
sium, lithium, and so forth—with a highly basic ion such as a hydroxyl ion (OH
formed by the combination of one or more of the alkaline metals—sodium, potas-
are often used synonymously. An
The terms
bases.
proteins in the other cells of the body are among the most important of the body’s
. The protein hemoglobin in the red blood cells and
as bases, because some of the amino acids that make up proteins have net negative
. The proteins in the body also function
to form H
. Likewise, HPO
to form H
. For example, HCO
). Likewise, carbonic acid (H
to as acids. An example is hydrochloric acid (HCl), which ionizes in water to form
A hydrogen ion is a single free proton released from a hydrogen atom. Molecules
various cell functions.
) concentration. Thus,
, which averages only 0.00004 mEq/L. Equally important, the
lar fluid (142 mEq/L) is about 3.5 million times as great as the normal concen-
is kept at a low level. For example, the concentration of sodium in extracellu-
Compared with other ions, the H
hydrogen concentration alter virtually all cell and body functions.
concentration. Therefore, changes in
Precisely Regulated
Hydrogen Ion Concentration Is
body fluids.
), one of the key components of acid-base control systems in the
secretion and renal reabsorption, production, and excretion of bicarbonate
concentration are discussed, with special emphasis on the control of renal
In this chapter, the various mechanisms that contribute to the regulation of
ple acid-base buffering mechanisms involving the blood, cells, and lungs that are
by the kidneys. There are also multi-
removal. However, precise control
as is true for other ions, the kidneys play a key role
from the body. And,
body. For instance, to achieve homeostasis, there
Regulation of Acid-Base Balance
C
H
A
P
T
E
R
3
0
383
Regulation of hydrogen ion (H
+
) balance is similar
in some ways to the regulation of other ions in the
must be a balance between the intake or production
of H
+
and the net removal of H
+
in regulating H
+
of extracellular fluid H
+
concentration involves
much more than simple elimination of H
+
essential in maintaining normal H
+
concentrations in both the extracellular and
the intracellular fluid.
H
+
H
+
ions (HCO
3
–
Precise H
+
regulation is essential because the activities of almost all enzyme
systems in the body are influenced by H
+
+
concentration of the body fluids normally
tration of H
+
normal variation in H
+
concentration in extracellular fluid is only about one mil-
lionth as great as the normal variation in sodium ion (Na
+
the precision with which H
+
is regulated emphasizes its importance to the
Acids and Bases—Their Definitions
and Meanings
containing hydrogen atoms that can release hydrogen ions in solutions are referred
hydrogen ions (H
+
) and chloride ions (Cl
–
2
CO
3
) ionizes
in water to form H
+
and bicarbonate ions (HCO
3
–
).
A base is an ion or a molecule that can accept an H
+
3
–
is a
base because it can combine with H
+
2
CO
3
4
=
is a base
because it can accept an H
+
2
PO
4
–
charges that readily accept H
+
base and alkali
alkali is a molecule
–
).

between 6.0 and 7.4. Hypoxia of the tissues and poor
. Depending on the type of cells, the
person can live more than a few hours is about 6.8, and
pH rises above 7.4. The lower limit of pH at which a
is 7.4, a person is considered to have
(Table 30–1). Because the normal pH of arterial blood
The normal pH of arterial blood is 7.4, whereas the
concentration, and a high pH
concentration; therefore, a low pH
From this formula, one can see that pH is inversely
(0.00000004 Eq/L). Therefore, the normal pH is
] is 40 nEq/L
For example,
the normal [H
scale, using pH units. pH is related to the actual H
because these small numbers are cumbersome, it is cus-
concentration normally is low, and
as 10 nEq/L to as high as 160 nEq/L without causing
conditions, the H
are only about 3 to 5 nEq/L, but under extreme
of about 0.00004 mEq/L (40 nEq/L). Normal variations
cussed earlier, the blood H
Normal Hydrogen Ion Concentration and pH of Body Fluids
base.
tion are weak acids and bases. The most important ones
. Most of the acids and bases in the extracel-
O). A typical weak base is
, which reacts
from a solution. A typical example is OH
and, therefore, quickly removes these
. A strong base is one that reacts rapidly and
with less vigor. An example is
therefore, release H
acids have less tendency to dissociate their ions and,
in solution. An example is HCl. Weak
Strong and Weak Acids and Bases.
acidosis.
, which is referred
from the body fluids, in
typical bases. For similar reasons, the term
to remove it from solution; they are, therefore,
The base portion of these molecules reacts quickly with
384
Unit V
The Body Fluids and Kidneys
H
+
alkalosis
refers to excess removal of H
+
contrast to the excess addition of H
+
to as
A strong acid is one that
rapidly dissociates and releases especially large
amounts of H
+
+
H
2
CO
3
strongly with H
+
–
with H
+
to form water (H
2
HCO
3
–
because it binds with H
+
much more weakly than
does OH
–
lular fluid that are involved in normal acid-base regula-
that we discuss in detail are H
2
CO
3
and bicarbonate
and Changes That Occur in Acidosis and Alkalosis.
As dis-
+
concentration is normally
maintained within tight limits around a normal value
+
concentration can vary from as low
death.
Because H
+
tomary to express H
+
concentration on a logarithm
+
con-
centration by the following formula (H
+
concentration
[H
+
] is expressed in equivalents per liter):
+
pH
= –log [0.00000004]
pH
= 7.4
related to the H
+
corresponds to a high H
+
corresponds to a low H
+
concentration.
pH of venous blood and interstitial fluids is about 7.35
because of the extra amounts of carbon dioxide (CO
2
)
released from the tissues to form H
2
CO
3
in these fluids
acidosis when the
pH falls below this value and to have alkalosis when the
the upper limit is about 8.0.
Intracellular pH usually is slightly lower than plasma
pH because the metabolism of the cells produces acid,
especially H
2
CO
3
pH of intracellular fluid has been estimated to range
blood flow to the tissues can cause acid accumulation
and decreased intracellular pH.
log H
pH
log
1
H
+
=
[ ]
= -
+
[ ]
ful of the acid-base regulatory systems.
hours to several days, they are by far the most power-
compared with the other defenses, over a period of
can eliminate the excess acid or base from the body.
kidneys,
slowly responding third line of defense, the
These
from the body.
therefore, H
The second line of defense, the
of a second to minimize these changes. Buffer systems
concentration, the
When there is a change in H
normal during acidosis or alkalosis.
excrete either acid or alkaline urine, thereby readjust-
kidneys,
uid; and (3) the
(and, therefore, H
respiratory center,
tion; (2) the
the body fluids,
chemical acid-base buffer systems of
alkalosis: (1) the
There are three primary systems that regulate the H
Buffers, Lungs, and Kidneys
Hydrogen Ion Concentration:
In the remainder of this chapter, we discuss the reg-
The H
cells of the stomach mucosa, as discussed in Chapter 64.
excreting acids or bases at variable rates.
cussed later, the kidneys play a major role in correcting
uid. As dis-
The pH of urine can range from 4.5 to 8.0, depending
on the acid-base status of the extracellular fl
abnormalities of extracellular fluid H
+
concentration by
An extreme example of an acidic body fluid is the
HCl secreted into the stomach by the oxyntic (parietal)
+
concentration in these cells is about 4 million
times greater than the hydrogen concentration in blood,
with a pH of 0.8.
ulation of extracellular fluid H
+
concentration.
Defenses Against Changes in
+
concentration in the body fluids to prevent acidosis or
which immediately combine with acid
or base to prevent excessive changes in H
+
concentra-
which regulates the
removal of CO
2
2
CO
3
) from the
extracellular fl
which can
ing the extracellular fluid H
+
concentration toward
+
buffer systems of the body fluids react within a fraction
do not eliminate H
+
from or add them to the body
but only keep them tied up until balance can be re-
established.
respiratory system,
also acts within a few minutes to eliminate CO
2
and,
2
CO
3
first two lines of defense keep the H
+
con-
centration from changing too much until the more
Although the kidneys are relatively slow to respond
Table 30–1
Urine
3
uid
1
uid
4.5
Venous blood
4.5
Arterial blood
4.0
Concentration (mEq/L)
pH
pH and H
+
Concentration of Body Fluids
H
+
Extracellular fluid
¥ 10
–5
7.40
¥ 10
–5
7.35
Interstitial fl
¥ 10
–5
7.35
Intracellular fl
¥ 10
–3
to 4
¥ 10
–5
6.0 to 7.4
¥ 10
–2
to 1
¥ 10
–5
4.5 to 8.0
Gastric HCl
160
0.8

, as
uid. NaHCO
salt, occurs predominantly as sodium bicarbonate
The second component of the system, bicarbonate
O to form H
with H
epithelial cells of the renal tubules, where CO
released; carbonic anhydrase is also present in the
dant in the walls of the lung alveoli, where CO
is present. This enzyme is especially abun-
This reaction is slow, and exceedingly small amounts
with H
, and (2) a bicarbonate salt, such as NaHCO
tion that contains two ingredients: (1) a weak acid,
The bicarbonate buffer system consists of a water solu-
Bicarbonate Buffer System
The action of acid-base buffers can perhaps best be
cause huge changes in body
mally is only about 0.00004 mEq/L. Without buffering,
example, about 80 milliequivalents of hydrogen is
amounts of acids produced by the body each day. For
The importance of the body
buffer. In this way, changes in H
shifts toward the left, and H
concentration decreases, the reaction
the buffer, as long as buffer is available. Conversely,
reaction is forced to the right, and more H
concentration increases, the
. When the H
In this example, a free H
The general form of the buffering reaction is
Buffering of Hydrogen Ions
Regulation of Acid-Base Balance
Chapter 30
385
in the Body Fluids
A buffer is any substance that can reversibly bind H
+
.
+
combines with the buffer to
form a weak acid (H buffer) that can either remain as
an unassociated molecule or dissociate back to buffer
and H
+
+
+
binds to
when the H
+
+
is released from the
+
concentration are
minimized.
fluid buffers can be
quickly realized if one considers the low concentration
of H
+
in the body fluids and the relatively large
either ingested or produced each day by metabolism,
whereas the H
+
concentration of the body fluids nor-
the daily production and ingestion of acids would
fluid H
+
concentration.
explained by considering the buffer system that is
quantitatively the most important in the extracellular
fluid—the bicarbonate buffer system.
H
2
CO
3
3
.
H
2
CO
3
is formed in the body by the reaction of CO
2
2
O.
of H
2
CO
3
are formed unless the enzyme carbonic
anhydrase
2
is
2
reacts
2
2
CO
3
.
H
2
CO
3
ionizes weakly to form small amounts of H
+
and HCO
3
–
.
(NaHCO
3
) in the extracellular fl
3
ionizes almost completely to form HCO
3
–
and Na
+
follows:
NaHCO
Na
HCO
3
+
3
+
-
¨
æ Æ
æ
æ
H C
H
HCO
2
+
3
3
+
-
¨
O
æÆ æ
æ
Æ
CO
H O
H CO
2
2
carbonic
anhydrase
2
3
+
¨ææ
ææ
æ Æ
ææ
æ
Buffer H
H Buffer
+
+
¨
æÆ æ
æ
æ Æ
æ
æ
solution, the
This equation indicates that in an H
For any acid, the concentration of the acid relative
From mass balance considerations, the concentrations
, are ionized to some extent.
All acids, including H
Quantitative Dynamics of the
expiration. The rise in blood HCO
of CO
levels in the blood to decrease, but the decreased CO
The net result, therefore, is a tendency for the CO
with H
it reacts with NaOH), causing more CO
time, the concentration of H
replaces the strong base NaOH. At the same
. Thus, the weak base
In this case, the OH
base, such as sodium hydroxide (NaOH), is added to
The opposite reactions take place when a strong
from the
ulates respiration, which eliminates the CO
O. The excess CO
, which in
O production. From these reactions, one
is formed, causing increased
As a result, more H
bicarbonate buffer solution, the increased H
When a strong acid such as HCl is added to the
, the H
Now, putting the entire system together, we have the
following:
Because of the weak dissociation of H
2
CO
3
+
concentration is extremely small.
+
released
from the acid (HCl
Æ H
+
+ Cl
–
) is buffered by HCO
3
–
.
≠H
+
+ HCO
3
–
Æ H
2
CO
3
Æ CO
2
+ H
2
O
2
CO
3
CO
2
and H
2
can see that H
+
from the strong acid HCl reacts with
HCO
3
–
to form the very weak acid H
2
CO
3
turn forms CO
2
and H
2
2
greatly stim-
2
extracellular fluid.
the bicarbonate buffer solution.
NaOH
+ H
2
CO
3
Æ NaHCO
3
+ H
2
O
–
from the NaOH combines with
H
2
CO
3
to form additional HCO
3
–
NaHCO
3
2
CO
3
decreases (because
2
to combine
2
O to replace the H
2
CO
3
.
2
2
in the blood inhibits respiration and decreases the rate
2
3
–
that occurs
is compensated for by increased renal excretion of
HCO
3
–
.
Bicarbonate Buffer System
2
CO
3
of H
+
and HCO
3
–
are proportional to the concentration
of H
2
CO
3
.
to its dissociated ions is defined by the dissociation
constant K
¢.
(1)
2
CO
3
amount of free H
+
is equal to
(2)
H CO
H
K
HCO
+
2
3
3
=
¢ ¥
-
H CO
H
HCO
¢ =
¥
-
K
+
3
2
3
H CO
HCO
H
2
3
+
3
+
-
¨
æÆ æ
æ
Æ
æ
æ Æ
+
≠
+
2
2
2
3
3
NaOH
Na
CO
H O
H CO
HCO
H
+
+
+
-
æ Æ
æ
æ
æ
2
2
2
3
CO
H O
H CO
H
HCO
+
Na
+
3
–
+
+
+
Ï Ì Ó
¨
æÆ æ
æ
æ Æ
æ
æ æ
¨
æÆ æ
æ
Æ
causes the pH to rise, shifting the acid-base balance
From the Henderson-Hasselbalch equation, it is
and with it, one can calculate the pH of a solution if
For the bicarbonate buffer system, the pK is 6.1, and
ator and denominator in the last term, using the law of
Rather than work with a negative logarithm, we can
Therefore,
of that equation, which yields
Therefore, we can express the H
similar manner.
The dissociation constant can be expressed in a
rather than in actual concentrations. Recall that pH is
As discussed earlier, it is
measured. Therefore, equa-
0.03 mmol/mm Hg at body temperature.This means that
conditions, the solubility coef
; under physiologic
of CO
. Fortunately, the amount
dissolved in solution. However, most clinical labo-
The dissociation constant (K) for equation 3 is only
. Therefore, equation 2
. However, the CO
The concentration of undissociated H
386
Unit V
The Body Fluids and Kidneys
2
CO
3
cannot be
measured in solution because it rapidly dissociates into
CO
2
and H
2
O or to H
+
and HCO
3
–
2
dissolved in the blood is directly proportional to the
amount of undissociated H
2
CO
3
can be rewritten as
(3)
about
1
/
400
of the dissociation constant (K
¢) of equation
2 because the proportionality ratio between H
2
CO
3
and
CO
2
is 1:400.
