1
True bacteria – Rods - Gram Negative Rods
Enteric Rods
Enterobacteriacease,Vibrio, Campylobacter,Helicobacter
Enterobacteriacease
General characters of Enterobacteriacease
(1) Numerous interrelated bacteria flora of intestine (GIT), some of them as normal
flora in the vagina (Klebsiella, Proteus)
(2) G
-
rods, non spore forming.
(3) Motile with peritrichate flagella, or non motile.
(4) Non acid fast.
(5) Ferment glucose with or without formation of gas.
(6) Nitrates + .
(7) Catalase + .
(8) Oxidase - .
(9) Facultative anaerobes, aerobes.
Classification of Enterobacteriacease
A- Based on action on lactose:- It’s an old method, practical value in diagnostic
bacteriology.
1- Lactose fermenter
e.g. Escherichia coli, Klebsiella,Citrobacter,Enterobacter
2- Late Lactose fermenter
e.g. Shigella sonnei, Serratia
3- Non Lactose fermentation
e.g. Proteus,Salmonella, Shigella , Hafnia
B- Modern taxonomical concept:-classified into tribes, genera and species by their
cultural and biochemical characters. The species are further classified into types,
biotypes, serotypes, bacteriophage type and colicin types. At present there are 5 tribes :
Tribe I: Escherichia
Tribe II:
Klebsielleae
Tribe III: Proteuae
Genus
Klebsiella
Genus
Escherichia
Enterobacter
Proteus
Edwardsiella
Hafina
Citrobacter
Serratia
Salmonella
Shigella
Tribe VI:
Erwinieae
Tribe V: Yersinae
Genus
Yersinia
Erwinia
2
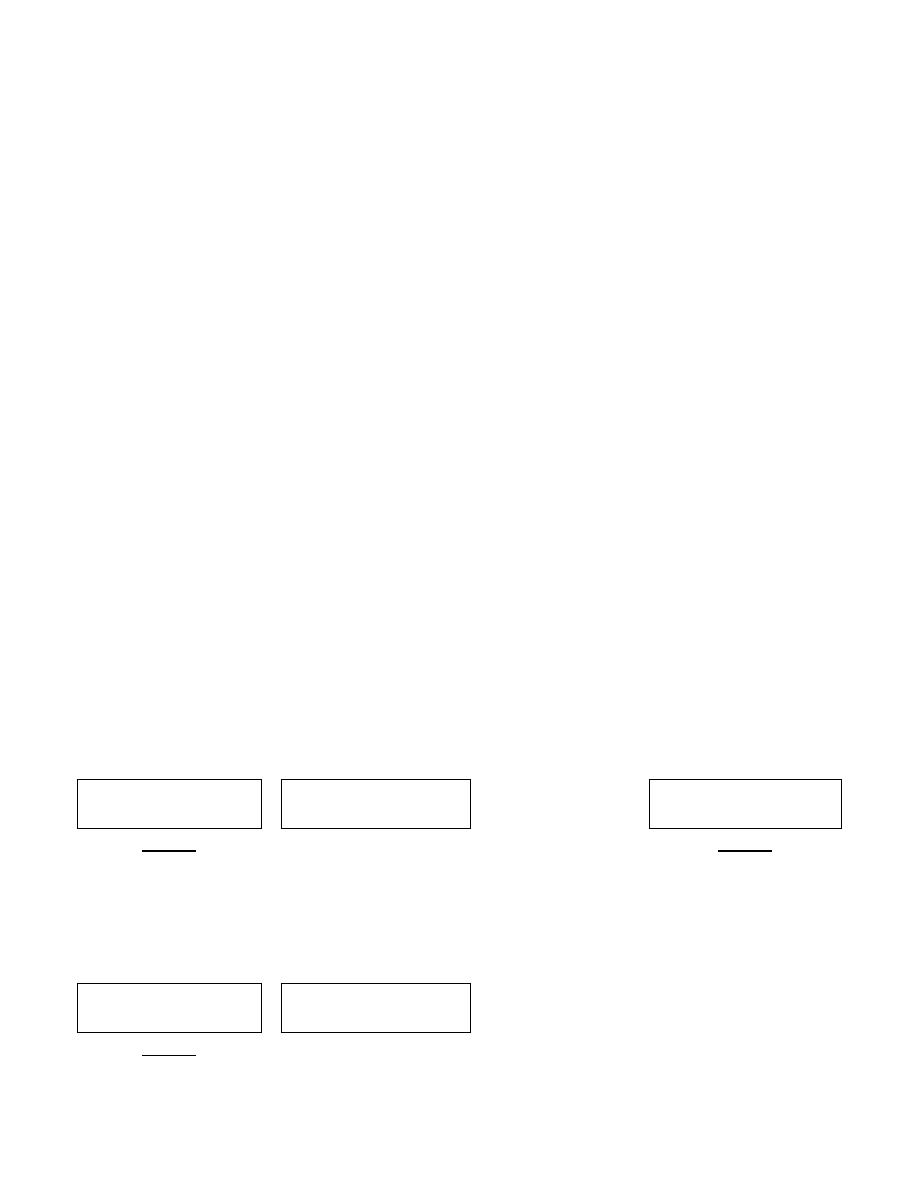
Escherichia coli
It lives only in human or animal intestine so detection of this genus in drinking
water is taken as evidence of recent pollution with human or animal excretation .
Antigenic structure
There are 4 types of antigens:
1- Somatic antigen (O antigen) = they are heat – stable, divided into 164 groups
designated as 1, 2, 3 ----
2- Surface antigen (K antigen) = they are heat-labile, divided into 3 types:
a. L antigen is destroyed by heating at 100
C for 1hs, and it’s capacity to combine
with antibodies is lost.
b. A antigen is capsular antigen and is heat-stable and is associated with well-
marked capsule.
c. B antigen is destroyed by heating at 100
C for 1hr, and it’s capacity to combine
with antibodies is present.
3- Flagellar antigen (H antigen) = they are heat – labile, about 75 types have been
described.
4- Fimbrial antigen (F antigen) = they are heat – labile and have no significance in
antigenic classification E. coli.
1
3
Toxins
E. coli produces endotoxin, and produces 2 types of exotoxins which are:
a) Enterotoxin which is heat labile and the mode of action is by raising level of cAMP
in the cells, causing excretion of fluid and electrolytes in the lumen of intestine.
The 2 types of E. coli enterotoxin have been:
1- Heat labile toxin LT: is of large molecular weight (80, 000) protein, which gets
inactivated by heating at 60
C for 10 mins and their action through mediaton of cAMP.
2- Heat stable toxin ST: is of low molecular weight (8000 – 8500) protein, which is not
destroyed by heating and their action through mediaton of cGMP (cyclic guanosine
monophosphate).
b) Hemolysin which may be
1- Heat labile and lethal for animals.
2-
Associated with bacterial cell .
