
Clinical biochemistry second stage prostaglandins Dr.Thana Alsewed
1
EICOSANOIDS
Eicosanoids: are Group of compounds containing 20 Carbon atoms
Examples of eicosanoids:
prostaglandins
prostacyclins
thromboxanes
leukotrienes
Prostaglandins and related compounds are collectively known as eicosanoids.Most are
produced from arachidonic acid, a 20-carbon polyunsaturated fatty acid (5,8,11,14-
eicosatetraenoic acid).
The eicosanoids are considered "local hormones."
-They have specific effects on target cells close to their site of formation. -They are
rapidly degraded, so they are not transported to distal sites within the body.
They have roles in:
Intercellular signaling
inflammation
fever
regulation of blood pressure
blood clotting
immune system modulation
control of reproductive processes & tissue growth
regulation of sleep/wake cycle.
Synthesis of prostaglandins and thromboxanes
The dietary precursor of the prostaglandins is the essential fatty acid, linoleic acid. it is
elongated and desaturated to arachidonic acid, the immediate precursor of the
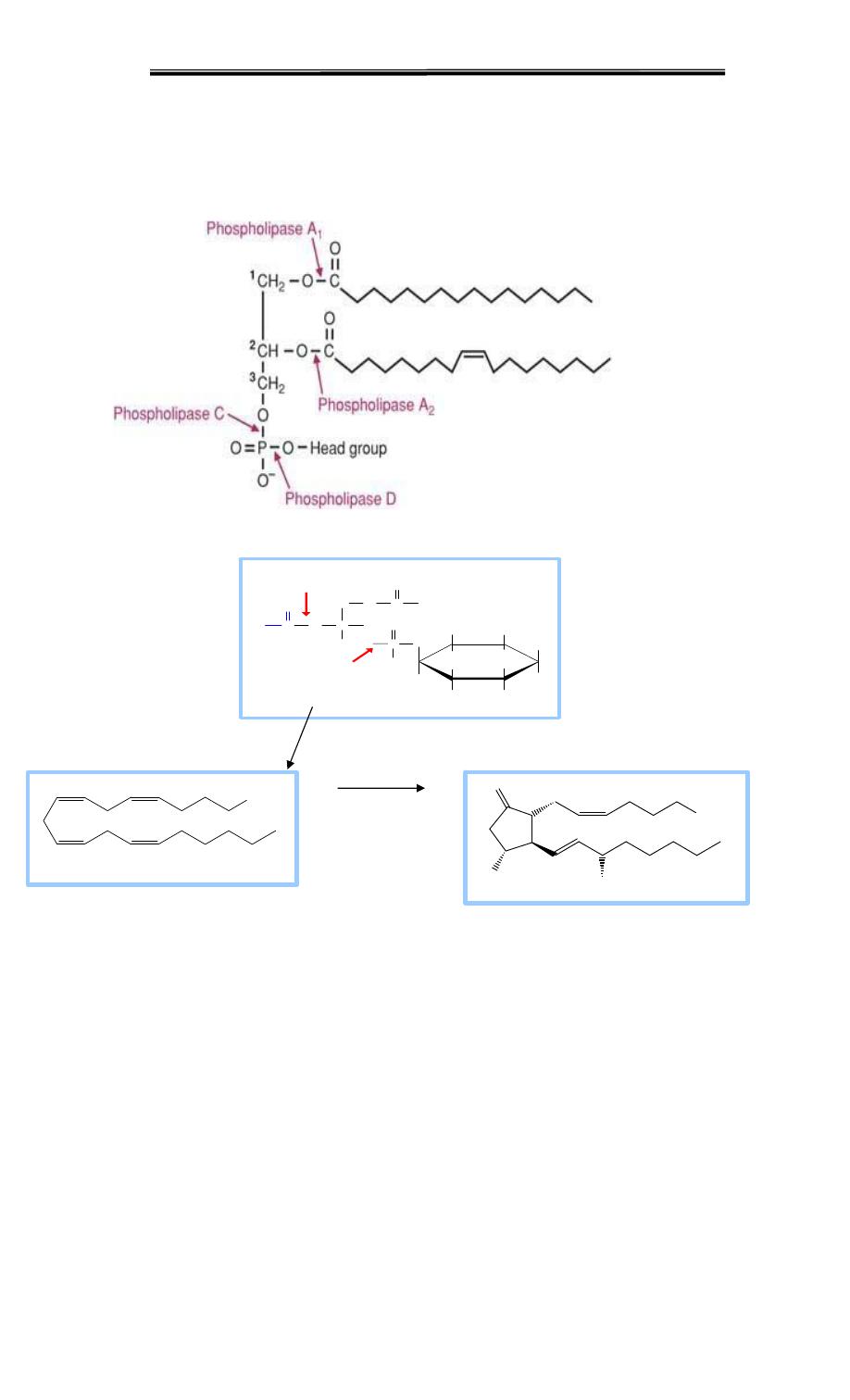
predominant class of prostaglandins Arachidonic acid is released from membrane-bound
phospholipids by phospholipase A2 in response to a variety of signals
The fatty acid arachidonate is often esterified to OH on C2 of glycerophospho-lipids,
especially phosphatidyl inositol Arachidonate is released from phospholipids by hydrolysis
catalyzed by Phospholipase A2. This enzyme hydrolyzes the ester linkage between a
Clinical biochemistry second stage prostaglandins Dr.Thana Alsewed
2
fatty acid and the OH at C2 of the glycerol backbone, releasing the fatty acid & a
lysophospholipid as products.
