
Chapter 13
Cardiovascular
system

Ch.13 - Part 1
Heart development
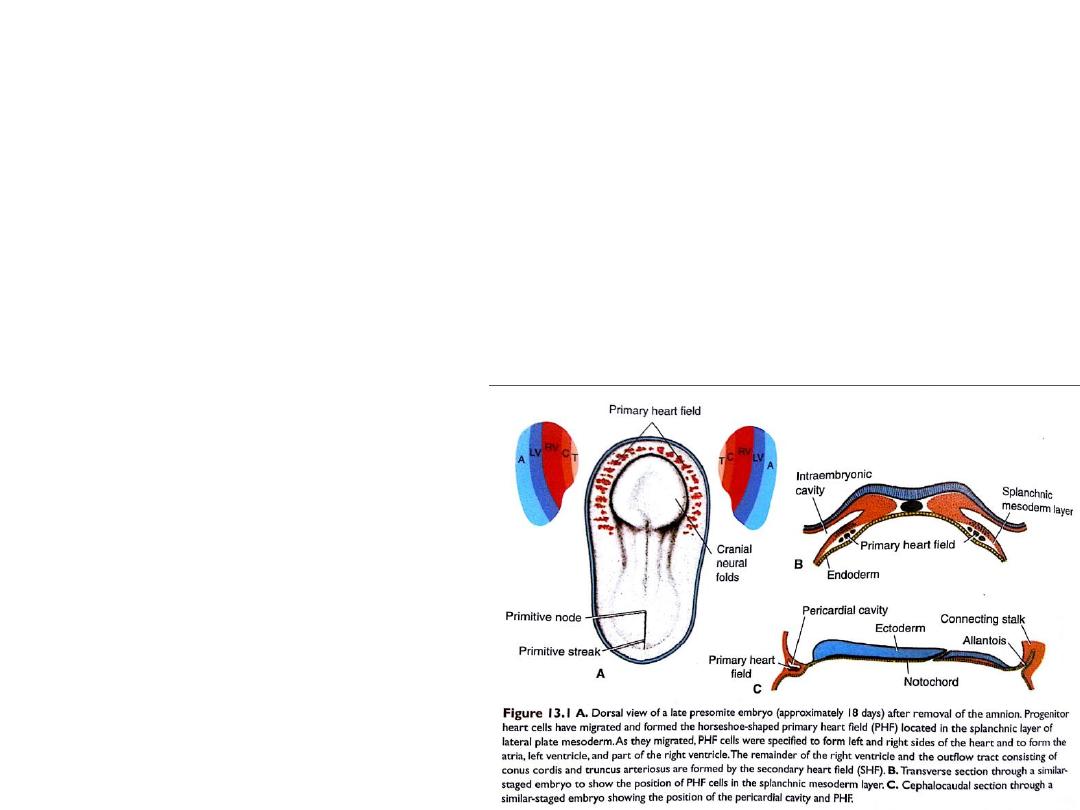
Establishment and patterning of the primary heart field
• The vascular system appears in the middle of the third week, when the embryo is
no longer able to satisfy its nutritional requirements by diffusion alone.
Progenitor heart cells lie in the epiblast, immediately near the cranial end
of the primitive streak.
From there, they migrate through the streak and into the splanchnic layer of
lateral plate mesoderm where they form a horseshoe-shaped cluster of cells
called: the primary heart field (PHF), cranial to the neural folds.
Migration of Progenitor heart cells
to form the PHF at days 16-18
They are specified on both sides
to become: atria, left ventricle
and most of right ventricle.
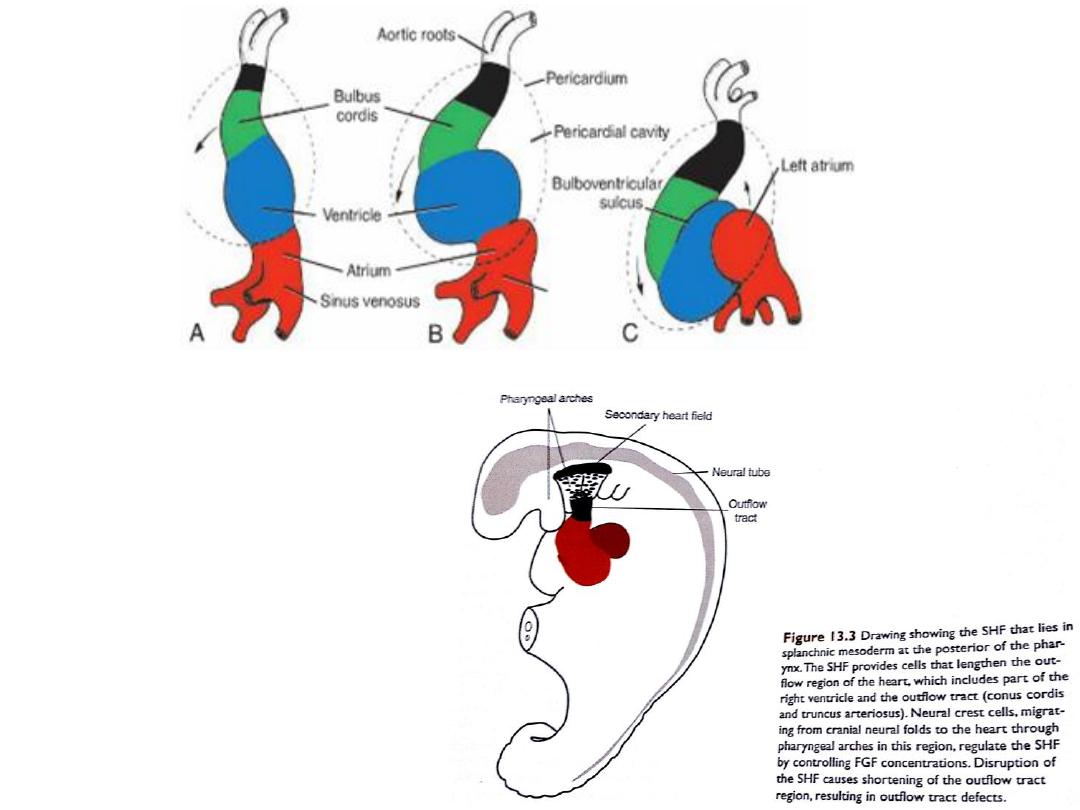
• The secondary heart field
(SHF): is the origin of the
remainder of the heart: part of
right ventricle, and outflow
tract (conus cordis & truncus
arteriosus).
• The SHF appears later (days 20-
21) in splanchnic mesoderm
ventral to posterior pharynx
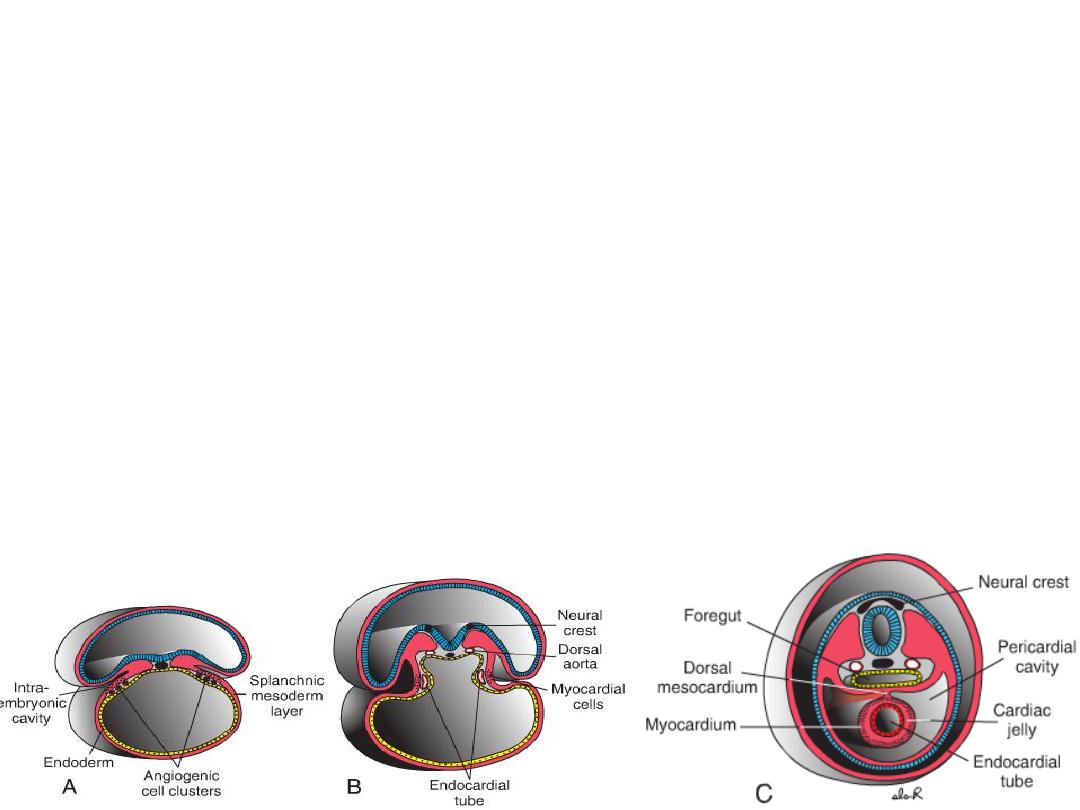
The PHF cells are induced by the underlying pharyngeal endoderm to form
cardiac myoblasts and blood islands that will form blood cells and
vessels by the process of vasculogenesis.
• With time, the islands unite and form a horseshoe-shaped endothelial-lined
tube (heart tube) surrounded by myoblasts.
• This region is the cardiogenic region, the intraembryonic cavity over it later
develops into the pericardial cavity.
• In addition to the cardiogenic region, other blood islands appear bilaterally,
parallel, and close to the midline of the embryonic shield. These islands
form a pair of longitudinal vessels, the dorsal aortae.
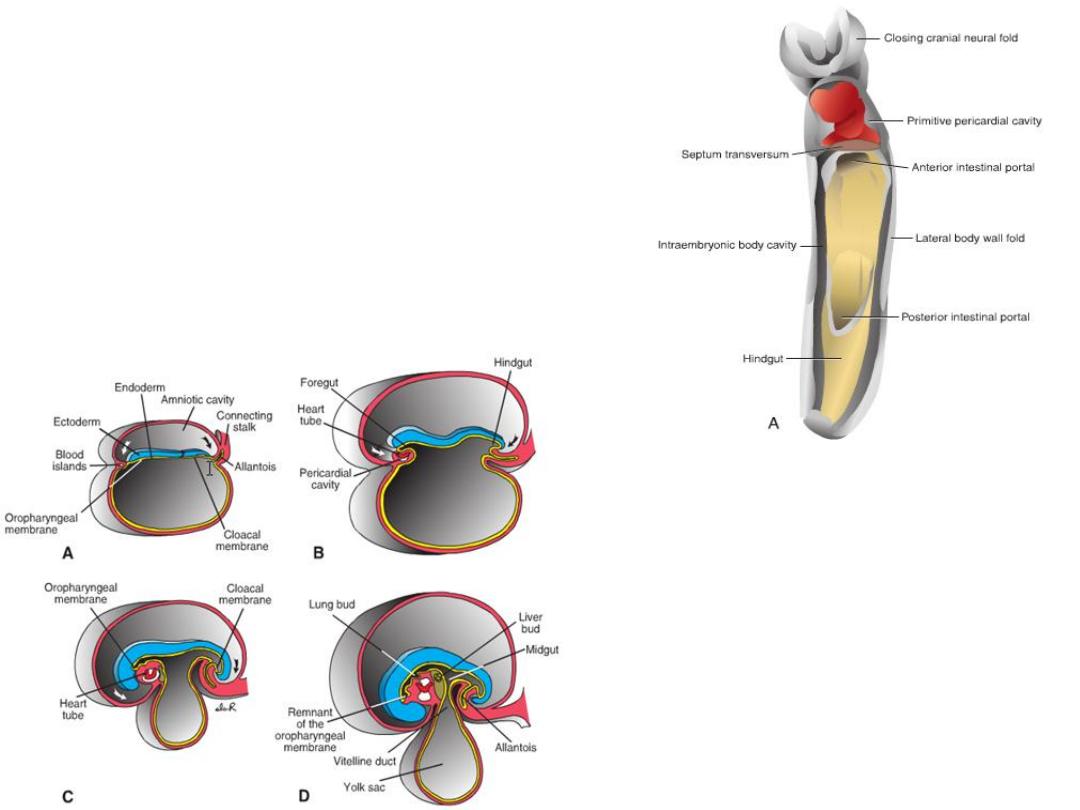
Thus, the heart becomes a continuous
expanded tube consisting of an inner
endothelial lining and an outer
myocardial layer.
It receives venous drainage at its caudal
pole and begins to pump blood out of
the first aortic arch into the dorsal aorta
at its cranial pole.
POSITION OF THE HEART TUBE
Cephalocaudal folding
– oropharyngeal membrane
pulled forward
– heart and pericardial cavity
move to the cervical region
and finally to the thorax.
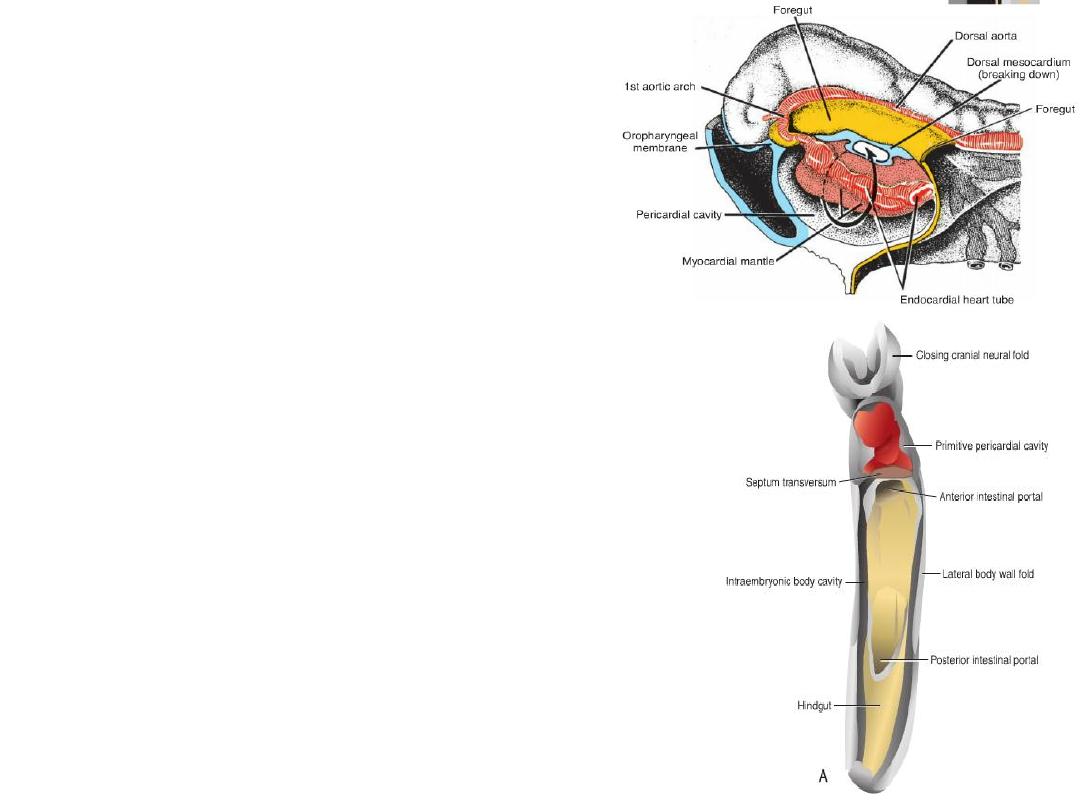
Dorsal mesocardium = attachment to the
dorsal side of the pericardial cavity, no
ventral mesocardium
Dorsal mesocardium disappears =
transverse pericardial sinus
Heart suspended in pericardial cavity by
cranial & caudal vessels
Origin of epicardium
Epicardium = from mesothelial cells on
the surface of the septum
transversum.
