluid loss of 1 liter for every liter
mouse could drink with impunity all the seawater it
wanted.
Requirements for Excreting a
and Hyperosmotic Renal Medulla
urine are (1) a
permeability of the distal tubules and collecting ducts
a high osmolarity of the
renal medullary interstitial fluid
osmotic gradient necessary for water reabsorption to
L day
1200 mOsm L
600 mOsm day
= 0 5
.

on, reducing the concentration inside the tubule and
thick ascending limb
3, step 1). Next, the active pump of
tubule (Figure 28
300 mOsm/L, the same as that leaving the proximal
becomes hyperosmotic. First, assume that the loop of
in mind, let us now discuss how the renal medulla
With these characteristics of the loop of Henle
Steps Involved in Causing Hyperosmotic Renal Medullary Inter-
ows toward the tip of the loop of Henle.
interstitium, and the tubular
renal medullary osmolarity. Therefore, water diffuses
the ascending limb, is very permeable to water, and the
s loop, in contrast to
The descending limb of Henle
water, adding further to the high solute concentration
s loop, which is also impermeable to
renal medullary interstitium. There is some passive
ow of water into the interstitium. Thus, the active
thick ascending limb is virtually impermeable to water,
uid. Because the
This pump is capable of establishing about a 200-
transport of potassium, chloride, and other ions from
The most important cause of the high medullary
inner medullary collecting ducts.
tubules, distal tubules, cortical collecting tubules, and
1, along with the characteristics of the proximal
istics of the loops of Henle are summarized in Table
The transport character-
Be Trapped in the Renal Medulla.
4. Diffusion of only small amounts of water from the
3. Facilitated diffusion of large amounts of urea
2. Active transport of ions from the collecting ducts
of potassium, chloride, and other ions out of the
1. Active transport of sodium ions and co-transport
The major factors that contribute to the buildup of
tion in the medulla is achieved, it is maintained by a
great excess of water. Once the high solute concentra-
pelvic tip of the medulla. This means that the renal
in the medulla of the kidney is much higher, increas-
282 mOsm/L.) The osmolarity of the interstitial
intermolecular attraction and repulsion, is about
corrected osmolar activity,
the plasma osmolarity. (As discussed in Chapter 25,
of the body is about 300 mOsm/L, which is similar to
The osmolarity of interstitial
Medullary Interstitium
osmotic renal medulla before it is excreted, also play
collecting ducts, which carry urine through the hyper-
nally, the
before returning to the renal cortex. And
the vasa recta, which also loop down into the medulla
renal pelvis. Paralleling the long loops of Henle are
the medulla before returning to the cortex. Some of
juxtamedullary nephrons,
. In the human, about 25 per
the specialized peritubular capillar-
The countercurrent mechanism depends on the
rent mechanism
This process involves the operation of the
but for now, what is the process by which renal
discuss the factors that control ADH secretion later,
degree of hyperosmolarity of the renal medulla. We
ability is limited by the level of ADH and by the
back into the blood. Thus, the urine concentrating
tium; from there it is carried away by the vasa recta
when ADH levels are high, water moves through the
collecting ducts normally is very hyperosmotic, so that
The renal medullary interstitium surrounding the
Chapter 28
Regulation of Extracellular Fluid Osmolarity and Sodium Concentration
351
tubular membrane by osmosis into the renal intersti-
medullary interstitial fluid becomes hyperosmotic?
countercur-
.
special anatomical arrangement of the loops of Henle
and the vasa recta,
ies of the renal medulla
cent of the nephrons are
with loops of Henle and vasa recta that go deeply into
the loops of Henle dip all the way to the tips of the
renal papillae that project from the medulla into the
fi
a critical role in the countercurrent mechanism.
Countercurrent Mechanism
Produces a Hyperosmotic Renal
fluid in almost all parts
the
which accounts for
fluid
ing progressively to about 1200 to 1400 mOsm/L in the
medullary interstitium has accumulated solutes in
balanced inflow and outflow of solutes and water in
the medulla.
solute concentration into the renal medulla are as
follows:
thick portion of the ascending limb of the loop
of Henle into the medullary interstitium
into the medullary interstitium
from the inner medullary collecting ducts into the
medullary interstitium
medullary tubules into the medullary interstitium,
far less than the reabsorption of solutes into the
medullary interstitium
Special Characteristics of Loop of Henle That Cause Solutes to
28–
osmolarity is active transport of sodium and co-
the thick ascending loop of Henle into the interstitium.
milliosmole concentration gradient between the
tubular lumen and the interstitial fl
the solutes pumped out are not followed by osmotic
fl
transport of sodium and other ions out of the thick
ascending loop adds solutes in excess of water to the
reabsorption of sodium chloride from the thin ascend-
ing limb of Henle’
of the renal medullary interstitium.
’
tubular fluid osmolarity quickly becomes equal to the
out of the descending limb of Henle’s loop into the
fluid osmolarity gradually
rises as it fl
stitium.
Henle is filled with fluid with a concentration of
–
the
on the loop of Henle is turned
Table 28–1
Summary of Tubule Characteristics—Urine Concentration
ADH
0
ADH
0
0
ADH
0
0
0
0
0
Thick ascending limb
Thin ascending limb
0
0
++
+
+
Thin descending limb
0
++
++
+
+
O
NaCl
Urea
Transport
Active NaCl
Permeability
H
2
Proximal tubule
+
+
++
Distal tubule
+
+
Cortical collecting
+
+
tubule
Inner medullary
+
+
++ADH
collecting duct
ADH, permeability to water or urea is increased by ADH.
, high level of active transport or permeability;
, moderate level of active
0, minimal level of active transport or permeability;
+
transport or permeability;
++
+
4). The early distal
about 100 mOsm/L (Figure 28
uid is dilute, with an osmolarity of only
cortex, the
When the tubular
Role of Distal Tubule and
arrived sodium chloride, thus
. The sodium chloride reabsorbed from
Thus, the repetitive reabsorption of sodium chloride
ity to 1200 to 1400 mOsm/L as shown in step 7
pumping of ions out of the thick ascending loop of
the concentration gradient established by the active
cient time,
in excess of water; with suf
These steps are repeated over and over, with the net
ows into the ascending limb, still
uid (step 6), and as the
500 mOsm/L (step 5). Then, once again, the
into the interstitium, with water remaining behind,
is in the ascending limb, additional ions are pumped
ow into the ascending limb. Once this
Henle from the proximal tubule, which causes the
ent, much less than that achieved by the countercur-
Henle. Thus, by itself, the active transport of sodium
out of the descending limb. The interstitial osmolarity
uid (step 2). The
raising the interstitial concentration; this pump estab-
352
Unit V
The Body Fluids and Kidneys
lishes a 200-mOsm/L concentration gradient between
the tubular fluid and the interstitial fl
limit to the gradient is about 200 mOsm/L because
paracellular diffusion of ions back into the tubule
eventually counterbalances transport of ions out of the
lumen when the 200-mOsm/L concentration gradient
is achieved.
Step 3 is that the tubular fluid in the descending limb
of the loop of Henle and the interstitial fluid quickly
reach osmotic equilibrium because of osmosis of water
is maintained at 400 mOsm/L because of continued
transport of ions out of the thick ascending loop of
chloride out of the thick ascending limb is capable of
establishing only a 200-mOsm/L concentration gradi-
rent system.
Step 4 is additional flow of fluid into the loop of
hyperosmotic fluid previously formed in the descend-
ing limb to fl
fluid
until a 200-mOsm/L osmotic gradient is established,
with the interstitial fluid osmolarity rising to
fluid in the
descending limb reaches equilibrium with the hyper-
osmotic medullary interstitial fl
hyperosmotic tubular fluid from the descending limb
of the loop of Henle fl
more solute is continuously pumped out of the tubules
and deposited into the medullary interstitium.
effect of adding more and more solute to the medulla
fi
this process
gradually traps solutes in the medulla and multiplies
Henle, eventually raising the interstitial fluid osmolar-
.
by the thick ascending loop of Henle and continued
inflow of new sodium chloride from the proximal
tubule into the loop of Henle is called the countercur-
rent multiplier
the ascending loop of Henle keeps adding to the newly
“multiplying” its con-
centration in the medullary interstitium.