Equation 3 is written in terms of the total amount of
CO
2
ratories measure the blood CO
2
tension (P
CO
2
) rather
than the actual amount of CO
2
2
in the blood is a linear function of P
CO
2
times
the solubility coefficient for CO
2
ficient for CO
2
is
0.03 millimole of H
2
CO
3
is present in the blood for each
millimeter of mercury P
CO
2
tion 3 can be rewritten as
(4)
Henderson-Hasselbalch Equation.
customary to express H
+
concentration in pH units
defined as pH
= –log H
+
.
pK
= –log K
+
concentration in
equation 4 in pH units by taking the negative logarithm
(5)
(6)
change the sign of the logarithm and invert the numer-
logarithms to yield
(7)
equation 7 can be written as
(8)
Equation 8 is the Henderson-Hasselbalch equation,
the molar concentration of HCO
3
–
and the P
CO
2
are
known.
apparent that an increase in HCO
3
–
concentration
0 03
.
log
pH
6
HCO
P
3
2
=
+
¥
-
.
1
co
0 03
pH
pK
HCO
P
3
2
=
+
¥
(
)
-
log
.
co
0 03
pH
pK
P
HCO
2
3
=
-
¥
(
)
-
log
.
co
0 03
log
log
log H
pK
-
= -
-
¥
(
)
-
P
HCO
+
2
3
.
co
0.03
P
H
K
HCO
+
2
3
=
¥
¥
(
)
-
co
H
K
CO
HCO
+
2
3
=
¥
-
Hasselbalch equation. When acid is added, it is buffered
increasing the pH, as is evident from the Henderson-
to CO
bonate buffer system. When base is added to the system,
components of the buffer system are equal, the pH of
log of 1, which is equal to 0. Therefore, when the two
equal, the right-hand portion of equation 8 becomes the
When the concentrations of these two components are
to CO
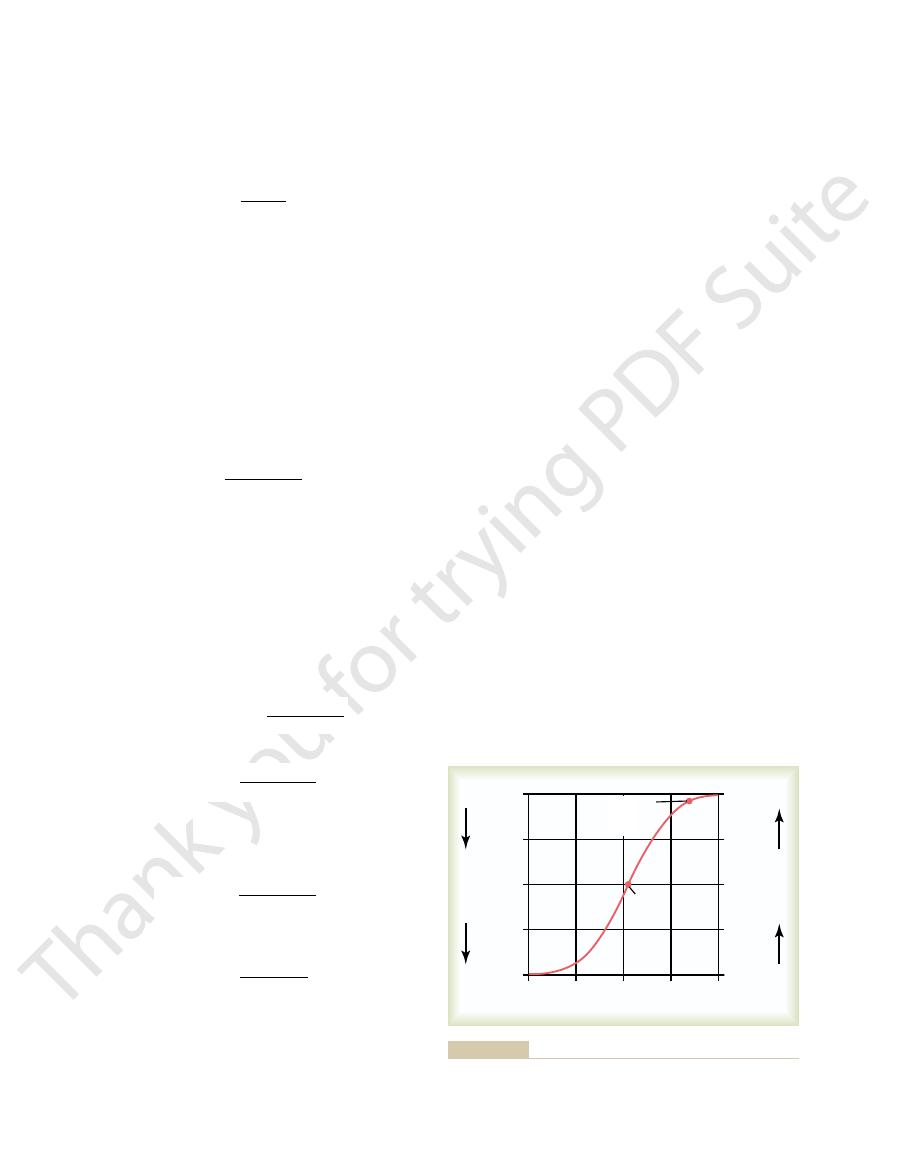
Figure 30
Bicarbonate Buffer System Titration Curve.
respiratory alkalosis.
by a decrease in P
respiratory acidosis,
bolic alkalosis.
bolic acidosis,
disorders. Therefore, acidosis caused by a primary
centration, they are referred to as
When disturbances of acid-base balance result from
impaired, thus altering either the bicarbonate concen-
lungs and the kidneys, and acid-base disorders occur
from the coordinated efforts of both of these organs, the
. Normal physiologic acid-base homeostasis results
plasma, and by decreasing respiration, the lungs elevate
from the
rate of respiration, the lungs remove CO
controlled by the rate of respiration.
by the kidneys, whereas the P
later,
uid. As discussed
uid, provides
The Henderson-Hasselbalch equation, in addition to
decrease, shifting the acid-base balance toward acidosis.
toward alkalosis. An increase in P
CO
2
causes the pH to
defining the determinants of normal pH regulation and
acid-base balance in the extracellular fl
insight into the physiologic control of acid and base
composition of the extracellular fl
the bicarbonate concentration is regulated mainly
CO
2
in extracellular fluid is
By increasing the
2
P
CO
2
when one or both of these control mechanisms are
tration or the P
CO
2
of extracellular fluid.
a primary change in extracellular fluid bicarbonate con-
metabolic acid-base
decrease in bicarbonate concentration is termed meta-
whereas alkalosis caused by a primary
increase in bicarbonate concentration is called meta-
Acidosis caused by an increase in P
CO
2
is called
whereas alkalosis caused
CO
2
is termed
–1 shows
the changes in pH of the extracellular fluid when the
ratio of HCO
3
–
2
in extracellular fluid is altered.
the solution is the same as the pK (6.1) of the bicar-
part of the dissolved CO
2
is converted into HCO
3
–
,
causing an increase in the ratio of HCO
3
–
2
and
Acid added
Per cent of buffer in form of
H
2
CO
3
and CO
2
0
25
50
75
100
Per cent of buffer in form of
HCO
3
-
Base added
0
25
50
75
100
8
7
6
5
4
pH
pK
Normal
operating
point in body
) are altered.
extracellular fluid when the percentages of buffer in the form of
Titration curve for bicarbonate buffer system showing the pH of
Figure 30–1
HCO
3
–
and CO
2
(or H
2
CO
3

become maximally effective.
For this reason, the buffer
changes in extracellular pH.
the pH in intracellular fluid to change when there are
diffuse through all the cell membranes.
, however, can rapidly
occurs in the red blood cells. CO
uid, except for rapid equilibrium that
through the cell membrane, although these ions
There is a slight amount of diffusion of H
uid pH changes.
uid, nevertheless changes approxi-
The pH of the cells, although slightly lower than in
within the cells.
body because of their high concentrations, especially
Intracellular Buffers
Proteins: Important
uid. Also, the pH of intracellular
uid does, bringing the operating
ing power of the phosphate system, and (2) the tubular
centrated in the tubules, thereby increasing the buffer-
reasons: (1) phosphate usually becomes greatly con-
important in the tubular fluids of the kidneys,
cellular buffer,
concentration of the bicarbonate buffer. Therefore,
uid is low, only about 8 per cent of the
buffering power. However, its concentration in the
The phosphate buffer system has a pK of 6.8, which
, causing only a slight increase in
base, NaH
In this case, a strong base, NaOH, is traded for a weak
buffer system, the OH
When a strong base, such as NaOH, is added to the
, and the decrease in pH is minimized.
The result of this reaction is that the strong acid, HCl,
HCl is added to a mixture of these two substances, the
. When a strong acid such as
The main elements of the phosphate buffer system
uids.
uid buffer, it plays a major
Phosphate Buffer System
by the lungs.
lungs, as discussed later. As a result of this regulation,
, are regulated, respectively, by the kidneys and the
that the two elements of the buffer system, HCO
body. This apparent paradox is due mainly to the fact
Despite these characteristics, the bicarbonate buffer
, are not great.
system, CO
low and the buffering power is poor. Second, the con-
. For this reason, this system operates on
bonate buffer system is 6.1. This means that there is
uid is about 7.4, whereas the pK of the bicar-
be powerful, for two reasons: First, the pH of the extra-
From the titration curve shown in Figure 30
Buffer.
changes the pH considerably.
system. With low concentrations of the buffers, only a
The absolute concentration of the buffers is also an
system has no more buffering power.
, the
these limits, the buffering power rapidly diminishes.
extends from a pH of about 5.1 to 7.1 units. Beyond
of the pK, which for the bicarbonate buffer system
the pH is near the pK of the system. The buffer system
system. This means that the change in pH for any given
part of the curve, where the pH is near the pK of the
Second, the buffer system is most effective in the central
1, several points are apparent. First,
curve in Figure 30
From the titration
to CO
, which is then converted into dissolved CO
Regulation of Acid-Base Balance
Chapter 30
387
by HCO
3
–
2
,
decreasing the ratio of HCO
3
–
2
and decreasing
the pH of the extracellular fluid.
“Buffer Power” Is Determined by the Amount and Relative Con-
centrations of the Buffer Components.
–
the pH of the system is the same as the pK when each
of the components (HCO
3
–
and CO
2
) constitutes 50 per
cent of the total concentration of the buffer system.
amount of acid or base added to the system is least when
is still reasonably effective for 1.0 pH unit on either side
And when all the CO
2
has been converted into HCO
3
–
or when all the HCO
3
–
has been converted into CO
2
important factor in determining the buffer power of a
small amount of acid or base added to the solution
Bicarbonate Buffer System Is the Most Important Extracellular
–1,
one would not expect the bicarbonate buffer system to
cellular fl
about 20 times as much of the bicarbonate buffer
system in the form of HCO
3
–
as in the form of dis-
solved CO
2
the portion of the buffering curve where the slope is
centrations of the two elements of the bicarbonate
2
and HCO
3
–
system is the most powerful extracellular buffer in the
3
–
and
CO
2
the pH of the extracellular fluid can be precisely con-
trolled by the relative rate of removal and addition of
HCO
3
–
by the kidneys and the rate of removal of CO
2
Although the phosphate buffer system is not impor-
tant as an extracellular fl
role in buffering renal tubular fluid and intracellular
fl
are H
2
PO
4
–
and HPO
4
=
hydrogen is accepted by the base HPO
4
=
and con-
verted to H
2
PO
4
–
.
HCl
+ Na
2
HPO
4
Æ NaH
2
PO
4
+ NaCl
is replaced by an additional amount of a weak acid,
NaH
2
PO
4
–
is buffered by the H
2
PO
4
–
to
form additional amounts of HPO
4
=
+ H
2
O.
NaOH
+ NaH
2
PO
4
Æ Na
2
HPO
4
+ H
2
O
2
PO
4
pH.
is not far from the normal pH of 7.4 in the body fluids;
this allows the system to operate near its maximum
extracellular fl
the total buffering power of the phosphate system in
the extracellular fluid is much less than that of the
bicarbonate buffering system.
In contrast to its rather insignificant role as an extra-
the phosphate buffer is especially
for two
fluid usually has a considerably lower pH than
the extracellular fl
range of the buffer closer to the pK (6.8) of the
system.
The phosphate buffer system is also important in
buffering intracellular fluid because the concentration
of phosphate in this fluid is many times that in the
extracellular fl
fluid is
lower than that of extracellular fluid and therefore is
usually closer to the pK of the phosphate buffer
system compared with the extracellular fluid.
Proteins are among the most plentiful buffers in the
the extracellular fl
mately in proportion to extracellular fl
+
and HCO
3
–
require several hours to come to equilibrium with the
extracellular fl
2
This diffusion
of the elements of the bicarbonate buffer system causes
systems within the cells help prevent changes in the
pH of extracellular fluid but may take several hours to
uids and blood, and the
metabolic processes. After it is formed, it diffuses from
Balances Metabolic Formation of CO
, thus also increasing H
concentration. Conversely, decreased
uid, which, by mass action,
tion by the lungs. An increase in ventilation eliminates
The second line of defense against acid-base distur-
Respiratory Regulation
The implication of this principle is that any condition
the bases of the three buffer systems.
, and A
, HA
, HA
respective acids, HA
time. This phenomenon is called the
uid, the
tions of all the systems. Therefore, whenever there is a
all work together, because H
uids. However, they
We have been discussing buffer systems as though they
in Equilibrium with the Same
in a Common Solution Are
Isohydric Principle: All Buffers
the cells, another factor that contributes to their
lular acid-base abnormalities.
However, except for the red blood cells, the slowness
most of this results from the intracellular proteins.
cal buffering of the body fluids is inside the cells, and
Approximately 60 to 70 per cent of the total chemi-
tant buffer, as follows:
In the red blood cell, hemoglobin (Hb) is an impor-
388
Unit V
The Body Fluids and Kidneys
with which H
+
and HCO
3
–
move through the cell mem-
branes often delays for several hours the maximum
ability of the intracellular proteins to buffer extracel-
In addition to the high concentration of proteins in
buffering power is the fact that the pKs of many of
these protein systems are fairly close to 7.4.
Hydrogen Ion Concentration
operated individually in the body fl
+
is common to the reac-
change in H
+
concentration in the extracellular fl
balance of all the buffer systems changes at the same
isohydric principle
and is illustrated by the following formula:
K
1
, K
2
, K
3
are the dissociation constants of three
1
2
3
1
, A
2
, A
3
are the
concentrations of the free negative ions that constitute
that changes the balance of one of the buffer systems
also changes the balance of all the others because the
buffer systems actually buffer one another by shifting
H
+
back and forth between them.
of Acid-Base Balance
bances is control of extracellular fluid CO
2
concentra-
CO
2
from extracellular fl
reduces the H
+
ventilation increases CO
2
+
con-
centration in the extracellular fluid.
