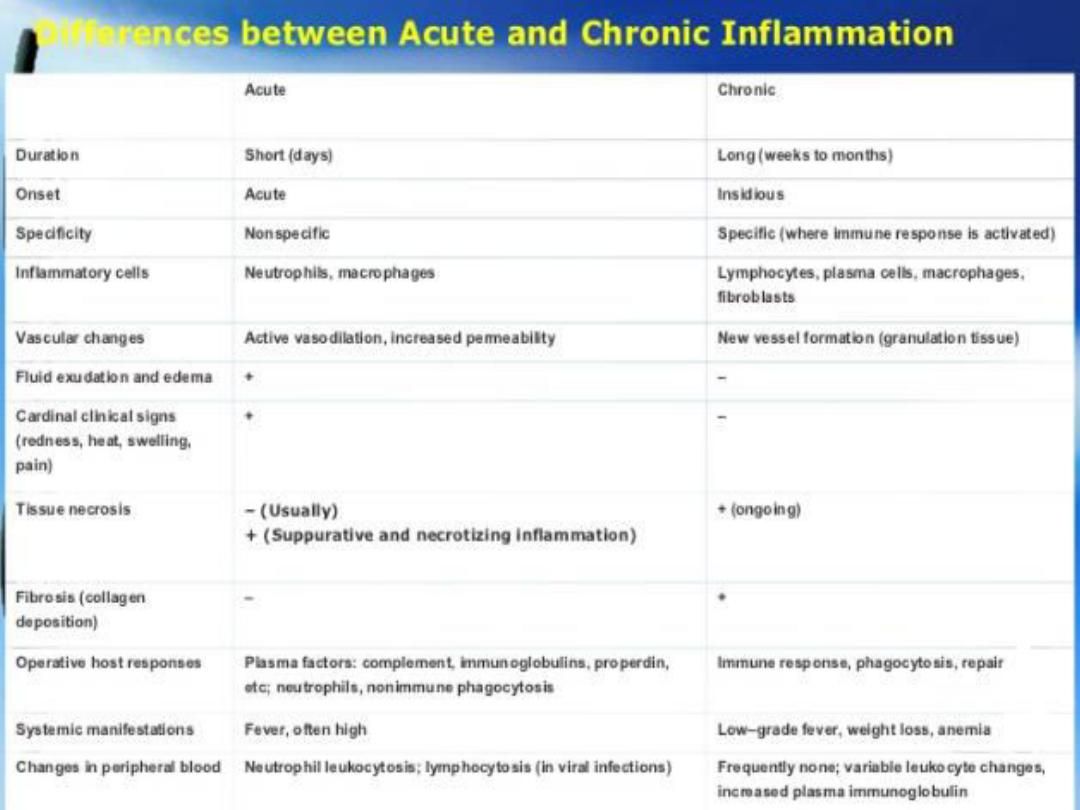
Inflammation

Inflammation
is a protective response intended to eliminate
the initial cause of cell injury as well as the
necrotic cells and tissues resulting from the
original insult

A double edge sword?
Although inflammation helps clear infections
and other noxious stimuli and initiates repair,
the inflammatory reaction and the subsequent
repair process can cause considerable harm
.

Injurious stimuli cause a protective
vascular connective tissue reaction
called “inflammation”
-Dilute
-Destroy
-Isolate
-Initiate repair

Definition: is the response of living
tissue to injurious agent.

Etiology:
1- Physical Agents: Mechanical trauma,
extremes of temperature, radiation.
2- Chemical Agents and Drugs: Industrial and
occupational hazards, such as asbestos.
3- Infectious Agents.
4- Tissue necrosis.
5- Immune reaction.
6- Foreign body.

Classification of inflammation:
1- Acute
2- Chronic
Acute inflammation:
Is a rapid host response that serves to
deliver
leukocytes
and
plasma proteins
,
such as antibodies, to sites of infection
or tissue injury.