Epicardium Coronary arteries:
endothelium & smooth muscles
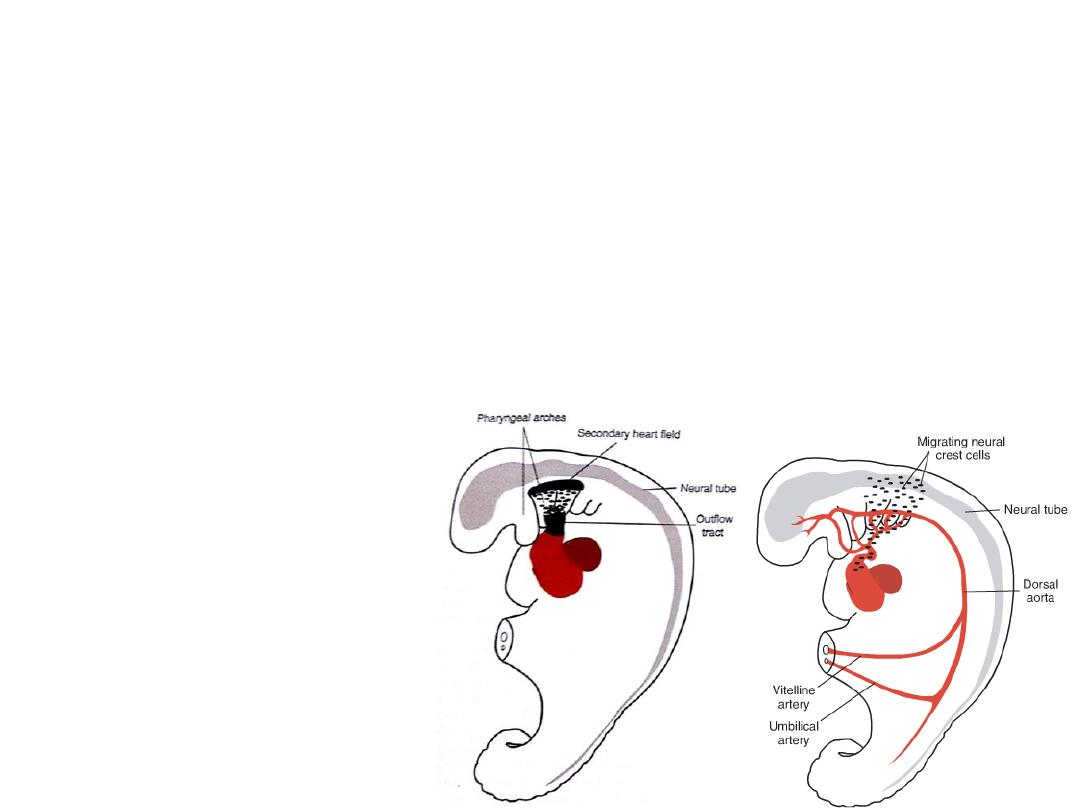
Formation of the cardiac loop
• The heart tube elongates as cells are added from the SHF to its cranial end
• This lengthening is important for formation of part of right ventricle,
outflow tract and for looping process
• If lengthening is inhibited outflow tract defects:
– VSD
– Tetralogy of Fallot
– Pulmonary atresia
– Pulmonary stenosis
• The SHF is regulated by neural
crest cells
• Neural crest cells pass nearby
SHF in the pharyngeal arches
as they migrate from hind
brain to septate the outflow
tract
Parts of the cardiac loop
1.
Atrial portion (common atrium)
2.
Early embryonic ventricle (left
ventricle)
3.
Bulbus cordis
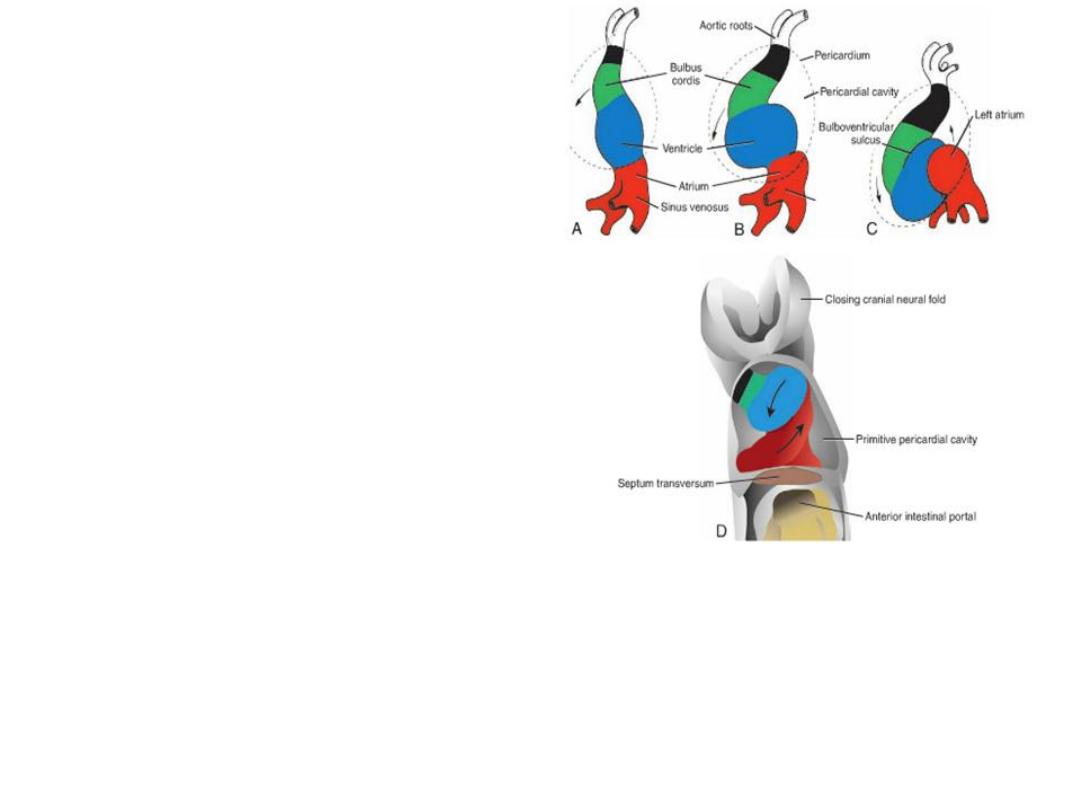
FORMATION OF THE CARDIAC LOOP
• Heart tube begins to loop at day 23
• Heart loop
– cranial end grows vertically and to the
right
– caudal end grows dorsally and to the left
– helps distinguish atria, ventricles, and
outflow tract as these regions expand.
• Looping is completed by day 28
• Common atrium = shifts inside pericardial cavity
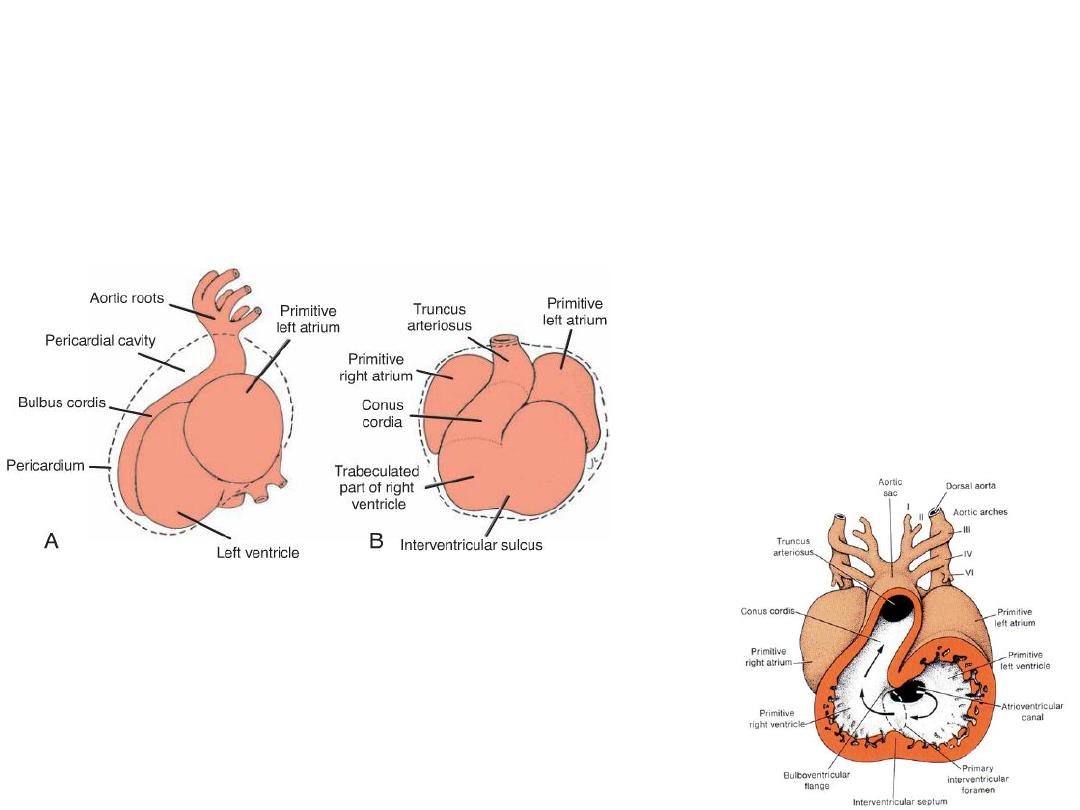
The bulbus cordis
• Proximal third = trabeculated part of right ventricle
• The mid portion = conus cordis = outflow tracts of both ventricles.
• The distal part = truncus arteriosus = roots and proximal portion of the aorta
and pulmonary artery.
• the atrioventricular canal , connects the common
atrium and the early embryonic ventricle .
Abnormalities
of Cardiac Looping
• Dextrocardia: in which the heart lies on the right side of the thorax instead of the
left
• Occurs when the heart loops to the left instead of the right.
• Induced during gastrulation when laterality is established, or later when cardiac
looping occurs.
• Dextrocardia occurs with situs inversus: a complete reversal of asymmetry in all
organs.
• Or may be associated with laterality sequences in which only some organ positions
are reversed.
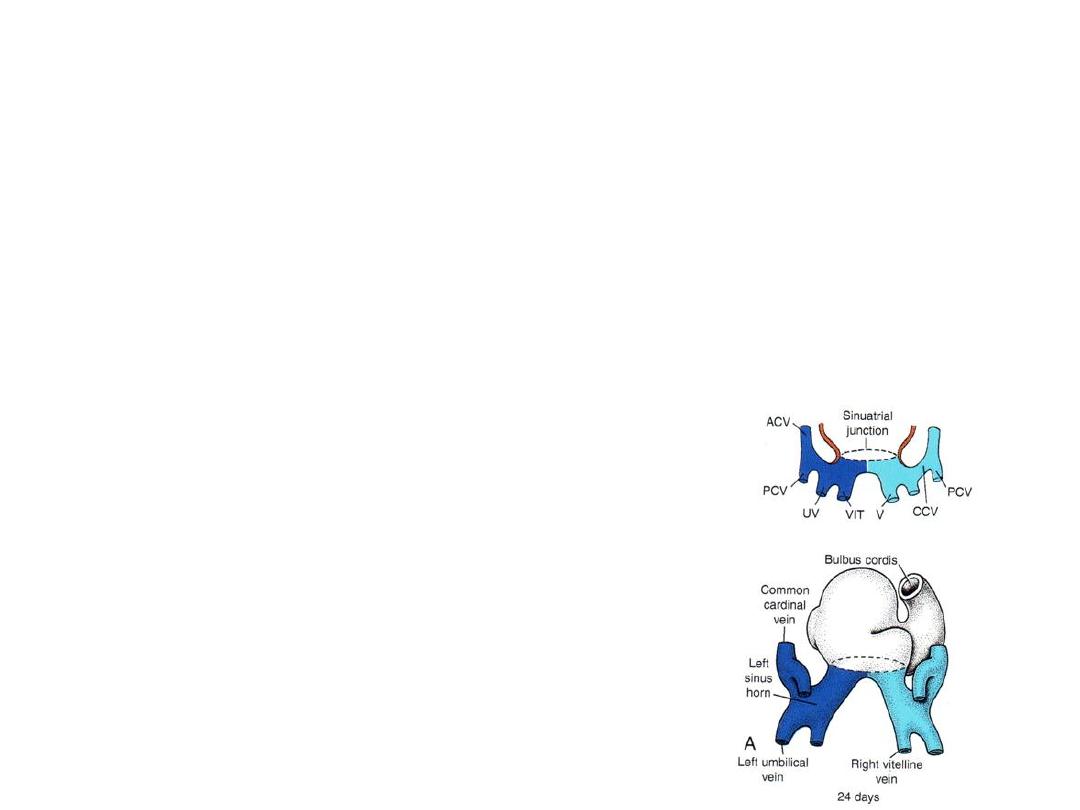
Development of Sinus venosus
• In middle of 4
th
week sinus venosus
receives blood from right and left
sinus horns
• Each horn receives blood from
1.
vitelline
2.
Umbilical
3.
common cardinal veins.
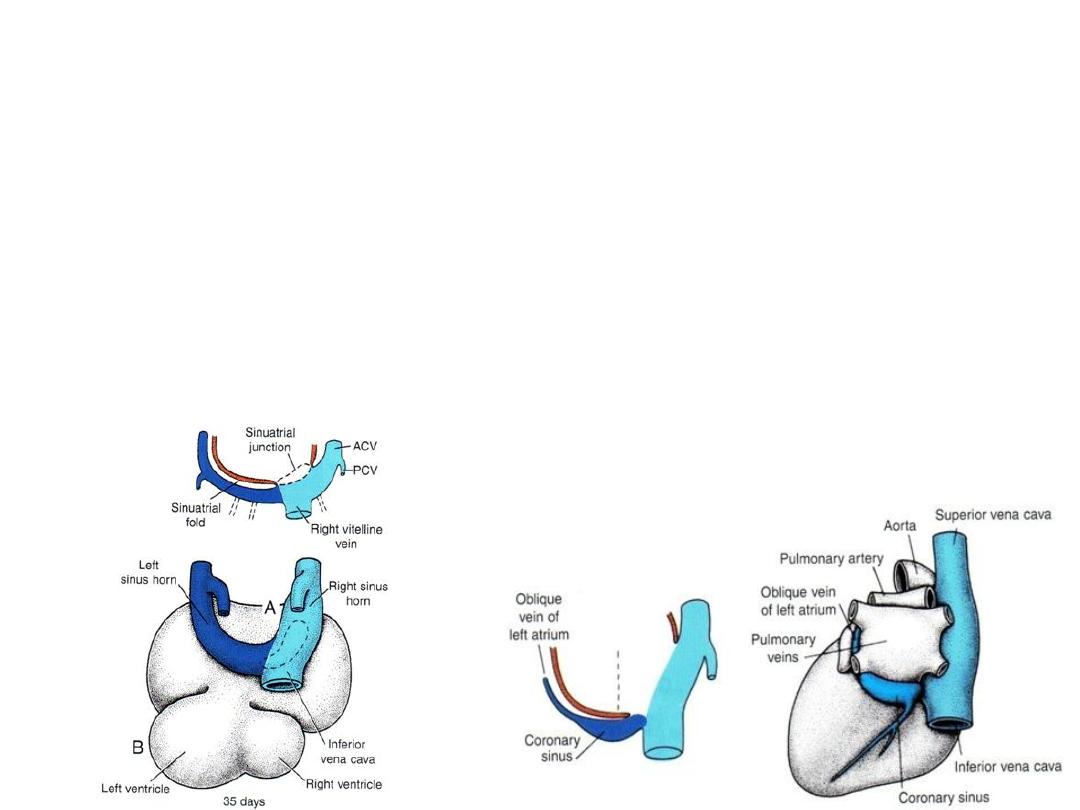
Left sinus horn: diminish
• Left-to-right shunts of blood in the venous system during 4
th
& 5
th
weeks
• Veins shift to right left sinus horn diminishes (remain only as oblique
vein of left atrium and the coronary sinus).
