
Chemistry
1
Osmosis -continue
Lec.4
By :Dr. Tamathir Abbas 20/12/2015
♣Colligative property Any property that depends on the number
of dissolved particles in a solvent.
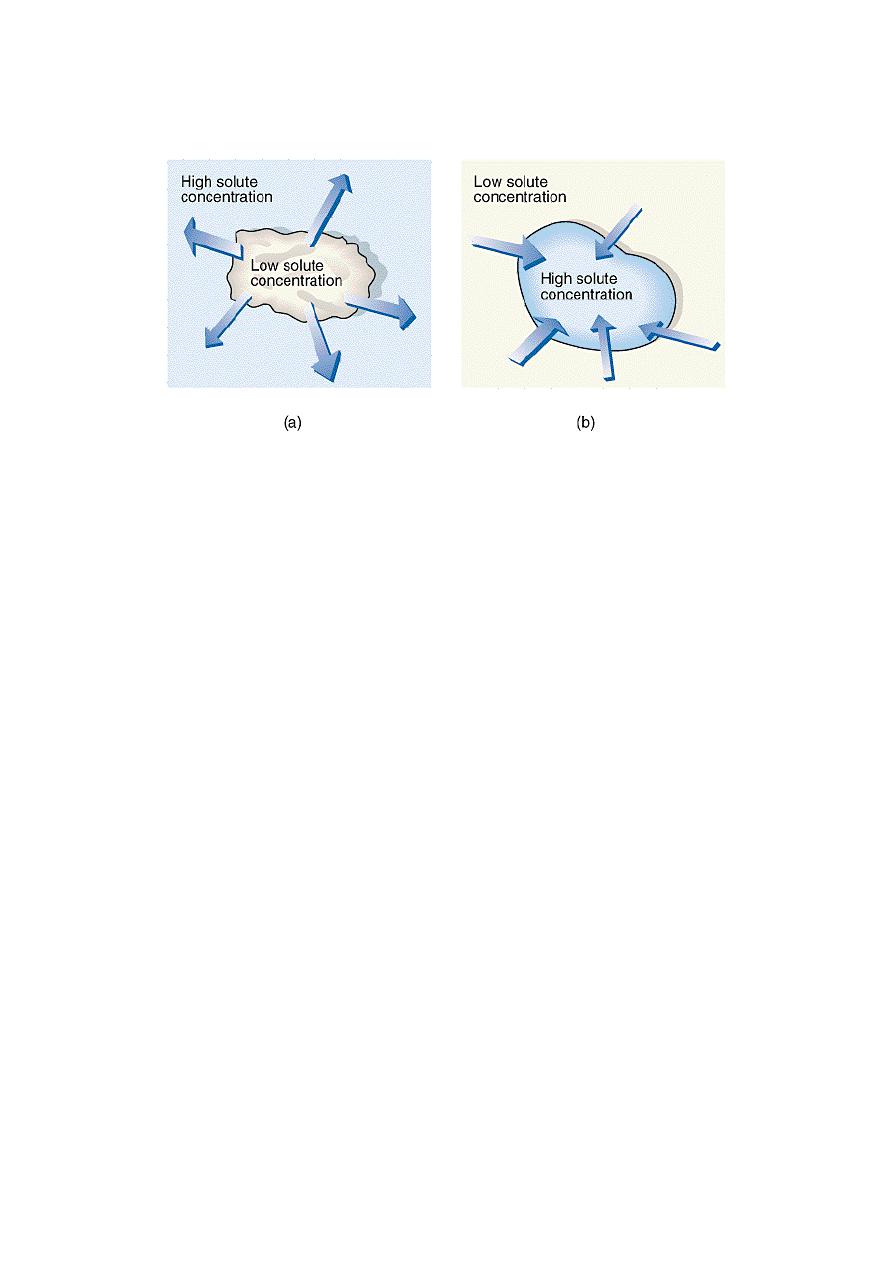
☻ Water moves to the solution that has the greater number of dissolved
particles (the more concentrated solution). This solution also has the
higher osmotic pressure. We can conclude that the greater the number of
particles, whether ions or molecules, in a solution, the greater its osmotic
pressure.
