
1
True bacteria – Rods - Gram positive rods
Non-spore forming bacteria
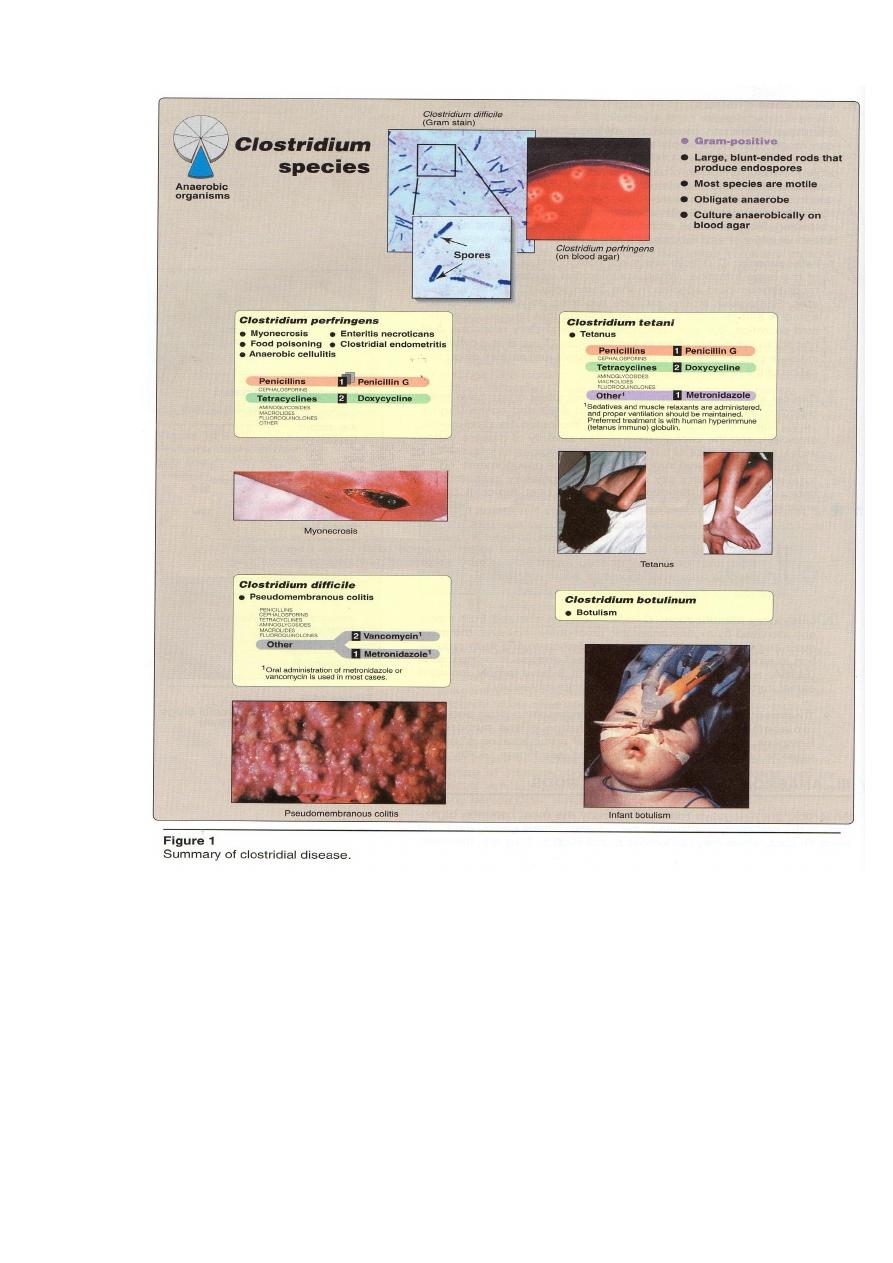
Anaerobic Rods-Clostridia
Clinically significant spps of Clostridia include:
1) C. perfringens which cause histotoxic (soft tissue and skin destructive)
infections (myonecrosis) and food poisoning.
2) C. tetani which causes tetanus.
3)C. botulinum which causes botulism.
4)C.difficile which causes pseudomembranous colitis associated with antibiotic
use.
General features of Clostridia
Physiology
C. use a variety of small organic molecules as pyruvate as the final electron
acceptors.
In vegetative state, C. are also damaged by O
2
. C. grow on enriched media in
the presence of a reducing agent such as cysteine or thioglycollate or in an O
2
-free,
gaseous atmosphere provided by sealed jar or an air evacuated glove box … etc.
