115
The genes on the X and Y chromosomes:
* 122 genes (May 2007) have been mapped to the Y chromosome.
- Many of the Y chromosome genes contain the instructions to make the baby develop as a male
rather than a female
- Without the presence of the Y chromosome genes, the baby will develop into a female
* In rare cases, a male baby is born with his cells containing 47 chromosomes: made up of 44
autosomes, two X chromosome copies and the Y chromosome (Klinefelter (XXY)
syndrome) Despite the presence of the two copies of the X chromosomes in the cells, the baby still
develops as a male because of the instructions issued by the Y chromosome genes
The genes on the X chromosome
* Unlike the Y chromosome, the X chromosome is ‘gene rich’ with 1021 genes
mapped to it (May 2007).
* Many of the X chromosome genes are very important for growth and
development;
e:
Exampl
- the genes that contain the instructions for a major protein in muscles
(dystrophin)
- several proteins that control clotting in the blood and
- a number of genes involved in the development of intelligence.
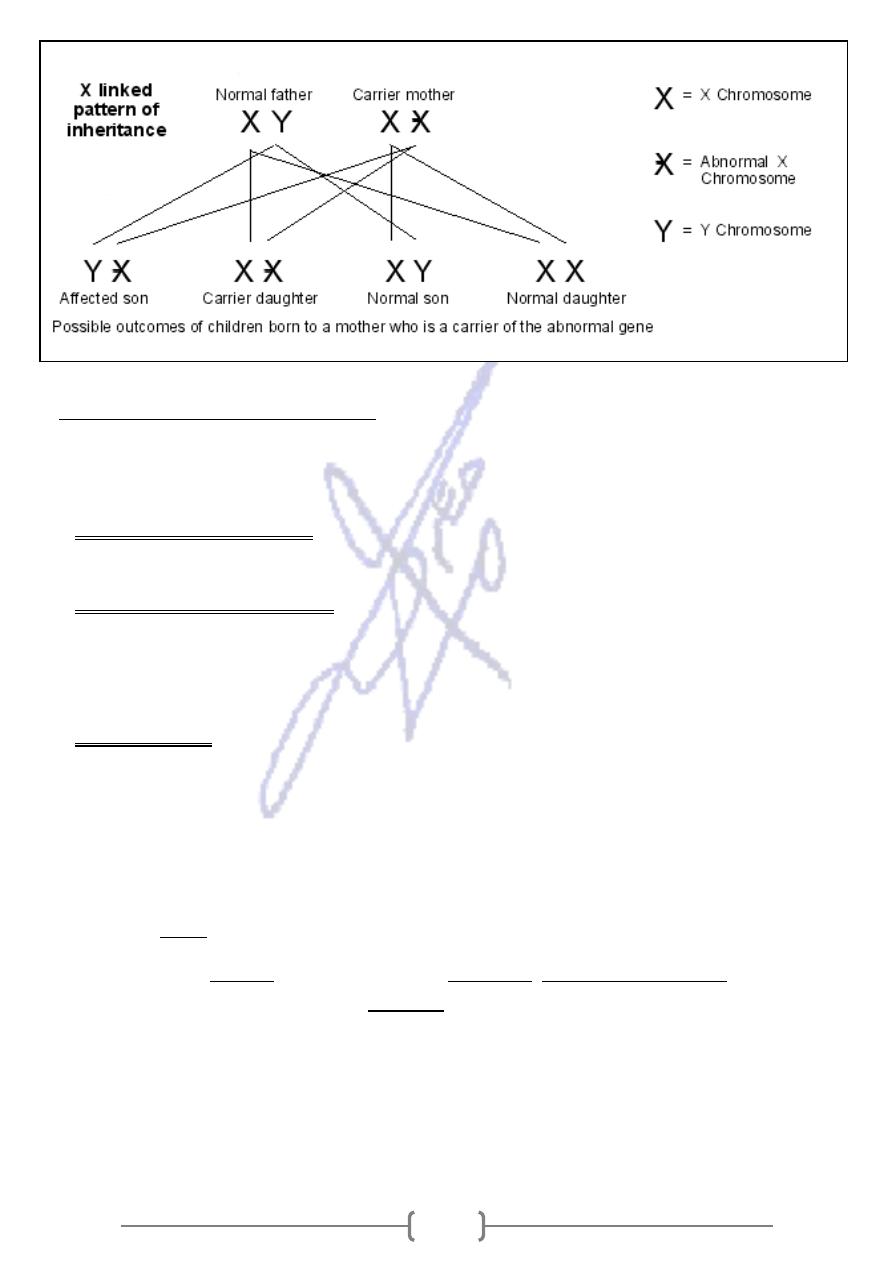
Sex linkage inheritance
* Sex linkage is the phenotypic expression of an allele related to the sex chromosome of the
individual.
