
1
True bacteria – Rods - Gram Negative Rods
Nonenteric Rods
Haemophilus, Bordetella, Pseudomonas,
Brucella, Yersinia pestis, Pasteurella
The organisms not part of a closely related family, they share two significant features:
1) All have a G
-
cell envelope , contain lipopolysaccharide (LPs) which is a virulence
factor.
2) All are aerobic, grow in the presence of oxygen cause infections at sites where O
2
tension is high.
It’s helpful to consider these organisms as
a) pathogen of human respiratory tract (Haemophilus, Bordetella, Legionella).
b) opportunistic pathogen (Pseudomonas).
c) pathogen of animals (as zoonotic organisms such as Brucella, Pasteurella,
Francisella, Bartonella, which human are accidental hosts. Yersinia pestis is
included in this group because it’s a nongastrointestinal G
-
rod.
Haemophilus
Haemophilus influenza, the major human pathogen of this genus. Serious invasive
H. influenzae disease is associated particularly with capsular type b (Hib), as virulence
factor, a pathogen of young children and in individuals of all age groups. Nontypeable
(unencapsulated) strains may cause pneumonia among the elderly and individuals with
chronic lung disease.
Epidemiology
H. influenzae is a normal flora of the upper respiratory tract, the conjunctiva and
genital tract in humans. Humans are the only natural hosts, and colonization begins
shortly after birth, with unencapsuiated strains and capsular type b being carried most
frequently. H. influenzae illnesses are usually sporadic in occurrence.
2
Pathogenesis
H. inftuenzae is transmitted by respiratory droplets. IgA protease degrades
secretory IgA, facilitating colonization of the upper respiratory tract mucosa → enter the
bloodstream and disseminate to distant sites. Diseases caused by H. influenzae,
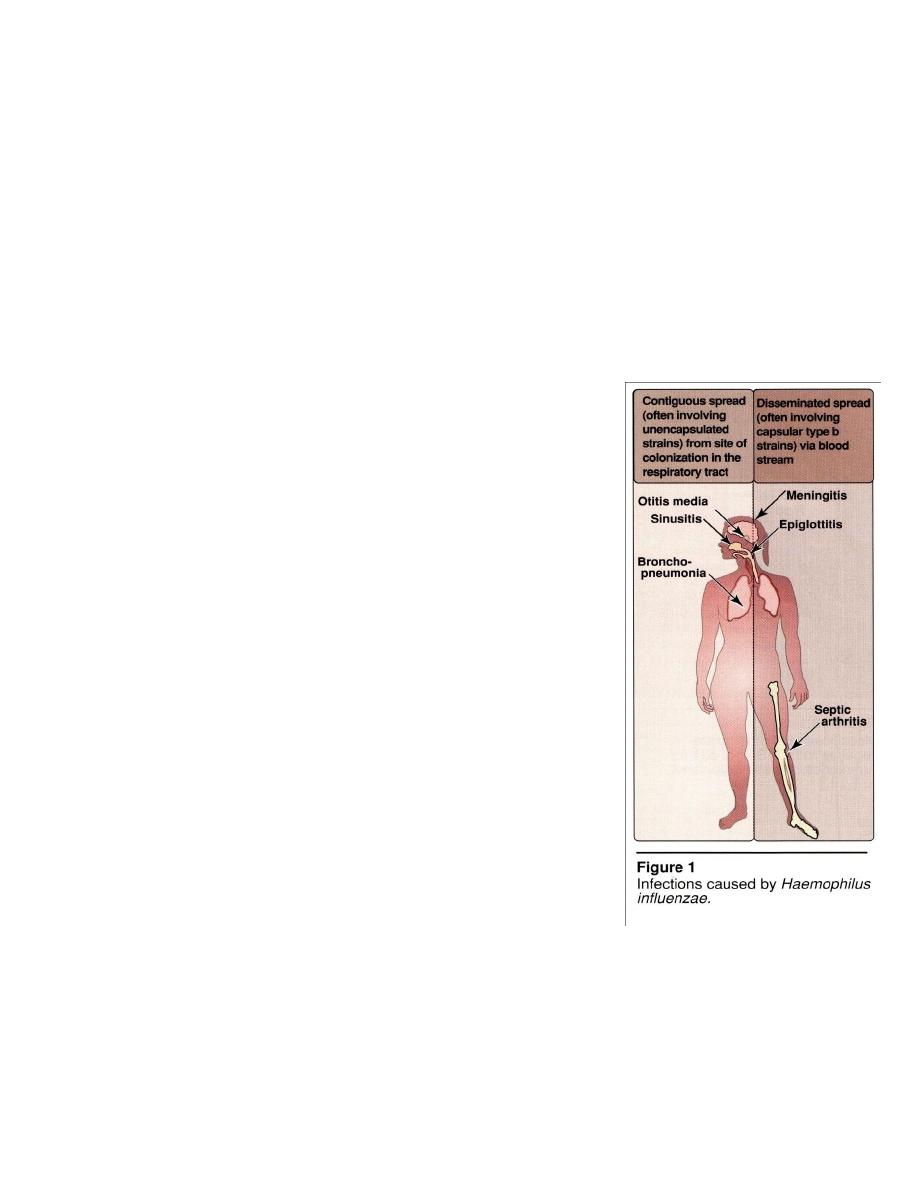
therefore, fall into two categories (Figure 1) :
1. Disorders such as otitis media, sinusitis, epiglottitis, and bronchopneumonia result
from contiguous spread of the organism,uncapsulated strain, from its site of
colonization in the respiratory tract.
