Adrenal Glands and its hormones ﺩ.ﺑﺎﻥ ﺟﺎﺑﺭ
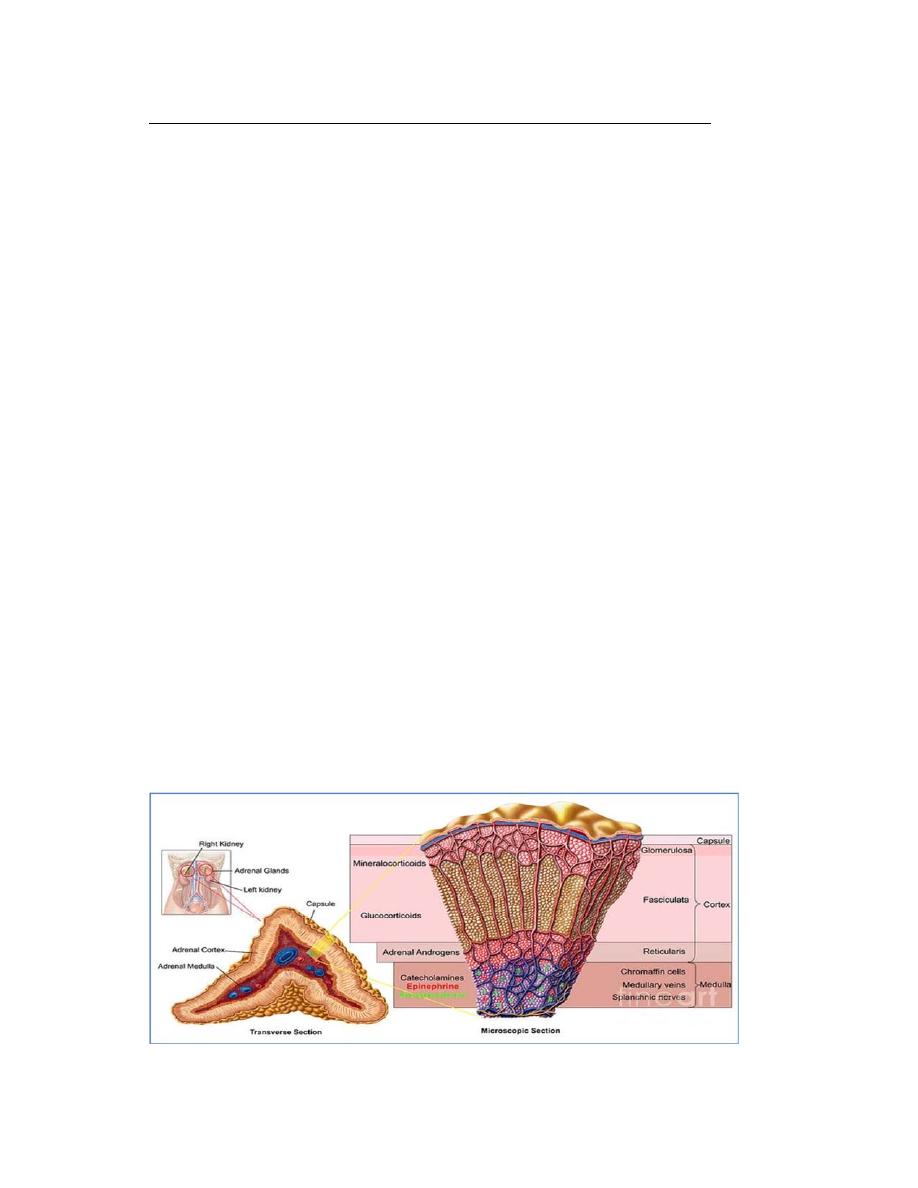
There are 2 adrenal glands that lie superiorly to each kidney. Each adrenal gland
composed of 2 parts : an adrenal cortex and an adrenal medulla.
The adrenal medulla is the inner portion and it secretes epinephrine and
norepinephrine when stimulated to do so by the sympathetic nervous system.
These two hormones are called catecholamines and are involved in the “fight or
flight”mechanism of the nervous system.
The adrenal cortex is the outer portion and secretes a variety of steroid
hormones called corticorsteroids (precursor is cholesterol).
About 80% of the adrenal gland is composed of the cortex which has three
layers or zones:
1) Zona glomerulosa – the outermost region or layer
2) Zona fasciculata – the middle and largest portion
3) Zona reticularis – the innermost layer.
All three layers secrete corticosteroids of which cholesterol is the common
precursor. On the basis of their primary actions, the adrenal steroids can be
divided into 3 categories:
a) mineralocorticoids – aldosterone, which influences mineral or electrolyte
balance; from z. glomerulosa
b) glucocorticoids – cortisol, which plays a role in glucose metabolism and
proteins and lipids as well; from z. fasciculata
c) sex hormones – identical to those produced by the gonads (male – testes;
female – ovaries); from z. reticularis
Histology of adrenal gland

Plasma transport of adrenal steroid hormones
Cortisol: binds to cortisol‐binding globulin in plasma with high affinity and to
albumin with low affinity (albumin binds all steroids). Aldosterone: No high
affinity binding protein is present in plasma so binds weakly to albumin and has
a shorter half‐life than cortisol as a result.
