
Esse
Esse
Esse
Esse
Essent
nt
nt
nt
ntials o
ials o
ials o
ials o
ials offfff
P H Y
P H Y
P H Y
P H Y
P H Y S I O L
S I O L
S I O L
S I O L
S I O L O
O
O
O
O G Y
G Y
G Y
G Y
G Y
fffffo
o
o
o
or D
r D
r D
r D
r Deeeeental St
ntal St
ntal St
ntal St
ntal Stud
ud
ud
ud
udeeeeents
nts
nts
nts
nts

Esse
Esse
Esse
Esse
Essent
nt
nt
nt
ntials o
ials o
ials o
ials o
ials offfff
P H Y
P H Y
P H Y
P H Y
P H Y S I O L
S I O L
S I O L
S I O L
S I O L O
O
O
O
O G Y
G Y
G Y
G Y
G Y
fffffo
o
o
o
or D
r D
r D
r D
r Deeeeental St
ntal St
ntal St
ntal St
ntal Stud
ud
ud
ud
udeeeeents
nts
nts
nts
nts
JAYPEE BROTHERS MEDICAL PUBLISHERS (P) LTD
Chennai • St Louis (USA) • Panama City (Panama) • London (UK) • New Delhi
Ahmedabad • Bengaluru • Hyderabad • Kochi • Kolkata • Lucknow • Mumbai • Nagpur
®
K Sembulingam
PhD
Shri Sathya Sai Medical College and Research Institute
Thiruporur-Guduvancherry Main Road, Nellikuppam, Tamil Nadu, India
Formerly at
MR Medical College, Gulbarga, Karnataka, India
Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India
School of Health Sciences, Universiti Sains Malaysia, Kelantan, Malaysia and
Sri Lakshmi Narayana Institute of Medical Sciences, Puducherry, India
Prema Sembulingam
PhD
Sathyabama University Dental College and Hospital
Jeppiaar Nagar, Old Mahabalipuram Road, Chennai
Tamil Nadu, India
Formerly at
MR Medical College, Gulbarga, Karnataka, India
Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India
School of Health Sciences, Universiti Sains Malaysia, Kelantan, Malaysia and
Sri Lakshmi Narayana Institute of Medical Sciences, Puducherry, India
Shri Sathya Sai Medical College and Research Institute, Nellikuppam, Tamil Nadu, India

Published by
Jitendar P Vij
Jaypee Brothers Medical Publishers (P) Ltd
Corporate office
4838/24 Ansari Road, Daryaganj, New Delhi - 110002, India, Phone: +91-11-43574357, Fax: +91-11-43574314
Registered office
B-3 EMCA House, 23/23B Ansari Road, Daryaganj, New Delhi - 110 002, India
Phones: +91-11-23272143, +91-11-23272703, +91-11-23282021
+91-11-23245672, Rel: +91-11-32558559, Fax: +91-11-23276490, +91-11-23245683
e-mail: jaypee@jaypeebrothers.com, Website: www.jaypeebrothers.com
Offices in India
•
Ahmedabad, Phone: Rel: +91-79-32988717, e-mail: ahmedabad@jaypeebrothers.com
•
Bengaluru, Phone: Rel: +91-80-32714073, e-mail: bangalore@jaypeebrothers.com
•
Chennai, Phone: Rel: +91-44-32972089, e-mail: chennai@jaypeebrothers.com
•
Hyderabad, Phone: Rel:+91-40-32940929, e-mail: hyderabad@jaypeebrothers.com
•
Kochi, Phone: +91-484-2395740, e-mail: kochi@jaypeebrothers.com
•
Kolkata, Phone: +91-33-22276415, e-mail: kolkata@jaypeebrothers.com
•
Lucknow, Phone: +91-522-3040554, e-mail: lucknow@jaypeebrothers.com
•
Mumbai, Phone: Rel: +91-22-32926896, e-mail: mumbai@jaypeebrothers.com
•
Nagpur, Phone: Rel: +91-712-3245220, e-mail: nagpur@jaypeebrothers.com
Overseas offices
•
North America office
,
USA, Ph: 001-636-6279734, e-mail: jaypee@jaypeebrothers.com,
anjulav@jaypeebrothers.com
•
Central America office, Panama City, Panama, Ph: 001-507-317-0160, e-mail: cservice@jphmedical.com
Website: www.jphmedical.com
•
Europe office, UK, Ph: +44 (0) 2031708910, e-mail: info@jpmedpub.com
Essentials of Physiology for Dental Students
© 2011, Jaypee Brothers Medical Publishers
All rights reserved. No part of this publication should be reproduced, stored in a retrieval system, or transmitted in
any form or by any means: electronic, mechanical, photocopying, recording, or otherwise, without the prior written
permission of the authors and the publisher.
This book has been published in good faith that the material provided by authors is original. Every effort is made
to ensure accuracy of material, but the publisher, printer and authors will not be held responsible for any inadvert-
ent error (s). In case of any dispute, all legal matters are to be settled under Delhi jurisdiction only.
First Edition:
2011
ISBN 978-93-5025-076-1
Typeset at
JPBMP typesetting unit
Printed at

To
Our beloved students

We, the authors of
Essentials of Medical Physiology are proud to bring out another textbook in
Physiology, titled
Essentials of Physiology for Dental Students. This is the outcome of requests,
wishes and friendly orders from different category of people including the dental and paramedical
students and faculties.
Physiology is different from other biomedical sciences as it deals with the functional aspects of
various systems in the living body along with the emphasis on the regulatory mechanism that maintain
the normalcy of the functions within narrow limits. It forms the strong foundation on which other
medical fields are constructed.
The primary aim of this book is to meet the needs of the dental, paramedical and health science
students precisely from the examination point of view, in getting knowledge of recent developments
in the field of physiology and in knowing the important applied aspects of various topics.
The descriptive diagrams are given in such a way that the students can easily understand and
reproduce them wherever necessary. The explanation of the topics is supported with the flow charts
and tables which make the reading a pleasure and stress-free.
In the starting of each chapter, we have included the topics that are to be learnt in that particular
chapter which will help the reader to remember the contents while revising the topic. At the end of
each section, the long questions and short questions are given for the follow-up of the topics.
This venture is possible only because of blessings of professors, best wishes and cooperation
of our friends and co-teachers and the students who know what they want and where to get them.
We are grateful and thankful to one and all for being the well wishers of us.
We wish to continue our services to the students’ community through this book. We are confident
that the opinions, comments and valuable suggestions from one and all coming across this book
will help us to improve it further to meet the needs of everyone who has Physiology as subject in
their career.
K Sembulingam
ksembu@yahoo.com
Prema Sembulingam
premsem@yahoo.com
Preface


Contents
Section 1: General Physiology
1. Cell ........................................................................................................................... 3
• Introduction ............................................................................................................ 3
• Structure of the Cell .............................................................................................. 4
• Cell Membrane ...................................................................................................... 4
• Cytoplasm .............................................................................................................. 6
• Organelles in Cytoplasm ...................................................................................... 6
• Organelles with Limiting Membrane .................................................................... 6
• Organelles without Limiting Membrane ............................................................. 10
• Nucleus ................................................................................................................ 11
• Cell Death ............................................................................................................ 12
2. Cell Junctions ........................................................................................................ 14
• Definition and Classification ................................................................................ 14
• Occluding Junction .............................................................................................. 14
• Communicating Junctions ................................................................................... 15
• Anchoring Junctions ............................................................................................ 15
3. Transport through Cell Membrane ...................................................................... 17
• Introduction .......................................................................................................... 17
• Basic Mechanism of Transport ........................................................................... 17
• Passive Transport ................................................................................................ 17
• Active Transport ................................................................................................... 20
4. Homeostasis ........................................................................................................... 25
• Introduction ........................................................................................................... 25
• Components of Homeostatic System .................................................................. 25
• Homeostasis and Various Systems of the Body .................................................. 26
Section 2: Blood and Body Fluids
5. Body Fluids ............................................................................................................ 33
• Introduction ........................................................................................................... 33
• Compartments of Body Fluids — Distribution of Body Fluids ............................. 33
• Composition of Body Fluids ................................................................................. 34
• Measurement of Body Fluid Volume .................................................................... 34
• Maintenance of Water Balance ............................................................................ 36
• Applied Physiology ............................................................................................... 36

x
Essentials of Physiology for Dental Students
6. Blood and Plasma Proteins ................................................................................. 38
• Blood .................................................................................................................... 38
• Plasma Proteins ................................................................................................... 40
7. Red Blood Cells ..................................................................................................... 43
• Introduction ........................................................................................................... 43
• Normal Value ........................................................................................................ 43
• Morphology of Red Blood Cells ........................................................................... 43
• Properties of Red Blood Cells .............................................................................. 44
• Lifespan of Red Blood Cells ................................................................................ 44
• Fate of Red Blood Cells ....................................................................................... 44
• Functions of Red Blood Cells .............................................................................. 45
• Variations in Number of Red Blood Cells ............................................................ 45
• Variations in Size of Red Blood Cells .................................................................. 47
• Variations in Shape of Red Blood Cells ............................................................... 47
• Hemolysis and Fragility of RBC ........................................................................... 47
8. Erythropoiesis ........................................................................................................ 49
• Definition .............................................................................................................. 49
• Site of Erythropoiesis ........................................................................................... 49
• Process of Erythropoiesis .................................................................................... 50
9. Hemoglobin ............................................................................................................ 54
• Introduction ........................................................................................................... 54
• Normal Hemoglobin Content ............................................................................... 54
• Functions of Hemoglobin ..................................................................................... 54
• Structure of Hemoglobin ...................................................................................... 55
• Types of Normal Hemoglobin .............................................................................. 55
• Abnormal Hemoglobin ......................................................................................... 55
• Abnormal Hemoglobin Derivatives ...................................................................... 55
• Synthesis of Hemoglobin ..................................................................................... 56
• Destruction of Hemoglobin .................................................................................. 56
10. Erythrocyte Sedimentation Rate and Packed Cell Volume ............................. 57
• Erythrocyte Sedimentation Rate .......................................................................... 57
• Packed Cell Volume ............................................................................................. 59
11. Anemia ..................................................................................................................... 60
• Introduction ........................................................................................................... 60
• Classification of Anemia ....................................................................................... 60
• Signs and Symptoms of Anemia .......................................................................... 63
12. White Blood Cells .................................................................................................. 64
• Introduction ........................................................................................................... 64
• Classification ........................................................................................................ 64
• Morphology of White Blood Cells ........................................................................ 64
• Normal Leukocyte Count ..................................................................................... 65
• Variations in Leukocyte Count ............................................................................. 65
• Lifespan of White Blood Cells .............................................................................. 66
• Properties of WBCs ............................................................................................. 66
• Functions of WBCs .............................................................................................. 68
• Leukopoiesis ........................................................................................................ 70
xi
Contents
13. Immunity.................................................................................................................. 71
• Definition and Types of Immunity ......................................................................... 72
• Development and Processing of Lymphocytes ................................................... 72
• Antigens ............................................................................................................... 73
• Development of Cell Mediated Immunity ............................................................. 74
• Development of Humoral Immunity ..................................................................... 76
• Natural Killer Cell ................................................................................................. 78
• Cytokines .............................................................................................................. 78
• Immune Deficiency Diseases .............................................................................. 78
• Autoimmune Diseases ......................................................................................... 79
14. Platelets ................................................................................................................... 80
• Introduction ........................................................................................................... 80
• Structure and Composition .................................................................................. 80
• Normal Count and Variations ............................................................................... 81
• Properties of Platelets .......................................................................................... 81
• Functions of Platelets ........................................................................................... 81
• Development of Platelets ..................................................................................... 82
• Lifespan and Fate of Platelets ............................................................................. 82
• Applied Physiology – Platelet Disorders .............................................................. 82
15. Hemostasis and Coagulation of Blood .............................................................. 83
• Hemostasis........................................................................................................... 84
• Definition of Blood Coagulation ........................................................................... 85
• Factors Involved in Blood Clotting ....................................................................... 85
• Sequence of Clotting Mechanism ........................................................................ 85
• Blood Clot ............................................................................................................. 88
• Anticlotting Mechanism in the Body ..................................................................... 88
• Anticoagulants ...................................................................................................... 88
• Physical Methods to Prevent Blood Clotting ........................................................ 90
• Procoagulants ...................................................................................................... 90
• Tests for Clotting .................................................................................................. 90
• Applied Physiology ............................................................................................... 91
16. Blood Groups and Blood Transfusion ............................................................... 93
• Introduction ........................................................................................................... 93
• ABO Blood Groups ............................................................................................... 93
• Rh Factor .............................................................................................................. 96
• Other Blood Groups ............................................................................................. 99
• Importance of knowing Blood Group ................................................................... 99
• Blood Transfusion ................................................................................................ 99
17. Reticuloendothelial System and Tissue Macrophage .................................... 101
• Definition and Distribution ................................................................................. 101
• Classification of Reticuloendothelial Cells ....................................................... 101
• Functions of Reticuloendothelial System ......................................................... 102
• Spleen ................................................................................................................ 103
18. Lymphatic System and Lymph .......................................................................... 104
• Lymphatic System ............................................................................................. 104
• Lymph Nodes ..................................................................................................... 104
• Lymph ................................................................................................................. 105

xii
Essentials of Physiology for Dental Students
19. Tissue Fluid and Edema ..................................................................................... 107
• Definition ............................................................................................................ 107
• Functions of Tissue Fluid ................................................................................... 107
• Formation of Tissue Fluid .................................................................................. 107
• Applied Physiology – Edema ............................................................................. 107
Section 3: Muscle Physiology
20. Classification of Muscles ................................................................................... 113
• Depending upon Striations ................................................................................ 113
• Depending upon Control ................................................................................... 113
• Depending upon Situation ................................................................................. 113
21. Structure of Skeletal Muscle .............................................................................. 116
• Muscle Mass ...................................................................................................... 116
• Muscle Fiber ....................................................................................................... 116
• Myofibril .............................................................................................................. 117
• Sarcomere .......................................................................................................... 117
• Contractile Elements (Proteins) of Muscle ........................................................ 118
• Sarcotubular System .......................................................................................... 119
• Composition of Muscle ....................................................................................... 120
22. Properties of Skeletal Muscle ............................................................................ 121
• Excitability .......................................................................................................... 121
• Contractility ......................................................................................................... 122
• Muscle Tone ....................................................................................................... 125
• Applied Physiology – Abnormalities of Muscle Tone ......................................... 125
23. Electrical and Molecular Changes during Muscular Contraction ................ 126
• Electrical Changes During Muscular Contraction .............................................. 126
• Molecular Changes During Muscular Contraction ............................................. 129
24. Neuromuscular Junction .................................................................................... 132
• Definition and Structure ...................................................................................... 132
• Neuromuscular Transmission ............................................................................ 133
• Neuromuscular Blockers .................................................................................... 134
• Motor Unit ........................................................................................................... 135
• Applied Physiology – Disorders of Neuromuscular Junction............................. 135
25. Smooth Muscle .................................................................................................... 136
• Distribution of Smooth Muscle ........................................................................... 136
• Structure of Smooth Muscle ............................................................................... 136
• Types of Smooth Muscle Fibers ........................................................................ 137
• Electrical Activity in Single Unit Smooth Muscle ................................................ 137
• Electrical Activity in Multiunit Smooth Muscle .................................................... 139
• Contractile Process in Smooth Muscle .............................................................. 139
• Control of Smooth Muscle .................................................................................. 140
Section 4: Digestive System
26. Introduction to Digestive System ...................................................................... 145
• Introduction ......................................................................................................... 145

xiii
Contents
• Functional Anatomy of the Digestive System .................................................... 145
• Wall of Gastrointestinal Tract ............................................................................. 146
• Nerve Supply to Gastrointestinal Tract .............................................................. 147
27. Mouth and Salivary Glands ................................................................................. 149
• Functional Anatomy of Mouth ............................................................................ 149
• Functions of Mouth ............................................................................................. 149
• Salivary Glands .................................................................................................. 149
• Properties and Composition of Saliva ............................................................... 151
• Functions of Saliva ............................................................................................. 151
• Regulation of Salivary Secretion ........................................................................ 153
• Effect of Drugs and Chemicals on Salivary Secretion ....................................... 155
• Applied Physiology ............................................................................................. 155
28. Stomach ................................................................................................................ 157
• Functional Anatomy of Stomach ........................................................................ 157
• Glands of Stomach ............................................................................................. 158
• Functions of Stomach ........................................................................................ 159
• Properties and Composition of Gastric Juice .................................................... 160
• Functions of Gastric Juice .................................................................................. 160
• Secretion of Gastric Juice .................................................................................. 161
• Regulation of Gastric Secretion ......................................................................... 162
• Applied Physiology ............................................................................................. 166
29. Pancreas ............................................................................................................... 168
• Functional Anatomy and Nerve Supply of Pancreas ......................................... 168
• Properties and Composition of Pancreatic Juice ............................................... 168
• Functions of Pancreatic Juice ............................................................................ 169
• Neutralizing Action of Pancreatic Juice ............................................................. 171
• Regulation of Pancreatic Secretion ................................................................... 172
• Applied Physiology ............................................................................................. 173
30. Liver and Gallbladder .......................................................................................... 174
• Functional Anatomy of Liver and Biliary System ............................................... 174
• Blood Supply to Liver ......................................................................................... 175
• Properties and Composition of Bile ................................................................... 176
• Formation of Bile ................................................................................................ 177
• Storage of Bile .................................................................................................... 177
• Bile Salts ............................................................................................................ 177
• Bile Pigments ..................................................................................................... 179
• Functions of Bile ................................................................................................. 180
• Functions of Liver ............................................................................................... 180
• Gallbladder ......................................................................................................... 181
• Regulation of Bile Secretion .............................................................................. 182
• Applied Physiology ............................................................................................. 183
31. Small Intestine ...................................................................................................... 186
• Functional Anatomy ............................................................................................ 186
• Intestinal Villi and Glands of Small Intestine...................................................... 186
• Properties and Composition of Succus Entericus ............................................. 187
• Functions of Succus Entericus .......................................................................... 187
• Functions of Small Intestine ............................................................................... 188
xiv
Essentials of Physiology for Dental Students
• Regulation of Secretion of Succus Entericus .................................................... 189
• Applied Physiology – Malabsorption .................................................................. 189
32. Large Intestine ..................................................................................................... 190
• Functional Anatomy of Large Intestine .............................................................. 190
• Secretions of Large Intestine ............................................................................. 190
• Functions of Large Intestine............................................................................... 191
• Applied Physiology ............................................................................................. 191
33. Movements of Gastrointestinal Tract ................................................................. 193
• Mastication ......................................................................................................... 193
• Deglutition .......................................................................................................... 193
• Movements of Stomach ..................................................................................... 196
• Filling and Emptying of Stomach ....................................................................... 196
• Vomiting .............................................................................................................. 197
• Movements of Small Intestine ............................................................................ 198
• Movements of Large Intestine ............................................................................ 200
• Defecation .......................................................................................................... 200
Section 5: Renal Physiology and Skin
34. Kidney.................................................................................................................... 205
• Introduction ......................................................................................................... 205
• Functions of Kidney............................................................................................ 205
• Functional Anatomy of Kidney ........................................................................... 206
35. Nephron and Juxtaglomerular Apparatus ........................................................ 208
• Introduction ......................................................................................................... 208
• Renal Corpuscle ................................................................................................. 208
• Tubular Portion of Nephron ................................................................................ 211
• Collecting Duct ................................................................................................... 213
• Juxtaglomerular Apparatus ................................................................................ 214
36. Renal Circulation ................................................................................................. 216
• Introduction ......................................................................................................... 216
• Renal Blood Vessels .......................................................................................... 216
• Measurement of Renal Blood Flow .................................................................... 217
• Regulation of Renal Blood Flow ........................................................................ 217
• Special Features of Renal Circulation ............................................................... 218
37. Urine Formation ................................................................................................... 219
• Introduction ......................................................................................................... 219
• Glomerular Filtration .......................................................................................... 220
• Tubular Reabsorption ......................................................................................... 223
• Tubular Secretion ............................................................................................... 226
• Summary of Urine Formation ............................................................................. 226
38. Concentration of Urine ....................................................................................... 227
• Introduction ......................................................................................................... 227
• Medullary Gradient ............................................................................................. 228
• Countercurrent Mechanism ................................................................................ 228
• Role of ADH ....................................................................................................... 230
• Summary of Urine Concentration ...................................................................... 231

xv
Contents
39. Acidification of Urine and Role of Kidney in Acid-Base Balance ................ 233
• Introduction ......................................................................................................... 233
• Secretion of Hydrogen Ions ............................................................................... 233
• Removal of Hydrogen Ions and Acidification of Urine ....................................... 234
40. Renal Function Tests .......................................................................................... 236
• Properties and Composition of Normal Urine .................................................... 236
• Renal Function Tests .......................................................................................... 236
• Examination of Urine — Urinalysis .................................................................... 236
• Examination of Blood ......................................................................................... 237
• Examination of Blood and Urine ........................................................................ 237
41. Micturition ............................................................................................................. 239
• Introduction ......................................................................................................... 239
• Functional Anatomy of Urinary Bladder ............................................................. 239
• Nerve Supply to Urinary Bladder and Sphincters .............................................. 239
• Filling of Urinary Bladder .................................................................................... 241
• Micturition Reflex ................................................................................................ 242
• Applied Physiology ............................................................................................. 243
42. Skin ....................................................................................................................... 244
• Structure of Skin ................................................................................................. 244
• Glands of Skin .................................................................................................... 245
• Functions of the Skin ......................................................................................... 247
43. Body Temperature ............................................................................................... 249
• Introduction ......................................................................................................... 249
• Body Temperature .............................................................................................. 249
• Heat Balance ...................................................................................................... 250
• Regulation of Body Temperature ....................................................................... 251
Section 6: Endocrinology
44. Introduction to Endocrinology .......................................................................... 257
• Introduction ......................................................................................................... 257
• Endocrine Glands ............................................................................................... 257
• Hormones ........................................................................................................... 258
• Hormonal Action ................................................................................................. 259
45. Pituitary Gland ...................................................................................................... 261
• Introduction ........................................................................................................ 261
• Anterior Pituitary ................................................................................................. 262
• Posterior Pituitary ............................................................................................... 266
• Applied Physiology—Disorders of Pituitary Gland ............................................. 268
46. Thyroid Gland ....................................................................................................... 274
• Introduction ........................................................................................................ 274
• Histology of Thyroid Gland ................................................................................. 274
• Hormones of Thyroid Gland ............................................................................... 275
• Synthesis of Thyroid Hormones ......................................................................... 275
• Storage of Thyroid Hormones ............................................................................ 276
• Release of Thyroid Hormones from the Thyroid Gland ..................................... 276
• Transport of Thyroid Hormones in the Blood ..................................................... 277

xvi
Essentials of Physiology for Dental Students
• Functions of Thyroid Hormones ......................................................................... 277
• Mode of Action of Thyroid Hormones ................................................................ 279
• Regulation of Secretion of Thyroid Hormones................................................... 279
• Applied Physiology —Disorders of Thyroid Gland ............................................. 280
• Thyroid Function Tests ....................................................................................... 282
47. Parathyroid Glands and Physiology of Bone .................................................. 283
• Introduction ......................................................................................................... 284
• Parathormone .................................................................................................... 284
• Applied Physiology — Disorders of Parathyroid Glands ................................... 286
• Calcitonin ........................................................................................................... 288
• Calcium Metabolism ........................................................................................... 288
• Phosphate Metabolism ...................................................................................... 291
• Physiology of Bone ............................................................................................ 292
48. Endocrine Functions of Pancreas .................................................................... 295
• Islets of Langerhans ........................................................................................... 296
• Insulin ................................................................................................................. 296
• Glucagon ............................................................................................................ 298
• Somatostatin ...................................................................................................... 298
• Pancreatic Polypeptide ...................................................................................... 299
• Regulation of Blood Sugar Level (Blood Glucose Level) .................................. 299
• Applied Physiology ............................................................................................. 300
49. Adrenal Cortex ..................................................................................................... 303
• Functional Anatomy of Adrenal Glands ............................................................. 303
• Hormones of Adrenal Cortex ............................................................................. 303
• Mineralocorticoids .............................................................................................. 304
• Glucocorticoids ................................................................................................... 306
• Adrenal Sex Hormones ...................................................................................... 309
• Applied Physiology ............................................................................................. 310
50. Adrenal Medulla ................................................................................................... 313
• Introduction ......................................................................................................... 313
• Hormones of Adrenal Medulla ........................................................................... 313
• Synthesis of Catecholamines ............................................................................ 313
• Metabolism of Catecholamines .......................................................................... 314
• Actions of Adrenaline and Noradrenaline .......................................................... 314
• Regulation of Secretion of Adrenaline and Noradrenaline ................................ 317
• Dopamine ........................................................................................................... 317
• Applied Physiology – Pheochromocytoma ........................................................ 317
51. Endocrine Functions of Other Organs ............................................................. 318
• Pineal Gland ....................................................................................................... 318
• Thymus ............................................................................................................... 318
• Kidneys ............................................................................................................... 319
• Heart ................................................................................................................... 320
52. Local Hormones ................................................................................................... 321
• Introduction ......................................................................................................... 321
• Local Hormones Synthesized in Tissues ........................................................... 321
• Local Hormones Produced in Blood .................................................................. 324

xvii
Contents
Section 7: Reproductive System
53. Male Reproductive System ................................................................................ 329
• Introduction to Male Reproductive System ........................................................ 329
• Primary Sex Organs in Males — Testes ............................................................ 329
• Accessory Sex Organs in Males ........................................................................ 331
• Functions of Testis ............................................................................................. 333
• Gametogenic Functions of Testis – Spermatogenesis ...................................... 333
• Endocrine Functions of Testis ............................................................................ 336
• Semen ................................................................................................................ 339
• Male Climacteric ................................................................................................. 340
• Applied Physiology ............................................................................................. 341
54. Female Reproductive System ............................................................................ 343
• Female Reproductive Organs ............................................................................ 343
• Ovarian Hormones ............................................................................................. 346
• Climacteric and Menopause .............................................................................. 349
55. Menstrual Cycle ................................................................................................... 350
• Introduction ......................................................................................................... 350
• Ovarian Changes during Menstrual Cycle ......................................................... 350
• Uterine Changes during Menstrual Cycle .......................................................... 354
• Changes in Cervix during Menstrual Cycle ....................................................... 356
• Changes in Vagina during Menstrual Cycle ....................................................... 356
• Regulation of Menstrual Cycle ........................................................................... 356
• Applied Physiology – Abnormal Menstruation ................................................... 357
56. Pregnancy, Mammary Glands and Lactation ................................................... 358
• Introduction ........................................................................................................ 358
• Fertilization of the Ovum .................................................................................... 358
• Sex Chromosomes and Sex Determination ...................................................... 358
• Implantation and Development of Embryo ........................................................ 359
• Placenta ............................................................................................................. 359
• Gestation Period ................................................................................................ 361
• Parturition ........................................................................................................... 361
• Pregnancy Tests ................................................................................................. 361
• Development of Mammary Glands .................................................................... 362
• Lactation ............................................................................................................. 363
57. Fertility Control .................................................................................................... 365
• Introduction ......................................................................................................... 365
• Rhythm Method (Safe Period) ........................................................................... 365
• Mechanical Barriers – Prevention of Entry of Sperm into Uterus ...................... 366
• Chemical Methods ............................................................................................. 366
• Oral Contraceptives (Pill Method) ...................................................................... 366
• Intrauterine Contraceptive Device (IUCD) – Prevention of
Fertilization and Implantation of Ovum .............................................................. 367
• Medical Termination of Pregnancy (MTP) – Abortion ........................................ 367
• Surgical Method (Sterilization) – Permanent Method ........................................ 367

xviii
Essentials of Physiology for Dental Students
Section 8: Cardiovascular System
58. Introduction to Cardiovascular System ........................................................... 371
• Cardiovascular System ..................................................................................... 371
• Heart ................................................................................................................... 371
• Actions of the Heart........................................................................................... 375
• Blood Vessels .................................................................................................... 375
• Divisions of Circulation ...................................................................................... 376
59. Properties of Cardiac Muscle ............................................................................ 377
• Excitability .......................................................................................................... 377
• Rhythmicity ......................................................................................................... 379
• Conductivity ........................................................................................................ 380
• Contractility ........................................................................................................ 381
60. Cardiac Cycle ....................................................................................................... 383
• Definition ............................................................................................................ 383
• Events of Cardiac Cycle .................................................................................... 383
• Subdivisions and Duration of Events of Cardiac Cycle ................................... 383
• Description of Atrial Events ............................................................................... 384
• Description of Ventricular Events ...................................................................... 384
61. Heart Sounds ........................................................................................................ 388
• Introduction ........................................................................................................ 388
• Description of Different Heart Sounds .............................................................. 388
• Methods of Study of Heart Sounds .................................................................. 390
• Cardiac Murmur ................................................................................................. 391
62. Electrocardiogram ............................................................................................... 392
• Definitions .......................................................................................................... 392
• Uses of ECG ...................................................................................................... 392
• Electrocardiographic Grid .................................................................................. 392
• ECG Leads ........................................................................................................ 393
• Waves of Normal Electrocardiogram ................................................................ 394
• Intervals and Segments of ECG ....................................................................... 396
63. Cardiac Output ..................................................................................................... 398
• Introduction ........................................................................................................ 398
• Definitions and Normal Values ......................................................................... 398
• Variations in Cardiac Output ............................................................................. 399
• Distribution of Cardiac Output .......................................................................... 399
• Factors Maintaining Cardiac Output ................................................................. 399
• Measurement of Cardiac Output ...................................................................... 401
64. Heart Rate ............................................................................................................. 404
• Heart Rate .......................................................................................................... 404
• Regulation of Heart Rate .................................................................................. 405
• Vasomotor Center – Cardiac Center ................................................................ 405
• Motor (Efferent) Nerve Fibers to Heart............................................................. 406
• Sensory (Afferent) Nerve Fibers from Heart .................................................... 407
• Factors Affecting Vasomotor Center – Regulation of Vagal Tone ................... 407

xix
Contents
65. Arterial Blood Pressure ...................................................................................... 411
• Definitions and Normal Values ......................................................................... 411
• Variations ........................................................................................................... 412
• Determinants of Arterial Blood Pressure – Factors Maintaining
Arterial Blood Pressure ..................................................................................... 412
• Regulation of Arterial Blood Pressure .............................................................. 413
• Nervous Mechanism for Regulation of Blood Pressure –
Short-term Regulation ....................................................................................... 414
• Renal Mechanism for Regulation of Blood Pressure –
Long-term Regulation ........................................................................................ 417
• Hormonal Mechanism for Regulation of Blood Pressure ................................ 418
• Local Mechanism for Regulation of Blood Pressure ....................................... 418
• Applied Physiology ............................................................................................ 419
66. Venous Pressure and Capillary Pressure ........................................................ 420
• Venous Pressure ............................................................................................... 420
• Capillary Pressure ............................................................................................. 421
• Regional Variations ............................................................................................ 421
67. Arterial Pulse and Venous Pulse ....................................................................... 422
• Arterial Pulse ..................................................................................................... 422
• Venous Pulse ..................................................................................................... 424
68. Regional Circulation ............................................................................................ 426
• Coronary Circulation .......................................................................................... 426
• Applied Physiology – Coronary Artery Disease ............................................... 427
• Cerebral Circulation ........................................................................................... 428
• Splanchnic Circulation ....................................................................................... 428
• Capillary Circulation .......................................................................................... 429
• Skeletal Muscle Circulation ............................................................................... 431
• Cutaneous Circulation ....................................................................................... 431
69. Fetal Circulation and Respiration ..................................................................... 432
• Introduction ........................................................................................................ 432
• Blood Vessels in Fetus ...................................................................................... 432
• Fetal Lungs ........................................................................................................ 433
• Changes in Circulation and Respiration after Birth –
Neonatal Circulation and Respiration ............................................................... 433
70. Hemorrhage, Circulatory Shock and Heart failure ......................................... 436
• Hemorrhage ....................................................................................................... 436
• Circulatory Shock .............................................................................................. 437
• Heart Failure ...................................................................................................... 437
71. Cardiovascular Adjustments during Exercise ................................................. 439
• Introduction ........................................................................................................ 439
• Types of Exercise .............................................................................................. 439
• Aerobic and Anaerobic Exercises ..................................................................... 439
• Severity of Exercise ........................................................................................... 440
• Effects of Exercise on Cardiovascular System ................................................ 440
xx
Essentials of Physiology for Dental Students
Section 9: Respiratory System and
Environmental Physiology
72. Respiratory Tract and Pulmonary Circulation ................................................. 445
• Introduction ........................................................................................................ 445
• Functional Anatomy of Respiratory Tract ......................................................... 445
• Respiratory Unit ................................................................................................. 446
• Nonrespiratory Functions of Respiratory Tract ................................................ 447
• Respiratory Protective Reflexes ....................................................................... 448
• Pulmonary Circulation ....................................................................................... 449
73. Mechanics of Respiration ................................................................................... 451
• Respiratory Movements ..................................................................................... 451
• Respiratory Pressures ....................................................................................... 453
• Compliance ........................................................................................................ 455
• Work of Breathing ............................................................................................. 455
74. Pulmonary Function Tests ................................................................................. 457
• Introduction ........................................................................................................ 457
• Lung Volumes .................................................................................................... 457
• Lung Capacities ................................................................................................. 458
• Vital Capacity ..................................................................................................... 459
• Forced Expiratory Volume (FEV) or Timed Vital Capacity .............................. 460
• Respiratory Minute Volume (RMV) ................................................................... 460
• Maximum Breathing Capacity (MBC) or
Maximum Ventilation Volume (MVV) ................................................................ 460
• Peak Expiratory Flow Rate (PEFR) .................................................................. 460
• Restrictive and Obstructive Respiratory Diseases ........................................... 461
75. Ventilation ............................................................................................................. 463
• Pulmonary Ventilation ........................................................................................ 463
• Alveolar Ventilation ............................................................................................ 463
• Dead Space ....................................................................................................... 464
• Ventilation–Perfusion Ratio ............................................................................... 464
• Inspired Air ......................................................................................................... 465
• Alveolar Air ......................................................................................................... 465
• Expired Air ......................................................................................................... 465
76. Exchange and Transport of Respiratory Gases .............................................. 466
• Exchange of Respiratory Gases in Lungs ....................................................... 466
• Exchange of Respiratory Gases at Tissue Level ............................................. 468
• Transport of Oxygen .......................................................................................... 469
• Transport of Carbon Dioxide ............................................................................. 471
77. Regulation of Respiration .................................................................................. 474
• Introduction ........................................................................................................ 474
• Nervous Mechanism .......................................................................................... 474
• Chemical Mechanism ........................................................................................ 478
78. Disturbances of Respiration .............................................................................. 480
• Apnea ................................................................................................................. 480
• Hyperventilation ................................................................................................. 480
• Hypoventilation .................................................................................................. 480
xxi
Contents
• Hypoxia .............................................................................................................. 481
• Oxygen Toxicity (Poisoning) .............................................................................. 483
• Hypercapnea ...................................................................................................... 483
• Hypocapnea ....................................................................................................... 483
• Asphyxia ............................................................................................................. 483
• Carbon Monoxide Poisoning ............................................................................. 486
79. High Altitude and Deep Sea Physiology .......................................................... 487
• High Altitude ....................................................................................................... 487
• Deep Sea Physiology ........................................................................................ 489
80. Effects of Exposure to Cold and Heat .............................................................. 492
• Effects of Exposure to Cold .............................................................................. 492
• Effects of Exposure to Heat .............................................................................. 493
81. Artificial Respiration ............................................................................................ 494
• Conditions when Artificial Respiration is Required .......................................... 494
• Methods of Artificial Respiration ....................................................................... 494
82. Effects of Exercise on Respiration ................................................................... 496
• Introduction ........................................................................................................ 496
• Effects of Exercise on Respiration ................................................................... 496
Section 10: Nervous System
83. Introduction to Nervous System ....................................................................... 501
• Divisions of Nervous System ............................................................................ 501
84. Neuron and Neuroglia ......................................................................................... 504
• Neuron ............................................................................................................... 504
• Neuroglia ............................................................................................................ 512
85. Receptors .............................................................................................................. 514
• Definition ............................................................................................................ 514
• Classification of Receptors ............................................................................... 514
• Properties of Receptors .................................................................................... 516
86. Synapse and Neurotransmitters ........................................................................ 519
• Definition ............................................................................................................ 519
• Classification of Synapse .................................................................................. 519
• Functions of Synapse ........................................................................................ 521
• Properties of Synapse ....................................................................................... 523
• Neurotransmitters .............................................................................................. 525
• Classification of Neurotransmitters ................................................................... 525
87. Reflex Activity ....................................................................................................... 526
• Definition and Significance of Reflexes ........................................................... 526
• Reflex Arc ........................................................................................................... 526
• Classification of Reflexes .................................................................................. 527
• Properties of Reflexes ....................................................................................... 530
• Reflexes in Motor Neuron Lesion ..................................................................... 531
88. Spinal Cord ........................................................................................................... 532
• Introduction ........................................................................................................ 532
• Internal Structure of Spinal Cord ...................................................................... 532

xxii
Essentials of Physiology for Dental Students
• Gray Matter of Spinal Cord ............................................................................... 532
• White Matter of Spinal Cord ............................................................................. 533
• Tracts in Spinal Cord ......................................................................................... 534
• Ascending Tracts of Spinal Cord ...................................................................... 534
• Descending Tracts of Spinal Cord .................................................................... 542
• Extrapyramidal Tracts ........................................................................................ 545
89. Somatosensory System and Somatomotor System ....................................... 547
• Somatosensory System .................................................................................... 547
• Somatomotor System ........................................................................................ 552
90. Physiology of Pain .............................................................................................. 555
• Introduction and Definition ................................................................................ 555
• Benefits of Pain Sensation ................................................................................ 555
• Components of Pain Sensation ........................................................................ 555
• Pathways of Pain Sensation ............................................................................. 556
• Visceral Pain ...................................................................................................... 556
• Referred Pain ..................................................................................................... 557
• Analgesia System .............................................................................................. 557
• Applied Physiology ............................................................................................ 557
91. Thalamus ............................................................................................................... 558
• Introduction ........................................................................................................ 558
• Thalamic Nuclei ................................................................................................. 558
• Functions of Thalamus ...................................................................................... 559
• Applied Physiology ............................................................................................ 560
92. Hypothalamus ...................................................................................................... 561
• Introduction ........................................................................................................ 561
• Nuclei of Hypothalamus .................................................................................... 562
• Functions of Hypothalamus .............................................................................. 562
• Applied Physiology – Disorders of Hypothalamus ........................................... 567
93. Cerebellum ............................................................................................................ 569
• Parts of Cerebellum........................................................................................... 569
• Divisions of Cerebellum .................................................................................... 570
• Vestibulocerebellum (Archicerebellum) ............................................................ 570
• Spinocerebellum (Paleocerebellum) ................................................................. 571
• Corticocerebellum (Neocerebellum) ................................................................. 572
• Applied Physiology – Cerebellar Lesions ......................................................... 574
94. Basal Ganglia ....................................................................................................... 575
• Introduction ........................................................................................................ 575
• Components of Basal Ganglia .......................................................................... 575
• Functions of Basal Ganglia ............................................................................... 576
• Applied Physiology – Disorders of Basal Ganglia ........................................... 577
95. Cerebral Cortex and Limbic System ................................................................. 579
• Introduction ........................................................................................................ 579
• Neocortex and Allocortex .................................................................................. 580
• Lobes of Cerebral Cortex .................................................................................. 580
• Cerebral Dominance ......................................................................................... 580
• Brodmann Areas ................................................................................................ 582

xxiii
Contents
• Frontal Lobe of Cerebral Cortex ....................................................................... 582
• Parietal Lobe ...................................................................................................... 585
• Temporal Lobe ................................................................................................... 586
• Occipital Lobe – Visual Cortex ......................................................................... 587
• Limbic System Or Limbic Lobe ......................................................................... 587
96. Reticular Formation ............................................................................................. 589
• Definition ............................................................................................................ 589
• Situation of Reticular Formation ....................................................................... 589
• Divisions of Reticular Formation ....................................................................... 589
• Functions of Reticular Formation ..................................................................... 589
97. Posture and Equilibrium ..................................................................................... 592
• Definition ............................................................................................................ 592
• Proprioceptors .................................................................................................... 592
• Basic Phenomena of Posture ........................................................................... 595
• Postural Reflexes .............................................................................................. 596
98. Vestibular Apparatus ........................................................................................... 599
• Introduction ........................................................................................................ 599
• Labyrinth ............................................................................................................ 599
• Functional Anatomy of Vestibular Apparatus ................................................... 600
• Receptor Organ in Vestibular Apparatus .......................................................... 600
• Nerve Supply to Vestibular Apparatus .............................................................. 602
• Functions of Vestibular Apparatus .................................................................... 602
• Applied Physiology ............................................................................................ 604
99. Electroencephalogram and Epilepsy ................................................................ 606
• Electroencephalogram ....................................................................................... 606
• Epilepsy .............................................................................................................. 607
100. Physiology of Sleep ............................................................................................ 609
• Definition ............................................................................................................ 609
• Sleep Requirement ............................................................................................ 609
• Physiological Changes During Sleep ............................................................... 609
• Types of Sleep ................................................................................................... 609
• Stages of Sleep and EEg Pattern ..................................................................... 611
• Mechanism of Sleep .......................................................................................... 611
101. Higher Intellectual Functions ............................................................................. 612
• Higher Intellectual Functions ............................................................................ 612
• Learning ............................................................................................................. 612
• Memory .............................................................................................................. 613
• Conditioned Reflexes ........................................................................................ 614
• Speech ............................................................................................................... 615
102. Cerebrospinal Fluid ............................................................................................. 617
• Introduction ........................................................................................................ 617
• Properties and Composition of CSF ................................................................. 617
• Formation of CSF .............................................................................................. 618
• Circulation of CSF ............................................................................................. 618
• Absorption of CSF ............................................................................................. 618
• Pressure Exerted by CSF ................................................................................. 618

xxiv
Essentials of Physiology for Dental Students
• Functions of CSF ............................................................................................... 619
• Collection of CSF .............................................................................................. 619
• Blood-Brain Barrier ............................................................................................ 619
• Blood – Cerebrospinal Fluid Barrier ................................................................. 620
• Applied Physiology — Hydrocephalus ............................................................. 620
103. Autonomic Nervous System .............................................................................. 621
• Introduction ........................................................................................................ 621
• Sympathetic Division ......................................................................................... 621
• Parasympathetic Division .................................................................................. 623
• Functions of ANS ............................................................................................... 623
• Neurotransmitters of ANS ................................................................................. 623
Section 11: Special Senses
104. Structure of the Eye ............................................................................................ 629
• Special Senses .................................................................................................. 629
• Functional Anatomy of the Eyeball ................................................................... 629
• Wall of the Eyeball ............................................................................................ 630
• Fundus Oculi or Fundus .................................................................................... 633
• Intraocular Fluids ............................................................................................... 634
• Intraocular Pressure .......................................................................................... 634
• Lens .................................................................................................................... 635
• Ocular Muscles .................................................................................................. 635
• Ocular Movements ............................................................................................. 636
• Applied Physiology ............................................................................................ 636
105. Visual Process and Field of Vision ................................................................... 638
• Visual Process ................................................................................................... 638
• Field of Vision .................................................................................................... 641
106. Visual Pathway ..................................................................................................... 643
• Introduction ........................................................................................................ 643
• Visual Receptors ................................................................................................ 643
• First Order Neurons ........................................................................................... 643
• Second Order Neurons ..................................................................................... 643
• Third Order Neurons ......................................................................................... 643
• Course of Visual Pathway ................................................................................. 643
• Applied Physiology ............................................................................................ 646
107. Pupillary Reflexes ................................................................................................ 647
• Introduction ........................................................................................................ 647
• Light Reflex ........................................................................................................ 647
• Accommodation ................................................................................................. 648
• Applied Physiology – Presbyopia ..................................................................... 650
108. Color Vision .......................................................................................................... 651
• Introduction ........................................................................................................ 651
• Visible Spectrum and Spectral Colors .............................................................. 651
• Cones and Color Vision –Young-helmholtz Trichromatic Theory .................... 652
• Applied Physiology – Color Blindness .............................................................. 652

xxv
Contents
109. Errors of Refraction ............................................................................................ 654
• Emetropoia and Ametropia ............................................................................... 654
• Anisometropia .................................................................................................... 655
• Astigmatism ....................................................................................................... 655
• Presbyopia ......................................................................................................... 656
110. Structure of Ear and Auditory Pathway ........................................................... 657
• External Ear ....................................................................................................... 657
• Middle Ear .......................................................................................................... 657
• Internal Ear ........................................................................................................ 659
• Auditory Pathway ............................................................................................... 661
111. Mechanism of Hearing and Auditory Defects .................................................. 663
• Introduction ........................................................................................................ 663
• Role of External Ear .......................................................................................... 664
• Role of Middle Ear ............................................................................................. 664
• Role of Inner Ear ............................................................................................... 664
• Electrical Events During the Process of Hearing............................................. 665
• Properties of Sound ........................................................................................... 665
• Appreciation of Pitch of the Sound – Theories of Hearing ............................. 665
• Appreciation of Loudness of Sound ................................................................. 666
• Localization of Sound ........................................................................................ 666
• Auditory Defects ................................................................................................ 666
112. Sensation of Taste ............................................................................................... 667
• Taste Buds ......................................................................................................... 667
• Pathway for Taste .............................................................................................. 668
• Taste Sensations and Chemical Constitutions ................................................. 669
• Taste Transduction ............................................................................................ 669
• Applied Physiology – Abnormalities of Taste Sensation ................................. 669
113. Sensation of Smell .............................................................................................. 670
• Olfactory Receptors ........................................................................................... 670
• Olfactory Pathway .............................................................................................. 670
• Generator Potential in Olfactory Receptor ....................................................... 670
• Classification of Odor ........................................................................................ 670
• Threshold for Olfactory Sensation .................................................................... 671
• Applied Physiology – Abnormalities of Olfactory Sensation ........................... 671
Index ....................................................................................................................... 673
General Physiology
1. Cell .......................................................................................... 3
2. Cell Junctions ........................................................................ 14
3. Transport through Cell Membrane......................................... 17
4. Homeostasis .......................................................................... 25
S E C T I O N
1
C H A P T E R S

n
INTRODUCTION
n
CELL
n
TISSUES
n
ORGANS
n
SYSTEMS
n
STRUCTURE OF THE CELL
n
CELL MEMBRANE
n
COMPOSITION
n
STRUCTURE
n
FUNCTIONS
n
CYTOPLASM
n
ORGANELLES IN CYTOPLASM
n
ORGANELLES WITH LIMITING MEMBRANE
n
ORGANELLES WITHOUT LIMITING MEMBRANE
n
NUCLEUS
n
STRUCTURE
n
FUNCTIONS
n
CELL DEATH
n
APOPTOSIS
n
NECROSIS
Cell
1
n
INTRODUCTION
n
CELL
Cell is defined as the structural and functional
unit of the living body because it has all the
characteristics of life.
n
TISSUES
The tissue is defined as the group of cells having
similar function. The tissues are classified into
four major types which are called the primary
tissues. The primary tissues include:

General Physiology
4
1. Muscle tissue – skeletal muscle, smooth
muscle and cardiac muscle
2. Nervous tissue – neurons and supporting
cells
3. Epithelial tissue – squamous, columnar and
cuboidal epithelial cells
4. Connective tissue – connective tissue proper,
cartilage, bone and blood.
n
ORGANS
An organ is defined as the structure that is
formed by two or more primary tissues.
Some organs are composed of all the four
types of primary tissues. The organs may be
tubular like intestine or hollow like stomach.
n
SYSTEMS
The system is defined as group of organs
functioning together to perform a specific
function of the body. For example, digestive
system is made out of groups of organs like
esophagus, stomach, intestine etc., which is
concerned with digestion of food particles.
n
STRUCTURE OF THE CELL
Each cell is formed by a cell body and a cell
membrane or plasma membrane that covers the
cell body. The important parts of the cell are
(Fig. 1-1)
a. Cell membrane
b. Nucleus
c. Cytoplasm with organelles
n
CELL MEMBRANE
The cell membrane is a protective sheath that
envelops the cell body. It separates the fluid
outside the cell called extracellular fluid (ECF)
and the fluid inside the cell called intracellular
fluid (ICF). It is a semipermeable membrane and
allows free exchange of certain substances
between ECF and ICF (Fig. 1-2).
n
COMPOSITION OF CELL MEMBRANE
The cell membrane is composed of three types
of substances:
FIGURE 1-1: Structure of the cell
1. Proteins (55%)
2. Lipids (40%)
3. Carbohydrates (5%).
n
STRUCTURE OF CELL MEMBRANE
The cell membrane is a unit membrane having
the ‘fluid mosaic model’ i.e., the membrane is a
fluid with mosaic of proteins (mosaic means
pattern formed by arrangement of different
colored pieces of stone, tile, glass or other such
materials) lipids and carbohydrates. The electron
microscopic study reveals three layers in the cell
membrane namely, one electron lucent lipid layer
in the center and two electron dense layers on
either side of the central layer. Carbohydrate
molecules are found on the surface of the cell
membrane.
Lipid Layer of Cell Membrane
It is a bilayered structure formed by a thin film
of lipids. It is fluid in nature and the portions of
the membrane along with the dissolved
substances move to all areas of the cell
membrane. The major lipids are:
a. Phospholipids
b. Cholesterol
1. Phospholipids
The phospholipid molecules are formed by
phosphorus and fatty acids. Each phospholipid
molecule resembles the headed pin in shape
(Fig. 1-3). The outer part of the phospholipid
molecule is the head portion which is water
soluble (hydrophilic) and the inner part is the tail
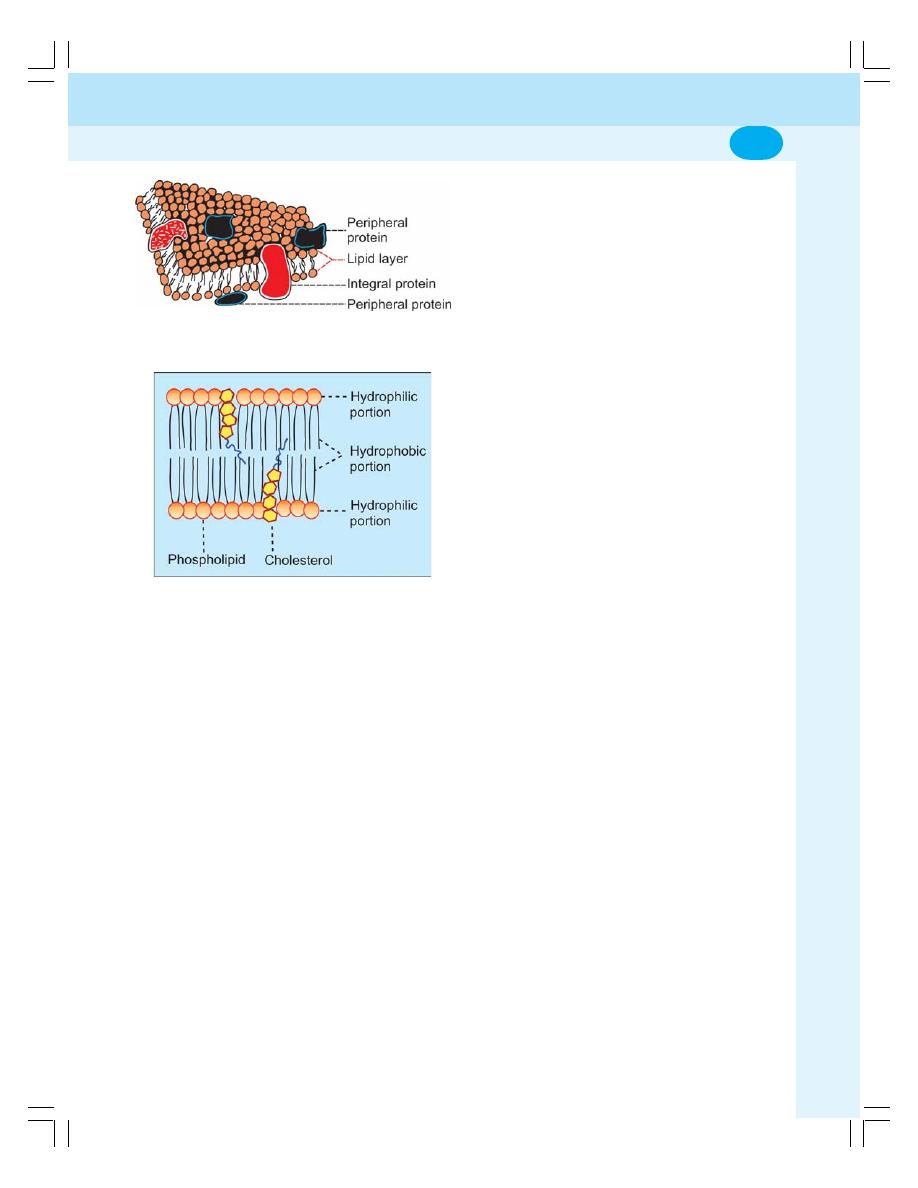
Chapter 1 Cell
5
portion that is not soluble in water (hydrophobic).
The hydrophobic tail portions meet in the center
of the membrane. The hydrophilic head portions
of outer layer face the ECF and those of the inner
layer face the cytoplasm.
2. Cholesterol
The cholesterol molecules are arranged in
between the phospholipid molecules. As
phospholipids are soft and oily in nature,
cholesterol helps to “pack” the phospholipids in
the membrane and maintain the structural
integrity of cell membrane.
Functions of lipid layer
The lipid layer is semi permeable in nature and
allows only the fat soluble substances like
oxygen, carbon dioxide and alcohol to pass
through it. It does not allow the water soluble
materials like glucose, urea and electrolytes to
pass through it.
Protein Layers of the Cell Membrane
The protein layers of the cell membrane are the
electron dense layers situated on either side of
the central lipid layer. The protein substances
present in these layers are mostly glycoproteins.
These protein molecules are classified into two
categories:
a. Integral proteins
b. Peripheral proteins
a. Integral proteins
The integral proteins, also known as trans-
membrane proteins, are tightly bound with the
cell membrane. These protein molecules pass
through the entire thickness of the cell membrane
from one side to the other side.
2. Peripheral proteins
The peripheral proteins, also known as peripheral
membrane proteins do not penetrate the cell
membrane but are embedded partially in the
outer and inner surfaces of the cell membrane.
These protein molecules are loosely bound with
the cell membrane and so dissociate readily from
the cell membrane.
Functions of protein layers
Functionally, the proteins in the cell membrane
exist in different forms such as integral proteins,
channel proteins, carrier proteins etc.
1. Integral proteins provide structural integrity
of the cell membrane
2. Channel proteins provide route for diffusion
of water soluble substances like glucose and
electrolytes
3. Carrier proteins help in transport of
substances across the cell membrane
4. Receptor proteins serve as receptor sites for
hormones and neurotransmitters
5. Enzymes: some of the protein molecules form
the enzymes which control chemical reactions
within the cell membrane
6. Antigens: Some proteins act as antigens and
induce the process of antibody formation.
FIGURE 1-3: Lipids of the cell membrane
FIGURE 1-2: Diagram of the cell membrane
General Physiology
6
Carbohydrates of the Cell Membrane
Carbohydrate molecules form a thin loose
covering over the entire surface of the cell mem-
brane called glycocalyx. Some carbohydrate
molecules are attached with proteins and form
glycoproteins and some are attached with lipids
and form glycolipids.
Functions of carbohydrates
1. The carbohydrate molecules are negatively
charged and do not permit the negatively
charged substances to move in and out of
the cell.
2. The glycocalyx from the neighboring cells
helps in the tight fixation of cells with one
another.
3. Some of the carbohydrate molecules form
the receptors for some hormones.
n
FUNCTIONS OF CELL MEMBRANE
1. Protective function: Cell membrane protects
the cytoplasm and the organelles present in
the cytoplasm.
2. Selective permeability: Cell membrane acts
as a semipermeable membrane which allows
only some substances to pass through it and
acts as a barrier for other substances.
3. Absorptive function: Nutrients are absorbed
into the cell through the cell membrane.
4. Excretory function: Metabolites and other
waste products from the cell are excreted out
through the cell membrane.
5. Exchange of gases: Oxygen enters the cell
from the blood and carbon dioxide leaves the
cell and enters the blood through the cell
membrane.
6. Maintenance of shape and size of the cell:
Cell membrane is responsible for the
maintenance of shape and size of the cell.
n
CYTOPLASM
The cytoplasm is the fluid present inside the cell.
It contains a clear liquid portion called cytosol
which contains various substances like proteins,
carbohydrates, lipids and electrolytes. Apart from
these substances, many organelles are also
present in cytoplasm. The cytoplasm is
distributed as peripheral ectoplasm just beneath
the cell membrane and inner endoplasm between
the ectoplasm and the nucleus.
n
ORGANELLES IN CYTOPLASM
All the cells in the body contain some common
structures called organelles in the cytoplasm.
Some organelles are bound by limiting
membrane and others do not have limiting
membrane (Table 1-1). The organelles carry out
the various functions of the cell (Table 1-2).
n
ORGANELLES WITH LIMITING
MEMBRANE
n
1. ENDOPLASMIC RETICULUM
Endoplasmic reticulum is made up of tubules and
microsomal vesicles. These structures form an
interconnected network which acts as the link
between the organelles and cell membrane.
Types of Endoplasmic Reticulum
The endoplasmic reticulum is of two types
namely, rough endoplasmic reticulum and
smooth endoplasmic reticulum.
TABLE 1-1: Cytoplasmic organelles
The organelles with limiting membrane
1. Endoplasmic reticulum
2. Golgi apparatus
3. Lysosome
4. Peroxisome
5. Centrosome and centrioles
6. Secretory vesicles
7. Mitochondria
8. Nucleus
The organelles without limiting membrane
1. Ribosomes
2. Cytoskeleton
Chapter 1 Cell
7
TABLE 1-2: Functions of cytoplasmic organelles
Organelles
Functions
Rough endoplasmic reticulum
1. Synthesis of proteins
2. Degradation of worn out organelles
Smooth endoplasmic reticulum
1. Synthesis of lipids and steroids
2. Role in cellular metabolism
3. Storage and metabolism of calcium
4. Catabolism and detoxification of toxic substances
Golgi apparatus
Processing, packaging, labeling and delivery of proteins and lipids
Lysosomes
1. Degradation of macromolecules
2. Degradation of worn out organelles
3. Removal of excess of secretory products.
4. Secretory function
Peroxisomes
1. Break down of excess fatty acids
2. Detoxification of hydrogen peroxide and other metabolic products
3. Oxygen utilization
4. Acceleration of gluconeogenesis
5. Degradation of purine to uric acid
6. Role in the formation of myelin
7. Role in the formation of bile acids
Centrosome
Movement of chromosomes during cell division
Mitochondria
1. Production of energy
2. Synthesis of ATP
3. Initiation of apoptosis
Ribosomes
Synthesis of proteins
Cytoskeleton
1. Determination of shape of the cell
2. Stability of cell shape
3. Cellular movements
Nucleus
1. Control of all activities of the cell
2. Synthesis of RNA
3. Sending genetic instruction to cytoplasm for protein synthesis
4. Formation of subunits of ribosomes
5. Control of cell division
6. Storage of hereditary information in genes (DNA)
Rough Endoplasmic Reticulum
Rough endoplasmic reticulum is the one to which
the granular ribosome is attached. This gives the
rough appearance and so, it is called the rough
endoplasmic reticulum. Attachment of the
granular ribosome also gives the beaded or
granular appearance and so it is also called
granular endoplasmic reticulum (Fig. 1-4).
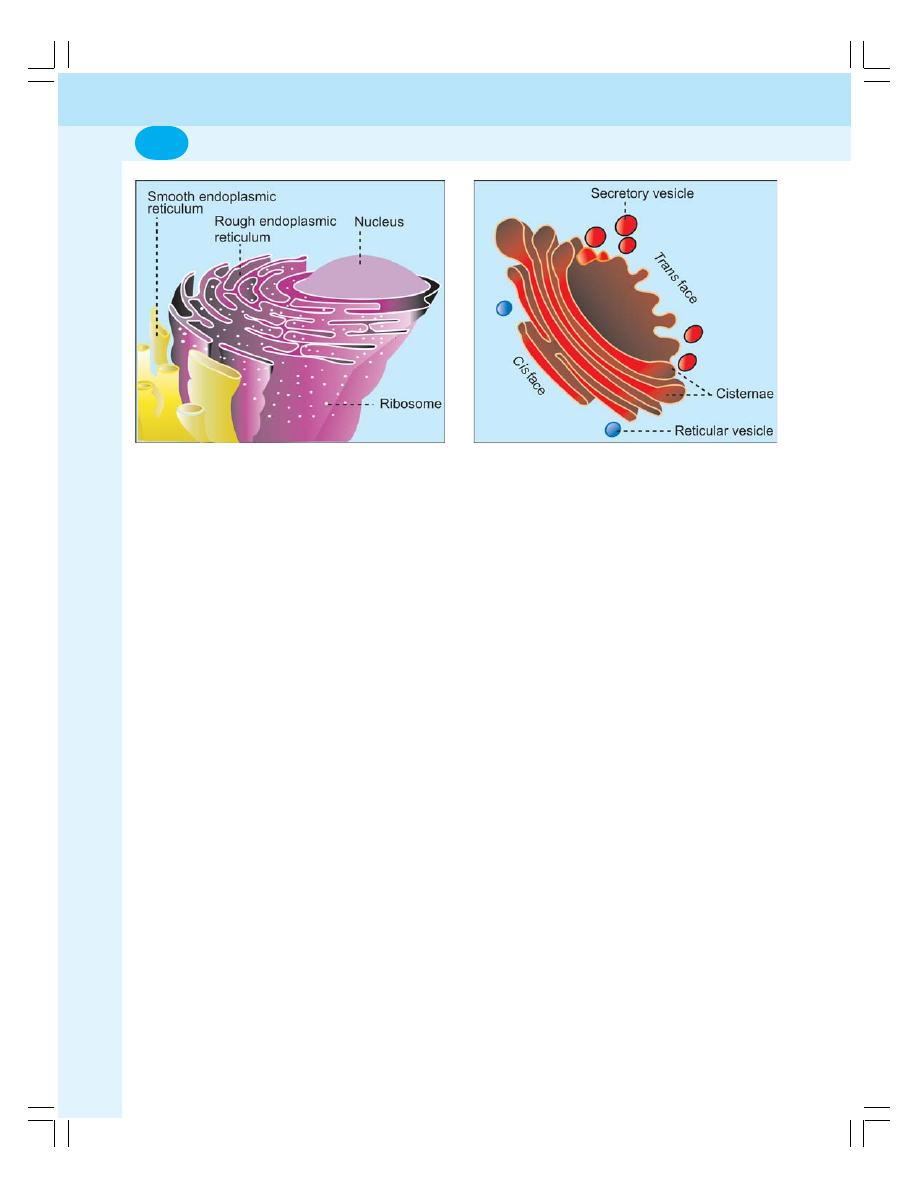
General Physiology
8
drugs and carcinogens (cancer producing
substances) in liver.
Rough endoplasmic reticulum and smooth
endoplasmic reticulum are interconnected and
continuous with one another. Depending upon
the activities of the cells, the rough endoplasmic
reticulum changes to smooth endoplasmic
reticulum and vice versa.
n
2. GOLGI APPARATUS
Golgi apparatus (Golgi body or Golgi complex)
is present in all the cells except red blood cells.
It consists of 5 to 8 flattened membranous sacs
called cisternae (Fig. 1-5).
The Golgi apparatus is situated near the
nucleus. It has two ends or faces namely, cis
face and trans face. The cis face is positioned
near the endoplasmic reticulum. The reticular
vesicles from endoplasmic reticulum enter the
Golgi apparatus through cis face. The trans face
is situated near the cell membrane. The
processed substances make their exit from Golgi
apparatus through trans face.
Functions of Golgi Apparatus
i.
It is concerned with the processing and
delivery of substances like proteins and lipids
to different parts of the cell.
ii. It functions like a post office because, it packs
the processed materials into the secretory
granules, secretory vesicles, and lysosomes
FIGURE 1-4: Endoplasmic reticulum
Functions of rough endoplasmic reticulum
It is concerned with the protein synthesis in the
cell, especially those secreted from the cell such
as insulin from
β cells of islets of Langerhans in
pancreas and antibodies in leukocytes.
It also plays an important role in degradation
of worn out cytoplasmic organelles like mito-
chondria. It wraps itself around the worn out
organelles and forms a vacuole which is often
called the autophagosome. It is digested by
lysosomal enzymes
Smooth Endoplasmic Reticulum
Smooth endoplasmic reticulum is also called as
agranular endoplasmic reticulum because of its
smooth appearance without the attachment of
ribosome. It is formed by many interconnected
tubules. So, it is also called tubular endoplasmic
reticulum.
Functions of smooth endoplasmic reticulum
i.
It is responsible for synthesis of cholesterol
and steroid
ii. It is concerned with various metabolic
processes of the cell because of the presence
of many enzymes on the outer surface
iii. It is concerned with the storage and
metabolism of calcium
iv. It is also concerned with catabolism and
detoxification of toxic substances like some
FIGURE 1-5: Golgi apparatus
Chapter 1 Cell
9
and dispatch them either out of the cell or to
another part of the cell.
iii. It also functions like a shipping department
of the cell because it sorts out and labels the
materials for distribution to their proper
destinations.
n
3. LYSOSOMES
These are small globular structures filled with
enzymes. These enzymes are synthesized in
rough endoplasmic reticulum and transported to
the Golgi apparatus. Here, these are processed
and packed in the form of small vesicles. Then,
these vesicles are pinched off from Golgi
apparatus and become the lysosomes.
Types of Lysosomes
Lysosomes are of two types:
i.
Primary lysosome which is pinched off from
Golgi apparatus. It is inactive in spite of having
the hydrolytic enzymes.
ii. Secondary lysosome which is active
lysosome formed by the fusion of a primary
lysosome with phagosome or endosome.
Functions of Lysosomes
i. Digestion of unwanted substances
With the help of hydrolytic enzymes like
proteases, lipases, amylases and nucleases,
lysosome digests and removes the unwanted
substances.
ii. Removal of excess secretory products in
the cells
Lysosomes in the cells of the secretory glands
play an important role in the removal of excess
secretory products by degrading the secretory
granules.
iii. Secretory function – Secretory lysosomes
Recently, lysosomes having secretory function
called secretory lysosomes are found in some
of the cells, particularly in the cells of immune
system. The conventional lysosomes are
modified into secretory lysosomes by combining
with secretory granules
Examples of secretory lysosomes:
a. In cytotoxic T lymphocytes and natural killer
(NK) cells, lysosomes secrete perforin and
granzymes which destroy both virus infected
cells and tumor cells.
b. In melanocytes, secretory lysosomes secrete
melanin.
c. In mast cells, secretory lysosomes secrete
serotonin which is an inflammatory mediator
n
4. PEROXISOMES
Peroxisomes are otherwise called as micro
bodies. These are pinched off from endoplasmic
reticulum. Peroxisomes contain some oxidative
enzymes such as catalase, urate oxidase and
D-amino acid oxidase.
Functions of Peroxisomes
Peroxisomes:
i.
Degrade the toxic substances like hydrogen
peroxide and other metabolic products by
means of detoxification
ii. Form the major site of oxygen utilization in
the cells
iii. Break down the excess fatty acids
iv. Accelerate gluconeogenesis from fats
v. Degrade purine to uric acid
vi. Participate in the formation of myelin and bile
acids.
n
5. CENTROSOME AND CENTRIOLES
The centrosome is situated near the center of
the cell close to the nucleus. It consists of two
cylindrical structures called centrioles which are
responsible for the movement of chromosomes
during cell division.
n
6. SECRETORY VESICLES
The secretory vesicles are globular structures,
formed in the endoplasmic reticulum, and
processed and packed in Golgi apparatus.
When necessary, the secretory vesicles rupture
and release the secretory substances into the
cytoplasm.
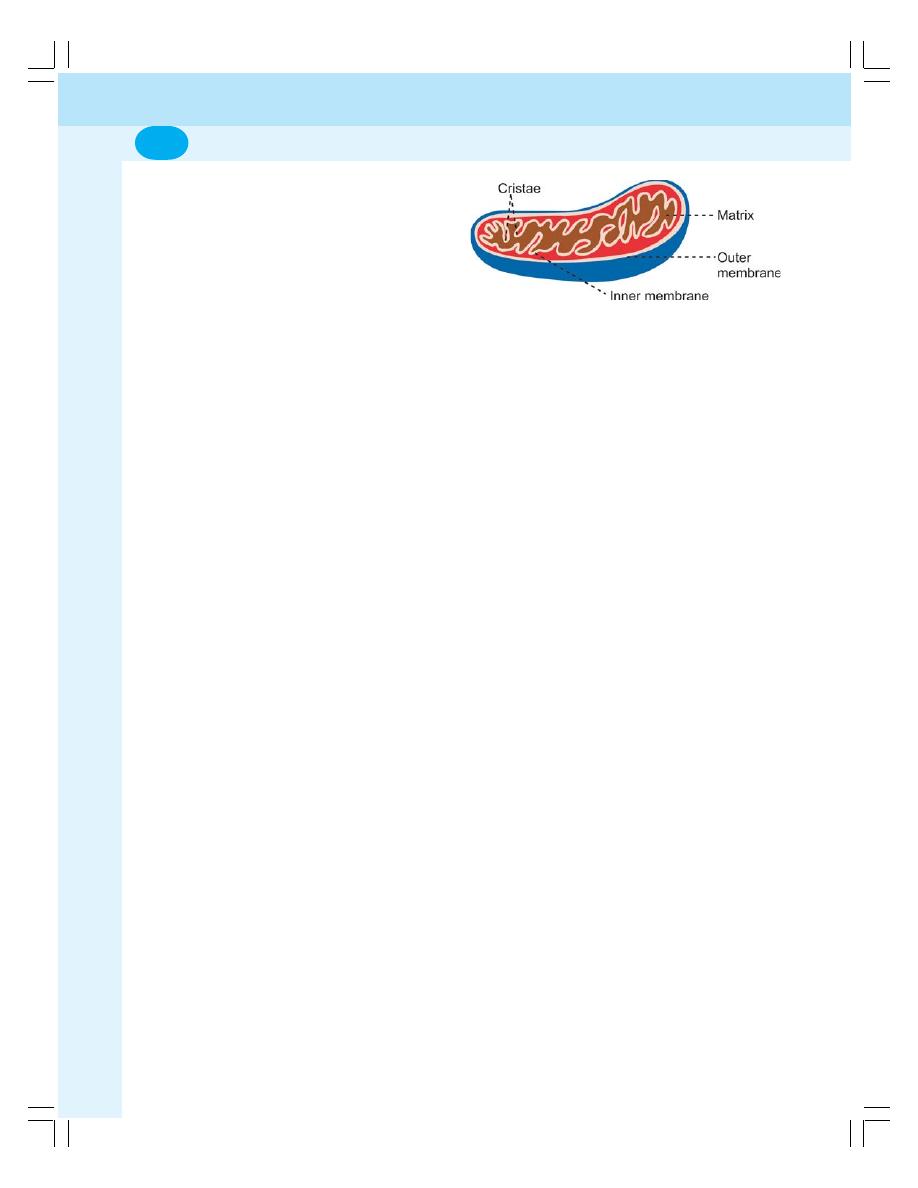
General Physiology
10
n
7. MITOCHONDRION
The mitochondrion (pleural = mitochondria) is a
rod or oval shaped structure with a diameter of
0.5 to 1
μ. It is covered by a double layered
membrane (Fig. 1-6). The outer membrane is
smooth and encloses the contents of
mitochondrion. It contains various enzymes such
as acetyl-CoA synthetase and glycerolphosphate
acetyl-transferase.
The inner membrane forms many folds called
cristae and covers the inner matrix space. The
cristae also contain many enzymes and other
protein molecules which are involved in
respiration and ATP synthesis. Because of these
functions, the enzymes and other protein
molecules in cristae are collectively known as
respiratory chain or electron transport system.
The mitochondria move freely in the
cytoplasm of the cell and are capable of
reproducing themselves. The mitochondria
contain their own DNA which is responsible for
many enzymatic actions.
Functions of Mitochondrion
i. Production of energy
The mitochondrion is called the ‘power house of
the cell’ because it produces the energy required
for the cellular functions. The energy is produced
by oxidation of the food substances like proteins,
carbohydrates and lipids by the oxidative
enzymes in cristae. During oxidation, water and
carbon dioxide are produced with release of
energy. The released energy is stored in
mitochondria and used later for synthesis of ATP.
ii. Synthesis of ATP
The components of respiratory chain in the
mitochondrion are responsible for the synthesis
of ATP by utilizing the energy through oxidative
phosphorylation. The ATP molecules defuse
throughout the cell from mitochondrion.
Whenever energy is needed for cellular activity,
the ATP molecules are broken down.
iii. Apoptosis
Mitochondria are involved in apoptosis (see
below) also.
n
ORGANELLES WITHOUT LIMITING
MEMBRANE
n
1. RIBOSOMES
The ribosomes are small granular structures with
a diameter of 15 nm. Some ribosomes are
attached to rough endoplasmic reticulum while
others are present as free ribosomes in the
cytoplasm. The ribosomes are made up of
proteins (35%) and RNA (65%). The RNA present
in ribosomes is called ribosomal RNA (rRNA).
Functions of Ribosomes
Ribosomes are called protein factories because
of their role in the synthesis of proteins.
Messenger RNA (mRNA) passes the genetic
code for protein synthesis from nucleus to the
ribosomes. The ribosomes, in turn arrange the
amino acids into small units of proteins. The
ribosomes attached with endoplasmic reticulum
are involved in the synthesis of proteins like the
enzymatic proteins, hormonal proteins, lysosomal
proteins and the proteins of the cell membrane.
The free ribosomes are responsible for the
synthesis of proteins in hemoglobin, peroxisome
and mitochondria.
n
2. CYTOSKELETON
The cytoskeleton of the cell is a complex network
that gives shape, support and stability to the cell.
It is also essential for the cellular movements
and the response of the cell to external stimuli.
The cytoskeleton consists of three major protein
components viz.
a. Microtubules
b. Intermediate filaments
c. Microfilaments.
FIGURE 1-6: Structure of mitochondrion
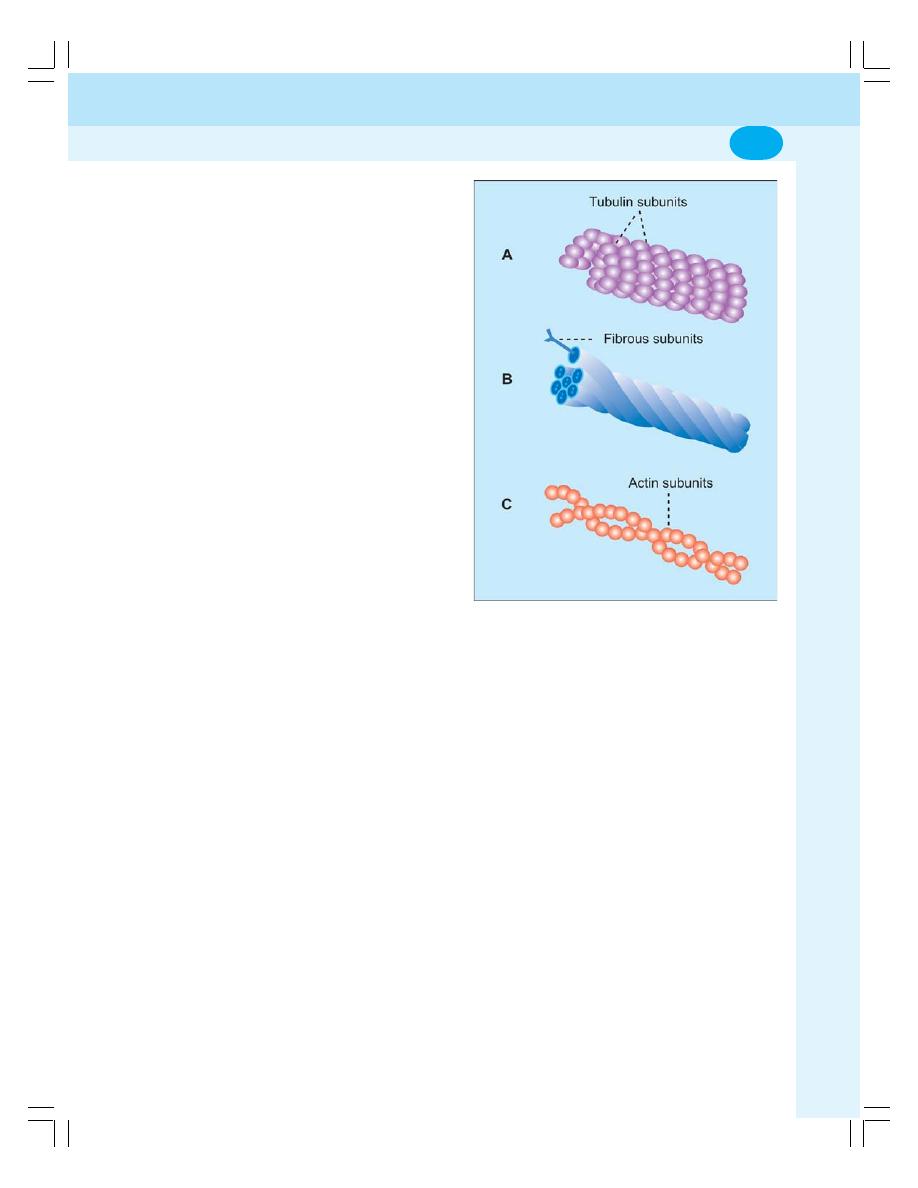
Chapter 1 Cell
11
Microtubules
Microtubules are straight and hollow tubular
structures formed by bundles of globular protein
called
α and β tubulin (Fig. 1-7 A).
Functions of microtubules
Microtubules:
i.
Determine the shape of the cell
ii. Give structural strength to the cell
iii. Act like conveyer belts which allow the move-
ment of granules, vesicles, protein molecules
and some organelles like mitochondria to
different parts of the cell
iv. Form the spindle fibers which separate the
chromosomes during mitosis
v. Responsible for the movements of centrioles
and the complex cellular structures like cilia.
Intermediate Filaments
The intermediate filaments form a network
around the nucleus and extend to the periphery
of the cell. These filaments are formed by fibrous
proteins (Fig. 1-7 B) and help to maintain the
shape of the cell. The adjacent cells are
connected by intermediate filaments by
desmosomes.
Microfilaments
Microfilaments are long and fine thread like
structures which are made up of non tubular
contractile proteins called actin and myosin. Actin
is more abundant than myosin.
Functions of microfilaments
Microfilaments:
i.
Give structural strength to the cell
ii. Provide resistance to the cell against the
pulling forces
iii. Responsible for cellular movements like
contraction, gliding and cytokinesis (partition
of cytoplasm during cell division).
n
NUCLEUS
Nucleus is present in those cells which divide
and produce enzymes. The cells with nucleus
FIGURE 1-7: A = Microtubules. B = Intermediate
filament. C = Microfilament of ectoplasm
are called eukaryotes and those without nucleus
are known as prokaryotes (e.g. red blood cells).
Prokaryotes do not divide or synthesize the
enzymes.
Most of the cells have only one nucleus
(uninucleated). Few types of cells like skeletal
muscle cells have many nuclei (multinucleated).
Generally the nucleus is located near the center
of the cell. It is mostly spherical in shape.
However, the shape and situation of nucleus vary
in different cells.
n
STRUCTURE OF NUCLEUS
Nuclear Membrane
The nucleus is covered by a double layered
membrane called nuclear membrane. It encloses
the fluid called nucleoplasm. Nuclear membrane
is porous and permeable in nature and it allows
nucleoplasm to communicate with the cytoplasm.
General Physiology
12
Nucleoplasm
It is a gel like ground substance and contains
large quantities of the genetic material in the form
of DNA. The DNA is made up of chromatin
threads. These chromatin threads become the
rod shaped chromosomes just before the cell
division.
Nucleoli
One or more nucleoli are present in each
nucleus. The nucleolus contains RNA and some
proteins, which are similar to those found in
ribosomes. The RNA is synthesized by
chromosomes and stored in the nucleolus.
n
FUNCTIONS OF NUCLEUS
Nucleus:
1. Controls all the activities of the cell
2. Synthesizes RNA
3. Forms subunits of ribosomes
4. Sends genetic instruction to the cytoplasm
for protein synthesis through mRNA
5. Controls the cell division through genes
6. Stores the hereditary information (in genes)
and transforms this information from one
generation of the species to the next.
n
CELL DEATH
The cell death occurs by two distinct processes:
1. Necrosis
2. Apoptosis.
n
APOPTOSIS
Apoptosis is defined as the programmed cell
death under genetic control. Originally apoptosis
(means ‘falling leaves’ in Greek) refers to the
process by which the leaves fall from trees in
autumn. It is also called ‘cell suicide’ since the
genes of the cell play a major role in the death.
This type of programmed cell death is a
normal phenomenon and it is essential for normal
development of the body.
Functional Significance of Apoptosis
The main function of apoptosis is to remove
unwanted cells without causing any stress or
damage to the neighboring cells. The functional
significance of apoptosis:
1. Plays a vital role in cellular homeostasis.
About 10 million cells are produced everyday
in human body by mitosis. An equal number
of cells die by apoptosis. This helps in cellular
homeostasis
2. Useful for removal of a cell that is damaged
by a virus or a toxin beyond repair
3. An essential event during the development
and in adult stage.
Examples:
i. A large number of neurons are produced
during the development of central
nervous system. But up to 50% of the
neurons are removed by apoptosis
during the formation of synapses
between neurons
ii. Apoptosis is responsible for the removal
of tissues of webs between fingers and
toes during developmental stage in fetus
iii. It is necessary for regression and
disappearance of duct systems during
sex differentiation in fetus (Chapter 53)
iv. The cell that looses the contact with
neighboring cells or basal lamina in the
epithelial tissue dies by apoptosis. This
is essential for the death of old
enterocytes shed into the lumen of
intestinal glands (Chapter 31)
v. It plays an important role in the cyclic
sloughing of the inner layer of
endometrium resulting in menstruation
(Chapter 55)
vi. Apoptosis removes the auto-aggressive
T cells and prevents autoimmune
diseases.
n
NECROSIS
Necrosis (means ‘dead’ in Greek) is the
uncontrolled and unprogrammed death of cells
Chapter 1 Cell
13
due to unexpected and accidental damage. It is
also called ‘cell murder’ because the cell is killed
by extracellular or external events. After necrosis,
the harmful chemical substances released from
the dead cells cause damage and inflammation
of neighboring tissues.
Causes for Necrosis
Common causes of necrosis are injury, infection,
inflammation, infarction and cancer. Necrosis is
induced by both physical and chemical events
such as heat, radiation, trauma, hypoxia due to
lack of blood flow, and exposure to toxins.
DEFINITION AND CLASSIFICATION
The connection between the cells or the contact
between the cell and extracellular matrix is called
the cell junction. It is also called as membrane
junction. It is generally classified into three types:
1. Occluding junction
2. Communicating junction
3. Anchoring junction
OCCLUDING JUNCTION
The junction which prevents the movement of
ions and molecules from one cell to another cell
is called the occluding junction. Tight junctions
belong to this category.
TIGHT JUNCTION
It is formed by the tight fusion of the cell
membranes from the adjacent cells. The area
of the fusion is very tight and forms a ridge. This
type of junction is present in the apical margins
of epithelial cells in intestinal mucosa, wall of
renal tubule, capillary wall and choroid plexus
(Fig. 2-1).
Functions of Tight Junctions
1. The tight junctions hold the neighboring cells
of the tissues firmly and thus provide strength
and stability to the tissues.
2. It provides the barrier or gate function by
which the interchange of ions, water and
macromolecules between the cells is
regulated.
3. It acts like a fence by preventing the lateral
movement of integral membrane proteins and
lipids from cell membrane
4. By the fencing function, the tight junctions
maintains the cell polarity by keeping the
Cell Junctions
2
DEFINITION AND CLASSIFICATION
OCCLUDING JUNCTIONS
TIGHT JUNCTION
COMMUNICATING JUNCTIONS
GAP JUNCTION
CHEMICAL SYNAPSE
ANCHORING JUNCTIONS
ADHERENS JUNCTIONS
FOCAL ADHESIONS
DESMOSOME
HEMIDESMOSOME
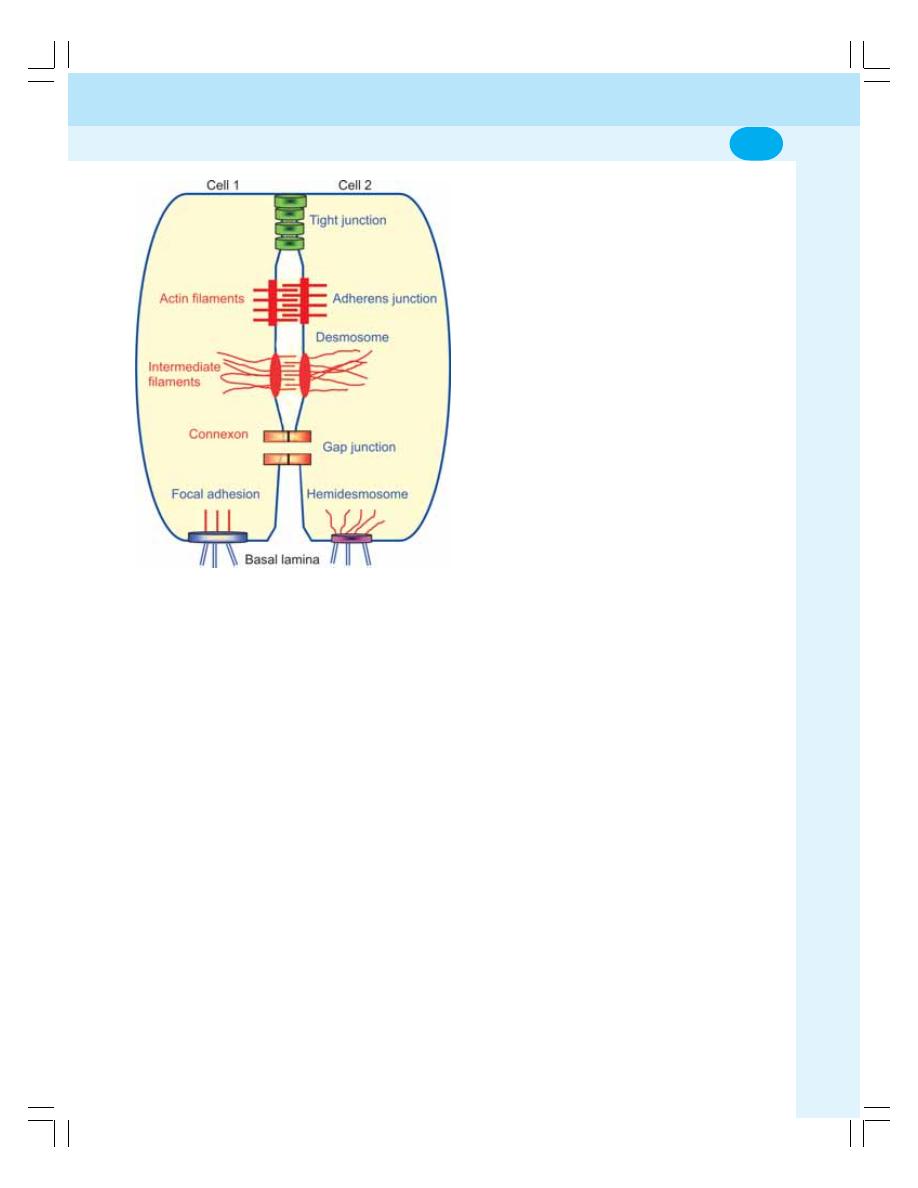
Chapter 2 Cell Junctions
15
proteins in the apical region of the cell
membrane.
5. Tight junctions in the brain capillaries form
the blood-brain barrier (BBB) which prevents
the entrance of many harmful substances
from the blood into the brain tissues.
COMMUNICATING JUNCTIONS
The junctions, which permit the movement of ions
and molecules from one cell to another cell, are
called communicating junctions. Gap junction
and chemical synapse are the communicating
junctions.
GAP JUNCTION OR NEXUS
The gap junction is also called nexus. It is present
in heart, basal part of epithelial cells of intestinal
mucosa, etc.
Structure of Gap Junction
The membranes of the two adjacent cells lie very
close to each other and the intercellular space
becomes a narrow channel. The cytoplasm of
the two cells is interconnected and the molecules
move from one cell to another cell through these
channels without having contact with ECF. The
channel is surrounded by 6 subunits of proteins
which are called connexins or connexons.
Functions of Gap Junction
1. The diameter of the channel in the gap
junction is about 1.5 to 3 nm. So, the
substances having molecular weight less than
1000 such as glucose also can pass through
this junction easily
2. It helps in the exchange of chemical
messengers between the cells
3. It helps in rapid propagation of action potential
from one cell to another cell.
CHEMICAL SYNAPSE
Chemical synapse is the junction between a
nerve fiber and a muscle fiber or between two
nerve fibers, through which the signals are
transmitted by the release of chemical transmitter
(Refer Chapter 86 for details).
ANCHORING JUNCTIONS
Anchoring junctions are the junctions, which
provide firm structural attachment between two
cells or between a cell and the extracellular
matrix. There are four types of anchoring
junctions
i.
Adherens junctions (cell to cell)
ii. Focal adhesions (cell to matrix)
iii. Desmosomes (cell to cell)
iv. Hemidesmosomes (cell to matrix)
ADHERENS JUNCTIONS
These are cell to cell junctions that is the
junctions found between the cells. The
connection occurs through the actin filaments.
Adherens junctions are present in the
intercalated discs of cardiac muscles (Chapter
58) and epidermis of the skin.
FIGURE 2-1: Different types of cell junctions
General Physiology
16
FOCAL ADHESIONS
These are cell to matrix junctions that is junctions
between the cell and the extracellular matrix. The
connection occurs through the actin filaments.
This type of junction is seen in epithelia of various
organs.
DESMOSOME
Desmosome is also cell to cell junction, but here
the membranes of the cells are thickened and
connected by intermediate filaments. So,
desmosome functions like tight junction. This
type of junction is found in areas subjected for
stretching such as the skin.
HEMIDESMOSOME
Hemidesmosome is also cell to matrix junction
and the connection is through intermediate
filaments. It is like half desmosome because
here, the membrane of only one cell thickens.
So, this is known as hemidesmosome or half
desmosome. Mostly, the hemidesmosome
connects the cells with their basal lamina.
INTRODUCTION
BASIC MECHANISM OF TRANSPORT
PASSIVE TRANSPORT
SIMPLE DIFFUSION
FACILITATED OR CARRIER MEDIATED DIFFUSION
FACTORS AFFECTING RATE OF DIFFUSION
SPECIAL TYPES OF PASSIVE TRANSPORT
OSMOTIC PRESSURE
ACTIVE TRANSPORT
MECHANISM OF ACTIVE TRANSPORT
CARRIER PROTEINS
SUBSTANCES TRANSPORTED BY ACTIVE TRANSPORT
TYPES OF ACTIVE TRANSPORT
PRIMARY ACTIVE TRANSPORT
SECONDARY ACTIVE TRANSPORT
SPECIAL CATEGORIES OF ACTIVE TRANSPORT
ENDOCYTOSIS
EXOCYTOSIS
TRANSCYTOSIS
Transport through Cell
Membrane
3
INTRODUCTION
Transport mechanism in the body is necessary
for the supply of essential substances like
nutrients, water, electrolytes, etc. to the tissues
and to remove the unwanted substances like
waste materials, carbon dioxide, etc. from the
tissues.
BASIC MECHANISM OF TRANSPORT
Two basic mechanisms for the transport of
substances across the cell membrane are:
1. Passive mechanism
2. Active mechanism.
PASSIVE TRANSPORT
The transport of the substances along the
concentration gradient or electrical gradient or
both (electrochemical gradient) is called passive
transport. Here, the substances move from the
region of higher concentration to the region
of lower concentration. It is also known as
diffusion or downhill movement. It does not need
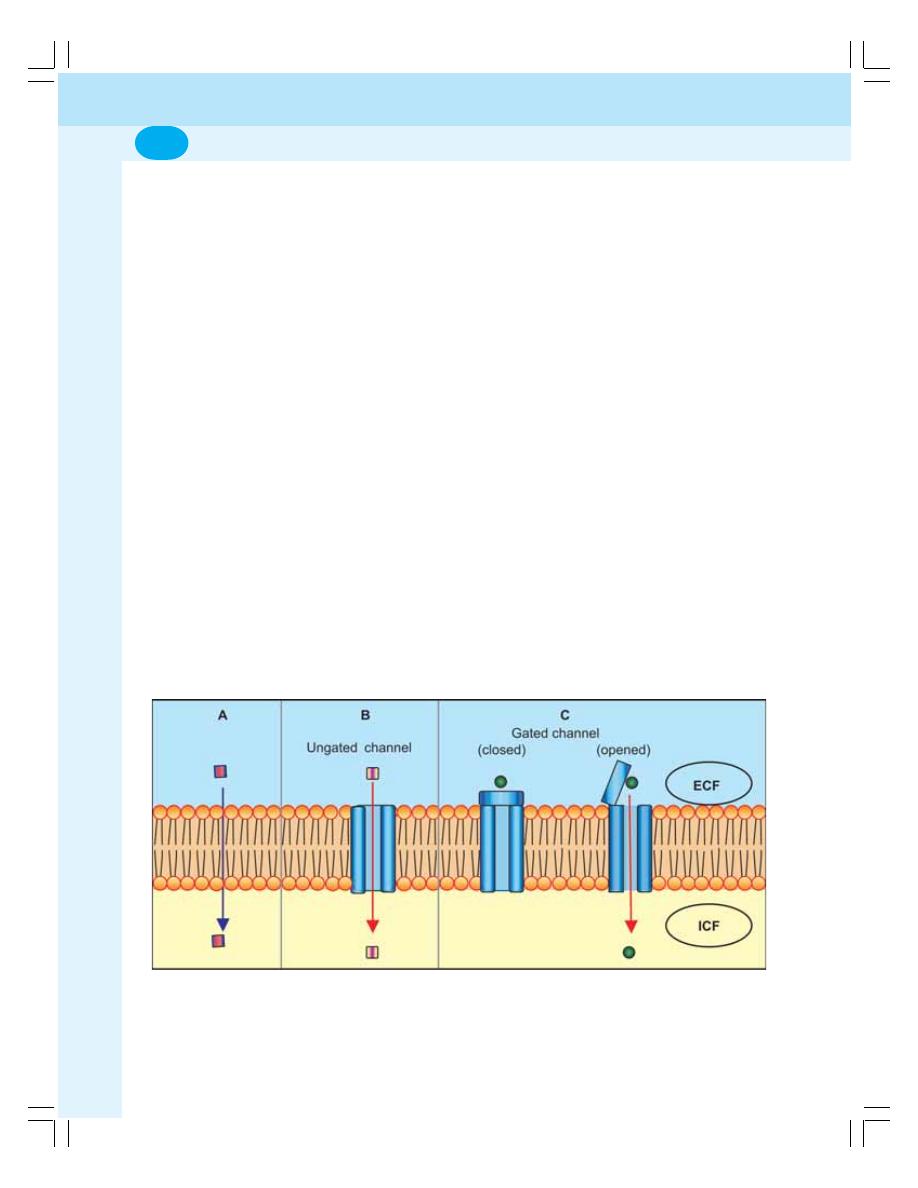
General Physiology
18
energy. Diffusion or passive transport is of two
types:
1. Simple diffusion
2. Facilitated diffusion.
SIMPLE DIFFUSION
Simple diffusion is of two types:
1. Simple diffusion through lipid layer
2. Simple diffusion through protein layer
Simple Diffusion through Lipid Layer
Lipid soluble substances like oxygen, carbon
dioxide and alcohol are transported by simple
diffusion through the lipid layer of the cell
membrane (Fig. 3-1A).
Simple Diffusion through Protein Layer
There are specific protein channels that extend
from cell membrane through which the simple
diffusion takes place. Water soluble substances
like electrolytes are transported through these
channels. These channels are selectively
permeable to only one type of ion. Accordingly,
the channels are named after the ions diffusing
through these channels like sodium channels,
potassium channels, etc.
Protein Channels
The protein channels are of two types:
1. Ungated channels which are opened
continuously
2. Gated channels which are closed all the time
and are opened only when required
(Fig. 3-1B).
Gated channels
The gated channels are divided into three
categories:
i. Voltage gated channels which open by
change in the electrical potential (Fig. 3-
1C). Examples are the calcium channels
present in neuromuscular junction (Chapter
24).
ii. Ligand gated channels that open in the
presence of hormonal substances (ligand).
Examples are the sodium channels which
are opened by acetylcholine in neuro-
muscular junction.
iii. Mechanically gated channels which are
opened by some mechanical factors like
pressure and force. Examples are the
sodium channels in pressure receptors
called Pacinian corpuscles.
FIGURE 3-1: Hypothetical diagram of simple diffusion through the cell membrane. A = Diffusion
through lipid layer. B = Diffusion through ungated channel. C = Diffusion through gated channel
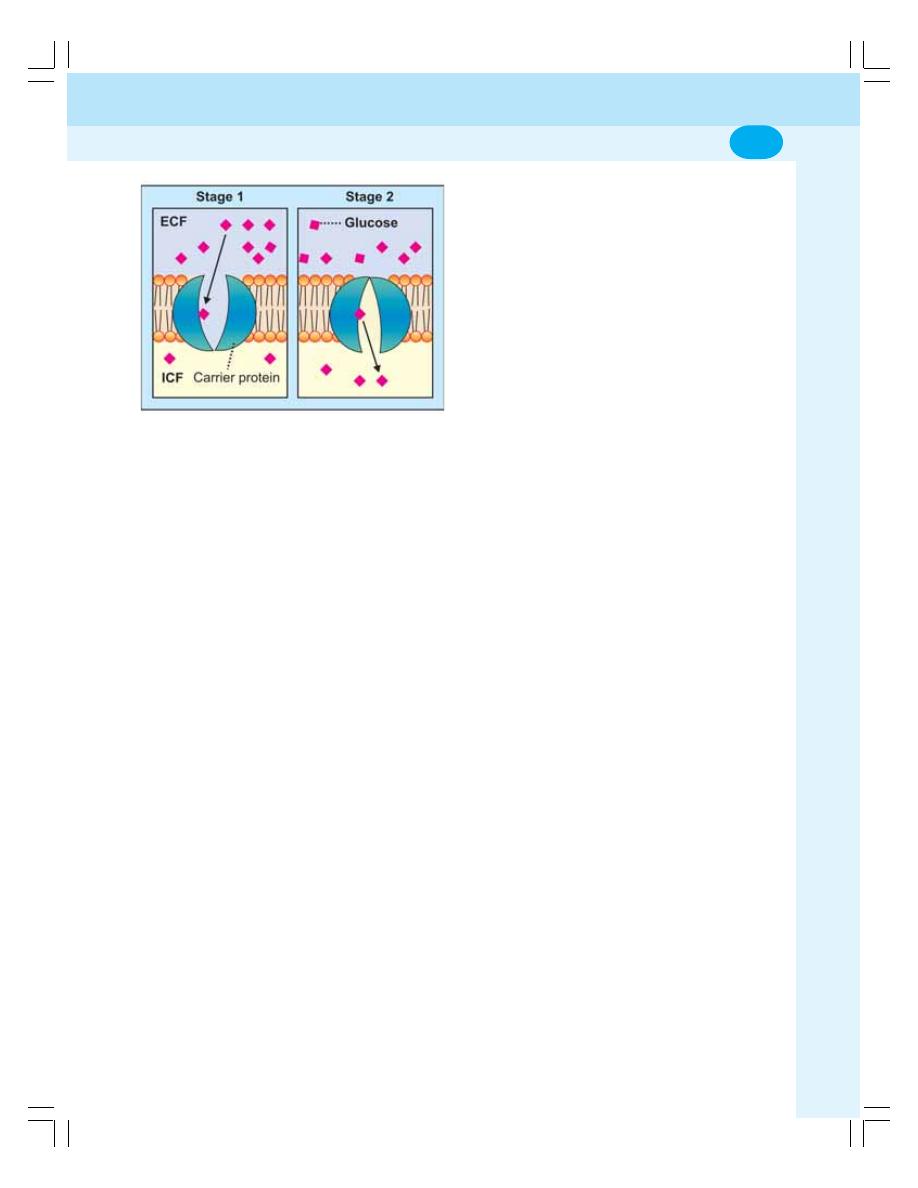
Chapter 3 Transport through Cell Membrane
19
FACILITATED OR CARRIER MEDIATED
DIFFUSION
In this type of diffusion, some carrier proteins help
the transport of substances. The water soluble
substances with larger molecules cannot pass
through the protein channels by simple diffusion.
Such substances are transported with the help
of carrier proteins. This type of diffusion is faster
than the simple diffusion. Glucose and amino
acids are transported by this method (Fig. 3-2).
FACTORS AFFECTING RATE OF
DIFFUSION
The rate of diffusion of substances through the
cell membrane is directly proportional to the
following factors:
1. Permeability of the cell membrane
2. Body temperature
3. Concentration gradient or electrical gradient
of the substance across the cell membrane
4. Solubility of the Substance
The rate of diffusion of substances through the
cell membrane is inversely proportional to the
following factors:
1. Thickness of the cell membrane
2. Charge of the ions
3. Size of the molecules.
SPECIAL TYPES OF PASSIVE
TRANSPORT
In additions to diffusion there are some special
types of passive transport viz.
1. Bulk flow
2. Filtration
3. Osmosis
Bulk Flow
The diffusion of large quantity of substances from
a region of high pressure to the region of low
pressure is known as bulk flow. Bulk flow is due
to the pressure gradient of the substance across
the cell membrane. The best example for this is
the exchange of gases across the respiratory
membrane in lungs (Chapter 76).
Filtration
The movement of water and solutes from an
area of high hydrostatic pressure to an area
of low hydrostatic pressure is called filtration.
The hydrostatic pressure is developed by the
weight of the fluid. Filtration process is seen
at the arterial end of the capillaries where
movement of fluid occurs along with dissolved
substances from blood into the interstitial fluid
(Chapter 19). It also occurs in glomeruli of
kidneys (Chapter 37).
Osmosis
Osmosis is the special type of diffusion. It is the
movement of water or any other solvent from an
area of lower concentration to an area of higher
concentration through a semipermeable
membrane (Fig. 3-3).
Osmosis is of two types:
i. Endosmosis by which water moves into
the cell
ii. Exosmosis by which water moves outside
the cell.
Osmotic Pressure
The pressure created by the solutes in a fluid is
called osmotic pressure. During osmosis, when
water or any other solvent moves from the area
of lower concentration to the area of higher
concentration, the solutes in the area of higher
FIGURE 3-2: Hypothetical diagram of facilitated
diffusion from higher concentration (ECF) to lower
concentration (ICF). Stage 1: Glucose binds with
carrier protein. Stage 2: Conformational change
occurs in the carrier protein and glucose is released
into ICF.
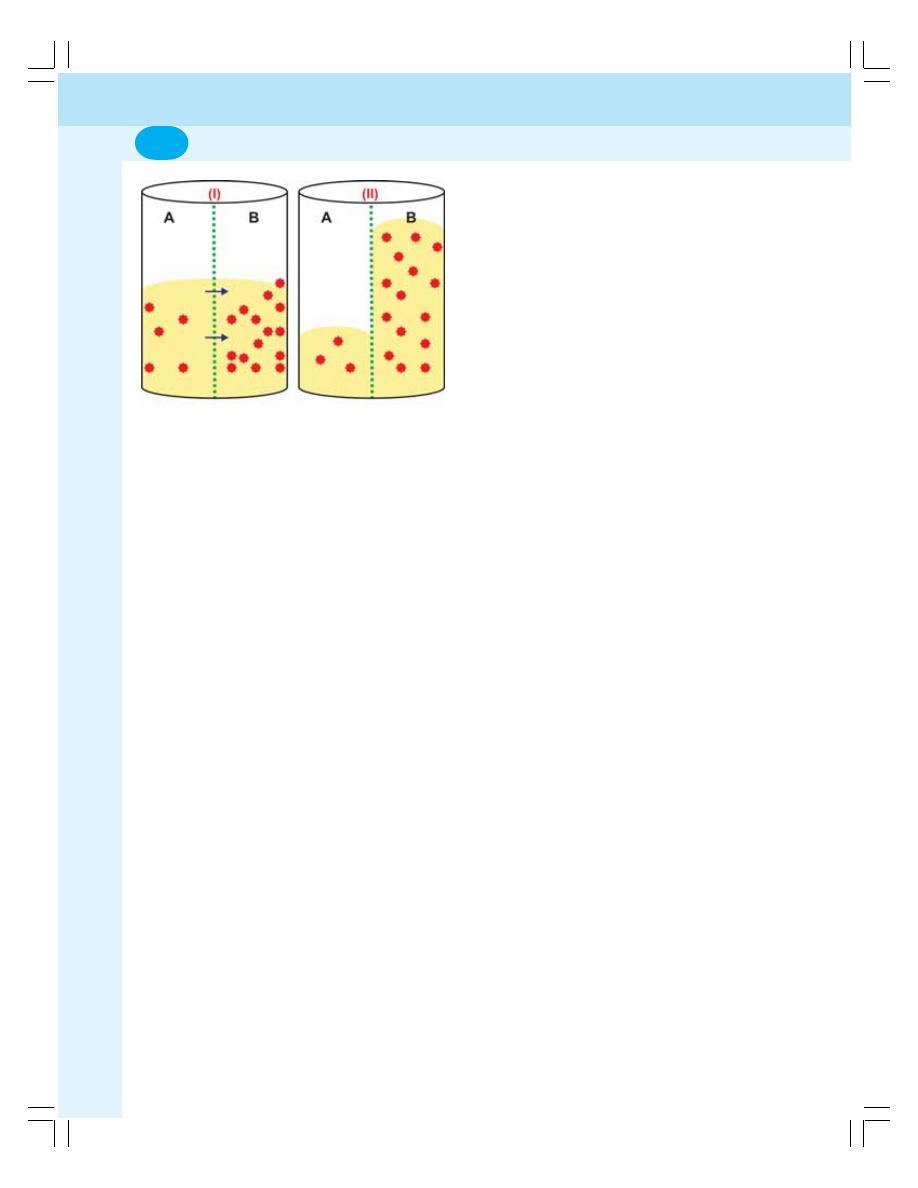
General Physiology
20
concentration get dissolved in the solvent. This
creates a pressure which is known as osmotic
pressure.
Colloidal Osmotic Pressure and Oncotic
Pressure
The osmotic pressure exerted by the colloidal
substances in the body is called the colloidal
osmotic pressure. And, the osmotic pressure
exerted by the colloidal substances (proteins) of
the plasma is known as oncotic pressure and it
is about 25 mm Hg.
ACTIVE TRANSPORT
Movement of substances against the chemical
or electrical or electrochemical gradient is called
active transport. It is also called uphill transport.
Active transport requires energy which is obtained
mainly by breakdown of ATP. It also needs a
carrier protein.
MECHANISM OF ACTIVE TRANSPORT
When a substance to be transported across the
cell membrane comes near the cell, it combines
with the carrier protein of the cell membrane and
forms substance – protein complex. This complex
moves towards the inner surface of the cell
membrane. Now, the substance is released from
the carrier proteins. The same carrier protein
moves back to the outer surface of the cell
membrane to transport another molecule of the
substance.
CARRIER PROTEINS
There are two types of carrier proteins:
1. Uniport
2. Symport or antiport
Uniport
The carrier protein that can carry only one
substance in a single direction is called uniport.
It is also known as uniport pump.
Symport and antiport
The carrier protein that transports two different
substances in the same direction is called
symport. The carrier protein that transports two
different substances in opposite directions is
called antiport.
SUBSTANCES TRANSPORTED BY
ACTIVE TRANSPORT
The actively transported substances are in ionic
form and nonionic form. The substances in ionic
form are sodium, potassium, calcium, hydrogen,
chloride and iodide. The substances in nonionic
form are glucose, amino acids and urea.
TYPES OF ACTIVE TRANSPORT
The active transport is of two types:
1. Primary active transport
2. Secondary active transport.
PRIMARY ACTIVE TRANSPORT
In primary active transport, the energy is liberated
directly from the breakdown of ATP. By this
method, the substances like sodium, potassium,
calcium, hydrogen and chloride are transported
across the cell membrane.
FIGURE 3-3: Osmosis. Red objects = solute. Yellow
shade = water. Green dotted line = semipermeable
membrane. In (I), concentration of solute is high in
the compartment B and low in compartment A. So,
water moves from A to B through semipermeable
membrane. In (II), entrance of water into B exerts
osmotic pressure
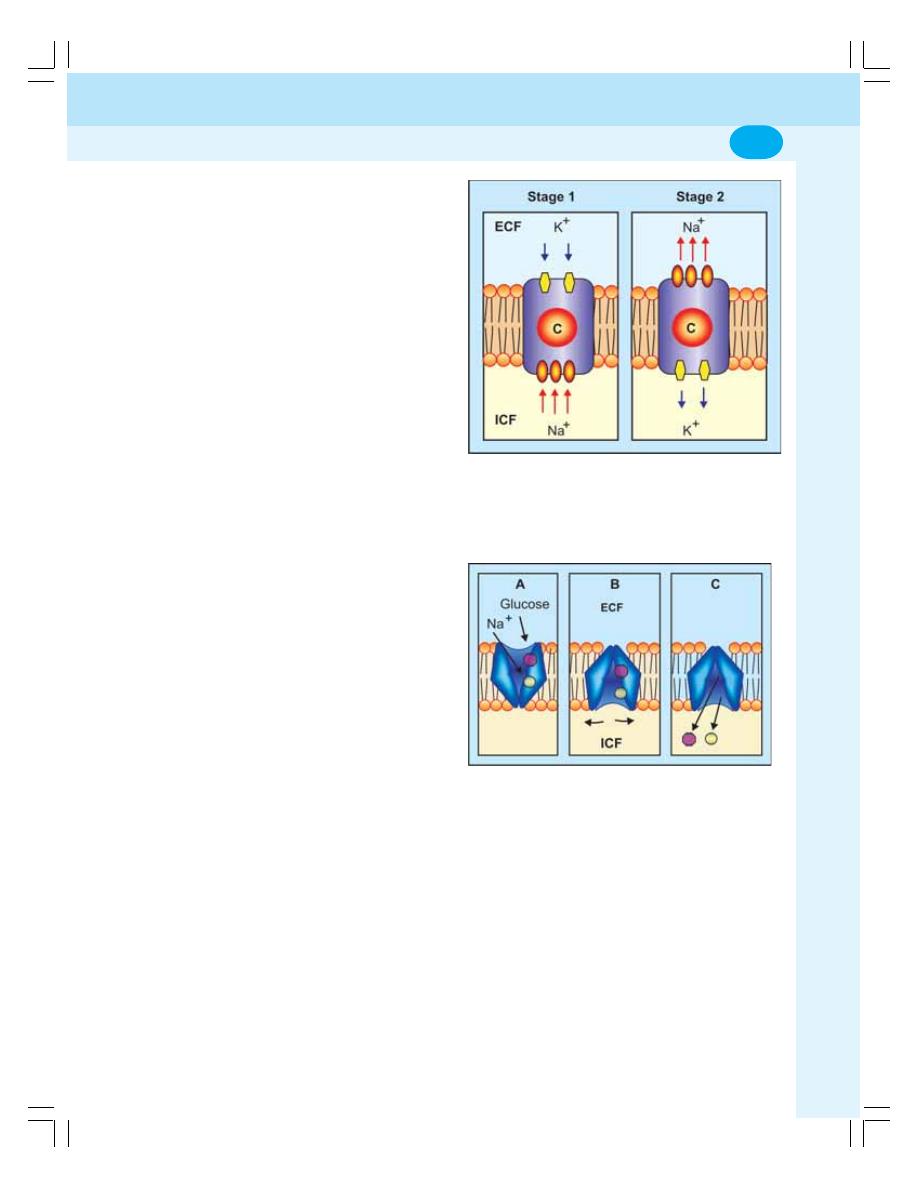
Chapter 3 Transport through Cell Membrane
21
Primary Active Transport of Sodium and
Potassium: Sodium-Potassium Pump
Sodium and potassium ions are transported
across the cell membrane by sodium-potassium
(Na
+
- K
+
) pump which is also called Na
+
- K
+
ATPase pump. This pump is formed by a carrier
protein and it is present in all cells of the body.
Three sodium ions from inside and two potassium
ions from outside get attached with the carrier
protein (Fig. 3-4, Stage1). Some conformational
change occurs in the carrier protein by which the
attachment with sodium ions faces the ECF and
the attachment with potassium ions faces the
ICF. Now the three sodium ions are released into
ECF and two potassium ions are released into
ICF (Fig. 3-4, Stage 2). It is responsible for the
establishment of resting membrane potential
(RMP) in the cell by distributing more sodium
ions outside and more potassium ions inside.
This action is called electrogenic activity of Na
+
-
K
+
pump
Transport of Calcium Ions
Calcium ions are actively transported from inside
to outside the cell by calcium pump with the help
of a separate carrier protein. The energy is
obtained from ATP.
Transport of Hydrogen Ions
Hydrogen ions are actively transported across the
cell membrane by hydrogen pump with the help
of another carrier protein. It also obtains energy
from ATP.
SECONDARY ACTIVE TRANSPORT
The transport of a substance with sodium ions
by a common carrier protein is called secondary
active transport. It is of two types:
1. Co-transport — transport of the substance in
the same direction along with sodium
2. Counter transport — transport of the sub-
stance in the opposite direction to that of
sodium
Sodium Co-transport
In this, along with sodium, another substance is
carried with the help of a carrier protein called
symport (the protein that transports two different
molecules in the same direction across the cell
membrane). Glucose, amino acids, chloride,
iodine, iron and urate ions are transported by this
method (Fig. 3-5).
Sodium Counter Transport
In this process, the substances are transported
across the cell membrane in exchange for sodium
ions by the carrier protein called antiport (the
FIGURE 3-4: Hypothetical diagram of sodium-
potassium pump. C = carrier protein. Stage 1: Three
Na
+
from ICF and two K
+
from ECF bind with ‘C’.
Stage 2: Conformational change occurs in ‘C’
followed by release of Na
+
into ECF and K
+
into ICF
FIGURE 3-5: Sodium co-transport. A = Na
+
and
glucose from ECF bind with carrier protein. B =
Conformational change occurs in the carrier protein.
C = Na
+
and glucose are released into ICF
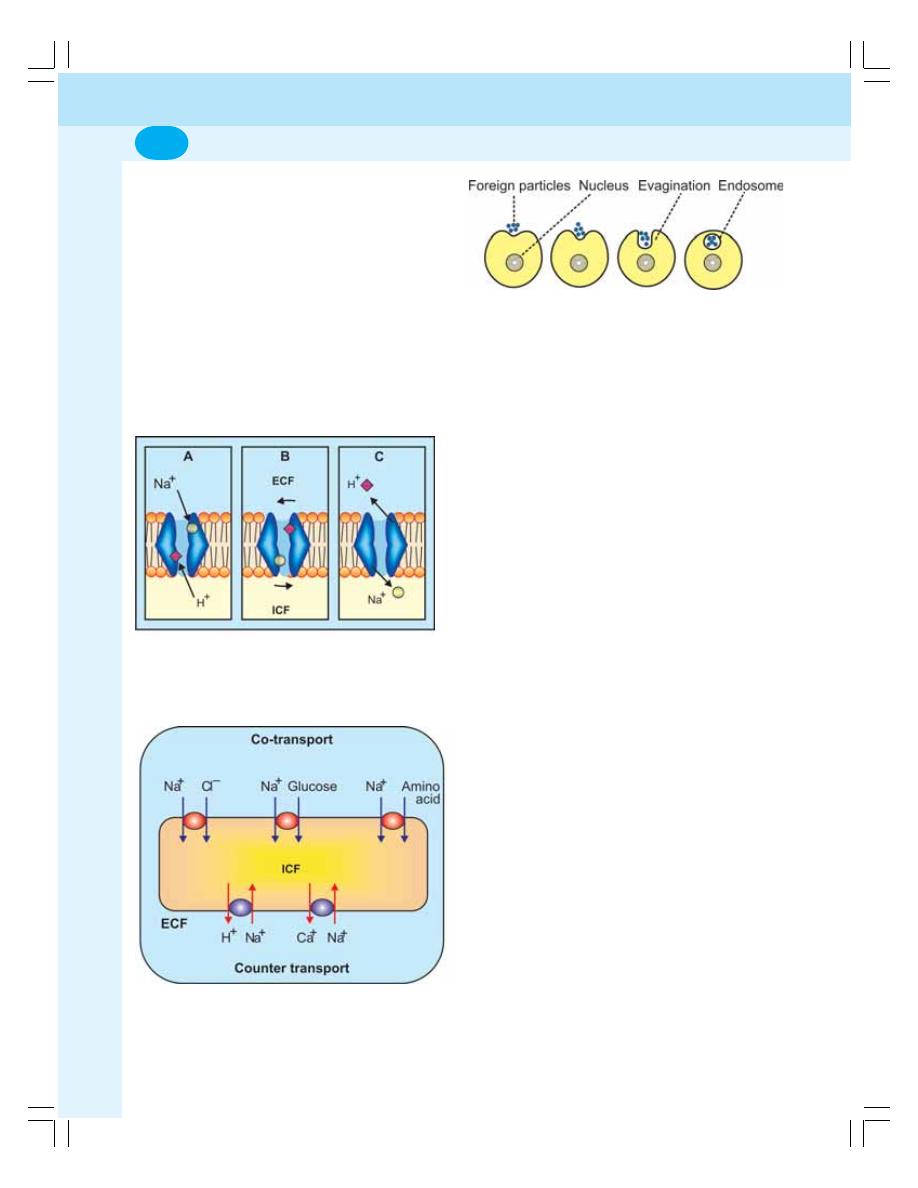
General Physiology
22
FIGURE 3-6: Sodium counter transport. A = Na
+
from
ECF and H
+
from ICF bind with carrier protein. B =
Conformational change occurs in the carrier protein.
C = Na
+
enters ICF and H
+
enters ECF
FIGURE 3-7: Sodium co-transport and counter
transport by carrier proteins
carrier protein that transports two different ions
or molecules in opposite direction across the cell
membrane). Examples of counter transport
systems are sodium-calcium counter transport
and sodium-hydrogen counter transport in the
tubular cells (Figs 3-6 and 3-7).
SPECIAL CATEGORIES OF ACTIVE
TRANSPORT
In addition to primary and secondary active
transport systems, some special categories of
active transport systems also exist in the body.
The special categories of active transport are:
I. Endocytosis
II. Exocytosis
III. Transcytosis.
ENDOCYTOSIS
Endocytosis is the transport mechanism by which
the macromolecules enter the cell. The
substances with larger molecules are called
macromolecules and these cannot pass through
the cell membrane either by active or by passive
transport mechanism. Such substances are
transported into the cell by endocytosis.
Endocytosis is of three types:
1. Pinocytosis
2. Phagocytosis
3. Receptor mediated endocytosis.
1. Pinocytosis
It is otherwise called the cell drinking. The
macromolecules like bacteria and antigens enter
the cells by pinocytosis.
Mechanism of pinocytosis
i. The macromolecules (in the form of
droplets of fluid) bind to the outer surface
of the cell membrane
ii. Now, the cell membrane evaginates and
engulfs the droplets
iv. The engulfed droplets are converted into
vesicles and vacuoles, which are called
endosomes (Fig. 3-8)
v. The endosome travels into the interior of
the cell
vi. The primary lysosome in the cytoplasm
fuses with the endosome and forms the
secondary lysosome
FIGURE 3-8: Process of pinocytosis
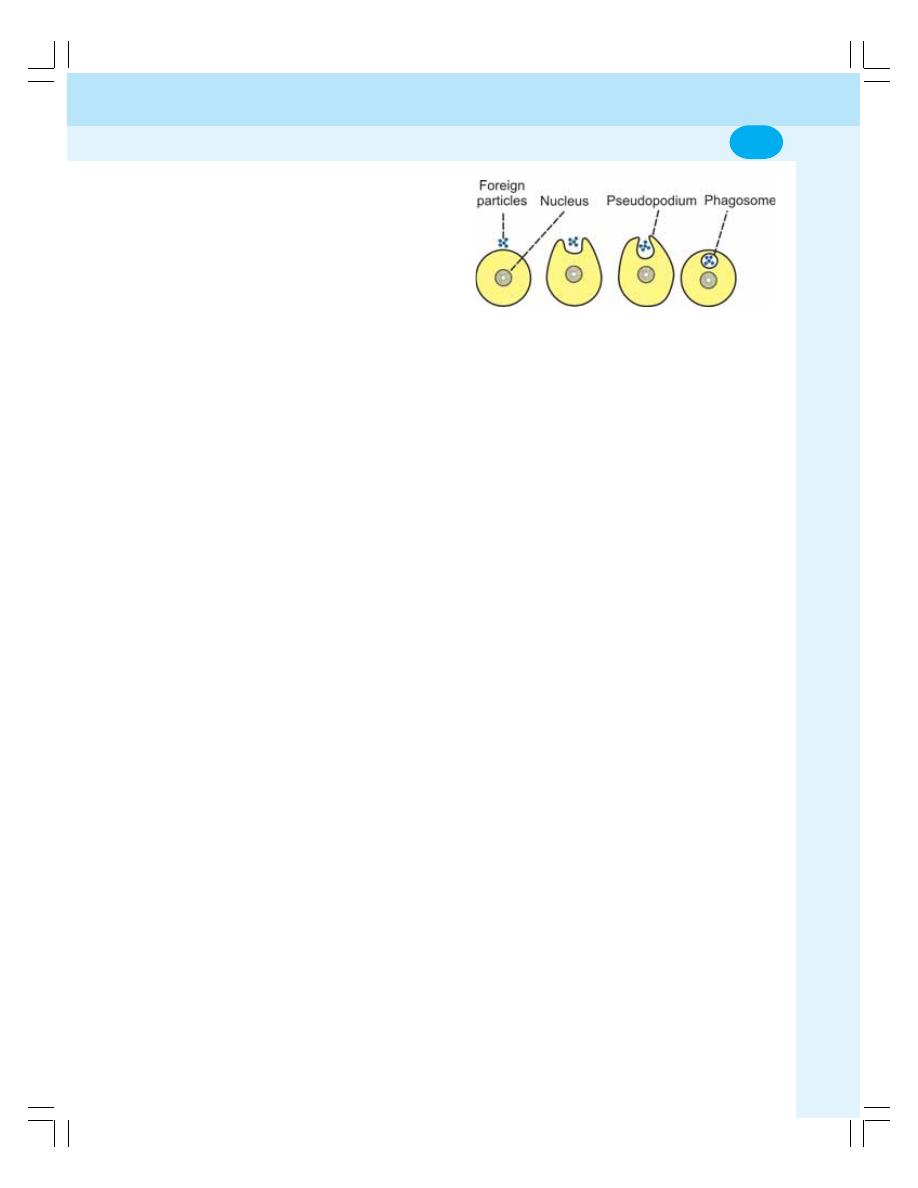
Chapter 3 Transport through Cell Membrane
23
vii. Now, hydrolytic enzymes present in the
secondary lysosome are activated resulting
in digestion and degradation of the
endosomal contents.
2. Phagocytosis
The process by which the particles larger than
the macromolecules are engulfed into the cells
is called phagocytosis or cell eating. Larger
bacteria, larger antigens and other larger foreign
bodies are taken inside the cell by means of
phagocytosis. Only few cells in the body like
neutrophils, monocytes and the tissue macro-
phages show phagocytosis. Among these cells,
the macrophages are the largest phagocytic cells.
Mechanism of phagocytosis
i. When the bacteria or the foreign body
enters the body, first the phagocytic cell
sends cytoplasmic extension (pseudopo-
dium) around the bacteria or the foreign
body
ii. Then, these particles are engulfed and are
converted into endosome like vacuole. The
vacuole is very large and it is usually called
the phagosome
iii. The phagosome travels into the interior of
the cell
iv. The primary lysosome fuses with this
phagosome and forms secondary
lysosome
v. The hydrolytic enzymes present in the
secondary lysosome are activated resulting
in digestion and degradation of the
phagosomal contents (Fig. 3-9).
3. Receptor Mediated Endocytosis
Transport of macromolecules which is mediated
by a receptor protein is called the receptor
mediated endocytosis. The surface of cell
membrane has some pits which contain a
receptor protein called clathrin. Together with a
receptor protein, each pit is called receptor coated
pit. The coated pits are involved in the receptor
mediated endocytosis.
FIGURE 3-9: Process of phagocytosis
Mechanism of receptor mediated endocytosis
i. The receptor mediated endocytosis is
induced by substances like ligand
(hormone) which bind to the receptors in
the coated pits and form the ligand-
receptor complexes
ii. The ligand-receptor complexes get
aggregated in the coated pits
iii. Then, the pit is detached from the cell
membrane and becomes the coated
vesicle. This coated vesicle forms the
endosome
vi. The endosome travels into the interior of
the cell (Fig. 3-10).
Receptor mediated endocytosis plays an
important role in the transport of various types
of macromolecules such as hormones, antibodies,
lipids, growth factors, toxins, bacteria and viruses.
EXOCYTOSIS
Exocytosis is the process by which the
substances are expelled from the cell. In this
process, the substances are extruded from the
cell without passing through the cell membrane.
This is the reverse of endocytosis.
Mechanism of exocytosis
Secretory substances from the cells are released
by exocytosis. The secretory substances of the
cell are stored in the form of secretory vesicles
in the cytoplasm. When required, the vesicles
move towards the cell membrane and get fused
with it. Later, the contents of the vesicles are
released out of the cell (Fig. 3-11).
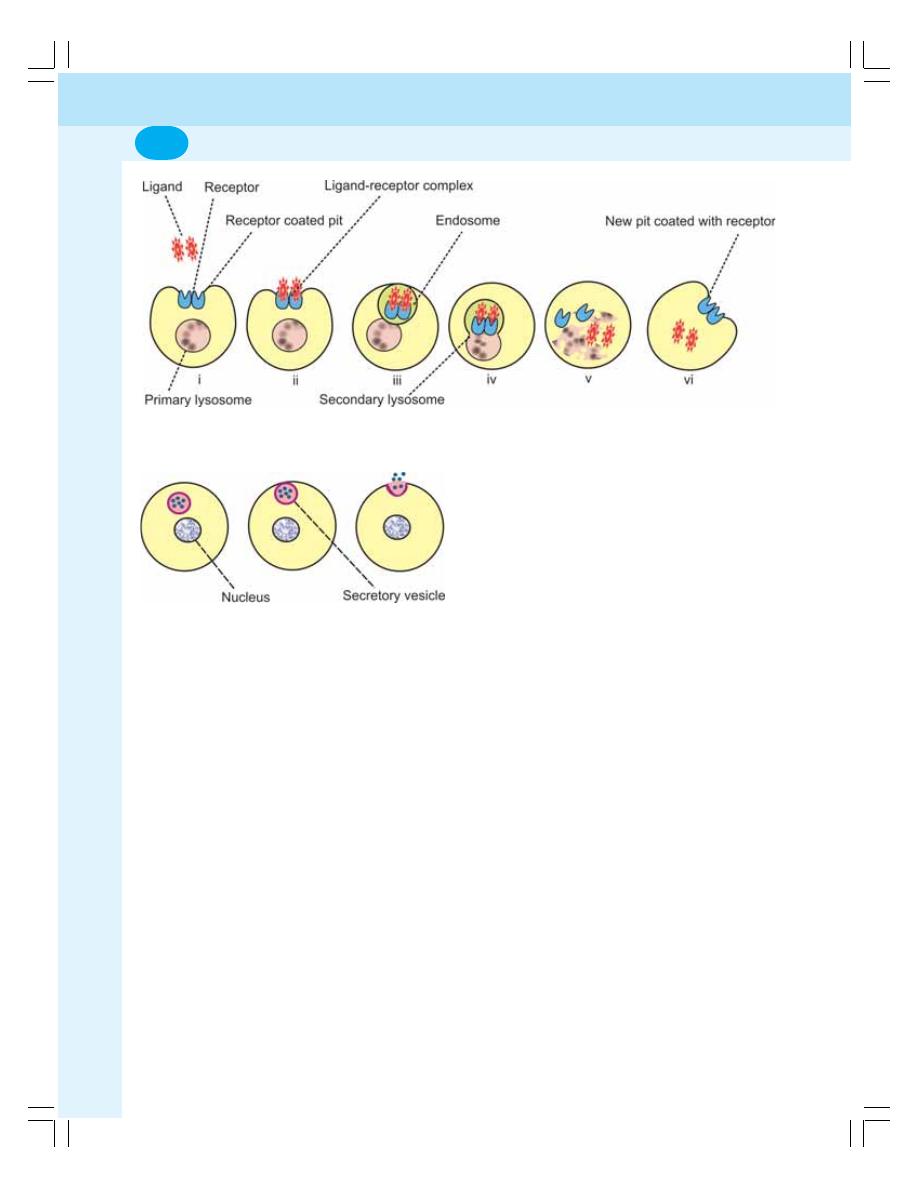
General Physiology
24
FIGURE 3-10: Mechanisms of receptor mediated endocytosis. The numbering of each figure
corresponds with the numbers used in the text
FIGURE 3-11: Exocytosis
TRANSCYTOSIS
Transcytosis is a transport mechanism in
which an extracellular macromolecule enters
through one side of a cell, migrates across
cytoplasm of the cell and exits through the
other side by means of exocytosis. Examples
are movement of proteins and pathogens like
HIV from capillary blood into interstitial fluid
through endothelial cells of the capillary.

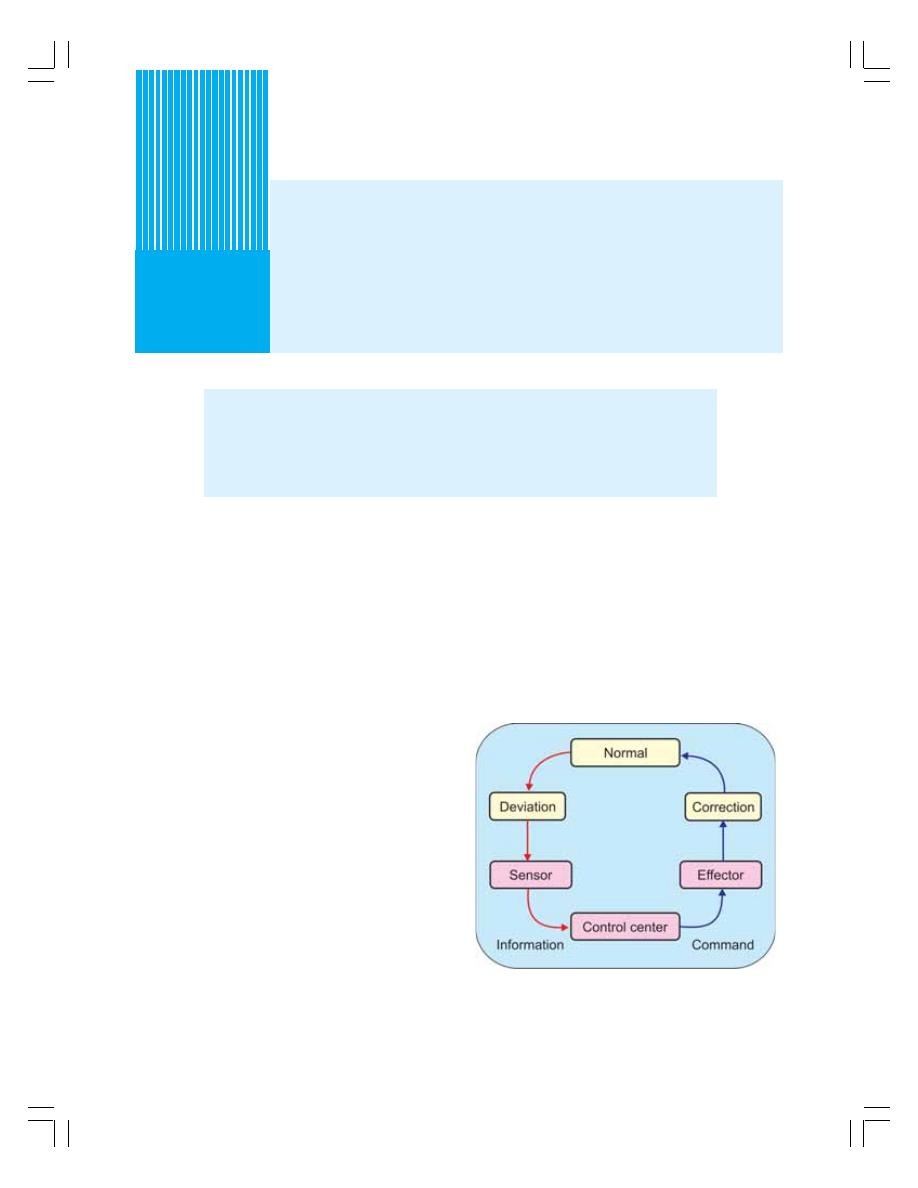
INTRODUCTION
COMPONENTS OF HOMEOSTATIC SYSTEM
HOMEOSTASIS AND VARIOUS SYSTEMS OF THE BODY
MECHANISM OF ACTION OF HOMEOSTATIC SYSTEM
Homeostasis
4
INTRODUCTION
"Homeostasis" means the maintenance of
constant internal environment. According to
Claude Bernard multicellular organisms
including man live in a perfectly organized and
controlled internal environment, which he called
"Milieu interieur". The word 'Homeostasis' was
introduced by Harvard Professor, Walter B
Cannon in 1930.
The internal environment in the body is the
ECF which contains nutrients, ions and all other
substances necessary for the survival of the cells
and in this environment the cells live. It includes
the blood and interstitial fluid.
For the operation of homeostatic mechanism,
the body must recognize the deviation of any
physiological activity from the normal limits.
Fortunately, body is provided with appropriate
detectors or sensors, which recognize the
deviation and alert the integrating center. The
integrating center immediately sends
information to the concerned effectors to either
accelerate or inhibit the activity so that the
normalcy is restored.
COMPONENTS OF HOMEOSTATIC
SYSTEM
The homeostatic system in the body acts through
self regulating devices, which operate in a cyclic
manner (Fig. 4-1). This cycle includes three
components:
FIGURE 4-1: Components of homeostatic system
General Physiology
26
1. Detectors or sensors, which recognize the
deviation
2. Control center to which the information
regarding deviation is transmitted
3. Effectors which receive the information from
the central control for correcting the deviation
The transmission of the message or
information may be an electrical process in the
form of impulses through nerves or a chemical
process in the form of mainly hormones through
blood and body fluids.
HOMEOSTASIS AND VARIOUS
SYSTEMS OF THE BODY
One or more systems are involved in homeostatic
mechanism of each function. Some of the
functions in which the homeostatic mechanism
is well established are given below.
1. The pH of the ECF has to be maintained
at the critical value of 7.4. The tissues
cannot survive if it is altered. Thus, the
decrease in pH (acidosis) or increase in
pH (alkalosis) affects the tissues markedly.
The respiratory system, blood and kidney
help in the regulation of pH.
2. The body temperature must be maintained
at 37.5°C. Increase or decrease in
temperature alters the metabolic activities
of the cells. The skin, respiratory system,
digestive system, excretory system,
skeletal muscles and nervous system are
involved in maintaining the temperature
within normal limits.
3. Adequate amount of nutrients must be
supplied to the cells for various activities
and growth of the tissues. Digestive
system and circulatory system play major
roles in the supply of nutrients.
4. Adequate amount of oxygen should be
supplied to the cells for the metabolic
processes and the carbon dioxide and other
metabolic end products must be removed
from the cells. Respiratory system and
excretory systems involved in these
activities.
5. Many hormones are essential for the
metabolism of nutrients and other
substances necessary for the cells. The
hormones are to be synthesized and
released from the endocrine glands in
appropriate quantities and, these
hormones must act on the body cells
appropriately. Otherwise, it leads to
abnormal signs and symptoms.
6. Water and electrolyte balance should be
maintained optimally. Otherwise it leads
to dehydration or water toxicity and
alteration in the osmolality of the body
fluids. Kidneys, skin, salivary glands and
gastrointestinal tract take care of this.
7. For all these functions, the blood, which
forms the major part of internal
environment, must be normal. It should
contain required number of normal red
blood cells and adequate amount of
plasma with normal composition. Only
then, it can transport the nutritive
substances, respiratory gases, metabolic
and other waste products.
8. Skeletal muscles also help in homeo-
stasis by helping the organism to move
around in search of food and protect the
organism from adverse surroundings of
damage and destruction.
9. The central nervous system which
includes brain and spinal cord also plays
an important role in homeostasis. The
sensory system detects the state of the
body or surroundings. The brain integrates
and interprets the pros and cons of these
information and commands the body to act
accordingly through motor system so that,
the body can avoid the damage.
10. The autonomic nervous system regulates
all the vegetative functions of the body
essential for homeostasis.
MECHANISM OF ACTION OF
HOMEOSTATIC SYSTEM
The homeostatic system acts through feedback
mechanism. Feedback is a process in which
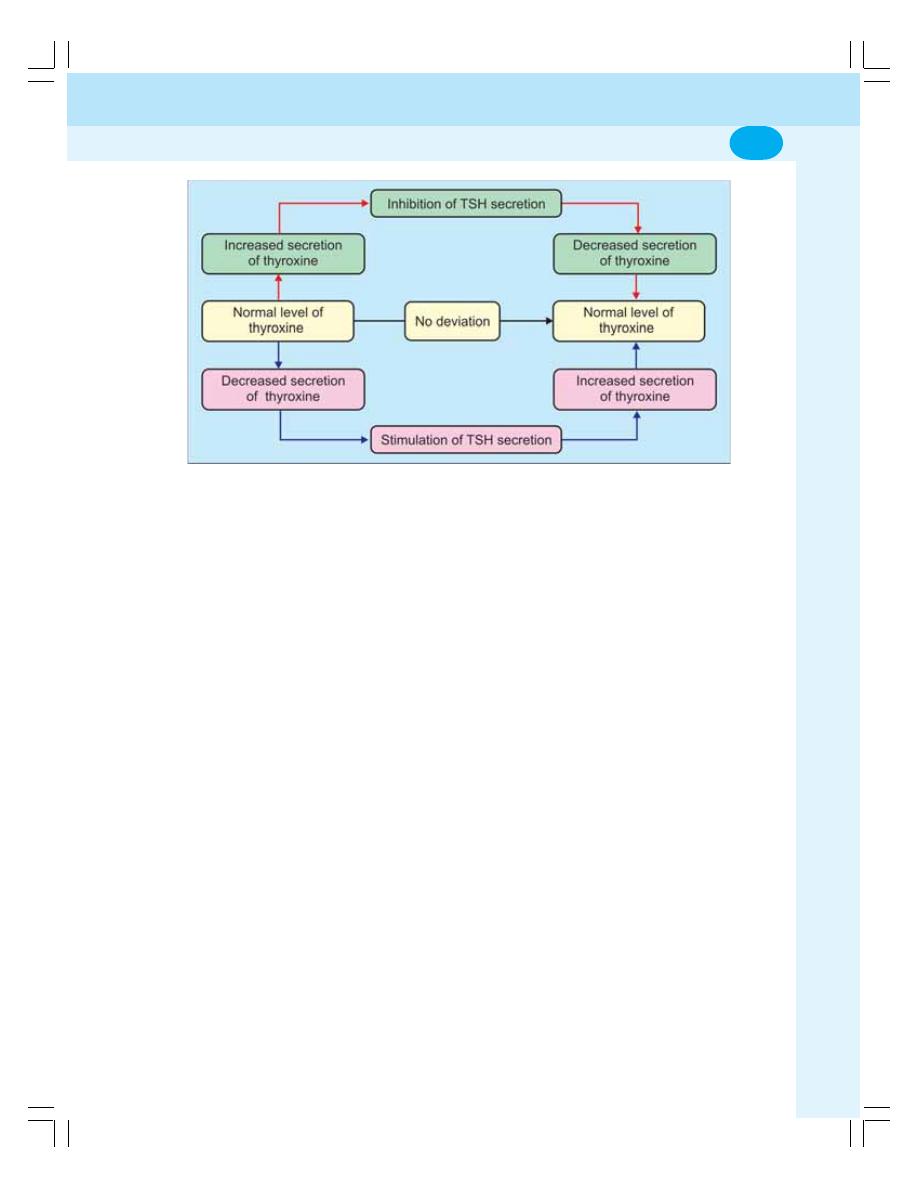
Chapter 4 Homeostasis
27
some proportion of the output signal of a system
is fed (passed) back to the input. There are two
types of feedback mechanisms:
1. Negative feedback mechanism
2. Positive feedback mechanism
Negative Feedback Mechanism
Negative feedback mechanism is the one by
which a particular system reacts in such a way
as to stop the change or reverse the direction of
change. After receiving a message, the effectors
send the inhibitory signals back to the system.
Now, the system stabilizes its own function either
by stopping the signals or by reversing the
signals.
For example, thyroid stimulating hormone
(TSH) released from pituitary gland stimulates
thyroid gland which in turn secretes thyroxine.
When thyroxin level increases in blood, it inhibits
the secretion of TSH from pituitary so that, the
secretion of thyroxine from thyroid gland
decreases (Fig. 4-2). On the other hand, if thyroxin
secretion is less, it induces pituitary gland to
release TSH. Now, TSH stimulates thyroid gland
to secrete thyroxine (Refer Chapter 46 for details).
Another example for negative feedback
mechanism is maintenance of water balance in
the body (Fig. 4-3).
Positive Feedback Mechanism
Positive feedback mechanism is the one in which
the system reacts in such a way as to amplify
(increase the intensity of) the change in the same
direction. Positive feedback is less common than
the negative feedback. However, it has its own
significance, particularly during emergency
conditions.
One of the positive feedbacks occurs during
the blood clotting. Blood clotting is necessary
to arrest bleeding during injury and it occurs in
three stages:
i. Formation of prothrombin activator
ii. Conversion of prothrombin into thrombin
iii. Conversion of fibrinogen into fibrin by
thrombin.
Thrombin formed in the second stage
stimulates the formation of more prothrombin
activator in addition to converting fibrinogen into
fibrin, (Fig. 4-4). It causes formation of more and
more amount of prothrombin activator so that the
blood clotting process is accelerated and blood
loss is prevented quickly (Chapter 15). Other
processes where positive feedback occurs are
milk ejection reflex (Chapter 45) and parturition
(Fig. 4-5) and both the processes involve
oxytocin secretion.
FIGURE 4-2: Negative feedback mechanism — secretion of thyroxine
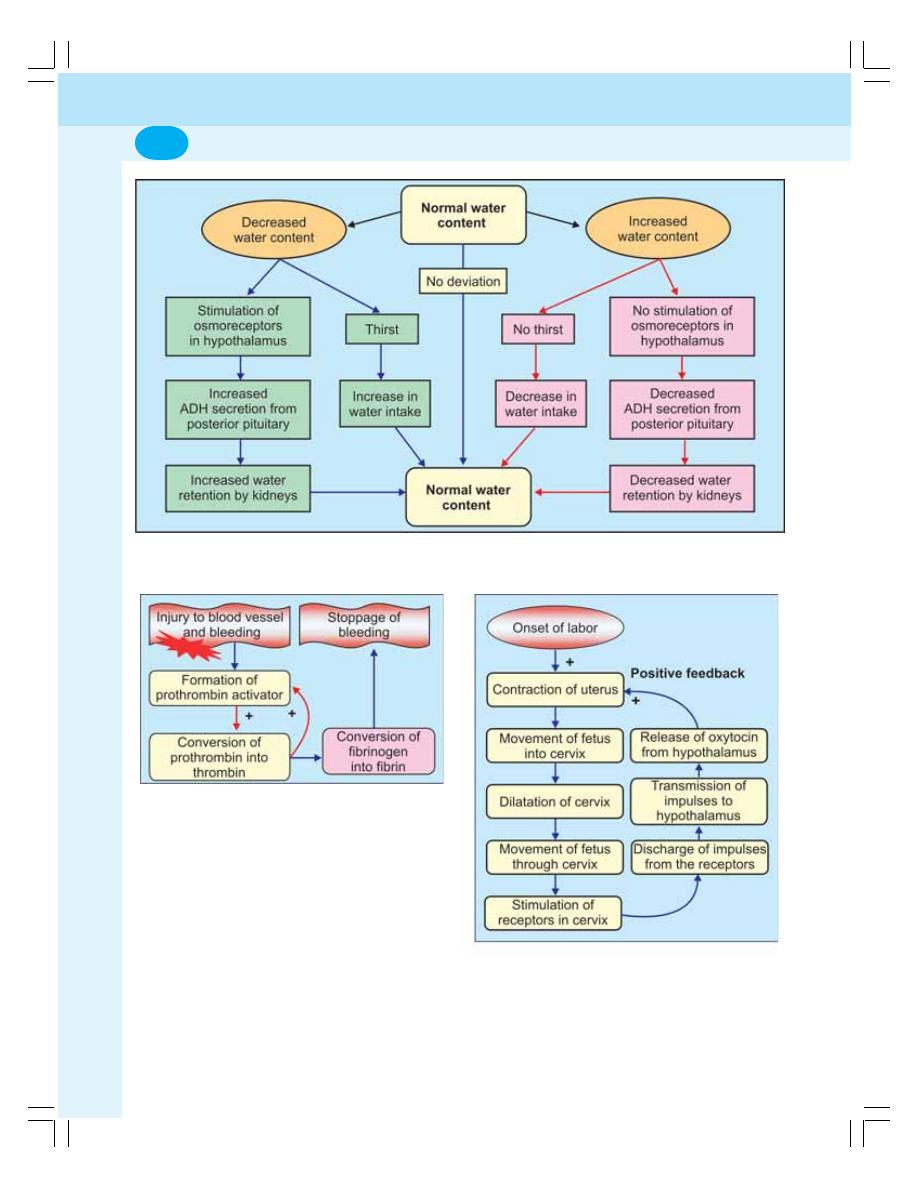
General Physiology
28
FIGURE 4-3: Negative feedback mechanism — maintenance of water balance
FIGURE 4-4: Positive feedback mechanism —
coagulation of blood. Once formed, thrombin
induces the formation of more prothrombin activator
FIGURE 4-5: Positive feedback mechanism —
parturition
Chapter 4 Homeostasis
29
QUESTIONS IN GENERAL PHYSIOLOGY
LONG QUESTIONS
1. Describe the mechanism of active transport
of substances through cell membrane.
2. Describe the mechanism of passive transport
of substances through cell membrane.
3. Explain the homeostasis in the body with
suitable examples.
SHORT QUESTIONS
1. Cell membrane.
2. Proteins of cell membrane.
3. Endoplasmic reticulum.
4. Ribosomes.
5. Mitochondria.
6. Golgi apparatus
7. Apoptosis
8. Tight junctions.
9. Gap junctions.
10. Passive transport.
11. Active transport.
12. Primary active transport.
13. Secondary active transport.
14. Sodium-potassium pump.
15. Facilitated diffusion or carrier mediated
diffusion.
16. Factors affecting diffusion.
17. Pinocytosis.
18. Phagocytosis.
19. Negative feedback.
20. Positive feedback
Questions in General Physiology
29

Blood and Body Fluids
5. Body Fluids ............................................................................ 33
6. Blood and Plasma Proteins ................................................... 38
7. Red Blood Cells ..................................................................... 43
8. Erythropoiesis ........................................................................ 49
9. Hemoglobin ........................................................................... 54
10. Erythrocyte Sedimentation Rate and Packed Cell Volume ... 57
11. Anemia .................................................................................. 60
12. White Blood Cells .................................................................. 64
13. Immunity ................................................................................ 71
14. Platelets ................................................................................. 80
15. Hemostasis and Coagulation of Blood .................................. 83
16. Blood Groups and Blood Transfusion .................................... 93
17. Reticuloendothelial System and Tissue Macrophage .......... 101
18. Lymphatic System and Lymph ............................................. 104
19. Tissue Fluid and Edema ...................................................... 107
S E C T I O N
2
C H A P T E R S

n
INTRODUCTION
n
COMPARTMENTS
n
COMPOSITION
n
MEASUREMENT
n
INDICATOR DILUTION METHOD
n
MEASUREMENT OF TOTAL BODY WATER
n
MEASUREMENT OF EXTRACELLULAR FLUID VOLUME
n
MEASUREMENT OF PLASMA VOLUME
n
MEASUREMENT OF BLOOD VOLUME
n
MEASUREMENT OF INTRACELLULAR FLUID VOLUME
n
MEASUREMENT OF INTERSTITIAL FLUID VOLUME
n
MAINTENANCE OF WATER BALANCE
n
APPLIED PHYSIOLOGY
n
DEHYDRATION
n
OVERHYDRATION OR WATER INTOXICATION
Body Fluids
5
n
INTRODUCTION
Body is formed by solids and fluids. The fluid part
is more than 2/3 of the whole body. Water forms
most of the fluid part of the body.
In human beings, the total body water (TBW)
varies from 45 to 75% of body weight. In a normal
young adult male, body contains 60 to 65% of
water and 35 to 40% of solids. In a normal young
adult female, the water is 50 to 55% and solids
are 45 to 50%. The total quantity of body water
in an average human being weighing about 70 kg
is about 40 L.
n
COMPARTMENTS OF BODY
FLUIDS — DISTRIBUTION OF
BODY FLUIDS
Compartments and distribution of body fluids with
the quantity is given in Table 5-1. Water moves
between different compartments (Fig. 5-1). TBW
(40 L) is distributed into two major fluid
compartments:
1. Intracellular fluid (ICF) forming 55% of the
total body water (22 L).
2. Extracellular fluid (ECF) forming 45% of the
total body water (18 L).

Blood and Body Fluids
34
n
COMPOSITION OF BODY FLUIDS
Body fluids contain water and solids. Solids are
organic and inorganic substances.
n
ORGANIC SUBSTANCES
Organic substances present in body fluids are
glucose, amino acids and other proteins, fatty
acids and other lipids, hormones and enzymes.
n
INORGANIC SUBSTANCES
The inorganic substances present in body fluids
are sodium, potassium, calcium, magnesium,
chloride, bicarbonate, phosphate and sulfate. The
differences between ECF and ICF are given in
the Table 5-1.
n
MEASUREMENT OF BODY FLUID
VOLUME
Volume of different compartments of the body
fluid is measured by indicator dilution method or
dye dilution method.
n
INDICATOR DILUTION METHOD
Principle
A known quantity of a substance such as a dye
is administered into a specific body fluid
compartment. These substances are called the
marker substances or indicators. After adminis-
tration into the fluid, the substance is allowed to
mix thoroughly with the fluid compartment. Then,
a sample of fluid is drawn and the concentration
FIGURE 5-1: Body fluid compartments and movement
of fluid between different compartments. Other fluids
= transcellular fluid, fluid in bones and fluid in
connective tissue
TABLE 5-1: Different compartments of body fluid
Substance
ECF
ICF
Sodium
142 mEq/L
10 mEq/L
Calcium
5 mEq/L
1 mEq/L
Potassium
4 mEq/L
140 mEq/L
Magnesium
3 mEq/L
28 mEq/L
Chloride
103 mEq/L
4 mEq/L
Bicarbonate
28 mEq/L
10 mEq/L
Phosphate
4 mEq/L
75 mEq/L
Sulfate
1 mEq/L
2 mEq/L
Proteins
2 g/dL
16 g/dL
Amino acids
30 mg/dL
200 mg/dL
Glucose
90 mg/dL
0 to 20 mg/dL
Lipids
0.5 g/dL
2 to 95 g/dL
Partial pressure
of oxygen
35 mm Hg
20 mm Hg
Partial pressure
of carbon
dioxide
46 mm Hg
50 mm Hg
Water
15 to 20 L (18) 20 to 25 L (22)
pH
7.4
7.0
Chapter 5 Body Fluids
35
of the marker substance is determined. The sub-
stances whose concentration can be determined
by using colorimeter or radioactive substances
are generally used as marker substances.
Formula to Measure the Body Fluid
Volume by Indicator Dilution Method
The quantity of fluid in the compartment is
measured by using the formula
M
V =
C
V = Volume of fluid in the compartment
M = Mass or total quantity of marker sub-
stance injected
C = Concentration of the marker substance in
the sample fluid
Correction factor: Some amount of marker
substance is lost through urine during distribution.
So, the formula is corrected as follows:
M – Amount of substance excreted
Volume =
C
Characteristics of Marker Substances
The dye or any substance used as a marker
substance should have the following qualities:
1. Must be nontoxic
2. Must mix with the fluid compartment
thoroughly within reasonable time
3. Should not be excreted rapidly
4. Should be excreted from the body completely
within reasonable time
5. Should not change the color of the body fluid
6. Should not alter the volume of body fluid.
n
MEASUREMENT OF TOTAL
BODY WATER
The marker substance for measuring TBW
should be distributed through all the com-
partments of body fluid. Such substances are:
1. Deuterium oxide
2. Tritium oxide
3. Antipyrine.
Deuterium oxide and tritium oxide mix with
fluids of all the compartments within few hours
after injection. Since plasma is part of total body
fluid, the concentration of marker substances can
be obtained from sample of plasma. And, the
formula for indicator dilution method is applied
to calculate total body water.
n
MEASUREMENT OF ECF VOLUME
ECF volume is measured by using the sub-
stances, which can pass through the capillary
membrane freely and remain only in the ECF but
not enter into the cell. Such marker substances
are:
1. Radioactive sodium, chloride, bromide, sul-
fate and thiosulfate
2. Nonmetabolizable saccharides like inulin,
mannitol, raffinose and sucrose.
When any of these substances is injected into
blood, it mixes with the fluid of all sub-
compartments of ECF within 30 minutes to
1 hour. The indicator dilution method is applied
to calculate ECF volume. Since ECF includes
plasma, the concentration of marker substance
can be obtained in the sample of plasma.
Some marker substances like sodium,
chloride, inulin and sucrose diffuse more widely
through all subcompartments of ECF. So, the
measured volume of ECF by using these sub-
stances is called sodium space, chloride space,
inulin space and sucrose space.
Example for Measurement of ECF Volume
Quantity of sucrose injected (M) : 150 mg
Urinary excretion of sucrose
: 10 mg
Concentration of sucrose in
plasma (C)
: 0.01 mg/ml
Mass – Amount lost in urine
Sucrose space =
Concentration of sucrose
in plasma
150 – 10 mg
140
=
=
0.01 mg/ml
0.01
Sucrose space = 14,000 ml
Therefore, the ECF volume = 14 L.

Blood and Body Fluids
36
n
MEASUREMENT OF PLASMA VOLUME
The substance, which binds with plasma proteins
strongly and diffuses into interstitium only in small
quantities or does not diffuse at all, is used to
measure plasma volume.
Measurement of Plasma Volume by
Indicator or Dye Dilution Technique
The principles and other details of this technique
are same as that of ECF volume. The dye which
is used to measure plasma volume is Evans blue
or T-1824.
Procedure: A small quantity of blood (3 to 4 ml)
is drawn from the subject and a known quantity
of the dye is added. This is used as control
sample in the procedure. Then, a known volume
of dye is injected intravenously. After 10 minutes,
a sample of blood is drawn. Then, another 4 sam-
ples of blood are collected at the interval of
10 minutes. All the 5 samples are centrifuged
and plasma is separated from the samples. In
each sample of plasma, the concentration of the
dye is measured by colorimetric method and the
average concentration is found. The subject’s
urine is collected and the amount of dye excreted
in the urine is measured.
Calculation
The plasma volume is determined by using the
formula,
Amount of dye injected –
Amount excreted
Volume =
Average concentration
of dye in plasma
n
MEASUREMENT OF BLOOD VOLUME
Measurement of total blood volume involves two
steps:
1. Determination of plasma volume
2. Determination of blood cell volume.
Plasma volume is determined by indicator
dilution technique as mentioned above. Blood cell
volume is determined by hematocrit value.
It is usually done by centrifuging the blood
and measuring the packed cell volume
(Chapter 10). PCV is expressed in percentage.
If this is deducted from 100, the percentage of
plasma is known. From this, and from the volume
of plasma, the amount of total blood is calculated
by using the formula
100 × Amount of plasma
Blood Volume =
100 – PCV
n
MEASUREMENT OF INTRACELLULAR
FLUID VOLUME
Intracellular fluid volume cannot be measured
directly. It is calculated from the values of volume
of total body water and ECF volume.
ICF volume = Total fluid volume – ECF volume.
n
MEASUREMENT OF INTERSTITIAL
FLUID VOLUME
Interstitial fluid volume also cannot be measured
directly. It is calculated from the values of ICF
volume and plasma volume.
Interstitial fluid volume = ICF volume – Plasma
volume.
n
MAINTENANCE OF WATER BALANCE
Body has several mechanisms which work
together to maintain the water balance. The
important mechanisms involve hypothalamus
(Chapters 4 and 92) and kidneys (Chapter 34).
n
APPLIED PHYSIOLOGY
n
DEHYDRATION
Definition
Significant decrease in water content of the body
is known as dehydration.
Classification
Basically dehydration is of three types:
1. Mild dehydration when fluid loss is about 5%
of total body fluids.

Chapter 5 Body Fluids
37
2. Moderate dehydration when fluid loss is about
10%.
3. Severe dehydration when fluid loss is about
15%.
Causes
1. Severe diarrhea and vomiting
2. Excess water loss through urine
4. Insufficient intake of water
5. Excess sweating
6. Use of laxatives or diuretics.
Signs and Symptoms
Mild and moderate dehydration
1. Dryness of the mouth
2. Excess thirst
3. Decrease in sweating
4. Decrease in urine formation.
Severe dehydration
1. Decrease in blood volume
2. Decrease in cardiac output
3. Cardiac shock.
Very severe dehydration
1. Damage of organs like brain, liver and kidneys
2. Mental depression and confusion
3. Renal failure
4. Coma.
n
OVERHYDRATION OR WATER
INTOXICATION
Definition
Overhydration, hyperhydration, water excess or
water intoxication is defined as the condition in
which body has too much water.
Causes
Overhydration occurs when more fluid is taken
than that can be excreted. It also develops in
some conditions such as heart failure, renal
disorders and hypersecretion of antidiuretic
hormone.
Signs and Symptoms
1. Behavioral changes
2. Drowsiness and inattentiveness
3. Nausea and vomiting
4. Sudden loss of weight followed by weakness
and blurred vision
5. Anemia, acidosis, cyanosis, hemorrhage and
shock
6. Muscular weakness, cramps and paralysis
7. Severe conditions of overhydration result
in:
i. Delirium (extreme mental condition char-
acterized by confused state and illusion)
ii. Seizures (sudden uncontrolled involuntary
muscular contractions)
iii. Coma (profound state of unconsciousness
in which the person fails to respond to ex-
ternal stimuli and cannot perform voluntary
actions).
n
BLOOD
n
PROPERTIES
n
COMPOSITION
n
FUNCTIONS
n
PLASMA PROTEINS
n
NORMAL VALUES
n
VARIATIONS IN PLASMA PROTEIN LEVEL
n
ORIGIN
n
PROPERTIES
n
FUNCTIONS
Blood and
Plasma Proteins
6
n
BLOOD
Blood is a connective tissue in fluid form. It is
considered as the fluid of life because it carries
oxygen from lungs to all parts of the body and
carbon dioxide from all parts of the body to the
lungs.
n
PROPERTIES OF BLOOD
1. Color: Blood is red in color. Arterial blood is
scarlet red because of more O
2
and venous
blood is purple red because of more CO
2
.
2. Volume: The average volume of blood in a
normal adult is 5 L. In newborn baby it is 450
ml. It increases during growth and reaches
5 L at the time of puberty. In females, it is
slightly less and is about 4.5 L. It is about
8% of the body weight in a normal young
healthy adult weighing about 70 kg.
3. Reaction and pH: Blood is slightly alkaline and
its pH in normal conditions is 7.4.
4. Specific gravity:
Specific gravity of total blood : 1.052 to 1.061
Specific gravity of blood cells : 1.092 to 1.101
Specific gravity of plasma
: 1.022 to 1.026
5. Viscosity: Blood is five times more viscous
than water. It is mainly due to red blood cells
and plasma proteins.
n
COMPOSITION OF BLOOD
Blood contains the blood cells which are called
formed elements and the liquid portion known
as plasma.
Blood Cells
Three types of cells are present in the blood:
1. Red blood cells (RBC) or erythrocytes
2. White blood cells (WBC) or leukocytes
3. Platelets or thrombocytes
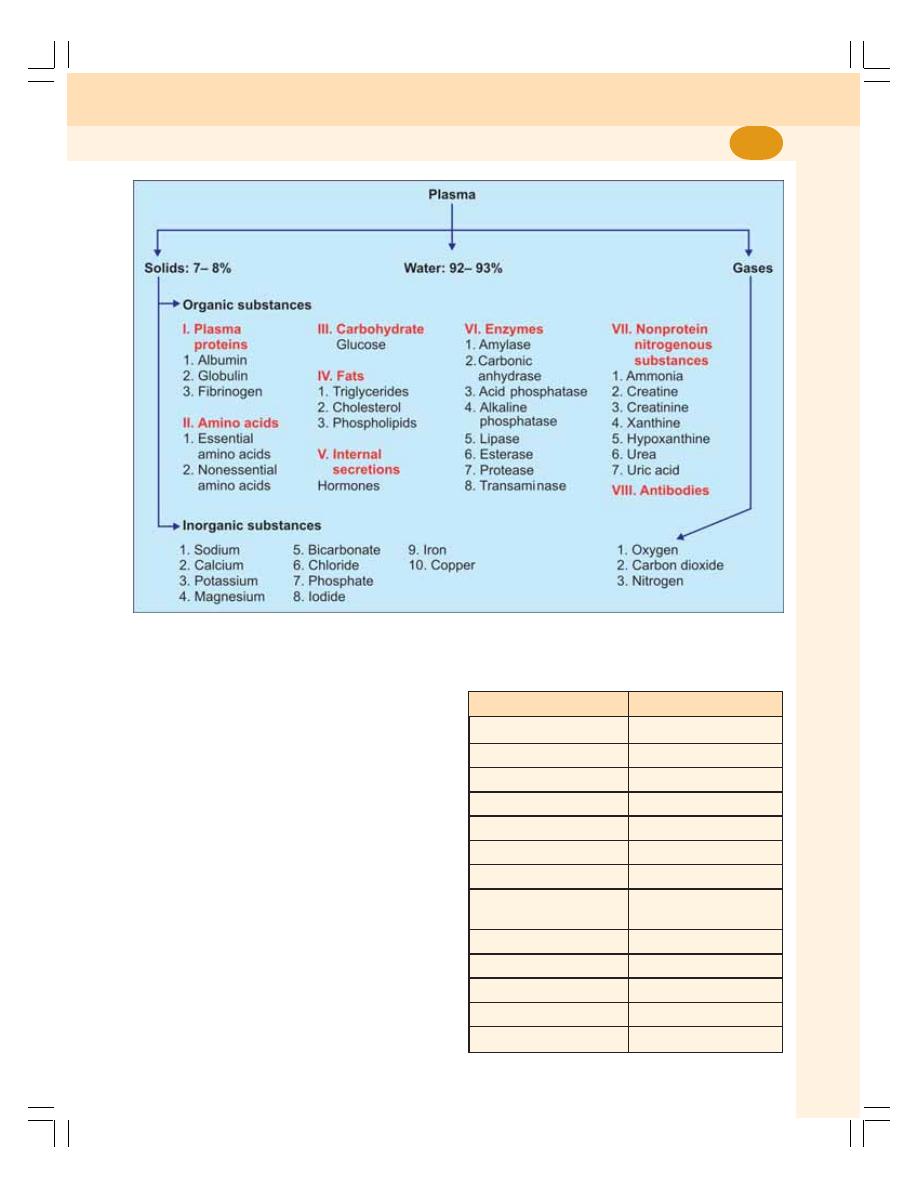
Chapter 6 Blood and Plasma Proteins
39
Plasma
Plasma is a straw colored clear liquid part of
blood. It contains 91 to 92% of water and 8 to
9% of solids. The solids are the organic and
inorganic substances (Fig. 6-1). Table 6-1 gives
the normal values of some important substances
in blood.
Serum
Serum is the clear straw colored fluid that oozes
out from the clot. When the blood is shed or
collected in a container, it clots because of the
conversion of fibrinogen into fibrin. After about
45 minutes, serum oozes out of the clot. For
clinical investigations, serum is separated from
blood cells by centrifuging. Volume of the serum
is almost the same as that of plasma (55%). It
is different from plasma only by the absence of
fibrinogen, i.e. serum contains all the other consti-
tuents of plasma except fibrinogen. Fibrinogen
FIGURE 6-1:
Composition of plasma
TABLE 6-1:
Normal values of some important
substances in blood
Substance
Normal value
Glucose
100 to 120 mg/dL
Creatinine
0.5 to 1.5
mg/dL
Cholesterol
Up to 200
mg/dL
Plasma proteins
6.4 to 8.3
g/dL
Bilirubin
0.5 to 1.5
mg/dL
Iron
50 to 150
μg/dL
Copper
100 to 200 mg/dL
Calcium
9 to 11
mg/dL
4.5 to 5.5
mEq/L
Sodium
135 to 145 mEq/L
Potassium
3.5 to 5.0
mEq/L
Magnesium
1.5 to 2.0
mEq/L
Chloride
100 to 110 mEq/L
Bicarbonate
22 to 26
mEq/L

Blood and Body Fluids
40
is absent in serum because it is converted into
fibrin during blood clotting.
Thus, the Serum = Plasma – Fibrinogen.
n
FUNCTIONS OF BLOOD
1. Nutrient Function
Nutritive substances like glucose, amino acids,
lipids and vitamins derived from digested food
are absorbed from gastrointestinal tract and
carried by blood to different parts of the body for
growth and production of energy.
2. Respiratory Function
Transport of respiratory gases is done by the
blood. It carries O
2
from alveoli of lungs to
different tissues and CO
2
from tissues to alveoli.
3. Excretory Function
Waste products formed in the tissues during
various metabolic activities are removed by blood
and carried to the excretory organs like kidney,
skin, liver, etc. for excretion.
4. Transport of Hormones and Enzymes
Hormones which are secreted by ductless (endo-
crine) glands are released directly into the blood.
The blood transports these hormones to their
target organs/tissues. Blood also transports
enzymes.
5. Regulation of Water Balance
Water content of the blood is freely interchange-
able with interstitial fluid. This helps in the
regulation of water content of the body.
6. Regulation of Acid-base Balance
The plasma proteins and hemoglobin act as
buffers and help in regulation of acid-base
balance.
7. Regulation of Body Temperature
Because of the high specific heat of blood, it is
responsible for maintaining the thermoregulatory
mechanism in the body, i.e. the balance between
heat loss and heat gain in the body.
8. Storage Function
Water and some important substances like
proteins, glucose, sodium and potassium are
constantly required by the tissues. All these
substances are present in the blood are taken
by the tissues during the conditions like star-
vation, fluid loss, electrolyte loss, etc.
9. Defensive Function
The WBCs in the blood provide the defense
mechanism and protect the body from the
invading organisms. Neutrophils and monocytes
engulf the bacteria by phagocytosis. Lympho-
cytes provide cellular and humoral immunity.
Eosinophils protect the body by detoxification,
disintegration and removal of foreign proteins
(Chapter 12).
n
PLASMA PROTEINS
The plasma proteins are:
1. Serum albumin
2. Serum globulin
3. Fibrinogen.
Globulin is of three types,
α-globulin, β-
globulin and
γ-globulin.
n
NORMAL VALUES
The normal values of the plasma proteins are:
Total proteins
:
7.3 g/dL (6.4-8.3 g/dL)
Serum albumin :
4.7 g/dL
Serum globulin :
2.3 g/dL
Fibrinogen
:
0.3 g/dL
Albumin/globulin Ratio
The ratio between plasma level of albumin and
globulin is called Albumin/Globulin (A/G) ratio.
It is an important indicator of some liver and
kidney diseases. Normal A/G ratio is 2:1.
n
VARIATIONS IN PLASMA PROTEIN
LEVEL
Hyperproteinemia
Hyperproteinemia is the elevation of all fractions
of plasma proteins.

Chapter 6 Blood and Plasma Proteins
41
It occurs in the following conditions:
1. Dehydration
2. Hemolysis
3. Acute infections
4. Respiratory distress syndrome
5. Excess of glucocorticoids
6. Leukemia
7. Rheumatoid arthritis
8. Alcoholism.
Hypoproteinemia
Hypoproteinemia is the decrease in all fractions
of plasma proteins.
It occurs in the following conditions:
1. Diarrhea
2. Hemorrhage
3. Burns
4. Pregnancy
5. Malnutrition
6. Prolonged starvation
7. Cirrhosis of liver
8. Chronic infections.
n
ORIGIN OF PLASMA PROTEINS
In embryonic stage, the plasma proteins are
synthesized by the mesenchyme cells. In adults,
the plasma proteins are synthesized mainly from
reticuloendothelial cells of liver and also from
spleen, bone marrow, disintegrating blood cells
and general tissue cells. Gamma globulin is
synthesized from B lymphocytes.
n
PROPERTIES OF PLASMA PROTEINS
Molecular Weight
Albumin
:
69,000
Globulin
: 1,56,000
Fibrinogen : 4,00,000
Specific Gravity
The specific gravity of the plasma proteins is
1.026.
Buffer Action
The acceptance of hydrogen ions is called buffer
action. The plasma proteins have 1/6 of total
buffering action of the blood.
n
FUNCTIONS OF PLASMA
PROTEINS
1. Role in Coagulation of Blood
Fibrinogen is essential for the coagulation of
blood (Chapter 15).
2. Role in Defense Mechanism of Body
The gamma globulins play an important role in
the defense mechanism of the body by acting
as antibodies. These proteins are also called
immunoglobulins (Chapter 13).
3. Role in Transport Mechanism
Plasma proteins are essential for the transport
of various substances in the blood. Albumin,
alpha globulin and beta globulin are responsible
for the transport of the hormones, enzymes, etc.
The alpha and beta globulins transport metals
in the blood.
4. Role in Maintenance of Osmotic
Pressure in Blood
Plasma proteins exert the colloidal osmotic
(oncotic) pressure. The osmotic pressure exerted
by the plasma proteins is about 25 mm Hg.
Since the concentration of albumin is more than
the other plasma proteins, it exerts maximum
pressure.
5. Role in Regulation of Acid-base Balance
Plasma proteins, particularly the albumin, play
an important role in regulating the acid-base
balance in the blood. This is because of the virtue
of their buffering action.

Blood and Body Fluids
42
6. Role in Viscosity of Blood
The plasma proteins provide viscosity to the
blood, which is important to maintain the blood
pressure. Albumin provides maximum viscosity
than the other plasma proteins.
7. Role in Erythrocyte Sedimentation
Rate (ESR)
Globulin and fibrinogen accelerate the tendency
of rouleaux formation by the red blood cells.
Rouleaux formation is responsible for ESR, which
is an important diagnostic and prognostic tool
(Chapter 7).
8. Role in Suspension Stability of
Red Blood Cells
During circulation, the red blood cells remain
suspended uniformly in the blood. This property
of the red blood cells is called the suspension
stability. Globulin and fibrinogen help in the
suspension stability of the red blood cells.
9. Role in Production of Trephone
Substances
Trephone substances are necessary for
nourishment of tissue cells in culture. These
substances are produced by leukocytes from the
plasma proteins.
10. Role As Reserve Proteins
During fasting, inadequate food intake or
inadequate protein intake, the plasma proteins
are utilized by the body tissues as the last source
of energy. The plasma proteins are split into
amino acids by the tissue macrophages. The
amino acids are taken back by blood and
distributed throughout the body to form cellular
protein molecules. Because of this, the plasma
proteins are called the reserve proteins.
n
INTRODUCTION
n
NORMAL VALUE
n
MORPHOLOGY
n
PROPERTIES
n
LIFESPAN
n
FATE
n
FUNCTIONS
n
VARIATIONS IN NUMBER
n
VARIATIONS IN SIZE
n
VARIATIONS IN SHAPE
n
HEMOLYSIS AND FRAGILITY
n
INTRODUCTION
Red blood cells (RBCs), also known as
erythrocytes are the non-nucleated formed
elements in the blood. The red color of the RBC
is due to the presence of hemoglobin.
n
NORMAL VALUE
The RBC count ranges between 4 and
5.5 millions/cu mm of blood. In adult males, it is
5 millions/cu mm and in adult females it is
4.5 millions/cu mm.
n
MORPHOLOGY OF RED BLOOD
CELLS
n
NORMAL SHAPE
Normally, the RBCs are disk-shaped and
biconcave (dumbbell-shaped). The central
portion is thinner and periphery is thicker. The
biconcave contour of RBCs has some
mechanical and functional advantages.
Advantages of Biconcave Shape of RBCs
1. It helps in equal and rapid diffusion of oxygen
and other substances into the interior of the
cell.
2. Large surface area is provided for absorption
or removal of different substances.
3. Minimal tension is offered on the membrane
when the volume of cell alters.
4. While passing through minute capillaries,
RBCs can squeeze through the capillaries
easily without getting damaged.
n
NORMAL SIZE
Diameter
: 7.2 μ (6.9 to 7.4 μ).
Red Blood Cells
7
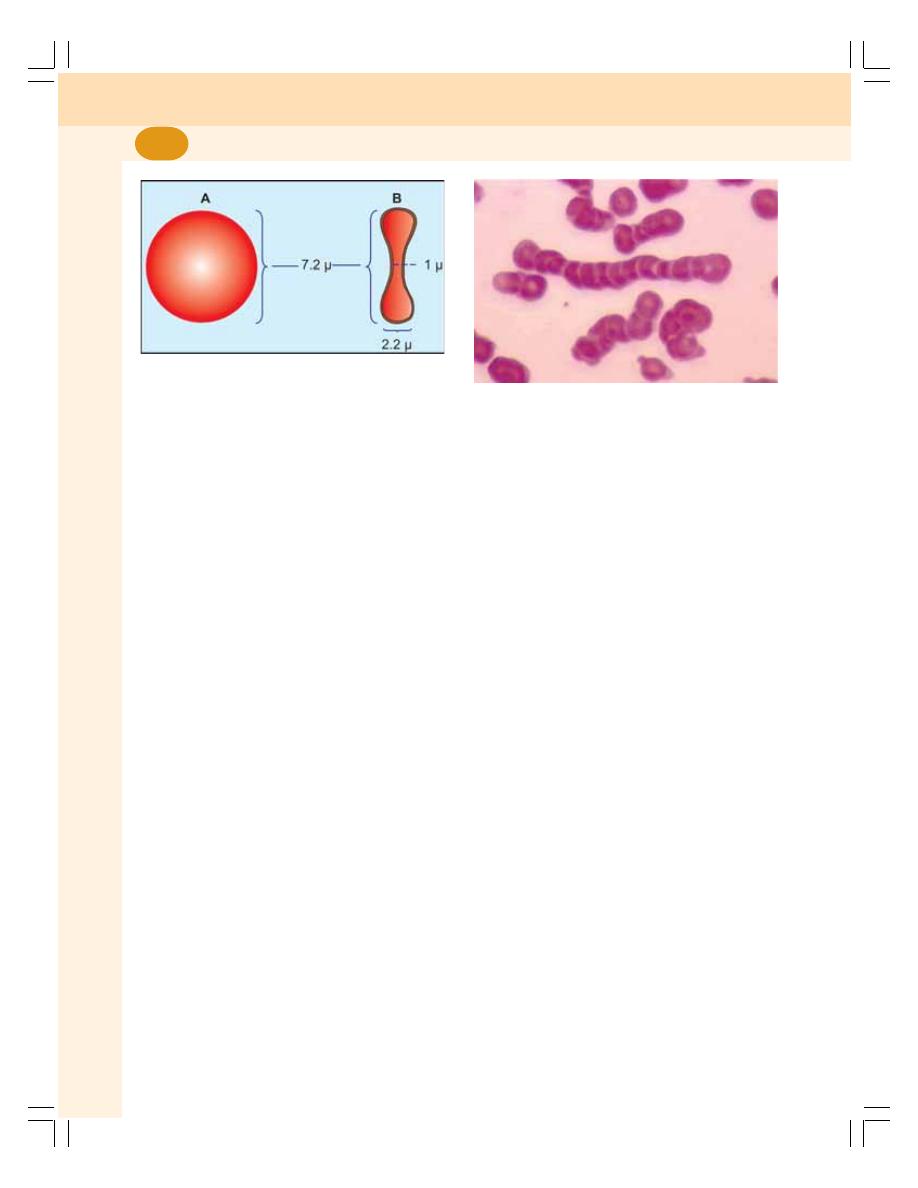
Blood and Body Fluids
44
Thickness
: At the periphery it is thicker with
2.2 μ and at the center it is thinner
with 1 μ (Fig. 7-1). The difference
in thickness is because of the
biconcave shape.
Surface area : 120 sq μ.
Volume
: 85 to 90 cu μ.
n
NORMAL STRUCTURE
RBC is non-nucleated cell. Because of the
absence of nucleus, the DNA is also absent.
Other organelles such as mitochondria and Golgi
apparatus also are absent in RBC. Since,
mitochondria are absent, the energy is produced
from glycolytic process.
n
PROPERTIES OF RED BLOOD CELLS
n
1. ROULEAUX FORMATION
When blood is taken out of the blood vessel, the
RBCs pile up one above another like the pile of
coins. This property of the RBCs is called
rouleaux (pleural = rouleau) formation (Fig. 7-2).
It is accelerated by plasma proteins, namely
globulin and fibrinogen.
n
2. SPECIFIC GRAVITY
The specific gravity of RBC is 1.092 to 1.101.
n
3. PACKED CELL VOLUME
Packed cell volume (PCV) is the volume of the
RBSc expressed in percentage. It is also called
hematocrit value. It is 45% of the blood and the
plasma volume is 55% (Chapter 10).
n
4. SUSPENSION STABILITY
During circulation, the RBCs remain suspended
or dispersed uniformly in the blood. This property
of the RBCs is called the suspension stability.
n
LIFESPAN OF RED BLOOD CELLS
Average lifespan of RBC is about 120 days.
After the lifetime, the senile (old) RBCs are
destroyed in reticuloendothelial system.
n
FATE OF RED BLOOD CELLS
When the RBCs become older (120 days), the
cell membrane becomes very fragile. So these
cells are destroyed while trying to squeeze
through the capillaries which have lesser or
equal diameter as that of RBC. The destruction
occurs mainly in the capillaries of spleen
because these capillaries are very much
narrow. So, the spleen is called graveyard of
RBCs.
The destroyed RBCs are fragmented and
hemoglobin is released from the fragmented
parts. Hemoglobin is degraded into iron, globin
and porphyrin. Iron combines with the protein
called apoferritin to form ferritin, which is stored
in the body and reused later. Globin enters the
protein depot for later use (Fig. 7-3). The
porphyrin is degraded into bilirubin which is
excreted by liver through bile (Chapter 30).
FIGURE 7-1:
Dimensions of RBC. A: Surface
view. B. Sectioned view
FIGURE 7-2:
Rouleaux formation
(Courtesy:
Dr Nivaldo Medeiros)
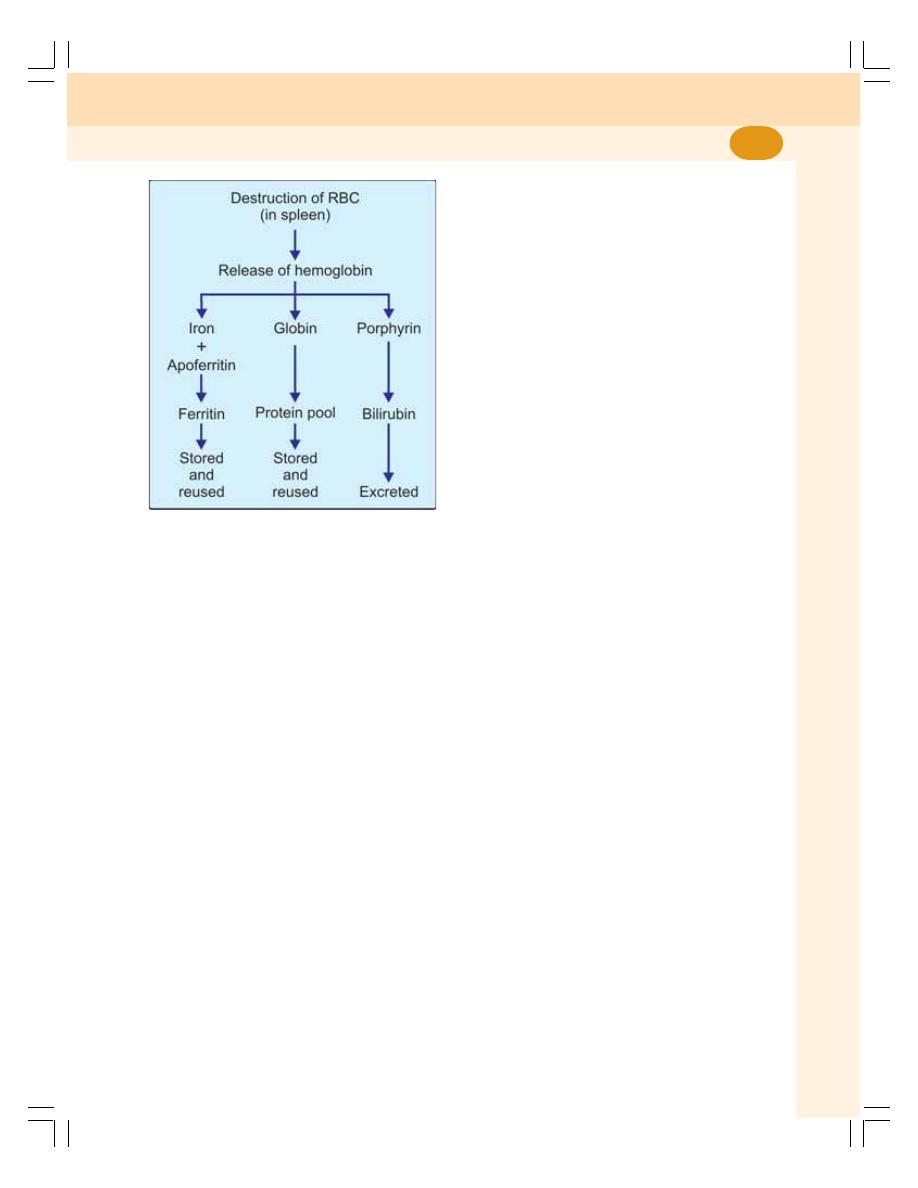
Chapter 7 Red Blood Cells
45
Daily 10% of senile RBCs are destroyed in
normal young healthy adults. It causes release
of about 0.6 g/dL of hemoglobin into the plasma.
From this 0.9 to 1.5 mg/dL bilirubin is formed.
n
FUNCTIONS OF RED BLOOD CELLS
1. Transport of O
2
from the Lungs to the
tissues
Hemoglobin combines with oxygen to form
oxyhemoglobin (Chapter 76).
2. Transport CO
2
from the Tissues to the
Lungs
Hemoglobin combines with carbon dioxide and
form carbhemoglobin.
3. Buffering Action in Blood
Hemoglobin functions as a good buffer. By this
action, it regulates the hydrogen ion
concentration and thereby plays a role in the
maintenance of acid-base balance.
4. In Blood Group Determination
RBCs carry the blood group antigens like A
antigen, B antigen and Rh factor. This helps in
determination of blood group and enables to
prevent the reactions due to incompatible blood
transfusion (Chapter 16).
n
VARIATIONS IN NUMBER OF
RED BLOOD CELLS
n
PHYSIOLOGICAL VARIATIONS
A. Increase in RBC Count — Polycythemia
Increase in the RBC count is known as
polycythemia. It occurs in both physiological and
pathological conditions. When it occurs in
physiological conditions it is called physiological
polycythemia. The increase in number during this
condition is marginal and temporary. It occurs
in the following conditions:
1. Age
At birth, the RBC count is 8 to 10 millions/cu mm
of blood. The count decreases within 10 days
after birth due to destruction of RBCs. This may
cause physiological jaundice in some newborn
babies. In infants and growing children, the RBC
count is more than in the adults.
2. Sex
Before puberty and after menopause, in females
the RBC count is similar to that in males. During
reproductive period of females, the count is less
than that of males (4.5 millions/cu mm).
3. High altitude
In people living in mountains (above 10,000 feet
from mean sea level), the RBC count is more
than 7 millions/cu mm. It is due to hypoxia
(decreased oxygen supply to tissues) in high
altitude. Hypoxia stimulates kidney to secrete a
hormone called erythropoietin which stimulates
the bone marrow to produce more RBCs
(Fig. 7-4).
4. Muscular exercise
RBC count increases after muscular exercise.
It is because of mild hypoxia which increases
the sympathetic activity and secretion of
FIGURE 7-3:
Fate of RBC
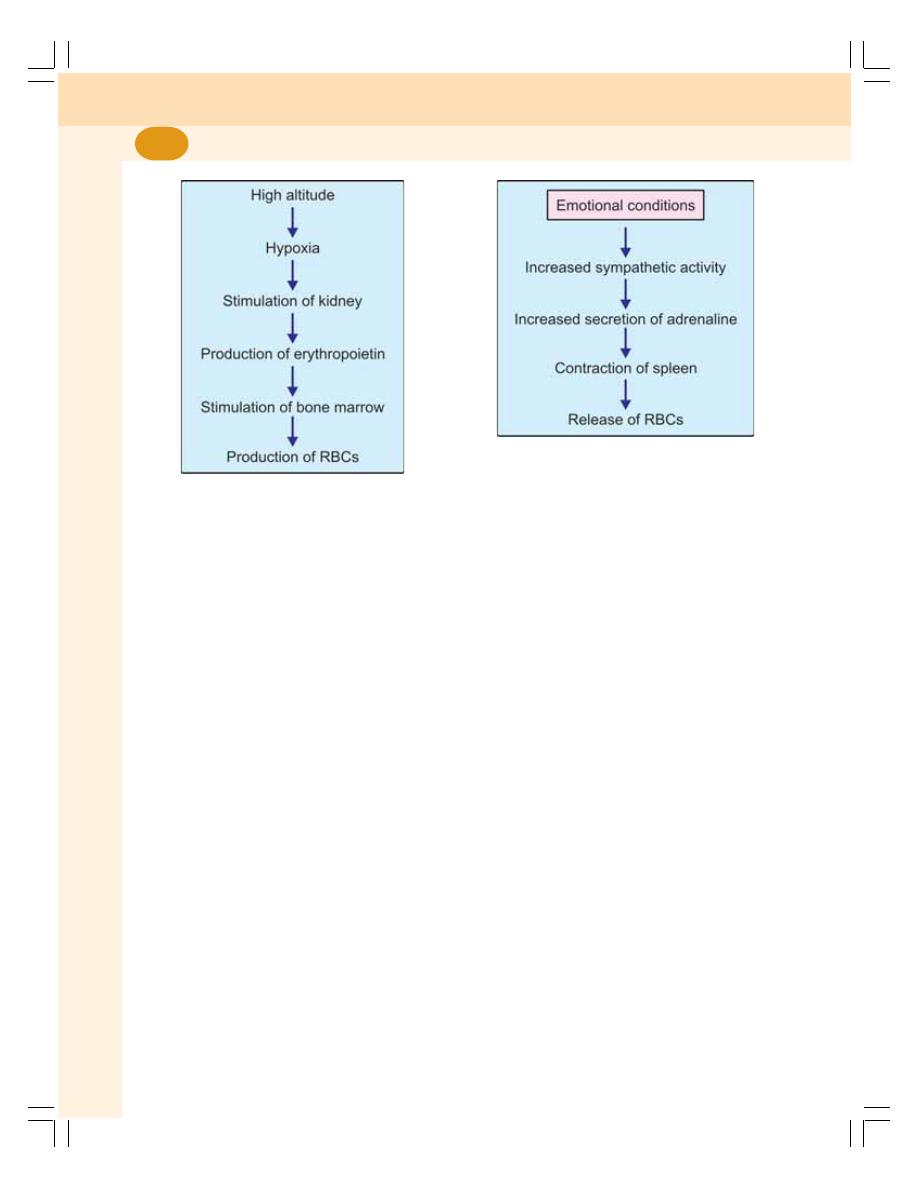
Blood and Body Fluids
46
adrenaline from adrenal medulla. Adrenaline
contracts spleen and RBCs are released into
blood. Hypoxia causes secretion of erythropoietin
which stimulates the bone marrow to produce
more RBCs
5. Emotional Conditions
The RBC count increases during the emotional
conditions such as anxiety. It is because of
increase in the sympathetic activity and
contraction of spleen (Fig. 7-5).
6. Increased environmental temperature
Generally increased temperature increases all
the activities in the body including production of
RBCs.
7. After meals
There is a slight increase in the RBC count after
taking meals. It is because of need for more
oxygen for metabolic activities.
B. Decrease in RBC Count
Decrease in RBC count occurs in the following
physiological conditions:
1. High Barometric Pressures
At high barometric pressures as in deep sea,
where the oxygen tension of blood is higher, the
RBC count decreases.
2. During Sleep
Generally all the activities of the body are
decreased during sleep including production of
RBCs.
3. Pregnancy
In pregnancy, the RBC count decreases. It is
because of increase in ECF volume. Increase
in ECF volume, increases the plasma volume
also resulting in hemodilution. So, there is a
relative reduction in the RBC count.
n
PATHOLOGICAL VARIATIONS
Pathological Polycythemia
Pathological polycythemia is the abnormal
increase in the RBC count. The count increases
above 7 millions/cu mm of the blood.
Polycythemia is of two types, the primary
polycythemia and secondary polycythemia.
Primary Polycythemia — Polycythemia Vera
Primary polycythemia is otherwise known as poly-
cythemia vera. It is a disease characterized by
FIGURE 7-4:
Physiological polycythemia in high
altitude
FIGURE 7-5:
RBC count in emotional conditions

Chapter 7 Red Blood Cells
47
persistent increase in RBC count above
14 millions/cu mm of blood. This is always
associated with increased WBC count above
24,000/cu mm of blood. Polycythemia vera
occurs because of red bone marrow malignancy.
Secondary Polycythemia
It is the pathological condition in which poly-
cythemia occurs because of diseases in some
other system such as:
1. Respiratory disorders like emphysema
2. Congenital heart disease
3. Ayerza’s disease — condition associated with
hypertrophy of right ventricle and obstruction
of blood flow to lungs
4. Chronic carbon monoxide poisoning
5. Poisoning by chemicals like phosphorus and
arsenic
6. Repeated mild hemorrhages.
All these conditions lead to hypoxia which
stimulates the release of erythropoietin.
Erythropoietin stimulates the bone marrow
resulting in increased RBC count.
Anemia
The abnormal decrease in RBC count is called
anemia. This is described in Chapter 11.
n
VARIATIONS IN SIZE OF RED
BLOOD CELLS
Under physiological conditions, the size of RBCs
in venous blood is slightly larger than those in
arterial blood. In pathological conditions, the
variations in size of RBCs are:
1. Microcytes
— smaller cells
2. Macrocytes
— larger cells
3. Anisocytosis — cells of different sizes.
Microcytes
Microcytes are present in:
i. Iron deficiency anemia
ii. Prolonged forced breathing
iii. Increased osmotic pressure in blood.
Macrocytes
Macrocytes are present in:
i. Megaloblastic anemia
ii. Muscular exercise
iii. Decreased osmotic pressure in blood.
Anisocytes
Anisocytes are found in pernicious anemia.
n
VARIATIONS IN SHAPE OF
RED BLOOD CELLS
The shape of RBCs is altered in many conditions
including different types of anemia:
1. Crenation: Shrinkage as in hypertonic
conditions
2. Spherocytosis: Globular form as in hypotonic
conditions
3. Elliptocytosis: Elliptical shape as in certain
types of anemia
4. Sickle cell: Crescentic shape as in sickle cell
anemia
5. Poikilocytosis: Unusual shapes due to
deformed cell membrane. The shape will be
of flask, hammer or any other unusual shape.
n
HEMOLYSIS AND FRAGILITY OF RBC
n
DEFINITION
Hemolysis
Hemolysis is the destruction of formed elements.
To define more specifically, it is the process,
which involves the breakdown of RBC and
liberation of hemoglobin.
Fragility
The susceptibility of RBC to hemolysis or
tendency to break easily is called fragility
(Fragile = easily broken).
Fragility is of two types:
i. Osmotic fragility which occurs due to
exposure to hypotonic saline.
ii. Mechanical fragility which occurs due to
mechanical trauma (wound or injury).

Blood and Body Fluids
48
Normally, old RBCs are destroyed in the
reticuloendothelial system. Abnormal hemolysis
is the process by which even younger RBCs are
destroyed in large number by the presence of
hemolytic agents or hemolysins.
n
PROCESS OF HEMOLYSIS
Normally, plasma and RBCs are in osmotic
equilibrium. When the osmotic equilibrium is
disturbed, the cells are affected. For example,
when the RBCs are immersed in hypotonic saline
the cells swell and rupture by bursting because
of endosmosis (Chapter 3). The hemoglobin is
released from the ruptured RBCs.
n
CONDITIONS WHEN HEMOLYSIS
OCCURS
1. Hemolytic jaundice
2. Antigen antibody reactions
3. Poisoning by chemicals or toxins.
n
HEMOLYSINS
Hemolysins or hemolytic agents are the
substances, which cause destruction of RBCs.
Hemolysins are of two types:
I. Chemical substances
II. Substances of bacterial origin or substances
found in body.
n
CHEMICAL SUBSTANCES
1. Alcohol
2. Benzene
3. Chloroform
4. Ether
5. Acids
6. Alkalis
7. Bile salts
8. Saponin
9. Chemical poisons like arsenial preparations,
carbolic acid, nitrobenzene and resin.
n
SUBSTANCES OF BACTERIAL ORIGIN
OR SUBSTANCES FOUND IN BODY
1. Toxic substances or toxins from bacteria such
as streptococcus, staphylococcus, tetanus
bacillus,
etc.
2. Venom of poisonous snakes like cobra
3. Hemolysins from normal tissues.
n
DEFINITION
n
SITE OF ERYTHROPOIESIS
n
IN FETAL LIFE
n
IN NEWBORN BABIES, CHILDREN AND ADULTS
n
PROCESS OF ERYTHROPOIESIS
n
STEM CELLS
n
CHANGES DURING ERYTHROPOIESIS
n
STAGES OF ERYTHROPOIESIS
n
FACTORS NECESSARY FOR ERYTHROPOIESIS
n
GENERAL FACTORS
n
MATURATION FACTORS
n
FACTORS NECESSARY FOR
HEMOGLOBIN FORMATION
n
DEFINITION
Erythropoiesis is the process of the origin,
development and maturation of erythrocytes.
Hemopoiesis is the process of origin,
development and maturation of all the blood
cells.
n
SITE OF ERYTHROPOIESIS
n
IN FETAL LIFE
In fetal life, the erythropoiesis occurs in different
sites in different periods:
1. Mesoblastic Stage
During the first two or three months (first
trimester) of intrauterine life, the RBCs are
produced from mesenchymal cells of yolk sac.
2. Hepatic Stage
During the next three months (second trimester)
of intrauterine life, RBCs are produced mainly
from the liver. Some cells are produced from the
spleen and lymphoid organs also.
3. Myeloid Stage
During the last three months (third trimester) of
intrauterine life, the RBCs are produced from red
bone marrow and liver.
n
IN NEWBORN BABIES, CHILDREN
AND ADULTS
1. Up to the age of 20 years:
RBCs are
produced from red bone marrow of all bones
2. After the age of 20 years:
RBCs are
produced from all the membranous bones
and ends of long bones.
Erythropoiesis
8
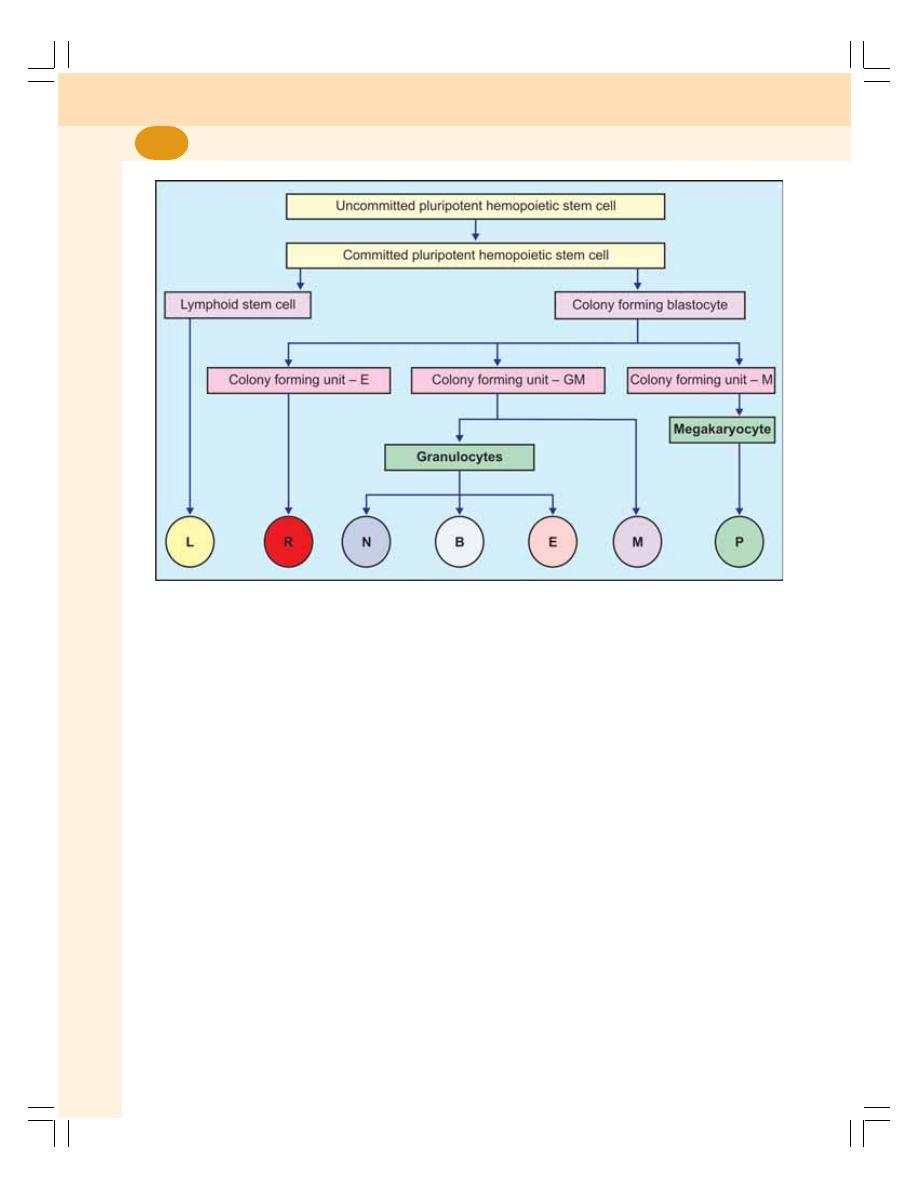
Blood and Body Fluids
50
n
PROCESS OF ERYTHROPOIESIS
n
STEM CELLS
RBCs develop from the hemopoietic stem cells
in the bone marrow. These cells are called
uncommitted pluripotent hemopoietic stem cells
(PHSC). PHSC are not designed to form a
particular type of blood cell; hence the name
uncommitted PHSC. When the cells are
designed to form a particular type of blood cell,
the uncommitted PHSCs are called committed
PHSC.
The committed PHSCs are of two types:
1. Lymphoid stem cells (LSC) which give rise
to lymphocytes and natural killer (NK) cells
2. Colony forming blastocytes, which give rise
to all the other blood cells except lympho-
cytes. When grown in cultures, these cells
form colonies hence the name colony forming
blastocytes.
The different units of colony forming cells are:
i. Colony forming Unit – Erythrocytes (CFU-
E) from which RBCs develop.
ii. Colony forming Unit – Granulocytes/
Monocytes (CFU-GM) from which
ganulocytes (neutrophils, basophils and
eosinophils) and monocytes develop.
iii. Colony forming Unit – Megakaryocytes
(CFU-M) from which platelets develop.
n
CHANGES DURING ERYTHROPOIESIS
When the cells of CFU-E pass through different
stages and finally become the matured RBCs,
four important changes are noticed.
1. Reduction in size of the cell (from the
diameter of 25 to 7.2
μ)
2. Disappearance of nucleoli and nucleus
3. Appearance of hemoglobin
4. Change in the staining properties of the
cytoplasm.
FIGURE 8-1:
Stem cells. L – Lymphocyte, R – Red blood cells, N – Neutrophil, B – Basophil,
E – Eosinophil, M – Monocyte, P – Platelets

Chapter 8 Erythropoiesis
51
n
STAGES OF ERYTHROPOIESIS
The various stages between CFU-E cells and
matured RBC are:
1. Proerythroblast
2. Early normoblast
3. Intermediate normoblast
4. Late normoblast
5. Reticulocyte
6. Matured erythrocyte.
1. Proerythroblast (Megaloblast)
Proerythroblast or megaloblast is very large in
size with a diameter of about 20 μ. A large
nucleus with two or more nucleoli and a
chromatin network is present. Hemoglobin is
absent. The cytoplasm is basophilic in nature.
The proerythroblast multiplies several times and
finally forms the cell of next stage called early
normoblast.
2. Early Normoblast
It is smaller than proerythroblast with a
diameter of about 15 μ. The nucleoli disappear
from the nucleus and condensation of
chromatin network occurs. The condensed
network becomes dense. The cytoplasm is
basophilic in nature. So, this cell is also called
basophilic erythroblast. This cell develops into
the next stage called intermediate normoblast
(Fig. 8-2).
3. Intermediate Normoblast
It is smaller than the early normoblast with a
diameter of 10 to 12 μ. The nucleus is still
present. But, the chromatin network shows
further condensation. This stage is marked by
the appearance of hemoglobin. Because of the
presence of small quantity of acidic hemoglobin,
the cytoplasm which is basophilic becomes
polychromatic, i.e. both acidic and basic in
nature. So this cell is called polychromophilic or
polychromatic erythroblast. This cell develops into
the next stage called late normoblast.
4. Late Normoblast
The diameter of the cell decreases further to
about 8 to 10 μ. Nucleus becomes very small
with very much condensed chromatin network
and is called ink spot nucleus. Quantity of
hemoglobin increases making the cytoplasm
almost acidophilic. So, the cell is now called
orthochromic erythroblast. At the end of late
normoblastic stage, just before it passes to the
next stage, the nucleus disintegrates and
disappears by the process called pyknosis. The
final remnant is extruded from the cell. Late
normoblast develops into the next stage called
reticulocyte.
5. Reticulocyte
It is slightly larger than matured RBC. It is
otherwise known as immature RBC. It is called
reticulocyte because, the reticular network or
reticulum that is formed from the disintegrated
organelles are present in the cytoplasm.
In newborn babies, the reticulocyte count is
2 to 6% of RBCs, i.e. 2 to 6 reticulocytes are
present for every 100 RBCs. The number of
reticulocytes decreases during the first week after
birth. Later, the reticulocyte count remains
constant at or below 1%. The number increases
whenever the erythropoietic activity increases.
Reticulocytes can enter the capillaries through
the capillary membrane from the site of
production by diapedesis.
6. Matured Erythrocyte
The cell decreases in size with the diameter of
7.2 μ. The reticular network disappears and the
cell becomes the matured RBC with biconcave
shape and hemoglobin but without nucleus. It
requires seven days for the proerythroblast to
become fully developed and matured RBC.
n
FACTORS NECESSARY FOR
ERYTHROPOIESIS
Development and maturation of erythrocytes
require many factors which are classified into 3
categories:
I. General factors
II. Maturation factors
III. Factors necessary for hemoglobin
formation.
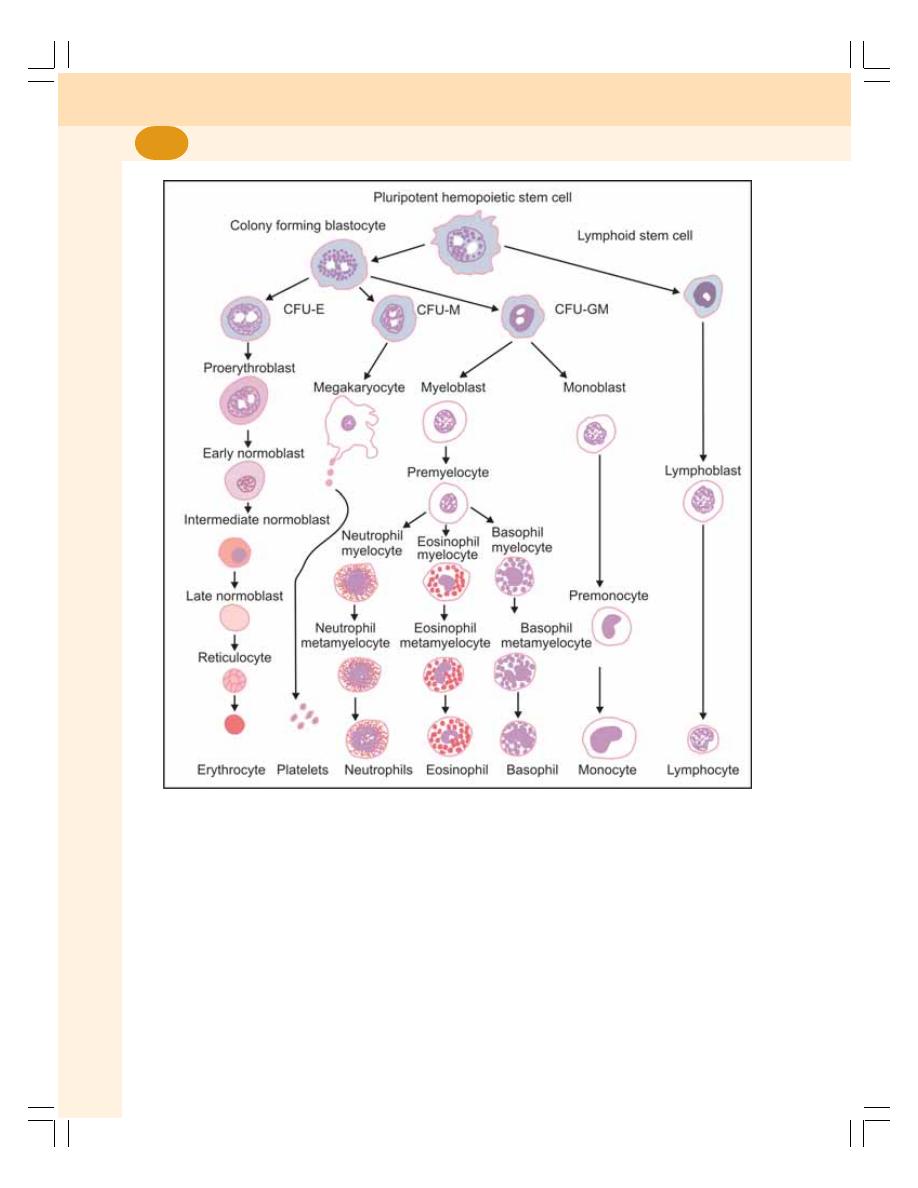
Blood and Body Fluids
52
n
GENERAL FACTORS
1. Erythropoietin
Erythropoietin is a hormone secreted mainly by
peritubular capillaries in the kidney and a small
quantity is also secreted from the liver and the
brain. Hypoxia is the stimulant for the secretion
of erythropoietin.
Erythropoietin promotes the following
processes:
i. Production of proerythroblasts from CFU-
E of the bone marrow
ii. Development of proerythroblasts into mat-
ured RBCs through the several stages
iii. Release of matured erythrocytes into
blood. Some reticulocytes are also
released along with matured RBCs.
FIGURE 8-2:
Stages of erythropoiesis. CFU-E = Colony forming unit – Erythrocyte,
CFU-M = Colony forming unit – Megakaryocyte, CFU-GM = Colony forming unit – Granulocyte/Monocyte

Chapter 8 Erythropoiesis
53
2. Thyroxine
Being a general metabolic hormone, thyroxine
accelerates the process of erythropoiesis at many
levels.
3. Hemopoietic Growth Factors
Hemopoietic growth factors or growth inducers
are the interleukins – 3, 6 and 11 and stem cell
factor (steel factor). Generally these factors
induce the proliferation of PHSCs.
4. Vitamins
The vitamins A, B, C, D and E are necessary
for erythropoiesis. Deficiency of these vitamins
causes anemia.
n
MATURATION FACTORS
Vitamin B
12
, intrinsic factor and folic acid are
necessary for the maturation of RBCs.
1. Vitamin B
12
(Cyanocobalamin)
Vitamin B
12
is essential for synthesis of DNA,
cell division and maturation in RBCs. It is also
called extrinsic factor as it is obtained mostly
from diet. It is also produced in the large
intestine by the intestinal flora. It is absorbed
from the small intestine in the presence of
intrinsic factor of Castle. Vitamin B
12
is stored
mostly in liver and in small quantity in muscle.
Its deficiency causes pernicious anemia
(macrocytic anemia) in which the cells remain
larger with fragile and weak cell membrane.
2. Intrinsic Factor of Castle
It is produced in gastric mucosa by the parietal
cells of the gastric glands. It is essential for the
absorption of vitamin B
12
from intestine. Absence
of intrinsic factor also leads to pernicious anemia
because of failure of vitamin B
12
absorption. The
deficiency of intrinsic factor occurs in conditions
like severe gastritis, ulcer and gastrectomy.
3. Folic Acid
Folic acid is also essential for the synthesis of
DNA. Deficiency of folic acid decreases the DNA
synthesis causing maturation failure. Here the
cells are larger and remain in megaloblastic
(proerythroblastic) stage which leads to
megaloblastic anemia.
n
FACTORS NECESSARY FOR
HEMOGLOBIN FORMATION
Various materials are essential for the formation
of hemoglobin in the RBCs such as:
1. First class proteins and amino acids of high
biological value — for the formation of globin.
2. Iron — for the formation of heme part of the
hemoglobin.
3. Copper — for the absorption of iron from GI
tract.
4. Cobalt and nickel — for the utilization of iron
during hemoglobin synthesis.
5. Vitamins: Vitamin C, riboflavin, nicotinic acid
and pyridoxine — for hemoglobin synthesis.
n
INTRODUCTION
n
NORMAL HEMOGLOBIN CONTENT
n
FUNCTIONS
n
STRUCTURE
n
TYPES OF NORMAL HEMOGLOBIN
n
ABNORMAL HEMOGLOBIN
n
ABNORMAL HEMOGLOBIN DERIVATIVES
n
SYNTHESIS
n
DESTRUCTION
n
INTRODUCTION
Hemoglobin (Hb) is the iron containing coloring
pigment of RBC. It forms 95% of dry weight of
RBC and 30 to 34% of wet weight. The molecular
weight of Hb is 68,000.
n
NORMAL HEMOGLOBIN CONTENT
Average Hb content in blood is 14 to 16 g/dL.
However, it varies depending upon age and sex
of the individual and the number of RBCs.
Age
At birth
: 25 g/dL
After 3rd month
: 20 g/dL
After 1 year
: 17 g/dL
From puberty onwards : 14-16 g/dL
At the time of birth and in infants and growing
children, Hb content is high because of increased
number of RBCs (Chapter 7).
Sex
In adult males
: 15 g/dL
In adult females
: 14.5 g/dL
n
FUNCTIONS OF HEMOGLOBIN
n
TRANSPORT OF RESPIRATORY GASES
The main function of Hb is the transport of
respiratory gases.
It transports:
i.
Oxygen from
lungs to tissues
ii. Carbon dioxide from tissues to lungs
(Chapter 76).
Hemoglobin
9

Chapter 9 Hemoglobin
55
n
BUFFER ACTION
Hb acts as a buffer and plays an important role
in acid-base balance.
n
STRUCTURE OF HEMOGLOBIN
Hb is a conjugated protein. It consists of a protein
called globin and an iron containing pigment
called heme.
Iron is present in an unstable ferrous (Fe
++
)
form. Heme part is called porphyrin. It is formed
by four pyrole rings (tetrapyrole). The iron is
attached to each pyrole ring and globin molecule.
Globin is made up of four polypeptide chains.
Among the four polypeptide chains, two are
α
chains and two are
β chains
n
TYPES OF NORMAL HEMOGLOBIN
Hb is of two types:
1. Adult Hb (Hb A)
2. Fetal Hb (Hb F)
Both the types of Hb differ from each other
structurally and functionally.
Structural Difference
In adult Hb, the globin contains two
α chains and
two
β chains. In fetal Hb, there are two α chains
and two
γ chains instead of β chains.
Functional Difference
Functionally, fetal Hb has more affinity for oxygen
than adult Hb. And, the oxygen hemoglobin
dissociation curve of fetal blood is shifted to left
(Chapter 76).
n
ABNORMAL HEMOGLOBIN
The abnormal types of Hb are produced because
of structural changes in the polypeptide chains
caused by mutation in the genes of the globin
chains. There are two categories of abnormal
Hb:
I.
Hemoglobinopathies
II. Hb in thalassemia and related disorders.
I.
Hemoglobinopathies
Hemoglobinopathy is a genetic disorder caused
by abnormal polypeptide chains of Hb.
Some of the hemoglobinopathies are Hb S,
C, E and M.
II. Hb in Thalassemia and Related Disorders
In thalassemia different types of abnormal Hb
are present. The polypeptide chains are
decreased, absent or abnormal. (Refer Chapter
11).
n
ABNORMAL HEMOGLOBIN
DERIVATIVES
Abnormal Hb formed by the combination of Hb
with some substances other than oxygen
and
carbon dioxide is called abnormal Hb derivative.
Abnormal Hb derivatives are formed by carbon
monoxide poisoning or due to the combination
of some drugs like nitrites, nitrates and
sulfonamides.
The abnormal hemoglobin derivatives are
carboxyhemoglobin, methemoglobin and
sulfhemoglobin. The high levels of abnormal Hb
derivatives in blood produce serious effects by
preventing the transport of oxygen. It results in
oxygen lack in tissues which may be fatal.
n
CARBOXYHEMOGLOBIN
Carboxyhemoglobin or carbon monoxyhemo-
globin is the abnormal Hb derivative formed by
the combination of carbon monoxide with Hb.
Carbon monoxide is a colorless and odorless
gas. Since Hb has 200 times more affinity for
carbon monoxide than oxygen, it hinders the
transport of oxygen resulting in tissue hypoxia
(Chapter 78).
Some of the sources of carbon monoxide are
charcoal burning, coal mines, deep wells,
underground drainage system, exhaust of
gasoline engines, gases from guns and other
weapons, heating system with poor or improper
ventilation, smoke from fire and tobacco
smoking.

Blood and Body Fluids
56
Signs and Symptoms of Carbon Monoxide
Poisoning
1. While breathing air with less than 1% of
carbon monoxide, the Hb saturation is
15 to 20% and mild symptoms like headache
and nausea appear.
2. While breathing air with more than 1% carbon
monoxide, the Hb saturation is 30 to 40%. It
causes severe symptoms like convulsions,
cardiorespiratory arrest, unconsciousness
and coma.
3. When Hb saturation increase above 50%,
death occurs.
n
METHEMOGLOBIN
Methemoglobin is the abnormal Hb derivative
formed when iron molecule of Hb is oxidized
from normal ferrous state to ferric state.
Methemoglobin is also called ferrihemoglobin.
Normal methemoglobin level is less than 3% of
total Hb.
Some of the sources of methemoglobin are
contaminated well waters with nitrates and
nitrites, match sticks, explosives, naphthalene
balls, irritant gases like nitrous oxide, etc.
n
SULFHEMOGLOBIN
Sulfhemoglobin is the abnormal Hb derivative
formed by the combination of hemoglobin with
hydrogen sulfide. It is caused by drugs such as
sulfonamides. Normal sulfhemoglobin level is
less than 1% of total Hb.
n
SYNTHESIS OF HEMOGLOBIN
Synthesis of Hb actually starts in proerythro-
blastic stage. However, Hb appears in the
intermediate normoblastic stage only. The
production of the Hb is continued until the stage
of reticulocyte. The heme portion of Hb is
synthesized in mitochondria. And the protein part
(globin) is synthesized in ribosomes.
n
SYNTHESIS OF HEME
Heme is synthesized from succinyl CoA and the
glycine in the mitochondria.
n
FORMATION OF GLOBIN
The polypeptide chains of globin are produced
in the ribosomes. There are four types of
polypeptide chains namely, alpha, beta, gamma
and delta chains. Each globin molecule is formed
by the combination of 2 pairs of chains. Adult
Hb contains two alpha chains and two beta
chains. Fetal Hb contains two alpha chains and
two gamma chains.
n
CONFIGURATION
Each polypeptide chain combines with one heme
molecule. Thus, after the complete configuration,
each Hb molecule contains 4 polypeptide chains
and 4 heme molecules.
n
SUBSTANCES NECESSARY FOR
HEMOGLOBIN SYNTHESIS
Various materials are essential for the formation
of Hb in the RBC (Refer Chapter 8 for details).
n
DESTRUCTION OF HEMOGLOBIN
After the lifespan of 120 days, the RBC is
destroyed in the reticuloendothelial system
particularly in spleen and the Hb is released into
plasma. Soon, the Hb is degraded in the
reticuloendothelial cells and split into globin, iron
and porphyrin.
Globin is utilized for the resynthesis of Hb.
Iron is stored in the body. Porphyrin is converted
into biliverdin. In human being, most of the
biliverdin is converted into bilirubin. Bilirubin and
biliverdin are together called the bile pigments
(Chapter 30).
n
ERYTHROCYTE SEDIMENTATION RATE
n
DEFINITION
n
DETERMINATION
n
NORMAL VALUES
n
SIGNIFICANCE OF DETERMINING
n
VARIATIONS
n
FACTORS AFFECTING
n
PACKED CELL VOLUME
n
DEFINITION
n
METHOD OF DETERMINATION
n
SIGNIFICANCE OF DETERMINING
n
NORMAL VALUES
n
VARIATIONS
n
ERYTHROCYTE SEDIMENTATION
RATE
n
DEFINITION
Erythrocyte Sedimentation Rate (ESR) is the rate
at which the erythrocytes settle down. Normally,
when the blood is in circulation, the RBCs remain
suspended uniformly. This is called suspension
stability of RBCs. If blood is mixed with an
anticoagulant and allowed to stand undisturbed
on a vertical tube, the red cells settle down due
to gravity with a supernatant layer of clear
plasma.
n
DETERMINATION OF ESR
There are two methods to determine ESR.
1. Westergren’s method
2. Wintrobe’s method.
Westergren’s Method
In this method, Westergren’s tube is used to
determine ESR. This tube is 300 mm long and
opened on both ends (Fig. 10-1A). It is marked
from 0 to 200 mm from above downwards. 1.6
mL of blood is mixed with 0.4 mL of 3.8 percent
sodium citrate (anticoagulant). The ratio of blood
and anticoagulant is 4:1. This blood is loaded in
the Westergren’s tube up to 0 mark above. The
tube is placed vertically in the Westergren’s stand
and left undisturbed. The reading is taken after
one hour.
Wintrobe’s Method
In this method, Wintrobe’s tube is used to
determine ESR. This tube is short and opened
on one end and closed on the other end
(Fig. 10-1B). It is 110 mm long with 3 mm bore.
Erythrocyte Sedimentation
Rate and Packed Cell
Volume
10
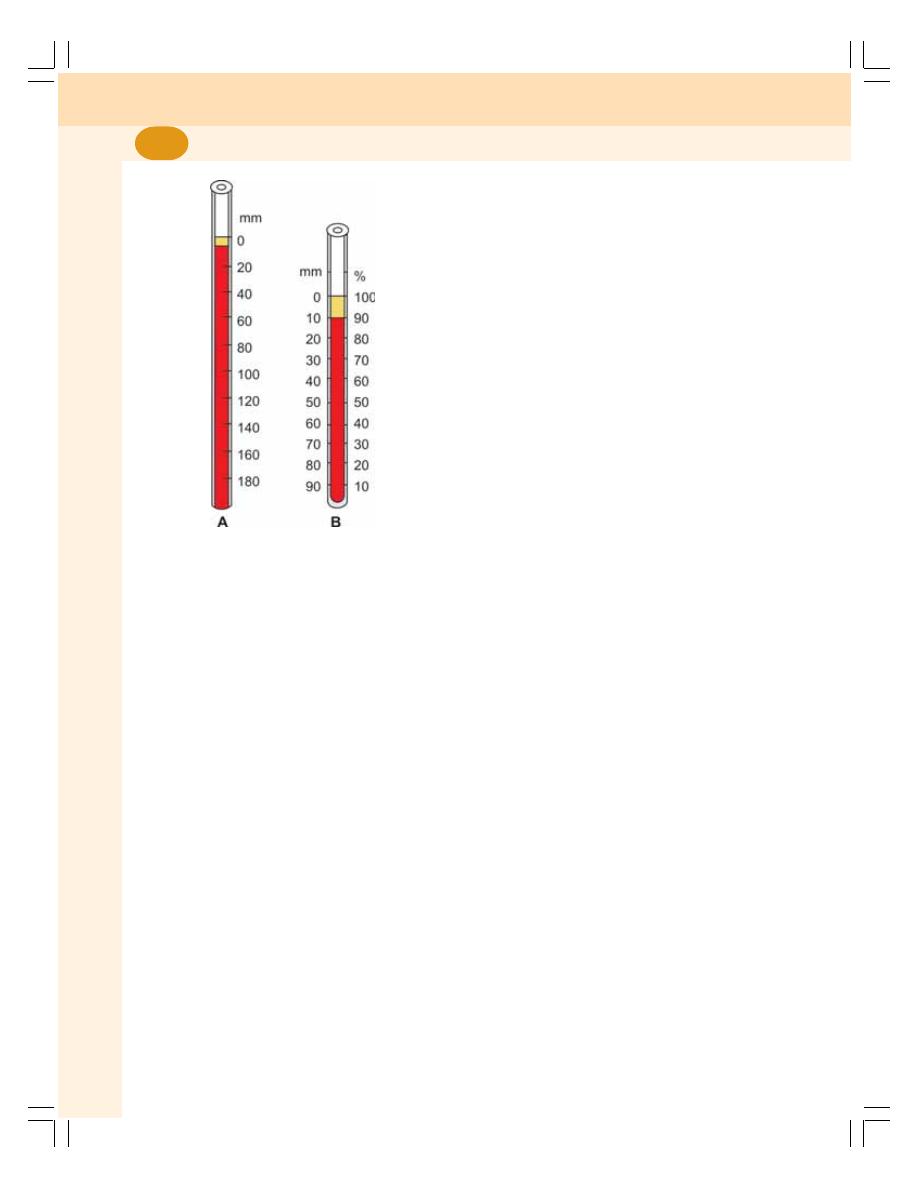
Blood and Body Fluids
58
It is used for determining ESR and PCV. It is
marked on both sides. On one side the marking
is from 0 to 100 (for ESR) and on other side from
100 to 0 (for PCV) from above downwards.
About one mL of blood is mixed with an
anticoagulant called ethylenediaminetetra acetic
acid (EDTA). The blood is loaded in the tube up
to ‘0’ mark above. The tube is placed on the
Wintrobe’s stand and left undisturbed. The
reading is taken after one hour.
n
NORMAL VALUES OF ESR
By Westergren’s Method
In males
: 3 to 7 mm in one hour
In females
: 5 to 9 mm in one hour
Infants
: 0 to 2 mm in one hour
By Wintrobe’s Method
In males
: 0 to 9 mm in one hour
In females
: 0 to 15 mm in one hour
Infants
: 0 to 5 mm in one hour
n
SIGNIFICANCE OF DETERMINING ESR
ESR is an easy and inexpensive test which helps
in diagnosis as well as prognosis. Prognosis
means monitoring the course of disease and
response of the patient to therapy. Determination
of ESR is especially helpful in assessing the
progress of patients treated for certain chronic
disorders such as pulmonary tuberculosis and
rheumatoid arthritis.
n
VARIATIONS OF ESR
Physiological Variation
1. Age: ESR is less in children and infants
because of more number of RBCs
2. Sex: It is more in females than in males
because of less number of RBCs
3. Menstruation: The ESR increases during
menstruation because of loss of blood and
RBCs
4. Pregnancy: From 3rd month to parturition,
ESR increases up to 35 mm in one hour
because of hemodilution.
Pathological Variation
ESR increases in the following diseases:
1. Tuberculosis
2. All types of anemia except sickle cell anemia
3. Malignant tumors
4. Rheumatoid arthritis
5. Rheumatic fever
6. Liver diseases.
ESR decreases in the following diseases:
1. Allergic conditions
2. Sickle cell anemia
3. Peptone shock
4. Polycythemia and
5. Severe leukocytosis.
n
FACTORS AFFECTING ESR
Following factors increase the ESR:
1. Specific gravity of RBC
2. Rouleaux formation
3. Increase in size of RBC
4. Decrease in RBC count
FIGURE 10-1: A = Westergren’s tube
B = Wintrobe’s tube
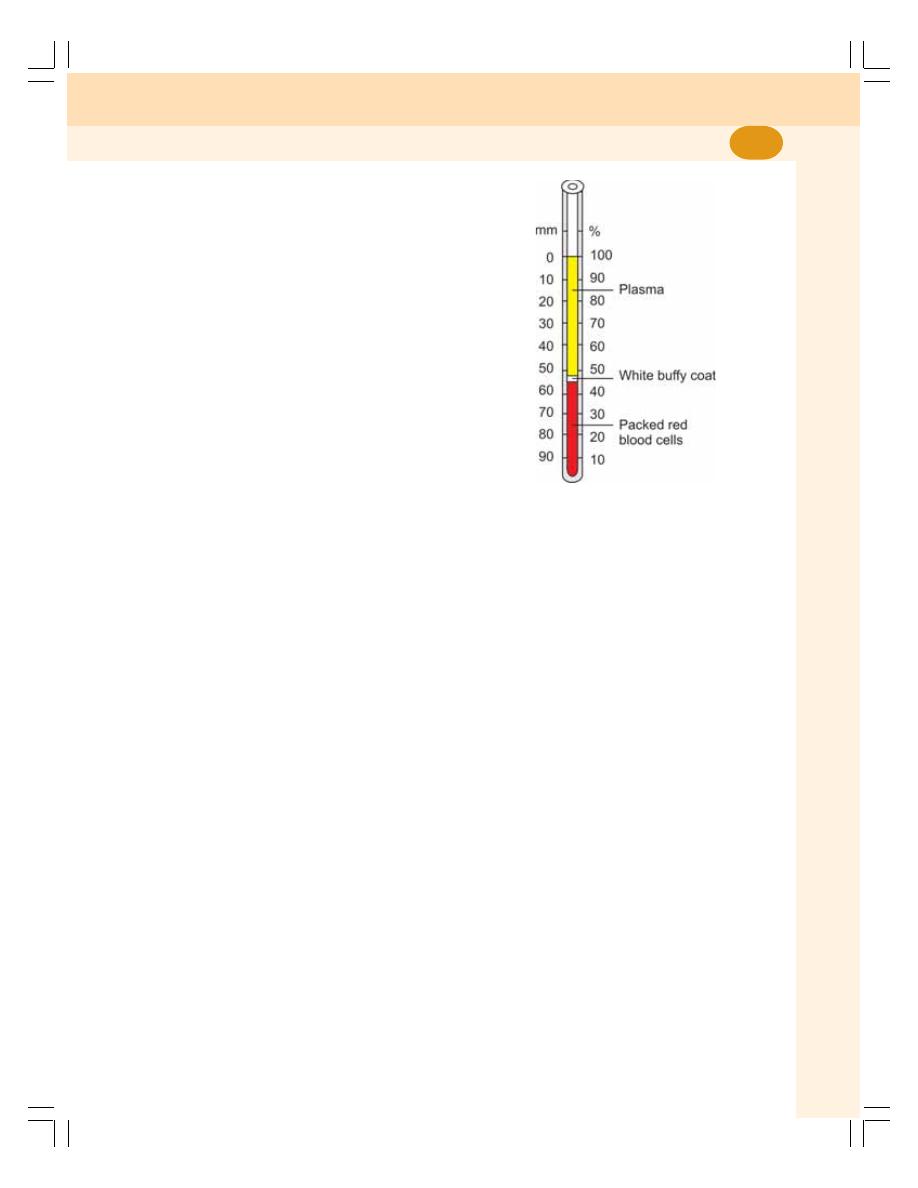
Chapter 10 Erythrocyte Sedimentation Rate and Packed Cell Volume
59
Following factors decrease the ESR:
1. Viscosity of blood
2. Increase in RBC count
n
PACKED CELL VOLUME
n
DEFINITION
Packed cell volume (PCV) is the volume of the
RBCs in the blood that is expressed in
percentage. It is also called hematocrit value.
n
METHOD OF DETERMINATION
Blood is mixed with the anticoagulant EDTA or
heparin and filled in Wintrobe’s tube up to the
100 or 0 mark above. The tube with the blood is
centrifuged at a speed of 3000 revolutions per
minute (rpm) for 30 minutes.
At the end of 30 minutes, the tube is taken
out and the reading is noted. The RBCs are
packed at the bottom and this is the PCV. The
plasma remains above this. In between the RBCs
and the plasma, there is a white buffy coat, which
is formed by white blood cells and the platelets
(see Fig. 10-2).
n
SIGNIFICANCE OF DETERMINING PCV
Determination of PCV helps in:
1. Diagnosis and treatment of anemia
2. Diagnosis and treatment of polycythemia
3. Determination of severity of dehydration and
recovery from dehydration after treatment
4. Decision of blood transfusion.
FIGURE 10-2: Packed cell volume
n
NORMAL VALUES OF PCV
Normal PCV:
In males
= 40 to 45%
In females = 38 to 42%
n
VARIATIONS IN PCV
PCV increases in:
1. Polycythemia
2. Dehydration
PCV decreases in:
1. Anemia
2. Cirrhosis of liver
3. Pregnancy
INTRODUCTION
CLASSIFICATION
SIGNS AND SYMPTOMS
Anemia
11
1. Hemorrhagic Anemia
Hemorrhage refers to excessive loss of blood
(Chapter 70). Anemia due to hemorrhage is
known as hemorrhagic anemia or blood loss
anemia. It occurs both in acute and chronic
hemorrhagic conditions.
Acute Hemorrhage
Acute hemorrhage means sudden loss of large
quantity of blood as in case of accidents. The
RBCs are normocytic and normochromic
(Table 11-2)
INTRODUCTION
Anemia is the blood disorder characterized by
the reduction in:
1. Red blood cell count
2. Hemoglobin content
3. Packed cell volume.
CLASSIFICATION OF ANEMIA
Anemia is classified by two methods:
A. Morphological classification
B. Etiological classification.
MORPHOLOGICAL CLASSIFICATION
Morphological classification depends upon the
size and color of RBC. Size of RBC is expressed
as mean corpuscular volume (MCV) and the
color is expressed as mean corpuscular hemo-
globin concentration (MCHC). By this method,
the anemia is classified into four types as given
in Table 11-1.
ETIOLOGICAL CLASSIFICATION
On the basis of the etiology (study of cause or
origin), the anemia is divided into five types:
TABLE 11-1: Morphological classification of anemia
Type of anemia
Size of RBC
Color of RBC
(MCV)
(MCHC)
Normocytic
normochromic
Normal
Normal
Normocytic
hypochromic
Normal
Less
Macrocytic
hypochromic
Large
Less
Microcytic
hypochromic
Small
Less

Chapter 11 Anemia
61
Chronic Hemorrhage
It refers to loss of blood over a long period of
time by internal or external bleeding as in
conditions like peptic ulcer, purpura, hemophilia
and menorrhagia. The RBCs are microcytic and
hypochromic (Table 11-2). It is because of
decrease in iron content.
2. Hemolytic Anemia
Hemolysis means destruction of RBCs. Anemia
due to excessive destruction of RBCs is called
hemolytic anemia. Hemolysis occurs because of
the following reasons (Table 11-2):
i. Liver failure
ii. Renal disorder
iii. Hypersplenism
iv. Burns
v. Infections like hepatitis, malaria and
septicemia
vi. Drugs such as penicillin, antimalarial
drugs and sulfa drugs
vii. Poisoning by chemical substances like
lead, coal and tar
viii. Presence of isoagglutinins like anti-Rh
ix. Autoimmune diseases such as rheu-
matoid arthritis and ulcerative colitis.
x. Hereditary factors
Hereditary Disorders
Sickle cell anemia
Sickle cell anemia is an inherited blood disorder
characterized by sickle shaped RBCs. It occurs
when a person inherits two abnormal genes (one
from each parent). It is also called hemoglobin
SS disease or sickle cell disease. It is common
in people of African origin.
In sickle cell anemia, hemoglobin becomes
abnormal with normal
α chains and abnormal β
chains. Because of this, RBCs attain sickle (cre-
scent) shape and become more fragile leading
to hemolysis (Table 11-2).
Thalassemia
Thalassemia is an inherited disorder characte-
rized by abnormal hemoglobin. In normal hemo-
globin, the number of
α and β chains is equal.
In thalassemia the number of these chains is not
equal. This causes the precipitation of the
polypeptide chains leading to defective formation
of RBCs or hemolysis of the matured RBCs.
It is also known as Cooley’s anemia or Medi-
terranean anemia. It is more common in Thailand
and to some extent in Mediterranean countries.
Thalassemia is of two types:
i.
α thalassemia
ii.
β thalassemia.
The
β thalassemia is very common among
these two.
3. Nutrition Deficiency Anemia
Anemia that occurs due to deficiency of a nutritive
substance necessary for erythropoiesis is called
nutrition deficiency anemia. Such substances are
iron, proteins and vitamins like C, B
12
and folic
acid. The types of nutrition deficiency anemia are:
Iron deficiency anemia
Iron deficiency anemia is the most common type
of anemia. It develops due to inadequate
availability of iron for hemoglobin synthesis. The
RBCs are microcytic and hypochromic (Table
11-2).
Protein deficiency anemia
Protein deficiency decreases the hemoglobin
synthesis and the RBCs become macrocytic and
hypochromic in nature (Table 11-2).
Vitamin B
12
deficiency — Pernicious anemia
Vitamin B
12
is a maturation factor for RBC and
deficiency of this causes pernicious anemia
which is also called Addison’s anemia. It occurs
because of less intake of vitamin B
12
or poor
absorption of vitamin B
12
. Vitamin B
12
is absor-
bed from the stomach with the help of intrinsic
factor of Castle which is secreted in the gastric
mucosa. Decrease in the production of intrinsic
factor causes poor absorption of vitamin B
12
.
RBCs are macrocytic and normochromic/
hypochromic (Table 11-2).

Blood and Body Fluids
62
Folic acid deficiency — Megaloblastic anemia
Folic acid is necessary for the maturation of RBC.
Deficiency of this leads to defective DNA
synthesis making the nucleus to remain
immature. The RBCs are megaloblastic and
hypochromic (Table 11-2).
4. Aplastic Anemia
Aplastic anemia is due to the bone marrow
disorder. The red bone marrow is reduced and
replaced by fatty tissues. In this condition, the
RBCs are normocytic and normochromic
(Table 11-2). It occurs in conditions such as
repeated exposure to X-ray or gamma ray
radiation, tuberculosis and viral infections like
hepatitis and HIV infections.
5. Anemia due to Chronic Diseases
Anemia occurs due to some chronic diseases
such as rheumatoid arthritis, tuberculosis and
Table 11-2: Etiological classification of anemia
Type of anemia
Causes
Morphology of RBC
Hemorrhagic anemia
1. Acute hemorrhage — acute loss of
Normocytic, normochromic
blood
2. Chronic hemorrhage — chronic loss
Microcytic, hypochromic
of blood
1. Liver failure
2. Renal disorder
3. Hypersplenism
4. Burns
5. Infections — malaria and septicemia
Normocytic normochromic
Hemolytic anemia
6. Drugs like penicillin, antimalarial
drugs and sulfa drugs
7. Poisoning by lead, coal and tar
8. Isoagglutinins — anti-Rh
Sickle cell anemia: Sickle
9. Hereditary disorders
shape and hypochromic
Thalassemia: Small, irregular
and hypochromic
1. Iron deficiency
Microcytic, hypochromic
Nutrition deficiency anemia
2. Protein deficiency
Macrocytic, hypochromic
3. Vitamin B
12
deficiency
Macrocytic, normochromic /
hypochromic
4. Folic acid deficiency
Megaloblastic, hypochromic
Aplastic anemia
Bone marrow disorder
Normocytic, normochromic
1. Rheumatoid arthritis
Anemia of chronic diseases
2. Tuberculosis
Normocytic, normochromic
3. Chronic renal failure

Chapter 11 Anemia
63
chronic renal failure. RBCs are normocytic and
normochromic (Table 11-2).
SIGNS AND SYMPTOMS OF
ANEMIA
SKIN AND MUCOUS MEMBRANE
The color of the skin and mucous membrane
becomes pale. Paleness is observed prominently
in buccal cavity, pharyngeal mucous membrane,
conjunctivae, lips, ear lobes, palm and nail bed.
Skin also looses the elasticity and becomes thin
and dry.
HAIR AND NAILS
Loss of hair is common with thinning and early
graying. The nails become brittle and easily
breakable.
CARDIOVASCULAR SYSTEM
There is increase in heart rate and cardiac output.
Heart is dilated and cardiac murmurs are pro-
duced. The velocity of blood flow is increased.
RESPIRATION
Rate and force of respiration increase. Some-
times, it leads to breathlessness and dyspnea
(difficulty in breathing). Oxygen hemoglobin
dissociation curve is shifted to right.
DIGESTION
Anorexia (loss of appetite), nausea, vomiting,
abdominal discomfort, and constipation are
common. In pernicious anemia, there is atrophy
of papillae in tongue. In aplastic anemia, necrotic
lesions appear in mouth and pharynx.
METABOLISM
Basal metabolic rate increases in severe anemia.
KIDNEY
Renal function is disturbed. Albuminuria is
common.
REPRODUCTIVE SYSTEM
In females, the menstrual cycle is disturbed.
There may be menorrhagia, oligomenorrhea or
amenorrhea (Chapter 55).
NEUROMUSCULAR SYSTEM
The common neuromuscular symptoms are
headache, lack of concentration, restlessness,
irritability, drowsiness, dizziness or vertigo espe-
cially when standing, increased sensitivity to cold
and fainting. Muscles become weak and the
patient feels lack of energy and fatigued quite
often and quite easily.
INTRODUCTION
CLASSIFICATION
MORPHOLOGY
NORMAL COUNT
VARIATIONS
LIFESPAN
PROPERTIES
FUNCTIONS
LEUKOPOIESIS
INTRODUCTION
White blood cells (WBCs) or leukocytes are the
colorless and nucleated formed elements of
blood (leuko = white or colorless). Compared to
RBCs, the WBCs are larger in size and lesser
in number. Yet functionally, these cells are as
important as RBCs and play very important role
in defense mechanism of body by acting like
soldiers and protecting the body from invading
organisms.
CLASSIFICATION
WBCs are classified into two groups depending
upon the presence or absence of granules in the
cytoplasm:
1. Granulocytes – with granules
2. Agranulocytes – without granules.
1. Granulocytes
Depending upon the staining property of
granules, the granulocytes are classified into
three types:
i. Neutrophils – granules take both acidic and
basic stains
ii. Eosinophils – granules take acidic stain
iii. Basophils – granules take basic stain.
2. Agranulocytes
Agranulocytes have plain cytoplasm without
granules. Agranulocytes are of two types:
i. Monocytes
ii. Lymphocytes.
MORPHOLOGY OF WHITE BLOOD
CELLS
NEUTROPHILS
Neutrophils are also known as polymorpho-
nuclear leukocytes because the nucleus is
multilobed. The number of lobes varies from 1
to 6 (Fig. 12-1). The granules are fine or small
in size. When stained with Leishman’s stain
(which contains acidic eosin and basic methylene
blue), the granules take both the stains equally.
White Blood Cells
12
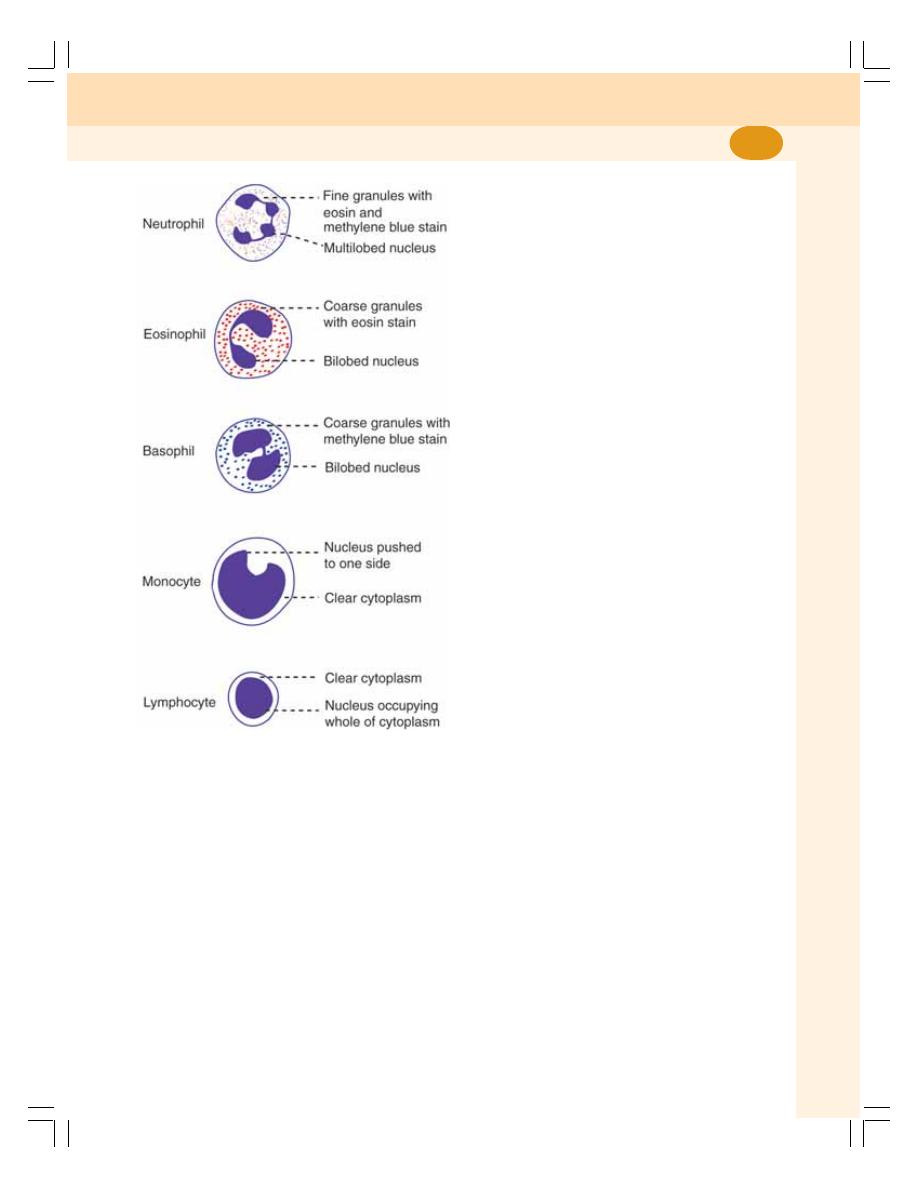
Chapter 12 White Blood Cells
65
So, the granules appear violet in color. The
diameter of cell is 10 to 12 μ. The neutrophils
are ameboid and phagocytic in nature.
EOSINOPHILS
Eosinophils have coarse (larger) granules in the
cytoplasm, which stain pink or red with eosin.
Normally, the nucleus is bilobed and spectacle
shaped. Rarely trilobed nucleus may be present.
The diameter of the cell varies between 10 and
14
μ.
BASOPHILS
Basophils also have coarse granules in the
cytoplasm and the granules stain purple blue with
methylene blue. Nucleus is bilobed. Diameter of
the cell is 8 to 10 μ.
MONOCYTES
Monocytes are the largest WBCs with diameter
of 14 to 18 μ. The cytoplasm is clear without
granules. The nucleus is round, oval, horseshoe
shaped, bean shaped or kidney shaped. The
nucleus is placed either in the center of the cell
or pushed to one side and a large amount of
cytoplasm is seen.
LYMPHOCYTES
Lymphocytes also do not have granules in the
cytoplasm. The nucleus is oval, bean shaped or
kidney shaped and occupies the whole of the
cytoplasm. A rim of cytoplasm may or may not
be seen.
Depending upon the size, the lymphocytes
are divided into two types:
i. Large lymphocytes – younger cells with a
diameter of 10 to 12 μ
ii. Small lymphocytes – older cells with a
diameter of 7 to 10 μ.
Depending upon the function, the
lymphocytes are divided into two types:
i. T lymphocytes – concerned with cellular
immunity
ii. B lymphocytes – concerned with humoral
immunity.
NORMAL LEUKOCYTE COUNT
1. Total WBC count (TC): 4,000 to 11,000/
cumm of blood
2. Differential WBC count (DC): Given in Table
12-1.
VARIATIONS IN LEUKOCYTE COUNT
WBC count varies both in physiological and
pathological conditions. Increase in WBC count
FIGURE 12-1:
Diagram of the cell membrane
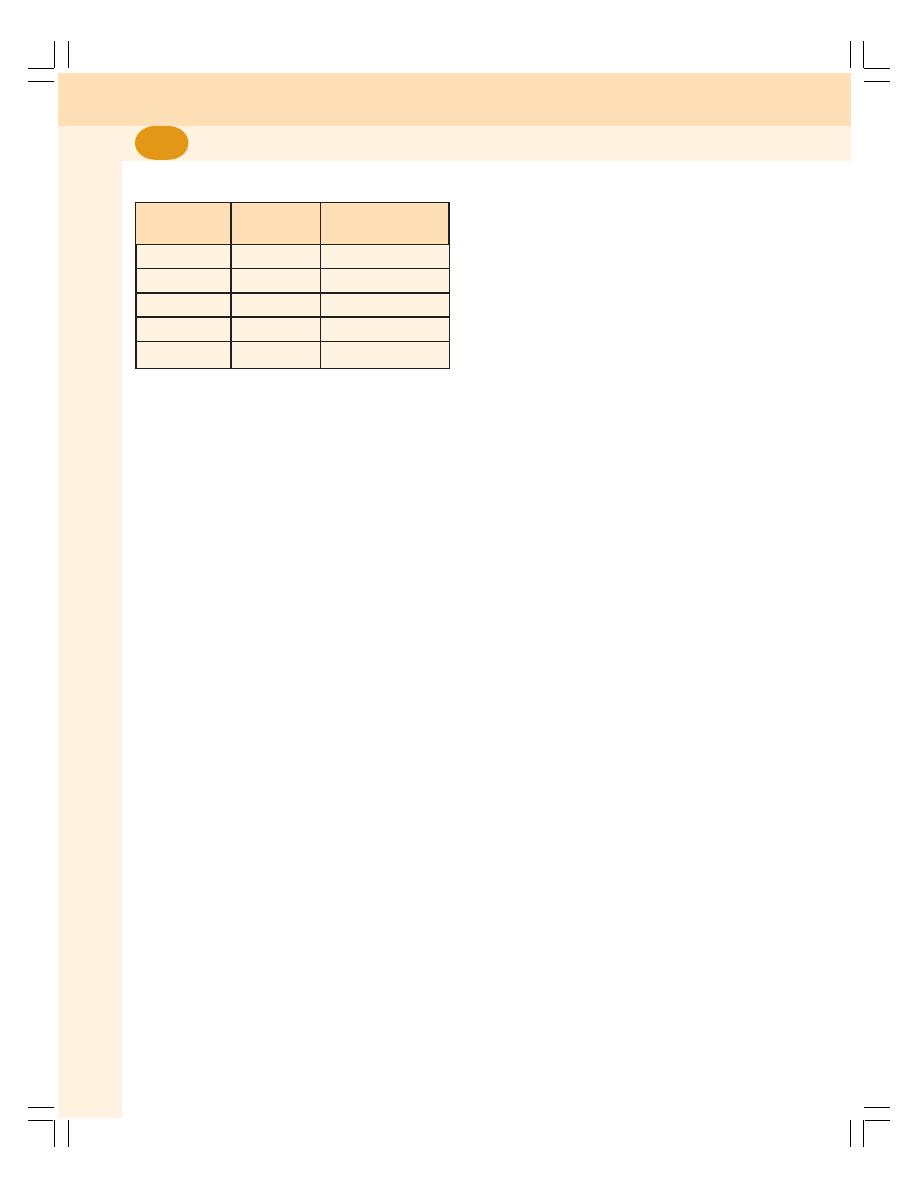
Blood and Body Fluids
66
is called leukocytosis and decrease in the count
is called leukopenia. The term leukopenia is
generally used only for pathological conditions.
PHYSIOLOGICAL VARIATIONS
1. Age: In infants and children, total WBC count
is more; it is about 20,000/cumm in infants
and about 10,000 to 15,000/cumm of blood
in children. In adults it ranges between 4000
and 11000/cumm of blood
2. Sex: Slightly more in males than in females
3. Diurnal variation: Minimum in early morning
and maximum in the afternoon
4. Exercise: Increases slightly
5. Sleep: Decreases slightly
6. Emotional conditions like anxiety: Increases
slightly
7. Pregnancy: Increases
8. Menstruation: Increases
9. Parturition: Increases
PATHOLOGICAL VARIATIONS
Leukocytosis
It occurs in the following pathological conditions:
1. Infections
2. Allergy
3. Common cold
4. Tuberculosis
5. Glandular fever.
Leukopenia
Leukopenia occurs in the following pathological
conditions:
1. Anaphylactic shock
2. Cirrhosis of liver
3. Disorders of spleen
4. Pernicious anemia
5. Typhoid and paratyphoid
6. Viral infections.
Leukemia
The leukemia is the condition, which is
characterized by abnormal and uncontrolled
increase in WBC count more than 1,000,000/
cumm. It is also called blood cancer.
However, all the WBCs may not increase at
a time. Leukocytosis occurs because of increase
in any one of the WBCs. The pathological
variations of different types of WBCs are given
in Table 12-2.
LIFESPAN OF WHITE BLOOD CELLS
Lifespan of WBCs is as follows:
Neutrophils
:
2 to 5 days
Eosinophils
:
7 to 12 days
Basophils
: 12 to 15 days
Monocytes
:
2 to 5 days
Lymphocytes
: ½ to 1 day
PROPERTIES OF WBCs
1. Diapedesis
Diapedesis is the process by which the WBCs
squeeze through the narrow blood vessels.
2. Ameboid Movement
Neutrophils, monocytes and lymphocytes show
amebic movement characterized by protrusion
of the cytoplasm and change in the shape.
3. Chemotaxis
Chemotaxis is the attraction of WBCs towards
the injured tissues by the chemical substances
released at the site of injury.
4. Phagocytosis
Neutrophils and monocytes engulf the foreign
bodies by means of phagocytosis (Chapter 3).
TABLE 12-1:
Normal values of different WBCs
Type of WBC Percentage
Absolute value
per cumm
Neutrophils
50 to 70
3000 to 6000
Eosinophils
2 to 4
150 to 450
Basophils
0 to 1
0 to 100
Monocytes
2 to 6
200 to 600
Lymphocytes
20 to 30
1500 to 2700

Chapter 12 White Blood Cells
67
TABLE 12-2:
Pathological variations in different types of WBCs
Disorder
Variation
Conditions
Neutrophilia
Increase in neutrophil count
1. Acute infections
2. Metabolic disorders
3. Injection of foreign proteins
4. Injection of vaccines
5. Poisoning with drugs, chemical, etc.
6. After acute hemorrhage
Neutropenia
Decrease in neutrophil count
1. Bone marrow disorders
2. Tuberculosis
3. Typhoid
4. Autoimmune diseases.
Eosinophilia
Increase in eosinophil count
1. Allergic conditions like asthma
2. Blood parasitism (malaria, filariasis)
3. Intestinal parasitism
4. Scarlet fever
Eosinopenia
Decrease in eosinophil count
1. Cushing’s syndrome
2. Bacterial infections
3. Stress
4. Prolonged administration of drugs like steroids,
ACTH and epinephrine
Basophilia
Increase in basophil count
1. Smallpox
2. Chickenpox
3. Polycythemia vera
Basopenia
Decrease in basophil count
1. Urticaria (skin disorder)
2. Stress
3. Prolonged exposure to chemotherapy or
radiation therapy
Monocytosis
Increase in monocyte count
1. Tuberculosis
2. Syphilis
3. Malaria
4. Kala-azar
Monocytopenia
Decrease in monocyte count
1. Prolonged use of prednisone
(immunosuppressant steroid)
Lymphocytosis
Increase in lymphocyte count
1. Diphtheria
2. Infectious hepatitis
3. Mumps
4. Malnutrition
5. Rickets
6. Syphilis
7. Thyrotoxicosis
8. Tuberculosis
Lymphocytopenia Decrease in lymphocyte count
1. AIDS
2. Hodgkin’s disease
3. Malnutrition
4. Radiation therapy
5. Steroid administration.

Blood and Body Fluids
68
FUNCTIONS OF WBCs
Generally, WBCs play an important role in
defense mechanism. These cells protect the
body from invading organisms or foreign bodies
either by destroying or inactivating them.
However, in defense mechanism, each type of
WBCs acts in a different way.
NEUTROPHILS
Along with monocytes, the neutrophils provide
the first line of defense against the invading
microorganisms. Neutrophils wander freely all
over the body through the tissue.
Mechanism of Action of Neutrophils
Neutrophils are released in large number from
the blood. At the same time, new neutrophils are
also produced from the progenitor cells. All the
neutrophils move by diapedesis towards the site
of infection by means of chemotaxis.
The chemotaxis occurs due to the attraction
by some chemical substances called
chemoattractants, which are released from the
infected area. After reaching the area, the
neutrophils surround the area and get adhered
to the infected tissues. The chemoattractants
increase the adhesive nature of neutrophils so
that all the neutrophils become sticky and get
attached firmly to the infected area. Each
neutrophil can hold about 15 to 20 microorga-
nisms at a time. Now, the neutrophils start
destroying the invaders. First, these cells engulf
the bacteria and then destroy them by means
of phagocytosis (Chapter 3).
Pus and Pus Cells
Pus is the whitish-yellow fluid formed in the
infected tissue. During the battle against the
bacteria, many WBCs are killed by the toxins
released from the bacteria. The dead cells are
collected in the center of infected area. The dead
cells together with plasma leaked from the blood
vessel, liquefied tissue cells and RBCs escaped
from damaged blood vessel (capillaries)
constitute the pus.
EOSINOPHILS
The eosinophils provide defense to the body by
acting against the parasitic infections and allergic
conditions like asthma. Eosinophils are res-
ponsible for detoxification, disintegration and
removal of foreign proteins.
Mechanism of Action of Eosinophils
The eosinophils attack the invading organisms
by secreting some special type of cytotoxic
substances. These substances become lethal
and destroy the parasites. Some of these
substances are:
1. Eosinophil peroxidase
2. Major basic protein (MBP)
3. Eosinophil cationic protein (ECP)
4. Eosinophil derived neurotoxin
5. Interleukin-4 and interleukin-5.
BASOPHILS
The basophils play an important role in healing
processes and acute hypersensitivity reactions
(allergy).
Mechanism of Action of Basophils
The basophils execute the functions by releasing
some important substances from their granules
such as:
1. Heparin which is essential to prevent the intra-
vascular blood clotting
2. Histamine, bradykinin and serotonin which
produce the acute hypersensitivity reactions
by causing vascular and tissue responses.
3. Proteases and myeloperoxidase that exag-
gerate the inflammatory responses
4. Interleukin-4 which accelerates inflammatory
responses and kill the invading organisms.
Mast Cell
Mast cell is a large tissue cell resembling the
basophil. Usually these cells are found along with
the blood vessels and do not enter the blood
stream. These cells are predominantly seen in
the areas such as skin, mucosa of the lungs and
digestive tract, mouth, conjunctiva and nose.
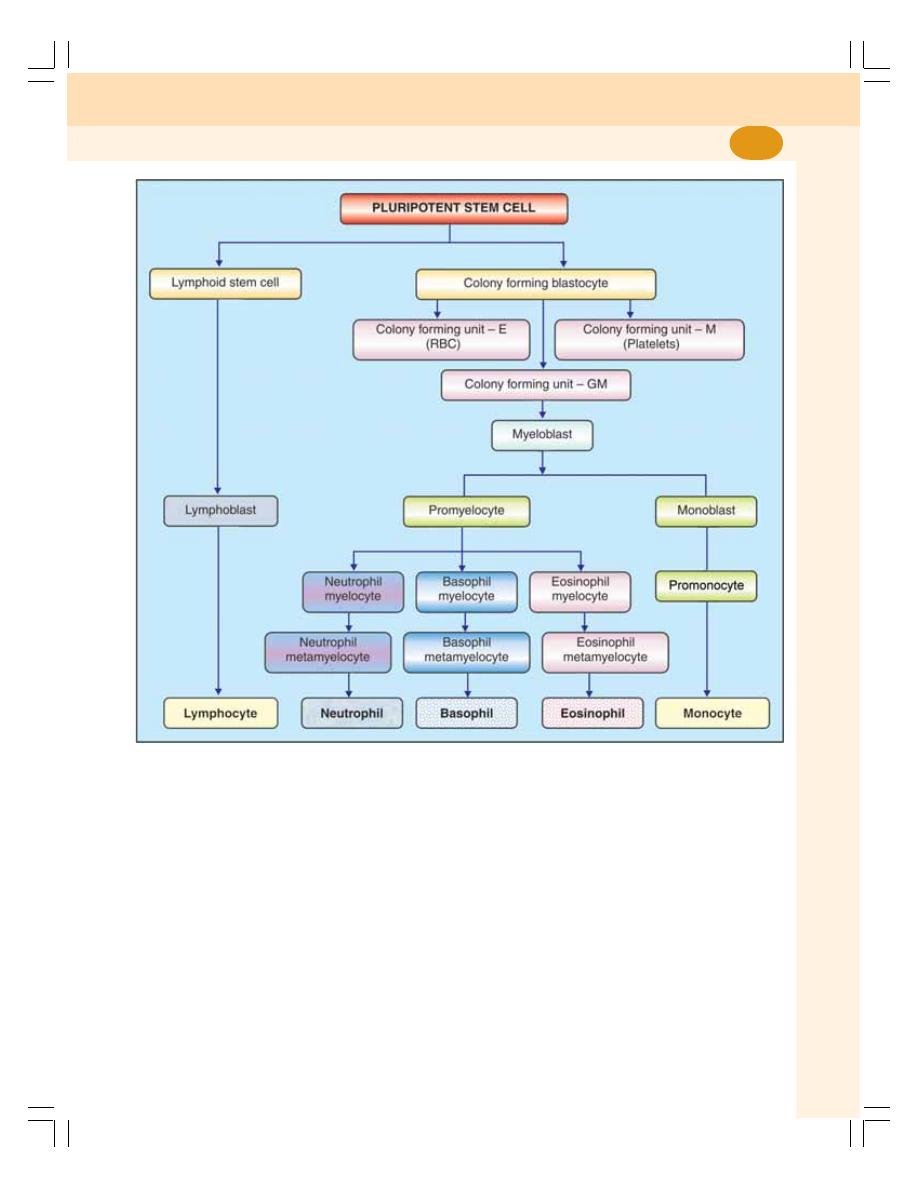
Chapter 12 White Blood Cells
69
Functions
The mast cells function along with basophils and
produce hypersensitivity reactions like allergy and
anaphylaxis. These cells act by secreting some
substances like histamine, heparin, serotonin,
hydrolytic enzymes, proteoglycans, chondroitin
sulphates, arachidonic acid derivatives such as
leukotriene C (LTC) and prostaglandin.
MONOCYTES
Monocytes are the largest cells among the
WBCs. Like neutrophils, monocytes also are
motile and phagocytic in nature. These cells
wander freely through all tissues of the body and
provide the first line of defense along with
neutrophils.
Monocytes are the precursors of the tissue
macrophages. The matured monocytes stay in
FIGURE 12-2:
Leukopoiesis

Blood and Body Fluids
70
the blood only for few hours. Afterwards these
cells enter the tissues from the blood and
become tissue macrophages. Examples of
tissue macrophages are Kupffer cells in liver,
alveolar macrophages in lungs and macro-
phages in spleen. The functions of macro-
phages are discussed in Chapter 17.
Monocytes act by secreting certain sub-
stances like interleukin-1 (IL-1), colony stimu-
lating factor (M-CSF) and platelet activating
factor (PAF).
LYMPHOCYTES
The lymphocytes are responsible for develop-
ment of immunity. Lymphocytes are classified
into two categories namely T lymphocytes and
B lymphocytes. The functions of these two types
of lymphocytes are explained in detail in
Chapter 13.
LEUKOPOIESIS
Leukopoiesis is the development and maturation
of WBCs (Fig. 12-2).
STEM CELLS
The committed pluripotent stem cell gives rise
to WBCs through various stages. The details are
given in Chapter 8.
FACTORS NECESSARY FOR
LEUKOPOIESIS
Leukopoiesis is influenced by hemopoietic
growth factors and colony stimulating factors.
Hemopoietic growth factors are discussed in
Chapter 8.
Colony Stimulating Factors
The colony stimulating factors (CSF) are pro-
teins which cause the formation of colony
forming blastocytes.
Colony stimulating factors are of three types:
1. Granulocyte CSF (G-CSF) secreted by
monocytes and endothelial cells
2. Granulocyte–Monocyte CSF (GM-CSF)
secreted by monocytes, endothelial cells and
T lymphocytes
3. Monocyte CSF (M-CSF) secreted by
monocytes and endothelial cells.
DEFINITION AND TYPES OF IMMUNITY
INNATE IMMUNITY OR NONSPECIFIC IMMUNITY
ACQUIRED IMMUNITY OR SPECIFIC IMMUNITY
DEVELOPMENT AND PROCESSING OF LYMPHOCYTES
T LYMPHOCYTES
B LYMPHOCYTES
ANTIGENS
DEFINITION AND TYPES
DEVELOPMENT OF CELL MEDIATED IMMUNITY
INTRODUCTION
ANTIGEN PRESENTING CELLS
ROLE OF HELPER T CELLS
ROLE OF CYTOTOXIC T CELLS
ROLE OF SUPPRESSOR T CELLS
ROLE OF MEMORY T CELLS
SPECIFICITY OF T CELLS
DEVELOPMENT OF HUMORAL IMMUNITY
INTRODUCTION
ROLE OF ANTIGEN PRESENTING CELLS
ROLE OF PLASMA CELLS
ROLE OF MEMORY B CELLS
ROLE OF HELPER T CELLS
ANTIBODIES
NATURAL KILLER CELL
CYTOKINES
IMMUNE DEFICIENCY DISEASES
CONGENITAL IMMUNE DEFICIENCY DISEASES
ACQUIRED IMMUNE DEFICIENCY DISEASES
AUTOIMMUNE DISEASES
Immunity
13

Blood and Body Fluids
72
DEFINITION AND TYPES OF
IMMUNITY
Immunity is defined as the capacity of the body
to resist the pathogenic agents. It is the ability
of the body to resist the entry of different types
of foreign bodies like bacteria, virus, toxic
substances, etc.
Immunity is of two types:
I.
Innate immunity
II. Acquired immunity.
INNATE IMMUNITY OR NONSPECIFIC
IMMUNITY
Innate immunity is the inborn capacity of the body
to resists the pathogens. By chance, if the
organisms enter the body, innate immunity
eliminates them before the development of any
disease.
This type of immunity represents the first line
of defense against any type of pathogens.
Therefore, it is also called nonspecific immunity.
Examples of innate immunity are:
1. Destruction of toxic substances or organisms
entering digestive tract through food by
enzymes in digestive juices
2. Destruction of bacteria by salivary lysozyme
3. Destruction of bacteria by acidity in urine and
vaginal fluid.
ACQUIRED IMMUNITY OR SPECIFIC
IMMUNITY
Acquired immunity is the resistance developed
in the body against any specific foreign body like
bacteria, viruses, toxins, vaccines or transplanted
tissues. So, this type of immunity is also known
as specific immunity.
It is the most powerful immune mechanism
that protects the body from invading organisms
or toxic substances. Lymphocytes are
responsible for acquired immunity (Fig. 13-1).
Types of Acquired Immunity
Two types of acquired immunity develop in the
body:
1. Cell mediated immunity or cellular immunity
2. Humoral immunity.
DEVELOPMENT AND PROCESSING
OF LYMPHOCYTES
In fetus, lymphocytes develop from bone marrow.
All the lymphocytes are released in the
circulation and are differentiated into two
categories:
1. T lymphocytes
2. B lymphocytes.
T LYMPHOCYTES
T lymphocytes are processed in thymus. The
processing occurs mostly during the period
between just before birth and few months after
birth.
Thymus secretes thymosin which
accelerates the proliferation and activation of
lymphocytes in thymus. It also increases the
activity of lymphocytes in lymphoid tissues.
Types of T Lymphocytes
During the processing, T lymphocytes are
transformed into four types:
1. Helper T cells or inducer T cells
2. Cytotoxic T cells or killer T cells
3. Suppressor T cells
4. Memory T cells.
Storage of T Lymphocytes
After the transformation, all the types of T
lymphocytes leave the thymus and are stored in
lymphoid tissues of lymph nodes, spleen, bone
marrow and the GI tract.
B LYMPHOCYTES
B lymphocytes were first discovered in the
bursa of Fabricius in birds hence the name B
lymphocytes. The bursa of Fabricius is a
lymphoid organ situated near the cloaca of
birds. The bursa is absent in mammals, and
the processing of B lymphocytes takes place
in bone marrow and liver.
Types of B Lymphocytes
After processing, the B lymphocytes are trans-
formed into two types:
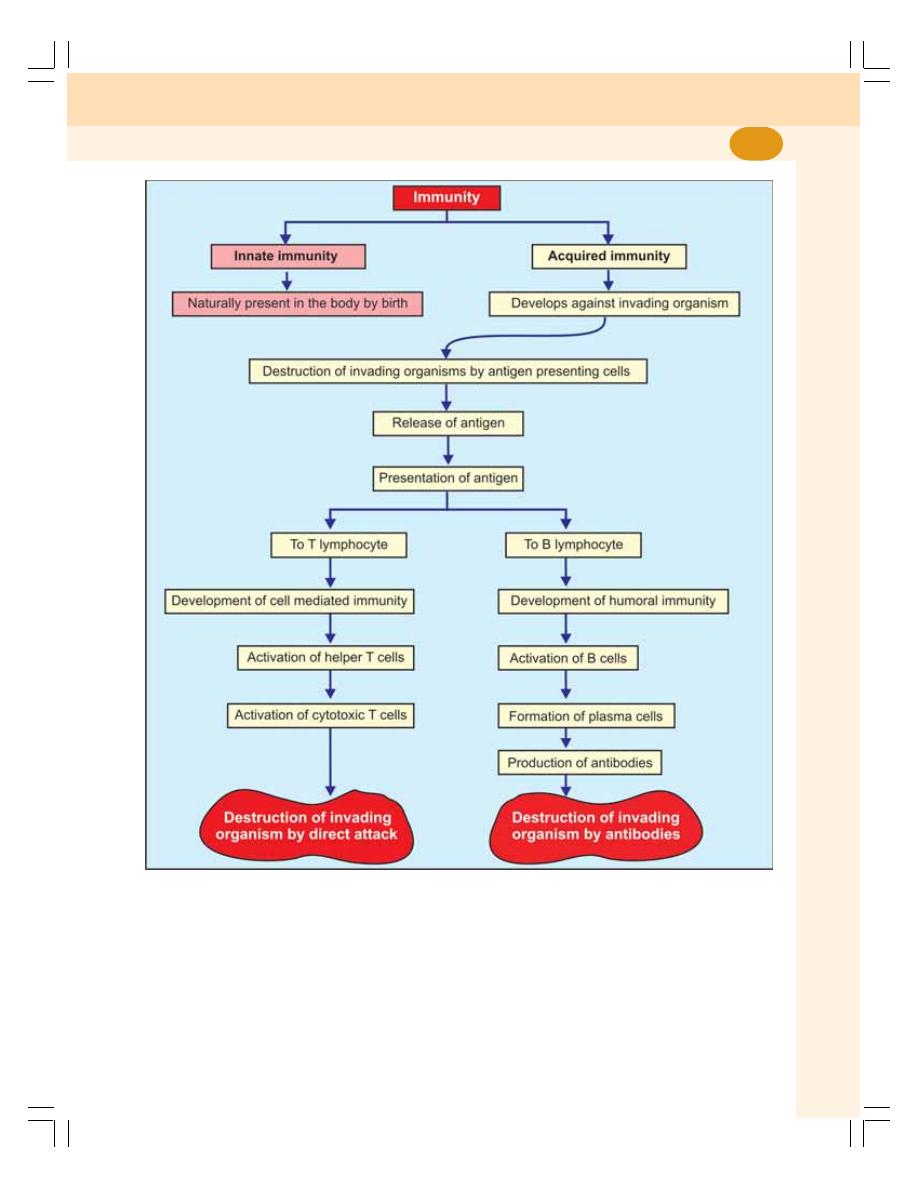
Chapter 13 Immunity
73
FIGURE: 13-1: Schematic diagram showing development of immunity
1. Plasma cells
2. Memory cells.
Storage of B Lymphocytes
After the transformation, B lymphocytes are
stored in the lymphoid tissues of lymph nodes,
spleen, bone marrow and the GI tract.
ANTIGENS
DEFINITION AND TYPES
Antigens are the substances, which induce
specific immune reactions in the body. The
antigens are mostly the conjugated proteins like
lipoproteins, glycoproteins and nucleoproteins.
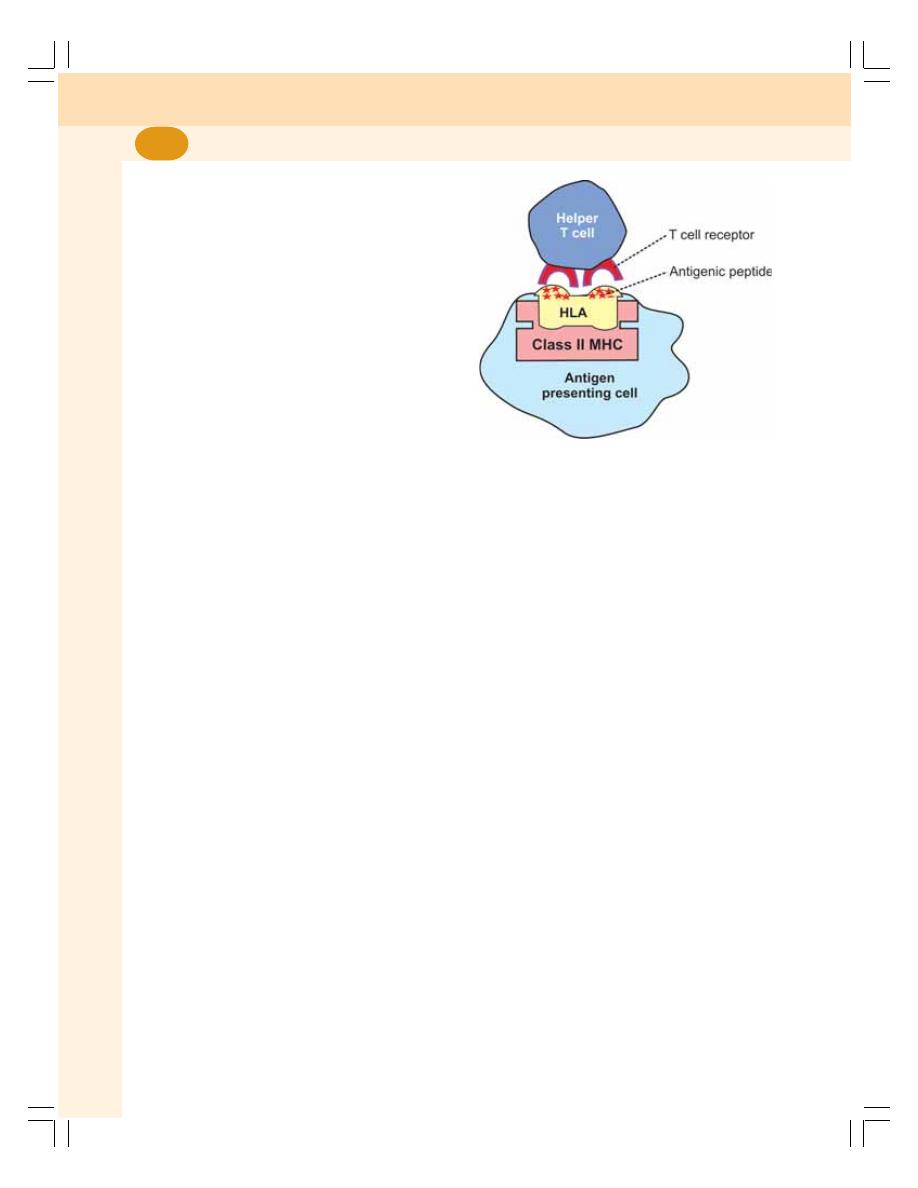
Blood and Body Fluids
74
Antigens are of two types:
1. Autoantigens or self antigens which are
present on the body's own cells like 'A' antigen
and 'B' antigen on the RBCs.
2. Foreign antigens or nonself antigens which
enter the body from outside.
DEVELOPMENT OF CELL
MEDIATED IMMUNITY
INTRODUCTION
The cell mediated immunity is offered by
T lymphocytes. It involves several types of cells
such as macrophages, T lymphocytes and natural
killer cells and hence the name cell mediated
immunity. It is also called cellular immunity or
T cell immunity. It does not involve antibodies.
Cellular immunity is the major defense
mechanism against infections by viruses, fungi
and few bacteria. It is also responsible for delayed
allergic reactions and rejection of transplanted
tissues.
Cell mediated immunity starts developing
when T cells come in contact with the antigens.
Usually, the invading microbial or nonmicrobial
organisms carry the antigenic materials. These
antigenic materials are released from invading
organisms and are presented to the helper T cells
by antigen presenting cells.
ANTIGEN PRESENTING CELLS
Antigen presenting cells are the special type of
cells in the body which induce the release of
antigenic materials from invading organisms and
later present these materials to the helper T cells.
Major antigen presenting cells are macrophages.
Dendritic cells in spleen, lymph nodes and skin
also function like antigen presenting cells.
Role of Antigen Presenting Cells
Invading foreign organisms are either engulfed by
macrophages through phagocytosis or trapped by
dendritic cells. Later, the antigen from these
organisms is digested into small peptides. The
antigenic peptide products are moved towards the
surface of the antigen presenting cells and loaded
on a genetic matter of the antigen presenting cells
called human leukocyte antigen (HLA). HLA is
present in the molecule of class II major
histocompatiblility complex (MHC) which is
situated on the surface of the antigen presenting
cells.
Presentation of Antigen
The antigen presenting cells present their class
II MHC molecules together with antigen bound
HLA to the helper T cells. This activates the helper
T cells through series of events (Fig. 13-2).
Sequence of Events during Activation of
Helper T Cells
1. Helper T cell recognizes the antigen bound
to class II MHC molecule which is displayed
on the surface of the antigen presenting cell.
It recognizes the antigen with the help of its
own surface receptor protein called T cell
receptor.
2. The recognition of the antigen by the helper
T cell initiates a complex interaction between
the helper T cell receptor and the antigen. This
reaction activates helper T cells.
3. At the same time macrophages (the antigen
presenting cells) release interleukin-1 which
FIGURE 13-2: Antigen presentation. The antigen
presenting cells present their class II MHC
molecules together with antigen bound HLA to the
helper T cells. MHC = Major histocompatiblility
complex.

Chapter 13 Immunity
75
facilitates the activation and proliferation of
helper T cells.
4. The activated helper T cells proliferate and the
proliferated helper T cells enter the circulation
for further actions.
5. Simultaneously, the antigen bound to class
II MHC molecules activates the B cells also
resulting in development of humoral immunity
(see below).
ROLE OF HELPER T CELLS
The helper T cells which enter the circulation
activate all the other T cells and B cells. The
helper T cells are of two types:
1. Helper-1 (TH1) cells
2. Helper-2 (TH2) cells.
Role of TH1 Cells
TH1 cells are concerned with cellular immunity
and secrete two substances:
i. Interleukin-2 which activates the other T
cells
ii. Gamma interferon which stimulates the
phagocytic activity of cytotoxic cells,
macrophages and natural killer (NK) cells.
Role of TH2 Cells
TH2 cells are concerned with humoral immunity
and secrete interleukin-4 and interleukin-5 which
are concerned with:
i. Activation of B cells
ii. Proliferation of plasma cells
iii. Production of antibodies by plasma cell
HLA = Human leukocyte antigen.
ROLE OF CYTOTOXIC T CELLS
The cytotoxic T cells that are activated by helper
T cells circulate through blood, lymph and
lymphatic tissues and destroy the invading
organisms by attacking them directly.
Mechanism of Action of Cytotoxic T Cells
1. The receptors situated on the outer membrane
of cytotoxic T cells bind the antigens or
organisms tightly with cytotoxic T cells.
2. Then, the cytotoxic T cells enlarge and
release cytotoxic substances like the
lysosomal enzymes which destroy the
invading organisms
3. Like this, each cytotoxic T cell can destroy
a large number of microorganisms one after
another.
Other Actions of Cytotoxic T Cells
1. The cytotoxic T cells also destroy cancer
cells, transplanted cells such as those of
transplanted heart or kidney or any other
cells, which are foreign bodies
2. Cytotoxic T cells destroy even body's own
tissues which are affected by the foreign
bodies, particularly the viruses. Many viruses
are entrapped in the membrane of affected
cells. The antigen of the viruses attracts the
T cells. And the cytotoxic T cells kill the
affected cells also along with viruses.
Because of this cytotoxic T cell is called killer
cell.
ROLE OF SUPPRESSOR T CELLS
The suppressor T cells are also called regulatory
T cells. These T cells suppress the activities of
the killer T cells. Thus, the suppressor T cells
play an important role in preventing the killer T
cells from destroying the body's own tissues
along with invaded organisms. The suppressor
cells suppress the activities of helper T cells also.
ROLE OF MEMORY T CELLS
Some of the T cells activated by an antigen do
not enter the circulation but remain in lymphoid
tissue. These T cells are called memory T cells.
In later periods, the memory cells migrate to
various lymphoid tissues throughout the body.
When the body is exposed to the same organism
for the second time, the memory cells identify
the organism and immediately activate the other
T cells. So, the invading organism is destroyed
very quickly. The response of the T cells is also
more powerful this time.

Blood and Body Fluids
76
SPECIFICITY OF T CELLS
Each T cell is designed to be activated only by
one type of antigen. It is capable of developing
immunity against that antigen only. This property
is called the specificity of T cells.
DEVELOPMENT OF HUMORAL
IMMUNITY
INTRODUCTION
Humoral immunity is the immunity mediated by
antibodies. Antibodies are secreted by B
lymphocytes and released into the blood and
lymph. The blood and lymph are the body fluids
(humours or humors in Latin). Since the B
lymphocytes provide immunity through humors,
this type of immunity is called humoral immunity
or B cell immunity.
The antibodies are the gamma globulins
produced by B lymphocytes. These antibodies
fight against the invading organisms. The humoral
immunity is the major defense mechanism
against the bacterial infection.
As in the case of cell mediated immunity, the
macrophages and other antigen presenting cells
play an important role in the development of
humoral immunity also.
ROLE OF ANTIGEN PRESENTING
CELLS
The ingestion of foreign organisms and digestion
of their antigen by the antigen presenting cells
are already explained.
Presentation of Antigen
The antigen presenting cells present their class
II MHC molecules together with antigen bound
HLA to B cells. This activates the B cells through
series of events.
Sequence of Events during Activation
of B Cells
1. The B cell recognizes the antigen bound to
class II MHC molecule which is displayed on
the surface of the antigen presenting cell. It
recognizes the antigen with the help of its own
surface receptor protein called B cell receptor.
2. The recognition of the antigen by the B cell
initiates a complex interaction between the
B cell receptor and the antigen. This reaction
activates B cells.
3. At the same time macrophages (the antigen
presenting cells) release interleukin-1 which
facilitates the activation and proliferation of B
cells.
4. The activated B cells proliferate and the
proliferated B cells carry out the further
actions.
5. Simultaneously the antigen bound to class II
MHC molecules activates the helper T cells
also resulting in development of cell mediated
immunity (already explained).
Transformation of B Cells
The proliferated B cells are transformed into two
types of cells:
1. Plasma cells
2. Memory cells.
ROLE OF PLASMA CELLS
The plasma cells destroy the foreign organisms
by producing the antibodies. Antibodies are
globulin in nature. The rate of the antibody
production is very high, i.e. each plasma cell
produces about 2000 molecules of antibodies per
second. The antibodies are also called
immunoglobulins.
The antibodies are released into lymph and
then transported into the circulation. The
antibodies are produced until the end of lifespan
of each plasma cell which may be from several
days to several weeks.
ROLE OF MEMORY B CELLS
Memory B cells occupy the lymphoid tissues
throughout the body. The memory cells are in
inactive condition until the body is exposed to
the same organism for the second time.

Chapter 13 Immunity
77
During the second exposure, the memory cells
are stimulated by the antigen and produce more
quantity of antibodies at a faster rate, than in the
first exposure. The antibodies produced during
the second exposure to the foreign antigen are
also more potent than those produced during first
exposure. This phenomenon forms the basic
principle of vaccination against the infections.
ROLE OF HELPER T CELLS
Helper T cells are simultaneously activated by
antigen. The activated helper T cells secrete two
substances called interleukin 2 and B cell growth
factor, which promote:
1. Activation of more number of B lymphocytes
2. Proliferation of plasma cells
3. Production of antibodies.
ANTIBODIES
An antibody is defined as a protein that is
produced by B lymphocytes in response to the
presence of an antigen. Antibody is globulin in
nature and it is also called immunoglobulin (Ig).
The immunoglobulins form 20 percent of the total
plasma proteins. The antibodies enter almost all
the tissues of the body.
Types of Antibodies
Five types of antibodies are identified:
1. IgA (Ig alpha)
2. IgD (Ig delta)
3. IgE (Ig epsilon)
4. IgG (Ig gamma)
5. IgM (Ig mu).
Among these antibodies, IgG forms 75
percent of the antibodies in the body.
Structure of Antibodies
Antibodies are gamma globulins and are formed
by two pairs of chains namely, one pair of heavy
or long chains and one pair of light or short chains.
Mechanism of Actions of Antibodies
The antibodies protect the body from the invading
organisms in two ways:
1. By direct actions
2. Through complement system.
1. Direct Actions of Antibodies
Antibodies directly inactivate the invading
organism by any one of the following methods:
i. Agglutination: In this, the foreign bodies
like RBCs or bacteria with antigens on
their surfaces are held together in a clump
by the antibodies.
ii. Precipitation: In this, the soluble antigens
toxin are converted into insoluble forms and
then precipitated.
iii. Neutralization: During this, the antibodies
cover the toxic sites of antigenic products.
iv. Lysis: In this, the antibodies rupture the
cell membrane of organisms and then
destroy them.
2. Actions of Antibodies through
Complement System
The complement system is the one that
enhances or accelerates various activities during
the fight against the invading organisms. It
contains plasma enzymes, which are identified
by numbers from C
1
to C
9
.
Functions of Different Antibodies
1. IgA plays a role in localized defense
mechanism in external secretions like tear
2. IgD is involved in recognition of the antigen
by B lymphocytes
3. IgE is involved in allergic reactions
4. IgG is responsible for complement fixation
5. IgM is also responsible for complement
fixation.

