
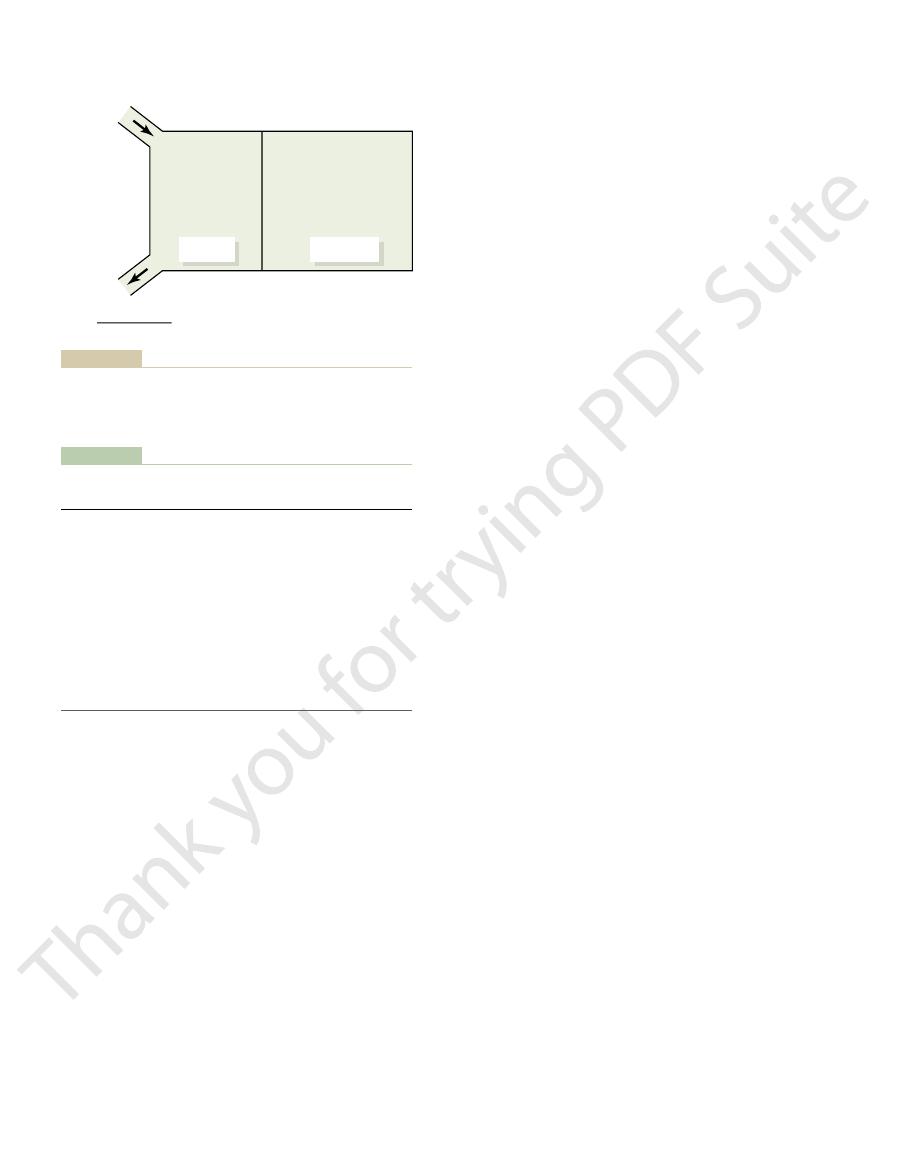
kalemia or as a source of potassium during hypokalemia. Thus, redistribution
over 98 per cent of the total body potassium is contained in the cells, they can
compartments also plays an important role in potassium homeostasis. Because
to wide variations in intake, as is also true for most other electrolytes.
of the potassium intake. Thus, the maintenance of normal potassium balance
responses.
(increased plasma potassium concentration). Likewise, a small loss of potassium
between 50 and 200 mEq/day; therefore, failure to rapidly rid the extracellular
is often as high as 50 milliequivalents, and the daily intake usually ranges
lents are in the extracellular fluid. Also, the potassium contained in a single meal
and 14 liters of extracellular fluid (20 per cent of body weight), about 3920 mil-
adult, who has about 28 liters of intracellular fluid (40 per cent of body weight)
and only 2 per cent in the extracellular fluid (Figure 29–1). For a 70-kilogram
concentration of only 3 to 4 mEq/L can cause cardiac arrhythmias, and higher
fluid potassium concentration. For instance, an increase in plasma potassium
0.3 mEq/L. This precise control is neces-
mally is regulated precisely at about 4.2 mEq/L,
Excretion and Potassium
Extracellular Fluid Volume
Control of Blood Volume and
Magnesium; Integration
Calcium, Phosphate, and
Renal Regulation of Potassium,
C
H
A
P
T
E
R
2
9
365
of Renal Mechanisms for
Regulation of Potassium
Concentration in
Extracellular Fluid
Extracellular fluid potassium concentration nor-
seldom rising or falling more than
±
sary because many cell functions are very sensitive to changes in extracellular
concentrations can lead to cardiac arrest or fibrillation.
A special difficulty in regulating extracellular potassium concentration is the
fact that over 98 per cent of the total body potassium is contained in the cells
liequivalents of potassium are inside the cells and only about 59 milliequiva-
fluid of the ingested potassium could cause life-threatening hyperkalemia
from the extracellular fluid could cause severe hypokalemia (low plasma potas-
sium concentration) in the absence of rapid and appropriate compensatory
Maintenance of potassium balance depends primarily on excretion by the
kidneys because the amount excreted in the feces is only about 5 to 10 per cent
requires the kidneys to adjust their potassium excretion rapidly and precisely
Control of potassium distribution between the extracellular and intracellular
serve as an overflow site for excess extracellular fluid potassium during hyper-
of potassium between the intra- and extracellular fluid compartments pro-
vides a first line of defense against changes in extracellular fluid potassium
concentration.
glucose raise extracellular osmolarity, causing cell
site effect. In diabetes mellitus, large increases in plasma
intracellular potassium concentration, thereby pro-
water out of the cells. The cellular dehydration increases
viduals with insulin deficiency. In rare instances, hyper-
extracellular fluid. Usually the hyperkalemia is mild, but
During prolonged exercise,
cell lysis.
extracellular compartment. This can cause significant
As cells are destroyed, the large amounts of potas-
Cell Lysis Causes Increased Extracellular Potassium Concentra-
(ATPase) pump. This in turn decreases cellular uptake
not completely understood, one effect of increased
extracellular fluid potassium concentration. Although
from the cells, whereas metabolic alkalosis decreases
sium concentration, in part by causing loss of potassium
and creates a tendency toward hyperkalemia.
propranolol, causes potassium to move out of the cells
-adrenergic receptor blockers, such as
-adrenergic receptors. Treatment of hyperten-
extracellular to the intracellular fluid, mainly by activa-
nephrine, can cause movement of potassium from the
Increased secretion of catecholamines, especially epi-
tion (Addison’s disease) often have clinically significant
Conversely, patients with deficient aldosterone produc-
movement of extracellular potassium into the cells.
invariably associated with hypokalemia, due in part to
aldosterone secretion (Conn’s syndrome) is almost
terone, which increases cell potassium uptake. Excess
however, can help to correct the hyperkalemia.
much greater than normal. Injections of insulin,
insulin deficiency owing to diabetes mellitus, the rise in
uptake after a meal is insulin. In people who have
cellular compartments.
until the kidneys can eliminate the excess. Table 29–1
the extracellular compartment. Fortunately, most of
cells. For example, absorption of 40 milliequivalents of
After ingestion of a normal meal, extracellular fluid
Regulation of Internal Potassium
366
Unit V
The Body Fluids and Kidneys
Distribution
potassium concentration would rise to a lethal level if
the ingested potassium did not rapidly move into the
potassium (the amount contained in a meal rich in veg-
etables and fruit) into an extracellular fluid volume of
14 liters would raise plasma potassium concentration
by about 2.9 mEq/L if all the potassium remained in
the ingested potassium rapidly moves into the cells
summarizes some of the factors that can influence the
distribution of potassium between the intra- and extra-
Insulin Stimulates Potassium Uptake into Cells.
One of the
most important factors that increase cell potassium
plasma potassium concentration after eating a meal is
Aldosterone Increases Potassium Uptake into Cells.
Increased
potassium intake also stimulates secretion of aldos-
hyperkalemia due to accumulation of potassium in
the extracellular space as well as to renal retention of
potassium.
b-Adrenergic Stimulation Increases Cellular Uptake of Potassium.
tion of
b
2
sion with
b
Acid-Base Abnormalities Can Cause Changes in Potassium Distri-
bution.
Metabolic acidosis increases extracellular potas-
the mechanisms responsible for the effect of hydrogen
ion concentration on potassium internal distribution are
hydrogen ion concentration is to reduce the activity
of the sodium-potassium adenosine triphosphatase
of potassium and raises extracellular potassium
concentration.
tion.
sium contained in the cells are released into the
hyperkalemia if large amounts of tissue are destroyed,
as occurs with severe muscle injury or with red blood
Strenuous Exercise Can Cause Hyperkalemia by Releasing Potas-
sium from Skeletal Muscle.
potassium is released from skeletal muscle into the
it may be clinically significant after heavy exercise in
patients treated with
b-adrenergic blockers or in indi-
kalemia after exercise may be severe enough to cause
cardiac arrhythmias and sudden death.
Increased Extracellular Fluid Osmolarity Causes Redistribution of
Potassium from the Cells to Extracellular Fluid.
Increased
extracellular fluid osmolarity causes osmotic flow of
moting diffusion of potassium out of the cells and
increasing extracellular fluid potassium concentration.
Decreased extracellular fluid osmolarity has the oppo-
Feces 8 mEq/day
K
+
intake
100 mEq/day
K
+
output
Urine 92 mEq/day
100 mEq/day
Extracellular
fluid K
+
4.2 mEq/L
x 14 L
Intracellular
fluid K
+
140 mEq/L
x 28 L
59 m Eq
3920 mEq
fluids, and potassium output from the body.
Normal potassium intake, distribution of potassium in the body
Figure 29–1
Table 29–1
Factors That Can Alter Potassium Distribution Between the
• Increased extracellular fluid
• Strenuous exercise
• Cell lysis
• Acidosis
• Alkalosis
(Addison’s disease)
-adrenergic stimulation
• Aldosterone deficiency
• Aldosterone
mellitus)
• Insulin
• Insulin deficiency (diabetes
])
(Increase Extracellular [K
into Cells
Factors That Shift K
Intra- and Extracellular Fluid
Factors That Shift K
+
+
Out of Cells
(Decrease Extracellular [K
+
+
])
•
b
•
b-adrenergic blockade
osmolarity

The cells in the late distal and cortical collecting
Collecting Tubules
Cells of Late Distal and Cortical
Potassium Secretion by Principal
ulate this process.
body. In the next section, we consider the basic mech-
reabsorbed or secreted, depending on the needs of the
collecting tubules, where potassium can be either
Thus, most of the day-to-day regulation of potas-
hypokalemia can develop.
With potassium intakes below this level, severe
sium in the distal segments of the nephron, and potas-
in potassium intake, there is net reabsorption of potas-
urinary potassium secretion. With extreme reductions
collecting tubules decreases, causing a reduction in
When potassium intake is reduced below normal,
trate, indicating a powerful mechanism for secreting
potassium diets, the rate of potassium excretion can
and collecting tubules. In fact, with extremely high
With high potassium intakes, the required extra
tubules.
body. With a normal potassium intake of 100 mEq/day,
other times be secreted, depending on the needs of the
segments, potassium can at times be reabsorbed or at
tubules and cortical collecting tubules. In these tubular
The most important sites for regulating potas-
Tubules.