Metabolism
The kidney filters free steroid hormone but reabsorbs ~90%. The liver converts
steroid hormones to hydrophilic metabolites by hydroxylation and conjugation
reactions (liver damage e.g. cirrhosis in alcoholics causes cortisol build up).
Mineralcorticoids from the zona glomerulosa
Aldosterone is the primary one and its most important effect is its action on
electrolyte balance. Its primary site of action is the distal tubules of the kidney
nephron (other sites:
salivary and sweat glands)
where it promotes Na+ retention into
the blood and enhances K+ elimination into the urine filtrate (
via stimulating
transcription of the Na/K ATPase protein )
. If Na+ is retained, H20 is osmotically attracted
to Na+ and therefore is retained as well. This increase in water in the blood
causes an increased blood volume. This is important in the longterm regulation
of blood pressure.
Without mineralocorticoids, death will occur due to large loss of plasma volume
that would be lead to circulatory shock. Other hormone deficiencies won’t cause
death directly such as this but problems associated could lead to premature
death.
Regulation of Aldosterone release or secretion
Aldosterone is released due to:
1) Activation of the renin‐angiotensin system in the kidneys and related to a
decrease in Na+ and decrease in BP.
2) Direct stimulation of the adrenal cortex by increase in blood K+ concentration
This zone is relatively independent of the anterior pituitary hormone influence
of ACTH. It may have a weak effect in releasing aldosterone but in general it
does not.
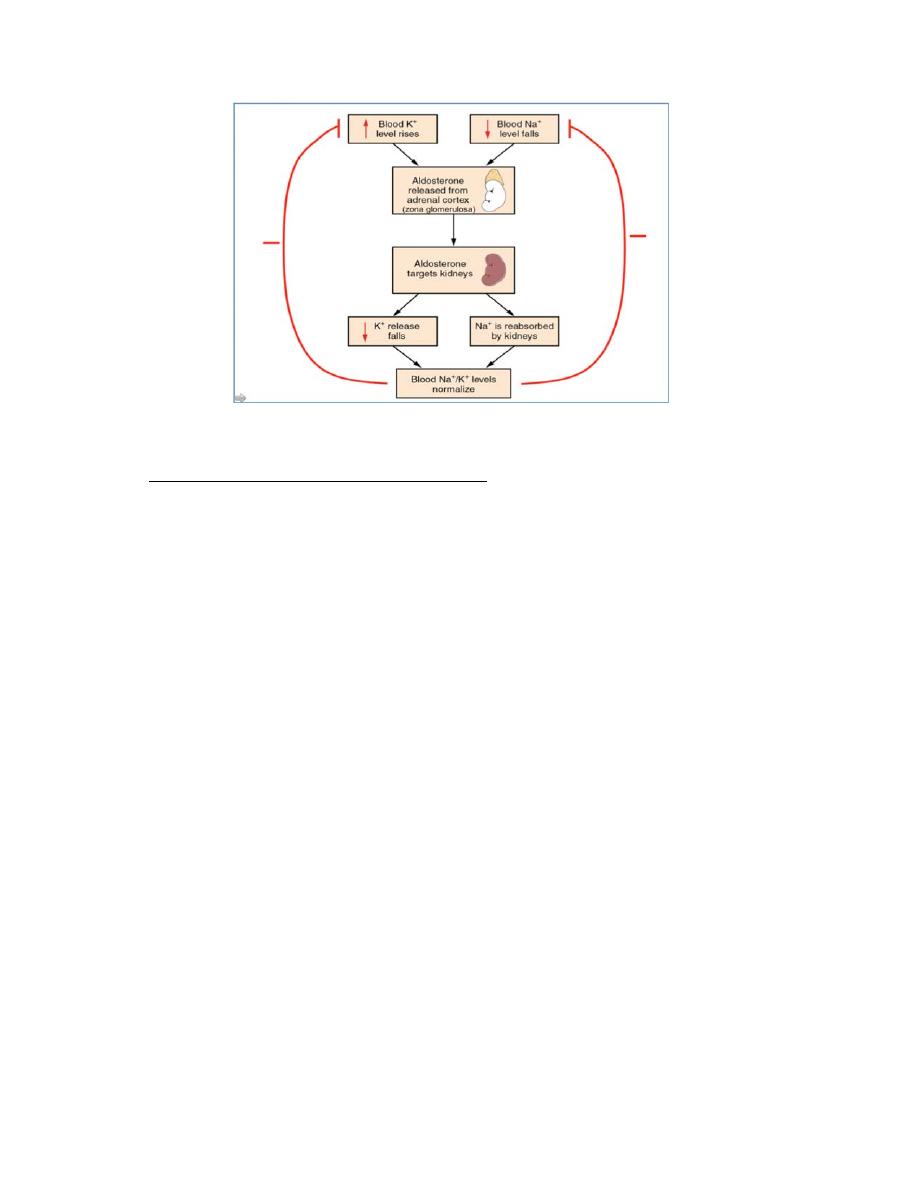
Regulation of Aldosterone secretion
Glucocorticoids from the zona fasciculata
The main glucocorticoid is cortisol from the z. fasciculata and it has an important
role in carbohydrate, fat and protein metabolism.
