~1
~
Third Stage Internal Medicine Dr. Fadhil
Clinical Immunology 4
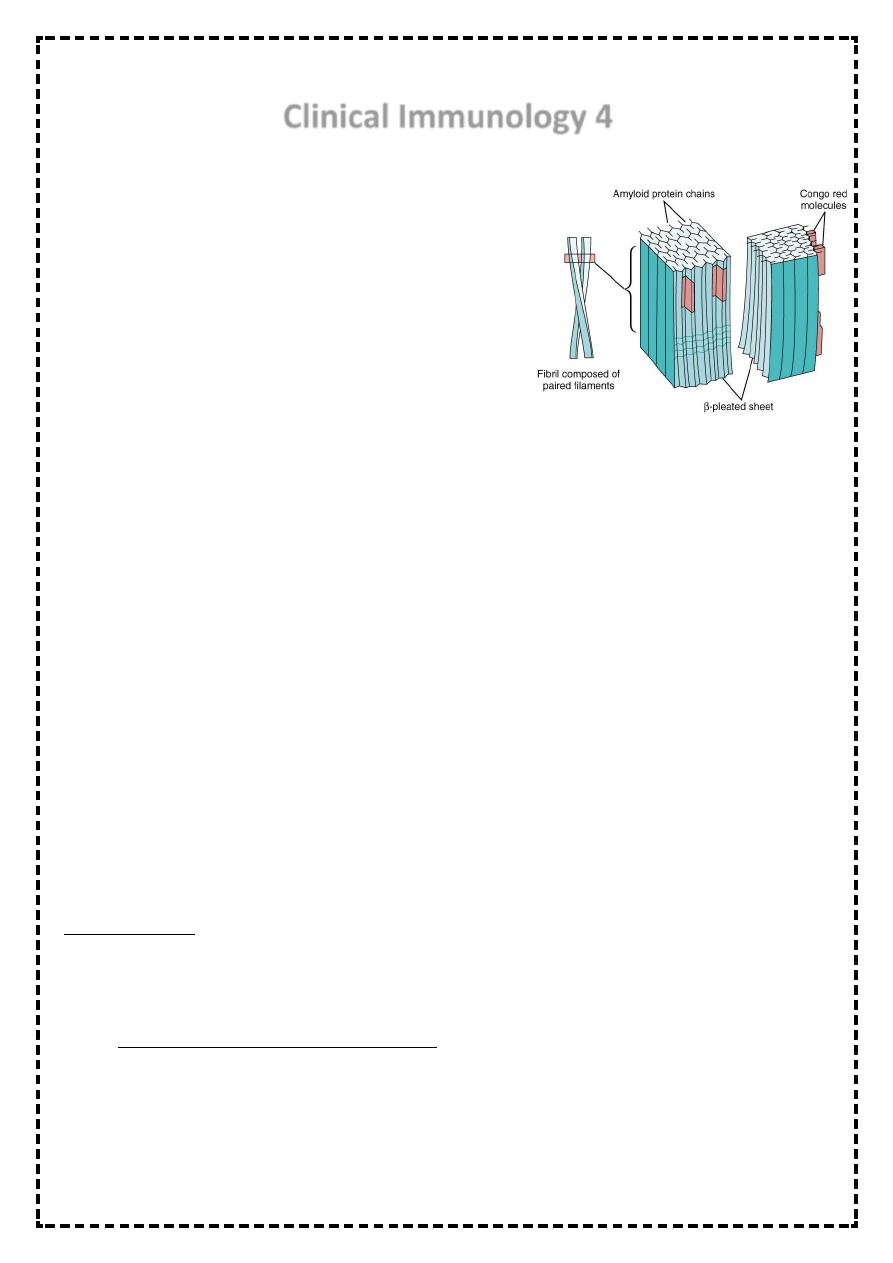
Amyloidosis
• Is a disorder of protein metabolism in which there
is an extracellular deposition of pathological
insoluble fibrillar proteins in organs and tissues.
Characteristically, the amyloid protein consist of B-
pleated sheets that are responsible for its
insolubility and resistance to proteolysis.
• Amyloidosis can be acquired or inherited.
Classification is based on the nature of precursor of plasma protein( at least 20) that form
the fibrillar deposit. The process for the production of the fibrils appears to be
multifactorial and differs amongst the various types of amyloid.
• This disease was named by Virchow in 1854 on the basis of color after staining with
iodine &sulfuric acid.
• All amyloid proteins share a unique fibrillar ultrastructure.
• amyloid proteins can be deposited locally or can involve virtually every organ system of
the body.
• Amyloid fibril deposition may have no clinical consequences or may lead to severe
pathophysiological changes. Often the disease falls between these two extremes.
• Regardless the etiology, the clinical diagnosis of amyloidosis is usually not made until the
disease is far advanced.
• The clinical manifestations of amyloidosis depends on the organ(s) affected.
• The diagnosis of amyloidosis should be considered in all cases of unexplained nephrotic
syndrome, cardiomyopathy, &peripheral neuropathy.
CLASSIFICATION
• Amyloid diseases are classified by etiology and type of protein deposited.
1- acquired systemic amyloidosis
• a- reactive(AA) (secondary) amyloidosis!
• These are due to amyloid formed from serum amyloid A(SAA), which is an acute phase
protein. It is, therefore, related to chronic inflammatory disorders and chronic infections.

