Clinical biochemistry second stage lecture 4 Dr.Thana Alsewedy
1
Amino Acid Metabolism (Degradation and Synthesis)
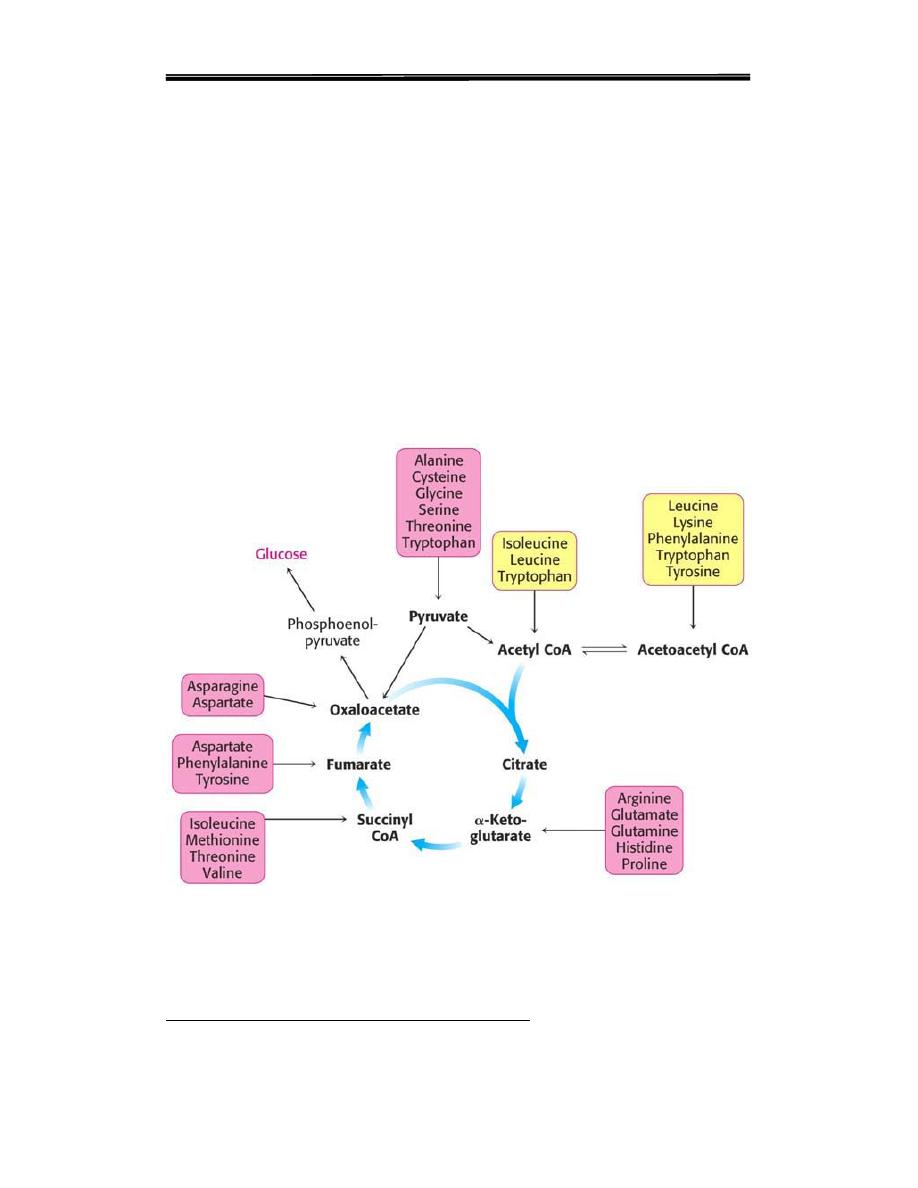
The fates of the carbon skeletons of amino acids after the removal of
the α-amino group.
The strategy of amino acid degradation is to transform the carbon
skeletons into major metabolic intermediates that can be converted into
glucose or oxidized by the citric acid cycle. The conversion pathways
range from extremely simple to quite complex. The carbon skeletons of
the diverse set of 20 fundamental amino acids are funneled into only
seven molecules and these intermediate produced from the metabolism
of aminoacid are;
acetyl CoA, pyruvate, oxaloacetate, α-ketoglutarate ,succinyl CoA, ,
Fumarate and acetoacetyl CoA.
