1
Phenols
Phenols are the organic compounds containing benzene ring
bonded to a hydroxyl group. They are also known as carbolic
acids
Nomenclature.
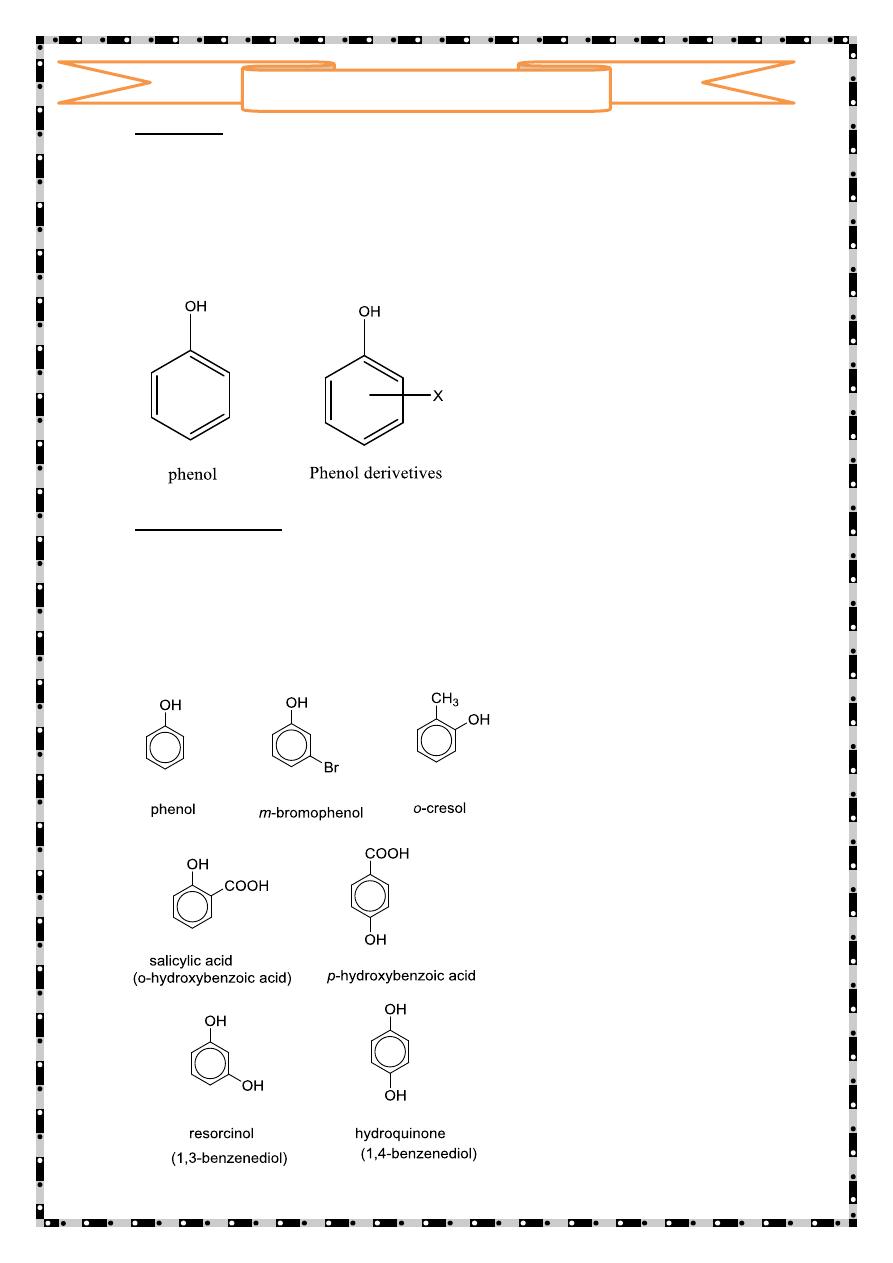
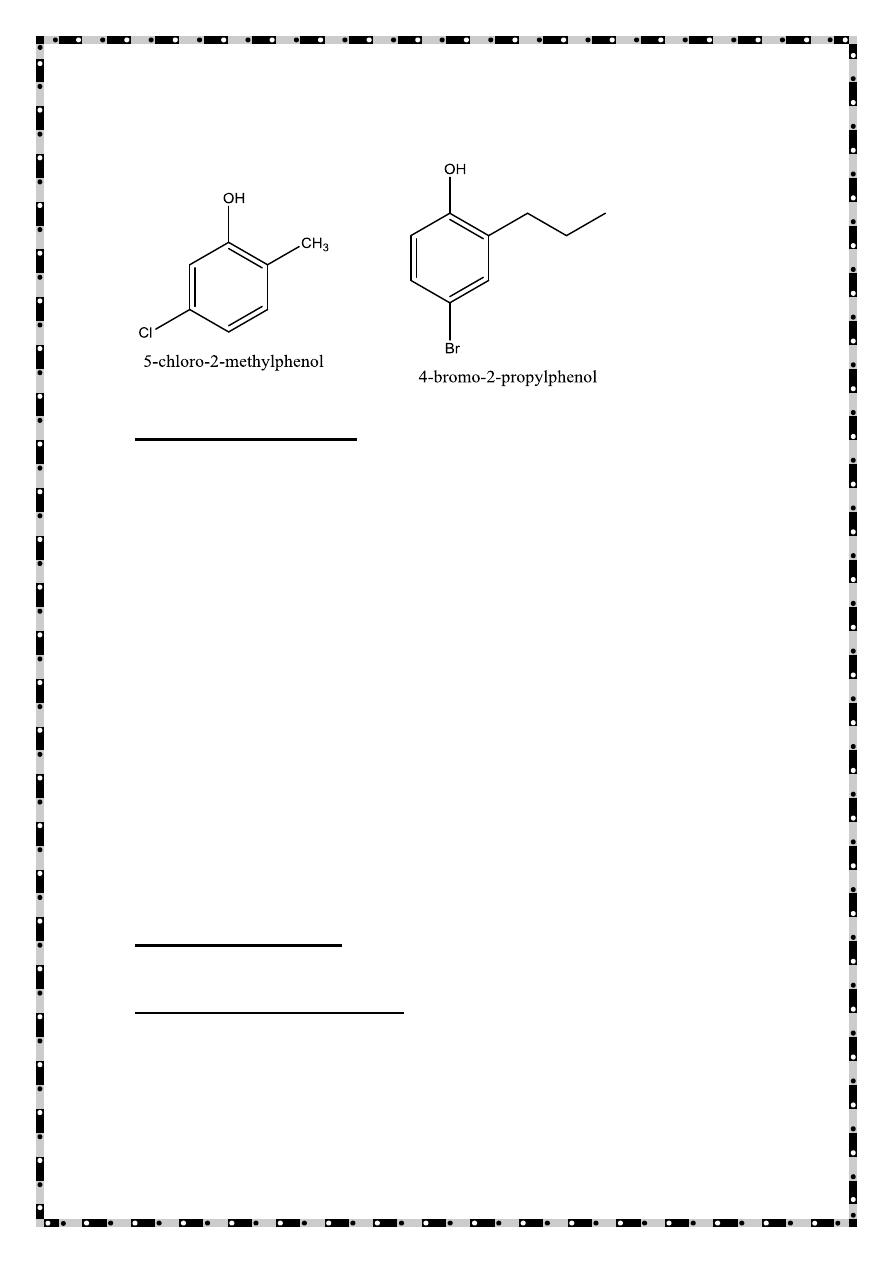
Phenols are usually named as substituted phenols. The methyl
phenols are given the special name, cresols. Some other phenols
are named as hydroxyl compounds.
Phenols
2
Physical Properties
Boiling point of phenols: Phenols generally have higher melting
point in comparison to other hydrocarbons having equal
molecular masses. This is due to the presence of intermolecular
hydrogen bonding between hydroxyl groups of phenol molecules.
In general, the melting point of phenols increases with increase
in the number of carbon atoms.
Solubility of phenols: Solubility of phenol in water is governed
by the hydroxyl group present. The hydroxyl group in phenol is
involved in the formation of intermolecular hydrogen bonding.