Right sinus horn: increase
• Left-to-right shunts of blood in the venous system during 4
th
& 5
th
weeks
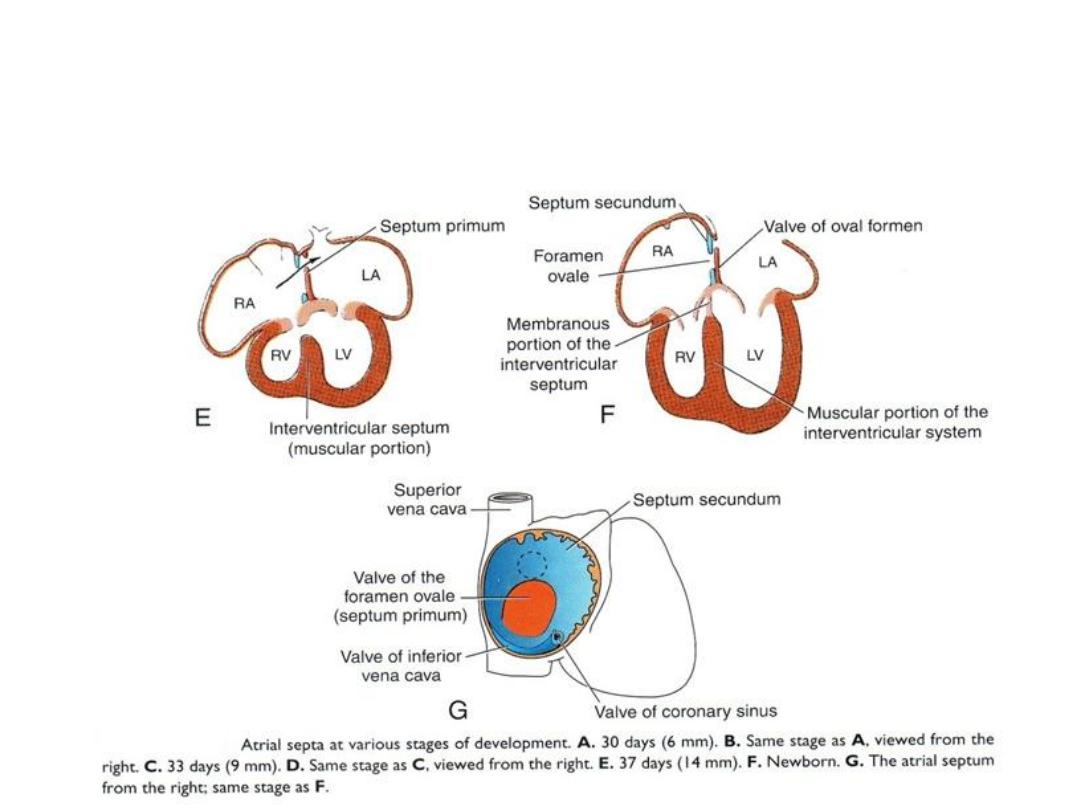
• Veins shift to rightright sinus horn increases in size
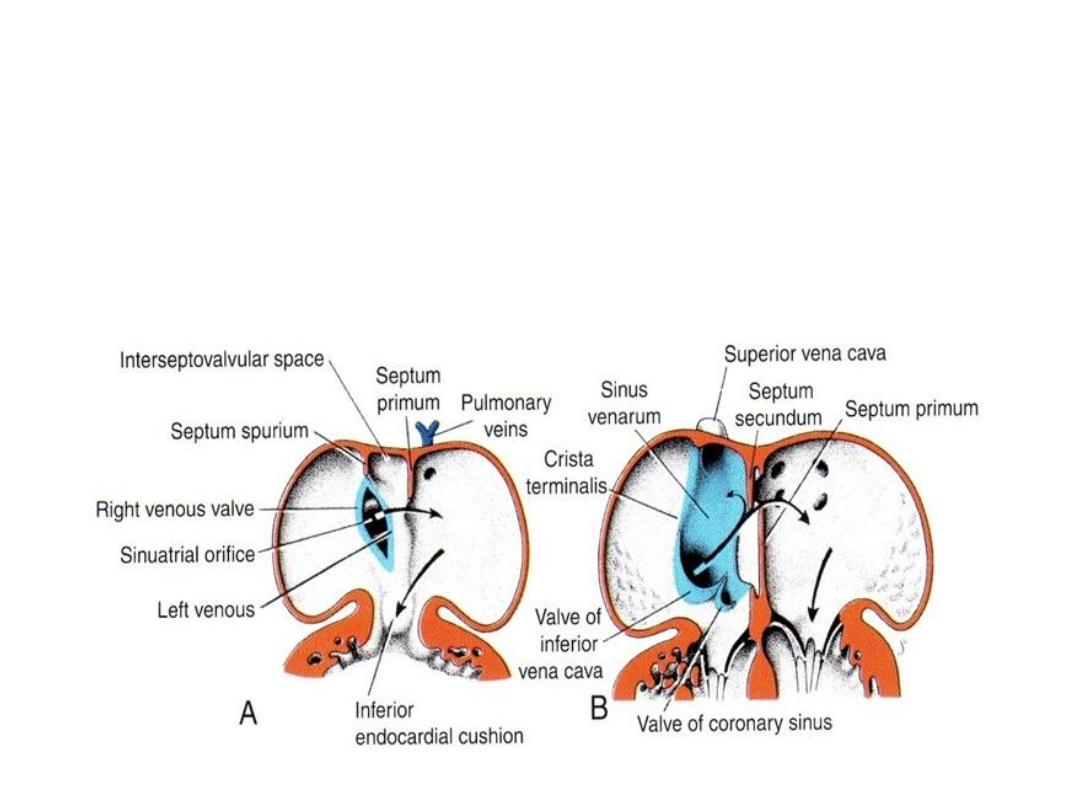
• Right sinus horn forms smooth-walled portion of the right atrium = sinus
venarum
• Trabeculated part of right atrium is derived from the original primitive
right atrium (right atrial appendage).
• Crista terminalis = dividing line between smooth [sinus venarum] and
trabeculated parts.
• Valves guard opening into smooth portion of right atrium = valve of the
inferior vena cava and valve of the coronary sinus.
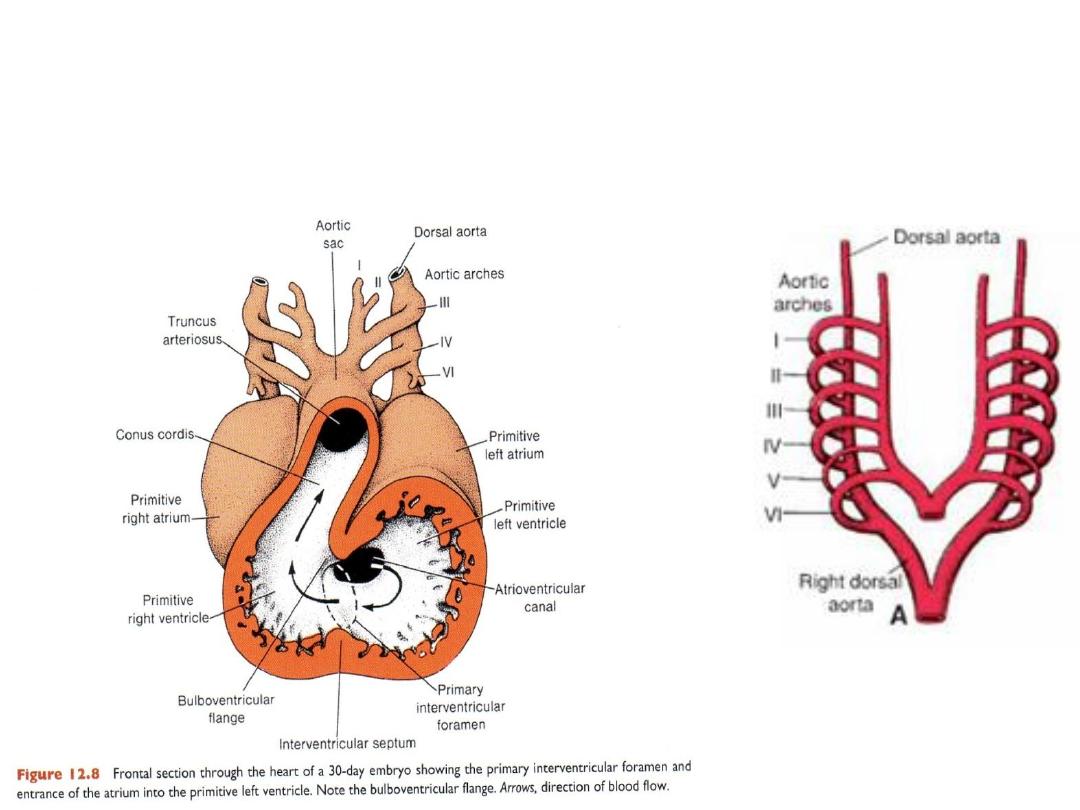
FORMATION OF THE CARDIAC SEPTA
• Endocardial cushions, develop in the atrioventricular and conotruncal regions.
• In these locations, they assist in formation of the atrial and
ventricular(membranous portion) septa, the atrioventricular canals and
valves, and the aortic and pulmonary channels.
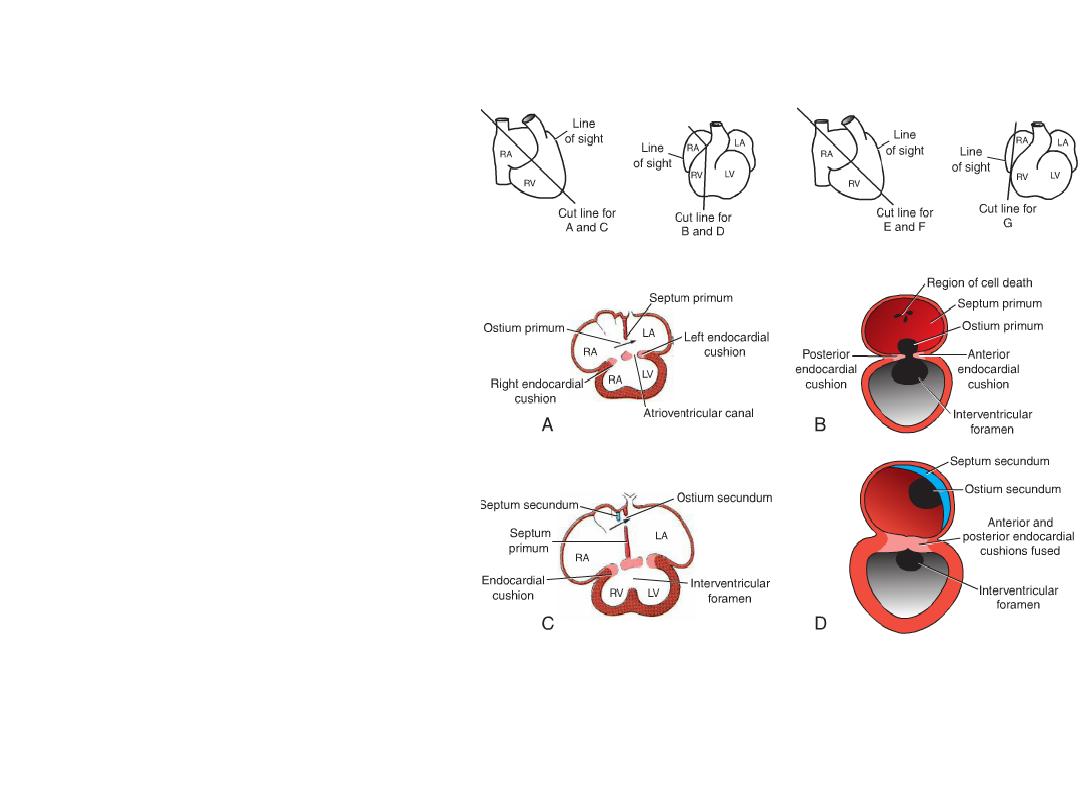
Septum Formation in the Common Atrium
• Atrial septum: septum primum
grows toward AV endocardial
cushions, but leaving ostium
primum.
• Closure of ostium primum
• Cell death in septum primum
creates a hole = ostium
secundum.
• Septum secundum grows toward
cushion, but never gets there =
forms valve over ostium
secundum = new opening =
foramen ovale.
• Septum primum = valve of the
foramen ovale.
• Probe patency of the foramen
ovale = valve does not close
completely at birth.
Septum Formation in the Common Atrium
Further Differentiation of the Atria
• Left atrium = smooth part = from
outgrowth of pulmonary veins
that later gets incorporated.
• Trabeculated left atrial
appendage = original left atrium.
• Right atrium= smooth part =
sinus venarum (right horn of the
sinus venosus).
