
DR.JAMAL
AB
1
PROTEIN SYNTHESIS INHIBITORS
A number of antibiotics exert their antimicrobial effects by targeting the bacterial
ribosome (30s and 50s subunits), which differs structurally from those of the
mammalian cytoplasmic ribosome therefore it will not affect the human cells except at
high levels of drugs which may cause toxic effects as a result of interaction with
mitochondrial ribosomes.
Protein synthesis inhibitors include:
1. Tetracyclines
2. Aminoglycosides
3. Macrolides
4. Chloramphenicol
5. Clindamycin
6. Quinupristin / dalfopristin
7. Linezolid
Tetracyclines
Tetracyclines are broad -spectrum antibiotics that consist of four fused rings.
Members:
Tetracycline, Doxycycline, Minocycline, Demeclocycline, Methacycline.
Mechanism of action:
Tetracyclines concentrate intracellularly in susceptible organisms. The drugs bind
reversibly to the 30S subunit of the bacterial ribosome. This action prevents binding of
tRNA to the mRNA–ribosome complex, thereby inhibiting bacterial protein synthesis
Antibacterial spectrum
The tetracyclines are bacteriostatic antibiotics effective against a wide variety of
organisms, including gram-positive and gram-negative bacteria, protozoa, spirochetes,
mycobacteria, and atypical species (see figure 39.3 in your book). They are commonly
used in the treatment of acne and Chlamydia infections (doxycycline).
Mechanism of resistance:
There are three types of tetracycline resistance:
1) Tetracycline efflux.
2) Ribosomal protection.
3) Tetracycline modification.
Pharmacokinetics
Administration: all are given orally; doxycycline and minocycline are available as oral
and intravenous (IV) preparations.
Absorption:
Well absorbed from the stomach and small intestine when taken orally.
Should not be taken with milk and milk products, which can bind with these
drugs and prevent their absorption. To prevent decreased absorption,
administer the tetracycline 1 hour before or 2 hours after meals.

DR.JAMAL
AB
2
Distribution:
Tetracyclines are distributed widely to tissues and body fluids.
They bind to tissues undergoing calcification e.g. teeth and bones.
Although all tetracyclines enter the CSF, levels are insufficient for therapeutic
efficacy, except for minocycline and doxycycline which is useful in eradicating
the meningococcal carrier state.
All tetracyclines cross the placental barrier, and concentrate in fetal bones and
dentition.
Fate:
Tetracycline is primarily excreted unchanged through the kidney
Doxycycline is primarily excreted unchanged through the bile and feces
(preferred in renally compromised patients).
Minocycline undergoes hepatic metabolism and excreted to a lesser extent
through the urine.
Adverse effects:
1. Gastric discomfort.
2. Effects on calcified tissue: Deposition in the bone and primary dentition occurs during
calcification in growing children. This causes discoloration and hypoplasia of the teeth
and a temporarily stunting of growth.
3. Fatal hepatotoxicity: in pregnant women who received high doses of tetracyclines,
especially if they were experiencing pyelonephritis.
4. Phototoxicity: Phototoxicity such as severe sunburn, occurs when a patient receiving
a tetracycline is exposed to sun or ultraviolet rays. This toxicity is encountered most
frequently with tetracycline, doxycycline, and demeclocycline.
5. Vestibular problems: Dizziness, nausea and vomiting .
6. Pseudotumor cerebri: Intracranial hypertension.
7. Superinfections.
8. Contraindications:
Renally impaired patients should not be treated with any of the tetracyclines
except doxycycline. Accumulation of tetracycline may aggravate preexisting
azotemia by interfering with protein synthesis, thus promoting amino acid
degredation.
The tetracyclines should not be employed in pregnant or breast feeding woman
or in children under 8 years of age.
GLYCYLCYCLINES
Tigecycline, a derivative of minocycline, is a broad-spectrum antimicrobial drug & is
indicated for the treatment of complicated skin and soft tissue infections, as well as
complicated intra-abdominal infections.
Mechanism of action
Tigecycline exhibits bacteriostatic action by reversibly binding to the 30S ribosomal
subunit and inhibiting protein synthesis.

DR.JAMAL
AB
3
Resistance
Resistance is primarily attributed to efflux pumps.
Pharmacokinetics
The primary route of elimination is hepatic.
Adverse effects
Nausea and vomiting.
Acute pancreatitis.
Elevations in liver enzymes and serum creatinine may also occur.
photosensitivity,
pseudotumor cerebri,
Discoloration of permanent teeth when used during tooth development.
Fetal harm when administered in pregnancy.
Tigecycline may decrease the clearance of warfarin and increase prothrombin
time. Therefore, the international normalized ratio should be monitored closely
when Tigecycline is coadministered with warfarin.
