
the juncture of the jugular and subclavian veins.
cholesterol, and 1 per cent apoprotein B. The chylomicrons are then transported
cipally of triglycerides, they also contain about 9 per cent phospholipids, 3 per cent
tract enter the chylomicrons. Thus, although the chylomicrons are composed prin-
to the lymphatic vessel walls.
to the outer surfaces of the chylomicrons. This leaves the remainder of the protein
ters are between 0.08 and 0.6 micron. A small amount of apoprotein B is adsorbed
chylomicrons,
enter the lymph as minute, dispersed droplets called
fatty acids. Then, while passing through the intestinal epithelial cells, the mono-
tinal lymph. During digestion, most triglycerides are split into monoglycerides and
tion of a few short-chain fatty acids, are absorbed from the intestines into the intes-
As explained in Chapter 65, almost all the fats in the diet, with the principal excep-
Gastrointestinal Tract by Lymph—The Chylomicrons
Transport of Triglycerides and Other Lipids from the
Transport of Lipids in the Body Fluids
an 18-carbon chain and is fully saturated with hydrogen atoms; (2)
(shown in the tristearin example above), which has
glycerol. The three fatty acids most commonly present in the triglycerides of the
Tristearin
with the utilization of triglycerides for energy, the following typical structure of the
Basic Chemical Structure of Triglycerides (Neutral Fat).
perform other cellular functions.
of triglycerides, are used to form the membranes of all cells of the body and to
However, some lipids, especially cholesterol, the phospholipids, and small amounts
metabolic processes, a function they share almost equally with the carbohydrates.
The triglycerides are used in the body mainly to provide energy for the different
many of the physical and chemical properties of other lipid substances.
sterol nucleus is synthesized from portions of fatty acid molecules, thus giving it
Although cholesterol does not contain fatty acid, its
acid, palmitic acid, is the following: CH
long-chain hydrocarbon organic acids. A typical fatty
fatty acids,
Chemically, the basic lipid moiety of the triglycerides
and (4) a few others of less importance.
cholesterol;
They include (1)
lipids.
C
H
A
P
T
E
R
6
8
840
Lipid Metabolism
Several chemical compounds in food and in the
body are classified as
neutral fat,
also known as triglycerides;
(2) phospholipids;
(3)
and the phospholipids is
which are simply
3
(CH
2
)
14
COOH.
Because most of this chapter deals
triglyceride molecule must be understood.
CH
3
—(CH
2
)
16
—COO—CH
2
|
CH
3
—(CH
2
)
16
—COO—CH
|
CH
3
—(CH
2
)
16
—COO—CH
2
Note that three long-chain fatty acid molecules are bound with one molecule of
human body are (1) stearic acid
oleic acid, which
also has an 18-carbon chain but has one double bond in the middle of the chain;
and (3) palmitic acid, which has 16 carbon atoms and is fully saturated.
glycerides and fatty acids are resynthesized into new molecules of triglycerides that
whose diame-
molecules projecting into the surrounding water and thereby increases the suspen-
sion stability of the chylomicrons in the lymph fluid and prevents their adherence
Most of the cholesterol and phospholipids absorbed from the gastrointestinal
upward through the thoracic duct and emptied into the circulating venous blood at

and phospholipids.
high-density lipoproteins,
phospholipids; and (4)
erides, leaving an especially high concentration of
teins,
low-density lipopro-
phospholipids are increased; (3)
removed, so that the concentrations of cholesterol and
lipoproteins,
lesterol and phospholipids; (2)
lipoproteins,
very low density
as measured in the ultracentrifuge: (1)
major types of lipoproteins, classified by their densities
are themselves very large lipoproteins, there are four
Aside from the chylomicrons, which
Types of Lipoproteins.
Protein
200
Triglycerides
160
Phospholipids
160
Cholesterol
180
per 100 ml of plasma—that is, 700 mg/dl. This can be
The total concentration
phospholipids,
triglycerides, cholesterol,
smaller than chylomicrons, but qualitatively similar
These are small particles—much
form of
have been removed from the blood, more than 95
In the postabsorptive state, after all the chylomicrons
Cholesterol and Phospholipids
Function in Transporting
conditions.
transport is extreme. This shows how variable the rate
fatty acid combine with each molecule of albumin, but
Under normal conditions, only about 3 molecules of
energy from carbohydrates.
conditions, the person derives little or no metabolic
cases of starvation and in diabetes; in both these
eightfold. Such a large increase occurs especially in
fatty acid concentration in the blood; in fact, the
2. Conditions that increase the rate of utilization
energy.
by the oxidation of transported free fatty acids,
calculate that at this rate, almost all the normal
new fatty acid every 2 to 3 minutes.
half the plasma fatty acid is replaced by
the blood, its rate of “turnover” is extremely
1. Despite the minute amount of free fatty acid in
latory system. Strangely enough, even this small amount
under resting conditions is about 15 mg/dl, which is a
The concentration of free fatty acids in the plasma
substances.
of (1) esters of glycerol, (2) cholesterol, or (3) other
nonesterified fatty acids,
with albumin molecules of the plasma proteins. Fatty
plasma, and the ionic portion combines immediately
On leaving fat cells, fatty acids ionize strongly in the
This is discussed later in the chapter.
and this also promotes rapid hydrolysis of triglycerides.
vated by several hormones from the endocrine glands,
Second, a
of triglycerides, the result is hydrolysis of triglycerides.
also available in insufficient quantities. Because this
-glycerophosphate,
the glucose breakdown products,
of glucose available to the fat cell is inadequate, one of
in promoting this hydrolysis. First, when the amount
This is achieved by hydrolysis of the triglycerides
acids.
tissue. It is transported mainly in the form of
be used elsewhere in the body to provide energy, it must
When fat that has been stored in the adipose tissue is to
in the Blood in Combination
“Free Fatty Acids” Are Transported
in the cells in the same way.
phospholipids; this, too, releases fatty acids to be stored
later in the chapter. The lipase also causes hydrolysis of
metabolic processes of the storage cells, as discussed
triglycerides, with new glycerol being supplied by the
these cells, the fatty acids are again synthesized into
of the adipose tissue and into the liver cells. Once inside
branes of the cells, immediately diffuse into the fat cells
The fatty acids, being highly miscible with the mem-
come in contact with the endothelial wall, thus releas-
is especially active in the capillary endothelium, where
This enzyme
lipoprotein lipase.
liver. Both adipose tissue and the liver contain large
and Fat Is Stored in Adipose Tissue and Liver Cells.
Chylomicron Triglycerides Are Hydrolyzed by Lipoprotein Lipase,
chylomicrons is removed mainly in the following way.
becomes clear again within a few hours. The fat of the
crons have a half-life of less than 1 hour, so the plasma
turbid and sometimes yellow. However, the chylomi-
of the large size of the chylomicrons, the plasma appears
rise to 1 to 2 per cent of the total plasma, and because
of fat, the chylomicron concentration in the plasma may
Chapter 68
Lipid Metabolism
841
Removal of the Chylomicrons
from the Blood
About 1 hour after a meal that contains large quantities
Most of the
chylomicrons are removed from the circulating blood as
they pass through the capillaries of adipose tissue or the
quantities of the enzyme
it hydrolyzes the triglycerides of chylomicrons as they
ing fatty acids and glycerol.
with Albumin
first be transported from the adipose tissue to the other
free fatty
back into fatty acids and glycerol.