Blood and Body Fluids
78
Specificity of B Lymphocytes
Each B lymphocyte is designed to be activated
only by one type of antigen. It is also capable of
producing antibodies against that antigen only.
This property of B lymphocyte is called specificity.
NATURAL KILLER CELL
Natural killer (NK) cell is a large granular cell with
indented nucleus. It is considered as the third
type of lymphocyte. It is not a phagocytic cell
but its granules contain hydrolytic enzymes which
causes lysis of cells of invading organisms.
Functions of NK Cell
The NK cell:
1. Destroys the viruses
2. Destroys the viral infected or damaged cells,
which might form tumors
3. Destroys the malignant cells and prevents
development of cancerous tumors
4. Secretes cytokines such as interleukin-2,
interferons, colony stimulating factor (GM-
CSF) and tumor necrosis factor- .
CYTOKINES
Cytokines are the hormone like small proteins
acting as intercellular messengers (cell signaling
molecules) by binding to specific receptors of
target cells. These nonantibody proteins are
secreted by WBCs and some other types of
cells. Their major function is the activation and
regulation of general immune system of the body.
Cytokines are distinct from the other cell
signaling molecules such as growth factors and
hormones. Cytokines are classified into several
types:
1. Interleukins
2. Interferons
3. Tumor necrosis factors
4. Chemokines
5. Defensins
6. Cathelicidins
7. Platelet activating factor
IMMUNE DEFICIENCY DISEASES
Immune deficiency diseases are group of
diseases in which some components of immune
system is missing or defective. Normally, the
defense mechanism protects the body from
invading pathogenic organism. When the defense
mechanism fails or becomes faulty (defective),
the organisms of even low virulence produce
severe disease. The organisms, which take
advantage of defective defense mechanism, are
called opportunists.
The immune deficiency diseases caused by
such opportunists are of two types:
1. Congenital immune deficiency diseases
2. Acquired immune deficiency diseases.
CONGENITAL IMMUNE DEFICIENCY
DISEASES
Congenital diseases are inherited and occur due
to the defects in B cell, or T cell or both. The
common examples are DiGeorge's syndrome
(due to absence of thymus) and severe combined
immune deficiency (due to lymphopenia or the
absence of lymphoid tissue).
ACQUIRED IMMUNE DEFICIENCY
DISEASES
Acquired immune deficiency diseases occur due
to infection by some organisms. The most
common disease of this type is acquired immune
deficiency syndrome (AIDS).
Acquired Immune Deficiency Syndrome
(AIDS)
It is an infectious disease caused by immune
deficiency virus (HIV). AIDS is the most common
problem throughout the world because of rapid
increase in the number of victims. Infection occurs
when a glycoprotein from HIV binds to surface
receptors of T lymphocytes, monocytes,
macrophages and dendritic cells leading to
destruction of these cells. It causes slow
progressive decrease in immune function
resulting in opportunistic infections of various

Chapter 13 Immunity
79
types. The common opportunistic infections, which
kill the AIDS patient are pneumonia and skin
cancer.
AUTOIMMUNE DISEASES
Autoimmune disease is defined as condition in
which the immune system mistakenly attacks
body's own cells and tissues. Normally, an antigen
induces the immune response in the body. The
condition in which the immune system fails to
give response to an antigen is called tolerance.
This is true with respect to body's own antigens
that are called self antigens or autoantigens.
Normally, body has the tolerance against self
antigen. However, in some occasions, the
tolerance fails or becomes incomplete against self
antigen. This state is called autoimmunity and
it leads to the activation of T lymphocytes or
production of autoantibodies from B lymphocytes.
The T lymphocytes (cytotoxic T cells) or
autoantibodies attack the body's normal cells
whose surface contains the self antigen or
autoantigen.
Common Autoimmune Diseases
1. Diabetes mellitus
2. Myasthenia gravis
3. Hashimoto's thyroiditis
4. Graves' disease
5. Rheumatoid arthritis.
INTRODUCTION
STRUCTURE AND COMPOSITION
NORMAL COUNT AND VARIATIONS
PROPERTIES
FUNCTIONS
DEVELOPMENT
LIFESPAN AND FATE
APPLIED PHYSIOLOGY — PLATELET DISORDERS
Platelets
14
INTRODUCTION
Platelets or thrombocytes are the formed ele-
ments of the blood. Platelets are small colorless,
non nucleated and moderately refractive bodies
which are considered to be the fragments of cyto-
plasm.
SIZE OF PLATELETS
Diameter : 2.5 μ (2 to 4 μ)
Volume
: 7.5 cuμ (7 to 8 cuμ).
SHAPE OF PLATELETS
Normally, platelets are of several shapes, viz.
spherical or rod shaped and become oval or disk
shaped when inactivated. Sometimes, the plate-
lets have dumb-bell shape, comma shape, cigar
shape or any other unusual shape.
STRUCTURE AND COMPOSITION
Platelets are constituted by cell membrane or
surface membrane, microtubules and cytoplasm.
CELL MEMBRANE
It is 6 nm thick and contains lipids in the form of
phospholipids, cholesterol and glycolipids,
carbohydrates as glycocalyx, and glycoproteins
and proteins.
MICROTUBULES
Microtubules form a ring around cytoplasm below
the cell membrane. Microtubules are made up
of proteins called tubulin. These tubules provide
structural support for the inactivated platelets to
maintain the disk-like shape.
CYTOPLASM
The cytoplasm of the platelets contains the cellu-
lar organelles, Golgi apparatus, endoplasmic
reticulum, mitochondria, microtubule, micro-
vessels, filaments and different types of granules.
Cytoplasm also contains some chemical
substances such as:

81
Chapter 14 Platelets
Proteins
1. Contractile proteins
i. Actin and myosin which are responsible
for contraction of platelets
ii. Thrombosthenin the third contractile pro-
tein which is responsible for clot retraction
2. von Willebrand factor: Responsible for adhe-
rence of platelets
3. Fibrin stabilizing factor: A clotting factor
4. Platelet derived growth factor (PDGF):
Responsible for repair of damaged blood
vessels and wound healing.
5. Platelet activating factor (PAF): Causes
aggregation of platelets during the injury of
blood vessels
6. Vitronectin (serum spreading factor): Pro-
motes adhesion of platelets and spreading
of cells in culture
7. Thrombospondin: Inhibits angiogenesis (for-
mation of new blood vessels).
Enzymes
1. ATPase
2. Enzymes necessary for synthesis of prostag-
landins.
Hormonal Substances
1. Adrenaline
2. 5-HT (serotonin)
3. Histamine.
Other Chemical Substances
1. Glycogen
2. Substances like blood group antigens
3. Inorganic substances—calcium, copper,
magnesium and iron.
Platelet Granules
Granules present in cytoplasm of platelets are
of two types, alpha granules and dense granules.
Alpha granules contain clotting factors V and XIII,
fibrinogen and platelet derived growth factor.
Dense granules contain nucleotides, serotonin,
phospholipid, calcium and lysosomes.
NORMAL COUNT AND VARIATIONS
Normal platelet count is 2,50,000. It ranges bet-
ween 2,00,000 and 4,00,000/cumm of blood.
PHYSIOLOGICAL VARIATIONS
1. Age: Platelets are less in infants (1,50,000-
2,00,000/cumm) and reaches normal level at
3rd month after birth.
2. Sex: There is no difference in the platelet
count between males and females. In
females, it is reduced during menstruation.
3. High altitude: Platelet count increases.
4. After meals: After taking food, the platelet
count increases.
PATHOLOGICAL VARIATIONS
Refer applied physiology of this chapter.
PROPERTIES OF PLATELETS
ADHESIVENESS
Adhesiveness is the property of sticking to a
rough surface. While coming in contact with any
rough surface the platelets are activated and stick
to the surface.
AGGREGATION (GROUPING OF
PLATELETS)
Aggregation is the grouping of platelets. Activated
platelets group together and become sticky.
AGGLUTINATION
Agglutination is the clumping together of platelets.
FUNCTIONS OF PLATELETS
1. ROLE IN BLOOD CLOTTING
The platelets are responsible for the formation
of intrinsic prothrombin activator. This substance
is responsible for the onset of blood clotting
(Chapter 15).
2. ROLE IN CLOT RETRACTION
In the blood clot, the blood cells including
platelets are entrapped in between the fibrin

Blood and Body Fluids
82
threads. The cytoplasm of platelets contains the
contractile proteins namely actin, myosin and
thrombosthenin which are responsible for clot
retraction (Chapter 15).
3. ROLE IN PREVENTION OF BLOOD
LOSS (HEMOSTASIS)
Platelets accelerate hemostasis by three ways:
i. Platelets secrete 5-HT, which causes the
constriction of blood vessels
ii. Due to the adhesive property, the platelets
seal the damage in blood vessels like
capillaries
iii. By formation of temporary plug also plate-
lets seal the damage in blood vessels
(Chapter 15).
4. ROLE IN REPAIR OF RUPTURED
BLOOD VESSEL
The platelet derived growth factor (PDGF) formed
in cytoplasm of platelets is useful for the repair
of the endothelium and other structures of the
ruptured blood vessels.
5. ROLE IN DEFENSE MECHANISM
By the property of agglutination, platelets encircle
the foreign bodies and destroy them by phago-
cytosis.
DEVELOPMENT OF PLATELETS
Platelets are formed from bone marrow. The
pluripotent stem cell gives rise to the CFU-M.
This develops into megakaryocyte. The cyto-
plasm of megakaryocyte form pseudopodium.
A portion of pseudopodium is detached to
form platelet, which enters the circulation
(Fig. 8-2).
Production of platelets is influenced by
thrombopoietin. Thrombopoietin is a glycoprotein
like erythropoietin. It is secreted by liver and
kidneys.
LIFESPAN AND FATE OF PLATELETS
Average lifespan of platelets is about 10 days.
Older platelets are destroyed by tissue macro-
phage system in spleen.
APPLIED PHYSIOLOGY –
PLATELET DISORDERS
THROMBOCYTOPENIA
Decrease in platelet count is called thrombo-
cytopenia. It leads to thrombocytopenic purpura
(Chapter 15). Thrombocytopenia occurs in the
following conditions:
i. Acute infections
ii. Acute leukemia
iii. Aplastic and pernicious anemia
iv. Chickenpox
v. Smallpox
vi. Splenomegaly
vii. Scarlet fever
viii. Typhoid
ix. Tuberculosis
x. Purpura.
THROMBOCYTOSIS
The increase in platelet count is called throm-
bocytosis. It occurs in the following conditions:
i. Allergic conditions
ii. Hemorrhage
iii. Bone fractures
iv. Surgical operations
v. Splenectomy
vi. Rheumatic fever
vii. Trauma (wound or injury or damage pro-
duced by external force).
THROMBOCYTHEMIA
It is the condition with persistent and abnormal
increase in platelet count. It occurs in:
i. Carcinoma
ii. Chronic leukemia
iii. Hodgkin’s disease.
GLANZMANN THROMBASTHENIA
It is an inherited hemorrhagic disorder caused
by structural or functional abnormality of plate-
lets. It leads to thrombasthenic purpura (Chapter
15).
HEMOSTASIS
DEFINITION
STAGES OF HEMOSTASIS
DEFINITION OF BLOOD COAGULATION
FACTORS INVOLVED IN BLOOD CLOTTING
SEQUENCE OF CLOTTING MECHANISM
ENZYME CASCADE THEORY
STAGE 1: FORMATION OF PROTHROMBIN ACTIVATOR
STAGE 2: CONVERSION OF PROTHROMBIN INTO THROMBIN
STAGE 3: CONVERSION OF FIBRINOGEN INTO FIBRIN
BLOOD CLOT
DEFINITION
CLOT RETRACTION
FIBRINOLYSIS
ANTICLOTTING MECHANISM IN THE BODY
ANTICOAGULANTS
HEPARIN
COUMARIN DERIVATIVES
EDTA
OXALATE COMPOUNDS
CITRATES
OTHER SUBSTANCES
PHYSICAL METHODS TO PREVENT BLOOD CLOTTING
PROCOAGULANTS
TESTS FOR CLOTTING
BLEEDING TIME
CLOTTING TIME
PROTHROMBIN TIME
APPLIED PHYSIOLOGY
BLEEDING DISORDERS
THROMBOSIS
Hemostasis and
Coagulation of Blood
15
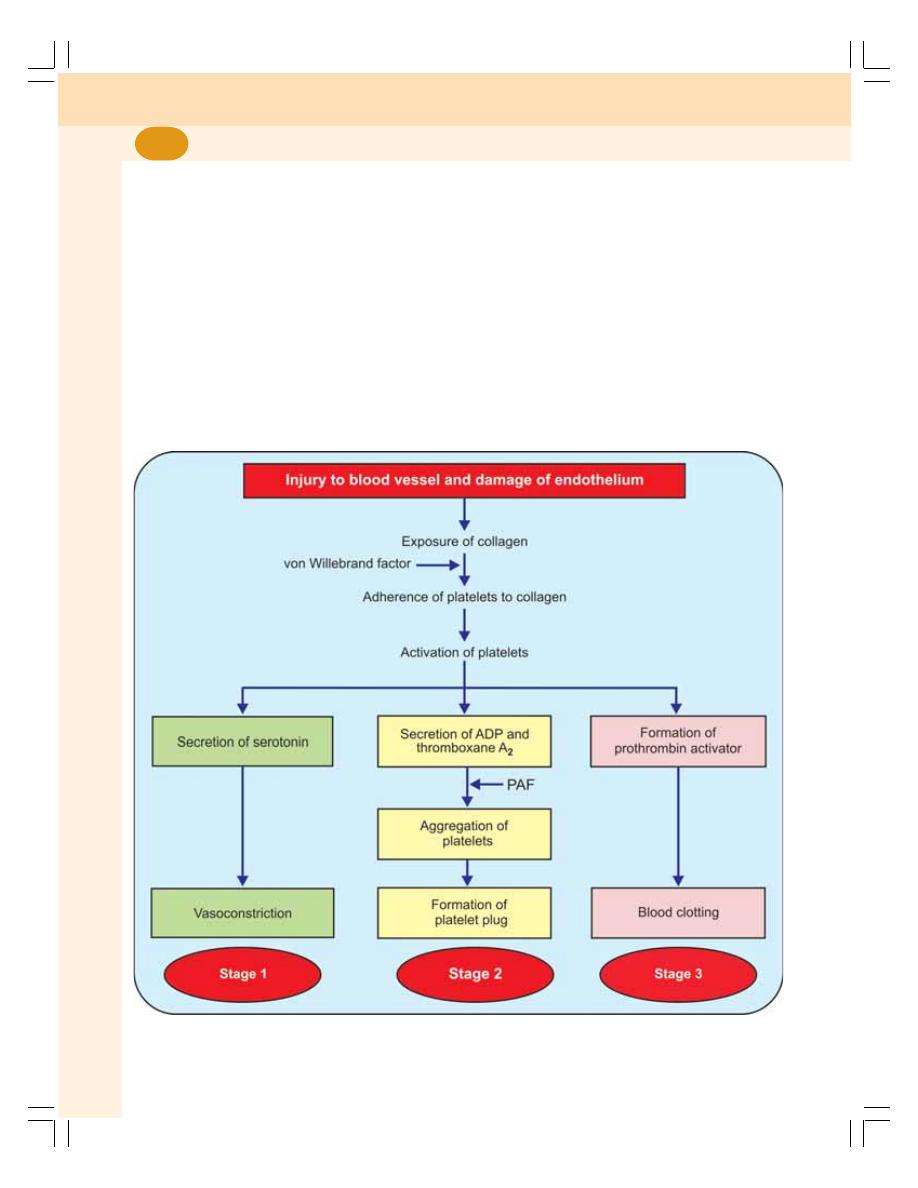
Blood and Body Fluids
84
HEMOSTASIS
DEFINITION
Hemostasis is defined as arrest or stoppage of
bleeding.
STAGES OF HEMOSTASIS
When a blood vessel is injured, the injury initiates
a series of reactions resulting in hemostasis. It
occurs in three stages:
1. Vasoconstriction
2. Platelet plug formation
3. Coagulation of blood.
1. Vasoconstriction
Immediately after injury, the blood vessel
constricts and decreases the loss of blood from
damaged portion. Usually, arterioles and small
arteries constrict. The vasoconstriction is purely
a local phenomenon. When the blood vessels are
cut, the endothelium is damaged and the collagen
is exposed. The platelets adhere to this collagen,
and get activated. The activated platelets secrete
serotonin and other vasoconstrictor substances
which cause constriction of the blood vessels
(Fig. 15-1). The adherence of platelets to the
collagen is accelerated by von Willebrand factor.
FIGURE 15-1: States of hemostasis. PAF = platelet activating factor.

Chapter 15 Hemostasis and Coagulation of Blood
85
2. Formation of Platelet Plug
The platelets get adhered to the collagen of
ruptured blood vessel and secrete ADP and
thromboxane A
2
. These two substances attract
more and more platelets and activate them. All
these platelets aggregate together and form a
loose temporary platelet plug or temporary
hemostatic plug, which closes the injured part
of the vessel and prevents further blood loss. The
platelet aggregation is accelerated by platelet
activating factor (PAF).
3. Coagulation of Blood
During this process, the fibrinogen is converted
into fibrin. The fibrin threads get attached to the
loose platelet plug, which blocks the ruptured part
of blood vessels and prevents further blood loss
completely.
DEFINITION OF BLOOD
COAGULATION
Coagulation or clotting is defined as the process
in which blood looses its fluidity and becomes a
jelly like mass few minutes after it is shed out
or collected in a container.
FACTORS INVOLVED IN BLOOD
CLOTTING
Coagulation of blood occurs through a series of
reactions due to the activation of a group of
substances. The substances necessary for
clotting are called clotting factors.
Thirteen clotting factors are identified:
Factor I
Fibrinogen
Factor II
Prothrombin
Factor III
Thromboplastin (Tissue factor)
Factor IV
Calcium
Factor V
Labile factor (Proaccelerin or
Accelerator globulin)
Factor VI
Presence has not been proved
Factor VII
Stable factor
Factor VIII Antihemophilic factor (Antihemophilic
globulin)
Factor IX
Christmas factor
Factor X
Stuart-Prower factor
Factor XI
Plasma thromboplastin antecedent
Factor XII
Hegman factor (Contact factor)
Factor XIII
Fibrin stabilizing factor (Fibrinase).
The clotting factors were named either after
the scientists who discovered them or as per the
activity except factor IX. Factor IX or Christmas
factor was named after the patient in whom it was
discovered.
SEQUENCE OF CLOTTING
MECHANISM
ENZYME CASCADE THEORY
Most of the clotting factors are proteins in the
form of enzymes. Normally, all the factors are
present in the form of inactive proenzyme. These
proenzymes must be activated into enzymes to
enforce clot formation. It is carried out by series
of proenzyme-enzyme conversion reactions. The
first one of the series is converted into an active
enzyme that activates the second one, which
activates the third one; this continues till the final
active enzyme thrombin is formed.
Enzyme cascade theory explains how various
reactions involved in the conversion of proenzymes
to active enzymes take place in the form of a
cascade. Cascade refers to a process that
occurs through a series of steps, each step
initiating the next, until the final step is reached.
Stages of Blood Clotting
In general, blood clotting occurs in three stages:
1. Formation of prothrombin activator
2. Conversion of prothrombin into thrombin
3. Conversion of fibrinogen into fibrin.
STAGE 1: FORMATION OF
PROTHROMBIN ACTIVATOR
Blood clotting commences with the formation of
a substance called prothrombin activator. Its
formation is initiated by substances produced
either within the blood itself or outside the
blood.
Thus, formation of prothrombin activator
occurs through two pathways:
A. Intrinsic pathway
B. Extrinsic pathway.

Blood and Body Fluids
86
Intrinsic Pathway for the Formation of
Prothrombin Activator
In this, the formation of prothrombin activator is
initiated by platelets, which are within the blood
itself (Fig. 15-2).
Sequence of events in intrinsic pathway
i. During the injury, the blood vessel is
ruptured. The endothelium is damaged and
collagen beneath the endothelium is
exposed
ii. When platelet comes in contact with
collagen of damaged blood vessel, it gets
activated and releases phospholipids
iii. When factor XII (Hegman factor) comes in
contact with collagen, it is converted into
activated factor XII in the presence of
kallikrein and high molecular weight
(HMW) kinogen
vi. The activated factor XII converts factor XI
into activated factor XI in the presence of
HMW kinogen
v. The activated factor XI activates factor IX
in the presence of factor IV (calcium)
vi. Activated factor IX activates factor X in the
presence of factor VIII and calcium
vii. Now the activated factor X reacts with
platelet phospholipid and factor V to form
prothrombin activator. This needs presence
of calcium ions
viii. Factor V is also activated by positive
feedback effect of thrombin (see below).
Extrinsic Pathway for the Formation of
Prothrombin Activator
In this, the formation of prothrombin activator is
initiated by the tissue thromboplastin which is
formed from the injured tissues.
Sequence of events in extrinsic pathway
i. The tissues that are damaged during injury
release factor III, i.e. tissue thromboplastin.
The thromboplastin contains proteins,
phospholipid and glycoprotein, which act
as proteolytic enzymes
ii. The glycoprotein and phospholipid
components of thromboplastin convert
factor X into activated factor X, in the
presence of factor VII
iii. The activated factor X reacts with factor
V and phospholipid component of tissue
thromboplastin to form prothrombin
activator. This reaction requires the
presence of calcium ions.
STAGE 2: CONVERSION OF
PROTHROMBIN INTO THROMBIN
Blood clotting is all about thrombin formation.
Once thrombin is formed, it definitely leads to
clot formation.
Sequence of Events in Stage 2
i. Prothrombin activator that is formed in
intrinsic and extrinsic pathways converts
prothrombin into thrombin in the presence
of calcium ions (Factor IV)
ii. Once formed, thrombin initiates the
formation of more thrombin molecules.
The initially formed thrombin activates
Factor V. Factor V in turn accelerates
formation of both extrinsic and intrinsic
prothrombin activator which converts
prothrombin into thrombin. This effect of
thrombin is called positive feedback effect
(Fig. 15-2).
STAGE 3: CONVERSION OF
FIBRINOGEN INTO FIBRIN
The final stage of blood clotting involves the
conversion of fibrinogen into fibrin by thrombin.
Sequence of Events in Stage 3
i. Thrombin converts fibrinogen into activated
fibrinogen which is called fibrin monomer.
ii. Fibrin monomer polymerizes with other
monomer molecules and form loosely
arranged strands of fibrin
iii. Later these loose strands are modified into
dense and tight fibrin threads by fibrin
stabilizing factor (factor XIII) in the
presence of calcium ions (Fig. 15-2). All
the tight fibrin threads are aggregated to
form a meshwork of stable clot.
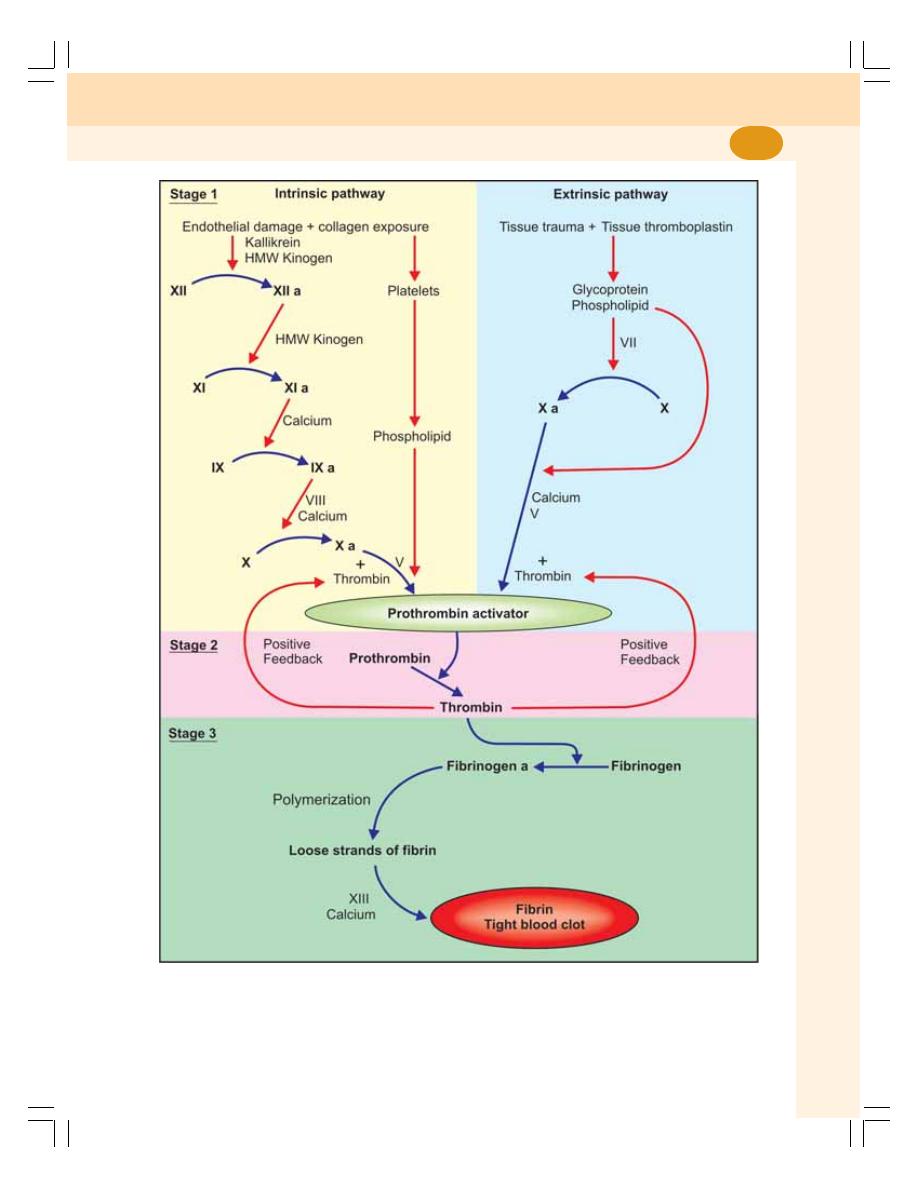
Chapter 15 Hemostasis and Coagulation of Blood
87
FIGURE 15-2: Stages of blood coagulation.
a = activated + = thrombin induces formation of more thrombin (positive feedback)

Blood and Body Fluids
88
BLOOD CLOT
DEFINITION
Blood clot is defined as the mesh of fibrin
entangling RBCs, WBCs and platelets.
CLOT RETRACTION
After the formation, the blood clot starts
contracting. And after about 30 to 45 minutes,
the straw colored serum oozes out of the clot.
The process involving the contraction of blood clot
and oozing of serum is called clot retraction.
The contractile proteins namely, actin, myosin
and thrombosthenin in the cytoplasm of platelets
are responsible for clot retraction.
FIBRINOLYSIS
The lysis of blood clot inside the blood vessel is
called fibrinolysis. It helps to remove the clot from
the lumen of the blood vessel. This process
requires a substance called plasmin or fibrinolysin.
Plasmin is formed from inactivated
glycoprotein called plasminogen. Plasminogen is
synthesized in liver and it is incorporated with
other proteins in the blood clot. Plasminogen is
converted into plasmin by tissue plasminogen
activator (t-PA), lysosomal enzymes and thrombin.
Plasmin causes lysis of clot by dissolving and
digesting the fibrin threads.
Significance of Lysis of Clot
In vital organs, particularly the heart, the blood
clot obstructs the minute blood vessel leading
to myocardial infarction. The lysis of blood clot
allows reopening of affected blood vessels and
prevents the development of infarction.
The fibrinolytic enzymes like streptokinase are
used for the lysis of blood clot during the
treatment in early stages of myocardial infarction.
ANTICLOTTING MECHANISM IN
THE BODY
Under physiological conditions, intravascular
clotting does not occur. It is because of the
presence of some physicochemical factors in the
body.
1. Physical Factors
i. Continuous circulation of blood
ii. Smooth endothelial lining of the blood
vessels.
2. Chemical Factors
i. Presence of natural anticoagulant called
heparin that is produced by the liver
ii. Production of thrombomodulin by
endothelium of the blood vessels (except
in brain capillaries). Thrombomodulin is a
thrombin binding protein. It binds with
thrombin and forms a thrombomodulin -
thrombin complex. This complex activates
protein-C. Activated protein-C along with
its cofactor protein-S inactivates Factor V
and Factor VIII. Inactivation of these two
clotting factors prevents clot formation
iii. All the clotting factors are in inactive state.
ANTICOAGULANTS
The substances, which prevent or postpone
coagulation of blood, are called anticoagulants.
Anticoagulants are of three types:
1. Anticoagulants used to prevent blood clotting
inside the body, i.e. in vivo
2. Anticoagulants used to prevent clotting of
blood that is collected from the body, i.e. in
vitro
3. Anticoagulants used to prevent blood clotting
both in vivo and in vitro.
1. HEPARIN
Heparin is a naturally produced anticoagulant in
the body. It is produced by mast cells which are
the wandering cells situated immediately outside
the capillaries in many tissues or organs that
contain more connective tissue. These cells are
abundant in liver and lungs. Basophils also
secrete heparin.
Heparin is a conjugated polysaccharide. The
commercial heparin is prepared from the liver and
other organs of animals. The commercial
preparation is available in liquid form or dry form
as sodium, calcium, ammonium or lithium salts.
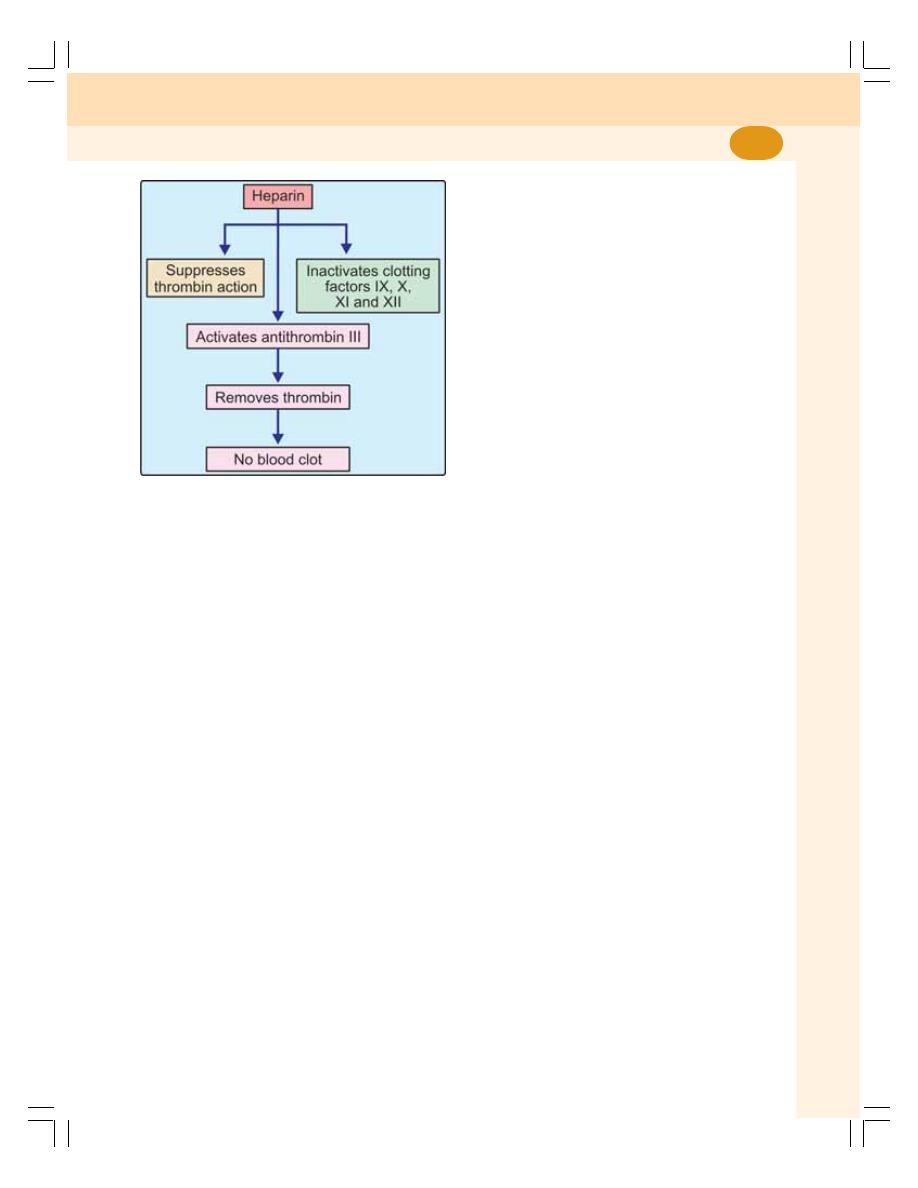
Chapter 15 Hemostasis and Coagulation of Blood
89
Mechanism of Action of Heparin
Heparin:
i. Prevents blood clotting by its antithrombin
activity. It directly suppresses the activity
of thrombin
ii. Combines with antithrombin III (a protease
inhibitor present in circulation) and removes
thrombin from circulation
iii. Activates antithrombin III
iv. Inactivates the active form of other clotting
factors like IX, X, XI and XII (Fig. 15-3).
Uses of Heparin
Heparin is used as an anticoagulant both in vivo
and in vitro.
Clinical use
Intravenous injection of heparin (0.5 to 1 mg/kg
body weight) postpones clotting for 3 to 4 hours
(until it is destroyed by the enzyme heparinase).
So, it is widely used as an anticoagulant in clinical
practice for many purposes such as:
i. To prevent intravascular blood clotting
during surgery
ii. During dialysis when blood is passed
through artificial kidney
iii. During cardiac surgery, that involves
passing the blood through heart lung
machine
iv. To preserve the blood before transfusion.
Use in the laboratory
Heparin is also used as anticoagulant in vitro
while collecting blood for various investigations.
Heparin is the most expensive anticoagulant.
2. COUMARIN DERIVATIVES
Dicoumoral and warfarin are the derivatives of
coumarin.
Mechanism of Action
The coumarin derivatives prevent blood clotting
by inhibiting the action of vitamin K. Vitamin K
is essential for the formation of various clotting
factors namely, II, VII, IX and X.
Uses
Dicoumoral and warfarin are the commonly used
oral anticoagulants in clinical practice (in vivo).
3. EDTA
Ethylenediaminetetra acetic acid (EDTA) is a
strong anticoagulant. It is available in two forms:
i. Disodium salt (Na
2
EDTA)
ii. Tripotassium salt (K
3
EDTA).
Mechanism of Action
These substances prevent blood clotting by
removing calcium from blood.
Uses
EDTA is used as an anticoagulant both in vivo
and in vitro.
i. It is administered intravenously in cases
of lead poisoning (in vivo)
ii. It is also used as an anticoagulant in the
laboratory (in vitro).
FIGURE 15-3: Mechanism of action of heparin

Blood and Body Fluids
90
4. OXALATE COMPOUNDS
Oxalate compounds prevent coagulation by
forming calcium oxalate, which is precipitated
later. Thus, these compounds reduce the blood
calcium level.
Earlier sodium and potassium oxalates were
used. Nowadays, mixture of ammonium oxalate
and potassium oxalate in the ratio of 3:2 is used.
Each salt is an anticoagulant by itself. But
potassium oxalate alone causes shrinkage of
RBCs. Ammonium oxalate alone causes
swelling of RBCs. But together, these
substances do not alter the cellular activity.
Mechanism of Action
Oxalate combines with calcium and forms
insoluble calcium oxalate. Thus, oxalate removes
calcium from blood and lack of calcium prevents
coagulation.
Uses
Oxalate compounds are used as in vitro
anticoagulants. Oxalate is poisonous so it cannot
be used in vivo.
5. CITRATES
Sodium, ammonium and potassium citrates are
used as anticoagulants.
Mechanism of Action
Citrate combines with calcium in blood to form
insoluble calcium citrate. Like oxalate, citrate also
removes calcium from blood and prevents
coagulation.
Uses
Citrates are used as an anticoagulant both in vivo
and in vitro.
i. Used to store blood in the blood bank. It
is available in two forms:
a. Acid citrate dextrose (ACD)
b. Citrate phosphate dextrose (CPD)
ii. Used in laboratory in vitro or RBC and
platelet counts.
6. OTHER SUBSTANCES WHICH
PREVENT BLOOD CLOTTING
Peptone, proteins from venom of copperhead
snake and hirudin (from leach) are the known
anticoagulants.
PHYSICAL METHODS TO
PREVENT BLOOD CLOTTING
The coagulation of blood is postponed or
prevented by the following physical methods:
1. COLD
Reducing the temperature to about 5°C
postpones coagulation of blood.
2. COLLECTING BLOOD IN A
CONTAINER WITH SMOOTH SURFACE
Collecting the blood in a container with smooth
surface like a silicon coated container prevents
clotting. The smooth surface inhibits the activation
of factor XII and platelets. So, the formation of
prothrombin activator is prevented.
PROCOAGULANTS
Procoagulants or hemostatic agents are the
substances, which accelerate the process of
blood coagulation. Procoagulants are:
1. Thrombin
2. Snake venom
3. Extracts of lungs and thymus
4. Sodium or calcium alginate
5. Oxidized cellulose
TESTS FOR CLOTTING
1. BLEEDING TIME
Bleeding time is the time interval from oozing of
blood after a cut or injury till arrest of bleeding.
Usually, it is determined by Duke method using
blotting paper or filter paper. Its normal duration
is 3-6 minutes. It is prolonged in purpura.
2. CLOTTING TIME
Clotting time is the time interval from oozing of
blood after a cut or injury till the formation of clot.

Chapter 15 Hemostasis and Coagulation of Blood
91
It is usually determined by capillary tube method.
Its normal duration is 3-8 minutes. And it is
prolonged in hemophilia.
3. PROTHROMBIN TIME
It is the time taken by blood to clot after adding
tissue thromboplastin to it. Blood is collected and
oxalated so that, the calcium is precipitated and
prothrombin is not converted into thrombin. Thus,
the blood clotting is prevented. Then a large
quantity of tissue thromboplastin with calcium is
added to this blood. Calcium nullifies the effect
of oxalate. The tissue thromboplastin activates
prothrombin and blood clotting occurs.
During this procedure, the time taken by blood
to clot after adding tissue thromboplastin is
determined. Prothrombin time indicates the total
quantity of prothrombin present in the blood.
The normal duration of prothrombin time is
about 12 seconds. It is prolonged in deficiency
of prothrombin and other factors like factors I, V,
VII and X. However, it is normal in hemophilia.
APPLIED PHYSIOLOGY
BLEEDING DISORDERS
Bleeding disorders are the diseases charac-
terized by prolonged bleeding time or clotting time.
The bleeding disorders are of three types:
1. Hemophilia
Hemophilia is a group of sex linked inherited blood
disorders characterized by prolonged clotting
time. In this disorder, males are affected and the
females are the carriers. Because of prolonged
clotting time, even a mild trauma causes excess
bleeding which can lead to death. Damage of
skin while falling or extraction of a tooth may
cause excess bleeding for few weeks. Easy
bruising and hemorrhage in muscles and joints
are also common in this disease.
Cause for hemophilia
Lack of prothrombin activator is the cause for
hemophilia. The formation of prothrombin activator
is affected due to the deficiency of factor VIII, IX
or XI.
Types of hemophilia
Depending upon the deficiency of the factor
involved, hemophilia is classified into three types:
i. Hemophilia A or classic hemophilia that
is due to the deficiency of factor VIII. 85
percent of people with hemophilia are
affected by hemophilia A.
ii. Hemophilia B or Christmas disease which
is due to the deficiency of factor IX. 15
percent of people with hemophilia are
affected by hemophilia B.
iii. Hemophilia C which is due to the
deficiency of factor XI. It is a very rare blood
disorder.
2. Purpura
It is a disorder characterized by prolonged
bleeding time. However, the clotting time is
normal. The characteristic feature of this
disease is spontaneous bleeding under the skin
from ruptured capillaries. It causes small tiny
hemorrhagic spots under the skin which are
called purpuric spots (purple colored patch like
appearance). That is why this disease is called
purpura.
Types and causes of purpura
The purpura is classified into different types
depending upon the causes.
Thrombocytopenic purpura
Thrombocytopenic purpura is due to the deficiency
of platelets (thrombocytopenia). In bone marrow
disease, platelet production is affected leading
to deficiency of platelets.
Idiopathic thrombocytopenic purpura
Purpura due to some unknown cause is called
idiopathic thrombocytopenic purpura. It is
believed that platelet count decreases due to the
development of antibodies against platelets,
which occurs after blood transfusion.

Blood and Body Fluids
92
Thrombasthenic purpura
It is due to structural or functional abnormality
of platelets. However, the platelet count is normal.
It is characterized by normal clotting time, normal
or prolonged bleeding time but defective clot
retraction.
3. von Willebrand Disease
von Willebrand disease is a bleeding disorder
characterized by excess bleeding even with a
mild injury. It is due to inherited deficiency of von
Willebrand factor which is a protein secreted by
endothelium of damaged blood vessels and
platelets. This protein is responsible for adherence
of platelets to endothelium of blood vessels during
hemostasis after an injury. It is also responsible
for the survival and maintenance of factor VIII in
plasma.
The deficiency of von Willebrand factor
suppresses platelet adhesion. It also causes
deficiency of factor VIII. This results in excess
bleeding which resembles the bleeding that
occurs during platelet dysfunction or hemophilia.
THROMBOSIS
Thrombosis or intravascular blood clotting refers
to coagulation of blood inside the blood vessels.
Normally, blood does not clot in the blood vessel
because of some factors which are already
explained. But some abnormal conditions can
cause thrombosis.
Causes of Thrombosis
1. Injury to blood vessels
2. Roughened endothelial lining
3. Sluggishness of blood flow
4. Agglutination of RBCs
5. Poisons like snake venom, mercury, and
arsenic compounds
6. Congenital absence of protein C.
Complications of Thrombosis
1. Thrombus
During thrombosis, lumen of blood vessels is
occluded. The solid mass of platelets, red cells
and/or clot, which obstructs the blood vessel, is
called thrombus. The thrombus formed due to
agglutination of RBC is called agglutinative
thrombus.
2. Embolism and embolus
Embolism is the process in which the thrombus
or part of it is detached and carried in bloodstream
and occludes the small blood vessels resulting
in arrests of blood flow to any organ or region of
the body. Embolus is the thrombus or part of it,
which arrests the blood flow. The obstruction of
blood flow by embolism is common in lungs
(pulmonary embolism), brain (cerebral embolism)
or heart (coronary embolism).
3. Ischemia
Insufficient blood supply to an organ or area of
body by the obstruction of blood vessels is called
ischemia. Ischemia results in tissue damage
because of hypoxia (lack of oxygen). Ischemia
also causes discomfort, pain and tissue death.
Death of body tissue is called necrosis.
4. Necrosis and infarction
Necrosis is a general term that refers to tissue
death caused by loss of blood supply, injury,
infection, inflammation, physical agents or
chemical substances.
Infarction means the tissue death due to loss
of blood supply. Loss blood supply is usually
caused by occlusion of an artery by thrombus
or embolus and sometimes by atherosclerosis
(Chapter 46). The area of tissue that undergoes
infarction is called infarct. Infarction commonly
occurs in heart, brain, lungs, kidneys and spleen.
INTRODUCTION
ABO BLOOD GROUPS
LANDSTEINER'S LAW
BLOOD GROUP SYSTEMS
ABO SYSTEM
DETERMINATION OF ABO GROUP
IMPORTANCE OF ABO GROUPS IN BLOOD TRANSFUSION
MATCHING AND CROSS MATCHING
INHERITANCE OF ABO AGGLUTINOGENS AND AGGLUTININS
TRANSFUSION REACTIONS DUE TO ABO INCOMPATIBILITY
Rh FACTOR
INHERITANCE OF Rh ANTIGEN
TRANSFUSION REACTIONS DUE TO Rh INCOMPATIBILITY
HEMOLYTIC DISEASE OF FETUS AND NEWBORN — ERYTHROBLASTOSIS FETALIS
OTHER BLOOD GROUPS
IMPORTANCE OF KNOWING BLOOD GROUP
BLOOD TRANSFUSION
INTRODUCTION
BLOOD SUBSTITUTES
EXCHANGE TRANSFUSION
AUTOLOGOUS BLOOD TRANSFUSION
Blood Groups and
Blood Transfusion
16
INTRODUCTION
Blood groups are determined by the presence of
antigen in RBC membrane. When blood from two
individuals is mixed, sometimes clumping
(agglutination) of RBCs occurs. This clumping is
because of the immunological reactions. But, why
clumping occurs in some cases and not in other
cases remained a mystery until the discovery of
blood groups by the Austrian Scientist, Karl
Landsteiner in 1901. He was honored with Nobel
Prize in 1930 for this discovery.
ABO BLOOD GROUPS
Determination of blood groups depends upon the
immunological reaction between antigen and
antibody. Landsteiner found two antigens on the
Blood and Body Fluids
94
surface of RBCs and named them as A antigen
and B antigen. These antigens are also called
agglutinogens because of their capacity to cause
agglutination of RBCS. He noticed the
corresponding antibodies or agglutinins in the
plasma and named them anti A or
α antibody and
anti B or
β antibody. However, a particular
agglutinogen and the corresponding agglutinin
cannot be present together. If present, it causes
clumping of the blood. Based on this, Landsteiner
classified the blood groups. Later it has become
the "Landsteiner's law" for grouping the blood.
LANDSTEINER'S LAW
Landsteiner's law states that:
1. If a particular antigen (agglutinogen) is present
in the RBCs, corresponding antibody
(agglutinin) must be absent in the serum.
2. If a particular antigen is absent in the RBCs,
the corresponding antibody must be present
in the serum.
Though the second part of Landsteiner's law
is a fact, it is not applicable to Rh factor.
BLOOD GROUP SYSTEMS
More than 20 genetically determined blood group
systems are known today. But, Landsteiner
discovered two blood group systems called ABO
system and Rh system. These two blood group
systems are the most important ones that are
determined before blood transfusions.
ABO SYSTEM
Based on the presence or absence of antigen A
and antigen B, blood is divided into four groups:
1. 'A' group
2. 'B' group
3. 'AB' group
4. 'O' group.
The blood having antigen A is called A group.
This group has
β antibody in the serum. The blood
with antigen B and
α antibody is called B group.
If both the antigens are present, the blood group
is called AB group and serum of this group does
not contain any antibody. If both antigens are
absent, the blood group is called O group and
both
α and β antibodies are present in the serum.
The antigens and antibodies present in different
groups of ABO system are given in Table 16-1.
Percentage of people among Asian and European
population belonging to different blood group is
given in Table 16-2.
"A" group has two subgroups namely "A
1
" and
"A
2
". Similarly, "AB" group has two subgroups
namely "A
1
B" and "A
2
B".
DETERMINATION OF THE ABO GROUP
Determination of the ABO group is also called
blood grouping, blood typing or blood matching.
Principle of Blood Typing — Agglutination
The blood typing is done on the basis of
agglutination. Agglutination means the
collection of separate particles like RBCs into
clumps or masses. Agglutination occurs if an
antigen is mixed with its corresponding
antibody which is called isoagglutinin.
Agglutination occurs when A antigen is mixed
with anti A or when B antigen is mixed with
anti B.
Requisites for Blood Typing
To determine the blood group of a person, a
suspension of his RBC and testing antisera are
TABLE 16-1: Antigen and antibody present in
ABO blood groups
Group
Antigen in RBC
Antibody in serum
A
A
Anti B (
β)
B
B
Anti A (
α)
AB
A and B
No antibody
O
No antigen
Anti A and Anti B
TABLE 16-2: Percentage of people having
different blood groups
Population
A
B
AB
O
Europeans
42
9
3
46
Asians
25
25
5
45
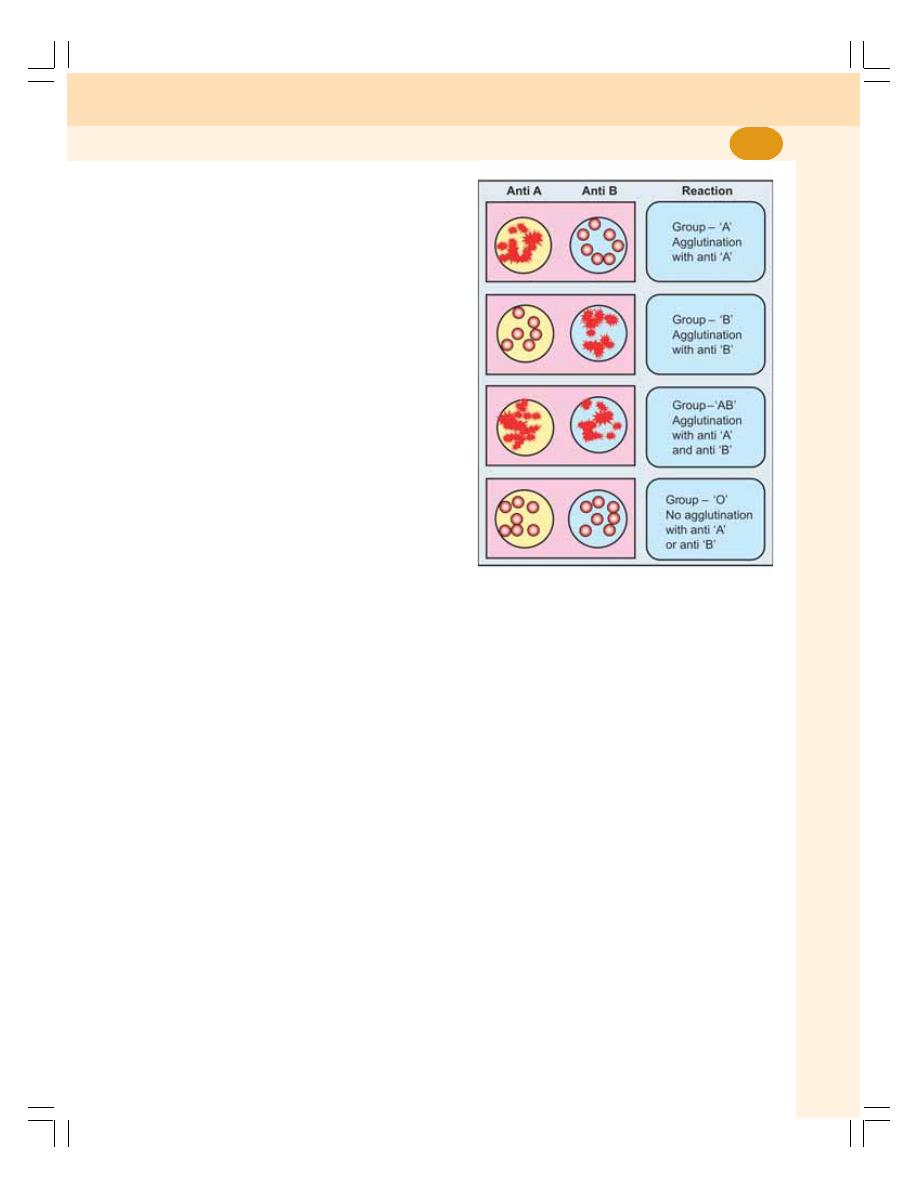
Chapter 16 Blood Groups and Blood Transfusion
95
required. Suspension of RBC is prepared by
mixing blood drops with isotonic saline (0.9%).
The test sera are:
1. Antiserum A, containing anti A
2. Antiserum B, containing anti B.
Procedure
1. One drop of antiserum A is placed on one end
of a glass slide (or a tile) and one drop of
antiserum B on the other end
2. One drop of RBC suspension is mixed with
each antiserum. The slide is slightly rocked
for 2 minutes. The presence or absence of
agglutination is observed by naked eyes and
if necessary it is confirmed by using micro-
scope
3. Presence of agglutination is confirmed by the
presence of thick masses (clumping) of RBCs
4. Absence of agglutination is confirmed by clear
mixture with dispersed RBCs.
Results
1. If agglutination occurs with antiserum A: The
antiserum A contains anti A or
α antibody. The
agglutination occurs if the RBC contains A
antigen. So, the blood group is A (Fig. 16-1).
2. If agglutination occurs with antiserum B: The
antiserum B contains anti B or
β antibody. The
agglutination occurs if the RBC contains B
antigen. So, the blood group is B.
3. If agglutination occurs with both antisera A
and B: The RBC contains both A and B
antigens to cause agglutination. And, the
blood group is AB.
4. If agglutination does not occur either with
antiserum A or antiserum B: The agglutination
does not occur if the RBC does not contain
any antigen. The blood group is O.
IMPORTANCE OF ABO GROUPS IN
BLOOD TRANSFUSION
During blood transfusion, only compatible blood
must be used. The one who gives blood is called
the donor and the one who receives the blood is
called recipient.
While transfusing the blood, antigen of the
donor and the antibody of the recipient are
considered. The antibody of the donor and antigen
of the recipient are ignored mostly.
Thus, RBC of "O" group has no antigen and
so agglutination does not occur with any other
group of blood. So, 'O' group blood can be given
to any blood group persons and the people of this
blood group are called universal donors.
The plasma of AB group blood has no
antibody. This does not cause agglutination of
RBC from any other group of blood. The people
of AB group can receive blood from any blood
group persons. So, people with this blood group
are called universal recipients.
MATCHING AND CROSS MATCHING
Blood matching (typing) is a laboratory test done
to determine the blood group of a person. When
the person needs blood transfusion, another test
called cross matching is done after the blood is
typed. It is done to find out whether the person's
body will accept the donor's blood or not.
For blood matching, RBC of the individual
(recipient) and test sera are used. Cross matching
is done by mixing the serum of the recipient and
FIGURE 16-1: Determination of blood group
Blood and Body Fluids
96
the RBCs of donor. Cross matching is always
done before blood transfusion. If agglutination of
RBCs from a donor occurs during cross matching,
the blood from that person is not used for
transfusion.
Matching = Recipient's RBC + Test sera
Cross matching = Recipient's serum +
Donor’s RBC
INHERITANCE OF ABO
AGGLUTINOGENS AND AGGLUTININS
Blood group of a person depends upon the two
genes inherited from each parent. Gene A and
gene B are dominant by themselves and gene
O is recessive. The inheritance of blood group
is represented schematically as given in
Table 16-3.
TRANSFUSION REACTIONS DUE TO
ABO INCOMPATIBILITY
Transfusion reactions are the adverse reactions
in the body which occur due to transfusion of
incompatible (mismatched) blood. The reactions
may vary from fever and hives (skin disorder
characterized by itching) to renal failure, shock
and death.
In mismatched transfusion, the transfusion
reactions occur between donor's RBC and
recipient's plasma. So, if the donor's plasma
contains antibody against recipient's RBC,
agglutination does not occur because these
antibodies are diluted in recipient's blood.
But, if recipient’s plasma contains antibodies
against donor’s RBCs, the immune system
launches a response against the new blood cells.
Donor RBCs are agglutinated and hemolyzed.
The hemolysis of RBCs results in release of
large amount of hemoglobin into the plasma. This
leads to the following complications.
1. Jaundice
Normally, hemoglobin released from destroyed
RBC is degraded and bilirubin is formed from it.
When the serum bilirubin level increases above
2 mg/dL jaundice occurs (Chapter 30).
2. Cardiac Shock
Simultaneously, the hemoglobin released into the
plasma increases the viscosity of blood. This
increases the workload on the heart leading to
heart failure.
3. Renal Shutdown
Dysfunction of kidneys is called renal shutdown.
The toxic substances from hemolyzed cells
cause constriction of blood vessels in kidney.
In addition, the toxic substances along with free
hemoglobin are filtered through glomerular
membrane and enter renal tubules. Because of
poor rate of reabsorption from renal tubules, all
these substances precipitate and obstruct the
renal tubule. This suddenly stops formation of
urine (anuria).
If not treated with artificial kidney, the person
dies within 10-12 days because of jaundice,
circulatory shock and more specifically due to
renal shutdown and anuria (Fig 16-2).
Rh FACTOR
Rh factor is an antigen present in RBC. The
antigen was discovered by Landsteiner and
Wiener. It was first discovered in rhesus monkey
and hence the name Rh factor. There are many
Rh antigens but only the D is more antigenic in
human.
The persons having D antigen are called Rh
positive and those without D antigen are called
TABLE 16-3: Inheritance of ABO group
Gene from
Group of the
Genotype
parents
offspring
A + A
A + O
A
AA or AO
B + B
B + O
B
BB or BO
A + B
AB
AB
O + O
O
OO

Chapter 16 Blood Groups and Blood Transfusion
97
Rh negative. Among Asian population, 85 percent
of people are Rh positive and 15 percent are Rh
negative.
Rh system is different from ABO group system
because, the antigen D does not have
corresponding natural antibody (anti D). However,
if Rh positive blood is transfused to a Rh negative
person for the first time, then anti D is formed in
that person. On the other hand, there is no risk
of complications if Rh positive person receives
Rh negative blood.
INHERITANCE OF Rh ANTIGEN
Rhesus factor is an inherited dominant factor. It
may be homozygous rhesus positive with DD or
heterozygous rhesus positive with Dd (Fig. 16-3).
Rhesus negative occurs only with complete
absence of D (i.e. with homozygous dd).
TRANSFUSION REACTIONS DUE TO
Rh INCOMPATIBILITY
When a Rh negative person receives Rh positive
blood for the first time, he is not affected much,
since the reactions do not occur immediately.
But, the Rh antibodies develop within one
month. The transfused RBCs, which are still
present in recipient's blood are agglutinated.
These agglutinated cells are lysed by
macrophages. So, a delayed transfusion
reaction occurs. But, it is usually mild and does
not affect the recipient. However, antibodies
developed in the recipient remain in the body
for ever. So, when this person receives Rh
positive blood for the second time, the donor
RBCs are agglutinated and severe transfusion
reactions occur immediately (Fig. 16-4). These
reactions are similar to the reactions of ABO
incompatibility (see above).
FIGURE 16-3: Inheritance of Rh antigen
FIGURE 16-2: Complications of mismatched
blood transfusion
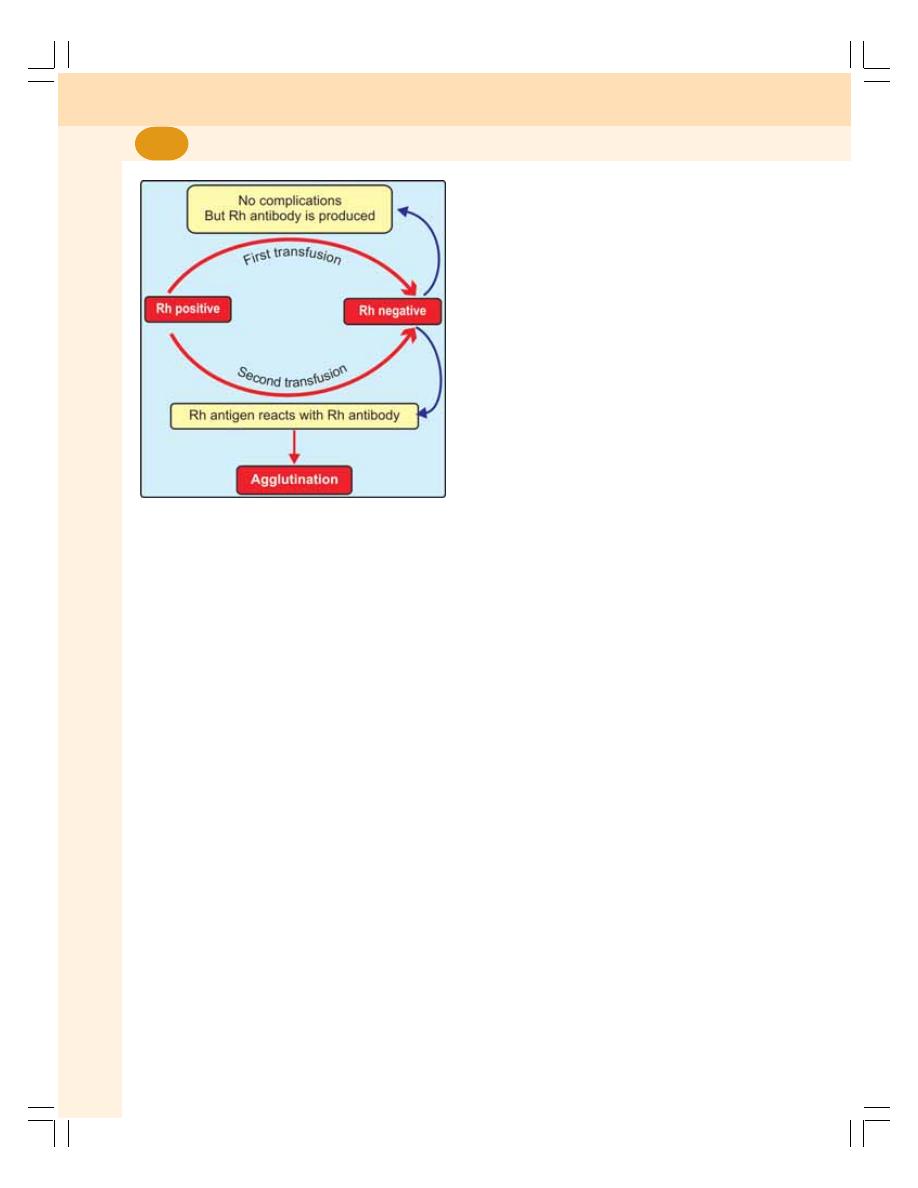
Blood and Body Fluids
98
HEMOLYTIC DISEASE OF FETUS AND
NEWBORN — ERYTHROBLASTOSIS
FETALIS
Hemolytic disease is the disease in fetus and
newborn characterized by abnormal hemolysis of
RBCs. It is due to Rh incompatibility, i.e. the
difference between the Rh blood group of the
mother and baby. Hemolytic disease leads to
erythroblastosis fetalis.
Erythroblastosis fetalis is a disorder in fetus
characterized by the presence of erythroblasts
in blood. When a mother is Rh negative and fetus
is Rh positive (the Rh factor being inherited from
the father), usually the first child escapes the
complications of Rh incompatibility. This is
because the Rh antigen cannot pass from fetal
blood into the mother's blood through the
placental barrier.
However, at the time of parturition (delivery of
the child) the Rh antigen from fetal blood may
leak into mother's blood because of placental
detachment. During postpartum period, i.e. within
a month after delivery, the mother develops Rh
antibody in her blood.
When the mother conceives for the second
time and if the fetus happens to be Rh positive
again, the Rh antibody from mother's blood
crosses placental barrier and enters the fetal
blood. Thus, the Rh antigen cannot cross the
placental barrier whereas Rh antibody can cross
it.
The Rh agglutinins which enter the fetus
cause agglutination of fetal RBCs resulting in
hemolysis.
The severe hemolysis in the fetus causes
jaundice. To compensate the hemolysis of more
and more number of RBCs, there is rapid
production of RBCs, not only from bone marrow,
but also from spleen and liver. Now, many large
and immature cells in proerythroblastic stage
are released into circulation. Because of this,
the disease is called erythroblastosis fetalis.
Ultimately due to excessive hemolysis severe
complications develop, viz.
1. Severe anemia
2. Hydrops fetalis
3. Kernicterus.
1. Severe Anemia
Excessive hemolysis results in anemia. And the
infant dies when anemia becomes severe.
2. Hydrops Fetalis
It is a serious condition in fetus characterized
by edema. Severe hemolysis results in the
development of edema, enlargement of liver and
spleen and cardiac failure. When this condition
becomes more severe it may lead to intrauterine
death of fetus.
3. Kernicterus
Kernicterus is the form of brain damage in infants
caused by severe jaundice. If the baby survives
anemia in erythroblastosis fetalis (see above)
then kernicterus develops because of high
bilirubin content.
Prevention or Treatment for Erythroblastosis
Fetalis
i. If mother is found to be Rh negative and
fetus is Rh positive, anti D (antibody
against D antigen) should be
administered to the mother at 28th and
FIGURE 16-4: Rh incompatibility

Chapter 16 Blood Groups and Blood Transfusion
99
34th weeks of gestation as prophylactic
measure. If Rh negative mother delivers
Rh positive baby, then anti D should be
administered to the mother within 48
hours of delivery. This develops passive
immunity and prevents the formation of
Rh antibodies in mother’s blood. So the
hemolytic disease of newborn does not
occur in a subsequent pregnancy.
ii. If the baby is born with erythroblastosis
fetalis, the treatment is given by means
of exchange transfusion (see below). Rh
negative blood is transfused into the infant
replacing infant's own Rh positive blood.
It will now take at least 6 months for the
infant's new Rh positive blood to replace
the transfused Rh negative blood. By this
time all the molecules of Rh antibody
derived from the mother get destroyed.
OTHER BLOOD GROUPS
In addition to ABO blood groups and Rh factor,
many more blood group systems were found.
However, these systems of blood groups do not
have much clinical importance.
Other blood groups include:
1. Lewis blood group
2. MNS blood groups
3. Auber groups
4. Diego group
5. Bombay group
6. Duffy group
7. Lutheran group
8. P group
9. Kell group
10. I group
11. Kidd group
12. Sulter XG group.
IMPORTANCE OF KNOWING
BLOOD GROUP
Nowadays, knowledge of blood group is very
essential medically, socially and judicially. The
importance of knowing blood group is:
1. Medically, it is important during blood
transfusions and in tissue transplants
2. Socially, one should know his or her own blood
group and become a member of the Blood
Donor's club so that he or she can be
approached for blood donation during
emergency conditions
3. It general among the couples, knowledge of
blood groups helps to prevent the
complications due to Rh incompatibility and
save the child from the disorders like
erythroblastosis fetalis
4. Judicially, it is helpful in medicolegal cases
to sort out parental disputes and as a
supporting evidence in identifying the criminals
BLOOD TRANSFUSION
INTRODUCTION
Blood transfusion is the process of transferring
blood or blood components from one person (the
donor) into the bloodstream of another person
(the recipient). Transfusion may be done as a
lifesaving procedure to replace blood cells or
blood products lost through bleeding.
Blood transfusion is essential in the
conditions like anemia, hemorrhage, trauma,
burns and surgery.
Precautions to be taken Before the
Transfusion of Blood
1. Donor must be healthy without any diseases
like syphilis, AIDS, etc.
2. Only compatible blood must be transfused
and Rh compatibility must be confirmed.
3. Both matching and cross-matching must be
done.
Precautions to be taken while Transfusing
Blood
1. Apparatus for transfusion must be sterile
2. The temperature of blood to be transfused
must be same as body temperature
3. The transfusion of blood must be slow. The
sudden rapid infusion of blood into the body
increases the load on the heart resulting in
many complications.

Blood and Body Fluids
100
BLOOD SUBSTITUTES
Substances infused in the body instead of whole
blood are known as blood substitutes. The
commonly used blood substitutes are human
plasma, 0.9 percent sodium chloride solution
(saline) and 5 percent glucose.
EXCHANGE TRANSFUSION
Exchange transfusion is the procedure which
involves removal of patient's blood and
replacement with fresh donor blood or plasma.
It is otherwise known as replacement
transfusion. It is carried out in conditions such
as severe jaundice, sickle cell anemia,
erythroblastosis fetalis, etc.
AUTOLOGOUS BLOOD TRANSFUSION
Autologous blood transfusion is the collection and
reinfusion of patient’s own blood. It is also called
self blood donation. The conventional transfusion
of blood that is collected from persons other than
the patient is called allogeneic or heterologous
blood transfusion.
DEFINITION AND DISTRIBUTION
RETICULOENDOTHELIAL SYSTEM OR MACROPHAGE SYSTEM
MACROPHAGE
CLASSIFICATION OF RETICULOENDOTHELIAL CELLS
FIXED RETICULOENDOTHELIAL CELLS – TISSUE MACROPHAGES
WANDERING RETICULOENDOTHELIAL CELLS
FUNCTIONS OF RETICULOENDOTHELIAL SYSTEM
SPLEEN
FUNCTIONS OF SPLEEN
DEFINITION AND DISTRIBUTION
RETICULOENDOTHELIAL SYSTEM OR
MACROPHAGE SYSTEM
Reticuloendothelial system or macrophage
system is the system of primitive phagocytic cells
which play important role in defense mechanism
of the body.
MACROPHAGE
Macrophage is a large cell derived from monocyte.
It has the property of phagocytosis. So, the
macrophage is also defined as a large phagocytic
cell.
CLASSIFICATION OF
RETICULOENDOTHELIAL CELLS
The reticuloendothelial cells are classified into
two types:
a. Fixed reticuloendothelial cells or tissue
macrophages
b. Wandering reticuloendothelial cells.
FIXED RETICULOENDOTHELIAL
CELLS – TISSUE MACROPHAGES
The fixed reticuloendothelial cells are also
called the tissue macrophages or fixed histio-
cytes because these cells are usually located
in the tissues.
The tissue macrophages are:
1. Reticuloendothelial cells in connective
tissues and in serous membranes like pleura,
omentum and mesentery
2. Endothelial cells of blood sinusoid in bone
marrow, liver, spleen, lymph nodes, adrenal
glands and pituitary glands. Kupffer’s cells in
liver belong to this category.
3. Cells in the reticulum of spleen, lymph node,
and bone marrow
Reticuloendothelial System
and Tissue Macrophage
17
17
17
17
17

Blood and Body Fluids
102
4. Meningocytes of meninges and microglia in
brain
5. Alveolar cells in lungs
6. Subcutaneous tissue cells.
WANDERING RETICULOENDOTHELIAL
CELLS
The wandering reticuloendothelial cells are also
called free histiocytes. There are two types of
wandering reticuloendothelial cells.
1. Free histiocytes of blood
i. Neutrophils
ii. Monocytes, which become macrophages
and migrate to the site of injury or infection
2. Free histiocytes of solid tissue
During emergency, the fixed histiocytes from
connective tissue and other organs become
wandering cells and enter the circulation.
FUNCTIONS OF
RETICULOENDOTHELIAL SYSTEM
Reticuloendothelial system plays an important
role in the defense mechanism of the body. Most
of the functions of the reticuloendothelial system
are carried out by the tissue macrophages.
The functions of tissue macrophages are:
1. Phagocytic Function
Macrophages are the large phagocytic cells,
which play an important role in defense of the
body by phagocytosis. When any foreign body
invades, macrophages ingest them by
phagocytosis and liberate the antigenic products
of the organism. The antigens activate the helper
T lymphocytes and B lymphocytes (Refer Chapter
13 for details).
The lysosomes of macrophages contain
proteolytic enzymes and lipases which digest the
bacteria and other foreign bodies.
2. Secretion of Bactericidal Agents
Tissue macrophages secrete many bactericidal
agents which kill the bacteria. The important
bactericidal agents of macrophages are the
oxidants. An oxidant is a substance that oxidizes
another substance. The oxidants secreted by
macrophages are:
i. Superoxide (O
2
–
)
ii. Hydrogen peroxide (H
2
O
2
)
iii. Hydroxyl ions (OH
–
).
3. Secretion of Interleukins
Tissue macrophages secrete interleukin-1, 6 and
12 which help in immunity.
4. Secretion of Tumor Necrosis Factors
Tissue macrophages secrete TNF-
α and TNF-β
which cause necrosis of tumor.
5. Secretion of Transforming Growth
Factor
Tissue macrophages secrete transforming
growth factor which plays an important role in
preventing rejection of transplanted tissues or
organs.
6. Secretion of Colony Stimulation Factor
Macrophages secrete the colony stimulation
factor (M-CSF) which accelerates growth of
granulocytes, monocytes and macrophages.
7. Secretion of Platelet Derived Growth
Factor
Tissue macrophages secrete the platelet derived
growth factor (PDGF), which accelerates repair
of damaged blood vessel and wound healing.
8. Removal of Carbon Particles and
Silicon
The macrophages ingest the substances like
carbon dust particles and silicon which enter the
body.
9. Destruction of Senile RBC
The reticuloendothelial cells, particularly those in
spleen destroy the senile RBCs and release
hemoglobin (Chapter 7).

Chapter 17 Reticuloendothelial System and Tissue Macrophage
103
10. Destruction of Hemoglobin
The hemoglobin released from broken senile
RBCs is degraded by the reticuloendothelial cells
(Chapter 9).
11. Hemopoietic Function
The reticuloendothelial cells also play an
important role in the production of blood cells.
SPLEEN
Spleen is the largest lymphoid organ in the body
and it is highly vascular. It also contains
reticuloendothelial cells.
FUNCTIONS OF SPLEEN
1. Formation of Blood Cells
The spleen plays an important role in the
hemopoietic function in embryo. During the
hepatic stage, spleen produces blood cells along
with liver. In myeloid stage, it produces the blood
cells along with liver and bone marrow.
2. Destruction of Blood Cells
The older RBCs, lymphocytes, and thrombocytes
are destroyed in the spleen. When the RBCs
become old (120 days), the cell membrane
becomes more fragile. The diameter of most of
the capillaries is less or equal to that of RBC.
The fragile old cells are destroyed while trying
to squeeze through the capillaries because
these cells cannot withstand the stress of
squeezing.
The destruction occurs mostly in the
capillaries of spleen because the splenic
capillaries have a thin lumen. So, the spleen is
known as graveyard of RBCs.
3. Blood Reservoir Function
In animals, spleen stores large amount of
blood. However, this function is not significant
in humans. But, a large number of RBCs are
stored in spleen. The RBCs are released from
spleen into circulation during the emergency
conditions like hypoxia and hemorrhage.
4. Role in Defense of Body
Spleen filters the blood by removing the
microorganisms. The macrophages in splenic
pulp destroy the micro-organisms and other
foreign bodies by phagocytosis. Spleen contains
about 25 percent of T lymphocytes and
15 percent of B lymphocytes and forms the site
of antibody production.
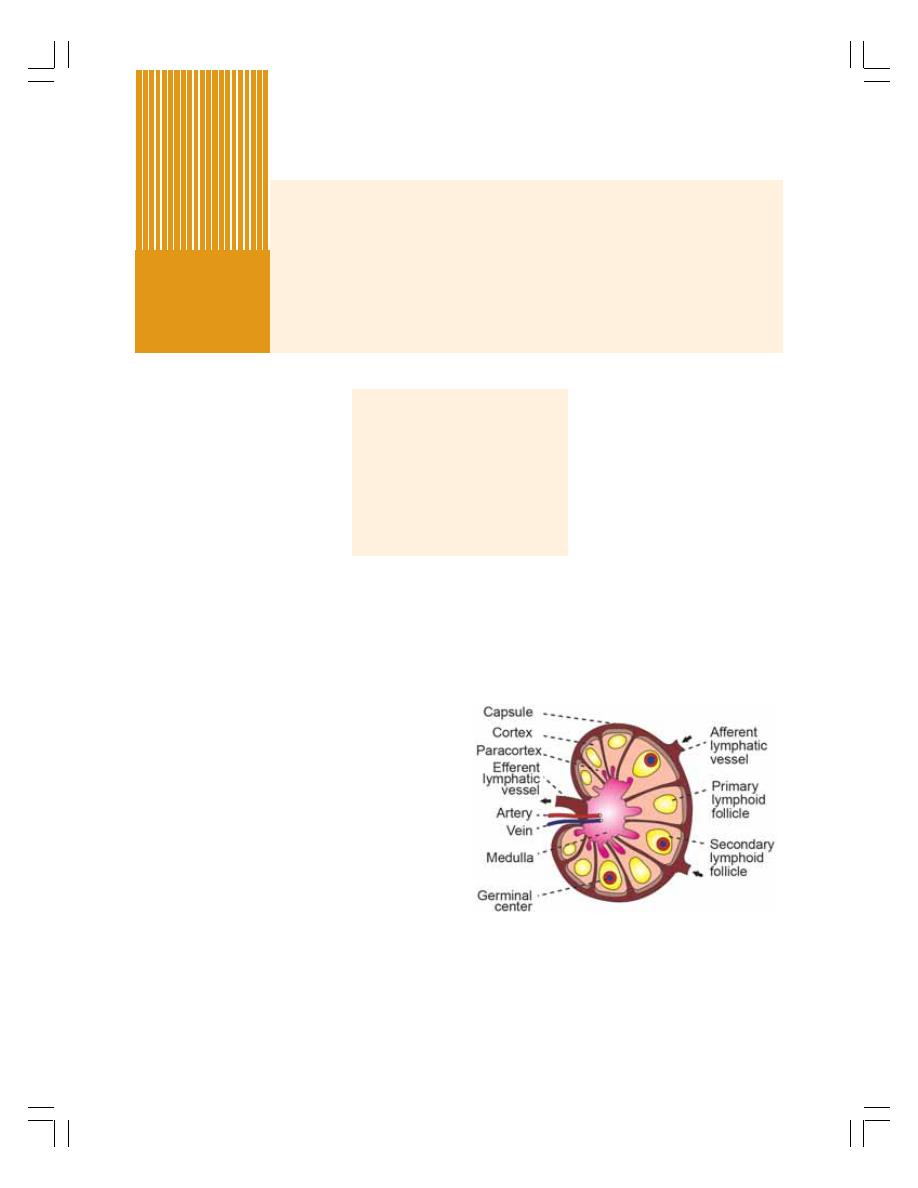
LYMPHATIC SYSTEM
LYMPH NODES
LYMPH
FORMATION
RATE OF FLOW
COMPOSITION
FUNCTIONS
LYMPHATIC SYSTEM
Lymphatic system is a closed system of lymph
channels or lymph vessels through which lymph
flows. It is an one way system and allows the
lymph flow from tissue spaces towards the
blood.
LYMPH NODES
Lymph nodes are small glandular structures
located in the course of lymph vessels. The
lymph nodes are also called lymph glands or
lymphatic nodes.
Each lymph node constitutes masses of
lymphatic tissue covered by a dense connective
tissue capsule. The structures are arranged in
three layers cortex, paracortex and medulla
(Fig. 18-1).
The lymph node receives lymph by one or
two lymphatic vessels called afferent vessels.
Afferent vessels divide into small channels.
Lymph passes through afferent vessels and small
channels and reaches the cortex. It circulates
through cortex, paracortex and medulla of the
lymph node. From medulla, the lymph leaves the
node via one or two efferent vessels.
The lymph nodes are present along the
course of lymphatic vessels in elbow, axilla, knee
and groin. The lymph nodes are also present in
certain points in abdomen, thorax and neck
where many lymph vessels join.
Lymphatic System and
Lymph
18
FIGURE 18-1: Structure of a lymph node
FUNCTIONS OF LYMPH NODES
Lymph nodes serve as filters which filter bacteria
and toxic substances from the lymph. The
functions of the lymph nodes are:
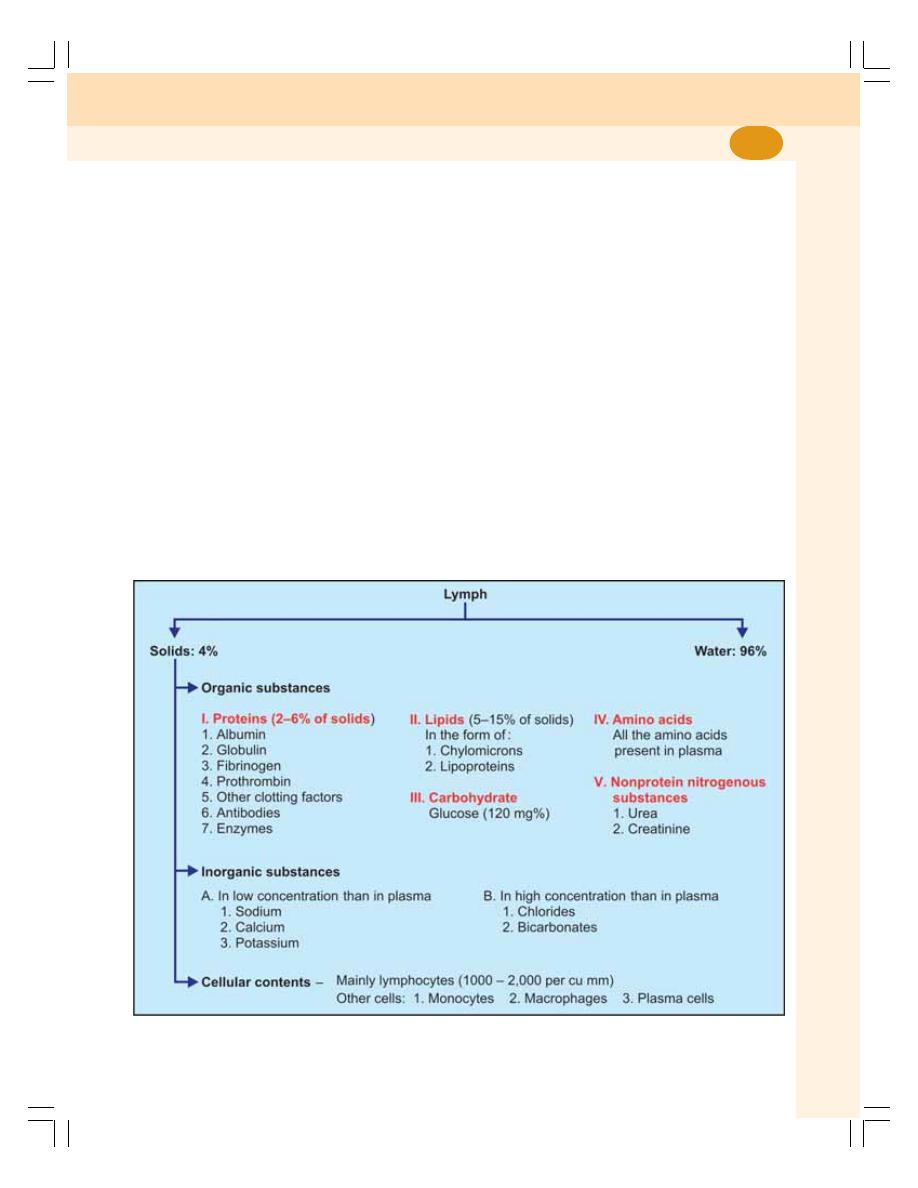
Chapter 18 Lymphatic System and Lymph
105
1. When lymph passes through the lymph
nodes, it is filtered, i.e. the water and
electrolytes are removed. But, the proteins
and lipids are retained in the lymph
2. The bacteria and other toxic substances are
destroyed by macrophages of lymph nodes.
Because of this, lymph nodes are called
defense barriers.
3. Bacteria are phagocytozed by the
macrophages of lymph node.
LYMPH
FORMATION
Lymph is formed from interstitial fluid, due to the
permeability of lymph capillaries. When blood
passes via blood capillaries in the tissues, 9/10th
of fluid passes into venous end of capillaries
from arterial end. And, the remaining 1/10th of
the fluid passes into lymph capillaries, which
have more permeability than blood capillaries.
So, when lymph passes through lymph
capillaries, the composition of lymph is more or
less similar to that of interstitial fluid including
protein content. Proteins present in the interstitial
fluid cannot enter the blood capillaries because
of their larger size. So, these proteins enter
lymph vessels, which are permeable to large
particles also.
RATE OF LYMPH FLOW
About 120 ml of lymph flows into blood per hour.
Out of this, about 100 ml/hour flows through
thoracic duct and 20 ml/hour flows through the
right lymphatic duct.
COMPOSITION OF LYMPH
Usually, lymph is a clear and colorless fluid. It is
formed by 96% water and 4% solids. Some blood
cells are also present in lymph (Fig. 18-2).
FIGURE 18-2: Composition of lymph

Blood and Body Fluids
106
FUNCTIONS OF LYMPH
1. The important function of lymph is to return
the proteins from tissue spaces into blood
2. Lymph flow plays an important role in
redistribution of fluid in the body
3. Through the lymph, the bacteria, toxins and
other foreign bodies are removed from
tissues
4. Lymph flow is responsible for the main-
tenance of structural and functional integrity
of tissue. Obstruction to lymph flow affects
various tissues particularly myocardium,
nephrons and hepatic cells
5. Lymph flow serves as an important route for
intestinal fat absorption. This is the reason
for the milky appearance of lymph after fatty
meal
6. It plays an important role in immunity by
transport of lymphocytes.
DEFINITION
DEFINITION
DEFINITION
DEFINITION
DEFINITION
FUNCTIONS OF TISSUE FLUID
FUNCTIONS OF TISSUE FLUID
FUNCTIONS OF TISSUE FLUID
FUNCTIONS OF TISSUE FLUID
FUNCTIONS OF TISSUE FLUID
FORMATION OF TISSUE FLUID
FORMATION OF TISSUE FLUID
FORMATION OF TISSUE FLUID
FORMATION OF TISSUE FLUID
FORMATION OF TISSUE FLUID
APPLIED PHYSIOLOGY – EDEMA
APPLIED PHYSIOLOGY – EDEMA
APPLIED PHYSIOLOGY – EDEMA
APPLIED PHYSIOLOGY – EDEMA
APPLIED PHYSIOLOGY – EDEMA
Tissue
Fluid and Edema
19
DEFINITION
Tissue fluid is the medium in which cells are
bathed. It is otherwise known as interstitial fluid.
It forms about 20% of ECF.
FUNCTIONS OF TISSUE FLUID
Because of the capillary membrane, there is no
direct contact between blood and cells. And, the
tissue fluid acts as a medium for exchange of
various substances between the cells and the
blood in the capillary loop. Oxygen and nutritive
substances diffuse from the arterial end of
capillary through the tissue fluid and reach the
cells. Carbon dioxide and waste materials diffuse
from the cells into the venous end of capillary
through this fluid.
FORMATION OF TISSUE FLUID
Formation of tissue fluid involves two processes:
1. Filtration
2. Reabsorption
FILTRATION
Tissue fluid is formed by the process of filtration.
Normally, the blood pressure (also called hydro-
static pressure) in arterial end of the capillary is
about 30 mm Hg. This hydrostatic pressure is
the driving force for filtration of water and other
substances from blood into tissue spaces
(Fig. 19-1).
REABSORPTION
The fluid filtered at the arterial end of capillaries
is reabsorbed back into the blood at the venous
end of capillaries. Here also, the pressure
gradient plays an important role. At the venous
end of capillaries, the hydrostatic pressure is
less (15 mm Hg) and the oncotic pressure is
more (25 mm Hg). Due to the pressure gradient
of 10 mm Hg, the fluid is reabsorbed along with
waste materials from the tissue fluid into the
capillaries. About 10% of filtered fluid enters the
lymphatic vessels.
Reabsorption at the venous end helps to
maintain the volume of tissue fluid.
APPLIED PHYSIOLOGY – EDEMA
DEFINITION
Edema is defined as the swelling caused by
excessive accumulation of fluid in tissues. It
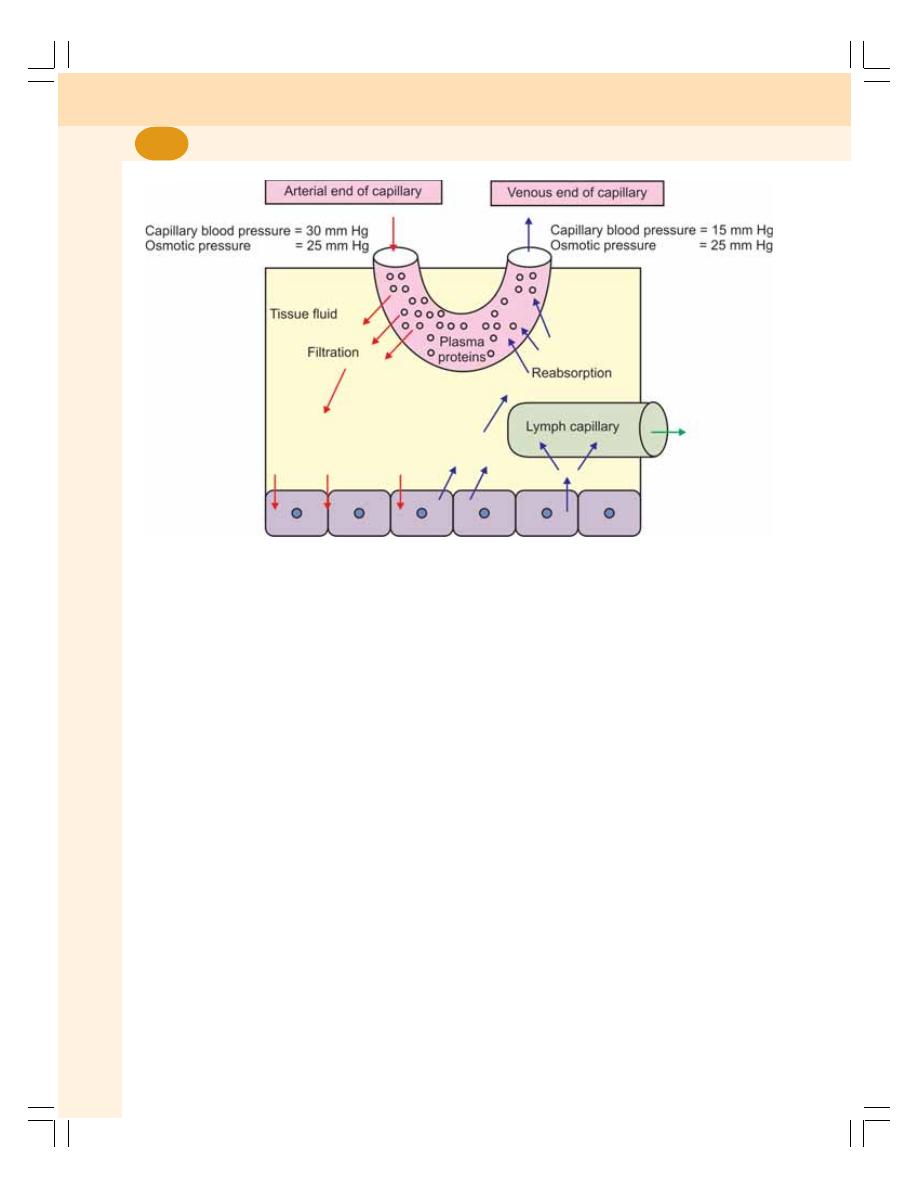
Blood and Body Fluids
108
may be generalized or local. Edema that involves
the entire body is called generalized edema.
Local edema is the one that occurs is specific
areas of the body such as abdomen, lungs and
extremities like feet, ankles and legs. The accu-
mulation of fluid may be inside or outside the cell.
TYPES OF EDEMA
Edema is classified into two types depending
upon the body fluid compartment where accu-
mulation of excess fluid occurs:
1. Intracellular edema
2. Extracellular edema.
Intracellular Edema
Intracellular edema is the accumulation of fluid
inside the cell. It occurs because of three rea-
sons:
i. Malnutrition
ii. Poor metabolism
iii. Inflammation of the tissues.
Extracellular Edema
Extracellular edema is defined as the accumu-
lation of fluid outside the cell. It occurs because
of abnormal leakage of fluid from capillaries into
interstitial space and obstruction of lymphatics
that prevents return of fluid from interstitial fluid
back into blood.
The common conditions which leads to extra-
cellular edema are:
1. Heart failure
2. Renal disease
3. Decreased amount of plasma proteins
4. Lymphatic obstruction
5. Increased endothelial permeability.
Pitting and Non-pitting Edema
Interstitial fluid is present in the form of a gel
that is almost like a semisolid substance. It is
because the interstitial fluid is not present as fluid
but is bound in a proteoglycan meshwork. It does
not allow any free space for the fluid movement.
FIGURE 19-1: Formation of tissue fluid. Plasma proteins remain inside the blood
capillary since the capillary membrane is not permeable to plasma proteins

Chapter 19 Tissue Fluid and Edema
109
When interstitial fluid volume increases,
most of the fluid becomes free fluid that is not
bound to proteoglycan meshwork. It flows
freely through tissue spaces, producing a
swelling called edema. This type of edema is
known as pitting edema because, when this area
is pressed with the finger, displacement of fluid
occurs producing a depression or pit. When the
finger is removed, the pit remains for few
seconds, sometimes as long as one minute, till
the fluid flows back into that area.
Edema also develops due to swelling of the
cells or clotting of interstitial fluid in the presence
of fibrinogen. This is called non-pitting edema
because, it is hard and a pit is not formed by
pressing.

Questions in Blood and Body Fluids
110
LONG QUESTIONS
1. What are the compartments of body fluid?
Enumerate the differences between ECF
and ICF and explain the measurement of
ECF volume.
2. What is indicator dilution technique? How
is it applied in the measurement of total
body water? Describe dehydration briefly.
3. Give a detailed account of erythropoiesis.
4. Define erythropoiesis. List the different
stages of erythropoiesis. Describe the
changes, which take place in each stage
and the factors necessary for erythro-
poiesis.
5. Describe the morphology, development
and functions of leukocytes.
6. Describe the development of cellular
mediated immunity.
7. Describe the development of humoral
immunity.
8. Define blood coagulation. Describe the
mechanisms involved in coagulation. Add
a note on anticoagulants.
9. Enumerate the factors involved in blood
coagulation and describe the intrinsic
mechanism of coagulation.
10. Give an account of extrinsic mechanism
of coagulation of blood. Give a brief des-
cription of bleeding disorders.
SHORT QUESTIONS
1. Dye or indicator dilution technique.
2. Measurement of total body water.
3. Measurement of ECF volume.
4. Measurement of ICF volume.
5. Measurement of blood volume.
6. Measurement of plasma volume.
7. Dehydration.
8. Water intoxication.
9. Functions of blood.
QUESTIONS IN BLOOD AND BODY FLUIDS
10. Plasma proteins.
11. Functions of RBCs.
12. Fate of RBCs.
13. Lifespan of RBCs.
14. Physiological variations of RBC count.
15. Polycythemia.
16. Factors necessary for erythropoiesis.
17. Destruction of hemoglobin.
18. Abnormal hemoglobin.
19. Abnormal hemoglobin derivatives.
20. Pernicious anemia.
21. Erythrocyte sedimentation rate.
22. Packed cell volume or hematocrit.
23. Anemia.
24. Hemolysins.
25. Types and morphology of WBCs.
26. Functions of WBCs.
27. T lymphocytes.
28. B lymphocytes.
29. Role of macrophages in immunity.
30. Immunoglobulins or antibodies.
31. Immune deficiency diseases.
32. Autoimmune diseases.
33. Platelets.
34. Hemostasis.
35. Fibrinolysis.
36. Tests for coagulation.
37. Anticoagulants.
38. Hemophilia.
39. Purpura.
40. Thrombosis.
41. ABO blood groups.
42. Rh factor.
43. Transfusion reactions.
44. Erythroblastosis fetalis.
45. Tissue macrophage.
46. Functions of spleen.
47. Lymph.
48. Lymph nodes.
49. Tissue fluid.
50. Edema.

Muscle Physiology
20. Classification of Muscles ..................................................... 113
21. Structure of Skeletal Muscle ................................................ 116
22. Properties of Skeletal Muscle .............................................. 121
23. Electrical and Molecular Changes during
Muscular Contraction .......................................................... 126
24. Neuromuscular Junction ...................................................... 132
25. Smooth Muscle .................................................................... 136
S E C T I O N
3
C H A P T E R S


DEPENDING UPON STRIATIONS
DEPENDING UPON CONTROL
DEPENDING UPON SITUATION
There are more than 600 muscles in our body.
Muscles perform many useful functions and help
us in doing everything in day to day life. Muscles
are classified by three different methods based
on different factors:
I.
Depending upon the presence or absence of
striations
II. Depending upon the control
III. Depending upon the function.
DEPENDING UPON STRIATIONS
Depending upon the presence or absence of the
cross striations, muscles are divided into two
groups:
1. Striated muscle
2. Nonstriated muscle.
Striated Muscle
Striated muscle is the muscle which has a large
number of cross striations (transverse lines).
Skeletal muscle and cardiac muscle belong to
this category.
Nonstriated Muscle
The muscle which does not have cross striations
is called nonstriated muscle. It is also called plain
muscle or smooth muscle. It is found in the wall
of the visceral organs.
DEPENDING UPON CONTROL
Depending upon control, the muscles are
classified into two types:
1. Voluntary muscle
2. Involuntary muscle.
Voluntary Muscle
Voluntary muscle is the muscle that is controlled
by the will. Skeletal muscles are the voluntary
muscles. These muscles are innervated by
somatic nerves.
Involuntary Muscle
The muscle that cannot be controlled by the will
is called involuntary muscle. Cardiac muscle and
smooth muscle are involuntary muscles. These
muscles are innervated by autonomic nerves.
DEPENDING UPON SITUATION
The muscles are classified into three types
depending upon the situation:
1. Skeletal muscle
2. Cardiac muscle
3. Smooth muscle.
Classification of Muscles
20

Muscle Physiology
114
The features of these muscles are given in
Table 20-1.
Skeletal Muscle
Skeletal muscle is situated in association with
bones forming the skeletal system. The skeletal
muscles form 40 to 50% of body mass and are
TABLE 20-1: Features of skeletal, cardiac and smooth muscle fibers
Features
Skeletal muscle
Cardiac muscle
Smooth muscle
Location
In association with bones
In the heart
In the visceral organs
Shape
Cylindrical and unbranched Branched
Spindle shaped unbranched
Length
1 to 4 cm
80 to 100
μ
50 to 200
μ
Diameter
10 to 100
μ
15 to 20
μ
2 to 5
μ
No. of Nucleus
More than one
One
One
Cross striations
Present
Present
Absent
Myofibrils
Present
Present
Absent
Sarcomere
Present
Present
Absent
Troponin
Present
Present
Absent
Sarcotubular system
Well developed
Well developed
Poorly developed
‘T’ tubules
Long and thin
Short and broad
Absent
Depolarization
Upon stimulation
Spontaneous
Spontaneous
Fatigue
Possible
Not possible
Not possible
Summation
Possible
Not possible
Possible
Tetanus
Possible
Not possible
Possible
Resting membrane
Stable
Stable
Unstable
potential
For trigger of contraction, Troponin
Troponin
Calmodulin
calcium binds with
Source of calcium
Sarcoplasmic reticulum
Sarcoplasmic
Extracellular
reticulum
Speed of contraction
Fast
Intermediate
Slow
Neuromuscular junction
Well defined
Not well defined
Not well defined
Action
Voluntary action
Involuntary action Involuntary action
Control
Only neurogenic
Myogenic
Neurogenic and myogenic
Nerve supply
Somatic nerves
Autonomic nerves Autonomic nerves
voluntary and striated. These muscles are
supplied by somatic nerves.
The fibers of the skeletal muscles are
arranged in parallel. In most of the skeletal
muscles, the muscle fibers are attached to
tendons on either end. The skeletal muscles are
anchored to the bones by the tendons.

Chapter 20 Classification of Muscles
115
Cardiac Muscle
Cardiac muscle forms the musculature of the
heart. These muscles are striated and involun-
tary. Cardiac muscles are supplied by autonomic
nerve fibers.
Smooth Muscle
Smooth muscle or visceral muscle is situated in
association with viscera. Smooth muscle is non-
striated and involuntary. Because of the absence
of cross striations it is called smooth or plain
muscle. It is supplied by autonomic nerve fibers.

MUSCLE MASS
MUSCLE FIBER
MYOFIBRIL
MICROSCOPIC STRUCTURE OF A MYOFIBRIL
SARCOMERE
ELECTRON MICROSCOPIC STUDY OF SARCOMERE
CONTRACTILE ELEMENTS (PROTEINS) OF MUSCLE
MYOSIN MOLECULE
ACTIN MOLECULE
TROPOMYOSIN
TROPONIN
SARCOTUBULAR SYSTEM
COMPOSITION OF MUSCLE
MUSCLE MASS
The muscle mass (or tissue) is made up of a large
number of individual muscle cells or myocytes.
The muscle cells are commonly called muscle
fibers because these cells are long and slender
in appearance. The skeletal muscle fibers are
multinucleated and arranged parallel to one
another with some connective tissue in between.
The muscle mass is separated from the
neighboring tissues by the thick fibrous tissue
layer known as fascia. Beneath the fascia, the
muscle is covered by a connective tissue sheath
called epimysium. In the muscle, the muscle
fibers are arranged in various groups called the
bundles or fasciculi. The connective tissue sheath
Structure of
Skeletal Muscle
21
that covers each fasciculus is called perimysium.
Each muscle fiber is covered by the connective
tissue layer called the endomysium (Fig. 21-1).
MUSCLE FIBER
Each muscle cell or muscle fiber is cylindrical in
shape. The length of the fiber is between 1 and
4 cm depending upon the length of the muscle.
The diameter of the muscle fiber varies from
10 to 100 μ. The muscle fibers are attached to
bone by a tough cord of connective tissue called
tendon.
Each muscle fiber is enclosed by a cell
membrane called plasma membrane that lies
beneath the endomysium. It is also called
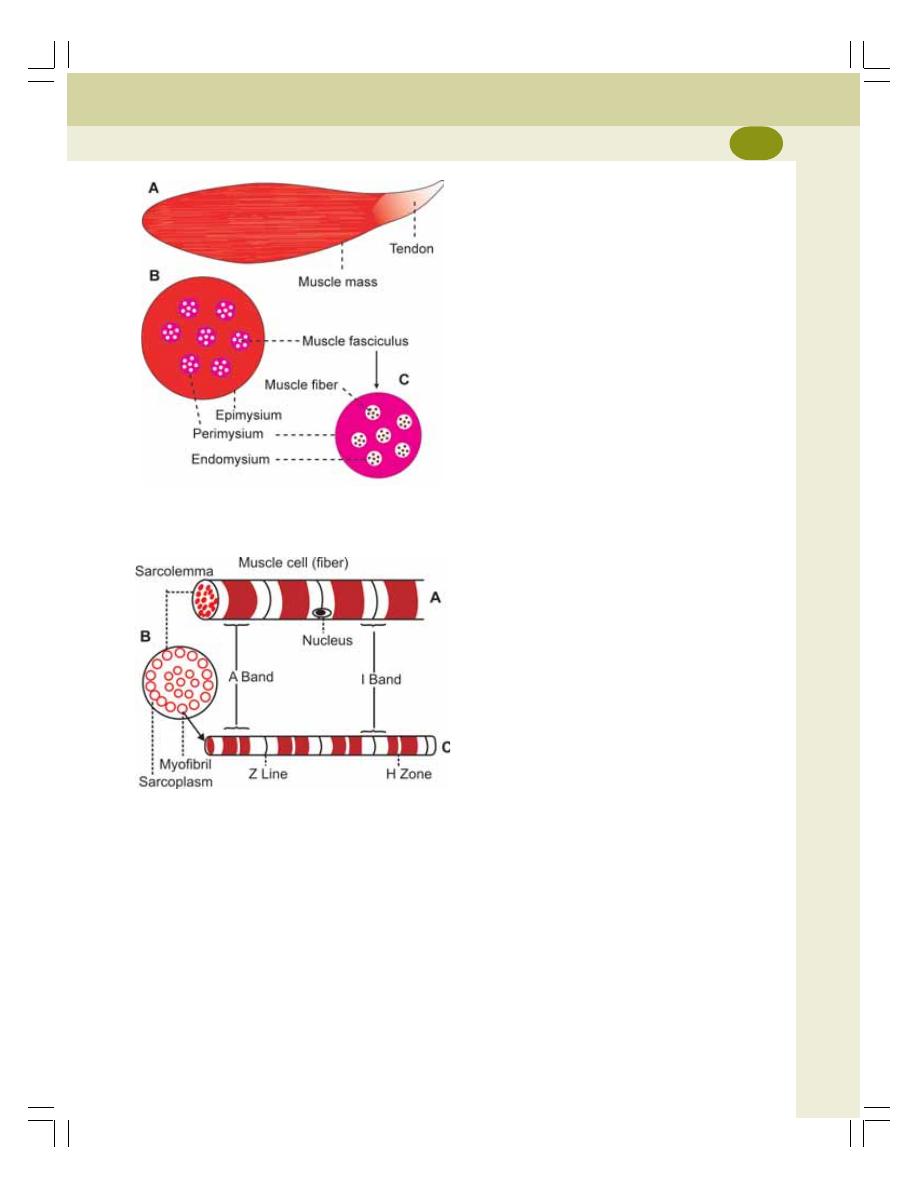
Chapter 21 Structure of Skeletal Muscle
117
MYOFIBRIL
Myofibrils or myofibrillae are the special
structures present only in muscle fibers. These
are the fine parallel filaments present in
sarcoplasm of the muscle cell. The myofibrils run
through the entire length of the muscle fiber.
MICROSCOPIC STRUCTURE
OF A MYOFIBRIL
Light microscopic studies show that, each
myofibril consists of a number of two alternating
bands. The two bands are:
1. Light band or ‘I’ band
2. Dark band or ‘A’ band.
Light Band or ‘I’ Band
The light band is called ‘I’ band because it is
isotropic to polarized light. When the polarized
light is passed through the muscle fiber at this
area the light rays are refracted at the same
angle.
Dark Band or ‘A’ Band
The dark band is called ‘A’ band because it is
anisotropic to polarized light. When the
polarized light is passed through the muscle
fiber at this area, the light rays are refracted at
different directions (An = not; iso = it; trops =
turning).
In an intact muscle fiber, ‘I’ band and ‘A’ band
of the adjacent myofibrils are placed side by
side. It gives the appearance of characteristic
cross striations in the muscle fiber.
I band is divided into two portions by a
narrow dark line called ‘Z’ line or ‘Z’ disk (in
German zwischenscheibe = between disks).
The ‘Z’ line is formed by a protein disk which
does not permit passage of light. The portion
of myofibril in between two ‘Z’ lines is called
sarcomere.
SARCOMERE
Definition
Sarcomere is the structural and functional unit
of the skeletal muscle.
FIGURE 21-2:
A = One muscle cell B = Cross-
section of one muscle cell C = One myofibril
FIGURE 21-1:
Diagram showing A = Skeletal muscle
mass B = Cross-section of muscle C = One muscle
fasciculus
sarcolemma (Fig. 21-2). The cytoplasm of the
muscle is known as sarcoplasm. Many structures
are embedded within the sarcoplasm:
1. Nuclei
2. Myofibril
3. Golgi apparatus
4. Mitochondria
5. Sarcoplasmic reticulum
6. Ribosomes
7. Glycogen droplets
8. Occasional lipid droplets.
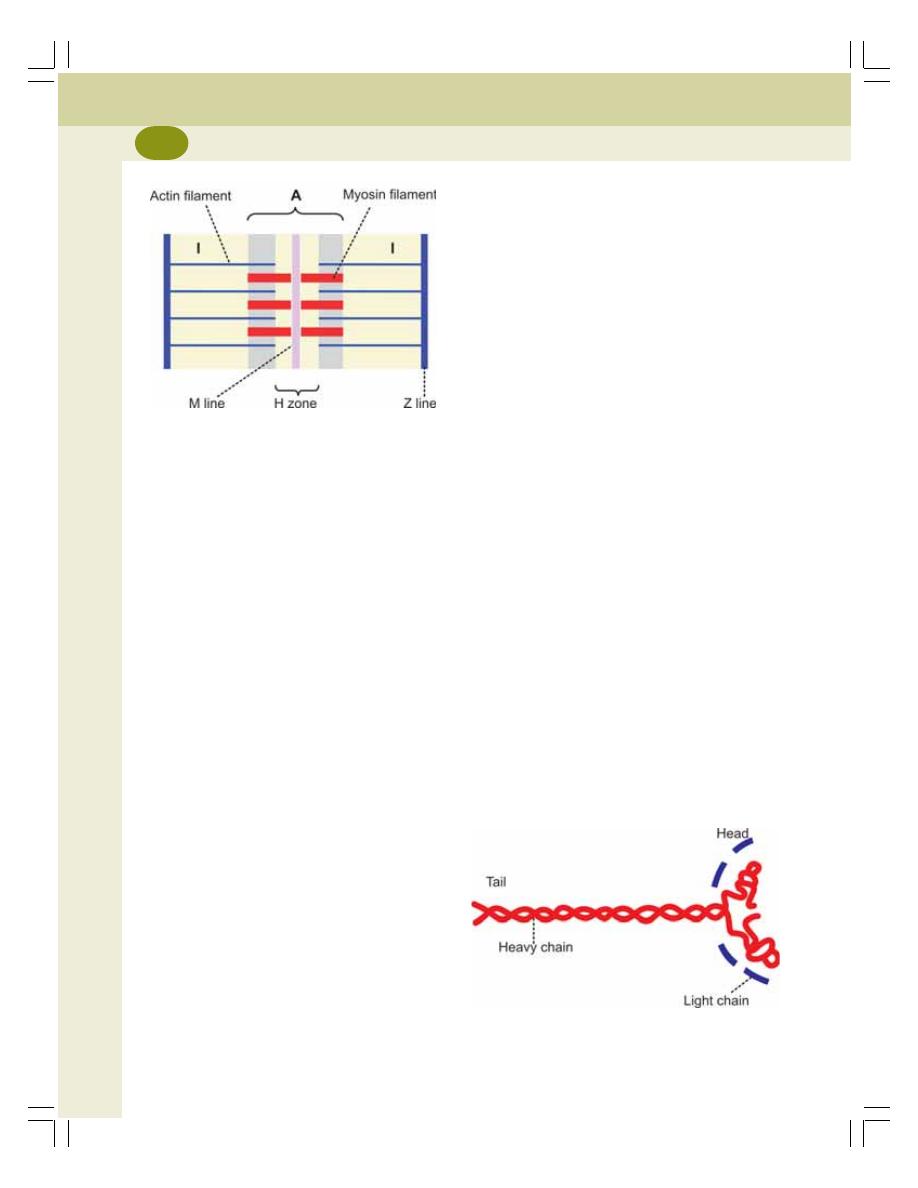
Muscle Physiology
118
Extent
Each sarcomere extends between two ‘Z’ lines
of myofibril. Thus, each myofibril contains many
sarcomeres arranged in series throughout its
length. When the muscle is in relaxed state, the
average length of each sarcomere is 2-3 microns.
Components
Each myofibril consists of alternate dark ‘A’
band and light ‘I’ band (Fig. 21-3). In the middle
of ‘A’ band, there is a light area called ‘H’ zone
(H = hell = light in German, H = Henson –
discoverer). In the middle of ‘H’ zone lies the
middle part of myosin filament. This is called
‘M’ line (in German mittel = middle). ‘M’ line is
formed by myosin binding proteins.
ELECTRON MICROSCOPIC STUDY
OF SARCOMERE
The electron microscopic studies reveal, that
the sarcomere consists of many thread like
structures called myofilaments. Myofilaments
are of two types:
1. Actin filaments
2. Myosin filaments.
Actin Filaments
Actin filaments are the thin filaments that extend
from either side of the ‘Z’ lines, run across ‘I’ band
and enter into ‘A’ band up to ‘H’ zone.
Myosin Filaments
Myosin filaments are thick filaments and are
situated in ‘A’ band.
Some lateral processes (projections) or cross
bridges arise from myosin filaments. These
bridges have enlarged structures called myosin
heads at their tips. The myosin heads attach
themselves to actin filaments. These heads pull
the actin filaments during contraction of the
muscle by means of a mechanism called sliding
mechanism or ratchet mechanism (Chapter 23).
CONTRACTILE ELEMENTS
(PROTEINS) OF MUSCLE
The myosin filaments are formed by protein
molecules called myosin molecules. The actin
filaments are formed by three types of proteins
called actin, tropomyosin and troponin. These four
proteins together constitute the muscle proteins
or the contractile elements of the muscle.
In addition to the contractile proteins, the sarco-
mere contains some more proteins (Fig. 21-8).
MYOSIN MOLECULE
Each myosin filament consists of about 200
myosin molecules. Myosin is a globulin which is
made up of 6 polypeptide chains. Out of these,
two are heavy chains and four are light chains.
The two heavy chains twist around each other to
form a double helix (Fig. 21-4). At one end, the
two chains remain twisted around one another
and form the tail portion. At the other end, both
the chains turn away in opposite directions and
FIGURE 21-3:
Sarcomere. A = A band,
I = I band
FIGURE 21-4:
Myosin molecule formed by two heavy
chains and four light chains of polypeptides
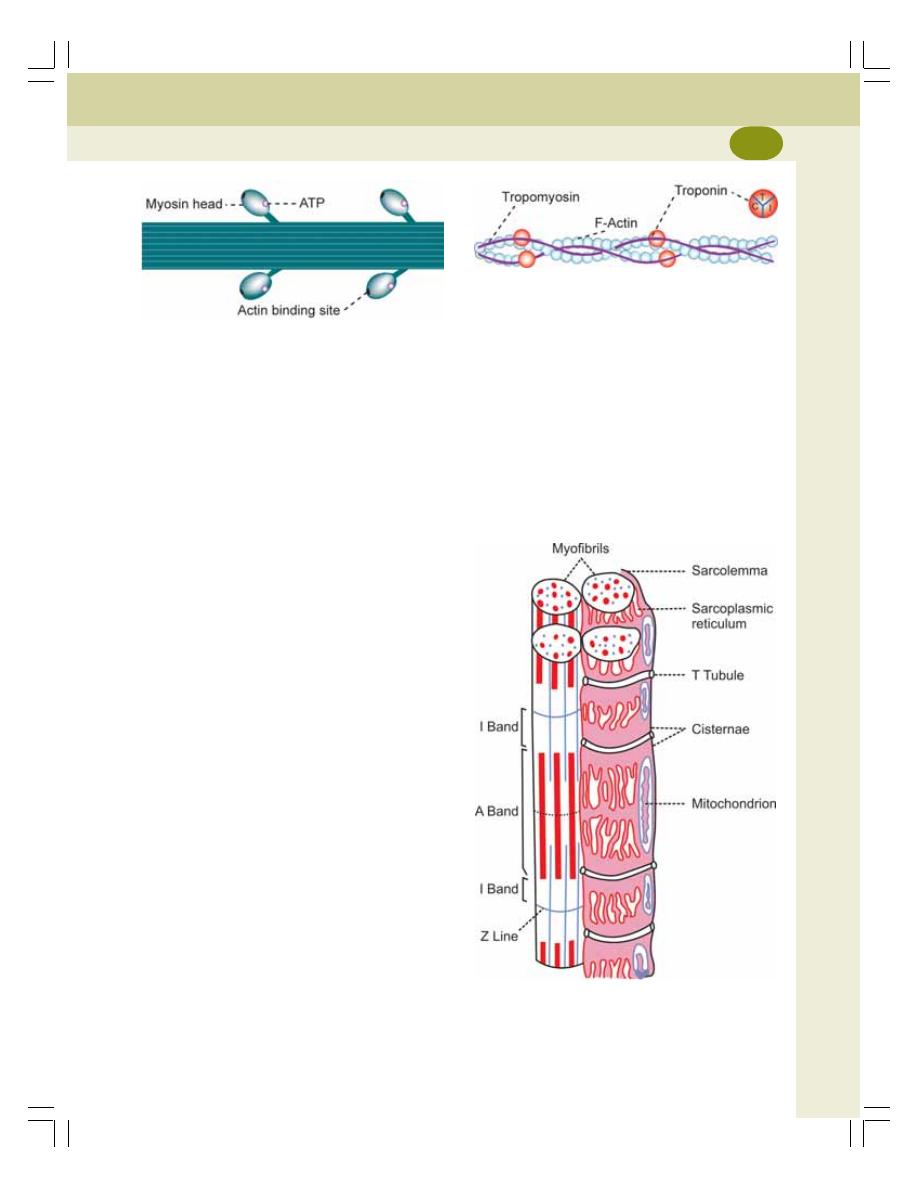
Chapter 21 Structure of Skeletal Muscle
119
form the globular head portion. To each part of
this head, are attached two light chains.
Each myosin head has two attachment sites.
One site is for actin filament and the other one
is for one ATP molecule (Fig. 21-5). In the central
part of the myosin filament, i.e. in the ‘H’ zone,
the myosin head is absent.
ACTIN MOLECULE
Actin molecules are the major constituents of the
thin actin filaments. Each actin molecule is called
F actin and it is derived from G actin. There are
about 300-400 actin molecules in each actin
filament. The actin molecules in the actin
filament are also arranged in the form of a double
helix. Each F actin molecule has an active site
to which the myosin head is attached (Fig. 21-6).
TROPOMYOSIN
There are about 40-60 tropomyosin molecules
situated along the double helix strand of actin
filament. In relaxed condition of the muscle, the
tropomyosin molecules cover all the active sites
of F actin molecules.
TROPONIN
It is formed by three subunits:
1. Troponin I – attached to F actin
2. Troponin T – attached to tropomyosin
3. Troponin C – attached to calcium ions.
SARCOTUBULAR SYSTEM
Sarcotubular system is a system of membranous
structures in the form of vesicles and tubules in
the sarcoplasm of the muscle fiber. It surrounds
the myofibrils embedded in the sarcoplasm.
FIGURE 21-5:
Diagram showing myosin filament
FIGURE 21-6:
Part of actin filament. Troponin
has three subunits, T, C and I
FIGURE 21-7:
Diagram showing the relation between
sarcotubular system and parts of sarcomere. Only few
myofilaments are shown in the myofibril drawn on the
right side of the diagram
The sarcotubular system is formed mainly by
two types of structures:
1. ‘T’ tubules
2. ‘L’ tubules or sarcoplasmic reticulum.
‘T’ Tubules
‘T’ tubules or transverse tubules are narrow
tubules formed by invagination of the sarco-
lemma. These tubules penetrate all the way from
one side of the muscle fiber to other side.
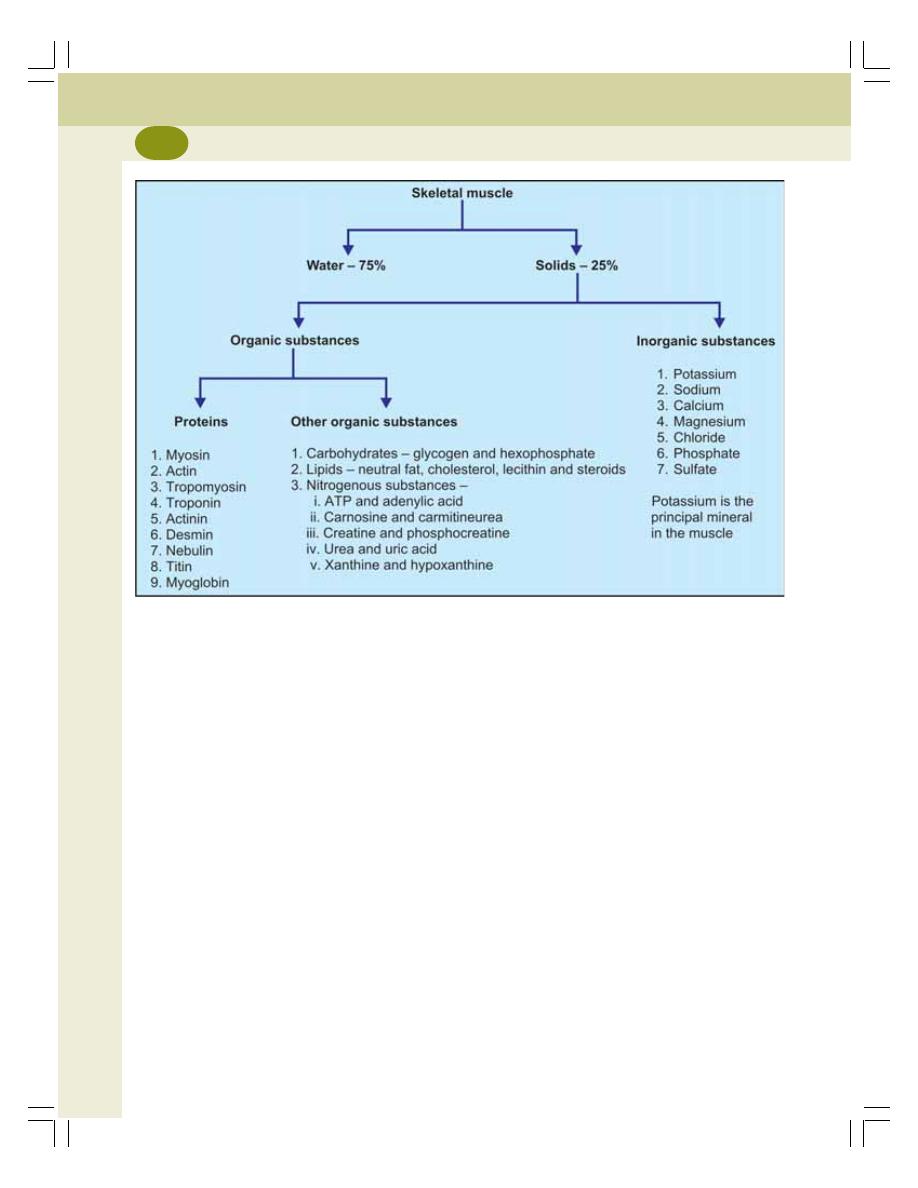
Muscle Physiology
120
Because of their origin from sarcolemma, the ‘T’
tubules open to the exterior of the muscle cell.
Therefore, the ECF runs through their lumen.
Function of ‘T’ Tubules
The ‘T’ tubules are responsible for rapid trans-
mission of impulse in the form of action potential
from sarcolemma to the myofibrils.
‘L’ Tubules or Sarcoplasmic Reticulum
The ‘L’ tubules or longitudinal tubules are the
closed tubules that run in long axis of the muscle
fiber forming sarcoplasmic reticulum. These
tubules form a closed tubular system around
each myofibril and do not open to exterior like
‘T’ tubules.
The ‘L’ tubules correspond to the endoplasmic
reticulum of other cells. At regular intervals,
throughout the length of the myofibrils, the ‘L’
tubules dilate to form a pair of lateral sacs called
terminal cisternae. Each pair of terminal cisternae
FIGURE 21-8:
Composition of skeletal muscle
is in close contact with ‘T’ tubule. The ‘T’ tubule
along with the cisternae on either side is called
the triad of skeletal muscle. Calcium ions are
stored in ‘L’ tubule and the amount of calcium
ions is more in cisternae (Fig. 21-7).
Functions of ‘L’ tubules
The ‘L’ tubules store a large quantity of calcium
ions. When the action potential reaches the
cisternae of ‘L’ tubule, the calcium ions are
released into the sarcoplasm. The calcium ions
trigger the processes involved in contraction of
the muscle. The process by which the calcium
ions cause contraction of muscle is called
excitation contraction coupling (Chapter 23).
COMPOSITION OF MUSCLE
The skeletal muscle is formed by 75% of water
and 25% of solids. Solids are 20% of proteins
and 5% of organic substances other than proteins
and inorganic substances (Fig. 21-8).

EXCITABILITY
DEFINITION
STIMULUS
CONTRACTILITY
TYPES OF CONTRACTION
RED MUSCLE AND PALE MUSCLE
FACTORS AFFECTING FORCE OF CONTRACTION
REFRACTORY PERIOD
MUSCLE TONE
DEFINITION
MAINTENANCE OF MUSCLE TONE
APPLIED PHYSIOLOGY— ABNORMALITIES OF MUSCLE TONE
EXCITABILITY
DEFINITION
Excitability is defined as the reaction or response
of a tissue to irritation or stimulation. It is a
physicochemical change.
STIMULUS
Stimulus is the change in environment. It is
defined as an agent or influence or act, which
brings about the response in an excitable tissue.
Types of Stimulus
There are four types of stimuli, which can excite
a living tissue:
1. Mechanical stimulus (Pinching)
2. Electrical stimulus (Electric shock)
3. Thermal stimulus (By applying heated glass
rod or icepiece)
4. Chemical stimulus (By applying chemical
substances like acids).
Electrical stimulus is commonly used for
experimental purposes.
Intensity of Stimulus
The intensity or strength of a stimulus is of five
types:
i.
Subminimal stimulus
ii. Minimal stimulus
iii. Submaximal stimulus
iv. Maximal stimulus
v. Supramaximal stimulus.
The stimulus whose strength (or voltage) is
sufficient to excite the tissue is called threshold
or liminal or minimal stimulus.
Properties of Skeletal Muscle
22

Muscle Physiology
122
CONTRACTILITY
Contractility is the response of the skeletal
muscle to a stimulus by change in either the
length or tension of the muscle fibers.
TYPES OF CONTRACTION
Muscular contraction is classified into two types
based on change in the length of muscle fibers
or tension of the muscle:
1. Isotonic contraction
2. Isometric contraction.
Isotonic Contraction
Isotonic contraction is the type of muscular
contraction in which the tension remains the
same and the length of the muscle fiber is altered
(Iso = same: Tonic = tension). Example is the
simple flexion of arm, where shortening of muscle
fibers occurs but the tension does not change.
Isometric Contraction
Isometric contraction is the type of muscular
contraction in which the length of muscle fibers
remains the same and the tension is increased.
Example is pulling any heavy object when
muscles become stiff and strained with
increased tension but the length does not
change.
RED MUSCLE AND PALE MUSCLE
Based on the contraction time, the skeletal
muscles are classified into two types, the red
(slow) muscles and pale (fast) muscles.
Similarly, the muscle fibers are also divided into
two types, type I and type II fibers. Type I fibers
(slow fibers or slow twitch fibers) have small
diameter. Type II fibers (fast fibers or fast twitch
fibers) have large diameter. Most of the skeletal
muscles in human beings contain both the types
of fibers.
Red Muscles
The muscles which contain large number of type
I fibers are called red muscles. These muscles
are also called slow muscles or slow twitch
muscles. The red muscles have longer
contraction time. Back muscles and
gastrocnemius muscles are red muscles.
Pale Muscles
The muscles which have large number of type
II fibers are called pale muscles. These muscles
are also called white muscles, fast muscles or
fast twitch muscles. The pale muscles have
shorter contraction time. Hand muscles and
ocular muscles are pale muscles.
The characteristic features of red and pale
muscles are given in Table 22-1.
FACTORS AFFECTING FORCE
OF CONTRACTION
The force of contraction of the skeletal muscle
is affected by the following factors:
A. Strength of stimulus
B. Number of stimulus
C. Temperature
D. Load.
A. Effect of Strength of Stimulus
Force of contraction is directly proportional to
strength of stimulus.
B. Effect of Number of Stimulus
The response of the muscle in the form of
contraction differs depending upon the number
of stimuli. If a single stimulus is given, the muscle
gives response only once. If two stimuli are given
with sufficient time interval it gives response
twice.
When a muscle is stimulated by multiple
stimuli, two types of effects are obtained
depending upon the frequency of stimuli:
1. Fatigue
2. Tetanus.
1. Fatigue
Definition
Fatigue is defined as the decrease in muscular
activity due to repeated stimuli with low
frequency. When the stimuli are applied

Chapter 22 Properties of Skeletal Muscle
123
continuously, after some time, the muscle does
not show any response to the stimulus. This
condition is called fatigue.
Causes for fatigue
i.
Exhaustion of acetylcholine in motor endplate
ii. Accumulation of metabolites like lactic acid
and phosphoric acid
iii. Lack of nutrients like glycogen
iv. Lack of oxygen.
Site (Seat) of fatigue
In the intact body, the sites of fatigue are in the
following order:
i.
Betz (pyramidal) cells in cerebral cortex
ii. Anterior gray horn cells (motor neurons) of
spinal cord
iii. Neuromuscular junction
iv. Muscle.
Recovery of the muscle after fatigue
The fatigue is a reversible phenomenon. The
fatigued muscle recovers if given rest and
nutrition.
Causes of recovery
i.
Removal of metabolites
ii. Formation of acetylcholine at the neuro-
muscular junction
iii. Re-establishment of normal polarized state
of the muscle
iv. Availability of nutrients
v. Availability of oxygen.
In the intact body, all the processes involved
in recovery are achieved by circulation itself.
2. Tetanus
Definition
Tetanus is defined as the sustained contraction
of muscle due to repeated stimuli with high
frequency. When the multiple stimuli are applied
at a higher frequency in such a way that the
successive stimuli fall during contraction period
of previous twitch, the muscle remains in state
of tetanus, i.e. all the contractions are fused. The
muscle relaxes only after the stoppage of
stimulus or when the muscle is fatigued.
TABLE 22-1: Features of red and pale muscles
Red (slow) muscle
Pale (fast) muscle
1.
Type I fibers are more
Type II fibers are more
2.
Myoglobin content is high. So, it is red
Myoglobin content is less. So, it is pale
3.
Sarcoplasmic reticulum is less extensive
Sarcoplasmic reticulum is more extensive
4.
Blood vessels are more extensive
Blood vessels are less extensive
5.
Mitochondria are more in number
Mitochondria are less in number
6.
Response is slow with long latent period
Response is rapid with short latent period
7.
Contraction is less powerful
Contraction is more powerful
8.
This muscle is involved in prolonged and
This muscle is not involved in prolonged and
continued activity as it undergoes sustained
continued activity as it relaxes immediately.
contraction
9.
Fatigue occurs slowly
Fatigue occurs quickly.
10.
Depends upon cellular respiration for ATP
Depends upon glycolysis for ATP production.
production

Muscle Physiology
124
If the frequency of stimuli is less, partial fusion
of contractions takes place leading to incomplete
tetanus or clonus.
Frequency of stimuli necessary to cause
tetanus and clonus
In gastrocnemius muscle of human being, the
frequency required to cause tetanus is 60/
second. And for clonus, the frequency of stimuli
necessary is 55/second.
C. Effect of Variations in Temperature
If the temperature of muscle is altered, the force
of contraction is also affected.
Warm temperature
At warm temperature of about 40°C, the force
of contraction increases because of the following
reasons:
1. The excitability of muscle increases
2. The chemical processes involved in muscular
contraction are accelerated
3. The viscosity of muscle decreases.
Cold temperature
At cold temperature of about 10°C, the force of
contraction decreases because of the following
reasons:
1. Excitability of muscle decreases
2. Chemical processes are slowed or delayed
3. Viscosity of the muscle increases.
High or hot temperature – Heat rigor
At high temperatures, heat rigor occurs in the
muscle. Rigor refers to shortening and stiffening
of muscle fibers. Heat rigor is the rigor that occurs
due to increased temperature above 60°C. The
cause of heat rigor is the coagulation of muscle
proteins actin and myosin. It is an irreversible
phenomenon.
Other types of rigors are:
1. Cold rigor that occurs due to the exposure
to severe cold. It is a reversible phenomenon
2. Calcium rigor which is due to increased
calcium content. It is also reversible
3. Rigor mortis which develops after death.
Rigor mortis
Rigor mortis refers to after death condition of the
body which is characterized by stiffness of
muscles and joints (Latin word rigor means stiff).
It occurs due to stoppage of aerobic respiration
which causes changes in the muscles.
Soon after death, the cell membrane
becomes highly permeable to calcium. So a
large number of calcium ions enters the muscle
fibers and promotes the formation of actomyosin
complex resulting in contraction of the muscles.
Few hours after death, all the muscles of body
undergo severe contraction and become rigid.
The joints also become stiff and locked.
Normally for relaxation, the muscle needs
to drive out the calcium which requires ATP.
But during continuous muscular contraction
and other cellular processes after death, the
ATP molecules are completely exhausted.
New ATP molecules cannot be produced
because of lack of oxygen. So in the absence
of ATP, the muscles remain in contracted state
until the onset of decomposition.
Medicolegal importance of rigor mortis
Rigor mortis is useful in determining the time of
death. Onset of stiffness starts between 10
minutes and 3 hours after death depending upon
the condition of the body and environmental
temperature at the time of death. If the body is
active or the environmental temperature is high
at the time of death, the stiffness sets in quickly.
The stiffness develops first in facial muscles
and then spreads to other muscles. The
maximum stiffness occurs around 12 to 24 hours
after death. The stiffness of muscles and joints
continues for 1 to 3 days.
Afterwards, the decomposition of the general
tissues starts. Now the lysosomal intracellular
hydrolytic enzymes like cathepsins and calpains
are released. These enzymes hydrolyze the
muscle proteins, actin and myosin resulting in
breakdown of actomyosin complex. It relieves the
stiffness of the muscles. This process is known
as resolution of rigor.

Chapter 22 Properties of Skeletal Muscle
125
D. Effect of Load
The load acting on muscle is of two types:
1. Afterload
2. Free load.
1. Afterload
Afterload is the load, that acts on the muscle after
the beginning of muscular contraction. Example
of afterload is lifting any object from the ground.
The load acts on muscles of arm only after lifting
the object off the ground, i.e. only after beginning
of the muscular contraction.
2. Free load
Free load is the load, which acts on the muscle
freely, even before the onset of contraction of
the muscle. It is otherwise called fore load.
Example of free load is filling water from a tap
by holding the bucket in hand.
Muscle in free loaded condition works better
than the muscle in after loaded condition. It is
because, in free loaded condition, the muscle
fibers are stretched and the initial length of
muscle fibers is increased. So, the force of
contraction and the work done by the muscles
are increased. It is in accordance with Frank-
Starling law.
Frank-Starling law
Frank-Starling law states that the force of
contraction is directly proportional to the initial
length of muscle fibers within physiological
limits.
REFRACTORY PERIOD
Refractory period is the period at which the
muscle does not show any response to a
stimulus. It is because already one action
potential is in progress and the muscle is in
depolarized state during this period. The muscle
is unexcitable to further stimulation until it is
repolarized. Refractory period is of two types:
1. Absolute refractory period
2. Relative refractory period
1. Absolute Refractory Period
Absolute refractory period is the period during
which the muscle does not show any response
at all, whatever may be the strength of stimulus.
2. Relative Refractory Period
Relative refractory period is the period, during
which the muscle shows some response if the
strength of stimulus is increased to maximum.
MUSCLE TONE
DEFINITION
Muscle tone is defined as continuous and partial
contraction of the muscles with certain degree
of vigor and tension. More details on muscle tone
are given in Chapter 97.
MAINTENANCE OF MUSCLE TONE
In Skeletal Muscle
Maintenance of tone in skeletal muscle is neuro-
genic. It is due to continuous discharge of
impulses from gamma motor neurons in anterior
gray horn of spinal cord. The gamma motor
neurons in spinal cord are controlled by higher
centers in brain.
In Cardiac Muscle
In cardiac muscle, maintenance of tone is purely
myogenic, i.e. the muscles themselves control
the tone. The tone is not under nervous control
in cardiac muscle.
In Smooth Muscle
In smooth muscle, tone is myogenic. It depends
upon calcium level and number of cross bridges.
APPLIED PHYSIOLOGY –
ABNORMALITIES OF
MUSCLE TONE
1. Hypertonia – increased muscle tone
2. Hypotonia – decreased muscle tone

ELECTRICAL CHANGES DURING MUSCULAR CONTRACTION
RESTING MEMBRANE POTENTIAL
ACTION POTENTIAL
ACTION POTENTIAL CURVE
IONIC BASIS OF ELECTRICAL EVENTS
GRADED POTENTIAL
MOLECULAR CHANGES DURING MUSCULAR CONTRACTION
ACTOMYOSIN COMPLEX
MOLECULAR BASIS OF MUSCULAR CONTRACTION
ELECTRICAL CHANGES DURING
MUSCULAR CONTRACTION
When the muscle is in resting condition, the
electrical potential is called resting membrane
potential. When the muscle is stimulated,
electrical changes occur which are collectively
called action potential.
RESTING MEMBRANE POTENTIAL
Resting membrane potential is the electrical
potential difference (voltage) across the cell
membrane (between inside and outside of the
cell) under resting condition. It is also called
membrane potential, transmembrane potential,
transmembrane potential difference or
transmembrane potential gradient.
Resting muscle shows negativity inside and
positivity outside. The condition of the muscle
during resting membrane potential is called
polarized state. In human skeletal muscle, the
resting membrane potential is –90 mV.
ACTION POTENTIAL
Action potential is defined as a series of electrical
changes that occur when the muscle or nerve
is stimulated.
Action potential occurs in two phases:
1. Depolarization
2. Repolarization.
Depolarization
Depolarization is the initial phase of action
potential in which the inside of the muscle
becomes positive and outside becomes
negative. That is, the polarized state (resting
membrane potential) is abolished resulting in
depolarization.
Repolarization
Repolarization is the phase of action potential
when the potential inside the muscle reverses
back to the resting membrane potential. That is,
Electrical and Molecular
Changes during Muscular
Contraction
23
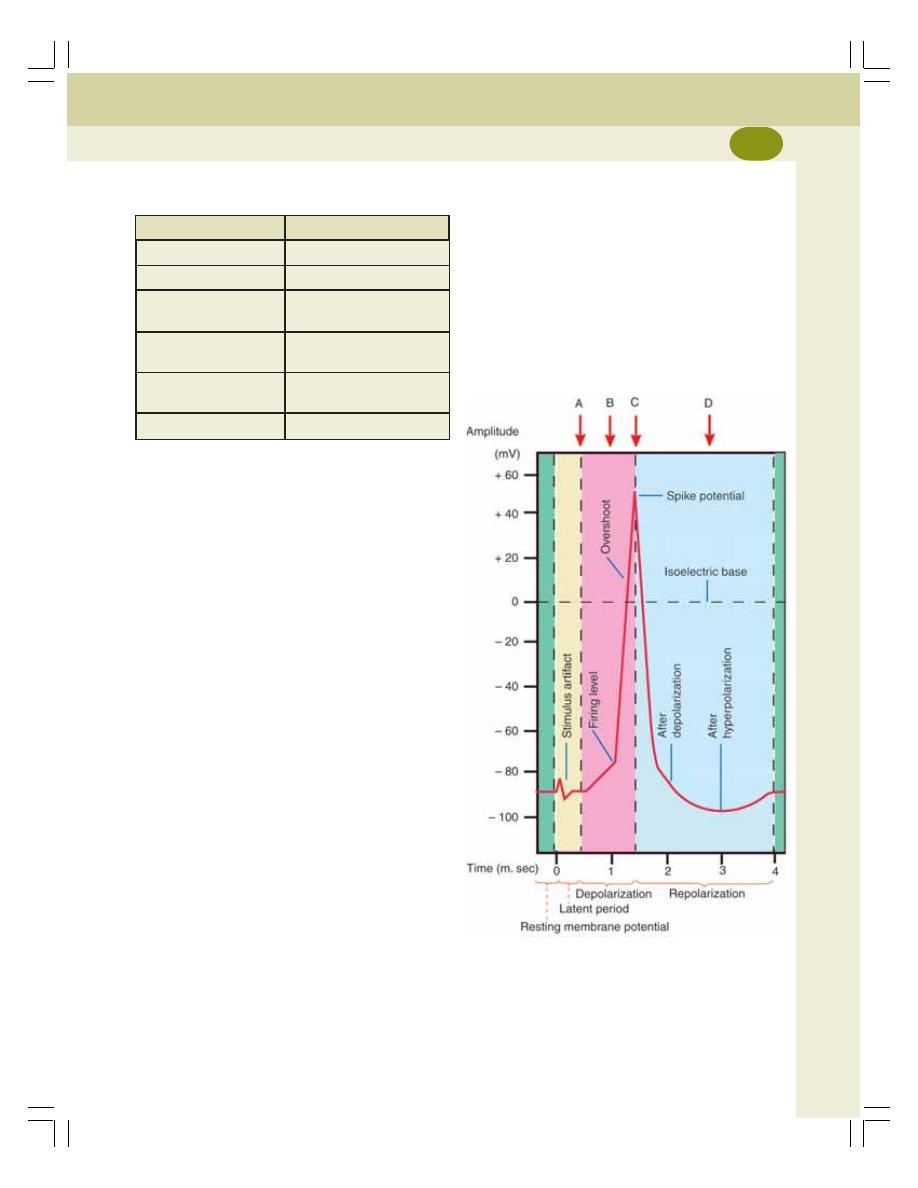
Chapter 23 Electrical and Molecular Changes during Muscular Contraction
127
the rate of depolarization increases suddenly.
The point at which, the depolarization increases
suddenly is called firing level.
Overshoot
From firing level, the curve reaches isoelectric
potential (zero potential) rapidly and then shoots
up (overshoots) beyond the zero potential
(isoelectric base) up to +55 mV. It is called
overshoot.
FIGURE 23-1: Action potential in a skeletal muscle
A = Opening of few Na
+
channels
B = Opening of many Na
+
channels
C = Closure of Na
+
channels and opening of K
+
channels
D = Closure of K
+
channels
TABLE 23-1: Properties of action potential and
graded potential
Action potential
Graded potential
Propagative
Non-propagative
Long distance signal
Short distance signal
Both depolarization
Only depolarization or
and repolarization
hyperpolarization
Obeys all or none law
Does not obey all or
none law
Summation is not
Summation is possible
possible
Has refractory period
No refractory period
within a short time after depolarization the interior
of muscle becomes negative and outside
becomes positive. So, the polarized state of the
muscle is re-established.
Properties of Action Potential
The properties of action potential are listed in
Table 23-1.
ACTION POTENTIAL CURVE
Stimulus Artifact
The resting membrane potential is recorded as
a straight baseline at –90 mV (Fig. 23-1). When
a stimulus is applied, there is a slight irregular
deflection of baseline for a very short period. This
is called stimulus artifact. The artifact is due to
leakage of current from stimulating electrode to
the recording electrode. The stimulus artifact is
followed by latent period.
Latent Period
This is the period when no change occurs in the
electrical potential. It is a very short period with
duration of 0.5 to 1 millisecond.
Firing Level and Depolarization
Depolarization starts after the latent period.
Initially, it is very slow. After the initial slow
depolarization for about 15 mV (up to –75 mV),

Muscle Physiology
128
Repolarization
When depolarization is completed (+55 mV), the
repolarization starts. Initially, the repolarization
occurs rapidly and then it becomes slow.
Spike Potential
The rapid rise in depolarization and the rapid fall
in repolarization are together called spike
potential. It lasts for 0.4 millisecond.
After Depolarization or Negative
after Potential
The rapid fall in repolarization is followed by a
slow repolarization. It is called after depola-
rization or negative after potential. Its duration
is 2 to 4 milliseconds.
After Hyperpolarization or Positive
after Potential
After reaching the resting level (–90 mV), it
becomes more negative beyond resting level.
This is called after hyperpolarization or positive
after potential. This lasts for more than 50
milliseconds. After this, the normal resting
membrane potential is restored slowly.
IONIC BASIS OF ELECTRICAL
EVENTS
Resting Membrane Potential
The development and maintenance of resting
membrane potential in a muscle fiber or a neuron
are carried out by movement of ions, which
produce ionic imbalance across the cell
membrane. This results in the development of
more positivity outside and more negativity inside
the cell.
The ionic imbalance is produced by two
factors:
1. Sodium-potassium pump
2. Selective permeability of cell membrane.
1. Sodium-potassium pump
Sodium and potassium ions are actively
transported in opposite directions across the cell
membrane by means of an electrogenic pump
called sodium-potassium pump. It moves three
sodium ions out of the cell and two potassium
ions inside the cell by using energy from
ATP. Since more positive ions (cations) are
pumped outside than inside, a net deficit of
positive ions occurs inside the cell. It leads to
negativity inside and positivity outside the cell.
More details of this pump are given Chapter 3.
2. Selective permeability of cell membrane
The permeability of cell membrane depends
largely on the transport channels. The transport
channels are selective for movement of some
specific ions. Most of the channels are gated
channels and the specific ions can move across
the membrane only when these gated channels
are opened.
Channels for major anions (negatively
charged substances) like proteins
However, channels for some of the negatively
charged large substances such as proteins
and negatively charged organic phosphate and
sulfate compounds are absent or closed. So,
such substances remain inside the cell and
play a major role in the development and
maintenance of negativity inside the cell
(resting membrane potential).
Channels for ions
In addition, the channels for three important ions,
sodium, chloride and potassium also play an
important role in maintaining the resting
membrane potential.
Action Potential
During the onset of depolarization, voltage gated
Na
+
channels open resulting in slow influx of Na
+
.
When depolarization reaches 7 to 10 mV, the
voltage gated Na
+
channels start opening at a
faster rate. It is called Na
+
channel activation.
When the firing level is reached, the influx of Na
+
is very great and it leads to overshoot.
But the Na
+
transport is short-lived. This is
because of rapid inactivation of Na
+
channels.

Chapter 23 Electrical and Molecular Changes during Muscular Contraction
129
Thus, the Na
+
channels open and close quickly.
At the same time, the K
+
channels start opening.
This leads to efflux of K
+
out of the cell, causing
repolarization.
Unlike the Na
+
channels, the K
+
channels
remain open for longer duration. These channels
remain opened for few more milliseconds after
completion of repolarization. It causes efflux of
more number of K
+
producing more negativity
inside. It is the cause for hyperpolarization.
GRADED POTENTIAL
Graded potential is a mild local change in the
membrane potential that develops in receptors,
synapse or neuromuscular junction when
stimulated. It is also called graded membrane
potential or graded depolarization. The graded
potential is distinct from the action potential and
the properties of these two potentials are given
in Table 23-1. In most of the cases, the graded
potential is responsible for the generation of
action potential. However, in some cases the
graded potential hyperpolarizes the membrane
potential (more negativity than resting membrane
potential).
The graded potentials include:
1. End plate potential in neuromuscular junction
(Chapter 24)
2. Receptor potential (Chapter 85)
4. Excitatory postsynaptic potential (Chapter 86)
5. Inhibitory postsynaptic potential (Chapter 86).
MOLECULAR CHANGES DURING
MUSCULAR CONTRACTION
ACTOMYOSIN COMPLEX
In the relaxed state of the muscle, the thin actin
filaments from the opposite ends of the
sarcomere are away from each other leaving a
broad ‘H’ zone.
During the contraction of the muscle, the actin
(thin) filaments glide over the myosin (thick)
filaments and form actomyosin complex.
MOLECULAR BASIS OF MUSCULAR
CONTRACTION
The molecular mechanism is responsible for
formation of actomyosin complex that results in
muscular contraction. It includes three stages:
1. Excitation contraction coupling
2. Role of troponin and tropomyosin
3. Sliding mechanism
1. Excitation Contraction Coupling
Excitation contraction coupling is the process that
occurs in between the excitation and contraction
of the muscle. This process involves series of
activities which are responsible for the contraction
of the excited muscle.
Stages of excitation contraction coupling
When the impulse passes through a motor
neuron and reaches the neuromuscular junction,
acetylcholine is released from motor endplate.
Acetylcholine causes opening of ligand gated
sodium channels. So, sodium ions enter the
neuromuscular junction. It leads to the
development of endplate potential. Endplate
potential causes generation of action potential
in the muscle fiber.
The action potential spreads over sarco-
lemma and also into the muscle fiber through
the ‘T’ tubules. The ‘T’ tubules are responsible
for the rapid spread of action potential into the
muscle fiber. When the action potential reaches
the cisternae of ‘L’ tubules, these cisternae are
excited. Now, the calcium ions stored in the
cisternae are released into the sarcoplasm. The
calcium ions from the sarcoplasm move towards
the actin filaments to produce the contraction.
Thus, the calcium ion forms the link or
coupling material between the excitation and
the contraction of muscle. Hence, the calcium
ions are said to form the basis of excitation
contraction coupling.
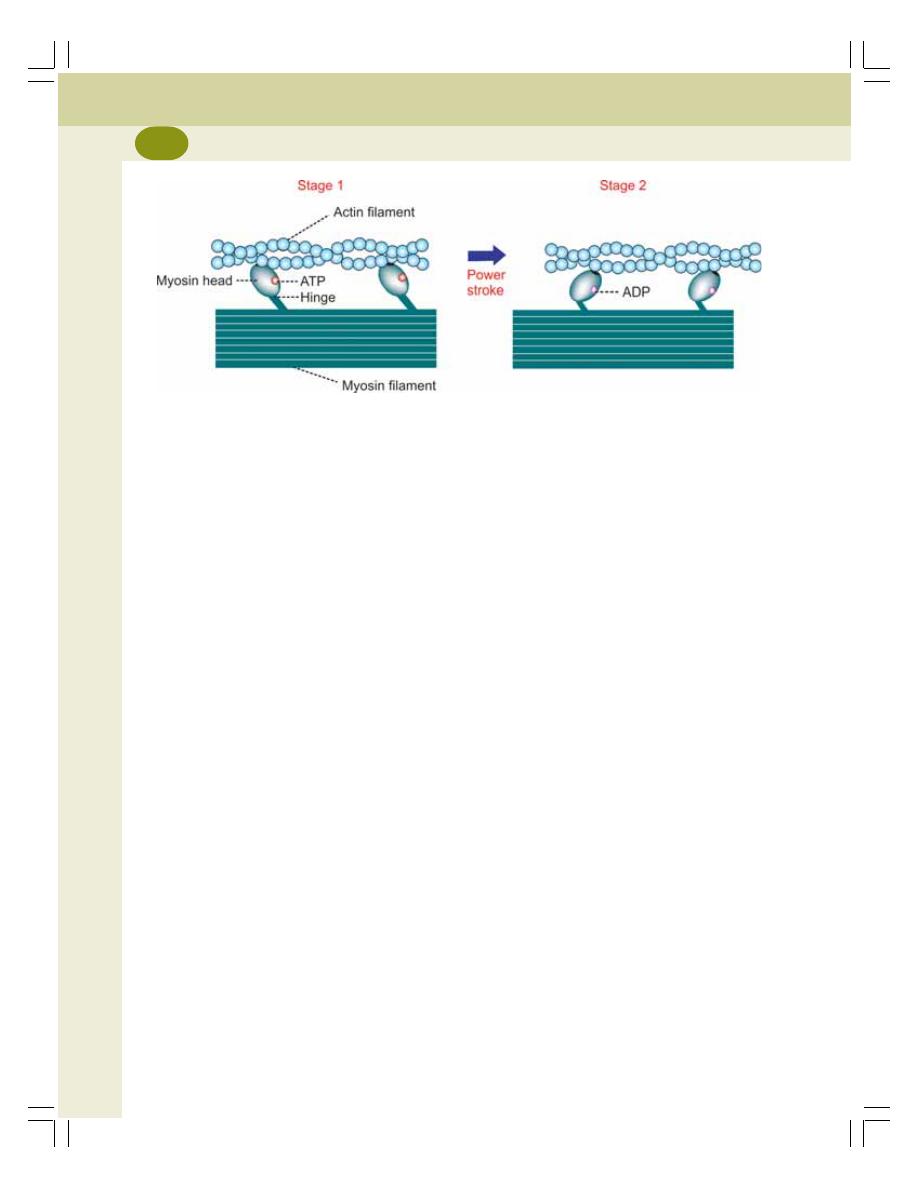
Muscle Physiology
130
2. Role of Troponin and Tropomyosin
Normally, the head of myosin molecules has a
strong tendency to get attached with active site
of F actin. However, in relaxed condition, the
active site of F actin is covered by the
tropomyosin. Therefore, the myosin head cannot
combine with actin molecule.
Large number of calcium ions, which are
released from ‘L’ tubules during the excitation of
the muscle, bind with troponin C. The loading of
troponin C with calcium ions produces some
change in the position of troponin molecule. It
in turn, pulls tropomyosin molecule away from
F actin. Due to the movement of tropomyosin,
the active site of F actin is uncovered and
immediately the head of myosin gets attached
to the actin.
3. Sliding Mechanism and Formation of
Actomyosin Complex – Sliding Theory
Sliding theory explains how the actin filaments
slide over myosin filaments and form the
actomyosin complex during muscular
contraction. It is also called ratchet theory or walk
along theory.
Each cross bridge from the myosin filaments
has got three components namely, a hinge, an
arm and a head.
After binding with active site of F actin, the
myosin head is tilted towards the arm so that
the actin filament is dragged along with it
(Fig. 23-2). This tilting of head is called power
stroke. After tilting, the head immediately breaks
away from the active site and returns to the
original position. Now, it combines with a new
active site on the actin molecule. And, tilting
movement occurs again. Thus, the head of
cross bridge bends back and forth and pulls the
actin filament towards the center of sarcomere.
In this way, all the actin filaments of both the
ends of sarcomere are pulled. So, the actin
filaments of opposite sides overlap and form
actomyosin complex. Formation of actomyosin
complex results in contraction of the muscle.
When the muscle shortens further, the actin
filaments from opposite ends of the sarcomere
approach each other. So, the ‘H’ zone becomes
narrow. And, the two ‘Z’ lines come closer with
reduction in length of the sarcomere. However,
the length of ‘A’ band is not altered. But, the length
of ‘I’ band decreases.
When the muscular contraction becomes
severe, the actin filaments from opposite ends
overlap and, the ‘H’ zone disappears.
Thus, during the contraction of the muscle,
the following changes occur in the sarcomere:
1. The length of all the sarcomeres decreases
as the ‘Z’ lines come close to each other
2. The length of the ‘I’ band decreases since
the actin filaments from opposite side overlap
FIGURE 23-2: Diagram showing power stroke by myosin head. Stage 1: Myosin head binds
with actin; Stage 2: Tilting of myosin head (power stroke) drags the actin filament
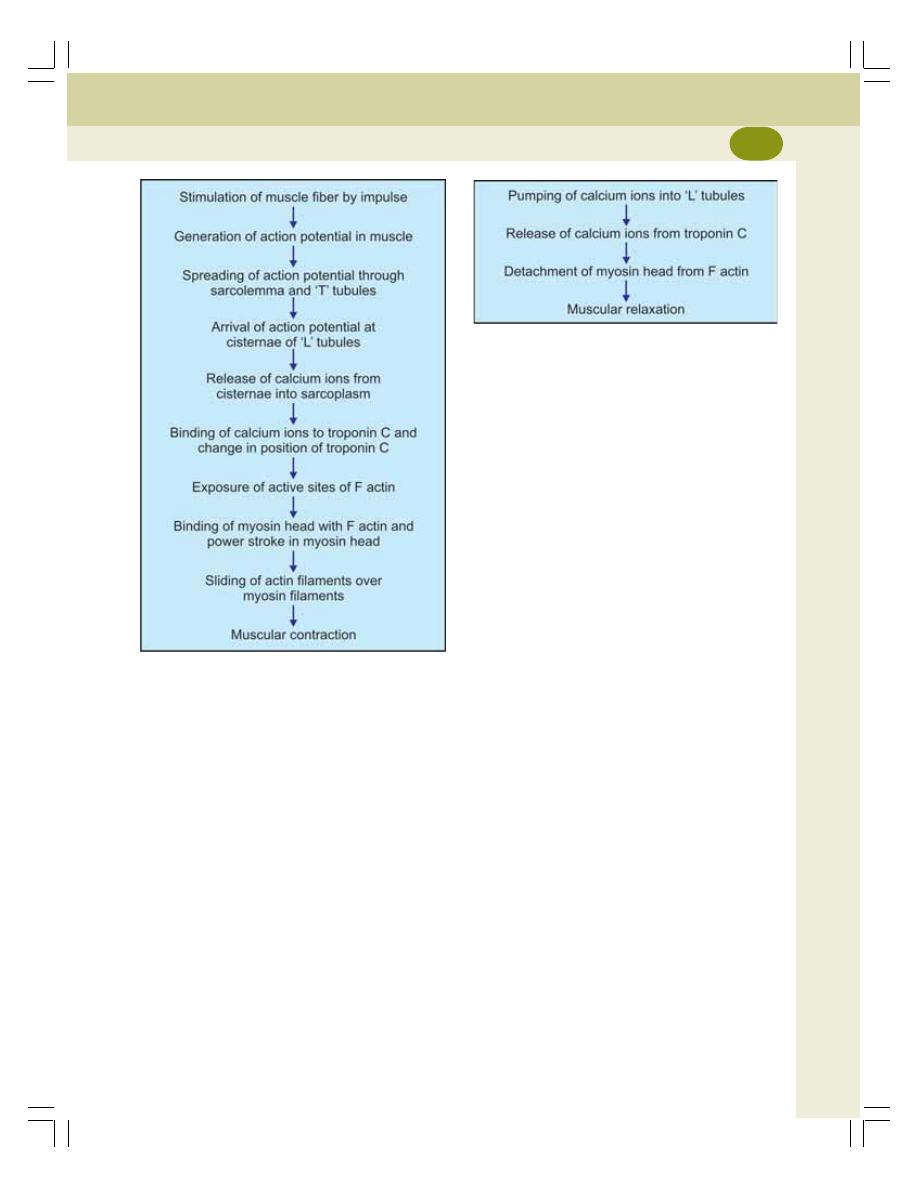
Chapter 23 Electrical and Molecular Changes during Muscular Contraction
131
FIGURE 23-3: Sequence of events during
muscular contraction
FIGURE 23-4: Sequence of events during
muscular relaxation
The head of myosin has a site for ATP.
Actually, the head itself can act as the enzyme
ATPase and catalyze the breakdown of ATP.
Even before the onset of contraction, an ATP
molecule binds with myosin head.
When tropomyosin moves to expose the
active sites, the head is attached to the active
site. Now ATPase cleaves ATP into ADP and
Pi, which remains in head itself. The energy
released during this process is utilized for
contraction.
When head is tilted, the ADP and Pi are
released and a new ATP molecule binds with
head. This process is repeated until the muscular
contraction is completed.
Relaxation of the Muscle
The relaxation of the muscle occurs when the
calcium ions are pumped back into the L tubules.
When calcium ions enter the L tubules, calcium
content in sarcoplasm decreases leading to the
release of calcium ions from the troponin. It
causes detachment of myosin from actin followed
by relaxation of the muscle (Fig. 23-4). The
detachment of myosin from actin obtains energy
from breakdown of ATP. Thus, the chemical
process of muscular relaxation is an active
process although the physical process is said to
be passive.
3. The ‘H’ zone either decreases or disappears
4. The length of ‘A’ band remains the same.
The summary of sequence of events during
muscular contraction is given in Fig. 23-3.
Energy for Muscular Contraction
The energy for movement of myosin head (power
stroke) is obtained by breakdown of adenosine
triphosphate (ATP) into adenosine diphosphate
(ADP) and inorganic phosphate (Pi).

DEFINITION AND STRUCTURE
NEUROMUSCULAR TRANSMISSION
RELEASE OF ACETYLCHOLINE
ACTION OF ACETYLCHOLINE
ENDPLATE POTENTIAL
MINIATURE ENDPLATE POTENTIAL
FATE OF ACETYLCHOLINE
NEUROMUSCULAR BLOCKERS
MOTOR UNIT
DEFINITION
NUMBER OF MUSCLE FIBERS IN MOTOR UNIT
APPLIED PHYSIOLOGY – DISORDERS OF NEUROMUSCULAR JUNCTION
Neuromuscular
Junction
24
DEFINITION AND STRUCTURE
DEFINITION
Neuromuscular junction is the junction between
the terminal branch of the nerve fiber and muscle
fiber.
STRUCTURE
Skeletal muscle fibers are innervated by the
motor nerve fibers. Each nerve fiber (axon)
divides into many terminal branches. Each
terminal branch innervates one muscle fiber
through the neuromuscular junction (Fig. 24-1).
Axon Terminal and Motor Endplate
Terminal branch of nerve fiber is called axon
terminal. When the axon comes close to the
muscle fiber, it loses the myelin sheath. So, the
axis cylinder is exposed. This portion of the axis
cylinder is expanded like a bulb which is called
motor endplate.
FIGURE 24-1: Longitudinal section
of neuromuscular junction
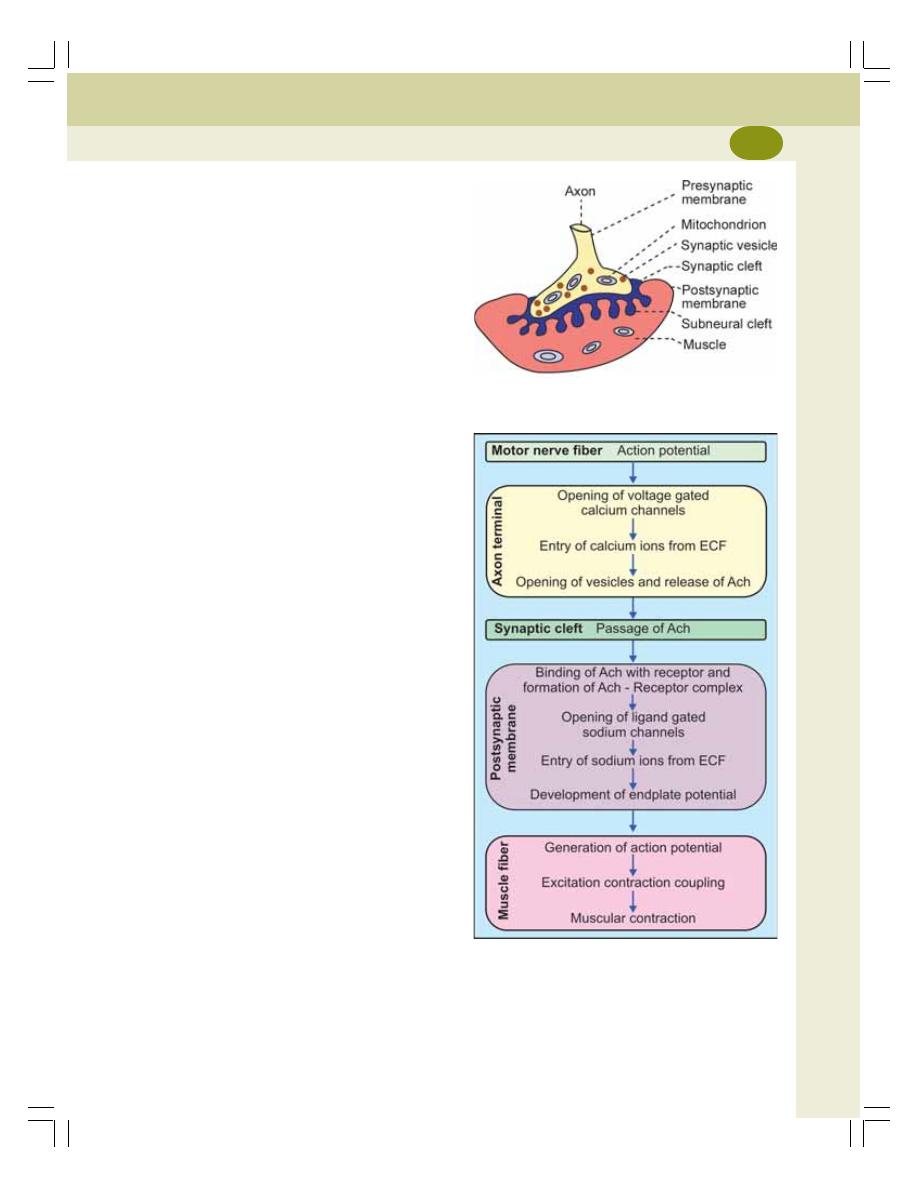
Chapter 24 Neuromuscular Junction
133
The axon terminal contains mitochondria and
synaptic vesicles. The synaptic vesicles contain
the neurotransmitter substance, acetylcholine.
The acetylcholine is synthesized by mitochondria
present in the axon terminal and stored in the
vesicles. The mitochondria contain ATP which
is the source of energy for the synthesis of
acetylcholine.
Synaptic Trough or Gutter
The motor endplate invaginates inside the
muscle fiber and forms a depression which is
known as synaptic trough or synaptic gutter. The
membrane of the muscle fiber below the motor
endplate is thickened.
Synaptic Cleft
The membrane of the nerve ending is called the
presynaptic membrane. The membrane of the
muscle fiber is called postsynaptic membrane.
The space between these two is called synaptic
cleft. The synaptic cleft contains basal lamina.
It is a thin layer of spongy reticular matrix through
which, the extracellular fluid diffuses. Large
quantity of an enzyme called acetylcho-
linesterase is attached to the matrix of basal
lamina.
Subneural Clefts
The postsynaptic membrane is the membrane
of the muscle fiber. It is thrown into numerous
folds called subneural clefts. The postsynaptic
membrane contains the receptors called nicotinic
acetylcholine receptors (Fig. 24-2).
NEUROMUSCULAR TRANSMISSION
Neuromuscular transmission is defined as the
transfer of information from motor nerve ending
to the muscle fiber through neuromuscular
junction. It is the mechanism by which the motor
nerve impulses initiate muscle contraction. A
series of events take place in the neuromuscular
junction during this process (Fig. 24-3).
FIGURE 24-2: Structure of neuromuscular junction
FIGURE 24-3: Sequence of events during
neuromuscular transmission.
Ach = Acetylcholine. ECF = Extracellular fluid

Muscle Physiology
134
1. Release of acetylcholine
2. Action of acetylcholine
3. Development of endplate potential
4. Development of miniature endplate potential
5. Destruction of acetylcholine.
1. RELEASE OF ACETYLCHOLINE
When the action potential reaches the axon
terminal, it opens the voltage gated calcium
channels in the membrane of the axon terminal.
Calcium ions enter the axon terminal from
extracellular fluid and cause bursting of the
vesicles. Now, acetylcholine is released from
the vesicles and diffuses through presynaptic
membrane and enters the synaptic cleft.
Each vesicle contains about 10,000 acetyl-
choline molecules. And, at a time, about 300
vesicles open and release acetylcholine.
2. ACTION OF ACETYLCHOLINE
After entering the synaptic cleft, the acetylcholine
molecules bind with nicotinic receptors present
in the postsynaptic membrane and form the
acetylcholine–receptor complex. It opens the
ligand gated channels for sodium in the
postsynaptic membrane. Now, sodium ions from
extracellular fluid enter the neuromuscular
junction through these channels. And there, the
sodium ions produce an electrical potential called
the endplate potential.
3. ENDPLATE POTENTIAL
Endplate potential is the change in the resting
membrane potential when an impulse reaches
the neuromuscular junction. The resting mem-
brane potential at the neuromuscular junction is
–90 mV. When sodium ions enter inside, slight
depolarization occurs up to –60 mV which is
called endplate potential.
The endplate potential is a graded potential
(Chapter 23) and it is not action potential. It is
nonpropagative. But it causes the development
of action potential in the muscle fiber.
4. MINIATURE ENDPLATE POTENTIAL
Miniature endplate potential is a weak endplate
potential in neuromuscular junction that is
developed by the release of a small quantity of
acetylcholine from axon terminal. And, each
quantum of this neurotransmitter produces a
weak miniature endplate potential. The amplitude
of this potential is only up to 0.5 mV.
Miniature endplate potential cannot produce
action potential in the muscle. When more and
more quanta of acetylcholine are released
continuously, the miniature endplate potentials
are added together and finally produce endplate
potential resulting in action potential in the
muscle.
5. FATE OF ACETYLCHOLINE
Acetylcholine released into the synaptic cleft is
destroyed very quickly within one millisecond by
the enzyme, acetylcholinesterase. However, the
acetylcholine is so potent, that even this short
duration of 1 millisecond is sufficient to excite
the muscle fiber. The rapid destruction of acetyl-
choline is functionally significant because it
prevents repeated excitation of the muscle fiber
and allows the muscle to relax.
Reuptake Process
Reuptake is a process in neuromuscular junction,
by which a degraded product of neurotransmitter
re-enters the presynaptic axon terminal where
it is reused. Acetylcholinesterase splits (de-
grades) acetylcholine into inactive choline and
acetate. Choline is taken back into axon terminal
from synaptic cleft by reuptake process. There,
it is reused in synaptic vesicle to form new acetyl-
choline molecule.
NEUROMUSCULAR BLOCKERS
Neuromuscular blockers are the drugs, which
can prevent the transmission of impulses from
nerve fiber to the muscle fiber through the neuro-

Chapter 24 Neuromuscular Junction
135
muscular junctions. Following are the neuro-
muscular blockers commonly used in surgery
and in research.
1. Curare
Curare prevents the neuromuscular transmission
by combining with acetylcholine receptors. So,
the acetylcholine cannot combine with the
receptors. And, the endplate potential cannot
develop. Since curare blocks the neuromuscular
transmission by acting on the acetylcholine
receptors, it is called receptor blocker.
2. Bungarotoxin
It is a toxin from the venom of deadly snakes. It
affects the neuromuscular transmission by
blocking the acetylcholine receptors.
3. Succinylcholine and Carbamylcholine
These drugs block the neuromuscular trans-
mission by acting like acetylcholine and keeping
the muscle in a depolarized state. But, these
drugs are not destroyed by cholinesterase. So,
the muscle remains in a depolarized state for a
long time.
4. Botulinum Toxin
It is derived from the bacteria Clostridium botu-
linum. It prevents release of acetylcholine from
axon terminal into the neuromuscular junction.
MOTOR UNIT
DEFINITION
The single motor neuron, its axon terminals and
the muscle fibers innervated by it are together
called motor unit. Each motor neuron activates
a group of muscle fibers through the axon
terminals. Stimulation of a motor neuron causes
contraction of all the muscle fibers innervated
by that neuron.
NUMBER OF MUSCLE FIBERS IN
MOTOR UNIT
The number of muscle fiber in each motor unit
varies. The number of muscle fiber is small in
the motor units of the muscles concerned with
fine, graded and precise movements. Examples
are:
Laryngeal muscles :
2 to 3 muscle fibers
per motor unit
Pharyngeal muscles :
2 to 6 muscle fibers
per motor unit
Ocular muscles
:
3 to 6 muscle fibers
per motor unit
The muscles concerned with crude or
coarse movements have motor units with large
number of muscle fibers. There are about 120
to 165 muscle fibers in each motor unit in these
muscles. Examples are the muscles of leg and
back.
APPLIED PHYSIOLOGY –
DISORDERS OF NEUROMUSCULAR
JUNCTION
The disorders of neuromuscular junction
includes:
1. Myasthenia gravis
2. Eaton-Lambert syndrome.
1. MYASTHENIA GRAVIS
Myasthenia gravis is an autoimmune disorder of
neuromuscular junction caused by antibodies to
cholinergic receptors. It is characterized by grave
weakness of the muscle due to the inability of
neuromuscular junction to transmit impulses from
nerve to the muscle.
2. EATON-LAMBERT SYNDROME
Eaton-Lambert syndrome is also an autoimmune
disorder of neuromuscular junction. It is caused
by antibodies to calcium channels in axon termi-
nal. This disease is characterized by features of
myasthenia gravis. In addition the patients have
blurred vision and dry mouth.
DISTRIBUTION
STRUCTURE
TYPES
ELECTRICAL ACTIVITY IN SINGLE UNIT SMOOTH MUSCLE
ELECTRICAL ACTIVITY IN MULTIUNIT SMOOTH MUSCLE
CONTRACTILE PROCESS
CONTROL OF SMOOTH MUSCLE
Smooth Muscle
25
DISTRIBUTION OF SMOOTH
MUSCLE
Smooth muscles are nonstriated (plain) and
involuntary muscles present in almost all the
organs in the form of sheets, bundles or sheaths
around other tissues. These muscles form the
major contractile tissues of various organs.
Smooth muscle fibers are present in the
following structures:
i. Wall of organs like esophagus, stomach
and intestine in gastrointestinal tract
ii. Ducts of digestive glands
iii. Trachea, bronchial tube and alveolar ducts
of respiratory tract
iv. Ureter, urinary bladder and urethra in
excretory system
v. Wall of the blood vessels in circulatory
system
vi. Arrector pilorum of skin
vii. Mammary glands, uterus, genital ducts,
prostate gland and scrotum in repro-
ductive system
viii. Iris and ciliary body of the eye.
STRUCTURE OF SMOOTH MUSCLE
Smooth muscle fibers are fusiform or
elongated cells. The nucleus is single and
elongated and it is centrally placed. Normally,
two or more nucleoli are present in the nucleus
(Fig. 25-1). Smooth muscle fibers are
generally very small, measuring 2 to 5
μ in
diameter and 50 to 200
μ in length. Smooth
muscle fibers are covered by connective
tissue. But the tendons are absent.
FIGURE 25-1: Smooth muscle fibers

Chapter 25 Smooth Muscle
137
Myofibrils and Sarcomere
Well defined myofibrils and sarcomere are
absent in smooth muscles. So the alternate dark
and light bands are absent. Absence of dark and
light bands gives the nonstriated appearance to
the smooth muscle.
Myofilaments and Contractile Proteins
The contractile proteins in smooth muscle fiber
are actin, myosin and tropomyosin. But troponin
or troponin like substance is absent.
Thick and thin filaments are present in
smooth muscle. However, these filaments are
not arranged in orderly fashion as in skeletal
muscle. Thick filaments are formed by myosin
molecules and have more number of cross
bridges than in skeletal muscle. Thin filaments
are formed by actin and tropomyosin molecules.
Dense Bodies
Dense bodies are the special structures of
smooth muscle fibers to which the actin and
tropomyosin molecules of thin filaments are
attached.
Sarcotubular System
Sarcotubular system in smooth muscle fibers is
in the form of network. ‘T’ tubules are absent and
‘L’ tubules are poorly developed (Table 20-1).
TYPES OF SMOOTH MUSCLE
FIBERS
Smooth muscle fibers are of two types:
1. Single unit or visceral smooth muscle fibers
2. Multiunit smooth muscle fibers.
SINGLE UNIT OR VISCERAL SMOOTH
MUSCLE FIBERS
Single unit smooth muscle fibers are the fibers
with interconnecting gap junctions. The gap
junctions allow rapid spread of action potential
throughout the tissue so that all the muscle fibers
show synchronous contraction as a single unit.
Single unit smooth muscle fibers are also called
visceral smooth muscle fibers.
The features of single unit smooth muscle
fibers:
i. The muscle fibers are arranged in sheets
or bundles
ii. The cell membrane of adjacent fibers
fuses at many points to form gap junc-
tions. Through the gap junctions, ions
move freely from one cell to the other.
Thus a functional syncytium is developed.
The syncytium contracts as a single unit.
In this way, the visceral smooth muscle
resembles cardiac muscle more than the
skeletal muscle.
The visceral smooth muscle fibers are in the
walls of the organs such as gastrointestinal
organs, uterus, ureters, respiratory tract, etc.
MULTIUNIT SMOOTH MUSCLE FIBERS
The multiunit smooth muscle fibers are the
muscle fibers without interconnecting gap junc-
tions. These smooth muscle fibers resemble the
skeletal muscle fibers in many ways. The
features of multiunit smooth muscle fibers:
i. The muscle fibers are individual fibers
ii. Each muscle fiber is innervated by a single
nerve ending
iii. Each muscle fiber has got an outer mem-
brane made up of glycoprotein, which
helps to insulate and separate the muscle
fibers from one another
iv. The control of these muscle fibers is
mainly by nerve signals
v. These smooth muscle fibers do not exhibit
spontaneous contractions.
The multiunit muscle fibers are in ciliary
muscles of the eye, iris of the eye, nictitating
membrane (in cat), arrector pili, and smooth
muscles of the blood vessels and urinary bladder.
ELECTRICAL ACTIVITY IN SINGLE
UNIT SMOOTH MUSCLE
Usually 30 to 40 smooth muscle fibers are simul-
taneously depolarized which leads to develop-
ment of self propagating action potential. It is
possible because of gap junctions and syncytial
arrangements of single unit smooth muscles.
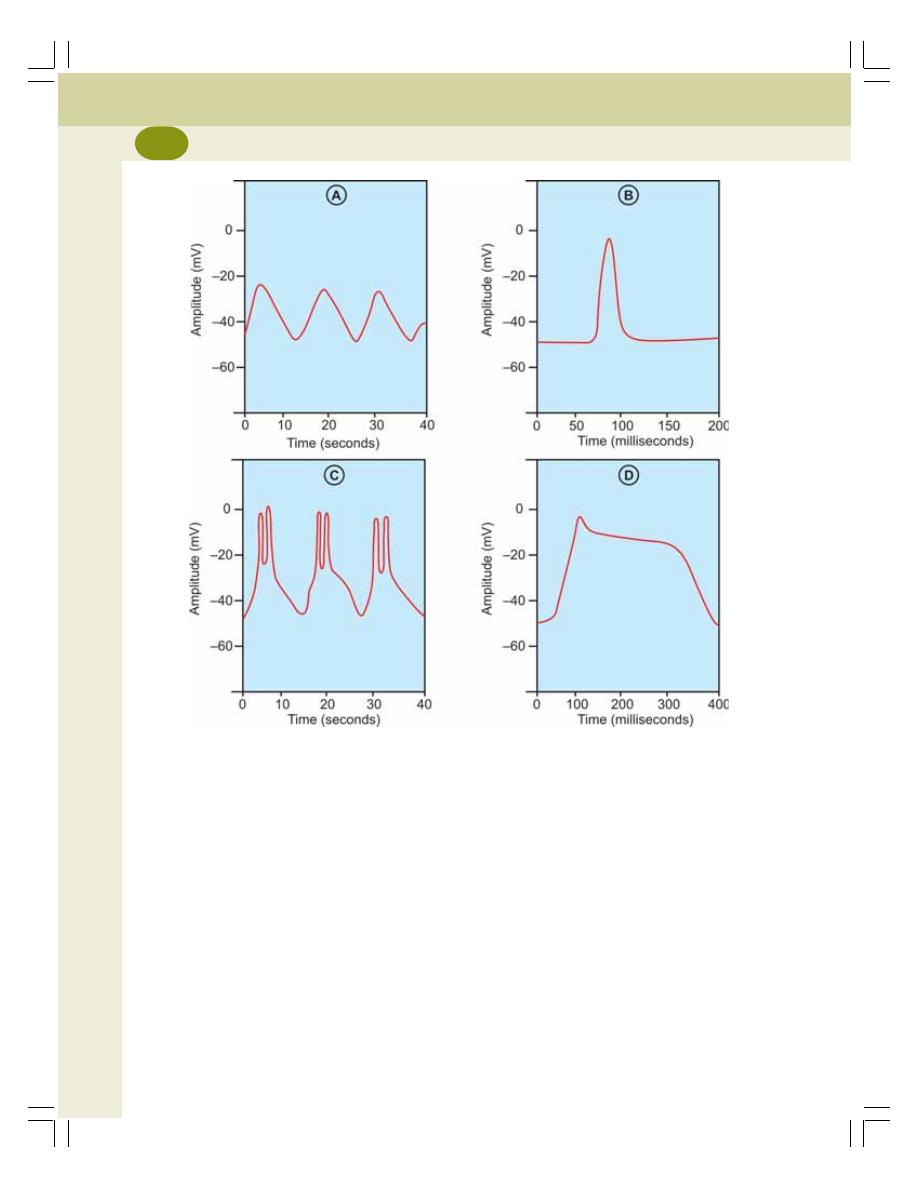
Muscle Physiology
138
RESTING MEMBRANE POTENTIAL
Resting membrane potential in visceral smooth
muscle is very much unstable and ranges
between –50 and –75 mV. Sometimes, it reaches
the low level of –25 mV.
CAUSE FOR UNSTABLE RESTING
MEMBRANE POTENTIAL – SLOW
WAVE POTENTIAL
The unstable is caused by the appearance of
some wave like fluctuations called slow waves.
The slow waves occur in a rhythmic fashion at
a frequency of 4 to 10 per minute with the
amplitude of 10 to 15 mV (Fig. 25-2). The cause
of the slow wave rhythm is not known. It is
suggested that it may be due to the rhythmic
modulations in the activities of sodium–
potassium pump. The slow wave is not action
potential and it cannot cause contraction of the
muscle. But it initiates the action potential (see
below).
ACTION POTENTIAL
Three types of action potential occur in visceral
smooth muscle fibers
FIGURE 25-2: Electrical activities in smooth muscle
A = Slow wave rhythm of resting membrane potential. B = Spike potential
C = Spike potential initiated by slow wave rhythm. D = Action potential with plateau

Chapter 25 Smooth Muscle
139
1. Spike potential
2. Spike potential initiated by slow wave rhythm
3. Action potential with plateau.
1. Spike Potential
The spike potential in visceral smooth muscle
is different from that in skeletal muscles. In
smooth muscle, the average duration of spike
potential varies between 30 and 50 milliseconds.
Its amplitude is very low and it does not reach
the isoelectric base. It is due to nervous and other
stimuli and it leads to contraction of the muscle.
2. Spike Potential Initiated by Slow Wave
Rhythm
Sometimes the slow wave rhythm of resting
membrane potential initiates the spike potentials,
which lead to contraction of the muscle. The
spike potentials appear rhythmically at a rate of
about one or two spikes at the peak of each slow
wave. These potentials initiated by the slow wave
rhythm cause rhythmic contractions of smooth
muscles. This type of potentials appears mostly
in smooth muscles, which are self excitatory and
contract themselves without any external stimuli.
So, the spike potentials initiated by slow wave
rhythm are otherwise called pacemaker waves.
The smooth muscles showing rhythmic contrac-
tions are present in some of the visceral organs
such as intestine.
3. Action Potential with Plateau
This type of action potential starts with rapid
depolarization as in the case of skeletal muscle.
But, repolarization does not occur immediately.
The muscle remains depolarized for long periods
of about 100 to 1000 milliseconds. This type of
action potential is responsible for sustained
contraction of smooth muscle fibers. After the
long depolarized state, slow repolarization
occurs.
TONIC CONTRACTION OF SMOOTH
MUSCLE WITHOUT ACTION POTENTIAL
The smooth muscles of some visceral organs
maintain a state of partial contraction called tonus
or tone. It is due to the tonic contraction of the
muscle that occurs without any action potential
or any stimulus. Sometimes, the tonic contraction
occurs due to the action of some hormones.
IONIC BASIS OF ACTION POTENTIAL
The important difference between the action
potential in skeletal muscle and smooth muscle
lies in the ionic basis of depolarization. In skeletal
muscle, the depolarization occurs due to opening
of sodium channels and entry of sodium ions
from extracellular fluid into the muscle fiber. But
in smooth muscle, the depolarization is due to
entry of calcium ions rather than sodium ions.
Unlike the fast sodium channels, the calcium
channels open and close slowly. It is responsible
for the prolonged action potential with plateau
in smooth muscles. The calcium ions play an
important role during the contraction of the
muscle.
ELECTRICAL ACTIVITY IN
MULTIUNIT SMOOTH MUSCLE
The electrical activity in multiunit smooth muscle
is different from that in the single unit smooth
muscle. The electrical changes leading to con-
traction of multiunit smooth muscle are triggered
by nervous stimuli. The nerve endings secrete
the neurotransmitters like acetylcholine and
noradrenaline. The neurotransmitters depolarize
the membrane of smooth muscle fiber slightly
leading to contraction. The action potential does
not develop. This type of depolarization is called
local depolarization of junctional potential. The
local depolarization travels throughout the entire
smooth muscle fiber and causes contraction. The
local depolarization is developed because the
multiunit smooth muscle fibers are too small to
develop action potential.
CONTRACTILE PROCESS IN
SMOOTH MUSCLE
Compared to skeletal muscles, in smooth mus-
cles, the contraction and relaxation processes
are slow.
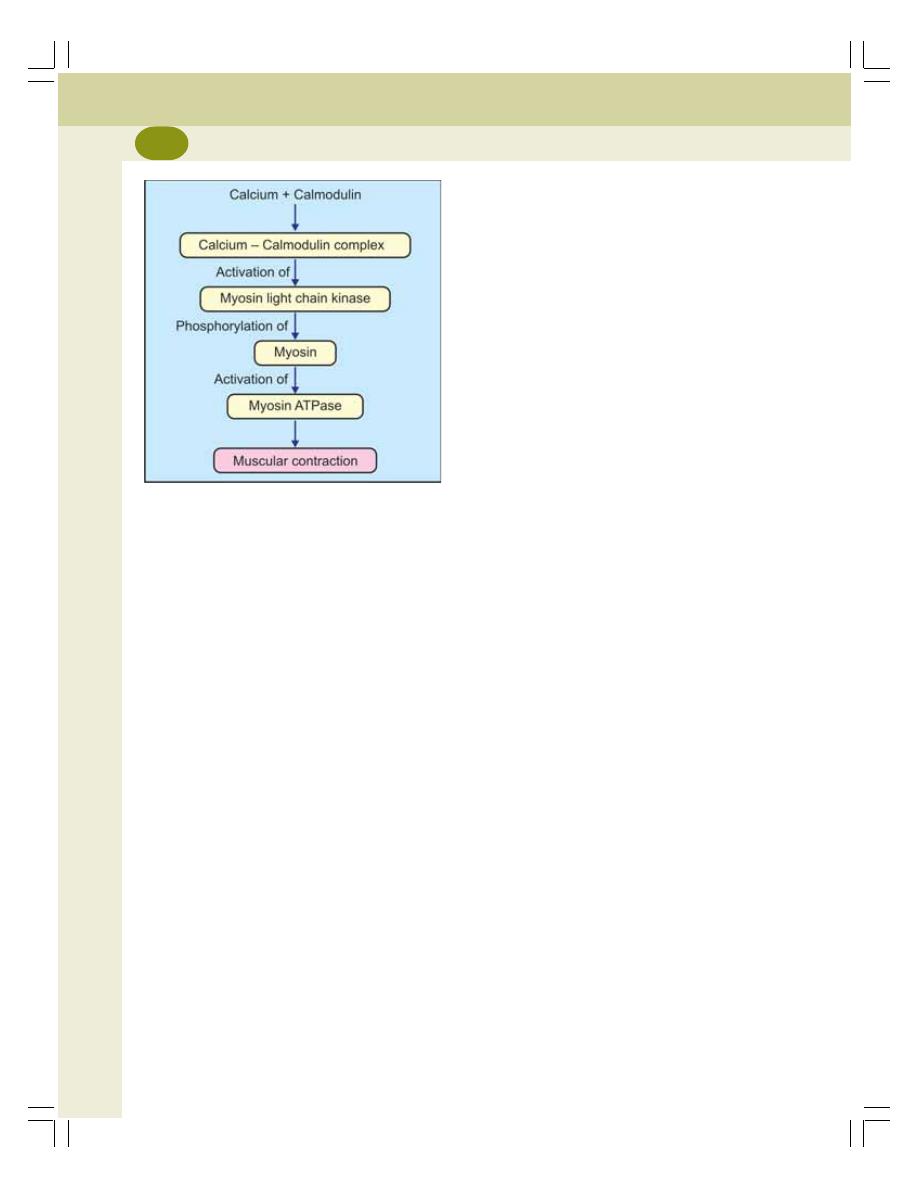
Muscle Physiology
140
MOLECULAR BASIS OF SMOOTH
MUSCLE CONTRACTION
The process of excitation and contraction is very
slow in smooth muscles is because of poor
development of L tubules (sarcoplasmic reti-
culum). So, the calcium ions, which are responsi-
ble for excitation contraction coupling, must be
obtained from the extracellular fluid. It makes the
process of excitation contraction coupling slow.
Calcium–Calmodulin Complex
The stimulation of ATPase activity of myosin in
smooth muscle is different from that in the
skeletal muscle. In smooth muscle, the myosin
has to be phosphorylated for the activation of
myosin ATPase. The phosphorylation of myosin
occurs in the following manner (Fig. 25-3).
Calcium which enters the sarcoplasm from the
extracellular fluid combines with a protein called
calmodulin and forms calcium–calmodulin
complex. It activates an enzyme called calmo-
dulin – dependent myosin light chain kinase.
This enzyme in turn causes phosphorylation of
myosin followed by activation of myosin
ATPase. Now, the sliding of actin filaments
starts.
The phosphorylated myosin gets attached to
the actin molecule for longer period. It is called
latch bridge mechanism and it is responsible for
the sustained contraction of the muscle with
expenditure of little energy.
The relaxation of the muscle occurs due to
the dissociation of calcium–calmodulin complex.
Length-Tension Relationship – Plasticity
Smooth muscle fibers have the property of plasti-
city. Plasticity is the adaptability of smooth muscle
fibers to a wide range of lengths. If the smooth
muscle fiber is stretched, it adapts to this new
length and contracts when stimulated. This adap-
tability exists to a wide range of lengths. Because
of this property, tension produced in the muscle
fiber is not directly proportional to resting length
of the muscle fiber. In other words, Starling’s law
is not applicable to smooth muscle. In skeletal
and cardiac muscles, Starling’s law is applicable
and the tension or force of contraction is directly
proportional to initial length of the muscle fibers.
CONTROL OF SMOOTH MUSCLE
Smooth muscle fibers are controlled by:
A. Nervous factors
B. Humoral factors.
NERVOUS FACTORS
Smooth muscles are supplied by both sympa-
thetic and parasympathetic nerves, which act
opposite to each other in controlling the activities
of smooth muscles. However, these nerves are
not responsible for the initiation of any activity
in smooth muscle.
HUMORAL FACTORS
The activity of smooth muscle is also controlled
by humoral factors which include hormones,
neurotransmitters and other humoral factors.
FIGURE 25-3: Molecular basis of
smooth muscle contraction

Questions in Muscle Physiology
141
LONG QUESTIONS
1. Explain the molecular basis of muscular
contraction.
2. Describe the electrical changes during
muscular contraction.
3. Explain the ionic basis of electrical events
during contraction of skeletal muscle.
4. Describe the neuromuscular junction with
a suitable diagram. Add a note on neuro-
muscular transmission.
SHORT QUESTIONS
1. Compare skeletal muscle and cardiac
muscle.
2. Compare skeletal muscle and smooth
muscle.
3. Sarcomere.
4. Contractile elements of the muscle.
5. Muscle proteins.
6. Sarcotubular system.
7. Sarcoplasmic reticulum.
8. Composition of muscle.
9. Differences between pale and red mus-
cles.
10. Heat rigor/rigor mortis.
11. Effects of repeated stimuli on skeletal
muscle.
12. Fatigue.
13. Tetanus.
14. Starling’s law of muscle.
15. Refractory period.
16. Muscle tone.
17. Resting membrane potential.
18. Action potential.
19. Graded potential.
20. Actomyosin complex.
21. Excitation contraction coupling.
22. Sliding theory of muscular contraction.
23. Electrical activity in smooth muscle.
24. Molecular basis of smooth muscular con-
traction.
25. Neuromuscular junction.
26. Neuromuscular transmission.
27. Endplate potential.
28. Neuromuscular blockers.
29. Motor unit.
30. Myasthenia gravis.
QUESTIONS IN MUSCLE PHYSIOLOGY

Digestive System
26. Introduction to Digestive System ......................................... 145
27. Mouth and Salivary Glands ................................................. 149
28. Stomach .............................................................................. 157
29. Pancreas ............................................................................. 168
30. Liver and Gallbladder .......................................................... 174
31. Small Intestine ..................................................................... 186
32. Large Intestine ..................................................................... 190
33. Movements of Gastrointestinal Tract ................................... 193
S E C T I O N
4
C H A P T E R S

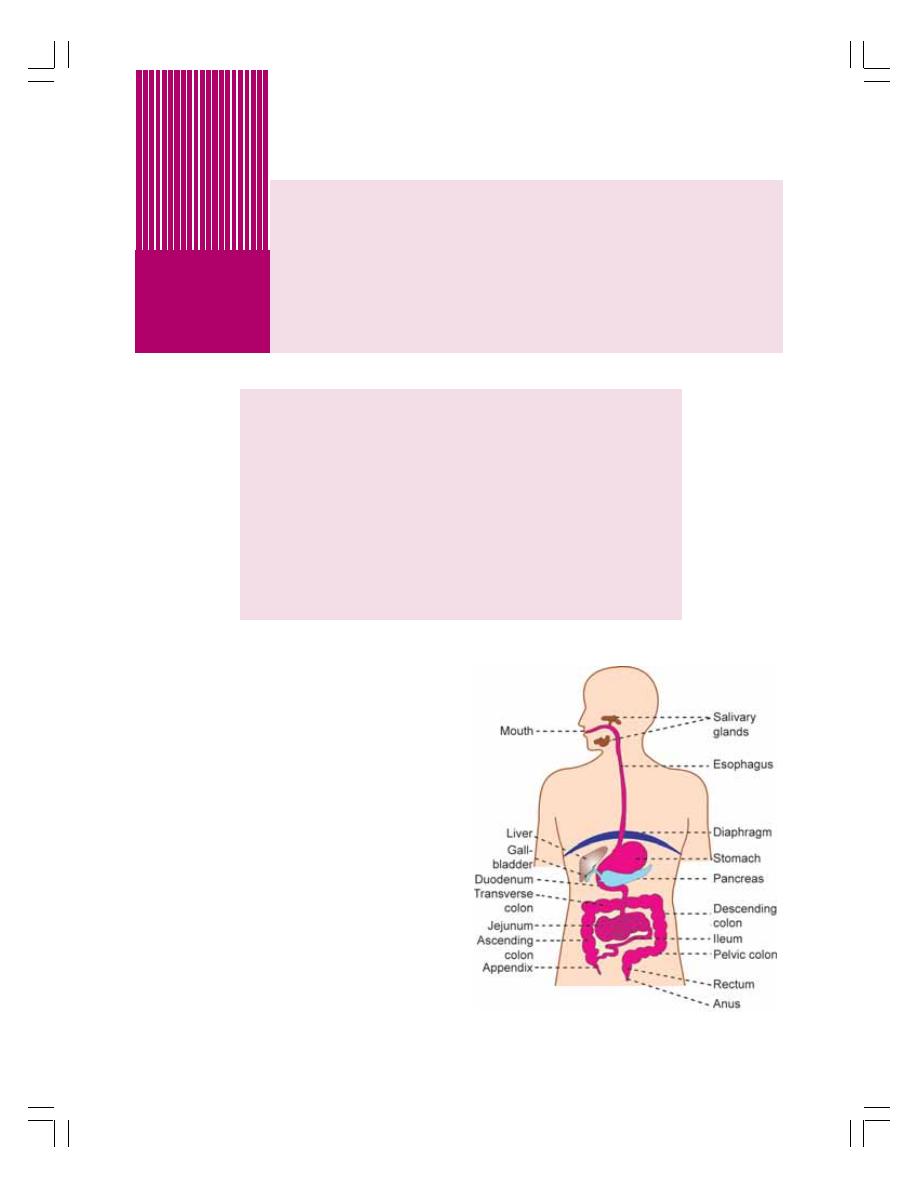
INTRODUCTION
FUNCTIONAL ANATOMY
WALL OF GASTROINTESTINAL TRACT
MUCUS LAYER
SUBMUCUS LAYER
MUSCULAR LAYER
SEROUS OR FIBROUS LAYER
NERVE SUPPLY TO GASTROINTESTINAL TRACT
INTRINSIC NERVE SUPPLY
EXTRINSIC NERVE SUPPLY
Introduction to
Digestive System
26
INTRODUCTION
Digestion is defined as the process by which food
is broken down into simple chemical substances
that can be absorbed and used as nutrients by
the body. Most of the substances in the diet
cannot be utilized as such. These substances
must be broken into smaller particles. Then only
these substances can be absorbed into blood
and distributed to various parts of the body for
utilization. The digestive system is responsible
for these functions.
FUNCTIONAL ANATOMY OF THE
DIGESTIVE SYSTEM
Digestive system is made up of gastrointestinal
tract (GI tract) or alimentary canal and accessory
organs, which help in the process of digestion
and absorption (Fig. 26-1). GI tract is a tubular
structure extending from the mouth up to anus
FIGURE 26-1: Gastrointestinal tract
Digestive System
146
with a length of about 30 feet. It opens to the
external environment on both ends. GI tract is
formed by two types of organs:
1. Primary digestive organs
2. Accessory digestive organs.
1. Primary Digestive Organs
Primary digestive organs are the organs where
actual digestion takes place. These organs are:
1. Mouth
2. Pharynx
3. Esophagus
4. Stomach
5. Small intestine
6. Large intestine.
2. Accessory Digestive Organs
Accessory digestive organs are the organs which
help the primary digestive organs in the process
of digestion. These organs are:
1. Teeth
2. Tongue
3. Salivary glands
4. Exocrine part of pancreas
5. Liver
6. Gallbladder.
WALL OF GASTROINTESTINAL
TRACT
In general, the wall of the GI tract is formed by
four layers which are from inside out:
1. Mucus layer
2. Submucus layer
3. Muscular layer
4. Serous or fibrous layer.
1. MUCUS LAYER
The mucus layer is the innermost layer of the
wall of GI tract. It is also called gastrointestinal
mucosa or mucous membrane. It faces the cavity
of GI tract.
The mucosa has three layers of structures:
i. Epithelial lining which is in contact with
contents of GI tract
ii. Lamina propria formed by connective
tissue
iii. Muscularis mucosa formed by smooth
muscle fibers.
2. SUBMUCUS LAYER
This is present in all parts of GI tract except
mouth and pharynx. This layer contains loose
collagen fibers, elastic fibers, reticular fibers and
few cells of connective tissue. Blood vessels,
lymphatic vessels and nerve plexus are present
in this layer.
3. MUSCULAR LAYER
This layer in lips, cheeks and wall of pharynx
have skeletal muscle fibers. The esophagus has
both skeletal and smooth muscle fibers. Wall of
the stomach and intestine is formed by smooth
muscle fibers.
The smooth muscle fibers in stomach are
arranged in three layers:
i. Inner oblique layer
ii. Middle circular layer
iii. Outer longitudinal layer.
The smooth muscle fibers in the intestine are
arranged in two layers:
i. Inner circular layer
ii. Outer longitudinal layer.
The smooth muscle fibers present in inner
circular layer of anal canal constitute internal anal
sphincter. The external anal sphincter is formed
by skeletal muscle fibers.
4. SEROUS OR FIBROUS LAYER
Outermost layer of the wall of GI tract is either
serous or fibrous in nature. The serous layer is
formed by connective tissue and mesoepithelial
cells. It is also called serosa or serous mem-
brane. It covers stomach, small intestine and
large intestine.
The fibrous layer is otherwise called fibrosa.
It is formed by connective tissue. It covers
pharynx and esophagus.
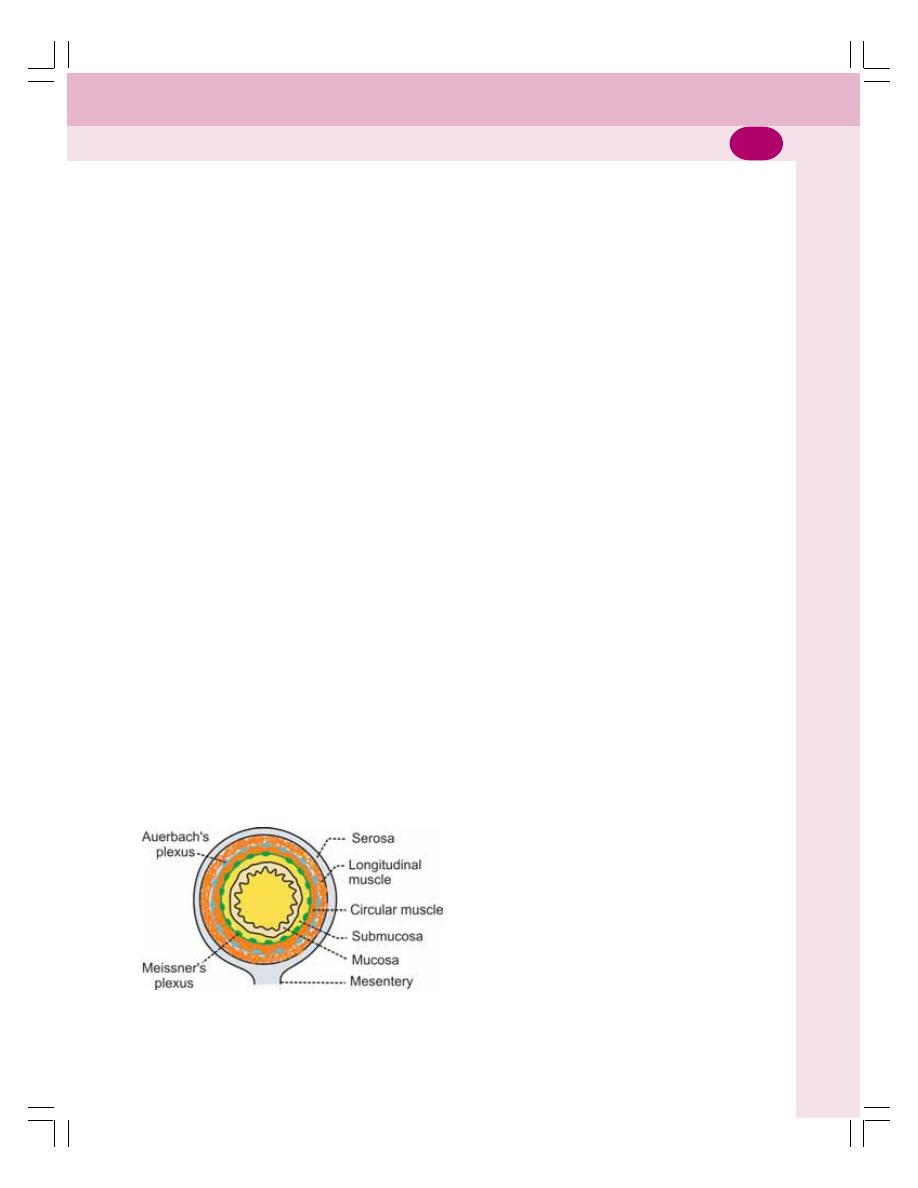
Chapter 26 Introduction to Digestive System
147
NERVE SUPPLY TO
GASTROINTESTINAL TRACT
GI tract has two types of nerve supply:
I. Intrinsic nerve supply
II. Extrinsic nerve supply.
INTRINSIC NERVE SUPPLY –
ENTERIC NERVOUS SYSTEM
The enteric nervous system is present within the
wall of GI tract from esophagus to anus. The
nerve fibers of this system are interconnected
and form two major networks called
1. Auerbach’s plexus
2. Meissner’s plexus.
These nerve plexus contain nerve cell bodies,
processes of nerve cells and the receptors. The
receptors in the GI tract are stretch receptors
and chemoreceptors. The enteric nervous sys-
tem is controlled by extrinsic nerves.
Auerbach’s Plexus
It is also known as myenteric nerve plexus. It is
present in between the inner circular muscle layer
and the outer longitudinal muscle layer
(Fig. 26-2).
Functions of Auerbach’s Plexus
The major function of this plexus is to regulate
the movements of GI tract. Some nerve fibers
of this plexus accelerate the movements by
secreting the excitatory neurotransmitter sub-
stances like acetylcholine, serotonin and
substance P. Other fibers of this plexus inhibit
the GI motility by secreting the inhibitory neuro-
transmitters such as vasoactive intestinal
polypeptide (VIP), neurotensin and enkephalin.
Meissner’s Nerve Plexus
Meissner’s plexus is otherwise called submucus
nerve plexus. It is situated in between the
muscular layer and submucosal layer of GI tract.
Functions of Meissner’s Plexus
The function of Meissner’s plexus is the regu-
lation of secretory functions of GI tract. And these
nerve fibers cause constriction of blood vessels
of GI tract.
EXTRINSIC NERVE SUPPLY
The extrinsic nerves that control the enteric
nervous system are from autonomic nervous
system. Both sympathetic and parasympathetic
divisions of autonomic nervous system innervate
the GI tract (Fig. 26-3).
Sympathetic Nerve Fibers
Preganglionic sympathetic nerve fibers to GI tract
arise from lateral horns of spinal cord between
fifth thoracic and second lumbar segments
(T5 – L2). From here, the fibers leave the spinal
cord, pass through the ganglia of sympathetic
chain without having any synapse and then
terminate in the celiac and mesenteric ganglia.
The postganglionic fibers from these ganglia are
distributed throughout the GI tract.
Functions of sympathetic nerve fibers
Sympathetic nerve fibers inhibit the movements
and decrease the secretions of GI tract by secre-
ting the neurotransmitter noradrenaline. It also
causes constriction of sphincters.
Parasympathetic Nerve Fibers
Parasympathetic nerve fibers to GI tract pass
through some of the cranial nerves and sacral
FIGURE 26-2: Structure of intestinal wall
with intrinsic nerve plexus
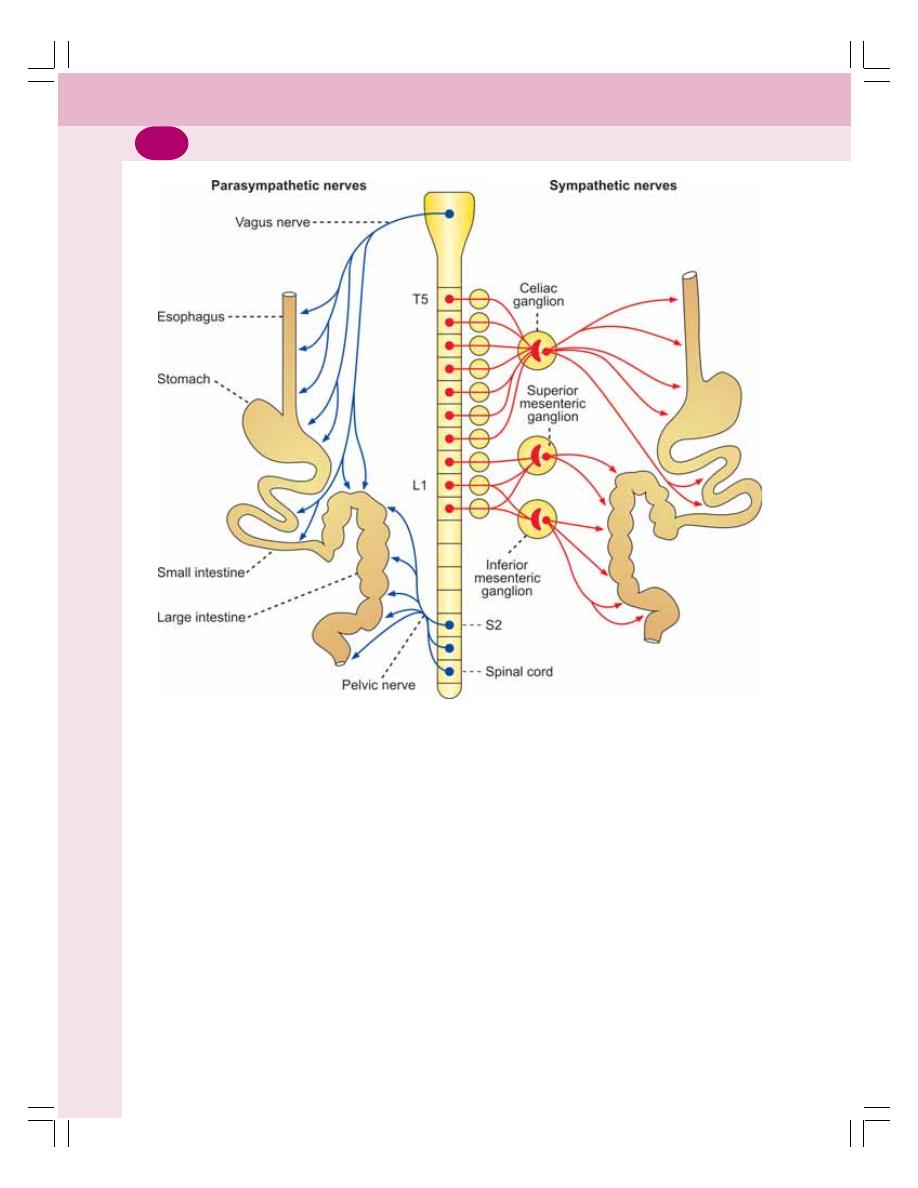
Digestive System
148
nerves. The preganglionic and postganglionic
parasympathetic nerve fibers to mouth and
salivary glands pass through facial and glosso-
pharyngeal nerves.
The preganglionic parasympathetic nerve
fibers to esophagus, stomach, small intestine and
upper part of large intestine pass through vagus
nerve. The preganglionic nerve fibers to lower
part of large intestine arise from second, third
and fourth sacral segments (S1, S2 and S3) of
FIGURE 26-3: Extrinsic nerve supply to GI tract. T5= 5th thoracic segment of spinal cord.
L1 = 1st lumbar segment of spinal cord; S2 = 2nd sacral segment of spinal cord
spinal cord and pass through pelvic nerve. All
these preganglionic parasympathetic nerve fibers
synapse with the postganglionic nerve cells in
the myenteric and submucus plexus.
Functions of parasympathetic nerve fibers
Parasympathetic nerve fibers accelerate move-
ments and increase the secretions of GI tract.
The neurotransmitter secreted by the para-
sympathetic nerve fibers is acetylcholine.
FUNCTIONAL ANATOMY OF MOUTH
FUNCTIONS OF MOUTH
SALIVARY GLANDS
PROPERTIES AND COMPOSITION OF SALIVA
FUNCTIONS OF SALIVA
REGULATION OF SALIVARY SECRETION
EFFECTS OF DRUGS AND CHEMICALS ON SALIVARY SECRETION
APPLIED PHYSIOLOGY
Mouth and
Salivary Glands
27
27
27
27
27
FUNCTIONAL ANATOMY OF
MOUTH
The mouth is otherwise known as oral cavity or
buccal cavity. It is formed by cheeks, lips and
palate. It encloses the teeth, tongue and salivary
glands. It opens anteriorly to the exterior through
lips and posteriorly through fauces into the
pharynx.
Digestive juice present in the mouth is saliva
which is secreted by the salivary glands.
FUNCTIONS OF MOUTH
The primary function of mouth is eating. It has
few other important functions also. The functions
of the mouth are:
1. Ingestion of food materials.
2. Chewing the food and mixing it with saliva.
3. Appreciation of the taste.
4. Transfer of food (bolus) to the esophagus by
swallowing.
5. Role in speech.
6. Social functions such as smiling and other
expressions.
SALIVARY GLANDS
In humans, the saliva is secreted by three pairs
of major (larger) salivary glands and some minor
(small) salivary glands in the oral and pharyngeal
mucous membrane. The major glands are:
1. Parotid glands
2. Submaxillary or submandibular glands
3. Sublingual glands.
PAROTID GLANDS
Parotid glands are the largest of all salivary glands
situated at the side of the face just below and in
front of the ear. Secretions from these glands are
emptied into the oral cavity by Stensen’s duct that
opens inside the cheek against the upper second
molar tooth (Fig. 27-1).
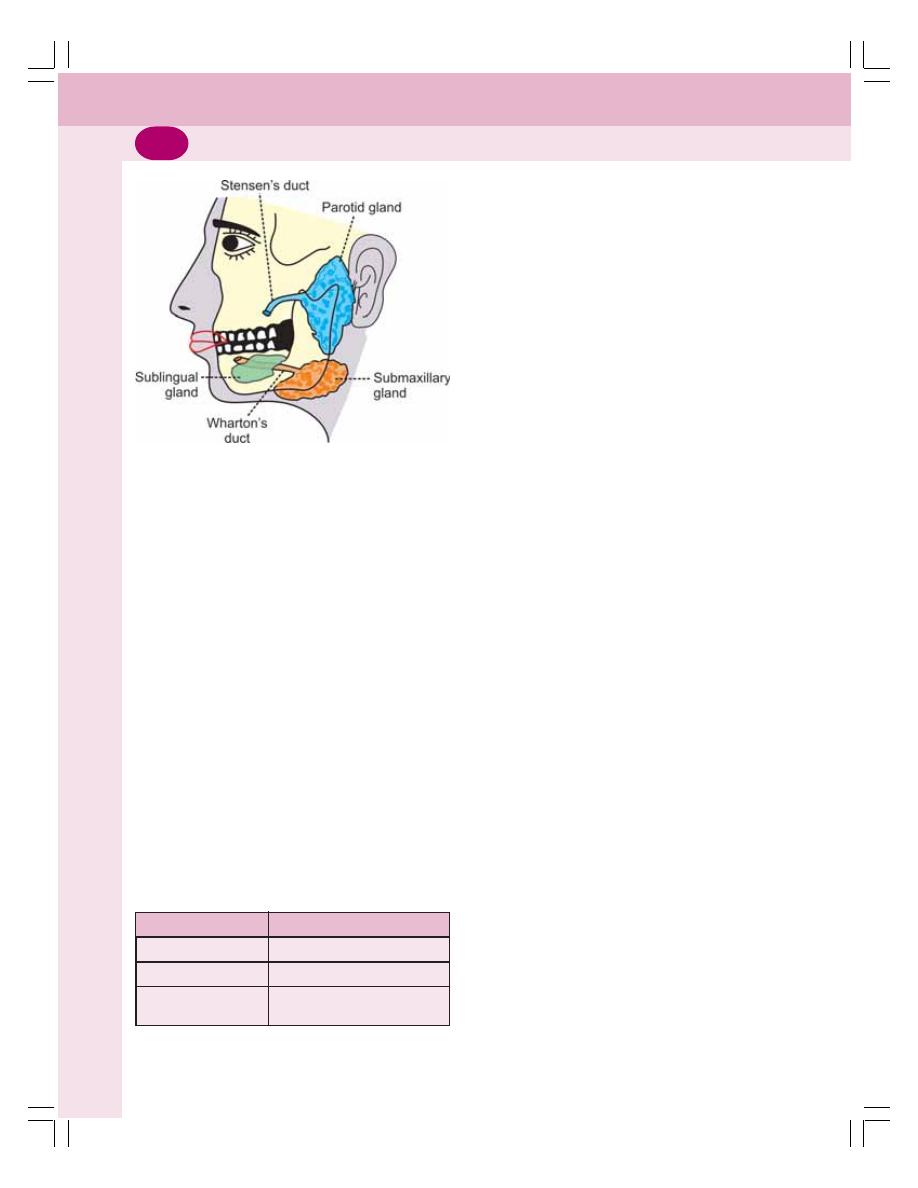
Digestive System
150
SUBMAXILLARY GLANDS
Submaxillary glands or submandibular glands
are located in submaxillary triangle medial to
mandible. Saliva from these glands is emptied
into the oral cavity by Wharton’s duct. The duct
opens at the side of frenulum of tongue by
means of a small opening on the summit of
papilla called caruncula sublingualis.
SUBLINGUAL GLANDS
Sublingual glands are the smallest salivary
glands situated in the mucosa at floor of mouth.
Saliva from these glands is poured into 5-15
small ducts called ducts of Ravinus. These
ducts open on small papillae beneath the
tongue. One of the ducts is larger and it is called
Bartholin’s duct (Table 27-1). It drains the
anterior part of the gland and opens on
caruncula sublingualis near the opening of
submaxillary duct.
MINOR SALIVARY GLANDS
1. Lingual mucus glands situated in posterior
1/3 of the tongue, behind circum vallate
papillae and at the tip and margins of tongue.
2. Lingual serous glands located near circum
vallate papillae and foliform papillae.
3. Buccal glands present between the mucous
membrane and buccinator muscle. Four to
five of these are larger and situated outside
buccinator around terminal part of parotid
duct. These glands are called molar glands.
4. Labial glands situated beneath the mucous
membrane around the orifice of mouth.
5. Palatal glands found beneath the mucous
membrane of the soft palate.
CLASSIFICATION OF SALIVARY
GLANDS
Salivary glands are classified into three types
based on the type of secretion.
1. Serous Glands
This type of gland is predominantly made up of
serous cells. These glands secrete thin and
watery saliva. Parotid glands and lingual serous
glands are serous glands.
2. Mucus Glands
This type of glands is made up of mainly the
mucus cells. These glands secrete thick, viscus
saliva with high mucin content. Lingual mucus
glands, buccal glands and palatal glands belong
to this type.
3. Mixed Glands
Mixed glands are made up of both serous and
mucus cells. Submandibular, sublingual and
labial glands are the mixed glands.
STRUCTURE AND DUCT SYSTEM OF
SALIVARY GLANDS
Salivary glands are made up of acini or alveoli.
Each acinus is formed by a small group of cells
which surround a central globular cavity. The
FIGURE 27-1: Major salivary glands
TABLE 27-1: Ducts of major salivary glands
Gland
Duct
Parotid gland
Stensen’s duct
Submaxillary gland
Wharton’s duct
Sublingual gland
Ducts of Ravinus/Bartholin’s
duct
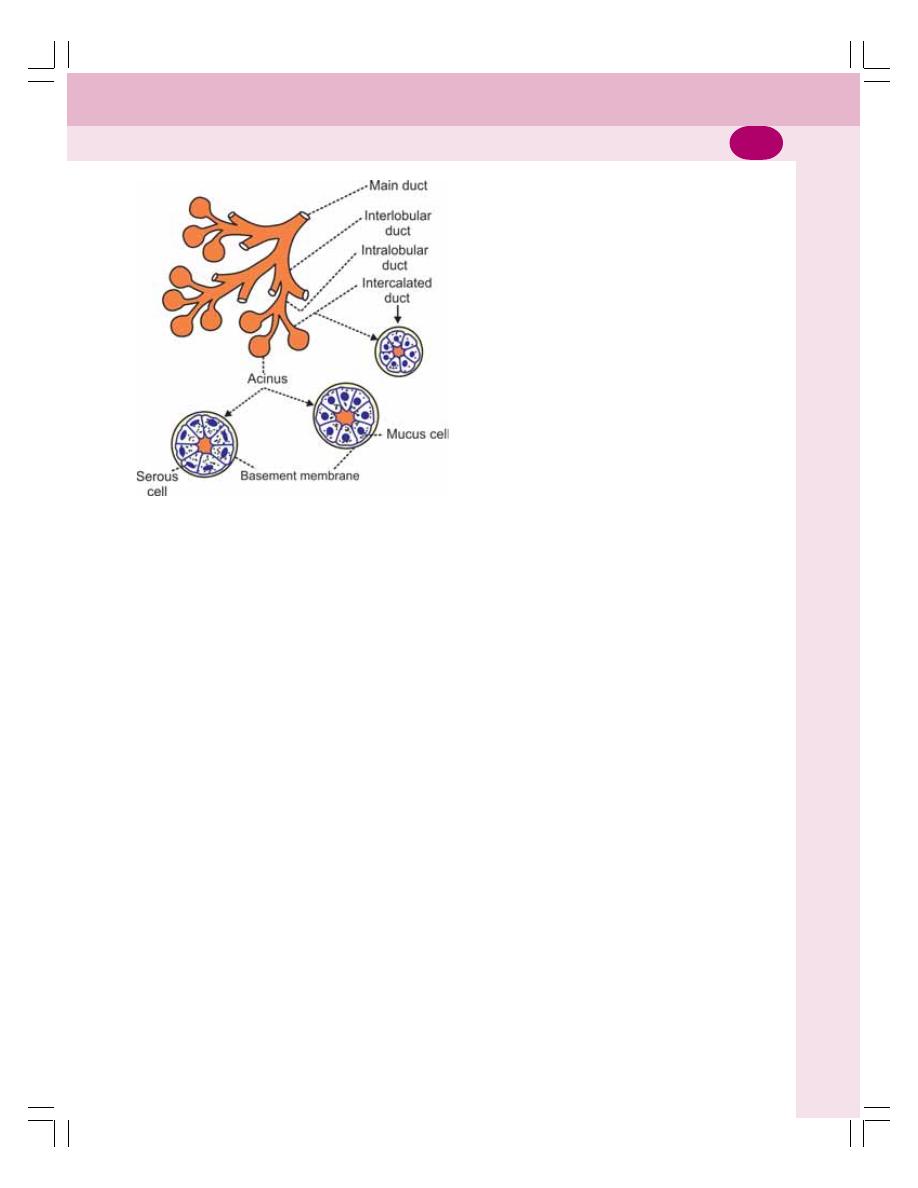
Chapter 27 Mouth and Salivary Glands
151
central cavity of each acinus is continuous with
the lumen of the duct. The fine duct draining each
acinus is called intercalated duct. Many
intercalated ducts join together to form
intralobular duct. Few intralobular ducts join to
form interlobular ducts, which unite to form the
main duct of the gland (Fig. 27-2). The gland with
this type of structure and duct system is called
racemose type (racemose = bunch of grapes).
PROPERTIES AND COMPOSITION
OF SALIVA
Properties of Saliva
1. Volume: 1000 to 1500 mL of saliva is secreted
per day and, it is approximately about 1 mL/
minute. Contribution by each major salivary
gland is:
i. Parotid glands: 25%
ii. Submaxillary glands: 70%
iii. Sublingual glands: 5%.
2. Reaction: Mixed saliva from all the glands is
slightly acidic with pH of 6.35 to 6.85.
3. Specific gravity: It ranges between 1.002 and
1.012.
4. Tonicity: Saliva is hypotonic to plasma.
Composition of Saliva
Mixed saliva contains 99.5% water and 0.5%
solids. Composition of saliva is given in
Figure 27-3.
FUNCTIONS OF SALIVA
Saliva is a very essential digestive juice. Since
it has many functions, its absence leads to many
inconveniences.
1. PREPARATION OF FOOD FOR
SWALLOWING
When food is taken into the mouth, it is mois-
tened and dissolved by saliva. The mucous
membrane of mouth is also moistened by saliva.
It facilitates chewing. By the movement of the
tongue, the moistened and masticated food is
rolled into a bolus. The mucin of saliva lubricates
the bolus and facilitates the swallowing.
2. APPRECIATION OF TASTE
Taste is a chemical sensation. Saliva by its sol-
vent action dissolves the solid food substances,
so that the dissolved substances can stimulate
the taste buds. The stimulated taste buds
recognize the taste.
3. DIGESTIVE FUNCTION
Saliva has three digestive enzymes namely,
salivary amylase, maltase and lingual lipase
(Table 27-2).
Salivary Amylase
Salivary amylase is a carbohydrate digesting
(amylolytic) enzyme. It acts on cooked or boiled
starch and converts it into dextrin and maltose.
Though starch digestion starts in the mouth,
major part of it occurs in the stomach because,
food stays only for a short time in the mouth.
The optimum pH necessary for the activation
of salivary amylase is 6. The salivary amylase
cannot act on cellulose. The enzyme maltase is
present only in traces in human saliva. It converts
maltose into glucose.
FIGURE 27-2: Diagram showing acini and
duct system in salivary glands
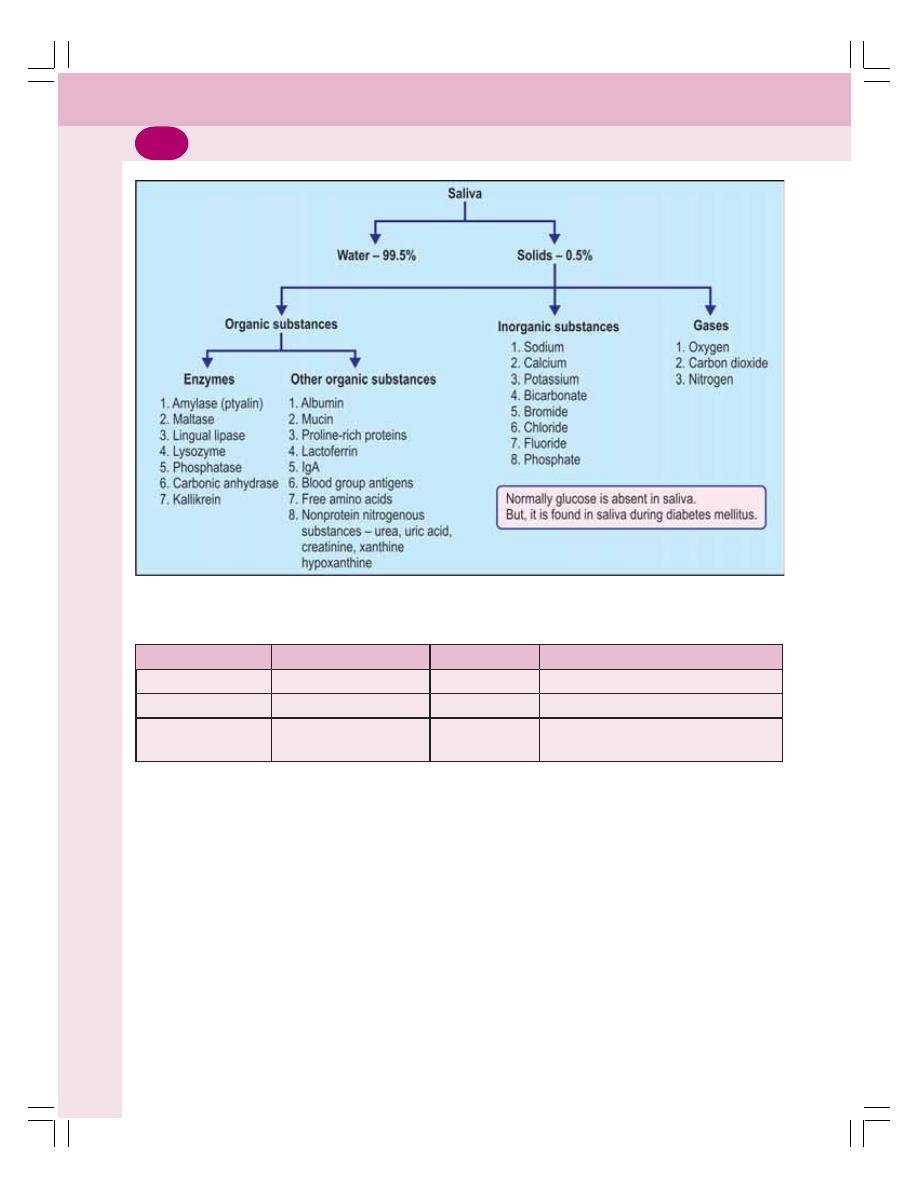
Digestive System
152
FIGURE 27-3: Composition of saliva
TABLE 27-2: Digestive enzymes of saliva
Enzyme
Source of secretion
Activator
Action
1. Salivary amylase
All salivary glands
Acid medium
Converts cooked starch into maltose
2. Maltase
Major salivary glands
Acid medium
Converts maltose into glucose
3. Lingual lipase
Lingual glands
Acid medium
Converts triglycerides of milk fat into
fatty acids and diacylglycerol
Lingual Lipase
Lingual lipase is lipid digesting (lipolytic)
enzymes. It digests milk fats (pre-emulsified fats).
It hydrolyzes triglycerides into fatty acids and
diacylglycerol (Table 27-2).
4. CLEANSING AND PROTECTIVE
FUNCTIONS
i. Due to the constant secretion of saliva,
the mouth and teeth are rinsed and kept
free off food debris, shed epithelial cells
and foreign particles. In this way, saliva
prevents bacterial growth by removing
materials, which may serve as culture
media for the bacterial growth
ii. The enzyme lysozyme of saliva kills some
bacteria such as staphylococcus, strepto-
coccus, and brucella
iii. The proline-rich proteins and lactoferrin
present in saliva possess antimicrobial
property. These proteins also protect the
teeth by stimulating enamel formation
iv. Saliva also contains secretory immuno-
globulin IgA which has antibacterial and
antiviral actions
v. Mucin present in the saliva protects the
mouth by lubricating the mucous mem-
brane of the mouth.

Chapter 27 Mouth and Salivary Glands
153
5. ROLE IN SPEECH
By moistening and lubricating soft parts of mouth
and lips, saliva helps in speech. If the mouth
becomes dry, articulation and pronunciation
become difficult.
6. EXCRETORY FUNCTION
Many substances, both organic and inorganic,
are excreted in saliva. It excretes substances like
mercury, potassium iodide, lead, and thiocyanate.
Saliva also excretes some viruses such as those
causing rabies and mumps.
In some pathological conditions, saliva
excretes certain substances, which are not found
in saliva under normal conditions such as glucose
in diabetes mellitus. In certain conditions, some
of the normal constituents of saliva are excreted
in large quantities. For example, excess urea is
excreted in saliva during nephritis, and excess
calcium is excreted during hyperparathyroidism.
7. REGULATION OF BODY
TEMPERATURE
In dogs and cattle, excessive dripping of saliva
during panting helps in loss of heat and regulation
of body temperature. However, in human being
sweat glands play major role in temperature
regulation and saliva does not play any role in
this function.
8. REGULATION OF WATER BALANCE
When the body water content decreases, salivary
secretion also decreases. This causes dryness
of the mouth and induces thirst. When the water
is taken, it quenches the thirst and restores the
body water content.
REGULATION OF SALIVARY
SECRETION
Salivary secretion is regulated only by nervous
mechanism. Autonomic nervous system is
involved in the regulatory function.
NERVE SUPPLY TO SALIVARY GLANDS
Salivary glands are supplied by parasympathetic
and sympathetic divisions of autonomic nervous
system.
PARASYMPATHETIC FIBERS
Parasympathetic Fibers to Submandibular
and Sublingual Glands
The parasympathetic preganglionic fibers to
submandibular and sublingual glands arise from
the superior salivatory nucleus situated in pons.
After taking origin from this nucleus, the
preganglionic fibers run through nervous
intermedius of Wrisberg, geniculate ganglion,
the motor fibers of facial nerve, chorda tympani
branch of facial nerve and lingual branch of
trigeminal nerve and finally reach the
submaxillary ganglion (Fig. 27-4).
The postganglionic fibers arise from this
ganglion and supply the submaxillary and
sublingual glands.
Parasympathetic Fibers to Parotid Gland
The parasympathetic preganglionic fibers to
parotid gland arise from inferior salivatory nucleus
situated in the upper part of medulla oblongata.
From here, the fibers pass through the tympanic
branch of glossopharyngeal nerve, tympanic
plexus and lesser petrosal nerve and end in otic
ganglion (Fig. 27-5).
The postganglionic fibers arise from otic
ganglion and reach the parotid gland by passing
through the auriculotemporal branch in mandi-
bular division of trigeminal nerve.
Function of Parasympathetic Fibers
When the parasympathetic fibers of salivary
glands are stimulated, a large quantity of watery
saliva is secreted with less amount of organic
constituents. It is because the parasympathetic
fibers activate the acinar cells and dilate the blood
vessels of salivary glands. The neurotransmitter
is acetylcholine.
SYMPATHETIC FIBERS
The sympathetic preganglionic fibers to salivary
glands arise from the lateral horns of first and
second thoracic segments of spinal cord. The
fibers leave the cord through the anterior nerve
roots and end in superior cervical ganglion of the
sympathetic chain.
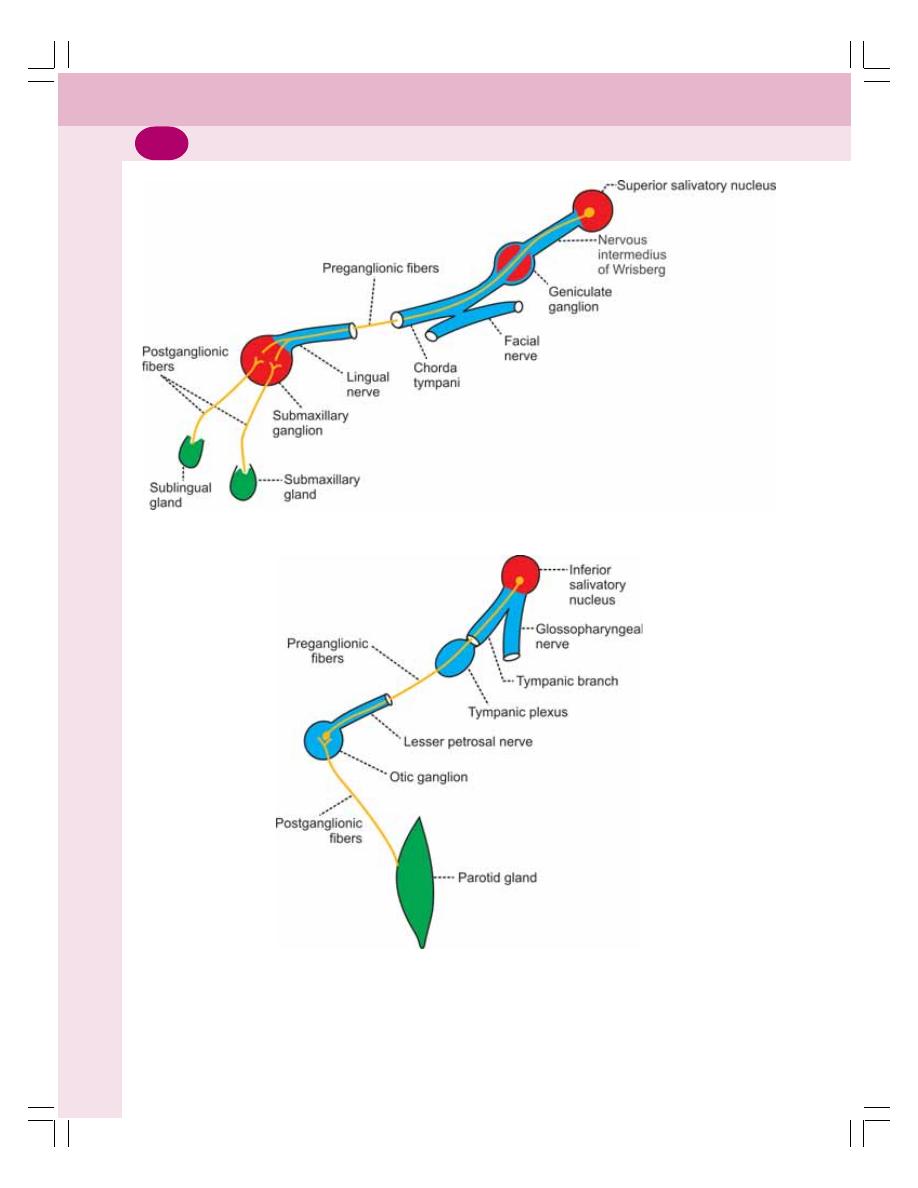
Digestive System
154
FIGURE 27-5: Parasympathetic nerve supply to parotid gland
FIGURE 27-4: Parasympathetic nerve supply to submaxillary and sublingual glands
The postganglionic fibers from this ganglion
are distributed to the salivary glands along the
nerve plexus around the arteries supplying the
glands.
Function of Sympathetic Fibers
The stimulation of sympathetic fibers causes less
secretion of saliva, which is thick and rich in

Chapter 27 Mouth and Salivary Glands
155
mucus. It is because these fibers activate the
acinar cells and cause vasoconstriction by
secreting noradrenaline.
REFLEX REGULATION OF SALIVARY
SECRETION
Salivary secretion is regulated by nervous
mechanism through reflex action. Salivary
reflexes are of two types:
1. Unconditioned reflex
2. Conditioned reflex.
1. Unconditioned Reflex
Unconditioned reflex is the inborn reflex that is
present since birth. It does not need any previous
experience. This reflex induces salivary secretion
when any substance is placed in the mouth. It
is due to the stimulation of nerve endings in the
mucous membrane of the oral cavity.
Examples:
i. When food is taken
ii. When any unpleasant or unpalatable
substance enters the mouth
iii. When the oral cavity is handled with
instruments by dentists.
2. Conditioned Reflex
Conditioned reflex is the one that is acquired by
experience and it needs previous experience
(Chapter 101). Presence of food in the mouth is
not necessary to elicit this reflex. The stimulus
for this reflex is the sight, smell, hearing or
thought of food. It is due to the impulses arising
from eyes, nose, ear, etc.
EFFECT OF DRUGS AND CHEMICALS
ON SALIVARY SECRETION
Substances which Increase the
Salivary Secretion
1. Sympathomimetic drugs like adrenaline and
ephedrine
2. Parasympathomimetic drugs like acetyl-
choline, pilocarpine, muscarine and physo-
stigmine
3. Histamine.
Substances which Decrease the
Salivary Secretion
1. Sympathetic depressants like ergotamine and
dibenamine
2. Parasympathetic depressants like atropine,
and scopolamine.
APPLIED PHYSIOLOGY
HYPOSALIVATION
The reduction in the secretion of saliva is called
hyposalivation. It is of two types, namely, the
temporary hyposalivation and the permanent
hyposalivation.
1. Temporary hyposalivation occurs in:
i. Emotional conditions like fear
ii. Fever
iii. Dehydration.
2. Permanent hyposalivation occurs in:
i. Obstruction of salivary duct (sialolithiasis)
ii. Congenital absence or hypoplasia of sali-
vary glands
iii. Paralysis of facial nerve (Bell’s palsy).
HYPERSALIVATION
The excess secretion of saliva is known as hyper-
salivation. The physiological condition when
hypersalivation occurs is pregnancy. Hyper-
salivation in pathological conditions is called
ptyalism, sialorrhea, sialism or sialosis.
Hypersalivation occurs in the following condi-
tions:
1. Decay of tooth or neoplasm (abnormal new
growth or tumor) in mouth or tongue – due
to continuous irritation of nerve endings in the
mouth
2. Disease of esophagus, stomach and intestine
3. Neurological disorders such as mental
retardation, cerebral stroke and parkinsonism
4. Some psychological and psychiatric condi-
tions
5. Nausea and vomiting.
OTHER DISORDERS
In addition to hyposalivation and hypersalivation,
salivary secretion is affected by other disorders
also which include:
Digestive System
156
1. Xerostomia
Xerostomia means dry mouth. It is also called
pasties or cottonmouth. It is due to hyposalivation
or absence of salivary secretion (aptyalism). The
causes of this disease are:
i. Dehydration or renal failure
ii. Sjögren’s syndrome (see below)
iii. Radiotherapy
iv. Trauma to salivary gland or their ducts
v. Side effect of drugs like antihistamines,
antidepressants, and, antiparkinsonian
drugs
vi. Shock
vii. After smoking marijuana (psychoactive
compound from the plant cannabis).
Xerostomia causes difficulties in mastication,
swallowing and speech. It also causes halitosis
(bad breath).
2. Drooling
Uncontrolled flow of saliva outside the mouth is
called drooling. It is often called ptyalism.
Drooling occurs because of excess produc-
tion of saliva in association with inability to retain
saliva within the mouth.
Drooling occurs in the following conditions:
i. During teeth eruption in children
ii. Upper respiratory tract infection or nasal
allergies in children
iii. Difficulty in swallowing
iv. Tonsillitis
v. Peritonsillar abscess.
3. Chorda Tympani Syndrome
Chorda tympani syndrome is the condition
characterized by sweating while eating. During
trauma or surgical procedure some of the para-
sympathetic nerve fibers to salivary glands may
be severed. And, during the regeneration some
of these nerve fibers, which run along with chorda
tympani branch of facial nerve may deviate and
join with the nerve fibers supplying sweat glands.
When the food is placed in the mouth, salivary
secretion is associated with sweat secretion.
4. Mumps
Mumps is the acute viral infection affecting the
parotid glands. The virus causing this disease
is paramyxovirus. It is common in children who
are not immunized. It occurs in adults also.
Features of mumps are puffiness of cheeks (due
to swelling of parotid glands), fever, sore throat
and weakness. Mumps affects meninges,
gonads and pancreas also.
5. Sjögren’s Syndrome
It is an autoimmune disorder in which the immune
cells destroy exocrine glands such as lacrimal
glands and salivary glands. Common symptoms
of this syndrome are dryness of the mouth due
to lack of saliva (xerostomia), persistent cough
and dryness of eyes. In severe conditions the
organs like kidneys, lungs, liver, pancreas,
thyroid, blood vessels and brain are affected.
■
■
■
■
■
FUNCTIONAL ANATOMY OF STOMACH
■
■
■
■
■
GLANDS OF STOMACH
■
■
■
■
■
FUNCTIONS OF STOMACH
■
■
■
■
■
PROPERTIES AND COMPOSITION OF GASTRIC JUICE
■
■
■
■
■
FUNCTIONS OF GASTRIC JUICE
■
■
■
■
■
DIGESTIVE FUNCTION
■
■
■
■
■
HEMOPOIETIC FUNCTION
■
■
■
■
■
PROTECTIVE FUNCTION – FUNCTION OF MUCUS
■
■
■
■
■
FUNCTIONS OF HYDROCHLORIC ACID
■
■
■
■
■
SECRETION OF GASTRIC JUICE
■
■
■
■
■
SECRETION OF PEPSINOGEN
■
■
■
■
■
SECRETION OF HYDROCHLORIC ACID
■
■
■
■
■
REGULATION OF GASTRIC SECRETION
■
■
■
■
■
METHODS OF STUDY
■
■
■
■
■
PHASES OF GASTRIC SECRETION
■
■
■
■
■
APPLIED PHYSIOLOGY
■
■
■
■
■
GASTRITIS
■
■
■
■
■
GASTRIC ATROPHY
■
■
■
■
■
PEPTIC ULCER
Stomach
28
■
FUNCTIONAL ANATOMY OF
STOMACH
Stomach is a hollow organ situated just below
the diaphragm on the left side in the abdominal
cavity. Volume of empty stomach is 50 ml.
Under normal conditions, it can expand to
accommodate 1 to 1.5 liters of solids and
liquids. However, it is capable of expanding still
further up to 4 liters.
■
■
■
■
■
PARTS OF STOMACH
In humans, stomach has four parts:
1. Cardiac region
2. Fundus
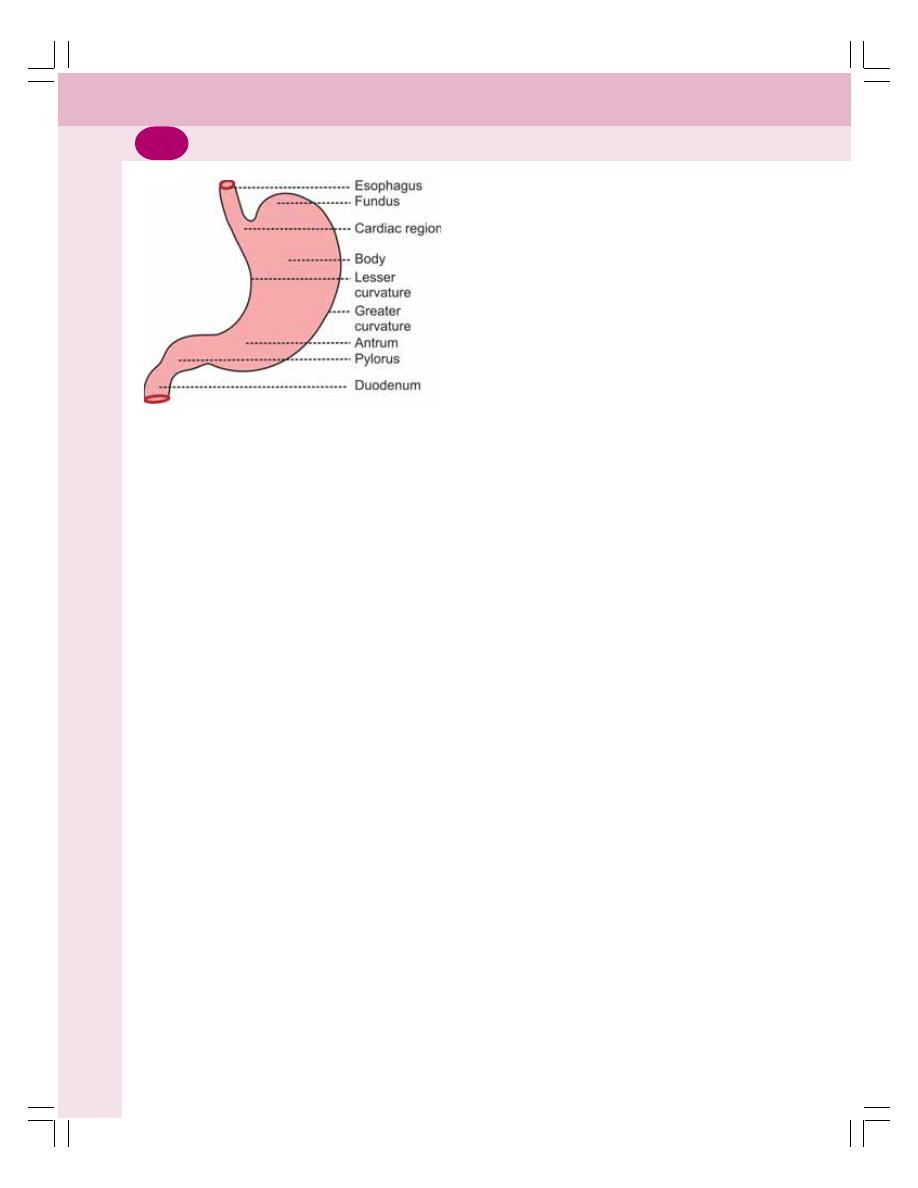
Digestive System
158
3. Body or corpus
4. Pyloric region.
1.
Cardiac Region
It is the upper part of the stomach where eso-
phagus opens. The opening is guarded by a
sphincter called cardiac sphincter which opens
only towards stomach. This portion is also known
as cardiac end.
2. Fundus
It is a small dome shaped structure. It is elevated
above the level of esophageal opening.
3.
Body or Corpus
It is the largest part of stomach forming about
75 to 80% of the whole stomach. It extends from
just below the fundus up to the pyloric region
(Fig. 28-1).
4.
Pyloric Region
The pyloric region has two parts, antrum and
pyloric canal. The body of the stomach ends in
antrum. The junction between body and antrum
is marked by an angular notch called incisura
angularis. Antrum is continued as the narrow
canal which is called pyloric canal or pyloric end.
Pyloric canal opens into first part of small intes-
tine called duodenum. The opening of pyloric
canal is guarded by a sphincter called pyloric
sphincter. It opens towards duodenum.
Stomach has two curvatures. The one on the
right side is lesser curvature and the one on the
left side is greater curvature.
■
■
■
■
■
STRUCTURE OF STOMACH WALL
The wall of the stomach is formed by four layers
of structures:
1. Outer serous layer formed by peritoneum
2. Muscular layer made up of three layers of
smooth muscle fibers namely, inner oblique,
middle circular and outer longitudinal layers
3. Submucus layer formed by areolar tissue,
blood vessels and lymph vessels
4. Inner mucus layer lined by mucus secreting
columnar epithelial cells. The gastric glands
are situated in this layer. The inner surface
of mucus layer is covered by 2 mm thick
mucus.
■
■
■
■
■
GLANDS OF STOMACH
Glands of the stomach or gastric glands are
tubular structures made up of different types of
cells. These glands open into the stomach cavity
through gastric pits.
■
■
■
■
■
CLASSIFICATION OF GLANDS OF
THE STOMACH
Gastric glands are classified into three types
depending upon their situation:
1. Fundic glands situated in body and fundus
of stomach. Fundic glands are also called
main gastric glands or oxyntic glands
2. Pyloric glands present in the pyloric part of
the stomach
3. Cardiac glands located in the cardiac region
of the stomach.
All the gastric glands open into the cavity of
stomach through gastric pits.
■
■
■
■
■
STRUCTURE OF GASTRIC GLANDS
Fundic Glands
The fundic glands are considered as the typical
gastric glands (Fig. 28-2). These glands are long
and tubular glands. Each gland has three parts
viz. body, neck and isthmus.
The cells present in the fundic glands are:
1. Chief cells or pepsinogen cells
2. Parietal cells or oxyntic cells
3. Mucus neck cells
4. Enterochromaffin (EC) cells
5. Enterochromaffin-like (ECL) cells.
FIGURE 28-1: Parts of stomach
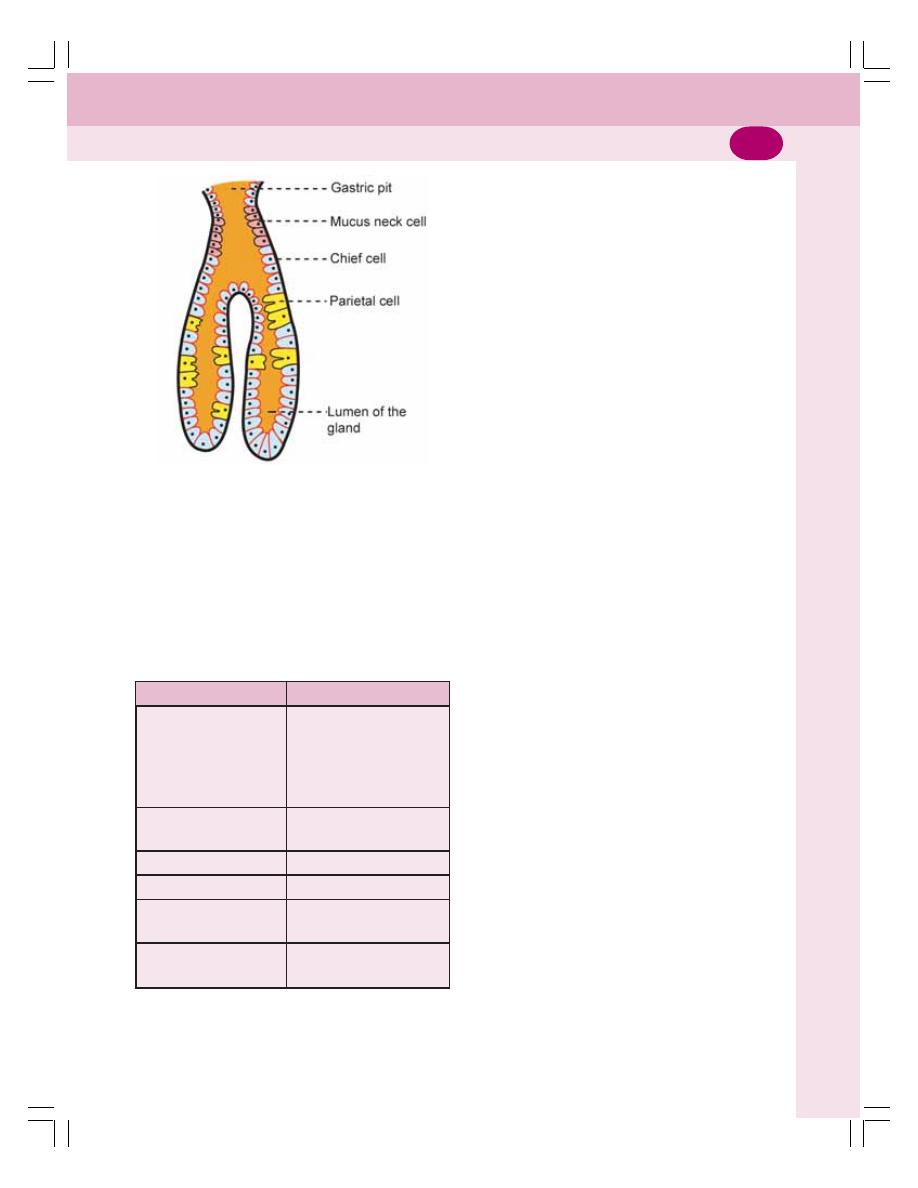
Chapter 28
Stomach
159
Secretions of these cells are given in Table
28-1.
Parietal cells are different from other cells of
the gland because of the presence of canaliculi
(singular = canaliculus). The parietal cells empty
their secretions into the lumen of the gland
through the canaliculi, whereas other cells empty
their secretions directly into lumen of the gland.
Pyloric Glands
The pyloric glands are short and tortuous in
nature. The cells that form the pyloric glands are
G cells, mucus cells, EC cells and ECL cells.
Cardiac Glands
Cardiac glands are also short and tortuous in
structure with many mucus cells. EC cells, ECL
cells and chief cells are also present in the
cardiac glands.
Enteroendocrine Cells
Enteroendocrine cells are the hormone secreting
cells present in the glands or mucosa of gastro-
intestinal tract particularly stomach and intestine.
The enteroendocrine cells present in gastric
glands are G cells, enterochromaffin cells and
enterochromaffin like cells.
■
■
■
■
■
FUNCTIONS OF STOMACH
■
■
■
■
■
1. MECHANICAL FUNCTION
i.
Storage Function
The food is stored in the stomach for a long
period, i.e. for 3 to 4 hours and emptied into the
intestine slowly. The maximum capacity of
stomach is up to 1.5 L. The slow emptying of
stomach provides enough time for proper diges-
tion and absorption of food substances in the
small intestine.
ii.
Formation of Chyme
The peristaltic movements of stomach mix the
bolus with gastric juice and convert it into the
semisolid material known as chyme.
■
■
■
■
■
2. DIGESTIVE FUNCTION
Refer functions of gastric juice.
TABLE 28-1: Secretory functions of
cells in gastric glands
Cell
Secretory products
Pepsinogen
Rennin
Chief cells
Lipase
Gelatinase
Urase
Parietal cells
Hydrochloric acid
Intrinsic factor of Castle
Mucus neck cells
Mucin
G cells
Gastrin
Enterochromaffin (EC)
Serotonin
cells
Enterochromaffin-like
Histamine
(ECL) cells
FIGURE 28-2: Gastric glands
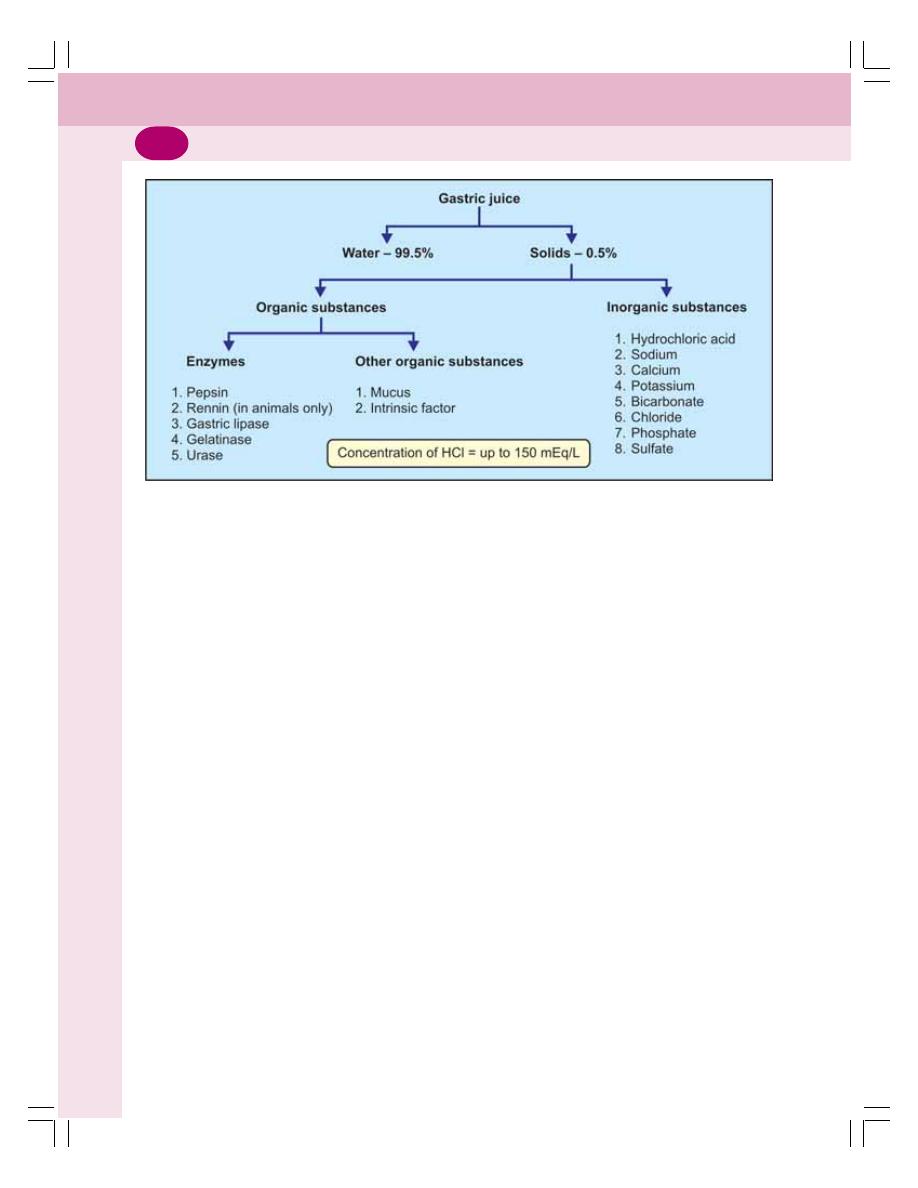
Digestive System
160
■
■
■
■
■
3. PROTECTIVE FUNCTION
Refer functions of gastric juice.
■
■
■
■
■
4. HEMOPOIETIC FUNCTION
Refer functions of gastric juice.
■
■
■
■
■
5. EXCRETORY FUNCTION
Many substances like toxins, alkaloids and
metals are excreted through gastric juice.
■
■
■
■
■
PROPERTIES AND COMPOSITION
OF GASTRIC JUICE
Gastric juice is the mixture of secretions from
different gastric glands.
Properties of Gastric Juice
Volume
: 1200 to 1500 mL/day
Reaction
: Gastric juice is highly acidic
with pH of 0.9 to 1.2 due to
hydrochloric acid
Specific gravity : 1.002 to 1.004
Composition of Gastric Juice
Gastric juice contains 99.5% of water and 0.5%
solids. The solids are organic and inorganic
substances. Refer Figure 28-3 for composition
of gastric juice.
■
■
■
■
■
FUNCTIONS OF GASTRIC JUICE
■
■
■
■
■
1. DIGESTIVE FUNCTION
The gastric juice acts mainly on proteins. The
proteolytic enzymes of the gastric juice are
pepsin and rennin (Table 28-2). Gastric juice also
contains some other enzymes like gastric lipase,
gelatinase, urase and gastric amylase.
Pepsin
Pepsin is secreted as inactive pepsinogen.
Pepsinogen is converted into pepsin by hydro-
chloric acid which is secreted by parietal cells.
The optimum pH for activation of pepsinogen is
below 6.
Action of pepsin
Pepsin converts proteins into proteoses, pep-
tones and polypeptides. Pepsin also causes
curdling and digestion of milk (casein).
Gastric Lipase
Gastric lipase is a weak lipolytic enzyme. It needs
acidic medium with pH is between 4 and 5 for
FIGURE 28-3: Composition of gastric juice
Chapter 28
Stomach
161
its action. But it becomes inactive when the pH
falls below 2.5. Gastric lipase acts on tributyrin
(butter fat) and hydrolyzes it into fatty acids and
glycerols.
Actions of Other Enzymes of
Gastric Juice
i. Gelatinase degrades gelatin and collagen
into peptides
ii. Urase acts on urea and produces ammo-
nia
iii. Gastric amylase degrades starch (but its
action is insignificant)
iv. Rennin curdles milk (present in animals
only).
■
■
■
■
■
2. HEMOPOIETIC FUNCTION
The intrinsic factor of Castle secreted by parietal
cells of gastric glands plays an important role in
erythropoiesis. It is necessary for absorption of
vitamin B
12
(which is called extrinsic factor) from
GI tract into the blood. Vitamin B
12
is an impor-
tant maturation factor during erythropoiesis.
Absence of intrinsic factor in gastric juice causes
deficiency of vitamin B
12
leading to pernicious
anemia (Chapter 11).
■
■
■
■
■
3. PROTECTIVE FUNCTION –
FUNCTION OF MUCUS
The mucus present in the gastric juice protects
gastric wall as mentioned below.
Mucus:
i. Protects the stomach wall from irritation
or mechanical injury by virtue of its high
viscosity
ii. Prevents the digestive action of pepsin on
gastric mucosa
iii. Protects the gastric mucosa from hydro-
chloric acid of gastric juice because of its
alkaline nature and its acid combining
power.
■
■
■
■
■
4. FUNCTIONS OF HYDROCHLORIC
ACID
Hydrochloric acid present in the gastric juice:
i. Activates pepsinogen into pepsin
ii. Kills some of the bacteria entering the
stomach along with food substances – this
action is called bacteriolytic action
iii. Provides acid medium which is necessary
for the actions of the hormones.
■
■
■
■
■
SECRETION OF GASTRIC JUICE
■
■
■
■
■
SECRETION OF PEPSINOGEN
Pepsinogen is synthesized from amino acids in
the ribosomes attached to endoplasmic reticulum
in chief cells. The pepsinogen molecules are
packed into zymogen granules by Golgi
apparatus.
When zymogen granule is secreted into sto-
mach from chief cells, the granule is dissolved
and pepsinogen is released into gastric juice.
TABLE 28-2: Digestive enzymes of gastric juice
Enzyme
Activator
Acts on
End products
1. Pepsin
Hydrochloric acid
Proteins
Proteoses, peptones and poly-
peptides
2. Gastric lipase
Acid medium
Triglycerides of butter
Fatty acids and glycerols
3. Gastric amylase
Acid medium
Starch
Dextrin and maltose (negligible
action)
4. Gelatinase
Acid medium
Gelatin and collagen
Peptides
of meat
5. Urase
Acid medium
Urea
Ammonia
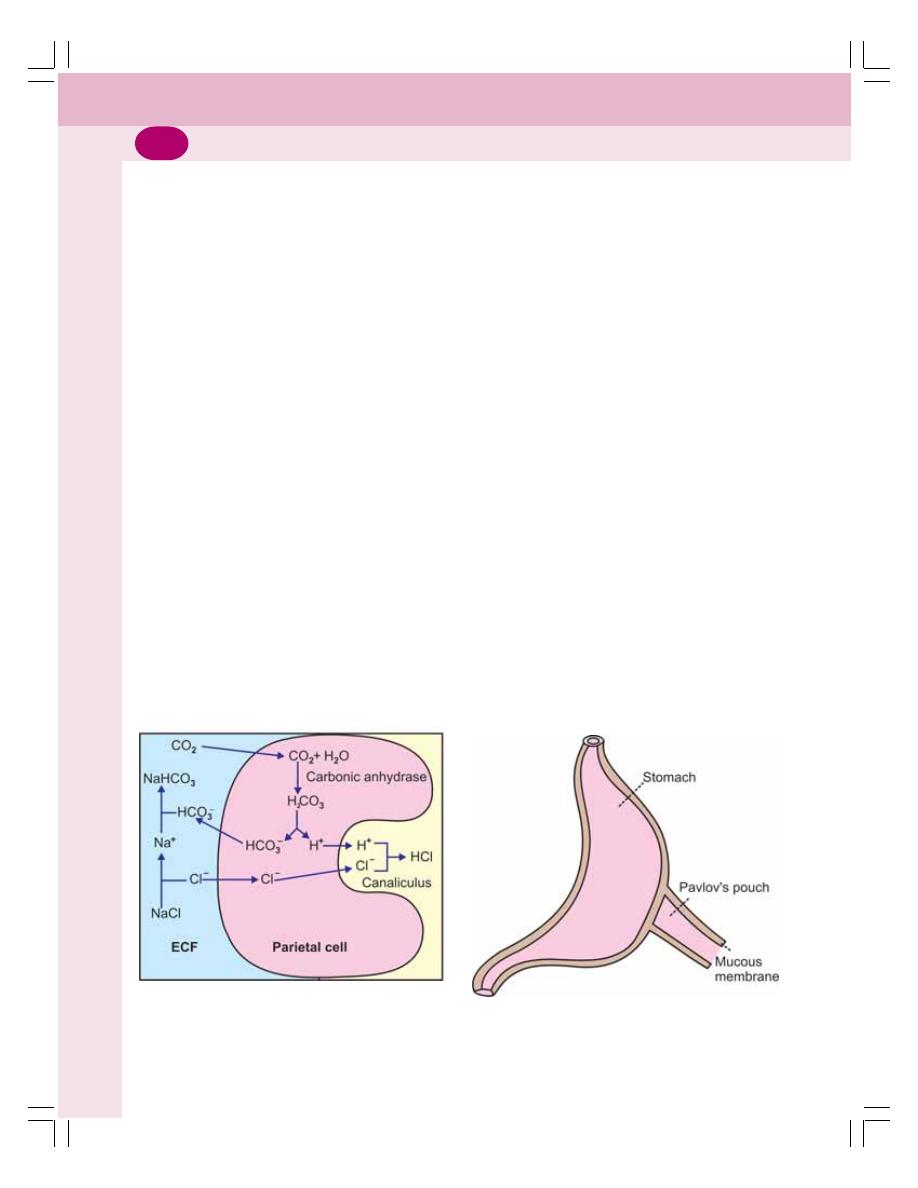
Digestive System
162
Pepsinogen is activated into pepsin by hydro-
chloric acid.
■
■
■
■
■
SECRETION OF HYDROCHLORIC ACID
Hydrochloric acid secretion is an active process
that takes place in the canaliculi of parietal cells
in gastric glands. The energy for this is derived
from oxidation of glucose
In the parietal cells, the carbon dioxide is
formed from metabolic activity. It is also derived
from blood. Carbon dioxide combines with water
to form carbonic acid in the presence of carbonic
anhydrase. This enzyme is present in high
concentration in parietal cells. Carbonic acid is
the most unstable compound and, immediately
it splits into hydrogen ion and bicarbonate ion.
The hydrogen ion is actively pumped into the
canaliculus of parietal cell.
Simultaneously, the chloride ion is also
pumped into canaliculus actively. The chloride
is derived from sodium chloride in the blood. Now,
the hydrogen ion combines with chloride ion to
form hydrochloric acid. To compensate the loss
of chloride ion, the bicarbonate ion from parietal
cell enters the blood and combines with sodium
to form sodium bicarbonate. Thus, the entire
process is summarized as (Fig. 28-4):
CO
2
+ H
2
O + NaCl
→
HCl + NaHCO
3
■
■
■
■
■
REGULATION OF GASTRIC
SECRETION
Regulation of gastric secretion and intestinal
secretion is studied by some experimental proce-
dures.
■
■
■
■
■
METHODS OF STUDY
1.
Pavlov’s Pouch
Pavlov’s pouch is a small part of the stomach
that is incompletely separated from the main
portion and made into a small bag like pouch
(Fig. 28-5). Pavlov’s pouch was designed by the
Russian scientist Pavlov in dog during his studies
on conditioned reflexes.
Procedure
To prepare a Pavlov’s pouch, stomach of an
anesthetized dog is divided into a larger part and
a smaller part by making an incomplete incision.
The mucous membrane is completely divided.
A small part of muscular coat called isthmus is
retained. The isthmus connects the two parts.
The cut edges of major portions are stitched.
The smaller part is also stitched, leaving a small
outlet. This outlet is brought out through the
abdominal wall and used to drain the pouch.
FIGURE 28-4: Secretion of hydrochloric
acid in parietal cell of gastric gland
FIGURE 28-5: Pavlov’s pouch

Chapter 28
Stomach
163
Nerve supply of Pavlov’s pouch
Pavlov’s pouch receives parasympathetic
(vagus) nerve fibers through isthmus and
sympathetic fibers through blood vessels.
Use of Pavlov’s pouch
Pavlov’s pouch is used to demonstrate the
different phases of gastric secretion particularly
the cephalic phase and used to demonstrate the
role of vagus in cephalic phase.
2.
Farrel and Ivy Pouch
This pouch is prepared by removing the part of
Pavlov’s pouch from the stomach and trans-
planting it in the subcutaneous tissue of abdo-
minal wall or thoracic wall in the same animal. It
is used for experimental purpose, when the new
blood vessels are developed.
Uses of Farrel and Ivy pouch
This pouch is useful to study the role of hormones
during gastric and intestinal phases of gastric
secretion.
3.
Sham Feeding
Sham feeding means the false feeding. It is
another experimental procedure devised by
Pavlov to demonstrate the regulation of gastric
secretion.
Procedure
i. A hole is made in the neck of an anesthe-
tized dog
ii. Esophagus is transversely cut. The cut
ends are drawn out through the hole in
the neck
iii. When the dog eats food, it comes out
through the cut end of the esophagus
iv. But the dog has the satisfaction of eating
the food. It is called sham feeding.
This experimental procedure is supported by
the preparation of Pavlov’s pouch with a fistula
from the stomach. The fistula opens to the
exterior and it is used to observe the gastric
secretion. The animal is used for experimental
purpose after a week’s time when healing is
completed.
Advantage of sham feeding
It is useful to demonstrate the secretion of gastric
juice during cephalic phase. In the same animal
after vagotomy, sham feeding does not induce
gastric secretion. It proves the role of vagus
nerve during cephalic phase.
■
■
■
■
■
PHASES OF GASTRIC SECRETION
Gastric juice is secreted in three different phases:
I. Cephalic phase
II. Gastric phase
III. Intestinal phase.
In human beings, a fourth phase called
interdigestive phase exists. All the phases are
regulated by neural mechanism or hormonal
mechanism or both.
■
■
■
■
■
CEPHALIC PHASE
Secretion of gastric juice by the stimuli arising
from head region (cephalus) is called cephalic
phase (Fig. 28-6). This phase is regulated by
nervous mechanism.
During this phase, the gastric secretion
occurs even without the presence of food in the
stomach. The quantity of the juice is less but it
is rich in enzymes and hydrochloric acid.
The nervous mechanism that regulates
cephalic phase operates through reflex action.
Two types of reflexes occur:
1. Unconditioned reflex
2. Conditioned reflex.
Unconditioned Reflex
Unconditioned reflex is the inborn reflex. When
food is placed in the mouth, it induces salivary
secretion (Chapter 27). Simultaneously, gastric
secretion also occurs.
Stages of the reflex action
i. The presence of food in the mouth stimu-
lates the taste buds and other receptors
in the mouth
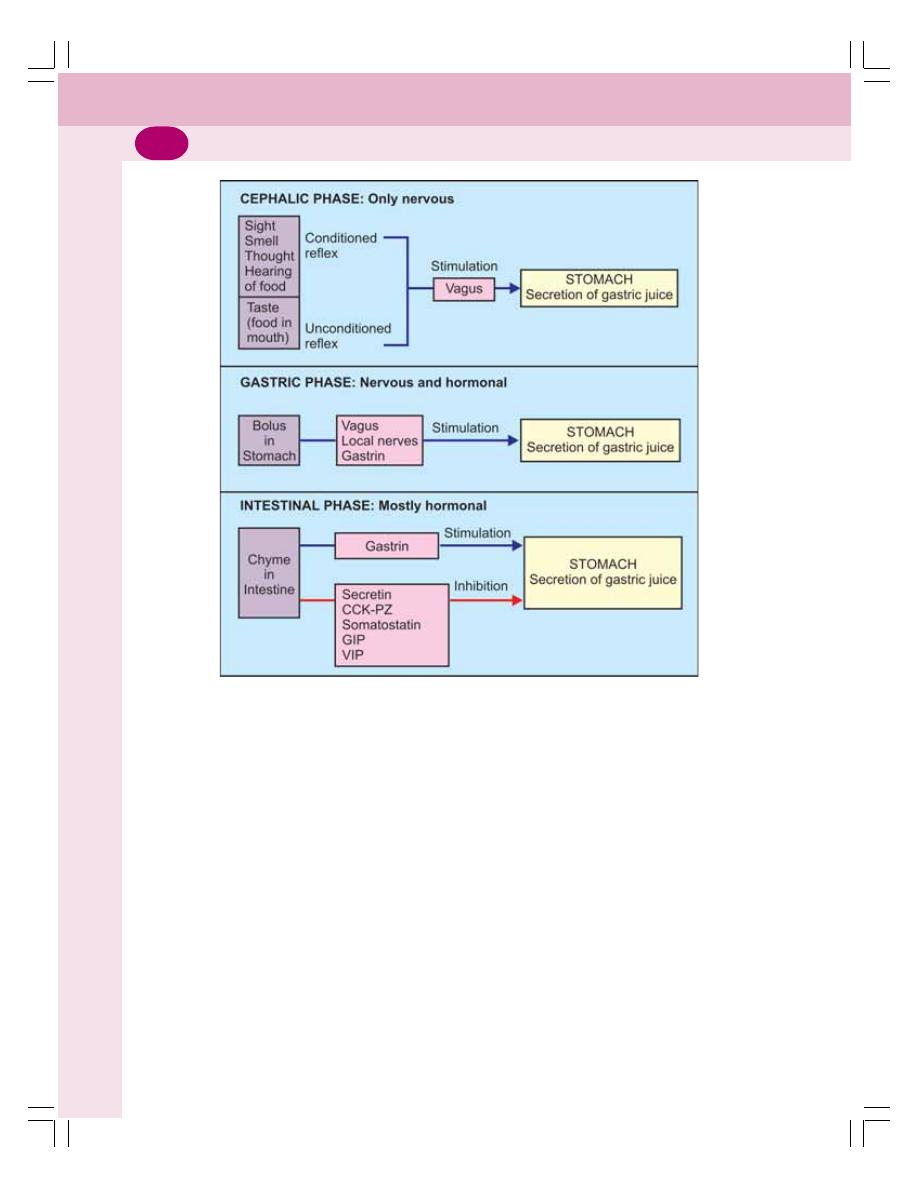
Digestive System
164
ii. The sensory (afferent) impulses from
mouth pass via afferent nerve fibers of
glossopharyngeal and facial nerves to
appetite center present in amygdala and
hypothalamus
iii. From here, efferent impulses pass
through dorsal nucleus of vagus and vagal
efferent nerve fibers to the wall of the
stomach
iv. Acetylcholine is secreted at the vagal
efferent nerve endings stimulates gastric
glands to increase the secretion.
This is experimentally proved by
Pavlov’s pouch and sham feeding.
Conditioned Reflex
Conditioned reflex is the reflex response acquired
by previous experience (Chapter 101). Presence
of food in the mouth is not necessary to elicit
this reflex. The sight, smell, hearing or thought
of food which induce salivary secretion also
induce gastric secretion.
Stages of reflex action
i. Impulses from the special sensory organs
(eye, ear and nose) pass through afferent
fibers of neural circuits to the cerebral
cortex. Thinking of food stimulates the
cerebral cortex directly
ii. From cerebral cortex the impulses pass
through dorsal nucleus of vagus and vagal
efferents and reach stomach wall
iii. The vagal nerve endings secrete acetyl-
choline. It stimulates the gastric glands to
increase its secretion.
FIGURE 28-6: Schematic diagram showing the regulation of gastric secretion

Chapter 28
Stomach
165
Conditioned reflex of gastric secretion is
proved by Pavlov’s pouch and bell dog experi-
ment (Chapter 101).
■
■
■
■
■
GASTRIC PHASE
The secretion of gastric juice when the food
enters the stomach is called gastric phase. This
phase is regulated by both nervous and hormonal
mechanisms. The gastric juice secreted during
this phase is rich in pepsinogen and hydrochloric
acid. The mechanisms involved in this phase are:
1. Nervous mechanism through local myenteric
reflex and vagovagal reflex
2. Hormonal mechanism through gastrin.
1.
Nervous Mechanism
Local myenteric reflex
Local myenteric reflex is elicited by stimulation
of myenteric nerve plexus in stomach wall. After
entering stomach, the food particles stimulate the
local nerve plexus (Chapter 26) present in the
wall of the stomach. These nerve fibers release
acetylcholine, which stimulates the gastric glands
to secrete a large quantity of gastric juice.
Simultaneously, acetylcholine stimulates G cells
to secrete gastrin (see below).
Vagovagal reflex
Vagovagal reflex is the reflex in which both
afferent and efferent vagal fibers are involved.
Presence of food in stomach stimulates the
sensory (afferent) nerve endings of vagus which
generate sensory impulses. The sensory
impulses are transmitted to the brainstem via
sensory fibers of vagus. Brainstem in turn sends
efferent impulses through the motor (efferent)
fibers of vagus back to stomach and cause
secretion of gastric juice. Since, both afferent and
efferent impulses pass through vagus, this reflex
is called vagovagal reflex.
2.
Hormonal Mechanism – Gastrin
Gastrin is a gastrointestinal hormone secreted
by the G cells which are present in pyloric glands
of stomach. Small amount of gastrin is also
secreted in mucosa of upper small intestine.
Gastrin is a polypeptide containing G14, G17 or
G34 amino acids.
Gastrin is released when food enters sto-
mach. The mechanism involved in the release
of gastrin may be the local nervous reflex or
vagovagal reflex. The nerve endings release the
neurotransmitter called gastrin releasing peptide
which stimulates the G cells to secrete gastrin.
Actions of gastrin on gastric secretion
Gastrin stimulates the secretion of pepsinogen
and hydrochloric acid by the gastric glands.
Experimental evidences of gastric phase
The nervous mechanism of gastric secretion
during gastric phase is proved by Pavlov’s pouch.
Hormonal mechanism of gastric secretion is
proved by Farrel and Ivy pouch (see above).
■
■
■
■
■
INTESTINAL PHASE
Intestinal phase is the secretion of gastric juice
when chyme enters the intestine. When chyme
enters the intestine initially the gastric secretion
increases and later it stops. Intestinal phase of
gastric secretion is under both nervous and hor-
monal control.
Initial stage of intestinal phase
The chyme entering intestine stimulates the
duodenal mucosa to release gastrin which is
transported to stomach through blood. There, it
increases gastric secretion.
Later stage of intestinal phase
After the initial increase, there is decrease or
complete stoppage of secretion of gastric juice.
Two factors are responsible for the inhibition:
1. Enterogastric reflex
2. GI hormones.
1.
Enterogastric reflex
It is a reflex that inhibits the secretion and
movements of stomach due to the distention or
Digestive System
166
irritation of intestinal mucosa. It is mediated by
myenteric nerve (Auerbach’s) plexus and vagus.
2.
GI hormones
The presence of chyme in the intestine
stimulates the secretion of many GI hormones
from intestinal mucosa and other structures. All
these hormones inhibit the gastric secretion.
Some of these hormones inhibit the gastric
motility also.
GI hormones which inhibit gastric secretion:
i.
Secretin: Secreted by the presence of acid
chyme in the intestine
ii.
Cholecystokinin: Secreted by the pre-
sence of chyme containing fats and amino
acids in intestine
iii.
Gastric inhibitory peptide (GIP): Secreted
by the presence of chyme containing
glucose and fats in the intestine
iv.
Vasoactive intestinal polypeptide (VIP):
Secreted by the presence of acidic chyme
in intestine
v.
Peptide YY: Secreted by the presence of
fatty chyme in intestine.
In addition to these hormones, pan-
creas also secretes a hormone called
somatostatin during intestinal phase. It
also inhibits gastric secretion.
The intestinal phase of gastric secretion is
demonstrated by Farrel and Ivy pouch.
■
■
■
■
■
INTERDIGESTIVE PHASE
Secretion of small amount of gastric juice in
between meals (or during period of fasting) is
called interdigestive phase. Gastric secretion
during this phase is mainly due to the hormones
like gastrin. This phase of gastric secretion is
demonstrated by Farrel and Ivy pouch.
■
■
■
■
■
APPLIED PHYSIOLOGY
■
■
■
■
■
1. GASTRITIS
Inflammation of gastric mucosa is called gastritis.
It may be acute or chronic.
Causes of Gastritis
i. Infection with bacterium
Helicobacter
pylori
ii. Excess consumption of alcohol
iii. Excess or long term administration
nonsteroidal anti-inflammatory drugs
(NSAIDs)
iv. Trauma by nasogastric tubes
v. Autoimmune disease.
Features
Features of gastritis are:
i. Abdominal upset or pain
ii. Nausea
iii. Vomiting
iii. Anorexia (loss of appetite)
iv. Indigestion
v. Discomfort or feeling of fullness in the
epigastric region
vi. Belching (process to relieve swallowed air
that is accumulated in stomach).
■
■
■
■
■
2. GASTRIC ATROPHY
Gastric atrophy is the condition in which the mus-
cles of the stomach shrink and become weak.
The gastric glands also shrink resulting in the
deficiency of gastric juice.
Cause
Gastric atrophy is caused by chronic gastritis and
autoimmune disease.
Features
Gastric atrophy causes achlorhydria (absence of
hydrochloric acid in gastric juice) and pernicious
anemia. Some patients develop gastric cancer.
■
■
■
■
■
3. PEPTIC ULCER
Ulcer means the erosion of the surface of any
organ due to shedding or sloughing of inflamed
necrotic tissue that lines the organ. Peptic ulcer
means an ulcer in the wall of stomach or duo-
denum caused by digestive action of gastric juice.
If peptic ulcer is found in stomach, it is called
gastric ulcer and if found in duodenum it is called
duodenal ulcer.

