
second stage
Lec-1
9/10/2016
biochemistry
د. أحمد يحيى
Bioenergetics and Biological Oxidation
Bioenergetics and Biological Oxidation
Adinosine triphosphate (ATP)
Bioenergetics and Biological Oxidation
Bioenergetics is the study of energy changes or thermodynamic accompanying biochemical
reactions, which takes place inside the body.
The reactions in general are accompanied by liberation of energy, they may require energy,
or neither gives nor requires energy.
Energy is necessary for biological system to perform the different biochemical and
physiological activities. This energy can be obtained from the breakdown of different
nutritional substances (Fuel compounds. lipids, carbohydrates and proteins).
This explain why in certain conditions e.g. during starvation for several days or weeks may
end with death and this can be accounted due to depletion of energy from all stores or
reserves of energy inside the body
.
In certain other conditions when there are defects in energy or other metabolic problems
may result in certain metabolic disorders as e.g. in case of Kwashiorkor and Marasmus in
which there are defect in energy supply and quantity and types of proteins.
On the other hand increased energy intake more than body requirement will result in obesity.
However, the energy release is regulated by thyroid hormones.
In normal condition, there is a fine control between two processes these are energy
requirement and energy releasing.
This control or link is very important in bioenergetics as it occur through ATP (Adenosine
triphosphate) which is made up of 3 parts
1)
A nitrogenous base (Adenine)
2)
A sugar (Ribose) &
3) 3 Phosphoric acid molecules as shown.
The two outer phosphate atoms are very important
in the bioenergetics as the bonds in these
phosphates are high energy ( ~ ).

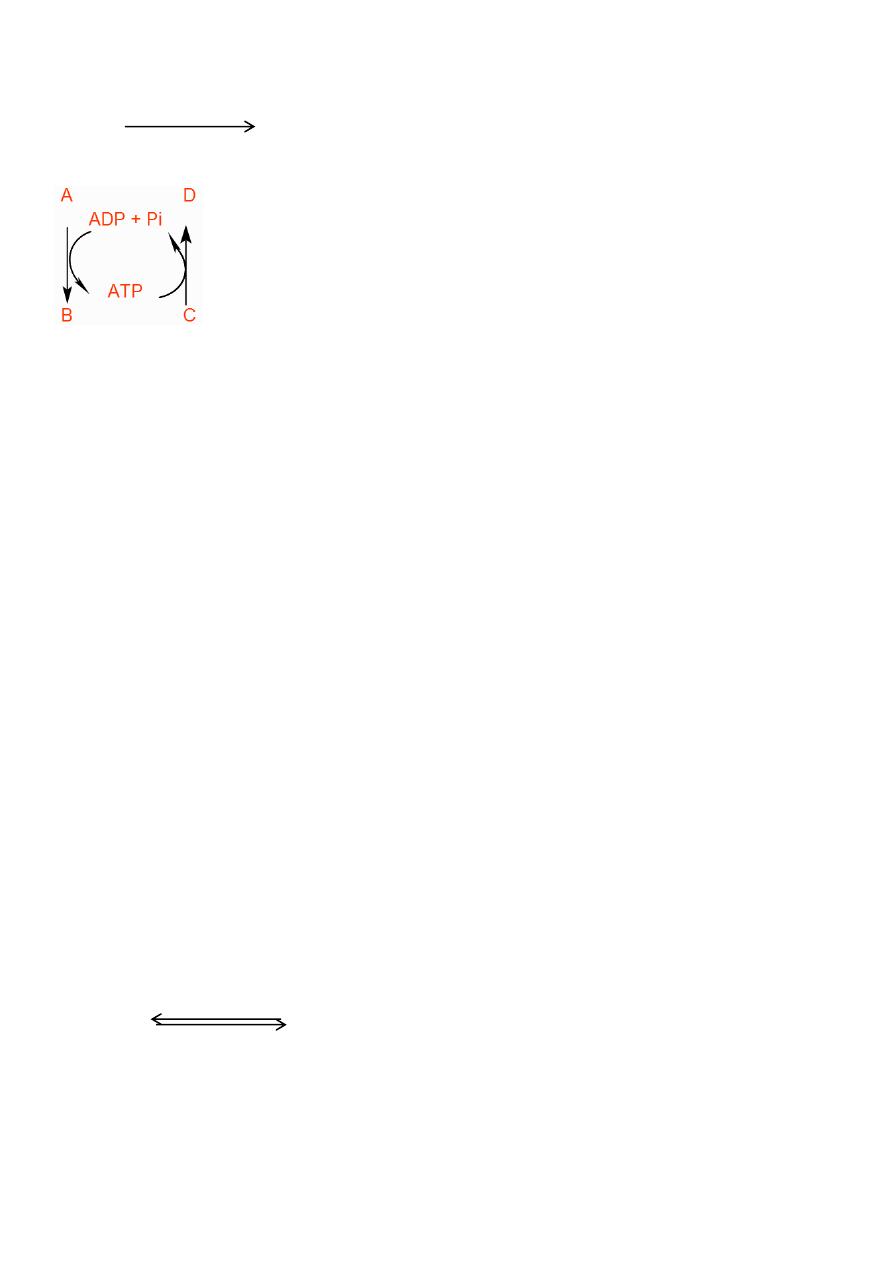
On hydrolysis of ATP, it gives adenosine diphosphate + Pi, in addition to energy.