At least two classes of stimuli play important roles
a
is
substance is required to maintain the glycerol portion
hormone-sensitive cellular lipase can be acti-
acids bound in this manner are called free fatty acids
or
to distinguish them from
other fatty acids in the plasma that exist in the form
total of only 0.45 gram of fatty acids in the entire circu-
accounts for almost all the transport of fatty acids from
one part of the body to another for the following
reasons:
rapid:
One can
energy requirements of the body can be provided
without using any carbohydrates or proteins for
of fat for cellular energy also increase the free
concentration sometimes increases fivefold to
as many as 30 fatty acid molecules can combine with a
single albumin molecule when the need for fatty acid
of lipid transport can be under different physiologic
Lipoproteins—Their Special
per cent of all the lipids in the plasma are in the
lipoprotein.
in composition—containing
and protein.
of lipoproteins in the plasma averages about 700 mg
broken down into the following individual lipoprotein
constituents:
mg/dl of plasma
which contain high concentrations of
triglycerides and moderate concentrations of both cho-
intermediate-density
which are very low density lipoproteins
from which a share of the triglycerides has been
which are derived from intermediate-density
lipoproteins by the removal of almost all the triglyc-
cholesterol and a moderately high concentration of
which
contain a high concentration of protein (about 50 per
cent) but much smaller concentrations of cholesterol

The fatty acid molecule is degraded in the
stance. Once inside the mitochondria, fatty acids split
their transport into the mitochondria. This is a carrier-
Therefore, the first step for the use of fatty acids is
Entry of Fatty Acids into Mitochondria.
processed further in the following way.
the fatty acids can be used for energy, they must be
glucose breakdown and is thus used for energy. Before
phosphate,
Glycerol, on entering the active tissue, is immediately
cells—can use fatty acids for energy.
some exceptions, such as brain tissue and red blood
will be oxidized to give energy. Almost all cells—with
transported in the blood to the active tissues, where they
glycerol. Then, both the fatty acids and the glycerol are
The first stage in using triglyc-
Hydrolysis of Triglycerides.
fatty acids released from the triglycerides for energy.
triglycerides, then stored, and used later in the form of
of carbohydrates is. In addition, many of the carbohy-
calories derived from carbohydrates. Therefore, the use
diet are derived from fats, which is almost equal to the
About 40 per cent of the calories in a typical American
of Adenosine Triphosphate
for Energy: Formation
Use of Triglycerides
cells.
fats, and their principal source is the liver. This desatu-
tissues of the body, because many structural elements of
triglycerides of adipose tissue.This capability of the liver
tissues of desaturating fatty acids, so that liver triglyc-
Also, the liver cells are much more capable than other
terol, which are continually synthesized by the liver.
The liver cells, in addition to containing triglycerides,
lipids are being used for energy.
tions, the total amount of triglycerides in the liver is
dation begin. Thus, under normal physiologic condi-
in the liver, where the initial stages of much of fat degra-
fatty acids in the blood, and redeposited as triglycerides
mobilized from the adipose tissue, transported as free
these conditions, large quantities of triglycerides are
instead of carbohydrates is being used for energy. In
mellitus, and (3) in any other condition in which fat
(1) during the early stages of starvation, (2) in diabetes
fatty acids, especially cholesterol and phospholipids.
proteins as well; and (3) synthesize other lipids from
mainly from carbohydrates, but to a lesser extent from
can be used for energy; (2) synthesize triglycerides,
The principal functions of the liver in lipid metabolism
Liver Lipids
not the same fat that was stored last month, thus empha-
fat cells are renewed about once every 2 to 3 weeks,
of the rapid exchange of fatty acids, the triglycerides in
erides of the fat cells to release free fatty acids. Because
activated by hormones, cause splitting of the triglyc-
from the chylomicrons and lipoproteins. Others, when
lipases are present in adipose tissue. Some of these
As discussed earlier, large quantities of
Tissue Lipases.
Exchange of Fat Between the Adipose Tissue and the Blood—
later in the chapter.
supplements the synthesis of fat in the liver, as discussed
acids and triglycerides from carbohydrates; this function
Fat cells can synthesize very small amounts of fatty
the cells.
in a liquid state. This is particularly important, because
melting point, thereby always allowing the fat to remain
the cell triglycerides, over a period of weeks, become
are exposed to prolonged cold, the fatty acid chains of
fat cells are generally in a liquid form. When the tissues
cent of the entire cell volume. Triglycerides inside the
The fat cells (adipocytes) of
provide heat insulation for the body, as discussed in
elsewhere in the body. A subsidiary function is to
The major function of adipose tissue is storage of
fat deposits,
The adipose
liver.
of the body, the
Adipose Tissue
the insides of arterial walls.
atherosclerosis,
periphery back to the liver. Later in the chapter, we
in the liver mainly to the adipose tissue, whereas the
port their lipid components in the blood. The very low
The primary function of the lipoproteins is to trans-
acids from the intestines.
triglycerides are synthesized. In addition, small quanti-
most of the plasma cholesterol, phospholipids, and
lipoproteins are formed in the liver, which is also where
Metabolism and Temperature Regulation
842
Unit XIII
Formation and Function of Lipoproteins.
Almost all the
ties of high-density lipoproteins are synthesized in the
intestinal epithelium during the absorption of fatty
density lipoproteins transport triglycerides synthesized
other lipoproteins are especially important in the dif-
ferent stages of phospholipid and cholesterol transport
from the liver to the peripheral tissues or from the
discuss in more detail special problems of cholesterol
transport in relation to the disease
which
is associated with the development of fatty lesions on
Fat Deposits
Large quantities of fat are stored in two major tissues
adipose tissue and the
tissue is usually called
or simply tissue fat.
triglycerides until they are needed to provide energy
Chapter 73.
Fat Cells (Adipocytes).
adipose tissue are modified fibroblasts that store almost
pure triglycerides in quantities as great as 80 to 95 per
either shorter or more unsaturated to decrease their
only liquid fat can be hydrolyzed and transported from
enzymes catalyze the deposition of cell triglycerides
which means that the fat stored in the tissues today is
sizing the dynamic state of storage fat.
are to (1) degrade fatty acids into small compounds that
Large quantities of triglycerides appear in the liver
determined to a great extent by the overall rate at which
contain large quantities of phospholipids and choles-
erides normally are much more unsaturated than the
to desaturate fatty acids is functionally important to all
all cells contain reasonable quantities of unsaturated
ration is accomplished by a dehydrogenase in the liver
of fats by the body for energy is as important as the use
drates ingested with each meal are converted into
erides for energy is their hydrolysis into fatty acids and
changed by intracellular enzymes into glycerol-3-
which enters the glycolytic pathway for
Degradation and oxi-
dation of fatty acids occur only in the mitochondria.
mediated process that uses carnitine as the carrier sub-
away from carnitine and are degraded and oxidized.
Degradation of Fatty Acids to Acetyl Coenzyme A by Beta-
Oxidation.

Chapter 67. The net reaction in the citric acid cycle for
of the mitochondria,
chemiosmotic oxidative system
dioxide and hydrogen atoms. The hydrogen is subse-
form citric acid, which then is degraded into carbon
Chapter 67), combining first with oxaloacetic acid to
The acetyl-CoA molecules
ecule at the same time, entirely separate from the
addition to the released acetyl-CoA molecules, four
original fatty acid molecule by another two carbons. In
still another acetyl-CoA molecule, thus shortening the
2 and progresses through equations 3, 4, and 5 to release
Next, this shorter fatty acyl-CoA enters into equation
molecule; this time, however, the molecule is two carbon
fatty acid molecule, and this forms a new fatty acyl-CoA
into the cell fluid. At the same time, another CoA mol-
Then, in equation 5, the right-hand two-carbon
is, the beta carbon becomes oxidized.
the fatty acyl-CoA binds with an oxygen molecule—that
4, the
A (CoA) to form fatty acyl-CoA. In equations 2, 3, and
oxidation process, note that in equation 1 the first step
To understand the essential steps in the beta-
process for degradation of fatty acids.
beta-oxidation
This process, which is shown in Figure 68–1, is called the
acetyl coenzyme A
Chapter 68
Lipid Metabolism
843
mitochondria by progressive release of two-carbon seg-
ments in the form of
(acetyl-CoA).
is combination of the fatty acid molecule with coenzyme
beta carbon (the second carbon from the right) of
portion of the molecule is split off to release acetyl-CoA
ecule binds at the end of the remaining portion of the
atoms shorter because of the loss of the first acetyl-CoA
from its terminal end.
atoms of hydrogen are released from the fatty acid mol-
acetyl-CoA.