Figure 1. overview of metabolic pathways of amino acid
GLUCOGENIC AND KETOGENIC AMINO ACIDS
Amino acids are grouped into two classes, based on whether or not their
carbon skeletons can be converted to glucose:

Clinical biochemistry second stage lecture 4 Dr.Thana Alsewedy
2
A. Glucogenic amino acids
Amino acids whose catabolism yields pyruvate or one of the
intermediates of the citric acid cycle are termed glucogenic or
glycogenic. These intermediates are substrates for gluconeogenesis
and, therefore, can give rise to the net formation of glucose or
glycogen in the liver and glycogen in the muscle.
B. Ketogenic amino acids
Amino acids whose yields either acetoacetate or its precursor, (acetyl
CoA or acetoacetyl CoA) are termed ketogenic. (Acetoacetate , 3-
hydroxybutyrate and acetone all are are called ketone body.) Leucine
and lysine are the only exclusively ketogenic amino acids found in
proteins. Their carbon skeletons are not substrates for gluconeogenesis
and, therefore, cannot give rise to the net formation of glucose or
glycogen
in
the
liver,
or
glycogen
in
the
muscle.
Figure 2.CATABOLISM OF THE CARBON SKELETONS OF AMINO ACIDS
The pathways by which amino acids are catabolized are conveniently
organized according to which one (or more) of the seven intermediates
listed above is produced from a particular amino acid.

Clinical biochemistry second stage lecture 4 Dr.Thana Alsewedy
3
A. Amino acids that form oxaloacetate
1.Asparagine is hydrolyzed by asparaginase, liberating ammonia and
aspartate
2.Aspartate loses its amino group by transamination to form oxaloacetate
(see Figure 3).
Figure 3.
Amino acids that form oxaloacetate
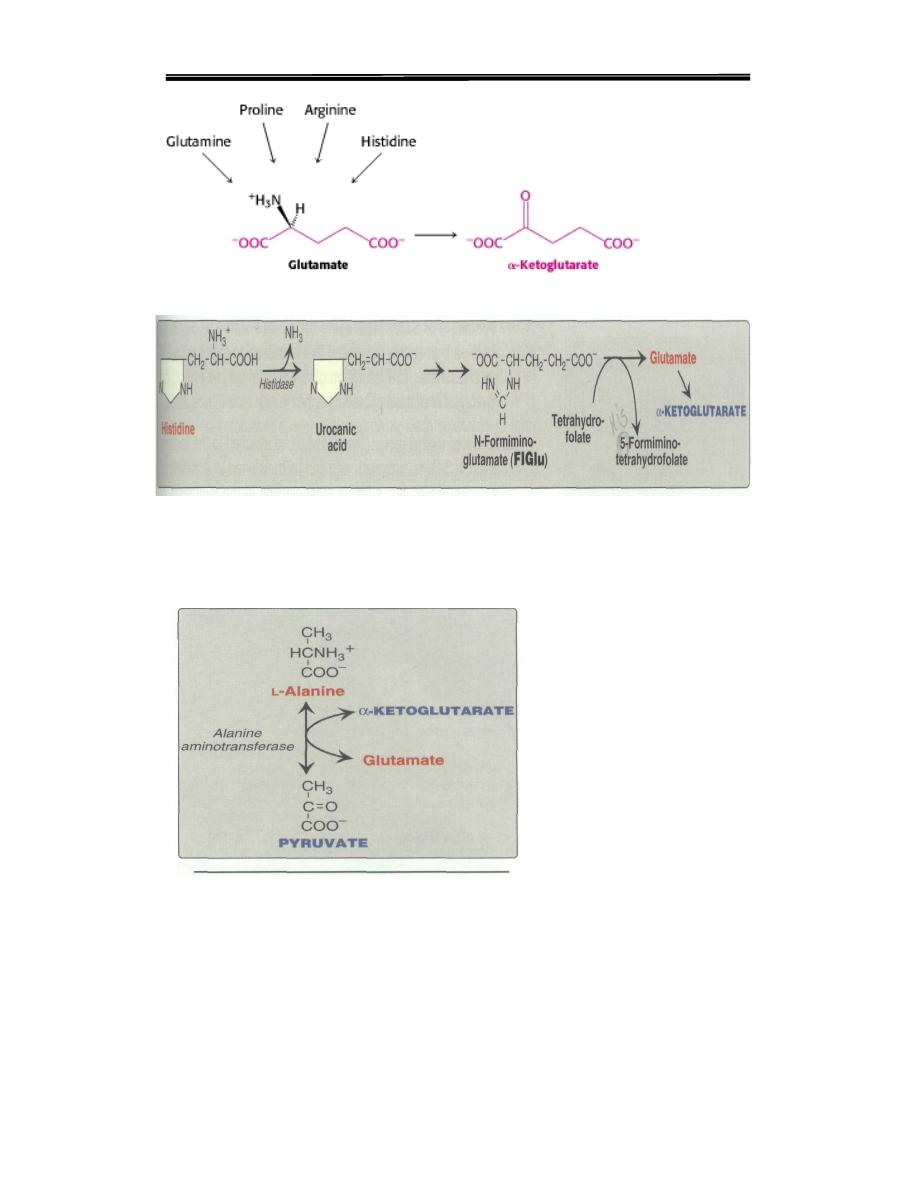
B. Amino acids that form α-ketoglutarate
1 .Glutamine is converted to glutamate and ammonia by the enzyme
Glutaminese Glutamate is converted to α-keto- glutarate by
transamination, or through oxidative deamination by glutamate
dehydrogenase
2. Proline is oxidized to glutamate
3.Arginine is cleaved by arginase to produce ornithine. [Note: This
reaction occurs primarily in the liver as part of the urea cycle
Ornithine is subsequently converted to α-ketoglutarate
4. Histidine is oxidatively deaminated by histidase
Clinical biochemistry second stage lecture 4 Dr.Thana Alsewedy
4
Amino acids that form α-ketoglutarate