Pulmonary Expiration of CO
2
2
CO
2
is formed continually in the body by intracellular
the cells into the interstitial fl
=
¥
=
¥
=
¥
H
K
HA
A
K
HA
A
K
HA
A
+
1
1
1
2
2
2
3
3
3
Hb
HHb
H
+
+
¨
æ Æ
æ
æ
¨
æÆ æ
æ
tion, doubling the ventilation rate raises the pH to
uid by about 0.23. If the pH
the rate of alveolar ventilation. Note that increasing
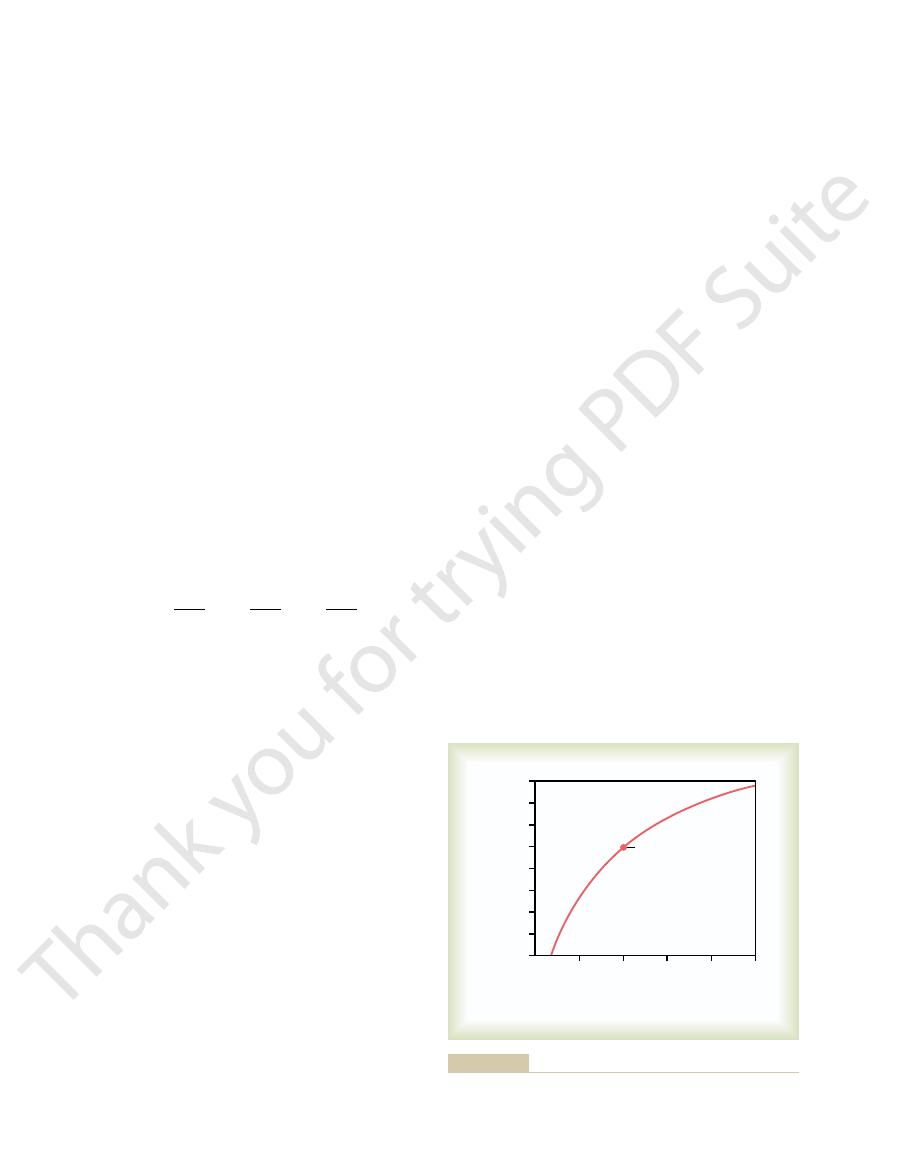
Figure 30
tion also increase, thereby lowering extracellular
increases, the H
As discussed previously, when CO
lower the alveolar ventilation rate, the higher the P
; conversely, the
alveolar ventilation, the lower the P
uid is the rate of alveolar ventilation. The higher the
Increasing Alveolar Ventilation
uid decreases. Therefore, changes in
is blown off from the lungs, and the P
. If the rate of pulmonary ventilation is increased,
Conversely, a decreased metabolic rate lowers the
increases,
of 40 mm Hg.
uid, corresponding to a P
atmosphere by pulmonary ventilation. About 1.2 mol/
owing blood transports it to the lungs, where it dif-
fl
fuses into the alveoli and then is transferred to the
L of dissolved CO
2
normally is in the extracellular
fl
co
2
If the rate of metabolic formation of CO
2
the Pco
2
of the extracellular fluid is likewise increased.
Pco
2
CO
2
co
2
in the
extracellular fl
either pulmonary ventilation or the rate of CO
2
for-
mation by the tissues can change the extracellular fluid
Pco
2
.
Decreases Extracellular Fluid
Hydrogen Ion Concentration and
Raises pH
If the metabolic formation of CO
2
remains constant,
the only other factor that affects Pco
2
in extracellular
fl
co
2
co
2
.
2
concentration
2
CO
3
concentration and H
+
concentra-
fluid pH.
–2 shows the approximate changes in
blood pH that are caused by increasing or decreasing
alveolar ventilation to about twice normal raises the
pH of the extracellular fl
of the body fluids is 7.40 with normal alveolar ventila-
Normal
0.5
1.0
1.5
2.0
2.5
pH change in body fluids
+
0.3
-
0.3
-
0.4
-
0.5
-
0.1
-
0.2
+
0.2
+
0.1
0
Rate of alveolar ventilation
(normal = 1)
decreased rate of alveolar ventilation, expressed as times normal.
Change in extracellular fluid pH caused by increased or
Figure 30–2
stances, the kidneys represent the sole remaining phys-
increased ventilation are blunted. In these circum-
Also, the ability to respond to metabolic aci-
acidosis.
uid and a tendency toward
; this causes a buildup of CO
severe emphysema, decreases the ability of the lungs
example, an impairment of lung function, such as
concentration. For
concentration. However,
in H
We have discussed thus far the role of the
Impairment of Lung Function Can Cause Respiratory Acidosis.
nism as by the chemical buffers.
uid combined. That is, one to two times as much
general, the overall buffering power of the respiratory
responding kidneys can eliminate the imbalance. In
Buffering Power of the Respiratory System.
response occurs within 3 to 12 minutes.
return the pH to a value of about 7.2 to 7.3. This
pH falls from 7.4 to 7.0, the respiratory system can
of 1 to 3. That is, if the H
feedback gain
50 and 75 per cent, corresponding to a
pH. Ordinarily, the respiratory mechanism for con-
Efficiency of Respiratory Control of Hydrogen Ion Concen-
becomes depressed, alveolar ventilation decreases, and
tration falls below normal, the respiratory center
tion back toward normal. Conversely, if H
and alveolar ventilation increases. This decreases the
above normal, the respiratory system is stimulated,
That is, whenever the H
concentration, the respi-
stimulates respiration, and because increased alveolar
ratory System.
stimulates the ventilation rate. Therefore, the respira-
) in the blood also decreases, which
concentration), the amount of oxygen
ventilation rate decreases, owing to an increase in pH
levels of pH. The reason for this is that as the alveolar
ventilation rate. As one can see from the graph, the
plasma pH rises above 7.4, this causes a decrease in the
7.4 to the strongly acidic value of 7.0. Conversely, when
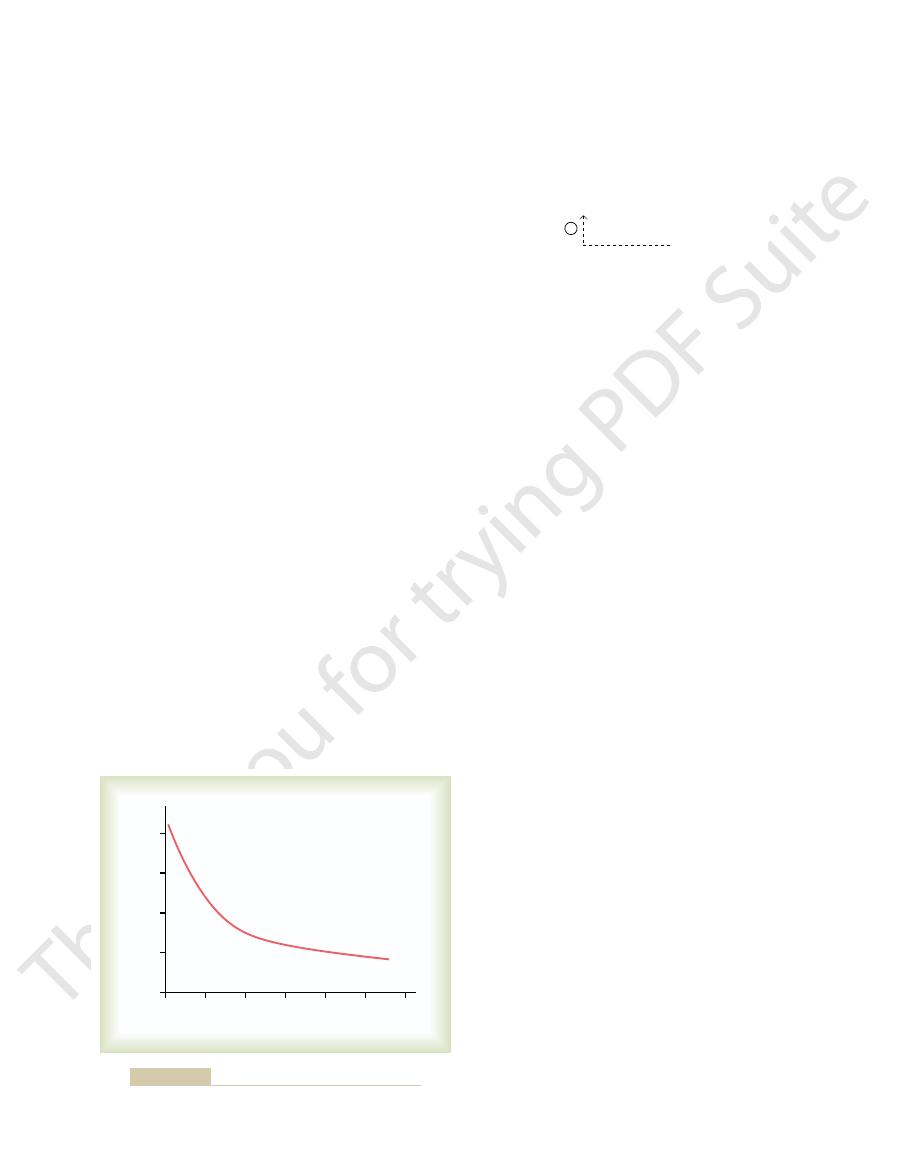
alveolar ventilation. Thus, Figure 30
uids, but the H
Alveolar Ventilation
times normal, one can easily understand how much the
can change markedly, from as low as 0 to as high as 15
the pH to 6.95. Because the alveolar ventilation rate
is, if the pH is 7.4 at a normal alveolar ventilation,
tion to one fourth normal reduces the pH by 0.45. That
about 7.63. Conversely, a decrease in alveolar ventila-
Regulation of Acid-Base Balance
Chapter 30
389
reducing the ventilation to one fourth normal reduces
pH of the body fluids can be changed by the respira-
tory system.
Increased Hydrogen Ion
Concentration Stimulates
Not only does the alveolar ventilation rate influence
H
+
concentration by changing the Pco
2
of the body
fl
+
concentration affects the rate of
–3 shows that the
alveolar ventilation rate increases four to five times
normal as the pH decreases from the normal value of
change in ventilation rate per unit pH change is much
greater at reduced levels of pH (corresponding to ele-
vated H
+
concentration) compared with increased
(decreased H
+
added to the blood decreases and the partial pressure
of oxygen (P
O
2
tory compensation for an increase in pH is not nearly
as effective as the response to a marked reduction in
pH.
Feedback Control of Hydrogen Ion Concentration by the Respi-
Because increased H
+
concentration
ventilation decreases the H
+
ratory system acts as a typical negative feedback con-
troller of H
+
concentration.
+
concentration increases
P
CO
2
in extracellular fluid and reduces H
+
concentra-
+
concen-
H
+
concentration increases back toward normal.
tration.
Respiratory control cannot return the H
+
concentration all the way back to normal when a dis-
turbance outside the respiratory system has altered
trolling H
+
concentration has an effectiveness between
+
concentration is suddenly
increased by adding acid to the extracellular fluid and
Respiratory reg-
ulation of acid-base balance is a physiologic type of
buffer system because it acts rapidly and keeps the H
+
concentration from changing too much until the slowly
system is one to two times as great as the buffering
power of all other chemical buffers in the extracellu-
lar fl
acid or base can normally be buffered by this mecha-
normal res-
piratory mechanism as a means of buffering changes
+
abnormalities of respi-
ration can also cause changes in H
+
to eliminate CO
2
2
in the
extracellular fl
respiratory
dosis is impaired because the compensatory reduc-
tions in P
CO
2
that would normally occur by means of
iologic mechanism for returning pH toward normal
after the initial chemical buffering in the extracellular
fluid has occurred.
≠[H
+
]
Æ ≠Alveolar ventilation
Ø
ØP
CO
2
–
7.0
7.1
7.2
7.3
7.4
7.5
7.6
Alveolar ventilation (normal = 1)
4
3
2
1
0
pH of arterial blood
Effect of blood pH on the rate of alveolar ventilation.
Figure 30–3
ows into the distal tubules and collecting ducts. In the
tubule, so that only a small amount of bicarbonate
bonate reabsorbed, an H
tion along the tubule. Keep in mind that for each bicar-
Henle. Figure 30
Renal Tubules
Secretion of Hydrogen Ions
discussed in the next few sections.
accomplished through the same basic mechanism, as
, and
, (2) reabsorption of filtered HCO
centration through three fundamental mechanisms: (1)
Thus, the kidneys regulate extracellular fluid H
uid. This reduces the extracellular
and produce new bicarbonate, which is added back to
In acidosis, the kidneys do not excrete bicarbonate
uid. Therefore, in alkalosis, the removal of
uid, this loss of
excretion of bicarbonate. Because HCO
ltered bicarbonate, thereby increasing the
concentration (alkalosis), the kidneys fail to reab-
When there is a reduction in the extracellular
uid each day.
body of the nonvolatile acids produced each day, for a
ltered bicarbonate. Then an additional
before it can be reabsorbed, 4320 mil-
secretion by the tubules.