Most Daily Variation in Potassium Excretion Is Caused by
imal tubule or loop of Henle.
these segments can influence potassium excretion, but
is reabsorbed. Changes in potassium reabsorption in
both the proximal tubule and the loop of Henle, a rel-
co-transported along with sodium and chloride. In
sium is reabsorbed in the loop of Henle, especially in
tubule. Another 25 to 30 per cent of the filtered potas-
potassium under normal conditions. About 65 per cent
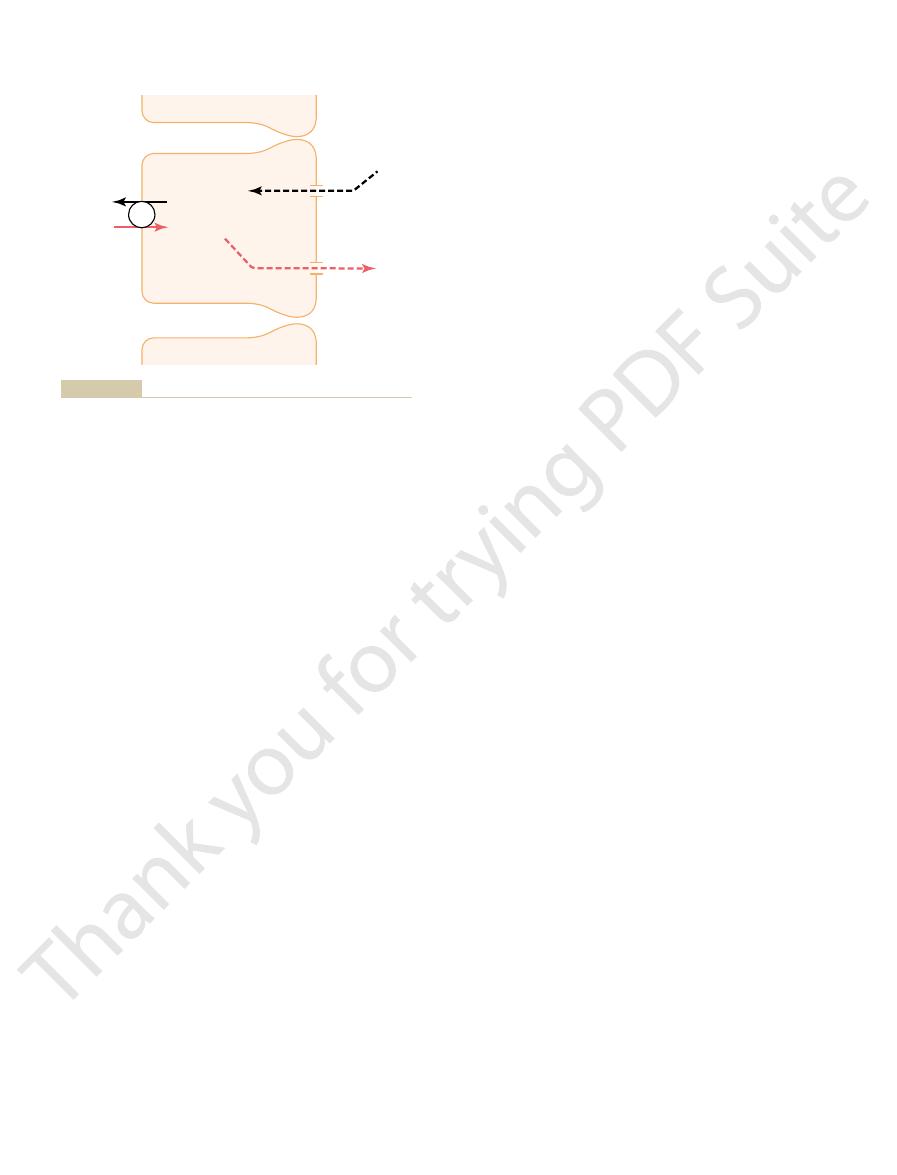
Figure 29–2 summarizes the tubular handling of
renal diseases, however, can cause serious potassium
tion is regulated. Severe decreases in GFR in certain
plasma potassium, 4.2 mEq/L); this rate of filtration is
about 756 mEq/day (GFR, 180 L/day multiplied by
the tubules. The normal rate of potassium filtration is
tubules, and (3) the rate of potassium secretion by
tion), (2) the rate of potassium reabsorption by the
renal processes: (1) the rate of potassium filtration
Potassium excretion is determined by the sum of three
Overview of Renal Potassium
Renal Regulation; Integration of Renal Mechanisms
Chapter 29
367
dehydration and movement of potassium from the cells
into the extracellular fluid.
Excretion
(GFR multiplied by the plasma potassium concentra-
usually relatively constant because of the autoregula-
tory mechanisms for GFR discussed previously and
the precision with which plasma potassium concentra-
accumulation and hyperkalemia.
of the filtered potassium is reabsorbed in the proximal
the thick ascending part where potassium is actively
atively constant fraction of the filtered potassium load
most of the day-to-day variation of potassium excre-
tion is not due to changes in reabsorption in the prox-
Changes in Potassium Secretion in Distal and Collecting
sium excretion are the principal cells of the late distal
the kidneys must excrete about 92 mEq/day (the
remaining 8 milliequivalents are lost in the feces).
About one third (31 mEq/day) of this amount of
potassium is secreted into the distal and collecting
excretion of potassium is achieved almost entirely by
increasing the secretion of potassium into the distal
exceed the amount of potassium in the glomerular fil-
potassium.
the secretion rate of potassium in the distal and
sium excretion can fall to 1 per cent of the potassium
in the glomerular filtrate (to less than 10 mEq/day).
sium excretion occurs in the late distal and cortical
anisms of potassium secretion and the factors that reg-
tubules that secrete potassium are called principal cells
65%
(491 mEq/day)
4%
(31 mEq/day)
12%
(92 mEq/day)
756 mEq/day
(180 L/day x 4.2 mEq/L)
27%
(204 mEq/day)
secreted into the different tubular seg-
much of the filtered load is reabsorbed or
capillaries. The percentages indicate how
the potassium filtered at the glomerular
the daily excretion is about 12 per cent of
ducts adds to the amount delivered, so that
the distal tubule. Secretion of potassium
8 per cent of the filtered load is delivered to
sorbed in the proximal tubule and in the
tion and secretion. Potassium is reab-
Renal tubular sites of potassium reabsorp-
Figure 29–2
ascending loop of Henle, so that only about
into the late distal tubules and collecting
ments.
which further stimulates potassium secretion, as dis-
membrane. (3) Increased potassium concentration
the epithelial cell; this reduces backleakage of potas-
membrane into the tubule. (2) Increased extracellular
tion, causing potassium to diffuse across the luminal
sium uptake across the basolateral membrane. This in
potassium ATPase pump, thereby increasing potas-
potassium secretion: (1) Increased extracellular fluid
There are three mechanisms by which increased
plasma potassium concentration, therefore, serves as
slightly less than the normal concentration. Increased
potassium concentration rises above about 4.1 mEq/L,
sium excretion, as shown in Figure 29–4. This effect
potassium concentration, leading to increases in potas-
The rate of potassium secre-
and (3) increased tubular flow rate.
potassium concentration, (2) increased aldosterone,
that influence secretion by these cells. The most impor-
collecting tubules, we will discuss the primary factors
Aldosterone, Tubular Flow Rate, and
Potassium Concentration,
Potassium Secretion: Plasma
tion, but under normal conditions it plays a small role
blood. This transporter is necessary to allow potassium
into the tubular lumen, and the potassium then diffuses
located in the luminal membrane. This transporter reab-
hydrogen-potassium ATPase
understood, one mechanism believed to contribute
sium in the late distal and collecting tubules. This
potassium depletion, there is a cessation of potassium
factors discussed later.
brane for potassium. These three determinants of
lumen, and (3) the permeability of the luminal mem-
ATPase pump, (2) the electrochemical gradient for
The
across the membrane.
potassium ions, thus allowing these ions to diffuse
the cell into the tubular lumen. The luminal membrane
cellular potassium concentration, which provides the
sodium-potassium ATPase pump creates a high intra-
from the interior of the cell into the tubular fluid. The
moves potassium to the interior of the cell. The second
membrane of the cell; this pump moves sodium out of
sodium-potassium ATPase pump in the basolateral
tubular lumen is a two-step process, beginning with
cells.
in these regions. Figure 29–3 shows the basic cellular
368
Unit V
The Body Fluids and Kidneys
and make up about 90 per cent of the epithelial cells
mechanisms of potassium secretion by the principal
Secretion of potassium from the blood into the
uptake from the interstitium into the cell by the
the cell into the interstitium and at the same time
step of the process is passive diffusion of potassium
driving force for passive diffusion of potassium from
of the principal cells is highly permeable to potassium.
One reason for this high permeability is that there are
special channels that are specifically permeable to
Control of Potassium Secretion by Principal Cells.
primary factors that control potassium secretion by the
principal cells of the late distal and cortical collecting
tubules are (1) the activity of the sodium-potassium
potassium secretion from the blood to the tubular
potassium secretion are in turn regulated by the
Intercalated Cells Can Reabsorb Potassium During Potassium
Depletion.
In circumstances associated with severe
secretion and actually a net reabsorption of potas-
reabsorption occurs through the intercalated cells;
although this reabsorptive process is not completely
is a
transport mechanism
sorbs potassium in exchange for hydrogen ions secreted
through the basolateral membrane of the cell into the
reabsorption during extracellular fluid potassium deple-
in controlling the excretion of potassium.
Summary of Factors That Regulate
Hydrogen Ion Concentration
Because normal regulation of potassium excretion
occurs mainly as a result of changes in potassium
secretion by the principal cells of the late distal and
tant factors that stimulate potassium secretion by the
principal cells include (1) increased extracellular fluid
One factor that decreases potassium secretion is
increased hydrogen ion concentration (acidosis).
Increased Extracellular Fluid Potassium Concentration Stimu-
lates Potassium Secretion.
tion in the late distal and cortical collecting tubules is
directly stimulated by increased extracellular fluid
is especially pronounced when extracellular fluid
one of the most important mechanisms for increasing
potassium secretion and regulating extracellular fluid
potassium ion concentration.
extracellular fluid potassium concentration raises
potassium concentration stimulates the sodium-
turn increases intracellular potassium ion concentra-
potassium concentration increases the potassium gra-
dient from the renal interstitial fluid to the interior of
sium ions from inside the cells through the basolateral
stimulates aldosterone secretion by the adrenal cortex,
cussed next.
Tubular
Tubular
ATP
Na
+
Na
+
K
+
K
+
Na
+
Na
+
Na
+
Na
+
K
+
K
+
Renal
interstitial
fluid
Renal
interstitial
fluid
lumen
lumen
Principal
cells
Principal
cells
0 mV
0 mV
-
50 mV
-
50 mV
-
70 mV
-
70 mV
K
+
K
+
Mechanisms of potassium secretion and sodium reabsorption by
Figure 29–3
the principal cells of the late distal and collecting tubules.