Thus, hydrogen bonds are formed between water and phenol
molecules which make phenol soluble in water. However the aryl
group attached to the hydroxyl group is hydrophobic in nature.
Thus, the solubility of phenol decreases with the increase in size
of aryl group
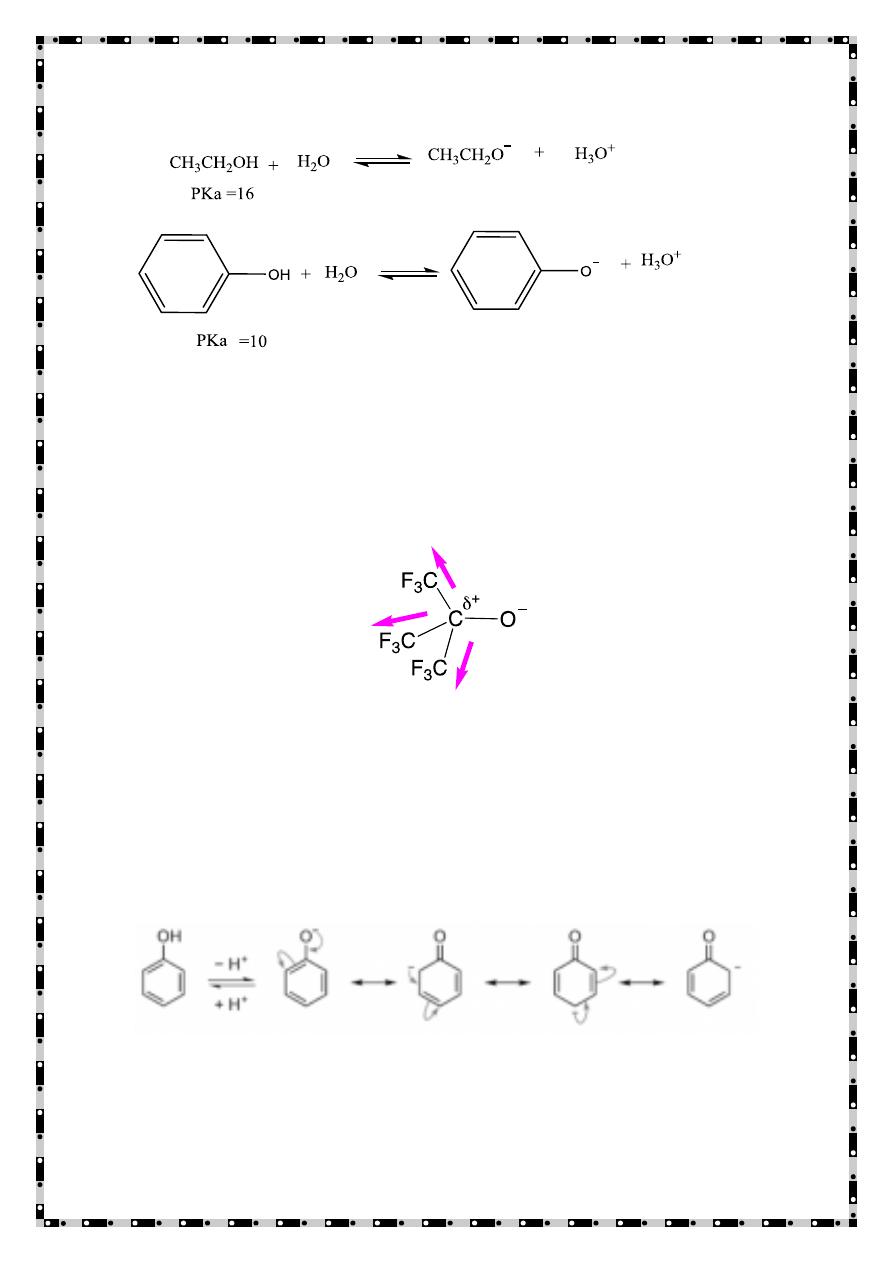
Acidity of Phenols
Phenols are more acidic than aliphatic alcohols
Factors that influence acidity:
1) Inductive effect
Presence of
Electron-withdrawing groups (such as F) makes an alcohol a
stronger acid by stabilizing the conjugate base (alkoxide)
3
Alcohol
CH
3
CH
2
OH, FCH
2
CH
2
OH F
2
CHCH
2
OH,F
3
CCH
2
OH,(F
3
C)
3
COH
PKa 16 , 14.4 , 13.3 , 12.4 , 5.4
Acidity:
(F
3
C
)3
COH>F
3
CCH
2
OH>F
2
CHCH
2
OH>FCH
2
CH
2
OH>CH
3
CH
2
OH
A benzene ring is generally considered electron withdrawing and stabilizes
the negative charge through inductive effects.