Pathogenesis
A- Gastroenteritis : Summer diarrhea occurs in children during second and third
summer of life in non epidemic form. Lactic acid produced from lactose fermentation
may cause irritation of the colon result in violent nausea, vomiting and diarrhea. There
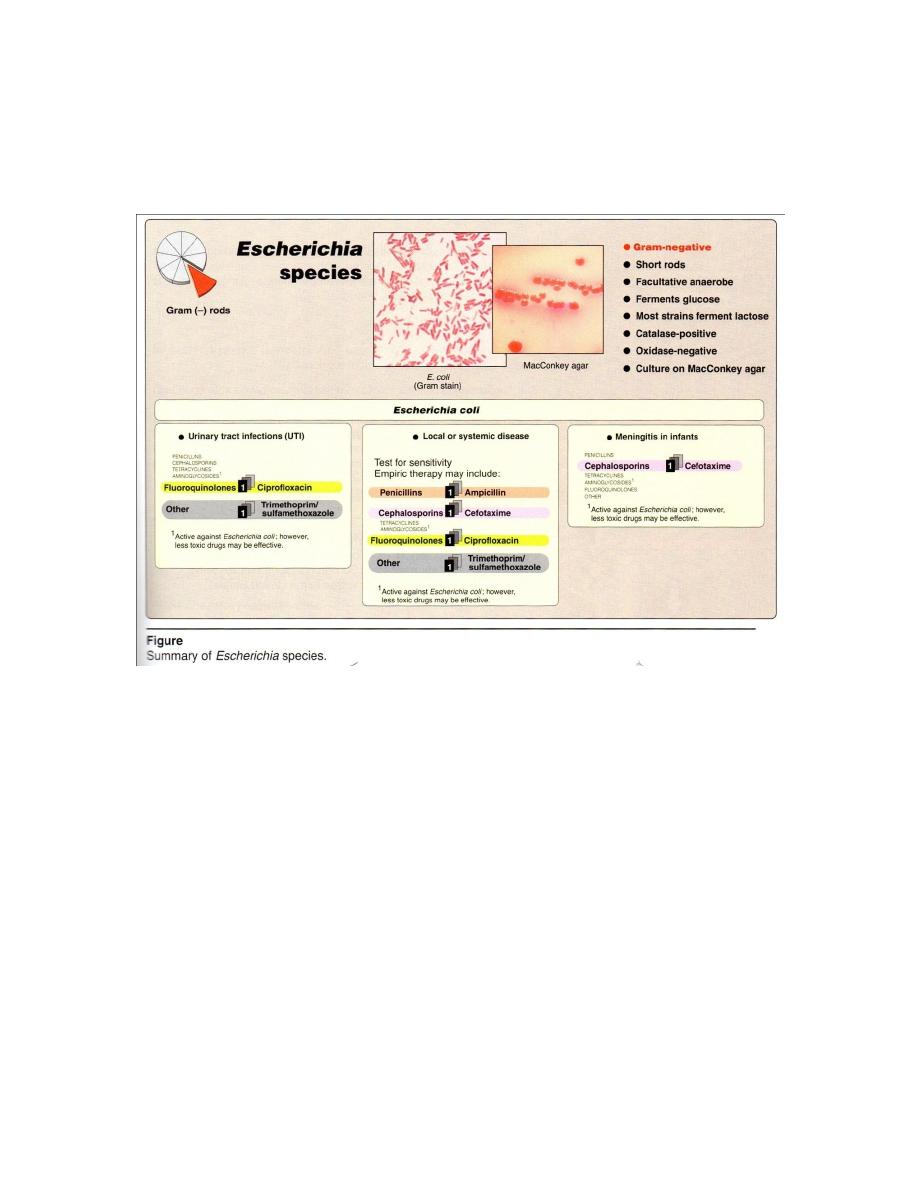
are 5 groups of E. coli, which causes diarrhea disease (Figure 2):
1- Enterotoxigenic E. coli also called ETEC: They produce heat labile and heat stable
enterotoxin and thus producing watery diarrhea in children and traveler’s diarrhea.
They posses surface properties called colonization factors which promote their
virulence, and this factors may be pilli or special types of protein K Ag. caused:
Mild or moderately sever childhood diarrhea in developing countries.
Cholera – life syndrome in adults living in areas where cholera is epidemic.
2

4
Traveler’s diarrhea in persons from developed country that visits developing
countries.
Outbreaks of diarrhea in newborn nurseries in developed countries.
Outbreaks of diarrhea due to fecal contamination of food and water in developing
countries.
2-Enteropathogenic E. coli also called EPEC: They are carrying B type of surface
(K Ag), caused watery diarrhea in babies less than 18 months old.The mechanism of
EPEC is there tight adherence to enterocytes resulting in the loss of microvilli and
cupping of enterocytes membrane to bacteria.
3- Enterohemorrhagic E. coli also called EHEC: there is no fever but haemorrhage is
marked(bloody diarrhea). EHEC produces a cytotoxin, it’s called verotoxin because it’s
effects on Vero cells in tissue culture.
4-Enteroinvasive E. coli also called EIEC: They invade intestinal epithelium like other
dysentery bacilli (bloody diarrhea),and it may be late Lac+ or Lac- and may be
anaerobic. They invasion of Hela cells in tissue culture .
5- Enteroadherent E. coli also called EAEC: cause watery diarrhea in children and in
patients infected with HIV.
B- Urinary tract infection (UTI) Infection may be precipitated by urinary obstruction
due to prostatic enlargement calculi and pregnancy. Strains carrying K Ag are
responsible for pyelonephritis while strains form cystitis lack K Ag. UTI causing by E.
coli binding to epithelial cells receptors by means of adhesion, such as uroepithial
adhesion assay has become one of the important parameters.
C- Pyogenic infection: e.g. wound infection, abscess, peritonitis, cholecystitis and
meningitis. It may cause septicemia.
D- Neonatal Meningitis: is a major cause of this disease occurring within the first
month of life. The K
1
Ag(capsular type, A type) is particularly associated with such
infections.
E. Nosocomial (hospital – acquired) infections: include sepsis, bacteremia, endotoxic
schock and pneumonia.
(The most common causes of neonatal meningitis
Group B Strep. + E. coli + Listeria.
The most common causes of neonatal sepsis with or without meningitis → Group B
Strep. + E. coli).
F- Septicemia: It’s one of the commonest causes of septicemia, causes fever,
hypotension, disseminated intravascular coagulation (endotoxin).Mortality is high.

5
Laboratory diagnosis
Haematological investigation: Total leukocytes count → within normal limits ,
but
in
tissue
invasion=
moderate
leukocytosis.
Differential
leukocyte
count → polymorphonuclear cells in tissue invasion.
Bacteriological investigation: Specimen → in UTI mid steam urine is collected
under aseptic condition examined for pus cell, RBC and bacteria. The count of the
organism should be more than 100000/ml in UTI, it’s called significant bacteriuria.
In acute diarrhea a sample of faces or a rectal swab is collected, pus may be
collected on sterile condition.
Microscopic examination : shows moderate to large numbers of pus cells and G
-
bacilli, motile and noncapsulated.
Macroscopic examination : culture in liquid broth → shows uniform turbidity . On
nutrient agar → colonies are circular 1-3 mm in diameter smooth, colourless, entire edge
with butyrous consistency and emulsified easily after 12 hs incubation. On MacConkey
agar (selective and differential media for all Enterobacteriaceae )→ colonies are pink
due to lactose fermentation. . EIEC strains often Lac- and may be detected on
MacConkey media ,while EHEC, unlike other strains of E coli, ferment sorbitol slowly
if at all, and may be detected on MacConkey sorbitol agar. On blood agar, some strains
may show
-hemolysis.
Biochemical tests: ferments Lactose, glucose, sucrose, maltose and mannitol
forming acid and gas. IMVC show + + - -, urease - , H
2
S – and nitrates +.
Serological tests → ELISA, Precipitin test, radioimmunoassay are other useful
tests to identity EPEC strains. Treatment of faecal matter with fluorescent – labeled O
group antisera is useful for early diagnosis of diarrhea.
DNA probes for different EPEC forms are quite reliable and useful.
It can survive for months in soil and water, it killed at 60
C in 20 minutes and
chloride(0.5 -1 part/million).
Treatment and Prevention
Treatment by:
(1)maintenance of fluid and electrolyte balance .
(2)antibiotics sensitivity testing is necessary to determine the appropriate choice
of drugs (Figure 1). It’s sensitive to sulfonamides, trimethoprim, tetracyclines,
chloramphenicol and aminoglycosides.
(3) Nitrofurantoin + nalidixic acid may be useful for treating UTI.
(4) In septicemia or serious infection is required to be started treated immediately
without waiting for drug sensitivity tests as gentamicin.

6
(5)Preparation of vaccine against E.coli colonization factor Ag (CFA) is being
considered( CFAII, IV and I).
Prevention by care in selection, preparation and consumption of food and water.
Klebsiella
It’s found in the mucosa of upper respiratory tract, intestine, and genitourinary tract.
Its G- rod short, thick, large ,non motile , capsulated. It’s growing on ordinary media
forming large mucoid colonies of varying degree of stickiness. They are Lac
+
on
MacConkey agar. Ferment carbohydrate forming acid and gas↑. IMVC are - - + +,
Urease +. Most spps are pathogenic and classification depends on biochemical
reactions which vary depends on capsular type, like spps below:
Klebsiella pneumonia
It causes labor pneumonia, sinusitis, otitis media, meningitis, and urinary tract
infections and bacteremia particularly in hospitalized patients.
K. pneumoniae and K. oxytoca cause necrotizing labor pneumonia in
individuals compromised by alcoholism, diabetes, or chronic obstructive pulmonary
disease.
Klebsiella ozaenae
It causes foul smelling nasal discharge (ozaena).Other strains cause
granulomatous lesion of nose in Mediterranean countries.
Klebsiella rhinosclermatis
Cause rhinoscleroma disease.
K. erogenes
Usually present in human intestine and isolated from faeces in small number than
E coli, so many survive longer out of intestine.
K. cloasa
It differs from other spps above in motility +, not always capsulated, liquefied
gelatin.
Citrobacter
It occurs as intestinal commensal in man. G-rods, motile, noncapsulated. Lac+,
citrate +, H
2
S+ . It has been isolated from enteric fever cases, and it cause UTI, gall
bladder infection and meningitis.