normal (blocks 3 and 4). Thus, this feedback mecha-
potassium excretion by the kidneys (block 2). The
blood level of aldosterone (block 1). The increase in
aldosterone secretion and, therefore, increases the
shown in Figure 29–6. In this feedback system, an
back system for regulating potassium excretion, as
The effect of potassium ion concentration to stimu-
nearly 0 to as high as 60 ng/100 ml, a concentration
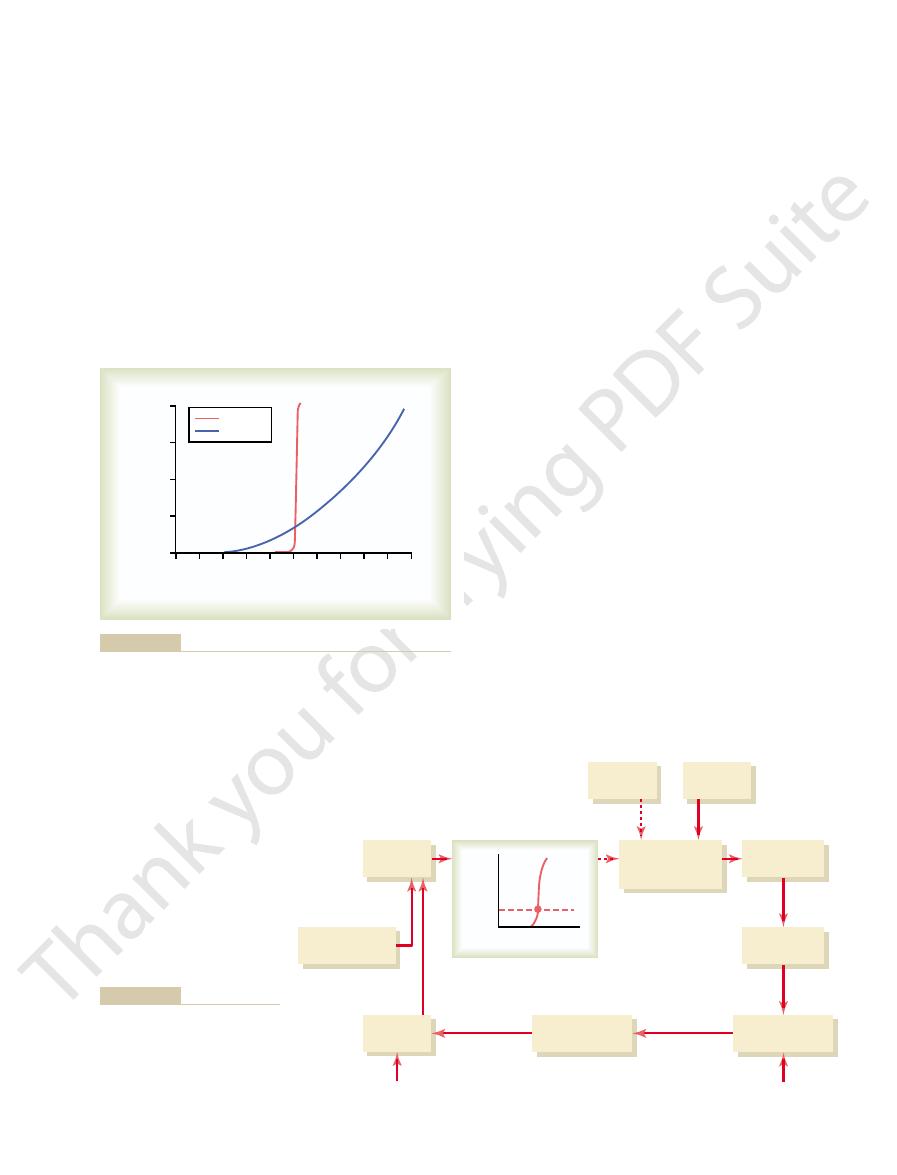
concentration. Figure 29–5 shows that an increase in
aldosterone-potassium control system, the rate of
feedback effect on the controller. In the case of the
systems, the factor that is controlled usually has a
excretion, as shown in Figure 29–4.
stimulating potassium secretion. Therefore, aldos-
Thus, aldosterone also has a powerful effect to control
is mediated through a sodium-potassium ATPase
the late distal tubules and collecting ducts. This effect
Renal Regulation; Integration of Renal Mechanisms
Chapter 29
369
Aldosterone Stimulates Potassium Secretion.
In Chapter 27,
we discuss the fact that aldosterone stimulates active
reabsorption of sodium ions by the principal cells of
pump that transports sodium outward through the
basolateral membrane of the cell and into the blood at
the same time that it pumps potassium into the cell.
the rate at which the principal cells secrete potassium.
A second effect of aldosterone is to increase the per-
meability of the luminal membrane for potassium,
further adding to the effectiveness of aldosterone in
terone has a powerful effect to increase potassium
Increased Extracellular Potassium Ion Concentration Stimu-
lates Aldosterone Secretion.
In negative feedback control
aldosterone secretion by the adrenal gland is con-
trolled strongly by extracellular fluid potassium ion
plasma potassium concentration of about 3 mEq/L can
increase plasma aldosterone concentration from
almost 10 times normal.
late aldosterone secretion is part of a powerful feed-
increase in plasma potassium concentration stimulates
blood aldosterone then causes a marked increase in
increased potassium excretion then reduces the extra-
cellular fluid potassium concentration back toward
nism acts synergistically with the direct effect of
1
2
3
5
Effect of extracellular
Effect of extracellular
Effect of aldosterone
Effect of aldosterone
Urinary potassium excretion
(times normal)
4
3
2
1
0
0
1
1
K
+
concentration
K
+
concentration
2
2
3
3
5
5
4
4
Extracellular potassium concentration
(mEq/L)
Extracellular potassium concentration
(mEq/L)
4
Plasma aldosterone (times normal)
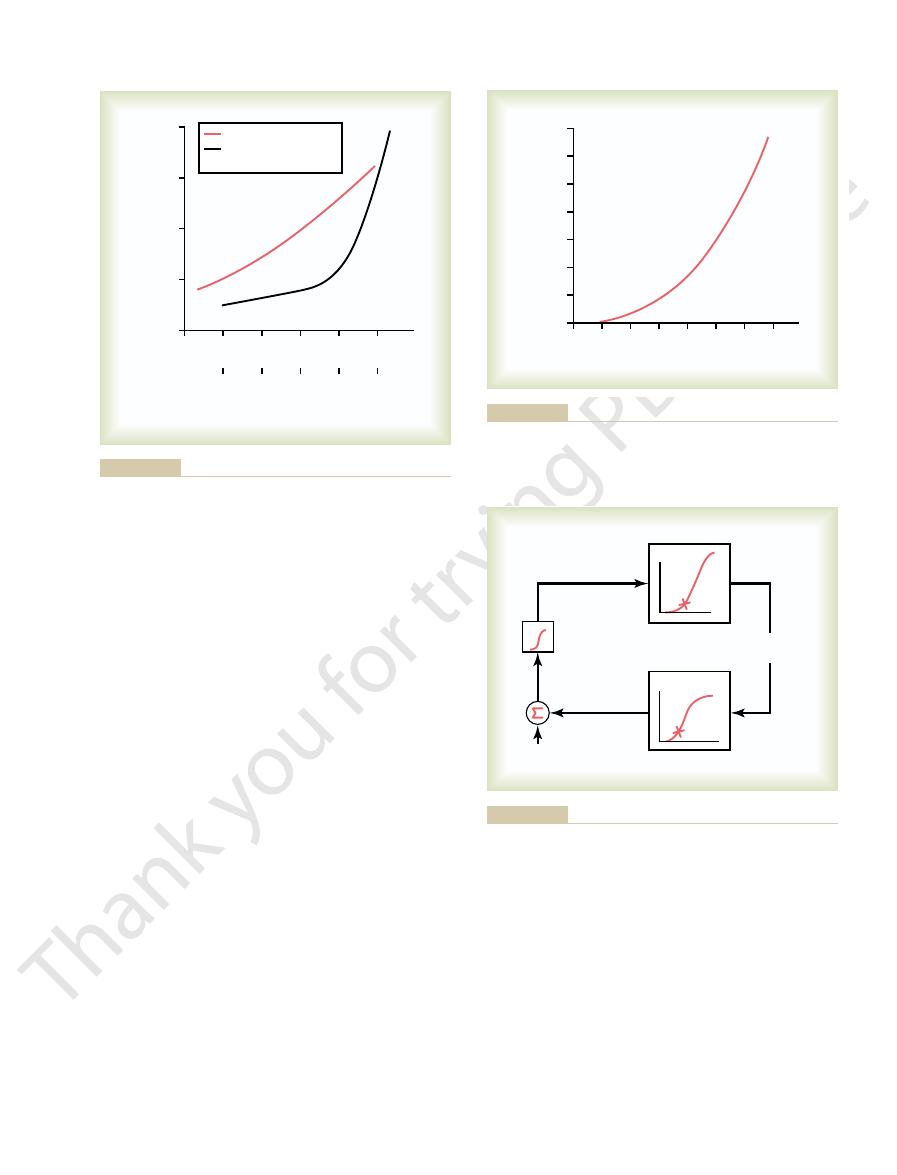
of aldosterone and plasma potassium on potassium excretion. Am
(Drawn from data in Young DB, Paulsen AW: Interrelated effects
secretion by the principal cells of the cortical collecting tubules.
urinary potassium excretion. These factors stimulate potassium
) on the rate of
red line
Effect of plasma aldosterone concentration (
Figure 29–4
) and extra-
cellular potassium ion concentration (black line
J Physiol 244:F28, 1983.)
3.5
4.0
5.0
4.5
6.0
3.0
6.5
Approximate plasma aldosterone
concentration (ng/100 ml plasma)
0
70
60
50
40
30
20
10
5.5
Serum potassium concentration (mEq/L)
Serum potassium concentration (mEq/L)
concentration cause large changes in aldosterone concentration.
aldosterone concentration. Note that small changes in potassium
Effect of extracellular fluid potassium ion concentration on plasma
Figure 29–5
Ald.
Ald.
1
1
K
+
K
+
4
4
3
3
Ald.
Ald.
2
2
K
+
excretion
K
+
excretion
K
+
excretion
K
+
excretion
K
+
concentration
K
+
concentration
Aldosterone
concentration
Aldosterone
concentration
+
+
-
-
K
+
intake
K
+
intake
sium concentration by aldosterone (Ald.).
Basic feedback mechanism for control of extracellular fluid potas-
Figure 29–6
fore, reduce urinary excretion of potassium. However,
decrease the rate of potassium secretion and, there-
aldosterone secretion, which by itself would tend to
example, with a high sodium intake, there is decreased
excretion during changes in sodium intake. For
The effect of increased tubular flow rate is especially
lated by increased tubular flow rate.
mized. Therefore, net potassium secretion is stimu-
continuously flushed down the tubule, so that the rise
increased tubular flow rate, the secreted potassium is
sium diffusion across the luminal membrane. With
increases, thereby reducing the driving force for potas-
tubular fluid, the luminal concentration of potassium
rate is as follows: When potassium is secreted into the
The mechanism for the effect of high-volume flow
by sodium depletion, reduces potassium secretion.
versely, a decrease in distal tubular flow rate, as caused
drug treatment, stimulates potassium secretion. Con-
with volume expansion, high sodium intake, or diuretic
. A rise in distal tubular flow rate, as occurs
Increased Distal Tubular Flow Rate Stimulates Potassium
aldosteronism (too much aldosterone) or Addison’s
systems, such as occurs in patients with either primary
aldosterone feedback system is blocked. A similar
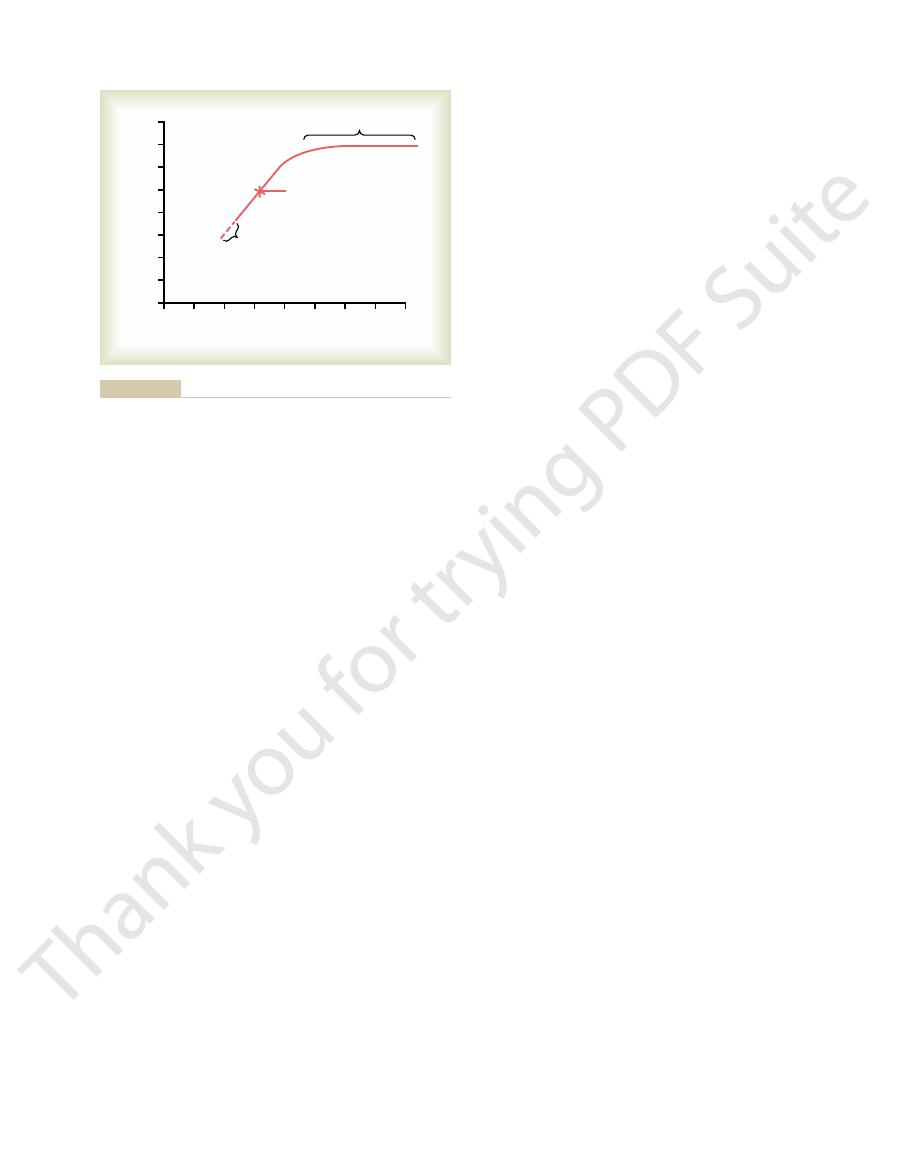
tration, from 3.8 to almost 4.7 mEq/L. Thus, control of
blocked, the same increases in potassium intake
When the aldosterone feedback system was
intake.
cisely controlled, despite large changes in potassium
functioning normally, potassium concentration is pre-
L. Thus, when the aldosterone feedback system is
crease in potassium concentration, from 4.2 to 4.3 mEq/
Note that in the normal animals, a sevenfold
neither increase nor decrease.