• Trabeculated right atrial
appendage = original right atrium
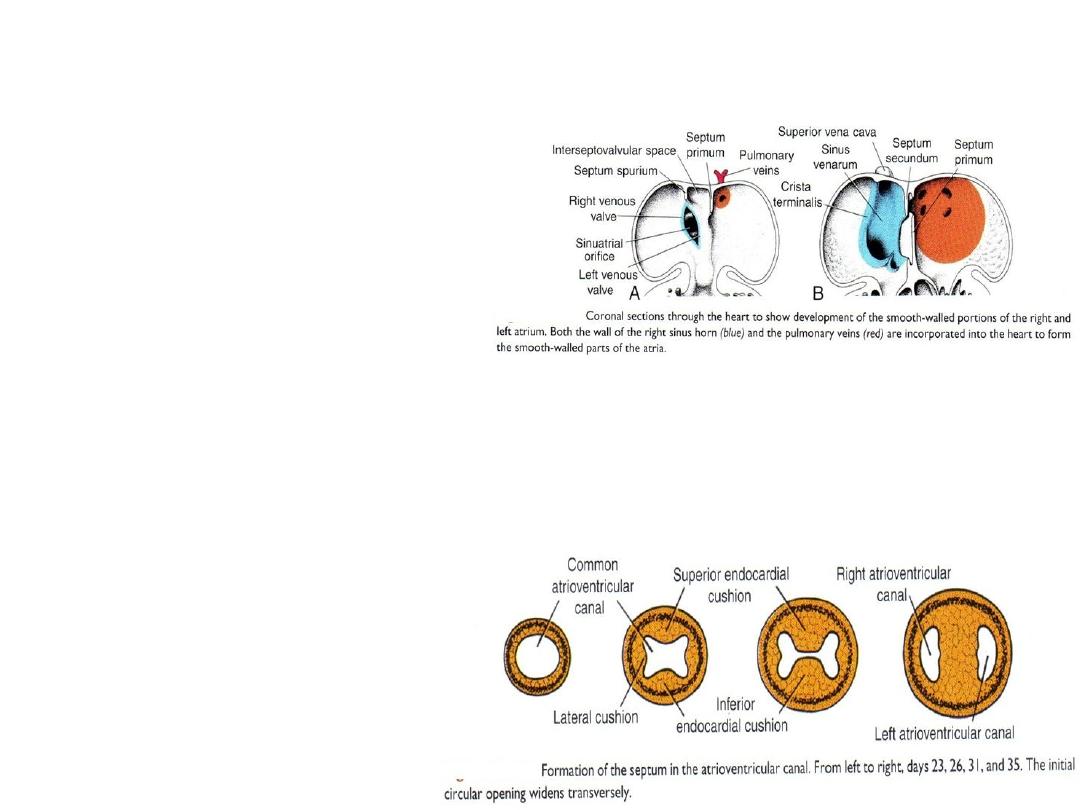
Septum Formation in the Atrioventricular Canal
• Atrioventricular junction
= canal = will be
separated into two
channels = right and left
AV canals.
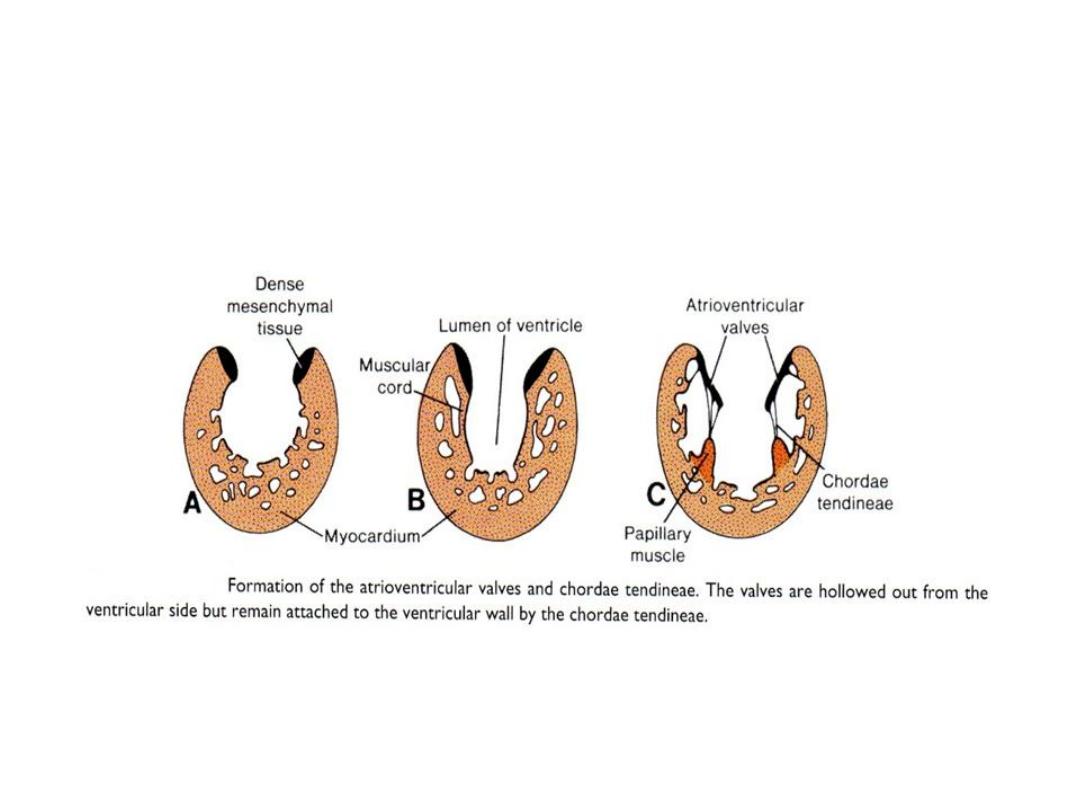
Atrioventricular Valves
• Each atrioventricular orifice is surrounded by local proliferations of
mesenchymal tissue which will transform into dense connective tissue.
• By effect of blood stream, valves and chordae tendineae will be formed.

Clinical Correlates
• Heart Defects: causes
1.
Chromosomal abnormalities:
trisomy 18.
genetic syndromes, including craniofacial abnormalities, such as
DiGeorge, Goldenhar, and Down syndromes.
2.
Environmental agents
3.
Genetic causes
4.
Multifactorial (environmental & genetic)
5.
Cardiovascular teratogens: rubella virus, thalidomide, retinoic acid
(accutane), alcohol, and many other compounds.
6.
Maternal diseases, such as insulin-dependent diabetes
7.
Mutations
Targets for genetic or teratogen-induced heart defects
1.
Heart progenitor cells from PHF & SHF
2.
Neural crest cells
3.
Endocardial cushions

Mutations
– mutations in the heart-specifying gene NKX2.5, on chromosome 5q35, can
produce atrial septal defects (secundum type), tetralogy of Fallot, and
atrioventricular conduction delays in an autosomal dominant fashion.
– Mutations in the TBX5 gene result in Holt-Oram syndrome, characterized
by preaxial (radial) limb abnormalities and atrial septal defects. Defects in
the muscular portion of the interventricular septum may also occur. Holt-
Oram syndrome is one of a group of heart-hand syndromes illustrating that
the same genes may participate in multiple developmental processes. For
example, TBX5 regulates forelimb development and plays a role in
septation of the heart. Holt-Oram syndrome is inherited as an autosomal
dominant trait with a frequency of 1/100,000 live births.
– Mutations in a number of genes regulating production of sarcomere
proteins cause hypertrophic-cardiomyopathy that may result in sudden
death in athletes and the general population. The disease is inherited as an
autosomal dominant. The result is cardiac hypertrophy due to disruption in
the organization of cardiac muscle cells (myocardial disarray) , which may
adversely affect cardiac output and/or conduction.
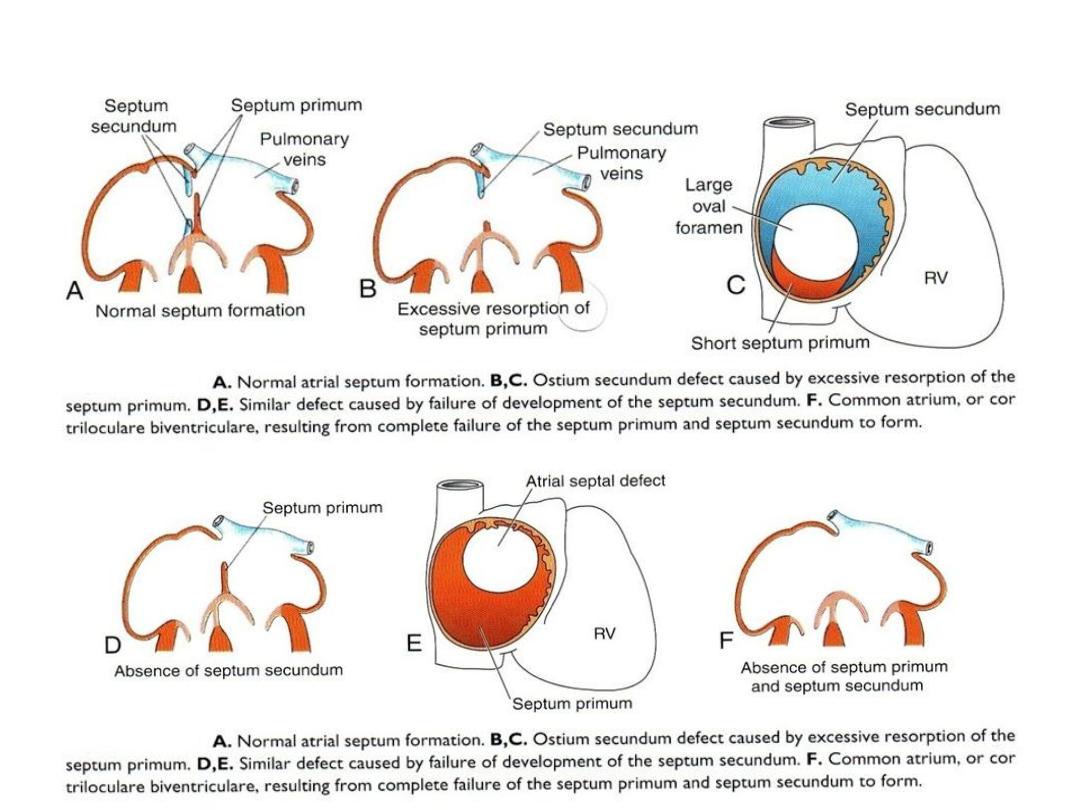
Atrial septal defect (ASD)
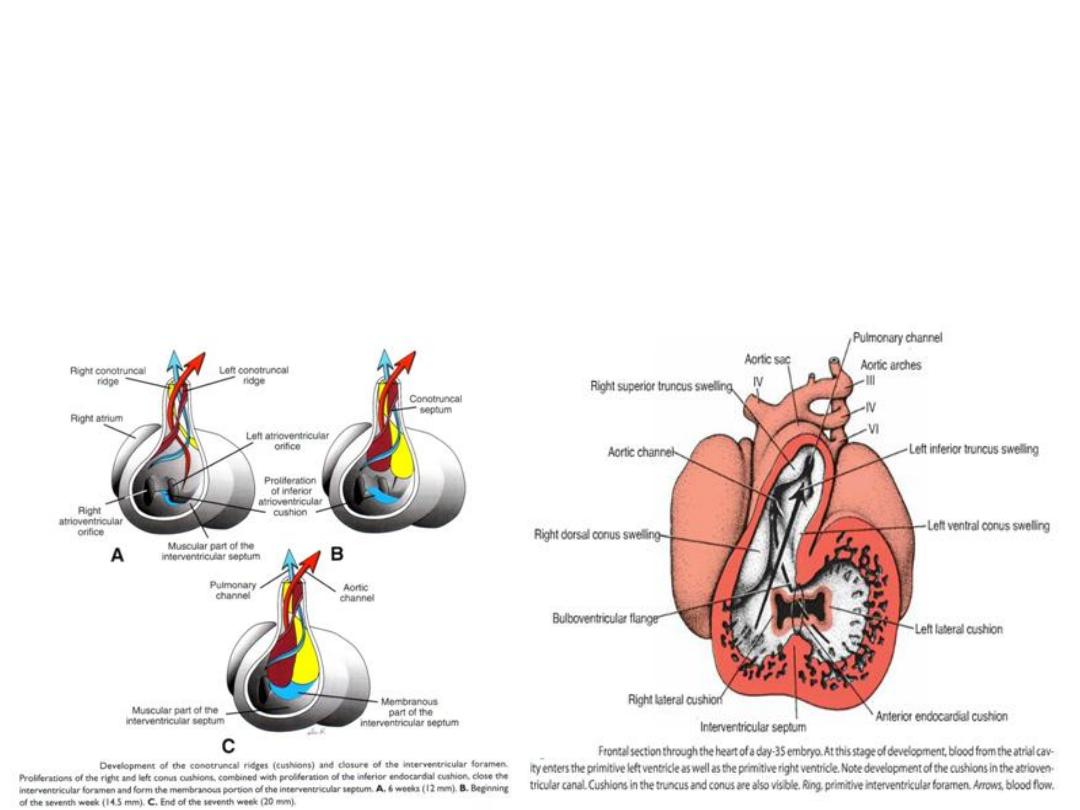
Septum formation in the truncus arteriosus and conus cordis
• In 5
th
week
• In truncus: truncus swellings (cushions)
• twist and fuse aortico-pulmonary septum
• Divide the truncus aortic & pulmonary channel
• In conus cordis: swellings outflow tracts of right & left ventricles
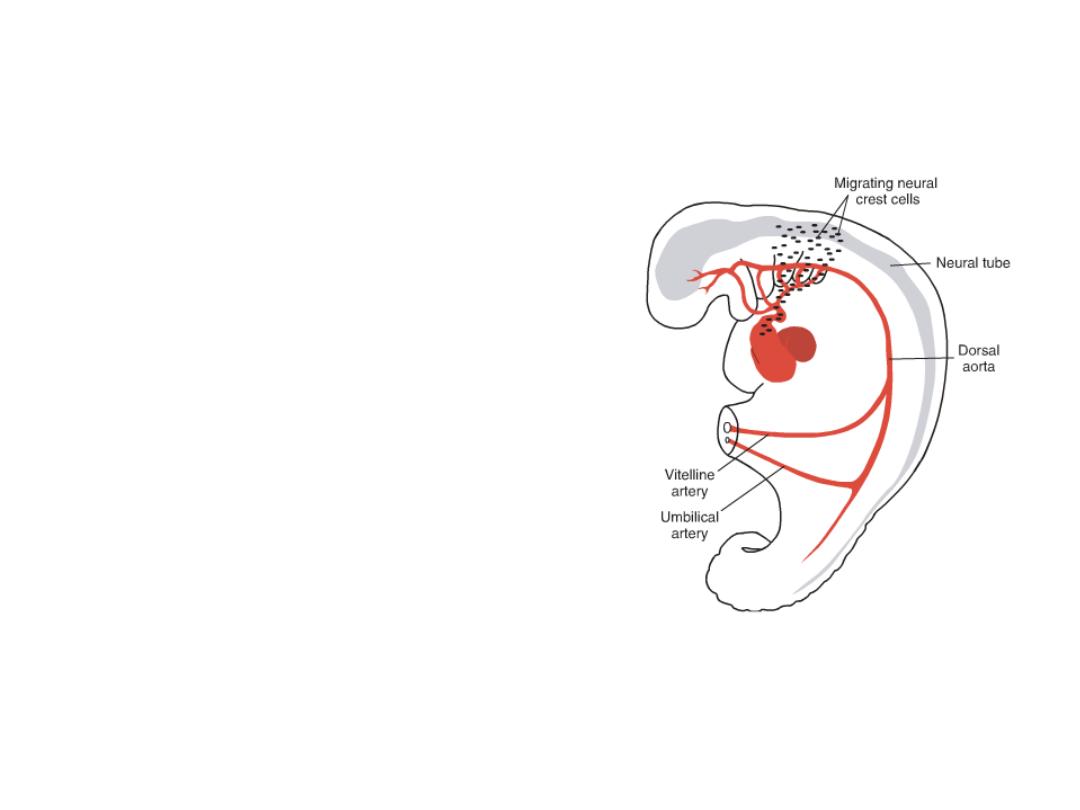
Neural crest cells
Originating in the edges of the neural folds in
the hindbrain region, migrate through
pharyngeal arches 3, 4, and 6 to the outflow
region of the heart, which they invade.
In this location, they contribute to
endocardial cushion formation in both the
conus cordis and truncus arteriosus.