7
Enterobacter
It is G- rods, motile, noncapsulated. Lac+, IMVC are - - + +, gelatin+. It found
in human and animal faces and in soil. They rarely cause disease in humans, but
frequently colonize hospitalized patients especially in association with antibiotic
treatment in dwelling catheters, or invasive procedures. It may infect burns,wounds
and the respiratory tract (causing pneumonia) or urinary tracts.
Serratia
It is G-rods, motile, noncapsulated. Late Lac+. It can cause extraintestinal
infections such as those of the lower respiratory and urinary tracts especially among
hospitalized patients, its opportunistic pathogen. It has been isolated from cases of
meningitis, endocarditis, septicemia and respiratory infection.
Proteus
Antigenic structure
A number of O and H antigen are produced in P. vulgaris .
Pathogenecity
UTI, pyogenic lesions like abscess, wound infection, respiratory tract infections,
diarrhea by P. morgani. P. spps common causes of uncomplicated as well as nosocomial
UTIs. Other extraintestinal infections such as wound infection, pneumonias, and
septicemias are associated with comporomised patients .
Laboratory diagnosis
Hematological investigation → leukocytosis with increase in PMN cells .
Specimens → urine, pus, sputum … etc
Microscopic examination : G
-
rods showing great variation in size, actively
motile , non capsulated.
Macroscopic examination : In broth media → shows uniform and moderate
turbidity, powdery deposit and ammonical odor after 18-24 hs.
On MacConkey agar → shows Lac
-
with fishing or bad odor. On nutrient agar
→ shows swarming motility best seen at 20
C, while P. vulgaris and P. mirabilis
swarm on solid media at 37
C after 12-18hs incubation. Swarming may be due to
progressive surface growth spreading from the edge of parent colony. This phenomena
does not occur on MacConkey agar.

8
Biochemical reactions of P. spps are:
Glucose
Lactose
Urease
H
2
S
I M V C
P. vulgaris
+
-
+
+
+ + - +d
P. mirabilis
+
-
+
+
- + -
P. reltegri
±
-
+
-
+ + - +
P. morgani
+d
-
+
-
+ + - -
It forms acid and gas from glucose (except P. reltegri). Deaminates
phenylalanine to phenyl pyruvic acid. urease + in few minutes. All of the them
nitrate +, Lactose –, MV are + - , H
2
S + only in P. vulgaris and P. mirabilis, Indole +
(except
P. mirabilis), Citrate + (except P. morgani),see the table above.
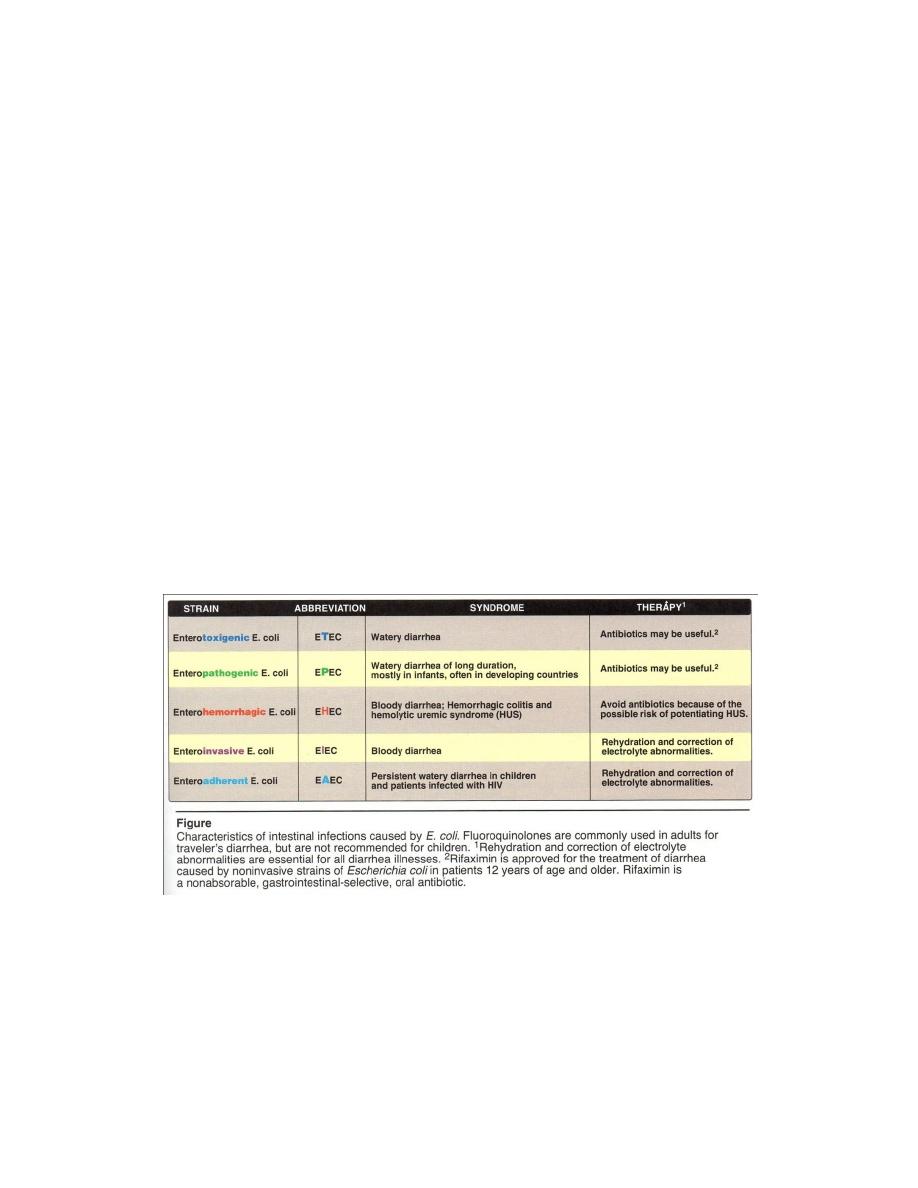
Salmonella
It’s found in intestine (man, animal , bird) and in contaminated food (egg and
meat) .Currently, all strains are grouped in a single spp: S. enterica which including the
clinically significant serotypes S. typhimurium and S. typhi.
Antigentic structure
1- Somatic antigen (O – Ag)
It’s phospholipid protein – polysaccharides complex,
stable by boiling ,alcohol and acid treatment. O - agglutination take place more
slowly, it’s less immunogenic, titer of O antibodies after infection or immunization
is lower than H – Ag.
2- Flagellar antigen (H- Ag)
is heat labile protein, destroyed by boiling, alcohol but
not by formaldehyde, it’s strongly immunogenic. H-Ag occurring in 2 phases:
phase I is specific designated as a, b, c, z, z
2
…etc
phase II is no specific designated as 1, 2, 3 or complex of e, n and x.
On the basis of somatic antigen (O-Ag), S. can be divided into 65 serogroups, each
group is designated as A, B, C and D .Species among each subgroup are recognized by
specific H-Ag (phases I and II).
3- Surface antigen(Vi antigen)
It’s labile antigen in agglutinable with O antiserum
because agglutinable after boiling or heating at 60
C for 1 h. It’s virulent to mice and
Vi Ab may provide protection, the resistance of Vi Ab indicates carrier state. It’s
present in S.typhi, S.paratyphi and S.dublin, and Citrobacter.

9
Epidemiology
S. typhi is human pathogen, whereas other strains are associated with animals and
foods (egg and poultry). Fecal/oral transmission occurs and may involve chronic
carriers. Young children ,elderly and individuals in crowded institutions are particularly
susceptible to S. infection .
Pathogenesis and clinical significance
Disease may be remain localized or become
systemic or disseminated. S. invade epithelial cells of the
small intestine , survive in phagocytic cell (Figure 1) . S.
infection can cause both intestinal and extraintestinal
disease (typoidal Salmonella). So it may produce 3 types
of lesion :
1- Enteric fever: it’s caused by S.typi 85% in India
S.paratyphi A (15-21%) and S.paratyphi B.
Infection is through ingestion, the bacilli may enter
the body through lymphoid of pharynx. In the gut, S.
attached with epithelial cells of intestinal villi and
penetrate lamina proprira and submucosa, they are
phagocytes by PMN or macrophages enter mesenteric
lymph nodes to multiply there
enter thoracic duct and
blood stream
bacteremia and S. are seeded in liver,
gall bladder, spleen bone marrow, lymph node, lung and
kidneys. In these organs further multiplication occur, there
are bacteremia and shows clinical symptoms.