Collecting Ducts in Excreting a
Concentrated Urine
fluid leaves the loop of Henle and
flows into the distal convoluted tubule in the renal
fl
–
300
300
300
300
300
300
300
300
300
300
300
300
400
400
400
300
300
300
300
200
200
200
200
2
400
400
400
300
400
400
400
200
200
200
200
400
300
400
400
300
300
400
400
200
200
400
400
3
4
1
350
500
500
300
350
500
500
150
150
300
300
6
700
300
1000
1200
300
700
1000
1200
100
500
800
1000
7
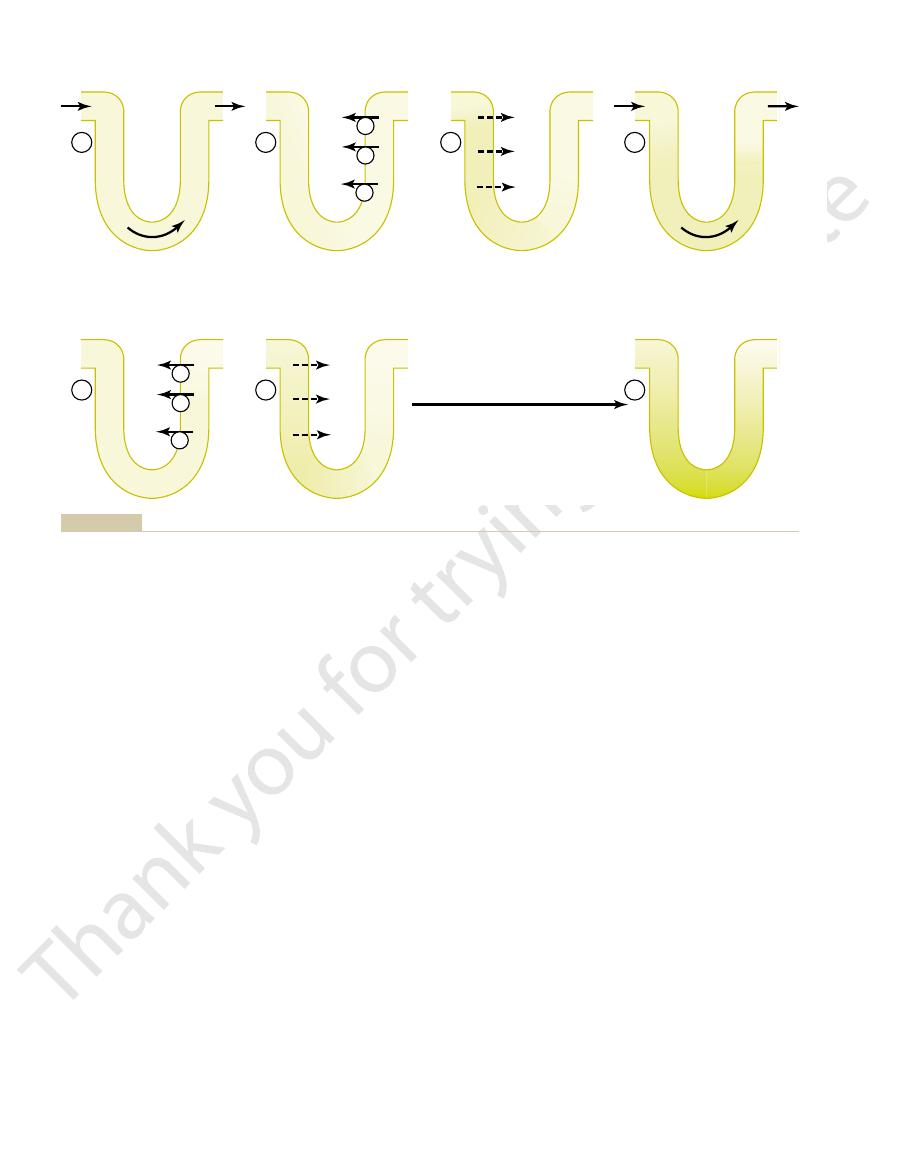
Repeat Steps 4-6
5
350
500
500
300
300
400
400
150
150
300
300
per liter.)
Countercurrent multiplier system in the loop of Henle for producing a hyperosmotic renal medulla. (Numerical values are in mill
Figure 28–3
iosmoles
(equal to the rate of urea production), despite the
centration increases markedly, returning the
have large reductions of GFR, the plasma urea con-
tration rate (GFR). In patients with renal disease who
determined mainly by two factors: (1) the concentra-
load of urea. In general, the rate of urea excretion is
impairment of urine concentrating ability.
whose protein intake and urea production are low.
people who ingest a high-protein diet, yielding large
The fundamental role of urea in contributing to
eventually, in the urine, even though urea is being
vated. The simultaneous movement of water and urea
collecting duct even more when ADH levels are ele-
urea transporters, UT-AI, is activated by ADH,
. One of these
into the renal interstitium. This diffusion is greatly
uid. This high concentration of
sorption takes place, causing an even higher concen-
inner medullary collecting ducts, still more water reab-
of the tubule. Then, as the tubular
1). In the presence of high concentrations of ADH,
these segments are impermeable to urea (see Table
cal collecting tubules, little urea is reabsorbed because
renal medulla is as follows: As water
The mechanism for reabsorption of urea into the
trations of ADH are high, large amounts of urea are
tubule. When there is water de
sodium chloride, urea is passively reabsorbed from the
is forming a maximally concentrated urine. Unlike
interstitium. However, urea contributes about 40
Thus far, we have considered only the contribution of
Renal Medullary Interstitium and to a
cits of body water.
the kidneys form a highly concentrated urine, excret-
3). Thus, by reabsorbing as much water as possible,
about 1200 mOsm/L (see Figure
the collecting ducts become permeable to water, so
venous blood. When high levels of ADH are present,
added to the cortex interstitium. The reabsorbed water
uid into the interstitium, but the total
lecting ducts, there is further water reabsorption from
owing peritubular capillaries.
tubule into the cortex interstitium, where it is swept
tubule becomes highly permeable to water, so that
high concentration of ADH, the cortical collecting
solutes and further dilutes the urine. When there is a
ADH, this segment is almost impermeable to water
the plasma concentration of ADH. In the absence of
ows into the cortical collecting tubule, the
atively impermeable to water.
segment, like the ascending loop of Henle, actively
Chapter 28
Regulation of Extracellular Fluid Osmolarity and Sodium Concentration
353
tubule further dilutes the tubular fluid because this
transports sodium chloride out of the tubule but is rel-
As fluid fl
amount of water reabsorbed is critically dependent on
and fails to reabsorb water but continues to reabsorb
large amounts of water are now reabsorbed from the
away by the rapidly fl
The fact that these large amounts of water are reab-
sorbed into the cortex, rather than into the renal
medulla, helps to preserve the high medullary intersti-
tial fluid osmolarity.
As the tubular fluid flows along the medullary col-
the tubular fl
amount of water is relatively small compared with that
is quickly carried away by the vasa recta into the
that the fluid at the end of the collecting ducts has
essentially the same osmolarity as the interstitial fluid
of the renal medulla—
28–
ing normal amounts of solutes in the urine while
adding water back to the extracellular fluid and com-
pensating for defi
Urea Contributes to Hyperosmotic
Concentrated Urine
sodium chloride to the hyperosmotic renal medullary
to 50 per cent of the osmolarity (500-600 mOsm/L) of
the renal medullary interstitium when the kidney
ficit and blood concen-
passively reabsorbed from the inner medullary col-
lecting ducts into the interstitium.
flows up the
ascending loop of Henle and into the distal and corti-
28–
water is reabsorbed rapidly from the cortical col-
lecting tubule and the urea concentration increases
rapidly because urea is not very permeant in this part
fluid flows into the
tration of urea in the fl
urea in the tubular fluid of the inner medullary col-
lecting duct causes urea to diffuse out of the tubule
facilitated by specific urea transporters
increasing transport of urea out of the inner medullary
out of the inner medullary collecting ducts maintains
a high concentration of urea in the tubular fluid and,
reabsorbed.
urine concentrating ability is evidenced by the fact that
amounts of urea as a nitrogenous “waste” product,
can concentrate their urine much better than people
Malnutrition is associated with a low urea concentra-
tion in the medullary interstitium and considerable
Recirculation of Urea from Collecting Duct to Loop of Henle
Contributes to Hyperosmotic Renal Medulla.
A person
usually excretes about 20 to 50 per cent of the filtered
tion of urea in the plasma and (2) the glomerular fil-
filtered
urea load and urea excretion rate to the normal level
reduced GFR.
NaCl
Urea
1200
1200
Medulla
Cortex
NaCl
300
100
300
600
1200
H
2
O
NaCl
NaCl
600
1200
Urea
H
2
O
NaCl
600
600
H
2
O
300
H
2
O
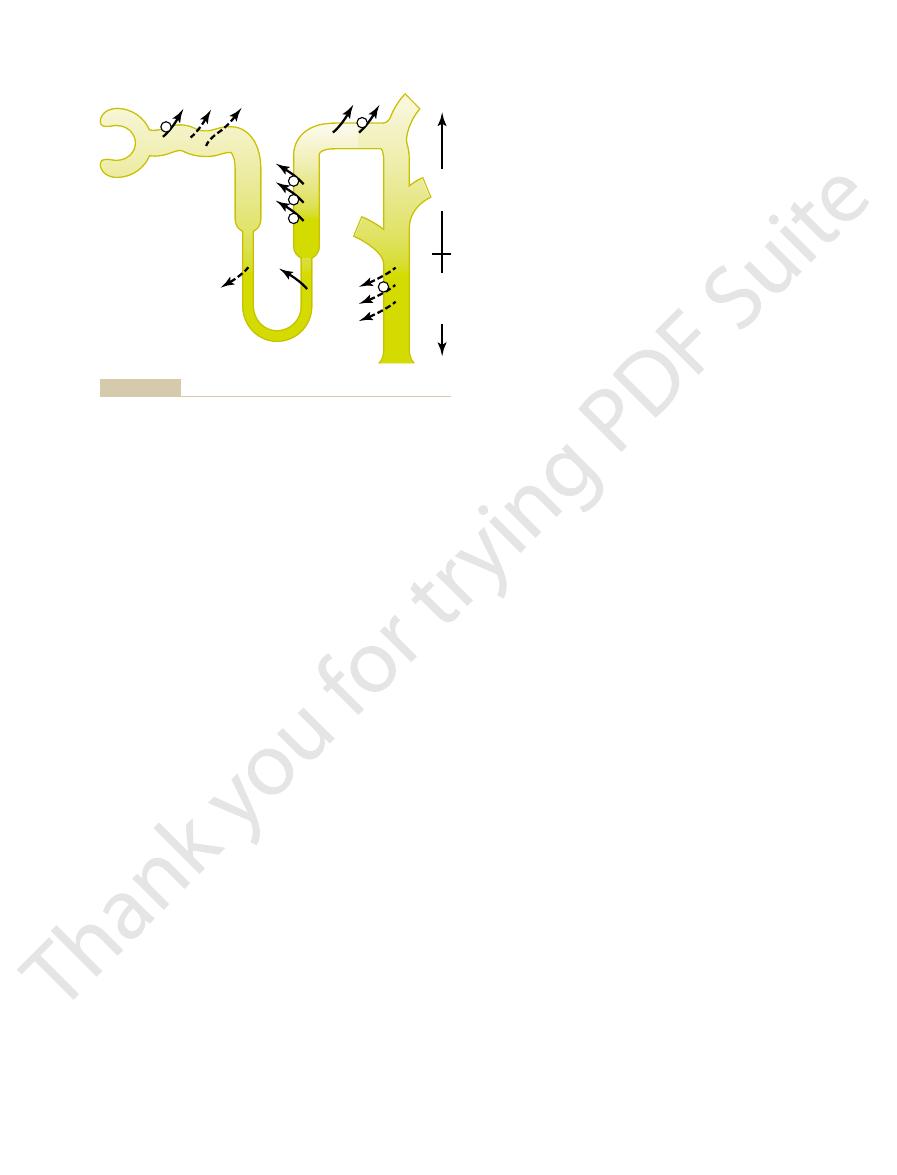
1200 mOsm/L. (Numerical values are in milliosmoles per liter.)