Abnormal migration, proliferation, or
differentiation of these cells results in
congenital malformations in this region,
such as tetralogy of Fallot, pulmonary
stenosis, persistent truncus arteriosus, and
transposition of the great vessels.
Since neural crest cells also contribute to
craniofacial development, it is not
uncommon to see facial and cardiac
abnormalities in the same individual.
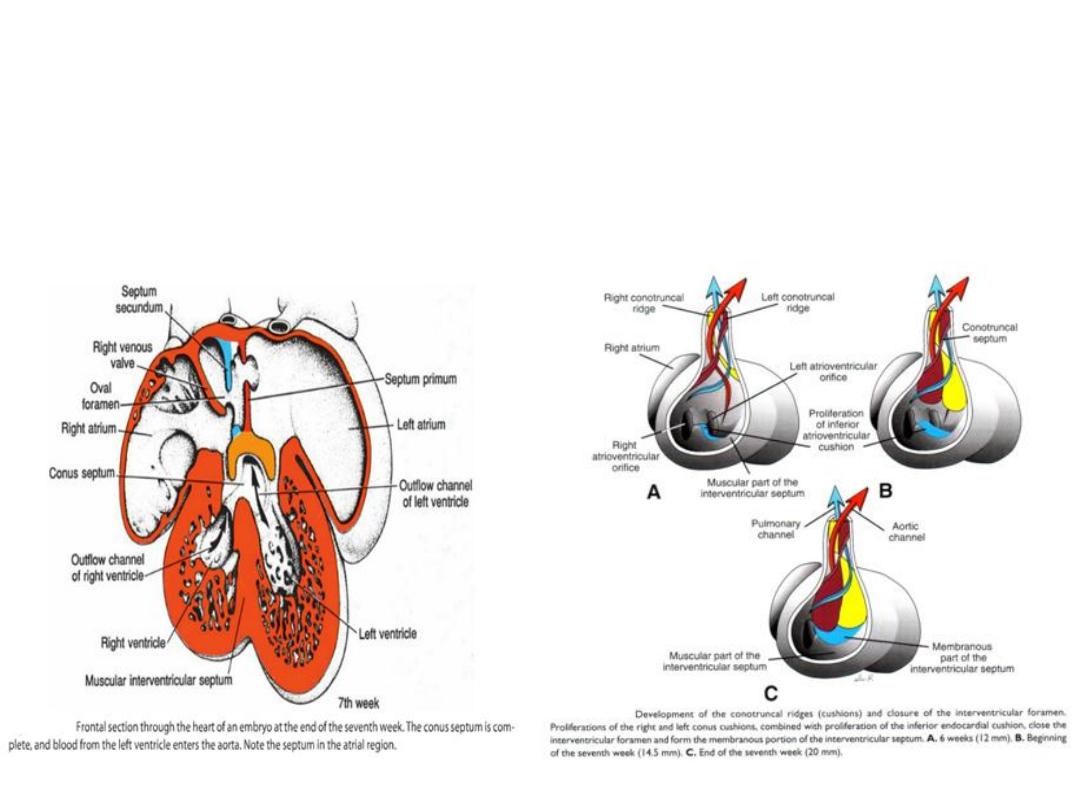
Septum formation in the ventricles
• Ventricular septation: created by growth of the muscular portion
(expansion of the ventricular chambers) and membranous portion (at top;
growth of the inferior endocardial cushion).
• Membranous portion = where most ventricular septal defects occur.
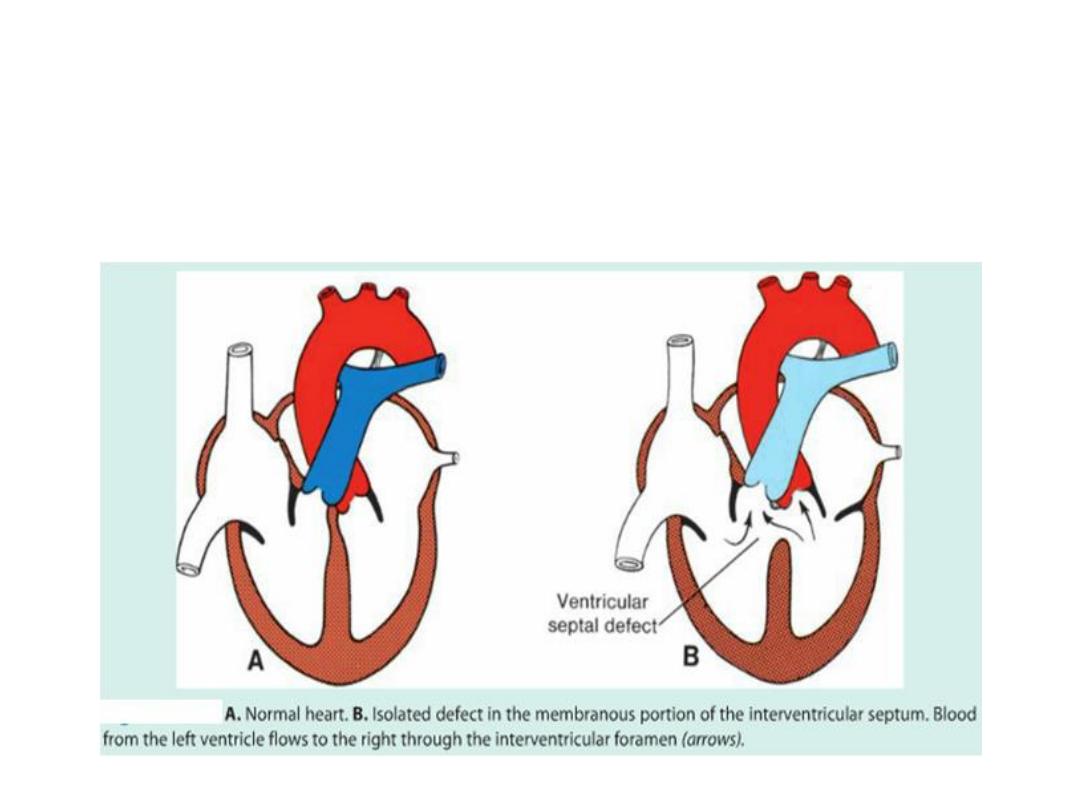
Clinical Correlates
• Heart Defects
• Ventricular septal defects (VSDs)
• Membranous ventricular septal defects (VSDs)
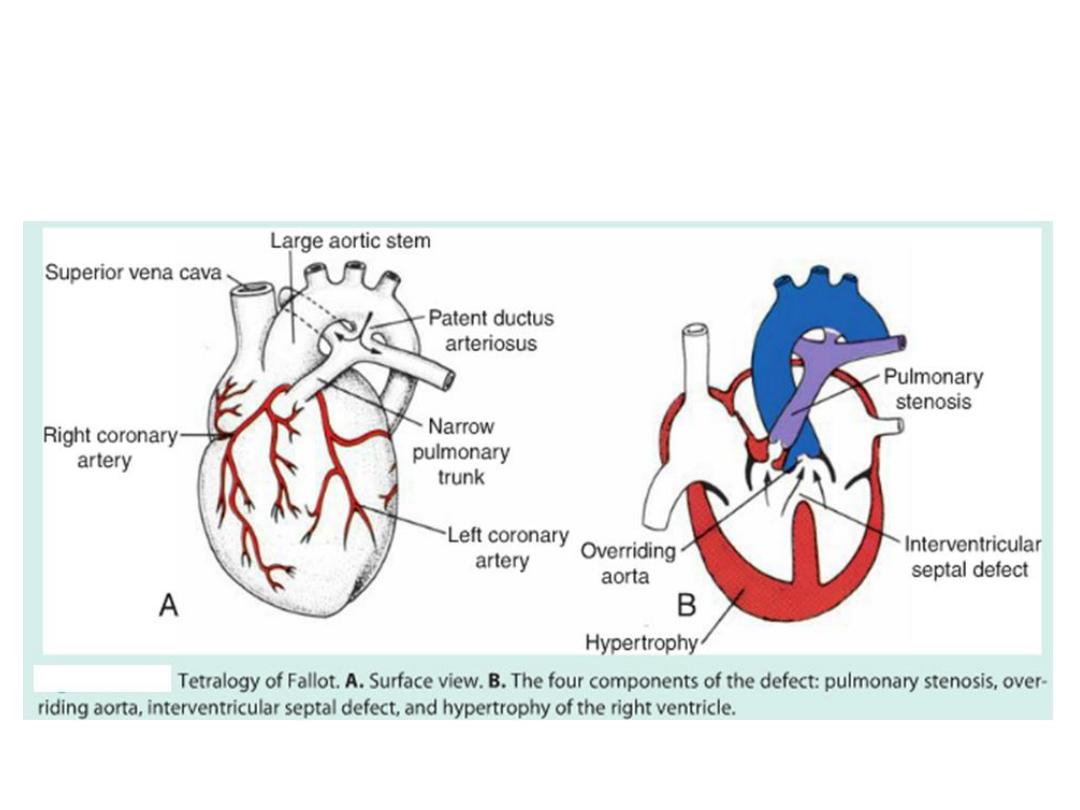
Tetralogy of Fallot
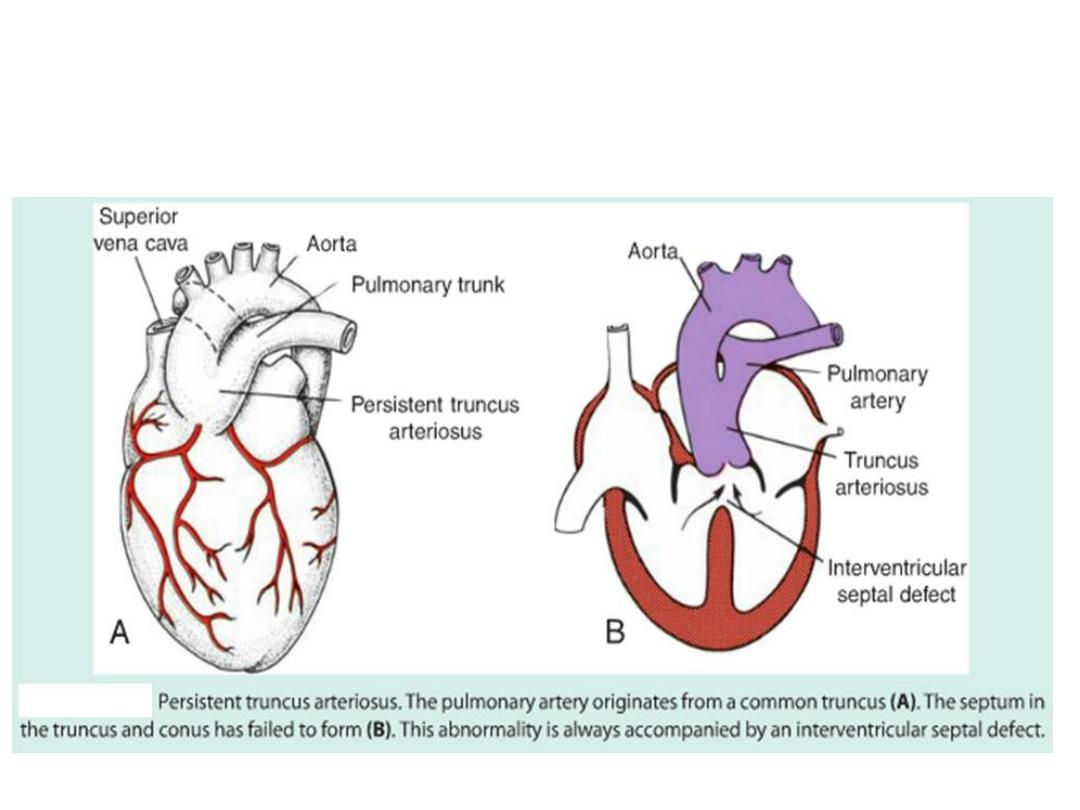
Persistent truncus arteriosus
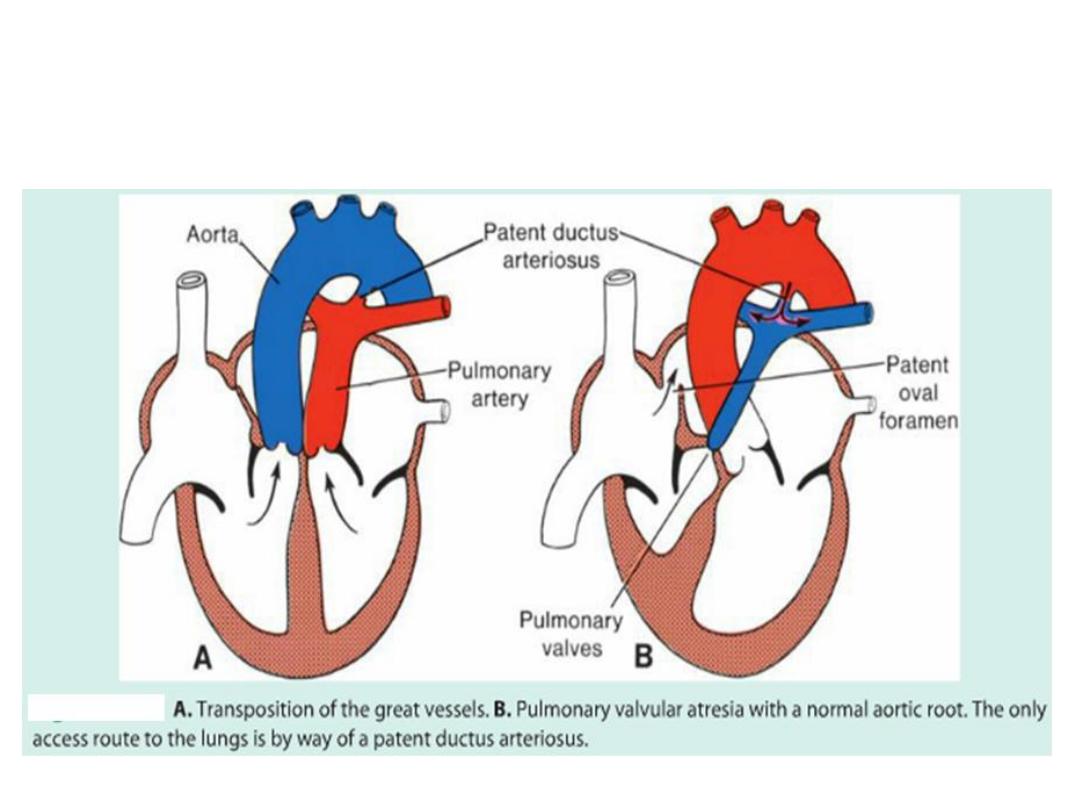
Transposition of the great vessels
Pulmonary stenosis

Ch.13 – Part 2
Vascular development
• Blood vessel development occurs by two mechanisms:
• (a) vasculogenesis: in which vessels arise by coalescence of angioblasts and
• (b) angiogenesis: whereby vessels sprout from existing vessels.
• The major vessels, including the dorsal aorta and cardinal veins, are formed
by vasculogenesis.
• The remainder of the vascular system then forms by angiogenesis.