Epidemiology
C. is a part of the vagina and intestinal flora in humans and other mammals,
found in soil, sewage, and aquatic settings, particularly those with high organic
content. A number of C. produce destructive and invasive infections when introduced
into tissues,by a break in the skin resulting from surgery or trauma,and presence
infectious processes is opportunistic and derives from the patient’s normal flora.
2
Clostridium perfringens
It is found in soil and the vegetative form as part of the normal flora of the
vagina, gastrointestinal (GI) tract. When introduced into tissue, its can cause
anaerobic cellulitis, and myonecrosis (gas gangrene). Some strains of C. perfringens
cause a common form of food poising.
3
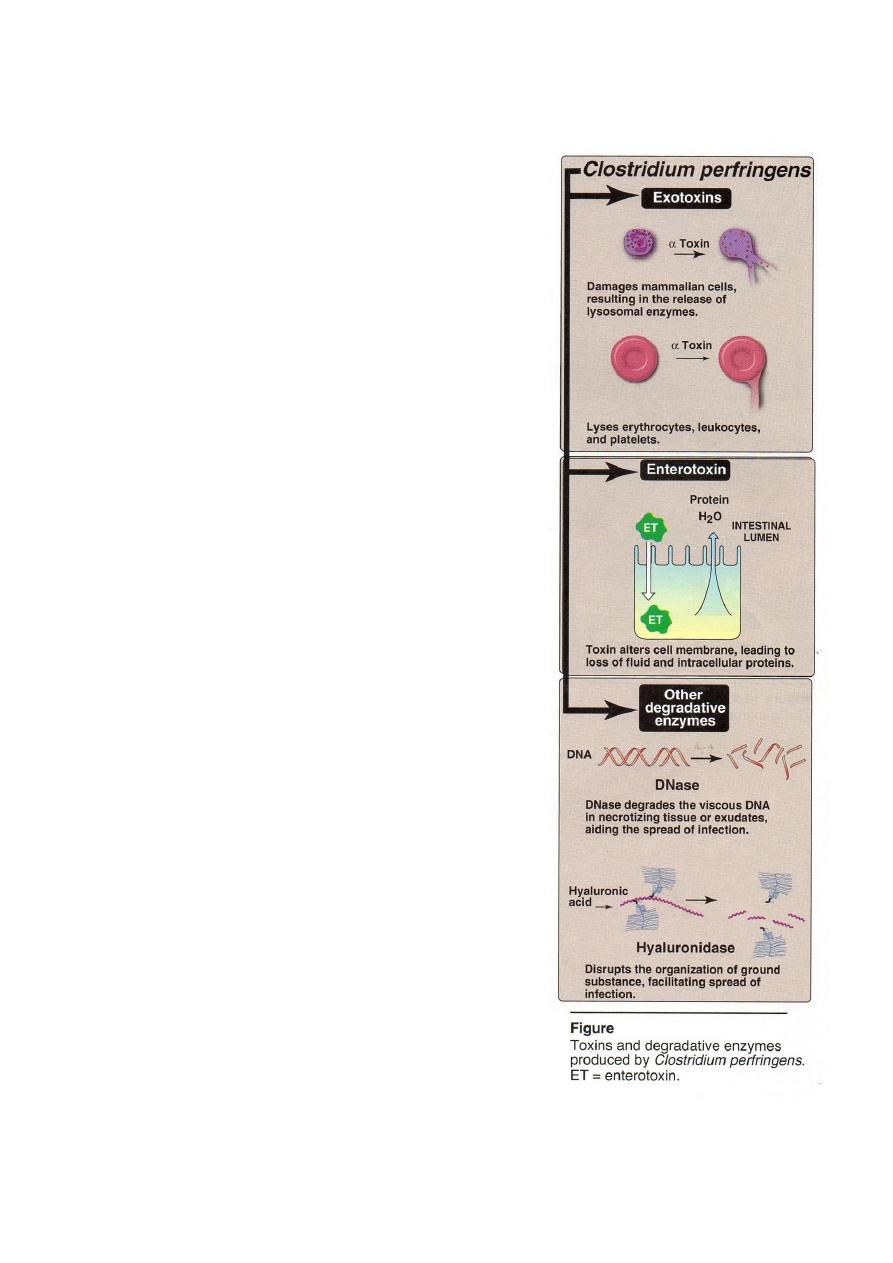
Pathogenesis
C. perfringens secretes a variety of exotoxins
enterotoxin, and hydrolytic Es that facilitate the
disease process (figure 2).