Overall, cortisol main effect is to increase concentration of blood glucose at the
expense of fat and proteins.
Metabolic effects of Cortisol
On carbohydrate (CHO)
1)
↑ Gluconeogenesis – this is the conversion of nonCHO precursors(aa)
into CHO within the liver. During periods of fasting, the liver delivers glycogen to
become glucose to maintain normal blood glucose levels for the brain. However,
if glycogen is depleted, then new glucose is made upon the stimulus of cortisol.
2)
Decrease utilization of glucose by cells everywhere else in the body
except the brain. So glucose more available to the brain.
Summary on CHOs: ↑gluconeogenesis and ↓ other cells’ uptake of glucose,
so cortisol caused a diabetogenic effect or ↑ blood glucose ' Adrenal diabetes'.
On Protein metabolism
1) Cortisol stimulates protein degradation in all cells except liver cells. So, by
breaking down a portion of muscle proteins into their amino acid components,

cortisol increases the concentration of blood amino acids. Now these amino
acids are available for gluconeogenesis at the liver.
2) Cortisol promotes formation of proteins(anabolism) by the liver which is
opposite to the rest of the body proteins. The liver makes plasma proteins so
these would be increased as well.
Summary on protein metabolism: ↓ muscle proteins, ↑ liver proteins.
On Lipid Metabolism: Cortisol increases lipolysis or increased fatty acids in the
blood from adipose tissue. These fatty acids can then be used for energy source
instead of glucose thereby conserving glucose for the brain.
Cortisol presence allows for permissiveness
Cortisol presence permits catecholamines to induce vasoconstriction. If not, a
person lacking cortisol, may go into circulatory shock (↓blood volume or blood
pressure) in a stressful situation that needs widespread vasoconstriction.
Anti‐inflammatory and Immunosuppressive Effect of cortisol
1) Anti‐inflammatory ‐ Synthetic glucocorticoids are being administered to
inhibit all steps in inflammation that are actually very destructive, such as in
rheumatoid arthritis. They act to decrease inflammation and swelling and
stabilize capillary membranes.
2) Immunosuppression – Corticosteroids are also given to inhibit the effects of
the immune system by knocking out of commission the white blood cells
responsible for antibody production and destruction of foreign cells. Useful in
allergic disorders and preventing organ transplant rejection.
Overuse and increased amounts of corticosteroids should be avoided and should
only be used sparingly.
Reasons why therapeutic use should be limited:
1) Persons using have limited ability to resist infections
2) Other undesirable effects can occur with the good ones such as: a)Gastric ulcers b)
↑blood pressure c) atherosclerosis d) menstrual irreg.
3) High levels of exogenous corticosteroids can lead to irreversible atrophy of the
cortisol‐secreting cells of the adrenal gland and later permanent inability of the body to
produce cortisol
.
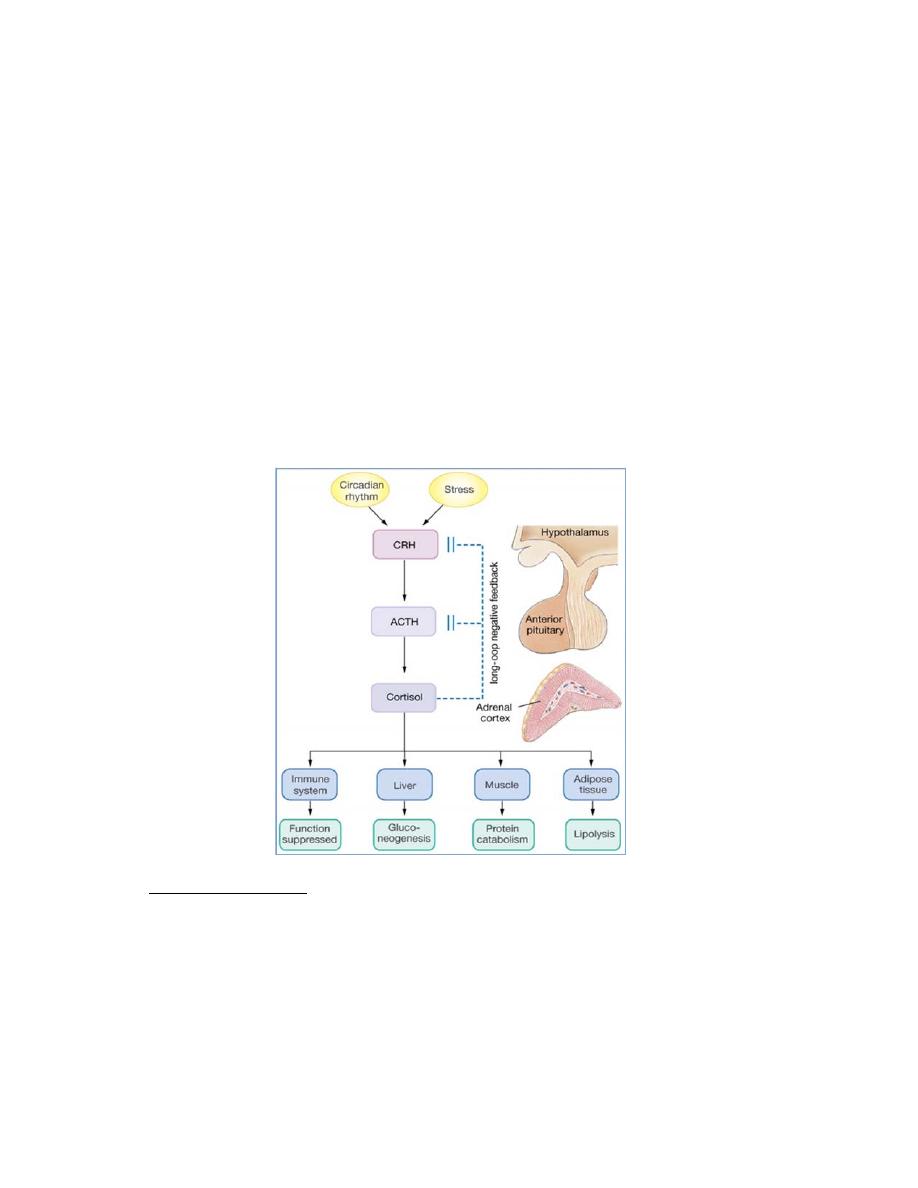
Regulation of Cortisol release
Two factors that can also influence the negative feedback are diurnal variation
in cortisol and stress.