Oxidation of Acetyl-CoA.
formed by beta-oxidation of fatty acids in the mito-
chondria enter immediately into the citric acid cycle (see
quently oxidized by the
which was also explained in
each molecule of acetyl-CoA is the following:
Citric acid cycle
CO
H + HCo-A + ATP + Oxaloacetic acid
CH COCo-A + Oxaloacetic acid
H O + ADP
3
2
2
+
æ
Æ
ææææææ
+
3
2
8
ATP.
acid molecule, making a
stearic acid. However, two high-energy bonds are con-
metabolized. Thus, a total of 148 molecules of ATP are
gen), one for each of the 9 acetyl-CoA molecules
rate from the ATP released by the oxidation of hydro-
of ATP are formed in the citric acid cycle itself (sepa-
from each molecule of stearic acid. Another 9 molecules
of ATP formed by the oxidation of hydrogen derived
gens. This makes 34 plus 105, or a total of 139 molecules
synthesized for each of the 70 NADH and H
avoprotein hydrogens, and 1.5 molecules of ATP are
1 molecule of ATP is synthesized for each of the 34
enter the oxidative system at different points, so that
the mitochondria, as discussed in Chapter 67, but they
These two groups of hydrogen atoms are oxidized in
as NADH and H
removed by nicotinamide adenine dinucleotide (NAD
avoproteins, and 70 are
acid molecule. Of this group, 34 are removed from
hydrogens. This makes a total of 104 hydrogen atoms
more hydrogen atoms are removed, making another 72
are subsequently degraded by the citric acid cycle, 8
addition, for each of the 9 molecules of acetyl-CoA that
molecules, 32 extra hydrogen atoms are removed. In
. Therefore, for every stearic
, NADH, and H
FADH
1, note that the 4 separate hydrogen
In Figure 68
Tremendous Amounts of ATP Are Formed by Oxidation of Fatty
triphosphate (ATP).
oxidation, liberating large amounts of adenosine
system of the mitochondria
chemiosmotic oxidative
metabolism of glucose. And the extra hydrogen atoms
CoA, their
Thus, after initial degradation of fatty acids to acetyl-
final breakdown is precisely the same as that
of the acetyl-CoA formed from pyruvic acid during the
are also oxidized by the same
that is used in carbohydrate
Acids.
–
atoms released each time a molecule of acetyl-CoA is
split from the fatty acid chain are released in the forms
2
+
fatty acid molecule that is split to form 9 acetyl-CoA
eventually released by the degradation of each stearic
the degrading fatty acids by fl
+
)
+
.
fl
+
hydro-
formed during the complete oxidation of 1 molecule of
sumed in the initial combination of CoA with the stearic
net gain of 146 molecules of
CH=CHCOCoA + H
(Fatty acyl-CoA) (Acetyl-CoA)
AMP
COCoA
CH=CHCOCoA + FADH
COCoA + CH
COCoA + CoA
COCoA + NADH + H
COCoA + NAD
COCoA + FAD
COOH + CoA + ATP
(1) RCH
2
CH
2
CH
2
(Fatty acid)
Thiokinase
Acyl dehydrogenase
Enoyl hydrase
dehydrogenase
Thiolase
b
-Hydroxyacyl
(2) RCH
2
CH
2
CH
2
(4) RCH
2
CHOHCH
2
+
RCH
2
COCH
2
+
(5) RCH
2
COCH
2
RCH
2
3
COCoA
RCH
2
CHOHCH
2
COCoA
RCH
2
2
RCH
2
CH
2
CH
2
+
+
Pyrophosphate
(Fatty acyl-CoA)
(Fatty acyl-CoA)
(3) RCH
2
2
O
Figure 68–1
Beta-oxidation of fatty acids to yield acetyl coenzyme A.

almost entirely of fat. In all these states, essentially no
occurs especially in starvation, in diabetes mellitus, and
Ketosis
ketone bodies.
The three compounds are called
ketosis,
uids; this condition
The con-
Ketosis in Starvation, Diabetes, and Other Diseases.
fusion into the cells.
the target cells, which allows almost instantaneous dif-
transport. The rapid transport of both these substances
actually transported, as is also true for free fatty acid
in the blood, large
plasma seldom rises above 3 mg/dl. Yet despite this
Normally, the acetoacetic acid and
oxidized for energy, as already explained.
formed. These in turn enter the citric acid cycle and are
tissues. Here they again diffuse into the cells, where
-hydroxybutyric acid, and
The acetoacetic acid,
-hydroxybutyric acid,
for energy. The chemical processes are the following:
to the other cells throughout the body, where it is used
acetoacetic acid, which is then transported in the blood
acid chains have been split into acetyl-CoA, two mole-
intrinsic metabolic processes. Instead, when the fatty
of lipids are being used for energy. However, the liver
occurs in the liver, especially when excessive amounts
in the Liver and Its Transport
Formation of Acetoacetic Acid
Metabolism and Temperature Regulation
844
Unit XIII
in the Blood
A large share of the initial degradation of fatty acids
uses only a small proportion of the fatty acids for its own
cules of acetyl-CoA condense to form one molecule of
Part of the acetoacetic acid is also converted into
b
and minute quantities are
converted into acetone in accord with the following
reactions:
b
acetone diffuse freely through the liver cell membranes
and are transported by the blood to the peripheral
reverse reactions occur and acetyl-CoA molecules are
b-hydroxybutyric
acid that enter the blood are transported so rapidly to
the tissues that their combined concentration in the
small concentration
quantities are
results from their high solubility in the membranes of
centrations of acetoacetic acid,
b-hydroxybutyric acid,
and acetone occasionally rise to levels many times
normal in the blood and interstitial fl
is called
because acetoacetic acid is a keto acid.
sometimes even when a person’s diet is composed
C
OH
C
CH
CH
CH
C
OH
C
CH
CH
3
2
O
O
Acetoacetic acid
Acetone
CH
3
2
OH
CH
3
3
O
+ 2H
-CO
2
O
b-Hydroxybutyric acid
Acetoacetic acid
Acetyl CoA
CH COCH COOH
HCo-A
CH COCo-A + H O
2
2
liver cells
other cells
3
2
3
2
-
æ
Æ
ææææ
æ
¨
æ
ææææ
æ
+
The
teins to the adipose tissue, where they are stored.
the adipose tissue itself. The triglycerides formed in the
the liver, but minute quantities are also synthesized in
In human beings, most triglyceride synthesis occurs in
form in the adipose tissue.
can be stored in the form of glycogen, the excess is
Whenever a greater quantity of carbohydrates enters
from Carbohydrates
Synthesis of Triglycerides
fats.
glucose, can derive 50 to 75 per cent of their energy from
by the cells. After a few weeks, even the brain cells,
clear, enhance the rate of acetoacetic acid metabolism
ketosis. Undoubtedly, several factors, none of which is
times live almost entirely on a fat diet, do not develop
not occur. For instance, the Inuit (Eskimos), who some-
than usual, and in this instance, ketosis normally does
a carbohydrate diet to an almost completely fat diet, a
When changing slowly from
criterion of ketosis.
ties in the expired air of the lungs. This gives the breath
substance, some of which is blown off in small quanti-
The acetone that is formed during ketosis is a volatile
20 times normal, thus leading to extreme acidosis, as
liver, the blood concentrations of acetoacetic acid and
entry of acetyl-CoA into the citric acid cycle, and when
ciency
processed in the citric acid cycle. Therefore, de
oxaloacetate
most important reason is the following: One of the prod-
the amount of ketone bodies that can be oxidized; the
to the cells. For several reasons, the cells are limited in
The ketone bodies pour out of the liver to be carried
acids is converted to ketone bodies.
energy and (2) to the liver cells, where much of the fatty
result, tremendous quantities of fatty acids become
the removal of fatty acids from the fat tissues. As a
secretion of glucagon by the pancreas, and decreased
tion of glucocorticoids by the adrenal cortex, increased
tion, several hormonal factors
of removal of fatty acids from adipose tissues; in addi-
of fats. We shall see later in the chapter that the unavail-
When carbohydrates are not used for energy, almost
glucose transport into the cells.
a high-fat diet because carbohydrates are not available,
carbohydrates are metabolized—in starvation and with
and in diabetes because insulin is not available to cause
all the energy of the body must come from metabolism
ability of carbohydrates automatically increases the rate
—such as increased secre-
secretion of insulin by the pancreas—further enhance
available (1) to the peripheral tissue cells to be used for
ucts of carbohydrate metabolism is the
that
is required to bind with acetyl-CoA before it can be
fi
of oxaloacetate derived from carbohydrates limits the
there is a simultaneous outpouring of large quantities
of acetoacetic acid and other ketone bodies from the
b-hydroxybutyric acid sometimes rise to as high as
explained in Chapter 30.
an acetone smell that is frequently used as a diagnostic
Adaptation to a High-Fat Diet.
person’s body adapts to use far more acetoacetic acid
which normally derive almost all their energy from
the body than can be used immediately for energy or
rapidly converted into triglycerides and stored in this
liver are transported mainly in very low density lipopro-
Conversion of Acetyl-CoA into Fatty Acids.