the renal medullary interstitial fluid in the papilla, which is about
is dilute but becomes concentrated as water is absorbed from the
(ADH) levels are high. Note that the fluid leaving the loop of Henle
Formation of a concentrated urine when antidiuretic hormone
Figure 28–4
distal tubules and collecting tubules. With high ADH levels, the
osmolarity of the urine is about the same as the osmolarity of
solute exchange across the vasa recta, there is little net
Thus, although there is a large amount of
toward the cortex, it becomes progressively less
the medullary interstitium. As blood ascends back
centration of about 1200 mOsm/L, the same as that of
blood reaches the tips of the vasa recta, it has a con-
loss of water into the interstitium. By the time the
becomes progressively more concentrated, partly
descends into the medulla toward the papillae, it
the blood, except for the plasma proteins. As blood
other capillaries, are highly permeable to solutes in
of the cortex and renal medulla. The vasa recta, like
6): Blood enters and leaves the
follows (Figure 28
The countercurrent exchange mechanism operates as
The vasa recta serve as countercurrent exchangers
This sluggish blood
ow.
, accounting for
The medullary blood flow is low
There are two special features of the renal
system, the solutes pumped into the renal medulla by
the kidney. Without a special medullary blood
of the Renal Medulla
Countercurrent Exchange in the Vasa
urea, and more urea is excreted in the urine.
levels of ADH, the inner medullary collecting ducts
When there is excess water in the body and low
is in short supply.
ucts that must be excreted by the kidneys, this mech-
This urea recirculation provides an additional mech-
excreted. Each time around the circuit contributes to
this way, urea can recirculate through these terminal
back down into the medullary collecting duct again. In
the distal tubule, the cortical collecting tubule, and
it passes upward through the ascending loop of Henle,
eventually diffuses into the thin loop of Henle, so that
into the medullary interstitium. A moderate share of
duct, the high tubular
uid concentration of urea. And as
of ADH are present, the reabsorption of water from
sorption occurs in these tubular segments. When the
tively impermeable to urea, and very little urea reab-
tubule, and the cortical collecting tubule are all rela-
The thick limb of the loop of Henle, the distal
loop of Henle from the medullary interstitium (Figure
segments of the loop of Henle, partly because of water
nearly as permeant as water. The concentration of urea
tered urea is reabsorbed, but even so, the tubular
In the proximal tubule, 40 to 50 per cent of the
354
Unit V
The Body Fluids and Kidneys
fil-
fluid
urea concentration increases because urea is not
continues to rise as the tubular fluid flows into the thin
reabsorption out of the descending loop of Henle but
also because of some secretion of urea into the thin
28–5).
kidney is forming a concentrated urine and high levels
the distal tubule and cortical collecting tubule further
raises the tubular fl
this urea flows into the inner medullary collecting
fluid concentration of urea
and specific urea transporters cause urea to diffuse
the urea that moves into the medullary interstitium
parts of the tubular system several times before it is
a higher concentration of urea.
anism for forming a hyperosmotic renal medulla.
Because urea is one of the most abundant waste prod-
anism for concentrating urea before it is excreted is
essential to the economy of the body fluid when water
have a much lower permeability to both water and
Recta Preserves Hyperosmolarity
Blood flow must be provided to the renal medulla to
supply the metabolic needs of the cells in this part of
flow
the countercurrent multiplier system would be rapidly
dissipated.
medullary blood flow that contribute to the preserva-
tion of the high solute concentrations:
1.
less than 5 per cent of the total renal blood fl
flow is sufficient to supply
the metabolic needs of the tissues but helps to
minimize solute loss from the medullary
interstitium.
2.
,
minimizing washout of solutes from the medullary
interstitium.
–
medulla by way of the vasa recta at the boundary
by solute entry from the interstitium and partly by
concentrated as solutes diffuse back out into the
medullary interstitium and as water moves into the
vasa recta.
fluid and
dilution of the concentration of the interstitial fluid at
each level of the renal medulla because of the U shape
Urea
500
550
300
300
Urea
Urea
Urea
20% remaining
100% remaining
50% remaining
100%
remaining
4.5
30
H
2
O
Cortex
Outer
medulla
Inner
medulla
15
7
30
4.5
tubules are indicated in the boxes.)
present. Percentages of the filtered load of urea that remain in the
antidiuresis, when large amounts of antidiuretic hormone are
urea. (Numerical values are in milliosmoles per liter of urea during
ducts, indicate that these segments are not very permeable to
the thick ascending loop of Henle to the medullary collecting
hyperosmolarity of the renal medulla. The heavy dark lines, from
helps to trap urea in the renal medulla and contributes to the
passes back into the collecting duct. The recirculation of urea
Henle, and then passes through the distal tubules, and finally
into the interstitial fluid. This urea diffuses into the thin loop of
Recirculation of urea absorbed from the medullary collecting duct
Figure 28–5
becomes very dilute, falling to a concentration of
the tubule into the medullary interstitium. Therefore,
sium, and other ions are actively transported from
to water, but large amounts of sodium, chloride, potas-
The thick part of the
Thick Ascending Loop of Henle.
prevent its washout from the renal medulla. This
also diffuses into the ascending limb, thereby return-
remains in the tubule. Some of the urea absorbed into
Thus, the tubular
removal from the descending loop of Henle, there is
uid, owing to water
sodium chloride. Because of the high concentration of
The thin ascending limb is
Thin Ascending Loop of Henle.
volume of dilute urine.
larity also becomes less concentrated. This is due
consequently, the descending loop tubular
formed, owing to low ADH concentrations, the med-
tration of ADH is high. When a dilute urine is being
urea. Therefore, the osmolarity of the
medulla. The descending limb is highly permeable to
descending loop of Henle, water is absorbed into the
Descending Loop of Henle.
ltrate, 300 mOsm/L.
osmosis. Therefore, the osmolarity of the
able to water, so that whenever solutes are reabsorbed,
However, the tubular membranes are highly perme-
electrolytes are reabsorbed in the proximal tubule.
Proximal Tubule.
nephron are shown in Figure 28
The changes in osmolarity and volume of the tubular
of the Tubules
Osmolarity in Different Segments
Mechanism and Changes in
with maximal levels of ADH, urine concentrating
uid. Even
mined not only by the level of ADH but also by the
urine concentrating ability. As discussed earlier,
out the hyperosmotic interstitium, thereby reducing
ing maximum urine concentrating ability. Large
ow, thereby
ing Ability.
Increased Medullary Blood Flow Can Reduce Urine Concentrat-
absorbed from the medullary tubules, and the high
laries. Thus, under steady-state conditions, the vasa
The U-shaped structure of the vessels minimizes
rent exchangers.
of the vasa recta capillaries, which act as countercur-
Chapter 28
Regulation of Extracellular Fluid Osmolarity and Sodium Concentration
355
Thus, the vasa recta do not create the
medullary hyperosmolarity, but they do prevent it from
being dissipated.
loss of solute from the interstitium but does not
prevent the bulk flow of fluid and solutes into the
blood through the usual colloid osmotic and hydro-
static pressures that favor reabsorption in these capil-
recta carry away only as much solute and water as is
concentration of solutes established by the counter-
current mechanism is maintained.
Certain vasodilators can markedly increase
renal medullary blood fl
“washing out”
some of the solutes from the renal medulla and reduc-
increases in arterial pressure can also increase the
blood flow of the renal medulla to a greater extent
than in other regions of the kidney and tend to wash
maximum concentrating ability of the kidney is deter-
osmolarity of the renal medulla interstitial fl
ability will be reduced if medullary blood flow
increases enough to reduce the hyperosmolarity in the
renal medulla.