Arterial System
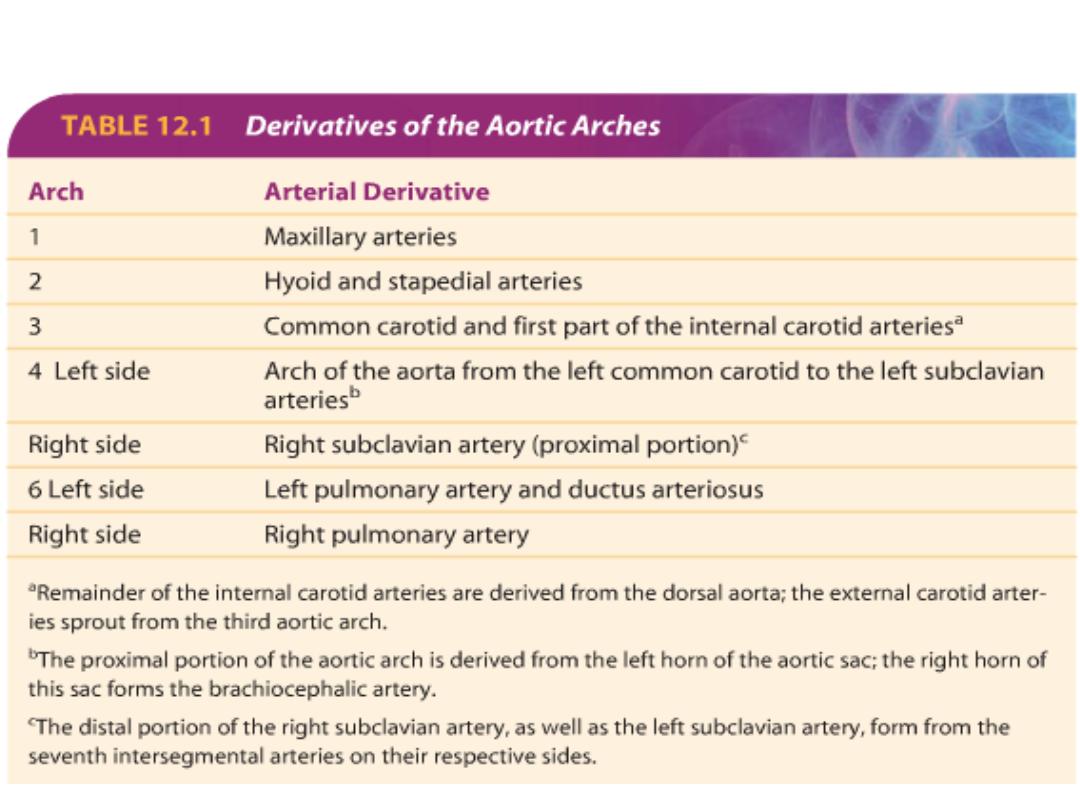
Aortic Arches
Aortic arches (5 pairs) associated with pharyngeal arches = arise
from aortic sac off the heart outflow tract, course through pharyngeal
arches.
Terminate in the right and left dorsal aortae.
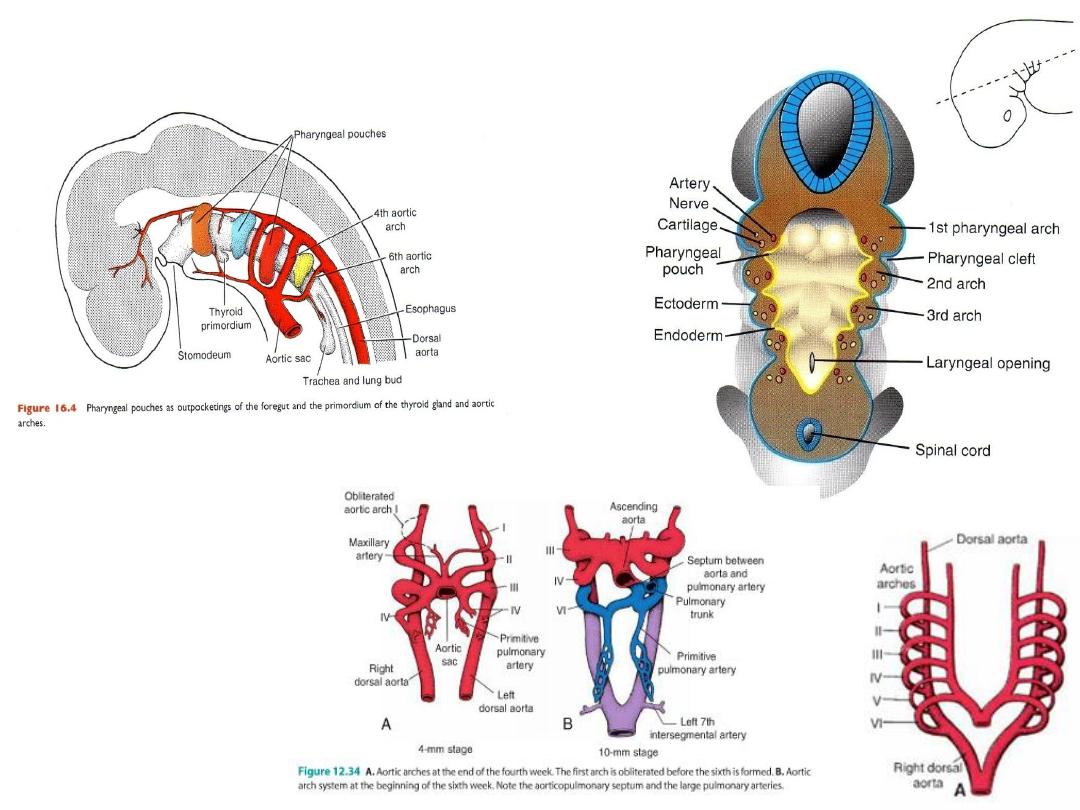
Pharyngeal Arches
The five arches are
numbered I, II, III,
IV, and VI (the 5
th
arch regresses).
Aortic arches undergo modification:
Aortic sac splits into:
R horn = brachiocephalic a
L horn = aortic arch
1st arch = mostly
disappears, leaves
maxillary artery
2nd arch = mostly
disappears, leaves
hyoid and stapedial
artery
3rd arch = carotid
system
4th arch = persists on
both sides =
subclavian and part of
the aortic arch
6th arch = forms
pulmonary arteries =
on left, the distal part
persists as the ductus
arteriosus
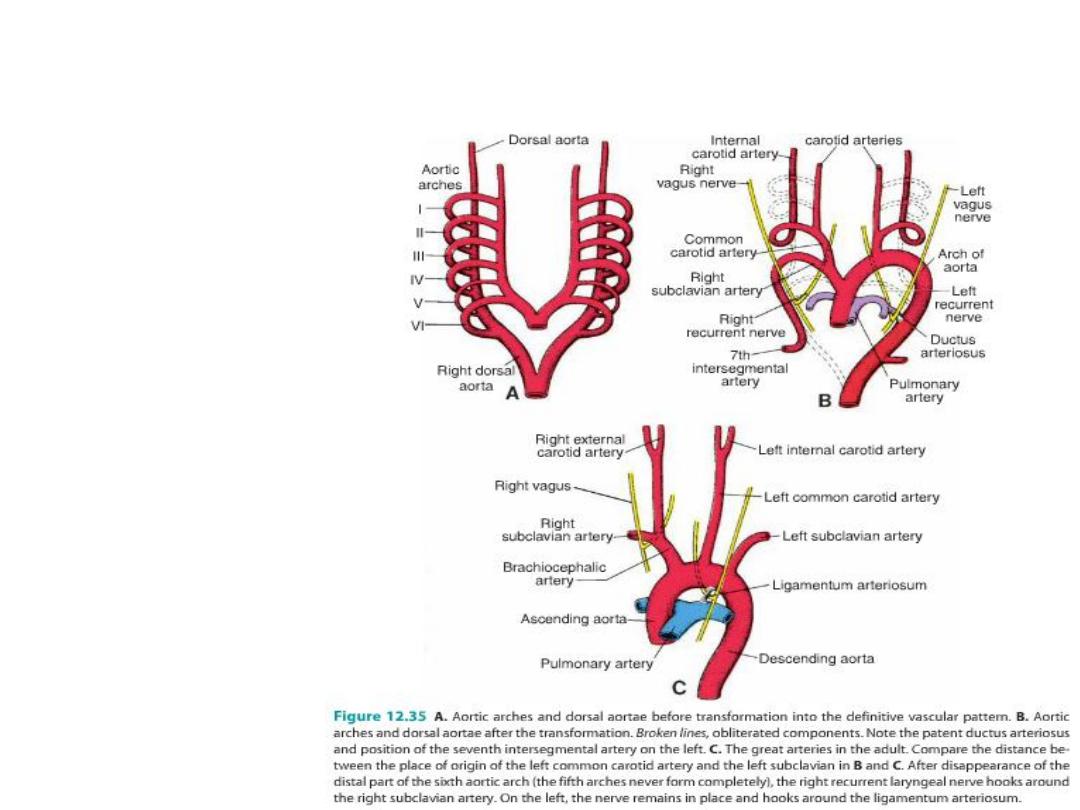
Derivatives of the aortic arches
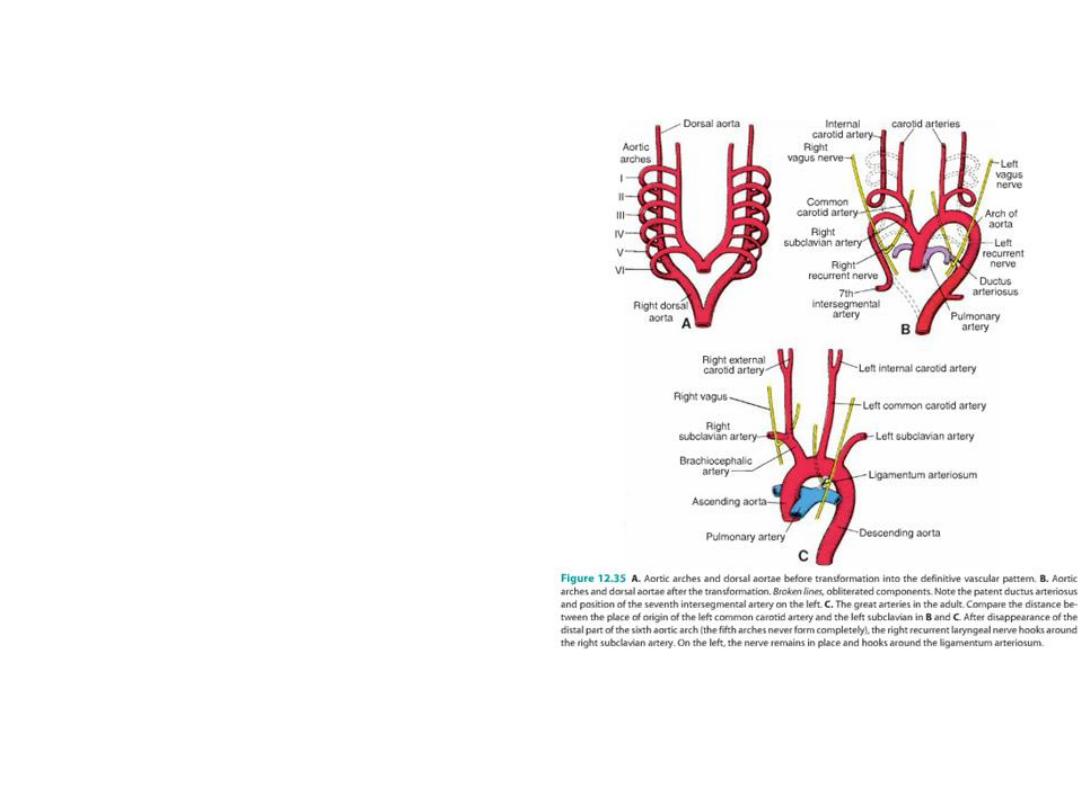
Recurrent laryngeal arteries
As a result of the caudal shift of the heart
and the disappearance of various portions
of the aortic arches, the course of the
recurrent laryngeal nerves becomes
different on the right and left sides.
Initially, these nerves, branches of the
vagus, supply the sixth pharyngeal arches.
When the heart descends, they hook
around the sixth aortic arches and ascend
again to the larynx, which accounts for their
recurrent course.
On the right, when the distal part of the
sixth aortic arch and the fifth aortic arch
disappear, the recurrent laryngeal nerve
moves up and hooks around the right
subclavian artery.
On the left, the nerve does not move up,
since the distal part of the sixth aortic arch
persists as the ductus arteriosus, which
later forms the ligamentum arteriosum.
Vitelline arteries
• Vitelline arteries supply the gut:
• Celiac foregut;
• superior mesenteric midgut;
• inferior mesenteric hindgut.
Umbilical arteries
• Umbilical arteries: join
common iliacs and ultimately
become internal iliacs and
medial umbilical ligaments.

Clinical Correlates
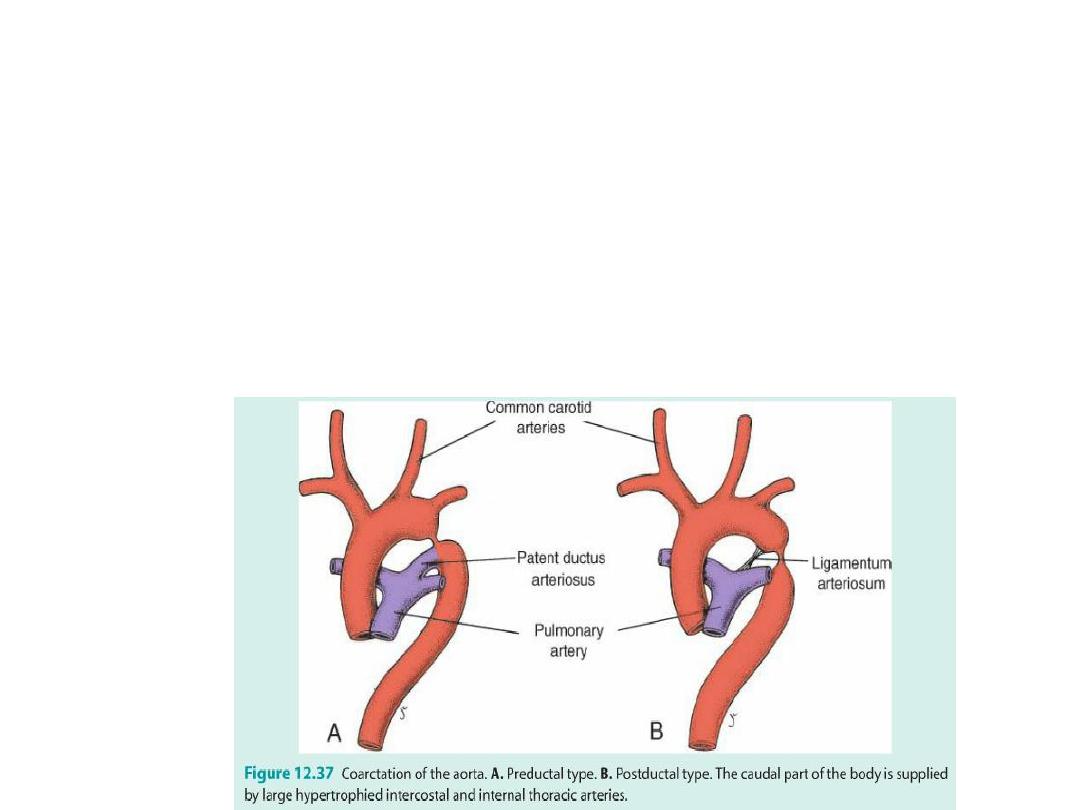
Arterial defects
(1) Patent ductus
• Under normal conditions, the ductus arteriosus obliterates after birth (by action of
lung bradykinin) ligamentum arteriosum.
