Acyanotic Heart Lesions
1. Shunt lesions (increased pulmonary flow)a. RV dominance: ASD (Secundum / venosus type), ASD with mitral stenosis .Coronary A-V fistula and PAPVC, partial AV canal defects (ASD primum).
b. LV or combined ventricular dominance (combined ventricular hypertrophy): VSD, PDA, complete AV canal defect, coronary arteriovenous fistula, VSD with left ventricle to RA shunt, VSD with AR, AP window and RSOV to RA/RV, ALCAPA.
2. No shunt lesions
a. Obstructive: Pulmonary stenosis, aortic stenosis, coarctation of aorta, cor triatriatum, peripheral pulmonary stenosis, congenital MS, pulmonary venous stenosis.
b. Non-obstructive: Aortic regurgitation, pulmonary regurgitation, idiopathic dilatation of pulmonary artery, mitral regurgitation
In acyanotic patients, the direction of the shunt is from left to right and the magnitude of the left-to-right shunt is determined by the size of the defect and the relative compliance of the right ventricle (RV) and left ventricle (LV).
Atrial Septal Defect
DEFINITIONDefect in interatrial septum in the region of fossa ovalis
producing communication between two atria is known as
secondum type of atrial septal defect. A patent foramen
ovale or a stretched foramen ovale is not a true atrial septal
defect because there is no loss of atrial tissue.
Pathology
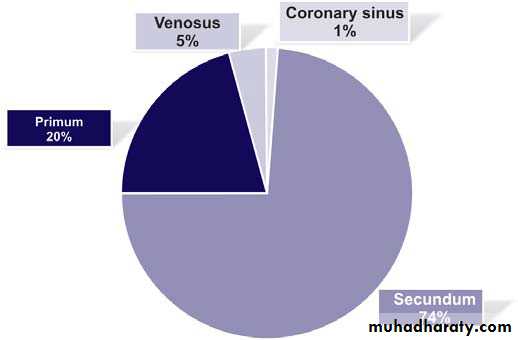
1. Three types of ASDs exist: secundum defect, primum defect, and sinus
venosus defect. Patent foramen ovale (PFO) does not ordinarily produce
intracardiac shunts.
2. Secundum ASDs represent 6% to 10% of all cardiac anomalies and are more frequent in females than males by about 2:1 .Ostium secundum defect is the most common type of ASD, accounting for 50% to 70% of all ASDs. This defect is present at the site of fossa ovalis, allowing left-to-right shunting of blood from the left atrium (LA) to the right atrium (RA) .(Anomalous pulmonary venous return is present in about 10% of cases.
3. Ostium primum defects occur in about 30% of all ASDs, if those that occur as part of complete ECD are included .Isolated ostium primum ASD occurs in about 15% of all ASDs.
.
4. Sinus venosus defect occurs in about 10% of all ASDs. The defect is most commonly located at the entry of the superior vena cava (SVC) into the RA (superior vena caval type) and rarely at the entry of the inferior vena cava (IVC) into the RA (inferior vena caval type).
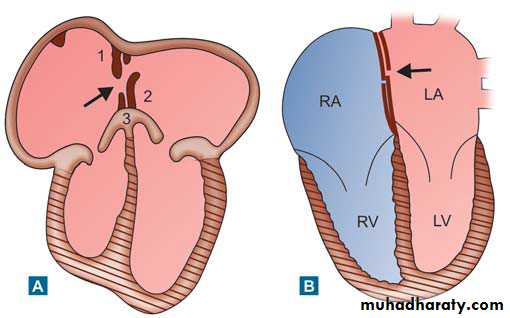
Schematic diagram showing development
of atrial septum: (A) Arrow indicates foramen ovale: 1—Septum secundum, 2—Septum primum, 3—Endocardial cushion (B) After birth, arrow shows closedforamen ovale by flap valve
Foramen Ovale
It is an interatrial communication that develops in-between third and fourth week of intrauterine life. It is a slit like opening in the septum primum, not classified as a type of septal defect.
Functional closure of the foramen ovale occurs postnatally as pressure in the left atrium exceeds that in the right atrium. As a result, the valve of the fossa ovalis is pressed against the limbus and forms a competent seal. During the first year of life, fibrous adhesions may develop between the limbus and valve and thereby produce a permanent anatomic seal and an imperforate atrial septum. In 25% to 30% of people, however, anatomic closure does not occur, and a potential interatrial channel persists through which blood or air may shunt whenever pressure in the right atrium exceeds that in the left atrium.