Summary of Urine Concentrating
fluid as it passes through the different parts of the
–7.
About 65 per cent of the filtered
water also diffuses through the tubular membrane by
fluid remains
about the same as the glomerular fi
As fluid flows down the
water but much less permeable to sodium chloride and
fluid flowing
through the descending loop gradually increases until
it is equal to that of the surrounding interstitial fluid,
which is about 1200 mOsm/L when the blood concen-
ullary interstitial osmolarity is less than 1200 mOsm/L;
fluid osmo-
partly to the fact that less urea is absorbed into the
medullary interstitium from the collecting ducts when
ADH levels are low and the kidney is forming a large
essentially impermeable to water but reabsorbs some
sodium chloride in the tubular fl
some passive diffusion of sodium chloride from the
thin ascending limb into the medullary interstitium.
fluid becomes more dilute as the
sodium chloride diffuses out of the tubule and water
the medullary interstitium from the collecting ducts
ing the urea to the tubular system and helping to
urea
recycling is an additional mechanism that contributes
to the hyperosmotic renal medulla.
ascending loop of Henle is also virtually impermeable
fluid in the thick ascending limb of the loop of Henle
about 100 mOsm/L.
Vasa recta
mOsm/L
Interstitium
mOsm/L
300
600
900
1200
H
2
O
600
H
2
O
1000
Solute
Solute
Solute
Solute
Solute
Solute
H
2
O
800
350
300
600
800
1000
600
800
1000
1200
in milliosmoles per liter.)
the U shape of the vasa recta capillaries. (Numerical values are
amounts of solutes would be lost from the renal medulla without
interstitial fluid and water diffuses back into the vasa recta. Large
ascending limb of the vasa recta, solutes diffuse back into the
of solutes from the renal interstitial fluid into the blood. In the
motic because of diffusion of water out of the blood and diffusion
the descending limb of the vasa recta becomes more hyperos-
Countercurrent exchange in the vasa recta. Plasma flowing down
Figure 28–6
large amounts of solute must be excreted, they must
amount of solute that must be excreted. Therefore, if
, which is dictated by the
nally, we should keep in mind that there is
This is accomplished by decreasing ADH secretion,
solutes to maintain the highly concentrated urine.
terone, which together cause avid sodium reabsorption
discussed in Chapter 29, low sodium intake stimulates
dehydration accompanied by low sodium intake. As
and creatinine. One condition in which this occurs is
other solutes, especially of waste products such as urea
. The hyperosmolarity of the urine in
sodium chloride
may not be obvious from this discussion. First,
There are several important points to consider that
centrating ability of the kidney.
medullary interstitium. This absorption of the urea
diffusion, much of the highly concentrated urea in
uid, and because the inner medullary collecting ducts
duced when ADH levels are high. Because water reab-
medullary interstitium (1200 to 1400 mOsm/L). Thus,
until osmotic equilibrium is reached, with the tubular
ADH, these ducts are highly permeable to water, and
rent mechanism. In the presence of large amounts of
depends on (1) ADH and (2) the osmolarity of the
The concentration of
Inner Medullary Collecting Ducts.
of ions from these segments.
cal collecting tubule; therefore, osmolarity decreases
excreted in the urine. In the absence of ADH, little
ducts, from which it is eventually reabsorbed or
concentration as water is reabsorbed. This allows most
this part of the nephron, resulting in increased urea
reabsorbed. Urea, however, is not very permeant in
able to water, and signi
high levels of ADH, these tubules are highly perme-
uid depends on the level of ADH. With
distal tubule and cortical collecting tubules, the osmo-
Late Distal Tubule and Cortical Collecting Tubules.
in the tubule.
Henle, so that further dilution of the tubular
The early distal tubule has proper-
Early Distal Tubule.
356
Unit V
The Body Fluids and Kidneys
ties similar to those of the thick ascending loop of
fluid
occurs as solutes are reabsorbed while water remains
In the late
larity of the fl
ficant amounts of water are
of the urea delivered to the distal tubule and collect-
ing tubule to pass into the inner medullary collecting
water is reabsorbed in the late distal tubule and corti-
even further because of continued active reabsorption
fluid in the inner medullary collecting ducts also
medullary interstitium established by the countercur-
water diffuses from the tubule into the interstitium
fluid having about the same concentration as the renal
a very concentrated but small volume of urine is pro-
sorption increases urea concentration in the tubular
fl
have specific urea transporters that greatly facilitate
the ducts diffuses out of the tubular lumen into the
into the renal medulla contributes to the high osmo-
larity of the medullary interstitium and the high con-
although sodium chloride is one of the principal
solutes that contributes to the hyperosmolarity of the
medullary interstitium, the kidney can, when needed,
excrete a highly concentrated urine that contains little
these circumstances is due to high concentrations of
formation of the hormones angiotensin II and aldos-
from the tubules while leaving the urea and other
Second, large quantities of dilute urine can be
excreted without increasing the excretion of sodium.
which reduces water reabsorption in the more distal
tubular segments without significantly altering sodium
reabsorption.
And fi
an obligatory urine volume
maximum concentrating ability of the kidney and the
be accompanied by the minimal amount of water
125 ml 44 ml
Ef
fect of
ADH
Osmolarity (mOsm/L)
Diluting segment
Late distal
Cortical
Medullary
1200
900
600
300
200
100
0
Proximal
tubule
25 ml
Distal
tubule
Collecting
tubule
and duct
Urine
20 ml
8 ml
0.2 ml
25 ml
Loop of Henle
ing along the different tubular
cal values indicate the approxi-
antidiuretic hormone (ADH) and
the presence of high levels of
the different tubular segments in
tubular fluid as it passes through
Figure 28–7
Changes in osmolarity of the
in the absence of ADH. (Numeri-
mate volumes in milliliters per
minute or in osmolarities in mil-
liosmoles per liter of fluid flow-
segments.)

positive.
free water,
in excess of solutes. Thus, water free of solutes, called
water clearance will be a positive value, denoting that
urine osmolarity is less than plasma osmolarity), free-
When the kidneys are forming a dilute urine (that is,
cits.
water back to the systemic circulation, as occurs during
excess of solutes, the kidneys are actually returning
1 ml/min. This means
rate is 1 ml/min and osmolar clearance is 2 ml/min,
Using the example discussed earlier, if urine
clearance is negative, excess solutes are being removed
water is being excreted by the kidneys; when free-water
kidneys. When free-water clearance is positive, excess
Thus, the rate of free-water clearance represents the
Free-
Assessed Using the Concept of “Free-Water Clearance.”
Relative Rates at Which Solutes and Water Are Excreted Can Be
being cleared of solute each minute.
(2.0 ml/min). This means that 2 milliliters of plasma are
0.6 mOsm/min divided by 300 mOsm/L, or 0.002 L/min
min), the rate of osmolar excretion is 0.6 mOsm/min
600 mOsm/L, and urine
plasma osmolarity is 300 mOsm/L, urine osmolarity is
is the plasma osmolarity. For example, if
rate, and P
is the urine osmolarity, V is the urine
volume of plasma cleared of solutes each minute, in
); this is the
expressed as the
The total clearance of solutes from the blood can be
is concentrated, solutes are excreted in excess of water.
excreted in excess of solutes. Conversely, when the urine
what independently. When the urine is dilute, water is
The process of concentrating or diluting the urine
“Free Water” and
liosmoles of solute must be excreted each day, this
necessary to excrete them. For example, if 1200 mil-
Chapter 28
Regulation of Extracellular Fluid Osmolarity and Sodium Concentration
357
requires at least 1 liter of urine if maximal urine con-
centrating ability is 1200 mOsm/L.
Quantifying Renal Urine
Concentration and Dilution:
Osmolar Clearances
requires the kidneys to excrete water and solutes some-
osmolar clearance (C
osm
the same way that clearance of a single substance is cal-
culated:
where U
osm
flow
osm
flow rate is 1 ml/min (0.001 L/
(600 mOsm/L
¥ 0.001 L/min) and osmolar clearance is
water clearance (C
H
2
O
) is calculated as the difference
between water excretion (urine flow rate) and osmolar
clearance:
rate at which solute-free water is excreted by the
from the blood by the kidneys and water is being
conserved.
flow
free-water clearance would be
-
that instead of water being cleared from the kidneys in
water defi
Thus, whenever urine osmolarity is greater
than plasma osmolarity, free-water clearance will be neg-
ative, indicating water conservation.
water is being removed from the plasma by the kidneys
“
” is being lost from the body and the plasma
is being concentrated when free-water clearance is
=
-
=
-
H O
osm
C
V C
V
U
V
P
osm
osm
2
¥
(
)
(
)
C
U
V
P
osm
osm
osm
=
¥
and tetracyclines (used as antibiotics), can impair the
urine concentrating ability. And certain drugs, such as
trolyte reabsorption by this segment, can compromise
loop of Henle, as occurs with diuretics that inhibit elec-
renal medulla. Also, impairment of the function of the
trating mechanism, especially those that damage the
formed, which tends to cause dehydration unless
ADH. In either case, large volumes of dilute urine are
kidneys. This abnormality can be due to either failure of
“nephrogenic” diabetes
This condition is referred to as
renal tubular segments cannot respond appropriately.
normal or elevated levels of ADH are present but the
There are circumstances in which
or orally, and rapidly restores urine output toward
Desmopressin can be given by injection, as a nasal spray,
permeability in the late distal and collecting tubules.
which acts selectively on V
istration of a synthetic analog of ADH,
The treatment for central diabetes insipidus is admin-
dehydration can rapidly occur.
scious (for example, because of a head injury), severe
intake is restricted, as can occur in a hospital setting
the large volume of dilute urine. However, if water
uid water do not occur. The primary abnormal-
as the person drinks enough water, large decreases in
excessive water is lost from the body; therefore, as long
nisms, discussed later in this chapter, are activated when
volumes that can exceed 15 L/day. The thirst mecha-
formation of a large volume of dilute urine, with urine
, results in the
“central” diabetes insipidus
dition, called
cannot reabsorb water in the absence of ADH, this con-
it can be congenital. Because the distal tubular segments
pituitary can be caused by head injuries or infections, or
inability to produce or release ADH from the posterior
collecting ducts to respond to ADH.
much ADH is present, maximal urine concentration
maximal urine concentrating ability. No matter how
Impairment of the countercurrent mechanism.
uid handling by the kidneys.
or too little ADH secretion results in abnormal
Inappropriate secretion of ADH.