Also S. invade tissue e.g. Payer’s patches and
lymphoid follicles of small intestine, the intestinal lesion
ulcerates and hemorrhage may occur.
S. produces endotoxin which produces toxic
symptoms like headache, anorexia, continuous fever and
congestion of mucus membrane. Incubation period is 10-
14 days the typical features are step ladder pyrexia,
palpable spleen and rose spots that fadeon pressure and
they appear in 2
nd
– 3
rd
weeks of infection, S.paratyphi A +
S. paratyphi B cause paratyphoid fever resembling enteric
fever.
10
2- Septicemia
It’s caused by S. cholera suis. It produce chills and spiked fever, local
lesions occur in various parts of the body producing osteomyelitis, pneumonia,
pulmonary abscess and meningitis … etc. The bowel is not invaded and fecal culture
is –ve.
3- Food poising
It by ingestion of contaminated food (e.g. meat and egg), caused by
S. typhimaurium, S. enteritides, S. newports etc … lncubation period is 12-48 hs. There
is fever, vomiting, diarrhea (mucous and blood in stool) there may be ulceration of
intestinal mucosa. There is no bacteremia.
Laboratory diagnosis
Specimens
:
For diarrhea = stools. For enteric fever = blood, bone marrow, urine,
stool, and tissue from typical rose spots, some cases from CSF.
Hematological investigation : Total leukocytes count in typhoid fever = leucopenia.
Differential leukocytes count = lymphocytosis and monocytosis.
Microscopic examination : G
-
rod, motile (except S.gallinarum and S.pullorm),
non capsulated, may have fimbriae (Figure 2).
Macroscopic examination : In broth media
shows uniform turbidity ,no
pellicle formation. Selenite F and tetrathionate
these broths are commonly used as
enrichment transport media.

11
On MacConkey agar (M.A.) and Deoxycholate citrate agar(D.C.A.)
Lac
-
colourless colonies (Figure 2). On Salmonella Shigella Agar (S.S.A)
colonies with
black center(while Shigella colonies without black center). Bismuth Sulphite
Agar(B.S.A.)
black colonies with metallic sheen dye ( H
2
S formation may appear in
colonies) S. paratypi A produce green colonies. On Blood agar
large colonies,
circular low convex, translucent, smooth and non – hemolytic (
- hemolytic).
Biochemical reactions: ferments glucose (acid + gas), Lac-, ferments mannitol +
maltose (acid + gas)(except S. typhi, which produce only acid no gas) I M V C
-+ - +
, urease - , H
2
S+
It’s can survive in ice, snow and water for months together, and resistante to
brilliant green ,malachite green ,bile salts , tetrathionate and selenium salt. It may be
killed by heating at 60
C for 15-20 min, pasteurization, boiling and chlorination.
Macroscopic examination steps :
1- Blood culture
Its + (5-10 ml blood of patient is collected and is transferred into
100 ml brain heart broth bottle then subculture to blood agar and MacConkey agar).
2- Clot culture
Its + (blood clot is cultured in 15 ml bile broth bottle (0.5% bile salt)).
3- Fecal culture
It’s + throughout, repeated cultures are required . It’s more useful in
cases that are on chloramphenicol. Plated on M.A. , D.C.A. and B.S.A.
4- Urine culture
It’s useful than blood and feces culture, it’s + ve in 2
nd
-3
rd
weeks.
Urine is used after centrifuged and sediments are inoculated into enriched and selective
media.
5- Other material =culture is + as bone marrow , rose sposts, pus from lesions, CSF,
sputum . Autopsy culture may be obtained from gall bladder, liver, spleen, mesenteric
lymph nodes.
6- Bile culture
It’s important for the detection of carriers and later stages of disease,
bile aspirated by duodenal tube is processed like fecal specimen.
Serological test : S. Abs appear at the end of first week, widal test is used to
measure H and O Abs in the sera of patients. Typhoid carrier may be detected as
follows:
1. Widal test may show raised Abs titer. 1 / 160 , 1 / 320 , 1 / 640 .
2. Vi agglutination test is + in a titer of 1/10 or more.
3. Several stool cultures may help .
4. Bile culture ,S. may be cultured from bile obtained after duodenal incubation.

12
Treatment and prevention
Antibiotics like chloramphenicol, flurazolidone, ampicillin trimethoprim
sulfamethoxazole, amoxicillin, cephalosporins, ceftriaxone, fluoroquinolones and
ciprofloxacin are used for enteric fever.
For gastroenteritis in uncompromised hosts, antibiotics therapy is not needed, and
may prolong the convalescent carrier state, which used cholecystectomy as treatment.
Prevention by proper sewage disposal, correct handling of food and good personal
hygiene.
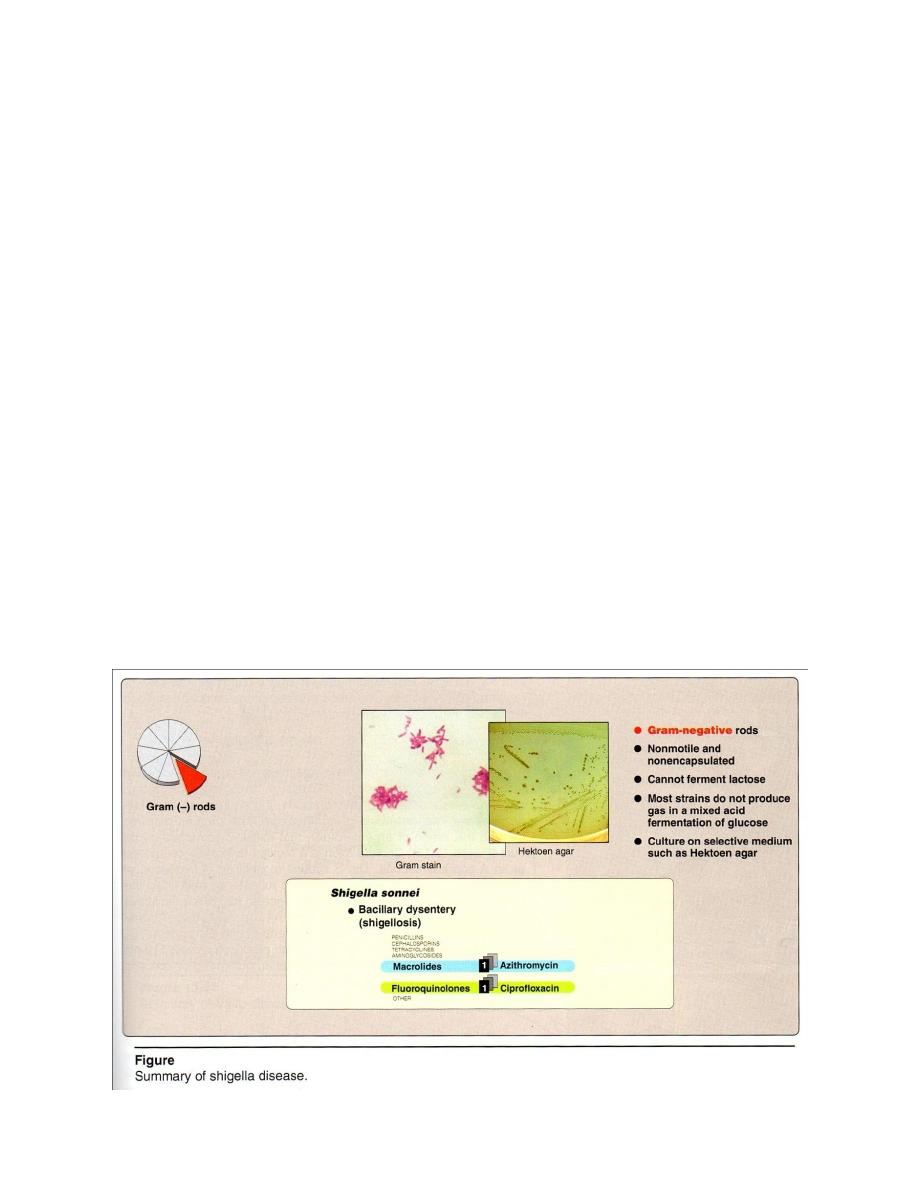
Shigella
Causes shigellosis (basillary dysentery) – a human intestinal disease that occurs
commonly among young children.
Epidemiology
It typically spread from 1 person to person with contaminated stools, serving as a
major source of organism. 2 Flies and contamined food or water . 3 crowding conditions
or poor sanitation ( due to low infectious dose fewer than 200 viable organisms ). The
forty serotypes of Shigella are organized into 4 groups (A, B, C, D) based on their
polysaccharide O-Ag. Group D (S. sonnei) is the serogroup found most commonly in
U.S.