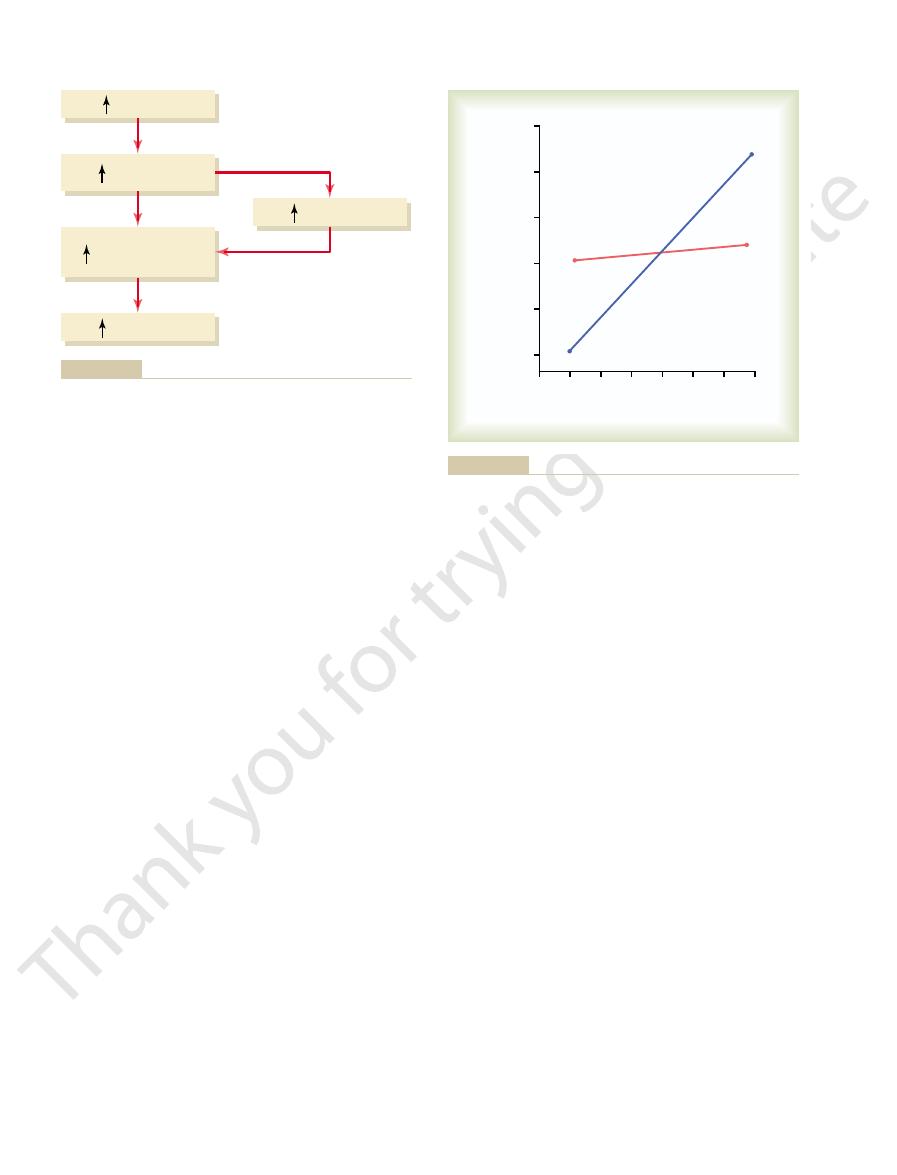
dogs under two conditions: (1) under normal condi-
centration is shown in Figure 29–8. In this experiment,
The special quantitative importance of the aldos-
increased, causing potassium loss by the kidneys and
aldosteronism), potassium secretion becomes greatly
versely, with excess aldosterone secretion (primary
concentration to rise to dangerously high levels. Con-
impaired, thus causing extracellular fluid potassium
Addison’s disease, renal secretion of potassium is
aldosterone secretion, as occurs in patients with
Blockade of Aldosterone Feedback System Greatly Impairs
raised (Figure 29–7).
370
Unit V
The Body Fluids and Kidneys
increased extracellular potassium concentration to
elevate potassium excretion when potassium intake is
Control of Potassium Concentration.
In the absence of
thus leading to hypokalemia.
terone feedback system in controlling potassium con-
potassium intake was increased almost sevenfold in
tions and (2) after the aldosterone feedback system
had been blocked by removing the adrenal glands and
placing the animals on a fixed rate of aldosterone infu-
sion so that plasma aldosterone concentration could
increase in potassium intake caused only a slight in-
caused a much larger increase in potassium concen-
potassium concentration is greatly impaired when the
impairment of potassium regulation is observed in
humans with poorly functioning aldosterone feedback
disease (too little aldosterone).
Secretion
in tubular potassium concentration becomes mini-
important in helping to preserve normal potassium
the high distal tubular flow rate that occurs with a high
sodium intake tends to increase potassium secretion
K
+
intake
K
+
excretion
Aldosterone
Plasma K
+
concentration
K
+
secretion
Cortical collecting
tubules
raising plasma aldosterone concentration.
lecting tubules and indirectly increases potassium secretion by
centration directly raises potassium secretion by the cortical col-
potassium excretion. Note that increased plasma potassium con-
Primary mechanisms by which high potassium intake raises
Figure 29–7
0
30
60
90
120 150
180
210
Plasma potassium concentration
(mEq/day)
3.8
4.8
4.6
4.4
4.2
4.0
Potassium intake (mEq/day)
Potassium intake (mEq/day)
Aldosterone
system
blocked
Aldosterone
system
blocked
Normal
Normal
Young.)
sium concentration was greatly impaired. (Courtesy Dr. David B.
that after blockade of the aldosterone system, regulation of potas-
after the aldosterone feedback had been blocked (
red line
potassium concentration under normal conditions (
Effect of large changes in potassium intake on extracellular fluid
Figure 29–8
) and
blue line). Note
. When extracellular fluid
decrease.
fluid. The bone, therefore, acts as a large reservoir
stored in the bone, with only about 1 per cent in the
Chapter 79.
major role in calcium homeostasis, as discussed in
the intestinal lumen. Therefore, the gastrointestinal
tions, fecal calcium excretion can exceed calcium
calcium excreted in the feces. Under certain condi-
intake is about 1000 mg/day, with about 900 mg/day of
occurs in the feces. The usual rate of dietary calcium
chloride, however, a large share of calcium excretion
over the long term. Unlike ions such as sodium and
As with other substances in the body, the intake of
calcium is bound to the plasma proteins. Therefore,
proteins. Conversely, in alkalosis, a greater amount of
teins. With acidosis, less calcium is bound to the plasma
that has biological activity at cell membranes. The
(5 mEq/L) exists in the ionized form, which is the form
cular excitability and can lead to cardiac arrhythmias.
spastic skeletal muscle contractions.
. This is characterized by
), the excitability of nerve and muscle
When calcium ion concentration falls to low levels
percentage points of its normal level, 2.4 mEq/L.
this chapter.
fore, calcium ion regulation is discussed only briefly in
parathyroid hormone (PTH) and calcitonin. There-
tration are discussed in detail in Chapter 79, along with
The mechanisms for regulating calcium ion concen-
Excretion and Extracellular
Control of Renal Calcium
Thus, chronic acidosis leads
potassium ATPase pump.
the secretion of potassium. This effect overrides
increases distal volume delivery, thereby stimulating
tubular sodium chloride and water reabsorption, which
excretion. The mechanism for this effect is due in part
several days, there is an increase in urinary potassium
With more prolonged acidosis, lasting over a period of
across the luminal membrane into the tubule.
activity of the sodium-potassium ATPase pump. This
losis) increases potassium secretion. The primary
low sodium intake, there is little change in potassium
little change in potassium excretion. Likewise, with a
flow rate, counterbalance each other, so that there is
Therefore, the two effects of high sodium intake,
(Figure 29–9), as discussed in the previous paragraph.
Renal Regulation; Integration of Renal Mechanisms
Chapter 29
371
decreased aldosterone secretion and the high tubular
excretion because of the counterbalancing effects of
increased aldosterone secretion and decreased tubular
flow rate on potassium secretion.
Acute Acidosis Decreases Potassium Secretion.
Acute
increases in hydrogen ion concentration of the extra-
cellular fluid (acidosis) reduce potassium secretion,
whereas decreased hydrogen ion concentration (alka-
mechanism by which increased hydrogen ion concen-
tration inhibits potassium secretion is by reducing the
in turn decreases intracellular potassium concentra-
tion and subsequent passive diffusion of potassium
to an effect of chronic acidosis to inhibit proximal
the inhibitory effect of hydrogen ions on the sodium-
to a loss of potassium, whereas acute acidosis leads to
decreased potassium excretion.
Calcium Ion Concentration
the endocrinology of the calcium-regulating hormones
Extracellular fluid calcium ion concentration
normally remains tightly controlled within a few
(hypocalcemia
cells increases markedly and can in extreme cases
result in hypocalcemic tetany
Hypercalcemia
(increased calcium concentration) depresses neuromus-
About 50 per cent of the total calcium in the plasma
remainder is either bound to the plasma proteins
(about 40 per cent) or complexed in the non-ionized
form with anions such as phosphate and citrate (about
10 per cent).