Chapter 28
Stomach
167
Causes
i. Increased peptic activity due to excessive
secretion of pepsin in gastric juice
ii. Hyperacidity of gastric juice
iii. Reduced alkalinity of duodenal content
iv. Decreased mucin content in gastric juice
or decreased protective activity in stomach
or duodenum
v. Constant physical or emotional stress
vi. Food with excess spices or smoking
(classical causes of ulcers)
vii. Long term use of NSAIDs (see above)
such as aspirin, ibuprofen, and naproxen
viii. Chronic inflammation due to
Helicobacter
pylori.
Features
The most common feature of peptic ulcer is
severe burning pain in epigastric region. In gastric
ulcer, pain occurs while eating or drinking. In
duodenal ulcer, pain is felt 1 or 2 hours after food
intake and during night.
Other symptoms accompanying pain are:
i. Nausea
ii. Vomiting
iii. Hematemesis (vomiting blood)
iv. Heartburn (burning pain in chest due to
regurgitation of acid from stomach into
esophagus)
v. Anorexia (loss of appetite)
vi. Loss of weight.
FUNCTIONAL ANATOMY AND NERVE SUPPLY OF PANCREAS
PROPERTIES AND COMPOSITION OF PANCREATIC JUICE
FUNCTIONS OF PANCREATIC JUICE
NEUTRALIZING ACTION OF PANCREATIC JUICE
REGULATION OF PANCREATIC SECRETION
APPLIED PHYSIOLOGY
FUNCTIONAL ANATOMY AND
NERVE SUPPLY OF PANCREAS
Pancreas is a dual organ having two functions,
the endocrine function and the exocrine function.
The endocrine function is concerned with
production of the hormones (Chapter 48). The
exocrine function is concerned with secretion of
digestive juice called pancreatic juice.
FUNCTIONAL ANATOMY OF
EXOCRINE PART OF PANCREAS
Exocrine part of pancreas is made up of acini
or alveoli like salivary glands. Each acinus has
a single layer of acinar cells with a lumen in the
center. The acinar cells contain zymogen
granules, which possess digestive enzymes.
A small duct arises from lumen of each
alveolus. Some of these ducts from neighboring
alveoli unite to form intralobular duct. All the
intralobular ducts unite to form the main duct of
pancreas called Wirsung’s duct. Wirsung’s duct
joins common bile duct to form ampulla of Vater
which opens into duodenum (see Fig. 30-3).
NERVE SUPPLY TO PANCREAS
Pancreas is supplied by both sympathetic and
parasympathetic fibers. The sympathetic fibers
are supplied through splanchnic nerve and
parasympathetic fibers are supplied through
vagus nerve.
PROPERTIES AND COMPOSITION
OF PANCREATIC JUICE
Properties of Pancreatic Juice
Volume
: 500 to 800 mL/day
Reaction
: Highly alkaline with pH of
8 to 8.3
Specific gravity : 1.010 to 1.018
Composition of Pancreatic Juice
Pancreatic juice contains 99.5% of water and
0.5% of solids. The solids are the organic and
inorganic substances. Composition of pancreatic
juice is given in Fig. 29-1.
The bicarbonate content is very high in
pancreatic juice. It is about 110 to 150 mEq/L
Pancreas
29
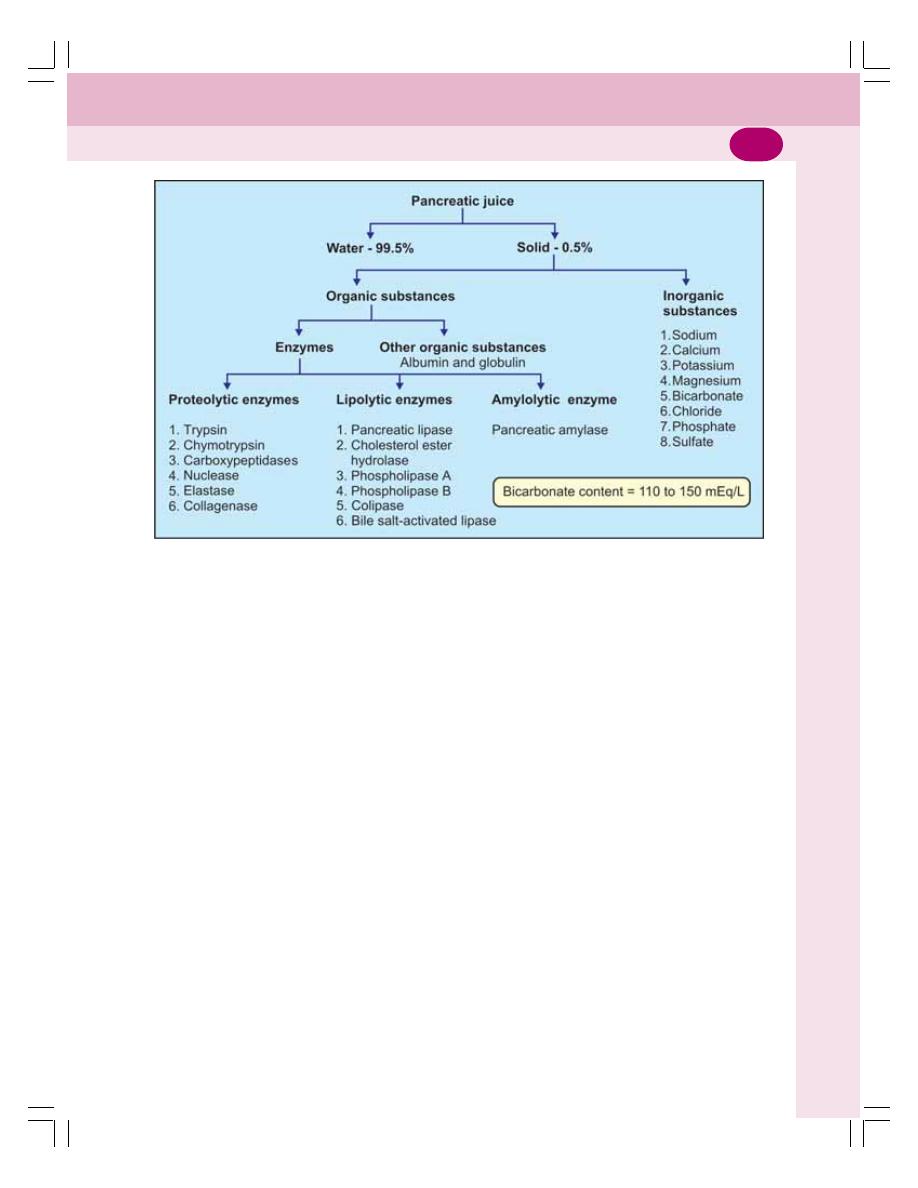
Chapter 29 Pancreas
169
against the concentration of 24 mEq/L in plasma.
This high concentration of bicarbonate is
responsible for the alkalinity of pancreatic juice.
FUNCTIONS OF PANCREATIC JUICE
Pancreatic juice has digestive functions and the
neutralizing action.
DIGESTIVE FUNCTIONS OF
PANCREATIC JUICE
Pancreatic juice plays an important role in the
digestion of proteins and lipids. It also has mild
action on carbohydrate digestion.
DIGESTION OF PROTEINS
The major proteolytic enzymes of pancreatic juice
are trypsin and chymotrypsin. Other proteolytic
enzymes are carboxypeptidases, nuclease,
elastase and collagenase.
1. Trypsin
Trypsin is a single polypeptide with a molecular
weight of 25,000. It contains 229 amino acids.
It is secreted as inactive trypsinogen which
is converted into active trypsin by enterokinase.
Enterokinase is also called enteropeptidase and
it is secreted by the brush bordered cells of
duodenal mucous membrane. Once formed,
trypsin itself activates trypsinogen by means of
autocatalytic or autoactive action.
Actions of trypsin
i.
Digestion of proteins: Trypsin is the most
powerful proteolytic enzyme. It is an
endopeptidase and breaks the interior bonds
of the protein molecules. And it converts
proteins into proteoses and polypeptides
ii. Curdling of milk – it converts caseinogens in
the milk into casein
iii. It accelerates blood clotting
iv. It activates other enzymes of pancreatic juice:
Chymotrypsinogen into chymotrypsin
Procarboxypeptidases into carboxy-
peptidases
Proelastase into elastase
Procolipase into colipase
v. Trypsin also activates collagenase, phos-
pholipase A and phospholipase B.
FIGURE 29-1: Composition of pancreatic juice
Digestive System
170
vi. Autocatalytic action — once formed, trypsin
itself converts trypsinogen into trypsin.
2. Chymotrypsin
Chymotrypsin is a polypeptide with a molecular
weight of 25,700 and 246 amino acids. It is
secreted as inactive chymotrypsinogen and
activated into chymotrypsin by trypsin.
Actions of chymotrypsin
i.
Digestion of proteins: Chymotrypsin is also
an endopeptidase and it breaks the proteins
into polypeptides
ii. Digestion of milk: Chymotrypsin digests
casein faster than trypsin. The combination
of both enzymes causes more rapid digestion
of milk.
iii. On blood clotting – no action.
3. Carboxypeptidases
The two carboxypeptidases are carboxy-
peptidase A and carboxypeptidase B. These are
secreted as procarboxypeptidase A and
procarboxypeptidase B. The inactive
procarboxypeptidases are activated into
carboxypeptidases by trypsin.
Actions of carboxypeptidases
Carboxypeptidases are exopeptidases and split
the polypeptides and other proteins into amino
acids.
4. Nucleases
The nucleases of pancreatic juice are
ribonuclease and deoxyribonuclease, which are
responsible for the digestion of nucleic acids.
These enzymes convert the ribonucleic acid
(RNA) and deoxyribonucleic acid (DNA) into
mononucleotides.
5. Elastase
Elastase is secreted as inactive proelastase and
is activated into active elastase by trypsin. It
digests the elastic fibers.
6. Collagenase
Collagenase is secreted as inactive
procollagenase and is activated into active
collagenase by trypsin. It digests collagen.
DIGESTION OF LIPIDS
The lipolytic enzymes present in pancreatic juice
are pancreatic lipase, cholesterol ester hydrolase,
phospholipase A, phospholipase B and a
coenzyme called colipase.
1. Pancreatic Lipase
Pancreatic lipase is a powerful lipolytic enzyme.
It digests the triglycerides into monoglycerides
and fatty acids. The activity of pancreatic lipase
is accelerated in the presence of bile. The
optimum pH required for activity of this enzyme
is 7 to 9.
Digestion of fat by pancreatic lipase requires
two more factors:
i.
Bile salts which are responsible for the
emulsification of fat prior to their digestion
ii. Colipase which is a coenzyme necessary for
the pancreatic lipase to hydrolyze the dietary
lipids. Colipase is secreted as an inactive
procolipase which activated into colipase by
trypsin.
About 80% of fat is digested by pancreatic
lipase. The deficiency or absence of this enzyme
leads to excretion of undigested fat in feces (see
below).
2. Cholesterol Ester Hydrolase
Cholesterol ester hydrolase or cholesterol
esterase converts cholesterol ester into free
cholesterol and fatty acid by hydrolysis.
3. Phospholipase A
It is activated by trypsin. Phospholipase A digests
phospholipids namely lecithin and cephalin and
converts them into lysolecithin and lysocephalin.
4. Phospholipase B
Phospholipase B is also activated by trypsin. This
enzyme converts lysolecithin and lysocephalin
into phosphoryl choline and free fatty acids.
Chapter 29 Pancreas
171
5. Colipase
Colipase is a small coenzyme which facilitates
the hydrolysis of fats by pancreatic lipase.
6. Bile Salt-activated Lipase
This enzyme has a weak lipolytic action. It digests
a variety of lipids like phospholipids, cholesterol
esters and triglycerides. Since it is activated bile
salt, it is known as bile salt-activated lipase
(Table 29-1).
DIGESTION OF CARBOHYDRATES
Pancreatic amylase is the amylolytic enzyme
present in pancreatic juice. Like salivary amylase,
the pancreatic amylase also converts starch into
dextrin and maltose.
NEUTRALIZING ACTION OF
PANCREATIC JUICE
When acid chyme enters intestine from stomach,
pancreatic juice with large quantity of
bicarbonate is released into intestine. Presence
of large quantity of bicarbonate ions makes the
pancreatic juice highly alkaline. This alkaline
pancreatic juice neutralizes acidity of chyme in
the intestine.
Neutralizing action is an important function
of pancreatic juice, because, it protects the
intestine from the destructive action of acid in
the chyme.
TABLE 29-1: Digestive enzymes of pancreatic juice
Enzyme
Activator
Acts on
End products
1. Trypsin
Enterokinase
Proteins
Proteoses and Polypeptides
Trypsin
2. Chymotrypsin
Trypsin
Proteins
Polypeptides
3. Carboxypeptidases
Trypsin
Polypeptides
Amino acids
4. Nucleases
Trypsin
RNA and DNA
Mononucleotides
5. Elastase
Trypsin
Elastin
Amino acids
6. Collagenase
Trypsin
Collagen
Amino acids
7. Pancreatic lipase
Alkaline medium
Triglycerides
Monoglycerides and fatty
acids
8. Cholesterol ester
Alkaline medium
Cholesterol ester
Cholesterol and fatty acids
hydrolase
9. Phospholipase A
Trypsin
Phospholipids
Lysophospholipids
10. Phospholipase B
Trypsin
Lysophospholipids
Phosphoryl choline and free
fatty acids
11. Colipase
Trypsin
Facilitates action of
- - -
trypsin
12. Bile salt – activated
Trypsin
Phospholipids
Lysophospholipids
lipase
Cholesterol esters
Cholesterol and fatty acids
Triglycerides
Monoglycerides and fatty
acids
13. Pancreatic amylase
- - -
Starch
Dextrin and maltose
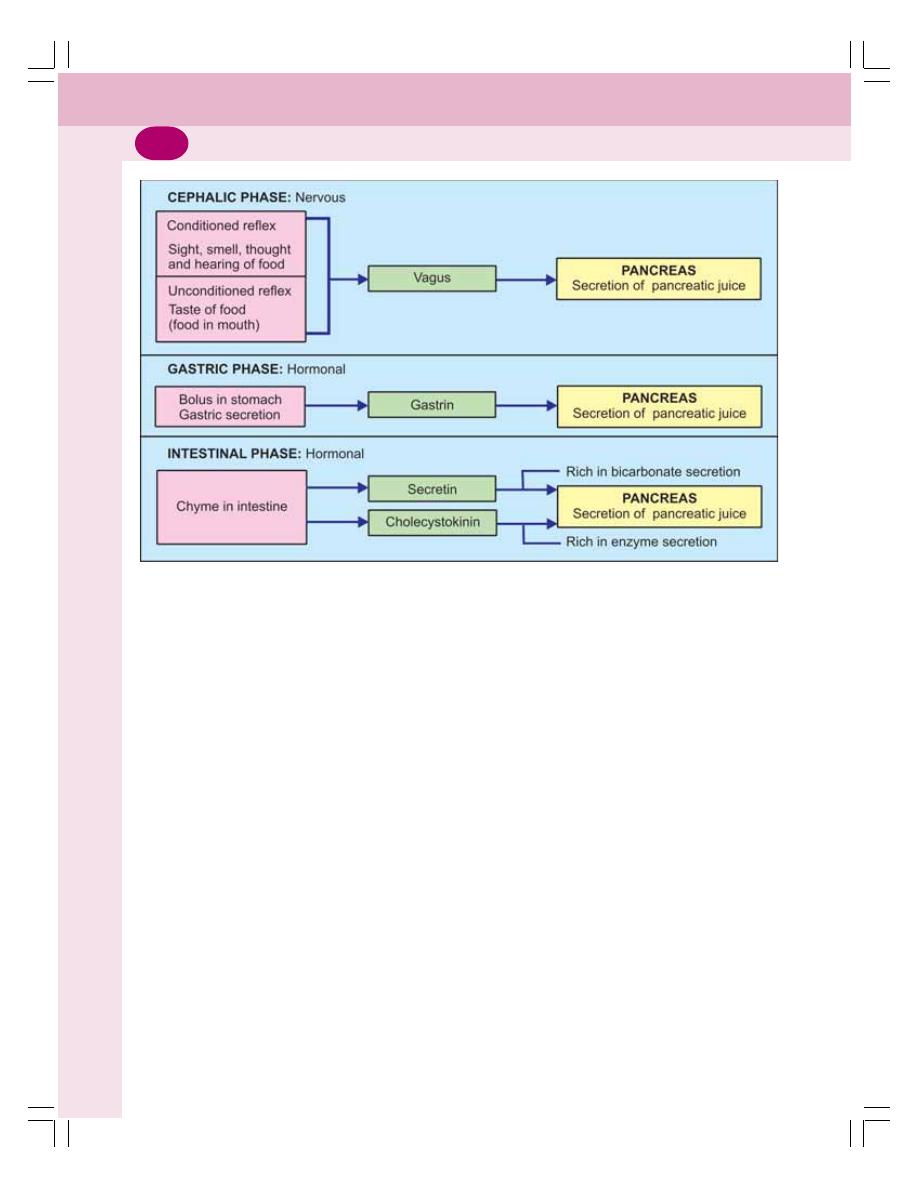
Digestive System
172
REGULATION OF PANCREATIC
SECRETION
The pancreatic secretion occurs in three stages:
1. Cephalic phase
2. Gastric phase
3. Intestinal phase.
Each phase is regulated by nervous
mechanism or hormonal mechanism or both.
1. CEPHALIC PHASE
As in case of gastric secretion, the cephalic
phase of pancreatic secretion is regulated by
nervous mechanism through reflex action. Two
types of reflexes occur:
1. Unconditioned reflex
2. Conditioned reflex.
Unconditioned Reflex
Unconditioned reflex is the inborn reflex. When
food is placed in the mouth, it induces salivary
secretion (Chapter 27), gastric secretion
(Chapter 28). Simultaneously it induces
pancreatic secretion also.
Conditioned Reflex
Conditioned reflex is the reflex response acquired
by previous experience (Chapter 101). Presence
of food in the mouth is not necessary to elicit
this reflex. The sight, smell, hearing or thought
of food which induce salivary secretion and
gastric secretion also induces pancreatic
secretion (Fig. 29-2).
The impulses from mouth (during
unconditioned reflex) or from the cerebral cortex
(during conditioned reflex) reach the dorsal
nucleus of vagus. From the dorsal nucleus of
vagus, the efferent impulses reach the
pancreas via efferent fibers of vagus nerve. The
vagal nerve endings release acetylcholine which
stimulates the acinar cells to release the
enzymes.
2. GASTRIC PHASE
Secretion of pancreatic juice when food enters
the stomach is known as gastric phase. This
phase of pancreatic secretion is under hormonal
control. The hormone involved is gastrin.
FIGURE 29-2: Schematic diagram showing the regulation of pancreatic secretion
Chapter 29 Pancreas
173
When food enters stomach, gastrin is
secreted from stomach (Chapter 28). When
gastrin is transported to pancreas through blood,
it stimulates the pancreatic secretion. The
pancreatic juice secreted during gastric phase
is rich in enzymes.
3. INTESTINAL PHASE
Intestinal phase is the secretion of pancreatic
juice when the chyme enters the intestine. This
phase is also under hormonal control.
When chyme enters the intestine, many
hormones are released. Some hormones
stimulate the pancreatic secretion and some
hormones inhibit the pancreatic secretion.
Hormones Stimulating Pancreatic
Secretion
i.
Secretin
ii. Cholecystokinin.
Secretin
Secretin is produced by S cells of mucous
membrane in duodenum and jejunum. It is
produced in an inactive prosecretin which is
activated into secretin by acid chyme.
The stimulant for the release and activation
of prosecretin is the acid chyme entering
intestine. The products of protein digestion also
stimulate the hormonal secretion.
Action of secretin
Secretin stimulates the secretion of watery
pancreatic juice which contains high con-
centration of bicarbonate ion.
Cholecystokinin
Cholecystokinin (CCK) is also called
cholecystokinin-pancreozymin (CCK-PZ). It is
secreted by I cells in duodenal and jejunal
mucosa. The stimulant for the release of this
hormone is the chyme containing digestive
products such as fatty acids, peptides and
amino acids.
Action of cholecystokinin
Cholecystokinin stimulates the secretion of
pancreatic juice rich in enzyme and less in
volume.
Hormones Inhibiting Pancreatic Secretion
i.
Pancreatic polypeptide – secreted by PP cells
in islets of Langerhans of pancreas
ii. Somatostatin – secreted by D cells in islets
of Langerhans of pancreas
iii. Peptide YY – secreted by intestinal mucosa
iv. Peptides like ghrelin and leptin.
APPLIED PHYSIOLOGY
PANCREATITIS
Pancreatitis is the inflammation of pancreatic
acini.
Causes of Pancreatitis
i.
Long-time consumption of low alcohol
ii. Congenital abnormalities of pancreatic duct
iii. Malnutrition (poor nutrition; mal = bad)
iv. Heavy alcohol intake
v. Gallstones.
Features of Pancreatitis
i.
Absence of pancreatic enzymes
ii. Steatorrhea
iii. Severe abdominal pain
iv. Nausea and vomiting
v. Loss of appetite and weight
vi. Fever
vii. Shock
STEATORRHEA
Steatorrhea is the formation of bulky, foul
smelling, frothy and clay colored stools with large
quantity of undigested fat because of impaired
digestion and absorption of fat.
Causes of Steatorrhea
1. Lack of pancreatic lipase
2. Liver disease affecting secretion of bile
3. Atrophy of intestinal villi.
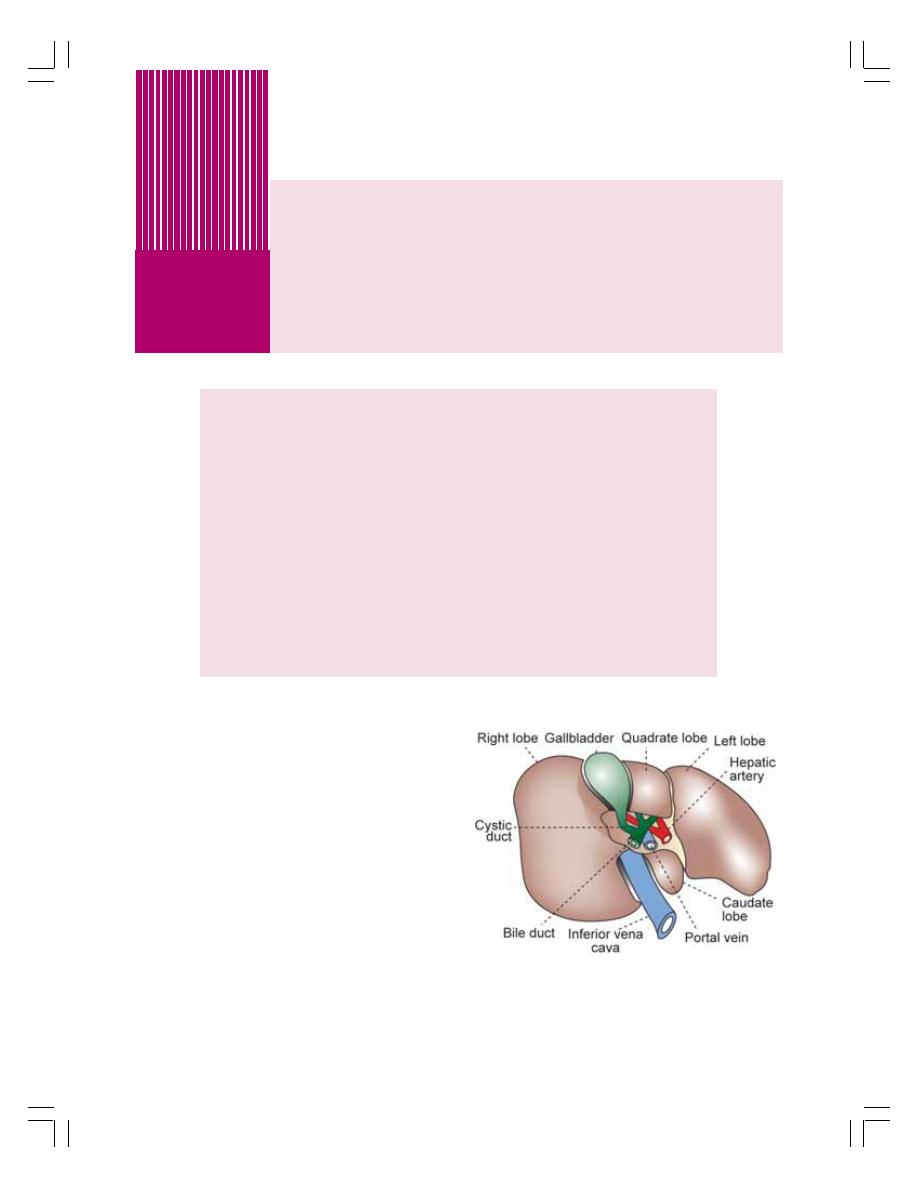
FUNCTIONAL ANATOMY OF LIVER AND BILIARY SYSTEM
BLOOD SUPPLY TO LIVER
PROPERTIES AND COMPOSITION OF BILE
FORMATION OF BILE
STORAGE OF BILE
BILE SALTS
BILE PIGMENTS
FUNCTIONS OF BILE
FUNCTIONS OF LIVER
GALLBLADDER
REGULATION OF BILE SECRETION
APPLIED PHYSIOLOGY
FUNCTIONAL ANATOMY OF LIVER
AND BILIARY SYSTEM
Liver is a dual organ having both secretory and
excretory functions. It is the largest gland in the
body weighing about 1.5 kg in man. It is located
in the upper and right side of the abdominal cavity
immediately beneath diaphragm.
LIVER
Liver is made up of many lobes called hepatic
lobes (Fig. 30-1). Each lobe consists of many
lobules called hepatic lobules.
The hepatic lobule is the structural and
functional unit of liver. It is a honeycomb like
structure and it is made up of liver cells called
hepatocytes. Hepatocytes are arranged in hepatic
plates. Each plate is made up of two columns
Liver and Gallbladder
30
FIGURE 30-1: Posterior surface of liver
of cells. In between the two columns of each plate
lies a bile canaliculus (Fig. 30-2).
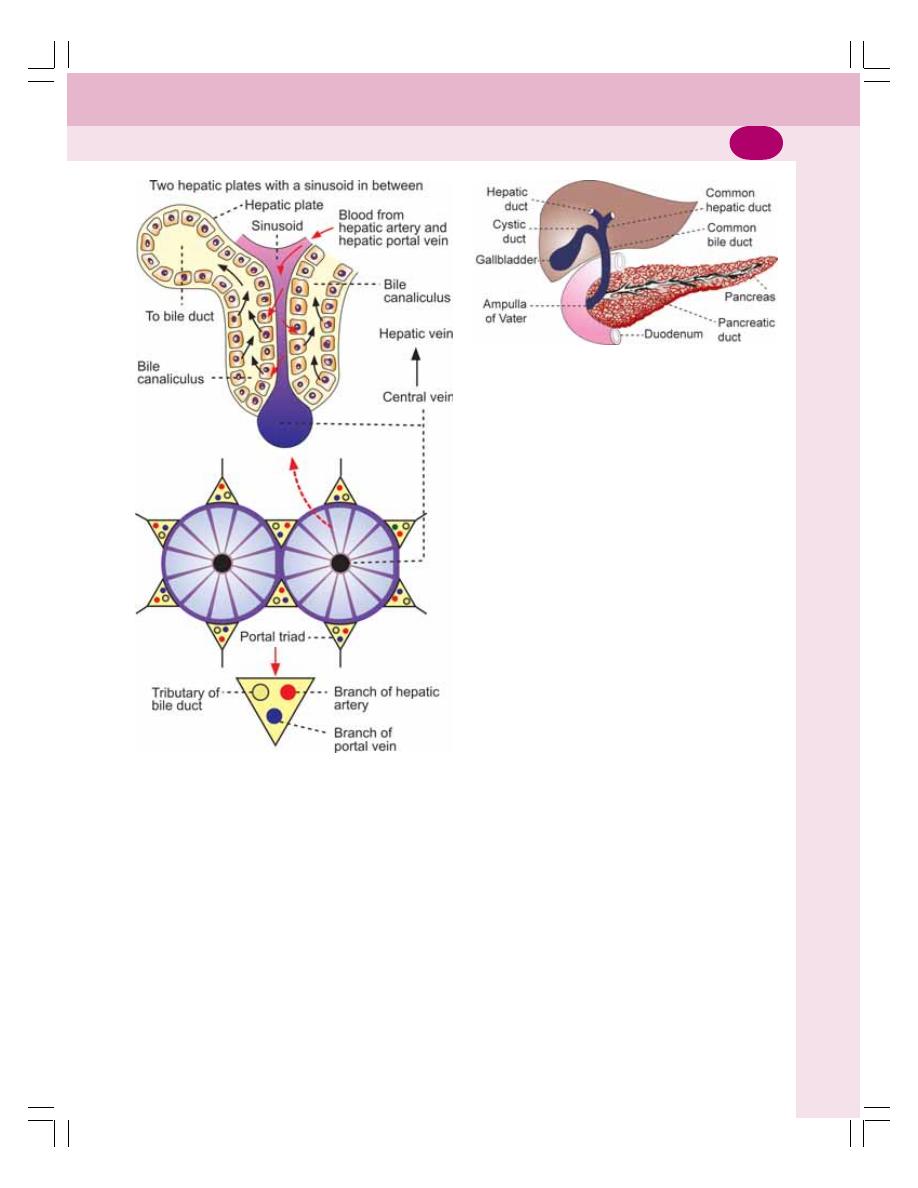
Chapter 30 Liver and Gallbladder
175
In between the neighboring plates a blood
space called sinusoid is present. Sinusoid is lined
by the endothelial cells. In between the
endothelial cells some special macrophages
called Kupffer’s cells are present.
Portal Triads
Each lobule is surrounded by many portal triads.
Each portal triad consists of three vessels:
1. A branch of hepatic artery
2. A branch of portal vein
3. A tributary of bile duct.
The branches of hepatic artery and portal vein
open into the sinusoid. Sinusoid opens into the
central vein. Central vein empties into hepatic
vein.
Bile is secreted by hepatic cells and emptied
into bile canaliculus. From canaliculus, the bile
enters the tributary of bile duct. The tributaries
of bile duct from canaliculi of neighboring lobules
unite to form small bile ducts. These small bile
ducts join together and finally form left and
right hepatic ducts which emerge out of liver.
BILIARY SYSTEM
Biliary system is also known as extrahepatic
biliary apparatus. It is formed by gallbladder and
the extrahepatic bile ducts (bile ducts outside the
liver). The right and left hepatic bile ducts which
come out of liver join to form common hepatic
duct. It unites with the cystic duct from gallbladder
to form common bile duct (Fig. 30-3).
The common bile duct unites with pancreatic
duct to form the common hepatopancreatic duct
or ampulla of Vater which opens into the
duodenum.
There is a sphincter called sphincter of Oddi
at the lower part of common bile duct before it
joins the pancreatic duct. It is formed by smooth
muscle fibers of common bile duct. It is normally
kept closed; so the bile secreted from liver enters
gallbladder where it is stored. Upon appropriate
stimulation the sphincter opens and allows flow
of bile from gallbladder into the intestine.
BLOOD SUPPLY TO LIVER
Liver receives the maximum blood supply of
about 1500 mL/min. It receives blood from two
sources namely the hepatic artery and portal vein
(Fig. 30-4).
FIGURE 30-2: Hepatic lobule
FIGURE 30-3: Biliary system
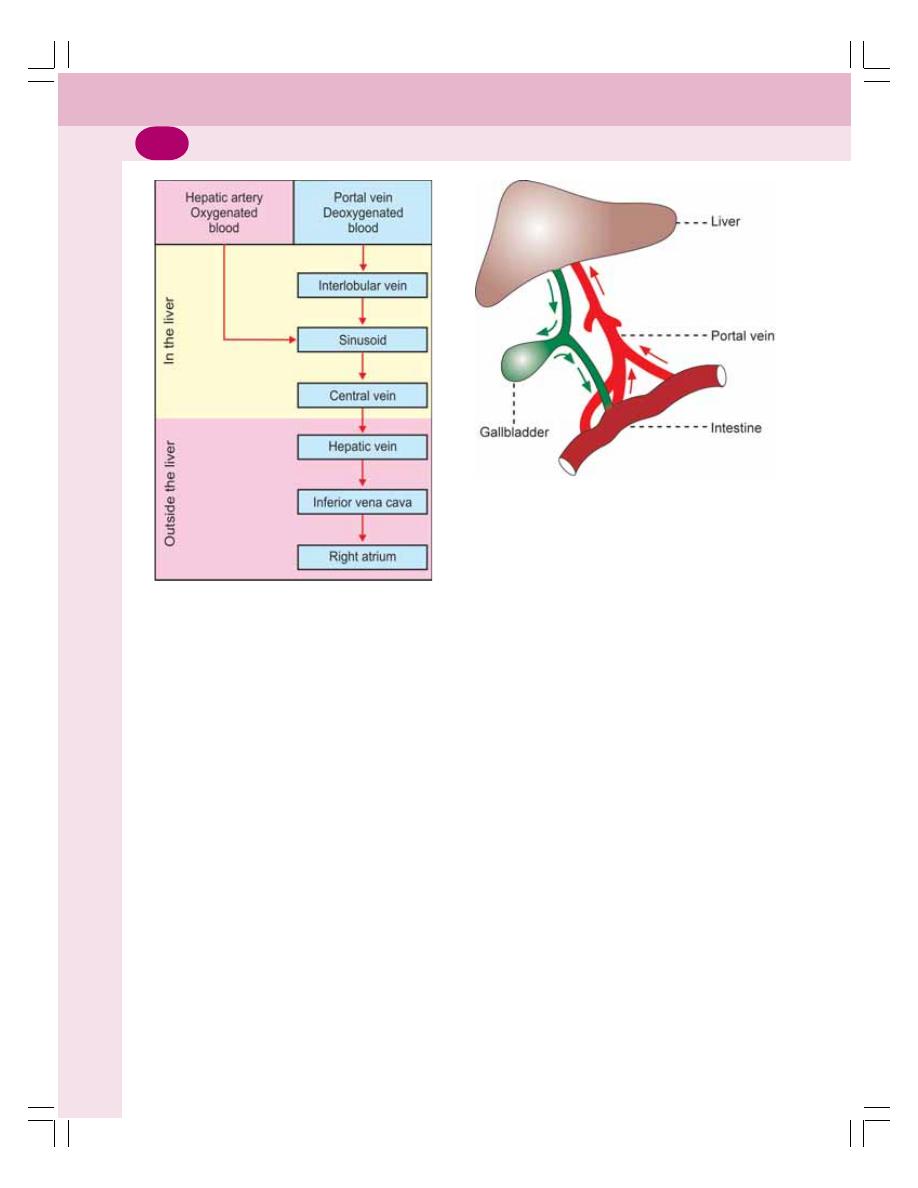
Digestive System
176
HEPATIC ARTERY
The hepatic artery arises directly from aorta and
supplies oxygenated blood to liver. After entering
the liver, the hepatic artery divides into many
branches. Each branch enters a portal triad.
PORTAL VEIN
The portal vein is formed by superior mesenteric
vein and splenic vein. It brings deoxygenated
blood from stomach, intestine, spleen and
pancreas. The portal blood is rich in
monosaccharides and amino acids. It also
contains bile salts, bilirubin, urobilinogen and GI
hormones. However, the oxygen content is less
in portal blood.
The flow of blood from intestine to liver
through portal vein is known as enterohepatic
circulation (Fig. 30-5).
The blood from hepatic artery mixes with
blood from portal vein in the hepatic sinusoids.
The hepatic cells obtain oxygen and nutrients
from the sinusoid.
HEPATIC VEIN
The substances synthesized by hepatic cells, the
waste products and carbon dioxide are
discharged into sinusoids. The sinusoids drain
them into the central vein of the lobule. The
central veins from many lobules unite to form
bigger veins which ultimately form hepatic veins
(right and left) which open into inferior vena cava.
PROPERTIES AND COMPOSITION
OF BILE
Bile is a golden yellow or greenish fluid. It enters
the digestive tract along with pancreatic juice
through the common opening called ampulla of
Vater.
Properties of Bile
Volume
: 800 to 1200 mL/day
Reaction
: Alkaline
pH
: 8 to 8.6
Specific gravity : 1.010 to 1.011
Composition of Bile
Bile contains 97.6% of water and 2.4% of solids.
Solids include organic and inorganic substances.
Refer Fig. 30-6 for details.
FIGURE 30-4: Schematic diagram of blood flow
through liver
FIGURE 30-5: Enterohepatic circulation
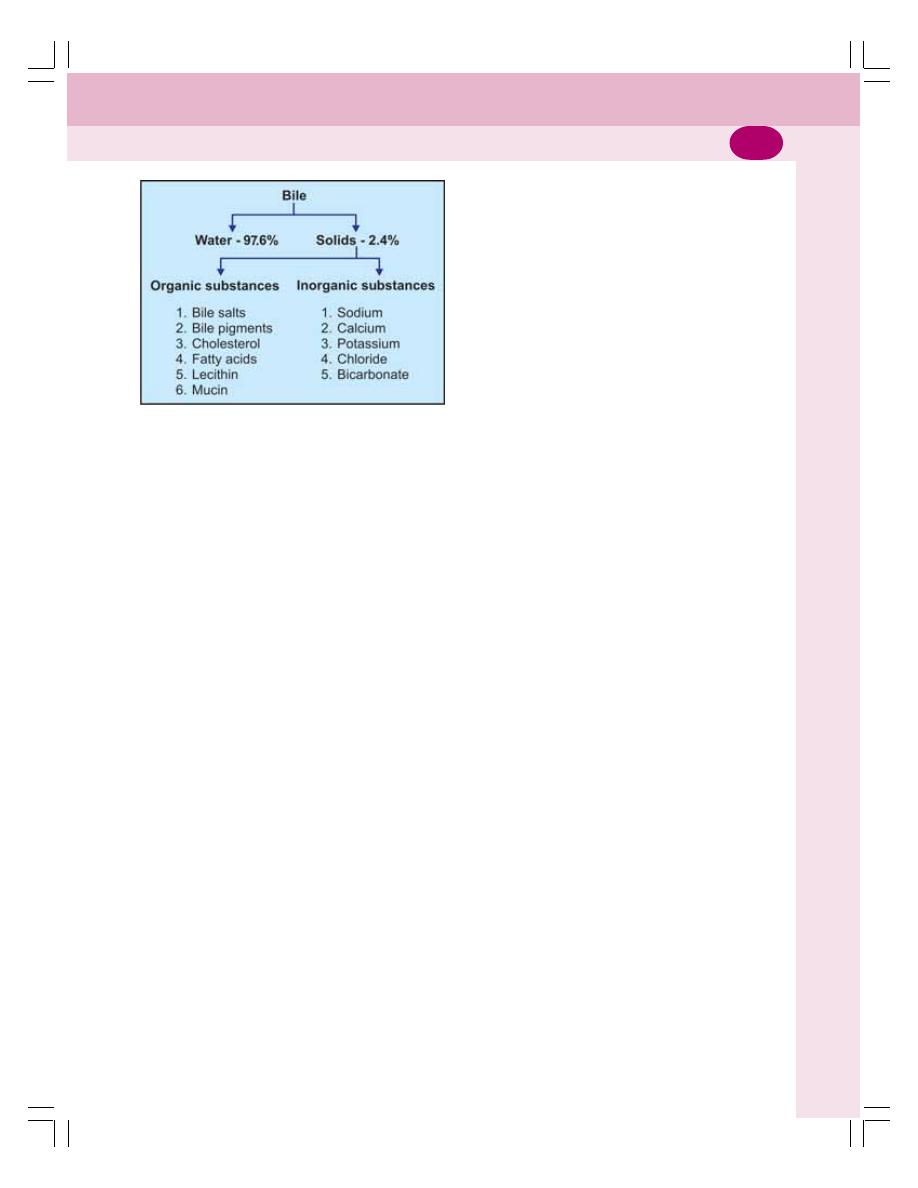
Chapter 30 Liver and Gallbladder
177
FORMATION OF BILE
Bile is secreted by hepatocytes. The initial bile
secreted by hepatocytes contains large quantity
of bile acids, bile pigments, cholesterol, lecithin
and fatty acids. From hepatocytes, bile passes
through canaliculi and hepatic ducts to reach
common hepatic duct. From here it may enter
the intestine or gallbladder.
Sodium, bicarbonate and water are added to
bile when it passes through the ducts. These
substances are secreted by the epithelial cells
of the ducts. The addition of sodium, bicarbonate
and water increases the total quantity of bile
(Fig. 30-8).
STORAGE OF BILE
Most of the bile from liver enters the gallbladder
where it is stored. It is released from gallbladder
into the intestine whenever it is required. When
bile is stored in gallbladder, it undergoes many
changes both in quality and quantity such as:
1. Volume is reduced because of absorption of
large amount of water and electrolytes
(except calcium and potassium)
2. Concentration of bile salts, bile pigments,
cholesterol, fatty acids and lecithin is
increased because of absorption of water
2. The pH is slightly decreased
3. Specific gravity is increased
4. Mucin is added (Table 30-1).
BILE SALTS
Bile salts are the sodium and potassium salts of
bile acids, which are conjugated with glycine or
taurine. Bile salts are formed in liver.
FORMATION OF BILE SALTS
Bile salts are formed from the primary bile acids
namely cholic acid and chenodeoxycholic acid
which are formed in liver and enter the intestine
through bile. Due to the bacterial action in the
intestine these primary bile acids are converted
into secondary bile acids:
Cholic acid
→ deoxycholic acid
Chenodeoxycholic acid
→ lithocholic acid
Secondary bile acids from intestine are
transported back to liver through enterohepatic
circulation. In the liver the secondary bile acids
are conjugated with glycine or taurine and form
conjugated bile acids namely glycocholic acid and
taurocholic acids. These bile acids combine with
sodium or potassium ions to form the salts,
sodium or potassium glycocholate and sodium
or potassium taurocholate.
ENTEROHEPATIC CIRCULATION OF
BILE SALTS
Enterohepatic circulation is the transport of
substances from small intestine to liver through
portal vein. About 90 to 95% of bile salts from
intestine are transported to liver through
enterohepatic circulation. The remaining 5 to
10% of the bile salts enter large intestine. Here
the bile salts are converted into deoxycholate and
lithocholate and excreted in feces.
FUNCTIONS OF BILE SALTS
The bile salts are required for digestion and
absorption of fats in the intestine. The functions
of bile salts are:
1. Emulsification of Fats
Emulsification is the process by which the fat
globules are broken down into minute droplets
and made in the form of a milky fluid called
emulsion. Emulsification of fats occurs in small
intestine by the action of bile salts.
FIGURE 30-6: Composition of bile
Digestive System
178
Fats cannot be digested directly by lipolytic
enzymes of GI tract, because the fats are
insoluble in water due to the surface tension. The
bile salts reduce the surface tension of the fats
due to their detergent action. Because of this,
the lipid granules are broken into minute particles
which can be easily digested by lipolytic enzymes.
The emulsification of fats by bile salts needs the
presence of lecithin from bile.
2. Absorption of Fats
Bile salts help in the absorption of digested fats
from intestine into blood. The bile salts combine
with fats and make complexes of fats called
micelles. The fats in the form of micelles can be
absorbed easily.
3. Choleretic Action
Bile salts stimulate the secretion of bile from liver.
This action is called choleretic action.
4. Cholagogue Action
Cholagogue is an agent, which causes
contraction of gallbladder and release of bile into
the intestine. Bile salts act as cholagogues
indirectly by stimulating the secretion of hormone
cholecystokinin. This hormone causes contra-
ction of gallbladder resulting in release of bile.
5. Laxative Action
Laxative is an agent which induces defecation.
Bile salts act as laxatives by stimulating peristaltic
movements of the intestine.
6. Prevention of Gallstone Formation
Bile salts prevent the formation of gallstone by
keeping the cholesterol and lecithin in solution.
In the absence of bile salts, cholesterol
precipitates along with lecithin and forms
gallstone.
TABLE 30-1: Differences between liver bile and gallbladder bile
Types of entities
Liver bile
Gallbladder bile
pH
8 to 8.6
7 to 7.6
Specific gravity
1010 to 1011
1026 to 1032
Water content
97.6%
89%
Solids
2.4%
11%
Organic substances
Bile salts
0.5 g/dL
6.0 g/dL
Bile pigments
0.05 g/dL
0.3 g/dL
Cholesterol
0.1 g/dL
0.5 g/dL
Fatty acids
0.2 g/dL
1.2 g/dL
Lecithin
0.05 g/dL
0.4 g/dL
Mucin
Absent
Present
Inorganic substances
Sodium
150 mEq/L
135 mEq/L
Calcium
4 mEq/L
22 mEq/L
Potassium
5 mEq/L
12 mEq/L
Chloride
100 mEq/L
10 mEq/L
Bicarbonate
30 mEq/L
10 mEq/L
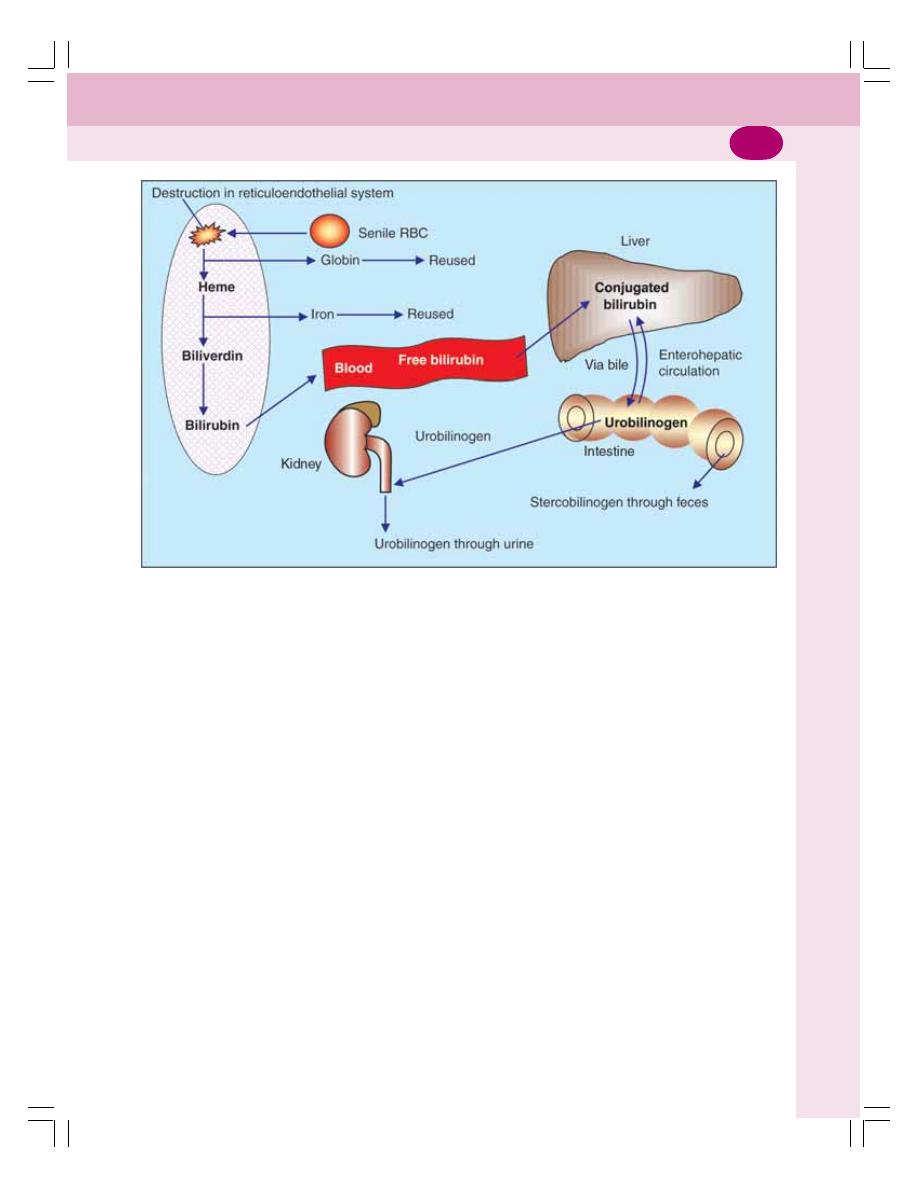
Chapter 30 Liver and Gallbladder
179
BILE PIGMENTS
Bile pigments are the excretory products in bile.
Bilirubin and biliverdin are the two bile pigments
and bilirubin is the major bile pigment in human
being.
The bile pigments are formed during the
breakdown of hemoglobin, which is released
from the destroyed RBCs in the
reticuloendothelial system (Fig. 30-7).
FORMATION AND EXCRETION OF
BILE PIGMENTS
Stages of formation and circulation of bile
pigments:
1. The senile erythrocytes are destroyed in reti-
culoendothelial system and hemoglobin is
released from them
2. The hemoglobin is broken into globin and
heme
3. Heme is split into iron and the pigment
biliverdin
4. The iron goes to iron pool and is reused
5. The first formed pigment biliverdin is reduced
to bilirubin
6. The bilirubin is released into blood from reti-
culoendothelial cells
7. The bilirubin circulating in the blood is called
free bilirubin or unconjugated bilirubin
8. Within few hours the free bilirubin is taken
up by the liver cells
9. In the liver, it is conjugated with glucuronic
acid to form conjugated bilirubin
10. Conjugated bilirubin is then excreted into
intestine through bile.
FATE OF CONJUGATED BILIRUBIN
Stages of excretion of conjugated bilirubin:
1. In the intestine 50% of the conjugated bilirubin
is converted into urobilinogen by intestinal
bacteria. First the conjugated bilirubin is
deconjugated into free bilirubin which is later
reduced into urobilinogen.
2. Remaining 50% of conjugated bilirubin
from intestine enters the liver through
FIGURE 30-7: Formation and circulation of bile pigments
Digestive System
180
enterohepatic circulation. From liver, it is re-
excreted in bile
3. Most of the urobilinogen from intestine enters
liver via enterohepatic circulation. Later, it is
re-excreted through bile
4. About 5% of urobilinogen is excreted by
kidney through urine. In urine, due to the
exposure to air, the urobilinogen is converted
into urobilin by oxidation
5. Some of the urobilinogen is excreted in feces
as stercobilinogen. In feces, stercobilinogen
is oxidized to stercobilin.
NORMAL PLASMA LEVELS OF
BILIRUBIN
The normal bilirubin (Total bilirubin) content in
plasma is 0.5 to 1.5 mg/dL. When it exceeds
1 mg/dL, the condition is called hyperbilirubi-
nemia. When it exceeds 2 mg/dL, jaundice
occurs.
FUNCTIONS OF BILE
Most of the functions of bile are due to the bile
salts.
1. DIGESTIVE FUNCTIONS
Refer functions of bile salts.
2. ABSORPTIVE FUNCTIONS
Refer functions of bile salts.
3. EXCRETORY FUNCTIONS
Bile pigments are the major excretory products
of the bile. The other substances excreted in bile
are:
i.
Heavy metals like copper and iron
ii. Some bacteria like typhoid bacteria
iii. Some toxins
iv. Cholesterol
v. Lecithin
vi. Alkaline phosphatase.
4. LAXATIVE ACTION
Bile salts act as laxatives (see above).
5. ANTISEPTIC ACTION
Bile inhibits the growth of certain bacteria in the
lumen of intestine by its natural detergent action.
6. CHOLERETIC ACTION
Bile salts have the choleretic action (see above).
7. MAINTENANCE OF pH IN
GASTROINTESTINAL TRACT
As the bile is highly alkaline, it neutralizes acid
chyme which enters the intestine from stomach.
Thus, an optimum pH is maintained for the action
of digestive enzymes.
8. PREVENTION OF GALLSTONE
FORMATION
Refer function of bile salts.
9. LUBRICATION FUNCTION
The mucin in bile acts as a lubricant for the
chyme in intestine.
10. CHOLAGOGUE ACTION
Bile salts act as cholagogues (see above).
FUNCTIONS OF LIVER
Liver is the largest gland and one of the vital
organs of the body. It performs many vital
metabolic and homeostatic functions, which are
summarized below.
1. METABOLIC FUNCTION
Liver is the organ where maximum metabolic
reactions are carried out such as metabolism of
carbohydrates, proteins, fats, vitamins and many
hormones.
2. STORAGE FUNCTION
Many substances like glycogen, amino acids,
iron, folic acid and vitamins A, B
12
, and D are
stored in liver.

Chapter 30 Liver and Gallbladder
181
3. SYNTHETIC FUNCTION
Liver produces glucose by gluconeogenesis. It
synthesizes all the plasma proteins and other
proteins (except immunoglobulins) such as
clotting factors, complement factors, and hor-
mone binding proteins. It also synthesizes
steroids, somatomedin and heparin.
4. SECRETION OF BILE
Liver secretes bile, which contains bile salts, bile
pigments, cholesterol, fatty acids and lecithin.
The functions of bile are mainly due to the
bile salts. The bile salts are required for digestion
and absorption of fats in the intestine. Bile helps
to carry away waste products and breakdown
fats, which are excreted through feces or urine.
5. EXCRETORY FUNCTION
Liver excretes cholesterol, bile pigments, heavy
metals (like lead, arsenic and bismuth), toxins,
bacteria and virus (like that of yellow fever)
through bile.
6. HEAT PRODUCTION
Liver is the organ where maximum heat is
produced because of the metabolic reactions.
7. HEMOPOIETIC FUNCTION
In fetus (hepatic stage), liver produces the blood
cells (Chapter 8). It stores vitamin B
12
necessary
for erythropoiesis and iron necessary for
synthesis of hemoglobin. Liver produces
thrombopoietin that promotes production of
thrombocytes.
8. HEMOLYTIC FUNCTION
The senile RBCs after the lifespan of 120 days
are destroyed by reticuloendothelial cells
(Kupffer’s cells) of liver.
9. INACTIVATION OF HORMONES
AND DRUGS
Liver catabolizes the hormones such as growth
hormone, parathormone, cortisol, insulin,
glucagon and estrogen. It also inactivates the
drugs particularly the fat soluble drugs. The fat
soluble drugs are converted into water soluble
substances, which are excreted through bile or
urine.
10. DEFENSIVE AND
DETOXIFICATION FUNCTIONS
The reticuloendothelial cells (Kupffer’s cells) of
the liver play an important role in the defense of
the body. Liver is also involved in the
detoxification of the foreign bodies.
i.
The foreign bodies such as bacteria or
antigens are swallowed and digested by
reticuloendothelial cells of liver by means of
phagocytosis
ii. The reticuloendothelial cells of liver are also
involved in production of some substances
like interleukins and tumor necrosis factors,
which activate the immune system of the body
(Chapter 13).
iii. Liver cells are involved in removal of toxic
property of various harmful substances. The
removal of toxic property of the harmful agent
is known as detoxification.
GALLBLADDER
The bile secreted from liver is stored in
gallbladder. The capacity of gallbladder is
approximately 50 mL. The gallbladder is not
essential for life. The removal of gallbladder
(cholecystectomy) is often done in patients
suffering from gallbladder dysfunction. After
cholecystectomy, patients do not suffer from any
major disadvantage. In some species, gallbladder
is absent.
FUNCTIONS OF GALLBLADDER
The major functions of gallbladder are the
storage and concentration of bile.
1. Storage of Bile
Bile is continuously secreted from liver. But it is
released into intestine only intermittently and
most of the bile is stored in gallbladder till it is
required.
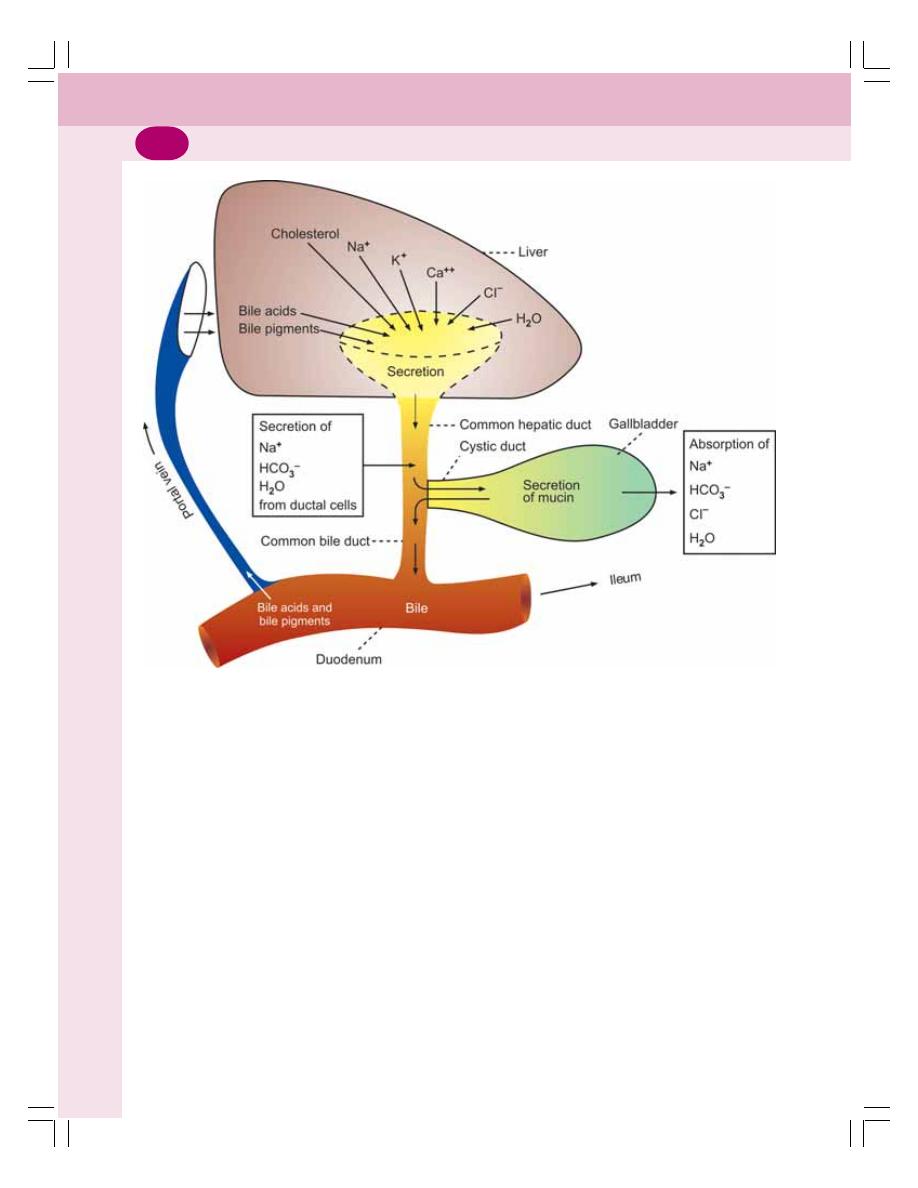
Digestive System
182
2. Concentration of Bile
Bile is concentrated while it is stored in
gallbladder. The mucosa of gallbladder rapidly
reabsorbs water and electrolytes except calcium
and potassium. But the bile salts, bile pigments,
cholesterol and lecithin are not reabsorbed. So,
the concentration of these substances in bile
increases 5 to 10 times.
3. Alteration of pH of Bile
The pH of bile decreases from 8 to 8.6 to 7 to
7.6 and it becomes less alkaline when it is stored
in gallbladder.
4. Secretion of Mucin
Gallbladder secretes mucin into the bile. Mucin
acts as a lubricant for movement of chyme in
the intestine.
5. Maintenance of Pressure in Biliary
System
Due to the concentrating capacity, gallbladder
maintains a pressure of about 7 cm H
2
O in biliary
system. This pressure in the biliary system is
essential for the release of bile into the intestine.
REGULATION OF BILE SECRETION
Bile secretion is a continuous process though
the amount may be less during fasting. It starts
increasing three hours after meals. The secretion
of bile from the liver and release of bile from the
gallbladder are influenced by some chemical
factors which are categorized into three groups:
1. Choleretics
2. Cholagogue
3. Hydrocholeretic agents.
FIGURE 30-8: Diagram showing the formation of bile from liver and changes taking place in the
composition of gallbladder bile

Chapter 30 Liver and Gallbladder
183
1. Choleretics
Substances, which increase the secretion of bile
from liver, are known as choleretics. The effective
choleretic agents are:
i.
Acetylcholine
ii. Secretin
iii. Cholecystokinin
iv. Acid chyme in intestine
v. Bile salts.
2. Cholagogues
Cholagogue is an agent, which increases the
release of bile from gallbladder into the intestine
by contracting the gallbladder. The common
cholagogues are:
i.
Bile salts
ii. Calcium
iii. Fatty acids
iv. Amino acids
v. Inorganic acids.
All these substances stimulate the secretion
of cholecystokinin, which, in turn causes
contraction of gallbladder and flow of bile into
intestine.
3. Hydrocholeretic Agents
Hydrocholeretic agent is a substance, which
causes secretion of bile from liver with large
amount of water and less amount of solids.
Hydrochloric acid is a hydrocholeretic agent.
APPLIED PHYSIOLOGY
JAUNDICE OR ICTERUS
Jaundice or icterus is the condition characterized
by yellow coloration of the skin, mucous
membrane and deeper tissues due to increased
bilirubin level in blood. The word jaundice is
derived from the French word “jaune” meaning
yellow.
The normal serum bilirubin level is 0.5 to 1.5
mg/dL. Jaundice occurs when bilirubin level
exceeds 2 mg/dL.
Types of Jaundice
Jaundice is classified into three types:
1. Prehepatic or hemolytic jaundice
2. Hepatic or hepatocellular jaundice
3. Posthepatic or obstructive jaundice.
1. Prehepatic or Hemolytic Jaundice
Hemolytic jaundice is the type of jaundice that
occurs because of excessive destruction of
RBCs resulting in increased blood level of free
(unconjugated) bilirubin. The function of liver is
normal. Since the quantity of bilirubin increases
enormously, the liver cells cannot excrete that
much bilirubin rapidly. So, it accumulates in the
blood resulting in jaundice.
Causes
Any condition that causes hemolytic anemia can
lead to hemolytic jaundice. The common causes
of hemolytic jaundice are:
i.
Liver failure
ii. Renal disorder
iii. Hypersplenism
iv. Burns
v. Infections such as malaria
vi. Hemoglobin abnormalities such as sickle cell
anemia or thalassemia
vii. Drugs or chemical substances causing red
cell damage
viii.Autoimmune diseases.
2. Hepatic or Hepatocellular or
Cholestatic Jaundice
This is the type of jaundice that occurs due to
the damage of hepatic cells. Because of the
damage, the conjugated bilirubin from liver
cannot be excreted and it returns to blood.
Causes
i.
Hepatitis or cirrhosis of liver
ii. Alcoholism
iii. Exposure to toxic materials.
Digestive System
184
3. Posthepatic or Obstructive or
Extrahepatic Jaundice
This type of jaundice occurs because of the
obstruction of bile flow at any level of the biliary
system. The bile cannot be excreted into small
intestine. So, bile salts and bile pigments enter
the circulation. The blood contains more amount
of conjugated bilirubin (Table 30-2).
Causes
i.
Gallstones
ii. Cancer of biliary system or pancreas.
HEPATITIS
Hepatitis is the liver damage characterized by
swelling and inadequate functioning of liver. It
TABLE 30-2: Features of different types of jaundice
Features
Prehepatic
Hepatic jaundice
Posthepatic jaundice
jaundice (Hemolytic)
(hepatocellular)
(Obstructive)
Cause
Excess breakdown of
Liver damage
Obstruction of bile ducts
RBCs
Type of bilirubin in blood
Unconjugated
Conjugated and
Conjugated
unconjugated
Urinary excretion of
Increases
Decreases
Decreases
urobilinogen
Absent in severe
obstruction
Fecal excretion of
Increases
Decreases
Absent
stercobilinogen
(pale feces)
(clay colored feces)
van den Bergh’s reaction
Indirect – positive
Biphasic
Direct – positive
Liver functions
Normal
Abnormal
Exaggerated
Blood picture
Anemia
Normal
Normal
Reticulocytosis
Abnormal RBC
Plasma albumin and
Normal
Albumin – increases
Normal
globulin
Globulin – increases
A : G ratio – decreases
Hemorrhagic tendency
Absent
Present due to lack of
vitamin K
Present due to
lack of vitamin K
is caused by several factors such as viral
infection, bacterial infection and excess alcohol.
Common features of hepatitis are fever,
nausea, vomiting, diarrhea, loss of appetite,
jaundice. Liver failure and death occur in severe
conditions.
CIRRHOSIS OF LIVER
Cirrhosis of liver refers to inflammation and
damage of parenchyma of liver resulting in
degeneration of hepatic cells and dysfunction of
liver. It is caused by infection, obstruction of biliary
system and liver enlargement due to intoxication.
Features of cirrhosis of liver are fever, nausea
and vomiting, jaundice, portal hypertension,
muscular weakness and wasting of muscles.
Coma occurs in advanced stages.

Chapter 30 Liver and Gallbladder
185
GALLSTONES
Definitions
Gallstone is a solid crystal deposit that is formed
by cholesterol, calcium ions and bile pigments
in the gallbladder or bile duct. Cholelithiasis is
the presence of gallstones in gallbladder.
Formation of Gallstones
Normally, cholesterol is water soluble. Under
some abnormal conditions, it precipitates resul-
ting in the formation of crystals in the mucosa
of gallbladder. Bile pigments and calcium are
attached to these crystals resulting in formation
of gallstones.
Causes for Gallstone Formation
1. Reduction in bile salts
2. Excess of cholesterol or disturbed chole-
sterol metabolism
4. Excess of calcium ions due to increased
concentration of bile
5. Damage or infection of gallbladder epithe-
lium
6. Obstruction of bile flow from the gall-
bladder.
Features
The common feature of gallstone is the pain in
stomach area or in upper right part of the belly
under the ribs. Other features include nausea,
vomiting, abdominal bloating and indigestion.
FUNCTIONAL ANATOMY
INTESTINAL VILLI AND GLANDS
PROPERTIES AND COMPOSITION OF SUCCUS ENTERICUS
FUNCTIONS OF SUCCUS ENTERICUS
FUNCTIONS OF SMALL INTESTINE
REGULATION OF SECRETION OF SUCCUS ENTERICUS
APPLIED PHYSIOLOGY
FUNCTIONAL ANATOMY
Small intestine is the part of GI tract extending
between the pyloric sphincter of stomach and
ileocecal valve, which opens into large intestine.
It is called small intestine because of its small
diameter compared to that of large intestine. But
it is longer than large intestine. Its length is about
6 meters.
The functional importance of small intestine
is absorption. Maximum absorption of digested
food products takes place in small intestine.
Small intestine consists of three portions:
1. Proximal part known as duodenum
2. Middle part known as jejunum
3. Distal part known as ileum.
INTESTINAL VILLI AND GLANDS
OF SMALL INTESTINE
INTESTINAL VILLI
The mucous membrane of small intestine is
covered by minute projections called villi. The
villi are lined by columnar cells, which are called
enterocytes. Each enterocyte gives rise to hair
like projections called microvilli. Within each
villus, there is a central channel called lacteal.
The lacteal opens into lymphatic vessels. It
contains blood vessels also.
CRYPTS OF LIEBERKÜHN OR
INTESTINAL GLANDS
The crypts of Lieberkühn or intestinal glands are
simple tubular glands of intestine. These glands
open into lumen of intestine between the villi. The
intestinal glands are lined by columnar cells. The
lining of each gland is continuous with epithelial
lining of the villi (Fig. 31-1).
Epithelial cells lining the intestinal glands
undergo division by mitosis at a faster rate. The
newly formed cells push the older cells upward
over the lining of villi. The cells which move to
villi are called enterocytes. The enterocytes
secrete the enzymes. The old enterocytes are
continuously shed into lumen along with
enzymes.
Small Intestine
31
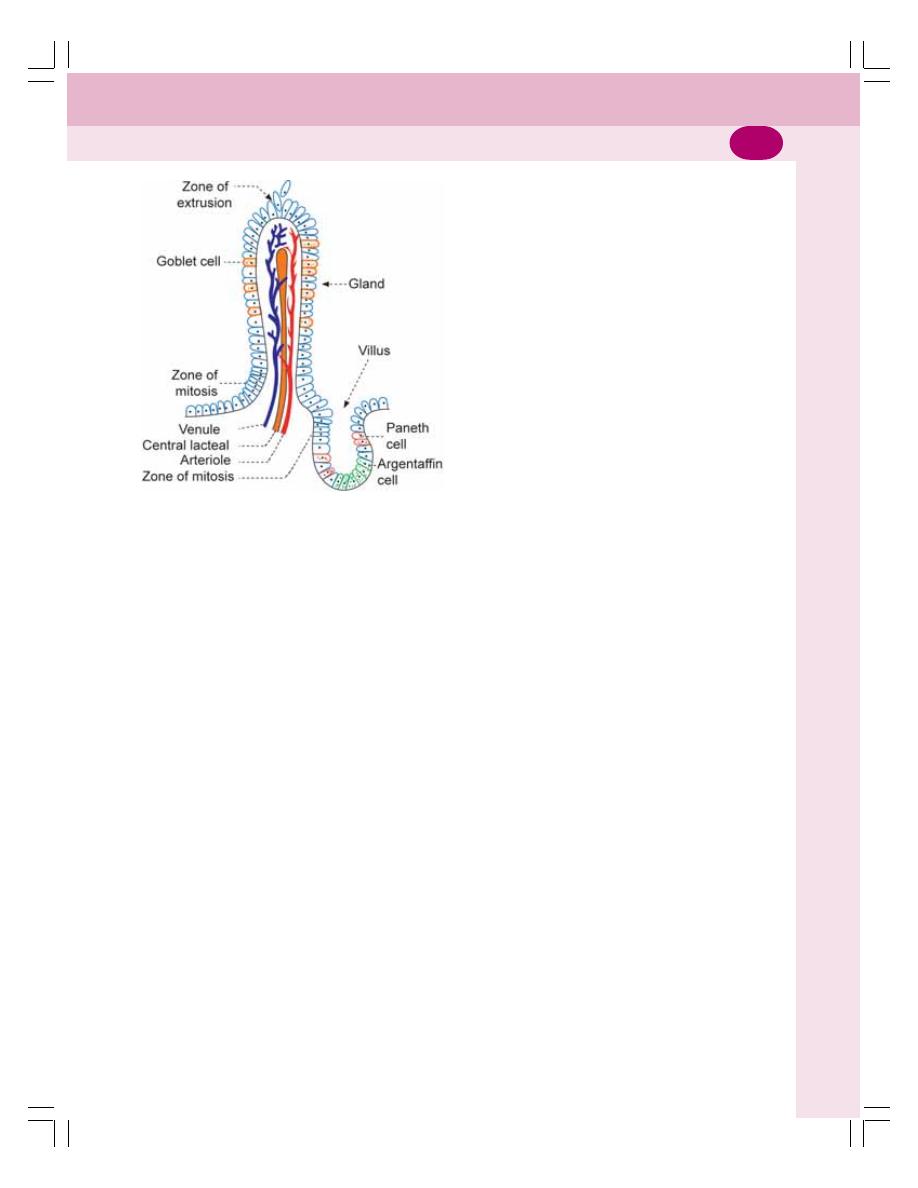
Chapter 31 Small Intestine
187
Three types of cells are interposed between
columnar cells of the glands:
1. Argentaffin cells which are otherwise known
as enterochromaffin cells. These cells secrete
intrinsic factor that is essential for the
absorption of vitamin B
12
2. Goblet cells which secrete mucus
3. Paneth cells which secrete the cytokines
called defensins.
BRUNNER’S GLANDS
In addition to intestinal glands, the first part of
duodenum contains some mucus glands, which
are called Brunner’s glands. Brunner’s gland
secretes mucus and traces of enzymes.
PROPERTIES AND COMPOSITION
OF SUCCUS ENTERICUS
Secretion from small intestine is called succus
entericus.
Properties of Succus Entericus
Volume
: 1800 mL/day
Reaction
: Alkaline
pH
: 8.3
Composition of Succus Entericus
The succus entericus contains water (99.5%) and
solids (0.5%). Solids include organic and
inorganic substances (Fig. 31-2). The
bicarbonate concentration is slightly high in
succus entericus.
FUNCTIONS OF SUCCUS
ENTERICUS
1. DIGESTIVE FUNCTION
The enzymes of succus entericus act on the
partially digested food and convert them into final
digestive products.
Proteolytic Enzymes
The proteolytic enzymes in succus entericus are
the peptidases which convert peptides into amino
acids (Fig. 31-2).
Amylolytic Enzymes
The carbohydrate splitting enzymes of succus
entericus are listed in Figure 31-2. Lactase,
sucrase and maltase convert the disaccharides
(lactose, sucrose and maltose) into two
molecules of monosaccharides (Table 31-1).
Dextrinase converts dextrin, maltose and
maltriose into glucose. Trehalase or trehalose
glucohydrolase causes hydrolysis of trehalose
(carbohydrate present in mushrooms and yeast)
and converts it into glucose.
Lipolytic Enzyme
Intestinal lipase acts on triglycerides and converts
them into fatty acids.
2. PROTECTIVE FUNCTION
i.
The mucus present in the succus entericus
protects the intestinal wall from the acid
chyme, which enters the intestine from stom-
ach; thereby it prevents the intestinal ulcer
ii. Paneth cells of intestinal glands secrete
defensins which are the antimicrobial
peptides.
FIGURE 31-1: Intestinal gland and villus
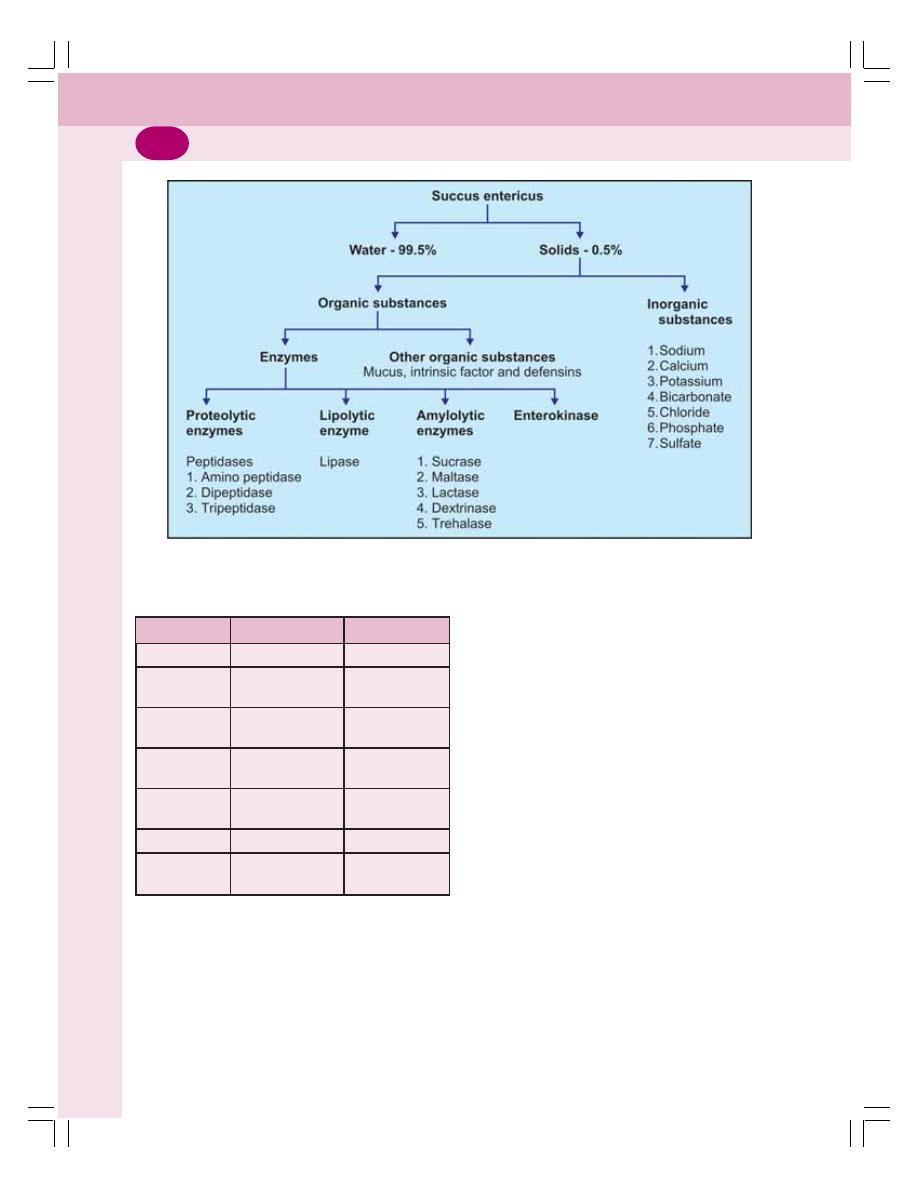
Digestive System
188
3. ACTIVATOR FUNCTION
The enterokinase present in intestinal juice
activates trypsinogen into trypsin. Trypsin, in turn
activates other enzymes (Chapter 29).
4. HEMOPOIETIC FUNCTION
The intrinsic factor of Castle, which is present
in the intestine, plays an important role in
erythropoiesis (Chapter 8).
5. HYDROLYTIC PROCESS
Intestinal juice helps in all the enzymatic reactions
of digestion.
FUNCTIONS OF SMALL INTESTINE
1. MECHANICAL FUNCTION
The mixing movements of small intestine help
in the thorough mixing of chyme with the digestive
juices like succus entericus, pancreatic juice and
bile.
2. SECRETORY FUNCTION
Small intestine secretes succus entericus,
enterokinase and the GI hormones.
FIGURE 31-2: Composition of succus entericus
TABLE 31-1: Digestive enzymes of succus
entericus
Enzyme
Substrate
End products
1. Peptidases
Peptides
Amino acids
2. Sucrase
Sucrose
Fructose and
glucose
3. Maltase
Maltose and
Glucose
maltriose
4. Lactase
Lactose
Galactose and
glucose
5. Dextrinase
Dextrin, maltose
Glucose
and maltriose
6. Trehalase
Trehalose
Glucose
7. Intestinal
Triglycerides
Fatty acids
lipase

Chapter 31 Small Intestine
189
3. HORMONAL FUNCTION
Small intestine secretes many GI hormones
such as secretin, cholecystokinin, etc. These
hormones regulate the movement of GI tract and
secretory activities of small intestine and
pancreas.
4. DIGESTIVE FUNCTION
Refer functions of succus entericus.
5. ACTIVATOR FUNCTION
Refer functions of succus entericus.
6. HEMOPOIETIC FUNCTION
Refer functions of succus entericus.
7. HYDROLYTIC FUNCTION
Refer functions of succus entericus.
8. ABSORPTIVE FUNCTIONS
The presence of villi and microvilli in small
intestinal mucosa increases the surface area of
the mucosa. This facilitates the absorptive
function of intestine.
The digested products of foodstuffs, proteins,
carbohydrates, fats and other nutritive
substances such as vitamins, minerals and water
are absorbed mostly in small intestine. From the
lumen of intestine, these substances pass
through lacteal of villi, cross the mucosa and
enter the blood directly or through lymphatics.
REGULATION OF SECRETION OF
SUCCUS ENTERICUS
The secretion of succus entericus is regulated
by both the nervous and hormonal mechanisms.
NERVOUS REGULATION
Stimulation of parasympathetic nerves causes
vasodilatation and increases the secretion of
succus entericus. Stimulation of sympathetic
nerves causes vasoconstriction and decreases
the secretion of succus entericus. But, the role
of these nerves in the regulation of intestinal
secretion in physiological conditions is uncertain.
However, the local nervous reflexes play an
important role in increasing the secretion of
intestinal juice. When chyme enters the small
intestine, the mucosa is stimulated by tactile
stimuli or irritation. It causes development of local
nervous reflexes, which stimulate the glands of
intestine.
HORMONAL REGULATION
When the chyme enters the small intestine, the
intestinal mucosa secretes enterocrinin, secretin
and cholecystokinin which promote the secretion
of succus entericus by stimulating the intestinal
glands.
APPLIED PHYSIOLOGY –
MALABSORPTION
Malabsorption is difficulty in the digestion or
absorption of nutrients from small intestine. It may
be the failure to absorb either the specific
substances such as proteins, carbohydrates, fats
and vitamins or some general nonspecific
substances of food. Malabsorption affects growth
and development of the body.

FUNCTIONAL ANATOMY
SECRETIONS OF LARGE INTESTINE
FUNCTIONS OF LARGE INTESTINE
APPLIED PHYSIOLOGY
Large Intestine
32
FUNCTIONAL ANATOMY OF LARGE
INTESTINE
The large intestine is also known as colon. It
extends from ileocecal valve up to anus
(Fig. 26-1). It consists of seven portions:
1. Cecum with appendix
2. Ascending colon
3. Transverse colon
4. Descending colon
5. Sigmoid colon or pelvic colon
6. Rectum
7. Anal canal.
The wall of large intestine is formed by four
layers of structures like any other part of the gut.
SECRETIONS OF LARGE INTESTINE
The large intestinal juice is a watery fluid with
pH of 8.0.
COMPOSITION OF LARGE
INTESTINAL JUICE
The large intestinal juice contains 99.5% of water
and 0.5% of solids (Fig. 32-1). Digestive enzymes
are absent and concentration of bicarbonate is
high in large intestinal juice.
FUNCTIONS OF LARGE INTESTINAL
JUICE
Neutralization of Acids
Strong acids formed by bacterial action in large
intestine are neutralized by the alkaline nature
of large intestinal juice. The alkalinity of this juice
is mainly due to the presence of large quantity
of bicarbonate.
FIGURE 32-1: Composition of large
intestinal juice

Chapter 32 Large Intestine
191
Lubrication Activity
The mucin present in secretion of large intestine
lubricates the mucosa of large intestine and the
bowel contents, so that, the movement of bowel
is facilitated.
The mucin also protects the mucous
membrane of large intestine by preventing the
damage caused by mechanical injury or chemical
substances.
FUNCTIONS OF LARGE INTESTINE
1. ABSORPTIVE FUNCTION
Large intestine plays an important role in the
absorption of various substances such as water,
electrolytes, organic substances like glucose,
alcohol and drugs like anesthetic agents,
sedatives and steroids.
2. FORMATION OF FECES
After the absorption of nutrients, water and other
substances, the unwanted substances in the
large intestine form feces. This is excreted out.
3. EXCRETORY FUNCTION
Large intestine excretes heavy metals like
mercury, lead, bismuth and arsenic through
feces.
4. SECRETORY FUNCTION
Large intestine secretes mucin and inorganic
substances like chlorides and bicarbonates.
5. SYNTHETIC FUNCTION
The bacterial flora of large intestine synthesizes
folic acid, vitamin B
12
and vitamin K. By this
function large intestine contributes in
erythropoietic activity and blood clotting
mechanism.
APPLIED PHYSIOLOGY
DIARRHEA
Diarrhea is the frequent and profuse discharge
of intestinal contents in loose and fluid form. It
occurs due to the increased movement of
intestine. It may be acute or chronic.
Causes
1. Intake of contaminated water or food, artificial
sweeteners found in food, spicy food, etc.
2. Indigestion
3. Infections by bacteria, viruses and parasites
4. Reaction to medicines such as antibiotics,
laxatives.
5. Intestinal diseases.
Features
Severe diarrhea results in loss of excess water
and electrolytes leading to dehydration and
electrolyte imbalance. Chronic diarrhea results
in hypokalemia and metabolic acidosis. Other
features of diarrhea are abdominal pain, nausea
and bloating (a condition in which the subject
feels the abdomen full and tight due to excess
intestinal gas).
CONSTIPATION
Failure of voiding of feces, which produces
discomfort, is known as constipation. It is due
to the lack of movements necessary for
defecation (Chapter 33). Due to the absence of
mass movement in colon, feces remain in the
large intestine for a long-time resulting in
absorption of fluid. So the feces become hard
and dry.
Causes
1. Lack of fiber or lack of liquids in diet
2. Irregular bowel habit
3. Spasm of sigmoid colon
4. Many types of diseases
5. Drugs like diuretics, pain relievers,
antihypertensive drugs antiparkinson drugs,
antidepressants and anticonvulsants
6. Dysfunction of myenteric plexus in large
intestine called megacolon
Megacolon is the condition characterized by
distension and hypertrophy of colon associated
with constipation. It is caused by the absence
Digestive System
192
or damage of ganglionic cells in myenteric plexus
which causes dysfunction of myenteric plexus.
It leads to accumulation of large quantity of feces
in colon. The colon is distended to a diameter
of 4-5 inches. It also results in hypertrophy of
colon.
APPENDICITIS
Appendix is a small, finger-like pouch projecting
from cecum of ascending colon. The inflam-
mation of appendix is known as appendicitis.
The cause for appendicitis is not known. It may
occur by viral infection of the GI tract or if the
connection between appendix and large intestine
is blocked.
Features
1. The main symptom of appendicitis is the pain,
which starts around the umbilicus and then
spreads to the lower right side of the
abdomen. The pain becomes severe within
6 to 12 hours
2. Nausea and vomiting
3. Constipation
4. Diarrhea
5. Low fever
6. Abdominal swelling
7. Loss of appetite
If not treated immediately, the appendix may
rupture and the inflammation will spread to the
whole body leading to severe complications,
sometimes even death.
MASTICATION
DEGLUTITION
MOVEMENTS OF STOMACH
FILLING AND EMPTYING OF STOMACH
VOMITING
MOVEMENTS OF SMALL INTESTINE
MOVEMENTS OF LARGE INTESTINE
DEFECATION
Movements of
Gastrointestinal Tract
33
33
33
33
33
MASTICATION
Mastication or chewing is the first mechanical
process in the GI tract by which the food
substances are torn or cut into small particles
and crushed or ground into a soft bolus.
The significances of mastication:
1. Breakdown of foodstuffs into smaller particles
2. Mixing of saliva with food substances
thoroughly
3. Lubrication and moistening of dry food by
saliva so that, the bolus can be easily
swallowed
4. Appreciation of taste of the food.
MUSCLES AND THE MOVEMENTS OF
MASTICATION
Muscles of mastication:
1. Masseter muscle
2. Temporal muscle
3. Pterygoid muscles
4. Buccinator muscle.
Movements involved in mastication:
1. Opening and closure of mouth
2. Rotational movements of jaw
3. Protraction and retraction of jaw.
CONTROL OF MASTICATION
Action of mastication is mostly a reflex process.
It is carried out voluntarily also. The center for
mastication is situated in medulla and cerebral
cortex. The muscles of mastication are supplied
by mandibular division of V cranial (trigeminal)
nerve.
DEGLUTITION
Definition
Deglutition or swallowing is the process by which
food passes from mouth into stomach.
Stages of Deglutition
Deglutition occurs in three stages:
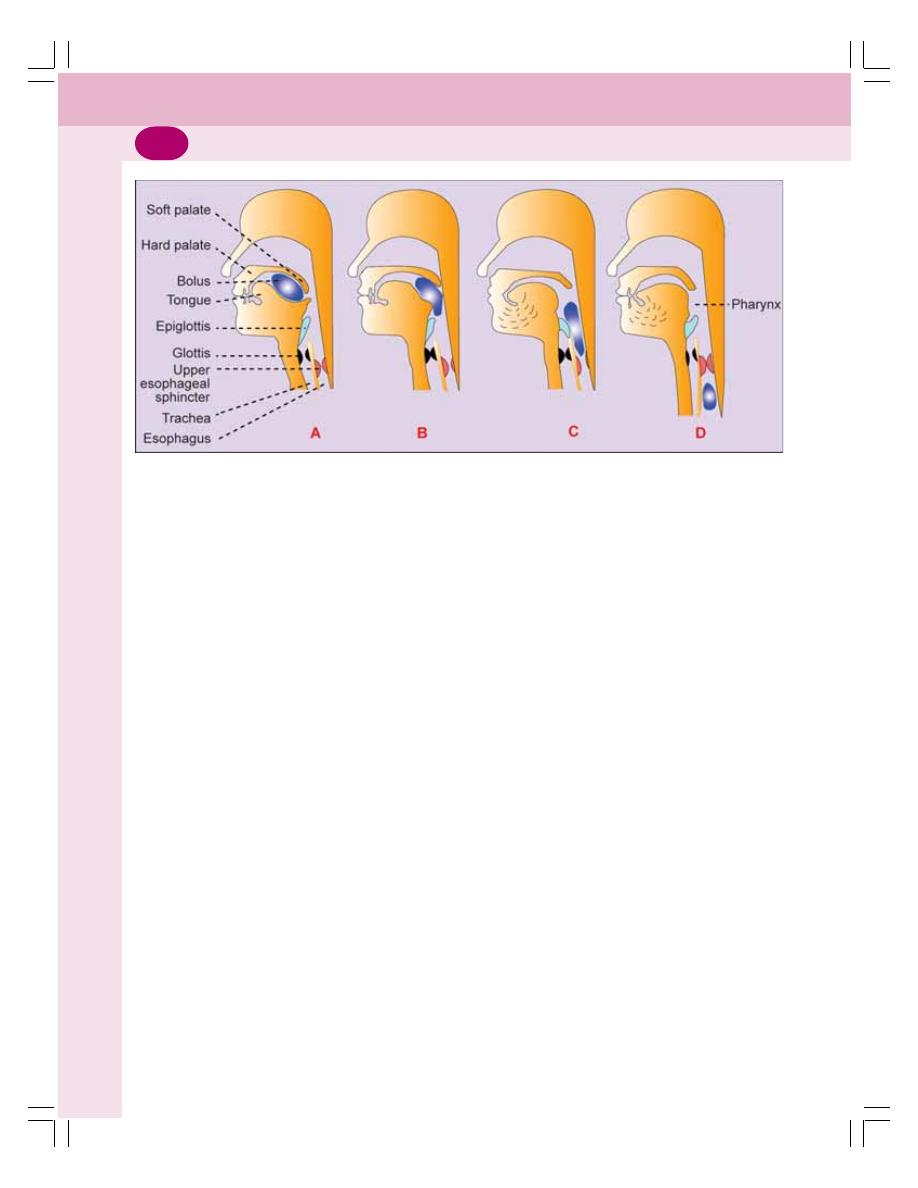
Digestive System
194
I.
Oral stage when food moves from mouth to
pharynx
II. Pharyngeal stage when food moves from
pharynx to esophagus
III. Esophageal stage when food moves from
esophagus to stomach.
ORAL STAGE OR FIRST STAGE
Oral stage is a voluntary stage. In this stage of
swallowing, the bolus from oral cavity passes into
the pharynx by means of series of actions
such as:
1. The bolus is placed over posterodorsal
surface of the tongue. It is called the
preparatory position
2. The anterior part of tongue is retracted and
depressed
3. The posterior part of tongue is elevated and
retracted against hard palate. This pushes the
bolus backwards into the pharynx
4. The forceful contraction of tongue against the
palate produces a positive pressure in the
posterior part of oral cavity. This pressure in
the oral cavity also pushes the food into
pharynx (Fig. 33-1).
PHARYNGEAL STAGE OR SECOND
STAGE
Pharyngeal stage is an involuntary stage. In this
stage, the bolus is pushed from pharynx into the
esophagus. The pharynx is a common passage
for food and air. It divides into larynx and
esophagus. Larynx lies anteriorly and continues
as respiratory passage. Esophagus lies behind
the larynx and continues as GI tract. Since
pharynx communicates with mouth, nose, larynx
and esophagus, during this stage of deglutition,
the bolus from the pharynx can enter into four
paths:
1. It can come back into mouth
2. It can go upwards into nasopharynx
3. It can move forwards into larynx
4. It can move downwards into esophagus.
However, due to various coordinated
movements, bolus is made to enter only into the
esophagus. The entrance of bolus through other
paths is prevented as follows:
1. Back into Mouth
Return of bolus back into the mouth is prevented
by:
i.
The position of tongue against the soft palate
(roof of the mouth)
ii. The high intraoral pressure developed by the
movement of tongue.
2. Upward into Nasopharynx
The movement of bolus into the nasopharynx
from pharynx is prevented by elevation of soft
palate along with its extension called uvula.
FIGURE 33-1: Stages of deglutition. A = Preparatory stage, B = Oral stage, C = Pharyngeal stage,
D = Esophageal stage

Chapter 33 Movements of Gastrointestinal Tract
195
3. Forward into Larynx
The movement of bolus into the larynx is
prevented by the following actions:
i.
Approximation of the vocal cords
ii. Forward and upward movement of larynx
iii. The backward movement of epiglottis to seal
the opening of the larynx (glottis)
iv. All these movements arrest respiration for a
few seconds. It is called deglutition apnea.
Deglutition apnea
Apnea refers to temporary arrest of breathing.
Deglutition apnea or swallowing apnea is the
arrest of breathing during deglutition.
4. Entrance of Bolus into Esophagus
Since the other three paths are closed for the
bolus, it has to pass only through the esophagus.
It occurs by the combined effects of various
factors:
i.
The upward movement of the larynx stretches
the opening of the esophagus
ii. Simultaneously, the upper 3 to 4 cm of
esophagus relaxes. This part of the
esophagus is formed by the cricopharyngeal
muscle and it is called upper esophageal
sphincter or pharyngoesophageal sphincter
iii. At the same time, the peristaltic contractions
start in the pharynx due to the contraction of
pharyngeal muscles
iv. Elevation of larynx also lifts the glottis away
from the food passage.
All the factors mentioned above act together
so that the bolus moves easily into the
esophagus. The whole process takes place within
1 to 2 seconds. And this process is purely
involuntary.
ESOPHAGEAL STAGE OR
THIRD STAGE
It is also an involuntary stage. In esophageal
stage, food from stomach enters esophagus. The
function of esophagus is to transport the bolus
from the pharynx to the stomach. The
movements of esophagus are specifically
organized for this function and the movements
are called peristaltic waves. Peristalsis means
a wave of contraction followed by the wave of
relaxation of muscle fibers of GI tract, which
travel in aboral direction (away from mouth). By
this type of movement, the contents are propelled
down along the GI tract.
Role of Lower Esophageal Sphincter
The distal 2 to 5 cm of esophagus acts like a
sphincter and it is called lower esophageal
sphincter. It is constricted always. When bolus
enters this part of the esophagus, this sphincter
relaxes so that the contents enter the stomach.
After the entry of bolus into the stomach, the
sphincter constricts and closes the lower end of
esophagus. The relaxation and constriction of
sphincter occur in sequence with the arrival of
peristaltic contractions of esophagus.
DEGLUTITION REFLEX
Though the beginning of swallowing is a voluntary
act, later it becomes involuntary and is carried
out by a reflex action called deglutition reflex. It
occurs during the pharyngeal and esophageal
stages.
Stimulus
When the bolus enters the oropharyngeal region,
the receptors present in this region are
stimulated.
Afferent Fibers
Afferent impulses from the oropharyngeal
receptors pass via the glossopharyngeal nerve
fibers to the deglutition center.
Center
The deglutition center is at the floor of the fourth
ventricle in medulla oblongata of brain.
Efferent Fibers
The impulses from deglutition center travel
through glossopharyngeal and vagus nerves
Digestive System
196
(parasympathetic motor fibers) and reach soft
palate, pharynx and esophagus. The glosso-
pharyngeal nerve is concerned with pharyngeal
stage of swallowing. The vagus nerve is
concerned with esophageal stage.
Response
The reflex causes upward movement of soft
palate to close nasopharynx and upward
movement of larynx to close respiratory passage
so that bolus enters the esophagus. Now the
peristalsis occurs in esophagus pushing the bolus
into stomach.
MOVEMENTS OF STOMACH
The movements of the stomach are:
1. Hunger contractions
2. Receptive relaxation
3. Peristalsis.
HUNGER CONTRACTIONS
Hunger contractions are the movements of empty
stomach. These contractions are related to the
sensations of hunger.
Hunger contractions are the peristaltic waves
superimposed over the contractions of gastric
smooth muscle as a whole. This type of peristaltic
waves is different from the digestive peristaltic
contractions. The digestive peristaltic
contractions usually occur in body and pyloric
parts of the stomach. But, the peristaltic
contractions of empty stomach involve the entire
stomach.
RECEPTIVE RELAXATION
Receptive relaxation is the relaxation of the upper
portion of the stomach when bolus enters the
stomach from esophagus. It involves the fundus
and upper part of the body of stomach. Its
significance is to accommodate the food easily
without much increase in pressure inside the
stomach. This process is called accommodation
of stomach.
PERISTALSIS OF STOMACH
When the food enters the stomach, the peristaltic
contraction or peristaltic wave appears with a
frequency of 3 per minute. It starts from the lower
part of the body of stomach, passes through the
pylorus till the pyloric sphincter.
Initially, the contraction appears as a slight
indentation on the greater and lesser curvatures
and travels towards pylorus. The contraction
becomes deeper while traveling. Finally, it ends
with the constriction of pyloric sphincter. Some
of the waves disappear before reaching the
sphincter. Each peristaltic wave takes about one
minute to travel from the point of origin to the
point of ending.
This type of peristaltic contraction is called
digestive peristalsis because it is responsible for
the grinding of food particles and mixing them
with gastric juice for digestive activities.
FILLING AND EMPTYING OF
STOMACH
FILLING OF STOMACH
While taking food, the food arranges itself in the
stomach in different layers. The first eaten food
is placed against the greater curvature in the
fundus and body of the stomach. The successive
layers of food particles lie nearer the lesser
curvature until the last portion of food eaten lies
near the upper end of lesser curvature adjacent
to cardiac sphincter.
EMPTYING OF STOMACH
Gastric emptying is the process by which the
chyme from stomach is emptied into intestine.
The food that is swallowed enters the stomach
and remains there for about 3 hours. During this
period, digestion takes place. The partly digested
food becomes the chyme.
Chyme
Chyme is the semisolid mass of partially digested
food that is formed in the stomach. It is acidic in

Chapter 33 Movements of Gastrointestinal Tract
197
nature. The acid chyme is emptied from stomach
into the intestine slowly with the help of peristaltic
contractions. It takes about 3 to 4 hours for
emptying of the chyme. This slow emptying is
necessary to facilitate the final digestion and
maximum (about 80%) absorption of the digested
food materials from small intestine. Gastric
emptying occurs due to the peristaltic waves in
the body and pyloric part of the stomach and
simultaneous relaxation of pyloric sphincter.
The gastric emptying is influenced by various
factors of the gastric content and food. The
factors which affect gastric emptying are:
1. Volume of Gastric Content
Gastric emptying is directly proportional to the
volume. If the content of stomach is more, a large
amount is emptied into the intestine rapidly.
2. Consistency of Gastric Content
Emptying of the stomach depends upon the
consistency (degree of density) of the contents.
Liquids, particularly the inert liquids like water
(which do not stimulate the stomach) leave the
stomach rapidly. Solids move out of stomach only
after being converted into fluid or semifluid.
Undigested solid particles are not easily emptied.
3. Chemical Composition
The chemical composition of the food also plays
an important role in the emptying of the stomach.
Carbohydrates are emptied rapidly than the
proteins. Proteins are emptied rapidly than the
fats.
4. pH of the Gastric Content
Gastric emptying is directly proportional to pH
of the chyme.
5. Osmolar Concentration of Gastric Content
The gastric content, which is isotonic to blood,
leaves the stomach rapidly than the hypotonic
or hypertonic content.
VOMITING
Vomiting or emesis is the abnormal emptying of
stomach and upper part of intestine through
esophagus and mouth.
CAUSES OF VOMITING
1. The presence of irritating contents in GI
tract
2. Mechanical stimulation of pharynx
3. Pregnancy
4. Excess intake of alcohol
5. Nauseating sight, odor or taste
6. Unusual stimulation of labyrinthine
apparatus as in the case of sea sickness,
air sickness, car sickness or swinging
7. Abnormal stimulation of sensory receptors
in other organs like kidney, heart,
semicircular canals or uterus
8. Drugs like antibiotics, opiates, etc.
9. Any GI disorder
10. Acute infection like urinary tract infection,
influenza, etc.
11. Metabolic disturbances like carbohydrate
starvation and ketosis (pregnancy),
uremia, ketoacidosis (diabetes) and
hypercalcemia.
MECHANISM OF VOMITING
Nausea
Vomiting is always preceded by nausea. Nausea
is unpleasant sensation which induces the desire
for vomiting. It is characterized by secretion of
large amount of saliva containing more amount
of mucus.
Retching
Strong involuntary movements in the GI tract start
even before actual vomiting and intensify the
feeling of vomiting. This condition is called
retching (try to vomit). And, vomiting occurs few
minutes after this.
Act of Vomiting
Act of vomiting involves series of movements that
takes place in GI tract.
Digestive System
198
The sequence of events:
1. Beginning of antiperistalsis which runs from
ileum towards the mouth through the intestine
pushing the intestinal contents into the
stomach within few minutes
2. Deep inspiration followed by temporary
cessation of breathing
3. Closure of glottis
4. Upward and forward movement of larynx and
hyoid bone
5. Elevation of soft palate
6. Contraction of diaphragm and abdominal
muscles with a characteristic jerk resulting
in elevation of intra-abdominal pressure
7. Compression of the stomach between
diaphragm and abdominal wall leading to rise
in intragastric pressure
8. Simultaneous relaxation of lower esophageal
sphincter, esophagus and upper esophageal
sphincter
9. Forceful expulsion of gastric contents
(vomitus) through esophagus, pharynx and
mouth.
All the movements during the act of vomiting
throw the vomitus (materials ejected during
vomiting) to the exterior through mouth. Some
of the movements play important roles by
preventing the entry of vomitus through other
routes and thereby prevent the adverse
effect of the vomitus on many structures.
Such movements are:
1. Closure of glottis and cessation of breathing
prevent entry of vomitus into the lungs
2. Elevation of soft palate prevents entry of
vomitus into the nasopharynx
3. Larynx and hyoid bone move upward and
forward and are placed in this position rigidly.
This causes the dilatation of throat which
allows free exit of vomitus.
VOMITING REFLEX
Vomiting is a reflex act. The sensory impulses
for vomiting arise from the irritated or distended
part of GI tract or other organs and are
transmitted to the vomiting center through vagus
and sympathetic fibers.
The vomiting center is situated bilaterally in
medulla oblongata near the nucleus tractus
solitarius.
Motor impulses from the vomiting center are
transmitted through V, VII, IX, X and XII cranial
nerves to the upper part of GI tract; and through
spinal nerves to diaphragm and abdominal
muscles.
MOVEMENTS OF SMALL INTESTINE
The movements of small intestine are essential
for mixing the chyme with digestive juices,
propulsion of food and absorption.
Four types of movements occur in small
intestine:
1. Mixing movements:
i.
Segmentation movements
ii. Pendular movements
2. Propulsive movements:
i.
Peristaltic movements
ii
Peristaltic rush
3. Peristalsis in fasting – Migrating motor
complex
4. Movements of villi.
MIXING MOVEMENTS
The mixing movements of small intestine are
responsible for proper mixing of chyme with
digestive juices like pancreatic juice, bile and
intestinal juice. The mixing movements of small
intestine are segmentation contractions and
pendular movements.
Segmentation Contractions
The segmentation contractions are the common
type of movements of small intestine, which occur
regularly or irregularly but in a rhythmic fashion.
So, these movements are also called rhythmic
segmentation contractions.
The contractions occur at regularly spaced
intervals along a section of intestine. The
segment of the intestine involved in each
contraction is about 1 to 5 cm long. The seg-
ments of intestine in between the contracted
segments are relaxed. The length of the relaxed
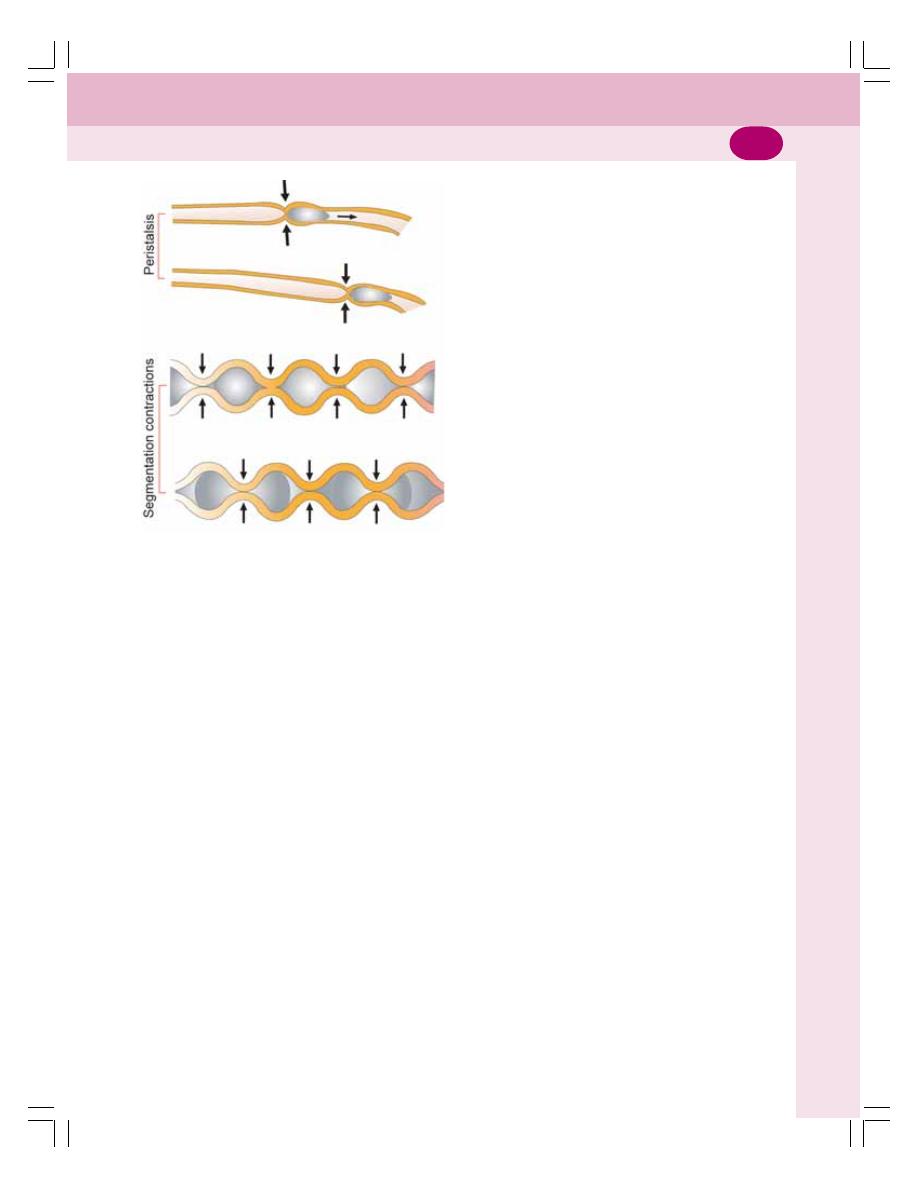
Chapter 33 Movements of Gastrointestinal Tract
199
segments is same as that of the contracted
segments. These alternate segments of
contraction and relaxation give appearance of
rings resembling the chain of sausages.
After sometime, the contracted segments are
relaxed and the relaxed segments are contracted
(Fig. 33-2). Therefore, the segmentation
contractions chop the chyme many times. This
helps in mixing of chyme with digestive juices.
Pendular Movement
Pendular movement is the sweeping movement
of small intestine resembling the movements of
pendulum of clock. Small portions of intestine
(loops) sweep forward and backward or upward
and downward. It is a type of mixing movement
noticed only by close observation.
It helps in mixing of chyme with digestive
juices.
PROPULSIVE MOVEMENTS
Propulsive movements are the movements of
small intestine which push the chyme in the
aboral direction through intestine. The propulsive
movements are peristaltic movements and
peristaltic rush.
Peristaltic Movements
Peristalsis is defined as the wave of contraction
followed by wave of relaxation, which travels in
aboral direction. The stimulation of smooth
muscles of intestine initiates the peristalsis. It
travels from point of stimulation in both directions.
But under normal conditions, the progress of
contraction in an oral direction is inhibited quickly
and the contractions disappear. Only the
contraction that travels in an aboral direction
persists.
Peristaltic Rush
Sometimes, the small intestine shows a
powerful peristaltic contraction. It is caused by
excessive irritation of intestinal mucosa or
extreme distention of the intestine. This type of
powerful contraction begins in duodenum and
passes through entire length of small intestine
and reaches the ileocecal valve within few
minutes. This is called peristaltic rush or rush
waves.
The peristaltic rush sweeps the contents of
intestine into the colon. Thus, it relieves the small
intestine off either irritants or excessive distention.
PERISTALSIS IN FASTING –
MIGRATING MOTOR COMPLEX
It is a type of peristaltic contraction, which occurs
in stomach and small intestine during the periods
of fasting for several hours. It is different from
the regular peristalsis because, a large portion
of stomach or intestine is involved in the
contraction. The contraction extends to about
20 to 30 cm of the stomach or intestine. This
type of movement occurs once in every 1½ to
2 hours.
It starts as a moderately active peristalsis in
the body of stomach and runs through the entire
length of small intestine. It travels at a velocity
of 6 to 12 cm/min. Thus, it takes about 10
minutes to reach the colon after taking origin from
stomach.
FIGURE 33-2: Movements of small intestine
Digestive System
200
Significance of Peristalsis in Fasting
The migrating motor complex sweeps the excess
digestive secretions into the colon and prevents
the accumulation of the secretions in stomach
and intestine. It also sweeps the residual
indigested materials into colon.
MOVEMENTS OF VILLI
The intestinal villi also shows movements
simultaneously along with intestinal movements.
It is because of the extension of smooth muscle
fibers of the intestinal wall into the villi.
The movements of villi are shortening and
elongation, which occur alternatively and help in
emptying lymph from the central lacteal into the
lymphatic system. The surface area of villi is
increased during elongation. This helps
absorption of digested food particles from the
lumen of intestine.
Movements of villi are caused by local
nervous reflexes, which are initiated by the
presence of chyme in small intestine.
MOVEMENTS OF LARGE INTESTINE
Large intestine shows sluggish movements. Still,
these movements are important for mixing,
propulsive and absorptive functions. Large
intestine shows two types of movements:
1. Mixing movements – Segmentation
contractions
2. Propulsive movements – Mass peristalsis.
MIXING MOVEMENTS –
SEGMENTATION CONTRACTIONS
Large circular constrictions, which appear in the
colon, are called mixing segmentation
contractions. The contractions occur at regular
distance in colon. The length of the portion of
colon involved in each contraction is nearly about
2.5 cm.
PROPULSIVE MOVEMENTS – MASS
PERISTALSIS
Mass peristalsis or mass movement propels the
feces from colon towards anus. Usually, this
movement occurs only a few times every day.
The duration of the mass movement is about 10
minutes in the morning before or after breakfast.
This is because of the neurogenic factors like
gastrocolic reflex (see below) and
parasympathetic stimulation.
DEFECATION
Voiding of feces is known as defecation. Feces
is formed in the large intestine and stored in
sigmoid colon. By the influence of an appropriate
stimulus, it is expelled out through the anus. This
is prevented by tonic constriction of anal
sphincters in the absence of the stimulus.
DEFECATION REFLEX
The mass movement drives the feces into
sigmoid or pelvic colon. In the sigmoid colon the
feces is stored. The desire for defecation occurs
when some feces enters rectum due to the mass
movement. Usually, the desire for defecation is
elicited by an increase in the intrarectal pressure
to about 20 to 25 cm H
2
O.
The usual stimulus for defecation is intake
of liquid like coffee or tea or water. But it differs
from person to person.
Act of Defecation
The act of defecation is preceded by voluntary
efforts like assuming an appropriate posture,
voluntary relaxation of external sphincter and the
compression of abdominal contents by voluntary
contraction of abdominal muscles.
Usually, the rectum is empty. During the
development of mass movement, the feces is
pushed into rectum and the defecation reflex is
initiated. The process of defecation involves the
contraction of rectum and relaxation of internal
and external anal sphincters.
The internal anal sphincter is made up of
smooth muscle and it is innervated by
parasympathetic nerve fibers via pelvic nerve.
The external anal sphincter is composed of
skeletal muscle and it is controlled by somatic
nerve fibers, which pass through pudendal nerve.
The pudendal nerve always keeps the external
sphincter constricted and the sphincter can relax
only when the pudendal nerve is inhibited.
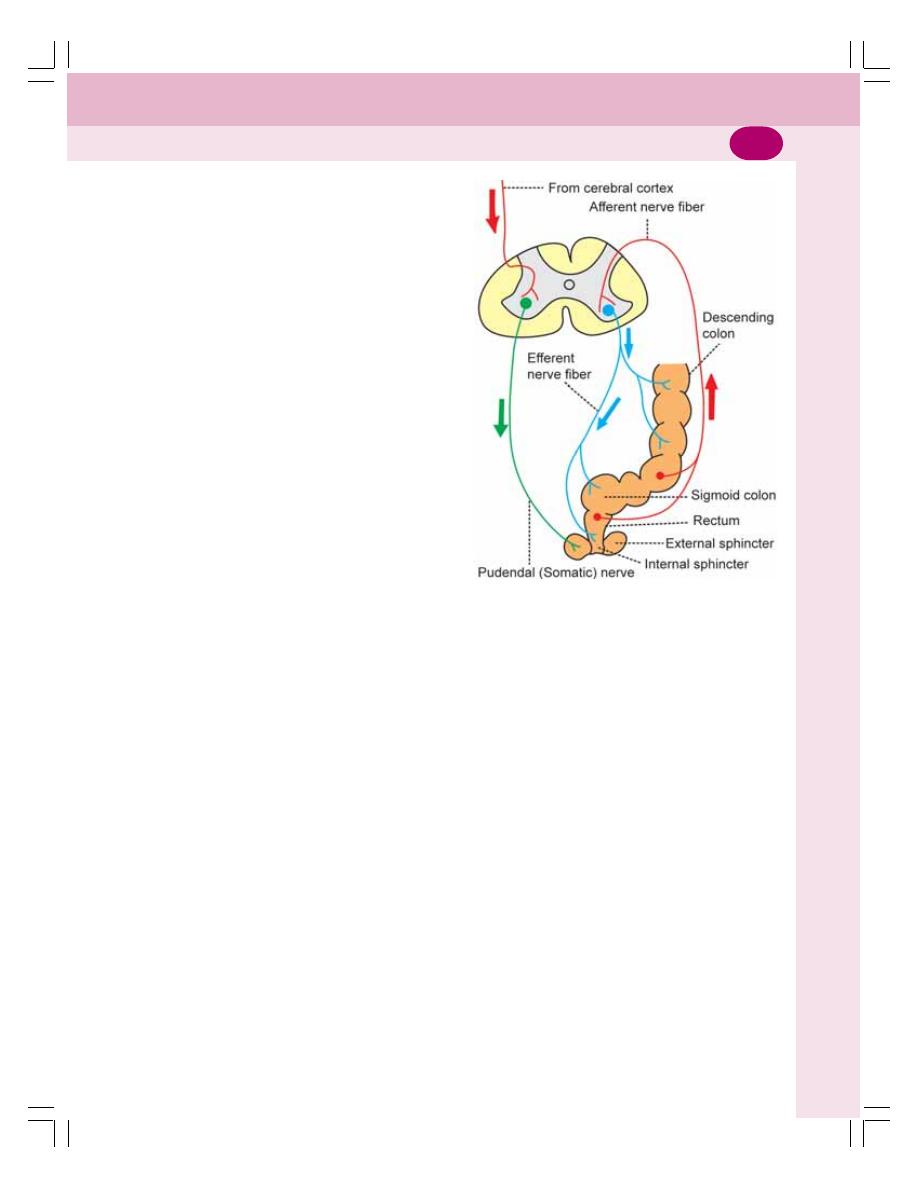
Chapter 33 Movements of Gastrointestinal Tract
201
Gastrocolic Reflex
Gastrocolic reflex is the contraction of rectum
followed by desire for defecation caused by
distention of stomach by food. It is mediated by
intrinsic nerve fibers of GI tract.
This reflex causes only a weak contraction
of rectum. But, it initiates defecation reflex.
PATHWAY FOR DEFECATION REFLEX
When rectum is distended due to the entry of
feces by mass movement, sensory nerve
endings are stimulated. The impulses from the
nerve endings are transmitted via afferent fibers
of pelvic nerve to the defecation center situated
in sacral segments (center) of spinal cord.
The center, in turn, sends motor impulses to
the descending colon, sigmoid colon and rectum
via efferent nerve fibers of pelvic nerve. The
motor impulses cause strong contraction of
descending colon, sigmoid colon and rectum and
relaxation of internal sphincter.
Simultaneously, voluntary relaxation of
external sphincter occurs. It is due to the inhibition
of pudendal nerve by impulses arising from
cerebral cortex (Fig. 33-3).
Failure of voiding of feces is called
constipation (Chapter 32).
FIGURE 33-3: Defecation reflex. Afferent and
efferent fibers of the reflex pass through pelvic
(parasympathetic) nerve. Voluntary control of
defecation is by pudendal (somatic) nerve.
Defecation center is in the sacral segments of spinal
cord
Questions in Digestive System
202
LONG QUESTIONS
1. What are the different types of salivary
glands? Describe the composition, functions
and regulation of secretion of saliva.
2. Describe the different phases of gastric
secretion with experimental evidences.
3. Explain the composition, functions and
regulation of secretion of pancreatic juice.
4. Describe the composition, functions and
regulation of secretion of bile. Enumerate the
differences between the liver bile and
gallbladder bile. Add a note on enterohepatic
circulation.
SHORT QUESTIONS
1. Properties and composition of saliva.
2. Functions of saliva.
3. Nerve supply to salivary glands.
4. Gastric glands.
5. Functions of stomach.
6. Properties and composition of gastric
juice.
7. Functions of gastric juice
8. Mechanism of secretion of hydrochloric
acid in stomach.
9. Pavlov’s pouch.
10. Sham feeding.
11. Cephalic phase of gastric secretion.
12. Gastrin.
13. Peptic ulcer.
QUESTIONS IN DIGESTIVE SYSTEM
14. Properties and composition of pancreatic
juice.
15. Functions of pancreatic juice.
16. Regulation of exocrine function of pan-
creas.
17. Steatorrhea.
18. Secretin.
19. Cholecystokinin.
20. Composition of bile.
21. Functions of bile.
22. Bile salts/bile pigments.
23. Enterohepatic circulation.
24. Functions of liver.
25. Differences between liver bile and gall-
bladder bile.
26. Functions of gallbladder.
27. Jaundice.
28. Succus entericus.
29. Functions of small intestine.
30. Functions of large intestine.
31. Mastication.
32. Swallowing.
33. Movements of stomach.
34. Filling and emptying of stomach.
35. Vomiting.
36. Movements of small intestine.
37. Movements of large intestine.
38. Defecation.
39. Constipation.
40. Diarrhea.

Renal Physiology and Skin
34. Kidney .................................................................................. 205
35. Nephron and Juxtaglomerular Apparatus ............................ 208
36. Renal Circulation ................................................................. 216
37. Urine Formation ................................................................... 219
38. Concentration of Urine ......................................................... 227
39. Acidification of Urine and Role of Kidney in
Acid-Base Balance .............................................................. 233
40. Renal Function Tests ........................................................... 236
41. Micturition ............................................................................ 239
42. Skin ..................................................................................... 244
43. Body Temperature ............................................................... 249
S E C T I O N
5
5
5
5
5
C H A P T E R S

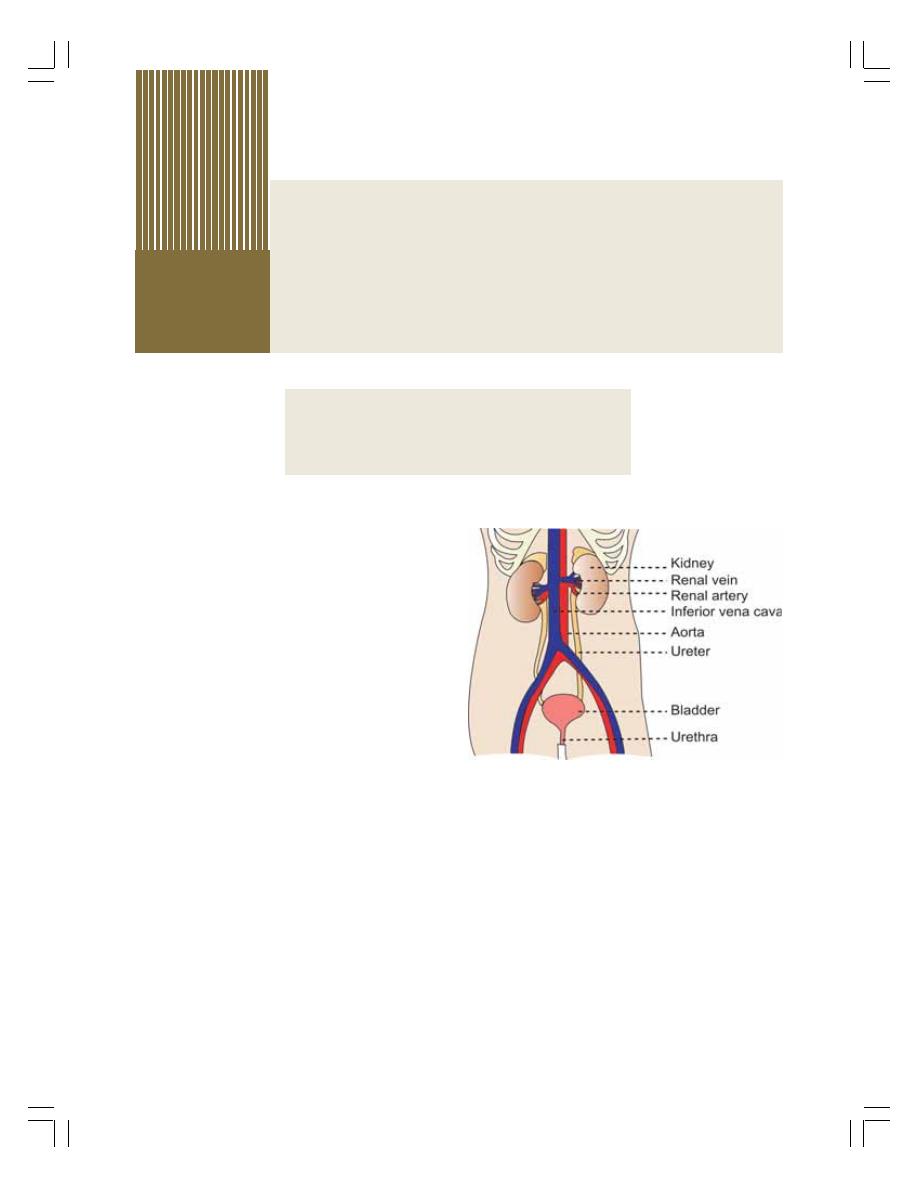
INTRODUCTION
INTRODUCTION
INTRODUCTION
INTRODUCTION
INTRODUCTION
FUNCTIONS OF KIDNEY
FUNCTIONS OF KIDNEY
FUNCTIONS OF KIDNEY
FUNCTIONS OF KIDNEY
FUNCTIONS OF KIDNEY
FUNCTIONAL ANATOMY OF KIDNEY
FUNCTIONAL ANATOMY OF KIDNEY
FUNCTIONAL ANATOMY OF KIDNEY
FUNCTIONAL ANATOMY OF KIDNEY
FUNCTIONAL ANATOMY OF KIDNEY
INTRODUCTION
Excretion is the process by which the unwanted
substances and metabolic wastes are elimi-
nated from the body.
Although various organs such as GI tract,
liver, skin and lungs are involved in removal of
wastes from the body, their excretory capacity
is limited. But, the renal system or urinary system
has maximum capacity of excretory function.
Renal system includes:
1. A pair of kidneys
2. Ureters
3. Urinary bladder
4. Urethra.
Kidneys produce the urine. Ureters transport
the urine to urinary bladder. Urinary bladder
stores urine until it is voided (emptied). Urine is
voided from bladder through urethra (Fig. 34-1).
FUNCTIONS OF KIDNEY
Kidneys perform
several vital functions besides
formation of urine. By excreting urine, kidneys
play the principal role in homeostasis. Thus,
the functions of kidneys are:
1. ROLE IN HOMEOSTASIS
The primary function of kidneys is homeostasis.
It is accomplished by the formation of urine.
During the formation of urine, kidneys regulate
various activities in the body, which are
concerned with homeostasis such as:
i.
Excretion of Waste Products
Kidneys excrete the unwanted waste products
which are formed during metabolic activities:
a. Urea – end product of amino acid metabolism
b. Uric acid – end product of nucleic acid
metabolism
c. Creatinine – end product of metabolism in
muscles
d. Bilirubin – end product of hemoglobin
degradation
Kidney
34
34
34
34
34
FIGURE 34-1: Urinary system
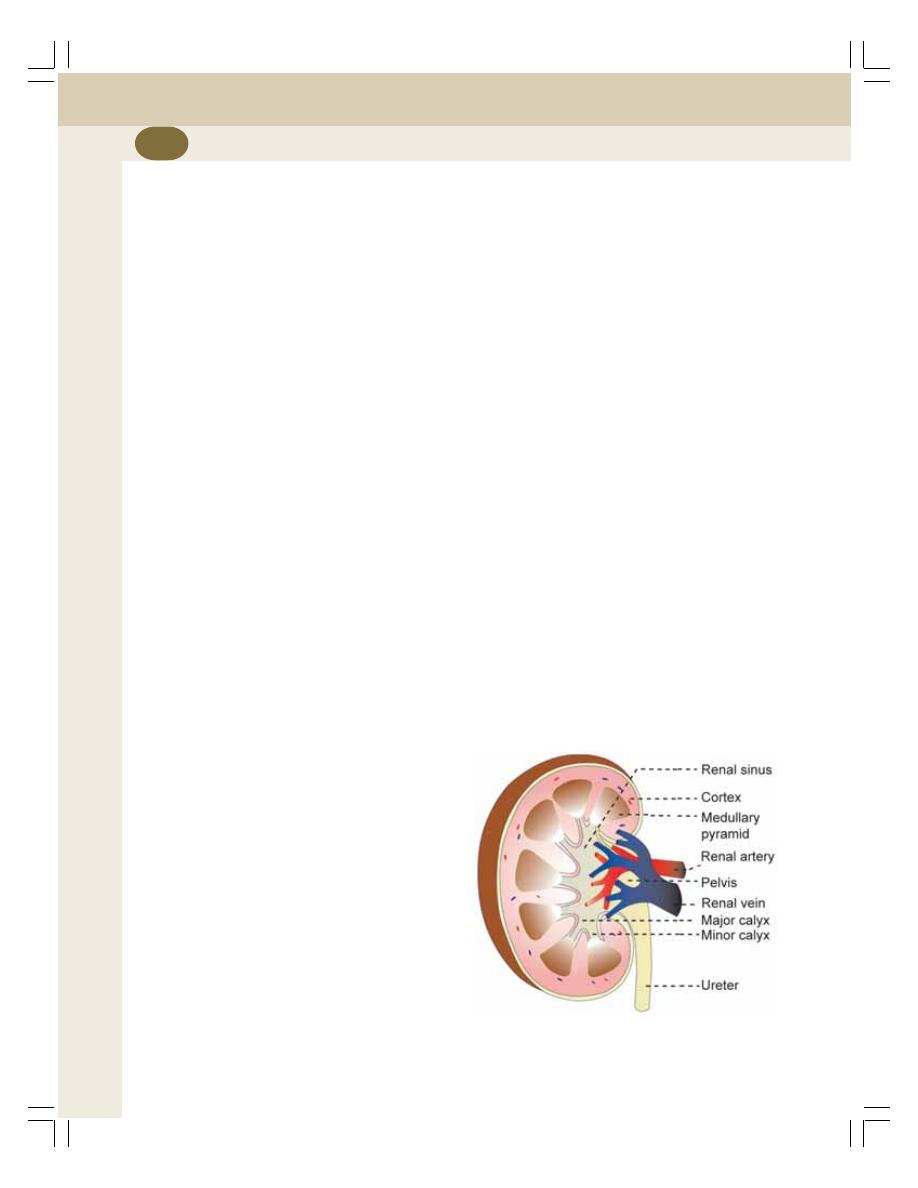
Renal Physiology and Skin
206
e. Products of metabolism of other substances
f.
Harmful foreign chemical substances like
toxins, drugs, heavy metals, pesticides, etc.
ii. Maintenance of Water Balance
Kidneys maintain the water balance in the body
by conserving water when it is decreased and
excreting water when it is excess in the body.
Refer Chapter 4 for details.
iii. Maintenance of Electrolyte Balance
Maintenance of electrolyte balance, especially
sodium is in relation to water balance. Kidneys
retain sodium if the osmolarity of body water
decreases and eliminate sodium when
osmolarity increases.
iv. Maintenance of Acid–Base Balance
The pH of the blood and body fluids should be
maintained within narrow range for healthy
living. It is achieved by the function of kidneys
(Chapter 39). Body is under constant threat to
develop acidosis, because of production of lot
of acids during metabolic activities. However, it
is prevented by kidneys, lungs and blood
buffers, which eliminate these acids. Among
these organs, kidneys play major role in
preventing acidosis.
2. HEMOPOIETIC FUNCTION
Kidneys stimulate the production of erythrocytes
by secreting erythropoietin. Erythropoietin is the
important stimulating factor for erythropoiesis
(Chapter 8). Kidney also secretes another factor
called thrombopoietin, which stimulates the
production of thrombocytes (Chapter 14).
3. ENDOCRINE FUNCTION
Kidneys secrete many hormonal substances in
addition to erythropoietin and thrombopoietin
(Chapter 51). The hormones secreted by kidneys
are:
i.
Erythropoietin
ii. Thrombopoietin
iii. Renin
iv. 1, 25-dihydroxycholecalciferol (calcitriol)
v. Prostaglandins.
4. REGULATION OF BLOOD
PRESSURE
Kidneys play an important role in long-term
regulation of arterial blood pressure (Chapter 65)
by two ways: by regulating ECF volume and
through renin-angiotensin mechanism.
5. REGULATION OF BLOOD
CALCIUM LEVEL
Kidneys play a role in the regulation of blood
calcium level by activating 1, 25-dihydroxycho-
lecalciferol into vitamin D. Vitamin D is necessary
for the absorption of calcium from intestine
(Chapter 51).
FUNCTIONAL ANATOMY OF
KIDNEY
Kidney is a compound tubular gland covered by
a connective tissue capsule. There is a
depression on the medial border of kidney
called hilum, through which renal artery, renal
veins, nerves and ureter pass.
DIFFERENT LAYERS OF KIDNEY
The components of kidney are arranged in three
layers (Fig. 34-2).
FIGURE 34-2: Longitudinal section of kidney

Chapter 34 Kidney
207
1. Outer cortex
2. Inner medulla
3. Renal sinus.
1. Outer Cortex
Cortex is dark and granular in appearance. It
contains renal corpuscles and convoluted
tubules. At intervals, cortical tissue penetrates
medulla in the form of columns, which are called
renal columns or columns of Bertini.
2. Inner Medulla
Medulla contains tubular and vascular structures
arranged in parallel radial lines. It is divided into
8 to 18 medullary or Malpighian pyramids.
3. Renal Sinus
Renal sinus consists of the following structures:
i.
Upper expanded part of ureter called renal
pelvis
ii. Subdivisions of pelvis – 2 or 3 major calyces
and about 8 minor calyces
iii. Branches of nerves and arteries and tri-
butaries of veins
iv. Loose connective tissues and fat.
PARENCHYMA OF KIDNEY
Parenchyma of kidney is made up of tubular
structures called uriniferous tubules. The uri-
niferous tubules are of two types:
1. Terminal or secretary tubules called neph-
rons, which are concerned with formation of
urine
2. Collecting ducts or tubules which are con-
cerned with transport of urine from nephrons
to pelvis of ureter.
The collecting ducts unite to form ducts of
Belini, which open into minor calyces through
papilla. Other details are given in Chapter 35.

INTRODUCTION
RENAL CORPUSCLE
SITUATION OF RENAL CORPUSCLE AND TYPES OF NEPHRON
STRUCTURE OF RENAL CORPUSCLE
TUBULAR PORTION OF NEPHRON
PROXIMAL CONVOLUTED TUBULE
LOOP OF HENLE
DISTAL CONVOLUTED TUBULE
COLLECTING DUCT
JUXTAGLOMERULAR APPARATUS
DEFINITION
STRUCTURE
FUNCTIONS
INTRODUCTION
Nephron is defined as the structural and
functional unit of kidney. Each kidney consists
of 1 to 1.3 millions of nephrons. The number of
nephrons decreases in old age.
Each nephron is formed by two parts
(Fig. 35-1):
1. A blind end called renal corpuscle or
Malpighian corpuscle
2. A tubular portion called renal tubule.
RENAL CORPUSCLE
The renal corpuscle is also known as Malpighian
corpuscle. It is a spheroidal and slightly flattened
structure with a diameter of about 200
μ. The
function of the renal corpuscle is the filtration of
blood which forms the first phase of urine
formation.
SITUATION OF RENAL CORPUSCLE
AND TYPES OF NEPHRON
Renal corpuscle is situated in the cortex of the
kidney either near the periphery or near the
medulla. Based on the situation of renal cor-
puscle, the nephrons are classified into two types:
1. Cortical nephrons or superficial nephrons
2. Juxtamedullary nephrons.
1. Cortical Nephrons
Cortical nephrons are the nephrons, which have
their corpuscles in the outer cortex of the kidney
near the periphery (Fig. 35-2). In human kidneys
85% nephrons are cortical nephrons.
2. Juxtamedullary Nephrons
Juxtamedullary nephrons are the nephrons
which have their corpuscles in the inner cortex
Nephron and
Juxtaglomerular Apparatus
35
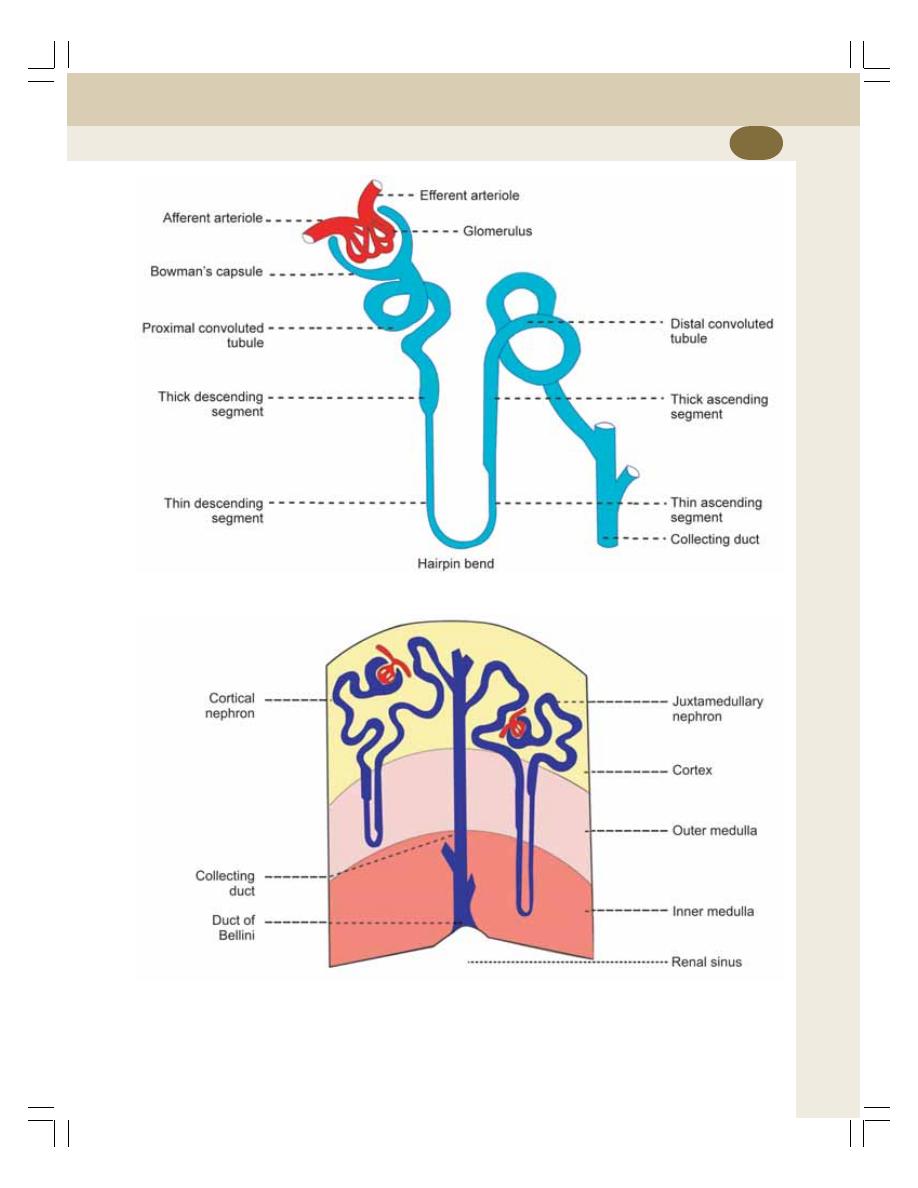
Chapter 35 Nephron and Juxtaglomerular Apparatus
209
FIGURE 35-1: Structure of nephron
FIGURE 35-2: Types of nephron
near medulla or corticomedullary junction
(Fig. 35-2).
The features of the two types of nephrons
are given in Table 35-1.
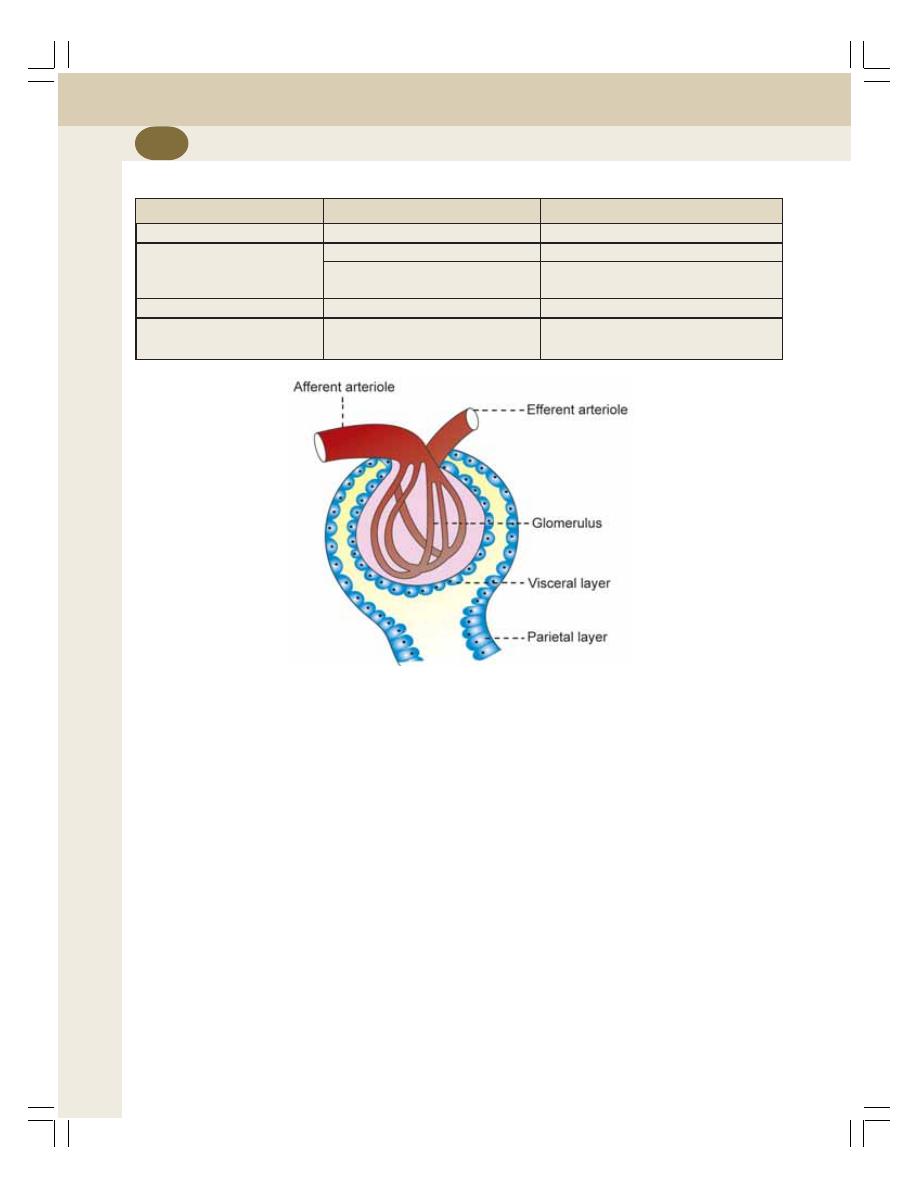
Renal Physiology and Skin
210
STRUCTURE OF RENAL CORPUSCLE
The renal corpuscle is formed by two portions:
1. Glomerulus
2. Bowman’s capsule
1. Glomerulus
Glomerulus is a tuft of capillaries enclosed by
Bowman’s capsule. These capillaries are
disposed between afferent arteriole and efferent
arteriole. Thus, the vascular system in the
glomerulus is purely arterial (Fig. 35-3).
The glomerular capillaries arise from the
afferent arteriole. After entering the Bowman’s
capsule, the afferent arteriole divides into many
small capillaries. These small capillaries are
arranged in irregular loops and form anasto-
mosis. All the smaller capillaries finally reunite
to form the efferent arteriole which leaves the
Bowman’s capsule.
The diameter of the efferent arteriole is less
than that of afferent arteriole. This difference in
diameter has functional significance.
The capillaries are made up of single layer
of endothelial cells which are attached to a
basement membrane. The endothelium has
many pores called fenestra or filtration pores. The
diameter of each pore is 0.1
μ. The presence of
the fenestra is the evidence of the filtration
function of the glomerulus.
2. Bowman’s Capsule
Bowman’s capsule encloses the glomerulus. The
structure of Bowman’s capsule is like a funnel
with filter paper. Its diameter is 200
μ.
It is formed by two layers:
1. The inner visceral layer
2. The outer parietal layer.
TABLE 35-1: Features of two types of nephron
Features
Cortical nephron
Juxtamedullary nephrons
Situation of renal corpuscle
Outer cortex near the periphery
Inner cortex near medulla
Loop of Henle
Short
Long
Hairpin bend penetrates only
Hairpin bend penetrates up to the
up to outer zone of medulla
tip of papilla
Blood supply to tubule
Peritubular capillaries
Vasa recta
Function
Formation of urine
Mainly the concentration of urine
and formation of urine
FIGURE 35-3: Renal corpuscle
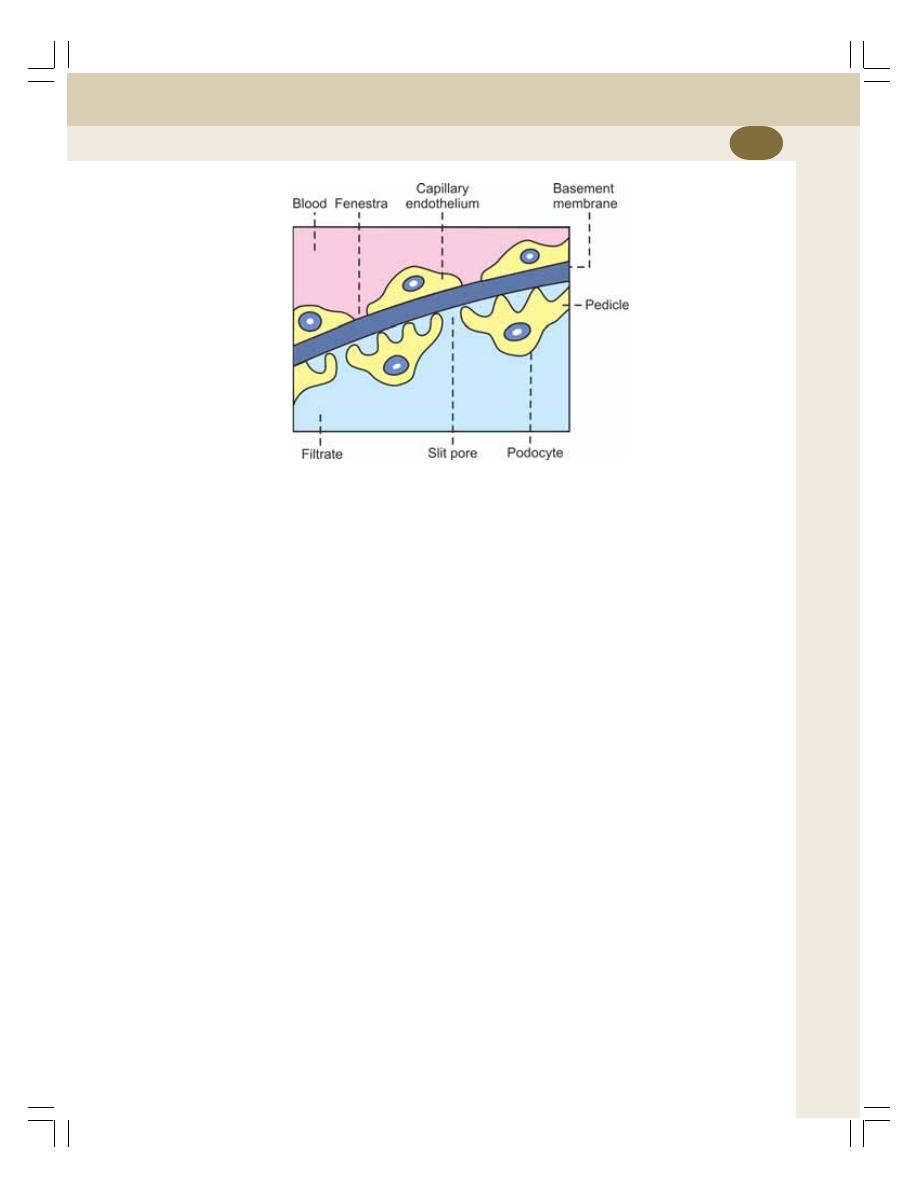
Chapter 35 Nephron and Juxtaglomerular Apparatus
211
Visceral layer covers the glomerular
capillaries. It is continued as the parietal layer
at the visceral pole. The parietal layer is continued
with the wall of the tubular portion of nephron.
The cleft like space between the visceral and
parietal layers is continued as the lumen of the
tubular portion.
Histology
Both the layers of Bowman’s capsule are
composed of a single layer of flattened epithe-
lial cells resting on a basement membrane. The
basement membrane of the visceral layer fuses
with the basement membrane of glomerular
capillaries on which the capillary endothelial
cells are arranged. Thus, the basement mem-
branes, which are fused together, form the
separation between the glomerular capillary
endothelium and the epithelium of visceral layer
of Bowman’s capsule.
The epithelial cells of the visceral layer fuse
with the basement membrane but the fusion is
not complete. Each cell is connected with the
basement membrane by cytoplasmic extensions
of epithelial cells called pedicles or feet. These
pedicles are arranged in an interdigitating manner
leaving small cleft like spaces in between. The
cleft like space is called slit pore. The epithelial
cells with pedicles are called podocytes
(Fig. 35-4).
TUBULAR PORTION OF NEPHRON
The tubular portion of nephron is the continuation
of Bowman’s capsule. It is made up of three
parts:
1. The proximal convoluted tubule
2. Loop of Henle
3. The distal convoluted tubule
PROXIMAL CONVOLUTED TUBULE
It is the coiled portion arising from Bowman’s
capsule. It is situated in the cortex. It is continued
as descending limb of loop of Henle. Length of
proximal convoluted tubule is 14 mm and the
diameter is 55
μ.
Histology
Proximal convoluted tubule is formed by single
layer of cuboidal epithelial cells. The special
feature of these cells is the presence of hair like
projections directed towards the lumen of the
tubule. Because of the presence of these pro-
jections, the epithelial cells are called brush
bordered cells.
FIGURE 35-4: Filtering membrane in renal corpuscle. It is formed by capillary endothelium on one
side (pink) and visceral layer of Bowman’s capsule (light blue) on the other side
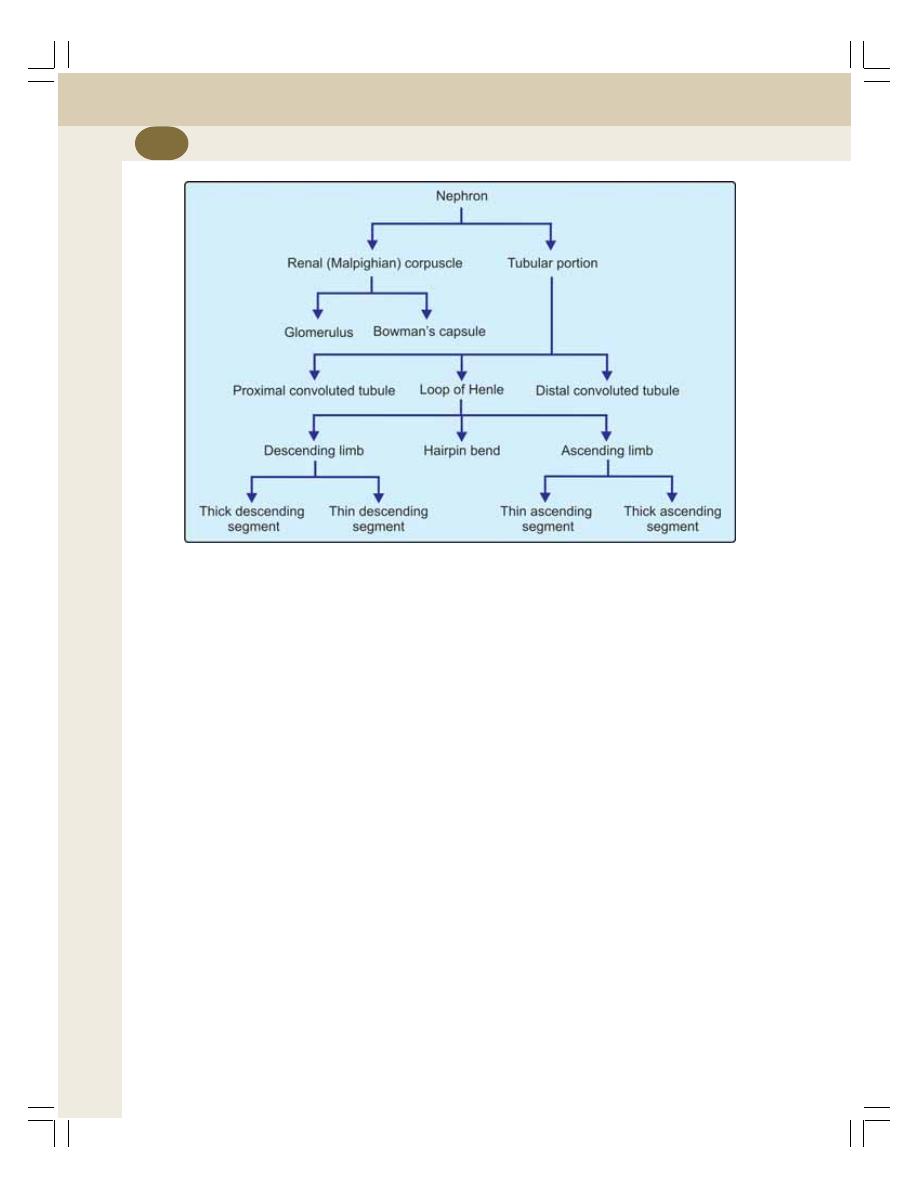
Renal Physiology and Skin
212
LOOP OF HENLE
Loop of Henle consists of:
i.
Descending limb
ii. Hairpin bend
iii. Ascending limb
Descending Limb
Descending limb of loop of Henle is made up of
thick descending segment and thin descending
segment. The thick descending segment is the
direct continuation of the proximal convoluted
tubule. It descends down into medulla. It has a
length of 6 mm and a diameter of 55
μ. The thick
descending segment of Henle’s loop is continued
as thin descending segment (Fig. 35-5).
Hairpin Bend
The thin descending segment is continued as
hairpin bend of the loop. The hairpin bend is
continued as the ascending segment of loop of
Henle.
Ascending Limb
Ascending limb of Henle’s loop has two parts,
thin ascending segment and thick ascending
segment. Thin ascending segment is the conti-
nuation of hairpin bend.
The total length of thin descending segment,
hairpin bend and thin ascending segment of
Henle’s loop 10 to 15 mm and the diameter is
15
μ.
The thin ascending segment is continued as
thick ascending segment. It is about 9 mm long
with a diameter of 30
μ. Thick ascending seg-
ment ascends to the cortex and continues as
distal convoluted tubule.
Length and Extent of Loop of Henle
The length and the extent of the loop of Henle
vary in different nephrons.
1. In cortical nephrons, it is short and the hairpin
bend penetrates only up to outer medulla
FIGURE 35-5: Parts of nephron
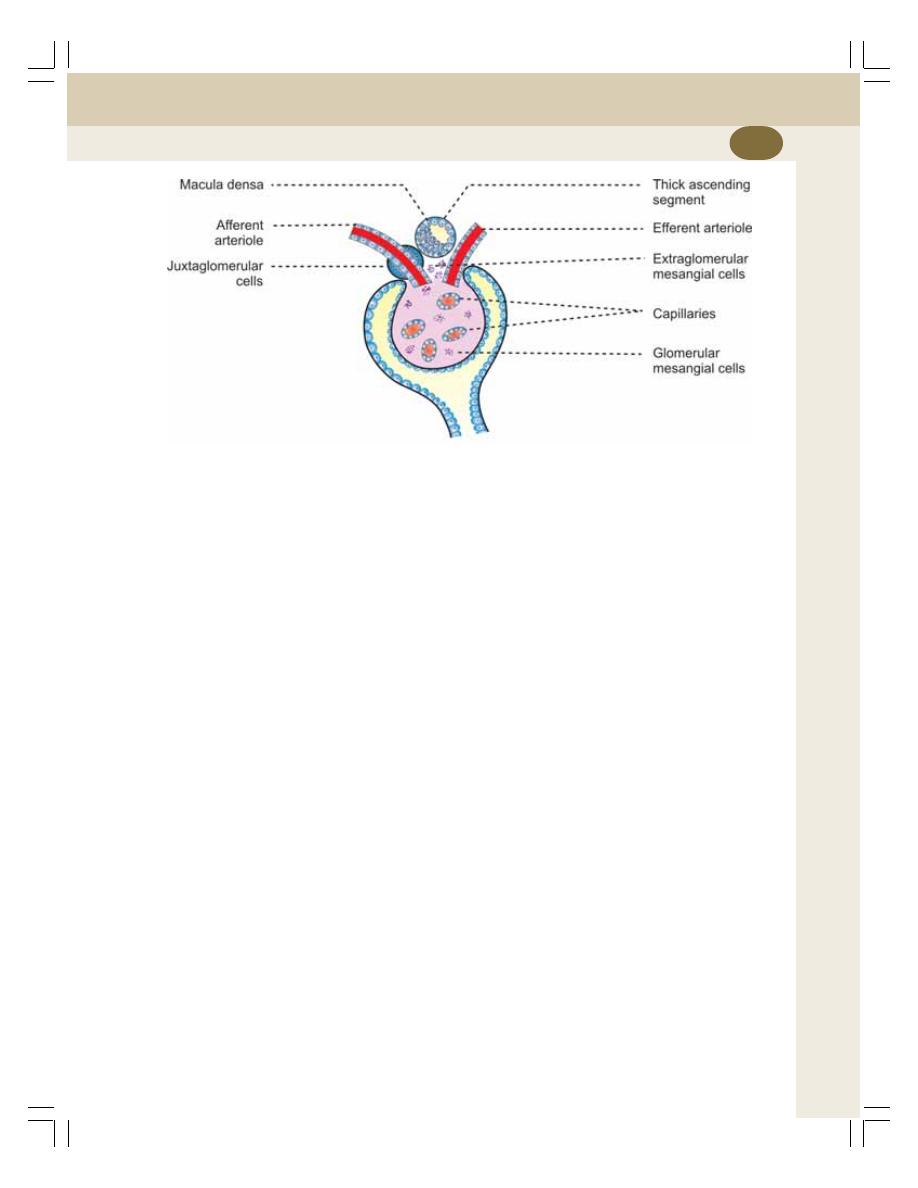
Chapter 35 Nephron and Juxtaglomerular Apparatus
213
2. In juxtamedullary nephrons, this is long and
the hairpin bend extends deep into the inner
medulla. In some nephrons it even runs up
to the papilla.
Histology
Thick descending segment is formed by brush
bordered cuboidal epithelial cells.
Thin descending segment, hairpin bend and
thin ascending segment are lined by flattened
epithelial cells without brush border. Thick
ascending segment is lined by cuboidal epithelial
cells without brush border.
The terminal portion of thick ascending
segment which runs between the afferent and
efferent arterioles of the same nephrons forms
the macula densa. Macula densa is the part of
juxtaglomerular apparatus (See below).
DISTAL CONVOLUTED TUBULE
It is the continuation of thick ascending segment
and occupies the cortex of kidney. It is continued
as collecting duct. The length of the distal
convoluted tubule is 14.5 to 15 mm. It has a
diameter of 22 to 50
μ.
Histology
Distal convoluted tubule is lined by single layer
of cuboidal epithelial cells without brush border.
The epithelial cells in distal convoluted tubule are
called intercalated cells (I cells).
COLLECTING DUCT
The distal convoluted tubule continues as the
initial or arched collecting duct, which is in cortex.
The lower part of the collecting duct lies in
medulla. Seven to ten initial collecting ducts unite
to form the straight collecting duct, which passes
through medulla.
The length of the collecting duct is 20 to 22
mm. The diameter of collecting duct varies
between 40 and 200
μ.
Histology
The collecting duct is formed by cuboidal or
columnar epithelial cells. The epithelial cells of
collecting duct are of two types:
1. The principal or P cells
2. Intercalated or I cells.
Passage of Urine
At the inner zone of medulla, the straight
collecting ducts from each medullary pyramid
unite to form papillary ducts or ducts of Bellini,
which open into the papilla. Papilla collects the
urine from each medullary pyramid and drains
into a minor calyx. Three or four minor calyces
unite to form one major calyx. Each kidney has
FIGURE 35-6: Juxtaglomerular apparatus

Renal Physiology and Skin
214
got about 8 minor calyces and 2 to 3 major
calyces. The major calyces open into the pelvis
of the ureter. The pelvis is the expanded portion
of ureter present in the renal sinus. Through
ureter, urine enters the urinary bladder.
JUXTAGLOMERULAR APPARATUS
DEFINITION
Juxtaglomerular apparatus is a specialized organ
situated near the glomerulus of each nephron
(juxta = near).
STRUCTURE OF
JUXTAGLOMERULAR APPARATUS
The juxta
glomerular apparatus is formed by three
different structures (Fig. 35-6):
1. Macula densa
2. Extraglomerular mesangial cells
3. Juxtaglomerular cells.
1. Macula Densa
Macula densa is the terminal portion of thick
ascending segment of Henle’s loop that runs in
between afferent and efferent arterioles of the
same nephron. Actually, it is very close to afferent
arteriole. In this part of thick ascending segment,
the cuboidal epithelial cells are tightly packed.
2. Extraglomerular Mesangial Cells
These cells are situated in the triangular region
bound by afferent arteriole, efferent arteriole and
macula densa. These cells are also called
agranular cells, lacis cells.
Glomerular mesangial cells
Glomerular mesangial cells or intraglomerular
mesangial cells are situated in between the
glomerular capillaries and form a cellular network
which supports the capillary loops. These cells
are contractile in nature and play an important
role in regulating the glomerular filtration.
The glomerular mesangial cells are also
phagocytic and secrete matrix of glomerular
interstitium, prostaglandins and cytokines.
3. Juxtaglomerular Cells
Juxtaglomerular cells are specialized smooth
muscle cells situated in the wall of afferent
arteriole just before it enters the Bowman’s
capsule. This part of the afferent arteriole is
thickened like a cuff and it is called polar cushion
or polkissen. Because of the presence of
secretary granules in their cytoplasm, the
juxtaglomerular cells are also called granular
cells.
FUNCTIONS OF JUXTAGLOMERULAR
APPARATUS
The primary function of juxtaglomerular
apparatus is the secretion of hormonal
substances. It also regulates the glomerular
blood flow and glomerular filtration rate.
1. Secretion of Renin
The juxtaglomerular cells secrete renin. Renin
is a peptide with 340 amino acids. Along with
angiotensins, renin forms the renin – angiotensin
system which is a hormone system that plays
an important role in the maintenance of blood
pressure (Chapter 65).
Renin–Angiotensin system
When renin is released into the blood, it acts on
angiotensinogen and converts it into angiotensin
I. Angiotensin I is converted into angiotensin II
by the activity of angiotensin converting enzyme
(ACE) secreted from lungs. Most of the
conversion of angiotensin I into angiotensin II
takes place in lungs.
Angiotensin II has a short half-life of about
1-2 minutes. Then it is degraded into angiotensin
III by angiotensinases which are present in RBCs
and vascular beds in many tissues. Finally,
angiotensin III is converted into angiotensin IV.
Actions of angiotensins
Angiotensin I
It is physiologically inactive and serves only as
the precursor of angiotensin II.

Chapter 35 Nephron and Juxtaglomerular Apparatus
215
Angiotensin II
Angiotensin II is the most active form. Its actions
are:
1. Angiotensin II increases arterial blood
pressure by causing vasoconstriction and
inhibiting baroreceptor reflex
2. It stimulates zona glomerulosa of adrenal
cortex to secrete aldosterone
3. Angiotensin II regulates glomerular filtration
4. It increases sodium reabsorption from renal
tubules
5. It increases water intake by stimulating the
thirst center
6. It increases secretion of antidiuretic hormone
(ADH) from hypothalamus.
Angiotensin III
Angiotensin III increases the blood pressure and
stimulates aldosterone secretion from adrenal
cortex.
Angiotensin IV
It also has adrenal cortical stimulating and
vasopressor activities.
2. Secretion of Other Substances
The extraglomerular mesangial cells of
juxtaglomerular apparatus secrete prostaglandin
(Chapter 51). In vitro secretion of cytokines like
IL-2 and TNF by the mesangial cells is observed
recently. Macula densa secretes thromboxane
A
2
.
3. Regulation of Glomerular Blood Flow
and Glomerular Filtration Rate
Macula densa of juxtaglomerular apparatus
plays an important role in the feedback
mechanism called tubuloglomerular feedback
mechanism, which regulates the renal blood flow
and glomerular filtration rate (Refer Chapter 37
for details).

INTRODUCTION
RENAL BLOOD VESSELS
MEASUREMENT OF RENAL BLOOD FLOW
REGULATION OF RENAL BLOOD FLOW
SPECIAL FEATURES OF RENAL CIRCULATION
Renal Circulation
36
INTRODUCTION
Blood vessels of kidneys are highly specialized
to facilitate the functions of the nephrons in the
formation of urine. Renal arteries supply the
blood to the kidneys.
In the adults, during resting conditions both
the kidneys receive 1,300 mL of blood per minute
or about 26% of the cardiac output. Kidneys are
the second organs to receive maximum blood
flow, the first organ being the liver which receives
1,500 mL per minute. The maximum blood supply
to kidneys has got the functional significance.
RENAL BLOOD VESSELS
Renal artery arises directly from abdominal aorta
and enters the kidney through the hilus. While
passing through renal sinus, the renal artery
divides into many segmental arteries, which
subdivide into interlobar arteries (Fig. 36-1).
Each interlobar artery passes in between the
medullary pyramids. At the base of the pyramid,
it turns and runs parallel to the base of pyramid
forming arcuate artery.
Each arcuate artery gives rise to interlobular
arteries. The interlobular arteries run through the
renal cortex perpendicular to arcuate artery. From
each interlobular artery, numerous afferent arte-
rioles arise.
The afferent arteriole enters the Bowman’s
capsule and forms glomerular capillary tuft. The
afferent arteriole divides into 4 or 5 large
capillaries. Each large capillary divides into small
capillaries, which form the loops. And, the
capillary loops unite to form the efferent arteriole,
which leaves the Bowman’s capsule.
The efferent arterioles form a second capillary
network called peritubular capillaries which
surround the tubular portions of the nephrons.
FIGURE 36-1: Renal blood vessels
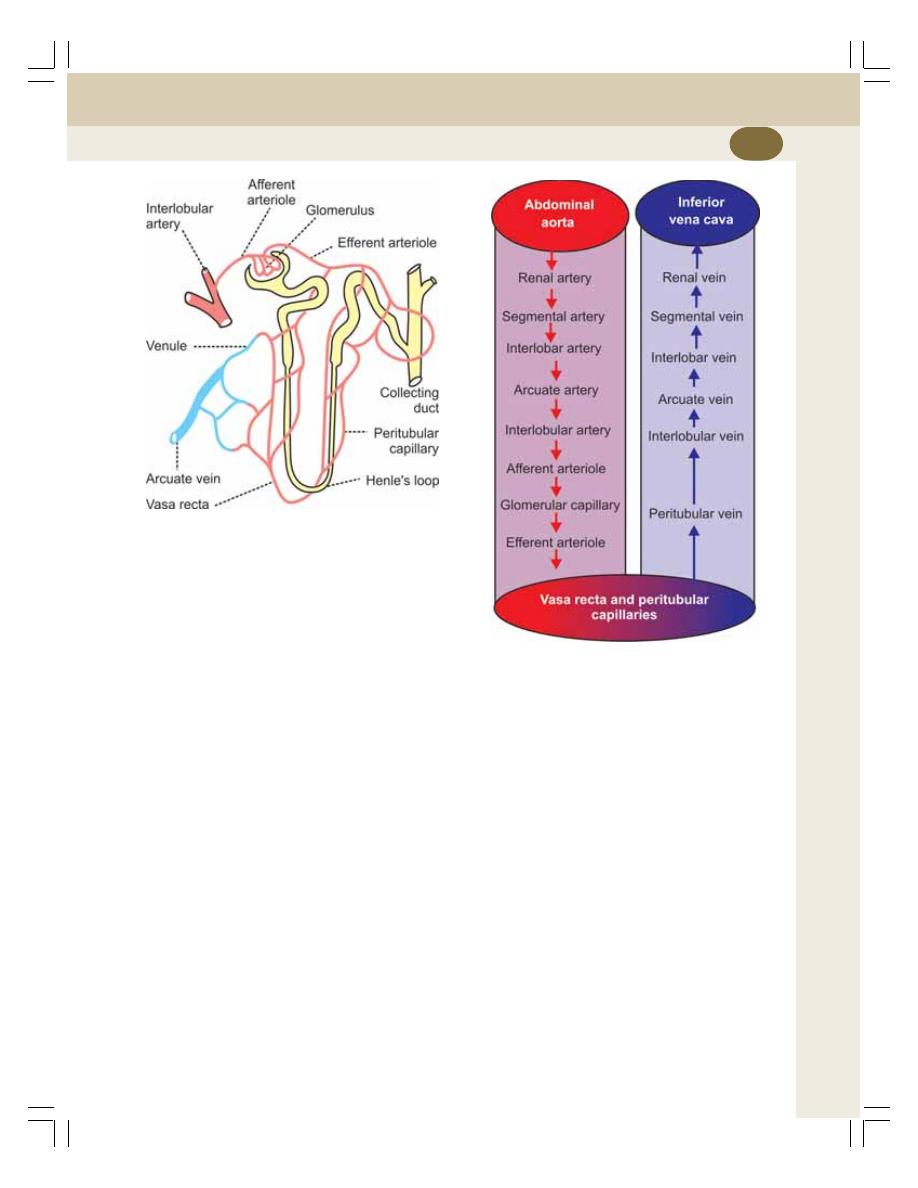
Chapter 36 Renal Circulation
217
Thus, the renal circulation forms a portal system
by the presence of two sets of capillaries –
glomerular capillaries and peritubular
capillaries.
The peritubular capillaries are found around
the tubular portion of cortical nephrons only. The
tubular portion of juxtamedullary nephrons are
supplied by some specialized capillaries called
vasa recta. Vasa recta arise directly from the
efferent arteriole of the juxtamedullary nephrons
and run parallel to the renal tubule into the
medulla and ascend up towards the cortex
(Fig. 36-2).
The peritubular capillaries and vasa recta
drain into the venous system. Venous system
starts with peritubular venules and continues as
interlobular veins, arcuate veins, interlobar veins,
segmental veins and finally the renal vein (Fig.
36-3).
Renal vein leaves the kidney through the hilus
and joins inferior vena cava.
MEASUREMENT OF RENAL BLOOD
FLOW
The blood flow to kidneys is measured by using
plasma clearance of para-aminohippuric acid
(Refer Chapter 40).
REGULATION OF RENAL BLOOD
FLOW
The regulation of renal blood flow is mostly by
autoregulation. The nerves innervating renal
blood vessels have no significant role in this.
AUTOREGULATION
The intrinsic ability of an organ to regulate its
own blood flow is called autoregulation. Auto-
regulation is present in some vital organs in the
body such as brain, heart and kidneys. It is highly
significant and more efficient in kidneys.
Renal Autoregulation
Renal autoregulation is important to maintain the
glomerular filtration rate (GFR). Blood flow to
kidneys remains normal even when the mean
FIGURE 36-2: Renal capillaries
FIGURE 36-3: Schematic diagram showing
renal blood flow

Renal Physiology and Skin
218
arterial blood pressure vary widely between 60
and 180 mm Hg. This helps to maintain normal
GFR.
Two mechanisms are involved in renal auto-
regulation:
1. Myogenic response
2. Tubuloglomerular feedback.
1. Myogenic Response
Whenever the blood flow to kidneys increases,
it stretches the elastic wall of the afferent arte-
riole. Stretching of vessel wall increases the flow
of calcium ions from extracellular fluid into the
cells. The influx of calcium ions leads to the con-
traction of smooth muscles in afferent arteriole
which causes constriction of afferent arteriole.
So, the blood flow is decreased.
2. Tubuloglomerular Feedback
Macula densa plays an important role in tubulo-
glomerular feedback which controls the renal
blood flow and GFR. Refer Chapter 37 for details.
SPECIAL FEATURES OF RENAL
CIRCULATION
The renal circulation has some special features
to cope up with the functions of the kidneys. Such
special features are:
1. The renal arteries arise directly from the
aorta. So the pressure in aorta is very high
and it facilitates a high blood flow to the renal
parenchyma.
2. Kidneys receive about 1,300 mL of blood per
minute, i.e. about 26% of cardiac output.
Kidneys are the second organs to receive
maximum blood flow, the first organ being the
liver which receives 1,500 mL per minute, i.e.
about 30% of cardiac output.
3. Whole amount of blood which flows to kidney
has to pass through the glomerular capillaries
before entering the venous system. Because
of this, the blood is completely filtered at the
renal glomeruli.
4. Renal circulation has a portal system, i.e. a
double network of capillaries namely glo-
merular capillaries and peritubular capillaries.
5. Renal glomerular capillaries form high pres-
sure bed with a pressure of 60 to 70 mm Hg.
It is much greater than the capillary pressure
elsewhere in the body, which is only about
25 to 30 mm Hg. High pressure is maintained
in the glomerular capillaries because the
diameter of afferent arteriole is more than that
of efferent arteriole. The high capillary
pressure augments glomerular filtration.
6. The peritubular capillaries form a low
pressure bed with a pressure of 8 to 10 mm
Hg. This low pressure helps tubular reabsorp-
tion.
7. The autoregulation of renal blood flow is well
established.

INTRODUCTION
GLOMERULAR FILTRATION
INTRODUCTION
GLOMERULAR FILTRATION RATE (GFR)
FILTRATION FRACTION
PRESSURES DETERMINING FILTRATION
FACTORS REGULATING (AFFECTING) GFR
TUBULAR REABSORPTION
INTRODUCTION
SELECTIVE REABSORPTION
MECHANISM OF REABSORPTION
SITE OF REABSORPTION
REGULATION OF TUBULAR REABSORPTION
TRANSPORT MAXIMUM – Tm VALUE
RENAL THRESHOLD
REABSORPTION OF IMPORTANT SUBSTANCES
TUBULAR SECRETION
INTRODUCTION
SUBSTANCES SECRETED IN DIFFERENT SEGMENTS OF RENAL TUBULES
SUMMARY OF URINE FORMATION
Urine Formation
37
INTRODUCTION
Urine formation is a blood cleansing function.
Normally, about 26% of cardiac output enters the
kidneys to get rid of unwanted substances.
Kidneys excrete the unwanted substances in
urine.
Normally, about 1 to 1.5 L of urine is formed
every day.
The mechanism of urine formation includes
three processes:
I. Glomerular filtration
II. Tubular reabsorption
III. Tubular secretion.
Among these three processes filtration is the
function of the glomerulus. Reabsorption and
secretion are the functions of tubular portion of
the nephron (Fig. 37-1).
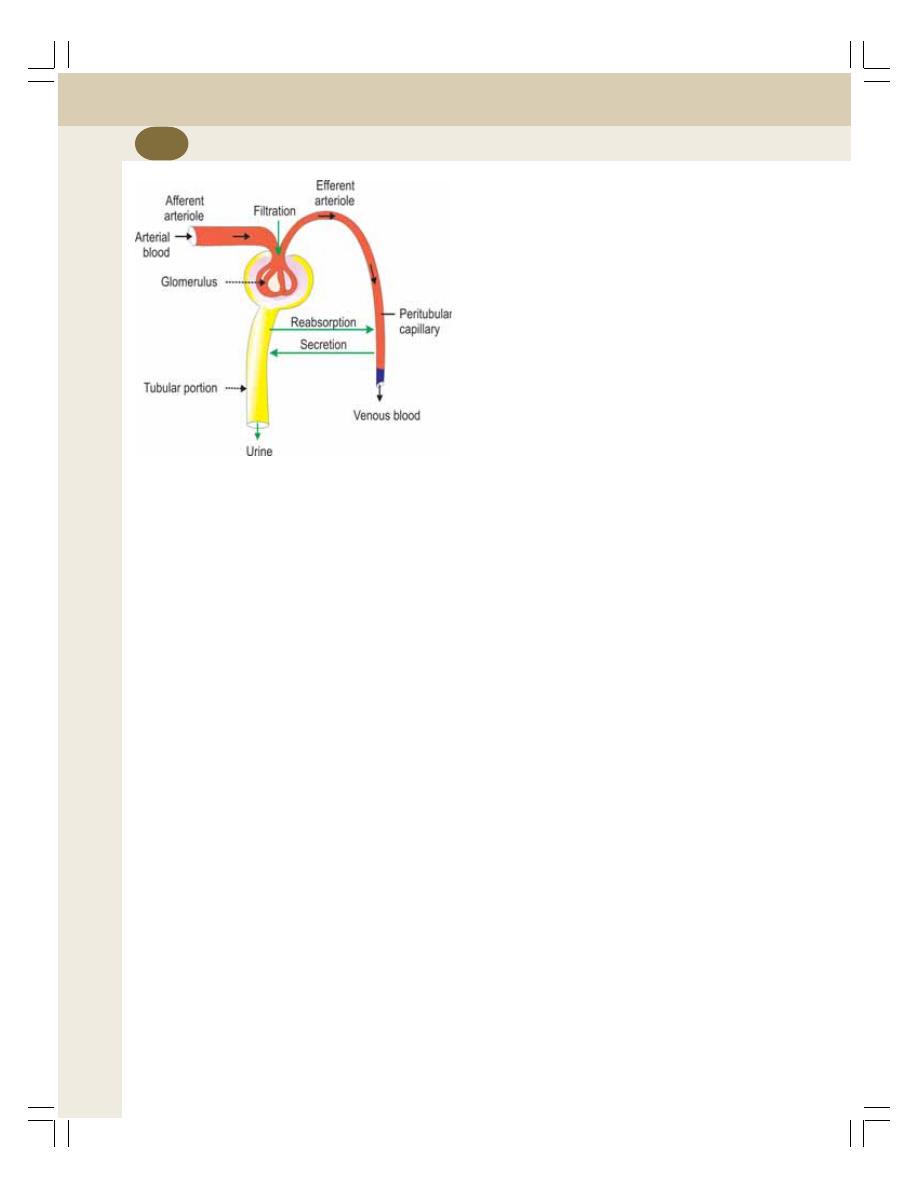
Renal Physiology and Skin
220
GLOMERULAR FILTRATION
INTRODUCTION
Glomerular filtration is the process by which the
blood that passes through glomerular capillaries
is filtered through the filtration membrane. It is
the first process of urine formation. The structure
of filtration membrane is well suited for this.
Filtration Membrane
It is formed by three layers:
1. The glomerular capillary membrane
2. Basement membrane
3. Visceral layer of Bowman’s capsule.
1. Glomerular Capillary Membrane
The glomerular capillary membrane is formed by
single layer of endothelial cells which are
attached to the basement membrane. The capi-
llary membrane has many pores called fenestra
or filtration pores with a diameter of 0.1
μ.
2. Basement Membrane
The basement membrane of glomerular capi-
llaries fuses with the basement membrane of
visceral layer of Bowman’s capsule. The base-
ment membrane separates the endothelium of
glomerular capillary and the epithelium of visceral
layer of Bowman’s capsule.
3. Visceral Layer of Bowman’s Capsule
This is composed of a single layer of flattened
epithelial cells resting on a basement membrane.
Each cell is connected with the basement
membrane by cytoplasmic extensions called
pedicles or feet. The pedicles are arranged in
an interdigitating manner leaving small cleft like
spaces in between. The cleft like space is called
slit pore. Filtration takes place through these slit
pores. The epithelial cells with pedicles are called
podocytes (Fig. 35-4).
Process of Glomerular Filtration
When the blood passes through the glomerular
capillaries, the plasma is filtered into the
Bowman’s capsule. All the substances of
plasma are filtered except plasma proteins. The
filtered fluid is called glomerular filtrate.
Ultrafiltration
The glomerular filtration is called ultrafiltration
because even the minute particles are filtered.
But, the plasma proteins are not filtered due to
their large molecular size. The protein molecules
are larger than the slit pores present in the
endothelium of capillaries. Thus, the glomerular
filtrate contains all the substances of plasma
except the plasma proteins.
GLOMERULAR FILTRATION RATE (GFR)
Glomerular filtration rate (GFR) is defined as the
total quantity of filtrate formed in all nephrons of
both the kidneys in the given unit of time.
The normal GFR is 125 mL per minute or
about 180 L per day.
FILTRATION FRACTION
Filtration fraction is the fraction (portion) of the
renal plasma which becomes the filtrate. It is the
ratio between renal plasma flow and glomerular
filtration rate. It is expressed in percentage.
FIGURE 37-1: Events of urine formation
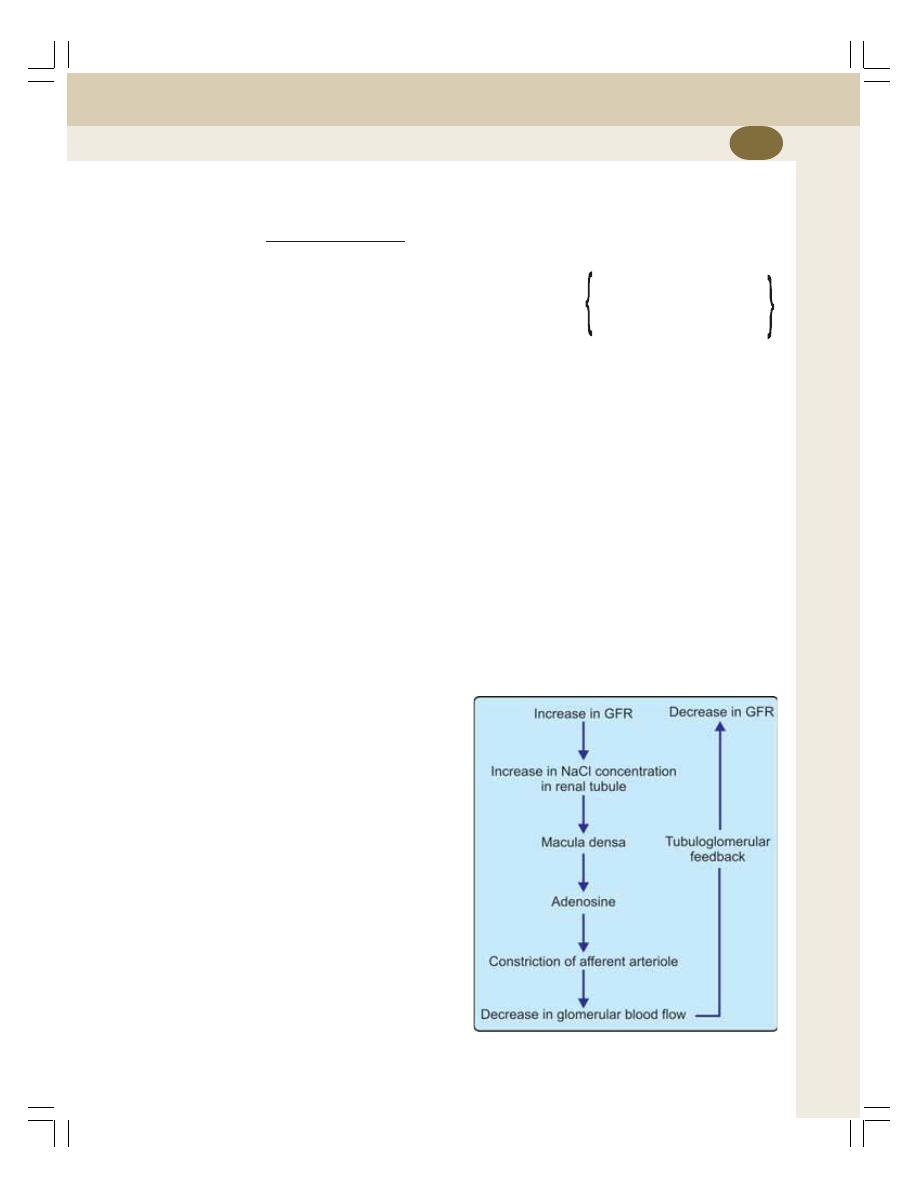
Chapter 37 Urine Formation
221
The normal filtration fraction varies from
15-20%.
GFR
Filtration fraction =
× 100
Renal plasma flow
125 ml/min
= —————— × 100
650 ml/min
=
19.2%.
The normal filtration fraction varies from
15 to 20%.
PRESSURES DETERMINING
FILTRATION
The pressures, which determine the GFR, are:
1. Glomerular capillary pressure
2. Colloidal osmotic pressure in the glomeruli
3. Hydrostatic pressure in the Bowman’s
capsule.
1. Glomerular Capillary Pressure
It is the pressure exerted by the blood in glo-
merular capillaries. It is about 60 mm Hg and,
varies between 45 and 70 mm Hg. Glomerular
capillary pressure is the highest capillary
pressure in the body. This pressure favors
glomerular filtration.
2. Colloidal Osmotic Pressure
It is exerted by plasma proteins in the glomeruli.
The plasma proteins are not filtered through the
glomerular capillaries and remain in the glo-
merular capillaries. These proteins develop the
colloidal osmotic pressure which is about 25 mm
Hg. It opposes glomerular filtration.
3. Hydrostatic Pressure in Bowman’s
Capsule
It is the pressure exerted by the filtrate in
Bowman’s capsule. It is also called capsular
pressure. It is about 15 mm Hg. It also opposes
glomerular filtration.
Net Filtration Pressure
Net filtration pressure is the balance between
pressure favoring filtration and pressures
opposing filtration. It is otherwise known as
effective filtration pressure or essential filtration
pressure.
The net filtration pressure =
Glomerular
Colloidal
Hydrostatic
capillary –
osmotic + pressure in
pressure
pressure
Bowman’s
capsule
= 60 – (25 + 15) = 20 mm Hg.
Normal net filtration pressure is about 20 mm
Hg, and, it varies between 15 and 20 mm Hg.
FACTORS REGULATING (AFFECTING)
GFR
1. Renal Blood Flow
It is the most important factor that is necessary
for glomerular filtration. GFR is directly propor-
tional to renal blood flow. The renal blood flow
itself is controlled by autoregulation. Refer pre-
vious chapter for details.
2. Tubuloglomerular Feedback
Tubuloglomerular feedback is the mechanism
that regulates GFR through renal tubule and
macula densa (Fig. 37-2). Macula densa of
FIGURE 37-2: Tubuloglomerular feedback

Renal Physiology and Skin
222
juxtaglomerular apparatus in the terminal portion
of thick ascending limb is sensitive to the sodium
chloride in the tubular fluid.
When glomerular filtrate passes through the
terminal portion of thick ascending segment,
macula densa acts like a sensor. It detects the
concentration of sodium chloride in the tubular
fluid and accordingly alters the glomerular blood
flow and GFR.
When the Concentration of Sodium Chloride
Increases in the Filtrate
When the concentration of sodium chloride
increases in the filtrate, macula densa releases
adenosine from ATP. Adenosine causes con-
striction of afferent arteriole. So the blood flow
through glomerulus decreases leading to
decrease in GFR.
When the Concentration of Sodium Chloride
Decreases in the Filtrate
When the concentration of sodium chloride
decreases in the filtrate, macula densa secretes
prostaglandin (PGE
2
), bradykinin and renin.
PGE
2
and bradykinin cause dilatation of
afferent arteriole. Renin induces the formation
of angiotensin II which causes constriction of
efferent arteriole. The dilatation of afferent arte-
riole and constriction of efferent arteriole leads
to increase in glomerular blood flow and GFR.
3. Glomerular Capillary Pressure
The GFR is directly proportional to glomerular
capillary pressure. The capillary pressure, in turn
depends upon the renal blood flow and arterial
blood pressure.
4. Colloidal Osmotic Pressure
The GFR is inversely proportional to colloidal
osmotic pressure which is exerted by plasma
proteins in the glomerular capillary blood. When
colloidal osmotic pressure increases as in case
of dehydration or increased plasma protein level,
GFR decreases. During hypoproteinemia, colloi-
dal osmotic pressure is low and GFR increases.
5. Hydrostatic Pressure in Bowman’s
Capsule
GFR is inversely proportional to this. The hydro-
static pressure in Bowman’s capsule increases
in conditions like obstruction of urethra and
edema of kidney beneath renal capsule.
6. Constriction of Afferent Arteriole
The constriction of afferent arteriole reduces the
blood flow to the glomerular capillaries which in
turn reduces GFR.
7. Constriction of Efferent Arteriole
If efferent arteriole is constricted, initially the GFR
increases because of stagnation of blood in the
capillaries. Later when all the substances are
filtered from this blood, further filtration does not
occur because, the efferent arteriolar constriction
prevents outflow of blood from glomerulus and
no fresh blood enters the glomerulus for filtration.
8. Systemic Arterial Pressure
Renal blood flow or GFR are not affected till the
mean arterial blood pressure is between 60 and
180 mm Hg. It is due to the autoregulatory
mechanism (Chapter 36). Variation in pressure
above 180 mm Hg or below 60 mm Hg affects
the renal blood flow and GFR accordingly
because the autoregulatory mechanism fails
beyond this range.
9. Sympathetic Stimulation
Afferent and efferent arterioles are supplied by
sympathetic nerves. The mild or moderate stimu-
lation of sympathetic nerves does not cause any
significant change either in renal blood flow or
GFR.
Strong sympathetic stimulation causes severe
constriction of the blood vessels by releasing the
neurotransmitter substance, noradrenaline. The
effect is more severe on the efferent arterioles
than on the afferent arterioles. So, initially there
is increase in filtration but later it decreases.

Chapter 37 Urine Formation
223
However, if the stimulation is continued for more
than 30 minutes, there is recovery of both renal
blood flow and GFR. It is because of reduction
in sympathetic neurotransmitter.
10. Surface Area of Capillary Membrane
GFR is directly proportional to the surface area
of the capillary membrane.
If the glomerular capillary membrane is affec-
ted as in the cases of some renal diseases, the
surface area for filtration decreases. So there is
reduction in GFR.
11. Permeability of Capillary Membrane
GFR is directly proportional to the permeability
of glomerular capillary membrane. In many
abnormal conditions like hypoxia, lack of blood
supply, presence of toxic agents, etc. the per-
meability of the capillary membrane increases.
In such conditions, even plasma proteins are
filtered and excreted in urine.
12. Contraction of Glomerular Mesangial
Cells
Glomerular mesangial cells are situated in
between the glomerular capillaries. Contraction
of these cells decreases surface area of capi-
llaries resulting in reduction in GFR.
13. Hormonal and Other Factors
Many hormones and other secretory factors alter
GFR by affecting the blood flow through glo-
merulus.
Factors increasing GFR by vasodilatation
i. Atrial natriuretic peptide
ii. Brain natriuretic peptide
iii. cAMP
iv. Dopamine
v. Endothelial derived nitric oxide
vi. Prostaglandin (PGE
2
).
Factors decreasing GFR by vasoconstriction
i. Angiotensin II
ii. Endothelines
iii. Noradrenaline
iv. Platelet activating factor
v. Platelet-derived growth factor
vi. Prostaglandin (PGF
2
).
TUBULAR REABSORPTION
INTRODUCTION
Tubular reabsorption is the process by which
water and other substances are transported from
renal tubules back to the blood. When the glo-
merular filtrate flows through the tubular portion
of nephron, both quantitative and qualitative
changes occur. Large quantity of water (more
than 99%), electrolytes and other substances are
reabsorbed by the tubular epithelial cells. The
reabsorbed substances move into the interstitial
fluid of renal medulla. And, from here, the sub-
stances move into the blood in peritubular capi-
llaries.
Since the substances are taken back into the
blood from the glomerular filtrate, the entire pro-
cess is called tubular reabsorption.
SELECTIVE REABSORPTION
Tubular reabsorption is known as selective
reabsorption because the tubular cells reabsorb
only the substances necessary for the body.
Essential substances such as glucose, amino
acids and vitamins are completely reabsorbed
from renal tubule. Whereas the unwanted
substances like metabolic waste products are
excreted through urine.
MECHANISM OF REABSORPTION
The basic transport mechanisms involved in
tubular reabsorption are of two types:
1. Active reabsorption
2. Passive reabsorption.
1. Active Reabsorption
Active reabsorption is the movement of mole-
cules against the electrochemical (uphill) gra-
dient. It needs liberation of energy which is
derived from ATP.

Renal Physiology and Skin
224
Substances reabsorbed actively
The substances reabsorbed actively from the
renal tubule are sodium, calcium, potassium,
phosphates, sulfates, bicarbonates, glucose,
amino acids, ascorbic acid, uric acid and ketone
bodies.
2. Passive Reabsorption
Passive reabsorption is the movement of mole-
cules along the electrochemical (downhill)
gradient. This process does not need energy.
Substances reabsorbed passively
The substances reabsorbed by passively are
chloride, urea and water.
SITE OF REABSORPTION
The reabsorption of the substances occurs in
almost all the segments of tubular portion of
nephron.
1. Substances Reabsorbed from Proximal
Convoluted Tubule
About 7/8 of the filtrate (about 88%) is reab-
sorbed in proximal convoluted tubule. The brush
border of the epithelial cell in proximal convolu-
ted tubule increases the surface area and
facilitates reabsorption.
Substances reabsorbed from proximal con-
voluted tubule are glucose, amino acids, sodium,
potassium, calcium, bicarbonates, chlorides,
phosphates, uric acid and water.
2. Substances Reabsorbed from Loop
of Henle
The substances reabsorbed from loop of Henle
are sodium and chloride.
3. Substances Reabsorbed from Distal
Convoluted Tubule
Sodium, calcium, bicarbonate and water are
reabsorbed from distal convoluted tubule.
REGULATION OF TUBULAR
REABSORPTION
Tubular reabsorption is regulated by three
factors:
1. Glomerulotubular balance
2. Hormonal factors
3. Nervous factors.
1. Glomerulotubular Balance
Glomerulotubular balance is the balance between
the filtration and reabsorption of solutes and
water in kidney. When GFR increases, the tubular
load of solutes and water in the proximal
convoluted tubule is increased. It is followed by
increase in the reabsorption of solutes and water.
This process helps in the constant reabsorption
of solute particularly sodium and water from renal
tubule.
Mechanism of Glomerulotubular Balance
Glomerulotubular balance occurs because of
osmotic pressure in the peritubular capillaries.
When GFR increases, more amount of plasma
proteins accumulate in the glomerulus. Conse-
quently, the osmotic pressure increases in the
blood by the time it reaches efferent arteriole and
peritubular capillaries. The elevated osmotic
pressure in the peritubular capillaries increases
reabsorption of sodium and water from the tubule
into the capillary blood.
2. Hormonal Factors
The hormones which regulate GFR are listed in
Table 37-1.
3. Nervous Factor
Activation of sympathetic nervous system
increases the tubular reabsorption (particularly
of sodium) from renal tubules. It also increases
the tubular reabsorption indirectly by stimulating
secretion of renin from juxtaglomerular cell. Renin
causes formation of angiotensin II which
increases the sodium reabsorption (Chapter 35).
Chapter 37 Urine Formation
225
TRANSPORT MAXIMUM – Tm VALUE
Tubular transport maximum or Tm is the rate at
which a substance is reabsorbed from the renal
tubule. For example, the transport maximum for
glucose, (TmG) is 375 mg/minute in adult males
and about 300 mg/minute in adult females.
RENAL THRESHOLD
Renal threshold is the plasma concentration at
which a substance appears first in urine. Every
substance has a threshold level in plasma or
blood. Below that threshold level, the substance
is completely reabsorbed and does not appear
in urine. When the concentration of that sub-
stance reaches the threshold, the excess amount
is not reabsorbed and, so it appears in urine. This
level is called the renal threshold of that sub-
stance.
For example, the renal threshold for glucose
is 180 mg/dL. That is, glucose is completely
reabsorbed from tubular fluid if its concentration
in blood is below 180 mg/dL. So, the glucose
does not appear in urine. When the blood level
of glucose reaches 180 mg/dL it is not reab-
sorbed completely and appears in urine.
REABSORPTION OF IMPORTANT
SUBSTANCES
Reabsorption of Sodium
From the glomerular filtrate, 99% of sodium is
reabsorbed. Two-thirds of sodium is reabsorbed
in proximal convoluted tubule and remaining
one-third in other segments (except descending
limb) and collecting duct.
Sodium reabsorption occurs:
i. In exchange for hydrogen ion by antiport
(sodium counterport protein) – in proximal
convoluted tubules
ii. Along with other substances like glucose
and amino acids by symport (sodium co-
transport protein) – in other segments and
collecting duct.
Reabsorption of Water
Reabsorption of water occurs from proximal and
distal convoluted tubules and in collecting duct.
Reabsorption of Water from Proximal
Convoluted Tubule – Obligatory Water
Reabsorption
Obligatory reabsorption is the type of water
reabsorption in proximal convoluted tubule, which
is secondary to sodium reabsorption. When
sodium is reabsorbed from the tubule, the osmo-
tic pressure decreases. It causes osmosis of
water from renal tubule.
Reabsorption of Water from Distal
Convoluted Tubule and Collecting Duct –
Facultative Water Reabsorption
Facultative reabsorption is the type of water
reabsorption in distal convoluted tubule and
collecting duct that occurs by the activity of
antidiuretic hormone (ADH). Normally, the distal
convoluted tubule and the collecting duct are not
permeable to water. But in the presence of
antidiuretic hormone (ADH), these segments
become permeable to water and so it is reab-
sorbed.
TABLE 37-1: Hormones regulating
tubular reabsorption
Hormone
Action
Aldosterone
Increases sodium reabsorption in
ascending limb, distal convoluted
tubule and collecting duct
Angiotensin II
Increases sodium reabsorption in
proximal tubule, thick ascending
limb, distal tubule and collecting
duct (mainly in proximal con-
voluted tubule)
Antidiuretic
Increases water reabsorption in
hormone
distal convoluted tubule and
collecting duct
Atrial natriuretic Decreases sodium reabsorption
factor
Brain natriuretic Decreases sodium reabsorption
factor
Parathormone
Increases reabsorption of calcium,
magnesium and hydrogen
Decreases phosphate
reabsorption
Calcitonin
Decreases calcium reabsorption

Renal Physiology and Skin
226
Mechanism of Action of Antidiuretic Hormone
ADH combines with V
2
receptors in the tubular
epithelial membrane and activates adenyl
cyclase, to form cyclic AMP. This cyclic AMP
increases the permeability of the tubules for
water by activating aquaporins which form the
water channels.
Aquaporins
Aquaporins (AQP) are the membrane proteins
which function as water channels. ADH increases
water reabsorption in distal convoluted tubules
and collecting ducts by regulating the aquaporins.
Reabsorption of Glucose
Glucose is completely reabsorbed in the proximal
convoluted tubule. It is transported by secondary
active transport (sodium co-transport) mecha-
nism. Glucose and sodium bind to a common
carrier protein in the luminal membrane of tubular
epithelium and enter the cell. The carrier protein
is called sodium-dependant glucose transporter
2 (SGLT 2). From tubular cell glucose is trans-
ported into medullary interstitium by another
carrier protein called glucose transporter 2
(GLUT 2).
Renal Threshold for Glucose
Renal threshold for glucose is 180 mg/dL in
venous blood. When the blood level reaches 180
mg/dL glucose is not reabsorbed completely and
appears in urine.
Tubular Maximum for Glucose (TmG)
In adult male TmG is 375 mg/minute and in adult
females it is about 300 mg/minute (see above).
Reabsorption of Bicarbonates
Bicarbonate is reabsorbed actively, mostly in
proximal tubule. It is reabsorbed in the form of
carbon dioxide.
Bicarbonate is mostly present as sodium
bicarbonate in the filtrate. Sodium bicarbonate
dissociates into sodium and bicarbonate ions in
the tubular lumen.
Sodium diffuses into tubular cell in exchange
of hydrogen. Bicarbonate combines with hydro-
gen to form carbonic acid. Carbonic acid dis-
sociates into carbon dioxide and water in the
presence of carbonic anhydrase. Carbon dioxide
and water enter the tubular cell.
In the tubular cells, carbon dioxide combines
with water to form carbonic acid. It immediately
dissociates into hydrogen and bicarbonate.
Bicarbonate from the tubular cell enters the
interstitium. There it combines with sodium to
form sodium bicarbonate.
TUBULAR SECRETION
INTRODUCTION
Tubular secretion is the process by which the
substances are transported from blood into
renal tubules. It is also called tubular excretion.
SUBSTANCES SECRETED IN
DIFFERENT SEGMENTS OF RENAL
TUBULES
1. Potassium is secreted actively by sodium-
potassium pump in proximal and distal
convoluted tubules and collecting ducts.
2. Ammonia is secreted in the proximal con-
voluted tubule.
3. Hydrogen ions are secreted in the proximal
and distal convoluted tubules. Maximum
hydrogen ion secretion occurs in proximal
tubule.
Thus, urine is formed in the nephron by the
processes of glomerular filtration, selective
reabsorption and tubular secretion.
SUMMARY OF URINE FORMATION
Urine formation takes place in three processes.
Glomerular Filtration
Plasma is filtered in glomeruli and the sub-
stances reach the renal tubules along with water
as filtrate.
Tubular Reabsorption
Ninety nine percent of filtrate is reabsorbed in
different segments of renal tubules.
Tubular Secretion
Some substances are secreted from blood into
the renal tubule.
With all these changes filtrate becomes urine.

INTRODUCTION
FORMATION OF DILUTE URINE
FORMATION OF CONCENTRATED URINE
MEDULLARY GRADIENT
MEDULLARY HYPEROSMOLARITY
DEVELOPMENT AND MAINTENANCE OF MEDULLARY GRADIENT
COUNTERCURRENT MECHANISM
COUNTERCURRENT FLOW
COUNTERCURRENT MULTIPLIER
COUNTERCURRENT EXCHANGER
ROLE OF ADH
SUMMARY OF URINE CONCENTRATION
INTRODUCTION
Osmolarity of glomerular filtrate is same as
that of plasma and it is 300 mOsm/L. But,
normally urine is concentrated and its osmolarity
is four times more than that of plasma, i.e.
1200 mOsm/L. Osmolarity of urine depends upon
two factors:
1. Water content in the body
2. Antidiuretic hormone.
FORMATION OF DILUTE URINE
Mechanism of urine formation is the same for
dilute urine and concentrated urine till the fluid
reaches the distal convoluted tubule. Whether it
has to be excreted as dilute urine or concen-
trated urine depends upon the water content
of the body.
If water content in body is more, kidney
excretes excess water making the urine dilute.
It is achieved by the inhibition of ADH secretion.
ADH is secreted by posterior pituitary
(Chapter 45). The stimulus for its secretion is
the decreased body fluid volume and/or increa-
sed sodium concentration (hyperosmolarity).
ADH increases the water reabsorption from distal
convoluted tubule and collecting duct resulting
in concentration of urine.
But when, the volume of body fluid increases
or the osmolarity of body fluid decreases, ADH
secretion stops. So water reabsorption from renal
tubules does not take place (see Fig. 38-3). This
leads to excretion of large amount of water in
urine making the urine dilute. It brings back the
normalcy of water content and osmolarity of body
fluids.
Concentration of Urine
38
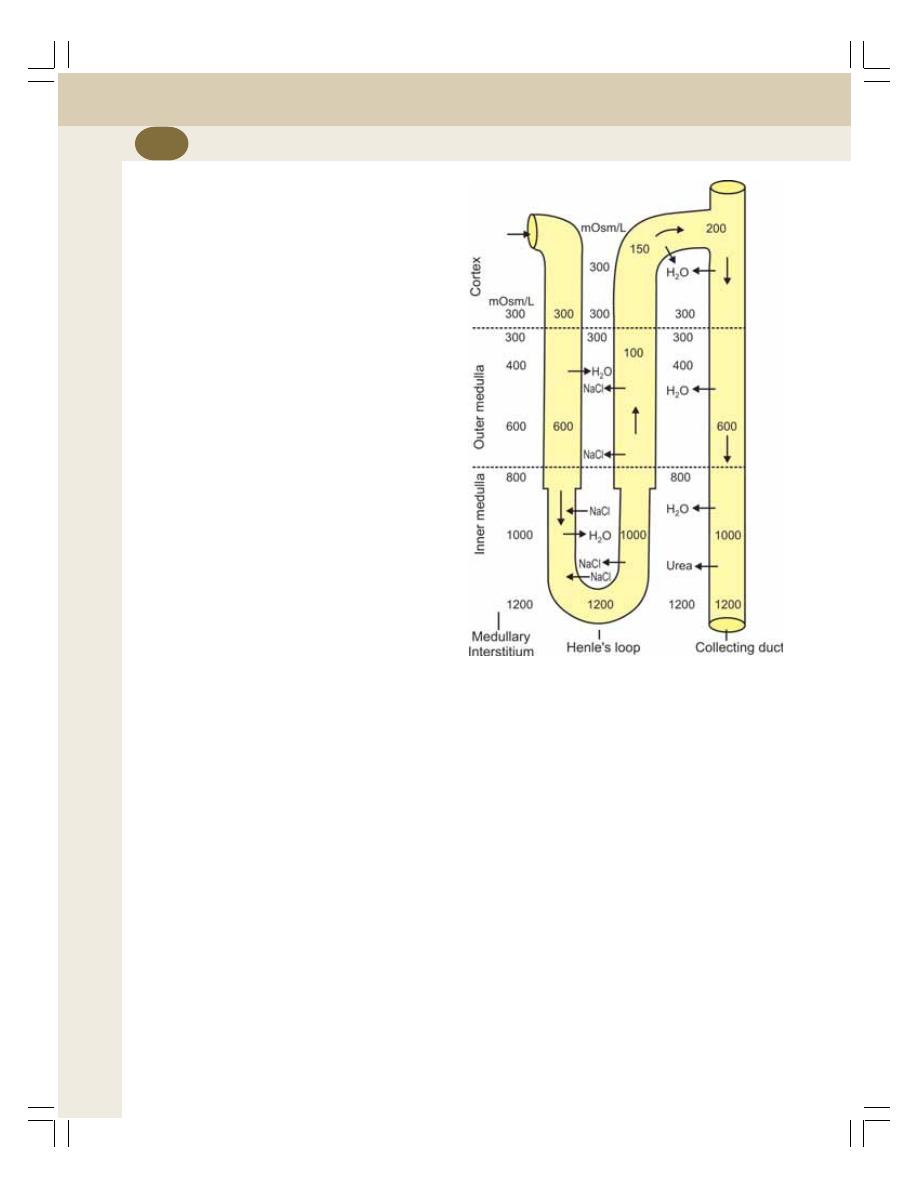
Renal Physiology and Skin
228
FORMATION OF CONCENTRATED
URINE
When the water content in body decreases,
kidney retains water and excretes concentrated
urine. Formation of concentrated urine is not as
simple as that of dilute urine. It involves two
important processes:
I.
Medullary gradient
II. Secretion of ADH.
MEDULLARY GRADIENT
MEDULLARY HYPEROSMOLARITY
The osmolarity of the cortical interstitial fluid is
isotonic, i.e. similar to that of plasma and it is
300 mOsm/L.
The osmolarity of medullary interstitial fluid
near the cortex also is 300 mOsm/L. However,
while proceeding from outer part towards the
inner part of medulla, it increases gradually
and, reaches the maximum at the inner most
part of medulla near renal sinus. Here, it is
1200 mOsm/L (Fig. 38-1).
This type of gradual increase in the osmolarity
of the medullary interstitial fluid is called the
medullary gradient. It plays an important role in
the concentration of urine.
DEVELOPMENT AND MAINTENANCE
OFMEDULLARY GRADIENT
Kidney has some unique anatomical arrange-
ments called countercurrent system, which are
responsible for the development and main-
tenance of medullary gradient and hyper-
osmolarity of interstitial fluid in the inner medulla.
COUNTERCURRENT MECHANISM
COUNTERCURRENT FLOW
A countercurrent system is a system of ‘U’
shaped tubules (tubes) in which, the flow of fluid
is in opposite direction in two limbs of the ‘U’
shaped tubules.
In kidney, the structures, which form the
counter current system, are the loop of Henle
and the vasa recta. In both, the direction of flow
of fluid in the descending limb is just opposite
to that in the ascending limb.
The loop of Henle forms the countercurrent
multiplier and, the vasa recta forms the
countercurrent exchanger.
COUNTERCURRENT MULTIPLIER
Loop of Henle
Loop of Henle functions as countercurrent
multiplier. It is responsible for the development
of hyperosmolarity of medullary interstitial fluid
and medullary gradient.
FIGURE 38-1: Countercurrent multiplier.
Numerical indicate osmolarity (mOsm/L)

Chapter 38 Concentration of Urine
229
Role of Loop of Henle in Development of
Medullary Gradient
The loop of Henle of juxtamedullary nephrons
plays a major role as countercurrent multiplier.
It is because the loop of juxtamedullary nephrons
is long and extends up to the deeper parts of
medulla.
The major cause for the hyperosmolarity of
medullary interstitial fluid is the active reab-
sorption of sodium, chloride and other solutes
from ascending limb of Henle’s loop into the
medullary interstitium. These solutes accumulate
in the medullary interstitium and increase the
osmolarity.
Now, due to the concentration gradient, the
sodium and chloride ions diffuse from medullary
interstitium into the descending limb of Henle’s
loop and reach the ascending limb again via
hairpin bend.
Thus, the sodium and chloride ions are
repeatedly recirculated between the descen-
ding limb and ascending limb of Henle’s loop
through medullary interstitial fluid leaving a small
portion to be excreted in the urine.
Apart from this there is regular addition of
more and more new sodium and chloride ions
into descending limb by constant filtration. Thus,
the reabsorption of sodium chloride from
ascending limb and addition of new sodium
chloride ions into the filtrate increase or multiply
the osmolarity of medullary interstitial fluid and
medullary gradient. Hence, it is called
countercurrent multiplier.
Other Factors Responsible for Hyper-
osmolarity of Medullary Interstitial Fluid
In addition to countercurrent multiplier action
provided by the loop of Henle, two more factors
are involved in hyperosmolarity of medullary
interstitial fluid.
1. Reabsorption of sodium from medullary part
of collecting duct into the medullary
interstitium, which adds to the osmolarity.
2. Urea recirculation: Urea is completely filtered
in the glomeruli. As it is a waste product, it is
not reabsorbed from the renal tubule. So, all
the filtered urea reach collecting duct. Now,
due to concentration gradient, urea diffuses
from the collecting duct into the inner
medullary interstitium. So, the osmolarity
increases in the inner medulla.
Due to the continuous diffusion, the
concentration of urea increases in the medullary
interstitium. Again, by concentration gradient,
urea enters the ascending limb. From here, it
passes through distal convoluted tubule and
reaches the collecting duct. From here, urea
enters the medullary interstitium and the cycle
repeats. By this way urea recirculates repea-
tedly, and helps to maintain the hyperosmolarity
in the inner medullary interstitium. Only a small
amount of urea is excreted in urine.
COUNTERCURRENT EXCHANGER
Vasa Recta
Vasa recta functions as countercurrent
exchanger. It is responsible for the maintenance
of the hyperosmolarity of medullary interstitial
fluid and the medullary gradient developed by
countercurrent multiplier (Fig. 38-2).
Role of Vasa Recta in the Maintenance of
Medullary Gradient
Vasa recta acts like countercurrent exchanger
because of its position. It is also ‘U’ shaped tubule
with a descending limb, hairpin bend and an
ascending limb. Vasa recta runs parallel to loop
of Henle. Its descending limb runs along the
ascending limb of Henle’s loop and its ascending
limb runs along with descending limb of Henle’s
loop.
The sodium chloride reabsorbed from
ascending limb of Henle’s loop enters the
medullary interstitium. From here it enters the
descending limb of vasa recta. Simultaneously
water diffuses from descending limb of vasa recta
into medullary interstitium.
The blood flows very slowly through vasa
recta. So, a large quantity of sodium chloride
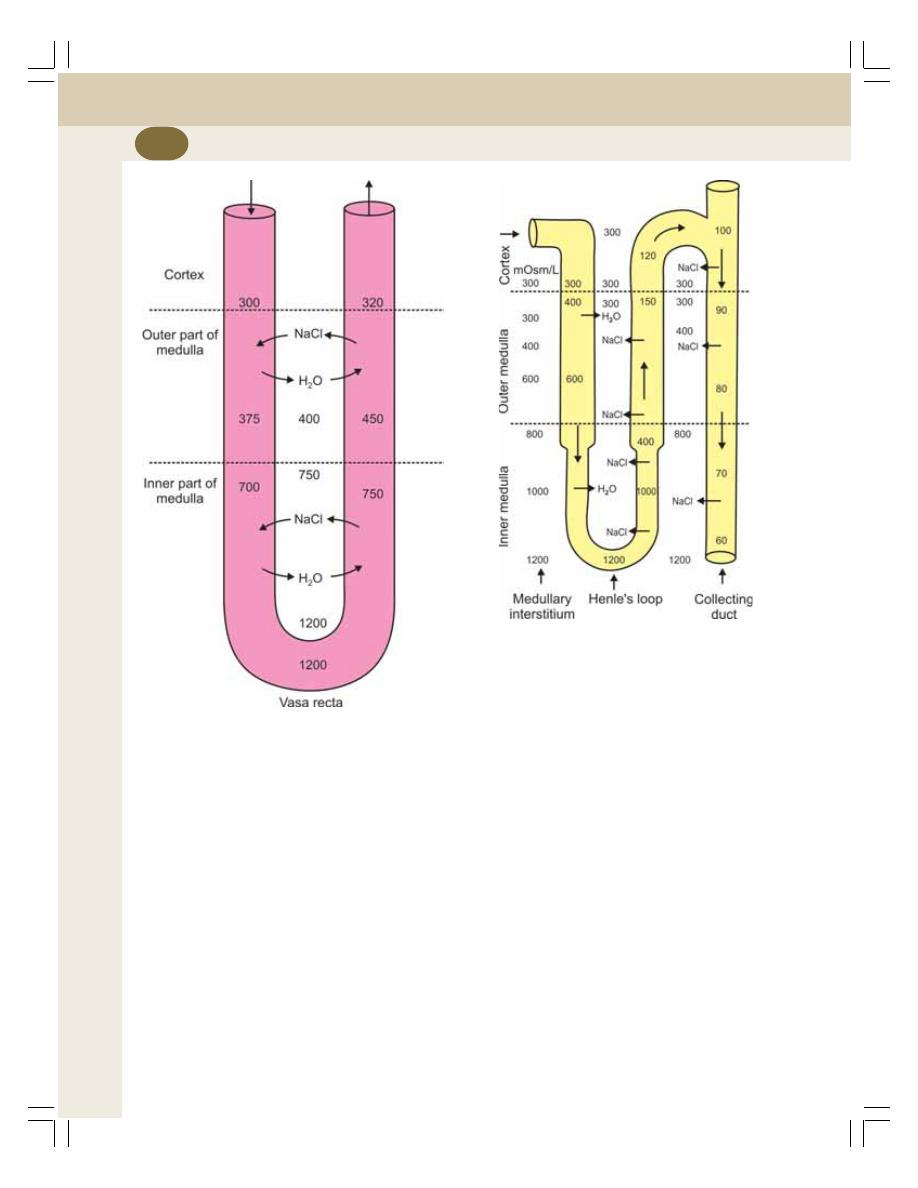
Renal Physiology and Skin
230
accumulates in descending limb of vasa recta
and flows slowly towards ascending limb. By the
time the blood reaches the ascending limb of
vasa recta, the concentration of sodium chloride
increases very much. This causes diffusion of
sodium chloride into the medullary interstitium.
Water from medullary interstitium enters the
ascending limb of vasa recta and the cycle is
repeated.
Thus, vasa recta retains sodium chloride in
the medullary interstitium and removes water
from it. So, the hyperosmolarity of medullary
interstitium is maintained.
Recycling of urea also occurs through vasa
recta. From medullary interstitium, along with
sodium chloride, urea also enters the descending
limb of vasa recta. When blood passes through
ascending limb of vasa recta, urea diffuses back
into the medullary interstitium along with sodium
chloride.
Thus, sodium chloride and urea are
exchanged for water between the ascending and
descending limbs of vasa recta, hence this
system is called countercurrent exchanger.
ROLE OF ADH
The final concentration of urine is achieved by
ADH. Normally, the distal convoluted tubule and
the collecting duct are not permeable to water.
In the presence of ADH, distal convoluted tubule
FIGURE 38-2: Countercurrent exchanger.
Numerical indicate osmolarity (mOsm/L)
FIGURE 38-3: Mechanism for the formation of dilute
urine. Numerical indicate osmolarity (mOsm/L)
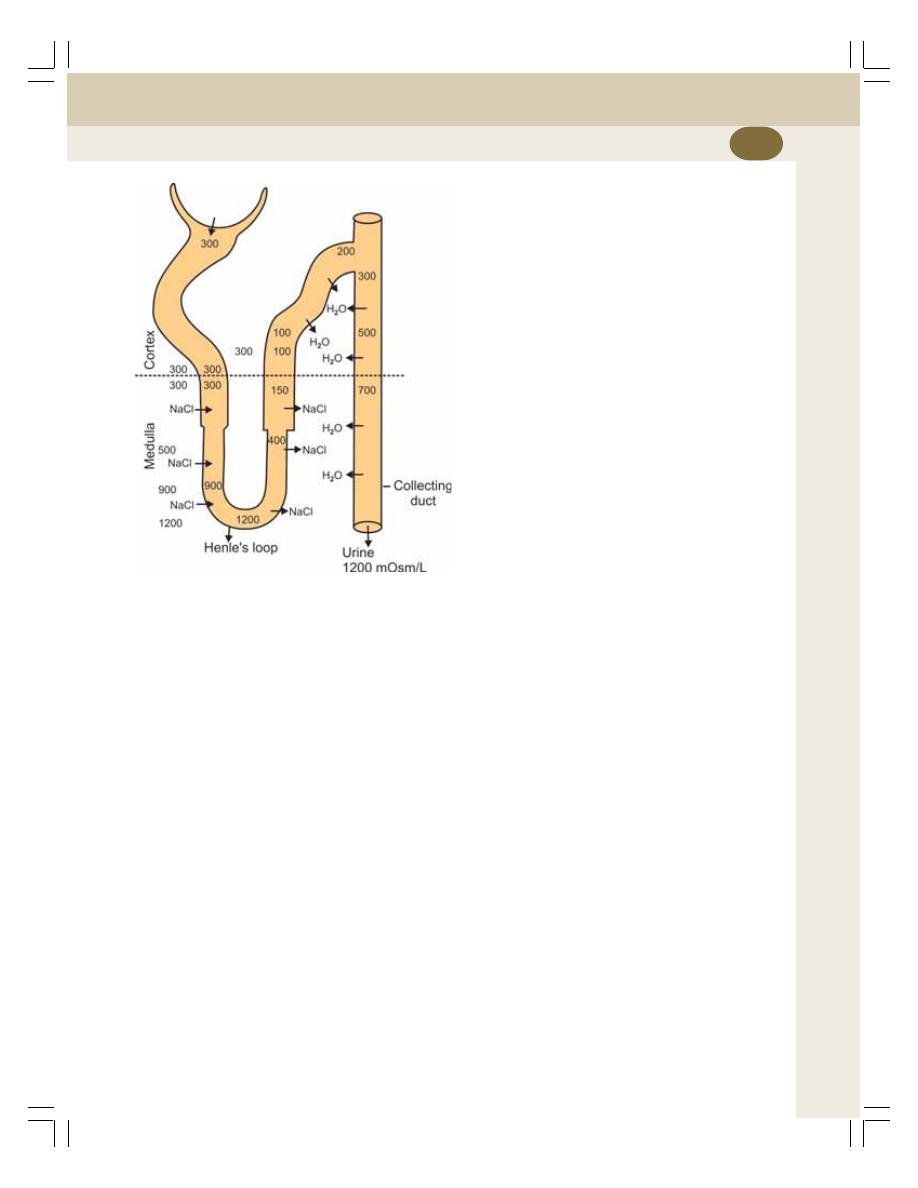
Chapter 38 Concentration of Urine
231
and collecting duct become permeable to water
resulting in water reabsorption. The water
reabsorption induced by ADH is called facultative
reabsorption of water (Refer Chapter 45 for
details).
A large quantity of water is removed from the
fluid while passing through distal convoluted
tubule and collecting duct. So, the urine becomes
hypertonic with an osmolarity of 1200 mOsm/L
(Fig. 38-4).
SUMMARY OF URINE
CONCENTRATION
When the glomerular filtrate passes through renal
tubule, its osmolarity is altered in different
segments as described below.
1. BOWMAN’S CAPSULE
The glomerular filtrate collected at the Bowman’s
capsule is isotonic to plasma. This is because it
contains all the substances of plasma except
proteins. The osmolarity of the filtrate at
Bowman’s capsule is 300 mOsm/L.
2. PROXIMAL CONVOLUTED TUBULE
When the filtrate flows through proximal
convoluted tubule, there is active reabsorption
of sodium and chloride followed by obligatory
reabsorption of water. So, the osmolarity of fluid
remains the same as in the case of Bowman’s
capsule, i.e. 300 mOsm/L. Thus, in proximal
convoluted tubules, the fluid is isotonic to plasma.
3. THICK DESCENDING SEGMENT
When the fluid passes from proximal convoluted
tubule into the thick descending segment, water
is reabsorbed from the tubule into outer medullary
interstitium by means of osmosis. It is due to the
increased osmolarity in the medullary interstitium,
i.e. outside the thick descending tubule. The
osmolarity of the fluid inside this segment is
between 450 and 600 mOsm/L. That means the
fluid is slightly hypertonic to plasma.
4. THIN DESCENDING SEGMENT OF
HENLE’S LOOP
As the thin descending segment of Henle’s loop
passes through the inner medullary interstitium
(which is increasingly hypertonic) more water is
reabsorbed.
This segment is highly permeable to water,
and so the osmolarity of tubular fluid becomes
equal to that of the surrounding medullary
interstitium.
In the short loops of cortical nephrons, the
osmolarity of fluid at the hairpin bend of loop
becomes 600 mOsm/L. And, in the long loops
of juxtamedullary nephrons, at the hairpin bend,
the osmolarity is1200 mOsm/L. Thus in this
segment, the fluid is hypertonic to plasma.
FIGURE 38-4: Role of ADH in the formation of
concentrated urine. ADH increases the permeability
for water in distal convoluted tubule and collecting
duct

Renal Physiology and Skin
232
5. THIN ASCENDING SEGMENT OF
HENLE’S LOOP
When the thin ascending segment of the loop
ascends upwards through the medullary region,
osmolarity decreases gradually.
Due to concentration gradient, sodium chlo-
ride diffuses out of tubular fluid and osmolarity
decreases to 400 mOsm/L. The fluid in this
segment is slightly hypertonic to plasma.
6. THICK ASCENDING SEGMENT
This segment is impermeable to water. But there
is active reabsorption of sodium and chloride
from this. Reabsorption of sodium decreases the
osmolarity of tubular fluid to a greater extent. The
osmolarity is between 150 and 200 mOsm/L. The
fluid inside becomes hypotonic to plasma.
7. DISTAL CONVOLUTED TUBULE
AND COLLECTING DUCT
In the presence of ADH, distal convoluted tubule
and collecting duct become permeable to water
resulting in water reabsorption and final
concentration of urine.
Reabsorption of large quantity of water
increases the osmolarity to 1200 mOsm/L (Fig.
34-4). The urine becomes hypertonic to plasma.

INTRODUCTION
SECRETION OF HYDROGEN IONS
REMOVAL OF HYDROGEN IONS AND ACIDIFICATION OF URINE
BICARBONATE MECHANISM
PHOSPHATE MECHANISM
AMMONIA MECHANISM
INTRODUCTION
Kidney plays an important role in maintenance
of acid–base balance by excreting hydrogen ions
and retaining bicarbonate ions.
Normally, urine is acidic in nature with a pH
of 4.5 to 6. The metabolic activities in the body
produce lot of acids (with lot of hydrogen ions)
which threaten to push the body towards
acidosis. However, kidneys prevent this by
excreting hydrogen ions (H
+
) and conserving
bicarbonate ions (HCO
3
–
).
About 4320 mEq of HCO
3
–
is filtered by the
glomeruli everyday. It is called filtered load of
HCO
3
–
. Excretion of this much HCO
3
–
through
urine will affect the acid–base balance of body
fluids. So, HCO
3
–
must be taken back from the
renal tubule by reabsorption.
The reabsorption of filtered HCO
3
–
occurs by
the secretion of H
+
in the renal tubules. About
4380 mEq of H
+
appear everyday in the renal
tubule by means of filtration and secretion. Not
all the H
+
are excreted in urine. Out of 4380 mEq,
about 4280 to 4330 mEq of H
+
is utilized for the
reabsorption of filtered HCO
3
–
. Only the
remaining 50 to 100 mEq is excreted. It results
in the acidification of urine.
SECRETION OF HYDROGEN IONS
H
+
is secreted in proximal convoluted tubule,
distal convoluted tubule and collecting duct.
Secretion of H
+
into the renal tubules occurs by
the formation of carbonic acid. Carbon dioxide
formed in the tubular cells combines with water
to form carbonic acid. Carbon dioxide enters the
cells from tubular fluid also. Carbonic anhydrase
is essential for the formation of carbonic acid.
This enzyme is available in large quantities in
the epithelial cells of the renal tubules. The
carbonic acid immediately dissociates into H
+
and
HCO
3
–
(Fig. 39-1). H
+
from the tubular cells is
secreted into proximal convoluted tubule, distal
convoluted tubule and collecting duct.
The distal convoluted tubule and collecting
duct have a special type of cells called
intercalated cells (I cells) that are involved in
handling hydrogen and bicarbonate ions.
Acidification of Urine and
Role of Kidney in
Acid–Base Balance
39
39
39
39
39
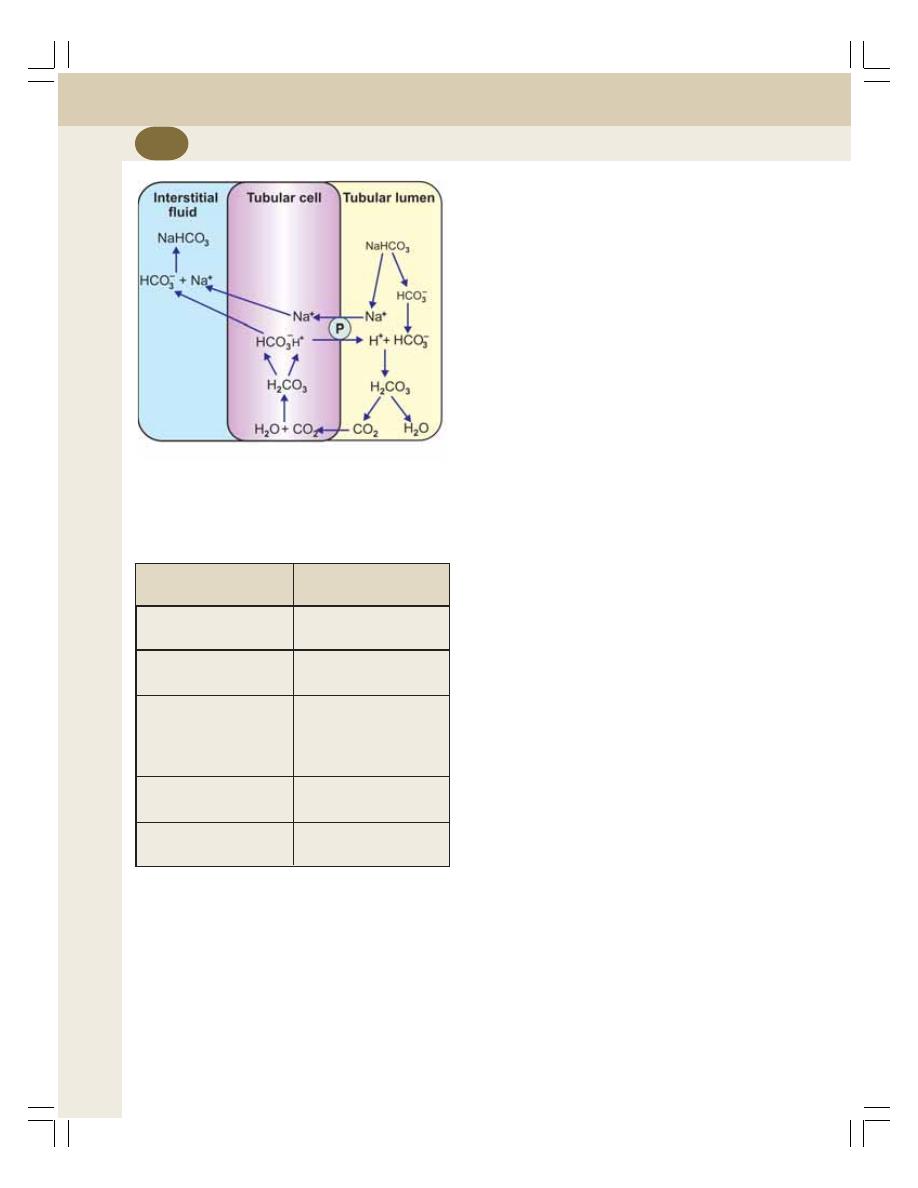
Renal Physiology and Skin
234
There are two mechanisms for the secretion
of H
+
:
1. Sodium-Hydrogen antiport pump
2. ATP driven proton pump.
SODIUM-HYDROGEN ANTIPORT PUMP
When sodium ion (Na
+
) is reabsorbed from the
tubular fluid into the tubular cell, H
+
is secreted
from the cell into the tubular fluid in exchange
for Na
+
. The sodium-hydrogen antiport pump
present in the tubular cells is responsible for the
exchange of Na
+
and H
+
. This type of sodium-
hydrogen counter transport occurs predomi-
nantly in distal convoluted tubule (Table 39-1).
ATP DRIVEN PROTON PUMP
This is an additional mechanism of H
+
secretion
in distal convoluted tubule and collecting duct.
This pump is operated by obtaining energy from
ATP.
REMOVAL OF HYDROGEN IONS
AND ACIDIFICATION OF URINE
Role of Kidney in Preventing Metabolic
Acidosis
Kidney plays an important role in preventing
metabolic acidosis by excreting H
+
. The excretion
of H
+
occurs by three mechanisms:
1. Bicarbonate mechanism
2. Phosphate mechanism
3. Ammonia mechanism.
BICARBONATE MECHANISM
All the HCO
3
–
filtered into the renal tubules is
reabsorbed. About 80% of it is reabsorbed in
proximal convoluted tubule; 15% in Henle’s loop
and 5% in distal convoluted tubule and collecting
duct. The reabsorption of HCO
3
–
utilizes the H
+
secreted into the renal tubules.
The H
+
secreted into the renal tubule,
combines with filtered HCO
3
–
forming carbonic
acid. Carbonic acid dissociates into carbon
dioxide and water in the presence of carbonic
anhydrase. Carbon dioxide and water enter the
tubular cell.
In the tubular cells, carbon dioxide combines
with water to form carbonic acid. It immediately
dissociates into H
+
and HCO
3
–
. HCO
3
–
from
the tubular cell enters the interstitium. Simul-
taneously Na
+
is reabsorbed from the renal tubule
under the influence of aldosterone. HCO
3
–
combines with Na
+
to form NaHCO
3
. Now, the
H
+
is secreted into the tubular lumen from the
cell in exchange for Na
+
(Fig. 39-1).
TABLE 39-1: Secretion and removal of hydrogen
ions in renal tubule
Mechanism
Segment of
renal tubule
Sodium-hydrogen
Distal convoluted tubule
pump
ATP driven proton
Distal convoluted tubule
pump
Collecting duct
Bicarbonate mechanism Proximal convoluted
tubule
Henle’s loop
Distal convoluted tubule
Phosphate mechanism
Distal convoluted tubule
Collecting duct
Ammonia mechanism
Proximal convoluted
tubule
FIGURE 39-1: Reabsorption of bicarbonate ions by
secretion of hydrogen ions in renal tubule. P = Sodium-
Hydrogen antiport pump
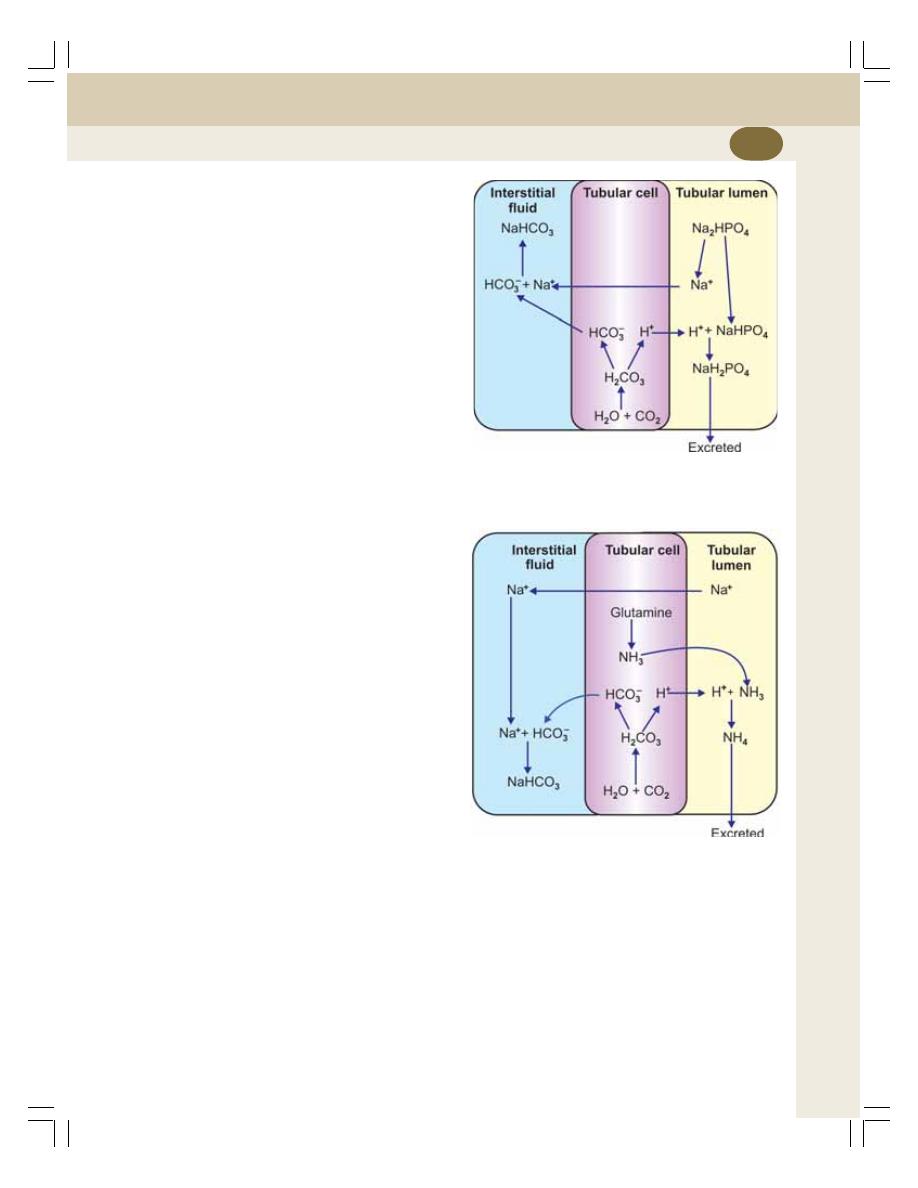
Chapter 39 Acidification of Urine and Role of Kidney in Acid–Base Balance
235
Thus, for every hydrogen ion secreted into
lumen of tubule, one bicarbonate ion is
reabsorbed from the tubule. In this way, kidneys
conserve the HCO
3
–
. The reabsorption of filtered
HCO
3
–
is an important factor in maintaining pH
of the body fluids.
PHOSPHATE MECHANISM
In the tubular cells, carbon dioxide combines with
water to form carbonic acid. It immediately
dissociates into H
+
and HCO
3
–
. HCO
3
–
from the
tubular cell enters the interstitium.
Simultaneously, Na
+
is reabsorbed from renal
tubule under the influence of aldosterone. Na
+
enters the interstitium and combines with HCO
3
–
.
The H
+
is secreted into the tubular lumen from
the cell in exchange for Na
+
(Fig. 39-2).
The H
+
, which is secreted into renal tubules,
reacts with phosphate buffer system. It combines
with sodium hydrogen phosphate to form sodium
dihydrogen phosphate. Sodium dihydrogen
phosphate is excreted in urine. The H
+
, which is
added to urine, makes it acidic. It happens mainly
in distal tubule and collecting duct because of
the presence of large quantity of sodium
hydrogen phosphate in these segments.
AMMONIA MECHANISM
This is the most important mechanism by which
kidneys excrete H
+
and make the urine acidic.
In the tubular epithelial cells, ammonia is formed
when the amino acid glutamine is converted into
glutamic acid in the presence of the enzyme
glutaminase. Ammonia is also formed by the
deamination of some of the amino acids such
as glycine and alanine (Fig. 39-3).
The ammonia (NH
3
) formed in tubular cells
is secreted into tubular lumen in exchange for
sodium ion. Here, it combines with H
+
to form
ammonium (NH
4
). The tubular cell membrane
is not permeable to ammonium. Therefore, it
remains in the lumen and combines with sodium
acetoacetate to form ammonium acetoacetate.
Ammonium acetoacetate is excreted through
urine. Thus, H
+
is added to urine in the form of
ammonium compounds resulting in acidification
of urine.
FIGURE 39-2: Excretion of hydrogen ions in
combination with phosphate ions
FIGURE 39-3: Excretion of hydrogen in
combination with ammonia
This process takes place mostly in the
proximal convoluted tubule because glutamine
is converted into ammonia in the cells of this
segment.
Thus, by excreting H
+
and conserving HCO
3
–
,
kidneys produce acidic urine and help to maintain
the acid–base balance of body fluids.

PROPERTIES AND COMPOSITION OF NORMAL URINE
RENAL FUNCTION TESTS
EXAMINATION OF URINE
EXAMINATION OF BLOOD
EXAMINATION OF BLOOD AND URINE
PROPERTIES AND COMPOSITION
OF NORMAL URINE
PROPERTIES OF URINE
Volume: 1000 to 1500 mL/day
Reaction: Slightly acidic with pH of 4.5 to 6
Specific gravity: 1.010 to 1.025
Color: Normally, urine is straw colored
Odor: Fresh urine has light aromatic odor. If
stored for some time, the odor becomes stronger
due to bacterial decomposition.
COMPOSITION OF URINE
Urine consists of water and solids. Solids include
organic and inorganic substances (Fig. 40-1).
RENAL FUNCTION TESTS
Renal function tests are the group of tests that
are performed to assess the functions of kidney.
The renal function tests are of three types:
I.
Examination of urine alone
II. Examination of blood alone
III. Examination of blood and urine.
EXAMINATION OF URINE —
URINANALYSIS
Routine Examination of Urine
During the routine examination of urine, the
following are determined:
i.
Specific gravity: Normally it is 1.010 to 1.025.
But, in some conditions like chronic nephritis,
it is decreased.
ii. Presence of normal constituents of urine in
abnormal quantity: Normally, substances like
water, salt, amino acids and creatinine are
excreted in urine either in greater or lesser
amount. But, if abnormally large amount is
excreted, it suggests some abnormal
functional status of kidney. If 4 to 5 liters of
water is excreted consistently per day, it is
suggestive of diabetes insipidus. Abnormally
low amount of water excretion indicates
nephritis. Abnormal amount of salts or
nutritive substances like amino acids appear
in urine during congenital tubular defects.
Abnormal albumin excretion occurs in
defective filtration. Abnormal amount of
glucose is excreted in diabetes mellitus.
Renal Function Tests
40

Chapter 40 Renal Function Tests
237
iii. Microscopic examination: This reveals the
presence of red blood cells, pus cells,
epithelial cells, casts and crystals which
suggests the renal pathology.
EXAMINATION OF BLOOD
The level of plasma proteins, urea, uric acid and
creatinine are determined in blood. The blood
level of these substances is altered in renal
failure.
EXAMINATION OF BLOOD AND
URINE
Plasma Clearance
Plasma clearance is defined as the amount of
plasma that is cleared off a substance in a given
unit of time. It is also known as renal clearance.
It is based on Fick’s principle.
The determination of clearance value for
certain substances helps in assessing the
following renal functions:
1. Glomerular filtration rate
2. Renal plasma flow
3. Renal blood flow.
To determine the plasma clearance of a
particular substance, measurement of the
following factors is required:
1. Volume of urine excreted
2. Concentration of the substance in urine
3. The concentration of the substance in blood.
The formula to calculate clearance value is
C =
UV
P
Where
C = Clearance
U = Concentration of the substance in urine
V = Volume of urine flow and
P = Concentration of the substance in
plasma.
1. Measurement of Glomerular Filtration
Rate
A substance that is completely filtered but
neither reabsorbed nor secreted should be used
to measure glomerular filtration rate (GFR). Inulin
is a substance that is completely filtered. And, it
is neither reabsorbed nor secreted. So, inulin is
the ideal substance used to measure GFR.
Inulin clearance
A known amount of inulin is injected into the
body. After sometime, the concentration of inulin
in plasma and urine and the volume of urine
excreted are estimated.
For example, the concentration of inulin in
urine is 125 mg/dL. The plasma concentration
is 1 mg/dL. The volume of urine output is
1 mL/min.
Thus,
Glomerular filtration rate =
UV
P
=
125 1
1
= 125 mL/min
2. Measurement of Renal Plasma Flow
To measure renal plasma flow, a substance,
which is filtered and secreted but not reabsorbed,
should be used. Such a substance is para-
aminohippuric acid (PAH). PAH clearance
indicates the amount of plasma passed through
kidneys.
A known amount of PAH is injected into the
body. After sometime, the concentration of PAH
FIGURE 40-1: Quantity of solids excreted
in urine (mMols/day)

Renal Physiology and Skin
238
in plasma and urine and the volume of urine
excreted are estimated.
For example, the concentration of PAH in
urine is 66 mg/dL. The plasma concentration is
0.1 mg/dL. The volume of urine output is 1 mL/
min. Thus,
Renal plasma flow =
=
= 660 mL/min
3. Measurement of Renal Blood Flow
To determine renal blood flow, value of two
factors is necessary:
i.
Renal plasma flow
ii. Percentage of plasma volume in the blood.
i. Renal plasma flow
Renal plasma flow is measured by using PAH
clearance.
ii. Percentage of plasma volume in the blood
The percentage of plasma volume is indirectly
determined by using PCV. For example, if PCV
is 45%, the plasma volume in the blood is
100 – 45 = 55%, i.e. 55 mL of plasma is present
in every 100 mL of blood.
Renal blood flow is calculated with the values
of renal plasma volume and % of plasma in blood
by using a formula given below.
Renal blood flow =
For example,
Renal plasma flow is 660 mL/min
Amount of plasma in blood is 55%
Renal blood flow
=
=
= 1200 mL/min

INTRODUCTION
FUNCTIONAL ANATOMY OF URINARY BLADDER
NERVE SUPPLY TO URINARY BLADDER AND SPHINCTER
SYMPATHETIC NERVE SUPPLY
PARASYMPATHETIC NERVE SUPPLY
SOMATIC NERVE SUPPLY
FILLING OF URINARY BLADDER
PROCESS OF FILLING
CYSTOMETROGRAM
MICTURITION REFLEX
APPLIED PHYSIOLOGY
INTRODUCTION
Micturition is a process by which urine is voided
from the urinary bladder. It is a reflex process.
However, in grown up children and adults, it can
be controlled voluntarily to some extent. The
functional anatomy and nerve supply of urinary
bladder are essential for the process of micturition.
FUNCTIONAL ANATOMY OF
URINARY BLADDER
Urinary bladder consists of the body, neck and
internal urethral sphincter. The smooth muscle
forming the body of bladder is called detrusor
muscle. At the posterior surface of the bladder
wall, there is a triangular area called trigone. At
the upper angles of this trigone, two ureters enter
the bladder.
The lower part of the bladder is narrow and
forms the neck. The distal end of the bladder is
guarded by internal urethral sphincter. This
sphincter is made up of detrusor muscle. It opens
towards urethra. At the distal end of urethra, there
is external urethral sphincter. It is made up of
skeletal muscle fibers. Therefore, it is responsible
for voluntary control of micturition.
NERVE SUPPLY TO URINARY
BLADDER AND SPHINCTERS
Urinary bladder and the internal sphincter are
supplied by sympathetic and parasympathetic
divisions of autonomic nervous system whereas,
the external sphincter is supplied by the somatic
nerve fibers (Fig. 41-1).
SYMPATHETIC NERVE SUPPLY
Preganglionic fibers of sympathetic nerve arise
from first two lumbar segments (L
1
and L
2
) of
spinal cord. After leaving spinal cord, the fibers
Micturition
41
41
41
41
41
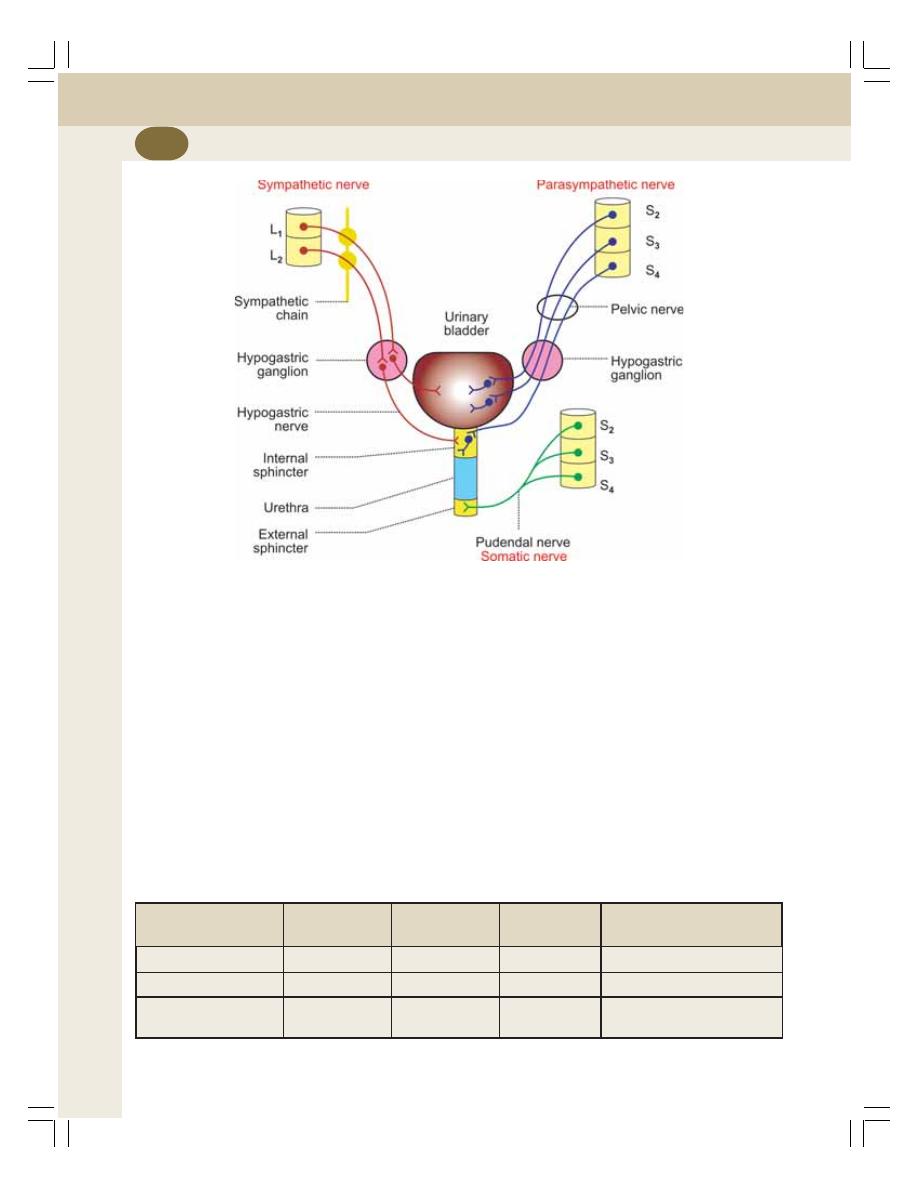
Renal Physiology and Skin
240
pass through lateral sympathetic chain without
any synapse in the sympathetic ganglia and
finally terminate in hypogastric ganglion. The
postganglionic fibers arising from this ganglion
form the hypogastric nerve, which supplies the
detrusor muscle and internal sphincter.
Function of Sympathetic Nerve
The stimulation of sympathetic nerve causes
relaxation of detrusor muscle and constriction of
the internal sphincter. It results in filling of urinary
bladder and so, the sympathetic nerve is called
nerve of filling.
PARASYMPATHETIC NERVE SUPPLY
The preganglionic fibers of parasympathetic
nerve form the pelvic nerve or nervous erigens.
Pelvic nerve fibers arise from second, third and
fourth sacral segments (S
2
, S
2
and S
3
) of spinal
cord. These fibers run through hypogastric
ganglion and synapse with postganglionic
neurons situated in close relation to urinary
bladder and internal sphincter (Table 41-1).
Function of Parasympathetic Nerve
The stimulation of pelvic (parasympathetic) nerve
causes contraction of detrusor muscle and
TABLE 41-1: Functions of nerves supplying urinary bladder and sphincters
Nerve
On detrusor
On internal
On external
Function
muscle
sphincter
sphincter
Sympathetic nerve
Relaxation
Constriction
Not supplied
Filling of urinary bladder
Parasympathetic nerve Contraction
Relaxation
Not supplied
Emptying of urinary bladder
Somatic nerve
Not supplied
Not supplied
Constriction
Voluntary control of
micturition
FIGURE 41-1: Nerve supply to urinary bladder and urethra
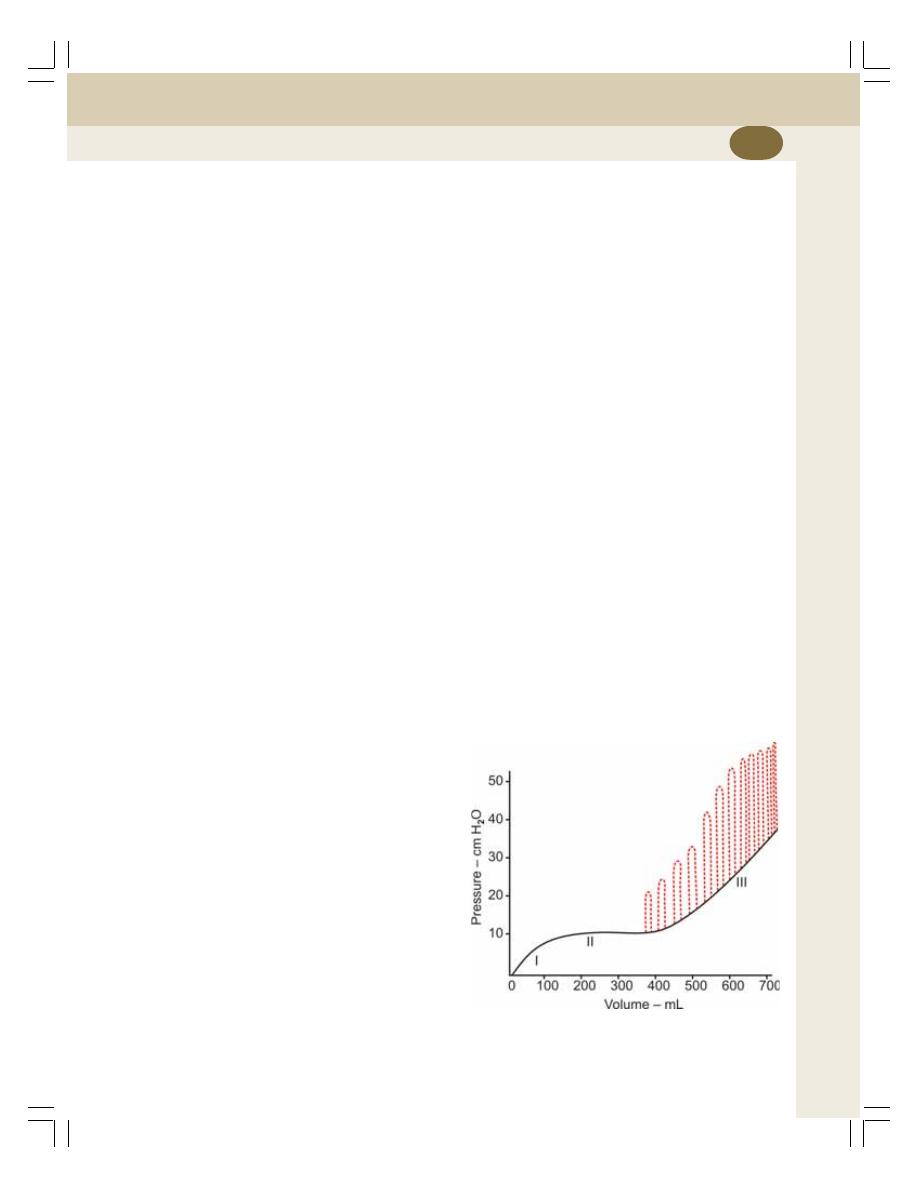
Chapter 41 Micturition
241
relaxation of the internal sphincter leading to
emptying of urinary bladder. So, the parasym-
pathetic nerve is called the nerve of emptying
or nerve of micturition.
The pelvic nerve has also the sensory fibers
which carry impulses from stretch receptors
present on the wall of the urinary bladder and
urethra to the central nervous system.
SOMATIC NERVE SUPPLY
The external sphincter is innervated by the
somatic nerve called the pudendal nerve. It
arises from second, third and fourth sacral
segments of the spinal cord.
Function of Pudendal Nerve
It maintains the tonic contraction of the skeletal
muscle fibers of the external sphincter and keeps
the external sphincter constricted always.
During micturition, this nerve is inhibited. It
causes relaxation of external sphincter leading
to voiding of urine. Thus, the pudendal nerve is
responsible for voluntary control of micturition.
FILLING OF URINARY BLADDER
PROCESS OF FILLING
Urine is continuously formed in the nephrons and
it is transported drop by drop through the ureters
into the urinary bladder. When urine collects in
the pelvis of ureter, the contraction sets up in
pelvis. The contraction is transmitted through rest
of the ureter in the form of peristaltic wave up to
trigone of the urinary bladder. The peristaltic wave
moves the urine into the bladder.
A reasonable volume of urine can be stored
in urinary bladder without any discomfort and
without much increase in pressure inside the
bladder (intravesical pressure). It is due to the
adaptation of detrusor muscle. The relationship
between the volume of urine and pressure in
urinary bladder is studied by cystometrogram.
CYSTOMETROGRAM
Definition
Cystometrogram is the graphical registration
(recording) of pressure changes in urinary
bladder in relation to volume of urine collected
in it.
Method of Recording Cystometrogram
A double lumen catheter is introduced into the
urinary bladder. One of the lumen is used to
infuse fluid into the bladder and the other one is
used to record the pressure changes by
connecting it to a suitable recording instrument.
First, the bladder is emptied completely. Then,
a known quantity of fluid is introduced into the
bladder at regular intervals. The intravesical
pressure developed by the fluid is recorded
continuously. A graph is obtained by plotting all
the values of volume and the pressure. This
graph is the cystometrogram (Fig. 41-2).
Description of Cystometrogram
Cystometrogram shows three segments.
Segment I
Initially, when the urinary bladder is empty, the
intravesical pressure is 0. When about 100 mL
of fluid is collected, the pressure rises sharply
to about 10 cm H
2
O.
Segment II
This segment shows the plateau, i.e. the
intravesical pressure remains more or less at
FIGURE 41-2: Cystometrogram. Dotted lines
indicate the contraction of detrusor muscle

Renal Physiology and Skin
242
10 cm H
2
O (level of segment I) without any
change even after introducing 300 to 400 mL of
fluid. It is because of adaptation of urinary bladder
by relaxation. It is in accordance with law of
Laplace.
Law of Laplace
According to this law, the pressure in a spherical
organ is inversely proportional to its radius, the
tone remaining constant. That is, if radius is
more, the pressure is less and if radius is less
the pressure is more, provided the tone remains
constant.
Urinary bladder obeys Laplace law. In the
bladder, the tension increases as the urine is
filled. At the same time, the radius also increases
due to relaxation of detrusor muscle. Because
of this, the pressure rise is almost zero.
When about 100 mL of urine is collected, the
pressure rises to about 10 cm H
2
O and now, the
desire for micturition occurs. The desire for
micturition is associated with a vague feeling in
the perineum. An additional volume of about
200 to 300 mL of urine can be collected in bladder
without much increase in pressure. However,
when total volume rises beyond 400 mL, the
pressure rises sharply and the urge for micturition
starts. Still voluntary control of micturition is
possible. And, beyond 600 to 700 mL of urine,
voluntary control starts failing.
Segment III
As the pressure increases with collection of 300-
400 mL of fluid, the contraction of detrusor mus-
cle becomes intense, increasing the conscious-
ness and the urge for micturition. Still, voluntary
control is possible. The voluntary control is
possible up to volume of 600 to 700 mL at which
the pressure rises to about 35 to 40 cm H
2
O.
When the intravesical pressure rises above
40 cm water, the contraction of detrusor muscle
becomes still more intense. And, voluntary control
of micturition is not possible. Now, pain sensation
develops and micturition should take place.
MICTURITION REFLEX
It is the reflex by which micturition occurs. This
reflex is elicited by the stimulation of stretch
receptors situated on the wall of urinary bladder
and urethra. When about 300 to 400 mL of urine
is collected in the bladder, the pressure inside
the bladder increases. This stretches the wall of
bladder resulting in stimulation of stretch
receptors and generation of sensory impulses.
The sensory (afferent) impulses from the
receptors reach the sacral segments of spinal
cord via the sensory fibers of pelvic (parasym-
pathetic) nerve. The motor (efferent) impulses
produced in spinal cord, travel through motor
fibers of pelvic nerve towards bladder and internal
sphincter. The motor impulses cause contraction
of detrusor muscle and relaxation of internal
sphincter so that, urine enters the urethra from
the bladder (Fig. 41-3).
Once urine enters urethra, the stretch
receptors in the urethra are stimulated and send
afferent impulses to spinal cord via pelvic nerve
fibers. These impulses inhibit pudendal nerve.
So, the external sphincter relaxes and micturition
occurs.
Once a micturition reflex begins, it is self-
regenerative, i.e. the initial contraction of bladder
further activates the receptors to cause still
further increase in sensory impulses from the
bladder and urethra. These impulses, in turn
cause further increase in reflex contraction of
bladder. The cycle continues repeatedly until the
force of contraction of bladder reaches the
maximum and the urine is voided out completely.
During micturition, the flow of urine is
facilitated by the increase in the abdominal
pressure due to the voluntary contraction of
abdominal muscles.
Higher Centers for Micturition
Spinal centers for micturition are present in sacral
and lumbar segments. These spinal centers are
regulated by higher centers which are of two
types:
1. Inhibitory centers which are situated in
midbrain and cerebral cortex
2. Facilitatory centers which are situated in pons
and cerebral cortex.
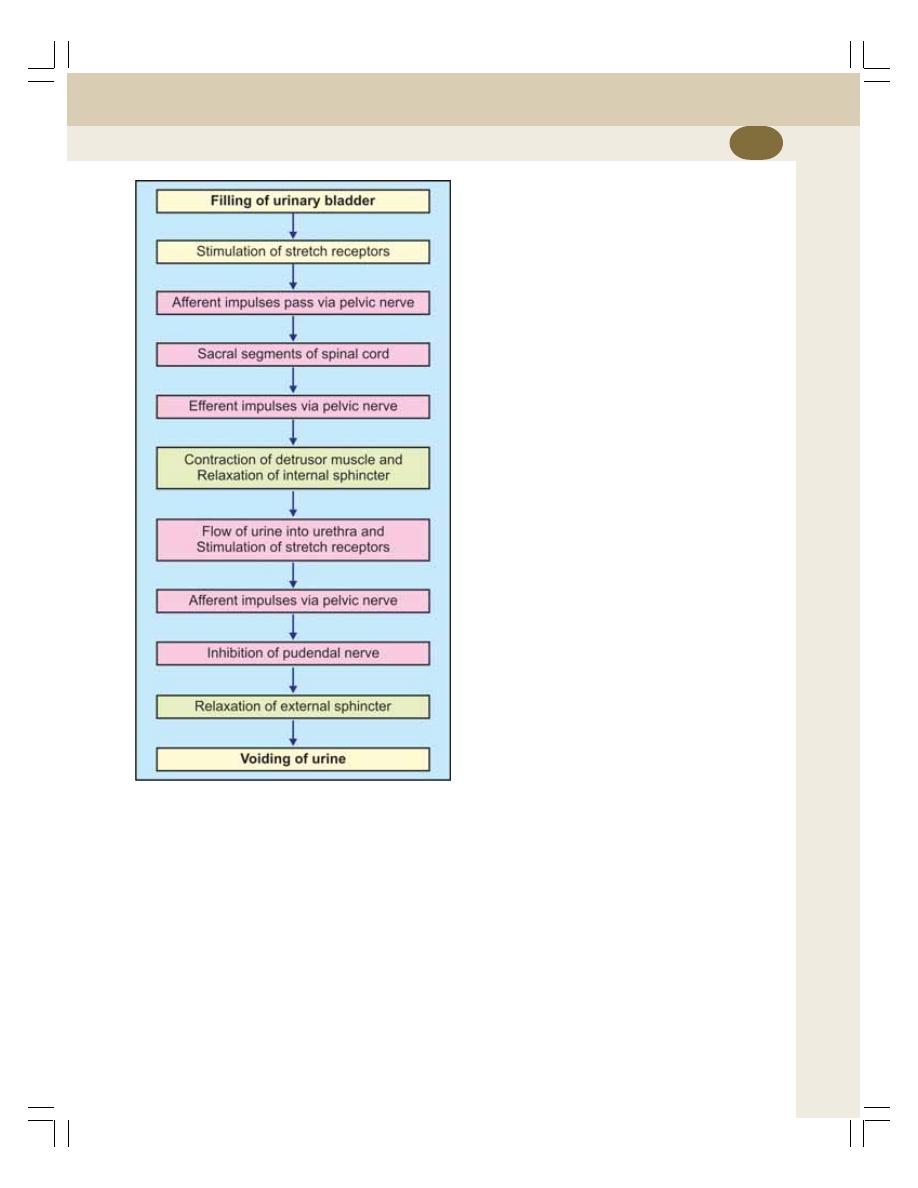
Chapter 41 Micturition
243
destruction of sensory (pelvic) nerve fibers of
urinary bladder.
Due to the destruction of sensory nerve
fibers, the bladder is filled up without any stretch
signals to spinal cord. Detrusor muscle looses
the tone and becomes flaccid. So, bladder is
completely filled with urine. Later, overflow
occurs in drops as and when urine enters the
bladder. It is called overflow incontinence or
overflow dribbling. It occurs in spinal injury and
syphilis.
2. AUTOMATIC BLADDER
Automatic bladder refers loss of voluntary control
of micturition. So, even with small amount of
urine collected in the urinary bladder, micturition
reflex occurs resulting in emptying of urine. This
occurs in transaction of spinal cord above the
sacral segments.
3. THE UNINHIBITED NEUROGENIC
BLADDER
It is the urinary bladder with frequent and
uncontrollable micturition caused by lesion in
midbrain.
The lesion in midbrain causes continuous
excitation of spinal micturition centers resulting
in frequent and uncontrollable micturition. Even
a small quantity of urine collected in bladder will
elicit the micturition reflex.
4. NOCTURNAL MICTURITION
Nocturnal micturition is the involuntary voiding
of urine during night. It is otherwise known as
enuresis or bed wetting. It occurs due to the
absence of voluntary control of micturition. It is
a common and normal process in infants and
children below 3 years. It is because of incomplete
myelination of motor nerve fibers of the bladder.
When myelination is complete, voluntary control
of micturition develops and bed wetting stops.
FIGURE 41-3: Micturition reflex
APPLIED PHYSIOLOGY
1. ATONIC BLADDER – EFFECT OF
DESTRUCTION OF SENSORY
NERVE FIBERS
Atonic bladder is the urinary bladder with loss
of tone in detrusor muscle. It is caused by
STRUCTURE OF SKIN
INTRODUCTION
EPIDERMIS
DERMIS
APPENDAGES OF SKIN
COLOR OF THE SKIN
GLANDS OF SKIN
SEBACEOUS GLANDS
SWEAT GLANDS
FUNCTIONS OF THE SKIN
STRUCTURE OF SKIN
INTRODUCTION
Skin is the largest organ of the body. It is not
uniformly thick. At some places, it is thick and
in some places, it is thin. The average thickness
of the skin is about 1 to 2 mm. In the sole of the
foot, palm of the hand and in the interscapular
region, it is considerably thick, measuring about
5 mm. In other areas of the body, the skin is thin.
It is thinnest over eyelids and penis measuring
about 0.5 mm only.
Skin is made up of two layers:
1. Outer epidermis
2. Inner dermis.
EPIDERMIS
The epidermis is the outer layer of skin. It is
formed by stratified epithelium, which consists
of 5 layers:
1. Stratum corneum
2. Stratum lucidum
3. Stratum granulosum
4. Stratum spinosum
5. Stratum germinativum
The important feature of epidermis is that, it
does not have blood vessels (Fig. 42-1). The
nutrition is provided to epidermis by the capillaries
of dermis.
DERMIS
Dermis is the inner layer of the skin. It is a
connective tissue layer made up of dense and
stout collagen fibers, fibroblasts and histiocytes.
Dermis is made up of 2 layers:
1. Superficial papillary layer
2. Deeper reticular layer.
APPENDAGES OF SKIN
The hair follicles with hairs, nails, sweat glands,
sebaceous glands and mammary glands are
considered as appendages of the skin.
Skin
42
42
42
42
42
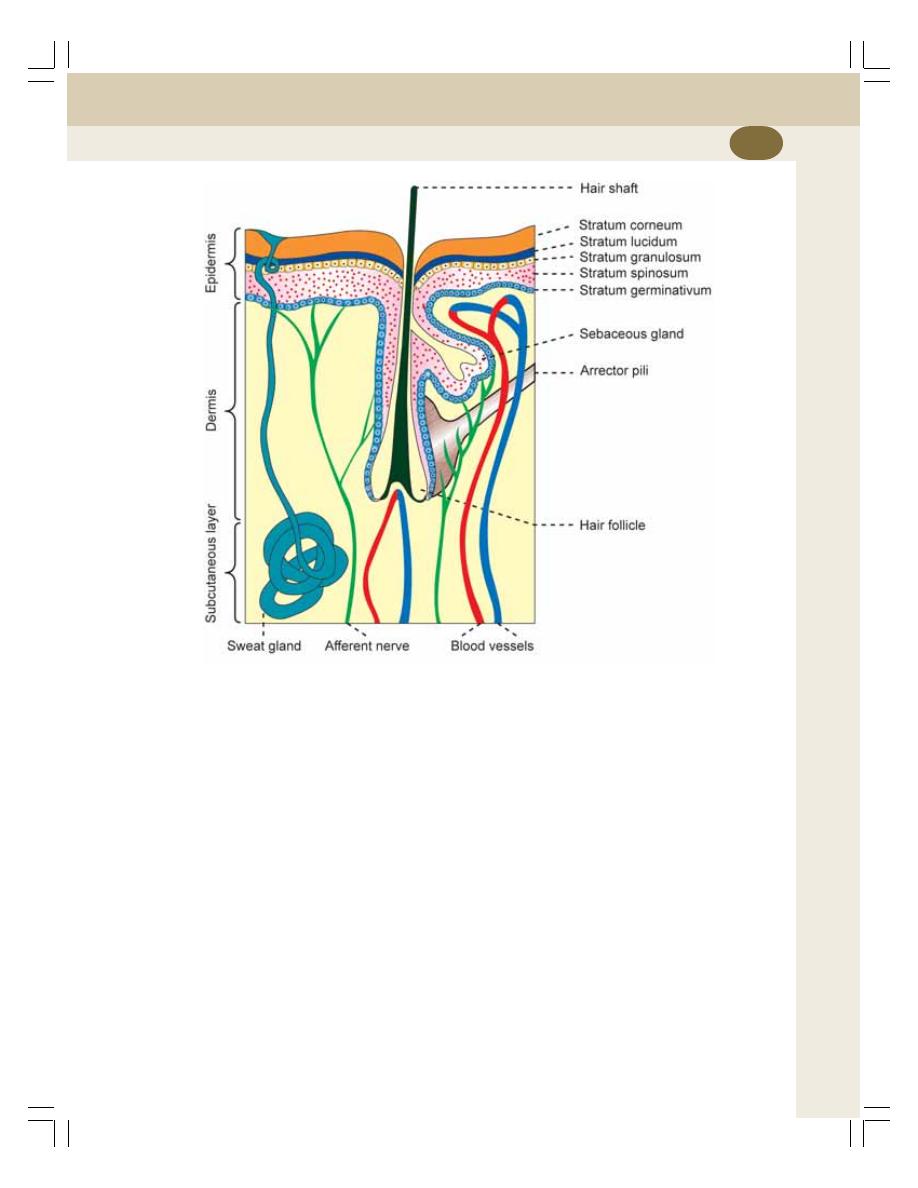
Chapter 42 Skin
245
COLOR OF THE SKIN
The color of the skin depends upon two important
factors:
1. Pigmentation of skin
2. Hemoglobin in the blood.
1. Pigmentation of the Skin
Cells of the skin contain a brown pigment
called melanin. Melanin is synthesized by
melanocytes which are present mainly in the
stratum germinativum and stratum spinosum of
epidermis. After synthesis, this pigment
spreads to the cells of the other layers.
Melanin
Melanin is the skin pigment and it forms the major
color determinant of human skin. Skin becomes
dark when melanin content increases. It is protein
in nature and it is synthesized from the amino
acid tyrosine via dihydroxyphenylalanine (DOPA).
2. Hemoglobin in Blood
The amount and the nature of hemoglobin that
circulates in the cutaneous blood vessels play
an important role in the coloration of the skin.
Skin becomes:
i. Pale when hemoglobin content decreases
ii. Pink when blood rushes to skin due to
cutaneous vasodilatation (blushing)
iii. Bluish during cyanosis which is caused
by excess amount of reduced hemoglobin.
GLANDS OF SKIN
The skin contains two types of glands, sebaceous
glands and the sweat glands.
FIGURE 42-1: Structure of skin

Renal Physiology and Skin
246
SEBACEOUS GLANDS
Sebaceous glands are simple or branched
alveolar glands situated in the dermis of the
skin. These glands are ovoid or spherical in
shape and open into the neck of the hair follicle
through a duct. In some areas like face, lips,
nipple, glans penis and labia minora the
sebaceous glands open directly into the exterior.
The sebaceous glands secrete an oily
substance called sebum.
Composition of Sebum
Sebum contains:
1. Free fatty acids
2. Triglycerides
3. Squalene
4. Sterols
5. Waxes
6. Paraffin.
Functions of Sebum
1. The free fatty acid content of the sebum has
antibacterial and antifungal actions. Thus, it
prevents the infection of skin by bacteria or
fungi
2. The lipid nature of sebum keeps the skin
smooth and oily. It protects the skin from
unnecessary desquamation and injury caused
by dryness
3. The lipids of the sebum prevent heat loss
from the body. It is particularly useful in cold
climate.
Activation of Sebaceous Glands at Puberty
Sebaceous glands are inactive till puberty. At the
time of puberty these glands are activated by sex
hormones in both males and females.
At the time of puberty particularly in males,
due to the increased secretion of sex hormones
especially dehydroepiandrosterone, the
sebaceous glands are stimulated suddenly. It
leads to the development of acne on the face.
Acne
Acne is the localized inflammatory condition of
the skin characterized by pimples on face, chest
and back. It occurs because of over activity of
sebaceous glands. Acne vulgaris is the common
type of acne that is developed during adolescence.
Acne disappears within few years when the
sebaceous glands become adapted to the sex
hormones.
SWEAT GLANDS
Sweat glands are of two types:
I. Eccrine glands
II. Apocrine glands.
Eccrine Glands
The eccrine glands are tubular glands distributed
throughout the body (Table 42-1). These glands
open out through the sweat pore.
Secretory Activity of Eccrine Glands
Eccrine glands function throughout life since
birth. These glands secrete a clear watery sweat.
The secretion increases during increase in
temperature and emotional conditions.
Eccrine glands play important role in
regulating the body temperature by secreting
sweat. Sweat contains water, sodium chloride,
urea and lactic acid.
Control of Eccrine Glands
Eccrine glands are under nervous control and
are supplied by sympathetic postganglionic
cholinergic nerve fibers, which secrete
acetylcholine. Stimulation of these nerves causes
secretion of sweat.
Apocrine Glands
Apocrine glands are situated only in certain areas
of the body like axilla, pubis, areola and
umbilicus. These glands are also tubular in nature
but open into the hair follicles.
Secretory Activity of Apocrine Glands
Apocrine sweat glands are nonfunctional till
puberty and start functioning only at the time of
puberty. In old age, the function of these glands
gradually declines.
The secretion of the apocrine glands is thick
and milky. At the time of secretion, it is odorless.

Chapter 42 Skin
247
When microorganisms grow in this secretion, a
characteristic odor develops in the regions where
apocrine glands are present. Secretion increases
only in emotional conditions.
The apocrine glands do not play any role in
temperature regulation like eccrine gland.
Control of Apocrine Glands
The apocrine glands are innervated by
sympathetic adrenergic nerve fibers. But, the
secretory activity is not under nervous control.
However, adrenaline from adrenal medulla
causes secretion by apocrine glands.
Glands of eyelids, glands of external auditory
meatus and mammary glands are the modified
apocrine glands.
FUNCTIONS OF THE SKIN
The primary function of skin is the protection of
organs. However, it has many other important
functions also.
1. PROTECTIVE FUNCTION
Skin forms the covering of all the organs of the
body and protects these organs from the
following factors:
i. Bacteria and toxic substances
ii. Mechanical blow
iii. Ultraviolet rays.
i.
Protection from Bacteria and Toxic
Substances
Skin covers the organs of the body and protects
the organs from having direct contact with
external environment. Thus, it prevents the
bacterial infection.
The lysozyme secreted in skin destroys the
bacteria. The stratum corneum of epidermis is
responsible for the protective function of skin.
This layer also offers resistance against toxic
chemicals like acids and alkalis.
ii. Protection from Mechanical Blow
The skin is not tightly placed over the underlying
organs or tissues. It is somewhat loose and
moves over the underlying subcutaneous
tissues. So, the mechanical impact of any blow
to the skin is not transmitted to the underlying
tissues.
iii. Protection from Ultraviolet Rays
Skin protects the body from ultraviolet rays of
sunlight. Exposure to sunlight or to any other
source of ultraviolet rays increases the
production of melanin pigment in skin. Melanin
absorbs ultraviolet rays. At the same time, the
thickness of stratum corneum increases. This
layer of epidermis also absorbs the ultraviolet
rays.
TABLE 42-1: Differences between eccrine and apocrine sweat glands
Features
Eccrine glands
Apocrine glands
1. Distribution
Throughout the body
Only in limited areas like axilla,
pubis, areola and umbilicus
2. Opening
Exterior through sweat pore
Into hair follicle
3. Period of functioning
Function throughout life
Start functioning only at puberty
4. Secretion
Clear and watery
Thick and milky
5. Regulation of body
Play important role in temperature Do not play any role in
temperature
regulation
temperature regulation
6. Conditions when secretion
During increased temperature
Only during emotional conditions
increases
and emotional conditions
7. Control of secretory activity
Under nervous control
Under hormonal control
8. Nerve supply
Sympathetic cholinergic fibers
Sympathetic adrenergic fibers

Renal Physiology and Skin
248
2. SENSORY FUNCTION
Skin is considered as the largest sense organ in
the body. It has many nerve endings, which form
the specialized cutaneous receptors (Chapter
85).
These receptors are stimulated by the
sensations of touch, pain, pressure or
temperature sensation and convey these
sensations to the brain via afferent nerves. At
the brain level, the perception of different
sensations occurs.
3. STORAGE FUNCTION
Skin stores fat, water, chloride and sugar. It can
also store blood by the dilatation of the
cutaneous blood vessels.
4. SYNTHETIC FUNCTION
Vitamin D
3
is synthesized in skin by the action
of ultraviolet rays from sunlight on cholesterol
(Chapter 47).
5. REGULATION OF BODY
TEMPERATURE
Skin plays an important role in the regulation of
body temperature. Excess heat is lost from the
body through skin by radiation, conduction,
convection and evaporation. Sweat glands of the
skin play active part in heat loss by secreting
sweat. The lipid content of sebum prevents loss
of heat from the body in cold environment. More
details are given in next chapter.
6. REGULATION OF WATER AND
ELECTROLYTE BALANCE
Skin regulates water balance and electrolyte
balance by excreting water and salts through
sweat.
7. EXCRETORY FUNCTION
Skin excretes small quantities of waste materials
like urea, salts and fatty substance.
8. ABSORPTIVE FUNCTION
Skin absorbs the fat soluble substances and
some ointments.
9. SECRETORY FUNCTION
Skin secretes sweat through sweat glands and
sebum through sebaceous glands. By secreting
sweat, skin regulates body temperature and
water balance. Sebum keeps the skin smooth
and moist.
INTRODUCTION
BODY TEMPERATURE
NORMAL BODY TEMPERATURE
TEMPERATURE AT DIFFERENT PARTS OF THE BODY
VARIATIONS OF BODY TEMPERATURE
HEAT BALANCE
HEAT GAIN OR HEAT PRODUCTION IN THE BODY
HEAT LOSS FROM THE BODY
REGULATION OF BODY TEMPERATURE
HEAT LOSS CENTER
HEAT GAIN CENTER
MECHANISM OF TEMPERATURE REGULATION
INTRODUCTION
The living organisms are classified into two
groups depending upon the maintenance
(regulation) of body temperature:
1. Homeothermic animals
2. Poikilothermic animals.
HOMEOTHERMIC ANIMALS
Homeothermic animals are the animals in which
the body temperature is maintained at a constant
level irrespective of the environmental tem-
perature. Birds and mammals including man
belong to this category. They are also called
warm blooded animals.
POIKILOTHERMIC ANIMALS
Poikilothermic animals are the animals in
which the body temperature is not constant. It
varies according to environmental tempe-
rature. Amphibians and reptiles are the
poikilothermic animals. These animals are also
called cold blooded animals.
BODY TEMPERATURE
Body temperature can be measured by placing
the clinical thermometer in different parts of the
body such as:
1. Mouth (oral temperature)
2. Axilla (axillary temperature)
3. Rectum (rectal temperature)
4. Over the skin (surface temperature).
NORMAL BODY TEMPERATURE
The normal body temperature in human is 37°C
(98.6°F) when measured by placing the clinical
thermometer in the mouth (oral temperature). It
Body Temperature
43
43
43
43
43

Renal Physiology and Skin
250
varies between 35.8°C and 37.3°C (96.4° and
99.1°F).
TEMPERATURE AT DIFFERENT
PARTS OF THE BODY
Axillary temperature is 0.3 to 0.6°C (0.5 to 1°F)
lower than the oral temperature. And, the rectal
temperature is 0.3 to 0.6°C (0.5 to 1°F) higher
than oral temperature. The superficial tempera-
ture (skin or surface temperature) varies between
29.5° and 33.9°C (85.1° and 93°F).
Core Temperature
Core temperature is the average temperature of
structures present in deeper part of the body. The
core temperature is always more than oral or
rectal temperature. It is about 37.8°C (100°F).
VARIATIONS OF BODY TEMPERATURE
Physiological Variations
1. Age
In infants, the body temperature varies in accor-
dance to environmental temperature for the first
few days after birth. It is because the temperature
regulating system does not function properly
during infancy. In children the temperature is
slightly (0.5°C) more than in adults because of
more physical activities. In old age, since the
heat production is less, the body temperature
decreases slightly.
2. Sex
In females, the body temperature is less because
of low basal metabolic rate when compared to
that of males. During menstrual phase it decrea-
ses slightly.
3. Diurnal variation
In early morning, the temperature is 1°C less.
In the afternoon, it reaches the maximum (about
1°C more than normal).
4. After meals
The body temperature rises slightly (0.5°C) after
meals.
5. Exercise
During exercise, the temperature raises due to
production of heat in muscles.
6. Sleep
During sleep, the body temperature decreases
by 0.5°C.
7. Emotion
During emotional conditions, the body temperature
increases.
8. Menstrual cycle
In females, immediately after ovulation, the
temperature rises (0.5° to 1°C) sharply. It
decreases (0.5°C) during menstrual phase.
Pathological Variations
Abnormal increase in body temperature is called
hyperthermia or fever and decreased body
temperature is called hypothermia.
HEAT BALANCE
Regulation of body temperature depends upon
the balance between heat produced in the body
and the heat lost from the body.
HEAT GAIN OR HEAT PRODUCTION
IN THE BODY
The various mechanisms involved in the
production of heat in the body are:
1. Metabolic Activities
The major portion of heat produced in the body
is due to the metabolism of foodstuffs. Heat
production is more during metabolism of fat.
About 9 calories of heat is produced during
metabolism of fats, when 1 liter of oxygen is
utilized. For the same amount of oxygen,
carbohydrate metabolism produces 4.7 calories
of heat. Protein metabolism produces 4.5
calories/liter. Liver is the organ in which
maximum heat is produced due to metabolic
activity.

Chapter 43 Body Temperature
251
2. Muscular Activity
Heat is produced in the muscle both at rest and
during activities. During rest, heat is produced
by muscle tone. About 80% of heat of activity is
produced by the activity of skeletal muscles.
3. Role of Hormones
Thyroxine and adrenaline increase the heat
production by accelerating the metabolic acti-
vities.
4. Radiation of Heat from the Environment
Body gains heat by radiation. It occurs when the
environmental temperature is higher than the
body temperature.
5. Shivering
Shivering refers to shaking of the body caused
by rapid involuntary contraction or twitching of
the muscles during exposure to cold. It is a
compensatory physiological mechanism in the
body, during which enormous heat is produced.
HEAT LOSS FROM THE BODY
Maximum heat is lost from the body through skin
and small amount of heat is lost through
respiratory system, kidney and GI tract. When
environmental temperature is less than body
temperature, heat is lost from the body. Heat loss
occurs by the following methods:
1. Conduction
Heat is lost from the surface of the body to
other objects such as chair or bed by means of
conduction.
2. Radiation
Sixty percent of heat is lost by means of radia-
tion, i.e. transfer of heat by infrared electro-
magnetic radiation from body to other objects
through the surrounding air.
3. Convection
Heat is conducted to the air surrounding the body
and then carried away by air currents, i.e.
convection.
4. Evaporation – Insensible Perspiration
Normally, a small quantity of water is
continuously evaporated from skin and lungs.
We are not aware of it. So it is called insensible
perspiration or insensible water loss. It is about
50 mL/hour. When body temperature increases,
more heat is lost by evaporation of more water.
5. Panting
Panting is the rapid shallow breathing associated
with dribbling of more saliva. In some animals
like dogs which do not have sweat glands, heat
is lost by evaporation of water from lungs and
saliva by means of panting.
REGULATION OF BODY
TEMPERATURE
The body temperature is regulated by
hypothalamus which sets the normal range of
body temperature. The set point under normal
physiological conditions is 37°C. Hypothalamus
has two centers which regulate the body
temperature (Fig. 43-1):
A. Heat loss center
B. Heat gain center.
HEAT LOSS CENTER
This center is situated in preoptic nucleus of
anterior hypothalamus. Neurons in preoptic
nucleus are heat sensitive nerve cells which are
called thermoreceptors. Stimulation of preoptic
nucleus results in cutaneous vasodilatation and
sweating. Removal or lesion of this nucleus
increases the body temperature.
HEAT GAIN CENTER
It is otherwise known as heat production center.
It is situated in posterior hypothalamic nucleus.
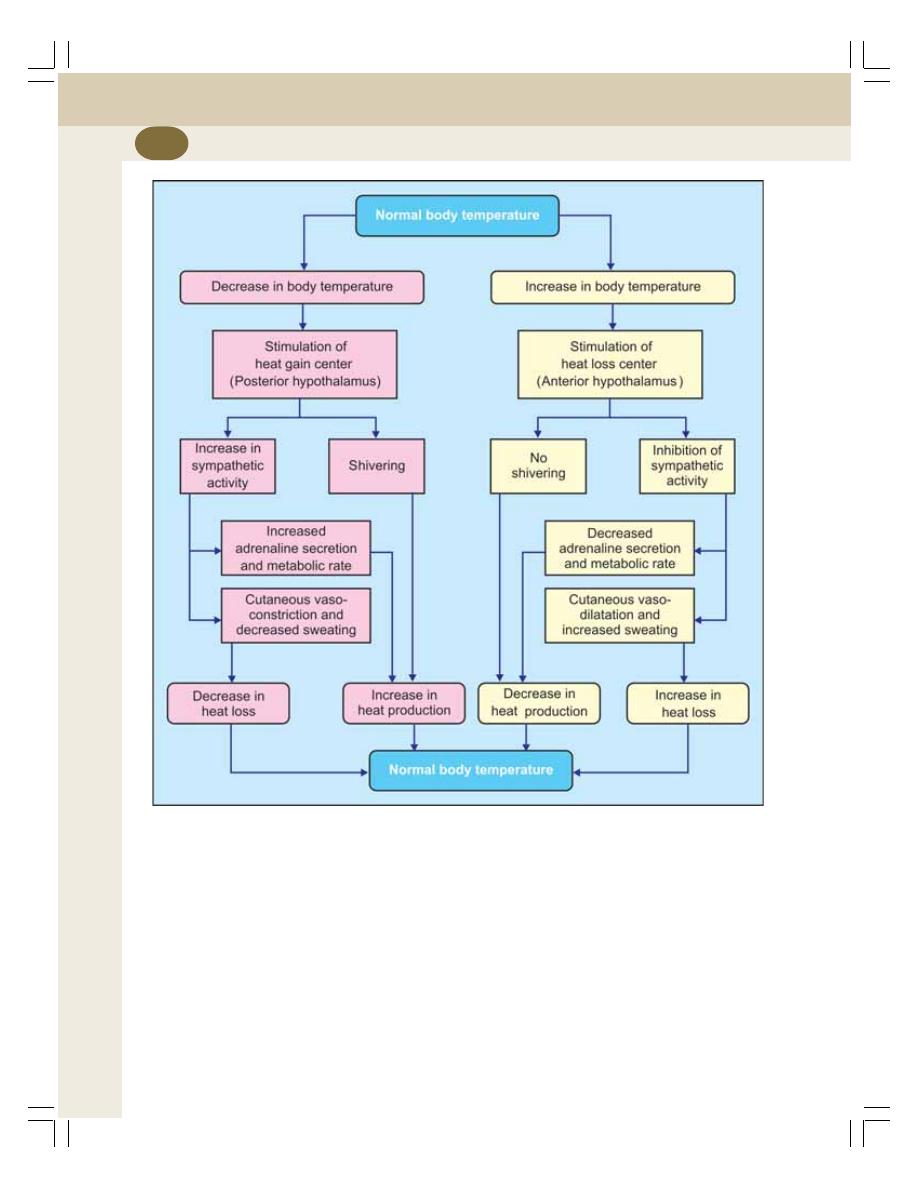
Renal Physiology and Skin
252
Stimulation of posterior hypothalamic nucleus
causes shivering. The removal or lesion of this
nucleus leads to fall in body temperature.
MECHANISM OF TEMPERATURE
REGULATION
When Body Temperature Increases
When body temperature increases, blood
temperature also increases. When blood with
increased temperature passes through hypo-
thalamus, it stimulates the thermoreceptors
present in the heat loss center in preoptic
nucleus. Now, the heat loss center brings the
temperature back to normal by two mechanisms:
1. Promotion of heat loss
2. Prevention of heat production
1.Promotion of heat loss
Heat loss center promotes heat loss from the
body by:
FIGURE 43-1: Regulation of body temperature

Chapter 43 Body Temperature
253
i. Increasing the secretion of sweat: When
sweat secretion increases, more water is
lost from skin along with heat
ii. Inhibiting the sympathetic centers in
posterior hypothalamus: This causes
cutaneous vasodilatation. Now, blood flow
through skin increases causing excess
sweating. It increases the heat loss
through sweat leading to decrease in body
temperature.
2. Prevention of heat Production
Heat loss center prevents heat production in the
body by inhibiting mechanisms involved in heat
production such as shivering and chemical
(metabolic) reactions.
When Body Temperature Decreases
When the body temperature decreases it is
brought back to normal by two mechanisms:
1. Prevention of heat loss
2. Promotion of heat production.
1. Prevention of heat loss
When body temperature decreases, the preoptic
thermoreceptors are not activated. So, the
posterior hypothalamus is not inhibited. This
causes cutaneous vasoconstriction. The blood
flow to skin decreases, and so the heat loss is
prevented.
2. Promotion of heat production
The heat production is promoted by two ways:
i. Shivering: The primary motor center for
shivering is situated in posterior hypo-
thalamus near the wall of the III ventricle.
When body temperature is low, this center
is activated by heat gain center and,
shivering occurs. Enormous heat is
produced during shivering due to severe
muscular activities.
ii. Increased metabolic reactions: The
sympathetic centers, which are activated
by heat gain center, stimulate secretion
of adrenaline and noradrenaline. These
hormones, particularly adrenaline increase
heat production by accelerating cellular
metabolic activities.
Simultaneously, hypothalamus secretes
thyrotropic releasing hormone. It causes release
of thyroid stimulating hormone from pituitary. It
in turn increases release of thyroxine from
thyroid. Thyroxine accelerates the metabolic
activities in the body and increases heat pro-
duction.
Chemical thermogenesis: It is the process in
which heat is produced in the body by metabolic
activities induced by hormones.

Renal Physiology and Skin
254
LONG QUESTIONS
1. Describe the process of urine formation.
2. What are the different stages of urine
formation? Explain the glomerular filtration.
3. Give an account of role of renal tubule in
the process of urine formation.
4. What is counter current mechanism?
Describe the anatomical and physiological
basis of counter current mechanism in
kidney.
5. Describe the mechanism involved in the
concentration of urine.
6. Give an account of micturition.
8. What is normal body temperature? Explain
heat balance and regulation of body
temperature. Add a note on fever.
SHORT QUESTIONS
1. Functions of kidney.
2. Structure of nephron.
3. Renal corpuscle.
4. Juxtaglomerular apparatus.
5. Renin–angiotensin system.
6. Peculiarities of renal circulation.
7. Autoregulation of renal circulation.
QUESTIONS IN RENAL PHYSIOLOGY AND SKIN
8. Glomerular filtration rate.
9. Effective filtration pressure in kidney.
10. Reabsorption of glucose in renal tubule.
11. Reabsorption of water in renal tubule.
12. Reabsorption of sodium in renal tubules.
13. Reabsorption of bicarbonate in renal
tubules.
14. Secretion in renal tubule.
15. Renal medullary gradient.
16. Counter current multiplier.
17. Counter current exchanger.
18. Actions of hormones on renal tubules.
19. Acidification of urine.
20. Plasma clearance.
21. Nerve supply to urinary bladder and sphinc-
ters.
22. Cystometrogram.
23. Micturition reflex.
24. Structure of skin.
25. Functions of skin.
26. Sebaceous glands.
27. Sweat glands.
28. Differences between eccrine glands and
apocrine glands.
29. Regulation of body temperature.
30. Heat balance.
Questions in Renal Physiology and Skin
254

Endocrinology
44. Introduction to Endocrinology .............................................. 257
45. Pituitary Gland ..................................................................... 261
46. Thyroid Gland ...................................................................... 274
47. Parathyroid Glands and Physiology of Bone ....................... 283
48. Endocrine Functions of Pancreas ....................................... 295
49. Adrenal Cortex .................................................................... 303
50. Adrenal Medulla .................................................................. 313
51. Endocrine Functions of Other Organs ................................. 318
52. Local Hormones .................................................................. 321
S E C T I O N
6
C H A P T E R S

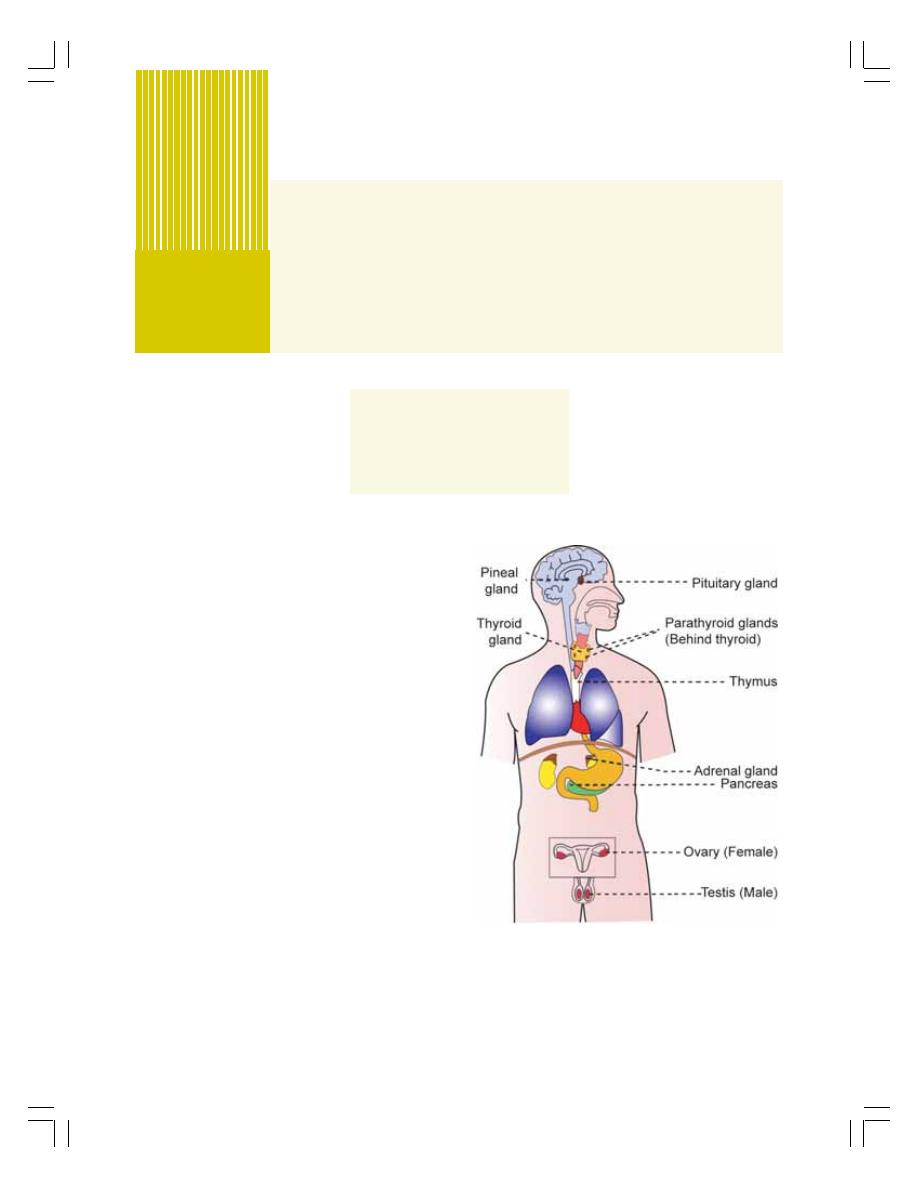
INTRODUCTION
ENDOCRINE GLANDS
HORMONES
HORMONAL ACTION
INTRODUCTION
All the physiological activities are regulated by
two major systems in the body.
1. Nervous system
2. Endocrine system.
These two systems interact with one another
and regulate the body functions. This section
deals with endocrine system and Section 10
deals with nervous system. Endocrine system
functions by secreting some chemical
substances called hormones.
ENDOCRINE GLANDS
Endocrine glands are the glands which
synthesize and release the classical hormones
into the blood. The endocrine glands are also
called ductless glands because the hormones
secreted by them are released directly into blood
without any duct.
Major endocrine glands are shown in
Fig. 44-1.
The hormones secreted by the major
endocrine glands are listed in Table 44-1.
The hormones secreted by the gonads are
given in Table 44-2.
Introduction to
Endocrinology
44
FIGURE 44-1: Major endocrine glands
The hormones secreted by other organs are
given in Table 44-3.
The local hormones are listed in Table 44-4.
Endocrinology
258
TABLE 44-2: Hormones secreted by gonads
Testis
1. Testosterone
2. Dihydrotestosterone
3. Androstenedion
Ovary
1. Estrogen
2. Progesterone
TABLE 44-1: Hormones secreted by major
endocrine glands
Anterior pituitary 1. Growth hormone (GH)
2. Thyroid stimulating hormone
(TSH)
3. Adrenocorticotropic hormone
(ACTH)
4. Follicle stimulating
hormone (FSH)
5. Luteinizing hormone (LH)
6. Prolactin
Posterior pituitary 1. Antidiuretic hormone (ADH)
2. Oxytocin
Thyroid gland
1. Thyroxine (T
4
)
2. Tri-iodothyronine (T
3
)
3. Calcitonin
Parathyroid gland 1. Parathormone
Pancreas — islets 1. Insulin
of Langerhans
2. Glucagon
3. Somatostatin
4. Pancreatic polypeptide
Adrenal cortex
Mineralocorticoids
1. Aldosterone
2. 11 deoxycorticosterone
Glucocorticoids
1. Cortisol
2. Corticosterone
Sex hormones
1. Androgens
2. Estrogen
3. Progesterone
Adrenal medulla
Catecholamines
1. Adrenaline (Epinephrine)
2. Noradrenaline
(Norepinephrine)
3. Dopamine
HORMONES
CLASSIFICATION OF HORMONES
Based on chemical nature the hormones are
classified into three types:
1. Steroid hormones
2. Protein hormones
3. Derivatives of the amino acid, called tyrosine.
Classification of hormones depending upon
their chemical nature are given in Table 44-5.
TABLE 44-4: Local hormones
1. Prostaglandins
2. Thromboxanes
3. Prostacyclin
4. Leukotrienes
5. Lipoxins
6. Acetylcholine
7. Serotonin
8. Histamine
9. Substance P
10. Heparin
11. Bradykinin
12. Gastrointestinal hormones
TABLE 44-3: Hormones secreted by other organs
Pineal gland
Heart
1. Melatonin
1. Arial natriuretic peptide
2. Brain natriuretic peptide
3. C-type natriuretic peptide
Thymus
Placenta
1. Thymosin
1. Human chorionic
2. Thymin
gonadotropin (hCG)
2. Human chorionic
somatomammotropin
Kidney
3. Estrogen
1. Erythropoietin
4. Progesterone
2. Renin
3. 1,25 dihydroxy-
cholecalciferol
(calcitriol)
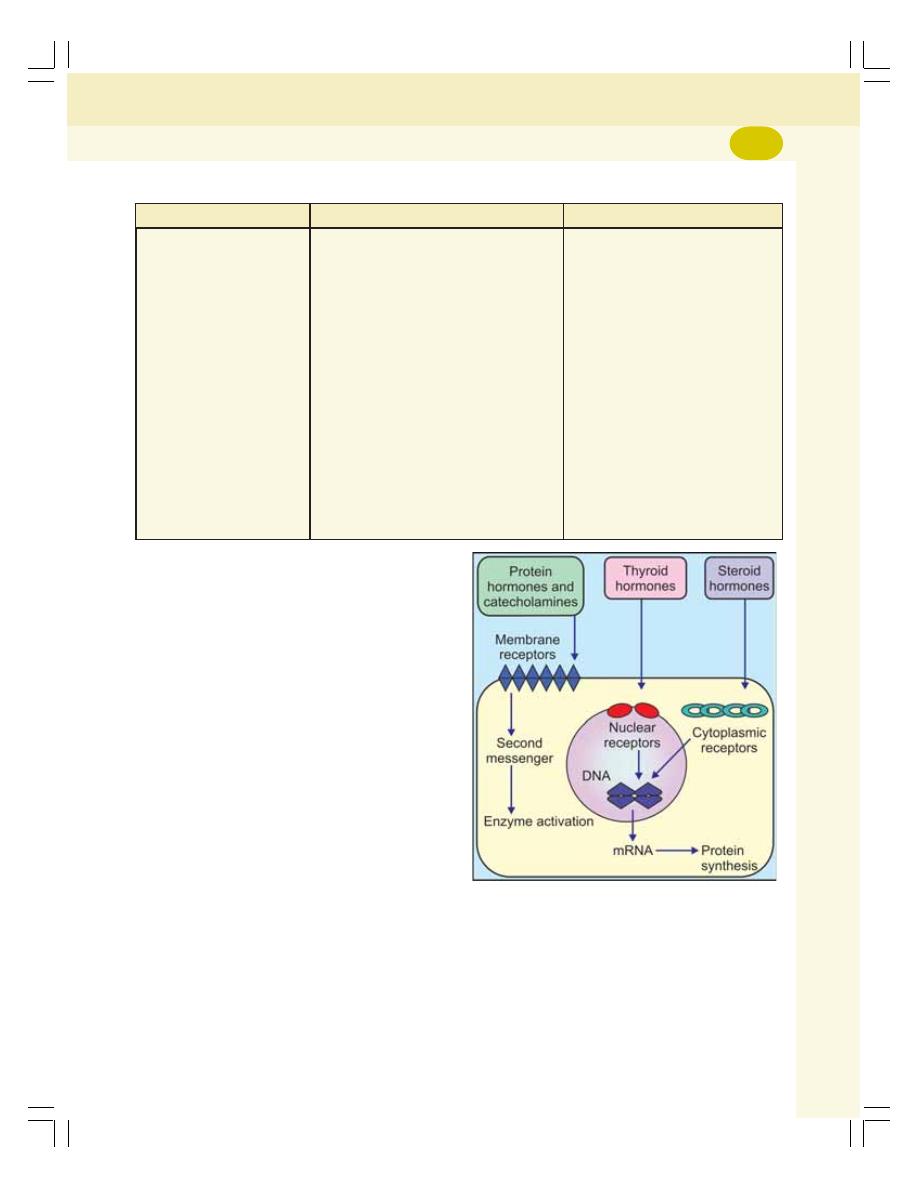
Chapter 44 Introduction to Endocrinology
259
HORMONAL ACTION
Hormone does not act directly on the cellular
structures. First it combines with receptors
present on the target cells and forms a hormone-
receptor complex. This hormone-receptor
complex induces various changes or reactions
in the target cells.
HORMONE RECEPTORS
The hormone receptors are the large proteins
present in the target cells. Each receptor is
specific for one single hormone, i.e. each
receptor can combine with only one hormone.
Situation of the Hormone Receptors
The hormone receptors are situated either in cell
membrane or cytoplasm or nucleus of the cells
as follows:
1. Cell membrane: Receptors of protein
hormones and adrenal medullary hormones
(catecholamines) are situated in the cell
membrane (Fig. 44-2)
2. Cytoplasm: Receptors of steroid hormones
are situated in cytoplasm of target cells
3. Nucleus: Receptors of thyroid hormones are
in the nucleus of the cell.
MECHANISM OF HORMONAL ACTION
On the target cell, the hormone–receptor complex
acts by any one of the following mechanisms:
1. By altering the permeability of the cell
membrane
2. By activating the intracellular enzyme
3. By activating the genes.
TABLE 44-5: Classification of hormones depending upon chemical nature
Steroids
Proteins
Derivatives of tyrosine
Aldosterone
Growth hormone (GH)
Thyroxine (T
4
)
11 deoxycorticosterone
Thyroid stimulating hormone (TSH)
Tri-iodothyronine (T
3
)
Cortisol
Adrenocorticotropic hormone (ACTH)
Adrenaline (Epinephrine)
Corticosterone
Follicle stimulating hormone (FSH)
Noradrenaline (Norepinephrine)
Testosterone
Luteinizing hormone (LH)
Dopamine
Dihydrotestosterone
Prolactin
Dehydroepiandrosterone
Antidiuretic hormone (ADH)
Androstenedione
Oxytocin
Estrogen
Parathormone
Progesterone
Calcitonin
Insulin
Glucagon
Somatostatin
Pancreatic polypeptide
Human chorionic gonadotropin (hCG)
Human chorionic somatomammotropin
FIGURE 44-2: Situation of hormonal receptors
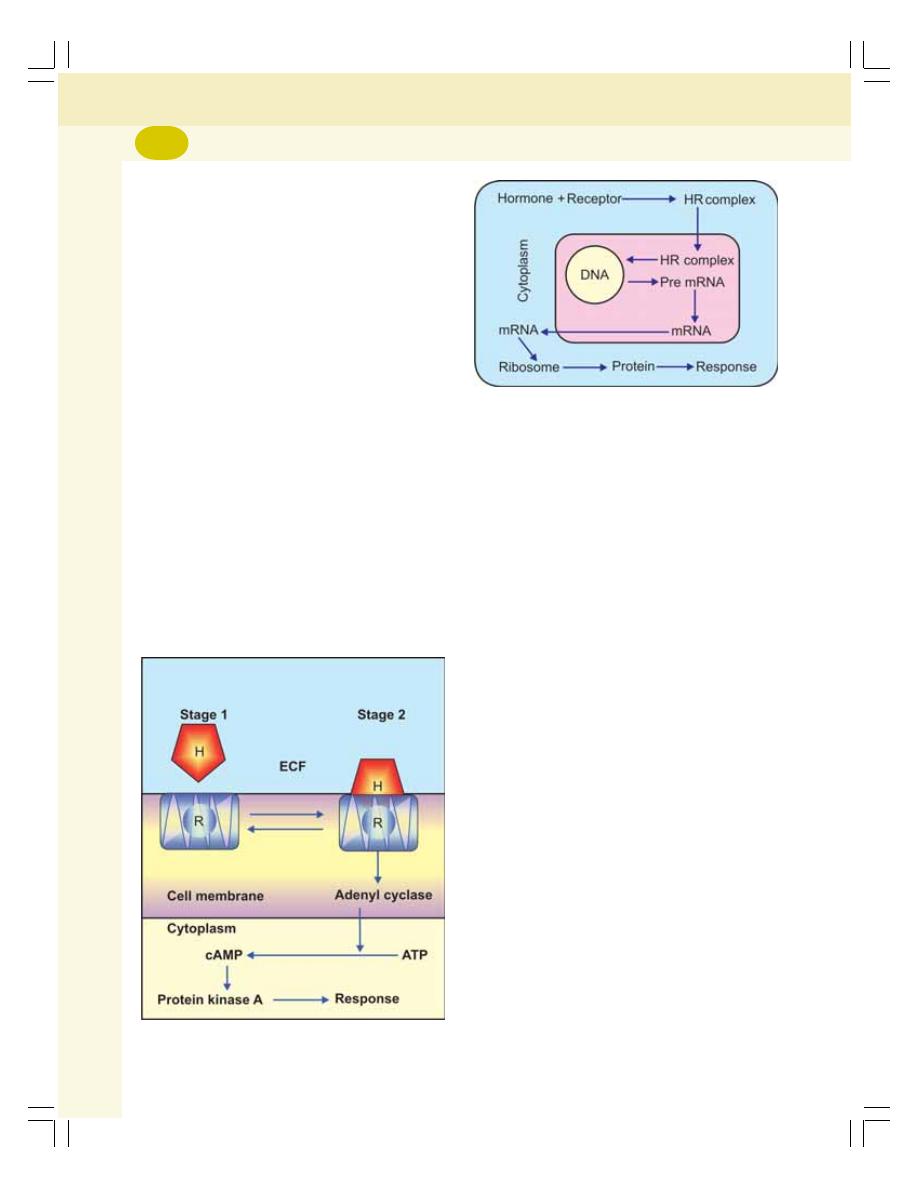
Endocrinology
260
1. By Altering the Permeability of Cell
Membrane
The neurotransmitter substances in a synapse
or neuromuscular junction act by changing the
permeability of postsynaptic membrane.
For example, in a neuromuscular junction,
when an impulse (action potential) reaches the
axon terminal of the motor nerve, acetylcholine
is released from the vesicles. Acetylcholine inc-
reases permeability of postsynaptic membrane
by opening the ligand gated sodium channels.
So, sodium ions enter the neuromuscular junction
from ECF through the channels. Sodium ions
alter the resting membrane potential so that,
endplate potential is developed.
2. By Activating the Intracellular Enzyme
The protein hormones and the catecholamines
act by activating the intracellular enzymes.
The hormone, which acts on a target cell, is
called first messenger or chemical mediator. This
hormone, in combination with the receptor forms
hormone-receptor complex. This in turn activates
the enzymes of the cell and causes the formation
of another substance called the second
messenger.
FIGURE 44-3: Mode of action of protein hormones
and catecholamines. H = Hormone, R = Receptor
FIGURE 44-4: Mode of action of steroid hormones.
Thyroid hormones also act in the similar way. But their
receptors are in the nucleus. HR = Hormone-receptor
complex
The second messenger produces the effects
of the hormone inside the cells. The most
common second messenger is adenosine mono-
phosphate (cyclic AMP or cAMP).
Sequence of events in the activation of
second messenger:
i. The hormone binds with the receptor in
the cell membrane and forms the hor-
mone-receptor complex which activates
the enzyme adenyl cyclase
ii. Adenyl cyclase converts the ATP of the
cytoplasm into cAMP. Cyclic AMP exe-
cutes the actions of hormone inside the
cell, by stimulating the enzymes like
protein kinase A (Fig. 44-3).
3. By Acting on Genes
Thyroid and steroid hormones act by activating
the genes of the target cells.
Sequence of events during activation of
genes:
i. The hormone enters the interior of the
cell and binds with receptor in cytoplasm
(steroid hormone) or in nucleus (thyroid
hormone) and forms hormone-receptor
complex
ii. This complex binds to DNA and increases
transcription of mRNA
iii. The mRNA moves out of nucleus and
reaches ribosomes and activates them
iv. The activated ribosomes produce large
quantities of proteins which produce the
physiological responses in the target cells
(Fig. 44-4).
INTRODUCTION
ANTERIOR PITUITARY
PARTS
HISTOLOGY
HORMONES
REGULATION
GROWTH HORMONE
OTHER HORMONES
POSTERIOR PITUITARY
PARTS
HISTOLOGY
HORMONES
ANTIDIURETIC HORMONE
OXYTOCIN
APPLIED PHYSIOLOGY – DISORDERS OF PITUITARY GLAND
HYPERACTIVITY OF ANTERIOR PITUITARY
HYPOACTIVITY OF ANTERIOR PITUITARY
HYPERACTIVITY OF POSTERIOR PITUITARY
HYPOACTIVITY OF POSTERIOR PITUITARY
HYPOACTIVITY OF ANTERIOR AND POSTERIOR PITUITARY
Pituitary Gland
45
INTRODUCTION
The pituitary gland is also known as hypophysis.
It is a small gland that lies at the base of the
brain. It is connected with the hypothalamus by
the pituitary stalk or hypophyseal stalk.
Pituitary gland is divided into two portions:
1. Anterior pituitary or adenohypophysis
2. Posterior pituitary or neurohypophysis.
Even though anterior pituitary and posterior
pituitary are situated in close approximation, both
are entirely different in their development,
structure and function.
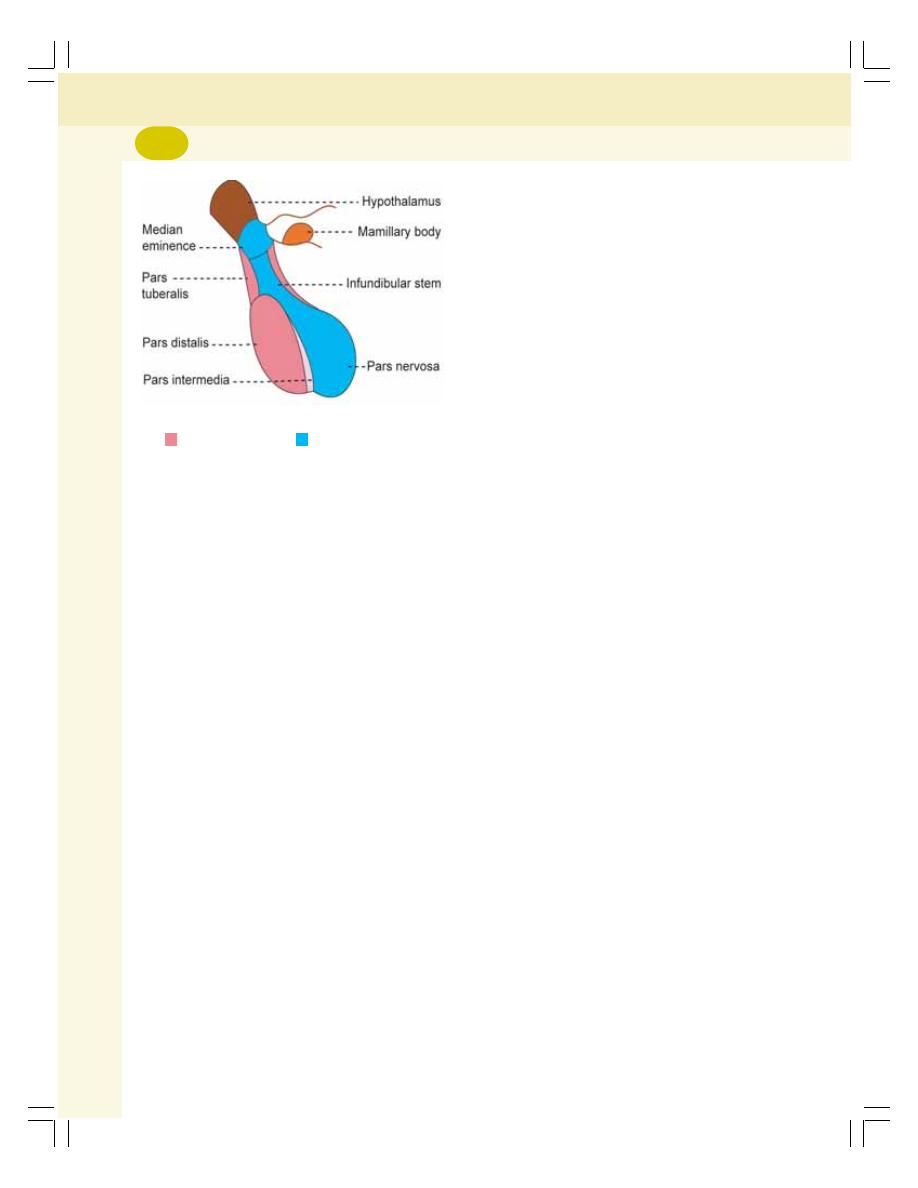
Endocrinology
262
ANTERIOR PITUITARY
PARTS
Anterior pituitary consists of three divisions
(Fig. 45-1):
1. Pars distalis
2. Pars tuberalis
3. Pars intermedia.
HISTOLOGY
Depending upon the staining property, the cells
of anterior pituitary are classified into two types:
1. Chromophobe cells which do not have
granules and stain poorly. These cells are not
secretory in nature
2. Chromophil cells which contain large granules
and are stained darkly. According to the
staining nature, the chromophil cells are of
two types, acidophilic cells or alpha cells and
basophilic cells or beta cells.
Based on the secretory nature the chromophil
cells are classified into five types:
i. Somatotropes which secrete growth
hormone
ii. Corticotropes which secrete adreno-
corticotropic hormone
iii. Thyrotropes which secrete thyroid
stimulating hormone
iv. Gonadotropes which secrete follicle stimu-
lating hormone and luteinizing hormone
v. Lactotropes which secrete prolactin.
Somatotropes and lactotropes are acidophilic
cells, whereas others are basophilic cells.
HORMONES SECRETED BY
ANTERIOR PITUITARY
Anterior pituitary is also known as the master
gland because it regulates many other endocrine
glands. Six hormones are secreted by the ante-
rior pituitary:
1. Growth hormone (GH) or somatotropic hor-
mone (STH)
2. Thyroid stimulating hormone (TSH) or
thyrotropic hormone
3. Adrenocorticotropic hormone (ACTH)
4. Follicle stimulating hormone (FSH)
5. Luteinizing hormone (LH in females) or
interstitial cell stimulating hormone (ICSH
in males)
6. Prolactin.
FSH and LH are together called gonadotropic
hormones or gonadotropins because of their
action on the gonads.
Recently, the hormone
β-lipotropin is found
to be secreted by anterior pituitary.
REGULATION OF SECRETION OF
ANTERIOR PITUITARY HORMONES
Secretion of anterior pituitary hormones is regu-
lated by hypothalamus. Hypothalamus secretes
some releasing and inhibitory hormones (factors)
which are transported from hypothalamus to
anterior pituitary through hypothalamo-hypo-
physeal portal vessels.
Releasing and Inhibitory Hormones
Secreted by Hypothalamus
1. Growth hormone releasing hormone (GHRH)
— stimulates the release of GH
2. Growth hormone releasing polypeptide
(GHRP) — stimulates the release of GHRH
and GH
3. Growth hormone inhibitory hormone (GHIH)
or somatostatin — inhibits GH release
4. Thyrotropic releasing hormone (TRH) —
stimulates the release of TSH
5. Corticotropin releasing hormone (CRH) —
stimulates the release of ACTH
FIGURE 45-1: Parts of pituitary gland
Adenohypophysis
Neurohypophysis

Chapter 45 Pituitary Gland
263
6. Gonadotropin releasing hormone (GnRH) —
the release of the gonadotropins — FSH and
LH
7. Prolactin inhibitory hormone (PIH) — inhibits
prolactin secretion.
GROWTH HORMONE
Growth hormone (GH) is secreted by the
acidophils of the anterior pituitary, which are also
known as somatotropes.
It is protein in nature having a single chain
polypeptide with 191 amino acids. GH is trans-
ported in blood by GH binding proteins (GHBPs).
The basal level of GH concentration in blood
of the normal adult is up to 300 g/dL and in
children it is about 500 ng/dL. Its daily output in
adults is 0.5 to 1.0 mg.
Actions of Growth Hormone
GH is responsible for the growth of almost all
tissues of the body, which are capable of growing.
It actually increases the size and number of
cells by increasing the mitotic division. GH also
causes specific differentiation of certain types of
cells like bone cells and muscle cells.
GH also acts on the metabolism of all the
three major types of foodstuffs in the body, viz.
proteins, lipids and carbohydrates.
1. On Metabolism
GH increases the synthesis of proteins, mobili-
zation of lipids and conservation of carbo-
hydrates.
A. On protein metabolism
GH accelerates the synthesis of protein by:
i. Increasing the amino acid transport through
the cell membrane.
ii. Increasing the RNA translation. Because of
this, the ribosomes are activated and more
proteins are synthesized.
iii. Increasing the transcription of DNA to RNA.
This, in turn accelerates the synthesis of
proteins in the cells.
iv. Decreasing the catabolism of protein.
v. Promoting the anabolism of proteins
indirectly by causing release of insulin
which has anabolic effect on proteins.
B. On fat metabolism
GH mobilizes fats from adipose tissue. Because
of this, the concentration of fatty acids increases
in the body fluids. These fatty acids are used for
the production of energy by the cells. So proteins
are spared.
During the utilization of fatty acids for the
production of energy, lot of acetoacetic acid is
produced by the liver and released into the body
fluids leading to ketosis. Sometimes excess
mobilization of fat from the adipose tissue causes
accumulation of fat in liver, resulting in fatty liver.
C. On carbohydrate metabolism
The main action of GH on carbohydrates is the
conservation of glucose.
The effects of GH on the carbohydrate
metabolism are:
i. Decrease in the peripheral utilization of
glucose for the production of energy.
ii. Increase in the deposition of glycogen in the
cells. Since, glucose is not utilized for energy
production by the cells, it is converted into
glycogen which is deposited in the cells.
iii. Decrease in the uptake of glucose by the
cells. As the deposition of glycogen
increases, the cells become saturated with
glycogen. Because of this, no more glucose
can enter the cells. So the blood glucose
level increases.
iv. Diabetogenic effect of GH: Hypersecretion
of GH increases blood glucose level
enormously. It causes continuous stimulation
of the
β cells in the islets of Langerhans in
pancreas and increases insulin secretion. In
addition to this, the GH also stimulates the
β cells of islets in pancreas directly and
causes secretion of insulin. Because of the
excess stimulation, the
β cells are burnt out
at one stage. This causes deficiency of
insulin, which leads to true diabetes mellitus
or full blown diabetes mellitus. This effect
of GH is called the diabetogenic effect.