1-Exotoxins: C. elaborates at least twelve exotoxins
designated by Greek letters (
-
- epsilon عand
iota) . The most important of these required for
virulence in tissue is α-toxin, which is a lecithinase
(phospholipase C) that degrades lecithin in
mammalian cell membrances causing lysis of
endothelial cells, erythrocytes, leukocytes and
platelets. Other C. perfringens extotoxins have
hemolytic or other cytotoxic and necrotic effects
either locally or when dispersed in the blood stream.
C. perfringens strains are grouped A
E
F
on the basis of their spectrum of exotoxins. Type A
strains, produce both α-toxin and enterotoxin, are
responsible for most human clostridial infections as
gas-gangerene ,also show lecithinase activity,that
gives positive Nagler’s Reaction(This test is useful
in rapid detection of C. perfringens in clinical
specimen and is inhibited by
-toxin – antitoxin
sera).
2- Enterotoxin: acts in the lower portion of the
small intestine. The molecule binds to receptors on
the epithelial cell surface and alters the cell
membrane, disrupting ion transport (in the ileum)
leading loss of fluid and intracellular proteins.
Enterotoxin (ET) – producing strains are
unusually heat resistant, the spores remaining viable
for longer than an hour at 100
C enhancing their
threat as food-borne pathogens.
3-Degradative enzymes: a variety of hydrolytic Es
including: DNases, hyaluronidase and collagenases
which liquefy tissue and promote the spread of
infection.
2

4
Eidemiology
Transmission
by contaminated food ,contamination with infected soil ,
contaminated instruments, severe and open wounds ,traumatic injuries, bowel
obstructions, bowel surgery and reduced blood supply to given tissue.
Clinical significance
The disease processes initiated by C. perfringens result from a combination of
infection + the production of exotoxins and/or enterotoxins + degradative enzymes.
1- Myonecrosis (gas gangrene)
C. spores are introduced into tissue by exogenous (contamination with infected
soil) or by endogenous (transfer from the intestinal tract) or by severe and open
wounds (such as compound fractures and other ischemia – producing injuries like car
accidents) are a prime predisposing condition.
-toxin and other exotoxins are secreted and extensive cell killing happened.
Production of Es facilitates the spread of infection. Fermentation of tissue
carbohydrates yield gas and an accumulation of gas bubbles in the subcutaneous
spaces produces a crinkling sensation on palpation, hence the name gas gangrene.
The exudates are foul smelling. As the disease progresses, the exotoxins being carried
by the circulation from damaged tissue to other organs such as shock, renal failure
and intravascular hemolysis, if untreated is fatal within days of the initiation of
gangrene.
2- Anaerobic cellulitis
Infection of connective tissue, along fascial planes does not involve muscle
tissue. Surgical intervention is unsuccessful, because of the rapid spread of infection.
3- Food poising
Nausea, abdominal cramp, diarrhea occurs 9 - 19 hours after eating
contaminated food with C. perfringens. Fever is absent and vomiting rare. A typical
episode of C. enterotoxin food poisoning involves →bad cooking that fails to
inactivate spores → holding the food for several hours under conditions , allow
bacterial germination several cycles of growth.
4- Enteritis necroticans
Necrotizing bowel disease with high mortality greater than 50% caused by it.
5- Clostridial endometritis
Is a complicated of incomplete abortion or the use of contaminated
instruments. Gangrenous infection of uterine tissue is followed by toxemia and
bacteremia.

5
Diagnostic laboratory tests
Diagnosis of C. myonecrosis or cellulitis rests on clinical impression.
Increasing pain at the site of prior injury or surgery, together with signs of systemic
toxicity and gas in the soft tissue, support the diagnosis of gas-gangrene.
Specimens = are collected from :
1- Muscles at the edge of affected area.
2- Exudate from area where infection appears more active.
3- Necrotic tissue and muscle fragment.
4- Suspected food and patient’s feces in food poisoning,.
Microscopic examination = G
+
rods , large, , capsulated, nonmotile.The
spores are oval , central or subterminal and not-bulging.
Macroscopic examination = cultured anaerobically on blood agar → grows
rapidly producing colonies with a unique double zone of hemolysis. Also cultured in
cooked meat media → carbohydrate fermentation as red broth.