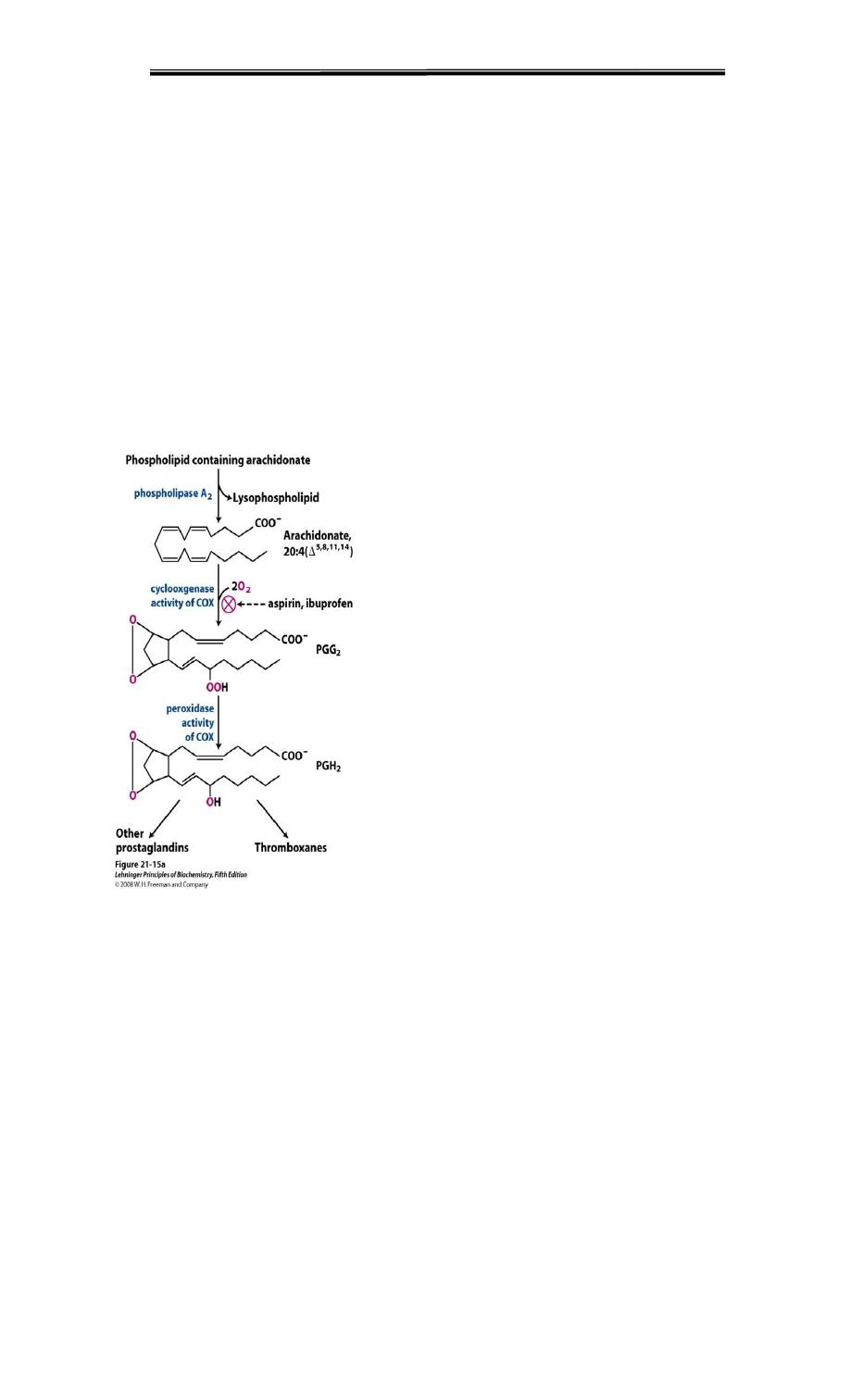
The free arachidonic acid is oxidized and cyclized in the ER by endoperoxide
synthase ( = PGH2 synthase)
This enzyme has two activities – cyclooxygenase (COX) which requires two molecules
of O2 and peroxidase which is dependent on reduced glutathione Initially yields PGH2
COOH
O
HO
OH
PGE
2
Arachidonic acid
COOH
P h o s p h a t i d y l i n o s i t o l
C
H
O
C H
2
O
H
2
C
O
C
R
1
O
C
R
2
O
P
O
O
O -
H
H
O H
O H
H
H
O H
O H
H
O H
H
S i t e o f c l e a v a g e b y
P h o s p h o l i p a s e A
2
S i t e o f c l e a v a g e b y
P h o s p h o l i p a s e C

Clinical biochemistry second stage prostaglandins Dr.Thana Alsewed
3
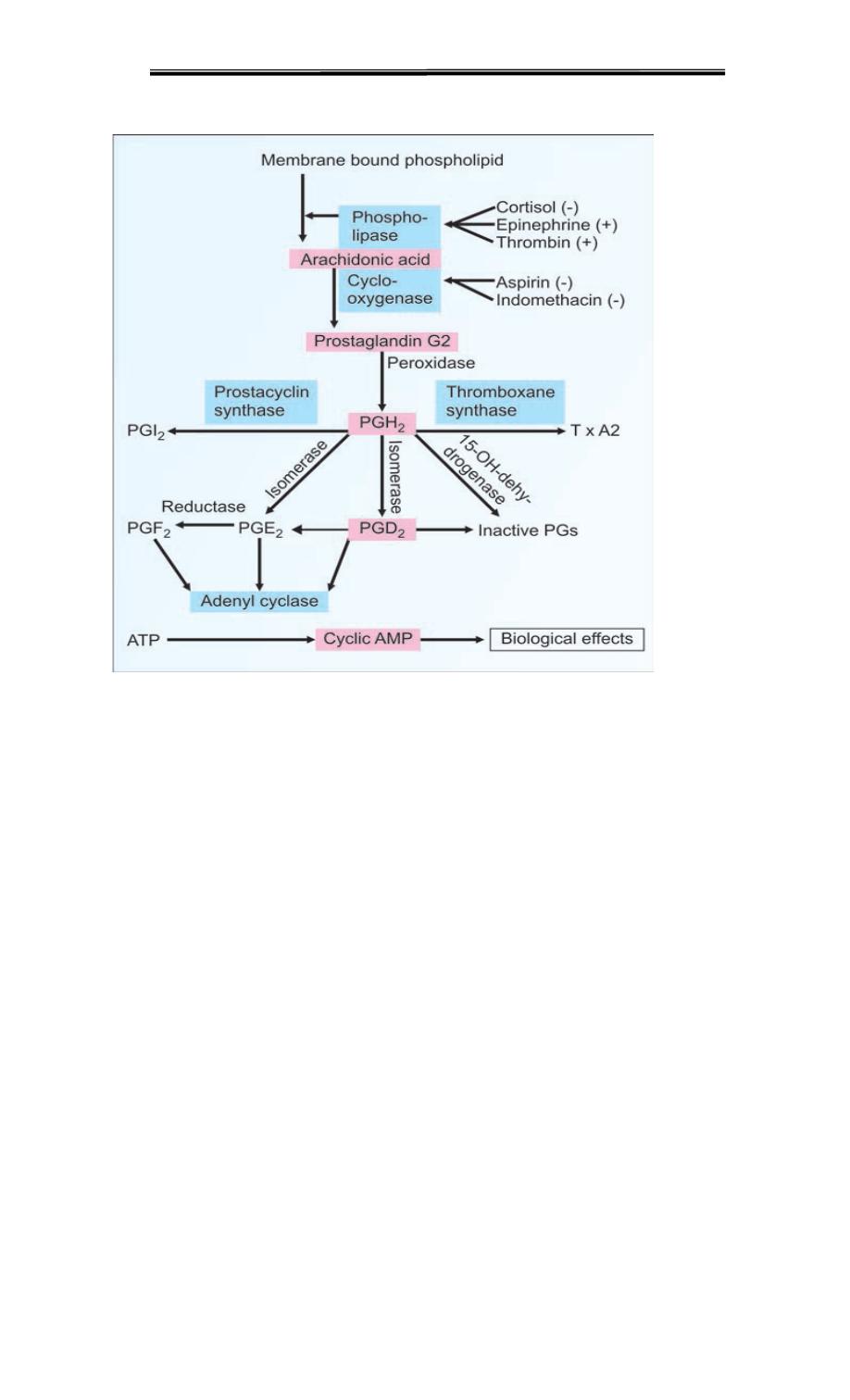
Subsequent steps lead to thromboxane A2 and various prostaglandins
Thromboxanes, like their name suggest, cause thrombosis; the most common
thromboxane is TXA2.
COX converts arachidonic acid into Prostaglandin H2, the next enzymes PG synthetase
and TX synthase, will yield other prostaglandins and thromboxanes, respectively.