- This mode of inheritance is in contrast to the inheritance of traits on autosomal
chromosomes, where both sexes have the same probability of inheritance.
- Since humans have many more genes on the X than the Y, there are many more X-linked
traits than Y-linked traits.
* Disease caused by genes on the X chromosome are said to be X-linked and specific
genetic conditions may result: hemophilia, muscular dystrophy and fragile X syndrome.
linked dominant
-
X
a few
although there are also
linked disease is recessive,
-
Most X
*
diseases.
116
development:
is a special feature of female
X inactivation
* In mammals, the female is the homozygous sex, with two X chromosomes (XX), while the male is
heterozygous, with one X and one Y chromosome (XY).
who first hypothesized that one X chromosome in each
Mary Lyon
r
In the early 1962s D
-
somatic cell of the female is inactivated.
- This would result in dosage compensation, that means an equalization of X-linked gene
products in males and females. This system of ‘switching off’ of one of the X chromosome copies is
seen in all mammals and is often called lyonization.
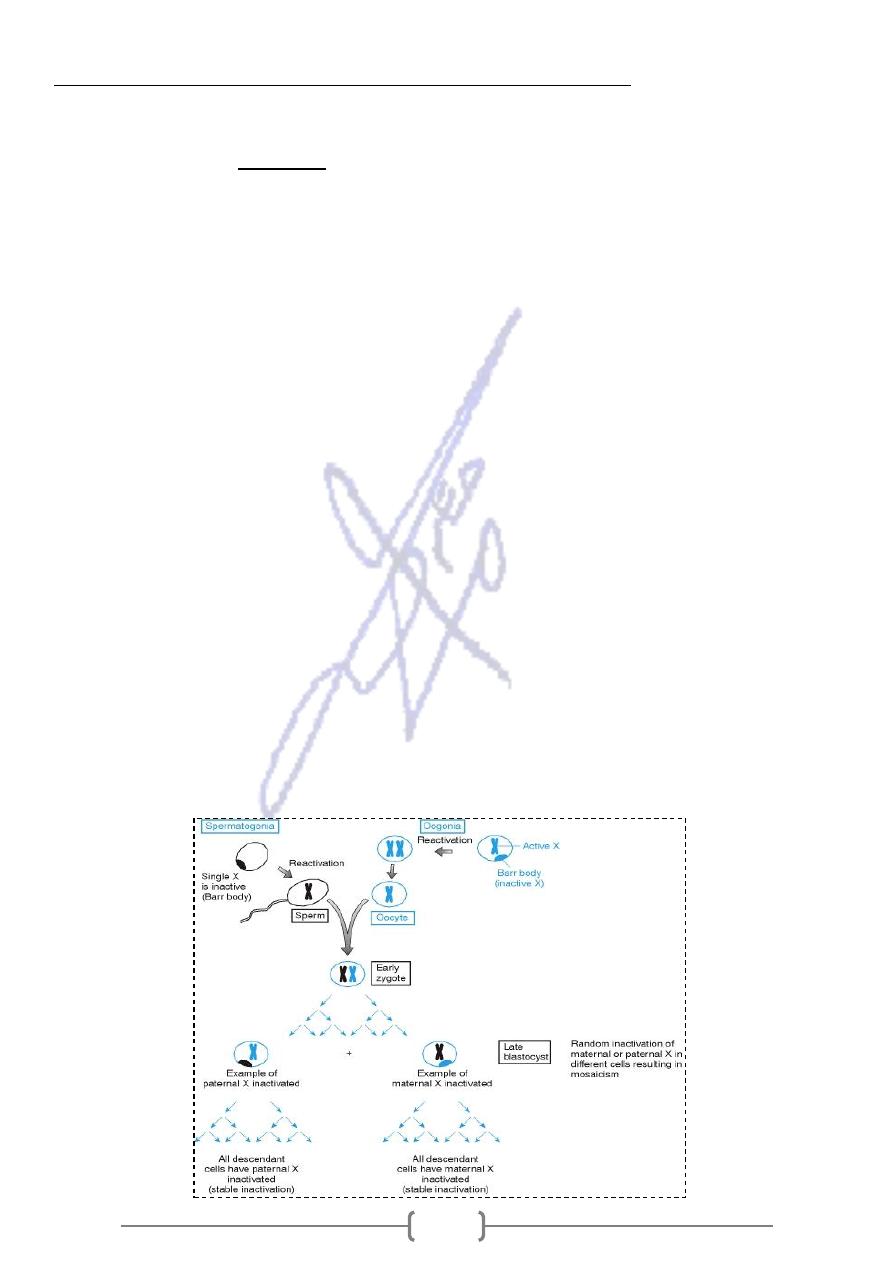
* The Lyon hypothesis stated that X inactivation occurs very early in female embryonic
development (at late blastocyst stage in humans),
-
that X chromosome contributed by the father is inactivated in some cells, where as in other
cells the X chromosome contributed by the mother is inactivated.
- Each cell chooses one of the two X chromosomes at random to be inactivated, so the
maternally and paternally derived X chromosomes will each be inactivated in about half of the
embryo's cells
* The maternal and paternal X chromosomes are both active in the zygote and in early
embryonic cells. X inactivation then take place, resulting in cells having either an active
paternal X or an active maternal X chromosome. Females are thus X chromosome
mosaics, as shown in the tissue sample at the bottom of the figure.
Once X chromosome is inactivated in a cell, it will remain inactive in all descendants of that cell
* As a result of X-inactivation, all normal females have two distinct populations of cells: one
population has an active paternally derived X- chromosome, and the other is an active
maternally derived X chromosome.
* With two populations of cells, females are mosaics for the X chromosome. Males are not
mosaics but are hemizygous for the X chromosome.