Biochemical reaction = Nagler’s Reaction + ( the B. are cultured on plates
containing 20% human serum, or egg yolk, produces opalescence in media due to LE
activity of
-toxin, which splits lipoproteins and librates lipids deposit around the
colony to give opalescence). Catalase - , gelatin + .
Animal pathogenicity = by injection of guinea pig, swelling of injected limb
appeared due to gas formation, muscles becomes pink. B. can recover from heart ,
spleen, liver and death occurs (24-48hr).
Treatment and Prevention
The key to both prevention and treatment of gas gangrene is
1- immediate removal of foreign material
2- removal dead tissue
3-exposure of the wound to O
2
.
4-Amputation of limb is required in many cases of gas-gangrene.
5-The administration of antibiotics in high dose, like penicillin and
tetracyclines (see figure 1).
Prevention of food poisoning by good cooking and good holding the food .
Prevention of clostridial endometritis by avoid useing of contaminated
instruments in delivery section .
6
Clostridium botulinum
C. botulinum causes botulism , which caused by the action of a neurotoxin,
causes a paralysis.
Epidemiology
C. botulinum is found in soil and aquatic sediments, and the spores
contaminate vegetables and meat or fish.The spore germinates → vegetative form,
produced → toxin elaborated in food →out breaks occur in families or other eating
groups.
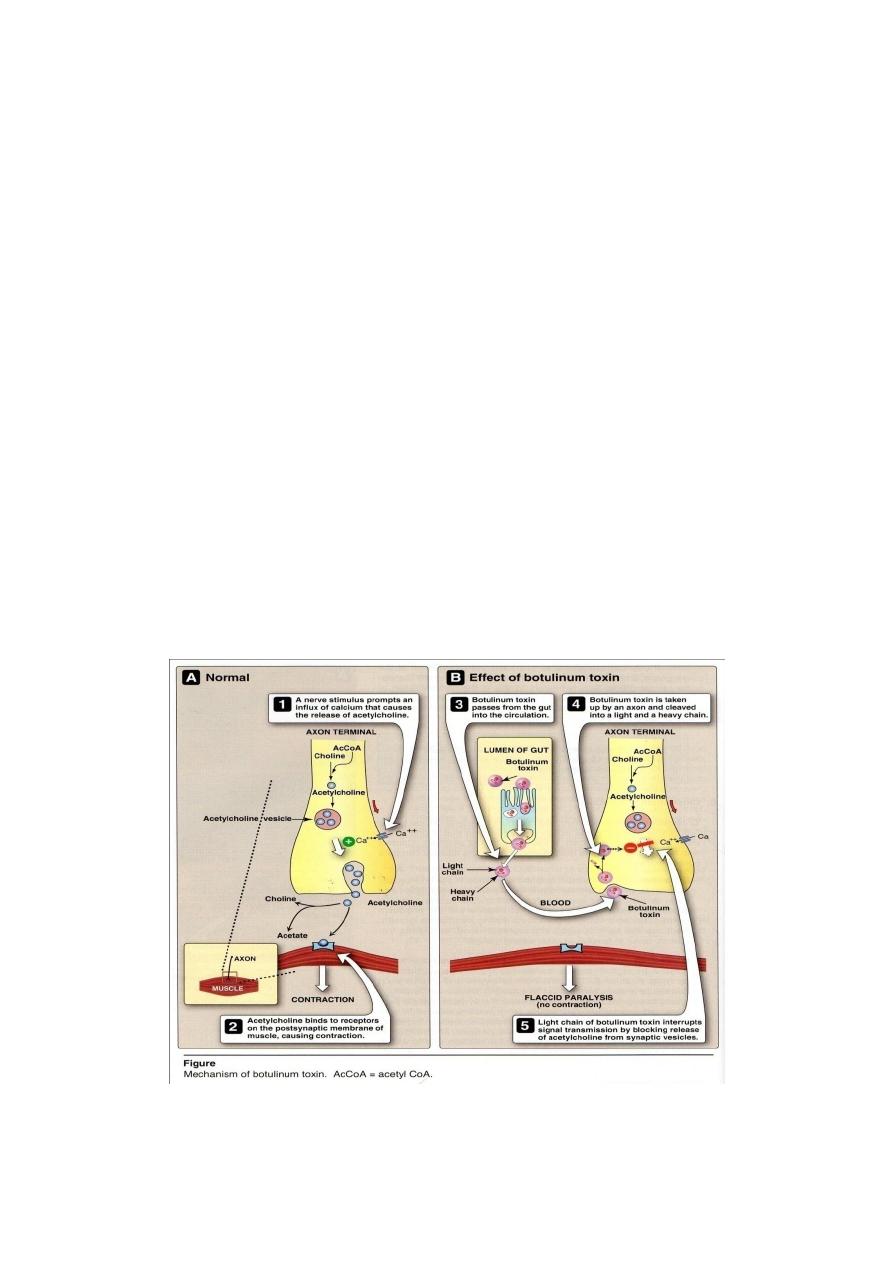
Pathogenesis
There are several types of botulinum toxin, designated A
G, but human
disease is almost caused by types A, B, or E. The botulinum serotypes and tetanus
toxin constitute a homologous set of proteins whose neurotoxicity arise from
proteolytic cleavage of specific synaptic vesicle peptides, causing subsequent failure
of neurotransmission. In contrast to tetanus toxin (which causes constant
contraction, spasms), botulinum toxins blocking the neuromuscular junction and
inhibiting release of the neurotransmitter, acetylcholine, preventing contraction and
causing paralysis (Figure 3).