2. Disorders such as meningitis, septic arthritis, and cellulitis result from invasion of
the bloodstream, followed by localization of H. influenza ,capsular type b
strains, in these and other areas of the body.
Clinical significance
H. influenzae has been a leading cause of bacterial
meningitis, primarily in infants and very young children,
frequently in conjunction with otitis media. A vaccine against
H. influenzae type b, administered to infants, has dramatically
decreased the frequency of such infections. Mortality from
meningitis is high in untreated patients, therapy reduces
mortality to about five percent.
Laboratory identification
Specimens = blood, CSF, or synovial fluid is
significant from pharyngeal cultures .
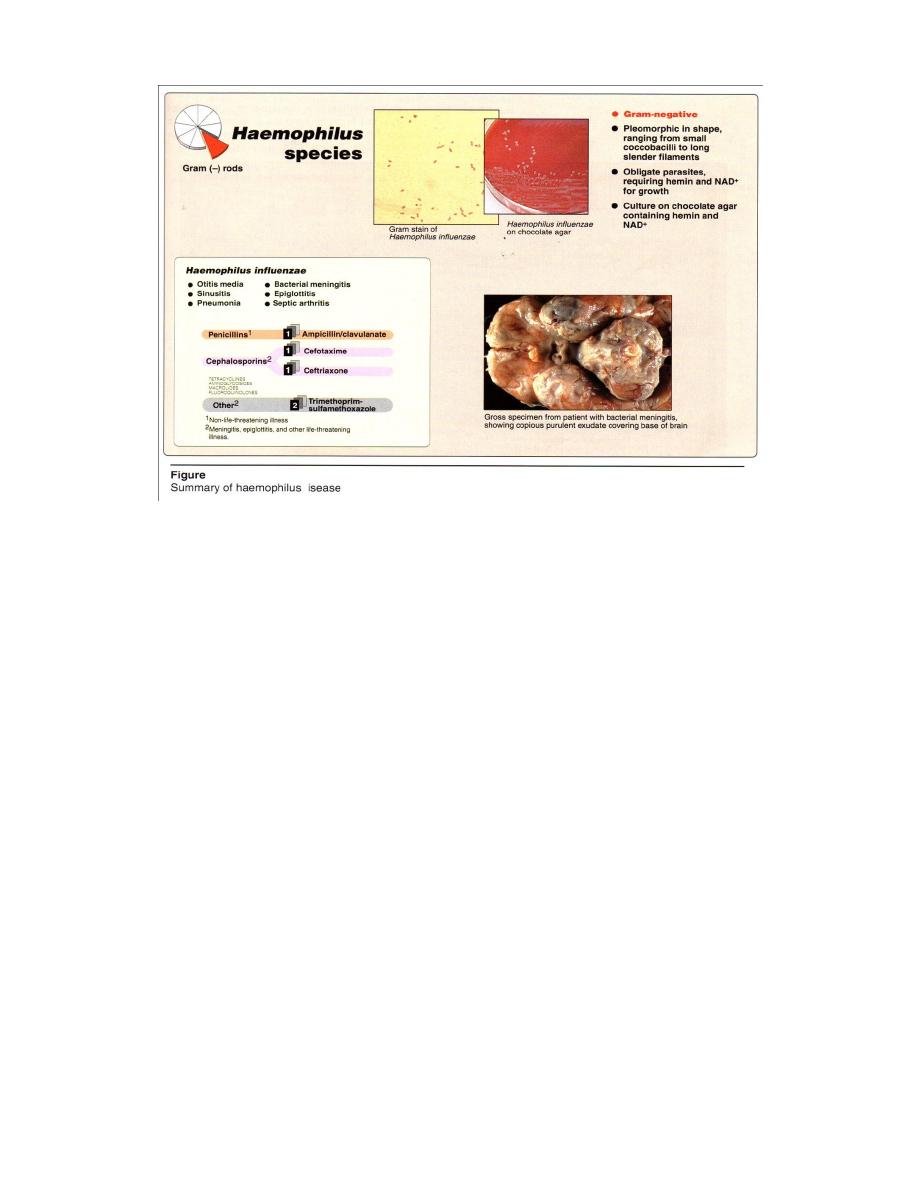
Microscopic examination = G- pleomorphic ranging
from coccobacilli to long slender filaments(Figure 2) may
produce a capsule or may be uncapsulated.