Figure 4.
Figure 5. Histidine metabolism
C. Amino acids that form pyruvate
1. Alanine loses its amino group by transamination to form pyruvate
Figure 6. transamination Alanine to form pyruvate
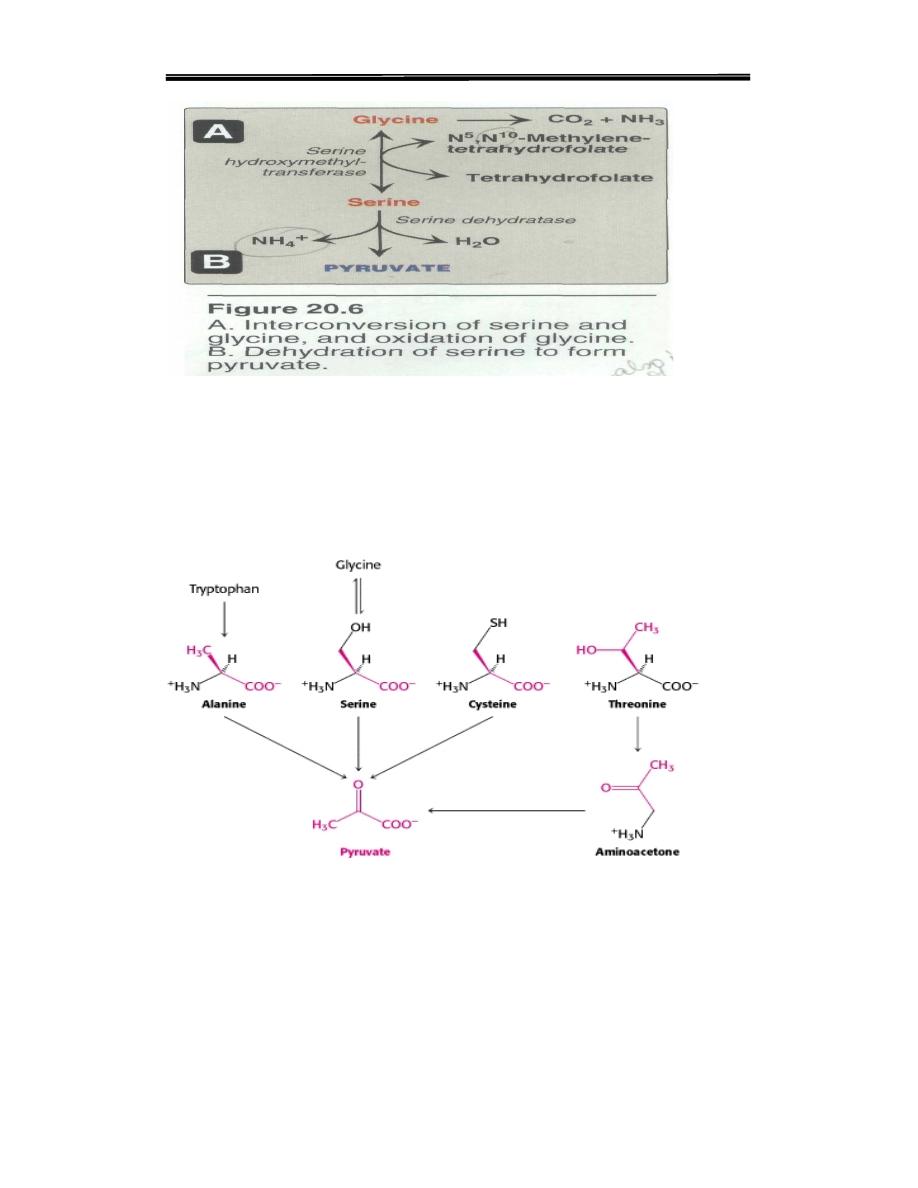
2. Serine can be converted to glycine
which requiring for (tetra hydro
folic acid
.Serine can also be converted to pyruvate by serine dehydratase
Clinical biochemistry second stage lecture 4 Dr.Thana Alsewedy
5
4. Cystine
undergoes desulfuration to yield pyruvate.
5. Threonine converted to pyruvate or to α-ketobutyrate, which forms
succinyl CoA.
6.Tryptophan converted to pyruvate
Figure7 .Amino acids that form pyruvate
D. Amino acids that form fumarate
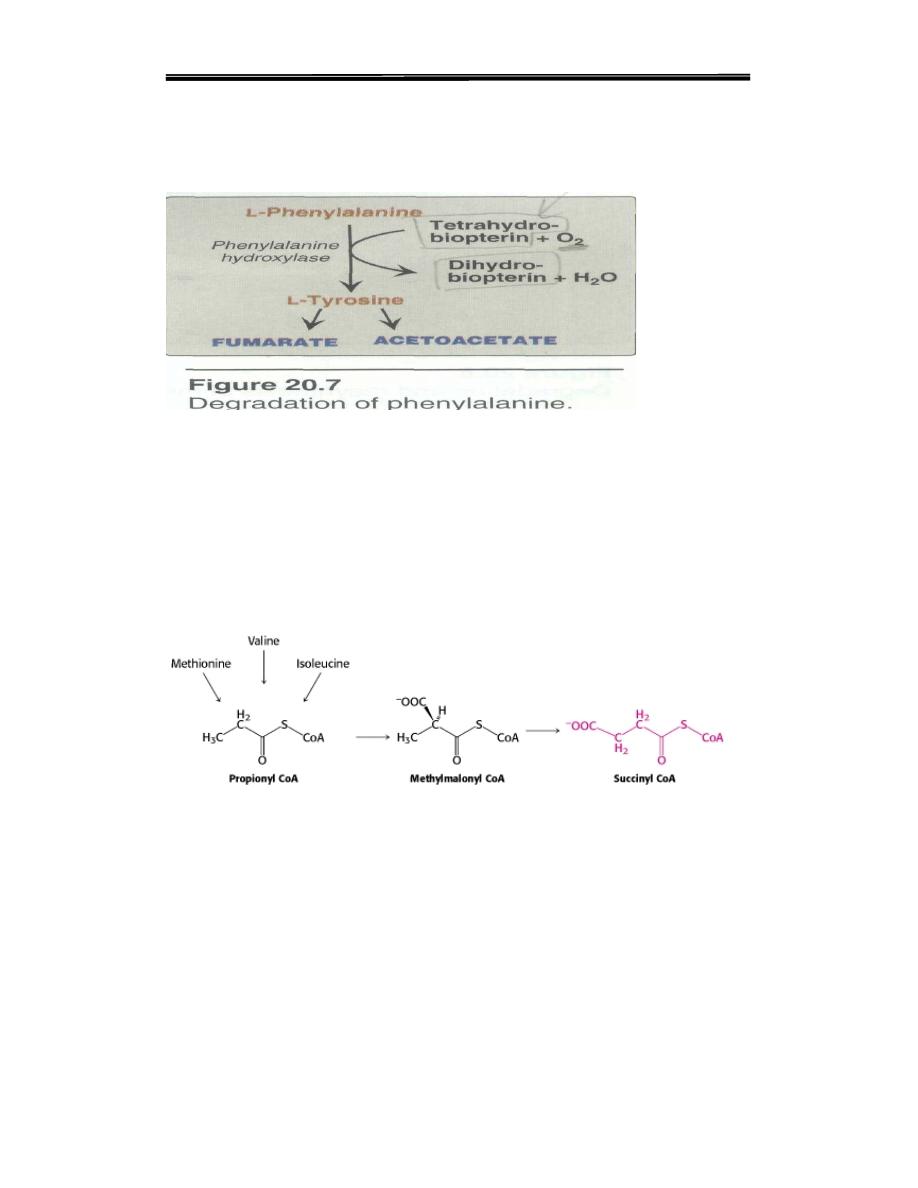
1. Phenylalanine and tyrosine: Hydroxylation of phenylalanine leads to
the formation of tyrosine (Figure 20.7). This reaction, catalyzed by
phenylalanine hydroxylase is the first reaction in the catabolism of
phenylalanine. Thus, the metabolism of phenylalanine and tyrosine
Clinical biochemistry second stage lecture 4 Dr.Thana Alsewedy
6
merge, leading ultimately to the formation of fumarate and acetoacetate.