ATP ADP + Pi + Energy (≈ 7Kcal)
This means it release the terminal inorganic phosphate and so it is converted to ADP+Pi. This
hydrolysis usually accompanied by liberation of energy or heat of about -7 Kcal (-30Kj)/ mol.
(- ve charge mean available energy while +ve charge mean required energy.
Under certain conditions further hydrolysis of ADP may occur resulting in the production of
AMP + Pi and this accompanied by liberation of almost the same quantity of energy.
ADP AMP + Pi + (≈7Kcal)
The phosphate atom in AMP is not considered as high-energy phosphate and so on hydrolysis
will not produce energy.
second stage
Lec-2
9/10/2016
biochemistry
د. أحمد يحيى
Bioenergetics and Biological Oxidation
Coupled Reactions
Biological Oxidation
Coupled Reactions:
The high-energy reaction (~), some times called energy favorable reaction or exergonic
reaction is usually coupled to another reaction e.g. if a compound A is converted to B in
certain biological system. If A is stable compound then B cannot appear in the medium unless
a catalyst** is present (Enzyme*) so an enzyme (E) is required
E
A B
* Enzyme: It is a catalyst in biological reactions. It is a protein in nature.
** Catalyst : It is certain substance, which help in increasing the speed of the reaction. It may
shear in the reaction and may suffer from physical changes but it return to its
original form at the end of the reaction.)
If this reaction is energy favorable then this reaction require another reaction to capture heat
or energy from the first reaction, otherwise it will dissipate. These reactions are called
coupled reactions and can be facilitated by the enzymes. Therefore, the enzyme may be
designed for the presence of ADP & Pi, which result in the formation of ATP.
E
ADP + Pi ATP
This reaction is a non favorable reaction (endergonic) and require energy, if we collect both
reactions in one equation
E
A + ADP + Pi B + ATP
Another example if a compound
E
C D
Where this reaction require an enzyme. If this reaction is non-favorable then it will require
an energy and so the enzyme in this case will provide ATP which will be hydrolyses to ADP+Pi
in addition to energy.
ATP ADP + Pi
When we collect both reactions:
E
C + ATP D + ADP + Pi
We can summarize the 2 reactions as follows
:
When A is changed to B energy is produced and captured by ADP+Pi and when C changed to
D it require energy and can be obtained from ATP.
However, all human and animals activities require energy, which can be available from the
breakdown of different fuel compounds in stepwise breakdown (Metabolism) in the cells and
any defect or fault will lead to certain disease due to abnormal metabolic disorder.
Exergonic and endergonic reactions are also terms used to describe the energy favorable and
energy required reactions respectively.
Exergonic or energy favorable reactions supply energy for the normal biochemical and
physiological functions of the body e.g. muscle contraction, nerve excitation, active
transport, synthetic reactions and so on.
Biological Oxidation:
About 1780 Lavoisier concluded that combustion processes must takes place in animal’s
organism. Since then biological oxidation has often been compared to combustion. Indeed
there is no difference in the product in both cases are CO2 + H2O + Energy.