Physiology
The direction in which blood flows through the defect primarily is related to the relative compliances of the ventricles. Generally, the right ventricle is more compliant than the left, resulting in less resistance to filling from the right atrium. In most situations, shunting is left to right.Clinical Features
Most infants with ASDs are asymptomatic, and the condition goes undetected. They may present at 6 to 8 weeks of age with a soft systolic ejection murmur and possibly a fixed and widely split S2. More recently, infants with heart murmurs have been referred earlier for echocardiographic evaluation so that the average age at which ASDs are being detected is about 6 months old. Older children with a moderate left-to-right shunt often are asymptomatic. Children with large left-to-right shunts are likely to complain of some fatigue and dyspnea. Growth failure is very uncommon. Rarely, ASDs in infants are associated with poor growth, recurrent lower respiratory tract infection, and heart failurePhysical examination
precordial bulge and a hyperdynamic cardiac impulse, especially in the older child and when the left-to-right shunt is large. Palpation of the precordium reveals a prominent systolic impulse. There are three important auscultatory features: (a) a typical wide and fixed splitting of the second heart sound, (b) a soft systolic ejection murmur at the second left intercostal space, and (c) an early to middiastolic murmur at the lower left sternal border. The term fixed refers to the constant time interval between A2 and P2 throughout the respiratory cycle. A delay in P2 is due, in part, to prolonged emptying of the right ventricle because of increased volume of blood to be ejected; additionally, considerable vasodilation of the pulmonary vasculature delays intra-arterial pulmonary tension necessary to close the pulmonary valve.Radiography Studies
1. Cardiomegaly with enlargement of the RA and right ventricle (RV) may be present.2. A prominent pulmonary artery (PA) segment and increased pulmonary vascular markings are seen when the shunt is significant
Electrocardiography
Right axis deviation of +90 to +180 degrees and mild right ventricular hypertrophy (RVH) or right bundle branch block (RBBB) with an rsR′ pattern in V1 are typical ECG findings. In about 50% of patients with sinus venosus ASD, the P axis is less than +30 degrees.Natural History
1. The defect may decrease in size in some patients. However, a more recent report indicates the overall rate of spontaneous closure to be 87%. In patients with an ASD smaller than 3 mm in size diagnosed before 3 months of age, spontaneous closure occurs in 100% of patients at 1½ years of age. Spontaneous closure occurs more than 80% of the time in patients with defects between 3 and 8 mm before 1½ years of age. An ASD with a diameter larger than 8 mm rarely closes
2. Most children with an ASD remain active and asymptomatic. Rarely, congestive heart failure (CHF) can develop in infancy.
3. If a large defect is untreated, CHF and pulmonary hypertension begin to develop in adults who are in their 20s and 30s, and it becomes common after
40 years of age
4. With or without surgery, atrial arrhythmias (flutter or fibrillation) may occur in adults. The incidence of atrial arrhythmias increases to as high as 13% in patients older than 40 years of age.
5. Infective endocarditis does not occur in patients with isolated ASDs.
Management
Medical1. Exercise restriction is unnecessary.
2. In infants with CHF, medical management (with a diuretic) is recommended because of its high success rate and posibility of spontaneous closure of the defect
Nonsurgical Closure
Nonsurgical closure using a catheter-delivered closure device has become a preferred method, provided the indications are met. Several closure devices that can be delivered through cardiac catheters have been shown to be safe and efficacious for secundum ASD closure.The use of the closure device may be indicated to close a secundum ASD measuring 5 mm or more in diameter (but less than 32 mm for Amplatzer device and a hemodynamically significant L-R shunt with clinical evidence of RV volume overload (i.e., Qp/Qs ratio of 1.5:1 or greater or RV enlargement).
There must be enough rim (4 mm) of septal tissue around the defect for appropriate placement of the device.
Surgical Closure
Indications and TimingSurgical closure is indicated only when device closure is not considered appropriate including deficient rims,severe tricuspid regurgetation,sinus venosus ASD,and contraindication to use of antiplatlets.