As discussed later, both the reabsorption of bicar-
sorbed from the tubules, thereby conserving the
L); under normal conditions, almost all this is reab-
volatile acids. Each day the kidneys
loss of bicarbonate in the urine, a task that is quanti-
is renal excretion. The kidneys must also prevent the
fore, cannot be excreted by the lungs. The primary
and, there-
from the metabolism of proteins.These acids are called
about 80 milliequivalents of nonvolatile acids, mainly
As discussed previously, each day the body produces
there will be a net loss of base.
versely, if more HCO
uid. Con-
ltered, there will
epithelial cells, thus removing acid from the blood. If
removes base from the blood. Large numbers of H
tubules, and if they are excreted into the urine, this
excrete acidic or basic urine is as follows: Large
The overall mechanism by which the kidneys
either an acidic or a basic urine. Excreting an acidic
The kidneys control acid-base balance by excreting
Renal Control of
390
Unit V
The Body Fluids and Kidneys
Acid-Base Balance
urine reduces the amount of acid in extracellular fluid,
whereas excreting a basic urine removes base from the
extracellular fluid.
numbers of HCO
3
–
are filtered continuously into the
+
are
also secreted into the tubular lumen by the tubular
more H
+
is secreted than HCO
3
–
is fi
be a net loss of acid from the extracellular fl
3
–
is filtered than H
+
is secreted,
nonvolatile because they are not H
2
CO
3
mechanism for removal of these acids from the body
tatively more important than the excretion of non-
filter about 4320
milliequivalents of bicarbonate (180 L/day
¥ 24 mEq/
primary buffer system of the extracellular fluid.
bonate and the excretion of H
+
are accomplished
through the process of H
+
Because the HCO
3
–
must react with a secreted H
+
to
form H
2
CO
3
liequivalents of H
+
must be secreted each day just to
reabsorb the fi
80 milliequivalents of H
+
must be secreted to rid the
total of 4400 milliequivalents of H
+
secreted into the
tubular fl
fluid
H
+
sorb all the fi
3
–
normally
buffers hydrogen in the extracellular fl
bicarbonate is the same as adding an H
+
to the extra-
cellular fl
HCO
3
–
raises the extracellular fluid H
+
concentration
back toward normal.
into the urine but reabsorb all the filtered bicarbonate
the extracellular fl
fluid H
+
concentration back toward normal.
+
con-
secretion of H
+
3
-
(3) production of new HCO
3
-
. All these processes are
and Reabsorption of
Bicarbonate Ions by the
Hydrogen ion secretion and bicarbonate reabsorption
occur in virtually all parts of the tubules except the
descending and ascending thin limbs of the loop of
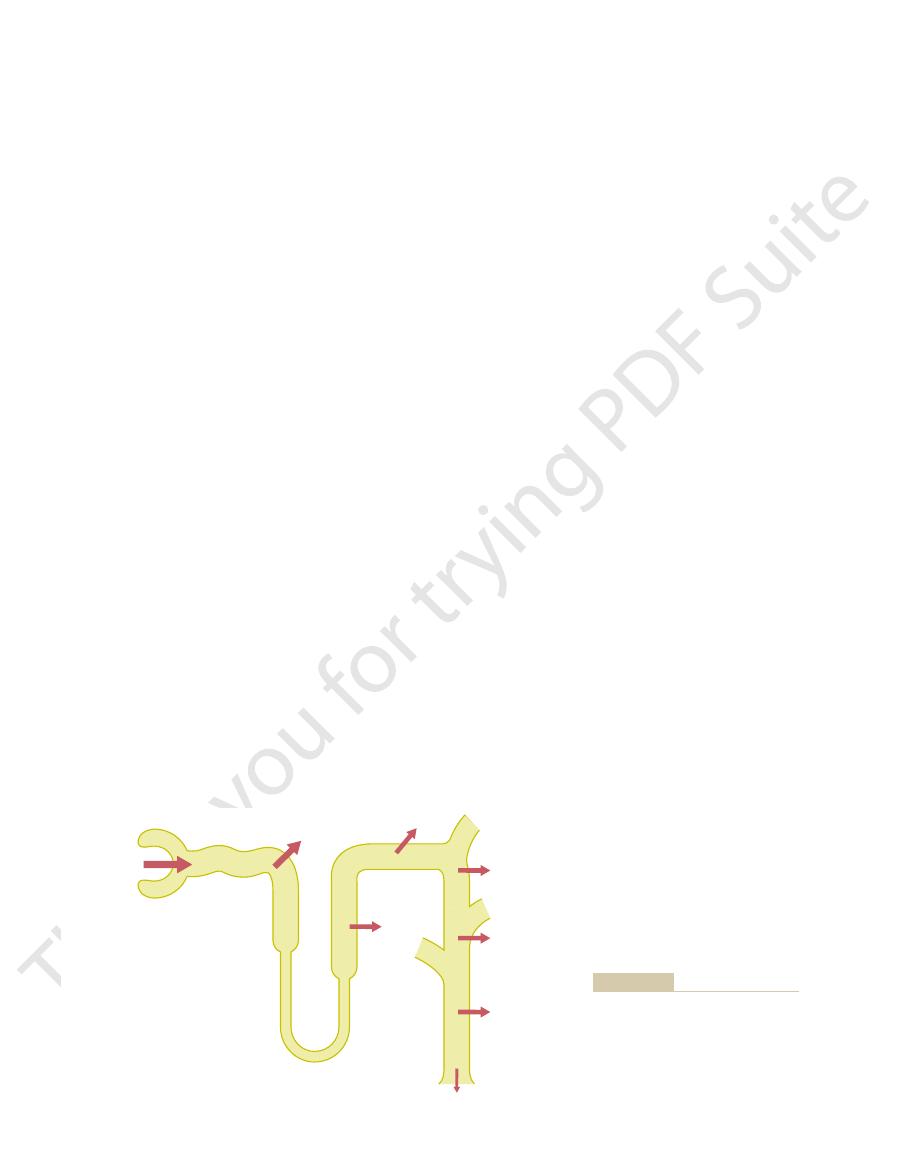
–4 summarizes bicarbonate reabsorp-
+
must be secreted.
About 80 to 90 per cent of the bicarbonate reab-
sorption (and H
+
secretion) occurs in the proximal
fl
85%
(3672 mEq/day)
>4.9%
(215 mEq/day)
(1 mEq/day)
4320 mEq/day
10%
(432 mEq/day)
reabsorbed per day under normal
tubular segments are shown, as well
percentages of the filtered load of
ent segments of the renal tubule. The
Reabsorption of bicarbonate in differ-
Figure 30–4
bicarbonate absorbed by the various
as the number of milliequivalents
conditions.
ltered into the tubules. The
from the tubules, although
The net effect of these reactions is
is also formed and released back
lial cells, an HCO
Thus, each time an H
exchange.
two mechanisms: (1) Na
peritubular capillary blood. The transport of HCO
; the
molecule. This
anhydrase, to generate a new H
O, under the in
fore, it instantly diffuses into the tubular cell, where it
can move easily across the tubular membrane; there-
O. The CO
secreted by the tubular cells. The H
This reabsorption of HCO
as shown in Figure 30
, which eventually becomes CO
directly reabsorbed. Instead, HCO
membranes of the renal tubular cells; therefore,
Hydrogen Ions in the Tubules
Filtered Bicarbonate Ions Are
secreted into the tubular lumen, an
and the peritubular capillary blood. The net result is
The HCO
basolateral membrane. The gradient for Na
lished by the sodium-potassium ATPase pump in the
with the carrier protein. The Na
same time, an H
in the luminal border of the cell membrane; at the
rior of the cell, it
sodium-hydrogen counter-transport. That is, when an
. The H
, which dissociates into HCO
O to form
carbonic anhydrase,
, under the in
epithelial cells. CO
achieves bicarbonate reabsorption. The secretory
Figure 30
lecting tubules and collecting ducts.
the tubules. This mechanism, however, does not estab-
bonate is reabsorbed in this manner, requiring about
lateral membrane. More than 90 per cent of the bicar-
adenosine triphosphatase (ATPase) pump in the baso-
This gradient is established by the sodium-potassium
exchanger protein, and the energy for H
5. This secondary active secretion of H
Figure 30
by sodium-hydrogen counter-transport, as shown in
segment of the ascending loop of Henle, and the early
The epithelial cells of the proximal tubule, the thick
in the Early Tubular Segments
by Secondary Active Transport
Hydrogen Ions Are Secreted
segments accomplish this task differently.
, but different tubular
and collecting duct. As discussed previously, the mech-
ltered bicarbonate is reabsorbed, and the remain-
thick ascending loop of Henle, another 10 per cent of
Regulation of Acid-Base Balance
Chapter 30
391
the fi
der of the reabsorption takes place in the distal tubule
anism by which bicarbonate is reabsorbed also
involves tubular secretion of H
+
distal tubule all secrete H
+
into the tubular fluid
–
+
is coupled with the transport of Na
+
into the cell at
the luminal membrane by the sodium-hydrogen
+
secretion
against a concentration gradient is derived from the
sodium gradient favoring Na
+
movement into the cell.
3900 milliequivalents of H
+
to be secreted each day by
lish a very high H
+
concentration in the tubular fluid;
the tubular fluid becomes very acidic only in the col-
–5 shows how the process of H
+
secretion
process begins when CO
2
either diffuses into the
tubular cells or is formed by metabolism in the tubular
2
fluence of the enzyme
combines with H
2
H
2
CO
3
3
–
and H
+
+
is secreted from the cell into the tubular lumen by
Na
+
moves from the lumen of the tubule to the inte-
first combines with a carrier protein
+
in the interior of the cells combines
+
moves into the cell
down a concentration gradient that has been estab-
+
move-
ment into the cell then provides the energy for moving
H
+
in the opposite direction from the interior of the
cell to the tubular lumen.
3
–
generated in the cell (when H
+
dissoci-
ates from H
2
CO
3
) then moves downhill across the
basolateral membrane into the renal interstitial fluid
that for every H
+
HCO
3
–
enters the blood.
Reabsorbed by Interaction with
Bicarbonate ions do not readily permeate the luminal
HCO
3
–
that is filtered by the glomerulus cannot be
3
–
is reabsorbed by
a special process in which it first combines with H
+
to
form H
2
CO
3
2
and H
2
O,
–5.
3
–
is initiated by a reaction
in the tubules between HCO
3
–
filtered at the glomeru-
lus and H
+
2
CO
3
formed then dissociates into CO
2
and H
2
2
recombines with H
2
fluence of carbonic
2
CO
3
H
2
CO
3
in turn dissociates to form HCO
3
–
and H
+
HCO
3
–
then diffuses through the basolateral mem-
brane into the interstitial fluid and is taken up into the
3
across the basolateral membrane is facilitated by
+
-HCO
3
–
co-transport and (2)
Cl
–
-HCO
3
–
+
is formed in the tubular epithe-
3
-
into the blood.
“reabsorption” of HCO
3
–
the HCO
3
–
that actually enters the extracellular fluid
is not the same as that fi
reabsorption of filtered HCO
3
–
does not result in net
secretion of H
+
because the secreted H
+
combines with
the filtered HCO
3
–
and is therefore not excreted.
HCO
3
-
+
H
+
HCO
3
-
+
H
+
Tubular cells
Tubular cells
Tubular
Tubular
ATP
Na
+
Na
+
K
+
K
+
Na
+
Na
+
Na
+
+
HCO
3
-
Na
+
+
HCO
3
-
H
+
H
+
+
+
CO
2
+
H
2
O
CO
2
+
H
2
O
H
2
CO
3
H
2
CO
3
H
2
O
H
2
O
Renal
interstitial
fluid
Renal
interstitial
fluid
lumen
lumen
Carbonic
anhydrase
Carbonic
anhydrase
CO
2
CO
2
CO
2
CO
2
H
2
CO
3
H
2
CO
3
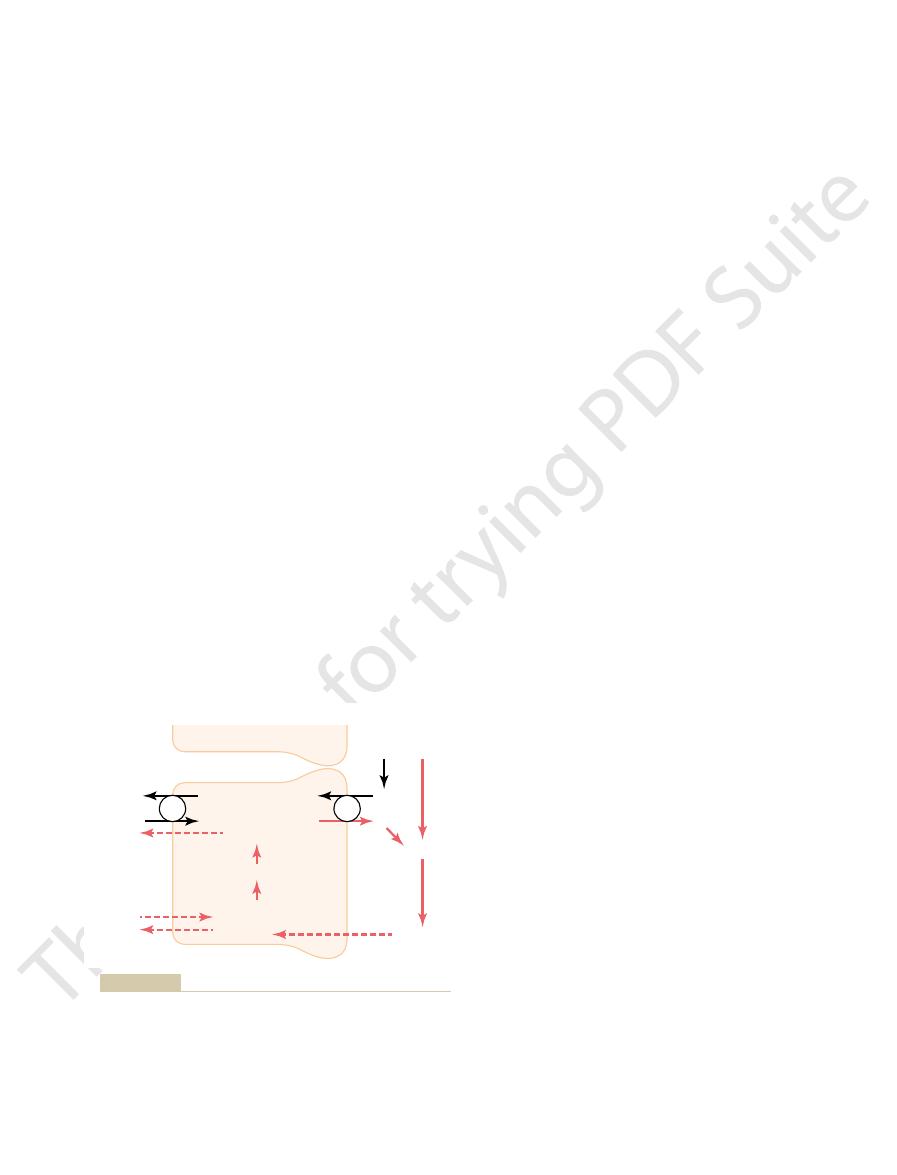
of hydrogen ion secretion occurs in the proximal tubule, the thick
reabsorption in exchange for hydrogen ions secreted. This pattern
sociates to form carbon dioxide and water; and (3) sodium ion
combination with hydrogen ions to form carbonic acid, which dis-
the renal tubule; (2) tubular reabsorption of bicarbonate ions by
Cellular mechanisms for (1) active secretion of hydrogen ions into
Figure 30–5
ascending segment of the loop of Henle, and the early distal
tubule.
metabolism each day, about 2667 liters of urine would
can be excreted. To excrete the
urine formed, a maximum of only about 0.03 mil-
mEq/L, or 0.03 mEq/L. Thus, for each liter of
is about 4.5, corresponding to an H
urine. The reason for this is that the minimal urine pH
uid, only a small part of the
When H
the Tubule—A Mechanism
and Ammonia Buffers in
Hydrogen Ions with Phosphate
limit of pH that can be achieved in normal kidneys.
uid to about 4.5, which is the lower
as 900-fold in the collecting tubules. This decreases the
However, H
can be reduced to only about 6.7, although large
about threefold to fourfold, and the tubular
imal tubules, H
tant in forming a maximally acidic urine. In the prox-
secreted, this mechanism is impor-
instead of by counter-transport, as occurs in the early
proximal tubules. The main difference is that H
is reabsorbed, similar to the process in the
hydrogen-ATPase mechanism. For each H
, which is secreted into the tubule by means of the
, which is reabsorbed into the blood, plus
into HCO
, and (2) the H
to form H
tubule and in the collecting tubules. Hydrogen ion
diphosphate.
derived from the breakdown of ATP to adenosine
ATPase. The energy required for pumping the H
c protein, a hydrogen-transporting
brane of the tubular cell, where H
6. It occurs at the luminal mem-
shown in Figure 30
The mechanism for primary active H
loop of Henle, and early distal tubule.
ferent from those discussed for the proximal tubule,
The characteristics of this transport are dif-
through the remainder of the tubular system, the
Distal and Collecting Tubules
Ions in the Intercalated Cells of Late
Primary Active Secretion of Hydrogen
and eventually excreted as salts. Thus, the basic mech-
passes into the urine. The excess H
In acidosis, there is excess H
alkalosis.
into the urine, which helps correct the metabolic
cannot be reabsorbed; therefore, the excess
urine, as occurs in metabolic alkalosis, the excess
When there is an excess of HCO
other urinary buffers, especially phosphate and
metabolism. As discussed later, most of this H
excreted in the urine. This excess H
The titration process is not quite exact because there
each other in the tubules.