2) Resonance effect
The benzene ring stabilizes the the phenoxide ion by resonance
delocalization of the negative charge
4
Substituent Effects on the Acidity of Phenols
Electron-donating substituents make a phenol less acidic by destabilizing
the phenoxide ion (resonance effect) .
Electron-withdrawing substituents make a phenol more acidic by
Stabilizing the phenoxide ion through delocalization of the negative
charge and through inductive effects.
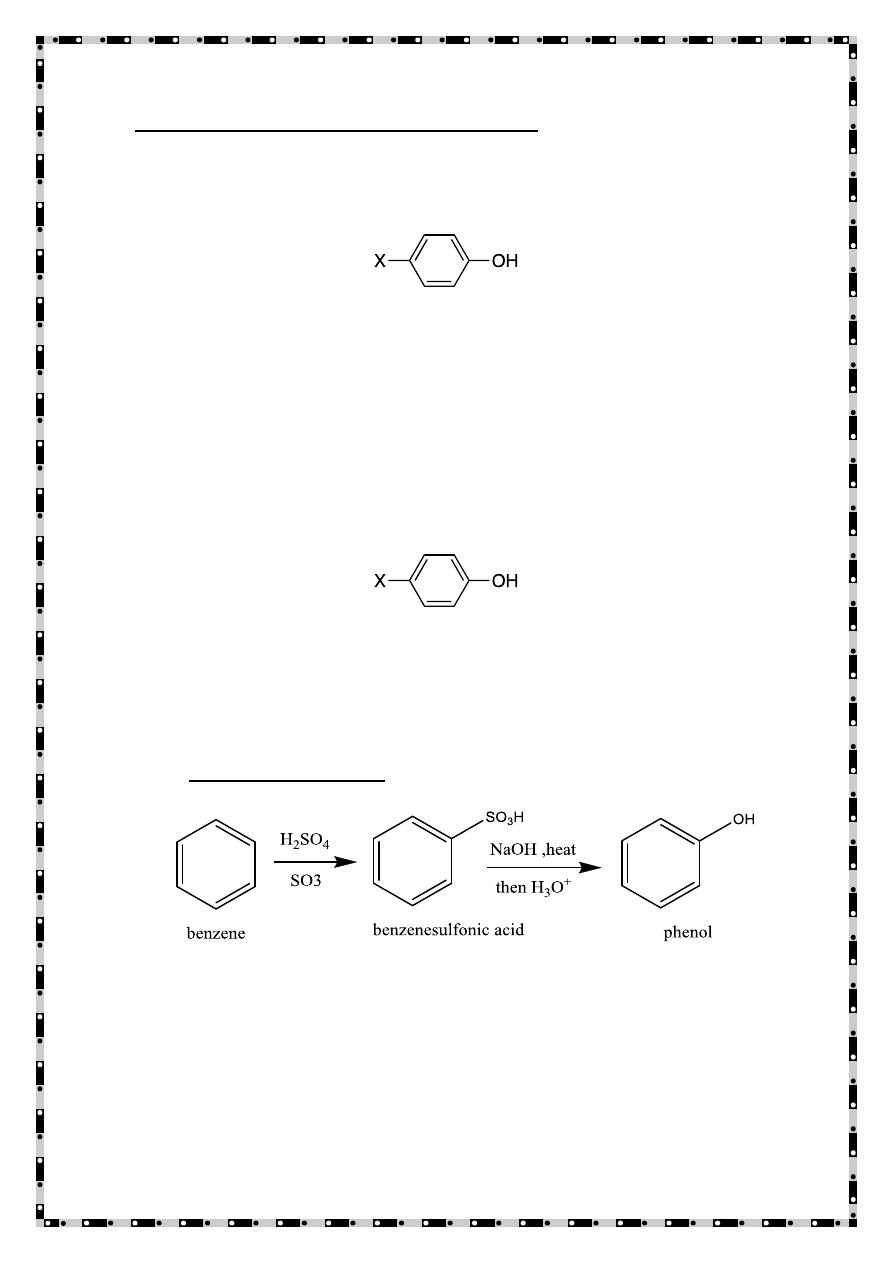
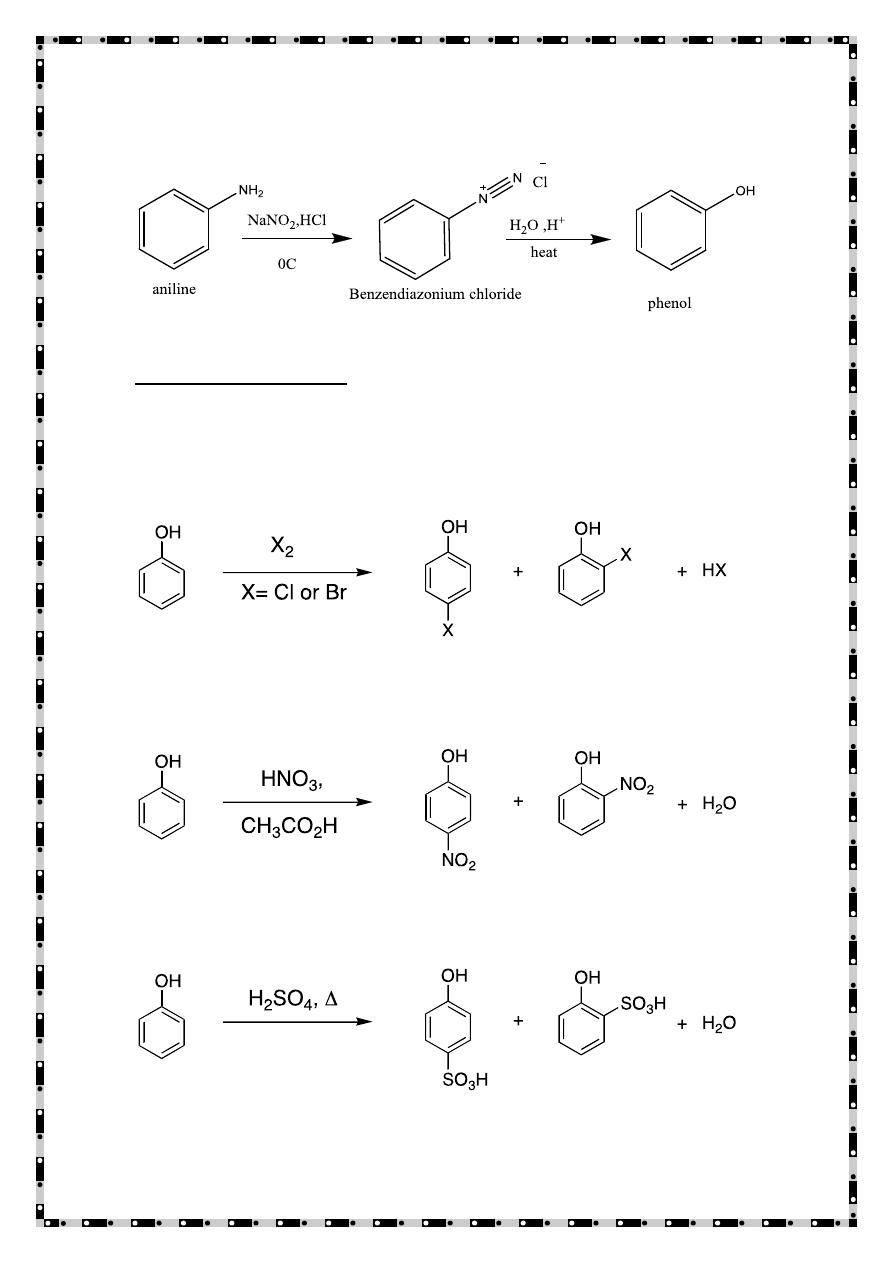
Prepration of Phenols
From benzene