1. A left-to-right shunt with a pulmonary-to-systemic blood flow ratio (Qp/Qs ratio) of 1.5:1 or greater is a surgical indication. Surgery is usually delayed until 2 to 4 years of age because the possibility of spontaneous closure exists.
2. If CHF does not respond to medical management, surgery is performed during infancy, again if device closure is considered inappropriate.
3. High pulmonary vascular resistance (PVR) (i.e., >10 units/m2, >7 units/m2 with vasodilators) may be a contraindication for surgery (or device closure).
Complete Endocardial Cushion Defect
Prevalence
Complete ECD (also known as AV canal defect, complete AV canal defect, or AV communis) occurs in 2% of all CHDs. Of patients with complete ECD, about 70% are children with Down syndrome. Of children with Down
syndrome, about 40% have CHDs, and 50% of the defects are ECD.
Pathology
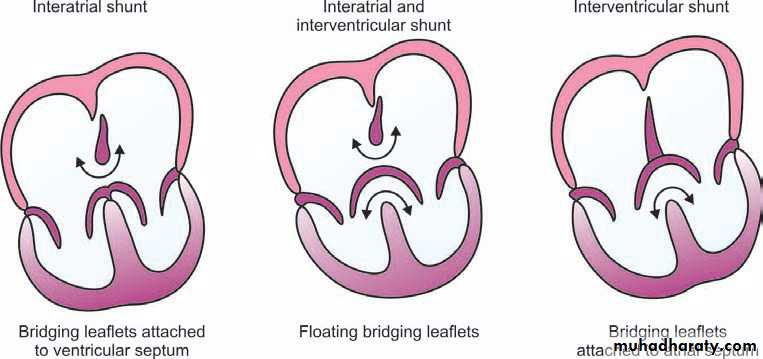
Abnormalities seen in complete ECD affect the structures normallyderived from the endocardial cushion tissue. Ostium primum ASD, VSD in the inlet ventricular septum, and clefts in the anterior mitral valve and the septal leaflet of the tricuspid valve (forming the common AV valve) are all present in the complete form of ECD .The combination of these defects may result in interatrial and interventricular shunts, LV-to-RA shunt, and AV valve regurgitation. Although rare, the entire atrial septum may be absent (common atrium). When two AV valve orifices are present without an interventricular shunt, the defect is called partial ECD or ostium primum
Whereas in complete ECD, a single valve orifice connects the atrial and ventricular chambers, in the partial form, there are separate mitral and tricuspid orifices. The common AV valve usually has five leaflets.
Clinical Manifestation
HistoryFailure to thrive, repeated respiratory infections, and signs of CHF are common.
Physical Examination
1. Infants with ECD are usually undernourished and have tachycardia and tachypnea (signs of CHF). This defect is common in infants with Down syndrome.
2. Hyperactive precordium with a systolic thrill at the lower left sternal border is common .
3. The S1 is accentuated. The S2 narrowly splits, and the P2 increases in intensity. A grade 3 to 4 of 6 holosystolic murmur is usually audible along the lower left sternal border. The systolic murmur may transmit well to the left axilla and be heard well at the apex when mitral regurgitation (MR) is significant.
A mid-diastolic rumble may be present at the lower left sternal border or at the apex as a result of relative stenosis of the tricuspid or mitral valve.
4. Signs of CHF (e.g., hepatomegaly, gallop rhythm) may be present.
Electrocardiography
1. “Superior” QRS axis with the QRS axis between −40 and −150 degrees is characteristic of the defect2. Most of the patients have a prolonged PR interval (first-degree AV block).
3. RVH or RBBB is present in all cases, and many
patients have LVH, too.
Radiography
Cardiomegaly is always present and involves all four cardiac chambers.Pulmonary vascular markings are increased and the main PA segment is prominent
Natural History
1. Patients with complete ECD develop heart failure 1 to 2 months after birth, and recurrent pneumonia is common.2. Without surgical intervention, most patients die by the age of 2 to 3 years.
3. In the latter half of the first year of life, survivors begin to develop pulmonary vascular obstructive disease. These survivors usually die in late childhood or as young adults.
.
Management
Medical
1. In small infants with CHF, anticongestive management with diuretics and ACE inhibitors should be started. Digoxin may also be used
2.Nutrition should be optimized.