Aminoglycosides
Aminoglycosides provide effective bactericidal activity against: gram -negative bacilli,
some aerobic gram -positive bacteria mycobacteria & some protozoa.
Members of the group:
Amikacin
Gentamicin
Neomycin
Streptomycin
Tobramycin
Pharmacokinetics
Because aminoglycosides are absorbed poorly from the GI tract, they’re usually given
parenterally. After I.V. or I.M. administration, aminoglycoside absorption is rapid and
complete.
Distribution
Aminoglycosides are distributed widely in extracellular fluid. They readily cross the
placental barrier, but don’t cross the blood -brain barrier
Metabolism and excretion
Aminoglycosides aren’t metabolized. They’re excreted primarily unchanged by the
kidneys.
Pharmacodynamics
Aminoglycosides act as bactericidal drugs thereby interrupting protein synthesis.
Resistance
Resistance to aminoglycosides occurs via:
1) Efflux pumps,
2) Decreased uptake, and/or
3) Modification and inactivation by plasmid-associated synthesis of enzymes.

DR.JAMAL
AB
4
Uses
Aminoglycosides are most useful in treating:
- Infections caused by gram -negative bacilli
- Serious nosocomial (hospital-acquired) infections, such as gram -negative
bacteremia (abnormal presence of microorganisms in the bloodstream),
peritonitis (inflammation of the peritoneum, the membrane that lines the
abdominal cavity), and pneumonia,
- In critically ill patients urinary tract infections (UTIs) caused by enteric bacilli that
are resistant to less toxic antibiotics, such as penicillins and cephalosporins
infections of the central nervous system and the eye (treated with local
instillation).
- Streptomycin is active against many strains of mycobacteria, including
Mycobacterium tuberculosis.
Adverse effects
1. Ototoxicity: Ototoxicity (vestibular and auditory) is directly related to high peak
plasma levels and the duration of treatment. The antibiotic accumulates in the
endolymph and perilymph of the inner ear. Deafness may be irreversible and has been
known to affect developing fetuses. Patients simultaneously receiving concomitant
ototoxic drugs, such as cisplatin or loop diuretics, are particularly at risk.
2. Nephrotoxicity: Ranging from mild, reversible renal impairment to severe, potentially
irreversible, acute tubular necrosis.
3. Neuromuscular paralysis: This adverse effect is associated with a rapid increase in
concentrations (for example, high doses infused over a short period.) or concurrent
administration with neuromuscular blockers. Patients with myasthenia gravis are
particularly at risk. Prompt administration of calcium gluconate or neostigmine can
reverse the block that causes neuromuscular paralysis.
4. Allergic reactions: Contact dermatitis is a common reaction to topically applied
neomycin.
Macrolides
Erythromycin was the 1st of macrolides to find clinical application, both as a drug of
choice and as an alternative to penicillin in individuals who are allergic to B-lactam
antibiotics. The newer members of this family are clarithromycin and azithromycin.
Pharmacokinetics
Because erythromycin is acid -sensitive, it must be buffered or have an enteric coating
to prevent destruction by gastric acid. Erythromycin is absorbed in the duodenum. It’s
distributed to most tissues and body fluids except, in most cases, for cerebrospinal fluid
(CSF). However, as a class, macrolides can enter the CSF when meninges are inflamed.
Metabolism and excretion
Erythromycin is metabolized by the liver and excreted in bile in high concentrations;
small amounts are excreted in urine. It also crosses the placental barrier and is secreted
in breast milk.
DR.JAMAL
AB
5
Pharmacodynamics:
Generally considered to be bacteriostatic, they may be bactericidal at higher
doses.
The macrolides bind irreversibly to a site on the 50S subunit of the bacterial
ribosome, thus inhibiting translocation steps of protein synthesis.
Adverse effects
1. Gastric distress and motility: especially with erythromycin, the others are better
tolerated
2. Cholestatic jaundice
3. Ototoxicity: Transient deafness has been associated with erythromycin, especially at
high dosages. Azithromycin has also been associated with irreversible sensorineural
hearing loss.
4. Contraindications:
Patients
with
hepatic
dysfunction
Patients with proarrhythmic
conditions or concomitant use
of proarrhythmic agents.