O. Therefore, it is said that HCO
form CO
almost equal, and they combine with each other to
is about 4320 mEq/day. Thus, the
secretion is about 4400 mEq/day, and the rate of
Under normal conditions, the rate of tubular
Tubules.
Bicarbonate Ions Are “Titrated” Against Hydrogen Ions in the
392
Unit V
The Body Fluids and Kidneys
H
+
fil-
tration by HCO
3
–
quantities of these two ions entering the tubules are
2
and H
2
3
–
and H
+
normally “titrate”
is usually a slight excess of H
+
in the tubules to be
+
(about 80 mEq/
day) rids the body of nonvolatile acids produced by
+
is
not excreted as free H
+
but rather in combination with
ammonia.
3
–
over H
+
in the
HCO
3
–
HCO
3
–
is left in the tubules and eventually excreted
+
relative to HCO
3
–
,
causing complete reabsorption of the bicarbonate;
the excess H
+
+
is
buffered in the tubules by phosphate and ammonia
anism by which the kidneys correct either acidosis or
alkalosis is incomplete titration of H
+
against HCO
3
–
,
leaving one or the other to pass into the urine and be
removed from the extracellular fluid.
Beginning in the late distal tubules and continuing
tubular epithelium secretes H
+
by primary active
transport.
+
secretion is
–
+
is transported
directly by a specifi
+
is
Primary active secretion of H
+
occurs in a special
type of cell called the intercalated cells of the late distal
secretion in these cells is accomplished in two steps:
(1) the dissolved CO
2
in this cell combines with H
2
O
2
CO
3
2
CO
3
then dissociates
3
–
H
+
+
secreted,
an HCO
3
–
+
moves
across the luminal membrane by an active H
+
pump
parts of the nephron.
Although the secretion of H
+
in the late distal tubule
and collecting tubules accounts for only about 5 per
cent of the total H
+
+
concentration can be increased only
fluid pH
amounts of H
+
are secreted by this nephron segment.
+
concentration can be increased as much
pH of the tubular fl
Combination of Excess
for Generating “New”
Bicarbonate Ions
+
is secreted in excess of the bicarbonate fil-
tered into the tubular fl
excess H
+
can be excreted in the ionic form (H
+
) in the
+
concentration
of 10
–4.5
liequivalent of free H
+
80 milliequivalents of nonvolatile acid formed by
have to be excreted if the H
+
remained free in
solution.
Tubular
Tubular
Tubular cells
Tubular cells
ATP
Cl
-
Cl
-
Cl
-
Cl
-
Cl
-
Cl
-
Carbonic
anhydrase
Carbonic
anhydrase
HCO
3
-
+
H
+
HCO
3
-
+
H
+
H
+
H
+
+
+
H
2
CO
3
H
2
CO
3
H
2
O
H
2
O
CO
2
CO
2
CO
2
CO
2
Renal
interstitial
fluid
Renal
interstitial
fluid
lumen
lumen
secreted along with the hydrogen ion.
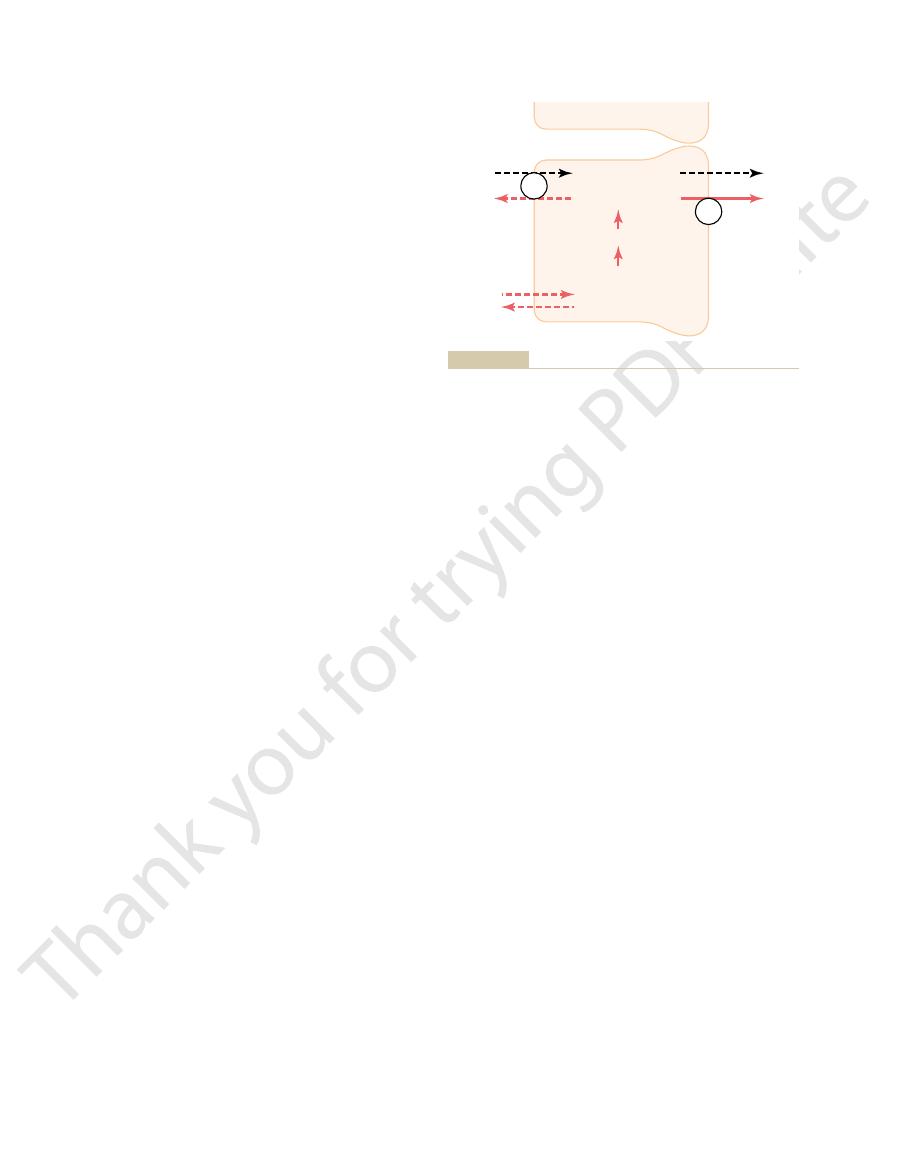
each hydrogen ion secreted, and a chloride ion is passively
membrane of the intercalated epithelial cells of the late distal and
Primary active secretion of hydrogen ions through the luminal
Figure 30–6
collecting tubules. Note that one bicarbonate ion is absorbed for
9). Here, H
(Figure 30
In the collecting tubules, the addition of NH
constitutes new bicarbonate.
generated by this process
metabolized in the proximal tubules, two NH
capillaries. Thus, for each molecule of glutamine
, into the inter-
brane, along with the reabsorbed Na
in exchange for sodium, which is reabsorbed. The
the tubular lumen by a counter-transport mechanism
. The NH
Once inside the cell, each molecule of glutamine is
the loop of Henle, and distal tubules (Figure 30
cells of the proximal tubules, thick ascending limb of
olism of amino acids in the liver. The glutamine deliv-
from glutamine, which comes mainly from the metab-
). Ammonium ion is synthesized
by the Ammonia Buffer System
and Generation of New Bicarbonate
. Therefore, much of the
phate is reabsorbed, and only about 30 to 40 mEq/day
Under normal conditions, much of the
. This demonstrates one of the
, the net effect is addition of a new
Therefore, whenever an H
by the blood, rather than merely a replace-
case, the HCO
excretion from that discussed previously. In this
There is one important difference in this sequence
), carrying with it
, it can be
to form H
and other tubular buffers. After the H
, any excess H
has been reabsorbed and is no longer avail-
. However, once all
uid, most of the
tubules is the same as described earlier. As long as
to the blood. The process of H
Figure 30
phosphate buffer system. Therefore, in the tubules, the
slightly acidic, and the urine pH is near the pK of the
is about 6.8. Under normal conditions, the urine is
uid buffer, it is much more effective
uid. Therefore, although phosphate is not an impor-
. Both become concentrated in the tubular
The phosphate buffer system is composed of HPO
and Generates New Bicarbonate
Phosphate Buffer System Carries
two sections, we discuss the mechanisms by which
uid in acidosis. In the next
, thereby helping to replenish the HCO
uid, the kidneys not
that can also enter the blood. Thus, when there
, and this results in the generation of new
in the urine, they combine with buffers other
secreted, as discussed earlier. But when there are
When H
weak buffer systems, such as urate and citrate, that are
phate buffer and ammonia buffer. There are other
uid. The most important buffers are phos-
The excretion of large amounts of H
Regulation of Acid-Base Balance
Chapter 30
393
+
(on occasion
as much as 500 mEq/day) in the urine is accomplished
primarily by combining the H
+
with buffers in the
tubular fl
much less important.
+
is titrated in the tubular fluid with HCO
3
–
,
this results in the reabsorption of one HCO
3
–
for each
H
+
excess H
+
than HCO
3
–
HCO
3
–
is excess H
+
in the extracellular fl
only reabsorb all the filtered HCO
3
–
but also generate
new HCO
3
–
3
–
lost from the extracellular fl
phosphate and ammonia buffers contribute to the gen-
eration of new HCO
3
–
.
Excess Hydrogen Ions into the Urine
4
=
and H
2
PO
4
–
fluid because of their relatively poor reabsorption and
because of the reabsorption of water from the tubular
fl
tant extracellular fl
as a buffer in the tubular fluid.
Another factor that makes phosphate important as
a tubular buffer is the fact that the pK of this system
phosphate buffer system normally functions near its
most effective range of pH.
–7 shows the sequence of events by which
H
+
is excreted in combination with phosphate buffer
and the mechanism by which new bicarbonate is added
+
secretion into the
there is excess HCO
3
–
in the tubular fl
secreted H
+
combines with HCO
3
–
the HCO
3
–
able to combine with H
+
+
can combine
with HPO
4
=
+
combines with HPO
4
=
2
PO
4
–
excreted as a sodium salt (NaH
2
PO
4
the excess hydrogen.
of H
+
3
–
that is generated in the tubular cell
and enters the peritubular blood represents a net gain
of HCO
3
–
ment of filtered HCO
3
–
.
+
secreted into the tubular lumen combines with a buffer
other than HCO
3
-
HCO
3
-
to the blood
mechanisms by which the kidneys are able to replen-
ish the extracellular fluid stores of HCO
3
–
.
filtered phos-
is available for buffering H
+
buffering of excess H
+
in the tubular fluid in acidosis
occurs through the ammonia buffer system.
Excretion of Excess Hydrogen Ions
A second buffer system in the tubular fluid that is even
more important quantitatively than the phosphate
buffer system is composed of ammonia (NH
3
) and the
ammonium ion (NH
4
+
ered to the kidneys is transported into the epithelial
–8).
metabolized in a series of reactions to ultimately form
two NH
4
+
and two HCO
3
–
4
+
is secreted into
HCO
3
–
is transported across the basolateral mem-
+
stitial fluid and is taken up by the peritubular
4
+
are
secreted into the urine and two HCO
3
–
are reabsorbed
into the blood. The HCO
3
–
4
+
to the
tubular fluids occurs through a different mechanism
–
+
is secreted by the tubular
Tubular
Tubular
Tubular cells
Tubular cells
ATP
Na
+
Na
+
K
+
K
+
Na
+
Na
+
Na
+
Na
+
Na
+
+
NaHPO
4
-
Na
+
+
NaHPO
4
-
H
+
+
NaHPO
4
-
H
+
+
NaHPO
4
-
HCO
3
-
HCO
3
-
HCO
3
-
+
H
+
HCO
3
-
+
H
+
+
+
NaH
2
PO
4
NaH
2
PO
4
H
2
CO
3
H
2
CO
3
H
2
O
H
2
O
Carbonic
anhydrase
Carbonic
anhydrase
CO
2
CO
2
CO
2
CO
2
Renal
interstitial
fluid
Renal
interstitial
fluid
lumen
lumen
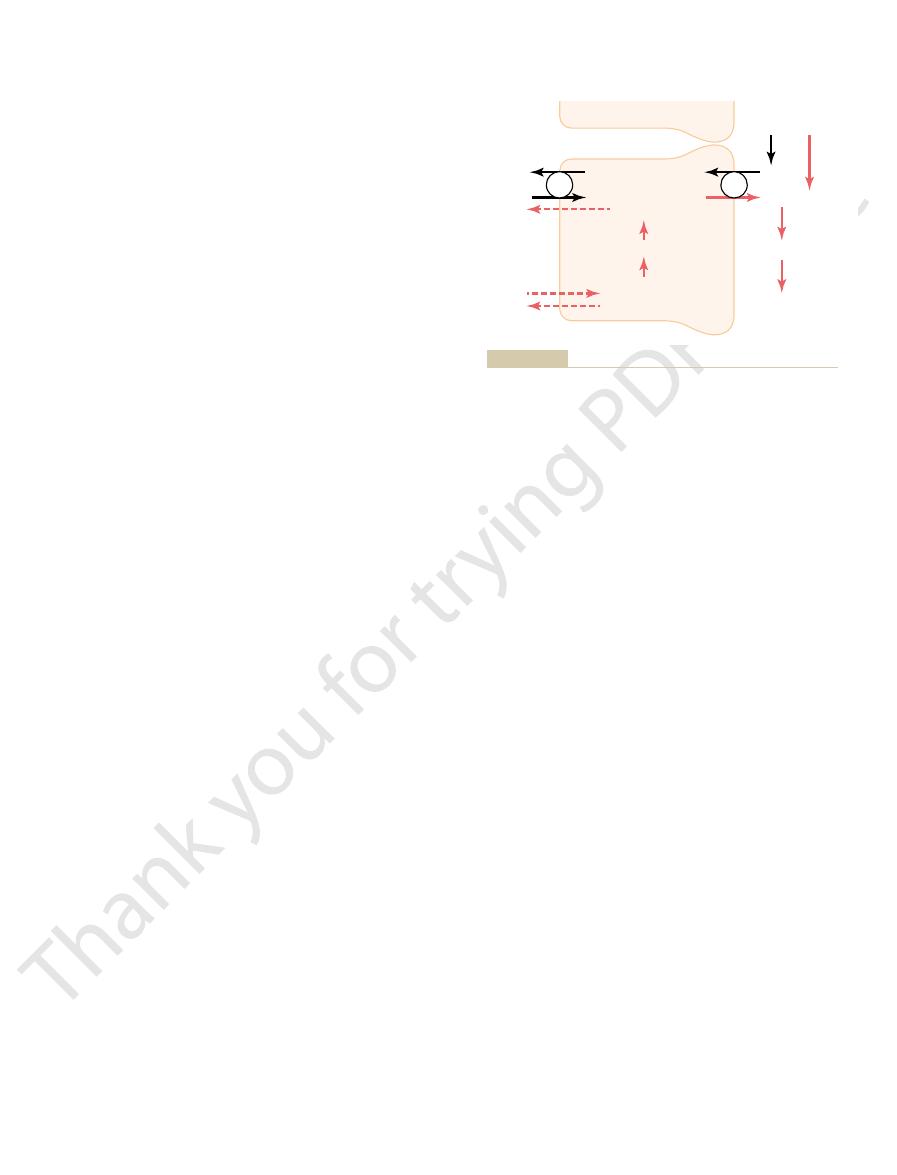
that reacts with a secreted hydrogen ion.