Type b capsule may be demonstrated directly in
CSF= either by the capsular swelling (quellung) reaction or
by immunofluorescent staining.
Macroscopic examination = On chocolate agar containing hemin and NAD for
growth.
Serological tests=Capsular antigen may be detected in CSF or other body fluids
using latex agglutination, immunoelectrophoresis, and radioimmune assay.
3
Treatment
A third generation cephalosporin (such as ceftriaxone or cefoiaxime) should be
started as soon as specimens have been taken for culture (see Figure 2). Antibiotic
sensitivity testing is necessary, because of emergence of strains resistant to antibiotics
(for example, strains with β-lactamase-mediated ampicillin resistance).In sinusitis, otitis
media, and other upper respratory tract infections are treated with trimethoprim-
sulfamethoxazole or ampicillin + clavulanate.
Prevention
By a vaccine against Hib and also reduces respiratory carriage of Hib, generally
given to children younger than two years. Rifampin is given prophylactically to
individuals in close contact with a patient infected with invasive H. influenza (for
example, meningitis).
Bordetella
Bordetella pertussis and B. parapertussis are the human pathogens of this genus,
causes pertussis, also known as whooping cough, is a highly contagious disease and a
significant cause of morbidity and mortality worldwide.
2
4
Epidemiology
The major mode of transmission is by droplets spread by coughing. The incidence
of whooping cough among different age-groups can vary depending on whether active
immunization of young children( 1-5years) is wide spread in the community.
Pathogenesis
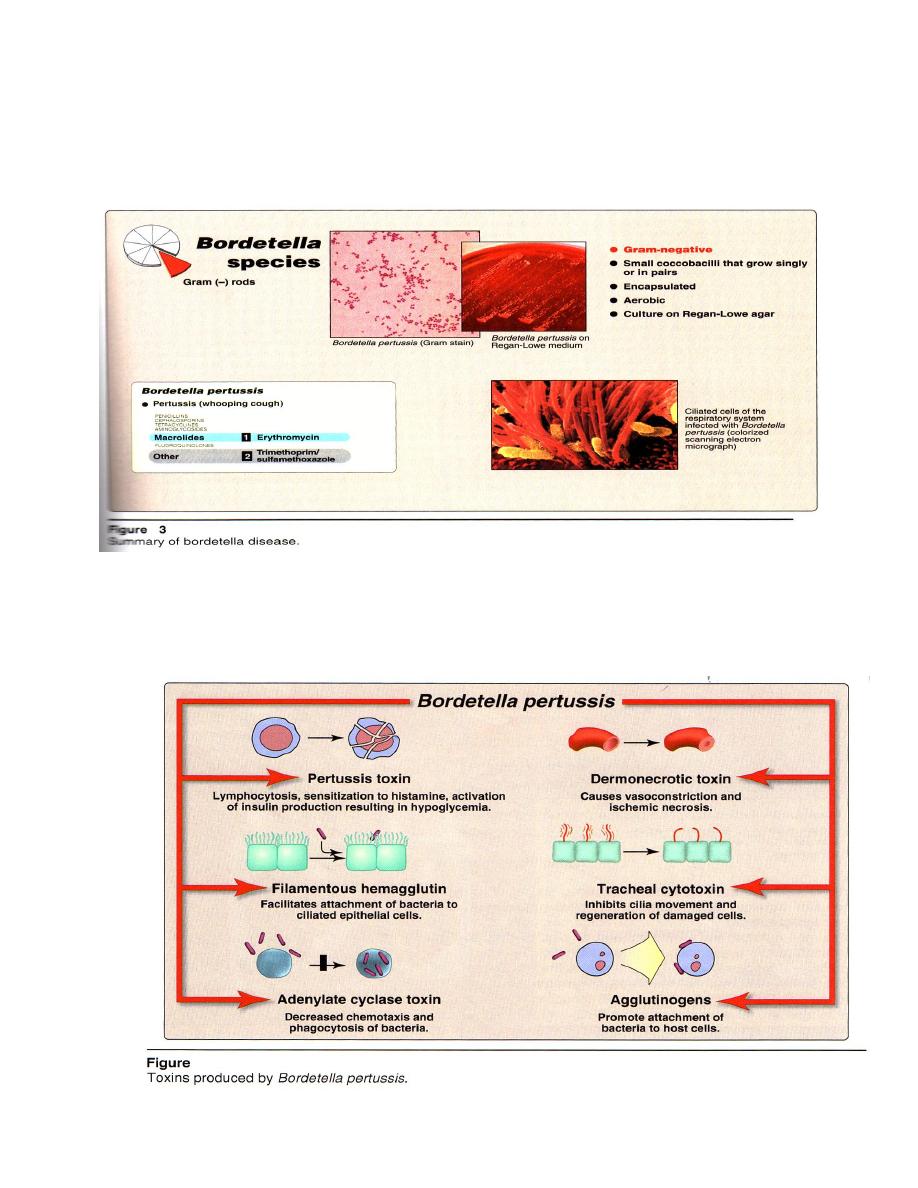
B. pertussis binds to ciliated epithelium in the upper respiratory tract (see Figure
3)and produce a variety of toxins and other virulence factors that interfere with ciliary
activity causing death of these cells (Figure 4).