first step
in the synthesis of triglycerides is conversion of
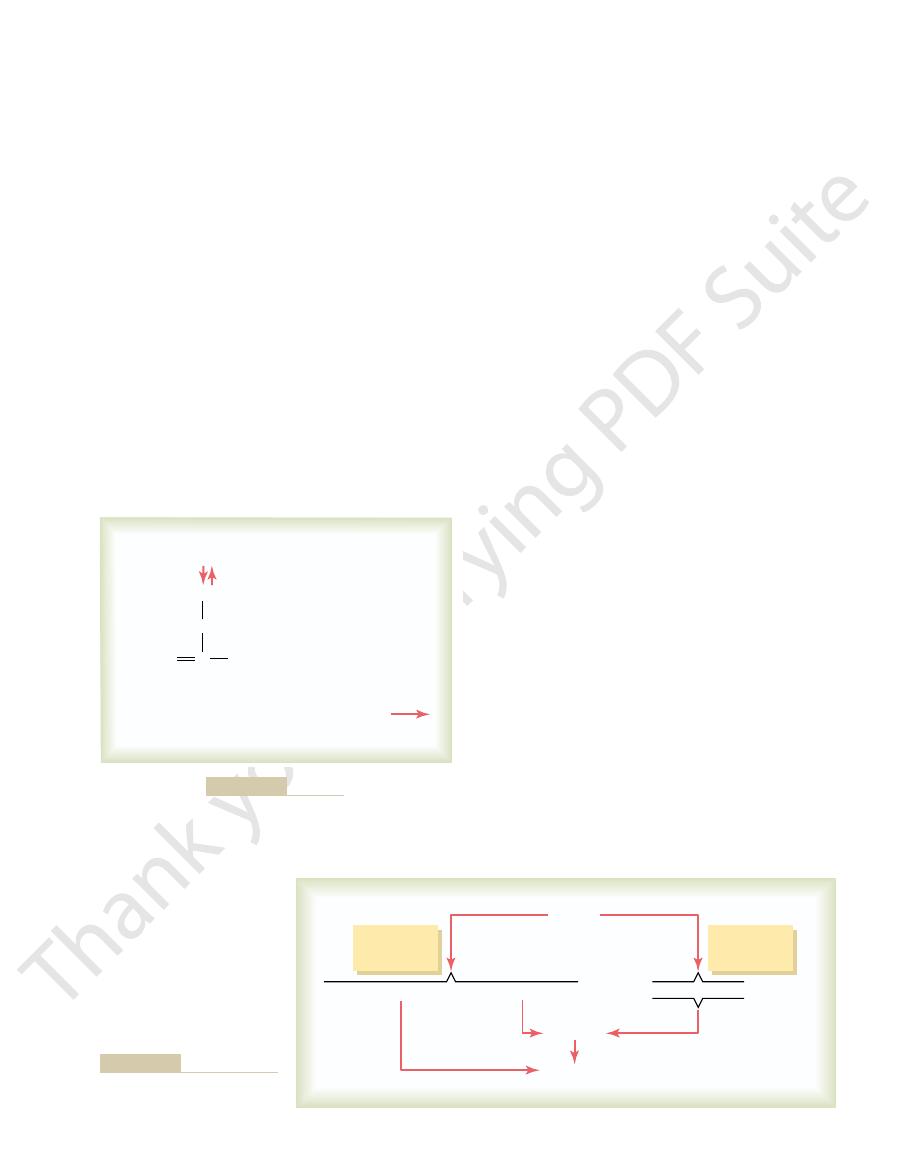
use as proteins, a large share of the excess is stored as
synthesized into triglycerides. Therefore, when people
discussed in Chapter 69. The acetyl-CoA can then be
Many amino acids can be converted into acetyl-CoA, as
from Proteins
Synthesis of Triglycerides
cult for the tissues to form triglycerides.
-glycerophosphate, which also
Second, lack of glucose in the fat cells greatly reduces
needed for fat synthesis can be derived from glucose.
factorily, so that little of the acetyl-CoA and NADPH
able, glucose does not enter the fat and liver cells satis-
for the following reasons: First, when insulin is not avail-
diabetes mellitus, fats are poorly synthesized, if at all,
When no insulin is available, as occurs in serious
an animal must be highly motile to survive.
carbohydrate, which is exceedingly important when
gain, a person can store several times as much
gram of glycogen. Therefore, for a given weight
2. Each gram of fat contains almost two and a half
as stored in the form of carbohydrate.
later use. Indeed, the average person has almost
can be stored. Therefore, fat synthesis provides a
put together. In contrast, many kilograms of fat
skeletal muscles, and all other tissues of the body
grams of glycogen can be stored in the liver, the
generally slight; a maximum of only a few hundred
1. The ability of the different cells of the body to
Fat synthesis from
triglycerides.
energy in the glucose is lost in the form of heat; the
eride synthesis, only about 15 per cent of the original
of glucose degradation. This mechanism is discussed in
-glycerophosphate, which
3, the glycerol portion of
As shown in Figure 68
in the body.
chain lengths of 14 carbon atoms or greater, a factor that
glycerol to form triglycerides. The enzymes that cause
grown to contain 14 to 18 carbon atoms, they bind with
Triglycerides.
-Glycerophosphate to Form
polymerization process.
and NADPH as the principal intermediates in the
2, using
two-step process shown in Figure 68
degradation described earlier. Instead, this occurs by the
acids. However, the synthesis of fatty acids from acetyl-
are actually large polymers of acetic acid, it is easy to
of glucose by the glycolytic system. Because fatty acids
Chapter 67, this occurs during the normal degradation
carbohydrates into acetyl-CoA. As explained in
Chapter 68
Lipid Metabolism
845
understand how acetyl-CoA can be converted into fatty
CoA is not achieved by simply reversing the oxidative
–
malonyl-
CoA
Combination of Fatty Acids with a
Once the synthesized fatty acid chains have
this conversion are highly specific for fatty acids with
controls the physical quality of the triglycerides stored
–
triglycerides is furnished by
a
is another product derived from the glycolytic scheme
Chapter 67.
Efficiency of Carbohydrate Conversion into Fat.
During triglyc-
remaining 85 per cent is transferred to the stored
Importance of Fat Synthesis and Storage.
carbohydrates is especially important for two reasons:
store carbohydrates in the form of glycogen is
means by which the energy of excess ingested
carbohydrates (and proteins) can be stored for
150 times as much energy stored in the form of fat
times the calories of energy contained by each
energy in the form of fat as in the form of
Failure to Synthesize Fats from Carbohydrates in the Absence of
Insulin.
the availability of
a
makes it diffi
have more proteins in their diets than their tissues can
fat.
9CoA
Malonyl-CoA
1 Acetyl-CoA
O
CoA
ADP
ATP
COCoA
(Acetyl-CoA carboxylase)
COOH
CH
3
+
CO
2
+
+
+
PO
4
–3
CH
2
C
+
+
16NADPH
+
16H
+
1 Steric acid
+
8CO
2
+
+
16NADP
+
+
7H
2
O
Step 1:
Step 2:
Malonyl-CoA
Figure 68–2
Synthesis of fatty acids.
Acetyl-CoA
Triglycerides
Glucose
Fatty acids
a
-Glycerophosphate
+
+
NADH
+
H
+
NADPH
+
H
+
Glycolytic
pathway
Pentose
phosphate
pathway
triglycerides from glucose.
Figure 68–3
Overall schema for synthesis of

cephalins,
lecithins,
The major types of body phospholipids are
Cholesterol
stores, resulting in severe obesity.
storage of fat continue normally. Such a one-way
adipose tissue by tissue lipase, while synthesis and
occurs. In at least one of these, the obesity is
adipose tissue, to be used later for energy.
or proteins, is then stored almost entirely as fat in the
for energy. The excess food, whether fats, carbohydrates,
y, it is caused by the ingestion of
balances, but brie
Obesity means deposition of excess fat in the body. This
hormone.