**If we measure the osmotic pressure of a 1 M aqueous sodium chloride
solution, we find that it is exactly twice that of a 1 M aqueous glucose
solution. The reason for this difference in osmotic pressure is that sodium
chloride is an electrolyte, whereas glucose is a nonelectrolyte.
☺An aqueous solution containing 1 mole of sodium chloride actually
contains 1 mole of sodium ions and 1 mole of chloride ions. A1M solution
of sodium chloride contains twice as many particles as an equal volume of a
1 M solution of glucose, a nonelectrolyte. As a result, its osmotic pressure
is exactly twice that of a 1 M glucose solution.
** The relative osmotic pressures of two solutions are extremely
important in living systems. In fact, they are so important that special
terms have been given to describe their relative osmotic pressure.
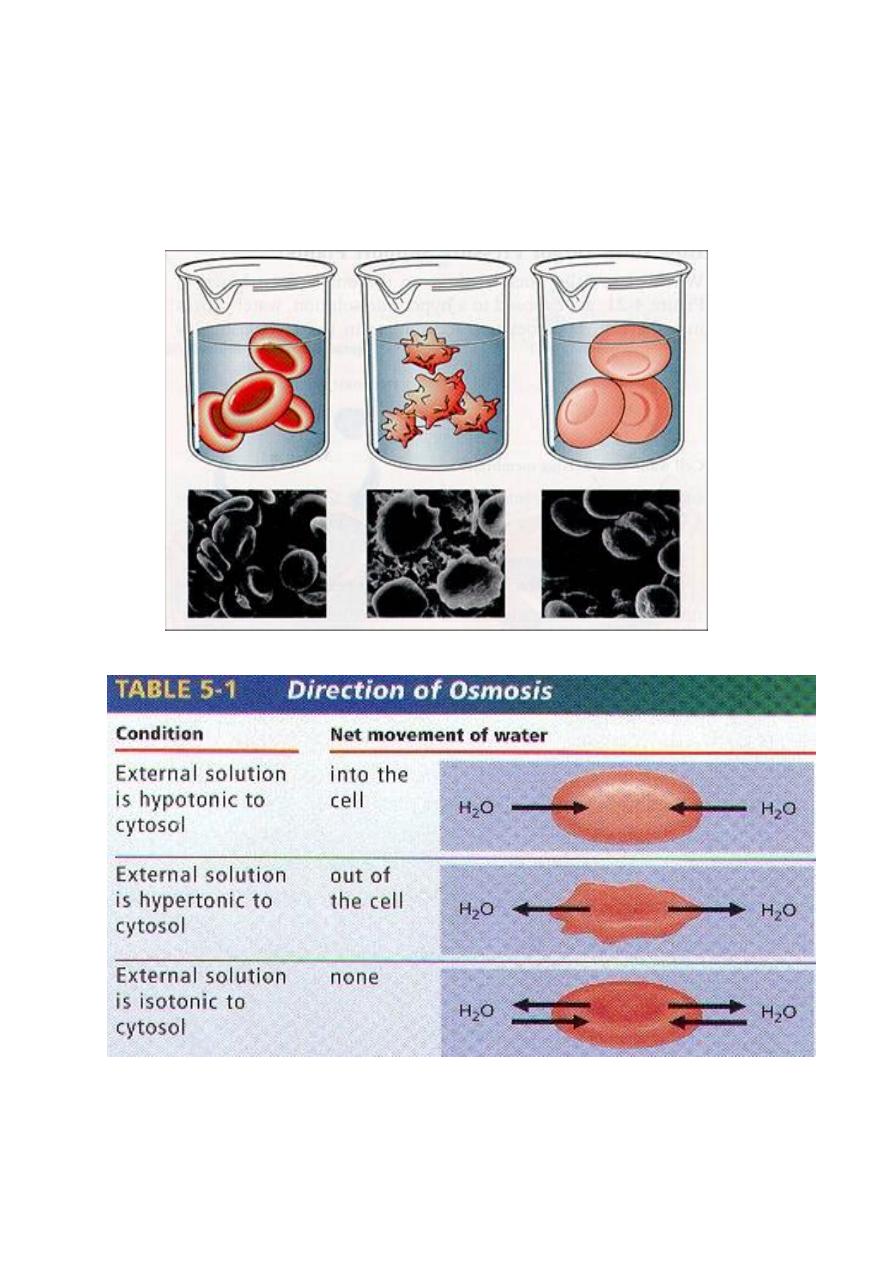
☼Isotonic solution :Two solutions that have the same osmotic pressure .