Disorders of Urinary
Concentrating Ability
An impairment in the ability of the kidneys to concen-
trate or dilute the urine appropriately can occur with
one or more of the following abnormalities:
1.
Either too much
fl
2.
A
hyperosmotic medullary interstitium is required for
is limited by the degree of hyperosmolarity of the
medullary interstitium.
3. Inability of the distal tubule, collecting tubule, and
Failure to Produce ADH: “Central” Diabetes Insipidus.
An
body fl
ity observed clinically in people with this condition is
when fluid intake is restricted or the patient is uncon-
desmopressin,
2
receptors to increase water
normal.
Inability of the Kidneys to Respond to ADH: “Nephrogenic”
Diabetes Insipidus.
insipidus because the abnormality resides in the
the countercurrent mechanism to form a hyperosmotic
renal medullary interstitium or failure of the distal and
collecting tubules and collecting ducts to respond to
fluid
intake is increased by the same amount as urine volume
is increased.
Many types of renal diseases can impair the concen-
lithium (used to treat manic-depressive disorders)
ability of the distal nephron segments to respond to
ADH.
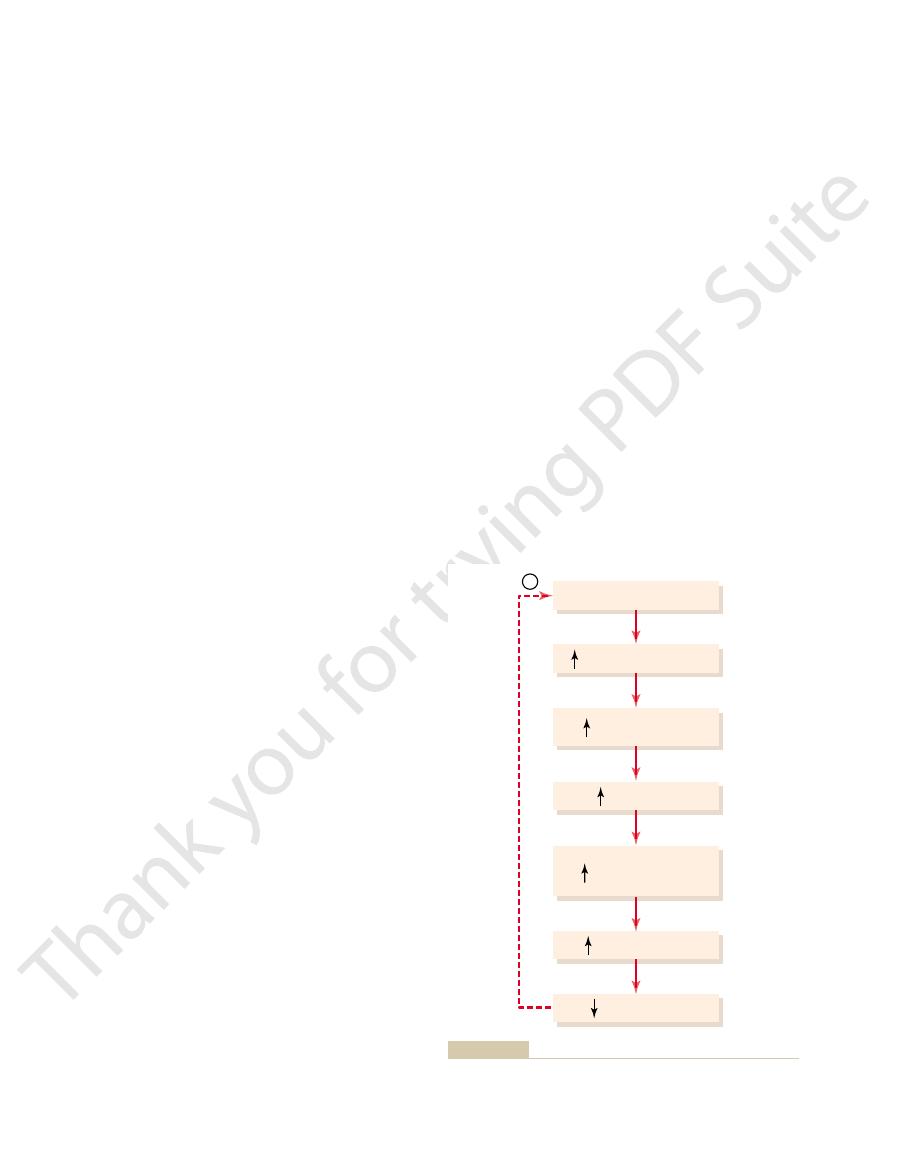
cells in the supraoptic nuclei, which then relay
re, sending nerve signals to additional nerve
2. Shrinkage of the osmoreceptor cells causes them
nuclei, to shrink.
, located in
1. An increase in extracellular
example, this feedback system operates as follows:
cit, for
ity. When osmolarity (plasma sodium concentration)
osmoreceptor-ADH feedback system for control of
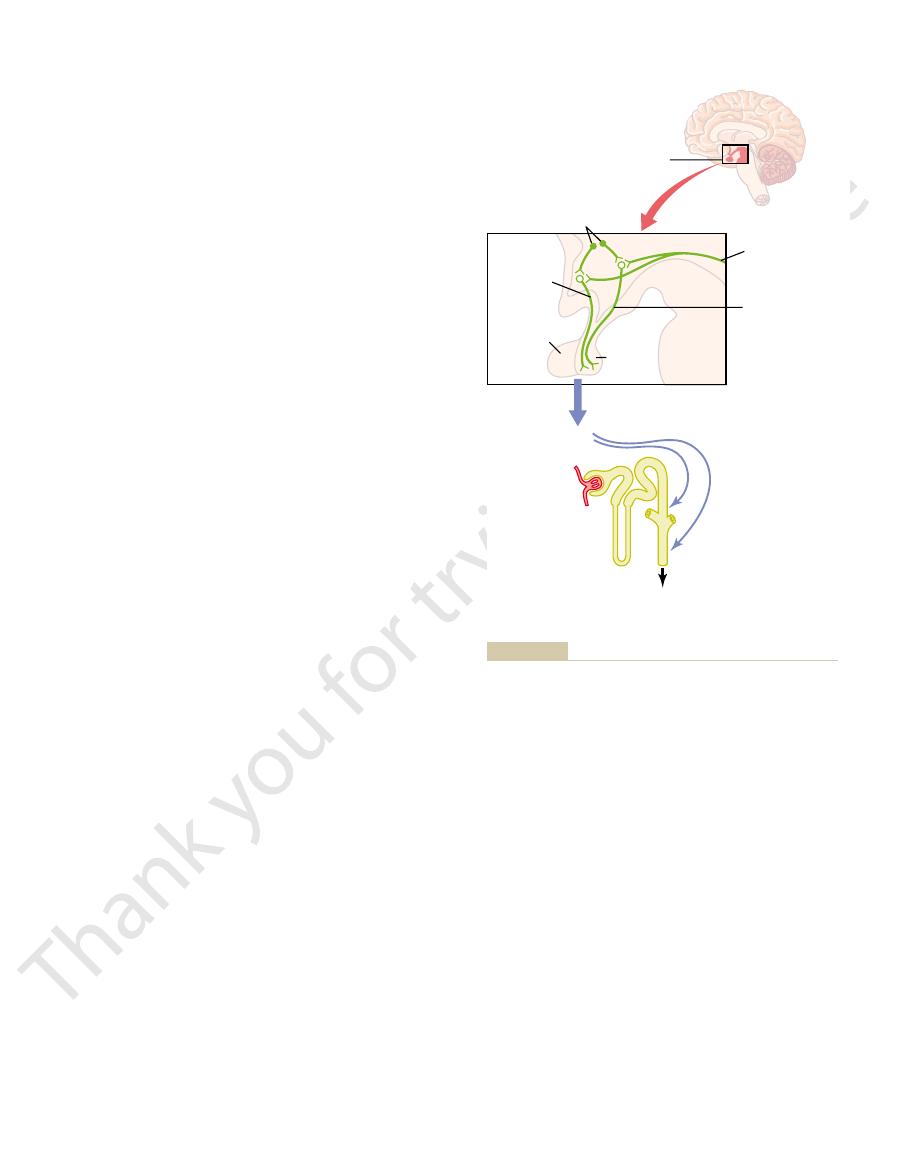
Figure 28
Osmoreceptor-ADH
uid: (1) the osmoreceptor-ADH system and
of sodium and water excretion by the kidneys, two
ion concentration at the same time.