4

5
Clinical significance
The incubation period for pertussis ranges from 1-3 weeks and can be divided
into two phases: catarrhal and paroxysmal.
1. Catarrhal phase: This phase begins with nonspecific symptoms such as rhinorrhea,
mild conjunctival infection, malaise, and or mlid fever, and then progresses to
include a dry, nonproductive cough.
2. Paroxysmal phase: This phase begins with worsening of the cough. The term
whooping cough derives from the paroxysms of coughing followed by a "whoop"
as the patient inspires rapidly. Large amounts of mucus may be produced. Paroxysms
may cause cyanosis and or end with vomiting. Pertussis typically causes leukocytosis
, 50,000 cells/µL (normal range = 4500-11,000 white blood cells/µL), with a
predominance of lymphocytes. Following the paroxysmal phase, convalescence
requires at least an additional three to four weeks. During this period, secondary
complications such as infections ( otitis media and pneumonia) and CNS dysfunction
( encephalopathy or seizures) may occur. Disease is generally most severe in infants.
Laboratory identification
Diagnosis may be made once the paroxysmal phase begins. Pertussis may be
suspected in an individual who has onset of catarrhal symptoms within 1-3 weeks .
Microscopic examination = G- small, encapsulated coccobacilli that grow singly
or in pairs .
Macroscopic examination = from the nasopharynx . The organism aerobic,
produces pinpoint colonies in 3-6 days on selective agar medium, Regan Lowe agar
contains blood and charcoal, which serves to absorb and or neutralize inhibitory
substances, and is supplemented with antibiotics to inhibit growth of normal flora.
Serologic tests for antibodies to B. pertussis are primarily useful for
epidemiologic surveys. Serotyped on the basis of cell-surface molecules termed
"agglutinogens'. More rapid diagnosis using a direct fluorescent antibody (DFA) test ,
in smears of nasopharyngeal specimens.
Treatment and prevention
Erythromycin is the drug of choice both as chemotherapy ( reduces both the
duration and severity of disease) and as chemoprophylaxis for household contacts (see
Figure 1). For erythromycin treatment failures, trimethoprim-sulfamethoxazole is an

6
alternative choice. Patients are most contagious during the catarrhal stage and
during the first two weeks after onset of coughing. Treatment of the infected
individuals during this period limits the spread of infection among household contacts.
Prevention by vaccine for infant two months old ,in combination with diphtheria
and tetanus toxoids (DTaP).
Pseudomonas
Pseudomonas aeruginosa, the primary human pathogen in this genus. It is
distributed in soil, water, plant, animals. Although it may colonize healthy human
without causing disease,its an opportunistic pathogen.
Pathogenesis
P.aeruginosa disease begins with attachment to and colonization of host tissue.
Pili on the P. mediate adherence + a capsule reduces the effectiveness of normal
clearance mechanisms + numerous toxins + extracellular products that support local
invasion and dissemination of it.
Virulence factors
Most strains produce :
(A) 2 exotoxins (exotoxin A + exoenzyme S)
(B) variety of cytotoxic substances, including proteases, phospholipases, rhamnolipids
and blue pigment (pyocyanin).
(C) exopolysaccharide, composed of D-mannuronic acid and L-glucuronic acid is
responsible for the mucoid phenotype.