X= -H
-
CH
3
-
OCH
3
-NH
2
pK
a
~ 10 10.3 10.2 10.5
X= -H
-
Cl -Br -NO
2
pK
a
~ 10
9.4 9.3 7.2
5
From aryl diazonium ion
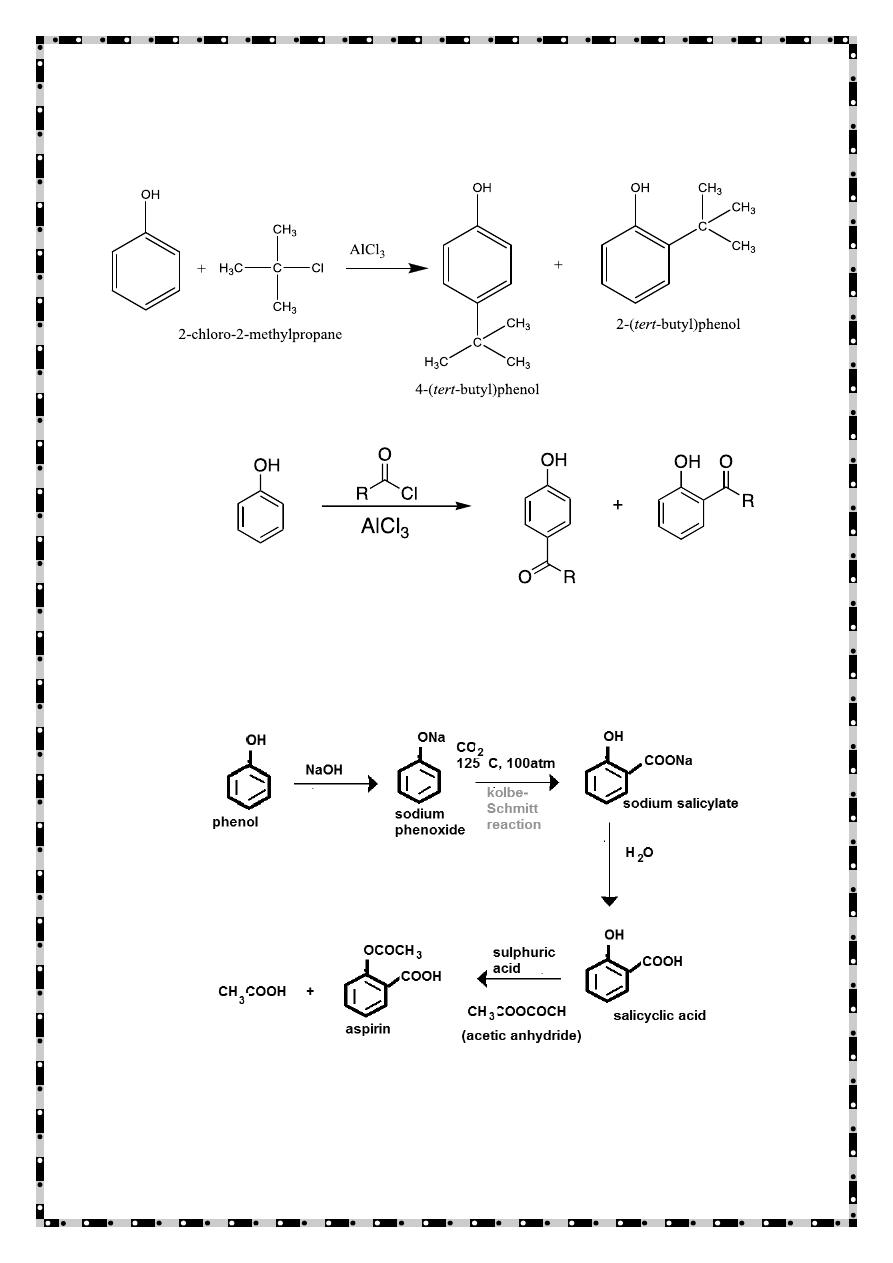
Reactions of Phenols
Electrophilic Aromatic Substitution
The hydroxyl group of phenols is a strong activator and o-p-director.