ment across the cell membrane. Consequently, we can
pressure under steady-state conditions. Therefore, the
most cell membranes, it exerts little
osmoles. However, because urea easily permeates
per cent of the extracellular osmoles, with glucose and
Normally, sodium ions and associated anions (pri-
percentage points of those measured directly.
glucose and urea, should be included. Such estimates
renal disease, the contribution of two other solutes,
be more exact, especially in conditions associated with
from the formula above to be about 298 mOsm/L. To
142 mEq/L, the plasma osmolarity would be estimated
For instance, with a plasma sodium concentration of
solute in the extracellular compartment, plasma osmo-
routinely measured. However, because sodium and its
In most clinical laboratories, plasma osmolarity is not
Plasma Sodium Concentration
compartments.
discussed in Chapter 25, these variables must be pre-
2 to 3 per cent. As
L, with an average concentration of about 142 mEq/L.
compartment. Plasma sodium concentration is nor-
Control of Extracellular Fluid
diuretic.
enhances renal sodium excretion, such as a thiazide
disorder. The hypernatremia can also be attenuated by
insipidus is to correct, if possible, the underlying renal
betes insipidus. The treatment for nephrogenic diabetes
desmopressin, the synthetic analog of ADH. Lack of a
358
Unit V
The Body Fluids and Kidneys
Nephrogenic diabetes insipidus can be distinguished
from central diabetes insipidus by administration of
prompt decrease in urine volume and an increase in
urine osmolarity within 2 hours after injection of
desmopressin is strongly suggestive of nephrogenic dia-
a low-sodium diet and administration of a diuretic that
Osmolarity and Sodium
Concentration
Regulation of extracellular fluid osmolarity and
sodium concentration are closely linked because
sodium is the most abundant ion in the extracellular
mally regulated within close limits of 140 to 145 mEq/
Osmolarity averages about 300 mOsm/L (about
282 mOsm/L when corrected for interionic attraction)
and seldom changes more than
±
cisely controlled because they determine the distribu-
tion of fluid between the intracellular and extracellular
Estimating Plasma Osmolarity from
associated anions account for about 94 per cent of the
larity (P
osm
) can be roughly approximated as
P
osm
= 2.1 ¥ Plasma sodium concentration
of plasma osmolarity are usually accurate within a few
marily bicarbonate and chloride) represent about 94
urea contributing about 3 to 5 per cent of the total
effective osmotic
sodium ions in the extracellular fluid and associated
anions are the principal determinants of fluid move-
discuss the control of osmolarity and control of sodium
Although multiple mechanisms control the amount
primary systems are especially involved in regulating
the concentration of sodium and osmolarity of extra-
cellular fl
(2) the thirst mechanism.
Feedback System
–8 shows the basic components of the
extracellular fluid sodium concentration and osmolar-
increases above normal because of water defi
fluid osmolarity
(which in practical terms means an increase in
plasma sodium concentration) causes the special
nerve cells called osmoreceptor cells
the anterior hypothalamus near the supraoptic
to fi
Water deficit
-
Extracellular osmolarity
Osmoreceptors
Plasma ADH
ADH secretion
(posterior pituitary)
H
2
O permeability in
distal tubules,
collecting ducts
H
2
O reabsorption
H
2
O excreted
for regulating extracellular fluid osmolarity in response to a water
Osmoreceptor-antidiuretic hormone (ADH) feedback mechanism
Figure 28–8
deficit.
uid osmolarity.
ring and secretion of ADH.
neurons. These cells send nerve signals to the supraop-
uid osmolarity; hence, the
In the vicinity of the AV3V region and the supraop-
thirst, and sodium appetite.
ulation by angiotensin II can alter ADH secretion,
pressure. Electrical stimulation of this region or stim-
of ADH secretion, thirst, sodium appetite, and blood
the AV3V region cause multiple de
control centers in the medulla of the brain. Lesions of
median preoptic nucleus,
. Between these two
, and at the infe-
. At the upper part of this region is a
AV3V region
, called the
anteroventral region of the third ventricle
osmolarity and ADH secretion is located along the
means for altering renal excretion of water.
severalfold within minutes, thereby providing a rapid
lus is rapid, so that plasma ADH levels can increase
Secretion of ADH in response to an osmotic stimu-
response to increased calcium entry. The released
entry. ADH stored in the secretory granules (also
impulses pass down these nerve endings, changing
lated by increased osmolarity or other factors, nerve
tips, terminating in the posterior pituitary gland. When
the posterior pituitary. Once ADH is synthesized, it is
nuclei. Both of these nuclei have axonal extensions to
, about
magnocellular (large) neurons that synthesize
sized and released. The hypothalamus contains two
amus and the pituitary gland, where ADH is synthe-
Figure 28
Hypothalamus and ADH Release
of dilute urine is formed. This in turn concentrates the
for water, less water is reabsorbed, and a large volume
formed, the renal tubules decrease their permeability
uid osmolarity, less ADH is
For example, with excess water ingestion and a
The opposite sequence of events occurs when the
other solutes continue to be excreted in the urine. This
Thus, water is conserved in the body while sodium and
concentrated urine.
5. The increased water permeability in the distal
collecting tubules, and medullary collecting ducts.
permeability of the late distal tubules, cortical
to the kidneys, where it increases the water
4. ADH enters the blood stream and is transported
nerve endings.
pituitary stimulate the release of ADH, which is
3. These action potentials conducted to the posterior
to the posterior pituitary.
Chapter 28
Regulation of Extracellular Fluid Osmolarity and Sodium Concentration
359
these signals down the stalk of the pituitary gland
stored in secretory granules (or vesicles) in the
nephron segments causes increased water
reabsorption and excretion of a small volume of
causes dilution of the solutes in the extracellular fluid,
thereby correcting the initial excessively concentrated
extracellular fluid.
extracellular fluid becomes too dilute (hypo-osmotic).
decrease in extracellular fl
body fluids and returns plasma osmolarity toward
normal.
ADH Synthesis in Supraoptic and
Paraventricular Nuclei of the
from the Posterior Pituitary
–9 shows the neuroanatomy of the hypothal-
types of
ADH in the supraoptic and paraventricular nuclei of
the hypothalamus
five sixths in the supraoptic
nuclei and about one sixth in the paraventricular
transported down the axons of the neurons to their
the supraoptic and paraventricular nuclei are stimu-
their membrane permeability and increasing calcium
called vesicles) of the nerve endings is released in
ADH is then carried away in the capillary blood of the
posterior pituitary into the systemic circulation.
A second neuronal area important in controlling
structure called the subfornical organ
rior part is another structure called the organum vas-
culosum of the lamina terminalis
organs is the
which has mul-
tiple nerve connections with the two organs as well as
with the supraoptic nuclei and the blood pressure
ficits in the control
tic nuclei are neuronal cells that are excited by small
increases in extracellular fl
term osmoreceptors has been used to describe these
tic nuclei to control their fi
It is also likely that they induce thirst in response to
increased extracellular fl
ADH
Urine:
decreased flow
and concentrated
Baroreceptors
Cardiopulmonary
receptors
Paraventricular
neuron
Osmoreceptors
Pituitary
Posterior
lobe
Anterior
lobe
Supraoptic
neuron
ADH is released.
(ADH) is synthesized, and the posterior pituitary gland, where
Neuroanatomy of the hypothalamus, where antidiuretic hormone
Figure 28–9

2. For example,
by various drugs and hormones, as shown in Table
Other Stimuli for ADH Secretion
osmolarity.
greatly enhances the ADH response to increased
plasma osmolarity. Decreased blood volume, however,
usual day-to-day regulation of ADH secretion during
play a major role in stimulating ADH secretion. The
decreases in blood volume, the cardiovascular re
volume, ADH levels rapidly increase. Thus, with severe
about 10 per cent. With further decreases in blood
contrast, after blood loss, plasma ADH levels do not
cient to increase ADH levels. By
For example, a change in plasma osmolarity of only
osmolarity than to similar changes in blood volume.
osmolarity stimulates ADH secretion. However, ADH
10, either a decrease in effec-
As shown in Figure 28
Osmolarity in Stimulating
uid reabsorption by the kidneys,
occurs during hemorrhage, increased ADH secretion
blood pressure and blood volume are reduced, such as
pressure and (2) decreased blood volume. Whenever
stimuli increase ADH secretion: (1) decreased arterial
Thus, in addition to increased osmolarity, two other
the tractus solitarius. Projections from these nuclei
atria. Afferent stimuli are carried by the vagus and
in the low-pressure regions, especially in the cardiac
culation, such as the aortic arch and carotid sinus, and
both of which are discussed in Chapter 18. These re
the cardiopulmonary reflexes
and (2)
ceptor reflexes
and/or blood volume, including (1) the
Blood Volume
of ADH Release by Decreased
tion of ADH and over thirst, as discussed later.
uid, exerting powerful control over the secre-
uid in this region. As a result, the osmoreceptors
tissue. This makes it possible for ions and other solutes
360
Unit V
The Body Fluids and Kidneys
Both the subfornical organ and the organum vascu-
losum of the lamina terminalis have vascular supplies
that lack the typical blood-brain barrier that impedes
the diffusion of most ions from the blood into the brain
to cross between the blood and the local interstitial
fl
rapidly respond to changes in osmolarity of the extra-
cellular fl
Cardiovascular Reflex Stimulation
Arterial Pressure and/or Decreased
ADH release is also controlled by cardiovascular
reflexes that respond to decreases in blood pressure
arterial barore-
,
flex
pathways originate in high-pressure regions of the cir-
glossopharyngeal nerves with synapses in the nuclei of
relay signals to the hypothalamic nuclei that control
ADH synthesis and secretion.
causes increased fl
helping to restore blood pressure and blood volume
toward normal.