☼ hypertonic solution A solution that has a higher osmotic pressure
than another solution.
☼ hypotonic solution A solution that has a lower osmotic pressure than
another solution.

Chemistry
2
Examples of each of these terms are given in Table 8-6.
**The plasma membranes of red blood cells behave as osmotic
membranes. The cells contain an aqueous fluid made up of dissolved
compounds. This fluid has an osmotic pressure determined by the
concentration of dissolved molecules and ions in the fluid. Osmosis
occurs when a red blood cell is placed in water. The solution inside the
cell is hypertonic compared to pure water, so water enters the cell. So
much water enters that the cell is ruptured. The rupture of red blood cells
in this way is called
hemolysis
. We say that the cells are
hemolyzed
.
** Osmosis also occurs when a red blood cell is placed in a concentrated
saline (sodium chloride) solution. But in this case, the solution inside the
cell is hypotonic compared to the saline solution and osmosis occurs in
the reverse direction.
**Water leaves the cell and passes into the solution. This causes the red
blood cell to shrivel and shrink. This process is called
crenation.
Chemistry
3
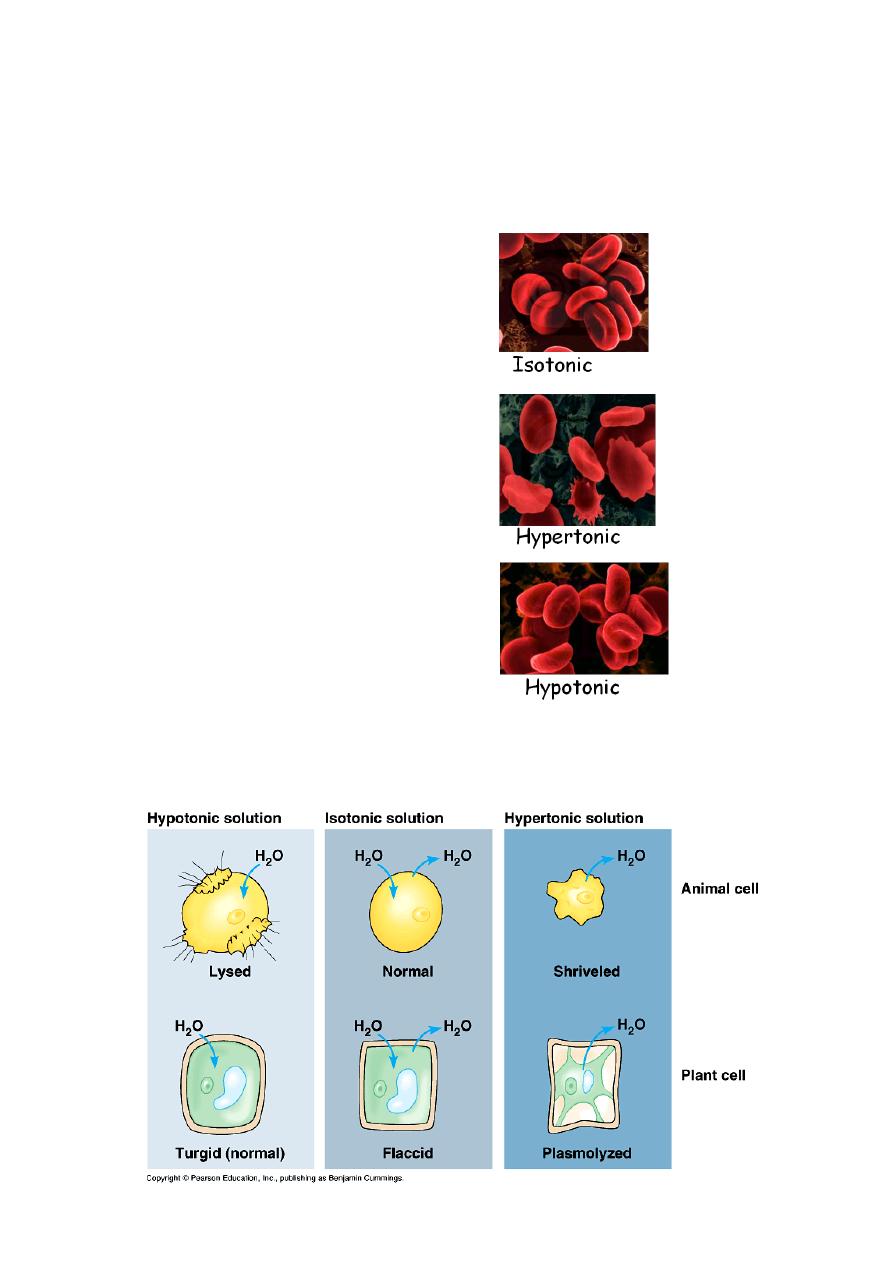
Crenation and Hemolysis
**A 0.95% saline solution is isotonic compared to the solution inside red
blood cells. Consequently, red blood cells placed in such a solution
undergo neither crenation nor hemolysis.