Endocrinology
264
2. On Bones
In embryonic stage, GH is responsible for the
differentiation and the development of bone
cells. In later stages, GH increases the growth
of the skeleton. It increases both the length as
well as the thickness of the bones.
In the bones, GH increases:
i. Protein synthesis by chondriocytes and
osteogenic cells
ii. Multiplication of chondrocytes and osteo-
genic cells
iii. Formation of new bones by converting
chondrocytes into osteogenic cells
iv. Increases the calcium absorption from intes-
tine. By this GH enhances the availability of
calcium for mineralization of bone matrix.
GH increases the length of the bones until
epiphysis fuses with the shaft. Usually fusion
occurs at puberty. After the epiphyseal fusion,
length of the bones cannot be increased.
However, it stimulates the osteoblasts strongly.
So, the bone continues to grow in thickness
throughout the life. Particularly, the membranous
bones such as jaw bone and skull bones
become thicker under the influence of GH.
Mode of Action of GH on Bones and
Metabolism
GH acts on bones, growth and protein meta-
bolism through a substance called somatomedin,
which is secreted by liver. GH stimulates the liver
to secrete somatomedin. Sometimes, in spite of
normal secretion of GH, growth is arrested
(dwarfism) due to the absence or deficiency of
somatomedin.
Somatomedin
Somatomedin is a polypeptide. There are two
types of somatomedins.
i. Insulin like growth factor-I (IGF-I), which is
also called somatomedin-C
ii. Insulin like growth factor-II.
Among the two somatomedins, the somato-
medin-C (IGF-I) is responsible for the action of
bones on bones and metabolism.
Regulation of GH Secretion
Secretion of GH is regulated by hypothalamus
and feedback control.
Role of hypothalamus in the secretion of GH
Hypothalamus regulates GH secretion by
releasing three hormones:
1. GHRH that increases the secretion of GH by
stimulating the somatotropes of anterior
pituitary
2. GHRP that promotes the release of GHRH
from hypothalamus and GH from pituitary
3. GHIH or somatostatin which inhibits the
secretion of GH.
These three hormones are transported from
hypothalamus to anterior pituitary by hypo-
thalamo-hypophyseal portal blood vessels.
Hypothalamus is in turn influenced by many
factors which cause increase or decrease in GH
secretion.
Factors which increase the GH secretion:
1. Hypoglycemia
2. Fasting
3. Starvation
4. Exercise
5. Stress and trauma
6. Initial stages of sleep.
Factors which decrease the GH secretion:
1. Hyperglycemia
2. Increase in free fatty acids in blood
3. Later stages of sleep.
Feedback control
GH secretion is under negative feedback control
(Chapter 4). Hypothalamus releases GHRH and
GHRP, which in turn promote the release of GH
from anterior pituitary. GH acts on various
tissues. It also activates the liver cells to secrete
somatomedin-C (IGF-I).
Now, the somatomedin-C increases the
release of GHIH from hypothalamus. GHIH in
turn inhibits release of GH from pituitary.
Somatomedin also inhibits the release of GHRP
from hypothalamus. It acts on pituitary directly
and inhibits the secretion of GH (Fig. 45-2).
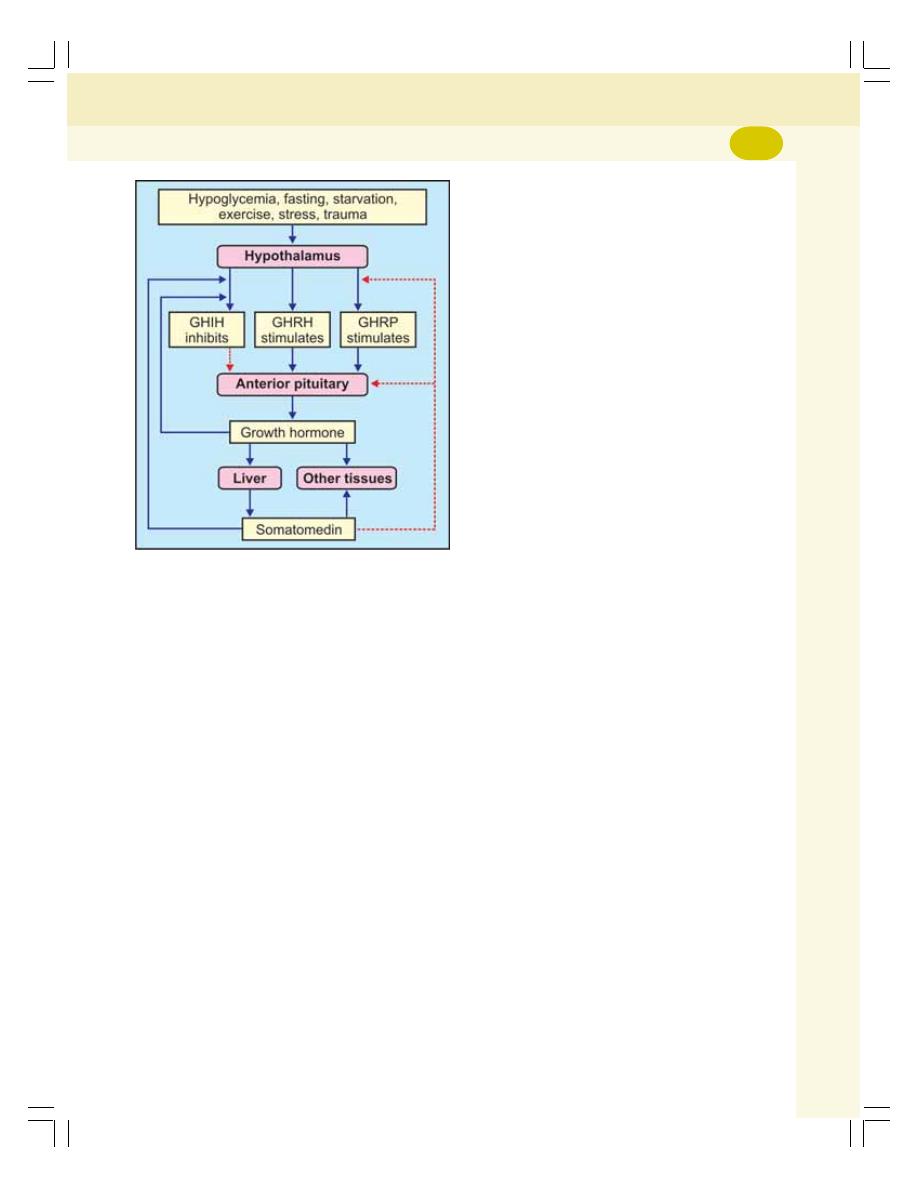
Chapter 45 Pituitary Gland
265
GH inhibits its own secretion by stimulating
the release of GHIH from hypothalamus. This
type of feedback is called short-loop feedback
control. Similarly, GHRH inhibits its own release
by short-loop feedback control.
Whenever, the blood level of GH decreases,
the GHRH is secreted from the hypothalamus.
It in turn causes secretion of GH from pituitary.
OTHER HORMONES OF ANTERIOR
PITUITARY
Thyroid Stimulating Hormone (TSH)
TSH is necessary for the growth and the
secretory activity of the thyroid gland.
Adrenocorticotropic Hormone (ACTH)
ACTH is necessary for the structural integrity and
the secretory activity of adrenal cortex.
FIGURE 45-2: Regulation of GH secretion. GHIH =
Growth hormone inhibitory hormone. GHRH = Growth
hormone releasing hormone. GHRP = Growth
hormone releasing polypeptide. Growth hormone
and somatomedin stimulate hypothalamus to
release GHIH. Somatomedin inhibits anterior pituitary
directly. Solid blue line = stimulation/secretion.
Dashed red line = inhibition
Follicle Stimulating Hormone (FSH)
Actions in males
In males, FSH acts along with testosterone and
accelerates the process of spermeogenesis.
Actions in females
1. It is responsible for the development of graa-
fian follicle from primordial follicle
2. It stimulates the theca cells of graafian follicle
and causes secretion of estrogen (refer
Chapter 54 for details)
3. Promotes aromatase activity in granulosa
cells resulting in conversion of androgens into
estrogen.
Luteinizing Hormone (LH)
Actions in males
In males, LH is known as interstitial cell stimu-
lating hormone (ICSH) because it stimulates the
interstitial cells of Leydig in testes. This hormone
is essential for the secretion of testosterone from
Leydig cells.
Actions in females
1. LH causes maturation of vesicular follicle
into graafian follicle along with follicle stimu-
lating hormone
2. It induces synthesis of androgens from theca
cells of growing follicle
3. It is responsible for ovulation
4. It is necessary for the formation of corpus
luteum
5. It activates the secretory functions of corpus
luteum.
Prolactin
Prolactin is necessary for the final preparation
of mammary glands for production and secretion
of milk.
β
β
β
β
β Lipotropin
It mobilizes fat from adipose tissue and promotes
lipolysis.
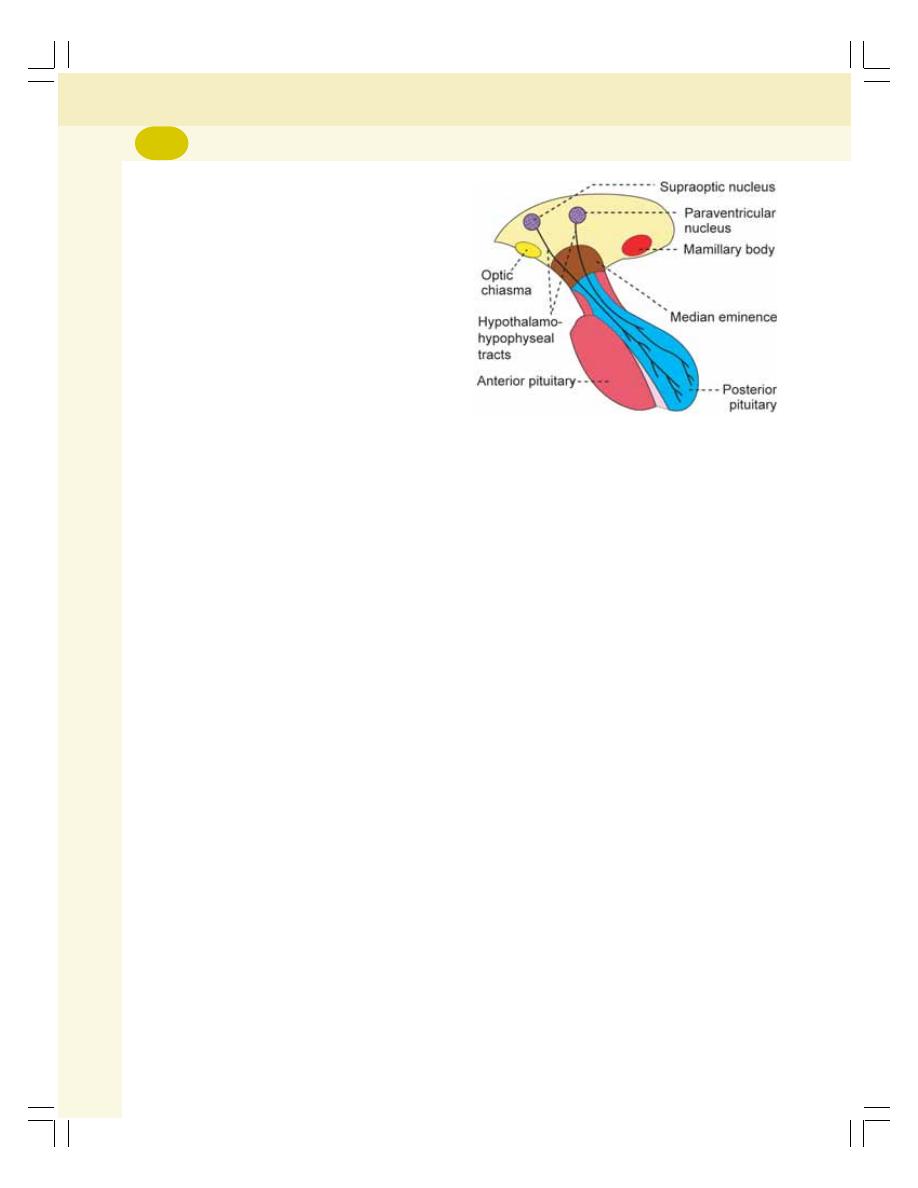
Endocrinology
266
POSTERIOR PITUITARY
PARTS
Posterior pituitary consists of three divisions:
1. The pars nervosa or infundibular process
2. Neural stalk or infundibular stem
3. The median eminence.
The pars tuberalis of anterior pituitary and the
neural stalk of posterior pituitary together form
the hypophyseal stalk.
HISTOLOGY
Posterior pituitary is made up of nerve cells called
pituicytes and unmyelinated nerve fibers.
Pituicytes act as supporting cells and do not
secrete any hormone. Neurohypophysis also has
numerous blood vessels, hyaline bodies,
neuroglial cells and mast cells.
HORMONES OF POSTERIOR
PITUITARY
Posterior pituitary hormones are:
1. Antidiuretic hormone (ADH) or vasopressin
2. Oxytocin.
Actually, the posterior pituitary does not
secrete any hormone. ADH and oxytocin are
synthesized in the hypothalamus. Hence, these
two hormones are called neurohormones.
ANTIDIURETIC HORMONE
ADH is secreted mainly by supraoptic nucleus
of hypothalamus and in small quantity by para-
ventricular nucleus. From here, this hormone is
transported to the posterior pituitary through the
nerve fibers of hypothalamo-hypophyseal tract
by means of axonic flow (Fig. 45-3).
Antidiuretic hormone is a polypeptide,
containing 9 amino acids.
Actions
The major function of ADH is retention of water
by acting on kidneys. It increases the facultative
reabsorption of water from distal convoluted
tubule and collecting duct in the kidneys
(Chapter 37).
ADH increases water reabsorption in the
tubular epithelial membrane by regulating the
water channel proteins called aquaporins
through V
2
receptors (Chapter 37).
Vasopressor Action
In large amount, the ADH shows vasoconstrictor
action in all parts of the body. Due to the vaso-
constriction, the blood pressure increases. ADH
acts on blood vessels through V
1A
receptors.
Regulation of Secretion
The secretion of ADH depends upon the volume
of body fluid and the osmolarity of the body
fluids.
The potent stimulants for ADH secretion are:
1. Decrease in the ECF volume
2. Increase in osmolar concentration in the ECF.
Role of osmoreceptors
The osmoreceptors are the receptors, which give
response to change in the osmolar concentration
of the blood. These receptors are situated in the
hypothalamus near supraoptic and paraven-
tricular nuclei. When osmolar concentration of
blood increases, the osmoreceptors are activated.
In turn, the osmoreceptors stimulate the
supraoptic and paraventricular nuclei which send
FIGURE 45-3: Hypothalamo-hypophyseal tracts

Chapter 45 Pituitary Gland
267
motor impulses to posterior pituitary through
the nerve fibers and cause release of ADH.
ADH causes reabsorption of water from the
renal tubules. This increases the volume of
the ECF and restores the normal osmolarity.
OXYTOCIN
Oxytocin is secreted mainly by the para-
ventricular nucleus and a small quantity is
secreted by the supraoptic nucleus in the
hypothalamus. And it is transported from
hypothalamus to posterior pituitary through the
nerve fibers of hypothalamo-hypophyseal tract.
In the posterior pituitary, the oxytocin is
stored in the nerve endings of hypothalamo-
hypophyseal tract. When suitable stimuli reach
the posterior pituitary from hypothalamus,
oxytocin is released into the blood. Oxytocin is
secreted in both males and females.
Oxytocin is a polypeptide, having 9 amino
acids.
Actions in Females
In females, oxytocin acts on mammary glands
and uterus.
Action of oxytocin on mammary glands
It causes ejection of milk from the mammary
glands. The ducts of the mammary glands are
lined by myoepithelial cells. Oxytocin causes
contraction of the myoepithelial cells and
squeezes the milk from alveoli of the mammary
glands to the exterior through the duct system
and nipple. The process by which the milk is
ejected from the alveoli of mammary glands is
called the milk ejection reflex or milk let down
reflex. It is one of the neuroendocrine reflexes.
Milk ejection reflex
Plenty of touch receptors are present on the
mammary glands, particularly around the nipple.
When the infant suckles mother’s nipple, the
touch receptors are stimulated and impulses are
discharged. Impulses from here are carried by
the somatic afferent nerve fibers and reach the
paraventricular and supraoptic nuclei of
hypothalamus.
Now, hypothalamus in turn, sends impulses
to the posterior pituitary through hypothalamo-
hypophyseal tract and cause release of oxyto-
cin into the blood. When the hormone reaches
the mammary gland, it causes contraction of
myoepithelial cells resulting in ejection of milk
from mammary glands (Fig. 45-4).
As this reflex is initiated by the nervous
factors and completed by the hormonal action,
it is called a neuroendocrine reflex. During this
reflex, large amount of oxytocin is released by
positive feedback mechanism.
Action on uterus
Oxytocin acts on pregnant uterus and nonpregnant
uterus.
On pregnant uterus
Throughout the period of pregnancy, oxytocin
secretion is inhibited by estrogen and proges-
terone. At the end of pregnancy, the secretion
of these two hormones decreases suddenly and
the secretion of oxytocin increases. Oxytocin
causes contraction of uterus and helps in the
expulsion of fetus.
During labor, large quantity of oxytocin is
released by means of positive feedback mecha-
nism, i.e. oxytocin induces contraction of uterus,
which in turn causes release of more amount of
oxytocin (Fig. 4-5).
The contraction of uterus during labor is
also a neuroendocrine reflex. Oxytocin also
stimulates the release of prostaglandins in the
placenta. The prostaglandins intensify the uterine
contraction induced by oxytocin.
On nonpregnant uterus
The action of oxytocin on nonpregnant uterus is
to facilitate the transport of sperms through
female genital tract up to fallopian tube by
producing the uterine contraction during the
sexual intercourse.
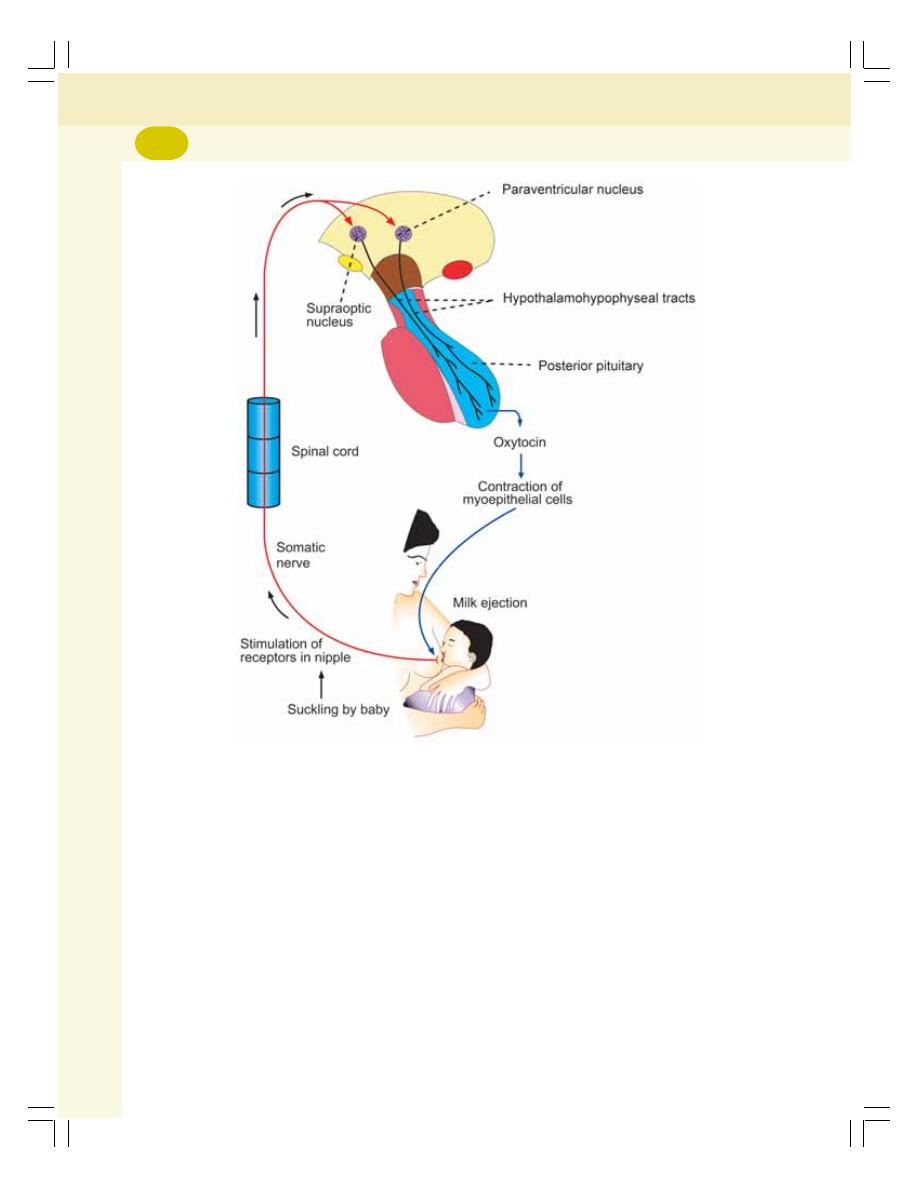
Endocrinology
268
During the sexual intercourse, the receptors
in the vagina are stimulated. The vaginal
receptors generate the impulses, which are
transmitted by somatic afferent nerves to the
paraventricular and supraoptic nuclei of hypo-
thalamus. When, these two nuclei are stimulated,
oxytocin is released, and transported by blood.
While reaching the female genital tract, the
hormone causes antiperistaltic contractions of
uterus towards the fallopian tube which acce-
lerate the transport of sperms. It is also a
neuroendocrine reflex.
The sensitivity of uterus to oxytocin is
accelerated by estrogen and decreased by
progesterone.
Action in Males
In males, the release of oxytocin increases
during ejaculation. It facilitates release of sperm
into urethra by causing contraction of smooth
muscle fibers in reproductive tract particularly
vas deferens.
Mode of Action of Oxytocin
Oxytocin acts on mammary glands and uterus
by activating G protein-coupled oxytocin receptor.
APPLIED PHYSIOLOGY—
DISORDERS OF PITUITARY GLAND
The disorders of pituitary gland are given in Table
45-1.
FIGURE 45-4: Milk ejection reflex

Chapter 45 Pituitary Gland
269
HYPERACTIVITY OF ANTERIOR
PITUITARY
1. Gigantism
Gigantism is the pituitary disorder charac-
terized by excess growth of the body. The
subjects look like the giants with average height
of about 7-8 feet.
Cause
Gigantism is due to hypersecretion of GH in
childhood or in the pre-adult life before the
fusion of epiphysis of bone with the shaft. It
occurs due to pituitary tumors.
Signs and symptoms
i. The general over growth of the person leads
to the development of a huge stature with a
height of more than 7 or 8 feet. The limbs
are disproportionately long
ii. The giants are hyperglycemic and they
develop glycosuria and pituitary diabetes.
The hyperglycemia causes constant
stimulation of
β cells of islets of Langerhans
in the pancreas and release of insulin.
However, the over activity of
β cells of
Langerhans in pancreas leads to degene-
ration of these cells and deficiency of insulin.
And, ultimately diabetes mellitus is developed
iii. The pituitary tumor itself causes constant
headache
iv. Pituitary tumor also causes visual dis-
turbances. It compresses the lateral fibers
of optic chiasma leading to bitemporal
hemianopia (Chapter 106).
2. Acromegaly
It is the disorder characterized by the
enlargement, thickening and broadening of
bones, particularly in the extremities of the body.
Cause
Acromegaly is due to hypersecretion of GH in
adults after the fusion of epiphysis with shaft of
the bone. Hypersecretion of GH is due to
adenomatous tumor of anterior pituitary
involving the acidophil cells.
Signs and symptoms
i. The striking facial features are protrusion
of supraorbital ridges, broadening of nose,
thickening of lips, thickening and wrinkles
formation on forehead, and protrusion of
lower jaw (prognathism). The face with these
features is called acromegalic or guerrilla
face (Fig. 45-5)
ii. Enlargement of hands and feet (Fig. 45-6)
with bowing of spine (kyphosis)
iii. The scalp is thickened and thrown into
folds or wrinkles like bulldog scalp. There is
general overgrowth of body hair
iv. The visceral organs such as lungs, heart,
liver and spleen are enlarged
v. Thyroid gland, parathyroid glands and the
adrenal glands show hyperactivity
vi. Hyperglycemia and glucosuria occur
resulting in diabetes mellitus
TABLE 45-1: Disorders of pituitary gland
Parts involved
Hyperactivity
Hypoactivity
Anterior pituitary
1. Gigantism
1. Dwarfism
2. Acromegaly
2. Acromicria
3. Acromegalic gigantism
3. Simmond’s disease
4. Cushing’s disease
Posterior pituitary
Syndrome of inappropriate
Diabetes insipidus
hypersecretion of ADH (SIADH)
Anterior and posterior pituitary
- - -
Dystrophia adiposogenitalis
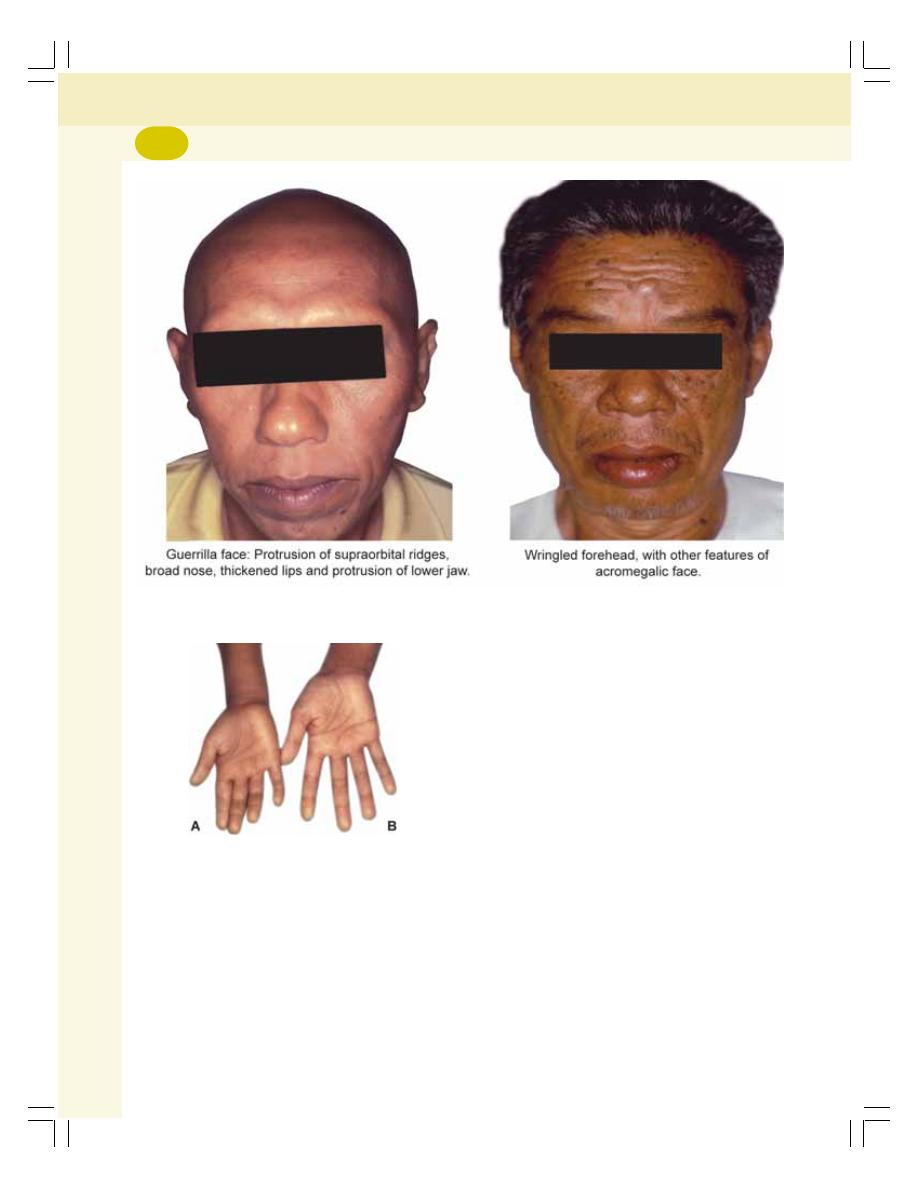
Endocrinology
270
vii. Hypertension
viii. Headache
xi. Visual disturbances—bitemporal hemianopia.
3. Acromegalic Gigantism
It is a rare disorder with symptoms of both
gigantism and acromegaly. Hypersecretion of GH
in children, before the fusion of epiphysis with
shaft of the bones causes gigantism. And, if
hypersecretion of the GH is continued even after
the fusion of epiphysis, the symptoms of
acromegaly also appear.
4. Cushing’s Disease
It is also a rare disease characterized by obesity.
The details are given in Chapter 49.
HYPOACTIVITY OF ANTERIOR
PITUITARY
1. Dwarfism
It is a pituitary disorder in children characterized
by the stunted growth.
Causes
Reduction in the GH secretion in infancy or early
childhood causes dwarfism. It occurs because
of the following reasons:
i. Deficiency of GHRH from hypothalamus
ii. Deficiency of somatomedin-C
FIGURE 45-5: Acromegaly (Courtesy: Prof Mafauzy Mohamad)
FIGURE 45-6: A. Normal hand; B. Acromegalic
hand (Courtesy: Prof Mafauzy Mohamad)

Chapter 45 Pituitary Gland
271
iii. Atrophy or degeneration of acidophilic cells
in the anterior pituitary
iv. Tumor of chromophobes: It is a nonfunction-
ing tumor, which compresses and destroys
the normal GH secreting cells
v. Panhypopituitarism: In this condition, there
is reduction in the secretion of all the
hormones of anterior pituitary gland. This
type of dwarfism is associated with other
symptoms due to the deficiency of other
anterior pituitary hormones.
Signs and symptoms
i. The primary symptom of hypopituitarism in
children is the stunted skeletal growth. The
maximum height of anterior pituitary dwarf
at the adult age is only about 3 feet
ii. But the proportions of different parts of the
body are almost normal. Only, the head
becomes slightly larger in relation to the body
iii. Pituitary dwarfs do not show any deformity
and their mental activity is normal with no
mental retardation
iv. Reproductive function is not affected, if there
is only GH deficiency. However, in
panhypopituitarism (see below), the dwarfs
do not obtain puberty due to deficiency of
gonadotropic hormones.
Laron dwarfism
Laron dwarfism is a genetic disorder that occurs
due to the presence of abnormal GH
secretagogue receptors.
Psychogenic dwarfism
Dwarfism occurs if the child is exposed to
extreme emotional deprivation or stress. The
short stature is because of deficiency of GH.
This type of dwarfism is called psychogenic
dwarfism, psychosocial dwarfism or stress
dwarfism.
Dwarfism in dystrophia adiposogenitalis
Dystrophia adiposogenitalis or Fröhlich’s
syndrome is a pituitary disorder (see below).
Dwarfism occurs if it develops in children.
2. Acromicria
It is a rare disease in adults characterized by the
atrophy of the extremities of the body.
Causes
Deficiency of GH in adults causes acromicria.
The secretion of GH decreases in the following
conditions:
i. Deficiency of GH releasing hormone from
hypothalamus
ii. Atrophy or degeneration of acidophilic cells
in the anterior pituitary
iii. Tumor of chromophobes: It is a non-
functioning tumor, which compresses and
destroys the normal cells secreting the GH
iv. Panhypopituitarism: In this condition, there
is reduction in the secretion of all the
hormones of anterior pituitary gland. Acro-
micria is associated with other symptoms
due to the deficiency of other anterior
pituitary hormones.
Signs and symptoms
i. Atrophy and thinning of extremities of the
body, (hands and feet) are the major symp-
toms in acromicria
ii. Acromicria is mostly associated with hypothy-
roidism and hyposecretion of adrenocortical
hormones
iii. The person becomes lethargic and obese
iv. There is loss of sexual functions.
3. Simmond’s Disease
It is a rare pituitary disease. It is also called
pituitary cachexia.
Causes
It occurs mostly in panhypopituitarism, i.e. hypo-
secretion of all the anterior pituitary hormones
due to the atrophy or degeneration of anterior
pituitary.
Symptoms
i. A major feature of Simmond’s disease is the
rapidly developing senile decay. Thus, a 30

Endocrinology
272
years old person looks like a 60 years old
person
ii. There is loss of hair over the body and loss
of teeth
iii. The skin on face becomes dry and wrinkled.
So, there is shrunken appearance of facial
features. It is the most common feature of
this disease.
HYPERACTIVITY OF POSTERIOR
PITUITARY
Syndrome of Inappropriate Hypersecretion
of Antidiuretic Hormone (SIADH)
SIADH is the disease characterized by loss of
sodium through urine due to hypersecretion of
ADH.
Causes
It occurs due to cerebral tumors, lung tumors and
lung cancers because the tumor cells and cancer
cells secrete ADH.
In normal conditions ADH decreases the urine
output by facultative reabsorption of water in
distal convoluted tubule and the collecting duct.
So, concentrated urine is formed with more
sodium and other ions and less water. This
decreases the osmolarity of plasma making it
hypotonic. The hypotonic plasma inhibits ADH
secretion resulting in restoration of plasma
osmolarity.
However, in SIADH secretion of ADH from
tumor or cancer cells is not inhibited by hypotonic
plasma. So there is continuous loss of sodium
resulting in persistent plasma hypotonicity.
Signs and symptoms
1. Loss of appetite
2. Weight loss
3. Nausea and vomiting
4. Headache
5. Muscle weakness, spasm and cramps
6. Fatigue
7. Restlessness and irritability.
In severe conditions, the patients die because
of convulsions and coma.
HYPOACTIVITY OF POSTERIOR
PITUITARY
Diabetes Insipidus
Diabetes insipidus is a posterior pituitary dis-
order characterized by excess excretion of water
through urine.
Causes
This disorder develops due to the deficiency
ADH which occurs in the following conditions:
i. Lesion (injury) or degeneration of supraoptic
and paraventricular nuclei of hypothalamus
ii. Lesion in hypothalamo-hypophyseal tract
iii. Atrophy of posterior pituitary
iv. Inability of renal tubules to give response to
ADH hormone. Such condition is called
nephrogenic diabetic insipidus (see below).
Signs and symptoms
i. Polyuria: Excretion of large quantity of dilute
urine with increased frequency of voiding is
called polyuria. Daily output of urine varies
between 4 and 12 liters. In the absence of
ADH, water is not reabsorbed from the renal
tubule and collecting duct leading to loss of
water through urine.
ii. Polydipsia: Intake of excess water is called
polydipsia. Loss of water due to polyuria
stimulates the thirst center in hypothalamus
resulting in intake of large quantity of water.
iii. Dehydration: In some cases, the thirst center
in the hypothalamus is also affected by the
lesion. Water intake decreases in these
patients and the loss of water through urine
is not compensated. So, dehydration
develops which may lead to death.
HYPOACTIVITY OF ANTERIOR AND
POSTERIOR PITUITARY
Dystrophia Adiposogenitalis
Dystrophia adiposogenitalis is a disease
characterized by obesity and hypogonadism
affecting mainly the adolescent boys. It is also
called Fröhlich’s syndrome or hypothalamic
eunuchism.

Chapter 45 Pituitary Gland
273
Causes
It is due to the hypoactivity of both anterior pitui-
tary and posterior pituitary. The common cause
of this disease is the tumor in pituitary gland and
hypothalamic regions concerned with food intake
and gonadal development.
Symptoms
Obesity is the common feature of this disorder.
Due to the abnormal stimulation of feeding
center, the person overeats and becomes obese.
Obesity is accompanied by sexual infantilism
(failure to develop secondary sexual characters)
or eunuchism. Dwarfism occurs if the disease
starts in growing age. In children, it is called
infantile or prepubertal type of Fröhlich’s
syndrome.
This disease develops in adults also. When
it occurs in adults, it is called adult type of
Fröhlich’s syndrome. In adults, the major symp-
toms are obesity and atrophy of sex organs.

INTRODUCTION
HISTOLOGY
HORMONES
SYNTHESIS OF THYROID HORMONES
STORAGE OF THYROID HORMONES
RELEASE OF THYROID HORMONES
TRANSPORT OF THYROID HORMONES IN THE BLOOD
FUNCTIONS OF THYROID HORMONES
MODE OF ACTION OF THYROID HORMONES
REGULATION OF SECRETION OF THYROID HORMONES
APPLIED PHYSIOLOGY — DISORDERS OF THYROID GLAND
THYROID FUNCTION TESTS
INTRODUCTION
Thyroid is an endocrine gland situated at the root
of the neck on either side of the trachea. It has
two lobes, which are connected in the middle by
an isthmus (Fig. 46-1). It weighs about 20 to
40 gm in adults. Thyroid is larger in females than
in males. The structure and the function of the
thyroid gland change in different stages of the
sexual cycle in females. Its function increases
slightly during pregnancy and lactation and
decreases during menopause.
HISTOLOGY OF THYROID GLAND
Thyroid gland is composed of large number of
closed follicles. The follicles are lined with
cuboidal epithelial cells, which are called the
Thyroid Gland
46
FIGURE 46-1:
Thyroid gland
follicular cells. The follicular cavity is filled with
a colloidal substance known as thyroglobulin
which is secreted by the follicular cells. Follicular
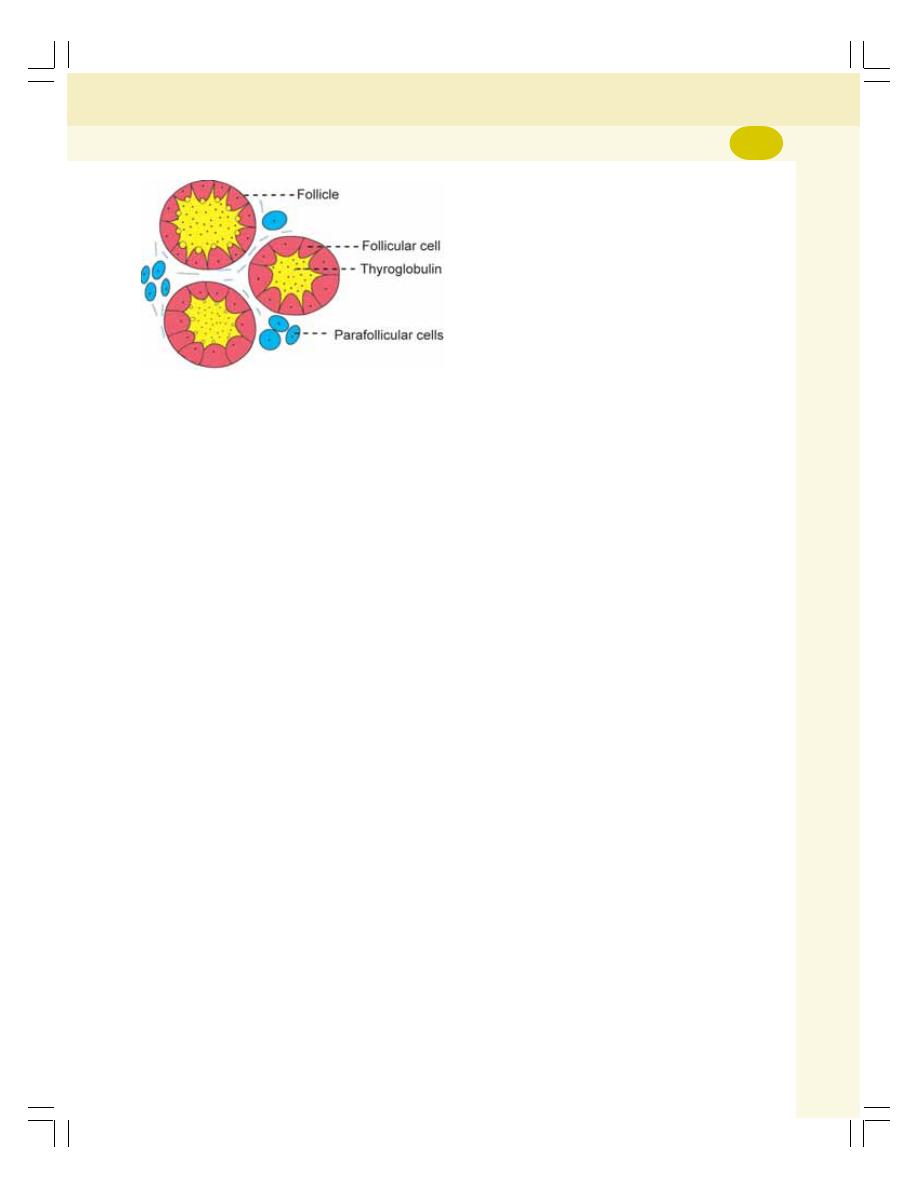
Chapter 46 Thyroid Gland
275
cells secrete tetraiodothyronine (T
4
or thyroxine)
and tri-iodothyronine (T
3
). In between the
follicles, the parafollicular cells are present
(Fig. 46-2). These cells secrete calcitonin.
HORMONES OF THYROID GLAND
Thyroid gland secretes three hormones:
1. Tetraiodothyronine – T
4
(thyroxine)
2. Tri-iodothyronine – T
3
3. Calcitonin.
T
4
is otherwise known as thyroxine and it
forms about 90% of the total secretion, whereas,
T
3
is only 9 to 10%. But the potency of T
3
is four
times more than that of T
4
.
SYNTHESIS OF THYROID HORMONES
Synthesis of thyroid hormones takes place in
thyroglobulin present in follicular cavity. Iodine
and tyrosine are essential for the formation of
thyroid hormones. Iodine is consumed through
diet. It is converted into iodide and absorbed from
GI tract. Tyrosine is also consumed through diet
and is absorbed from the GI.
For the synthesis of normal quantities of
thyroid hormones, approximately 1 mg of iodine
is required per week or about 50 mg per year.
To prevent iodine deficiency, common table salt
is iodized with one part of sodium iodide to every
100,000 parts of sodium chloride.
Various stages involved in the synthesis of
thyroid hormones are:
1. Thyroglobulin synthesis
2. Iodide trapping or iodide pump
3. Oxidation of iodide
4. Iodination of tyrosine
5. Coupling reactions.
1. Thyroglobulin Synthesis
The endoplasmic reticulum and Golgi apparatus
in the follicular cells of the thyroid gland
synthesize and secrete a thyroglobulin conti-
nuously. Each thyroglobulin molecule contains
140 tyrosine molecules. After synthesis, the
thyroglobulin is stored in the follicle.
2. Iodide Trapping or Iodide Pump
Iodide is transported actively from the blood into
the follicular cell against the electrochemical
gradient by a process called iodide trapping.
Iodide is pumped with sodium into the follicular
cell by sodium-iodide symport pump. From here,
iodide is transported into the follicular cavity by
an iodide-chloride pump.
3. Oxidation of the Iodide
Iodide must be oxidized to elementary iodine
because only iodine is capable of combining with
tyrosine to form thyroid hormones. The oxidation
of iodide into iodine occurs inside the follicular
cells in the presence of thyroid peroxidase.
4. Iodination of Tyrosine
The combination of iodine with tyrosine is known
as iodination. It takes place in the follicle within
thyroglobulin. First, iodine is released from
follicular cells into the follicular cavity where it
binds with thyroglobulin. This process is called
organification of thyroglobulin. In the thyro-
globulin, iodine combines with tyrosine which is
already present there.
Binding of iodine (I) with tyrosine is acce-
lerated by the enzyme iodinase which is secreted
by the follicular cells (Fig. 46-3). Iodination of
tyrosine occurs in several stages. Tyrosine is
iodized first into monoiodotyrosine (MIT) and later
into di-iodotyrosine (DIT). MIT and DIT are called
the iodotyrosine residues.
FIGURE 46-2:
Histology of thyroid gland
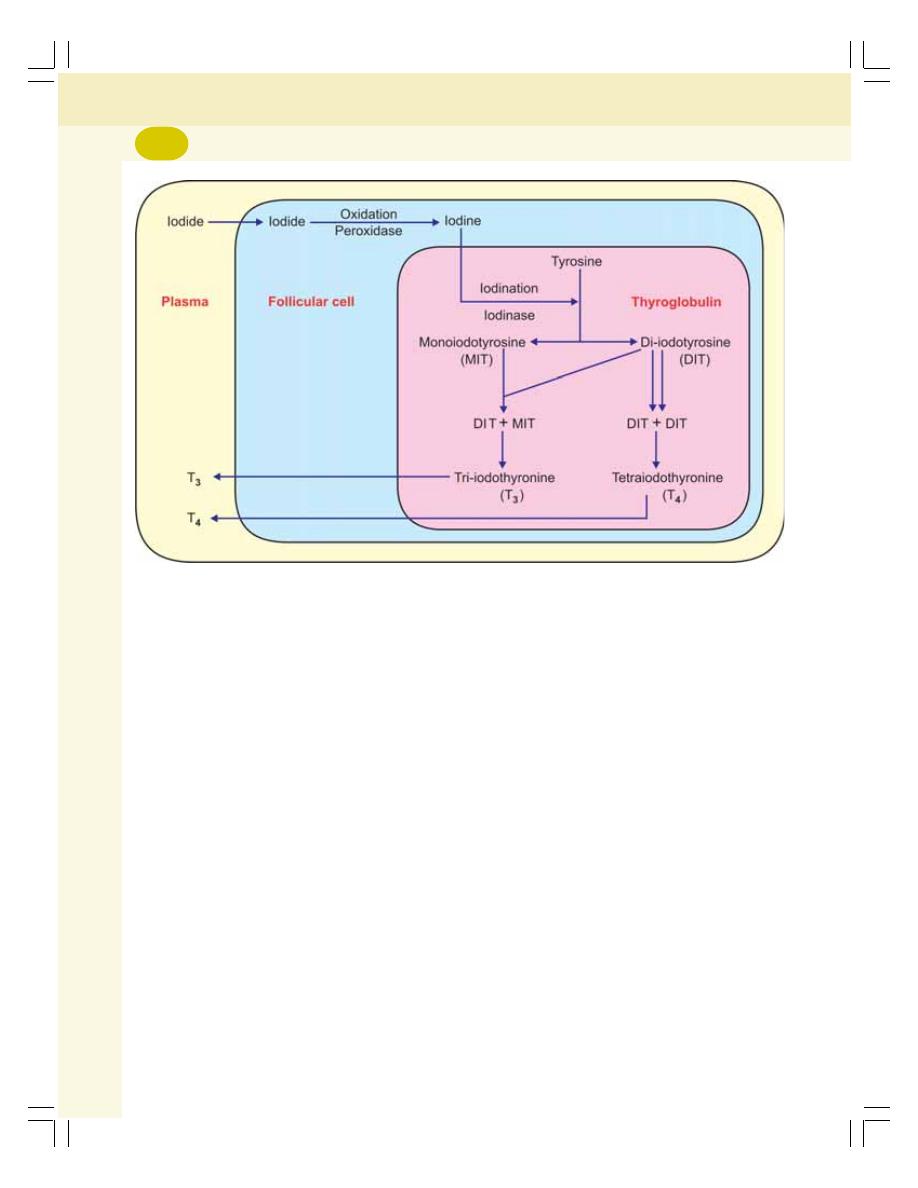
Endocrinology
276
5. Coupling Reactions
The iodotyrosine residues get coupled with one
another through coupling reactions. The coupling
occurs in different configurations to give rise to
different thyroid hormones:
i. One molecule of DIT and one molecule of
MIT combine to form tri-iodothyronine (T
3
)
ii. Sometimes one molecule of MIT and one
molecule of DIT combine to produce another
form of T
3
called reverse T
3
or rT
3
. Reverse
T3 is only 1% of thyroid output
iii. Two molecules of DIT combine to form
tetraiodothyronine (T
4
) which is thyroxine.
Tyrosine + I = Monoiodotyrosine (MIT)
MIT + I
= Di-iodotyrosine (DIT)
DIT + MIT
= Tri-iodothyronine (T
3
)
MIT + DIT
= Reverse T
3
DIT + DIT
= Tetraiodothyronine or
Thyroxine (T
4
)
STORAGE OF THYROID HORMONES
After synthesis, the thyroid hormones remain in
the form of vesicles within thyroglobulin. In
combination with thyroglobulin, the thyroid
hormones can be stored for several months.
And, thyroid gland is unique in this, as it is the
only endocrine gland that can store its hormones
for a long period of about 4 months. So, when
the synthesis of thyroid hormone stops, the signs
and symptoms of deficiency do not appear for
about 4 months.
RELEASE OF THYROID HORMONES
FROM THE THYROID GLAND
Thyroglobulin itself is not released into the
bloodstream. On the other hand, the hormones
are first cleaved from the thyroglobulin.
Only T
3
and T
4
are released into the blood.
In the peripheral tissues T
4
is converted into T
3
.
A small amount of reverse T
3
is also formed. But
reverse T
3
is biologically inactive.
The MIT and DIT are not released into blood.
These iodotyrosine residues are deiodinated
by an enzyme called iodotyrosine deiodinase
resulting in release of iodine. The iodine is
reutilized by the follicular cells for synthesis of
FIGURE 46-3:
Synthesis of thyroid hormones

Chapter 46 Thyroid Gland
277
thyroid hormones. During congenital absence of
iodotyrosine deiodinase, MIT and DIT are
excreted in urine and the symptoms of iodine
deficiency develop.
TRANSPORT OF THYROID
HORMONES IN THE BLOOD
The normal plasma level of total T3 is 0.12 mg/dL
and that of total T
4
is 8 mg/dL. The thyroid
hormones are transported in the blood in com-
bination with three types of plasma proteins.
1. Thyroxine binding globulin (TBG)
2. Thyroxine binding prealbumin (TBPA)
3. Albumin.
FUNCTIONS OF THYROID
HORMONES
Thyroid hormones have two major effects on the
body:
I. To increase the overall metabolic rate in the
body
II. To stimulate growth in children.
The actions of thyroid hormones are:
1. ON BASAL METABOLIC RATE
Thyroxine increases the metabolic activities of
almost all tissues of the body except brain, retina,
spleen, testes and lungs. It increases the basal
metabolic rate (BMR) by increasing the oxygen
consumption of the tissues. The action that
increases the BMR is called calorigenic action.
2. ON PROTEIN METABOLISM
Thyroid hormones increase synthesis of proteins.
Thyroxine accelerates protein synthesis by
increasing:
i. Translation of RNA in the cells
ii. Transcription of DNA to RNA
iii. Activity of mitochondria
iv. Activity of cellular enzymes.
Though thyroxine increases protein syn-
thesis, it also causes catabolism of proteins.
3. ON CARBOHYDRATE
METABOLISM
Thyroxine stimulates almost all processes
involved in the metabolism of carbohydrate.
It increases:
i. Absorption of glucose from GI tract
ii. Glucose uptake by the cells, by accelerating
transport of glucose through cell membrane
iii. Breakdown of glycogen into glucose
iv. Gluconeogenesis.
4. ON FAT METABOLISM
Thyroxine decreases the fat storage by mobi-
lizing it from adipose tissues and fat depots. The
mobilized fat is converted into free fatty acid and
transported by blood. Thus, thyroxine increases
the free fatty acid level in blood.
5. ON PLASMA AND LIVER FATS
Even though there is increase in the blood level
of free fatty acids, thyroxine specifically decrea-
ses the cholesterol, phospholipids and triglyceride
levels in the plasma. So, in hyposecretion of thy-
roxine, the cholesterol level in plasma increases
resulting in atherosclerosis.
Thyroxine also increases deposition of fats
in the liver leading to fatty liver. Thyroxine dec-
reases plasma cholesterol level by increasing
its excretion from liver cells into bile. Cholesterol
enters the intestine through bile and then it is
excreted through the feces.
6. ON VITAMIN METABOLISM
Thyroxine increases the formation of many
enzymes. Since, the vitamins form the essential
parts of the enzymes, it is believed that the
vitamins may be utilized during the formation of
the enzymes. Hence, vitamin deficiency is
possible during hypersecretion of thyroxine.
7. ON BODY TEMPERATURE
Thyroid hormone increases the heat production
in the body by accelerating various cellular
metabolic processes and increasing BMR.
8. ON GROWTH
Thyroid hormones have general and specific
effects on growth. Lack of thyroxine arrests the
growth and increase in thyroxine secretion
accelerates the growth of the body especially in
growing children. At the same time, the closure

Endocrinology
278
of epiphysis occurs at an early age under the
influence of thyroxine. So, the height of the
individual may be slightly less.
Thyroxine is more important to promote
growth and development of the brain during
fetal life and the first few years of postnatal
life. Lack of thyroid hormones at this period
leads to mental retardation.
9. EFFECT ON BODY WEIGHT
Thyroxine is essential for maintaining body
weight. Increase in thyroxine secretion decreases
the body weight and fat storage; and decrease
in thyroxine secretion increases the body weight
because of fat deposition.
10. EFFECT ON BLOOD
Thyroxine increases the production of RBCs. It
is one of the important general factors necessary
for erythropoiesis. Thus, thyroxine increases
erythropoietic activity and blood volume.
11. ON CARDIOVASCULAR SYSTEM
Thyroxine increases overall activity of cardio-
vascular system:
i. On Heart
Thyroxine acts directly on heart and increases
rate and force of contraction.
ii. On Blood Vessels
Thyroxine causes vasodilatation by increasing
the metabolic activity. During metabolic activity,
production of metabolites is increased. The
metabolites cause vasodilatation and increase
the blood flow.
iii. On Arterial Blood Pressure
Thyroxine increases systolic blood pressure by
increasing rate and force of contraction of the
heart, blood volume and cardiac output. At
the same time it decreases diastolic pressure
by it vasodilator effect. So only the pulse
pressure increases and the mean pressure is
not altered.
12. EFFECT ON RESPIRATION
Thyroxine increases the rate and force of
respiration indirectly. The increased metabolic
rate (caused by thyroxine) increases the demand
for oxygen and formation of excess carbon
dioxide. These two factors stimulate the
respiratory centers to increase the rate and force
of respiration.
13. ON GASTROINTESTINAL TRACT
Generally, thyroxine increases the appetite and
food intake. It also increases the secretions and
movements of GI tract.
14. ON CENTRAL NERVOUS SYSTEM
Thyroxine is very essential for the development
and maintenance of normal functioning of the
central nervous system.
i.
On Development of Central Nervous
System
Thyroxine is very important to promote growth
and development of the brain during fetal life
and during the first few years of postnatal life.
Thyroid deficiency in infants results mental
retardation.
ii. On the Normal Function of Central
Nervous System
Thyroxine is a stimulating factor for the brain so
normal functioning of the brain needs the
presence of thyroxine. Thyroxine also increases
the blood flow to brain.
Thus, during the hypersecretion of thyroxine
there is excess stimulation of the central ner-
vous system. So, the person is likely to have
extreme nervousness and may develop psycho-
neurotic problems such as anxiety complexes,
excess worries. Hyposecretion of thyroxine leads
to lethargy and somnolence (excess sleep).
15. ON SKELETAL MUSCLE
Thyroxine is essential for the normal activity
of the skeletal muscles. Slight increase in
thyroxine level makes the muscles to work with

Chapter 46 Thyroid Gland
279
more vigor. But, hypersecretion of thyroxine
causes weakness of the muscles due to the
catabolism of proteins. Lack of thyroxine
makes the muscles more sluggish.
16. ON SLEEP
Normal thyroxine level is essential to maintain
normal sleep. Hypersecretion of thyroxine
causes excessive stimulation of the muscles
and central nervous system. So, the person feels
tired, exhausted, and feels like sleeping. But,
the person cannot sleep because of the stimu-
latory effect of thyroxine on neurons. On the
other hand, hyposecretion of thyroxine causes
somnolence.
17. ON SEXUAL FUNCTION
Normal thyroxine level is essential for normal
sexual function. In men, hypothyroidism leads
to complete loss of libido (sexual drive). And
hyperthyroidism leads to impotence.
In women, hypothyroidism causes menorr-
hagia and polymenorrhea (Chapter 55). In some
women, it causes irregular menstruation and
occasionally amenorrhea. Hyperthyroidism in
women leads to oligomenorrhea and sometimes
amenorrhea (Chapter 55).
18. ON OTHER ENDOCRINE GLANDS
Because of its metabolic effects, thyroxine
increases the demand for secretion of other
endocrine glands.
MODE OF ACTION OF THYROID
HORMONES
Thyroid hormones act by activating the genes
(Chapter 44).
REGULATION OF SECRETION OF
THYROID HORMONES
The secretion of thyroid hormones is controlled
by anterior pituitary and hypothalamus through
feedback mechanism (Fig. 46-4).
ROLE OF PITUITARY GLAND
Thyroid Stimulating Hormone
Thyroid stimulating hormone (TSH) secreted by
anterior pituitary is the major factor regulating
the synthesis and release of thyroid hormones.
TSH is a peptide hormone with one a chain
and one
β chain. Normal plasma level of TSH is
approximately 2 U/mL.
Actions of TSH
TSH increases:
1. The number of thyroid cells, which are
cuboidal in nature and, then it converts them
into columnar cells and causes the
development of thyroid follicles
2. The size and secretory activity of the cells
3. The iodide pump and iodide trapping in the
cells
4. The thyroglobulin secretion into the follicles
5. Iodination of tyrosine and coupling to form the
hormones
6. Proteolysis of the thyroglobulin, by which,
release of hormone is enhanced and the
colloidal substance is decreased.
Mode of Action of TSH
TSH acts through cyclic AMP mechanism.
ROLE OF HYPOTHALAMUS
Hypothalamus regulates thyroid secretion by
controlling TSH secretion through thyrotropic
releasing hormone (TRH) from hypothalamus.
From hypothalamus, TRH is transported through
the hypothalamo-hypophyseal portal vessels
to the anterior pituitary. After reaching the pituitary
gland, the TRH causes the release of TSH.
FEEDBACK CONTROL
Thyroid hormones regulate their own secretion
through negative feedback control by inhibiting
the release of TRH from hypothalamus and TSH
from anterior pituitary (Fig. 46-4).
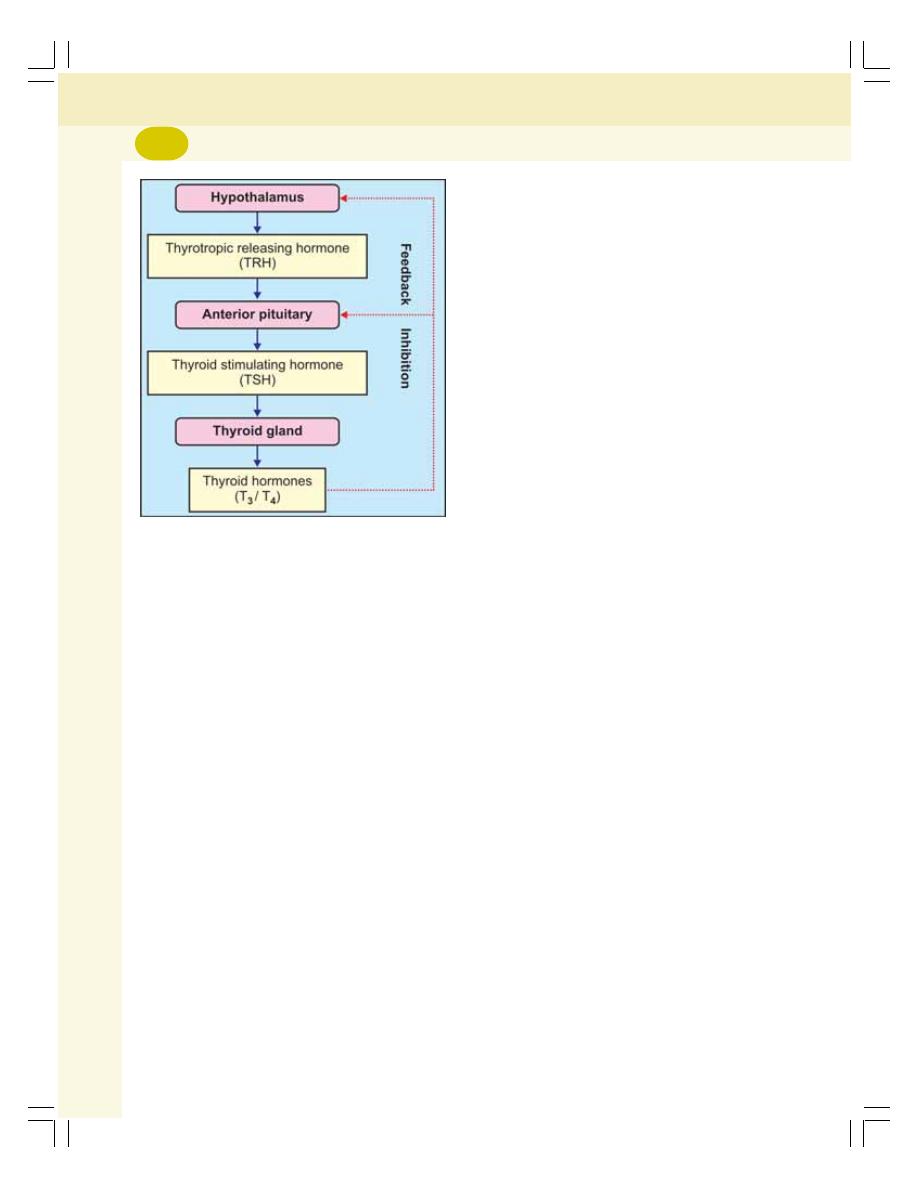
Endocrinology
280
ROLE OF IODIDE
Iodide is an important factor regulating the
synthesis of thyroid hormones. When the dietary
level of iodine is moderate, the blood level of
thyroid hormones is normal. However, when
iodine intake is high, the enzymes necessary
for synthesis of thyroid hormones are inhibited
by iodide itself resulting in suppression of
hormone synthesis.
APPLIED PHYSIOLOGY—
DISORDERS OF THYROID GLAND
1. HYPERTHYROIDISM
Causes for Hyperthyroidism
i. Graves’ disease
Graves’ disease is an autoimmune disease.
Normally, thyroid stimulating hormone (TSH)
combines with surface receptors of thyroid cells
and causes the synthesis of thyroid hormones.
In Graves’ disease the B lymphocytes (plasma
cells) produce autoimmune antibodies called
thyroid stimulating autoantibodies. These
antibodies act like TSH by binding with mem-
brane receptors of TSH and activating cAMP
system of the thyroid follicular cells. This results
in hypersecretion of thyroid hormones.
ii. Thyroid adenoma
Sometimes, a localized tumor develops in the
thyroid tissue. It is known as thyroid adenoma
and it secretes large quantities of thyroid
hormones.
Signs and Symptoms of Hyperthyroidism
i. Intolerance to heat because production of
more heat during increased basal metabolic
rate caused by hyperthyroidism
ii. Increased sweating due to vasodilatation
iii. Decreased body weight due to fat
mobilization
iv. Diarrhea due to increased motility of GI tract
v. Muscular weakness due to excess protein
catabolism
vi. Neuronal disturbances such as nervous-
ness, extreme fatigue, inability to sleep,
mild tremor in hands and psychoneurotic
symptoms such as hyperexcitability,
extreme anxiety or worry
vii. Toxic goiter
viii. Oligomenorrhea or amenorrhea
ix. Exophthalmos
x. Polycythemia
xi. Tachycardia and atrial fibrillation
xii. Systolic hypertension
xiii. Cardiac failure.
Exophthalmos
Protrusion of eye balls is called exophthalmos.
Most, but not all hyperthyroid patients develop
some degree of protrusion of eyeballs.
Causes for exophthalmos
Exophthalmos in hyperthyroidism is due to the
edematous swelling of the retro-orbital tissues
and degenerative changes in the extraocular
muscles. Severe exophthalmic conditions lead
to blindness because of two reasons:
i. Protrusion of the eyeball stretches and
damages the optic nerve resulting in
blindness or
ii. Due to the protrusion of eyeballs, the eyelids
cannot be closed completely while blinking
FIGURE 46-4:
Regulation of secretion of
thyroid hormones

Chapter 46 Thyroid Gland
281
or during sleep. So, the constant exposure
of eyeball to atmosphere causes dryness
of the cornea leading to irritation and
infection. It finally results in ulceration of the
cornea leading to blindness.
2. HYPOTHYROIDISM
Decreased secretion of thyroid hormones is
called hypothyroidism. Hypothyroidism leads to
myxedema in adults and cretinism in children.
Myxedema
It is the hypothyroidism in adults characterized
by generalized edematous appearance.
Causes for myxedema
Myxedema occurs due to diseases of thyroid
gland, genetic disorder or iodine deficiency. In
addition, it is also caused by deficiency of thyroid
stimulating hormone or thyrotropic releasing
hormone.
Signs and symptoms of myxedema
Typical feature of this disorder is an edematous
appearance throughout the body. It is associa-
ted with the following symptoms:
i. Swelling of the face
ii. Bagginess under the eyes
iii. Nonpitting type of edema, i.e. when pressed,
it does not make pits and the edema is hard
iv. Atherosclerosis: It is the hardening of the
walls of arteries because of accumulation
of fat. In myxedema it occurs because of
increased plasma level of cholesterol which
leads to deposition of cholesterol on the
walls of the arteries.
Atherosclerosis produces arteriosclerosis
which refers to thickening and stiffening of arterial
wall. Arteriosclerosis causes hypertension.
Other general features of hypothyroidism in
adults are:
i. Anemia
ii. Fatigue and muscular sluggishness
iii. Extreme somnolence with sleeping up to 14
to 16 hours per day
iv. Menorrhagia and polymenorrhea
v. Decreased cardiovascular functions such
as reduction in rate and force of contraction
of the heart, cardiac output and blood
volume
vi. Increase in body weight
vii. Constipation
viii. Mental sluggishness
ix. Depressed hair growth
x. Scaliness of the skin
xi. Frog like husky voice
xii. Cold intolerance.
Cretinism
Cretinism is the hypothyroidism in children
characterized by stunted growth.
Causes for cretinism
Cretinism occurs due to congenital absence of
thyroid gland, genetic disorder or lack of iodine
in the diet.
Features of cretinism
i. A newborn baby with thyroid deficiency may
appear normal at the time of birth because
thyroxine might have been supplied from
mother. But a few weeks after birth, the baby
starts developing the signs like sluggish
movements and croaking sound while crying.
Unless treated immediately, the baby will be
mentally retarded permanently.
ii. Skeletal growth is more affected than the soft
tissues. So, there is stunted growth with
bloated body. The tongue becomes so big,
that it hangs down with dripping of saliva.
The big tongue obstructs swallowing and
breathing. The tongue produces characte-
ristic guttural breathing that may sometimes
choke the baby.
Cretin vs dwarf
A cretin is different from pituitary dwarf. In
cretinism, there is mental retardation and the
different parts of the body are disproportionate.
Whereas, in dwarfism, the development of
nervous system is normal and the parts of the
body are proportionate (Fig. 46-5). The
reproductive function is affected in cretinism but
in dwarfism, it may be normal.

Endocrinology
282
mechanism, hypothalamus and anterior pituitary
are stimulated. It increases the secretion of TRH
and TSH. The TSH then causes the thyroid cells
to secrete tremendous amounts of thyroglobulin
into the follicle. As there are no hormones to be
cleaved, the thyroglobulin remains as it is and,
gets accumulated in the follicles of the gland.
This increases the size of gland.
ii. Idiopathic nontoxic goiter
It is the goiter due to unknown cause. Enlarge-
ment of thyroid gland occurs even without iodine
deficiency. The exact cause is not known.
Some foodstuffs contain goiterogenic
substances (goitrogens) such as goitrin. These
substances contain antithyroid substances like
propylthiouracil. Goitrogens suppress the syn-
thesis of thyroid hormones. Therefore, TSH
secretion increases resulting in enlargement of
the gland. Such goitrogens are found in vege-
tables like turnips and cabbages. Soybean also
contains some amount of goitrogens.
The goitrogens become active only during low
iodine intake.
THYROID FUNCTION TESTS
The following tests are commonly done to
assess the functional status of thyroid gland:
1. Measurement of concentration of T
3
and T
4
in plasma
2. Measurement of measurement of TRH and
TSH in plasma
3. Measurement of basal metabolic rate.
FIGURE 46-5:
Cretinism (3 months old baby)
(Courtesy: Prof Mafauzy Mohamad)
3. GOITER
Goiter means enlargement of the thyroid gland.
It occurs both in hypothyroidism and hyper-
thyroidism.
Goiter in Hyperthyroidism — Toxic Goiter
Toxic goiter is the enlargement of thyroid gland
with increased secretion of thyroid hormones
caused by thyroid tumor.
Goiter in Hypothyroidism — Nontoxic
Goiter
Nontoxic goiter is the enlargement of thyroid
gland without increase in hormone secretion. It
is also called hypothyroid goiter (Fig. 46-6).
Based on the cause, the nontoxic hypothyroid
goiter is classified into two types:
i. Endemic colloid goiter
ii. Idiopathic nontoxic goiter.
i. Endemic colloid goiter
It is the nontoxic goiter caused by iodine
deficiency. It is also called iodine deficiency
goiter. Iodine deficiency occurs when intake is
less than 50 μg /day. Because of lack of iodine,
there is no formation of hormones. By feedback
FIGURE 46-6:
Nontoxic goiter
(Courtesy: Prof Mafauzy Mohamad)
INTRODUCTION
PARATHORMONE
ACTIONS OF PARATHORMONE
REGULATION OF PARATHORMONE SECRETION
APPLIED PHYSIOLOGY — DISORDERS OF PARATHYROID GLANDS
HYPOPARATHYROIDISM — HYPOCALCEMIA
HYPERPARATHYROIDISM — HYPERCALCEMIA
PARATHYROID FUNCTION TESTS
CALCITONIN
ACTIONS OF CALCITONIN
REGULATION OF CALCITONIN SECRETION
CALCIUM METABOLISM
IMPORTANCE
NORMAL VALUE
TYPES
SOURCE
DAILY REQUIREMENTS
ABSORPTION AND EXCRETION
REGULATION
PHOSPHATE METABOLISM
IMPORTANCE
NORMAL VALUE
REGULATION
PHYSIOLOGY OF BONE
FUNCTIONS OF BONE
CELL TYPES OF BONE
BONE REMODELING
APPLIED PHYSIOLOGY — DISEASES OF BONE
Parathyroid Glands and
Physiology of Bone
47
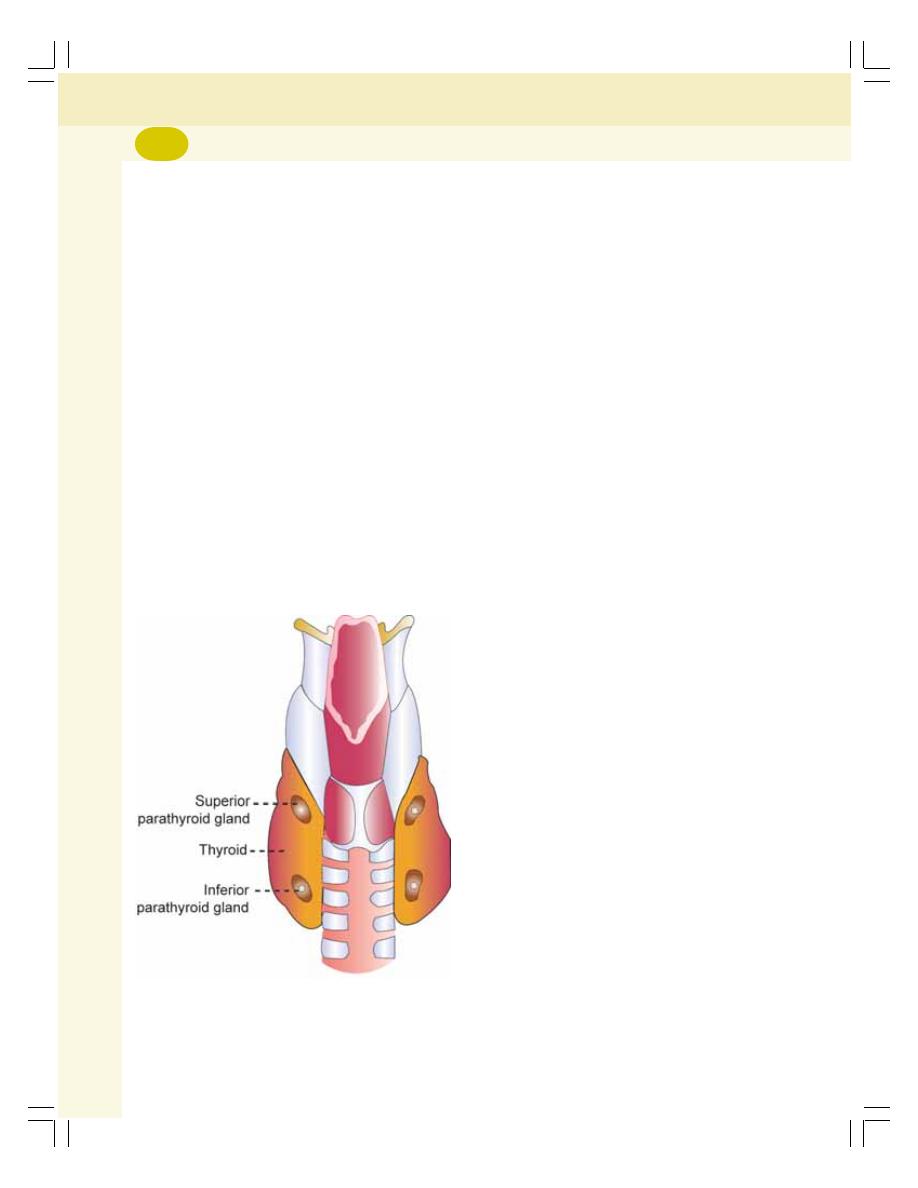
Endocrinology
284
INTRODUCTION
There are four parathyroid glands located
immediately behind thyroid gland at the upper
and lower poles (Fig. 47-1). The parathyroid
glands are very small in size measuring about
6 mm long, 3 mm wide and 2 mm thick with dark
brown color.
Histology: Each parathyroid gland is made up
of chief cells and oxyphil cells. The chief cells
secrete parathormone. The function of the
oxyphil cell is not known. It is believed that
oxyphil cells are the degenerated chief cells.
Parathormone is essential for the main-
tenance of blood calcium level within a very
narrow critical level.
PARATHORMONE
Parathormone (PTH) is secreted by the chief
cells of the parathyroid glands. It is protein in
nature having 84 amino acids. The normal
plasma level of PTH is about 1.5 to 5.5 ng/dL.
ACTIONS OF PARATHORMONE
PTH maintains the blood calcium level and blood
phosphate level.
On Blood Calcium Level
The primary action of PTH is to maintain the
blood calcium level within the critical range of
9 to 11 mg/dL. The blood calcium level has to
be maintained critically because, it is very
important for many of the activities in the body.
PTH maintains the blood calcium level by
acting on:
1. Bones
2. Kidneys
3. GI tract.
1. On bone
PTH increases resorption of calcium from the
bones by acting on osteoblasts, osteocytes and
osteoclasts of the bone.
PTH increases the permeability of the
membranes of osteoblasts and osteocytes for
calcium ions. So calcium ions move from these
bone cells into the blood.
PTH stimulates osteoclasts and causes
release of proteolytic enzymes and some acids
such as citric acid and lactic acid. All these
substances digest or dissolve the organic matrix
of the bone, releasing the calcium ions into the
plasma.
2. On kidneys
PTH increases the reabsorption of calcium from
distal convoluted tubule and proximal part of
collecting duct into the plasma. It also increases
the formation of 1,25-dihydroxycholecalciferol
(activated form of vitamin D) from 25-hydroxy-
cholecalciferol in kidneys which is necessary for
absorption of calcium form GI tract.
3. On gastrointestinal tract
PTH increases the absorption of calcium from
GI tract by increasing the formation of 1,25-
dihydroxycholecalciferol in the kidneys.
FIGURE 47-1: Parathyroid glands on the
posterior surface of thyroid gland
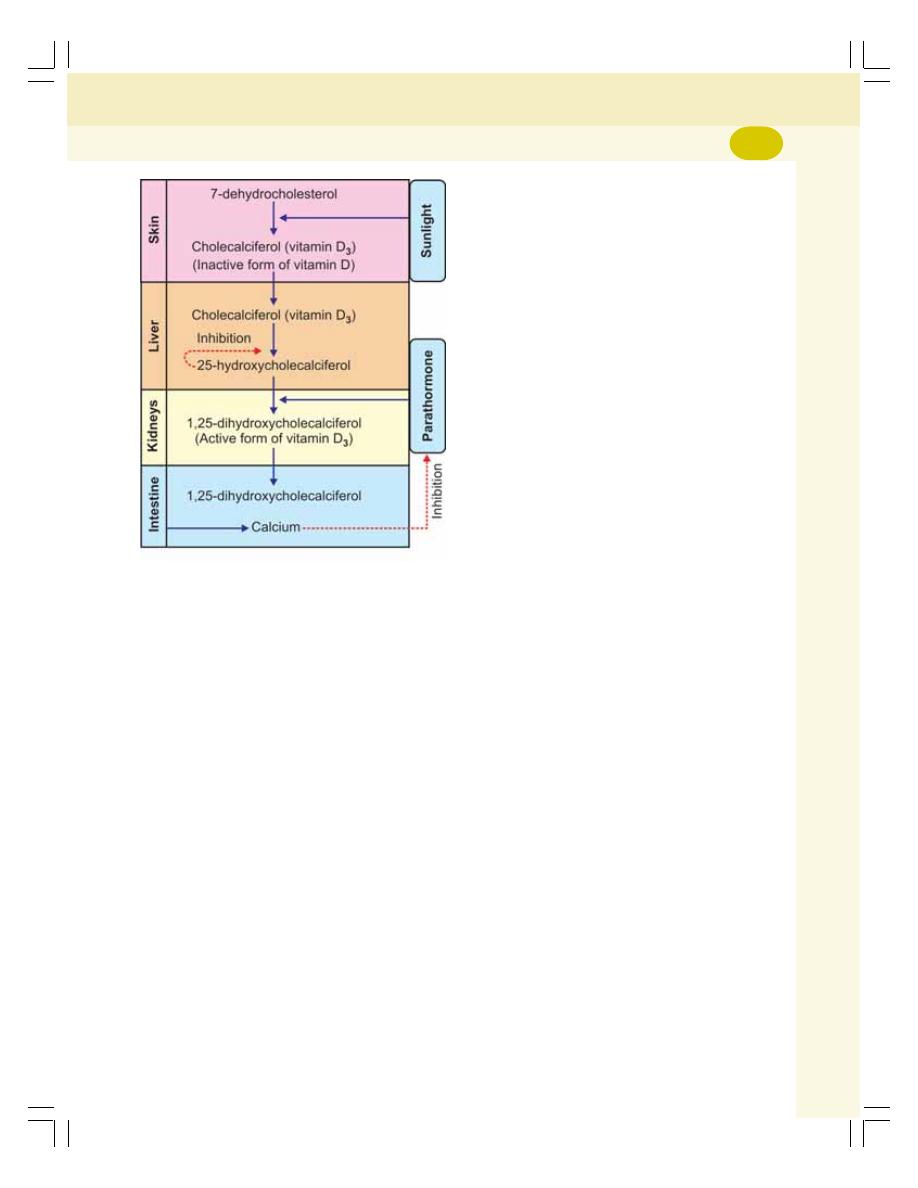
Chapter 47 Parathyroid Glands and Physiology of Bone
285
Activation of vitamin D: There are various forms
of vitamin D but, the most important one is
vitamin D
3
. It is also known as cholecalciferol.
Vitamin D
3
is synthesized in the skin from
7-dehydrocholesterol by the action of ultraviolet
rays from the sunlight. It is also obtained from
dietary sources. The activation of vitamin D
3
occurs in two steps (Fig. 47-2).
In the first step, cholecalciferol (vitamin D
3
)
is converted into 25-hydroxycholecalciferol in the
liver. This process is limited and is inhibited by
25-hydroxycholecalciferol itself by feedback
mechanism.
In the second step, 25-hydroxycholecalciferol
is converted into 1,25-dihydroxycholecalciferol
(calcitriol) in kidney. And, it is the active form of
vitamin D
3
. This step needs the presence of
PTH.
The 1,25-dihydroxycholecalciferol increases
the absorption of calcium and phosphate from
intestine.
On Blood Phosphate Level
PTH decreases blood level of phosphate by
acting on:
1. Bones
2. Kidneys
3. GI tract.
1. On bone
PTH increases the phosphate absorption from
bones.
2. On kidneys
Phosphaturic action: Phosphaturic action is the
effect of PTH by which phosphate is excreted in
urine. PTH inhibits reabsorption of phosphate
from renal tubules so that excretion of phosphate
through urine increases.
3. On gastrointestinal tract
PTH increases the formation of 1,25-dihydro-
xycholecalciferol in the kidneys. This vitamin in
turn increases the absorption of phosphate along
with calcium.
Mode of Action of PTH
On the target cells, PTH executes its action
through cAMP.
REGULATION OF PARATHORMONE
SECRETION
Blood level of calcium is the main factor that
regulates the secretion of PTH. Blood phos-
phate level also influences PTH secretion.
Blood Level of Calcium
PTH secretion is inversely proportional to blood
calcium level. Increase in blood calcium level
decreases PTH secretion.
Blood Level of Phosphate
PTH secretion is directly proportional to blood
phosphate level. Whenever the blood level of
phosphate increases, it combines with ionized
FIGURE 47-2: Schematic diagram showing
activation of vitamin D
Endocrinology
286
calcium to form calcium hydrogen phosphate.
This decreases ionized calcium level in blood
which stimulates PTH secretion.
APPLIED PHYSIOLOGY —
DISORDERS OF
PARATHYROID GLANDS
The disorders of parathyroid glands are of two
types:
I. Hypoparathyroidism
II. Hyperparathyroidism.
HYPOPARATHYROIDISM —
HYPOCALCEMIA
Hypoparathyroidism leads to hypocalcemia
(decrease in blood calcium level).
Causes for Hypoparathyroidism
1. Surgical removal of parathyroid glands
(parathyroidectomy)
2. Removal of parathyroid glands during surgical
removal of thyroid gland (thyroidectomy)
3. Autoimmune disease
4. Deficiency of receptors for PTH in the target
cells. In this, the PTH secretion is normal or
increased but the hormone cannot act on the
target cells. This condition is called
pseudohypoparathyroidism.
Hypocalcemia and Tetany
Hypoparathyroidism causes hypocalcemia by
decreasing the resorption of calcium from bones.
It causes neuromuscular hyperexcitability
resulting in hypocalcemic tetany. Normally, tetany
occurs when blood calcium level falls below
6 mg/dL from its normal value of 9.4 mg/dL.
Hypocalcemic Tetany
Tetany is an abnormal condition characterized
by painful muscular spasm (involuntary
contraction of muscle or group of muscles)
particularly in feet and hand. It is because of
hyperexcitability of nerves and skeletal muscles
due to calcium deficiency.
The signs and symptoms of hypocalcemic
tetany:
1. Hyper-reflexia and convulsions
The increased neural excitability results in hyper-
reflexia (overactive reflex actions) and convulsive
muscular contractions.
2. Carpopedal spasm
Carpopedal spasm is the spasm (violent and
painful muscular contraction) in hand and feet
that occurs due to hypocalcemia. During the
spasm, the hand shows a peculiar attitude with
flexion at wrist joint and metacarpophalangeal
joints, adduction of the thumb, and extension of
interphalangeal joints (Fig. 47-3).
3. Laryngeal stridor
Stridor means noisy breathing. Laryngeal stridor
means a loud crowing sound during inspiration
which occurs mainly due to laryngospasm
(involuntary contraction of laryngeal muscles).
Laryngeal stridor is a common feature of
hypocalcemic tetany.
4. Cardiovascular changes
i. Dilatation of the heart
ii. Prolonged duration of ST segment and QT
interval in ECG
iii. Arrhythmias (irregular heartbeat)
iv. Hypotension
v. Heart failure.
FIGURE 47-3: Carpopedal spasm

Chapter 47 Parathyroid Glands and Physiology of Bone
287
5. Other features
i. Decreased permeability of the cell mem-
brane
ii. Dry skin with brittle nails
iii. Hair loss
iv. Seizures
v. Signs of mental retardation in children or
dementia in adults (Chapter 101).
When the calcium level falls below 4 mg/dL
it becomes fatal. During such severe hypo-
calcemic conditions, tetany occurs so quickly that
a person develops spasm of different groups of
muscles in the body. Worst affected are the
laryngeal and bronchial muscles which develop
respiratory arrest resulting in death.
Latent Tetany
Latent or subclinical tetany is the neuromuscular
hyperexcitability due to hypocalcemia that
develops before the onset of tetany. It is charac-
terized by general weakness and cramps in feet
and hand. The hyperexcitability in these patients
is detected by some signs, which do not appear
in normal persons.
1. Trousseau’s sign
It is the spasm of the hand that is developed after
3 minutes of arresting the blood flow to lower
arm and hand. The blood flow to lower arm and
hand is arrested by inflating the blood pressure
cuff 20 mm Hg above the patient’s systolic
pressure.
2. Chvostek’s sign
Chvostek’s sign is the twitch of the facial muscles
caused by a gentle tap over the facial nerve in
front of the ear. It is due to the hyperirritability of
facial nerve.
3. Erb sign
Hyperexcitability of the skeletal muscles even to
a mild electrical stimulus is called Erb sign. It is
also called Erb-Westphal sign.
HYPERPARATHYROIDISM—
HYPERCALCEMIA
Hyperparathyroidism results in hypercalcemia
(increase in blood calcium level).
Causes of Hyperparathyroidism
1. Tumor in parathyroid glands
2. Compensatory hypertrophy of parathyroid
glands in response to hypocalcemia which
occurs due to other pathological conditions
such as chronic renal failure, vitamin D
deficiency and rickets
3. Hyperplasia (abnormal increase in the
number of cells) of all the parathyroid glands.
Hypercalcemia
Hypercalcemia is the increase in plasma calcium
level. It occurs in hyperparathyroidism because
of increased resorption of calcium from bones.
The common signs and symptoms of hyper-
calcemia:
i. Depression of the nervous system
ii. Sluggishness of reflex activities
iii. Reduced ST segment and QT interval in
ECG
iv. Lack of appetite
v. Constipation.
The depressive effects of hypercalcemia are
noticed when the blood calcium level increases
to 12 mg/dL. The condition becomes severe with
15 mg/dL and it becomes lethal when blood
calcium level reaches 17 mg/dL.
The other effects of hypercalcemia:
i. Bone diseases: Bone diseases like osteitis
fibrosa cystica develop.
ii. Parathyroid poisoning: It is the condition
characterized by severe manifestations
that occur when blood calcium level rises
above 15 mg/dL along with increase in
phosphate level leading to formation of
calcium-phosphate crystals. The calcium-
phosphate crystals may be deposited in
the tubules of the kidneys, thyroid gland,
alveoli of lungs, gastric mucosa and in the

Endocrinology
288
wall of the arteries. Calcium deposition
results in dysfunction of these organs.
Renal stones are formed when it is
deposited in kidney.
PARATHYROID FUNCTION TESTS
1. Measurement of blood calcium level
2. Chvostek’s sign and Trousseau’s sign for
hypoparathyroidism.
CALCITONIN
Source of Secretion
Calcitonin is secreted by the parafollicular cells
or clear cells (C cells) situated amongst the
follicles in thyroid gland.
Chemistry and Plasma Level
It is a polypeptide chain with 32 amino acids. Its
molecular weight is about 3,400. Plasma level
of calcitonin is 1 to 2 ng/L.
ACTIONS OF CALCITONIN
1. On Blood Calcium Level
Calcitonin plays an important role in controlling
the blood calcium level. It decreases the blood
calcium level and thereby counteracts para-
thormone.
Calcitonin reduces the blood calcium level by
acting on bones, kidneys and intestine.
i. On bones
Calcitonin stimulates osteoblastic activity and
facilitates the deposition of calcium on bones.
At the same time, it suppresses the activity of
osteoclasts and inhibits the resorption of calcium
from bones. It inhibits even the development of
new osteoclasts in bones.
ii. On kidney
Calcitonin increases the excretion of calcium
through urine, by inhibiting the reabsorption of
calcium from the renal tubules.
iii. On intestine
It prevents the absorption of calcium from
intestine into the blood.
2. On Blood Phosphate Level
With respect to calcium, calcitonin is an
antagonist to PTH. But it has similar actions of
PTH with respect to phosphate. It decreases
the blood level of phosphate by acting on bones
and kidneys.
i. On bones
Calcitonin inhibits the resorption of phosphate
from bone and stimulates deposition of
phosphate on bones.
ii. On kidney
Calcitonin increases the excretion of phosphate
through urine, by inhibiting phosphate reab-
sorption from renal tubules.
REGULATION OF CALCITONIN
SECRETION
High calcium content in plasma stimulates the
calcitonin secretion through a calcium receptor
in parafollicular cells. Gastrin also is known to
stimulate release of calcitonin.
CALCIUM METABOLISM
IMPORTANCE OF CALCIUM
Calcium is very essential for many activities in
the body such as:
1. Teeth and bone formation
2. Neuronal activity
3. Skeletal muscle activity
4. Cardiac activity
5. Smooth muscle activity
6. Secretory activity of the glands
7. Cell division and growth
8. Coagulation of blood.
NORMAL VALUE
In a normal young healthy adult, there is about
1100 g of calcium in the body. It forms about 1.5%
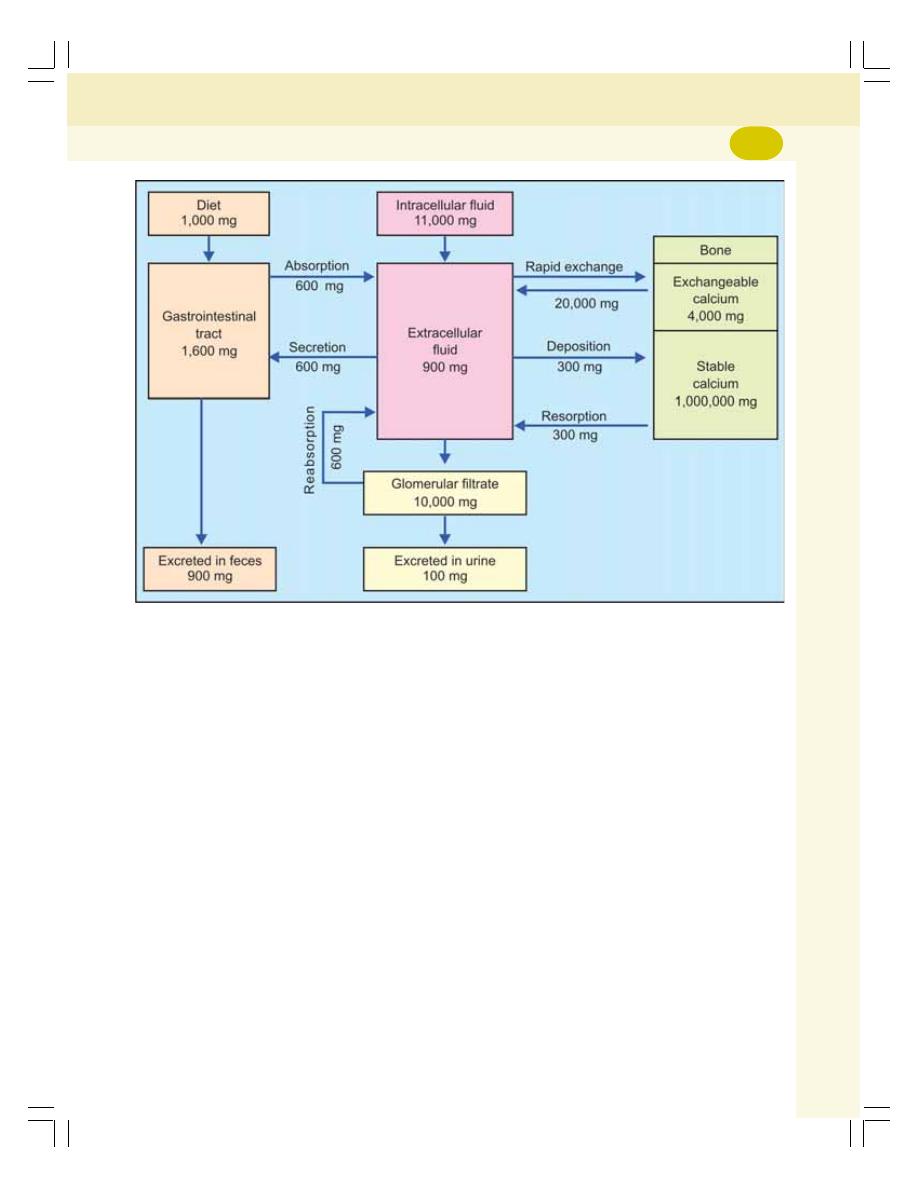
Chapter 47 Parathyroid Glands and Physiology of Bone
289
of total body weight. Ninety nine percent of calcium
is present in the bones and teeth and the rest is
present in the plasma. The normal blood calcium
level ranges between 9 and 11 mg/dL.
TYPES OF CALCIUM
Calcium in Plasma
Calcium is present in three forms in plasma:
i. Ionized or diffusible calcium
ii. Nonionized or nondiffusible calcium
iii. Calcium bound to albumin.
Ionized calcium is found freely in the plasma
and it forms about 50% of plasma calcium. It is
essential for the vital functions like neuronal
activity, muscle contraction, cardiac activity,
secretions in the glands, blood coagulation, etc.
About 8 to 10% of plasma calcium is present in
nonionic form such as calcium bicarbonate.
About 40 to 42% of calcium is bound with plasma
protein particularly, albumin.
Calcium in Bones
Calcium is constantly removed from bone and
deposited in bone. The process of calcium
metabolism is explained schematically in
Fig. 47-4.
SOURCE OF CALCIUM
1. Dietary Source
Calcium is available in several foodstuffs such
as milk, cheese, vegetables, meat, egg, grains,
sugar, coffee, tea, chocolate, etc.
2. From Bones
Besides dietary calcium, blood also gets calcium
from bones by resorption.
FIGURE 47-4: Schematic diagram showing calcium metabolism. The values belong to adults
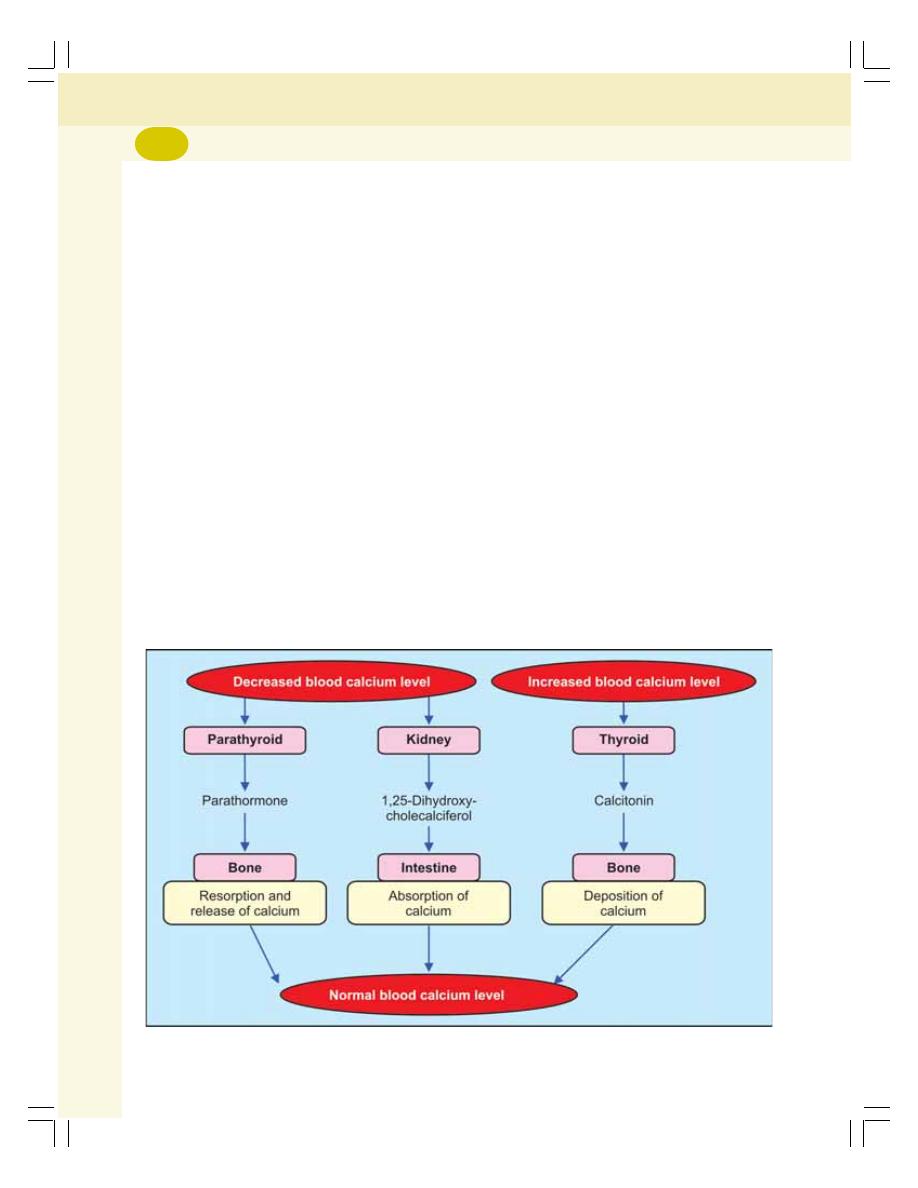
Endocrinology
290
DAILY REQUIREMENTS OF CALCIUM
1
to
3 years
=
500 mg
4
to
8 years
=
800 mg
9
to
18 years
= 1300 mg
19
to
50 years
= 1000 mg
51 years and above
= 1200 mg
Pregnant ladies and lactating mothers = 1300 mg
ABSORPTION AND EXCRETION OF
CALCIUM
Calcium taken through dietary sources is
absorbed from the GI tract into blood and distri-
buted to various parts of the body. Depending
upon the blood level, the calcium is either
deposited in the bone or removed from the bone
(resorption). Calcium is excreted from the body
through urine and feces.
Absorption from GI Tract
Calcium is absorbed from duodenum by carrier
mediated active transport and from the rest of
the small intestine by facilitated diffusion.
Vitamin D is essential for the absorption of
calcium from GI tract.
FIGURE 47-5: Schematic diagram showing regulation of blood calcium level
Excretion
While passing through the kidney, a large quantity
of calcium is filtered in the glomerulus. From the
filtrate, 98 to 99% of calcium is reabsorbed from
renal tubules into the blood and only a small
quantity is excreted through urine.
Most of the filtered calcium is reabsorbed in
the distal convoluted tubules and proximal part
of collecting duct. In distal convoluted tubule
parathormone increases the reabsorption. In
collecting duct vitamin D increases the reabsorp-
tion and calcitonin decreases reabsorption.
About 1000 mg of calcium is excreted daily.
Out of this 900 mg is excreted through feces and
100 mg through urine.
REGULATION OF BLOOD CALCIUM
LEVEL
Blood calcium level is regulated mainly by three
hormones (Figs 47-5 and 47-6):
1. Parathormone
2. 1,25-dihydroxycholecalciferol (calcitriol)
3. Calcitonin.
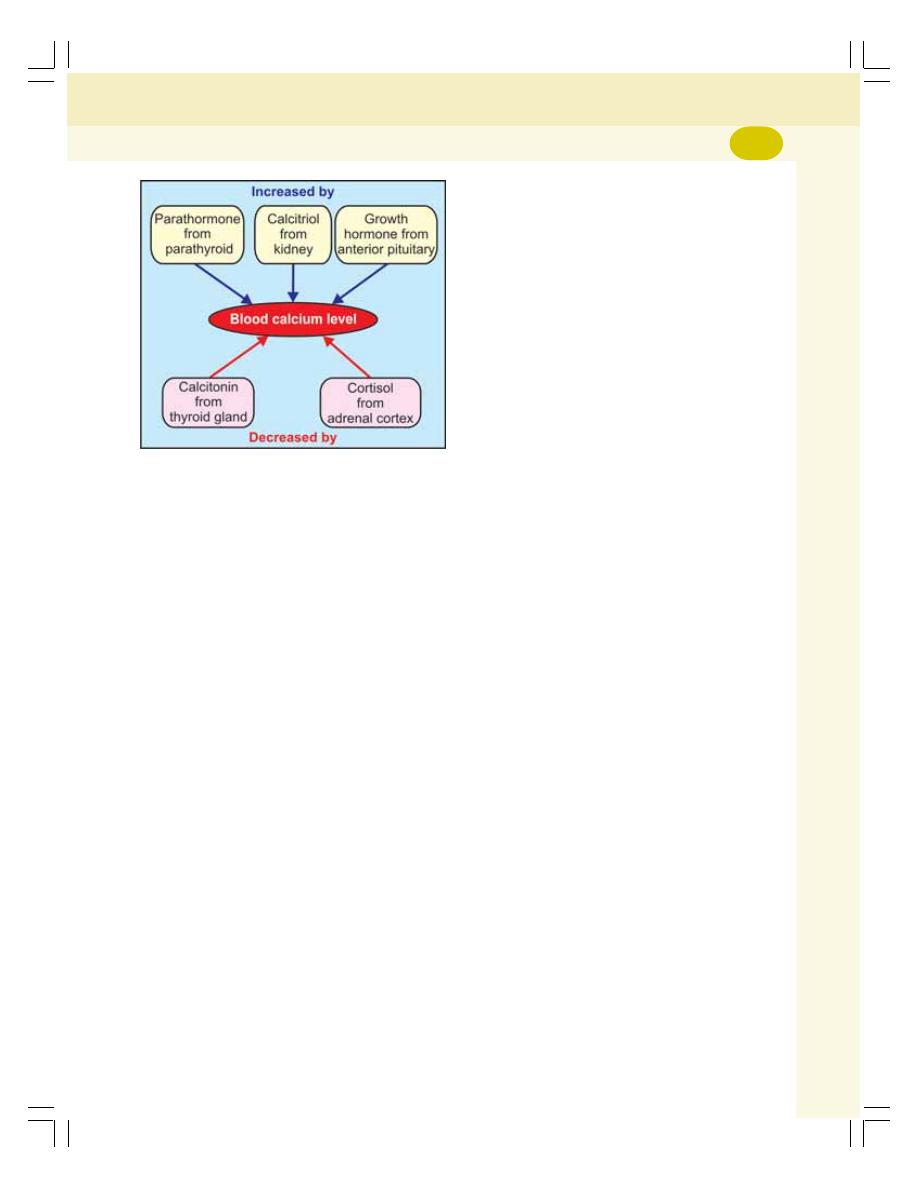
Chapter 47 Parathyroid Glands and Physiology of Bone
291
1. Parathormone
It is a protein hormone secreted by parathyroid
gland and its main function is to increase the
blood calcium level by mobilizing calcium from
bone (resorption) (see above for details).
2. 1,25-Dihydroxycholecalciferol — Calcitriol
It is a steroid hormone synthesized in kidney. It
is the activated form of vitamin D. Its main action
is to increase the blood calcium level by
increasing the calcium absorption from the small
intestine (see above for details).
3. Calcitonin
It is a protein hormone secreted by parafollicular
cells of thyroid gland. It is a calcium lowering
hormone. It reduces the blood calcium level
mainly by decreasing bone resorption (see above
for details).
Effects of Other Hormones
In addition to the above mentioned three
hormones, growth hormone and glucocorticoids
also influence the calcium level.
1. Growth hormone
It increases the blood calcium level by increa-
sing the intestinal calcium absorption.
2. Glucocorticoids
Glucocorticoids (cortisol) decrease blood
calcium by inhibiting intestinal absorption and
increasing the renal excretion of calcium.
PHOSPHATE METABOLISM
Phosphorus (P) is an essential mineral that is
required by every cell in the body for normal
function. Phosphorus is present in many food
substances, such as peas, dried beans, nuts,
milk, cheese and butter. Inorganic phosphorus
(Pi) is in the form of the phosphate (PO
4
). The
majority of the phosphorus in the body is found
as phosphate. Phosphorus is also the body’s
source of phosphate. In the body, phosphate is
the most abundant intracellular anion.
IMPORTANCE OF PHOSPHATE
1. Phosphate is an important component of
many organic substances such as, ATP, DNA,
RNA and many intermediates of metabolic
pathways
2. Along with calcium it forms an important
constituent of bone and teeth
3. It forms a buffer in the maintenance of acid–
base balance.
NORMAL VALUE
Total amount of phosphate in the body is 500 to
800 g. Though it is present in every cell of the
body, 85 to 90% of body’s phosphate is found in
the bones and teeth. Normal plasma level of
phosphate is 4 mg/dL.
REGULATION OF PHOSPHATE LEVEL
Phosphorus is taken through dietary sources. It
is absorbed from the GI tract into blood and
distributed to various parts of the body. While
passing through the kidney, a large quantity of
phosphate is excreted through urine. Phosphate
homeostasis depends upon three processes:
1. Absorption from gastrointestinal tract
2. Resorption from bone
3. Excretion through urine.
FIGURE 47-6: Effect of hormones on blood
calcium level
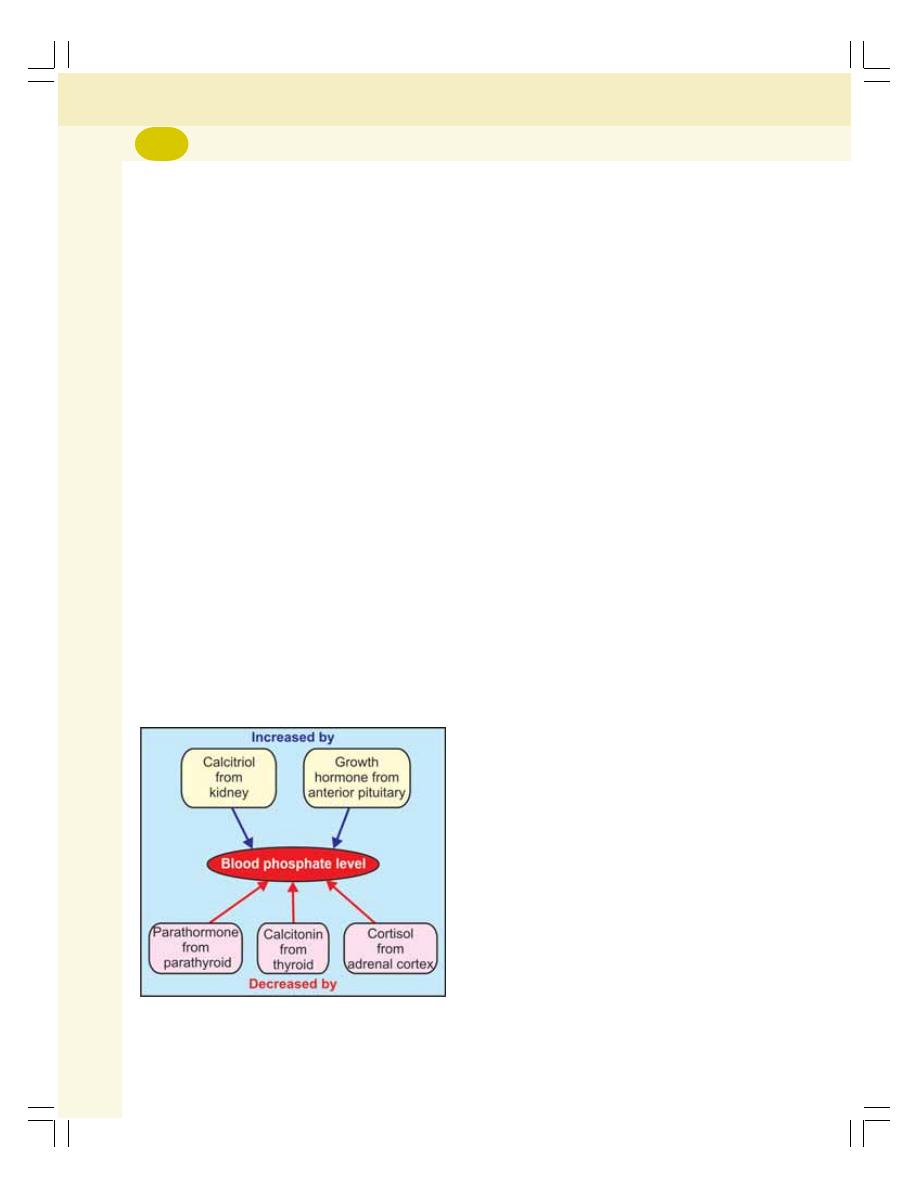
Endocrinology
292
These three processes are regulated by three
hormones:
1. Parathormone
2. Calcitonin
3. 1,25-dihydroxycholecalciferol (calcitriol).
1. Parathormone
Parathormone stimulates resorption of phos-
phate from bone, and increases its urinary
excretion. It also increases the absorption of
phosphate from gastrointestinal tract through
calcitriol. The overall action of parathormone
decreases the plasma level of phosphate.
2. Calcitonin
Calcitonin also decreases the plasma level of
phosphate by inhibiting bone resorption and
stimulating urinary excretion.
3. 1,25-Dihydroxycholecalciferol —
Calcitriol
This hormone increases absorption of phosphate
from small intestine (Fig. 47-7).
Effects of Other Hormones
In addition to the above mentioned three
hormones, growth hormone, and glucocorticoids
also influence the phosphate level.
1. Growth hormone
It increases the blood phosphate level by
increasing the intestinal phosphate absorption.
2. Glucocorticoids
Glucocorticoids (cortisol) decrease blood phos-
phate by inhibiting intestinal absorption and
increasing the renal excretion of phosphate.
PHYSIOLOGY OF BONE
Bone or osseous tissue is a specialized rigid
connective tissue that forms the skeleton. It
consists of special type of cells and tough
intercellular matrix of ground substance. The
matrix is formed by organic substances like
collagen and it is strengthened by the deposition
of mineral salts like calcium phosphate and
calcium carbonate. Throughout life, the bone is
renewed by the process of bone formation and
bone resorption.
FUNCTIONS OF BONE
1. Protective function – protects the soft tissues
and vital organs of the body
2. Mechanical function – supports the body and
brings out various movements of the body
3. Metabolic function – metabolism and
homeostasis of calcium and phosphate in the
body
4. Hemopoietic function – red bone marrow in
the bones is the site of production of blood
cells.
CELL TYPES OF BONE
Bone has three major types of cells:
1. Osteoblasts
2. Osteocytes
3. Osteoclasts.
1. Osteoblasts
Osteoblasts are the bone cells that are con-
cerned with bone formation. These cells are
situated in the outer surface of bone, the marrow
cavity and epiphyseal plate. The osteoblasts
arise from the giant multinucleated primitive
cells called the osteoprogenitor cells.
FIGURE 47-7: Effect of hormones on blood
phosphate level

Chapter 47 Parathyroid Glands and Physiology of Bone
293
Functions of osteoblasts
Osteoblasts
i. Are responsible for the synthesis of bone
matrix
ii. Are rich in the enzymes alkaline phos-
phatase, which is necessary for deposition
of calcium in the bone matrix (calcification)
iii. Synthesize the proteins called matrix
glaprotein and osteopontin, which are
involved in the calcification.
Fate of osteoblasts
After taking part in bone formation, the osteo-
blasts differentiate into osteocytes, which are
trapped inside the lacunae of calcified bone.
2. Osteocytes
Osteocytes are the cells concerned with main-
tenance of bone. Osteocytes are small flattened
and rounded cells embedded in the bone
lacunae. These bone cells are the main cells of
developed bone and are derived from the
matured osteoblasts.
Functions of osteocytes
i. Help to maintain the bone as living tissue
because of their metabolic activity
ii. Maintain the exchange of calcium bet-
ween the bone and ECF.
3. Osteoclasts
Osteoclasts are the bone cells that are concerned
with bone resorption. Osteoclasts are the giant
phagocytic multinucleated cells found in the
lacunae of bone matrix. These bone cells are
derived from hemopoietic stem cells via mono-
cytes (CFU-M).
Functions of osteoclasts
i. Responsible for bone resorption during
bone remodeling
ii. Synthesis and release of lysosomal
enzymes necessary for bone resorption
into the bone resorbing compartment.
BONE REMODELING
Bone remodeling is a dynamic lifelong process
in which old bone is resorbed and new bone is
formed. The process of remodeling extends for
about 100 days in compact bone and about
200 days in spongy bone.
Bone remodeling includes two processes:
1. Bone resorption: Destruction of entire bone
matrix and removal of calcium (osteoclastic
activity). Osteoclasts are responsible for this
2. Bone formation: Development and minerali-
zation of new matrix (osteoblastic activity).
Osteoblasts are responsible for this.
Significance of Bone Remodeling
In children
1. Thickness of bone increases
2. Bone obtains strength in proportion to the
growth
3. Shape of the bone is re-altered in relation to
the growth of the body.
In adults
1. It is responsible for the maintenance of
toughness of bone
2. Ensures the mechanical integrity of skeleton
throughout life
3. Plays important role in calcium homeostasis.
APPLIED PHYSIOLOGY — DISEASES
OF BONE
1. Osteoporosis
Osteoporosis is the bone disease characterized
by the loss of bone matrix and minerals. The
meaning of the word osteoporosis is ‘porous
bones’. It occurs due to excessive bone resorp-
tion and decreased bone formation.
The loss of bone matrix and minerals leads
to loss of bone strength associated with archi-
tectural deterioration of bone tissue. Ultimately,
the bones become fragile with high-risk of
fracture. Commonly affected bones are vertebrae
and hip. Osteoporosis is common in women after
60 years.

Endocrinology
294
2. Rickets
Rickets is the bone disease in children charac-
terized by inadequate mineralization of bone
matrix. It occurs due to vitamin D deficiency.
Vitamin D deficiency develops due to insuffi-
ciency in diet or due to inadequate exposure to
sunlight.
The deficiency of vitamin D affects the
reabsorption of calcium and phosphorus from
renal tubules resulting in calcium deficiency. It
causes inadequate mineralization of epiphyseal
growth plate in growing bones. This defect
produces various manifestations.
Manifestations of rickets
i. Collapse of chest wall: Due to the
flattening of sides of thorax with projecting
sternum called pigeon chest, chicken
chest or pectus carinatum
ii. Rachitic rosary: A visible swelling where
the ribs join their cartilages
iii. Kyphosis: The excess curvature of upper
back bone with convexity backward
(forward bending or forward curvature)
iv. Lordosis: The excess forward curvature
of back bone in lumbar region
v. Scoliosis: The lateral curvature of spine
vi. Bowing of hands and legs
vii. Enlargement of liver and spleen
viii. Tetany in advanced stages. The patient
may die because of tetany involving the
respiratory muscles.
3. Osteomalacia
The rickets in adults is called osteomalacia or
adult rickets. It occurs because of deficiency of
vitamin D. It also occurs due to prolonged
damage of kidney (renal rickets).
The characteristic features of osteomalacia:
i. Vague pain
ii. Tenderness in bones and muscles
iii. Myopathy leading to waddling gait (gait
means the manner of walking). In
waddling gait, the feet are wide apart and
walk resembles that of a duck.
iv. Occasional hypoglycemic tetany.
ISLETS OF LANGERHANS
INSULIN
ACTIONS
MODE OF ACTION
REGULATION OF SECRETION
GLUCAGON
ACTIONS
MODE OF ACTION
REGULATION OF SECRETION
SOMATOSTATIN
ACTIONS
MODE OF ACTION
REGULATION OF SECRETION
PANCREATIC POLYPEPTIDE
ACTIONS
MODE OF ACTION
REGULATION OF SECRETION
REGULATION OF BLOOD SUGAR LEVEL
NORMAL BLOOD SUGAR LEVEL
ROLE OF LIVER IN THE MAINTENANCE OF BLOOD SUGAR LEVEL
ROLE OF INSULIN IN THE MAINTENANCE OF BLOOD SUGAR LEVEL
ROLE OF GLUCAGON IN THE MAINTENANCE OF BLOOD SUGAR LEVEL
ROLE OF OTHER HORMONES IN THE MAINTENANCE OF BLOOD SUGAR
LEVEL
APPLIED PHYSIOLOGY
HYPOACTIVITY — DIABETES MELLITUS
HYPERACTIVITY — HYPERINSULINISM
Endocrine Functions
of Pancreas
48

Endocrinology
296
ISLETS OF LANGERHANS
The endocrine function of pancreas is performed
by the islets of Langerhans. Human pancreas
contains about 1 to 2 million islets.
Islets of Langerhans consist of four types of
cells:
1. A cells or
α cells which secrete glucagon
2. B cells or
β cells which secrete insulin
3. D cells or
δ cells which secrete somatostatin
4. F cells or PP cells which secrete pancreatic
polypeptide.
INSULIN
Insulin is secreted by B cells or the
β cells in the
islets of Langerhans of pancreas. Insulin is a
polypeptide with 51 amino acids. It has two amino
acid chains called
α and β chains which are
linked by disulfide bridges. The
α chain of insulin
contains 21 amino acids, and
β chain contains
30 amino acids.
Basal level of insulin in plasma is 10 μU/mL.
ACTIONS
Insulin is the important hormone that is concer-
ned with regulation of carbohydrate metabolism
and blood sugar level. It is also concerned with
metabolism of proteins and fats.
1. On Carbohydrate Metabolism
Insulin is the only antidiabetic hormone secreted
in the body, i.e. it is the only hormone in the body
that reduces blood sugar level. Insulin reduces
the blood sugar level by its following actions on
carbohydrate metabolism are:
i. Increases transport and uptake of glucose
by the cells
Insulin facilitates the transport of glucose from
the blood into the cells by increasing the per-
meability of cell membrane to glucose. Insulin
stimulates the rapid uptake of glucose by all the
tissues particularly liver, muscle and adipose
tissues. However, insulin is not required for
glucose uptake in some tissues like brain
(except hypothalamus), renal tubules, mucous
membrane of intestine and RBCs. Insulin also
increases the number of glucose transporters
called GLUT 4 in the cell membrane.
ii. Promotes peripheral utilization of glucose
Insulin promotes the peripheral utilization of
glucose. In the presence of insulin, the glucose
which enters the cell is oxidized immediately. The
rate of utilization depends upon intake of glucose.
iii. Promotes storage of glucose — glycogenesis
Insulin promotes the rapid conversion of glucose
into glycogen (glycogenesis), which is stored in
muscle and liver. Thus, glucose is stored in these
two organs in the form of glycogen. Insulin
activates the enzymes, which are necessary for
glycogenesis. In liver, when glycogen content
increases beyond its storing capacity, insulin
causes conversion of glucose into fatty acids.
iv. Inhibits glycogenolysis
Insulin prevents the breakdown of glycogen into
glucose in muscle and liver.
v. Inhibits gluconeogenesis
Insulin prevents gluconeogenesis, i.e. the
formation of glucose from proteins.
Thus, insulin decreases the blood sugar level
by:
i. Facilitating transport and uptake of
glucose by the cells
ii. Increasing peripheral utilization of glu-
cose
iii. Increasing the storage of glucose by
converting it into glycogen in liver and
muscle
iv. Inhibiting glycogenolysis
v. Inhibiting gluconeogenesis.
2. On Protein Metabolism
Insulin facilitates the synthesis and storage of
proteins and inhibits the cellular utilization of
proteins by:
i. Facilitating the transport of amino acids
into the cell from blood. Insulin actually,

Chapter 48 Endocrine Functions of Pancreas
297
increases the permeability of cell mem-
brane for amino acids
ii. Accelerating the synthesis of proteins by
influencing the transcription of DNA and
by increasing the translation of mRNA
iii. Preventing the catabolism of proteins by
decreasing the activity of cellular enzy-
mes, which act on proteins
iv. Preventing the conversion of proteins into
glucose.
Thus, insulin is responsible for conservation
and storage of proteins in the body.
3. On Fat Metabolism
Insulin stimulates the synthesis of fat. It also
increases the storage of fat in the adipose tissue.
Actions of insulin on fat metabolism are:
i. Synthesis of fatty acids and triglycerides
Insulin promotes the transport of excess glucose
into cells particularly the liver cells. This glucose
is utilized for the synthesis of fatty acids and
triglycerides. Insulin promotes the synthesis of
lipids by activating the enzymes which convert:
a. Glucose into fatty acids
b. Fatty acids into triglycerides.
ii. Transport of fatty acids into adipose tissue
Insulin facilitates the transport of fatty acids into
the adipose tissue.
iii. Storage of fat
Insulin promotes the storage of fat in adipose
tissue by inhibiting the enzymes, which degrade
the triglycerides.
4. On Growth
Along with growth hormone, insulin promotes
growth of body by its anabolic action on proteins.
It enhances the transport of amino acids into
the cells and synthesis of proteins in the cells. It
also has the protein-sparing effect, i.e. it causes
conservation of proteins by increasing the
glucose utilization by the tissues.
MODE OF ACTION
On the target cells, insulin binds with the
receptor protein and forms the insulin-receptor
complex. This executes the action by activating
the intracellular enzyme system.
REGULATION OF SECRETION
Insulin secretion is mainly regulated by blood
glucose level. In addition, other factors like amino
acids, lipid derivatives, gastrointestinal and
endocrine hormones and autonomic nerve fibers
also stimulate insulin secretion.
1. Role of Blood Glucose Level
When the blood glucose level is normal (80 to
100 mg/dL), the rate of insulin secretion is low
(up to 10 μU/minute). When the blood glucose
level increases between 100 to 120 mg/dL, the
rate of insulin secretion rises rapidly to 100 μU
/minute. When the blood glucose level rises
above 200 mg/dL, the rate of insulin secretion
also rises very rapidly up to 400 μU /minute.
2. Role of Proteins
The excess amino acids in blood also stimulate
insulin secretion.
3. Role of Lipid Derivatives
The
β ketoacids such as acetoacetate also
increase insulin secretion.
4. Role of Gastrointestinal Hormones
Insulin secretion is increased by some of the
gastrointestinal hormones such as gastrin,
secretin, cholecystokinin, and GIP.
5. Role of Endocrine Hormones
The diabetogenic hormones like glucagon,
growth hormone, and cortisol increase the blood
sugar level which, in turn, stimulate insulin
secretion indirectly. The prolonged hyper-
secretion of these hormones causes exhaustion
of
β cells resulting in diabetes mellitus.

Endocrinology
298
6. Role of Autonomic Nerves
The stimulation of parasympathetic nerve to the
pancreas (right vagus) increases insulin
secretion.
GLUCAGON
Glucagon is secreted from A cells or
α cells in
the islets of Langerhans of pancreas. It is also
secreted from A cells of stomach and L cells of
intestine. Glucagon is a polypeptide with
29 amino acids.
ACTIONS
Actions of glucagon are antagonistic to those of
insulin. It increases the blood sugar level and
peripheral utilization of lipids and facilitates the
conversion of proteins into glucose.
1. On Carbohydrate Metabolism
Glucagon increases the blood glucose level by
increasing glycogenolysis and gluconeogenesis
in liver and releasing glucose into the blood.
2. On Protein Metabolism
Glucagon increases transport of amino acids into
liver cells. The amino acids are utilized for
gluconeogenesis.
3. On Fat Metabolism
Glucagon shows lipolytic and ketogenic actions.
It increases lipolysis by increasing the release
of free fatty acids from adipose tissue and
making them available for peripheral utilization.
The lipolytic activity of glucagon, in turn, promotes
ketogenesis (formation of ketone bodies) in liver.
4. Other Actions
Glucagon:
i. Inhibits the secretion of gastric juice
ii. Increases the secretion of bile from liver.
MODE OF ACTION
On the target cells (mostly liver cells) glucagon
causes formation of cyclic AMP which brings out
the actions of glucagon.
REGULATION OF SECRETION
The secretion of glucagon is controlled mainly
by blood glucose and amino acid levels in the
blood.
1. Role of Blood Glucose Level
The important factor that regulates the secretion
of glucagon is the decrease in blood glucose
level. When blood glucose level decreases below
80 mg/dL of blood,
α cells of islets of Langerhans
are stimulated and more glucagon is released.
The glucagon in turn increases the blood glucose
level. On the other hand, when the blood sugar
level increases,
α cells are inhibited and the
secretion of glucagon decreases.
2. Role of Amino Acid Level in Blood
Increase in amino acid level in blood stimulates
the secretion of glucagon. Glucagon, in turn,
converts the amino acids into glucose.
3. Role of Other Factors
Factors which increase glucagon secretion:
i. Exercise
ii. Stress
iii. Gastrin
iv. Cholecystokinin
v. Cortisol.
Factors which inhibit glucagon secretion:
i. Somatostatin
ii. Insulin
iii. Free fatty acids
iv. Ketones.
SOMATOSTATIN
Somatostatin is secreted from hypothalamus,
D cells (
δ cells) in islets of Langerhans of pan-
creas and D cells in stomach and upper part of
small intestine. Somatostatin is a polypeptide.
ACTIONS
1. Somatostatin acts within islets of Langerhans
and, inhibits
α and β cells, i.e. it inhibits the
secretion of both glucagon and insulin

Chapter 48 Endocrine Functions of Pancreas
299
2. It decreases the motility of stomach, duode-
num and gallbladder
3. It reduces the secretion of gastrointestinal
hormones gastrin, CCK, GIP and VIP
4. Hypothalamic somatostatin inhibits secretion
of GH and TSH from anterior pituitary. That
is why, it is also called growth hormone
inhibitory hormone (GHIH).
MODE OF ACTION
Somatostatin brings out its actions through
cAMP.
REGULATION OF SECRETION
The secretion of pancreatic somatostatin is
stimulated by glucose, amino acids and chole-
cystokinin. The tumor of D cells of islets of
Langerhans causes hypersecretion of soma-
tostatin. It leads to hyperglycemia and other
symptoms of diabetes mellitus.
The secretion of somatostatin in GI tract
increases by the presence of chyme containing
glucose and proteins in stomach and small
intestine.
PANCREATIC POLYPEPTIDE
Pancreatic polypeptide is secreted by F cells
or PP cells in the islets of Langerhans of pan-
creas. It is also found in small intestine. It is a
polypeptide with 36 amino acids.
ACTIONS
The exact physiological action of pancreatic
polypeptide is not known. It is believed to
increase the secretion of glucagon from
α cells
in islets of Langerhans.
MODE OF ACTION
Pancreatic polypeptide brings out its actions
through cAMP.
REGULATION OF SECRETION
Secretion of pancreatic polypeptide is stimu-
lated by the presence of chyme containing more
proteins in the small intestine.
REGULATION OF BLOOD SUGAR
LEVEL (BLOOD GLUCOSE LEVEL)
NORMAL BLOOD SUGAR LEVEL
In normal persons, blood sugar level is
controlled within a narrow range. In the early
morning after overnight fasting, the blood sugar
level is low ranging between 70 and 110 mg/dL
of blood. Between first and second hour after
meals (postprandial), the blood sugar level rises
to 100 to 140 mg/dL. The sugar level in the blood
is brought back to normal at the end of second
hour after the meals.
The blood sugar regulating mechanism is
operated through liver and muscle by the
influence of the pancreatic hormones insulin
and glucagon. Many other hormones are also
involved in the regulation of blood sugar level.
Among all the hormones, insulin is the only
hormone that reduces the blood sugar level
and it is called the antidiabetogenic hormone.
The hormones, which increase blood sugar
level, are called diabetogenic hormones or anti-
insulin hormones.
Necessity of Regulation of Blood Glucose
Level
Regulation of blood sugar (glucose) level is very
essential because, glucose is the only nutrient
that is utilized for energy by many tissues such
as brain tissues, retina and germinal epithelium
of the gonads.
ROLE OF LIVER IN THE
MAINTENANCE OF BLOOD SUGAR
LEVEL
Liver serves as an important glucose buffer
system. When blood sugar level increases after
a meal, the excess glucose is converted into
glycogen and stored in liver. Afterwards, when
blood sugar level falls, the glycogen in liver is
converted into glucose and released into the
blood. The storage of glycogen and release of
glucose from liver are mainly regulated by insulin
and glucagon.

Endocrinology
300
ROLE OF INSULIN IN THE
MAINTENANCE OF BLOOD SUGAR
LEVEL
Insulin decreases the blood sugar level and it
is the only antidiabetic hormone available in the
body (Refer the actions on insulin on carbo-
hydrate metabolism in this chapter).
ROLE OF GLUCAGON IN THE
MAINTENANCE OF BLOOD SUGAR
LEVEL
Glucagon increases the blood sugar level (Refer
actions of glucagon on carbohydrate metabolism
in this chapter).
ROLE OF OTHER HORMONES IN THE
MAINTENANCE OF BLOOD SUGAR
LEVEL
The other hormones which increase the blood
sugar level are:
1. Growth hormone (Chapter 45)
2. Thyroxine (Chapter 46)
3. Cortisol (Chapter 49)
4. Adrenaline (Chapter 50).
Thus, liver helps to maintain the blood sugar
level by storing glycogen when blood glucose
level is high after meals; and by releasing
glucose, when blood sugar level is low after 2
to 3 hours of food intake. Insulin helps to control
the blood sugar level, especially after meals.
Glucagon and other hormones help to maintain
the blood sugar level by raising it in between the
meals.
APPLIED PHYSIOLOGY
HYPOACTIVITY — DIABETES MELLITUS
Diabetes mellitus is a metabolic disorder
characterized by high blood sugar (glucose) level
associated with other manifestations. In most of
the cases, the diabetes mellitus develops due
to the deficiency of insulin.
Types of Diabetes Mellitus
Diabetes mellitus is of two types, Type I and
Type II. The differences between the two types
are given in Table 48-1.
Type I Diabetes Mellitus
Type I diabetes mellitus is due to the deficiency
of insulin. So it is also called insulin dependent
diabetes mellitus (IDDM). Type I diabetes mellitus
may occur at any age of life but, it usually occurs
before 40 years of age. When it occurs at infancy
(due to congenital disorder) or in childhood, it is
called juvenile diabetes.
Causes of type I diabetes mellitus
1. Degeneration of
β cells in the islets of
Langerhans of pancreas
2. Destruction of
β cells by viral infection
3. Congenital disorder of
β cells
4. Destruction of
β cells during autoimmune
diseases.
Type II Diabetes Mellitus
It is due to the absence or deficiency of insulin
receptors. It usually occurs after 40 years hence,
it is called maturity onset diabetes mellitus. This
type of diabetes mellitus is also called noninsulin
dependent diabetes mellitus (NIDDM).
Causes for type II diabetes mellitus
In this type of diabetes the structure and function
of
β cells and the blood level of insulin are normal.
But the insulin receptors are reduced in number
or absent in the body. The major causes for
type II diabetes are:
1. Hereditary disorders
2. Other endocrine disorders.
Diabetes mellitus associated with other
endocrine disorders
Diabetes is very common in some of the
endocrine disorders like gigantism, acromegaly,
and Cushing’s syndrome. The hyperglycemia in
these conditions causes excess stimulation of
β cells. The constant and excess stimulation, in
turn causes burning out and degeneration of
β
cells. The
β cell exhaustion leads to permanent
diabetes mellitus. This type of diabetes mellitus
is called secondary diabetes.
Chapter 48 Endocrine Functions of Pancreas
301
Signs and Symptoms of Diabetes Mellitus
Various manifestations of diabetes mellitus
develop because of three major setbacks of
insulin deficiency:
1. Increased blood sugar level (300 to
400 mg/dL) due to reduced utilization by
tissue
2. Mobilization of fats from adipose tissue for
energy purpose, leading to elevated fatty acid
content in blood. This causes deposition of
fat on the wall of arteries and development
of atherosclerosis
3. Depletion of proteins from the tissues.
Following are the signs and symptoms of
diabetes mellitus:
1. Glucosuria
Loss of glucose in urine is known as glucosuria.
Normally, glucose does not appear in urine.
When glucose level rises above 180 mg/dL in
blood, glucose appears in urine. It is the renal
threshold level for glucose.
2. Osmotic diuresis
Diuresis due to osmotic effects is called osmotic
diuresis. The excess glucose in the renal tubules
develops osmotic effect. The osmotic effect
decreases the reabsorption of water from renal
tubules resulting in diuresis. It leads to polyuria
and polydipsia.
3. Polyuria
Excess urine formation with increase in frequency
of voiding urine is called polyuria. It is due to the
osmotic diuresis caused by increase in blood
sugar level.
4. Polydipsia
The increase in water intake is called polydipsia.
The excess loss of water decreases water
content and increases salt content in the body.
This stimulates the thirst center in hypothalamus.
Thirst center in turn increases the intake of water.
TABLE 48-1:
Differences between type I and type II diabetes mellitus
Features
Type I (IDDM)
Type II (NIDDM)
Age of onset
Usually before 40 years
Usually after 40 years
Major cause
Lack of insulin
Lack of insulin receptor
Insulin deficiency
Yes
Partial deficiency
Immune destruction of
β cells
Yes
No
Involvement of other endocrine disorders
No
Yes
Hereditary cause
Yes
May or may not be
Need for insulin
Always
Not in initial stage
May require in later stage
Insulin resistance
No
Yes
Control by oral hypoglycemic agents
No
Yes
Symptoms appear
Rapidly
Slowly
Body weight
Usually thin
Usually overweight
Stress induced obesity
No
Yes
Ketosis
Yes
May or may not be

Endocrinology
302
5. Polyphagia
Polyphagia means the intake of excess food. It
is very common in diabetes mellitus.
6. Asthenia
The loss of strength is called asthenia. The body
becomes very weak. There is loss of energy.
Asthenia is because of protein depletion which
is caused by lack of insulin.
7. Acidosis
During insulin deficiency glucose cannot be
utilized by the peripheral tissues for energy. So,
a large amount of fat is broken down to release
energy. It causes the formation of excess
ketoacids leading to acidosis.
8. Acetone breathing
In cases of severe ketoacidosis, acetone is
expired in the expiratory air, giving the charac-
teristic acetone or fruity breath odor. It is a life-
threatening condition of severe diabetes.
9. Kussmaul breathing
Kussmaul breathing is the increase in rate and
depth of respiration caused by severe acidosis.
10. Circulatory shock
The osmotic diuresis leads to dehydration, which
causes circulatory shock. It occurs only in severe
diabetes.
11. Coma
Due to Kussmaul breathing, a large amount of
carbon dioxide is lost during expiration. It leads
to drastic reduction in the concentration of
bicarbonate ions causing severe acidosis and
coma. It occurs in severe cases of diabetes
mellitus.
Increase in blood sugar level develops hyper-
osmolarity of plasma which also leads to coma.
It is called hyperosmolar coma.
Complications of Diabetes Mellitus
Prolonged hyperglycemia in diabetes mellitus
causes dysfunction and injury of many tissues
resulting in some complications such as:
1. Cardiovascular complications like hyperten-
sion and myocardial infarction
2. Degenerative changes in retina called diabetic
retinopathy
3. Degenerative changes in kidney known as
diabetic nephropathy
4. Degeneration of autonomic and peripheral
nerves called diabetic neuropathy.
Diagnostic Tests for Diabetes Mellitus
Diagnosis of diabetes mellitus includes the
determination of:
1. Fasting blood sugar
2. Postprandial blood sugar
3. Glucose tolerance test (GTT)
4. Glycosylated (glycated) Hb.
HYPERACTIVITY — HYPERINSULINISM
Hyperinsulinism is the hypersecretion of insulin.
Cause of Hyperinsulinism
Hyperinsulinism occurs due to the tumor of
β cells in the islets of Langerhans.
Signs and Symptoms of Hyperinsulinism
1. Hypoglycemia
The blood sugar level falls below 50 mg/dL.
2. Manifestations of central nervous system
Manifestations of central nervous system occur
when the blood sugar level decreases. All the
manifestations are together called neuroglyco-
penic symptoms.
Initially, the activity of neurons increases
resulting in nervousness, tremor all over the body
and sweating. If not treated immediately, it leads
to clonic convulsions and unconsciousness.
Slowly, the convulsions cease and coma occurs
due to damage of neurons.
FUNCTIONAL ANATOMY OF ADRENAL GLANDS
HORMONES OF ADRENAL CORTEX
MINERALOCORTICOIDS
FUNCTIONS
MODE OF ACTION
REGULATION OF SECRETION
GLUCOCORTICOIDS
FUNCTIONS
MODE OF ACTION
REGULATION OF SECRETION
ADRENAL SEX HORMONES
APPLIED PHYSIOLOGY
HYPERACTIVITY OF ADRENAL CORTEX
HYPOACTIVITY OF ADRENAL CORTEX
Adrenal Cortex
49
FUNCTIONAL ANATOMY OF
ADRENAL GLANDS
There are two adrenal glands. Each gland is
situated on the upper pole of each kidney.
Because of the situation, adrenal glands are
otherwise called suprarenal glands. Each gland
is made of two parts, the adrenal cortex and
adrenal medulla. Adrenal cortex is the outer
portion constituting 80% of the gland. Adrenal
medulla is the central portion of gland constituting
20%.
Adrenal cortex is formed by three distinct
layers of structures (Fig. 49-1).
1. Zona glomerulosa – outer layer
2. Zona fasciculata – middle layer
3. Zona reticularis – inner layer
HORMONES OF ADRENAL CORTEX
The hormones secreted by adrenal cortex are
collectively known as adrenocortical hormones
or corticosteroids. Based on their functions the
corticosteroids are classified into three groups:
1. Mineralocorticoids
2. Glucocorticoids
3. Sex hormones.
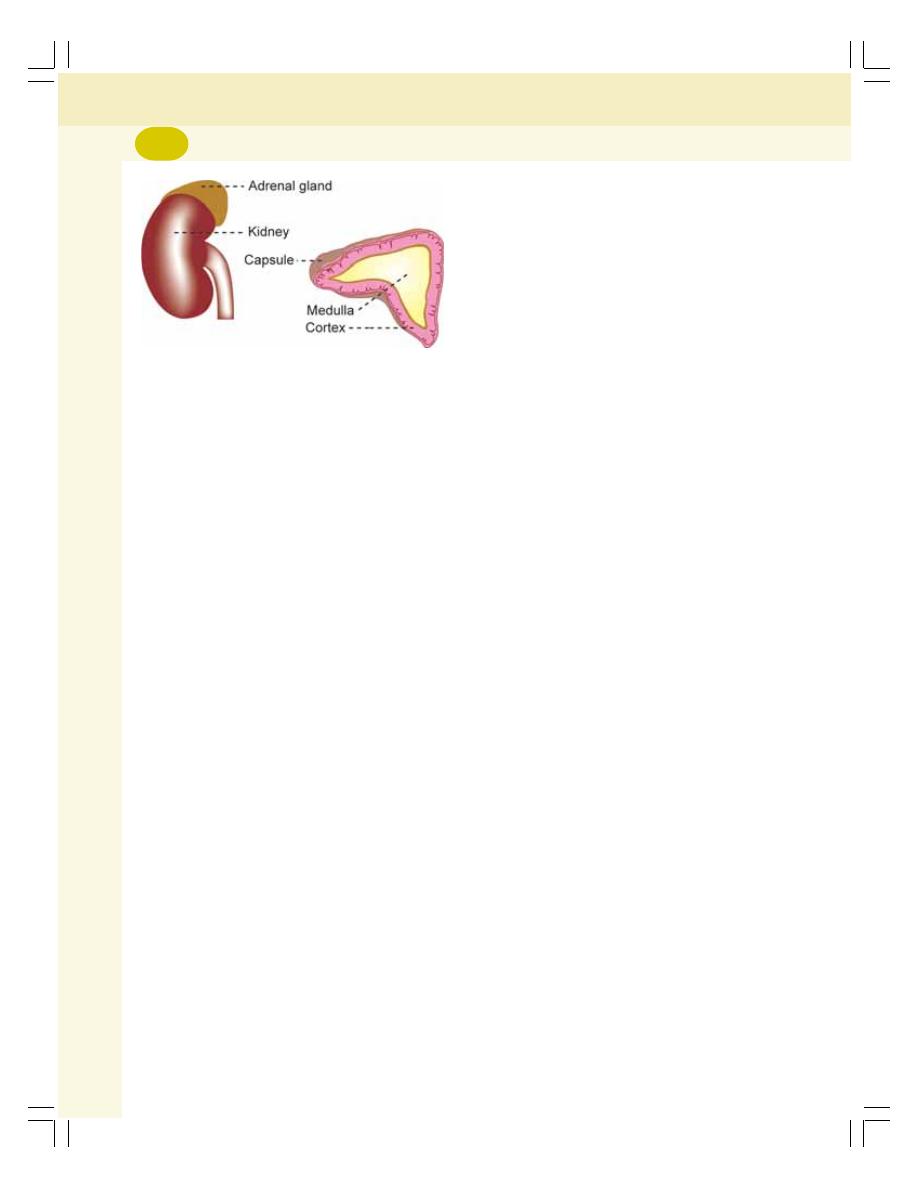
Endocrinology
304
MINERALOCORTICOIDS
Mineralocorticoids are the corticosteroids that act
on the minerals (electrolytes) particularly sodium
and potassium. The mineralocorticoids are
secreted by zona glomerulosa of adrenal cortex.
Mineralocorticoids are:
1. Aldosterone
2. 11-Deoxycorticosterone.
Mineralocorticoids are C
21
steroids having
21 carbon atoms. Plasma level of aldosterone
and 11-Deoxycorticosterone is 0.006 μg/dL.
FUNCTIONS OF
MINERALOCORTICOIDS
Ninety percent of mineralocorticoid activity is
provided by aldosterone.
Life Saving Hormone
Aldosterone is very essential for life and it is
usually called life saving hormone because, the
total loss of this hormone causes death within
3 days to 2 weeks. It is mainly because of loss
of mineralocorticoids which are essential to
maintain the osmolarity and volume of ECF.
Actions of aldosterone are:
1. On Sodium Ions
Aldosterone increases reabsorption of sodium
from distal convoluted tubule and the collecting
duct in kidney.
2. On Extracellular Fluid Volume
When sodium ions are reabsorbed from the renal
tubules, almost an equal amount of water is also
reabsorbed. So the net result is the increase in
ECF volume.
Even though aldosterone increases the
sodium reabsorption from the renal tubules, the
concentration of sodium in the body does not
increase very much because of simultaneous
reabsorption of water.
But still, there is possibility for mild increase
in concentration of sodium in the blood (mild
hypernatremia). It induces thirst leading to intake
of water which again increases the ECF volume
and blood volume.
3. On Blood Pressure
Increase in ECF volume and the blood volume
finally leads to increase in blood pressure.
Aldosterone escape or escape phenomenon
Aldosterone escape refers to escape of the
kidney from salt-retaining effects of excess
secretion of aldosterone as in the case of pri-
mary hyperaldosteronism.
Mechanism of aldosterone escape
When aldosterone level increases, there is
excess retention of sodium and water. This
increases the ECF volume and blood pressure.
Aldosterone induced high blood pressure
decreases the ECF volume through two types
of reactions:
i. It stimulates secretion of atrial natriuretic
peptide (ANP) from atrial muscles of the
heart: ANP causes excretion of sodium in
spite of increase in aldosterone secretion
ii. It causes pressure diuresis (excretion of
excess salt and water by high blood
pressure) through urine. This decreases the
salt and water content in ECF in spite of
hypersecretion of aldosterone (Fig. 49-2).
Because of aldosterone escape, edema does
not occur in primary hyperaldosteronism.
FIGURE 49-1:
Adrenal gland
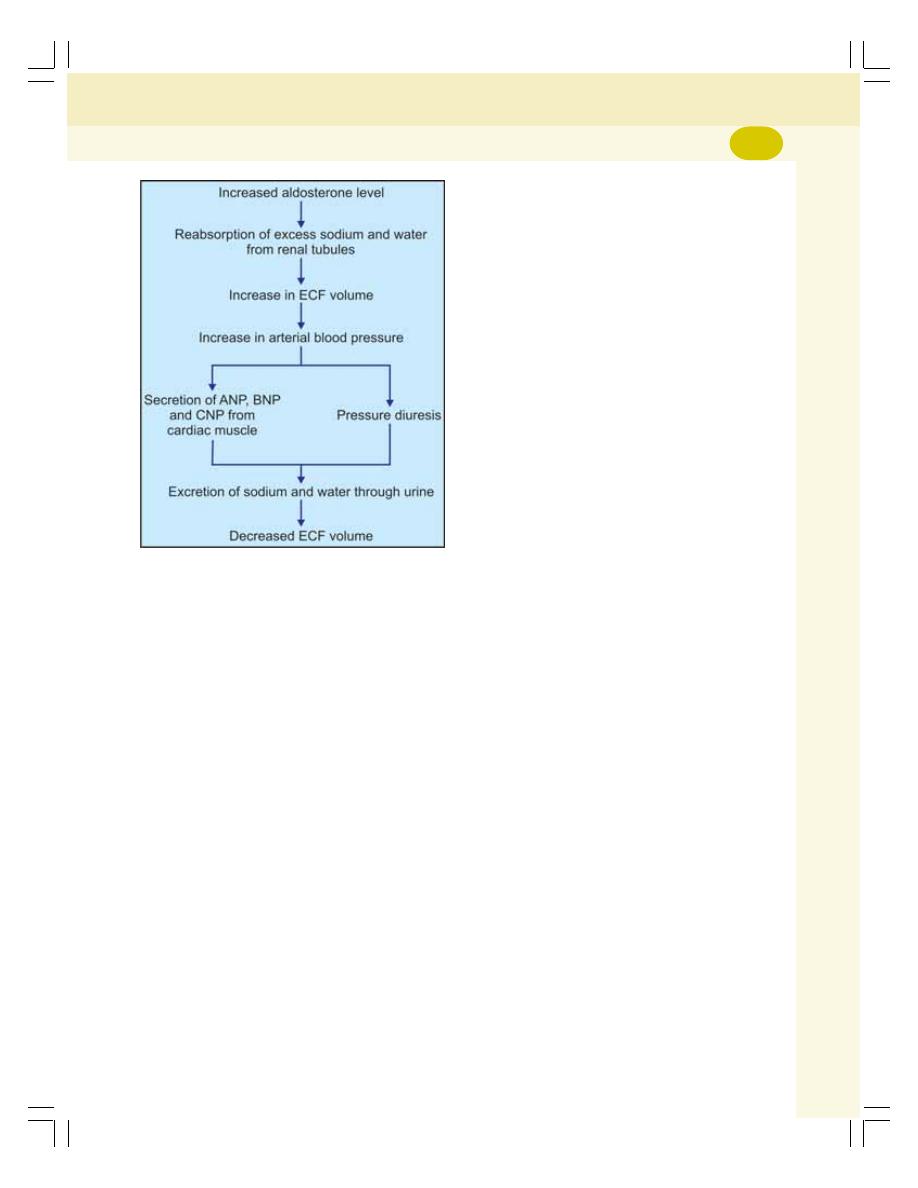
Chapter 49 Adrenal Cortex
305
4. On Potassium Ions
Aldosterone increases the potassium excretion
through the renal tubules.
5. On Hydrogen Ion Concentration
While increasing the sodium reabsorption from
the renal tubules, aldosterone causes tubular
secretion of hydrogen ions which is essential to
maintain acid–base balance in the body.
6. On Sweat Glands and Salivary Glands
Aldosterone has almost the similar effect on
sweat glands and salivary glands as it shows on
renal tubules. Sodium is reabsorbed from sweat
glands under the influence of aldosterone, thus
the loss of sodium from the body is prevented.
Same effect is shown on saliva also. Thus,
aldosterone helps conservation of sodium in the
body.
FIGURE 49-2:
Aldosterone escape. ANP = atrial
natriuretic peptide. BNP = brain natriuretic peptide.
CNP = C-type natriuretic peptide.
7. On Intestine
Aldosterone increases sodium absorption from
the intestine, especially from the colon and pre-
vents loss of sodium through feces.
MODE OF ACTION
Mineralocorticoids act through the messenger
RNA mechanism.
REGULATION OF SECRETION
Aldosterone secretion is regulated by four impor-
tant factors (Fig. 49-3). The stimulatory agents
for aldosterone secretion are given below in
the order of their potency:
1. Increase in potassium ion concentration in
ECF
2. Decrease in sodium ion concentration in ECF
3. Decrease in ECF volume
4. Adrenocorticotropic hormone.
Increase in the concentration of potassium
ions is the most effective stimulant for aldo-
sterone secretion. It acts directly on zona glo-
merulosa and increases the secretion of
aldosterone. Decrease in sodium ion concen-
tration and ECF volume stimulates aldosterone
secretion through renin-angiotensin mechanism.
Renin secreted from juxtaglomerular apparatus
of kidney acts on angiotensinogen in the plasma
and converts it into angiotensin I, which is
converted into angiotensin II by converting
enzyme (ACE) secreted by lungs. Angiotensin
II acts on the zona glomerulosa to secrete more
aldosterone. Aldosterone, in turn, increases the
retention of sodium and water and excretion of
potassium leading to increase in the sodium ion
concentration and ECF volume.
Now, the increased sodium ion concentration
and the ECF volume inhibit the juxtaglomerular
apparatus and stop the release of renin. So,
angiotensin II is not formed and release of
aldosterone from adrenal cortex is stopped.
Adrenocorticotropic hormone mainly stimu-
lates the secretion of glucocorticoids. It has only
a mild stimulating effect on aldosterone secretion.
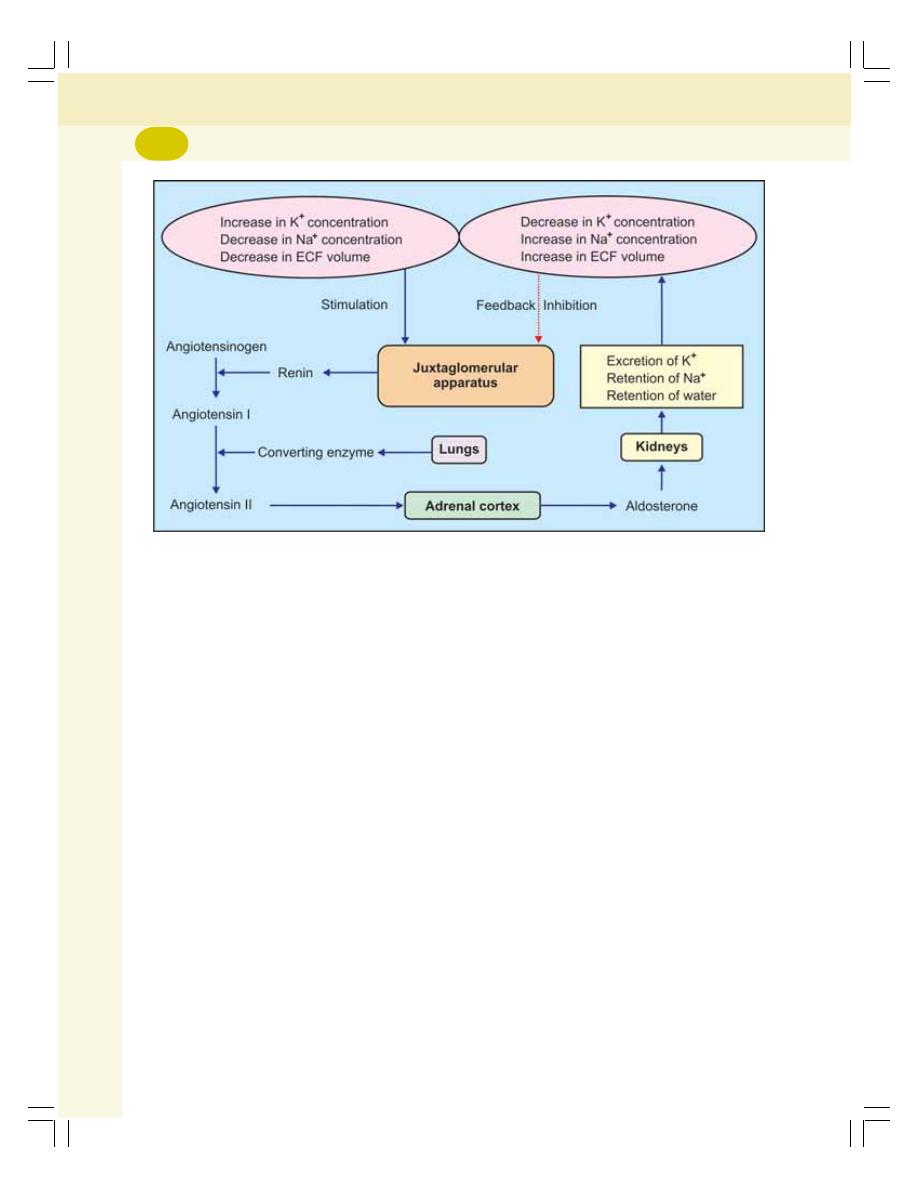
Endocrinology
306
GLUCOCORTICOIDS
Glucocorticoids are the corticosteroids which act
mainly on glucose metabolism. Glucocorticoids
are secreted mainly by zona fasciculata of
adrenal cortex. A small quantity of glucocorticoids
is also secreted by zona reticularis.
Glucocorticoids are:
1. Cortisol
2. Corticosterone
3. Cortisone.
Glucocorticoids are C
21
steroids having
21 carbon atoms. The plasma level of cortisol
is 13.9 μg/dL and that of corticosterone is
0.4 μg/dL.
FUNCTIONS OF GLUCOCORTICOIDS
Cortisol or hydrocortisone is more potent and it
has 95% of glucocorticoid activity. Corticosterone
is less potent showing only 4% of glucocorticoid
activity. Cortisone with 1% activity is secreted in
minute quantity.
Life Protecting Hormone
Like aldosterone, cortisol is also essential for
life but in a different way. Aldosterone is a life
saving hormone, whereas cortisol is a life pro-
tecting hormone because, it helps to withstand
the stress and trauma in life.
Glucocorticoids have metabolic effects on
carbohydrates, proteins, fats and water. These
hormones also show mild mineralocorticoid effect.
1. On Carbohydrate Metabolism
Glucocorticoids increase the blood glucose level
by two ways:
i. By promoting gluconeogenesis in liver
from amino acids
ii. By inhibiting glucose uptake and utilization
by peripheral cells
2. On Protein Metabolism
Glucocorticoids promote catabolism of proteins
leading to decrease in cellular proteins and
FIGURE 49-3:
Regulation of aldosterone secretion

Chapter 49 Adrenal Cortex
307
increase in plasma amino acids and protein
content in liver by the following methods:
i. Glucocorticoids decrease the protein in
the body cells except liver cells by
accelerating protein catabolism and
release of amino acids from the tissues
ii. Glucocorticoids increase the transport of
amino acids into hepatic cells. In hepatic
cells, the amino acids are used for
synthesis of proteins, plasma proteins
and for gluconeogenesis.
Thus, glucocorticoids cause mobilization of
proteins from tissues other than liver.
3. On Fat Metabolism
Glucocorticoids cause mobilization and redis-
tribution of fats. The actions on fats are:
i. Mobilization of fatty acids from adipose
tissue
ii. Increasing the concentration of fatty acids
in blood
iii. Increasing the utilization of fat for energy.
By increasing the utilization of fats for energy
release, glucocorticoids cause the formation of
a large amount of ketone bodies. It is called
ketogenic effect of glucocorticoids.
4. On Water Metabolism
Glucocorticoids accelerate the excretion of water
and play an important role in the maintenance
of water balance.
5. On Mineral Metabolism
Glucocorticoids enhance the retention of sodium
and to a lesser extent increase the excretion of
potassium. Glucocorticoids decrease blood
calcium by inhibiting absorption of calcium from
intestine and increasing the excretion of calcium
through urine.
6. On Bone
Glucocorticoids stimulate the bone resorption
(osteoclastic activity) and inhibit bone formation
and mineralization (osteoblastic activity).
7. On Muscles
Glucocorticoids cause catabolism of proteins
from muscle.
8. On Blood Cells
Glucocorticoids decrease the number of cir-
culating eosinophils by increasing the destruction
of eosinophils in reticuloendothelial cells. These
hormones also decrease the number of baso-
phils, and lymphocytes and, increase the number
of circulating neutrophils, RBCs and platelets.
9. On Vascular Response
Presence of glucocorticoids is essential for the
constrictor action of adrenaline and noradre-
naline. In adrenal insufficiency, the blood vessels
fail to respond to adrenaline and noradrenaline
leading to vascular collapse.
10. On Central Nervous System
Glucocorticoids are essential for normal
functioning of nervous system. Insufficiency of
these hormones causes personality changes like
irritability and lack of concentration.
11. Permissive Action of Glucocorticoids
Permissive action of glucocorticoids refers to
execution of actions of some hormones only in
the presence of glucocorticoids. Examples are:
i. Calorigenic effects of glucagon
ii. Lipolytic effects of catecholamines
iii. Pressor effects of catecholamines
iv. Bronchodilator effect of catecholamines.
12. On Resistance to Stress
The exposure to any type of stress, either phy-
sical or mental, increases the secretion of ACTH.
ACTH in turn increases glucocorticoid secretion.
The increase in glucocorticoid level is very
essential for survival, as it offers high resistance
to the body against stress.
It is assumed that the glucocorticoids
enhance the resistance by the following ways:
i. Immediate release and transport of amino
acids from tissues to liver cells for
synthesis of new proteins and other

Endocrinology
308
substances which are essential to
withstand the stress
ii. Release of fatty acids from cells for
production of more energy during stress
iii. Enhancement of vascular reactivity to
catecholamines and fatty acid mobilizing
action of catecholamines, which are
necessary to withstand the stress
iv. Prevention of severity of other changes
in the body caused by stress.
13. Anti-inflammatory Effects
Inflammation is defined as a localized protective
response induced by injury or destruction of
tissues. When the tissue is injured by mechanical
or chemical factors, some substances are
released from the affected area, which produce
series of changes in the affected area.
Glucocorticoids prevent the inflammatory
changes in the injured or infected tissues by:
i. Inhibiting the release of proteolytic
enzymes responsible for inflammation
ii. Preventing rush of blood to the injured
area by enhancing vasoconstrictor action
of catecholamines
iii. Inhibiting migration of leukocytes into the
affected area
iv. Preventing loss of fluid from plasma into
the affected tissue by decreasing the
permeability of capillaries
v. Reducing the reactions of tissues by
suppressing T cells and other leukocytes
In addition to preventing inflammatory
reactions, if inflammation has already started, the
glucocorticoids cause an early resolution of
inflammation and rapid healing.
14. Anti-allergic Actions
Corticosteroids prevent the various reactions in
allergic conditions as in the case of inflammation.
15. Immunosuppressive Effects
Glucocorticoids suppress the immune system of
the body by decreasing the number of circulating
T lymphocytes. It is done by suppressing
lymphoid tissues (lymph nodes and thymus) and
proliferation of T cells. Glucocorticoids also
prevent release of interleukin-2 by T cells.
Thus, hypersecretion or excess use of
glucocorticoids decreases the immune reactions
against all foreign bodies entering the body. It
leads to severe infection causing death.
The immunological reactions, which are
common during organ transplantation, may
cause rejection of the transplanted tissues.
Glucocorticoids are used to suppress the immu-
nological reactions, because of their immuno-
suppressive action.
MODE OF ACTION
Glucocorticoids act through the messenger RNA
mechanism.
REGULATION OF SECRETION
Anterior pituitary regulates glucocorticoid sec-
retion by secreting ACTH. ACTH secretion is
regulated by hypothalamus through corticotropin
releasing factor (CRF).
Role of Anterior Pituitary — ACTH
Anterior pituitary controls the activities of adre-
nal cortex by secreting ACTH. ACTH is secreted
by the basophilic chromophilic cells of anterior
pituitary. It is a single chained polypeptide with
39 amino acids. Its concentration in plasma is
3 ng/dL.
ACTH is mainly concerned with the regulation
of cortisol secretion. It plays only a minor role in
the regulation of mineralocorticoid secretion.
Actions
ACTH is necessary for the structural integrity and
the secretory activity of adrenal cortex. It has
other functions also.
Actions of ACTH on adrenal cortex
(adrenal actions)
1. Maintenance of structural integrity and vas-
cularization of zona fasciculata and zona
reticularis of adrenal cortex. In hypophysec-
tomy, these two layers in the adrenal cortex
are atrophied
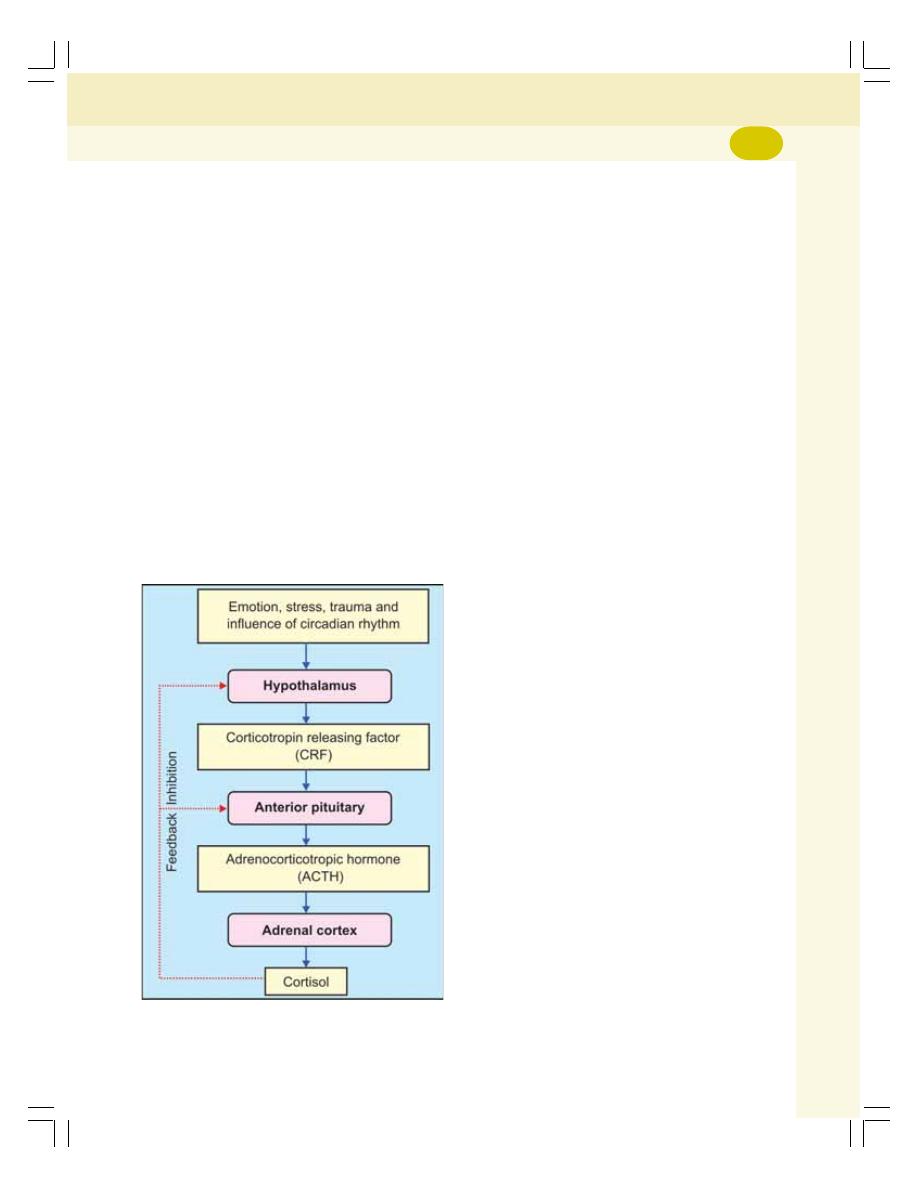
Chapter 49 Adrenal Cortex
309
2. Conversion of cholesterol into pregnenolone,
which is the precursor of glucocorticoids.
Thus, adrenocorticotropic hormone is res-
ponsible for synthesis of glucocorticoids
3. Release of glucocorticoids
4. Prolongation of glucocorticoid action on
various cells.
Other (nonadrenal) actions of ACTH
1. Mobilization of fats from tissues
2. Melanocyte stimulating effect. Because of
structural similarity with melanocyte stimu-
lating hormone, ACTH shows melanocyte
stimulating effect. It causes darkening of
skin by acting on melanophores which are
the cutaneous pigment cells containing
melanin.
Mode of action of ACTH
ACTH acts by the formation of cyclic AMP.
Role of Hypothalamus
Hypothalamus also plays an important role in
the regulation of cortisol secretion by controlling
the ACTH secretion through corticotropin
releasing factor (CRF). It is also called
corticotropin releasing hormone. CRF reaches
the anterior pituitary through the hypothalamo-
hypophyseal portal vessels.
CRF stimulates the corticotropes of anterior
pituitary and causes synthesis and release of
ACTH.
CRF secretion is induced by several factors
such as emotion, stress, trauma and circadian
rhythm. CRF in turn, causes release of ACTH,
which induces glucocorticoid secretion.
Feedback Control
Cortisol regulates its own secretion through
negative feedback control by inhibiting the
release of CRF from hypothalamus and ACTH
from anterior pituitary (Fig. 49-4).
ADRENAL SEX HORMONES
Adrenal sex hormones are secreted mainly by
zona reticularis. Zona fasciculata secretes small
quantities of sex hormones. Most of the hor-
mones are male sex hormones (androgens). But
small quantities of estrogen and progesterone
are also secreted by adrenal cortex. The
androgens secreted by adrenal cortex are:
1. Dehydroepiandrosterone
2. Androstenedione
3. Testosterone.
Dehydroepiandrosterone is the most active
adrenal androgen.
The androgens, in general, are responsible
for masculine features of the body (Chapter 53).
But in normal conditions, the adrenal androgens
have insignificant physiological effects, because
of the low amount of secretion both in males and
females.
In congenital hyperplasia of adrenal cortex
or tumor of zona reticularis, an excess quantity
of androgens is secreted. In males, it does not
produce any special effect because, a large
FIGURE 49-4:
Regulation of cortisol secretion
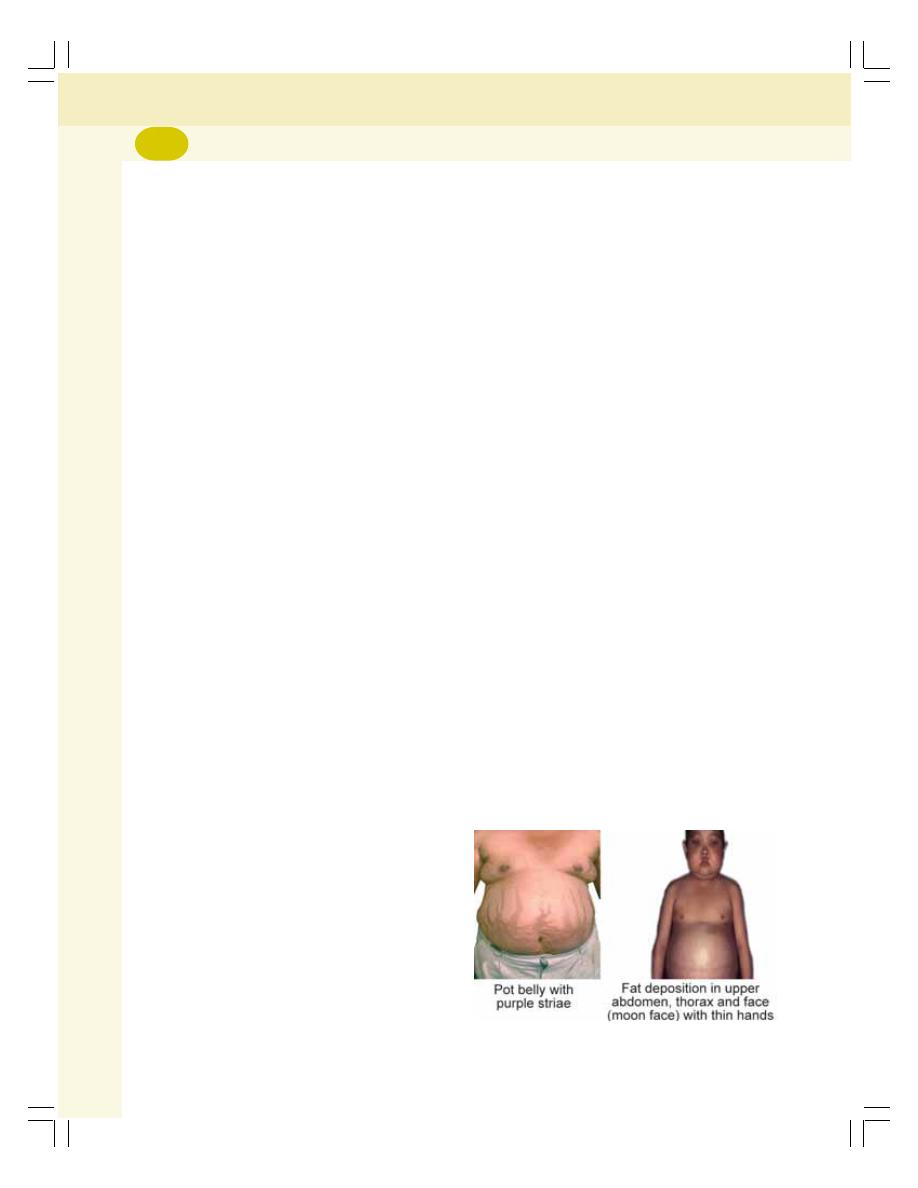
Endocrinology
310
quantity of androgens is produced by testes also.
But in females, the androgens produce masculine
features. Some of the androgens are converted
into testosterone. Testosterone is responsible for
the androgenic activity in adrenogenital syndrome
or congenital adrenal hyperplasia.
APPLIED PHYSIOLOGY
HYPERACTIVITY OF ADRENAL
CORTEX
1. Cushing’s syndrome
2. Hyperaldosteronism
3. Adrenogenital syndrome.
1. Cushing’s Syndrome
Cushing’s syndrome is a disorder characterized
by obesity.
Causes
Cushing’s syndrome is due to the hypersecretion
of glucocorticoids, particularly cortisol. It may be
due to either pituitary origin or adrenal origin.
If it is due to pituitary origin it is known as
Cushing’s disease. If it is due to adrenal origin it
is called Cushing’s syndrome. Generally, these
two terms are used interchangeably.
Pituitary origin
Increased secretion of ACTH causes hyperplasia
of adrenal cortex leading to hypersecretion of
cortisol.
Adrenal origin
Cortisol secretion is increased by:
i. Tumor or carcinoma in zona fasciculata
of adrenal cortex
ii. Prolonged treatment with exogenous
glucocorticoids
iii. Prolonged treatment with high dose of
ACTH
Signs and Symptoms
i. Characteristic feature of this disease is
the disproportionate distribution of body
fat resulting in some abnormal features:
a. Moon face: The edematous facial
appearance due to fat accumulation
and retention of water and salt
b. Torso: Accumulation of fat in chest and
abdomen. Arms and legs are very slim
in proportion to torso (torso means
trunk of the body)
c. Buffalo hump: Due to fat deposit on
the back of neck and shoulder
d. Pot belly: Due to fat accumulation in
upper abdomen (Fig. 49-5).
ii. Purple striae: Reddish purple stripes on
abdomen due to three reasons:
a. Stretching of abdominal wall by excess
subcutaneous fat
b. Rupture of subdermal tissues due to
stretching
c. Deficiency of collagen fibers due to
protein catabolism.
iii. Thinning of extremities
iv. Thinning of skin and subcutaneous
tissues due to protein catabolism
v. Darkening of skin on neck (aconthosis)
vi. Pigmentation of skin – hypersecretion of
ACTH which has got melanocyte stimu-
lating effect
vii. Facial redness (facial plethora)
viii. Facial hair growth (hirsutism)
ix. Weakening of muscles because of protein
depletion
x. Bone resorption and osteoporosis due to
protein depletion. Bone becomes sus-
ceptible to easy fracture
xi. Hyperglycemia due to gluconeogenesis
(from proteins) and inhibition of peripheral
FIGURE 49-5:
Cushing’s syndrome
(Courtesy: Prof Mafauzy Mohamad)

Chapter 49 Adrenal Cortex
311
utilization of glucose. Hyperglycemia leads
to glucosuria and adrenal diabetes
xii. Hypertension by the mineralocorticoid
effects of glucocorticoids – retention of
sodium and water results in increase in
ECF volume and blood volume leading to
hypertension
xiii. Immunosuppression resulting in sus-
ceptibility for infection
xiv. Poor wound healing.
2. Hyperaldosteronism
Increased secretion of aldosterone is called
hyperaldosteronism.
Causes
Depending upon the causes, hyperaldosteronism
is classified into two types:
i. Primary hyperaldosteronism which occurs
due to tumor in zona glomerulosa of
adrenal cortex. It is otherwise known as
Conn’s syndrome
ii. Secondary hyperaldosteronism which
occurs due to extra-adrenal causes such
as congestive cardiac failure, nephrosis,
toxemia of pregnancy and cirrhosis of
liver.
Signs and Symptoms
i. Increase in ECF volume and blood volume
ii. Hypertension due to increase in ECF
volume and blood volume
iii. Severe depletion of potassium. Prolonged
depletion of potassium causes renal
damage. The kidneys fail to produce
concentrated urine. It leads to polyuria and
polydipsia
iv. Muscular weakness due to potassium
depletion
v. Metabolic alkalosis due to secretion of
large amount of hydrogen ions into renal
tubules. Metabolic alkalosis reduces blood
calcium level causing tetany.
3. Adrenogenital Syndrome
Under normal conditions, adrenal cortex
secretes small quantities of androgens which do
not have any significant effect on sex organs or
sexual function. However, secretion of abnormal
quantities of adrenal androgens develops
adrenogenital syndrome.
Causes
It is due to the tumor of zona reticularis in
adrenal cortex.
Symptoms
Adrenogenital syndrome is characterized by the
tendency for the development of secondary
sexual character of opposite sex.
In females, increased secretion of androgens
causes development of male secondary sexual
characters. The condition is called adrenal
virilism.
In males, the tumor of estrogen secreting
cells produces more than normal quantity of
estrogens resulting in symptoms such as femi-
nization, gynecomastia (enlargement of breast)
and atrophy of testis.
HYPOACTIVITY OF ADRENAL
CORTEX
1. Addison’s disease or chronic adrenal
insufficiency
2. Congenital adrenal hyperplasia.
1. Addison’s Disease or Chronic Adrenal
Insufficiency
It is the failure of adrenal cortex to secrete
corticosteroids. It is classified into three types:
i. Primary Addison’s disease that occurs
due to adrenal cause
ii. Secondary Addison’s disease which is
due to failure of anterior pituitary to
secrete ACTH
iii. Tertiary Addison’s disease which is due
failure of hypothalamus to secrete CRF.
Causes for Primary Addison’s Disease
i. Atrophy or destruction of adrenal cortex
ii. Malignancy of adrenal cortex

Endocrinology
312
iii. Congenital failure to secrete cortisol
iv. Adrenalectomy and failure to take
hormone therapy.
Signs and Symptoms
The signs and symptoms develop in Addison’s
disease because of deficiency of both cortisol
and aldosterone. The common signs and
symptom are:
i. Pigmentation of skin and mucous
membrane
ii. Muscular weakness
iii. Dehydration with loss of sodium
iv. Hypotension
v. Decrease in size of the heart
vi. Hypoglycemia
vii. Nausea, vomiting and diarrhea
viii. Loss of body weight
ix. Susceptibility to any type of infection
x. Inability to withstand any stress resulting
in Addisonian crisis (see below).
Addisonian Crisis or Adrenal Crisis or
Acute Adrenal Insufficiency
It is a common symptom of Addison’s disease
characterized by sudden collapse associated
with an increase in need for large quantities of
glucocorticoids. The condition becomes fatal if
not treated in time.
Causes
i. Exposure to even mild stress
ii. Hypoglycemia due to fasting
iii. Trauma
iv. Surgical operation
v. Sudden withdrawal of glucocorticoid
treatment.
2. Congenital Adrenal Hyperplasia
It is a congenital disorder characterized by
increase in size of adrenal cortex. Size
increases due to abnormal increase in the
number of steroid secreting cortical cells.
Causes
Even though the size of the gland increases,
cortisol secretion decreases. It is because of
the congenital deficiency of the enzymes
necessary for the synthesis of cortisol, parti-
cularly, 21-hydroxylase.
Lack of this enzyme reduces the synthesis
of cortisol. It, in turn, increases the secretion of
ACTH from pituitary by feedback mechanism.
ACTH stimulates the adrenal cortex causing
hyperplasia with the accumulation of lipid
droplets. Cortisol cannot be synthesized because
of lack of 21-hydroxylase. Therefore, due to the
constant simulation of adrenal cortex by ACTH,
the secretion of androgens increases. It results
in sexual abnormalities such as virilism.
Symptoms
The characteristic features of adrenal hyperplasia
are virilism and excess body growth.
In boys
Adrenal hyperplasia produces a condition known
as macrogenitosomia praecox (Fig. 49-6). The
features of this condition are:
i. Precocious body growth, causing stocky
appearance called Infant Hercules
ii. Precocious sexual development with
enlarged penis even at age of 4 years.
In girls
In girls, adrenal hyperplasia produces masculi-
nization. It is otherwise called virilism. In some
cases of genetic disorders, the female child is
born with external genitalia of male type. This
condition is called pseudohermaphroditism.
FIGURE 49-6:
Congenital adrenal hyperplasia
(Macrogenitosomia praecox)
(Courtesy: Prof Mafauzy Mohamad)
INTRODUCTION
HORMONES OF ADRENAL MEDULLA
SYNTHESIS OF CATECHOLAMINES
METABOLISM OF CATECHOLAMINES
ACTIONS OF ADRENALINE AND NORADRENALINE
REGULATION OF SECRETION OF ADRENALINE AND NORADRENALINE
DOPAMINE
APPLIED PHYSIOLOGY – PHEOCHROMOCYTOMA
Adrenal Medulla
50
INTRODUCTION
Medulla is the inner part of the adrenal gland and
it forms 20% of mass of adrenal gland. It is made
up of interlacing cords of cells known as chro-
maffin cells, pheochrom cells or chromophil cells.
These cells contain fine granules which are
stained brown by potassium dichromate. The
chromaffin cells are of two types:
1. Adrenaline secreting cells (90%)
2. Noradrenaline secreting cells (10%).
HORMONES OF ADRENAL MEDULLA
Adrenal medullary hormones are the amines
derived from catechol and so these hormones
are called catecholamines. Three catechola-
mines are secreted by medulla:
1. Adrenaline or epinephrine
2. Noradrenaline or norepinephrine
3. Dopamine.
PLASMA LEVEL OF CATECHOLAMINES
1. Adrenaline
:
3
μg/dL
2. Noradrenaline
: 30
μg/dL
3. Dopamine
:
3.5 μg/dL
SYNTHESIS OF CATECHOLAMINES
Catecholamines are synthesized from the amino
acid tyrosine in the chromaffin cells of adrenal
medulla (Fig. 50-1). These hormones are formed
from phenylalanine also. But phenylalanine has
to be converted into tyrosine.
Stages of synthesis of catecholamines:
1. Formation of tyrosine from phenylalanine in
the presence of enzyme phenylalanine hydro-
xylase
2. Uptake of tyrosine from blood into the chro-
maffin cells of adrenal medulla by active
transport
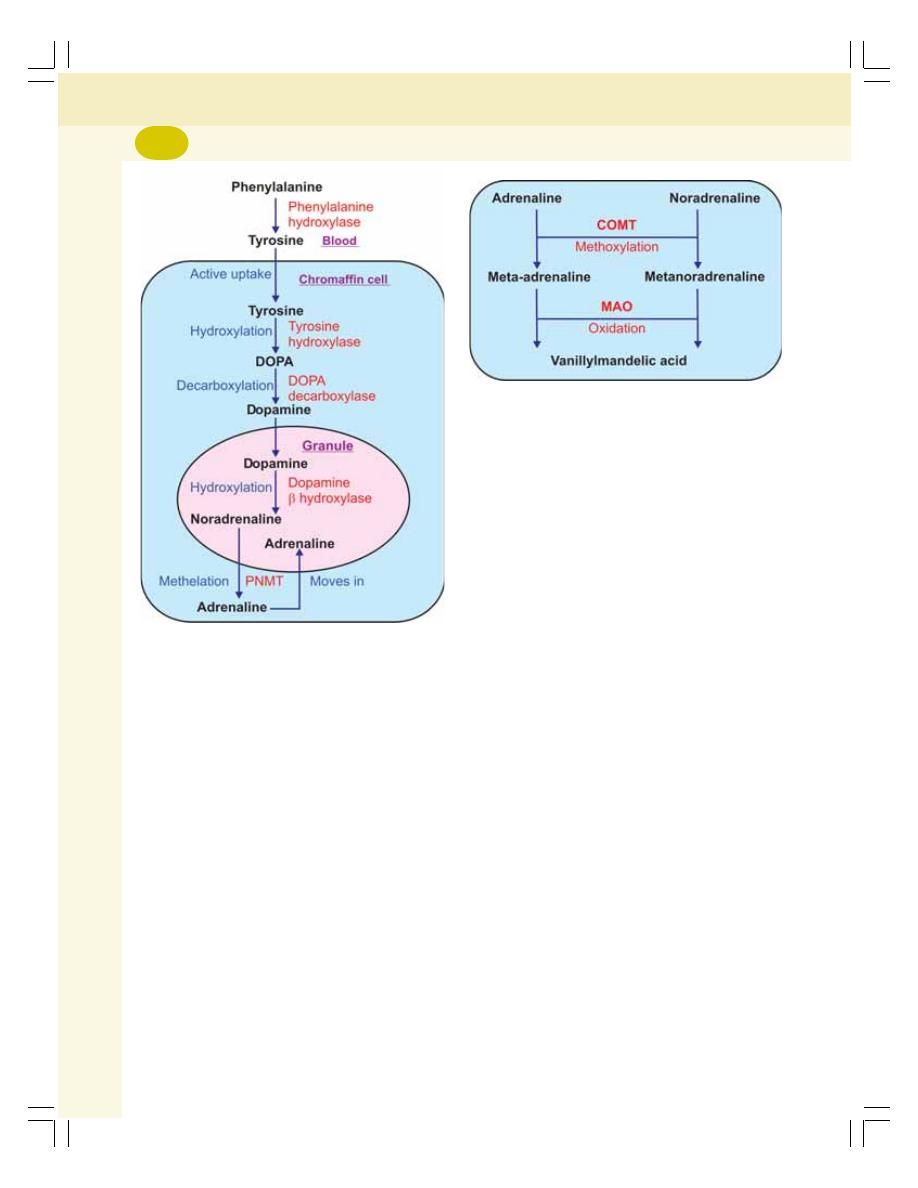
Endocrinology
314
3. Conversion of tyrosine into dihydroxyphenyl-
alanine (DOPA) by hydroxylation in the
presence of tyrosine hydroxylase
4. Decarboxylation of DOPA into dopamine by
DOPA decarboxylase
5. Entry of dopamine into granules of chromaffin
cells
6. Hydroxylation of dopamine into noradrenaline
by the enzyme dopamine beta hydroxylase
7. Release of noradrenaline from granules into
the cytoplasm
8. Methylation of noradrenaline into adrenaline
by the most important enzyme called phenyl-
ethanolamine-N-methyltransferase (PNMT).
PNMT is present in chromaffin cells.
METABOLISM OF CATECHOLAMINES
Eighty five percent of noradrenaline is taken up
by the sympathetic adrenergic neurons. The
biological inactivation (degradation) and removal
of remaining 15% of noradrenaline and adre-
naline (Fig. 50-2) occurs in the following manner:
1. Adrenaline is methoxylated into meta-adre-
naline. Noradrenaline is methoxylated into
metanoradrenaline. The methoxylation occurs
in the presence of ‘Catechol-O-Methyl-
transferase’ (COMT). Meta-adrenaline and
metanoradrenaline are together called meta-
nephrines
2. Then, oxidation of metanephrines into
vanillyl-mandelic acid (VMA) occurs by
monoamine oxidase (MAO)
3. Catecholamines are removed from body
through urine in three forms:
i. 15% as free adrenaline and free noradre-
naline
ii. 50% as free or conjugated meta-adre-
naline and meta noradrenaline
iii. 35% as VMA.
ACTIONS OF ADRENALINE AND
NORADRENALINE
Adrenaline and noradrenaline stimulate the ner-
vous system. Adrenaline has significant effects
FIGURE 50-1:
Synthesis of catecholamines.
PNMT = Phenyl- ethanolamine–N–methyltransferase.
DOPA = Dihydroxyphenylalanine
FIGURE 50-2:
Metabolism of catecholamines
COMT = Catechol – O – methyltransferase
MAO = Monoamine oxidase

Chapter 50 Adrenal Medulla
315
on metabolic functions and both adrenaline and
noradrenaline have significant effects on cardio-
vascular system.
MODE OF ACTION OF ADRENALINE
AND NORADRENALINE –
ADRENERGIC RECEPTORS
Adrenaline and noradrenaline execute their
actions by binding with receptors called adre-
nergic receptors which are present in the target
organs.
Adrenergic receptors are of two types:
1. Alpha adrenergic receptors
2. Beta adrenergic receptors.
Alpha receptors and, beta receptors are
divided into beta
1
and beta
2
receptors. Refer
Table 50-1 for their mode of action and response.
ACTIONS
The effects of adrenaline and noradrenaline on
various target organs depend upon the type of
receptors present in the cells of the organs.
Adrenaline acts through both alpha and beta
receptors equally. Noradrenaline acts mainly
through alpha receptors and occasionally through
beta receptors.
1. On Metabolism (via Alpha and Beta
Receptors)
Adrenaline influences the metabolic functions
more than noradrenaline.
i. General metabolism: Adrenaline increa-
ses oxygen consumption and carbon
dioxide removal. It increases basal meta-
bolic rate. So, it is said to be a calorigenic
hormone
ii. Carbohydrate metabolism: Adrenaline
increases the blood glucose level. It is by
increasing the glycogenolysis in liver and
muscle. So, a large quantity of glucose
enters the circulation
iii. Fat metabolism: Adrenaline causes mobi-
lization of free fatty acids from adipose
tissues. Catecholamines need the pre-
sence of glucocorticoids for this action.
2. On Blood (via Beta Receptors)
Adrenaline decreases blood coagulation time. It
increases RBC count in blood by contracting
smooth muscles of splenic capsule and releasing
RBCs from spleen into circulation.
3. On Heart (via Beta Receptors)
Adrenaline has stronger effects on heart than nor-
adrenaline. It increases overall activity of the
heart, i.e.
i. Heart rate (chronotropic effect)
ii. Force of contraction (inotropic effect)
iii. Excitability of heart muscle (bathmotropic
effect)
iv. Conductivity in heart muscle (dromotropic
effect).
4. On Blood Vessels (via Alpha and Beta2
Receptors)
Noradrenaline has strong effects on blood
vessels. It causes constriction of blood vessels
TABLE 50-1:
Adrenergic receptors
Receptor
Mode of Action
Response
Alpha
1
receptor
Activates IP
3
through phospholipase C
Mediates more of noradrenaline actions
than adrenaline actions
Alpha
2
receptor
Inhibits adenyl cyclase and cAMP
Beta
1
receptor
Activates adenyl cyclase and cAMP
Mediates actions of adrenaline and
noradrenaline equally
Beta
2
receptor
Activates adenyl cyclase and cAMP
Mediates more of adrenaline actions than
noradrenaline actions

Endocrinology
316
throughout the body via alpha receptors. So it
is called ‘General vasoconstrictor’. The vasocon-
strictor effect of noradrenaline increases total
peripheral resistance.
Adrenaline also causes constriction of blood
vessels. However, it causes dilatation of blood
vessels in skeletal muscle, liver and heart through
beta
2
receptors. So, the total peripheral resis-
tance is decreased by adrenaline.
5. On Blood Pressure (via Alpha and
Beta Receptors)
Adrenaline increases systolic blood pressure by
increasing the force of contraction of the heart
and cardiac output. But, it decreases diastolic
blood pressure by reducing the total peripheral
resistance. Noradrenaline increases diastolic
pressure due to general vasoconstrictor effect
by increasing the total peripheral resistance. It
also increases the systolic blood pressure to a
slight extent by its actions on heart. The action
of catecholamines on blood pressure needs the
presence of glucocorticoids.
Thus, hypersecretion of catecholamines leads
to hypertension.
6. On Respiration (via Beta
2
Receptors)
Adrenaline increases rate and force of respi-
ration. Adrenaline injection produces apnea,
which is known as adrenaline apnea. It also
causes bronchodilation.
7. On Skin (via Alpha and Beta
2
Receptors)
Adrenaline causes contraction of arrector pili. It
also increases the secretion of sweat.
8. On Skeletal Muscle (via Alpha and
Beta
2
Receptors)
Adrenaline causes severe contraction and quick
fatigue of skeletal muscle. It increases glyco-
genolysis and release of glucose from muscle
into blood. It also causes vasodilatation in
skeletal muscles.
9. On Smooth Muscle (via Alpha and Beta
Receptors)
Catecholamines cause contraction of smooth
muscles in the following organs:
i. Splenic capsule
ii. Sphincters of GI tract
iii. Arrector pili of skin
iv. Gallbladder
v. Uterus
vi. Dilator pupillae of iris
vii. Nictitating membrane of cat.
Catecholamines cause relaxation of smooth
muscles in some organs like:
i. Nonsphincteric part of GI tract (esopha-
gus, stomach and intestine)
ii. Bronchioles
iii. Urinary bladder.
10. On Central Nervous System (via Beta
Receptors)
Adrenaline increases the activity of brain.
Adrenaline secretion increases during ‘fight or
flight reactions’ after exposure to stress. It enhan-
ces the cortical arousal and other facilitatory
functions of central nervous system.
11. Other Effects of Catecholamines
i. On salivary glands (via alpha and beta
2
receptors) – cause vasoconstriction in
salivary gland leading to mild increase in
salivary secretion
ii. On sweat glands (via beta
2
receptors) –
increase the secretion of apocrine sweat
glands
iii. On lacrimal glands (via alpha receptors)
– increase the secretion of tears
iv. On ACTH secretion (via alpha receptors)
– adrenaline increases ACTH secretion
v. On nerve fibers (via alpha receptors) –
adrenaline decreases the latency of action
potential in the nerve fibers, i.e. electrical
activity is accelerated
vi. On renin secretion (via beta receptors) –
increase the secretion of renin from juxta-
glomerular apparatus of the kidney.

Chapter 50 Adrenal Medulla
317
REGULATION OF SECRETION OF
ADRENALINE AND NORADRENALINE
Adrenaline and noradrenaline are secreted from
adrenal medulla in small quantities even during
rest. During stress conditions, due to sympatho-
adrenal discharge, a large quantity of catecho-
lamines is secreted. These hormones prepare
the body for fight or flight reactions.
Catecholamine secretion increases in expo-
sure to cold and hypoglycemia also.
DOPAMINE
Dopamine is secreted by adrenal medulla. The
type of cells secreting this hormone is not known.
Dopamine is also secreted by dopaminergic
neurons in some areas of brain particularly, basal
ganglia. In brain, this hormone acts as a neuro-
transmitter.
The injected dopamine produces the following
effects:
1. Vasoconstriction by releasing norepinephrine
2. Vasodilatation in mesentery
3. Increase in heart rate via beta receptors
4. Increase in systolic blood pressure. Dopa-
mine does not affect diastolic blood pressure.
Deficiency of dopamine in basal ganglia pro-
duces nervous disorder called Parkinsonism
(Chapter 94).
APPLIED PHYSIOLOGY –
PHEOCHROMOCYTOMA
Pheochromocytoma is a condition characterized
by hypersecretion of catecholamines.
Cause
Pheochromocytoma is caused by tumor of chro-
mophil cells in adrenal medulla. It is also caused
rarely by tumor of sympathetic ganglia (extra
adrenal pheochromocytoma).
Signs and Symptoms
The characteristic feature of pheochromocytoma
is hypertension. This type of hypertension is
known as endocrine or secondary hypertension.
Other features are:
1. Anxiety
2. Chest pain
3. Fever
4. Headache
5. Hyperglycemia
6. Metabolic disorders
7. Nausea and vomiting
8. Palpitation
9. Polyuria and glucosuria
10. Sweating and flushing
11. Tachycardia
12. Weight loss.
PINEAL GLAND
THYMUS
KIDNEYS
HEART
Endocrine Functions of
Other Organs
51
PINEAL GLAND
SITUATION AND STRUCTURE
Pineal gland is otherwise called epiphysis. It is
a small cone shaped structure. In human, it is
about 10 mm long. Pineal gland is located in dien-
cephalic area of brain above the hypothalamus.
In human, pineal gland has two types of cells:
1. Parenchymal cells, which are large epithelial
cells
2. Neuroglial cells.
In adults, the pineal gland is calcified. But,
the epithelial cells exist and secrete the hormonal
substance.
FUNCTIONS
Pineal gland has two functions:
1. It controls the sexual activities in animals by
regulating the seasonal fertility. However, the
pineal gland plays little role in regulating the
sexual functions in human being.
2. The parenchymal cells of pineal gland secrete
a hormonal substance called melatonin.
Melatonin
Melatonin is secreted by the parenchymal cells
of pineal gland. It is an indole (N-acetyl-5 metho-
xytryptamine).
Actions
Melatonin acts mainly on gonads. Its action differs
from species to species. In some animals, it
stimulates the gonads while in other animals it
inhibits the gonads.
In humans, it inhibits the onset of puberty by
inhibiting the gonads.
Diurnal variation in melatonin secretion
Melatonin secretion is more in darkness than in
daylight. In animals the secretion of melatonin
varies according to activities in different periods
of the day, i.e. circadian rhythm. Hypothalamus
is responsible for the circadian fluctuations of
melatonin secretion.
THYMUS
SITUATION
It is situated in front of trachea below the thyroid
gland. Thymus is small in newborn infants and
gradually enlarges till puberty, and then decrea-
ses in size.
FUNCTIONS
Thymus has lymphoid function and endocrine
function. It plays an important role in development
of immunity in the body. It has two functions:

Chapter 51 Endocrine Functions of Other Organs
319
1. Processing the T lymphocytes
2. Endocrine function.
1. Processing the T Lymphocytes
Thymus plays an essential role in the develop-
ment of immunity by processing the T lym-
phocytes (Chapter 13). The lymphocytes, which
are produced in bone marrow, are processed in
thymus into T lymphocytes. It occurs during the
period between three months before birth and
three months after birth. So, the removal of
thymus 3 months after birth will not affect the
cell mediated immunity.
2. Endocrine Function of Thymus
Thymus secretes two hormones:
i. Thymosin
ii. Thymin.
Thymosin
Thymosin is a peptide. It accelerates lympho-
poiesis and proliferation of T lymphocytes.
Thymin
It is also called thymopoietin. It suppresses the
neuromuscular activity by inhibiting acetylcholine
release. Hyperactivity of thymus causes myasthe-
nia gravis.
KIDNEYS
Kidneys secrete five hormonal substances:
1. Erythropoietin
2. Thrombopoietin
3. Renin
4. 1,25-Dihydroxycholecalciferol (calcitriol)
5. Prostaglandins.
Recently, it is discovered that kidney secretes
small quantity of C-type natriuretic peptide (see
below).
1. ERYTHROPOIETIN
Erythropoietin is secreted by endothelial cells of
peritubular capillaries in the kidney. It is a gly-
coprotein with 165 amino acids.
Erythropoietin stimulates the bone marrow
and causes erythropoiesis. More details are given
in Chapter 8.
2. THROMBOPOIETIN
Thrombopoietin is a glycoprotein. It is secreted
by kidneys and liver. It stimulates production of
platelets.
3. RENIN
Renin is secreted by granular cells of juxtaglo-
merular apparatus of the kidney.
Actions of Renin
When renin is released into the blood, it acts on
a specific plasma protein called alpha
2
globulin.
It is also called angiotensinogen or renin sub-
strate.
Renin converts angiotensinogen into angio-
tensin I which is converted into angiotensin II by
a converting enzyme. The other details of renin
and angiotensin II are given in Chapter 35.
4. 1,25-DIHYDROXYCHOLECAL-
CIFEROL – CALCITRIOL
Formation of 1,25-Dihydroxycholecalciferol
1,25-dihydroxycholecalciferol is otherwise
known as calcitriol or activated vitamin D. It is
formed from cholecalciferol which is present in
skin and intestine. The cholecalciferol (vitamin
D3) from skin or intestine is converted into
25-hydroxy-cholecalciferol in liver. This in turn,
is activated into 1,25-dihydroxycholecalciferol by
parathor-mone in kidney (refer Chapter 47).
Action of 1,25-Dihydroxycholecalciferol
The activated vitamin D plays an important role
in the maintenance of blood calcium level. It acts
on the intestinal epithelium and enhances
absorption of calcium from intestine into the
blood. Details are given in Chapter 47.
5. PROSTAGLANDINS
The prostaglandins secreted from kidney are
PGA
2
and PGE
2
. These hormones are secreted

Endocrinology
320
by juxtaglomerular cells and type I interstitial
cells present in medulla of kidney. The pro-
staglandins decrease the blood pressure by
systemic vasodilatation, diuresis and natriuresis.
Details of prostaglandins are given in Chapter
52.
HEART
Heart secretes the hormones atrial natriuretic
peptide and brain natriuretic peptide. Recently
another peptide called C-type natriuretic peptide
is found in heart.
ATRIAL NATRIURETIC PEPTIDE
Atrial natriuretic peptide (ANP) is a polypeptide
with 28 amino acids. It is secreted by atrial
musculature of the heart. Recently, it is found in
hypothalamus of brain also. However, its action
in brain is not known.
ANP is secreted during overstretching of
atrial muscles in conditions like increase in blood
volume. ANP in turn increases excretion of
sodium (followed by water excretion) through
urine and helps in the maintenance of ECF
volume and blood volume. It also lowers blood
pressure.
Effect of ANP on Sodium Excretion
ANP increases excretion of sodium ions through
urine by:
1. Increasing glomerular filtration rate
2. Inhibiting sodium reabsorption from distal
convoluted tubules and collecting ducts
3. Increasing the secretion of sodium into the
renal tubules.
Escape phenomenon
Thus, ANP is responsible for escape pheno-
menon, and prevention of edema in primary
hyperaldosteronism in spite of increased ECF
volume (Refer Chapter 52 for details).
Effect of ANP on Blood Pressure
ANP decreases the blood pressure by:
1. Vasodilatation
2. Inhibiting renin secretion from juxtaglomerular
apparatus
3. Inhibiting vasoconstrictor effect of angiotensin
II
4. Inhibiting vasoconstrictor effects of cate-
cholamines.
BRAIN NATRIURETIC PEPTIDE
Brain natriuretic peptide (BNP) is also called
B-type natriuretic peptide. It is a polypeptide with
32 amino acids. It is secreted by the cardiac
muscle. It is also secreted in some parts of brain.
The stimulant for its secretion is not known.
BNP has same actions of ANP (see above).
On brain, its actions are not known.
C-TYPE NATRIURETIC PEPTIDE
C-type natriuretic peptide (CNP) is the newly
discovered peptide hormone. It is a 22 amino
acid peptide. Initially, it was identified in brain.
Now it is known to be secreted by several tissues
which include myocardium, endothelium of blood
vessels, gastrointestinal tract and kidneys. The
functions of this hormone are not fully studied.
It is believed that it has similar action of atrial
natriuretic peptide.
INTRODUCTION
LOCAL HORMONES SYNTHESIZED IN TISSUES
PROSTAGLANDINS AND ITS RELATED HORMONES
OTHER LOCAL HORMONES SYNTHESIZED IN TISSUES
LOCAL HORMONES SYNTHESIZED IN TISSUES
Local Hormones
52
INTRODUCTION
Local hormones are the substances which act
on the same area of their secretion or in imme-
diate neighborhood. The endocrine hormones are
secreted in one place but execute their actions
on some other remote place.
Local hormones are produced in tissues and
blood. These hormones are usually released in
an inactive form and are activated by some
conditions or substances.
Local hormones are classified into two types:
I. Hormones synthesized in tissues
II. Hormones synthesized in blood.
LOCAL HORMONES SYNTHESIZED
IN TISSUES
The local hormones synthesized in the tissues
are:
A. Prostaglandins and related substances
B. Other local hormones synthesized in tissues.
PROSTAGLANDINS AND ITS
RELATED HORMONES
Prostaglandins and other hormones which are
derived from arachidonic acid are collectively
called eicosanoids. The eicosanoids are:
1. Prostaglandins
2. Thromboxanes
3. Prostacyclin
4. Leukotrienes
5. Lipoxins.
1. Prostaglandins
Prostaglandins were first discovered and
isolated from human semen. However, now it is
believed that almost all the tissues of the body
including renal tissues synthesize prostaglandins.
Pro-staglandins are unsaturated fatty acids with
a cyclopentane ring and 20 carbon atoms.
Types
A variety of prostaglandins are identified. Active
forms of prostaglandins are PGA
2
, PGD
2
, PGE
2
,
and PGF
2
.
Actions
i. On blood: Prostaglandins accelerate the
capacity of RBCs to pass through minute
blood vessels
ii. On blood vessels: PGE
2
causes vaso-
dilatation

Endocrinology
322
iii. On GI tract: The prostaglandins reduce
gastric secretion. In experimental animals,
prostaglandins inhibit the formation of
peptic ulcer.
iv. On respiratory system: PGE
2
causes
bronchodilatation.
v. On lipids: Some of the prostaglandins
inhibit the release of free fatty acids from
adipose tissue.
vi. On nervous system: In brain, prosta-
glandins control or alter the actions of
neurotransmitters.
vii. On reproduction: Prostaglandins play an
important role in regulating the reproduc-
tive cycle. These hormones also cause
degeneration of corpus luteum (luteolysis).
Prostaglandins increase the velocity of
sperm transport in female genital tract.
Prostaglandins (PGE
2
) play an impor-
tant role during parturition and facilitate
labor by increasing the force of uterine
contractions.
When injected intra-amniotically during
pregnancy, prostaglandins induce abor-
tion. When injected during last stages of
pregnancy, the prostaglandins induce
labor.
viii. On kidney: The prostaglandins stimulate
juxtaglomerular apparatus and enhance
the secretion of renin, dieresis and natri-
uresis.
2. Thromboxanes
Thromboxanes are derived from arachidonic
acid. Thromboxanes are of two types:
i. Thromboxane A
2
which is secreted in
platelets
ii. Thromboxane B
2
the metabolite of throm-
boxane A
2
.
The thromboxane A
2
causes vasoconstriction.
It plays an important role in hemostasis by acce-
lerating aggregation of platelets. It also acce-
lerates the clot formation.
3. Prostacyclin
Prostacyclin is also a derivative of arachidonic
acid. It is produced in the endothelial cells and
smooth muscle cells of blood vessels.
It causes vasodilatation and inhibits platelet
aggregation.
4. Leukotrienes
Leukotrienes are derived from arachidonic acid
via 5-hydroperoxy eicosatetraeonic acid
(5-HETE). Leukotrienes are the mediators of
allergic responses. These hormones also pro-
mote inflammatory reactions.
The release of leukotrienes increases when
some allergic agents combine with antibodies
like IgE.
The leukotrienes cause:
i. Bronchiolar constriction
ii. Arteriolar constriction
iii. Vascular permeability
iv. Attraction of neutrophils and eosinophils
towards the site of inflammation.
5. Lipoxins
Lipoxins are also derived from arachidonic acid
via 15-hydroperoxy eicosatetraeonic acid (15-
HETE). Lipoxins are of two types namely, Lipoxin
A and Lipoxin B.
Lipoxin A causes dilation of minute blood ves-
sels. Both the types inhibit the cytotoxic effects
of killer T cells.
OTHER LOCAL HORMONES
SYNTHESIZED IN TISSUES
In addition to prostaglandins and related hor-
monal substances, tissues secrete some more
hormones which are listed below.
1. Acetylcholine
2. Serotonin
3. Histamine
4. Substance P
5. Heparin
6. Leptin
7. GI hormones.
1. Acetylcholine
Acetylcholine is the cholinergic neurotransmitter.
It is the transmitter substance at neuromuscular
junction. It is also released by following nerve
endings:

Chapter 52 Local Hormones
323
i. Preganglionic parasympathetic nerve
ii. Postganglionic parasympathetic nerve
iii. Preganglionic sympathetic nerve
iv. Postganglionic sympathetic cholinergic
nerves such as:
a. Nerves supplying eccrine sweat glands
b. Sympathetic vasodilator nerves in
skeletal muscle
v. Nerves in amacrine cells of retina
Acetylcholine is also secreted by mast cell,
gastric mucosa, lungs and brain.
Acetylcholine produces the excitatory function
of synapse by opening the sodium channels.
Acetylcholine is very quick in action. It is also
destroyed immediately after executing the action
by the enzyme acetylcholinesterase. This enzyme
is present in basal lamina of the synaptic cleft.
Acetylcholine activates smooth muscles in GI
tract, urinary tract and skeletal muscles. It inhibits
cardiac function and causes vasodilatation.
2. Serotonin
It is otherwise known as 5-hydroxytryptamine.
Serotonin is secreted in the following structures:
i. Hypothalamus
ii. Limbic system
iii. Cerebellum
iv. Spinal cord
v. Retina
vi. GI tract
vii. Lungs
viii. Platelets
Serotonin is an inhibitory substance. It inhibits
impulses of pain sensation in posterior gray horn
of spinal cord. It causes mood depression and
sleep. It also causes vasoconstriction.
3. Histamine
It is secreted in nerve endings of hypothalamus,
limbic cortex and other parts of cerebral cortex.
It is an excitatory neurotransmitter substance
secreted in spinal cord.
Histamine is also released from tissues during
allergic condition, inflammation or damage. It
causes vasodilatation and enhances the capillary
permeability for fluid and plasma proteins from
blood into the affected tissues. So, the accumu-
lation of fluid with proteins develops local edema.
In GI tract, histamine increases the motility.
4. Substance P
Substance P is the neurotransmitter for pain. It
is secreted by nerve endings (first order neurons
of pain pathway) in spinal cord and retina.
It is also the neurotransmitter substance in
GI tract. The presence of chyme in duodenum
causes secretion of substance P. In GI tract, it
increases the mixing and propulsive movements
of small intestine.
5. Heparin
Heparin is produced in mast cells. Mast cells are
the wandering cells present immediately outside
the capillaries in large number of tissues or
organs, which contain more connective tissue.
These wandering cells are abundant in liver and
lungs. Basophils also secrete heparin. Heparin
is a naturally produced anticoagulant (Refer
Chapter 15 for other details).
6. Leptin
Leptin (in Greek it means thin) is a protein hor-
mone with 167 amino acids. It is secreted by
adipocytes in adipose tissues.
Leptin plays an important role in controlling
the adipose tissue and food intake. Leptin acts
on hypothalamus and inhibits the feeding cen-
ter resulting in stoppage of food intake (Chapter
92). At the same time it also stimulates the
metabolic reactions involved in utilization of fat
stored in adipose tissue for energy. Thus, the
circulating leptin level informs the brain about
the energy storage and the necessity to regulate
food intake and metabolic reactions.
7. Gastrointestinal Hormones
i. Gastrin (Chapter 28)
ii. Secretin (Chapter 29)
iii. Cholecystokinin (Chapter 29)
iv. Gastric inhibitory peptide – GIP (Chapter
28)
v. Vasoactive intestinal polypeptide – VIP
(Chapter 28)
vi. Pancreatic polypeptide (Chapter 29)
vii. Somatostatin (Chapter 29)
viii. Peptide YY (Chapter 29)

Endocrinology
324
LOCAL HORMONES PRODUCED IN
BLOOD
The local hormones produced in the blood are:
A. Serotonin
B. Angiotensinogen
C. Kinins.
Serotonin is described above. Angioten-
sinogen is explained in Chapter 35.
KININS
Kinins are protein hormones circulating in blood.
There are two types of kinins:
1. Bradykinin
2. Kallidin.
Actions of bradykinin
Bradykinin:
1. Dilates blood vessels and decreases the
blood pressure. It is considered as a potent
vasodilator
2. Increases the blood flow throughout the body
by its vasodilator action
3. Increases permeability of capillaries during
inflammatory conditions resulting in edema
in the affected area
4. Stimulates pain receptors
5. Causes contraction of smooth muscles of
intestine.
Action of kallidin
Kallidin is also a vasodilator hormone.

Chapter 52 Local Hormones
325
QUESTIONS IN ENDOCRINOLOGY
LONG QUESTIONS
1. Enumerate the hormones secreted by
pituitary gland. Describe the actions and
regulation of secretion of growth hormone.
Write in brief about effects of hyper-
secretion of anterior pituitary gland.
2. Describe the synthesis, storage, release,
transport, functions and regulation of
secretion of thyroid hormones.
3. Explain the functions and regulation of
secretion of parathormone. Add a note on
the disorders of parathormone secretion.
4. Explain the regulation of blood calcium
level. Add a note on tetany.
5. Enlist the hormones secreted by pan-
creas. Explain the functions and regulation
of secretion of insulin.
6. Describe in detail the regulation of blood
sugar level.
7. Classify the hormones secreted by
adrenal cortex. Explain the actions and
regulation of secretion of cortisol.
8. Enumerate the corticosteroids. Describe
the actions and regulation of secretion of
aldosterone.
9. What are catecholamines? Explain the
synthesis, metabolism, actions and
regulation of secretion of catecholamines.
SHORT QUESTIONS
1. Growth hormone.
2. Thyroid stimulating hormone.
3. Adrenocorticotropic hormone.
4. Gonadotropins.
5. Somatomedin.
6. Oxytocin.
7. Antidiuretic hormone.
8. Milk ejection/neuroendocrine reflex.
9. Gigantism.
10. Acromegaly.
11. Dwarfism.
12. Acromicria.
13. Simmond’s disease.
14. Fröhlich’s syndrome.
15. Disorders of posterior pituitary gland.
16. Diabetes insipidus.
17. Synthesis of thyroid hormones.
18. Thyroglobulin.
19. Thyroxine.
20. Hyperthyroidism/Graves’ disease.
21. Hypothyroidism.
22. Goiter.
23. Cretinism.
24. Myxedema.
25. Parathormone.
26. Tetany.
27. Hypercalcemia/hypocalcemia.
28. Insulin.
29. Glucagon.
30. Somatostatin.
31. Diabetes mellitus.
32. Hyperinsulinism.
33. Cortisol.
34. Nonmetabolic actions of cortisol.
35. Aldosterone.
36. Aldosterone escape.
37. Adrenal androgens.
38. Cushing’s syndrome or disease.
39. Hyperaldosteronism.
40. Endocrine function of heart.
41. Adrenogenital syndrome.
42. Addison’s disease.
43. Synthesis of catecholamines.
44. Actions of catecholamines.
45. Dopamine.
46. Pheochromocytoma.
47. Functions of pineal gland.
48. Functions of thymus.
49. Prostaglandins.
50. Acetylcholine.
Questions in Endocrinology
325


Reproductive System
53. Male Reproductive System.................................................. 329
54. Female Reproductive System ............................................. 343
55. Menstrual Cycle ................................................................... 350
56. Pregnancy, Mammary Glands and Lactation....................... 358
57. Fertility Control .................................................................... 365
S E C T I O N
7
C H A P T E R S

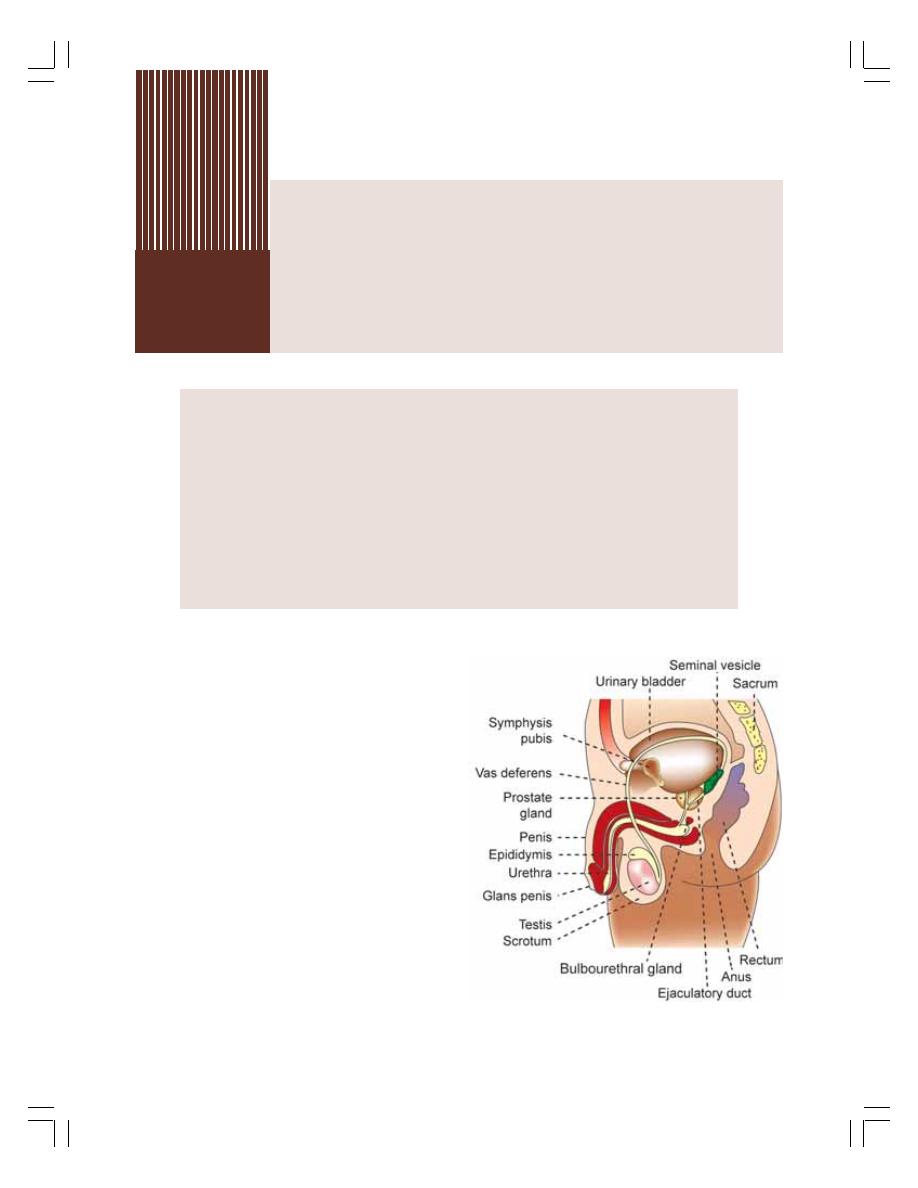
INTRODUCTION
PRIMARY SEX ORGANS IN MALES – TESTES
ACCESSORY SEX ORGANS IN MALES
FUNCTIONS OF TESTIS
GAMETOGENIC FUNCTIONS OF TESTIS – SPERMATOGENESIS
ENDOCRINE FUNCTIONS OF TESTIS
SEMEN
MALE CLIMACTERIC
APPLIED PHYSIOLOGY
Male
Reproductive System
53
53
53
53
53
INTRODUCTION TO MALE
REPRODUCTIVE SYSTEM
Reproductive system ensures the continuation
of the species. The organs of the reproductive
system are of two groups namely internal
reproductive organs and external genital organs.
Gonads are the main organs which produce the
gametes i.e., sperm and ovum. A pair of testes
(singular – testis) produces sperms in males and
a pair of ovaries produces ovum in females.
Male reproductive system includes the pri-
mary sex organs and accessory sex organs.
PRIMARY SEX ORGANS
IN MALES – TESTES
Testis is the primary sex organ or gonad in males.
There are two testes in almost all the species.
The testes are ovoid or walnut shaped bodies
located in the sac like structure called scrotum
(Fig. 53-1)
FIGURE 53-1:
Male reproductive system
and other organs of pelvis
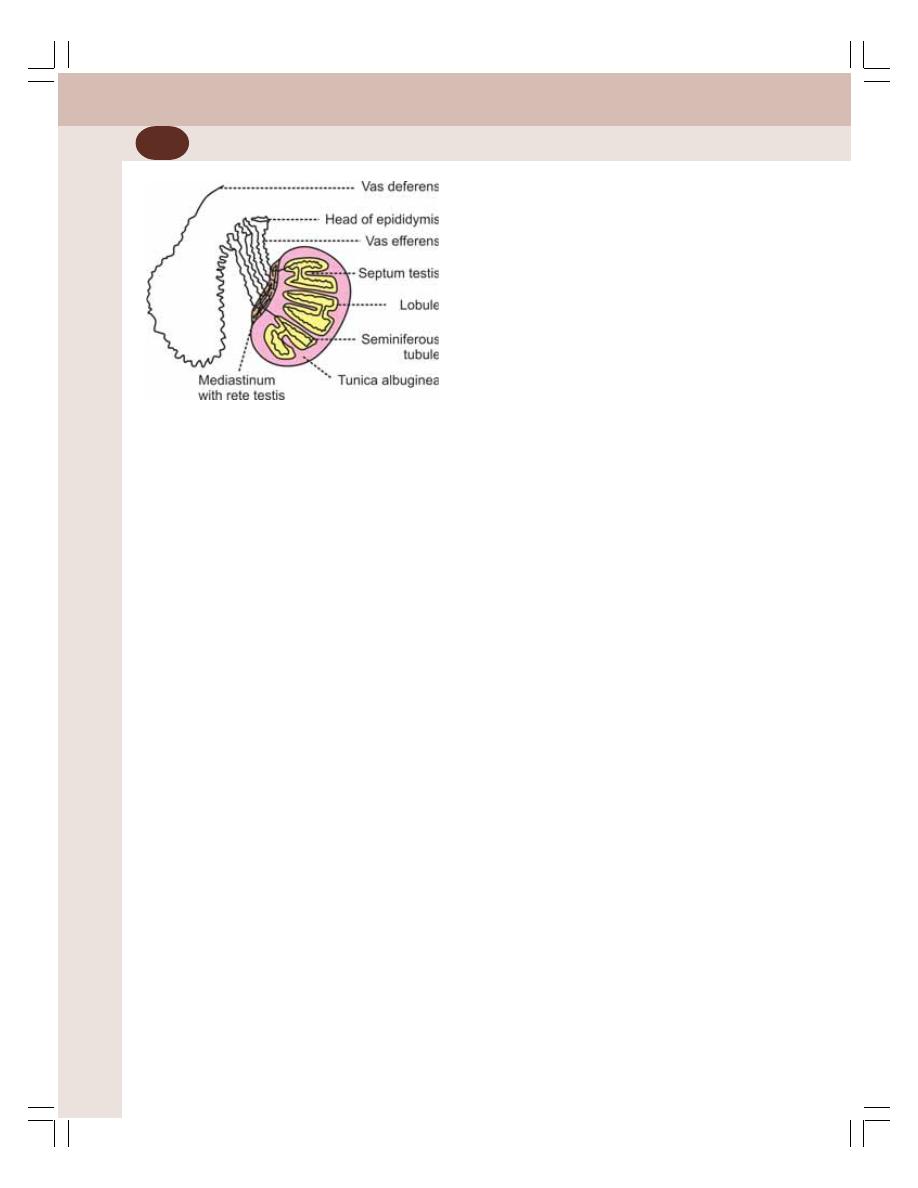
Reproductive System
330
COVERINGS OF TESTIS
Each testis is enclosed by three coverings.
1. Tunica vasculosa which is the innermost
covering. It is made up of connective tissue
and it is rich in blood vessels.
2. Tunica albuginea which is the middle cover-
ing. It is a dense fibrous capsule.
3. Tunica vaginalis which is the outermost cover-
ing. It is formed by visceral and parietal layers.
The tunica albuginea on the posterior surface
of testis is thickened to form the mediastinum
testis. From this, the connective tissue septa
called septula testis radiate into testis and bind
with tunica albuginea at various points. By this,
the interior of testis is divided into a number of
pyramidal lobules, with bases directed towards
the periphery and the apices towards the
mediastinum (Fig. 53-2).
The septula do not form complete partition.
Because of this, the lobules of testis anastomose
with one another at many places.
FUNCTIONAL ANATOMY OF TESTIS
Each testis has about 200 to 300 lobules. Each
lobule contains 1 to 4 coiled tubules known as
the seminiferous tubules. Seminiferous tubules
continue as the vas efferens, which form the
epididymis. It is continued as vas deferens. The
terminal portion of vas deferens is called ampulla
(Fig. 53-3).
Seminiferous Tubules
Seminiferous tubules are thread like convoluted
tubular structures in which the spermatozoa or
sperms are produced. There are about 400 to
600 seminiferous tubules in each testis. The
length of each seminiferous tubule is between
30 and 70 cm. The diameter of the tubules is
between 150 and 300 μ. The seminiferous
tubules are surrounded and supported by
interlobular connective tissue (Fig. 53-2).
The wall of the seminiferous tubule is formed
by three layers:
1. The outer capsule or tunica propria
2. A thin homogeneous basement membrane
3. The stratified epithelium which consists of two
types of cells:
i. Spermatogenic cells or germ cells
ii. Supporting cells called Sertoli cells.
Spermatogenic Cells
The spermatogenic cells or germ cells present
in seminiferous tubules are the precursor cells
of spermatozoa. These cells lie in between Sertoli
cells and are arranged in an orderly manner in
4 to 8 layers. In children, the spermatogenic cells
are in the primitive stage called spermatogonia.
With onset of puberty, these cells develop into
sperms through different stages.
Sertoli Cells
Sertoli cells are the large and tall irregular
columnar cells present in seminiferous tubule.
The spermatogenic cells are attached to Sertoli
cells by means of cytoplasmic connection.
Functions of Sertoli cells
Sertoli cells:
1. Support and nourish the spermatogenic cells
till the spermatozoa are released from them
2. Secretes the enzyme aromatase which con-
verts androgens into estrogen
3. Secrete androgen binding protein (ABP)
which is essential for testosterone activity
particularly in spermatogenesis.
4. Secrete estrogen binding protein (EBP)
FIGURE 53-2:
Structure of testis
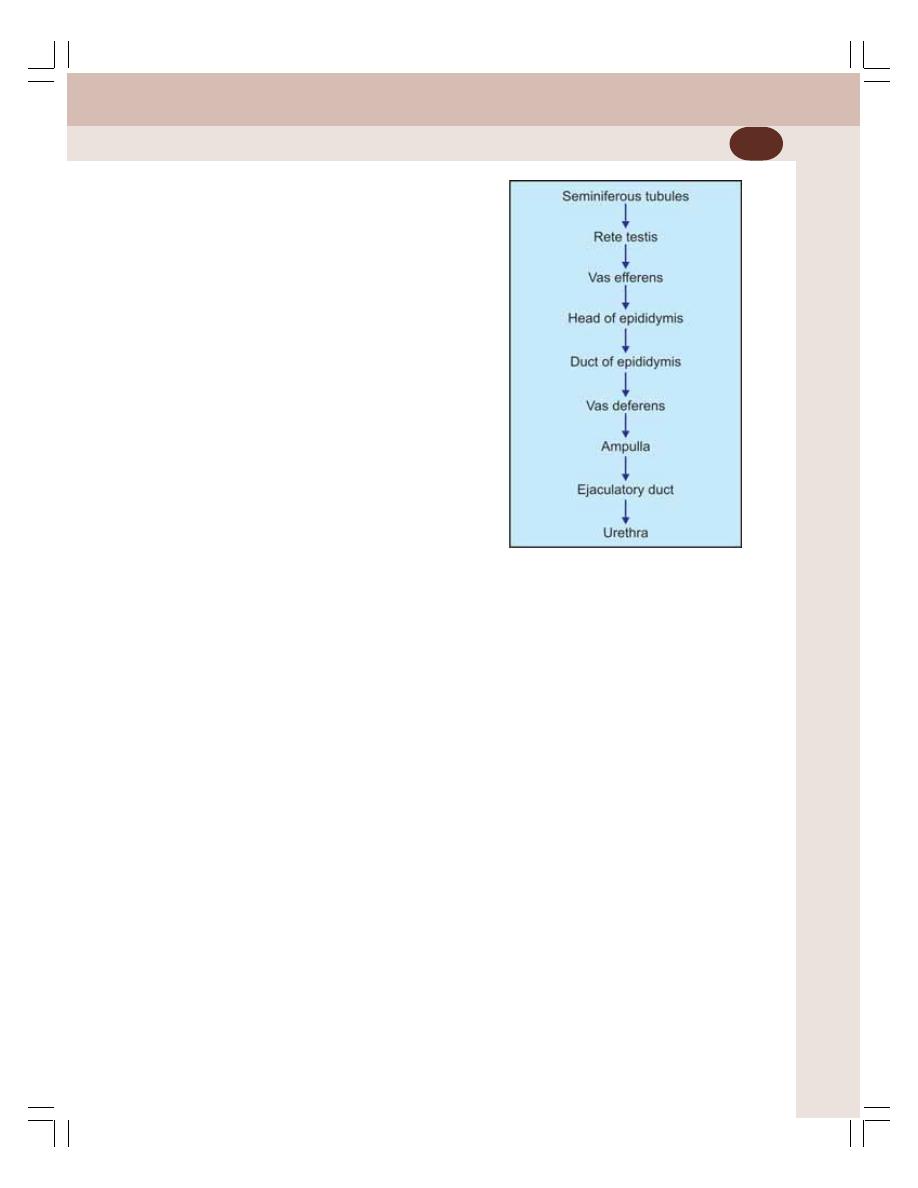
Chapter 53 Male Reproductive System
331
5. Secrete inhibin which inhibits the release of
FSH from anterior pituitary
6. Secrete activin which increases FSH release
7. Secrete Müllerian regression factor (MRF) in
fetal testes. MRF is also called Müllerian inhi-
biting substance (MIS). MRF is responsible
for the regression of Müllerian duct during sex
differentiation in fetus (see below).
Blood-Testis Barrier
Blood-testis barrier is a mechanical barrier that
separates blood from seminiferous tubules of the
testes. It is formed by tight junctions between
the adjacent Sertoli cells near the basal mem-
brane of seminiferous tubule.
Blood-testes barrier protects the seminiferous
tubules and spermatogenic cells by preventing
the entry of toxic substances from blood into
testis. At the same time it permits nutritive and
other essential substances necessary for sper-
matogenic cells.
Rete Testis
Each seminiferous lobules open into a network
of thin walled channels called the rete testis.
Vas Efferens
From rete testis, 8 to 15 tubules called vas
efferens arise. Vas efferens join together and
form the head of epididymis and then converge
to form duct of epididymis (Fig. 53-3).
Epididymis
The duct of epididymis is an enormously con-
voluted tubule with a length of about 4 meters.
It begins at head, where it receives vas efferens.
Vas Deferens
At the caudal pole of testis, epididymis turns
sharply upon itself and continues as vas deferens
without any definite demarcation.
Interstitial Cells of Leydig
Interstitial cells of Leydig are the hormone secre-
ting cells of the testes situated in between the
seminiferous tubules.
ACCESSORY SEX ORGANS IN MALES
Accessory sex organs in males are:
1. Seminal vesicles
2. Prostate gland
3. Urethra
4. Penis.
SEMINAL VESICLES
Seminal vesicles are the paired glands situated
in lower abdomen on either side of prostate
gland behind urinary bladder. Each seminal
vesicle is a hollow sac of irregular shape. It is
lined by complexly folded mucous membrane
which secretes seminal fluid.
FIGURE 53-3:
Pathway for
passage of sperms

Reproductive System
332
Seminal fluid is added to semen in the eja-
culatory duct through ampulla of vas deferens.
The ejaculatory duct passes through prostate to
form internal urethra.
Properties and Composition of
Seminal Fluid
The seminal fluid is mucoid and viscous in
nature. It is neutral or slightly alkaline in reaction.
It adds to the bulk of semen as it forms 60% of
total semen. The seminal vesicles secrete seve-
ral important substances. Refer Fig. 53-7 for the
products of seminal fluid.
Functions of Seminal Fluid
1. Nutrition to sperms
The fructose and other nutritive substances in
seminal fluid are utilized by sperms after being
ejaculated into female genital tract.
2. Clotting of semen
As soon as semen is ejaculated it is clotted
because of conversion of fibrinogen of seminal
fluid into fibrin. Clotting of semen is essential for
holding the sperms in uterine cervix.
3. On fertilization
The prostaglandin of seminal fluid enhances
fertilization of ovum by the following processes:
i. Increasing the receptive capacity of cervi-
cal mucosa for sperms
ii. Causing reverse peristaltic movement of
uterus and fallopian tubes. This, in turn,
increases the rate of transport of sperms
in female genital tract during coitus.
PROSTATE GLAND
Human prostate gland weighs about 40 g. It is
formed by 20 to 30 separate secretory glands,
which open separately into the urethra. Prostate
secretes prostatic fluid.
Properties and Composition of Prostatic Fluid
The prostate fluid is a thin, milky and alkaline
fluid. It forms 30% of total semen. Refer Fig. 53-
7 for the products secreted by prostate gland.
Functions of Prostatic Fluid
1. Maintenance of sperm motility
The prostatic fluid provides optimum pH for the
motility of sperms. Generally, sperms are non-
motile at a pH of less than 6.0. There are two
factors which decrease the pH and motility of
sperm:
i. Metabolic end products from sperm which
make the fluid in vas deferens acidic
ii. Vaginal secretions in females are highly
acidic with a pH of 3.5 to 4.0.
The prostatic secretion neutralizes the acidity
and maintains a pH of 6.0 to 6.5. At this pH, the
sperms become motile and the chances of ferti-
lization are enhanced.
2. Clotting of semen
The clotting enzymes present in prostatic fluid
convert fibrinogen (from seminal vesicles) into
clot.
3. Lysis of clot
The clot is dissolved by fibrinolysin of the
prostatic fluid so that, the sperms become motile.
URETHRA
Urethra has two parts namely, internal urethra
and external urethra. Internal urethra is the
continuation of ejaculatory duct. Internal urethra
passes through penis as external urethra. Urethra
contains mucus glands throughout its length,
which are called glands of Littre. The bilateral
bulbourethral glands also open into the urethra.
PENIS
Penis is the male genital organ. Urethra passes
through penis and opens to the exterior. Penis
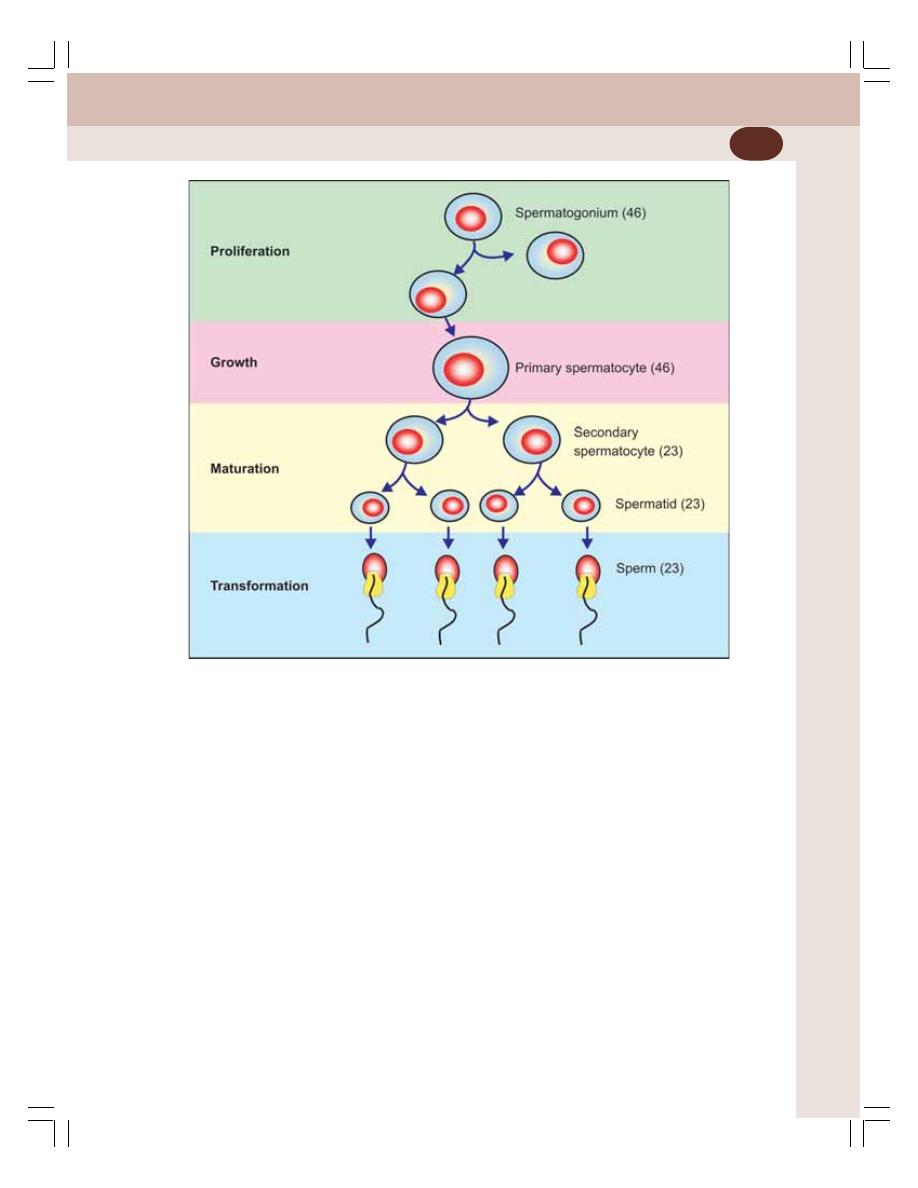
Chapter 53 Male Reproductive System
333
is formed by three erectile tissue masses, i.e.
a paired corpora cavernosa and an unpaired
corpus spongiosum. The corpus spongiosum
surrounds the urethra and terminates distally to
form glans penis.
FUNCTIONS OF TESTIS
Testis performs two functions:
1. Gametogenic function by which gametes are
produced in the gonads
2. Endocrine function by which male sex hor-
mones are secreted.
GAMETOGENIC FUNCTIONS OF
TESTIS – SPERMATOGENESIS
Spermatogenesis is the process by which the
male gametes called spermatozoa (sperms) are
formed from the primitive spermatogenic cells
(spermatogonia) in the testis (Fig. 53-4). It takes
74 days for the formation of sperm from a pri-
mitive germ cell.
STAGES OF SPERMATOGENESIS
Spermatogenesis occurs in four stages:
1. Stage of proliferation
2. Stage of growth
3. Stage of maturation
4. Stage of transformation.
1. Stage of Proliferation
Each spermatogonium contains diploid number
(23 pairs) of chromosomes. One member of each
pair is derived from mother and the other one
from the father. The 23 pairs include 22 pairs of
FIGURE 53-4:
Spermatogenesis. Number in parenthesis indicate chromosomal number

Reproductive System
334
autosomal chromosomes and one pair of sex
chromosomes. The sex chromosomes are one
X chromosome and one Y chromosome.
During the proliferative stage, spermatogonia
divide by mitosis without any change in chro-
mosomal number. In man, there are usually
seven generations of spermatogonia. During this
stage, the spermatogonia migrate along with
Sertoli cells towards the lumen of seminiferous
tubule.
The last generation of spermatogonia enters
the stage of growth as primary spermatocyte.
2. Stage of Growth
In this stage, the primary spermatocyte grows
into a large cell. Apart from growth, there is no
other change in spermatocyte during this stage.
3. Stage of Maturation
After reaching the full size, each primary sperma-
tocyte quickly undergoes meiotic or maturation
division, which occurs in two phases:
First phase
In the first phase each primary spermatocyte
divides into two secondary spermatocytes. The
significance of the first meiotic division is that
each secondary spermatocyte receives only the
haploid or half the number of chromosomes.
23 chromosomes include 22 autosomes and an
X or a Y chromosome.
Second phase
During this phase, each secondary spermatocyte
undergoes second meiotic division resulting in
two smaller cells called spermatids. Each
spermatid has haploid number of chromosomes.
4. Stage of Transformation
There is no further division. The spermatids are
transformed into matured spermatozoa (sperms).
Transformation occurs in two stages.
i. Spermeogenesis
It is the process by which spermatids become
matured spermatozoa. The changes taking place
during this stage are:
i. Condensation of nuclear material
ii. Formation of acrosome, mitochondrial
spiral filament and tail structures
iii. Removal of unwanted quantity of cyto-
plasm.
ii. Spermination
Spermination is the process by which the matu-
red sperms are released from Sertoli cells into
the lumen of seminiferous tubules. Structure of
sperm is explained later in this chapter.
ROLE OF SERTOLI CELLS IN
SPERMATOGENESIS
Sertoli cells:
1. Support and nourish the germ cells
2. Provide hormonal substances necessary for
spermatogenesis
3. Secrete androgen binding protein (ABP)
which is essential for testosterone activity,
particularly on spermatogenesis
4. Release sperms into lumen of seminiferous
tubules (spermination).
ROLE OF HORMONES IN
SPERMATOGENESIS
Spermatogenesis is influenced by many
hormones which act either directly or indirectly:
Table 53-1 gives the hormones essential for
each stage of spermatogenesis. The hormones
necessary for spermatogenesis are:
1. FSH
2. LH
3. GH
4. Testosterone
5. Estrogen
6. Inhibin
7. Activin.
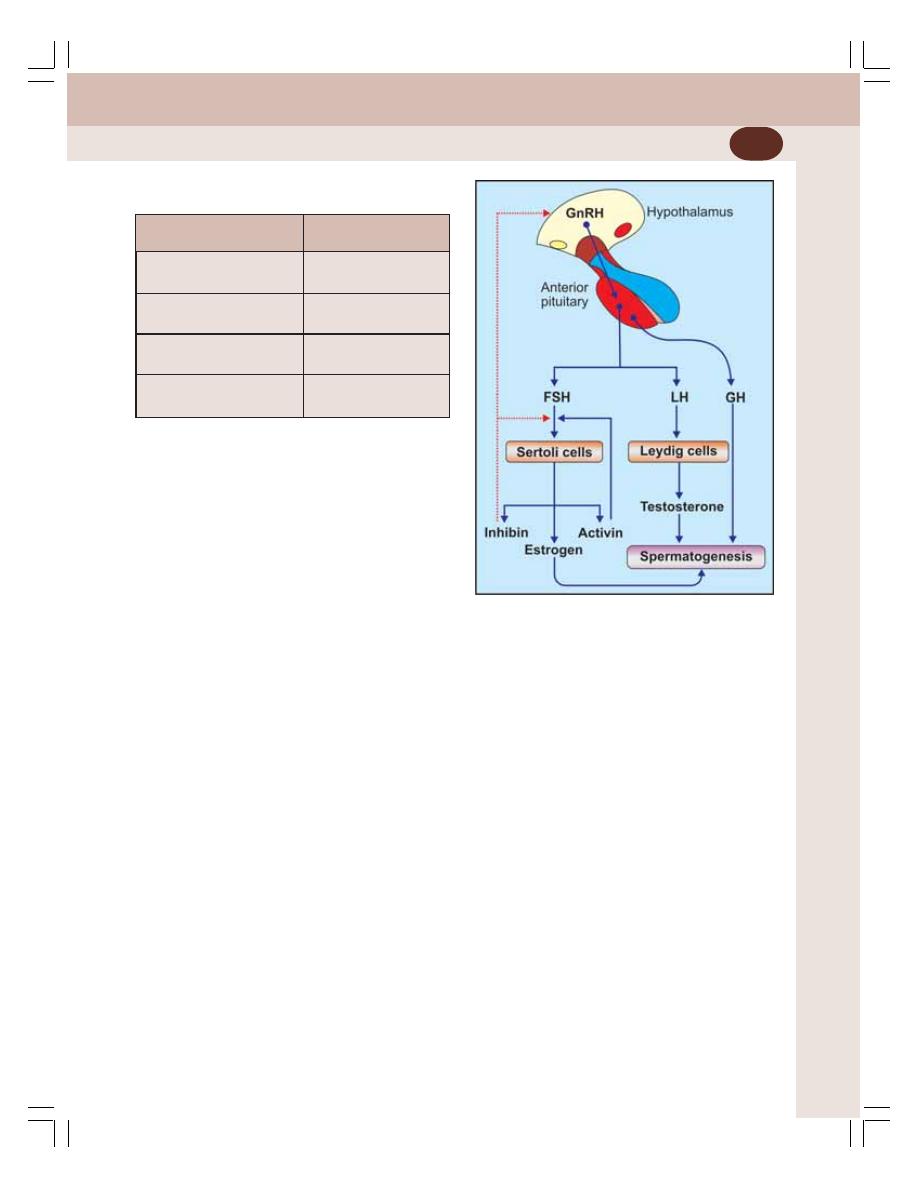
Chapter 53 Male Reproductive System
335
6. Inhibin
Inhibin is a peptide hormone and serves as a
transforming growth factor. It is secreted by Ser-
toli cells. In females, it is secreted by granulosa
cells of ovarian follicles. Its secretion is stimu-
lated by FSH.
Inhibin inhibits FSH secretion through feed-
back mechanism leading to decrease in the pace
of spermatogenesis.
7. Activin
It is also a peptide hormone secreted in gonads
along with inhibin. The exact location of its secre-
tion in testis is not known. It is suggested that it
is secreted by Sertoli cells and Leydig cells.
Activin has opposite actions of inhibin. It
increases secretion of FSH and accelerates
spermatogenesis.
FIGURE 53-5:
Role of hormones in spermatogenesis
Blue arrow = Stimulation. Red dotted arrow = Inhibition
TABLE 53-1:
Hormones necessary
for spermatogenesis
Stage of
Hormones necessary
spermatogenesis
1. Stage of proliferation
FSH
Growth hormone
2. Stage of growth
Testosterone
Growth hormone
3. Stage of maturation
Testosterone
Growth hormone
4. Stage of transformation Testosterone
Estrogen
1. FSH
FSH is responsible for the initiation of sperma-
togenesis. It binds with Sertoli cells and
spermatogonia and induces the proliferation of
spermatogonia. It also stimulates formation of
estrogen and androgen binding protein from
Sertoli cells (Fig. 53-5).
2. LH
In males this hormone is called interstitial cell
stimulating hormone. It is essential for the secre-
tion of testosterone from Leydig cells.
3. GH
Growth hormone is essential for the general
metabolic processes in testis. It is also
necessary for proliferation of spermatogonia. In
pituitary dwarfs, the spermatogenesis is severely
affected.
4. Testosterone
Testosterone is responsible for sequence of
remaining stages in spermatogenesis. It is also
responsible for maintenance of spermato-
genesis. Testosterone activity is largely influen-
ced by androgen binding protein.
5. Estrogen
It is formed from testosterone in Sertoli cells. It
is necessary for spermeogenesis.

Reproductive System
336
OTHER FACTORS AFFECTING
SPERMATOGENESIS
Spermatogenesis is also influenced by some
other factors:
1. Increase in the body temperature prevents
spermatogenesis. It occurs in cryptorchidism
(see below). Normally, the temperature in the
scrotum is about 2°C less than the body
temperature. But in cryptorchidism the testes
are in the abdomen where the temperature
is always higher than that of scrotum. In-
crease in temperature stops spermatogene-
sis.
2. Infectious diseases such as mumps cause
degeneration of seminiferous tubules and
absence of spermatogenesis.
ENDOCRINE FUNCTIONS OF TESTIS
Testis secretes male sex hormones which are
collectively called the androgens. Androgens
secreted by testis are:
1. Testosterone
2. Dihydrotestosterone
3. Androstenedione.
The androgens are secreted in large quanti-
ties by interstitial cells of Leydig in testes and in
small quantity by zona reticularis in adrenal
cortex.
Androgens are steroid hormones synthesized
from cholesterol or acetate. Testosterone is a
C19 steroid. The plasma level of testosterone
in an adult male varies between 300 and 700
ng/dL. In adult female the testosterone level is
30 to 60 mg/dL.
FUNCTIONS OF TESTOSTERONE
In general, testosterone is secreted in fetal life
and adult life. But, in childhood, practically no
testosterone is secreted approximately until
10 to 12 years of age. Afterwards, the
testosterone secretion starts and, it increases
rapidly at the onset of puberty and lasts through
most of the remaining part of life. The secretion
starts decreasing after 40 years and becomes
almost zero by the age of 90 years.
Functions of Testosterone in Fetal Life
The fetal testes begin to secrete testosterone
at about 2nd to 4th month of fetal life. Testo-
sterone performs three functions in fetus:
1. Sex differentiation in fetus
2. Development of accessory sex organs
3. Descent of the testes.
1. Sex differentiation in fetus
Testosterone is responsible for the sex differen-
tiation of fetus. Fetus has two genital ducts:
i. Müllerian duct which gives rise to female
accessory sex organs such as vagina,
uterus and fallopian tube
ii. Wolffian duct which gives rise to male
accessory sex organs such as epididymis,
vas deferens and seminal vesicles.
If testosterone is secreted from the genital
ridge of the fetus at about 7th week of intrauterine
life, the Müllerian duct system disappears and
male sex organs develop from Wolffian duct.
In addition to testosterone, Müllerian regres-
sion factor (MRF) secreted by Sertoli cells is also
responsible for regression of Müllerian duct.
In the absence of testosterone, Wolffian duct
regresses and female sex organs develop from
Müllerian duct.
2. Development of accessory sex organs and
external genitalia
Testosterone is also essential for the growth of
the external genitalia viz. penis and scrotum and
other accessory sex organs namely genital ducts,
seminal vesicles and prostate.
3. Descent of testes
Initially, testes are developed in the abdominal
cavity and are later pushed down into the
scrotum through inguinal canal just before birth.
The process by which testes enter the scrotum
is called the descent of testes. Testosterone is
necessary for descent of testes.
Cryptorchidism
Cryptorchidism is a congenital disorder
characterized by the failure of one or both the

Chapter 53 Male Reproductive System
337
testes to descent from abdomen into scrotum.
In such case, the testes are called undescended
testes. Administration of testosterone or
gonadotropic hormones (which stimulate Leydig
cells) causes descent of testes. Surgery is
required if the inguinal canal is narrow. Males
with untreated testes are prone for testicular
cancer.
Functions of Testosterone in Adult Life
Testosterone has two important functions in
adult:
1. Effect on sex organs
2. Effect on secondary sexual characters.
1. Effect on sex organs
Testosterone increases the size of penis,
scrotum and the testes after puberty. All these
organs are enlarged at least 8 folds between the
onset of puberty and the age of 20 years, under
the influence of testosterone. Testosterone is
also necessary for spermatogenesis.
2. Effect on secondary sexual characters
Secondary sexual characters are the physical
and behavioral characteristics that distinguish
the male from female. These characters appear
at the time of puberty. Testosterone is res-
ponsible for the development of secondary
sexual characters in males.
The secondary sexual characters in males
are:
i. Effect on muscular growth
Testosterone increases the muscle mass due
to its anabolic effects on proteins. It accelerates
transport of amino acids into the muscle cells,
synthesis of proteins and storage of proteins in
the muscles. It also decreases breakdown of
proteins.
ii. Effect on bone growth
After puberty, testosterone increases the
thickness of bones by increasing the matrix
content and calcium deposition. The increase
in matrix content in bones is because of its
anabolic effects on protein. The deposition of
calcium is secondary to the increase in bone
matrix.
In addition to increase in the size and
strength of bones, testosterone also causes
early fusion of epiphyses of long bones with
shaft. So, if testes are removed before puberty,
the fusion of epiphyses is delayed and the height
of the person increases.
iii. Effect on shoulder and pelvic bones
Testosterone causes broadening of shoulders
and it has a specific effect on pelvis which
results in:
a. Lengthening of pelvis
b. Funnel like shape of pelvis
c. Narrowing of pelvic outlet.
Thus, pelvis in males is different from that of
females, which is broad and round or oval in
shape.
iv. Effect on skin
Testosterone increases the thickness of skin and
ruggedness of subcutaneous tissue by
increasing the deposition of proteins in skin. It
also increases the quantity of melanin pigment,
which is responsible for the deepening of the
skin color.
Testosterone enhances the secretory activity
of sebaceous glands. So, at the time of puberty,
when the body is exposed to sudden increase
in testosterone secretion, the excess secretion
of sebum leads to development of acne on the
face. After few years, the skin gets adapted to
testosterone secretion and, acne disappears.
v. Effect on hair distribution
The testosterone causes male type of hair distri-
bution on the body, i.e. hair growth over the
pubis, along linea alba up to umbilicus, on face,
on chest and other parts of the body such as
back and limbs. In males, the pubic hair has the
base of the triangle downwards whereas in it is
upwards. Testosterone decreases the hair
growth on the head and may cause baldness if
there is genetic background.
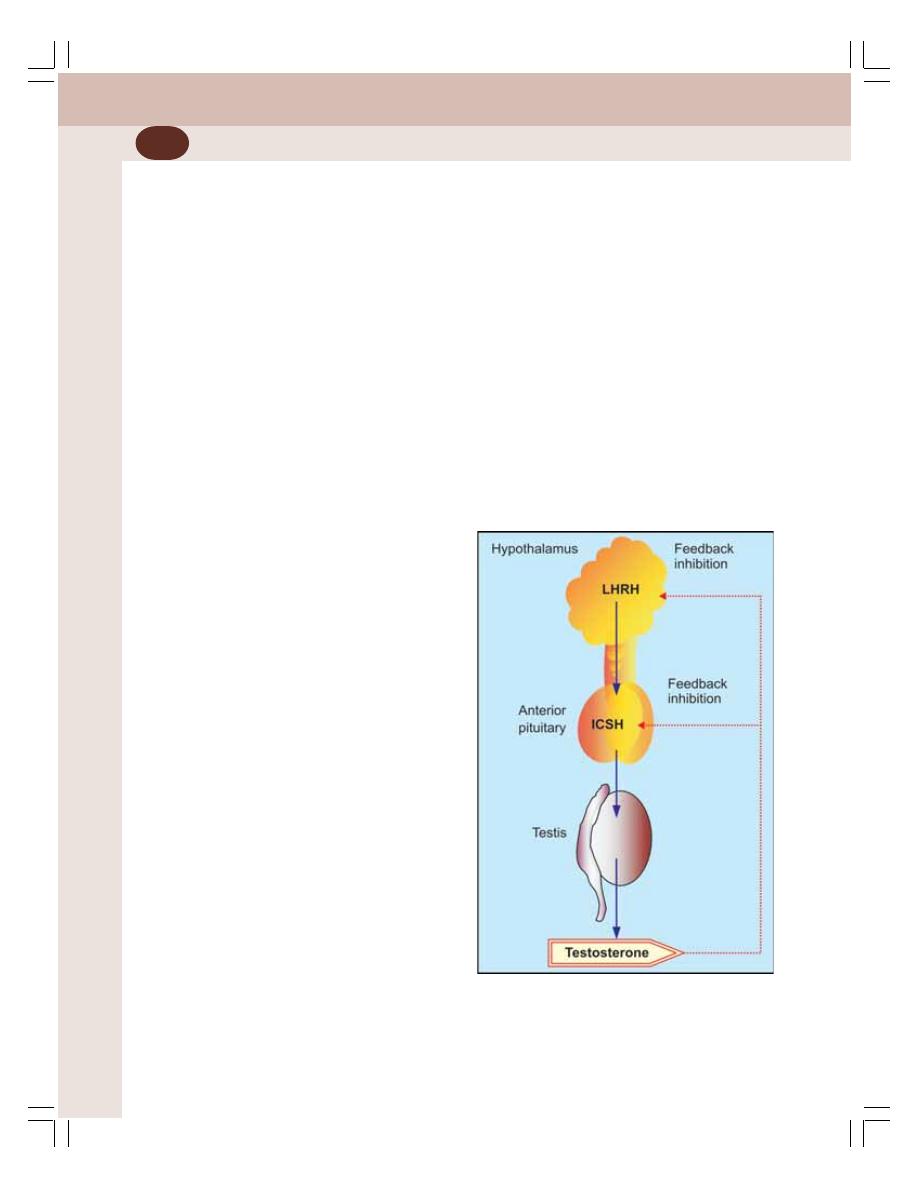
Reproductive System
338
vi. Effect on voice
At the time of adolescence, the boys have a
cracking voice. It is because of the testosterone
effect which causes:
a. Hypertrophy of laryngeal muscles
b. Enlargement of larynx and lengthening
c. Thickening of vocal cords.
Later, the cracking voice changes gradually
into a typical adult male voice with a bossing
sound.
vii. Effect on basal metabolic rate
At the time of puberty and earlier part of adult
life, the testosterone increases the basal
metabolic rate to about 5 to 10% by its anabolic
effects on protein metabolism.
viii. Effect on electrolyte and water balance
Testosterone increases the sodium reabsorption
from renal tubules along with water. It leads to
increase in ECF volume.
ix. Effect on blood
Testosterone has got erythropoietic action. So,
after puberty, testosterone causes mild
increase in RBC count. It also increases the
blood volume by increasing the water retention
and ECF volume.
MODE OF ACTION OF TESTOSTERONE
Testosterone acts via genes.
REGULATION OF TESTOSTERONE
SECRETION
In Fetus
During fetal life, the testosterone secretion from
testis is stimulated by human chorionic gona-
dotropin, which has the properties similar to those
of luteinizing hormone. Human chorionic gona-
dotropin stimulates the development of Leydig
cells in the fetal testes and promotes testosterone
secretion.
In Adults
LH or ICSH stimulates the Leydig cells and the
quantity of testosterone secreted is directly pro-
portional to the amount of LH available.
Secretion of LH from anterior pituitary gland
is stimulated by LHRH from hypothalamus.
Feedback Control
Testosterone regulates its own secretion by
negative feedback mechanism. It acts on hypo-
thalamus and inhibits the secretion of LHRH.
When LHRH secretion is inhibited, LH is not
released from anterior pituitary resulting in
stoppage of testosterone secretion from testes.
On the other hand, when testosterone production
is low, lack of inhibition of hypothalamus leads
to secretion of testosterone through LHRH and
LH (Fig. 53-6).
FIGURE 53-6:
Regulation of testosterone secretion
LHRH = Luteinizing hormone releasing hormone
ICSH = Interstitial cell stimulating hormone
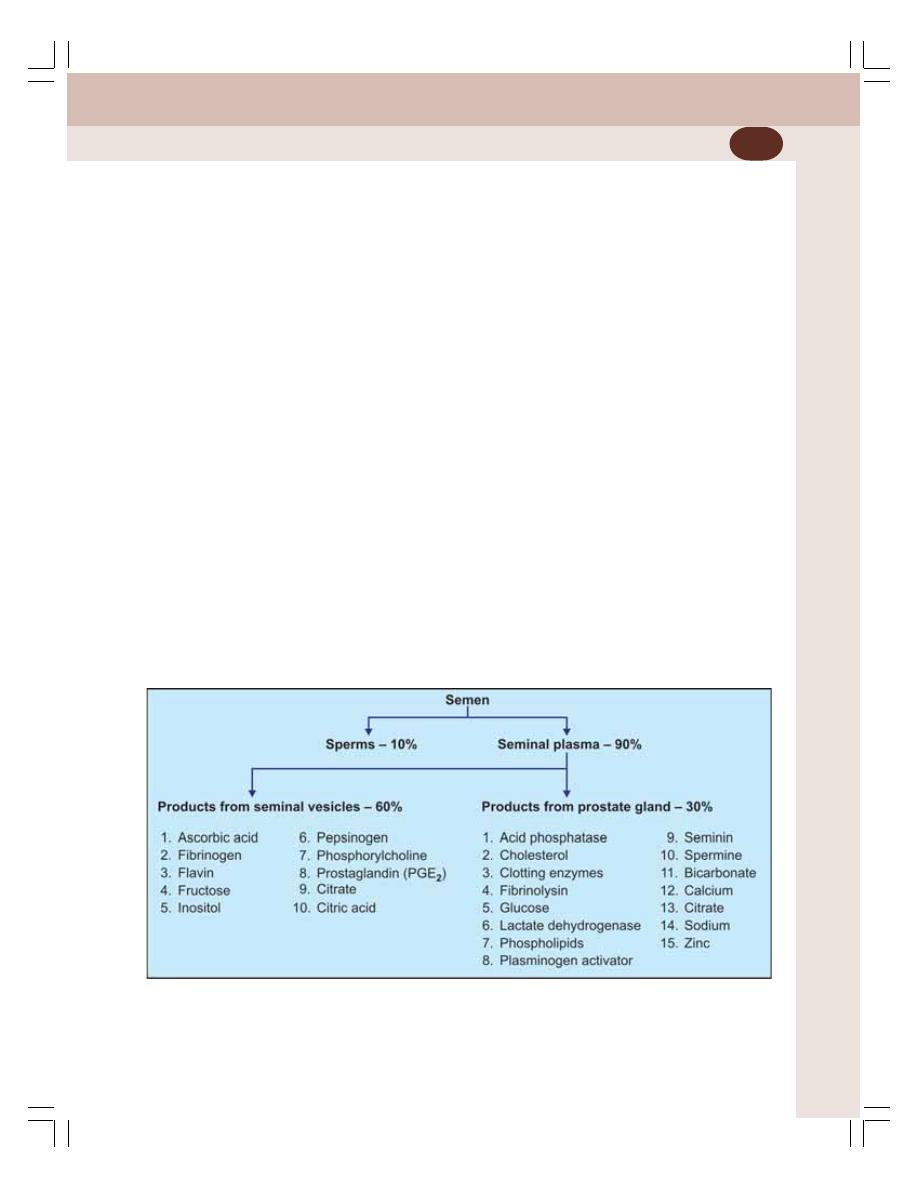
Chapter 53 Male Reproductive System
339
SEMEN
Semen is a white or grey fluid that contains
spermatozoa (sperms). It is the collection of
fluids from testes, seminal vesicles, prostate
gland and bulbourethral glands. Semen is
discharged during sexual act and the process
of discharge of semen is called ejaculation.
Testes contribute sperms. The prostate
secretion gives milky appearance to semen. And,
the secretions from seminal vesicles and
bulbourethral glands provide mucoid consistency
to semen.
At the time of ejaculation, human semen is
liquid in nature. Immediately, it coagulates and
after some time it becomes liquid again.
The fibrinogen secreted from seminal vesicle
is converted into a weak coagulum by the clotting
enzymes secreted from prostate gland. The coa-
gulum is liquefied after about 30 minutes, as it
is lysed by fibrinolysin. Fibrinolysin is the
activated form of profibrinolysin produced in
prostate gland.
When semen is ejaculated, the sperms are
nonmotile due to the viscosity of coagulum. When
the coagulum dissolves, the sperms become
motile.
PROPERTIES OF SEMEN
1. Specific gravity : 1.028
2. Volume
: 2 to 6 mL per ejaculation
3. Reaction
: It is alkaline with a pH of
7.5. The alkalinity is due to
the secretions from pro-
state gland.
COMPOSITION OF SEMEN
Semen contains 10% sperms and 90% of fluid
part which is called seminal plasma. The seminal
plasma contains the products from seminal
vesicle and prostate gland (Fig. 53-7). It also has
small amount of secretions from the mucus
glands, particularly the bulbourethral glands.
SPERM
Sperm or spermatozoon (pleural = spermatozoa)
is the male reproductive cell, developed in the
testis. The total count of sperm is about 100 to
150 million/mL of semen. Sterility occurs when
the sperm count falls below 20 millions/mL.
Though the sperms can be stored in male
genital tract for longer periods, after ejaculation
FIGURE 53-7:
Composition of semen
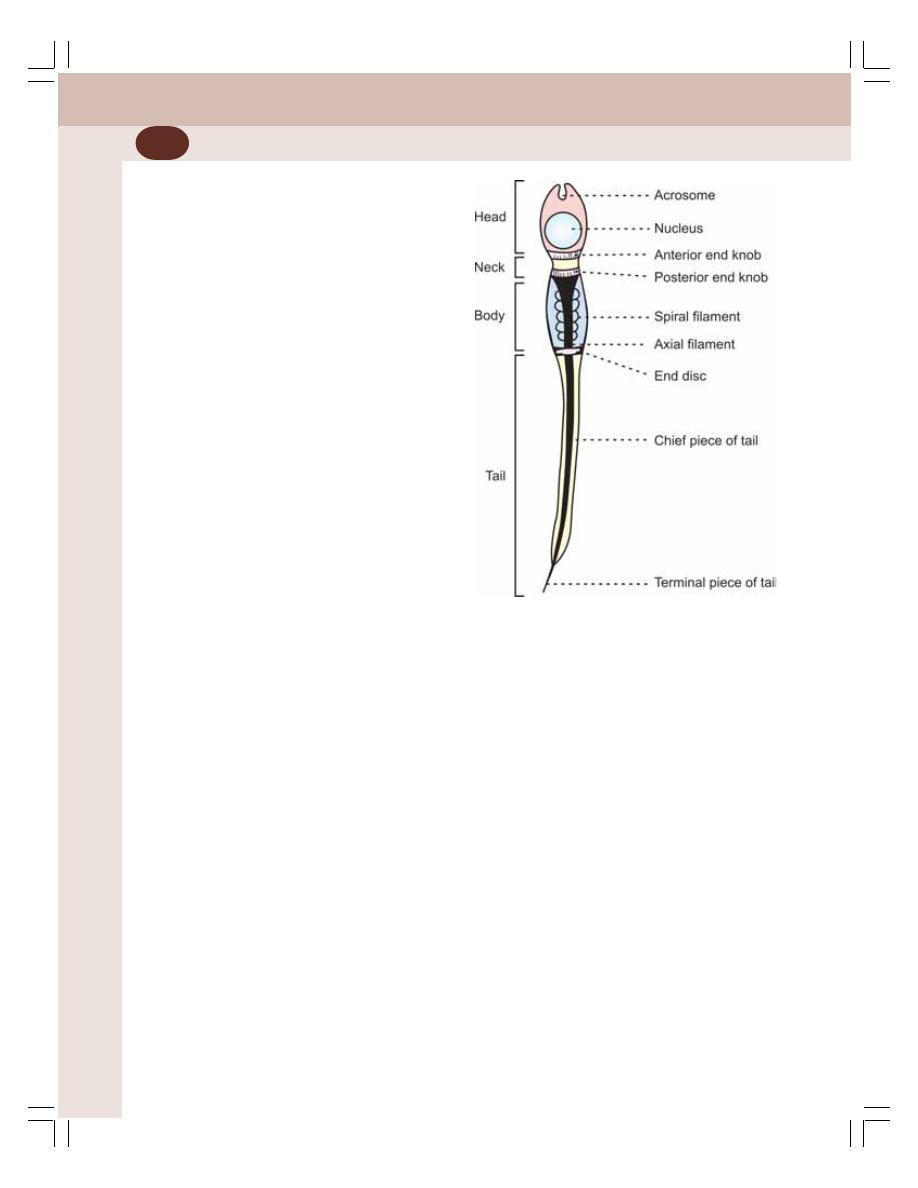
Reproductive System
340
the survival time is only about 24 to 48 hours at
a temperature equivalent to body temperature.
The rate of motility of sperm in female genital
tract is about 3 mm/minute. The sperms reach
the fallopian tube in about 30 to 60 minutes after
sexual intercourse. The uterine contractions
during sexual act facilitate the movement of
sperms.
Structure of Sperm
The matured sperm is 60 μ long. Each sperm
consists four parts:
1. Head
2. Neck
3. Body
4. Tail.
1. Head
Head of sperm is oval in shape (in front view),
with a length of 3 to 5 μ and width of up to 3 μ.
The anterior portion of head is thin (Fig. 53-8).
The head is formed by thin cytoplasm with
a condensed nucleus and it is covered by a thin
cell membrane. The anterior two thirds of the
head is like a thick cap and it is called acrosome.
Acrosome develops from Golgi apparatus and
it is made up of mucopolysaccharide and acid
phosphatase. It also contains hyaluronidase and
proteolytic enzymes which are essential for the
sperm to fertilize the ovum.
2. Neck
The head is connected to the body by a short
neck. Its anterior end is formed by thick disk
shaped anterior end knob, which is also called
proximal centriole. The posterior end is formed
by another similar structure known as posterior
end knob. It gives rise to the axial filament of
body.
Often, the neck and body of sperm are
together called midpiece.
3. Body
It is cylindrical with a length of 5 to 9 μ and the
thickness of 1 μ. The body of the sperm consists
of a central core called axial filament covered
by thin cytoplasmic capsule.
The axial filament starts from posterior end
knob of the neck. It passes through the body and
a perforated disc called end disc or end ring
centriole. Finally, the axial filament reaches the
tail as axial thread.
In the body, the axial filament is surrounded
by a closely wound spiral filament consisting of
mitochondria.
4. Tail
The tail of the sperm consists of two segments:
i. The chief or main piece of tail which is
enclosed by cytoplasmic capsule and has
an axial thread. It is 40 to 50 μ long
ii. The terminal or end piece of tail that has
only the axial filament.
MALE CLIMACTERIC
Male andropause or climacteric is the condition
in men characterized by emotional and physical
changes in the body due to low androgen level
FIGURE 53-8:
Human sperm

Chapter 53 Male Reproductive System
341
with aging. It is also called viropause. After the
age of 50, testosterone secretion starts declining
because of decrease in number and secretory
activity of Leydig cells.
APPLIED PHYSIOLOGY
EFFECTS OF EXTIRPATION OF TESTES
The removal of testes is called castration. The
effects of castration depend upon the age when
testes are removed.
1. Effects of Extirpation of Testes before
Puberty – Eunuchism
If a boy looses the testes before puberty, he
continues to have infantile sexual characters
throughout life. This condition is called eunu-
chism. The height of the person is slightly more
but the bones are weak and thin. The muscles
become weak and shoulder remains narrow.
The sex organs do not increase in size, and,
the male secondary sexual characters do not
develop. The voice remains like that of a child.
There is abnormal deposition of fat on but-
tocks, hip, pubis and breast, resembling the
feminine distribution.
2. Effects of Extirpation of Testes
Immediately after Puberty
If testes are removed after puberty, some of the
male secondary sexual characters revert to those
of a child and other masculine characters are
retained.
Sex organs are depressed. Seminal vesicles
and prostate undergo atrophy. Penis remains
smaller. Voice remains mostly masculine but
other secondary sexual characters like
masculine hair distribution, musculature and
thickness of bones are lost. There may be loss
of sexual desire and sexual activities.
3. Effect of Extirpation of Testes in Adults
Removal of testis in adults does not cause loss
of secondary sexual characters. But, accessory
sex organs start degenerating. The sexual desire
is not totally lost. Erection occurs but ejaculation
is rare because of degeneration of accessory sex
organs and lack of sperms.
HYPERGONADISM IN MALES
Hypergonadism is the condition characterized by
hypersecretion of sex hormones from gonads.
Cause
Hypergonadism in males is mainly due to the
tumor of Leydig cells. It is common in prepubertal
boys who develop precocious pseudo puberty.
Symptoms
There is rapid growth of musculature and bones.
But, the height of the person is less because of
early closure of epiphysis. There is excess deve-
lopment of sex organs and secondary sexual
characters.
The tumors also secrete estrogenic hor-
mones which cause gynecomastia (the enlarge-
ment of breasts).
HYPOGONADISM IN MALES
Hypogonadism is a condition characterized by
reduction in the functional activity of gonads.
Causes
The hypogonadism in males is due to various
abnormalities of testes:
1. The congenital non-functioning of testes
2. Under developed testes due to absence of
human chorionic gonadotropins in fetal life
3. Cryptorchidism associated with partial or total
degeneration of testes
4. Castration
5. Absence of androgen receptors in testes
6. Disorder of gonadotropes (cells secreting
gonadotropins) in anterior pituitary
7. Hypothalamic disorder.
Signs and Symptoms
The clinical picture of male hypogonadism
depends upon whether the testicular deficiency
develops before or after puberty.