Oxidation is defined today very generally as a loss of electrons (e). Oxidation of molecular
hydrogen can therefore be formulated as follows
H
2
-
2e
-
2H
+
……1
An oxidizing agent must accept the electrons. If we use e.g. a ferric salt, the equation
becomes:
H2 + 2 Fe
+++
= 2H
+
+ 2Fe
++
………. 2

Molecular oxygen can act as an oxidizing agent similarly by picking up either 2 or 4 electrons:
+2H+
O2 + 2 e- = O2-2 H2O2 …3
+4H+
O2 + 4 e- = 2O-2 2H2O ……4
Therefore, Oxidation is the removal of electrons and reduction is the gain of electrons.
Oxidation is always accompanied by reduction of an electron acceptor. Also,
dehydrogenation mean oxidation of a compound.
In chemical oxidation O2 can oxidizes the compound directly while in biological system O2 is
not directly oxidizes the compound but it shear in oxidation at the final stage, certain
enzymes and coenzymes* are responsible for oxidation instead of oxygen.
*Coenzyme: It is a prosthetic group in the enzyme molecule it is non-protein organic
substance, which inter really in the reaction but cannot catalyse the reaction
without the enzyme. They usually contain certain member of the B-complex
vitamin.
The sequence of the enzymes and carriers responsible for the transport of reducing
equivalent (e) from substrate* to molecular oxygen is known as respiratory chain or electron
transport chain or mitochondrial transport system, which is imbedded in the inner
membrane of the mitochondria.
*Substrate: is the substance upon which the enzymes act on.
This will carry the electrons on a series of carriers and when pass from one carrier to another
the first is oxidized and the next is reduced and the final one is oxygen. At the end, the final
compound is converted to energy in addition to H2O + CO2, which are excreted to the
outside.
Many enzymes and coenzymes participate in biological oxidation some of them are members
of the respiratory chain in the mitochondria. These are called oxido-reductases. They include:
1. Oxidases
2.
Dehydrogenases: a. aerobic dehydrogenases
b. Anaerobic dehydrogenases:
dependent on Nicotiamide (NAD&NADP)
dependent on flavine (FAD&FMN)
3.
Cytochromes
4.
Hydroperoxidases: Peroxidase , Catalases
5. Oxygenases: Mono , Di
6. CoQ (ubiquinone)

second stage
Lec-3
9/10/2016
biochemistry
د. أحمد يحيى
Bioenergetics and Biological Oxidation
Oxidases:
They act by the removal of hydrogen from one substrate to another and the substrate will be
oxidized. They use only oxygen as hydrogen acceptor. Water is always the product of this
reaction.
Dehydrogenases:
a- aerobic dehydrogenases: they catalyses removal of hydrogen from certain
substrates and can use oxygen or other compounds as hydrogen acceptor,
H2O2 is always the product.
•
Aerobic dehydrogenases usually contain flavoprotein as coenzyme i.e.
coenzyme containing FAD or FMN.
•
FAD: Flavine adnine dinucleotide
•
FMN: Flavine mononucleotide.
•
(Containing riboflavin or vit B2)
•
Riboflavine or Vit B2 : Consist of ribitol and flavine ,
available in all vegetables and milk , deficiency cause
inflammation of the tongue and angles of the
mouth(Chilosis).
b- Anaerobic dehydrogenases: catalysis the oxidation of substrate but can not use oxygen as
hydrogen acceptor.
dependent on Nicotiamide (NAD&NADP) : They contain in their structure the vitamin
niacin or vit B3. Niacine or Vit B3 (Nicotinic acid) is a non toxic part of a toxic alkaloid
present in tobacco (nicotin) present in milk and leafy vegetables. Diffeciency causes
Pellagra( or 3 d)(Dermatitis, Dementia, and diarrhea. NAD is a member the respiratory
chain of the mitochondria. The active part of NAD that participates in the reaction is
the nicotinamide.
dependent on Riboflavin:
The active part is riboflavin or vitB2 e.g.
of this coenzyme is FAD and FMN.
Cytochromes:
They are members of respiratory chain. They function as carries of electrons from CoQ to
cytochrome oxidase.
The cytochromes contain iron as the active part in the center of porphyrin (Cytochrome).