1)Cortisol levels are highest in the morning and lowest at night. This is primarily
related to the sleep‐wake cycle. They will be reversed in one who works nightly
and sleeps daily. This information is particularly important to know: 1) when
blood sample is taken; 2) the sleep cycle of person being sampled; and 3) the
effect of surgery time of day in helping the individual handle the stress.
2)Stress can greatly affect levels of cortisol in the blood. It can override, for
example the hypothalamic‐pituitary axis of negative feedback. The magnitude of
the increase of blood cortisol is proportional to the intensity of the stressful
stimulation. More stress – more cortisol. Less stress‐ less cortisol.
Adrenal Androgens
Adrenal Androgens is produced and released from the adrenal cortex zona
reticularis. DHEA is a weak androgen which stimulates axillary/pubic hair
development at puberty, and libido. Release of DHEA is stimulated by ACTH. At
most times DHEA is a very minor component of adrenal secretions.
Pathologies associated with Adrenal cortex hormone secretion
A) Oversecretion of Adrenal Cortex Hormones
1) 1
o
Hyperaldosteronism – Conn’s syndrome –hypersecreting adrenal tumor of
zona glomerulosa; ↑ aldosterone with no nega ve feedback. High Na+
(hypernatremia) and low plasma K+(hypokalemia) which leads to↑↑ BP
2) 2
o
Hyperaldosteronism – produced by any condition that causes a chronic
reduction in arterial blood flow to the kidneys, thereby excessively activating the
renin‐angiotensin system. Ex. Is atherosclerotic narrowing of the renal arteries.
Similar results as in above.
Diagnosis :
Electrolyte : Hypernatraemia – Hypokalaemica
Plasma aldosterone : renin activity ratio
saline infusion test : if plasma aldosterone remains high ‐‐ primary type
Imaging techniques – CT scan – MRI (adenoma or hyperplasia )
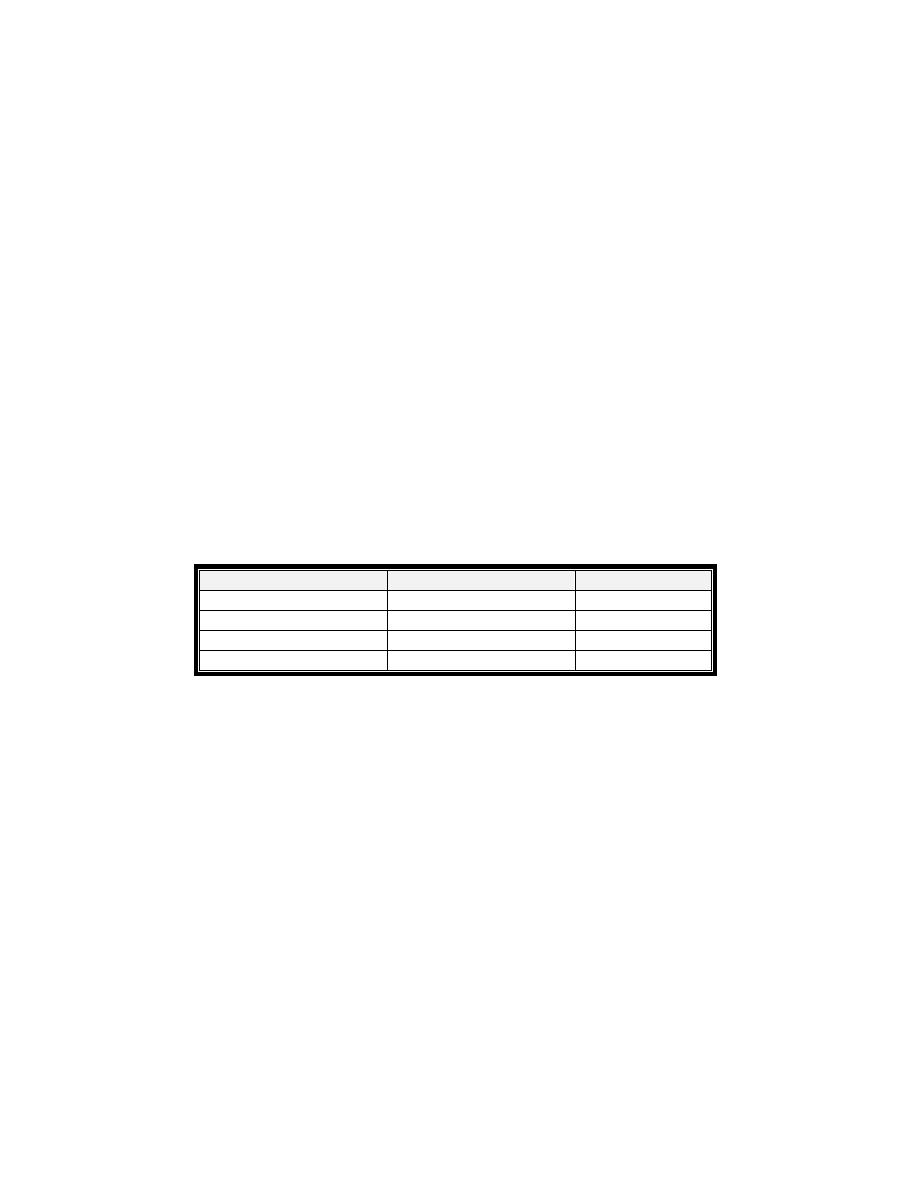
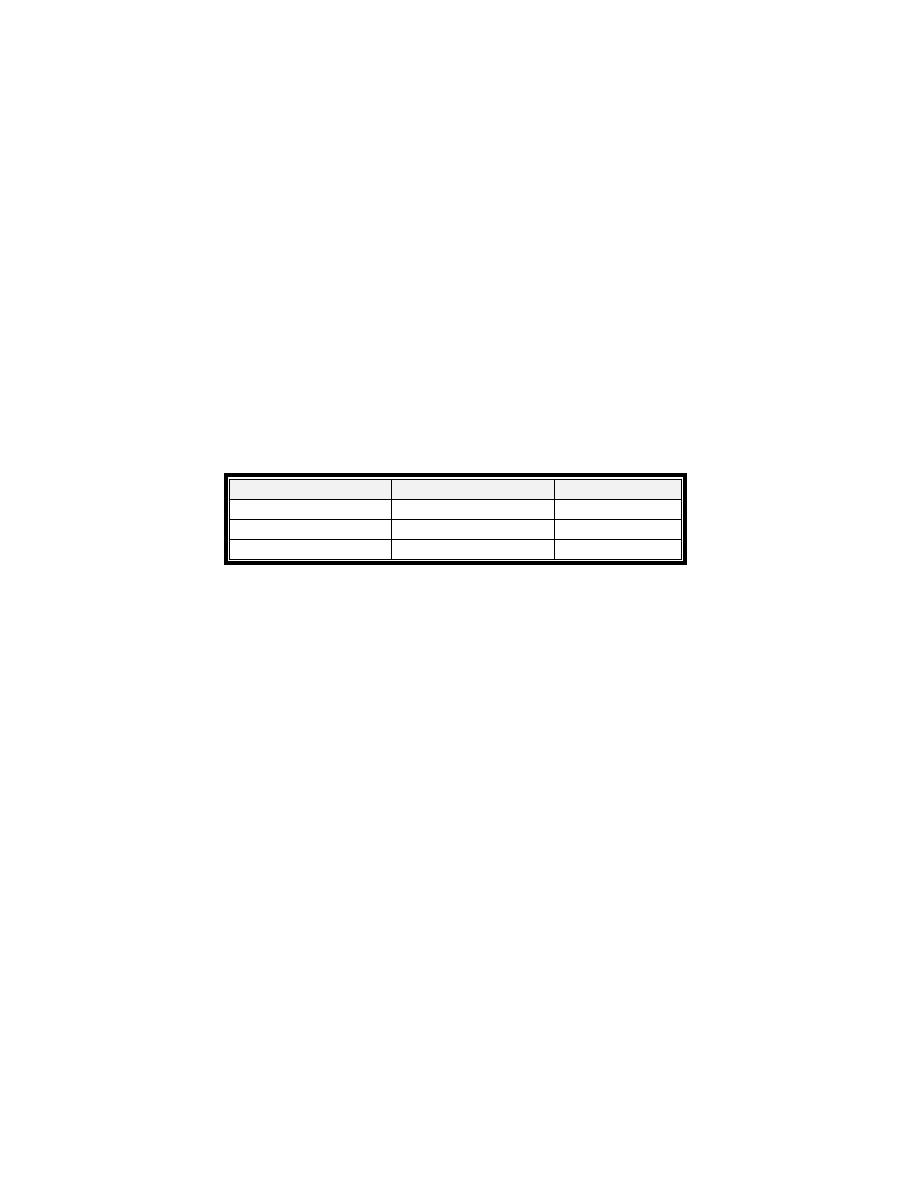
Primary
Secondary
potassium
Low
Low
Sodium
High
High
Rennin
Low
High
Aldosterone
High
High
Treatment : Tumour – Remove surgically while in Bilateral adrenal hyperplasia
treated by Spironolactone.
