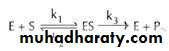محاضرات الكيمياء الحياتية كلية طب الاسنان / جامعة كركوك المرحلة الثانية د. نوال عبدالله مرتضى Enzymes
Chemical Nature of Enzymes
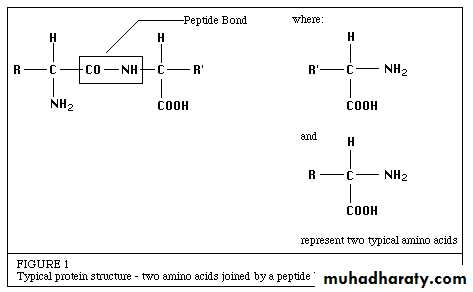
All known enzymes are proteins. They are high molecular weight compounds (biologic polymers) made up principally of chains of amino acids linked together by peptide bonds. See Figure 1.
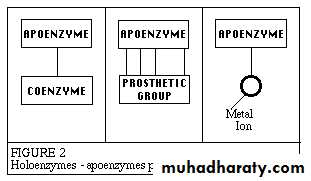
Enzymes catalyze the chemical reactions that make life as we know it possible. It can be denatured and precipitated with salts, solvents and other reagents. They have molecular weights ranging from 10,000 to 2,000,000. Many enzymes require the presence of other compounds ; cofactors - before their catalytic activity can be exerted. This entire active complex is referred to as the holoenzyme; i.e., apoenzyme (protein portion) plus the cofactor (coenzyme, prosthetic group or metal-ion-activator) is called the holoenzyme.
Apoenzyme + Cofactor = Holoenzyme
Apoenzymea protein that forms an active enzyme system by combination with a coenzymeand determines the specificity of this system for a The cofactor may be:
1- A coenzyme - a non-protein organic substance which is dialyzable, thermo stable and loosely attached to the protein part.
2- A prosthetic group - an organic substance which is dialyzable and thermo stable which is firmly attached to the protein or apoenzyme portion.
3- A metal-ion-activator - these include K+, Fe++, Fe+++, Cu++, Co++, Zn++, Mn++, Mg++, Ca++ and Mo.
A prosthetic group: is a tightly bound, specific non - polypeptide unit required for the biological function of some proteins. The prosthetic group may be organic (such as a vitamin, sugar, or lipid) or inorganic (such as a metal ion), but is not composed of amino acids. Prosthetic groups are bound tightly to proteins and may even be attached through a covalent bond, as opposed to coenzymes, which are loosely bound. In enzymes, prosthetic groups are often involved in the active site, playing an important role in the functions of enzymes.
Mode of action :
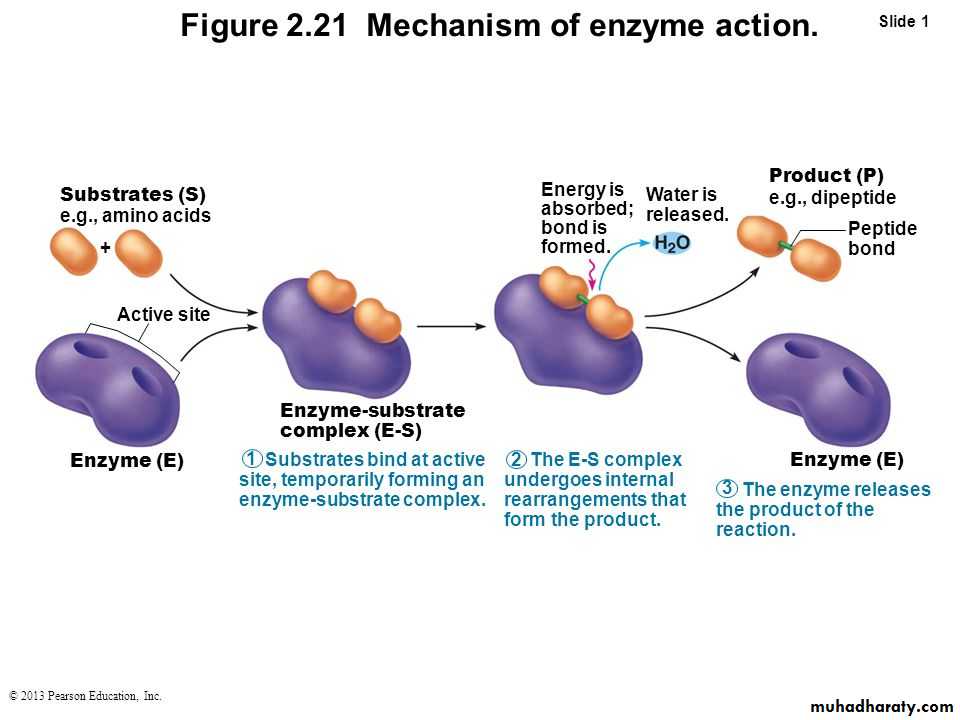
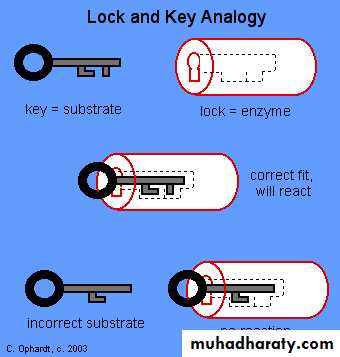
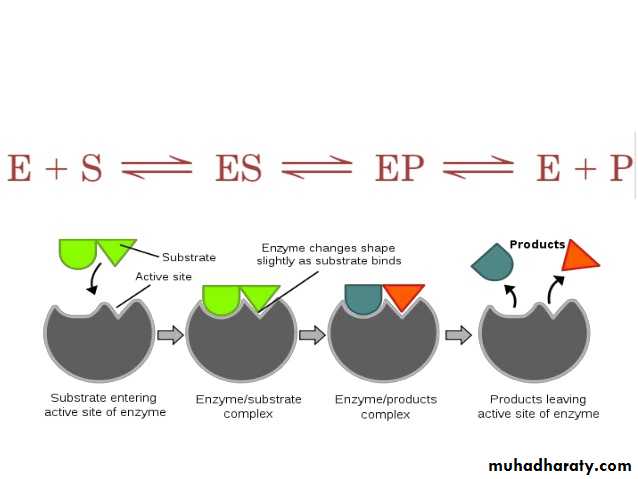
Enzymes enable chemical reactions to occur at cooler temperatures by reducing the amount of activation energy required to break the bonds of the reactant molecules. Each enzyme is very selective in the reaction it catalyzes, this feature is based on the ability of the enzyme to recognize the shape of the certain reactant molecule - substrate. There is a special region on the enzyme that has same shape and chemistry as the substrate, so the substrate fits perfectly into this docking station, the enzyme embraces it slightly and catalyzes the reaction. When the product is released, the enzyme is ready to accept another molecule of particular shape. There are two common models