). Note that a new bicarbonate ion is returned to the
Buffering of secreted hydrogen ions by filtered phosphate
Figure 30–7
(NaHPO
4
–
blood for each NaHPO
4
–
Bicarbonate excretion
Thus, the
reaction is 9.2, and titration with NaOH to a pH of 7.4
, because the pK of the ammonia-ammonium
phate and other organic buffers. The titratable acid
. Therefore, the number of mil-
ltrate. This titration reverses the events that occurred
pH of normal plasma, and the pH of the glomerular
with a strong base, such as NaOH, to a pH of 7.4, the
The amount of titrat-
The rest of the nonbicarbonate, non-NH
and phosphate. Therefore, the
ously, the primary sources of nonbicarbonate urinary
nonbicarbonate urinary buffers. As discussed previ-
to the blood). In alkalosis, the loss of
This number indicates how rapidly the kidneys are
follows.
net excretion of acid or net addition
Based on the principles discussed earlier, we can quan-
Acid-Base Excretion
new bicarbonate during chronic acidosis.
This also pro-
chronic acidosis, the dominant mechanism by which
Therefore, with
increase to as much as 500 mEq/day.
chronic acidosis,
generated by the kidneys. However,
normal conditions,
buffering; a decrease in H
stimulates renal glutamine metabolism and, therefore,
excreted, a new HCO
For each
tubular lumen and eliminated in the urine.
, the NH
to form NH
with NH
; therefore, once the H
diffuse into the tubular lumen. However, the luminal
, which can easily
, which is then excreted. The col-
to form NH
membrane into the lumen, where it combines with
394
Unit V
The Body Fluids and Kidneys
NH
3
4
+
lecting ducts are permeable to NH
3
membrane of this part of the tubules is much less per-
meable to NH
4
+
+
has reacted
3
4
+
4
+
is trapped in the
NH
4
+
3
-
is generated and added to
the blood.
Chronic Acidosis Increases NH
4
+
Excretion.
One of the most
important features of the renal ammonium-ammonia
buffer system is that it is subject to physiologic control.
An increase in extracellular fluid H
+
concentration
increases the formation of NH
4
+
and new HCO
3
–
to be
used in H
+
+
concentration
has the opposite effect.
Under
the amount of H
+
elimi-
nated by the ammonia buffer system accounts for
about 50 per cent of the acid excreted and 50 per cent
of the new HCO
3
–
with
the rate of NH
4
+
excretion can
acid is eliminated is excretion of NH
4
+
.
vides the most important mechanism for generating
Quantifying Renal
titate the kidneys’
or elimination of bicarbonate from the blood as
Bicarbonate excretion is calculated as the urine flow
rate multiplied by urinary bicarbonate concentration.
removing HCO
3
–
from the blood (which is the same as
adding an H
+
HCO
3
–
helps return the plasma pH toward normal.
The amount of new bicarbonate contributed to
the blood at any given time is equal to the amount of
H
+
secreted that ends up in the tubular lumen with
buffers are NH
4
+
amount of HCO
3
–
added to the blood (and H
+
excreted
by NH
4
+
) is calculated by measuring NH
4
+
excretion (urine flow rate multiplied by urinary NH
4
+
concentration).
4
+
buffer
excreted in the urine is measured by determining a
value known as titratable acid.
able acid in the urine is measured by titrating the urine
fi
in the tubular lumen when the tubular fluid was
titrated by excreted H
+
liequivalents of NaOH required to return the urinary
pH to 7.4 equals the number of milliequivalents of H
+
added to the tubular fluid that combined with phos-
measurement does not include H
+
in association with
NH
4
+
does not remove the H
+
from NH
4
+
.
net acid excretion by the kidneys can be
assessed as
Net acid excretion
= NH
4
+
excretion
+ Urinary
titratable acid –
Glutamine
Glutamine
Tubular
Tubular
Cl
-
Cl
-
+
Cl
-
+
Cl
-
Na
+
Na
+
Na
+
Na
+
Renal
interstitial
fluid
Renal
interstitial
fluid
lumen
lumen
Proximal
tubular cells
Proximal
tubular cells
Glutamine
Glutamine
Glutamine
Glutamine
2HCO
3
-
2HCO
3
-
2NH
4
+
2NH
4
+
NH
4
+
NH
4
+
NH
4
+
NH
4
+
are returned
are produced and secreted and two HCO
is secreted into the lumen by a
) by proximal
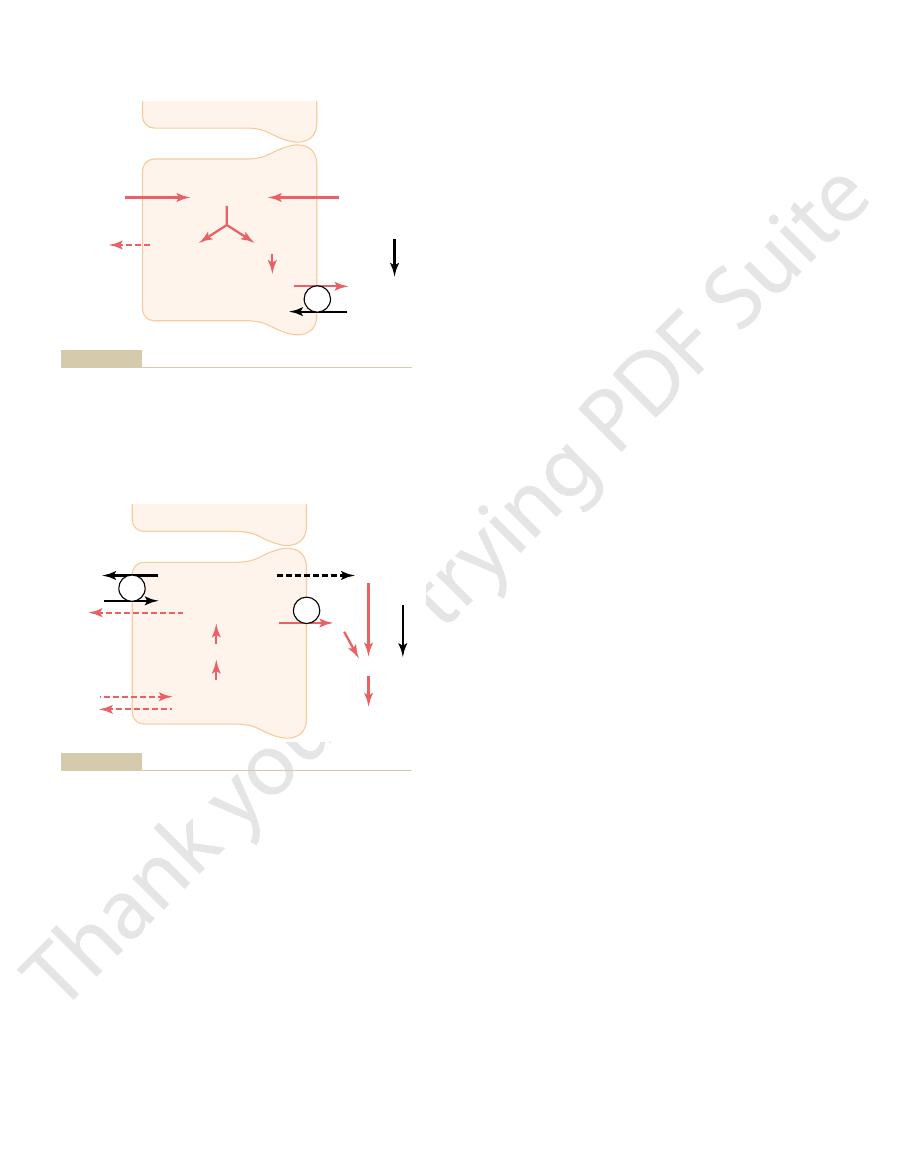
Production and secretion of ammonium ion (NH
Figure 30–8
4
+
tubular cells. Glutamine is metabolized in the cell, yielding NH
4
+
and bicarbonate. The NH
4
+
sodium-NH
4
+
pump. For each glutamine molecule metabolized,
two NH
4
+
3
–
to the blood.
Tubular
Tubular
ATP
ATP
K
+
K
+
Carbonic
anhydrase
Carbonic
anhydrase
Na
+
Na
+
NH
3
NH
3
Cl
-
Cl
-
Renal
interstitial
fluid
Renal
interstitial
fluid
lumen
lumen
Collecting
tubular cells
Collecting
tubular cells
HCO
3
-
+
H
+
HCO
3
-
+
H
+
NH
3
NH
3
NH
4
+
+ Cl
-
NH
4
+
+ Cl
-
H
+
H
+
+
+
H
2
CO
3
H
2
CO
3
H
2
O
H
2
O
CO
2
CO
2
CO
2
CO
2
tubular cells and returned to the blood.
is formed in the
excreted, a new HCO
excreted. For each NH
reacts with secreted hydrogen ions to form NH
lecting tubules. Ammonia diffuses into the tubular lumen, where it
Buffering of hydrogen ion secretion by ammonia (NH
Figure 30–9
3
) in the col-
4
+
, which is then
4
+
3
–

reabsorption and tends to cause acidosis.
losis. Hyperkalemia decreases H
the renal tubular cells. This, in turn, stimulates H
proximal tubule. A decreased plasma potassium con-
secretion, with hypokalemia stimu-
lecting tubules. Therefore, extracellular
(2) increased aldosterone levels, which stimulate H
exchanger in the renal tubules, and
angiotensin II levels, which directly stimulate the activ-
through multiple mechanisms, including (1) increased
uid volume, may also
Therefore, factors that stimulate Na
proximal tubule and thick ascending loop of Henle.
base balance. For example, H
reabsorption. Some of
Table 30
in both respiratory and metabolic alkalosis.
concentration per se, as occurs
, as occurs in respiratory alkalosis, or
The decreased H
The tubular cells usually respond to a decrease in H
amounts of bicarbonate added back to the blood. This
uid and, consequently, increased
s syndrome, can cause excessive secretion of H
Therefore, oversecretion of aldosterone, as occurs in
terone secretion. Aldosterone stimulates the secretion
. The
cells, which in turn stimulates the secretion of H
of the tubular cells,
The increased P
of the blood, as occurs in respiratory acidosis,
The tubular cells respond directly to an increase in
secretion by
acid, thereby contributing large amounts of new
urine in alkalosis. During acidosis, the tubular H
therefore, there is no new HCO
excretion. In this condition, titratable acid and
reabsorption, enabling the kidneys to increase HCO
In alkalosis, tubular secretion of H
ltered, and there must be enough
ostasis. Under normal conditions, the kidney tubules
able acid formation. Therefore, the rate of H
As discussed earlier, H
Regulation of Renal Tubular Hydrogen
is generated by the kidneys.
This
alkalosis, there is a negative net acid secretion.
Therefore, in
excretion increases.
to 0, whereas HCO
In alkalosis, titratable acid and NH
back to the blood as more NH
Therefore, in acidosis, there is a net
tion, thereby removing acid from the blood. The net
markedly, especially because of increased NH
in the body. In acidosis, the net acid excretion increases
the blood. To maintain acid-base balance, the net acid
The reason we subtract bicarbonate excretion is that
Regulation of Acid-Base Balance
Chapter 30
395
the loss of HCO
3
–
is the same as the addition of H
+
to
excretion must equal the nonvolatile acid production
4
+
excre-
acid excretion also equals the rate of net HCO
3
–
addi-
tion to the blood.
addition of HCO
3
–
4
+
and
urinary titratable acid are excreted.
4
+
excretion drop
3
–
means that there is a net loss of HCO
3
–
from the blood
(which is the same as adding H
+
to the blood) and that
no new HCO
3
–
Ion Secretion
+
secretion by the tubular
epithelium is necessary for both HCO
3
–
reabsorption
and generation of new HCO
3
–
associated with titrat-
+
secre-
tion must be carefully regulated if the kidneys are to
effectively perform their functions in acid-base home-
must secrete at least enough H
+
to reabsorb almost all
the HCO
3
–
that is fi
H
+
left over to be excreted as titratable acid or NH
4
+
to rid the body of the nonvolatile acids produced each
day from metabolism.
+
must be reduced
to a level that is too low to achieve complete HCO
3
–
3
–
ammonia are not excreted because there is no excess
H
+
available to combine with nonbicarbonate buffers;
3
–
added to the
+
secretion must be increased sufficiently to reabsorb
all the filtered HCO
3
–
and still have enough H
+
left
over to excrete large amounts of NH
4
+
and titratable
HCO
3
–
to the total body extracellular fluid. The
most important stimuli for increasing H
+
the tubules in acidosis are (1) an increase in P
CO
2
of the extracellular fluid and (2) an increase in H
+
concentration of the extracellular fluid (decreased
pH).
P
CO
2
with an increase in the rate of H
+
secretion as follows:
CO
2
raises the P
CO
2
causing increased formation of H
+
in the tubular
+
second factor that stimulates H
+
secretion is an
increase in extracellular fluid H
+
concentration
(decreased pH).
A special factor that can increase H
+
secretion under
some pathophysiologic conditions is excessive aldos-
of H
+
by the intercalated cells of the collecting duct.
Conn’
+
into the tubular fl
usually causes alkalosis in patients with excessive
aldosterone secretion.
+
concentration (alkalosis) by reducing H
+
secretion.