Pathogenesis and clinical significance
It’s invade and destroy the mucosa of the large intestine. Infection rarely
penetrates to deeper layers of the intestine, and does not lead to bacteremia (Figure 1).
1
13
An exotoxin with enterotoxic and cytotoxic properties has been isolated from
these organisms, and it’s toxicity may play a secondary role in development of intestinal
lesions.
It’s cause classic dysentery, characterized by bloody diarrhea , mucus and
painful abdominal cramping. The disease is most severe in the very young and elderly
individuals,in whom shigellosis may lead to severe dehydration and some time death.
Laboratory identification
Microscopic examination : G- rod, non motile , noncapsulated(Figure 2) .
Macroscopic examination : Cultured from stools using differential selective media
like Hektoen agar , or media specific for intestinal pathogens such as MacConkey’s
agar or Deoxycholate citrate agar ( DCA) on which they form colorless colonies due to
Lac - , only S. sonnei give pale pink colonies which ferment lactose late( Late Lac+)
on both media . On Salmonella Shigella Agar,colorless colonies without black center.
Biochemical reactions → glucose + acid only , nitrate +, Lac
-
(except S. sonnei),
I M VC are + + - - (like E coli), H
2
S -,uerase-, Catalase +, mannitol +.
Treatment and prevention
Antibiotics (ciprofloxacin, azithromycin) can used.
Prevention by
protection of the water and food supply and personal hygiene are
important (Figure 2) .
2
14
Hafina
It is also intestinal commensal. G-rods, motile, noncapsulated. Lac- . IMVC are
- - + +.
Note:
Klebsiella , Enterobacter, Proteus , Serratia are opportunistic pathogen,so
sensitivity test necessary to determine the appropriate antibiotics.
VIBRIOS
They are closely related to the family Enterobacteriaceae.
Antigentic structure
O and H Ags are both present, but only O Ags are useful in distinguishing
epidemic cholera. Pathogenic Vibrios include :
(1) V. cholerae, serogroup O1 strains that are associated with epidemic cholera.
(2) non-O
1
V.cholerae and related strains that cause sporadic cases of cholera-like and
other illness, is resemble to V. cholera in morphologically and biochemically, not
agglutinable with O antiserum of V. cholera .
(3) V. parahaemolyticus and other halophilic vibrios, which cause gasteroenteritis and
extraintestinal infections, also causes food poisoning .
Epidemiology
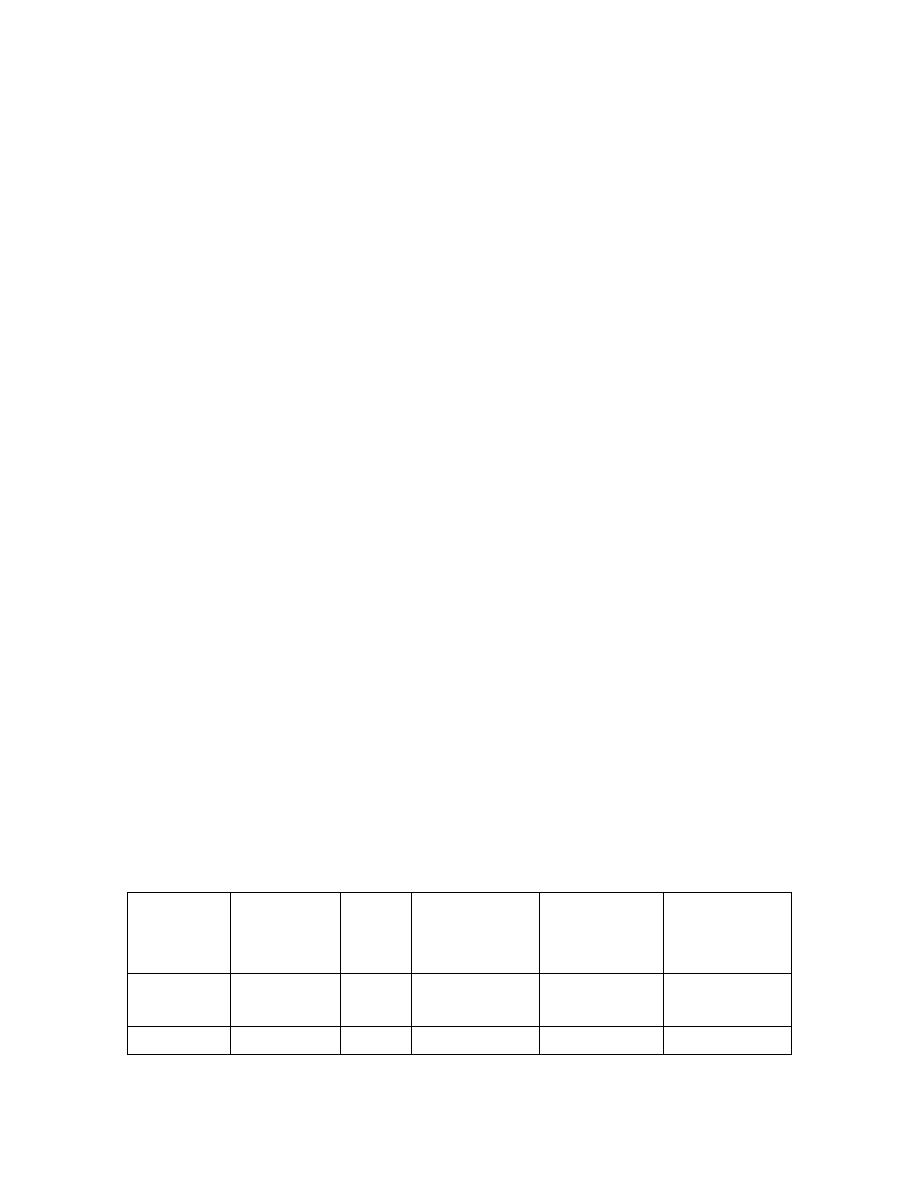
V. choleraeis transmitted by contaminated water and food. There are 2 biotypes
(subdivisions) of V. cholerae spps : classic and El Tor. The El Tor strain, in contrast to
classic strains, is distinguished by the production of hemolysin, higher carriage rates,
and the ability to survive in water for long periods. (see the table below) .
Hemolysis
Vp
Chick
erythrocyte
agglutination
Polymyxin
B-sensitivity
Group IV
phage
susceptibility
Classical
cholera
-
-
-
+
+
El Tor
+
+
+
-
-
15
Vibro El Tor was the cause of epidemics of V. in south east Asian, and is related to
people with blood group O. Outbreak of both strains have been associated with raw
or undercooked seafood harvested from contaminated waters.