The effects of the different hormones on metabolism
uence of this hormone. The
of fat, which is believed to result indirectly from an
Finally,
activating hormone-sensitive lipase. Therefore, growth
Growth hormone
a great extent that ketosis results. Corticotropin and
s disease, fats are frequently mobilized to such
long periods, as occurs in the endocrine condition called
nephrine or a similar lipase. When corticotropin and
glucocorticoids.
be released by the anterior pituitary gland, and this
mobilization and utilization in a similar manner.
increased. Other types of stress that activate the sym-
rises as much as eightfold, and the use of these fatty
and mobilization of fatty acids. Sometimes the free fatty
cells, and this causes rapid breakdown of triglycerides
eride lipase,
cise, as a result of sympathetic stimulation. These two
utilization is that observed during heavy exercise. This
noted here.
insulin lack,
cant effects on fat utilization. Some important
carbohydrates.
but also decreases fat storage, which further shifts the
caused by the absence of carbohydrates. This not only
adipose tissue. Among the most important of these is
used for energy in place of carbohydrates.
direction, and fat is mobilized from the adipose cells and
not available. The equilibrium shifts in the opposite
converted to fat for storage.
acts as a fat-sparer but also increases fat stores. In fact,
Thus, an excess of carbohydrates in the diet not only
automatically causing increased synthesis of fatty acids.
drates are being used, these intermediates increase,
mediates of the citric acid cycle. When excess carbohy-
acetyl-CoA carboxylase,
malonyl-CoA. The rate of this reaction is controlled
rst step, which is the rate-limiting step, in the synthesis
conversion of carbohydrates to fats is the following: The
ate for the conversion of acetyl-CoA into fatty acids.
in the adipose tissue, thus creating conditions appropri-
degraded. This effect is caused partially by the large
Second, when carbohydrates are available in excess,
automatically inhibits the use of fatty acids for energy.
metabolism, the availability of large amounts of glucose
are available to be used for energy. Because
consequently, only minute quantities of fatty acids
result, the equilibrium between free fatty acids and
free fatty acids in the form of stored triglycerides. As a
available), the excess
other.When excess quantities of
fatty acids. They are in constant equilibrium with each
forms: stored triglycerides and small quantities of free
lowing: The fats in adipose tissue cells are present in two
carbohydrates. One of the most important is the fol-
There are several reasons for this
are used preferentially over triglycerides for energy.
carbohydrates are available in the body, carbohydrates
When excess quantities of
Carbohydrates Are Available.
Release from Triglycerides
Regulation of Energy
Metabolism and Temperature Regulation
846
Unit XIII
Carbohydrates Are Preferred over Fats for Energy When Excess
“fat-sparing” effect of
a-glycerophosphate are
present (which occurs when excess carbohydrates are
a-glycerophosphate binds the
triglycerides shifts toward the stored triglycerides;
a-
glycerophosphate is an important product of glucose
fatty acids are synthesized more rapidly than they are
quantities of acetyl-CoA formed from the carbohy-
drates and by the low concentration of free fatty acids
An even more important effect that promotes the
fi
of fatty acids is carboxylation of acetyl-CoA to form
primarily by the enzyme
the
activity of which is accelerated in the presence of inter-
all the excess carbohydrates not used for energy or
stored in the small glycogen deposits of the body are
Acceleration of Fat Utilization for Energy in the Absence of Car-
bohydrates.
All the fat-sparing effects of carbohydrates
are lost and actually reversed when carbohydrates are
Also important are several hormonal changes that
take place to promote rapid fatty acid mobilization from
a marked decrease in pancreatic secretion of insulin
reduces the rate of glucose utilization by the tissues
equilibrium in favor of fat metabolism in place of
Hormonal Regulation of Fat Utilization.
At least seven of
the hormones secreted by the endocrine glands have
signifi
hormonal effects on fat metabolism—in addition to
discussed in the previous paragraph—are
Probably the most dramatic increase that occurs in fat
results almost entirely from release of epinephrine and
norepinephrine by the adrenal medullae during exer-
hormones directly activate hormone-sensitive triglyc-
which is present in abundance in the fat
acid concentration in the blood of an exercising person
acids by the muscles for energy is correspondingly
pathetic nervous system can also increase fatty acid
Stress also causes large quantities of corticotropin to
causes the adrenal cortex to secrete extra quantities of
Both corticotropin and glucocorticoids
activate either the same hormone-sensitive triglyceride
lipase as that activated by epinephrine and norepi-
glucocorticoids are secreted in excessive amounts for
Cushing’
glucocorticoids are then said to have a ketogenic effect.
has an effect similar to but weaker
than that of corticotropin and glucocorticoids in
hormone can also have a mild ketogenic effect.
thyroid hormone causes rapid mobilization
increased overall rate of energy metabolism in all cells
of the body under the infl
resulting reduction in acetyl-CoA and other intermedi-
ates of both fat and carbohydrate metabolism in the
cells is a stimulus to fat mobilization.
are discussed further in the chapters dealing with each
Obesity
subject is discussed in Chapter 71 in relation to dietary
fl
greater amounts of food than can be used by the body
Strains of rats have been found in which hereditary
obesity
caused by ineffective mobilization of fat from the
process causes progressive enhancement of the fat
Phospholipids and
Phospholipids
and sphingomyelin; their typical chemical
the liver, but all other cells of the body form at least
endogenous cho-
formed in the cells of the body, called
exogenous cholesterol,
each day from the gastrointestinal tract, which is called
form of cholesterol esters.
esters with fatty acids. Indeed, about 70 per cent of the
soluble in water. It is speci
intestinal lymph. It is highly fat soluble but only slightly
5, is present in the diets of all people, and it can be
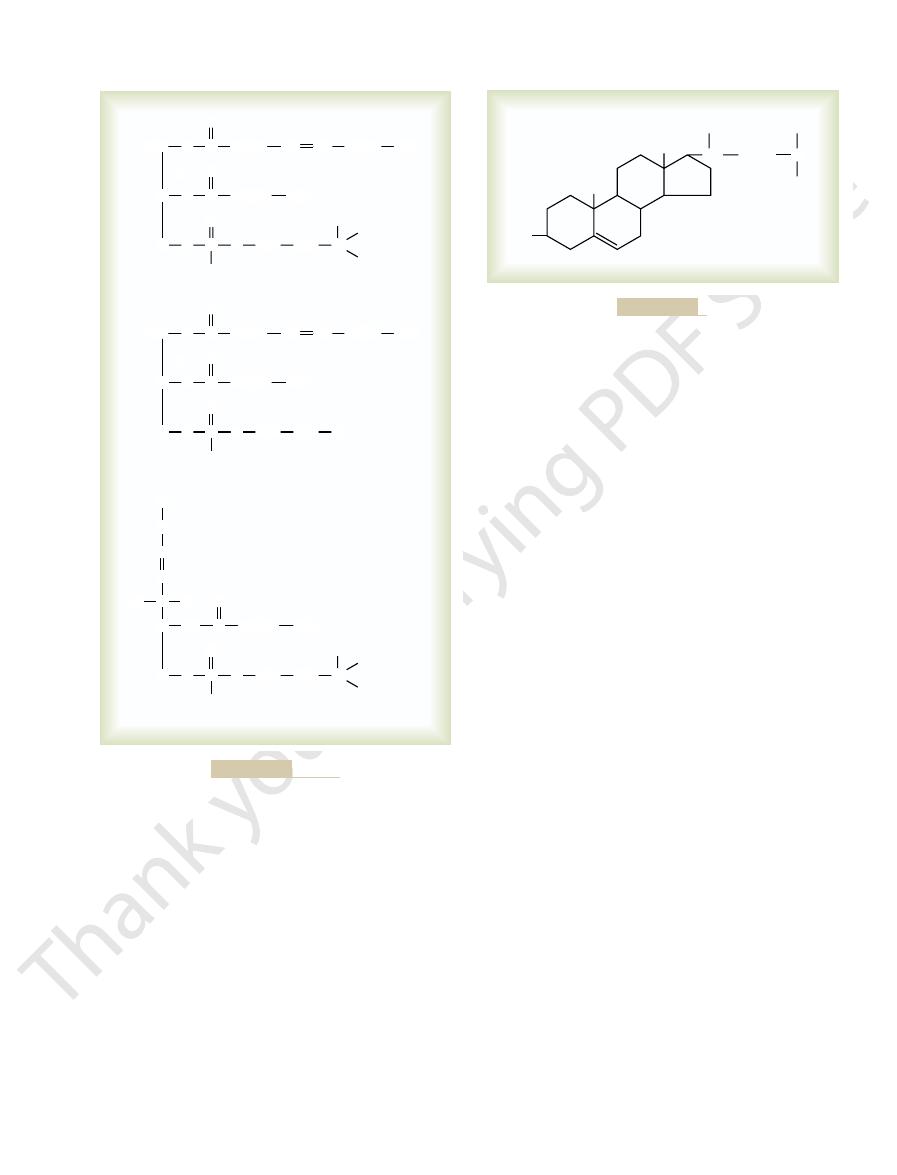
Cholesterol, the formula of which is shown in Figure
cells throughout the body, as discussed in the next
the tissues. (5) Perhaps the most important of all the
bers. (4) Phos-
nervous system; this substance acts as an electrical insu-
ting process, is composed mainly of one of the cephalins.