** There is a very important practical reason for worrying about the
osmotic pressure of the fluid inside a red blood cell compared to that of
the cell's environment. Patients often must be fed intravenously. To
prevent damage to their red blood cells, the concentration of the solution
must be controlled so that neither hemolysis nor crenation occurs.
Therefore, the concentration of the solution must match closely the
concentration of all of the particles within the red blood cells. In other
words, the solution to be given a patient intravenously must be isotonic
with blood.
☻There is a very important practical reason for worrying about the
osmotic pressure of the fluid inside a red blood cell compared to that of
the cell's environment.
♣Patients often must be fed intravenously. To prevent damage to their red
blood cells, the concentration of the solution must be controlled so that
neither hemolysis nor crenation occurs.
Chemistry
4
♦Therefore, the concentration of the solution must match closely the
concentration of all of the particles within the red blood cells. In other
words, the solution to be given a patient intravenously must be isotonic
with blood.
Chemistry
5
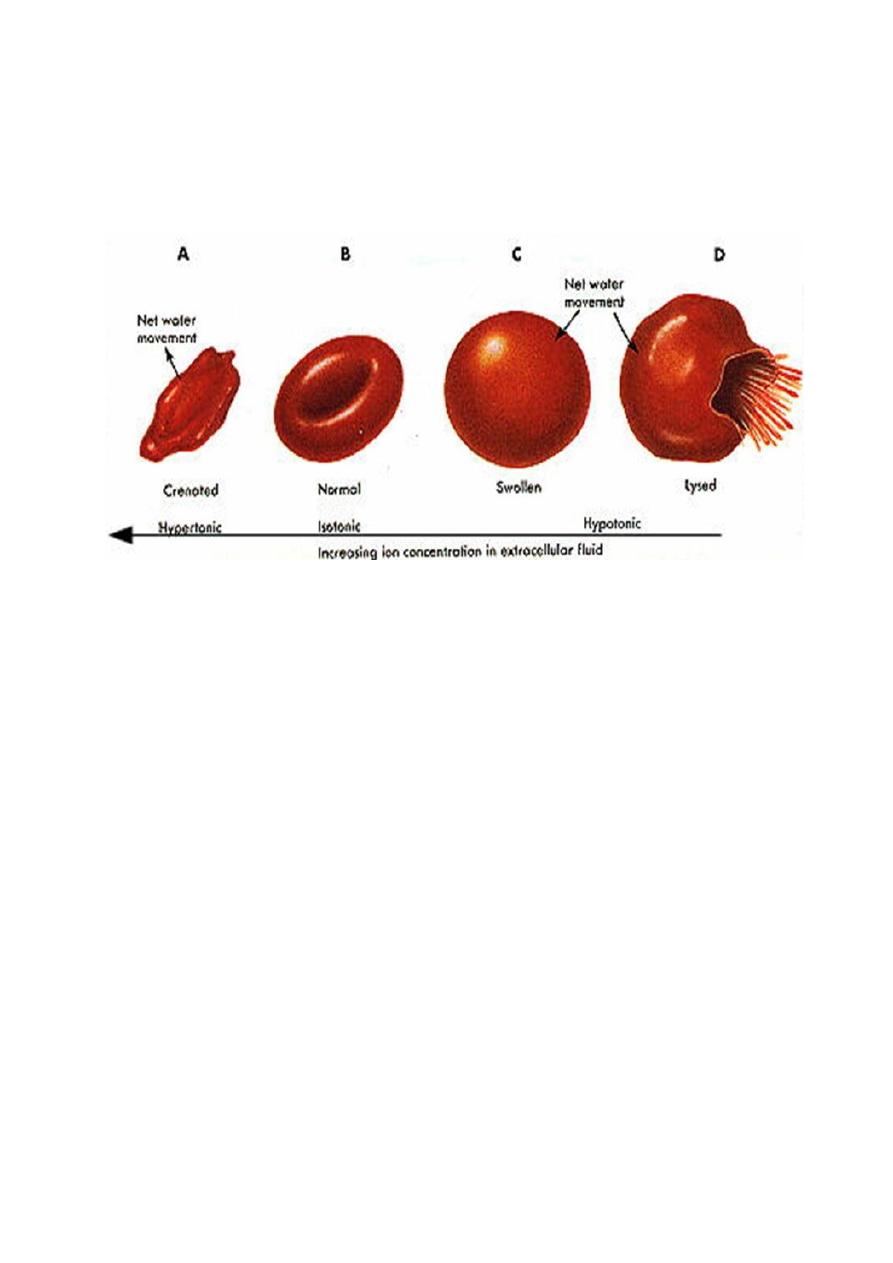
Osmosis in Red Blood Cells
• Observe sheep RBCs via a wet mount of the sample
• Aliquot one drop the following solutions with a ½ drop of RBC to
a slide
0.9% saline
10% NaCl
Distilled water
How living cells react to changes in the solute concentrations of their
environments
Chemistry
6
Effect of Water on RBC

Chemistry
7
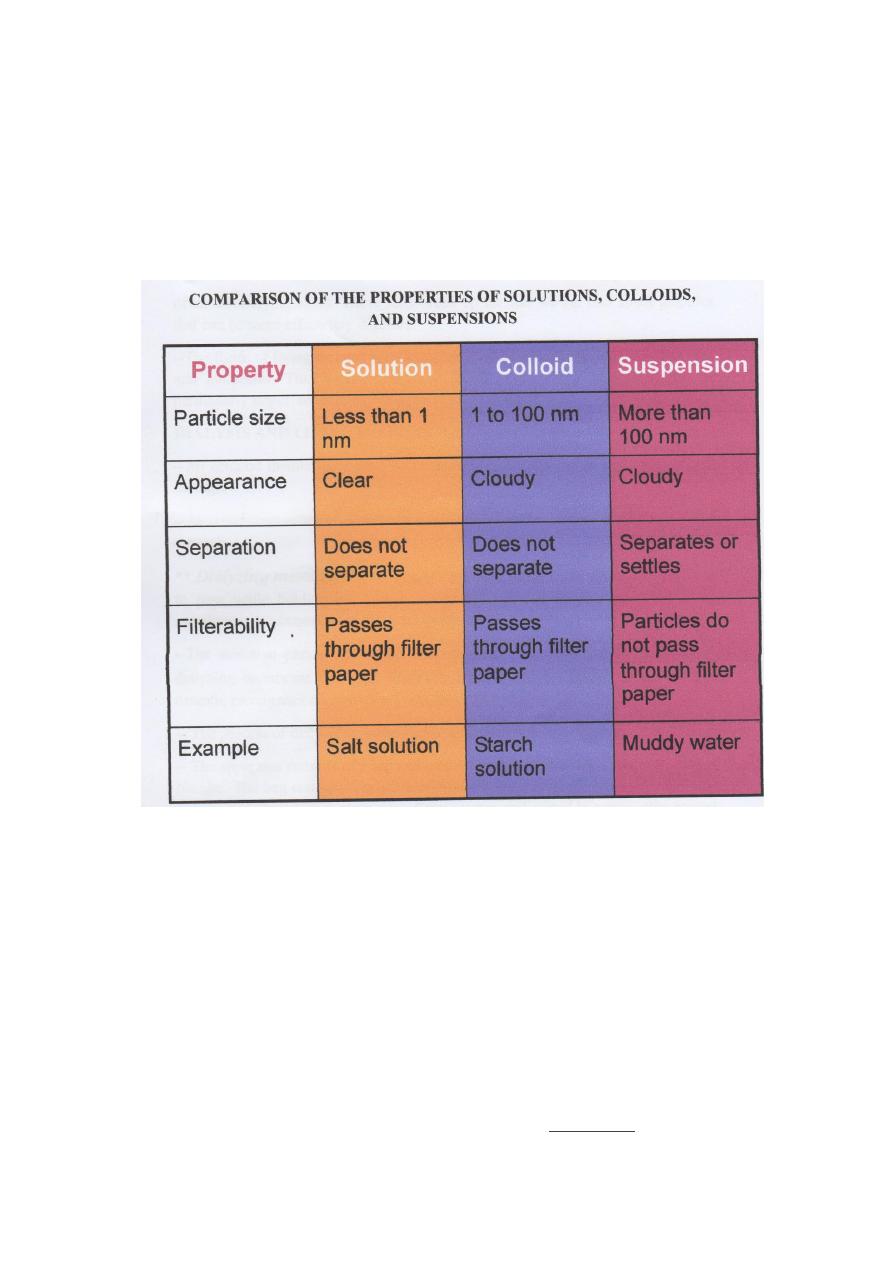
COLLOIDS AND GOLLOIDAL DISPERSIONS
-- A colloid: Matter containing particles of the sizes of clusters range
from 1 to 100 nm.
