1
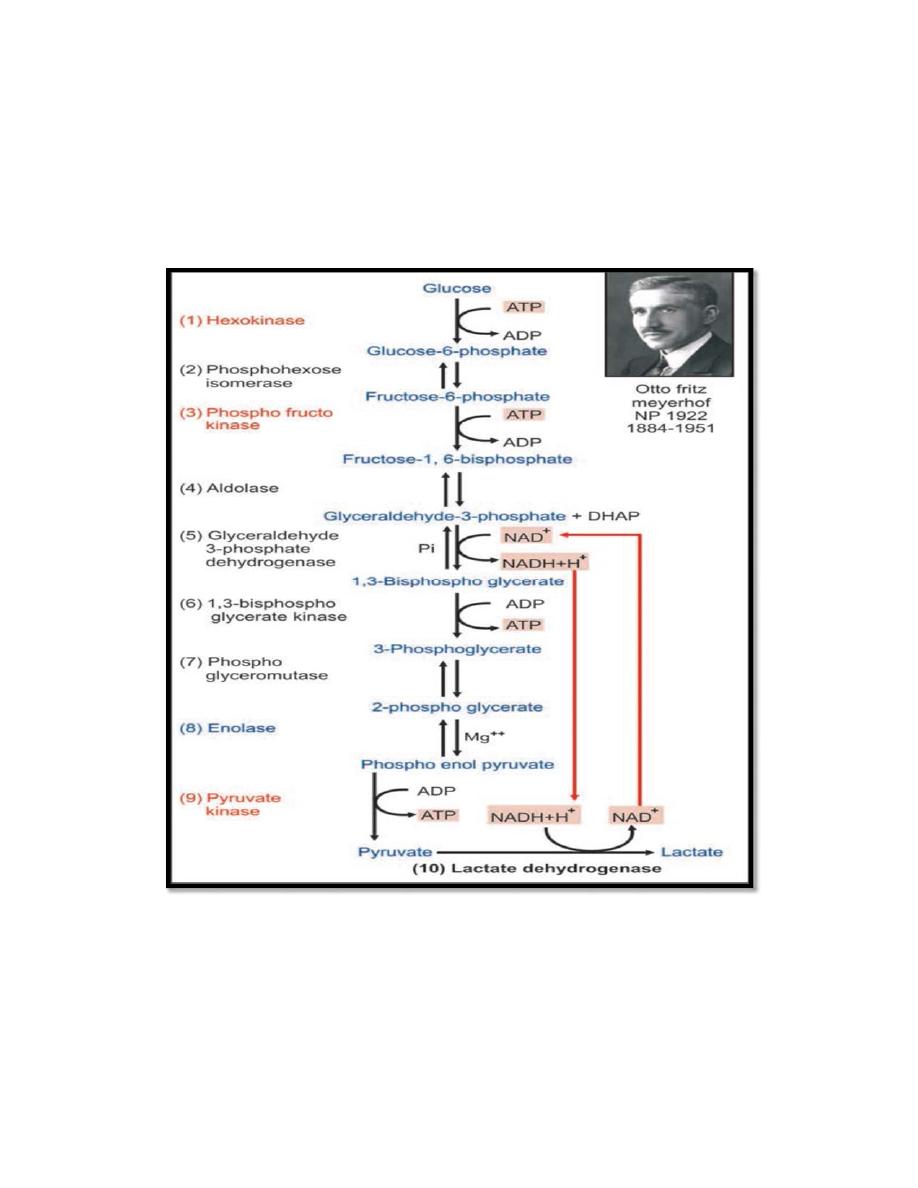
GLYCOLYSIS
In the pathway of glycolysis, glucose (6 carbons molecule) is split into
two pyruvate (3-carbon molecule) under aerobic conditions; or lactate
under anaerobic conditions, along with production of a small quantity of
energy.
Summary of glycolysis (Embden-Meyerhof pathway)
The whole reaction is summarized as
Glucose + 2 Pi + 2 ADP --> 2 Lactate + 2 ATP
glucokinase
2
Notes
Steps 1, 3 and 9 are key enzymes; these reactions are
irreversible. Steps 5, 6 and 9 produce energy. Steps 5 and 10 are
coupled for regeneration of NAD+.