117
* In each body cell (somatic cell) of the developing baby girl, one of the X
chromosomes becomes very shortened and condensed so that most of its
genes are not able to be ‘read’ by the cells. An examination of female cells
under a microscope reveals a dark body in the cell (called a Barr body) or sex
chromatin body near the membrane of the interphase nucleus which is
.
inactivated X chromosome
Linked Recessive Inheritance:
-
X
* X-linked recessive traits are expressed in all heterogametics and homogametics that are
homozygous for the recessive allele.
- Because females inherit two copies of the X chromosome, they can be homozygous for a
disease allele at a given locus, heterozygous, or homozygous for the normal allele at the locus
- In females, an X-linked recessive trait behaves much like an autosomal recessive trait.
However only one X chromosome is active in an individual somatic cell. This means that about
half of the cells in a heterozygous female will express the disease allele and half will express the
normal allele. Thus as with autosomal recessive trait, the heterozygote will produce about
50% of the normal level of the gene product, and this is sufficient for a normal phenotype
* The situation is different in males, who are hemizygous for the X chromosome.
* If a male inherits a recessive disease gene on the X chromosome, he will be affected by the
disease because the Y chromosome does not carry a normal allele to compensate for the
effects of the disease gene.
- In males they express the trait when they inherit one mutant allele, gene frequency (q). In
contrast, a female must inherit two mutant alleles, a less frequent event since the mutant allele
is rare in the population.
- The incidence of recessive X-linked phenotypes in females is the square of that in
males (q
2)
. If 1 in 20 males in a human population are green color blind , then 1 in 400
females in the population are expected to be color blind, gene frequency= (
1
/
20
)*(
1
/
20
)
* Since a father can transmit only a Y chromosome to his son, X- linked genes are not
passed from father to son.
normal
phenotypically
le can be transmitted through a series of
linked disease alle
-
An X
*
, causing the appearance of “skipped” generations.
heterozygous females
transmit it to
who act as carriers
,
all his daughters
to
affected father
The gene is passed from an
*
approximately half of their sons, who are affected.