Pathogenesis
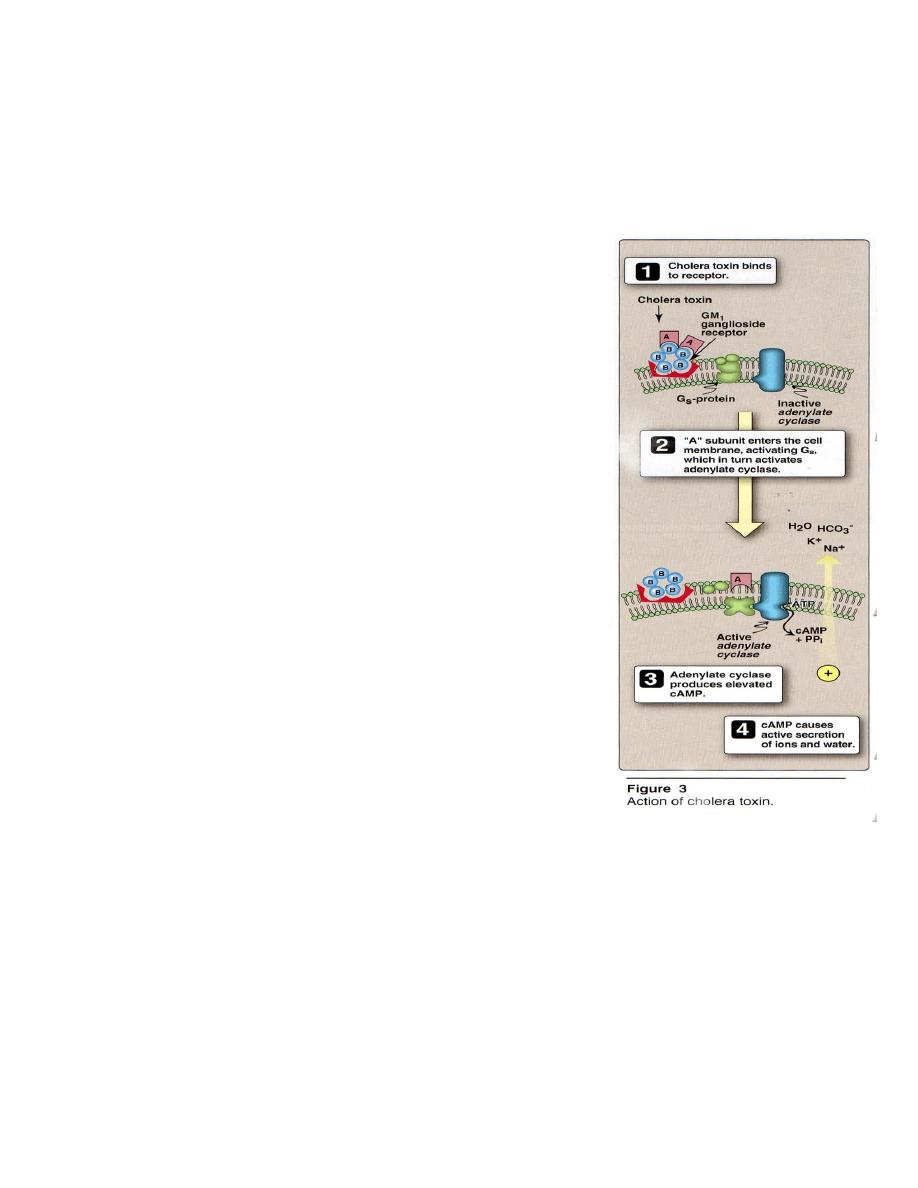
Following ingestion, V. cholera infects the small
intestine. Adhesion factor(s) are important for colonization
and virulence. V. are noninvasive and causes disease
through the action of an enterotoxin (Figure 3), which is a
multimeric protein composed of an A and a B subunit. The
B subunit (consisting of 5 identical monomers) binds to the
receptor of cells lining the intestine. The A subunit has 2
components: A
2
, which facilitates penetration of the cell
membrane, and A
1
, which actives adenylate cyclase
produces elevated levels of cAMP, causes secretion of ions
and water to the lumen of the intestine.
Clinical significance
Cholera is characterized by massive loss of fluid and
electrolytes from the body. After an incubation period
ranging from hours-few days, show watery diarrhea (rice-
water stools) beings. Untreated, death from severe
dehydration causing hypovolemic shock may occur in hours
to days, and the death rate may exceed 50%.
Non-O1 V. cholera and other non-halophilic Vibrios
causes sporadic cases of cholera indistinguishable from that
caused by V. cholera serotype O1. They also cause milder
illness comparable to that caused by enterotoxigenic E coli
(ETEC).
Laboratory identification
Hematological investigation
:
It’s not diagnostically significant in early stages.
However, there may be increase in, packed cell volume PCV and Hb.
Specimens : stools is may be collected immediately or if delay transport special
media used (Venkat Raman medium, VR) ,in this medium V. remain viable for weeks
but do not multiply. In case of rectal swab, trypticase taurocholate tellurite broth (pH
9.2) or alkaline peptone water broth is used.
16
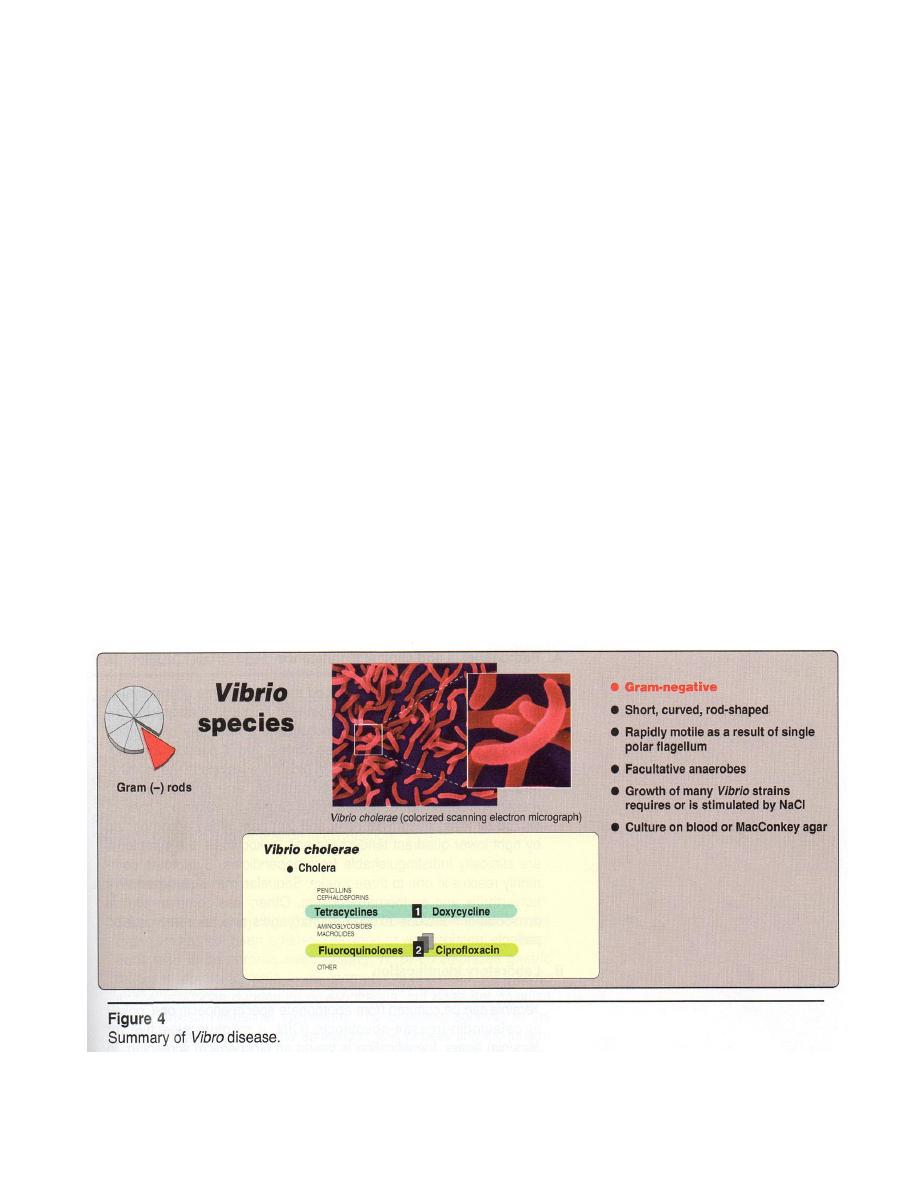
Microscopic examination : G - rod thin, short, curved comma –shaped , occur
singly or as S-shape semicircular pairs, rapidly motile by a single polar flagellum (this
contrasts with the peritrichous flagella of the motile Enterobacteriacea) noncapsulated .
Macroscopic examination : The growth of many Vibrio strains either requires
or stimulated by NaCl (halophilic bacteria) . On B.A
- hemolytic
then
-
hemolytic later. Specimen is inoculated in Mansur’s media. Thiosulfate- Citrate-Bile
salts- Sucrose (TCBS) medium can enhance isolation.
Biochemical reactions : Lac - Oxidase +, nitrate +, glucose, sucrose, mannose,
arabinose + acid only . Indole +, gelatin +, Urease - .
Cholera red reaction
+ (adding H
2
SO
4
to culture of V. cholera in peptone
water broth
red pink colour due to formation of nitrosoindole) .
Serological investigation : it’s little useful as slide agglutination with O group .
Treatment and prevention:
1 Patients with suspected cholera need to be treated prior to laboratory
confirmation because death can occur within hours.
2 Replacement of fluids and electrolytes in preventing shock.
3 Antibiotics as doxycycline, tetracycline (figure 4).
Prevention needs good personal hygiene + good cooking of foods + Vaccine E1
Tor type vaccine offers protection about 90-100% lasts for 3 years.