COXare present as Isozymes
COX1 is made constitutively in most tissues, and is required for maintenance of healthy
gastric tissue, renal homeostasis, and platelet aggregation
COX2 is inducible in a limited number of tissues in response to products of activated
immune and inflammatory cells
.
Clinical biochemistry second stage prostaglandins Dr.Thana Alsewed
4
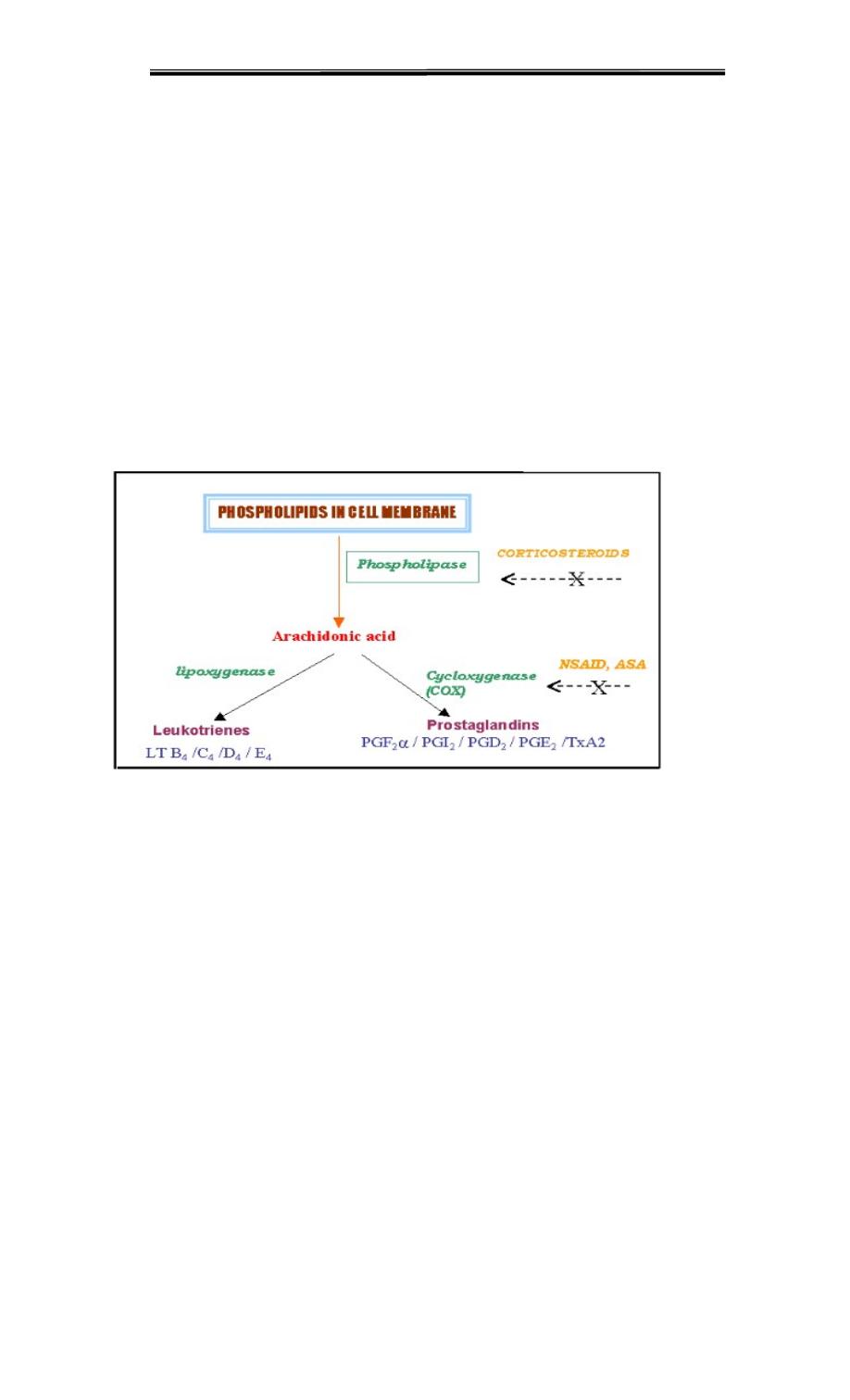
Inhibition of prostaglandin synthesis :
The synthesis of prostaglandins can be inhibited by a number of unrelated compounds.
For example, Cortisol (a steroidal anti-inflammatory agent) inhibits phospholipase A2
activity and, therefore, the precursor of the prostaglandins, arachidonic acid, isnot
available .
Aspirin, indomethacin, and phenylbutazone (all nonsteroidal anti-inflammatory agents
or NSAIDS inhibit cyclooxygenase, and, therefore, prevent the synthesis of the parent
prostaglandin, so low-dose aspirin
low-dose aspirin therapy used to lower the risk of stroke and heart attacks by