Changes in plasma hydrogen ion concentration can
influence the degree of calcium binding to plasma pro-
patients with alkalosis are more susceptible to hypocal-
cemic tetany.
calcium must be balanced with the net loss of calcium
ingestion because calcium can also be secreted into
tract and the regulatory mechanisms that influence
intestinal calcium absorption and secretion play a
Almost all the calcium in the body (99 per cent) is
extracellular fluid and 0.1 per cent in the intracellular
for storing calcium and as a source of calcium when
extracellular fluid calcium concentration tends to
One of the most important regulators of bone uptake
and release of calcium is PTH
Na
+
intake
Aldosterone
-
+
GFR
Unchanged K
+
excretion
Distal tubular
flow rate
K
+
secretion
Cortical collecting
ducts
Proximal
tubular Na
+
reabsorption
change in potassium excretion.
sodium diet counterbalance each other, so that there is little
increase potassium secretion. The opposing effects of a high-
fluid delivery to the cortical collecting duct, which tends to
tubules. However, the high-sodium diet simultaneously increases
tends to decrease potassium secretion by the cortical collecting
that a high-sodium diet decreases plasma aldosterone, which
Effect of high sodium intake on renal excretion of potassium. Note
Figure 29–9
of phosphate is present in the glomerular filtrate,
about 0.1 mM/min. When less than this amount
explained as follows: The renal tubules have a normal
Regulation of Renal Phosphate
in Table 29–2.
reabsorption in the distal tubule.
bolic acidosis and inhibited by metabolic alkalosis.
reducing calcium excretion. The opposite occurs with
calcium reabsorption by the renal tubules, thereby
in plasma phosphate stimulates PTH, which increases
is the plasma concentration of phosphate. An increase
decreased blood pressure, calcium excretion decreases
Conversely, with extracellular volume contraction or
sequently, increased urinary excretion of calcium.
also reduction in calcium reabsorption and, con-
parallels sodium and water reabsorption. Therefore,
In the proximal tubule, calcium reabsorption usually
distal tubules.
versely, reduction of PTH promotes calcium excretion
which reduces urinary excretion of calcium. Con-
thick ascending loops of Henle and distal tubules,
PTH, there is increased calcium reabsorption in the
calcium reabsorption is PTH. With increased levels of
tion, calcium excretion by the kidneys decreases as a
intake is eliminated in the feces. With calcium deple-
excretion, although much of the increase of calcium
calcium intake, there is also increased renal calcium
adjusted to meet the body’s needs. With an increase in
As is true with the other ions, calcium excretion is
lecting tubules. This pattern of reabsorption is similar
25 to 30 per cent is reabsorbed in the loop of Henle,
filtered calcium is reabsorbed in the proximal tubule,
tered calcium being excreted. About 65 per cent of the
by the tubules, with only about 1 per cent of the fil-
calcium can be filtered at the glomerulus. Normally,
Therefore, only about 50 per cent of the plasma
proteins or complexed with anions such as phosphate.
ionized, with the remainder being bound to the plasma
kidneys but not secreted, the rate of renal calcium
by the Kidneys
Control of Calcium Excretion
cussed elsewhere, and the remainder of this section
29–10). The control of gastrointestinal calcium reab-
(Figure
intestinal reabsorption of calcium; and (3) by directly
ulating activation of vitamin D, which then increases
effects: (1) by stimulating bone resorption; (2) by stim-
Thus, PTH regulates
The most important regulator of calcium reabsorption
excretion by the gastrointestinal tract and the kidneys.
supply of calcium. Therefore, over the long term, the
The bones, however, do not have an inexhaustible
new bone formation. Thus, the day-to-day regulation
that almost no bone resorption now occurs; instead,
concentration is elevated, PTH secretion decreases, so
calcium levels back toward normal. When calcium ion
calcium into the extracellular fluid, thereby returning
bones) and, therefore, to release large amounts of
levels to promote increased secretion of PTH. This
calcium concentration falls below normal, the parathy-
372
Unit V
The Body Fluids and Kidneys
roid glands are directly stimulated by the low calcium
hormone then acts directly on the bones to increase
the resorption of bone salts (release of salts from the
excess calcium is deposited in the bones because of
of calcium ion concentration is mediated in large part
by the effect of PTH on bone resorption.
intake of calcium must be balanced with calcium
at both of these sites is PTH.
plasma calcium concentration through three main
increasing renal tubular calcium reabsorption
sorption and calcium exchange in the bones is dis-
focuses on the mechanisms that control renal calcium
excretion.
Because calcium is both filtered and reabsorbed in the
excretion is calculated as
Renal calcium excretion
= Calcium filtered
- Calcium reabsorbed
Only about 50 per cent of the plasma calcium is
about 99 per cent of the filtered calcium is reabsorbed
and 4 to 9 per cent is reabsorbed in the distal and col-
to that for sodium.
result of enhanced tubular reabsorption.
One of the primary controllers of renal tubular
by decreasing reabsorption in the loops of Henle and
in instances of extracellular volume expansion or
increased arterial pressure—both of which decrease
proximal sodium and water reabsorption—there is
primarily because of increased proximal tubular
reabsorption.
Another factor that influences calcium reabsorption
reduction in plasma phosphate concentration.
Calcium reabsorption is also stimulated by meta-
Most of the effect of hydrogen ion concentration on
calcium excretion results from changes in calcium
A summary of the factors that are known to influ-
ence calcium excretion by the renal tubules is shown
Excretion
Phosphate excretion by the kidneys is controlled
primarily by an overflow mechanism that can be
transport maximum for reabsorbing phosphate of
Vitamin D
3
activation
PTH
[Ca
++
]
Intestinal Ca
++
reabsorption
Renal Ca
++
reabsorption
Ca
++
release
from bones
concentration mediated by parathyroid hormone (PTH) and
Compensatory responses to decreased plasma ionized calcium
Figure 29–10
vitamin D.

excretion by the kidneys is determined by intake. To
that matter—is that under steady-state conditions,
sodium excretion—or excretion of any electrolyte, for
Matched to Intake Under Steady-State
Sodium Excretion Is Precisely
the amount of extracellular water, so that osmolality
the ADH-thirst mechanisms are functioning normally,
(ADH)-thirst mechanisms are also operative. When
fluid volume, provided the antidiuretic hormone
volume, we also consider the factors that regulate the
conditions.
volume regulation is usually placed on the kidneys,
mechanisms. Therefore, the burden of extracellular
person’s habits rather than by physiologic control
In most cases, salt and fluid intakes are dictated by a
Mechanisms for Control
tion, (2) extracellular volume expansion, and (3)
bances lead to increased magnesium excretion: (1)
are not well understood, but the following distur-
The mechanisms that regulate magnesium excretion
collecting tubules.
the filtered load of magnesium is reabsorbed. Only a
tion is the loop of Henle, where about 65 per cent of
the filtered magnesium. The primary site of reabsorp-
mainly by changing tubular reabsorption. The proxi-
the body, including activation of many enzymes, its
almost nil during magnesium depletion. Because mag-
nesium in the glomerular filtrate.
of magnesium, or 125 to 150 mg/day. The kidneys
absorbed magnesium, about one half the daily intake
magnesium balance, the kidneys must excrete this
absorbed by the gastrointestinal tract. To maintain
to 300 mg/day, but only about one half of this intake is
The normal daily intake of magnesium is about 250
0.8 mEq/L.
is bound to plasma proteins. Therefore, the free
tration is about 1.8 mEq/L, more than one half of this
fluid. Although the total plasma magnesium concen-
in the bones. Most of the rest resides within the cells,
More than one half of the body’s magnesium is stored
Excretion and Extracellular
Control of Renal Magnesium
among phosphate, PTH, and calcium are discussed in
. These interrelations
is lost in the urine.
port maximum for phosphate by the renal tubules,
from the bone salts, and (2) PTH decreases the trans-
promotes bone resorption, thereby dumping large
phate concentration through two effects: (1) PTH
the tendency for phosphate to spill over into the urine.
transport maximum for phosphate, thereby reducing
in phosphate can, over time, increase the reabsorptive
influence phosphate excretion. For instance, a diet low
continual excretion of phosphate into the urine.
maintained above 1 mM/L, a level at which there is
and meat, the concentration of phosphate is usually
assuming a GFR of 125 ml/min. Because most people
a tubular load of phosphate of about 0.1 mM/min,
rises above a threshold of about 0.8 mM/L, which gives
Therefore, phosphate normally begins to spill into the
When more than this is present, the
Renal Regulation; Integration of Renal Mechanisms
Chapter 29
373
essentially all the filtered phosphate is reabsorbed.
excess is excreted.
urine when its concentration in the extracellular fluid
ingest large quantities of phosphate in milk products
Changes in tubular phosphate reabsorption can also
PTH can play a significant role in regulating phos-
amounts of phosphate ions into the extracellular fluid
so that a greater proportion of the tubular phosphate
Thus, whenever plasma PTH is
increased, tubular phosphate reabsorption is decreased
and more phosphate is excreted
more detail in Chapter 79.
Magnesium Ion Concentration
with less than 1 per cent located in the extracellular
ionized concentration of magnesium is only about
normally excrete about 10 to 15 per cent of the mag-
Renal excretion of magnesium can increase
markedly during magnesium excess or can decrease to
nesium is involved in many biochemical processes in
concentration must be closely regulated.
Regulation of magnesium excretion is achieved
mal tubule usually reabsorbs only about 25 per cent of
small amount (usually less than 5 per cent) of the
filtered magnesium is reabsorbed in the distal and
increased extracellular fluid magnesium concentra-
increased extracellular fluid calcium concentration.
Integration of Renal
of Extracellular Fluid
Extracellular fluid volume is determined mainly by the
balance between intake and output of water and salt.
which must adapt their excretion of salt and water to
match intake of salt and water under steady-state
In discussing the regulation of extracellular fluid
amount of sodium chloride in the extracellular fluid,
because changes in extracellular fluid sodium chloride
content usually cause parallel changes in extracellular
a change in the amount of sodium chloride in the
extracellular fluid is matched by a similar change in
and sodium concentration are maintained relatively
constant.
Conditions
An important consideration in overall control of
Table 29–2
Metabolic acidosis
Metabolic alkalosis
Factors That Alter Renal Calcium Excretion
Ø Calcium Excretion
≠ Calcium Excretion
≠ Parathyroid hormone (PTH)
Ø PTH
Ø Extracellular fluid volume
≠ Extracellular fluid volume
Ø Blood pressure
≠ Blood pressure
≠ Plasma phosphate
Ø Plasma phosphate
Vitamin D
3

therefore, decreases formation of angiotensin II and
after a short time delay, suppresses renin release and,
enhanced because the increased blood pressure also,
these factors. With chronic increases in blood pressure,
as angiotensin II, ADH, or aldosterone, because pres-
pathetic nervous system or of various hormones, such
threefold increase in urinary sodium output. This
urinary sodium output. Note that acute increases in
Figure 29–11 shows the effect of arterial pressure on
as “pressure natriuresis” in the following discussion.
occur in parallel, we refer to these mechanisms simply
sure. Because pressure diuresis and natriuresis usually
discussed in Chapter 19, this feedback between the
mechanisms, respectively. As
fluid balance, is the effect of blood pressure on sodium
volume, as well as for the maintenance of sodium and
Diuresis in Maintaining Body
Natriuresis and Pressure
Importance of Pressure
keep in mind, however, that all these feedback mech-
also to control extracellular fluid volume. We should
equal sodium intake. In the next few sections, we
hormones, that eventually return sodium excretion to
changes in blood pressure and changes in various
other feedback mechanisms come into play, such as
urine sodium and water excretion. When this happens,
the way back to normal, changes in either GFR or
these same intrarenal feedbacks.
and return of GFR toward normal. Likewise, abnor-
, in which increased sodium chloride delivery to
back
, and (2)
much of the extra sodium chloride filtered, called
compensations: (1) increased tubular reabsorption of
tubules, which in turn leads to at least two intrarenal
fever), this raises sodium chloride delivery to the
mized by various buffering mechanisms. For example,
reabsorption, changes in urinary excretion are mini-
of water and electrolytes.
and GFR usually are regulated precisely, so that excre-
volume and sodium excretion. Tubular reabsorption
GFR, would also lead to dramatic changes in urine
tion, in the absence of compensatory adjustments of
volumes. Similarly, small changes in tubular reabsorp-
volume, if tubular compensations did not occur; this
(to 189 L/day) would cause a 9 L/day increase in urine
excretion. For example, a 5 per cent increase in GFR
L/day. Thus, small changes in GFR or tubular reab-
sorption is 178.5 L/day, and urine excretion is 1.5
GFR normally is about 180 L/day, tubular reab-
Tubular
The two variables that influence sodium and water
Tubular Sodium Reabsorption Rates
Altering Glomerular Filtration or
Sodium Excretion Is Controlled by
balance with intake.
vascular collapse within a few days. Thus, one can view
tion or loss of electrolytes and fluid, causing cardio-
compensations, however, are necessary because a
body that may, in the long run, be damaging. These
ations of sympathetic nervous system activity. These
pressure, changes in circulating hormones, and alter-
adjustments must be invoked, such as changes in blood
intrarenal compensations are exhausted, systemic
cellular fluid volume or other systemic adjustments.
severe, sodium balance may be achieved mainly by
a few days.
major changes in kidney function, balance between
ingested. Therefore, even with disturbances that cause
maintain life, a person must, over the long term,
374
Unit V
The Body Fluids and Kidneys
excrete almost precisely the amount of sodium
intake and output of sodium usually is restored within
If disturbances of kidney function are not too
intrarenal adjustments with minimal changes in extra-
But when perturbations to the kidneys are severe and
adjustments are costly in terms of overall homeostasis
because they cause other changes throughout the
sustained imbalance between fluid and electrolyte
intake and excretion would quickly lead to accumula-
the systemic adjustments that occur in response to
abnormalities of kidney function as a necessary trade-
off that brings electrolyte and fluid excretion back in
excretion are the rates of filtration and the rates of
reabsorption:
Excretion
= Glomerular filtration -
reabsorption
sorption potentially can cause large changes in renal
would quickly cause catastrophic changes in body fluid
tion by the kidneys can be exactly matched to intake
Even with disturbances that alter GFR or tubular
if the kidneys become greatly vasodilated and GFR
increases (as can occur with certain drugs or high
glomerulotubular balance
macula densa feed-
the distal tubule causes afferent arteriolar constriction
malities of tubular reabsorption in the proximal tubule
or loop of Henle are partially compensated for by
Because neither of these two mechanisms operates
perfectly to restore distal sodium chloride delivery all
tubular reabsorption can lead to significant changes in
review how these mechanisms operate together to
control sodium and water balance and in so doing act
anisms control renal excretion of sodium and water by
altering either GFR or tubular reabsorption.