Etiology of Acute Inflammation:
Acute inflammatory reactions may be triggered
by a variety of stimuli:
1
• Infections
(bacterial, viral, fungal, parasitic) and
microbial toxins.
2
• Tissue necrosis
from any cause, including ischemia (as
in a myocardial infarct), trauma, and physical and
chemical injury.
3
• Foreign bodies
(splinters, dirt, sutures) typically elicit
inflammation because they cause traumatic tissue injury
or carry microbes.
4
• Immune reactions
(also called hypersensitivity
reactions).
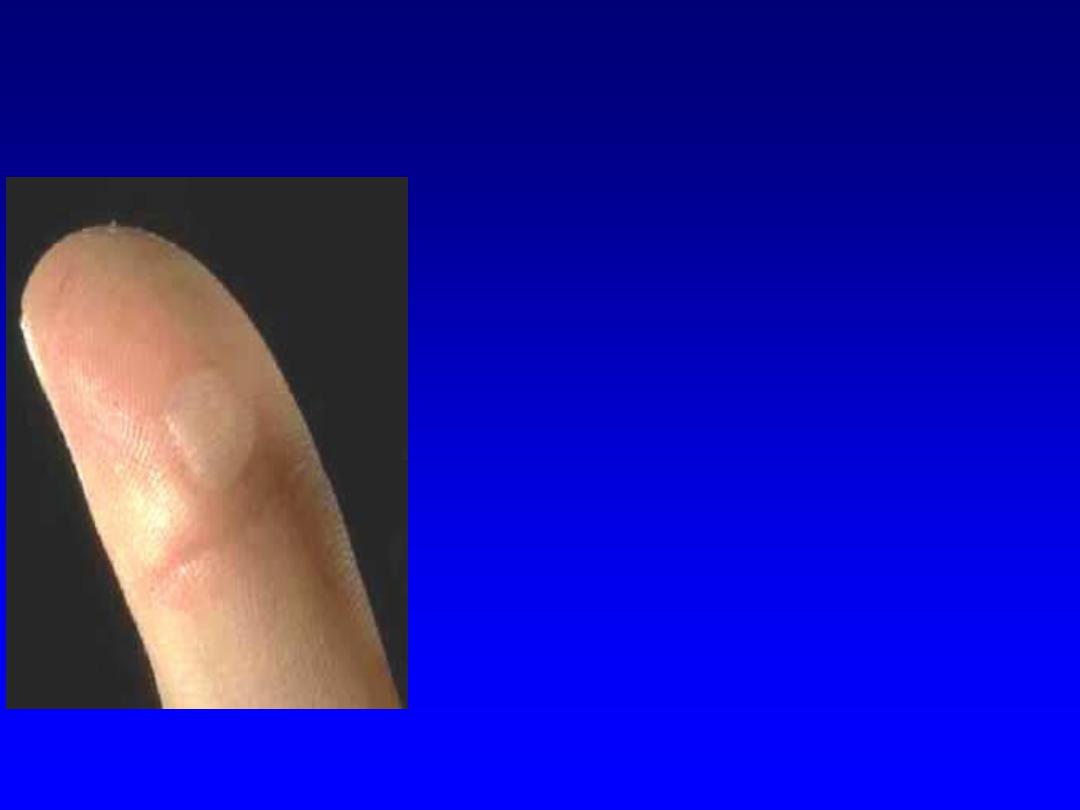
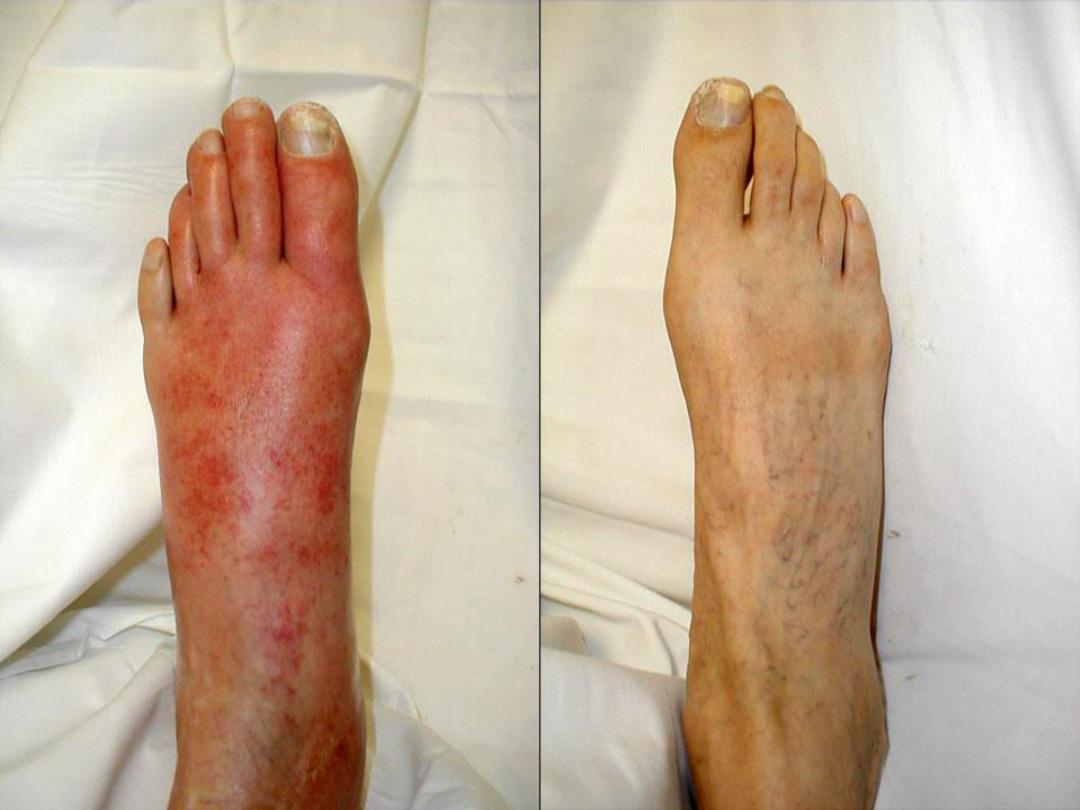
Cardinal Signs of Inflammation
Redness : Hyperaemia.
Warm : Hyperaemia.
Pain : Nerve, Chemical mediators
Swelling : Exudation
Loss of Function: Pain
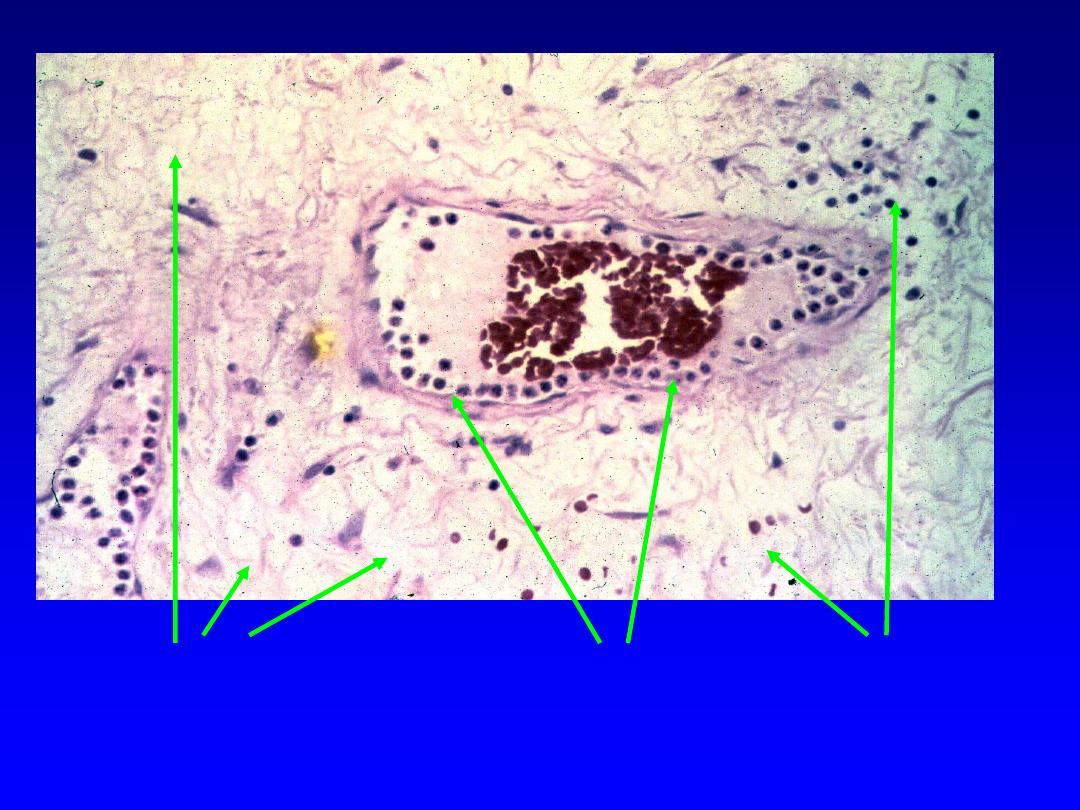
Tissue oedema
Neutrophil margination …. And emigration

Changes of acute inflammation:
A- Vascular changes: which includes:
(1) Changes in vascular caliber and blood flow:
First:
there is a transient constriction of arterioles, lasting
a few seconds.
Second:
vasodilation which involves the
arterioles
and
then leads to
opening of new capillary beds
, the result is
increased blood flow, which is the cause of heat and
redness (erythema) at the site of inflammation,
vasodilation is induced by the action of several mediators,
notably histamine and nitric oxide (NO).
Third:
slowing of blood flow (stasis) as a result of
the loss
of fluid
,
increased vessel diameter
, concentration of red
cells in small vessels, and increased viscosity of the
blood.


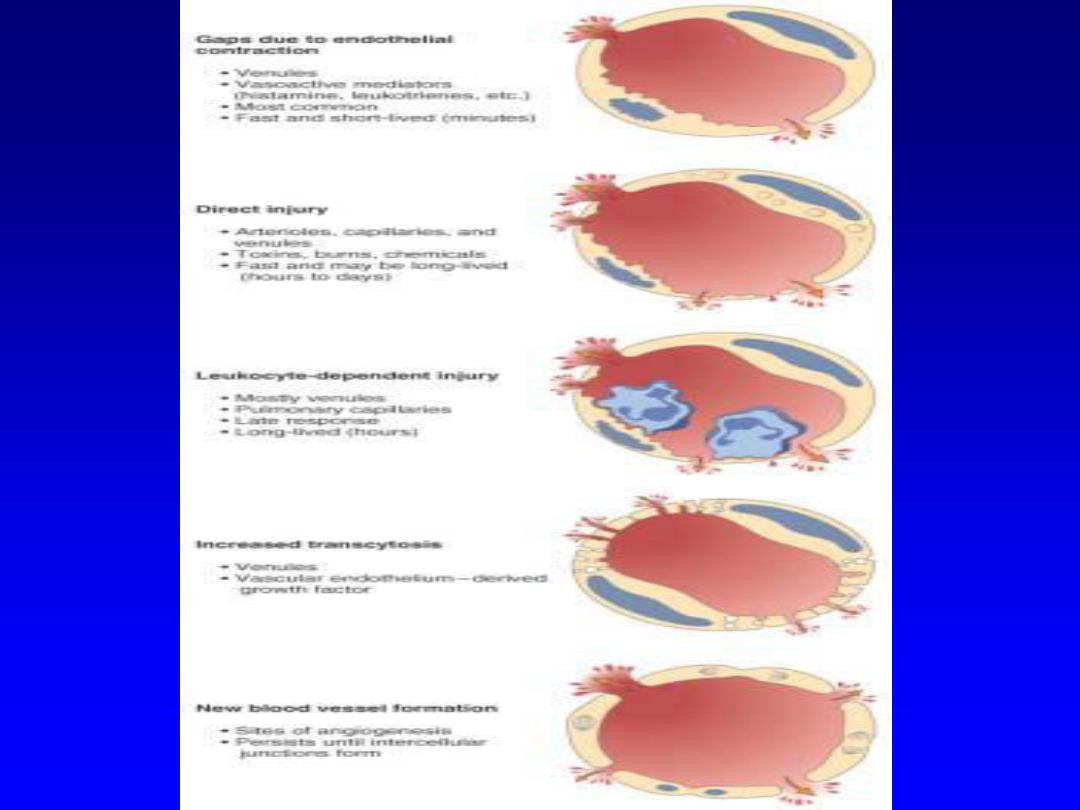
(2) Changes in the in vascular structure: (increased
vascular permeability)
Increased vascular permeability leads to the
escape of a protein-rich exudate into the
extravascular tissue, causing edema. Several
mechanisms are responsible for the increased
vascular permeability:
B- Cellular changes: which includes
(1) Emigration of the leukocytes from the
microcirculation:
Leukocyte Adhesion to Endothelium.
Leukocyte Migration through Endothelium.
(2) Accumulation of the leukocytes in the
focus of injury:
Chemotaxis of Leukocytes.
(3) Activation of the leukocytes to
eliminate the offending agent:
Recognition of Microbes and Dead Tissues
Removal of the Microbes and Dead Tissues