Reproductive System
342
Before puberty
The features of hypogonadism are similar to
those developed due to extirpation of testes
before puberty, which are described above.
After puberty
The symptoms are similar to those developed
due to the removal of testes after puberty (see
above).
In adults
The same symptoms, which develop after
extirpation of testis, occur in this condition.
Hypogonadism caused by testicular disorders
increases the gonadotropin secretion and the
condition is called hypergonadotropic hypo-
gonadism. Hypogonadism that occurs due to
deficiency of gonadotropins (pituitary or hypo-
thalamic disorder) is called hypogonadotropic
hypogonadism.
Dystrophia adiposogenitalis
It is the disorder characterized by obesity and
hypogonadism in adolescent boys. It is also
called Fröhlich’s syndrome or hypothalamic
eunuchism. Refer Chapter 45 for details.
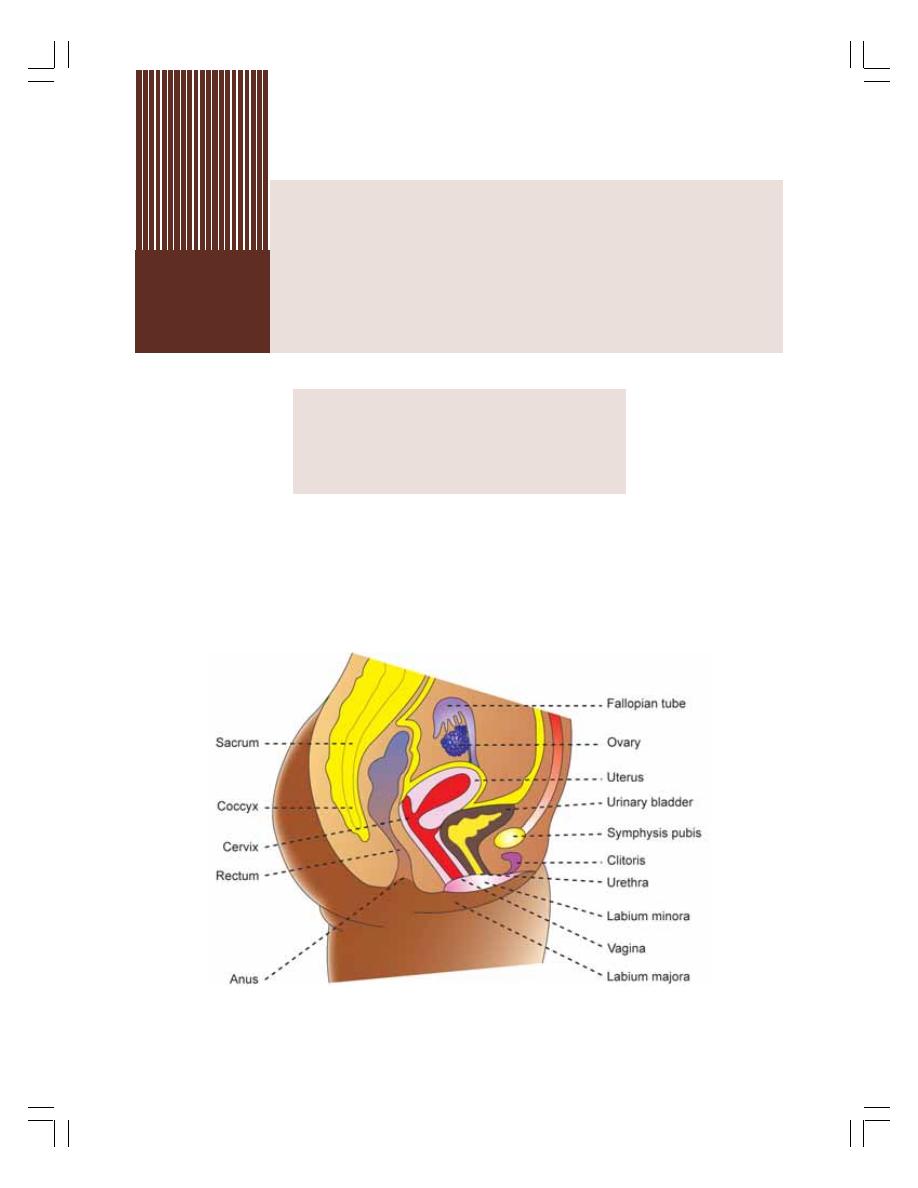
FEMALE REPRODUCTIVE ORGANS
SEXUAL LIFE IN FEMALES
OVARIAN HORMONES
CLIMACTERIC AND MENOPAUSE
Female
Reproductive System
54
FEMALE REPRODUCTIVE ORGANS
Female reproductive system comprises primary
sex organs and accessory sex organs (Figs
54-1 and 54-2).
PRIMARY SEX ORGANS – OVARIES
The primary sex organs are a pair of ovaries,
which produce eggs or ova and secrete female
sex hormones, the estrogen and progesterone.
FIGURE 54-1: Female reproductive organs and other organs of pelvis
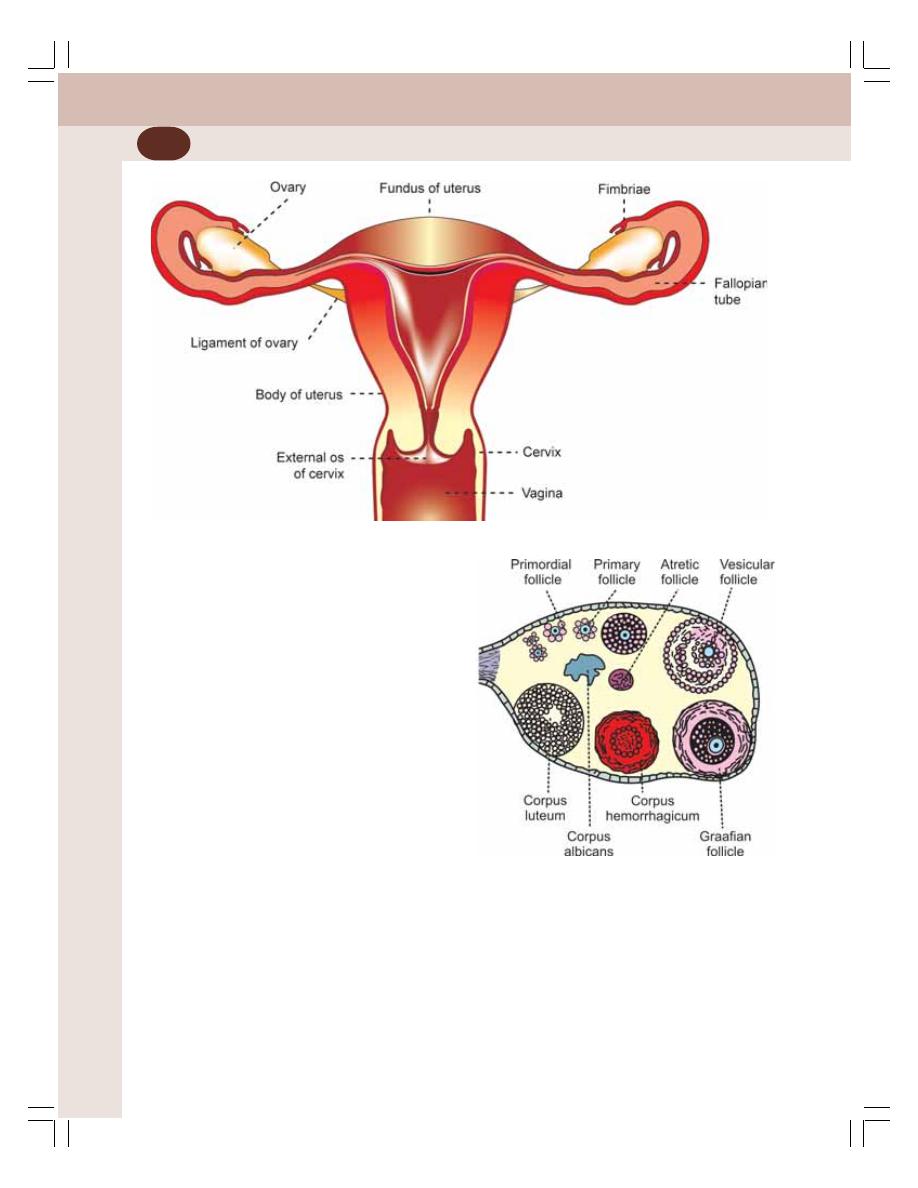
Reproductive System
344
Ovaries are flattened ovoid bodies with
dimensions of 4 cm in length, 2 cm in width and
1 cm in thickness. On cross section, each ovary
shows two zones:
1. Medulla
2. Cortex.
Medulla
The medulla is otherwise known as zona
vasculosa. It is the inner portion of the ovary. It
has the stroma of loose connective tissues. It
contains blood vessels, lymphatics, nerve fibers
and bundles of smooth muscle fibers near the
hilum.
Cortex
It is the outer broader portion and has compact
cellular layers. Cortex is lined by the germinal
epithelium underneath a fibrous layer known as
tunica albuginea. The cortex consists of ovarian
follicles at different stages, connective tissue and
interstitial.
In the intrauterine life, the outer part of cortex
contains germinal epithelium, which is derived
from the germinal ridges. When the fetus deve-
lops, the germinal epithelium gives rise to a
number of primordial ova. The primordial ova
move towards the inner substance of cortex. A
layer of granulose cells from the ovarian stroma
surround the ova. The primordial ovum along with
granulosa cells is called the primordial follicle
(Fig. 54-3).
FIGURE 54-2: Female reproductive system
FIGURE 54-3: Ovarian follicles and
corpus luteum
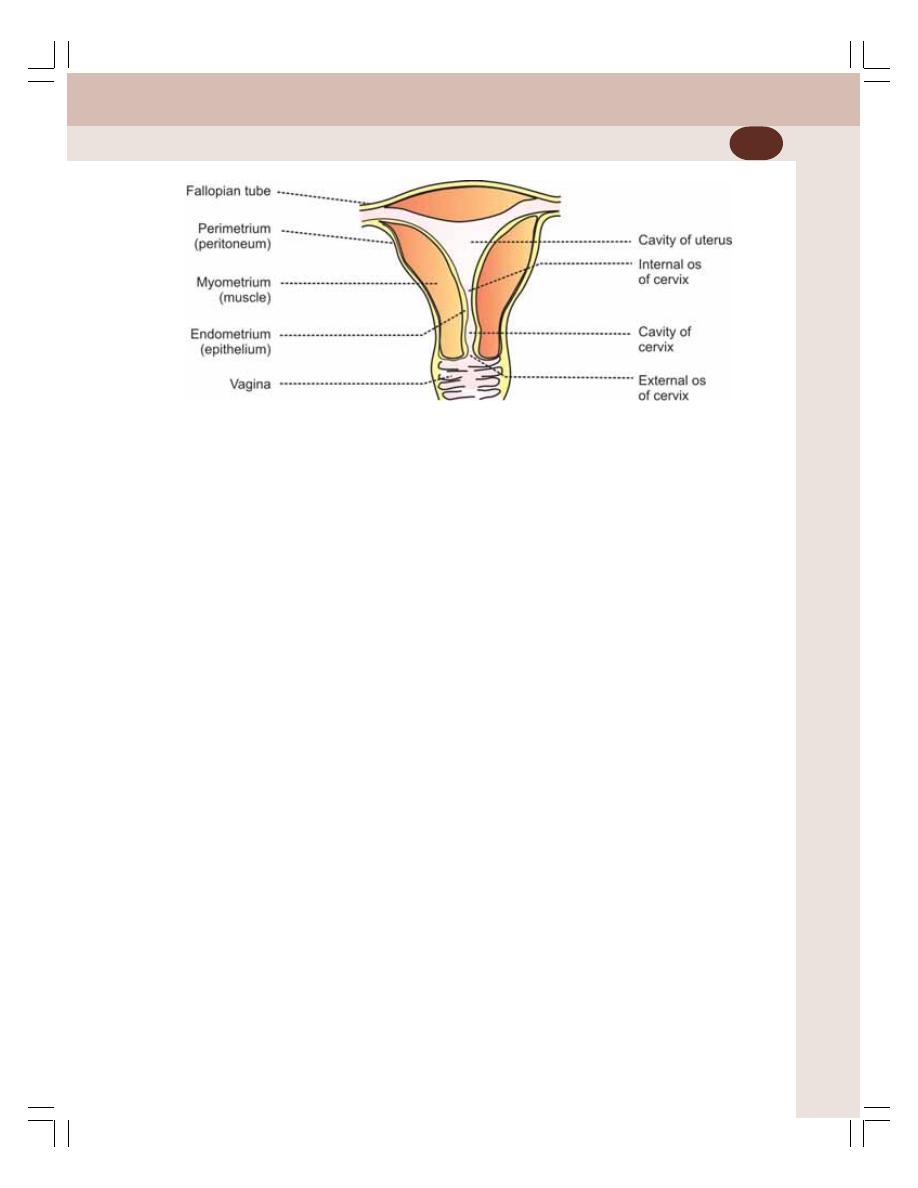
Chapter 54 Female Reproductive System
345
At 7th or 8th month of intrauterine life, about
6 million primordial follicles are found in the
ovary. But, at the time of birth, only 1 million
primordial follicles are seen in both the ovaries
and, the rest of the follicles degenerate. At the
time of puberty, the number decreases further
to about 3,00,000 to 4,00,000. After menarche,
during every menstrual cycle, one of the follicles
matures and releases its ovum. During every
menstrual cycle, only one ovum is released from
any one of the ovaries.
During every cycle, many of the follicles
degenerate and become atretic follicles which
disappear without leaving any scar.
Functions of Ovary
1. Secretion of female sex hormones
2. Oogenesis
3. Menstrual cycle.
ACCESSORY SEX ORGANS
The accessory sex organs in females are:
1. A system of genital ducts that includes fallo-
pian tubes, uterus, cervix and vagina
2. External genitalia which are labia majora,
labia minora and clitoris.
The mammary glands are not the female
genital organs but are the important glands of
female reproductive system.
Uterus
Uterus or womb is a hollow muscular organ with
a thick wall. It lies in the pelvic cavity in between
rectum and urinary bladder on its posterior side.
The central cavity of uterus opens into vagina
through cervix. On either side at its upper part,
the fallopian tubes open. Uterus communicates
with peritoneal cavity through fallopian tubes. The
dome-shaped part of the body which lies above
a plane passing through the points of entrance
of the fallopian tubes is known as the fundus.
There is a constriction almost at the middle
of uterus called isthmus. It divides the uterus into
two portions:
1. The portion above the isthmus called the body
of uterus
2. Portion below the isthmus called cervix.
Structure of uterus
Uterus is made up of 3 layers:
1. Outer serous layer derived from peritoneum
(Fig. 54-4)
2. Middle muscular layer or myometrium made
up of smooth muscle fibers
3. Inner mucus layer or endometrium made up
of ciliated columnar epithelial cells, connective
tissue and uterine glands.
FIGURE 54-4: Section of uterus

Reproductive System
346
Cervix
Cervix is the lower constricted part of uterus. It
is divided into two portions:
1. Upper supravaginal portion which communi-
cates with body of uterus through internal os
(orifice) of cervix
2. Lower vaginal portion which communicates
with vagina through external os.
Vagina
Vagina is a short tubular organ. It is lined by
mucous membrane which is formed by stratified
epithelial cells.
OVARIAN HORMONES
Ovary secretes the female sex hormones estro-
gen and progesterone. Ovary also secretes few
more hormones namely, inhibin, relaxin and small
quantities of androgens.
ESTROGEN
In a normal nonpregnant female, estrogen is
secreted in large quantity by theca interna cells
of ovarian follicles and in small quantity by
corpus luteum of the ovaries. A small quantity
of estrogen is also secreted by adrenal cortex.
In pregnancy, a large amount of estrogen is
secreted by the placenta.
Estrogen is a C
18
steroid and it is present in
three forms in plasma:
1.
β estradiol
2. Estrone
3. Estriol.
The quantity and potency of
β estradiol are
more than those of estrone and estriol.
The plasma level of estrogen in females at
normal reproductive age varies during different
phases of menstrual cycle. In follicular phase, it
is 30 to 200 pg/mL (Fig. 55-4). In normal adult
male estrogen level is 12 to 34 pg/mL.
Functions of Estrogen
The major function of the estrogen is to promote
cellular proliferation and tissue growth in the
sexual organs and in other tissues related to
reproduction.
Effects of estrogen are:
1. Effect on ovarian follicles
Estrogen promotes the growth of ovarian
follicles by increasing the proliferation of the
follicular cells. It also increases the secretory
activity of theca cells (Refer chapter 55 for
details).
2. Effect on uterus
Estrogen produces the following changes in
uterus:
i. Enlargement of uterus to about double of
its childhood size by the proliferation of
endometrial cells
ii. Increase in the blood supply to endo-
metrium
iii. Deposition of glycogen and fats in endo-
metrium
iv. Proliferation and dilatation of blood
vessels of endometrium
v. Proliferation and dilatation of the endo-
metrial glands, which become more
tortuous with increased blood flow
vi. Increase in the spontaneous activity of
the uterine muscles and their sensitivity
to oxytocin
vii. Increase in the contractility of the uterine
muscles due to increase in actomyosin
concentration.
All these changes prepare uterus for preg-
nancy.
3. Effect on fallopian tubes
Estrogen:
i. Acts on the mucosal lining of the fallopian
tubes and increases the number and size
of the epithelial cells, especially the
ciliated epithelial cells lining the fallopian
tubes
ii. Increases the activity of the cilia, so that
the movement of the ovum in the fallopian
tube is facilitated
iii. Enhances the proliferation of glandular
tissues in fallopian tubes.
All these changes are necessary for fertili-
zation of ovum.

Chapter 54 Female Reproductive System
347
4. Effect on vagina
Estrogen:
i. Changes the vaginal epithelium from
cuboidal into stratified type. The stratified
epithelium is more resistant to trauma and
infection
ii. Increases the layers of the vaginal epi-
thelium by proliferation
iii. Reduces the pH of vagina making it more
acidic.
All these changes are necessary for preven-
tion of certain common vaginal infections such
as gonorrheal vaginitis. Such infections can be
cured by administration of estrogen.
5. Effect on secondary sexual characters
Estrogen is responsible for the development of
secondary sexual characters in females.
The secondary sexual characters in female
are:
i. Hair distribution: Hair develops in the
pubic region and axilla. In females, pubic
hair has the base of the triangle upwards.
Body hair growth is less. Scalp hair grows
profusely.
ii. Skin: Estrogen renders softness and
smoothness to the skin. It also increases
the vascularity of the skin
iii. Body shape: The shoulders become
narrow, hip broadens, the thighs converge,
and the arms diverge. The fat deposition
increases in the breasts and buttocks
iv. Pelvis: Estrogen has a specific effect on
pelvis which results in:
a. Broadening of pelvis with increased
transverse diameter
b. The round or oval-shape of pelvis
c. Round or oval-shaped pelvic outlet.
Thus, pelvis in females is different from
that of males, which is funnel-shaped.
iv. Voice: The larynx remains in prepubertal
stage, which produces high pitch voice.
6. Effect on breast
Estrogen causes:
i. Development of stromal tissues of breasts
ii. Growth of an extensive ductile system
iii. Deposition of fat in the ductile system
All these effects prepare the breasts for
lactation.
7. Effect on bones
Estrogen increases osteoblastic activity. So, at
the time of puberty, the growth rate increases
enormously. But, at the same time, estrogen
causes early fusion of the epiphysis with the
shaft. This effect is much stronger in the females
than the similar effect of testosterone in males.
As a result, the growth of the females usually
ceases few years earlier than in the males.
In old age, the estrogen is not secreted or it
becomes scanty. It leads to osteoporosis in which
the bones become extremely weak and fragile.
And, because of this, the bones are highly
susceptible for fractures (Chapter 47).
8. Effect on metabolism
i. On protein metabolism: Estrogen induces
anabolism of proteins by which it in-
creases the total body protein.
ii. On fat metabolism: Estrogen causes
deposition of fat in the subcutaneous
tissues, breasts, buttocks and thighs. The
overall specific gravity of the female body
is considerably lesser than that of males
because of the fat deposition.
9. Effect on electrolyte balance
Estrogen causes sodium and water retention
from the renal tubules. This effect is normally
insignificant but in pregnancy, it becomes more
significant.
Mode of Action of Estrogen
The estrogen acts through genes.
Regulation of Estrogen Secretion
The secretion of estrogen is regulated by FSH
released from anterior pituitary. The release of
FSH is stimulated by gonadotropic releasing
hormone (GnRH) secreted from hypothalamus.
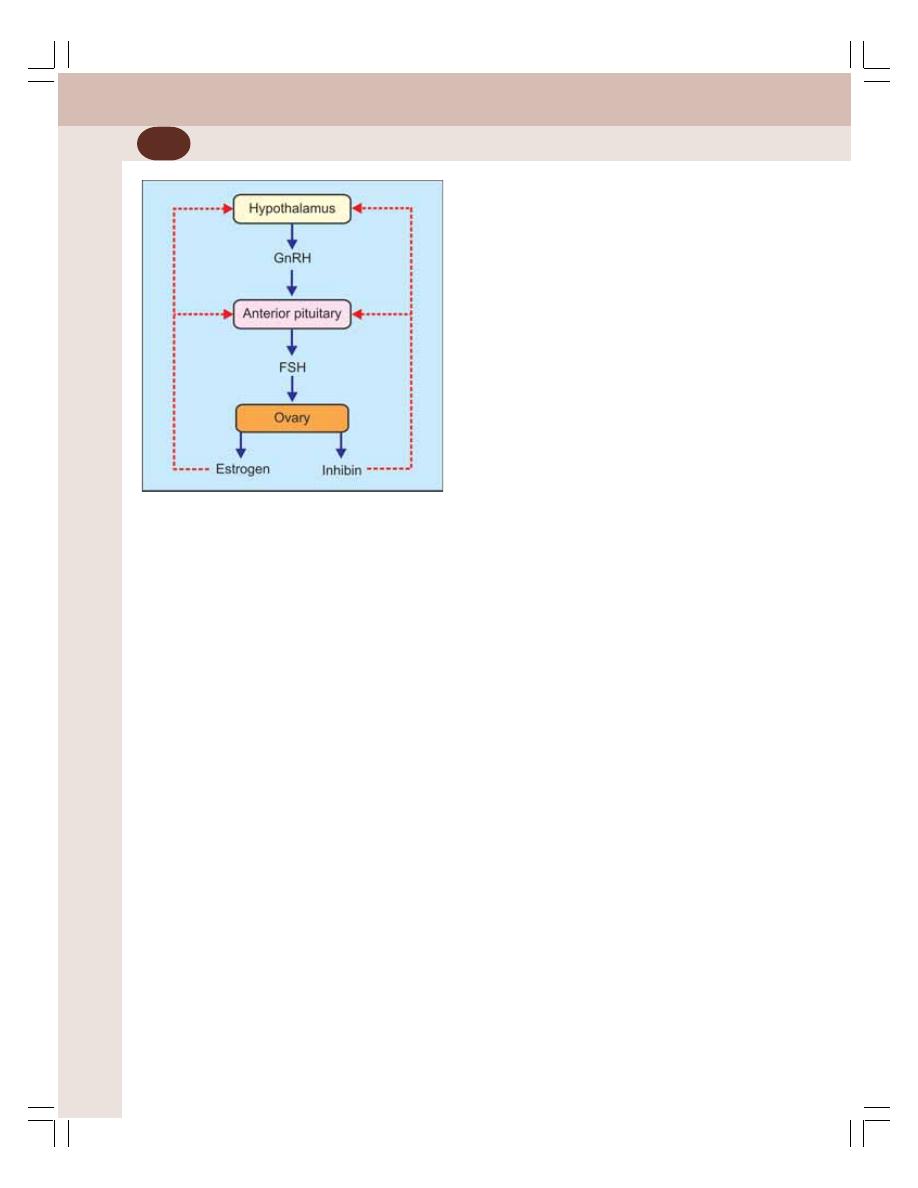
Reproductive System
348
FSH stimulates the secretory activities of
theca and granulosa cells. Estrogen inhibits sec-
retion of FSH and GnRH by negative feedback.
Inhibin secreted by granulosa cells (Chapter 55)
also decreases estrogen secretion by inhibiting
secretion of FSH and GnRH (Fig. 54-5).
PROGESTERONE
A small quantity of progesterone is secreted by
theca interna cells of ovaries during the first half
of menstrual cycle, i.e. during follicular stage. But,
a large quantity of progesterone is secreted
during the latter half of each menstrual cycle, i.e.
during secretory phase by the corpus luteum.
Small amount of progesterone is secreted from
adrenal cortex also.
During pregnancy, large amount of progeste-
rone is secreted by the corpus luteum in the first
trimester. In the second trimester corpus luteum
degenerates. Placenta secretes large quantity of
progesterone in second and third trimesters.
Progesterone is a C
21
steroid. The plasma
level of progesterone in females at normal
reproductive age varies during different phases
of menstrual cycle. In follicular phase, it is about
0.9 ng/mL (Fig. 55-4). In normal adult male
progesterone level is 0.3 ng/mL.
Functions of Progesterone
Progesterone is concerned mainly with the final
preparation of the uterus for pregnancy and the
breasts for lactation. The effects of progesterone
are:
1. Effect on fallopian tubes
Progesterone promotes secretory activities of
mucosal lining of the fallopian tubes. The secre-
tions of fallopian tubes are necessary for nutrition
of the fertilized ovum while it is in fallopian tube
before implantation.
2. Effect on uterus
Progesterone promotes secretory activities of
uterine endometrium during the secretory phase
of the menstrual cycle. Thus, uterus is prepared
for implantation of the fertilized ovum.
Progesterone:
i. Increases the thickness of the endo-
metrium by increasing the number and
size of the cells
ii. Increases the size of uterine glands and
these glands become more tortuous
iii. Increases secretory activities of epithelial
cells of uterine glands
iv. Increases the deposition of lipid and gly-
cogen in the stromal cells of endometrium
v. Increases the blood supply to endo-
metrium
vi. Decreases the frequency of uterine con-
tractions during pregnancy. Because of
this, the expulsion of the implanted ovum
is prevented.
3. Effect on cervix
Progesterone increases thickness of cervical
mucosa and thereby inhibits transport of sperm
into uterus. This effect is utilized in the contra-
ceptive actions of mini pills.
4. Effect on mammary glands
Progesterone promotes the development of the
lobules and alveoli of the mammary glands by
proliferating and enlarging the alveolar cells. It
also makes the breasts secretory in nature. It
FIGURE 54-5: Regulation of estrogen secretion.
Red dashed lines indicate inhibition

Chapter 54 Female Reproductive System
349
makes the breasts to swell by increasing the
secretory activity and fluid accumulation in the
subcutaneous tissue.
5. Effect on hypothalamus
Progesterone inhibits the release of LH from
hypothalamus through feedback effect. This
effect is utilized for its contraceptive action.
6. Thermogenic effect
Progesterone increases the body temperature
after ovulation. The mechanism of thermogenic
action is not known. It is suggested that pro-
gesterone increases the body temperature by
acting on hypothalamic centers for temperature
regulation.
7. Effect on respiration
During luteal phase of menstrual cycle and during
pregnancy, progesterone increases the venti-
lation via respiratory center. This results in
decreased PCO
2
in the alveoli.
8. Effect on electrolyte balance
Progesterone increases reabsorption of sodium
and water from the renal tubules. However, in
large doses, it is believed to cause excretion of
sodium and water. This may be due to an indirect
effect, i.e. progesterone combines with the same
receptors, which bind with aldosterone. So, the
action of aldosterone is blocked leading to
excretion of sodium and water.
Mode of Action of Progesterone
Like estrogen, progesterone also acts through
genes.
Regulation of Secretion of Progesterone
LH from anterior pituitary activates the corpus
luteum to secrete progesterone. Secretion of LH
is influenced by the gonadotropic releasing hor-
mone secreted in hypothalamus. Progesterone
inhibits release of LH from anterior pituitary by
negative feedback.
CLIMACTERIC AND MENOPAUSE
Climacteric is the period in old age when repro-
ductive system undergoes changes due to the
decreased secretion of sex hormones estrogen
and progesterone. It occurs at the age of 45 to
55. In females, climacteric is accompanied by
meno-pause.
Menopause is defined as the period charac-
terized by the permanent cessation of mens-
truation. Normally, it occurs at the age of 45 to
55 years.
In some women, the menstruation stops
suddenly. In others, the menstrual flow dec-
reases gradually during every cycle and finally
it stops. Sometimes irregular menstruation
occurs with lengthening or shortening of the
period with less or more flow.
Early menopause may occur because of
surgical removal of ovaries (ovariectomy) or
uterus (hysterectomy) as a part of treatment for
abnormal menstruation. Usually, females with
short menstrual cycle attain menopause earlier
than the females with longer cycle. Cigarette
smoking causes earlier onset of menopause.
CHANGES DURING MENOPAUSE –
POSTMENOPAUSAL SYNDROME
Postmenopausal syndrome is the group of
symptoms that appear in women immediately
after menopause. It is characterized by certain
physical, physiological and psychological chan-
ges. The symptoms start appearing soon after
the ovaries stop functioning.
The cause for the symptoms is the lack of
estrogen and progesterone. The symptoms may
persist till the body gets acclimatized to the
absence of estrogen and progesterone.
The symptoms do not appear in all women.
Some women develop mild symptoms and some
women develop severe symptoms. The symp-
toms last for few months to few years.
Most of the women manage it very well. But,
about 15% of the women need treatment. In
many cases, psychotherapy works very well. If
it fails, hormone replacement therapy is given.
INTRODUCTION
OVARIAN CHANGES DURING MENSTRUAL CYCLE
UTERINE CHANGES DURING MENSTRUAL CYCLE
CHANGES IN CERVIX DURING MENSTRUAL CYCLE
CHANGES IN VAGINA DURING MENSTRUAL CYCLE
REGULATION OF MENSTRUAL CYCLE
APPLIED PHYSIOLOGY – ABNORMAL MENSTRUATION
Menstrual Cycle
55
INTRODUCTION
DEFINITION
The cyclic events that take place in a rhythmic
fashion during the reproductive period of a
woman’s life is called menstrual cycle. The
menstrual cycle starts at the age of 12 to
15 years, which marks the onset of puberty. The
commencement of menstrual cycle is called
menarche. The menstrual cycle ceases at the
age of 45 to 50 years. The permanent cessation
of menstrual cycle in old age is called meno-
pause.
DURATION OF MENSTRUAL CYCLE
The duration of menstrual cycle is usually 28
days. But, under physiological conditions, it may
vary between 20 and 40 days.
CHANGES DURING MENSTRUAL CYCLE
During each menstrual cycle, series of changes
occur in ovary and accessory sex organs. All
these changes which take place simultaneously
are divided into 4 groups:
I. Ovarian changes
II. Uterine changes
III. Vaginal changes
IV. Changes in cervix.
OVARIAN CHANGES DURING
MENSTRUAL CYCLE
The changes in the ovary during each menstrual
cycle occur in two phases.
A. Follicular phase
B. Luteal phase.
FOLLICULAR PHASE
Follicular phase extends from the 5th day of the
cycle until the time of ovulation, which takes
place on 14th day. During this phase develop-
ment of ovarian follicles and maturation of ovum
take place.
Ovarian Follicles
Ovarian follicles are present the stroma of cortex.
Each follicle consists of the ovum surrounded
by epithelial cells namely granulosa cells. The

Chapter 55 Menstrual Cycle
351
follicles gradually grow into a matured follicle
through various stages:
1. Primordial Follicle
At the time of puberty, both the ovaries contain
about 4,00,000 primordial follicles. The diameter
of the primordial follicle is about 15 to 20 μ and
that of ovum is about 10 μ. Each primordial follicle
has an ovum which is incompletely surrounded
by the granulosa cells (Fig. 54-3). These cells
are believed to provide nutrition to the ovum
during childhood.
Granulosa cells also secrete the oocyte
maturation inhibiting factor which keeps the
ovum in the immature stage. All the ova present
in the ovaries are formed before birth. No new
ovum is developed after birth.
At the onset of puberty, under the influence
of FSH and LH the primordial follicles start
growing through various stages.
2. Primary Follicle
The primordial follicle becomes the primary
follicle, when the ovum is completely surroun-
ded by the granulosa cells. During this stage the
follicle and the ovum inside the follicle increase
in size. The diameter of the follicle increases to
30 to 40 μ and that of ovum increases to about
20 μ. The follicle is not covered by a definite
connective tissue capsule.
The characteristic changes taking place
during the development of primary follicles are:
i. Proliferation of granulosa cells and
increase in size of the follicle
ii. Increase in size of ovum
iii. Onset of formation of connective tissue
capsule around the follicle.
The primary follicle develops into vesicular
follicle.
3. Vesicular Follicle
Under the influence of FSH, about 6-12 primary
follicles start growing and develop into the
vesicular follicles. The changes, which take place
during development of vesicular follicles are:
a. Changes in granulosa cells
b. Changes in ovum
c. Formation of capsule.
a. Changes in granulosa cells
i. First, the proliferation of granulosa cells
occurs
ii. A cavity called follicular cavity or antrum
is formed in between the granulosa cells
iii. Antrum is filled with a serous fluid called
the liquor folliculi
iv. Ovum is pushed to one side and it is
surrounded by granulosa cells which
forms the germ hill or cumulus oophorus
v. Granulosa cells which line the antrum
form membrana granulosa
vi. Cells of germ hill become columnar and
form corona radiata.
b. Changes in ovum
i. First, the ovum increases in size and its
diameter increases to 100 to 150 μ
ii. Nucleus becomes larger and vesicular
iii. Cytoplasm becomes granular
iv. Thick membrane is formed around the
ovum which is called zona pellucida
v. A narrow cleft called perivitelline space
appears between ovum and zona pellu-
cida.
c. Formation of capsule
The spindle cells from the stroma of ovarian
cortex are modified and form a covering sheath
around the follicle. The covering sheath is known
as follicular sheath or theca folliculi.
The theca folliculi divides into two layers:
i. Theca interna: It is the inner vascular
layer with loose connective tissue and
epithelial cells with lipid granules. The
epithelial cells become secretory in nature
and start secreting the female sex
hormones, especially estrogen which is
released into the fluid of antrum.
ii. Theca externa: It is the outer layer of the
follicular capsule and consists of thickly
packed fibers and spindle shaped cells.
After about 7th day of menstrual cycle, one
of the vesicular follicles outgrows the others
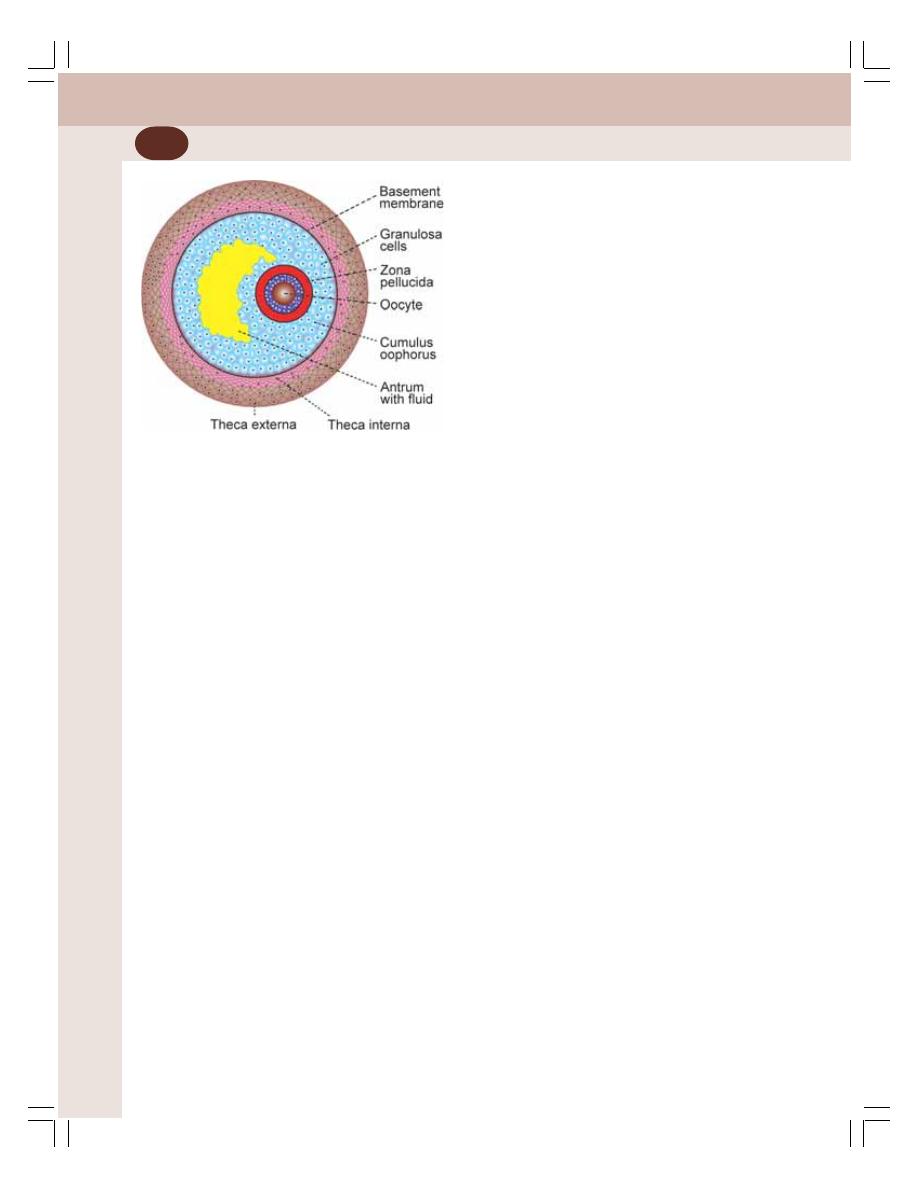
Reproductive System
352
and becomes the dominant follicle. It develops
further to form graafian follicle. The other
vesicular follicles degenerate by means of
apoptosis.
4. Graafian Follicle
Graafian follicle is the matured ovarian follicle
with maturing ovum. It is named after the Dutch
physician and anatomist Regnier De Graff
(Fig. 55-1). Many changes take place during the
development of graafian follicle:
i. The size of the follicle increases to about
10 to 12 mm
ii. At one point, the follicle encroaches upon
tunica albuginea and protrudes upon the
surface of the ovary. This protrusion is
called stigma. At the stigma, the tunica
albuginea becomes thin
iii. Follicular cavity becomes larger and
distended with fluid
iv. Ovum attains maximum size
v. Zona pellucida becomes thick
vi. The corona radiata becomes prominent
vii. Small spaces filled with fluid appear
between the cells of germ hill outside the
corona radiata. These spaces weaken the
attachment of the ovum to the follicular
wall
viii. Theca interna becomes prominent. Its
thickness becomes double with formation
of rich capillary network
ix. On 14th day of menstrual cycle, the graa-
fian follicle is ready for the process of
ovulation.
OVULATION
Ovulation is the process in which there is rupture
of graafian follicle with consequent discharge of
ovum into abdominal cavity. This occurs after the
maturity of follicle. It is influenced by LH. The
ovulation occurs usually on 14th day of menstrual
cycle in a normal cycle of 28 days. The ovum
enters the fallopian tube.
Process of Ovulation
Ovulation is a gradual process that occurs in
different stages:
1. Rupture of graafian follicles takes place at
the stigma
2. Follicular fluid oozes out
3. Germ hillock is freed from wall
4. Ovum is expelled out into the abdominal
cavity along with some amount of fluid and
granulosa cells
5. From abdominal cavity, the ovum enters the
fallopian tube through the fimbriated end.
The ovum becomes haploid before or during
ovulation by the formation of polar bodies. After
ovulation, the ovum is viable only for 48 hours.
So fertilization should take place during this
period.
After fertilization, the ovum is called zygote.
From the fallopian tube, the zygote reaches the
uterus on 3rd day after ovulation. And, the implan-
tation of the zygote in the uterine wall occurs on
6th or 7th day.
If fertilization does not occur, the ovum de-
generates. Generally, only one ovum is released
from one of the ovaries.
Determination of Ovulation Time
Different methods are available to determine the
ovulation time:
1. Determination of basal body temperature:
There is a slight fall in the basal temperature
just prior to ovulation. And, the temperature
increases after ovulation. The alteration in the
temperature is very mild and it is about ± 0.3
to 0.5°C.
FIGURE 55-1:
Graafian follicle
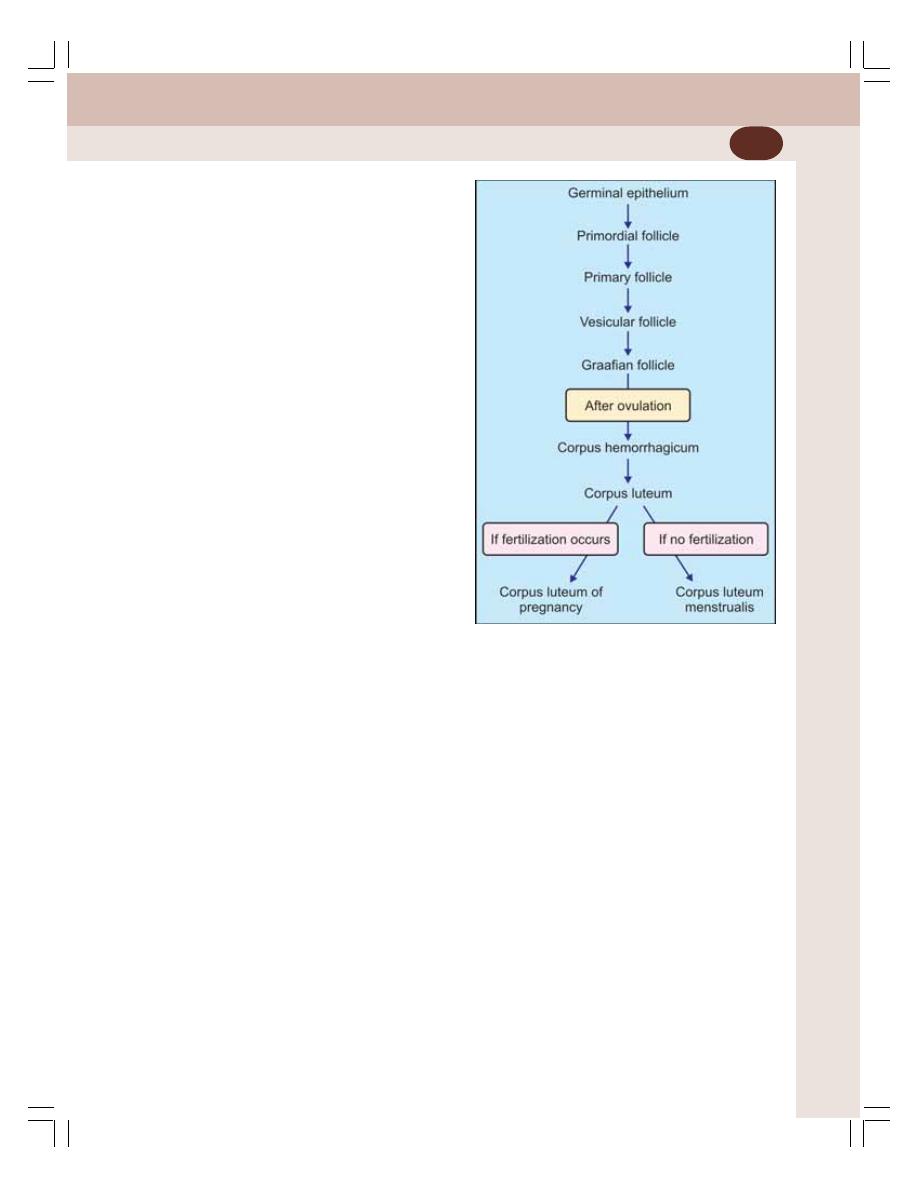
Chapter 55 Menstrual Cycle
353
2. Determination of hormonal excretion in
urine
: At the time of ovulation, there is an
increase in the urinary excretion of metabolic
end products of estrogen and progesterone.
3. Determination of hormonal level in plasma:
Plasma level of FSH, LH, estrogen and pro-
gesterone is altered at the time of ovulation
and after ovulation.
4. Ultrasound scanning: Process of ovulation is
observed by ultrasound scanning.
5. Cervical mucus pattern: When the cervical
mucus spread on a slide is examined under
microscope, it shows a fern pattern. This
pattern disappears after ovulation.
Significance of Determining Ovulation Time
Family planning by rhythm method may be well
adopted by determination of ovulation time
(Chapter 57).
LUTEAL PHASE
This phase extends between 15th and 28th day
of menstrual cycle. During this phase corpus
luteum is developed and hence this phase is
called luteal phase (Fig. 55-2).
Corpus Luteum
Corpus luteum is a glandular yellow body
developed from the ruptured graafian follicle
after the release of ovum. It is also called yellow
body.
Development of Corpus Luteum
Soon after the rupture of graafian follicle and
release of ovum, the follicle is filled with blood.
Now the follicle is called corpus hemorrhagicum.
The blood clots slowly. The corpus hemorr-
hagicum is transformed into a corpus luteum.
In the corpus luteum, the granulosa cells and
theca interna cells are transformed into lutein
cells namely granulosa lutein cells and theca
lutein cells by accumulation of fine lipid
granules and the yellowish pigment granules.
The yellowish pigment granules give the
characteristic yellow color to corpus luteum.
Functions of Corpus Luteum
1. Secretion of hormones
The corpus luteum acts as a temporary endo-
crine gland. It secretes large quantity of proges-
terone and small amount of estrogen. LH
influences the secretion of these two hormones.
2. Maintenance of pregnancy
If pregnancy occurs, corpus luteum maintains the
pregnancy for about three months of pregnancy
till placenta starts secreting estrogen and
progesterone. Abortion occurs if corpus luteum
becomes inactive or removed before third month
of pregnancy, i.e. before placenta starts secreting
the hormones.
Fate of Corpus Luteum
Fate of corpus luteum depends upon whether
the ovum is fertilized or not.
FIGURE 55-2:
Ovarian follicles
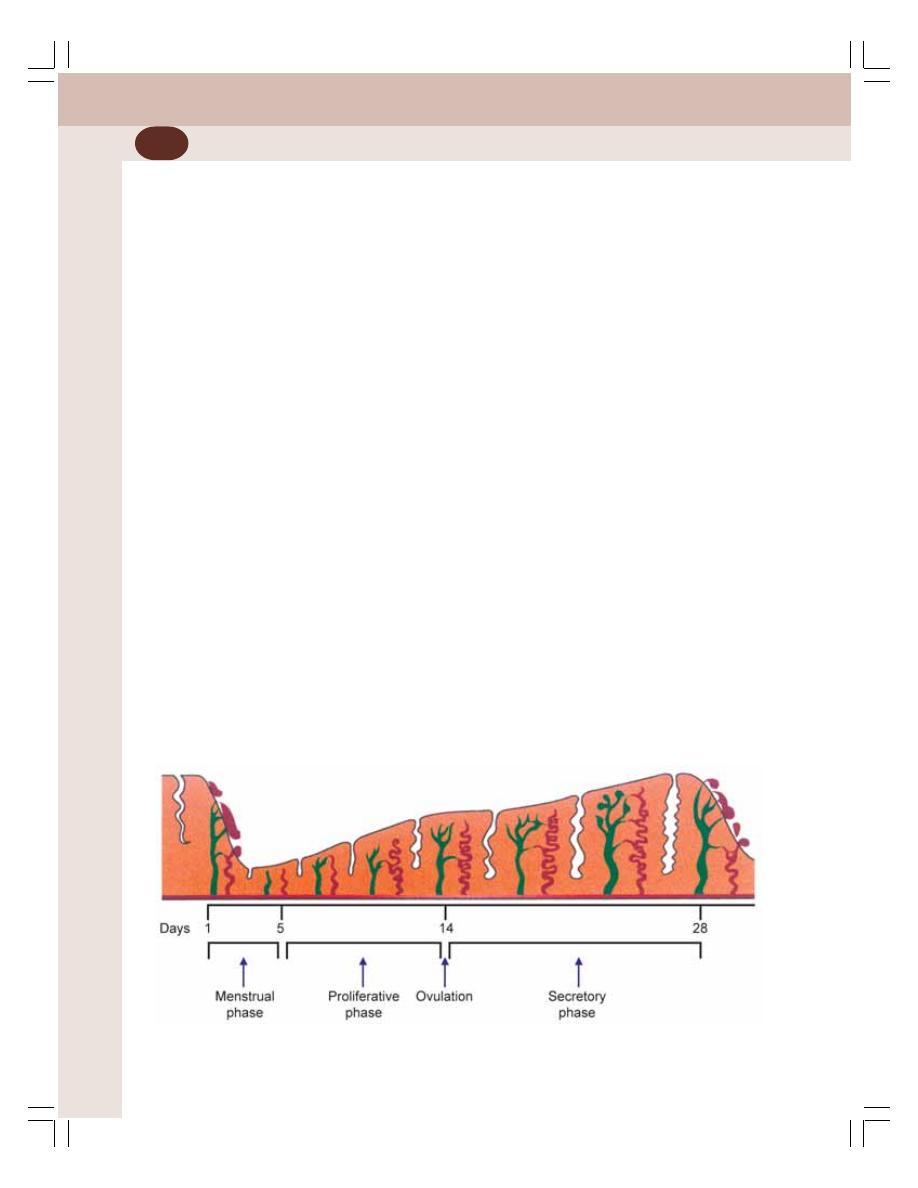
Reproductive System
354
1. If the ovum is not fertilized
If fertilization does not take place, the corpus
luteum reaches the maximum size about one
week after ovulation. During this period, it se-
cretes large quantity of progesterone with small
quantity of estrogen. Then, it degenerates into
the corpus luteum menstrualis. The cells
decrease in size and the corpus luteum becomes
smaller and involuted. Afterwards, the corpus
luteum menstrualis is transformed into a whitish
scar called corpus albicans. The process by
which corpus luteum undergoes regression is
called luteolysis.
2. If ovum is fertilized
If ovum is fertilized and pregnancy occurs, the
corpus luteum persists and increases in size. It
attains a diameter of 20 to 30 mm and it is trans-
formed into corpus luteum graviditatis (verum)
or corpus luteum of pregnancy. It remains in the
ovary for 3 to 4 months. During this period, it
secretes large amount of progesterone with small
quantity of estrogen, which are essential for the
maintenance of pregnancy. After 3 to 4 months,
placenta starts secreting these hormones and
corpus luteum degenerates.
UTERINE CHANGES DURING
MENSTRUAL CYCLE
During each menstrual cycle, along with ovarian
changes, uterine changes also occur simul-
taneously. The changes in uterus take place in
three phases:
A. Menstrual phase
B. Proliferative phase
C. Secretory phase.
MENSTRUAL PHASE
After ovulation, if pregnancy does not occur, the
thickened endometrium is shed or desquamated.
This desquamated endometrium is expelled out
through vagina along with some blood and tissue
fluid. The process of shedding and exit of uterine
lining along with blood and fluid is called
menstruation or menstrual bleeding. It lasts for
about 4 to 5 days (Fig. 55-3). This period is called
menstrual phase or menstrual period.
The day when bleeding starts is considered
as the first day of the menstrual cycle. Two days
before onset of bleeding, that is on 26th or 27th
day of the previous cycle, there is sudden reduc-
tion in the release of estrogen and progesterone
from ovary. Decreased level of these two hor-
mones is responsible for menstruation.
Changes in Endometrium during
Menstrual Phase
1. Lack of estrogen and progesterone cau-
ses sudden involution of endometrium and
reduction in the thickness of endometrium
up to 65% of original thickness
FIGURE 55-3:
Uterine changes during menstrual cycle

Chapter 55 Menstrual Cycle
355
2. During the next 24 hours, the tortuous
blood vessels in the endometrium undergo
severe constriction.
3. The vasoconstriction leads to hypoxia
which results in necrosis of the endo-
metrium, rupture of blood vessels and
oozing of blood
5. The outer layer of the necrotic endo-
metrium is separated and passes out
along with blood
7. This process is continued for about
24 to 36 hours
8. Within 48 hours after the reduction in the
secretion of estrogen and progesterone,
the superficial layers of endometrium are
completely desquamated
9. The desquamated tissues and the blood
in the endometrial cavity initiate the con-
traction of uterus
10. Uterine contractions expel the blood along
with desquamated uterine tissues to the
exterior through vagina.
During normal menstruation, about 35 mL of
blood along with 35 mL of serous fluid is
expelled. The blood clots as soon as it oozes
into the uterine cavity. The fibrinolysin causes
lysis of clot in uterine cavity itself so that, the
expelled menstrual fluid does not clot. However,
in the pathological conditions involving uterus,
the lysis of blood clot does not occur. So the
menstrual fluid comes out with blood clot.
Menstruation stops between 3rd and 7th day
of menstrual cycle. At the end of menstrual
phase, the thickness of endometrium is only
about 1 mm. This is followed by proliferative
phase.
PROLIFERATIVE PHASE
Proliferative phase extends usually from 5 to
14th day of menstruation, i.e. between the day
when menstruation stops and the day of
ovulation. It corresponds to the follicular phase
of ovarian cycle.
At the end of menstrual phase, only a thin
layer (1 mm) of endometrium remains as, most
of the endometrial stroma is desquamated.
Changes in Endometrium during
Proliferative Phase
1. The endometrial cells proliferate rapidly
2. The epithelium reappears on the surface of
endometrium within the first 4 to 7 days
3. The uterine glands start developing within the
endometrial stroma
4. Blood vessels also appear in the stroma
5. The proliferation of endometrial cells occurs
continuously so that the endometrium rea-
ches the thickness of 3 to 4 mm at the end
of proliferative phase.
All these uterine changes during proliferative
phase occur because of the influence of estrogen
released from ovary. On 14th day, ovulation
occurs under the influence of LH. This is followed
by secretory phase.
SECRETORY PHASE
Secretory phase extends between 15th and 28th
day of the menstrual cycle, i.e. between the day
of ovulation and the day when menstruation of
next cycle commences.
After ovulation, corpus luteum is developed
in the ovary. It secretes a large quantity of pro-
gesterone along with a small amount of
estrogen. Estrogen causes further proliferation
of cells in uterus, so that, the endometrium
becomes more thick. Progesterone causes
further enlargement of endometrial stroma and
further growth of glands.
Under the influence of progesterone, the
endometrial glands commence their secretory
function. Many changes occur in the endo-
metrium before commencing the secretory
function.
Changes in Endometrium during
Secretory Phase
1. The glands of the endometrium become more
tortuous.
2. The cytoplasm of stromal cells increases
because of the deposition of glycogen and
lipids
3. Many new blood vessels appear within endo-
metrial stroma and blood supply to endo-
metrium increases.

Reproductive System
356
Actually, secretory phase is the preparatory
period during which, the uterus is prepared for
implantation of ovum. At the end of secretory
phase, the thickness of endometrium is 5 to
6 mm. All these uterine changes during secretory
phase occur due to the influence of estrogen and
progesterone. Estrogen is responsible for repair
of damaged endometrium and growth of the
glands. Progesterone is responsible for further
growth of these structures and secretory
activities in the endometrium.
If a fertilized ovum is implanted during this
phase and, if the implanted ovum starts deve-
loping into a fetus, further changes occur in the
uterus for the survival of the developing fetus.
If the implanted ovum is unfertilized or if
pregnancy does not occur, menstruation occurs
after this phase and a new cycle begins.
CHANGES IN CERVIX DURING
MENSTRUAL CYCLE
The mucous membrane of cervix also shows
cyclic changes during different phases of men-
strual cycle.
Proliferative Phase
Under the influence of estrogen, during proli-
ferative phase, the mucous membrane of cervix
becomes thinner and more alkaline. It helps in
the survival and motility of spermatozoa.
Secretory Phase
Because of actions of progesterone during
secretory phase, the mucus membrane of cervix
becomes more thick and adhesive.
CHANGES IN VAGINA DURING
MENSTRUAL CYCLE
Proliferative Phase
The epithelial cells of vagina are cornified.
Estrogen released from ovary is responsible for
the cornification of vaginal epithelial cells.
Secretory Phase
Vaginal epithelium proliferates due to the actions
of progesterone. The vaginal epithelium is
infiltrated with leukocytes. These two changes
increase the resistance for infection.
REGULATION OF MENSTRUAL CYCLE
Menstrual cycle is regulated hormones of hypo-
thalamo-pituitary-ovarian axis.
HORMONES INVOLVED IN REGULATION
Hormones involved in regulation of menstrual
cycle are:
1. Hypothalamic hormone – GnRH
2. Anterior pituitary hormones – FSH and LH
3. Ovarian hormones – Estrogen and proges-
terone.
Hypothalamic Hormone
GnRH from hypothalamus triggers the cyclic
changes during menstrual cycle by stimulating
secretion of FSH and LH from anterior pituitary.
Anterior Pituitary Hormones
FSH and LH secreted from anterior pituitary
modulate the ovarian and uterine changes by
acting directly and/or indirectly via ovarian
hormones. FSH stimulates the recruitment and
growth of immature ovarian follicles. LH triggers
ovulation and sustains corpus luteum.
Secretion of FSH and LH is under the influ-
ence of GnRH.
Ovarian Hormones
Estrogen and progesterone which are secreted
by follicle and corpus luteum show many activities
during menstrual cycle. Ovarian follicle secretes
large quantity of estrogen and corpus luteum
secretes large quantity of progesterone.
Estrogen secretion reaches the peak twice
in each cycle; once during follicular phase just
before ovulation and another one during luteal
phase (Fig. 55-4). On the other hand proges-
terone is virtually absent during follicular phase
till prior to ovulation. But it plays a critical role
during luteal phase.
Estrogen is responsible for the growth of
follicles. Both the steroids act together to produce
the changes in uterus, cervix and vagina.
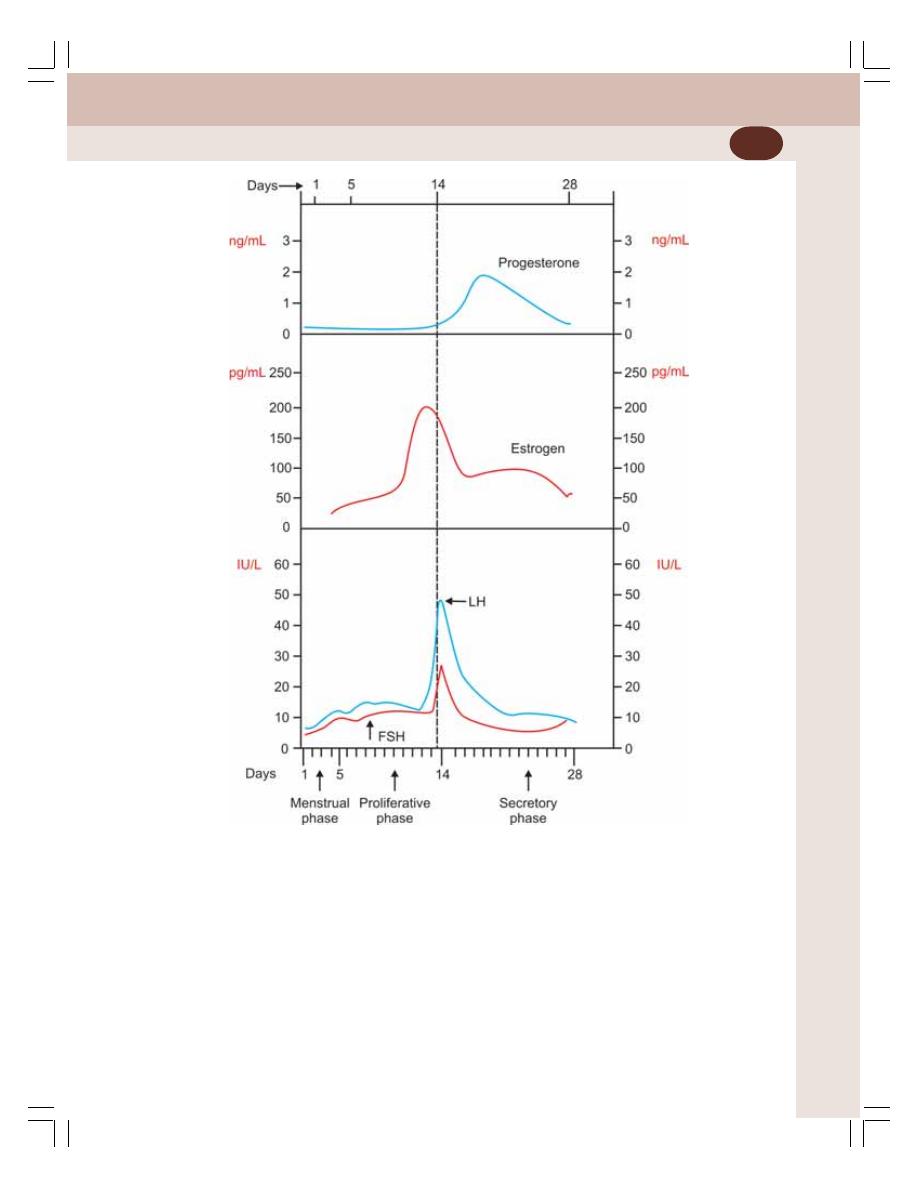
Chapter 55 Menstrual Cycle
357
FIGURE 55-4:
Hormonal level during menstrual cycle
Both the ovarian hormones are under the
influence of GnRH which acts via FSH and LH.
In addition, the secretion of GnRH, FSH and LH
is regulated by ovarian hormones.
APPLIED PHYSIOLOGY –
ABNORMAL MENSTRUATION
1. Amenorrhea: Absence of menstruation
2. Hypomenorrhea: Decreased menstrual blee-
ding
3. Menorrhagia: Excess menstrual bleeding
4. Oligomenorrhea: Decreased frequency of
menstrual bleeding
5. Polymenorrhea: Increased frequency of men-
struation
6. Dysmenorrhea: Menstruation with pain
7. Metrorrhagia: Uterine bleeding in between
menstruations.
INTRODUCTION
FERTILIZATION OF THE OVUM
SEX CHROMOSOMES AND SEX DETERMINATION
IMPLANTATION AND DEVELOPMENT OF EMBRYO
PLACENTA
GESTATION PERIOD
PARTURITION
PREGNANCY TESTS
DEVELOPMENT OF MAMMARY GLANDS
LACTATION
Pregnancy, Mammary
Glands and Lactation
56
INTRODUCTION
Ovum is released from graafian follicle of ovary
into the abdominal cavity at the time of ovulation.
From abdominal cavity the ovum enters one of
the fallopian tubes via fimbriated end.
FERTILIZATION OF THE OVUM
Fertilization refers to fusion (union) of male and
female gamates (sperm and ovum) to form a new
offspring.
Ovum is released into abdominal cavity during
ovulation. If sexual intercourse occurs at this time
and semen is ejaculated in the vagina, the
sperms travel through the vagina and uterus to
reach the fallopian tube. Among 200 to 300
millions of sperms entering female genital tract,
only one succeeds in fertilizing the ovum.
During fertilization, the sperm enters the ovum
by penetrating granulosa cells present around
the ovum. It is facilitated by hyaluronidase and
proteolytic enzymes present in the acrosome of
sperm.
SEX CHROMOSOMES AND SEX
DETERMINATION
SEX CHROMOSOMES
All the dividing cells in the body have 23 pairs of
chromosomes. Among the 23 pairs, 22 pairs are
called somatic chromosomes or autosomes. The
remaining one pair of chromosomes is called sex
chromosomes. Sex chromosomes are X and Y
chromosomes.
SEX DETERMINATION
Sex chromosomes are responsible for sex deter-
mination. During fertilization of ovum, 23 chromo-
somes from ovum and 23 chromosomes from

Chapter 56 Pregnancy, Mammary Glands and Lactation
359
the sperm unite together to form the 23 pairs (46)
of chromosomes in the fertilized ovum. Now, sex
determination occurs. Ovum contains the X chro-
mosome. Sperm has either X chromosome or
Y chromosome. When the ovum is fertilized by
a sperm with X chromosome, the child will be
female with XX chromosome. And, if the ovum
is fertilized by a sperm with Y chromosome, the
sex of the child will be male with XY chromo-
some. So, the sex of the child depends upon the
male partner.
Role of testosterone in sex differentiation is
explained in Chapter 53.
IMPLANTATION AND
DEVELOPMENT OF EMBRYO
Implantation is the process by which the fertilized
ovum implants (fixes itself or gets attached) in
the endometrial lining of uterus. After the ferti-
lization, the ovum is known as zygote. The zygote
takes three to five days to reach the uterine cavity
from fallopian tube. While travelling through the
fallopian tube, the zygote receives its nutrition
from the secretions of fallopian tube.
After reaching the uterus, the developing
zygote remains freely in the uterine cavity for
two to four days before it is implanted. Just
before implantation, the zygote develops into
morula.
Already uterus is prepared by progesterone
secreted from the corpus luteum during
secretory phase of menstrual cycle. After
implantation, morula develops into embryo.
Placenta develops between morula and
endometrium.
PLACENTA
Placenta is a temporary membranous vascular
organ that develops in females during preg-
nancy. It is expelled after child birth. Placenta
forms a link between the fetus and mother. It is
considered as an anchor for the growing fetus.
It is not only the physical attachment between
the fetus and mother, but also forms the
physiological connection between the two.
Placenta is implanted in the wall of the uterus.
It is formed from both embryonic and maternal
tissues. So, it consists of two parts namely the
fetal part and the mother’s part. It is connected
to the fetus by umbilical cord which contains
blood vessels and connective tissue.
The delivery of fetus is followed by the
expulsion of placenta. After expulsion of the
placenta, the umbilical cord is cut. The site of
the attachment of placenta in the center of the
anterior abdomen of fetus is called naval or
umbilicus.
FUNCTIONS OF PLACENTA
1. Nutritive Function
The various nutritive substances, electrolytes and
hormones necessary for the development of
fetus diffuse from the mother’s blood into the fetal
blood through placenta.
2. Excretory Function
The metabolic end products and other waste
products from the fetal body are excreted into
the mother’s blood through placenta.
3. Respiratory Function
Fetal lungs are nonfunctioning and placenta
forms the respiratory organ for fetus. Oxygen
necessary for fetus is received by diffusion from
the maternal blood and, carbon dioxide from the
fetal blood diffuses into the mother’s blood
through placenta.
4. Endocrine Function
Hormones secreted by placenta are:
1. Human chorionic gonadotropin
2. Estrogen
3. Progesterone
4. Human chorionic somatomammotropin
5. Relaxin.
1. Human Chorionic Gonadotropin
Human chorionic gonadotropin (hCG) is a glyco-
protein.
Actions of hCG:
i. On corpus luteum: hCG is responsible for
the preservation and the secretory activity

Reproductive System
360
of corpus luteum. Progesterone and estro-
gen secreted by corpus luteum are essen-
tial for the maintenance of pregnancy.
Deficiency or absence of hCG during the
first two months of pregnancy leads to
termination of pregnancy (abortion),
because of involution of corpus luteum.
ii. On fetal testes: Action of hCG on fetal
testes is similar to that of LH in adults. It
stimulates the interstitial cells of Leydig
and causes secretion of testosterone
which is necessary for the development
of sex organs in male fetus.
2. Estrogen
Placental estrogen is similar to ovarian estrogen
in structure and function.
Actions of placental estrogen
i. On uterus: Causes enlargement of the
uterus so that, the growing fetus can be
accommodated
ii. On breasts: Responsible for the enlarge-
ment of the breasts and growth of the duct
system in the breasts
iii. On external genitalia: Causes enlarge-
ment of the female external genitalia
iv. On pelvis: Relaxes pelvic ligaments. It
facilitates the passage of the fetus through
the birth canal at the time of labor.
3. Progesterone
Placental progesterone is similar to ovarian pro-
gesterone in structure and function.
Actions of placental progesterone
i. On endometrium of uterus: Accelerates
the proliferation and development of deci-
dual cells in the endometrium of uterus.
The decidual cells are responsible for the
supply of nutrition to the embryo in the
early stage
ii. On the movements of uterus: Inhibits the
contraction of muscles in the pregnant
uterus. It is an important function of
progesterone as it prevents expulsion of
fetus during pregnancy
iii. On breasts: Causes enlargement of
breasts and growth of duct system of the
breasts. Progesterone is responsible for
further development and preparation of
mammary glands for lactation.
4. Human Chorionic Somatomammotropin
Human chorionic somatomammotropin (hCS) is
a protein hormone secreted from placenta. It is
often called placental lactogen. It acts like pro-
lactin and growth hormone secreted from pitui-
tary. So, it is believed to act on mammary glands
and to enhance the growth of fetus by influencing
the metabolic activities. It increases the amount
of glucose and lipids in the maternal blood which
are transferred to fetus.
Actions of hCS
i. On breasts: In experimental animals,
administration of hCS causes enlarge-
ment of mammary glands and induces
lactation. That is why, it is named as
mammotropin. However, the action of this
hormone on the breasts of pregnant
women is not known
ii. On protein metabolism: hCS acts like GH
on protein metabolism. It causes anabo-
lism of proteins and accumulation of pro-
teins in the fetal tissues. Thus, the growth
of fetus is enhanced
iii. On carbohydrate metabolism: It reduces
the peripheral utilization of glucose in the
mother leading to availability of large
quantity of glucose to the growing fetus
iv. On lipid metabolism: It mobilizes fat from
the adipose tissue of the mother. A large
amount of free fatty acid is made available
as the source of energy in the mother’s
body. It compensates the loss of glucose
from the mother’s blood to fetus.
5. Relaxin
Relaxin is a polypeptide which is secreted by
corpus luteum. It is also secreted in large quantity
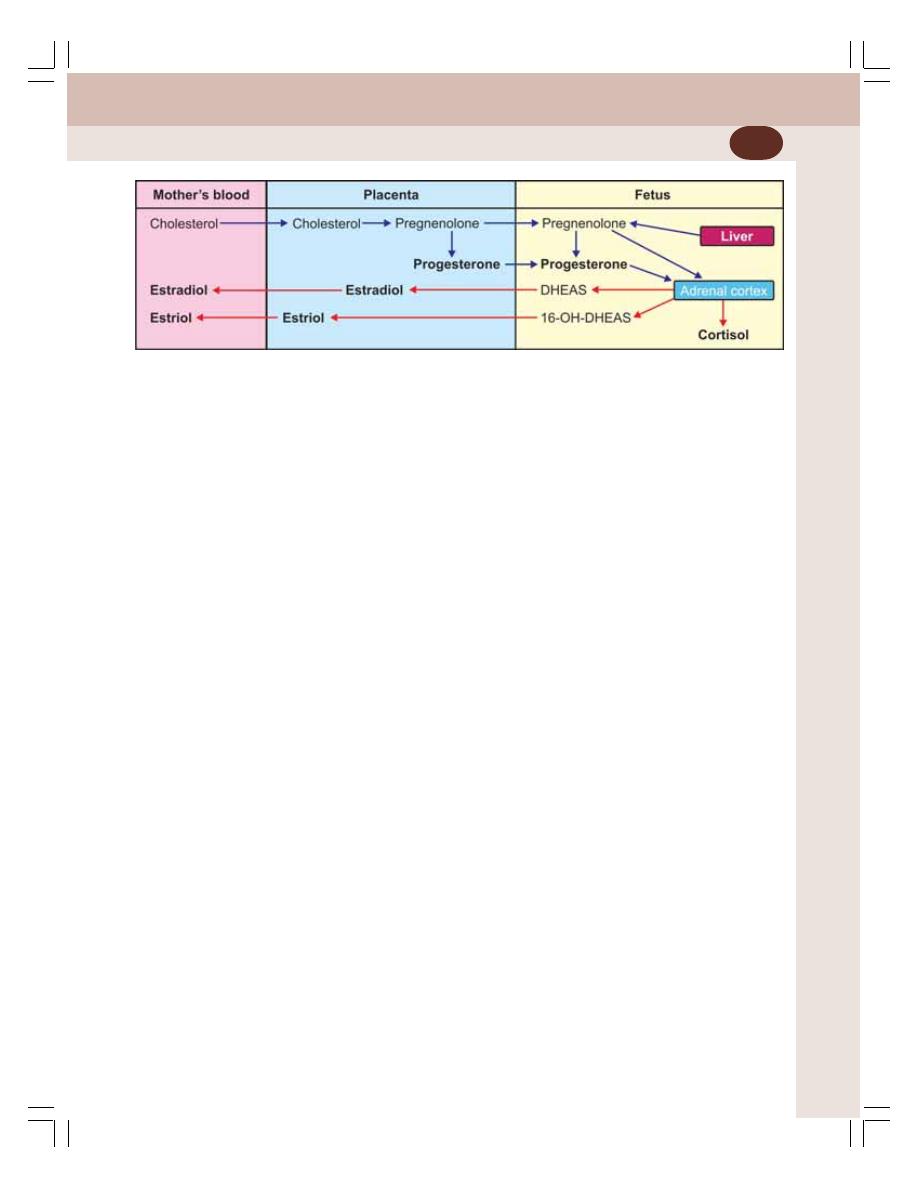
Chapter 56 Pregnancy, Mammary Glands and Lactation
361
by placenta and mammary glands at the time of
labor.
Fetoplacental Unit
Fetoplacental unit refers to the interaction bet-
ween fetus and placenta in the formation of
steroid hormones. The interaction between fetus
and placenta occurs because, some of the
enzymes involved in steroid synthesis present
in fetus are absent in placenta and, those
enzymes which are absent in fetus are present
in placenta.
Due to this interaction during synthesis of
steroid hormones, fetus and placenta are to-
gether called fetoplacental unit (Fig. 56-1).
GESTATION PERIOD
Gestation period refers to the pregnancy period.
The average gestation period is about 280 days
or 40 weeks from the date of last menstrual
period (LMP). Traditionally it is calculated as 10
lunar months. However, in terms of modern
calendar it is calculated as 9 months and 7 days.
If the menstrual cycle is normal 28 day cycle,
the fertilization of ovum by the sperm occurs on
14th day after last menstrual period. Thus, the
actual duration of human pregnancy is 280 – 14
= 266 days. If the pregnancy ends before 28th
week, it is referred as miscarriage.
PARTURITION
Parturition is the expulsion or delivery of the fetus
from the mother’s body. It occurs at the end of
pregnancy. The process by which the delivery
of fetus occurs is called labor. It involves various
actions, like contraction of uterus, dilatation of
cervix and opening of vaginal canal.
STAGES OF PARTURITION
Parturition occurs in three stages:
First Stage
First, the strong uterine contractions called labor
contractions commence. The labor contractions
arise from the fundus of uterus and move down-
wards so that the head of fetus is pushed against
the cervix. It results in dilatation of cervix and
opening of vaginal canal. This stage extends for
a variable period of time.
Second Stage
In this stage, the fetus is delivered out from uterus
through cervix and vaginal canal. This stage lasts
for about one hour.
Third Stage
During this stage, the placenta is detached from
the decidua and is expelled out from uterus. It
occurs within 10 to 15 minutes after the delivery
of the child.
PREGNANCY TESTS
Pregnancy test is the test used to detect or
confirm pregnancy. The basis of pregnancy tests
is to determine the presence of the human
chorionic gonadotropin (hCG) in the urine of
woman suspected for pregnancy. Both biological
FIGURE 56-1: Fetoplacental unit
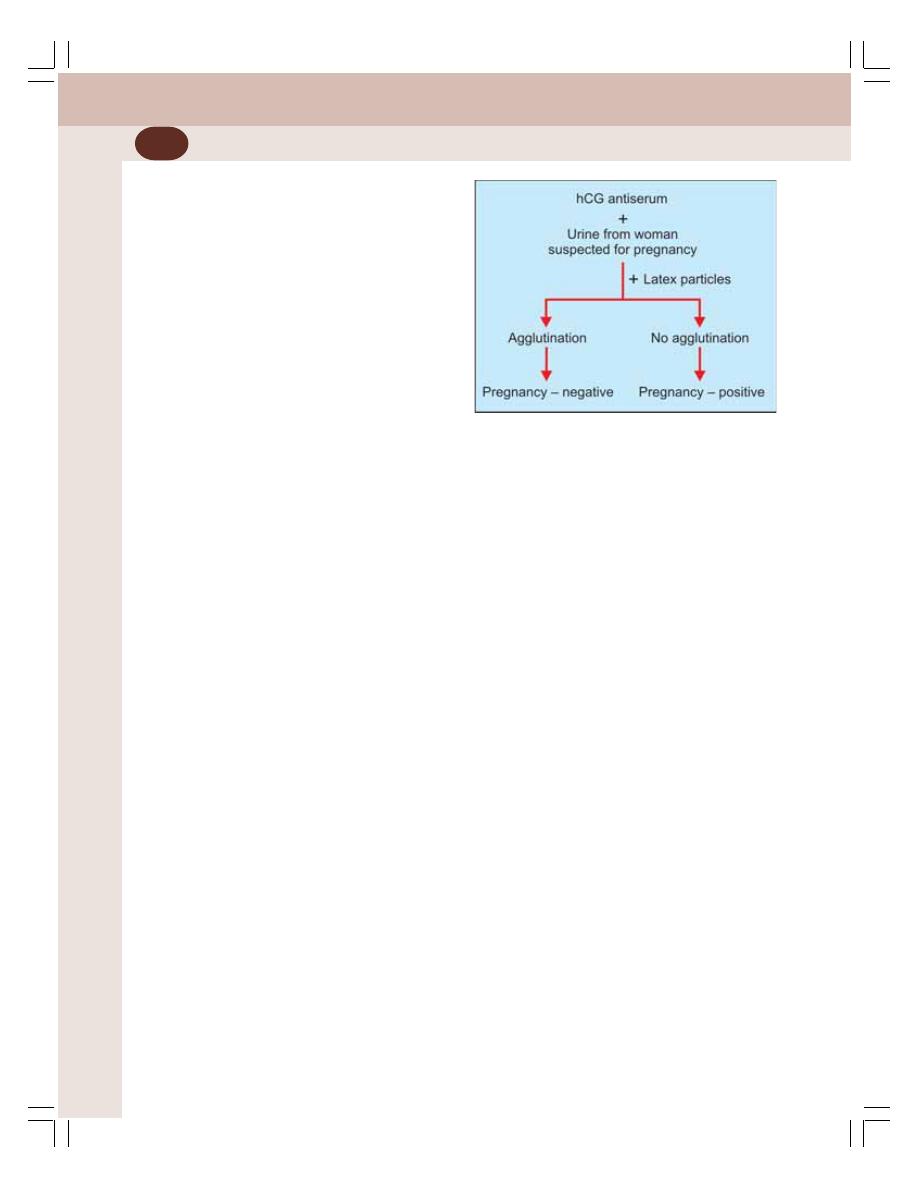
Reproductive System
362
and immunological tests are available to
determine the presence of hCG in the urine of
the pregnant woman. However, biological tests
for pregnancy are replaced by immunological
tests because of several disadvantages.
IMMUNOLOGICAL TESTS
Immunological tests are more accurate and the
result is obtained quickly within few minutes.
These tests are based on double antigen anti-
body reactions. The most commonly performed
immunological test is known as Gravindex test.
Principle
Principle is to determine the agglutination of
sheep RBCs coated with hCG. Latex particles
could also be used instead of sheep RBCs.
Requisites
1. Antiserum from rabbit
Urine from a pregnant woman is collected and
hCG is isolated. This hCG is injected into a rabbit.
The rabbit develops antibodies against hCG.
The antibodies are called hCG antibody or anti
hCG. The rabbit’s blood is obtained and serum
is separated. The serum containing hCG anti-
body is called rabbit antiserum or hCG anti-
serum. It is readily available in the market.
2. Red blood cells from sheep
The RBCs are obtained from sheep’s blood and
are coated with pure hCG obtained from urine
of the pregnant women. Nowadays, instead of
sheep’s RBCs, the rubberized synthetic particles
called the latex particles are used.
3. Urine
Fresh urine sample of the woman, who needs
to confirm pregnancy, is collected.
Procedure
1. One drop of hCG antiserum is taken on a
glass slide. One drop of urine from the woman
who wants to confirm pregnancy is added to
this and both are mixed well. If urine contains
hCG, all the antibodies of antiserum are used
up for agglutination of hCG molecules. The
agglutination of hCG molecules by the
antiserum is not visible because it is colorless
2. Now, one drop of latex particles is added to
this and mixed.
Observation and Result
If the urine contains hCG, it is agglutinated by
the antibodies of the antiserum and, all the
antibodies are fully used up. No free antibody is
available. Later when latex particles are added,
these particles are not agglutinated because
the free antibody is not available. Thus, the
absence of agglutination of latex particles
confirms pregnancy.
If the urine without hCG is mixed with anti-
serum, the antibodies are freely available. When
the latex particles are added, the antibodies
cause agglutination of these latex particles. The
agglutination of latex particles can be seen
clearly even with naked eye. Thus, the presence
of agglutination of latex particles indicates that,
the woman is not pregnant (Fig. 56-2).
DEVELOPMENT OF MAMMARY
GLANDS
AT BIRTH
At the time of birth, the mammary gland is rudi-
mentary and consists of only a tiny nipple and
few radiating ducts from it.
FIGURE 56-2: Immunological test for pregnancy

Chapter 56 Pregnancy, Mammary Glands and Lactation
363
AT CHILDHOOD
Till puberty, there is no difference in the structure
of mammary gland between male and female.
AT PUBERTY
At the time of puberty and afterwards there is a
vast change in the structure of female mammary
gland due to hormonal influence. The beginning
of changes in the mammary gland is called thel-
arche. It occurs at the time of puberty, just before
menarche (Chapter 55). At puberty, there is
growth of duct system and formation of glandular
tissue. Progressive enlargement occurs, which
is also due to the deposition of fat.
DURING PREGNANCY
During pregnancy, the mammary glands enlarge
to a great extent accompanied by marked chan-
ges in structure. During first half of pregnancy,
the duct system develops further with appea-
rance of many new alveoli. No milk is secreted
by the gland now.
During the second half, there is enormous
growth of glandular tissues and the develop-
ment is completed for the production of milk just
before the end of gestation period.
ROLE OF HORMONES IN GROWTH
OF MAMMARY GLANDS
Various hormones are involved in the
development and growth of breasts at different
stages.
1. Estrogen causes growth and branching of
duct system and accumulation of fat in
breasts
2. Progesterone stimulates the development
of glandular tissues and stroma of mammary
glands
3. Prolactin is necessary for milk secretion. It
also accelerates growth of mammary glands
during pregnancy by causing proliferation of
epithelial cells of alveoli
4. Placental hormones namely estrogen and
progesterone cause further development of
mammary glands during pregnancy by
stimulating the proliferation of ducts and
glandular cells.
5. Other hormones such as growth hormone,
thyroxine, cortisol and relaxin enhance the
overall growth and development of mammary
glands in all stages.
LACTATION
Lactation means synthesis, secretion and ejec-
tion of milk. It involves two processes:
A. Milk secretion
B. Milk ejection.
MILK SECRETION
Synthesis of milk by alveolar epithelium and its
passage through the duct system is called milk
secretion. This process occurs in two phases:
1. Initiation of milk secretion or lactogenesis
2. Maintenance of milk secretion or galacto-
poiesis.
1. Initiation of Milk Secretion or Lactogenesis
Although small amount of milk secretion occurs
at later months of pregnancy, a free flow of milk
occurs only after the delivery of the child. The
milk which is secreted initially before parturition
is called colostrum.
Colostrum is lemon yellow in color and it is
rich in protein (particularly globulins) and salts.
But its sugar content is low. It contains almost
all the components of milk except fat.
Role of hormones in lactogenesis
During pregnancy, particularly in later months,
large quantity of prolactin is secreted. But the
activity of this hormone is suppressed by estro-
gen and progesterone secreted by placenta.
Because of this, lactation is prevented during
pregnancy.
Immediately after the delivery of the baby and
expulsion of placenta, there is sudden loss of
estrogen and progesterone. Now, the prolactin
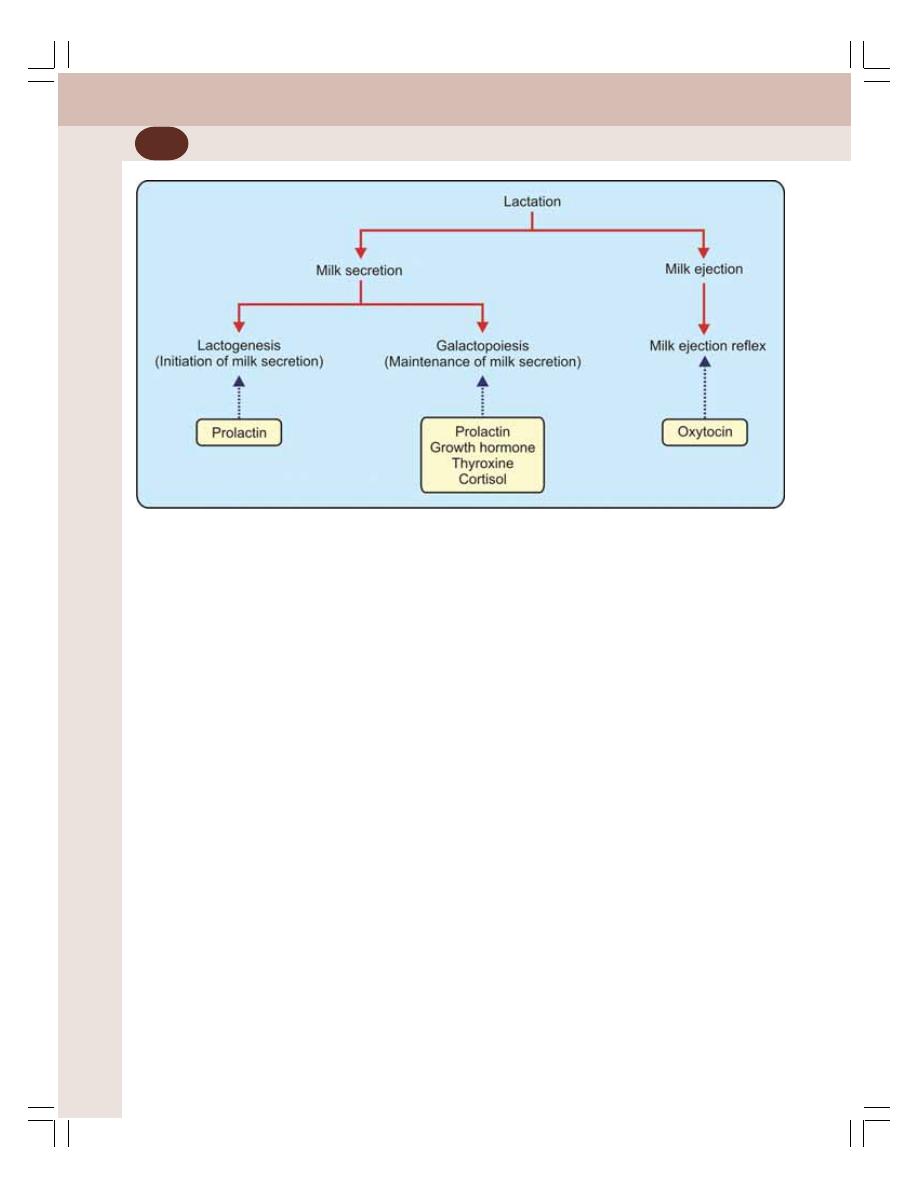
Reproductive System
364
is free to exert its action on breasts and to pro-
mote lactogenesis.
2. Maintenance of Milk Secretion or
Galactopoiesis
The galactopoiesis occurs up to 7 to 9 months
after delivery of child provided feeding the baby
with mother’s milk is continued till then. In fact,
the milk production is continued only if feeding
the baby is continued.
Role of hormones in galactopoiesis
Galactopoiesis depends upon prolactin secretion.
Other hormones like growth hormone, thyroxine
and cortisol are essential for continuous supply
FIGURE 56-3: Process of lactation and role of hormones
of glucose, amino acids, fatty acids, calcium and
other substances necessary for the milk pro-
duction (Fig. 56-3).
MILK EJECTION
Milk ejection is the discharge of milk from mam-
mary gland. It depends upon suckling exerted
by the baby and on contractile mechanism in
breast, which expels milk from alveoli into the
ducts.
Milk ejection is a reflex phenomenon. It is
called milk ejection reflex or milk let down reflex.
It is a neuroendocrine reflex.
Milk Ejection Reflex
It is explained in Chapter 45.
INTRODUCTION
RHYTHM METHOD (SAFE PERIOD)
MECHANICAL BARRIERS – PREVENTION OF ENTRY OF SPERM INTO
UTERUS
CHEMICAL METHODS
ORAL CONTRACEPTIVES (PILL METHOD)
INTRAUTERINE CONTRACEPTIVE DEVICE (IUCD)
MEDICAL TERMINATION OF PREGNANCY (MTP) – ABORTION
SURGICAL METHOD (STERILIZATION) – PERMANENT METHOD
Fertility Control
57
INTRODUCTION
Fertility control is the use of any method or device
to prevent pregnancy. It is also called birth control,
family planning, or contraception. The fertility
control techniques may be temporary or perma-
nent. Several methods are available for fertility
control.
RHYTHM METHOD (SAFE PERIOD)
Rhythm method of fertility control is based on
the time of ovulation. After ovulation, i.e. on the
14th day of menstrual cycle, the ovum is fertilized
during its passage through fallopian tubes. Its
viability is only for 2 days after ovulation, and
should be fertilized within this period.
The sperms survive only for about 24 to
48 hours after ejaculation in the female genital
tract. If sexual intercourse occurs during this
period, i.e. few days before and few days after
ovulation, there is chance of pregnancy. This
period is called the dangerous period. Pregnancy
can be avoided if there is no sexual intercourse
during this period. The prevention of pregnancy
by avoiding sexual mating during this period is
called rhythm method.
The periods, when pregnancy does not occur
are 4 to 5 days after menstrual bleeding and 5
to 6 days before the onset of next cycle. These
periods are together called safe period.
Advantages and Disadvantages
It is one of the most successful methods of
fertility control provided the woman knows the
exact day of ovulation. However, it is not a
successful method because of various reasons.
Basic knowledge about the menstrual cycle is
necessary to determine the day of ovulation. Self
restrain is essential to avoid sexual intercourse.
Because of the practical difficulties, this method
is not popular.

Reproductive System
366
MECHANICAL BARRIERS –
PREVENTION OF ENTRY OF SPERM
INTO UTERUS
Mechanical barriers are used to prevent the entry
of sperm into uterine cavity. These barriers are
called condoms. The male condom is a leak
proof sheath, made of latex. It covers the penis
and does not allow entrance of semen into the
female genital tract during coitus.
In females, the commonly used condom is
cervical cap or diaphragm. It covers the cervix
and prevents entry of sperm into uterus.
CHEMICAL METHODS
Chemical substances, which destroy the
sperms, are applied in female genital tract
before coitus. Destruction of sperms is called
spermicidal action. The spermicidal substances
are available in the form of foam tablet, jelly,
cream and paste.
ORAL CONTRACEPTIVES
(PILL METHOD)
The oral contraceptives are the drugs taken by
mouth (pills) to prevent pregnancy. These pills
prevent pregnancy by inhibiting maturation of
follicles and ovulation. This leads to alteration
of normal menstrual cycle. The menstrual cycle
becomes the anovulatory cycle.
This method of fertility control is called pill
method and pills are called contraceptive pills
or birth control pills. These pills contain synthetic
estrogen and progesterone.
Contraceptive pills are of three types:
1. Classical or combined pills
2. Sequential pills
3. Minipills or micropills.
1. CLASSICAL OR COMBINED PILLS
The classical or combined pills contain a mode-
rate dose of synthetic estrogen like ethinyl estra-
diol or mestranol and a mild dose of synthetic
progesterone like norethindrone or norgestral.
The pills are taken daily from 5 to 25th day
of menstrual cycle. The withdrawal of the pills
after 25th day causes menstrual bleeding. The
intake of pills is resumed again after 5th day of
the next cycle.
Mechanism of Action
During the continuous intake of the pills, there
is relatively large amount of estrogen and
progesterone in the blood. It suppresses the
release of gonadotropins, FSH and LH from
pituitary by means of feedback mechanism. The
lack of FSH and LH prevents the maturation of
follicle, and ovulation. In addition, progesterone
increases the thickness of mucosa in cervix,
which is not favorable for transport of sperm.
When the pills are withdrawn after 21 days the
menstrual flow starts.
2. SEQUENTIAL PILLS
Sequential pills contain a high dose of estrogen
along with moderate dose of progesterone.
These pills are taken in two courses.
i. Daily for 15 days from 5 to 20th day of
the menstrual cycle and then
ii. During the last 5 days, i.e. 23 to 28th day.
Sequential pills also prevent ovulation.
3. MINIPILLS OR MICROPILLS
The minipills contain a low dose of only proges-
terone and are taken throughout the menstrual
cycle. It prevents pregnancy without affecting
ovulation. The progesterone increases the thick-
ness of cervical mucosa, so that the transport
of sperms is inhibited. It also prevents implan-
tation of ovum.
DISADVANTAGES AND ADVERSE
EFFECTS OF ORAL CONTRACEPTIVES
About 40% of women who use contraceptive pills
may have minor transient side effects. However,
long-term use of oral contraceptives causes
some serious side effects.
LONG-TERM CONTRACEPTIVES
To avoid taking pills daily, the long-term contra-
ceptives are used. These contraceptives are in

Chapter 57 Fertility Control
367
the form of implants containing mainly proges-
terone. The implants which are inserted beneath
the skin release the drug slowly and prevent ferti-
lity for 4 to 5 years. Though it seems to be
effective, it may produce amenorrhea.
INTRAUTERINE CONTRACEPTIVE
DEVICE (IUCD) – PREVENTION OF
FERTILIZATION AND IMPLANTATION
OF OVUM
The fertilization and the implantation of ovum are
prevented by inserting some object made from
metal or plastic into uterine cavity. Such object
is called intrauterine contraceptive device (IUCD).
MECHANISM OF ACTION OF IUCD
IUCD prevents fertilization and implantation of
the ovum. The IUCD with copper content has
spermicidal action also. The IUCD which is
loaded with synthetic progesterone slowly relea-
ses progesterone. Progesterone causes thick-
ening of cervical mucus and prevents entry of
sperm into uterus.
The common intrauterine contraceptive de-
vice is Lippe’s loop, which is ‘S’ shaped and made
of plastic and copper T which is made up of
copper. It is inserted into the uterine cavity by
using some special applicator.
DISADVANTAGES OF IUCD
IUCD has some disadvantages. It has the ten-
dency to:
1. Cause heavy bleeding in some women
2. Promote infection
3. Come out of uterus accidentally.
MEDICAL TERMINATION OF
PREGNANCY (MTP) – ABORTION
The abortion is done during first few months of
pregnancy. This method is called medical termi-
nation of pregnancy (MTP). There are three ways
of doing MTP.
1. DILATATION AND CURETTAGE
(D AND C)
In this method, the cervix is dilated and the
implanted ovum or zygote is removed.
2. VACUUM ASPIRATION
The implanted ovum is removed by vacuum
aspiration method. This is done up to 12 weeks
of pregnancy.
3. ADMINISTRATION OF
PROSTAGLANDIN
Administration of prostaglandin like PGE
2
and
PGF
2
intravaginally increases uterine contrac-
tions resulting in abortion.
SURGICAL METHOD
(STERILIZATION) – PERMANENT
METHOD
Permanent sterility is obtained by surgical
methods. It is also called sterilization.
TUBECTOMY
In tubectomy, the fallopian tubes are cut and both
the cut ends are ligated. It prevents entry of ovum
into uterus. The operation is done through vaginal
orifice in the postpartum period. During other
periods, it is done by abdominal incision. Tubec-
tomy is done quickly (in few minutes) by using a
laparoscope.
Though tubectomy causes permanent sterility,
if necessary recanalization of fallopian tube can
be done using plastic tube by another surgical
procedure.
VASECTOMY
In vasectomy, the vas deferens is cut and the
cut ends are ligated. So the sperms cannot enter
the ejaculatory duct and the semen is devoid of
sperms. It is done by surgical procedure with
local anesthesia. If necessary, the recanali-
zation of vas deferens can be done with plastic
tube.

LONG QUESTIONS
1. Describe the functions of testis and
regulation of testicular functions.
2. Describe the actions and regulation of
secretion of testosterone.
3. What are the female sex hormones?
Explain their actions.
4. What is menstrual cycle? Explain the
ovarian changes taking place during
menstrual cycle.
5. Describe the uterine changes during men-
strual cycle.
SHORT QUESTIONS
1. Spermatogenesis.
2. Sertoli cells.
3. Testosterone.
4. Cryptorchidism.
5. Secondary sexual characters in males.
6. Semen.
7. Effects of removal of testes.
QUESTIONS IN REPRODUCTIVE SYSTEM
8. Estrogen.
9. Progesterone.
10. Follicle stimulating hormone.
11. Luteinizing hormone.
12. Gonadotropins.
13. Secondary sexual characters in females.
14. Ovarian follicles.
15. Ovulation.
16. Corpus luteum.
17. Functions of placenta.
18. Pregnancy tests.
19. Role of hormones in lactation.
20. Prolactin.
21. Milk ejection reflex.
22. Safe period/Rhythm method.
23. Oral contraceptives.
24. MTP.
25. Tubectomy.
26. Contraceptive methods in males.
27. Condoms.
28. IUCD.
29. Vasectomy.
30. Contraceptive methods in females.
Questions in Reproductive System
368

Cardiovascular System
58. Introduction to Cardiovascular System................................ 371
59. Properties of Cardiac Muscle .............................................. 377
60. Cardiac Cycle ...................................................................... 383
61. Heart Sounds ...................................................................... 388
62. Electrocardiogram ............................................................... 392
63. Cardiac Output .................................................................... 398
64. Heart Rate ........................................................................... 404
65. Arterial Blood Pressure ....................................................... 411
66. Venous Pressure and Capillary Pressure............................ 420
67. Arterial Pulse and Venous Pulse ......................................... 422
68. Regional Circulation ............................................................ 426
69. Fetal Circulation and Respiration ........................................ 432
70. Hemorrhage, Circulatory Shock and Heart Failure ............. 436
71. Cardiovascular Adjustments during Exercise ...................... 439
S E C T I O N
8
C H A P T E R S

CARDIOVASCULAR SYSTEM
HEART
ACTIONS OF THE HEART
BLOOD VESSELS
DIVISIONS OF CIRCULATION
Introduction to
Cardiovascular System
58
CARDIOVASCULAR SYSTEM
Cardiovascular system is made up of heart and
blood vessels. Heart pumps the blood into the
blood vessels. Blood vessels circulate the blood
throughout the body and transport nutrients and
oxygen to the tissues and remove carbon dioxide
and waste products from the tissues.
HEART
Heart is a muscular organ that pumps blood
throughout the circulatory system. It situated in
between the two lungs in the mediastinum. It is
made up of four chambers – two atria and two
ventricles. The musculature is more and thick
in the ventricles than in the atria. The force of
contraction of the heart depends upon the mus-
cles.
RIGHT SIDE OF THE HEART
Right side of the heart has two chambers, the
upper right atrium and lower right ventricle. Right
atrium is a thin walled and low pressure chamber.
It has got the pacemaker known as sinoatrial
node that produces cardiac impulses and atrio-
ventricular node that conducts the impulses to
the ventricles. It receives venous (deoxygena-
ted) blood via two large veins:
1. Superior vena cava that returns the venous
blood from the head, neck and upper limbs
2. Inferior vena cava that returns the venous
blood from lower parts of the body (Fig. 58-1).
Right atrium communicates with the right
ventricle through the tricuspid valve. Venous
blood from the right atrium enters the right
ventricle through this valve.
From the right ventricle, pulmonary artery
arises. It carries the venous blood from right ven-
tricle to the lungs. In the lungs, the deoxygenated
blood is oxygenated.
LEFT SIDE OF THE HEART
Left side of the heart has two chambers, the
upper left atrium and lower left ventricle. Left
atrium is a thin walled and low pressure
chamber. It receives oxygenated blood from the
lungs through pulmonary veins. This is the only
excep-tion in the body where an artery carries
venous blood and vein carries the arterial blood.
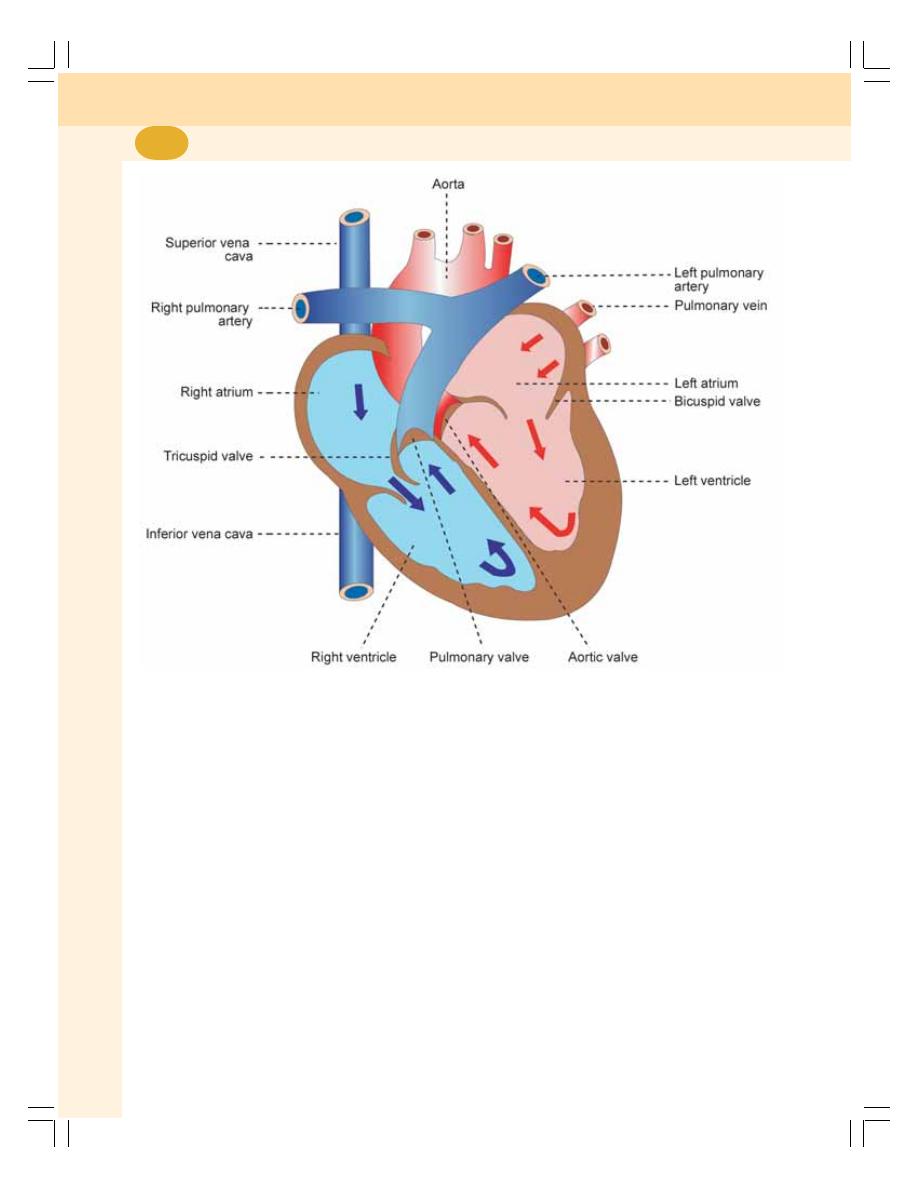
Cardiovascular System
372
Blood from left atrium enters the left ventricle
through the mitral valve (bicuspid valve). Wall
of the left ventricle is very thick. Left ventricle
pumps the arterial blood to different parts of the
body through systemic aorta.
SEPTA OF THE HEART
Right and left atria of the heart are separated
from one another by interatrial septum. The
ventricles are separated from one another by
interventricular septum.
LAYERS OF WALL OF THE HEART
Heart is made up of three layers of tissues:
1. Outer pericardium
2. Middle myocardium
3. Inner endocardium.
PERICARDIUM
Pericardium is the outer covering of the heart. It
is made up of two layers
i. Outer parietal pericardium which forms a
strong protective sac around the heart
ii. Inner visceral pericardium or epicardium
that covers myocardium.
These two layers are separated by a space
called pericardial cavity which contains a thin film
of fluid.
MYOCARDIUM
Myocardium is the middle layer of the wall of the
heart and it is formed by cardiac muscle fibers.
It forms the bulk of the heart and it is responsible
for the pumping action of the heart. Refer Chapter
20 for features of cardiac muscles.
FIGURE 58-1:
Section of the heart

Chapter 58 Introduction to Cardiovascular System
373
Myocardium is formed by three types of
cardiac muscle fibers:
i. Muscle fibers which form the contractile
unit of the heart
ii. Muscle fibers which form pacemaker
iii. Muscle fibers which form the conductive
system.
i.
Muscle Fibers which Form the
Contractile Unit of the Heart
These cardiac muscle fibers are striated fibers
and are similar to the skeletal muscles in
structure. But, unlike the skeletal muscle fibers,
the cardiac muscle fibers are involuntary in
nature.
The cardiac muscle fiber is covered by
sarcolemma. It has a centrally placed nucleus.
The myofibrils are embedded in the sarcoplasm.
The sarcomere of the cardiac muscle has muscle
proteins namely, actin, myosin, troponin and
tropomyosin. The cardiac muscles also have
sarcotubular system like that of skeletal muscle.
The important difference between skeletal
muscle and cardiac muscle is that the cardiac
muscle fiber is branched and the skeletal muscle
is not branched.
Intercalated disk
Intercalated disk is a tough double membranous
structure situated at the junction between the
branches of neighboring cardiac muscle fibers.
It is formed by the fusion of the membrane of
the cardiac muscle branches (Fig. 58-2).
The intercalated disks form adherens junc-
tions which play an important role in contraction
of the muscle as a single unit (Chapter 2).
Syncytium
The structure of cardiac muscle is considered
as a syncytium. The word syncytium refers to
the tissue in which there is cytoplasmic continuity
between the adjacent cells. However, in cardiac
muscle there is no continuity of the cytoplasm
and the muscle fibers are separated from each
other by cell membrane. But at the sides,
membranes of the adjacent muscle fibers fuse
together to form gap junctions which facilitates
the rapid conduction of electrical activity from
one fiber to another. This makes the cardiac
muscle fibers act like a single unit referred as
physiological syncytium.
The syncytium in human heart has two por-
tions, atrial syncytium and ventricular syncytium
which are connected by atrioventricular ring.
ii. Muscle Fibers which Form the Pacemaker
Some of the muscle fibers of the heart are
modified into a specialized structure known as
pacemaker. The muscle fibers forming the
pacemaker have less striation.
Pacemaker
Pacemaker is structure in the heart that gene-
rates the impulses for heart beat. It is formed
by the pacemaker cells called P cells. Sinoatrial
(SA) node forms the pacemaker in human heart.
Details of pacemaker are given in next chapter.
iii. Muscle Fibers which Form the
Conductive System
The conductive system of the heart is formed
by the modified cardiac muscle fibers. The impul-
ses from SA node are transmitted to the atria
directly. However, the impulses are transmitted
to the ventricles, through various components of
conducting system which are given in the next
chapter.
FIGURE 58-2:
Cardiac muscle fibers
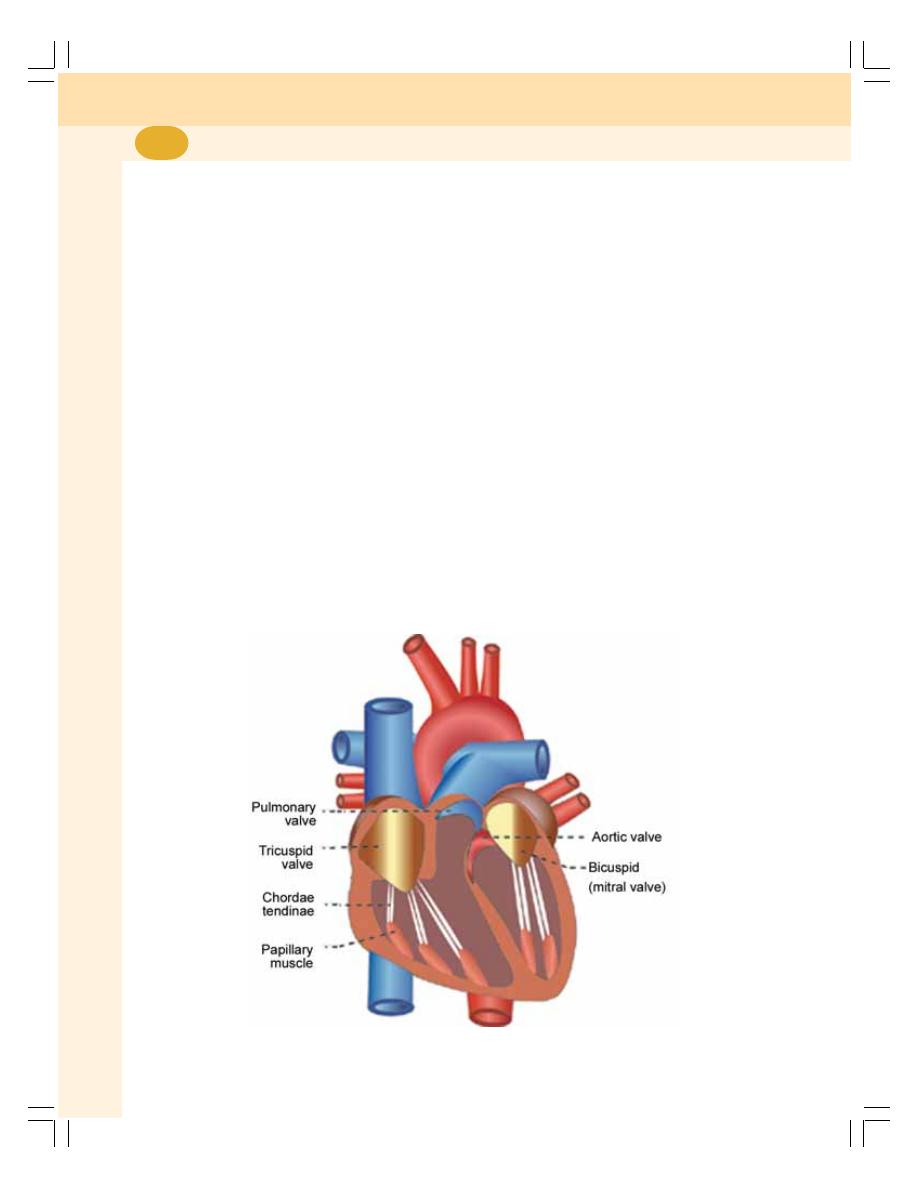
Cardiovascular System
374
ENDOCARDIUM
Endocardium is the inner most layer of the heart
wall. It is a thin, smooth and glistening mem-
brane. It is formed by a single layer of endothelial
cells lining the inner surface of the heart. Endo-
cardium continues as endothelium of the blood
vessels.
VALVES OF THE HEART
There are four valves in human heart. Two of
the valves are in between the atria and the
ventricles called atrioventricular valves. The
other two are the semilunar valves, placed at the
opening of the blood vessels arising from the
ventricles, i.e. systemic aorta and pulmonary
artery. The valves of the heart permit the flow of
blood through the heart in only one direction.
Atrioventricular Valves
Left atrioventricular valve is otherwise known as
mitral valve or bicuspid valve. It is formed by two
valvular cusps or flaps (Fig. 58-3). Right atrio-
ventricular valve is known as tricuspid valve and
it is formed by three cusps.
The brim of the atrioventricular valves is
attached to the atrioventricular ring, which is the
fibrous connection between the atria and
ventricles. The cusps of the valves are attached
to the papillary muscles by means of chordae
tendinae. The papillary muscles arise from the
inner surface of the ventricles. The papillary mus-
cles play an important role in closure of the cusps
and in preventing the back flow of blood from
ventricle to atria during ventricular contraction.
Atrioventricular valves open only towards
ventricles and prevent the backflow of blood into
atria.
Semilunar Valves
The semilunar valves are present at the
openings of systemic aorta and pulmonary artery
and are known as aortic valve and pulmonary
valve respectively. Because of the half moon
shape, these two valves are called semilunar
valves. The semilunar valves are made up of
three flaps.
The semilunar valves open only towards the
aorta and pulmonary artery and prevent the
backflow of blood into the ventricles.
FIGURE 58-3:
Valves of the heart

Chapter 58 Introduction to Cardiovascular System
375
ACTIONS OF THE HEART
The actions of the heart are classified into four
types:
1. Chronotropic action
2. Inotropic action
3. Dromotropic action
4. Bathmotropic action.
CHRONOTROPIC ACTION
Chronotropic action is the frequency of heartbeat
or heart rate. It is of two types:
i. Tachycardia or increase in heart rate
ii. Bradycardia or decrease in the heart rate.
INOTROPIC ACTION
Force of contraction of heart is called inotropic
action. It is of two types:
i. Positive inotropic action or increase in the
force of contraction
ii. Negative inotropic action or decrease in
the force of contraction.
DROMOTROPIC ACTION
Dromotropic action is the conduction of impulse
through heart. It is of two types:
i. Positive dromotropic action or increase in
the velocity of conduction
ii. Negative dromotropic action or decrease
in the velocity of conduction
BATHMOTROPIC ACTION
Bathmotropic action is the excitability of cardiac
muscle. It is also of two types:
i. Positive bathmotropic action or increase
in the excitability of cardiac muscle
ii. Negative bathmotropic action or the
decrease in the excitability of cardiac
muscle.
Regulation of Actions of the Heart
All the actions of the heart are continuously
regulated. It is essential for the heart to cope up
with the needs of the body. All the actions are
altered by the stimulation of nerves supplying the
heart or some hormones or hormonal substances
secreted in the body.
BLOOD VESSELS
The vessels of circulatory system are divided into
arterial system and venous system.
ARTERIAL SYSTEM
The arterial system comprises the aorta, arteries
and arterioles. The walls of the aorta and arteries
are formed by three layers.
1. Outer tunica adventitia, which is made up of
connective tissue layer. It is the continuation
of fibrous layer of parietal pericardium
2. Middle tunica media, which is formed by
smooth muscles
3. Inner tunica intima, which is made up of
endothelium. It is the continuation of endo-
cardium.
The arterial branches become narrower and
their walls become thinner while reaching the
periphery. The aorta has got the maximum
diameter of about 25 mm. The diameter of the
arteries is gradually decreased and at the end
arteries it is about 4 mm. It further decreases to
30 μ in the arterioles and ends up with 10 μ in
the terminal arterioles. The resistance (peripheral
resistance) is offered to the blood flow in the
arterioles and so these vessels are called resis-
tant vessels.
The arterioles are continued as capillaries
which are small, thin walled vessels having a
diameter of about 5 to 8 μ. The capillaries are
functionally very important because, the ex-
change of materials between the blood and the
tissues occurs through these vessels.
VENOUS SYSTEM
From the capillaries venous system starts and
it includes the venules, veins and vena cavae.
The capillaries end in the venules. The venules
are smaller vessels with thin muscular wall than
the arterioles. The diameter of the venules is
about 20 μ. At a given time, large quantity of
blood is held in venules and so the venules are
called capacitance vessels. The venules are
continued as veins, which have the diameter of
5 mm. The veins form superior and inferior vena
cavae which have a diameter of about 30 mm
(Table 58-1).
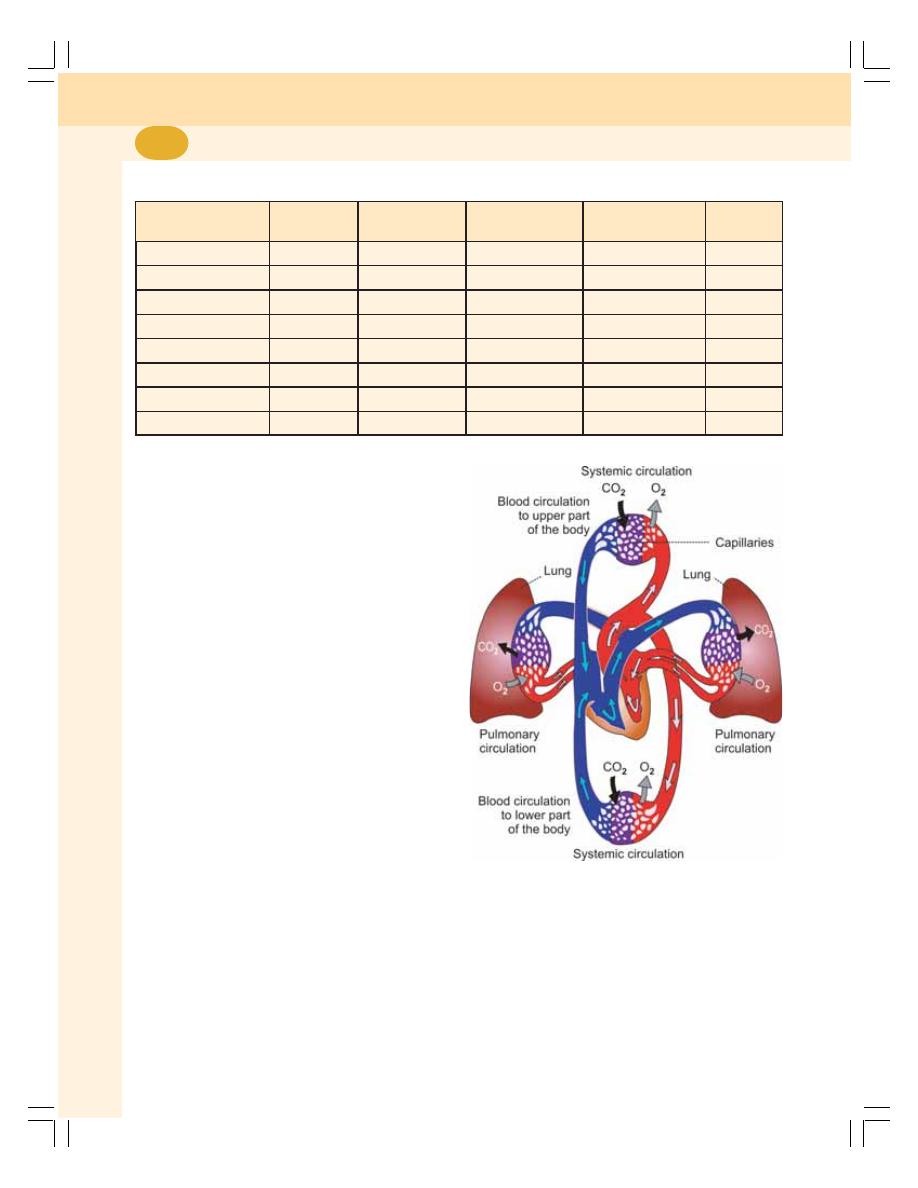
Cardiovascular System
376
FIGURE 58-4:
Systemic and
pulmonary circulation
The walls of the veins and vena cavae are
made up of inner endothelium, elastic tissues,
smooth muscles and outer connective tissue
layer. In the veins and vena cavae, the elastic
tissue is less but the smooth muscle fibers are
more.
DIVISIONS OF CIRCULATION
Blood flows through two divisions of circulating
system:
1. Systemic circulation
2. Pulmonary circulation.
SYSTEMIC CIRCULATION
It is otherwise known as greater circulation
(Fig. 58-4). The blood pumped from left ventricle
passes through a series of blood vessels of arte-
rial system and reaches the tissues. Exchange
of various substances between blood and the
tissues takes place in the capillaries. After the
exchange of substances in the capillaries, the
blood enters the venous system and returns to
right atrium and then the right ventricles.
PULMONARY CIRCULATION
It is otherwise called lesser circulation. Blood is
pumped from right ventricle to lungs through
pulmonary artery. The exchange of gases occurs
between blood and alveoli of the lungs through
TABLE 58-1:
Structural and dimensional differences between different blood vessel walls
Blood vessel
Diameter
Thickness of
Elastic tissue
Smooth muscle
Fibrous
the wall
fibers
tissue
Aorta
25 mm
2 mm
More
Less
More
Artery
4 mm
1 mm
More
More
Moderate
Arteriole
30 μ
6 μ
Moderate
More
Moderate
Terminal arteriole
10 μ
2 μ
Less
More
Moderate
Capillary
8 μ
0.5 μ
Absent
Absent
Moderate
Venule
20 μ
1 μ
Absent
Absent
Less
Vein
5 mm
0.5 mm
Less
More
Moderate
Vena cava
30 mm
1.5 mm
Less
More
More
pulmonary capillary membrane. The oxygenated
blood returns to left atrium through the pulmonary
veins.
Thus, the left side of the heart contains oxy-
genated or arterial blood and the right side of
the heart contains the venous blood.
EXCITABILITY
DEFINITION
ELECTRICAL POTENTIALS IN CARDIAC MUSCLE
IONIC BASIS OF ACTION POTENTIAL
SPREAD OF ACTION POTENTIAL THROUGH CARDIAC MUSCLE
RHYTHMICITY
DEFINITION
PACEMAKER
ELECTRICAL POTENTIAL IN SA NODE
CONDUCTIVITY
CONDUCTIVE SYSTEM IN HUMAN HEART
VELOCITY OF IMPULSES AT DIFFERENT PARTS OF THE CONDUCTIVE
SYSTEM
CONTRACTILITY
ALL OR NONE LAW
STAIRCASE PHENOMENON
SUMMATION OF SUBLIMINAL STIMULI
REFRACTORY PERIOD
Properties of
Cardiac Muscle
59
EXCITABILITY
DEFINITION
Excitability is defined as the ability of a living
tissue to give response to a stimulus. In all the
tissues, the initial response to a stimulus is the
electrical activity in the form of action potential.
It is followed by mechanical activity in the form
of contraction, secretion, etc.
ELECTRICAL POTENTIALS IN
CARDIAC MUSCLE
Refer Chapter 23 for basics of electrical poten-
tials in the muscle.
Resting Membrane Potential
The resting membrane potential in:
Single cardiac muscle fiber : – 85 to – 95 mV
SA node
: – 55 to – 60 mV
Purkinje fibers
: – 90 to –100 mV.
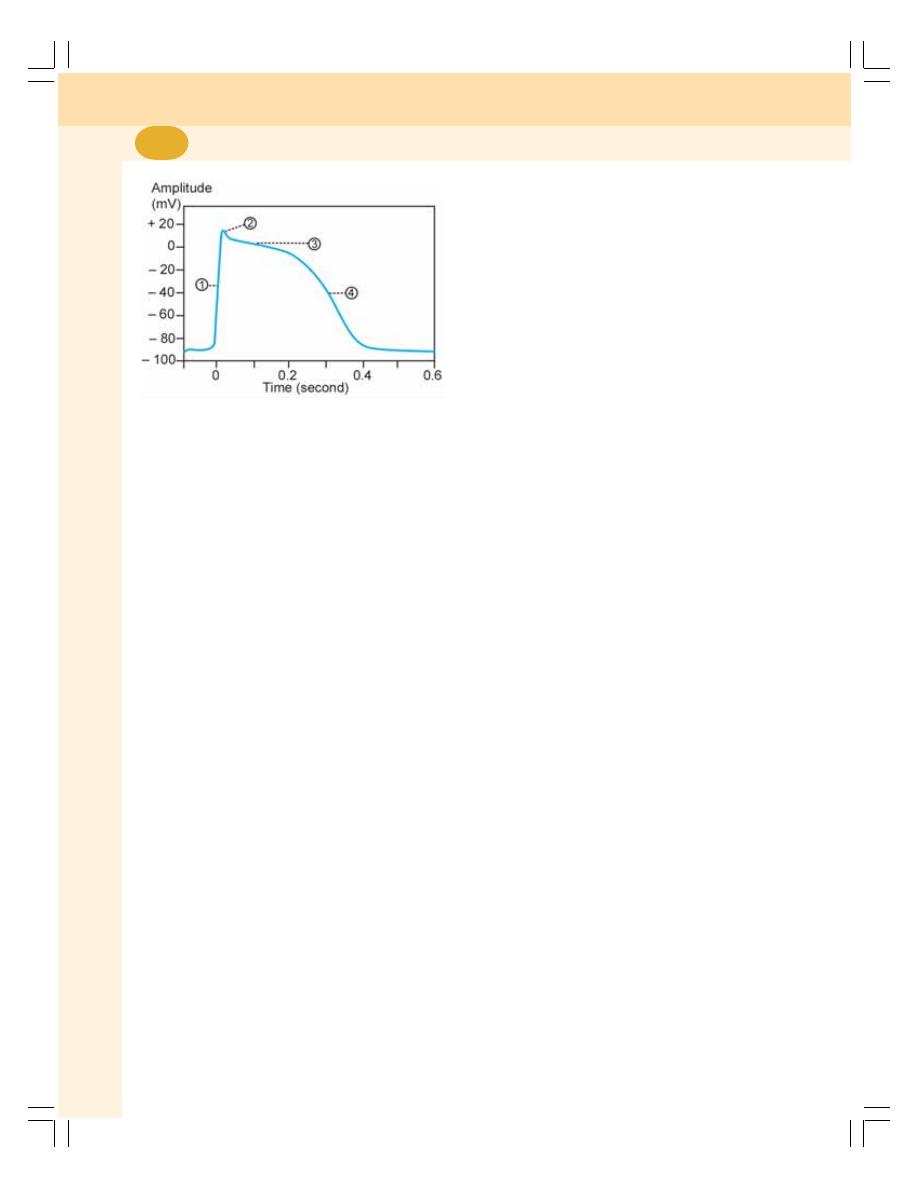
Cardiovascular System
378
Action Potential
Action potential in a single cardiac muscle fiber
occurs in 4 phases:
1. Initial depolarization
2. Initial repolarization
3. A plateau – final depolarization
4. Final repolarization.
Approximate duration of action potential in
cardiac muscle is 250 to 350 msec (0.25 to
0.35 sec).
1. Initial Depolarization
Initial depolarization is very rapid and it lasts for
about 2 msec. The amplitude of the depolari-
zation is about + 20 mV (Fig. 59-1).
2. Initial Repolarization
Immediately after depolarization, there is an
initial rapid repolarization for a short period of
about 2 msec. The end of rapid repolarization is
represented by a notch.
3. Plateau – Final Depolarization
Afterwards, the muscle fiber remains in the
depolarized state for sometime before further
repolarization. It forms the plateau (stable period)
in the action potential curve. The plateau lasts
for about 200 msec (0.2 sec) in atrial muscle
fibers and for about 300 msec (0.3 sec) in
ventricular muscle fibers. Due to the long plateau
in action potential, the contraction time is longer
in cardiac muscle by about 5 to 15 times than
in skeletal muscle.
4. Final Repolarization
Final repolarization occurs after the plateau. It
is a slow process and it lasts for about 50 to
80 msec (0.05 to 0.08 sec) before the re-
establishment of resting membrane potential.
IONIC BASIS OF ACTION POTENTIAL
1. Initial depolarization is due to opening of fast
sodium channels and the rapid influx of
sodium ions as in the case of skeletal muscle
fiber
2. Initial repolarization is due to the transient
(short duration) opening of potassium
channels and efflux of a small quantity of
potassium ions from the muscle fiber.
Simultaneously, the fast sodium channels
close suddenly and slow sodium channels
open resulting in slow influx of a low quantity
of sodium ions.
3. Plateau (final depolarization) is because of
the opening of calcium channels. These
channels are kept opened for a longer period
and cause influx of large number of calcium
ions. Already the slow sodium channels are
opened through which slow influx of sodium
ions continues. The entry of both calcium and
sodium ions is responsible for prolonged
depolarization, i.e. plateau.
4. Final repolarization is due to increase in
efflux of potassium ions increases.
Restoration of resting membrane potential
At the end of final repolarization, all the sodium
ions, which entered the cell throughout the
process of action potential move out of the cell
and potassium ions move inside by sodium-
potassium pump. Simultaneously, the excess
of calcium ions, which entered the muscle fiber
also move out through sodium-calcium pump.
Thus, the resting membrane potential is restored.
FIGURE 59-1: Action potential in ventricular muscle.
1 = Depolarization, 2 = Initial rapid repolarization,
3 = Plateau, 4 = Final repolarization

Chapter 59 Properties of Cardiac Muscle
379
SPREAD OF ACTION POTENTIAL
THROUGH CARDIAC MUSCLE
The action potential spreads through the
cardiac muscle very rapidly. It is because of the
presence of gap junctions between the cardiac
muscle fibers. The gap junctions are permeable
junctions and allow free movement of ions. Due
to this, the action potential spreads rapidly from
one muscle fiber to another fiber.
The action potential is transmitted from atria
to ventricles through the fibers of specialized
conductive system, which is explained later in
this chapter.
RHYTHMICITY
DEFINITION
Rhythmicity is the ability of a tissue to produce
its own impulses regularly. It is more
appropriately named as autorhythmicity. It is
also called self excitation. The property of
rhythmicity is present in all the tissues of the
heart. However, heart has a specialized
excitatory structure from which the discharge of
impulses is rapid. This specialized structure is
called pacemaker. From this, the impulses
spread to other parts through the specialized
conductive system.
PACEMAKER
Pacemaker is defined as the part of the heart
from which the impulses for heartbeat are
produced normally. It is formed by the
pacemaker cells called P cells. In mammalian
heart, the pacemaker is sinoatrial node
(SA node).
SA Node
SA node is a small strip of modified cardiac
muscle situated in the superior part of lateral wall
of right atrium, just below the opening of superior
vena cava. The fibers of this node do not have
contractile elements. These fibers are continuous
with fibers of atrial muscle, so that the impulses
from the SA node spread rapidly through atria.
Other parts of heart like AV node, atria and
ventricle also can produce the impulses and
function as pacemaker. Still SA node is called
the pacemaker because the rate of production
of impulse (rhythmicity) is more in SA node than
in other parts. It is about 70 to 80/minute.
Spread of Impulses from SA Node
The mammalian heart has got a specialized
conductive system by which, the impulses from
SA node spreads to other parts of the heart (see
below).
Rhythmicity of Other Parts of the Heart
Though the SA node is the pacemaker in mam-
malian heart, other parts of the heart also have
the property of rhythmicity. The rhythmicity of
different parts:
1. AV node
: 40 to 60/minute
2. Atrial muscle
: 40 to 60/minute
3. Purkinje fibers
: 35 to 40/minute
4. Ventricular muscle : 20 to 40/minute
ELECTRICAL POTENTIAL IN SA NODE
Resting Membrane Potential — Pacemaker
Potential
Pacemaker potential is the unstable resting
membrane potential in SA node. It is also called
prepotential.
The electrical potential in SA node is different
from that of other cardiac muscle fibers. In the
SA node each impulse triggers the next impulse.
It is mainly due to the unstable resting membrane
potential.
The resting membrane potential in SA node
has a negativity of – 55 to – 60 mV. It is different
from the negativity of – 85 to – 95 mV in other
cardiac muscle fibers.
Action Potential
The depolarization starts very slowly and the
threshold level of –40 mV is reached very
slowly. After the threshold level, rapid depolari-
zation occurs up to +5 mV. It is followed by
rapid repolarization. Once again, the resting
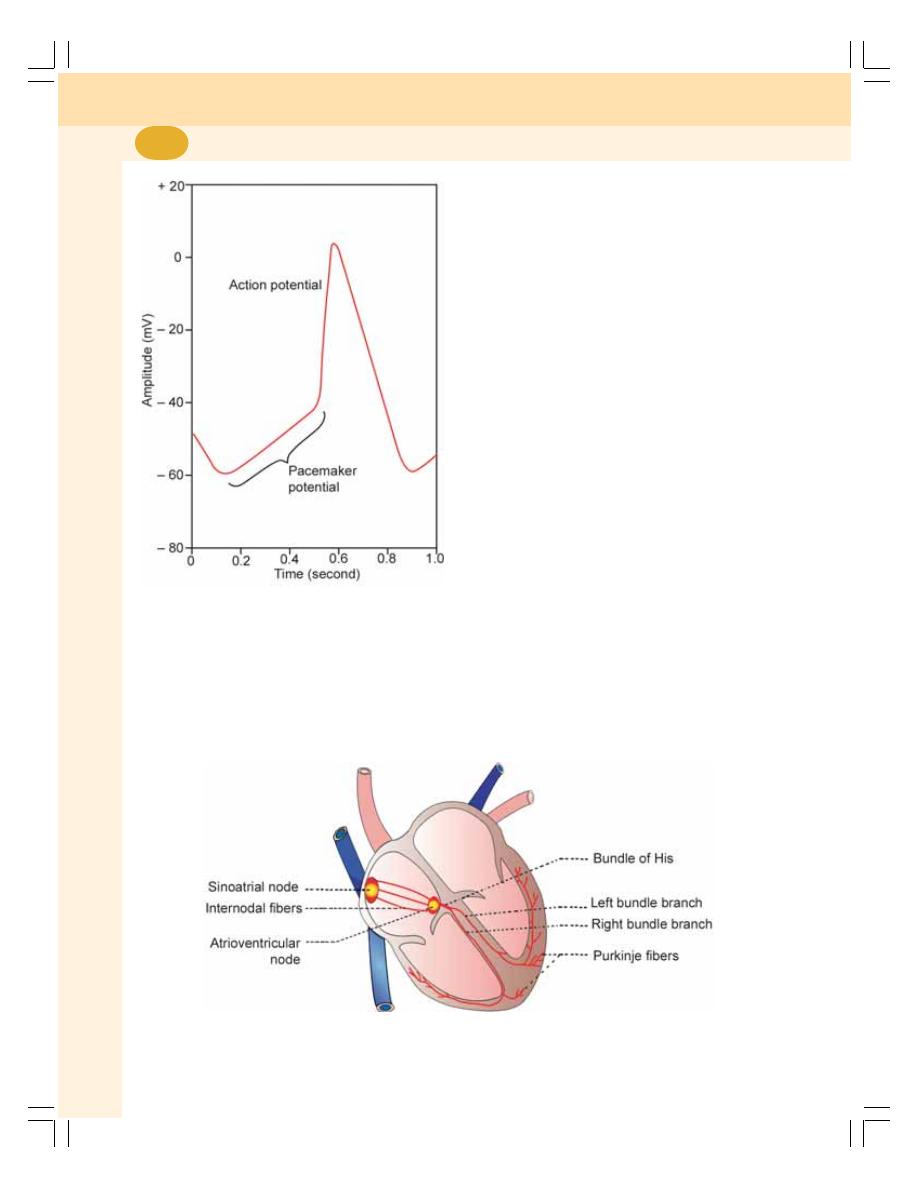
Cardiovascular System
380
membrane potential becomes unstable and
reaches the threshold level slowly (Fig. 59-2).
CONDUCTIVITY
Human heart has a specialized conductive
system through which the impulses from SA node
are transmitted to all other parts of the heart
(Fig. 59-3).
CONDUCTIVE SYSTEM IN HUMAN
HEART
The conductive system of the heart is formed
by the modified cardiac muscle fibers. The con-
ductive tissues of the heart are also called the
junctional tissues. The conductive system in
human heart comprises:
1. AV node
2. Bundle of His
3. Right and left bundle branches
4. Purkinje fibers.
SA node is situated in right atrium just below
the opening of superior vena cava. AV node is
situated in right posterior portion of intra-atrial
septum. The impulses from SA node are con-
ducted throughout right and left atria. The
impulses also reach the AV node via some
specialized fibers called intermodal fibers. There
are three types of intermodal fibers:
1. Anterior internodal fibers of Bachman
2. Middle internodal fibers of Wenckebach
3. Posterior internodal fibers of Thorel.
All these fibers from SA node converge on
AV node and interdigitate with fibers of AV
node. From AV node, the bundle of His arises.
It divides into right and left bundle branches
which run on either side of the interventricular
septum. From each branch of Bundle of His,
FIGURE 59-2: Pacemaker potential
FIGURE 59-3: Sinoatrial node and conductive system of the heart
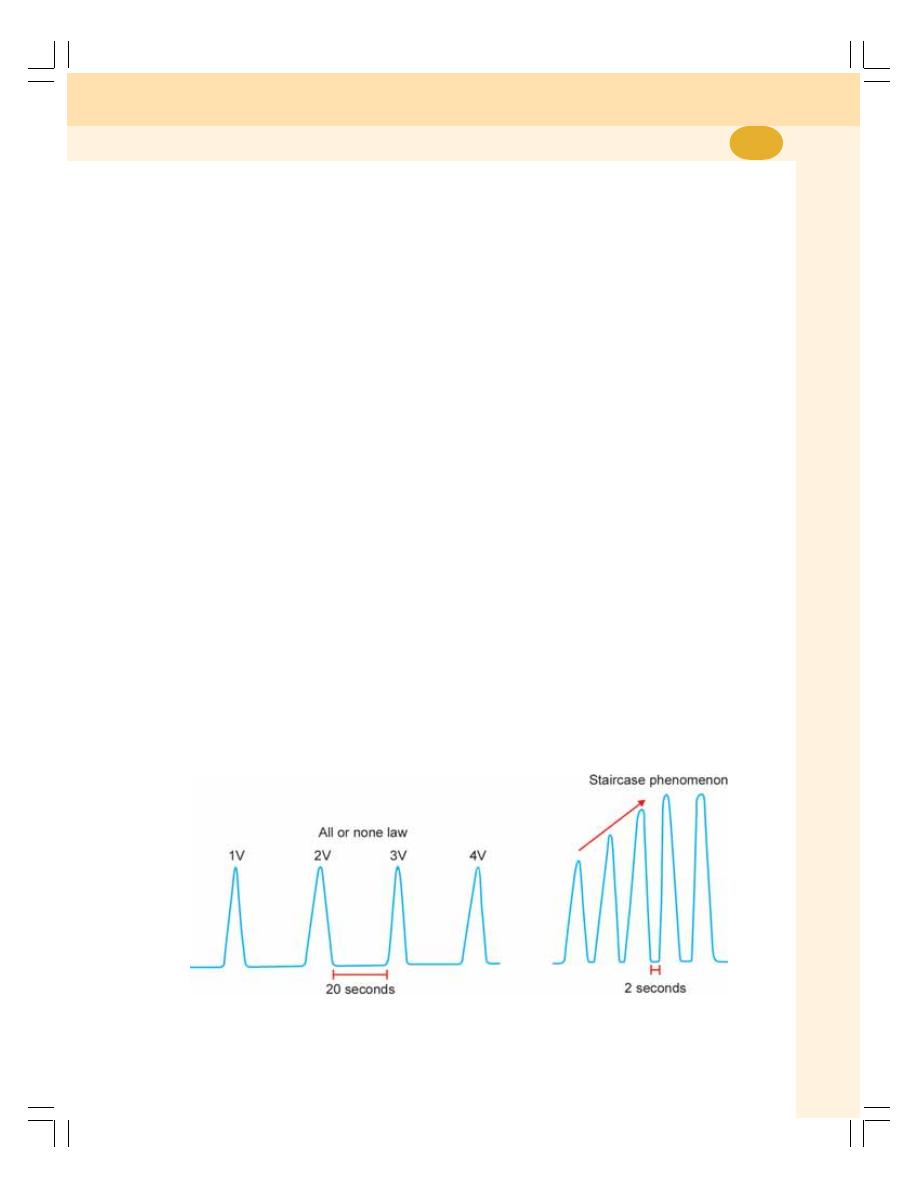
Chapter 59 Properties of Cardiac Muscle
381
many Purkinje fibers arise and spread all over
the ventricular myocardium.
VELOCITY OF IMPULSES AT DIFFERENT
PARTS OF THE CONDUCTIVE SYSTEM
1. Atrial muscle fibers : 0.3
meter/second
2. Internodal fibers
: 1.0
meter/second
3. AV node
: 0.05 meter/second
4. Bundle of His
: 0.12 meter/second
5. Purkinje fibers
: 4.0
meter/second
6. Ventricular muscle
: 0.5
meter/second
fibers
Thus, the velocity of impulses is maximum
in Purkinje fibers and minimum at AV node.
CONTRACTILITY
Contractility is ability of the tissue to shorten in
length (contraction) after receiving a stimulus.
Various factors affect the contractile properties
of the cardiac muscle.
The contractile properties are:
ALL OR NONE LAW
According to all or none law, when a stimulus is
applied, whatever may be the strength, the
whole cardiac muscle gives maximum response
or it does not give any response at all. Below
the threshold level, i.e. if the strength of stimulus
is not adequate, the muscle does not give
response.
Cause for All or None Law
All or none law is applicable to whole cardiac
muscle. It is because of syncytial arrangement
of cardiac muscle. In the case of skeletal muscle,
all or none law is applicable only to a single
muscle fiber.
STAIRCASE PHENOMENON
When the ventricle is stimulated successively
(at a short interval of two seconds) without
changing the strength, the force of contraction
increases gradually for the first few contractions,
and then it remains same. Gradual increase in
the force of contraction is called staircase
phenomenon.
Cause for Staircase Phenomenon
The staircase phenomenon occurs because of
the beneficial effect which facilitates the force
of successive contraction. So, there is a gradual
increase in force of contraction (Fig. 59-4).
SUMMATION OF SUBLIMINAL STIMULI
When a stimulus with a subliminal strength is
applied, the heart does not show any response.
When few stimuli with same subliminal strength
are applied in succession, the heart shows res-
ponse by contraction. It is due to the summation
of the stimuli.
FIGURE 59-4: All or none law and staircase phenomenon in cardiac muscle

Cardiovascular System
382
REFRACTORY PERIOD
Refractory period is the period in which the mus-
cle does not show any response to a stimulus.
It is of two types:
1. Absolute refractory period
2. Relative refractory period.
Absolute Refractory Period
Absolute refractory period is the period during
which the muscle does not show any response
at all, whatever may be the strength of the sti-
mulus. It is because, the depolarization occurs
during this period. So a second depolarization
is not possible.
Relative Refractory Period
The relative refractory period is the period during
which the muscle shows response if the
strength of stimulus is increased to maximum.
It is the stage at which the muscle is in
repolarizing state.
Refractory Period in Cardiac Muscle
Cardiac muscle has a long refractory period
compared to that of skeletal muscle. The
absolute refractory period extends throughout
contraction period of cardiac muscle. It is for 0.27
sec and relative refractory period extends during
first half of relaxation period which is about 0.26
sec. So, the total refractory period is 0.53 sec.
Significance of Long Refractory Period in
Cardiac Muscle
Long refractory period in cardiac muscle has
three advantages:
1. Summation of contractions does not occur
2. Fatigue does not occur
3. Tetanus does not occur.
DEFINITION
EVENTS OF CARDIAC CYCLE
SUBDIVISIONS AND DURATION OF EVENTS OF CARDIAC CYCLE
DESCRIPTION OF ATRIAL EVENTS
DESCRIPTION OF VENTRICULAR EVENTS
DEFINITION
Cardiac cycle is defined as the sequence of
coordinated events in the heart which are
repeated during every heartbeat in a cyclic
manner. Each heartbeat consists of two major
periods called systole and diastole. Systole is
the contraction of the cardiac muscle and diastole
is the relaxation of cardiac muscle.
EVENTS OF CARDIAC CYCLE
The events of cardiac cycle are classified into
two divisions:
1. Atrial events which constitute atrial systole
and atrial diastole
2. Ventricular events which constitute ventricular
systole and ventricular diastole.
However, in clinical practice, the term ‘systole’
refers to ventricular systole and ‘diastole’ refers
to ventricular diastole.
SUBDIVISIONS AND DURATION OF
EVENTS OF CARDIAC CYCLE
When the heart beats at the normal rate of 72/
minute, the duration of each cardiac cycle is
about 0.8 second.
ATRIAL EVENTS
1. Atrial systole = 0.11 (0.1) sec
2. Atrial diastole = 0.69 (0.7) sec
VENTRICULAR EVENTS
The duration of ventricular systole is 0.27 second
and that of diastole is 0.53 second. Generally,
ventricular systole is divided into two subdivi-
sions and ventricular diastole is divided into five
subdivisions. The subdivisions and the duration
of ventricular events are:
Ventricular Systole
Time (sec)
1. Isometric contraction
= 0.05
2. Ejection period
= 0.22
0.27
Ventricular Diastole
1. Protodiastole
= 0.04
2. Isometric relaxation
= 0.08
3. Rapid filling
= 0.11
4. Slow filling
= 0.19
5. Last rapid filling or atrial systole
= 0.11
0.53
Cardiac Cycle
60
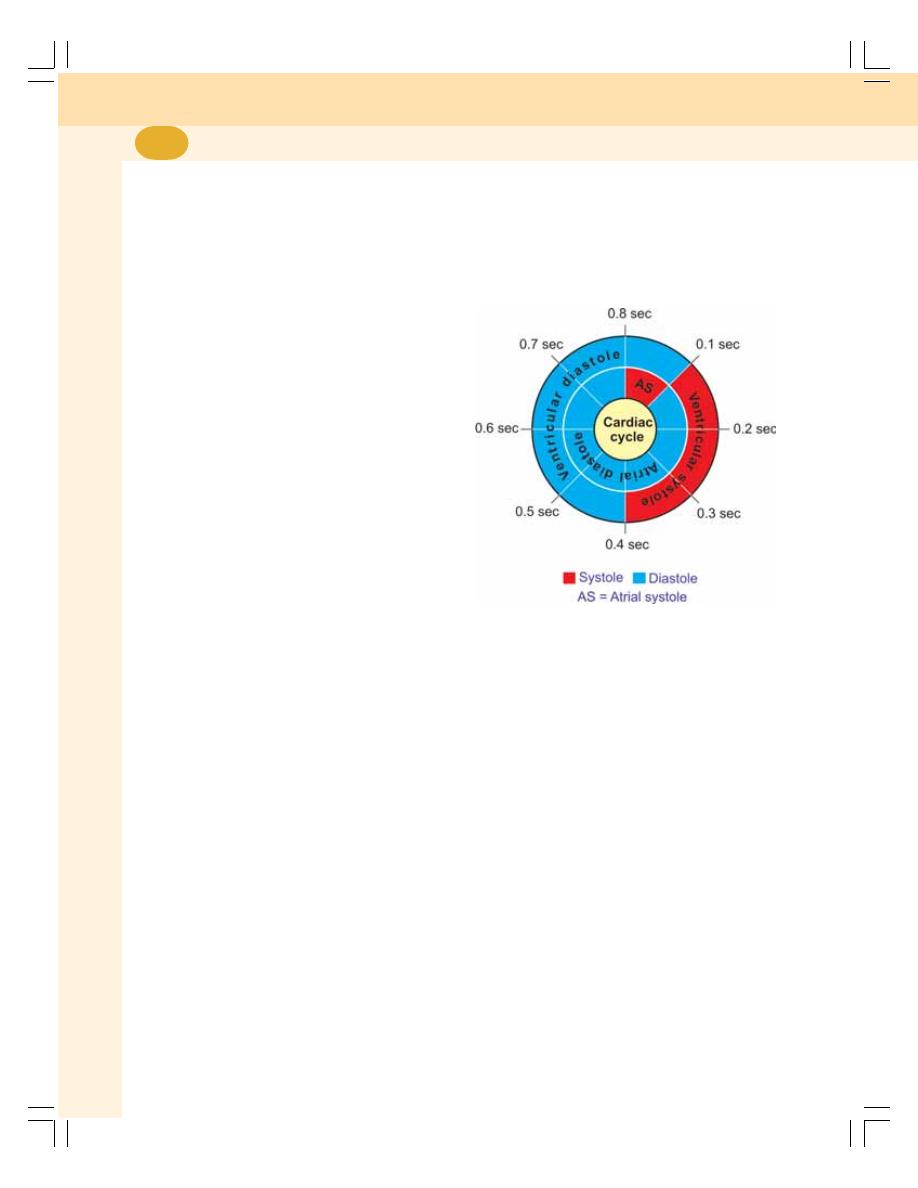
Cardiovascular System
384
The total duration of ventricular events is
0.27 + 0.53 = 0.8 second.
Among the atrial events, atrial systole
occurs during the last phase of ventricular
diastole. Atrial diastole is not considered as a
separate phase, since it coincides with whole
of ventricular systole and earlier part of
ventricular diastole.
DESCRIPTION OF ATRIAL EVENTS
For the sake of better understanding, the
description of events of cardiac cycle is
commenced with atrial systole.
ATRIAL SYSTOLE
Atrial systole is also known as second or last
rapid filling phase or presystole. It is considered
as the last phase of ventricular diastole. Its
duration is 0.11 second.
During this period, only a small amount, i.e.
10% of blood is forced from atria into ventricles.
Atrial systole is not essential for the maintenance
of circulation. Many persons with atrial fibrillation
survive for years, without suffering from
circulatory insufficiency. However, such persons
feel difficult to cope up with physical stress like
exercise.
During atrial systole, the intra-atrial pressure
increases. Intraventricular pressure and ventri-
cular volume also increase but slightly.
Fourth heart sound
Contraction of atrial musculature causes
production of fourth heart sound.
ATRIAL DIASTOLE
After atrial systole, the atrial diastole starts.
Atrial diastole lasts for about 0.7 sec (accurate
duration is 0.69 sec). This long atrial diastole
is necessary because, this is the period during
which atrial filling takes place. Right atrium
receives deoxygenated blood from all over the
body through superior and inferior vena cavae.
Left atrium receives oxygenated blood from
lungs through pulmonary veins.
Atrial Events vs Ventricular Events
Out of 0.7 sec of atrial diastole, first 0.3 sec
(0.27 sec accurately) coincides with ventricular
systole. So, the heart relaxes as a whole for 0.4
sec. Figure 60-1 shows the correlation between
atrial and ventricular events of cardiac cycle.
FIGURE 60-1: Atrial and ventricular events of
cardiac cycle
DESCRIPTION OF VENTRICULAR
EVENTS
VENTRICULAR SYSTOLE
1. Isometric Contraction
Isometric contraction is the type of muscular
contraction characterized by increase in tension
without any change in the length of muscle
fibers. Isometric contraction of ventricular
muscle is also called isovolumetric contraction.
Isometric contraction period in cardiac cycle
is the first phase of ventricular systole. It lasts
for 0.05 second. Immediately after atrial systole,
the atrioventricular valves are closed due to
increase in ventricular pressure. The semilunar
valves are already closed. Now, the ventricles
contract as closed cavities in such a way that,
there is no change in the volume of ventricular
chambers or in the length of muscle fibers. Only,
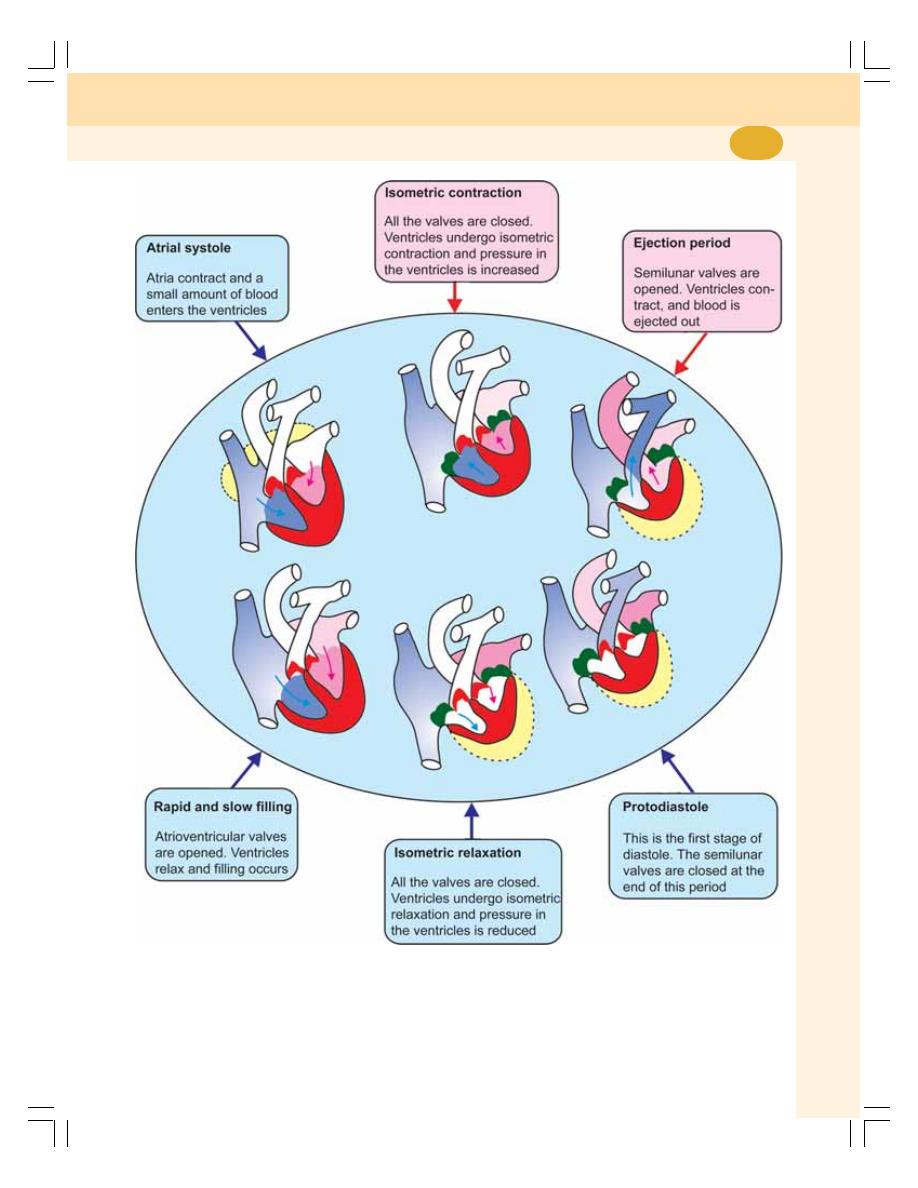
Chapter 60 Cardiac Cycle
385
the tension increases in ventricular musculature
leading to a sharp increase in intraventricular
pressure (Fig. 60-2).
First heart sound
Closure of atrioventricular valves at the begin-
ning of this phase produces first heart sound.
FIGURE 60-2: Events of cardiac cycle
Cardiovascular System
386
Significance of isometric contraction
During isometric contraction period, the ventri-
cular pressure increases greatly (Table 60-1).
When this pressure increases above the
pressure in the aorta and pulmonary artery, the
semilunar valves open. Thus, the pressure rise
in the ventricle caused by isometric contraction
is responsible for opening of semilunar valves
leading to ejection of blood from the ventricles
into aorta and pulmonary artery.
2. Ejection Period
Due to the opening of semilunar valves and the
contraction of ventricles, the blood is ejected
out of both the ventricles. Hence, this period
is called ejection period. The duration of this
period is 0.22 second.
Ejection period is of two stages:
1. First stage is called the rapid ejection period.
Immediately after the opening of semilunar
valves, a large amount of blood is rapidly
ejected from both the ventricles. It lasts for
0.13 second.
2. Second stage is called the slow ejection
period. During this stage, the blood is ejected
slowly with much less force. The duration of
this period is 0.09 second.
VENTRICULAR DIASTOLE
1. Protodiastole
It is the first stage of ventricular diastole hence
the name protodiastole. Duration of this period
is 0.04 second. During this period the pressure
in ventricles drops due to ejection of blood. At
the end of this period intraventricular pressure
becomes less than the pressure in aorta and
pulmonary artery. This causes closure of
semilunar valves. The atrioventricular valves are
already closed (see above). No other change
occurs in the heart during this period. Thus,
protodiastole indicates only the end of systole
and beginning of diastole.
Second heart sound
Closure of semilunar valves during this phase
produces second heart sound.
2. Isometric Relaxation
Isometric relaxation is the type of muscular
relaxation characterized by decrease in tension
without any change in the length of muscle fibers.
Isometric relaxation of ventricular muscle is also
called isovolumetric relaxation.
During isometric relaxation period, once again
all the valves of the heart are closed (Fig. 60-2).
Now, both the ventricles relax as closed cavities
without any change in volume or length of the
muscle fiber. The intraventricular pressure
decreases during this period. Duration of
isometric relaxation period is 0.08 second.
Significance of isometric relaxation
During isometric relaxation period, the ventri-
cular pressure decreases greatly. When the
ventricular pressure becomes less than the
pressure in the atria, the atrioventricular valves
open. This leads to ventricular filling.
3. Rapid Filling
When AV valves are opened, there is a sudden
rush of blood from atria into ventricles. So this
period is called the first rapid filling period.
Filling during this period occurs without atrial
systole. About 70% of filling takes place during
this phase which lasts for 0.11 second.
Third heart sound
Rushing of blood into ventricles during this
phase causes production of third heart sound
(Fig. 60-3).
TABLE 60-1: Pressure changes
during cardiac cycle
Area
Maximum
Minimum
pressure
pressure
Left atrium
7 to 8 mm Hg
0 to 2 mm Hg
Right atrium
5 to 6 mm Hg
0 to 2 mm Hg
Left ventricle
120 mm Hg
5 mm Hg
Right ventricle
25 mm Hg
2 to 3 mm Hg
Systemic aorta
120 mm Hg
80 mm Hg
Pulmonary artery
25 mm Hg
7 to 8 mm Hg
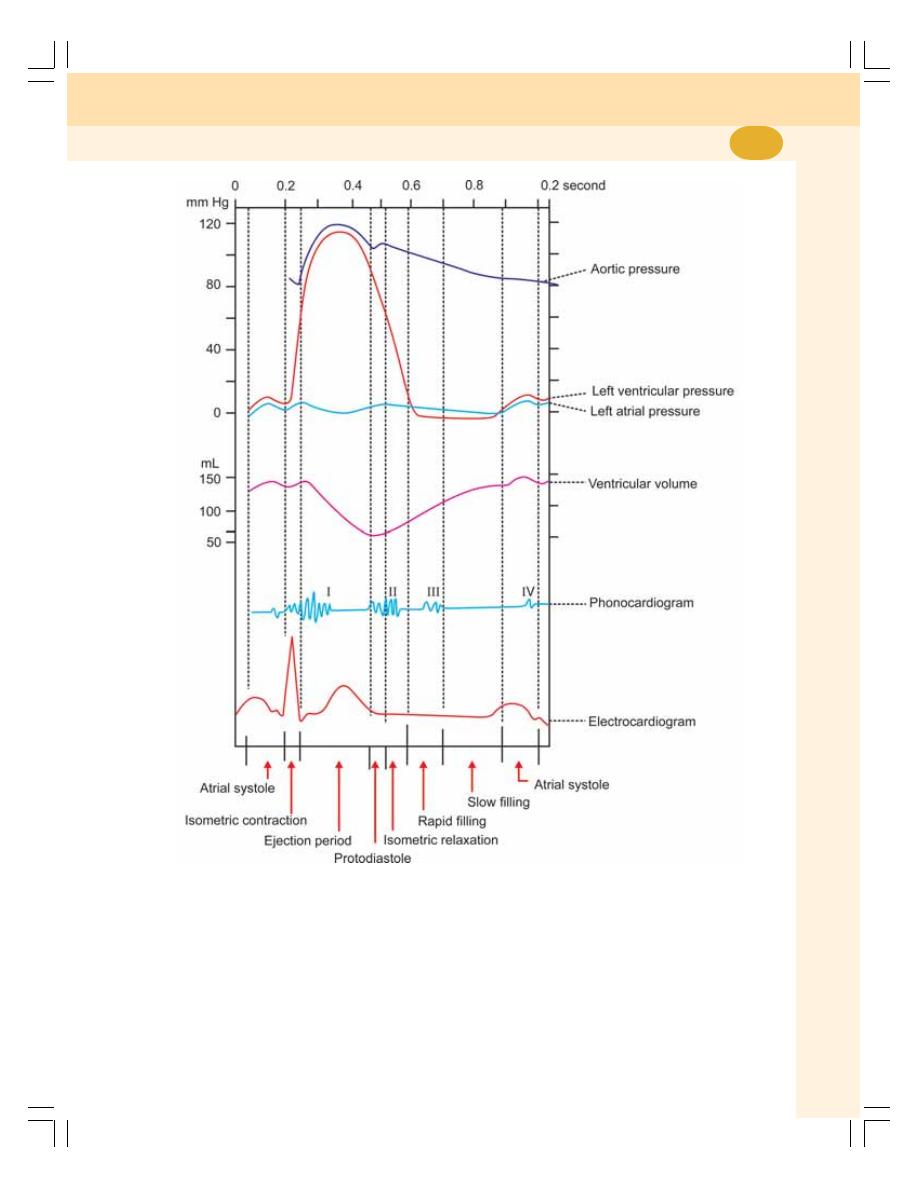
Chapter 60 Cardiac Cycle
387
4. Slow Filling
After the sudden rush of blood, the ventricular
filling becomes slow. Now, it is called the slow
filling. It is also called diastasis. Filling during
this phase also occurs without atrial systole.
About 20% of filling occurs in this phase.
Duration of slow filling phase is 0.19 second.
FIGURE 60-3: Comprehensive diagram showing ECG, phonocardiogram,
pressure changes and volume changes during cardiac cycle
5. Last Rapid Filling or
Atrial Systole
After slow filling period, the atria contract and
push a small amount of blood into the ventricles.
About 10% of ventricular filling takes place
during this period.
INTRODUCTION
DESCRIPTION OF DIFFERENT HEART SOUNDS
FIRST HEART SOUND
SECOND HEART SOUND
THIRD HEART SOUND
FOURTH HEART SOUND
METHODS OF STUDY OF HEART SOUNDS
CARDIAC MURMUR
INTRODUCTION
Heart sounds are the sounds produced by the
mechanical activities of the heart during each
cardiac cycle. Generally, heart sounds are
produced by:
1. Flow of blood through the chambers of the
heart
2. Contraction of cardiac muscle
3. Closure of valves of the heart.
The heart sounds are heard by placing the
ear over the chest or by using a stethoscope or
microphone. These sounds are also recorded
graphically.
Four heart sounds are produced during each
cardiac cycle. The first and second heart sounds
are called classical heart sounds. These sounds
are more prominent and resemble the spoken
words ‘LUB’ (or LUBB) and ‘DUB’ (or DUP)
respectively. These two heart sounds are heard
by using the stethoscope.
IMPORTANCE OF HEART SOUNDS
The study of heart sounds has important
diagnostic value in clinical practice because
the alteration in the heart sounds indicates the
cardiac diseases involving the valves of the
heart.
DESCRIPTION OF DIFFERENT
HEART SOUNDS
FIRST HEART SOUND
First heart sound is heard during isometric
contraction period and earlier part of ejection
period (Table 61-1).
Causes
The major cause for first heart sound is the
sudden and synchronous (simultaneous) closure
of atrioventricular valves. In addition to this, the
ejection of blood from ventricles into aorta and
Heart Sounds
61
Chapter 61 Heart Sounds
389
pulmonary artery and contraction of cardiac
muscles also contribute in the production of the
first heart sound.
Characteristics
The first heart sound is a long, soft and low
pitched sound. It resembles the spoken word
‘LUBB’. The duration of this sound is 0.10 to 0.17
second. Its frequency is 25 to 45 cycles/second.
First Heart Sound and ECG
First heart sound coincides with peak of ‘R’ wave
in ECG.
SECOND HEART SOUND
The second heart sound is produced at the end
of protodiastolic period.
Cause
The second heart sound is produced due to
the sudden and synchronous closure of the
semilunar valves.
Characteristics
The second heart sound is a short, sharp and
high pitched sound. It resembles the spoken
word ‘DUBB’ (or DUP). The duration of the
second heart sound is 0.10 to 0.14 seconds. Its
frequency is 50 cycles/second.
Second Heart Sound and ECG
The second heart sound coincides with the ‘T’
wave in ECG. Sometimes, it may precede the
‘T’ wave or it may commence after the peak of
‘T’ wave.
TABLE 61-1: Heart sounds
Features
First heart
Second heart
Third heart
Fourth heart
sound
sound
sound
sound
Occurs during
Isometric
Protodiastole and
Rapid filling phase
Atrial systole
contraction period
part of isometric
and part of ejection
relaxation
period
Cause
Closure of
Closure of
Rushing of blood
Contraction of
atrioventricular
semilunar valves
into ventricle
atrial musculature
valves
Characteristics
Long, soft and low
Short, sharp and
Low pitched
Inaudible sound
pitched.
high pitched.
Resembles the
Resembles the
word ‘LUB’
word ‘DUB’
Duration (sec)
0.10 to 0.17
0.10 to 0.14
0.07 to 0.10
0.02 to 0.04
Frequency
25 to 45
50
1 to 6
1 to 4
(cycles per sec)
Relation with ECG
Coincides with
Precedes or
Between ‘T’ wave
Between ‘P’ wave
peak of R’ wave
appears 0.09
and ‘P’ wave
and ‘Q’ wave
second after
peak of ‘T’ wave
No. of vibrations in
9 to 13
4 to 6
1 to 4
1 to 2
phonocardiogram

Cardiovascular System
390
THIRD HEART SOUND
The third heart sound is a low pitched sound that
is produced during rapid filling period of the
cardiac cycle. Usually, the third heart sound is
inaudible by stethoscope and it can be heard only
by using microphone.
Cause
Third heart sound is produced by the rushing of
blood into ventricles during rapid filling phase.
Characteristics
Third heart sound is a short and low pitched
sound. The duration of this sound is 0.07 to 0.10
second. Its frequency is 1 to 6 cycles/second.
Conditions when Third Heart Sound
becomes Audible by Stethoscope
Third heart sound can be heard by stethoscope
in children and athletes. Pathological condi-
tions when third heart sound becomes loud and
audible by stethoscope are aortic regurgitation,
cardiac failure and cardiomyopathy with dilated
ventricles.
When third heart sound is heard by
stethoscope the condition is called triple heart
sound. Third heart sound is usually heard best
with the bell of stethoscope placed at the apex
beat area when the patient is in left lateral
decubitus (lying on left side) position.
Third Heart Sound and ECG
It appears between ‘T’ and ‘P’ waves of ECG.
FOURTH HEART SOUND
Normally the fourth heart sound is an inaudible
sound. It becomes audible only in pathological
conditions. It is studied only by graphical
recording that is by phonocardiography. This
sound is produced during atrial systole (late
diastole) and it is considered as the physiologic
atrial sound.
Cause
Fourth heart sound is produced by contraction
of atrial musculature during atrial systole.
Characteristics
Fourth heart sound is a short and low pitched
sound. The duration of this sound is 0.02 to 0.04
second. And its frequency is 1 to 4 cycles/second.
Conditions when Fourth Heart Sound
becomes Audible
Fourth heart sound becomes audible by
stethoscope when the ventricles become stiff.
Ventricular stiffness occurs in conditions like
ventricular hypertrophy, long standing hyper-
tension and aortic stenosis. To overcome the
ventricular stiffness, the atria contract forcefully
producing audible fourth heart sound.
When fourth heart sound is heard by
stethoscope the condition is called triple heart
sound. It is usually heard best with the bell of
stethoscope placed at the apex beat area when
the patient is in supine or left semilateral
position.
Fourth Heart Sound and ECG
Fourth heart sound coincides with the interval
between the end of ‘P’ wave and the onset of
‘Q’ wave.
METHODS OF STUDY OF HEART
SOUNDS
Heart sounds are studied by three methods:
1. By using stethoscope
2. By using microphone
3. By phonocardiogram.
BY USING STETHOSCOPE —
AUSCULTATION AREAS
The first and second heart sounds are heard on
the auscultation areas by using the stethoscope.
The chest piece of the stethoscope is placed
over 4 areas on the chest, which are called
auscultation areas. The four auscultation areas
are:
i. Mitral Area (Bicuspid Area)
It is in the left 5th intercostal space about 10
cm away from the midline (midclavicular line).

Chapter 61 Heart Sounds
391
The sound produced by the closure of mitral
valve (first heart sound) is heard well on this
area. This area is also called apex beat area
because apex beat is felt in this area. Apex beat
is the thrust of the apex of the ventricles against
the chest wall during systole.
ii. Tricuspid Area
This area is on the xiphoid process. The sound
produced by the closure of tricuspid valve (first
heart sound) is transmitted well into this area.
iii. Pulmonary Area
The pulmonary area is on the left 2nd intercostal
space close to sternum. Sound produced by the
closure of pulmonary valve (second heart sound)
is heard well on this area.
iv. Aortic Area
This area is over the right 2nd intercostal space
close to the sternum. On this area, the sound
produced by the closure of aortic valve (second
heart sound) is heard well.
The first heart sound is best heard in mitral
and tricuspid areas. However, it is heard in other
areas also but the intensity is less. Similarly, the
second heart sound is best heard in pulmonary
and aortic areas. It is also heard in other areas
with less intensity.
BY MICROPHONE
A highly sensitive microphone is placed over the
chest. The heart sounds are amplified by means
of an amplifier and heard by using a loudspeaker.
First, second and third heart sounds are heard
by this method.
BY PHONOCARDIOGRAM
Phonocardiography is the technique used to
record the heart sounds. Phonocardiogram is the
graphical record of the heart sounds. It is done
by placing an electronic sound transducer over
the chest. This transducer is connected to a
recording device like polygraph. All the four heart
sounds can be recorded in phonocardiogram. It
helps to analyze the frequency of the sound
waves (Fig. 60-3).
CARDIAC MURMUR
Cardiac murmur is the abnormal or unusual heart
sound heard by stethoscope along with normal
heart sounds. Cardiac murmur is also called
abnormal heart sound or cardiac bruit. The
abnormal sound is produced because of the
change in the pattern of blood flow. Normally,
blood flows in stream line through the heart and
the blood vessels. However, during the abnormal
conditions like valvular diseases, the blood flow
becomes turbulent. It produces the cardiac
murmur.
The cardiac murmur is heard by placing the
chest piece of the stethoscope over the
auscultatory areas. The murmur due to disease
of a particular valve is heard well over the
auscultatory area of that valve. Sometimes, the
murmur is felt by palpation as “thrills”. In some
patients, the murmur is heard without any aid
even at a distance of few feet away from the
patient.
Valvular diseases are of two types:
1. Stenosis or narrowing of the heart valve: The
blood flows rapidly with turbulence through
the narrow orifice of the valve resulting in
murmur.
2. Incompetence or weakening of the heart
valve: When the valve becomes weak, it
cannot close properly. It causes back flow of
blood resulting in turbulence. This disease is
also called regurgitation or valvular insuffi-
ciency.
CLASSIFICATION OF MURMUR
Cardiac murmur is classified into three types:
1. Systolic murmur produced during systole of
the heart
2. Diastolic murmur produced during diastole of
the heart
3. Continuous murmur produced continuously.
DEFINITIONS
USES OF ECG
ELECTROCARDIOGRAPHIC GRID
ECG LEADS
WAVES OF NORMAL ECG
INTERVALS AND SEGMENTS OF ECG
DEFINITIONS
Electrocardiography
Electrocardiography is the technique by which
the electrical activities of the heart are studied.
Electrocardiograph
Electrocardiograph is the instrument (ECG
machine) by which the electrical activities of
the heart are recorded.
Electrocardiogram
Electrocardiogram (ECG) is the record or
graphical registration of electrical activities of
the heart, which occur prior to the onset of
mechanical activities. It is the summed electrical
activity of all the cardiac muscle fibers recorded
from the surface of the body.
USES OF ECG
ECG is useful in determining and diagnosing
the following:
1. Heart rate
2. Heart rhythm
3. Abnormal electrical conduction
4. Poor blood flow to heart muscle (ischemia)
5. Heart attack
6. Coronary artery disease
7. Hypertrophy of heart chambers.
ELECTROCARDIOGRAPHIC GRID
The paper that is used for recording ECG is
called ECG paper. The electrocardiograph or
ECG machine amplifies the electrical signals
produced from the heart and records these
signals on a moving ECG paper. ECG grid refers
to the markings (lines) on ECG paper. The ECG
paper has horizontal and vertical lines at regular
intervals of 1 mm. Every 5th line (5 mm) is
thickened.
DURATION
The duration of different ECG waves is denoted
by the vertical lines.
Interval between two thick lines (5 mm)
= 0.2 second.
Interval between two thin lines (1 mm)
= 0.04 second.
Electrocardiogram
62
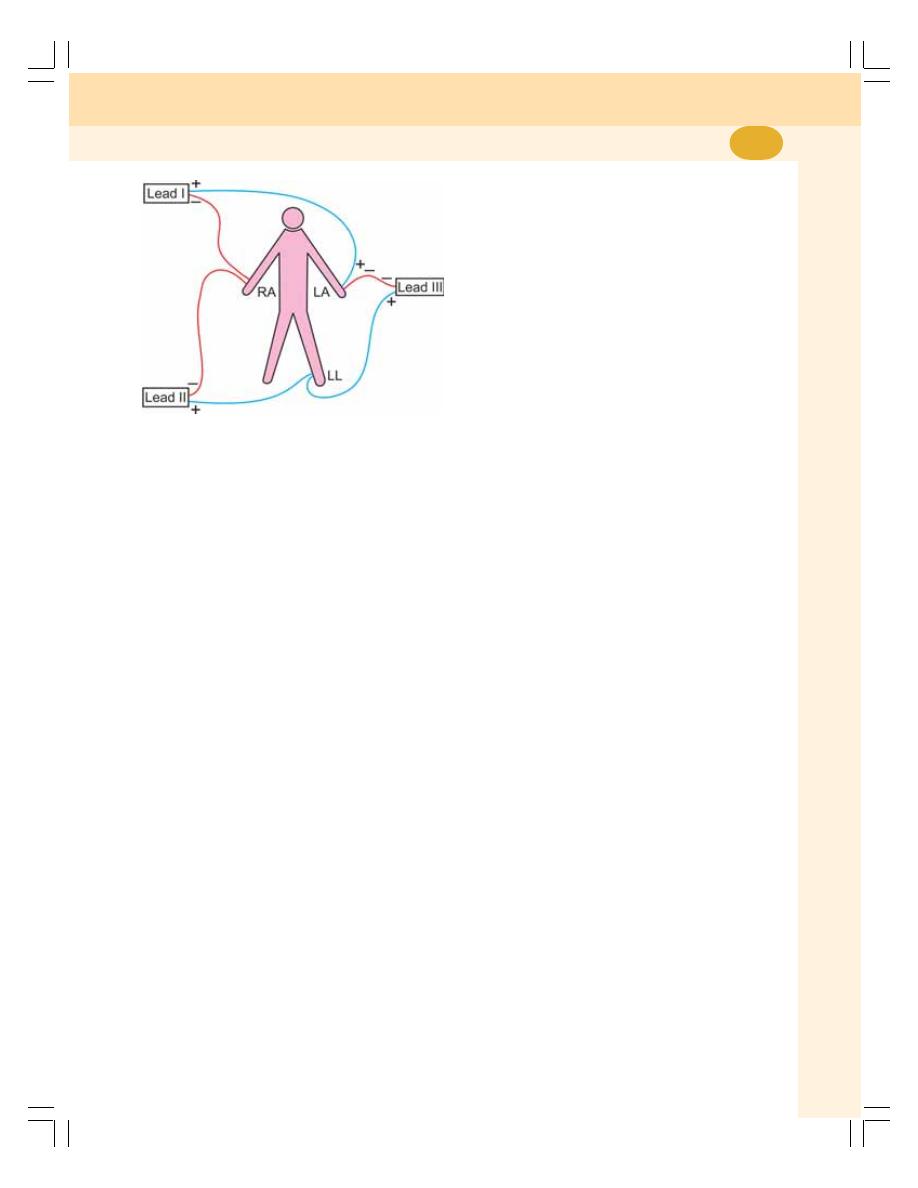
Chapter 62 Electrocardiogram
393
ECG is recorded in 12 leads which are
generally classified into two categories.
A. Bipolar leads
B. Unipolar leads
BIPOLAR LIMB LEADS
Bipolar limb leads are otherwise known as
standard limb leads. Two limbs are connected
to obtain these leads and both the electrodes
are active recording electrodes, i.e. one electrode
is positive and the other one is negative (Fig. 62-1).
There are three standard limb leads:
Limb lead I
Limb lead II
Limb lead III
Lead I
Lead I is obtained by connecting right arm and
left arm. The right arm is connected to the
negative terminal of the instrument and the left
arm is connected to the positive terminal.
Lead II
Lead II is obtained by connecting right arm and
left leg. The right arm is connected to the
negative terminal of the instrument and the left
leg is connected to the positive terminal.
Lead III
Lead III is obtained by connecting left arm and
left leg. The left arm is connected to the negative
terminal of the instrument and the left leg is
connected to the positive terminal.
UNIPOLAR LEADS
Here, one electrode is active electrode and the
other one is an indifferent electrode. The active
electrode is positive and the indifferent electrode
is serving as a composite negative electrode.
The unipolar leads are of two types:
1. Unipolar limb leads
2. Unipolar chest leads.
FIGURE 62-1: Position of electrodes for standard limb
leads. RA = Right arm. LA = Left arm. LL = Left leg
AMPLITUDE
The amplitude of ECG waves is denoted by
horizontal lines.
Interval between two thick lines (5 mm)
= 0.5 mV.
Interval between two thin lines (1 mm)
= 0.1 mV.
SPEED OF THE PAPER
The movement of paper can be adjusted in two
speeds, 25 mm/second and 50 mm/second.
Usually, the speed of the paper during recording
is fixed at 25 mm/second. If the heart rate is very
high, the speed of the paper is changed to 50
mm/second.
ECG LEADS
ECG is recorded by placing series of electrodes
on the surface of the body. These electrodes are
called ECG leads and are connected to the ECG
machine.
The electrodes are fixed on the limbs. Usually
right arm, left arm and left leg are chosen. The
heart is said to be in the center of an imaginary
equilateral triangle drawn by connecting the roots
of these three limbs. This triangle is called
Einthoven’s triangle. The electrical potential
generated from the heart appears simul-
taneously on the roots of these three limbs.
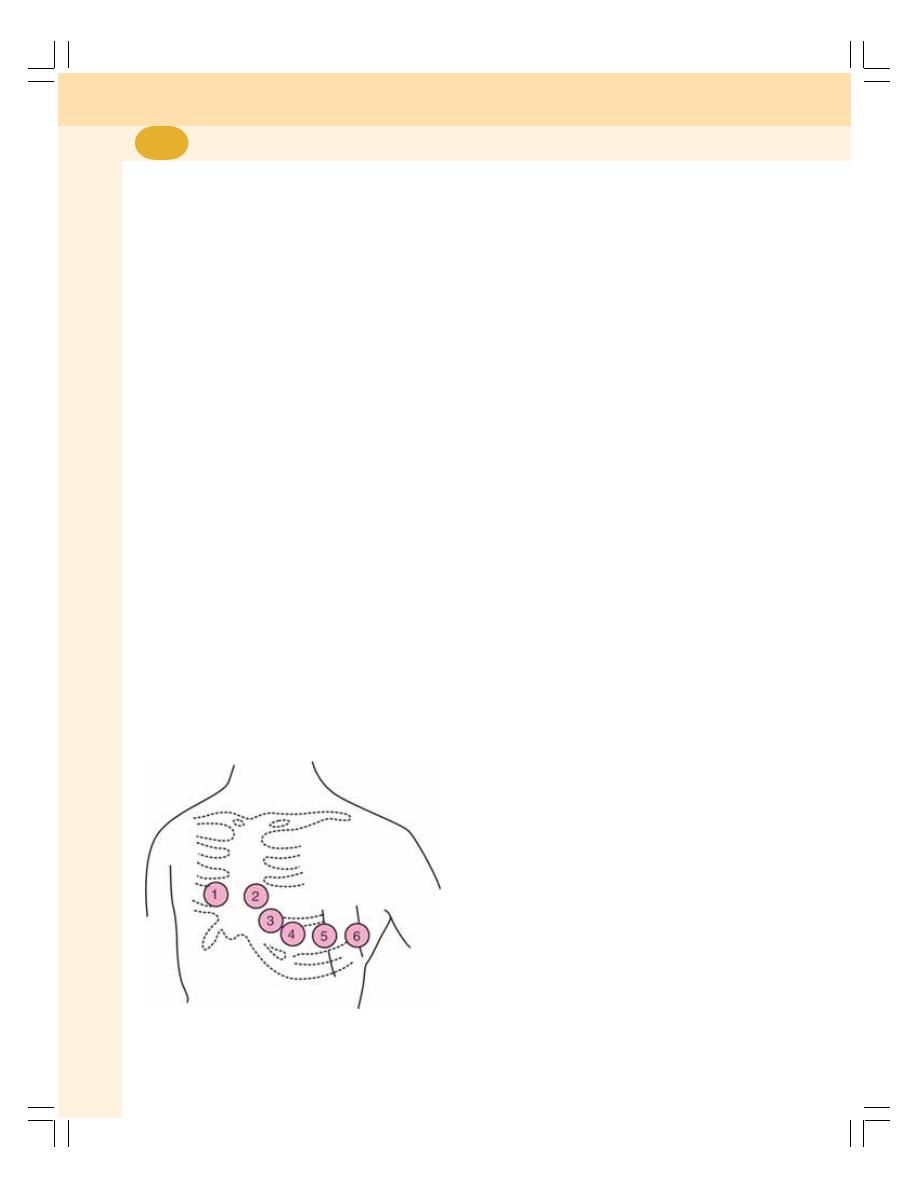
Cardiovascular System
394
1. Unipolar Limb Leads
These leads are also called augmented limb
leads. The active electrode is connected to one
of the limbs. The indifferent electrode is obtained
by connecting the other two limbs through a
resistance.
Unipolar limb leads are of three types:
i. aVR lead in which the active electrode is
from right arm
ii. aVL lead in which the active electrode is
from left arm
iii. aVF lead in which the active electrode is
from left leg (foot).
2. Unipolar Chest Leads
Chest leads are also called precardial leads.
The indifferent electrode is obtained by
connecting the three limbs – left arm, left leg and
right arm through a resistance of 5000 ohms.
The active electrode is placed on six points over
the chest (Fig. 62-2). This electrode is known
as the chest electrode and the six points over
the chest are called V
1
, V
2
, V
3
, V
4
, V
5
, and V
6
.
V indicates vector, which shows the direction of
flow of current.
Position of chest leads:
V
1
: Over 4th intercostal space near right sternal
margin
V
2
: Over 4th intercostal space near left sternal
margin
V
3
: In between V
2
and V
4
V
4
: Over left 5th intercostal space on the mid
clavicular line
V
5
: Over left 5th intercostal space on the
anterior axillary line
V
6
: Over left 5th intercostal space on the mid
axillary line.
WAVES OF NORMAL
ELECTROCARDIOGRAM
A normal ECG consists of waves, complexes,
intervals and segments. The waves of ECG
recorded by Limb Lead II are considered as the
typical waves. Normal electrocardiogram has the
following waves namely P, Q, R, S and T (Table
62-1 and Figs 62-3 and 62-4)). Einthoven had
named the waves of ECG starting from the
middle of the English alphabets (P) instead of
starting from the beginning (A).
The major complexes in ECG are:
1. ‘P’ wave, the atrial complex
2. ‘QRS’ complex, the initial ventricular complex
3. ‘T’ wave, the final ventricular complex.
4. ‘QRST’, the ventricular complex.
‘P’ WAVE
It is a positive wave and the first wave in ECG.
It is also called atrial complex.
Cause
‘P’ wave is a positive wave produced due to the
depolarization of atrial musculature. Atrial
repolarization is not recorded as a separate wave
in ECG because it merges with QRS complex.
Duration
0.1 second.
Amplitude
0.1 to 0.12 mV.
‘QRS’ Complex
It is also called the initial ventricular complex.
‘Q’ wave is a small negative wave. It is continued
as the tall ‘R’ wave, which is a positive wave. ‘R’
FIGURE 62-2: Position of electrodes for chest
leads (V
1
to V
6
)
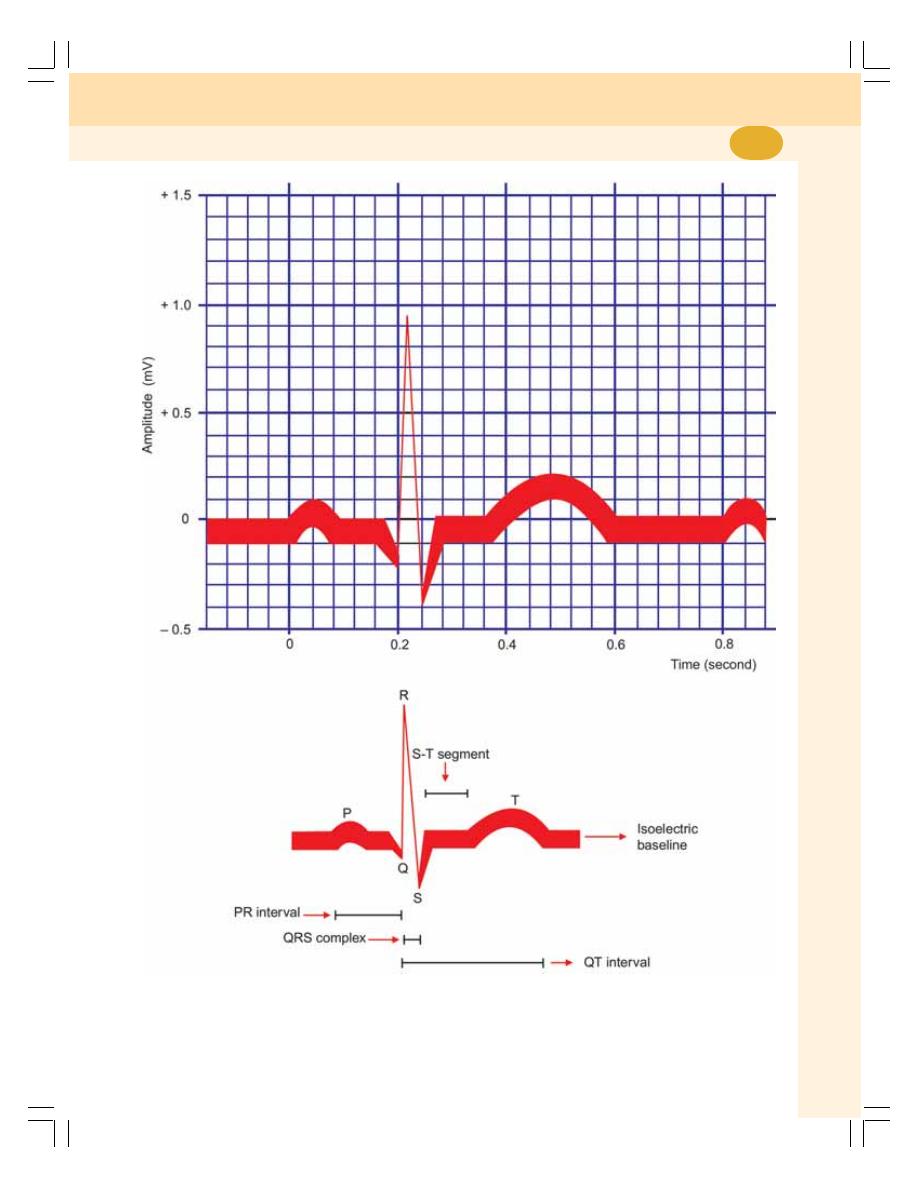
Chapter 62 Electrocardiogram
395
FIGURE 62-3: Waves of normal ECG

Cardiovascular System
396
wave is followed by a small negative wave, the
‘S’ wave.
Cause
‘QRS’ complex is due to depolarization of
ventricular musculature. ‘Q’ wave is due to the
depolarization of basal portion of interventricular
septum. ‘R’ wave is due to the depolarization of
apical portion of interventricular septum and
apical portion of ventricular muscle. And, ‘S’ wave
is due to the depolarization of basal portion of
ventricular muscle near the atrioventricular ring.
Duration
0.08 to 0.10 second.
Amplitude
‘Q’ wave: 0.1 to 0.2 mV.
‘R’ wave: 1 mV.
‘S’ wave: 0.4 mV.
‘T’ WAVE
It is the final ventricular complex and is a positive
wave.
Cause
‘T’ wave is due to the repolarization of ventricular
musculature.
Duration
0.2 second.
Amplitude
0.3 mV.
‘U’ WAVE
‘U’ wave is not always seen. It is also an
insignificant wave in ECG. It is supposed to be
due to repolarization of papillary muscle.
INTERVALS AND SEGMENTS
OF ECG
‘P-R’ INTERVAL
It is the interval between the onset of ‘P’ wave
and the onset of ‘Q’ wave.
‘P-R’ interval signifies the atrial depolariza-
tion and conduction of impulses through AV node.
It shows the duration of conduction of the
impulses from the SA node to ventricles through
atrial muscle and AV node.
It is represented by the short isoelectric (zero
voltage) period after the end of ‘P’ wave and
onset of ‘Q’ wave. It denotes the time taken for
the passage of depolarization within AV node.
Duration
The normal duration is 0.18 second and varies
between 0.12 and 0.2 second. If it is more than
0.2 second, that signifies the delay in the
conduction of impulse from SA node to the
ventricles. Usually, the delay occurs in the AV
node. So it is called the AV nodal delay.
‘Q-T’ INTERVAL
It is the interval between the onset of ‘Q’ wave
and the end of ‘T’ wave.
‘Q-T’ interval indicates the ventricular depo-
larization and ventricular repolarization, i.e. it
signifies the electrical activity in ventricles.
FIGURE 62-4: 12 – lead ECG
(Courtesy: Dr Atul Luthra)
Chapter 62 Electrocardiogram
397
Duration
Between 0.4 and 0.42 second.
‘S-T’ SEGMENT
The time interval between the end of ‘S’ wave
and the onset of ‘T’ wave is called ‘S-T’ segment.
It is an isoelectric period.
J Point
The point where ‘S-T’ segment starts is called
‘J’ point. It is the junction between the QRS
complex and ‘S-T’ segment.
TABLE 62-1: Waves of normal ECG
Wave/Segment
From - To
Cause
Duration (second)
Amplitude (mV)
P wave
— — —
Atrial depolarization
0.1
0.1 to 0.12
QRS complex
Onset of Q wave to Ventricular depolarization 0.08 to 0.10
Q = 0.1 to 0.2
the end of S wave
R = l
S = 0.4
T wave
— — —
Ventricular repolarization
0.2
0.3
P-R interval
Onset of P wave to Atrial depolarization and
0.18 (0.12 to 0.2)
— — —
onset of Q wave
conduction through AV
node
Q-T interval
Onset of Q wave
Electrical activity in
0.4 to 0.42
— — —
and end of T wave
ventricles
S-T segment
End of S wave and
Isoelectric
0.08
— — —
onset of T wave
Duration of ‘S-T’ Segment
0.08 second.
‘R-R’ INTERVAL
‘R-R’ interval is the time interval between two
consecutive ‘R’ waves. ‘R-R’ interval signifies the
duration of one cardiac cycle.
Duration
The normal duration of ‘R-R’ interval is 0.8
second.
INTRODUCTION
DEFINITIONS AND NORMAL VALUES
STROKE VOLUME
MINUTE VOLUME
CARDIAC INDEX
VARIATIONS IN CARDIAC OUTPUT
PHYSIOLOGICAL VARIATIONS
PATHOLOGICAL VARIATIONS
DISTRIBUTION OF CARDIAC OUTPUT
FACTORS MAINTAINING CARDIAC OUTPUT
VENOUS RETURN
FORCE OF CONTRACTION
HEART RATE
PERIPHERAL RESISTANCE
MEASUREMENT OF CARDIAC OUTPUT
INTRODUCTION
Cardiac output is the amount of blood pumped
from each ventricle. Usually, it refers to the left
ventricular output through aorta. Cardiac output
is the most important factor in cardiovascular
system, because, the rate of blood flow through
different parts of the body depends upon the
cardiac output.
DEFINITIONS AND NORMAL
VALUES
Usually, cardiac output is expressed in three
ways:
1. Stroke volume
2. Minute volume
3. Cardiac index.
However, in routine clinical practice cardiac
output refers to minute volume.
1. STROKE VOLUME
It is the amount of blood pumped out by each
ventricle during each beat.
Normal value: 70 mL (60 to 80 mL) when the
heart rate is normal (72/minute).
2. MINUTE VOLUME
Minute volume is the amount of blood pumped
out by each ventricle in one minute. It is the
product of stroke volume and heart rate:
Cardiac Output
63

Chapter 63 Cardiac Output
399
Minute volume = Stroke volume × Heart rate
Normal value: 5 liters/ ventricle/ minute.
3. CARDIAC INDEX
Cardiac index is the minute volume expressed
in relation to square meter of body surface area.
It is defined as the amount of blood pumped out
per ventricle/minute/ square meter of the body
surface area.
Normal value: Cardiac index = 2.8 ± 0.3 liters/
square meter of body surface area/ minute.
(In an adult, the average body surface area is
1.734 square meter and normal minute volume
is 5 liters/minute).
VARIATIONS IN CARDIAC OUTPUT
PHYSIOLOGICAL VARIATIONS
1. Age: In children, cardiac output is less
because of less blood volume. The
cardiac index is more than in adults
because of less body surface area
2. Sex: In females, cardiac output is less
Cardiac index is more than in males,
because of less body surface area
3. Body build: Greater the body build, more
is the cardiac output
4. Diurnal variation: Cardiac output is low in
early morning and increases in day time
5. Environmental temperature: Moderate
change in temperature does not affect
cardiac output. Increase in temperature
above 30°C raises cardiac output
6. Emotional conditions: Anxiety, appre-
hension and excitement increase cardiac
output about 50 to 100%
7. After meals: During the first one hour after
taking meals, cardiac output increases
8. Exercise: Cardiac output increases during
exercise
9. High altitude: In high altitude, the cardiac
output increases
10. Posture: While changing from recumbent
to upright position, the cardiac output
decreases
11. Pregnancy: During the later months of
pregnancy, cardiac output increases by
40%
12. Sleep: Cardiac output is slightly decreased
or unaltered during sleep.
PATHOLOGICAL VARIATIONS
Conditions when Cardiac Output
Increases
1. Fever
2. Anemia
3. Hyperthyroidism.
Conditions when Cardiac Output
Decreases
1. Hypothyroidism
2. Atrial fibrillation
3. Heart block
4. Congestive cardiac failure
5. Shock
6. Hemorrhage.
DISTRIBUTION OF CARDIAC
OUTPUT
The whole amount of blood pumped out by right
ventricle goes to lungs. But, the blood pumped
by left ventricle is distributed to different parts
of the body. The fraction of cardiac output
distributed to a particular region or organ
depends upon the metabolic activities of that
region or organ. The distribution of blood
pumped out of left ventricle is:
Liver
: 1500 mL = 30%
Kidneys
: 1300 mL = 26%
Skeletal muscles
:
900 mL = 18%
Brain
:
800 mL = 16%
Skin, bone and GI tract :
300 mL =
6%
Heart
:
200 mL =
4%
Total
: 5000 mL = 100%
The heart, which pumps the blood to all the
other organs, receives the least amount of blood.
FACTORS MAINTAINING CARDIAC
OUTPUT
Cardiac output is maintained (determined) by four
factors:
1. Venous return
2. Force of contraction
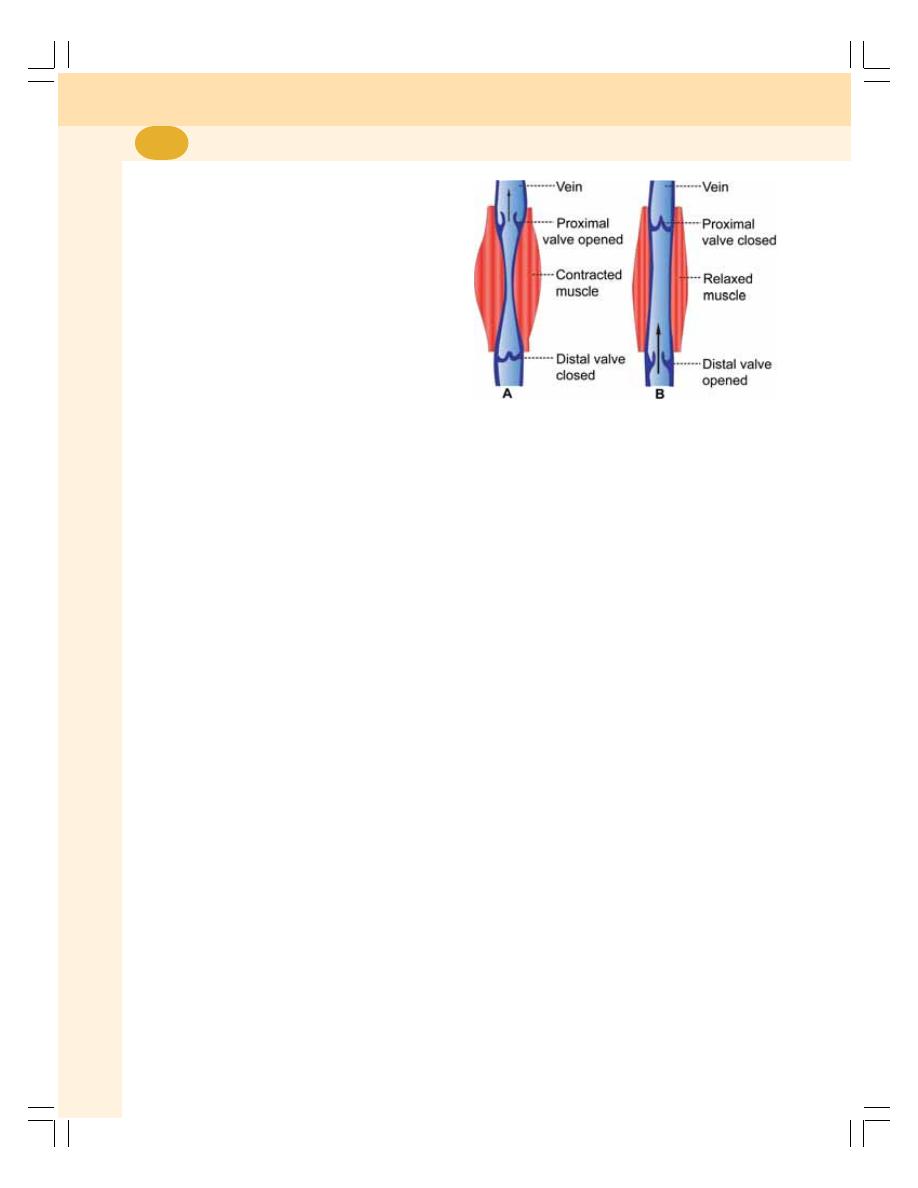
Cardiovascular System
400
3. Heart rate
4. Peripheral resistance.
1. VENOUS RETURN
Venous return is the amount of blood, which is
returned to the heart from different parts of the
body. When it increases, the ventricular filling
and cardiac output are increased. Thus, cardiac
output is directly proportional to venous return
provided the other factors (force of contraction,
heart rate and peripheral resistance) remain
constant.
Venous return in turn depends upon
respiratory pump and muscle pump.
i.
Respiratory Pump
Respiratory pump is the respiratory activity that
helps return of the blood back to heart during
inspiration. It is also called abdominothoracic
pump. During inspiration, thoracic cavity expands
and makes the intrathoracic pressure more
negative. It increases the diameter of inferior
vena cava resulting in increased venous return.
At the same time, descent of diaphragm
increases the intra-abdominal pressure which
compresses abdominal veins and pushes the
blood upward towards the heart and thereby the
venous return is increased.
Respiratory pump is much stronger in forced
respiration and in severe muscular exercise.
ii. Muscle Pump
Muscle pump is the muscular activity that helps
return of the blood back to heart. When muscular
activity increases the venous return is more.
When the skeletal muscles contract the vein
located in between the muscles is compressed.
The valve of the vein proximal to the contracting
muscles (Fig. 63-1A) is opened and the blood
is propelled towards the heart. The valve of the
vein distal to the muscles is closed by the back
flow of blood.
During the relaxation of the muscles
(Fig. 63-1B), the valve proximal to the muscles
closes and prevents the back flow of the blood.
And the valve distal to the muscles opens and
allows the blood to flow upwards.
2. FORCE OF CONTRACTION
The cardiac output is directly proportional to the
force of contraction provided the other three
factors remain constant. Force of contraction
depends upon diastolic period and ventricular
filling. Frank-Starling law of heart is applicable
to this.
According to Frank-Starling law, the force of
contraction of heart is directly proportional to the
initial length of muscle fibers before the onset
of contraction.
The force of contraction also depends upon
preload and after load.
Preload
Preload is the stretching of the cardiac muscle
fibers at the end of diastole just before
contraction. Preload depends upon venous
return and ventricular filling. During diastolic
period due to the ventricular filling, the ventricular
pressure increases. This causes stretching of
muscle fibers resulting in increase in their length.
The length of the muscle fibers determines the
force of contraction and cardiac output.
The force of contraction of heart and cardiac
output are directly proportional to preload.
Afterload
Afterload is the force against which the ventricles
must contract and eject the blood. The force is
determined by the arterial pressure. At the end
of isometric contraction period, the semilunar
FIGURE 63-1: Mechanism of muscle pump

Chapter 63 Cardiac Output
401
valves are opened and blood is ejected into the
aorta and pulmonary artery. So the pressure
increases in these two vessels. Now, the
ventricles have to work against this pressure for
further ejection. Thus, the afterload for left
ventricle is determined by aortic pressure and
afterload for right ventricular pressure is
determined by pressure in pulmonary artery.
The force of contraction of heart and cardiac
output are inversely proportional to afterload.
A. During contraction of the muscle. B. During
relaxation of the muscle.
3. HEART RATE
Cardiac output is directly proportional to heart
rate provided the other three factors remain
constant. Moderate change in heart rate does
not alter the cardiac output. If there is a marked
increase in heart rate, cardiac output is
increased.
If there is marked decrease in heart rate,
cardiac output is decreased.
4. PERIPHERAL RESISTANCE
Peripheral resistance is the resistance offered
to blood flow at the peripheral blood vessels.
Peripheral resistance is the resistance or load
against which the heart has to pump the blood.
So, the cardiac output is inversely proportional
to peripheral resistance.
The resistance is offered at arterioles. So, the
arterioles are called resistant vessels. In the body,
the maximum peripheral resistance is offered at
the splanchnic region.
MEASUREMENT OF CARDIAC
OUTPUT
The methods used to measure cardiac output
are:
1. By using Fick’s principle
2. Indicator (dye) dilution technique
3. Thermodilution technique
4. Ultrasonic Doppler transducer technique
5. Doppler echocardiography
6. Ballistocardiography.
1. By Using Fick’s Principle
According to this principle, the amount of a
substance taken up by an organ (or by the whole
body) or given out in a unit of time is the product
of amount of blood flowing through the organ and
the arteriovenous difference of the substance
across the organ.
Amount of
Amount of
Arteriovenous
substance
= blood
× difference
taken or given
flow/minute
The Fick’s principle is modified to measure the
cardiac output or a part of cardiac output (amount
of blood to an organ). Thus, cardiac output or
the amount of blood flowing through an organ
in a given unit of time is determined by the
formula given below.
By using Fick’s principle, cardiac output is
measured in two ways:
i. By using oxygen consumption
ii. By using carbon dioxide given out.
Measurement of Cardiac Output by Using
Oxygen Consumption
Fick’s principle is used to measure cardiac
output by determining the amount of oxygen
consumed in the body in a given period of time
and dividing this value by the arteriovenous
difference across the lungs.
Oxygen consumption: To measure the
amount of oxygen consumed, a respirometer is
used.
Oxygen content in arterial blood: For
determining the oxygen content in arterial
blood, blood is collected from any artery. Oxygen
content is determined by blood gas analysis.
Oxygen content in venous blood: For
determining the oxygen content of venous blood,
only mixed venous blood is used, since oxygen
content is different in different veins. The mixed
venous blood is collected from right atrium or
pulmonary artery. It is done by introducing a
catheter through basilar vein of forearm. Oxygen
is determined from this blood by blood gas
analysis.
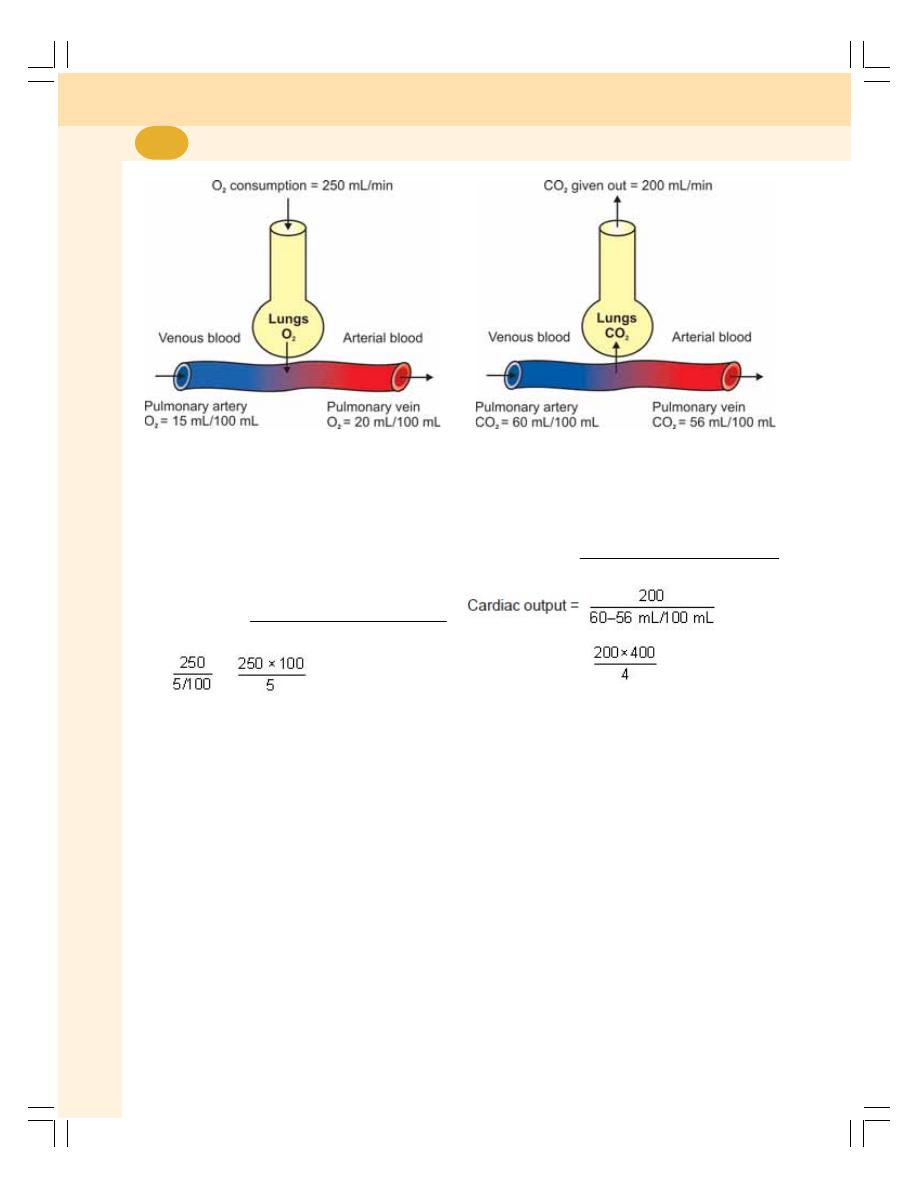
Cardiovascular System
402
FIGURE 63-2: Oxygen consumption
Calculation
For example, in a subject the following data are
obtained (Fig. 63-2):
O
2
consumed (by lungs) = 250 mL/minute
O
2
content in arterial blood = 20 mL/100 mL
O
2
content in venous blood = 15 mL/100 mL
Cardiac output =
2
2
O consumed (in mL/minute)
Arteriovenous O difference
=
=
= 5000 mL/minute
5 mL of oxygen is taken by 100 mL of blood
while passing through the lungs. Thus, 250 mL
of oxygen is taken by 5000 mL of blood. Since,
cardiac output is equivalent to the amount of
blood passing through pulmonary circulation, the
cardiac output = 5 liters/minute.
Measurement of Cardiac Output by Using
Carbon Dioxide
The cardiac output is also measured by knowing
the arteriovenous difference of carbon dioxide
and amount of carbon dioxide given out from
lungs (Fig. 63-3).
Calculation
For example, in a subject
FIGURE 63-3: Carbon dioxide given out
CO
2
removed by lungs = 200 mL/minute
CO
2
content in arterial blood = 56 mL/100 mL
CO
2
content in venous blood = 60 mL/100 mL
Cardiac output =
2
2
CO given out (in mL/minute)
Arteriovenous CO difference
=
= 5000 mL = 5 liters/minute
Since, cardiac output is equal to amount of
blood passing through lungs (pulmonary
circulation), the cardiac output = 5 liters/minute.
2. Indicator (Dye) Dilution Method
The indicator dilution technique is described in
detail in Chapter 5. Marker substance used to
measure cardiac output is lithium chloride.
3. Thermodilution Technique
Cardiac output can also be measured by
thermodilution technique or thermal indicator
method. This method is the modified indicator
dilution method. It is the popular method to
measure cardiac output.

Chapter 63 Cardiac Output
403
In this method, a known volume of cold sterile
solution is injected into the right atrium by using
a catheter. Cardiac output is measured by
determining the resultant change in the blood
temperature in pulmonary artery. For this purpose,
two thermistors (temperature transducers) are
used. One of them is placed in the inferior vena
cava and the second one is placed in pulmonary
artery.
4. Esophageal Doppler Transducer
Technique
This technique involves insertion of a flexible
probe into mid thoracic part of esophagus. A
pulse wave ultrasonic Doppler transducer is fixed
at the tip of the probe. This transducer calculates
the velocity of blood flow in descending aorta
(refer ultrasonic Doppler flow meter for details).
The diameter of aorta is determined by echo-
cardiography (see below). Cardiac output is
calculated by using the values of velocity of blood
flow and diameter of aorta.
5. Doppler Echocardiography
Doppler echocardiography is a method for
detecting the direction and velocity of moving
blood within the heart. This is also a popular
method to measure cardiac output.
6. Ballistocardiographic Method
Ballistocardiography is the technique to record
the movements of the body caused by ballistic
recoil associated with contraction of heart and
ejection of blood. It is based on Newton’s third
law of motion (for every action there is an equal
and opposite reaction). When heart pumps blood
into aorta and pulmonary artery, a recoiling force
is exerted against heart and the body. It is similar
to that of ballistic recoil when a bullet is fired from
a riffle.
Pulsations due to this ballistic recoil can be
recorded graphically by making the subject to lie
on a suspended bed movable in the long axis of
the body. The cardiac output is determined by
analyzing the graph obtained.
HEART RATE
NORMAL HEART RATE
TACHYCARDIA
BRADYCARDIA
REGULATION OF HEART RATE
VASOMOTOR CENTER
VASOCONSTRICTOR AREA
VASODILATOR AREA
SENSORY AREA
MOTOR (EFFERENT) NERVE FIBERS TO HEART
PARASYMPATHETIC NERVE FIBERS
SYMPATHETIC NERVE FIBERS
SENSORY (AFFERENT) NERVE FIBERS FROM HEART
FACTORS AFFECTING VASOMOTOR CENTER – REGULATION OF VAGAL
TONE
IMPULSES FROM HIGHER CENTERS
IMPULSES FROM RESPIRATORY CENTERS
IMPULSES FROM BARORECEPTORS
IMPULSES FROM CHEMORECEPTORS
IMPULSES FROM RIGHT ATRIUM
IMPULSES FROM OTHER AFFERENT NERVES
HEART RATE
NORMAL HEART RATE
Normal heart rate is 72/minute. It ranges between
60 and 80 per minute.
TACHYCARDIA
Tachycardia is the increase in the heart rate
above 100/minute.
Physiological conditions when tachycardia
occurs are:
1. Childhood
Heart Rate
64

Chapter 64 Heart Rate
405
2. Exercise
3. Pregnancy
4. Emotional conditions such as anxiety.
Pathological conditions when tachycardia occurs
are:
1. Fever
2. Anemia
3. Hypoxia
4. Hyperthyroidism
5. Hypersecretion of catecholamines
6. Cardiomyopathy
7. Valvular heart diseases.
BRADYCARDIA
Bradycardia is the decrease in the heart rate
below 60/minute.
Physiological conditions when bradycardia
occurs are:
1. Sleep
2. Athletic heart.
Pathological conditions when bradycardia
occurs are:
1. Hypothermia
2. Hypothyroidism
3. Heart attack
4. Congenital heart disease
5. Degenerative process of aging
6. Obstructive jaundice
7. Increased intracranial pressure.
REGULATION OF HEART RATE
Heart rate is maintained within normal range
constantly. It is subjected for variation during
normal physiological conditions such as
exercise, emotion, etc. However, under physio-
logical conditions, the altered heart rate is quickly
brought back to normal. Heart rate is regulated
by the nervous mechanism which consists of
three components:
I. Vasomotor center
II. Motor (efferent) nerve fibers to the heart
III. Sensory (afferent) nerve fibers from the
heart.
VASOMOTOR CENTER – CARDIAC
CENTER
Vasomotor center is the nervous center that
regulates the heart rate. It also regulates the
blood pressure. Earlier it was called the cardiac
center.
Vasomotor center is bilaterally situated in the
reticular formation of medulla oblongata and the
lower part of the pons.
Vasomotor center has three areas:
1. Vasoconstrictor area
2. Vasodilator area
3. Sensory area.
VASOCONSTRICTOR AREA –
CARDIOACCELERATOR CENTER
Situation
It is situated in the reticular formation of medulla
in the floor of the IV ventricle and it forms the
lateral portion of vasomotor center. It is otherwise
known as pressor area or cardioaccelerator
center.
Function
This area increases the heart rate by sending
accelerator impulses to heart through sympa-
thetic nerves. It also causes constriction of blood
vessels.
VASODILATOR AREA –
CARDIOINHIBITORY CENTER
Situation
It is also situated in the reticular formation of
medulla oblongata in the floor of IV ventricle. It
forms the medial portion of vasomotor center. It
is also called depressor area or cardioinhibitory
center.
Function
This area decreases the heart rate by sending
inhibitory impulses to the heart through vagus
nerve. It also causes dilatation of blood vessels.
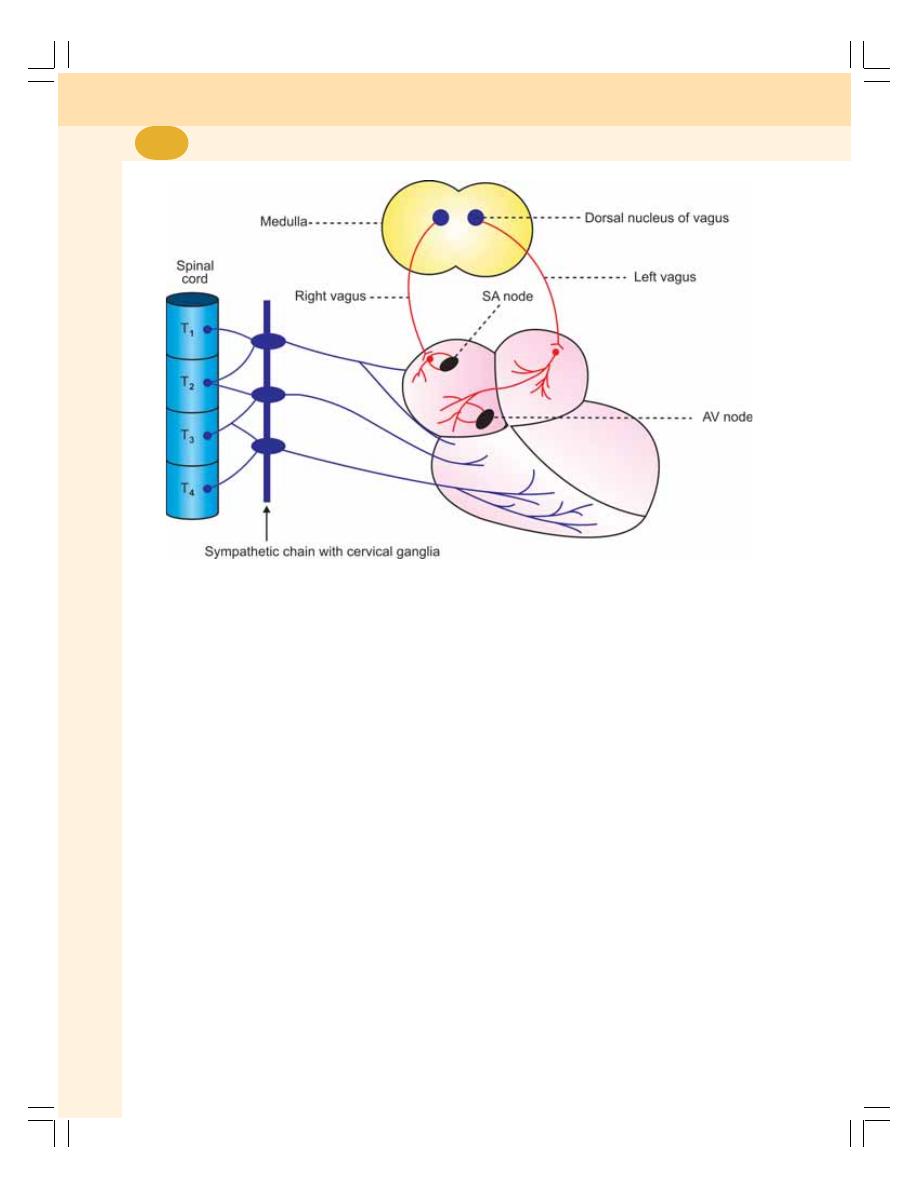
Cardiovascular System
406
SENSORY AREA
It is in the posterior part of vasomotor center,
which lies in nucleus of tractus solitarius in
medulla and pons. This area receives sensory
impulse via glossopharyngeal nerve and vagus
nerve from periphery, particularly, from the
baroreceptors. In turn, this area controls the
vasoconstrictor and vasodilator areas.
MOTOR (EFFERENT) NERVE
FIBERS TO HEART
Heart receives efferent nerves from both the
divisions of autonomic nervous system.
Parasympathetic fibers arise from the medulla
oblongata and pass through vagus nerve. The
sympathetic fibers arise from upper thoracic
(T
1
to T
4
) segments of spinal cord (Fig. 64-1).
PARASYMPATHETIC NERVE FIBERS
Origin
The parasympathetic nerve fibers supplying
heart arise from the dorsal nucleus of vagus
situated in the floor of the fourth ventricle in
medulla oblongata.
Distribution
The preganglionic parasympathetic nerve fibers
from dorsal nucleus of vagus reach the heart
and terminate on postganglionic neurons. The
postganglionic fibers from these neurons
innervate heart muscle. Most of the fibers from
right vagus terminate in SA node. Remaining
fibers supply the atrial muscles and AV node.
Most of the fibers from left vagus supply AV
node and some fibers supply the atrial muscle
and SA node. Ventricles do not receive the
vagus nerve supply.
Function
The vagus nerve is cardioinhibitory in function
and carries inhibitory impulses from vasodilator
area to the heart.
Vagal Tone
Vagal tone is the continuous stream of inhibitory
impulses arising from vasodilator area. Heart
FIGURE 64-1: Nerve supply to heart

Chapter 64 Heart Rate
407
rate is kept under control because of vagal tone.
The impulses from vasodilator area pass through
vagus nerve, reach the heart and exert inhibitory
effect on heart. The heart rate is inversely
proportional to vagal tone.
SYMPATHETIC NERVE FIBERS
Origin
The preganglionic fibers of the sympathetic
nerves to heart arise from lateral grey horns of
the first 4 thoracic (T
1
to T
4
) segments of the
spinal cord.
Course and Distribution
The preganglionic fibers reach the superior,
middle and inferior cervical sympathetic ganglia
situated in the sympathetic chain. The inferior
cervical sympathetic ganglion fuses with first
thoracic sympathetic ganglion forming stellate
ganglion.
From these ganglia, the postganglionic
fibers arise. The postganglionic fibers form
superior, middle and inferior cervical sympa-
thetic nerves. The superior sympathetic nerve
innervates larger arteries and base of the heart.
The middle one supplies the rest of the heart.
The inferior nerve serves as sensory (afferent)
nerve from the heart.
Function
The sympathetic nerves are cardioaccelerator
in function and carry cardioaccelerator impulses
from vasoconstrictor area to the heart.
Sympathetic Tone
Sympathetic tone or cardioaccelerator tone is the
continuous stream of impulses produced by the
vasoconstrictor area. The impulses pass through
sympathetic nerves and accelerate the heart rate.
Under normal conditions, the vagal tone is
dominant over sympathetic tone. Whenever
vagal tone is reduced or abolished, the sympa-
thetic tone becomes powerful.
SENSORY (AFFERENT) NERVE
FIBERS FROM HEART
The afferent (sensory) nerve fibers from the heart
pass through the inferior cervical sympathetic
nerve. These nerve fibers carry sensations of
stretch and pain from the heart to the brain via
spinal cord.
FACTORS AFFECTING
VASOMOTOR CENTER –
REGULATION OF VAGAL TONE
The vasomotor center regulates the cardiac
activity by receiving impulses from different
sources in the body. After receiving the impulses
from different sources, the vasodilator area
alters the vagal tone and modulates the activities
of the heart. The various sources from which
the impulses reach the vasomotor center are:
1. IMPULSES FROM HIGHER
CENTERS
The vasomotor center is mainly controlled by the
impulses from the higher centers in the brain.
The higher centers are the following:
Cerebral Cortex
Area 13 in cerebral cortex is concerned with
emotional reactions of the body. During emo-
tional conditions, this area sends inhibitory
impulses to the vasodilator area. This causes
reduction in vagal tone leading to cardio-
acceleration.
Hypothalamus
Hypothalamus influences the heart rate via
vasomotor center. Stimulation of posterior and
lateral hypothalamic nuclei causes tachycardia.
Stimulation of preoptic and anterior nuclei causes
bradycardia.
2. IMPULSES FROM RESPIRATORY
CENTERS
In forced breathing, heart rate increases during
inspiration and decreases during expiration. This
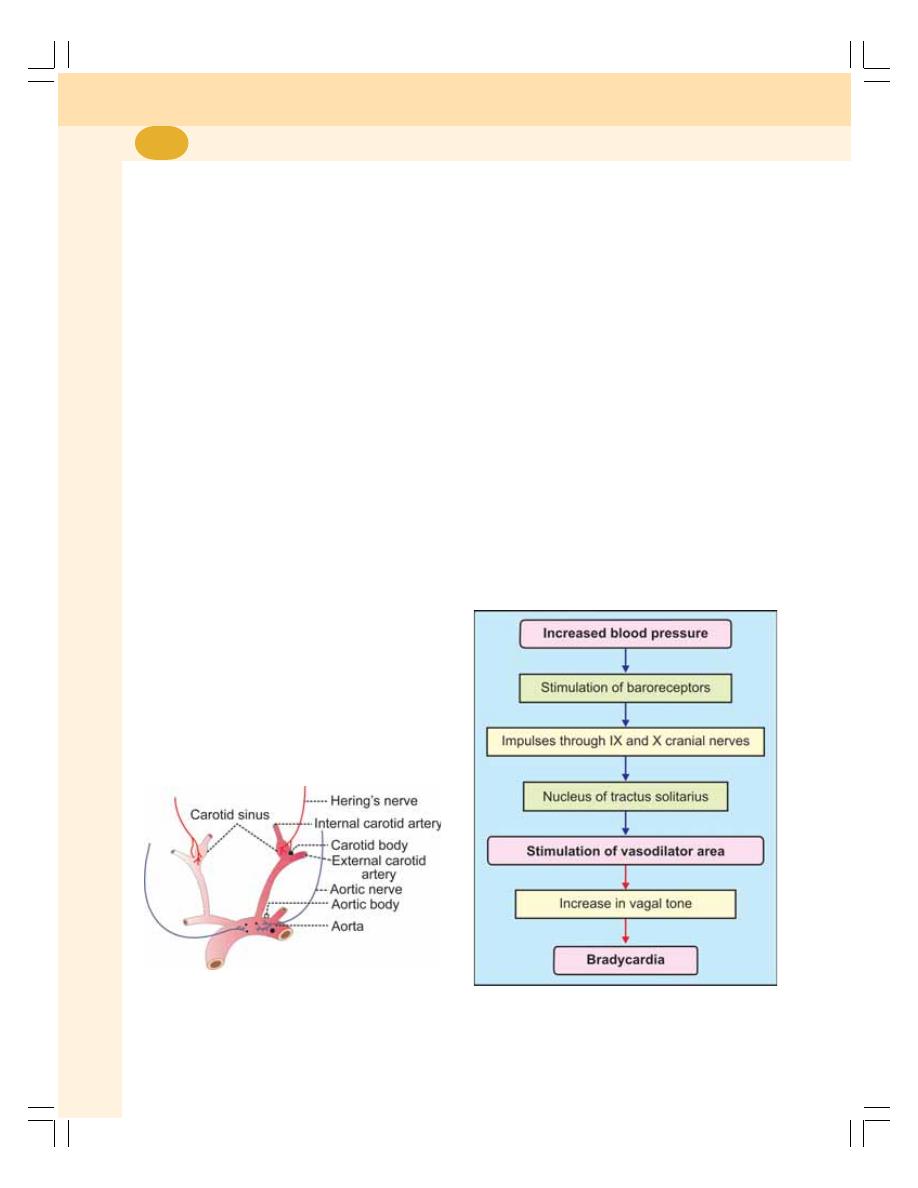
Cardiovascular System
408
FIGURE 64-2: Nerve supply to baroreceptors and
chemoreceptors
variation is called respiratory sinus arrhythmia.
This is common in some children and in some
adults even during quiet breathing.
3. IMPULSES FROM
BARORECEPTORS – MAREY’S
REFLEX
Baroreceptors
The baroreceptors or pressoreceptors are the
receptors, which give response to change in
blood pressure.
Situation
Baroreceptors are or of two types, carotid
baroreceptors and aortic baroreceptors. Carotid
baroreceptors are situated in the carotid sinus,
which is present in the wall of the internal carotid
artery near the bifurcation of common carotid
artery. The aortic baroreceptors are situated in
the wall of the arch of aorta.
Nerve Supply
Carotid baroreceptors are supplied by Hering’s
nerve, which is the branch of glossopharyngeal
(IX cranial) nerve. The aortic baroreceptors are
supplied by aortic nerve, which is a branch of
vagus (X cranial) nerve (Fig. 64-2). The nerve
fibers from the baroreceptors reach the nucleus
of tractus solitarius situated adjacent to vaso-
motor center.
Function – Marey’s Reflex
The baroreceptors regulate the heart rate
through a reflex called Marey’s reflex. The
stimulus for this reflex is increase in blood
pressure.
Marey’s reflex is a cardioinhibitory reflex that
decreases heart rate when blood pressure
increases. Whenever, the blood pressure
increases, the aortic and carotid baroreceptors
are stimulated and stimulatory impulses are
sent to nucleus of tractus solitarius via Hering’s
nerve and aortic nerve (afferent nerves). Now,
the nucleus of tractus solitarius stimulates the
vasodilator area, which in turn increases the
vagal tone leading to decrease in heart rate
(Fig. 64-3).
When pressure is less, the baroreceptors are
not stimulated. So, no impulses go to the nucleus
of tractus solitarius and heart rate is not
decreased.
Thus, the heart rate is inversely proportional
to blood pressure.
FIGURE 64-3: Marey’s (cardioinhibitory) reflex
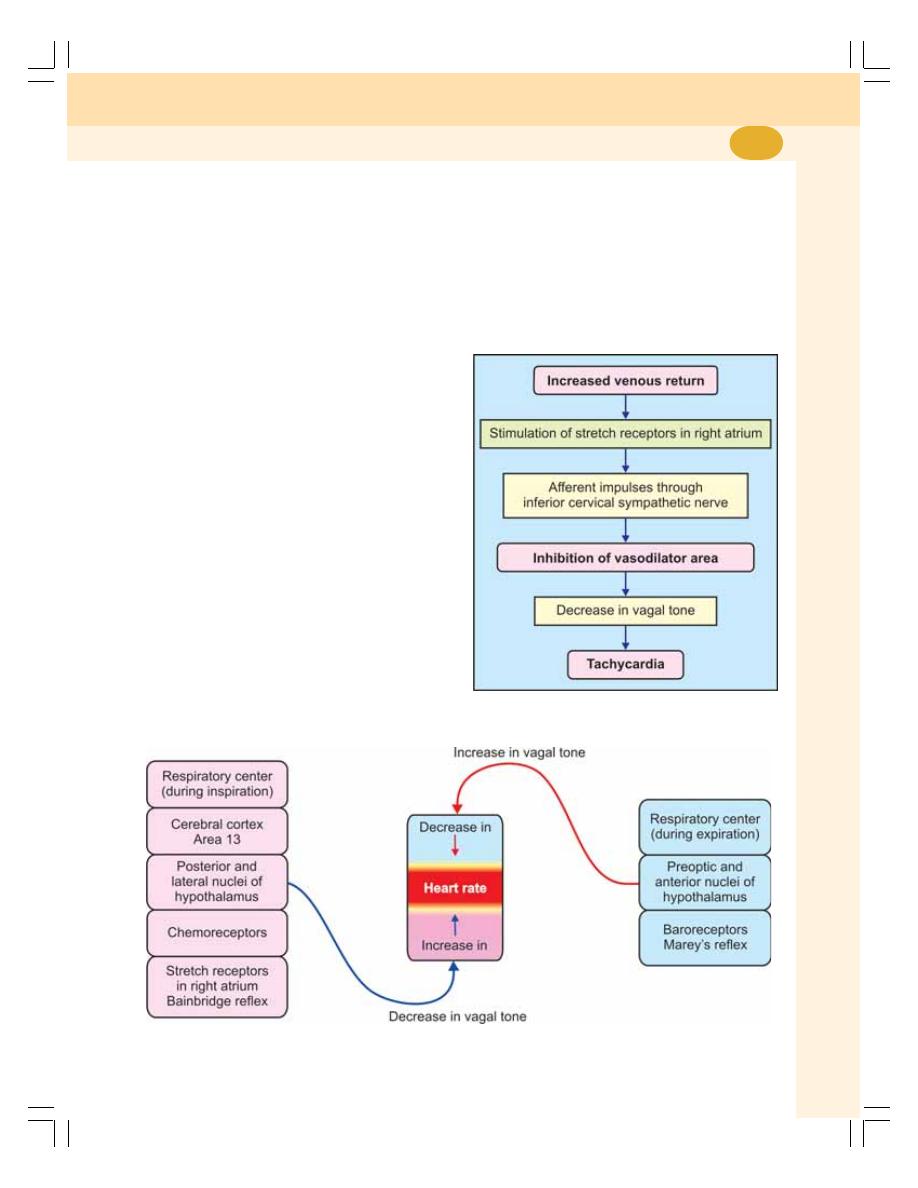
Chapter 64 Heart Rate
409
Marey’s law
According to Marey’s law, the pulse rate (which
represents heart rate) is inversely proportional
to blood pressure.
The baroreceptors produce the Marey’s
reflex only during resting conditions. So, in many
conditions such as exercise, there is an increase
in both blood pressure and heart rate.
4. IMPULSES FROM
CHEMORECEPTORS
Chemoreceptors
Chemoreceptors are receptors giving response
to change in chemical constituents of blood,
particularly oxygen, carbon dioxide and hydrogen
ion concentration.
Situation
Peripheral chemoreceptors are situated in the
carotid body and aortic body adjacent to
baroreceptors.
Nerve Supply
The chemoreceptors in the carotid body are
supplied by Hering’s nerve, which is the branch
of glossopharyngeal nerve and those in aortic
body are supplied by the aortic branch of vagus
nerve (Fig. 64-2).
FIGURE 64-4: Bainbridge (cardioaccelerator)
reflex
Function
Whenever there is hypoxia, hypercapnea, and
increased hydrogen ions concentration in the
blood, the chemoreceptors are stimulated and
inhibitory impulses are sent to vasodilator area.
Vagal tone decreases and heart rate increases.
The chemoreceptors play a major role in main-
taining respiration than the heart rate.
FIGURE 64-5: Factors regulating vagal tone and heart rate

Cardiovascular System
410
Sinoaortic Mechanism and Buffer Nerves
Sinoaortic mechanism is the mechanism of bar-
oreceptors and chemoreceptors in carotid and
aortic regions that regulates heart rate, blood
pressure and respiration. The nerves from these
receptors are called buffer nerves.
5. IMPULSES FROM RIGHT ATRIUM –
BAINBRIDGE REFLEX
Bainbridge reflex is a cardioaccelerator reflex
that increases the heart rate when venous
return is increased. Since, this reflex arises
from right atrium, it is also called right atrial
reflex.
There are some stretch receptors in the wall
of right atrium. When venous return increases,
the right atrium is distended. The right atrial
distention stimulates the stretch receptors. The
stretch receptors, in turn, send inhibitory
impulses through inferior cervical sympathetic
nerve to vasodilator area of vasomotor center.
The vasodilator area is inhibited resulting in
decrease in vagal tone and increase in heart
rate (Fig. 64-4).
6. IMPULSES FROM OTHER
AFFERENT NERVES
Stimulation of sensory nerves produces varying
effects.
Examples:
i. Stimulation of receptors in nasal mucous
membrane causes bradycardia. The
impulses from nasal mucous membrane
pass via the branches of V cranial nerve
and decrease the heart rate.
ii. Most of the painful stimuli cause
tachycardia and some cause bradycardia.
The impulses are transmitted via pain
nerve fibers (Fig. 64-5).
DEFINITIONS AND NORMAL VALUES
VARIATIONS
DETERMINANTS OF ARTERIAL BLOOD PRESSURE
REGULATION OF ARTERIAL BLOOD PRESSURE
NERVOUS MECHANISM
RENAL MECHANISM
HORMONAL MECHANISM
LOCAL MECHANISM
APPLIED PHYSIOLOGY
DEFINITIONS AND NORMAL VALUES
Arterial blood pressure is defined as the lateral
pressure exerted by the column of blood on the
wall of arteries. Arterial blood pressure is
expressed in four different terms:
1. Systolic blood pressure
2. Diastolic blood pressure
3. Pulse pressure
4. Mean arterial blood pressure.
1. SYSTOLIC BLOOD PRESSURE
Systolic blood pressure (systolic pressure) is
defined as the maximum pressure exerted in the
arteries during systole of the heart. The normal
systolic pressure is 120 mm Hg. It ranges
between 110 and 140 mm Hg.
2. DIASTOLIC BLOOD PRESSURE
Diastolic blood pressure (diastolic pressure) is
defined as the minimum pressure in the arteries
during diastole of the heart. The normal diastolic
pressure is 80 mm Hg. It varies between 60 and
80 mm Hg.
3. PULSE PRESSURE
Pulse pressure is the difference between the
systolic pressure and diastolic pressure.
Normally, it is 40 mm Hg (120 to 80).
4. MEAN ARTERIAL BLOOD PRESSURE
It is the average pressure existing in the arteries.
It is not the arithmetic mean of systolic and
diastolic pressures. It is the diastolic pressure
plus one-third of pulse pressure. To determine
the mean pressure, the diastolic pressure is
considered than the systolic pressure because
the diastolic period of cardiac cycle is longer
(0.53 second) than the systolic period
(0.27 second). Normal mean arterial pressure
is 93 mm Hg (80 + 13 = 93).
Arterial Blood Pressure
65

Cardiovascular System
412
VARIATIONS
PHYSIOLOGICAL VARIATIONS
1. Age
Arterial blood pressure increases as age
advances.
The systolic pressure in different age:
In newborn
: 40 mm Hg
After 15 days : 70 mm Hg
After 1 month : 90 mm Hg
At puberty
: 20 mm Hg
At 50 years
: 140 mm Hg
At 70 years
: 160 mm Hg
At 80 years
: 180 mm Hg
The diastolic pressure in different age:
At puberty
: 80 mm Hg
At 50 years
: 85 mm Hg
At 70 years
: 90 mm Hg
At 80 years
: 95 mm Hg
2. Sex
In females, up to the period of menopause, the
arterial pressure is about 5 mm Hg less than in
males of same age. After menopause, the
pressure in females becomes equal to that in
males of same age.
3. Body Built
The pressure is more in obese persons than in
lean persons.
4. Diurnal Variation
In early morning, the pressure is slightly low. It
gradually increases and reaches the maximum
at noon. It becomes low in evening.
5. After Meals
The arterial blood pressure is increased for few
hours after meals due to increase in cardiac
output.
6. During Sleep
Usually, the pressure is reduced up to 15 to
20 mm Hg during deep sleep. However, it
increases slightly during sleep associated with
dreams.
7. Emotional Conditions
During excitement or anxiety, the blood pressure
is increased due to release of adrenaline.
8. After Exercise
After moderate exercise, systolic pressure
increases by 20 to 30 mm Hg above the basal
level due to increase in force of contraction and
stroke volume. Normally, diastolic pressure is not
affected by moderate exercise. It is because the
diastolic pressure depends upon peripheral
resistance, which is not altered by moderate
exercise.
After severe muscular exercise, the systolic
pressure rises by 40 to 50 mm Hg above the
basal level. But, the diastolic pressure reduces
because the peripheral resistance decreases in
severe muscular exercise. More details are given
in Chapter 71.
PATHOLOGICAL VARIATIONS
Pathological variations of arterial blood pressure
are hypertension and hypotension. Refer applied
physiology of this chapter for details.
DETERMINANTS OF ARTERIAL
BLOOD PRESSURE – FACTORS
MAINTAINING ARTERIAL BLOOD
PRESSURE
Some factors are necessary for the maintenance
of normal blood pressure, which are called local
factors, mechanical factors or determinants of
blood pressure. These factors are divided into
two types:
I.
Central factors which are pertaining to the
heart:
1. Cardiac output
2. Heart rate
II. Peripheral factors which are pertaining to
blood and blood vessels:
1. Peripheral resistance
2. Blood volume
3. Venous return

Chapter 65 Arterial Blood Pressure
413
4. Elasticity of blood vessels
5. Velocity of blood flow
6. Diameter of blood vessels
7. Viscosity of blood.
CENTRAL FACTORS
1. Cardiac Output
Systolic pressure is directly proportional to
cardiac output. Whenever the cardiac output
increases, the systolic pressure is increased and,
when cardiac output is less, the systolic pressure
is reduced. The cardiac output increases in
muscular exercise, emotional conditions, etc. So
in these conditions the systolic pressure is
increased. In conditions like myocardial infarc-
tion, the cardiac output decreases resulting in
fall in systolic pressure.
2. Heart Rate
Moderate changes in heart rate do not affect
arterial blood pressure much. However, marked
alteration in the heart rate affects the blood
pressure by altering cardiac output (Chapter 63).
PERIPHERAL FACTORS
1. Peripheral Resistance
It is the resistance offered to the blood flow at
the periphery. The resistance is offered at
arterioles, which are called the resistant vessels.
This is the important factor, which maintains
diastolic pressure. The diastolic pressure is
directly proportional to peripheral resistance.
When peripheral resistance increases, diastolic
pressure is increased and when peripheral
resistance decreases, the diastolic pressure is
decreased.
2. Blood Volume
Blood pressure is directly proportional to blood
volume. Blood volume maintains the blood
pressure through the venous return and cardiac
output. If the blood volume increases, there is
increase in venous return and cardiac output
resulting in elevation of blood pressure.
3. Venous Return
Blood pressure is directly proportional to venous
return. When venous return increases, there is
increase in ventricular filling and cardiac output
resulting in elevation of arterial blood pressure.
4. Elasticity of Blood Vessels
Blood pressure is inversely proportional to the
elasticity of blood vessels. Due to the elastic
property, the blood vessels are distensible and
are able to maintain the pressure. When the
elastic property is lost, the blood vessels become
rigid (arteriosclerosis) and pressure increases as
in old age. The deposition of cholesterol, fatty
acids and calcium ions cause rigidity of blood
vessels (atherosclerosis) leading to increased
blood pressure.
5. Velocity of Blood Flow
The pressure in a blood vessel is directly
proportional to the velocity of blood flow. If the
velocity of the blood flow increases, the resis-
tance is increased. So, the pressure is increased.
6. Diameter of Blood Vessels
The arterial blood pressure is inversely
proportional to diameter of the blood vessel. If
the diameter decreases, the peripheral resistance
increases leading to increase in the pressure.
7. Viscosity of Blood
Arterial blood pressure is directly proportional to
the viscosity of blood. When viscosity of blood
increases, the frictional resistance is increased
and this increases the pressure.
REGULATION OF ARTERIAL
BLOOD PRESSURE
Arterial blood pressure varies even under
physiological conditions. However, immediately
it is brought back to normal level because of the
presence of well organized regulatory mecha-
nisms in the body. Body has four such regulatory
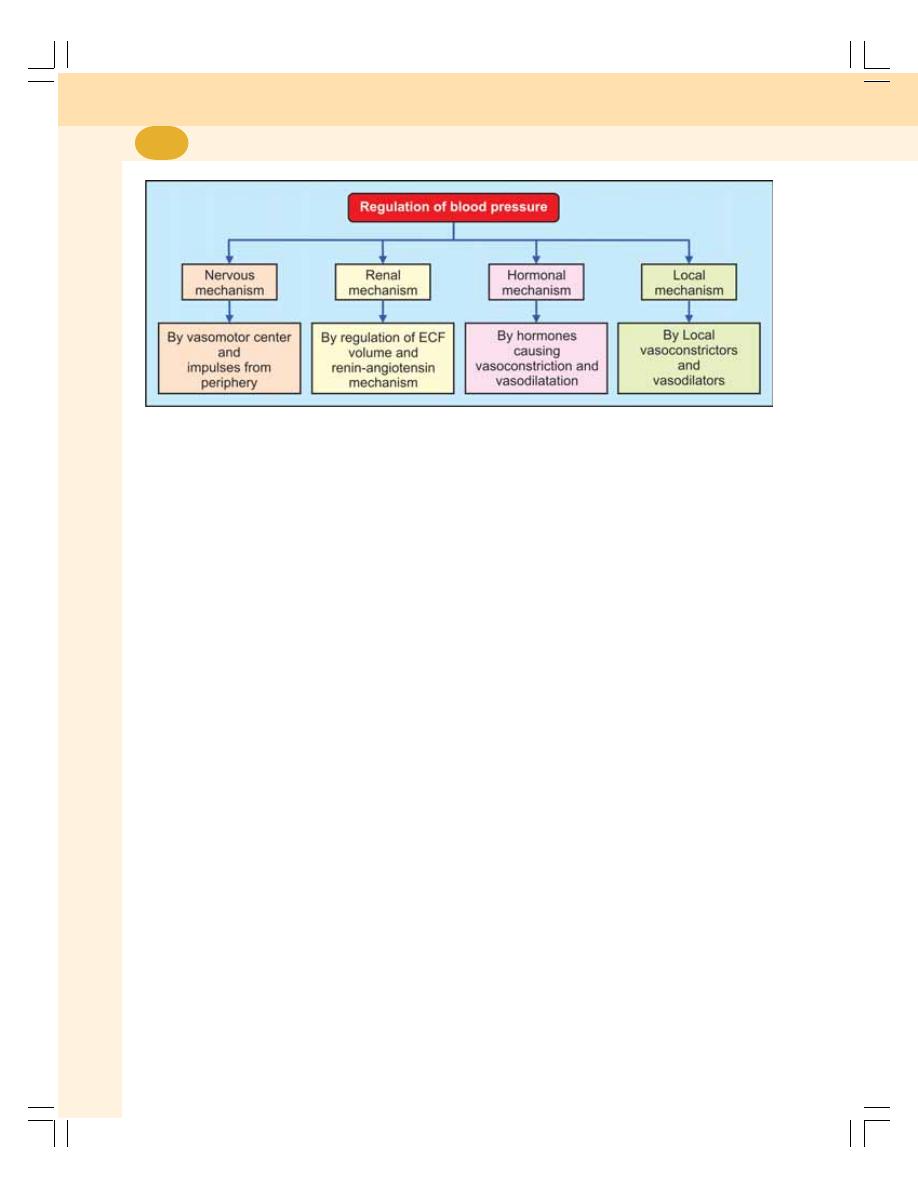
Cardiovascular System
414
FIGURE 65-1: Regulation of blood pressure
mechanisms to maintain the blood pressure
within normal limits (Fig. 65-1):
I. Nervous mechanism or short-term
regulatory mechanism
II. Renal mechanism or long-term regulatory
mechanism
III. Hormonal mechanism
IV. Local mechanism.
NERVOUS MECHANISM FOR
REGULATION OF BLOOD
PRESSURE – SHORT-TERM
REGULATION
The nervous regulation is rapid among all the
mechanisms involved in the regulation of arterial
blood pressure. When the blood pressure alters,
the nervous system brings the pressure back
to normal within few minutes. Although nervous
mechanism is quick in action, it operates only
for a short period and then it adapts to the new
pressure. Hence, it is called short-term regu-
lation. The nervous mechanism regulating the
arterial blood pressure operates through the
vasomotor system.
VASOMOTOR SYSTEM
The vasomotor system includes three com-
ponents:
1. Vasomotor center
2. Vasoconstrictor fibers
3. Vasodilator fibers.
1. Vasomotor Center
Vasomotor center is bilaterally situated in the
reticular formation of medulla oblongata and the
lower part of the pons.
Vasomotor center consists of three areas:
i. Vasoconstrictor area
ii. Vasodilator area
iii. Sensory area.
i. Vasoconstrictor area
It is also called the pressor area. It forms the
lateral portion of vasomotor center. Vaso-
constrictor area sends impulses to blood vessels
through sympathetic vasoconstrictor fibers. So,
the stimulation of this area causes vasoconstric-
tion and rise in arterial blood pressure. This area
is also concerned with acceleration of heart rate
(Chapter 64).
ii. Vasodilator area
It is otherwise called depressor area. It forms
the medial portion of vasomotor center. This area
suppresses the vasoconstrictor area and causes
vasodilatation. It is also concerned with cardio-
inhibition (Chapter 64).
iii. Sensory area
It is in the nucleus of tractus solitarius, which is
situated in the posterolateral part of medulla and
pons. This area receives sensory impulses via

Chapter 65 Arterial Blood Pressure
415
glossopharyngeal and vagal nerves from the
periphery, particularly, from the baroreceptors.
Sensory area in turn, controls the vasocons-
trictor and vasodilator areas.
2. Vasoconstrictor Fibers
The vasoconstrictor fibers belong to the
sympathetic division of autonomic nervous
system. These fibers cause vasoconstriction by
the release of the neurotransmitter substance,
noradrenaline.
Vasomotor tone
Vasomotor tone is the continuous discharge of
impulses from vasoconstrictor center through
the vasoconstrictor fibers. The vasomotor tone
plays an important role in regulating the pressure
by producing a constant partial state of
constriction of the blood vessels. Thus, the
arterial blood pressure is directly proportional to
the vasomotor tone. The vasomotor tone is also
called sympathetic vasoconstrictor tone or
sympathetic tone.
3. Vasodilator Fibers
Vasodilator fibers are of three types:
i. Parasympathetic vasodilator fibers
ii. Sympathetic vasodilator fibers
iii. Antidromic vasodilator fibers.
i. Parasympathetic vasodilator fibers
These vasodilator fibers cause dilatation of blood
vessels by releasing the chemical mediator,
acetylcholine.
ii. Sympathetic vasodilator fibers
Some of the sympathetic fibers cause vasodila-
tation in certain areas by secreting acetylcholine.
Such fibers are called sympathetic vasodilator
or sympathetic cholinergic fibers. The sympa-
thetic cholinergic fibers, which supply the blood
vessels of skeletal muscles are important in
increasing the blood flow to muscles by vaso-
dilatation during conditions like exercise.
iii. Antidromic vasodilator fibers
Normally, the impulses produced by a cutaneous
receptor (like pain receptor) pass through
sensory nerve fibers. But, some of these impul-
ses pass through the other branches of the axon
in the opposite direction and reach the blood
vessels supplied by these branches. These
impulses now dilate the blood vessels. It is called
the antidromic or axon reflex. And, the nerve
fibers are called antidromic vasodilator fibers.
MECHANISM OF ACTION OF
VASOMOTOR CENTER IN THE
REGULATION OF BLOOD PRESSURE
The vasomotor center regulates the arterial
blood pressure by causing vasoconstriction or
vasodilatation. However, its actions depend upon
the impulses it receives from other structures
such as baroreceptors, chemoreceptors, higher
centers and respiratory centers. Among these
structures, baroreceptors and chemoreceptors
play a major role in the short-term regulation of
blood pressure.
1. Baroreceptor Mechanism
The baroreceptors are the receptors, which give
response to change in blood pressure.
Refer Chapter 64 for details of baroreceptors.
Functions
When blood pressure increases: When arterial
blood pressure rises rapidly, the baroreceptors
are activated and send stimulatory impulses to
nucleus of tractus solitarius through glosso-
pharyngeal and vagus nerves. Now, the nucleus
of tractus solitarius acts on both vasoconstrictor
area and vasodilator areas of vasomotor center.
It inhibits the vasoconstrictor area and excites
the vasodilator area.
The inhibition of vasoconstrictor area reduces
vasomotor tone. Reduction in vasomotor tone
causes vasodilatation resulting in decreased
peripheral resistance. The simultaneous
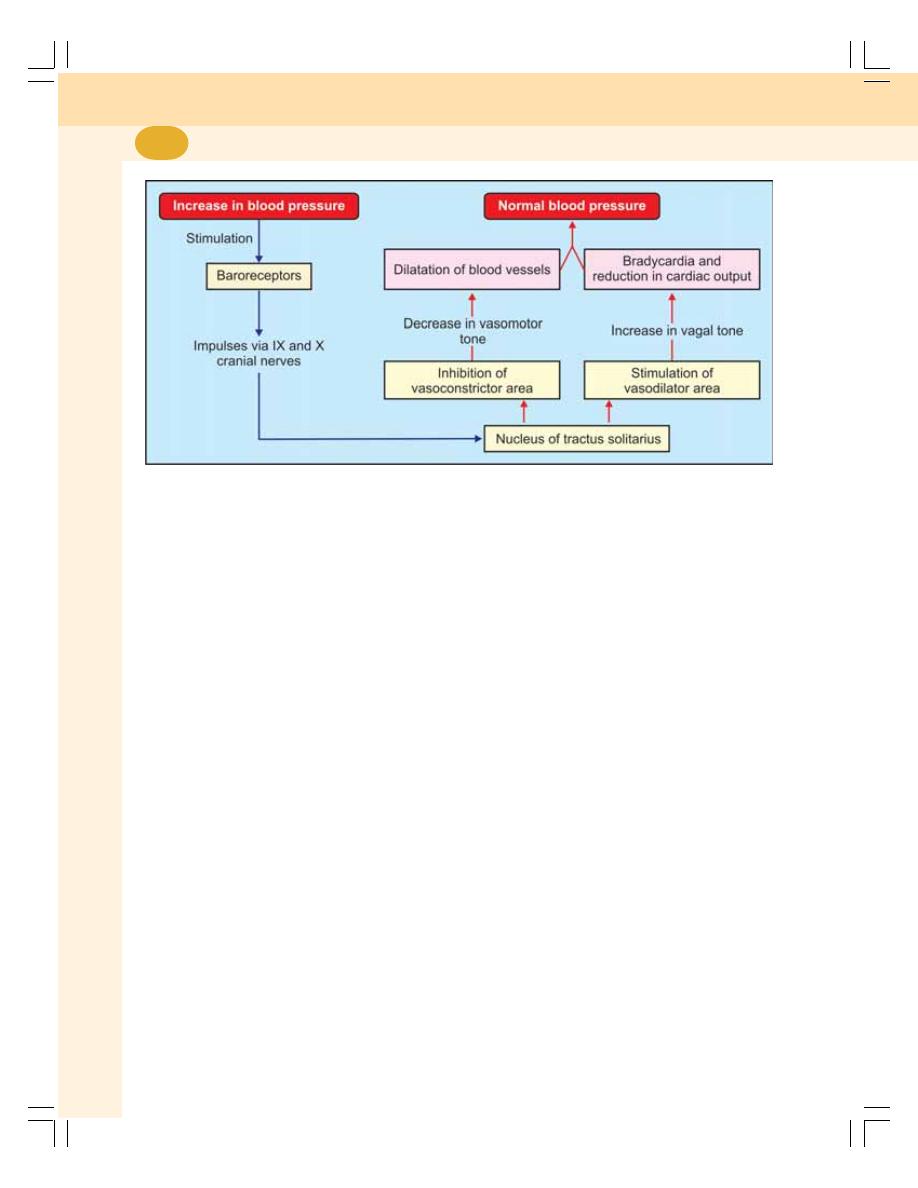
Cardiovascular System
416
excitation of vasodilator center increases vagal
tone (Chapter 64). This decreases the rate and
force of contraction of heart leading to reduction
in cardiac output. These two factors, i.e.
decreased peripheral resistance and reduced
cardiac output bring the arterial blood pressure
back to normal level (Fig. 65-2).
When blood pressure decreases: The fall in
arterial blood pressure or the occlusion of
common carotid arteries decreases the pressure
in carotid sinus. This causes inactivation of
baroreceptors. Now, there is no inhibition of
vasoconstrictor center or excitation of vasodilator
center. Therefore, the blood pressure rises.
2. Chemoreceptor Mechanism
Chemoreceptors are the receptors giving
response to change in chemical constituents of
blood. Peripheral chemoreceptors influence the
vasomotor center. Refer Chapter 64 for details
of peripheral chemoreceptors are situated in the
carotid body and aortic body (Chapter 64).
Function
Peripheral chemoreceptors are sensitive to
lack of oxygen, excess of carbon dioxide and
hydrogen ion concentration in blood. Whenever
blood pressure decreases, the blood flow
decreases resulting in decreased oxygen
content and excess of carbon dioxide and
hydrogen ion. These factors stimulate the
chemoreceptors, which send impulses to
stimulate the vasoconstrictor center. The blood
pressure rises and blood flow increases.
Chemoreceptors play a major role in main-
taining respiration rather than blood pressure
(Chapter 77).
Sinoaortic mechanism
Mechanism of action of baroreceptors and
chemoreceptors in carotid and aortic region
constitute sinoaortic mechanism. The nerves
from the baroreceptors and chemoreceptors are
called buffer nerves because these nerves
regulate the heart rate (Chapter 64), blood
pressure and respiration (Chapter 77).
3. Higher Centers
The vasomotor center is also controlled by the
impulses from the two higher centers in the
brain.
i. Cerebral cortex
Area 13 in cerebral cortex is concerned with
emotional reactions. During emotional conditions,
this area sends impulses to vasomotor center.
The vasomotor center is activated, the vasomotor
tone is increased and the pressure rises.
FIGURE 65-2: Regulation of blood pressure by baroreceptor mechanism

Chapter 65 Arterial Blood Pressure
417
ii. Hypothalamus
Stimulation of posterior and lateral nuclei of
hypothalamus causes vasoconstriction and
increase in blood pressure. Stimulation of
preoptic area causes vasodilatation and
decrease in blood pressure. The impulses from
hypothalamus are mediated via vasomotor
center.
4. Respiratory Centers
During the beginning of expiration, arterial blood
pressure increases slightly, i.e. by 4 to 6
mm Hg. And it decreases during later part of
expiration and during inspiration. It is because
of two factors:
i. Radiation of impulses from respiratory
centers towards vasomotor center at
different phases of respiratory cycle
ii. Pressure changes in thoracic cavity
leading to alteration of venous return and
cardiac output.
RENAL MECHANISM FOR
REGULATION OF BLOOD
PRESSURE – LONG-TERM
REGULATION
The kidneys play an important role in the long-
term regulation of arterial blood pressure.
Kidneys regulate arterial blood pressure by
two ways:
1. By regulation of ECF volume
2. Through renin-angiotensin mechanism.
BY REGULATION OF
EXTRACELLULAR FLUID VOLUME
When the blood pressure increases, kidneys
excrete large amounts of water and salt,
particularly sodium by means of pressure
diuresis and pressure natriuresis. Pressure
diuresis is the excretion of large quantity of water
in urine because of increased blood pressure.
Even a slight increase in blood pressure doubles
the water excretion. Pressure natriuresis is the
excretion of large quantity of sodium in urine.
Because of diuresis and natriuresis, there is
decrease in the ECF volume and blood volume,
which in turn brings the arterial blood pressure
back to normal level.
When blood pressure decreases, the
reabsorption of water from renal tubules is
increased. This in turn, increases ECF volume,
blood volume and cardiac output resulting in
restoration of blood pressure.
THROUGH RENIN-ANGIOTENSIN
MECHANISM
The details about source of renin secretion,
formation of angiotensin and conditions when
renin is secreted are described in Chapter 35.
Actions of Angiotensin II
When blood pressure and ECF volume
decrease, renin secretion from kidneys is
increased. It converts angiotensinogen into
angiotensin I. This is converted into angiotensin
II by ACE (angiotensin converting enzyme).
Angiotensin II acts in two ways to restore the
blood pressure:
i. It causes constriction of arterioles in the
body so that the peripheral resistance is
increased, and blood pressure rises. In
addition, angiotensin II causes con-
striction of afferent arterioles in kidneys
so that the glomerular filtration reduces.
This results in retention of water and salts.
This increases ECF volume to normal
level. This in turn increases the blood
pressure to normal level.
ii. Simultaneously, angiotensin II stimulates
the adrenal cortex to secrete aldosterone.
This hormone increases reabsorption of
sodium from renal tubules. Sodium reab-
sorption is followed by water reabsorption
resulting in increased ECF volume and
blood volume. It increases the blood
pressure to normal level (Fig. 65-3).
Actions of Angiotensin III and
Angiotensin IV
Like angiotensin II, the angiotensins III and IV
also increase the blood pressure and stimulate
adrenal cortex to secrete aldosterone
(Chapter 35).
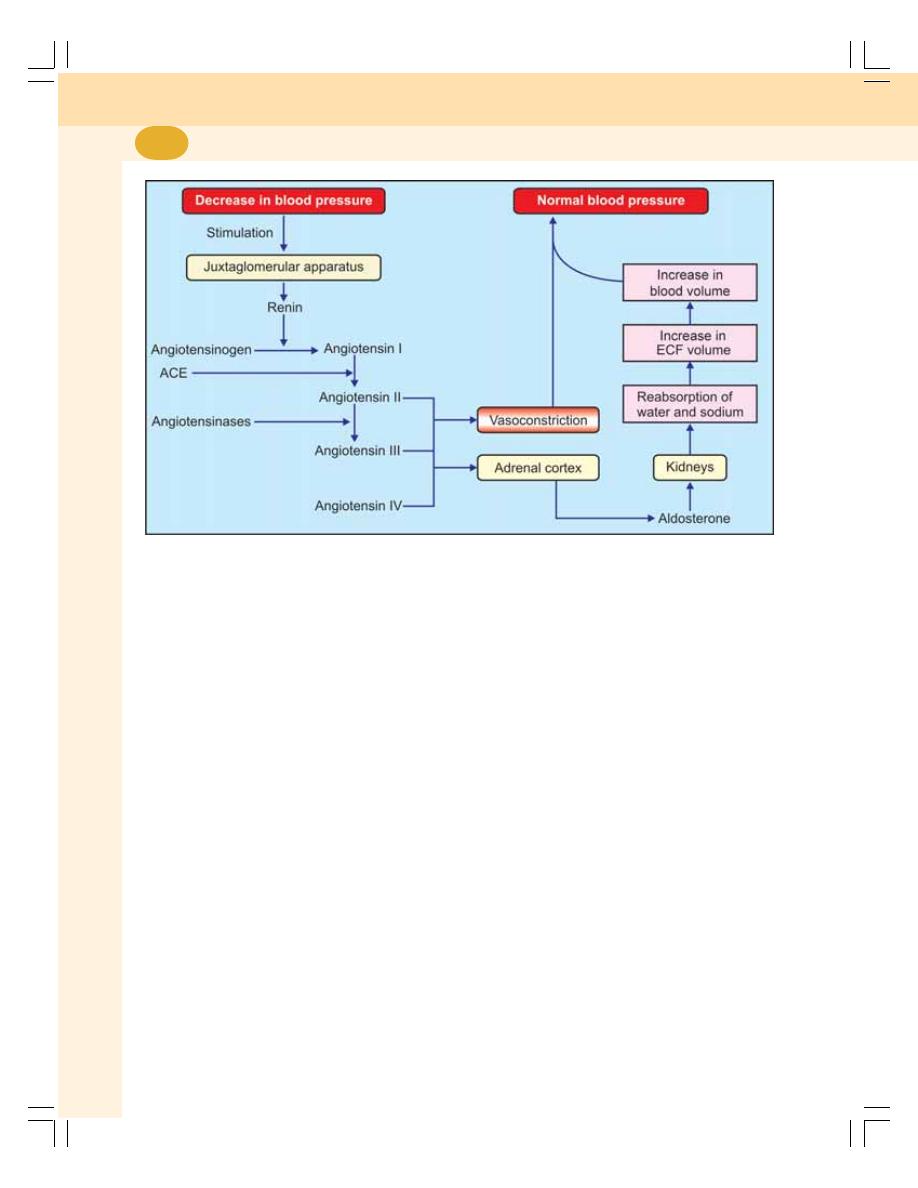
Cardiovascular System
418
FIGURE 65-3: Regulation of blood pressure by renin-angiotensin mechanism.
ACE = Angiotensin converting enzyme
HORMONAL MECHANISM FOR
REGULATION OF BLOOD
PRESSURE
Many hormones are involved in the regulation
of blood pressure.
Hormones which Increase the Blood
Pressure
1. Adrenaline
2. Noradrenaline
3. Thyroxine
4. Aldosterone
5. Vasopressin
6. Angiotensin
7. Serotonin.
Hormones which Decrease the Blood
Pressure
1. Vasoactive intestinal polypeptide (VIP)
2. Bradykinin
3. Prostaglandin
4. Histamine
5. Acetylcholine
6. Atrial natriuretic peptide
7. Brain natriuretic peptide
8. C-type natriuretic peptide.
LOCAL MECHANISM FOR
REGULATION OF BLOOD
PRESSURE
In addition to nervous, renal and hormonal
mechanisms, some local substances also
regulate the blood pressure. The local sub-
stances regulate the blood pressure by
vasoconstriction or vasodilatation.
LOCAL VASOCONSTRICTORS
The local vasoconstrictor substances are of
vascular endothelial origin and are known as
endothelins (ET). Endothelins are peptides with
21 amino acids. Endothelins are produced by
stretching of blood vessels. These peptides act
by activating phospholipase, which in turn
activates the prostacyclin and thromboxane A
2
.
These two substances cause constriction of
blood vessels and increase in blood pressure.

Chapter 65 Arterial Blood Pressure
419
LOCAL VASODILATORS
The local vasodilators are of two types:
1. Vasodilators of metabolic origin such as
carbon dioxide, lactate, hydrogen ions and
adenosine
2. Vasodilators of endothelial origin such as nitric
oxide (NO).
APPLIED PHYSIOLOGY
The pathological variations of arterial blood
pressure are:
I. Hypertension
II. Hypotension.
HYPERTENSION
Definition
Hypertension is defined as the persistent high
blood pressure. Clinically, when the systolic
pressure remains elevated above 150 mm Hg
and diastolic pressure remains elevated above
90 mm Hg, it is considered as hypertension. If
there is increase only in systolic pressure, it is
called systolic hypertension.
Types of Hypertension
Hypertension is divided into two types:
1. Primary hypertension
2. Secondary hypertension.
1. Primary hypertension or essential
hypertension
Primary hypertension is the elevated blood
pressure in the absence of any underlying
disease. It is also called essential hypertension.
The arterial blood pressure is increased because
of increased peripheral resistance, which occurs
due to some unknown cause.
2. Secondary hypertension
Secondary hypertension is the high blood
pressure due to some underlying disorders. The
different forms of secondary hypertension are:
i. Cardiovascular hypertension that is
produced due to the cardiovascular
disorders such as atherosclerosis
(hardening of blood vessels by fat
deposition) and coarctation (narrowing) of
aorta
ii. Endocrine hypertension which is due to
hyperactivity of some endocrine glands
such as pheochromocytoma, hyper-
aldosteronism and Cushing’s syndrome
iii. Renal hypertension that is caused by renal
diseases like glomerulonephritis and
stenosis of renal arteries
iv. Neurogenic hypertension which is deve-
loped by nervous disorders such as
increased intracranial pressure and lesion
in tractus solitarius
v. Hypertension during pregnancy which is
due to toxemia of pregnancy.
HYPOTENSION
Definition
Hypotension is the low blood pressure. When the
systolic pressure is less than 90 mm Hg, it is
considered as hypotension.
Types
1. Primary hypotension
2. Secondary hypotension.
1. Primary hypotension
Primary hypotension is the low blood pressure
that develops in the absence of any underlying
disease and develops due to some unknown
cause. It is also called essential hypotension.
Frequent fatigue and weakness are the
common symptoms of this condition. However,
the persons with primary hypotension are not
easily susceptible to heart or renal disorders.
2. Secondary hypotension
It is the hypotension that occurs due to some
underlying diseases. The diseases which cause
hypotension are:
i. Myocardial infarction
ii. Hypoactivity of pituitary gland
iii. Hypoactivity of adrenal glands
iv. Tuberculosis
v. Nervous disorders.
VENOUS PRESSURE
DEFINITION AND NORMAL VALUES
EFFECT OF RESPIRATION ON VENOUS PRESSURE
CAPILLARY PRESSURE
DEFINITION AND NORMAL VALUES
REGIONAL VARIATIONS
VENOUS PRESSURE
DEFINITION AND NORMAL VALUES
Venous pressure is the pressure exerted by the
contained blood in the veins. The pressure in
vena cava and right atrium is called central
venous pressure. And the pressure in peripheral
veins is called peripheral venous pressure.
Pressure is not the same in all the veins. It
varies in different veins in the extremities of the
body and also varies from central veins to
peripheral veins.
Venous Pressure in the Extremities of the
Body
Venous pressure is less in the parts of the body
above the level of the heart and it is more in parts
below the level of the heart.
Pressure in jugular vein: 5.1 mm Hg (6.9 cm
H
2
O).
Pressure in dorsal venous arch of foot: 13.2
mm Hg (17.9 cm H
2
O).
(1 mm Hg pressure = 1.359 cm H
2
O
pressure).
Venous Pressure in Central and
Peripheral Veins
Pressure is greater in peripheral veins than in
central veins.
Pressure in antecubital vein: 7.1 mm Hg (9.6
cm H
2
O).
Pressure in superior vena cava: 4.6 mm Hg
(6.2 cm H
2
O).
EFFECT OF RESPIRATION ON
VENOUS PRESSURE
The effect of respiration on venous pressure is
demonstrated by some procedures which
exaggerate these effects on venous pressure.
Such procedures are Valsalva maneuver and
Müeller’s maneuver.
Valsalva Maneuver or Valsalva Experiment
Valsalva maneuver is the forced expiratory effort
with closed glottis. It is performed by attempting
to exhale forcibly while keeping the mouth and
nose closed.
Venous Pressure and
Capillary Pressure
66

Chapter 66 Venous Pressure and Capillary Pressure
421
During this maneuver, the intrathoracic
pressure increases greatly and causes the
following effects:
1. Compression of central vein in thorax
2. Accumulation of blood in peripheral veins like
veins of neck, face and limbs leading to
increase in peripheral venous pressure to
about 30 cm H
2
O
3. Decrease in the venous return to right atrium
4. Decrease in central venous pressure.
Valsalva maneuver is used as a diagnostic
tool to evaluate the cardiovascular disorders.
Müeller’s Maneuver or Müeller’s
Experiment
Müeller’s maneuver or experiment is the forced
inspiratory effort with closed glottis. It is
performed by attempting to inhale forcibly while
keeping the mouth and nose closed. It is also
called reverse Valsalva maneuver.
During this maneuver, the intrathoracic
pressure decreases greatly (becomes more
negative) and causes the following effects:
1. Dilatation of right atrium and central vein
because of increase in negative intrathoracic
pressure
2. Rapid emptying of blood from peripheral veins
into the central veins
3. Increase in central venous pressure and
decrease in the peripheral venous pressure.
The peripheral venous pressure falls below
3 to 4 cm H
2
O.
Müeller’s maneuver is used to evaluate upper
respiratory tract problems and sleep apnea
syndrome.
CAPILLARY PRESSURE
DEFINITION AND NORMAL VALUES
Capillary pressure is the pressure exerted by the
blood contained in capillary. It is also called
capillary hydrostatic pressure.
Capillary pressure is responsible for the
exchange of various substances between blood
and interstitial fluid through capillary wall.
Capillary pressure varies depending upon the
function of the organ or the region of the body.
Generally, the pressure in the arterial end of
capillary is about 30 to 32 mm Hg and in venous
end it is 15 mm Hg.
REGIONAL VARIATIONS
The capillary pressure varies in different organs
particularly in kidneys and lungs. The regional
variation in capillary pressure is in relation to the
physiological activities of the particular region.
So, it has some functional significance.
Capillary Pressure in Kidney
In kidney, the glomerular capillary pressure is
high. It is about 60 mm Hg. This high capillary
pressure is responsible for glomerular filtration.
Capillary Pressure in Lungs
In lungs, the pulmonary capillary pressure is low.
It is about 7 mm Hg. It favors exchange of gases
between blood and alveoli.
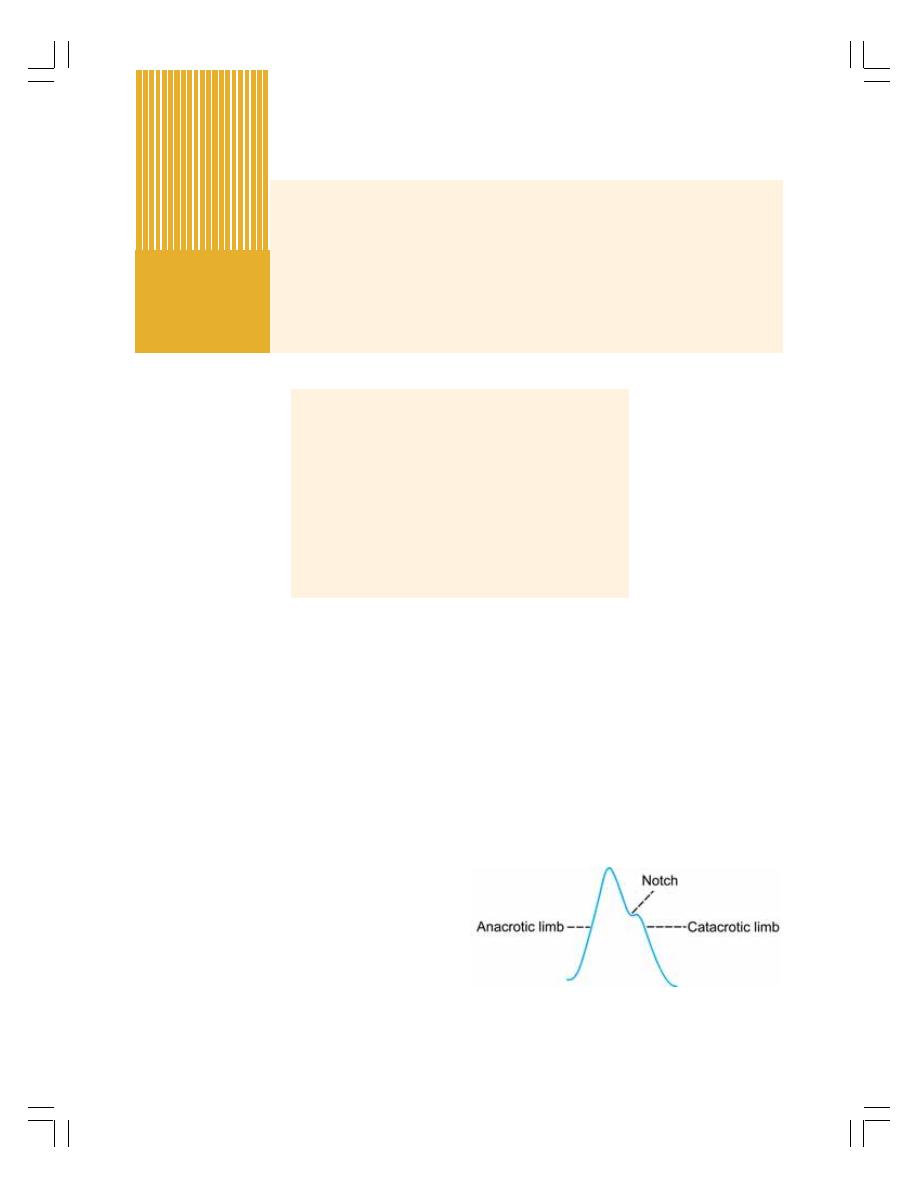
ARTERIAL PULSE
INTRODUCTION
ARTERIAL PULSE TRACING
PULSE POINTS
EXAMINATION OF RADIAL PULSE
VENOUS PULSE
INTRODUCTION
EXAMINATION OF VENOUS PULSE
JUGULAR VENOUS PULSE TRACING
ARTERIAL PULSE
INTRODUCTION
The arterial pulse is defined as the pressure
changes transmitted in the form of waves
through the arterial wall and blood column from
heart to the periphery.
When heart contracts the blood is ejected into
aorta with great force. It causes distension of this
blood vessel and a rise in pressure. A pressure
wave is produced on the elastic wall of the aorta.
It travels rapidly from the heart and can be felt
after a brief interval, at any superficial peripheral
artery like radial artery at wrist.
Pulse rate is the accurate measure of heart
rate except in conditions like pulses deficit.
Velocity of Transmission of Pulse
The average velocity at which the pulse wave is
transmitted varies between 7 and 9 meters/
second. Pulse wave travels faster than blood
flow. The maximum velocity of blood flow in the
body (in larger arteries) is only 50 cm/second.
ARTERIAL PULSE TRACING
The arterial pulse is recorded by using
polygraph. The pulse recorded in radial artery
or femoral artery is the typical peripheral pulse
(Fig. 67-1). The peripheral pulse tracing has
three main features.
Arterial Pulse and
Venous Pulse
67
FIGURE 67-1: Radial pulse tracing

Chapter 67 Arterial Pulse and Venous Pulse
423
1. Anacrotic Limb
It is ascending limb or upstroke. It is also called
primary wave. It is due to the rise in pressure
during systole.
2. Catacrotic Limb
It is descending limb or downstroke. It is due to
the fall in pressure during diastole.
3. Catacrotic Notch
In the upper part of the catacrotic limb of pulse
tracing, a small notch appears. It is known as
catacrotic notch or incisura. This notch is
produced by the backflow of blood during the
closure of semilunar valves at the beginning of
diastolic period, which produces slight increase
in the pressure.
4. Pre and Postcatacrotic Waves
The wave appearing before the notch is called
precatacrotic wave. The wave appearing after
the notch is called postcatacrotic wave.
PULSE POINTS
Usually, the pulse is palpated on the radial artery
because it is easily approachable and placed
superficially. However, arterial pulse can be felt
in different areas on the body. These areas are
called pulse points.
1. Temporal pulse – over the temple in front
of the ear on superficial temporal artery
2. Facial pulse – on facial artery at the angle
of jaw
3. Carotid pulse – in the neck along the
anterior border of sternocleidomastoid
muscle on common carotid artery
4. Axillary pulse – in axilla on axillary artery
5. Brachial pulse – in cubital fossa along
medial border of biceps muscle on
brachial artery
6. Radial pulse – over the thumbside of wrist
between tendons of brachioradialis and
flexor carpi radialis muscles on radial
artery
7. Ulnar pulse – over the little fingerside of
wrist on ulnar artery
8. Femoral pulse – in the groin on femoral
artery
9. Popliteal pulse – behind knee in the
popliteal fossa on popliteal artery
10. Dorsalis pedis pulse – over the dorsum
of the foot on dorsalis pedis artery
11. Tibialis pulse – over the back of the ankle
behind medial malleolus on posterior tibial
artery.
EXAMINATION OF RADIAL PULSE
Examination of pulse is a valuable clinical
procedure. Pulse represents the heartbeat. By
examining pulse, important information regarding
cardiac function such as rate of contraction,
rhythmicity, etc. can be obtained. In addition, an
experienced physician can determine the mean
arterial pressure by hardness of pulse and its
amplitude.
Pulse is examined by placing the tips of three
fingers, index finger, middle finger and ring finger
on the artery. While examining the pulse, the
following features are observed:
1. Rate
2. Rhythm
3. Character
4. Volume
5. Condition of blood vessel wall
6. Delayed pulse.
1. Rate
The number of pulse per minute is pulse rate. It
has to be counted at least for 30 seconds. Pulse
rate in adults is 72/minute.
2. Rhythm
The regularity of pulse is known as rhythm.
Under normal conditions, the pulse appears at
regular intervals. The rhythm of the pulse
becomes irregular in conditions like atrial
fibrillation. The irregular rhythm of pulse is of
two types, regularly irregular and irregularly
irregular.
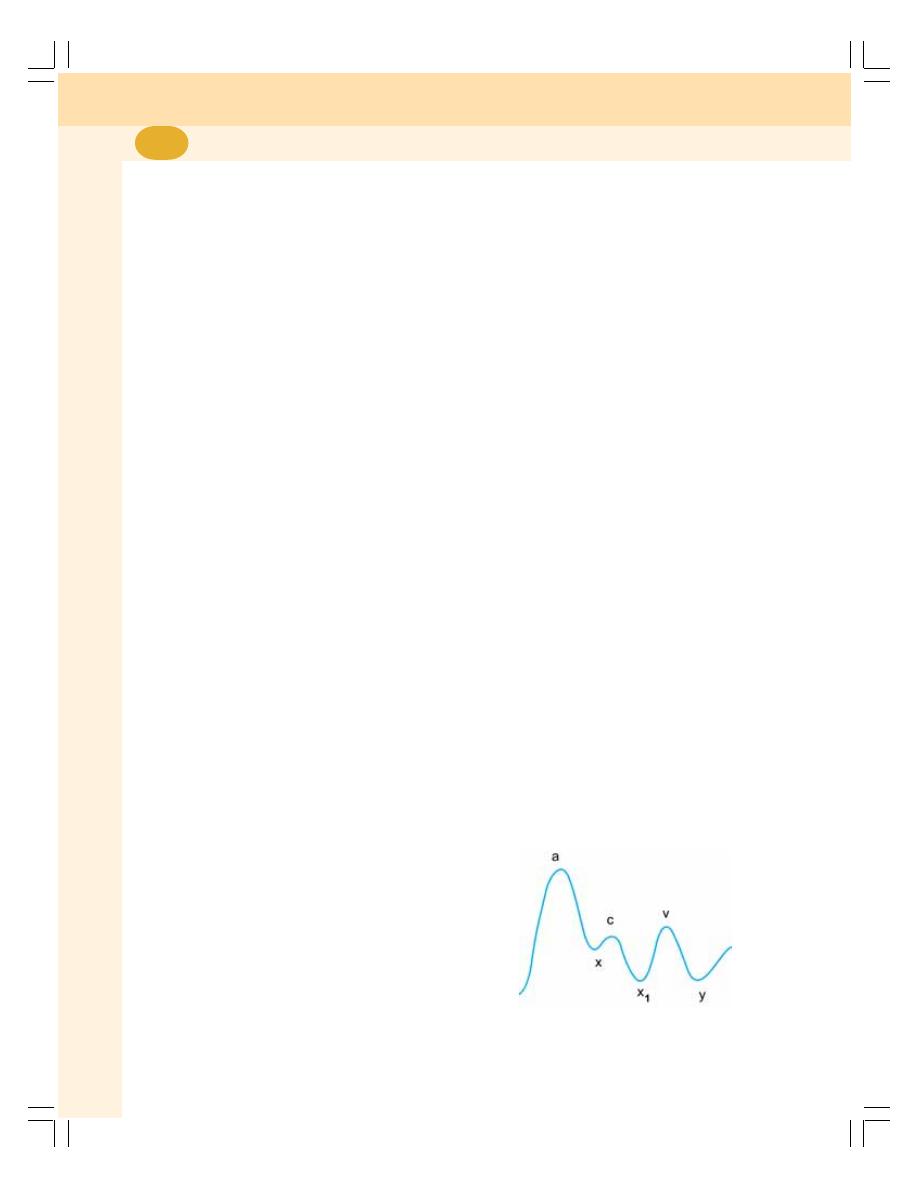
Cardiovascular System
424
3. Character
The character of the pulse is observed while
examining the pulse. It denotes the tension on
the vessel wall produced by the waves of pulse.
4. Volume
It is the determination of the movement of the
vessel wall produced by the transmission of
pulse wave. It is also a measure of pulse
pressure. It depends upon the condition of the
blood vessel.
5. Condition of Wall of the Blood Vessel
It is assessed by feeling and rolling the radial
artery against the underlying bones. Normally,
the wall of the vessel is not palpable in children
and young adults. However, in old age the wall
of the vessel becomes rigid and palpable. In
abnormal conditions like arteriosclerosis, it is felt
as a hard rope.
6. Delayed Pulse
Sometimes the arrival of pulse in certain
peripheral arteries is delayed. It is an important
feature to be noted because it is useful in
diagnosis of certain diseases. For example, while
palpating radial pulse and femoral pulse simul-
taneously, there is a short delay in the arrival of
femoral pulse wave. It is called femoral delay,
radial femoral delay or radiofemoral delay.
VENOUS PULSE
INTRODUCTION
Venous pulse is defined as the pressure
changes transmitted in the form of waves from
right atrium to the veins near the heart. Venous
pulse is observed only in larger veins near the
heart such as jugular vein.
Evaluation of the venous pulse is an integral
part of the physical examination because it
reflects right atrial pressure and hemodynamic
events in right atrium. Venous pulse recording
is used to determine the rate of atrial contraction,
just as the record of arterial pulse is used to
determine the rate of ventricular contraction.
In addition, many phases of cardiac cycle can
be recognized by means of venous pulse tracing.
It is the simple and accurate method to measure
duration of different phases in diastole. It also
represents the atrial pressure changes taking
place during cardiac cycle.
EXAMINATION OF VENOUS PULSE
Inspection of jugular vein pulsations is routinely
done by bedside examination of neck veins. It
provides valuable information about the cardiac
function.
To observe the pulsation of the internal
jugular vein, the head of the subject is tilted
upwards at 45º. However, in patients with
increased venous pressure, the head should be
tilted as much as 90°. The pulsations of jugular
vein can be noticed when light is passed across
the skin overlying internal jugular vein with
relaxed neck muscles. Simultaneous palpation
of the left carotid artery helps the examiner
confirm the venous pulsations.
JUGULAR VENOUS PULSE TRACING
The recording of jugular venous pulse is also
called phlebogram. It is similar to intra-atrial
pressure curve (Fig. 67-2).
Phlebogram also has three positive waves —
a, c, v and three negative waves — x, x
1
, y.
‘a’ Wave
It is the first wave and is a positive wave. It is
due to rise in atrial pressure during atrial systole.
FIGURE 67-2: Phlebogram

Chapter 67 Arterial Pulse and Venous Pulse
425
‘x’ Wave
This negative wave is due to fall of pressure in
atrium and coincides with atrial diastole and
beginning of ventricular systole.
‘c’ Wave
This positive wave occurs due to rise in atrial
pressure during isometric contraction period.
During this period the atrioventricular valves
bulge into the atria and increase the pressure in
the atria slightly.
‘x
1
’ Wave
It is a negative wave and it is due to fall in
pressure during ejection period. During ejection
period, the atrioventricular ring is pulled towards
ventricles causing fall in atrial pressure.
‘v’ Wave
This positive wave is due to rise in atrial pressure.
The pressure increases because of atrial filling
(venous return). It is obtained during isometric
relaxation period or during atrial diastole.
‘y’ Wave
This negative wave denotes fall in pressure in
atria. It is due to the opening of atrioventricular
valve and emptying of blood into the ventricle. It
appears during rapid and slow filling periods. ‘y’
wave is followed by ‘a’ wave and the cycle is
repeated.
CORONARY CIRCULATION
CEREBRAL CIRCULATION
SPLANCHNIC CIRCULATION
CAPILLARY CIRCULATION
SKELETAL MUSCLE CIRCULATION
CUTANEOUS CIRCULATION
CORONARY CIRCULATION
DISTRIBUTION OF CORONARY
BLOOD VESSELS
Coronary Arteries
Heart muscle is supplied by two coronary
arteries, the right and left coronary arteries,
which are the first branches of aorta. The arteries
encircle the heart in the manner of a crown
hence the name coronary arteries (Latin word
corona = crown).
Branches of coronary arteries
The coronary arteries divide and subdivide into
smaller branches, which run all along the surface
of the heart. The smaller branches are called
epicardiac arteries and give rise to further
smaller branches known as final arteries or
intramural vessels. The final arteries run at right
angles through the heart muscle near the inner
aspect of wall of the heart.
Venous Drainage
The venous drainage from the heart muscle is
by three types of vessels:
1. Coronary sinus: It is the larger vein draining
75% of total coronary flow. It drains blood
from left side of the heart and opens into right
atrium near tricuspid valve
2. Anterior coronary veins: The anterior
coronary veins drain blood from right side of
the heart and open directly into right atrium
3. Thebesian veins: Thebesian veins drain
deoxygenated blood from myocardium
directly into the concerned chamber of the
heart.
Physiological Shunt
Physiological shunt is the diverted route
(diversion) through which the venous blood is
mixed with arterial blood. The deoxygenated
blood flowing from thebesian veins into cardiac
chambers makes up part of normal physiological
shunt. The other component of physiological
Regional Circulation
68
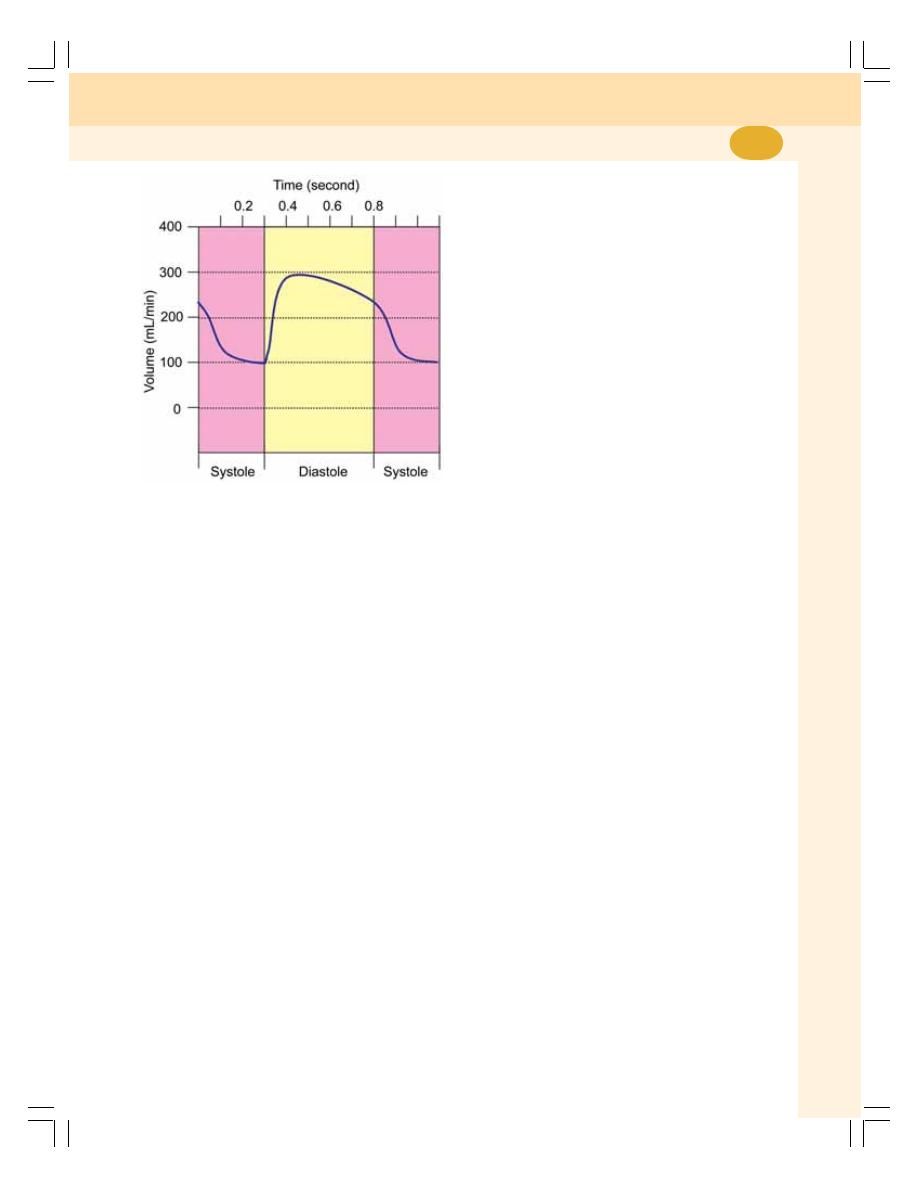
Chapter 68 Regional Circulation
427
shunt is the drainage of deoxygenated blood
from bronchial circulation into pulmonary vein
without being oxygenated (Chapter 72).
NORMAL CORONARY BLOOD FLOW
Normal blood flow through coronary circulation
is about 200 mL/minute. It forms 4% of cardiac
output. It is about 65 to 70 mL/minute/100 g of
cardiac muscle.
PHASIC CHANGES CORONARY
BLOOD FLOW
The blood flow through coronary arteries is not
constant. It decreases during systole and
increases during diastole (Fig. 68-1).
During contraction, the coronary blood
vessels are compressed and blood flow is
reduced. During diastole, the compression is
released and the blood vessels are distended.
So, the blood flow is increased.
APPLIED PHYSIOLOGY –
CORONARY ARTERY DISEASE
Coronary artery disease (CAD) is the heart
disease that is caused by inadequate blood
supply to cardiac muscle due to occlusion of
coronary artery. It is also called coronary heart
disease.
Coronary Occlusion
Coronary occlusion means the partial or
complete obstruction of the coronary artery. The
occlusion occurs because of atherosclerosis, a
condition associated with deposition of choles-
terol on the walls of the artery. In due course,
this part of the arterial wall becomes fibrotic and
it is called atherosclerotic plague. The plague
is made up of cholesterol, calcium and other
substances from blood. Because of the athero-
sclerotic plague the lumen of the coronary artery
becomes narrow. In severe conditions, the artery
is completely occluded.
Smaller blood vessels are occluded by the
thrombus or part of atherosclerotic plague
detached from coronary artery. This thrombus
or part of the plague is called embolus.
Myocardial Ischemia
Myocardial ischemia is the reaction of a part of
myocardium in response to hypoxia. Hypoxia
develops when blood flow to a part of myocar-
dium decreases severely due to occlusion of a
coronary artery.
When the ischemia is mild due to obstruction
of smaller blood vessel, the blood flow can be
restored by rapid development of coronary
collateral arteries.
Necrosis
Necrosis refers to death of cells or tissues by
injury or disease in a localized area. When
coronary occlusion is severe involving larger
blood vessels, the severe ischemia leads to
necrosis of myocardium. Necrosis is irreversible.
Myocardial Infarction – Heart Attack
Myocardial infarction is the necrosis of
myocardium caused by insufficient blood flow
due to embolus, thrombus or vascular spasm.
It is also called heart attack. In myocardial
FIGURE 68-1: Phasic changes in coronary
blood flow

Cardiovascular System
428
infarction, death occurs rapidly due to ventricular
fibrillation.
Common symptoms of myocardial infarction
are:
1. Cardiac pain
2. Nausea
3. Vomiting
4. Palpitations
5. Difficulty in breathing
6. Extreme weakness
7. Sweating
8. Anxiety.
Cardiac Pain – Angina Pectoris
Cardiac pain is the chest pain that is caused by
myocardial ischemia. It is also called angina
pectoris. It is the common manifestation of
coronary artery disease. The pain starts beneath
the sternum and radiates to the surface of left
arm and left shoulder. The cardiac pain is called
referred pain since it is felt over the body away
from the heart. It is because, heart and left arm
develop from the same dermatomal segment in
embryo.
CEREBRAL CIRCULATION
IMPORTANCE
Brain tissues need adequate blood supply
continuously. Stoppage of blood flow for
5 seconds leads to unconsciousness, and for
5 minutes leads to irreparable damage to the
brain cells.
CEREBRAL BLOOD VESSELS
Brain receives blood from the basilar artery and
internal carotid artery. The branches from
these arteries form circle of Willis. The venous
drainage is by sinuses, which open into internal
jugular vein.
NORMAL CEREBRAL BLOOD FLOW
Normally, brain receives 750 to 800 mL of blood
per minute. It is about 15 to 16% of total cardiac
output and about 50 to 55 mL/100 grams of brain
tissue per minute.
APPLIED PHYSIOLOGY – STROKE
Definition
Stroke is the sudden death of neurons in
localized area of brain due to inadequate blood
supply. It is characterized by reversible or
irreversible paralysis with other symptoms.
Stroke is also called cardiovascular accident
(CVA) or brain attack.
Causes
1. Heart disease
2. Hypertension
3. High cholesterol in blood
4. High blood sugar – diabetes mellitus
5. Heavy smoking
6. Heavy alcohol consumption.
Symptoms
Symptoms of stroke depend upon the area of
brain that is damaged. Generally, stroke causes
dizziness, loss of consciousness, coma or death.
Other features of stroke are:
1. Weakness
2. Numbness or paralysis particularly on one
side of the body
3. Impairment of speech
4. Emotional disturbances
5. Loss of coordination
6. Loss of memory.
SPLANCHNIC CIRCULATION
INTRODUCTION
The splanchnic or visceral circulation constitutes
three portions:
1. Mesenteric circulation supplying blood to GI
tract
2. Splenic circulation supplying blood to spleen
3. Hepatic circulation supplying blood to liver.
The unique feature of splanchnic circulation
is that, the blood from mesenteric bed and

Chapter 68 Regional Circulation
429
spleen forms a major amount of blood flowing
to liver. Blood flows to liver from GI tract and
spleen through portal system.
MESENTERIC CIRCULATION
Distribution of Blood Flow
Stomach — 35 mL/100 gm/minute
Intestine — 50 mL/100 gm/minute
Pancreas — 80 mL/100 gm/minute
SPLENIC CIRCULATION
Importance of Splenic Circulation
Spleen is the main reservoir for blood. Due to
the dilatation of blood vessels, a large amount
of blood is stored in spleen. And the constriction
of blood vessels by sympathetic stimulation
releases blood into circulation.
Storage of Blood
In spleen, two structures are involved in storage
of blood namely, splenic venous sinuses and
splenic pulp.
The small arteries and arterioles open directly
into the venous sinuses. When spleen expands,
the sinuses swell and large quantity of blood is
stored. The capillaries of splenic pulp are highly
permeable. So, most of the blood cells pass
through capillary membrane and are stored in
the pulp. The venous sinuses and the pulp are
lined with reticuloendothelial cells.
HEPATIC CIRCULATION
Hepatic Blood Vessels
Liver receives blood from two sources:
1. Hepatic artery from aorta
2. Portal vein from mesenteric and splenic
vascular bed.
More details are given in Chapter 30.
Normal Blood Flow
Liver receives maximum amount of blood as
compared to any other organ in the body since,
most of the metabolic activities are carried out
in the liver. The blood flow to liver is 1,500 mL/
minute, which forms 30% of cardiac output. It is
about 100 mL/100 g of tissue/minute.
Normally, about 1100 mL of blood flows
through portal vein and remaining 400 mL of
blood flows through hepatic artery. However,
portal vein carries only about 25% of oxygen to
liver. It is because it carries the blood, which has
already passed through the blood vessels of GI
tract where oxygen might have been used.
Hepatic artery transports 75% of oxygen to the
liver.
CAPILLARY CIRCULATION
MICROCIRCULATION
Microcirculation refers to the flow of blood
through the minute blood vessels such as
arterioles, capillaries and venules. Capillary
circulation forms the major part of micro-
circulation. The capillaries are formed by single
layer of endothelial cells which are wrapped
around by pericytes.
FEATURES OF CAPILLARIES
Capillaries arise from arterioles and form the
area for the actual function of circulatory system,
i.e. exchange of materials between blood and
tissues. Structurally, capillaries are very narrow
and short. However, quantitatively, these vessels
outnumber the other blood vessels. About ten
billion capillaries are present in the body.
Each capillary lies in a very close proximity
to the cells of the tissues at a distance of about
20 to 30 mm. This enables easy and rapid
exchange of substances between blood and the
tissues through interstitial fluid.
PATTERN OF CAPILLARY SYSTEM
Capillaries are disposed between arterioles and
venules. From the arterioles, the meta-arterioles
take origin (Fig. 68-2). From meta-arterioles, two
types of capillaries arise:
1. Preferential channels
2. True capillaries.
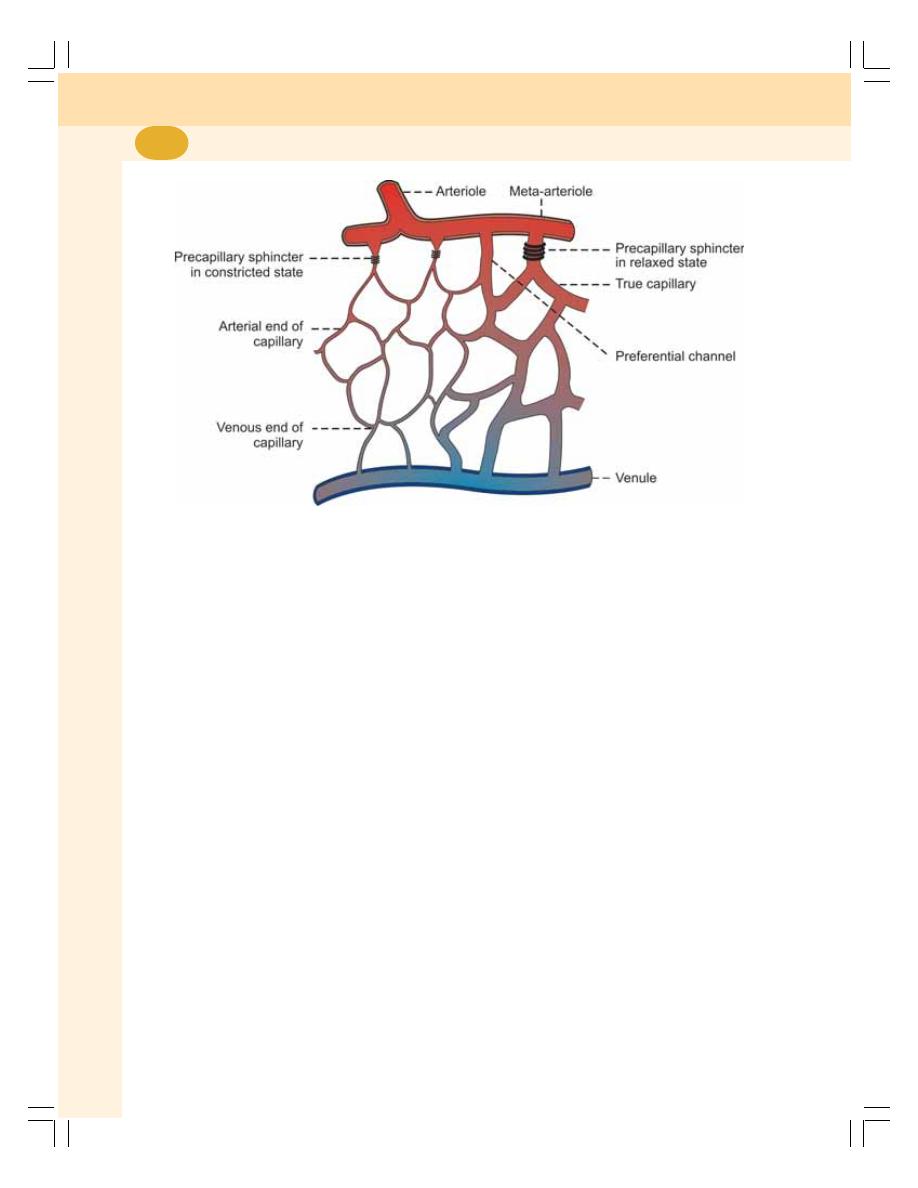
Cardiovascular System
430
1. Preferential Channels
The preferential channels or continuous
capillaries have same diameter as meta-
arterioles. After arising from the meta-arterioles,
the preferential channels form a network and
finally join the venules.
2. True Capillaries
The diameter of the true capillaries is less than
that of the meta-arterioles. Arising from meta-
arterioles, the true capillaries also form a network
and join the venules.
At the beginning of true capillaries, there is
an encircling of smooth muscle fibers. It functions
as a sphincter; so it is known as precapillary
sphincter. It controls the blood flow through true
capillaries.
ANATOMICAL AND PHYSIOLOGICAL
SHUNTS
Anatomical Shunt
Anatomical shunt is the direct link between
arterioles and venules. It is also called
arteriovenous shunt. Flow of blood through the
capillaries where exchange of nutrients, gases
and other substances takes place is called
nutritional flow. The flow of blood through
anatomical shunt is called non-nutritional flow.
Non-nutritional blood flow occurs in many tissues
of the body particularly during resting conditions
when metabolic activities are low.
Physiological Shunt
Physiological shunt is the link between arterial
and venous side of circulation provided by meta-
arteriole. Many tissues of the body such as
muscles do not have anatomical shunts.
However, the meta-arteriole in these tissues
acts as the physiological shunt between arterial
and venous sides of the circulation. The non-
nutritional blood flow occurs through physio-
logical shunt under resting conditions.
Shunt in Capillaries vs Shunt in Heart
Physiological shunt in capillaries is different from
physiological shunt in heart. In capillaries the
oxygenated blood flows towards deoxygenated
blood. But in heart, the deoxygenated blood
flows towards the oxygenated blood (see above).
FIGURE 68-2: Capillary bed

Chapter 68 Regional Circulation
431
PECULIARITIES OF CAPILLARY
BLOOD FLOW
1. The blood does not pass through capillary
system continuously. It is because of the
alternate constriction and dilatation of meta-
arterioles and alternate opening and closure
of precapillary sphincters
2. The direction of blood flow through capillaries
is not fixed as in the case of other blood
vessels. The blood may flow in opposite
direction in two adjacent capillaries
3. In capillaries, blood flows as a single pile or
single row of blood cells. In other blood
vessels, the blood flows in either axial stream
containing mainly blood cells or peripheral
stream containing plasma
4. Under resting conditions, most of the
capillaries lie in collapsed state. Only during
activity, all the capillaries open up and
increase the vascularity
5. The amount of blood flowing through capillary
system throughout the body is very low. It is
only about 150 mL/minute
6. The velocity of blood flow is least in
capillaries. It is only about 0.5 to 1 mm/
second. It facilitates exchange of substances
between capillaries and tissues.
FUNCTIONS OF CAPILLARIES
The most important function of capillaries is the
exchange of substances between blood and
tissues. Oxygen, nutrients and other essential
substances enter the tissues from capillary blood;
carbon dioxide, metabolites and other unwanted
substances are removed from the tissues by
capillary blood. Exchange of materials across the
capillary endothelium occurs primarily by
diffusion. It also occurs by means of filtration and
pinocytosis.
SKELETAL MUSCLE CIRCULATION
BLOOD FLOW TO SKELETAL MUSCLES
During resting condition, blood flow to skeletal
muscle is 4 to 7 mL/100 gram/minute. During
exercise, it increases to about 100 mL/100 gram/
minute.
MUSCULAR CONTRACTION AND
BLOOD FLOW
During contraction of the muscle, the blood
vessels are compressed and the blood flow
decreases. And during the relaxation of the
muscle, the compression of the blood vessels
is relieved and the blood flow increases.
In severe muscular exercise, the blood flow
increases in between the muscular contractions.
CUTANEOUS CIRCULATION
ARCHITECTURE OF CUTANEOUS
BLOOD VESSELS
1. The arterioles arising from the smaller
arteries reach the dermis
2. After taking origin, the arterioles turn
horizontally and give rise to meta-arterioles
3. From meta-arterioles, hairpin shaped capillary
loops arise. The arterial limb of the loop
ascends vertically and turns to form a venous
limb, which descends down
4. After reaching the base of dermis, few venous
limbs of neighboring papillae unite to form the
collecting venule
5. The collecting venules anastomose with one
another to form the subpapillary venous
plexus
6. The subpapillary plexus runs horizontally and
drain into deeper veins.
FUNCTIONS OF CUTANEOUS
CIRCULATION
Cutaneous blood flow performs two functions:
1. The supply of nutrition to skin
2. The loss of heat from the body and regulation
of body temperature.
BLOOD FLOW TO SKIN
Under normal conditions, the blood flow to skin
is about 250 mL/square meter/minute. When the
body temperature increases, cutaneous blood
flow increases up to 2800 mL/sq. meter/minute
because of cutaneous vasodilatation.
INTRODUCTION
BLOOD VESSELS IN FETUS
FETAL LUNGS
CHANGES IN CIRCULATION AND RESPIRATION AFTER BIRTH –
NEONATAL CIRCULATION AND RESPIRATION
INTRODUCTION
Fetal circulation is different from that of adults
because of the presence of placenta. Since fetal
lungs are nonfunctioning, placenta is responsible
for exchange of gases between fetal blood and
mother’s blood. So the blood from right ventricle
is diverted to placenta.
The development of heart is completed at
fourth week of intrauterine life and, it starts
beating at the rate of 65 per minute. Along with
heart, the blood vessels also develop. The heart
rate gradually increases and reaches the
maximum rate of about 140 beats per minute
just before birth.
Fetus is connected with the mother through
the placenta. Fetal blood passes to placenta
through umbilical vessels and the maternal blood
runs through uterine vessels. These two sets of
blood vessels lie in close proximity in the placenta
through which the exchange of substances takes
place between mother’s blood and fetal blood.
However, there is no direct admixture of maternal
and fetal blood (Fig. 69-1).
BLOOD VESSELS IN FETUS
As the fetal lungs are nonfunctioning, there is
no necessity of large amount of blood to be
pumped into lungs. Instead, the fetal heart
pumps large quantity of blood into the placenta
for exchange of substances. From placenta, the
umbilical veins collect the blood, which has more
oxygen and nutrients. The umbilical vein passes
through liver. Some amount of blood is supplied
to liver from umbilical vein. However, a large
quantity of blood is diverted from umbilical vein
into the inferior vena cava through ductus
venosus. Liver receives blood from portal vein
also.
In liver, the oxygenated blood mixes slightly
with deoxygenated blood and enters the right
atrium via inferior vena cava. From right atrium,
major portion of blood is diverted into left atrium
via foramen ovale. Foramen ovale is an opening
in intra-atrial septum.
Blood from the upper part of the body enters
the right atrium through superior vena cava.
From right atrium, blood enters right ventricle.
Fetal Circulation and
Respiration
69
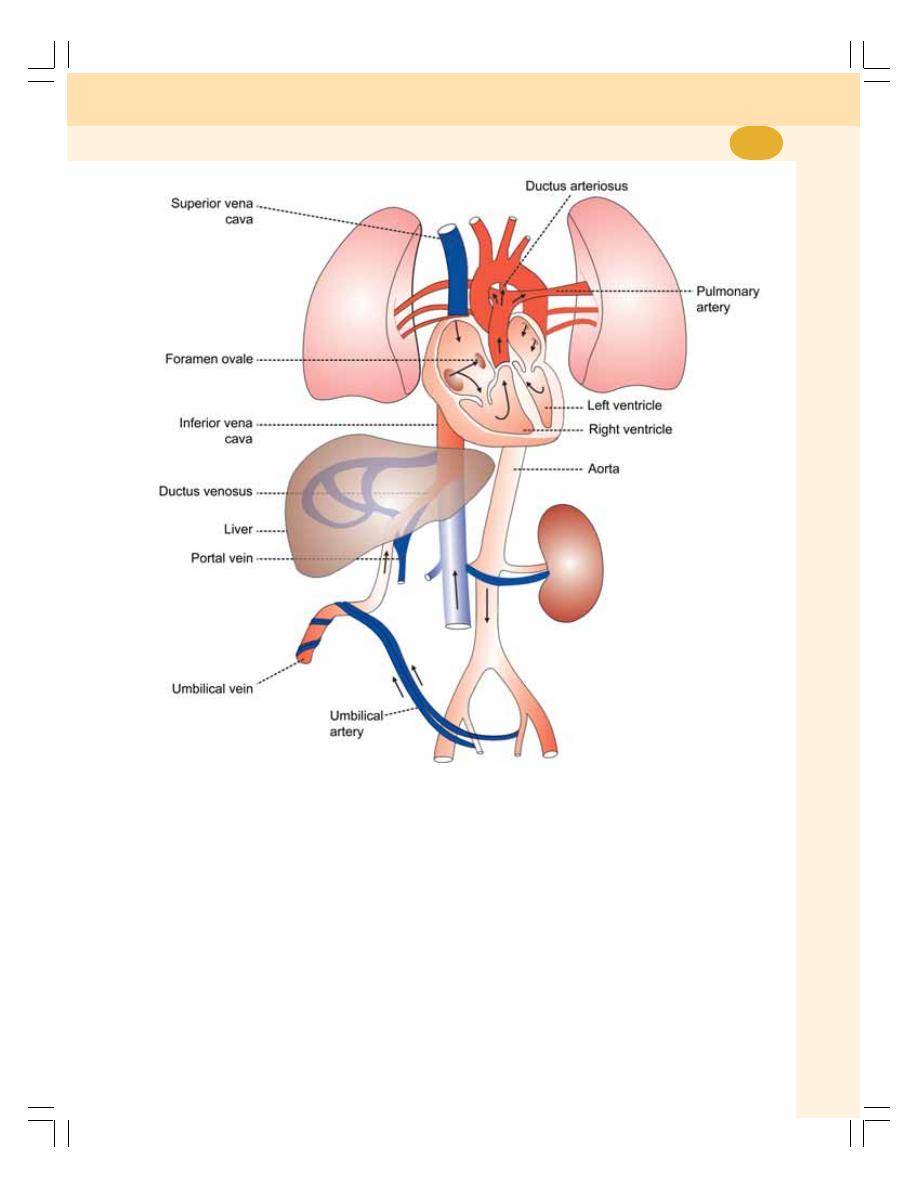
Chapter 69 Fetal Circulation and Respiration
433
From here, blood is pumped into pulmonary
artery. From pulmonary artery, blood enters the
systemic aorta through ductus arteriosus. Only
a small quantity of blood is supplied to fetal
lungs. Blood from left ventricle is pumped into
aorta. Fifty percent of blood from aorta reaches
the placenta through umbilical arteries.
FETAL LUNGS
Pulmonary vascular resistance is very high in
fetus because of non functioning of lungs. It is
the resistance offered to blood flow through
pulmonary vascular bed. The high resistance
increases the pressure in the blood vessels of
lungs. Because of the high pressure, the blood
is diverted from pulmonary artery into aorta via
ductus arteriosus.
CHANGES IN CIRCULATION AND
RESPIRATION AFTER BIRTH –
NEONATAL CIRCULATION AND
RESPIRATION
1. FIRST BREATH OF THE CHILD
When fetus is delivered and umbilical cord is
cut and tied, the lungs start functioning. When
FIGURE 69-1: Fetal circulation
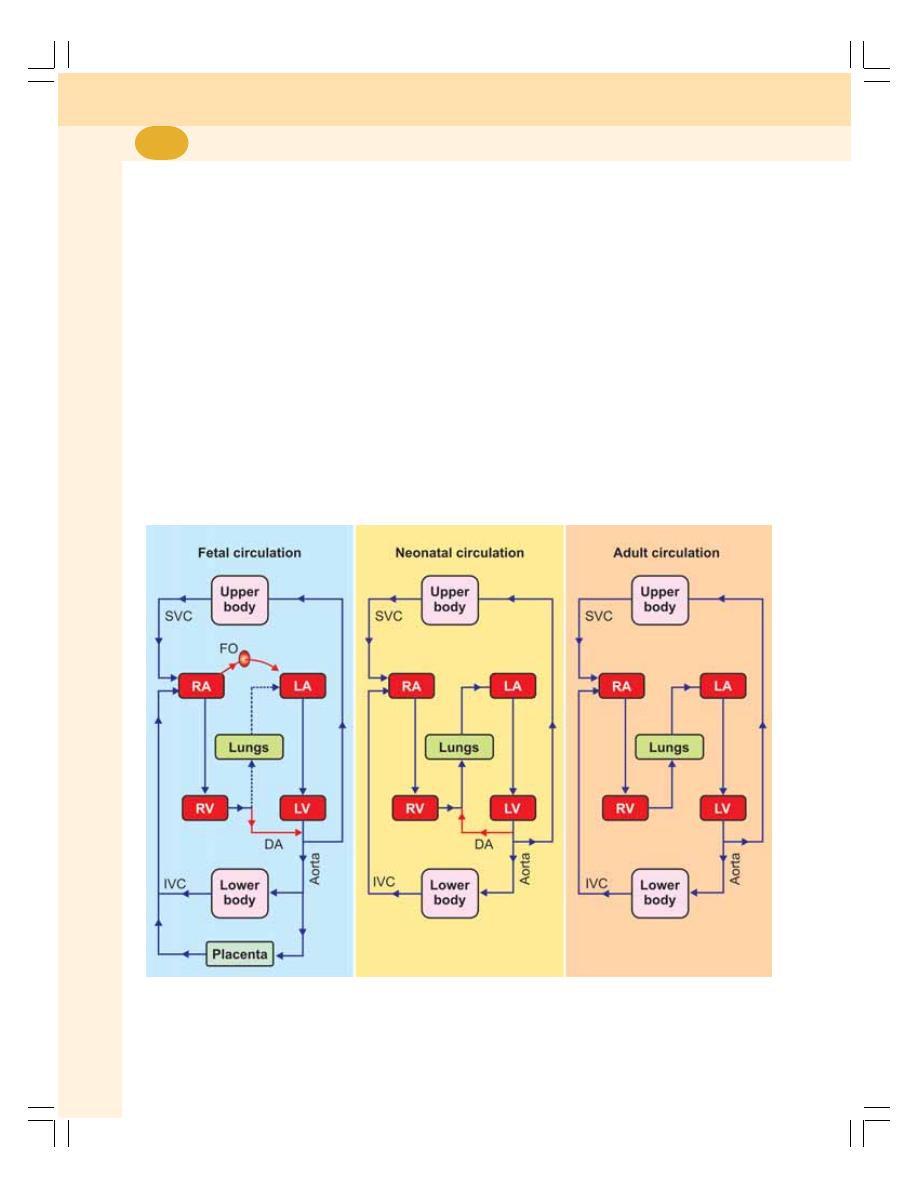
Cardiovascular System
434
placental blood flow is cut off, there is sudden
hypoxia and hypercapnia. Now, the respiratory
center is strongly stimulated by these two factors
and, the respiration starts. Initially, there is
gasping, which is followed by normal respiration.
2. FLOW OF BLOOD TO LUNGS
Lungs expand during the first breath of the infant.
The expansion of lungs causes immediate
reduction in the pulmonary vascular resistance
and a sudden fall in pressure in the blood vessels
of lungs. Therefore, the blood flow from
pulmonary artery to lungs increases.
3. CLOSURE OF FORAMEN OVALE
When blood starts flowing through the pulmonary
circulation, the oxygenated blood from the lungs
returns to left atrium. It causes increase in the
left atrial pressure. Simultaneously, due to
stoppage of blood from placenta, pressure in
inferior vena cava is decreased. It leads to fall
in right atrial pressure. Thus, the pressure in right
atrium is less and the pressure in left atrium is
already high. This causes the closure of foramen
ovale. Within few days after birth, the foramen
ovale closes completely and fuses with the atrial
wall.
4. REVERSAL OF BLOOD FLOW IN
DUCTUS ARTERIOSUS
In fetus, since pulmonary arterial pressure is very
high, the blood passes from pulmonary artery
into aorta via ductus arteriosus. However, in
neonatal life, since the systemic arterial pressure
FIGURE 69-2: Fetal, neonatal and adult circulation. RA = Right atrium. LA = Left atrium. RV = Right ventricle.
LV = Left ventricle. FO = Foramen ovale. DA = Ductus arteriosus. SVC = Superior vena cava. IVC = Inferior
vena cava. Dashed blue line (Fetal circulation) indicates flow of very less quantity of blood

Chapter 69 Fetal Circulation and Respiration
435
is more than pulmonary arterial pressure, the
blood passes in opposite direction in ductus
arteriosus, i.e. from systemic aorta into
pulmonary aorta (Fig. 69-2). The reversed flow
in ductus arteriosus is heard as continuous
murmur in infants.
5. CLOSURE OF DUCTUS VENOSUS
Due to the contraction of smooth muscle near
junction between umbilical vein and ductus
venosus, the constriction and closure of ductus
venosus occurs. Later, the ductus venosus
becomes fibrous band.
6. CLOSURE OF DUCTUS ARTERIOSUS
The ductus arteriosus starts closing due to
narrowing. It closes completely after two days
and the adult type of circulation starts. In some
rare cases, the ductus arteriosus does not close.
It remains intact producing a continuous murmur.
The condition with intact ductus arteriosus is
known as patent ductus arteriosus.
HEMORRHAGE
DEFINITION
TYPES AND CAUSES
EFFECTS OF HEMORRHAGE
CIRCULATORY SHOCK
DEFINITION
MANIFESTATIONS OF SHOCK
HEART FAILURE
DEFINITION
CAUSES
SIGNS AND SYMPTOMS
HEMORRHAGE
DEFINITION
Hemorrhage is the excess loss of blood due to
the rupture of blood vessels.
TYPES AND CAUSES OF
HEMORRHAGE
Hemorrhage occurs due to various reasons.
Based on the cause, hemorrhage is classified
into five categories:
1. Accidental hemorrhage
2. Capillary hemorrhage
3. Internal hemorrhage
4. Postpartum hemorrhage
5. Hemorrhage due to premature detachment
of placenta.
1. Accidental Hemorrhage
It occurs in road accidents and industrial
accidents, which are very common in the
developed and developing countries.
2. Capillary Hemorrhage
Capillary hemorrhage is the bleeding due to the
rupture of blood vessels, particularly capillaries.
It is very common in brain (cerebral hemorrhage)
and heart during cardiovascular diseases.
3. Internal Hemorrhage
Internal hemorrhage is the bleeding in viscera.
It is caused by rupture of blood vessels in the
viscera.
Hemorrhage, Circulatory
Shock and Heart Failure
70

Chapter 70 Hemorrhage, Circulatory Shock and Heart Failure
437
4. Postpartum Hemorrhage
Excess bleeding that occurs immediately after
labor (delivery of the baby) is called postpartum
hemorrhage.
5. Hemorrhage Due to Premature
Detachment of Placenta
In some cases, the placenta is detached from
the uterus of mother before the due date of
delivery causing severe hemorrhage.
EFFECTS OF HEMORRHAGE
Many effects are observed during and after
hemorrhage. The effects are different in acute
hemorrhage and chronic hemorrhage.
Acute Hemorrhage
Acute hemorrhage is the sudden loss of large
quantity of blood. It occurs in conditions like
accidents. Decreased blood volume in acute
hemorrhage causes hypovolemic shock.
Chronic Hemorrhage
Chronic hemorrhage is the loss of blood either
by internal or by external bleeding over a long
period of time. Internal bleeding occurs in
conditions like ulcer. External bleeding occurs
in conditions like hemophilia and excess vaginal
bleeding (menorrhagia). Chronic hemorrhage
produces different types of effects such as
anemia.
Compensatory Effects
After hemorrhage, series of compensatory
reactions develop in the body to cope up with
the blood loss. Some of the compensatory
reactions take place immediately after
hemorrhage and others at a later period.
CIRCULATORY SHOCK
DEFINITION
Shock is a general term that refers to the
depression or suppression of body functions
produced by any disorder. Circulatory shock
refers to the shock developed by inadequate
blood flow throughout the body. It is a life-
threatening condition and if the affected person
is not treated immediately it may result in death.
MANIFESTATIONS OF SHOCK
The characteristic feature of all types of
circulatory shock is the insufficient blood flow
to the tissues particularly the brain. The blood
flow decreases due to the reduction in cardiac
output. Following are the manifestations of
circulatory shock:
1. When cardiac output reduces, the arterial
blood pressure drops down
2. Low blood pressure produces reflex
tachycardia and reflex vasoconstriction
3. The pulse also becomes feeble
4. The velocity of the blood flow decreases
resulting in stagnant hypoxia
5. Skin becomes pale and cold
6. Cyanosis in many parts of the body,
particularly ear lobes and fingertips
8. Decrease in renal blood flow, GFR and
urinary output
9. Acceleration of metabolic activities of
myocardium resulting in accumulation of
excess lactic acid and acidosis
11. Acidosis decreases the efficiency of
myocardium leading to further reduction
in cardiac output
12. So, the blood flow to vital organs is
severely affected
13. The lack of blood flow to the brain tissues
produces ischemia resulting in fainting
and irreparable damage to the brain.
14. Finally the damage of brain tissues and
cardiac arrest kill the victim.
HEART FAILURE
DEFINITION
Heart failure or cardiac failure is the condition in
which the heart looses the ability to pump
sufficient amount of blood to all parts of the body.
Heart failure may involve left ventricle or right
ventricle or both. It may be acute or chronic.