These virulence factors depends upon the site and nature infectionas:
Proteases play role in corneal ulceration.
Exotoxin and proteases in burn infections and septicemia.
Alginate and quorum sensing molecules in chronic pulmonary colonization.
Pyochelin and fluorescein (pyoverdin) act as important bacterial siderphores.
Fluorescein in vivo ,allows P. aeruginosa to compete with transferrin (mammalin
iron-binding proteins).
Pyocyanin in infected wounds.
Exotoxin A is similar to dipheria toxin subunit A.

7
Clinical significance
It’s a major cause of nosocomial infections (hospital – acquired), such as
nosocomial pneumonia, nosocomial urinary tract infections, surgical site infections,
infection of severe burns, and infections of patients undergoing either chemotherapy for
neoplastic disease or antibiotic therapy. P.aeruginosa causes both localized and systemic
illness . Individuals must at risk include those with impaired immune defenses.
1- Localized infections: ( lead disseminated infection by invade blood vessel walls).
These may occur in the eye ( following trauma), ear (external otitis or swimmer’s ear,
particularly in elderly diabetic patients or trauma patients) skin (wound sepsis, pustular
rashes occurring in epidemics associated with use of contaminated whirlpools, hot tubs,
swimming pools). Urinary tract (in hospitalized patients who have been subjected to
catheterization, instrumentation, surgery or renal transplantation). Respiratory tract
(pneumonia in individuals with chronic lung disease, congestive heart failure or cystic
fibrosis, and in patients who have been incubated or are on ventilators).
Gastrointestinal tract (infection range from mild diarrheal illness in children to severe,
necrotizing enterocolitis in infants and neutropenic cancer patients). Central nervous
system (CNS, meningitis, brain abscesses association with trauma, surgery, or tumors of
the head or neck).
2- Systemic infections: include bacteremia (in compromised patients), secondary
pneumonia, bone and joint infections (in IV drug users and patients with urinary tract
or pelvic infections), endocarditis (in IV drug users and patients with prosthetic heart
valves), CNS and skin/soft tissue infections.
So, P.aeruginosa is feared because (1) it can cause severe hospital – acquired
infections, especially in immunocompromised hosts (2) it’s often antibiotic resistant,
complicating the choice of therapy.
Laboratory identification
Microscopic examination = G
-
rods, motile has polar flagella, encapsulated .
Macroscopic examination = obligately aerobic (it oxidizes carbohydrates). Nutritional
requirements are minimal so it’s can grow, on a wide variety of organic substrate, in
laboratory water baths, hot tubs, wet IV tubing and other water-containing vessels. This
explains why the organism is responsible for so many nosocomial infections.