+
secretion results from decreased
extracellular P
CO
2
from a decrease in H
+
–2 summarizes the major factors that influ-
ence H
+
secretion and HCO
3
–
these are not directly related to the regulation of acid-
+
secretion is coupled
to Na
+
reabsorption by the Na
+
-H
+
exchanger in the
+
reabsorption,
such as decreased extracellular fl
secondarily increase H
+
secretion.
Extracellular fluid volume depletion stimulates
sodium reabsorption by the renal tubules and
increases H
+
secretion and HCO
3
–
reabsorption
ity of the Na
+
-H
+
+
secretion by the intercalated cells of the cortical col-
fluid volume
depletion tends to cause alkalosis due to excess H
+
secretion and HCO
3
–
reabsorption.
Changes in plasma potassium concentration can
also influence H
+
lating and hyperkalemia inhibiting H
+
secretion in the
centration tends to increase the H
+
concentration in
+
secretion and HCO
3
–
reabsorption and leads to alka-
+
secretion and HCO
3
–
Table 30–2
Factors That Increase or Decrease H
+
Secretion and HCO
3
-
Secretion and
Decrease H
Reabsorption by the Renal Tubules
Increase H
+
+
Secretion and
HCO
3
–
Reabsorption HCO
3
–
Reabsorption
≠ P
CO2
Ø P
CO2
≠ H
+
,
Ø HCO
3
–
Ø H
+
,
≠ HCO
3
–
Ø Extracellular fluid volume
≠ Extracellular fluid volume
≠ Angiotensin II
Ø Angiotensin II
≠ Aldosterone
Ø Aldosterone
Hypokalemia
Hyperkalemia

losis, HCO
and is, therefore, excreted in the urine. Thus, in alka-
uid. The net effect of this is an excess of
bolic or respiratory abnormalities, there is still an
in Renal Tubular Fluid
concentration), as is evident from the Henderson-
increases, causing a rise in pH (a decrease in H
to CO
opposite to those that occur in acidosis. In alkalosis,
The compensatory responses to alkalosis are basically
and Increased Excretion
Secretion of Hydrogen Ions
Alkalosis—Decreased Tubular
Renal Correction of
bicarbonate to the extracellular fluid, helps minimize
, and renal compensation, which, by adding new
tions include increased ventilation rate, which reduces
However, in this case, the primary abnormality is a
metabolic acidosis,
, thereby returning the
The rise in HCO
the extracellular fluid by the kidneys.
, caused by the addition of new bicarbonate to
, which is the initial cause of the acidosis.
concentration, and an increase in
there is a reduction in pH, an increase in
acidosis,
cussed in the next section. Note that in
respiratory and metabolic alkalosis, which are dis-
Table 30
helping to correct the acidosis.
, also
acidosis is metabolically mediated, additional com-
the extracellular pH and corrects the acidosis. If the
with the Henderson-Hasselbalch equation, helps raise
of the bicarbonate buffer system, which, in accordance
uid. This increases the bicarbonate part
Thus, with chronic acidosis, the increased secretion
; this, in turn, contributes up to 500 mEq/day of
can be excreted in the urine, mainly in the form of
severe chronic acidosis, as much as 500 mEq/day of H
uid. With
, which
regardless of whether it is respiratory or metabolic,
As discussed previously, with chronic acidosis,
, which stimulates H
In respiratory acidosis, the excess H
This decreased
over HCO
In metabolic acidosis, an excess of H
dosis, the kidneys reabsorb all the
. Thus, in aci-
renal tubules, causing complete reabsorption of HCO
uid. As a result, there is an excess of H
in Renal Tubular Fluid
respiratory acidosis.
, the acidosis is referred
metabolic acidosis.
, the acidosis is
uid decreases, thereby decreasing pH. If this ratio
to CO
Hasselbalch equation, we can see that acidosis occurs
Referring to equation 8,
the Henderson-
, we can
Hydrogen Ions and Addition
Increased Excretion of
Renal Correction of Acidosis—
396
Unit V
The Body Fluids and Kidneys
of Bicarbonate Ions to the
Extracellular Fluid
Now that we have described the mechanisms by which
the kidneys secrete H
+
and reabsorb HCO
3
–
explain how the kidneys readjust the pH of the extra-
cellular fluid when it becomes abnormal.
when the ratio of HCO
3
–
2
in the extracellular
fl
decreases because of a fall in HCO
3
–
referred to as
If the pH falls
because of an increase in P
CO
2
to as
Acidosis Decreases the Ratio of
HCO
3
-
/H
+
Both respiratory and metabolic acidosis cause a
decrease in the ratio of HCO
3
–
to H
+
in the renal
tubular fl
+
in the
3
–
and still leaving additional H
+
available to combine
with the urinary buffers NH
4
+
and HPO
4
=
filtered HCO
3
–
and
contribute new HCO
3
–
through the formation of NH
4
+
and titratable acid.
+
3
–
occurs in the tubular fluid primarily because of
decreased filtration of HCO
3
-
.
filtration
of HCO
3
–
is caused mainly by a decrease in the extra-
cellular fluid concentration of HCO
3
–
.
+
in the tubular
fluid is due mainly to the rise in extracellular fluid
P
CO
2
+
secretion.
there is an increase in the production of NH
4
+
further contributes to the excretion of H
+
and the addi-
tion of new HCO
3
–
to the extracellular fl
+
NH
4
+
new HCO
3
–
that is added to the blood.
of H
+
by the tubules helps eliminate excess H
+
from
the body and increases the quantity of HCO
3
–
in the
extracellular fl
pensation by the lungs causes a reduction in P
CO
2
–3 summarizes the characteristics associ-
ated with respiratory and metabolic acidosis as well as
respiratory
extracellular fluid H
+
P
CO
2
The
compensatory response is an increase in plasma
HCO
3
-
3
–
helps offset the increase in P
CO
2
plasma pH toward normal.
In
there is also a decrease in pH
and a rise in extracellular fluid H
+
concentration.
decrease in plasma HCO
3
–
. The primary compensa-
P
CO
2
the initial fall in extracellular HCO
3
-
concentration.
of Bicarbonate Ions
the ratio of HCO
3
–
2
in the extracellular fluid
+
Hasselbalch equation.
Alkalosis Increases the Ratio of
HCO
3
-
/H
+
Regardless of whether the alkalosis is caused by meta-
increase in the ratio of HCO
3
–
to H
+
in the renal
tubular fl
HCO
3
–
that cannot be reabsorbed from the tubules
3
–
is removed from the extracellular fluid by
Table 30–3
≠
Ø
≠
≠≠
Ø
≠
Ø
ØØ
≠
Ø
ØØ
Ø
Ø
≠
≠≠
≠
Normal
7.4
40 mEq/L
40 mm Hg
24 mEq/L
pH
H
Characteristics of Primary Acid-Base Disturbances
+
P
CO
2
HCO
3
–
Respiratory acidosis
Respiratory alkalosis
Metabolic acidosis
Metabolic alkalosis
, whereas metabolic disorders are initiated by an increase or decrease in
). Note that
The primary event is indicated by the double arrows (
≠≠ or ØØ
respiratory acid-base disorders are initiated by an increase or decrease in
P
CO
2
HCO
3
–
.

tents alone would cause loss of acid and a tendency
Vomiting of gastric con-
Vomiting of Intestinal Contents.
serious and can cause death, especially in young
urine. This form of metabolic acidosis is particularly
from the body, which has the same
large amounts of bicarbonate, and diarrhea results in
The gastrointestinal secretions normally contain
feces.
cause of metabolic acidosis.
s syndrome.
impair tubular function, such as Fanconi
of renal tubular acidosis include chronic renal failure,
uids. Some causes
are excreted, so that there is
an alkaline urine. In these cases, inadequate amounts of
establish a normal acidic urine, causing the excretion of
in the urine, or (2)
sorption, causing loss of HCO
types: (1) impairment of renal tubular HCO
, or both. These disorders are generally of two
This type of acidosis results from
Renal Tubular Acidosis.
bolic acidosis are the following.
uids. Some speci
uids, which has the same effect as adding an acid to the
infusion of acids, and (4) loss of base from the body
excess quantities of metabolic acids in the body, (3)
acids normally formed in the body, (2) formation of
causes: (1) failure of the kidneys to excrete metabolic
uids. Metabolic acidosis can result from several general
The term
Metabolic Acidosis Results from
alkalosis. Again, the major means for compensation are
content of the air stimulates respiration, which causes
when a person ascends to high altitude. The low oxygen
person becomes alkalotic.
pathological conditions. However, a psychoneurosis can
the lungs. Rarely does this occur because of physical
from Increased Ventilation and
Respiratory Alkalosis Results
for the disorder.
the kidneys, which require several days to compensate
In respiratory acidosis, the compensatory responses
alveolar air, can cause respiratory acidosis.
brane surface area, as well as any factor that interferes
pneumonia, emphysema, or decreased pulmonary mem-
medulla oblongata can lead to respiratory acidosis.Also,
For example, damage to the respiratory center in the
acidosis.
an abnormality in respiration, it is called
sulting in acidosis. Because the acidosis is caused by
concentration, thus re-
uid. This causes
From the previous discussion, it is obvious that any
by Decreased Ventilation and
Respiratory Acidosis Is Caused
Acid-Base Disorders
excretion, which helps compen-
, and
tions are decreased ventilation, which raises P
In metabolic alkalosis, the primary compensa-
urine.
to react with, and it is excreted in the
uid. The excess HCO
uid pH toward normal. In addition, the
tion rate, which increases P
This is
The cause of metabolic alkalosis, however, is a rise in
metabolic alkalosis,
caused by increased renal excretion of HCO
Therefore, the compensatory
rection of the alkalosis.
reabsorbed and is excreted in the urine. This results in
Therefore, the HCO
uid. Consequently, there is not
secretion by the renal tubules. The
The reduction in P
, caused by hyperventi-
sis,
piratory and metabolic alkalosis. In
Table 30
uid. This helps return the H
renal excretion, which has the same effect as adding an
Regulation of Acid-Base Balance
Chapter 30
397
H
+
to the extracellular fl
+
concentration and pH back toward normal.
–3 shows the overall characteristics of res-
respiratory alkalo-
there is an increase in extracellular fluid pH and a
decrease in H
+
concentration. The cause of the alkalo-
sis is a decrease in plasma P
CO
2
lation.
CO
2
then leads to a decrease
in the rate of H
+
decrease in H
+
secretion reduces the amount of H
+
in
the renal tubular fl
enough H
+
to react with all the HCO
3
–
that is filtered.
3
–
that cannot react with H
+
is not
a decrease in plasma HCO
3
–
concentration and cor-
response to a primary reduction in P
CO
2
in respiratory
alkalosis is a reduction in plasma HCO
3
-
concentration,
3
-
.
In
there is also an increase in
plasma pH and a decrease in H
+
concentration.
the extracellular fluid HCO
3
–
concentration.
partly compensated for by a reduction in the respira-
CO
2
and helps return the
extracellular fl
increase in HCO
3
–
concentration in the extracellular
fluid leads to an increase in the filtered load of HCO
3
–
,
which in turn causes an excess of HCO
3
–
over H
+
secreted in the renal tubular fl
3
–
in the tubular fluid fails to be reabsorbed because
there is no H
+
CO
2
increased renal HCO
3
-
sate for the initial rise in extracellular fluid HCO
3
-
concentration.
Clinical Causes of
Increased P
CO
2
factor that decreases the rate of pulmonary ventilation
also increases the P
CO
2
of extracellular fl
an increase in H
2
CO
3
and H
+
respiratory
Respiratory acidosis can occur from pathological
conditions that damage the respiratory centers or
that decrease the ability of the lungs to eliminate CO
2
.
obstruction of the passageways of the respiratory tract,
with the exchange of gases between the blood and the
available are (1) the buffers of the body fluids and (2)
Decreased P
CO
2
Respiratory alkalosis is caused by overventilation by
occasionally cause overbreathing to the extent that a
A physiologic type of respiratory alkalosis occurs
excess loss of CO
2
and development of mild respiratory
the chemical buffers of the body fluids and the ability
of the kidneys to increase HCO
3
–
excretion.
Decreased Extracellular Fluid
Bicarbonate Concentration
metabolic acidosis refers to all other types of
acidosis besides those caused by excess CO
2
in the body
fl
addition of metabolic acids to the body by ingestion or
fl
body fl
fic conditions that cause meta-
a defect in renal secretion of H
+
or in reabsorption of
HCO
3
–
3
–
reab-
3
–
inability of the renal tubular H
+
secretory mechanism to
titratable acid and NH
4
+
net accumulation of acid in the body fl
insufficient aldosterone secretion (Addison’s disease),
and several hereditary and acquired disorders that
’
Diarrhea.
Severe diarrhea is probably the most frequent
The cause of this acidosis is
the loss of large amounts of sodium bicarbonate into the
the loss of HCO
3
–
effect as losing large amounts of bicarbonate in the
children.

alkalosis.
acidosis, whereas a pH greater than 7.4 indicates
is acidosis or alkalosis. A pH less than 7.4 indicates
ining the pH, one can determine whether the disorder
10. By exam-
several steps, as shown in Figure 30
The diagnosis of simple acid-base disorders involves
plasma bicarbonate concentration, and P
three measurements from an arterial blood sample: pH,
proper diagnosis. The simple acid-base disorders
Disorders
Clinical Measurements and
monohydrochloride.
gerous. Another substance used occasionally is
highly toxic, and this procedure can be dan-
occasionally is infused intravenously, but NH
tion in the acidic direction. Ammonium chloride
reaction liberates HCl, which immediately reacts with
portion is converted by the liver into urea. This
chloride is absorbed into the blood, the ammonia
can be administered by mouth. When the ammonium
ammonium chloride
For the treatment of alkalosis,
the molecules are metabolized in the body, leaving the
The lactate and gluconate portions of
sodium gluconate.
are often used instead, such as
physiologic effects of such treatment, other substances
intravenously, but because of the potentially dangerous
toward normal. Sodium bicarbonate can also be infused
the bicarbonate buffer system, thereby increasing pH
can be ingested by mouth. The sodium
To neutralize excess acid, large amounts of
cumstances, various agents can be used to neutralize the
impaired lung function or kidney failure. In these cir-
cult, especially in chronic diseases that cause
the condition that caused the abnormality. This is often
The best treatment for acidosis or alkalosis is to correct
or Alkalosis
Treatment of Acidosis
ulcer.
bicarbonate, for the treatment of gastritis or peptic
alkalosis is ingestion of alkaline drugs, such as sodium
pyloric sphincter muscles.
losis. This type of alkalosis occurs especially in neonates
stomach mucosa. The net result is a loss of acid from the
nal contents, causes loss of the HCl secreted by the
tents alone, without vomiting of the lower gastrointesti-
Vomiting of the gastric con-
Vomiting of Gastric Contents.
metabolic alkalosis.
its increased excretion by the kidneys and, therefore,
lecting tubules. This increased secretion of H
alkalosis develops. As discussed previously, aldosterone
are secreted by the adrenal glands, a mild metabolic
When large amounts of aldosterone
development of alkalosis, characterized by increased
bicarbonate reabsorption. These changes lead to the
secretion, the enhanced sodium reabsorption also
from these parts of the nephrons.
distal and collecting tubules. This leads to increased
along the tubules, usually causing increased
as follows.
dosis, but some of the causes of metabolic alkalosis are
from the body, this results in metabolic alkalosis. Meta-
When there is excess retention of HCO
Metabolic Alkalosis Is Caused
associated with severe metabolic acidosis.
uids. Thus, chronic renal failure can be
kidneys. In addition, the decreased glomerular
markedly, there is a buildup of the anions of weak acids
. When kidney function declines
cylics (aspirin) and methyl alcohol (which forms formic
certain acidic poisons. Some of these include acetylsali-
ingested in normal foods. However, severe metabolic
times as much as 500 mmol/day.
large amounts of acid are excreted in the urine, some-
acidosis. In an attempt to compensate for this acidosis,
acid levels can rise very high, causing severe metabolic
glucose. With severe diabetes mellitus, blood acetoacetic
some of the fats are split into acetoacetic acid, and this
use of glucose for metabolism is prevented. Instead,
cient insulin, the normal
diabetes). In the absence of suf
acidosis.
occurs, causes loss of bicarbonate and results in meta-
deeper in the gastrointestinal tract, which sometimes
highly acidic. However, vomiting large amounts from
398
Unit V
The Body Fluids and Kidneys
toward alkalosis because the stomach secretions are
bolic acidosis in the same way that diarrhea causes
Diabetes Mellitus.