-- Sometimes intermolecular attractions between molecules cause several
hundred to several thousand of them to cluster together. The sizes of
these clusters range from 1 to 100 nm.
--A uniform dispersion of a colloid in water is called a colloidal
dispersion. This dispersion is similar to a solution in that the particles do
not settle out on standing. However, a colloidal dispersion usually
appears cloudy, and its particles are large enough to be photographed
with the aid of an electron microscope.
**Dispersed substance: The colloid in a colloidal dispersion .
** Dispersing substance: The continuous matter in which the colloid is
dispersed.
** The dispersed and dispersing substances can be liquids, solids, or
gases. They can combine in nine different ways to form colloidal
dispersions containing two components. Only eight of these nine possible
combinations are known. A mixture of two gases cannot be a colloidal
dispersion because the particles of a gas are individual molecules.
-- As the molecules form clusters, the gas changes to a liquid. The eight
types of colloidal dispersion are given in Table 8-7 with examples.
Chemistry
8
--Many compounds of high molecular weight in living systems form
colloidal dispersions rather than solutions in water. Starch and proteins
are examples of such compounds.
**If colloids are clusters of molecules, why don't the clusters increase
in size until they get large enough to settle out?
** The reason is that the particles in most stable colloidal dispersions all
have the same electrical charge. These charges can be caused by
adsorption of ions to the surface of the participles, or the large particles
themselves can be charged. As a result, the participles repel each other
and cannot form particles large enough to settle out.
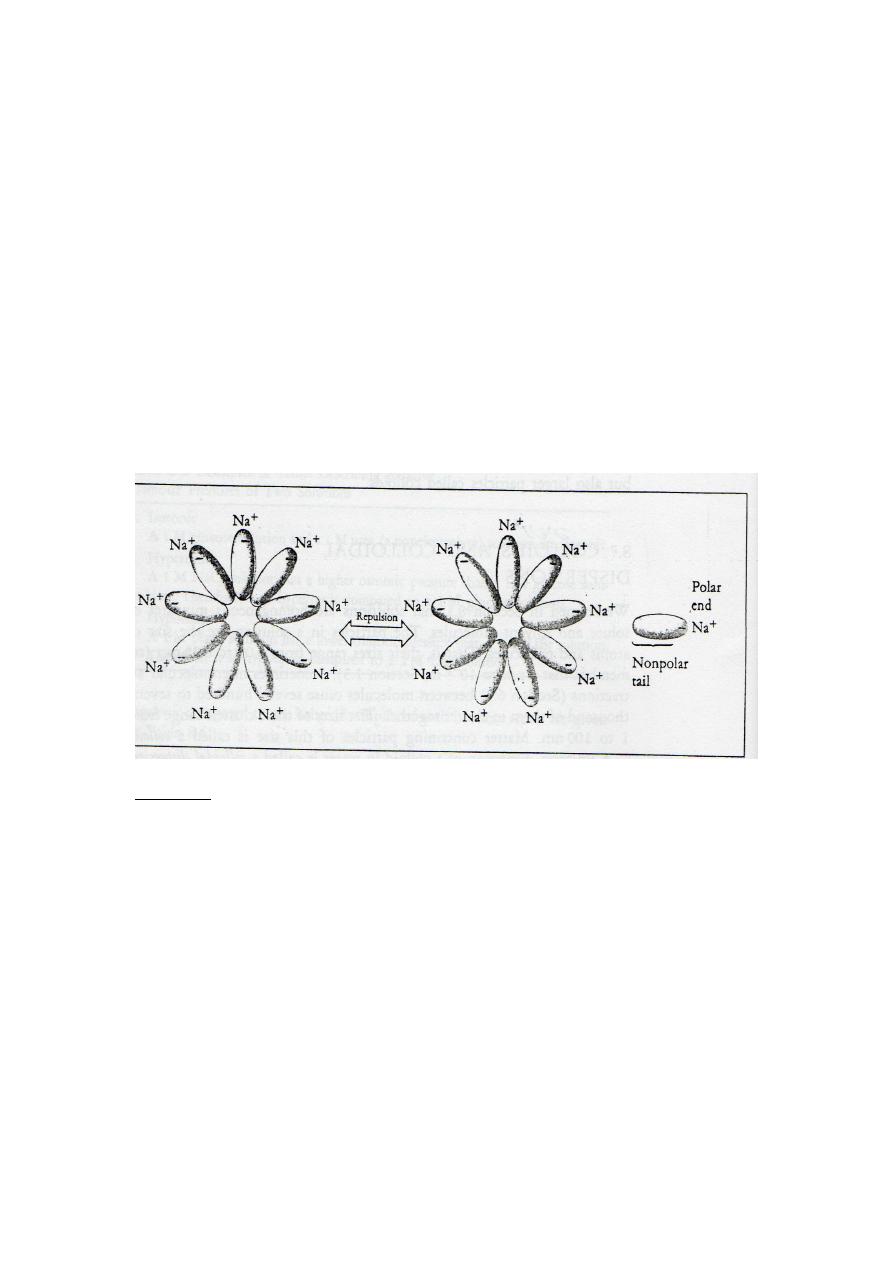
** This repulsion between colloids in water is shown in Figure 8-7.
Fig.8-7.