Sodium and Fluid Balance
One of the most basic and powerful mechanisms for
control of blood volume and extracellular fluid
and water excretion—called the pressure natriuresis
and pressure diuresis
kidneys and the circulatory system also plays a domi-
nant role in long-term blood pressure regulation.
Pressure diuresis refers to the effect of increased
blood pressure to raise urinary volume excretion,
whereas pressure natriuresis refers to the rise in
sodium excretion that occurs with elevated blood pres-
blood pressure of 30 to 50 mm Hg cause a twofold to
effect is independent of changes in activity of the sym-
sure natriuresis can be demonstrated in an isolated
kidney that has been removed from the influence of
the effectiveness of pressure natriuresis is greatly
increased intake, and further accumulation of fluid
8. The increased fluid excretion balances the
output by way of pressure diuresis. The steepness
7. An increased arterial pressure increases urine
pressure.
6. An increased cardiac output raises arterial
5. An increased pressure gradient for venous return
4. An increase in mean circulatory filling pressure
circulatory filling pressure.
3. An increase in blood volume raises mean
extracellular fluid volume. As discussed later, the
accumulates in the blood and interstitial spaces,
2. As long as fluid intake exceeds urine output, fluid
fluid in the body.
1. An increase in fluid intake (assuming that sodium
fluid volume, and arterial pressure as follows:
to minimize changes in blood volume, extracellular
During changes in sodium and fluid intake, this feed-
volume, cardiac output, arterial pressure, and urine
pressure control. The extracellular fluid volume, blood
output, as shown in Figure 29–12. This is the same
The effect of increased blood pressure to raise urine
Fluid Volumes and Arterial Pressure
Fluid Feedback for Regulating Body
Pressure Natriuresis and Diuresis Are
reabsorption of sodium, thereby amplifying the direct
aldosterone. As discussed previously, decreased levels
Renal Regulation; Integration of Renal Mechanisms
Chapter 29
375
of angiotensin II and aldosterone inhibit renal tubular
effects of increased blood pressure to raise sodium and
water excretion.
Key Components of a Renal-Body
output is part of a powerful feedback system that oper-
ates to maintain balance between fluid intake and
mechanism that is discussed in Chapter 19 for arterial
output are all controlled at the same time as separate
parts of this basic feedback mechanism.
back mechanism helps to maintain fluid balance and
accompanies the fluid intake) above the level of
urine output causes a temporary accumulation of
causing parallel increases in blood volume and
actual increases in these variables are usually
small because of the effectiveness of this
feedback.
raises the pressure gradient for venous return.
elevates cardiac output.
of the normal pressure natriuresis relation
indicates that only a slight increase in blood
pressure is required to raise urinary excretion
severalfold.
is prevented.
80 100 120 140 160 180 200
0
20 40 60
Urinary sodium or volume
output (times normal)
0
8
6
4
2
Chronic
Acute
Arterial pressure (mm Hg)
Arterial pressure (mm Hg)
pressure.
output than those measured during acute increases in arterial
in arterial pressure cause much greater increases in sodium
by the kidneys (pressure natriuresis). Note that chronic increases
Acute and chronic effects of arterial pressure on sodium output
Figure 29–11
Arterial
pressure
Extracellular
fluid volume
Blood
volume
Mean circulatory
filling pressure
Venous
return
Vascular
capacity
Rate of change of
extracellular
fluid volume
Arterial pressure
Renal fluid
excretion
Nonrenal
fluid loss
Fluid
intake
Cardiac
output
Heart strength
Total peripheral
resistance
negative effects.
effects, and dashed lines indicate
volume, and arterial pressure.
mechanism for control of
Basic renal–body fluid feedback
Figure 29–12
blood volume, extracellular fluid
Solid lines indicate positive

spaces act as an “overflow” reservoir for excess fluid,
Thus, under normal conditions, the interstitial
the tissues, is lost once the tissues become highly
factor against edema, owing to a rising interstitial
pressure rising much more. In other words, the safety
spaces become compliant, and large amounts of fluid
tive value to become positive, the tissue interstitial
remains in the blood. This occurs because once the
30 to 50 per cent above normal, almost all the addi-
When the extracellular fluid volume rises more than
remainder is distributed to the interstitial spaces.
in the blood and increases the blood volume. The
renal output of fluid, about 20 to 30 per cent of it stays
and the distribution that occurs in edema states. When
Figure 29–14 shows the normal distribution of fluid
uted to the interstitial spaces.
. In all these conditions, an unusually high
spaces and blood can vary greatly. As discussed in
There are circumstances, however, in which the dis-
usually are controlled simultaneously.
fore, blood volume and extracellular fluid volume
between the interstitial spaces and the plasma. There-
into the blood, but it rapidly becomes distributed
in parallel with each other. Ingested fluid initially goes
From Figure 29–12 it is apparent that blood volume
Spaces and Vascular System
normal despite the low red blood cell mass.
the plasma volume will simply make up the difference,
when there is deficiency of erythropoietin or other
ties of red blood cell volume remain, such as occurs
cells and plasma proteins in the blood. If abnormali-
In this case, fluid is retained by the kidneys, and other
there is a loss of whole blood because of hemorrhage.
The same control mechanisms operate whenever
control of blood volume.
pressure causes a large change in urine output. These
in blood pressure, and (3) a slight change in blood
volume causes a marked change in cardiac output, (2)
this is the following: (1) a slight change in blood
extreme changes in daily fluid intake. The reason for
By studying Figure 29–12, one can see why the blood
Extracellular Fluid Volume Regulation
Precision of Blood Volume and
tion or other inescapable losses.
ficient to make up for fluid losses caused by evapora-
variations in daily intake of water and electrolytes,
strated in Figure 29–13, which shows that changes in
volume. The effectiveness of this mechanism in pre-
blood pressure, blood volume, or extracellular fluid
large decrease in urine output, thereby allowing fluid
lar fluid volume, as well as reduced arterial pressure.
dency toward decreased blood volume and extracellu-
intake falls below normal. In this case, there is a ten-
The opposite sequence of events occurs when fluid
pressure.
extracellular fluid volume, cardiac output, and arterial
modated with only slight changes in blood volume,
pressure diuresis mechanism is operating effectively,
intake. As long as kidney function is normal and the
Thus, the renal-body fluid feedback mechanism
376
Unit V
The Body Fluids and Kidneys
operates to prevent continuous accumulation of salt
and water in the body during increased salt and water
large changes in salt and water intake can be accom-
Even a small decrease in blood pressure causes a
balance to be maintained with minimal changes in
venting large changes in blood volume is demon-
blood volume are almost imperceptible despite large
except when intake becomes so low that it is not suf-
volume remains almost exactly constant despite
a slight change in cardiac output causes a large change
factors work together to provide effective feedback
parallel processes occur to reconstitute the red blood
factors needed to stimulate red blood cell production,
and the overall blood volume will return essentially to
Distribution of Extracellular
Fluid Between the Interstitial
and extracellular fluid volume are usually controlled
tribution of extracellular fluid between the interstitial
Chapter 25, the principal factors that can cause accu-
mulation of fluid in the interstitial spaces include (1)
increased capillary hydrostatic pressure, (2) decreased
plasma colloid osmotic pressure, (3) increased perme-
ability of the capillaries, and (4) obstruction of lym-
phatic vessels
proportion of the extracellular fluid becomes distrib-
between the interstitial spaces and the vascular system
small amounts of fluid accumulate in the blood as a
result of either too much fluid intake or a decrease in
tional fluid goes into the interstitial spaces and little
interstitial fluid pressure rises from its normally nega-
then pour into the tissues without interstitial fluid
fluid pressure that counteracts fluid accumulation in
compliant.
2
3
4
5
6
7
8
Blood volume (liters)
0
6
5
4
3
2
1
0
1
Blood volume
Normal range
Death
Daily fluid intake
(water and electrolytes) (L/day)
Daily fluid intake
(water and electrolytes) (L/day)
normal range of daily fluid intakes.
volume. Note that blood volume remains relatively constant in the
Approximate effect of changes in daily fluid intake on blood
Figure 29–13
pressures and body fluid volumes.
Thus, changes in activity of the renin-angiotensin
arterial blood pressure that would otherwise occur.
normal, increased levels of angiotensin II cause
Conversely, when sodium intake is reduced below
wise occur when sodium intake increases.
The net result is to minimize the rise in extracellular
increasing the kidneys’ excretion of sodium and water.
tubular reabsorption of sodium and water, thus
Chapter 27, a reduced level of angiotensin II decreases
ing tubular reabsorption of sodium, as explained in
decreased angiotensin II formation.
Because
above normal, renin secretion is decreased, causing
balances. That is, when sodium intake is elevated
angiotensin II formation, and this in turn contributes
excretion is angiotensin II. Changes in sodium and
One of the body’s most powerful controllers of sodium
Role of Angiotensin II In Controlling
contains large amounts of salt and water.
Also, reflex inhibition of renal sympathetic activity
that occurs in acute conditions such as hemorrhage.
and aortic arch. All these reflexes together play an
rial pressure, further activation of the sympathetic
increase tubular reabsorption. And if the reduction in
and aldosterone formation, both of which further
tubular reabsorption of salt and water; and (3) stimu-
rioles, with resultant decreased GFR; (2) increased
and water excretion: (1) constriction of the renal arte-
activity, which has several effects to decrease sodium
system. This in turn increases renal sympathetic nerve
and other low-pressure regions of the thorax decrease,
orrhage, the pressures in the pulmonary blood vessels
For example, when blood volume is reduced by hem-
of extracellular fluid volume under some conditions.
innervation, changes in sympathetic activity can alter
Control of Renal Excretion: Arterial
fluid volumes, as discussed later.
response to day-to-day challenges. However, abnor-
fluid volume, and arterial pressure that occur in
minimizing the changes in blood volume, extracellular
diuresis mechanisms, making them more effective in
and, therefore, renal excretion of salt and water. These
In Chapter 27, we discuss the nervous and hormonal
Fluid Feedback Control
Effectiveness of Renal-Body
Factors Increase the
Nervous and Hormonal
the capillary membranes.
volume are controlled simultaneously, but the quanti-
To summarize, extracellular fluid volume and blood
edema and cardiac failure.
culation, protecting the cardiovascular system against
causes edema, as explained in Chapter 25, but it also
sometimes increasing in volume 10 to 30 liters. This
Renal Regulation; Integration of Renal Mechanisms
Chapter 29
377
acts as an important overflow release valve for the cir-
dangerous overload that could lead to pulmonary
tative amounts of fluid distribution between the
interstitium and the blood depend on the physical
properties of the circulation and the interstitial spaces
as well as on the dynamics of fluid exchange through
factors that influence GFR and tubular reabsorption
nervous and hormonal mechanisms usually act in
concert with the pressure natriuresis and pressure
malities of kidney function or of the various nervous
and hormonal factors that influence the kidneys can
lead to serious changes in blood pressure and body
Sympathetic Nervous System
Baroreceptor and Low-Pressure
Stretch Receptor Reflexes
Because the kidneys receive extensive sympathetic
renal sodium and water excretion as well as regulation
causing reflex activation of the sympathetic nervous
lation of renin release and increased angiotensin II
blood volume is great enough to lower systemic arte-
nervous system occurs because of decreased stretch of
the arterial baroreceptors located in the carotid sinus
important role in the rapid restitution of blood volume
may contribute to the rapid elimination of excess fluid
in the circulation that occurs after eating a meal that
Renal Excretion
fluid intake are associated with reciprocal changes in
greatly to the maintenance of body sodium and fluid
angiotensin II has several important effects in increas-
fluid volume and arterial pressure that would other-
sodium and water retention and oppose reductions in
system act as a powerful amplifier of the pressure
natriuresis mechanism for maintaining stable blood
0
5
10
15
20
25
30
35
Blood volume (liters)
0
8
7
6
5
4
3
2
1
Edema
Normal value
Normal value
Death
40
Extracellular fluid volume (liters)
Extracellular fluid volume (liters)
interstitial spaces, and edema results.
occurs, the additional extracellular fluid volume resides in the
also showing the failure of blood volume to continue rising when
volume, showing a nearly linear relation in the normal range but
Approximate relation between extracellular fluid volume and blood
Figure 29–14
the extracellular fluid volume becomes excessive. When this
in the urine.
reabsorption and potassium secretion. Therefore, the
in the cortical collecting tubules. The increased sodium
Aldosterone increases sodium reabsorption, especially
Role of Aldosterone in Controlling
this effect, and sodium excretion is once again restored
sodium and water, but the fall in blood pressure offsets
enzyme inhibitor is administered, there is initial loss of
mation, as occurs when an angiotensin-converting
sure. Conversely, after blockade of angiotensin II for-
ing the sodium- and water-retaining effects of the
kidney output of sodium and water, thereby overcom-
increase in extracellular fluid volume. This also initi-
occurs with a renin-secreting tumor of the kidney,
that with large increases in angiotensin II levels, as
volume or blood volume. The reason for this is
sodium- and water-retaining hormones in the body,
Extracellular Fluid Volume Because Increased Arterial Pressure
angiotensin II receptor antagonists.