(2) Thromboplastin, which is needed to initiate the clot-
of transport of cholesterol and other lipids can occur.
of most of these; in their absence, serious abnormalities
phospholipids are the following: (1) Phospholipids
Specific Uses of Phospholipids.
mation of some cephalins.
lecithin molecule. Also,
lecithin, because choline is the nitrogenous base of the
thesized in the body, is needed for the formation of
choline,
For instance,
are needed for the formation of some phospholipids.
tion increases. Also, certain speci
deposited in the liver, the rate of phospholipid forma-
rate of fat metabolism because, when triglycerides are
The rate of phospholipid formation is governed to
Probably 90 per cent are formed in the liver cells; sub-
in essentially all cells of the body, although certain cells
membranes and intracellular membranes.
body for various structural purposes, such as in cell
transported in lipoproteins, and used throughout the
properties are similar because they are all lipid soluble,
phospholipids are somewhat variant, their physical
nitrogenous base. Although the chemical structures of
one phosphoric acid radical, and they usually contain a
4. Phospholipids
formulas are shown in Figure 68
Chapter 68
Lipid Metabolism
847
–
always contain one or more fatty acid molecules and
Formation of Phospholipids.
Phospholipids are synthesized
have a special ability to form great quantities of them.
stantial quantities are also formed by the intestinal
epithelial cells during lipid absorption from the gut.
some extent by the usual factors that control the overall
fic chemical substances
either obtained in the diet or syn-
inositol is needed for the for-
Several functions of the
are an important constituent of lipoproteins in the
blood and are essential for the formation and function
(3) Large quantities of sphingomyelin are present in the
lator in the myelin sheath around nerve fi
pholipids are donors of phosphate radicals when these
radicals are needed for different chemical reactions in
functions of phospholipids is participation in the for-
mation of structural elements—mainly membranes—in
section of this chapter in connection with a similar func-
tion for cholesterol.
Cholesterol
68–
absorbed slowly from the gastrointestinal tract into the
fically capable of forming
cholesterol in the lipoproteins of the plasma is in the
Formation of Cholesterol.
Besides the cholesterol absorbed
an even greater quantity is
lesterol. Essentially all the endogenous cholesterol that
circulates in the lipoproteins of the plasma is formed by
O
O
N
O
O
N
C
O
O
(CH
2
)
7
(CH
2
)
7
CH
3
CH
CH
H
2
C
P
+
O
CH
2
OH
CH
2
CH
3
CH
3
CH
3
H
2
C
C
O
O
(CH
2
)
16
CH
3
HC
C
O
O
(CH
2
)
7
(CH
2
)
7
CH
3
CH
CH
H
2
C
P
+
H
3
O
CH
2
OH
CH
2
H
2
C
C
O
O
(CH
2
)
16
CH
3
HC
P
O
O
O
CH
2
CH
CH
OH
CH
2
HC
H
C
NH
O
(CH
2
)
16
CH
3
HC
N
+
CH
CH
3
CH
3
H
C
HO
(CH
2
)
12
CH
3
A lecithin
A cephalin
Sphingomyelin
Typical phospholipids.
Figure 68–4
CH
HO
CH
CH
3
(CH
2
)
3
CH
3
CH
3
CH
3
CH
3
Cholesterol.
Figure 68–5

of the vessel wall, and
the endothelium, enter the
). The monocytes cross
at the site of injury (Figure 68
endothelium occurs, circulating monocytes and lipids
to the endothelium. After damage to the vascular
adhesion of macromolecules, platelets, and monocytes
. This, in turn,
of all sizes.
, in contrast, is a general
arterial walls.
Atherosclerosis
properties.
rates measured in months or years. For instance, their
uids.
lipids, cholesterol, and certain insoluble proteins. The
and some proteins. Thus, the physical integrity of cells
soluble in water must be available. In general, the only
For membranes to be formed, substances that are not
membranes.
organelles of all cells. It is also known that the
Chapter 2, it was pointed out that large quantities of
mainly membranes, in all cells of the body. In
with their function of forming specialized structures,
The previously mentioned uses of phospholipids and
Cellular Structural Functions of
400 milliliters.
skin; without this protection, the amount of evaporation
otherwise easily penetrate the body. Also, these lipid
agents, because cholesterol and the other skin lipids are
the skin highly resistant to the absorption of water-
corneum of the skin. This, along with other lipids, makes
endocrinology.
mones from them, as discussed in the chapters on
These glands can
testosterone.
adrenocortical hormones,
and absorption of fats.
substances to form bile salts, which promote digestion
explained in Chapter 70, this is conjugated with other
per cent of cholesterol is converted into cholic acid. As
body is to form cholic acid in the liver. As much as 80
Specific Uses of Cholesterol in the Body.
substances.
These effects are probably caused mainly by
blood cholesterol concentration, whereas excess
Lack of insulin
present-day dietary strategy.
mechanism of this effect is unknown, despite the
concentration a slight to moderate amount. The
3. Ingestion of fat containing highly
important, if not more important, to maintain a diet
cholesterol concentration, it is usually just as
of cholesterol. Therefore, to decrease the blood
results from increased fat deposition in the liver,
cholesterol concentration 15 to 25 per cent. This
2. A
markedly.
diet, although the response of individuals differs
in plasma cholesterol concentration. As a result,
reductase, thus providing an intrinsic feedback
of cholesterol, 3-hydroxy-3-methylglutaryl CoA
slightly. However, when cholesterol is ingested,
each day
amount of cholesterol ingested
1. An increase in the
secreted by the adrenal cortex, the ovaries, and the
in the liver; and (3) many important steroid hormones
(2) cholic acid, which is the basis of the bile acids formed
acetyl-CoA. In turn, the sterol nucleus can be modi
This is synthesized entirely from multiple molecules of
The basic structure of cholesterol is a sterol nucleus.
tially composed of this substance.
some cholesterol, which is consistent with the fact that
Metabolism and Temperature Regulation
848
Unit XIII
many of the membranous structures of all cells are par-
fied
by means of various side chains to form (1) cholesterol;
testes (these hormones are discussed in later chapters).
Factors That Affect Plasma Cholesterol Concentration—Feed-
back Control of Body Cholesterol.
Among the important
factors that affect plasma cholesterol concentration are
the following:
increases the plasma concentration
the rising concentration of cholesterol inhibits the
most essential enzyme for endogenous synthesis
control system to prevent an excessive increase
plasma cholesterol concentration usually is not
changed upward or downward more than
±15 per
cent by altering the amount of cholesterol in the
highly saturated fat diet increases blood
which then provides increased quantities of
acetyl-CoA in the liver cells for the production
low in saturated fat as to maintain a diet low in
cholesterol.
unsaturated fatty
acids usually depresses the blood cholesterol
fact that this observation is the basis of much
4.
or thyroid hormone increases the
thyroid hormone decreases the concentration.