(1) Emigration of the leukocytes from
the microcirculation.
Leukocyte Adhesion to Endothelium.
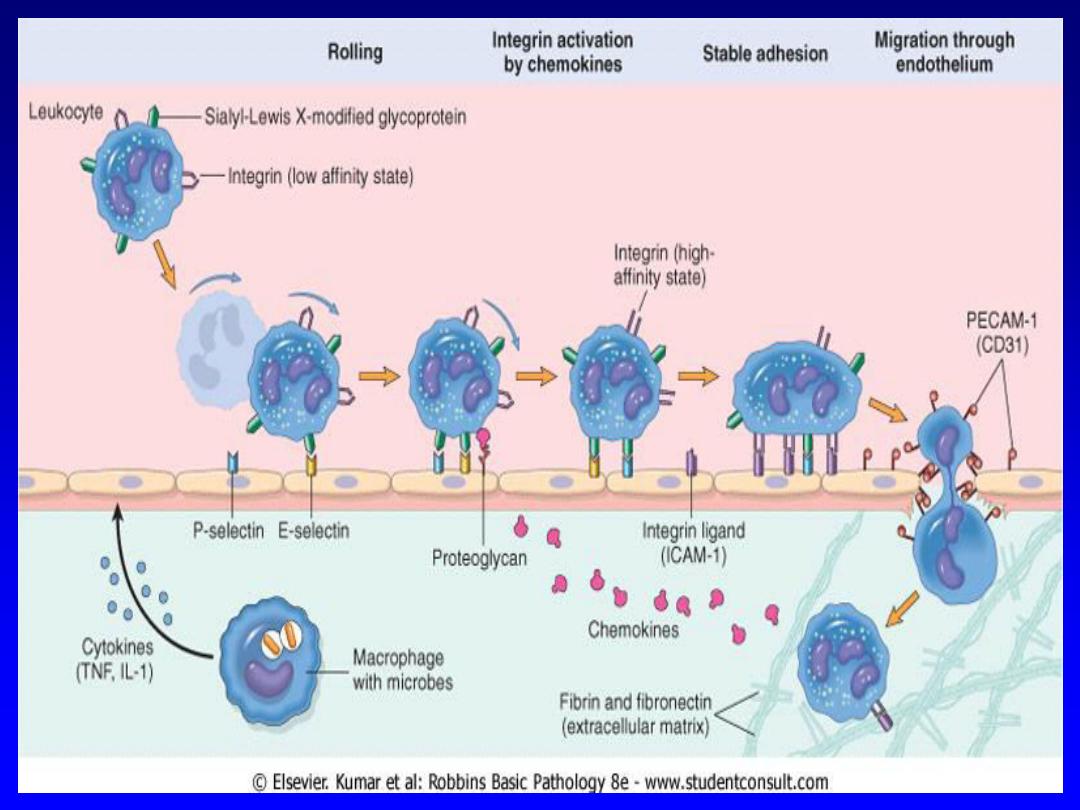
Margination:
is the process of leukocyte
redistribution, because blood flow slows
early in inflammation (stasis), more white
cells assume a peripheral position along
the endothelial surface.

Rolling:
is the process of leukocytes adhesion
transiently to the endothelium, detach and bind
again, thus rolling on the vessel wall.
Rolling is mediated by a family of proteins called
selectins (adhesion molecules). There are three
types of selectins: one expressed on leukocytes
(L-selectin), one on endothelium (E-selectin), and
one on platelets and on endothelium (P-selectin).
Adhesion:
is the process of leukocytes adhesion
firmly to the endothelium, firm adhesion is
mediated by a family of proteins called integrins
(VLA-4, LFA-1 and Mac-1)

Leukocyte Migration through Endothelium.
The next step is migration of the leukocytes through
the endothelium, called transmigration or diapedesis.
Transmigration of leukocytes occurs mainly in
postcapillary venules. Chemokines act on the
adherent leukocytes and stimulate the cells to migrate
through interendothelial spaces toward the chemical
concentration gradient, (toward the site of injury or
infection where the chemokines are being produced),
after traversing the endothelium, leukocytes penetrate
the basement membrane, by secreting collagenases,
and enter the extravascular tissue.

(2) Accumulation of the leukocytes in the
focus of injury. Chemotaxis of Leukocytes.
After exiting the circulation, leukocytes emigrate
in tissues toward the site of injury by
a process called chemotaxis, which is defined
as locomotion oriented along a chemical
gradient.

Both exogenous and endogenous
substances can act as chemoattractants.
The most common exogenous agents are
bacterial products.
Endogenous chemoattractants include
several chemical mediators:
(1) Cytokines (e.g., IL-8).
(2) Components of the complement
system, particularly C5a
(3) Arachidonic acid (AA) metabolites,
mainly leukotriene B4 (LTB4).

All these chemotactic agents bind to specific
receptors on the surface of leukocytes result in
increased cytosolic calcium, with actin and
myosin changes at the leading edge of the cell.
The leukocyte moves by extending filopodia
that pull the back of the cell in the direction of
extension, the net result is that leukocytes
migrate toward the inflammatory stimulus in the
direction of the gradient of locally produced
chemoattractants.

The nature of the leukocyte infiltrate varies with the:
1- Age of the inflammatory response.
In most forms of acute inflammation neutrophils
predominate in the inflammatory infiltrate during the
first 6 to 24 hours and are replaced by monocytes in
24 to 48 hours. After entering tissues, neutrophils are
short-lived; they undergo apoptosis and disappear
after 24 to 48 hours.
Monocytes survive longer and proliferate in the
tissues, and thus become the dominant population in
chronic inflammatory reactions.

2- Type of stimulus.
In
certain infections
—for example, those
produced by Pseudomonas bacteria
— the
cellular infiltrate is dominated by continuously
recruited neutrophils for several days.
In viral infections
, lymphocytes may be the first
cells to arrive.
In some
hypersensitivity reactions
, eosinophils
may be the main cell type.

(3) Activation of the leukocytes to eliminate the
offending agent.
Recognition of Microbes and Dead Tissues
Leukocytes express several receptors that recognize
external stimuli:
• Receptors for microbial products.
• G protein–coupled receptors.
• Receptors for opsonins: Leukocytes express
receptors for proteins that coat microbes. The
process of coating a microbe, to target it for
ingestion (phagocytosis) is called opsonization,
and substances that do this are opsonins.
These substances include antibodies, complement
proteins.


Removal of the Microbes and Dead
Tissues
Recognition of microbes or dead cells by
the receptors induces leukocytes for
destruction of microbes by phagocytosis
and intracellular killing.

Phagocytosis and intracellular killing,
involves three sequential steps:
(1) Attachment of the particle to be ingested by the
leukocyte
(2) Engulfment, with subsequent formation of a
phagocytic vacuole; by extensions of the
cytoplasm (pseudopods) around the microbe, and
the formation of a vesicle (phagosome) that
encloses the particle. The phagosome then fuses
with a lysosomal granule, resulting in discharge of
the granule's contents into the phagolysosome

(3) Killing or degradation of the ingested material
within neutrophils and macrophages,
microbial killing is accomplished largely by
reactive oxygen species (ROS, also called
reactive oxygen intermediates) and reactive
nitrogen species and action of other substances
in leukocyte granules such as enzymes (
elastase) , lysozyme, which hydrolyzes the bond
found in the coat of all bacteria.


Termination (Control) of the Acute
Inflammatory Response
Acute inflammation, needs tight controls to
minimize the damage.
1- Inflammation declines simply because the
mediators of inflammation have short half-lives, and
are degraded after their release.
2- Neutrophils also have short half-lives in tissues
and die by apoptosis within a few hours after leaving
the blood.
3- There are a variety of stop signals that serve to
terminate the inflammation, including transforming
growth factor-
β (TGF-β) and IL-10.