The Fe atom will oscillate from ferric to ferrous (Fe
+3
and Fe
+2
) during oxidation and reduction.
When these enzymes catalyse the oxidation, the metabolite will be oxidized and Fe atom will
be reduced. At least five cytochromes were isolated in the respiratory chain of the
mitochondria. These are cytochrome b, c1, c, a & a3.
Hydroperoxidases:
They are group of enzymes act mainly using hydrogen peroxide (H2O2) as a substrate
hydolysing it to H2O and O2.
Oxygenases:
They are group of enzymes catalysis the incorporation of oxygen into substrate molecule.
They are two types:
a)
Dioxygenases:
b)
Monooxygenases:
CoQ or ubiquinone:
This coenzyme is present in the inner mitochondrial membrane. It is a member of the
respiratory chain, and has the property of electron carrier

second stage
Lec-4
9/10/2016
biochemistry
د. أحمد يحيى
Bioenergetics and Biological Oxidation
• Respiration and respiratory chain
• Sequence of Redox system in the respiratory chain:
• Redox occurs in the Mitochondria:
• Sites of ATP production
• Oxidative Phosphorylation
Respiration and respiratory chain:
The mitochondrion in each cell is regarded as the power house. Inside the mitochondria,
there are series of carrier, which carry the electrons (also called reducing equivalents). This
system is called respiratory chain or mitochondrial transport system or electron transport
chain. This chain or system is imbedded in the inner mitochondrial membrane arranged in
certain manner through increasing redox potential.
The reason for this arrangement may be due to that NAD which is the first member of the
respiratory chain which accept H from the substrate specific dehydrogenase ) if it is directly
react with oxygen then large amount of energy will be produced. This energy may be larger
than the ability of a single coupling reaction and so energy may dissipate.
Instead it is subdivided into small individual energy so NADH2 does not react directly with
oxygen but in a series of intermediate steps with the possibility of coupling each step with
other reaction thus keeping much of the energy formed.
As the energy of biological system is in the form of ATP so the respiratory chain coupled with
phosphorylation i.e. with the formation of ATP from ADP+Pi and the whole process is called