3) Hypersecretion of cortisol, Cushing’s syndrome – increase cortisol cause
excess glucose level which lead to deposit of fat in strange position “moonface”,
“buffalo hump” (fat above the shoulder blades ). Diabetogenic effect occurs and
the conditonis called an adrenal diabetes. It Could be due to a) oversecretion of
CRH or ACTH causing overstimulation of the adrenal cortex (Cushing’s Disease );
or, b)adrenal tumors that uncontrollably secrete cortisol independent of ACTH;
or, c)ACTH‐secreting tumors located in places other than the pituitary, most
commonly the lung.
Diagnosis :
Assessment of circadian rhythm in cortisol secretion : In Cushing’s
syndrome : rhythum is loss
Measuring 24‐hour urinary free cortisol Level .
Low dose Dexamethasone suppression test :
oral Dexametason given 6 hourly for
2 days then blood for plasma cortisol messured : if plasma cortisol suppress mean normal
if no suppression of Pl. cortisol mean Cushing's syndrome
Plasma ACTH : Elevated mean secondary type (pituitary dependent or
ectopic secretion of ACTH
CRH Test – Differentiate ectopic ACTH secretion and Cushing’s disease. In
Cushing’s disease – plasma ACTH and cortisol increases over baseline while
Ectopic ACTH or adrenal tumour – no response
Imaging :CT scan/MRI .
Primary
Secondary
Cortisol
High
High
ACTH
Low
High
CRH
Low
High
Treatment : surgical excision of Adrenal adenoma and Adrenal Carcinoma .In
Cushing’s disease treated by transphenoidal hyposectomy. Or by Drug ( block
cortisol synthesis ) – metyrapone.
4) Adrenal Androgen Hypersecretion – excess adrenal androgen secretion could
either be a virilizing adenoma in females with too much testosterone produced.
In males, it may show up as a feminizing adenoma with too much estrogen but is
very rare. If ↑androgens occur in prepubertal boys, it may cause premature
secondary sex characteristics.
B) Insufficiency of Adrenal Cortex Hormones
1) Primary adrenal insufficiency – called Addison’s Disease – Hyposecretion of all
hormones of the cortex ; due to 1)idiopathic atrophy of the adrenal gland 2)
autoimmune that is attacking the adrenal cortex. cortisol ↓ as well as
aldosterone↓, ACTH ↑(darkens skin due to ↑MSH as well from the same
precursor and cell), lethargy, poor response to stress. Patient had Hypoglycemic,
hyperkalemia and hyponatremia with hypotension and if aldosterone low
enough, can be very life‐threatening.
↑CRH →↑ACTH→(atrophied adrenal gland) ↓cortisol ,↓aldosterone.
2) Secondary adrenal insufficiency – This may be due to a pituitary or
hypothalamus . abnormality with only a decrease in cortisol. Aldosterone not
affected. ↓ ACTH →↓cortisol only
Diagnosis:
Decrease Plasma cortisol concentration
Plasma ACTH measurement : To differentiate between primary and
secondary adrenal failure ; Primary insufficiency ‐ ↑ACTH . Secondary ‐
↓ACTH .
CRH stimulation test • To differentiate between secondary adrenal
insufficiency due to pituitary or hypothalamic dis. ;Results : • Pituitary
disease – no response • Hypothalamic lesions – positive response.
ACTH stimulation test: give injection of ACTH If cortisol increase mean
secondary type.
Plasma Renin And Aldosterone : in primary type , ↓ aldosterone level
with ↑ rennin level.