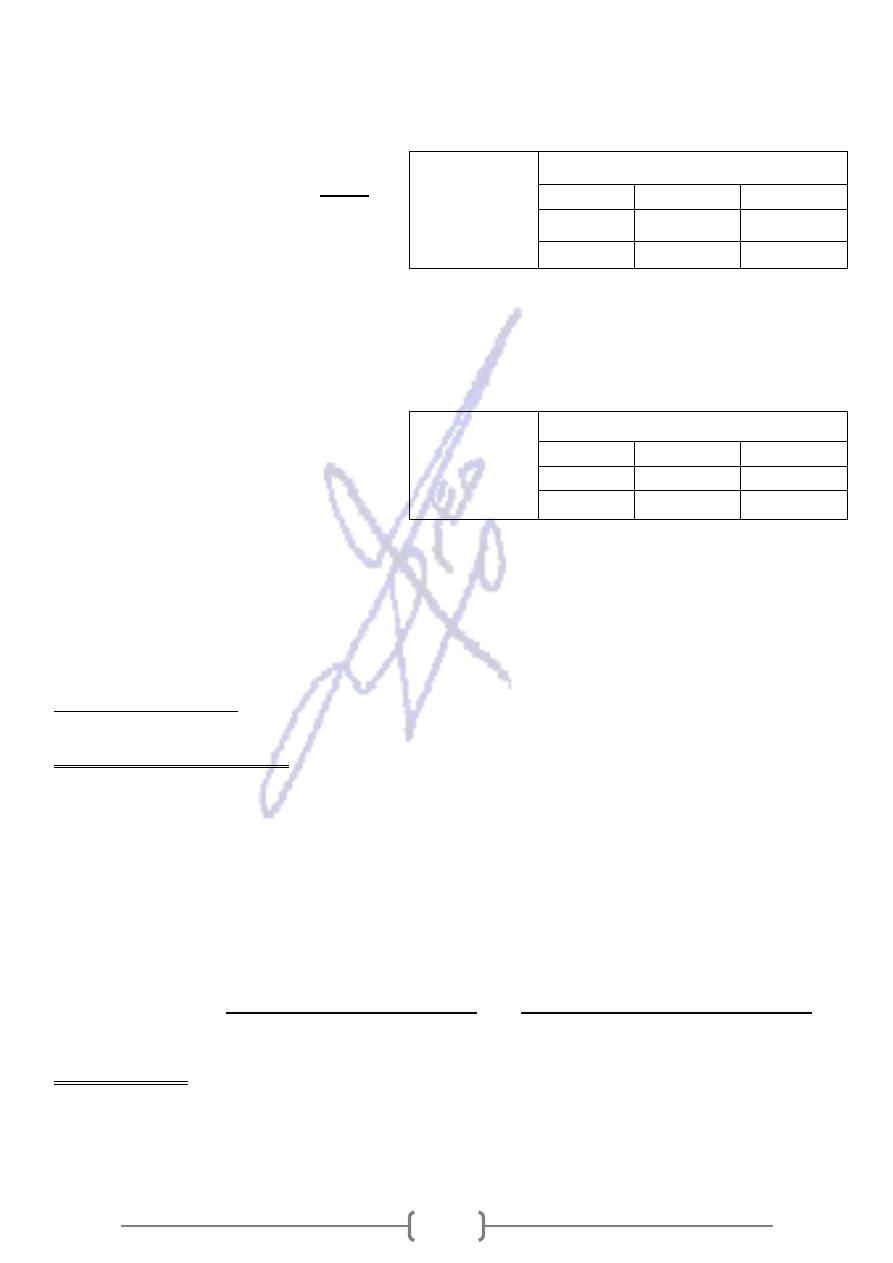
Punnett square representation of the mating of a
ressecive
linked
-
who carries an X
heterozygous female
. X1 chromosome with
normal male
a
with
disease gene
normal allele; X2 chromosome with disease allele.
Daughters: 50% normal, 50% carriers.