Cardiovascular System
438
Acute Heart Failure
Acute heart failure refers to sudden and rapid
onset of signs and symptoms of abnormal heart
functions. Its symptoms are severe initially.
However, the symptoms last for a very short-time
and the condition improves rapidly.
Chronic Heart Failure
Chronic heart failure is the heart failure that is
characterized by the symptoms that appear
slowly over a period of time and become worst
gradually.
Congestive Heart Failure
It is a general term used to describe heart failure
resulting in accumulation of fluid in lungs and
other tissues. When heart is not able to pump
blood through aorta, the blood remains in heart.
It results in dilatation of the chambers and
accumulation of blood in veins (vascular
congestion). This condition is also manifested
by fluid retention and pulmonary edema.
CAUSES OF HEART FAILURE
The common causes of heart failure are:
1. Coronary artery disease
2. Defective heart valves
3. Arrhythmia (abnormal heartbeat)
4. Cardiac muscle disease such as cardio-
myopathy
5. Hypertension
6. Congenital heart disease
7. Diabetes
8. Hyperthyroidism
9. Anemia
10. Lung disorders
11. Inflammation of cardiac muscle
(myocarditis) due to viral infection, drugs,
alcohol, etc.
SIGNS AND SYMPTOMS OF HEART
FAILURE
Signs and Symptoms of Chronic Heart
Failure
1. Fatigue and weakness
2. Rapid and irregular heartbeat
3. Shortness of breathing
4. Fluid retention and weight gain
5. Loss of appetite, nausea and vomiting
6. Cough
8. Chest pain, if developed by myocardial
infarction.
Signs and Symptoms of Acute Heart
Failure
The signs and symptoms of acute heart failure
may be same as chronic heart failure. But the
signs and symptoms appear suddenly and
severely. When heart starts to fail suddenly, the
fluid accumulates in lungs causing pulmonary
edema. It results in sudden and severe short-
ness of breath, cough with pink, foamy mucus
and heart palpitations. It may lead to sudden
death, if not attended immediately.
INTRODUCTION
TYPES OF EXERCISE
AEROBIC AND ANAEROBIC EXERCISES
SEVERITY OF EXERCISE
EFFECTS OF EXERCISE
INTRODUCTION
During exercise, there is an increase in
metabolic needs of body tissues, particularly
the muscles. Various adjustments, which take
place in the body, are aimed at
1. Supply of nutrients and oxygen to muscles
and other tissues involved in exercise
2. Prevention of increase in body temperature.
TYPES OF EXERCISE
Exercise is generally classified into two types
depending upon the type of muscular contraction.
1. Dynamic exercise
2. Static exercise.
DYNAMIC EXERCISE
The dynamic exercise involves isotonic mus-
cular contraction and keeps the joints and
muscles moving. Examples are swimming,
bicycling, walking, etc. External work is involved
in this type of exercise. The shortening of muscle
fibers against load is called external work.
In this type of exercise, the heart rate, force
of contraction, cardiac output and systolic blood
pressure increase. However, the diastolic blood
pressure is unaltered or decreased. It is
because, during dynamic exercise, the peri-
pheral resistance is unaltered or decreased.
STATIC EXERCISE
Static exercise involves isometric muscular
contraction without movement of joints.
Example is pushing heavy object. This is a type
of exercise without the performance of external
work. During this exercise, apart from increase
in heart rate, force of contraction, cardiac output
and systolic blood pressure, the diastolic blood
pressure also increases. It is because of
increase in peripheral resistance during static
exercise.
AEROBIC AND ANAEROBIC
EXERCISES
Based on the type of metabolism (energy
producing process) involved, the exercise is
classified into two types:
1. Aerobic exercise
2. Anaerobic exercise.
Cardiovascular Adjustments
during Exercise
71

Cardiovascular System
440
AEROBIC EXERCISE
Aerobic means ‘with air’ or ‘with oxygen’. The
energy is obtained by utilizing nutrients in the
presence of oxygen. Aerobic exercise involves
activities with lower intensity, which is performed
for longer period. At the beginning, the body
obtains energy by burning glycogen stored in
liver. After about 20 minutes, when stored
glycogen is exhausted, the body starts burning
fat. Body fat is converted into glucose, which is
utilized for energy.
Examples of aerobic exercise:
1. Fast walking
2. Jogging
3. Running
4. Bicycling
5. Skiing
6. Skating
7. Hockey
8. Soccer
9. Tennis
10. Badminton
11. Swimming
12. Rowing.
ANAEROBIC EXERCISE
Anaerobic means ‘without air ’ or ‘without
oxygen’. Body obtains energy by burning
glycogen stored in the muscles without oxygen.
Anaerobic exercise involves exertion for short
periods followed by periods of rest. It uses the
muscles at high intensity and a high rate of work
for a short period.
Burning glycogen without oxygen liberates
lactic acid. Accumulation of lactic acid leads to
fatigue. Therefore, this type of exercise cannot
be performed for longer period. And a recovery
period is essential before going for another burst
of anaerobic exercise. Anaerobic exercise helps
to increase the muscle strength.
Examples of anaerobic exercise:
1. Pull ups
2. Push ups
3. Weightlifting
4. Sprinting
5. Any other rapid burst of strenuous exercise.
SEVERITY OF EXERCISE
The cardiovascular and other changes in the
body depend upon the severity of exercise also.
Based on severity, the exercise is classified into
three types:
1. Mild exercise
2. Moderate exercise
3. Severe exercise.
1. MILD EXERCISE
It is the very simple form of exercise like slow
walking. Little or no change occurs in cardio-
vascular system during mild exercise.
2. MODERATE EXERCISE
Moderate exercise does not involve strenuous
muscular activity and it can be performed for a
longer period. Exhaustion does not occur at the
end of moderate exercise. The examples of this
type of exercise are fast walking and slow
running.
3. SEVERE EXERCISE
Severe exercise involves strenuous muscular
activity and it can be performed only for short
duration. Fast running for a distance of 100 or
400 meters is the best example of this type of
exercise. Complete exhaustion occurs at the end
of severe exercise.
EFFECTS OF EXERCISE ON
CARDIOVASCULAR SYSTEM
1. ON BLOOD
Red blood cell count increases because of
release of erythropoietin from juxtaglomerular
apparatus due to hypoxia. The pH of blood
decreases due to increased carbon dioxide
content.
2. ON BODY FLUIDS
More heat is produced during exercise and the
thermoregulatory system is activated. This in turn,

Chapter 71 Cardiovascular Adjustments during Exercise
441
causes secretion of large amount of sweat
leading to:
i. Fluid loss
ii. Reduced blood volume
iii. Hemoconcentration
iv. Sometimes, severe exercise leads to
dehydration.
3. ON HEART RATE
Heart rate increases during exercise. Even the
thought of exercise or preparation for exercise
increases the heart rate. It is because of impulses
from cerebral cortex to medullary centers, which
reduces vagal tone.
In moderate exercise, the heart rate increases
to 180 beats/minute. In severe muscular exercise
it reaches 240 to 260 beats/minute. The
increased heart rate during exercise is mainly
vagal withdrawal and increase in sympathetic
tone.
4. ON CARDIAC OUTPUT
Cardiac output increases up to 20 liters/minute
in moderate exercise and up to 35 liters/minute
during severe exercise. The increase in cardiac
output is directly proportional to the increase in
the amount of oxygen consumed during exercise.
5. ON VENOUS RETURN
Venous return increases during exercise because
of muscle pump, respiratory pump and splan-
chnic vasoconstriction.
6. ON BLOOD FLOW TO SKELETAL
MUSCLES
There is increase in the amount of blood flowing
to skeletal muscles during exercise. In resting
condition, the blood supply to the skeletal
muscles is 3 to 4 mL/100 gram of the muscle/
minute. It increases up to 60 to 80 mL in
moderate exercise and up to 90 to 120 mL in
severe exercise.
During the muscular activity, stoppage of
blood flow occurs when the muscles contract. It
is because of compression of blood vessels
during contraction. And in between the contrac-
tions, the blood flow increases.
7. ON BLOOD PRESSURE
During moderate isotonic exercise, the systolic
pressure is increased. It is due to increase in
heart rate and stroke volume. Diastolic pressure
is not altered because peripheral resistance is
not affected during moderate isotonic exercise.
In severe exercise involving isotonic muscular
contraction, the systolic pressure enormously
increases but the diastolic pressure decreases.
The decrease in diastolic pressure is because
of the decrease in peripheral resistance.
Decrease in peripheral resistance is due to
vasodilatation caused by metabolites.
During exercise involving isometric contrac-
tion, the peripheral resistance increases. So, the
diastolic pressure also increases along with
systolic pressure.
Blood Pressure after Exercise
After exercise, the blood pressure falls below the
resting level. It is because of vasodilatation
caused by metabolic end products accumulated
in muscles during exercise. However, the
pressure returns to resting level quickly as soon
as the metabolic end products are removed from
muscles.

Questions in Cardiovascular System
442
LONG QUESTIONS
1. Define cardiac cycle. Describe various events
of cardiac.
2. Define electrocardiogram. Describe the
waves, segments and intervals of normal
ECG. Add a note on ECG leads.
3. Give the definitions, normal values and
variations of cardiac output. Explain the
factors regulating cardiac output.
4. What is cardiac output? Enumerate the
various methods to measure cardiac output
and, explain the measurement of cardiac
output by applying Fick’s principle.
5. Describe the innervation of heart and the
regulation of heart rate.
6. Define arterial blood pressure. Describe the
nervous regulation (short-term) of arterial
blood pressure.
7. Describe renal mechanism of (long-term)
regulation of arterial blood pressure.
SHORT QUESTIONS
1. Action potential in cardiac muscle.
2. Pacemaker.
3. Conductive system in heart.
4. Isometric contraction period.
5. Heart sounds.
6. Waves of normal ECG.
7. ECG leads.
8. Peripheral resistance.
9. Fick’s principle.
10. Cardiac centers.
11. Nerve supply to heart.
12. Vagal tone.
13. Baroreceptors.
14. Chemoreceptors.
15. Bainbridge reflex.
16. Determinants of arterial blood pressure.
17. Vasomotor center.
18. Vasomotor tone.
19. Renal regulation of blood pressure.
20. Renin-angiotensin mechanism.
21. Hypertension.
22. Venous pressure.
23. Capillary pressure.
24. Arterial pulse.
25. Phlebogram/venous pulse.
26. Capillary circulation (microcirculation).
27. Effect of exercise on blood pressure.
QUESTIONS IN CARDIOVASCULAR SYSTEM

Respiratory System and
Environmental Physiology
72. Respiratory Tract and Pulmonary Circulation ...................... 445
73. Mechanics of Respiration .................................................... 451
74. Pulmonary Function Tests ................................................... 457
75. Ventilation ............................................................................ 463
76. Exchange and Transport of Respiratory Gases .................. 466
77. Regulation of Respiration .................................................... 474
78. Disturbances of Respiration ................................................ 480
79. High Altitude and Deep Sea Physiology .............................. 487
80. Effects of Exposure to Cold and Heat ................................. 492
81. Artificial Respiration ............................................................. 494
82. Effects of Exercise on Respiration ...................................... 496
S E C T I O N
9
C H A P T E R S

INTRODUCTION
FUNCTIONAL ANATOMY OF RESPIRATORY TRACT
RESPIRATORY UNIT
NONRESPIRATORY FUNCTIONS OF RESPIRATORY TRACT
RESPIRATORY PROTECTIVE REFLEXES
PULMONARY CIRCULATION
Respiratory Tract and
Pulmonary Circulation
72
INTRODUCTION
Respiration is the process by which oxygen is
taken in and carbon dioxide is given out. The
normal respiratory rate in adults is 12 to
16/minute.
TYPES OF RESPIRATION
Respiration is often classified into two types:
1. External respiration that involves exchange
of respiratory gases, i.e. oxygen and carbon
dioxide between lungs and blood.
2. Internal respiration which involves exchange
of gases between blood and tissues.
PHASES OF RESPIRATION
Respiration occurs in two stages:
1. Inspiration during which the air enters the
lungs from atmosphere
2. Expiration during which the air leaves the
lungs.
FUNCTIONAL ANATOMY OF
RESPIRATORY TRACT
Respiratory tract is the anatomical structure
through which air moves in and out. It consists
of nose, pharynx, larynx, trachea, bronchi and
lungs (Fig. 72-1).
Pleura
Each lung is enclosed by a bilayered serous
membrane called pleura or pleural sac. The two
layers of pleura are the visceral and parietal
layers. Visceral (inner) layer lines the surface of
the lungs. At hilum, it is continuous with parietal
(outer) layer, which is attached to the wall of the
thoracic cavity.
The narrow space in between the two layers
of pleura is called intrapleural space or pleural
cavity. Its space contains a thin film of pleural
fluid which is involved in the creating the negative
pressure called intrapleural pressure within
intrapleural space.
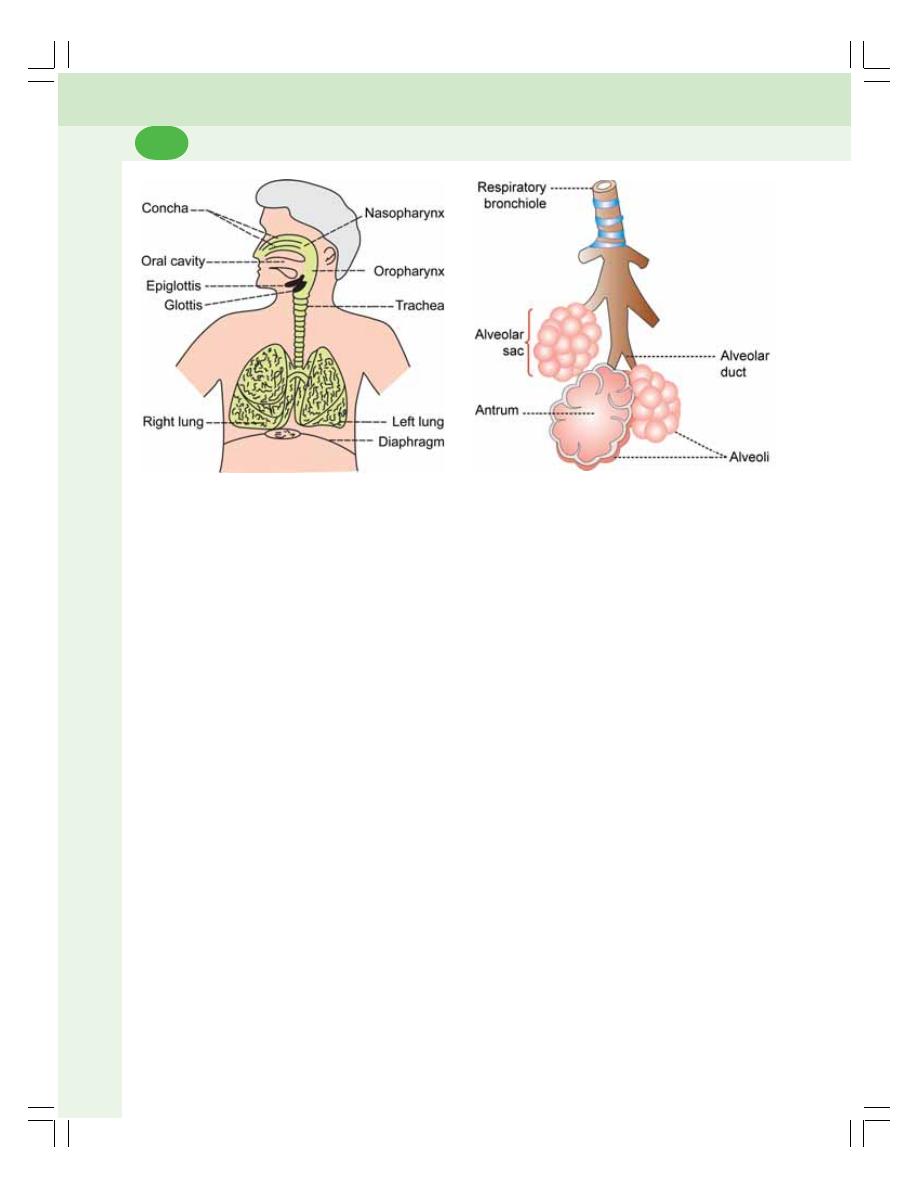
Respiratory System and Environmental Physiology
446
Tracheobronchial Tree
The trachea and bronchi are together called
tracheobronchial tree. It forms a part of air
passage.
The trachea bifurcates into two main or
primary bronchi called right and left bronchi.
Each primary bronchus enters the lungs and
divides into secondary bronchi. The secondary
bronchi divide into tertiary bronchi. In right lung,
there are ten tertiary bronchi and, in left lung,
there are eight tertiary bronchi.
The tertiary bronchi divide several times with
reduction in length and diameter into many
generations of bronchioles. When the diameter
of bronchioles becomes 1 mm or less, it is called
terminal bronchiole. Terminal bronchiole conti-
nues or divides into respiratory bronchiole, which
has a diameter of 0.5 mm.
Generally, the respiratory tract is divided into
two parts:
1. Upper respiratory tract which includes all the
structures from nose up to vocal cords
2. Lower respiratory tract that includes trachea,
bronchi and lungs.
RESPIRATORY UNIT
Lung parenchyma is formed by respiratory unit
that forms the terminal portion of respiratory tract.
Respiratory unit is defined as the structural and
functional unit of lung. The exchange of gases
occurs only in this part of the respiratory tract.
STRUCTURE OF RESPIRATORY UNIT
The respiratory unit starts from the respira-
tory bronchioles (Fig. 72-2). Each respiratory
bronchiole divides into alveolar ducts. Each
alveolar duct enters an enlarged structure
called the alveolar sac. The space inside the
alveolar sac is called antrum. Alveolar sac
consists of a cluster of alveoli. Few alveoli are
present in the wall of alveolar duct also.
Thus, respiratory unit includes:
1. Respiratory bronchioles
2. Alveolar ducts
3. Alveolar sacs
4. Antrum
5. Alveoli.
Each alveolus is like a pouch with the dia-
meter of about 0.2 to 0.5 mm. It is lined by
epithelial cells called alveolar cells or
pneumocytes. Alveolar cells are of two types:
i. Type I alveolar cells which form the site
of gaseous exchange between alveolus
and blood
ii. Type II alveolar cells which secrete the
alveolar fluid and surfactant.
FIGURE 72-2: Respiratory unit
FIGURE 72-1: Respiratory tract

Chapter 72 Respiratory Tract and Pulmonary Circulation
447
RESPIRATORY MEMBRANE
Respiratory membrane is the structure through
which the exchange of gases occurs. It separates
air in the alveoli from the blood in capillary. It is
formed by the alveolar membrane and capillary
membrane. Respiratory membrane has a surface
area of 70 sq. meters and thickness of 0.5
microns. The structure of respiratory membrane
is explained in Chapter 76 (Fig. 76-1).
NONRESPIRATORY FUNCTIONS OF
RESPIRATORY TRACT
Besides the primary function of gaseous
ex-change, the respiratory tract is involved in
several nonrespiratory functions of the body.
1. OLFACTION
Olfactory receptors present in the mucous mem-
brane of nostril are responsible for olfactory
sensation.
2. VOCALIZATION
Along with other structures, larynx forms the
speech apparatus.
3. PREVENTION OF DUST PARTICLES
The dust particles, which enter the nostrils from
air, are prevented from reaching the lungs by
filtration action of the hairs in nasal mucous
membrane. The small particles, which escape
the hairs, are held by the mucus secreted by
the nasal mucous membrane. Those dust
particles, which escape the nasal hairs and nasal
mucous membrane, are removed by the
phagocytic action of the macrophages in the
alveoli. The particles which escape the protective
mechanisms in nose and alveoli are thrown out
by cough reflex and sneezing reflex
4. DEFENSE MECHANISM
The defense functions of the lungs are performed
by their own defenses and by the presence of
various types of cells in the mucous membrane
lining the alveoli of lungs. These cells are leuko-
cytes, macrophages, mast cells, natural killer
cells and dendritic cells.
i.
Lung’s Own Defenses
The epithelial cells lining the air passage secrete
some innate immune factors called defensins
and cathelicidins. These substances are the
antimicrobial peptides which play an important
role in lung’s natural defenses.
ii. Defense through Leukocytes
The leukocytes, particularly the neutrophils and
lymphocytes present in the alveoli of lungs
provide defense mechanism against bacteria
and virus. The neutrophils kill the bacteria by
phagocytosis. Lymphocytes develop immunity
against bacteria.
iii. Defense through Macrophages
Macrophages engulf the dust particles and the
pathogens, which enter the alveoli and thereby
act as scavengers in lungs. Macrophages are
also involved in the development of immunity
by functioning as antigen presenting cells.
iv. Defense through Mast Cell
Mast cell produces the allergic reaction.
v. Defense through Natural Killer Cell
Natural killer (NK) cell destroys the micro-orga-
nisms like viruses and the viral infected or
damaged cells, which may form the tumors. It
also destroys the malignant cells and prevents
development of cancerous tumors.
vi. Defense through Dendritic Cells
Dendritic cells in the lungs function as antigen
presenting cells.
5. MAINTENANCE OF WATER BALANCE
Respiratory tract plays a role in water loss
mechanism. During expiration, water evaporates
through the expired air and some amount of
body water is lost by this process.

Respiratory System and Environmental Physiology
448
6. REGULATION OF BODY
TEMPERATURE
During expiration, along with water, heat is also
lost from the body. Thus, respiratory tract plays
a role in heat loss mechanism.
7. REGULATION OF ACID–BASE
BALANCE
Lungs play a role in maintenance of acid–base
balance of the body by regulating the carbon
dioxide content in blood. Carbon dioxide is pro-
duced during various metabolic reactions in the
tissues of the body. When it enters the blood,
carbon dioxide combines with water to form
carbonic acid. Since carbonic acid is unstable,
it splits into hydrogen and bicarbonate ions.
CO
2
+ H
2
O
→ H
2
CO
3
→ H
+
+ HCO
3
–
The entire reaction is reversed in lungs when
carbon dioxide is removed from blood into the
alveoli of lungs.
H
+
+ HCO
3
–
→ H
2
CO
3
→ CO
2
+ H
2
O
As carbon dioxide is a volatile gas, it is practi-
cally blown out by ventilation.
8. ANTICOAGULANT FUNCTION
Mast cells in lungs secrete heparin. Heparin is
an anticoagulant and it prevents the intravascular
clotting.
9. SECRETION OF ANGIOTENSIN
CONVERTING ENZYME
Endothelial cells of the pulmonary capillaries
secrete the angiotensin converting enzyme
(ACE). It converts the angiotensin I into active
angiotensin II which plays an important role in
the regulation of ECF volume and blood pressure
(Chapter 35).
10.SYNTHESIS OF HORMONAL
SUBSTANCES
Lung tissues are also known to synthesize the
hormonal substances, prostaglandins, acetyl-
choline and serotonin which have many physio-
logical actions in the body including regulation
of blood pressure (Chapter 52).
RESPIRATORY PROTECTIVE
REFLEXES
Respiratory protective reflexes are the reflexes
that protect the lungs and air passage from
foreign particles. The respiratory protective refle-
xes are:
COUGH REFLEX
Cough is a modified respiratory process
characterized by forced expiration. It is the pro-
tective reflex that occurs because of irritation of
respiratory tract and some other areas such as
external auditory canal. Cough is produced
mainly by irritant agents.
Mechanism
Cough begins with deep inspiration followed by
forced expiration with closed glottis. This
increases the intrapleural pressure above
100 mm Hg. Then, glottis opens suddenly with
explosive outflow of air at a high velocity. The
velocity of the airflow may reach 960 km/hour.
It causes expulsion of irritants out of the
respiratory tract.
SNEEZING REFLEX
Sneezing is also a modified respiratory process
characterized by forced expiration. It is the pro-
tective reflex caused by irritation of nasal mucous
membrane. Irritation of the nasal mucous mem-
brane occurs because of dust particles, debris,
mechanical obstruction of the airway, and excess
fluid accumulation in the nasal passages.
Mechanism
Sneezing starts with deep inspiration, followed
by forceful expiratory effort with opened glottis
resulting in expulsion of irritant agents out of
respiratory tract.

Chapter 72 Respiratory Tract and Pulmonary Circulation
449
SWALLOWING (DEGLUTITION) REFLEX
Swallowing is a respiratory protective reflex that
prevents entrance of food particles into the air
passage during swallowing.
While swallowing of the food, the respiration
is arrested for a while. The temporary arrest of
respiration is called apnea. The arrest of breath-
ing during swallowing is called swallowing apnea
or deglutition apnea. It takes place during pharyn-
geal stage, i.e. II stage of deglutition and prevents
entry of food particles into the respiratory tract.
Refer Chapter 33 for details.
PULMONARY CIRCULATION
PULMONARY BLOOD VESSELS
Pulmonary blood vessels include pulmonary
artery which carries deoxygenated blood to
alveoli of lungs and bronchial artery which
supply oxygenated blood to other structures of
lungs (see below).
Pulmonary Artery
Pulmonary artery supplies deoxygenated blood
pumped from right ventricle to alveoli of lungs
(pulmonary circulation). After leaving the right
ventricle, it divides into right and left branches.
Each branch enters the corresponding lung
along with primary bronchus. After entering the
lung, the branch of the pulmonary artery divides
into small vessels and finally forms the capillary
plexus that is in intimate relationship to alveoli.
Capillary plexus is solely concerned with alveolar
gas exchange. Oxygenated blood from the
alveoli is carried to left atrium by one pulmonary
vein from each side.
Bronchial Artery
Bronchial artery arises from descending thoracic
aorta. It supplies arterial blood to bronchi,
connective tissue and other structures of lung
stroma, visceral pleura and pulmonary lymph
nodes. Venous blood from these structures is
drained by two bronchial veins from each side.
However, the blood from distal portion of
bronchial circulation is drained directly into the
tributaries of pulmonary veins.
Physiological Shunt
Physiological shunt is a diversion through which
the venous blood is mixed with arterial blood.
The deoxygenated blood flowing from bronchial
circulation into pulmonary veins without
being oxygenated makes up part of normal
physiological shunt. The other component of
physiological shunt is the drainage of deoxy-
genated blood from thebesian veins into cardiac
chambers directly (Chapter 68).
CHARACTERISTIC FEATURES OF
PULMONARY BLOOD VESSELS
1. The pulmonary artery has a thin wall and it
has only about 1/3 of thickness of the syste-
mic aortic wall. The wall of other pulmonary
blood vessels is also thin
2. The pulmonary blood vessels are highly
elastic and more distensible
3. The smooth muscle coat is not well developed
in the pulmonary blood vessels
4. True arterioles have less smooth muscle
fibers
5. Pulmonary capillaries are larger than
systemic capillaries.
6. Vascular resistance in pulmonary circulation
is very less; it is only one tenth of systemic
circulation
7. Pulmonary vascular system is a low pressure
system (see below)
8. Pulmonary artery carries deoxygenated blood
from heart to lungs and pulmonary veins carry
oxygenated blood from lungs to heart
9. Physiological shunt is present.
PULMONARY BLOOD FLOW
The lungs receive the whole amount of blood that
is pumped out from right ventricle. The output

Respiratory System and Environmental Physiology
450
of blood per minute is same in both the right and
left ventricle. It is about 5 liters.
PULMONARY BLOOD PRESSURE
The pulmonary blood pressure is less than
systemic blood pressure because the pulmonary
blood vessels are more distensible than systemic
blood vessels. Thus, the entire pulmonary
vascular system is a low pressure bed.
Pulmonary Arterial Pressure
Systolic pressure
: 25 mm Hg
Diastolic pressure
: 10 mm Hg
Mean arterial pressure
: 15 mm Hg
Pulmonary Capillary Pressure
The pulmonary capillary pressure is about 7 mm
Hg.
RESPIRATORY MOVEMENTS
RESPIRATORY PRESSURES
COMPLIANCE
WORK OF BREATHING
Mechanics of
Respiration
73
73
73
73
73
RESPIRATORY MOVEMENTS
INTRODUCTION
During normal quiet breathing, inspiration is the
active process and expiration is the passive
process. During inspiration, thoracic cage
enlarges and lungs expand so that air enters the
lungs easily. During expiration, the thoracic cage
and lungs decrease in size and attain the
preinspiratory position so that air leaves the
lungs easily.
MUSCLES OF RESPIRATION
Muscles involved in inspiratory movements are
known as inspiratory muscles and the muscles
involved in expiratory movements are called
expiratory muscles. However, the respiratory
muscles are generally classified into two types:
1. Primary or major respiratory muscles which
are responsible for change in size of thoracic
cage during normal quiet breathing
2. Accessory respiratory muscles that help
primary respiratory muscles during forced
respiration.
Inspiratory Muscles
Primary inspiratory muscles are diaphragm and
external intercostal muscles. Accessory inspi-
ratory muscles are sternocleidomastoid, scaleni,
anterior serrati, elevators of scapulae and
pectorals.
Expiratory Muscles
Primary expiratory muscles are the internal
intercostal muscles. Accessory expiratory mus-
cles are the abdominal muscles.
MOVEMENTS OF THORACIC CAGE
Inspiration causes enlargement of thoracic
cage. Thoracic cage enlarges because of
increase in all diameters, viz. anteroposterior,
transverse and vertical diameters. Increase in
anteroposterior and transverse diameters occurs
due to the elevation of ribs. The vertical diameter
of thoracic cage is increased by the descent of
diaphragm.
In general, the change in the size of thoracic
cavity occurs because of the movements of four
units of structures.

Respiratory System and Environmental Physiology
452
1. Thoracic lid
2. Upper costal series
3. Lower costal series
4. Diaphragm.
1. Thoracic Lid
The thoracic lid is formed by manubrium sterni
and the first pair of ribs. Movement of thoracic
lid increases the anteroposterior diameter of
thoracic cage.
2. Upper Costal Series
The upper costal series is constituted by second
to sixth pair of ribs. Upper costal series increases
the anteroposterior and transverse diameter of
the thoracic cage by pump handle movement and
bucket handle movement.
Pump handle movement
During inspiration, there is elevation of upper
costal series of ribs and upward and forward
movement of sternum. This movement is called
pump handle movement. It increases antero-
posterior diameter of the thoracic cage.
Bucket handle movement
Simultaneously, the central portions of these ribs
(arches of ribs) move upwards and outwards to
a more horizontal position. This movement is
called bucket handle movement and it increases
the transverse diameter of thoracic cage.
3. Lower Costal Series
It is formed by the seventh to tenth pair of ribs.
Movement of lower costal series increases the
transverse diameter of the thoracic cage. These
ribs also show bucket handle movement by
swinging outward and upward.
The eleventh and twelfth pairs of ribs are the
floating ribs, which are not involved in changing
the size of thoracic cage.
4. Diaphragm
Movement of diaphragm increases the vertical
diameter of thoracic cage. Normally, before
inspiration the diaphragm is dome-shaped with
convexity facing upwards. During inspiration, due
to the contraction of muscle fibers the central
tendinous portion is drawn downwards so the
diaphragm is flattened and increases the vertical
diameter of the thoracic cage.
MOVEMENTS OF LUNGS
During inspiration, due to the enlargement of
thoracic cage, the negative pressure is increased
in the thoracic cavity. It causes expansion of the
lungs. During expiration, the thoracic cavity
decreases in size to the preinspiratory position.
The pressure in the thoracic cage also comes
back to the preinspiratory level. It compresses
the lung tissues so that, the air is expelled out
of lungs.
Collapsing Tendency of Lungs
The lungs are under constant threat to collapse
even under resting conditions because of certain
factors.
Factors causing collapsing tendency of lungs
Two factors are responsible for the collapsing
tendency of lungs
1. Elastic property of lung tissues which show
constant recoiling tendency and try to
collapse the lungs
2. Surface tension exerted on the surface of the
alveolar membrane by the fluid secreted from
alveolar epithelium.
Fortunately, there are some factors which
save the lungs from collapsing.
Factors preventing collapsing tendency of lungs
In spite of the elastic property of the lungs and
the surface tension in the alveoli of lungs, the
collapsing tendency of lungs is prevented by two
factors:
1. Intrapleural pressure which is always
negative (see below). Because of negativity,
it keeps the lungs expanded and prevents
the collapsing tendency of lungs produced
by the elastic tissues
2. Surfactant secreted in alveolar epithelium. It
reduces surface tension and prevents the
collapsing tendency produced by surface
tension.

Chapter 73 Mechanics of Respiration
453
Surfactant
Pulmonary surfactant is a surface acting material
that decreases the surface tension on the
alveolar membrane. It is secreted by two types
of cells:
1. Type II alveolar epithelial cells in the lungs
2. Clara cells, which are situated in the bron-
chioles.
Chemistry
Surfactant is a lipoprotein complex formed by
lipids especially phospholipids, proteins and ions.
The phospholipid dipalmitoylphosphatidylcholine
(DPPC) is the major component of surfactant.
Functions
1. The surfactant reduces the surface tension
in the alveoli of lungs and prevents the collap-
sing tendency of lungs. The phospholipid
molecule in the surfactant is responsible for
this
2. The surfactant is responsible for stabilization
of the alveoli, which is necessary to withstand
the collapsing tendency.
3. It plays an important role in the inflation of
lungs after birth. In fetus, lungs are solid and
not expanded. First breath starts soon after
birth. Although the respiratory movements are
attempted by the infant, the lungs tend to
collapse repeatedly. And, the presence of
surfactant in the alveoli prevents the lungs
from collapsing.
4. The hydrophilic proteins in surfactant play a
role in defense in the lungs by destroying the
bacteria and viruses.
Effect of deficiency of surfactant – respiratory
distress syndrome
Deficiency or absence of surfactant in infants
causes collapse of lungs. This condition is called
respiratory distress syndrome or hyaline mem-
brane disease. The deficiency of surfactant
occurs in adults also and it is called adult res-
piratory distress syndrome (ARDS).
RESPIRATORY PRESSURES
Two types of pressures are exerted in the tho-
racic cavity and the lungs during the process of
respiration:
1. Intrapleural pressure or intrathoracic
pressure
2. Intra-alveolar pressure or intrapulmonary
pressure.
INTRAPLEURAL PRESSURE
Definition
The intrapleural pressure is the pressure existing
in pleural cavity, that is, in between the visceral
and parietal layers of pleura. It is exerted by the
suction of the fluid that lines the pleural cavity
(Fig. 73-1). It is also called intrathoracic pressure
since it is exerted in the whole of thoracic cavity.
Normal Values
Respiratory pressures are expressed in relation
to atmospheric pressure which is 760 mm Hg.
Intrapleural pressure is always negative.
The normal values are:
1. At the end of normal inspiration: – 6 mm Hg
(760 – 6 = 754 mm Hg)
2. At the end of normal expiration: – 2 mm Hg
(760 – 2 = 758 mm Hg)
3. At the end of forced inspiration: – 30 mm Hg.
Cause for Negativity of Intrapleural Pressure
The pleural cavity is always lined by a thin layer
of fluid that is secreted by the visceral layer of
pleura. This fluid is constantly pumped from the
pleural cavity into the lymphatic vessels. The
pumping of fluid creates the negative pressure
in the pleural cavity.
Significance of Intrapleural Pressure
Throughout the respiratory cycle intrapleural
pressure remains lower than intra-alveolar
pressure. This keeps the lungs always inflated.
The intrapleural pressure has two important
functions:
i. It prevents the collapsing tendency of
lungs
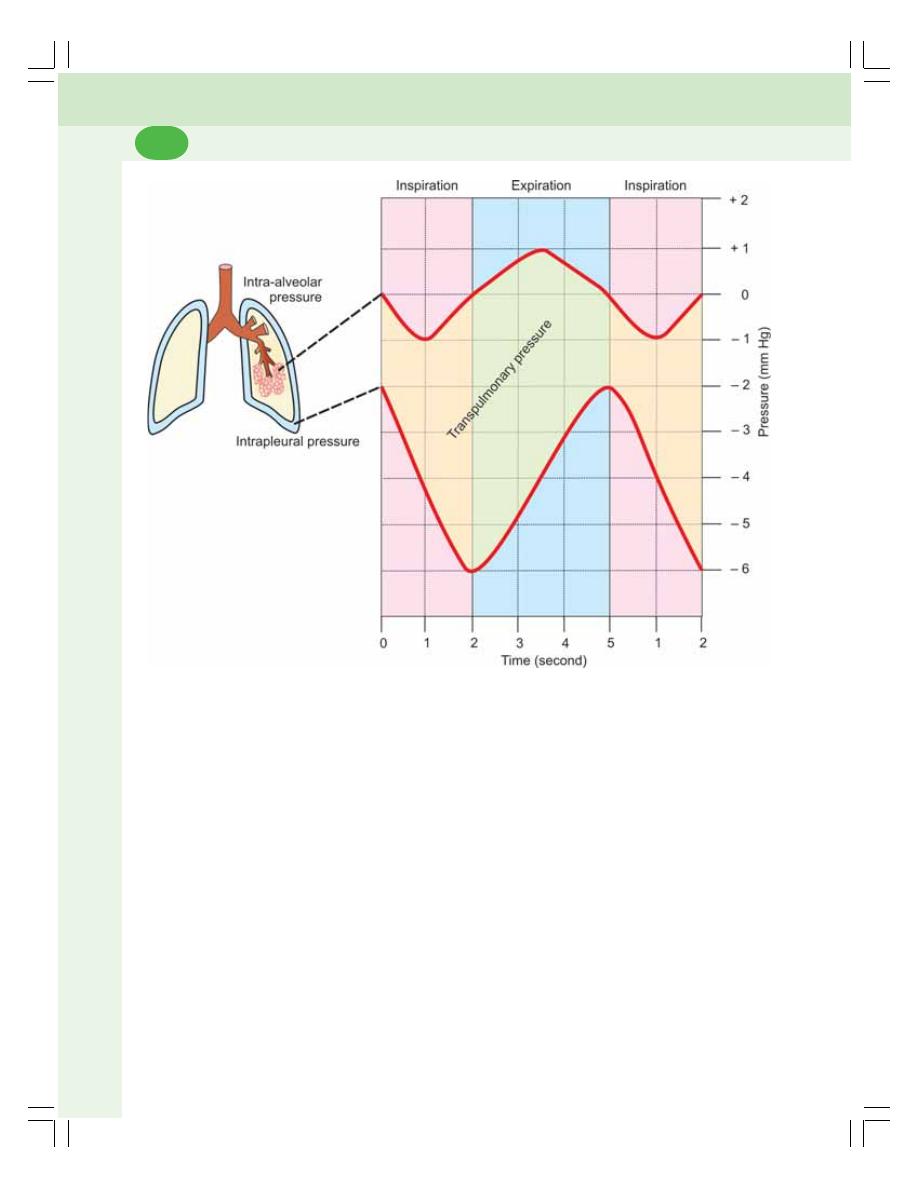
Respiratory System and Environmental Physiology
454
ii. It causes dilatation of vena cava and
larger veins in thorax. Also, the negative
pressure acts like suction pump and pulls
the venous blood from lower part of body
towards the heart against gravity. Thus,
the intrapleural pressure is responsible for
the venous return. So, it is called the respi-
ratory pump for venous return (Chapter
63).
INTRA-ALVEOLAR PRESSURE
Definition
Intra-alveolar pressure is the pressure existing
in the alveoli of the lungs. It is also known as
intrapulmonary pressure.
Normal Values
Normally, intra-alveolar pressure becomes
negative during inspiration and positive during
expiration.
The normal values are:
1. During normal inspiration: – 1 mm Hg
(760 – 1 = 759 mm Hg)
2. During normal expiration: + 1 mm Hg
(760 + 1 = 761 mm Hg)
3. At the end of inspiration and expiration: Equal
to atmospheric pressure (760 mm Hg)
Significance of Intra-alveolar Pressure
i. It causes flow of air in and out of alveoli.
During inspiration, the intra-alveolar
FIGURE 73-1: Changes in respiratory pressures during inspiration and expiration
‘0’ indicates the normal atmospheric pressure (760 mm Hg)
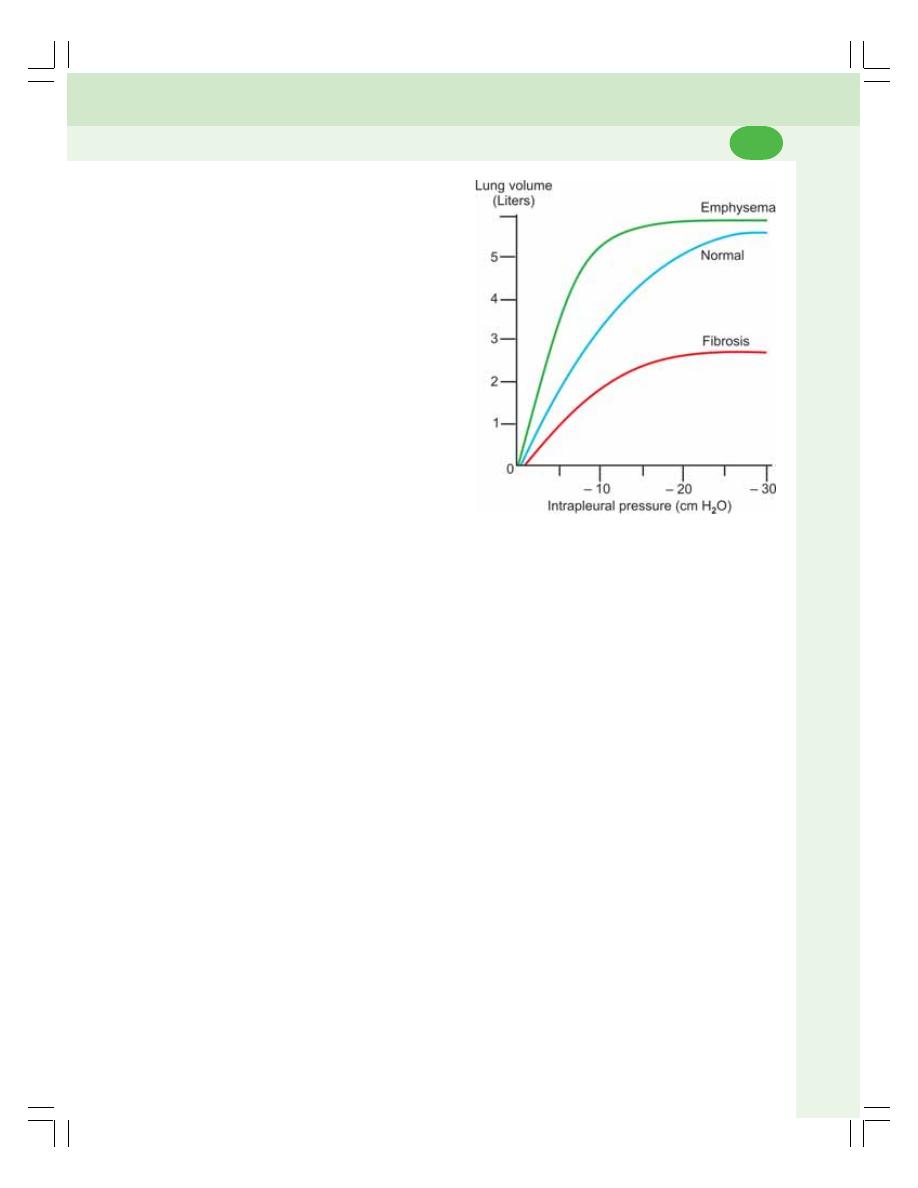
Chapter 73 Mechanics of Respiration
455
pressure becomes negative, so the
atmospheric air enters the alveoli. And,
during expiration, the air is expelled out
of alveoli
ii. It also helps in the exchange of gases
between the alveolar air and the blood.
Transpulmonary Pressure
Transpulmonary pressure is the difference bet-
ween intra-alveolar pressure and intrapleural
pressure.
COMPLIANCE
DEFINITION
Compliance is the ability of the lungs and thorax
to expand or it is the expansibility of lungs and
thorax. It is defined as the change in volume per
unit change in the pressure. Determination of
compliance is useful as it is the measure of
stiffness of lungs. Stiffer the lungs, less is the
compliance.
NORMAL VALUES
The compliance is expressed in relation to
respiratory pressures.
Compliance in Relation to
Intra-alveolar Pressure
Compliance is the volume increase in lungs per
unit increase in the intra-alveolar pressure.
1. Compliance of lungs and thorax together: 130
mL/1 cm H
2
O pressure
2. Compliance of lungs alone: 220 mL/1 cm H
2
O
pressure.
Compliance in Relation to
Intrapleural Pressure
Compliance is the volume increase in lungs per
unit decrease in the intrapleural pressure.
1. Compliance of lungs and thorax together =
100 mL/1 cm H
2
O pressure
2. Compliance of lungs alone = 200 mL/1 cm
H
2
O pressure.
Thus, if lungs are removed from thorax, the
expansibility (compliance) of lungs alone is doub-
led. It is because of the absence of the inertia
and the restriction exerted by the structures of
thoracic cage, which interfere with expansion of
lungs.
Variation in Compliance
Compliance increases in physiological and
pathological conditions.
1. In old age, lung compliance increases due
to loss of elastic property of lung tissues
2. In emphysema, lung compliance increases
because of damage of alveolar membrane
(Fig. 73-2).
Compliance decreases in pathological con-
ditions such as:
1. Deformities of thorax like kyphosis and sco-
liosis
2. Paralysis of respiratory muscles
3. Pleural effusion
4. Fibrosis
5. Abnormal thorax.
WORK OF BREATHING
The work done by the respiratory muscles during
breathing to overcome the resistance in the
FIGURE 73-2: Variations in lung compliance
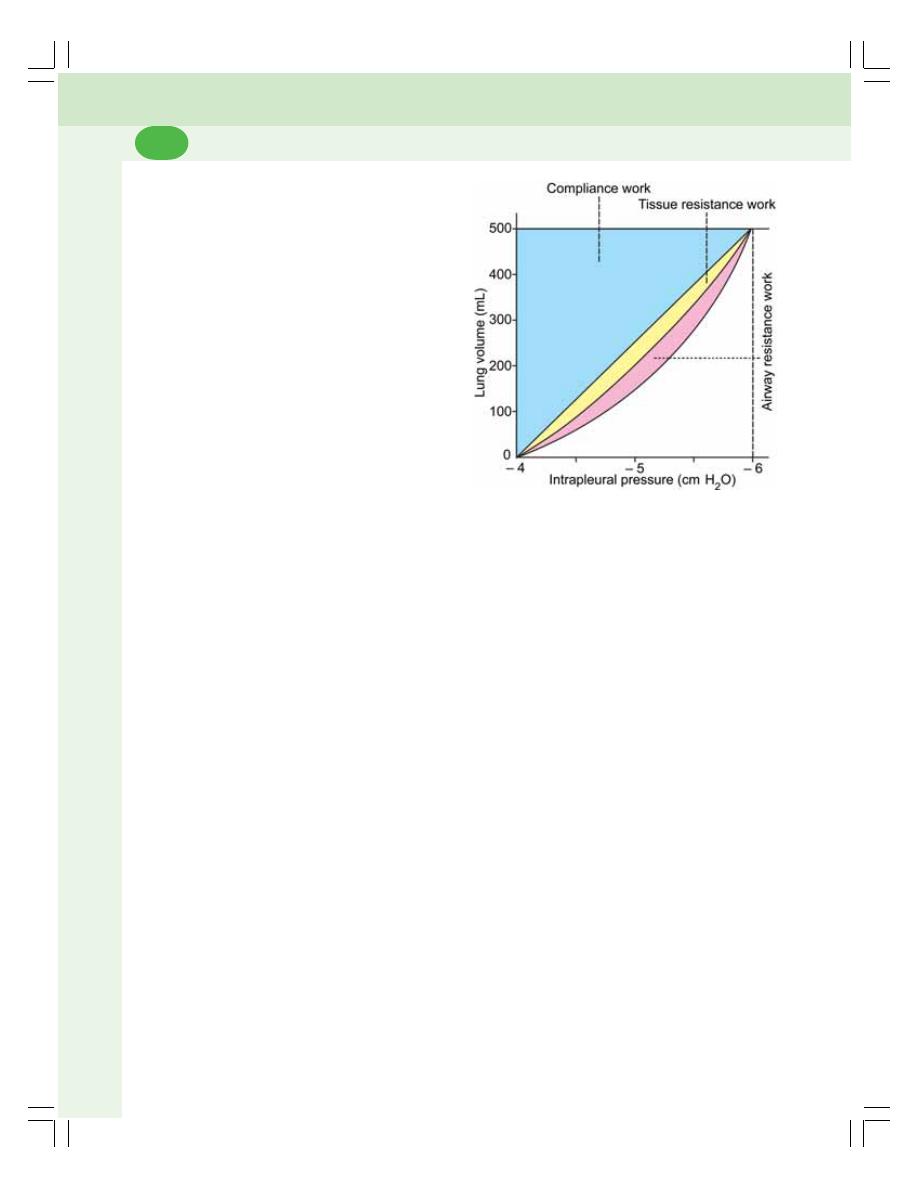
Respiratory System and Environmental Physiology
456
FIGURE 73-3: Work of breathing
thorax and respiratory tract is known as work of
breathing.
WORK DONE BY RESPIRATORY
MUSCLES
During the respiratory processes, inspiration is
active process and the expiration is a passive
process. So, during quiet breathing, the respi-
ratory muscles perform the work only during
inspiration and not during expiration.
The energy obtained during the work of
breathing is utilized to overcome three types of
resistance:
1. Airway resistance
2. Elastic resistance of lungs and thorax
3. Nonelastic viscous resistance.
1. Airway Resistance
Airway resistance is the resistance offered to the
passage of air through respiratory tract. The work
done to overcome this is called airway resistance
work.
2. Elastic Resistance of Lungs and Thorax
The work done to overcome this elastic resis-
tance is called compliance work.
3. Nonelastic Viscous Resistance
The work done to overcome this viscous resis-
tance is called the tissue resistance work. The
above factors are explained by the curve that
shows the relation between lung volume and
pleural pressure (Fig. 73-3).
INTRODUCTION
LUNG VOLUMES
TIDAL VOLUME
INSPIRATORY RESERVE VOLUME
EXPIRATORY RESERVE VOLUME
RESIDUAL VOLUME
LUNG CAPACITIES
INSPIRATORY CAPACITY
VITAL CAPACITY
FUNCTIONAL RESIDUAL CAPACITY
TOTAL LUNG CAPACITY
VITAL CAPACITY
FORCED EXPIRATORY VOLUME OR TIMED VITAL CAPACITY
RESPIRATORY MINUTE VOLUME
MAXIMUM BREATHING CAPACITY OR MAXIMUM VENTILATION VOLUME
PEAK EXPIRATORY FLOW RATE
RESTRICTIVE AND OBSTRUCTIVE RESPIRATORY DISEASES
INTRODUCTION
Pulmonary function tests or lung function tests
are useful in assessing the functional status of
the respiratory system. These tests involve
measurement of lung volumes and capacities.
The air in lung is classified into two divisions:
I. Lung volumes
II. Lung capacities.
Pulmonary function tests are carried out
mostly by using spirometer (Fig. 74-1). The
graphical recording of lung volumes and
capacities is called spirogram (Fig. 74-2).
LUNG VOLUMES
Lung volumes are the static volumes of air
breathed by an individual. The lung volumes are
of four types.
1. TIDAL VOLUME (TV)
Tidal volume is the volume of air breathed in and
out of lungs in a single normal quiet respiration.
Tidal volume signifies the normal depth of
breathing.
Normal value = 500 mL (0.5 L)
Pulmonary Function Tests
74
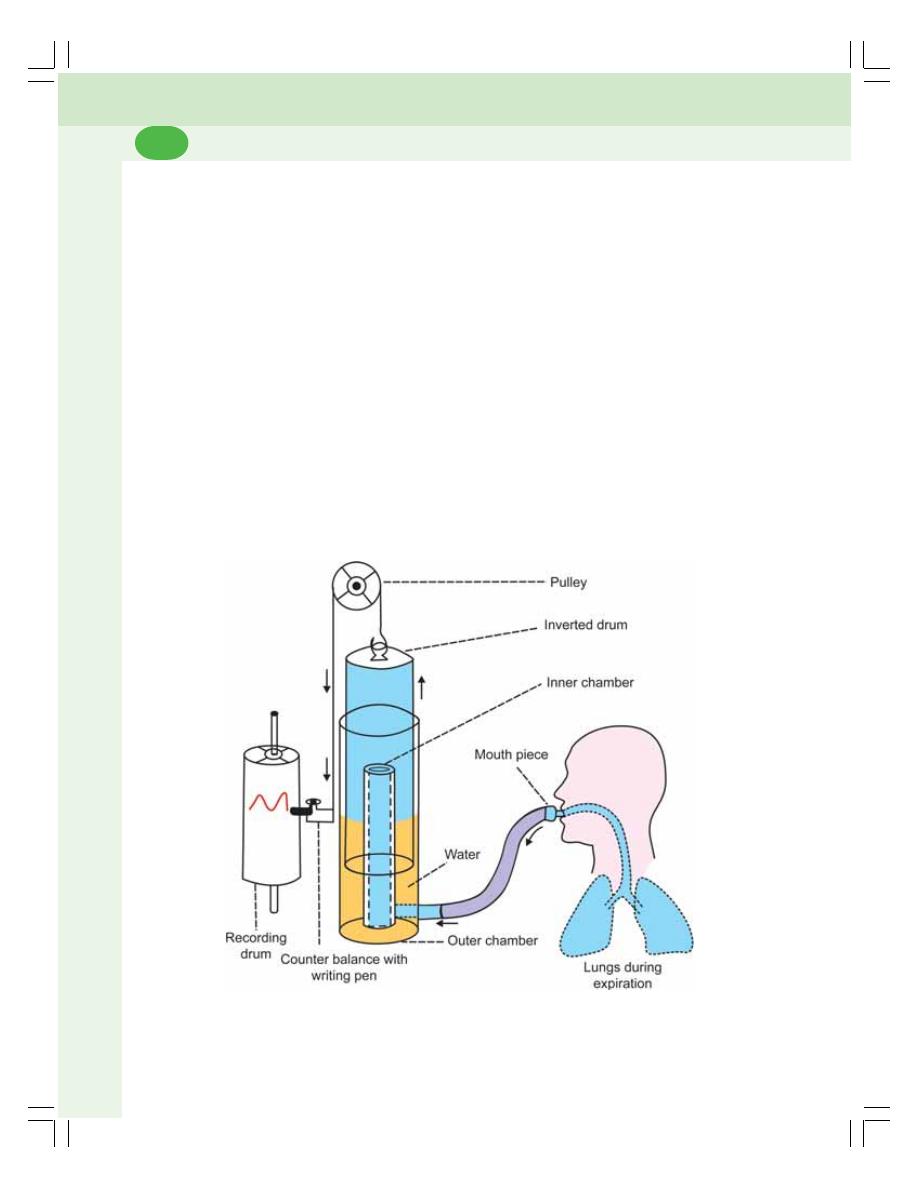
Respiratory System and Environmental Physiology
458
2. INSPIRATORY RESERVE VOLUME
(IRV)
Inspiratory reserve volume is an additional
volume of air that can be inspired forcefully after
the end of normal inspiration.
Normal value = 3300 mL (3.3 L).
3. EXPIRATORY RESERVE VOLUME
(ERV)
Expiratory reserve volume is the additional
volume of air that can be expired out forcefully,
after normal expiration.
Normal value = 1000 mL (1 L).
4. RESIDUAL VOLUME (RV)
Residual volume is the volume of air remaining
in the lungs even after forced expiration.
Normally, lungs cannot be emptied completely
even by forceful expiration. Some quantity of air
always remains in the lungs even after the forced
expiration. Residual volume helps to aerate the
blood in between breathing and during expi-
ration.
Normal value = 1200 mL (1.2 L)
LUNG CAPACITIES
Lung capacities are the combination of two or
more lung volumes. Lung capacities are of four
types.
1. INSPIRATORY CAPACITY (IC)
Inspiratory capacity is the maximum volume of
air that is inspired after normal expiration (end
expiratory position). It includes tidal volume and
inspiratory reserve volume (Figs. 74-2).
IC = TV + IRV
= 500 + 3300 = 3800 mL
FIGURE 74-1: Spirometer. During expiration, the air enters the spirometer from lungs. The inverted
drum moves up and the pen draws a downward curve on the recording drum
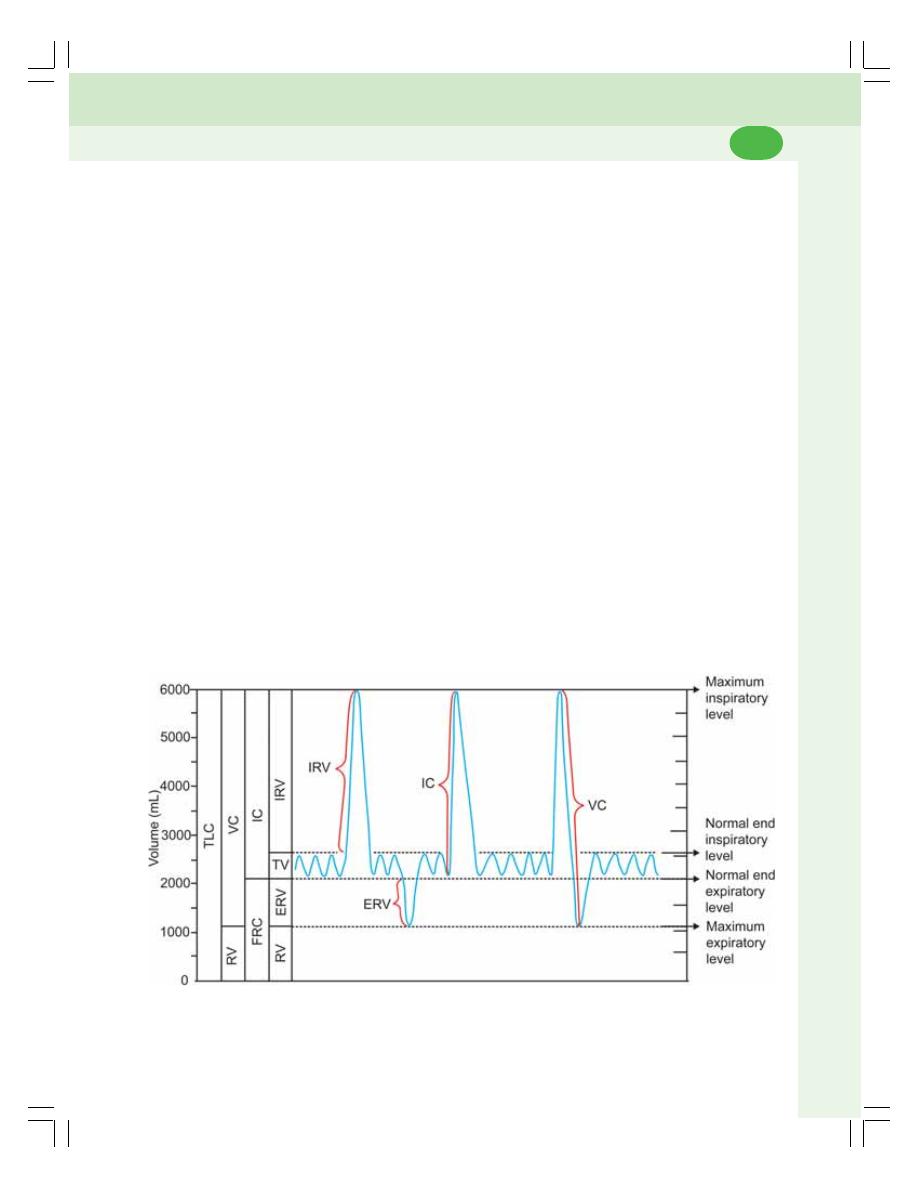
Chapter 74 Pulmonary Function Tests
459
2. VITAL CAPACITY (VC)
It is the maximum volume of air that can be
expelled out forcefully after a deep (maximal)
inspiration. Vital capacity includes inspiratory
reserve volume, tidal volume and expiratory
reserve volume.
VC = IRV + TV + ERV
= 3300 + 500 + 1000 = 4800 mL
3. FUNCTIONAL RESIDUAL CAPACITY
(FRC)
It is the volume of air remaining in the lungs after
normal expiration (after normal tidal expiration).
Functional residual capacity includes expiratory
reserve volume and residual volume.
FRC = ERV + RV
= 1000 + 1200 = 2200 mL
4. TOTAL LUNG CAPACITY (TLC)
Total lung capacity is the volume of air present
in the lungs after a deep (maximal) inspiration.
It includes all the volumes.
TLC = IRV + TV + ERV + RV
= 3300 + 500 + 1000 + 1200 = 6000 mL
VITAL CAPACITY
DEFINITION AND NORMAL VALUE
Definition and normal value of vital capacity are
already given.
VARIATIONS OF VITAL CAPACITY
Physiological Variations
i. Sex: In females, vital capacity is less than
in males
ii. Body built: Vital capacity is slightly more
in heavily built persons
iii. Posture: Vital capacity is more in standing
position and less in lying position
iv. Athletes: Vital capacity is more in athletes
v. Occupation: Vital capacity is decreased
in people with sedentary jobs. It is
increased in persons who play musical
wind instruments such as bugle and flute.
Pathological Variations
Vital capacity is reduced in the following
respiratory diseases:
i. Asthma
ii. Emphysema
FIGURE 74-2: Spirogram. TV = Tidal volume. IRV = Inspiratory reserve volume. ERV = Expiratory reserve
volume. RV = Residual volume. IC = Inspiratory capacity. FRC = Functional residual capacity. VC = Vital
capacity. TLC = Total lung capacity

Respiratory System and Environmental Physiology
460
iii. Weakness or paralysis of respiratory
muscle
iv. Pulmonary congestion
v. Pneumonia
vi. Pulmonary edema
vii. Pulmonary tuberculosis.
FORCED EXPIRATORY VOLUME
(FEV) OR TIMED VITAL CAPACITY
DEFINITION
Forced expiratory volume (FEV) is the volume
of air, which can be expired forcefully in a given
unit of time (after a deep inspiration). It is also
called timed vital capacity.
FEV
1
: Volume of air expired forcefully in
1 second
FEV
2
: Volume of air expired forcefully in
2 seconds
FEV
3
: Volume of air expired forcefully in
3 seconds.
NORMAL VALUES
FEV in persons with normal respiratory functions
is as follows:
FEV
1
= 83% of total vital capacity
FEV
2
= 94% of total vital capacity
FEV
3
= 97% of total vital capacity
After 3rd second = 100% of total vital capacity.
SIGNIFICANCE OF DETERMINING
FEV
The vital capacity may be almost normal in some
of the respiratory diseases. However, the FEV
has great diagnostic value, as it is decreased
significantly in some respiratory diseases. For
example, it is very much decreased in the
obstructive diseases like asthma and
emphysema. It is slightly reduced in some of the
restrictive respiratory diseases like fibrosis
(Fig. 74-3).
RESPIRATORY MINUTE VOLUME
(RMV)
Respiratory minute volume is the volume of air
breathed in and out of lungs every minute. It is
the product of tidal volume (TV) and respiratory
rate (RR).
RMV = TV × RR
= 500 × 12 = 6000 mL
Normal respiratory minute volume is 6 L. It
increases in physiological conditions such as
voluntary hyperventilation, exercise and
emotional conditions. It is reduced in respiratory
diseases.
MAXIMUM BREATHING CAPACITY
(MBC) OR MAXIMUM VENTILATION
VOLUME (MVV)
Maximum breathing capacity (MBC) is the
maximum volume of air which can be breathed
in and out of lungs by forceful respiration
(hyperventilation = increase in rate and force of
respiration) per minute. It is also called maxi-
mum ventilation volume (MVV).
Normal value in adult male, it is 150 to 170
L/minute and, in females, it is 80 to 100 L/min.
MBC is reduced in respiratory diseases.
PEAK EXPIRATORY FLOW RATE
(PEFR)
Peak expiratory flow rate (PEFR) is the
maximum rate at which the air can be expired
after a deep inspiration. It is measured by
Wright’s peak flow meter or a mini peak flow
meter.
Normal value is 400 L/minute.
SIGNIFICANCE OF DETERMINING
PEFR
Determination of peak expiratory flow rate is
useful for assessing the respiratory diseases
especially to differentiate the obstructive and
restrictive diseases. Generally, PEFR is reduced
in all type of respiratory disease. However, the
reduction is more significant in the obstructive
diseases than in the restrictive diseases.
Thus, in restrictive diseases, the PEFR is 200
L/minute and in obstructive diseases, it is only
100 L/minute.
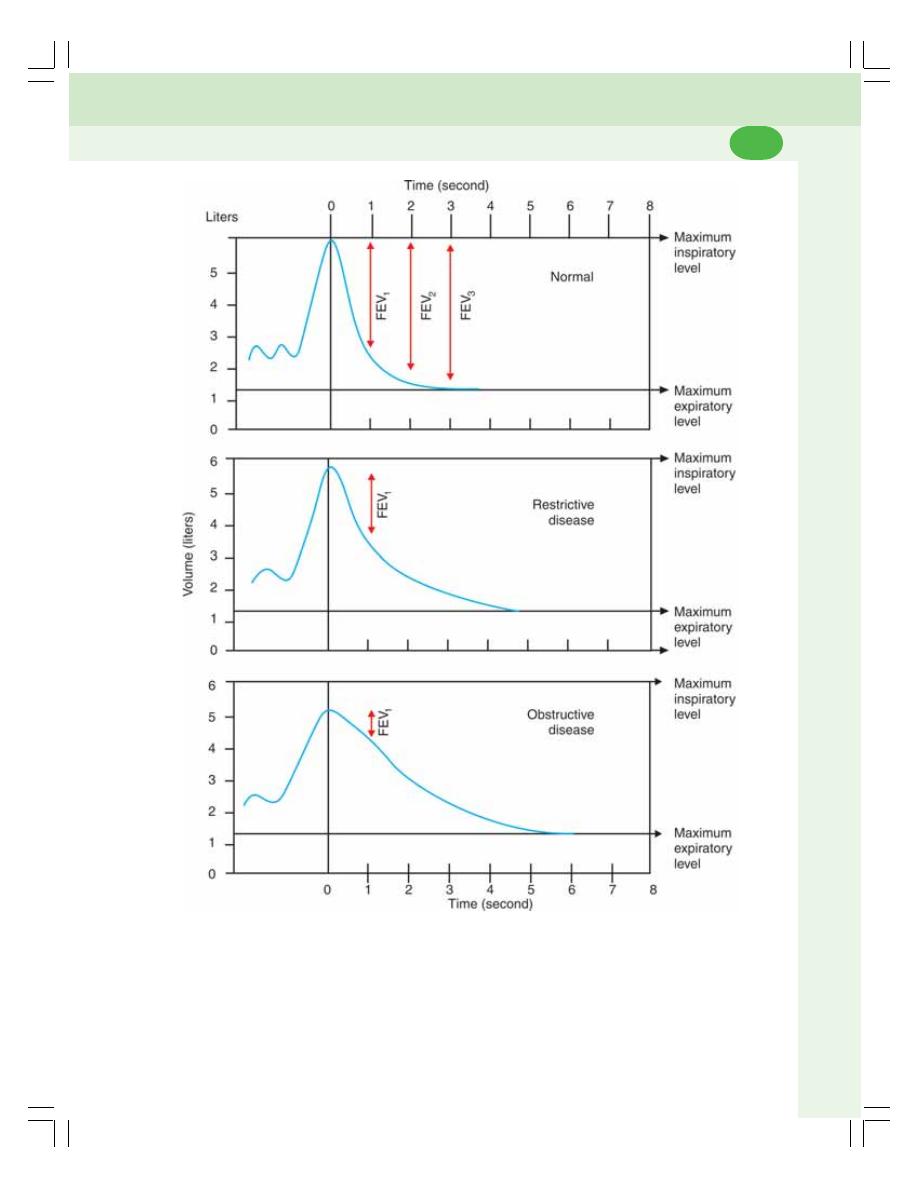
Chapter 74 Pulmonary Function Tests
461
FIGURE 74-3: Forced expiratory volume
RESTRICTIVE AND OBSTRUCTIVE
RESPIRATORY DISEASES
The diseases of respiratory tract are classified
into two types:
1. Restrictive respiratory disease
2. Obstructive respiratory disease.
These two types of respiratory diseases are
determined by lung functions tests, particularly
FEV.

Respiratory System and Environmental Physiology
462
TABLE 74-1: Restrictive and obstructive respiratory diseases
Type
Disease
Structures involved
Restrictive respiratory diseases
Poliomyelitis
CNS
Myasthenia gravis
CNS and thoracic cavity
Flail chest (broken ribs)
Thoracic cavity
Paralysis of diaphragm
CNS
Spinal cord diseases
CNS
Pleural effusion
Thoracic cavity
Obstructive respiratory diseases
Asthma
Lower respiratory tract
Chronic bronchitis
Emphysema
Cystic fibrosis
Laryngotracheobronchitis
Upper respiratory tract
Epiglottis
Tumors
Severe cough and cold with phlegm
RESTRICTIVE RESPIRATORY DISEASE
Restrictive respiratory disease is the abnormal
respiratory condition characterized by difficulty
in inspiration. The expiration is not affected.
Restrictive respiratory disease may be because
of abnormality of lungs, thoracic cavity or/and
nervous system.
OBSTRUCTIVE RESPIRATORY
DISEASE
Obstructive respiratory disease is the abnormal
respiratory condition characterized by difficulty
in expiration. The obstructive and respiratory
diseases are listed in Table 74-1.
PULMONARY VENTILATION
DEFINITION
NORMAL VALUE AND CALCULATION
ALVEOLAR VENTILATION
DEFINITION
NORMAL VALUE AND CALCULATION
DEAD SPACE
DEFINITION
TYPES
NORMAL VALUE AND MEASUREMENT
VENTILATION–PERFUSION RATIO
DEFINITION
NORMAL VALUE AND CALCULATION
SIGNIFICANCE
VARIATIONS
INSPIRED AIR
ALVEOLAR AIR
EXPIRED AIR
PULMONARY VENTILATION
DEFINITION
Pulmonary ventilation is the volume of air moving
in and out of lungs per minute in quiet breathing.
It is also called respiratory minute volume (RMV).
NORMAL VALUE AND CALCULATION
Normal value of pulmonary ventilation is
6 L/minute. It is the product of tidal volume (TV)
and the rate of respiration (RR). It is calculated
by the formula:
Pulmonary ventilation
= Tidal volume × Respiratory rate
= 500 mL × 12/minute
= 6,000 mL = 6 L/minute
ALVEOLAR VENTILATION
DEFINITION
Alveolar ventilation is the amount of air utilized
for gaseous exchange every minute. Alveolar
ventilation is different from pulmonary ventilation.
In pulmonary ventilation, 6 L of air moves in and
Ventilation
75
Respiratory System and Environmental Physiology
464
out of lungs in every minute. But the whole
volume of air is not utilized for exchange of
gases. The volume of air subjected for exchange
of gases is the alveolar ventilation. The air
trapped in the respiratory passage (dead space)
does not take part in gaseous exchange.
NORMAL VALUE AND CALCULATION
Normal value of alveolar ventilation is 4,200 mL
(4.2 L)/ minute.
It is calculated by the formula given below.
Alveolar
Tidal
Dead
Respiratory
ventilation
volume
space
rate
= (500 – 150) × 12
= 4,200 mL = 4.2 L/minute
DEAD SPACE
DEFINITION
Dead space is defined as the part of the
respiratory tract, where gaseous exchange
does not take place. The air present in the dead
space is called dead space air.
TYPES OF DEAD SPACE
Dead space is of two types:
I. Anatomical dead space
II. Physiological dead space.
Anatomical Dead Space
It includes nose, pharynx, trachea, bronchi and
branches of bronchi up to terminal bronchioles.
Physiological Dead Space
Physiological dead space includes the
anatomical dead space plus two additional
volumes.
1. The air in the alveoli, which are nonfunction-
ing. In some of the respiratory diseases,
alveoli do not function because of dysfunction
or destruction of alveolar membrane
2. The air in the alveoli, which do not receive
adequate blood flow. Gaseous exchange
does not take place during inadequate blood
supply.
NORMAL VALUE AND
MEASUREMENT OF DEAD SPACE
Under normal conditions, the physiological dead
space is equal to anatomical dead space. It is
because, all the alveoli are functioning and all
alveoli receive adequate blood flow in normal
conditions. The volume of normal dead space
is 150 mL.
In respiratory disorders, which affect the
pulmonary blood flow or the alveoli, the dead
space increases. It is associated with reduction
in alveolar ventilation.
The dead space is measured by single breath
nitrogen washout method.
VENTILATION–PERFUSION RATIO
DEFINITION
The ventilation–perfusion ratio is the ratio of
alveolar ventilation and the amount of blood that
perfuse the alveoli.
It is expressed as V
A
/Q
Where,
V
A
is alveolar ventilation
Q is the blood flow (perfusion)
NORMAL VALUE AND CALCULATION
Normal Value
Normal value of ventilation–perfusion ratio is
about 0.84.
Calculation
Alveolar ventilation is calculated by the formula:
Alveolar
Tidal
Dead
Respiratory
ventilation
volume
space
rate
= (500–150) × 12
= 4,200 mL/minute
Blood flow through alveoli
(Pulmonary blood flow)
= 5,000 mL/minute
Therefore,
Ventilation–perfusion ratio
=
4,200
5,000
= 0.84
Chapter 75 Ventilation
465
SIGNIFICANCE OF VENTILATION–
PERFUSION RATIO
The ventilation–perfusion ratio signifies the
gaseous exchange. It is affected if there is any
change in alveolar ventilation or in blood flow.
VARIATIONS IN VENTILATION–
PERFUSION RATIO
Physiological Variation
1. Ratio increases, if ventilation increases
without any change in blood flow
2. Ratio decreases if blood flow increases
without any change in ventilation
Pathological Variation
In chronic obstructive pulmonary diseases
(COPD), the ventilation is affected because of
destruction of alveolar membrane. So, the
ventilation–perfusion ratio reduces greatly.
INSPIRED AIR
Inspired air is the atmospheric air, which is
inhaled during inspiration. The composition of
inspired air is given in Table 75-1.
ALVEOLAR AIR
Alveolar air is the air present in the alveoli of
lungs and it is collected by Haldane-Priestly tube.
Alveolar air is different from the inspired air.
Differences between alveolar air and inspired
air are:
1. The alveolar air is partially replaced by the
atmospheric air during each breath
2. Oxygen diffuses from the alveolar air into
pulmonary capillaries constantly
3. Carbon dioxide diffuses from pulmonary blood
into alveolar air constantly
4. The dry atmospheric air is humidified, while
passing through respiratory passage just
before entering the alveoli (Table 75-1).
RENEWAL
The alveolar air is constantly renewed. The rate
of renewal is slow during normal breathing.
During each breath, out of 500 mL of tidal volume
only 350 mL of air enters the alveoli and the
remaining quantity of 150 mL (30%) becomes
dead space air. Hence, the amount of alveolar
air replaced by new atmospheric air with each
breath is only about 70% of the total alveolar air.
Thus,
Alveolar air =
350
500
×100 = 70%
EXPIRED AIR
Expired air is the amount of air that is exhaled
during expiration. It is a combination of dead
space air and alveolar air. Expired air is collected
by using Douglas bag.
The concentration of gases in expired air is
somewhere between inspired air and alveolar
air. The composition of expired air is given in
Table 75-1.
TABLE 75-1: Composition of alveolar air, inspired air and expired air
Air
Inspired
Alveolar air
Expired air
(atmospheric) air
Gas
Content
Partial
Content
Partial
Content
Partial
(mL %)
pressure
(mL %)
pressure
(mL %)
pressure
(mm Hg)
(mm Hg)
(mm Hg)
Oxygen
20.84
159.00
13.60
104.00
15.70
120.00
Carbon dioxide
0.04
0.30
5.30
40.00
3.60
27.00
Nitrogen
78.62
596.90
74.90
569.00
74.50
566.00
Water vapor etc.
0.50
3.80
6.20
47.00
6.20
47.00
Total
100.00
760.00
100.00
760.00
100.00
760.00
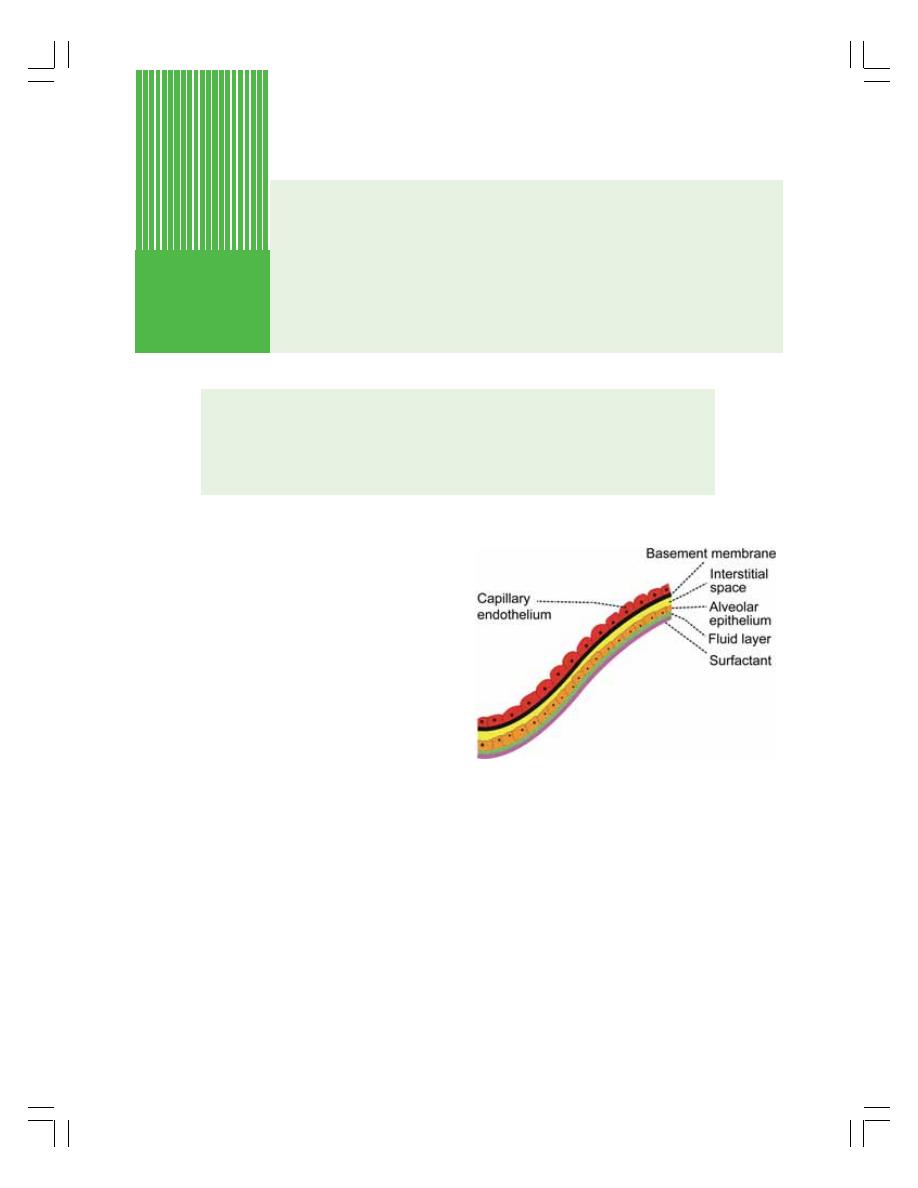
EXCHANGE OF RESPIRATORY GASES IN LUNGS
EXCHANGE OF RESPIRATORY GASES AT TISSUE LEVEL
TRANSPORT OF OXYGEN
TRANSPORT OF CARBON DIOXIDE
EXCHANGE OF RESPIRATORY
GASES IN LUNGS
In the lungs, exchange of respiratory gases takes
place between the alveoli and the blood. The
exchange of gases occurs through bulk flow
diffusion (Chapter 3).
Respiratory unit is the structure through
which the exchange of gases between blood and
alveoli takes place. Refer Chapter 72 for details.
RESPIRATORY MEMBRANE
Exchange of respiratory gases takes place
through respiratory membrane. It is formed by
the epithelium of the respiratory unit and
endothelium of pulmonary capillary. The
epithelium of the respiratory unit is a very thin
layer (Chapter 72). Since the capillaries are in
close contact with this membrane, the alveolar
air is in close proximity to capillary blood. This
facilitates the gaseous exchange between air
and blood (Fig. 76-1).
The respiratory membrane is formed by
different layers of structures belonging to the
alveoli and capillaries. The different layers of
respiratory membrane are as follows from within
outside:
From Alveolar Portion
1. Monomolecular layer of surfactant, which
spreads over the surface of the fluid lining
of alveoli
2. A thin layer of fluid that lines the alveoli
3. The alveolar epithelial layer, which is
composed of thin epithelial cells resting on
a basement membrane
In between Alveolar Portion and Capillary
Portion
4. An interstitial space
Exchange and Transport of
Respiratory Gases
76
FIGURE 76-1: Structure of respiratory membrane
Chapter 76 Exchange and Transport of Respiratory Gases
467
From Capillary Portion
5. Basement membrane of capillary
6. Capillary endothelial cells.
The average diameter of pulmonary capillary
is only 8
μ, which means that the red blood cells
with a diameter of 7.4
μ actually squeeze through
the capillaries. Therefore, the membrane of red
blood cells is in close contact with capillary wall.
This facilitates the quick exchange of oxygen
and carbon dioxide between the blood and
alveoli.
DIFFUSING CAPACITY
The diffusing capacity is defined as the volume
of gas that diffuses through the respiratory
membrane each minute for a pressure gradient
of 1 mm Hg.
Diffusing Capacity for Oxygen and Carbon
Dioxide
Diffusing capacity for oxygen is 21 mL/minute/1
mm Hg. Diffusing capacity for carbon dioxide is
400 mL/minute/1 mm Hg. Thus, the diffusing
capacity for carbon dioxide is about 20 times
more than that of oxygen.
Factors Affecting Diffusing Capacity
1. Pressure gradient
Diffusing capacity is directly proportional to the
pressure gradient. Pressure gradient is the
difference between the partial pressure of a
gas in the alveoli and pulmonary capillary blood
(see below). It is the major factor which affects
the diffusing capacity.
2. Solubility of gas in fluid medium
Diffusing capacity is directly proportional to
solubility of the gas. If the solubility of a gas is
more in the fluid medium, a large number of
molecules dissolve in it and diffuse easily.
3. Total surface area of respiratory membrane
Diffusing capacity is directly proportional to
surface area of respiratory membrane. The
surface area of respiratory membrane in each
lung is about 70 sq. m. If the total surface area
of respiratory membrane decreases, the
diffusing capacity for the gases is decreased.
4. Molecular weight of the gas
Diffusing capacity is inversely proportional to
molecular weight of the gas. If the molecular
weight is more, the density is more and the rate
of diffusion is less.
5. Thickness of respiratory membrane
Diffusion is inversely proportional to the thick-
ness of respiratory membrane. More the
thickness of respiratory membrane less is the
diffusion. It is because the distance through
which the diffusion takes place is long.
DIFFUSION OF OXYGEN
Entrance of Oxygen from Atmospheric Air
into the Alveoli
The partial pressure of oxygen in the
atmospheric air is 159 mm Hg and in the alveoli,
it is 104 mm Hg. Because of the pressure
gradient of 55 mm Hg, oxygen easily enters from
atmospheric air into the alveoli (Table 76.1).
Diffusion of Oxygen from Alveoli into the
Blood
When the blood is flowing through the pulmonary
capillary, RBC is exposed to oxygen only for
0.75 sec at rest and only for 0.25 sec during
severe exercise. So the diffusion of oxygen must
be quicker and effective. Fortunately, this is
possible because of pressure gradient.
The partial pressure of oxygen in the
pulmonary capillary is 40 mm Hg and in the
alveoli, it is 104 mm Hg. The pressure gradient
is 64 mm Hg. It facilitates the diffusion of oxygen
from alveoli into the blood (Fig. 76-2).
DIFFUSION OF CARBON DIOXIDE
Diffusion of Carbon Dioxide from Blood
into Alveoli
The partial pressure of carbon dioxide in alveoli
is 40 mm Hg whereas in the blood it is 46 mm Hg.
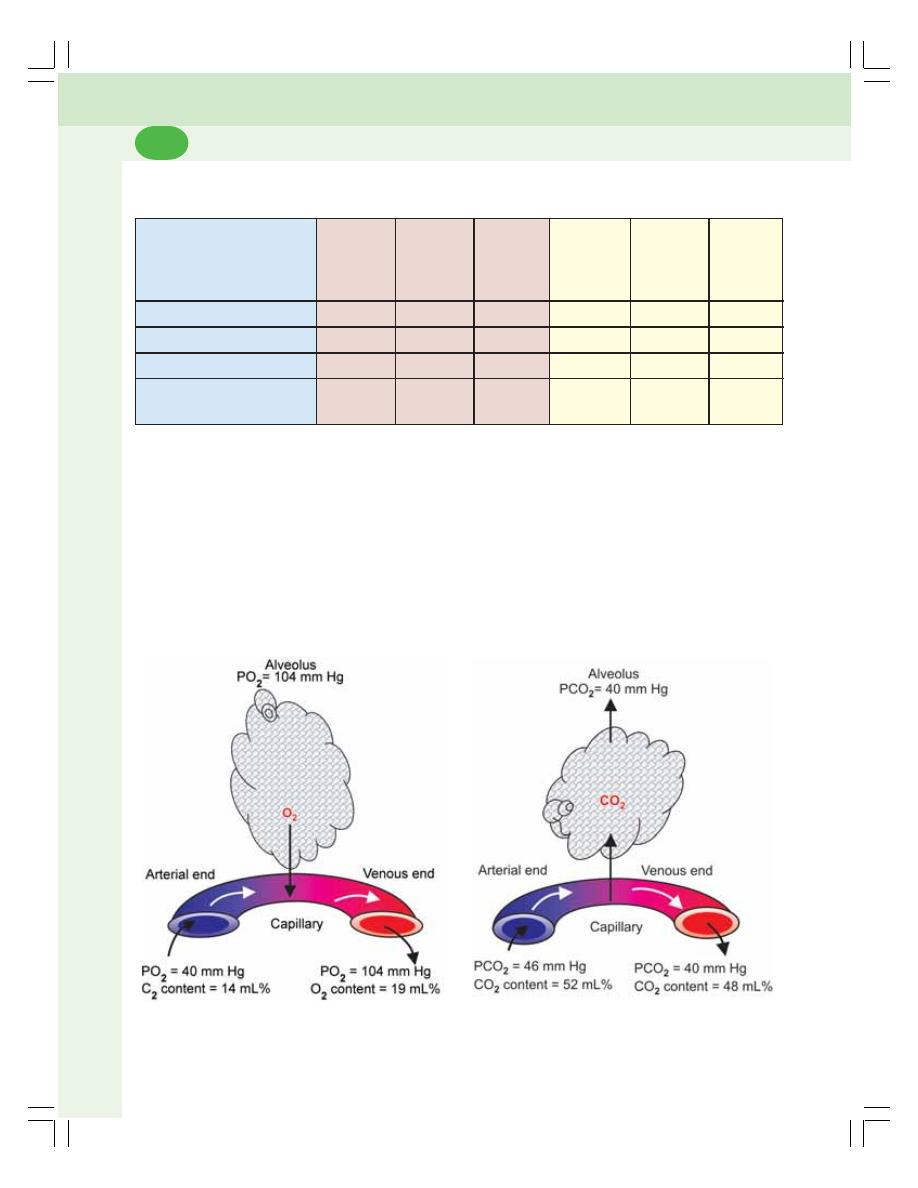
Respiratory System and Environmental Physiology
468
FIGURE 76-2: Diffusion of oxygen from alveolus
to pulmonary capillary
FIGURE 76-3: Diffusion of carbon dioxide from
pulmonary capillary to alveolus
The pressure gradient of 6 mm Hg is responsible
for the diffusion of carbon dioxide from blood into
the alveoli (Fig. 76-3).
Diffusion of Carbon Dioxide from the
Alveoli into the Atmospheric Air
In the atmospheric air, the partial pressure of
carbon dioxide is very insignificant and is only
about 0.3 mm Hg whereas, in the alveoli, it is
40 mm Hg. So, carbon dioxide enters passes to
atmosphere from alveoli easily.
EXCHANGE OF RESPIRATORY
GASES AT TISSUE LEVEL
DIFFUSION OF OXYGEN FROM
BLOOD INTO THE TISSUES
The partial pressure of oxygen in the venous end
of pulmonary capillary is 104 mm Hg. However,
TABLE 76-1: Partial pressure and content of oxygen and carbon dioxide in alveoli,
capillaries and tissue
Gas
Arterial
Alveoli
Venous
Arterial
Tissue
Venous
end of
end of
end of
end of
pulmonary
pulmonary
systemic
systemic
capillary
capillary
capillary
capillary
PO
2
(mm Hg)
40
104
104
95
40
40
Oxygen content (mL %)
14
–
19
19
–
14
PCO
2
(mm Hg)
46
40
40
40
46
46
Carbon dioxide content
52
–
48
48
–
52
(mL %)
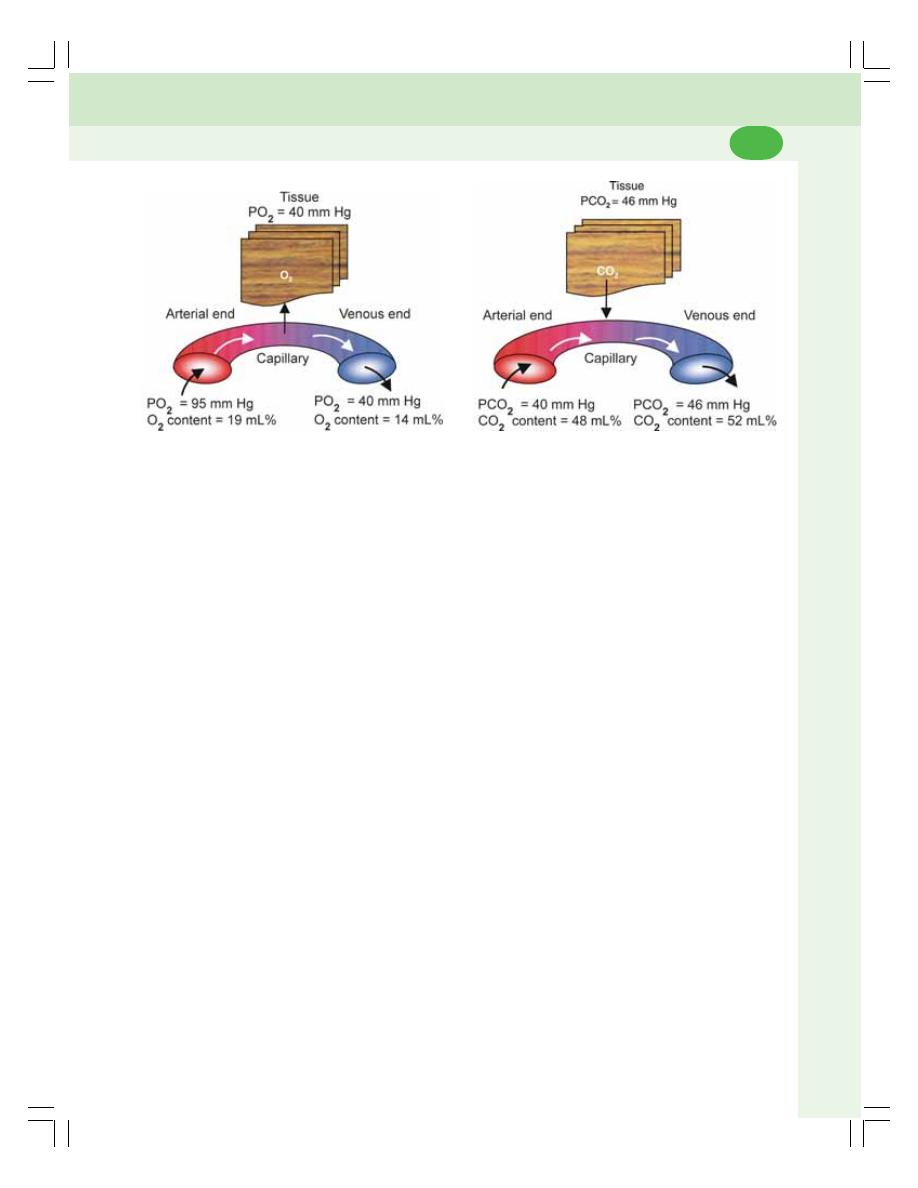
Chapter 76 Exchange and Transport of Respiratory Gases
469
the partial pressure of oxygen in the arterial end
of systemic capillary is only 95 mm Hg. It may
be because of the physiological shunt in lungs
(Chapter 72). About 2% of blood reaches the
heart without being oxygenated.
The average oxygen tension in the tissues
is 40 mm Hg. It is because of continuous
metabolic activity and constant utilization of
oxygen. Thus, a pressure gradient of about
55 mm Hg exists between capillary blood and
the tissues so that oxygen can easily diffuse into
the tissues (Fig. 76-4).
The oxygen content in arterial blood is
19 mL% and, in the venous blood, it is 14 mL%.
Thus, the diffusion of oxygen from blood to the
tissues is 5 mL/100 mL of blood.
DIFFUSION OF CARBON DIOXIDE
FROM TISSUES INTO THE BLOOD
Due to the continuous metabolic activity, carbon
dioxide is produced constantly in the cells of the
tissues. So, the partial pressure of carbon
dioxide is high in the cells and is about 46 mm
Hg. The partial pressure of carbon dioxide in
arterial blood is 40 mm Hg. The pressure
gradient of 6 mm Hg is responsible for the
diffusion of carbon dioxide from tissues to the
blood (Fig 76-5).
The carbon dioxide content in arterial blood
is 48 mL%. And, in the venous blood, it is
52 mL%. So, the diffusion of carbon dioxide
FIGURE 76-4: Diffusion of oxygen from capillary
to tissue
FIGURE 76-5: Diffusion of carbon dioxide from
tissue to capillary
from tissues to the blood is 4 mL/100 mL of
blood.
TRANSPORT OF OXYGEN
Oxygen is transported from alveoli to the tissue
by the blood in two forms:
1. As simple physical solution
2. In combination with hemoglobin.
TRANSPORT OF OXYGEN AS SIMPLE
SOLUTION
Oxygen dissolves in water of plasma and is trans-
ported in this physical form. The amount of
oxygen transported in this way is very negligible.
It is only 0.3 mL/100 mL of plasma. It is about
3% of total oxygen in blood.
IN COMBINATION WITH
HEMOGLOBIN
Oxygen combines with hemoglobin in blood and
is transported as oxyhemoglobin. The transport
of oxygen in this form is important because,
maximum amount (97%) of oxygen is transported
by this method.
Oxygen combines with hemoglobin only as
a physical combination. It is only oxygenation
and not oxidation. This type of combination of
oxygen with hemoglobin has got some
advantages. Oxygen can be readily released
from hemoglobin when it is needed.
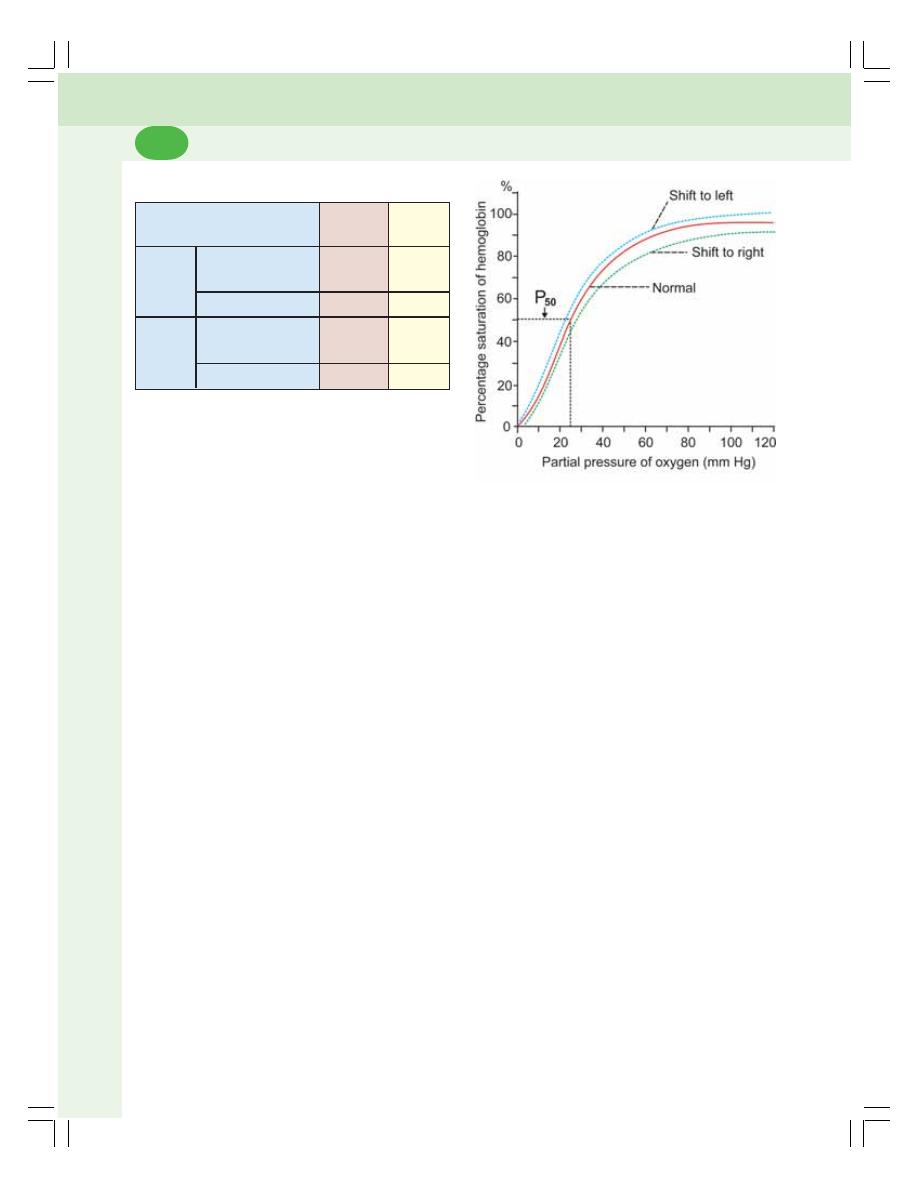
Respiratory System and Environmental Physiology
470
Normal Oxygen Hemoglobin Dissociation
Curve
Under normal conditions, the oxygen hemoglobin
dissociation curve is ‘S’ shaped or sigmoid
shaped (Fig. 76-6). The lower part of the curve
indicates dissociation of oxygen from
hemoglobin. The upper part of the curve indicates
the acceptance of oxygen by hemoglobin
depending upon the partial pressure of oxygen.
P
50
P
50
is the partial pressure of oxygen at which
hemoglobin saturation with oxygen is 50%.
When the partial pressure of oxygen is 25 to
27 mm Hg, the hemoglobin is saturated to about
50%. That is, the blood contains 50% of oxygen.
At 40 mm Hg of partial pressure of oxygen, the
saturation is 75%. It becomes 95% when the
partial pressure of oxygen is 100 mm Hg.
Factors Affecting Oxygen Hemoglobin
Dissociation Curve
The oxygen hemoglobin dissociation curve is
shifted to left or right by various factors:
I. Shift to left indicates acceptance
(association) of oxygen by hemoglobin
II. Shift to right indicates dissociation of
oxygen from hemoglobin.
TABLE 76-2: Gases in arterial and venous blood
Gas
Arterial
Venous
blood
blood
Oxygen
Partial pressure
95
40
(mm Hg)
Content (mL %)
19
14
Carbon
Partial pressure
40
46
dioxide
(mm Hg)
Content (mL %)
48
52
FIGURE 76-6: Oxygen hemoglobin dissociation
curve
Oxygen combines with the iron in heme part
of hemoglobin.
Oxygen Carrying Capacity of Blood
The oxygen carrying capacity of blood is amount
of oxygen transported by blood. One gram of
hemoglobin carries 1.34 mL of oxygen. It is called
oxygen carrying capacity of hemoglobin. The
normal hemoglobin content in blood is 15 g%.
So, the blood with 15 g% of hemoglobin should
carry 20.1 mL% of oxygen, i.e. 20.1 mL of oxygen
in 100 mL of blood. But, the blood with 15 g% of
hemoglobin carries only 19 mL% of oxygen, i.e.
19 mL of oxygen is carried by 100 mL of blood
(Table 76-2). The oxygen carrying capacity of
blood is only 19 mL% because the hemoglobin
is not fully saturated with oxygen. It is saturated
only for about 95%.
OXYGEN HEMOGLOBIN
DISSOCIATION CURVE
Oxygen hemoglobin dissociation curve is the
curve that demonstrates the relationship
between partial pressure of oxygen and the
percentage saturation of hemoglobin with
oxygen. It explains the affinity of hemoglobin for
oxygen.
Normally in the blood, hemoglobin is
saturated with oxygen only up to 95%. The
saturation of hemoglobin with oxygen depends
upon the partial pressure of oxygen. When the
partial pressure of oxygen is more, hemoglobin
accepts oxygen and when the partial pressure
of oxygen is less, hemoglobin releases oxygen.
Chapter 76 Exchange and Transport of Respiratory Gases
471
I. Shift to right
The oxygen hemoglobin dissociation curve is
shifted to right in the following conditions:
1. Decrease in partial pressure of oxygen
2. Increase in partial pressure of carbon dioxide
(Bohr’s effect)
3. Increase in hydrogen ion concentration and
decrease in pH (acidity)
4. Increased body temperature
5. Excess of 2,3-diphosphoglycerate (DPG)
which a byproduct carbohydrate metabolism
present in red blood corpuscles.
II. Shift to left
Shift of the oxygen hemoglobin dissociation curve
to left occurs in the following conditions:
1. In fetal blood because, fetal hemoglobin has
got more affinity for oxygen than the adult
hemoglobin.
2. Decrease in hydrogen ion concentration and
increase in pH (alkalinity).
Bohr’s Effect
Bohr’s effect is the effect by which the presence
of carbon dioxide decreases the affinity of
hemoglobin for oxygen. In the tissues, due to
continuous metabolic activities, the partial
pressure of carbon dioxide is very high and it
enters the blood. The presence of carbon dioxide
in blood decreases the affinity of hemoglobin for
oxygen so that and oxygen is released from the
blood to the tissues. The oxygen dissociation
curve is shifted to right.
TRANSPORT OF CARBON DIOXIDE
Carbon dioxide is transported by the blood from
tissues to the alveoli. The partial pressure and
content of carbon dioxide in arterial blood and
venous blood are given in Table 76-2. Carbon
dioxide is transported in the blood in four ways.
1. As dissolved form – 7%
2. As carbonic acid – negligible
3. As bicarbonates – 63%
4. As carbamino compounds – 30%.
TRANSPORT OF CARBON DIOXIDE
AS DISSOLVED FORM
Carbon dioxide diffuses into blood and dissolves
in the fluid of plasma forming a simple solution.
Only about 3 mL/100 mL of plasma of carbon
dioxide is transported as dissolved state. It is
about 7% of total carbon dioxide in the blood.
TRANSPORT OF CARBON DIOXIDE
AS CARBONIC ACID
Part of dissolved carbon dioxide in plasma
combines with the water to form carbonic acid.
This reaction is very slow and the transport of
carbon dioxide in this form is negligible.
TRANSPORT OF CARBON DIOXIDE
AS BICARBONATE
About 63% of carbon dioxide is transported as
bicarbonate. From plasma, the carbon dioxide
enters the RBCs. In the RBCs, carbon dioxide
combines with water to form carbonic acid. The
reaction inside RBCs is very rapid. The rapid
formation of carbonic acid inside the RBCs is
due to the presence of an enzyme called
carbonic anhydrase. This enzyme accelerates
the reaction. Carbonic anhydrase is present only
inside the RBCs and not in the plasma. That is
why the carbonic acid formation is at least 200
to 300 times more in the RBCs than in plasma.
The carbonic acid is very unstable. Almost
all carbonic acid (99.9%) formed in RBCs,
dissociates into bicarbonate and hydrogen ions.
The concentration of bicarbonate ions in RBC
increases more and more. Due to concentration
gradient, bicarbonate ions diffuse through the
cell membrane into the plasma.
Chloride Shift or Hamburger Phenomenon
Chloride shift or Hamburger phenomenon is the
exchange of a chloride ion for a bicarbonate ion
across the erythrocyte membrane.
Chloride shift occurs when carbon dioxide
enters the blood from tissues. In plasma, plenty
of sodium chloride is present. It dissociates into
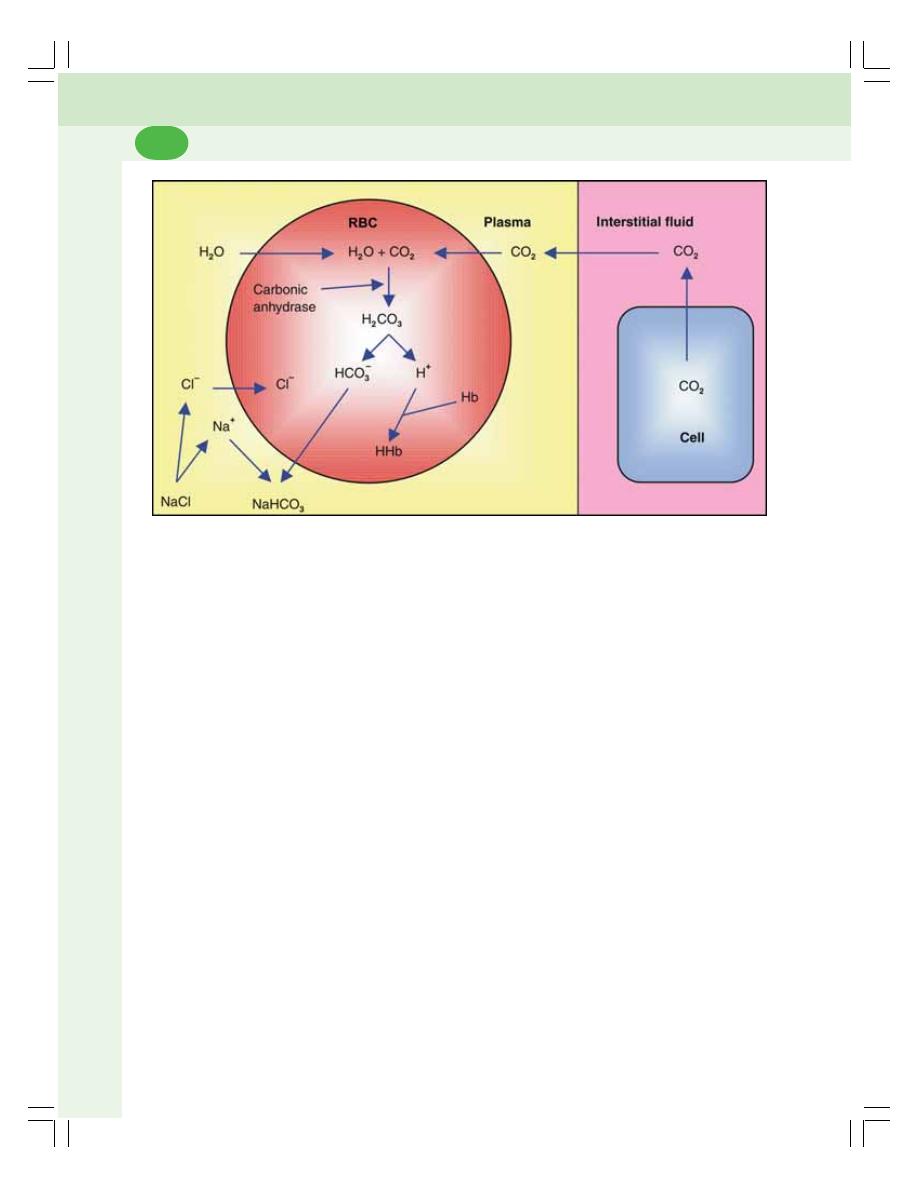
Respiratory System and Environmental Physiology
472
sodium and chloride ions (Fig. 76-7). When the
negatively charged bicarbonate ions move out
of RBC into the plasma, the negatively charged
chloride ions move into the RBC in order to
maintain the electrolyte equilibrium (ionic
balance).
Reverse Chloride Shift
Reverse chloride shift is the process by which
the chloride ions are moved back into plasma
from RBC. This occurs in lungs.
When the blood reaches the alveoli, sodium
bicarbonate in the plasma dissociates into
sodium and bicarbonate ions. Bicarbonate ion
moves into the RBC. It makes chloride ion to
move out of the RBC into the plasma, where it
combines with sodium and forms sodium
chloride.
TRANSPORT OF CARBON DIOXIDE
AS CARBAMINO COMPOUNDS
About 30% of carbon dioxide is transported as
carbamino compounds. Carbon dioxide is
transported in blood in combination with
hemoglobin and plasma proteins. Carbon
dioxide combines with hemoglobin to form
carbamino hemoglobin or carbhemoglobin.
And, it combines with plasma proteins to form
carbamino proteins. The carbamino hemoglobin
and carbamino proteins are together called
carbamino compounds.
The carbon dioxide combines with proteins
or hemoglobin with a loose bond so that, carbon
dioxide is easily released into alveoli, where the
partial pressure of carbon dioxide is low. Thus,
the combination of carbon dioxide with proteins
and hemoglobin is a reversible one. The amount
of carbon dioxide is transported in combination
with plasma proteins is very less compared to
the amount transported in combination with
hemoglobin. It is because, the quantity of
proteins in plasma is only half of the quantity of
hemoglobin.
CARBON DIOXIDE DISSOCIATION
CURVE
Carbon dioxide is transported in blood as physical
solution and in combination with water, plasma
proteins and hemoglobin. The amount of carbon
FIGURE 76-7: Transport of carbon dioxide in blood in the form of bicarbonate and chloride shift
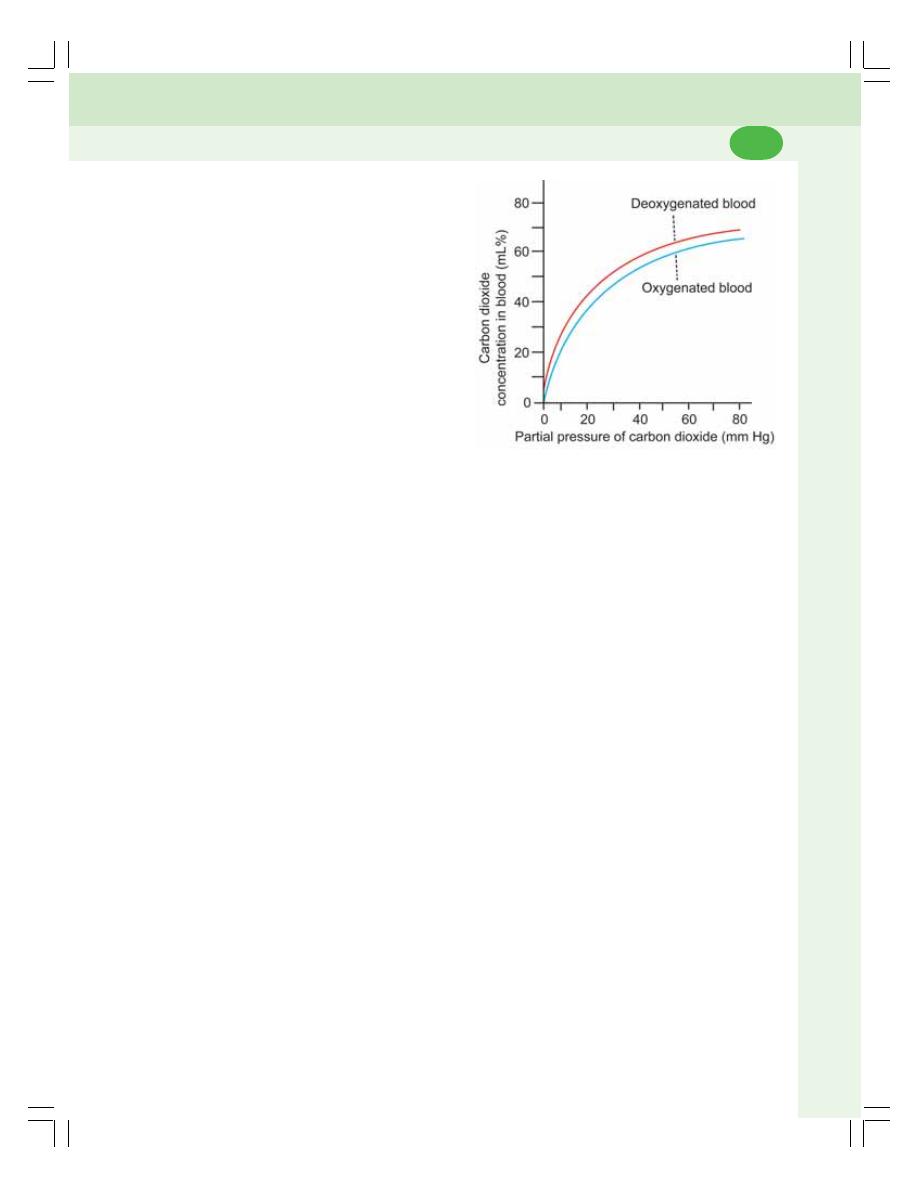
Chapter 76 Exchange and Transport of Respiratory Gases
473
dioxide combining with blood depends upon the
partial pressure of carbon dioxide.
Carbon dioxide dissociation curve is the
curve that demonstrates the relationship
between the partial pressure of carbon dioxide
and the quantity of carbon dioxide that combines
with blood.
Normal Carbon Dioxide Dissociation
Curve
The normal carbon dioxide dissociation curve
shows that the carbon dioxide content in the
blood is 48 mL% when the partial pressure of
carbon dioxide is 40 mm Hg and, it is 52 mL%
when the partial pressure of carbon dioxide is
48 mm Hg. The carbon dioxide content becomes
70 mL% when the partial pressure is about 100
mm Hg (Fig. 76-8).
Haldane Effect
Haldane effect is the effect by which combina-
tion of oxygen with hemoglobin displaces carbon
dioxide from hemoglobin. The excess of oxygen
FIGURE 76-8: Carbon dioxide dissociation curve
content in blood causes shift of the carbon
dioxide dissociation curve to the right.
Significance of Haldane effect
Haldane’s effect is essential for release of
carbon dioxide from blood into the alveoli of
lungs and uptake of oxygen by the blood.
INTRODUCTION
NERVOUS MECHANISM
RESPIRATORY CENTERS
CONNECTIONS OF RESPIRATORY CENTERS
INTEGRATION OF RESPIRATORY CENTERS
FACTORS AFFECTING RESPIRATORY CENTERS
CHEMICAL MECHANISM
CENTRAL CHEMORECEPTORS
PERIPHERAL CHEMORECEPTORS
INTRODUCTION
Respiration is a reflex process. But it can be
controlled voluntarily also. Voluntary arrest of
respiration (voluntary apnea) is possible only for
a short period of about 40 seconds. However,
by practice, breathing can be withheld for a long
period. At the end of that period, the person is
forced to breathe.
However, normally, the quiet regular
breathing takes place because of regulatory
mechanisms.
Respiration is regulated by two mechanisms:
A. Nervous or neural mechanism
B. Chemical mechanism.
NERVOUS MECHANISM
Nervous mechanism that regulates respiration
includes respiratory centers, afferent nerves and
efferent nerves.
RESPIRATORY CENTERS
Respiratory centers are group of neurons, which
control the rate, rhythm and force of respiration.
These centers are bilaterally situated in reticular
formation of the brainstem (Fig. 77-1). Depen-
ding upon the situation in the brainstem, the
respiratory centers are classified into two
groups:
I.
Medullary centers which are made up of:
1. Dorsal respiratory group of neurons
2. Ventral respiratory group of neurons
II. Pontine centers which are:
1. Pneumotaxic center
2. Apneustic center.
MEDULLARY CENTERS
1. Dorsal Respiratory Group of Neurons
Dorsal respiratory group of neurons are
responsible for basic rhythm of respiration (see
Regulation of Respiration
77
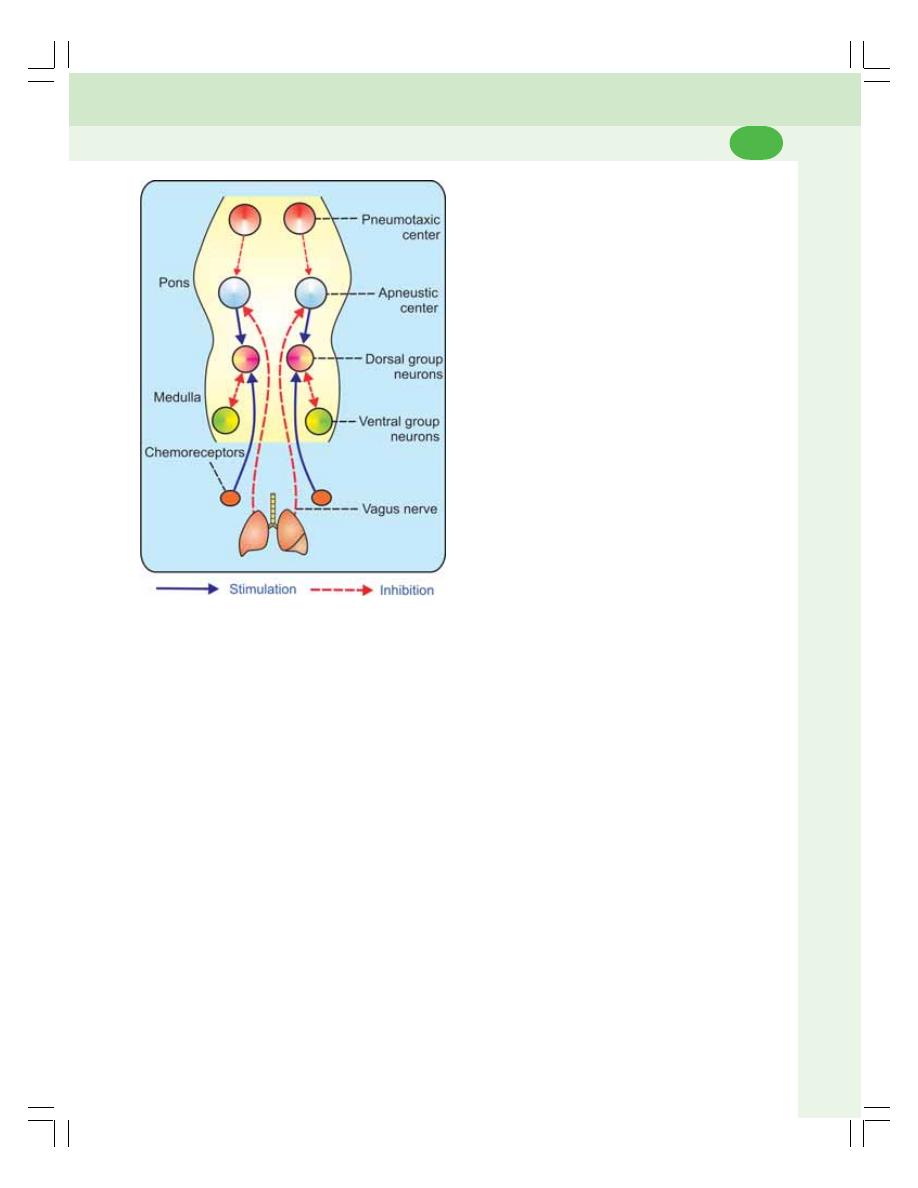
Chapter 77 Regulation of Respiration
475
below for details). Electrical stimulation of these
neurons in animals causes contraction of
inspiratory muscles and prolonged inspiration.
2. Ventral Respiratory Group of Neurons
Ventral respiratory group of neurons are inactive
during quiet breathing and become active during
forced breathing. During forced breathing, these
neurons stimulate both inspiratory muscles and
expiratory muscles.
Electrical stimulation of the inspiratory neu-
rons in ventral group causes contraction of
inspiratory muscles and prolonged inspiration.
Stimulation of expiratory neurons causes
contraction of expiratory muscles and prolonged
expiration.
PONTINE CENTERS
1. Pneumotaxic Center
The pneumotaxic center controls the medullary
respiratory centers, particularly the dorsal group
neurons. It acts through apneustic center. The
pneumotaxic center inhibits the apneustic center
so that the dorsal group of neurons is inhibited.
Because of this inspiration stops and expiration
starts. Thus, the pneumotaxic center influences
the switching between inspiration and expiration.
The pneumotaxic center increases the respi-
ratory rate by reducing the duration of inspiration.
Stimulation of pneumotaxic center causes
prolongation of expiration by inhibiting the dorsal
respiratory group of neurons through apneustic
center.
2. Apneustic Center
The apneustic center increases the depth of
inspiration by acting directly on the dorsal group
neurons.
The stimulation of apneustic center causes
apneusis. Apneusis is an abnormal pattern of
respiration or breathing irregularity characterized
by prolonged inspiration followed by short,
inefficient expiration.
CONNECTIONS OF RESPIRATORY
CENTERS
Efferent Pathway
The nerve fibers from the respiratory centers
leave brainstem and descend in spinal cord and
terminate on the motor neurons in the anterior
horn cells of cervical and thoracic segments of
spinal cord. From the motor neurons of spinal
cord two sets of nerve fibers arise:
1. Phrenic nerve fibers (C
3
– C
5
) which supply
the diaphragm
2. The intercostal nerve fibers (T
1
– T
11
) which
supply the external intercostal muscles.
Afferent Pathway
Impulses from peripheral chemoreceptors and
baroreceptors are carried to the respiratory
centers by the branches of glossopharyngeal
and vagus nerves. Vagal nerve fibers also carry
impulses from the stretch receptors of lungs to
the respiratory centers.
Thus, the respiratory centers receive afferent
impulses from different parts of the body and,
modulate the movements of thoracic cage and
lungs accordingly through efferent nerve fibers.
FIGURE 77-1: Nervous regulation of respiration

Respiratory System and Environmental Physiology
476
INTEGRATION OF RESPIRATORY
CENTERS
Role of Medullary Centers
Rhythmic discharge of inspiratory impulses
Dorsal respiratory group neurons maintain the
normal rhythm of respiration by rhythmic
discharge of impulses (action potentials). These
impulses are transmitted to the respiratory
muscles by phrenic and intercostal nerves.
Inspiratory Ramp
Inspiratory ramp is the pattern of discharge from
dorsal respiratory group neurons characterized
by steady increase in amplitude of the action
potential. To start with, the amplitude of the action
potential is low due to the activation of only few
neurons. Later, more and more neurons are
activated leading to gradual increase in the
amplitude of the action potential in a ramp
fashion. The impulses of this type of firing from
dorsal group neurons are called inspiratory ramp
signals.
The impulses from dorsal group of neurons
are produced only for a period of 2 seconds
during which inspiration occurs. After 2 seconds,
the ramp signals stop abruptly and do not appear
for another 3 seconds. The switching off ramp
signals causes expiration. At the end of 3
seconds, the inspiratory ramp signals reappear
in the same pattern, and the cycle is repeated.
Significance of inspiratory ramp signals
The significance of inspiratory ramp signals is
that there is a slow and steady inspiration so that,
the filling of lungs with air is also steady.
Role of Pontine Centers
Pontine respiratory centers regulate the medu-
llary centers. The apneustic center accelerates
the activity of dorsal group of neurons and the
stimulation of this center, causes prolonged
inspiration.
The pneumotaxic center inhibits the
apneustic center and restricts the duration of
inspiration.
FACTORS AFFECTING RESPIRATORY
CENTERS
The respiratory centers regulate the respiratory
movements, by receiving impulses from various
sources in the body.
1. Impulses from Higher Centers
Higher centers alter the respiration by sending
impulses directly to the dorsal group neurons.
The impulses from various parts of cerebral
cortex such as anterior cingulate gyrus, olfactory
tubercle and posterior orbital gyrus inhibit the
respiration. The impulses from motor area and
Sylvian area of cerebral cortex cause forced
breathing.
2. Impulses from Stretch Receptors of
Lungs: Hering-Breuer Reflex
Hering-Breuer reflex is a protective reflex that
restricts the inspiration and prevents over
stretching of lung tissues. It is initiated by the
stimulation of stretch receptors of air passage.
Stretch receptors give response to stretch of
the tissues. During inspiration, there is stretching
of lungs due to entrance of air resulting in
stimulation of stretch receptors. The impulses
from stretch receptors pass through vagal
afferent fibers to respiratory centers and inhibit
the dorsal group neurons. So inspiration stops
and expiration starts (Fig. 77-2). Thus, the
overstretching of lung tissues is prevented.
However, Hering-Breuer reflex does not ope-
rate during quiet breathing. It operates, only when
the tidal volume increases beyond 1000 mL.
This reflex is also called Hering-Breuer
inflation reflex since it occurs due to inflation of
lungs during inspiration. The reverse of this reflex
is called Hering-Breuer deflation reflex and it
takes place during expiration. During expiration
as the stretching of lungs is abolished, the
deflation of lungs occurs.
3. Impulses from ‘J’ Receptors of Lungs
‘J’ receptors are juxtacapillary receptors which
are present on the wall of the alveoli and having
close contact with the pulmonary capillaries.
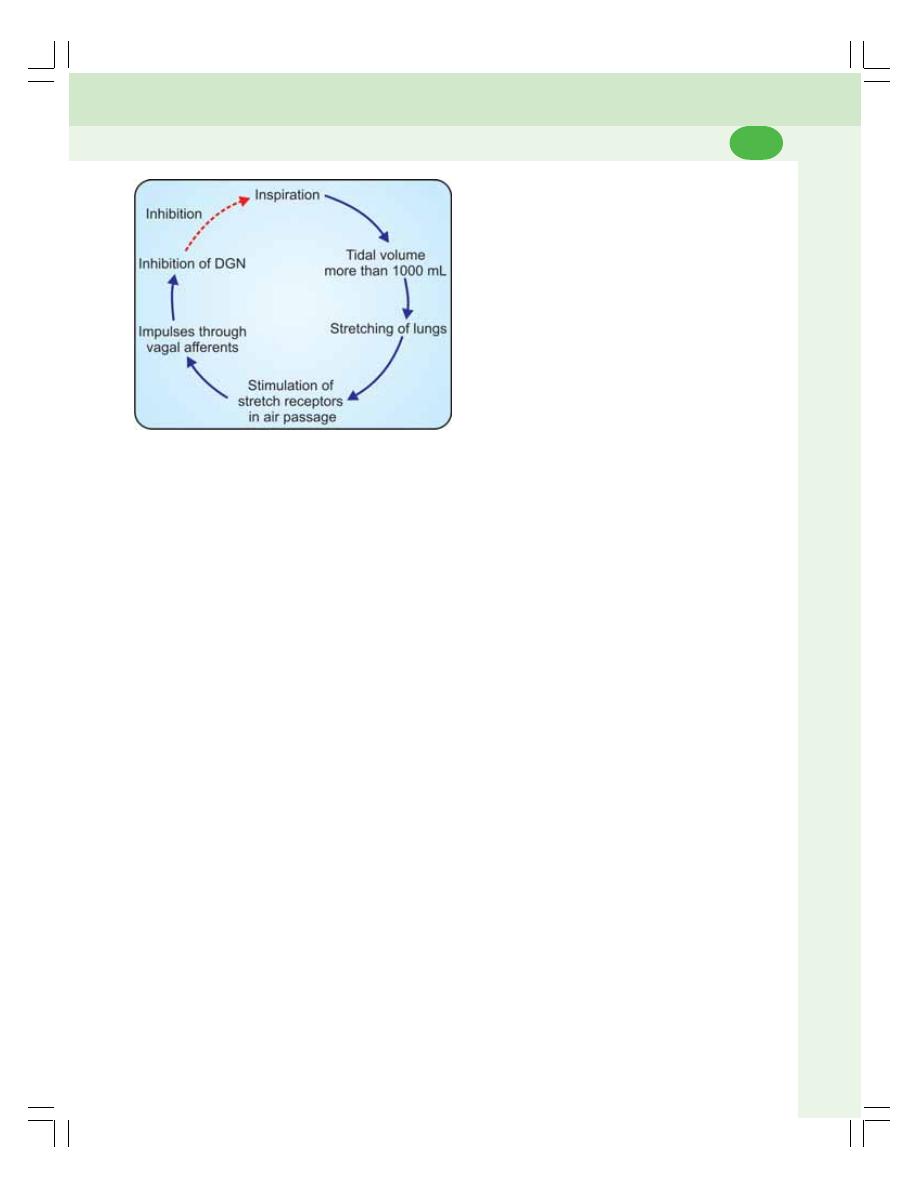
Chapter 77 Regulation of Respiration
477
The stimulation of the ‘J’ receptors produces
a reflex response, which is characterized by
apnea. Apnea is followed by hyperventilation.
The role of ‘J’ receptors in physiological
conditions is not clear. However, these receptors
are responsible for hyperventilation in the patients
affected by pulmonary congestion and left heart
failure.
4. Impulses from Irritant Receptors of
Lungs
Besides stretch receptors, there is another type
of receptors in the bronchi and bronchioles, called
irritant receptors. The irritant receptors are
stimulated by irritant chemical agents such as
ammonia and sulfur dioxide.
Stimulation of irritant receptors produces
reflex hyperventilation along with broncho-
spasm. Hyperventilation along with broncho-
spasm prevents further entry of harmful agents
into the alveoli.
5. Impulses from Baroreceptors
The baroreceptors are the receptors which give
response to change in blood pressure. Refer
Chapter 64 for details of baroreceptors.
Function
Whenever arterial blood pressure increases,
baroreceptors are activated and send inhibitory
impulses to vasomotor center in medulla
oblongata. This causes decrease in blood
pressure and inhibition of respiration. However,
in physiological conditions, the role of
baroreceptors in regulation of respiration is
insignificant.
6. Impulses from Chemoreceptors
Chemoreceptors play an important role in the
chemical regulation of respiration. The details of
the chemoreceptors and chemical regulation of
respiration are explained later in this chapter.
7. Impulses from Proprioceptors
Proprioceptors are the receptors, which give res-
ponse to the change in the position of the body.
These receptors are situated in joints, tendons
and muscles. The proprioceptors are stimulated
during the muscular exercise and, send impulses
to brain particularly, the cerebral cortex through
somatic afferent nerves. Cerebral cortex in turn
causes hyperventilation by sending impulses to
the medullary respiratory centers.
8. Impulses from Thermoreceptors
Thermoreceptors are the cutaneous receptors,
which give response to change in the
environmental temperature. There are two types
of temperature receptors, namely, the receptors
for cold and the receptors for warmth. When the
body is exposed to cold or when cold water is
applied over the body, the cold receptors are
stimulated and, send impulses to cerebral cortex
via somatic afferent nerves. Cerebral cortex in
turn stimulates the respiratory centers and
causes hyperventilation.
9. Impulses from Pain Receptors
The pain receptors are those which give
response to pain stimulus. Whenever pain
FIGURE 77-2: Hering-Breuer inflation reflex. DGN =
Dorsal respiratory group of neurons. Dashed red arrow
indicates inhibition
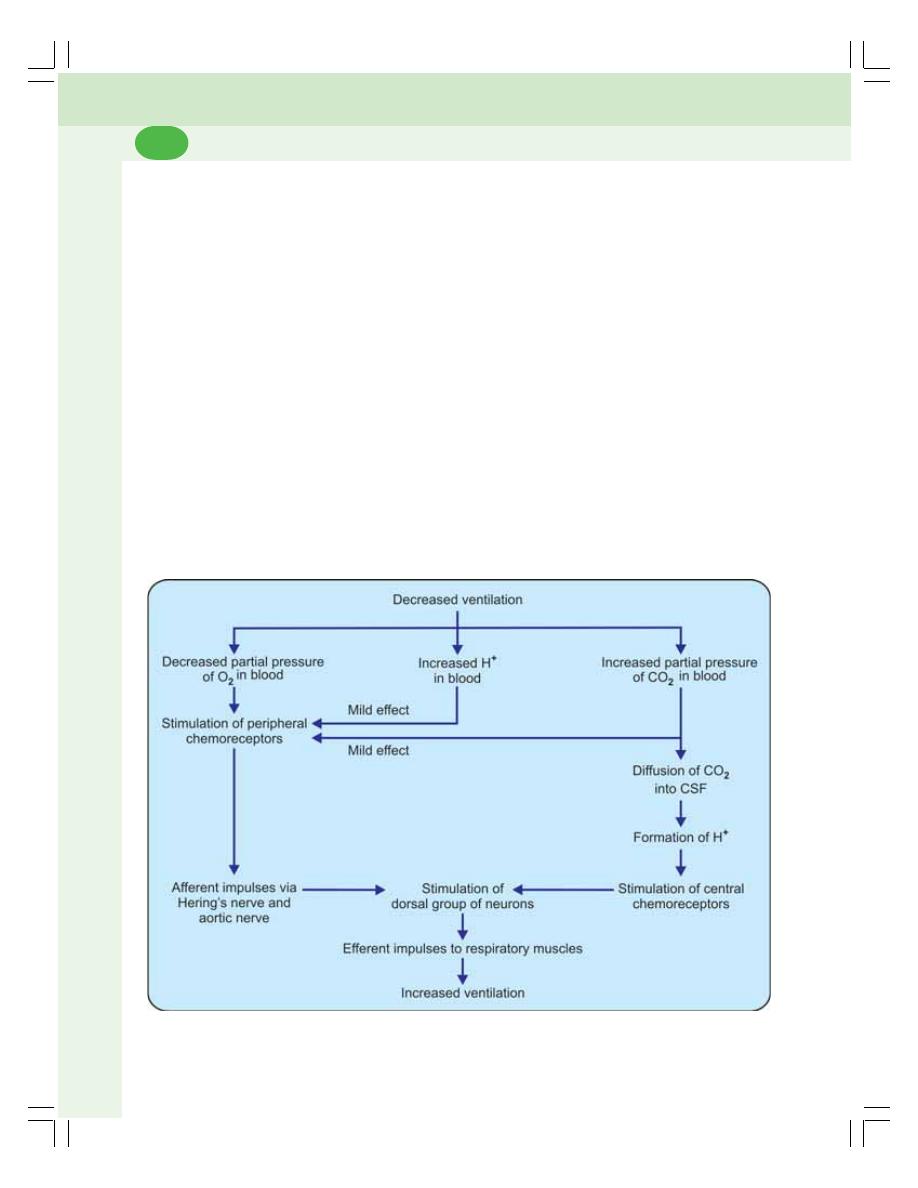
Respiratory System and Environmental Physiology
478
receptors are stimulated, the impulses are sent
to the cerebral cortex via somatic afferent
nerves. Cerebral cortex in turn stimulates the
respiratory centers and causes hyperventilation.
CHEMICAL MECHANISM
The chemical mechanism of regulation of
respiration is operated through the chemo-
receptors which give response to chemical
changes in blood such as:
1. Hypoxia (decreased PO
2
)
2. Hypercapnea (increased PCO
2
)
3. Increased hydrogen ion concentration.
Types of Chemoreceptors
Chemoreceptors are classified into two groups:
1. Central chemoreceptors
2. Peripheral chemoreceptors.
CENTRAL CHEMORECEPTORS
The chemoreceptors present in the brain are
called the central chemoreceptors. These
chemoreceptors are situated in medulla
oblongata, close to dorsal respiratory group of
neurons.
Mechanism of Action
The main stimulant for the central chemo-
receptors is the increased hydrogen ion
concentration.
However, if hydrogen ion concentration
increases in the blood, it cannot stimulate the
central chemoreceptors because, the hydrogen
ions from blood cannot cross the bloodbrain
barrier and blood cerebrospinal fluid barrier.
On the other hand, if carbon dioxide
increases in the blood, it can easily cross the
blood-brain barrier and blood cerebrospinal
fluid barrier and enter the interstitial fluid of brain
FIGURE 77-3: Chemical regulation of respiration
Chapter 77 Regulation of Respiration
479
or the cerebrospinal fluid. There, the carbon
dioxide combines with water to form carbonic
acid. Since carbonic acid is unstable, it
immediately dissociates into hydrogen ion and
bicarbonate ion (Fig. 77-3).
CO
2
+ H
2
O
→ H
2
CO
3
→ H
+
+ HCO
3
–
The hydrogen ions stimulate the central
chemoreceptors. Chemoreceptors in turn send
stimulatory impulses to dorsal respiratory group
of neurons causing increased ventilation
(increased rate and force of breathing). Because
of this, the excess carbon dioxide is washed out
and the respiration is brought back to normal.
PERIPHERAL CHEMORECEPTORS
Chemoreceptors present in the carotid and aortic
region are called peripheral chemoreceptors.
Refer Chapter 64 for details.
Mechanism of Action
Reduction in partial pressure of oxygen is the
most potent stimulant for the peripheral
chemoreceptors. Whenever, the partial pressure
of oxygen decreases, the chemoreceptors are
stimulated and send impulses through aortic and
Hering’s nerves. These impulses reach the
respiratory centers, particularly the dorsal group
of neurons and stimulate them. Dorsal group of
neurons send stimulatory impulses to respiratory
muscles resulting in increased ventilation. This
provides enough oxygen and rectifies the lack
of oxygen.
The peripheral chemoreceptors are mildly
sensitive to the increased partial pressure of
carbon dioxide and increased hydrogen ion
concentration.
APNEA
HYPERVENTILATION
HYPOVENTILATION
HYPOXIA
OXYGEN TOXICITY (POISONING)
HYPERCAPNEA
HYPOCAPNEA
ASPHYXIA
DYSPNEA
PERIODIC BREATHING
CYANOSIS
CARBON MONOXIDE POISONING
APNEA
Apnea is defined as temporary arrest of
breathing. Apnea can also be produced volun-
tarily which is called breath holding or voluntary
apnea. The breath holding time is known as
apnea time. It is about 40 to 60 seconds in a
normal person, after a deep inspiration.
Apnea occurs in the following conditions:
1. Voluntary effort (voluntary apnea or breath
holding)
2. After hyperventilation
3. During deglutition (deglutition apnea - Chapter
33)
4. During stimulation of vagus nerve in animals
(vagal apnea)
5. After injection of adrenaline (adrenaline
apnea)
6. Sleep apnea (apnea during sleep).
HYPERVENTILATION
Hyperventilation means increased pulmonary
ventilation due to forced breathing. Both rate and
force of breathing are increased.
Hyperventilation occurs in conditions like
exercise. Voluntarily hyperventilation also can be
produced.
HYPOVENTILATION
Hypoventilation is the decrease in pulmonary
ventilation caused by decrease in rate or force
of breathing.
Disturbances of Respiration
78
Chapter 78 Disturbances of Respiration
481
Hypoventilation occurs when respiratory
centers are suppressed, or by administration of
some drugs. It occurs during partial paralysis of
respiratory muscles also.
HYPOXIA
Hypoxia is the reduced availability of oxygen to
the tissues.
CLASSIFICATION AND CAUSES OF
HYPOXIA
Four important factors which lead to hypoxia are:
1. Oxygen tension in arterial blood
2. Oxygen carrying capacity of blood
3. Velocity of blood flow
4. Utilization of oxygen by the cells.
On the basis of these factors, hypoxia is
classified into four types (Table 78-1):
I. Hypoxic hypoxia
II. Anemic hypoxia
III. Stagnant hypoxia
IV. Histotoxic hypoxia.
Each type of hypoxia may be acute or
chronic. Simultaneously, two or more types of
hypoxia may be present.
I. Hypoxic Hypoxia
Hypoxic hypoxia means the decreased oxygen
content in the blood. It is also called arterial
hypoxia.
Causes for hypoxic hypoxia
i. Low oxygen tension in inspired (atmospheric)
air
ii. Respiratory disorders
iii. Cardiac disorders.
Characteristic features of hypoxic hypoxia
It is characterized by reduced oxygen tension in
arterial blood. All other features remain normal
(Table 78-1).
II. Anemic Hypoxia
Anemic hypoxia is the condition characterized
by the inability of blood to carry enough amount
of oxygen. The oxygen availability is normal. But
the blood is not able to take up sufficient amount
of oxygen due to anemic condition.
Causes of anemic hypoxia
Any condition that causes anemia can cause
anemic hypoxia. It occurs because of the
following conditions:
i. Decreased number of RBCs
ii. Decreased hemoglobin content in the blood
iii. Formation of altered hemoglobin
iv. Combination of hemoglobin with gases other
than oxygen and carbon dioxide.
Characteristic features of anemic hypoxia
Anemic hypoxia is characterized by the inability
of blood to carry sufficient oxygen. All other
features remain normal (Table 78-1).
III. Stagnant Hypoxia
It is the hypoxia caused by decreased velocity
of blood flow. It is otherwise called hypokinetic
hypoxia.
Causes of stagnant hypoxia
Stagnant hypoxia occurs mainly due to reduction
in velocity of blood flow. The velocity of blood
flow decreases in the following conditions:
i. Congestive cardiac failure
ii. Hemorrhage
iii. Surgical shock
iv. Vasospasm
v. Thrombosis
vi. Embolism.
Characteristic features of stagnant hypoxia
The characteristic feature of stagnant hypoxia
is the decreased velocity of blood flow. All other
features remain normal (Table 78-1).
Respiratory System and Environmental Physiology
482
IV. Histotoxic Hypoxia
It is the type of hypoxia produced by the inability
of tissues to utilize oxygen.
Causes for histotoxic hypoxia
Histotoxic hypoxia occurs due to cyanide or
sulfide poisoning. These substances destroy the
cellular oxidative enzymes. So, even if oxygen
is supplied, the tissues are not in a position to
utilize it.
Characteristic features of histotoxic hypoxia
Here, the tissues are not able to use the oxygen
even if it is delivered. All other features remain
normal (Table 78-1).
EFFECTS OF HYPOXIA
Acute and severe hypoxia leads to unconscious-
ness. If not treated immediately, brain death
occurs. Chronic hypoxia produces various
symptoms in the body.
1. Effects on Blood
Hypoxia stimulates the secretion of erythro-
poietin from kidney. Erythropoietin increases
production of RBCs. Thus, the oxygen carrying
capacity of blood is improved by increase in RBC
count and hemoglobin content.
2. Effects on Cardiovascular System
Initially, due to the reflex stimulation of cardiac
and vasomotor centers, there is increase in
rate and force of contraction of heart, cardiac
output and blood pressure. Later, there is
reduction in the rate and force of contraction of
heart. Cardiac output and blood pressure are
also decreased.
3. Effects on Respiration
Initially, the respiratory rate is increased due to
chemoreceptor reflex. Because of this large
amount of carbon dioxide is washed out leading
to alkalemia. Later, the respiration tends to be
shallow and periodic. Finally, the rate and force
of breathing are reduced to a great extent due
to the failure of respiratory centers.
4. Effects on Digestive System
Hypoxia is associated with loss of appetite,
nausea and vomiting. Mouth becomes dry and
there is a feeling of thirst.
5. Effects on Kidney
Juxtaglomerular apparatus of kidney secretes
erythropoietin. Alkaline urine is excreted.
6. Effects on Central Nervous System
In mild hypoxia, the symptoms are similar to
those of alcoholic intoxication.
The individual is depressed, apathic with
general loss of self control. The person becomes
talkative, quarrelsome, ill tempered and rude.
The subject starts shouting, singing or crying.
There is disorientation, and loss of
discriminative ability and loss of power of
judgment. Memory is impaired. Weakness, lack
TABLE 78-1: Characteristic features of different types of hypoxia
Features
Hypoxic
Anemic
Stagnant
Histotoxic
hypoxia
hypoxia
hypoxia
hypoxia
1. PO
2
in arterial blood
Reduced
Normal
Normal
Normal
2. O
2
carrying capacity of blood
Normal
Reduced
Normal
Normal
3. Velocity of blood flow
Normal
Normal
Reduced
Normal
4. Utilization of O
2
by tissues
Normal
Normal
Normal
Reduced
5. Efficacy of O
2
therapy
100%
75%
< 50%
Not useful
Chapter 78 Disturbances of Respiration
483
of coordination and fatigue of muscles are
common in hypoxia.
If hypoxia is acute and severe, there is
sudden loss of consciousness. If not treated
immediately, coma occurs which leads to
death.
TREATMENT FOR HYPOXIA —
OXYGEN THERAPY
The best treatment for hypoxia is oxygen
therapy, i.e. treating the affected person with
oxygen. Pure oxygen or oxygen combined with
another gas is administered.
Efficacy of Oxygen Therapy in Different
Types of Hypoxia
Oxygen therapy is not effective equally in all
types of hypoxia. The value of oxygen therapy
depends upon the type of hypoxia. In hypoxic
hypoxia, the oxygen therapy is 100% useful. In
anemic hypoxia, oxygen therapy is moderately
effective to about 70%. In stagnant hypoxia, the
effectiveness of oxygen therapy is less than
50%. In histotoxic hypoxia, the oxygen therapy
is not useful at all. It is because, even if oxygen
is delivered, the cells cannot utilize oxygen.
OXYGEN TOXICITY (POISONING)
Oxygen toxicity is the increased oxygen content
in tissues beyond certain critical level. It is also
called oxygen poisoning. It occurs because of
breathing pure oxygen with high pressure of 2-3
atmospheres (hyperbaric oxygen).
EFFECTS OF OXYGEN TOXICITY
1. Lung tissues are affected first with
tracheobronchial irritation and pulmonary
edema
2. The metabolic rate increases in all the body
tissues and the tissues are burnt out by
excess heat. The heat also destroys
cytochrome system leading to damage of
tissues
3. When brain is affected, first hyperirritability
occurs. Later, it is followed by increased
muscular twitching, ringing in ears and
dizziness
4. Finally, the toxicity results in convulsions,
coma and death.
HYPERCAPNEA
Hypercapnea is the increased carbon dioxide
content of blood. It occurs in conditions, which
leads to blockage of respiratory pathway as in
case of asphyxia. It also occurs while breathing
air containing excess carbon dioxide content.
EFFECTS OF HYPERCAPNEA
1. Excess stimulation of respiratory centers
leading to dyspnea
2. Reduction in pH of blood
3. Increase in heart rate and blood pressure
4. Headache, depression and laziness
5. Muscular rigidity, tremors and convulsions
6. Giddiness and loss of consciousness.
HYPOCAPNEA
Hypocapnea is the decreased carbon dioxide
content in blood. It occurs in conditions
associated with hypoventilation.
EFFECTS OF HYPOCAPNEA
1. Rate and force of respiration decrease
2. The pH of blood increases leading to res-
piratory alkalosis
3. Calcium concentration decreases resulting in
tetany
4. Dizziness, mental confusion, muscular twit-
ching and loss of consciousness
ASPHYXIA
Asphyxia is the condition characterized by
combination of hypoxia and hypercapnea due
to obstruction of air passage. It develops due
to acute obstruction of air passage in conditions
like strangulation, hanging and drowning.

Respiratory System and Environmental Physiology
484
EFFECTS OF ASPHYXIA
The effects of asphyxia develop in three stages:
1. Stage of hyperpnea
2. Stage of convulsions
3. Stage of collapse.
1. Stage of Hyperpnea
Hyperpnea is the first stage of asphyxia. It
extends for about 1 minute. In this stage,
breathing becomes deep and rapid. It is due to
the powerful stimulation of respiratory centers
by accumulation of carbon dioxide. Hyperpnea
is followed by dyspnea and cyanosis. The eyes
become more prominent.
2. Stage of Convulsions
This stage is characterized mainly by
convulsions (uncontrolled involuntary muscular
contractions). Duration of this stage is less than
one minute. The following effects develop in this
stage due to the effect of hypercapnea on brain
and spinal cord:
i. Expiratory efforts become more violent
ii. Generalized convulsions appear
iii. Heart rate increases
iv. Arterial blood pressure greatly increases
v. Consciousness is lost.
3. Stage of Collapse
This stage lasts for about three minutes. The
effects of this stage are:
i. Depression of brain centers due to lack of
oxygen. So, the convulsions disappear
ii. Respiratory gasping occurs with stretching
of the body and opening of mouth as if
gasping for breath
iii. Dilatation of pupils
iv. Reduction in heart rate
v. Loss of all reflexes
vi. Increase in the duration between the gasps
vii. Finally, the death.
All together, asphyxia extends only for
5 minutes. The person can be saved by timely
help such as relieving the respiratory obstruction,
good aeration, etc. Otherwise, death occurs.
DYSPNEA
Dyspnea means difficulty in breathing. It is
otherwise called the air hunger. Normally, the
breathing goes on without consciousness. When
the breathing enters the consciousness and
produces discomfort, it is called dyspnea.
Dyspnea is also defined as “a consciousness
of necessity for increased respiratory effort”.
Physiologically, dyspnea occurs during
severe muscular exercise. The pathological
conditions when dyspnea occurs are respiratory,
cardiac and metabolic disorders.
PERIODIC BREATHING
Periodic breathing is the abnormal or uneven
respiratory rhythm. It is of two types:
1. Cheyne-Stokes breathing
2. Biot’s breathing.
CHEYNE-STOKES BREATHING
Cheyne-Stokes breathing is the periodic
breathing characterized by rhythmic hyperpnea
and apnea. It is the most common type of
periodic breathing. It is marked by two alternate
patterns of respiration:
i.
Hyperpneic period
ii. Apneic period
Hyperpneic Period — Waxing and Waning
of Breathing
To begin with, the breathing is shallow. The force
of respiration increases gradually and reaches
the maximum (hyperpnea). Then, it decreases
gradually and reaches minimum and is followed
by apnea. The gradual increase followed by
gradual decrease in force of respiration is called
waxing and waning of breathing (Fig. 78-1).
Apneic Period
When, the force of breathing is reduced to
minimum, cessation of breathing occurs for a
short period. It is again followed by hyperpneic
period and the cycle is repeated.

Chapter 78 Disturbances of Respiration
485
ii. During cardiac failure
iii. During renal diseases
iv. Poisoning by narcotics
v. In premature infants.
BIOT’S BREATHING
It is another form of periodic breathing charac-
terized by period of apnea and hyperpnea. There
is no waxing and waning of breathing (Fig. 78-1).
After apneic period, hyperpnea occurs abrup-
tly.
Causes of Abrupt Apnea and Hyperpnea
Due to apnea, carbon dioxide accumulates and
it stimulates the respiratory centers leading to
hyperventilation. During hyperventilation, lot of
carbon dioxide is washed out. So, the respi-
ratory centers are not stimulated and apnea
occurs.
Conditions when Biot’s Breathing Occurs
Biot’s breathing occurs only in pathological
conditions such as nervous disorders.
CYANOSIS
Cyanosis is defined as diffused bluish coloration
of skin and mucous membrane. It is due to the
presence of large amount of reduced hemoglobin
in the blood.
Cyanosis is distributed all over the body. But,
it is more marked in certain regions such as lips,
cheeks, ear lobes, nose and fingertips above the
base of the nail.
CONDITIONS WHEN CYANOSIS
OCCURS
1. Any condition which leads to arterial hypoxia
and stagnant hypoxia.
2. Conditions when altered hemoglobin like
methemoglobin or sulfhemoglobin is formed.
3. Conditions like polycythemia when blood flow
is slow.
FIGURE 78-1: Periodic breathing
Causes for waxing and waning
Initially, during forced breathing, large quantity
of carbon dioxide is washed out from blood
leading to inactivation of respiratory centers. It
causes apnea. During apnea, there is accu-
mulation of carbon dioxide with reduction in
oxygen tension resulting in activation of res-
piratory centers. This causes gradual increase
in the force of breathing to the maximum. And
the cycle is repeated.
Conditions when Cheyne-Stokes
Breathing Occurs
Cheyne-Stokes breathing occurs in both
physiological and pathological conditions.
Physiological conditions when Cheyne-Stokes
breathing occurs
i. During deep sleep
ii. In high altitude
iii. After prolonged voluntary hyperventilation
iv. In newborn babies
v. After severe muscular exercise.
Pathological conditions when Cheyne-Stokes
breathing occurs
i. During increased intracranial pressure

Respiratory System and Environmental Physiology
486
CARBON MONOXIDE POISONING
Carbon monoxide is a dangerous gas. The
common sources for carbon monoxide are
exhaust of gasoline engines, coal mines, gases
from guns, deep wells and underground drainage
system.
EFFECTS OF CARBON MONOXIDE
Carbon monoxide is a dangerous because it
displaces oxygen from hemoglobin by binding
with same site in hemoglobin for oxygen. So,
oxygen transport and oxygen carrying capacity
of the blood are decreased.
SYMPTOMS OF CARBON MONOXIDE
POISONING
The symptoms of carbon monoxide poisoning
depend upon the concentration of this gas in air:
1. While breathing air with 1% of carbon mono-
xide, mild symptoms like headache and
nausea appear
2. While breathing air containing carbon mono-
xide more than 1% causes convulsions,
cardiorespiratory arrest, loss of conscious-
ness and coma.
3. High carbon monoxide content in air causes
death.
HIGH ALTITUDE
BAROMETRIC PRESSURE AND PARTIAL PRESSURE OF OXYGEN AT
DIFFERENT ALTITUDES
CHANGES IN THE BODY AT HIGH ALTITUDE
MOUNTAIN SICKNESS
ACCLIMATIZATION
DEEP SEA PHYSIOLOGY
BAROMETRIC PRESSURE AT DIFFERENT DEPTHS
EFFECT OF HIGH BAROMETRIC PRESSURE — NITROGEN NARCOSIS
DECOMPRESSION SICKNESS
HIGH ALTITUDE
Any altitude above 8000 ft from mean sea level
is called high altitude. People can ascend up to
this level without any adverse effect. The
different altitudes are given in Table 79-1.
At high altitudes, the barometric pressure is
low. However, the amount of oxygen available
in the atmosphere is same as it is at the sea
level. Due to low barometric pressure, the partial
pressure of gases, particularly oxygen decreases
leading to hypoxia.
The carbon dioxide in high altitude is very
much negligible and it does not create any
problem.
BAROMETRIC PRESSURE AND
PARTIAL PRESSURE OF OXYGEN AT
DIFFERENT ALTITUDES
The barometric pressure decreases at different
altitudes and, accordingly the partial pressure
of oxygen also decreases leading to various
effects on the body. Barometric pressure and
partial pressure of oxygen at different altitudes
and their common effects on the body are given
in Table 79-2.
CHANGES IN THE BODY AT HIGH
ALTITUDE
When a person is exposed to high altitude
particularly by rapid ascent, the various systems
in the body cannot cope with the lowered oxygen
tension and, the effects of hypoxia start. Besides,
hypoxia, other factors such as expansion of
gases, fall in atmospheric temperature and light
rays are also responsible for the changes in the
functions of the body at high altitude.
MOUNTAIN SICKNESS
Definition
Mountain sickness is the condition characte-
rized by adverse effects of hypoxia at high
High Altitude and Deep Sea
Physiology
79

Respiratory System and Environmental Physiology
488
altitude. It is commonly developed in persons
going to high altitude for the first time. It occurs
within a day in these persons before they get
acclimatized to the altitude.
Symptoms
In mountain sickness, the symptoms occur
mostly in digestive system, cardiovascular
system, respiratory system and nervous system.
The symptoms of mountain sickness are:
1. Digestive system
Loss of appetite, nausea and vomiting occur
because of expansion of gases in the gastro-
intestinal tract.
2. Cardiovascular system
Heart rate increases.
3. Respiratory system
Pulmonary blood pressure increases due to
increased blood flow. Blood flow increases
because of vasodilatation induced by hypoxia.
Increased pulmonary blood pressure results in
pulmonary edema which casus breathlessness.
4. Nervous system
The symptoms of nervous system are headache,
depression, disorientation, irritability, lack of
sleep, weakness and fatigue.
TABLE 79-1: Barometric pressure, partial pressure of oxygen and
common effects at different altitudes
Altitude
Barometric Partial
Common effects
(feet)
pressure
pressure
(mm Hg)
of oxygen
(mm Hg)
Sea Level 760
159
––––––
5,000
600
132
No hypoxia
10,000
523
110
Mild symptoms of hypoxia start appearing
15,000
400
90
Moderate hypoxia develops with following symptoms:
— Reduction in visual acuity
— Effects on mental functions:
— Improper judgment and
— Feeling of over confidence
20,000
349
73
Severe hypoxia appears with cardiorespiratory symptoms such as:
— Increase in heart rate and cardiac output
— Increase in respiratory rate and respiratory minute volume
This is the highest level for permanent inhabitants
25,000
250
62
This is the critical altitude for survival
— Hypoxia becomes severe
— Breathing oxygen becomes essential
29,628
235
49
This is the height of Mount Everest
30,000
226
47
Symptoms become severe even with oxygen
50,000
87
18
Hypoxia becomes more severe even with pure oxygen
Chapter 79 High Altitude and Deep Sea Physiology
489
Treatment
Mountain sickness is treated by oxygen therapy.
ACCLIMATIZATION
Definition
Acclimatization refers to the adaptations or the
adjustments by the body in high altitude. While
staying at high altitudes for several days to
several weeks, a person slowly gets adapted or
adjusted to the low oxygen tension so that,
hypoxic effects are reduced. It enables the
person to ascent further.
Changes during Acclimatization
The various changes during acclimatization help
the body to cope with the adverse effects of
hypoxia at high altitude. Following changes occur
in the body during acclimatization:
1. Changes in blood
During acclimatization, the RBC count increases
and packed cell volume rises from the normal
value of 45% to about 59%. The hemoglobin
content in the blood rises from 15 g% to 20 g%.
So, the oxygen carrying capacity of the blood is
increased. Thus, more oxygen can be carried to
tissues in spite of hypoxia.
Increase in RBC count, packed cell volume
and hemoglobin content is due to erythropoietin
that is released from juxtaglomerular apparatus
of kidney
2. Changes in cardiovascular system
Overall activity of cardiovascular system is
increased in high altitude. There is increase in
rate and force of contraction of heart, cardiac
output and blood pressure. Hypoxia induced
vasodilatation increases the vascularity in the
body. So, blood flow to the vital organs such as
heart, brain, muscles, etc. increases.
3. Respiratory system
i. Pulmonary ventilation increases up to 65%
due to the stimulation of chemoreceptors.
This helps the person to ascend several
thousand feet
ii. Pulmonary hypertension develops due to
increased cardiac output, and pulmonary
blood flow
iii. Diffusing capacity of gases increases in the
alveoli due to the increase in pulmonary
blood flow and pulmonary ventilation. It
enables more diffusion of oxygen in blood.
4. Changes in tissues
Both in human beings and animals residing at
high altitudes permanently, the cellular oxidative
enzymes involved in metabolic reactions are
more than in the inhabitants at sea level.
Even, when a sea level inhabitant stays at
high altitude for certain period, the amount of
oxidative enzymes is not increased. So, the
elevation in the amount of oxidative enzymes
occurs only in fully acclimatized persons. An
increase in the number of mitochondria is
observed in these persons.
DEEP SEA PHYSIOLOGY
In high altitude, the problem is with low
atmospheric (barometric) pressure. In deep sea
or mines, the problem is with high barometric
pressure. The increased pressure decreases
the volume of gases and produces compression
effect on the body and internal organs.
BAROMETRIC PRESSURE AT
DIFFERENT DEPTHS
At sea level, the barometric pressure is 760 mm
Hg, which is referred as 1 atmosphere. At the
depth of every 33 feet (about 10 m), the pressure
increases by one atmosphere. Thus, at the depth
of 33 feet, the pressure is two atmospheres. It
is due to the air above water and the weight of
water itself. The pressure at different depths is
given in Table 79-2.
EFFECT OF HIGH BAROMETRIC
PRESSURE — NITROGEN NARCOSIS
Narcosis refers to unconsciousness or stupor
(lethargy with suppression of sensations and
feelings) produced by drugs. Nitrogen narcosis
means narcotic effect produced by nitrogen at
high pressure.
Respiratory System and Environmental Physiology
490
Nitrogen narcosis is common in deep sea
divers who breathe compressed air (air under
high pressure). Breathing compressed air (air
under high pressure) is essential for a deep sea
diver or an underwater tunnel worker. It is to
equalize the surrounding high pressure acting on
thoracic wall and abdomen.
Symptoms
The first symptom starts appearing at a depth
of 120 feet. The person becomes very jovial and
careless without understanding the seriousness
of the conditions. Other symptoms are given in
Table 79-2.
Mechanism
Nitrogen is soluble in fat. During compression
by high barometric pressure in deep sea,
nitrogen escapes from blood vessels and gets
dissolved in the fat present in various parts of
the body, especially the neuronal membranes.
The dissolved nitrogen acts like an anesthetic
agent suppressing the neuronal excitability.
Nitrogen remains in dissolved form in the fat till
the person remains in the deep sea. When he
ascends up, decompression sickness develops.
DECOMPRESSION SICKNESS
Definition
Decompression sickness is the disorder that
occurs when a person returns rapidly to normal
surroundings (atmospheric pressure) from the
area of high atmospheric pressure like deep sea.
It is also known as dysbarism, compressed air
sickness, caisson disease, bends or diver’s palsy.
Cause
The high barometric pressure at deep sea
leads to compression of gases in the body.
Compression reduces the volume of gases.
Among the respiratory gases, oxygen is
utilized by tissues. Carbon dioxide can be expired
out. But, nitrogen being an inert gas is neither
utilized nor expired. When it is compressed, it
escapes from blood vessels and enters the
organs. As it is fat soluble, it gets dissolved in
the fat of the tissues and tissue fluids.
As long as the person remains in deep sea,
nitrogen remains in solution and does not cause
any problem. But, if the person ascends rapidly
and returns to atmospheric pressure, nitrogen
is decompressed and escapes from the tissues
and forms bubbles. The bubbles obstruct the
blood vessels and produce decompression
sickness.
Symptoms
The symptoms of decompression sickness are
mainly due to the escape of nitrogen from the
tissues in the form of bubbles. The symptoms
of decompression sickness are:
1. Pain in joints, numbness, tingling itching and
muscle cramps due to the presence of
bubbles in myelin sheath of sensory nerve
fibers
2. Coronary ischemia due to occlusion of
coronary arteries by bubbles
TABLE 79-2: Barometric pressure and the effects
at different depth
Depth
Atmospheric
Effects on the
(feet)
Pressure
subject
Sea Level
1
—-
33
2
—-
66
3
—-
100
4
Symptoms of nitrogen
narcosis appear
133
5
Lack of concentration
Becomes jovial and
careless
166
6
Starts feeling drowsy
200
7
Feels fatigued, weak
and careless
233
8
Looses power of
judgment.
Unable to do skilled
work
266
9
Becomes unconscious
Barometric pressure: 1 atmosphere = 760 mm Hg
Chapter 79 High Altitude and Deep Sea Physiology
491
3. Damage of brain or spinal cord because of
obstruction of blood vessels by the bubbles
4. Dizziness, paralysis of muscle, shortness of
breath and choking
5. Finally, fatigue and severe pain leading to
unconsciousness and death.
Prevention
Decompression sickness is prevented by taking
proper precautionary measures. While returning
to mean sea level, the ascent should be very
slow with short stay at regular intervals. The
stepwise ascent allows nitrogen to come back
to the blood without forming bubbles. It prevents
the decompression sickness.
Treatment
If a person is affected by decompression
sickness, first recompression should be done.
It is done by keeping the person in a recom-
pression chamber. Then, he is brought back to
atmospheric pressure by reducing the pressure
slowly. Oxygen therapy may be useful.
EFFECTS OF EXPOSURE TO COLD
HEAT PRODUCTION
PREVENTION OF HEAT LOSS
EFFECTS OF EXPOSURE TO SEVERE COLD
EFFECTS OF EXPOSURE TO HEAT
HEAT EXHAUSTION
DEHYDRATION EXHAUSTION
HEAT CRAMPS
HEATSTROKE
Effects of Exposure to
Cold and Heat
80
EFFECTS OF EXPOSURE TO COLD
During exposure to cold, the body temperature
is maintained by two mechanisms (Chapter 43).
A. Heat production
B. Prevention of heat loss.
HEAT PRODUCTION
When the body is exposed to cold, the heat is
produced by the following activities:
1. By Increased Metabolic Activities
The heat gain center in hypothalamus is
stimulated during exposure to cold. It causes
secretion of adrenaline and noradrenaline by
activating sympathetic centers. These hormones,
especially adrenaline increase heat production
by accelerating cellular metabolic activities.
2. By Shivering
Shivering is the increased involuntary muscular
activity with slight vibration of the body in
response to fear, onset of fever or exposure to
cold. Shivering occurs when the body
temperature falls to about 25°C (77°F). During
exposure to cold, the heat gain center activates
the motor center for shivering situated in poste-
rior hypothalamus and, shivering occurs.
Enormous heat is produced during shivering due
to severe muscular activities.
PREVENTION OF HEAT LOSS
When the body is exposed to cold, the heat gain
center in the posterior nucleus of hypothalamus
is stimulated. It activates the sympathetic centers
in posterior hypothalamus resulting in cutaneous
vasoconstriction and decrease in blood flow. Due
to decrease in cutaneous blood flow, sweat sec-
retion is decreased and heat loss is prevented.
EFFECTS OF EXPOSURE TO SEVERE
COLD
Exposure of body to severe cold leads to death
if quick remedy is not provided. The survival time

Chapter 80 Effects of Exposure to Cold and Heat
493
depends upon the temperature of the environ-
ment.
If a person is exposed to ice cold water, i.e.
0°C for 20 to 30 minutes, the body temperature
falls below 25°C (77°F) and the person can
survive if he is placed immediately in hot water
tub with a temperature of 43°C (110°F). The
survival time at 9°C (28°F) is about 1 hour and
the survival time at 15.5°C (60°F) is about 5
hours.
The effects of exposure of body to extreme cold
are:
1. Loss of temperature regulating capacity
2. Frostbite.
Loss of Temperature Regulating Capacity
The temperature regulating capacity of
hypothalamus is affected when the body
temperature reduces to about 34.4°C (94°F).
The hypothalamus totally looses the power of
temperature regulation when body temperature
falls below 25°C (77°F). Shivering does not
occur.
In addition to loss of hypothalamic function,
the metabolic activities are also suppressed.
Sleep or coma develops due to depression of
the central nervous system.
Frostbite
Frostbite is the freezing of the surface of the
body when it is exposed to cold. It occurs due
to sluggishness of blood flow. Most commonly
the exposed areas such as ear lobes and digits
of hands and feet are affected. Frostbite is
common in mountaineers. Prolonged exposure
will lead to permanent damage of the cells
followed by thawing and gangrene (death and
decay of tissues) formation.
EFFECTS OF EXPOSURE TO HEAT
HEAT EXHAUSTION
Heat exhaustion is the body’s response to
excess loss of water and salt through sweat
caused by exposure to hot environmental
conditions. In fact it is the warning that body is
getting too hot. Heat exhaustion results in loss
of consciousness and collapse.
DEHYDRATION EXHAUSTION
Prolonged exposure to heat results in dehydra-
tion. It is due to excessive sweating. Dehydration
leads to fall in cardiac output, and blood pressure.
Collapse occurs if treatment is not given imme-
diately.
HEAT CRAMPS
Severe painful cramps occur due to reduction
in the quantity of salts and water as a result of
increased sweating during the continuous expo-
sure to heat.
HEATSTROKE
Heatstroke
Heatstroke is an abnormal increase in body
temperature that occurs during exposure to
extreme heat. It is characterized by increase in
body temperature above 41°C (106°F)
accompanied by some physical and neurological
symptoms. Compared to other effects of
exposure to heat such as heat exhaustion and
heat cramps, heatstroke is very severe and often
becomes fatal if not treated immediately. The
hypothalamus looses the power of regulating
body temperature.
Sunstroke is the heatstroke that is caused
by prolonged exposure to sun during summer
in desert or tropical areas.
Features
The common features of heatstroke are nausea,
vomiting, dizziness, headache, abdominal pain,
difficulty in breathing, vertigo, confusion, muscle
cramps, convulsions, paralysis and unconscious-
ness. If immediate and vigorous treatment is not
given, the damage of brain tissues occurs, resul-
ting in coma and death.
Treatment
The person affected by heatstroke must be
treated before the damage of organs. Immediate
cooling of the body is the usual treatment.
CONDITIONS WHEN ARTIFICIAL RESPIRATION IS REQUIRED
METHODS OF ARTIFICIAL RESPIRATION
MANUAL METHODS
MECHANICAL METHODS
Artificial Respiration
81
CONDITIONS WHEN ARTIFICIAL
RESPIRATION IS REQUIRED
Artificial respiration is required whenever there
is arrest of breathing without cardiac failure. The
arrest of breathing occurs in the following condi-
tions:
1. Accidents
2. Drowning
3. Gas poisoning
4. Electric shock
5. Anesthesia.
The tissues of brain, particularly the tissues
of cerebral cortex are affected by irreversible
changes if oxygen supply is stopped for 5 minu-
tes. So, the artificial respiration (resuscitation)
must be started quickly without any delay, before
the development of cardiac failure.
The purpose of artificial respiration is to venti-
late the alveoli and to stimulate the respiratory
centers.
METHODS OF ARTIFICIAL
RESPIRATION
The methods of artificial respiration are of two
types:
1. Manual methods
2. Mechanical methods.
MANUAL METHODS
Manual methods of artificial respiration can be
applied quickly without waiting for the availability
of any mechanical aids.
The person affected must be provided with
clear air. The clothes around neck and chest
regions must be loosened. Mouth, face and
throat should be cleared of mucus, saliva, foreign
particles, etc. The tongue must be drawn for-
ward and, it must be prevented from falling
posteriorly which may cause airway obstruction.
There are two manual methods:
i. Mouth to mouth method
ii. Holger Nielson method.
Mouth to Mouth Method
The subject is kept in supine position. The
resuscitator (the person who gives artificial
respiration) kneels at the side of the subject. By
keeping the thumb on subject’s mouth, the lower
jaw is pulled downwards. The nostrils of the
subject are closed with the thumb and index
finger of the other hand.
The resuscitator then takes a deep breath and
exhales into the subject’s mouth forcefully. The
volume of air exhaled must be twice the normal
tidal volume. This expands the subject’s lungs.

Chapter 81 Artificial Respiration
495
Then, the resuscitator removes his mouth from
that of the subject. Now, a passive expiration
occurs in the subject due to the elastic recoil of
the lungs. The procedure is repeated at a rate
of 12 to 14 times a minute, till normal respiration
is restored.
Mouth to mouth method is the most effective
manual method because, the carbon dioxide in
expired air from the resuscitator can directly
stimulate the respiratory centers and facilitates
the onset of respiration. The only disadvantage
is that, the close contact between the mouths
of resuscitator and the subject may not be
acceptable for various reasons.
Holger Nielsen Method or Back
Pressure Arm Lift Method
The subject is placed in the prone position with
head turned to one side. The hands are placed
under the cheeks with flexion at elbow joint
and abduction of arms at the shoulders. The
resuscitator kneels beside the head of the
subject. By placing the palm of the hands over
the back of the subject, the resuscitator bends
forward with straight arms (without flexion at
elbow) and applies pressure on the back of the
subject.
The weight of the resuscitator and the pres-
sure on the back of the subject compresses his
chest and expels air from the lungs. Later, the
resuscitator leans back. At the same time, he
draws the subject’s arm forward by holding it
just above elbow.
This procedure causes expansion of thoracic
cage and flow of air into the lungs. The move-
ments are repeated at the rate of 12 per minute,
till the normal respiration is restored.
MECHANICAL METHODS
Mechanical methods of artificial respiration
become necessary when the subject needs
artificial respiration for long periods. It is essen-
tial during the respiratory failure due to paralysis
of respiratory muscles or any other cause. The
mechanical methods are of two types:
i. Drinker’s method
ii. Ventilation method.
Drinker’s Method
The machine used in this method is called iron
lung chamber or tank respirator. The equipment
has an airtight chamber made of iron or steel.
The subject is placed inside this chamber with
the head outside the chamber.
By means of some pumps, the pressure
inside the chamber is made positive and nega-
tive alternately. During the negative pressure in
the chamber, the subject’s thoracic cage
expands and inspiration occurs. And, during
positive pressure, the expiration occurs.
By using the tank respirator, the patient can
survive for a longer time, even up to the period
of one year till the natural respiratory functions
are restored.
Ventilation Method
A rubber tube is introduced into the trachea of
the patient through the mouth. By using a pump,
air or oxygen is pumped into the lungs with
pressure intermittently. When air is pumped,
inflation of lungs occurs. When it is stopped,
expiration occurs and the cycle is repeated.
The apparatus used for ventilation is called
ventilator.
INTRODUCTION
EFFECTS OF EXERCISE ON RESPIRATION
PULMONARY VENTILATION
DIFFUSING CAPACITY FOR OXYGEN
CONSUMPTION OF OXYGEN
OXYGEN DEBT
VO
2
MAX
Effects of Exercise
on Respiration
82
INTRODUCTION
Muscular exercise brings about a lot of changes
on various systems of the body. The degree of
changes depends upon the severity of exercise.
Refer Chapter 71 for types and severity of
exercise.
EFFECTS OF EXERCISE ON
RESPIRATION
EFFECT ON PULMONARY VENTILATION
Normal pulmonary ventilation is 6 L/minute. In
moderate exercise, it increases to about 60 L/
minute. In severe muscular exercise, it rises still
further up to 100 liters/minute.
EFFECT ON DIFFUSING CAPACITY
FOR OXYGEN
The diffusing capacity for oxygen is about
21 mL/minute at resting condition. It rises to
45 to 50 mL/minute during moderate exercise
due to increase in blood flow through the
pulmonary capillaries.
EFFECT ON CONSUMPTION OF OXYGEN
The oxygen consumed by the tissues,
particularly the skeletal muscles is increased
during exercise because of increased metabolic
activities.
OXYGEN DEBT
Oxygen debt is the extra amount oxygen required
by the muscles during recovery from severe mus-
cular exercise. After a period of severe muscular
exercise, the amount of oxygen consumed is
greatly increased. The oxygen required is more
than the quantity available to the muscle.
So, an extra amount of oxygen must be made
available in the body after the severe muscular
exercise. The oxygen debt is about six times

Chapter 82 Effects of Exercise on Respiration
497
497
more than the amount of oxygen consumed
under resting conditions.
VO
2
MAX
VO
2
Max is the amount of oxygen consumed
under maximal aerobic metabolism. It is the
product of maximal cardiac output and maximal
amount of oxygen consumed by the muscle.
In a normal active and healthy male, the
VO
2
Max is 35 to 40 mL/kg body weight/minute.
In females, it is 30 to 35 mL/kg/minute. There is
an increase of VO
2
Max by 50% during exer-
cise.

LONG QUESTIONS
1. Explain the transport of oxygen in blood.
2. Explain the transport of carbon dioxide in
blood.
3. Describe the nervous regulation of res-
piration.
4. Describe the chemical regulation of res-
piration.
5. What is hypoxia? Describe the types,
causes and effects of hypoxia. Add a note
on oxygen therapy.
SHORT QUESTIONS
1. Respiratory unit.
2. Respiratory membrane.
3. Nonrespiratory functions of respiratory
tract/lungs.
4. Characteristic features of pulmonary
circulation.
5. Surfactant.
6. Respiratory pressures.
7. Compliance.
8. Work of breathing.
9. Lung volumes/capacities/spirogram
10. Vital capacity.
11. Forced expiratory volume.
12. Alveolar ventilation.
QUESTIONS IN RESPIRATORY SYSTEM AND ENVIRONMENTAL
PHYSIOLOGY
13. Dead space.
14. Oxygen hemoglobin dissociation curve.
15. Carbon dioxide dissociation curve.
16. Bohr’s effect.
17. Haldane effect.
18. Chloride shift.
19. Diffusing capacity.
20. Exchange of gases between alveoli and
blood.
21. Exchange of gases between blood and
tissues.
22. Respiratory centers.
23. Inspiratory ramp.
24. Hering-Breuer reflex.
25. Chemoreceptors.
26. Apnea.
27. Hypoxia.
28. Asphyxia.
29. Dyspnea.
30. Periodic breathing.
31. Cyanosis.
32. Mountain sickness.
33. Acclimatization.
34. Decompression sickness.
35. Effects of sudden exposure to cold.
36. Effects of sudden exposure to heat.
37. Artificial respiration.
38. Respiratory changes during exercise.
Questions in Respiratory System and Environmental Physiology
498

Nervous System
83. Introduction to Nervous System ........................................ 501
84. Neuron and Neuroglia ....................................................... 504
85. Receptors .......................................................................... 514
86. Synapse and Neurotransmitters........................................ 519
87. Reflex Activity .................................................................... 526
88. Spinal Cord........................................................................ 532
89. Somatosensory System and Somatomotor System.......... 547
90. Physiology of Pain ............................................................. 555
91. Thalamus .......................................................................... 558
92. Hypothalamus ................................................................... 561
93. Cerebellum ........................................................................ 569
94. Basal Ganglia .................................................................... 575
95. Cerebral Cortex and Limbic System ................................. 579
96. Reticular Formation ........................................................... 589
97. Posture and Equilibrium .................................................... 592
98. Vestibular Apparatus ......................................................... 599
99. Electroencephalogram and Epilepsy ................................. 606
100. Physiology of Sleep........................................................... 609
101. Higher Intellectual Functions ............................................. 612
102. Cerebrospinal Fluid ........................................................... 617
103. Autonomic Nervous System……………………………… .. 621
S E C T I O N
10
C H A P T E R S

DIVISIONS OF NERVOUS SYSTEM
CENTRAL NERVOUS SYSTEM
PERIPHERAL NERVOUS SYSTEM
Introduction to
Nervous System
83
DIVISIONS OF NERVOUS SYSTEM
Nervous system controls all the activities of the
body. It is quicker than the other control system
in the body namely, the endocrine system.
Primarily, the nervous system is divided into two
parts.
1. Central nervous system
2. Peripheral nervous system.
CENTRAL NERVOUS SYSTEM
The central nervous system (CNS) includes brain
and spinal cord. It is formed by neurons and the
supporting cells called neuroglia. The structures
of brain and spinal cord are arranged in two
layers, namely, the gray matter and white matter.
The gray matter is formed by nerve cell bodies
and the proximal parts of nerve fibers arising from
the nerve cell body. The white matter is formed
by nerve fibers.
In brain the white matter is centrally placed
and gray matter is in the outer part. In spinal cord
white matter is in the outer part and gray matter
is in the inner part.
Brain is situated in the skull. It is continued
as spinal cord in the vertebral canal through the
foramen magnum of the skull bone. Brain and
spinal cord are surrounded by three layers of
meninges called the outer dura mater, middle
arachnoid mater and inner pia mater. The space
between the arachnoid mater and pia mater is
known as subarachnoid space. This space is
filled with a fluid called cerebrospinal fluid
(CSF). The brain and spinal cord are actually
suspended in CSF. The important parts of brain
and segments of spinal cord are shown in
Figure 83-1.
Parts of Brain
Brain consists of three major divisions:
1. Prosencephalon
2. Mesencephalon
3. Rhombencephalon
1. Prosencephalon
It is otherwise known as forebrain. It is further
divided into two parts:
i. Telencephalon which includes cerebral
hemispheres, basal ganglia, hippo-
campus and amygdaloid nucleus.
ii. Diencephalon which consists of thalamus,
hypothalamus, metathalamus and sub-
thalamus.
2. Mesencephalon
It is also known as midbrain.
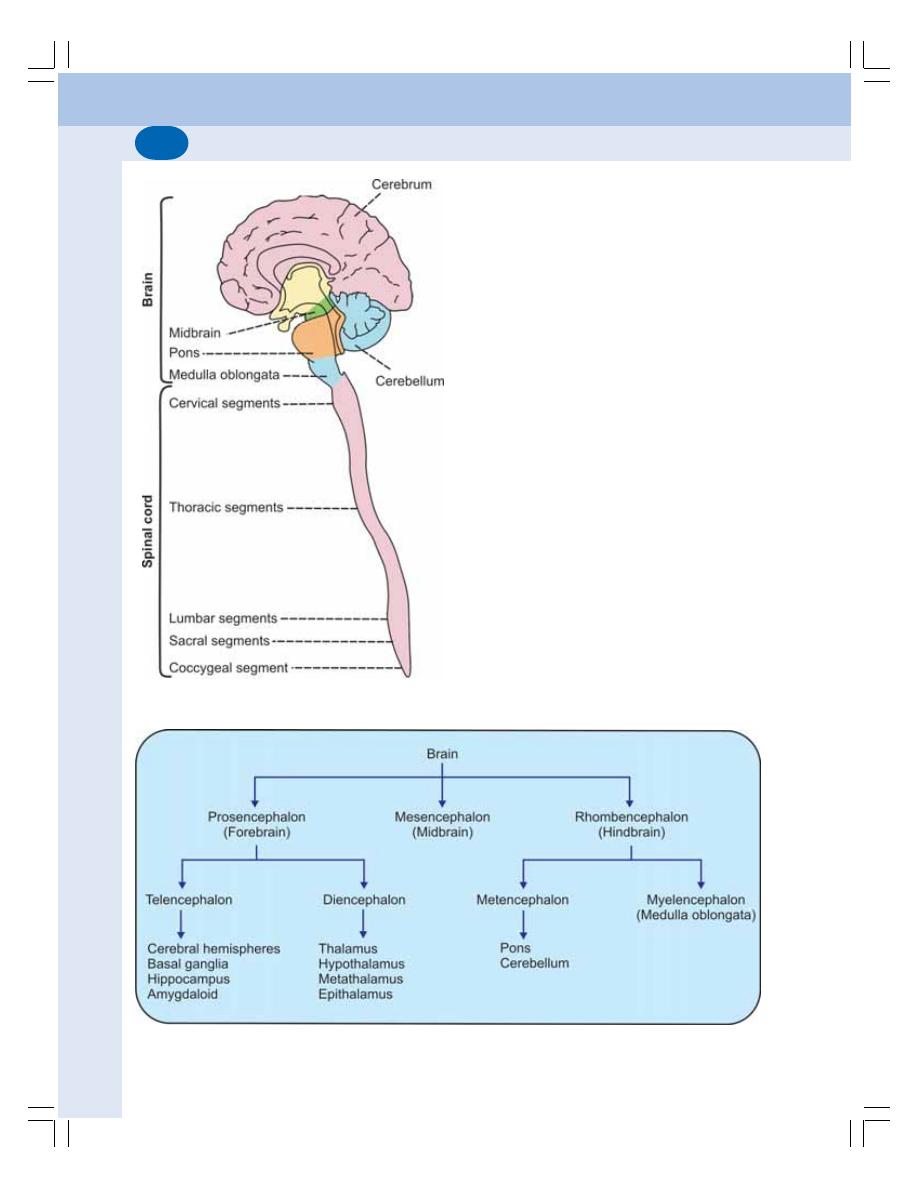
Nervous System
502
3. Rhombencephalon
Rhombencephalon or hindbrain is subdivided into
two portions:
i. Metencephalon formed by pons and
cerebellum
ii. Myelencephalon or medulla oblongata
(Fig. 83-2).
Midbrain, pons and medulla oblongata are
together called the brainstem.
PERIPHERAL NERVOUS SYSTEM
The peripheral nervous system (PNS) is formed
by the neurons and their processes present in
all regions of the body. It consists of cranial
nerves arising from brain and spinal nerves
arising from the spinal cord. It is again divided
into two subdivisions:
1. Somatic nervous system
2. Autonomic nervous system.
1. Somatic Nervous System
The somatic nervous system is concerned with
somatic functions. It includes the nerves
supplying the skeletal muscles. Somatic
nervous system controls the movements of
the body by acting on the skeletal muscles
(Fig. 83-3).
FIGURE 83-1: The parts of central nervous
system
FIGURE 83-2: The parts of brain
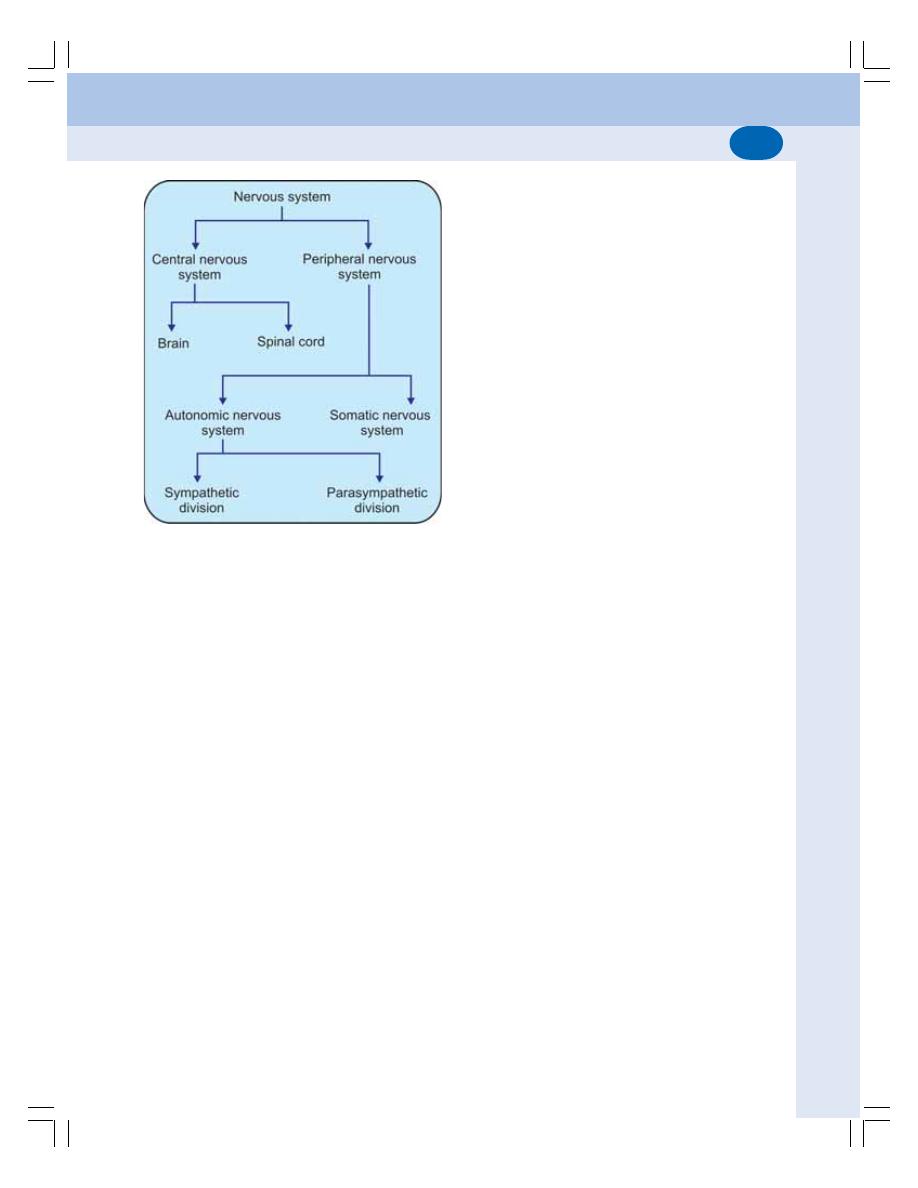
Chapter 83 Introduction to Nervous System
503
2. Autonomic Nervous System
The autonomic nervous system is concerned with
regulation of visceral or vegetative functions. So,
it is otherwise called vegetative or involuntary
nervous system. The autonomic nervous system
consists of two divisions, sympathetic division
and parasympathetic division.
FIGURE 83-3: Organization of nervous system
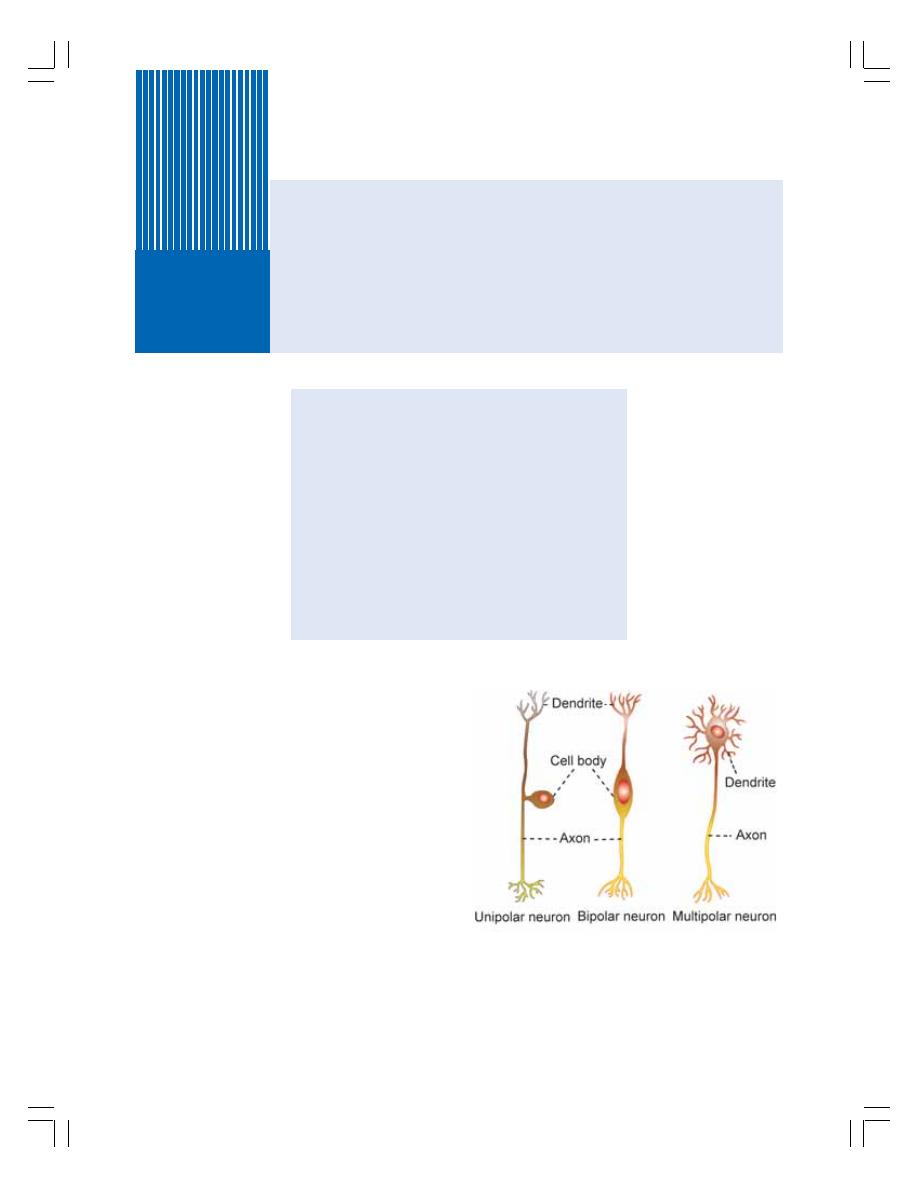
NEURON
CLASSIFICATION OF NEURON
STRUCTURE OF NEURON
NEUROTROPHINS
CLASSIFICATION OF NERVE FIBERS
PROPERTIES OF NERVE FIBERS
DEGENERATION OF NERVE FIBERS
REGENERATION OF NERVE FIBERS
NEUROGLIA
DEFINITION
CLASSIFICATION
Neuron and Neuroglia
84
NEURON
Neuron is defined as the structural and func-
tional unit of the nervous system. It is otherwise
called nerve cell. Neuron is like any other cell
in the body having nucleus and all the organelles
in the cytoplasm. However, it is different from
other cells by two ways:
1. Neuron has branches or processes called
axon and dendrites
2. Neuron does not have centrosome; so it can-
not undergo division.
CLASSIFICATION OF NEURON
The neurons are classified by three different
methods.
I. Depending upon number of poles
II. Depending upon function
III. Depending upon length of the axon.
Depending upon Number of Poles
Based on the number of poles from which the
nerve fibers arise, neurons are divided into three
types:
FIGURE 84-1: Types of neuron

Chapter 84 Neuron and Neuroglia
505
1. Unipolar neurons that have only one pole
from which, both the axon and dendrite arise
(Fig. 84-1)
2. Bipolar neurons which have two poles. Axon
arises from one pole and dendrites arise from
the other pole.
3. Multipolar neurons which have many poles.
One of the poles gives rise to the axon and,
all the other poles give rise to dendrites.
Depending upon Function
On the basis of function, the nerve cells are
classified into two types:
1. Motor neurons or efferent neurons which
carry the motor impulses from central
nervous system to the peripheral effector
organs like muscles, glands, blood vessels,
etc.
2. Sensory neurons or afferent neurons which
carry the sensory impulses from periphery to
the central nervous system.
Depending upon Length of Axon
Depending upon the length of axon, neurons are
divided into two types:
1. Golgi type I neurons that have long axons.
The cell body of these neurons is in central
nervous system and their axons reach the
remote peripheral organs
2. Golgi type II neurons that have short axons.
These neurons are present in cerebral cortex
and spinal cord.
STRUCTURE OF NEURON
Each neuron is made up of three parts:
1. Nerve cell body
2. Dendrite
3. Axon.
The dendrite and axon together form the
processes of neuron (Fig. 84-2). In general, the
dendrites are short processes and the axons are
long processes. The dendrites and axons are
usually called nerve fibers.
Nerve Cell Body
The nerve cell body is also known as soma or
perikaryon. It is irregular in shape and, it is
constituted by a mass of cytoplasm called
neuroplasm which is covered by a cell mem-
brane. The cytoplasm contains a large nucleus,
Nissl bodies, neurofibrils, mitochondria and Golgi
apparatus. Nissl bodies and neurofibrils are
found only in nerve cell and not in other cells.
Nucleus
Each neuron has one nucleus which is centrally
placed in the nerve cell body. The nucleus has
one or two prominent nucleoli. The nucleus does
not contain centrosome. So, the nerve cell cannot
multiply like the other cells.
Nissl bodies
Nissl bodies or Nissl granules are small baso-
philic granules found in cytoplasm of neurons
and are named after the discoverer. These
bodies are present in the soma except in axon
hillock. Nissl bodies are called tigroid sub-
stances since these bodies are responsible for
the tigroid or spotted appearance of soma after
suitable stain-ing. The Nissl granules flow into
the dendrites from soma, but not into axon. So,
the dendrites are distinguished from axons by
the presence of Nissl granules under micro-
scope.
The Nissl bodies are membranous organelles
containing ribosomes. So, these bodies are con-
cerned with synthesis of proteins in the neurons.
The proteins formed in soma are transported to
the axon by axonal flow.
Neurofibrils
Neurofibrils are thread like structures present in
the form of network in the soma and the nerve
FIGURE 84-2: Structure of a neuron
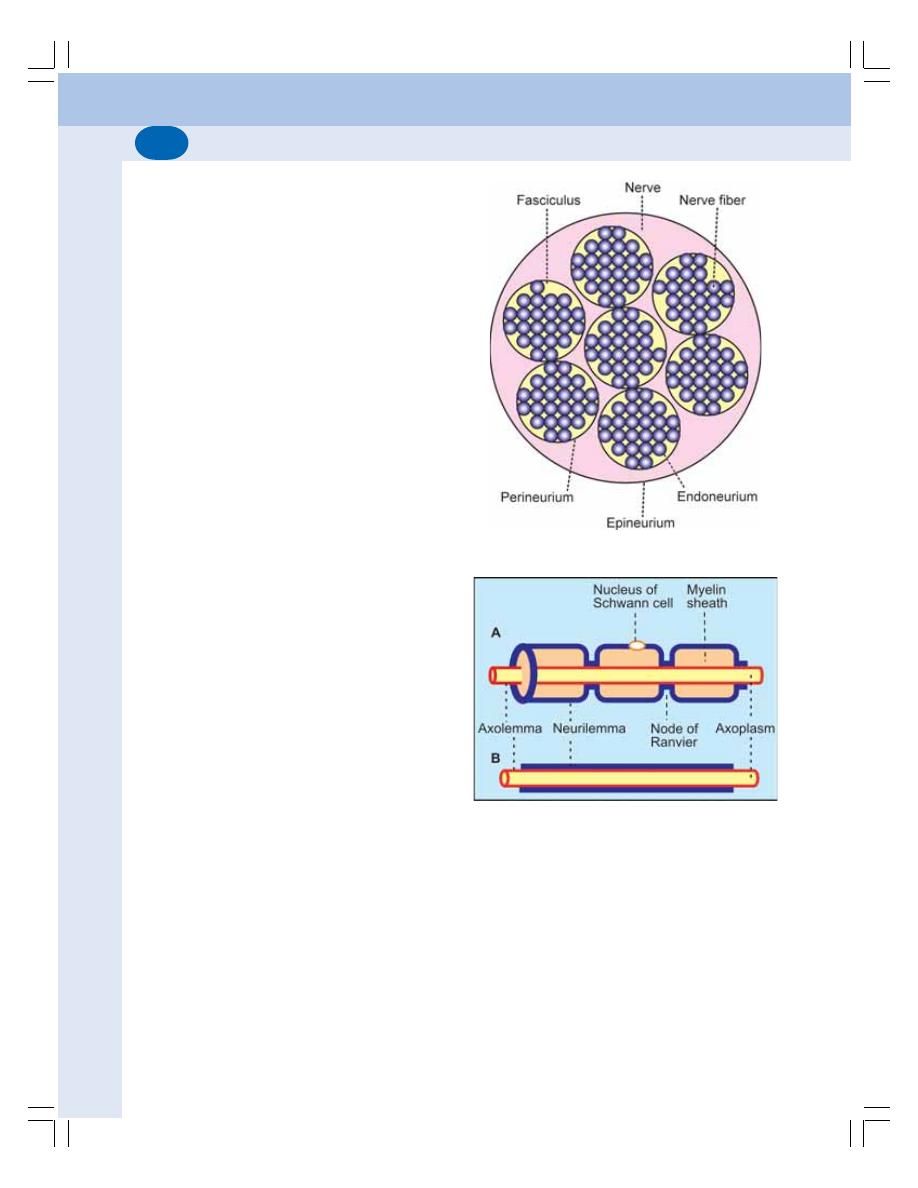
Nervous System
506
processes. Presence of neurofibrils is another
characteristic feature of the neurons.
Mitochondria
The mitochondria are present in the soma and
in axon. As other cells, the mitochondria form
the powerhouse of the nerve cell, where ATP is
produced (Chapter 1).
Golgi apparatus
Golgi apparatus of the nerve cell body is similar
to that of other cells. It is concerned with pro-
cessing and packaging of proteins into granules
(Chapter 1).
Dendrite
The dendrite is the branched process of the
neuron and it is branched repeatedly. The den-
drite may be present or absent. If present, it may
be one or many in number. The dendrite has
Nissl granules and neurofibrils.
Dendrite is conductive in nature. It transmits
impulses towards the nerve cell body.
Axon
The axon is longer than dendrite. Each neuron
has only one axon. The axon arises from axon
hillock of the nerve cell body. The axon extends
for a long distance away from the nerve cell body.
The length of the longest axon is about one
meter.
Organization of nerve
Many axons together form a bundle called
fasciculus. Many fasciculi together form a nerve.
The whole nerve is covered by tubular sheath,
which is formed by areolar membrane. This
sheath is called epineurium. Each fasciculus is
covered by perineurium and each nerve fiber
(axon) is covered by endoneurium (Fig. 84-3).
Internal structure of axon — Axis cylinder
The axon has a long central core of cytoplasm
called axoplasm. The axoplasm is covered by
the tubular sheath like membrane called
FIGURE 84-3: Cross section of a nerve
FIGURE 84-4: A. Myelinated nerve fiber
B. Non-myelinated nerve fiber
axolemma which is the continuation of the cell
membrane of nerve cell body. The axoplasm
along with the axolemma is called the axis
cylinder of the nerve fiber (Fig. 84-4).
Axoplasm contains mitochondria, neurofibrils
and axoplasmic vesicles. But, Nissl bodies are
absent in the axon. The axis cylinder of the nerve
fiber is covered by a membrane called neuri-
lemma (see below).

Chapter 84 Neuron and Neuroglia
507
Nonmyelinated nerve fiber
The nerve fiber described above is the non-
myelinated nerve fiber which is not covered by
myelin sheath.
Myelinated nerve fiber
The nerve fibers which are insulated by myelin
sheath are called myelinated nerve fibers.
Myelin Sheath
Myelin sheath is a thick lipoprotein sheath that
insulates the myelinated nerve fiber. Myelin
sheath is not a continuous sheath. It is absent
at regular intervals. The area where the myelin
sheath is absent is called node of Ranvier. The
segment of the nerve fiber between two nodes
is called internode. Myelin sheath is responsible
for the white color of the nerve fibers.
Chemistry of myelin sheath
Myelin sheath is formed by concentric layers of
proteins alternating with lipids. The lipids are
cholesterol, lecithin and cerebroside
(sphingomyelin).
Formation of myelin sheath — Myelinogenesis
The formation of myelin sheath around the axon
is called the myelinogenesis. It is formed by
Schwann cells in neurilemma.
Functions of myelin sheath
1. Faster conduction: Myelin sheath is responsi-
ble for faster conduction of impulse through
the nerve fibers. In the myelinated nerve
fibers, the impulses jump from one node to
another node by saltatory conduction.
2. Insulating capacity: Myelin sheath has a high
insulating capacity. Because of this quality,
the myelin sheath restricts the nerve impulse
within the single nerve fiber, and prevents the
stimulation of neighboring nerve fibers.
Neurilemma
Neurilemma is a thin membrane which surrounds
the axis cylinder. It is also called neurilemmal
sheath or sheath of Schwann. It contains Sch-
wann cells, which have flattened and elongated
nuclei. The cytoplasm is thin and modified to form
the thin sheath of neurilemma.
One nucleus is present in each internode of
the axon. The nucleus is situated between myelin
sheath and neurilemma.
In nonmyelinated nerve fiber, the neurilemma
continuously surrounds axolemma. In myelinated
nerve fiber, it covers the myelin sheath. At the
node of Ranvier (where myelin sheath is absent),
the neurilemma invaginates and runs up to axo-
lemma in the form of a finger like process.
Functions of neurilemma
In nonmyelinated nerve fiber, the neurilemma
serves as a covering membrane. In myelinated
nerve fiber, it is necessary for the formation of
myelin sheath (myelinogenesis).
NEUROTROPHINS — NEUROTROPHIC
FACTORS
Neurotrophins or neurotrophic factors are the
protein substances, which play important role in
growth and functioning of nervous tissue.
Neurotrophins are secreted by many tissues in
the body particularly muscles, neuroglial cells
and neurons.
Nerve Growth Factor
Nerve growth factor (NGF) is an important
neurotrophin found in many peripheral tissues.
It promotes early growth and development of
neurons.
The commercial preparation of NGF extrac-
ted from snake venom and submaxillary glands
of male mouse is used to treat many nervous
disorders such as Alzheimer’s disease, neuron
degeneration in aging and neuron regeneration
in spinal cord injury.
CLASSIFICATION OF NERVE FIBERS
The nerve fibers are classified by different
methods. The basis of classification differs in
each method. Nerve fibers are classified by six
methods:
Nervous System
508
1. Depending upon structure
2. Depending upon distribution
3. Depending upon origin
4. Depending upon function
5. Depending upon secretion of neurotransmitter
6. Depending upon diameter and conduction of
impulse (Erlanger-Gasser classification).
1. Depending upon Structure
Based on the structure, the nerve fibers are
classified into two types:
i. Myelinated nerve fibers that are covered
by myelin sheath
ii. Nonmyelinated nerve fibers which are not
covered by myelin sheath.
2. Depending upon Distribution
Nerve fibers are classified into two types on the
basis of the distribution:
i. Somatic nerve fibers which supply the
skeletal muscles of the body
ii. Visceral or autonomic nerve fibers which
supply internal organs of the body.
3. Depending upon Origin
On the basis of origin, the nerve fibers are divided
into two types:
i. Cranial nerves arising from brain
ii. Spinal nerves arising from spinal cord.
4. Depending upon Function
Functionally, the nerve fibers are of two types:
i. Sensory or afferent nerve fibers which
carry sensory impulses from different
parts of the body to the central nervous
system
ii. Motor or efferent nerve fibers which carry
motor impulses from central nervous sys-
tem to different parts of the body.
5. Depending upon Secretion of
Neurotransmitter
Depending upon the neurotransmitter substance
secreted, the nerve fibers are divided into two
types:
i. Adrenergic nerve fibers that secrete
noradrenaline
ii. Cholinergic nerve fibers that secrete
acetylcholine.
6. Depending upon Diameter and
Conduction of Impulses
Erlanger and Gasser classified the nerve fibers
into three major types on the basis of diameter
of the fibers and the rate of conduction of impul-
ses:
1. Type A nerve fibers
2. Type B nerve fibers
3. Type C nerve fibers.
Among these fibers, type A nerve fibers are
the thickest fibers and type C nerve fibers are
the thinnest fibers. Type A nerve fibers are
divided into four subtypes. Except ‘C’ type of
fibers, all the nerve fibers are myelinated.
The velocity of impulse through a nerve fiber
is directly proportional to the thickness of the
fibers. The different types of nerve fibers along
with diameter and velocity of conduction are
given in the Table 84-1.
PROPERTIES OF NERVE FIBERS
Excitability
Excitability is defined as the physiochemical
change that occurs in a tissue when a stimulus
is applied.
The stimulus is defined as an external agent,
which produces excitability in the tissues. When
TABLE 84-1: Types of nerve fibers
Type
Diameter (
μ)
Velocity of
conduction
(meters/second)
A alpha
12 to 24
70 to 120
A Beta
6 to 12
30 to
70
A gamma
5 to
6
15 to
30
A delta
2 to
5
12 to
15
B
1 to
2
3 to
10
C
< 1.5
0.5 to
2
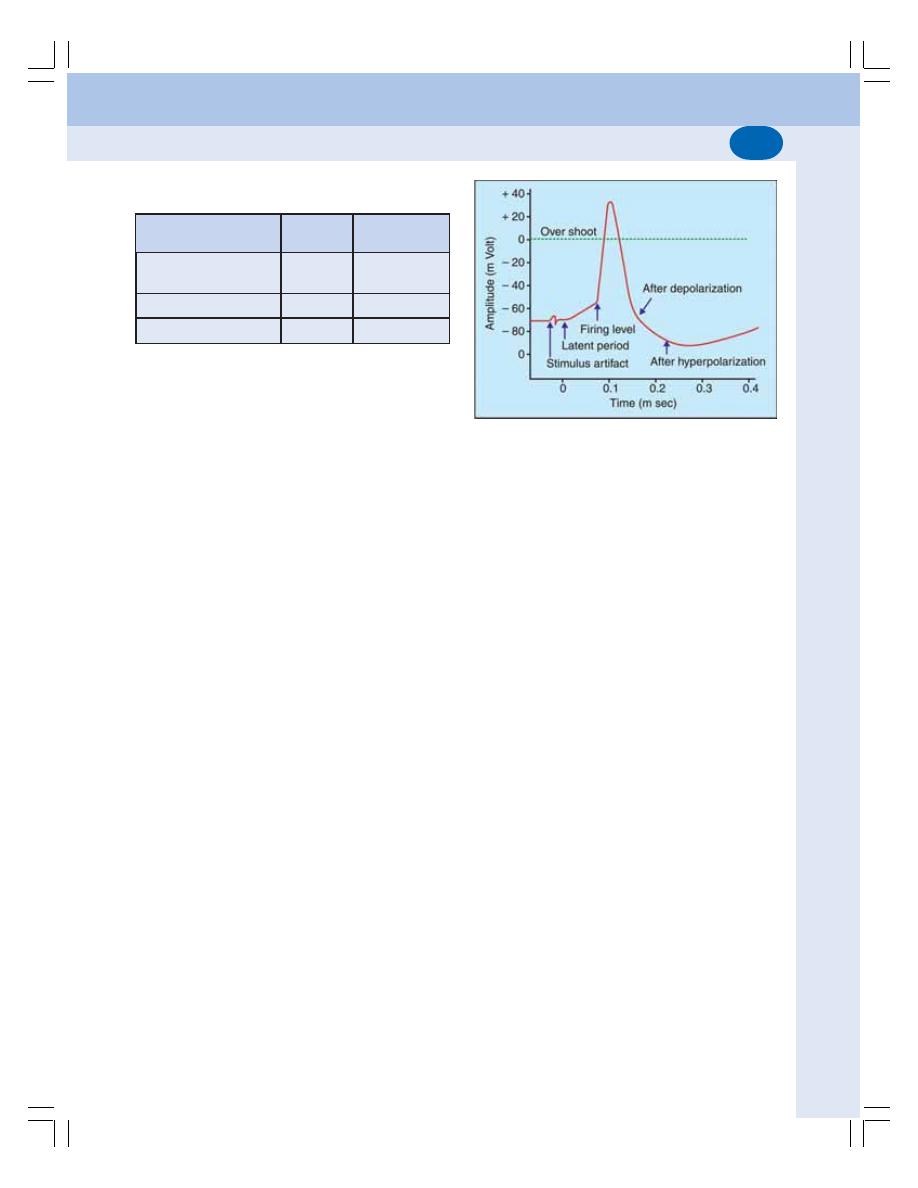
Chapter 84 Neuron and Neuroglia
509
the nerve fiber is stimulated action potential
develops.
Action potential or nerve impulse
The action potential in a nerve fiber is similar to
that in a muscle, except for some minor diffe-
rences (Table 84-2). The action potential in a
skeletal muscle fiber is described in Chapter 23.
The resting membrane potential in the nerve
fiber is –70 mV. The firing level is at –55 mV.
Depolarization ends at +35 mV (Fig. 84-5).
Usually, the action potential starts in the initial
segment of nerve fiber.
Conductivity
Conductivity is the ability of nerve fibers to
transmit the impulse from the area of stimula-
tion to the other areas. The action potential is
transmitted through the nerve fiber as nerve
impulse. Normally in the body, the action
potential is transmitted through the nerve fiber
in only one direction.
Mechanism of conduction of action potential
The depolarization occurs first at the site of
stimulation in the nerve fiber. It causes depo-
larization of the neighboring areas. Like this,
depolarization travels throughout the nerve fiber.
Depolarization is followed by repolarization.
Conduction through myelinated nerve
fiber — Saltatory conduction
Saltatory conduction is the form of conduction
of nerve impulse in which, the impulse jumps
from one node to another. Conduction of impulse
through a myelinated nerve fiber is about 50
times faster than through a nonmyelinated fiber.
It is because the action potential jumps from
one node to another node of Ranvier instead
of travelling through the entire nerve fiber
(Fig. 84-6).
Mechanism of saltatory conduction
The myelin sheath is not permeable to ions. So,
the entry of sodium from extracellular fluid into
nerve fiber occurs only in the node of Ranvier,
where the myelin sheath is absent. It causes
depolarization in the node, and not in the inter-
node. Thus, the depolarization occurs at succes-
sive nodes. So, the action potential jumps from
one node to another. Hence, it is called saltatory
conduction (saltare = jumping).
Refractory Period
Refractory period is the period at which the
nerve does not give any response to a stimulus.
Refractory period is of two types:
1. Absolute refractory period
Absolute refractory period is the period during
which the nerve does not show any response at
all, whatever may be the strength of stimulus.
2. Relative refractory period
It is the period, during which the nerve fiber
shows response, if the strength of stimulus is
increased to maximum.
TABLE 84-2: Differences between electrical
potential in nerve fiber and muscle fiber
Event
Nerve
Skeletal
fiber
muscle fiber
Resting membrane
– 70 mV
– 90 mV
potential
Firing level
– 55 mV
– 75 mV
End of depolarization
+ 35 mV
+ 55 mV
FIGURE 84-5: Action potential in nerve fiber
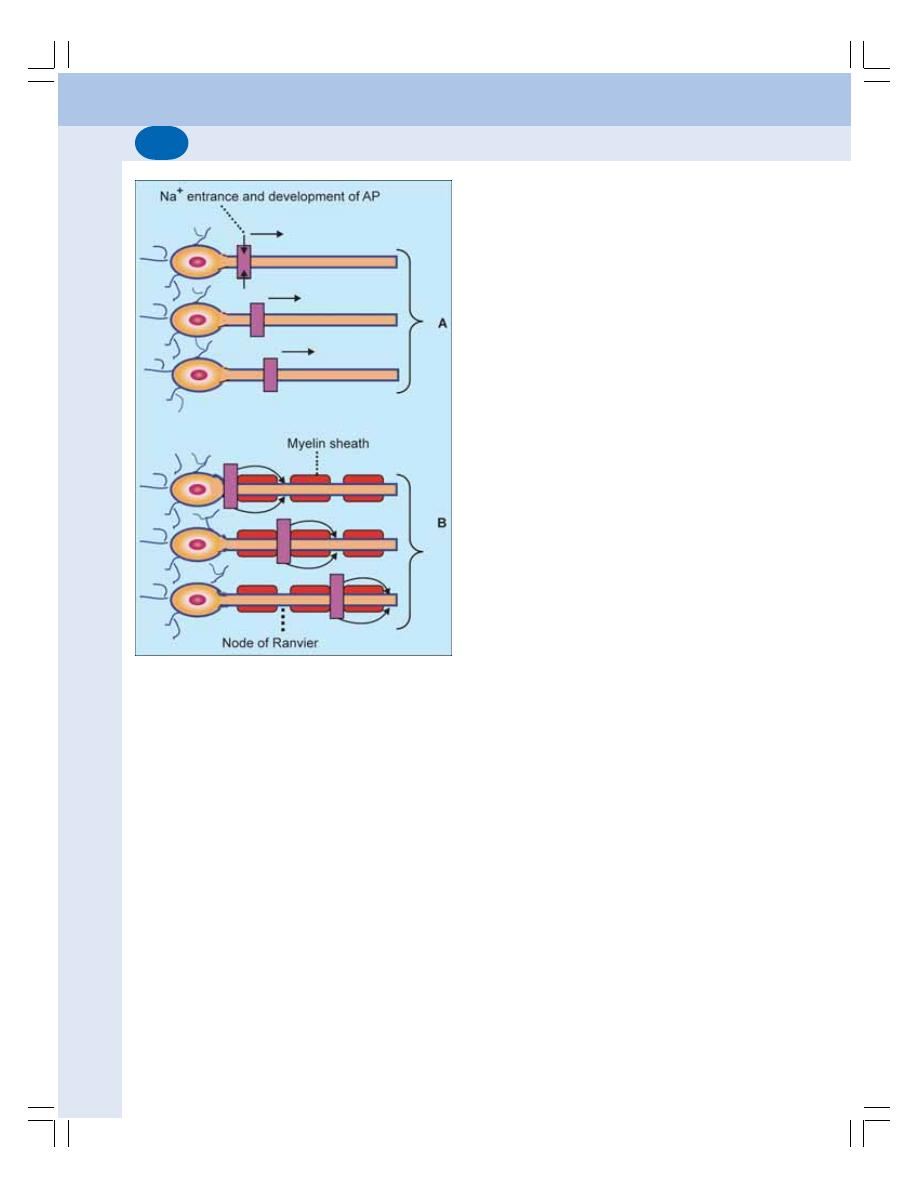
Nervous System
510
Absolute refractory period corresponds to
the period from the time when firing level is
reached till the time when 1/3 of repolarization
is completed. The relative refractory period
extends through rest of the repolarization period.
Summation
When one subliminal stimulus is applied, it does
not produce any response in the nerve fiber
because, the subliminal stimulus is very weak.
However, if two or more subliminal stimuli are
applied within a short interval of about 0.5 m sec,
the response is produced. It is because the
subliminal stimuli are summed up together to
become strong enough to produce the response.
This phenomenon is known as summation.
Adaptation
While stimulating a nerve fiber continuously, the
excitability of the nerve fiber is greater in the
beginning. Later the response decreases slowly
and finally the nerve fiber does not show any
response at all. This phenomenon is known as
adaptation or accommodation.
The causes for adaptation are:
1. When a nerve fiber is stimulated continuously,
depolarization occurs continuously
2. The continuous depolarization inactivates the
sodium pump and increases the efflux of
potassium ions.
Infatigability
A nerve fiber cannot be fatigued, even if it is
stimulated continuously for a long time. The
reason for this is the nerve fiber can conduct only
one action potential at a time. At that time, it is
completely refractory and does not conduct
another action potential.
All or None Law
All or none law states that when a nerve is
stimulated by a stimulus it gives maximum res-
ponse or does not give response at all. Refer
Chapter 59 for more details on all or none law.
DEGENERATION OF NERVE FIBERS
When a nerve fiber is injured, various changes
occur in the nerve fiber and nerve cell body. All
these changes are together called the de-
generative changes. The injury occurs due to
the obstruction of blood flow, local injection of
toxic substances, crushing of nerve fiber or the
transection of the fiber.
Degenerative Changes in the Neuron
Degeneration refers to deterioration or impair-
ment or pathological changes of an injured
tissue. When a peripheral nerve fiber is injured,
the degenerative changes occur in the nerve cell
FIGURE 84-6: Mode of conduction
through nerve fibers
A. Nonmyelinated nerve fiber — Continuous conduc-
tion B. Myelinated nerve fiber — Saltatory conduction:
Impulse jumps from node to node. AP = Action potential

Chapter 84 Neuron and Neuroglia
511
body and the nerve fiber same neuron and the
adjoining neuron. Accordingly, the degenerative
changes are classified into three types:
1. Wallarian degeneration
2. Retrograde degeneration
3. Transneural degeneration.
Wallerian or Orthograde Degeneration
Wallerian or orthograde degeneration is the
pathological change that occurs in the distal cut
end of nerve fiber (axon). It is named after the
discoverer, Waller. Wallerian degeneration starts
within 24 hours of injury. The change occurs
throughout the length of distal part of nerve fiber
simultaneously.
1. Axis cylinder swells and breaks up into small
pieces. After few days, the broken pieces
appear as debris in the space occupied by
axis cylinder (Fig. 84-7).
2. The myelin sheath is slowly disintegrated into
fat droplets. The changes in myelin sheath
occur from 8th to 35th day.
3. The neurilemmal sheath is unaffected, but
the Schwann cells multiply rapidly. The
macrophages invade from outside. The
macrophages remove the debris of axis
cylinder and the fat droplets of disintegrated
myelin sheath. So, the neurilemmal tube
becomes empty. Later it is filled by the
cytoplasm of Schwann cell. All these changes
take place for about 2 months from the day
of injury.
Retrograde Degeneration
Retrograde degeneration is the pathological
changes which occur in the nerve cell body and
axon proximal to the cut end.
FIGURE 84-7: Degeneration and regeneration of nerve fiber
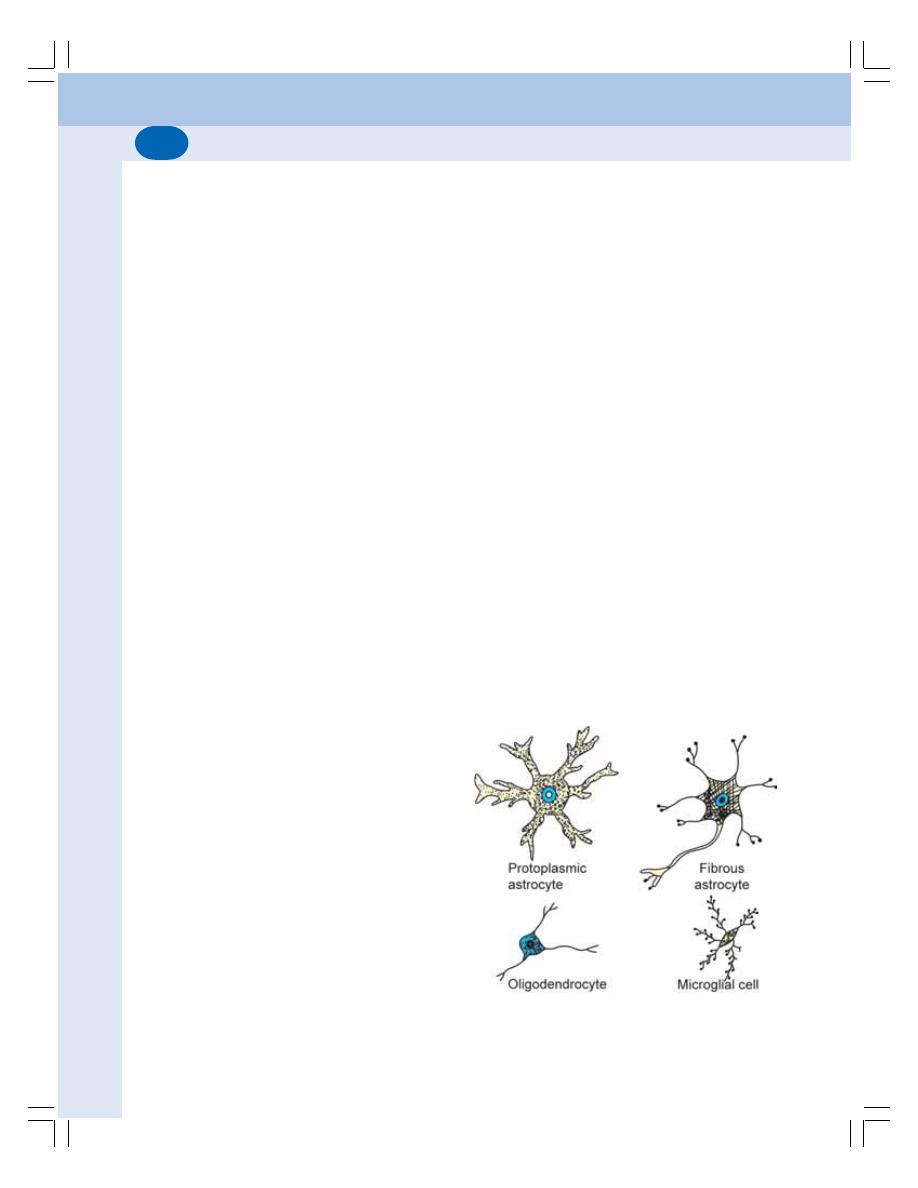
Nervous System
512
Transneuronal Degeneration
If an afferent nerve fiber is cut, the degenerative
changes occur in the neuron with which the
afferent nerve fiber synapses. It is called trans-
neuronal degeneration.
REGENERATION OF NERVE FIBER
The term regeneration refers to regrowth of lost
or destroyed part of a tissue. The injured and
degenerated nerve fiber can regenerate. It starts
as early as 4th day after injury, but, becomes
more effective only after 30 days and is com-
pleted in about 80 days.
Criteria for Regeneration
Regeneration is possible only if certain criteria
are fulfilled by the degenerated nerve fiber:
1. The gap between the cut ends of the nerve
should not exceed 3 mm
2. The neurilemma should be present
3. The nucleus must be intact
4. The two cut ends should remain in the same
line.
Stages of Regeneration
1. First, some pseudopodia like extensions
called fibrils grow from the proximal cut end
of the nerve and move towards the distal cut
end of the nerve fiber
2. Some of the fibrils enter the neurilemmal tube
of distal end and form axis cylinder
3. Schwann cells line up in the neurilemmal
tube and guide the fibrils into the tube
4. The axis cylinder is formed inside the neuri-
lemmal tube in about 3 months after injury
5. The myelin sheath is formed by Schwann
cells slowly and it is completed in one year
6. In the nerve cell body, first the Nissl granules
appear followed by Golgi apparatus
7. The cell looses the excess fluid. The nucleus
occupies the central portion
Though the anatomical regeneration occurs
in the nerve, the functional recovery occurs after
a long period.
NEUROGLIA
DEFINITION
Neuroglia or the glia (glia = glue) is the
supporting cell of the nervous system. The
neuroglial cells are non-excitable and do not
transmit nerve impulse (action potential). So,
these cells are also called non-neural cells or
glial cells.
CLASSIFICATION OF NEUROGLIAL
CELLS
The neuroglial cells are distributed in central
nervous system (CNS) as well as peripheral
nervous system (PNS).
Central Neuroglial Cells
The neuroglial cells in CNS are of three types:
1. Astrocytes
2. Microglia
3. Oligodendrocytes.
1. Astrocytes
Astrocytes are star shaped neuroglial cells pre-
sent in all the parts of the brain (Fig. 84-8).
Astocytes are of two types, fibrous astrocytes
and protoplasmic astrocytes.
FIGURE 84-8: Neuroglial cells in CNS

Chapter 84 Neuron and Neuroglia
513
Astrocytes:
i. Twist around the nerve cells and form the
supporting network in brain and spinal
cord
ii. Form the blood-brain barrier and thereby
regulate the entry of substances from
blood into brain tissues (Chapter 102)
iii. Maintain the chemical environment of ECF
around CNS neurons
iv. Provide calcium and potassium and regu-
late neurotransmitter level in synapses
2. Microglia
Microglia are the smallest neuroglial cells. These
cells are derived from monocytes and enter the
tissues of nervous system from blood. These
phagocytic cells migrate to the site of infection
or injury and are often called the macrophages
of CNS.
Microglia engulf and destroy the micro-
organisms and cellular debris by means of
Phagocytosis.
3. Oligodendrocytes
Oligodendrocytes are neuroglial cells which
produce myelin sheath around nerve fibers in
CNS.
Oligodendrocytes provide myelination around
the nerve fibers in CNS where Schwann cells
are absent
Peripheral Neuroglial Cells
The neuroglial cells in PNS are of two types:
1. Schwann cells
2. Satellite cells
1. Schwann Cells
Schwann cells are the major glial cells in
PNS.
Schwann cells provide myelination (insula-
tion) around the nerve fibers in PNS. These cells
remove cellular debris during regeneration by
their phagocytic activity.
2. Satellite Cells
Satellite cells are the glial cells present on the
exterior surface of PNS neurons.
Satellite cells provide physical support to the
PNS neurons.
DEFINITION
CLASSIFICATION
EXTEROCEPTORS
INTEROCEPTORS
PROPERTIES
SPECIFICITY OF RESPONSE
ADAPTATION — SENSORY ADAPTATION
RESPONSE TO INCREASE IN THE STRENGTH OF STIMULUS
SENSORY TRANSDUCTION
RECEPTOR POTENTIAL
Receptors
85
DEFINITION
Receptors are the sensory (afferent) nerve
endings that terminate in the periphery as bare
unmyelinated nerve endings or in the form of
specialized capsulated structures. When
stimulated, receptors produce a series of
impulses which are transmitted through the
afferent nerves.
Actually receptors function like a transducer.
Transducer is a device, which converts one form
of energy into another.
So, the receptors are often defined as the
biological transducers which convert various
forms of energy (stimuli) in the environment into
action potentials in nerve fiber.
CLASSIFICATION OF RECEPTORS
Generally, the receptors are classified into two
types:
I. Exteroceptors
II. Interoceptors.
EXTEROCEPTORS
Exteroceptors are the receptors which give
response to stimuli arising from outside the body.
The exteroceptors are divided into three
groups.
1. Cutaneous Receptors
The receptors situated in the skin are called the
cutaneous receptors. Cutaneous receptors are
also called mechanoreceptors because of their
response to mechanical stimuli such as touch,
pressure and pain (Fig. 85-1). Touch and
pressure receptors give response to vibration
also. The different types of cutaneous receptors
are given in Figure 85-2.
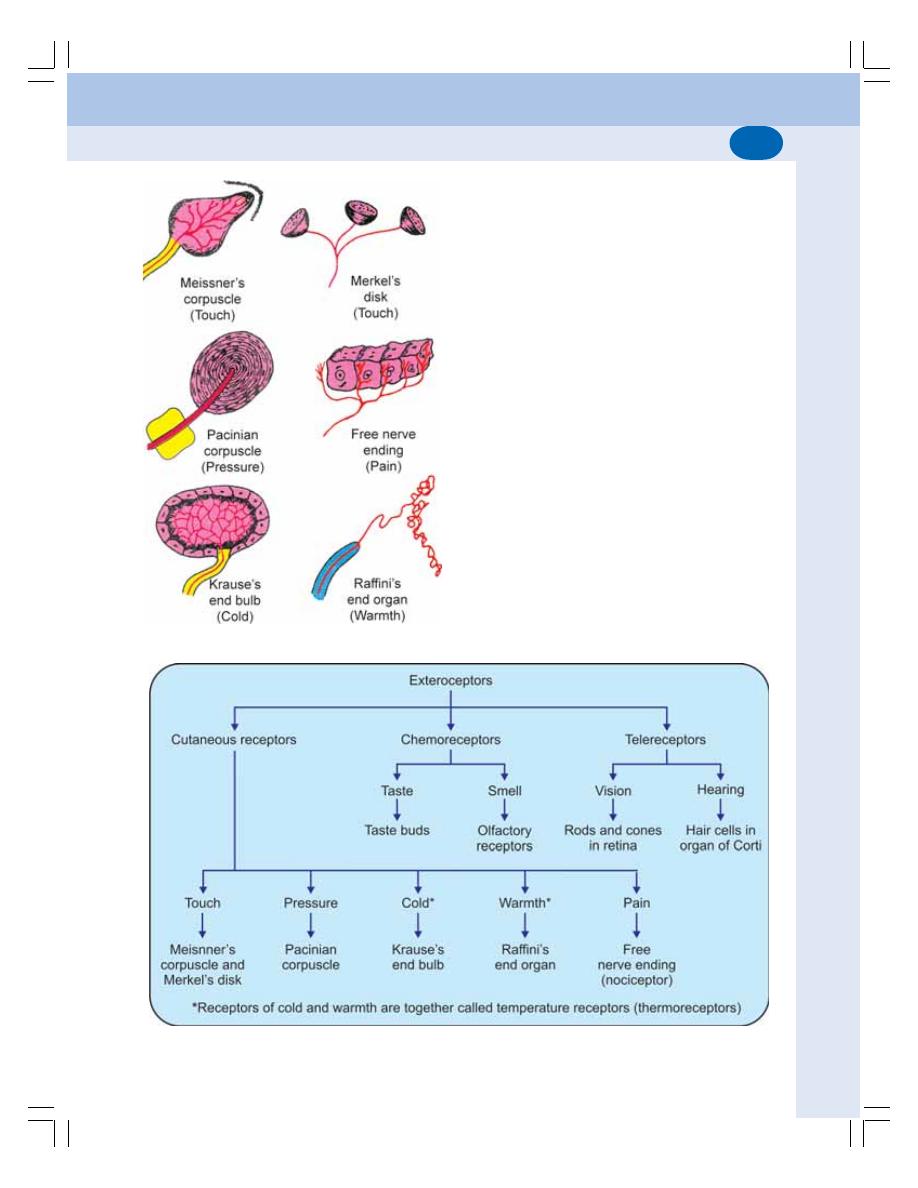
Chapter 85 Receptors
515
2. Chemoreceptors
The receptors, which give response to chemical
stimuli, are called the chemoreceptors.
Examples are given in Figure 85-2.
3. Telereceptors
Telereceptors are the receptors that give
response to stimuli arising away from the
body. These receptors are also called the
distance receptors. Examples are given in
Figure 85-2.
INTEROCEPTORS
Interoceptors are the receptors which give
response to stimuli arising from within the body.
Interoceptors are of two types:
1. Visceroceptors
Receptors situated in the viscera are called
visceroceptors. Visceroceptors are listed in
Figure 85-3.
FIGURE 85-1: Cutaneous receptors
FIGURE 85-2: Exteroceptors
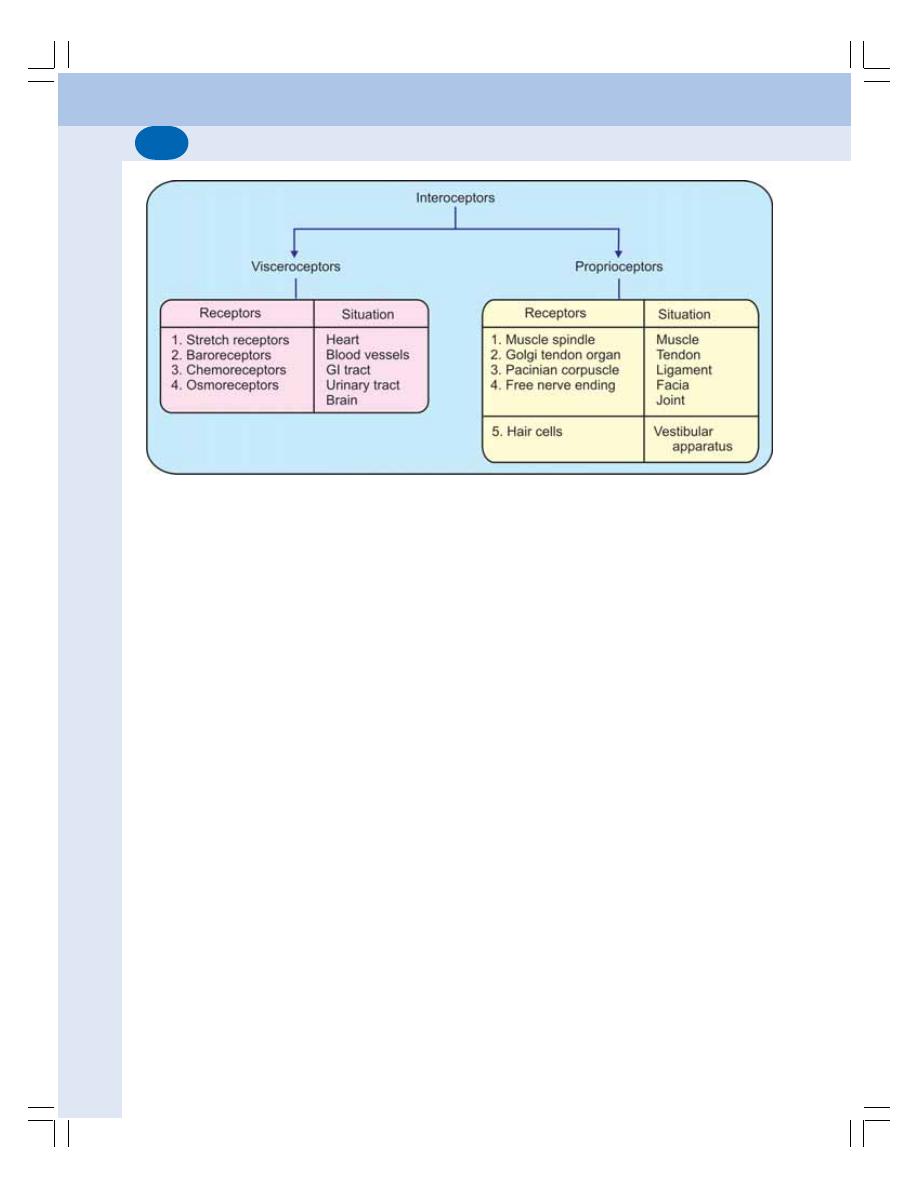
Nervous System
516
2. Proprioceptors
Proprioceptors are the receptors which give
response to change in the position of different
parts of the body (Chapter 97). Proprioceptors
are listed in Figure 85-3.
PROPERTIES OF RECEPTORS
1. SPECIFICITY OF RESPONSE —
MÜLLER'S LAW
Specificity of response or Müller's law refers to
the response given by a particular type of
receptor to a specific sensation. For example,
pain receptors give response only to pain
sensation. Similarly, temperature receptors give
response only to temperature sensation.
Specificity of response is also called doctrine of
specific nerve energies.
2. ADAPTATION — SENSORY
ADAPTATION
Adaptation is the decrease in discharge of
sensory impulses when a receptor is stimulated
continuously with constant strength. It is also
called sensory adaptation or desensitization.
Depending upon adaptation time, the receptors
are divided into two types:
FIGURE 85-3:Interoceptors
i. Phasic receptors, which get adapted
rapidly. Touch and pressure receptors are
the phasic receptors
ii. Tonic receptors, which adapt slowly.
Muscle spindle, pain receptors and cold
receptors are the tonic receptors.
3. RESPONSE TO INCREASE IN THE
STRENGTH OF STIMULUS
During the stimulation of a receptor, if the
response given by the receptor is to be doubled,
the strength of stimulus must be increased 100
times. This phenomenon is called Weber-
Fechner law, which states that the change in
response of a receptor is directly proportional
to the logarithmic increase in the intensity of
stimulus.
4. SENSORY TRANSDUCTION
Sensory transduction in a receptor is a process
by which the energy (stimulus) in the environ-
ment is converted into electrical impulses (action
potentials) in nerve fiber (transduction =
conversion of one form of energy into another).
When a receptor is stimulated, it gives
response by sending information about the
stimulus to CNS. Series of events occur to carry
out this function such as the development of
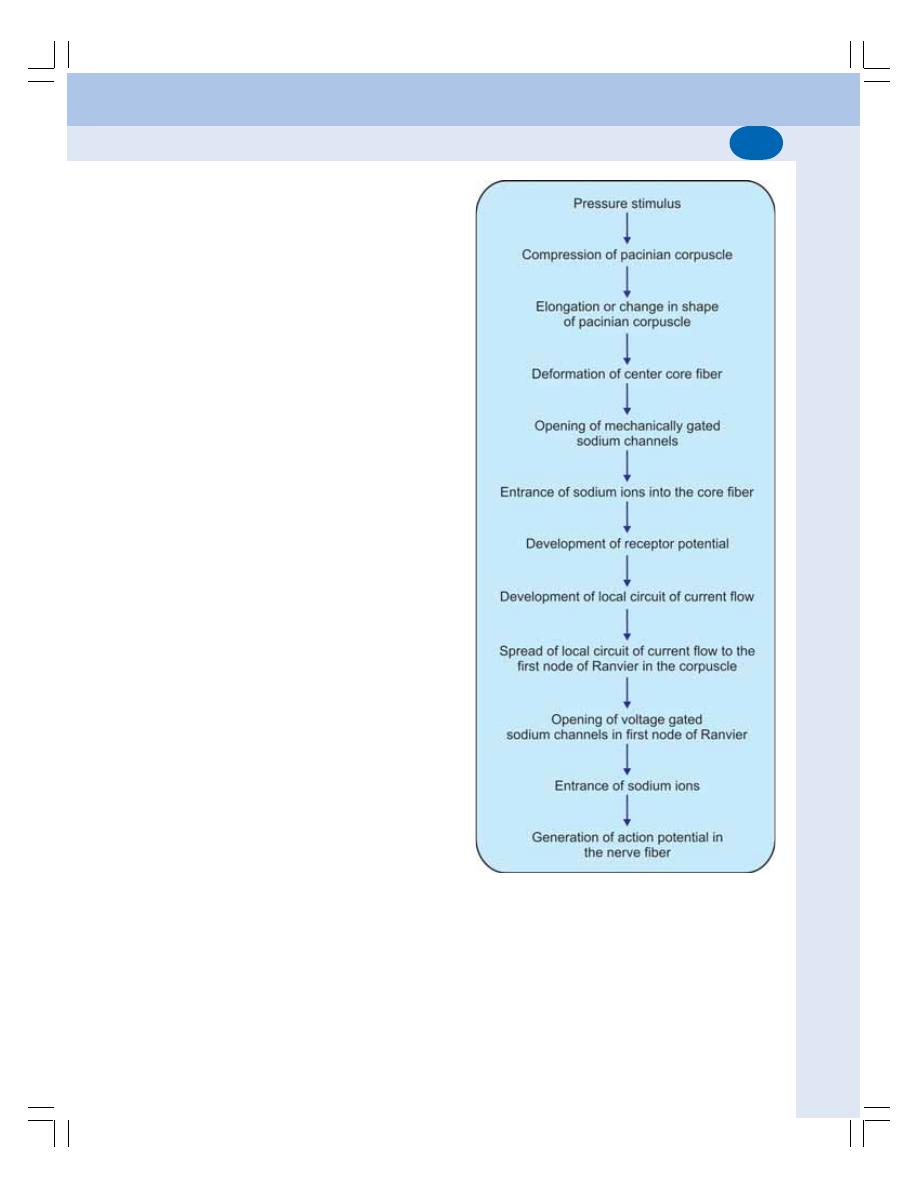
Chapter 85 Receptors
517
receptor potential in the receptor cell and
development of action potential in the sensory
nerve.
The sensory transduction varies depending
upon the type of receptor. For example, the
chemoreceptor converts chemical energy into
action potential in the sensory nerve fiber. The
touch receptor converts mechanical energy into
action potential in the sensory nerve fiber.
5. RECEPTOR POTENTIAL
Receptor potential is a nonpropagated trans-
membrane potential difference that develops
when a receptor is stimulated. It is also called
generator potential. The receptor potential is
short lived and hence, it is called transient
receptor potential.
Receptor potential is not action potential. It
is a graded potential (Chapter 23). It is similar
to excitatory postsynaptic potential (EPSP) in
synapse, endplate potential in neuromuscular
junction and electrotonic potential in the nerve
fiber.
Properties of Receptor Potential
Receptor potential has two important properties:
i. Receptor potential is nonpropagated. It is
confined within the receptor itself
ii. It does not obey all or none law.
Significance of Receptor Potential
When the receptor potential is sufficiently strong
(when the magnitude is about 10 mV), it causes
development of action potential in the sensory
nerve.
Mechanism of Development of Receptor
Potential
The pacinian corpuscles are generally used to
study the receptor potential because of their
large size and anatomical configuration. Pacinian
corpuscles give response to pressure stimulus.
When pressure stimulus is applied, the Pacinian
corpuscle is compressed. This compression
causes elongation or change in shape of the
corpuscle. The change in shape of the corpuscle
leads to the deformation of central fiber of the
corpuscle. This results in the opening of
mechanically gated sodium channels (Chapter
3). So, the positively charged sodium ions enter
the interior of fiber. This produces a mild depo-
larization, i.e. receptor potential.
FIGURE 85-4:Schematic diagram showing develop-
ment of receptor potential and generation of action
potential in the nerve fiber

Nervous System
518
Generation of Action Potential in the
Nerve Fiber
The receptor potential causes development of
a local circuit of current flow which spreads along
the unmyelinated part of the nerve fiber within
the corpuscle.
When this local circuit of current reaches the
first node of Ranvier within the corpuscle, it
causes opening of voltage gated sodium
channels and entrance of sodium ions into the
nerve fiber. This leads to the development of
action potential in the nerve fiber (Fig. 85-4).
DEFINITION
CLASSIFICATION
ANATOMICAL CLASSIFICATION
FUNCTIONAL CLASSIFICATION
FUNCTIONAL ANATOMY
FUNCTIONS
EXCITATORY SYNAPSE
INHIBITORY SYNAPSE
PROPERTIES
ONE WAY CONDUCTION – BELL-MAGENDIE LAW
THE SYNAPTIC DELAY
FATIGUE
SUMMATION
ELECTRICAL PROPERTY
NEUROTRANSMITTERS
DEFINITION
CLASSIFICATION
Synapse and
Neurotransmitters
86
DEFINITION
Synapse is the junction between the two
neurons. It is not the anatomical continuation.
But, it is only a physiological continuity between
two nerve cells.
CLASSIFICATION OF SYNAPSE
Synapse is classified by two methods,
anatomical classification and functional
classification.
ANATOMICAL CLASSIFICATION
Synapse is formed by axon of one neuron
ending on the cell body, dendrite or axon of the
next neuron. Depending upon the ending of
axon, the synapse is classified into three types
(Fig. 86-1):
1. Axoaxonic synapse in which axon of one
neuron terminates on axon of another neuron
2. Axodendritic synapse in which axon of one
neuron terminates on dendrite of another
neuron
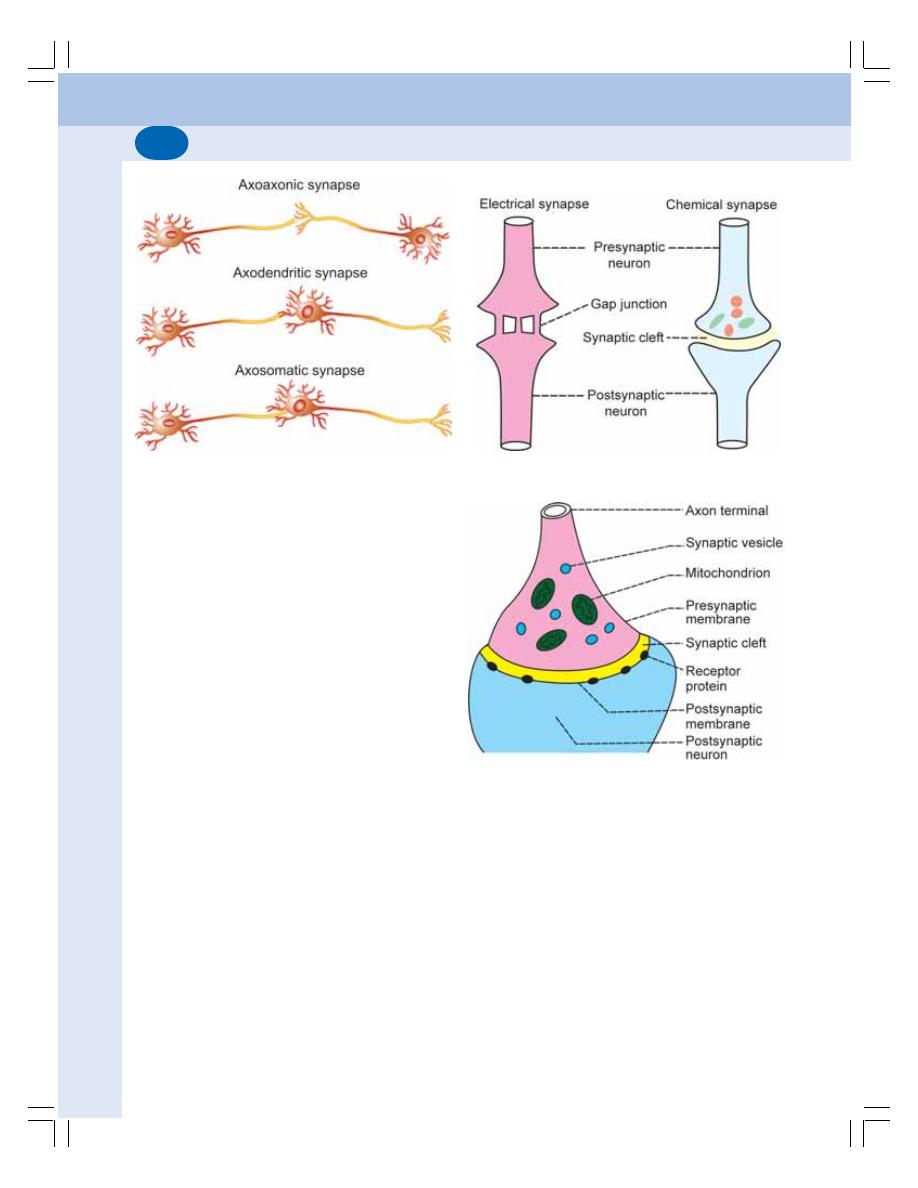
Nervous System
520
3. Axosomatic synapse in which axon of one
neuron ends on soma (cell body) of another
neuron.
FUNCTIONAL CLASSIFICATION
Function classification depends upon of mode
of impulse transmission. On this basis, synapse
is classified into two types:
1. Electrical Synapse
Electrical synapse is the synapse in which the
physiological continuity between the presynaptic
and the postsynaptic neurons is provided by the
gap junction between these two neurons
(Fig. 86-2). There is direct exchange of ions
between the two neurons though the gap
junction. So, the action potential reaching the
terminal portion of presynaptic neuron directly
enters the postsynaptic neuron.
2. Chemical Synapse
Chemical synapse is the junction between a
nerve fiber and a muscle fiber or between two
nerve fibers, through which the signals are
transmitted by the release of chemical
transmitter. In the chemical synapse, there is no
continuity between the presynaptic and post-
synaptic neurons. These two neurons are
separated by a space called synaptic cleft
between the two neurons.
FUNCTIONAL ANATOMY OF
CHEMICAL SYNAPSE
The functional anatomy of a chemical synapse
is shown in Figure 86-3. The neuron from which
the axon arises is called the presynaptic neuron
and the neuron on which the axon ends is called
postsynaptic neuron. The axon of the presynaptic
neuron divides into many small branches before
forming the synapse. The branches are known
as presynaptic axon terminals.
FIGURE 86-2: Electrical and chemical synapse
FIGURE 86-3: Structure of chemical synapse
FIGURE 86-1: Anatomical synapses
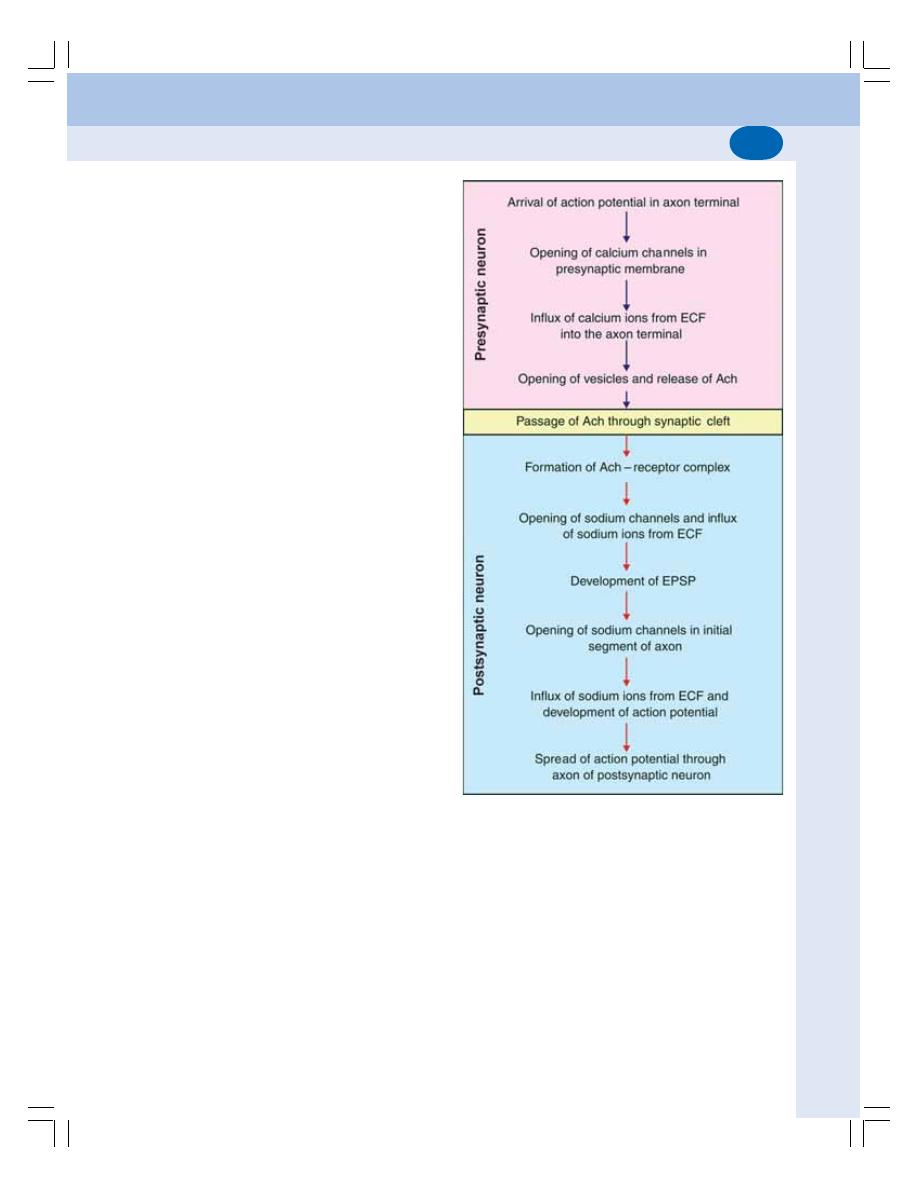
Chapter 86 Synapse and Neurotransmitters
521
Axon terminal has membrane known as
presynaptic membrane. The presynaptic terminal
has two important structures:
i. Mitochondria, which help in the synthesis
of neurotransmitter substances
ii. Synaptic vesicles, which store neuro-
transmitter substance.
The membrane of the postsynaptic neuron
is called postsynaptic membrane. It contains
some receptor proteins. The small space in
between the presynaptic membrane and the
postsynaptic membrane is called synaptic cleft.
The basal lamina of this cleft contains
cholinesterase, which destroys acetylcholine.
FUNCTIONS OF SYNAPSE
The function of the synapse is to transmit the
impulses from one neuron to another. However,
some synapses inhibit the impulses.
Accordingly, synapse is divided into two types:
1. Excitatory synapses, which transmit the
impulses — excitatory function
2. Inhibitory synapses, which inhibit the trans-
mission of impulses — inhibitory function.
EXCITATORY SYNAPSE
Excitatory synapse transmits the impulses from
presynaptic neuron to postsynaptic neuron by the
development of excitatory postsynaptic potential.
Excitatory Postsynaptic Potential
Excitatory postsynaptic potential (EPSP) is the
nonpropagated electrical potential that develops
during the process of synaptic transmission.
When the action potential reaches the pre-
synaptic axon terminal, the voltage gated
calcium channels at the presynaptic membrane
are opened. Now, the calcium ions enter the
axon terminal from ECF (Fig. 86-4).
The calcium ions cause the release of neuro-
transmitter substance from the vesicles by
means of exocytosis. The common neurotrans-
mitter in synapse is acetylcholine.
The neurotransmitter passes through
presynaptic membrane and synaptic cleft and
reaches the postsynaptic membrane. Now, it
binds with the receptor protein present in the
postsynaptic membrane to form the neuro-
transmitter-receptor complex.
The neurotransmitter-receptor complex
causes opening of ligand gated sodium
channels. Now, the sodium ions from ECF enter
the cell body of postsynaptic neuron. As the
FIGURE 86-4: Sequence of events during synaptic
transmission. Ach = Acetylcholine. ECF = Extracellular
fluid. EPSP = Excitatory postsynaptic potential