Surgical
Indications
The presence of complete ECD indicates the need for surgery because an important hemodynamic derangement is usually present. Most of these infants have CHF that is unresponsive to medical therapy, and some have elevated PVR.
Timing
Although timing varies among institutions and with the hemodynamics of the defect, most centers perform the repair at 2 to 4 months of age. Early surgical repair is especially important for infants with Down syndrome with complete ECD because of their known tendency to develop early pulmonary vascular obstructive disease.
Ventricular Septal Defect
PrevalenceVentricular septal defect is the most common form of CHD and accounts for 20% to 30% of all such defects, not including those occurring as part of cyanotic CHDs.
DEFINITION
Ventricular septal defect (VSD) is a congenital defect(hole) in the interventricular septum, which connects both ventricles of the heart
Pathology
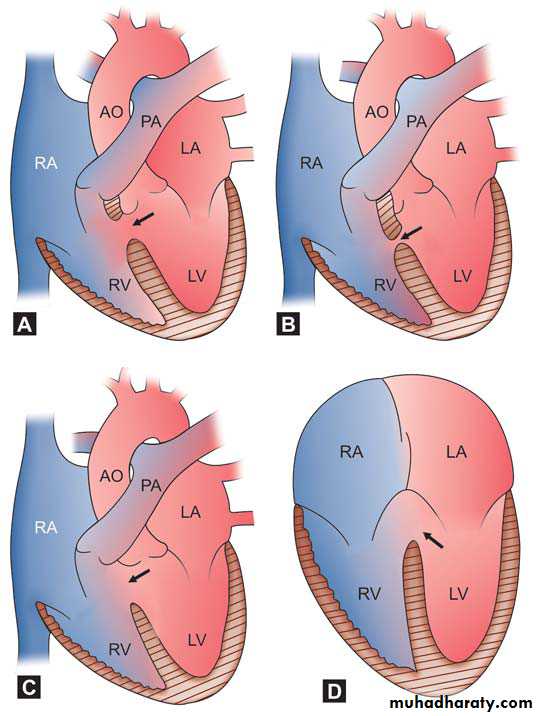
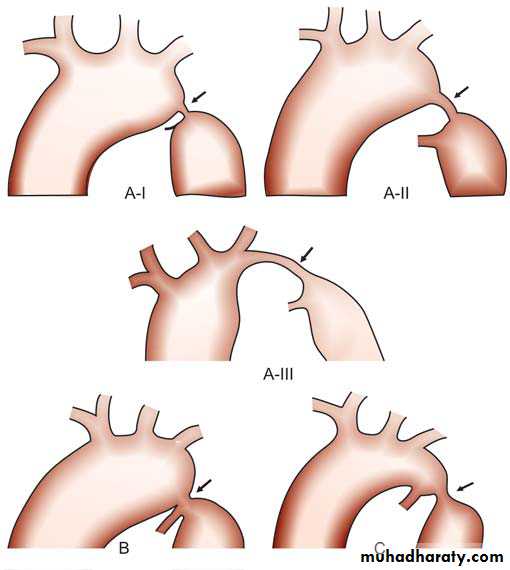
The ventricular septum may be divided into a small membranous portion and a large muscular portion. The muscular septum has three components: the inlet septum, the trabecular septum, and the outlet (infundibular or conal) septum. The trabecular septum (also simply called the muscular septum) is further divided into anterior, posterior, mid, and apical portions. Therefore, VSD may be classified as a membranous, inlet, outlet (or infundibular), midtrabecular (or midmuscular), anterior trabecular (or anterior muscular, posterior trabecular (or posterior muscular), and apical muscular defect.Schematic diagrams of different types of
VSDs. Arrow showing (A) Sub-aortic (Perimembranous),
(B) Muscular, (C) Subpulmonic (supracristal), and (D) Inlet
type
a. The membranous septum is a relatively small area immediately beneath the aortic valve. The membranous defect involves varying amounts of muscular tissue adjacent to the membranous septum (perimembranous VSD). Perimembranous defects are most common (70%).
b. Outlet (infundibular or conal) defects account for 5% to 7% of all VSDs in the Western world and about 30% in Far Eastern countries. The defect is located within the outlet (conal) septum, and part of its rim is formed by the aortic and pulmonary annulus. An aortic leaflet can prolapse through the VSD and cause aortic insufficiency (It has been called a supracristal, conal, subpulmonary, or subarterial defect.