Phenylalanine and tyrosine are, therefore, both glucogenic and ketogenic.
E. Amino acids that form succinyl CoA:
Succinyl CoA is a point of entry for some of the carbon atoms of methio-
nine, isoleucine, and valine. Propionyl CoA and then methylmalonyl CoA
are intermediates in the breakdown of these three nonpolar amino acids
The mechanism for the interconversion of propionyl CoA and
methylmalonyl CoA. This pathway from propionyl CoA to succinyl CoA
is also used in the oxidation of fatty acids that have an odd number of
carbon atoms and this reaction required vitamin B 12 .
Figure 8. Amino acids that form succinyl CoA
Methionine metabolism
Methionine
is sulfur containing, essential, glucogenic
amino acid Degradation of methionine results in the synthesis of cysteine.
Methionine is one of amino acids that form succinyl CoA.
Metabolism of sulfur containing amino acids may be studied under the
following major headings:
A. Activation of methionine and transmethylation
B. Conversion of methionine to cysteine
C. Degradation of cysteine
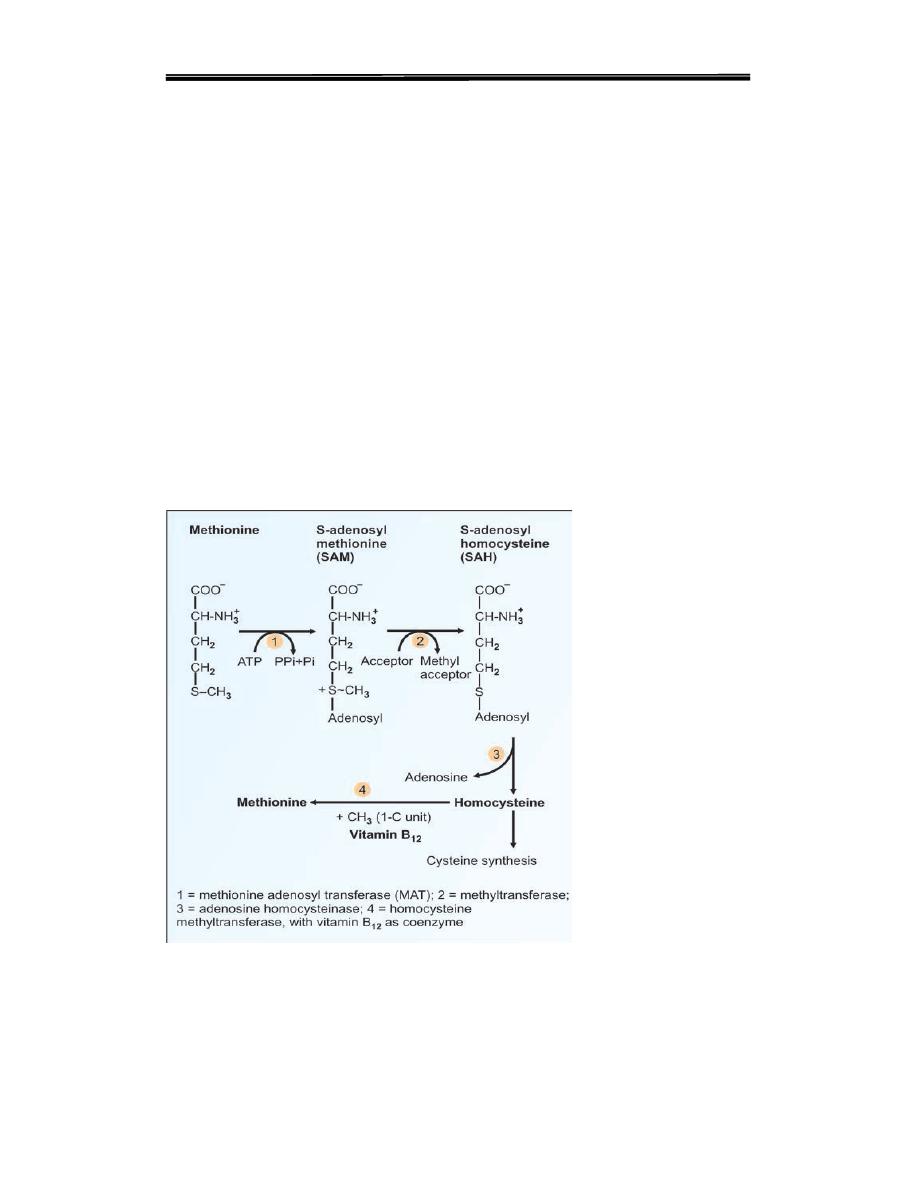
1. Activation of methionine to SAM: In the major pathway, methionine
is activated to‘active methionine' or S-adenosyl methionine(SAM). The
Clinical biochemistry second stage lecture 4 Dr.Thana Alsewedy
7
adenosyl group is transferred to the sulfur atom. (Step 1, Fig. 15.14). This
is done by the enzyme, methionine adenosyl transferase (MAT). The
SAM is the main source of methyl groups in the body.
2. Methyl transfer. In methionine, the thio-ether linkage (C–S–C) is very
stable. In SAM, due to the presence of a high energy bond, the methyl
group is labile, and may be transferred easily to other acceptors (Step 2,
Fig. 15.14; and Table15.1).
3. Homocysteine. After donation of the methyl group from SAM, S-
adenosyl homocysteine is formed
and
The resulting
adenosylhomocysteine is hydrolyzed to
homocysteine
From the S-
adenosyl homocysteine(SAH), the adenosyl group is removed to form
homocysteine, which is the higher homologue of cysteine (Step 3, Fig.
15.14).
4. Methionine synthesis. Homocysteine can be converted to methionine
by addition of a methyl group. This methyl group is donated from one
carbon pool( tetrahydrofolate) with the help of vitamin B12 (and this
called
Activated Methyl Cycle
.of methionine
Step4, Fig15.14..