of enzyme action. In the first model, the lock-and-key model, a protein called an enzyme "the lock" binds with another substance called a substrate "the key" and causes the lock to break down after forming an enzyme - substrate complex. The model is so named because substrates are very specific to individual enzymes. The second model is called the induced-fit model. It is pretty much the same as the first, except it is understood that the enzyme undergoes a conformational change before binding with the substrate. Enzymes are able to facilitate reactions at lower than usual temperatures because they lower the activation energy requirements of many biological reactions, acting as organic catalysts. This catalysis often requires "helper" substances called co-enzymes and cofactors. The rate at which enzymes work is determined by the type of enzyme, the amount of substrate present, and the amount of enzyme present. Some molecules can inhibit enzyme function by imitating the substrate or causing the enzyme to change shape. Mechanism of action:
"Lock and key" model
To explain the observed specificity of enzymes, in 1894 Emil Fischer proposed that both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. This is often referred to as "the lock and key" model. This early model explains enzyme specificity, but fails to explain the stabilization of the transition state that enzymes achieve.Induced fit model
In 1958, Daniel Koshland suggested a modification to the lock and key model: since enzymes are rather flexible structures, the active site is continuously reshaped by interactions with the substrate as the substrate interacts with the enzyme. As a result, the substrate does not simply bind to a rigid active site; the amino acid side - chains that make up the active site are molded into the precise positions that enable the enzyme to perform its catalytic function. In some cases, such as glycosidases, the substrate molecule also changes shape slightly as it enters the active site. The active site continues to change until the substrate is completely bound, at which point the final shape and charge distribution is determined. Induced fit may enhance the fidelity of molecular recognition in the presence of competition and noise via the conformational proofreading mechanism.Specificity of Enzymes:
One of the properties of enzymes that makes them so important as diagnostic and research tools is the specificity they exhibit relative to the reactions they catalyze. A few enzymes exhibit absolute specificity; that is, they will catalyze only one particular reaction. Other enzymes will be specific for a particular type of chemical bond or functional group. In general, there are four distinct types of specificity:1- Absolute specificity ( absolute, high or substrate specificity):- the enzyme will catalyze only one reaction and it acts on one substrate e.g.