The steps 1, 2 and 3 are called the preparatory phase, The next
steps are together called the energy producing phase.
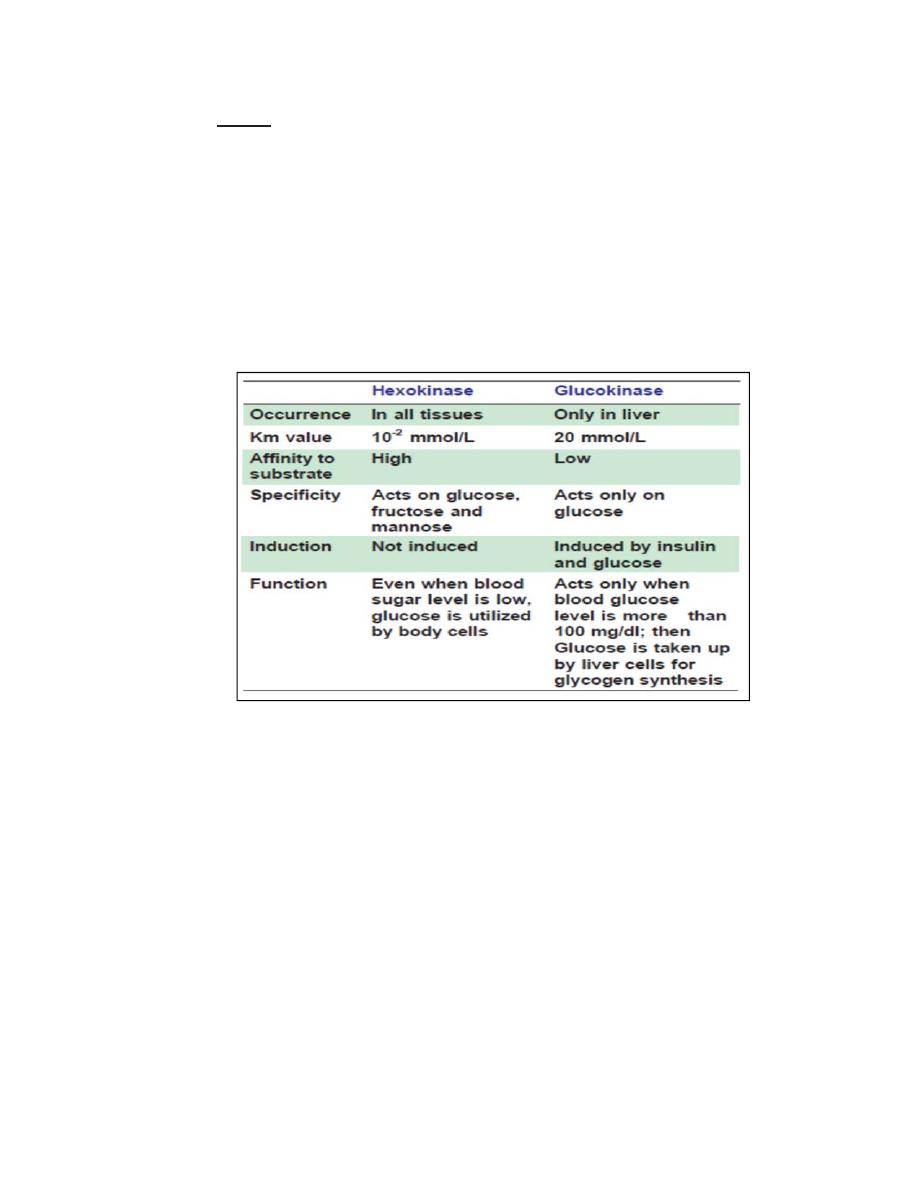
Hexokinase and glucokinase may be considered as iso-enzymes;
their properties are compared in Table below. Glucokinase is
under the influence of insulin; but hexokinase is not.
The phosphorylation of glucose to glucose-6-phosphate traps it
within the cells to be metabolized.
