CALIBRATION CURVE
For accurate work, standard solutions should be included with each determination. Variations in chemical reactions of new batches of reagents and in instrument behavior combine to cause variability in the absorbance ‘A’ of samples. It is useful to document the successiveabsorbance ‘A’ readings of the standard for quality control purposes.
Furthermore, when Beer-Lambert law is obeyed, calibration curve is prepared for the range of concentrations intended to be covered in practice.This curve is prepared by taking several dilutions of the
substance to be measured, running the experiment as described, then plotting absorbance against concentration on a graph paper. When running the experiment, the concentration of the unknown is found from this curve..
Preparation of calibration curve
An alternative procedure to find the concentration of ‘test’ of
unknown protein sample is to prepare a calibration curve, then read the concentration of the unknownProcedure
Three known concentrations of protein standard will be provided.Place 3 ml of each concentration in a glass tube and label (St1, St2 & St3).
Place 3 ml of distilled water in another tube and label B. Add 5 ml of
Biuret colour reagent to all tubes, mix, incubate for 30 min. at room temp.
or for 10 min. at 37 °C water bath. Read the absorbance of the standard
and blank by the spectrophotometer at 540 nm against distilled water.
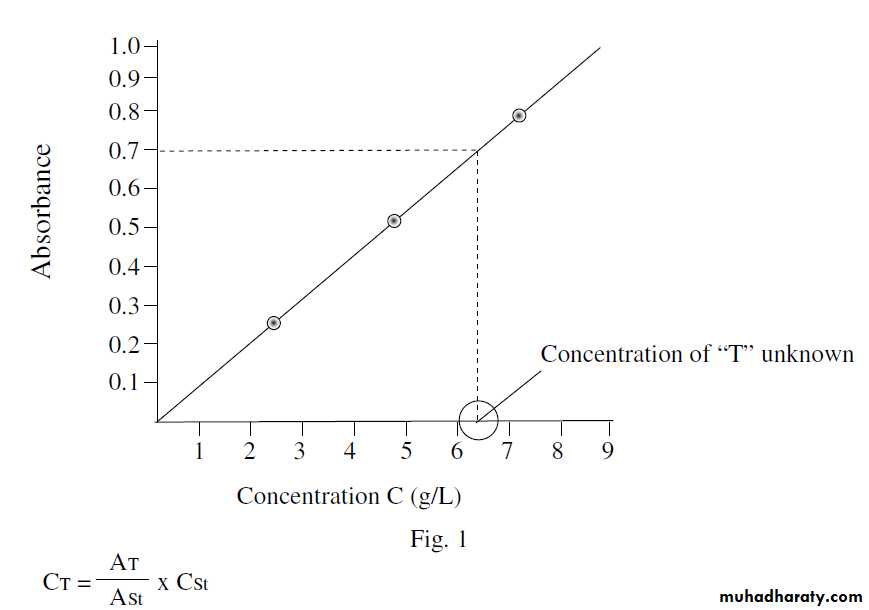
Subtract ‘B’ reading from each reading of the standards, then construct a calibration or standard curve with absorbance on Y-axis and concentrations in g/L ml on X-axis as in fig. (1). Find the concentration of the unknown solution ‘T’ from the curve .
3- Unknowns: the above standards can be given as unknowns or any
other dilution could be made.
Reagents
1- Biurt reagent (as for total protein).2- Protein standards : 2.5, 5.0, 7.5 g/L. They are equivalent to: 37.5,
75.0, 102.5 g/L in serum.
Prepare stock std: 5gm bovine albumin in 100 ml H2O or (50 g/L).
a. Take 12.5 ml of stock and dilute to 250 ml with DW. This
will give 2.5 g/L.
b. Take 25 ml of stock and dilute to 250 ml with DW. This will
give 5.0 g/L.
c. Take 37.5 ml of stock and dilute to 250 ml with DW. This
will give7.5 g/L.
3- Unknowns: the above standards can be given as unknowns or any other dilution could be made.
other dilution could be made.