Diabetes mellitus is caused by lack of
insulin secretion by the pancreas (type I diabetes) or
by insufficient insulin secretion to compensate for
decreased sensitivity to the effects of insulin (type II
fi
is metabolized by the tissues for energy in place of
Ingestion of Acids.
Rarely are large amounts of acids
acidosis occasionally results from the ingestion of
acid when it is metabolized).
Chronic Renal Failure
in the body fluids that are not being excreted by the
filtration
rate reduces the excretion of phosphates and NH
4
+
,
which reduces the amount of bicarbonate added back
to the body fl
by Increased Extracellular Fluid
Bicarbonate Concentration
3
–
or loss of H
+
bolic alkalosis is not nearly as common as metabolic aci-
Administration of Diuretics (Except the Carbonic Anhydrase
Inhibitors).
All diuretics cause increased flow of fluid
flow in the
reabsorption of Na
+
Because the sodium reabsorption here is coupled with
H
+
leads to an increase in H
+
secretion and an increase in
extracellular fluid bicarbonate concentration.
Excess Aldosterone.
promotes extensive reabsorption of Na
+
from the distal
and collecting tubules and at the same time stimulates
the secretion of H
+
by the intercalated cells of the col-
+
leads to
extracellular fluid and development of metabolic alka-
who have pyloric obstruction caused by hypertrophied
Ingestion of Alkaline Drugs.
A common cause of metabolic
diffi
excess acid or base in the extracellular fluid.
sodium
bicarbonate
bicarbonate is absorbed from the gastrointestinal tract
into the blood and increases the bicarbonate portion of
sodium lactate and
sodium in the extracellular fluid in the form of sodium
bicarbonate and thereby increasing the pH of the fluid
toward normal.
the buffers of the body fluids to shift the H
+
concentra-
4
+
is
lysine
Analysis of Acid-Base
Appropriate therapy of acid-base disorders requires
described previously can be diagnosed by analyzing
CO
2
.
–
increased above the normal value of 40 mm Hg. This is
is absent, and P
However, the respiratory compensation that would
the patient is acidotic, and there appears to be a meta-
50 mm Hg. In this example,
7 mEq/L, and plasma P
lowing values: pH 7.15, plasma HCO
Hg to 25 mm Hg.
acidosis, with appropriate respiratory compensation
25 mm Hg. With these values, one can look at the
concentration 12.0 mEq/L, and plasma P
patient yields the following values: pH 7.30, plasma
For example, assume that the arterial plasma from a
an acid-base disorder.
mind, the acid-base diagrams can be used as a quick
is a simple acid-base disorder. With this reservation in
always
be a mixed acid-base disorder.
lie outside the shaded area, this suggests that there may
Conversely, if the values for pH, bicarbonate, or P
suggests that there is a simple acid-base disturbance.
ratory disorders. If a value is within the shaded area, this
response, which is 6 to 12 hours for the ventilatory com-
When using this diagram, one must assume that suf-
tory disorders.
within the normal range. The shaded areas of the
balch equation. The central open circle shows normal
concentration, and P
acid-base diagram, pH, HCO
of acidosis or alkalosis, as well as its severity. In this
11. This diagram can be used to determine the type
to use an acid-base nomogram, as shown in Figure
mixed acidosis. This could occur, for example, in a
Therefore, this disorder would be categorized as a
, one would suspect a respiratory compo-
. However, if the low plasma
compensation, a low P
concentration and, after appropriate respiratory
ated, this would also be accompanied by a low plasma
rized as acidotic. If the disorder was metabolically medi-
For example, a patient with low pH would be catego-
more underlying causes for the acid-base disturbance.
This means that there are two or
acid-base disorder.
mixed
this occurs, the abnormality is referred to as a
panied by appropriate compensatory responses. When
In some instances, acid-base disorders are not accom-
Use of the Acid-Base Nomogram
Complex Acid-Base Disorders and
, and increased P
sis, one would expect to find increased pH, increased
concentration in the plasma. In simple metabolic alkalo-
, and decreased HCO
increased pH, decreased P
simple respiratory alkalosis, one would expect to find
Therefore, in
metabolic component to the alkalosis.
, there must be a
respiratory component to the alkalosis. If the rise in pH
, there must be a
that there is an increase in plasma pH. If the increase in
sis involve the same basic steps. First, alkalosis implies
The procedures for categorizing the types of alkalo-
concentration, and a reduction in P
sis, one would expect to find a low pH, a low plasma
Therefore, in simple metabolic acido-
pensation, in contrast to respiratory acidosis, in which
component to the acidosis. In simple metabolic acidosis,
concentration, there must be a metabolic
concentration. Therefore, if a low pH is associated with
in plasma pH. However, with metabolic acidosis, the
For metabolic acidosis, there would also be a decrease
, and increased
be reduced plasma pH, increased P
Therefore, the
component to the acidosis. After renal compensation,
is increased, there must be a respiratory
, it is 24 mEq/L. If the
about 40 mm Hg, and for HCO
concentration. The normal value for P
The second step is to examine the plasma P
Regulation of Acid-Base Balance
Chapter 30
399
CO
2
and
HCO
3
–
CO
2
is
3
–
disorder has been characterized as acidosis and the
plasma P
CO
2
the plasma HCO
3
–
concentration in respiratory acidosis
would tend to increase above normal.
expected values for a simple respiratory acidosis would
CO
2
plasma HCO
3
–
concentration after partial renal
compensation.
primary abnormality is a decrease in plasma HCO
3
–
a low HCO
3
–
the P
CO
2
is reduced because of partial respiratory com-
P
CO
2
is increased.
HCO
3
-
CO
2
after
partial respiratory compensation.
pH is associated with decreased P
CO
2
is associated with increased HCO
3
–
CO
2
3
-
plasma HCO
3
-
CO
2
.
for Diagnosis
HCO
3
–
CO
2
pH and low HCO
3
–
concentration are associated with
elevated P
CO
2
nent to the acidosis as well as a metabolic component.
patient with acute HCO
3
–
loss from the gastrointestinal
tract because of diarrhea (metabolic acidosis) who also
has emphysema (respiratory acidosis).
A convenient way to diagnose acid-base disorders is
30–
3
–
CO
2
values intersect according to the Henderson-Hassel-
values and the deviations that can still be considered
diagram show the 95 per cent confidence limits for the
normal compensations to simple metabolic and respira-
ficient time has elapsed for a full compensatory
pensations in primary metabolic disorders and 3 to 5
days for the metabolic compensations in primary respi-
CO
2
It is important to recognize that an acid-base value
within the shaded area does not
mean that there
means of determining the specific type and severity of
HCO
3
–
CO
2
diagram and find that this represents a simple metabolic
that reduces the P
CO
2
from its normal value of 40 mm
A second example would be a patient with the fol-
3
–
concentration
CO
2
bolic component because the plasma HCO
3
–
concentra-
tion is lower than the normal value of 24 mEq/L.
normally reduce P
CO
2
CO
2
is slightly
Metabolic
Respiratory
Arterial blood sample
pH?
Alkalosis
Acidosis
>7.4
<7.4
HCO
3
-
>24 mEq/L
Pco
2
<40 mm Hg
Pco
2
>40 mm Hg
Pco
2
<40 mm Hg
HCO
3
-
<24 mEq/L
HCO
3
-
>24 mEq/L
HCO
3
-
<24 mEq/L
Pco
2
>40 mm Hg
Respiratory
Respiratory
compensation
Respiratory
compensation
Metabolic
Renal
compensation
Renal
compensation
of the figure, one should suspect a mixed acid-base disorder.
responses are markedly different from those shown at the bottom
Analysis of simple acid-base disorders. If the compensatory
Figure 30–10
bolic acidosis.
4. By calculating the anion gap,
are shown in Table 30
. Some examples of metabolic aci-
ketoacids, is associated with an increased plasma anion
nonvolatile acids (besides HCl), such as lactic acid or
culated anion gap. Metabolic acidosis caused by excess
, there must be increased levels of
hyperchloremic metabolic acidosis.
anion gap will remain normal, and this is often referred
, the
increase to maintain electroneutrality. If plasma Cl
sodium concentration is unchanged, the concentration
is reduced. If the plasma
dosis, the plasma HCO
different causes of metabolic acidosis. In metabolic aci-
The plasma anion gap is used mainly in diagnosing
cations, and the anion gap ranges between 8 and
albumin, phosphate, sulfate, and other organic anions.
potassium, and the major unmeasured anions are
unmeasured cations include calcium, magnesium, and
or if unmeasured cations fall. The most important
The anion gap will increase if unmeasured anions rise
108
24
[Cl
[HCO
and unmeasured cations, and is estimated as
. The
, and the anions are usually Cl
measured in the clinical laboratory. The cation normally
However, only certain cations and anions are routinely
fore, there is no real
must be equal to maintain electrical neutrality. There-
The concentrations of anions and cations in plasma
Use of Anion Gap to Diagnose
base disorders.
ate concentrations. In a clinical setting, the patient
, and plasma bicarbon-
tributing to abnormal pH, P
The acid-base diagram serves as a quick way to assess
400
Unit V
The Body Fluids and Kidneys
consistent with a mixed acid-base disturbance con-
sisting of metabolic acidosis as well as a respiratory
component.
the type and severity of disorders that may be con-
CO
2
’s
history and other physical findings also provide impor-
tant clues concerning causes and treatment of the acid-
Acid-Base Disorders
“anion gap” in the plasma.
measured is Na
+
–
and
HCO
3
–
“anion gap” (which is only a diagnostic
concept) is the difference between unmeasured anions
Plasma anion gap
= [Na
+
] –
3
–
] –
–
]
= 144 –
–
= 10 mEq/L
Usually the unmeasured anions exceed the unmeasured
16 mEq/L.
3
–
of anions (either Cl
–
or an unmeasured anion) must
–
increases in proportion to the fall in plasma HCO
3
–
to as
If the decrease in plasma HCO
3
–
is not accompanied
by increased Cl
–
unmeasured anions and therefore an increase in the cal-
gap because the fall in HCO
3
–
is not matched by an
equal increase in Cl
–
dosis associated with a normal or increased anion gap
–
one can narrow some of the potential causes of meta-
Table 30–4
Metabolic Acidosis Associated with Normal or Increased
Aspirin (acetylsalicylic acid)
Addison
Chronic renal failure
Carbonic anhydrase inhibitors
Lactic acidosis
Renal tubular acidosis
Diabetes mellitus (ketoacidosis)
Diarrhea
(Normochloremia)
(Hyperchloremia)
Increased Anion Gap
Normal Anion Gap
Plasma Anion Gap
’s disease
poisoning
Methanol poisoning
Ethylene glycol poisoning
Starvation
110
120
100 90
80
70
60
50
40
7.40
7.50
7.60
7.70
7.80
Arterial plasma [HCO
3
-
] (mEq/L)
20
24
28
32
36
40
44
48
52
56
60
16
12
8
4
0
7.30
7.20
7.10
35
30
25
20
15
10
7.00
Arterial blood pH
Chronic
respiratory
acidosis
Metabolic
alkalosis
Acute
respiratory
alkalosis
Metabolic
acidosis
Acute
respiratory
acidosis
Chronic
respiratory
alkalosis
Normal
Pco
2
(mm Hg)
Pco
2
(mm Hg)
Base Disorders in the Kidney, 3rd
base disorder. (Adapted from
lying outside the shaded areas,
respiratory disorders. For values
for the normal compensations
show the approximate limits
shaded areas in the nomogram
status in normal people. The
approximate limits for acid-base
central open circle shows the
arterial blood pH, arterial plasma
Figure 30–11
Acid-base nomogram showing
HCO
3
–
, and P
CO
2
values. The
caused by simple metabolic and
one should suspect a mixed acid-
Cogan MG, Rector FC Jr: Acid-
ed. Philadelphia: WB Saunders,
1986.)

Endocr Metab Disord 4:343, 2003.
White NH: Management of diabetic ketoacidosis. Rev
York: Raven Press, 2000, pp 2055-2072.
Physiology and Pathophysiology, 3rd ed. New
metabolic alkalosis. In Seldin DW, Giebisch G (eds): The
Wesson DE, Alpern RJ, Seldin DW: Clinical syndromes of
duct. J Nephrol 15(Suppl 5):S112, 2002.
Wagner CA, Geibel JP: Acid-base transport in the collecting
balance by the lung. Nephron Physiol 93:61, 2003.
Madias NE, Adrogue HJ: Cross-talk between two organs:
285:F811, 2003.
acid and base in humans. Am J Physiol Renal Physiol
Lemann J Jr, Bushinsky DA, Hamm LL: Bone buffering of
Laffey JG, Kavanagh BP: Hypocapnia. N Engl J Med 347:43,
Nephrol 13:2178, 2002.
Karet FE: Inherited distal renal tubular acidosis. J Am Soc
dosis. J Am Soc Nephrol 13:2171, 2002.
Igarashi I, Sekine T, Inatomi J, Seki G: Unraveling the molec-
s loop. Ann Rev Physiol 56:623, 1994.
Good DW: Ammonium transport by the thick ascending
York: Raven Press, 2000, pp 2015-2054.
Physiology and Pathophysiology, 3rd ed. New
homeostasis. In Seldin DW, Giebisch G (eds): The
Gennari FJ, Maddox DA: Renal regulation of acid-base
proton transfer pathways. Physiol Rev 83:475, 2003.
Decoursey TE: Voltage-gated proton channels and other
ulation. J Nephrol 15(Suppl 5):S88, 2002.
along the loop of Henle: molecular mechanisms and reg-
Capasso G, Unwin R, Rizzo M, et al: Bicarbonate transport
(ed): The Kidney, 6th ed. Philadelphia: WB Saunders, 2000,
cation mechanisms. In Brenner BM
Alpern RJ: Renal acidi
Regulation of Acid-Base Balance
Chapter 30
401
References
fi
pp 455-519.
Kidney—
limb of Henle’
ular pathogenesis of isolated proximal renal tubular aci-
2002.
how the kidney responds to disruption of acid-base
Kidney—