changes in the degree of activation of specific
enzymes responsible for the metabolism of lipid
By far the most
abundant nonmembranous use of cholesterol in the
A small quantity of cholesterol is used by (1) the
adrenal glands to form
(2)
the ovaries to form progesterone and estrogen, and (3)
the testes to form
also synthesize their own sterols and then form hor-
A large amount of cholesterol is precipitated in the
soluble substances and to the action of many chemical
highly inert to acids and to many solvents that might
substances help prevent water evaporation from the
can be 5 to 10 liters per day (as occurs in burn patients
who have lost their skin) instead of the usual 300 to
Phospholipids and Cholesterol—
Especially for Membranes
cholesterol are of only minor importance in comparison
phospholipids and cholesterol are present in both the
cell membrane and the membranes of the internal
ratio
of membrane cholesterol to phospholipids is especially
important in determining the fluidity of the cell
substances in the body that are not soluble in water
(besides the inorganic substances of bone) are the lipids
everywhere in the body is based mainly on phospho-
polar charges on the phospholipids also reduce the
interfacial tension between the cell membranes and
the surrounding fl
Another fact that indicates the importance of phos-
pholipids and cholesterol for the formation of structural
elements of the cells is the slow turnover rates of
these substances in most nonhepatic tissues—turnover
function in brain cells to provide memory processes
is related mainly to their indestructible physical
Atherosclerosis is a disease of the large and intermedi-
ate-sized arteries in which fatty lesions called athero-
matous plaques develop on the inside surfaces of the
Arteriosclerosis
term that refers to thickened and stiffened blood vessels
One abnormality that can be measured very early
in blood vessels that later become atherosclerotic is
damage to the vascular endothelium
increases the expression of adhesion molecules on
endothelial cells and decreases their ability to release
nitric oxide and other substances that help prevent
(mostly low-density lipoproteins) begin to accumulate
–6A
intima
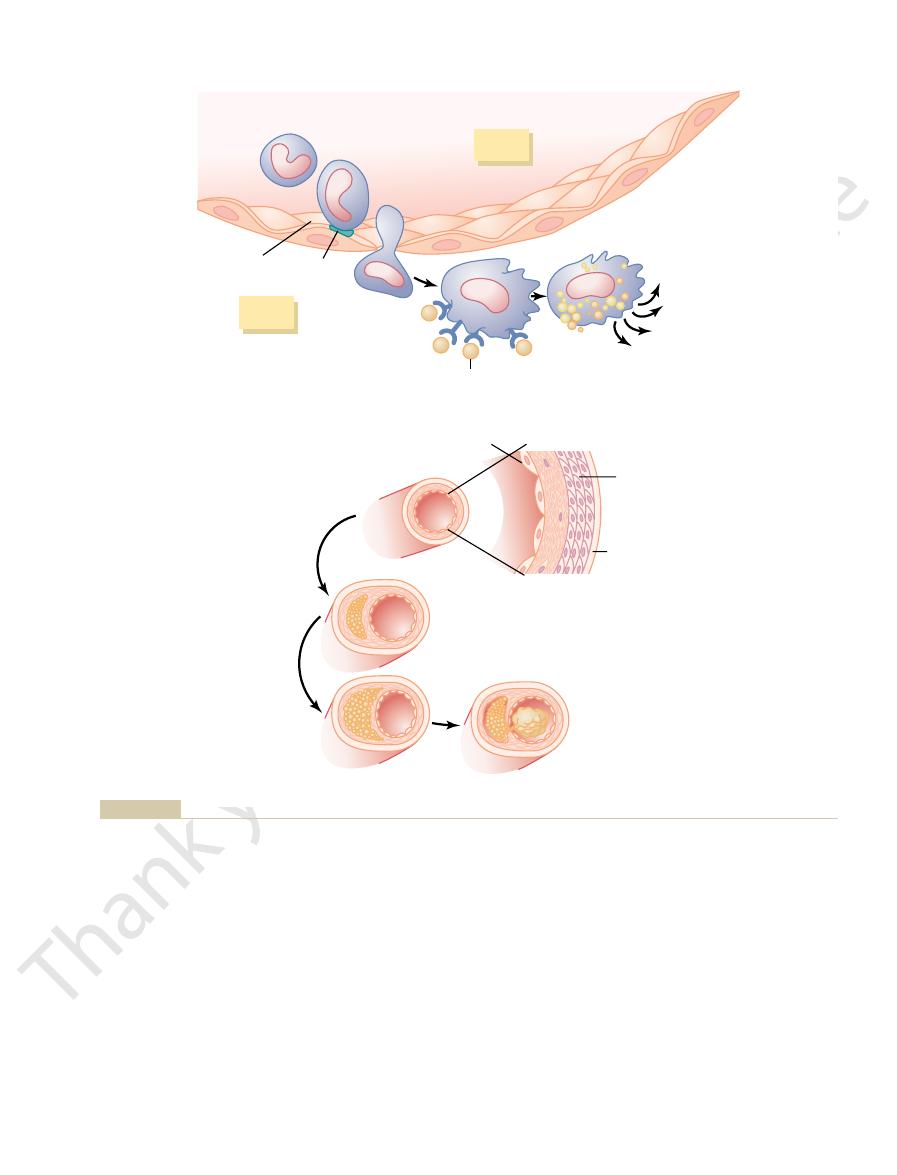
Chapter 68
Lipid Metabolism
849
Adhesion
molecule
Damaged
endothelium
Lipoprotein
particle
Normal
artery
Lipid
droplets
Macrophage
foam cell
Growth/
inflammatory
factors
Receptor
Arterial
intima
Arterial
lumen
Blood monocyte
Monocyte
adhered to
epithelium
Monocyte
migrating
into intima
Intima
Endothelium
Media
Smooth
muscle
cells
Adventitia
Thrombosis
of a ruptured
plaque
Large
plaque
Small
plaque
A
B
that can make the arteries rigid tubes. Both of these
lipids of the plaques, leading to bony-hard calci
arteries become stiff and unyielding. Still later, calcium
without occlusion, the
ow, sometimes completely occluding the vessel. Even
arterial wall. The lipid deposits plus the cellular prolif-
). Also, the macrophages release substances that
proliferate to form larger and larger plaques (see Figure
With time, the fatty streaks grow larger and coalesce,
giving the macrophages a foamlike appearance. These
ingest and oxidize the accumulated lipoproteins,
macrophages,
ulate and form a thrombus. (Modified from Libby P: Inflammation in atherosclerosis. Nature 420:868, 2002.)
ry to coag-
to grow larger and accumulate lipids. Eventually, the plaque could occlude the vessel or rupture, causing the blood in the arte
Additional accumulation of macrophages and growth of the intima cause the plaque
cause inflammation and growth of the intimal layer.
macrophage then ingests and oxidizes lipoprotein molecules, becoming a macrophage foam cell. The foam cells release substances
The monocyte then migrates through the endothelium into the intimal layer of the arterial wall and is transformed into a macrop
Attachment of a monocyte to an adhesion molecule on a damaged endothelial cell of an artery.
Development of atherosclerotic plaque.
Figure 68–6
A,
hage. The
that
B,
differentiate to become
which then
macrophage foam cells then aggregate on the blood
vessel and form a visible fatty streak.
and the surrounding fibrous and smooth muscle tissues
68–6B
cause inflammation and further proliferation of smooth
muscle and fibrous tissue on the inside surfaces of the
eration can become so large that the plaque bulges into
the lumen of the artery and greatly reduces blood
fl
fibroblasts of the plaque eventu-
ally deposit extensive amounts of dense connective
tissue; sclerosis (fibrosis) becomes so great that the
salts often precipitate with the cholesterol and other
fications

atherosclerosis. Most of the cholesterol formed in the
diabetes develops; and (4) avoiding cigarette smoking.
if hypertension does develop; (3) effectively controlling
healthy diet and being physically active, or effectively
content; (2) preventing hypertension by maintaining a
weight, being physically active, and eating a diet that
The most important measures to protect against the
Prevention of Atherosclerosis
the incidence of atherosclerosis. The precise mecha-
density lipoprotein called lipoprotein(a), containing an
icals in the blood that damage the vessel walls. About
can lead to atherosclerosis, perhaps by forming free rad-
To add to the complexity of atherosclerosis, experi-
in the plasma. Others, such as hypertension, lead to
might be protective.
atherogenic or, conversely, that female sex hormones
rable age, suggesting that male sex hormones might be
In early and middle adulthood, men are more likely
stroke, and kidney disease.
atherosclerosis, which in turn may lead to heart attack,
do occur together, greatly increasing their risk for
overweight and obese patients, these three risk factors
increase the risk of developing atherosclerosis. In many
artery disease is increased almost 20-fold, suggesting
are all present, the risk for atherosclerotic coronary
when hypertension, diabetes mellitus, and hyperlipemia
artery disease is increased by more than eightfold. And
diabetes mellitus occur together, the risk for coronary
oping coronary artery disease. When hypertension and
on average, more than a twofold increased risk of devel-
twofold. Likewise, a person with diabetes mellitus has,
Hypertension, for example, increases the risk for
cigarette smoking.
and (5)
diabetes mellitus,
obesity,
terol and lipoproteins, atherosclerosis still develops.
high-density to low-density lipoproteins, the likelihood
Consequently, when a person has a high
protect against the development of atherosclerosis.
nism is true or not, high-density lipoproteins do help
to be deposited in arterial walls. Whether this mecha-
teins. It is believed that high-density lipoproteins can
rotic blockage of blood vessels throughout the body.
to 1000 mg/dl, levels that are four to six times normal.
plasma increases immensely.
plasma cholesterol. As a result, the number of very low
rampage, producing new cholesterol; it is no longer
or low-density lipoproteins. Without this absorption, the
tors, the liver cannot absorb either intermediate-density
s cells. In the absence of these recep-
This is a disease in which
throughout their arterial systems.
because of their vegetarian diet. Simply feeding these
An interesting example occurs in rabbits, which
levels of low-density lipoproteins.
extent, eating excess cholesterol may also raise plasma
the daily diet, obesity, and physical inactivity. To a lesser
several factors, including eating highly saturated fat in
lipoproteins. The plasma concentration of these high-
The Roles of Cholesterol
Basic Causes of Atherosclerosis
also the kidneys, liver, gastrointestinal tract, limbs, and
of the body, especially the brain (causing strokes), but
coronary arteries. The remaining one third are caused
Europe are due to vascular disease. About two thirds of
ow in the artery.
bus or embolus formation (see Chapter 36), leading to
can cause blood clots to develop, with resultant throm-
owing blood, their rough surfaces
walls, they are easily ruptured. Also, where the plaques
bility, and because of the degenerative areas in their
arteries.