P.aeruginosa can be isolated by plating on a variety of media( Fig. 1). On nutrient agar
produces diffusible blue - green pigments (pyocyanin) or yellow – green fluorescent
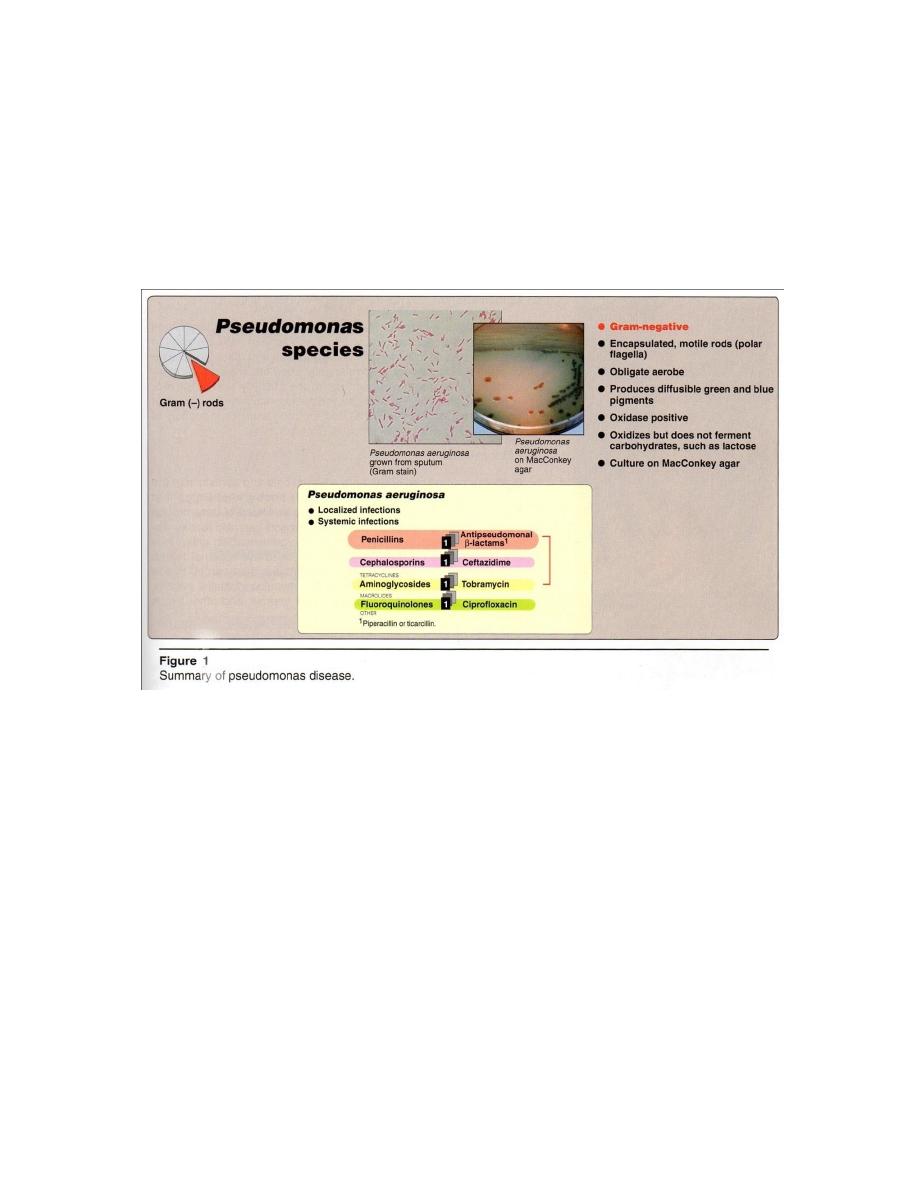
8
pigment (fluorescein or pyoverdin) or pyoruben (red)/ melanin (brown), with fruity odor
(sweet grape like) from the culture . On MacConkey agar
Lac
+
as oxidizes but does
not ferment.
Biochemical tests = Catalase +, oxidase +, nitrate - , glucose + (oxidation) .
Serological test = serum Abs against P. have no place in diagnosis except in
cystic fibrosis or chronic obstructive pulmonary disease, where Ab levels increasing
correlate directly with immune-mediated lung damage.
Treatment and prevention
It’s difficult to find antibiotics effective against P.aeruginosa because of
(1)its rapid development of resistance mutations.
(2)its own innate mechanisms of antibiotic resistance.
P. infections typically occur in patients with impaired defenses, so, aggressive
antimicrobial therapy is generally required (Figure 1) combination of 2 bactericidal
antibiotics such as aminoglycoside + an antipseudomonal
-lactam, or a quinolone.
Brucella
Brucella are primarily pathogens of animals, is a zoonosis . It’s includes
B. abortus
cattle B. melitensis
goats and sheep B. ovis
sheep
B. suis
swine B. canis
dogs

9
All but B. ovis are known to cause disease in humans . Its facultative
intracellular parasites that can survive and multiply within host phagocytes(Figure 2).
Epidemiology
Brucellosis is a chronic infection in animals. It localize in reproductive
organs (male and female) and release in large numbers in milk, urine, and the placenta
and other tissues discharged during delivery or spontaneous abortion. Causes in animals
sterility or abortion. Transmission to human occurs as a result of 1 direct contact with
infected animal tissue 2 ingestion of unpasteurized milk or milk products. Person to
person transmission is rare.
Pathogenesis
It typically enter the body through = cuts in the skin or the gastrointestinal
(GI) tract or Inhalation of infected aerosols among abattoir workers, veterinarians and
farmers. Once the organism entry
transported by the lymphatic system
to the
regional lymph nodes
multiply
by the blood to organs that are involved in the
reticuloendothelial system, including liver, spleen, kidneys, bone marrow and other
lymph nodes.

10
Antigenic structure
LPS and cell wall Ag are the major virulence factor. Somatic Ag (O Ag) has to
components A and M . B abortus contain 20 times as much (A and M). B. melitensis
contain 20 times as much (M and A). B. suis has intermediate Ag pattern.
Ag cross reaction occurs between Brucella and V. cholerae. In addition, L -Ag
has been demonstrated that resemble the Vi-Ag of Salmonella . Phage typing = one at
strain phage is Tb, is specific as lysis strains having character of B. abortus., which is of
great value in identification Brucella.