• Patent ductus arteriosus: no obliteration after birth
Coarctation of aorta
(2) Coarctation of aorta: preductal and postductal = blood finds ways around the
block.
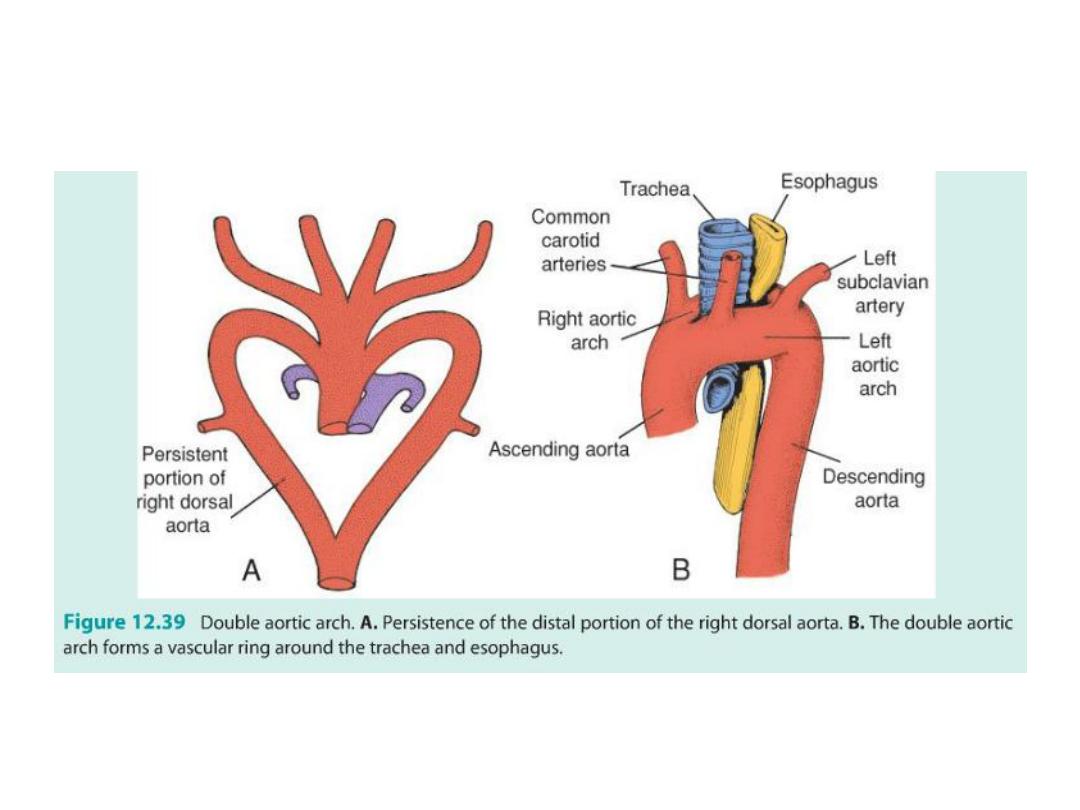
Double aortic arch
(3) Double aortic arch = difficulty swallowing = both parts of dorsal aorta
remain

Venous System
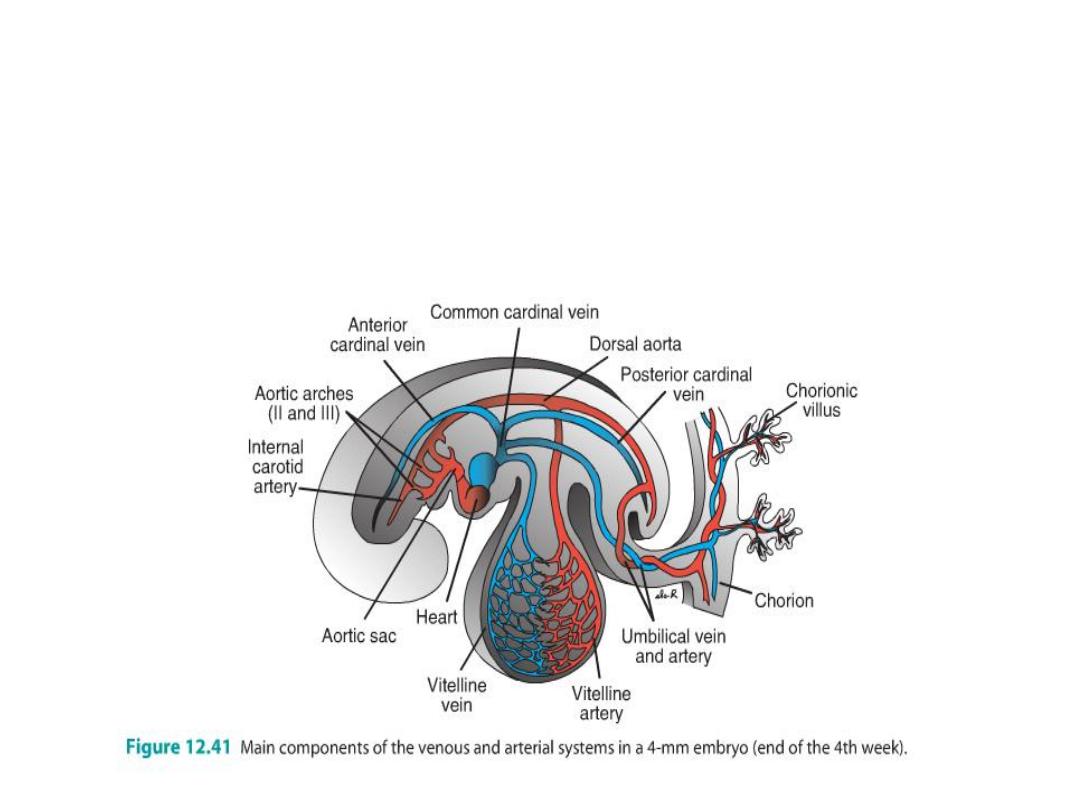
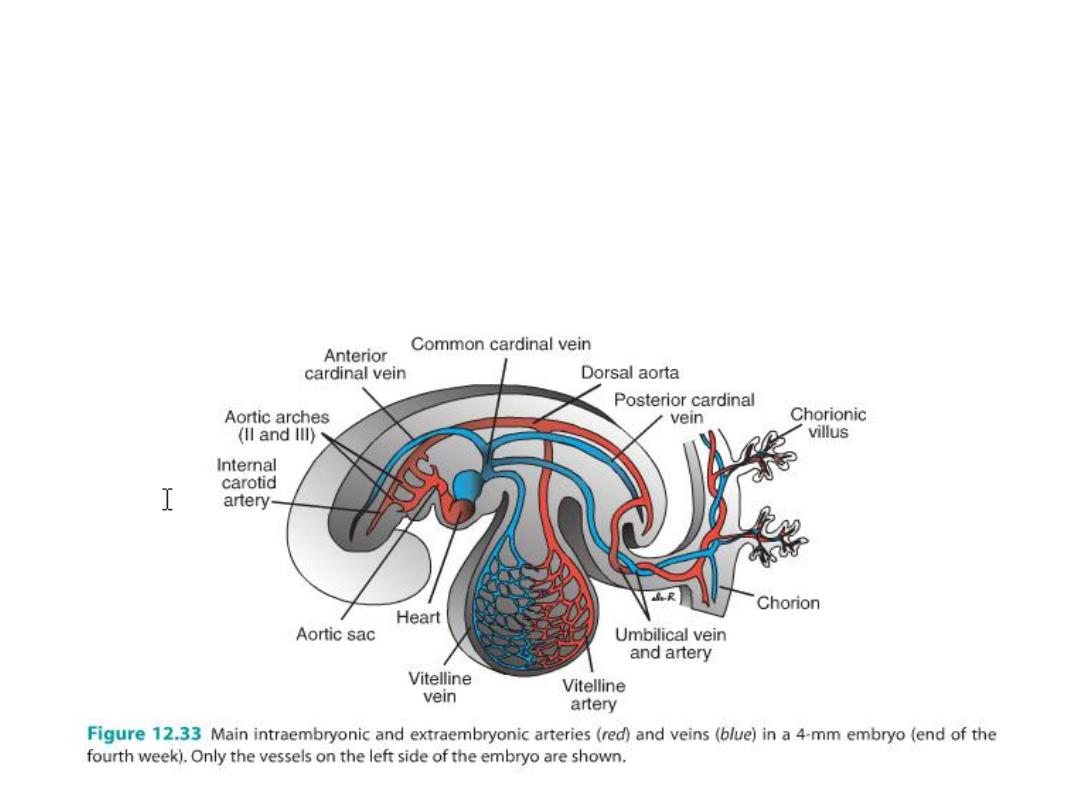
• In the fifth week, three pairs of major veins:
• (a) the vitelline veins, or omphalomesenteric veins, carrying blood from the yolk
sac to the sinus venosus;
• (b) the umbilical veins, originating in the chorionic villi and carrying oxygenated
blood to the embryo; and
• (c) the cardinal veins, draining the body of the embryo proper.
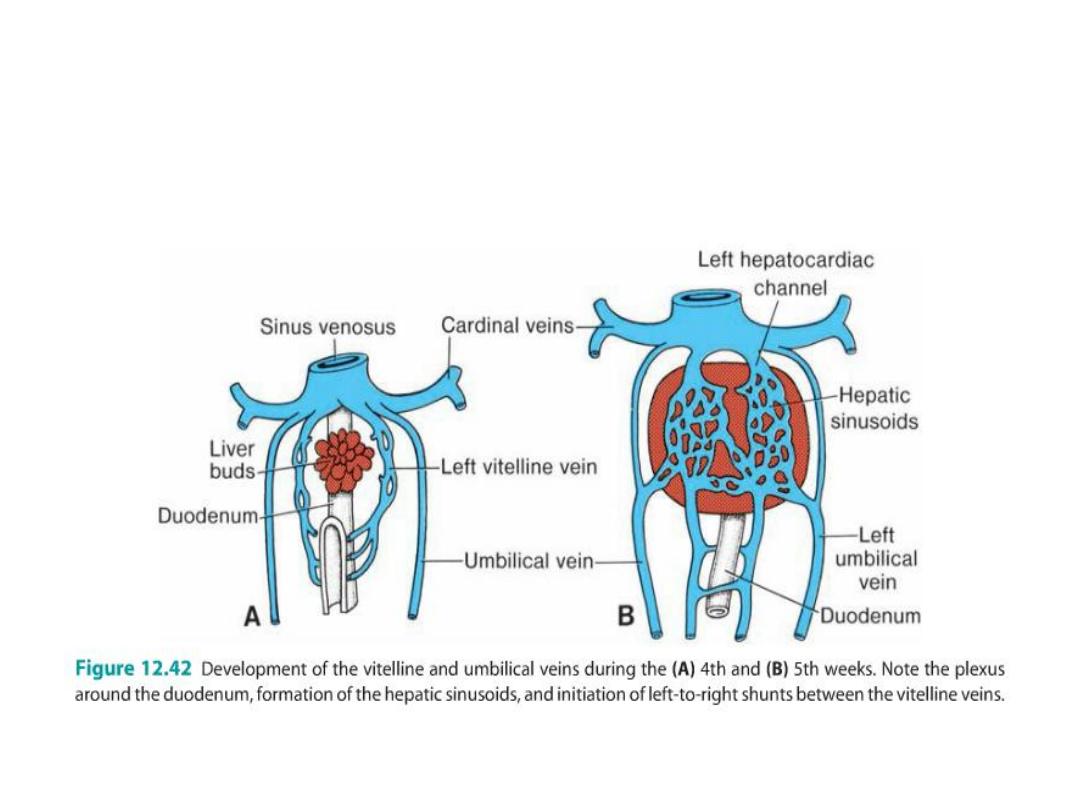
Vitelline Veins
• Vitelline veins septum transversum hepatic sinusoids
• Left to right shifting of blood enlargement of the right vitelline vein
(right hepatocardiac channel) hepatocardiac portion of the inferior
vena cava.
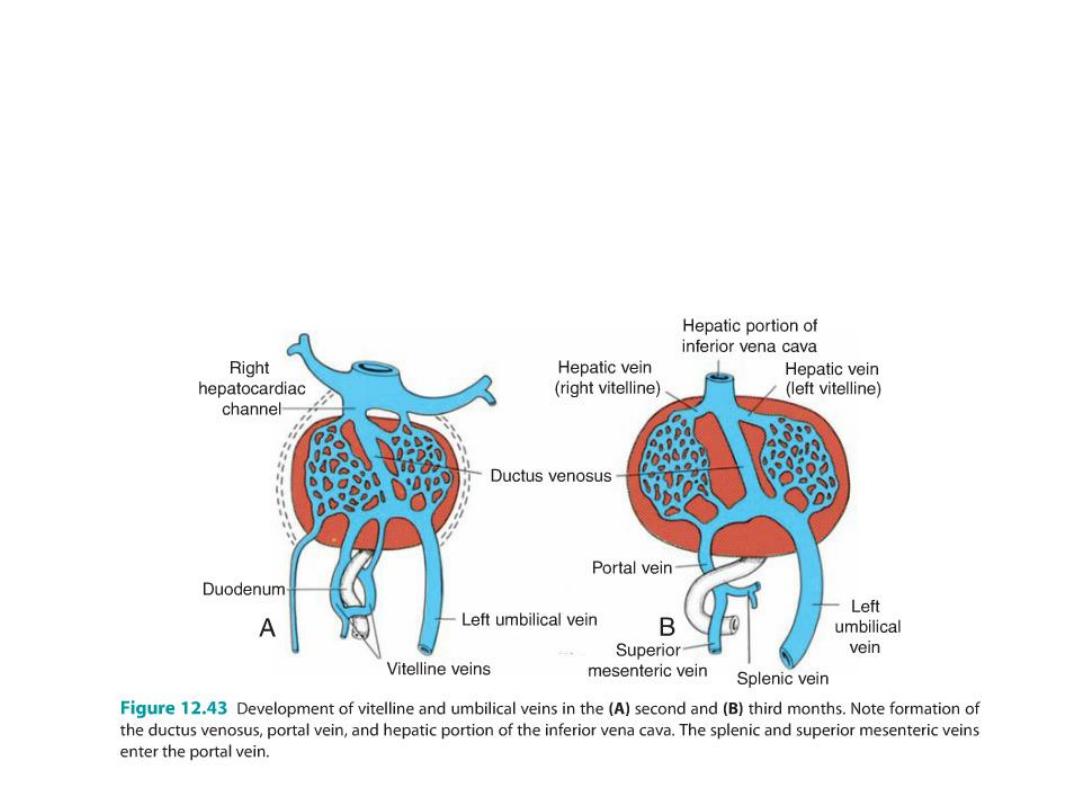
• Left vitelline vein disappears
• Right vitelline vein becomes: superior mesenteric vein and portal vein

Umbilical Veins
• The right umbilical vein disappear
• The left umbilical vein is the only one to carry blood from the placenta to
the liver
• Ductus venosus: direct communication forms between the left
umbilical vein and the right hepatocardiac channel,
• Afterbirth, the left umbilical vein ligamentum teres hepatis
• Ductus venosus ligamentum venosum
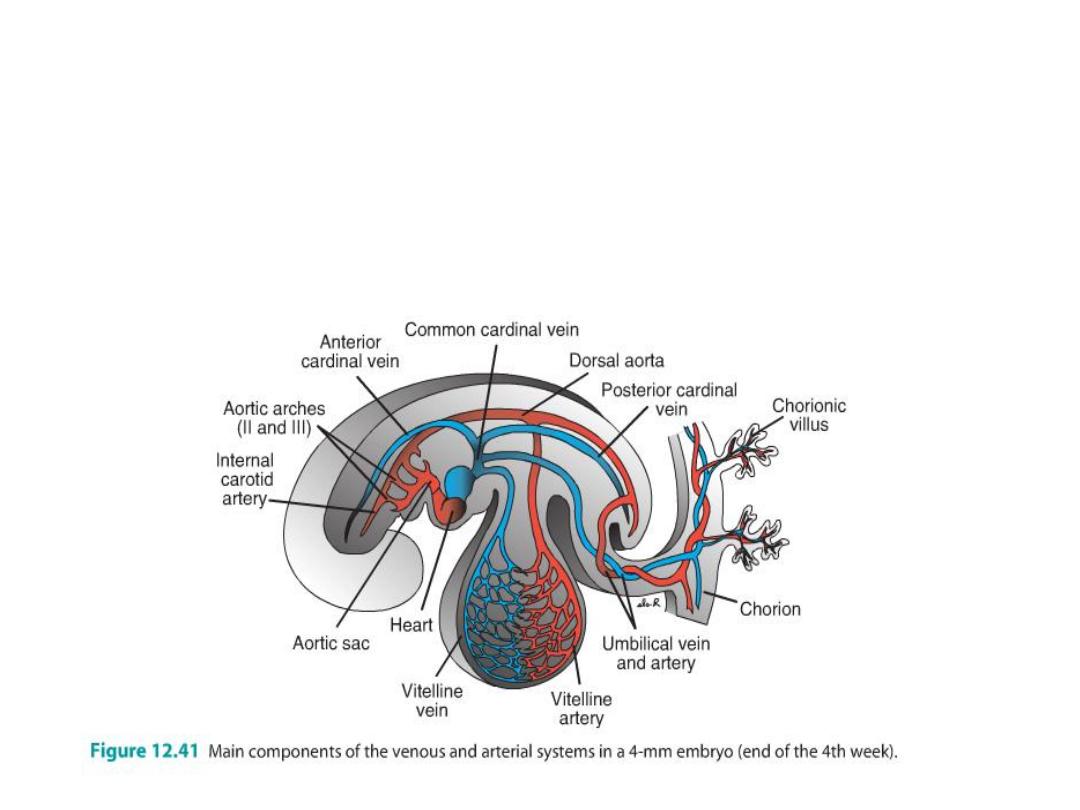
Cardinal Veins
• Anterior cardinal veins, drain the cephalic part of the embryo, and
• Posterior cardinal veins, drain the rest of the embryo.