3

7
Clinical Significance
1- Classic botulism
Is a food poisoning in which a patient first begins to experience difficulties in
focusing vision, swallowing and other cranial nerve functions, 12-36 hs after
ingesting toxin containing food but not necessarily viable organisms. There is no
fever or sign of sepsis. A progressive paralysis of striated muscle groups develops
and mortality is about 15 %, reach to respiratory paralysis.Toxin absorbed from
small intestine
blood
peripheral nerves
2- Infant botulism or floppy baby syndrome
Any supplements, such as honey contaminated with C. botulinum spores may
transmit the C. colonizes to the large bowel of infants 3 - 24 weeks of age, the toxin
produced is slowly absorbed. Constipation, feeding problems, lethargy and poor
muscle tone are common early signs, lead to sudden infant death syndrome, but
recovery is the usual outcome following prolonged treatment .
3- Wound botulism
A rare form occurs when a wound becomes contaminated with the C. and
toxin is absorbed from that site.
Diagnostic laboratory tests
Microscopic examination = G
+
rods, large, noncapsulated, motile. The spores
are oval ,central or subterminal and bulging.
Macroscopic examination = identified by standard anaerobic methods. In
cooked meat broth → protolytic as black broth.
Biochemical reaction = Nagler’s Reaction - , Catalase - , gelatin + .
Animal pathogenicity = With standard animal pathogenicity method ,toxin is
also identifiable in serum, stool, and food .
Treatment and Prevention
Antitoxin( which neutralizes unbound botulinum toxin) should be administered
as soon as possible. A trivalent (A, B, E) horse antiserum is available from the
Centers for Disease Control (CDC), and mechanical ventilation may be required.
In wound botulism , the infection can be treated with penicillin or other antibiotics.
Prevention by inactivated toxin at boiling temperature(100
C) for 20 mints,
although killing of botulinal spores requires autoclaving.
8
Clostridium tetani
The introduction of C. tetani spores into small wounds and contaminated soil is
probably a common occurrence. But a combination of the extreme O
2
sensitivity of
vegetative C. tetani + immunization against its exotoxin, make the resulting disease
tentanus rare in developed countries.
In U.S. the disease is seen in older individuals who have not received their
immunization regularly and whose immunity has waned.
Epidemiology
C. tetani spores are common in garden, barnyard and
manures and the infection done by: 1-puncture wound ,
2- severe burns , 3- surgery, 4-injection of drugs
often contain spores. Introduced foreign bodies or small areas
of cell killing create the spores to germinate and grow.