Sequelae of Acute Inflammation
there are four main possible sequelae of acute
inflammation:
●
Resolution:
complete resolution occurs following
short-lived tissue injury in which there has been little
tissue damage. The bacterium may be neutralized,
killed and cleared by the acute inflammatory response
and the affected tissues return entirely to normal. This
occurs in some acute bacterial infections and is the
ideal outcome.
●
Abscess formation:
This is characteristically seen
with certain pyogenic organisms such as staphylococci.
An abscess may discharge spontaneously or require
drainage by surgical intervention.

Healing by fibrosis and scar formation:
Healing by fibrosis and scar formation
occurs when substantial tissue destruction is
seen during the acute inflammation. The
damaged tissues are unable to regenerate and
are replaced by fibrous tissue.
● Progression to chronic inflammation


Factors affecting outcome of acute
inflammation
1. Severity of tissue damage
2. Capacity of cells to divide
3. Type of agent causing damage
4. The responsiveness of the host
5. Site involved

Morphologic PATTERNS
of Acute INFLAMMATION
•Serous
(watery)
•Fibrinous
(hemorrhagic,
rich in FIBRIN)
•Suppurative
(PUS)
•Ulcerative

BLISTER, “Watery”, i.e., SEROUS
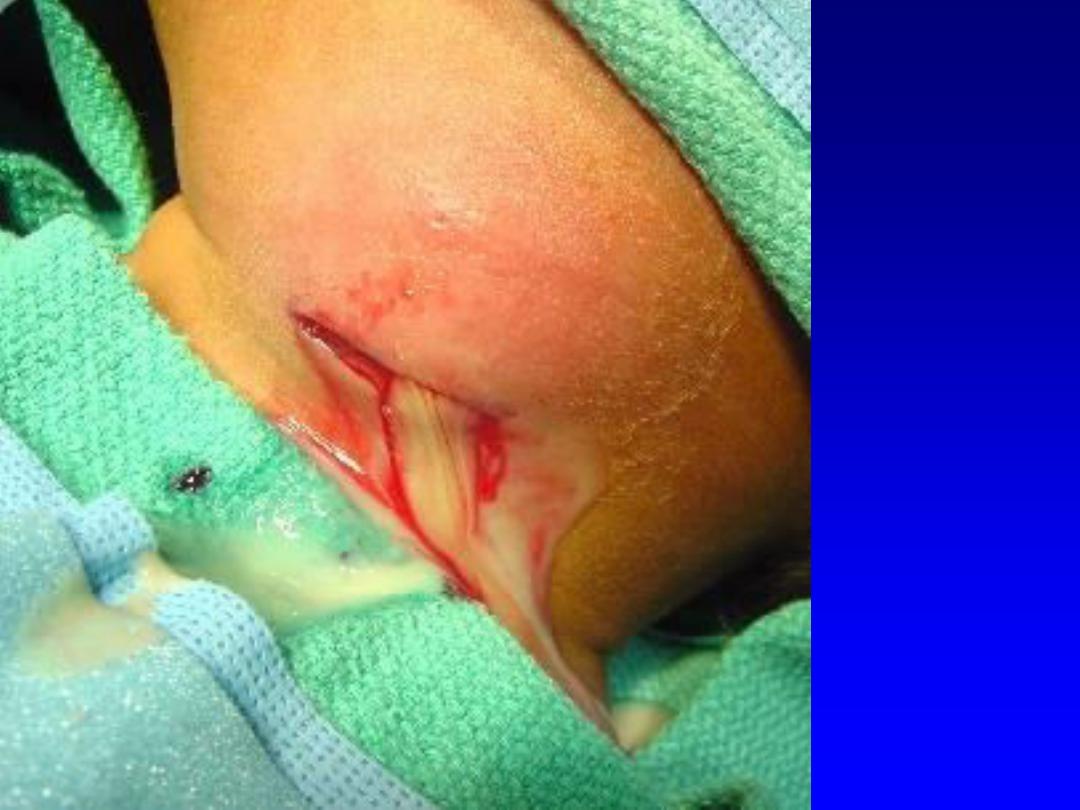
PUS
=
PURULENT
ABSCESS
=
OF
PUS
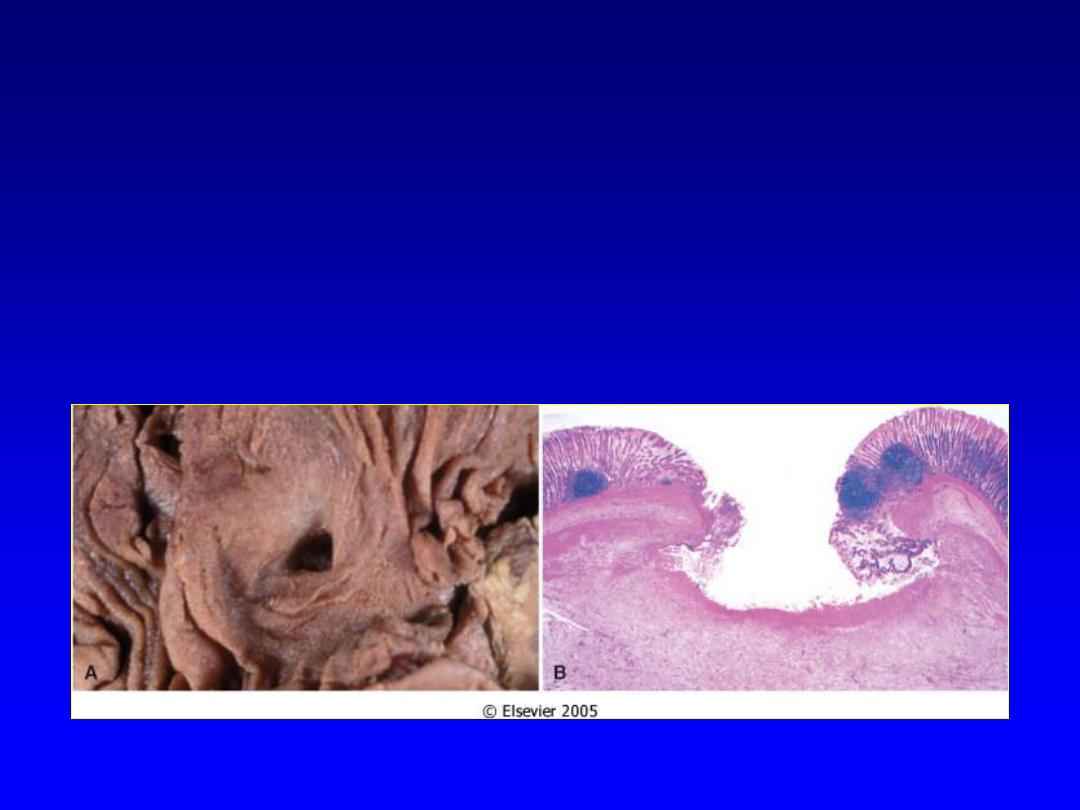
Ulcerative
• Necrotic and eroded epithelial surface
• Underlying acute and chronic
inflammation
• Trauma, toxins, vascular insufficiency