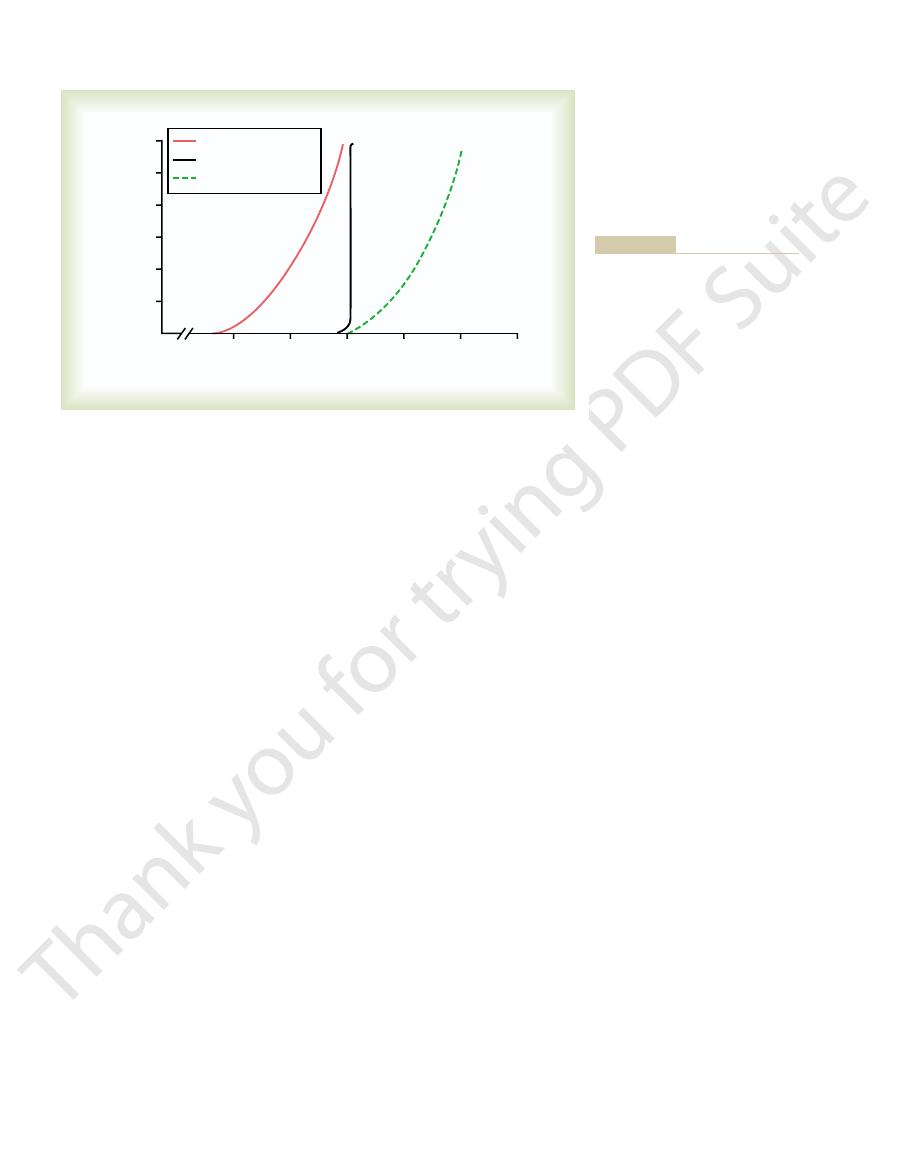
arterial pressures. This shift of pressure natriuresis
pressures; this indicates an enhanced ability of the
29–15) or an angiotensin II receptor antagonist, the
angiotensin-converting enzyme inhibitor (see Figure
When angiotensin II formation is blocked with an
ing the kidneys’ ability to excrete salt and water.
The use of drugs to block the effects of angiotensin
sis and makes arterial pressure very salt sensitive, as
pressure to rise as much as 50 mm Hg. Thus, the inabil-
sodium, the same rise in sodium intake causes blood
few millimeters of mercury in arterial pressure,
sodium balance. For example, in most people, a 10-fold
raised, much greater increases in arterial pressure are
nearly as steep. Therefore, when sodium intake is
renin secretion, the pressure natriuresis curve is not
(high angiotensin II curve), as occurs in some hyper-
In contrast, when angiotensin levels cannot be
effective is shown in Figure 29–15. Note that when the
The importance of angiotensin II
378
Unit V
The Body Fluids and Kidneys
Importance of Angiotensin II in Increasing Effectiveness of
Pressure Natriuresis.
in making the pressure natriuresis mechanism more
angiotensin control of natriuresis is fully functional,
the pressure natriuresis curve is steep (normal curve),
indicating that only minor changes in blood pressure
are needed to increase sodium excretion when sodium
intake is raised.
decreased in response to increased sodium intake
tensive patients who have impaired ability to decrease
needed to increase sodium excretion and maintain
increase in sodium intake causes an increase of only a
whereas in subjects who cannot suppress angiotensin
II formation appropriately in response to excess
ity to suppress angiotensin II formation when there is
excess sodium reduces the slope of pressure natriure-
discussed in Chapter 19.
II has proved to be important clinically for improv-
renal–pressure natriuresis curve is shifted to lower
kidneys to excrete sodium because normal levels of
sodium excretion can now be maintained at reduced
provides the basis for the chronic blood pressure–
lowering effects in hypertensive patients of the
angiotensin-converting enzyme inhibitors and
Excessive Angiotensin II Does Not Cause Large Increases in
Counterbalances Angiotensin-Mediated Sodium Retention.
Although angiotensin II is one of the most powerful
neither a decrease nor an increase in circulating
angiotensin II has a large effect on extracellular fluid
the high angiotensin II levels initially cause sodium
and water retention by the kidneys and a small
ates a rise in arterial pressure that quickly increases
angiotensin II and re-establishing a balance between
intake and output of sodium at a higher blood pres-
to normal.
Renal Excretion
reabsorption is also associated with increased water
net effect of aldosterone is to make the kidneys retain
sodium and water but to increase potassium excretion
80
100
120
140
160
Sodium intake and output
(times normal)
12
10
8
6
4
2
0
60
Angiotensin blockade
Normal
High angiotensin II
Arterial pressure (mm Hg)
lower blood pressures.
mation shifts pressure natriuresis to
very sensitive to changes in sodium
natriuresis, making blood pressure
mation decrease the slope of pressure
renal–pressure natriuresis curve. Note
angiotensin II formation on the
formation and effect of blocking
Effect of excessive angiotensin II
Figure 29–15
that high levels of angiotensin II for-
intake. Blockade of angiotensin II for-

changes in blood volume during various disturbances,
Changes in ANP levels probably help to minimize
compensate for the excess blood volume.
increased excretion of salt and water, which helps to
ducts. These combined actions of ANP lead to
cardiac atria, ANP enters the circulation and acts on
from excess blood volume. Once released by the
appears to be overstretch of the atria, which can result
muscle fibers. The stimulus for release of this peptide
, released by the cardiac atrial
regulation. One of the most important of the natri-
lular fluid volume. However, several different natri-
and water-retaining hormones in controlling extracel-
Thus far, we have discussed mainly the role of sodium-
in Controlling Renal Excretion
Role of Atrial Natriuretic Peptide
arterial pressure.
to water is prevented, the inability to secrete ADH
enough water to maintain fluid balance. If free access
This is almost always compensated for by ingestion of
lar fluid in the urine through pressure natriuresis.
same time, the small increase in blood pressure that
the kidneys dilutes the extracellular sodium, and at the
. The
high ADH levels can cause severe reduc-
sure, although
Thus, high levels of ADH do not cause major
which ADH levels may be elevated severalfold.
, in
inappropriate ADH syndrome
also elevated by less than 10 mm Hg. The same is true
more than 5 to 10 per cent, and the arterial pressure is
nism. After several days of ADH infusion, the blood
to this increased volume, much of the excess volume
fluid volume. As the arterial pressure rises in response
of ADH into animals initially causes renal retention of
extracellular fluid volume. Infusion of large amounts
extracellular fluid volume, excessive levels of ADH
Although ADH is important in regulating
Extracellular Fluid Volume but Large Decreases in Sodium Con-
volume.
the kidneys, thus helping to rid the body of the excess
Conversely, when there is excess extracellular volume,
both extracellular fluid volume and arterial pressure.
tion in the distal and collecting tubules, the same
nizes the action of ADH to promote water reabsorp-
effects of ADH are blocked with a drug that antago-
fluid volume and arterial pressure. However, if the
otherwise occur. Water deprivation for 24 to 48 hours
deprivation, which strongly elevates plasma levels of
salt. This effect is especially important during water
As discussed in Chapter 28, ADH plays an important
Water Excretion
Role of ADH in Controlling Renal
urine output of salt and water.
terone, the volume depletion may be severe unless the
low blood pressure. In the complete absence of aldos-
in extracellular fluid volume, and a tendency toward
is increased excretion of sodium and water, reduction
secrete enough aldosterone (Addison’s disease), there
In patients with adrenal insufficiency who do not
diuresis that occur when the arterial pressure rises.
presence of high levels of aldosterone. The primary
sodium equal to the daily intake, despite continued
rises sufficiently, the kidneys “escape” from the sodium
in arterial blood pressure. When the arterial pressure
retention, the extracellular fluid volume rises by about
are transient. After 1 to 3 days of sodium and water
(Conn’s syndrome), the increased sodium reabsorp-
aldosterone or excessive formation of aldosterone, as
reabsorption, when there is excessive infusion of
during variations in salt intake.
the kidneys to excrete larger amounts of sodium. Thus,
formation decreases tubular reabsorption, allowing
with high sodium intake, suppression of aldosterone
to the maintenance of sodium balance. Conversely,
reduction in urinary sodium excretion and, therefore,
aldosterone secretion, which in turn contributes to the
angiotensin II. That is, with reduction in sodium intake,
The function of aldosterone in regulating sodium
Renal Regulation; Integration of Renal Mechanisms
Chapter 29
379
balance is closely related to that described for
the increased angiotensin II levels that occur stimulate
changes in aldosterone formation also aid the pressure
natriuresis mechanism in maintaining sodium balance
During Chronic Oversecretion of Aldosterone, Kidneys
“Escape” from Sodium Retention as Arterial Pressure Rises.