c. Inlet (or AV canal) defects account for 5% to 8% of all VSDs. The defect is located posterior and inferior to the perimembranous defect beneath the septal leaflet of the tricuspid valve
Muscular: 5%-20%
Central: mid-muscular, may have multiple apparent channels on RV side and coalesce to single defect on LV sideApical: multiple apparent channels on RV side may be single defect on LV side as with central defect
Marginal: along RV septal junction
Swiss cheese septum: large number of muscular defects
Clinical Manifestations
History1. With a small VSD, the patient is asymptomatic with normal growth and development.
2. With a moderate to large VSD, delayed growth and development, decreased exercise tolerance, repeated pulmonary infections, and CHF are relatively common during infancy.
3. With long-standing pulmonary hypertension, a history of cyanosis and a decreased level of activity may be present.
Physical Examination
1. Infants with small VSDs are well developed and acyanotic. Before 2 or 3 months of age, infants with large VSDs may have poor weight gain or show signs of CHF.
2. A systolic thrill may be present at the lower left sternal border. Precordial bulge and hyperactivity are present with a large-shunt VSD.
3. The intensity of the P2 is normal with a small shunt and moderately
increased with a large shunt. The S2 is loud and single in patients with
pulmonary hypertension or pulmonary vascular obstructive disease. A grade 2 to 5 of 6 systolic murmur is audible at the lower left sternal border .It may be holosystolic(pansystolic murmur) or early systolic.
Electrocardiography
1. With a small VSD, the ECG findings are normal.2. With a moderate VSD, left ventricular hypertrophy (LVH) and occasional left atrial hypertrophy (LAH) may be seen, in some cases mild or moderate elevation of right ventricular pressure can result in right ventricular hypertrophy, which is evident in lead V4R or V1 as an rsR pattern with the increasing in amplitude with increasing right ventricular pressure.
3. With a large defect, the ECG shows biventricular hypertrophy (BVH) with or without LAH 4. If pulmonary vascular obstructive disease develops, the ECG shows RVH only.
Echocardiography
Two-dimensional and Doppler echocardiographic studies can identify the number, size, and exact location of the defect; estimate pulmonary artery pressure .identify other associated defects; and estimate the magnitude of the shunt.Radiography
1. Cardiomegaly of varying degrees is present and involves the left atrium (LA), left ventricle (LV), and sometimes right ventricle(RV). Pulmonary vascular markings increase.The degree of cardiomegaly and the increase in pulmonary vascular markings directly relate to the magnitude of the left-to-right shunt
2. In pulmonary vascular obstructive disease, the main pulmonary artery and the hilar PAs enlarge noticeably, but the peripheral lung fields are ischemic. The heart size is usually normal.
Natural History.
1. Spontaneous closure of perimembranous and muscular VSDs can occur. It occurs more frequently with small defects and during the first 6 months of life. About 60% of small to moderate muscular VSDs close spontaneously but not after 8 years of age. About 35% of small perimembranous VSDs close spontaneously but not after 5 years of age. These VSDs do not become bigger with age; rather, they decrease in size. However, inlet defects and outlet (infundibular) defects do not become smaller or close spontaneously.2. CHF develops in infants with large VSDs but usually not until 6 to 8 weeks of age.
3. Pulmonary vascular obstructive disease may begin to develop as early as 6 to 12 months of age in patients with large VSDs, but the resulting right-toleft shunt usually does not develop until the teenage years.
4. Infundibular stenosis may develop in some infants with large defects and result in a decrease in the magnitude of the left-to-right shunt (i.e., acyanotic TOF), with an occasional occurrence of a right-to-left shunt.
COMPLICATIONS
Small VSD
common complication of small VSD is infective endocarditis,
mainly beyond 2 years of age.