Halogenation: Phenols are so highly activated that they often react with
Br
2
and Cl
2
without a catalyst.
Nitration
4-nitrophenol
Sulfonation
4-hydroxybenzenesulfonic acid
6
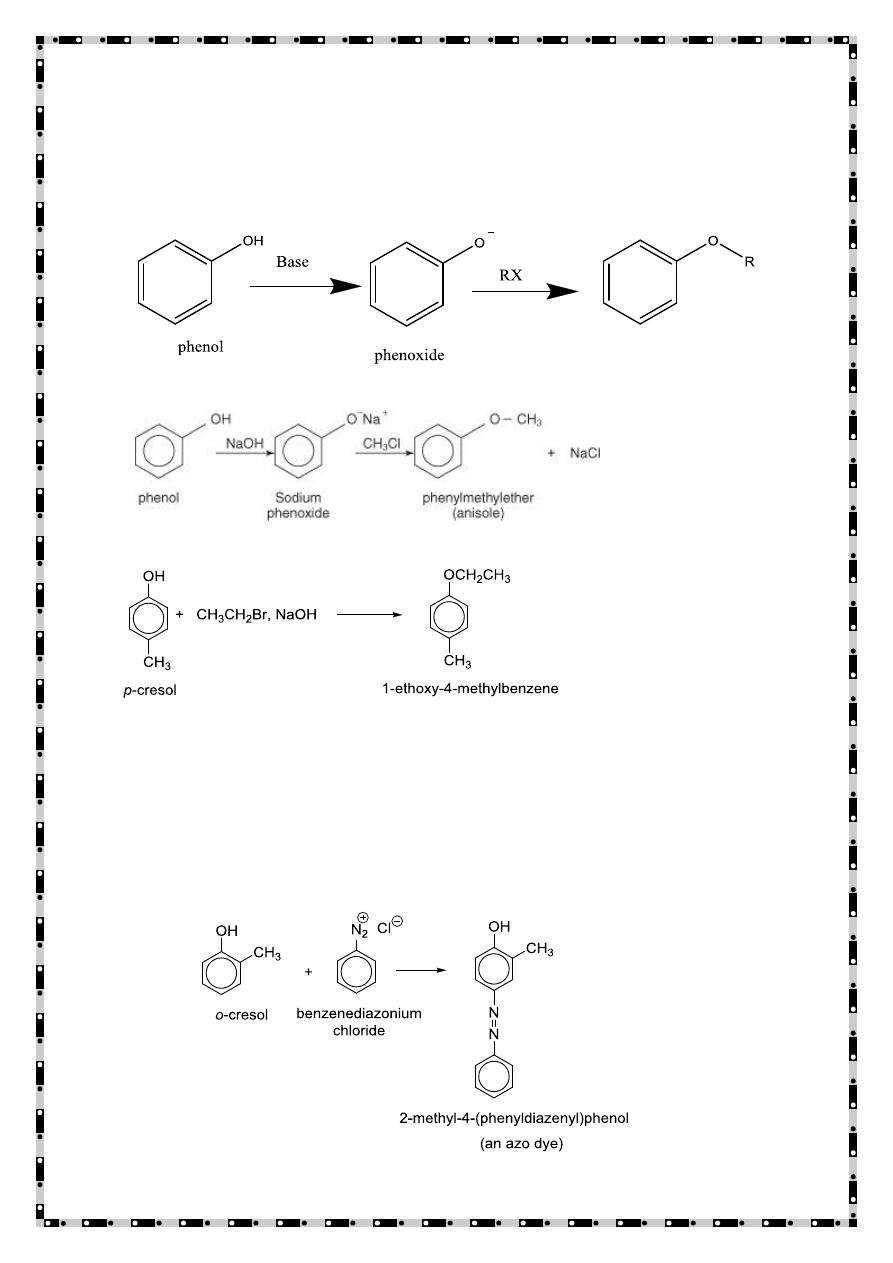
Friedel-Crafts alkylation
Friedel-Crafts acylation
The Kolbe-Schmitt Reaction (Carboxylation of Phenols)
Synthesis of salicylic acid (o-hydroxybenzoic acid) from phenol.