17
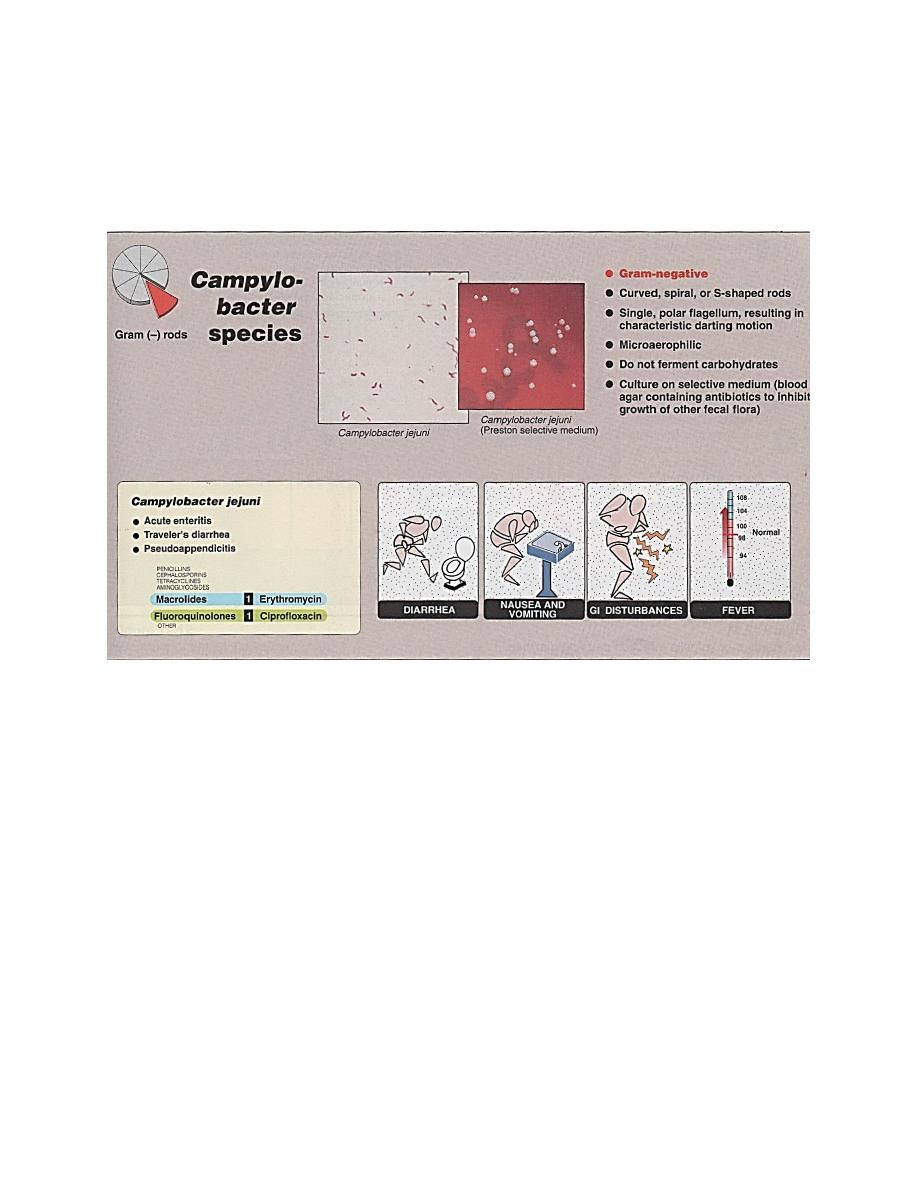
Campylobacter
It is a zoonosis disease (animal→human) ,was known by Vibrio fetus discussed
with Vibrios ,but recently differently (its Guanin≡Cytocin ratio lower than Vibro) led to
new group named Campylobacter (GC ratio 30-35% while Vibro 40-53%). Many strains
like C. jejuni, C.fetus, C.coli.
Epidemiology
C. is as commensals of many different vertebrate spps, including mammals and
fowl, both wild and domestic, serve as reservoirs of infection. C. is transmitted to
humans by:1-The fecal/oral route through direct contact.
2-Exposure to contaminated meat (poultry).
3-Contaminated water supplies.
Pathogenesis and clinical significance
Its infect the intestine and can cause ulcerative,
inflammatory lesions in the jejunum, ileum or colon
produce heat-labile exotoxin .So may cause both
intestinal and extraintestinal disease. C.jejuni cause:
1.Acute enteritis following 1-7 days incubation
(figure 1), lasts days to several weeks and generally is
self-limiting. Symptoms may be both systemic (fever,
headache, myalgia) + intestinal (abdominal cramping,
diarrhea may or may not be bloody).
2.Travelers diarrhea.
3. Pseudoappendicitis (appendicitis without
inflammation).
Fig 1 Forms of f bacterial food poisoning.

18
4.Bacterima in infants and the elderly (in host compromise). Complication include:
septic abortion, reactive arthritis, Guillain-Barre syndrome.
C. fetus cause endovascular infection and C. N. S. infection.
Laboratory identification
Specimens : feces using selective media and microaerophilic conditions. Because
of their small size, they are not retained by bacteriologic filters that hold back other
bacteria, so filtration of the fecal suspension may enhance recovery rate.
Microscopic examination : G- rod, curved spiral or S-shaped organisms
resemble vibrios, but it does not requires NACL in their growth . Its motile with a
single polar flagellum provides the organism with its characteristic darting motility.
Macroscopic examination : microaerophilic (require lower concentrations of
O
2
), use a respiratory pathway and do not ferment carbohydrates they require 3-6%
O
2
, 10% Co
2
hydrogen and nitrogen, need differential media like blood agar containing
antibiotics to inhibit growth of other fecal flora (like vancomycin, trimethoprim,
cycloheximde, polymyxin B) → γ-hemolysis.
Biochemical tests : H2S +, DNA hydrolysis to differentiate spps, hypopiruate
hydrolysis, oxidase +, catalase±, nitrate+, urease-.
Serological tests : Somatic, flagellar and capsular antigens all contribute to the
numerous serotype. Serotyping with O Ag by Indirect hemagglutination test , Cell wall
composition and Phage typing (bacteriocin typing).
Treatment and prevention
Using electrolyte and fluid replacement for diarrhea. For severe symptoms (high
fever, bloody diarrhea) antibiotics should be administered (figure 2). For C.jejuni
ciprofloxacin or erythromycin is used. For C.fetus ampicillin or third generation
cephalosporin for 4 weeks in C.N.S infection and for endovascular infection requires 4
weeks treatment with gentamycin.
19
Prevention by cooking of contaminated foods (poultry) + pasteurization of milk
and milk products is essential + surfaces used to prepare raw meat or poultry should be
disinfected before using them for uncooked foods, such as salads.
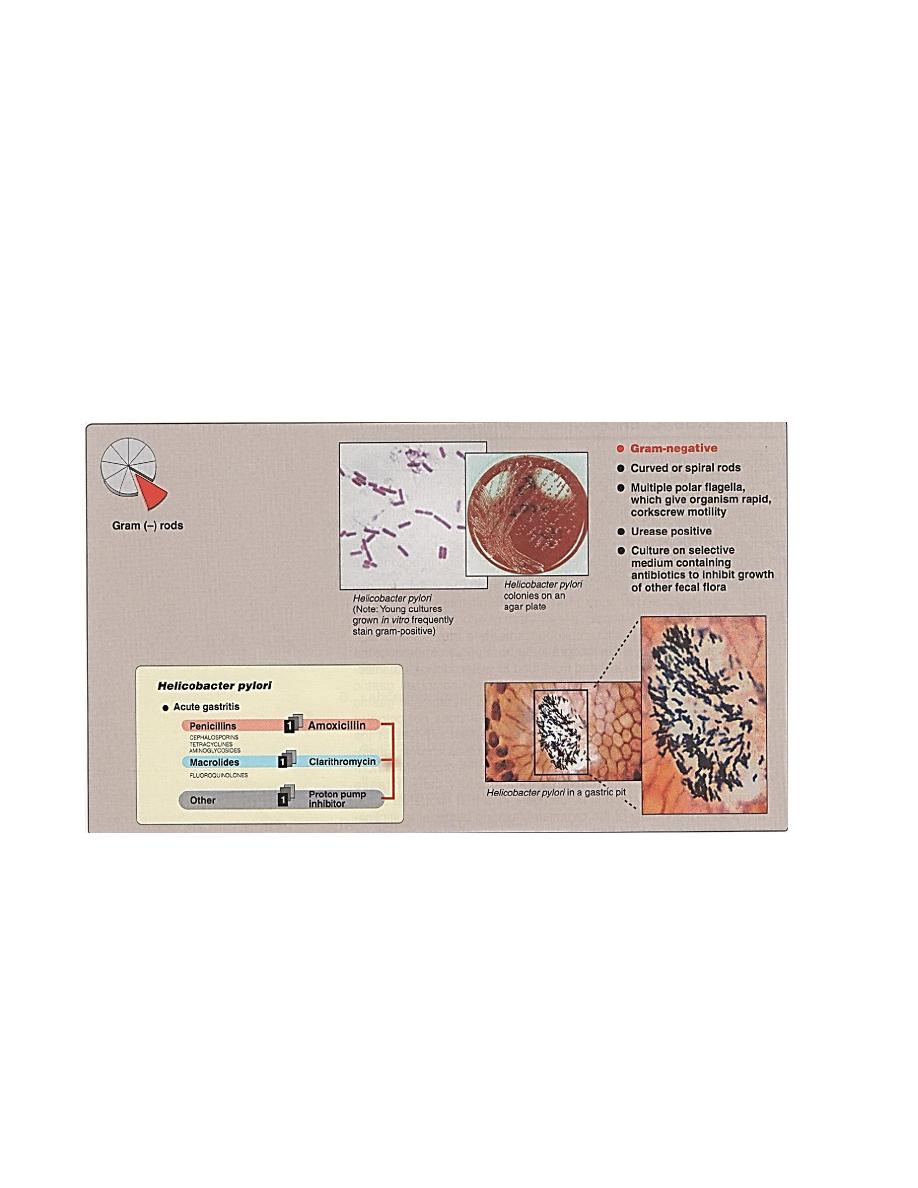
Helicobacter
Previously known by Campylobacter pylori ,recently it has been excluded from
C.geneus and has been renamed H.pyloridis because of these characters:
1- Bears multiple unipolar flagella,as corkscrew motion.