Large VSD
Complications in Large VSD are (i) CHF, (ii) arrhythmias,
(iii) infective endocarditis, (iv) Eisenmenger’s complex,(v)repeated chest infection
Management
Medical1. Treatment of CHF, if it develops, is indicated with diuretics (with or
without) digoxin for 2 to 4 months to see if growth failure can be improved. Some centers do not use digoxin. Addition of spironolactone may be helpful to minimize potassium loss from diuretics.Concomitant use of an afterload reducing agent, such as captopril, has gained popularity. Angiotensin-converting enzyme
(ACE) inhibitors may raise the serum potassium level, and spironolactone or potassium supplementation should be discontinued. Frequent feedings of high-calori formulas, either by nasogastric tube or oral feeding also can be effective.
Anemia if present, should be corrected by oral iron therapy. These measures often allow delay of surgical treatment and may promote spontaneous reduction or closure of the VSD.
2. No exercise restriction is required in the absence of pulmonary
hypertension.
3. Nonsurgical transcatheter closure of selected VSDs is possible in selected patients when the defect is not too close to cardiac valves and when it is difficult to access surgically.
\Surgical
.`Indications for surgical repair in infancy are uncontrolled congestive heart failure, including growth failure or recurrent respiratory infection. Large defects, even in the absence of symptoms, are repaired prior to 2 years of age (often prior to 1 year) if associated with elevated pulmonary artery pressure. Surgery generally is recommended for older, asymptomatic children with normal pulmonary pressure if the pulmonary-to-systemic flow ratio is >2:1. Defects associated with significant left ventricular dilation are often considered appropriate for repair.
There is a lower threshold for operation in patients with VSD and associated aortic insufficiency, especially if the latter is related to aortic cusp prolapse
Coarctation of the Aorta
Prevalence
Coarctation of the aorta occurs in 8% to 10% of all cases of CHD. It is more common in males than in females (male-to-female ratio of 2 to 1). Among patients with Turner’s syndrome, 30% have COA.
Pathology
1. The usual location of COA is juxtaductal, just distal to the left subclavian artery; less often it is proximal to the origin of the left subclavian artery.2. The most common associated anomaly is bicuspid aortic valve, which occurs in more than 50% and up to 85% of all patients with COA. In symptomatic infants with COA, other associated cardiac defects such as aortic hypoplasia, abnormal aortic valve, VSD, and mitral valve anomalies are often present. The coarctation is almost always juxtaductal (i.e., located opposite the entry of the ductus arteriosus)
Symptomatic Infants
Clinical ManifestationsHistory
Poor feeding, dyspnea, or signs of acute circulatory shock may develop in the first 6 weeks of life. The newborn discharge examination may have been normal as a result of incomplete obliteration of the aortic end of the ductus, which would permit blood flow to the descending aorta. After ductal obliteration, the aortic lumen narrows with loss of the space provided by the aortic end of the ductus.
Physical Examination
1. Infants with COA are pale and experience varying degrees of respiratory distress. Oliguria or anuria, general circulatory shock, and severe acidemia are common.2. Peripheral pulses may be weak and thready as a result of CHF. A blood pressure differential may become apparent only after improvement of cardiac function with administration of rapidly acting inotropic agents.
3. The S2 is single and loud; a loud S3 gallop is usually present. No heart murmur is present in 50% of sick infants. A nonspecific ejection systolic murmur is audible over the precordium. The heart murmur may become louder after treatment
Electrocardiography
A normal or rightward QRS axis and RVH or right bundle branch block (RBBB) are present in most infants with COA rather than LVH; LVH is seen in older children
Radiography
Marked cardiomegaly and pulmonary edema or pulmonary venous congestion are usually present.Natural History
About 20% to 30% of all patients with COA develop CHF by 3 month of age.If COA is undetected and untreated in a symptomatic infant, early death may result from CHF and renal shutdown.
Management
Medical
1. In symptomatic neonates, PGE1 infusion should be started to promote ductal patency and establish flow to the descending aorta and the kidneys.
2. Intensive anticongestive measures with short-acting inotropic agents (e.g.,dopamine, dobutamine), diuretics, and oxygen should be started.
Metabolic disturbances (e.g., acidosis and hypoglycemia) should be recognized and treated promptly.
4. When the patient is stabilized, either surgical repair or balloon procedure should be performed because the improvement from anticongestive measures is usually temporary.
Asymptomatic Infants and Children
Clinical ManifestationsMost children are asymptomatic. Occasionally, a child complains of weakness or pain (or both) in the legs after exercise.