Quantitative Importance of
Cardiovascular Reflexes and
ADH Secretion
–
tive blood volume or an increase in extracellular fluid
is considerably more sensitive to small changes in
1 per cent is suffi
change appreciably until blood volume is reduced by
flexes
simple dehydration is effected mainly by changes in
ADH secretion can also be increased or decreased by
other stimuli to the central nervous system as well as
28–
nausea is a potent stimulus for
5
10
15
20
0.1
AVP
AVP
Plasma ADH
(pg/ml)
Per cent change
P
= 2.5
D
Osm + 2.0
P
= 1.3 e
–
7 vol.
0
5
10
15
20
25
30
35
40
50
45
0
Isotonic volume depletion
Isovolemic osmotic increase
By copyright permission of the
lality and volume in regulating vasopressin secretion in the rat. J
Brennan TJ, Nelson AE, Robertson GL: The role of blood osmo-
called arginine vasopressin (AVP). (Redrawn from Dunn FL,
volume on the level of plasma (P) antidiuretic hormone (ADH), also
The effect of increased plasma osmolarity or decreased blood
Figure 28–10
Clin Invest 52(12):3212, 1973.
American Society of Clinical Investigation.)
Table 28–2
Nicotine
Clonidine (antihypertensive drug)
Morphine
Alcohol
Drugs:
Drugs:
Increase ADH
Decrease ADH
Regulation of ADH Secretion
≠ Plasma osmolarity
Ø Plasma osmolarity
Ø Blood volume
≠ Blood volume
Ø Blood pressure
≠ Blood pressure
Nausea
Hypoxia
Cyclophosphamide
Haloperidol (dopamine blocker)

person would continue to drink more and more, even-
were not temporarily relieved after drinking water, the
tributed throughout the body. If the thirst sensation
After a person drinks water, 30 to 60 minutes may be
The ability of animals and humans to
mechanisms is short-lived; the desire to drink is com-
stomach can relieve thirst. However, relief of thirst
thirst; for instance, simple in
drinking, although the relief is only temporary. Also,
into the blood, partial relief of thirst occurs after
. For example, in animals that have an esophageal
uid osmolarity.
immediately after drinking water, even though the
can elicit the sensation of thirst. As a result,
sure toward normal, along with the other actions
with hypovolemia and low blood pressure, its effect on
angiotensin II diffuse into the tissues. Because
outside the blood-brain barrier, and peptides such as
losum of the lamina terminalis. These regions are
A third important stimulus for thirst is angiotensin II
in plasma osmolarity. This probably occurs because of
osmolarity. Thus, blood volume loss by hemorrhage
obvious: it helps to dilute extracellular
the sensation of thirst. The value of this response is
, thereby stimulating
cellular fluid osmolarity, which causes intracellular
thirst. One of the most important is
Table 28
this response.
the AV3V region, is intimately involved in mediating
, which lies immediately
promote drinking. It is likely that the
release.
the same way that the osmoreceptors stimulate ADH
osmoreceptors to activate the thirst mechanism, in
ing behavior. These cells almost certainly function as
The neurons of the thirst center respond to injec-
lasts. All these areas together are called the
area that, when stimulated electrically, causes immedi-
motes ADH release also stimulates thirst. Located
9, the same area along
Referring again to Figure 28
Central Nervous System Centers
scious desire for water.
tion also increase thirst, which is de
Many of the same factors that stimulate ADH secre-
mechanism, maintains precise control of extracellular
anism, which, together with the osmoreceptor-ADH
nal tract. Fluid intake is regulated by the thirst mech-
uid intake, however, is necessary to coun-
through the osmoreceptor-ADH feedback system.
The kidneys minimize
Role of Thirst in Controlling
is due in part to inhibition of ADH release.
, inhibit ADH release. The
some drugs, such as
stimulate ADH release, whereas
times normal after vomiting. Also, drugs such as
ADH release, which may increase to as much as 100
Chapter 28
Regulation of Extracellular Fluid Osmolarity and Sodium Concentration
361
nico-
tine and morphine
alcohol
marked diuresis that occurs after ingestion of alcohol
Extracellular Fluid Osmolarity
and Sodium Concentration
fluid loss during water deficits
Adequate fl
terbalance whatever fluid loss does occur through
sweating and breathing and through the gastrointesti-
fluid osmolarity and sodium concentration.
fined as the con-
for Thirst
–
the anteroventral wall of the third ventricle that pro-
anterolaterally in the preoptic nucleus is another small
ate drinking that continues as long as the stimulation
thirst
center.
tions of hypertonic salt solutions by stimulating drink-
Increased osmolarity of the cerebrospinal fluid in
the third ventricle has essentially the same effect to
organum vascu-
losum of the lamina terminalis
beneath the ventricular surface at the inferior end of
Stimuli for Thirst
–3 summarizes some of the known stimuli for
increased extra-
dehydration in the thirst centers
fluids and
returns osmolarity toward normal.
Decreases in extracellular fluid volume and arterial
pressure also stimulate thirst by a pathway that is inde-
pendent of the one stimulated by increased plasma
stimulates thirst even though there might be no change
neutral input from cardiopulmonary and systemic
arterial baroreceptors in the circulation.
.
Studies in animals have shown that angiotensin II acts
on the subfornical organ and on the organum vascu-
angiotensin II is also stimulated by factors associated
thirst helps to restore blood volume and blood pres-
of angiotensin II on the kidneys to decrease fluid
excretion.
Dryness of the mouth and mucous membranes of the
esophagus
a thirsty person may receive relief from thirst almost
water has not been absorbed from the gastrointestinal
tract and has not yet had an effect on extracellular
fl
Gastrointestinal and pharyngeal stimuli influence
thirst
opening to the exterior so that water is never absorbed
gastrointestinal distention may partially alleviate
flation of a balloon in the
sensations through gastrointestinal or pharyngeal
pletely satisfied only when plasma osmolarity and/or
blood volume returns to normal.
“meter” fluid
intake is important because it prevents overhydration.
required for the water to be reabsorbed and dis-
tually leading to overhydration and excess dilution of
Table 28–3
Increase Thirst
Decrease Thirst
Control of Thirst
≠ Osmolarity
Ø Osmolarity
Ø Blood volume
≠ Blood volume
Ø Blood pressure
≠ Blood pressure
≠ Angiotensin
Ø Angiotensin II
Dryness of mouth
Gastric distention

12. This
the experiment of Figure 28
This relative unimportance of aldosterone in regulat-
conditions.
, except under extreme
Therefore,
sodium concentration. Although these hormones
kidneys, one might mistakenly infer that they also
versely, with high sodium intake, decreased formation of
may be reduced to as low as 10 per cent of normal. Con-
prevent large sodium losses, even though sodium intake
sodium reabsorption by the kidneys and, therefore,
is low, increased levels of these hormones stimulate
reabsorption by the renal tubules. When sodium intake
As discussed in Chapter 27, both angiotensin II and
Sodium Concentration
Aldosterone in Controlling
Role of Angiotensin II and
osmolarity.
occur. In the absence of the ADH-thirst mechanisms,
after blocking the total ADH-thirst system, relatively
controlled; thus, when sodium intake is increased
and thirst mechanisms fail simultaneously, plasma
gastrointestinal losses. However, if both the ADH
water losses caused by respiration, sweating, or
effectiveness, as long as there is enough
When either the ADH or the thirst mechanism fails,
are both functioning normally.
centration as long as the ADH and thirst mechanisms
osmolarity reasonably constant. Figure 28
intake, these feedback systems are able to keep plasma
Even with additional challenges, such as high salt
tion, despite the constant challenges of dehydration.
In a healthy person, the osmoreceptor-ADH and thirst
Mechanisms in Controlling
Integrated Responses of
and volume toward normal. In this way, the extracel-
intake, which restores extracellular
. Thus, even small increases
activated, causing a desire to drink water. This is called
about 2 mEq/L above normal, the thirst mechanism is
ity. When the sodium concentration increases only
tendency for dehydration, with resultant increased
sweating from the skin. Therefore, there is always a
olism. Water is also lost by evaporation from the lungs
uid, even in a dehydrated person, to rid the body of
The kidneys must continually excrete at least some
of Drinking
Threshold for Osmolar Stimulus
uids. Experimental studies have repeatedly
362
Unit V
The Body Fluids and Kidneys
the body fl
shown that animals drink almost exactly the amount
necessary to return plasma osmolarity and volume to
normal.
fl
excess solutes that are ingested or produced by metab-
and the gastrointestinal tract and by evaporation and
extracellular fluid sodium concentration and osmolar-
the threshold for drinking
in plasma osmolarity are normally followed by water
fluid osmolarity
lular fluid osmolarity and sodium concentration are
precisely controlled.