Nervous System
522
sodium ions are positively charged, the resting
membrane potential inside the cell body
becomes slightly positive and a mild depola-
rization develops. This type of mild depolarization
is called EPSP.
EPSP is confined only to the synapse. It is a
graded potential (Chapter 23). It is similar to
receptor potential and endplate potential.
Properties of EPSP
EPSP has two properties.
1. It is nonpropagated
2. It does not obey all or none law.
Significance of EPSP
The EPSP is not transmitted into the axon of
postsynaptic neuron. However, it causes
development of action potential in the axon.
When the EPSP is strong enough, it causes
the opening of voltage gated sodium channels
in the initial segment of axon. Now, due to the
entrance of sodium ions, the depolarization
occurs in the initial segment of axon and thus,
the action potential develops. From here, the
action potential spreads to other segment of the
axon.
INHIBITORY SYNAPSE
Inhibitory synapse does not transmit the impul-
ses from presynaptic neuron to postsynaptic
neuron. Inhibition of synaptic transmission is
classified into three types:
1. Postsynaptic inhibition
2. Presynaptic inhibition
3. Renshaw cell inhibition.
1. Postsynaptic Inhibition
Postsynaptic inhibition is the type of synaptic
inhibition that occurs due to the release of an
inhibitory neurotransmitter from presynaptic
terminal instead of an excitatory neurotransmitter
substance. It is also called direct inhibition. The
inhibitory neurotransmitter develops inhibitory
post synaptic potential (IPSP) instead of EPSP.
The inhibitory neurotransmitters are gamma
amino butyric acid (GABA), dopamine and
glycine.
Action of GABA — Development of IPSP
IPSP is the electrical potential in the form of
hyperpolarization that develops during post-
synaptic inhibition. The inhibitory neurotrans-
mitter substance acts on postsynaptic mem-
brane by binding with receptor. The transmitter
– receptor complex opens the ligand gated
potassium channels instead of sodium channels.
Now, the potassium ions which are available in
plenty in the cell body of postsynaptic neuron
move to ECF. Simultaneously, chloride channels
also open and chloride ions (which are more in
ECF) move inside the cell body of postsynaptic
neuron. The exit of potassium ions and influx
of chloride ions cause more negativity inside,
leading to hyperpolarization. The hyperpolarized
state of the synapse inhibits synaptic trans-
mission.
2. Presynaptic Inhibition
It is the synaptic inhibition which occurs because
of the failure of presynaptic axon terminal to
release the excitatory neurotransmitter substance.
It is also called indirect inhibition.
3. Renshaw Cell Inhibition
It is the type of synaptic inhibition which is
caused by Renshaw cells in spinal cord.
Renshaw cells are small motor neurons scat-
tered among the large a motor neurons in
anterior gray horn of spinal cord (Chapter 88).
The motor nerve fibers to effector organs arise
from a motor neurons. Some of these fibers
send collateral fibers to Renshaw cells.
When the motor neurons send motor impul-
ses to effector organs, some of the impulses
reach the Renshaw cell by passing through
collaterals. Now, the Renshaw cell is stimulated.
In turn, it sends inhibitory impulses to a motor
neurons so that, the discharge from a motor
neurons is reduced (Fig. 86-5).
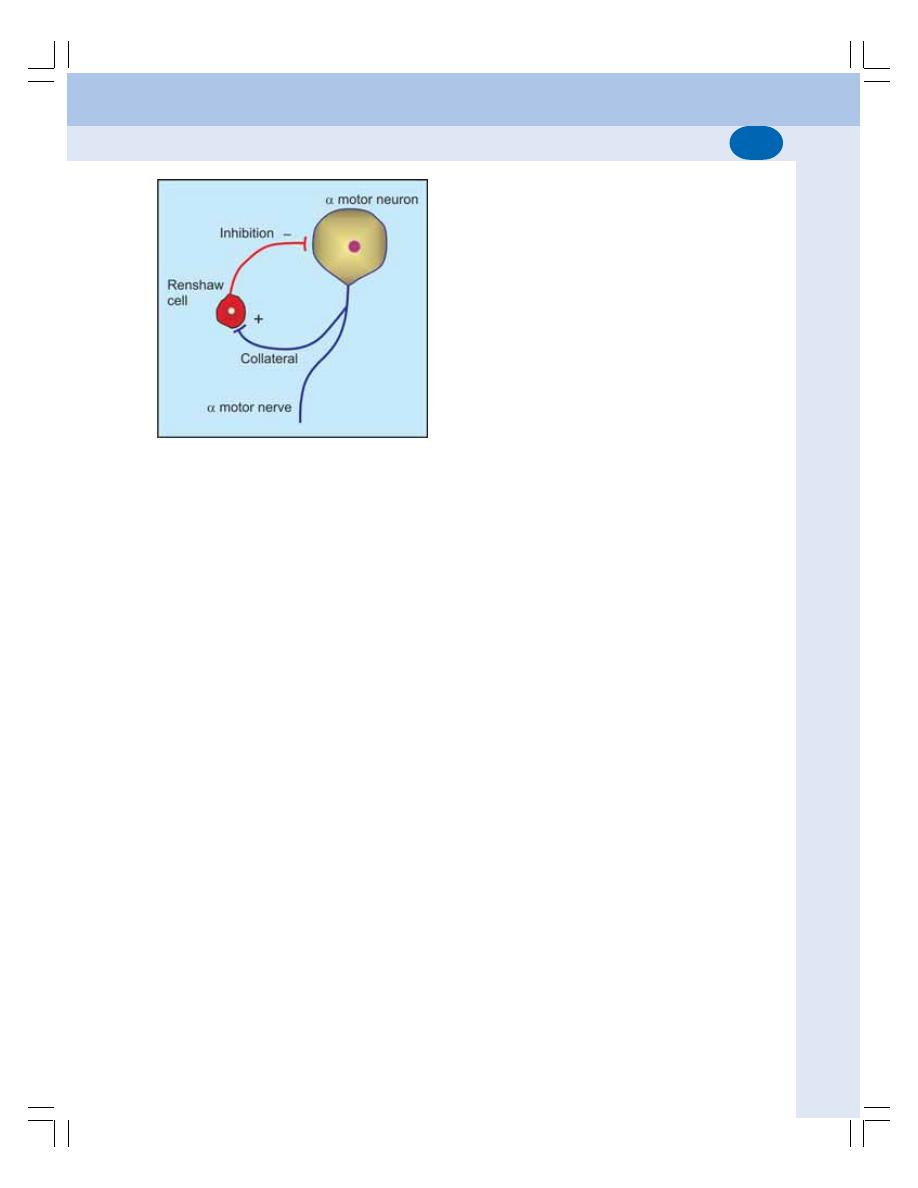
Chapter 86 Synapse and Neurotransmitters
523
Significance of synaptic inhibition
The synaptic inhibition in CNS limits the number
of impulses going to muscles and enables the
muscles to act properly and appropriately.
PROPERTIES OF SYNAPSE
1. ONE WAY CONDUCTION – BELL-
MAGENDIE LAW
According to Bell-Magendie law, the impulses
are transmitted only in one direction in synapse,
i.e. from presynaptic neuron to postsynaptic
neuron.
2. THE SYNAPTIC DELAY
Synaptic delay is a short delay that occurs during
the transmission of impulses through the
synapse. It is due to the time taken for:
i. Release of neurotransmitter
ii. Passage of neurotransmitter from axon
terminal to postsynaptic membrane
iii. Action of the neurotransmitter to open the
ionic channels in postsynaptic membrane.
The normal duration of synaptic delay is
0.3 to 0.5 msec. The synaptic delay is one
of the causes for reaction time of the reflex
activity.
FIGURE 86-5: Renshaw cell inhibition
Significance of determining synaptic delay
Determination of synaptic delay helps to find out
whether the pathway for a reflex is mono-
synapatic or polysynaptic.
3. FATIGUE
During continuous muscular activity, the synapse
forms the seat of fatigue along with the Betz cells
present in the motor area of the frontal lobe of
the cerebral cortex (Refer Chapter 22 for details
of fatigue). The fatigue at the synapse is due to
the depletion of neurotransmitter substance,
acetylcholine.
Depletion of acetylcholine occurs by two
factors:
i. Soon after the action, acetylcholine is
destroyed by acetylcholinesterase
ii. Due to continuous action, new acetyl-
choline is not synthesized.
These two factors lead to depletion of acetyl-
choline resulting in fatigue.
4. SUMMATION
It is the fusion of effects or progressive increase
in the excitatory postsynaptic potential (EPSP)
in postsynaptic neuron when many presynaptic
excitatory terminals are stimulated simul-
taneously or when single presynaptic terminal is
stimulated repeatedly. The increased EPSP
triggers the action potential in the initial segment
of the axon of postsynaptic neuron (Fig. 86-6).
Summation is of two types:
i. Spatial Summation which occurs when
many presynaptic terminals are stimu-
lated simultaneously
ii. Temporal summation which occurs when
one presynaptic terminal is stimulated
repeatedly (Fig. 86-6).
5. ELECTRICAL PROPERTY
The electrical properties of the synapse are the
EPSP and IPSP, which are already described in
this chapter.
Nervous System
524
TABLE 86-1: Neurotransmitters
Group Name
Site of secretion
Action
GABA
Cerebral cortex, cerebellum, basal ganglia, retina and spinal cord
Inhibitory
Glycine
Forebrain, brainstem, spinal cord and retina
Inhibitory
Glutamate
Cerebral cortex, brainstem, and cerebellum
Excitatory
Aspartate
Cerebellum, spinal cord and retina
Excitatory
Noradrenaline
Postganglionic adrenergic sympathetic nerve endings,
Excitatory
cerebral cortex, hypothalamus, basal ganglia, brainstem,
and
locus ceruleus and spinal cord
Inhibitory
Adrenaline
Hypothalamus, thalamus and spinal cord
Excitatory
and
Inhibitory
Dopamine
Basal ganglia, hypothalamus, limbic system, neocortex, retina and
Inhibitory
sympathetic ganglia
Serotonin
Hypothalamus, limbic system, cerebellum, spinal cord, retina,
Inhibitory
GI tract, lungs and platelets
Histamine
Hypothalamus, cerebral cortex, GI tract and mast cells
Excitatory
Nitric oxide
Many parts of CNS, neuromuscular junction and GI tract
Excitatory
Acetylcholine
Preganglionic parasympathetic nerve endings
Excitatory
Postganglionic parasympathetic nerve endings
Preganglionic sympathetic nerve endings
Postganglionic sympathetic cholinergic nerve endings
Neuromuscular junction, cerebral cortex, hypothalamus, basal
ganglia, thalamus, hippocampus and amacrine cells of retina
Others
Amines
Aminoacids
TABLE 86-2: Excitatory and inhibitory neurotransmitters
Excitatory
Inhibitory
Neurotransmitters with excitatory
neurotransmitters
neurotransmitters
and inhibitory actions
1. Acetylcholine
1. GABA
1. Noradrenaline
2. Nitric oxide
2. Glycine
2. Adrenaline
3. Histamine
3. Dopamine
4. Glutamate
4. Serotonin
5. Aspartate
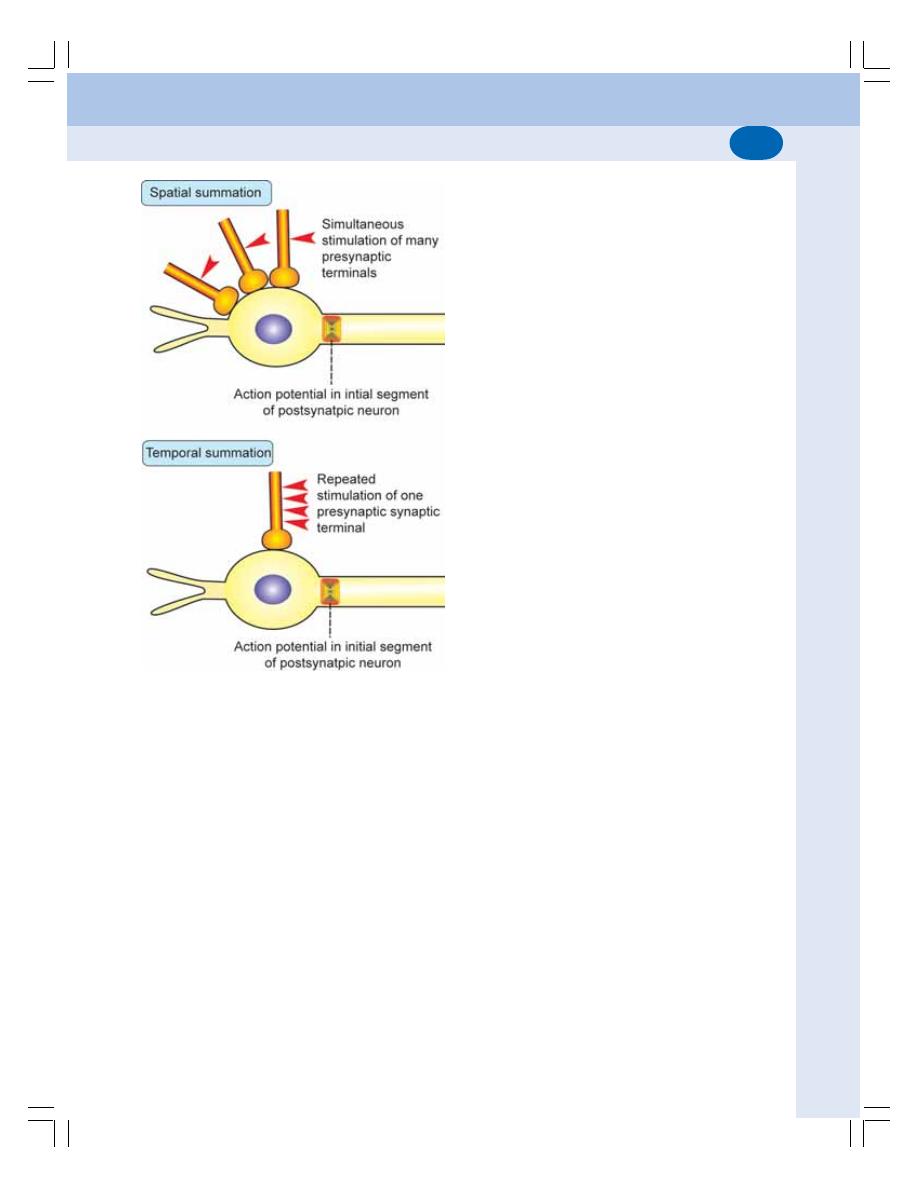
Chapter 86 Synapse and Neurotransmitters
525
FIGURE 86-6: Spatial and temporal summation
NEUROTRANSMITTERS
DEFINITION
Neurotransmitter is a chemical substance that
acts as the mediator for the transmission of nerve
impulse from one neuron to another neuron
through a synapse.
CLASSIFICATION OF
NEUROTRANSMITTERS
Depending Upon Chemical Nature
Depending upon chemical nature, neuro-
transmitters are classified into three groups
(Table 86-1):
1. Amino acids
2. Amines
3. Others
Depending Upon Function
Depending upon function, neurotransmitters are
classified into two types:
1. Excitatory neurotransmitters which are
responsible for the conduction of impulse
2. Inhibitory neurotransmitters which inhibit the
conduction of impulse
Details of neurotransmitters are given in the
Tables 86-1 and 86-2.
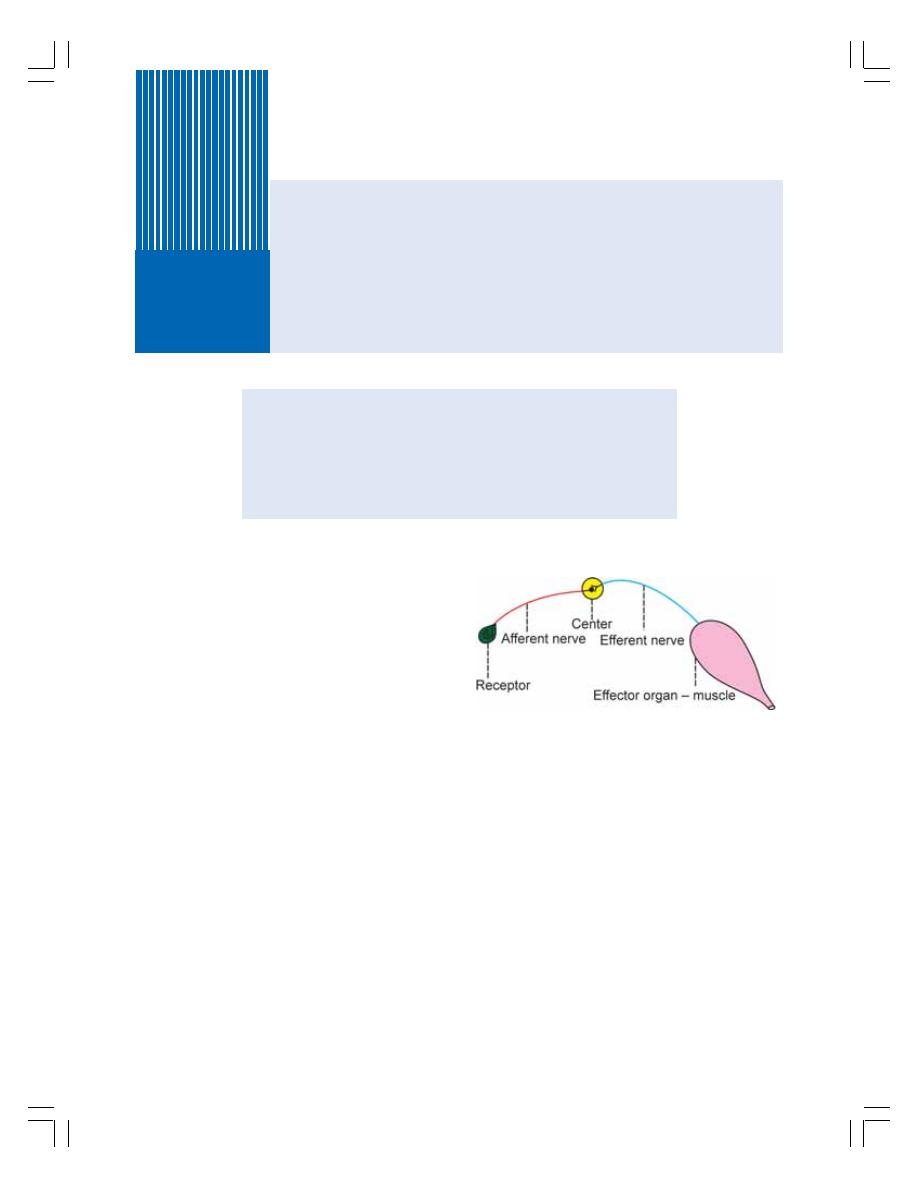
DEFINITION AND SIGNIFICANCE OF REFLEXES
REFLEX ARC
CLASSIFICATION OF REFLEXES
PROPERTIES OF REFLEXES
REFLEXES IN MOTOR NEURON LESION
Reflex Activity
87
DEFINITION AND SIGNIFICANCE OF
REFLEXES
Reflex activity is the response to a peripheral
nervous stimulation that occurs without our
consciousness. It is a type of protective mecha-
nism and it protects the body from irreparable
damages.
For example, when the hand is placed on a
hot object, it is withdrawn immediately. When a
very bright light is thrown into the eyes, eyelids
are closed and pupil is constricted to prevent
the damage of retina by the entrance of
excessive light into the eyes.
REFLEX ARC
Reflex arc is the anatomical nervous pathway
for a reflex action. A simple reflex arc includes
five components (Fig. 87-1).
1. Receptor
It is the end organ, which receives the stimulus.
When the receptor is stimulated, impulses are
generated in afferent nerve.
2. Afferent Nerve
Afferent or sensory nerve transmits sensory
impulses from the receptor to the center.
3. Center
The center is located in the brain or spinal cord.
The center receives the sensory impulses via
afferent nerve fibers and in turn, it generates
appropriate motor impulses.
4. Efferent Nerve
Efferent or motor nerve transmits motor impulses
from the center to the effector organ.
FIGURE 87-1: Simple reflex arc

Chapter 87 Reflex Activity
527
5. Effector Organ
The effector organ is the structure such as the
muscle or gland where the activity occurs in
response to the stimulus.
Afferent and efferent nerve fibers may be
connected directly to the center. In some places,
one or more neurons are interposed between
these nerve fibers and the center. Such neurons
are called connector neurons or internuncial
neurons or interneurons.
CLASSIFICATION OF REFLEXES
Reflexes are classified by five different methods.
I. DEPENDING UPON WHETHER
INBORN OR ACQUIRED
1. Unconditioned Reflexes or Inborn Reflexes
Unconditioned reflexes are the natural reflexes
which are present since the time of birth hence
the name inborn reflexes. Such reflexes do not
require previous learning, training, or condi-
tioning. The best example is the secretion of
saliva when a drop of honey is kept in the mouth
of a newborn baby for the first time. The baby
does not know the taste of the honey but still
saliva is secreted.
2. Conditioned Reflexes or Acquired
Reflexes
Conditioned or acquired reflexes are the reflexes
that are developed after conditioning or training.
These reflexes are not inborn but acquired after
birth. Such reflexes need previous learning,
training, or conditioning. The example is the
secretion of saliva by the sight, smell, thought
or hearing of a known edible substance.
II. DEPENDING UPON THE SITUATION
OF THE CENTER
1. Cerebellar Reflexes
Cerebellar reflexes are the reflexes which have
the center in cerebellum.
2. Cortical Reflexes
Cortical reflexes are the reflexes that have the
center in cerebral cortex.
3. Midbrain Reflexes
Midbrain reflexes are the reflexes which have
the center in midbrain.
4. Bulbar or Medullary Reflexes
Bulbar or medullary reflexes are the reflexes
which have the center in medulla oblongata.
5. Spinal Reflexes
Reflexes having their center in the spinal cord
are called spinal reflexes.
III. DEPENDING UPON THE PURPOSE
— FUNCTIONAL SIGNIFICANCE
1. Protective Reflexes or Flexor Reflexes
The protective reflexes are the reflexes which
protect the body from nociceptic (harmful)
stimuli. These reflexes are also called withdrawal
reflexes or flexor reflexes. Protective reflexes
involve flexion at different joints hence the name
flexor reflexes.
2. Antigravity Reflexes or Extensor Reflexes
Antigravity reflexes are the reflexes that protect
the body against the gravitational force. These
reflexes are also called the extensor reflexes
because, the extensor muscles contract during
these reflexes resulting in extension at joints.
IV. DEPENDING UPON THE NUMBER
OF SYNAPSE
1. Monosynaptic Reflexes
Reflexes having only one synapse in the reflex
arc are called monosynaptic reflexes. Stretch
reflex is the best example for monosynaptic
reflex and it is elicited due to the stimulation of
muscle spindle.
Nervous System
528
TABLE 87-1: Superficial mucous membrane reflexes
Reflex
Stimulus
Response
Afferent Nerve
Center
Efferent Nerve
1. Corneal reflex
Irritation of cornea
Blinking of eye
V cranial nerve
Pons
VII cranial nerve
(closure of
eyelids)
2. Conjunctival
Irritation of
Blinking of eye
V cranial nerve
Pons
VII cranial nerve
reflex
conjunctiva
3. Nasal reflex
Irritation of nasal
Sneezing
V cranial nerve
Motor
X cranial nerve
(sneezing
mucous
nucleus
and upper
reflex)
membrane
of V
cervical
cranial
nerves
nerve
4. Pharyngeal
Irritation of
Retching or
IX cranial nerve Nuclei of
X cranial nerve
reflex
pharyngeal
gagging
X cranial
mucous
(opening of
nerve
membrane
mouth)
5. Uvular reflex
Irritation of uvula
Raising of uvula IX cranial nerve Nuclei of
X cranial nerve
X cranial
nerve
2. Polysynaptic Reflexes
Reflexes having more than one synapse in the
reflex arc are called polysynaptic reflexes. Flexor
reflexes (withdrawal reflexes) are the polysynap-
tic reflexes.
V. DEPENDING UPON CLINICAL BASIS
Depending upon clinical basis reflexes are
classified into four types:
1. Superficial Reflexes
Superficial reflexes are the reflexes, which are
elicited from the surface of the body. The super-
ficial reflexes are of two types, mucous mem-
brane reflexes (Table 87-1) and skin reflexes
(Table 87-2).
2. Deep Reflexes
The deep reflexes are elicited from the deeper
structures beneath the skin like tendon. These
reflexes are otherwise known as tendon reflexes.
The details of these are given in Table 87-3.
3. Visceral Reflexes
Visceral reflexes are the reflexes arising from
the pupil and the visceral organs.
Visceral reflexes are:
i.
Pupillary reflexes in which, the size of pupil
is altered (Chapter 107)
ii. Oculocardiac reflex in which heart rate decre-
ases due to the pressure applied over eyeball
iii. Carotid sinus reflex in which the pressure
over carotid sinus in neck due to tight collar
decreases heart rate and blood pressure.
4. Pathological Reflexes
Pathological reflexes are the reflexes that are
elicited only in pathological conditions. Three
pathological reflexes are well known.
i. Babinski’s sign
The abnormal plantar reflex is called Babinski’s
sign. It is also called Babinski’s reflex or pheno-
menon. In the normal plantar reflex, a gentle
scratch over the outer edge of the sole of the
foot causes plantar flexion and adduction of all
Chapter 87 Reflex Activity
529
TABLE 87-3: Deep reflexes
Reflex
Stimulus
Response
Center – Spinal
segments involved
1. Jaw jerk
Tapping middle of the chin
Closure of mouth
Pons - V Cranial
with slightly opened mouth
nerve
2. Biceps jerk
Percussion of biceps tendon
Flexion of forearm
C5, C6
3. Triceps jerk
Percussion of triceps tendon
Extension of forearm
C6, to C8
4. Supinator jerk or
Percussion of tendon over
Supination and flexion
C7, C8
radial periosteal
distal end (styloid process)
of forearm
reflex
of radius
5. Wrist tendon or
Percussion of wrist tendons
Flexion of corresponding
C8, T1
finger flexion reflex
finger
6. Knee jerk or
Percussion of patellar
Extension of leg
L 2, To L4
patellar tendon
ligament
reflex
7. Ankle jerk or
Percussion of Achilles tendon
Plantar flexion of foot
L 5 to S2
Achilles tendon
reflex
TABLE 87-2: Superficial cutaneous reflexes
Reflex
Stimulus
Response
Center – spinal
segments
involved
1. Scapular reflex
Irritation of skin at the
Contraction of scapular muscles
C5 to T1
interscapular space
and drawing in of scapula
2. Upper abdominal
Stroking the abdominal wall Ipsilateral contraction of abdo-
T6 to T9
reflex
below the costal margin
minal muscle and movement of
umbilicus towards the site of
stroke
3. Lower abdominal
Stroking the abdominal wall Ipsilateral contraction of abdo-
T10 to T12
reflex
at umbilical and iliac level
minal muscle and movement of
umbilicus towards the site of
stroke
4. Cremasteric
Stroking the skin at upper
Elevation of testicles
L1, L2
reflex
and inner aspect of thigh
5. Gluteal reflex
Stroking the skin over glutei Contraction of glutei
L4 to S1,2
6. Plantar reflex
Stroking the sole
Plantar flexion and adduction
L5 to S2
of toes
7. Bulbocavernous
Stroking the dorsum of
Contraction of bulbocavernous
S3, S4
reflex
glans penis
8. Anal reflex
Stroking the perianal region Contraction of anal sphincter
S4, S5

Nervous System
530
toes. But in Babinski’s sign, there is dorsiflexion
of great toe and fanning of other toes.
Babinski’s sign is present in upper motor
neuron lesion. Physiological conditions when
Babinski’s sign is present are infancy and deep
sleep. It is present in infants because of non-
myelination of pyramidal tracts.
ii. Clonus
Clonus is a series of rapid and repeated invo-
luntary jerky movements, which occur while
eliciting a deep reflex. It occurs in upper motor
neuron lesion. When a deep reflex is elicited in
a normal person, the contractions of a muscle
or group of muscles are smooth and continuous.
But in upper motor neuron lesion clonus occurs.
It is because of hypertonicity of muscles and
exaggeration of deep reflexes. Clonus is well
seen in calf muscles producing ankle clonus and
quadriceps producing patella clonus.
iii. Pendular movements
Pendular movements are the slow oscillatory
movements (instead of brisk movements) that
are developed while eliciting a tendon jerk. The
pendular movements are very common while
eliciting the knee jerk in patients affected by
cerebellar lesion.
PROPERTIES OF REFLEXES
1. ONE WAY CONDUCTION
(BELL-MAGENDIE LAW)
During any reflex activity, the impulses are
transmitted in only one direction through the
reflex arc as per Bell-Magendie law. The impul-
ses pass from receptors to the center and then
from center to effector organ.
2. REACTION TIME
Reaction time is the time interval between
application of stimulus and the onset of reflex.
It depends upon the length of afferent and
efferent nerve fibers, velocity of impulse through
these fibers and central delay. Central delay is
the delay at the synapse. It is also called synaptic
delay.
3. SUMMATION
Refer Chapter 86 for details of summation. The
summation in reflex action is of two types.
i.
Spatial Summation
When two afferent nerve fibers supplying a
muscle are stimulated separately with subliminal
stimulus, there is no response. But the muscle
contracts when both the nerve fibers are stimu-
lated together with same strength of stimulus.
It is called spatial summation.
ii. Temporal Summation
When one nerve fiber is stimulated repeatedly
with subliminal stimuli, these stimuli are summed
up to give response in the muscle. It is called
temporal summation.
Thus, both spatial summation and temporal
summation play an important role in the facili-
tation of responses during the reflex activity.
4. RECRUITMENT
Recruitment is defined as the successive acti-
vation of additional motor units with progressive
increase in force of muscular contraction.
When an excitatory nerve is stimulated for a
long time, there is a gradual increase in the
response of reflex activities. It is due to the
activation of more and more motor neurons.
Recruitment is similar to the effect of temporal
summation.
5. AFTER DISCHARGE
After discharge is the persistence or continuation
of response for some time even after cessation
of stimulus. When a reflex action is elicited
continuously for some time, and then the stimu-
lation is stopped, the reflex activity (contraction)
will be continued for some time even after the
stoppage of the stimulus. It is because of the
discharge of impulses from the center even after

Chapter 87 Reflex Activity
531
stoppage of stimulus. The internuncial neurons
are responsible for after discharge.
6. REBOUND PHENOMENON
The reflex activities can be forcefully for some
time. But, when the inhibition is suddenly
removed, the reflex activity becomes more
forceful than before inhibition. It is called rebound
phenomenon. The reason for this state of over
excitation is not known.
7. FATIGUE
When a reflex activity is continuously elicited
for a long time, the response is reduced slowly
and at one stage, the response does not occur.
This type of failure to give response to the
stimulus is called fatigue. The center or the
synapse of the reflex arc is the first seat of
fatigue.
REFLEXES IN MOTOR NEURON
LESION
UPPER MOTOR NEURON LESION
During upper motor neuron lesion, all the super-
ficial reflexes are lost. The deep reflexes are
exaggerated and the Babinski’s sign is present
(Chapter 89).
LOWER MOTOR NEURON LESION
During lower motor lesion, all the superficial and
deep reflexes are lost (Chapter 89).
INTRODUCTION
GRAY MATTER
WHITE MATTER
TRACTS IN SPINAL CORD
ASCENDING TRACTS
DESCENDING TRACTS
Spinal Cord
88
INTRODUCTION
The spinal cord lies loosely in the vertebral canal.
It extends from foramen magnum where it is
continuous with medulla oblongata, above and
up to the lower border of first lumbar vertebra
below.
Segments of Spinal Cord
Spinal cord is made up of 31 segments:
Cervical segments
=
8
Thoracic segments
=
12
Lumbar segments
=
5
Sacral segments
=
5
Coccygeal segment
=
1
In fact, the spinal cord is a continuous
structure. The appearance of the segment is
given by the nerves arising from the spinal cord
which are called spinal nerve.
Spinal Nerves
The segments of spinal cord correspond to the
31 pairs of spinal nerves in a symmetrical
manner: The spinal nerves are:
Cervical spinal nerves
=
8
Thoracic spinal nerves
=
12
Lumbar spinal nerves
=
5
Sacral spinal nerves
=
5
Coccygeal nerve
=
1
Nerve Roots
Each spinal nerve is formed by an anterior
(ventral) root and a posterior (dorsal) root. Both
the roots on either side leave the spinal cord
through the corresponding intervertebral
foramina.
INTERNAL STRUCTURE OF SPINAL
CORD
The neural substance of spinal cord is divided
into inner gray matter and outer white matter
(Fig. 88-1).
GRAY MATTER OF SPINAL CORD
Gray matter of the spinal cord is the collection
of nerve cell bodies, dendrites and parts of
axons. It is placed centrally in the form of wings
of the butterfly and it resembles the letter H.
Exactly in the center of gray matter, there is a
canal called the spinal canal.
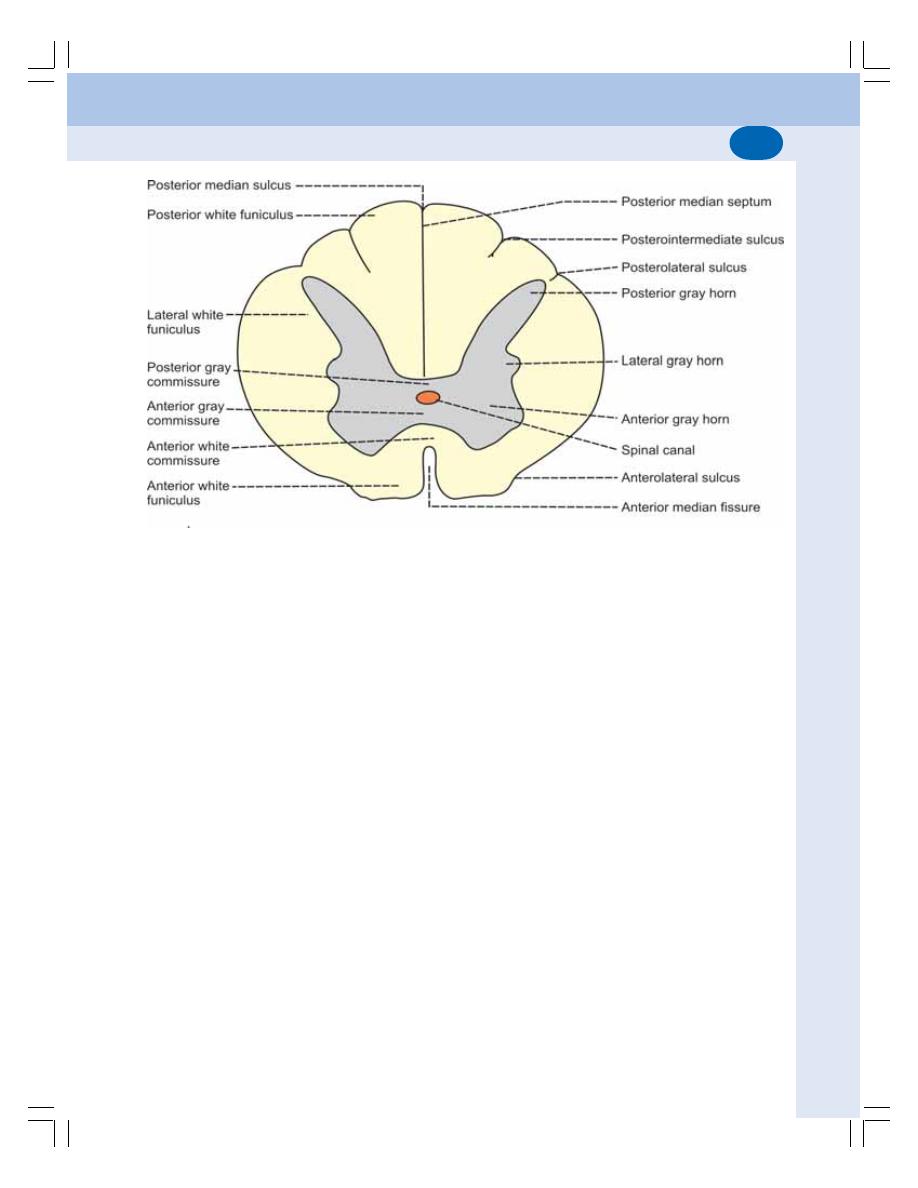
Chapter 88 Spinal Cord
533
The ventral and the dorsal portions of each
lateral half of gray matter are called ventral
(anterior) and dorsal (posterior) gray horns
respectively. In addition, the gray matter forms
a small projection in between the anterior and
posterior horns in all thoracic and first two lumbar
segments. It is called the lateral gray horn. The
part of the gray matter anterior to central canal
is called the anterior gray commissure and the
part of gray matter posterior to the central canal
is called the posterior gray commissure.
Neurons in Gray Horn
Clusters of neurons are present in gray matter
in the form of nuclei.
Nuclei in posterior gray horn
The posterior gray horn contains the nuclei of
sensory neurons which receive impulses from
various receptors of the body through posterior
nerve root fibers. Sensory neurons are of four
types (Fig. 88-2).
1. Marginal nucleus
2. Substantia gelatinosa of Rolando
3. Chief sensory nucleus
4. Clarke’s nucleus.
Nuclei in lateral gray horn
Lateral gray horn has intermediolateral nucleus.
The neurons of this nucleus give rise to
sympathetic preganglionic fibers, which leave
the spinal cord through the anterior nerve root.
Intermediolateral nucleus extends between T1
and L2 segments of spinal cord.
Nuclei in anterior gray horn
Anterior gray horn contains the nuclei of lower
motor neurons which are involved in motor
function. Lower motor neurons are of three types.
1. Alpha motor neurons
2. Gamma motor neurons
3. Renshaw cells
WHITE MATTER OF SPINAL CORD
White matter of spinal cord surrounds the gray
matter. It is formed by the bundles of nerve fibers.
The anterior median fissure and the posterior
FIGURE 88-1: Section of spinal cord – thoracic segment
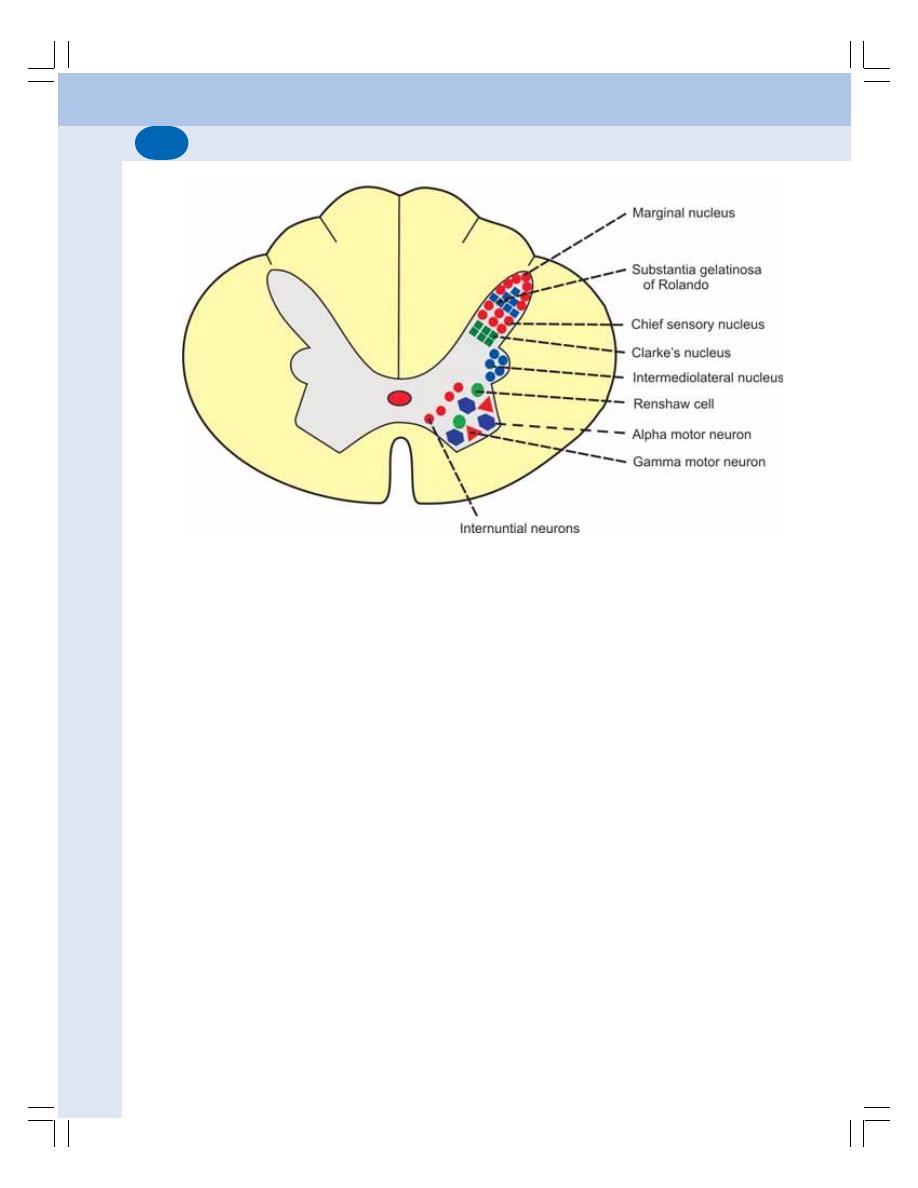
Nervous System
534
median septum divide the entire mass of white
matter into two lateral halves. The band of white
matter lying in front of anterior gray commissure
is called the anterior white commissure.
Each half of the white matter is divided by
the fibers of anterior and posterior nerve roots
into three white columns or funiculi:
1. Anterior or ventral white column or funiculus
2. Lateral white column or funiculus
3. Posterior or dorsal white column or funiculus
TRACTS IN SPINAL CORD
Tracts of the spinal cord are collections of nerve
fibers passing through the spinal cord. Spinal
tracts are divided into two main groups:
I. Short tracts which connect different parts
of spinal cord itself.
II. Long tracts which connect the spinal cord
with other parts of central nervous
system.
Long tracts are of two types:
1. Ascending tracts which carry sensory
impulses from the spinal cord to brain
2. Descending tracts, which carry motor
impulses from brain to the spinal cord.
ASCENDING TRACTS OF SPINAL
CORD
The ascending tracts of spinal cord carry the
impulses of various sensations to the brain.
The pathway for each sensation is formed by
two or three groups of neurons:
1. First order neurons
2. Second order neurons
3. Third order neurons
First Order Neurons
First order neurons receive sensory impulses
from the receptors and send them to sensory
neurons present in the posterior gray horn of
spinal cord through their fibers. The nerve cell
bodies of these neurons are located in the
posterior nerve root ganglion that lies outside
the spinal cord.
Second Order Neurons
The second order neurons are the sensory
neurons present in the posterior gray horn. The
fibers from these neurons form the ascending
tracts of spinal cord. These fibers carry sensory
FIGURE 88-2: Nuclei in gray horn of spinal cord – thoracic segment
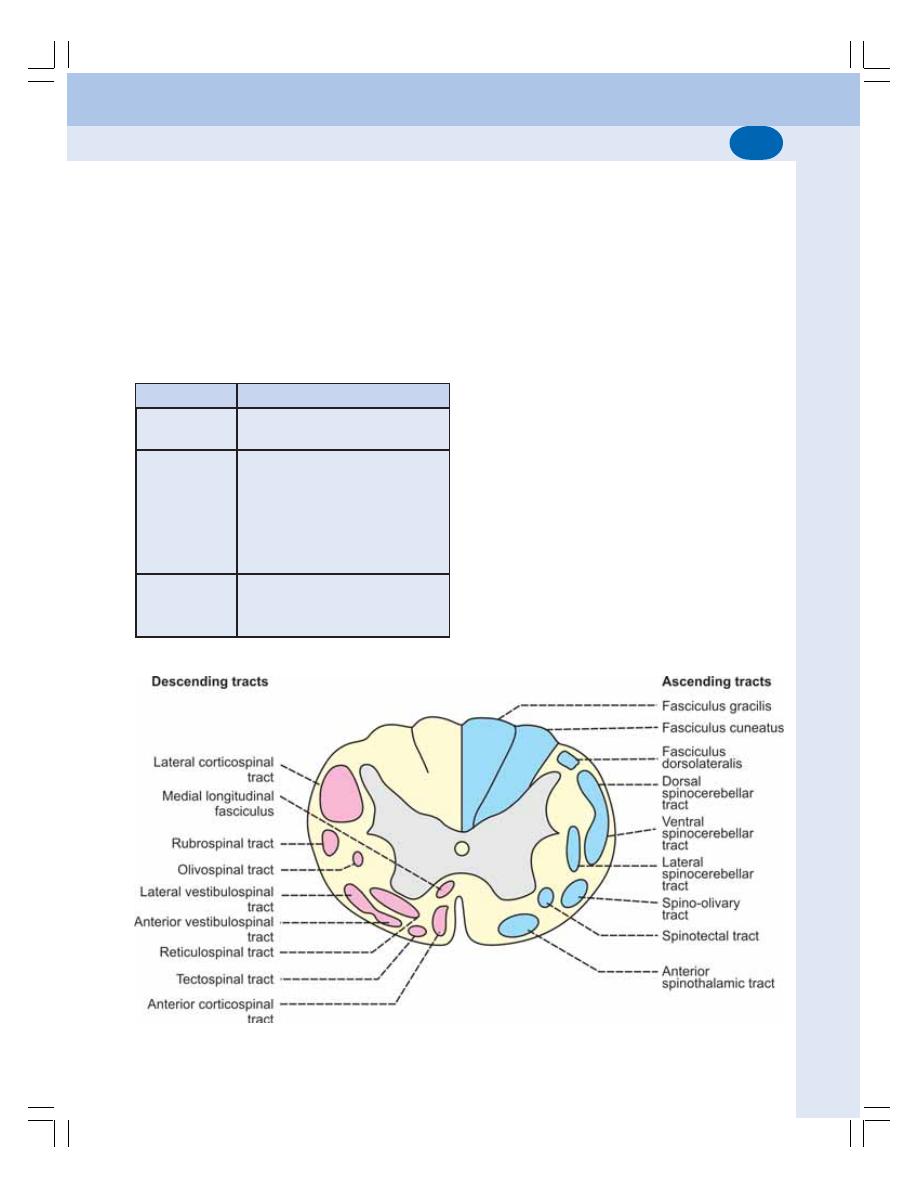
Chapter 88 Spinal Cord
535
impulses from spinal cord to different brain areas
below cerebral cortex (subcortical areas) such
as thalamus, cerebellum etc.
All the ascending tracts are formed by fibers
of second order neurons of the sensory pathways
except the ascending tracts in the posterior white
column which are formed by the fibers of first
order neurons.
TABLE 88-1: List of ascending tracts of spinal
cord
White column
Tract
Anterior white
1. Anterior spinothalamic tract
column
Lateral white
2. Lateral spinothalamic tract
column
3. Ventral spinocerebellar tract
4. Dorsal spinocerebellar tract
5. Spinotectal tract
6. Spinoreticular tract
7. Spinoolivary tract
8. Spinovestibular tract
Posterior white
9. Fasciculus gracilis
column
10. Fasciculus cuneatus
11. Comma tract of Schultze
Third Order Neurons
Third order neurons are in the subcortical areas.
The fibers of these neurons carry the sensory
impulses from subcortical areas to cerebral
cortex.
The ascending tracts situated in different
white columns are listed in Table 88-1. The
features of the ascending tracts are given in
Table 88-2.
1. ANTERIOR SPINOTHALAMIC
TRACT
Anterior spinothalamic tract is formed by the
fibers of second order neurons of the pathway
for crude touch sensation (Figs 88-3 and 88-4).
This tract is situated in anterior white column.
Origin
The fibers of anterior spinothalamic tract arise
from cells of chief sensory nucleus of posterior
gray horn which form the second order neurons.
The first order neurons are situated in the
posterior nerve root ganglia. These neurons
receive the impulses from the pressure
FIGURE 88-3: Tracts of spinal cord
Nervous System
536
TABLE 88-2:
Ascending tract
s of spinal cord
Situation
T
ract
Origin
Course
T
ermination
Function
Anterior
white
1.
Anterior
spinothalamic
Chief
sensory
Crossing in spinal cord
V
entral
posterolateral
Crude
touch
sensation
column
tract
nucleus
forms spinal lemniscus
nucleus
of
thalamus
Lateral white
2.
Lateral
spinothalamic
Subst
antia
Crossing in spinal cord
V
entral
posterolateral
Pain
and
temperature
column
tract
gelatinosa
forms spinal lemniscus
nucleus
of thalamus
sensations
3.
V
entral spinocerebellar
Marginal
nucleus
Crossing
in spinal cord
Anterior
lobe of
Subconscious
tract
cerebellum
kinesthetic sensations
4.
Dorsal spinocerebellar
Clarke’
s
nucleus
Uncrossed fibers
Anterior
lobe of
Subconscious
tract
cerebellum
kinesthetic sensations
5.
S
pinotect
al
tract
Chief
sensory
Crossing in spinal cord
Superior
colliculus
S
pinovisual reflex
nucleus
6.
Fasiculus
dorsolateralis
Posterior nerve
Component of lateral
Subst
antia
gelatinosa
Pain and temperature
root ganglion
spinothalamic
tract
sensations
7.
S
pinoreticular tract
Intermediolateral
Crossed and uncrossed
Reticular
formation
Consciousness
and
nucleus
fibers
of brainstem
awareness
8.
S
pinoolivary tract
Nonspecific
Uncrossed fibers
Olivary
nucleus
Proprioception
9.
S
pinovestibular tract
Nonspecific
Crossed and uncrossed
Lateral vestibular
Proprioception
fibers
nucleus
Posterior white
10.
Fasciculus
gracilis
Posterior nerve
Uncrossed
fibers
Nucleus gracilis in
T
actile
sensation
column
root ganglia
No
synap
se
in
spinal
medulla
T
actile localization
cord
T
actile discrimination
V
ibratory sensation
Conscious kinesthetic
sensation
1
1.
Fasciculus
cuneatus
Posterior
nerve
Uncrossed
fibers
Nucleus cuneatus in
S
tereognosis
root ganglia
No
synap
se in
spinal
medulla
cord
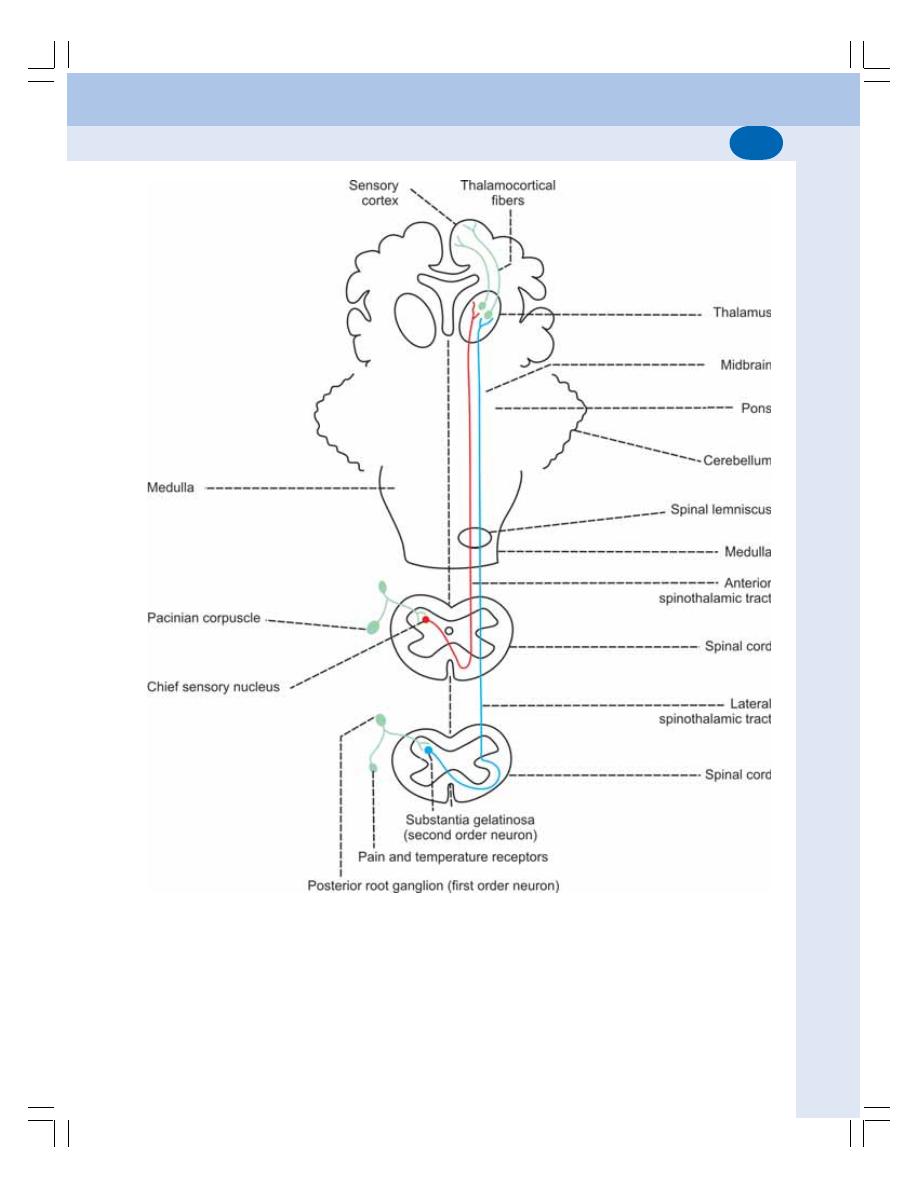
Chapter 88 Spinal Cord
537
FIGURE 88-4: Spinothalamic tracts and pathways for crude touch, pain and temperature sensations. Anterior
spinothalamic tract (red) carries crude touch sensation. Lateral spinothalamic tract (blue) carries pain and
temperature sensations
receptors. The axons of the first order neurons
reach the chief sensory nucleus through the
posterior nerve root.
Course
This tract contains crossed fibers. After taking
origin, these fibers cross obliquely in the anterior

Nervous System
538
white commissure and enter the anterior white
column of opposite side. Here, the fibers ascend
through other segments of spinal cord and
brainstem (medulla, pons and midbrain) and
reach thalamus.
Termination
The fibers of anterior spinothalamic tract
terminate in the ventral posterolateral nucleus
of thalamus. The fibers from thalamic nucleus
carry the impulses to somesthetic area (sensory
cortex) of cerebral cortex.
Function
This tract carries impulses of crude touch
(protopathic) sensation. The bilateral lesion of
this tract leads to loss of crude touch sensation.
The unilateral lesion of this tract causes loss of
crude touch sensation in the opposite side below
the level of lesion (because fibers of this tract
cross to the opposite side in spinal cord).
2. LATERAL SPINOTHALAMIC TRACT
Lateral spinothalamic tract is formed by the
fibers from the second order neurons (Fig. 88-4).
This tract is situated in the lateral white column.
Origin
The fibers of lateral spinothalamic tract take
origin from marginal nucleus and substantia
gelatinosa of Rolando.
Course
This tract has crossed fibers. After origin the
fibers of this tract cross the mid line, reach the
lateral column of opposite side and ascend. All
the fibers pass through medulla, pons and
midbrain reach thalamus.
Termination
The fibers of lateral spinothalamic tract termi-
nate in the ventral posterolateral nucleus of
thalamus. From here, third order neuron fibers
relay to the somesthetic area (sensory cortex)
of cerebral cortex.
Function
The fibers of this tract carry impulses of pain
and thermal sensations. The bilateral section of
this tract leads to total loss of pain and
temperature sensations on both the sides below
the level of lesion. The unilateral lesion or
sectioning of the lateral spinothalamic tract
causes loss of pain and temperature sensations
below the level of lesion in the opposite side.
3. VENTRAL SPINOCEREBELLAR
TRACT
Ventral spinocerebellar tract is also known as
Gower’s tract, indirect spinocerebellar tract or
anterior spinocerebellar tract. It is constituted by
the fibers of second order (Fig. 88-5). This tract
is situated in lateral white column.
Origin
The fibers of this tract arise from the marginal
nucleus in posterior gray horn. Neurons of marginal
nucleus form the second order neurons.
The first order neurons in the posterior root
ganglia receive the impulses of proprioception
from the proprioceptors in muscle, tendon and
joints. The fibers from the neurons of posterior
root ganglia reach the marginal nucleus through
posterior nerve root.
Course
This tract contains both crossed and uncrossed
fibers. Majority of the fibers from the marginal
nucleus cross the midline and ascend in lateral
white column of opposite side. Some fibers
ascend in the lateral white column of the same
side. All the fibers reach the cerebellum by
ascending through other spinal segments,
medulla, pons, midbrain and superior cerebellar
peduncle.
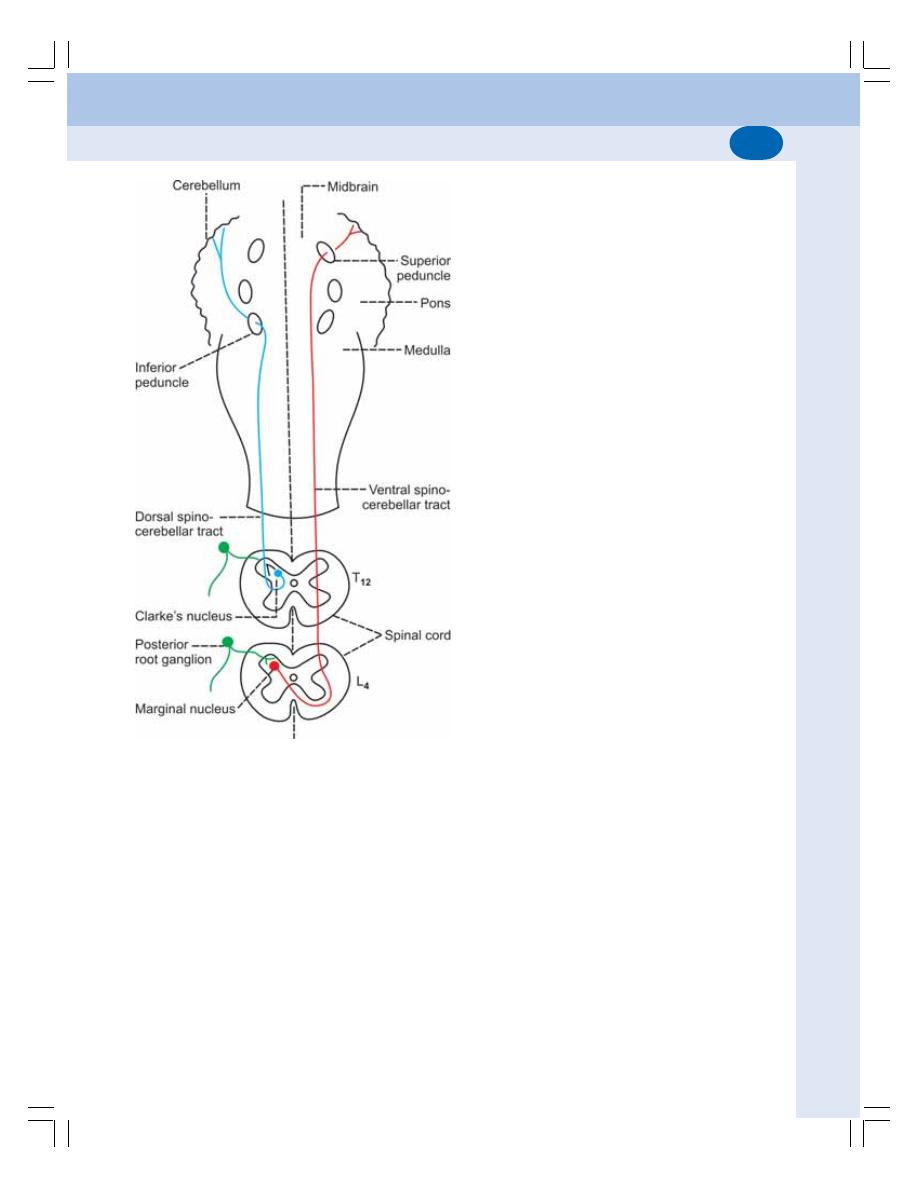
Chapter 88 Spinal Cord
539
Termination
These fibers terminate in the anterior lobe of
cerebellum.
Function
This tract carries the impulses of subconscious
kinesthetic sensation (proprioceptive impulses
from muscles, tendons and joints). The impulses
of subconscious kinesthetic sensation are also
called nonsensory impulses. The lesion of this
tract leads to loss of subconscious kinesthetic
sensation in the opposite side.
4. DORSAL SPINOCEREBELLAR
TRACT
It is otherwise called Flechsig’s tract, direct
spinocerebellar tract or posterior spinocerebellar
tract. It is formed by the second order neuron
fibers. The first order neurons are in the posterior
nerve root ganglia (Fig. 88-5). It is situated in
the lateral white column.
Origin
Fibers of this tract arise from the Clarke’s
nucleus in posterior gray matter. First
appearance of the fibers is in the upper lumbar
segments. From lower lumbar and sacral
segments, the impulses are carried upwards by
the dorsal nerve roots to the upper lumbar
segments.
Course
This tract is formed by uncrossed fibers. The
axons from neurons of Clarke’s nucleus run to
lateral column of same side ascend through other
spinal segments and reach medulla oblongata.
From here, the fibers reach the cerebellum
through inferior cerebellar peduncle.
Termination
The fibers of this tract end in the cortex of anterior
lobe of cerebellum along with ventral spino-
cerebellar tract fibers.
Function
Along with ventral spinocerebellar tract, the
dorsal spinocerebellar tract carries the impulses
of subconscious kinesthetic sensation, which are
known as nonsensory impulses. Unilateral loss
of the subconscious kinesthetic sensation occurs
in lesion of this tract on the same side, as this
tract has uncrossed fibers.
5. SPINOTECTAL TRACT
The spinotectal tract is considered as a
component of anterior spinothalamic tract. It is
constituted by the fibers of second order
neurons. It is situated in the lateral white column.
FIGURE 88-5: Spinocerebellar tracts and pathway
for subconscious kinesthetic sensation

Nervous System
540
Origin
Fibers of this tract originate from the chief
sensory nucleus. First appearance of the fibers
is in upper lumbar segments.
Course
This tract contains crossed fibers. After taking
origin, the fibers cross to opposite lateral column.
Then, the fibers ascend to the midbrain along
with anterior spinothalamic tract.
Termination
The fibers of spinotectal tract end in the superior
colliculus in midbrain.
Function
This tract is concerned with spinovisual reflex.
6. FASCICULUS DORSOLATERALIS
It is otherwise called tract of Lissauer. It is
considered as a component of lateral spino-
thalamic tract. And, it is constituted by the fibers
of first order neurons. This tract is situated in
the lateral white column.
Origin
It is formed by the fibers arising from the cells
of posterior root ganglia and enters the spinal
cord through the lateral division of posterior
nerve root.
Course
This tract contains uncrossed fibers. After
entering the spinal cord, the fibers pass up-
wards or downwards for few segments on the
same side and synapse with cells of substantia
gelatinosa of Rolando. Axons from these cells
(second order neurons) join the lateral
spinothalamic tract.
Function
The fibers of the dorsolateral fasciculus carry
impulses of pain and thermal sensations.
7. SPINORETICULAR TRACT
Spinoreticular tract is formed by the fibers of
second order neurons. It is situated in
anterolateral white column.
Origin
The fibers of this tract arise from intermediolateral
nucleus.
Course
This tract consists of crossed and uncrossed
fibers. After taking origin, some of the fibers
cross the midline and then ascend upwards.
Remaining fibers ascend up in the same side
without crossing.
Termination
All the fibers terminate in the reticular formation
of brainstem.
Function
The fibers of the spinoreticular tract are the
components of ascending reticular activating
system and are concerned with consciousness
and awareness.
8. SPINO-OLIVARY TRACT
This tract is situated in anterolateral part of white
column. Origin of the fibers of this tract is not
specific. However, the fibers terminate in the
olivary nucleus of medulla oblongata. From here,
the neurons project into cerebellum. This tract
is concerned with proprioception.
9. SPINOVESTIBULAR TRACT
The spinovestibular tract is situated in the lateral
white column of the spinal cord. The fibers of
this tract arise from all the segments of spinal
cord and terminate on the lateral vestibular
nucleus. This tract is also concerned with
proprioception.
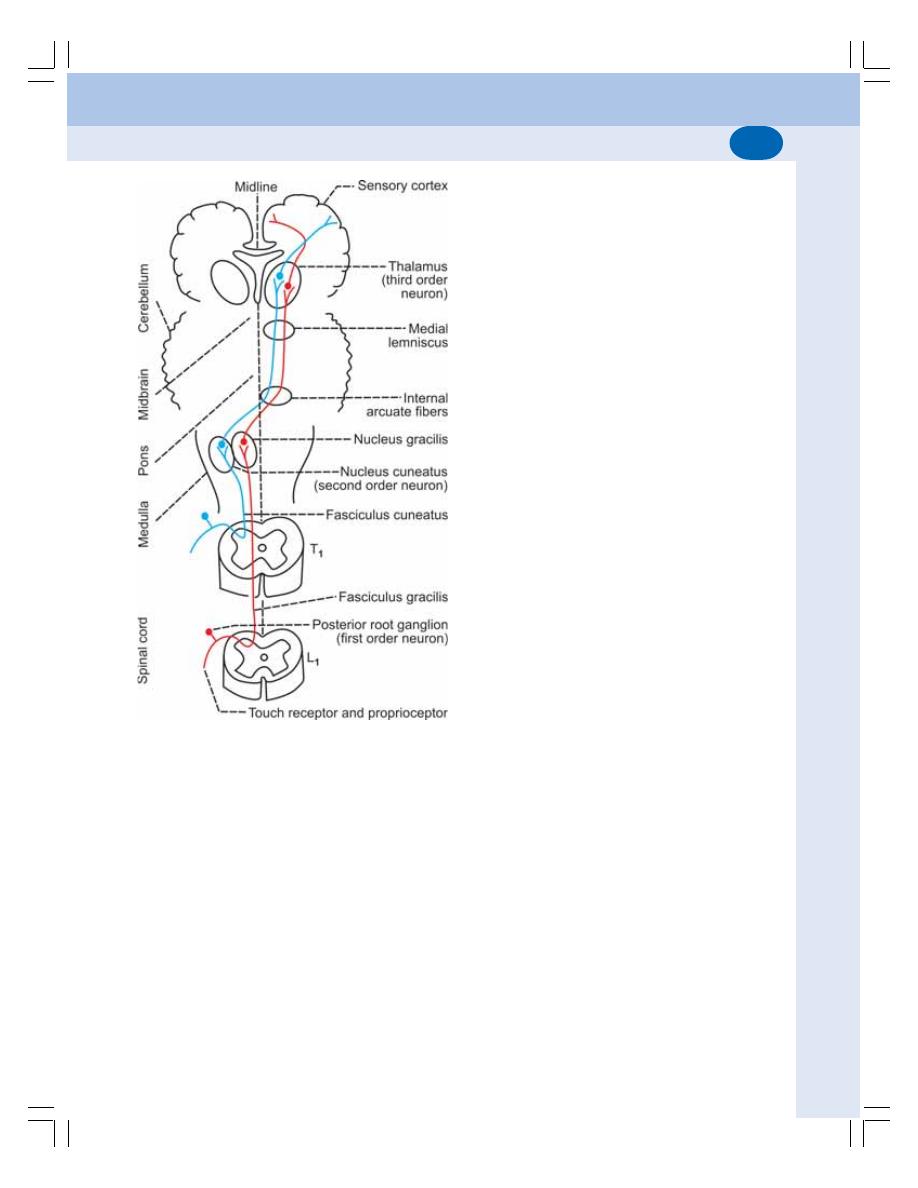
Chapter 88 Spinal Cord
541
10. FASCICULUS GRACILIS (TRACT
OF GOLL) AND FASCICULUS
CUNEATUS (TRACT OF BURDACH)
These two tracts are together called ascending
posterior column tracts. These tracts are formed
by the fibers from posterior root ganglia. Thus,
both the tracts are constituted by the fibers of
first order neurons of the sensory pathway
(Fig. 88-6).
These two tracts are situated in posterior
white column of spinal cord hence the name
posterior column tracts. In the cervical and upper
thoracic segments of spinal cord, the posterior
white column is divided into medial fasciculus
gracilis and lateral fasciculus cuneatus.
Origin
Fibers of these two tracts are the axons of first
order neurons. The cell body of these neurons
is in the posterior root ganglia and, the fibers form
the medial division (bundle) of the posterior nerve
root.
Course
After entering the spinal cord, the fibers ascend
through the posterior white column. These fibers
do not synapse in the spinal cord.
The fasciculus gracilis contains the fibers
from the lower extremities and lower parts of the
body, i.e. from sacral, lumbar and lower thoracic
ganglia of posterior nerve root. Fasciculus
cuneatus contains fibers from upper part of the
body, i.e. from upper thoracic and cervical
ganglia of posterior nerve root.
Termination
These two tracts terminate in the medulla
oblongata. The fibers of fasciculus gracilis
terminate in the nucleus gracilis and the fibers
of fasciculus cuneatus terminate in the nucleus
cuneatus. The cells of these medullary nuclei
form the second order neurons.
The axons of the second order neurons form
the internal arcuate fibers. The internal arcuate
fibers from both the sides cross the midline
forming sensory decussation and then ascend
through pons and midbrain as medial lemniscus.
The fibers of medial lemniscus terminate in
ventral posterolateral nucleus of thalamus. From
here, fibers of the third order neurons relay to
sensory area of cerebral cortex.
Functions
The tracts of the posterior white column convey
impulses of following sensations:
i. Fine (epicretic) tactile (touch) sensation
ii. Tactile localization: It is the ability to locate
the area of skin where the tactile stimulus
is applied with closed eyes
FIGURE 88-6: Ascending tracts in posterior white
column of spinal cord and pathway for – (i) Fine touch
sensation, (ii) Tactile localization, (iii) Tactile
discrimination, (iv) Vibratory sensation, (v) Conscious
kinesthetic sensation and (vi) Stereognosis
Nervous System
542
iii. Tactile discrimination (two point
discrimination): It is the ability to recognize
the two stimuli applied over the skin
simultaneously with closed eyes
iv. Sensation of vibration: It is the ability to
perceive the vibrations (from a vibrating
tuning fork placed over bony prominence)
conducted to deep tissues through skin
v. Conscious kinesthetic sensation: It is the
sensation or awareness of various
muscular activities in different parts of the
body
vi. Stereognosis: It is the ability to recognize
the known objects by touch with closed
eyes.
Effect of Lesion
The lesion in the fibers of these tracts or lesion
in the posterior white column leads to the
following symptoms on the same side below the
lesion:
i. Loss of fine tactile sensation. However,
crude touch sensation is normal
ii. Loss of tactile localization
iii. Loss of two point discrimination
iv. Loss of sensation of vibration
v. Astereognosis: It is the inability to
recognize known objects by touch while
closing the eyes
vi. Lack of ability to differentiate the weight
of different objects
vii. Loss of proprioception: It is inability to
appreciate the position and movement of
different parts of the body
viii. Sensory ataxia or posterior column ataxia:
It is the condition characterized by
uncoordinated, slow and clumsy voluntary
movements because of the loss of
proprioception.
DESCENDING TRACTS OF SPINAL
CORD
The descending tracts of the spinal cord are
formed by motor nerve fibers arising from brain
and descend into the spinal cord. These tracts
carry motor impulses from brain to spinal cord.
The descending tracts of the spinal cord are of
two types:
I.
Pyramidal tracts
II. Extrapyramidal tracts.
The descending tracts are listed in Table 88-
3. The features of the descending are given in
Table 88-4.
PYRAMIDAL TRACTS
The pyramidal tracts were the first tracts to be
found in man. These tracts of the spinal cord are
concerned with voluntary motor activities of the
body. These tracts are otherwise known as
corticospinal tracts. There are two corticospinal
tracts, the anterior corticospinal tract and lateral
corticospinal tract.
While running from cerebral cortex towards
spinal cord, the fibers of these two tracts give
the appearance of a pyramid on the upper part of
anterior surface of medulla oblongata (Fig. 88-7)
and hence the name pyramidal tracts.
Origin
Fibers of pyramidal tracts arise from the following
nerve cells in the cerebral cortex:
i. Giant cells or Betz cells or pyramidal cells
situated in area 4 (primary motor area) of
frontal lobe
ii. Premotor area (area 6) and supplementary
motor areas
iii. Other parts of frontal lobe
iv. Somatosensory areas of parietal lobe.
TABLE 88-3: List of descending tracts of spinal
cord
Type
Tract
Pyramidal tracts
1. Anterior corticospinal tract
2. Lateral corticospinal tract
Extrapyramidal
1. Medial longitudinal fasciculus
tracts
2. Anterior vestibulospinal tract
3. Lateral vestibulospinal tract
4. Reticulospinal tract
5. Tectospinal tract
6. Rubrospinal tract
7. Olivospinal tract
Chapter 88 Spinal Cord
543
TABLE 88-4:
D
escending tract
s of spinal cord
T
ract
Situation
Origin
Course
Function
1.
Anterior corticospinal tract
Anterior
white
Motor
and
somato-
Uncrossed
fibers
i.
Control
of
volunt
a
ry
column
sensory areas of
movement
s
cerebral
cortex
ii
.
Form upper motor neurons
2.
Lateral corticospinal tract
Lateral
white
Motor
and
somato-
Crossed fibers
column
sensory areas of
cerebral cortex
1.
Medial longitudinal
Anterior
white
V
estibular
nucleus
Uncrossed
fibers
i.
Coordination of reflex ocular
fasciculus
column
Reticular formation
Extend up to upper
movement
s
Superior
colliculus
cervical
segment
s
ii
.
Integration of movement
s of
and cells of Cajal
eyes and neck
2.
Anterior
vestibulospinal
Anterior white
Medial
vestibular
Uncrossed
fibers
i.
Maintenance of muscle tone
tract
column
nucleus
Extend up to upper
and
posture
thoracic
segment
s
ii
.
Maintenance of position of
head and body during
acceleration
3.
Lateral vestibulospinal
Lateral
white
Lateral
vestibular
Mostly
uncrossed
tract
column
nucleus
Extend to all segments
4.
Reticulospinal
tract
Anterior
white
Reticular
formation
Mostly
uncrossed
i.
Coordination of volunt
ary and
fa
s
c
ic
u
lu
s
of pons and medulla
Extend
up
to
thoracic
reflex
movements
segment
s
ii.
Control of muscle tone
iii.
Control of respiration and
diameter of blood vessels
5.
T
ectospinal
tract
Anterior
white
Superior
colliculus
Crossed
fibers
Control of movement of head
column
Extend
up
to
lower
in response to visual and
cervical segment
s
auditory impulses
6.
Rubrospinal
tract
Lateral white
Red nucleus
Crossed
fibers
Facilit
atory influence on flexor
column
E
x
te
n
d
u
p
t
o
t
h
o
ra
c
ic
muscle tone
segment
s
7.
Olivospinal
tract
Lateral white
Inferior olivary nucleus
Mostly
crossed
Control
of
movement
s
due
to
column
Extent – not
clear
proprioception
T
ermination – Fibers of all the tract
s terminate in motor neurons situated in the anterior gray horn of spinal cord.
Extrapyramidal tracts
Pyramidal tract
s
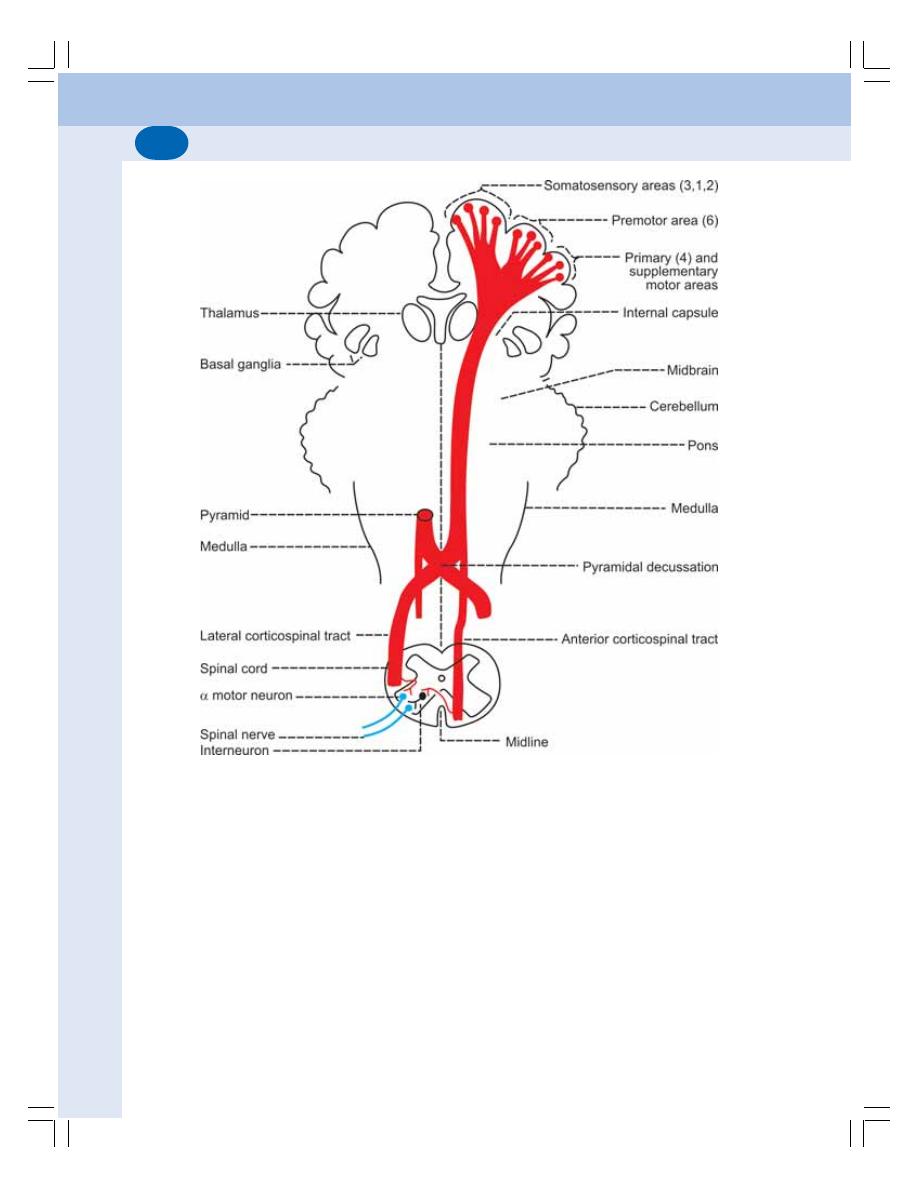
Nervous System
544
Course
After taking origin, the nerve fibers run downwards
through cerebral hemisphere and converge in the
form of a fan like structure called corona radiata.
Then the fibers descend down through
internal capsule, midbrain and pons. In the upper
part of medulla these fibers give the appearance
of a pyramid. In the lower part of medulla, 80%
of fibers from each side cross to the opposite
side. While crossing the midline, the fibers of both
sides form the pyramidal decussation.
After crossing and forming pyramidal
decussation, these fibers descend through the
posterior part of lateral white column of the spinal
cord as crossed pyramidal tract or lateral
corticospinal tract or indirect corticospinal tract.
The remaining 20% of fibers do not cross to
the opposite side but descend down through the
anterior white column of the spinal cord as
uncrossed pyramidal tract or anterior corti-
cospinal tract or direct corticospinal tract.
Termination
All the fibers of pyramidal tracts terminate in the
motor neurons of anterior gray horn. The axons
of the motor neurons leave the spinal cord as
FIGURE 88-7: Pyramidal tracts

Chapter 88 Spinal Cord
545
spinal nerves through anterior nerve roots and
supply the skeletal muscles.
The neurons giving origin to the fibers of
pyramidal tract are called the upper motor
neurons. The motor neurons in the spinal cord
are called the lower motor neurons.
Function
The pyramidal tracts are concerned with
voluntary movements of the body. Fibers of the
pyramidal tracts transmit motor impulses from
motor area of cerebral cortex to the anterior
motor neurons of the spinal cord. These two
tracts are responsible for fine, skilled movements.
The lesion in the neurons of motor cortex and
the fibers of pyramidal tracts is called the upper
motor neuron lesion. Effects of upper motor
lesion are given in the next Chapter.
EXTRAPYRAMIDAL TRACTS
The descending tracts of spinal cord other than
pyramidal tracts are called extrapyramidal tracts.
Extrapyramidal tracts are listed in Table 88-3.
1. MEDIAL LONGITUDINAL
FASCICULUS
Origin
The fibers of this tract take origin from brainstem.
It situated in anterior white column of the spinal
cord.
Course
After entering the spinal cord from the brainstem,
the fibers descend through anterior white column
of the same side. In the spinal cord, this tract
runs along with anterior vestibulospinal tract.
Termination
The fibers of this tract terminate in anterior motor
neurons of the spinal cord along with fibers of
anterior vestibulospinal tract.
Function
This tract helps in the coordination of reflex ocular
movements and the integration of ocular and
neck movements. Reflex ocular movements and
reflex neck movements are affected in the lesion
of this tract.
2. ANTERIOR VESTIBULOSPINAL
TRACT
Origin
The fibers of this tract arise from the medial
vestibular nucleus in medulla oblongata. It is
situated in the anterior white column.
Course
The fibers of this tract run down from medulla
into the anterior column of spinal cord. All the
fibers are uncrossed.
Termination
Along with fibers of lateral vestibulospinal tract,
the fibers of this tract terminate in anterior motor
neurons directly or through internuncial neurons.
Function
The function of this tract is explained along with
the function of lateral vestibulospinal tract.
3. LATERAL VESTIBULOSPINAL
TRACT
Origin
The fibers of this tract take origin from the lateral
vestibular nucleus in medulla. This tract occupies
the lateral white column of spinal cord.
Course
From medulla, most of the fibers descend directly
through lateral column.
Termination
The fibers of this tract terminate in the anterior
motor neurons.
Functions
The vestibulospinal tracts are concerned with
adjustment of position of head and body during

Nervous System
546
angular and linear acceleration. During the lesion
of these tracts the adjustment of head and body
becomes difficult while walking.
4. RETICULOSPINAL TRACT
Origin
Fibers of this tract arise from the reticular
formation of pons and medulla. These fibers
descend in anterior column and to some extend
in the anterior part of lateral column. The
reticulospinal tract is situated in the anterior white
column.
Termination
The fibers of reticulospinal tract terminate in the
gamma motor neurons of anterior gray horn.
Functions
The reticulospinal tract is concerned with control
of movements and maintenance of muscle tone,
respiration and diameter of blood vessels. Lesion
of this tract causes disturbances in respiration,
blood pressure, movements of body and muscle
tone.
5. TECTOSPINAL TRACT
Origin
The nerve fibers of this tract arise from superior
colliculus of midbrain. This tract is situated in
the anterior white column of the spinal cord.
Course
After taking origin from the superior colliculus, the
fibers cross the midline in the dorsal tegmental
decussation and descend in anterior column.
Termination
The fibers of this tract terminate in the anterior
motor neurons of the spinal cord.
Function
This tract is responsible for the movement of
head in response to visual and auditory stimuli.
6. RUBROSPINAL TRACT
Origin
The fibers of this tract arise from red nucleus in
midbrain. The rubrospinal tract is situated in the
lateral white column.
Course
After arising from the red nucleus, the fibers cross
the midline and descend into spinal cord through
the reticular formation of pons and medulla.
Termination
The fibers of rubrospinal tract end in the anterior
motor neurons of the spinal cord.
Function
This tract exhibits facilitatory influence upon the
flexor muscle tone.
7. OLIVOSPINAL TRACT
Origin
The nerve fibers of this tract take origin from the
inferior olivary nucleus present in the medulla
oblongata. The olivospinal tract is present in the
lateral white column.
Termination
The fibers of this tract terminate in the anterior
motor neurons of the spinal cord.
Function
This tract is involved in reflex movements arising
from the proprioceptors.
SOMATOSENSORY SYSTEM
DEFINITION AND TYPES OF SENSATIONS
TYPES OF SOMATIC SENSATIONS
SENSORY PATHWAYS
SENSORY FIBERS OF TRIGEMINAL NERVE
APPLIED PHYSIOLOGY
SOMATOMOTOR SYSTEM
MOTOR ACTIVITIES OF THE BODY
SOMATOMOTOR SYSTEM
CLASSIFICATION OF MOTOR PATHWAYS
UPPER MOTOR NEURON AND LOWER MOTOR NEURON
APPLIED PHYSIOLOGY
Somatosensory System and
Somatomotor System
89
SOMATOSENSORY SYSTEM
DEFINITION AND TYPES OF
SENSATIONS
Somatosensory system is defined as sensory
system associated with different parts of the
body. It is also defined as the faculty of bodily
perception of various sensations.
Sensations are of two types:
1. Somatic sensations
2. Special sensations.
1. Somatic Sensations
Somatic sensations are the sensations arising
from skin, muscles, tendons and joints. These
sensations have specific receptors, which
respond to a particular type of stimulus.
2. Special Sensations
Special sensations are the complex sensations
for which the body has some specialized sense
organs. The special sensations are usually called
special senses. Sensations of vision, hearing,
taste and smell are the special sensations.
This chapter deals with somatic sensations.
TYPES OF SOMATIC SENSATIONS
Generally, somatic sensations are classified into
three types:
A. Epicretic sensations
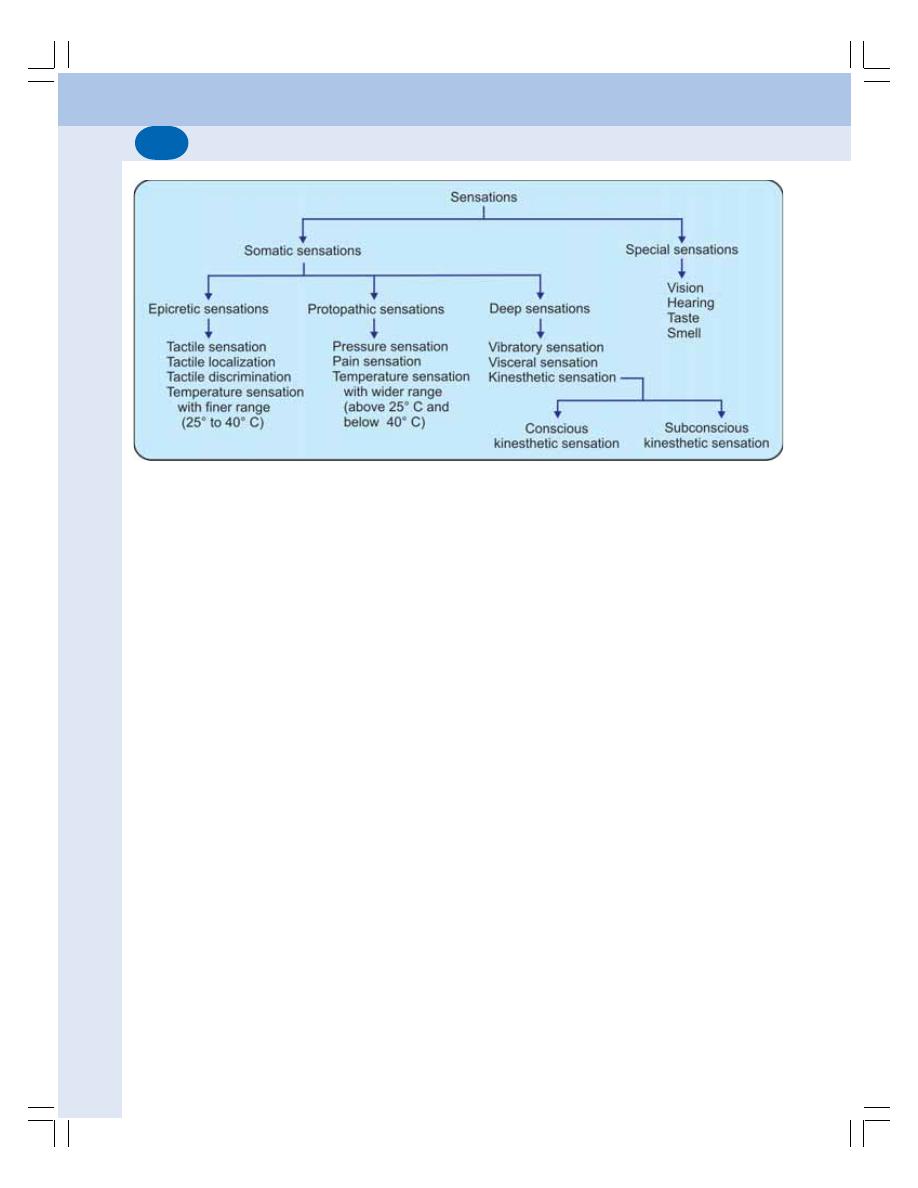
Nervous System
548
B. Protopathic sensations
C. Deep sensations (Fig. 89-1).
A. Epicretic Sensations
Epicretic sensations are the mild or light sensa-
tions. Such sensations are perceived more
accurately. Epicretic sensations are:
1. Fine touch or tactile sensation
2. Tactile localization
3. Tactile discrimination
4. Temperature sensation with finer range
between 25 and 40°C.
B. Protopathic Sensations
Protopathic sensations are the crude sensations.
Protopathic sensations are:
1. Pressure sensation
2. Pain sensation
3. Temperature sensation with a wider range,
i.e. above 40°C and below 25°C.
C. Deep Sensations
Deep sensations are the sensations arising
from the deeper structures beneath the skin and
the visceral organs. The deep sensations are
classified into three types:
1. Sensation of vibration or pallesthesia
2. Kinesthetic sensation or kinesthesia:
Sensation of position and movements of
FIGURE 89-1: Classification of sensations
different parts of the body. This sensation
arises from the proprioceptors present in
muscles, tendons, joints and ligaments.
The kinesthetic sensation is of two types.
i. Conscious kinesthetic sensation
ii. Subconscious kinesthetic sensation. The
impulses of this sensation are called non-
sensory impulses.
3. Visceral sensations arising from viscera.
SENSORY PATHWAYS
The nervous pathways of the sensations are
called the sensory pathways. These pathways
carry the impulses from the receptors in different
parts of the body to the centers in brain.
The sensory pathways are of two types:
1. Pathways of somatosensory system
2. Pathways of viscerosensory system.
The pathways of somatosensory system
convey the information from the sensory
receptors in skin, skeletal muscles and joints.
The pathways of this system are constituted by
somatic nerve fibers called somatic afferent
nerve fibers.
The pathways of viscerosensory system
convey the information from the receptors of
the viscera. The pathways of this system are
constituted by visceral or autonomic fibers.
This chapter deals mainly with the somato-
sensory system.
Chapter 89 Somatosensory System and Somatomotor System
549
TABLE 89-1
:
Sensory p
athways
Sensation
Receptor
First order
Second order
Third
order
Center
neuron in
neuron in
neuron in
Fine
touch
Meissner
’s
corpuscles
Posterior nerve root
Nucleus
gracilis
and
V
entral
posterolateral
Sensory
cortex
T
actile
localization
and Merkel’s disk
ganglion – fibers form
Nucleus
cuneatus
–
nucleus
of
thalamus
T
actile
discrimination
Fasciculus gracilis and
Fibers
form
internal
V
ibratory sensation
Fasciculus
cuneatus
arcuate fibers
S
tereognosis
Pressure
Pacinians
corpuscle
Posterior nerve root
Chief
sensory
V
entral
posterolateral
Sensory
cortex
Crude touch
ganglion
nucleus
–
fibers
nucleus of thalamus
form anterior
spinothalamic tract
T
emperature
W
armth-Raf
fini’
s
Posterior nerve root
Subst
antia gelatinosa –
V
entral
posterolateral
Sensory
cortex
end bulb
ganglion
fibers
form
lateral
nucleus of thalamus
Cold – Krause’
s end bulb
spinothalamic
tract
Conscious
Proprioceptors
–
Posterior nerve root
Nucleus
gracilis
and
V
entral
posterolateral
Sensory
cortex
kinesthetic
Muscle
spindle
ganglion – fibers form
Nucleus
cuneatus
–
nucleus
of
thalamus
sensation
Golgi
tendon
Fasciculus gracilis and
Fibers
form
internal
app
aratus
Fasciculus cuneatus
arcuate
fibers
Subconscious
Proprioceptors
–
Posterior nerve root
Nucleus of Clarke and
Anterior
lobe
kinesthetic
Muscle spindle
ganglion
Marginal nucleus –
of
cerebellum
sensation
Golgi
tendon
fibers form dorsal and
—
—
—
app
aratus
ventral spinocerebellar
tract
s
Pain
Free
nerve
endings
Posterior nerve root
Fast p
ain – marginal
V
entral
posterolateral
Sensory
cortex
ganglion
nucleus in spinal
cord
nucleus of thalamus
Fast p
ain –
A
δ
fibers
Slow pain
–
substantia
reticular formation
Slow p
ain – C fibers
gelatinosa
of
Rolando
and
midbrain
Fibers form lateral
spinothalamic tract
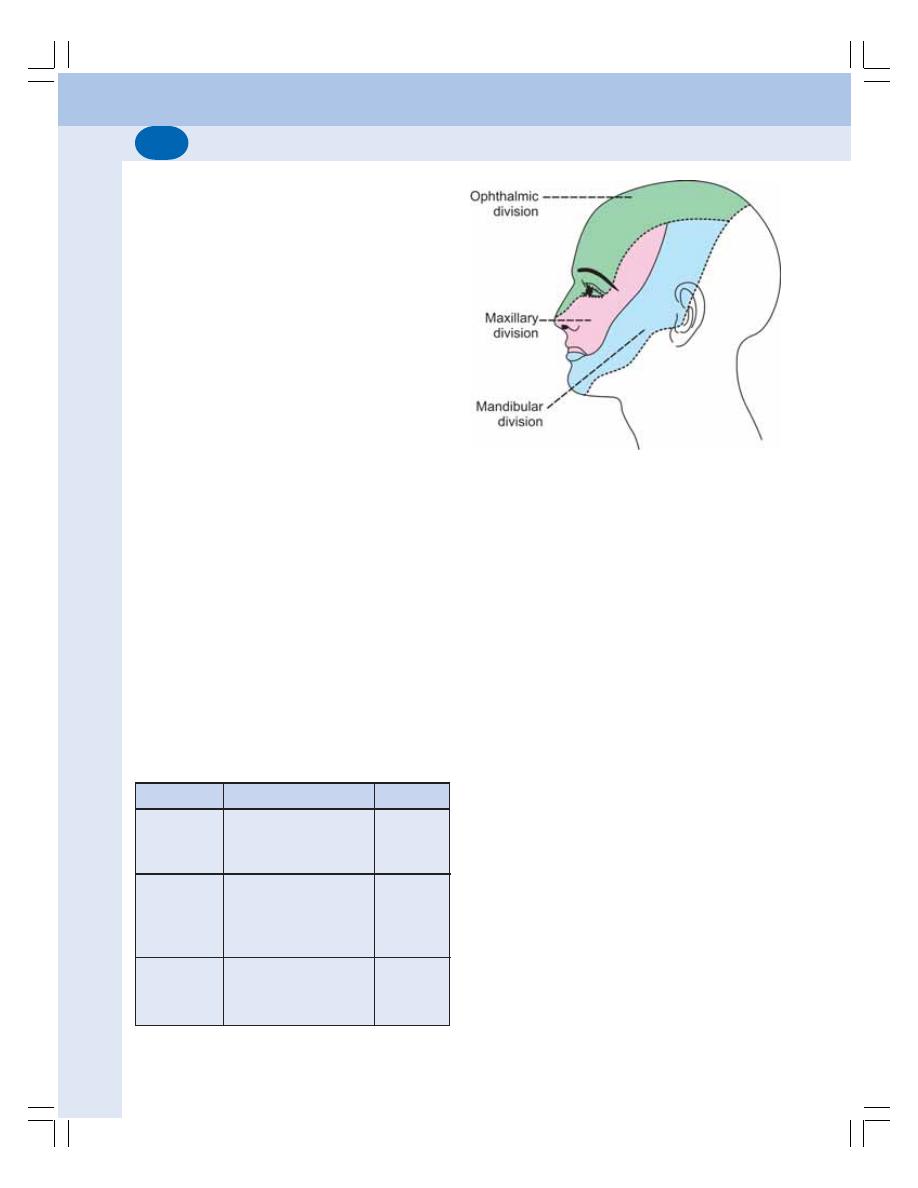
Nervous System
550
Somatosensory Pathways
Each sensory pathway is constituted by two or
three groups of neurons:
1. First order neurons
2. Second order neurons
3. Third order neurons.
The details of these neurons are given in
Chapter 88. The pathways of some of the
sensations like kinesthetic sensation have only
first and second order neurons.
The details of the pathways are given in Table
89-1. The diagrams of the pathways are given
in the previous chapter along with ascending
tracts of spinal cord.
SENSORY FIBERS OF TRIGEMINAL
NERVE
Trigeminal nerve is a mixed cranial nerve. It is
the chief sensory nerve for face and the motor
nerve for muscles of mastication. Trigeminal
nerve carries somatosensory information from
face, teeth, periodontal tissues (tissues around
teeth), oral cavity, nasal cavity, cranial dura mater
and major part of scalp to sensory cortex. It also
conveys proprioceptive impulses from the
extrinsic muscles of the eyeball. The functions
of three divisions of trigeminal nerve are listed
in Table 89-2.
TABLE 89-2: Functions of three divisions of
trigeminal nerve
Division
Areas supplied
Function
Ophthalmic
Forehead
Sensory
Eye
Front portion of nose
Sensory
Maxillary
Upper teeth, gums
and lip
Lower eyelid
Sides of nose
Mandibular
Lower teeth,
Sensory
gums and lip
Jaw
Motor
FIGURE 89-2: Cutaneous distribution (sensory) of
the three divisions of trigeminal nerve
Origin
The sensory fibers of trigeminal nerve arise from
the trigeminal ganglion situated near temporal
bone. The peripheral processes of neurons in
this ganglion form three divisions of trigeminal
nerve namely, ophthalmic, mandibular and
maxillary divisions. The cutaneous distribution
of the three divisions of trigeminal nerve is shown
in Figure 89-2.
The central processes from the neurons of
trigeminal ganglion enter the pons in the form
of sensory root.
Termination
After reaching the pons, the fibers of sensory
root divide into two groups namely, descending
fibers and ascending fibers. The descending
fibers terminate on primary sensory nucleus and
spinal nucleus of trigeminal nerve.
The ascending fibers of the sensory root
terminate in the mesencephalic nucleus of
trigeminal nerve situated in the brainstem above
the level of primary sensory nucleus (Fig. 89-3).
Central Connections
Majority of fibers from the primary sensory
nucleus and spinal nucleus of trigeminal nerve
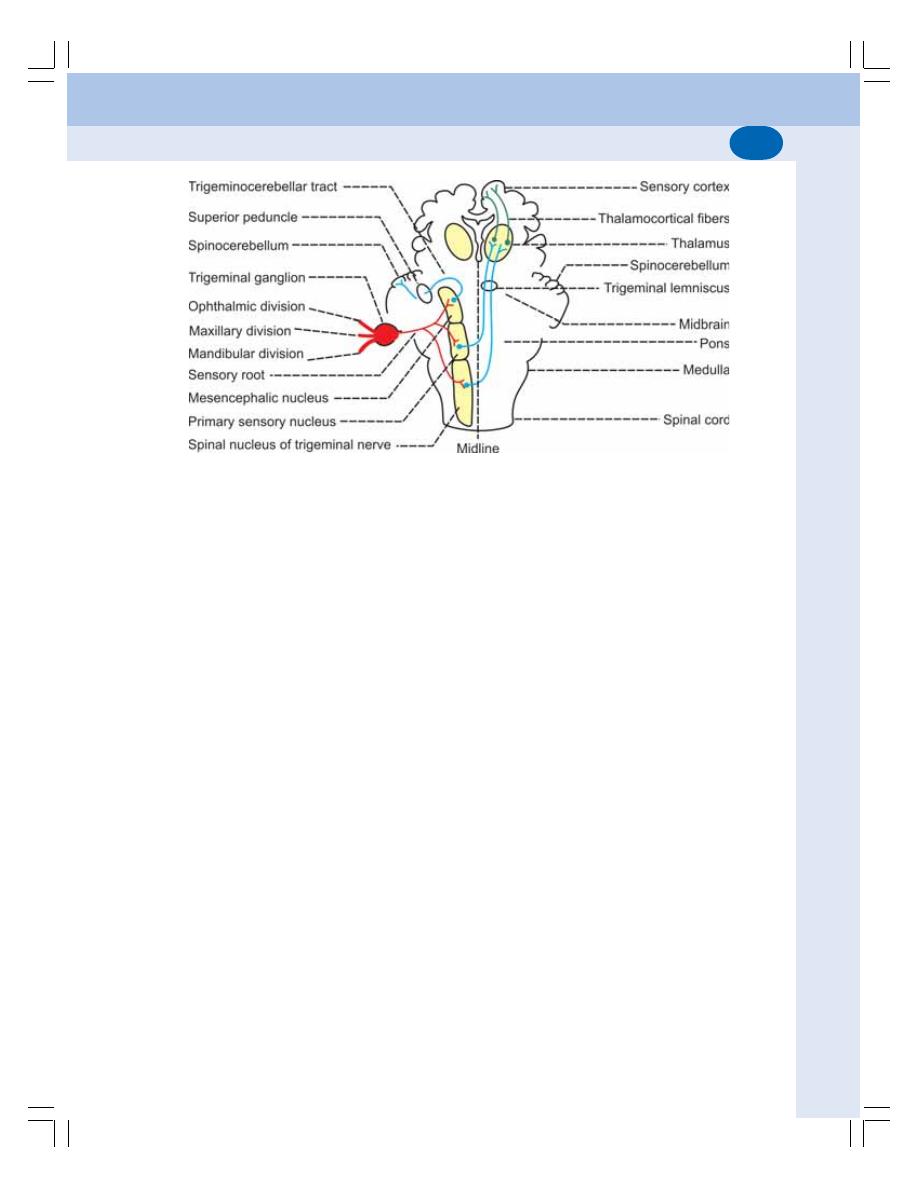
Chapter 89 Somatosensory System and Somatomotor System
551
ascend in the form of trigeminal lemniscus and
terminate in the ventral posteromedial nucleus
of thalamus in the opposite side. Remaining
fibers from these two nuclei terminate on the
thalamic nucleus of the same side. From
thalamus, the fibers reach the somatosensory
areas of cerebral cortex.
The primary sensory nucleus and the spinal
nucleus of trigeminal nerve relay the sensations
of touch, pressure, pain and temperature from
the regions mentioned above.
The fibers from mesencephalic nucleus form
the trigeminocerebellar tract that enters
spinocerebellum via the superior cerebellar
peduncle of the same side. This nucleus
conveys the proprioceptive impulses from the
facial muscles, muscles of mastication and
ocular muscles.
APPLIED PHYSIOLOGY
Lesions in sensory pathway affect the sensory
functions of the body:
1. Anesthesia: Loss of all sensations
2. Hyperesthesia: Increased sensitivity to
sensory stimuli
3. Hypoesthesia: Reduction in the sensitivity
to sensory stimuli
4. Hemiesthesia: Loss of all sensations in
one side of the body
5. Paresthesia: Abnormal sensations such
as tingling, burning, prickling and
numbness
6. Hemiparesthesia: Abnormal sensations in
one side of the body
7. Dissociated anesthesia: Loss of some
sensations while other sensations are
intact
8. General anesthesia: Loss of all sensa-
tions with loss of consciousness produced
by anesthetic agents
9. Local anesthesia: Loss of sensations in
a restricted area of the body
10. Spinal anesthesia: Loss of sensations,
due to lesion in spinal cord or induced by
anesthetic agents injected beneath the
coverings of spinal cord
11. Tactile anesthesia: Loss of tactile sensa-
tions
12. Tactile hyperesthesia: Increased sensitivity
to tactile stimuli
13. Analgesia: Loss of pain sensation
14. Hyperalgesia: Increased sensitivity to pain
stimulus
15. Paralgesia: Abnormal pain sensation
FIGURE 89-3: Diagrammatic representation of trigeminal pathway. Trigeminal lemniscus carries impulses
of touch, pressure, pain and temperature sensations to somatosensory cortex. The trigeminocerebellar tract
carries proprioceptive impulses to spinocerebellum

Nervous System
552
16. Thermoanesthesia or thermanesthesia or
thermanalgesia: Loss of thermal sensation
17. Pallanesthesia: Loss of sensation of
vibration
18. Astereognosis: Loss of ability to recognize
any known object with closed eyes due
to loss of cutaneous sensations
19. Illusion: Mental depression due to
misinterpretation of a sensory stimulus
20. Hallucination: Feeling of a sensation
without any stimulus.
SOMATOMOTOR SYSTEM
MOTOR ACTIVITIES OF THE BODY
The motor activities of the body are divided into
two types:
1. The activities of skeletal muscles which are
involved in the posture and movement
2. The activities of smooth muscles, cardiac
muscles and other tissues, which are involved
in the functions of various visceral organs.
The activities of the skeletal muscles
(voluntary functions) are controlled by the
somatomotor system, which is constituted by the
somatic motor nerve fibers. The activities of
tissues or the visceral organs (involuntary
functions) are controlled by the visceral or
autonomic nervous system, which is constituted
by the sympathetic and parasympathetic
systems. Autonomic nervous system is
described in Chapter 103.
This chapter deals with somatomotor system.
TYPES OF MOVEMENTS
The movements of the body depend upon the
different groups of skeletal muscles. Various
types of movements or the motor activities
brought about by these muscles are:
1. Execution of smooth, precise and accurate
voluntary movements
2. Coordination of movements responsible for
skilled activities
3. Coordination of movements responsible for
maintenance of posture and equilibrium.
All these motor activities are controlled by
different parts of the nervous system, which are
together called the motor system.
The motor system includes spinal cord and
its nerves, cranial nerves, brainstem, cerebral
cortex, cerebellum and basal ganglia. The
neuronal circuits between these parts of the
nervous system which are responsible for the
motor activities are called the motor pathways.
CLASSIFICATION OF MOTOR
PATHWAYS
Motor pathways are divided into pyramidal and
extrapyramidal tracts.
Pyramidal Tracts
The pyramidal tracts are those fibers, which form
the pyramids in the upper part of medulla.
Pyramidal tracts are the anterior and lateral
corticospinal tracts. These tracts control the
voluntary movements of the body (Chapter 88).
Extrapyramidal Tracts
Motor pathways other than pyramidal tracts are
known as extrapyramidal tracts. Details of theses
tracts are given in Chapter 88. The extrapyramidal
tracts are concerned with regulation of tone,
posture and equilibrium.
UPPER MOTOR NEURON AND
LOWER MOTOR NEURON
The neurons of the motor system are divided
into upper motor neurons and lower motor
neurons depending upon their location and
termination.
Upper Motor Neuron
Upper motor neurons are the neurons in the
higher centers of brain, which control the lower
motor neurons. There are three types of upper
motor neurons:
1. Motor neurons in the cerebral cortex. The
fibers of these neurons form corticospinal
(pyramidal) and corticobulbar tracts
Chapter 89 Somatosensory System and Somatomotor System
553
2. Neurons in the basal ganglia and brainstem
nuclei
3. Neurons in the cerebellum.
The motor neurons in the cerebral cortex,
which give origin to pyramidal tracts, belong to
the pyramidal system and the remaining motor
neurons belong to extrapyramidal system.
Lower Motor Neuron
Lower motor neurons are the anterior gray horn
cells in the spinal cord and the motor neurons
of the cranial nerve nuclei situated in brainstem,
which innervate the muscles directly.
The lower motor neurons constitute the “Final
common pathway” of motor system. The lower
motor neurons are under the influence of the
upper motor neurons.
APPLIED PHYSIOLOGY
Effects of Lesion of Motor Neurons
The effects of lesions of upper motor neurons
and lower motor neurons are given in Table 89-3.
The effects of lower motor neuron lesion are the
loss of muscle tone and flaccid paralysis. The
effects of upper motor neuron lesion depend
upon the site:
1. The lesion in pyramidal system causes
hypertonia and spastic paralysis
2. Lesion in basal ganglia produces hypertonia
and rigidity involving both flexor and extensor
muscles
3. Lesion in cerebellum causes hypotonia,
muscular weakness and incoordination of
movements.
Paralysis
Paralysis is defined as the complete loss of
strength and functions of muscle group or a limb.
Causes for paralysis
Common causes for paralysis are trauma, tumor,
stroke, cerebral palsy (condition caused by brain
injury immediately after birth) and neurodege-
nerative diseases.
TABLE 89-3: Effects of upper motor neuron lesion and lower motor neuron lesion
Effects
Upper motor neuron
Lower motor neuron
lesion
lesion
1. Muscle tone
Hypertonia
Hypotonia
2. Paralysis
Spastic type of paralysis
Flaccid type of paralysis
3. Wastage of muscle
No wastage of muscle
Wastage of muscle occurs
4. Superficial reflexes
Lost
Lost
5. Plantar reflex
Abnormal plantar reflex –
Plantar reflex – absent
Babinski’s sign
6. Deep reflexes
Exaggerated
Lost
7. Clonus
Present
Not present
8. Electrical activity
Normal
Absent
9. Muscles affected
Groups of muscles are
Individual muscles are
affected
affected
10. Fascicular twitch in EMG
Absent
Present
Clinical observation
Clinical
confir
-
mation
Nervous System
554
Types of paralysis
The paralysis of the muscles in the body
depends upon the type and location of motor
TABLE 89-4: Types of paralysis
Paralysis
Definition
Causes
Monoplegia
Paralysis of one limb
Isolated damage of central nervous system
or peripheral nervous system
Diplegia
Paralysis of both the upper limbs or
Isolated damage of brain
both the lower limbs
Hemiplegia
Paralysis of upper limb and lower limb
Lesion in motor cortex and corticospinal
on one side of the body
tracts in posterior limb of internal capsule
on the side opposite the paralysis
Paraplegia
Paralysis of both the lower limbs
Injury to lower part of spinal cord
Quadriplegia or
Paralysis of all the four limbs
Injury to upper part of spinal cord (shoulder
Tetraplegia
level or above at which the motor nerves
of upper limbs leave the spinal cord)
neurons affected by lesion. Different types of
paralysis are given in Table 89-4.
INTRODUCTION AND DEFINITION
BENEFITS OF PAIN SENSATION
COMPONENTS OF PAIN SENSATION
PATHWAYS OF PAIN SENSATION
VISCERAL PAIN
REFERRED PAIN
ANALGESIA SYSTEM
APPLIED PHYSIOLOGY
Physiology of Pain
90
INTRODUCTION AND DEFINITION
Pain is defined as an unpleasant and emotional
experience associated with or without actual
tissue damage. The pain sensation is described
in many ways like sharp, pricking, electric, dull
ache, shooting, cutting, stabbing, etc. Often it
induces crying and fainting.
It is produced by real or potential injury to the
body. Often it is expressed in terms of injury. For
example, pain produced by fire is expressed as
burning sensation; pain produced by severe
sustained contraction of skeletal muscles is
expressed as cramps.
BENEFITS OF PAIN SENSATION
Pain is an important sensory symptom. Though
it is an unpleasant sensation, it has protective
or survival benefits such as:
1. It gives warning signal about the existence
of a problem or threat. It also creates the
awareness of injury
2. It prevents further damage by causing reflex
withdrawal of the body from the source of
injury
3. It forces the person to rest or to minimize the
activities thus enabling the rapid healing of
the injured part
4. It urges the person to take required treatment
to prevent major damage.
COMPONENTS OF PAIN SENSATION
A pain stimulus produces two pain sensations
1. Fast pain
2. Slow pain.
Fast pain is the first sensation whenever a
pain stimulus is applied. It is experienced as a
bright, sharp and localized pain sensation. The
fast pain is followed by the slow pain which is
experienced as a dull, diffused and unpleasant
pain.
The receptors for both the components of
pain are the same, i.e. the free nerve endings.
But, the afferent nerve fibers are different. The

Nervous System
556
fast pain sensation is carried by A
δ fibers and
the slow pain sensation is carried by C type of
nerve fibers.
PATHWAYS OF PAIN SENSATION
Pain sensation from various parts of body is
carried to brain by different pathways which are:
1. Pathway from skin and deeper structures
2. Pathway from face
3. Pathway from viscera
4. Pathway from pelvic region.
PATHWAY OF PAIN SENSATION
FROM SKIN AND DEEPER
STRUCTURES
Receptors
The receptors of pain sensation are the free
nerve endings which are distributed throughout
the body.
First Order Neurons
First order neurons are the cells in the posterior
nerve root ganglia which receive the impulses
of pain sensation from the pain receptors
through their dendrites. These impulses are
transmitted to spinal cord through the axons of
these neurons.
Fast pain fibers
Fast pain sensation is carried by A
δ type afferent
fibers which synapse with neurons of marginal
nucleus in the posterior gray horn.
Slow pain fibers
Slow pain sensation is carried by C type afferent
fibers which synapse with neurons of substantia
gelatinosa of Rolando in the posterior gray horn
(Fig. 88-4).
Second Order Neurons
The neurons of marginal nucleus and substantia
gelatinosa of Rolando form the second order
neurons. Fibers from these neurons ascend in
the form of the lateral spinothalamic tract.
Third Order Neurons
The third order neurons are in thalamic nucleus,
reticular formation. Axons from these neurons
reach the sensory area of cerebral cortex.
Center for Pain Sensation
The center for pain sensation is in the postcentral
gyrus of parietal cortex. Fibers reaching hypotha-
lamus are concerned with arousal mechanism
due to pain stimulus.
PATHWAY OF PAIN SENSATION
FROM FACE
Pain sensation from face is carried by trigeminal
nerve (Chapter 89).
PATHWAY OF PAIN SENSATION
FROM VISCERA
The pain sensation from thoracic and abdominal
viscera is transmitted by sympathetic (thora-
columbar) nerves. Pain from esophagus, trachea
and pharynx is carried by vagus and glosso-
pharyngeal nerves.
PATHWAY OF PAIN SENSATION
FROM PELVIC REGION
Pain sensation from deeper structures of pelvic
region is conveyed by sacral parasympathetic
nerves.
VISCERAL PAIN
Pain from viscera is unpleasant. It is poorly
localized.
CAUSES OF VISCERAL PAIN
1. Ischemia: The substances released during
ischemic reactions like bradykinin and
proteolytic enzymes stimulate the pain
receptors of viscera.

Chapter 90 Physiology of Pain
557
2. Chemical stimuli: The chemical substances
like acidic gastric juice leaks from ruptured
ulcers into peritoneal cavity and produce
pain.
3. Spasm of hollow organs: Spastic contraction
of smooth muscles in gastrointestinal tract
and other hollow organs of viscera cause pain
by stimulating the free nerve endings.
4. Overdistention of hollow organs also causes
pain.
REFERRED PAIN
DEFINITION
Referred pain is the pain that is perceived at a
site adjacent to or away from the site of origin.
The deep pain and some visceral pain are
referred to other areas. But, the superficial pain
is not referred.
EXAMPLES OF REFERRED PAIN
1. Cardiac pain is felt at the inner part of left
arm and left shoulder
2. Pain in ovary is referred to umbilicus
3. Pain from testis is felt in abdomen
4. Pain in diaphragm is referred to right shoulder
5. Pain in gallbladder is referred to epigastric
region
6. Renal pain is referred to loin.
MECHANISM OF REFERRED PAIN
Dermatomal Rule
According to dermatomal rule, pain is referred
to a structure, which is developed from the same
dermatome from which the pain producing
structure is developed.
A dermatome includes all the structures or
parts of the body, which are innervated by
afferent nerve fibers of one dorsal root. For
example, the heart and inner aspect of left arm
originate from the same dermatome. So, the pain
in heart is referred to left arm.
ANALGESIA SYSTEM
Analgesia system means the pain control
system. The body has its own analgesia system
in brain which provides a short term relief from
pain. It is also called endogenous analgesia
system. It includes gray matter surrounding the
III ventricle and aqueduct of Sylvius and reticular
formation of brainstem.
The analgesia system has got its own path-
way through which it blocks the synaptic trans-
mission of pain sensation in spinal cord and
suppresses the pain sensation. In fact analgesic
drugs such as opioids act through this system
and provide a controlled pain relief.
GATE CONTROL THEORY
Gate control theory explains the pain sup-
pression. According to this theory, the pain
stimuli transmitted by afferent pain fibers are
blocked by gate mechanism located at the
posterior gray horn of spinal cord. If the gate is
opened, pain is felt. If the gate is closed, pain
is suppressed. Brain also plays some important
role in the gate control system of the spinal cord.
Significance of Gate Control
Thus, the gating of pain at spinal level is similar
to presynaptic inhibition. It forms the basis for
relief of pain through rubbing, massage techni-
ques, application of ice packs, acupuncture and
electrical analgesia. All these techniques relieve
pain by stimulating the release of endogenous
pain relievers (opioid peptides) which close the
gate and block the pain signals.
APPLIED PHYSIOLOGY
1. Analgesia (loss of pain sensation)
2. Hyperalgesia (increased sensitivity to pain
sensation)
3. Paralgesia (abnormal pain sensation).
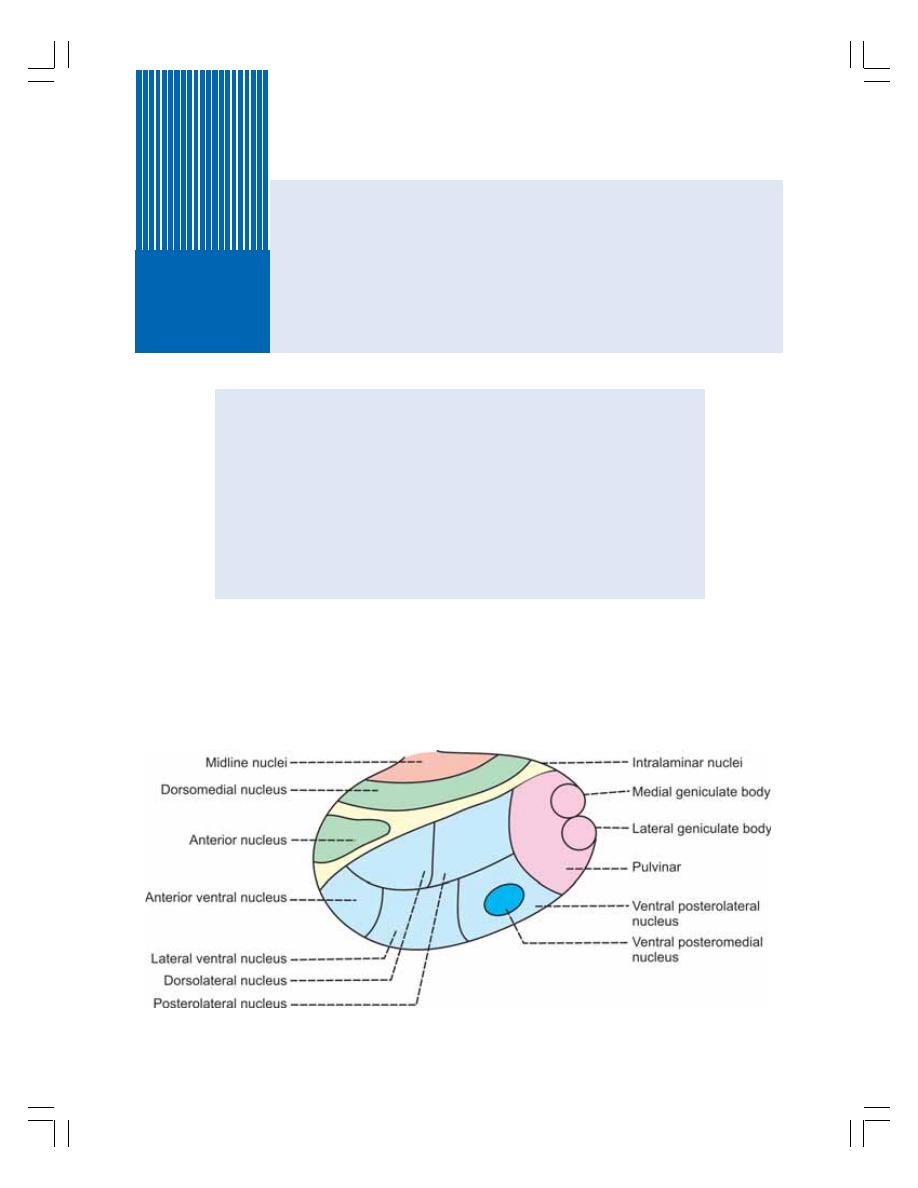
INTRODUCTION
THALAMIC NUCLEI
FUNCTIONS OF THALAMUS
RELAY CENTER FOR SENSATIONS
CENTER FOR PROCESSING OF SENSORY INFORMATION
CENTER FOR DETERMINING QUALITY OF SENSATIONS
CENTER FOR SEXUAL SENSATIONS
ROLE IN AROUSAL AND ALERTNESS REACTIONS
APPLIED PHYSIOLOGY
Thalamus
91
INTRODUCTION
Thalamus is a large ovoid mass of gray matter,
situated bilaterally in diencephalon. Both thalami
form 80% of diencephalon (Fig. 91-1).
THALAMIC NUCLEI
Thalamus on each side is divided into five main
nuclear groups by means of internal medullary
septum.
FIGURE 91-1: Thalamic nuclei. Red = Midline nuclei, Yellow = Intralaminar nuclei,
Green = Medial mass of nuclei, Blue = Lateral mass of nuclei, Pink = Posterior group of nuclei

Chapter 91 Thalamus
559
1. MIDLINE NUCLEI
It is a group of small nuclei, situated on the medial
surface of thalamus near midline.
2. INTRALAMINAR NUCLEI
The intralaminar nuclei are smaller nuclei present
in the medullary septum of the thalamus.
3. MEDIAL MASS OF NUCLEI
Medial mass of nuclei is situated medial to
septum and it comprises two nuclei.
1. Anterior nucleus
2. Dorsomedial nucleus.
4. LATERAL MASS OF NUCLEI
This group of nuclei is situated lateral to septum.
Lateral mass of nuclei is again divided into two
subgroups:
a. Dorsal group of lateral mass with two nuclei:
1. Dorsolateral nucleus
2. Posterolateral nucleus
b. Ventral group of lateral mass with three nuclei:
1. Anterior ventral nucleus
2. Lateral ventral nucleus.
3. Posteroventral nucleus. It consists of two
parts:
i. Ventral posterolateral nucleus
ii. Ventral posteromedial nucleus.
5. POSTERIOR GROUP OF NUCLEI
It is the continuation of lateral mass of nuclei. It
has two subgroups:
a. Pulvinar
b. Metathalamus which consists of two struc-
tures:
1. Medial geniculate body
2. Lateral geniculate body.
FUNCTIONS OF THALAMUS
Thalamus is primarily concerned with somatic
functions and it plays little role in the visceral
functions. The various functions of thalamus are:
1. RELAY CENTER FOR SENSATIONS
Thalamus forms the relay center for the sensa-
tions. The impulses of almost all the sensations
reach the thalamic nuclei, particularly in the
ventral posterolateral nucleus. After being
processed in the thalamus, the impulses are
carried to cerebral cortex through thalamocortical
fibers.
2. CENTER FOR PROCESSING OF
SENSORY INFORMATION
Thalamus forms the major center for processing
the sensory information. All the peripheral
sensory impulses reaching thalamus are inte-
grated and modified before being sent to specific
areas of cerebral cortex. This function of thala-
mus is usually called the processing of sensory
information.
Functional Gateway for Cerebral Cortex
Almost all the sensations are processed in
thalamus before reaching cerebral cortex. Very
little information of somatosensory function is
sent directly to cerebral cortex without being
processed by the thalamic nuclei. Because of
this function, thalamus is usually called a “Func-
tional gateway” for cerebral cortex.
3. CENTER FOR DETERMINING
QUALITY OF SENSATIONS
Thalamus is also the center for determining the
quality of sensations, that is, to determine the
affective nature of sensations. Usually the sen-
sations have two qualities:
i. The discriminative nature
ii. The affective nature.
i.
The Discriminative Nature
It is the ability to recognize the type, location and
other details of the sensations and it is the func-
tion of cerebral cortex.
ii. The Affective Nature
The affective nature is the capacity to determine
whether a sensation is pleasant or unpleasant
and agreeable or disagreeable. Determining the
affective nature of sensations is the function of
thalamus.

Nervous System
560
4. CENTER FOR SEXUAL SENSATIONS
Thalamus forms the center for perception of
sexual sensations.
5. ROLE IN AROUSAL AND
ALERTNESS REACTIONS
Because of its connections with nuclei of reti-
cular formation, thalamus plays an important role
in arousal and alertness reactions.
6. CENTER FOR REFLEX ACTIVITY
Since the sensory fibers relay here, thalamus
forms the center for many reflex activities.
7. CENTER FOR INTEGRATION OF
MOTOR ACTIVITY
Through the connections with cerebellum and
basal ganglia, thalamus serves as a center for
integration of motor functions.
APPLIED PHYSIOLOGY
THALAMIC SYNDROME
Thalamic syndrome is the neurological disease
caused by lesion of thalamus. Lesion occurs
because of blockage (due to thrombosis) in the
thalamogeniculate branch of posterior cerebral
artery. The symptoms are:
1. Loss of Sensations
Loss of all sensations (anesthesia) occurs as the
sensory relay system in thalamus is affected.
2. Astereognosis
Astereognosis is the loss of ability to recognize
a known object by touch with closed eyes. It is
due to the loss of tactile and kinesthetic sen-
sations in thalamic syndrome.
3. Ataxia
Ataxia is the incoordination of voluntary move-
ments.
4. Thalamic Phantom Limb
Thalamic phantom limb is the inability to locate
the position of a limb with closed eyes. The
patient will search for the limb in air.
5. Amelognosia
It is the illusion felt by the patient that his limb is
absent.
6. Pain Sensation
Spontaneous pain occurs often. The pain may
be so intense, that it even resists the action of
powerful sedatives like morphine. Sometimes,
the patient feels pain even in the absence of pain
stimulus.
7. Involuntary Movements
Thalamic syndrome is always associated with
some involuntary motor movements.
a. Athetosis (slow writhing and twisting move-
ments)
b. Chorea (quick jerky involuntary movements)
c. Intention tremor: Tremor is defined as rapid
alternate rhythmic and involuntary movement
of flexion and extension in the joints of fingers
and wrist or elbow. Intention tremor is the
tremor that develops while attempting to do
any voluntary act. Intention tremor is the
common feature of thalamic syndrome.
8. Thalamic Hand or Athetoid Hand
It is the abnormal attitude of the hand in thalamic
lesion. It is characterized by moderate flexion at
wrist and hyperextension of all fingers.
INTRODUCTION
NUCLEI
FUNCTIONS
SECRETION OF POSTERIOR PITUITARY HORMONES
CONTROL OF ANTERIOR PITUITARY
CONTROL OF ADRENAL CORTEX
CONTROL OF ADRENAL MEDULLA
REGULATION OF AUTONOMIC NERVOUS SYSTEM
REGULATION OF HEART RATE
REGULATION OF BLOOD PRESSURE
REGULATION OF BODY TEMPERATURE
REGULATION OF HUNGER AND FOOD INTAKE
REGULATION OF WATER BALANCE
REGULATION OF SLEEP AND WAKEFULNESS
ROLE IN BEHAVIOR AND EMOTIONAL CHANGES
REGULATION OF SEXUAL FUNCTION
REGULATION OF RESPONSE TO SMELL
ROLE IN CIRCADIAN RHYTHM
APPLIED PHYSIOLOGY – DISORDERS OF HYPOTHALAMUS
DIABETES INSIPIDUS
DYSTROPHIA ADIPOSOGENITALIS
LAURENCE-MOON-BIEDL SYNDROME
NARCOLEPSY
CATAPLEXY
Hypothalamus
92
INTRODUCTION
Hypothalamus is a diencephalic structure. It is
situated just below thalamus in the ventral part
of diencephalon. It is formed by groups of nuclei
scattered in the walls and floor of third ventricle.
It extends from optic chiasma to mamillary body.
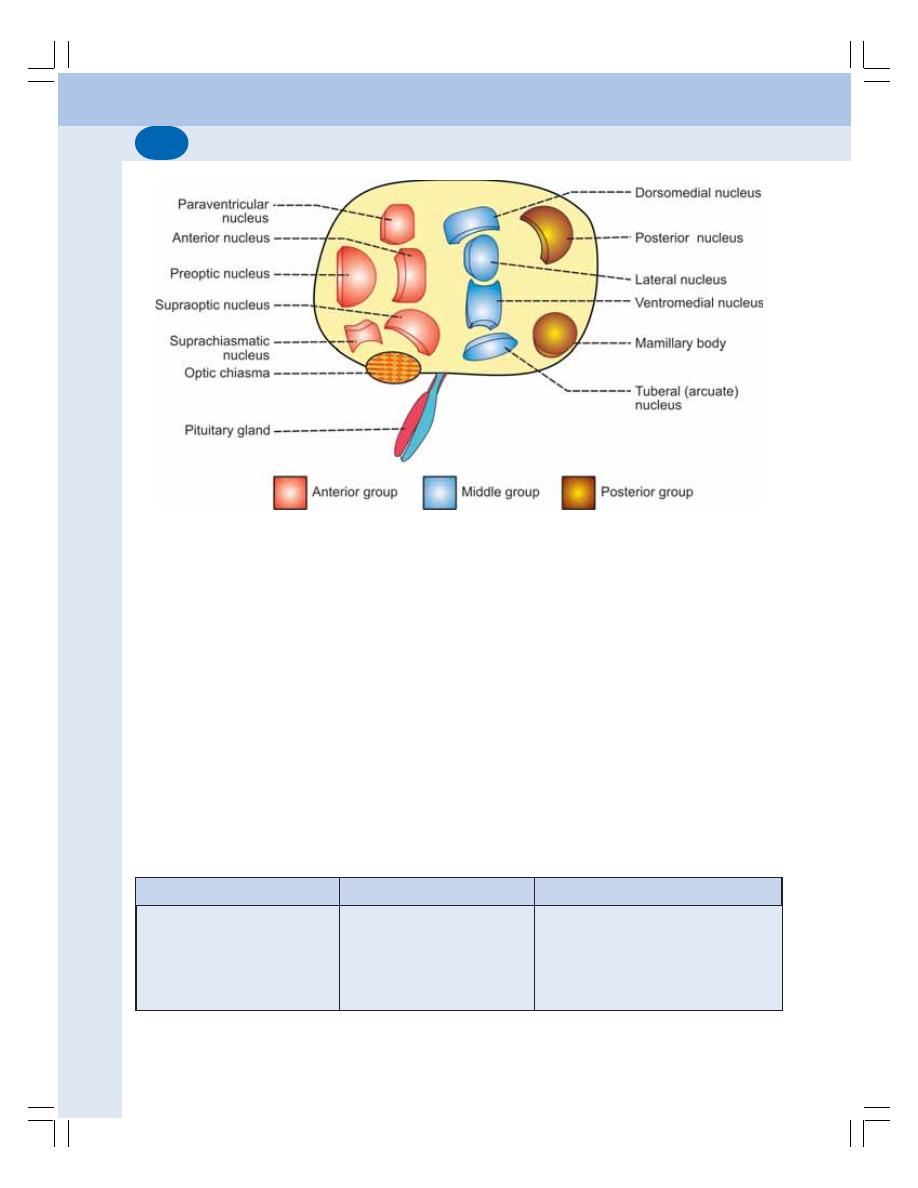
Nervous System
562
NUCLEI OF HYPOTHALAMUS
The nuclei of hypothalamus are divided into three
groups:
1. Anterior or preoptic group
2. Middle or tuberal group
3. Posterior or mamillary group.
Nuclei of each group are listed in Table 92-1
and represented diagrammatically in Fig. 92-1.
FUNCTIONS OF HYPOTHALAMUS
Hypothalamus is the important part of the brain
concerned with homeostasis of the body. It
regulates many vital functions of the body
like endocrine functions, visceral functions,
metabolic activities, hunger, thirst, sleep,
wakefulness, emotion, sexual functions, etc
(Table 92-2).
1. SECRETION OF POSTERIOR
PITUITARY HORMONES
Posterior pituitary hormones namely, antidi-
uretic hormone (ADH) and oxytocin are secreted
by supraoptic and paraventricular nuclei of
hypothalamus. These two hormones are
transported by means of axonic or axoplasmic
flow through the fibers of hypothalamo-
hypophyseal tracts to the posterior pituitary
(Refer Chapter 45 for details).
TABLE 92-1: Nuclei of hypothalamus
Anterior or Preoptic group
Middle or Tuberal group
Posterior or Mamillary group
1. Preoptic nucleus
1. Dorsomedial nucleus
1. Posterior nucleus
2. Paraventricular nucleus
2. Ventomedial nucleus
2. Mamillary body
3. Anterior nucleus
3. Lateral nucleus
4. Supraoptic nucleus
4. Arcuate (tuberal) nucleus
5. Suprachiasmatic nucleus
FIGURE 92-1: Nuclei of hypothalamus

Chapter 92 Hypothalamus
563
2. CONTROL OF ANTERIOR PITUITARY
Hypothalamus controls the secretions of anterior
pituitary gland by secreting releasing hormones
and inhibitory hormones. It secretes seven
hormones.
i. Growth hormone releasing hormone
(GHRH)
ii. Growth hormone releasing polypeptide
(GHRP)
iii. Growth hormone inhibitory hormone
(GHIH) or somatostatin
iv. Thyrotropic releasing hormone (TRH)
v. Corticotropin releasing hormone (CRH)
vi. Gonadotropin releasing hormone (GnRH)
vii. Prolactin inhibitory hormone (PIH).
These hormones are transported from
hypothalamus to the anterior pituitary by the
hypothalamo-hypophyseal portal blood vessels
(Refer Chapter 45 for details).
3. CONTROL OF ADRENAL CORTEX
Hypothalamus controls adrenal cortex through
anterior pituitary. Anterior pituitary regulates the
adrenal cortex by secreting adrenocorticotropic
hormone (ACTH). ACTH secretion is in turn
regulated by corticotropic releasing hormone
(CRH) which is secreted by the paraventricular
nucleus of hypothalamus (Refer Chapter 49 for
details).
4. CONTROL OF ADRENAL MEDULLA
Dorsomedial and posterior hypothalamic nuclei
are excited by emotional stimuli. These
hypothalamic nuclei, in turn, send impulses to
adrenal medulla through sympathetic fibers and
cause release of catecholamines, which are
essential to cope up with emotional stress
(Chapter 50).
5. REGULATION OF AUTONOMIC
NERVOUS SYSTEM
Hypothalamus controls the autonomic nervous
system (ANS). The sympathetic division of ANS
is regulated by posterior and lateral nuclei of
hypothalamus. The parasympathetic division of
ANS is controlled by anterior group of nuclei.
The influences of cerebral cortex on ANS are
executed through hypothalamus (Chapter 103).
6. REGULATION OF HEART RATE
Hypothalamus regulates heart rate through
vasomotor center in the medulla oblongata.
Stimulation of posterior and lateral nuclei of
hypothalamus increases the heart rate.
Stimulation of preoptic and anterior nuclei
decreases the heart rate (Chapter 64).
7. REGULATION OF BLOOD PRESSURE
Hypothalamus regulates the blood pressure by
acting on the vasomotor center. Stimulation of
posterior and lateral hypothalamic nuclei
increases arterial blood pressure and stimula-
tion of preoptic area decreases the blood
pressure (Chapter 65).
8. REGULATION OF BODY
TEMPERATURE
The body temperature is regulated by hypo-
thalamus which sets the normal range of body
temperature. The set point under normal
physiological conditions is 37°C. Hypothalamus
has two centers which regulate the body
temperature:
i. Heat loss center that is present in preoptic
nucleus of anterior hypothalamus
ii. Heat gain center that is situated in
posterior hypothalamic nucleus.
Regulation of body temperature is explained
in Chapter 43.
9. REGULATION OF HUNGER AND
FOOD INTAKE
Food intake is regulated by two centers present
in hypothalamus:
i. Feeding center
ii. Satiety center.
Feeding Center
Feeding center is in the lateral hypothalamic
nucleus. In experimental conditions, the
stimulation of this center in animals leads to
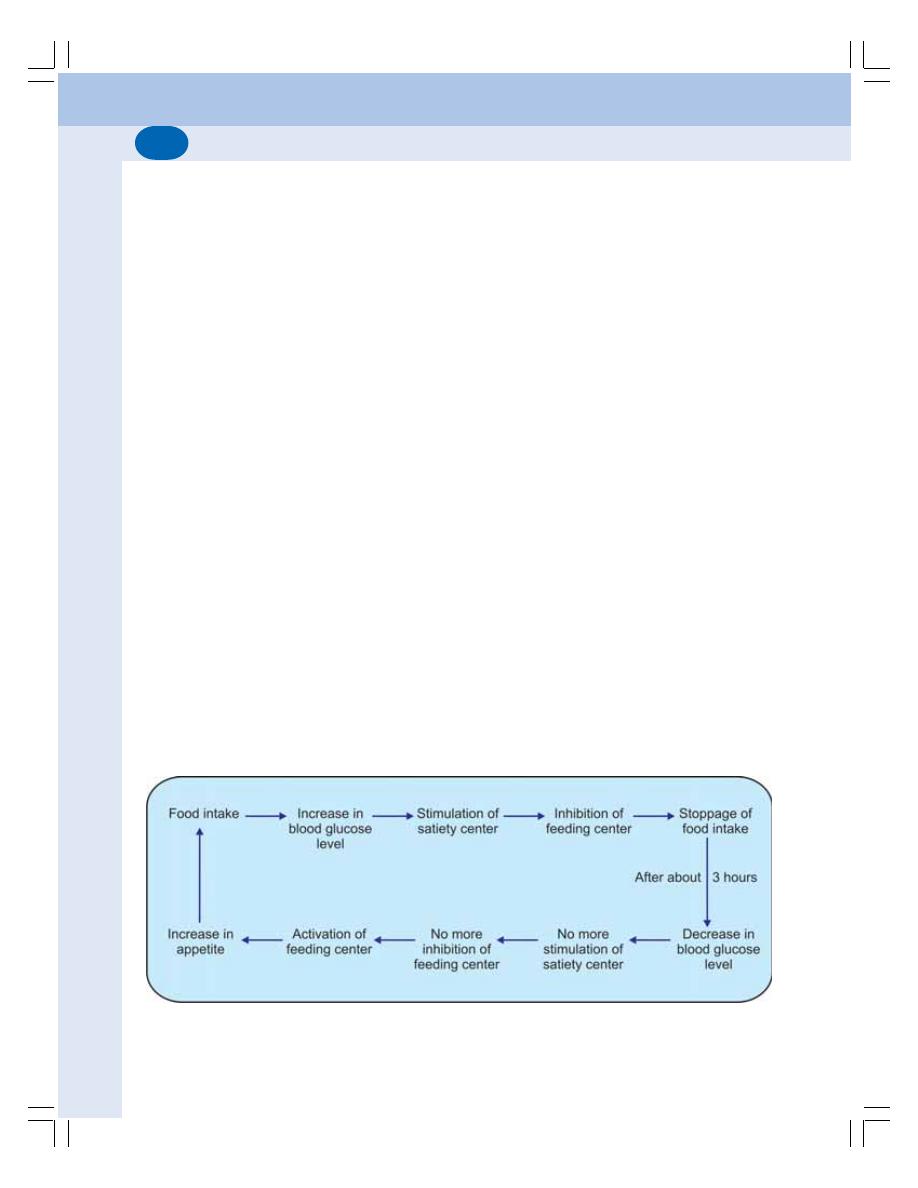
Nervous System
564
uncontrolled hunger and increased food intake
(hyperphagia) resulting in obesity. The destruc-
tion of feeding center leads to loss of appetite
(anorexia) and the animal refuses to take food.
Normally feeding center is always active. That
means it has the tendency to induce food intake
always.
Satiety Center
Satiety center is in the ventromedial nucleus of
the hypothalamus. Stimulation of this nucleus in
animals causes total loss of appetite and
cessation of food intake. Destruction of satiety
center leads to hyperphagia and the animal
becomes obese. This type of obesity is called
hypothalamic obesity.
Satiety center plays important role in
regulation of food intake by temporary inhibition
of feeding center after food intake.
Mechanism of Regulation of Food Intake
Under normal physiological conditions appetite
and food intake are well balanced and continues
in a cyclic manner. Feeding center and satiety
center of hypothalamus are responsible for
regulation of appetite and food intake. These
hypothalamic centers are regulated by the
following mechanisms:
i. Glucostatic mechanism
ii. Lipostatic mechanism
iii. Peptide mechanism
iv. Hormonal mechanism
v. Thermostatic mechanism.
i. Glucostatic Mechanism
The cells of the satiety center function as
glucostats or glucose receptors. The glucostats
are stimulated by increased blood glucose level
during food intake. This develops the feeling of
‘fullness’. The satiety center in turn, inhibits the
feeding center resulting in stoppage of food
intake.
After few hours of food intake, the blood
glucose level decreases and satiety center
becomes inactive. So, the feeding center is no
longer inhibited. Now it becomes active and
increases the appetite and induces food intake.
After taking food, once again blood glucose
level increases and the cycle is repeated
(Fig. 92-2).
ii. Lipostatic Mechanism
Leptin is a peptide secreted by adipocytes (cells
of adipose tissue). It plays an important role in
controlling the food intake and adipose tissue
volume. The details of leptin are given in
Chapter 52.
When the volume of adipose tissues
increases, adipocytes secrete and release a
large quantity of leptin into the blood. While
circulating through brain, leptin acts on
hypothalamus and inhibits the feeding center
FIGURE 92-2: Glucostatic mechanism

Chapter 92 Hypothalamus
565
resulting in loss of appetite and stoppage of food
intake.
iii. Peptide Mechanism
Some peptides regulate the food intake either
by stimulating or inhibiting the feeding center
directly or indirectly.
The peptides which increase the food intake
are:
a. Ghrelin
b. Neuropeptide Y.
Peptides which decrease food intake are:
a. Leptin
b. Peptide YY.
iv. Hormonal Mechanism
Some of the endocrine hormones and GI
hormones inhibit the food intake by acting
through hypothalamus. Such hormones are:
a. Somatostatin
b. Oxytocin
c. Glucagon
d. Pancreatic polypeptide
e. Cholecystokinin.
v. Thermostatic Mechanism
Food intake is inversely proportional to body
temperature. So in fever, the food intake is
decreased due to the influence of preoptic
thermoreceptors (see above) on feeding center.
10. REGULATION OF WATER
BALANCE
Hypothalamus regulates water content of the
body by two mechanisms:
i. Thirst mechanism
ii. ADH mechanism.
i. Thirst Mechanism
Thirst center is in the lateral nucleus of
hypothalamus. There are some osmoreceptors
in the areas adjacent to thirst center. When
the ECF volume decreases, the osmolality of
ECF is increased. If the osmolarity increases by
1 to 2%, the osmoreceptors are stimulated.
Osmoreceptors in turn, activate the thirst center
and thirst sensation is initiated. Now, the person
feels thirsty and drinks water. Water intake
increases ECF volume and decreases the
osmolality.
ii. ADH Mechanism
Simultaneously, when the volume of ECF
decreases with increased osmolality, the
supraoptic nucleus is stimulated and ADH is
released. ADH causes retention of water by
facultative reabsorption in the renal tubules. It
increases the ECF volume and brings the
osmolality back to the normal level. On the
contrary, when ECF volume is increased, the
supraoptic nucleus is not stimulated and ADH
is not secreted. In the absence of ADH, more
amount of water is excreted through urine and
the volume of ECF is brought back to normal.
11. REGULATION OF SLEEP AND
WAKEFULNESS
Mamillary body in the posterior hypothalamus
is considered as the wakefulness center. Stimu-
lation of mamillary body causes wakefulness and
its lesion leads to sleep. Stimulation of anterior
hypothalamus also leads to sleep.
12. ROLE IN BEHAVIOR AND
EMOTIONAL CHANGES
The behavior of animals and human beings is
mostly affected by two responding systems in
hypothalamus and other structures of limbic
system. These two systems act opposite to one
another.
The responding systems are concerned with
the affective nature of sensations, i.e. whether
the sensations are pleasant or painful. These
two qualities are called the Reward (satisfaction)
and punishment (aversion or avoidance). Hypo-
thalamus has two centers for behavior and
emotional changes are:
i. Reward center
ii. Punishment center.
Nervous System
566
Reward Center
It is situated in medial forebrain bundle and
ventromedial nucleus of hypothalamus. Electrical
stimulation of these areas in animals pleases
or satisfies the animals.
Punishment Center
It is situated in posterior and lateral nuclei of
hypothalamus. Electrical stimulation of these
nuclei in animals leads to pain, fear, defense,
escape reactions and other elements of
punishment.
Role of Reward and Punishment Centers
The importance of the reward and punishment
centers lies in the behavioral pattern of the
individuals. Almost all the activities of day to day
life depend upon reward and punishment. While
doing something, if the person is rewarded or
TABLE 92-2: Functions of hypothalamus
Functions
Action/Center
Nuclei/Parts involved
1. Control of anterior pituitary
Releasing hormones
Discrete areas
Inhibitory hormones
2. Secretion of posterior
Oxytocin
Paraventricular nucleus
pituitary hormones
ADH
Supraoptic nucleus
3. Control of adrenal cortex
CRH
Paraventricular nucleus
4. Control of adrenal medulla
Catecholamines during
Posterior and dorsomedial nuclei
emotion
5. Regulation of ANS
Sympathetic
Posterior and lateral nuclei
Parasympathetic
Anterior nuclei
6. Regulation of heart rate
Acceleration
Posterior and lateral nuclei
Inhibition
Preoptic area
7. Regulation of blood pressure
Pressor effect
Posterior and lateral nuclei
Depressor effect
Preoptic area
8. Regulation of body temperature
Heat gain center
Posterior hypothalamus
Heat loss center
Anterior hypothalamus
9. Regulation of hunger and
Feeding center
Lateral nucleus
food intake
Satiety center
Ventromedial nucleus
10. Regulation of water intake
Thirst center
Lateral nucleus
Water retention by ADH
Supraoptic nucleus
11. Regulation of sleep and
Sleep
Anterior hypothalamus
wakefulness
Wakefulness
Mamillary body
12. Regulation of behavior and
Reward center
Ventromedial nucleus
emotion
Punishment center
Posterior and lateral nuclei
13. Regulation of sexual function
Sexual cycle
Tuberal and posterior nuclei
14. Regulation of response to smell
Autonomic responses
Posterior hypothalamus
15. Role in circadian rhythm
Rhythmic changes
Suprachiasmatic nucleus

Chapter 92 Hypothalamus
567
feels satisfied, he or she continues to do so. If
the person feels punished or unpleasant, he or
she stops doing so. Thus, these two centers play
an important role in the development of the
behavioral pattern of a person.
Rage
Rage refers to violent and aggressive emotional
expression with extreme anger. It is common in
animals when punishment centers in
hypothalamus are stimulated. The reactions of
rage are expressed by developing a defense
posture which includes:
i. Extension of limbs with lifting of tail
ii. Hissing and spitting
iii. Piloerection
iv. Wide opening of eyeballs with pupillary
dilatation
v. Severe savage attack even by mild
provocation.
Sham Rage
Sham rage means false rage. It is an extreme
emotional condition that resembles rage and
occurs in some pathological conditions in
humans. Sham rage is due to release of
hypothalamus from the inhibitory influence of
cortical control.
13. REGULATION OF SEXUAL
FUNCTION
In animals, hypothalamus plays an important role
in regulating sexual functions by secreting
gonadotropin releasing hormones. Arcuate and
posterior hypothalamic nuclei are involved in the
regulation of sexual functions.
14. ROLE IN RESPONSE TO SMELL
Posterior hypothalamus along with other
structures like hippocampus and brainstem
nuclei is responsible for the autonomic respon-
ses of body to olfactory stimuli. The responses
include feeding activities and emotional
responses like fear, excitement and pleasure.
15. ROLE IN CIRCADIAN RHYTHM
Circadian rhythm is the regular recurrence of
physiological processes or activities which occur
in cycles of 24 hours. It is also called diurnal
rhythm. The term circadian is a Latin word
meaning ‘around the day’.
The circadian rhythm occurs in response to
recurring daylight and darkness. The cyclic
changes taking place in various physiological
processes are set by means of a hypothetical
internal clock that is often called biological clock.
The suprachiasmatic nucleus of hypo-
thalamus plays an important role in setting the
biological clock by its connection with retina via
retinohypothalamic fibers. Through the efferent
fibers it sends circadian signals to different parts
and maintains the circadian rhythm of sleep,
hormonal secretion, thirst, hunger, appetite, etc.
Whenever body is exposed to a new pattern
of daylight/ darkness rhythm, the biological clock
is reset provided the new pattern is regular.
Accordingly, the circadian rhythm also changes.
APPLIED PHYSIOLOGY –
DISORDERS OF HYPOTHALAMUS
Following disorders develop in hypothalamic
lesion that occurs due to tumors, encephalitis or
ischemia.
1. DIABETES INSIPIDUS
Diabetes insipidus is the condition characteri-
zed by excretion of large quantity of water
through urine. Refer Chapter 45 for details.
2. DYSTROPHIA ADIPOSOGENITALIS
It is characterized by obesity and sexual
infantilism, associated with dwarfism (if the
condition occurs during growing period). It is also
called Fröhlich’s syndrome. Refer Chapter 45
for details.

Nervous System
568
3. LAURENCE-MOON-BIEDL
SYNDROME
This disorder of hypothalamus is characterized
by moon face (facial contours become round by
hiding the bony structures), obesity, polydactylism
(having one or more extra fingers or toes), mental
retardation and hypogenitalism.
5. NARCOLEPSY
Narcolepsy is a hypothalamic disorder with
abnormal sleep pattern. There is sudden attack
of uncontrollable desire for sleep and, the person
suddenly falls asleep. It occurs in the daytime.
6. CATAPLEXY
It is the sudden uncontrolled outbursts of
emotion associated with narcolepsy. Due to
emotional outburst like anger, fear or excitement,
the person becomes completely exhausted
with muscular weakness. The attack is brief and
last for few seconds to a few minutes. The
consciousness is not lost.
PARTS
VERMIS
CEREBELLAR HEMISPHERES
DIVISIONS
VESTIBULOCEREBELLUM
COMPONENTS
FUNCTIONS
SPINOCEREBELLUM
COMPONENTS
FUNCTIONS
CORTICOCEREBELLUM
COMPONENTS
AFFERENT–EFFERENT CIRCUIT
FUNCTIONS
APPLIED PHYSIOLOGY – CEREBELLAR LESIONS
DISTURBANCES IN TONE AND POSTURE
DISTURBANCES IN EQUILIBRIUM
DISTURBANCES IN MOVEMENTS
PARTS OF CEREBELLUM
Cerebellum consists of a narrow, worm like
central body called vermis and two lateral lobes,
the right and left cerebellar hemispheres
(Fig. 93-1). The part of vermis on the upper
surface of cerebellum is known as superior
vermis and the vermis on the under surface of
cerebellum is called inferior vermis.
VERMIS
The vermis of cerebellum is formed by nine
parts. The parts of superior vermis and inferior
vermis are listed in Table 93-1.
Nodulus and flocculi are together called
flocculonodular lobe. On either side of pyramid,
there is another extension named parafloccu-
lus.
Cerebellum
93
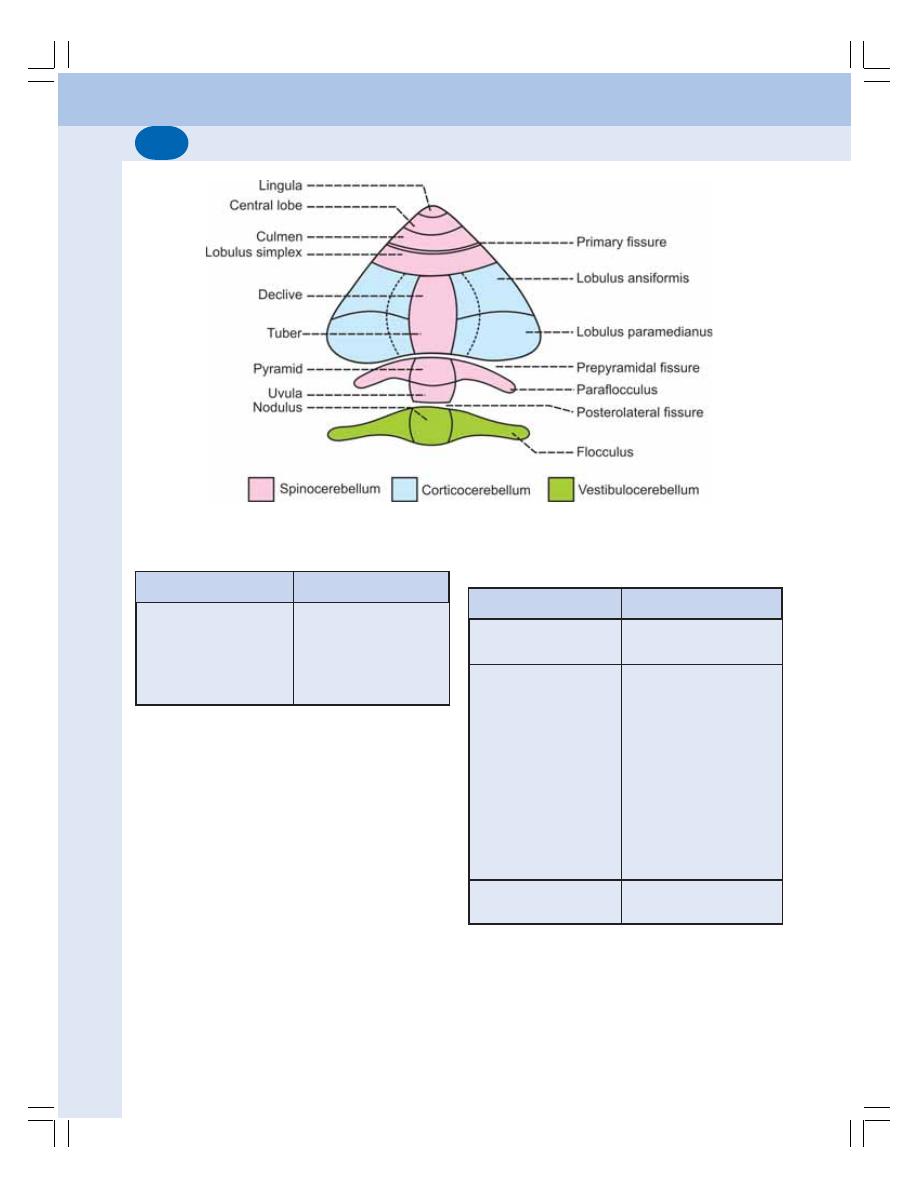
Nervous System
570
FIGURE 93-1: Parts and functional divisions of cerebellum
CEREBELLAR HEMISPHERES
The cerebellar hemispheres are the extended
portions on either side of the vermis. Each
hemisphere has two portions.
1. Lobulus ansiformis or ansiform lobe
2. Lobulus paramedianus or paramedian lobe
DIVISIONS OF CEREBELLUM
Based on the functions, the cerebellum is divided
into three divisions:
1. Vestibulocerebellum
2. Spinocerebellum
3. Corticocerebellum
TABLE 93-1: Parts of superior and inferior vermis
Superior vermis
Inferior vermis
1. Lingula
2. Central lobe
3. Culmen
4. Lobulus simplex
5. Declive
6. Tuber
7. Pyramid
8. Uvula
9. Nodulus
VESTIBULOCEREBELLUM
(ARCHICEREBELLUM)
This part of cerebellum is connected with the
vestibular apparatus and so it is known as
TABLE 93-1: Parts of superior and inferior vermis
Superior vermis
Inferior vermis
1. Lingula
2. Central lobe
3. Culmen
4. Lobulus simplex
5. Declive
6. Tuber
7. Pyramid
8. Uvula
9. Nodulus
TABLE 93-2: Components of divisions of
cerebellum
Division
Components
Vestibulocerebellum
Flocculonodular lobe
(Nodulus and Flocculi)
Spinocerebellum
Lingula
Central lobe
Culmen
Lobulus simplex
Declive
Tuber
Pyramid
Uvula
Paraflocculi
Medial portions of
cerebral hemispheres
Corticocerebellum
Lateral portions of
cerebral hemispheres
Chapter 93 Cerebellum
571
vestibulocerebellum. Since, vestibulocerebe-
llum is the phylogenetically oldest part of
cerebellum, it is also called archicerebellum.
COMPONENTS OF
VESTIBULOCEREBELLUM
Vestibulocerebellum includes the flocculo-
nodular lobe that is formed by the nodulus of
vermis and its lateral extensions called flocculi
(Fig. 93-1 and Table 93-2).
FUNCTIONS OF
VESTIBULOCEREBELLUM
Vestibulocerebellum regulates tone, posture
and equilibrium by receiving impulses from
vestibular apparatus regarding gravity and
movements (Table 93-3).
Mechanism of Action of Vestibulocerebellum
Normally, the vestibular nuclei of brain stem
facilitate the movements of trunk, neck and limbs.
The medullary reticular formation inhibits the
muscle tone.
After receiving information from vestibular
apparatus, the vestibulocerebellum inhibits both
vestibular nuclei and medullary reticular
formation. As a result, the movements of neck,
trunk and limbs are checked and the muscle
tone increases. Because of these effects, any
disturbance in posture and equilibrium is
corrected.
In the lesion of vestibulocerebellum, there is
reduction of muscle tone (hypotonia) and failure
to maintain posture and equilibrium.
SPINOCEREBELLUM
(PALEOCEREBELLUM)
Spinocerebellum or paleocerebellum is
connected with spinal cord and hence the name.
It forms the major receiving area of cerebellum
for sensory inputs. Spinocerebellum is also
phylogenetically older part of cerebellum
COMPONENTS OF SPINOCEREBELLUM
Spinocerebellum consists of medial portions of
cerebellar hemisphere, paraflocculi and parts of
vermis, viz. lingula, central lobe, culmen, lobulus
simplex, declive, tuber, pyramid and uvula
(Fig. 93-1 and Table 93-2).
FUNCTIONS OF SPINOCEREBELLUM
Spinocerebellum regulates tone, posture and
equilibrium by receiving sensory impulses form
tactile receptors, proprioceptors, visual receptors
and auditory receptors. It also receives the
cortical impulses via pontine nuclei.
TABLE 93-3: Functions of cerebellum
Functions
Mechanism
Division of
cerebellum involved
1. Regulation of tone,
By receiving impulses from vestibular apparatus
Vestibulocerebellum
posture and equilibrium
By receiving impulses from proprioceptors in
Spinocerebellum
muscles, tendons and joints, tactile receptors,
visual receptors and auditory receptors
2. Regulation of coordinated
By:
Corticocerebellum
movements
i.
Damping action
(Neocerebellum)
ii.
Control of ballistic movements
iii.
Timing and programming the movements
iv.
Servomechanism
v.
Comparator function
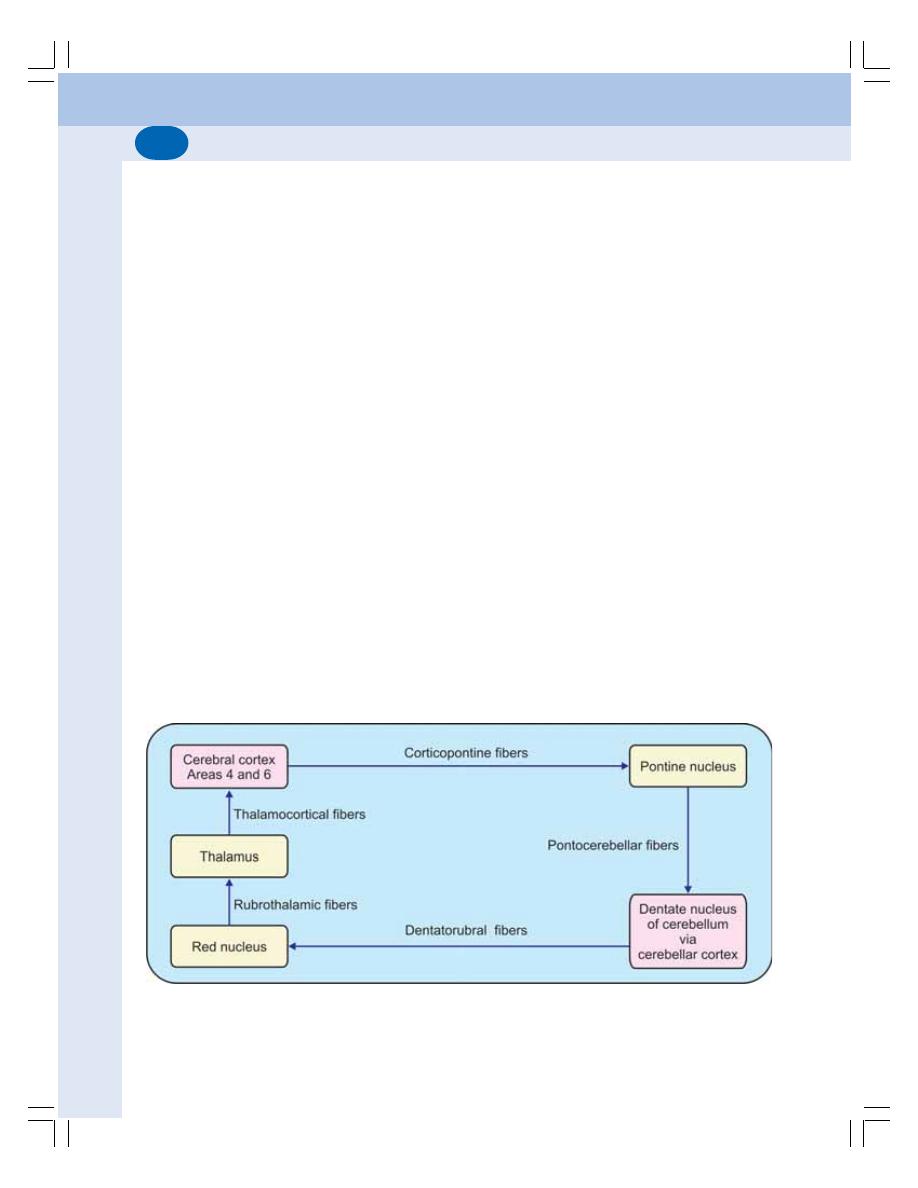
Nervous System
572
Spinocerebellum facilitates the discharge
from gamma motor neurons. Increased discharge
from gamma motor neurons increases the
muscle tone. The lesion in spinocerebellum
causes stoppage of discharge from the gamma
motor neurons resulting in hypotonia and
disturbances in posture.
Spinocerebellum also receives impulses from
optic and auditory pathway and helps in
adjustment of posture and equilibrium in
response to visual and auditory impulses.
CORTICOCEREBELLUM
(NEOCEREBELLUM)
Corticocerebellum is largest part of cerebellum.
Because of its connection with cerebral cortex,
it is called corticocerebellum or cerebro-
cerebellum. It is phylogenetically newer part of
cerebellum. So, it is also called neocerebellum.
It is concerned with planning, programming and
coordination
COMPONENTS OF
CORTICOCEREBELLUM
Corticocerebellum includes the lateral portions
of cerebellar hemispheres (Fig. 93-1 and Table
93-2).
AFFERENT–EFFERENT CIRCUIT
(CEREBRO-CEREBELLO-CEREBRAL
CONNECTIONS)
It is a neuronal pathway through which corti-
cocerebellum controls the voluntary movements.
Fibers from motor areas 4 and 6 in frontal
lobe of cerebral cortex enter the pontine nuclei.
These fibers are called corticopontine fibers (Fig
93-2). From pontine nuclei, the pontocerebellar
fibers arise and pass through middle cerebellar
peduncle of the opposite side and terminate in
the cerebellar cortex. This pathway is called the
cerebropontocerebellar tract.
The cerebellar cortex is, in turn, connected
to the dentate nucleus. Fibers from the dentate
nucleus pass via superior cerebellar peduncle
and end in red nucleus of opposite side. These
fibers are called dentatorubral fibers. From red
nucleus, the rubrothalamic fibers go to thalamus.
Thalamus is connected to areas 4 and 6 in motor
cortex of cerebrum by thalamocortical fibers. This
pathway is called dentatorubrothalamocortical
tract.
FUNCTIONS OF CORTICOCEREBELLUM
Corticocerebellum is concerned with the
integration and regulation of well coordinated
FIGURE 93-2: Schematic representation of cerebro–cerebello–cerebral circuit

Chapter 93 Cerebellum
573
muscular activities. It is because of its afferent-
efferent connection with cerebral cortex through
the cerebro-cerebello-cerebral circuit. Apart from
its connections with cerebral cortex, cerebellum
also receives feedback signals from the muscles
through the nerve fibers of proprioceptors.
Mechanism of Action of Corticocerebellum
1. Damping action
Damping action refers to prevention of
exaggerated muscular activity. This helps in
making the voluntary movements smooth and
accurate. All the voluntary muscular activities are
initiated by motor areas of cerebral cortex.
Simultaneously, corticocerebellum receives
impulses from motor cortex as well as feedback
signals from the muscles as soon as the
muscular activity starts.
Corticocerebellum, in turn, sends information
(impulses) to cerebral cortex to discharge only
appropriate signals to the muscles and to cut
off any extra impulses. Because of this damping
action of corticocerebellum, the exaggeration of
muscular activity is prevented and the
movements become smooth and accurate.
Literally, the word damping means any effect that
decreases the amplitude of mechanical
oscillation.
2. Control of ballistic movements
Ballistic movements are the rapid alternate
movements, which take place in different parts
of the body while doing any skilled or trained
work like typing, cycling, dancing, etc. Cortico-
cerebellum plays an important role in preplan-
ning the ballistic movements during learning
process.
3. Timing and programming the movements
Corticocerebellum plays an important role in
timing and programming the movements
particularly during learning process. While using
a typewriter or while doing any other fast skilled
work, a chain of movements occur rapidly in a
sequential manner. During the learning process
of these skilled works, corticocerebellum plans
the various sequential movements. It also plans
schedule of time duration of each movement and
the time interval between movements. All the
information from corticocerebellum are
communicated to sensory motor area of cerebral
cortex and stored in the form of memory. So,
after the learning process is over, these activities
are executed easily and smoothly in sequential
manner.
4. Servomechanism
Servomechanism is the correction of any
disturbance or interference while performing
skilled work. Once the skilled works are learnt,
the sequential movements are executed without
any interruption. Cerebellum lets the cerebral
cortex to discharge the signals, which are
already programmed and stored at sensory
motor cortex, and, does not interfere much.
However, if there is any disturbance or inter-
ference, the corti-cocerebellum immediately
influences the cortex and corrects the move-
ments.
5. Comparator function
The comparator function of the corticocere-
bellum is responsible for the integration and
coordination of the various muscular activities.
On one side, cerebellum receives the
information from cerebral cortex regarding the
cortical impulses which are sent to the muscles.
On the other side, it receives the feedback
information (proprioceptive impulses) from the
muscles regarding their actions under the
instruction of cerebral cortex.
By receiving the messages from both ends,
corticocerebellum compares the cortical
commands for muscular activity and the actual
movements carried out by the muscles. If any
correction is to be done, then, corticocerebellum
sends instructions (impulses) to the motor
cortex.
Accordingly, the cerebral cortex corrects or
modifies the signals to muscles, so that the
movements become accurate, precise and
smooth. This function of corticocerebellum is
known as comparator function.

Nervous System
574
Simultaneously, it also receives impulses
from tactile receptors, eye and ear. Such addi-
tional information facilitates the comparator
function of corticocerebellum.
APPLIED PHYSIOLOGY –
CEREBELLAR LESIONS
Cerebellar lesions occur due to tumor, abscess,
injury, excess alcohol ingestion. In general,
during cerebellar lesions, there are disturbances
in posture, equilibrium and the movements. In
unilateral lesion, symptoms appear on the
affected side because cerebellum controls the
same (ipsilateral) side of the body.
DISTURBANCES IN TONE AND
POSTURE
1. Atonia or Hypotonia
Atonia is the loss of tone and hypotonia is
reduction in tone of the muscle. Atonia or
hypotonia occurs because of the loss of
facilitatory impulses from cerebellum to gamma
motor neurons in the spinal cord.
2. Attitude
Attitude of the body changes in unilateral lesion
of the cerebellum. The changes in the attitude
are:
i. Rotation of head towards the opposite
side (unaffected side)
ii. Lowering of shoulder on the same side
iii. Abduction of leg on the affected side. The
leg is rotated outward
iv. The weight of the body is thrown on the
leg of unaffected side. So, the trunk is bent
with concavity towards the affected side.
3. Deviation Movement
It is the lateral deviation of arms when both the
arms are stretched and held in front of the body
with closed eyes. In bilateral lesion, both the
arms deviate and in unilateral lesion arm of the
affected side deviates.
4. Effect on Deep Reflexes
Pendular movements occur while eliciting a
tendon jerk particularly the knee jerk (Chapter
87).
DISTURBANCES IN EQUILIBRIUM
While Standing
While standing, the legs are spread to provide
a broad base. And, the body sways side-to-side
with the oscillations of the head.
While Moving – Gait
Gait means the manner of walking. In cerebellar
lesion, a staggering, reeling and drunken like gait
is observed.
DISTURBANCES IN MOVEMENTS
1. Ataxia – lack of coordination of movements.
2. Asynergia – lack of coordination between
different groups of muscles
3. Asthenia – weakness, easy fatigability and
slowness of muscles
4. Dysmetria – inability to check the exact
strength and duration of muscular
contractions required for any voluntary act.
While reaching for an object, the arm may
overshoot (hypermetria) or it may fall short
(hypometria) of the object
5. Intention tremor – tremor that occurs while
attempting to do any voluntary act. Refer
Chapter 91 for details of tremor
6. Astasia – unsteady voluntary movements
7. Nystagmus – to and fro movement of eyeball
(Chapter 98)
8. Rebound phenomenon – when the patient
attempts to do a movement against a
resistance, and if the resistance is suddenly
removed, the limb moves forcibly in the
direction in which the attempt was made
9. Dysarthria – disturbance in speech
10. Adiadochokinesis – inability to do rapid
alternate successive movements such as
supination and pronation of arm.
INTRODUCTION
COMPONENTS
CORPUS STRIATUM
SUBSTANTIA NIGRA
SUBTHALAMIC NUCLEUS OF LUYS
FUNCTIONS
CONTROL OF MUSCLE TONE
CONTROL OF MOTOR ACTIVITY
CONTROL OF REFLEX MUSCULAR ACTIVITY
CONTROL OF AUTOMATIC ASSOCIATED MOVEMENTS
ROLE IN AROUSAL MECHANISM
APPLIED PHYSIOLOGY – DISORDERS
PARKINSON’S DISEASE
WILSON’S DISEASE
CHOREA
ATHETOSIS
CHOREOATHETOSIS
HUNTINGTON’S DISEASE
HEMIBALLISMUS
KERNICTERUS
INTRODUCTION
Basal ganglia are the scattered masses of gray
matter submerged in subcortical substance of
cerebral hemisphere (Fig. 94-1). Basal ganglia
form the part of extrapyramidal system, which
is concerned with integration and the regulation
of motor activities.
COMPONENTS OF BASAL GANGLIA
Basal ganglia include three primary components:
1. Corpus striatum
2. Substantia nigra
3. Subthalamic nucleus of Luys.
Basal Ganglia
94
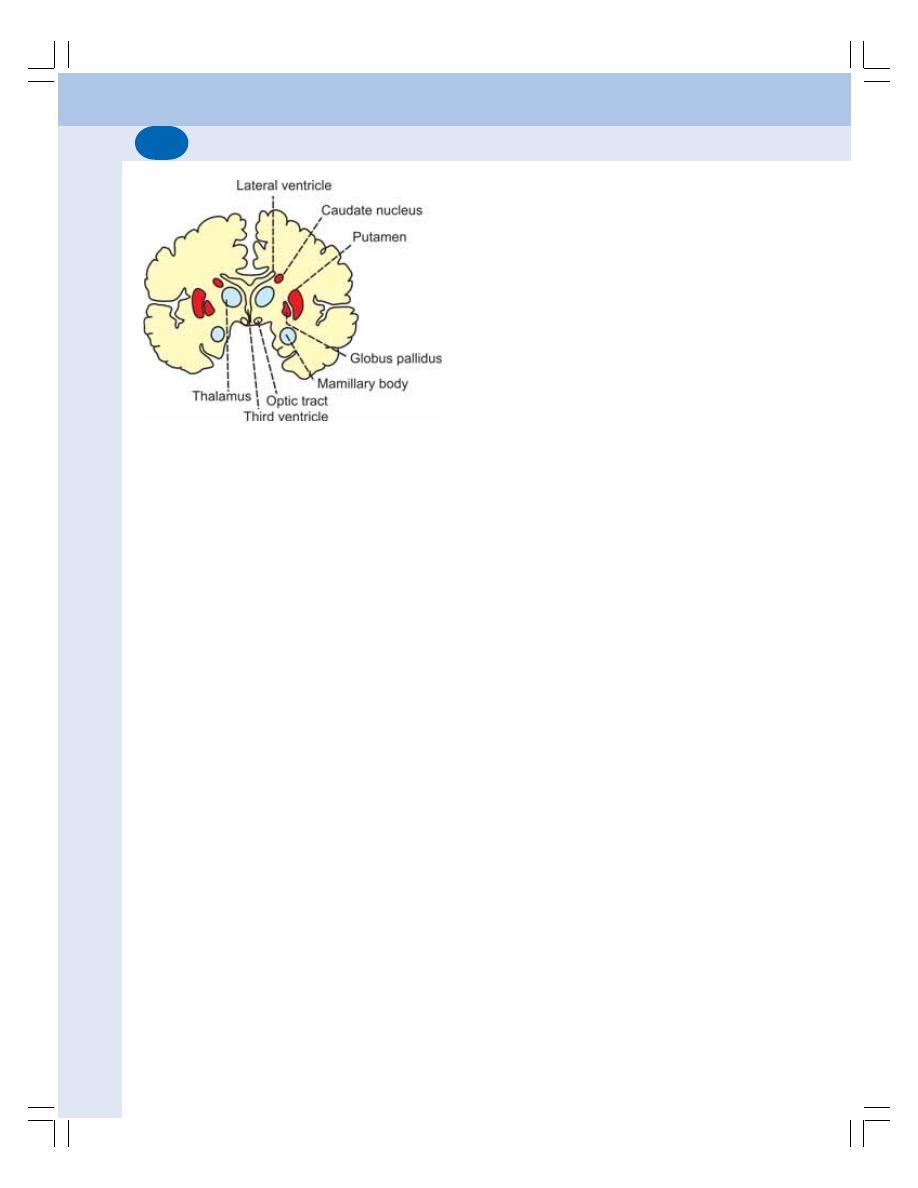
Nervous System
576
CORPUS STRIATUM
It is a mass of gray matter situated at the base
of cerebral hemispheres in close relation to the
thalamus. It has two parts:
i.
Caudate nucleus
ii. Lenticular nucleus which is divided into
two portions:
a. Putamen
b. Globus pallidus.
SUBSTANTIA NIGRA
Substantia nigra is situated below red nucleus.
It is made up of large pigmented and small
nonpigmented cells. The pigment contains high
quantity of iron.
SUBTHALAMIC NUCLEUS OF LUYS
This nucleus is situated lateral to red nucleus
and dorsal to substantia nigra.
FUNCTIONS OF BASAL GANGLIA
The basal ganglia form the part of extrapyramidal
system, which is concerned with motor activities.
The various functions of basal ganglia are:
1. CONTROL OF MUSCLE TONE
Basal ganglia control the muscle tone. In fact
the gamma motor neurons of spinal cord are
responsible for the tone of the muscles. Basal
ganglia decrease muscle tone by inhibiting the
gamma motor neurons through descending
inhibitory reticular system in brainstem.
2. CONTROL OF MOTOR ACTIVITY
i.
Regulation of Voluntary Movements
Voluntary motor activities are initiated by cerebral
cortex. However, these movements are
controlled by basal ganglia. During lesions of
basal ganglia, the control mechanism is lost and
so the movements become inaccurate and
awkward.
Basal ganglia control the motor activities
because of the nervous (neuronal) circuits
between basal ganglia and other parts of brain
involved in motor activity.
ii. Regulation of Conscious Movements
Basal ganglia regulate the conscious move-
ments. This function of basal ganglia is also
known as the cognitive control of activity. For
example, when a stray dog barks at a man,
immediately the person, understands the
situation, turns away and starts running.
iii. Regulation of Subconscious Movements
Basal ganglia regulate the subconscious
movements which take place during trained
motor activities, i.e. skilled activities such as
writing the learnt alphabet, paper cutting, nail
hammering, etc.
3. CONTROL OF REFLEX MUSCULAR
ACTIVITY
Some of the reflex muscular activities,
particularly visual and labyrinthine reflexes are
important in the maintenance of posture. Basal
ganglia are responsible for the coordination and
integration of impulses for these reflex activities.
4. CONTROL OF AUTOMATIC
ASSOCIATED MOVEMENTS
Automatic associated movements are the
movements in the body, which take place along
with some motor activities. Examples are the
FIGURE 94-1: Basal ganglia

Chapter 94 Basal Ganglia
577
swing of the arms while walking, appropriate
facial expressions while talking or doing any
work. Basal ganglia are responsible for the
automatic associated movements.
5. ROLE IN AROUSAL MECHANISM
Globus pallidus and red nucleus are involved in
arousal mechanism because of their connec-
tions with reticular formation. Extensive lesion
in globus pallidus causes drowsiness, leading
to sleep.
APPLIED PHYSIOLOGY –
DISORDERS OF BASAL GANGLIA
1. PARKINSON’S DISEASE
The Parkinson’s disease is a slow progressive
degenerative disease of nervous system
associated with destruction of dopamine
producing cells in brain. It is named after the
discoverer James Parkinson. It is also called
parkinsonism or paralysis agitants.
Causes of Parkinson’s Disease
Parkinson’s disease occurs due to lack of
dopamine caused by damage of basal ganglia.
It is mostly due to the destruction of substantia
nigra and the nigrostrial pathway, which has
dopaminergic fibers. Damage of basal ganglia
usually occurs because of the following causes:
i. Viral infection of brain like encephalitis
ii. Cerebral arteriosclerosis
iii. Injury to basal ganglia
iv. Destruction or removal of dopamine in basal
ganglia. It occurs mostly due to long term
treatment with antihypertensive drugs like
reserpine. Parkinsonism due to the drugs
is known as drug-induced Parkinsonism
v. Unknown causes: Parkinsonism can occur
because of the destruction of basal ganglia
due to some unknown causes. This type
of Parkinsonism is called idiopathic
Parkinsonism.
Signs and Symptoms of Parkinson’s Disease
Parkinson’s disease develops very slowly and
the early signs and symptoms may be unnoticed
for months or even for years. Often the
symptoms start with a mild noticeable tremor in
just one hand. When the tremor becomes
remarkable the disease causes slowing or
freezing of movements followed by rigidity.
Common signs and symptoms of Parkinson’s
disease are:
1. Tremor
Refer Chapter 91 for details of tremor. In
Parkinson’s disease, static tremor or resting
tremor occurs during rest. But it disappears while
doing any work. It is also called drum beating
tremor, as the movements are similar to beating
a drum. The thumb moves rhythmically over the
index and middle fingers. These movements are
called pill rolling movements.
2. Slowness of movements
Over the time, the movements start slowing
down (bradykinesia) and it takes a long time
even to perform a simple task. Gradually the
patient becomes unable to initiate the voluntary
activity (akinesia) or the voluntary movements
are reduced (hypokinesia). It is because of
hypertonicity of the muscles.
3. Poverty of movements
Poverty of movements is the loss of all automatic
associated movements. Because of absence of
the automatic associate movements, the body
becomes statue like. The face becomes mask
like, due to absence of appropriate expressions
like blinking and smiling.
4. Rigidity
Stiffness of muscles occurs in limbs resulting
in rigidity of limbs. The muscular stiffness occurs
because of increased muscle tone which is due
to the removal of inhibitory influence on gamma

Nervous System
578
motor neurons. It affects both flexor and
extensor muscles equally. So, the limbs become
more rigid like pillars. The condition is called lead
pipe rigidity. In later stages the rigidity extends
to neck and trunk.
5. Gait
Gait refers to manner of walking. The patient
looses the normal gait. Gait in Parkinson’s
disease is called festinant gait. The patient walks
quickly in short steps by bending forward as if
he is going to catch up the center of gravity.
6. Speech Problems
Many patients develop speech problems. They
may speak very softly or sometimes rapidly. The
words are repeated many times. Finally the
speech becomes slurred and they hesitate to
speak.
7. Emotional changes
The persons affected by Parkinson’s disease are
often upset emotionally.
8. Dementia
In later stages, some patients develop dementia
(Chapter 101).
2. WILSON’S DISEASE
Wilson’s disease is an inherited disorder
characterized by excess of copper in the body
tissues. It is also known as progressive
hepatolenticular degeneration. This disease
develops due to damage of the lenticular
nucleus. In addition to symptoms of Parkinson’s
disease, liver failure and damage to the central
nervous system occur.
3. CHOREA
It is an abnormal involuntary movement. Chorea
means rapid jerky movements. It mostly involves
the limbs. It is due to lesion in caudate nucleus
and putamen.
4. ATHETOSIS
It is another type of abnormal involuntary
movement, which refers to slow rhythmic and
twisting movements. It is because of the lesion
in caudate nucleus and putamen.
5. CHOREOATHETOSIS
It is the condition characterized by aimless
involuntary muscular movements. It is due to
combined effects of chorea and athetosis.
6. HUNTINGTON’S DISEASE
Huntington’s disease is an inherited progressive
neural disorder due to the degeneration of
neurons secreting GABA in corpus striatum and
substantia nigra. It is characterized by chorea,
hypotonia and dementia.
7. HEMIBALLISMUS
It is a disorder characterized by violent
involuntary abnormal movements on one side
of the body involving mostly the arm. While
walking, the arm swings widely. Hemiballism
occurs due to degeneration of subthalamic
nucleus of Luys.
8. KERNICTERUS
Kernicterus is a form of brain damage in infants
caused by severe jaundice. Basal ganglia are
the mainly affected parts of brain. Refer Chapter
16 for details.
INTRODUCTION
NEOCORTEX AND ALLOCORTEX
LOBES OF CEREBRAL CORTEX
CEREBRAL DOMINANCE
BRODMANN AREAS
FRONTAL LOBE
PRECENTRAL CORTEX
PREFRONTAL CORTEX OR ORBITOFRONTAL CORTEX
APPLIED PHYSIOLOGY – FRONTAL LOBE SYNDROME
PARIETAL LOBE
SOMESTHETIC AREA I
SOMESTHETIC AREA II
SOMESTHETIC ASSOCIATION AREA
APPLIED PHYSIOLOGY
TEMPORAL LOBE
PRIMARY AUDITORY AREA
AUDITOPSYCHIC AREA
AREA FOR EQUILIBRIUM
APPLIED PHYSIOLOGY – TEMPORAL LOBE SYNDROME
OCCIPITAL LOBE
AREAS OF VISUAL CORTEX
APPLIED PHYSIOLOGY
LIMBIC SYSTEM
COMPONENTS
FUNCTIONS
Cerebral Cortex and
Limbic System
95
INTRODUCTION
The cerebral cortex consists of two hemispheres
with area of 2.2 sqm. The two cerebral hemi-
spheres are separated by a deep vertical fissure
(deep furrow or groove). The separation is
complete anteriorly and posteriorly. But in the
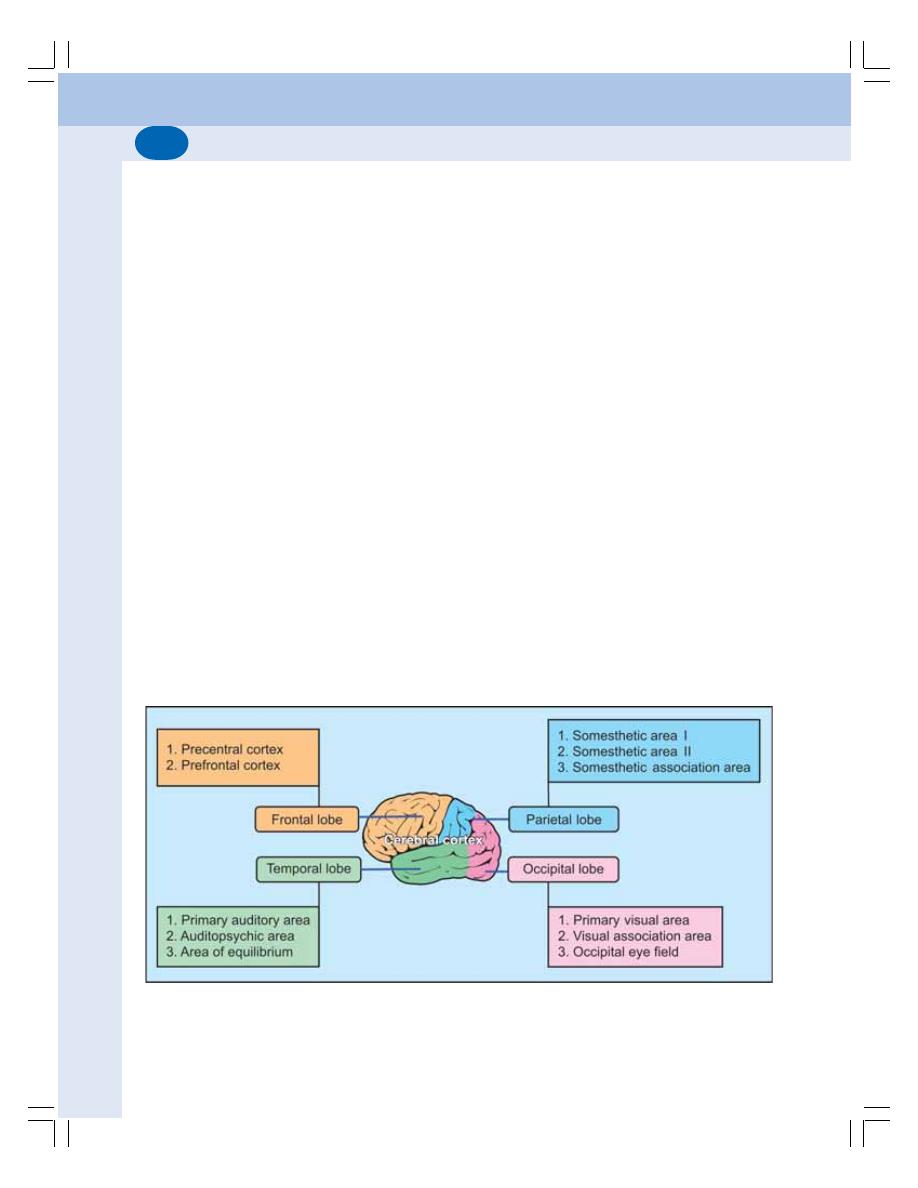
Nervous System
580
middle portion, the fissure extends only up to
corpus callosum which the broad band of com-
missural fibers, connecting the two hemispheres.
The surface of the cerebral cortex is characte-
rized by complicated pattern of sulci (singular =
sulcus) and gyri (singular = gyrus). Sulcus is a
slight depression or groove and gyrus is a raised
ridge.
Cerebral cortex is formed by outer gray
matter and inner white matter. It has different
types of nerve cells along with their processes
and neuroglia which are arranged in six layers.
It is not uniform throughout. It is thickest at the
precentral gyrus and thinnest at the frontal and
occipital poles.
NEOCORTEX AND ALLOCORTEX
The part of the cerebral cortex that has all six
layers of structures is called neocortex. It is also
called isocortex or neopallium. It is the phylo-
genetically new structure of cerebral cortex. Neo-
cortex forms the major portion of cerebral cortex.
The remaining part of cerebral cortex has less
than six layers of structures. This part of the
cortex is called allocortex. It includes archicortex
and paleocortex that form the part of limbic
system.
LOBES OF CEREBRAL CORTEX
In each hemisphere, there are three surfaces
lateral, medial and inferior surfaces. The neo-
cortex of each cerebral hemisphere consists of
four lobes (Figs 95-1 to 95-3):
1. Frontal lobe
2. Parietal lobe
3. Occipital lobe
4. Temporal lobe.
The lobes of each hemisphere are demarked
by four main fissures and sulci:
1. Central sulcus or Rolandic fissure between
frontal and parietal lobes
2. Parieto-occipital sulcus between parietal and
occipital lobe
3. Sylvian fissure or lateral sulcus between
parietal and temporal lobes
4. Callosomarginal fissure between temporal
lobe and limbic area.
CEREBRAL DOMINANCE
Cerebral dominance is defined as the dominance
of one cerebral hemisphere over the other in the
control of cerebral functions. The two cerebral
hemispheres are not functionally equivalent.
Cerebral dominance is related to handed-
ness, i.e. preference of the individual to use right
FIGURE 95–1: Parts of cerebral cortex
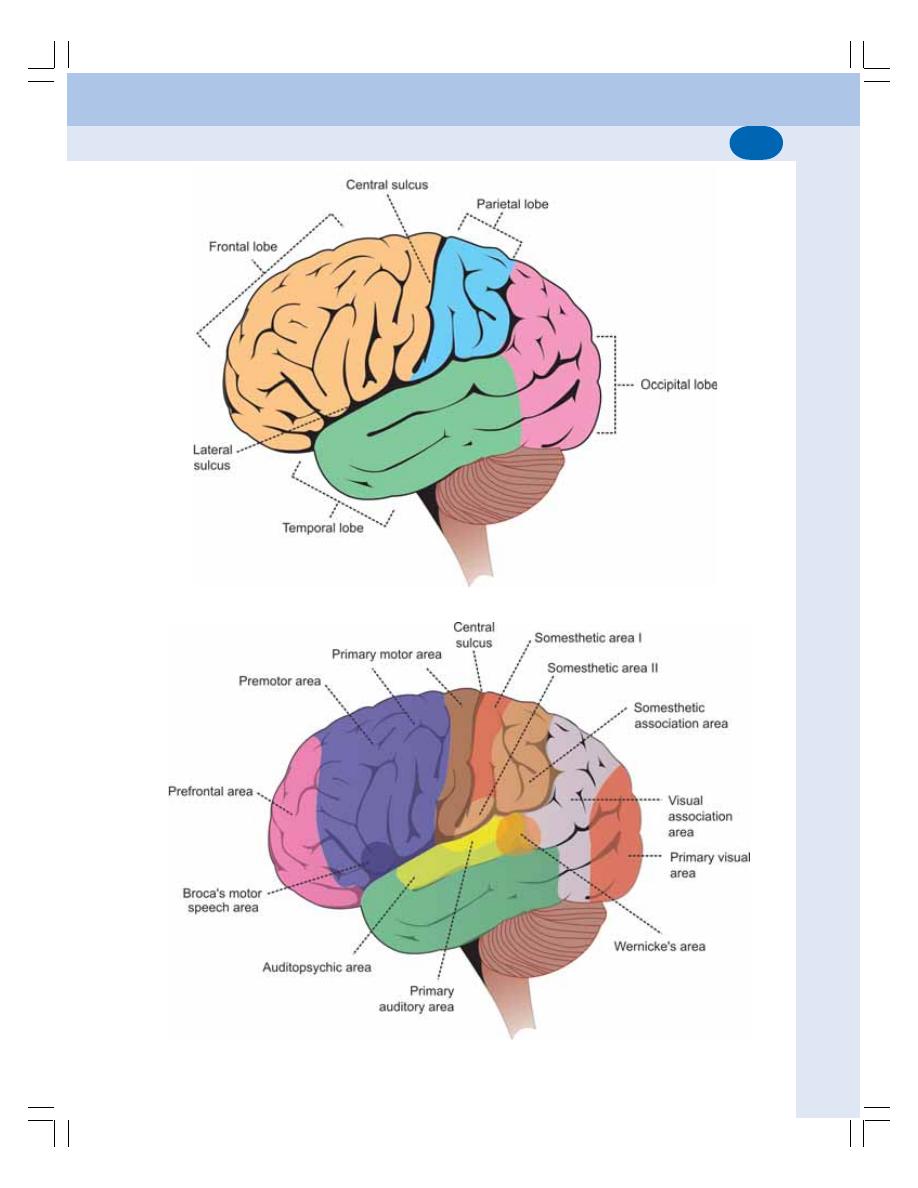
Chapter 95 Cerebral Cortex and Limbic System
581
FIGURE 95-2: Lobes of cerebral cortex
FIGURE 95-3: Functional regions on lateral surface of cerebral cortex
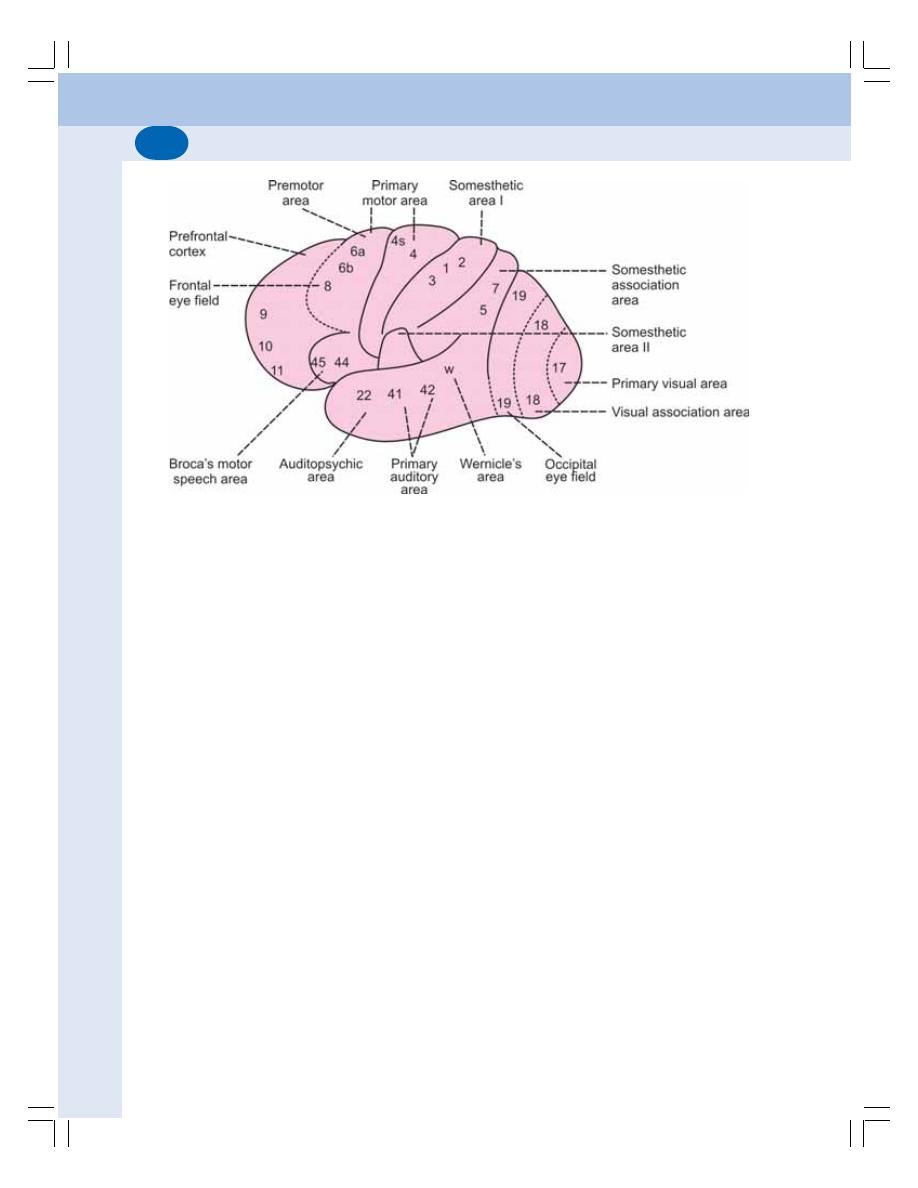
Nervous System
582
or left hand. More than 90% of people are right
handed. In these individuals, the left hemisphere
is dominant and it controls the analytical process
and language related functions such as speech,
reading and writing. Hence, the left hemisphere
of these persons is called dominant or
categorical hemisphere.
BRODMANN AREAS
Brodmann area is a region of cerebral cortex
defined on the basis of organization of neurons.
These areas were originally defined and num-
bered Korbinian Brodmann. Some of these
areas were given specific names based on their
functions.
FRONTAL LOBE OF CEREBRAL
CORTEX
The frontal lobe forms one-third of the cortical
surface. It extends from frontal pole to the central
sulcus and limited below by the lateral sulcus.
The frontal lobe of cerebral cortex is divided into
two parts:
I. Precentral cortex situated posteriorly
II. Prefrontal cortex situated anteriorly.
PRECENTRAL CORTEX
The posterior part of frontal lobe is called
precentral cortex. It includes the lip of central
sulcus, whole of precentral gyrus and posterior
portions of superior, middle and inferior frontal
gyri. It also extends to the medial surface.
This part is also called excitomotor cortex or
area, since the stimulation of different points in
this area causes activity of discrete skeletal
muscle. Precentral cortex is further divided into
three functional areas (Fig. 95-3):
1. Primary motor area
2. Premotor area
3. Supplementary motor area.
1. Primary Motor Area
It extends throughout the precentral gyrus and
the adjoining lip of central sulcus. The areas 4
and 4S are present here (Figs 95-4 and 95-5).
Functions of primary motor area
The primary motor area is concerned with initia-
tion of voluntary movements and speech.
FIGURE 95-4: Lateral surface of cerebral cortex
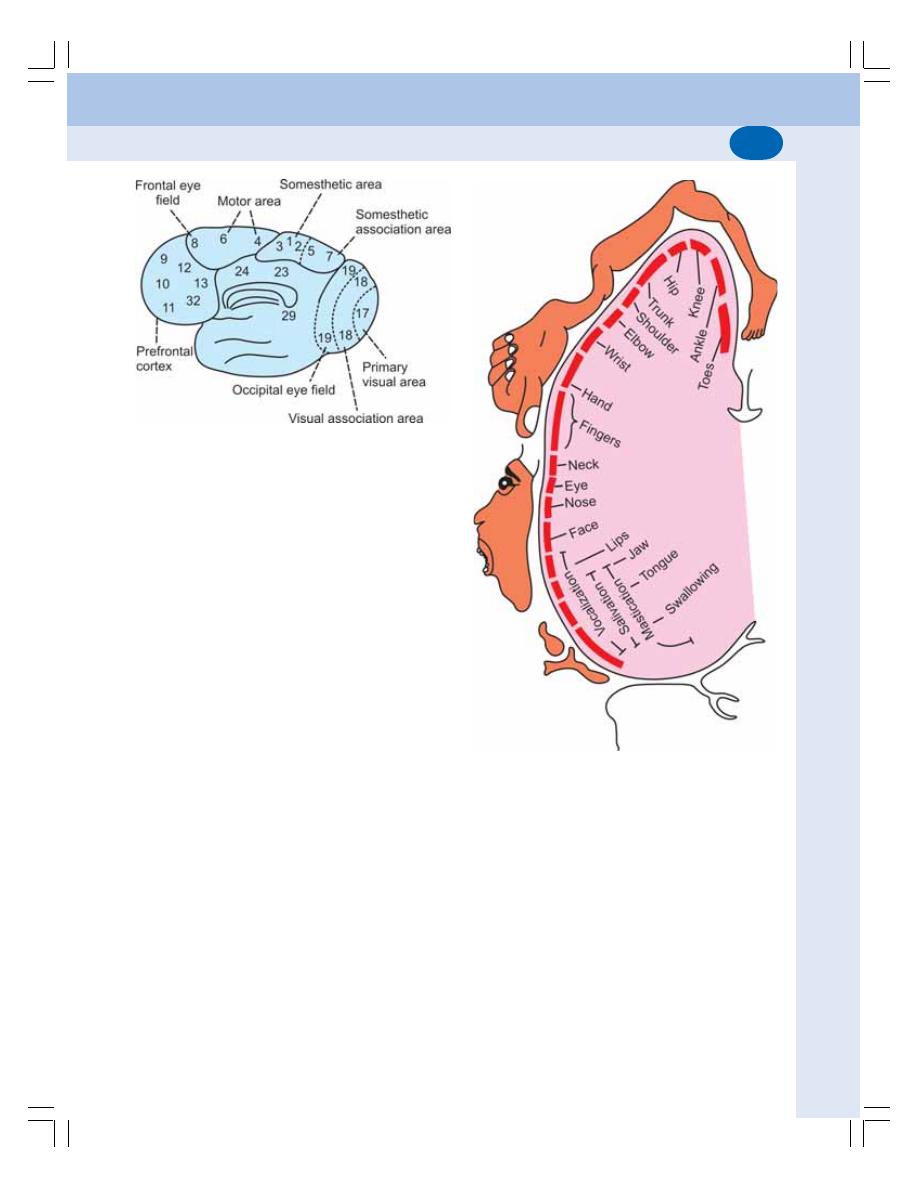
Chapter 95 Cerebral Cortex and Limbic System
583
Area 4S
Area 4S is called suppressor area. It forms a
narrow strip anterior to area 4. It scrutinizes and
suppresses the extra impulses produced by area
4 and prevents exaggeration of movements.
2. Premotor Area
This has areas 6, 8, 44 and 45. The premotor
area is anterior to primary motor area in the pre-
central cortex.
FIGURE 95-6: Topographical arrangement
(homunculus) of motor areas in cerebral cortex
FIGURE 95-5: Medial surface of cerebral cortex
Area 4
It is a tapering strip of area situated in precentral
gyrus of frontal lobe (Figs 95-4 and 95-5). Area
4 is the center for movement, as it sends all
efferent (corticospinal) fibers of primary motor
area. Through the fibers of corticospinal tracts,
area 4 activates the lower motor neurons in the
spinal cord. It activates both
α motor neurons
and
γ motor neurons simultaneously by the
process called coactivation.
Activation of
α motor neurons causes con-
traction of extrafusal fibers of the muscles.
Activation of
γ motor neurons causes contraction
of intrafusal fibers leading to increase in muscle
tone.
Localization – homunculus
The muscles of various parts of the body are
represented in area 4 in an inverted way from
medial to lateral surface. The lower parts of body
are represented in medial surface and upper
parts of the body are represented in the lateral
surface. The order of representation from medial
to lateral surface – toes, ankle, knee, hip, trunk,
shoulder, arm, elbow, wrist, hand fingers and
face. However, parts of the face are not represen-
ted in inverted manner (Fig. 95-6).

Nervous System
584
Functions of premotor area
The premotor area is concerned with control of
postural movements.
Area 6
Area 6 is in the posterior portions of superior,
middle and inferior frontal gyri. It is subdivided
into 6a and 6b. It gives origin to some of the
pyramidal tract fibers.
Area 6 has two functions:
i. It is concerned with coordination of
movements initiated by area 4. It helps
to make the skilled movements more
accurate and smooth
ii. It is believed to be the cortical center for
extrapyramidal system.
Area 8
Area 8 is called frontal eye field. It lies anterior
to area 6 in the precentral cortex. The frontal eye
field is concerned with conjugate movement of
eyeballs.
Areas 44 and 45 – Broca’s Area
The Broca’s area is the motor area for speech.
It is present in left hemisphere (dominant
hemisphere) of right handed persons and in
the right hemisphere of left handed persons. It
is a special region of premotor cortex situated
in inferior frontal gyrus.
Broca’s area is responsible for movements
of tongue, lips and larynx, which are involved in
speech.
3. Supplementary Motor Area
It is situated in medial surface of frontal lobe
rostral to primary motor area.
Function of supplementary motor area
The exact function of this area is not understood
clearly. It is suggested that it is concerned with
coordinated skilled movements.
PREFRONTAL CORTEX OR
ORBITOFRONTAL CORTEX
It is the anterior part of frontal lobe of cerebral
cortex, in front of areas 8 and 44. It occupies
the medial, lateral and inferior surfaces and
includes orbital gyri, medial frontal gyrus and the
anterior portions of superior, middle and inferior
frontal gyri.
Areas present in prefrontal cortex are 9, 10,
11, 12, 13, 14, 23, 24, 29 and 32. Areas 12, 13,
14, 23, 24, 29 and 32 are in medial surface. Areas
9, 10 and 11 are in lateral surface.
Area 13 is concerned with emotional
reactions.
Functions of Prefrontal Cortex
1. It forms the center for the higher functions
like emotion, learning, memory and social
behavior. Short term memories are registered
here
2. It is the center for planned actions
3. It is the seat of intelligence. So, it is also called
the organ of mind
4. It is responsible for the personality of the
individuals
5. It is responsible for the various autonomic
changes during emotional conditions,
because of its connections with hypotha-
lamus and brainstem.
APPLIED PHYSIOLOGY – FRONTAL
LOBE SYNDROME
The injury or ablation of prefrontal cortex leads
to a condition called frontal lobe syndrome. The
features of this syndrome are:
1. Emotional instability: There is lack of restraint
leading to hostility, aggressiveness and
restlessness
2. Lack of concentration and lack of fixing atten-
tion
3. There is lack of initiation and difficulty in
planning any course of action
4. Impairment of recent memory occurs. How-
ever, the memory of remote events is not lost
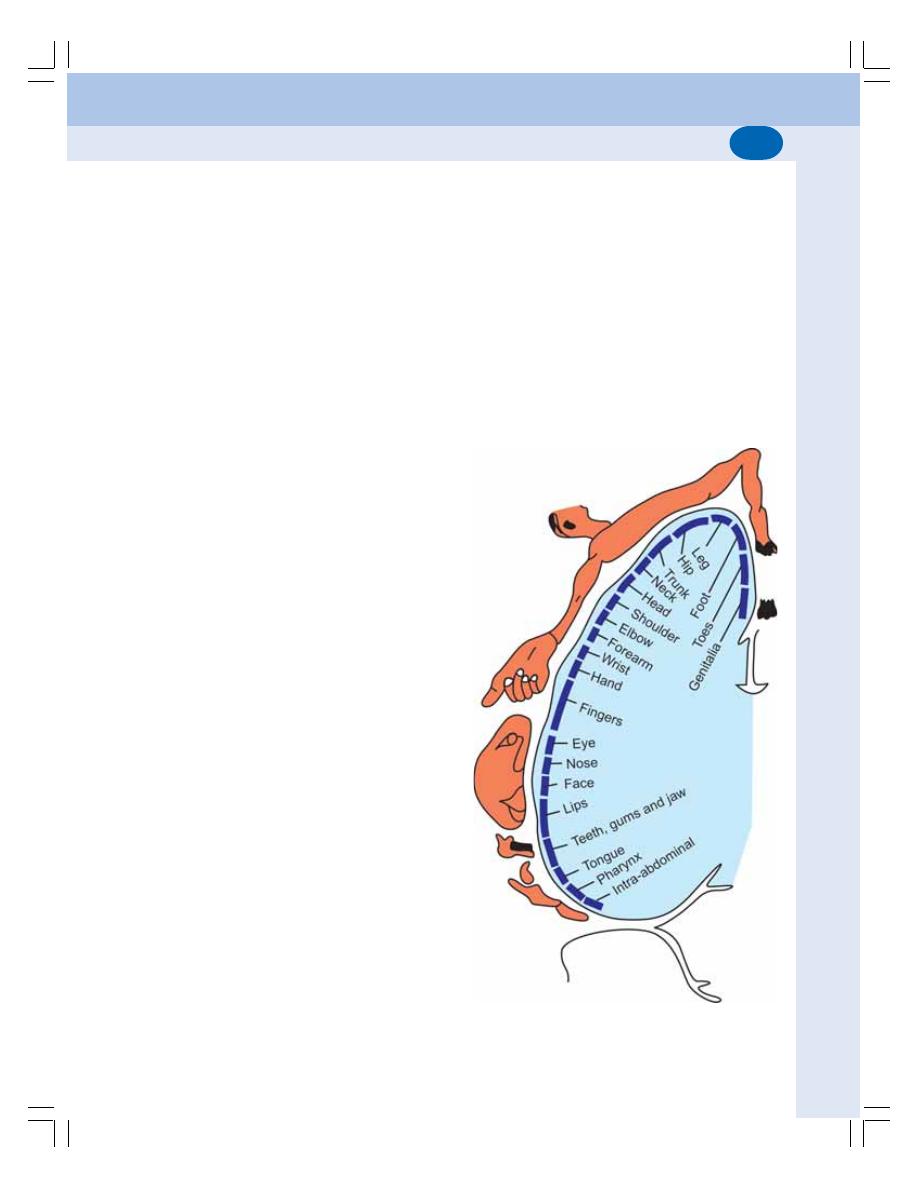
Chapter 95 Cerebral Cortex and Limbic System
585
5. Loss of moral and social sense is common
and there is loss of love for family and
friends
6. There is failure to realize the seriousness of
the condition. The subject has the sense of
well being and also has flight of ideas
7. Apart from behavioral changes, there are
some functional abnormalities also
i. Hyperphagia (increased food intake)
ii. Loss of control over sphincter of the uri-
nary bladder or rectum
iii. Disturbances in orientation
iv. Slight tremor.
PARIETAL LOBE
Parietal lobe extends from central sulcus and
merges with occipital lobe behind and temporal
lobe below. This lobe is separated from occipital
lobe by parieto-occipital sulcus and from
temporal lobe by Sylvian sulcus. Parietal lobe
is divided into three functional areas:
A. Somesthetic area I
B. Somesthetic area II
C. Somesthetic association area.
In addition to these three areas, a part of sen-
sory motor area is also situated in parietal lobe
(see below).
SOMESTHETIC AREA I
It is also called somatosensory area I or primary
somesthetic or primary sensory area. It is
present in the posterior lip of central sulcus, in
the postcentral gyrus and in the paracentral
lobule.
Areas
This part of parietal lobe has three areas which
are called areas 3, 1 and 2. The anterior part of
this forms area 3 and posterior part forms areas
1 and 2.
Localization – Homunculus
The different sensory areas of the body are
represented in postcentral gyrus (primary
sensory area) in an inverted manner as in the
motor area. The toes are represented in lowest
part of medial surface, legs at the upper border
of hemispheres, then from above downwards
knee, thigh, hip, trunk, upper limb, neck and
face. The representation of face is not inverted.
The representation of parts of face from above
downwards is eyelids, nose, cheek, upper lip and
lower lip (Fig. 95-7).
Functions
1. The somesthetic area I is responsible for
perception and integration of cutaneous and
kinesthetic sensations. It receives sensory
impulses from cutaneous receptors (touch,
FIGURE 95-7: Topographical arrangement
(homunculus) of sensory areas in cerebral cortex

Nervous System
586
pressure, pain, temperature) and proprio-
ceptors of opposite side through thalamic
radiation. Area 1 is concerned with sensory
perception. The areas 2 and 3 are involved
in the integration of these sensations.
2. This area sends sensory feedback to the
premotor area. It is also concerned with the
movements of head and eyeballs.
3. Discriminative functions: In addition to
perception of cutaneous and kinesthetic
sensation, this area is also responsible for
recognizing the discriminative features of
sensations.
SOMESTHETIC AREA II
It is situated in postcentral gyrus below the area
of face of somesthetic area I. A part of this is
buried in Sylvian sulcus. It is also known as
somatosensory area II.
This area receives sensory impulses from
somesthetic area I and from thalamus directly.
Though the exact role of this area is not clear, it
is concerned with perception of sensation. Thus,
the sensory parts of body have two represen-
tations — in somesthetic area I and area II.
SOMESTHETIC ASSOCIATION AREA
This area is situated posterior to postcentral
gyrus, above the auditory cortex and in front of
visual cortex. It has two areas – 5 and 7. It is
concerned with synthesis of various sensations
perceived by somesthetic area I. Thus, the some-
sthetic association area forms the center for
combined sensations like stereognosis. The
lesion of this area causes astereognosis.
Sensorymotor Area
The sensory area of cortex is not limited to post-
central gyrus in parietal lobe. It extends anteriorly
into motor area in precentral gyrus of frontal lobe.
Similarly, the motor area is extended from pre-
central gyrus posteriorly into postcentral gyrus.
Thus, the precentral and postcentral gyri are
knit together by association neurons and are
functionally interrelated. So, this area is called
sensorymotor area.
The function of sensory motor area is to store
the timing and programming of various
sequential movements of complicated skilled
movements which are planned by neocerebellum
(Chapter 93).
APPLIED PHYSIOLOGY
Lesion or ablation of parietal lobe (sensory
cortex) results in the following disturbances:
1. Contralateral disturbance of cutaneous
sensations
2. Disturbances in kinesthetic sensations
3. Loss of tactile localization and discrimination.
TEMPORAL LOBE
Temporal lobe of cerebral cortex includes three
functional areas
A. Primary auditory area
B. Auditopsychic area
C. Area for equilibrium.
PRIMARY AUDITORY AREA
Primary auditory area includes:
1. Area 41
2. Area 42
3. Wernicke’s area.
Areas 41 and 42 are situated in anterior
transverse gyrus and lateral surface of superior
temporal gyrus. Wernicke’s area is in upper part
of superior temporal gyrus posterior to areas 41
and 42.
Functions
Primary auditory area is concerned with percep-
tion of auditory impulses, analysis of pitch and
determination of intensity and source of sound.
Areas 41 and 42 are concerned only with the
perception of auditory impulses. Wernicke’s area
is responsible for the interpretation of sound. It
carries out this function with the help of audi-
topsychic area (area 22).
AUDITOPSYCHIC AREA
It is the area 22 and it occupies the superior
temporal gyrus. This area is concerned with

Chapter 95 Cerebral Cortex and Limbic System
587
interpretation of auditory sensation along with
Wernicke’s area.
AREA FOR EQUILIBRIUM
This area is in the posterior part of superior
temporal gyrus. It is concerned with the main-
tenance of equilibrium of the body. Stimulation
of this area causes dizziness, swaying, falling
and feeling of rotation.
APPLIED PHYSIOLOGY – TEMPORAL
LOBE SYNDROME
Temporal lobe syndrome is otherwise known as
Kluver-Bucy syndrome. It is observed in animals,
particularly monkeys after the bilateral ablation
of temporal lobe along with amygdaloid and
uncus. It occurs in human beings during bilateral
lesions of these structures. The manifestations
of this syndrome are:
1. Aphasia: Disturbance in speech
2. Auditory disturbances: Such as frequent
attacks of tinnitus, auditory hallucinations
with sounds like buzzing, ringing or humming.
Tinnitus means noise in the ear. Hallucination
means feeling of a particular type of sensa-
tion without any stimulus
3. Disturbances in smell and taste sensations
4. Dreamy states: The patients are not aware
of their own activities and, have the feeling
of unreality
5. Visual hallucinations associated with hemi-
anopia.
OCCIPITAL LOBE – VISUAL CORTEX
Occipital lobe is also called the visual cortex.
AREAS OF OCCIPITAL LOBE
The occipital lobe consists of three functional
areas:
1. Primary visual area – area 17
2. Visual association area – area 18
3. Occipital eye field – area 19.
Functions
1. Primary visual area – area 17 is concerned
with perception of visual impulses
2. Visual association area – area 18 is con-
cerned with interpretation of visual impulses
3. Occipital eye field – area 19 is concerned with
movement of eyes.
APPLIED PHYSIOLOGY
Lesion in the upper or lower part of visual cortex
results in hemianopia. Bilateral lesion leads to
total blindness. Refer Chapter 106 for details.
LIMBIC SYSTEM OR LIMBIC LOBE
Limbic system or limbic lobe is a complex
system of cortical and subcortical structures that
form a ring around the hilus of cerebral hemi-
sphere (Fig. 95-8). Limbus means ring.
COMPONENTS
Structures of limbic system are classified into
four groups:
I. Achicortical structures
II. Paleocortical structures
III. Juxtallocortical structures
IV. Subcortical structures.
The structures of each group are given in
Fig. 95-9.
FUNCTIONS
1. Olfaction
The pyriform cortex and amygdaloid nucleus form
the olfactory centers.
2. Regulation of Endocrine Glands
Hypothalamus plays an important role in regu-
lation of endocrine secretion (Chapter 45).
3. Regulation of Autonomic Functions
Hypothalamus plays an important role in regula-
ting the autonomic functions (Chapter 92) such
as heart rate, blood pressure, water balance and
body temperature.
4. Regulation of Food Intake
Along with amygdaloid complex, the feeding cen-
ter and satiety center present in hypothalamus
regulate food intake (Chapter 92).
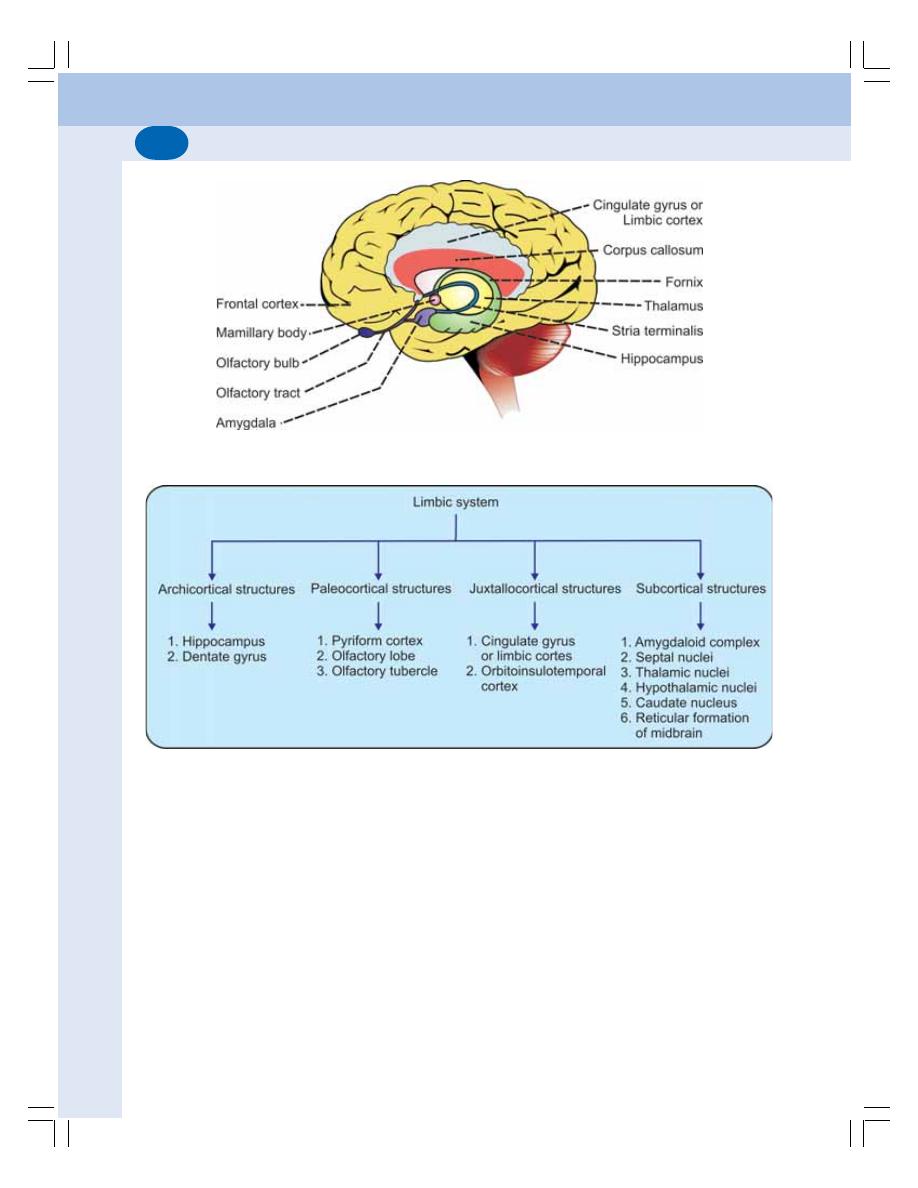
Nervous System
588
FIGURE 95-9: Components of limbic system
FIGURE 95-8: Major components of limbic system
5. Control of Circadian Rhythm
Hypothalamus is taking major role in the circadian
fluctuations of various physiological activities.
6. Regulation of Sexual Functions
Hypothalamus is responsible for maintaining
sexual functions.
7. Role in Emotional State
The emotional state of a person is maintained
by hippocampus along with hypothalamus.
8. Role in Memory
Hippocampus and Papez circuit play an
important role in memory (Chapter 101).
9. Role in Motivation
Reward and punishment centers present in
hypothalamus and other structures of limbic
system are responsible for motivation and the
behavior pattern of human beings (Chapter 92).
Refer Chapter 92 for details of the hypo-
thalamic functions.
DEFINITION
SITUATION
DIVISIONS
FUNCTIONS
ASCENDING RETICULAR ACTIVATING SYSTEM (ARAS)
DESCENDING RETICULAR SYSTEM
Reticular Formation
96
DEFINITION
Reticular formation is a diffused mass of neurons
and nerve fibers forming an ill-defined meshwork
of reticulum in the central portion of the brain-
stem.
SITUATION OF RETICULAR
FORMATION
The reticular formation is situated in brainstem.
It extends downwards into spinal cord and
upwards up to thalamus and subthalamus.
DIVISIONS OF RETICULAR
FORMATION
Reticular formation is divided into three divisions
based on the location in brainstem:
1. Medullary reticular formation
2. Pontine reticular formation
3. Midbrain reticular formation.
Each division of reticular formation has its
own collection of nuclei.
FUNCTIONS OF RETICULAR
FORMATION
Based on functions, the reticular formation along
with its connections is divided into two systems.
I. Ascending reticular activating system
II. Descending reticular system.
ASCENDING RETICULAR ACTIVATING
SYSTEM (ARAS)
ARAS begins in lower part of brainstem, extends
upwards through pons, midbrain, thalamus and
finally projects throughout the cerebral cortex.
It projects into cerebral cortex via subthalamus
and thalamus.
The ARAS receives fibers from the sensory
pathways via long ascending spinal tracts
(Fig. 96-1).
Functions of ARAS
1. ARAS is concerned with arousal pheno-
menon, alertness, maintenance of attention
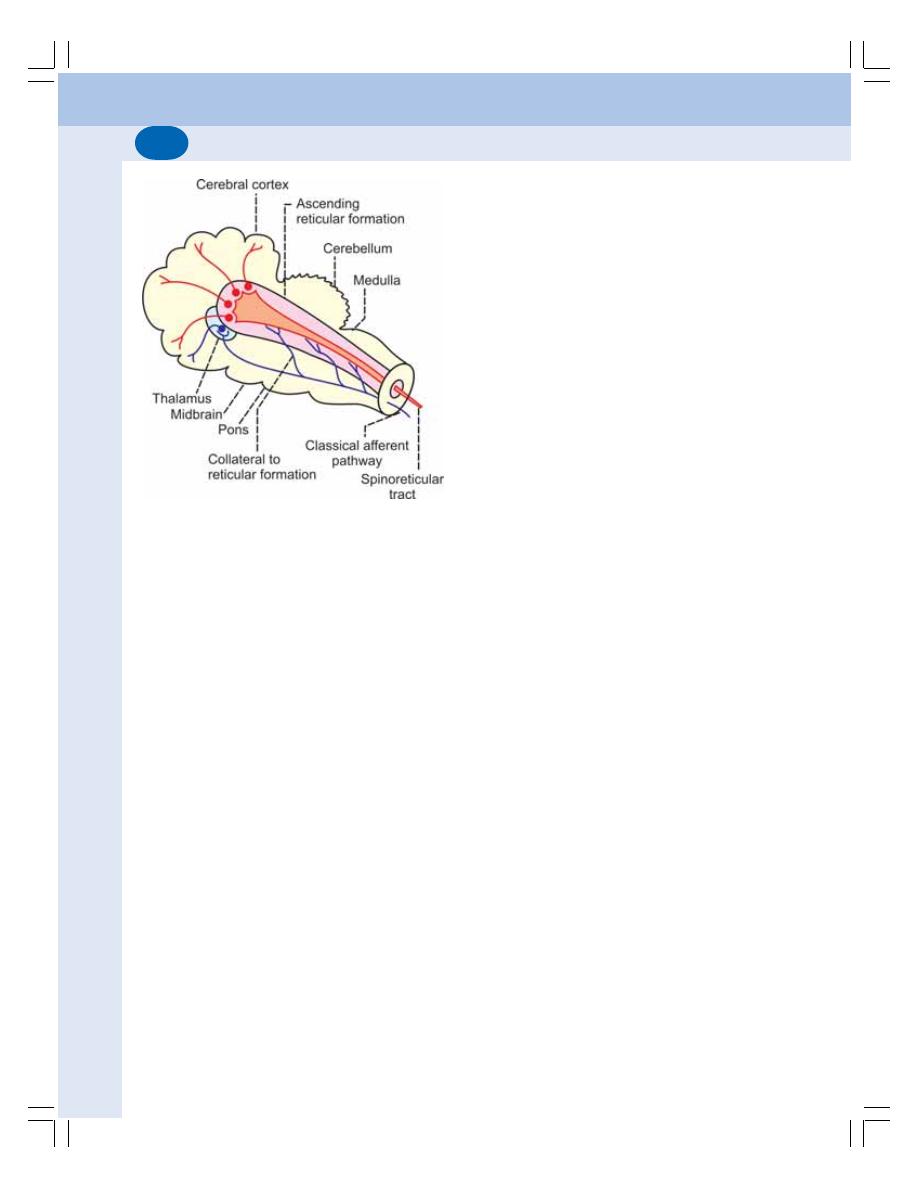
Nervous System
590
and wakefulness. Hence, it is called ascen-
ding reticular activating system. Stimulation
of midbrain reticular formation produces
wakefulness by generalized activation of
entire brain including cerebral cortex, thala-
mus, basal ganglia and brainstem. Any type
of sensory impulses such as impulses of
proprioception, pain, auditory, visual, taste,
and olfactory sensations cause sudden
activation of the ARAS producing arousal
phenomenon in animals and human beings.
Even the impulses of visceral sensations
activate this system. The sympathetic stimu-
lation and adrenaline cause arousal by
affecting midbrain
2. ARAS also causes emotional reactions
3. ARAS plays an important role in regulating
the learning processes and the development
of conditioned reflexes.
Mechanism of Action of ARAS
The impulses of all the sensations reach the
cerebral cortex through two channels:
1. Classical or specific sensory pathways
2. Ascending reticular activating system or
nonspecific specific pathway.
1. Classical or specific sensory pathways
Classical sensory pathways are the pathways
which transmit the sensory impulses from recep-
tors to cerebral cortex via thalamus. Some of
the pathways carry impulses of a particular
sensation only. For example, the auditory
stimulus transmitted by the auditory pathway
reaches the auditory cortex via thalamus and
causes perception of sound. Such classical
sensory pathways are called specific sensory
pathways.
2. Ascending reticular activating system or
nonspecific sensory pathway
All the sensory pathways send collaterals to
diffused areas of ARAS. It also receives afferents
from spinal cord directly in the form of spino-
reticular tract. ARAS in turn sends the impulses
to almost all the areas of cerebral cortex and
other parts of brain. Hence, this pathway is called
the nonspecific sensory pathway.
The nonspecific projection of ARAS into the
cortex is responsible for the arousal, alertness
and wakefulness. The sensory impulses trans-
mitted directly to cortex via classical pathway
causes perception of only the particular sen-
sation. Whereas, the impulses transmitted to
cortex via ARAS do not cause the perception
of any particular sensation but cause the gene-
ralized activation of almost all the areas of cere-
bral cortex and other parts of brain. This leads
to reactions of arousal, alertness and wake-
fulness.
The ARAS in turn is controlled by the feed-
back signals from cerebral cortex. Also, an inhibi-
tory system controls the activities of ARAS. The
inhibitory system involves posterior hypothala-
mus, intralaminar and anterior thalamic nuclei
and medullary area at the level of tractus soli-
tarius.
The tumor or lesion in ARAS leads to sleeping
sickness or coma. The impact of head injury on
ARAS also causes coma.
FIGURE 96-1: Ascending reticular formation
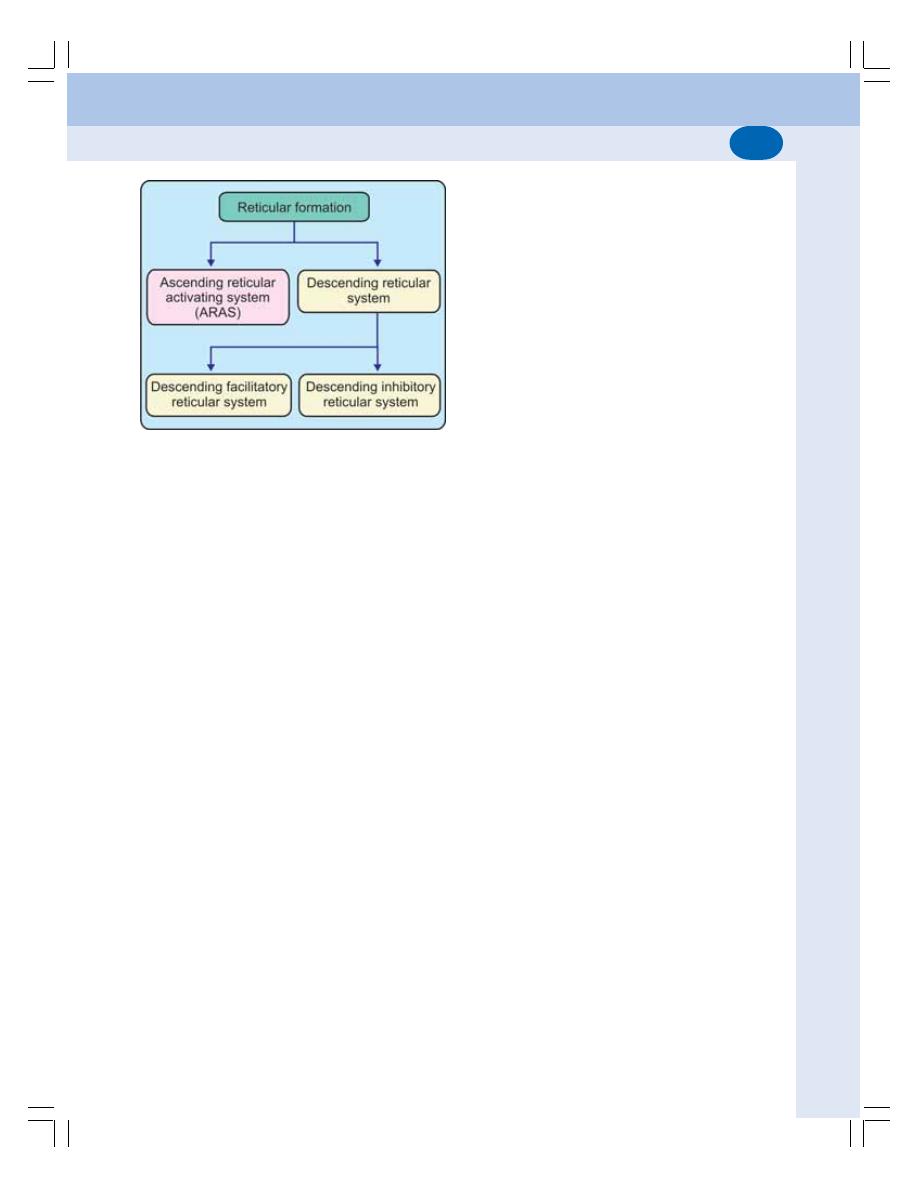
Chapter 96 Reticular Formation
591
DESCENDING RETICULAR SYSTEM
The descending reticular system includes reti-
cular formation in brainstem, the reticulospinal
tract and reticular formation in spiral cord.
It modifies the activities of spinal motor
neurons. Functionally, descending reticular sys-
tem is divided into two subdivisions (Fig. 96-2):
I. Descending facilitatory reticular system
II. Descending inhibitory reticular system.
Descending Facilitatory Reticular System
Descending facilitatory reticular system is
present in upper and lateral reticular formation.
Its functions are:
FIGURE 96-2: Functional divisions of
reticular formation
1. Facilitation of somatomotor activities by:
i. Exciting the gamma motor neurons in
spinal cord and increasing muscle tone
ii. Accelerating movements of the body
iii. Causing wakefulness and alertness.
2. Facilitation of vegetative functions:
Descending facilitatory reticular system is
the center for facilitation of the autonomic
functions such as cardiac function, blood
pressure, respiration, gastrointestinal func-
tion and body temperature.
Descending Inhibitory Reticular System
Descending inhibitory reticular system is
located in a small area in lower and medial
reticular formation. Its functions are:
1. Control of somatomotor activities by:
i. Inhibiting gamma motor neurons of spinal
cord and decreasing muscle tone
ii. Inhibiting the
α motor neurons of spinal
cord and producing smooth and accurate
voluntary movements
iii. Controlling the reflex movements.
2. Control of vegetative functions:
The descending inhibitory reticular system
is the center for inhibition of several auto-
nomic functions such as cardiac function,
blood pressure, respiration, gastrointestinal
function and body temperature.
DEFINITION
PROPRIOCEPTORS
MUSCLE SPINDLE
GOLGI TENDON ORGAN
PACINIAN CORPUSCLE
FREE NERVE ENDING
BASIC PHENOMENA OF POSTURE
MUSCLE TONE
STRETCH REFLEX
POSTURAL REFLEXES
CLASSIFICATION OF POSTURAL REFLEXES
STATIC REFLEXES
STATOKINETIC REFLEXES
Posture and Equilibrium
97
97
97
97
97
DEFINITION
Subconscious adjustment of tone in the different
muscles in relation to every movement is known
as the posture. The significance of posture is
to make the movement smooth and accurate
and to maintain the line of gravity constant or
to keep the body in equilibrium with the line of
gravity. Posture is not the active movement. It
is the passive movement associated with
redistribution of tone in different groups of related
muscles.
Proprioceptors play a major role in the main-
tenance of posture and equilibrium.
PROPRIOCEPTORS
Proprioceptors are the receptors, which give
response to change in the position of different
parts of the body. These receptors are also called
kinesthetic receptors.
Proprioceptors are situated in labyrinth, mus-
cles, tendon of the muscles, joints, ligaments and
fascia.
Different proprioceptors are:
1. Muscle spindle
2. Golgi tendon organ
3. Pacinian corpuscle
4. Free nerve ending
5. Proprioceptors in labyrinth
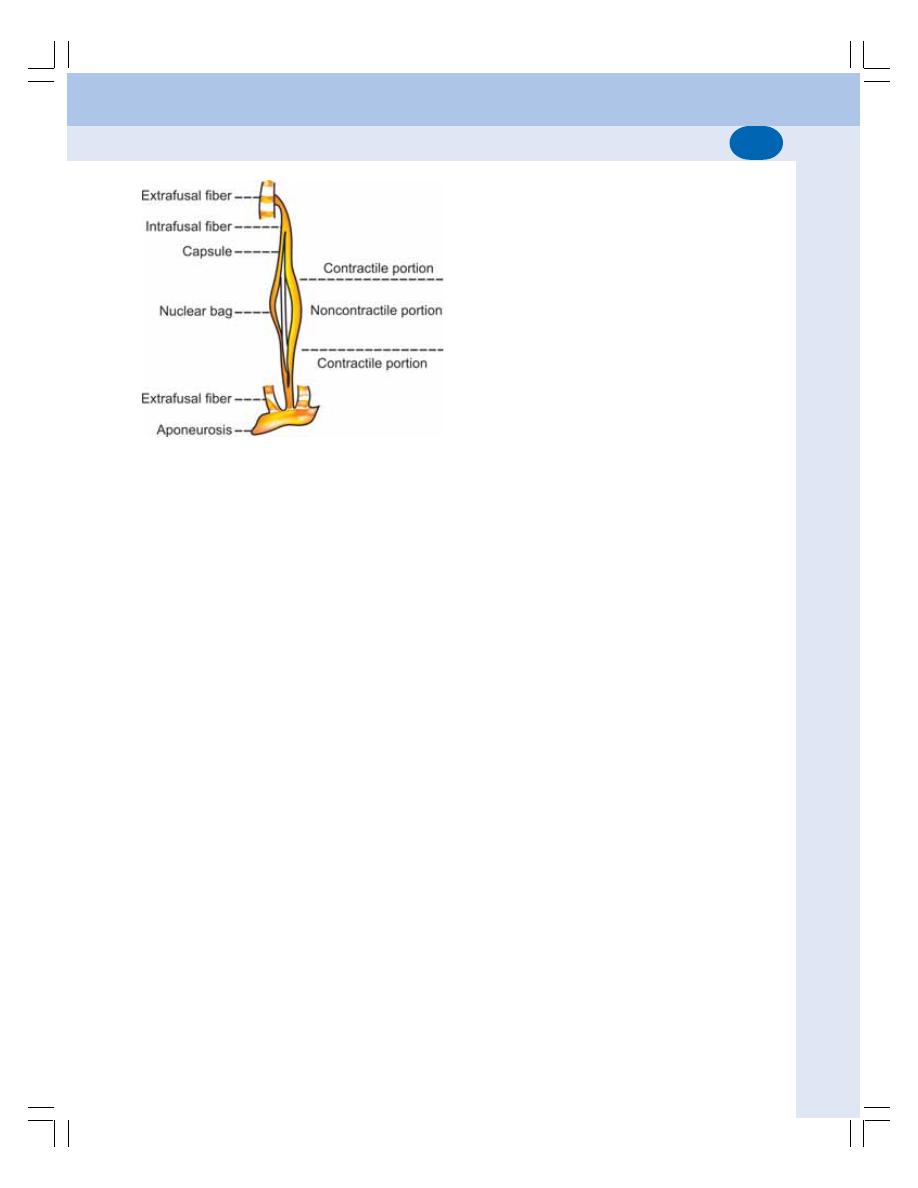
593
Chapter 97 Posture and Equilibrium
Proprioceptors in labyrinth are described in
the next chapter.
MUSCLE SPINDLE
Muscle spindle is a spindle shaped proprioceptor
situated in the skeletal muscle. It is formed by
modified skeletal muscle fibers called intrafusal
muscle fibers.
Structure of Muscle Spindle
The muscle spindle has a central bulged portion
and two tapering ends. Each muscle spindle is
formed by 5 to 12 intrafusal muscle fibers. All
these fibers are enclosed by a capsule, which
is formed by connective tissue. The intrafusal
fibers are attached to the capsule on either end.
The capsule is attached to either side of
extrafusal fibers or the tendon of the muscle.
Thus, the intrafusal fibers are placed parallel to
the extrafusal fibers.
The intrafusal fibers are thin and striated
(Fig. 97-1). The central portion of the intrafusal
fibers does not contract because it has only few
or no actin and myosin filaments. So, this portion
acts only as a receptor. Only the end portion of
the intrafusal fibers can contract. The discharge
from the gamma motor neurons causes the
contraction of the intrafusal fibers.
Types of Intrafusal Fibers
The muscle spindle is formed by two types of
intrafusal fibers.
1. Nuclear bag fiber
The central portion of this fiber is enlarged like
a bag and contains many nuclei. Hence, it is
called the nuclear bag fiber.
2. Nuclear chain fiber
In this fiber, the central portion is not bulged and
the nuclei are arranged in the center in the form
of a chain. The nuclear chain fiber is attached
to the side of end portion of the nuclear bag fiber.
Nerve Supply to Muscle Spindle
The muscle spindle is innervated by both
sensory and motor nerves. It is the only receptor
in the body, which has both sensory and motor
nerve supply.
Sensory nerve supply
Each muscle spindle receives two types of sen-
sory nerve fibers:
1. Primary sensory nerve fiber: It belongs to type
I
α (Aα) nerve fiber. Each sensory (afferent)
nerve fiber has two branches. One of the
branches supplies the central portion of nu-
clear bag fiber (Fig. 97-2). The other branch
ends in the central portion of the nuclear chain
fiber. The branches end in the form of rings
around the central portion of the nuclear bag
and nuclear chain fibers. Therefore, these
nerve endings are called annulospiral end-
ings.
2. Secondary sensory nerve fiber: It is a type
II (A
β) nerve fiber. It innervates only the
nuclear chain fiber and ends near the end
portion of nuclear chain fiber like the petals
of the flower. So, the nerve ending is called
the flower spray ending.
Motor nerve supply
Motor nerve fiber supplying the muscle spindle
belongs to gamma motor neuron (A
γ) type.
FIGURE 97-1: Muscle spindle
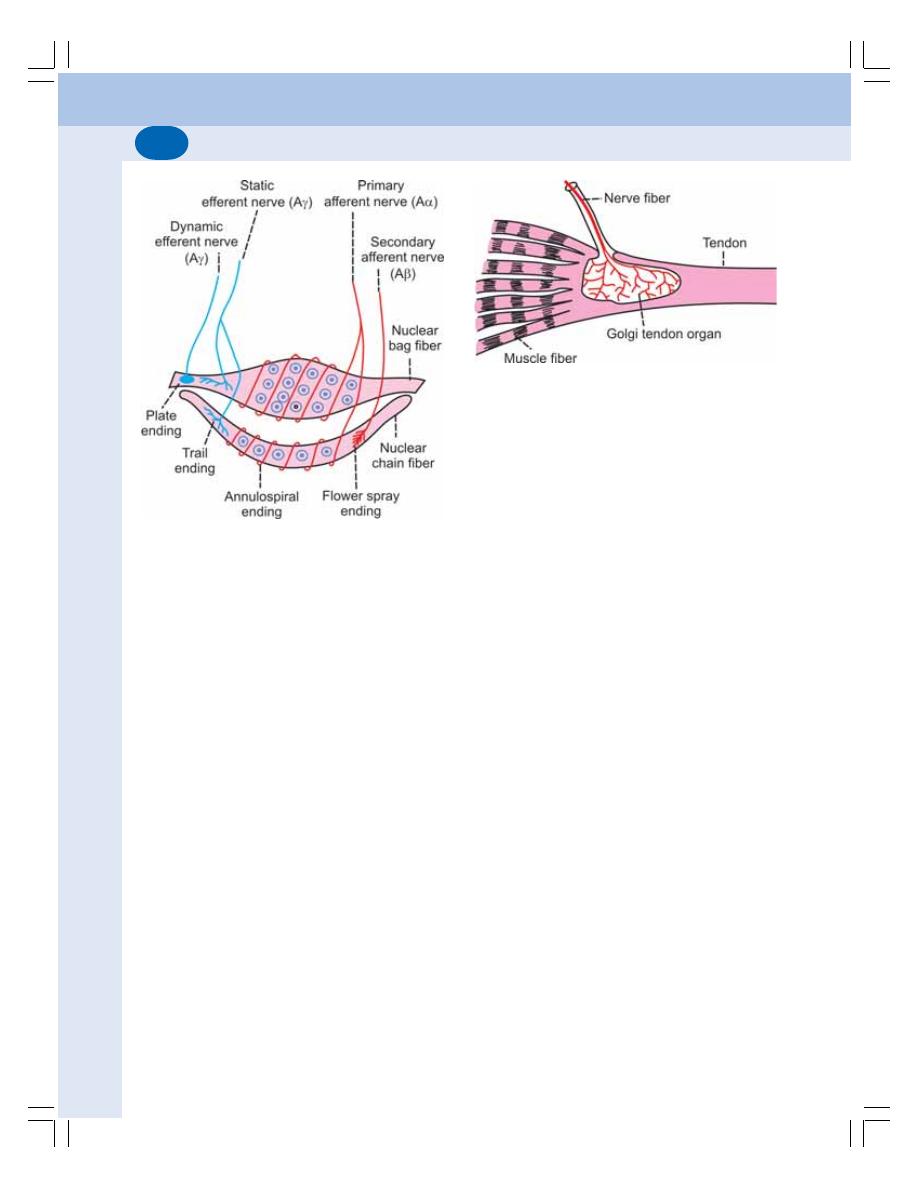
594
Nervous System
1. Motor nerve supply to nuclear bag fiber: The
gamma motor nerve fiber supplying nuclear
bag fiber ends as motor end plate. This nerve
ending is called plate ending. Functionally, it
is known as dynamic gamma efferent (motor)
nerve fiber.
2. Motor nerve supply to nuclear chain fiber:
The gamma motor nerve fiber supplying the
nuclear chain fiber divides into many bran-
ches, which form a network called trail
ending. Functionally, it is known as static
gamma efferent (motor) nerve fiber. Some-
times, it gives a branch to nuclear bag fiber
also.
Functions of Muscle Spindle
Muscle spindle gives response to change in the
length of the muscle. It detects how much the
muscle is being stretched and sends this infor-
mation to central nervous system via sensory
nerve fibers. The information is processed in
central nervous system to determine the position
of different parts of the body.
By detecting the change in length of the mus-
cle, the spindle plays an important role in stretch
reflex and maintenance of muscle tone (see
below).
GOLGI TENDON ORGAN
Golgi tendon organ is situated in the tendon of
skeletal muscle near the attachment of extra-
fusal fibers. It is placed in series between the
muscle fibers and the tendon. Golgi tendon
organ is formed by a group of nerve endings
covered by a connective tissue capsule
(Fig. 97-3).
Nerve Supply to Golgi Tendon Organ
The sensory nerve fiber supplying Golgi tendon
organ belongs to Ib type.
Functions of Golgi Tendon Organ
The Golgi tendon organ gives response to the
change in the force or tension developed in the
skeletal muscle during contraction.
PACINIAN CORPUSCLE
Pacinian corpuscle is a mechanoreceptor that
senses pressure and vibration. It is situated in
the deeper layers of skin. It is also situated in
the tissues surrounding the joints such as fascia
over the muscle, tendons, and joint capsule. The
pacinian corpuscles situated in these tissues
send information about joint position to central
nervous system.
FIGURE 97-3: Golgi tendon apparatus
FIGURE 97-2: Nerve supply to muscle spindle. Red
= Afferent (sensory) nerve fibers. Blue = Efferent
(motor) nerve fibers. Letters in parenthesis indicate
the type of nerve fibers
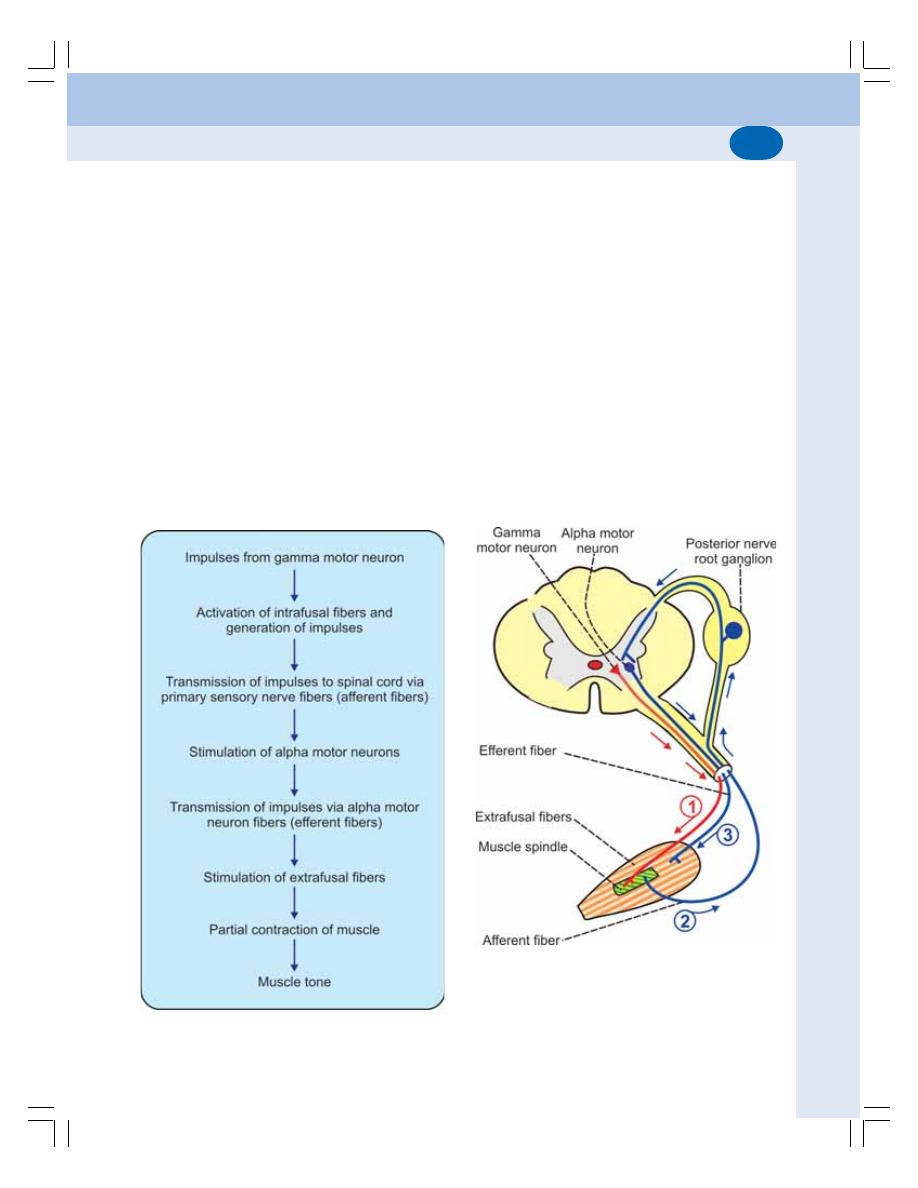
595
Chapter 97 Posture and Equilibrium
FREE NERVE ENDING
Free nerve ending is the receptor for pain sen-
sation situated in skin, muscles, tendon, fascia
and joints. It is stimulated during some specific
joint positions. In turn, it sends information about
joint position to central nervous system.
BASIC PHENOMENA OF POSTURE
The basic phenomena for maintenance of pos-
ture are the muscle tone and stretch reflex.
MUSCLE TONE
Definition
Muscle tone is defined as the state of continuous
and passive partial contraction of the muscle with
certain vigor and tension. It is also called tonus.
It is also defined as resistance offered by the
muscle to stretch.
Significance of Muscle Tone
Muscle tone plays an important role in main-
tenance of posture. Change in muscle tone
enables movement of different parts of the body.
Muscle tone is present in all the skeletal
muscles. However, it is more in the antigravity
muscles such as extensors of lower limb, trunk
muscles and neck muscles.
Development of Muscle Tone
Gamma motor neurons and muscle spindle are
responsible for the development and main-
tenance of muscle tone (Figs 97-4 and 97-5).
FIGURE 97-4: Schematic diagram showing
development of muscle tone
FIGURE 97-5: Development of muscle tone.
1. Impulses from
γ motor neuron stimulate muscle
spindle. 2. Afferent impulses from muscle spindle to
α motor neuron. 3. Efferent impulses from α motor
neuron produce contraction of extrafusal fibers and
develop muscle tone

596
Nervous System
The muscle tone is purely a reflex process.
This reflex is a spinal segmental reflex. It is deve-
loped by continual synchronous discharge of
motor impulses from the gamma motor neurons
present in the anterior gray horn of the spinal
cord.
Regulation of Muscle Tone
Though the muscle tone is developed by
discharges form gamma motor neurons, it is
maintained continuously and regulated by some
supraspinal centers situated in different parts
of brain. Some of these centers increase the
muscle tone by sending facilitatory impulses
while other centers decrease the muscle tone
by inhibitory impulses.
STRETCH REFLEX
Stretch reflex is the reflex contraction of muscle
when it is stretched. It is also called myotatic
reflex. It is a monosynaptic reflex and the quick-
est of all the reflexes. The extensor muscles,
particularly the antigravity muscles exhibit a
severe and prolonged contraction during stretch
reflex.
Stimulation of muscle spindle elicits the
stretch reflex. The intrafusal muscle fibers are
situated parallel to the extrafusal muscle fibers
and are attached to the tendon of the muscle
by means of capsule. So, stretching of the
muscle causes stretching of the muscle spindle
also. This stimulates the muscle spindle and it
discharges the sensory impulses. These
impulses are transmitted via the primary and
secondary sensory nerve fibers to the alpha
motor neurons in spinal cord. Alpha motor
neurons in turn send motor impulse to muscles
through their fibers and cause contraction of
extrafusal fibers.
Stretch reflex is the basic reflex involved in
maintenance of posture. It is particularly respon-
sible to maintain the body in an upright position.
POSTURAL REFLEXES
Postural reflexes are the reflexes which are
responsible for the maintenance of posture. The
afferent impulses for the maintenance of posture
arise from proprioceptors, vestibular apparatus
and retina of the eye and reach the centers in
central nervous system. The centers, which
maintain the posture, are located at different
levels of central nervous system particularly
cerebral cortex, cerebellum, brainstem and
spinal cord. These centers send motor impulses
to the different groups of skeletal muscles so
that appropriate movements occur to maintain
the posture.
CLASSIFICATION OF POSTURAL
REFLEXES
The postural reflexes are generally classified into
two groups:
A. Static reflexes
B. Statokinetic reflexes
STATIC REFLEXES
Static reflexes are the postural reflexes that main-
tain posture at rest. Static reflexes are of four
types:
I. General static reflexes or righting reflexes
II. Local static reflexes or supporting reflexes
III. Segmental static reflexes
IV. Statotonic or attitudinal reflexes
I.
General Static Reflexes or Righting
Reflexes
General static reflexes are otherwise called
righting reflexes because these reflexes help to
maintain an upright position of the body. Righting
reflexes help to govern the orientation of the
head in space, position of the head in relation
to the body and appropriate adjustment of the
limbs and eyes in relation to the position of the
head, so that upright position of the body is
maintained.
When a cat, held with its back downwards,
is allowed to fall through the air, it lands upon its
paws, with the head and body assuming the
normal attitude in a flash. A fish resists any
attempt to turn it from its normal position and if
it is placed in water upon its back, it flips almost
instantly into the normal swimming position. All
these actions occur because of the righting
reflexes.
597
Chapter 97 Posture and Equilibrium
The righting reflexes consist of a chain of
reactions which occur one after another in an
orderly sequence. Each reflex causes the deve-
lopment of the succeeding one.
The righting reflexes are divided into five
types:
1. Labyrinthine righting reflexes acting upon the
neck muscles
2. Neck righting reflexes acting upon the body
3. Body righting reflexes acting upon the head
4. Body righting reflexes acting upon the body
5. Optical righting reflexes.
The first four reflexes are easily demonstrated
on a thalamic animal or a normal animal, which
is blindfolded.
Sequential events of righting reflexes
1. When the animal is placed upon its back, the
labyrinthine reflexes acting upon the neck
muscles turn the head into its normal position
in space, in relation to the body
2. The proprioceptive reflexes of the neck
muscles then bring the body into its normal
position in relation to the position of head
3. When resting upon a rigid support, these
reflexes are reinforced by the body righting
reflexes on head as well as on the body
4. If the animal happens to be a labyrinthec-
tomized one, then it makes an attempt to
recover its upright position as a result of
operation of the optical righting reaction. If
the optical righting reflexes are abolished by
covering the eyes, the righting ability is lost.
Optical righting reflexes are also demons-
trated in 3 or 4 weeks old baby. When laid down
on belly, i.e. prone position, the baby tries to raise
the head to a vertical position.
Centers for righting reflexes
The centers for the first four righting reflexes are
in the red nucleus situated in midbrain. The
center for the optical righting reflexes is in the
occipital lobe of cerebral cortex (Table 97-1).
II. Local Static Reflexes or Supporting
Reflexes
Local static reflexes or the supporting reactions
support the body in different positions against
the gravity and also protect the limbs against
hyperextension or hyperflexion.
The supporting reactions are classified into
two types:
1. Positive supporting reflexes
2. Negative supporting reflexes.
TABLE 97-1: Static postural reflexes
Reflex
Center
General static reflexes
1. Labyrinthine righting reflexes acting upon
(Righting reflexes)
the neck muscles
2. Neck righting reflexes acting upon body
Red nucleus in
3. Body righting reflexes acting upon head
midbrain
4. Body righting reflexes acting upon body
5. Optical righting reflexes
Occipital lobe
Local static reflexes
1. Positive supporting reflexes
Spinal cord
2. Negative supporting reflexes
Segmental static reflexes
1. Crossed extensor reflex
Spinal cord
Statotonic or attitudinal
1. Tonic labyrinthine and neck reflexes acting
Medulla oblongata
reflexes
on limbs
2. Labyrinthine and neck reflexes acting
upon eyes

598
Nervous System
1. Positive supporting reflexes
Positive supporting reflexes are the reactions,
which help to fix the joints and make the limbs
rigid like pillars, so that limbs can support the
weight of the body against gravity.
The positive supporting reflexes are deve-
loped while standing. The body is supported
against gravity while standing by the simul-
taneous reflex contractions of both extensor and
flexor muscles and other opposing muscles. The
impulses for these reflexes arise from proprio-
ceptors present in the muscles, joints and ten-
dons and the exteroceptors, particularly pressure
receptors present in deeper layers of the skin
of sole.
2. Negative supporting reflexes
Relaxation of the muscles and unfixing of the
joints enable the limbs to flex and move to a
new position. It is called negative supporting
reaction. It is brought about by raising the leg
off the ground and plantar flexion of toes and
ankle. When the leg is lifted off the ground, the
exteroceptive impulses are stopped. When the
toes and ankle joints are plantar flexed, the
stretch stimulus for the plantar muscles is
stopped. It causes unlocking of the limbs and
facilitates new movement.
The centers for the supporting reflexes are
located in the spinal cord.
III. Segmental Static Reflexes
The segmental static reflexes are essential for
walking. During walking, in one leg, the flexors
are active and the extensors are inhibited. On
the opposite leg, the flexors are inhibited and
extensors are active. Thus, the flexors and ex-
tensors of the same limb are not active simul-
taneously. It is known as crossed extensor reflex.
It is due to the reciprocal inhibition and the neural
mechanism responsible for this reflex is called
reciprocal innervation.
The centers for these reflexes are situated
in the spinal cord.
IV. Statotonic or Attitudinal Reflexes
Statotonic or attitudinal reflexes are developed
according to the attitude of the body and are of
two types:
1. Tonic labyrinthine and neck reflexes acting
on the limbs
2. Labyrinthine and neck reflexes acting upon
the eyes.
1. Tonic labyrinthine and neck reflexes acting
on the limbs
These reflexes maintain the movements of
limbs in accordance to the position of the head.
When the head of an animal is dorsiflexed, all
the four limbs are extended and, when the head
is ventriflexed, all the four limbs are flexed.
2. Labyrinthine and neck reflexes acting
upon the eyes
According to the changes in the position of the
head and neck, the eyes also move. These refle-
xes arise from labyrinth and neck muscles.
Turning the head downward causes upward
movement of the eyes. The eyes remain in this
position as long as the position of the head is
retained.
The centers for the statotonic reflexes are
present in the medulla oblongata.
STATOKINETIC REFLEXES
Statokinetic reflexes are the postural reflexes
that maintain posture during movement. These
reflexes are concerned with both angular
(rotatory), and linear (progressive) movements.
The vestibular apparatus is responsible for
these reflexes (Chapter 98).
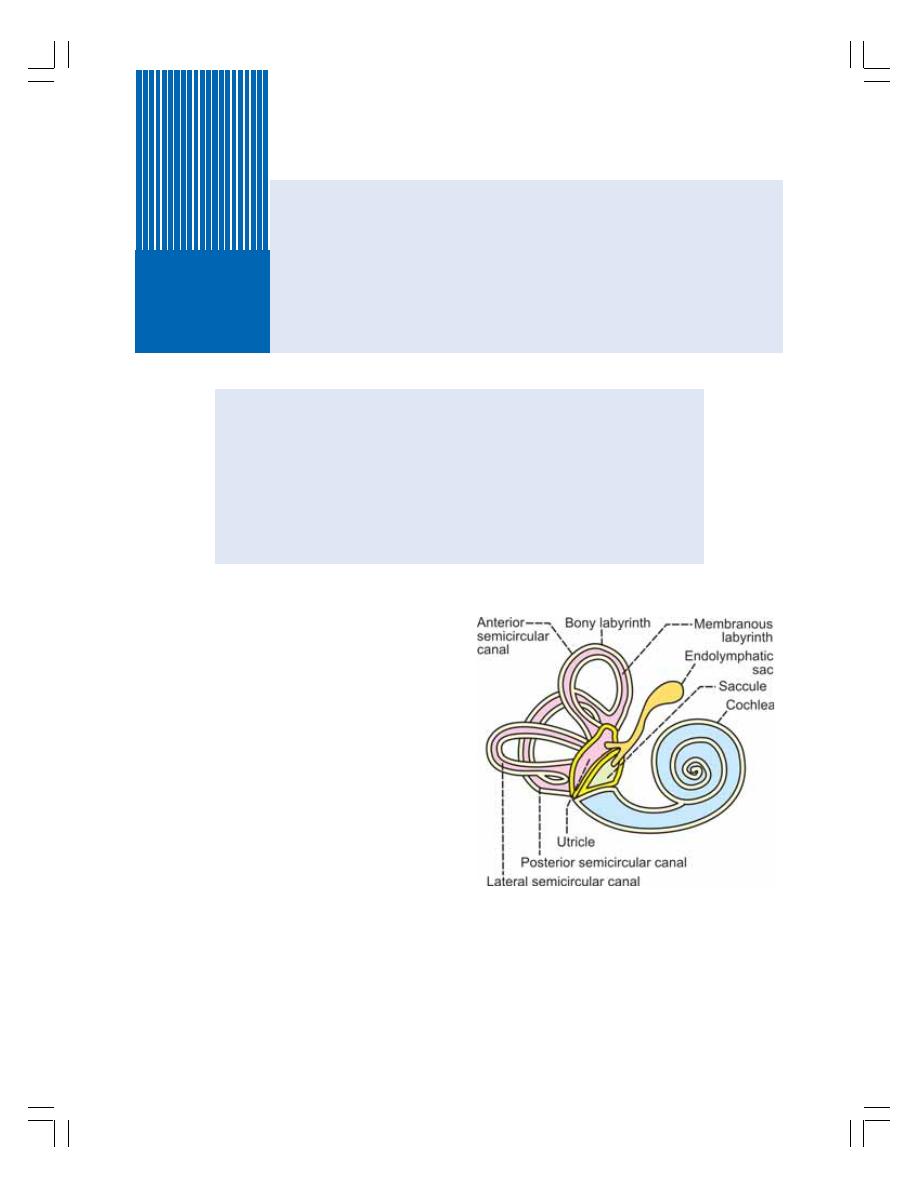
INTRODUCTION
LABYRINTH
FUNCTIONAL ANATOMY OF VESTIBULAR APPARATUS
RECEPTOR ORGAN OF VESTIBULAR APPARATUS
NERVE SUPPLY TO VESTIBULAR APPARATUS
FUNCTIONS OF VESTIBULAR APPARATUS
APPLIED PHYSIOLOGY
Vestibular Apparatus
98
INTRODUCTION
Vestibular apparatus is the part of labyrinth or
inner ear. It plays an important role in maintaining
posture and equilibrium through statokinetic
reflexes. The other part of labyrinth is the
cochlea, which is concerned with sensation of
hearing.
LABYRINTH
Labyrinth (inner ear) consists of two structures,
bony labyrinth and membranous labyrinth.
Bony labyrinth is a series of cavities or
channels present in the petrous part of temporal
bone. Membranous labyrinth is situated inside
bony labyrinth (Fig. 98-1). The space between
bony labyrinth and membranous labyrinth is filled
with a fluid called perilymph which is similar to
ECF in composition.
The membranous labyrinth consists of two
portions:
1. Cochlea which is concerned with sensation
of hearing (Chapter 110)
2. Vestibular apparatus which is concerned with
posture and equilibrium.
The membranous labyrinth is filled with a fluid
called endolymph which is similar to ICF in
composition.
FIGURE 98-1: Labyrinth
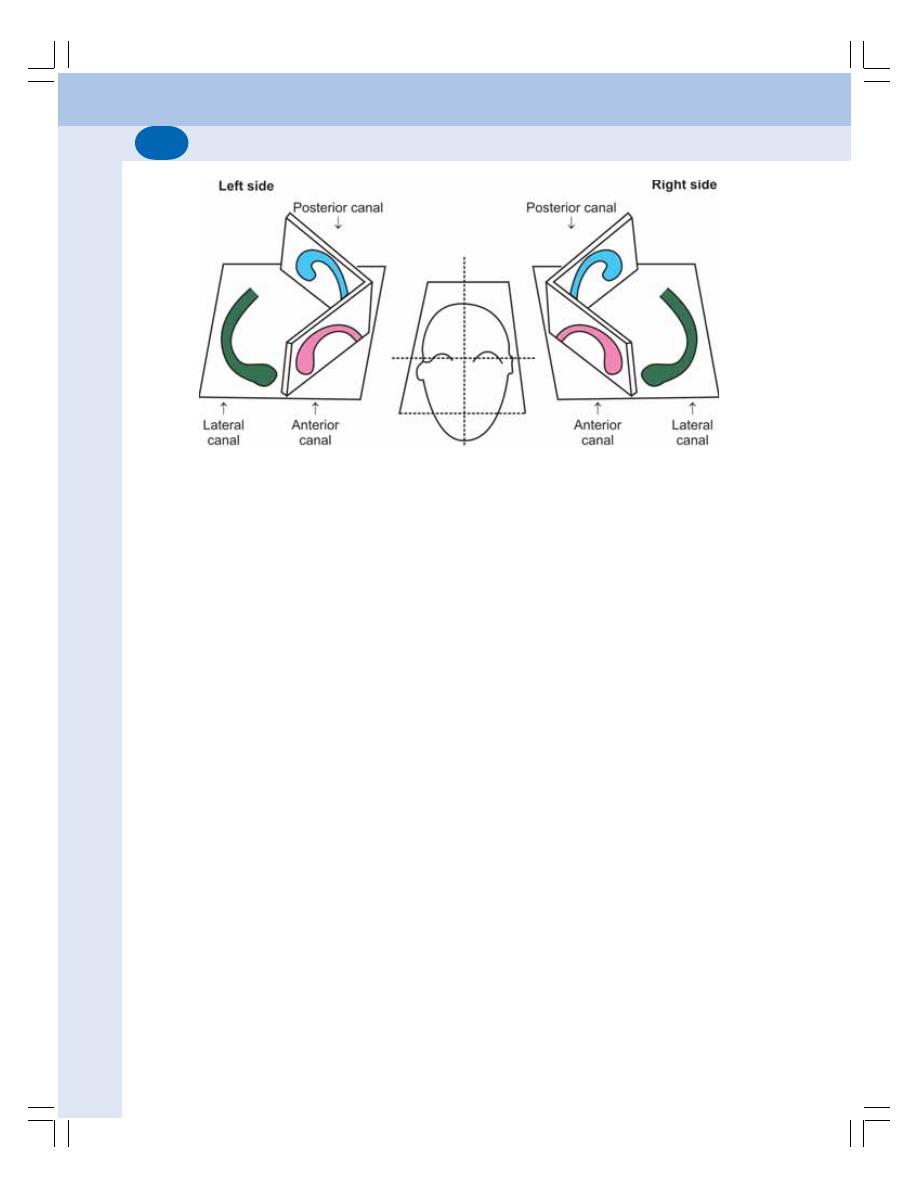
Nervous System
600
FUNCTIONAL ANATOMY OF
VESTIBULAR APPARATUS
Vestibular apparatus is formed by three semi-
circular canals and otolith organ or vestibule.
SEMICIRCULAR CANALS
The semicircular canals are:
1. Anterior or superior canal
2. Posterior canal
3. Lateral or horizontal or external canal.
The anterior and posterior canals are situated
vertically and the lateral canal is situated in
horizontal plane (Fig. 98-2).
Ampulla
There are two ends for each semicircular canal.
One end is narrow and the other end is enlarged.
The enlarged end is called ampulla. The ampulla
contains the receptor organ of semicircular
canals known as crista ampullaris. The ampulla
of all the three canals and narrow end of
horizontal canal open directly into the utricle. The
narrow ends of anterior and posterior canals
open into the utricle jointly, by forming the
common crus. Thus, all the three semicircular
canals open into the utricle by means of five
openings. Utricle opens into saccule.
OTOLITH ORGAN
Otolith organ or vestibule is formed by utricle and
saccule.
RECEPTOR ORGAN IN
VESTIBULAR APPARATUS
The receptor organ in semicircular canal is called
crista ampullaris and that in otolith organ is called
macula. These receptor organs contain the pro-
prioceptors.
RECEPTOR ORGAN IN SEMICIRCULAR
CANAL – CRISTA AMPULLARIS
Crista ampullaris is situated inside the ampulla
of semicircular canals. The crest is formed by a
receptor epithelium (neuroepithelium) which
consists of hair cells and supporting cells
(Fig. 98-3).
Hair Cells
Hair cells are the receptor cells (proprioceptors)
of crista ampullaris. There are two types of hair
cells, type I and type II hair cells. Hair cells of
semicircular canals, utricle and saccule receive
both afferent and efferent nerve terminals.
FIGURE 98-2: Position of semicircular canals
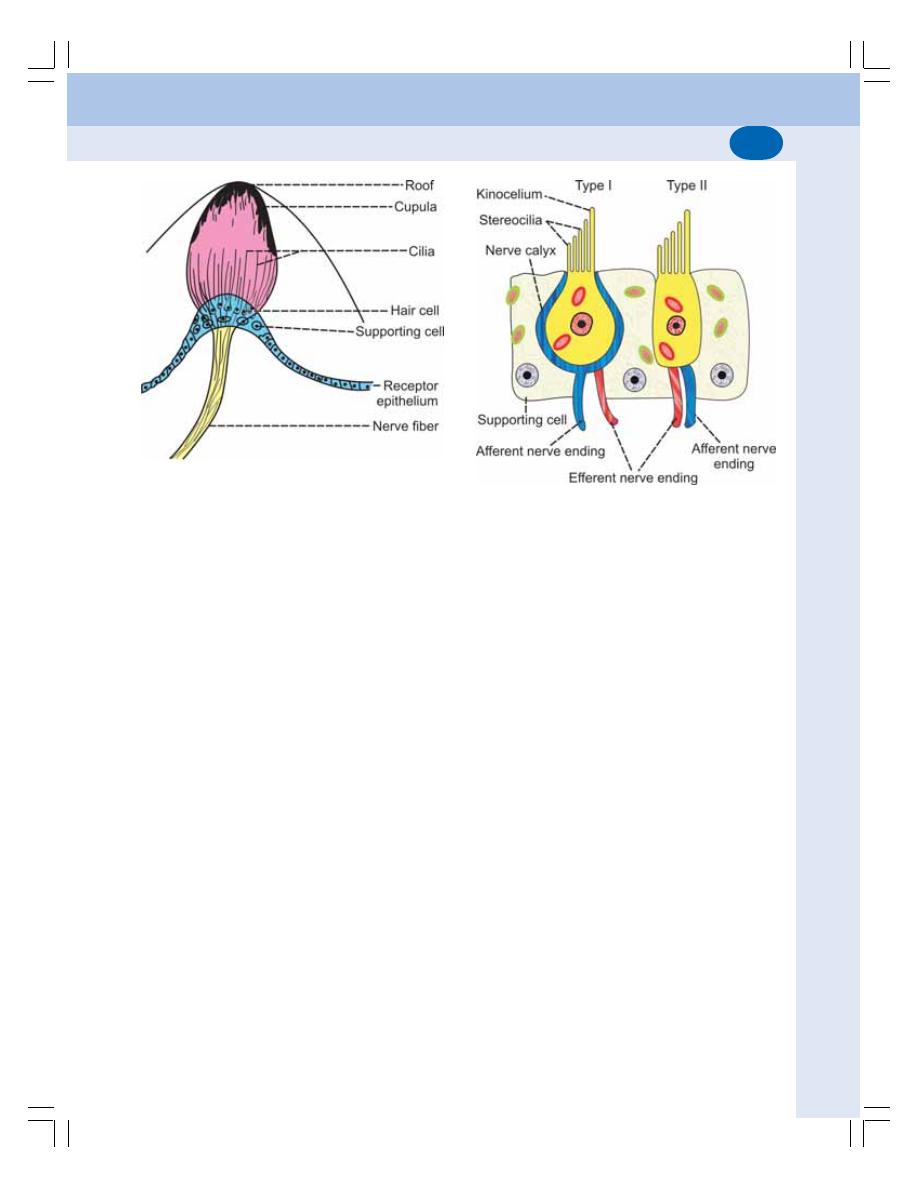
Chapter 98 Vestibular Apparatus
601
Type I hair cells
Type I hair cells are flask shaped. The afferent
nerve terminates in the form of a calyx that
surrounds the cell body. The efferent nerve
terminal ends on the surface of the calyx.
Type II hair cells
These cells have a cylindrical or test tube
shape. Both afferent and efferent nerve fibers
terminate on the surface cell body without
forming calyx.
Cilia of hair cells
The apex of each hair cell has a cuticular plate.
From this plate, about 40 to 60 cilia arise which
are called stereocilia. Each stereocilium is
attached at its tip to the neighboring taller one
by means of a fine process called tip link.
Because of the tip links, all the stereocilia are
held together. One of the cilia is very tall which
is named as kinocilium (Fig. 98-4).
Cupula
From crista ampullaris, a domeshaped gelati-
nous structure extends up to the roof of the
ampulla. It is known as cupula. The cupula
encloses the cilia of hair cells. The cilia of hair
cells are projected in the cupula.
RECEPTOR ORGAN IN OTOLITH
ORGAN – MACULA
The receptor organ in otolith organ is called
macula. Like crista ampullaris, the macula is also
formed by neuroepithelium and supporting cells.
The neuroepithelium of macula also has two
types of hair cells, the type I and type II hair cells
(Fig. 98-5).
Otolith Membrane
Like crista ampullaris, macula is also covered
by a gelatinous membrane called otolith mem-
brane. It is a flat structure and not dome shaped
like cupula. The stereocilia and kinocelium of
each hair cell are embedded in otolith mem-
brane. Otolith membrane contains some crys-
tals, which are called ear dust, otoconia or
statoconia. The otoconia are mainly constituted
by calcium carbonate.
Situation of Macula
In utricle, the macula is situated in horizontal
plane, so that the cilia from hair cells are in
FIGURE 98-4: Hair cells of vestibular apparatus
FIGURE 98-3: Crista ampullaris
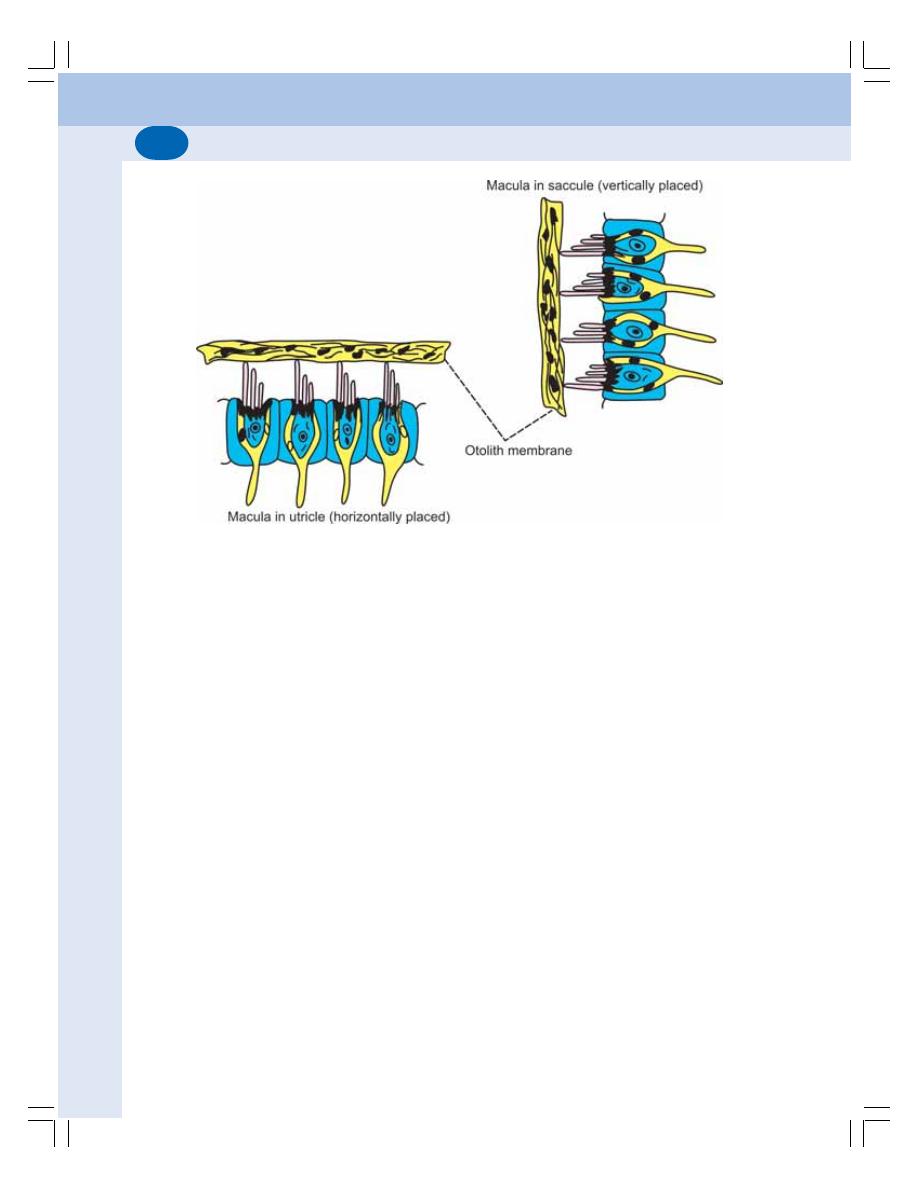
Nervous System
602
vertical direction. In saccule, the macula is in
vertical plane and the cilia are in horizontal
direction.
NERVE SUPPLY TO VESTIBULAR
APPARATUS
The impulses from the hair cells of crista ampul-
laris and maculae are transmitted to medulla
oblongata and other parts of central nervous
system through the fibers of vestibular division
of vestibulocochlear (VIII cranial) nerve.
The first order neurons of the sensory path-
way are bipolar in nature. The dendrites of the
bipolar cells have close contact with the basal
part of hair cells. The axons of the first order
neurons (bipolar cells) form the vestibular division
of vestibulocochlear nerve.
The hair cells also have efferent nerve fiber
which controls the hair cells.
FUNCTIONS OF VESTIBULAR
APPARATUS
The receptors of semicircular canals give res-
ponse to rotatory movements or angular accele-
ration of the head. And, the receptors of utricle
and saccule give response to linear acceleration
of head.
Thus, the vestibular apparatus is responsible
for detecting the position of head during different
movements. It also causes the reflex adjust-
ments in the position of eyeball, head and body
during postural changes.
FUNCTIONS OF SEMICIRCULAR
CANALS
Semicircular canals are concerned with angular
(rotatory) acceleration. Semicircular canals
sense the rotational movement. Each semicir-
cular canal is sensitive to rotation in a particular
plane.
Superior Semicircular Canal
Superior semicircular canal gives response to
rotation in anteroposterior plane (transverse
axis), i.e. front to back movements like nodding
the head while saying ‘Yes – yes’.
Horizontal Semicircular Canal
This semicircular canal gives response to
rotation in horizontal plane (vertical axis), i.e. side
FIGURE 98-5: Macula in otolith organ
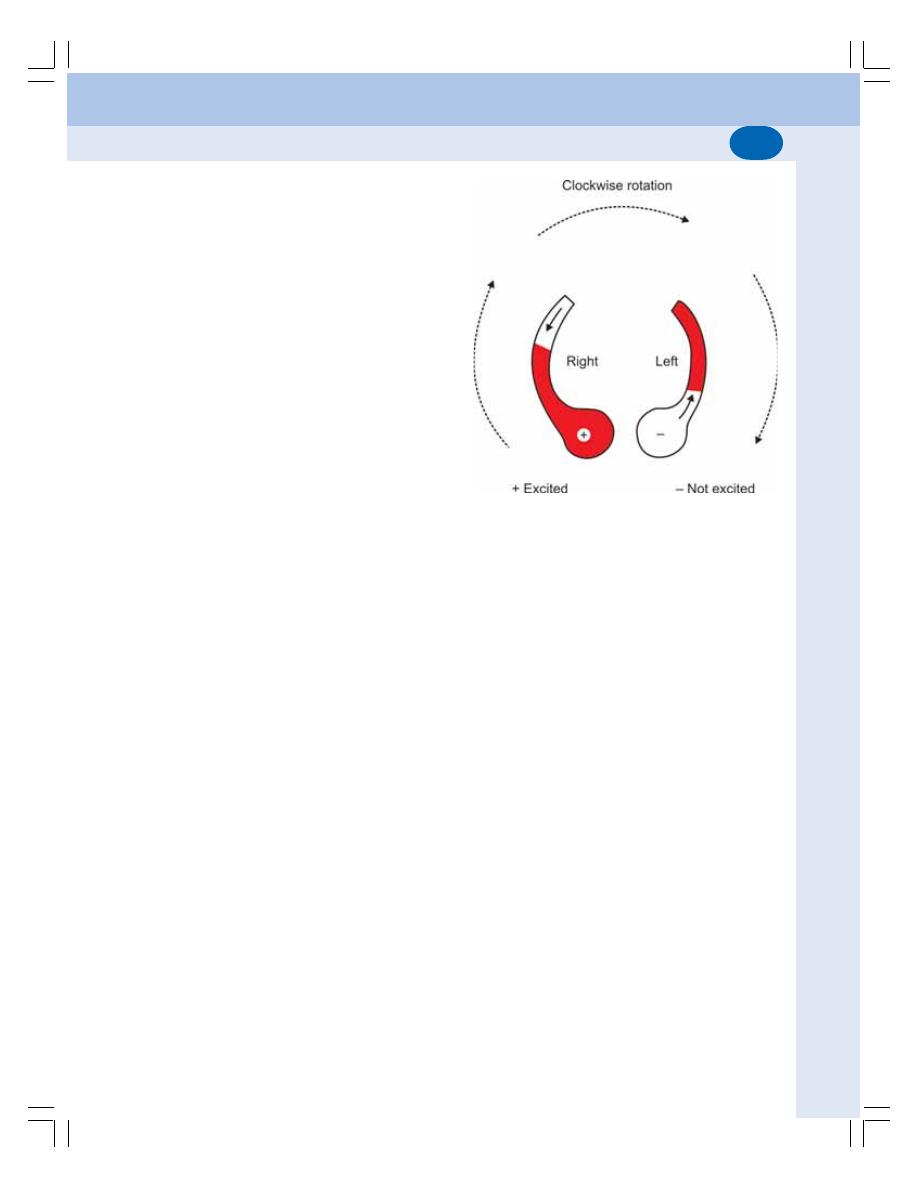
Chapter 98 Vestibular Apparatus
603
to side movements (left to right or right to left)
like shaking the head while saying ‘No – no’.
Posterior Semicircular Canal
This semicircular canal gives response to rotation
in the vertical plane (anteroposterior axis) by
which head is rotated from shoulder to shoulder.
Mechanism of Stimulation of Receptor
Cells in Semicircular Canal
At the beginning of rotation, the receptor cells
are stimulated by the movement of endolymph
inside the semicircular canals. However, the
receptors are stimulated only at the beginning
and at the stoppage of rotatory movements. And
during rotation at a constant speed, these recep-
tors are not stimulated.
When a person rotates in clockwise direction
in horizontal plane (vertical axis), horizontal canal
moves in clockwise direction. But, there is no
corresponding movement of endolymph inside
the canal at the beginning of rotation. Because
of the inertia, endolymph remains static. This
phenomenon causes relative displacement of
endolymph in the direction opposite to that of
the rotation of head. That is, the fluid is pushed
in anticlockwise direction.
Thus, in the right horizontal semicircular
canal, the endolymph flows towards the ampulla
and, in the left canal, the fluid moves away from
the ampulla (Fig. 98-6). The movement of endo-
lymph in semicircular canal, in turn causes
corresponding movement of gelatinous cupula.
Thus, in the right horizontal canal, the cupula
moves towards the ampulla. Whereas in the left
canal the cupula moves away from ampulla. In
any semicircular canal, when cupula moves
towards the ampulla, the stereocilia of hair cells
are pushed towards kinocilium leading to stimu-
lation of hair cells. When the cupula moves away
from ampulla, the stereocilia are pushed away
from kinocilium and hair cells are not stimulated.
Electrical Potential in Hair Cells –
Mechanotransduction
Mechanotransduction is a type of sensory trans-
duction (Chapter 85) in the hair cell (receptor)
FIGURE 98-6: Movement of fluid and excitation of
crista ampullaris in right horizontal semicircular canal
during clockwise rotation
by which the mechanical energy (movement of
cilia in hair cell) caused by stimulus is converted
into action potentials in the vestibular nerve fiber.
The resting membrane potential in hair cells
is about – 60 mV. The movement of stereocilia
of hair cells towards kinocilium causes deve-
lopment of mild depolarization in hair cells up
to – 50 mV which is called receptor potential.
The receptor potential in hair cells causes
generation of action potential in nerve fibers distri-
buted to hair cells.
Movement of stereocilia in the opposite direc-
tion (away from kinocilium) causes hyperpolari-
zation of hair cells which stops generation of
action potential in the nerve fibers (Fig. 98-7).
Nystagmus
Nystagmus is the rhythmic oscillatory involuntary
movements of eyeball. It is common during rota-
tion. It is due to the natural stimulatory effect of
vestibular apparatus during rotational accele-
ration.
Vestibulo-ocular reflex and nystagmus
The nystagmus is a reflex phenomenon that
occurs in order to maintain the visual fixation.
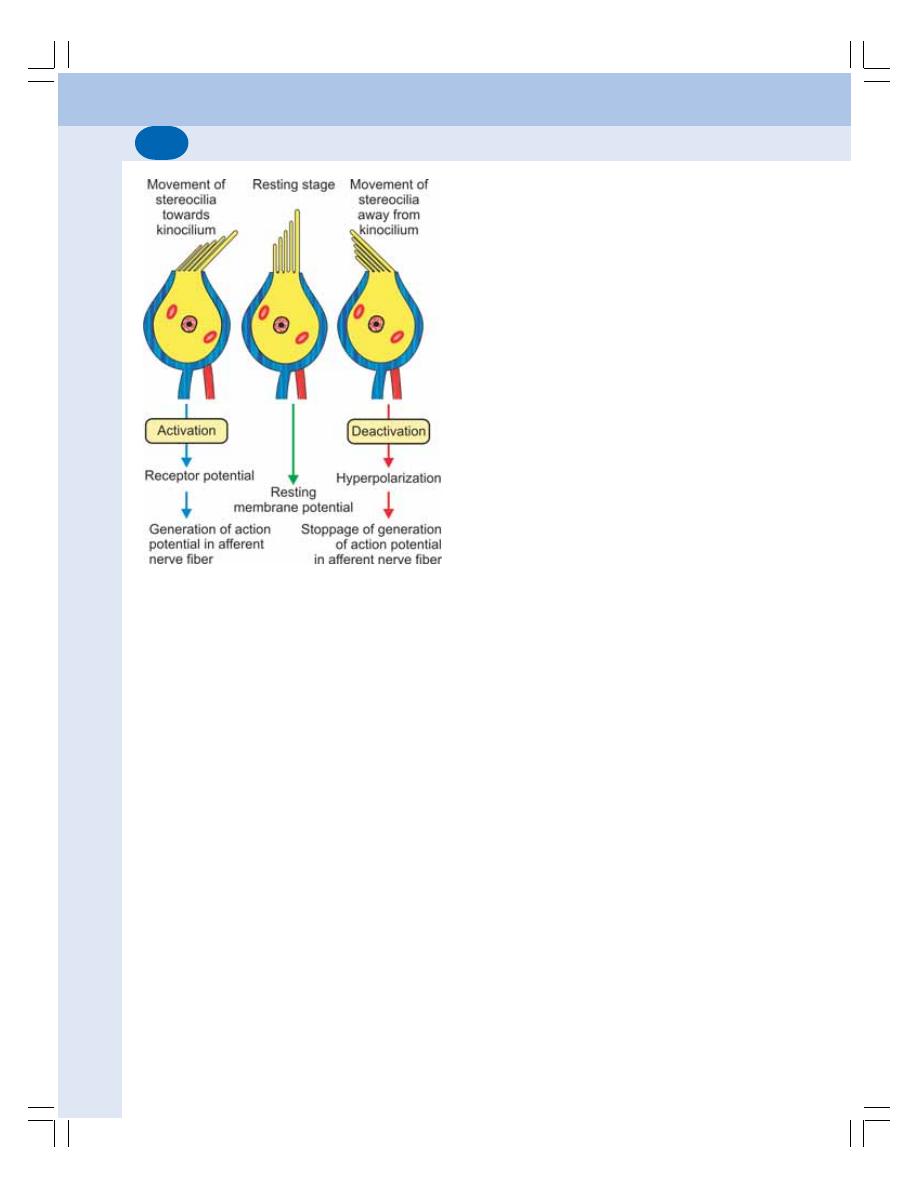
Nervous System
604
Since the movements of eyeballs occur in res-
ponse to the stimulation of vestibular apparatus
this reflex is called the vestibulo-ocular reflex.
FUNCTION OF OTOLITH ORGAN
Otolith organ is concerned with linear accele-
ration and detects acceleration in both horizontal
and vertical planes. Utricle responds during
horizontal acceleration and saccule responds
during vertical acceleration.
Function of Utricle
The position of hair cells of macula helps utricle
to respond to horizontal acceleration. In the
utricle, the macula is situated in horizontal plane
with the hair cells in vertical plane (Fig. 98-5).
While moving horizontally, because of inertia the
otoconia move in opposite direction and pull the
cilia of hair cells resulting in stimulation of hair
cells.
For example, when the body moves forward,
the otoconia fall back in otolith membrane and
pull the cilia of hair cells backward. Pulling of
cilia causes stimulation of hair cells. Hair cells
send information (impulses) to vestibular,
cerebellar and reticular centers. These centers
in turn send instructions to various muscles to
maintain equilibrium of the body during the
forward movement.
Function of Saccule
Macula of saccule is situated in vertical plane
with the cilia of hair cells in horizontal plane.
While moving vertically, as in the case of utricle,
the otoconia of saccule move in opposite
direction and pull the cilia resulting in stimulation
of hair cells.
For example, while climbing up, the otoconia
move down by pulling the cilia downwards. It
stimulates the hair cells which in turn send
information to the brain centers. And the action
follows as in the case of movement in horizontal
plane.
APPLIED PHYSIOLOGY
LABYRINTHECTOMY
Removal of labyrinthine apparatus on both sides
leads to complete loss of equilibrium. The
equilibrium could be maintained only by visual
sensation. The postural reflexes are severely
affected. There is loss of hearing sensation too.
Removal of labyrinthine apparatus on one
side causes less effect on postural reflexes.
However, severe autonomic symptoms such as
nausea, vomiting and diarrhea occur.
FIGURE 98-7: Mechanotransduction in hair cell of
vestibular apparatus. During activation, receptor
potential develops in hair cell. It causes development
of action potential in afferent nerve fiber.