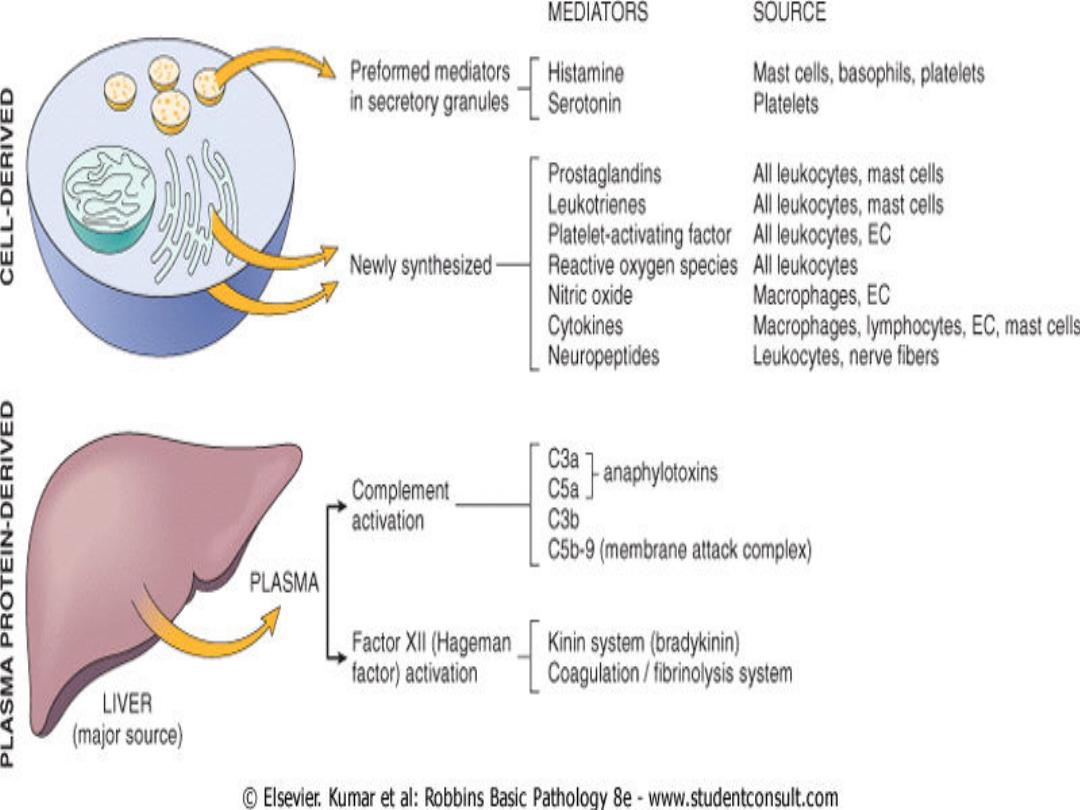
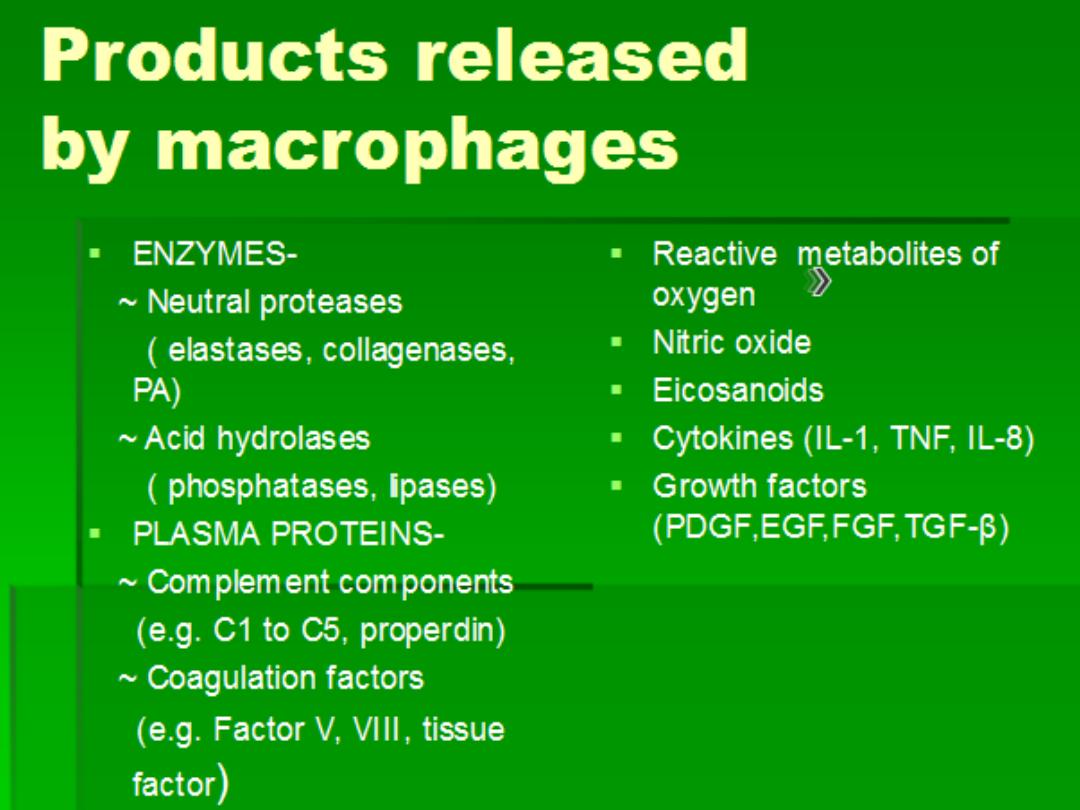
Chemical mediators of inflammation
• Mediators may be produced
locally by
cells at the site of inflammation,
• or may be
circulating in the plasma as
inactive precursors that are activated at the site of
inflammation

Chronic Inflammation
Chronic inflammation is inflammation of
prolonged duration (weeks or months)
in which
inflammation, tissue injury, and attempts at
repair coexist, in varying combinations.

CAUSES OF CHRONIC
INFLAMMATION
1.Persistent Infection
2. Immune-mediated inflammatory
diseases
3. Toxic Agents

Causes of chronic inflammation
• Acute inflammation
:
– Progressive: osteomyelitis
– Recurrent: cholycystitis, gastritis.
• Primary: (
abinitio
) abinitio means
"from the
beginning“
• Contents
– TB, fungal inf.
– HSR.
• Persistent factor
: foreign bodies

Primary Chronic Inflammation
It is the cause of tissue damage in some of the most
common and disabling human diseases, such as
Rheumatoid arthritis, atherosclerosis, tuberculosis,
and pulmonary fibrosis.
It has also been implicated in the progression of
cancer and in diseases once thought to be purely
degenerative, such as Alzheimer disease.