Sons: 50% normal, 50% affected
Mother
Father
X
2
X
1
X
1
X
2
X
1
X
1
X
1
X
2
Y
X
1
Y
Y
118
- the heterozygous mother is called "carrier" because she has one copy of the recessive allele.
sons will have 50% probability to be affected 50% of unaffected daughters will become carriers like
their mother
Punnett square representation of the
male
with a
normal female
mating of a
who is affected by an X- linked recessive
disease.
X
1
chromosome with normal allele;
X
2
chromosome with disease allele.
Daughters: 100% carriers. Sons: 100% normal.
the affected father has one X-linked recessive gene allele,the mother is homozygous for the allele
only daughters (all) will be affected.
Punnett square representation of the
mating of a carrier female with a male
affected with an X- linked recessive gene
disease.
X
1
chromosome with normal allele;
X
2
chromosome with disease allele.
Daughters: 50% affected, 50%carriers. Sons: 50% normal, 50% affected disease.
The affected mother is hetrozygous with one copy of the recessive allele : both daughters and
sons will have 50% probability to be affected.
Linked Diseases
-
X
* Over 400 human traits and diseases seem to be encoded by genes on the X chromosome.
was the first human trait proven to be due to a gene on X
blindness:
green color
-
1. Red
chromosome.
* Males are more severely affected than females; in the case of red-green color blindness, Females
who have one copy of the mutant gene (that is, are heterozygous or carriers) are not at all affected
* Among offspring of carrier mothers, 50% of their sons are affected, whereas 50% of their
daughters are carriers.
* Affected fathers cannot pass their mutant X chromosome to their sons, but do pass it to all of
their daughters, who thereby are carriers.
A number of other well-known human conditions behave in this manner, including the two
phosphate dehydogenase
-
6
-
glucose
, and
Duchenne muscular dystrophy
hemophilia,
forms of
deficiency that predisposes to hemolytic anemia.
2. Hemophilia A
* Hemophilia A is caused by deficient in factor VIII, a key component of the clotting
cascade. Fibrin formation is affected, resulting in prolonged and often sever bleeding from
wounds and hemorrhages in the joints and muscles. Spontaneous bleeding may occur.
Mother
Father
X
1
X
1
X
1
X
2
X
1
X
2
X
2
X
1
Y
X
1
Y
Y
Mother
Father
X
2