oxidative phosphorylation.
Sequence of Redox system in the respiratory chain:
The arrangement of component enzymes and coenzymes in the respiratory chain depend on
the redox potential. The most negative potential is that of NAD so it is the first member of
the RC, which can receive H from the substrate and become reduced.
The hydrogen is then transferred to the next member of the RC (FP) and so the first member
(NADH2) is reoxidised and the second one is reduced and become FPH2 then the H will be
transferred to the third (CoQ) and so flavoprotein will be reoxidised.
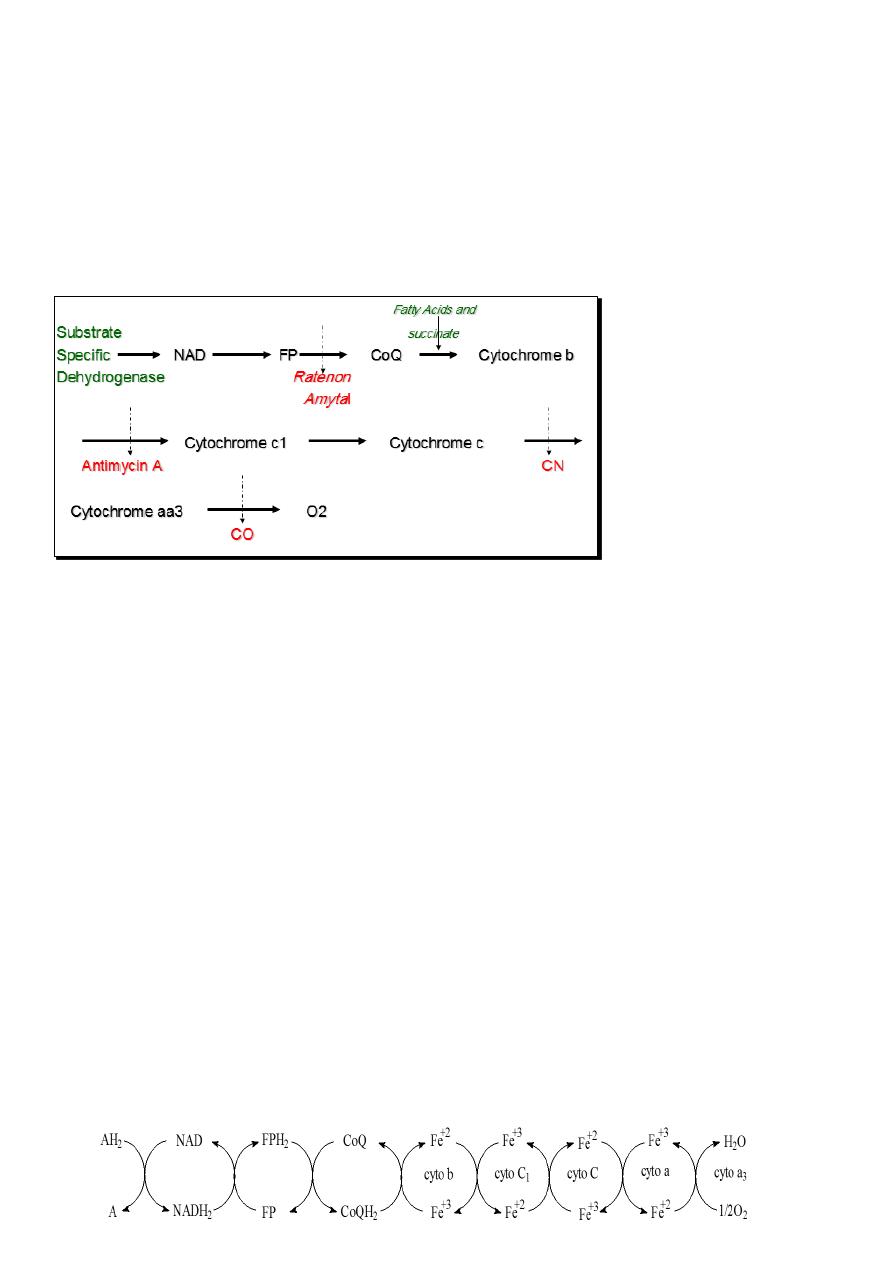
CoQ is also a point or a site for the entry of hydrogen from the dehydrogenation of succinate
and fatty acids. The next member of the RC does the reoxidation of CoQ, which is the
cytochrome system that acts by change of valence of iron. From this point, only electrons are
transferred.
The cytochromes are arranged also according to their redox potential in the sequence shown
where cytochrome aa3 transfer the electrons to oxygen, which immediately pick up 2H+ to
produce water.
Certain inhibitors like
Ratenon and Amytal,
antimycin A, Cyanide
and CO can block the
redox at the sites
shown.
Redox occurs in the Mitochondria:
Any oxidation-reduction reaction is accompanied by transfer of electrons between the two
systems.
This happen in the mitochondria as pair of electrons are given to NAD which is the first
member of the RC from different intermediate like glutamate, pyrovate, α-keto acids by
different dehydrogenases systems.
This pair of electrons then flows from the first member of the RC (NAD) to the second
member (FP) and so on until the final component of the RC giving its pair of (e) to oxygen
forming water.
There are certain intermediate which cannot give their pair of electrons to the first member
(NAD) instead they give it to CoQ and this is due to charge on the compound.
When the electrons go to CoQ, they also flow in the same direction of the chain and finally
reach oxygen to produce water and energy.
Therefore, the electrons can pass to the RC either through NAD or through CoQ. It is clear
that the transfer of electrons from one member to another in the RC is an oxidation-
reduction process. The following scheme shows the redox in the mitochondria.