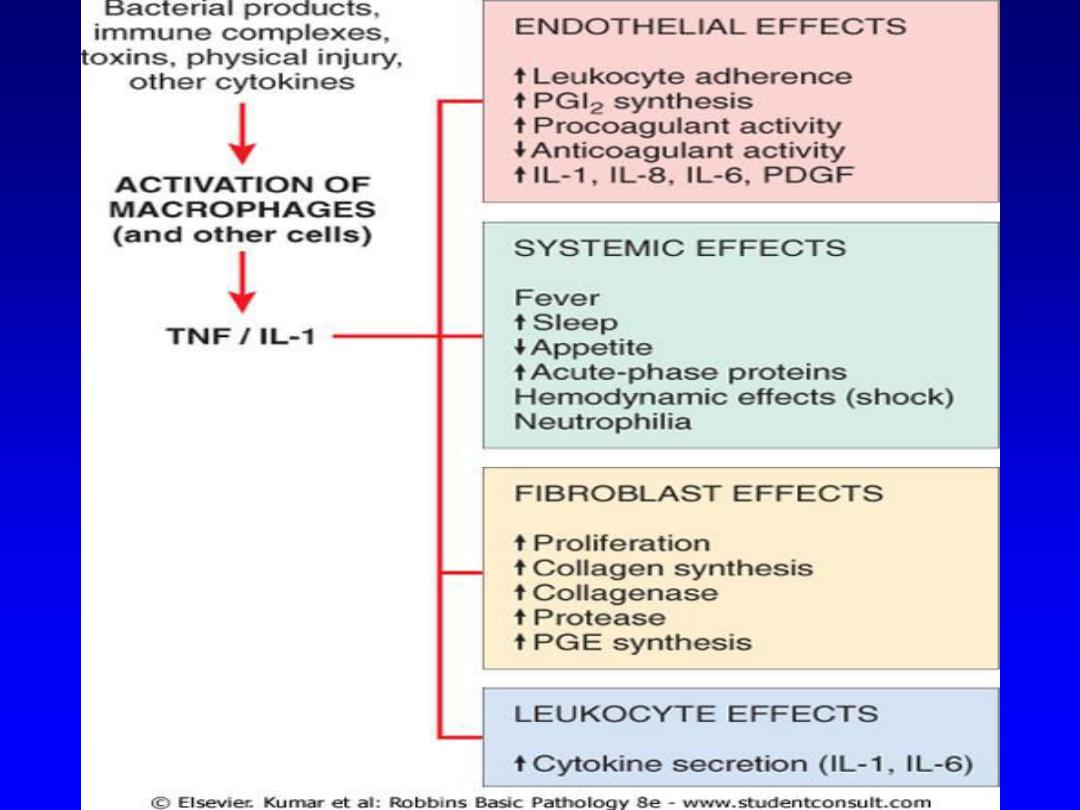
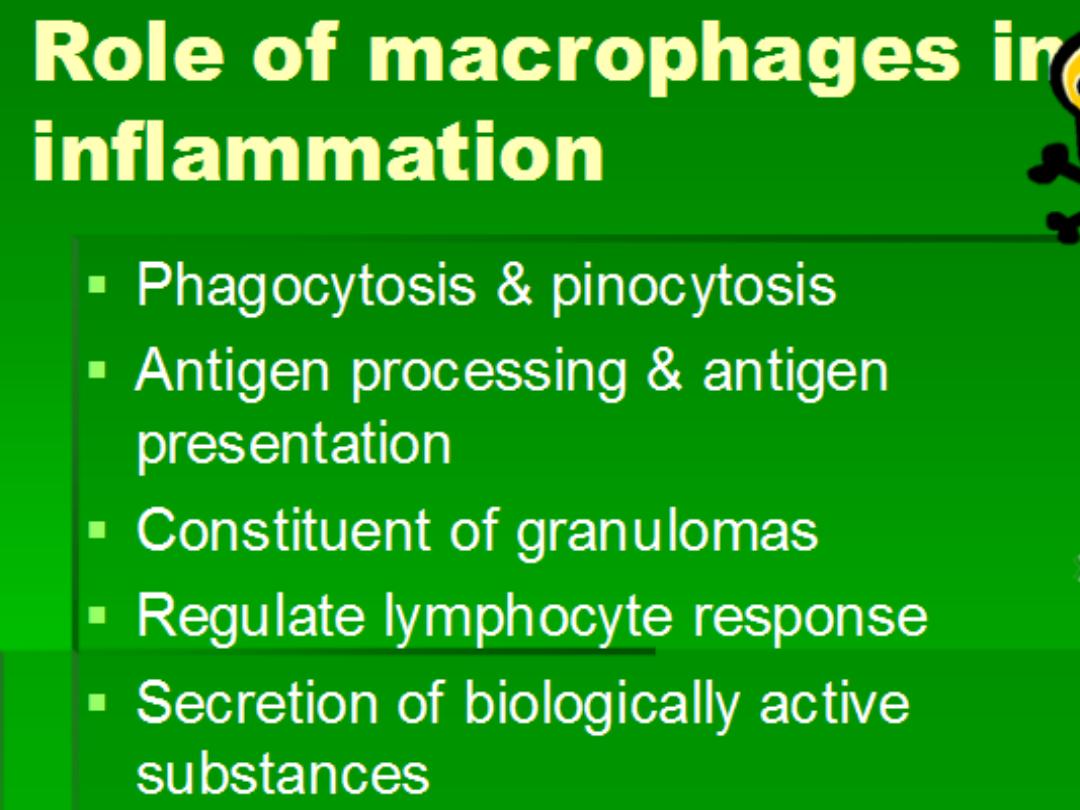
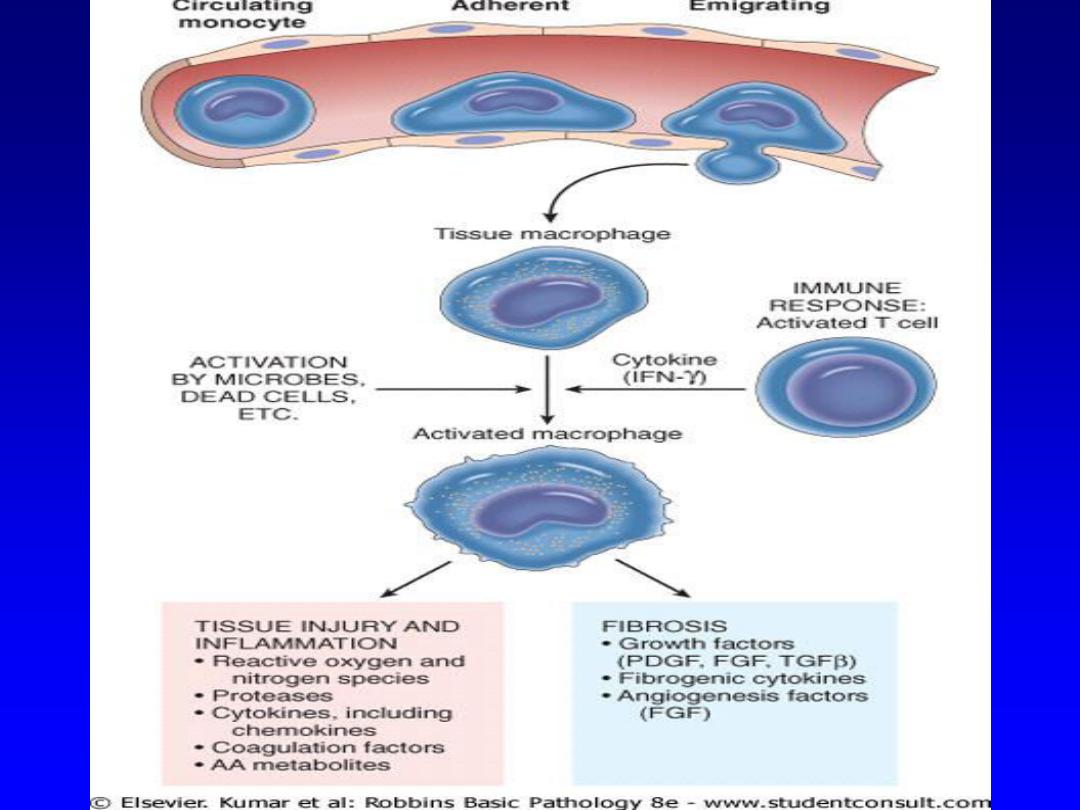
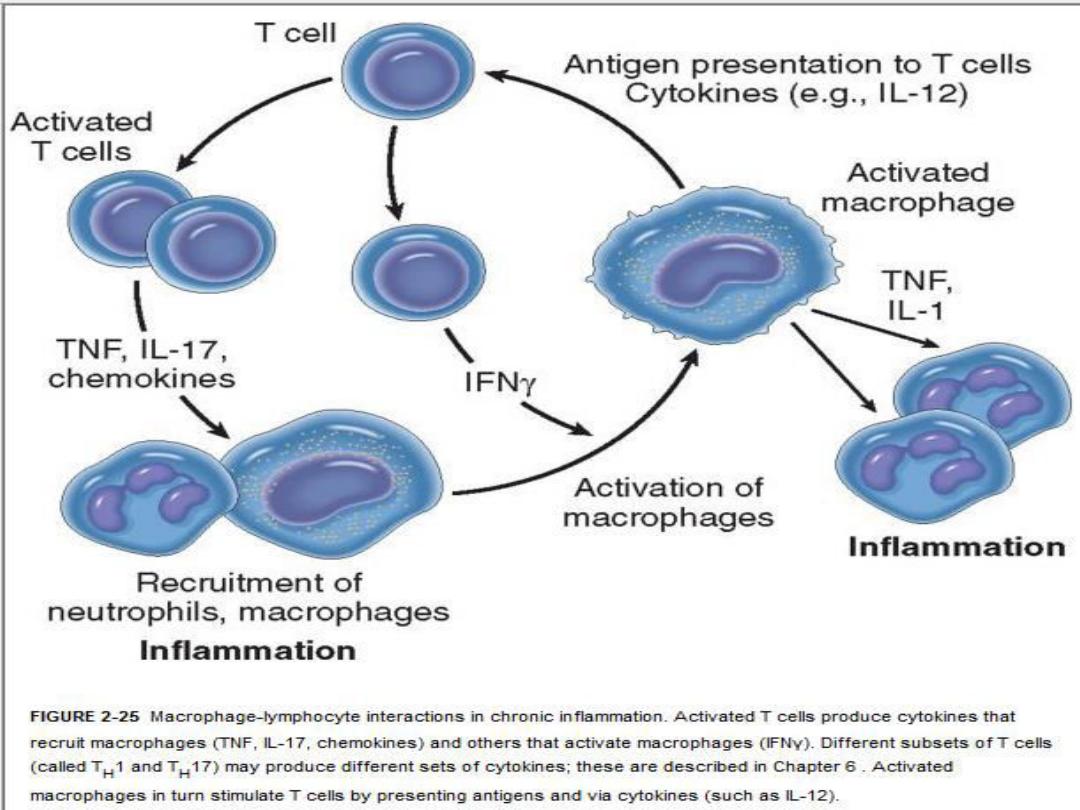
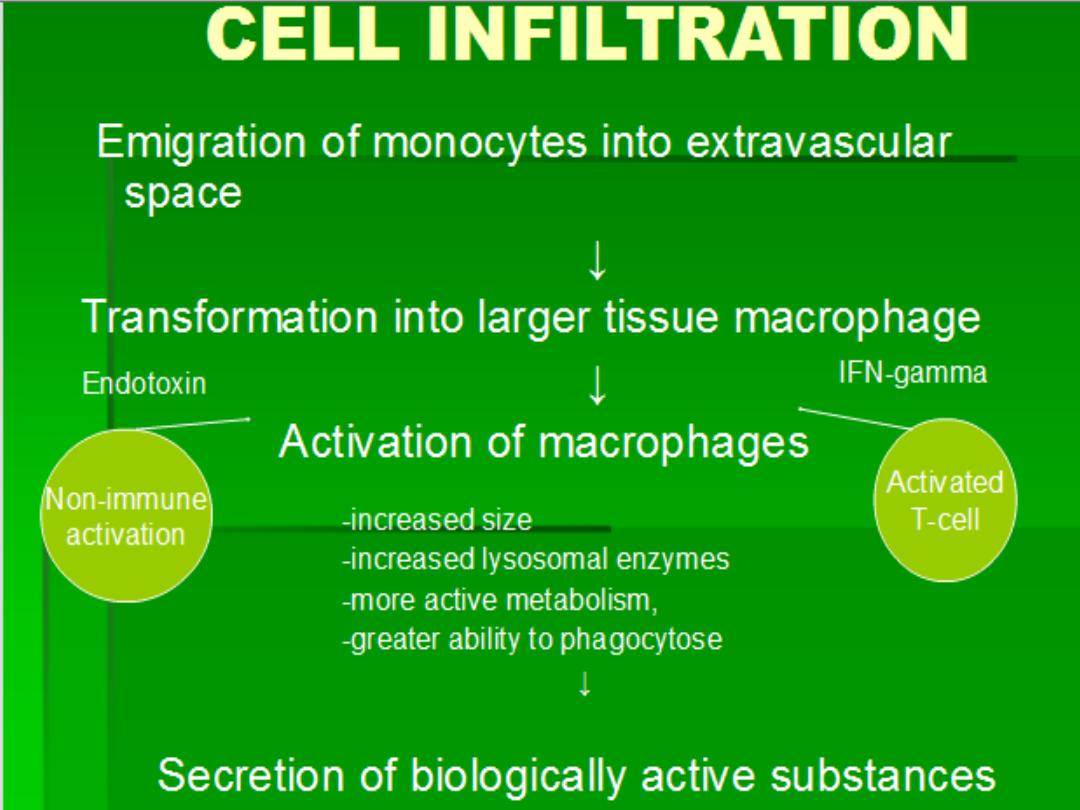
Mechanism of chronic inf
Macrophages
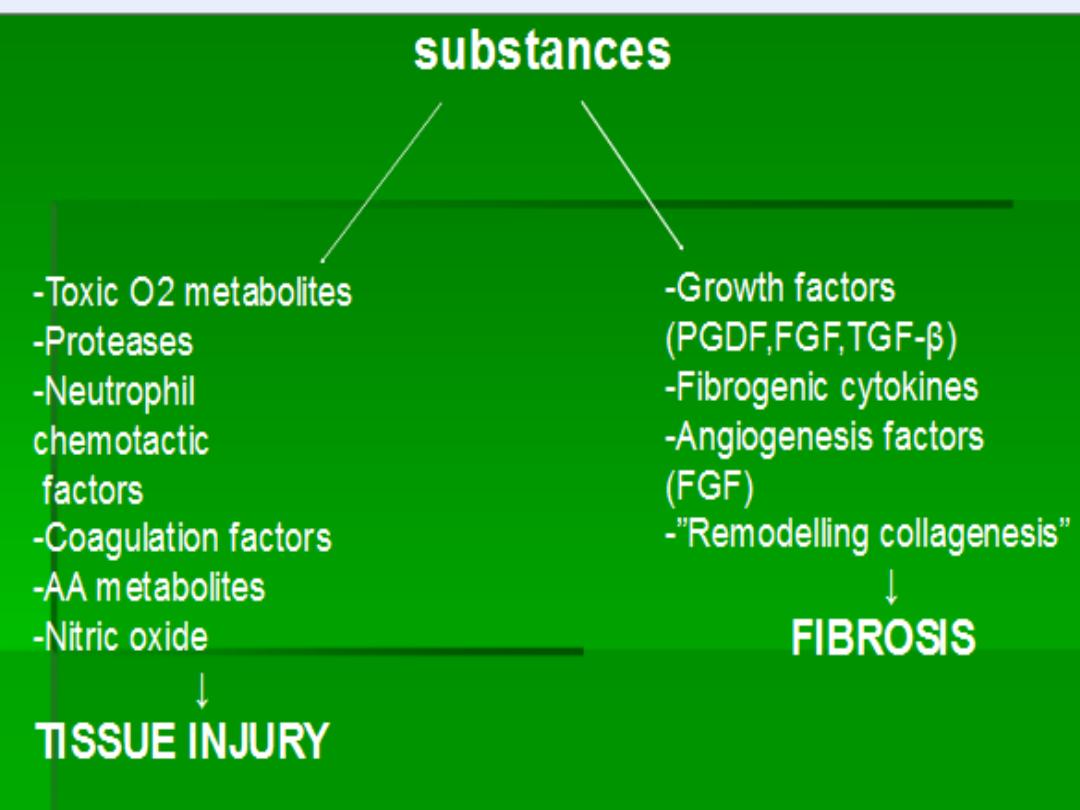
Cytokines
Free
radicals
Enzymes
NO
TGF-B
FGE
GCSF
+
EGF
Angiogenesis
Fibrosis
Granulation tissue
Healing
Activation