A- Uricase, which acts only on uric acid.
B- Arginase, which acts only on arginine.
C- Carbonic anhydrase, which acts only on carbonic acid.
D- Lactase, which acts only on lactose.
E- Sucrase, which acts only on sucrose.
F- Maltase, which acts only on maltase.
2- Moderate, structural or group specificity - the enzyme will act only on molecules that have specific functional groups, such as amino, phosphate and methyl groups. The enzyme is specific not only to the type of bond but also to the structure surrounding it.
a- Pepsin: is an endopeptidase that hydrolyzes central peptide bonds in which the amino group belongs to aromatic amino acids e.g. phenyl alanine, tyrosine and tryptophan.
b- Trypsin: is an endopeptidase that hydrolyzes central peptide bonds in which the amino group belongs to basic amino acids e.g. arginine, lysine and histidine.
c- Chymotrypsin: is an endopeptidase that hydrolyzes central peptide
bonds in which the carboxyl group belongs to aromatic amino acids.
d- Aminopeptidase: is an exopeptidase that hydrolyzes peripheral peptide bond at the amino terminal (end) of polypeptide chain.
e- Carboxypeptidase: is an exopeptidase that hydrolyzes peripheral peptide bond at the carboxyl terminal of polypeptide chain.
3- Linkage specificity (Relative, low or bond specificity): the enzyme will act on a particular type of chemical bond regardless of the rest of the molecular structure. The enzyme acts on substrates that are similar in structure and contain the same type of bonds e.g.
a- Amylase: acts on α 1-4 glycosidic, bonds in starch, dextrin and glycogen.
b- Lipase
e: hydrolyzes ester bonds in different triglycerides.
4- Stereochemical specificity - the enzyme will act on a particular steric or optical isomer. In this type of specificity, the enzyme is specific not only to the substrate but also to its optical configuration e.g.
A- L amino acid oxidase acts only on L amino acids.
B- D amino acid oxidase acts only on D amino acids.
C- α - glycosidase acts only on α – glycosidic bonds, which are present in starch, dextrin and glycogen.
D- β – glycosidase acts only on β – glycosidic bonds that are present in cellulose.
Phenylethanolamine N-methyltransferase (PNMT): is an enzyme found in the adrenal medulla that converts norepinephrine (noradrenaline) to epinephrine (adrenaline).
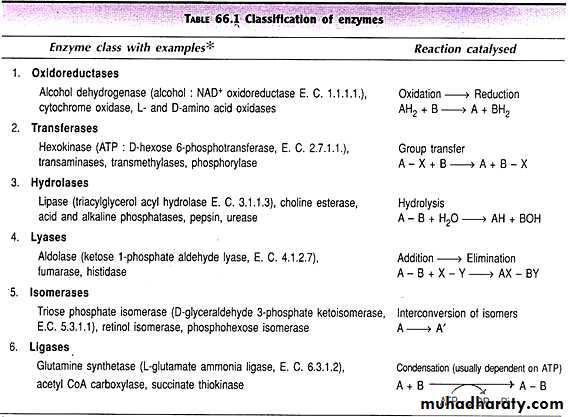
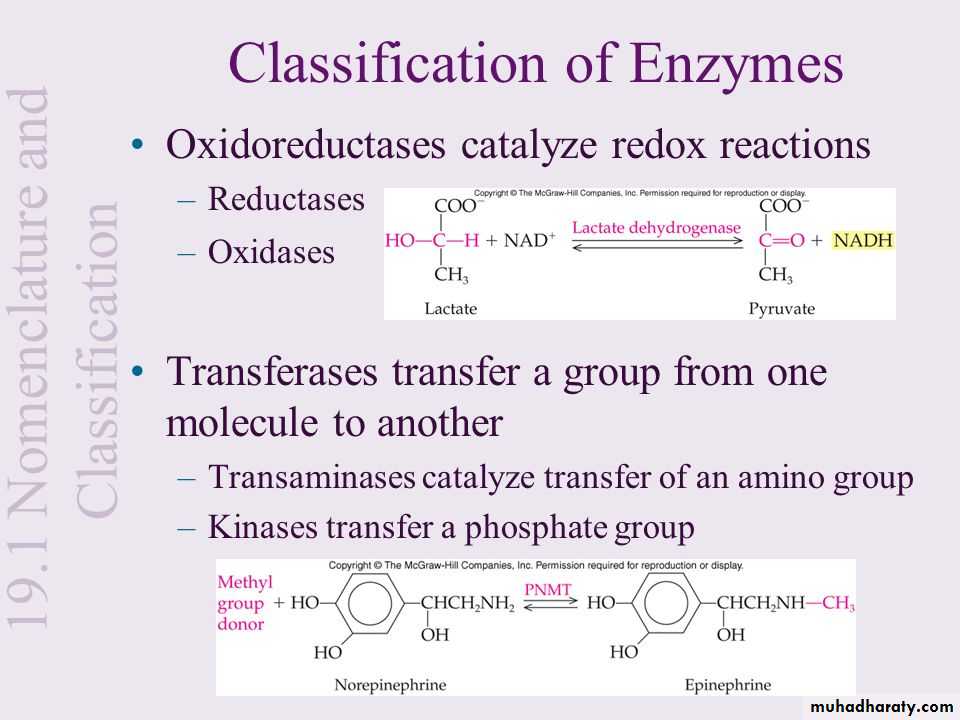
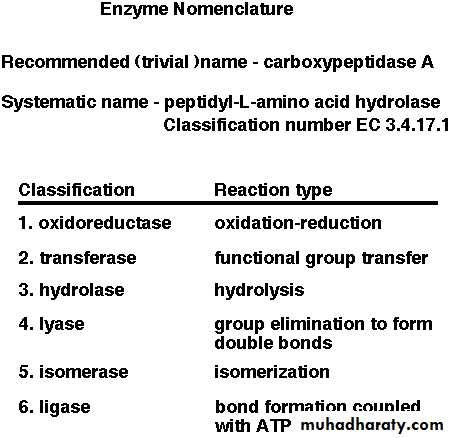
1- Oxidoreductases: are classified as EC 1 in the EC number classification of enzymes. Oxidoreductases can be further classified into 22 subclasses:
EC 1.1 includes oxidoreductases that act on the CH-OH group of donors (alcohol oxidoreductases).
EC 1.2 includes oxidoreductases that act on the aldehyde or oxo group of donors.
EC 1.3 includes oxidoreductases that act on the CH-CH group of donors (CH-CH oxidoreductases).
EC 1.4 includes oxidoreductases that act on the CH-NH2 group of donors (Amino acid oxidoreductases, Monoamine oxidase).
EC 1.5 includes oxidoreductases that act on CH-NH group of donors.
EC 1.6 includes oxidoreductases that act on NADH or NADPH.
EC 1.7 includes oxidoreductases that act on other nitrogenous compounds as donors.
EC 1.8 includes oxidoreductases that act on a sulfur group of donors. EC 1.9 includes oxidoreductases that act on a heme group of donors.
EC 1.10 includes oxidoreductases that act on diphenols and related substances as donors.
2- Transferases:
Group(s) transferred
Examples
EC number
single-carbon groups
methyltransferase and formyltransferase
EC 2.1
aldehyde or ketone groups
transketolase and transaldolase
EC 2.2
acyl groups or groups that become alkyl groups during transfer
acyltransferase
EC 2.3
glycosyl groups, as well as hexoses and pentoses
glycosyltransferase, hexosyltransferase, and pentosyltransferase
EC 2.4
alkyl or aryl groups, other than methyl groups
riboflavin synthase and chlorophyll synthase
EC 2.5
nitrogenous groups
transaminase, and oximino transferase
EC 2.6
phosphorus-containing groups; subclasses are based on the acceptor (e.g. alcohol, carboxyl, etc.)
phosphotransferase, polymerase, and kinase
EC 2.7
sulfur-containing groups
sulfurtransferase and sulfo transferase
EC 2.8
selenium-containing groups
selenotransferase
EC 2.9
molybdenum or tungsten
molybdenumtransferase and tungsten
transferase
EC 2.10
3- Hydrolase:
a hydrolase or hydrolytic enzyme is an enzyme that catalyzes the hydrolysis of a chemical bond. For example, an enzyme that catalyzed the following reaction is a hydrolase:
A–B + H2O → A–OH + B–H
Hydrolases are classified as EC 3 in the EC number classification of enzymes. Hydrolases can be further classified into several subclasses, based upon the bonds they act upon:
EC 3.1: ester bonds (esterases: nucleases, phosphodiesterases, lipase, phosphatase.
EC 3.2: sugars (DNA glycosylases, glycoside hydrolase).
EC 3.3: ether bonds.
EC 3.4: peptide bonds (Proteases/peptidases).
EC 3.5: carbon-nitrogen bonds, other than peptide bonds.
EC 3.6: acid anhydrides (acid anhydride hydrolases, including helicases and GTPase).