X
1
X
2
X
2
X
2
X
1
X
2
X
2
Y
X
1
Y
Y
119
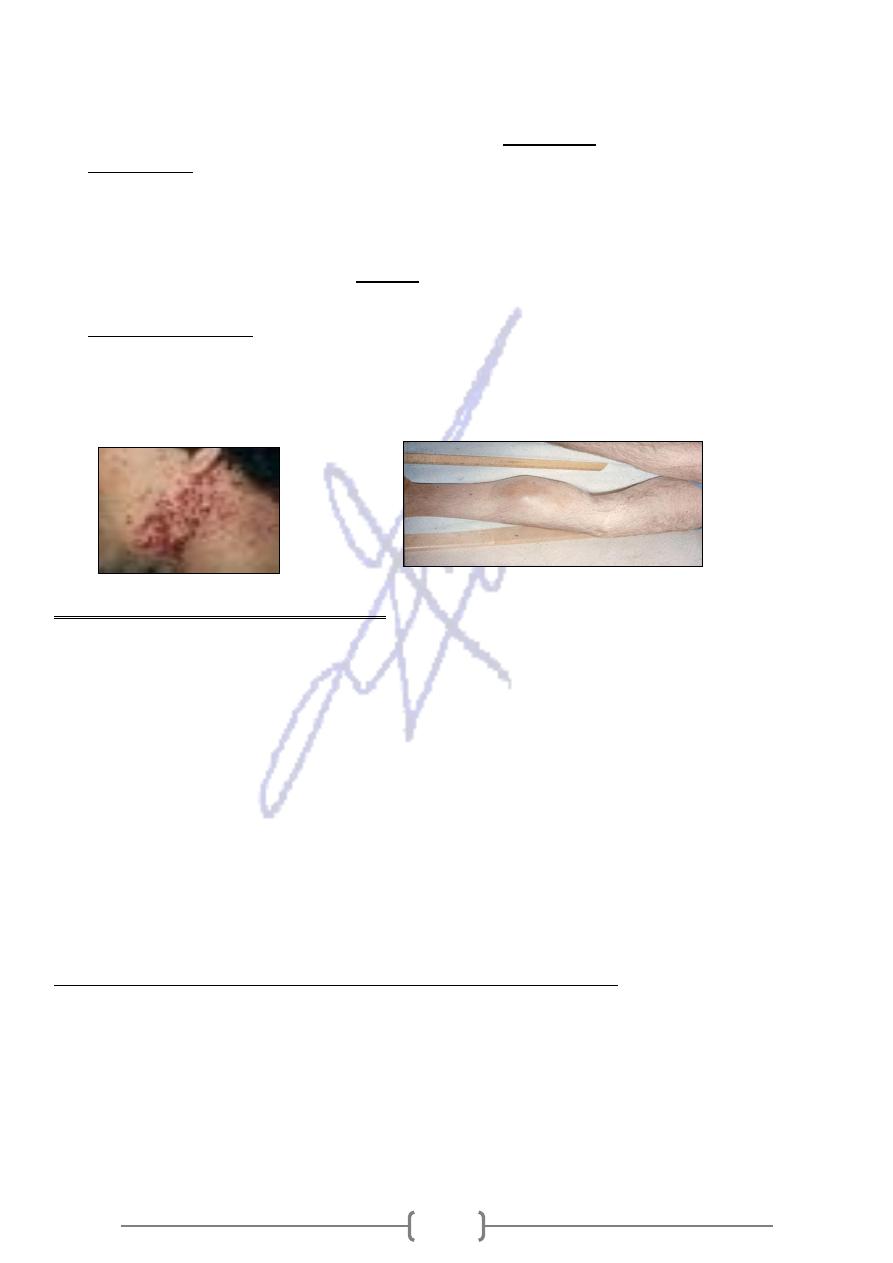
* Hemarthroses (bleeding into the joints) are common in joints such as the ankles,
knees, hips, and elbows, resulting in swelling and impaired function.
factor.
coagulation
Hereditary bleeding disorder caused by deficiency of a
-
, lack of factor VIII causes classic hemophilia, affect approximately 1 in
hemophilia A
In
-
5000 to 1 in 10000 males worldwide.
- Other types are caused by deficiency of factor IX or XI. The first two are transmitted by sex-
linked heredity; the third has dominant inheritance and occurs in females as well as males.
or even death. Large bruises of the skin and soft
anemia
Hemophilia characterized by severe
-
tissue are often seen, usually following injury. There may also be bleeding in the mouth, nose,
.
gastrointestinal tract
and
Treatment: Administration of donor- derived factor VIII.
- Drawback: Because infusion contained plasma products from different donors, it was
frequently contaminated by viruses, like hepatitis B and C, also HIV
3. Duchenne Muscular Dystrophy (DMD)
* A progressive weakness and loss of muscle. Muscle tissue degenerates and regenerates
randomly and is replaced by scar tissue and fat.
* Muscles contain a protein called dystrophin, which is necessary for muscles to function
properly.
- People with DMD have a shortage of dystrophin in their muscles due to faulty gene. The lack of
dystrophin leads to muscle fiber damage and a gradual weakening of the muscles.
- The symptoms of DMD are usually seen before the age 5 years, all skeletal muscle degenerates
eventually and most patients with DMD are confined to a wheelchair by 11 years of age.
- The heart and respiratory musculature become impaired, and death results from respiratory or
cardiac failure. Survival beyond age 25 years is uncommon.
- It affects 1 of every 3500 males
- There is no specific treatment Physical therapy, and corrective surgery may help
ry the gene?
Can Duchenne's muscular dystrophy affect women who car
• Girls and women who carry the DMD gene are usually well and have no symptoms of DMD
themselves.
• A small number of women 8-10% carrying the DMD gene may develop some muscle weakness
themselves
121
Linked Dominant Inheritance
-
X
Are fewer in number and prevalence than the X- linked recessive diseases.