2- Produce large quantity of urease.
3- Has smooth cell surface.
4- Cell wall fatty acid composition
5- rRNA sequencing and DNA base composition is differ
Figure 2 Summary of Campylobacter disease.
20
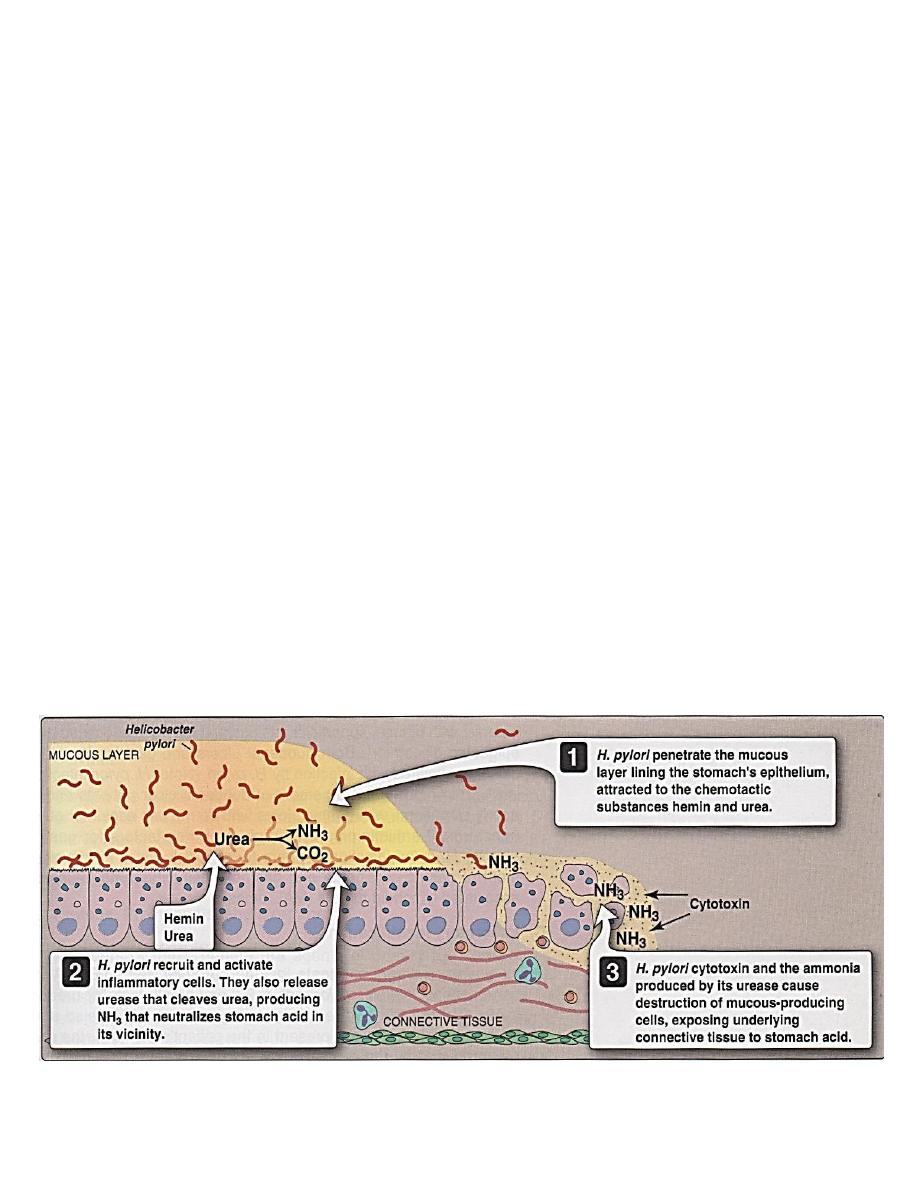
Toxins and enzymes production
Toxins and enzymes produced by H. may account for mucosal injury in absence
of actual bacterial invasion, are:
1- Protease with endopeptiase causing degradation of gastric mucin.
2- Cytotoxin factor causing intracellular vaculation.
3- Urease production responsible for metabolism of urea to ammonia and CO
2
,causing gastric mucosal damage. This ammonia produced may →:
A. Directly toxic to epithelial cells (see figure 4).
B. Indirectly cause tissue injury by allowing hydrogen ion back diffusion.
Pathogenesis
H. spps are unusual in their ability to colonize the stomach where low ph
normally protects against bacterial infection.
Transmission of H. pylori is thought to be from person to person, the H. has not
been isolated from food or water. Untreated infections tend to be chronic, even
lifelong.
Figure 3
Summary of Helicobacter disease.
21
It colonize gastric mucosal (epithelial) cells in the stomach and metaplastic gastric
epithelium in the duodenum or esophagus but does not colonize the rest of the intestinal
epithelium. It survives in the mucosa layer causes chronic inflammation of the mucosa
(figure 4), its noninvasive, recruits and actives inflammatory cells. Urease released by
H. pylori→ NH3 that
(1)
neutralize stomach acid in the vicinity of H.→ Multiplication
of H.
(2)
both cause injury and potentiate the effects of a cytotoxic produced by H.→
destruction of mucous-producing cells, exposing underlying connective tissue to
stomach acid→ ulceration.
Clinical significance
Initial infection with H. pylori causes acute gastric, sometime with diarrhea lasts
about 1 week, then become chronic, with diffuse, superficial gastritis associated with
epigastric discomfort. Both duodenal ulcers and gastric ulcers are closely correlated
with infection by H. pylori (in 95% of duodenal ulcer + in all patients with gastric
ulcers, who do not use aspirin or other nonsteroidal antiflammatory drugs,both a risk
factors for gastric ulcers). H. pylori infection appears to be a risk factor for development
of gastric carcinoma and gastric bacteria-cell lymphoma [mucosa-associated
lymphoid tumors (MALTmas)].
Figure 4 Helicobacter pylori infection, resulting in ulceration of the stomach

22
Laboratory Identification
A-Noninvasive diagnostic tests include :
1- Serologic tests→ ELISA for serum Abs to H. pylori.
2- Breath tests for urease→ administering radioactively label urea by mouth, if H.
present in patients stomach the urease produced split the urea→
NH
3
+Co
2
↑(radioactively labeled and exhaled).
B-Invasive diagnostic tests include:
Gastric biopsy specimens by endoscopy, also to detect H. histologically.
bacteriologically. Microscopic examination : G- curved or spiral rods (figure 3),
corkscrew motility resulting from multiple polar flagella.
Macroscopic examination : microaerophilic (10% Co
2
), need addition of blood
or other animal fluids to better growth. On blood agar → 36hr incubation show fine
colonies reaching maximum size in 2-4 days, colonies circular, cloudy, glistening with
entirc edge, butyrous consistency, slightly bluish greytinge. Selective media → Brewer’s
sodium thioglycolate, Skirrow’s medium, Butzler medium, and Smibert medium.
Biochemical tests →urease+, alkaline phosphates+, catalase+.
Treatment and prevention
Combination therapy with 2 or more antibiotics such as amoxicillin +
clarithromycin + omeprazole (a proton pump inhibitor) (figure 3).