7
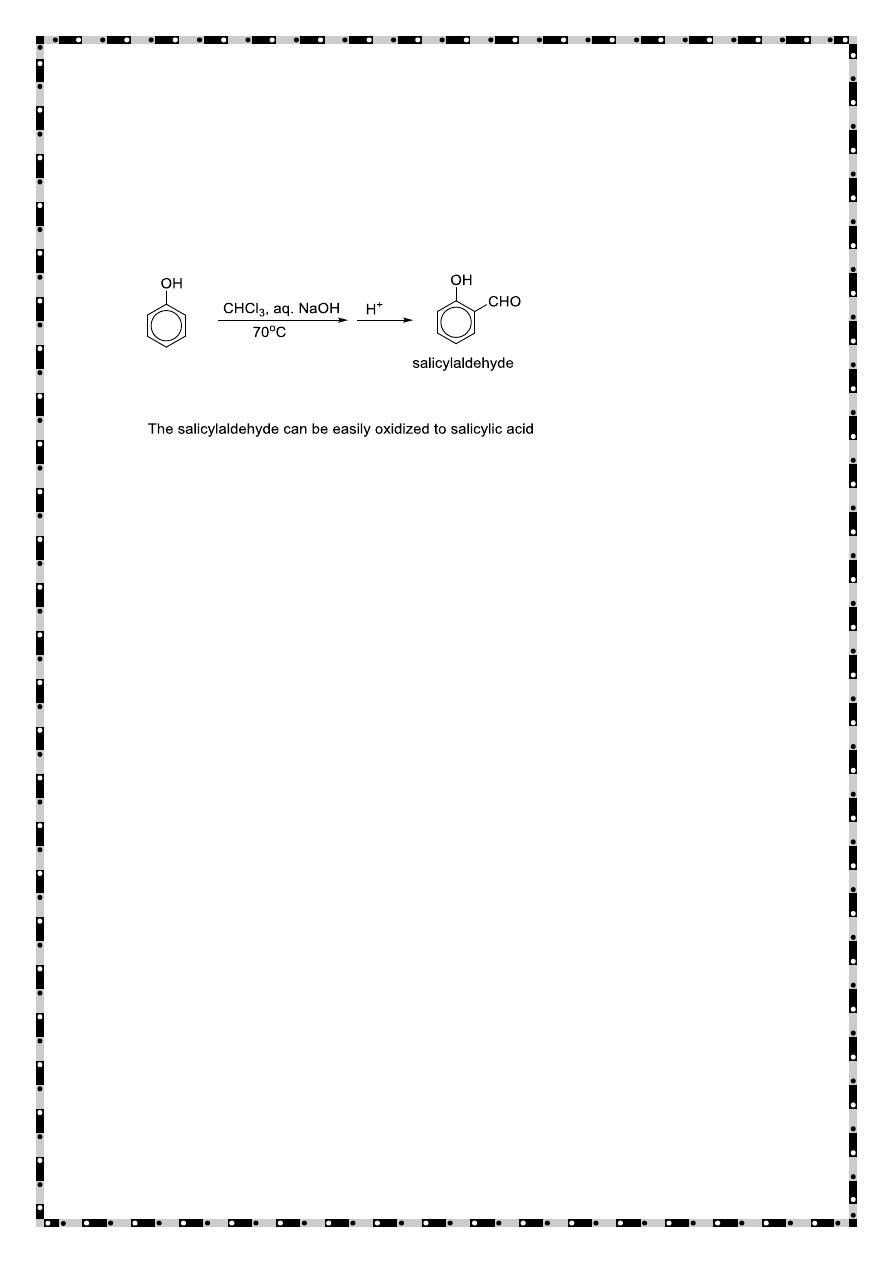
Preparation of Aryl Ethers
The phenoxide ion is agood nucleophile and reacts with 1° and 2° alkyl
halides and tosylates afford aryl ethers (Williamson ether synthesis)
Coupling with diazonium salts
(EAS with the weak electrophile diazonium)
8
Reimer-Tiemann reaction