An example:
the kidneys are impaired in their
a disease in which
Hypophosphatemic rickets:
1.
ability to reabsorb phosphate. This results in abnormal ossification of the bones.
a disorder characterized by abnormal skin
Incontinentia pigmenti type I:
2.
pigmentation, conical or missing teeth, and ocular and neurological abnormalities.
This disorder is seen only in females.
It is thought that hemizygous males are so severely affected, they die inutero.
a neurodevelopment disorder seen in 1/10000 to 1/ 15000 females and
Rett Syndrome:
3.
in smaller proportion of males (some males are lost inutero, while others survive).
It characterized by mental retardation, and gait ataxia.
As with autosomal dominant diseases, an individual need inherit only a single copy
of an X- linked dominant disease gene to manifest the disorder
Because females have two X chromosomes, either can carry the disease gene, they
as commonly affected as males (unless the disorder is lethal in males).
twice
are about
must
All of their daughters
.
their sons
transmit the trait to
cannot
Affected father
affected.
inherit the disease gene, so all are
Affected females are usually heterozygotes and thus have a 50% chance of passing
the disease allele to their daughters and sons.
121
Linked Inheritance
-
Y
The Y chromosome contains relatively few genes. The Y- chromosome cannot carry
any genes whose function is important for health, because females are perfectly
normal without any Y- linked genes.
Thus any Y- linked genes must code either for non-essential characters or for male
specific functions includes the gene that initiates differentiation of the embryo into
a male. Any defects in Y chromosome associated with male sexual dysfunction.
Transmission of Y- linked traits is strictly from father to sons.
Mitochondrial inheritance gives a recognizable matrilineal pedigree pattern
Mitochondrial mutations are a significant cause of human genetic disease.
The mitochondrial genome is small but highly mutable compared to nuclear DNA. The
than that of nuclear DNA. This is
10 times higher
s about
mutation rate of mtDNA i
caused by:
a lack of DNA repair mechanisms in the mtDNA, and
possibly by damage from free oxygen radicals released during the oxidative
phosphorylation process.
Mitochondrial inheritance
Mitochondrial encoded diseases have two unusual features:
Matrilineal inheritance and
Frequent heteroplasmy( a single cell can harbor some molecules that have an
mtDNA mutation and other molecules that do not).
In some patients with a mitochondrial disease, every mitochondrial genome carries
the causative mutation is (homoplasmy).
but is passed on only
both sexes,
Thus a mitochondrial inherited condition can affect
.
affected mothers
by
So small number of maternal mtDNA molecules give rise to all the mitochondrial DNA
of the child.
Each tissue type requires a certain amount of mitochondria produced ATP for normal
function. Organ systems with large ATP requirements and high thresholds tend to be
the ones most seriously affected by mitochondrial diseases.
For example, the central nervous system (CNS) consumes about 20% of the body’s
ATP production and therefore affected by mtDNA mutation.
122
disorders can be classified according to the type of mutation that causes
Mitochondrial
them.
pair substitutions, which brings change in single amino
-
(base
Missense mutations
1.
acid) in protein- coding mtDNA genes cause one of the best- known mtDNA diseases
called,
A. “Leber’s hereditary optic neuropathy” (LHON)
Characterized by sudden loss of vision and irreversible loss of sight as a result of optic
nerve death.
Vision loss typically begins in the third decade of life.
Heteroplasmy is rare in LHON, so expression tend to be uniform.
. (the cause of male affected is
all affected patients are male
unexpectedly almost
unknown, possibly LHON requires both a mitochondrial and X- linked mutation).
B. Mitochondrial Encephalomyopathy (MELAS) caused by a single- base tRNA mutation
in mitochondria, it is heteroplasmic.
Example:
mtDNA mutation due to insertion or deletion:
2.
1. Kearns- Sayre disease (muscle weakness, cerebellar damage, and heart failure)
2. Pearson syndrome (infantile pancreatic insufficiency, and lactic acidosis).
Mitochondrial mutations are also involved in some common human diseases.
A mitochondrial deletion causes a form of deafness,
MELAS mutation is seen in 1-2% of individuals with Type 2 DM, and
mtDNA defects may also be associated with some cases of Alzheimer disease.