decreasing formation thrombi.
Inhibition of prostaglandin by Aspirin

Clinical biochemistry second stage prostaglandins Dr.Thana Alsewed
5
Inhibition of prostaglandin by steroid
Synthesis of leukotrienes
, Leukotrienes are a family of eicosanoid inflammatory mediators produced in
leukocytes by the oxidation of arachidonic acid by the enzyme arachidonate 5-
lipoxygenase. Leukotrienes are produced in platelets, leukocytes, mast cells, and heart
and lung vascular tissues; they are involved in creating allergic responses and
inflammation. Leukotriene synthesis is not inhibited by NSAIDs.
Leukotrienes use lipid signaling to convey information to either the cell producing them
(autocrine signaling) or neighboring cells (paracrine signaling) in order to regulate
immune responses. Leukotriene production is usually accompanied by the production
of histamine and prostaglandins, which also act as inflammatory mediators.
One of their roles (specifically, leukotriene D
4
) is to trigger contractions in the smooth
muscles lining the bronchioles; their overproduction is a major cause of inflammation in
asthma and allergic rhinitis. Leukotriene antagonists are used to treat these disorders by
inhibiting the production of Leukotrienes .
Clinical biochemistry second stage prostaglandins Dr.Thana Alsewed
6
Leukotrienes have roles in inflammation. They are produced in areas of inflammation
in blood vessel walls as part of the pathology of Aatherosclerosis.Some leukotrienes
act via specific G-protein coupled receptors (GPCRs) in the plasma membrane.
Synthesis of leukotrienes
Arachidonic acid is converted to a variety of leukotrienes involving a family of
lipoxygenasesLipoxygenases are not affected by NSAIDS. Leukotrienes are mediators
of allergic response and inflammation. [Note: Inhibitors of 5-lipoxygenase and
leukotriene receptor antagonists are used in the treatment of asthma
Clinical biochemistry second stage prostaglandins Dr.Thana Alsewed
7
Pathway of synthesis of different type of prostoglandin