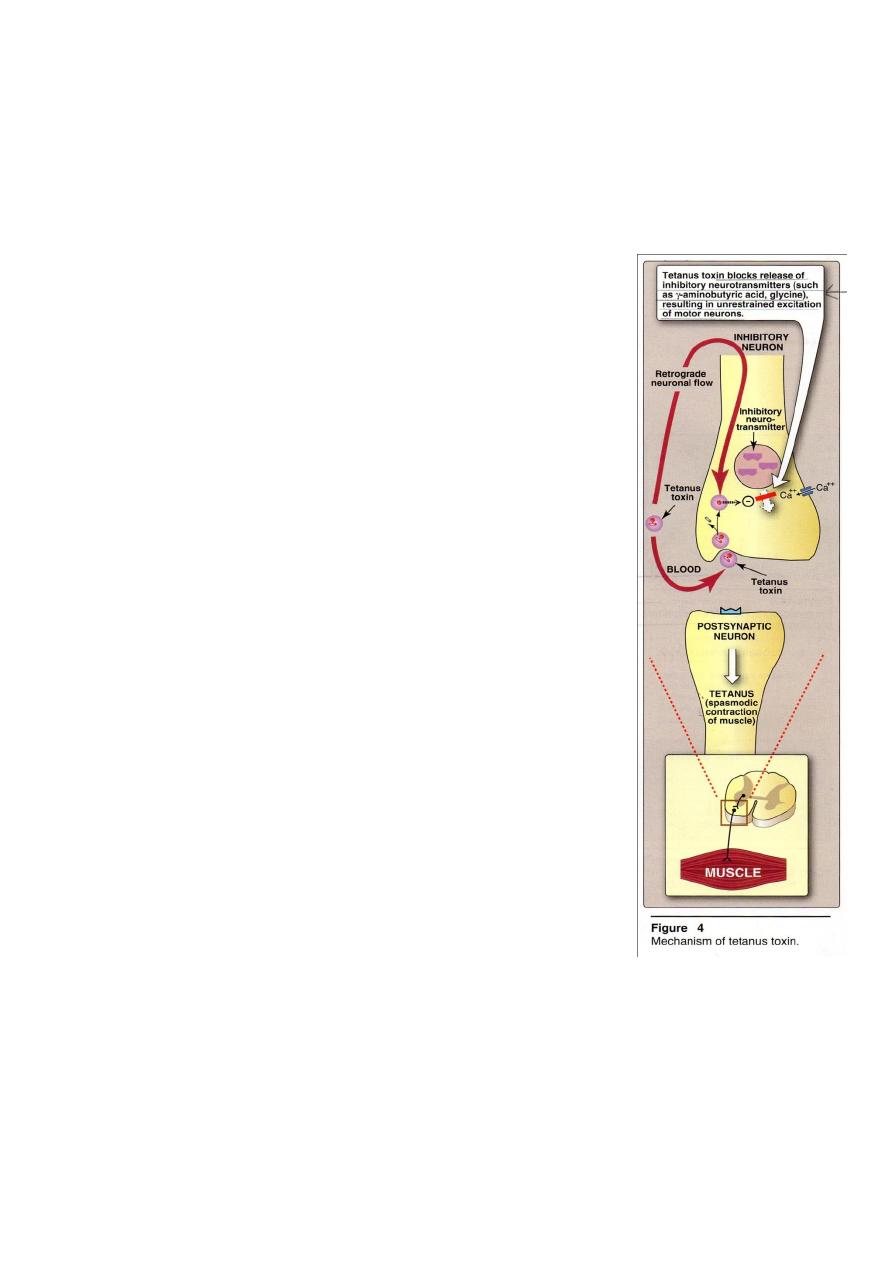
Pathogenesis
Tetanus toxin, called tetanospasmin, is transported from
an infected locus by neuronal flow or blood,consist of 2 chains
held together by a disulfide bond. The heavy fragment (B):
binding to neurons and cell penetration of the light fragment
(A). The (A) fragment: blocks neurotransmitter release at
inhibitory synapses and causes severe prolonged muscle
spasms (Figure 4).
Clinical Significance
1-Tetanus: an incubation period varying from 4-10 days to
several weeks. A shorter period is associated with more severe
disease and wounds closer to the brain .Tetanus presents as a
spastic paralysis, in which
(1) muscle spasms often involve the site of infection.
(2) the jaw muscles are affected, so that the mouth
cannot open (“lock jaw”)
(3) gradually, other muscles become involved and any external stimulus (e.g.
noise or bright light) precipitates a painful spasm and convulsions
(4) paralysis of chest muscles leading to respiratory failure, in young children
and olderly persons then death occurs in 15-60 % of cases
(5)in some cases, the spasm may be powerful and cause bones to break.
2-Umbilical tetanus: is of the umbilical cord of the infants born at home and treated
with unsterile instruments. Small amount of toxin is able to cause the disease because
it’s potent.