Metabolism and Temperature Regulation
850
Unit XIII
later stages of the disease are called “hardening of the
”
Atherosclerotic arteries lose most of their distensi-
protrude into the fl
a sudden blockage of all blood fl
Almost half of all deaths in the United States and
these deaths are caused by thrombosis of one or more
by thrombosis or hemorrhage of vessels in other organs
so forth.
—
and Lipoproteins
Increased Low-Density Lipoproteins.
An important factor
in causing atherosclerosis is a high blood plasma con-
centration of cholesterol in the form of low-density
cholesterol low-density lipoproteins is increased by
normally have low plasma cholesterol concentrations
animals large quantities of cholesterol as part of their
daily diet leads to serious atherosclerotic plaques
Familial Hypercholesterolemia.
the person inherits defective genes for the formation of
low-density lipoprotein receptors on the membrane sur-
faces of the body’
cholesterol machinery of the liver cells goes on a
responsive to the feedback inhibition of too much
density lipoproteins released by the liver into the
Patients with full-blown familial hypercholes-
terolemia have blood cholesterol concentrations of 600
Many of these people die before age 20 because of
myocardial infarction or other sequelae of atheroscle-
Role of High-Density Lipoproteins in Preventing Atherosclerosis.
Much less is known about the function of high-density
lipoproteins compared with that of low-density lipopro-
actually absorb cholesterol crystals that are beginning
ratio of
of developing atherosclerosis is greatly reduced.
Other Major Risk Factors
for Atherosclerosis
In some people with perfectly normal levels of choles-
Some of the factors that are known to predispose to ath-
erosclerosis are (1) physical inactivity and
(2)
(3) hypertension, (4) hyperlipidemia,
atherosclerotic coronary artery disease by at least
that these factors interact in a synergistic manner to
to develop atherosclerosis than are women of compa-
Some of these factors cause atherosclerosis by
increasing the concentration of low-density lipoproteins
atherosclerosis by causing damage to the vascular en-
dothelium and other changes in the vascular tissues that
predispose to cholesterol deposition.
mental studies suggest that excess blood levels of iron
one quarter of all people have a special type of low-
additional protein, apoprotein(a), that almost doubles
nisms of these atherogenic effects have yet to be
discovered.
development of atherosclerosis and its progression to
serious vascular disease are (1) maintaining a healthy
contains mainly unsaturated fat with a low cholesterol
controlling blood pressure with antihypertensive drugs
blood glucose with insulin treatment or other drugs if
Several types of drugs that lower plasma lipids and
cholesterol have proved to be valuable in preventing
liver is converted into bile acids and secreted in this

Rev Physiol 65:333, 2003.
Unger RH: The physiology of cellular liporegulation. Annu
exercise. Acta Physiol Scand 178:443, 2003.
Spriet LL, Watt MJ: Regulatory mechanisms in the interac-
agents? Circulation 109(21 Suppl 1):II18, 2004.
CoA reductase inhibitors: statins as antiin
ammation, immunity, and HMG-
Schonbeck U, Libby P: In
144:5166, 2003.
activated protein kinase, and adiposity. Endocrinology
Ruderman NB, Saha AK, Kraegen EW: Malonyl CoA, AMP-
human skeletal muscle. News Physiol Sci 19:92, 2004.
Roden M: How free fatty acids inhibit glucose utilization in
orders. Annu Rev Physiol 64:477, 2002.
Rinaldo P, Matern D, Bennett MJ: Fatty acid oxidation dis-
Physiol Rev 83:1069, 2003.
Osterud B, Bjorklid E: Role of monocytes in atherogenesis.
ammation in atherosclerosis. Nature 420:868,
Libby P: In
6A):3S, 2004.
for management of dyslipidemia. Am J Med 116(Suppl
LaRosa JC, Gotto AM Jr: Past, present, and future standards
thesis in the liver. J Clin Invest 109:1125, 2002.
Horton JD, Goldstein JL, Brown MS: SREBPs: activators of
Annu Rev Physiol 65:697, 2003.
Hilgemann DW: Getting ready for the decade of the lipids.
Biol 24:213, 2004.
lipoproteins: where the action is.Arterioscler Thromb Vasc
Havel RJ, Hamilton RL: Hepatic catabolism of remnant
go: hormone-sensitive lipase. Curr Opin Lipidol 14:289,
Haemmerle G, Zimmermann R, Zechner R: Letting lipids
109:433, 2004.
nition. Circulation
and Blood Institute/American Heart Association confer-
metabolic syndrome: report of the National Heart, Lung,
Grundy SM, Brewer HB Jr, Cleeman JI, et al: De
Coll Cardiol 43:717, 2004.
heart disease: a working group report and update. J Am
Gotto AM Jr, Brinton EA: Assessing low levels of high-
30:121, 2004.
of mitochondrial dysfunction in NASH. Diabetes Metab
Fromenty B, Robin MA, Igoudjil A, et al: The ins and outs
Lipidol 14:389, 2003.
Fielding BA, Frayn KN: Lipid metabolism. Curr Opin
folded proteins. Lancet 363:1139, 2004.
ammation, cholesterol, and mis-
s disease: in
Casserly I, Topol E: Convergence of atherosclerosis and
blood. Proc Natl Acad Sci U S A 96:11041, 1999.
trols the cholesterol content of membranes, cells, and
Brown MS, Goldstein JL: A proteolytic pathway that con-
density lipoproteins. Annu Rev Med 54:321, 2003.
Assmann G, Nofer JR: Atheroprotective effects of high-
heart attacks.
ity from atherosclerotic heart disease. Therefore, appro-
plasma, there is about a 2 per cent decrease in mortal-
In general, studies show that for each 1 mg/dl
who have increased plasma cholesterol levels.
tion. These drugs are now widely used to treat patients
atherosclerosis, such as attenuating vascular in
in plasma levels of low-density lipoproteins. The statins
the liver, usually causing a 25 to 50 per cent reduction
of cholesterol. This inhibition decreases cholesterol syn-
, a rate-limiting enzyme in the synthesis
hydroxymethylglutaryl-coenzyme A (HMG-
tion, thereby reducing cholesterol synthesis by the liver.
atherogenic plaques.
stituent of many breakfast cereals, increases the pro-
lesterol to be converted into new bile acids. Thus, simply
circulating blood. This causes far more of the liver cho-
and used over and over again in the bile. Therefore, any
form into the duodenum; then, more than 90 per cent of
Chapter 68
Lipid Metabolism
851
these same bile acids is reabsorbed in the terminal ileum
agent that combines with the bile acids in the gastroin-
testinal tract and prevents their reabsorption into the
circulation can decrease the total bile acid pool in the
eating oat bran, which binds bile acids and is a con-
portion of liver cholesterol that forms new bile acids
rather than forming new low-density lipoproteins and
Resin agents can also be used to
bind bile acids in the gut and increase their fecal excre-
Another group of drugs called statins competitively
inhibits
CoA) reductase
thesis and increases low-density lipoprotein receptors in
may also have other beneficial effects that help prevent
flamma-
decrease in low-density lipoprotein cholesterol in the
priate preventive measures are valuable in decreasing
References
Alzheimer’
fl
density lipoprotein cholesterol as a risk factor in coronary
finition of
ence on scientific issues related to defi
2003.
the complete program of cholesterol and fatty acid syn-
fl
2002.
fl
flammatory
tion between carbohydrate and lipid oxidation during