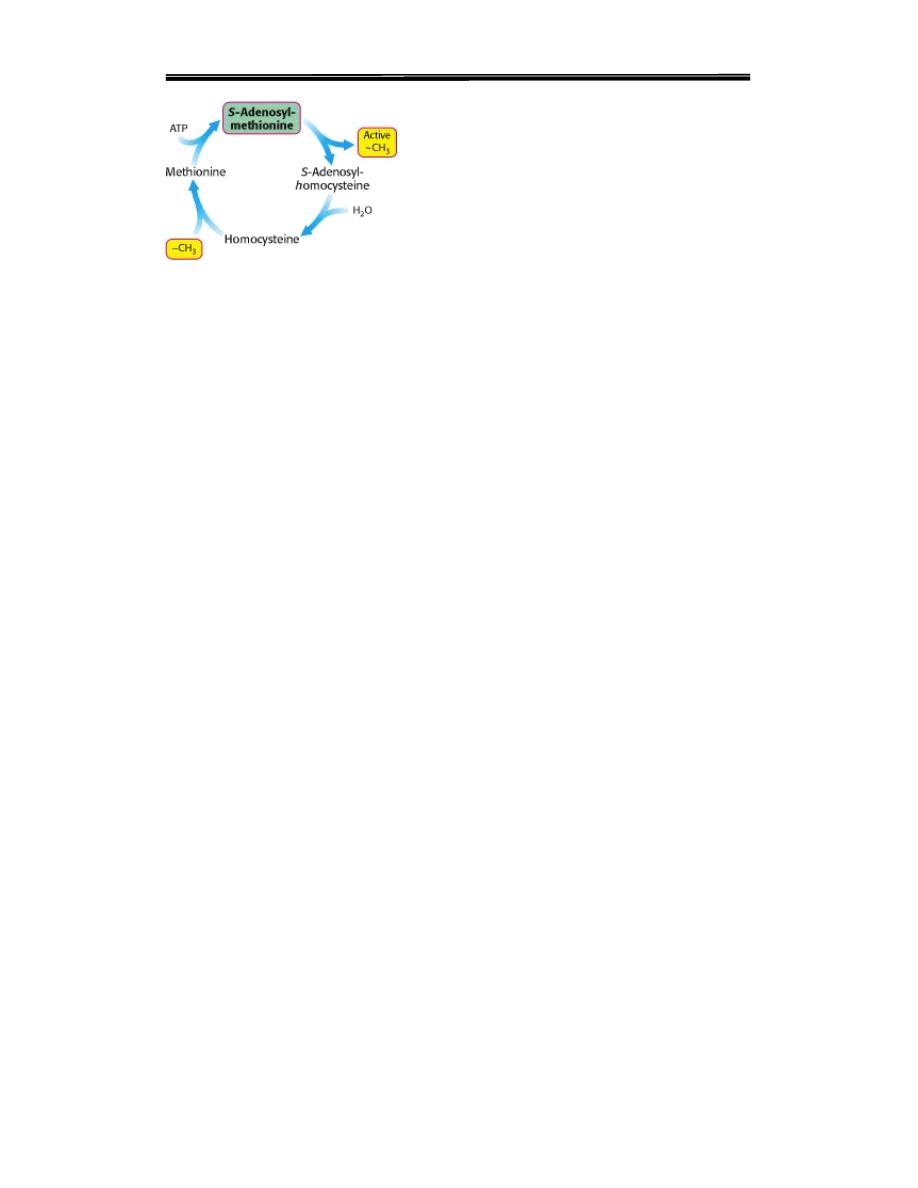
Clinical biochemistry second stage lecture 4 Dr.Thana Alsewedy
8
Activated Methyl Cycle
.of methionine The methyl group of methionine is activated by
the formation of S-adenosylmethionine
F. Other amino acids that form succinyl CoA
Degradation of valine, isoleucine, and threonine also results in the
production of succinyl TCA cycle intermediate and glucogenic
compound.