Physical Examination
1. Patients grow and develop normally.
2. Arterial pulses in the leg are either absent or weak and delayed. There is hypertension in the arm or the leg systolic pressure is equal to or lower than the arm systolic pressure. In normal children, the oscillometric systolic pressure in the thigh or calf is 5 to 10 mm Hg higher than that in the arm.
With use of the auscultatory method, the leg systolic pressure may be as
much as 20 mm Hg higher than in the arm in normal children .
3. A systolic thrill may be present in the suprasternal notch. The S2 splits
normally, and the A2 is accentuated. An ejection click is frequently audible at the apex or at the base (or both), which may originate in the associated bicuspid aortic valve or from systemic hypertension.
An ejection systolic murmur grade 2 to 4 of 6 is heard at the upper right sternal border and mid or lower left sternal border.
Electrocardiography
Leftward QRS axis and LVH are commonly found. The ECG is normal in approximately 20% of patients.
Radiography
1. The heart size may be normal or slightly enlarged.
2. Dilatation of the ascending aorta may be seen.
3. An E-shaped indentation on the barium-filled esophagus or a “3 sign” on overpenetrated films suggests COA.
4. Rib notching between the fourth and eighth ribs may be seen in older children but rarely in children younger than 5 years of age.
Natural History
1. LV failure, rupture of the aorta, intracranial hemorrhage (i.e., rupture of a berry aneurysm of the arterial circle of Willis), hypertensive encephalopathy, and hypertensive cardiovascular disease are rare complications seen in adulthood.2. A bicuspid aortic valve may cause stenosis or regurgitation with age.
Management
Medical
1. Children with mild COA should be watched regularly for hypertension in the arm and for increasing pressure differences between the arm and leg. Reduced BP readings in the lower extremities may be caused by femoral artery injuries resulting from previous surgeries or interventional procedures.
2. Balloon angioplasty for native unoperated coarctation is controversial, although most centers perform balloon dilatation for recurrent coarctation.
Some centers continue to use the balloon procedure for the native COA, but other centers prefer a surgical approach.
I
Surgical
Indications and Timing1. Significant narrowing of the aorta with pressure gradient greater than 20 to 30 mm Hg is considered an indication for surgery in asymptomatic children.
2. The preferred age for surgery varies from center to center; some centers prefer ages of 2 and 3 years, and others prefer delaying it until 4 to 5 years of age. early surgery (i.e., before 1 year of age)
appears to increase the chance of recoarctation .risk of late recurrence of coarctation is low if the surgery is performed after 2 years of age.
Procedures
1. Through a left thoracotomy incision, extended resection of the coarctation segment and end-to-end anastomosis is the procedure of choice for discrete COA in children
2. Occasionally, subclavian artery aortoplasty or circular or patch grafts may be performed.
Patent Ductus Arteriosus
Prevalence
Patent ductus arteriosus occurs in 5% to 10% of all CHDs, excluding premature infants. It is more common in females than in males (male-to female ratio of 1:3). PDA is a common problem in premature infants
Pathology
1. There is a persistent patency of a normal fetal structure between the pulmonary artery and the descending aorta, that is, about 5 to 10 mm distal to the origin of the left subclavian artery.2. The ductus is usually cone shaped with a small orifice to the PA, which is restrictive to blood flow. The ductus may be short or long, straight, or tortuous.
Clinical Manifestations
History1. Patients are usually asymptomatic when the ductus is small.
2. A large-shunt PDA may cause a lower respiratory tract infection,
atelectasis, and CHF
3. Exertional dyspnea may be present.
Physical Examination 1. Tachycardia and tachypnea may be present in infants with CHF.
2. Bounding peripheral pulses with wide pulse pressure.
3. With a large shunt, the precordium is hyperactive. A systolic thrill may be present at the upper left sternal border. The P2 is usually normal, but its intensity may be accentuated if pulmonary hypertension is present. A grade 1 to 4 of 6 continuous (“machinery”) murmur is best audible at the left infraclavicular area or upper left sternal border.
Electrocardiography
The ECG findings in PDA are similar to those in VSD. A normal ECG or LVH is seen with small to moderate PDA. BVH is seen with large PDA. If
pulmonary vascular obstructive disease develops, RVH is present.
Radiography
Radiographs are also similar to those of VSD.