~2
~
• Clinical features depend on the nature of underlying disorder. Chronic inflammatory
disorders include rheumatoid arthritis, inflammatory bowel disease and untreated
familial Mediterranean fever.
• In developing countries it is still associated with infectious diseases such as tuberculosis,
bronchiectasis and osteomyelitis. AA amyloidosis often presents with chronic kidney
disease, with hepatomegaly and splenomegaly. Macroglossia is not a feature and cardiac
involvement is rare.
• The degree of renal failure correlates with SAA level in a more favourable out come in
patients with low normal levels.
• b-light chain amyloid. (AL, primary) seen in patients with Myeloma and plasmacytoma.
• It manifested as restrictive cardiomyopathy&
• peripheral neuropathy.
• c- Dialysis related amyloidosis:
• This is due to B2- microglobulin producing amyloid fibrils in chronic dialysis patients. It
frequently presents with carpal tunnel syndrome. It occurs 5-10 years after dialysis.
• dialysis associated(AB2M) amyloidosis.
• Occurs 5-10 years of dialysis
• d- senile systemic amyloidosis:
• Usually asymptomatic& occurs in patients over the age of 70 years.
2-Familial(hereditary)amyloidosis: these are autosomally dominant transmitted diseases
where the mutant protein forms amyloid fibrils, starting usually in the middle age.
• hereditary systemic amyloidosis
• An autosomal dominant disorder& manifested as
• peripheral& autonomic neuropathy& cardiomyopathy.
• Examples include disorders such as familial amyloidosis polyneuropathy (FAP),
cardiomyopathy or the nephrotic syndrome. Major foci of FAP occur in Portugal, japan
and Sweden.
• Local amyloidosis:
• Deposits of amyloid fibril of various types can be localized to various organs or tissues(
skin, heart or brain).
• The brain is a common site of amyloid deposition.
• Intracerebral and cerebrovascular amyloid deposits are seen in Alzheimer’s disease

~3
~
Diagnosis
• the diagnosis is established by
biopsy which may be of an affected
organ , rectum or subcutaneous fat.
• The pathognomonic histological
feature is apple-green birefringence
of amyloid deposits when stained
with Congo red dye and viewed
under polarized light.
MANAGEMENT
• the aims of treatment are to support the function of the affected organ and in acquired
amyloidosis to prevent further amyloid deposition through treatment of the primary
cause.
• Genetic counseling is an important aspect of treatment in heredofamilial amyloidosis.
• Liver transplantation had been carried out since 1990 for patients with familial amyloid-
polyneuropathy(FAP).
• Recent trials have indicated that a prednisolone/melphalan/
• Colchicine's program can prolongs the life.
Autoimmune diseases
• Result from a failure of self-tolerance
• Immunological tolerance is specific unresponsiveness to an antigen
• All individuals are tolerant of their own (self) antigens
• these disorders are chronic and usually irreversible
• incidence: 5%-7% of population, higher frequencies in women, increases with age
Immunological tolerance
• This is the process by which the immune system distinguishes self from foreign tissues.
• Central tolerance occurs during lymphocyte development in the thymus and bone
marrow.
• T&B lymphocytes that recognize self antigens are deleted before they develop into fully
immunocompetent cells.
• Some autoreactive cells inevitably evade deletion &escaping to the peripheral circulation.
These cells are controlled through peripheral tolerance mechanisms.

~4
~
• These include the suppression of autoreactive cells by 'regulatory' T-cells& the generation
of the hyporesponsivness 'anergy‘ in lymphocytes.
• Failure of any of these tolerance mechanisms may result in the development of
autoimmune disease.
Factors predisposing to autoimmune disease
• Both genetic and environmental factors contribute to the development of autoimmune
disease.
• The most important genetic determinants of autoimmune susceptibility are the HLA
genes, reflecting there importance in shaping of lymphocyte responses.
• Several environmental factors can trigger autoimmunity in genetically susceptible
individuals.
• The most widely studied of these is infection, as occurs in acute rheumatic fever fallowing
streptococcal infection or reactive arthritis fallowing bacterial infection.
• A number of mechanisms have been postulated including cross- reactivity between the
infectious pathogen and self determinants(molecular mimicry), and release of
sequestered antigens which are not usually visible to the immune system from damaged
tissue.
• Occasionally the development of autoimmune disease is a side effect of drug treatment,
for example:
the metabolic products of the anesthetic drug halothane bind to liver enzymes, resulting
in a structurally novel protein.
• This is recognized as new(foreign) antigen by the immune system causing hepatic
necrosis.
HLA-association in autoimmune disease
• Disease HLA-ASSOCIATION
Ankylosing spondylitis B27
Type-1 diabetes DR3/4
Rheumatoid arthritis DR4
Grave's disease DR3
Myasthenia gravis DR3

~5
~
CLASSIFICATION OF AUTOIMMUNE DISEASE
• TYPE DISEASE
• Organ specific
• Immune response Grave's disease
• Directed against Addison's dis.
• Localized antigens Pernicious
anemia
Type-1 diabetes
Pemphigus vulgaris
• Idiopathic thrombocytopenic
purpura
• Autoimmune hemolytic anemia
Myasthenia gravis
Rheumatoid arthritis
Dermatomyositis
primary biliary
cirrhosis
• multisystem
• Immune response Systemic sclerosis
directed to Mixed connective
widespread tissue disease
target antigens SLE
*******************
Treatment of autoimmune diseases
– Treatment aimed at:
• Killing dividing cells
– Immunosuppressant
• Controlling T cell signaling
~6
~
– Cyclosporin
• Anti-inflammatory medications
– Cortisone-like steroids
• Replacement therapy
– Insulin