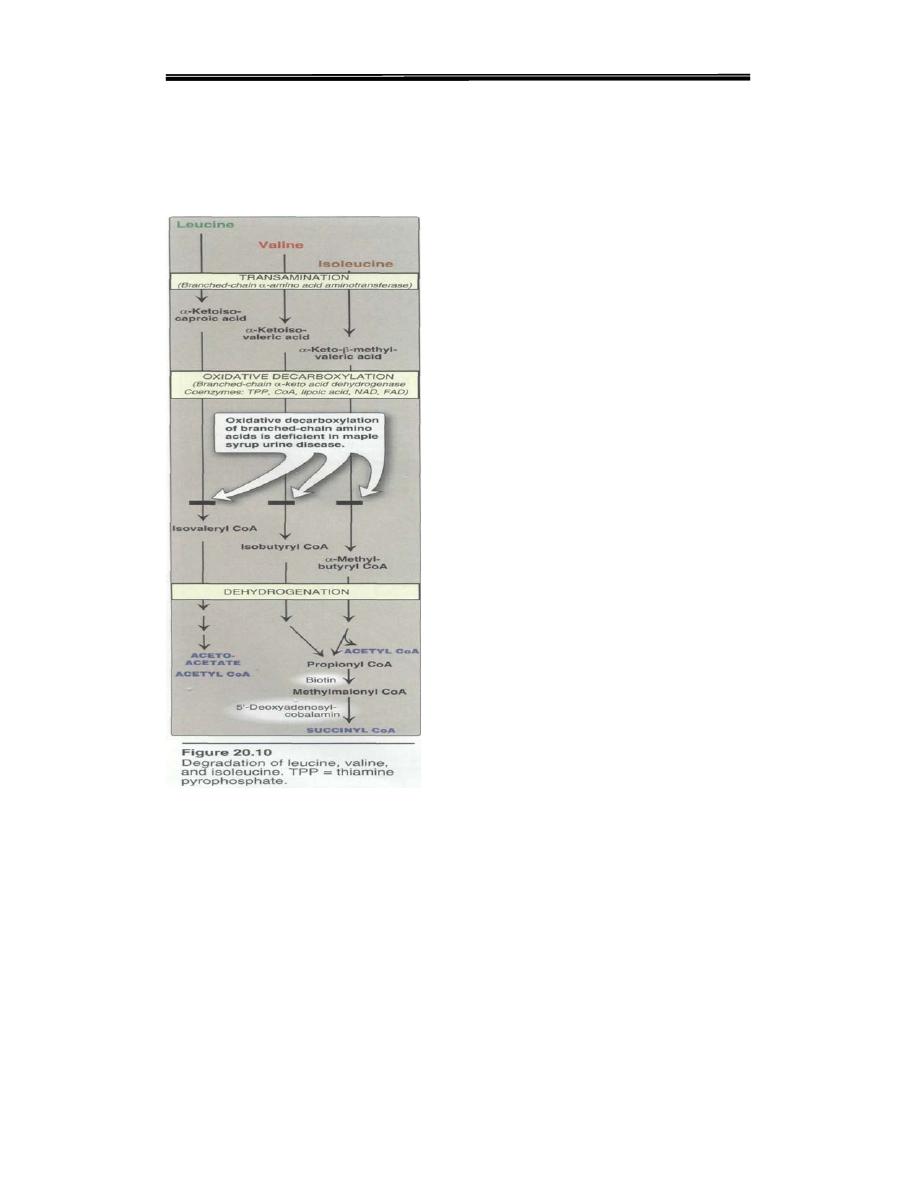
1. Valine and isoleucine are branched-chain amino acids that yield
succinyl CoA (Figure 20.10).
2. Threonine is dehydrated to α-ketobutyrate, which is converted to
propionyl CoA, the precursor of succinyl CoA [Note:Threonine can
also be converted to pyruvate.]
G. Amino acids that form acetyl CoA or acetoacetyl CoA
Leucine, isoleucine, lysine, and tryptophan form acetyl CoA or
acetoacetyl CoA directly, without pyruvate serving as ketogenic
As mentioned previously, phenylalanine and tyrosine also give rise to
acetoacetate during their catabolism. Therefore,there are a total of six
ketogenic amino acids.
The branched-chain amino acids, isoleucine, leucine, and valine
1. Leucine is exclusively ketogenic in its catabolism, forming acetylCoA
and acetoacetate
2.Lysine, an exclusively ketogenic amino acid
3.Isoleucine is both ketogenic and glucogenic
WHEN THE BRANCHED CHAIN AMINO ACIDS VALINE,
ISOLEUCINE, AND LEUCINE ARE DEGRADED IN EXTRA-
HEPATIC TISSUES THEY SHARE TWO COMMON ENZYMES:
branched-chain aminotransferase
branched-chain a-ketoacid dehydrogenase complex
Clinical biochemistry second stage lecture 4 Dr.Thana Alsewedy
9
While much of the catabolism of amino acids takes place in the
liver, the branched chain amino acids are oxidized as primary fuels in
muscle, adipose, kidney, and brain tissues.