The enzyme phosphofructokinase (PFK) is an allosteric,
inducible, regulatory enzyme. It is an important key enzyme of this
pathway. This is an activation process, the energy being derived by
hydrolysis of ATP. This irreversible step is the rate limiting
reaction in glycolysis.
The energy of bisphospho glycerate (1,3-BPG) is trapped to
synthesize one ATP molecule with the help of bisphospho
glycerate kinase. This is an example of substrate level
phosphorylation (where energy is trapped directly from the
substrate without the help of the complicated electron transport

3
chain reactions). When energy is trapped by oxidation of reducing
equivalents such as NADH, it is called oxidative phosphorylation.
In the 5th step, for each molecule of glucose entering in the
pathway, two molecules of NAD+ are reduced to NADH. The
availability of co-enzymes inside a cell is limited. Therefore, this
step becomes a bottleneck in the whole reaction sequence.
For smooth operation of the pathway, the NADH is to be reconverted to
NAD+ by oxidative phosphorylation which needs oxygen. However,
during exercise, there is lack of oxygen so this reconversion is not
possible, Therefore, the cell has to couple some other reaction in which
NAD+ is regenerated in the cytoplasm itself hence, pyruvate is reduced to
lactate; the NAD+ thus generated is reutilized for uninterrupted operation
of the pathway. But when oxygen is in plenty, the two NADH molecules,
generated in the glyceraldehyde- 3-phosphate dehydrogenase reaction
(step 5), can enter the mitochondrial electron transport chain for complete
oxidation as each NADH provides 3 ATPs.
In RBCs, there are no mitochondria (where oxidative phosphorylation
occurs) hence RBCs derive energy only through anaerobic glycolysis,
where the end product is lactic acid.
Enolase (step 8) requires Mg++, fluoride irreversibly inhibit this
enzyme by removing magnesium ions. Thus, fluoride will stop the
whole glycolysis. So when taking blood for glucose estimation,
fluoride is added to blood. If not, glucose is metabolized by the
blood cells and lower blood glucose values are obtained (incorrect
result).
Factors Regulating Glycolysis
A. Glucokinase enzyme is active mainly in liver and has a high Km for