EC 3.7: carbon-carbon bonds.
EC 3.8: halide bonds.
EC 3.9: phosphorus-nitrogen bonds.
EC 3.10: sulphur-nitrogen bonds.
4- Lyase
a lyase is an enzyme that catalyzes the breaking (an "elimination" reaction) of various chemical bonds by means other than hydrolysis (a "substitution" reaction) and oxidation, often forming a new double bond or a new ring structure. The reverse reaction is also possible (called a "Michael addition”). For example, an enzyme that catalyzed this reaction would be a lyase:ATP → cAMP + PPi
Lyases differ from other enzymes in that they require only one substrate for the reaction in one direction, but two substrates for the reverse reaction.
Lyases are classified as EC 4 in the EC number classification of enzymes. Lyases can be further classified into seven subclasses:
EC 4.1 includes lyases that cleave carbon-carbon bonds, such as decarboxylases (EC 4.1.1), aldehyde lyases (EC 4.1.2), oxo acid lyases (EC 4.1.3), and others (EC 4.1.99).)
EC 4.2 includes lyases that cleave carbon-oxygen bonds, such as dehydratases.
EC 4.3 includes lyases that cleave carbon-nitrogen bonds.
EC 4.4 includes lyases that cleave carbon-sulfur bonds.
EC 4.5 includes lyases that cleave carbon-halide bonds.
EC 4.6 includes lyases that cleave phosphorus-oxygen bonds, such as adenylyl cyclase and guanylyl cyclase.
EC 4.99 includes other lyases, such as ferrochelatase.
5- Isomerase:
Isomerases are a general class of enzymes which convert a molecule from one isomer to another. Isomerases can either facilitate intramolecular rearrangements in which bonds are broken and formed. The general form of such a reaction is as follows:
A–B → B–A
There is only one substrate yielding one product. This product has the same molecular formula as the substrate but differs in bond connectivity or spatial arrangements. Isomerases catalyze reactions across many biological processes, such as in glycolysis and carbohydrate metabolism.
6- Ligase:
a ligase is an enzyme that can catalyze the joining of two large molecules by forming a new chemical bond, usually with accompanying hydrolysis of a small pendant chemical group on one of the larger molecules or the enzyme catalyzing the linking together of two compounds, e.g., enzymes that catalyze joining of C-O, C-S, C-N, etc. In general, a ligase catalyzes the following reaction:
Ab + C → A–C + b
or sometimes
Ab + cD → A–D + b + c + d + e + f
where the lowercase letters signify the small, dependent groups. Ligase can join two complementary fragments of nucleic acid and repair single stranded breaks that arise in double stranded DNA during replication.
Enzyme kinetics and Km value:
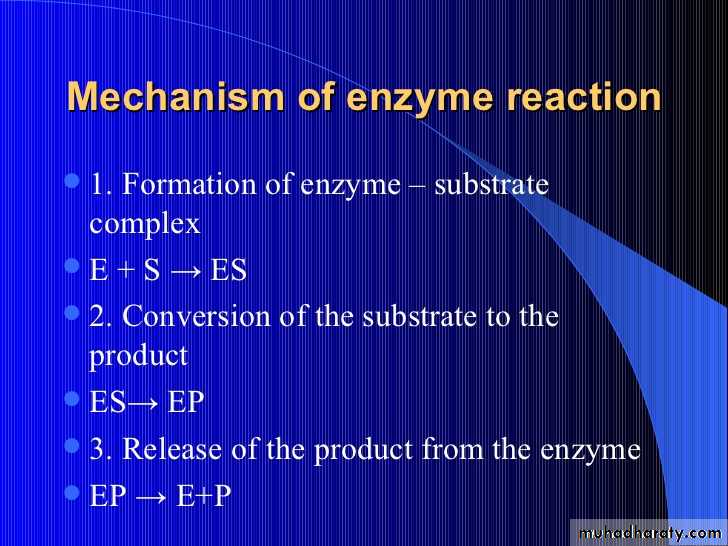
The enzyme (E) and substrate (S) combine with each other to form an unstable enzyme-substrate complex (ES) for the formation of product (P).
Here k1, k2 and k3 represent the velocity constants for the respective reactions, as indicated by arrows. Km, the Michaelis - Menten constant (or Brig’s and Haldane’s constant), is given by the formula:
The following equation is obtained after suitable algebraic manipulation.