1. Chest radiographs may be normal with a small-shunt PDA.
2. Cardiomegaly of varying degrees occurs in moderate-to large-shunt PDA with enlargement of the LA, LV, and ascending aorta. Pulmonary vascular markings are increased.
3. With pulmonary vascular obstructive disease, the heart size becomes
normal, with a marked prominence of the PA segment and hilar vessels.
Natural History
1. Unlike PDA in premature infants, spontaneous closure of a PDA is rare in full-term infants and children. This is because the PDA in term infants results from a structural abnormality of the ductal smooth muscle rather than a decreased responsiveness of the ductal smooth muscle to oxygen.2. CHF or recurrent pneumonia develops if the shunt is large.
3. Pulmonary vascular obstructive disease may develop if a large PDA with pulmonary hypertension is left untreated.
4. Although rare, an aneurysm of PDA may develop and possibly rupture in adult life.
Medical
Unlike in premature infants with PDAs, indomethacin is ineffective in term infants and should not be used.Medical Management
Patients having small PDA are asymptomatic and lead a normal life except the risk of developing infective endarteritis. Previously it was left as such but now a days PDA is closed either by catheter closure (device or coil closure) or by surgical ligation mainly to prevent the deadly complication of infective endarteritis. Infants with CHF are
treated with decongestive therapy before closure of ductus.
.No exercise restriction is needed in the absence of pulmonary
hypertension
. Nonsurgical occlusion of PDA has become a standard of care at many centers except in patients with very low birth weight..
Small ductus (<3 mm in diameter) are closed by various kinds
of coils and larger ones by the Amplatzer PDA device.
For larger PDA but smaller than 12 mm in diameter, specialized devices, such as the Amplatzer duct occluder, are available for catheter-based closure. The devices are implanted antegrade from the femoral vein.
Although original recommendations from the manufacturer exclude
patients who weigh less than 6 kg, successful use in infants as small as 2.5 kg has been reported. There is a 98% or greater closure rate at 6 months with minimal complications and no mortality.
Surgical Closure
Surgical closure is reserved for patients in whom a nonsurgical closure technique is not considered applicable.Patent Ductus Arteriosus in Preterm Neonates
PrevalenceClinical evidence of PDA appears in 45% of infants with a birth weight less than 1750 g and in about 80% of infants with a birth weight less than 1200 g.
Significant PDA with CHF occurs in 15% of premature infants with a birth weight less than 1750 g and in 40% to 50% of those with a birth weight less than 1500 g.
Pathophysiology
1. PDA is a special problem in premature infants who are recovering from hyaline membrane disease. With improvement in oxygenation, the PVR falls rapidly, but the ductus remains patent because its responsiveness to oxygen is immature in premature newborns. The resulting large left to-right shunt makes the lung stiff, and weaning the infant from the ventilator and oxygen therapy becomes difficult.2. Premature infants with significant left-to-right shunt may suffer from the consequences of prolonged hypoperfusion to many organs, which may include intracranial hemorrhage, renal dysfunction, myocardial ischemia, and necrotizing enterocolitis. Early recognition and appropriate management of PDA are keys to improving the prognosis of these infants.
Clinical Manifestations
The history is important in suspecting a significant PDA in a premature neonate. Typically, a premature infant with hyaline membrane disease shows some improvement during the first few days after birth. This is followed by an inability to wean the infant from the ventilator or a need to increase ventilator settings or oxygen requirements in 4- to 7-day-old premature infants. Episodes of apnea or bradycardia may be the initial sign of PDA ininfants who are not on ventilators.
Management
For symptomatic infants, either pharmacologic or surgical closure of the ductus is indicated. A small PDA that does not cause symptoms should be followed medically for 6 months without surgical ligation because of the possibility of spontaneous closure.Medical
1. Fluid restriction to 120 mL/kg per day and a diuretic (e.g., furosemide, 1mg/kg two to three times a day) may be tried for 24 to 48 hours, but these regimens have a low success rate. Digoxin is not used because it has little hemodynamic benefit
2. Pharmacologic closure of the PDA can he achieved with indomethacin (a prostaglandin synthetase inhibitor). One popular approach is to give indomethacin (Indocin) 0.2 mg/kg intravenously every 12 hours for up to three doses in selected cases. A second course of indomethacin treatment is occasionally necessary to achieve adequate ductal closure.