MORPHOLOGIC FEATURES
Chronic inflammation is characterized by:
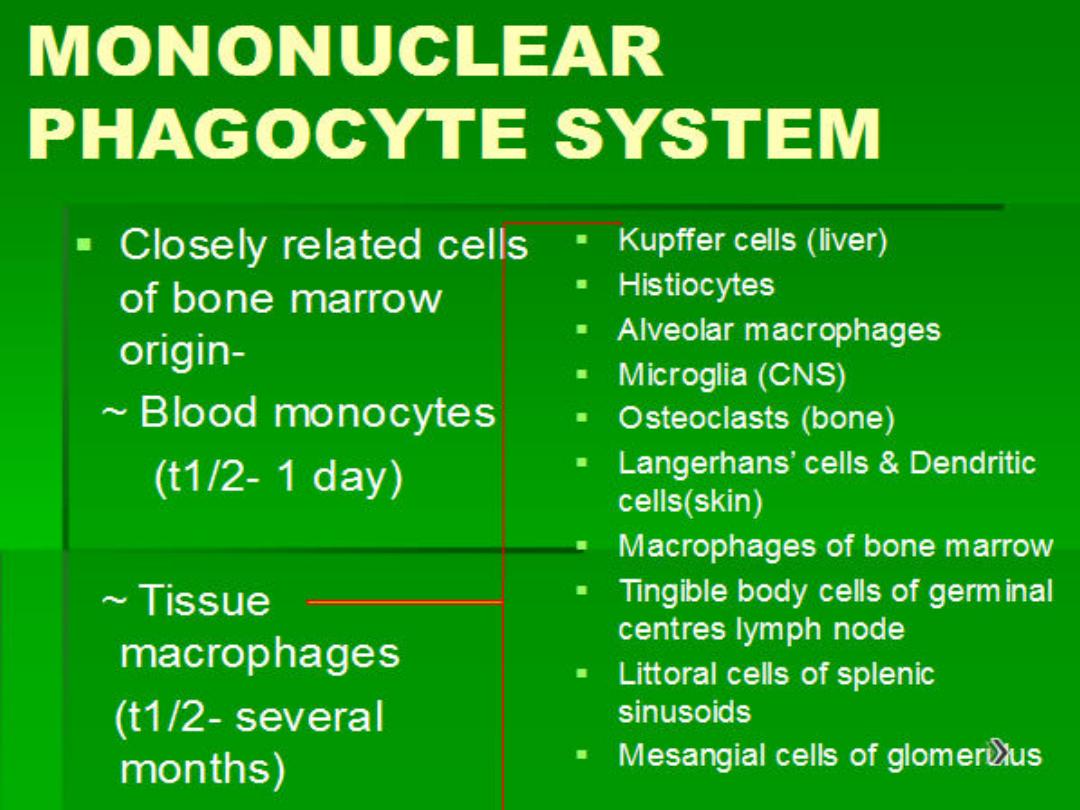
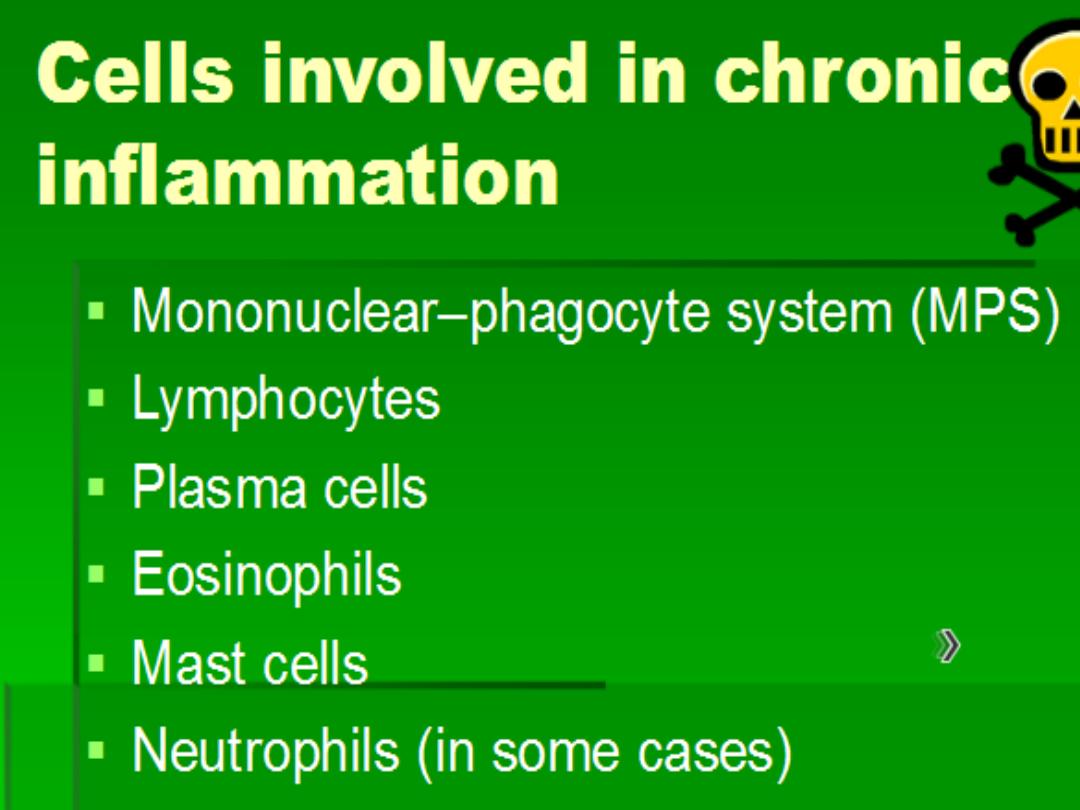
1. Infiltration with mononuclear cells
, which include
macrophages, lymphocytes, and plasma cells
2. Tissue destruction
, induced by the persistent
offending agent or by the inflammatory cells
3.
Attempts at healing by connective tissue
replacement of damaged tissue
, accomplished by
proliferation of small blood vessels
(
angiogenesis
)
and, in particular,
fibrosis
.