• anterior and posterior c v join before entering the sinus horn and form the
short common cardinal veins.
• During the fourth week, the cardinal veins form a symmetrical system.
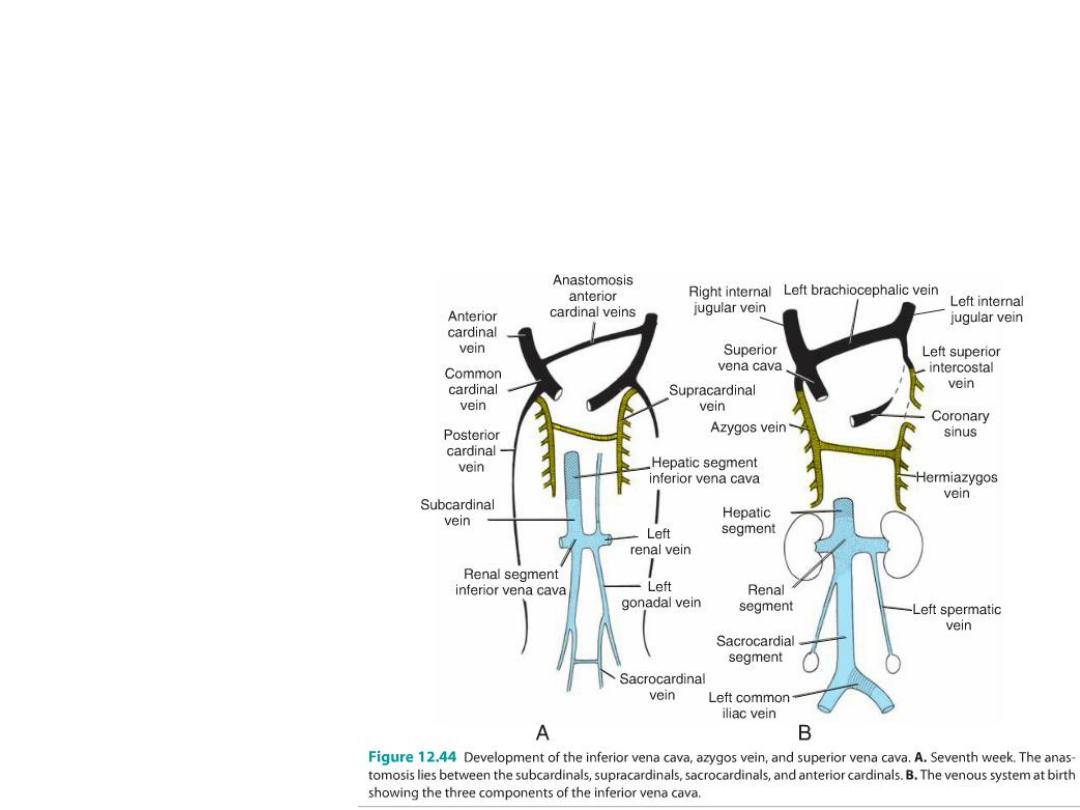
• As organs form new veins develop to drain them and all shift to the right
• (a) the subcardinal veins, drain kidneys;
• (b) the sacrocardinal veins, drain the lower extremities; and
• (c) the Supracardinal veins, drain the body wall by way of the intercostal
veins.
• L braciocephalic =
anastomosis between ant
card v.
• Sup vena cava = R common
and R ant cardinal v.
• L renal v = anastomosis
between subcardinal v.
• L common iliac v =
anastomosis of sacrocardinal
v.
• inf vena cava = Sacrocardinal
and subcardinal on R
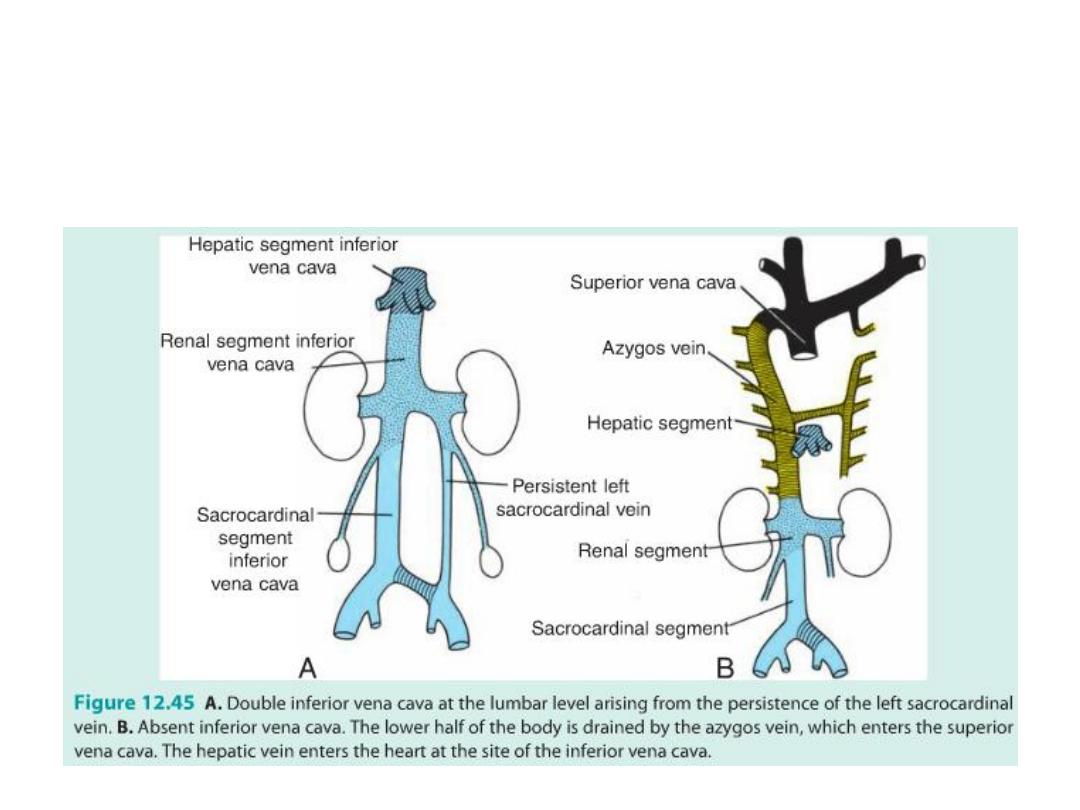
• The complicated caval system is characterized by many abnormalities,
such as double inferior and superior vena cava and left superior vena
cava

Circulatory changes at birth
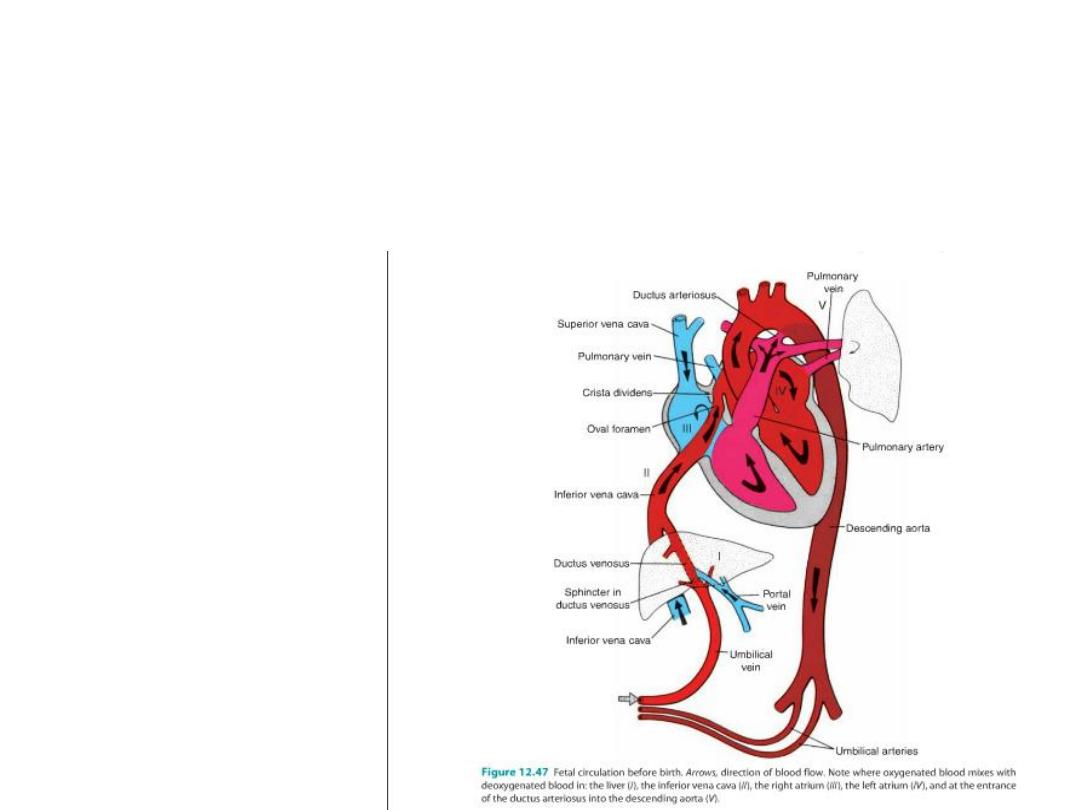
Before birth: blood flow in fetus = umbilical v from placenta (oxygenated)
to ductus venosus (by passes liver) to inf vena cava to R atrium to L
atrium through foramen ovale to L ventricle to aorta to umbilical arteries to
placenta (little blood goes to R ventricle because pulmonary circulation
not functional = lungs not working.
Any blood in R ventricle goes out pulmonary vessel and through ductus
arteriosus into aorta.)
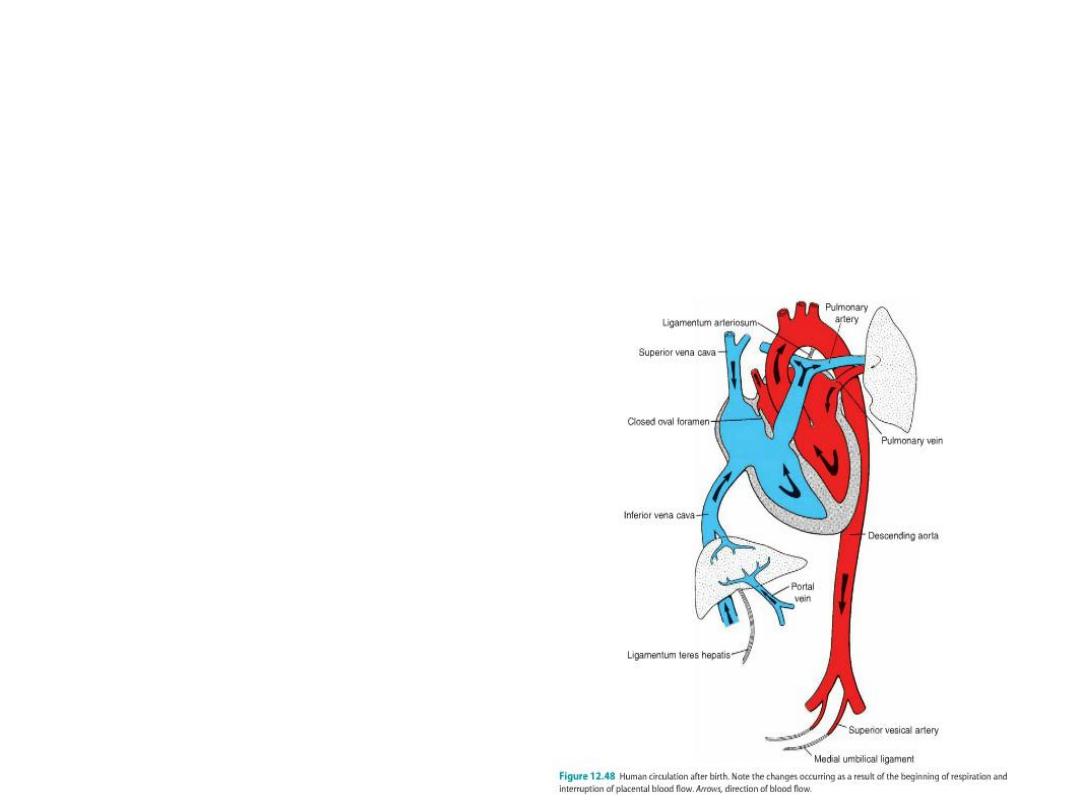
After birth:
•Sphincter regulating flow in ductus venosus closes (ligamentum
venosum),
•Umbilical arteries close (medical umbilical ligaments),
•Umbilical v closes (lig teres),
•Ductus arteriosus closes (bradykinin from lungs causes closure = lig
arteriosum),
• Foramen ovale closes.