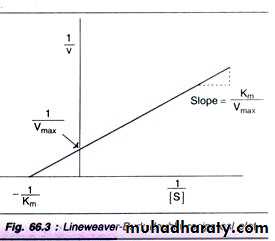
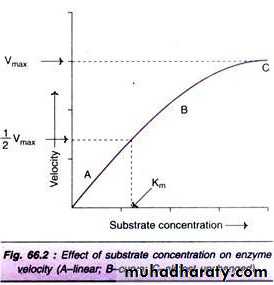
where v = Measured velocity, Vmax = Maximum velocity, S = Substrate concentration, Km = Michaelis - Menten constant. Km or the Michaelis - Menten constant: is defined as the substrate concentration (expressed in moles / lit) to produce half - maximum velocity in an enzyme catalyzed reaction. It indicates that half of the enzyme molecules (i.e. 50%) are bound with the substrate molecules when the substrate concentration equals the Km value. Km value is a constant and a characteristic feature of a given enzyme. It is a representative for measuring the strength of ES complex. A low Km value indicates a strong affinity between enzyme and substrate, whereas a high Km value reflects a weak affinity between them. For Line weaver-Burk double reciprocal plot:
For the determination of Km value, the substrate saturation curve (Fig. 66.2) is not very accurate since Vmax is approached asymptotically. By taking the reciprocals of the equation (1), a straight line graphic representation is obtained. The Line weaver - Burk plot is shown in Fig. 66.3. It is much easier to calculate the Km from the intercept on x-axis which is -(1/Km). Further, the double reciprocal plot is useful in understanding the effect of various inhibitions. majority of enzymes, the Km values are in the range of 10-5 to 10-2 moles.
Factors Affecting Enzyme Function
1- Effect of concentrations.a- Enzyme concentration.
b- Substrate concentration.
c- Product Concentration.
2- Temperature.
3- pH.
4- Salinity.
5- Activators.
6- Inhibitors.
1- Effect of concentrations.
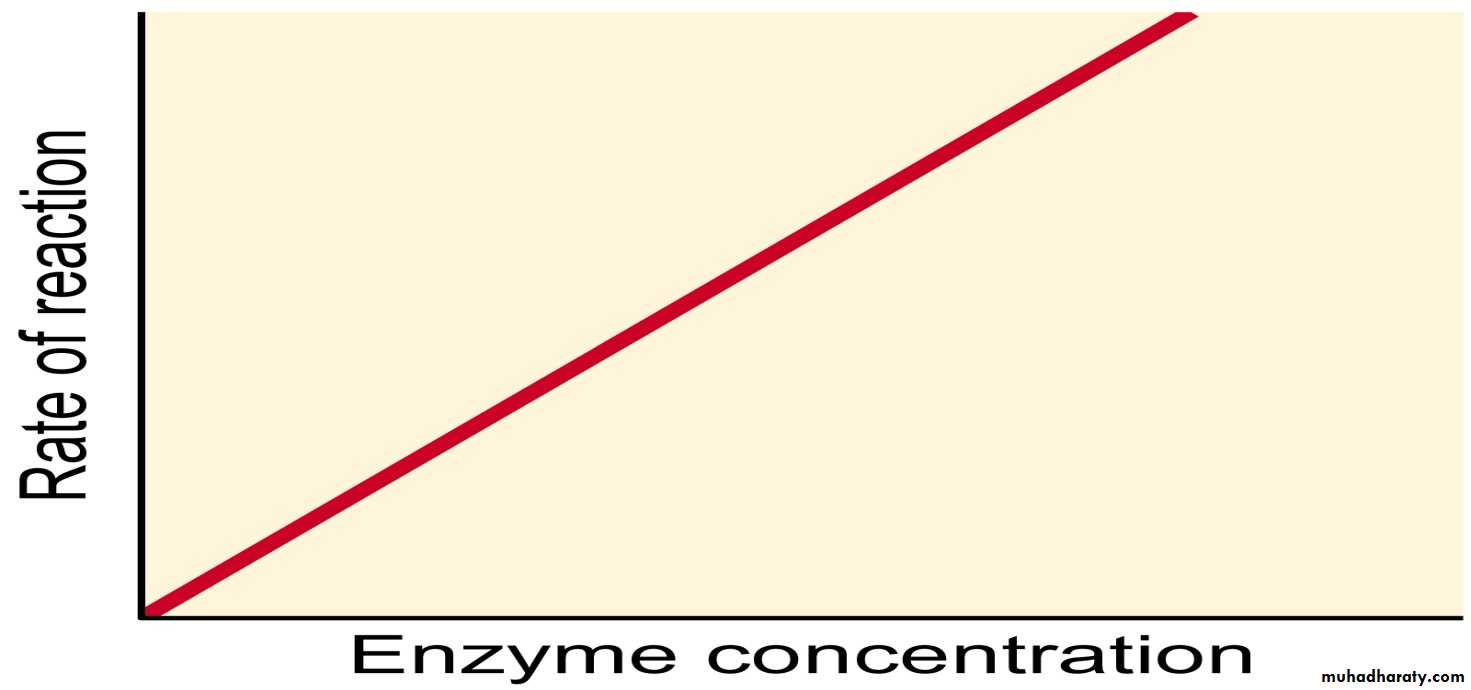
a- Enzyme Concentration: Increasing enzyme concentration will increase the rate of reaction, as more enzymes will be colliding with substrate molecules. However, this too will only have an effect up to a certain concentration, where the enzyme concentration is no longer the limiting factor.