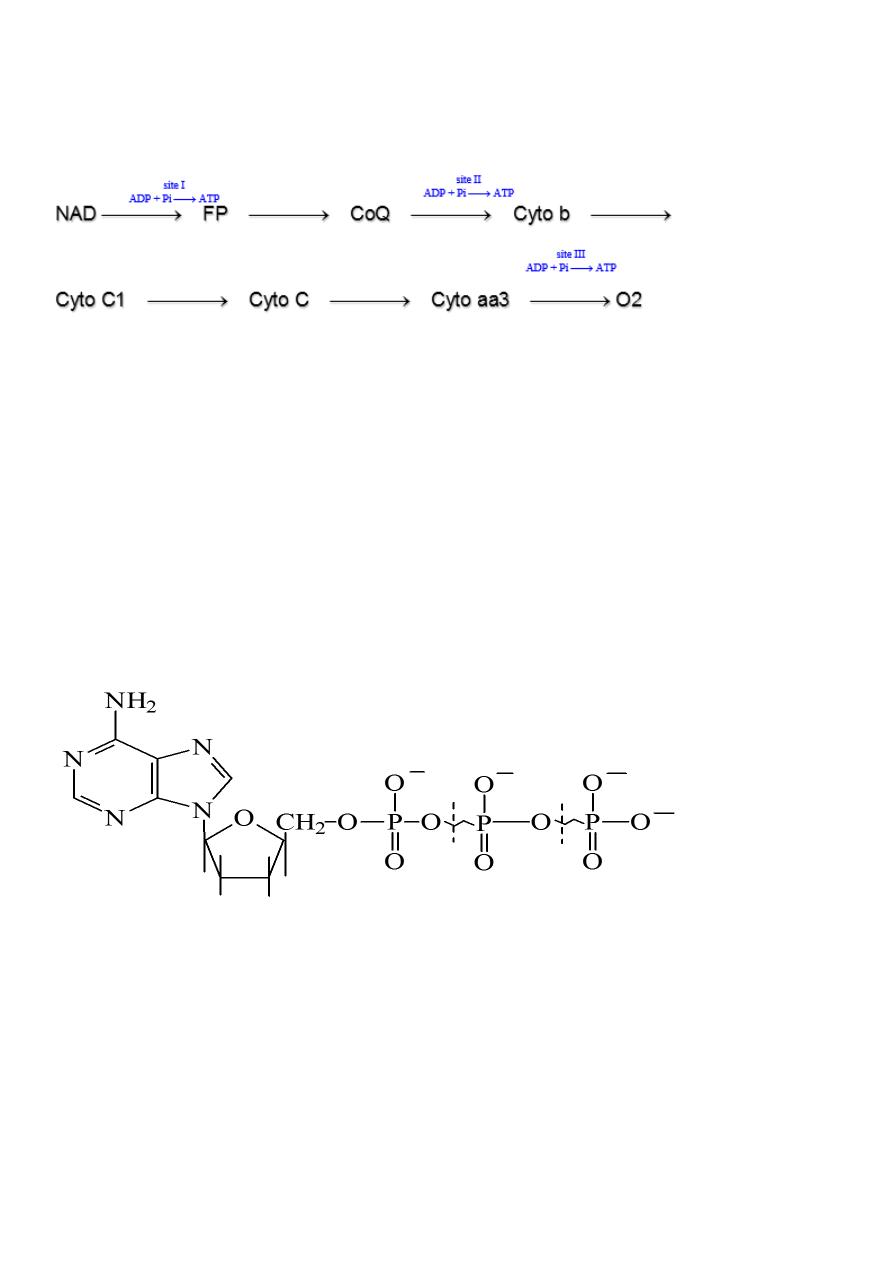
Sites of ATP production:
Each pair of electrons when it enter the chain from the beginning till react with oxygen then
one high energy product (ATP) is produced in 3 sites between NADH2 and FP, CoQ or
ubiquinone and cytochrome b and Cytochrome aa3 and O2.
Therefore 3 molecules of ATP are produced from the cycle when started from the beginning
(NAD) to the end. While when the electrons enter the RC from CoQ then 2 ATP are formed
as it miss or bypass the first site of synthesis of ATP.
Oxidative Phosphorylation:
The importance of RC is because free energy of oxidation of some of the individual steps are
trapped or stored in the form of ATP using ADP+Pi in each site. This process is termed
oxidative phosphorylation or RC phosphorylation.
It is found that 1 ATP can arise for each pair of electrons (or hydrogen) transferred at each
of 3 sites between NADH2 and FP, ubiquinone and Cyto b and Cyto aa3 and oxygen.
ATP
The high energy compound, ATP speaks:
I am the energy currency of the cell!
Continuous consumption and regeneration is my role;
Without me, all biochemical functions come to a standstill;
Existence of life is unimaginable without my will