Clinical significance
The incubation period for B. infections ranges from 5 days – several months, or
several weeks. Symptoms are nonspecific and flulike (malaise, fever, sweats, anorexia,
GI symptoms, headache, and back pains) and depression. Untreated patients may
develop an undulating pattern of fever (temperatures repeatedly rise then fall, hence the
name undulant fever is the traditional name for brucellosis). Brucellosis may involve
any of a variety of organ system including GI tract, skeletal, neurologic cardiovascular
and pulmonary systems.
Laboratory identification
The nonspecific symptoms may not point to a diagnosis of brucellosis, a
detailed history is important, including the patients’ occupation+ exposure to
animals+ travel to countries where Brucella infections is prevalent + ingestion of
contaminated foods.
Specimen : blood and other body fluids or from tissue.
Hematological investigation = Total leukocyte count shows leukocytosis in
early acute phase of disease. Differential leukocyte count shows lymphocytosis
Microscopic examination = G- small coccobacilli ,arranged singular or in
pairs (Figure 2), nonmotile, uncapsulated .
Macroscopic examination = In liquid media , uniform turbidity, in old culture
there may be powdery deposits (a tryptose broth or trypticase – soya broth).
On Blood culture , most defined method for brucellosis = Colonies may
appear in 4-5 days longer times and these cultures are routinely examined for up to one
month before declared –ve, its strict aerobic, addition of 10% CO
2
improves the growth
of B. abortus, B. melitensis.

11
On nutrient agar = Colonies are small, moist, translucent and glistening with
butyrous consistency. On liver infusion agar = after 48-72 hs. colonies similar to in N.A.
On MacConkey agar = after 7 days, colonies Lac
-
.
Biochemical reactions : catalase +ve, oxidase +ve, urease +ve, nitrate +ve, no
carbohydrate is fermented.
Basic Fuchsine and thionin dyes which can be used to differentiate spp. (B
abortus and B melitensis growth in basic Fuchsine 1: 50.000, while B. suis growth in
thionin 1:250.000.
Serological diagnosis : such as
Agglutination test: it’s +ve about week , titer 1/100 or more indicates active
infection. Slide agglutination method as Rose Bengal test ( the titer is 1/80 ).
Radio immunoassay and ELISA
to distinguish acute brucellosis.
2-mercaptoethanol test and complement fixation test
gives good result with
chronic disease.
Indirect immunoflorescent test
specific and sensitive.
Indirect hemaglutination test
very sensitive.
Skin test (Brucillin test)
non specific used in chronic cases, give an indicator for
post or present infection.
Rapid plate agglutination test.
Treatment
Combination of doxycycline + gentamicin (or streptomycin) is used (Figure
2)for six weeks .
Yersina
The genus Yersinia is a member of the family Enterobacteriaceae , includes three
species of medical importance: Y.enterocolitica and Y. pseudotuberculosis, both
pathogens of the GI tract (enteric) and Y. pestis, the etiologic agent of bubonic
plague(nonenteric).
Y. enterocolitica and Y. pseudotuberculosis
Pathogenesis
Infection occurs by ingestion of contaminated food with domestic animals,
abattoirs, or raw meat (especially pork).

12
Clinical significance
Y. enterocolitica is uncommon cause of enterocolitis in the United States, and
pseudotuberculosis is even rarer. Infection results in ulcerative lesions in the terminal
ileum, necrotic lesions in Peyer patches, and enlargement of mesenteric lymph nodes.
Other, less common clinical presentations include exudative pharyngitis and, in
compromised patients, septicemia.
Laboratory identification
Microscopic examination = G -rod ,motile when grown at 25°C but not at 37°C,
and noncapsulated.
Macroscopic examination = In contrast to pathogenic Enterobacteriaceae, these
strains of Yersinia grow well at room temperature as well as at 37°C.
On MacConkey or Cefsulodin-Irgasan-Novobiocin agar (CIN, selective medium
for Yersinia). Most strains are Lac - .
Identification is based on biochemical screening. In the absence of a positive
culture, serologic tests for anti-Yersinia antibodies may assist in diagnosis.
Treatment and prevention
Antibiotic therapy (for example, with ciprofloxacin or trimethoprim-
sulfamethoxazole) is essential for systemic disease (Figure 5).
Prevention by limit contamination of meat, ensuring its proper handling and
preparation.

13
Yersina pestis
Causes plague, rather than enteric disease and therefore, is being discussed
separately from the rest of the Yersina family.
Epidemiology
Plague is a zoonosis with worldwide distribution(rats, prairie dogs and ground
squirrels). Household pets, particularly cats in plague-enzootic areas, may also become
infected. Plague is characteristically transmitted by (1) fleas, from the infected animal
reservoir, humans are generally accidental and dead-end hosts. (2) by ingestion of
contaminated animal tissue.(3) by the respiratory (pneumonic plague), either when
reach the lung and establish a secondary pneumonia, or following exposure to
respiratory secretions from a patient or animal with plague pneumonia .