glucose and low affinity. Hence, glucokinase can act only when there is
adequate glucose supply. Hexokinase with low km and high affinity can
phosphorylate glucose even at lower concentrations so that glucose is
made available to brain, cardiac and skeletal muscle.
Glucokinase can act only when there is plenty of glucose. Thus, when the
supply of glucose is limited, glucose is made available to brain and
muscles. Insulin increases GK activity whereas glucagon inhibits it.
B. Pyruvate Kinase enzyme catalyses an irreversible step and is a
regulatory enzyme of glycolysis. When energy is plenty in the cell,
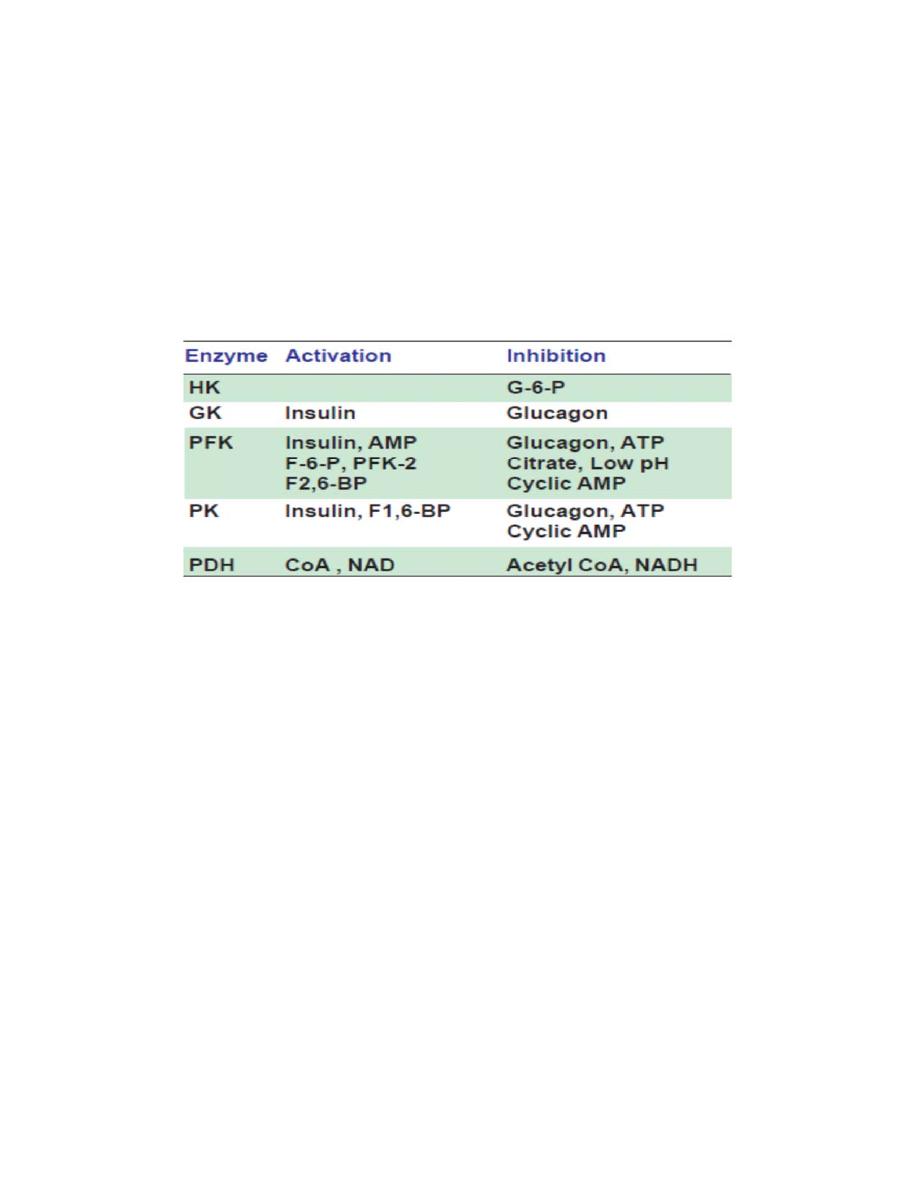
4
glycolysis is inhibited; Pyruvate kinase is inactive in the phosphorylated
state.
Insulin favors glycolysis by activating the above two key glycolytic
enzymes (PK and GK).
Glucagon and glucocorticoids
inhibit glycolysis and favor
gluconeogenesis.
C. PF K enzyme as mention above
Regulatory enzymes of glycolysis
Significance of the Glycolysis Pathway
1. It is the only pathway that is taking place in all the cells (cytoplasm) of
the body.
2. Glycolysis is the only source of energy in erythrocytes.
3. In strenuous exercise, when muscle tissue lacks enough oxygen,
anaerobic glycolysis forms the major source of energy for muscles.
4. The glycolytic pathway may be considered as the preliminary step
before complete oxidation.
5. The glycolytic pathway provides carbon skeletons for synthesis of non-
essential amino acids as well as glycerol part of fat (glycerol is required
which can be derived from glucose through DHAP also glycerol portion
of the neutral fat can enter into glycolytic or gluconeogenic pathways at
step 4).
6. Most of the reactions of the glycolytic pathway are reversible, which
are also used for gluconeogenesis.
Clinical Applications of Glycolytic Enzymes
1. Lactic acidosis may be seen in hypoxia, shock, pulmonary failure,
alcohol abuse and diabetes mellitus .
5
2. Deficiency of glycolytic enzymes. These conditions are rare, out of
which pyruvate kinase deficiency and hexokinase deficiency are
comparatively common. These deficiency states can lead to hemolytic
anemia, because energy depleted RBCs are destroyed. Inherited aldolase
deficiency also causes hemolysis. In PFK deficiency, muscle weakness is
seen.