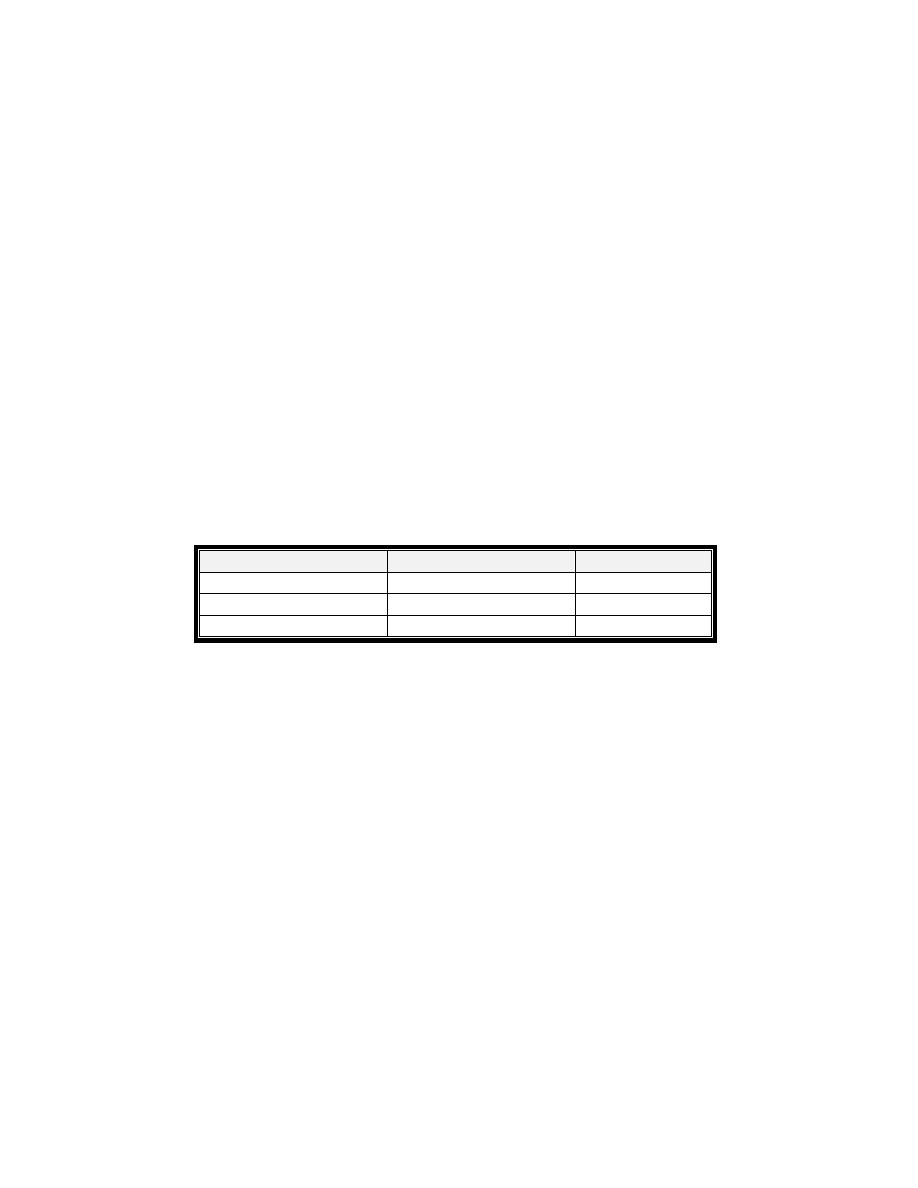
Primary
Secondary
Cortisol
Low
Low
Aldosterone
Low
Normal
ACTH
High
Low
Treatment :
Hormone replacement (Life‐long replacement therapy) : Hydrocortisone and
9α‐fludrocortisone • Secondary adrenocortical insufficiency : definitive
treatment e.g. surgical removal of a pituitary tumour.
ADRENAL CRISIS
:acute adrenal insufficiency , Medical emergency , Acute in
onset; can be fatal if not promptly recognized and treated .
Clinical features : Severe hypovolaemia , Dehydration , Shock , Hypoglycaemia ,
possible mental confusion and loss of consciousness.
Causes : Precipitated by stress like infection, trauma or surgery in patients with
incipient adrenal failure.
Treatment : Resuscitation e.g. IV fluids, IV glucose. • IV hydrocortisone 100mg
which should be continued daily until the patient can take oral medication.

The Adrenal Medulla ‐ responses to acute stress
The adrenal medulla hormones are amino acid in nature
. It synthesis in the
cytoplasm of chromaffin cells ; tyrosine is converted to DOPA by tyrosine hydroxylase; DOPA to
dopamine by DOPA decarboxylase; dopamine is then pumped into granules and is converted to
noradrenaline by dopamine hydroxylase; noradrenaline is then stored or pumped out of the
granule for conversion to adrenaline (80% of total) by phenyl‐N‐methyl transferase (PNMT) in
the cytoplasm.
Adrenal is then pumped into granules for storage and release
when stimulated by sympathetic neuron.
Receptors: Adrenaline and noradrenaline act at adrenergic receptors on cell
membrane.
ß receptors (cAMP coupled); ß1 (heart, fat); ß2 bronchi, blood vessels
(vasodilator skeletal muscle).
alpha receptors (PLC coupled): alpha 1, all blood vessels (vasoconstrictor), gut
sphincters, alpha 2 presynaptic terminals.
Relative potency of adrenaline and noradrenaline at receptors:
ß1 A=NA; ß2 A>>NA; alpha NA>A.
Physiological function
Any stressfull stimuli which activate the sympathetic nervous system – e.g. low
blood pressure, haemorrhage, pain which stimulate adrenal medulla . The
adrenal medulla contributes 10% of the total sympathetic nervous system
response to stress so thus it is not vital.
Cardiovascular system, adrenaline, cause increases heart rate and force of
contraction via ß 1. it stimulates vasodilation in skeletal muscle (ß 2), and
vasoconstriction in skin (alpha1). Noradrenaline increases mean arterial pressure
Respiratory system, adrenaline increase dilation of the bronchi and bronchioles
via ß2 receptors and increase respiratory rate by effects in the CNS.
GI tract, adrenaline acts to cause: inhibition of peristalsis, relaxation of gut
smooth muscle and contraction of gut sphincters (alpha 1).