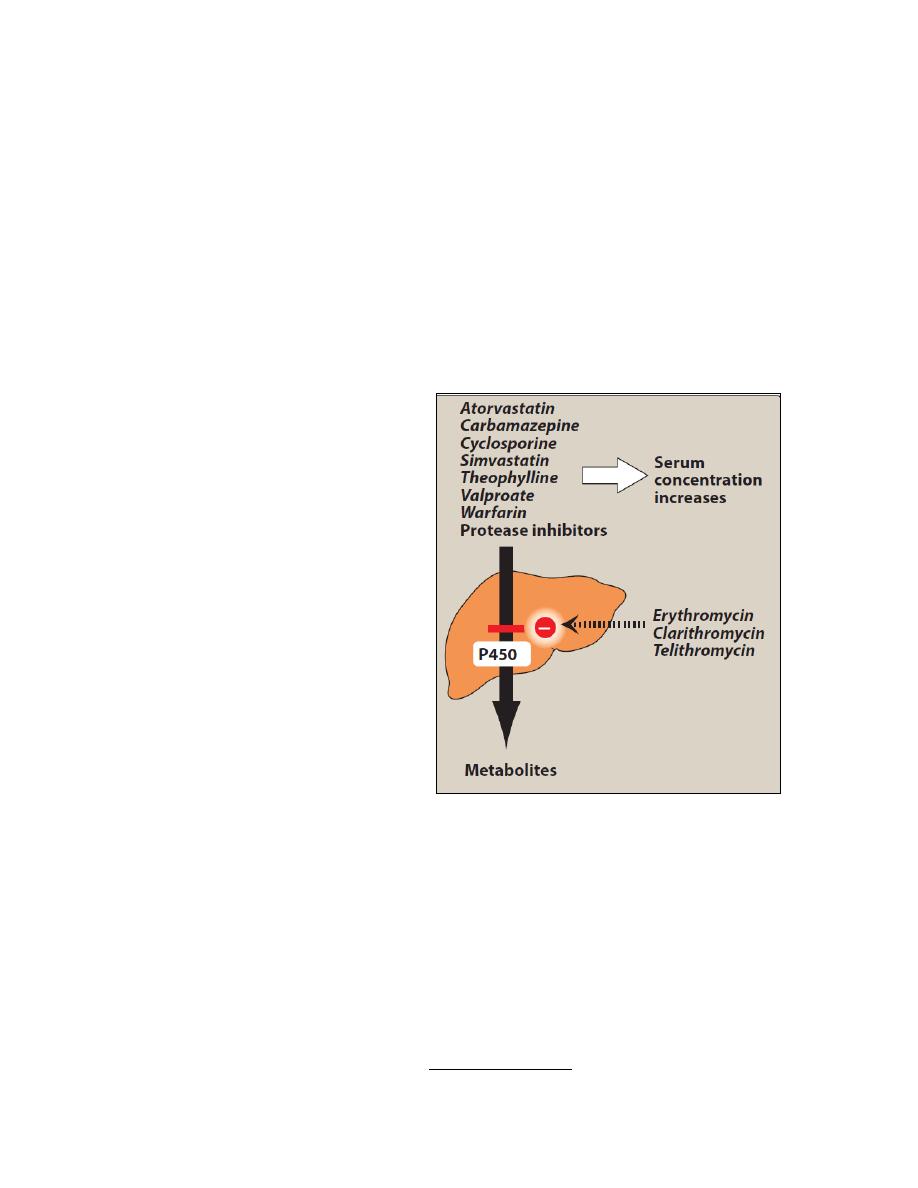
5. Drug interactions: Erythromycin,
telithromycin,
and
clarithromycin
inhibit the hepatic metabolism of a
number of drugs, which can lead to
toxic
accumulation
of
these
compounds (Figure). An interaction
with digoxin may occur. In this case,
the antibiotic eliminates a species of
intestinal
flora
that
ordinarily
inactivates digoxin, thus leading to
greater reabsorption of the drug from
the enterohepatic circulation.
Chloramphenicol:
The use of chloramphenicol, a broad-spectrum antibiotic, is restricted to life-threatening
infections for which no alternatives exist.
Mechanism of Action:
The drug binds to the bacterial 50s ribosomal subunit and inhibits protein synthesis.
At high levels; protein synthesis in mammalian mitochondrial ribosome may be inhibited
producing bone marrow toxicity.
Antibacterial Spectrum:
Chloramphenicol is active against many types of microorganisms including chlamydiae,
rickettsiae, spirochetes, and anaerobes. The drug is primarily bacteriostatic, but
depending on the dose and organism, it may be bactericidal.

DR.JAMAL
AB
6
Pharmacokinetics:
Chloramphenicol is completely absorbed via the oral route, widely distributed
throughout the body and readily enters the normal CSF. The drug inhibits the hepatic
cytochrome P450 system. Excretion of the drug depends on its conversion in the liver to
a glucuronide, which is then secreted by the renal tubule. Chloramphenicol is also
secreted into breast milk.
Adverse Effects:
The clinical use of chloramphenicol is limited to life-threatening infections because of
the serious adverse effects associated with its administration.