Although aldosterone has powerful effects on sodium
occurs in patients with tumors of the adrenal gland
tion and decreased sodium excretion by the kidneys
10 to 15 per cent and there is a simultaneous increase
and water retention and thereafter excrete amounts of
reason for the escape is the pressure natriuresis and
person is allowed to eat large amounts of salt and
drink large amounts of water to balance the increased
role in allowing the kidneys to form a small volume of
concentrated urine while excreting normal amounts of
ADH that in turn increase water reabsorption by the
kidneys and help to minimize the decreases in extra-
cellular fluid volume and arterial pressure that would
normally causes only a small decrease in extracellular
period of water deprivation causes a substantial fall in
decreased ADH levels reduce reabsorption of water by
Excess ADH Secretion Usually Causes Only Small Increases in
centration.
seldom cause large increases in arterial pressure or
water and a 10 to 15 per cent increase in extracellular
is excreted because of the pressure diuresis mecha-
volume and extracellular fluid volume are elevated no
for patients with
increases of either body fluid volume or arterial pres-
tions in extracellular sodium ion concentration
reason for this is that increased water reabsorption by
does occur causes loss of sodium from the extracellu-
In patients who have lost their ability to secrete
ADH because of destruction of the supraoptic nuclei,
the urine volume may become 5 to 10 times normal.
may lead to marked reductions in blood volume and
uretic hormones may also contribute to volume
uretic hormones is a peptide referred to as atrial natri-
uretic peptide (ANP)
the kidneys to cause small increases in GFR and
decreases in sodium reabsorption by the collecting

decreased arterial pressure. The fall in pressure causes
29–12), which leads to decreased cardiac output and
reduces mean circulatory filling pressure (see Figure
capacity. An increase in vascular capacity initially
Increased Blood Volume Caused by
pressure to normal. This allows even the weakened
volume, which helps to return cardiac output and blood
congenital abnormalities of the heart, an important
In myocardial failure, heart valvular disease, and
occurs, the kidneys continue to retain volume until the
enough to restore urine output to normal. When this
weakened, arterial pressure will not be able to increase
pumping adequately. However, if the heart is greatly
rial pressure virtually all the way to normal, and sodium
Indeed, if the heart failure is not too severe, the rise in
fore, the kidneys retain volume in an attempt to return
itself causes the kidneys to retain salt and water. There-
nervous systems. In addition, the low blood pressure
the renin-angiotensin, aldosterone, and sympathetic
activates multiple sodium-retaining systems, especially
consequently, decreases arterial pressure. This in turn
29–12. Initially, heart failure reduces cardiac output and,
this can be understood by re-examination of Figure
times increases by 200 per cent or more. The reason for
15 to 20 per cent, and extracellular fluid volume some-
In congestive heart failure, blood volume may increase
Extracellular Fluid Volume Caused
Increased Blood Volume and
abnormalities.
can cause large increases in both of these variables.
sonably constant, there are abnormal conditions that
Fluid Volume
Volume and Extracellular
Large Increases in Blood
levels.
sodium intake is increased. The opposite changes take
and suppression of sodium- and water-retaining
Thus, the combined activation of natriuretic systems
, especially ANP,
aldosterone secretion, thus further reducing
Also, reduced angiotensin II decreases
volume expansion, decreases tubular sodium
, caused by
pressure natriuresis.
volume expansion, raise sodium excretion through
, caused by
increase in salt and water intake.
This mechanism is most important in the first few
atrium and the pulmonary blood vessels. Signals
Activation of low pressure receptor reflexes
sodium excretion. These mechanisms include the
increase in extracellular fluid volume. It is mainly this
cumulative sodium balance, which causes a slight
intake. The time delay results in a small increase in the
increased, sodium output initially lags slightly behind
High Sodium Intake Suppresses Antinatriuretic Systems and
dietary sodium intake. As discussed previously, the
The integration of the different control systems that
normal, despite continued excess of ANP.
hours, this effect is overcome by a slight decrease in
cause slight decreases in blood volume. In less than 24
natriuresis. For example, infusions of large amounts of
changes in blood pressure, acting through pressure
excessive production of ANP or even complete lack of
such as increased salt and water intake. However,
380
Unit V
The Body Fluids and Kidneys
ANP does not cause major changes in blood volume
because these effects can easily be overcome by small
ANP initially raise urine output of salt and water and
blood pressure that returns urine output toward
Integrated Responses to
Changes in Sodium Intake
regulate sodium and fluid excretion under normal
conditions can be summarized by examining the
homeostatic responses to progressive increases in
kidneys have an amazing capability to match their
excretion of salt and water to intakes that can range
from as low as one tenth of normal to as high as 10
times normal.
Activates Natriuretic Systems.
As sodium intake is
small increase in extracellular fluid volume that trig-
gers various mechanisms in the body to increase
following:
1.
that
originate from the stretch receptors of the right
from the stretch receptors go to the brain stem
and there inhibit sympathetic nerve activity to the
kidneys to decrease tubular sodium reabsorption.
hours—or perhaps the first day—after a large
2. Small increases in arterial pressure
3. Suppression of angiotensin II formation
increased arterial pressure and extracellular fluid
reabsorption by eliminating the normal effect of
angiotensin II to increase sodium reabsorption.
tubular sodium reabsorption.
4. Stimulation of natriuretic systems
contributes further to increased sodium excretion.
systems leads to an increase in sodium excretion when
place when sodium intake is reduced below normal
Conditions That Cause
Despite the powerful regulatory mechanisms that main-
tain blood volume and extracellular fluid volume rea-
Almost all of these conditions result from circulatory
by Heart Diseases
the arterial pressure and cardiac output toward normal.
blood volume can often return cardiac output and arte-
excretion will eventually increase back to normal,
although there will remain excess extracellular fluid
volume and blood volume to keep the weakened heart
person develops severe circulatory congestion and
eventually dies of pulmonary edema.
circulatory compensation is an increase in blood
heart to maintain a life-sustaining level of cardiac
output.
Increased Capacity of Circulation
Any condition that increases vascular capacity will also
cause the blood volume to increase to fill this extra
salt and water retention by the kidneys until the blood

Nephrol 10(Suppl 12):s258, 1999.
ulation: the overriding dominance of the kidney. J Am Soc
Hall JE: Angiotensin II and long-term arterial pressure reg-
2000, pp 1009-1046.
and Pathophysiology, 3rd ed. New York: Raven Press,
Seldin DW, Giebisch G (eds): The Kidney—Physiology
system: renal mechanisms and circulatory homeostasis. In
Hall JE, Brands MW: The renin-angiotensin-aldosterone
kidneys and body fluids. Science 252:1813, 1991.
Guyton AC: Blood pressure control—special role of the
2000, pp 1811-1840.
and Pathophysiology, 3rd ed. New York: Raven Press,
Seldin DW, Giebisch G (eds): The Kidney—Physiology
Guise TA, Mundy GR: Disorders of calcium metabolism. In
sure natriuresis. Curr Hypertens Rep 4:15, 2002.
Granger JP, Alexander BT, Llinas M: Mechanisms of pres-
potassium transport. Pflugers Arch 446:289, 2003.
Giebisch G, Hebert SC, Wang WH: New aspects of renal
62:1498, 2002.
Giebisch GH: A trail of research on potassium. Kidney Int
York: Raven Press, 2000, pp 1153-1174.
Kidney—Physiology and Pathophysiology, 3rd ed. New
and salt appetite. In Seldin DW, Giebisch G (eds): The
Fitzsimmons JT: Physiology and pathophysiology of thirst
Physiol Rev 72:231, 1992.
Cowley AW Jr: Long-term control of arterial pressure.
84:169, 2004.
roendocrine control of body fluid metabolism. Physiol Rev
Antunes-Rodrigues J, de Castro M, Elias LL, et al: Neu-
capacity.
rial pressure are restored to normal. In some cases,
decreased plasma volume. That is, the kidneys continue
from the circulation, the renal responses are similar to
ascites.
leakage of fluid and proteins into the peritoneal cavity,
portal vascular bed, which also contributes to the
This in turn raises capillary pressure throughout the
impedes the flow of portal blood through the liver.
of fibrous tissue in the liver structure, which greatly
proteins. Cirrhosis is also associated with large amounts
results from destruction of the liver cells, thus reducing
rhosis, the reduction in plasma protein concentration
liver as in nephrotic syndrome, except that in liver cir-
by the Kidneys
by the Liver and Sodium Retention
restore the plasma proteins.
into the tissues of the body. The net result is massive
becomes further diluted, causing still more fluid to leak
water retention, the plasma protein concentration
However, because of the large amount of sodium and
system. The kidneys continue to retain sodium and
aldosterone, and possibly the sympathetic nervous
interstitial fluid, including activation of various sodium-
edema and decreases the plasma volume.
of fluid into the various tissues, which in turn causes
colloid osmotic pressure falls to low levels. This causes
decreased plasma protein concentration, the plasma
to less than one-third normal. As a consequence of the
plasma protein can be lost in the urine each day, some-
permeability of the glomerulus. Thirty to 50 grams of
In nephrotic syndrome, the
nephrotic syndrome.
edema are reviewed in Chapter 25. One of the most
The general mechanisms that lead to extracellular
Sodium Retention by the Kidneys
however, leaks into the interstitium, causing further
blood volume toward normal. Much of the extra fluid,
similar to the response after hemorrhage. That is, the
volume. The kidneys’ response to these conditions is
into the interstitium, which tends to decrease the blood
remains normal or even slightly reduced. These condi-
There are several conditions in which extracellular fluid
Blood Volume
Volume but with Normal
Large Increases in
vascular capacity. In these cases, salt and water are
have large varicose veins of the legs, which in rare
volume 15 to 25 per cent. Similarly, in patients who
of the woman’s body regularly increases the blood
ity of the uterus, placenta, and other enlarged organs
For example, in pregnancy the increased vascular capac-
volume increases sufficiently to fill the extra capacity.
Renal Regulation; Integration of Renal Mechanisms
Chapter 29
381
instances may hold up to an extra liter of blood,
the blood volume simply increases to fill the extra
retained by the kidneys until the total vascular bed is
filled enough to raise blood pressure to the level
required to balance renal output of fluid with daily
intake of fluid.
Conditions That Cause
Extracellular Fluid
volume becomes markedly increased but blood volume
tions are usually initiated by leakage of fluid and protein
kidneys retain salt and water in an attempt to restore
edema.
Nephrotic Syndrome—Loss of
Plasma Proteins in Urine and
important clinical causes of edema is the so-called
glomerular capillaries leak large amounts of protein
into the filtrate and the urine because of an increased
times causing the plasma protein concentration to fall
the capillaries all over the body to filter large amounts
Renal sodium retention in nephrotic syndrome
occurs through multiple mechanisms activated by
leakage of protein and fluid from the plasma into the
retaining systems such as the renin-angiotensin system,
water until plasma volume is restored nearly to normal.
fluid retention by the kidneys until tremendous extra-
cellular edema occurs unless treatment is instituted to
Liver Cirrhosis—Decreased
Synthesis of Plasma Proteins
A similar sequence of events occurs in cirrhosis of the
the ability of the liver to synthesize enough plasma
a condition called
Once fluid and protein are lost
those observed in other conditions associated with
to retain salt and water until plasma volume and arte-
plasma volume may actually increase above normal
because of increased vascular capacity in cirrhosis;
the high pressures in the portal circulation can
greatly distend veins and therefore increase vascular
References

Endocr Rev 6:24, 1985.
Young DB: Analysis of long-term potassium regulation.
potassium regulation. Am J Physiol 255: F811, 1988.
Young DB: Quantitative analysis of aldosterone’s role in
. Annu Rev Physiol 64:845, 2002.
Warnock DG. Renal genetic disorders related to K
Kidney, 6th ed. Philadelphia: WB Saunders, 2000, pp 520-
magnesium and phosphate. In: Brenner BM (ed): The
Suki WN, Lederer ED, Rouse D: Renal transport of calcium
2000, pp 1551-1574.
and Pathophysiology, 3rd ed. New York: Raven Press,
Seldin DW, Giebisch G (eds): The Kidney—Physiology
Rosa R, Epstein FH: Extrarenal potassium metabolism. In
Physiol 65:531, 2003.
Na/Pi transporter in the proximal tubule. Annu Rev
Murer H, Hernando N, Forster I, Biber J: Regulation of
382
Unit V
The Body Fluids and Kidneys
574.
+
and
Mg
2
+