Metabolic effect , adrenaline increases metabolite availability. In liver:
promotes glycogenolysis, gluconeogenesis, release of glucose into the
circulation. skeletal muscle: promotes glycogenolysis .fat: stimulates lipolysis
Central nervous system: it causes arousal via actions in the brainstem
Pathologies associated with Adrenal medulla hormone secretion
If the adrenal medulla is removed the adrenal medulla stress response is
compensated for by the remainder of the sympathetic system. Tumours of the
adrenal medulla (phaeochromocytoma) constantly secrete catecholamines
causing hypertension, tremor, anxiety, forceful heartbeat.
Treatment : Either surgical option requires or adrenoceptor blocker like
phenoxybenzamine
The body response to stress
The stressor is the agent inducing the response of the body while stress is the
state induced by the stressor. The stimuli that can induce a stress response are
1) Physical ‐ trauma, surgery, intense heat or cold 2) Chemical ‐ ↓O2 supply,
acid‐base imbalance3) Physiological – heavy exercise, pain, hemorrhagic shock
4) Psychological or emotional – anxiety (exams), fear 5) Social –changes in
lifestyle.
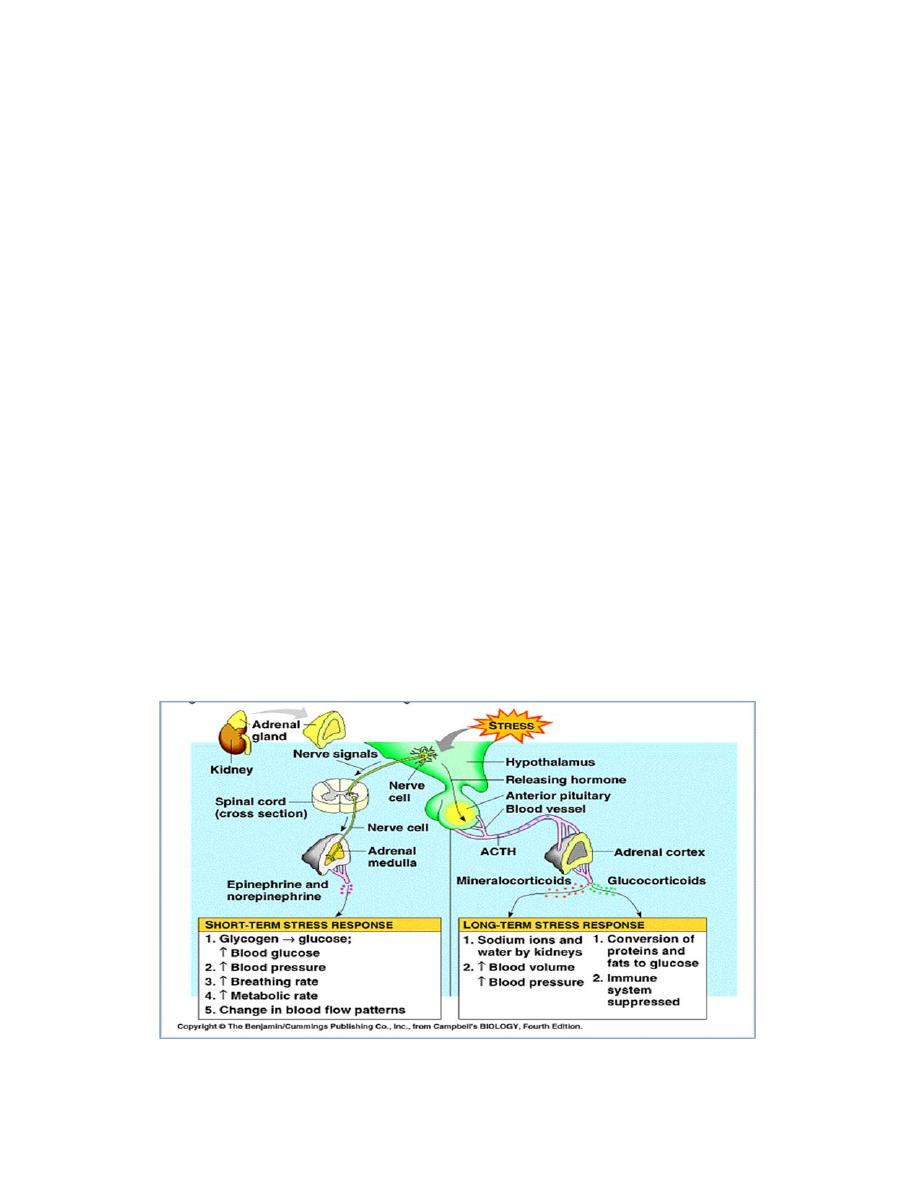
The hypothalamus in the brain is in charge of the stress response. When a stress
response is triggered, it sends signals to two other structures: the pituitary
gland, and the adrenal medulla. These short term responses, are produced by
The Fight or Flight Response via the Sympathomedullary Pathway (SAM). Long
term stress is regulated by the Hypothalamic Pituitary‐Adrenal (HPA) system.
The body response to acute and chronic stress