Colloids formed by attractions between complex molecules. One end of
each individual molecule has a negative charge (balanced by a sodium ion), and the
other end is a long nonpolar tail. The long tails are held together by hydrophobic
attractions. The negatively charged ends form the surface of a sphere. Adjacent
colloids are repelled by their identical charge..
Other colloids are stabilized in water by the action of a third
substance called an emulsifying agent. An example is a mixture of oil and
water. Oil is immiscible with water. However, if we add soap to the
mixture, the oil is emulsified by the soap. The soap is the emulsifying
agent.
--Soap breaks up the oil into small drops. The soap molecules form a
negatively charged layer on the surface of each oil drop. This causes the
oil drops to repel each other, and they disperse throughout the water. Bile
Chemistry
9
salts are another example of an emulsifying agent. These salts break up
the fats we eat into small globules that can be more effectively digested.
--The fluids of living systems are a complex mixture of colloids and
dissolved ions and molecules. The behavior of these fluids in the body is
vital to life. A particularly important property of these fluids is dialysis.
DIALYSIS AND LIVING SYSTEMS
—An osmotic membrane allows water molecules, but not solute particles,
to pass through.
** Diatyzing membranes : are membranes that allow small molecules and
ions to pass while holding back large molecules and colloidal particles. .
Plasma membranes are examples of such membranes.
—The selective passage of small molecules and ions in either direction by a
dialyzing membrane is called dialysis. Dialysis differs from
osmosis in that
osmotic membranes allow only solvent molecules to pass.
—The process of dialysis is shown by the apparatus in Figure 8-8.