Alternate fates of pyruvate:
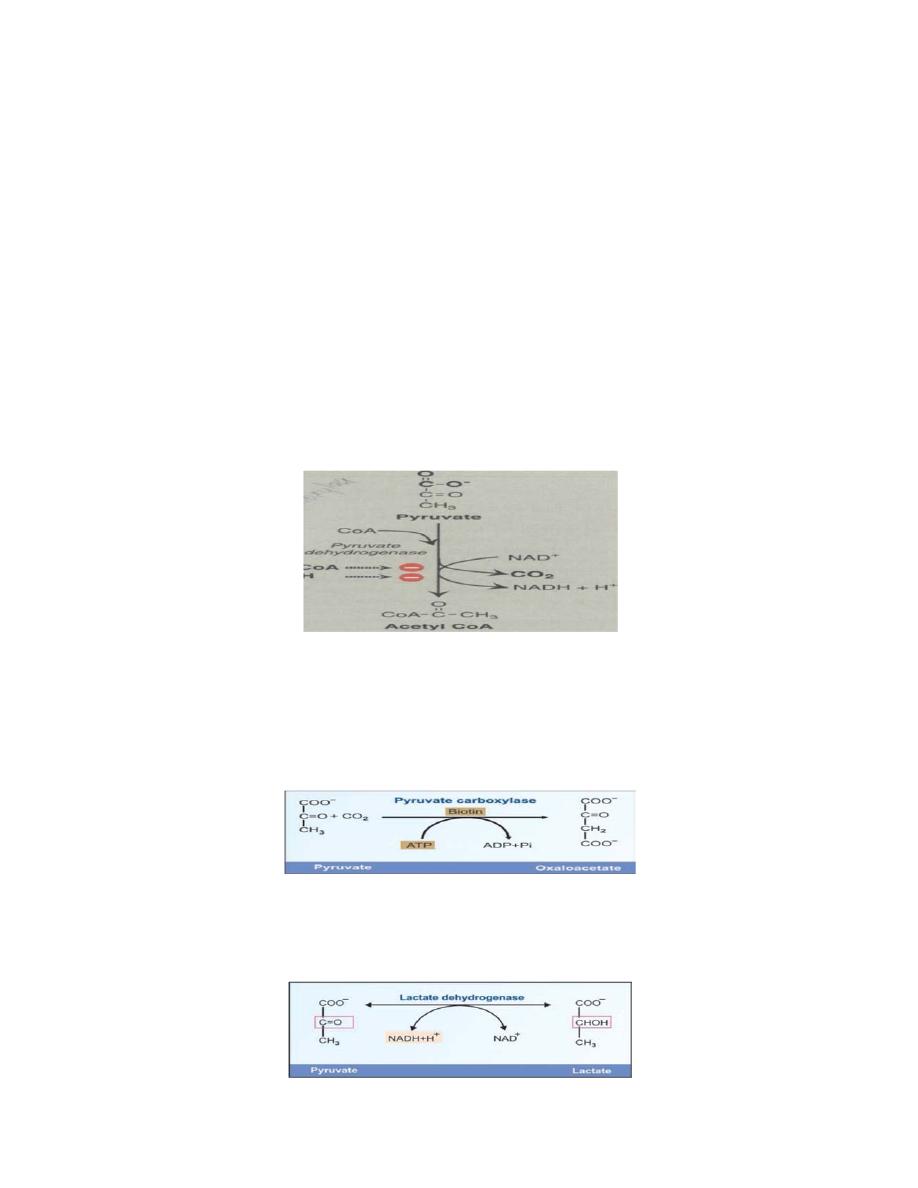
A. Oxidative decarboxylation of pyruvate
Oxidative decarboxylation of pyruvate by pyruvate dehydrogenase
complex is an important pathway in tissues with a high oxidative
capacity, such as cardiac muscle. Pyruvate dehydrogenase irreversibly
converts pyruvate, the end product of glycolysis, into acetyl CoA, a major
fuel for the tricarboxylic acid cycle.
B. Carboxylation of pyruvate to oxaloacetate
Carboxylation of pyruvate to oxaloacetate (OAA) by Pyruvate
carboxylase is a biotin-dependent reaction. This reaction is important
because it replenishes the citric acid cycle intermediates, and provides
substrate for gluconeogenesis.
C. Reduction of pyruvate to lactate
Reduction of pyruvate to lactate by lactate dehydrogenase under
anaerobic condition