Pathogenesis
Organisms are carried by the lymphatic system from the site of inoculation to
lymph nodes, ingested by phagocytes, resistant to intracellular killing by phagocytes
and instead may multiply . The bacteria released from lysed phagocytes have
synthesized a new envelope antigen that confers increased resistance to phagocytosis.
The affected lymph nodes display hemorrhagic necrosis accompanied by high
concentrations of both polymorphonuclear leukocytes and extracellular bacteria.
Hematogenous spread of bacteria to other organs or tissues may occur, resulting in
additional hemorrhagic lesions at these sites.
6

14
Clinical significance
Plague may present several clinically different pictures. Most common is the
bubonic septicemic form. Pneumonic plague may develop during epidemics. Less
common are plague meningitis , cutaneous plague and pharyngitis.
1. Bubonic (septicemic) plague: by a flea ingests a blood meal from infected animal.
Y. pestis produces a coagulase that causes the blood to clot in the flea's foregut and
multiplies in this environment. When the flea next attempts to feed, it regurgitates
these bacteria from its foregut into the new animals skin. The incubation period is
generally 2-8 days. Nonspecific symptoms, such as high fever, chills. headache,
myalgia, and weakness that proceeds to prostration. Within a short time, a
characteristic= painful bubo develops. Buboes (swellings comprised of one or more
infected nodes and surrounding edema that led to the term "bubonic plague") are
typically located in the groin, axillae or on the neck. As the disease proceeds, blood
pressure generally drops, potentially leading to septic shock and death. Other
manifestations include pustules or vesicles containing leukocytes and Y. pestis
(ingestion of contaminated meat or exposure to airborne bacilli can result in primary
lesions in the pharynx, produce a severe tonsillitis and cervical buboes).
2. Pneumonic plague: If plague bacilli reach the lungs, they cause purulent pneumonia
that, if untreated, is rapidly fatal. It is also highly contagious, and the organisms
cause pneumonic plague directly when inhaled.
3. Plague meningitis: dissemination of organisms to the meninges occur following
incompletely treated bubonic plague or without, or prior to, development of a bubo.
Laboratory identification
Diagnosis may be made on the basis of clinical presentation.
Specimen = aspirate from a bubo or from CSF or sputum in the case of meningitis
or pneumonic .
Microscopic examination = G- small rod that stains bipolarly (see Figure 6)
capsulated, non motile .
Macroscopic examination = on CIN , MacConkey and blood agars= colonies
grow somewhat more slowly than those of other Enterobacteriaceae.

15
Treatment and prevention
Streptomycin is the drug of choice; gentamicin and doxycycline are alternatives
(Figure 6). For plague meningitis, chloramphenicol offers good penetration into the
CSF. Prevention by
(1) vaccine for those at high risk of acquiring plague.
(2) For individuals in enzootic areas, efforts to minimize exposure to rodents and fleas .
(3)Sick or dead rodents should never be touched with bare hands.
Pasteurella
Pasteurella colonize mammals and birds, both domestic and wild. Its a zoonosis.
The major human pathogen in this genus is Pasteurella multocida, which can cause
either disease or asymptomatic infections. Virulence factors include capsule and
endotoxin.
Epidemiology
The majority of Pasteurella infections in humans are soft tissue infections that
follow an animal bite or cat scratch. A smaller fraction of human Pasteurella
infections occur either following a non-bite animal exposure, or in the absence of any
known animal exposure. The source of Pasteurella in the latter infections is suspected
to be nasopharyngeal colonization of the patient.
Clinical significance
P. multocida infection should be suspected in cases of acute, painful cellulitis that
develop within 24 hours of an animal bite or cat scratch, or an infected animal licking an
open wound. Soft tissue infections are characterized by the rapid onset of acute local
inflammation within hours of the bite or scratch. Lesions often begin to drain within one
to two days. Manifestations of P. multocida infection include lymphangitis,
lymphadenitis, fever, and local complications such as osteomyelitis or arthritis.
Laboratory identification
Pasteurella are G - coccobacilli or rods , bipolar staining , some strains are
encapsulated.Laboratory diagnosis ( in non-bite / scratch-associated cases) by culturing
on blood agar, γ- hemolytic , and performing appropriate biochemical tests.
Treatment
(1)soft tissue infections, wounds should be cleansed, irrigated, and debrided.
(2)deep infections require surgical drainage
(3)Prolonged antibiotic treatment ,penicillin is the drug of choice.