Chapter 98 Vestibular Apparatus
605
MOTION SICKNESS
Motion sickness is defined as the syndrome of
physiological response during movement (travel)
to which the person is not adapted. It can occur
while traveling in any form of vehicle like auto-
mobile, ship, aircraft or spaceship. The motion
sickness that occurs while traveling in a water-
craft is called seasickness.
Cause
Motion sickness is due to excessive and repeated
stimulation of vestibular apparatus
Symptoms
1. Nausea and vomiting
2. Sweating
3. Diarrhea
4. Excess salivation
5. Discomfort
8. Headache
9. Disorientation
The responses of motion sickness can be
prevented by avoiding greasy and bulky food
before travel and by taking antiemetic drugs
(drugs preventing nausea and vomiting).
ELECTROENCEPHALOGRAM
DEFINITION
METHOD OF RECORDING EEG
WAVES OF EEG
ECG DURING SLEEP
EPILEPSY
DEFINITION
TYPES OF EPILEPSY
Electroencephalogram and
Epilepsy
99
ELECTROENCEPHALOGRAM
DEFINITION
Electroencephalography is the study of electrical
activities of brain. Electroencephalogram (EEG)
is the graphical recording of electrical activities
of brain. EEG is useful in the diagnosis of
neurological disorders such as epilepsy and
sleep disorders.
METHOD OF RECORDING EEG
Electroencephalograph is the instrument used to
record EEG. The electrodes called scalp elec-
trodes from the instrument placed over unopened
skull or over the brain after opening the skull, or
by piercing into the brain.
WAVES OF EEG
EEG has three types of waves (Fig. 99-1):
1. Alpha waves
2. Beta waves
3. Delta waves.
In children, in addition to these waves, theta
waves appear.
Alpha Waves
Alpha waves are rhythmical waves, which
appear at a frequency of 8 to 12 waves/second
with the amplitude of 50 μV. The alpha waves
are synchronized regular waves.
Alpha waves are obtained in inattentive brain
or mind as in drowsiness, light sleep or narcosis
with closed eyes. These waves are abolished by
any type of stimuli or mental effort and diminished
when eyes are opened.
Alpha waves are most marked in parieto-
occipital area.
Alpha block
Alpha block is the replacement of synchronized
alpha waves in EEG by desynchronized and low
voltage waves when the eyes are opened. The
desynchronized waves do not have specific
frequency. It occurs due to any form of sensory
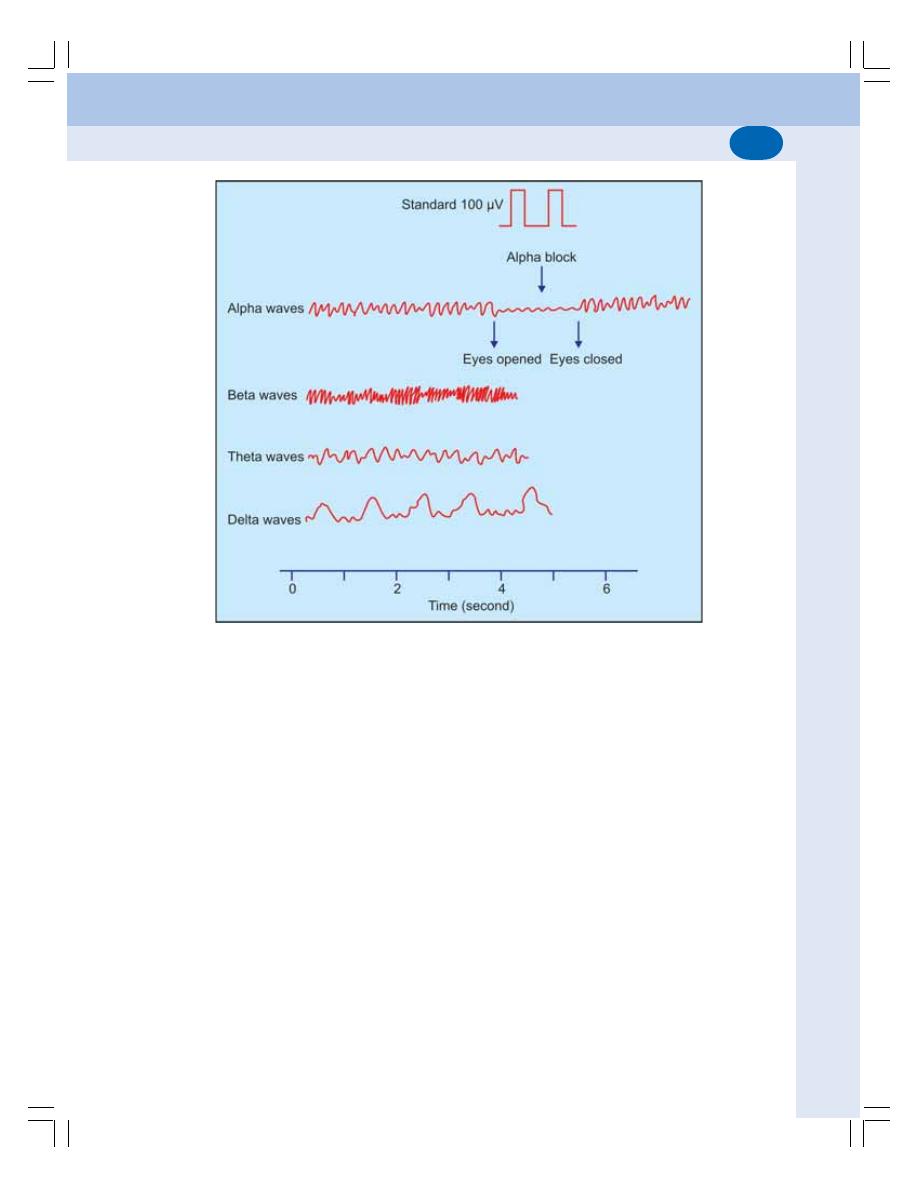
Chapter 99 Electroencephalogram and Epilepsy
607
stimulation or mental concentration, such as
solving arithmetic problems.
Beta Waves
Beta waves are high frequency waves of 15 to
60/second. But, their amplitude is low, i.e. 5 to
10 μV. Beta waves are desynchronized waves
and are recorded during mental activity or mental
tension or arousal state. These waves are not
affected by opening the eyes.
Delta Waves
Delta waves are low frequency and high ampli-
tude waves. Frequency of these waves is 1 to
5/second and the amplitude is 20 to 200 μV.
Delta waves are common in early childhood
during waking hours. In adults, these waves
appear mostly during deep sleep.
Theta Waves
Theta waves are obtained generally in children
below 5 years of age. These waves are of low
frequency and low voltage waves. The frequency
of theta waves is 4 to 8/second and the amplitude
is about 10 μV.
EEG DURING SLEEP
The changes in the EEG pattern during sleep
are described in Chapter 100.
EPILEPSY
DEFINITION
Epilepsy is a brain disorder characterized by
convulsive seizures or loss of consciousness or
both. Convulsion refers to uncontrolled involun-
tary muscular contractions. Convulsive seizure
FIGURE 99-1:
Waves of EEG

Nervous System
608
means sudden attack of uncontrolled involuntary
muscular contractions. It occurs due to paroxys-
mal (sudden and usually recurring periodically)
uncontrolled discharge of impulses from neurons
of brain particularly cerebral cortex.
The person with epilepsy remains normal in
between seizures. The epileptic attack develops
only when the excitability of the neuron is increa-
sed, causing excessive neuronal discharge. The
persons affected by epilepsy are known as epi-
leptics.
TYPES OF EPILEPSY
Epilepsy is divided into two categories:
1. Generalized epilepsy
2. Localized epilepsy.
Generalized Epilepsy
Generalized epilepsy is the type of epilepsy that
occurs due to excessive discharge of impulses
from all parts of the brain. It is also called general
onset seizure or general onset epilepsy.
Generalized epilepsy is subdivided into three
types:
1. Grand mal
2. Petit mal
3. Psychomotor epilepsy.
Grand mal
Grand mal is characterized by sudden loss of
consciousness followed by convulsion. Just
before the onset of convulsions, the person feels
the warning sensation in the form of some
hallucination. It is called epileptic aura.
In EEG recording, fast waves with a
frequency of 15 to 30 per second are seen during
initial stage. Later slow and large waves appear.
In between seizures, the EEG shows delta
waves in all types of epileptics.
The cause of grand mal epilepsy is the ex-
cess neural activity in all parts of the brain.
Petit mal
In this type of epilepsy, the person becomes
unconscious suddenly without any warning. The
unconsciousness lasts for a very short period
of 3 to 30 seconds. Convulsions do not occur.
However, the muscles of face show twitch like
contractions and there is blinking of eyes.
Afterwards, the person recovers automatically
and becomes normal. The frequency of attack
may be once in many months or many attacks
may appear in rapid series. It usually occurs in
late childhood and disappears completely at the
age of 30 or above.
The EEG recording shows slow and large
waves during the attack. Each wave is followed
by a sharp spike. Delta waves appear in between
the seizures.
The causes of petit mal are head injury,
stroke, brain tumor and brain infection.
Psychomotor epilepsy
It is characterized by emotional outbursts such
as abnormal rage, sudden anxiety, fear or dis-
comfort. There is amnesia or a confused mental
state for some period. Some persons have the
tendency to attack others bodily or rub their own
face vigorously. In most cases, the persons are
not aware of their activities.
The EEG recordings show low frequency
rectangular waves, ranging between 2 and 4 per
second.
The causes of the psychomotor epilepsy are
the abnormalities in temporal lobe and tumor in
hypothalamus and other regions of limbic system
like amygdala and hippocampus.
Localized Epilepsy
The epilepsy that occurs because of excessive
discharge of impulses from one part of brain is
called localized epilepsy. The contractions
usually start in the mouth region and spread
down towards the legs. This type of seizure is
also known as Jacksonian epilepsy.
Localized epilepsy is caused by brain tumor.
DEFINITION
SLEEP REQUIREMENT
PHYSIOLOGICAL CHANGES DURING SLEEP
TYPES OF SLEEP
STAGES OF SLEEP AND EEG PATTERN
MECHANISM OF SLEEP
APPLIED PHYSIOLOGY – SLEEP DISORDERS
Physiology of Sleep
100
DEFINITION
Sleep is the natural periodic state of rest for mind
and body with closed eyes characterized by
partial or complete loss of consciousness. Loss
of consciousness leads to decreased response
to external stimuli and decreased body move-
ments. The depth of sleep is not constant
throughout the sleeping period. It varies in
different stages of sleep.
SLEEP REQUIREMENT
Sleep requirement is not constant. However, the
average sleep requirement per day at different
age groups is:
1. Newborn infants
— 18 to 20 hours
2. Growing children
— 12 to 14 hours
3. Adults
—
7 to
9 hours
4. Old persons
—
5 to
7 hours
PHYSIOLOGICAL CHANGES
DURING SLEEP
1. Plasma volume decreases by about 10%
2. Heart rate reduces to about 45 to 60
beats/min
3. Systolic pressure falls to about 90 to 110
mm Hg. If sleep is disturbed by exciting
dreams, the pressure is elevated above
130 mm Hg
4. Rate and force of respiration are decrea-
sed. Cheyne-Stokes type of periodic
breathing may develop
5. Salivary secretion decreases during
sleep. Contraction of empty stomach is
more vigorous
6. Urine formation decreases. The specific
gravity of urine increases
7. Sweat secretion increases
8. Lacrimal secretion decreases
9. Muscle tone reduces
10. Some reflexes particularly the knee jerk,
are abolished. Babinski’s sign becomes
positive.
TYPES OF SLEEP
The sleep is of two types:
1. Rapid eye movement sleep or REM sleep
2. Non-rapid eye movement sleep, NREM sleep
or non-REM sleep.
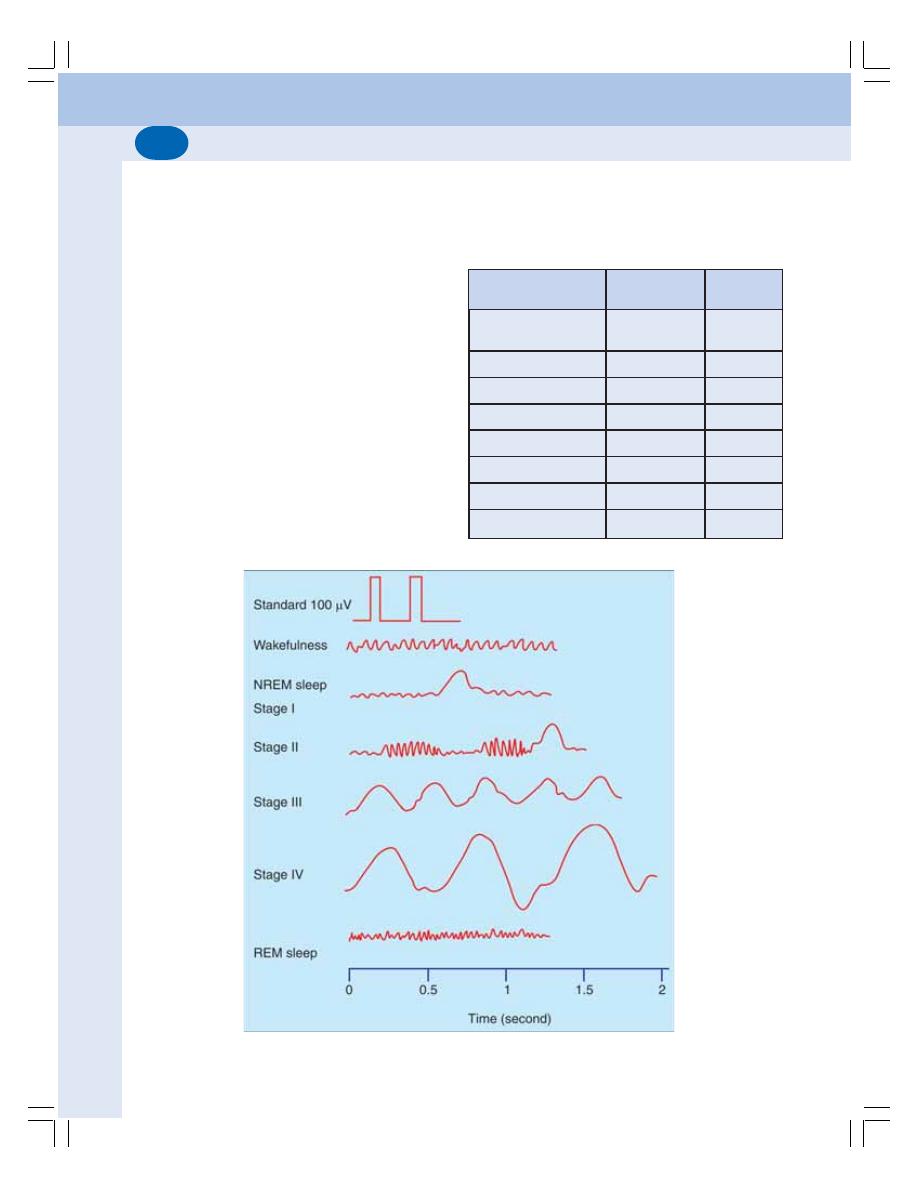
Nervous System
610
TABLE 100-1:
REM sleep and non-REM sleep
Characteristics
REM sleep
Non REM
sleep
1. Rapid eye
Present
Absent
movement
2. Dreams
Present
Absent
3. Muscle twitching
Present
Absent
4. Heart rate
Fluctuating
Stable
5. Blood pressure
Fluctuating
Stable
6. Respiration
Fluctuating
Stable
7. Body temperature Fluctuating
Stable
8. Neurotransmitter
Noradrenaline serotonin
FIGURE 100-1:
EEG during wakefulness, different stages of NREM sleep and REM sleep
1. RAPID EYE MOVEMENT SLEEP
(REM SLEEP)
REM sleep is the type of sleep associated with
rapid conjugate movements of the eyeballs
which occurs frequently. Though the eyeballs
move, the sleep is deep. So, it is also called
paradoxical sleep. It occupies about 20 to 30%
of sleeping period. Functionally, REM sleep is
very important because, it plays an important role
in consolidation of memory. Dreams occur during
this period.
2. NON-RAPID EYE MOVEMENT SLEEP
(NREM OR NON-REM SLEEP)
NREM sleep is the type of sleep without the
movements of eyeballs. It is also called slow
wave sleep. The dreams do not occur in this type
of sleep and it occupies about 70 to 80% of total
sleeping period. The non-REM sleep is followed
by REM sleep.
The differences between the two types of
sleep are given in Table 100-1.

Chapter 100 Physiology of Sleep
611
STAGES OF SLEEP AND EEG
PATTERN
RAPID EYE MOVEMENT SLEEP
During REM sleep, the EEG shows irregular
waves with high frequency and low amplitude.
These waves are desynchronized waves.
NON-RAPID EYE MOVEMENT SLEEP
NREM sleep is divided into four stages, based
on the EEG pattern. During the stage of wake-
fulness, i.e. while lying down with closed eyes
and relaxed mind, the alpha waves of EEG
appear. When the person proceeds to drowsy
state, the alpha waves diminish (Fig. 100-1).
Stage I: Stage of Drowsiness
Alpha waves are diminished and abolished. EEG
shows only low voltage fluctuations and infre-
quent delta waves.
Stage II: Stage of Light Sleep
It is characterized by spindle bursts at a fre-
quency of 14 per second, superimposed by low
voltage delta waves.
Stage III: Stage of Medium Sleep
During this stage, the spindle bursts disappear.
Frequency of delta waves decreases to 1 or
2 per second and amplitude increases to about
100 μV.
State IV: Stage of Deep Sleep
Delta waves become more prominent with low
frequency and high amplitude.
MECHANISM OF SLEEP
Sleep occurs due to the activity of some sleep
inducing centers in brain.
SLEEP CENTERS
Complex pathways between the reticular for-
mation of brainstem, diencephalon and cerebral
cortex are involved in the onset and main-
tenance of sleep. However, two centers are loca-
ted in brainstem, which induce sleep:
1. Raphe nucleus which is responsible for non-
REM sleep
2. Locus ceruleus which is responsible for REM
sleep
Inhibition of ascending reticular activating
system also results in sleep.
HIGHER INTELLECTUAL FUNCTIONS
LEARNING
DEFINITION
TYPES
MEMORY
DEFINITION
TYPES
PHYSIOLOGICAL BASIS
APPLIED PHYSIOLOGY
CONDITIONED REFLEXES
DEFINITION
TYPES
SPEECH
DEFINITION
MECHANISM
NERVOUS CONTROL
APPLIED PHYSIOLOGY
Higher Intellectual
Functions
101
HIGHER INTELLECTUAL
FUNCTIONS
Higher intellectual functions are very essential
to make up the human mind. These functions
are also called higher brain or cortical functions.
Cerebral cortex is responsible for these functions.
The important higher intellectual functions are
learning, memory, conditioned reflexes and
speech.
LEARNING
DEFINITION
Learning is defined as the process by which new
information is acquired.
TYPES OF LEARNING
Learning is of two types:
1. Non-associative learning
2. Associative learning.

Chapter 101 Higher Intellectual Functions
613
1. Non-associative Learning
It involves response of a person to only one type
of stimulus. It is based on two factors:
i. Habituation
Habituation means getting used to something to
which a person is constantly exposed. When a
person is exposed to a stimulus repeatedly, he
starts ignoring the stimulus slowly. During the first
experience, the event (stimulus) is novel and
evokes a response. However, it evokes less
response when it is repeated. Finally, the person
is habituated to the event and ignores it.
ii. Sensitization
Sensitization means a state in which the body
becomes more sensitive to a stimulus. When a
stimulus is applied repeatedly, habituation
occurs. But if the same stimulus is combined
with another type of stimulus, which may be
pleasant or unpleasant, the person becomes
more sensitive to the original stimulus.
For example, a woman gets habituated to
different sounds around her and sleep is not
disturbed by these sounds. However, she
suddenly wakes up when her baby cries
because she is sensitized to the crying sound
of her baby.
2. Associative Learning
It involves learning about relations between two
or more stimuli at a time. The classic example
of associative learning is the conditioned reflex
(see below).
MEMORY
DEFINITION
Memory is defined as the ability to recall the past
experience. It is also defined as retention of
learned materials.
TYPES OF MEMORY
Memory is classified into two types:
1. Explicit memory
2. Implicit memory.
1. Explicit Memory
Explicit memory is otherwise known as
declarative memory or recognition memory. It
is defined as the memory that involves conscious
recollection of past experience. It consists of
memories regarding the events which occurred
in the external world around us. The information
stored may be about a particular event that
happened at a particular time and place.
Examples: Recollection of a birthday party
celebrated three days ago; the events taken
place while taking breakfast, etc.
Explicit memory involves hippocampus and
medial part of temporal lobe.
2. Implicit Memory
Implicit memory is otherwise known as
nondeclarative memory or skilled memory. It is
defined as the memory in which past experience
is utilized without conscious awareness. It helps
to perform various skilled activities properly. For
example, cycling, driving, playing tennis, dancing,
typing, etc. are performed automatically without
awareness.
Implicit memory involves the sensory and
motor pathways.
Memory is also classified into:
1. Short term memory
2. Long term memory
1. Short Term Memory
Short term memory is the recalling the events
that happened very recently, i.e. within hours or
days. It is also known as recent memory. For
example, telephone number that is known today
may be remembered till tomorrow. If it is not
recalled repeatedly, it may be forgotten on third
day.

Nervous System
614
2. Long Term Memory
It is otherwise called remote memory. It is the
recalling of the events of weeks, months, years
or sometimes lifetime. Examples are recalling
first day of schooling, birthday celebration of
previous year, picnic enjoyed last week, etc.
PHYSIOLOGICAL BASIS OF MEMORY
Memory is stored in brain by the alteration of
synaptic transmission between the neurons
involved in memory. Storage of memory may be
facilitated or habituated.
Facilitation
It is the process by which the memory storage
is enhanced. It involves increase in synaptic
transmission and increased postsynaptic activity.
Habituation
It is the process by which the memory storage
is attenuated (attenuation = decrease in strength,
effect or value). It involves reduction in synaptic
transmission and slow stoppage of postsynaptic
activity.
Basis for Short Term Memory
Basic mechanism of memory is the development
of new neuronal circuits by the formation of new
synapses and facilitation of synaptic trans-
mission. The number of presynaptic terminals
and the size of the terminals are also increased.
Basis for Long Term Memory
When the neuronal circuit is reinforced by cons-
tant activity, the memory is consolidated and
encoded into different areas of the brain. This
encoding makes memory a permanent or long
term memory.
Sites of Encoding
Hippocampus and the Papez circuit (the closed
circuit between hippocampus, thalamus,
hypothalamus and corpus striatum) are the main
sites for memory encoding. Frontal and parietal
areas are also involved in memory storage.
Consolidation of Memory
The process by which a short term memory is
crystallized into a long term memory is called
memory consolidation
APPLIED PHYSIOLOGY –
ABNORMALITIES OF MEMORY
1. Amnesia – loss of memory
2. Dementia – progressive deterioration of intell-
ect, emotional control and social behavior
and associated with loss of memory
3. Alzheimer’s Disease – progressive neuro-
degenerative disease due to death of neurons
in brain.
CONDITIONED REFLEXES
DEFINITION
Conditioned reflex is the acquired reflex that
requires learning, memory and recall of previous
experience. It forms the basis of learning.
The unconditioned reflex is the inborn reflex
which does not need previous experience.
TYPES OF CONDITIONED REFLEXES
The conditioned reflexes are of two types:
A. Classical conditioned reflexes
B. Instrumental conditioned reflexes.
Classical Conditioned Reflexes
The Classical conditioned reflexes are those
reflexes, which are established by a conditioned
stimulus followed by an unconditioned stimulus.
Method of study – Pavlov’s bell-dog
experiments
The classical conditioned reflexes are
demonstrated by the classical bell-dog
experiments (salivary secretion experiments)
done by Ivan Pavlov and his associates.
In dogs, the duct of parotid gland or sub-
mandibular gland was taken outside through
cheek or chin respectively and the salivary
secretion was measured in drops by means of
an electrical recorder.

Chapter 101 Higher Intellectual Functions
615
Types of classical conditioned reflexes
Classical conditioned reflexes are classified into
two groups:
I.
Positive or excitatory conditioned reflexes
II. Negative conditioned reflexes.
I. Positive conditioned reflexes
Positive conditioned reflexes are of three types:
1. Primary conditioned reflex: It is the reflex
developed with one unconditioned stimulus
and one conditioned stimulus. The dog is fed
with food (unconditioned stimulus). Simul-
taneously a flash of light (conditioned
stimulus) is also shown. Both the stimuli are
repeated for some days. After the
development of reflex, the flash of light
(conditioned stimulus) alone causes salivary
secretion without food (unconditioned
stimulus).
2. Secondary conditioned reflex: It is the reflex
developed with one unconditioned stimulus
and two conditioned stimuli. After
establishment of a conditioned reflex with one
conditioned stimulus, another conditioned
stimulus is applied along with the first one.
For example, the animal is fed with food
(unconditioned reflex) and simultaneously a
flash of light (first conditioned stimulus), and
a bell sound (second conditioned stimulus)
are applied. After the development of the
reflex, the second conditioned stimulus – the
bell sound alone can cause salivary secretion
3. Tertiary conditioned reflex: In this reflex, a
third conditioned stimulus is added and, the
reflex is established. But, the reflex with more
than three conditioned stimuli is not possible.
II. Negative conditioned reflexes
In negative conditioned reflexes the established
conditioned reflexes are inhibited by some
factors. For example, some disturbing factors
like sudden entrance of a stranger or sudden
noise can abolish the conditioned reflex and
inhibit salivary secretion.
Instrumental or Operant Conditioned
Reflexes
The instrumental or operant conditioned reflexes
are the reflexes in which the behavior of the
person is instrumental. This type of reflexes is
developed by the conditioned stimulus followed
by a reward or punishment.
For example, if the animal is rewarded by a
banana by pressing a bar, the animal repeatedly
presses the bar. If the animal is given a tasty
food along with electric shock, the animal starts
avoiding that food.
The instrumental conditioned reflexes play an
important role during the learning processes of
a child. These conditioned reflexes are also
responsible for behavior pattern of an individual.
SPEECH
DEFINITION
Speech is defined as the expression of thoughts
by production of articulate sound, bearing a
definite meaning. When a sound is produced
verbally, it is called the speech. If it is expressed
by visual symbols, it is known as writing.
MECHANISM OF SPEECH
Speech depends upon the coordinated activities
of central speech apparatus and peripheral
speech apparatus. The central speech apparatus
consists of higher centers, i.e. the cortical and
subcortical centers. The peripheral speech
apparatus includes larynx or sound box, pharynx,
mouth, nasal cavities, tongue and lips.
NERVOUS CONTROL OF SPEECH
Speech is controlled by the following cortical
areas.
A. Motor Areas
1. Broca’s area
It is area 44. It is also called speech center. It
is situated in lower part of lateral surface of pre-
frontal cortex. This area controls the movements
of structures concerned with vocalization.

Nervous System
616
2. Upper frontal motor area
It is situated in the paracentral gyrus over the
medial surface of the cerebral hemisphere. It
controls the coordinated movements concerned
with writing.
B. Sensory Areas
1. Auditopsychic area
Auditopsychic area is situated in the superior
temporal gyrus. It is concerned with storage of
memories of spoken words.
2. Visuopsychic area
It is present in angular gyrus of the parietal
cortex. It is concerned with storage of memories
of the visual symbols.
C. Wernicke’s Area
This area is situated in the upper part of temporal
lobe. It is responsible for understanding the
auditory and visual information about any word.
APPLIED PHYSIOLOGY – DISORDERS
OF SPEECH
1. Aphasia
Aphasia is the loss or impairment of speech. It
is due to damage of speech centers which
occurs during stroke, head injury, cerebral
tumors, brain infections and degenerative
disease like Parkinson’s disease.
Head’s classification of aphasia
Henry Head has classified aphasia into four
types:
1. Verbal aphasia: Disability in the formation of
words
2. Syntactical aphasia: Inability to arrange words
in proper sequence
3. Semantic aphasia: Inability to recognize the
significance of words
4. Nominal aphasia: Difficulty in naming the
object due to failure in recognizing the
meaning of wards.
2. Dysarthria or Anarthria
Dysarthria or anarthria is the difficulty or inability
to speak because of paralysis of muscles
involved in articulation. The spoken and written
words are understood. It is caused by damage
of brain areas or the nerves that control muscles
involved in speech. It occurs in conditions like
stroke, brain injury and degenerative disease.
3. Dysphonia
Dysphonia is a voice disorder characterized by
hoarseness and a sore or dry throat. Hoarseness
means the difficulty in producing sound while
trying to speak or a change in the pitch or
loudness of voice. It occurs due to diseases of
vocal cords or larynx.
4. Stammering
Stammering or shuttering is a speech disorder
in which the normal flow of speech is disturbed
by repetitions or stoppage of sound and words.
It is associated with some unusual facial and
body movements. Stammering is due to genetic
factors, brain damage, neurological disorders or
anxiety.
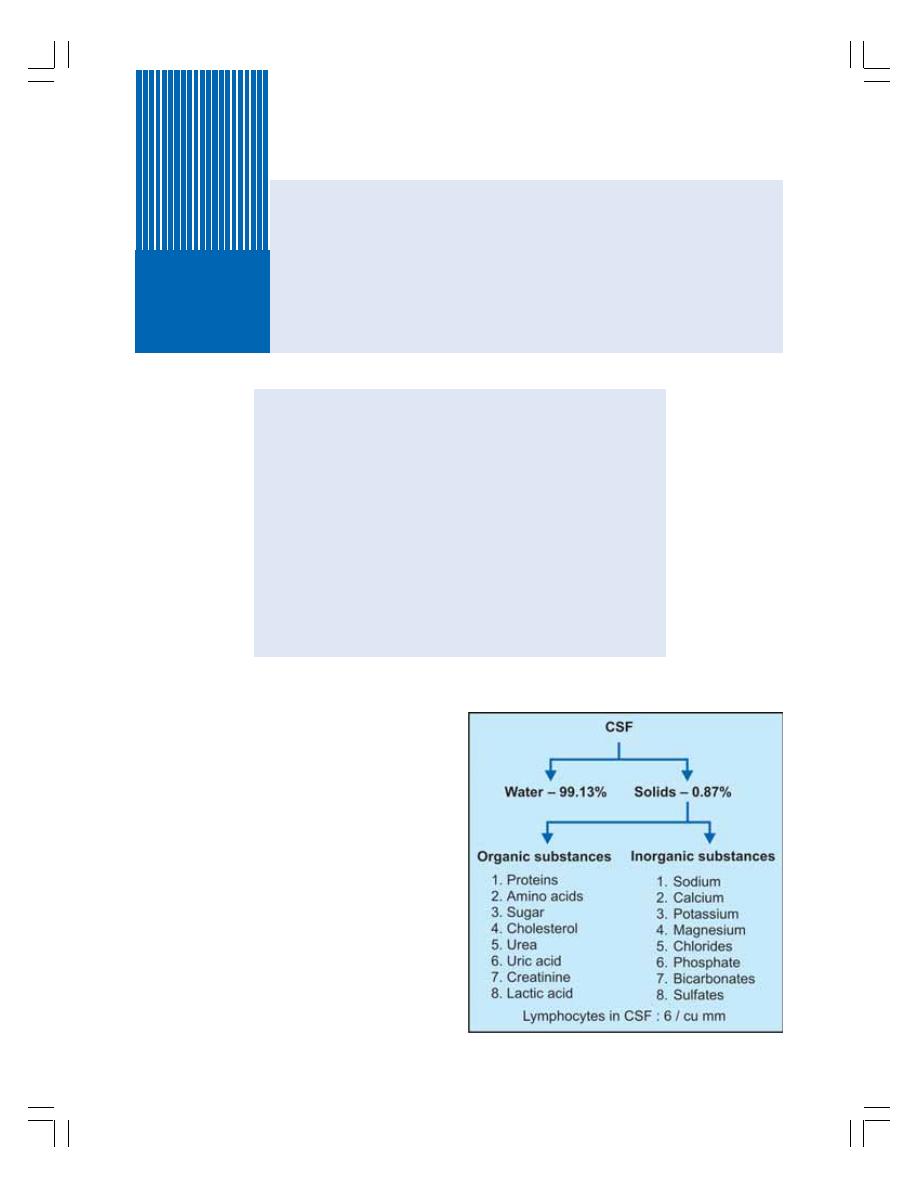
INTRODUCTION
PROPERTIES AND COMPOSITION
FORMATION
CIRCULATION
ABSORPTION
PRESSURE EXERTED BY CSF
FUNCTIONS
COLLECTION
BLOOD-BRAIN BARRIER
BLOOD – CEREBROSPINAL FLUID BARRIER
APPLIED PHYSIOLOGY
INTRODUCTION
Cerebrospinal fluid (CSF) is the clear, colorless
and transparent fluid that circulates through
ventricles of brain, subarachnoid space and
central canal of spinal cord. It is a part of ECF.
PROPERTIES AND COMPOSITION
OF CSF
Properties
Volume
:
150 ml
Rate of formation
:
0.3 ml per minute
Specific gravity
:
1.005
Reaction
:
Alkaline.
Composition
Composition of CSF is given in Fig. 102-1. CSF
also contains some lymphocytes which are
added when it flows in the spinal cord.
Cerebrospinal Fluid
102
FIGURE 102-1: Composition of cerebrospinal fluid
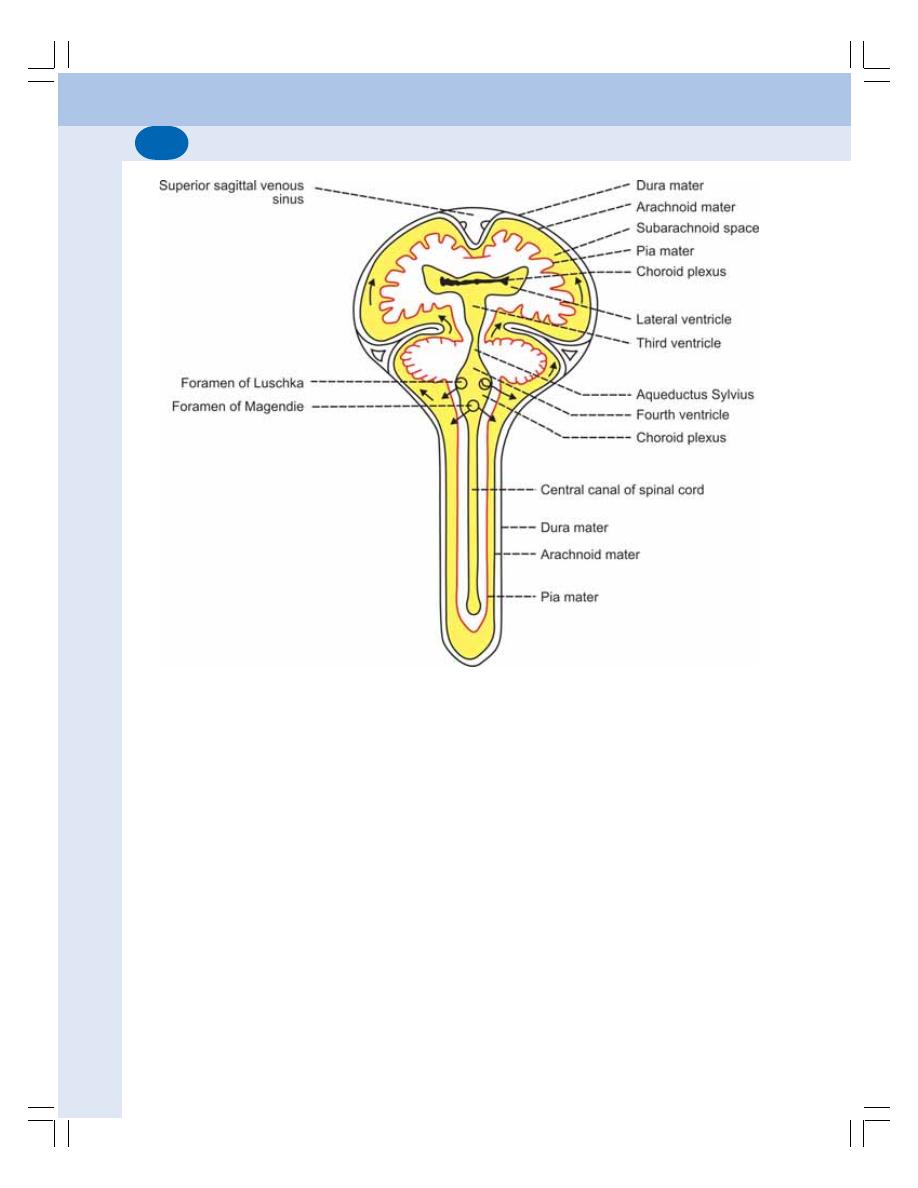
Nervous System
618
FORMATION OF CSF
CSF is formed by the choroid plexuses (tuft of
capillaries) situated within the ventricles.
It
is
formed by the process of secretion which
involves active transport.
CIRCULATION OF CSF
Major quantity of CSF is formed in the lateral
ventricles and passes through the foramen of
Monro into the third ventricle (Figs 102.2
and 102.3). From here, it passes to the fourth
ventricle through aqueductus Sylvius. From
fourth ventricle, it enters into the cisterna magna
and cisterna lateralis through foramen of
Magendie (central opening) and foramen of
Luschka (lateral opening). The cisternal fluid
circulates through the subarachnoid space over
spinal cord and cerebral hemispheres.
ABSORPTION OF CSF
CSF is mostly absorbed by the arachnoid villi
into dural sinuses and spinal veins. Small
amount is absorbed into cervical lymphatics
and perivascular spaces. The mechanism of
absorption is by filtration. Normally, about 500
ml of CSF is formed everyday and an equal
amount is absorbed.
PRESSURE EXERTED BY CSF
The pressure exerted by CSF varies in different
position, viz.
Lateral recumbent position = 10 to 18 cm of H
2
O
Lying position
= 13 cm of H
2
O
Sitting position
= 30 cm of H
2
O.
Certain events like coughing, crying and
compression of internal jugular vein increase the
pressure.
FIGURE 102-2: Circulation of cerebrospinal fluid
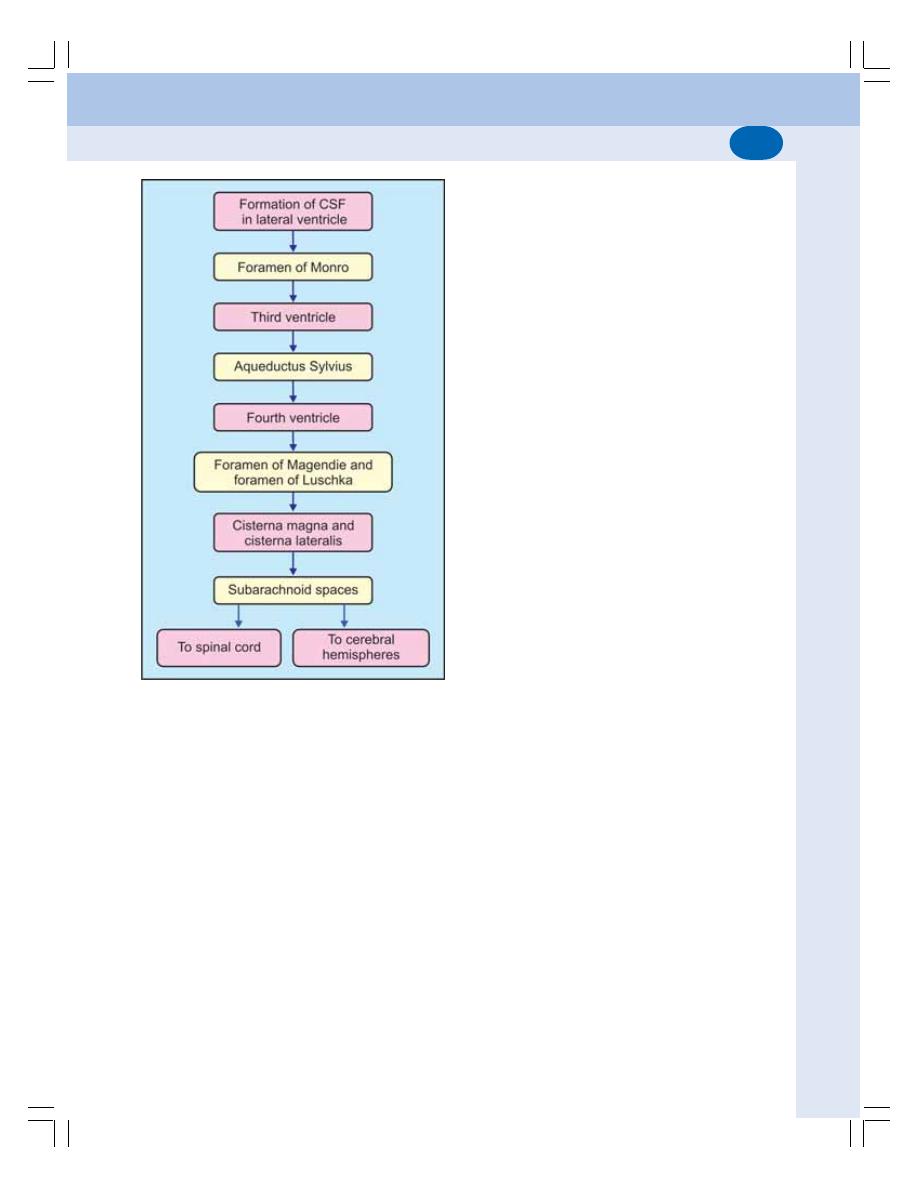
Chapter 102 Cerebrospinal Fluid
619
FUNCTIONS OF CSF
1. Protective Function
CSF acts as fluid buffer and protects the brain
from shock. Since, the specific gravity of brain
and CSF is more or less same, brain floats in
CSF. When head receives a blow, CSF acts like
a cushion and prevents the movement of brain
against the skull bone and thereby prevents the
damage of brain.
2. Regulation of Cranial Content Volume
Regulation of cranial content volume is essential
because, if the cranial contents increase in
volume, the brain may be affected. The increase
in cranial content volume is prevented by greater
absorption of CSF.
3. Medium of Exchange
CSF is the medium through which many sub-
stances, particularly the nutritive substances
and waste materials are exchanged between
blood and brain tissues.
COLLECTION OF CSF
CSF is collected either by cisternal puncture or
lumbar puncture. In cisternal puncture, the CSF
is collected by passing a needle between the
occipital bone and atlas, so that it enters the
cisterna magna. In lumbar puncture, the needle
is introduced into the subarachnoid space in the
lumbar region, between the third and fourth
lumbar spines.
BLOOD-BRAIN BARRIER
Blood-brain barrier (BBB) is a neuroprotective
structure that prevents the entry of many sub-
stances and pathogens into the brain tissues
from blood. The barrier exists in the capillary
membrane of all parts of the brain except in
some areas of hypothalamus.
BBB is formed by tight junctions in the
endothelial cells of the brain capillaries. The
cytoplasmic foot processes of astrocytes
(neuroglial cells) develop around the capillaries
and reinforce the barrier.
FUNCTIONS OF BLOOD-BRAIN
BARRIER
BBB acts as both a mechanical barrier and a
transport mechanisms. It prevents harmful
chemical substances and permits metabolic and
essential materials into the brain tissues.
Substances which can pass through
Blood-Brain Barrier
1. Oxygen
2. Carbon dioxide
FIGURE 102-3: Schematic diagram of CSF
circulation

Nervous System
620
3. Water
4. Glucose
5. Amino acids
6. Electrolytes
7. Lipid soluble drugs such as L–dopa, 5-HT and
tetracycline
8. Lipid soluble anesthetic gases such as ether
and nitrous oxide
9. Other lipid soluble substances.
Substances which cannot pass through
Blood-Brain Barrier
1. Injurious chemical agents
2. Pathogens such as bacteria
3. Drugs such as penicillin and the cate-
cholamines
4. Bile pigments.
BLOOD–CEREBROSPINAL FLUID
BARRIER
It is the barrier between the blood and
cerebrospinal fluid that exists at the choroid
plexus. The function of this barrier is similar to
that of the BBB. It does not allow the movement
of many substances from blood to cerebrospinal
fluid. It allows the movement of only those
substances, which are allowed by BBB.
APPLIED PHYSIOLOGY –
HYDROCEPHALUS
The abnormal accumulation of CSF in the skull
associated with enlargement of head is called
hydrocephalus. Hydrocephalus along with
increased intracranial pressure causes
headache and vomiting. In severe conditions, it
leads to atrophy of brain, mental weakness and
convulsions.
INTRODUCTION
SYMPATHETIC DIVISION
PARASYMPATHETIC DIVISION
FUNCTIONS
NEUROTRANSMITTERS
Autonomic Nervous System
103
INTRODUCTION
The autonomic nervous system (ANS) is pri-
marily concerned with the regulation of visceral
or vegetative functions of the body. So, it is
also called vegetative or involuntary nervous
system.
DIVISIONS OF ANS
Autonomic nervous system is divided into two
divisions:
1. Sympathetic division
2. Parasympathetic division.
The differences between both the divisions
of ANS are given in Table 103-1.
SYMPATHETIC DIVISION
It is otherwise called thoracolumbar outflow
because, the preganglionic neurons are situated
in lateral gray horns of 12 thoracic and first two
lumbar segments of spinal cord. The fibers
arising from here are called preganglionic
fibers. The preganglionic fibers leave the spinal
cord through anterior nerve root and white rami
communicants, and terminate in the post-
ganglionic neurons, which are situated in the
sympathetic ganglia.
Sympathetic division supplies smooth muscle
fibers of all the visceral organs such as blood
vessels, heart, lungs, glands, gastrointestinal
organs, etc.
SYMPATHETIC GANGLIA
The ganglia of sympathetic division are classified
into three groups:
I.
Paravertebral or sympathetic chain ganglia
II. Prevertebral or collateral ganglia
III. Terminal or peripheral ganglia.
I.
Paravertebral or Sympathetic Chain
Ganglia
Paravertebral or sympathetic chain ganglia are
present on either side of vertebral column. These
ganglia are connected with each other by
longitudinal fibers to form the sympathetic
chains (Fig. 103-1). Both the chains extend
from skull to coccyx.
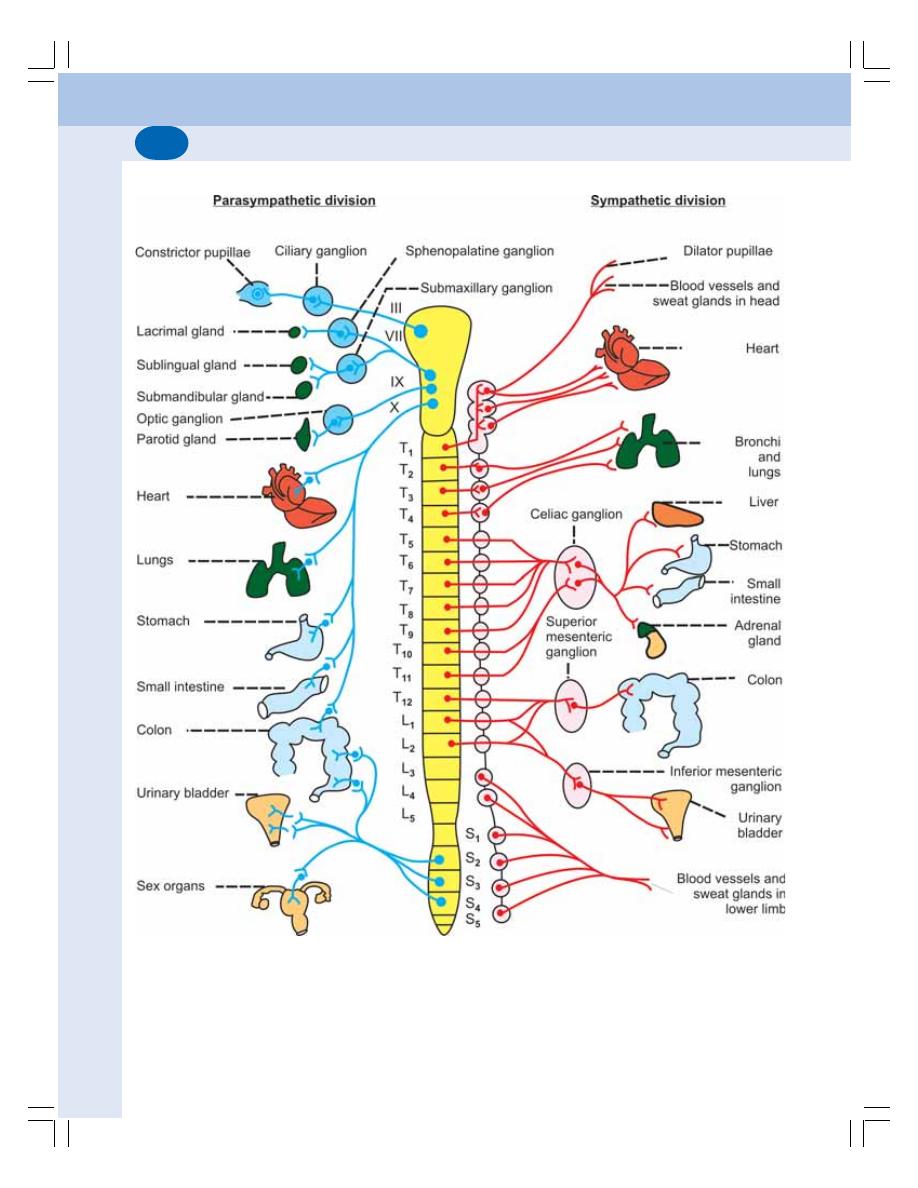
Nervous System
622
FIGURE 103-1: Autonomic nervous system

Chapter 103 Autonomic Nervous System
623
Ganglia of the sympathetic chain (trunk) on
each side are divided into four groups:
1. Cervical ganglia –
8 in number
2. Thoracic ganglia – 12 in number
3. Lumbar ganglia
–
5 in number
4. Sacral ganglia
–
5 in number.
II. Prevertebral or Collateral Ganglia
Prevertebral ganglia are situated in thorax,
abdomen
and pelvis in relation to aorta and its
branches.
The prevertebral ganglia are:
1. Celiac ganglion
2. Superior mesenteric ganglion
3. Inferior mesenteric ganglion.
The prevertebral ganglia receive pre-
ganglionic fibers from T
5
to L
2
segments. The
postganglionic fibers from these ganglia supply
the visceral organs of thorax, abdomen and
pelvis.
III. Terminal or Peripheral Ganglia
Terminal ganglia are situated within or close to
structures innervated by them. Heart, bronchi,
pancreas and urinary bladder are innervated by
the terminal ganglia.
Sympathoadrenergic System
Sympathoadrenergic system is a functional and
phylogenetic unit that includes sympathetic
division and adrenal medulla. Adrenal medulla
is a modified sympathetic ganglion.
PARASYMPATHETIC DIVISION
The parasympathetic division of ANS is
otherwise called craniosacral outflow because,
the fibers of this division arise from brainstem
and sacral segments of spinal cord. The cranial
portion of parasympathetic division innervates
the blood vessels of the head and neck and
many thoracoabdominal visceral organs.
The sacral portion of parasympathetic
division innervates the smooth muscles for-
ming the walls of viscera and the glands such
as large intestine, liver, spleen, kidneys, bladder,
genitalia, etc.
CRANIAL NERVES OF
PARASYMPATHETIC DIVISION
The cranial nerves of the parasympathetic
division are:
1. Oculomotor (III) nerve
2. Facial (VII) nerve
3. Glossopharyngeal (IX) nerve
4. Vagus (X) nerve.
The fibers of sacral outflow arise from
second to fourth sacral (S
2
to S
4
) segments of
spinal cord.
FUNCTIONS OF ANS
The ANS is concerned with regulation of
vegetative functions, which are beyond volun-
tary control. By controlling the various vegetative
functions, ANS plays an important role in
maintaining the constant internal environment
(homeostasis).
Almost all the visceral organs are supplied
by both sympathetic and parasympathetic
divisions of ANS and, the two divisions produce
antagonistic effects on each organ. When the
fibers of one division supplying to an organ is
sectioned or affected by lesion, the effects of
fibers from other division on the organ become
more prominent.
The actions of the sympathetic and para-
sympathetic fibers on various structures are given
in Table 103-1.
NEUROTRANSMITTERS OF ANS
The different nerve fibers of ANS execute the
functions by releasing some neurotransmitter
substances.
SYMPATHETIC FIBERS
1. Preganglionic fibers: Acetylcholine (Ach)
2. Postganglionic noradrenergic fibers: Nora-
drenaline
3. Postganglionic cholinergic fibers: Ach
Nervous System
624
The postganglionic sympathetic cholinergic
nerve fibers supply sweat glands and blood
vessels in heart and skeletal muscle.
TABLE 103-1: Actions of sympathetic and parasympathetic divisions of ANS
Effector organ
Sympathetic division
Parasympathetic
division
1. Eye
Ciliary muscle
Relaxation
Contraction
Pupil
Dilatation
Constriction
2. Lacrimal glands
Decrease in secretion
Increase in secretion
3. Salivary secretion
Decrease in secretion and
Increase in secretion
vasoconstriction
and vasodilatation
4. Gastrointestinal tract
Motility
Inhibition
Acceleration
Secretion
Decrease
Increase
Sphincters
Constriction
Relaxation
Smooth muscles
Relaxation
Contraction
5. Gallbladder
Relaxation
Contraction
6. Urinary bladder
Detrusor muscle
Relaxation
Contraction
Internal sphincter
Constriction
Relaxation
7. Sweat glands
Increase in secretion
-------
8. Heart rate and force
Increase
Decrease
9. Blood vessels
Constriction of all blood
Dilatation
vessels except those in
heart and skeletal muscle
10. Bronchioles
Dilatation
Constriction
PARASYMPATHETIC FIBERS
1. Preganglionic fibers: Ach
2. Postganglionic fibers: Ach

Chapter 103 Autonomic Nervous System
625
LONG QUESTIONS
1. What is synapse? Explain the structure,
functions and properties of synapse.
2. Define and classify reflex action. Explain
reflex arc and the properties of reflexes.
3. Name the ascending tracts of the spinal
cord and, explain spinothalamic tracts.
4. What are the tracts of Spinal cord?
Describe the spinocerebellar tracts.
5. Give an account of tracts in the posterior
white funiculus of spinal cord.
6. Enumerate the descending tracts of spinal
cord. Describe in detail the pyramidal
tracts. Write a note on the effects of upper
and lower motor neuron lesions.
7. What are the thalamic nuclei? Describe
the functions and effects of lesions of
thalamus.
8. Name the hypothalamic nuclei. Explain
the functions and effects of lesions of
hypothalamus.
9. What are the different divisions of
cerebellum? Explain the functions of each
division. Add a note on cerebellar lesions.
10. What are the components of basal
ganglia? Give an account of functions and
disorders of basal ganglia.
11. Name lobes of cerebral cortex? Describe
the functions of each lobe. Add a note on
frontal lobe syndrome.
SHORT QUESTIONS
1. Structure of neuron.
2. Myelin sheath.
3. Classification of nerve fibers.
4. Properties of nerve fibers.
5. Action potential in nerve fiber.
6. Saltatory conduction.
7. Wallarian degeneration.
8. Neuroglia.
QUESTIONS IN NERVOUS SYSTEM
9. Cutaneous receptors.
10. Generator (receptor) potential.
11. EPSP.
12. IPSP.
13. Synaptic transmission.
14. Reflex arc.
15. Properties of reflexes.
16. Superficial reflexes.
17. Deep reflexes.
18. Babinski’s sign.
19. Upper/lower motor neuron lesion.
20. Pathway for fine touch sensations.
21. Pathway for pressure sensation.
22. Pathway for temperature sensations.
23. Pathway for conscious kinesthetic
sensations.
24. Pathway for subconscious kinesthetic
sensations.
25. Pathway for pain sensations.
26. Functions of thalamus.
27. Thalamic syndrome.
28. Functions of hypothalamus.
29. Regulation of food intake.
30. Disorders of hypothalamus.
31. Corticocerebellum (Neocerebellum).
32. Spinocerebellum (Paleocerebellum).
33. Vestibulocerebullum
34. Functions of basal ganglia.
35. Parkinsonism.
36. Frontal lobe of cerebral cortex.
37. Parietal lobe (or sensory areas) of cere-
bral cortex.
38. Functions of limbic system.
39. Muscle spindle.
40. Muscle tone
41. Righting reflexes.
42. Semicircular canal.
43. Otolith organ.
44. Motion sickness.
45. EEG.
Questions in Nervous System
625

Nervous System
626
46. Epilepsy.
47. EEG pattern during sleep.
48. REM and non-REM sleep.
49. Learning.
50. Memory.
51. Conditioned reflexes.
52. Speech disorders.
53. CSF.
54. Blood-brain barrier.
55. Role of ANS in the regulation of cardio-
vascular functions.
56. Role of ANS in the regulation of gastro-
intestinal activity.
57. Functions of sympathetic division of ANS.
58. Functions of parasympathetic division of
ANS.
Questions in Nervous System
626

Special Senses
104. Structure of the Eye ............................................................. 629
105. Visual Process and Field of Vision ...................................... 638
106. Visual Pathway .................................................................... 643
107. Pupillary Reflexes ................................................................ 647
108. Color Vision ......................................................................... 651
109. Errors of Refraction ............................................................. 654
110. Structure of Ear and Auditory Pathway ................................ 657
111. Mechanism of Hearing and Auditory Defects ...................... 663
112. Sensation of Taste ............................................................... 667
113. Sensation of Smell .............................................................. 670
S E C T I O N
11
C H A P T E R S

SPECIAL SENSES
FUNCTIONAL ANATOMY OF THE EYEBALL
WALL OF THE EYEBALL
FUNDUS OCULI
INTRAOCULAR FLUIDS
INTRAOCULAR PRESSURE
LENS
OCULAR MUSCLES
OCULAR MOVEMENTS
APPLIED PHYSIOLOGY
SPECIAL SENSES
Special senses or special sensations are the
complex sensations which involve specialized
sense organs. These sensations are different
from somatic sensations that arise from skin,
muscles, tendons and joints (Chapter 89).
Special senses are:
1. Sensation of vision
2. Sensation of hearing
3. Sensation of taste
4. Sensation of smell.
FUNCTIONAL ANATOMY OF THE
EYEBALL
MORPHOLOGY
Human eyeball (bulbus oculi) is approximately
globe shaped with a diameter of about 24 mm.
It is slightly flattened from above downwards.
The center of anterior curvature of the eyeball
Structure of the Eye
104
is called the anterior pole, and the center of
posterior curvature is called the posterior pole.
The line joining the two poles is called optic axis.
The line joining a point in cornea little medial to
anterior pole and the fovea centralis situated
lateral to posterior pole is known as visual axis.
The light rays pass through the visual axis of
eyeball (Fig. 104-1).
ORBITAL CAVITY
Except the anterior 1/6, the eyeball is situated
in the bony orbital cavity or eye socket. A thick
layer of areolar tissue is interposed between the
bone and the eye. It serves as a cushion to pro-
tect the eyeball from external force. Eyeballs are
attached to orbital cavity by ocular muscles.
EYELIDS
Eyelids protect the eyeball from foreign particles
coming in contact with its surface and cutoff the
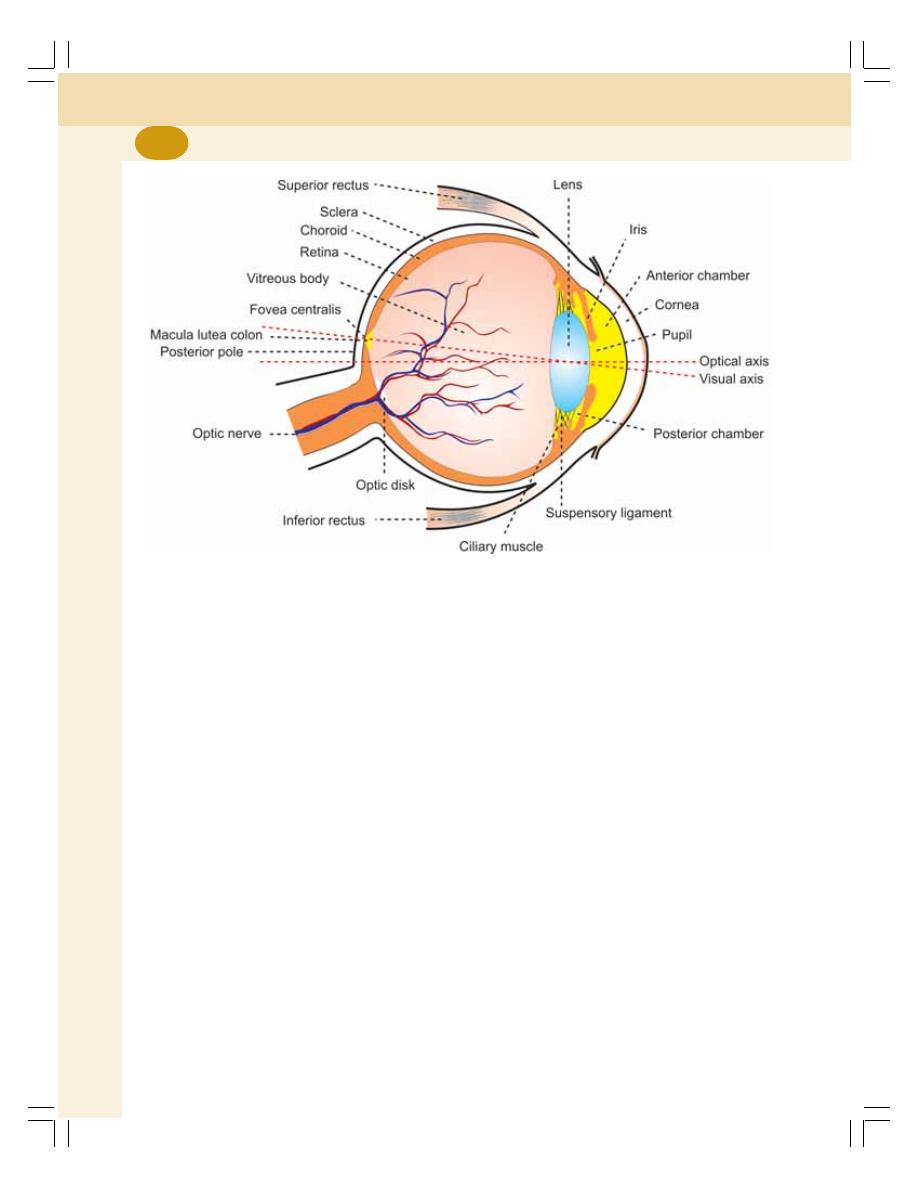
Special Senses
630
light during sleep. The eyelids are opened and
closed voluntarily as well as reflexly.
The margins of eyelids have sensitive hairs
called the cilia. Each cilium arises from a follicle,
which is surrounded by a sensory nerve plexus.
When the dust particle comes in contact with cilia,
these sensory nerves are activated resulting in
rapid blinking of eyelids. It prevents the dust
particles from reaching the eyeball.
The opening between the two eyelids is called
palpebral fissure. In adults, it is about 25 mm
long. Its width is about 12 to 15 mm when
opened.
CONJUNCTIVA
It is a thin mucous membrane, which covers the
exposed part of the eye. After covering the ante-
rior surface, the conjunctiva is reflected into the
inner surfaces of the eyelids. The part of
conjunctiva covering the eyeball is called the
bulbar portion. The part covering the eyelid is
called the palpebral portion.
LACRIMAL GLAND
The lacrimal gland is situated in the shelter of
bone, forming the upper and outer border of wall
of the eye socket. From the lacrimal gland, tear
flows over the surface of conjunctiva and drains
into nose via lacrimal ducts, lacrimal sac and
nasolacrimal duct. Tear is a hypertonic fluid. Due
to its continuous washing and lubrication, the
conjunctiva is kept moist and is protected from
infection. Tear also contains lysozyme that kills
bacteria.
WALL OF THE EYEBALL
The wall of the eyeball is composed of three
layers namely outer, middle and inner layers
(Fig. 104-2).
OUTER LAYER OR TUNICA EXTERNA
OR TUNICA FIBROSA
The outer layer preserves the shape of eyeball.
The anterior 1/6 is transparent and is known as
FIGURE 104-1: Structure of eyeball
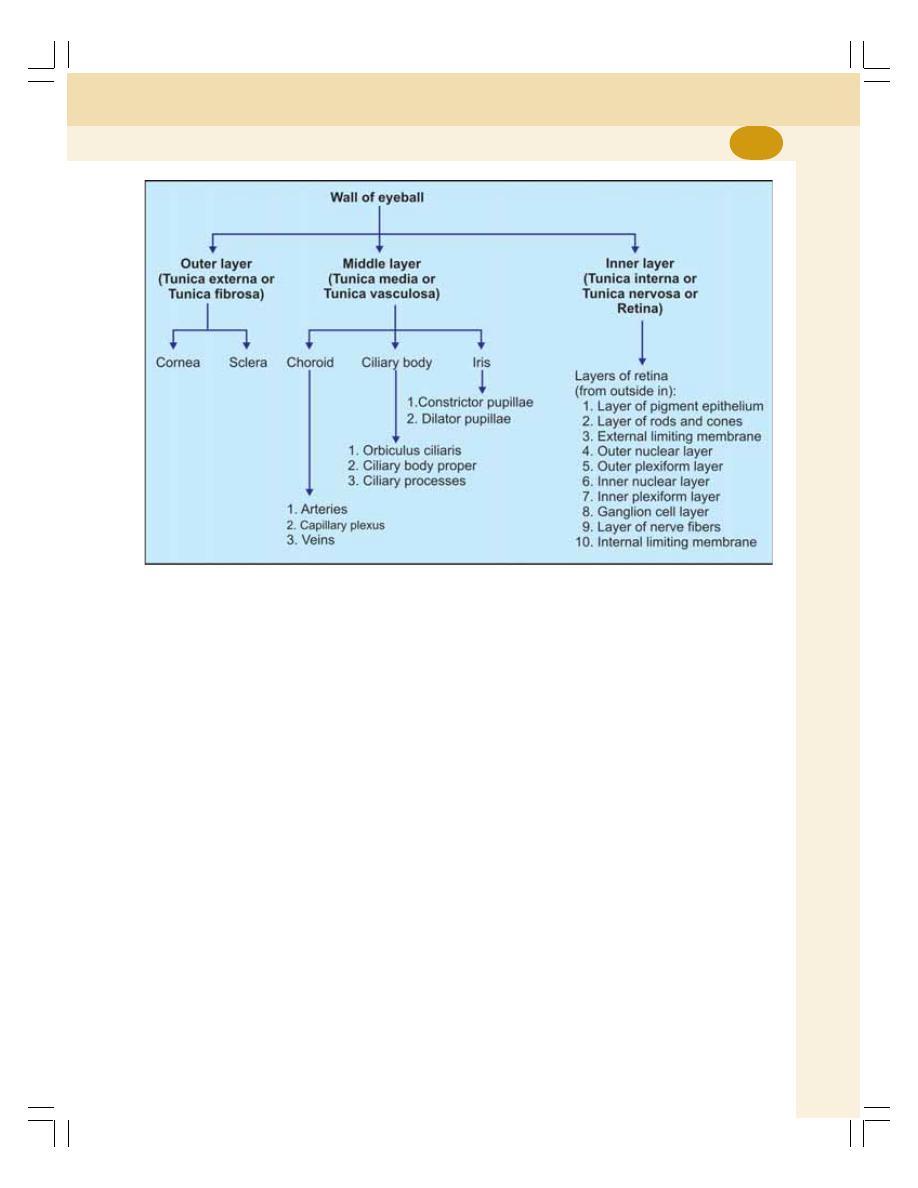
Chapter 104 Structure of the Eye
631
cornea. It covers the iris and the pupil. It is
continuous with the sclera. The posterior 5/6 of
this coat is tough, fibrous and opaque and it is
called the sclera.
MIDDLE LAYER OR TUNICA MEDIA
OR TUNICA VASCULOSA
The middle layer surrounds the eyeball com-
pletely except for a small opening in front known
as the pupil. This layer comprises three struc-
tures.
1. Choroid
2. Ciliary body
3. Iris.
1. Choroid
Choroid is the thin vascular layer of eyeball
situated between sclera and retina. It forms
posterior 5/6 of middle layer. The choroid is
extended anteriorly up to the insertion of ciliary
muscle (the level of ora serrata). Choroid is
composed of a rich capillary plexus, numerous
small arteries and veins.
2. Ciliary Body
Ciliary body is the thickened anterior part of
middle layer of eye situated between choroid and
iris. It is situated in front of ora serrata.
It is in the form of a ring. Its outer surface is
separated from the sclera by perichoroidal space.
The inner surface of the ciliary body faces the
vitreous body and lens. The suspensory liga-
ments from the lens are attached to ciliary body.
The anterior surface of ciliary body faces towards
the center of cornea. From the surface, the iris
arises. Ciliary body has three parts:
i. Orbiculus ciliaris
ii. Ciliary body proper
iii. Ciliary processes.
3. Iris
Iris is the thin colored curtain like structure of
eyeball. It forms the anterior most part of middle
layer. It is like a thin circular diaphragm, placed
in front of the lens. It has a circular opening in
the center called pupil. Iris is a muscular structure
and has two muscles:
FIGURE 104-2: Wall of the eyeball
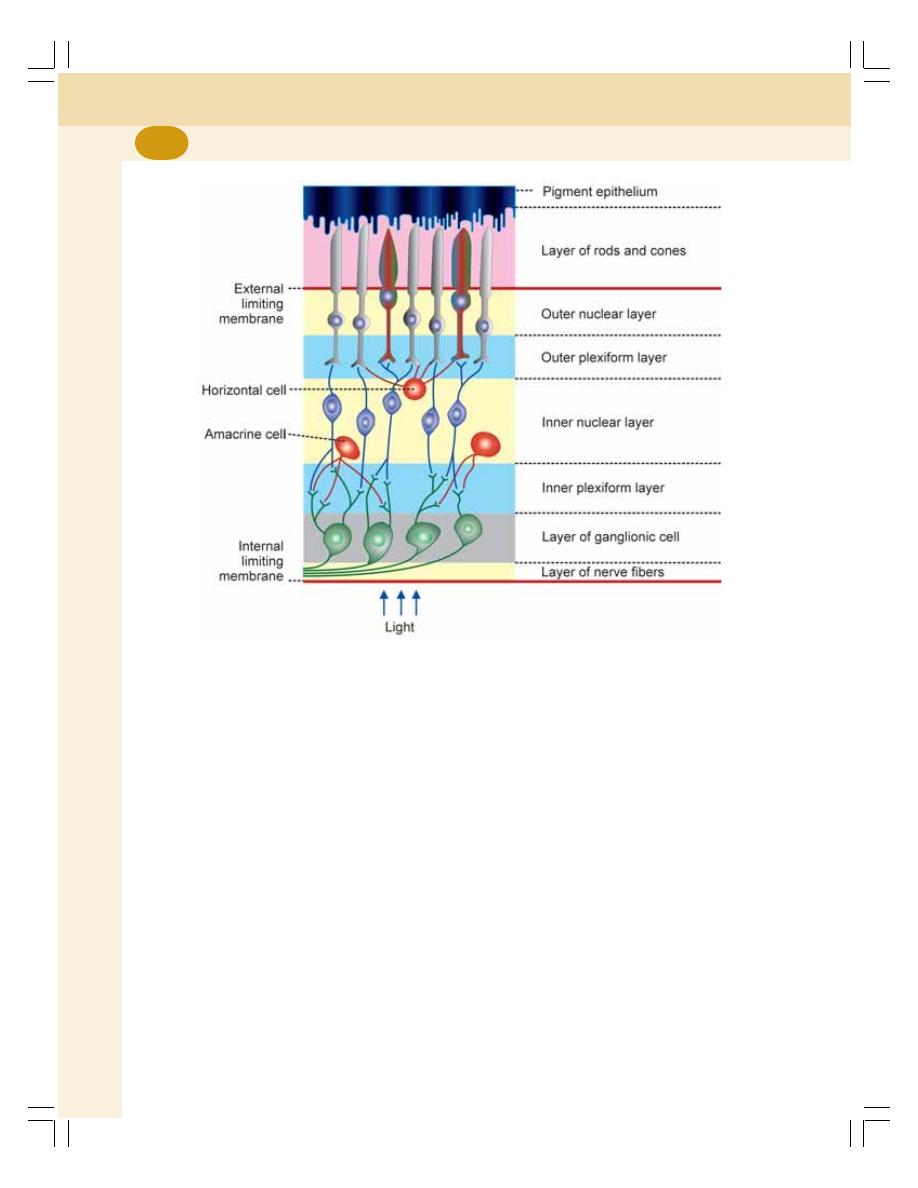
Special Senses
632
i. Constrictor papillae or sphincter pupillae.
Contraction of this muscle causes con-
striction of pupil
ii. Dilator papillae or pupillary dilator muscle.
Contraction of this muscle causes dila-
tation of pupil.
The activities of these muscles of iris increase
or decrease the diameter of the pupil and regu-
late the amount of light entering the eye. Thus,
iris acts like the diaphragm of a camera.
Iris separates the space between cornea and
lens into two chambers namely, the anterior and
posterior chambers. Both the chambers com-
municate with each other through pupil. The
lateral border of anterior chamber is angular in
shape. It is called iris angle or angle of anterior
chamber.
INNER LAYER OR TUNICA INTERNA
OR TUNICA NERVOSA OR RETINA
Retina is the light sensitive membrane that
forms the innermost layer of eyeball. It extends
from the margin of optic disk to just behind the
ciliary body. Here, it ends abruptly as a dentated
border known as ora serrata. Retina has the
receptors of vision. Structurally, retina is made
up of 10 layers (Fig. 104-3).
1. Layer of pigment epithelium
2. Layer of rods and cones
3. External limiting membrane
4. Outer nuclear layer
5. Outer plexiform layer
6. Inner nuclear layer
7. Inner plexiform layer
8. Ganglion cell layer
FIGURE 104-3: Layers of retina

Chapter 104 Structure of the Eye
633
9. Layer of nerve fibers
10. Internal limiting membrane.
1. Layer of Pigment Epithelium
It is the outermost layer situated adjacent to
choroid. It is a single layer of hexagonal epithelial
cells which contain the pigment melanin.
2. Layer of Rods and Cones
This layer lies between pigment epithelial layer
and external limiting membrane. The rods and
cones are the light sensitive portions of the visual
receptor cells, the rod cells and the cone cells.
The receptor cells are arranged in a parallel
fashion and are perpendicular to the inner sur-
face of the eyeball.
3. External Limiting Membrane
It is a thin layer, formed by the chief supporting
elements of retina called the Müller’s fibers.
4. Outer Nuclear Layer
The fibers and granules of rods and cones are
present in this layer. The granules of rods and
cones contain nucleus.
5. Outer Plexiform Layer
This layer contains reticular meshwork formed
by the terminal fibers of rods and cones and the
dendrites from bipolar cells, situated in the inner
nuclear layer.
6. Inner Nuclear Layer
The inner nuclear layer contains small oval sha-
ped flattened bipolar cells. The axons of the
bipolar cells go inside and synapse with
dendrites of ganglionic cells in the inner plexiform
layer. The dendrites synapse with fibers of rods
and cones in the outer plexiform layer. This layer
also contains nuclei of Müller’s supporting fibers
and some association neurons called horizontal
cells and amacrine cells.
7. Inner Plexiform Layer
This layer of retina consists of synapses between
dendrites of ganglionic cells and axons of bipolar
cells.
8. Ganglion Cell Layer
Multipolar cells are present in this layer. The
axons from ganglion cells are in the inner surface
of the retina. These axons form the optic nerve.
The dendrites of the ganglion cells synapse with
axons of bipolar cells in the inner plexiform layer.
9. Layer of Nerve Fibers
It is formed by nonmyelinated axons of
ganglionic cells. After taking origin, the axons
run horizontally to a short distance. Afterwards,
the fibers converge towards the optic disk and
form the optic nerve.
10. Internal Limiting Membrane
It is the inner most layer of retina and it separates
retina from the vitreous body. It a hyaline mem-
brane formed by the opposition of expanded
ends of Müller’s fibers.
FUNDUS OCULI OR FUNDUS
Fundus oculi or fundus is the posterior part of
interior of eyeball (Fig. 104-4). It is examined by
ophthalmoscope. It has two important structures:
1. Optic disk
2. Macula lutea with fovea centralis.
OPTIC DISK – BLIND SPOT
Optic disk is a pale disk situated near the center
of the posterior wall of eyeball. It is formed by
the convergence of axons from ganglion cells,
while forming the optic nerve. The optic disk
contains all the layers of retina except rods and
cones. Therefore, it is insensitive to light, i.e. the
object is not seen if the image falls upon this
area. Because of this, the optic disk is known
as blind spot.
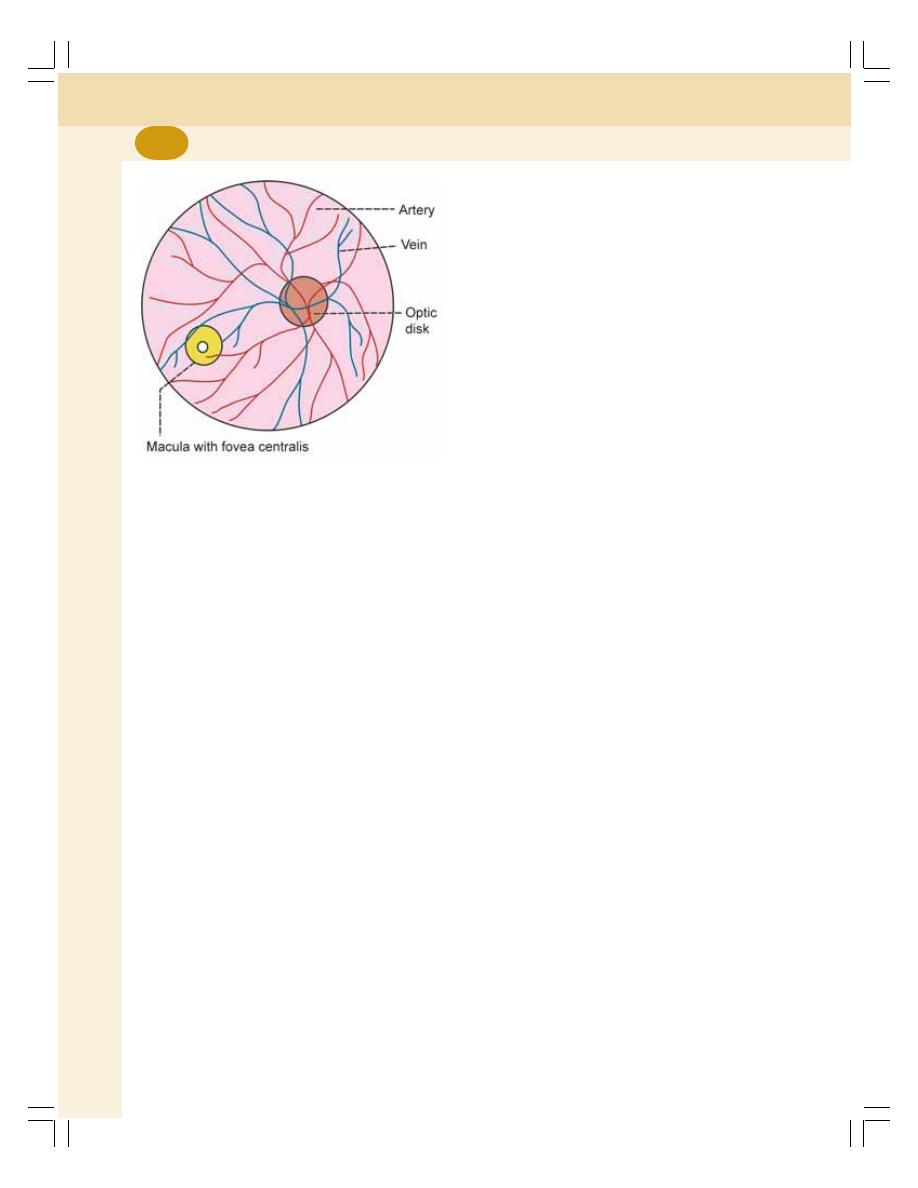
Special Senses
634
MACULA LUTEA
Macula lutea is a small yellowish area situated
lateral to optic disk in retina. It is also called yellow
spot. The yellow color of macula lutea is due to
the presence of a yellow pigment. Macula lutea
has fovea centralis in its center.
Fovea Centralis
Fovea centralis is a minute depression in the
center of macula lutea. The fovea is the region
of the most acute vision because it contains only
cones. When one looks at an object, the eyeballs
are directed towards the object, so that, the
image of that object falls on the fovea of each
eye and the person can see the object very
clearly. It is known as foveal vision.
The vision in other parts of retina is called
peripheral or extrafoveal vision. It is less
sensitive and enables the subject to gain only a
dim and an ill-defined impression of
surroundings.
INTRAOCULAR FLUIDS
Two types of fluids are present in the eye:
1. Vitreous humor
2. Aqueous humor.
VITREOUS HUMOR
Vitreous humor or vitreous body is a viscous fluid
present behind the lens in the space between
the lens and retina. It is a highly viscous and
gelatinous substance. It is formed by a fine
fibrillar network of proteoglycan molecules.
Vitreous humor helps maintain the shape of the
eyeball.
AQUEOUS HUMOR
It is a thin fluid present in front of retina. It fills
the space between the lens and cornea. This
space is divided into anterior and posterior
chambers by iris. Both the chambers
communicate with each other through pupil.
Aqueous humor is formed by ciliary pro-
cesses. After formation, aqueous humor reaches
the posterior chamber. From here it reaches the
anterior chamber via pupil.
From anterior chamber, the aqueous humor
passes through the angle between cornea and
iris, meshwork of trabeculae and canal of
Schlemm and reaches the venous system via
anterior ciliary vein.
Functions of aqueous humor
Aqueous humor:
1. Maintains shape of the eyeball
2. Maintains the intraocular pressure
3. Provides nutrients, oxygen and electrolytes
to the avascular structures like lens and
cornea
4. Removes metabolic end products from lens
and cornea.
INTRAOCULAR PRESSURE
Intraocular pressure is the measure of fluid pre-
ssure in the eye exerted by aqueous humor. The
normal intraocular pressure varies between 12
and 20 mm Hg. It is measured by tonometer.
When intraocular pressure increases to about
60 to 70 mm Hg, glaucoma occurs. Refer Applied
Physiology in this chapter for details.
FIGURE 104-4: Fundus oculi
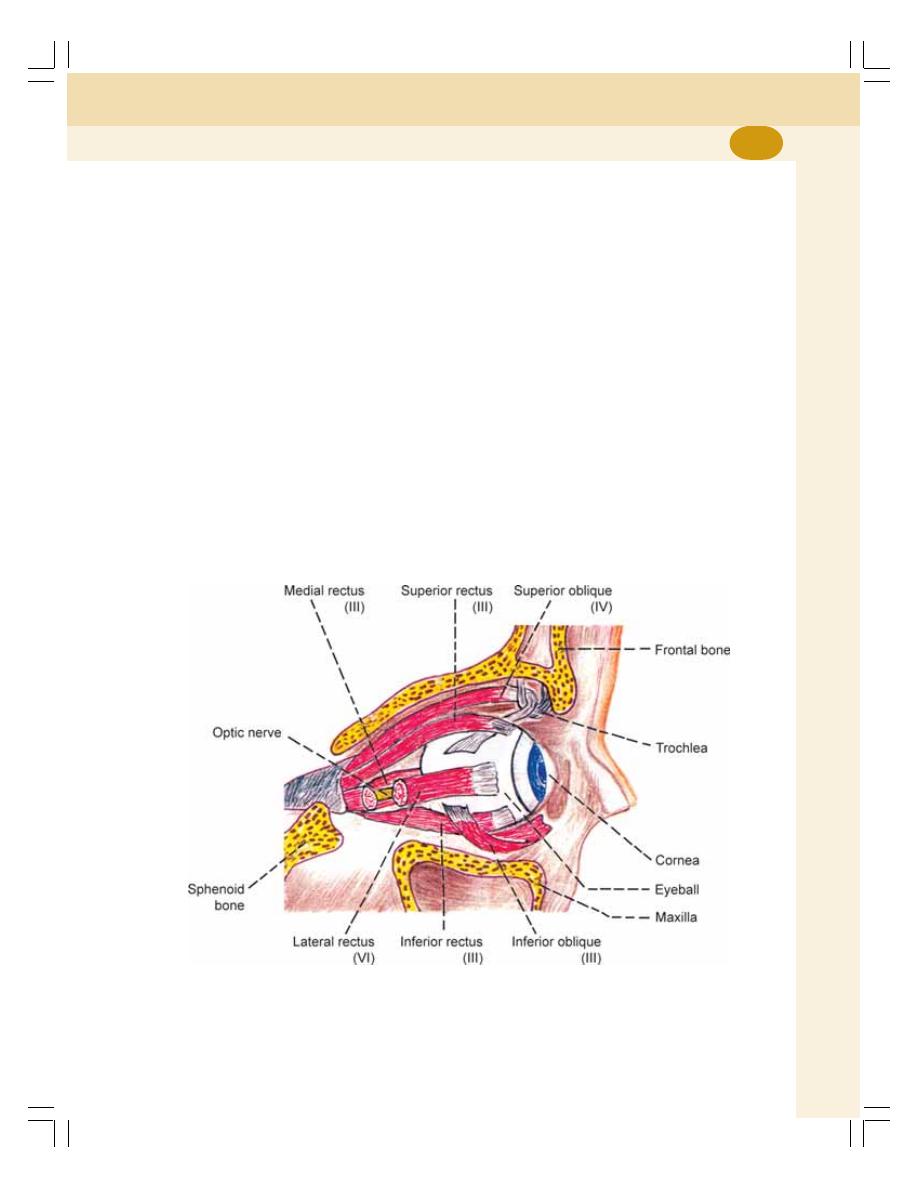
Chapter 104 Structure of the Eye
635
LENS
The lens of the eyeball is crystalline in nature.
It is situated behind the pupil. It is a biconvex,
transparent and elastic structure. It is avascular
and receives its nutrition mainly from the
aqueous humor.
Lens refracts light rays and helps to focus
the image of the objects on retina. The focal
length of human lens is 44 mm and its refractory
power is 23D.
Lens is supported by the suspensory liga-
ments (zonular fibers) which are attached with
ciliary bodies.
CHANGES IN THE LENS DURING
OLD AGE
In old age, the elastic property of lens is decrea-
sed due to the physical changes in lens and its
capsule. It causes presbyopia.
In old age, lens becomes opaque and this
condition is called cataract.
OCULAR MUSCLES
MUSCLES OF THE EYEBALL
The muscles of the eyeball are of two types:
1. Intrinsic muscles
2. Extrinsic muscles.
1. Intrinsic Muscles
The intrinsic muscles are formed by smooth
muscle fibers and are controlled by the
autonomic nerves. The intrinsic muscles of the
eye are constrictor pupillae, dilator pupillae and
ciliary muscle.
2. Extrinsic Muscles
The extrinsic muscles are formed by skeletal
muscle fibers and are controlled by the somatic
FIGURE 104-5: Extrinsic muscles of eyeball. Numbers in
parenthesis indicate the cranial nerve supplying the muscle
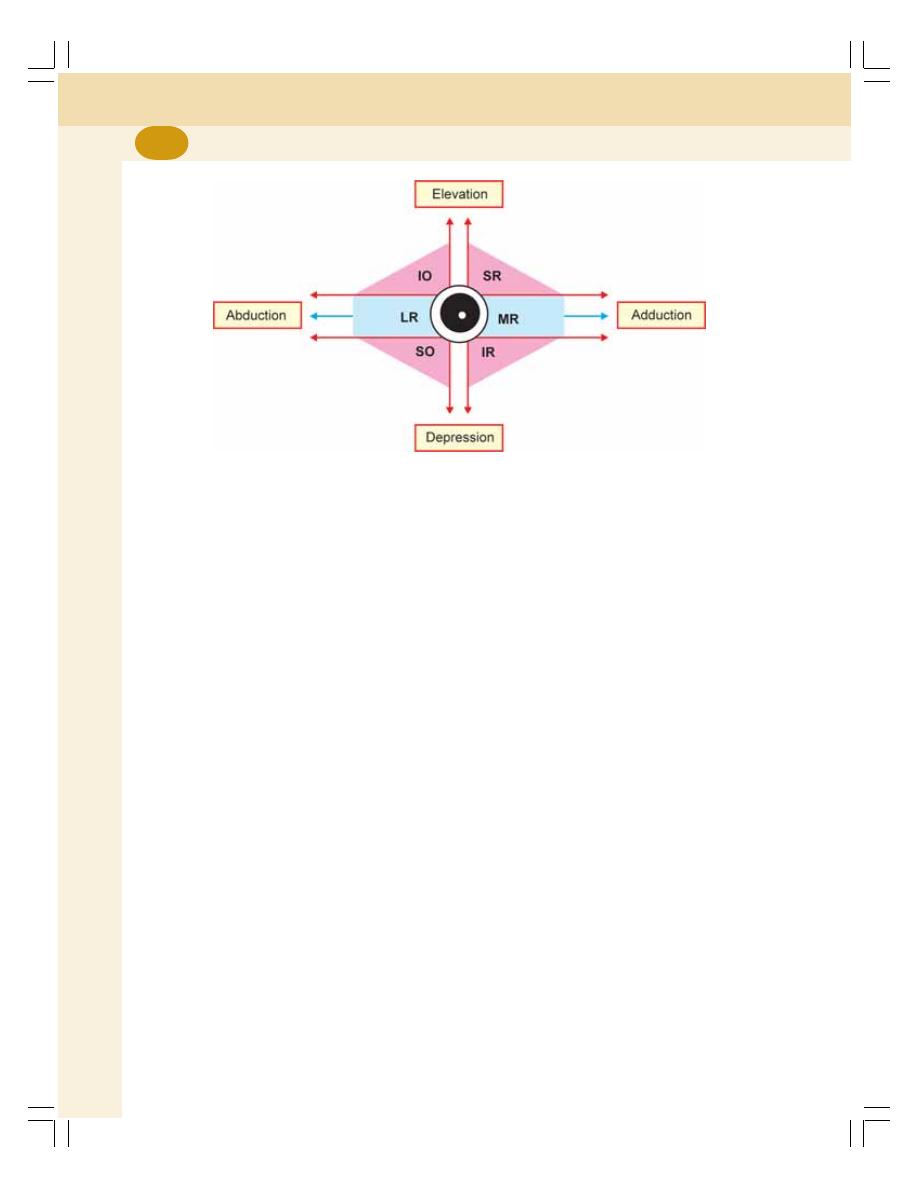
Special Senses
636
nerves. The eyeball moves within the orbit by six
extrinsic skeletal muscles (Fig. 104-5). One end
of each muscle is attached to the eyeball and
the other end to the wall of orbital cavity. There
are four straight muscles (rectus) and two oblique
muscles:
1. Superior rectus
2. Inferior rectus
3. Medial or internal rectus
4. Lateral or external rectus
5. Superior oblique
6. Inferior oblique.
INNERVATION OF OCULAR MUSCLES
Innervation of Intrinsic Muscles
The intrinsic muscles of eyeball are innervated
by both sympathetic and parasympathetic divi-
sions of autonomic nervous system.
Innervation of Extrinsic Muscles
The extrinsic muscles of the eyeball are inner-
vated by somatic motor nerve fibers. The somatic
nerve fibers arise from the cranial nerve nuclei
in brainstem and reach the ocular muscles via
three cranial nerves:
FIGURE 104-6: Diagram showing the movements of right eye. MR = Medial rectus. SO = Superior
oblique. LR = Lateral rectus. IO = Inferior oblique. SR = Superior rectus. IR = Inferior rectus
1. Oculomotor (third) nerve which supplies
superior rectus, inferior rectus, medial rectus
(internal rectus) and Inferior oblique
2. Trochlear (fourth) nerve which supplies the
superior oblique
3. Abducent (sixth) nerve which supplies the
lateral rectus (external rectus).
OCULAR MOVEMENTS
The eyeball moves or rotates within the orbital
socket in any of the three primary axes, vertical
(abduction and adduction), transverse (elevation
and depression) and anteroposterior axis (extor-
sion and intorsion). Refer Fig. 104-6 and Table
104-1 for details.
APPLIED PHYSIOLOGY
GLAUCOMA
Glaucoma is a disease characterized by
increase in intraocular pressure above 60 mmHg
resulting in damage of optic nerve and blindness.
Intraocular pressure increases due to the
blockage in the drainage of aqueous humor.
Chapter 104 Structure of the Eye
637
CATARACT
Cataract is the opacity or cloudiness in the
natural lens of the eye. It is the major cause of
blindness worldwide. When the lens becomes
cloudy, light rays cannot pass through it easily,
and vision is blurred. Cataract develops in old
age after 55 to 60 years.
The lens is situated within the sealed
capsule. The old cells die and accumulate within
the capsule. Over years, the accumulation of
cells is associated with accumulation of fluid and
denaturation of the proteins in the lens fibers
causing cloudiness of lens and blurred image.
TABLE 104-1: Muscles taking part
in ocular movements
Movement
Primary muscle
Secondary
muscle
1. Abduction
Lateral rectus
Superior oblique
Inferior oblique
2. Adduction
Medial rectus
Superior rectus
Inferior rectus
3. Elevation
Superior rectus
Inferior oblique
4. Depression Inferior rectus
Superior oblique
5. Extorsion
Inferior oblique
Inferior rectus
6. Intorsion
Superior oblique
Superior rectus
VISUAL PROCESS
INTRODUCTION
IMAGE FORMING MECHANISM
NEURAL BASIS OF VISUAL PROCESS
CHEMICAL BASIS OF VISUAL PROCESS
ACUITY OF VISION
FIELD OF VISION
DEFINITION
BINOCULAR AND MONOCULAR VISION
DIVISIONS OF VISUAL FIELD
CORRESPONDING RETINAL POINTS
BLIND SPOT
VISUAL FIELD AND RETINA
MAPPING OF VISUAL FIELD
Visual Process and
Field of Vision
105
VISUAL PROCESS
INTRODUCTION
Visual process is the series of actions that take
place during visual perception. When the image
of an object is focused on retina, the energy in
visual spectrum is converted into electrical
potentials (impulses) by rods and cones of retina
through some chemical reactions. The impulses
from rods and cones reach the cerebral cortex
through optic nerve. And, the sensation of vision
is produced in cerebral cortex. Thus, process
of visual sensation is explained on the basis of
image formation, and neural and chemical
phenomena.
IMAGE FORMING MECHANISM
While looking at an object, the light rays from
the object are refracted and brought to a focus
upon retina. The image falls on the retina in an
inverted position and reversed side to side. In
spite of this, the object is seen in an upright
position. It is because of the role played by
cerebral cortex.
The light rays are refracted by the lens and
cornea. The refractory power is measured in
diopter (D). A diopter is the reciprocal of focal
length expressed in meters.
The focal length of cornea is 24 mm and
refractory power is 42D. The focal length of lens
is 44 mm and refractory power is 23D.

Chapter 105 Visual Process and Field of Vision
639
NEURAL BASIS OF VISUAL PROCESS
The retina has the visual receptors which are
also called photoreceptors. The photo receptors
are rods and cones. There are about 6 million
cones and 12 million rods in the human eye. The
distribution of the photoreceptors varies in
different areas of retina. Fovea has only cones
and no rods. While proceeding from fovea
towards the periphery of retina, the rods increase
and the cones decrease in number. At the
periphery of the retina, only rods are present and
cones are absent.
Functions of Rods
Rods are very sensitive to light and have a low
threshold. So, the rods are responsible for dim
light vision or night vision or scotopic vision. But,
rods do not take part in resolving the details and
boundaries of objects (visual acuity) or the color
of the objects (color vision). The vision by rod is
black, white or in the combination of black and
white namely, gray. Therefore, the colored objects
appear faded or grayish in twilight.
Functions of Cones
Cones have high threshold for light stimulus. So,
the cones are sensitive only to bright light.
Therefore, the cone cells are called receptors
of bright light vision or photopic vision or day
light vision. The cones are also responsible for
acuity of vision and the color vision.
CHEMICAL BASIS OF VISUAL PROCESS
Photosensitive pigments present in rods and
cones are concerned with chemical basis of
visual process. The chemical reactions involved
in these pigments lead to the development of
electrical activity in retina and generation of
impulses (action potentials) which are trans-
mitted through optic nerve. The photochemical
changes in the visual receptors are called Wald’s
visual cycle.
Rhodopsin
Rhodopsin is the photosensitive pigment of rod
cells. Rhodopsin is made up of a protein called
opsin and a chromophore. The opsin present in
rhodopsin is known as scotopsin. Chromophore
is a chemical substance that develops color in
the cell. The chromophore present in the rod cells
is called retinal. The retinal is the aldehyde of
vitamin A or retinol.
Photochemical Changes in Rhodopsin
During exposure to light, rhodopsin is bleached
and it is split into retinine and the protein called
opsin through various intermediate photochemi-
cal reactions. The metarhodopsin produced
during these reactions is the activated rhodopsin.
It is responsible for development of receptor
potential in rod cells.
Phototransduction
Visual transduction or phototransduction is the
process by which the light energy is converted
into receptor potential in visual receptors.
The resting membrane potential in other
sensory receptor cells is usually between –70
and –90 mV. However, in the visual receptors
in dark, the negativity is reduced and the resting
membrane potential is about –40 mV. When
light falls on retina, the rhodopsin is converted
into metarhodospsin which causes mild hyper-
polarization which is called receptor potential in
the rod cells.
Thus, the process of receptor potential in
visual receptors is different from that of other
sensory receptors. When other sensory receptors
are excited, the electrical response is in the form
of depolarization. But, in visual receptors, the
response is in the form of hyperpolarization.
Significance of Hyperpolarization
The hyperpolarization in rod cells leads to the
development of response in bipolar cells and
ganglionic cells so that the action potentials are
transmitted to cerebral cortex via optic pathway.
Photosensitive Pigment in Cone Cells
The photosensitive pigment in the cone cells are
porpyropsin, iodopsin and cyanopsin. Only one
of these pigments is present in each cone. Each

Special Senses
640
type of cone pigment is sensitive to a particular
light and the maximum response is shown at a
particular light and wavelength.
The processes involved in phototransduction
in cone cells are similar to those in the rod cells.
Dark Adaptation
Dark adaption is the process by which the
person is able to see the objects in dim light. If
a person enters a dim lighted room (darkroom)
from a bright lighted area, he is blind for some
time, i.e. he cannot see any object. After
some time his eyes get adapted and he starts
seeing the objects slowly. The maximum duration
for dark adaptation is about 20 minutes.
Causes for dark adaptation
1. Resynthesis of rhodopsin: The time required
for dark adaptation is partly determined by
the time to resynthesize rhodopsin. In bright
light, much of the pigment is being bleached
(broken down). But in dim light, it requires
some time for the regeneration of certain
amount of rhodopsin, which is necessary for
optimal rod function.
2. Dilatation of pupil: The dilatation of pupil
during dark adaptation allows more and
more light to enter the eye.
Light Adaptation
Light adaptation is the process in which eyes
get adapted to bright light. When a person enters
a bright lighted area from a dim lighted area,
he feels discomfort due to the dazzling effect
of bright light. After some time, when the eyes
become adapted to light, he sees the objects
around him without any discomfort. It is the mere
disappearance of dark adaptation. The
maximum period for light adaptation is about
5 minutes.
Causes for light adaptation
1. Reduced sensitivity of rods during light
adaptation due to the breakdown of
rhodopsin
2. Constriction of pupil which reduces quantity
of light rays entering the eye.
Night Blindness
Night blindness is defined as the loss of vision
in dim light. It is otherwise called nyctalopia or
defective dim light (scotopic) vision.
Causes of night blindness
It is due to the deficiency of vitamin A, which is
essential for the function of rods. The deficiency
of vitamin A which occurs because of:
1. The diet containing less amount of vitamin A
2. Decreased absorption of vitamin A from the
intestine.
Initially, vitamin A deficiency causes defec-
tive rod function. Prolonged deficiency leads to
anatomical changes in rods and cones, and
finally the degeneration of other retinal layers
occurs. So, retinal function can be restored, only
if treatment is given with vitamin A before the
visual receptors start degenerating.
ACUITY OF VISION
Definition
Acuity of vision is the ability of the eye to
determine the precise shape and details of the
object. It is also called visual acuity. Cones of
the retina are responsible for acuity of vision.
Visual acuity is highly exhibited in fovea centralis,
which contains only cones. It is greatly reduced
during the refractory errors.
Test for Acuity of Vision
Acuity of vision is tested for distant vision as
well as near vision. If there is any difficulty in
seeing the distant object or the near object, the
defect is known as error of refraction. The
refractive errors are described separately in
Chapter 109.
Distant vision
Snellen’s chart is used to test the acuity of vision
for distant vision in the diagnosis of refractive
errors of the eye.
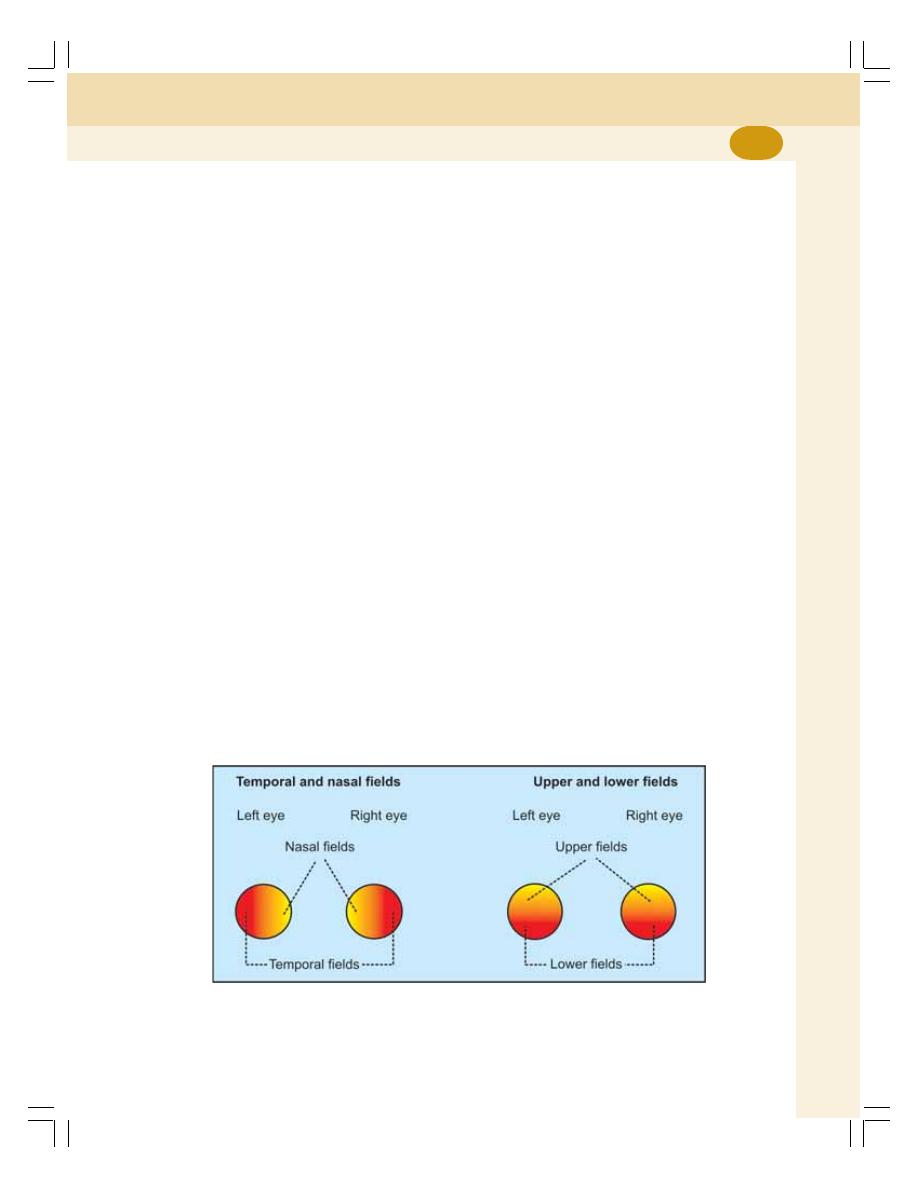
Chapter 105 Visual Process and Field of Vision
641
Near vision
Jaeger’s chart is used to test the visual acuity
for near vision.
FIELD OF VISION
DEFINITION
The part of the external world seen by one eye
when it is fixed in one direction is called field of
vision or visual field of that eye.
BINOCULAR AND MONOCULAR VISION
Binocular Vision
Binocular vision is the vision in which both the
eyes are used together so that a portion of
external world is seen by the eyes together. In
humans and some animals, the eyeballs are
placed in front of the head. So, the visual fields
of both the eyes overlap. Because of this a
portion of the external world is seen by both the
eyes.
Monocular Vision
It is the vision in which each eye is used sepa-
rately. In some animals like dog, rabbit and
horse, the eyeballs are present at the sides of
head. So, the visual fields of both eyes overlap
to a very small extent. Because of this, different
portion of the external world is seen by each eye.
DIVISIONS OF VISUAL FIELD
The visual field of human eye has an angle of
160° in horizontal meridian and 135° in vertical
meridian. The visual filed is divided into four parts:
1. Temporal field
2. Nasal field
3. Upper field
4. Lower field.
Temporal and Nasal Fields
The visual field of each eye is divided into two
unequal parts namely, outer or temporal field
and the inner or nasal field by a vertical line
passing through the fixation point (Fig. 105-1).
The fixation point is the meeting point of visual
axis with the object.
The temporal part of visual field extends up
to about 100° but the nasal part extends only up
to 60° because it is restricted by nose.
Upper and Lower Fields
The visual field of each eye is also divided into
an upper field and a lower field by a horizontal
line passing through the fixation point. The extent
of the upper field is about 60° as it is restricted
by upper eyelid and orbital margin. The extent
of lower field is about 75°. It is restricted by
cheek. Thus, the visual field is restricted in all
the sides except in the temporal part.
FIGURE 105-1: Divisions of visual field

Special Senses
642
CORRESPONDING RETINAL POINTS
Corresponding retinal points are the area in
retina of both eyes on which the light rays from
the object falls. It occurs in the binocular vision.
The two images developed on retina of both eyes
are fused into a single sensation. So, we see
the objects with single image.
Diplopia
Diplopia means double vision. Normal single
sensation is because of the ocular muscles,
which direct the axes of the eyes in such a way,
that the light rays from the object fall upon the
corresponding points of both retinas. If the light
rays do not fall on the corresponding retinal
points, diplopia occurs.
BLIND SPOT
Blind spot is the small area of retina where visual
receptors are absent. The optic disk in the retina
does not have any visual receptors and, if the
image of any object falls on the optic disk, the
object cannot be seen. So this part of the retina
is blind hence the name blind spot.
Normally, the darkness in the visual field due
to the blind spot does not cause any incon-
venience because, the fixation of each eye is at
different angles. Even when one eye is closed
or blind, the person is not aware of blind spot.
However, one can recognize blind spot by some
experimental procedures.
VISUAL FIELD AND RETINA
The light rays from different halves of each visual
field do not fall on the same halves of the retina.
The light rays from temporal part of visual field
of an eye fall on the nasal half of retina of that
eye. Similarly, the light rays from nasal part of
visual field fall on the temporal half of retina of
the same side.
MAPPING OF VISUAL FIELD
The shape and extent of visual field is mapped
out by means of an instrument called perimeter.
The visual field is also determined by Bjerrum
screen or by confrontation test.
INTRODUCTION
VISUAL RECEPTORS
FIRST ORDER NEURONS
SECOND ORDER NEURONS
THIRD ORDER NEURONS
COURSE OF VISUAL PATHWAY
APPLIED PHYSIOLOGY
Visual Pathway
106
INTRODUCTION
Visual pathway or optic pathway is the nervous
pathway that carries the retinal impulses to
cerebral cortex. In binocular vision, the light rays
from temporal (outer) half of visual field (Chapter
105) fall upon the nasal part of corresponding
retina. Light rays from nasal (inner) half of visual
field fall upon the temporal part of retina.
VISUAL RECEPTORS
Rods and cones, which are present in the retina
of eye, form the visual receptors. Fibers from
the visual receptors synapse with dendrites of
bipolar cells of inner nuclear layer of retina.
FIRST ORDER NEURONS
First order neurons (primary neurons) are bipolar
cells in the retina. Axons from the bipolar cells
synapse with dendrites of ganglionic cells.
SECOND ORDER NEURONS
Second order neurons (secondary neurons) are
the ganglionic cells in ganglionic cell layer of
retina. The axons of the ganglionic cells form
optic nerve. The optic nerve leaves the eye and
terminates in lateral geniculate body.
THIRD ORDER NEURONS
The third order neurons are in the lateral geni-
culate body. Fibers arising from here reach the
visual cortex.
COURSE OF VISUAL PATHWAY
The visual pathway consists of six components:
1. Optic nerve
2. Optic chiasma
3. Optic tract
4. Lateral geniculate body
5. Optic radiation
6. Visual cortex.
1. OPTIC NERVE
It is formed by the axons of ganglionic cells
(Fig. 106-1). Optic nerve leaves the eye through
optic disk. The fibers from temporal part of retina
are in lateral part of the nerve and carry the
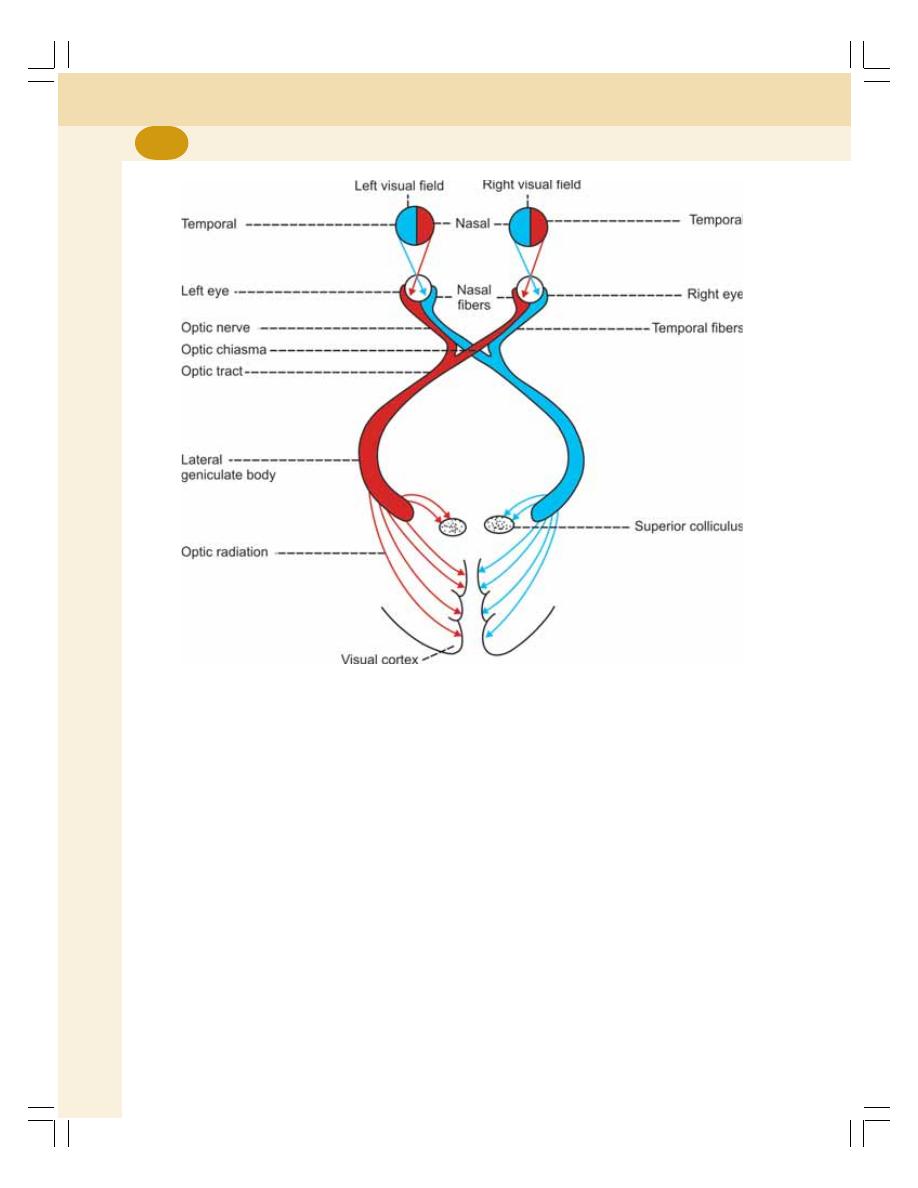
Special Senses
644
impulses from nasal half of visual field of same
eye. The fibers from nasal part of retina are in
medial part of the nerve and carry the impulses
from temporal half of visual field of same eye.
2. OPTIC CHIASMA
The medial fibers of each optic nerve cross the
midline and join the uncrossed lateral fibers of
opposite side to form the optic tract (Fig. 106-1).
The area of crossing of the optic nerve fibers is
called optic chiasma.
3. OPTIC TRACT
It is formed by uncrossed fibers of optic nerve
on the same side and crossed fibers of optic
nerve from the opposite side. All the fibers of
optic tract run backward and outward and
terminate in the lateral geniculate body in
thalamus. Few fibers just pass through medial
geniculate body and run towards superior
colliculus in midbrain.
Due to crossing of medial fibers in optic
chiasma, the left optic tract carries impulses from
temporal part of left retina and nasal part of right
retina, i.e. it is responsible for vision in nasal
half of left visual field and temporal half of right
visual field. The right optic tract contains fibers
from nasal half of left retina and temporal half
of right retina. It is responsible for vision in
temporal half of left visual field and nasal half
of right visual field.
4. LATERAL GENICULATE BODY
Majority of the fibers of optic tract terminate in
lateral geniculate body, which forms the sub-
cortical center for visual sensation. From here,
FIGURE 106-1: Visual pathway
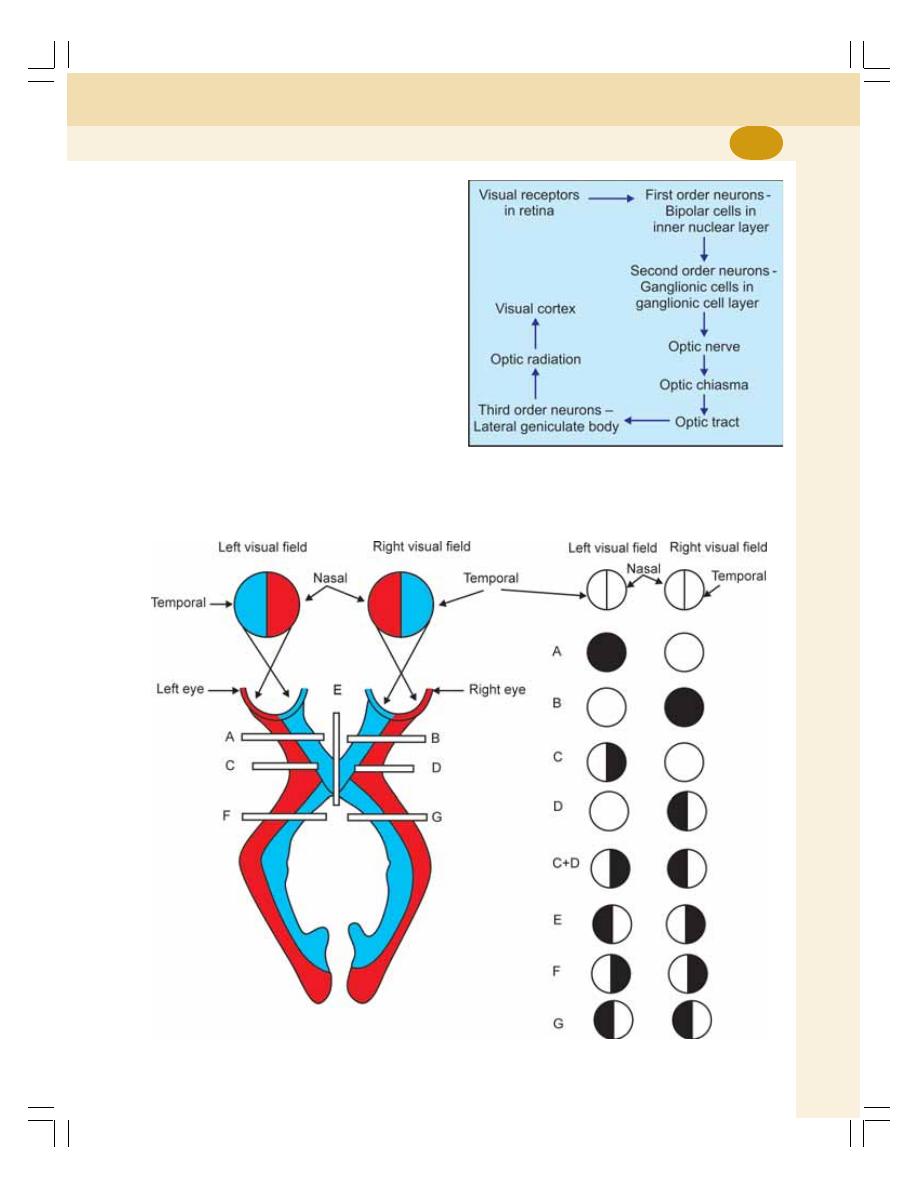
Chapter 106 Visual Pathway
645
the geniculocalcarine tract or optic radiation ari-
ses. This tract is the last relay of visual pathway.
Some of the fibers from optic tract do not
synapse in lateral geniculate body but, pass
through it and terminate in one of the following
centers:
i. The superior colliculus which is concer-
ned with reflex movements of eyeballs
and head in response to optic stimulus
ii. Pretectal nucleus which is concerned with
light reflexes
iii. Supraoptic nucleus of hypothalamus
which is concerned with the retinal control
of pituitary.
5. OPTIC RADIATION
Fibers from lateral geniculate body pass through
internal capsule and form optic radiation. Optic
radiation ends in visual cortex (Fig. 106-2).
FIGURE 106-3: Effects of lesions of optic pathway. Dark shade in circles indicates blindness
FIGURE 106-2: Schematic representation
of visual pathway

Special Senses
646
6. VISUAL CORTEX
The primary cortical center for vision is called
visual cortex that is located on the medial sur-
face of occipital lobe. It forms the walls and lips
of calcarine fissure in medial surface of occipital
lobe.
There is definite localization of retinal pro-
jections upon visual cortex. In fact, the point to
point projection of retina upon visual cortex is
well established. The peripheral retinal represen-
tation occupies the anterior part of visual
cortex. The macular representation occupies the
posterior part of visual cortex near the occipital
pole.
Areas of Visual Cortex and their Function
Three areas are present in visual cortex:
i. Primary visual area (area 17) which is
concerned with perception of visual impul-
ses
ii. Visual association area (area 18) which
is concerned with interpretation of visual
impulses
iii. Occipital eye field (area 19) which is
concerned with movement of eyes.
APPLIED PHYSIOLOGY
The injury to any part of optic pathway causes
visual defect and the nature of defect depends
upon the location and extent of injury. The loss
of vision in one visual field is known as anopia.
Loss of vision in one half of visual field is called
hemianopia (Fig. 106-3).
Hemianopia is classified into two types:
1. Homonymous hemianopia: Loss of vision in
the same halves of both visual fields
2. Heteronymous hemianopia: Loss of vision in
opposite halves of visual field.
EFFECT OF LESION AT DIFFERENT
LEVELS OF VISUAL PATHWAY
1. Lesion of left optic nerve – total blindness
(anopia) of left eye (Fig. 106-3: A)
2. Lesion of right optic nerve – total blindness
(anopia) of right eye (Fig. 106-3: B)
3. Lesion of lateral fibers in left side of optic
chiasma – left nasal hemianopia
(Fig. 106-3: C)
4. Lesion of lateral fibers in right side of optic
chiasma – right nasal hemianopia
(Fig. 106-3: D)
5. Lesion of lateral fibers in both sides of optic
chiasma – binasal hemianopia (Fig. 106-3:
C + D)
6. Lesion of medial fibers in optic chiasma –
bitemporal hemianopia (Fig. 106-3: E)
7. Lesion of left optic radiation – right homo-
nymous hemianopia (Fig. 106-3: F)
8. Lesion of right optic radiation – left homo-
nymous hemianopia (Fig. 106-3: G).
INTRODUCTION
LIGHT REFLEX
DIRECT LIGHT REFLEX
INDIRECT LIGHT REFLEX
PATHWAY FOR LIGHT REFLEX
CILIOSPINAL REFLEX
ACCOMMODATION
DEFINITION
MECHANISM OF ACCOMMODATION
ACCOMMODATION REFLEX
PATHWAY FOR ACCOMMODATION REFLEX
APPLIED PHYSIOLOGY – PRESBYOPIA
Pupillary Reflexes
107
INTRODUCTION
Pupillary reflexes are the visceral reflexes, which
alter the size of pupil. Pupillary reflexes are
classified into three types:
1. Light reflex
2. Ciliospinal reflex
3. Accommodation reflex.
LIGHT REFLEX
It is the reflex in which the pupil constricts when
light is flashed into the eyes. It is also called
pupillary light reflex. Light reflex is of two types:
DIRECT LIGHT REFLEX
Direct light reflex or direct pupillary light reflex
is constriction of pupil in an eye when light is
thrown into that eye.
INDIRECT LIGHT REFLEX
Indirect light reflex or consensual light reflex is
constriction of pupil in both eyes when light is
thrown into one eye.
PATHWAY FOR LIGHT REFLEX
Afferent Pathway
The pathway for light reflex is slightly deviated
from visual pathway. When light falls on the eye,
the visual receptors are stimulated. The afferent
(sensory) impulses from the receptors pass
through the optic nerve, optic chiasma and optic
tract. At the midbrain level, few fibers get sepa-
rated from the optic tract and synapse on the
neurons of pretectal nucleus, which lies close
to the superior colliculus.
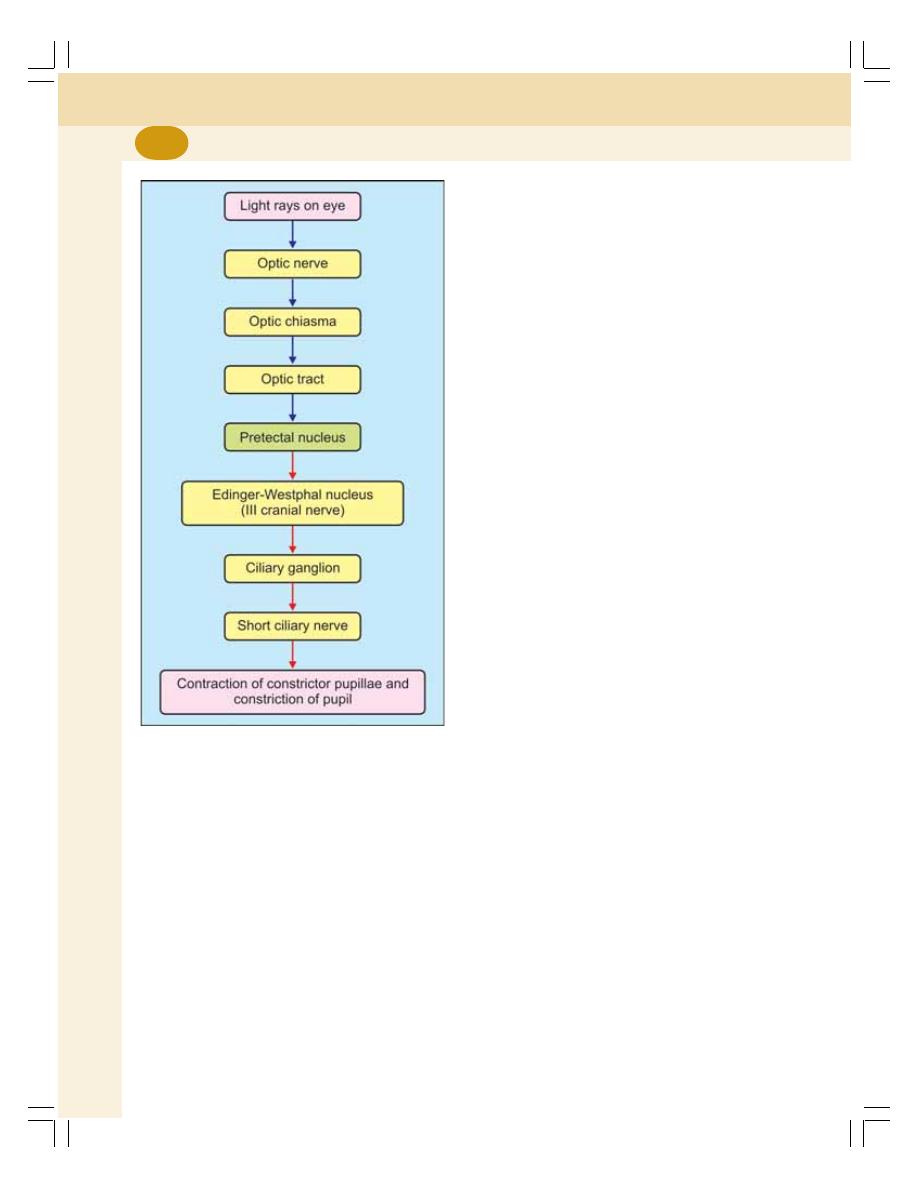
Special Senses
648
Center
The pretectal nucleus of midbrain forms the
center for light reflexes.
Efferent Pathway
The efferent (motor) impulses from this nucleus
are carried by short fibers to Edinger-Westphal
nucleus (parasympathetic nucleus) of
oculomotor nerve (third cranial nerve) in
midbrain. From Edinger-Westphal nucleus, the
preganglionic fibers pass through oculomotor
nerve and reach the ciliary ganglion. The
postganglionic fibers arising from the ciliary
ganglion pass through the short ciliary nerves
and reach the eyeball. These fibers cause
contraction of constrictor pupillae muscle of iris
(Fig. 107-1) resulting in constriction of pupil.
CILIOSPINAL REFLEX
Ciliospinal reflex is the dilatation of pupil in eyes
caused by painful stimulation of skin over the
neck. It is due to the contraction of dilator
pupillae muscle. Sensory impulses pass through
cutaneous afferent nerve. The center is in first
thoracic spinal segment. The efferent impulses
pass through sympathetic fibers and reach
dilator pupillae.
ACCOMMODATION
DEFINITION
Accommodation is the adjustment of the eye to
see either near or distant objects clearly. It is the
process, by which light rays from near objects
or distant objects are brought to a focus on the
sensitive part of retina. It is achieved by various
adjustments made in the eyeball.
MECHANISM OF ACCOMMODATION
Light rays from distant objects are approximately
parallel and are less refracted while getting
focused on retina. But, the light rays from near
objects are divergent. So, to be focused on
retina, these light rays should be refracted
(converged) to a greater extent.
Accommodation in near vision occurs by
means of three adjustments made in the eye-
balls:
1. Increase in anterior curvature of the lens so
that the refractory power of lens is increased
2. Convergence of both eyeballs which brings
the retinal images on to the corresponding
points
3. Constriction of pupil that causes:
i. Increase in the visual acuity
ii. Reduction in the quantity of light entering
eye
iii. Increase in the depth of focus through
more central part of lens as its convexity
is increased.
FIGURE 107-1: Pathway for light reflex
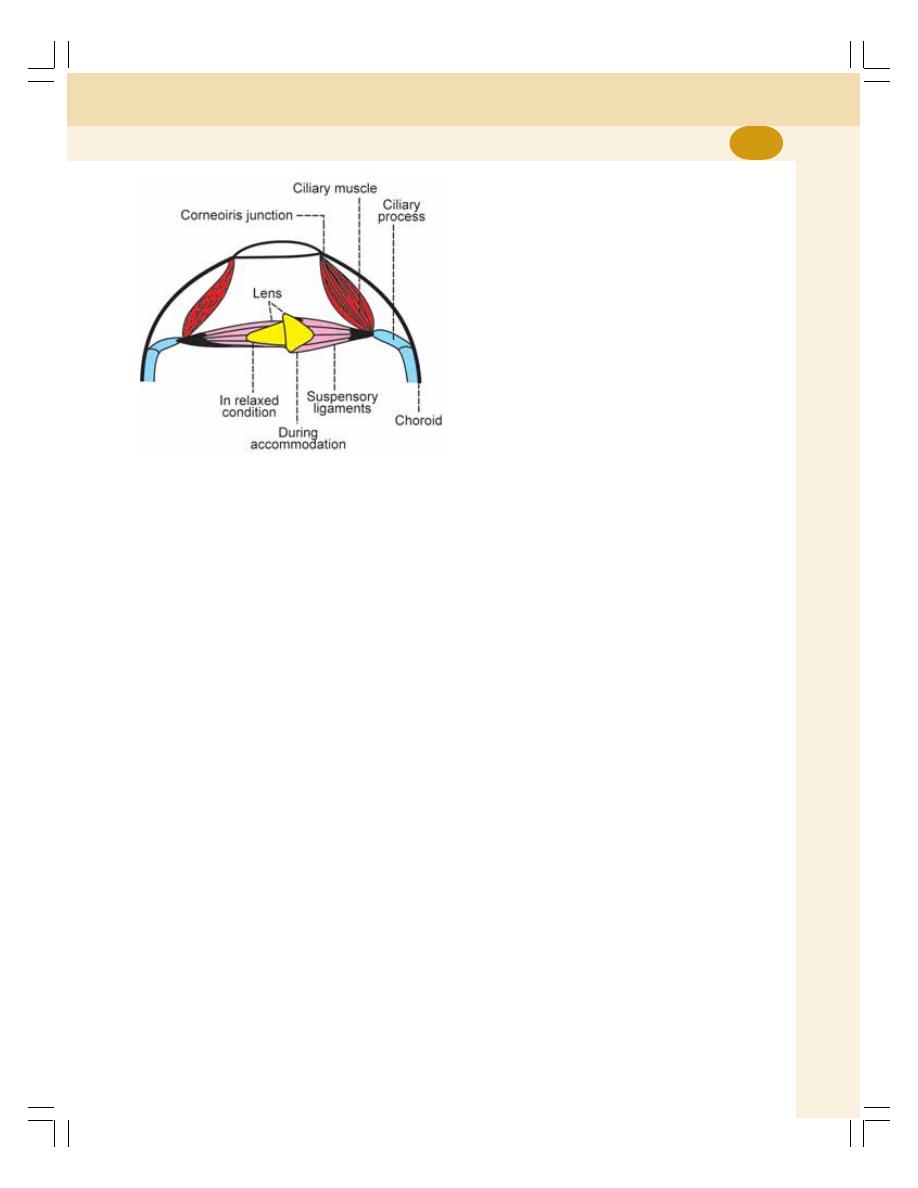
Chapter 107 Pupillary Reflexes
649
Young-Helmholtz Theory
This theory describes how the curvature of lens
increases and thereby, the refractive power of
lens is enhanced. When the eyes are fixed on
a distant object (distant vision) lens is flat due
to the traction of suspensory ligaments which
extend from the capsule of lens and are attached
to the ciliary processes. The ciliary processes
are attached to choroid through the ciliary muscle
(Fig. 107-2).
When the vision is shifted from the dis-
tant object to a near object (near vision), ciliary
muscle contracts and draws the choroid forward.
The ciliary processes are brought closer to lens.
The suspensory ligaments are slackened. Now,
the tension on the lens is released. The lens,
due to its elastic property, bulges forward. The
anterior curvature (convexity) of lens increases
greatly. A very little change occurs in posterior
curvature.
In resting eye, the intraocular pressure sets
up tension in choroids and pulls the ciliary pro-
cesses backward and outward. The suspensory
ligaments are tensed up and the lens becomes
flat.
FIGURE 107-2: Accommodation
ACCOMMODATION REFLEX
Accommodation is a reflex action. When a per-
son looks at a near object after seeing a far
object, three adjustments are made in the eye-
balls:
1. Convergence of the eyeballs due to contrac-
tion of the medial recti
2. Constriction of the pupil due to the contraction
of constrictor pupillae of iris
3. Increase in the anterior curvature of the lens
due to contraction of the ciliary muscle.
Thus, the accommodation reflex involves
both skeletal muscle (medial recti) and smooth
muscle (ciliary muscle and sphincter pupillae).
PATHWAY FOR ACCOMMODATION
REFLEX
Afferent Pathway
Visual impulses from retina pass through the
optic nerve, optic chiasma, optic tract, lateral
geniculate body and optic radiation to visual
cortex (area 17) of occipital lobe. From here, the
association fibers carry the impulses to frontal
lobe (Fig. 107-3).
Center
The center for accommodation lies in frontal eye
field (area 8) that is situated in the frontal lobe
of cerebral cortex.
Efferent Pathway
1. Efferent fibers to ciliary muscle and sphincter
pupillae: From area 8, the corticonuclear
fibers pass via internal capsule to the
Edinger-Westphal nucleus of III cranial nerve.
From here, the preganglionic fibers pass
through the third cranial nerve to the ciliary
ganglion. The postganglionic fibers from
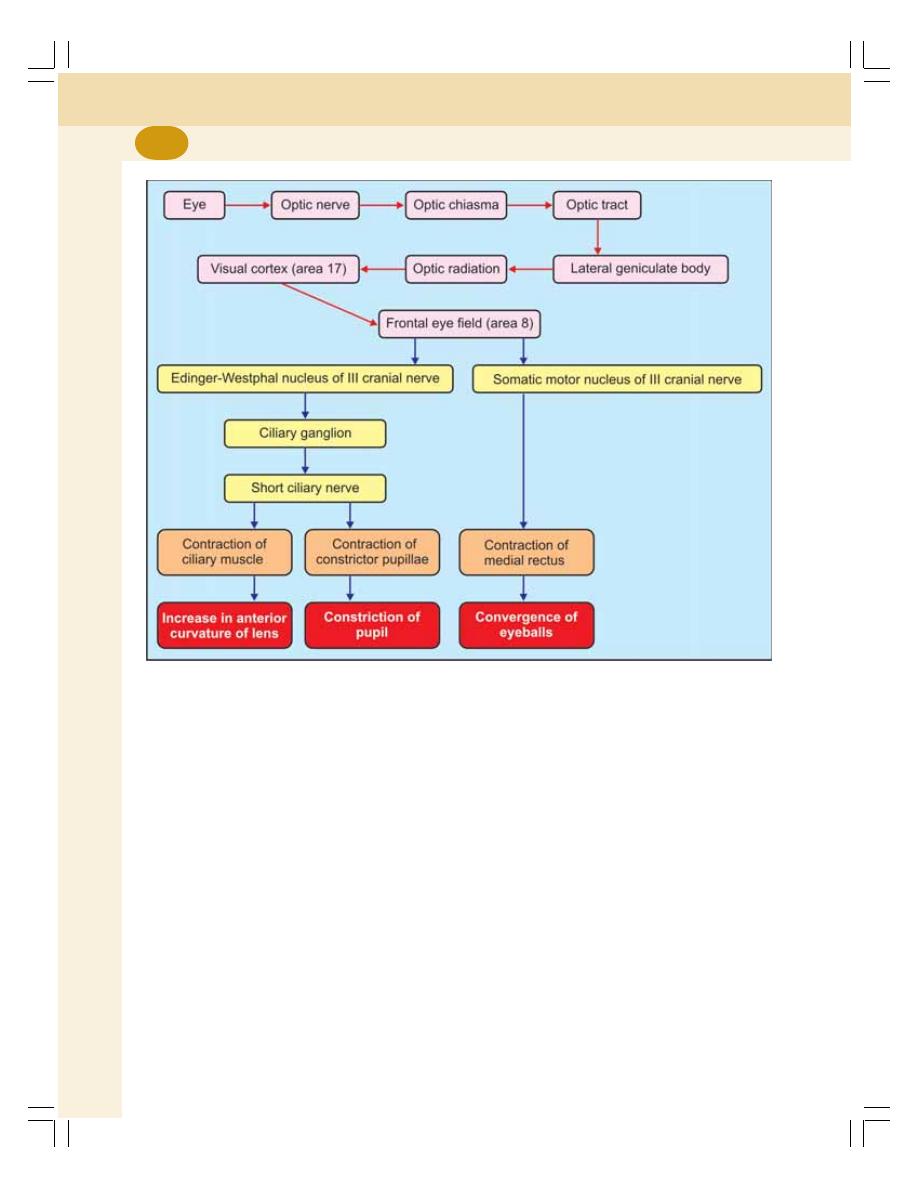
Special Senses
650
ciliary ganglion pass via the short ciliary
nerves and supply the ciliary muscle and the
constrictor pupillae.
2. Efferent fibers to medial rectus: Some of the
fibers from frontal eye field terminate in the
somatic motor nucleus of oculomotor nerve.
The fibers from the motor nucleus supply the
medial rectus.
FIGURE 107-3: Pathway for accommodation reflex
APPLIED PHYSIOLOGY –
PRESBYOPIA
In old age, the amplitude of accommodation is
decreased and the near point is away from the
eye. So the near objects cannot be seen clearly.
This condition is called presbyopia. Details are
given in Chapter 109.
INTRODUCTION
VISIBLE SPECTRUM AND SPECTRAL COLORS
CONES AND COLOR VISION – YOUNG-HELMOLTZ TRICHROMATIC
THEORY
APPLIED PHYSIOLOGY – COLOR BLINDNESS
Color Vision
108
INTRODUCTION
The human eye can recognize about 150
different colors in the visible spectrum. The dis-
crimination and appreciation of colors depend
upon the ability of cones in retina.
VISIBLE SPECTRUM AND SPECTRAL
COLORS
SPECTRAL COLORS
When the sunlight or white light is passed
through a glass prism, it is separated into
different colors. The series of colored light
produced by the prism is called the visible
spectrum and the colors that form the spectrum
are called the spectral colors. The spectral colors
are red, orange, yellow, green, blue, indigo and
violet (ROYGBIV or VIBGYOR). In the spectrum,
the colors occupy the position according to their
wavelengths. Wavelength is the distance
between two identical points in the wave of light
energy. Accordingly, red has got the maximum
wavelength of about 8,000 Å and the violet has
got the minimum wavelength of about 3,000 Å.
The light rays longer than the red are called
infrared rays or the heat waves and the rays
shorter than violet are called the ultraviolet rays.
But, these two extraordinary types of rays do
not evoke the sensation of vision.
EXTRASPECTRAL COLORS
Extraspectral colors are the colors other than
those present in visible spectrum. These colors
are formed by the combination of two or more
spectral colors. For example, purple is the
combination of violet and red. Pink is the combi-
nation of red and white.
PRIMARY COLORS
The primary colors are those, which when com-
bined together produce the white. The primary
colors are red, green and blue. These three
colors in equal proportion give white.
COMPLEMENTARY COLORS
Complementary colors are the pair of two colors
which produce white when mixed or combined

Special Senses
652
in proper proportion. Examples of comple-
mentary colors are red and greenish blue;
orange and cyan blue; yellow and indigo blue;
violet and greenish yellow; and purple and green.
CONES AND COLOR VISION –
YOUNG-HELMHOLTZ
TRICHROMATIC THEORY
According to Young- Helmholtz theory, retina has
three types of cones and each cone is supplied
by a separate fiber of optic nerve. Each cone
has its own photosensitive pigment and gives
response to one of the primary colors namely,
red, green and blue. The different color sensa-
tions are produced by the stimulation of various
combinations of the three types of cones. White
is perceived by equal stimulation of all three
types of cones.
APPLIED PHYSIOLOGY – COLOR
BLINDNESS
Color blindness is the failure to appreciate one
or more colors. It is common in 8% of males
and only in 0.4% of females, as mostly the color
blindness is an inherited sex linked recessive
character. In addition to hereditary conditions,
color blindness occurs due to acquired
conditions also such as ocular diseases or injury
or disease of retina.
CLASSIFICATION OF COLOR
BLINDNESS
Based on Young-Helmholtz trichromatic theory,
color blindness is classified into three types.
1. Monochromatism
2. Dichromatism
3. Trichromatism.
1. Monochromatism
Monochromatism is the condition characterized
by total inability to perceive color. It is also called
total color blindness or achromatopsia. Mono-
chromatism is very rare. The persons with
monochromatism are called monochromats. The
retina of monochromats is totally insensitive to
color and they see the whole spectrum in only
black, white and different shades of gray. So,
their vision is similar to black and white
photography.
2. Dichromatism
Dichromatism is the color blindness in which the
subject can appreciate only two colors. Persons
with this defect are called dichromats. They can
match the entire spectrum of colors by only two
primary colors because the receptors for third
color are defective. The defects are classified
into three groups:
i. Protanopia
Protanopia is the type of dichromatism caused
by the defect in the receptor of first primary color,
i.e. red. So, the red color cannot be appreciated.
The persons having protanopia are called pro-
tanopes. They use blue and green to match the
colors. Thus, they confuse red with green.
ii. Deuteranopia
It is the dichromatism caused due to the defect
in the receptor of the second primary color, i.e.
green. Deuteranopes use blue and red colors and
they cannot appreciate green color.
iii. Tritanopia
It is the dichromatism caused due to the defect
in the receptor of third primary color, i.e. blue.
Tritanopes use red and green colors and they
cannot appreciate blue color.
3. Trichromatism
Trichromatism is the color blindness in which
the intensity of one of the primary colors cannot
be appreciated correctly though the affected
persons are able to perceive all the three colors.
The persons with this defect are called
trichromats. Even the dark shades of one
particular color look dull for them. Trichromatism
is classified into three types:

Chapter 108 Color Vision
653
i. Protanomaly
Protanomaly is the type of trichromatism in which
the perception for red is weak. So to appreciate
the red color, the person requires more intensity
of red than a normal person.
ii. Deuteranomaly
Deuteranomaly is the trichromatism in which
the perception for green is weak.
iii. Tritanomaly
It is the trichromatism with weak perception for
blue.
TESTS FOR COLOR BLINDNESS
Color blindness is determined by using:
1. Ishihara’s color charts
2. Colored wool
3. Edridge-Green lantern
EMETROPIA AND AMETROPIA
MYOPIA OR SHORT SIGHTEDNESS
HYPERMETROPIA OR LONG SIGHTEDNESS
ANISOMETROPIA
ASTIGMATISM
PRESBYOPIA
Errors of Refraction
109
EMETROPOIA AND AMETROPIA
Emmetropia is the vision with lens having normal
refractive power and eye is called emmetropic
eye. Any deviation in the refractive power from
normal condition which leads to inadequate
focusing on retina is called ametropia and the
eye is called ametropic eye. The defect is due
to the change in shape of the eyeball.
Ametropia is of two types:
1. Myopia
2. Hypermetropia.
MYOPIA OR SHORT SIGHTEDNESS
Myopia is the eye defect characterized by the
inability to see the distant object. It is otherwise
called short sightedness because the person can
see near objects clearly but not the distant
objects (Fig. 109-1 and Table 109-1).
Cause
In myopia, the refractive power of the lens is
usually normal. But, the anteroposterior diameter
of the eyeball is abnormally long. Therefore, the
image is brought to a focus a little in front of
retina. In other words, the refractory power of
lens is too strong for the length of eyeball. The
light rays, after coming to a focus, disperse again
so, a blurred image is formed upon retina.
Correction
In myopic eye, in order to form a clear image
on the retina, the light rays entering the eye must
be divergent and not parallel. Thus, the myopic
eye is corrected by using biconcave lens. The
light rays are diverged by the concave lens
before entering the eye (Fig. 109-1).
HYPERMETROPIA OR LONG
SIGHTEDNESS
Hypermetropia is the eye defect characterized
by the inability to see the near object. It is other-
wise known as long sightedness because the
person can see the distant objects clearly but
not the near objects. It is also called hyperopia.
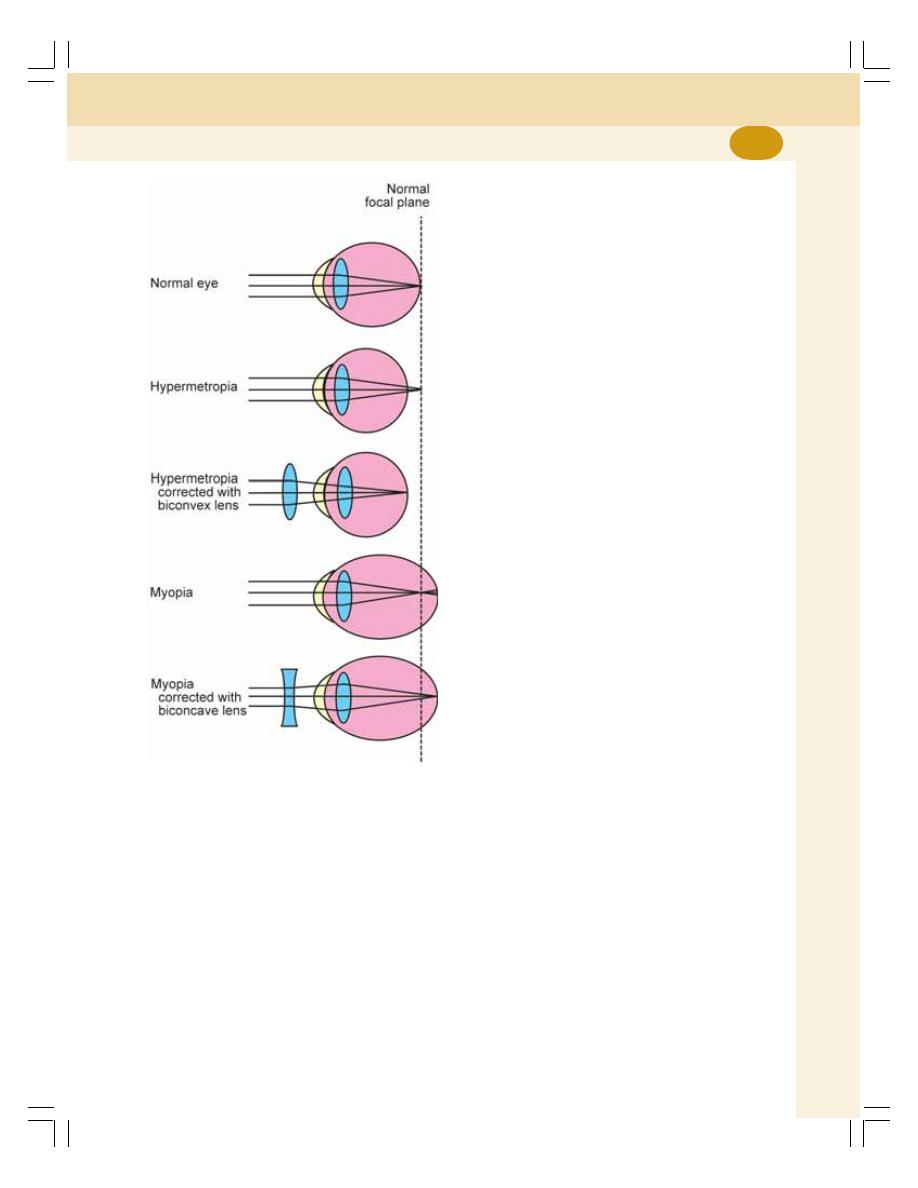
Chapter 109 Errors of Refraction
655
FIGURE 109-1: Errors of refraction
Cause
Hypermetropia is due to decreased anteropos-
terior diameter of the eyeball. So, even though
the refractive power of the lens is normal, the
light rays are not converged enough to form a
clear image on retina, i.e. the light rays are
brought to a focus behind retina. It causes a
blurred image of near objects. Hypermetropia
occurs in childhood, if the eyeballs fail to develop
to the correct size. It is common in old age also.
Correction
Hypermetropia is corrected by using biconvex
lens. The light rays are converged by convex
lens before entering the eye (Fig. 109-1).
ANISOMETROPIA
Anisometropia is the condition in which the two
eyes have unequal refractive power. It is
corrected by using different appropriate lens for
each eye (Table 109-1).
ASTIGMATISM
Astigmatism is the condition in which the light
rays are not brought to a sharp point upon retina.
It is the common optical defect present in all
eyes. When it is moderate, it is known as
physiological astigmatism. When it is well
marked, it is considered abnormal. For example,
the stars appear as small dots of light to a person
with normal eye. But in astigmatism, the stars
appear as radiating short lines of light (A = not;
stigma = point).
CAUSE OF ASTIGMATISM
The light rays pass through all meridians of a
lens. In a normal eye, lens has approximately
same curvature in all meridians. So, the light rays
are refracted almost equally in all meridians and
brought to a focus.
If the curvature is different in different
meridians viz. vertical, horizontal and oblique,
the refractive power is also different in different
meridians. The meridian with greater curvature
refracts the light rays more strongly than the
other meridians. So, these light rays are brought
to a focus in front of the light rays, which pass
through other meridians. Such irregularity of
curvature of lens causes astigmatism.
TYPES OF ASTIGMATISM
Astigmatism is of two types.
1. Regular astigmatism
2. Irregular astigmatism.
Special Senses
656
focus on near objects with age. It is due to the
gradual reduction in the amplitude of accommo-
dation. Presbyopia starts developing after middle
age and progresses as the age advances (pres-
byos = old; ops = eye). In presbyopia, the distant
vision is unaffected. Only the near vision is
affected. The near point is away from eye. In
presbyopia, the anterior curvature of lens does
not increase during near vision. So, the light rays
from near objects are not brought to a focus on
retina.
CAUSES OF PRESBYOPIA
1. Decreased elasticity of lens is because of the
physical changes in lens and its capsule
during old age. So, the anterior curvature is
not increased during near vision.
2. Decreased convergence of eyeballs due to
the concomitant weakness of ocular muscles
in old age.
CORRECTION OF PRESBYOPIA
Presbyopia is corrected by using biconvex lens.
TABLE 109-1: Errors of refraction
Type of error
Cause
Correction
Myopia
Increase in anteroposterior diameter of the eyeball
Biconcave lens
Hypermetropia
Decrease in anteroposterior diameter of the eyeball
Biconvex lens
Anisometropia
Difference in refractive power of both eyes
Separate lens
(biconcave or biconvex)
for each eye as required
Astigmatism
Refractory power of lens is different in different
meridians
Regular astigmatism Refractory power of lens is unequal in different
meridians but uniform in one single meridian
Cylindrical lens
Irregular astigmatism Refractory power of lens is unequal in different
meridians as well as in different points in
same meridian
Presbyopia
Loss of elasticity in lens and weakness of ocular
muscles due to old age
Biconvex lens
1. Regular Astigmatism
In this type of astigmatism, the refractive power
is unequal in different meridians because of
alteration of curvature in one meridian. But, it is
uniform in all points throughout the affected
meridian.
2. Irregular Astigmatism
Here, the refractive power is unequal not only in
different meridians, but it is also unequal in
different points of same meridian.
CORRECTION OF ASTIGMATISM
Astigmatism is corrected by using cylindrical
glass lens having the convexity in the meridians
corresponding to that of lens of eye having a
lesser curvature, i.e. if the horizontal curvature
of lens is less, the person should use cylindrical
glass lens with the convexity in horizontal
meridian.
PRESBYOPIA
Presbyopia is the condition characterized by
progressive decrease in the ability of eyes to
EXTERNAL EAR
AURICLE OR PINNA
EXTERNAL AUDITORY MEATUS
MIDDLE EAR
AUDITORY OSSICLES
AUDITORY MUSCLES
EUSTACHIAN TUBE
INTERNAL EAR
COCHLEA
COMPARTMENTS OF COCHLEA
ORGAN OF CORTI
AUDITORY PATHWAY
INTRODUCTION
RECEPTORS
FIRST ORDER NEURONS
SECOND ORDER NEURONS
THIRD ORDER NEURONS
CORTICAL AUDITORY CENTERS
APPLIED PHYSIOLOGY – EFFECT OF LESION
Structure of Ear and
Auditory Pathway
110
EXTERNAL EAR
The ear consists of three parts namely, external
ear, middle ear and internal ear (Fig. 110-1). The
external ear is formed by two parts:
1. Auricle or pinna
2. External auditory meatus.
MIDDLE EAR
The middle ear or tympanic cavity is situated
within the temporal bone. It is separated from
external auditory meatus by a thin semitrans-
parent membrane called tympanic membrane
(Fig. 110-2).
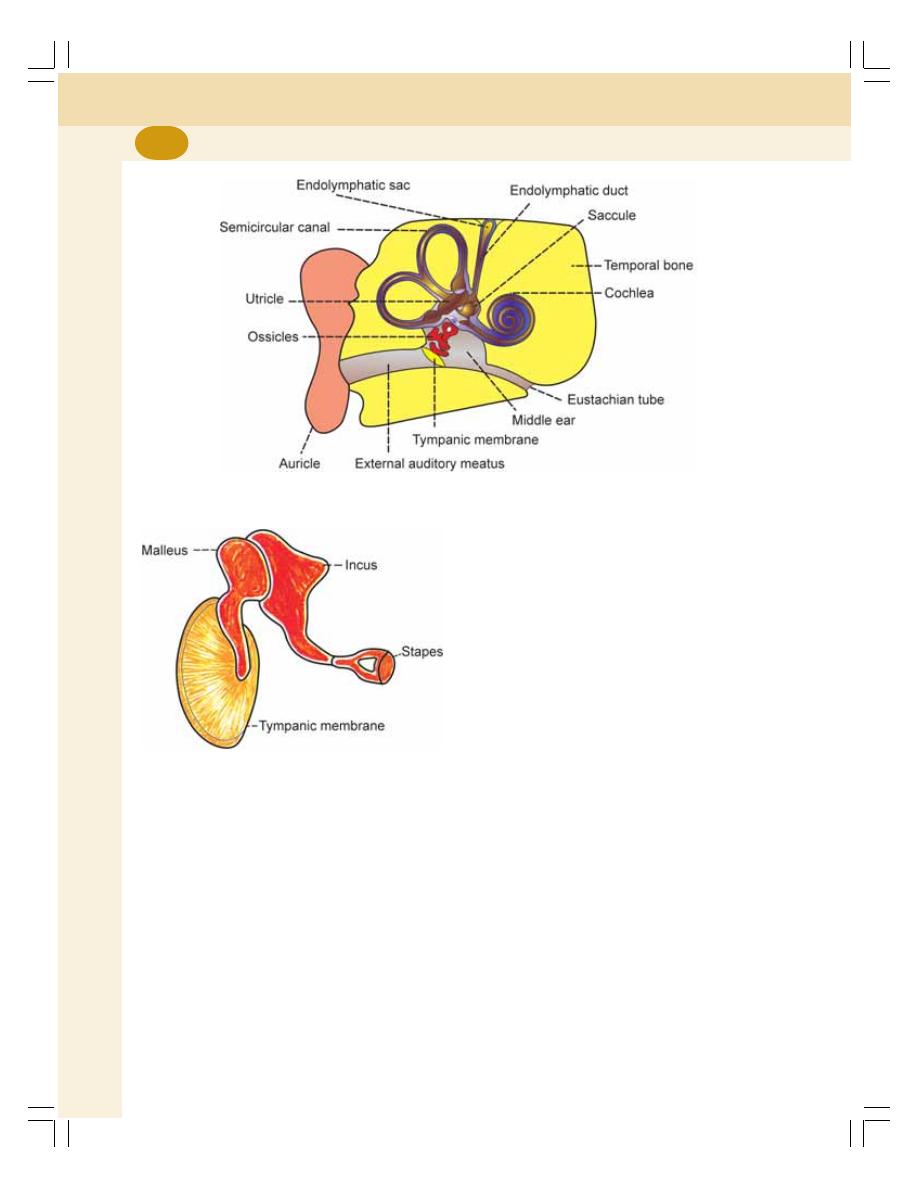
Special Senses
658
Middle ear consists of the following
structures:
1. Auditory ossicles
2. Auditory muscles
3. Eustachian tube.
AUDITORY OSSICLES
The auditory ossicles are the three miniature
bones, which are arranged in the form of a chain
extending across the middle ear from the
tympanic membrane to oval window (Fig. 110-2).
The auditory ossicles are:
1. Malleus
2. Incus
3. Stapes.
1. Malleus
It is otherwise called hammer. It has a handle,
head and neck. The handle is otherwise known
as manubrium. It is attached to the tympanic
membrane. The head or capitullum articulates
with the body of next bone incus.
2. Incus
Incus is also known as anvil. It looks like a
premolar tooth. Incus has a body, one long
process and one short process. Anterior surface
of the body articulates with head of malleus. The
tip of the long process is like a knob, called
lenticular process and it articulates with the next
bone, stapes.
3. Stapes
Stapes is also called stirrup. It is the smallest
bone present in the body. It has a head, neck,
anterior crus, posterior crus and a footplate.
Head articulates with incus. Footplate fits into
the oval window.
FIGURE 110-1: Diagram showing the structure of ear
FIGURE 110-2: Tympanic membrane and
auditory ossicles

Chapter 110 Structure of Ear and Auditory Pathway
659
AUDITORY MUSCLES
Two skeletal muscles are attached to the ossi-
cles:
1. Tensor tympani
2. Stapedius.
1. Tensor Tympani
Tensor tympani muscle arises from cartilagi-
nous portion of eustachian tube. Its tendon is
inserted on manubrium of malleus which is in
turn attached to tympanic membrane.
Tensor tympani muscle pulls and keeps the
tympanic membrane stretched constantly.
2. Stapedius
Stapedius is the smallest skeletal muscle in
human body with a length of just over 1 mm. It
arises from interior pyramid of tympanic cavity.
Its tendon is inserted into the posterior surface
of neck of stapes.
Stapedius prevents excess movements of
stapes. When it contracts, it pulls the neck of
stapes backwards and reduces the movement
of footplate against the fluid in cochlea.
EUSTACHIAN TUBE
Eustachian tube or the auditory tube connects
the middle ear with posterior part of nose and
forms the passage of air between middle ear
and atmosphere. So, the pressure on both sides
of tympanic membrane is equalized.
INTERNAL EAR
The internal ear or labyrinth is a membranous
structure, enclosed by a bony labyrinth in petrous
part of temporal bone. It consists of the sense
organs of hearing and equilibrium. The sense
organ for hearing is the cochlea. And, the sense
organ for equilibrium is the vestibular apparatus.
Vestibular apparatus is already explained in
Chapter 98.
COCHLEA
Cochlea is a coiled structure like a snail’s shell
(cochlea = snail’s shell). It consists of two
structures:
1. Central conical axis formed by spongy bone
called modiolus
2. Bony spiral canal, which winds around the
modiolus.
COMPARTMENTS OF COCHLEA
Two membranous partitions called basilar
membrane and vestibular membrane divide the
spiral canal of cochlea into three compartments.
The compartments of spiral canal of cochlea
are:
i. Scala vestibuli
ii. Scala tympani
iii. Scala media.
All the three compartments are filled with
fluid. Scala vestibuli and scala tympani contain
perilymph. The scala media is filled with
endolymph.
i. Scala vestibuli
Scala vestibuli lies above the scala media. It
arises from oval window (fenestra vestibuli)
which is closed by the footplate of stapes. It
follows the osseous canal up to its apex. At the
apex, it communicates with the scala tympani
through a small canal called helicotrema.
ii. Scala tympani
It lies below the scala media. It is parallel to scala
vestibuli and ends at the round window. The
round window is closed by a strong thin mem-
brane known as secondary tympanic membrane.
iii. Scala media
Scala media is otherwise called cochlear duct.
It ends blindly at the apex and at the base of
cochlea. The sensory part of cochlea called
organ of Corti is situated on the upper surface
of basilar membrane (Fig. 110-3).
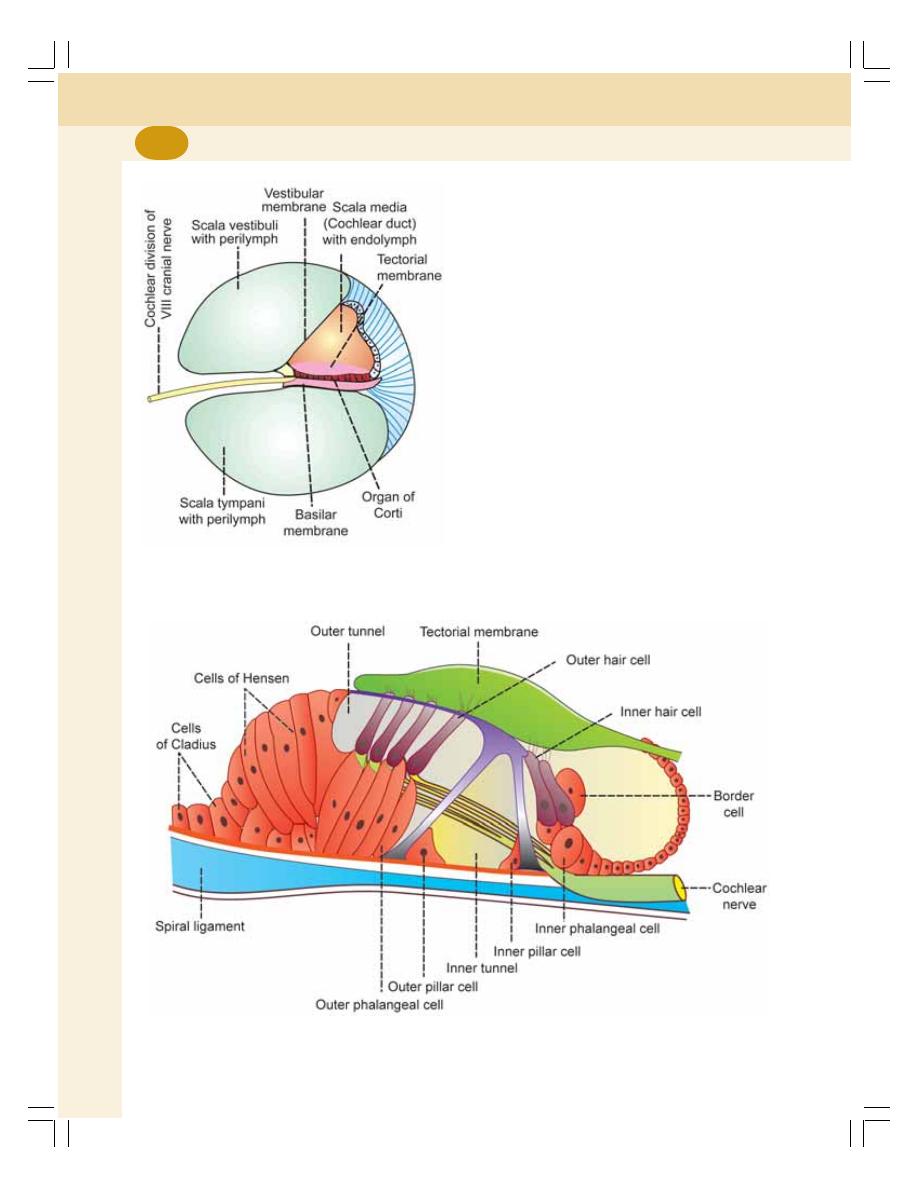
Special Senses
660
FIGURE 110-4: Organ of Corti
FIGURE 110-3: Cross section of
spiral canal of cochlea
ORGAN OF CORTI
Organ of Corti is the receptor organ for hearing.
It is the neuroepithelial structure in cochlea
(Fig. 110-4). It rests upon the lip of spiral lamina
and the basilar membrane. It extends through-
out the cochlear duct, except for a short distance
on either end. The roof of the organ of Corti is
for-med by gelatinous tectorial membrane.
Structure
The organ of Corti is made up of the sensory
elements, called the hair cells and various
supporting cells. All the cells of organ of Corti
are arranged in order from center towards
periphery of the cochlea:
1. Border cells
2. Inner hair cells
3. Inner phalangeal cells
4. Inner pillar cells
5. Outer pillar cells
6. Outer phalangeal cells
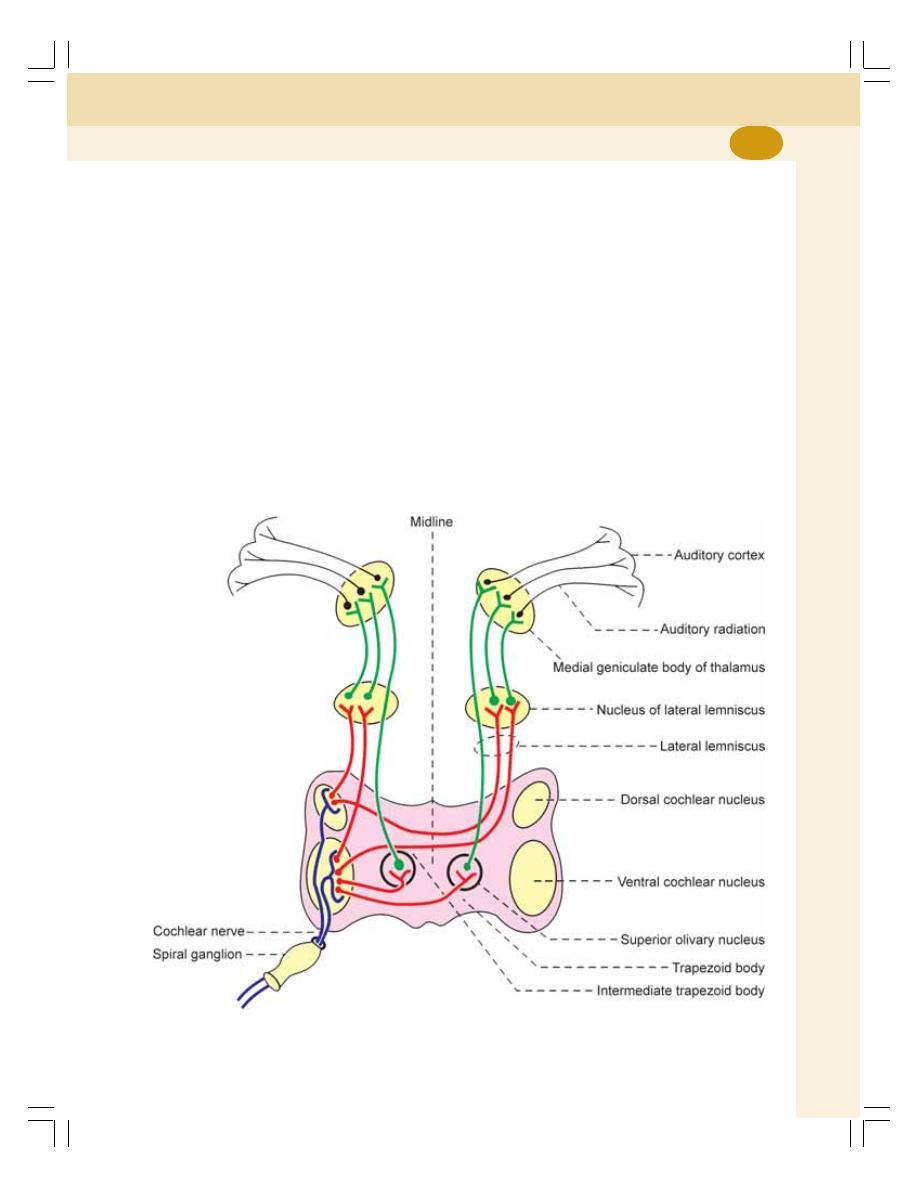
Chapter 110 Structure of Ear and Auditory Pathway
661
7. Outer hair cells
8. Cells of Hensen
9. Cells of Claudius
10. Tectorial membrane and lamina reticularis.
Hair Cells of Organ of Corti
The hair cells in organ of Corti are the receptors
of the auditory sensation. The hair cells are of
two types, outer hair cells and inner hair cells.
The surface of the hair cells bears a cuticular
plate and a number of short stiff hairs which are
called stereocilia. Each hair cell has about
100 stereocilia. One of the stereocilia is
larger and it is called kinocilium. The stereocilia
are in contact with the tectorial membrane.
Sensory nerve fibers are distributed around the
hair cells.
AUDITORY PATHWAY
INTRODUCTION
The fibers of auditory pathway pass through
cochlear division of vestibulocochlear nerve (VIII
cranial nerve). It is also known as auditory nerve.
RECEPTORS
The outer and inner hair cells in organ of Corti
are the receptors of the auditory sensation. The
afferent nerve fibers which innervate the hair
cells form the auditory nerve (see below).
FIRST ORDER NEURONS
The first order neurons of the auditory pathway
are the bipolar cells of spiral ganglion situated
in the modiolus of cochlea (Fig. 110-5).
FIGURE 110-5: Auditory pathway. Blue = First order neuron. Red = Second order neuron.
Green =Third order neuron. Black = Auditory radiation

Special Senses
662
The dendrites of the bipolar cells are
distributed around the hair cells of organ of
Corti. Their axons leave ear as cochlear nerve
fibers and enter medulla oblongata. Immediately
after entering the medulla oblongata, the fibers
divide into two groups which end on ventral and
dorsal cochlear nuclei of the same side in
medulla oblongata.
SECOND ORDER NEURONS
The neurons of dorsal and ventral cochlear nuclei
in the medulla oblongata form the second order
neurons of auditory pathway. The axons of the
second order neurons run in four different direc-
tions:
1. First group of fibers cross the midline and run
to the opposite side to form trapezoid body
and go to the superior olivary nucleus.
2. Second group of the fibers terminate at the
superior olivary nucleus of same side via
trapezoid body of the same side
3. Third group of fibers run in the lateral lemnis-
cus of the same side and terminate in the
nucleus of lateral lemniscus
4. Fourth group of fibers cross the midline as
intermediate trapezoid fibers and join the
nucleus of lateral lemniscus of opposite side.
THIRD ORDER NEURONS
Third order neurons are in the superior olivary
nuclei and nucleus of lateral lemniscus. The
fibers from here end in medial geniculate body
which forms the subcortical auditory center.
Fibers from medial geniculate body go to the
temporal cortex, via internal capsule as auditory
radiation.
CORTICAL AUDITORY CENTERS
The cortical auditory centers are in the temporal
lobe of cerebral cortex. The auditory areas are
area 41, area 42 and Wernicke’s area.
Areas 41 and 42 are the primary auditory
areas which are concerned with the perception
of auditory impulses. Wernicke’s area is res-
ponsible for the analysis and interpretation of
sound with the help of auditopsychic area.
APPLIED PHYSIOLOGY – EFFECT OF
LESION
1. Lesion of cochlear nerve causes deafness
2. Unilateral lesion of auditory pathway above
the level of cochlear nuclei causes dimini-
shed hearing
3. Degeneration of hair cells in organ of Corti
leads to gradual loss of hearing that is com-
mon in old age
4. Lesion in superior olivary nucleus results in
poor localization of sound.

INTRODUCTION
ROLE OF EXTERNAL EAR
ROLE OF MIDDLE EAR
ROLE OF TYMPANIC MEMBRANE
ROLE OF AUDITORY OSSICLES
ROLE OF EUSTACHIAN TUBE
ROLE OF INNER EAR
TRAVELING WAVE
EXCITATION OF HAIR CELLS
ELECTRICAL EVENTS DURING THE PROCESS OF HEARING
SOUND TRANSDUCTION
RECEPTOR POTENTIAL
PROPERTIES OF SOUND
APPRECIATION OF PITCH OF THE SOUND – THEORIES OF HEARING
APPRECIATION OF LOUDNESS OF SOUND
LOCALIZATION OF SOUND
AUDITORY DEFECTS
CONDUCTION DEAFNESS
NERVE DEAFNESS
Mechanism of Hearing and
Auditory Defects
111
INTRODUCTION
The sound waves travel through the external
auditory meatus and produce vibrations in the
tympanic membrane. The vibrations from tym-
panic membrane travel through malleus and
incus and reach the stapes resulting in the
movement of stapes. The movements of stapes
produce vibrations in the fluids of cochlea and
which stimulate the hair cells in the organ of
Corti. This, in turn, causes the generation of
action potential (auditory impulses) in the
auditory nerve fibers. When the auditory
impulses reach the cerebral cortex, the
perception of hearing occurs.

Special Senses
664
ROLE OF EXTERNAL EAR
External ear directs the sound waves towards
the tympanic membrane. The sound waves pro-
duce pressure changes over the surface of
tympanic membrane.
ROLE OF MIDDLE EAR
ROLE OF TYMPANIC MEMBRANE
Due to the pressure changes produced by
sound waves, the tympanic membrane vibrates,
i.e. it moves in and out of middle ear. Thus, the
tym-panic membrane acts as a resonator that
pro-duces the vibration of sound.
ROLE OF AUDITORY OSSICLES
The vibrations set up in tympanic membrane
are transmitted through the malleus and incus
and reach the stapes, causing to and fro
movement of stapes against oval window and
against the perilymph present in scala vestibuli
of cochlea.
Impedance Matching
Impedance matching is the process, by which
the tympanic membrane and auditory ossicles
convert the sound energy into the mechanical
vibrations in the fluid of internal ear with
minimum loss of energy by matching the
impedance offered by the fluid.
Impedance means obstruction or opposition
to the passage of sound waves. When sound
waves reach the inner ear, the fluid (perilymph)
in cochlea offers impedance, i.e. the fluid resists
the transmission of sound due to its own inertia.
Tympanic membrane and the auditory ossicles
effectively reduce the sound impedance which
is called the impedance matching.
Significance of impedance matching
Impedance matching is the most important
function of middle ear. Because of impedance
matching the sound waves (stimuli) are trans-
mitted to cochlea with minimum loss of intensity.
Without impedance matching conductive deaf-
ness occurs.
Types of Conduction
Conduction of sound from external ear to internal
ear through middle ear occurs by three routes:
1. Ossicular conduction
2. Air conduction
3. Bone conduction.
1. Ossicular conduction
Ossicular conduction is the conduction of sound
waves through middle ear by auditory ossicles.
This is the normal way of conduction of the
sound waves through middle ear.
2. Air conduction
It is the conduction of sound waves through air
in middle ear. It occurs when the auditory
ossicles are diseased.
3. Bone conduction
It is the conduction of sound waves by bones.
When middle ear is affected, bone conduction
occurs. In this type of conduction, the sound
waves are transmitted to cochlear fluid by the
vibrations set up in the skull bones.
ROLE OF EUSTACHIAN TUBE
The Eustachian tube is not concerned with
hearing directly. However, it is responsible for
equalizing the pressure on either side of
tympanic membrane.
ROLE OF INNER EAR
TRAVELING WAVE
The movement of footplate of stapes against
oval window causes movement of perilymph in
scala vestibuli. The fluid does not move all the
way from oval window towards round window
through the helicotrema. It immediately hits the
vestibular membrane near oval window and
displaces the fluid in scala media (Fig. 111-1).
This causes bulging of basal portion of basilar
membrane towards scala tympani.

Chapter 111 Mechanism of Hearing and Auditory Defects
665
The elastic tension developed in the bulged
portion of basilar membrane initiates a wave
called travelling wave. It travels along basilar
membrane towards the helicotrema like that of
arterial pulse wave.
EXCITATION OF HAIR CELLS
The stereocilia of hair cells in organ of Corti are
embedded in tectorial membrane. The hair cells
are tightly fixed by cuticular lamina reticularis and
the pillar cells Corti. When the traveling wave
produces vibration of basilar membrane, all
these structures move as a single unit. It causes
movements of stereocilia leading to excitement
of hair cells and generation of receptor potential.
ELECTRICAL EVENTS DURING THE
PROCESS OF HEARING
SOUND TRANSDUCTION
Sound transduction is a type of sensory trans-
duction (Chapter 85) in the hair cell (receptor)
by which the energy (movement of cilia in hair
cell) caused by sound is converted into action
potentials in the auditory nerve fiber.
RECEPTOR POTENTIAL OR COCHLEAR
MICROPHONIC POTENTIAL
Receptor potential or cochlear microphonic
potential is the mild depolarization that is develo-
ped in the hair cells of cochlea when sound
waves are transmitted to internal ear. The rest-
ing membrane potential in hair cells is about
– 60 mV.
Receptor potential in the hair cells causes
generation of action potential in auditory nerve
fibers.
PROPERTIES OF SOUND
Sound has two basic properties:
1. The pitch which depends upon the frequency
of sound waves. Frequency of sound is
expressed in hertz. The frequency of sound
audible to human ear lies between 20 and
20,000 Hz or cycles/second. The range of
greatest sensitivity lies between 2,000 and
3,000 Hz (cycles/second).
2. The loudness or intensity which depends
upon the amplitude of sound waves. It is
expressed in decibel (dB). The threshold
intensity of sound wave is not constant. It
varies in accordance to the frequency of the
sound.
APPRECIATION OF PITCH OF THE
SOUND – THEORIES OF HEARING
Though many theories are postulated to explain
the mechanism by which the pitch of the sound
is appreciated only few theories are accepted
so far. The accepted theories are:
1. Place Theory
According to this theory, the nerve fibers from
different portions (places) of organ of Corti on
basilar membrane give response to sounds of
different frequency. Accordingly, the corres-
ponding nerve fiber from organ of Corti gives
information to the brain regarding the portion of
organ of Corti that is stimulated.
2. Traveling Wave Theory
This theory explains how the traveling wave is
generated in the basilar membrane. The gene-
ration, movement and disappearance of travel-
FIGURE 111-1: Diagrammatic representation of
cochlea. The arrows show displacement of fluid

Special Senses
666
ing wave are already described earlier in this
chapter.
APPRECIATION OF LOUDNESS OF
SOUND
Appreciation of loudness of sound depends upon
the activities of auditory nerve fibers.
When the loudness of sound increases, it
produces longer vibrations which spread over
longer area of basilar membrane. This activates
large number of hair cells and recruits more
number of auditory nerve fibers. So, the fre-
quency of action potential is also increased.
LOCALIZATION OF SOUND
Sound localization is the ability to detect the
source from where the sound is produced or the
direction through which the sound wave is
traveling. It is important for survival and it helps
to protect us from moving objects such as
vehicles. Cerebral cortex and medial geniculate
body are responsible for localization of sound.
AUDITORY DEFECTS
The auditory defects may be either partial or
complete. The auditory defects are of two types:
1. Conduction deafness
2. Nerve deafness.
1. CONDUCTION DEAFNESS
Conduction deafness occurs due to impairment
in the transmission of sound waves in external
ear or middle ear.
Causes of Conduction Deafness
i. Obstruction of external auditory meatus
with dry wax or foreign bodies
ii. Thickening of tympanic membrane due to
infection
iii. Perforation of tympanic membrane due to
inequality of pressure on either side
iv. Inflammation of middle ear (otitis media)
v. Fixation of footplate of stapes against
oval window (otosclerosis).
2. NERVE DEAFNESS
Nervous deafness is caused by damage of any
structure in cochlea such as hair cell, organ of
Corti, basilar membrane or cochlear duct or the
lesion in auditory pathway.
Causes of Nerve Deafness
i. Degeneration of hair cells
ii. Damage of cochlea by prolonged expo-
sure to loud noise
iii. Tumor affecting VIII cranial nerve.
TASTE BUDS
TASTE BUDS
TASTE BUDS
TASTE BUDS
TASTE BUDS
PATHWAY FOR TASTE
PATHWAY FOR TASTE
PATHWAY FOR TASTE
PATHWAY FOR TASTE
PATHWAY FOR TASTE
PRIMARY TASTE SENSATIONS
PRIMARY TASTE SENSATIONS
PRIMARY TASTE SENSATIONS
PRIMARY TASTE SENSATIONS
PRIMARY TASTE SENSATIONS
DISCRIMINATION OF DIFFERENT TASTE SENSATIONS
DISCRIMINATION OF DIFFERENT TASTE SENSATIONS
DISCRIMINATION OF DIFFERENT TASTE SENSATIONS
DISCRIMINATION OF DIFFERENT TASTE SENSATIONS
DISCRIMINATION OF DIFFERENT TASTE SENSATIONS
TASTE SENSATIONS AND CHEMICAL CONSTITUTIONS
TASTE SENSATIONS AND CHEMICAL CONSTITUTIONS
TASTE SENSATIONS AND CHEMICAL CONSTITUTIONS
TASTE SENSATIONS AND CHEMICAL CONSTITUTIONS
TASTE SENSATIONS AND CHEMICAL CONSTITUTIONS
TASTE TRANSDUCTION
TASTE TRANSDUCTION
TASTE TRANSDUCTION
TASTE TRANSDUCTION
TASTE TRANSDUCTION
APPLIED PHYSIOLOGY
APPLIED PHYSIOLOGY
APPLIED PHYSIOLOGY
APPLIED PHYSIOLOGY
APPLIED PHYSIOLOGY
TASTE BUDS
Taste buds are the sense organs for taste or
gustatory sensation. The taste buds are ovoid
bodies with a diameter of 50 to 70 μ.
SITUATION OF TASTE BUD
Most of the taste buds are present on the
papillae of tongue. Some taste buds are situa-
ted in the mucosa of epiglottis, palate, pharynx
and proximal part of esophagus. Three types of
papillae are located on the tongue:
1. Filiform papillae
2. Fungiform papillae
3. Circumvallate papillae.
1. Filiform Papillae
Filiform papillae are small and conical shaped
papillae situated over the dorsum of tongue.
These papillae contain less number of taste buds
(only a few).
2. Fungiform Papillae
Fungiform papillae are round in shape and are
situated over the anterior surface of tongue near
the tip. Numerous fungiform papillae are pre-
sent. The number of taste buds in each is
moderate (up to 10).
3. Circumvallate Papillae
Circumvallate papillae are large structures
arranged in ‘V’ shape on the posterior part of
tongue and are many in number. Each papilla
contains many taste buds (up to 100).
STRUCTURE OF TASTE BUD
The taste bud is a bundle of taste receptor cells,
with supporting cells embedded in the epithelial
covering of the papillae (Fig. 112-1). Each taste
bud contains about 40 cells, which are the
Sensation of Taste
112
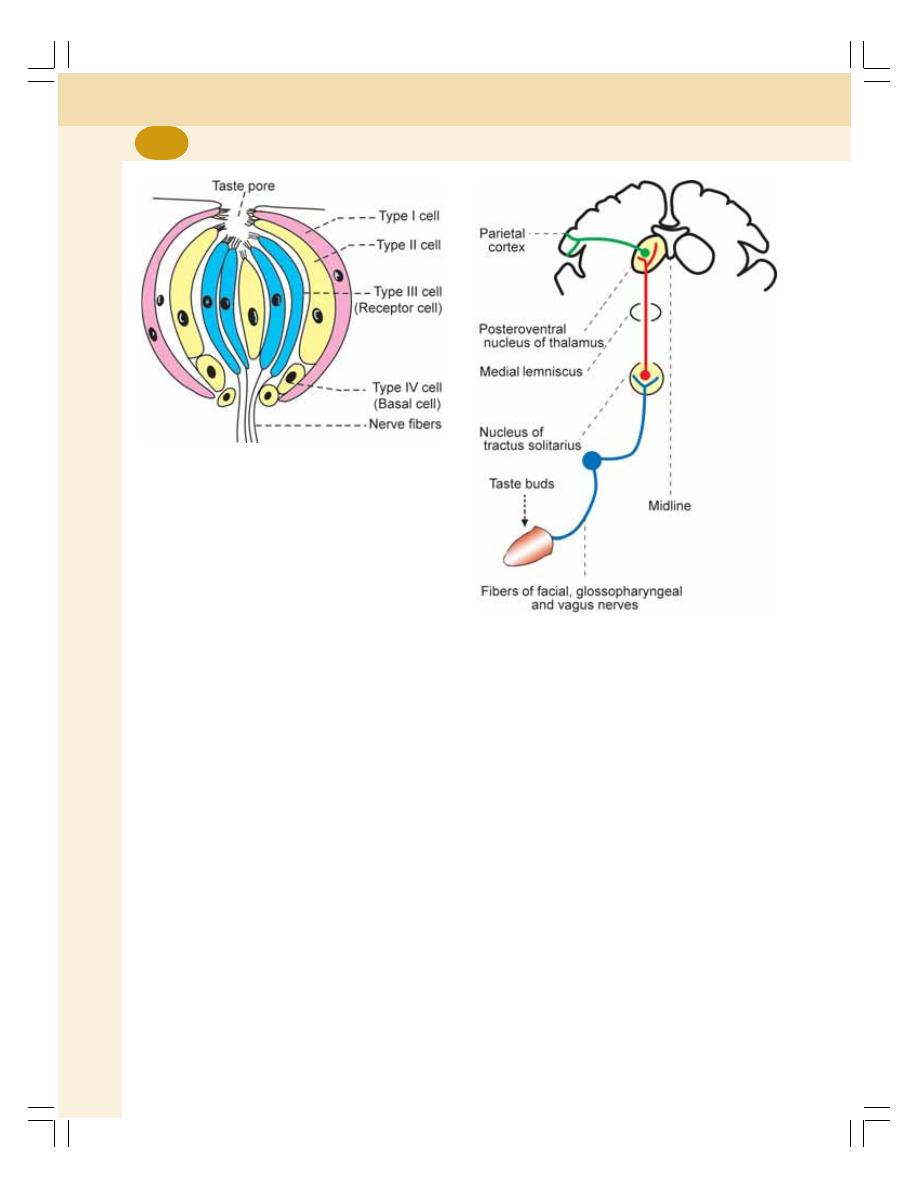
Special Senses
668
dendrites of the neurons are distributed to the
taste buds. After arising from taste buds, the
fibers run along the following nerves (Fig. 112-2):
1. Chorda tympani fibers of facial nerve, which
run from anterior two-third of tongue
2. Glossopharyngeal nerve fibers, which run
from posterior one-third of the tongue
3. Vagal fibers, which run from taste buds in
other regions.
Axons of the first order neurons run together
in medulla oblongata and terminate in the
nucleus of tractus solitarius.
Second Order Neurons
Second order neurons are in the nucleus of
tractus solitarius. Axons of the second order
neurons run through medial lemniscus and
terminate in posteroventral nucleus of thalamus.
FIGURE 112-1:
Taste bud
FIGURE 112-2:
Pathway for taste sensation
modified epithelial cells. The cells of taste bud
are divided into four groups:
1. Type I cells or sustentacular cells
2. Type II cells
3. Type III cells
4. Type IV cells or basal cells.
Type I cells and type IV cells are supporting
cells. Type III cells are the taste receptor cells.
Function of type II cell is unknown. Type I, II and
III cells have projections called microvilli. The
microvilli project into an opening in the epithe-
lium covering the tongue. The opening is called
taste pore. All the cells of taste bud are
surrounded by epithelial cells.
PATHWAY FOR TASTE
Receptors
Receptors for taste sensation are the type III
cells of taste buds. Each taste bud is innervated
by about 50 sensory nerve fibers and each nerve
fiber supplies at least 5 taste buds.
First Order Neurons
First order neurons of taste pathway are in the
nuclei of three different cranial nerves. The

Chapter 112 Sensation of Taste
669
Third Order Neurons
The third order neurons are in the posteroventral
nucleus of thalamus. The axons from the third
order neurons project into cerebral cortex.
Taste Center
The center for taste sensation is in the opercular
insular cortex (lower part of postcentral gyrus)
in parietal lobe of cerebral cortex.
PRIMARY TASTE SENSATIONS
The primary or fundamental taste sensations
are divided into five types:
1. Sweet
2. Salt
3. Sour
4. Bitter
5. Umami.
Man can perceive more than 100 different
tastes. Other taste sensations are just the
combination of two or more primary sensations.
Sometimes, the taste sensation is combined
with other sensations like pain (ginger) or tem-
perature (flavor).
TASTE SENSATIONS AND
CHEMICAL CONSTITUTIONS
1. SWEET TASTE
Sweet taste is produced mainly by organic
substances like monosaccharides, polysacch-
arides, glycerol, alcohol, aldehydes, ketones and
chloroform. The inorganic substances, which
produce sweet taste are lead and beryllium.
2. SALT TASTE
Salt taste is produced by chlorides of sodium,
potassium and ammonium, nitrates of sodium
and potassium. Some sulfates, bromides and
iodides also produce salt taste.
3. SOUR TASTE
Sour taste is produced by hydrogen ions in acids
and acid salts.
4. BITTER TASTE
Bitter taste is produced by organic substances
like quinine, strychnine, morphine, glucosides,
picric acid and bile salts and inorganic subs-
tances like salts of calcium, magnesium and
ammonium.
5. UMAMI
Umami is the recently recognized taste
sensation. Umami is a Japanese word meaning
‘delicious’. Receptors of this taste sensation
respond to monosodium glutamate which is a
common ingredient in Asian food.
TASTE TRANSDUCTION
Taste transduction is the process in which taste
receptor converts chemical energy into action
potentials in the taste nerve fiber. Receptors of
taste sensation are chemoreceptors, which are
stimulated by substances dissolved in mouth by
saliva. The dissolved substances act on the
microvilli of taste receptors exposed in the taste
pore. It causes the development of receptor
potential in the receptor cells. This in turn, is
responsible for the generation of action potential
in the sensory neurons.
APPLIED PHYSIOLOGY –
ABNORMALITIES OF TASTE
SENSATION
1. Ageusia – loss of taste sensation
2. Hypogeusia – decrease in the taste sensation
3. Taste blindness – inability to recognize
substances by taste due to genetic disorder
4. Dysgeusia – disturbance in the taste
sensation like hallucinations of taste.
OLFACTORY RECEPTORS
OLFACTORY PATHWAY
GENERATOR POTENTIAL IN OLFACTORY RECEPTOR
CLASSIFICATION OF ODOR
THRESHOLD FOR OLFACTORY SENSATION
APPLIED PHYSIOLOGY
OLFACTORY RECEPTORS
Olfactory receptors are situated in olfactory
mucous membrane that lines upper part of
nostril. The olfactory mucous membrane
consists of 10 to 20 millions of olfactory recep-
tor cells supported by the sustentacular cells.
The mucosa also contains mucus secreting
Bowman’s glands (Fig. 113-1).
The olfactory receptor cell is a bipolar neuron.
The dendrite of this neuron is short. The
expanded end of the dendrite is called olfactory
rod. From the rod, about 10 to 12 cilia arise. Cilia
are nonmyelinated with a length of 2 μ and a
diameter of 0.1 μ. The cilia project to the
surface of olfactory mucous membrane.
The mucus secreted by Bowman’s glands
continuously lines the olfactory mucosa. The
mucus contains some proteins, which increase
the actions of odoriferous substances on
receptor cells.
OLFACTORY PATHWAY
Axons of the bipolar olfactory receptors pierce
the cribriform plate of ethmoid bone and reach
the olfactory bulb. Here, the axons synapse
with dendrites of mitral cells. Different groups
of these synapses form globular structures,
called olfactory glomeruli.
The axons of mitral cells leave the olfactory
bulb and form olfactory tract. The olfactory tract
runs backwards and ends in olfactory cortex.
The olfactory cortex includes the structures,
which form a part of limbic system. The struc-
tures are anterior olfactory nucleus, prepyriform
cortex, olfactory tubercle and amygdala.
GENERATOR POTENTIAL IN
OLFACTORY RECEPTOR
The odoriferous substance stimulates the
olfactory receptors resulting in generation of
receptor potential.
The receptor potential causes generation of
action potential in the axon of the bipolar neuron.
CLASSIFICATION OF ODOR
The odor is classified into various types:
1. Aromatic or resinous odor – camphor,
lavender, clove and bitter almonds
Sensation of Smell
113
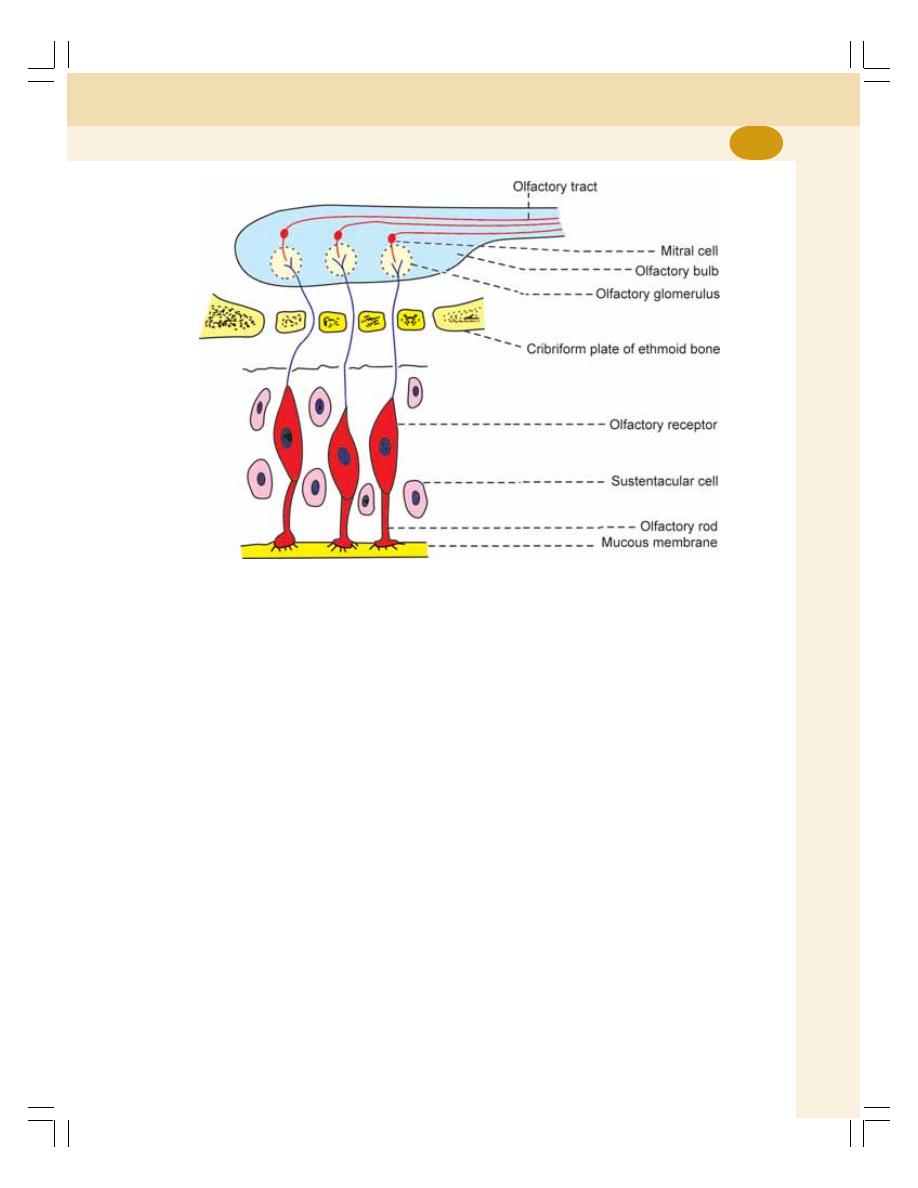
Chapter 113 Sensation of Smell
671
2. Ambrosial odor – musk
3. Burning odor – burning feathers, tobacco,
roasted coffee and meat
4. Ethereal odor – fruits, ethers and bees wax
5. Fragrant or balsamic odor – flowers and
perfumes
6. Garlic odor – garlic, onion, and sulfur
7. Goat odor – caproic acid, and sweet cheese
8. Nauseating odor – decayed vegetables and
feces
9. Repulsive odor – bed bug.
THRESHOLD FOR OLFACTORY
SENSATION
Ethyl ether
: 5.8
mg/L of air
Chloroform
: 3.3
mg/L of air
Peppermint oil
: 0.02
mg/L of air
FIGURE 113-1:
Olfactory mucous membrane and pathway for olfactory sensation
Butyric acid
: 0.009
mg/L of air
Artificial musk
: 0.00004
mg/L of air
Methyl mercaptan : 0.0000004 mg/L of air
Thus, the methyl mercaptan produces olfac-
tory sensation even at a low concentration of
0.0000004 mg/L of air.
APPLIED PHYSIOLOGY –
ABNORMALITIES OF OLFACTORY
SENSATION
1. Anosmia – total loss of sensation of smell
2. Hyposmia – reduced ability to recognize and
to detect any odor
3. Hyperosmia or olfactory hyperesthesia –
increased or exaggerated olfactory sen-
sation.

LONG QUESTIONS
1. Draw a diagram of visual pathway and explain
it. Add a note on hemianopia.
2. Explain the auditory pathway with suitable
diagram. Add a note on auditory defects.
3. Explain the mechanism of hearing.
SHORT QUESTIONS
1. Retina.
2. Ocular movements.
3. Visual receptors.
4. Aqueous humor.
5. Intraocular pressure.
6. Dark adaptation.
7. Light adaptation.
8. Nyctalopia.
9. Effects of lesion in optic pathway.
10. Accommodation reflex.
11. Presbyopia.
12. Color blindness.
13. Errors of refraction.
14. Auditory ossicles.
15. Cochlea/organ of Corti.
16. Role of middle ear in hearing /
functions of middle ear.
17. Auditory defects.
18. Taste buds.
19. Taste pathway.
20. Olfactory pathway.
QUESTIONS IN SPECIAL SENSES
Questions in Special Senses
672
Index
Symbols
11-Deoxycorticosterone 304
1,25-Dihydroxycholecalciferol 284, 291
1,25-Dihydroxycholecalciferol —
calcitriol 285, 292, 319
15-hydroperoxy eicosatetraeonic acid
(15-HETE) 322
2,3-diphosphoglycerate 471
25-hydroxycholecalciferol 285
5-hydroperoxy eicosatetraeonic acid
(5-HETE) 322
A
‘A’ Band 117
A cells 298
Abdominal aorta 216
Abdominal muscles 451
Abducent (sixth) nerve 636
Abnormal heart sound 391
Abnormal menstruation
applied physiology 357
ABO incompatibility 96
ABO system 94
Accelerator globulin 85
Acclimatization 489
Accommodation 648, 649
applied physiology 650
definition 648
mechanism 648
pathway 649
reflex 649
Acetone breathing 302
Acetyl-CoA synthetase 10
Acetylcholine 134, 322, 623
Acetylcholine receptors 133
Acetylcholinesterase 323, 523
Achlorhydria 166
Achromatopsia 652
Acid phosphatase 340
Acid–base balance 206, 233, 448
Acidophilic cells 262
Acidosis 302
Acne 246, 337
Aconthosis 310
Acquired immune deficiency
diseases 78
Acquired immune deficiency
syndrome (AIDS) 78
Acromegalic gigantism 270
Acromegaly 269
Acromicria 271
Acrosome 340
ACTH 308, 309
actions 309
Mode of action 309
Actin filaments 118
Action molecule 119
Action potential 126-128, 138, 139,
378, 379
cardiac muscle 378
iIonic basis 128, 139, 378
properties 127
skeletal muscle 126
smooth muscle 138
Action potential curve 127
stimulus artifact 127
Action potential with plateau 139
Active transport 20, 21, 22
mechanism 20
primary 20
secondary 21
special categories 22
substances transported 20
Activin 331
Actomyosin complex 129, 130
Acuity of vision 640
Acupuncture 557
Acute adrenal insufficiency 312
Addisonian crisis 312
Addison’s disease 311
Adenohypophysis 261
Adenosine mono-phosphate cyclic
AMP (cAMP) 260
ADH 230
ADH mechanism 565
Adherens junctions 15
Adhesiveness 81
Adiadochokinesis 574
Adipocytes 564
Adipose tissue 323, 564
Adrenal cortex 303, 310
applied physiology 310
functional anatomy 303
hormones 303
hyperactivity 310
hypoactivity 311
Adrenal crisis 312
Adrenal glands 303
cortex 303
functional anatomy 303
medulla 313
Adrenal medulla
hormones 313
Adrenal medulla 313
Adrenal sex hormones 309
Adrenal virilism 311
Adrenaline 313, 314, 315
actions 315
mode of action 315
Adrenaline 155, 317
regulation of secretion 317
Adrenaline apnea 316, 480
Adrenergic receptors 315
Adrenocorticotropic hormone
262, 265, 305
Adrenogenital syndrome 311
Adult 55
Afferent circuit 572
After depolarization 128
After discharge 530
After hyperpolarization 128
Afterload 125, 400
Ageusia 669
Agglutination 81, 94
Agglutinin 94
Agglutinogen 94
Aggregation 81
Agranular cells, lacis cells 214
Agranulocytes 64
Air conduction 664
Air hunger 484
Airway resistance 456
Airway resistance work 456
Akinesia 577
Alanine 235
Aldosterone 304
Aldosterone escape 305
Alimentary canal 145
All or none law 381
Alpha block 606
Alpha cells 262
Alpha motor neurons 533
Alpha waves 606
Alveolar air 465
Alveolar cells 446
Alveolar ducts 446
Alveolar epithelial cells 453

674
Essentials of Physiology for Dental Students
Alveolar sacs 446
Alveoli 446
Alzheimer’s disease 507, 614
Amacrine cells 633
Amelognosia 560
Ametropia 654
Ametropic eye 654
Amino tyrosine 245
Aminohippuric acid (PAH) 237
Ammonia 235, 477
Ammonia mechanism 234, 235
Ammonium 235
Amnesia 614
Ampulla 600
Ampulla of Vater 168, 175
Amygdala 670
Amygdaloid 587
Amygdaloid complex 587
Amygdaloid nucleus 501
Amylase 151, 171
pancreatic 171
salivary 151
Anacrotic limb 423
Anal canal 190
Analgesia 551, 557
Analgesia system 557
Anarthria 616
Anatomical shunt 430
Anchoring junctions 15
Androgen binding protein 334
Androgens 309
Androstenedione 309, 336
Anemia 60, 61, 62, 63
aplastic anemia 62
classification 60
classification of anemia 62
due to chronic diseases 62
folic acid deficiency 62
hemolytic 61
hemorrhagic 60
iron deficiency 61
megaloblastic 62
nutrition deficiency 61
pernicious 61
protein deficiency 61
sickle cell 61
signs and symptoms 63
thalassemia 61
Anermia
vitamin B12 deficiency 61
Anesthesia 551, 560
Angina pectoris 428
Angiotensin 417
Angiotensin converting enzyme
417, 448
Angiotensin converting enzyme (ACE)
214
Angiotensin II 417
Angiotensinases 214
Angiotensins 214
Anisocytes 47
Anisometropia 655
Anisometropia 656
Annulospiral endings 593
Anopia 646
Anorexia 63, 166, 564
Anosmia 671
Ansiform lobe 570
Antecubital vein 420
Anterior white column 534
Anterior ciliary vein 634
Anterior gray commissure 533, 534
Anterior median fissure 533
Anterior nucleus 559, 562
Anterior olfactory nucleus 670
Anterior pituitary 262
hormones secreted 262
Anterior serrati 451
Anterior white commissure 534
Anti-insulin hormones 299
Antibodies 77
structure 77
types 77
Anticoagulants 88
Antidiabetic hormone 296
Antidiuretic hormone 266
actions 266
Antidiuretic hormone 562
Antidromic 415
Antidromic vasodilator fibers 415
Antiemetic drugs 605
Antigen presenting cells 74
Antigens 73
definition 73
types 73
Antihemophilic factor 85
Antihypertensive drugs 577
Antiport 20
Antipyrine 35
Antiseptic action 180
Antiserum 95
Antrum 446
Anuria 96
Anvil 658
Aortic area 391
Aortic body 409
Aortic nerve 408
Apex beat 391
Apex beat area 391
Aphasia 587, 616
Apnea 195, 480
Apnea time 480
Apneic period 484
Apneusis 475
Apneustic center 475
Apocrine glands 246, 247
Apoferritin 44
Apoptosis 10, 12
Appendicitis 192
Appendix 190, 192
Aproferritin 45
Aptyalism 156
Aquaporins 226
Aqueductus sylvius 618
Aqueous humor 634
Arachidonic acid 321
Arachnoid mater 501
ARAS 589
Arche 363
Arcuate artery 216
Arcuate nucleus 562
Area for Equilibrium 587
Areas 1-8, 13, 17-19, 22, 41, 42, 44,
45, 407, 416, 583-587, 662
Argentaffin cells 187
Aromatase 265
Arousal mechanism 577
Arrhythmia 438
Arterial blood pressure 411-413
after exercise 441
applied physiology 419
determinants 412
during exercise 441
long term regulation 417
regulation 413
variations 412
Arterial pulse 422
Arterial pulse tracing 422
Arterial system 375
Arteries 375
Arterioles 375
Arterior blood pressure
definitions 411
normal values 411
Arteriosclerosis 281, 413
Arteriovenous shunt 430
Artificial musk 671
Ascending limb 212
Asphyxia 483
Astasia 574
Astereognosis 542, 552, 560
Asthenia 302, 574
Astigmatism 655, 656
Astrocytes 512
Asynergia 574
Ataxia 560, 574
Atherosclerosis 281, 413, 427
Atherosclerotic plague 427
Athetoid hand 560
Athetosis 560, 578
Atonia 574
Atonic bladder 243
applied physiology 243
ATP 10
synthesis 10
ATP driven proton pump 234
Atrial diastole 384
Atrial muscle 379
Atrial natriuretic peptide 304, 320
Atrial systole 384, 387

675
Index
Atrioventricular valves 374
Atropine, 155
Attitudinal reflexes 598
Auditopsychic area 586, 616
Auditory defects 666
Auditory disturbances 587
Auditory hallucinations 587
Auditory muscles 659
Auditory ossicles 658, 664
Auditory pathway 661
Auerbach’s plexus 147
Auricle 657
Auscultation areas 390
Autocatalytic action 170
Autoimmune diseases 79
Autoimmunity 79
Automatic associated movements 576
Automatic bladder 243
Autonomic nervous system 563, 621,
623
divisions 621
functions 623
neurotransmitters 623
parasympathetic division 623
sympathetic division 621
Autonomic nervous system regulation
563
Autoregulation 217
renal 217
Autosomes 358
AV node 379
Axial filament 340
Axillary temperature 249, 250
Axis cylinder 506
Axolemma 506
Axon 506
Axon reflex 415
Axon terminal 132, 520
Axoplasm 506
Axoplasmic flow 562
Axoplasmic vesicles 506
Ayerza’s disease 47
B
B cells 296
B estradiol 346
B Lipotropin 262, 265
Babinski’s sign 528, 530
Bactericidal agents 102
Bain bridge reflex 410
Ballistic movements 573
Ballistocardiography 403
Barometric pressure 489
at different altitudes 487
at different depths 489
Barometric pressure 487
Baroreceptor mechanism 415
Baroreceptors 408, 415, 477
Bartholin’s duct 150
Basal ganglia 575, 576, 577
applied physiology 577
components 575
functions 576
Basal ganglia 501
Basal metabolic rate 277
Basilar artery 428
Basilar membrane 659
Basopenia 67
Basophilia 67
Basophilic cells 262
Basophilic erythroblast 51
Basophils 65, 66, 68, 323
Bathmotropic 375
BBB 619
Bed bug 671
Bed wetting 243
Behavior 565
Behavioral pattern 566
Belching 166
Bell-dog experiments 614
Bell-Magendie law 523, 530
Bell’s palsy 155
Bends 490
Beta cells 262
Beta waves 607
Betz cells 123, 542
Bicarbonate mechanism 234
Biconcave lens 654
Biconvex 655
Bicuspid area 390
Bicuspid valve 374
Bile 176, 177, 180, 182
composition 176
concentration 182
formation 177
functions 180
properties 176
regulation of secretion 182
storage 177
Bile acids 177
Bile canaliculus 175
Bile pigments 56, 179
excretion 179
formation 179
Bile salt – activated lipase 171
Bile salts 177
enterohepatic circulation 177
formation 177
functions 177
Biliary system 174, 175
Bilirubin 44, 45, 56, 179, 180
conjugated 179
fate 179
normal plasma levels 180
unconjugated 179
Biliverdin 56, 179
Binocular vision 641
Biological clock 567
Biological transducers 514
Biot’s breathing 485
Bipolar cells 633, 661
Bipolar limb leads 393
Birth control 365
Bitter almonds 670
Bitter taste 669
Bjerrum screen 642
Bleeding disorders 91
von Willebrand disease 92
Bleeding time 90
Blind spot 633, 642
Blindness 587, 646
Bloating 191
Blood 38, 40, 299
cells 38
composition 38
functions 40
properties 38
specific gravity 38
viscosity 38
volume 38
Blood calcium level 284
Blood clot 88
Blood clotting 85
factors involved 85
sequence 85
Blood coagulation 85
definition 85
Blood flow 429
intestine 429
lungs 449
pancreas 429
splenic circulation 429
stomach 429
to lungs 449
to skeletal muscles 441
Blood flow 449
spleen 429
Blood flow to 431
heart 427
skeletal muscles 431
skin 431
Blood gas analysis 401
Blood glucose level 297, 299
normal 299
regulation 299
Blood groups 93
Blood matching 95
Blood phosphate level 285
Blood pressure 414, 563
regulation 563
short term regulation 414
Blood sugar level 299
maintenance 299
Blood transfusion 99, 100
autologous 100
exchange transfusion 100
Blood typing 94
Blood vessels 375

676
Essentials of Physiology for Dental Students
Blood volume 36, 413
measurement 36
Blood–cerebrospinal fluid barrier 620
Blood-brain barrier 15, 619
Blood-testis barrier 331
Body temperature 563
Body fluids 33, 34
compartments 33
composition 34
distribution 33
measurement 34
Body surface area 399
Body temperature 249, 250, 251
normal 249
regulation 251, 252, 563
variations 250
Body temperature 249, 252
Bohr’s effect 471
Bolus 193
Bone 292
cell types 292
functions 292
physiology 292
Bone 292, 293
applied physiology 293
formation 293
osteoporosis 293
remodeling 293
resorption 293
rickets 294
Bone conduction 664
Bony spiral canal 659
Border cells 660
Botulinum toxin 135
Bowman’s capsule 210
Bowman’s glands 670
Bradycardia 375, 405
Bradykinesia 577
Bradykinin 222, 324, 556
Brain 501
Parts 501
Brain attack 428
Brain natriuretic peptide 320
Brainstem 502
Breath holding 480
Bright light vision 639
Broca’s area 584, 615
Brodmann areas 582
Bronchi 446
Bronchial artery 449
Bronchioles 446
Brunner’s glands 187
Brush bordered cells 211
Buccal cavity 149
Buccal glands 150
Buccinator muscle 193
Bucket handle movement 452
Buffalo hump 310
Buffer nerves 410
Bulbourethral glands 332, 339
Bulbus oculi 629
Bulk flow 19
Bulldog scalp 269
Bundle of His 380
Bungarotoxin 135
Bursa of fabricius 72
Butyric acid 671
C
c-type natriuretic peptide 320
Cabbages 282
Caisson disease 490
Calcitonin 275, 288, 291, 292
actions 288
plasma level 288
regulation of secretion 288
Calcitriol 291
Calcium 21, 289, 290
absorption and excretion 290
daily requirements 290
in bones 289
in plasma 289
regulation 290
source of 289
transport 21
types 289
Calcium in the body 288
Calcium ions 21
Calcium metabolism 288
Calcium pump 21
Calcium rigor 124
Calcium–calmodulin complex 140
Callosomarginal fissure 580
Calpains 124
Calyx 601
Camphor 670
Canal of Schlemm 634
Canaliculus 162
Capillaries
features 429
functions 431
Capillaries 375, 429, 431
Capillary circulation 429
Capillary hydrostatic pressure 421
Capillary pressure 421
definition 421
normal values 421
regional variations 421
Capillary system
pattern 429
Caproic acid 671
Carbamylcholine 135
Carbon dioxide 467, 471
diffusing capacity 467
diffusion 467
transport 471
Carbon dioxide dissociation curve 472
Carbon monoxide poisoning 486
Carbonic acid 162, 234, 471
Carbonic anhydrase 162, 234, 471
Carboxyhemoglobin 55
Carboxypeptidases 170, 171
Carcinoma 82
Cardiac bruit 391
Cardiac center 405
Cardiac cycle 383
atrial events 384
duration 383
subdivisions 383
ventricular events 384
Cardiac cycle 383
Cardiac failure 437
Cardiac glands 158, 159
Cardiac index 399
Cardiac murmur 391
Cardiac muscle 114, 377
electrical potentials 377
properties 377
Cardiac muscle fiber 373
Cardiac output 398, 399, 401, 413
definitions 398
distribution 399
factors maintaining 399
measurement 401
normal values 398
variations 399
Cardiac pain 428, 557
Cardiac shock 96
Cardioaccelerator center 405
Cardioaccelerator reflex 410
Cardioinhibitory center 405
Cardioinhibitory reflex 408
Cardiovascular accident 428
Cardiovascular system 371
Carotid body 409
Carpopedal spasm 286
Carrier proteins 5, 20
Caruncula sublingualis 150
Cascade 85
Casein 169
Caseinogens 169
Castration 341
Catacrotic limb 423
Catacrotic notch 423
Cataplexy 568
Cataract 635, 637
Catecholamines 313, 314
actions 314
metabolism 314
plasma level 313
synthesis 313
Catecholamines 313
Cathelicidins 78, 447
Cathepsins 124
Catheter 401
Caudate nucleus 576
Cecum 190
Celiac ganglion 623

677
Index
Cell 3, 4
membrane 4
structure 4
Cell mediated immunity 74
Cell death 12
cell junction 14
Cells of claudius 661
Cells of Hensen 661
Central lobe 570
Central sulcus 580
Centrioles 9
Centrosome 7, 9
Cephalic phase 163, 172
Cerebellar cortex 572
Cerebellar hemispheres 569, 570
Cerebellar lesions 574
Cerebello-cerebral connections 572
Cerebellum 569, 570, 572
applied physiology 574
corticocerebellum neocerebellum
572
divisions 570
paleocerebellum 571
parts 569
spinocerebellum 571
vestibulocerebellum
archicerebellum 570
Cerebellum 571
Cerebral blood flow 428
applied physiology 428
normal 428
Cerebral blood vessels 428
Cerebral circulation 428
Cerebral cortex 580, 582
allocortex 580
lobes 580
neocortex 580
Cerebral cortex
archicortex 580
excitomotor cortex 582
frontal lobe 582
limbic lobe 587
occipital lobe 587
orbitofrontal 584
paleocortex 580
parietal lobe 585
precentral cortex 582
prefrontal 584
temporal lobe 586
visual cortex 587
Cerebral cortex 584
Cerebral dominance 580
Cerebral embolism 92
Cerebral hemispheres 501, 579
Cerebral palsy 553
Cerebro-cerebello-cerebral circuit 573
Cerebro-cerebral connections 572
Cerebroside 507
Cerebrospinal fluid 617
absorption 618
circulation 618
collection 619
composition 617
formation 618
functions 619
properties 617
Cerebrospinal fluid
applied physiology 620
Cervical cap 366
Cervical ganglia 623
Cervical mucus pattern 353
Cervix 345, 346, 348
Channel proteins 5
Chemical thermogenesis 253
Chemoattractants 68
Chemokines 78
Chemoreceptor mechanism 416
Chemoreceptors 409, 416, 477, 478,
479, 669
central 478
peripheral 479
Chemotaxis 66
Chenodeoxycholic acid 177
Chest electrode 394
Chewing 151, 193
Cheyne-Stokes breathing 484
Chicken chest 294
Chief cells 158, 159, 284
Chief sensory nucleus 533, 535
Chloride shift 471, 472
reverse 472
Chloroform 671
Cholagogue action 178, 180
Cholagogues 183
Cholecalciferol 285
Cholecystectomy 181
Cholecystokinin 166, 172, 173
Cholecystokinin-pancreozymin
(CCK-PZ) 173
Cholelithiasis 185
Choleretic action 178, 180
Choleretics 183
Cholesterol ester hydrolase 170
Cholesterol ester hydrolase 171
Cholic acid 177
Cholinesterase 521
Chorda tympani 153, 668
Chorda tympani syndrome 156
Chorea 560, 578
Choreoathetosis 578
Choroid 631
Choroid plexuses 618
Christmas factor 85
Chromaffin cells 313
Chromophil cells 262, 313
Chromophobe cells 262
Chromophore 639
Chromosomes 333
Chronic adrenal insufficiency 311
Chronic obstructive pulmonary
diseases (COPD) 465
Chronotropic action 375
Chvostek’s sign 287
Chyme 159, 196
Chymotrypsin 170, 171
Chymotrypsinogen 170
Cilia 630
Ciliary body 631
Ciliary body proper 631
Ciliary ganglion 648
Ciliary processes 631
Ciliospinal reflex 648
Circadian rhythm 567
Circle of Willis 428
Circulation 376
pulmonary 376
systemic 376
Circulatory shock 302, 437
definition 437
manifestations 437
Circulatory system 371
Cis face 8
Cisterna lateralis 618
Cisterna magna 618
Cisternae 120
Cisternal puncture 619
Citrates 90
Clara cells 453
Clarke’s nucleus 533, 539
Classical 366, 590
Claude Bernard 25
Clonus 124, 530
Clot retraction 88
Clotting 90
tests for 90
Clotting time 90
Clove 670
Co-transport 21
Coactivation 583
Coagulum 339
Cobalt 53
Cochlea 659
Cochlear duct 659
Cochlear microphonic potential 665
Cognitive control 576
Cold rigor 124
Colipase 170, 171
Collagenase 170, 171
Collateral ganglia 623
Collecting duct 213
Colloidal osmotic pressure 20, 221
Colon 190
Colony forming blastocytes 50
Colony forming unit 50
Colony stimulating 70
Colony stimulation factor 102
Color blindness 652, 653

678
Essentials of Physiology for Dental Students
Color blindness 652
classification 652
tests 653
Colored wool 653
Colostrum 363
Columns of Bertini 207
Coma 37, 302
Combined pills 366
Common bile duct 168, 175
Common hepatic duct 175
Common hepatopancreatic duct 175
Communicating junctions 15
Comparator function 573
Complement system 77
functions of 77
Complementary colors 651
Compliance 455
Compliance work 456
Compressed air sickness 490
Conditioned reflex 155, 164, 172
Conditioned reflexes 614, 615
classical 614
definition 614
instrumental 615
negative 615
operant 615
positive 615
types 614
Conditioned stimulus 615
Conduction 251
Conductive system 373
Conductivity 380
Cones 639
Confrontation test 642
Congenital adrenal hyperplasia 312
Conjunctiva 630
Connective tissue 4
Conscious kinesthetic sensation 542
Conscious movements 576
regulation 576
Constipation 191
Constrictor papillae 632, 648
Continuous murmur 391
Contraception 365
Contraceptive pills 366
Contractile proteins 118, 137
Contractile proteins 118
Contractility 381
Contraction 122
isometric 122
isotonic 122
Control 576
muscle tone 576
Convection 251
Converting enzyme 305
Convulsion 286, 484, 607
Convulsive seizure 607
Copper 53
Core temperature 250
Cornea 631
Corona radiata 544
Coronary arteries 426
Coronary artery disease (CAD) 427
Coronary blood flow 427
applied physiology 427
normal 427
phasic changes 427
Coronary blood vessels 426
Coronary circulation 426
Coronary collateral arteries 427
Coronary embolism 92
Coronary occlusion 427
Coronary sinus 426
Corpora cavernosa 333
Corpus albicans 354
Corpus luteum
fate 353
Corpus luteum 353
definition 353
development 353
functions 353
Corpus luteum graviditatis 354
Corpus spongiosum 333
Corpus striatum 576
Corresponding antibody 94
Corresponding retinal points 642
Cortex 207
Cortical auditory centers 662
Cortical nephron 210
Corticomedullary junction 209
Corticosterone 306
Corticotropes 262, 309
Corticotropin releasing factor 309
Corticotropin releasing hormone 262,
309
Cortisol 306, 309
secretion 309
Cortisone 306
Cottonmouth 156
Cough reflex 448
Coumarin derivatives 89
Counter transport 21
Countercurrent exchanger 229
Countercurrent flow 228
Countercurrent mechanism 228
Countercurrent multiplier 228
Cranial content volume 619
Crenation 47
Cretin vs dwarf 281
Cretinism 281
Crista Ampullaris 600
Cross bridges 118
Cross matching 95
Crude touch 538
Cryptorchidism 336
Crypts of Lieberkühn 186
CSF 617
Culmen 570
Cumulus oophorus 351
Cupula 601
Curare 135
Cushing’s disease 270, 310
Cushing’s syndrome 310
Cutaneous blood vessels 431
Cutaneous circulation 431
Cutaneous reflexes 529
Cyanide poisoning 482
Cyanocobalamin 53
Cyanopsin 639
Cyanosis 485
Cylindrical glass 656
Cystic duct 175
Cystometrogram 241
Cytochrome system 483
Cytokines 78, 215
Cytoplasm 6
Cytoplasmic
organelles 6
Cytoskeleton 7, 10
Cytotoxic T cells 75
D
D and C 367
D cells 298
Damping action 573
Dark adaption 640
Day light vision 639
Dead space 464
definition 464
measurement 464
normal value 464
types 464
Deafness 662, 666
Declive 570
Decompression sickness 490
Deep reflexes 529
Deep sea 489
Defecation 191, 200
Defecation reflex 200, 201
Defensins 78, 447
Deglutition 193
definition 193
stages 193
Deglutition apnea 195, 480
Deglutition reflex 195
Dehydration 36, 272
Dehydration exhaustion 493
Dehydroepiandrosterone 309
Delirium 37
Delta waves 607
Dementia 578, 614
Dendrite 506
Dendritic cells 447
Dense bodies 137
Dentate nucleus 572
Dentatorubrothalamocortical tract 572
Deoxycholic acid 177
Deoxyribonuclease 170
Depolarization 126, 127
Depressor area 405

679
Index
Dermatomal rule 557
Dermatome 557
Dermis 244
Descent of testes 336
Desmosome 16
Detoxification 181
Detrusor muscle 239
Deuteranomaly 653
Deuteranopes 652
Deuteranopia 652
Deuterium oxide 35
Deviation movement 574
Dextrinase 187, 188
Di-iodotyrosine (DIT) 275
Diabetes insipidus 272, 567
Diabetes mellitus 79, 300, 301
Diabetogenic hormones 299
Diapedesis 66
Diaphragm 451, 452
Diarrhea 191
Diastasis 387
Diastole 383
Diastolic blood pressure 411
Diastolic murmur 391
Dichromatism 652
Dichromats 652
Dicoumoral 89
Diencephalon 501, 558
Diffusing capacity 467, 496
Diffusion 18, 19
facilitated 19
factors affecting 19
simple 18
DiGeorge's syndrome 78
Digestion 145
Digestive functions 169
Digestive organs 146
Digestive system 145
functional anatomy 145
gastrointestinal tract 145
Dihydrotestosterone 336
Dihydroxyphenyl-alanine (DOPA) 314
Dilatation and curettage 367
Dilator papillae 632
Dilator pupillae muscle 648
Dim light vision 639
Diopter 638
Diplegia 554
Diplopia 642
Disodium salt 89
Dissociated anesthesia 551
Distal convoluted tubule 213
Diver’s palsy 490
Doctrine of specific nerve energies 516
Dominant hemisphere 582
DOPA decarboxylase 314
Dopamine 313, 317
Doppler Echocardiography 403
Dorsal nucleus of vagus 406
Dorsal respiratory group of neurons
474
Dorsal venous arch 420
Dorsolateral nucleus 559, 562
Double vision 642
Douglas bag 465
Dreamy states 587
Drinker’s method 495
Dromotropic 375
Drooling 156
Drum beating tremor 577
DUBB 389
Ducts of Belini 207
Ducts of Ravinus 150
Ductus arteriosus 433, 434
Ductus venosus 432, 435
Duke method 90
Duodenal ulcer 166
Duodenum 186
Dura mater 501
Dwarfism 270, 271
causes 270
signs and symptoms 271
Dwarfism 271
in dystrophia adiposogenitalis 271
laron 271
psychogenic 271
Dye dilution method 34
Dysarthria 574, 616
Dysbarism 490
Dysgeusia 669
Dysmenorrhea 357
Dysmetria 574
Dysphonia 616
Dyspnea 484
Dystrophia adiposogenitalis 272, 342,
567
E
Ear 657, 659
external 657
internal 659
middle 657
Ear dust 601
Eaton-Lambert syndrome 135
EC cells 159
Eccrine glands 246, 247
ECD
leads 393
ECF volume 35
measurement 35
ECG 392, 393, 396
definitions 392
intervals 396
segments 396
uses 392
waves 394
ECL cells 159
Edema 108
definition 107
extracellular edema 108
intracellular edema 108
non-pitting 108
non-pitting edema 108
pitting 108
types 108
Edinger-Westphal nucleus 648, 649
Edridge-Green lantern 653
EEG 606
Effector organ 527
Efferent circuit 572
Eicosanoids 321
Einthoven 394
Ejection period 386
Elastase 170, 171
Elastic resistance 456
Electrical analgesia 557
Electrocardiogram 392
Electrocardiograph 392
Electrocardiographic grid 392
Electrocardiography 392
Electroencephalogram 606
definition 606
method of recording EEG 606
waves 606
Electronic sound transducer 391
Elevators of scapulae 451
Elliptocytosis 47
Embolism 92
Embolus 92, 427
Embryo
implantation 359
Embryo 359
Emmetropia 654
Emmetropic eye 654
Emotional changes 565
Emulsification of fat 170
Emulsification of fats 177
Emulsion 177
End disc 340
End ring centriole 340
Endocardium 374
Endocrine glands 257
Endocrinology 257
Endocytosis 22, 23
receptor mediated 23
Endolymph 599, 659
Endometrium 345, 354
Endomysium 116
Endoneurium 506
Endopeptidase 169, 170
Endoplasmic reticulum 6, 7
Endosomes 22
Endothelins 418
Endothelium 375
Endplate potential 129, 134
miniature 134
Enteric nervous system 147
Enterochromaffin (EC) cells 158, 159
Enterochromaffin cells 159, 187

680
Essentials of Physiology for Dental Students
Enterochromaffin-like (ECL) cells
158, 159
Enterocrinin 189
enterocytes 186
Enteroendocrine cells 159
Enterogastric reflex 165
Enterohepatic circulation 176
Enterokinase 169, 188
Enteropeptidase 169
Enuresis 243
Enzyme cascade theory 85
Eosinopenia 67
Eosinophil cationic protein 68
Eosinophil derived neurotoxin 68
Eosinophil peroxidase 68
Eosinophilia 67
Eosinophils 65, 66, 68
Ephedrine 155
Epicardiac arteries 426
Epicardium 372
Epidermis 244
Epididymis 330, 331
Epilepsy 607, 608
definition 607
generalized 608
grand mal 608
localized 608
psychomotor 608
types 608
Epimysium 116
Epinephrine 313
Epineurium 506
Epithelial tissue 4
Equilibrium 574
disturbances 574
Erb sign 287
Errors of refraction 654
Erythroblastosis fetalis 98
Erythrocyte sedimentation rate 57, 58
factors affecting 58
variations 58
Erythrocyte sedimentation rate 58
definition 57
determination 57
normal values 58
Erythrocytes 43
Erythropoiesis 49, 50, 51
definition 49
Erythropoiesis 52
changes during 50
factors necessary for 51
process of 50
site of 49
stages of 51
Erythropoietin 52, 206, 319
Escape phenomenon 304, 320
Esophageal Doppler transducer 403
Esophageal stage 195
Estriol 346
Estrogen 346, 347, 360
functions 346
mode of action 347
plasma level 346
regulation of secretion 347
secretion 347
Estrogen binding protein 330
Estrone 346
Ethyl ether 671
Ethylenediaminetetra acetic acid
(EDTA) 58, 89
Eunuchism 341
Eustachian tube 659, 664
Exchange in lungs 466
Excitability 121, 377, 508
Excitation contraction coupling 129
Excretion 205
Exercise 439, 440
aerobic 440
anaerobic 440
cardiovascular adjustments 439
effects on cardiovascular system
440
severity of 440
types 439
Exocytosis 23
Exopeptidases 170
Exophthalmos 280
Expiration 445
Expiratory muscles 451
Expiratory reserve volume 458
Exposure to cold 492
Exposure to heat 493
External anal sphincter 146, 200
External auditory meatus 657
External intercostal muscles 451
External limiting membrane 633
External urethral sphincter 239
External work 439
Exteroceptors 515
Extracellular fluid 33
Extrafoveal vision 634
Extrafusal fibers 593
Extrahepatic biliary apparatus 175
Extraspectral colors 651
Extrinsic factor 161
Extrinsic nerve supply 147
Extrinsic pathway 86, 87
Eyeball 629, 630
applied physiology 636
functional anatomy 629
wall 630
Eyelids 629
F
F actin 119
Facial nerve 153
Facial plethora 310
Facilitation 614
Facultative reabsorption 225
fallopian tubes 345, 346, 348
Family planning 365
Farrel and Ivy pouch 163
Fascia 116
Fasciculata secretes 309
Fasciculus cuneatus 536, 541
Fasciculus dorsolateralis 540
Fasciculus gracilis 536, 541
Fasiculus dorsolateralis 536
Fatigue 122, 382
Fatty liver 277
Feces 191
Feedback mechanism 27
negative 27
positive 27
Feeding center 563
Female 343
accessory sex organs 345
reproductive system 343
secondary sexual characters 347
Fenestra 210, 211
Fenestra vestibuli 659
Ferrihemoglobin 56
Ferritin 44, 45
Fertility control 365
Fetal 55
Fetal circulation 432
Fetal lungs 432, 433
Fetoplacental unit 361
Fetus 432
blood vessels 432
Fever 250
Fibrin 86
Fibrin monomer 86
Fibrin stabilizing factor 81, 85
Fibrinogen 86, 332
Fibrinolysin 332, 355
Fibrinolysis 88
Fibroblasts 244
Fibrosa 146
Fick’s principle 237, 401
Field of vision 641
Filtration 19
Filtration fraction 220
Filtration membrane 220
Filtration pores 210
Final common pathway 553
Firing level 127
First breath 433
First class proteins 53
First order neurons 534
Fistula 163
Flechsig’s tract 539
Flocculi 569
Flocculonodular lobe 569
Flower spray ending 593
Flowers 671
Fluid mosaic model 4
Focal adhesions 16
Focal length of cornea 638
Focal length of lens 638

681
Index
Folic acid 53
Follicle stimulating hormone 262, 265
follicular cells 274
Follicular phase 350
Follicular sheath 351
Food intake 563, 564
regulation 564
Foramen magnum 501, 532
Foramen of Luschka 618
Foramen of Magendie 618
Foramen of Monro 618
Foramen ovale 432, 434
Forced expiratory volume (FEV) 460
Fourth ventricle 618
Fovea centralis 634
Foveal vision 634
Fragility 47
Frank-Starling law 125, 400
Free load 125
Free nerve ending 515, 595
Fröhlich’s syndrome 272, 342, 567
Frontal eye field 584
Frontal lobe syndrome 584
Frostbite 493
Functional residual capacity 459
Fundic glands 158
Fundus 633
Fundus oculi 633
G
G actin 119
G cells 159
Gait 294, 574, 578
drunken like gait 574
festinant gait 578
myopathy 294
waddling gait 294
Galactopoiesis 364
Gallbladder 181
functions 181
Gallstones 185
Gamma amino butyric acid (GABA) 522
Gamma interferon 75
Gamma motor neurons 533, 576, 593
Ganglionic cells 633
Gap junction 15
Garlic 671
Gasping 484
Gastric 172
Gastric atrophy 166
Gastric content 197
Gastric glands 158
Gastric inhibitory peptide (GIP) 166
Gastric juice 160, 161, 162
composition 160
functions 160
properties 160
regulation of secretion 162
secretion 161
Gastric phase 165, 172
Gastric secretion 163
phases of 163
Gastric ulcer 166
Gastrin 165, 172
Gastritis 166
Gastrocolic reflex 200, 201
Gastrointestinal hormones 323
Gastrointestinal tract 146, 193
movements 193
nerve supply 147
Gastrointestinal tract 145, 147
functional anatomy 145
wall 146
Gate control theory 557
Gated channels 18
Gelatinase 161
General anesthesia 551
General static reflexes 596
General vasoconstrictor 316
Generator potential 517
Geniculate ganglion 153
Germ cells 330
Germ hill 351
Germinal ridges 344
Gestation period 361
Ghrelin 565
GI hormones 166, 189
Giant cells 542
Giants 269
Gigantism 269
Glands 150
Glans penis 333
Glaucoma 634, 636
Glia 512
Glial cells 512
Globin 55, 56
Globus pallidus 576
Glomerular capillaries 210
Glomerular capillary 216
Glomerular capillary membrane 220
Glomerular capillary pressure 221
Glomerular filtration 220
Glomerular filtration rate 220, 237
measurement 237
Glomerulotubular balance 224
Glomerulus 210
Glossopharyngeal nerve 153, 408,
409, 668
Glottis 195
Glucagon 298
actions 298
mode of action 298
regulation of secretion 298
Glucocorticoid 308
Glucocorticoids 306, 308
functions 306
mode of action 308
plasma level 306
regulation of secretion 308
Gluconeogenesis 296
Glucose 296, 299
promotes peripheral 296
Glucose receptors 564
Glucose transporter 2 (GLUT 2) 226
Glucostatic mechanism 564
Glucostats 564
Glucosuria 301
Glutamic acid 235
Glutaminase 235
Glutamine 235
Glycerolphosphate acetyl-transferase
10
Glycine 235
Glycocalyx 6
Glycocholate 177
Glycocholic acid 177
Glycogenesis 296
Glycogenolysis 296
Goblet cell 187
Goiter 282
nontoxic goiter 282
toxic goiter 282
Goitrin 282
Goitrogens 282
Golgi apparatus 7, 8, 340
Golgi tendon organ 594
Gonadotropes 262
Gonadotropic hormones 262
Gonadotropin releasing hormone 263
Gower’s tract 538
Graafian follicle 352
Graded potential 127, 129
Granular cells 214
Granulocytes 64
Granulosa cells 351
Graves' disease 79, 280
Graveyard of RBCs 44, 103
Gravindex test 362
Gravitational force 527
Gray horns 533
Greater circulation 376
Growth 263, 264, 297
Growth hormone 262, 263
actions 263
basal level 263
diabetogenic effect 263
mode of action 264
regulation 264
Growth hormone inhibitory hormone
262
Growth hormone releasing hormone
262
Growth hormone releasing polypep-
tide 262
Guerrilla face 269
Gynecomastia 311, 341
Gyri 580

682
Essentials of Physiology for Dental Students
H
‘H’ zone 118
Habituation 613, 614
Hair cells 600, 661
organ of Corti 661
Hairpin bend 212
Haldane effect 473
Haldane-priestly tube 465
Halitosis 156
Hallucination 552
Hamburger phenomenon 471
Hammer 658
Hashimoto's thyroiditis 79
hCG antibody 362
Hearing 665
theories 665
Hearing mechanism 663
Heart 371, 372, 374, 375, 380, 406,
407
actions 375
conductive system 380
hormones 320
hormones secreted by 320
left side 371
motor nerve fibers 406
regulation of actions 375
right side 371
sensory nerve fibers 407
septa 372
valves 374
wall 372
Heart 320, 371, 372
Heart attack 427
Heart failure 437, 438
acute 438
chronic 438
congestive 438
definition 437
Heart rate 404, 405, 413, 441, 563
during exercise 441
normal 404
rregulation 405, 563
Heart sounds 388, 390
description 388
importance 388
methods of study 390
Heartbeat 383
Heartburn 167
Heat cramps 493
Heat exhaustion 493
Heat gain 250
Heat gain center 251, 563
Heat loss 251
Heat loss center 251, 563
Heat production 250
Heat rigor 124
Heatstroke 493
Hegman factor 86
Hegman factor (Contact factor) 85
Helicobacter pylori 166
Helicotrema 659, 664
Helper T cells 75
Hematemesis 167
Hematocrit value 59
Heme 56
Hemianopia 587, 646
Hemiballismus 578
Hemidesmosome 16
Hemiesthesia 551
Hemiparesthesia 551
Hemiplegia 554
Hemoglobin 54, 56
content 54
normal 54
Hemoglobin 54, 55, 56
abnormal 55
derivatives 55
destruction 56
functions 54
structure 55
synthesis 56
types 55
Hemoglobinopathies 55
Hemolysins 48
Hemolysis 47, 48
Hemophilia 91
Hemopoietic growth factors 53
Hemorrhage 60, 436, 437
acute 437
causes 436
chronic 437
definition 436
effects 437
types 436
Hemostasis 82, 84
Henson 118
Heparin 88, 323
Heparinase 89
Hepatic artery 176
hepatic bile ducts 175
Hepatic cells 175
Hepatic duct 175
Hepatic jaundice (hepatocellular) 184
Hepatic lobes 174, 175
Hepatic lobules 174
Hepatic plate sinusoid 175
Hepatic plates 174
Hepatic sinusoids 176
Hepatic vein 176
Hepatitis 184
Hepatocytes 174
Hering-Breuer reflex 476
Hering’s nerve 408, 409
Hertz 665
High altitude 487
High molecular weight 86
Higher intellectual functions 612
Hippocampus 501, 613, 614
Hirsutism 310
Histamine 155, 323
Histiocytes 102, 244
Histology 284
Hodgkin’s disease 82
Holger Nielsen method 495
Homeostasis 25, 205, 562
Homeostatic system 26
Homeothermic animals 249
Homunculus 585
motor 583
sensory 585
Homunculus 583
Horizontal cells 633
Hormonal action 259
mechanism 259
Hormone 259
receptors 259
Hormone replacement therapy 349
Hormone–receptor complex 259
Hormones 258
classification 258, 259
secreted by gonads 258
secreted by major glands 258
Hormones 259
Human chorionic gonadotropin 338,
359, 361
Human chorionic somatomammotropin
360
Human leukocyte antigen (HLA) 74
Humoral
immunity 76
Hunger 563
Hunger contractions 196
Huntington’s disease 578
Hyaline membrane disease 453
Hyaluronidase 340
Hydorgen
transport 21
Hydrocephalus 620
Hydrochloric acid 161, 162
secretion 162
Hydrocholeretic agents 183
Hydrogen 21
Hydrogen ions 233
secretion 233
Hydrogen peroxide 102
Hydrogen pump 21
Hydrops fetalis 98
Hydrostatic pressure in Bowman’s 221
Hydroxyl ions 102
Hyper-reflexia 286
Hyperactivity 302
Hyperaldosteronism 311
primary 311
secondary 311
Hyperalgesia 551, 557
hyperbaric oxygen 483
Hypercalcemia 287
Hypercapnea 483
Hyperesthesia 551
Hyperinsulinism 302

683
Index
Hypermetria 574
Hypermetropia 654, 656
Hyperopia 654
Hyperosmia 671
Hyperosmolarity 227
Hyperparathyroidism 287
Hyperphagia 564
Hyperplasia 287
Hyperpneic period 484
Hyperpolarization 129, 603, 639
Hyperproteinemia 40
Hypertension 419
Hyperthermia 250
Hyperthyroidism 280
Hypertonia 125
Hyperventilation 480
Hypoactivity 300
Hypocalcemia 286
Hypocapnea 483
Hypoesthesia 551
Hypogastric ganglion 240
Hypogenitalism 568
Hypogeusia 669
Hypoglycemia 302
Hypogonadotropic hypogonadism 342
Hypokinesia 577
Hypometria 574
Hypoparathyroidism 286
Hypophyseal stalk 266
Hypoproteinemia 41
Hyposmia 671
Hypotension 419
Hypothalamic eunuchism 272, 342
Hypothalamo-hypophyseal portal
blood vessels 264, 563
Hypothalamo-hypophyseal portal
vessels 262
Hypothalamo-hypophyseal tract
266, 267, 562
Hypothalamus 501, 562, 566, 567
applied physiology 567
functions 562, 566
nuclei 562
Hypothermia 250
Hypothyroidism 281
Hypotonia 125, 574
Hypoventilation 480
Hypoxia 481, 482, 483
anemic 481
causes 481
classification 481
effects of 482
histotoxic 482
hypoxic 481
stagnant 481
treatment for 483
Hysterectomy 349
I
‘I’ band 117
Ice packs 557
Icterus 183
Ileum 186
Illusion 552
Image forming mechanism 638
Immune deficiency diseases 78
Immunity 72, 73
acquired immunity 72
definition 72
innate immunity 72
types 72
Immunoglobulin (Ig) 77
Impedance 664
Impedance matching 664
Implantation 359
Incompetence of heart valve 391
Incus 658
Indicator (Dye) dilution method 402
Indicator dilution method 34
Indicators 34
Infarct 92
Infarction 92
Inferior mesenteric ganglion 623
Inferior oblique 636
Inferior rectus 636
Inferior salivatory nucleus 153
Inferior vena cava 176
Inflammation 308
Infrared rays 651
Infundibular process 266
Infundibular stem 266
Inhibin 331
Inhibitory hormones 262, 563
Inhibitory post synaptic potential
(IPSP) 522
Inotropic 375
Insensible perspiration 251
Inspiration 445
Inspiratory capacity 458
Inspiratory muscles 451
Inspiratory ramp 476
Inspiratory reserve volume 458
Inspired air 465
Insulin 296
actions 296
basal level 296
mode of action 297
regulation of secretion 297
Insulin dependent diabetes mellitus
(IDDM) 300
Insulin like growth factor 264
Integral proteins 5
Intention tremor 560, 574
interatrial septum 372
Intercalated disk 373
Intercalated duct 151
Intercostal nerve 475
Interdigestive phase 166
Interferons 78
interleukin-1 70
Interleukin-2 75
Interleukin-4 68
interleukin-5 68
Interleukins 78
Interlobar arteries 216
Interlobular ducts 151
Intermediate filaments 11
Intermediate trapezoid fibers 662
Intermediolateral nucleus 533
Internal anal sphincter 146, 200
Internal carotid artery 428
Internal intercostal muscles 451
Internal limiting membrane 633
Internal urethral sphincter 239
Internodal fibers of Bachman 380
Internodal fibers of Thorel 380
Internodal fibers of Wenckebach 380
Internode 507
Interoceptors 516
Interstitial cells of Leydig 331
Interstitial fluid volume 36
measurement 36
Interventricular septum 372
Intestinal glands 186
Intestinal lipase 188
Intestinal phase 165, 173
Intestinal villi 186
Intra-abdominal pressure 400
Intra-alveolar pressure 454
Intracellular fluid 33
Intracellular fluid volume 36
measurement 36
Intrafusal muscle fibers 593
Intralaminar nuclei 559
Intralobular duct 168
Intramural vessels 426
Intraocular fluids 634
Intraocular pressure 453, 634
Intrapleural space 445
Intrapulmonary pressure 454
Intrathoracic pressure 400, 453
Intravesical pressure 241
Intrinsic factor of castle 53, 161, 188
Intrinsic nerve supply 147
Intrinsic pathway 86, 87
Inulin clearance 237
Involuntary functions 552
Involuntary nervous system
503, 621
Iodide pump 275
Iodide trapping 275
Iodine 275
Iodopsin 639
Iodotyrosine deiodinase 276
Iodotyrosine residues 275
Ipsilateral 574

684
Essentials of Physiology for Dental Students
Iris 631
Iris angle 632
Iron 53, 55
Ischemia 92, 556
Ishihara’s color charts 653
Islets of Langerhans 296
Isoagglutinin 94
Isoelectric base 127
Isometric contraction 384
Isometric muscular contraction 439
Isometric relaxation 386
Isotonic muscular contraction 439
Isovolumetric contraction 384
Isovolumetric relaxation 386
Isthmus 274
IUCD 367
Ivan pavlov 614
J
‘J’ receptors 476
Jaeger’s chart 641
James Parkinson 577
Jaundice 96, 183, 184
cholestatic 183
extrahepatic 184
hemolytic 183
hepatic 183
hepatocellular 183
obstructive 184
posthepatic 184
prehepatic 183
types 183
Jejunum 186
Jugular vein 420
Jugular venous pulse 424
Juxtacapillary receptors 476
Juxtaglomerular apparatus 214
definition 214
functions 214
structure 214
Juxtaglomerular cells 214
Juxtamedullary nephrons 210
K
kallidin 324
kallikrein 86
Karl Landsteiner 93
Kernicterus 98, 578
Ketoacidosis 197
ketosis 197
Kidney 205, 206, 207
different layers 206
functional anatomy 206
functions 205
parenchyma 207
Kidneys
blood flow 216
blood vessels 216
Kidneys 216
Kinesthesia 548
Kinins 324
Kinocilium 601, 661
Kinogen 86
Kluver-Bucy syndrome 587
Krause’s end bulb 515
Kupffer’s cells 101, 175, 181
Kussmaul breathing 302
Kyphosis 269, 294
L
‘L’ tubules 120
Labial glands 150
Labile factor 85
Labyrinth 599, 659
Lacrimal gland 630
Lactase 187, 188
Lactation 363
Lacteal 186, 187
Lactoferrin 152
Lactogenesis 363
Lactotropes 262
Lamina reticularis 661
Landsteiner’s law 94
Laparoscope 367
Laplace law 242
Large intestinal juice 190
composition 190
functions 190
Large intestine
applied physiology 191
functionsl anatomy 190
functions 191
movements 200
secretions 190
Large intestine 191, 200
Laryngeal muscles 135
Laryngeal stridor 286
Laryngospasm 286
Latent period 127
Latent tetany 287
Lateral geniculate body 559, 644
Lateral lemniscus 662
Lateral nucleus 562
Lateral or external rectus 636
Lateral sulcus 580
Lateral ventricles 618
Lateral white column 534
Latex particles 362
Laurence-Moon-Biedl syndrome 568
Lavender 670
Laxative action 178, 180
Lead pipe rigidity 578
Learning 612
associative 613
definition 612
non-associative 613
types 612
Learning 613
Lecithin 507
Left atrium 372
Left ventricle 372
Length-tension relationship 140
Lens 635
Lenticular nucleus 576
Lenticular process 658
Leptin 323, 564, 565
Lesser petrosal nerve 153
Leukemia 66
Leukocytes 64
Leukocytosis 66
Leukopenia 66
Leukopoiesis 70
Leukotrienes 321, 322
Life protecting hormone 306
Life saving hormone 304
Ligand gated channels 18
Light adaptation 640
Light reflex 647, 648
Limbic system 587
components 587
functions 587
Limbic system 670
Lingual 150
Lingula 570
Lipase 152, 160, 170
gastric 160
intestinal 187
lingual 152
pancreatic 170
Lipase 187
Lipolytic enzyme 152, 170
Lipostatic mechanism 564
Lipoxins 321, 322
Lithocholic acid 177
Liver 174, 175, 180
applied physiology 183
blood supply 175
cirrhosis 184
functional anatomy 174
functions 180
Lobulus ansiformis 570
Lobulus paramedianus 570
Lobulus simplex 570
Local anesthesia 551
Local hormones 258, 321
Local myenteric reflex 165
Local static reflexes 597
Local vasoconstrictors 418
Localization 583
motor 583
sensory 585
Locus ceruleus 611
Long sightedness 654
Loop of Henle 212
Lordosis 294
Lower costal series 452
Lower motor neuron 553
Lower motor neuron lesion 531, 553
LUBB 389
Lumbar ganglia 623

685
Index
lumbar puncture 619
Lung 457
function tests 457
Lung capacities 458
Lung chamber 495
Lung function tests 457
Lung volumes 457
Lungs 452, 476, 477
collapsing tendency 452
irritant receptors 477
‘J’ receptors 476
movements 452
stretch receptors 476
Luteal phase 353
Luteinizing hormone 262, 265
Luteolysis 322, 354
Lymph 105, 106
composition 105
flow 105
formation 105
functions 106
Lymph nodes 104
functions 104
Lymphatic system 104
Lymphatic vessels 104
Lymphocytes 65, 66, 70, 72
B lymphocytes 72
development 72
processing 72
T lymphocytes 72
Lymphocytopenia 67
Lymphocytosis 67
Lymphoid stem cells (LSC) 50
Lysocephalin 170
Lysolecithin 170
Lysosomes 7, 9
Lysozyme 152, 247
M
‘M’ line 118
Macrocytes 47
Macrogenitosomia praecox 312
Macrophage 101
Macrophage system 101
Macrophages 447
Macula 601
Macula densa 213, 214, 221
Macula lutea 634
Major basic protein 68
Major calyces 207
Major histocompatiblility complex
(MHC) 74
Malabsorption 189
Male condom 366
Males 329, 331, 337, 340, 341
accessory sex organs 331
climacteric 340
hypergonadism 341
hypogonadism 341
primary sex organs 329
scrotum 329
testes 329
reproductive system 329
gametes 329
gonads 329
testes 329
testis 329
secondary sexual characters 337
seminal vesicles 331
Malleus 658
Malnutrition 173
Malpighian corpuscle 208
Malpighian pyramids 207
Maltase 187, 188
Mamillary body 562
Mammary glands 267, 345, 348, 362
development 362
role of hormones 363
Manual methods 494
Marey’s law 408
Marey’s reflex 408
Marginal nucleus 533, 538, 556
Marijuana 156
Marker substances 34
Mass peristalsis 200
Masseter muscle 193
Mast cell 68, 323, 447
Mastication 193
movements involved 193
movements of 193
muscles 193
muscles of 193
Maturation factors 53
Maturity onset diabetes mellitus 300
Maximum breathing capacity 460
Maximum ventilation volume 460
Mean arterial blood pressure 411
Mean corpuscular hemoglobin
concentration (MCHC) 60
Mean corpuscular volume (MCV) 60
Meat 671
Mechanical methods 495
Mechanically gated channels 18
Mechanotransduction 603
Medial geniculate body 559, 662
Medial lemniscus 541, 668
Medial longitudinal fasciculus 543, 545
Medial or internal rectus 636
Median eminence 266
Median septum 534
Mediastinum 371
Mediastinum testis 330
Medical termination of pregnancy 367
Medulla 207
Medullary gradient 228
Medullary hyperosmolarity 228
medullary interstitium 229
Megacolon 191
Megakaryocytes 50
Megaloblast 51
Meissner’s corpuscle 515
Meissner’s nerve plexus 147
Melanin 245
Melatonin 318
Memory 613, 614
applied physiology 614
consolidation 614
definition 613
explicit 613
implicit 613
long term 614
physiological basis 614
short term 613
types 613
Memory T cells 75, 76
Menarche 350
Meninges 501
Meningocytes 102
Menopause 349, 350
Menorrhagia 357
Menstrual bleeding 354
Menstrual cycle 350, 354, 356
changes in cervix during 356
changes during 350
definition 350
duration 350
ovarian changes 350
regulation 356
uterine changes 354
Menstrual phase 354
Menstruation 354
Mental retardation 271, 281, 568
Merkel’s disk 515
Mesangial cells 214
Mesencephalic nucleus 550
Mesencephalon 501
Mesenchymal cells 49
Mesenteric circulation 428, 429
Meta-arterioles 430, 431
Metarhodopsin 639
Metathalamus 501, 559
Metencephalon 502
Methemoglobin 56
Methyl mercaptan 671
Metrorrhagia 357
Microcirculation 429
Microcytes 47
Microfilaments 11
Microglia 513
Microphone 391
Micropills 366
Microtubules 11
Microvilli 186
Micturition 239
Micturition reflex 242
Midbrain 501
Midline nuclei 559
Migrating motor complex 199
Milieu interieur 25

686
Essentials of Physiology for Dental Students
Milk 169, 170, 363
synthesis 363
Milk ejection 364
Milk ejection reflex 27, 267, 364
Milk let down reflex 267
Milk secretion 363
Mineralocorticoids 304
functions 304
plasma level 304
Mineralocorticoids 304
mode of action 305
regulation of secretion 305
Minipills 366
Minor calyces 207
Minute volume 398
Mitochondria 7, 133
Mitochondrion 10
Mitral area 390
Mitral cells 670
Mitral valve 374
Mixing movements 198, 200
Modiolus 659
Molar glands 150
Monochromatism 652
Monochromats 652
Monocular vision 641
Monocytes 65, 66, 69
Monocytopenia 67
Monocytosis 67
Monoiodotyrosine (MIT) 275
Mononucleotides 170
Monoplegia 554
Monosodium glutamate 669
Monosynaptic reflex 596
Moon face 310, 568
Morula 359
Motion sickness 605
Motor activities 552, 576
control 576
Motor endplate 132, 594
Motor pathways 552
Motor unit 135
Mountain sickness 487
Mouth 149
functional anatomy 149
functions 149
Mouth to mouth method 494
Movements 574
Movements 545, 552, 573, 577
coordination of 552
disturbances 574
poverty 577
skilled 545
timing and programming 573
types 552
voluntary 545
MTP 367
Mucin 182
Mucous membrane reflexes 528
Mucus cells 159
Mucus layer 146
Mucus neck cells 158, 159
Müeller’s maneuver 421
Müllerian duct 336
Müllerian regression factor (MRF) 331,
336
Müller’s fibers 633
Müller's law 516
specificity of response 516
Müller's law 516
Müller’s supporting fibers 633
Multipolar cells 633
Mumps 156
Muscarine 155
Muscle 113-115, 118, 122, 131, 136
cardiac 115
classification 113
contractile elements 118
involuntary 113
muscles (slow) 122
nonstriated 113
pale 122
pale (fast) muscles 122
proteins 118
red 122
relaxation 131
skeletal 114
smooth 115, 136
striated 113
voluntary 113
Muscle fiber 116, 137
Muscle mass 116
Muscle pump 400
Muscle spindle 593, 594
functions 594
nerve supply 593
structure 593
Muscle tissue 4
Muscle tone 125, 595, 596
definition 595
development 595
regulation 596
significance 595
Muscular contraction 126, 129, 131
electrical changes 126
energy 131
molecular basis 129
molecular changes 129
Muscular layer 146
Musk 671
Myasthenia gravis 79, 135
Myelencephalon 502
Myelin 507
Myelinogenesis 507
Myenteric nerve plexus 147
Myocardial infarction 427
Myocardial ischemia 427
Myocarditis 438
Myocardium 372
Myoepithelial cells 267
Myofibril 117, 137
Myofilaments 137
Myogenic response 218
Myometrium 345
Myopia 654, 656
Myosin filaments 118
Myosin light chain kinase 140
Myosin molecule 118
Myotatic reflex 596
Myxedema 281
N
Narcolepsy 568
Narcosis 489
Natriuresis 320
Natural killer cell 78
Nausea 197
Necrosis 12, 92, 427
Negative after potential 128
Neopallium
isocortex 580
Neoplasm 155
Nephrogenic diabetic insipidus 272
Nephron 208, 211
cortical 208
defined 208
juxtamedullary 208
tubular portion 211
types 208
Nerve cell 504
Nerve cell body 505
Nerve fiber 512
classification 507
myelinated 507
nonmyelinated 507
refractory period 509
regeneration 512
Nerve fiber 507
action potential 509
adaptation 510
all or none law 510
autonomic 508
cranial nerves 508
degeneration 510
Erlanger and Gasser classified 508
infatigability 510
motor 508
myelinated 508
nonmyelinated 508
properties 508
resting membrane potential 509
sensory 508
somatic 508
spinal nerves 508
summation 510
visceral 508
Nerve fibers 507, 508, 510
Nerve growth factor 507

687
Index
Nerve impulse 509
action potential 509
conductivity 509
Nerve of filling 240
Nerve of micturition 241
Nerve roots 532
Nervous intermedius of wrisberg 153
Nervous system 501, 502, 503
autonomic 503
central 501
peripheral 502
somatic 502
Nervous tissue 4
Net filtration pressure 221
Neural stalk 266
Neurilemma 506, 507
Neurilemmal tube 512
Neurodegenerative disease 614
Neuroendocrine reflex 364
Neuroepithelium 601
Neurofibrils 505
Neuroglia 501, 512
Neurohypophysis 266
Neuromuscular blockers 134
Neuromuscular junction 132
definition 132
structure 132
Neuromuscular junction
disorders 135
Neuromuscular transmission 133
Neuron 505
bipolar 505
classification 504
definition 504
Golgi type i 505
Golgi type ii 505
motor 505
multipolar neurons 505
sensory 505
structure 505
unipolar 505
Neuron 504
Neuropeptide Y 565
Neuroplasm 505
Neurotransmitters 524, 525
Neurotrophic factors 507
Neurotrophins 507
Neutropenia 67
Neutrophilia 67
Neutrophils 64, 66, 68
Newton’s third law of motion 403
Nexus 15
Nickel 53
Nicotinic acid 53
Night blindness 640
Night vision 639
Nipple 362
Nissl bodies 505
Nitric oxide (NO) 419
Nitrogen narcosis 489
Nitrogen washout method 464
Nocturnal micturition 243
Node of Ranvier 507
Nodulus 569, 570
Non-nutritional blood flow 430
Nonelastic viscous resistance 456
Nonmetabolizable saccharides 35
Nonspecific sensory pathway 590
Nonsteroidal anti-inflammatory drugs
(NSAIDs) 166
Noradrenaline
actions 315
mode of action 315
regulation of secretion 317
Noradrenaline 313, 314, 317, 623
Norepinephrine 313
Normoblast 51
Nuclear bag fiber 593
Nuclear chain fiber 593
Nuclear membrane 11
Nucleases 170, 171
Nucleoli 12
Nucleoplasm 12
Nucleus 7, 11
Nucleus of lateral lemniscus 662
Nucleus of trigeminal nerve 550
Nutritional flow 430
Nyctalopia 640
Nystagmus 574, 603
O
Obesity 564
Obligatory reabsorption 225
Occipital eye field 587, 646
Occluding junction 14
Ocular movements 636
Ocular movements 637
Ocular muscles 135, 635
Oculomotor (third) nerve 636
Odor 670
Odoriferous substance 670
Olfactory cortex 670
Olfactory glomeruli 670
Olfactory hyperesthesia 671
Olfactory mucous membrane 670
Olfactory pathway 670
Olfactory receptors 670
Olfactory sensation 671
applied physiology 671
threshold 671
Olfactory sensation 671
Olfactory tract 670
Olfactory tubercle 670
Oligodendrocytes 513
Oligomenorrhea 357
Ollecting ducts 207
Oncotic pressure 20
One way conduction 523, 530
Onion 671
Opioid peptides 557
Opsin 639
Optic axis 629
Optic chiasma 644
Optic disk 633
Optic nerve 643
Optic pathway 643
Optic radiation 645
Optic tract 644
Ora serrata 631, 632
Oral cavity 149
Oral contraceptives 366
Oral stage 194
Oral temperature 249, 250
Orbiculus ciliaris 631
Orbital cavity 629
Organ 4
Organ of Corti 660
Orthograde degeneration 511
Osmosis 19, 20
Osmotic diuresis 301
Osmotic pressure 19
Ossicular conduction 664
Osteoblasts 292, 293
Osteocytes 293
Osteomalacia 294
Osteoprogenitor cells 292
Otic ganglion 153
Otitis media 666
Otoconia 601
Otolith membrane 601
Otolith organ 600
Otosclerosis 666
Oval window 658
Ovarian follicles 350
Ovariectomy 349
Ovaries 343-345
functions 345
hormones 346
primary sex organs 343
structure 344
Overactive reflex actions 286
Overflow dribbling 243
Overflow incontinence 243
Overhydration 37
Overshoot 127
Ovulation 352
Ovulation time 352
Ovum 358
fertilization 358
Ovum 351, 358
Oxalate compounds 90
Oxidants 102
Oxidative enzymes 489
Oxygen 467, 469
diffusing capacity 467
diffusion 467
oxygen carrying capacity 470
oxygen hemoglobin dissociation
curve 470

688
Essentials of Physiology for Dental Students
oxyhemoglobin 469
transport 469
Oxygen debt 496
Oxygen poisoning 483
Oxygen therapy 483
Oxygen toxicity 483
Oxyntic cells 158
Oxyntic glands 158
Oxyphil cells 284
Oxytocin 267, 268
actions 267
mode of action 268
P
P cells 379
P wave 397
‘P’ wave 394
P-R interval 397
‘P-R’ interval 396
P50 470
Pacemaker 373, 379
Pacemaker cells 379
Pacemaker potential 379
Pacinian corpuscle 515, 594
Pacinian corpuscles 517
Packed cell volume 59
definition 59
method of determination 59
normal values 59
significance 59
variations 59
Packed cell volume 44
PAH clearance 237
Pain 538, 555
Pain receptors 477
Pain relievers 557
Pain sensation 555, 556, 560
applied physiology 557
benefits 555
center 556
components 555
definition 555
pathways 556
referred 557
spontaneous 560
visceral 556
Pain sensation 556, 557
Palatal glands 150
Pale muscle 123
Pallanesthesia 552
Pallesthesia 548
Palpebral fissure 630
Pancreas 168, 295, 296
applied physiology 300
endocrine functions 295, 296
exocrine part 168
functional anatomy 168
nerve supply 168
Pancreatic amylase 171
Pancreatic duct 175
Pancreatic juice 168, 169, 171, 172
composition 168
functions 169
neutralizing action 171
properties 168
properties 168
regulation of secretion 172
Pancreatic lipase 171
Pancreatic polypeptide 299
actions 299
mode of action 299
regulation of secretion 299
Pancreatic polypeptide 173
Pancreatitis
applied physiology 173
Paneth cell 187
Panhypopituitarism 271
Panting 251
Papez circuit 614
Papilla 207
Papillary muscles 374
Paraffin 246
Paralgesia 551, 557
Paralysis 553, 554
types 554
Paralysis agitants 577
Paramedian lobe 570
Paraplegia 554
Parasympathetic vasodilator fibers 415
Parasympathomimetic drugs 155
Parathormone 284, 285, 291, 292
actions 284
mode of action 285
plasma level 284
regulation of secretion 285
Parathyroid function tests 288
Parathyroid glands 284, 286
applied physiology 286
Parathyroid poisoning 287
Parathyroidectomy 286
Paraventricular nucleus 562
Paravertebral ganglia 621
Paresthesia 551
Parietal cells 158, 159, 162
Parieto-occipital sulcus 580, 585
Parkinsonism 577
Parkinson’s disease 577
Parotid glands 149
Pars distalis 262
Pars intermedia 262
Pars tuberalis 262
Parturition 27, 28, 98, 361
Passive transport 19
special types 19
Pasties 156
Patent ductus arteriosus 435
Pavlov 162
Pavlov’s pouch 162
Peak expiratory flow rate (PEFR) 460
Pectorals 451
Pectus carinatum 294
Pedicles 211, 220
Pelvic nerve 200, 240
Pendular movements 199, 530, 574
Penis 332
Peppermint oil 671
Pepsin 160
Pepsinogen 161
Pepsinogen cells 158
Peptic ulcer 166
Peptidases 188
Peptidases 187
Peptide mechanism 565
Peptide YY 166, 173, 565
Perfumes 671
Pericardial cavity 372
Pericardium 372
Perichoroidal space 631
Perilymph 599, 659
Perimeter 642
Perimysium 116
Perineurium 506
Periodic breathing 484
Periodontal tissues 550
Peripheral ganglia 623
Peripheral proteins 5
Peripheral resistance 413
Peripheral resistance 401
Peristalsis in fasting 199
Peristaltic contraction 196
Peristaltic movements 199
Peristaltic rush 199
Peritoneum 345
Peritubular capillaries 216
Peritubular capillary 217
Perivitelline space 351
Permissive action 307
Peroxisomes 7, 9
Petit mal 608
PGE2 222
Phagocytosis 23, 66, 102
Phagosome 23
Phalangeal cells 660
Pharyngeal muscles 135
Pharyngeal stage 194
Phenyl-ethanolamine-N-
methyltransferase (PNMT) 314
Phenylalanine 313
Pheochrom cells 313
Pheochromocytoma 317
Phlebogram 424
Phonocardiogram 391
Phonocardiography 391
Phosphate
importance 291
normal value 291
regulation 291
Phosphate buffer system 235
Phosphate mechanism 234, 235
Phosphate metabolism 291

689
Index
Phosphaturic action 285
Phospholipase A 170, 171
Phospholipase B 170, 171
Phospholipid
dipalmitoylphosphatidylcholine
453
Phospholipids 4
Phosphoryl choline 170
Photopic vision 639
Photoreceptors 639
Photosensitive pigments 639
Phototransduction 639
Phrenic nerve 475
Physiological shunt 426, 430, 449
in capillaries 430
in heart 430
Physiological shunt 430
Physo-stigmine 155
Pia mater 501
Pigeon chest 294
Pill rolling movements 577
Pillar cells 660
Pilocarpine 155
Pineal gland 318
Pinna 657
Pinocytosis 22
Pituicytes 266
Pituitary 262
anterior 262
Pituitary cachexia 271
Pituitary gland 261, 268, 269
applied physiology 268
disorders 269
posterior 266
Pituitary tumor 269
Place theory 665
Placenta 359
functions 359
Placenta 359, 432
Plasma 39
Plasma cells 76
Plasma clearance 237
Plasma membrane 4
Plasma protein 40
albumin/globulin ratio 40
normal values 40
variations 40
Plasma proteins 40, 41
albumin 40
functions 41
globulin 40
origin 41
properties 41
Plasma thromboplastin antecedent 85
Plasma volume 36, 238
measurement 36
Plasmin 88
Plasminogen 88
Plasticity 140
Plateau 378
Platelet 81
granules 81
Platelet activating factor 70, 78, 81, 85
Platelet derived growth factor (PDGF)
82, 102
Platelet plug 85
Platelets 80-82
applied physiology 82
composition 80
development 82
disorders 82
fate 82
functions 81
lifespan 82
normal count 81
properties 81
shape 80
size 80
structure 80
variations 81
Pleura 445
Pleural cavity 445
Pleural sac 445
Pneumotaxic center 475
Podocytes 211, 220
Poikilocytosis 47
Poikilothermic animals 249
Polarized light 117
Polychromatic erythroblast 51
Polycythemia 46
Polycythemia vera 46
Polydactylism 568
Polydipsia 272, 301
Polygraph 391
Polymenorrhea 357
Polypeptide chains 55
Polypeptides 169
Polyphagia 302
Polyuria 272, 301
Pontine nuclei 572
Pontocerebellar fibers arise 572
Porphyrin 44, 45, 55
Porpyropsin 639
Portal triad 175
Portal vein 176
Positive after potential 128
Positive feedback 86
Postcatacrotic wave 423
Posterior 340, 533
Posterior column ataxia 542
Posterior end knob 340
Posterior gray commissure 533
Posterior nerve root ganglia 535
Posterior nucleus 562
Posterior white column 534
Posterolateral nucleus 559
Posthepatic jaundice (obstructive) 184
Postmenopausal syndrome 349
Postsynaptic membrane 521
Postsynaptic neuron 520
Postural reflexes 596
Postural reflexes 597
Posture and equilibrium 592
definition 592
Pot belly 310
Power house of the cell 10
Power stroke 130
Pre-emulsified fats 152
Precatacrotic wave 423
Precocious body growth 312
Precocious sexual development 312
Preferential channels 430
Pregnancy test 361
Prehepatic jaundice (hemolytic) 184
Preload 400
Premotor area 542, 583
Preoptic nucleus 562
Preparatory position 194
Prepyriform cortex 670
Presbyopia 635, 650, 656
Pressor area 405
Presynaptic inhibition 522
Presynaptic membrane 521
Presynaptic neuron 520
Pretectal nucleus 645
Pretectal nucleus 648
Primary auditory area 586
Primary colors 651
Primary follicle 351
Primary motor area 582
Primary sensory nerve fiber 593
Primary sensory nucleus 550
Primary visual area 587, 646
Primordial follicle 351
Proaccelerin 85
Procarboxypeptidase 170
Procoagulants 90
Procollagenase 170
Proelastase 170
Proenzymes 85
Proerythroblast 51
Progesterone 348, 349, 360
functions 348
mode of action 349
plasma level 348
regulation of secretion 349
Prognathism 269
Progressive hepatolenticular
degeneration 578
Prolactin 262, 265
Prolactin inhibitory hormone 263
Proliferative phase 355
Proline-rich proteins 152
Proprioceptors 477, 592
Propulsive movements 199, 200
Prosencephalon 501
Prostacyclin 321, 322
Prostaglandins 215, 319, 321, 367

690
Essentials of Physiology for Dental Students
Prostate gland 332
Prostatic fluid 332
composition 332
functions 332
properties 332
Protanomaly 653
Protanopes 652
Protanopia 652
Protein channels 18
Protein-sparing effect 297
Proteoglycan 108
proteoglycan meshwork 109
Proteolytic enzymes 169
Proteoses 169
Prothrombin 85
Prothrombin activator 85
Prothrombin time 91
Protodiastole 386
Proximal centriole 340
Proximal convoluted tubule 211
Pseudohermaphroditism 312
Pterygoid muscles 193
Ptyalism 155, 156
Puberty 337
Pudendal nerve 200, 241
Pulmonary area 391
Pulmonary arterial pressure 450
Pulmonary artery 449
pulmonary artery arises 371
Pulmonary blood flow 449
Pulmonary capillary pressure 450
Pulmonary circulation 449
pulmonary blood vessels 449
Pulmonary congestion 477
Pulmonary embolism 92
Pulmonary function 457
Pulmonary veins 371
Pulmonary ventilation 496
Pulse points 423
Pulse pressure 411
Pulvinar 559
Pump 21
Pump handle movement 452
Punishment center 566
Pupil 632
Pupillary dilator muscle 632
Pupillary reflexes 647
Purkinje fibers 379, 381
Purpura 91, 92
idiopathic thrombocytopenic 91
thrombasthenic 92
thrombocytopenic 91
Purpuric spots 91
Pus 68
Pus cells 68
Putamen 576
Pyknosis 51
Pyloric glands 158, 159
Pyramid 544, 570
Pyramidal cells 542
Pyramidal decussation 544
Pyridoxine 53
Pyriform cortex 587
Pyrole rings 55
Q
‘Q-T’ interval 396, 397
‘QRS’ complex 394, 397
Quadriplegia 554
R
‘R-r’ interval 397
Rachitic rosary 294
Radial pulse 423
Radiation 251
Raffini’s end organ 515
Rage 567
Raphe nucleus 611
Rapid filling 386
Reaction time 530
Rebound phenomenon 531, 574
Recanalization 367
Receptive relaxation 196
Receptor blocker 135
Receptor potential 517
Receptor proteins 5
Receptors 514, 516, 592
chemoreceptors 515
classification 514
cutaneous 514
definition 514
distance receptors 515
exteroceptors 514
interoceptors 515
kinesthetic 592
phasic receptors 516
pressure 514
properties 516
proprioceptors 516
telereceptors 515
tonic receptors 516
touch 514
visceroceptors 515
Recompression 491
Rectal temperature 249, 250
Rectum 190
Red blood cells 43, 44, 45, 47
morphology 43
normal value 43
Red blood cells 43, 47
fate 44
fragility 47
functions 45
hemolysis 47
lifespan 44
properties 44
structure 44
suspension stability 44
variations in number 45
variations in shape 47
variations in size 47
Red bone marrow 49
Red muscle 123
Referred pain 428
Reflex 526, 528, 530
antigravity 527
bulbar 527
carotid sinus 528
cerebellar 527
classification 527
conditioned 527
cortical 527
deep 528
definition 526
fatigue 531
flexor 527
in motor neuron 531
medullary 527
midbrain 527
monosynaptic 527
oculocardiac 528
pathological 528
plantar 528
polysynaptic 528
properties 530
protective 527
pupillary 528
recruitment 530
reflex arc 526
spinal 527
summation 530
superficial 528
tendon 528
unconditioned 527
visceral 528
withdrawal 527
Refractory period 125, 382
cardiac muscle 382
nerve fiber 509
skeletal muscle 125
types 382
Refractory power 638
Regional circulation 426
Relaxin 360
Releasing hormones 262, 563
Renal artery 216
Renal blood flow 217, 221, 238
measurement 217, 238
regulation 217
Renal corpuscle 208
Renal function tests 236
Renal plasma flow 237
measurement 237
Renal shutdown 96
Renal sinus 207, 216
Renal system 205
Renal threshold 225
Renal vein 217
Renin 214, 319

691
Index
Renin–angiotensin system 214
Renin-angiotensin mechanism 417
Rennin 161
Renshaw cell inhibition 522
Renshaw cells 533
Repolarization 126, 128
Reproductive system 327
female 327
male 327
Respiratory distress syndrome 453
Reserve proteins 42
Residual volume 458
Resistant vessels 401
Respiration 445
phases 445
types 445
Respiration
445, 451, 474, 480, 494, 496
artificial 494
definition 445
disturbances 480
effects of exercise 496
mechanics 451
muscles of 451
regulation 474
Respiratory bronchioles 446
Respiratory centers 474, 476
factors affecting 476
integration 476
medullary 474
pontine 475
Respiratory diseases 461
Respiratory distress syndrome 453
Respiratory gases 466, 468
exchange 466
exchange at tissue level 468
transport 466
Respiratory membrane 447, 466
Respiratory minute volume 460, 463
Respiratory movements 451
Respiratory pressures 453
Respiratory pump 400, 454
Respiratory rate 445
Respiratory sinus arrhythmia 408
Respiratory tract 445-447
functional anatomy 445
lower 446
nonrespiratory functions 447
upper 446
Respiratory unit 446, 466
Respirometer 401
Resting membrane potential 126, 128,
138, 377
ionic basis 128
skeletal muscle 126
smooth muscle 138
Resting tremor 577
Resuscitation 494
Resuscitator 494
Retching 197
Rete testis 331
Reticular formation 589, 591
ascending reticular activating
system 589
definition 589
descending reticular system 591
divisions 589
functions 589
situation 589
Reticulocyte 51
Reticuloendothelial 101
Reticuloendothelial cells 101
Retina 632
Retinal 639
Retinol 639
Retro-orbital tissues 280
Retrograde degeneration 511
Reuptake process 134
Reward center 566
Rh factor 96
Rh incompatibility 97
Rhesus monkey 96
Rheumatoid arthritis 79
Rhodopsin 639
Rhombencephalon 502
Rhythm method 365
Rhythmicity 379
Riboflavin 53
Ribonuclease 170
Ribosomes 7
Rickets 294
Right atrial reflex 410
Right atrium 371
Right ventricle 371
Righting reflexes 596
Rigidity 577
Rigor 124
Rigor mortis 124
Roasted coffee 671
Rods 639
Rolandic fissure 580
Rouleaux formation 44
S
S-T segment 397
SA node 379, 380
electrical potential 379
Saccule 600, 604
Sacral ganglia 623
Safe period 365
Saliva 151, 155
applied physiology 155
composition 151
effect of drugs 155
functions 151
hypersalivation 155
hyposalivation 155
properties 151
regulation of secretion 153
Salivary glands 149, 150, 153
classification 150
duct system 150
minor 150
nerve supply 153
structure 150
sublingual glands 150
submandibular glands 150
submaxillary glands 150
Salt taste 669
Saltatory conduction 509
Sarcolemma 117, 373
Sarcomere 117, 137
Sarcoplasm 117, 373
Sarcoplasmic reticulum 120
Sarcotubular system 119, 137, 373
Satellite cells 513
Satiety center 564
Sausages 199
Scala media 659
Scala tympani 659
Scala vestibuli 659
Scaleni 451
Scalp electrodes 606
Schwann cells 507, 512, 513
Sclera 631
Scoliosis 294
Scopolamine 155
Scotopic vision 639
Scotopsin 639
Sea sickness 605
Sebaceous glands 246, 337
Sebum 246, 337
Second messenger 260
Second order neurons 534
Secondary sensory nerve fiber 593
Secondary sexual characters 337
Secondary tympanic membrane 659
Secretin 166, 172, 173
Secretory phase 355
Secretory vesicles 9
Segmental static reflexes 598
Segmentation contractions 198, 200
Seizures 37
Selective reabsorption 223
Semen 332, 339
composition 339
properties 339
Semicircular canals 600
Semilunar valves 374
Seminal fluid 332
composition 332
functions 332
properties 332
Seminiferous tubules 330
Senile decay 271
Sensation of vibration 542
Sensations 538, 547-549
applied physiology 551
conscious kinesthetic 549

692
Essentials of Physiology for Dental Students
crude touch 549
deep 548
definition 547
epicretic 548
fine touch 548, 549
kinesthetic 548, 549
of vibration 548
pain 549
pressure 549
protopathic 548
quality 559
somatic 547
special 547
subconscious kinesthetic 549
tactile 548
temperature 549
types 547
vibratory 549
visceral 548
Sensations 548, 551, 559
Sensitization 613
Sensory adaptation 516
adaptation 516
Sensory area 414
Sensory ataxia 542
Sensory pathways 548
Sensory transduction 516
Sensorymotor area 586
Septula testis radiate 330
Sequential pills 366
Serotonin 323, 324
Sertoli cells 330
Serum 39
Servomechanism 573
Sex chromosomes 358
Sex determination 358
Sex differentiation in fetus 336
Sexual infantilism 567
Sham feeding 163
Sham rage 567
Sheath of Schwann 507
Shivering 251, 492
Shock 437
Short ciliary nerves 648
Short sightedness 654
Sialism 155
Sialolithiasis 155
Sialorrhea 155
Sialosis 155
Sickle cell 47
Silicon 102
Simmond’s disease 271
Sinoaortic mechanism 410, 416
Sinoatrial node 371, 379
Sinusoid 175
Sjögren’s syndrome 156
Skeletal muscle 116, 121
properties 121
structure 116
Skeletal muscle 114, 120
composition 120
Skilled activities 576
Skin 244, 245, 247, 310
appendages 244
color 245
functions 247
glands 245
pigmentation 245
pigmentation of 310
structure 244
Skull 501
Sleep 565, 609, 611
centers 611
definition 609
mechanism 611
non-rapid eye movement sleep
(NREM or non-REM slee 610
physiological changes 609
rapid eye movement sleep (REM
sleep) 610
regulation 565
requirement 609
stages 611
types 609
Sleep apnea 480
Sliding mechanism 130
Sliding theory 130
Slit pore 211, 220
Slow filling 387
Slow wave potential 138
Slow wave rhythm 139
Slow waves 138
Small intestine 186, 188, 198
applied physiology 189
functional anatomy 186
functions 188
glands 186
movements 198, 199
villi 186
Small intestine 199
Smell 670
Smooth muscle 136, 137, 139, 140
distribution 136
electrical activity 137
multiunit 137
structure 136
types 137
Smooth muscle 114, 136, 137, 140
contractile process 139
control 140
molecular basis 140
single unit 137
tonic contraction 139
Sneezing reflex 448
Snellen’s chart 640
Sodium 225
reabsorption 225
Sodium co-transport 21
Sodium counter transport 21
Sodium-dependant glucose trans-
porter 226
Sodium-hydrogen antiport pump 234
Sodium-hydrogen pump 234
Sodium-iodide symport pump 275
Sodium-potassium 21
Sodium-potassium pump 21, 128
Somatic chromosomes 358
Somatic nerve fibers 239
Somatic sensations 547
Somatomedin 264
Somatomedin-C 264
Somatomotor system 552
Somatosensory pathways 550
Somatosensory system 547
Somatostatin 298
actions 298
mode of action 299
regulation of secretion 299
Somatostatin 166, 173
Somatotropes 262
Somatotropic hormone 262
Somesthetic area 585
Somesthetic association area 586
Somnolence 279
Sound 665, 666
localization 666
loudness/intensity 665
pitch/frequency 665
properties 665
Sound transduction 665
Sour taste 669
Soybean 282
Spasm 286
Special sensations 629
Special senses 629
Specific sensory pathways 590
Spectral colors 651
Speech 615, 616
applied physiology 616
definition 615
mechanism 615
nervous control 615
Speech apparatus 447, 615
Sperm 339, 340
structure 340
total count 339
Spermatids 334
Spermatocyte 334
Spermatogenesis 333-335
activin 335
cryptorchidism 336
estrogen 335
FSH 335
GH 335
inhibin 335
LH 335
mumps 336
role of hormones 334
role of Sertoli cells 334

693
Index
stages 333
testosterone 335
Spermatogenic cells 330
Spermatogonia 334
Spermatogonium 333
Spermatozoa 334
Spermatozoon 339
Spermeogenesis 334
Spermicidal action 366
Spermicidal substances 366
Spermination 334
Spherocytosis 47
Sphincter of Oddi 175
Sphincter pupillae 632
Sphingomyelin 507
Spike potential 128, 139
Spinal anesthesia 551
Spinal canal 532
Spinal cord 501, 532, 533, 534, 542
ascending tracts 534
descending tracts 542
gray matter 532
internal structure 532
segments 532
white matter 533
Spinal nerve 532
Spiral ganglion 661
Spirogram 457
Spirometer 457
Splanchnic circulation 428
Splanchnic region 401
Spleen 44, 103
functions 103
Splenic pulp
hepatic blood vessels 429
hepatic circulation 429
Splenic pulp 429
hepatic artery 429
portal vein 429
Splenic vein 176
Squalene 246
Stable factor 85
Stage of hyperpnea 484
Staircase phenomenon 381
Stammering 616
Stapedius 659
Stapes 658
Static reflexes 596
Static tremor 577
Statoconia 601
Statokinetic reflexes 598
Statotonic 598
Steatorrhea 173
Stem cells 50, 70, 293
Stenosis of heart valve 391
Stensen’s duct 149
Stercobilin 180
Stercobilinogen 180
Stereocilia 601, 661, 665
Stereocilium 601
Stereognosis 542
Sterilization 367
Sternocleidomastoid 451
Sternum 391
Sterols 246
Stethoscope 390
Stimulus 121, 122
intensity 121
number 122
strength 122
types 121
Stirrup 658
Stomach 157, 159, 196
applied physiology 166
cardiac region 158
corpus 158
emptying 196
filling of 196
functional anatomy 157
functions 159
fundus 158
movements 196
parts 157
peristalsis 196
pyloric region 158
wall 158
Stratum corneum 244
Stratum germinativum 244, 245
Stratum granulosum 244
Stratum lucidum 244
Stratum spinosum 244
Streptokinase 88
Stress 307
Stretch reflex 596
Stridor 286
Stroke 428
Stroke volume 398
Stuart-Prower factor 85
Stunted growth 270
Subarachnoid space 501, 618
Subconscious kinesthetic sensation
539
Subconscious movements 576
regulation 576
Subliminal stimulI 381
Submaxillary ganglion 153
Submucus layer 146
Submucus nerve plexus 147
Subneural clefts 133
Subpapillary plexus 431
Substance P 323
Substantia gelatinosa of Rolando
533, 538, 556
Substantia nigra 576
Subthalamic nucleus of Luys 576
Subthalamus 501
Succinylcholine 135
Succus entericus 187, 189
regulation of secretion 189
Succus entericus 187
composition 187
functions 187
properties of 187
Sucrase 187, 188
Sulci 580
Sulfhemoglobin 56
Sulfide poisoning 482
Sulfur 671
Sulfur dioxide 477
Summation 381, 382
Sunstroke 493
Superior colliculus 645
Superior mesenteric ganglion 623
Superior mesenteric vein 176
Superior oblique 636
Superior olivary nucleus 662
Superior rectus 636
Superior salivatory nucleus 153
Superior vena cava 371, 420
Superoxide 102
Supplementary motor area 584
Supporting reactions 597
Supporting reflexes 597
Suppressor area 583
Suppressor T cells 75
Suprachiasmatic nucleus 562
Supraoptic nucleus 562, 645
Suprarenal glands 303
Surface temperature 249
Surface tension 452
Surfactant 452
Suspension stability of RBCs 57
Sustentacular cells 670
Swallowing reflex 449
Sweat glands 246
Sweating 253
Sweet cheese 671
Sweet taste 669
Sylvian fissure 580
Sylvian sulcus 585
Sympathetic chain ganglia 621
Sympathetic ganglia 621
Sympathetic tone 407
Sympathetic vasoconstrictor fibers 414
Sympathetic vasodilator fibers 415
Sympathoadrenergic system 623
Sympathomimetic drugs 155
Symport 20
Synapse 15, 519-523
axoaxonic 519
axodendritic 519
axosomatic 520
chemical 520
classification 519
definition 519
electrical 520
electrical property 523
excitatory 521
excitatory postsynaptic potential 521

694
Essentials of Physiology for Dental Students
fatigue 523
functions 521
inhibitory 522
properties 523
summation 523
Synapse 519, 520
Synaptic cleft 133, 521
Synaptic delay 523
Synaptic gutter 133
Synaptic trough 133
Synaptic vesicles 133, 521
Syncytium 137, 373
cardiac muscle 373
Syndrome of inappropriate hyperse-
cretion of antidiuretic hormone
(SIADH) 272
System 4
Systole 383
Systolic blood pressure 411
Systolic murmur 391
T
T lymphocytes 319
processing 319
‘T’ tubules 119
T wave 397
T-1824 36
Tachycardia 375, 404
Tactile anesthesia 551
Tactile discrimination 542
Tactile hyperesthesia 551
Tactile localization 541, 549
Tank respirator 495
Taste blindness 669
Taste buds 667
basal cells 668
circumvallate papillae 667
filiform papillae 667
fungiform papillae 667
gustatory sensation 667
papillae of tongue 667
sustentacular cells 668
taste pore 668
taste receptor cells 667
Taste hallucinations 669
Taste pathway 668
Taste sensation 667, 669
applied physiology 669
Taste sensations and chemical
constitutions 669
Taste transduction 669
Taurocholate 177
Taurocholic acids 177
Tectorial membrane 661
Telencephalon 501
Temporal bone 657
Temporal lobe syndrome 587
Temporal muscle 193
Tendon 116
Tensor tympani 659
Terminal ganglia 623
Testes 341
applied physiology 341
effects of extirpation 341
Testis 330, 333, 336
coverings 330
descent 336
endocrine functions 336
functional anatomy 330
functions 333
gametogenic functions 333
Testosterone 309, 336, 338
functions 336
mode of action 338
regulation of secretion 338
Tetanus 123, 382
Tetany 286
Tetraiodothyronine – T4 (thyroxine) 275
Tetraplegia 554
Tetrapyrole 55
Thalamic hand 560
Thalamic nucleus 551
Thalamic phantom limb 560
Thalamic syndrome 560
Thalamus 501, 558, 559
applied physiology 560
functions 559
nuclei 558
Thalassemia 55
Thebesian veins 426
Theca externa 351
Theca folliculi 351
Theca interna 351
Thermal sensations 538
Thermanalgesia 552
Thermanesthesia 552
Thermoanesthesia 552
Thermodilution technique 402
Thermoreceptors 251, 447
Thermostatic mechanism 565
Theta waves 607
Third order neurons 535
Third ventricle 618
Thirst mechanism 565
Thoracic cage 451, 452
Thoracic ganglia 623
Thoracic lid 452
Thoracolumbar outflow 621
Threshold 121
Threshold stimulus 121
Thrombasthenia 82
Glanzmann 82
Thrombocythemia 82
Thrombocytosis 82
Thromboplastin 85, 86
Thrombopoietin 319
Thrombosis 92
Thrombospondin 81
Thrombosthenin 81
Thromboxane A2 85
Thromboxanes 321, 322
Thrombus 92, 427
Thymin 319
Thymosin 319
Thymus 318
Thyroglobulin 275
Thyroid adenoma 280
Thyroid function tests 282
Thyroid gland 274, 275, 280
applied physiology 280
histology 274
hormones 275
Thyroid hormones 275-277, 279
functions 277
mode of action 279
regulation of secretion 279
storage 276
synthesis 275, 276
transport 277
Thyroid stimulating hormone 262, 279
Thyroidectomy 286
Thyrotropes 262
Thyrotropic releasing hormone 262
Thyroxine 275, 277
Thyroxine binding globulin (TBG) 277
Thyroxine binding prealbumin (TBPA)
277
Tidal volume 457
Tight junction 14
Timed vital capacity 460
Tinnitus 587
Tissue 3
Tissue factor 85
Tissue fluid 107
definition 107
formation 107
functions 107
Tissue macrophages 101
Tissue plasminogen activator 88
Tissue resistance work 456
Tm value 225
TmG 225
Tobacco 671
Torso 310
Total body water 33, 35
measurement 35
Total lung capacity 459
Touch sensation 541
Trabeculae 634
Trachea 446
Tracheobronchial tree 446
Tract 535, 536, 538-543, 545, 546
anterior corticospinal 543
anterior spinothalamic 535, 536
anterior vestibulospinal 543, 545
corticospinal 542
dorsal spinocerebellar 536, 539
extrapyramidal 545
lateral corticospinal 543
lateral spinothalamic 536, 538

695
Index
lateral vestibulospinal 543, 545
of Goll 541
of Lissauer 540
olivospinal 543, 546
pyramidal 542
reticulospinal 543, 546
rubrospinal 543, 546
spino-olivary 536, 540
spinoreticular 536, 540
spinotectal 536, 539
spinovestibular 536, 540
tectospinal 543, 546
ventral spinocerebellar 536, 538
Tracts in spinal cord 534
Tractus solitarius 408, 668
Trail ending 594
Trans face 8
Transcytosis 24
Transducer 514
Transduction 516
sensory 516
Transforming growth factor 102
Transient receptor potential 517
Transmembrane potential 126
Transmitter-receptor complex 521
Transneuronal degeneration 512
Transport 17, 20
active 20
basic mechanism 17
passive 17
Transpulmonary pressure 455
Trapezoid body 662
Trauma 47, 82
Traveling wave 664
Traveling wave theory 665
Trehalase 187, 188
Trehalose 187
Trehalose glucohydrolase 187
Tremor 560, 577
Trephone substances 42
Tri-iodothyronine – T3 275
Triad of skeletal muscle 120
Trichromatism 652
Trichromats 652
Tricuspid area 391
Tricuspid valve 374
Trigeminal ganglion 550
Trigeminal lemniscus 551
Trigeminal nerve 153, 550
Trigone 239
Triple heart sound 390
Tripotassium salt 89
Tritanomaly 653
Tritanopia 652
Tritium oxide 35
Trochlear (fourth) nerve 636
Tropomyosin 119, 130
Troponin 119, 130
Trousseau’s sign 287
True capillaries 430
Trypsin 169, 171
Trypsinogen 169
Tubectomy 367
Tuber 570
Tubular reabsorption 223
Tubular secretion 226
Tubular transport maximum 225
Tubulin 80
Tubuloglomerular feedback 218, 221
Tumor necrosis factors 78, 102
Tunica adventitia 375
Tunica albuginea 330, 344
Tunica externa 630
Tunica fibrosa 630
Tunica interna 632
Tunica intima 375
Tunica media 375, 631
Tunica nervosa 632
Tunica vaginalis 330
Tunica vasculosa 330, 631
Tympanic cavity 657
Tympanic membrane 657, 664
Tympanic plexus 153
Tyrosine 275, 313
U
‘U’ wave 396
Ulcer 166
Ultrafiltration 220
Ultraviolet rays 247, 651
Umami 669
Umbilical arteries 433
Umbilical vein 432
Unconditioned reflex 155, 163, 172
Unconditioned stimulus 615
Ungated channels 18
Uninhibited neurogenic bladder 243
Unipolar chest leads 394
Unipolar leads 393
Unipolar limb leads 394
Uniport 20
Universal donors 95
Universal recipients 95
Upper costal series 452
Upper motor neuron 552
Upper motor neuron lesion 541, 545,
553
Urase 161
Ureter 207
Ureters 205
Urethra 205, 239, 332
Urinalysis 236
Urinary bladder 205, 239, 241
filling 241
functional anatomy 239
nerve supply 239
Urinary system 205
Urine 219, 227, 233, 236
acidification 233
composition 236
examination 236
formation 219
normal constituents 236
osmolarity 227
Urine 227, 236
Urine formation 219, 226
summary 226
Uriniferous tubules 207
Urobilinogen 179
Uterus 345, 346, 348
Utricle 600, 604
Uvula 194, 570
V
V1A receptors 266
V2 receptors 226, 266
Vagal apnea 480
Vagal fibers 668
Vagal tone 406
Vagina 345, 346, 347
Vagovagal reflex 165
Vagus nerve 406, 409
Valsalva maneuver 420
Van den Bergh’s reaction 184
Vas deferens 330, 331
Vas efferens 331
Vasa recta 217, 229, 230
Vascular congestion 438
Vasectomy 367
Vasoactive intestinal polypeptide (VIP)
166
Vasoconstrictor area 405, 414
Vasoconstrictor fibers 415
Vasodilator area 405, 414
Vasodilator fibers 415
Vasomotor center 405, 414
Vasomotor system 414
Vasomotor tone 415
Vegetative nervous system 621
Veins 375
Vena 375
Vena cavae 375
Venous pressure 420
definition 420
normal values 420
Venous pressure
effect of respiration 420
Venous pulse 424
Venous return 400, 413
Venous system 375
Ventilation 463
alveolar 463
pulmonary 463
Ventilation method 495
Ventilation–perfusion ratio 464
Ventilator 495
Ventomedial nucleus 562
Ventral posteromedial nucleus 551
Ventral respiratory group of neurons
475

696
Essentials of Physiology for Dental Students
Ventricular diastole 386
Ventricular hypertrophy 390
Ventricular muscle 379
Ventricular stiffness 390
Ventricular systole 384
Venules 375
Vermis 569
Vertebral canal 501, 532
Verum 354
Vesicular follicle 351
Vestibular apparatus 570, 600, 602
applied physiology 604
functional anatomy 600
functions 602
nerve supply 602
receptor organ 600
Vestibular membrane 659
Vestibulo-ocular reflex 603
Vestibulocochlear nerve 602
Villi 200
Virilism 312
Visible spectrum 651
Visual acuity 639, 640
Visual association area 587, 646
Visual axis 629
Visual cortex 646
Visual field 641
Visual hallucinations 587
Visual pathway 643
applied physiology 646
course 643
Visual process 638
Visual receptors 639, 643
Visual transduction 639
Visuopsychic area 616
Vital capacity 459
Vitamin A 639, 640
deficiency 640
Vitamin B12 53
Vitamin C 53
vitamin D 285
activation 285
Vitamin D3 248, 285
Vitreous humor 634
Vitronectin 81
VO2 max 497
Vocalization 447
Voltage gated channels 18
Voluntary apnea 474, 480
Voluntary functions 552
Vomiting 197
causes 197
mechanism 197
Vomiting reflex 198
Vomitus 198
Von Willebrand disease 92
Von Willebrand factor 81, 84, 92
W
Wakefulness 565
regulation 565
Wald’s visual cycle 639
Wallerian 511
Walter B 25
Walter B cannon 25
Warfarin 89
Waste products 205
Water 225
reabsorption 225
Water balance 565
regulation 565
Water intoxication 37
Waxes 246
Waxing and waning of breathing 484
Weber-Fechner law 516
Wernicke’s area 586, 616, 662
Westergren’s method 57
Westergren’s tube 57
Wharton’s duct 150
White blood cells 64, 66
classification 64
functions 68
lifespan 66
morphology 64
normal count 65
properties 66
variations 65
White blood cells (WBCs) 64
White buffy coat 59
White column 534
Wiener 96
Wilson’s disease 578
Wintrobe’s method 57
Wintrobe’s tube 57
Wirsung’s duct 168
Wolffian duct 336
Work of breathing 455
X
Xerostomia 156
Xiphoid process 391
Y
Yellow spot 634
Yolk sac 49
Young-Helmholtz theory 649, 652
Z
‘Z’ line 117
Zero potential 127
Zero voltage 396
Zona fasciculata 303, 306
Zona glomerulosa 303, 304
Zona pellucida 351
Zona reticularis 303, 306, 309
Zona vasculosa 344
Zwischenscheibe 117
Zygote 359
Zymogen granules 168