1. GIT upsets.
2. Overgrowth of Candida albicans may appear on mucous membrane.
3. Anemia; which may be:
a. Hemolytic anemia occurs in patients with low levels of glucose-6- phosphate
dehydrogenase.
b. Reversible anemia, which is apparently dose-related and occurs concomitantly
with therapy.
c. Aplastic anemia, which although rare is idiosyncratic and usually fatal. It's
independent of dose and may occur after therapy has been ceased.
4. Gray Baby Syndrome: occurs in neonates if the dosage regimen of chloramphenicol is
not properly adjusted. Neonates have a low capacity to glucuronylate the antibiotic and
they've underdeveloped renal function. The drug accumulates to the levels that
interfere with the function of mitochondrial ribosomes. This leads to depressed
breathing, cardiovascular collapse, cyanosis and death.
5. Interactions: Chloramphenicol inhibits some of the hepatic metabolism of many drugs
such warfarin and phenytoin, thereby elevating their concentration and potentiating
their effects.
Clindamycin:
Mechanism of action and resistance: similar to those of erythromycin.
Uses: used primarily in the treatment of infections caused by gram-positive organisms,
including MRSA and streptococcus, and anaerobic bacteria.
Administration: Clindamycin is available in both IV and oral formulations, but use of the
oral form is limited by gastrointestinal intolerance.
Distribution: It distributes well into all body fluids except CSF. Penetration into bone
occurs even in the absence of inflammation.
Metabolism: Clindamycin undergoes extensive oxidative metabolism to inactive
products and is primarily excreted into the bile. Low urinary elimination limits its clinical
utility for urinary tract infections
The most serious adverse effect is potentially fatal pseudomembranous colitis caused
by overgrowth of Clostridium difficile, which elaborates necrotizing toxins. Oral
administration of either metronidazole or vancomycin is usually effective in controlling
this serious problem.
Metronidazole is the drug of choice to treat pseudomembranous colitis.

DR.JAMAL
AB
7
QUINUPRISTIN/DALFOPRISTIN
Quinupristin/dalfopristin is a mixture of two streptogramins in a ratio of 30 to 70,
respectively. Due to significant adverse effects, the drug is normally reserved for the
treatment of severe vancomycin-resistant Enterococcus faecium (VRE) in the absence of
other therapeutic options.
A. Mechanism of action
Each component of this combination drug binds to a separate site on the 50S bacterial
ribosome.
B. Antibacterial spectrum
The combination drug is active primarily against gram-positive cocci, including those
resistant to other antibiotics. Its primary use is in the treatment of E. faecium infections,
including VRE strains. The drug is not effective against E. faecalis.
Pharmacokinetics
Quinupristin/dalfopristin is injected intravenously
Levels in the CSF are low. Both compounds undergo hepatic metabolism, with excretion
mainly in the feces.
Adverse effects
a. Venous irritation commonly occurs when quinupristin/dalfopristin is
administered through a peripheral rather than a central line.
b. Hyperbilirubinemia.
c. Arthralgia and myalgia.
d. Quinupristin/dalfopristin inhibits the cytochrome P450 isoenzyme, and
concomitant administration with drugs that are metabolized by this pathway
may lead to toxicities.
LINEZOLID
Linezolid is a synthetic oxazolidinone developed to
combat resistant gram-positive organisms, such as methicillin-resistant
Staphylococcus aureus, VRE, and penicillin-resistant streptococci.
Mechanism of action
Linezolid binds to the bacterial ribosome 50S site,
Antibacterial spectrum
The antibacterial action of linezolid is directed primarily against grampositive organisms,
such as staphylococci, streptococci, and enterococci, as well as Corynebacterium species
and Listeria monocytogenes. It is also moderately active against Mycobacterium
tuberculosis and may be used against drug-resistant strains.
Pharmacokinetics
Linezolid is given orally or intravenously.
The drug is widely distributed throughout the body.
It is metabolized via oxidation & excreted both by renal and nonrenal routes.
No dose adjustments are required for renal or hepatic dysfunction.

DR.JAMAL
AB
8
Adverse effects
The most common adverse effects are
gastrointestinal upset, nausea, diarrhea,
headache, and
Rash.
Thrombocytopenia has been reported, mainly in patients taking the drug for
longer than 10 days.
Linezolid possesses nonselective monoamine oxidase activity and may lead to
serotonin syndrome if given concomitantly with large quantities of tyramine-
containing foods, selective serotonin reuptake inhibitors, or monoamine oxidase
inhibitors. The condition is reversible when the drug is discontinued.
Irreversible peripheral neuropathies and optic neuritis (causing blindness) have
been associated with greater than 28 days of use, limiting utility for extended-
duration treatments.