- Denaturation: is a permanent structural change in a protein that results in a loss of its biological properties. In other words, some chemical bonds break, and the active site is destroyed.
Human enzymes: can denature in individuals running a high fever. -
Some arctic: fish have enzymes that denature at 10ºC.-
The enzymes: of some thermophilic bacteria denature at 85ºC.-
b- Substrate concentration:
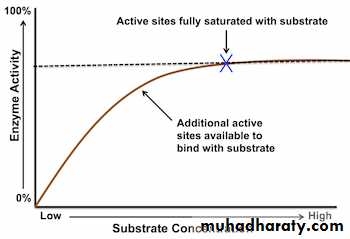
At low substrate concentration the reaction proceeds slowly. This is because there are not enough substrate molecules to occupy all of the active sites on the enzyme. As substrate concentration increases, the rate increases because there are more enzyme substrate complexes formed. At point x, however, increasing the substrate concentration will have no further effect on the rate of reaction. This is because all of the enzyme’s active sites are now occupied by substrate molecules – increasing the substrate concentration further will have no effect, because no more enzyme substrate complexes can form. The rate of reaction now depends on the turnover rate of the enzyme, i.e. the number of substrate molecules transformed by one molecule of enzyme per second.
c- Effect of Product Concentration:
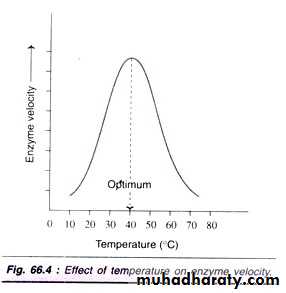
The accumulation of reaction products generally decreases the enzyme velocity. For certain’ enzymes, the products combine with the active site of enzyme and form a loose complex and, thus, inhibit the enzyme activity. In the living system, this type of inhibition is generally prevented by a quick removal of products formed.2- Effect of Temperature: Enzymes have an optimum temperature – this is the temperature at which they work most rapidly. Below the optimum temperature, increasing temperature will increase the rate of the reaction. This is because temperature increases the kinetic energy of the system, effectively increasing the number of collisions between the substrate and the enzyme’s active site. Temperatures above the optimum will lead to denaturation. This occurs because the hydrogen bonds and disulphide bridges which maintain the shape of the active site are broken. Thus, enzyme substrate complexes can no longer be fo rmed.
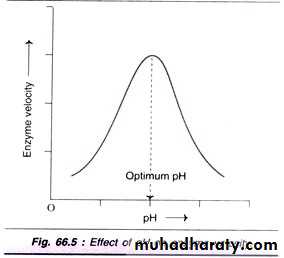
3- Effect of pH: Increase in the hydrogen ion concentration (pH) considerably influences the enzyme activity and a bell-shaped curve is normally obtained. Each enzyme has an optimum pH at which the velocity is maximum. Most of the enzymes of higher organisms show optimum activity around neutral pH (6-8). There are, however, many exceptions like pepsin (1-2), acid phosphatase (4-5) and alkaline phosphatase (10-11) for optimum pH. As with temperature, each enzyme has an optimum pH. If pH increases or decreases much beyond this optimum, the ionisation of groups at the active site and on the substrate may change, effectively slowing or preventing the formation of the enzyme substrate complex. At extreme pH, the bonds which maintain the tertiary structure – hence the active site – are disrupted and the enzyme is irreversibly denatured. Since most human enzymes are intracellular, most have a pH optimum of 7.3-7.4. However, pepsin, which works in the acidic environment of the stomach, has an optimum of 2.4.
4- Effect of time:
At the beginning, the rate of reaction increases but by time the rate of reaction decreases due to :
a- Depletion of substrate.
b- Accumulation of end product.
c- Change in PH of the reaction, becomes different from the optimum PH of the enzyme.