A, Chronic inflammation in the lung, showing all
three
characteristic
histologic features: (1) collection of chronic inflammatory cells (*), (2)
destruction of parenchyma (normal alveoli are replaced by spaces
lined by cuboidal epithelium, arrowheads), and (3) replacement by
connective tissue (fibrosis, arrows).





GRANULOMATOUS INFLAMMATION
Granulomatous inflammation
is a distinctive
pattern of chronic inflammation that is
encountered in a limited number of infectious
and some noninfectious conditions.
Immune reactions are usually involved in the
development of granulomas
.
A granuloma is a cellular attempt to contain an
offending agent that is difficult to eradicate.
In this attempt there is often strong activation of
T lymphocytes leading to macrophage
activation, which can cause injury to normal
tissues
.


A granuloma is a focus of chronic
inflammation
consisting of
a microscopic aggregation of macrophages
that are transformed into
epithelium-like
cells
,
surrounded by a collar of
mononuclear leukocytes,
principally
lymphocytes .
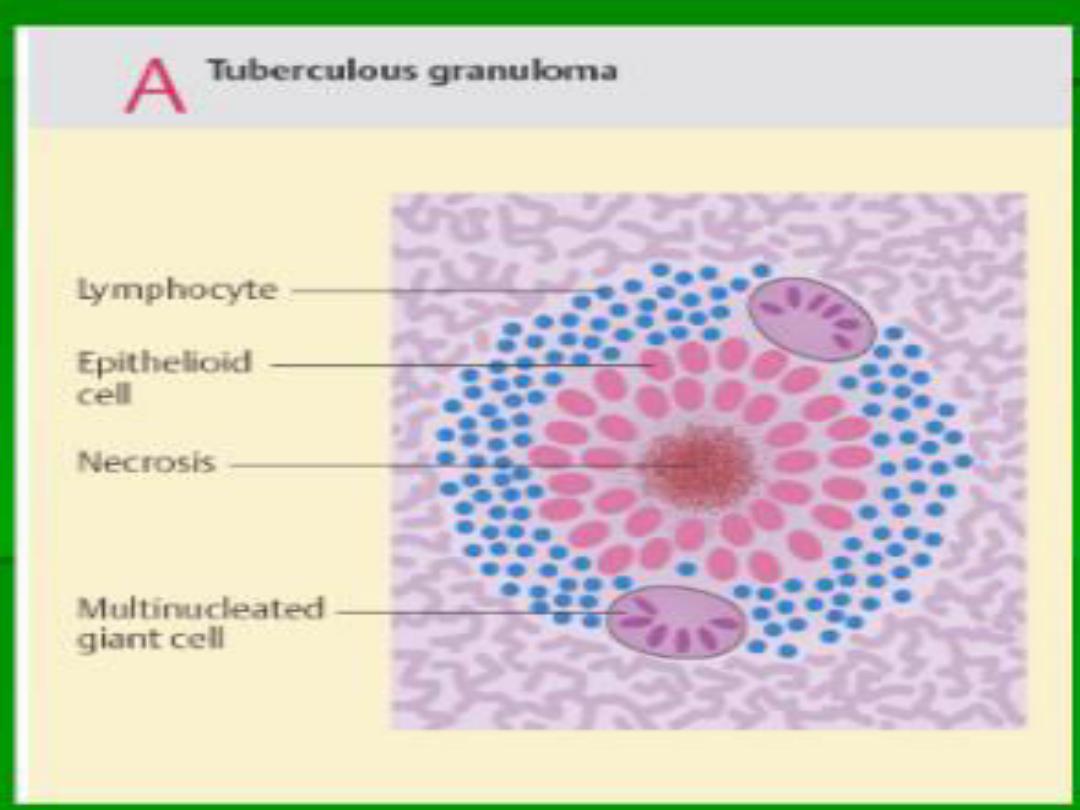

Giant Cells
Older granulomas
develop an
enclosing rim
of
fibroblasts and connective tissue
.
Frequently,
epithelioid cells fuse to form
giant
cells
in the periphery
or
sometimes in the
center of granulomas.
These giant cells may attain diameters of
40
to 50
μm
.
They have a large mass of
cytoplasm containing
20 or more small
nuclei
arranged either
peripherally
(Langhans-type giant cell)
or
haphazardly
(
foreign body
–type giant cell
).

Types of Granulomas
I. Foreign body granulomas
II. Immune granulomas

Foreign body granulomas
Incited by relatively inert foreign bodies.
Typically, foreign body granulomas form
around material
that are large enough to
preclude phagocytosis by a single
macrophage and do not incite any specific
inflammatory or immune response.
The foreign material can usually be identified
in the center of the granuloma,
particularly if
viewed with
polarized light
, in which it
appears
refractile.

Immune granulomas
Caused by agents that are capable of
inducing an immune response which
produces granulomas usually when
the inciting agent is poorly degradable
or particulate.


Methods of identification of Specific etiologic
agent in Granulomatous diseases
1.
Special stains for organisms
(e.g.,
acid-fast stains for tubercle bacilli
),
2.
Culture methods
(e.g., in
tuberculosis and fungal diseases
),
3.
Molecular techniques
(e.g.,
the polymerase chain reaction in tuberculosis
)
,
4.
Serologic studies
(e.g., in
syphilis
).