Osmoreceptor-ADH and Thirst
Extracellular Fluid Osmolarity and
Sodium Concentration
mechanisms work in parallel to precisely regulate
extracellular fluid osmolarity and sodium concentra-
–11 shows
that an increase in sodium intake to as high as six times
normal has only a small effect on plasma sodium con-
the other ordinarily can still control extracellular
osmolarity and sodium concentration with reasonable
fluid intake
to balance the daily obligatory urine volume and
sodium concentration and osmolarity are very poorly
large changes in plasma sodium concentration do
no other feedback mechanism is capable of ad-
equately regulating plasma sodium concentration and
Extracellular Fluid Osmolarity and
aldosterone play an important role in regulating sodium
these hormones permits the kidneys to excrete large
amounts of sodium.
Because of the importance of angiotensin II and
aldosterone in regulating sodium excretion by the
play an important role in regulating extracellular fluid
increase the amount of sodium in the extracellular fluid,
they also increase the extracellular fluid volume by
increasing reabsorption of water along with the sodium.
angiotensin II and aldosterone have little
effect on sodium concentration
ing extracellular fluid sodium concentration is shown by
–
figure shows the
effect on plasma sodium concentration of changing
Normal
ADH and
thirst
systems
blocked
0
30
60
90
120
150
180
Plasma sodium concentration (mEq/L)
Sodium intake (mEq/day)
136
152
148
144
140
feedback systems. (Courtesy Dr. David B. Young.)
). Note that control of extra-
and after the antidiuretic hormone (ADH) and thirst feedback
red line
sodium concentration in dogs under normal conditions (
Effect of large changes in sodium intake on extracellular fluid
Figure 28–11
)
systems had been blocked (blue line
cellular fluid sodium concentration is poor in the absence of these

Am J Physiol Regul Integr Comp Physiol 284:R1355, 2003.
ow.
Cowley AW Jr, Mori T, Mattson D, Zou AP: Role of renal
and salt appetite at the same time.
animals. Also, circulatory re
to be involved, because lesions in this region frequently
neuronal centers in the AV3V region of the brain seem
gous to that of the thirst mechanism. Some of the same
The neuronal mechanism for salt appetite is analo-
ciency. These are the same major stimuli that elicit thirst.
or blood pressure, associated with circulatory insuffi-
decreased sodium concentration; both of these changes
ciency of aldosterone secretion,
instance, there is de
s disease. In this
of sodium, such as occurs in Addison
ciency
urally eat a low-sodium diet, but salt craving may also
This is particularly important in herbivores, which nat-
ciency in the body.
cient. There is also a regulatory component
ostasis, and there is evidence that our usual high sodium
function normally on 10 to 20 mEq/day. Thus, most
200 mEq/day, even though humans can survive and
necessary for homeostasis. In fact, the average sodium
lizations, sodium intake is almost always greater than
sodium excretion and sodium intake. In modern civi-
and Volume
for Controlling Extracellular
with a functional ADH-thirst mechanism. Even so, the
cantly, even
Thus, there are extreme situations in which plasma
conditions.
exes. This leads to a
pressure, which can activate the thirst mechanism
reductions in plasma sodium concentration. One of the
dous loss of sodium by the kidneys, which can lead to
secretion or total lack of aldosterone), there is tremen-
patients with Addison
Under extreme conditions, caused by complete loss of
extremely high levels of aldosterone, the plasma sodium
, who have
ulating sodium concentration under normal conditions.
toward normal. The ADH-thirst system far overshad-
tion, which tends to dilute the extracellular
increased water intake or increased plasma ADH secre-
anism is functional, any tendency toward increased
Second, as long as the ADH-thirst mech-
concentrations.
tubules, leading to increases in extracellular
cussed earlier, angiotensin II and aldosterone increase
effect on plasma sodium concentration. First, as dis-
There are two primary reasons why changes in
conducted after blocking angiotensin II formation, with
well regulated. The same type of experiment has been
feedback system, plasma sodium concentration can be
changed only about 1 to 2 per cent in either case. This
intake was increased sixfold, plasma concentration
change upward or downward. Note that when sodium
Chapter 28
Regulation of Extracellular Fluid Osmolarity and Sodium Concentration
363
sodium intake more than sixfold under two conditions:
(1) under normal conditions and (2) after the aldos-
terone feedback system has been blocked by removing
the adrenal glands and infusing the animals with aldos-
terone at a constant rate so that plasma levels could not
indicates that even without a functional aldosterone
the same result.
angiotensin II and aldosterone do not have a major
both sodium and water reabsorption by the renal
fluid
volume and sodium quantity but little change in sodium
plasma sodium concentration is compensated for by
fluid back
ows the angiotensin II and aldosterone systems for reg-
Even in patients with primary aldosteronism
concentration usually increases only about 3 to 5 mEq/L
above normal.
aldosterone secretion because of adrenalectomy or in
’s disease (severely impaired
reasons for this is that large losses of sodium eventually
cause severe volume depletion and decreased blood
through the cardiovascular refl
further dilution of the plasma sodium concentration,
even though the increased water intake helps to mini-
mize the decrease in body fluid volumes under these
sodium concentration may change signifi
ADH-thirst mechanism is by far the most powerful
feedback system in the body for controlling extracellu-
lar fluid osmolarity and sodium concentration.
Salt-Appetite Mechanism
Fluid Sodium Concentration
Maintenance of normal extracellular fluid volume and
sodium concentration requires a balance between
intake for individuals in industrialized cultures eating
processed foods usually ranges between 100 and
people eat far more sodium than is necessary for home-
intake may contribute to cardiovascular disorders such
as hypertension.
Salt appetite is due in part to the fact that animals and
humans like salt and eat it regardless of whether they
are salt-defi
to salt appetite in which there is a behavioral drive to
obtain salt when there is sodium defi
be important in humans who have extreme defi
’
fi
which causes excessive loss of sodium in the urine and
leads to decreased extracellular fluid volume and
elicit the desire for salt.
In general, the two primary stimuli that are believed to
increase salt appetite are (1) decreased extracellular fluid
sodium concentration and (2) decreased blood volume
affect both thirst and salt appetite simultaneously in
flexes elicited by low blood
pressure or decreased blood volume affect both thirst
References
NO production in the regulation of medullary blood fl
90
120 150 180
210
0
30
60
110
Plasma sodium concentration
(mEq/L)
Sodium intake (mEq/L)
100
120
130
140
150
Normal
Aldosterone system blocked
Aldosterone system blocked
aldosterone feedback control. (Courtesy Dr. David B. Young.)
). Note that sodium concentration is maintained relatively
and after the aldosterone feedback system had been blocked
red line
sodium concentration in dogs under normal conditions (
Effect of large changes in sodium intake on extracellular fluid
Figure 28–12
)
(blue line
constant over this wide range of sodium intakes, with or without

4:177, 2003.
Verbalis JG: Diabetes insipidus. Rev Endocr Metab Disord
Behav 77:731, 2002.
thirst: similarities and dissimilarities in signals. Physiol
Stricker EM, Sved AF: Controls of vasopressin secretion and
brane Biol 191:149, 2003.
Sands JM: Molecular mechanisms of urea transport. J Mem-
York: Raven Press, 2000.
Physiology and Pathophysiology, 3rd ed. New
its regulation. In Seldin DW, Giebisch G (eds): The
Sands JM, Layton HE: Urine concentrating mechanism and
New York: Raven Press, 2000.
Physiology and Pathophysiology, 3rd ed.
The Kidney
Robertson GL: Vasopressin. In Seldin DW, Giebisch G (eds):
Regul Integr Comp Physiol 284:R1153, 2003.
current exchange in the renal medulla. Am J Physiol
Pallone TL, Turner MR, Edwards A, Jamison RL: Counter-
kidney: from molecules to medicine. Physiol Rev 82:205,
Nielsen S, Frokiaer J, Marples D, et al: Aquaporins in the
Annu Rev Physiol 63:607, 2001.
Morello JP, Bichet DG: Nephrogenic diabetes insipidus.
uid intake. News Physiol Sci 19:1, 2004.
McKinley MJ, Johnson AK: The physiological regulation of
medicine. J Clin Invest 109:1395, 2002.
nels: atomic structure molecular dynamics meet clinical
Kozono D, Yasui M, King LS, Agre P: Aquaporin water chan-
Physiol Renal Physiol 284:F433, 2003.
hyaluronan as a mechano-osmotic transducer. Am J
tion of solutes in the renal inner medulla: interstitial
Knepper MA, Saidel GM, Hascall VC, Dwyer T: Concentra-
Physiol Rev 78:583, 1998.
Fitzsimons JT: Angiotensin, thirst, and sodium appetite.
Physiol Renal Physiol 278:F257, 2000.
solute recovery by inner medullary vasa recta. Am J
Edwards A, Delong MJ, Pallone TL: Interstitial water and
18:1, 2003.
that concentrates urine in the papilla. News Physiol Sci
Dwyer TM, Schmidt-Nielsen B: The renal pelvis: machinery
364
Unit V
The Body Fluids and Kidneys
thirst and fl
2002.
—
Kidney—