5- Effect of salinity: Salinity may be important not just in solubility, but in promoting enzyme activity. Higher salinity may promote binding of a hydrophobic substrate to an enzyme, or of hydrophobic residues to each other within the enzyme to ensure optimal folding for enzymatic activity. 6- Effect of Activators: Some molecules bind to the enzyme molecule and consequently increase the reaction rate. These are known as activator molecules, Like certain inorganic metallic cations ; Mg2+, Mn2+, Zn2+, Ca2+, Co2+, Cu2+, Na+, K+ etc. for their optimum activity. Rarely, anions are also needed for enzyme activity e.g. chloride ions (CI–) activate salivary amylase, calcium ions (Ca+2) activate thrombokinase enzyme (thrombokinase - an enzyme liberated from blood platelets that converts prothrombin into thrombin as blood starts to clot). In the conjugated protein enzymes that need coenzyme the increase in the coenzyme concentration causes an increase in the rate of enzyme action.
7- Effect of Inhibitors:
Some molecules bind to the enzyme or the substrate or the enzyme - substrate complex and lower the reaction rate. These are known as inhibitor molecules. There are two types of inhibitors; 1) Reversible inhibitors and 2) Irreversible inhibitors.
1) Reversible inhibitors include:
a) competitive inhibition: the substrate and inhibitor cannot bind to the enzyme at the same time.
b) uncompetitive inhibition: the inhibitor binds only to the substrate-enzyme complex.
c) non-competitive inhibition: the binding of the inhibitor to the enzyme reduces its activity but does not affect the binding of substrate.
d) mixed inhibition: the inhibitor can bind to the enzyme at the same time as the enzyme's substrate.
2) Reversible inhibitors: Traditionally reversible enzyme inhibitors have been classified as competitive, uncompetitive, or non-competitive, according to their effects on Km and Vmax. These different effects result from the inhibitor binding to the enzyme E, to the enzyme–substrate complex ES, or to both, respectively.
Isoenzymes: (also known as isozymes or more generally as multiple forms of enzymes) are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. These enzymes usually display different kinetic parameters (e.g. different KM values), or different regulatory properties. Isozymes or Isoenzymes are proteins with different structure which catalyze the same reaction. Frequently they are oligomers made with different polypeptide chains, so they usually differ in regulatory mechanisms and in kinetic characteristic. In biochemistry, isozymes (or isoenzymes) are isoforms (closely related variants) of enzymes. In many cases, they are coded for by homologous genes that have diverged over time. Allozymes represent enzymes from different alleles of the same gene, and isozymes represent enzymes from different genes that process or catalyse the same reaction, the two words are usually used interchangeably.
Co-enzyme function and important
Coenzymes are a type of cofactor and they are bound to enzyme active sites to aid with their proper functioning. Coenzymes which are directly involved and altered in the course of chemical reactions are considered to be a type of secondary substrate. This is because they are chemically changed as a result of the reaction unlike enzymes. However unlike the primary substrates, coenzymes can be used by a number of different enzymes and as such are not specific. For example hundreds of enzymes are able to use the coenzyme NAD. The function of coenzymes is to transport groups between enzymes. Chemical groups include hydride ions which are carried by coenzymes such as NAD, phosphate groups which are carried by coenzymes such as ATP and acetyl groups which are carried by coenzymes such as coenzyme A. Coenzymes which lose or gain these chemical groups in the course of the reaction are often reformed in the same metabolic pathway. For example NAD+ used in glycolysis and the citric acid cycle is replaced in the electron transport chain of respiration.How are coenzymes made?
Due to the importance of coenzymes in chemical reactions, and due to the fact that they are used up and chemically altered by reactions, coenzymes must be continually regenerated. For example, synthesis of B vitamins is a complex, step wise process because the B vitamins have chiral centres which are complicated to synthesise. Coenzymes that are produced from B vitamins are especially important to the proper functioning of enzymes involved with regulation of metabolism and with the release of energy from food. Important B vitamins that are used as large components of coenzymes include riboflavin, niacin, biotin, pantothenic acid, B6, folate and B12. For example riboflavin, or vitamin B2 is used as a large component of FAD and FADH, and niacin is an important component of NAD and NADH. However vitamins cannot be made by the body, but instead they must be consumed in the diet. Therefore vitamins are essential components of the diet. Although the human body uses more than its own body weight in ATP, not as much of the vitamin that is used to produce the coenzyme is needed to be consumed. This is because the body is able to use the vitamins very instensively through regeneration.