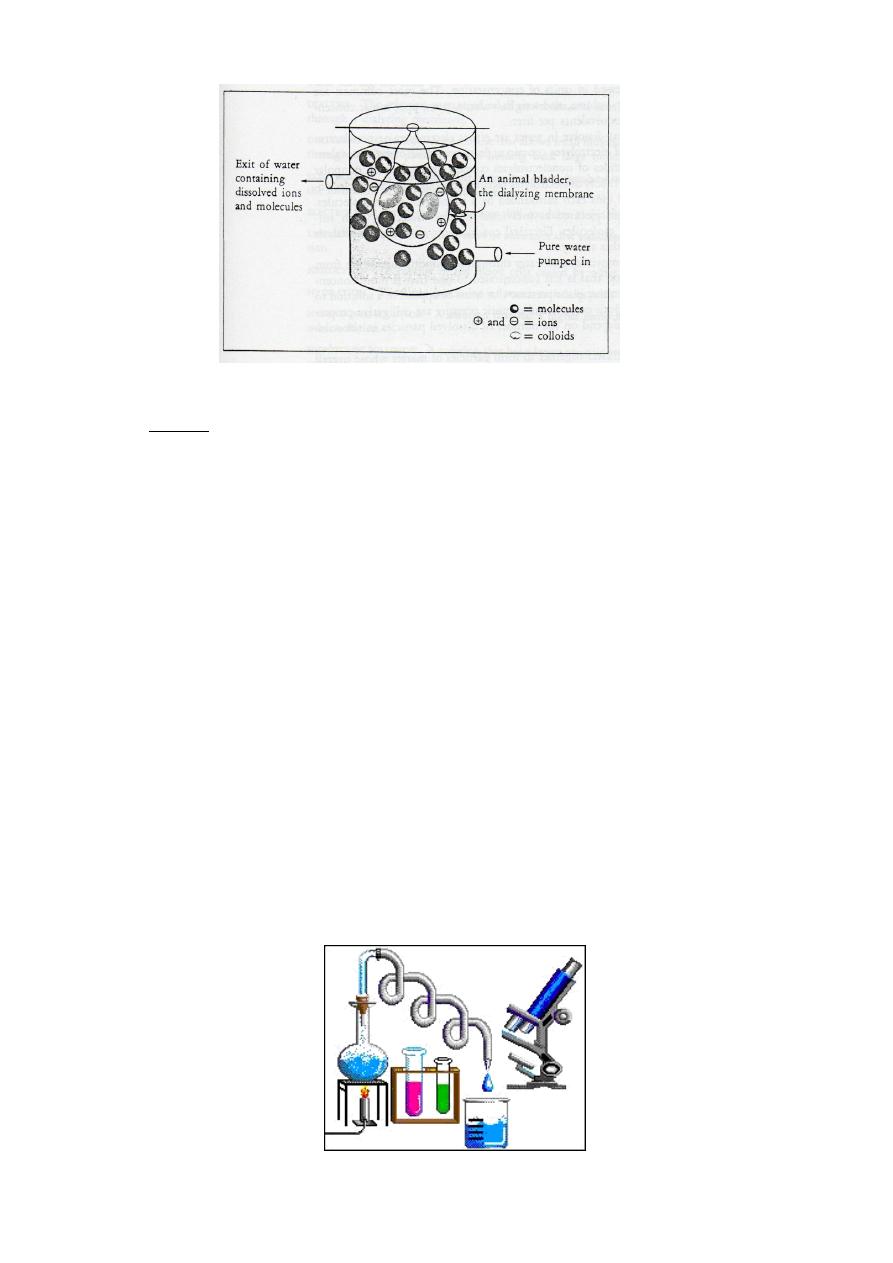
Chemistry
11
Fig. 8-8. Dialysis apparatus. Dissolved molecules and ions pass through the dialyzing
membrane, but colloids do not.
♣ The apparatus consists of a bag made of a dialyzing membrane such as an animal
bladder. The bag contains a mixture of colloids and dissolved molecules and
ions. The bag is placed in a container of pure water and water is continually
passed through the membrane. The water carries the ions and molecules
through the membrane, leaving the colloids behind. The ability of dialyzing
membranes to allow the passage of only selected substances is extremely
important to living systems.
- The kidneys are an example of organs in the body that use dialysis to
maintain the solute and electrolyte balance of the blood. The main purpose
of the kidneys is to cleanse the blood by removing the waste products of
metabolism and control the concentrations of electrolytes. The kidneys do
this job very efficiently.
~ Approximately 180 L of blood are purified daily in a 68-kg (150-lb)
adult. Approximately 99 percent of the total volume processed is retained,
and the remaining 1 percent is eliminated as urine. Part of the purification
of blood occurs by dialysis
.
The End