9
Antigenic structure
Ten types of Cl. tetani are differentiated by the “flagellar antigen”, but they
share the same "somatic antigen". Cl. tetani produces 3 types of toxins:
1-Hemolysin (Tetanolysis) is heat-labile and O
2
– labile, and is active against
R.B.Cs of animals
2-Neutrotoxin (Tetanospasmin) is heat-labile and O
2
– stable, can get toxoided
in presence of low concentration of formaldehyde. It’s antigenic and neutralized
by antitoxin.
3-Non-spasmogenic peripherally active neurotoxin.
Diagnostic laboratory tests
Diagnosis based largely on clinical findings because treatment must be
initiated immediately .
Microscopic examination =, G
+
rod, large long slender rod, noncapsulated,
motile.The spore is spherical terminal bulging as dram stick appearance .
Macroscopic examination = diagnosis by culture is more dependable. In
cooked meat broth → slightly proteolytic as black broth . On blood agar →
swarming growth
- hemolysin which later develops into
- hemolysin .
Biochemical reactions = indole + , catalase - , gelatin +
Animal pathogenicity : Mouses are used. Symptoms appear in 12 – 24 hours
then the animal dies with 2 days.
Treatment
Treatment must be initiated immediately .
1-administration of antitoxin(to neutralize any toxin in blood).
2-Treatment with human hyperimmune globulin (tetanus immune ) is preferred or
horse antitoxin is used, as toxoid to gives active immunization by stimulating the
antitoxins in the body.
3- give penicillin or tetracycline,large doses.
4-Remove dead tissue or foreign bodies from wounds is necessary and maintenance
of ventilation.
5-Antispasmatic drugs should be used in case of muscle spasm.
Prevention
1- Active immunization with tetanus toxiod ,administered a triple vaccine (DPT)
begin with infant 1-3 month old 3 doses/ weekly
1-4 years later.
2- immunizations with diphtheria + tetanus toxoids(Td) given every 10 years
throughout life are recommended( Ab levels gradually decline in older individuals, so
they lose protection).

10
3-Tetanus immunoglobulin (with human antitoxin) can be used to give immediate
passive immunity to injury victims with no history of immunization.
4-Antitoxin + toxoid, administered in different areas of the body, can be given
simultaneously.
5- Spores can be killed by autoclaving at 121
C for 20 min, 1% iodine solution and
H
2
O
2
kill spores within few minutes.
6-Neonatal umbilical cord tetanus can be prevented by activity immunization
pregnant females who have no previous immunization and the newborne infant will
then have natural passive immunity at time of birth to reduce the chance of
developing umbilical tetanus.
Clostridium difficile
Diarrhea, a common complication of antimicrobial drug treatment, can range
from loose stools to Pseudo Membranous Colitis (PMC) (Figure 1). C. difficile is
responsible for about one fourth of antibiotic associated diarrheas (AAD) in
hospitalized patients and all cases of PMC. After its introduction to a site the
environment
dust + bedding + toilets – becomes contaminated with spores which
are easily colonized and are a higher risk for developing the intestinal effect of
antibiotic treatments
Pathogenesis
C. difficile is a minor component of the normal flora of the large intestine,but
when antimicrobial treatment suppresses spps in this community, C. difficile
proliferates.
Pathogenic strains produce 2 toxic = Toxin A is an enterotoxin that causes
excessive fluid secretion, but also (cytotoxic activity) stimulates an inflammatory
response and has some cytopathic effect in tissue culture. Toxin B is a cytotoxin in
tissue culture, it disrupts protein synthesis and causes disorganization of the
cytoskeleton.
Clinical Significance
All antimicrobial drugs have been reported as predisposing to clostridial AAD
and colitis (Figure5) and the most commonly implicated are clindamycin,
amoxicillin, ampicillin, and the cephalosporins.
The severity of disease varies from mild diarrhea + inflammation of the large
intestine + PMC. The pseudomembranous exudate, composed of mucus, fibrin,
inflammatory cells and cell debris overlying an ulcerated epithelium, is best
demonstrated by endoscopy.

11
Laboratory Identification
Specimens = watery or bloody stools.
Microscopic examination = G+ rods, large blunt ended,noncapsulated motile
with spherical subterminal notbulging spores.
Macroscopic examination = identified by routine anaerobic procedures.
Serology tests= ELISA test for exotoxins A and B have largely replaced
cytotoxicity assays( immunologic tissue culture).
Treatment
1-Discontinuance of the predisposing drug.
2-fluid replacement.
3-Oral administration of metronidazole or vancomycin is added.

12
