
DIABETES MELLITUS

•
Diabetes mellitus is a clinical syndrome characterised by an increase in plasma
blood glucose (hyperglycaemia).
•
Diabetes has many causes but is most commonly due to type 1 or type 2
diabetes.
Type 1 diabetes
• is caused by autoimmune
destruction of insulin-
producing cells (β cells)
in the pancreas, resulting
in absolute insulin
deficiency
Type 2 diabetes
• is characterised by
resistance to the action of
insulin and an inability to
produce sufficient insulin
to overcome this ‘insulin
resistance’.

•
Hyperglycaemia results in both acute and long-term
problems. Acutely, high glucose and lack of insulin can result
in marked symptoms, metabolic decompensation and
hospitalisation.
•
Chronic hyperglycaemia is responsible for diabetes-specific
‘microvascular’ complications affecting the eyes
(retinopathy), kidneys (nephropathy) and feet (neuropathy).

NORMAL GLUCOSE AND FAT METABOLISM
•
Blood glucose is tightly regulated and maintained within a narrow range. This is
essential for ensuring a continuous supply of glucose to the central nervous system.
•
The brain has little capacity to store energy in the form of glycogen or triglyceride
and the blood–brain barrier is largely impermeable to fatty acids, so the brain
depends on the liver for a constant supply of glucose for oxidation and hence
generation of adenosine triphosphate (ATP).
•
Glucose homeostasis is achieved through the coordinated actions of multiple organs,
but mainly reflects a balance between the entry of glucose into the circulation from
the liver, supplemented by intestinal absorption of glucose after meals, and the
uptake of glucose by peripheral tissues, particularly skeletal muscle and brain.

NORMAL GLUCOSE AND FAT METABOLISM
After ingestion of a meal containing carbohydrate,
normal blood glucose levels are maintained by:
Suppression
of hepatic
glucose
production
Stimulation of
hepatic
glucose
uptake
Stimulation of
glucose
uptake by
peripheral
tissues
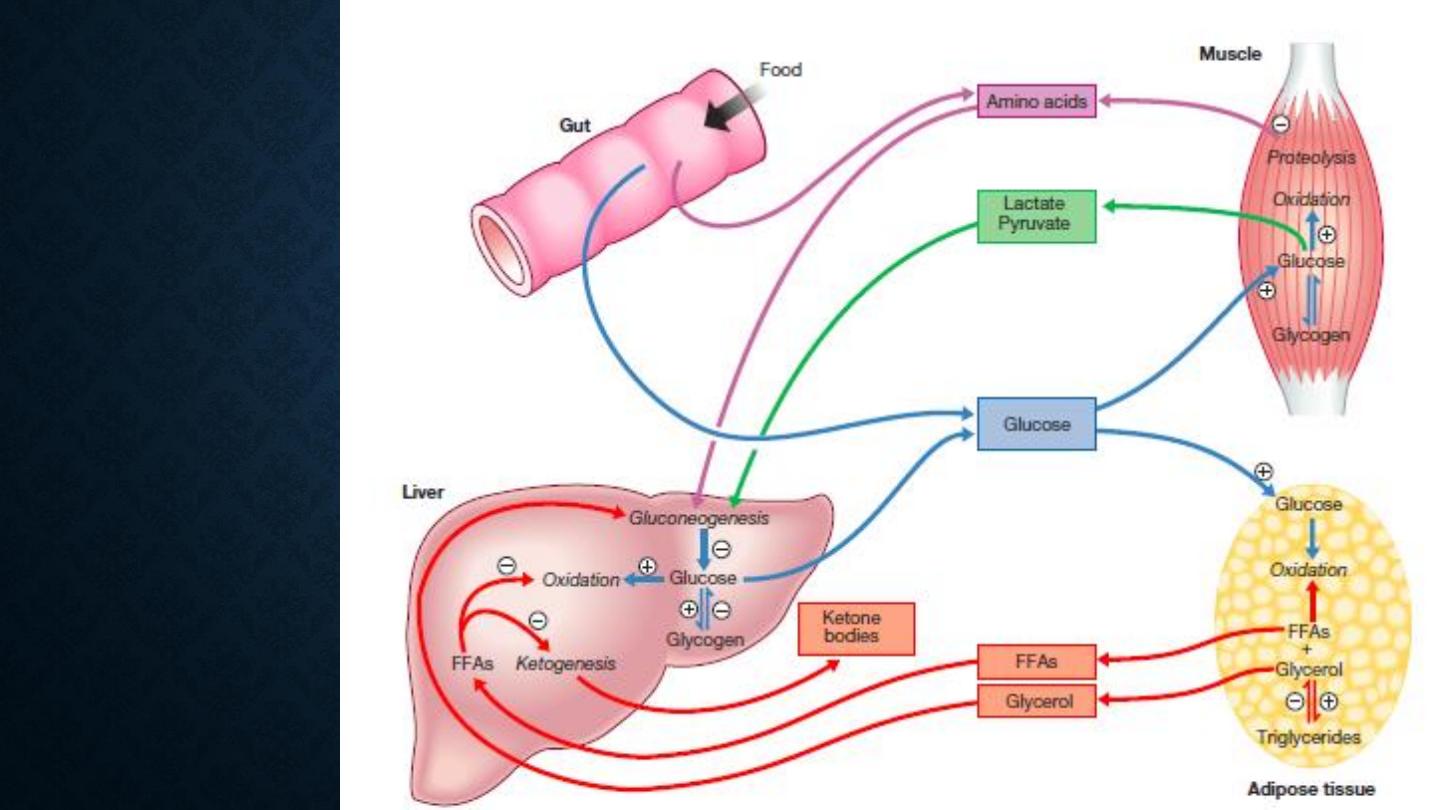
Major metabolic
pathways of
fuel metabolism
and the actions
of insulin

•
Insulin, the primary regulator of glucose metabolism and
storage is secreted from pancreatic β cells into the portal
circulation in response to a rise in blood glucose
•
A number of other factors released from the gut following
food intake can augment insulin release, including amino
acids and hormones such as glucagon-like peptide 1 (GLP-1)
and gastrointestinal peptide (GIP).
•
As a result, insulin release is greater when glucose is
administered by mouth than when the same rise in plasma
glucose is achieved by intravenous glucose infusion, a
phenomenon termed the ‘incretin’ effect

•
The post-prandial rise in portal vein insulin and glucose,
together with a fall in portal
•
When intestinal glucose absorption declines between meals,
portal vein insulin and glucose concentrations fall while
glucagon levels rise. This leads to increased hepatic glucose
output via gluconeogenesis and glycogen breakdown.
•
The liver now resumes net glucose production and glucose
homeostasis is maintained.
•
The main substrates for gluconeogenesis are glycerol and
amino acids, their use in production of energy leads to
ketosis
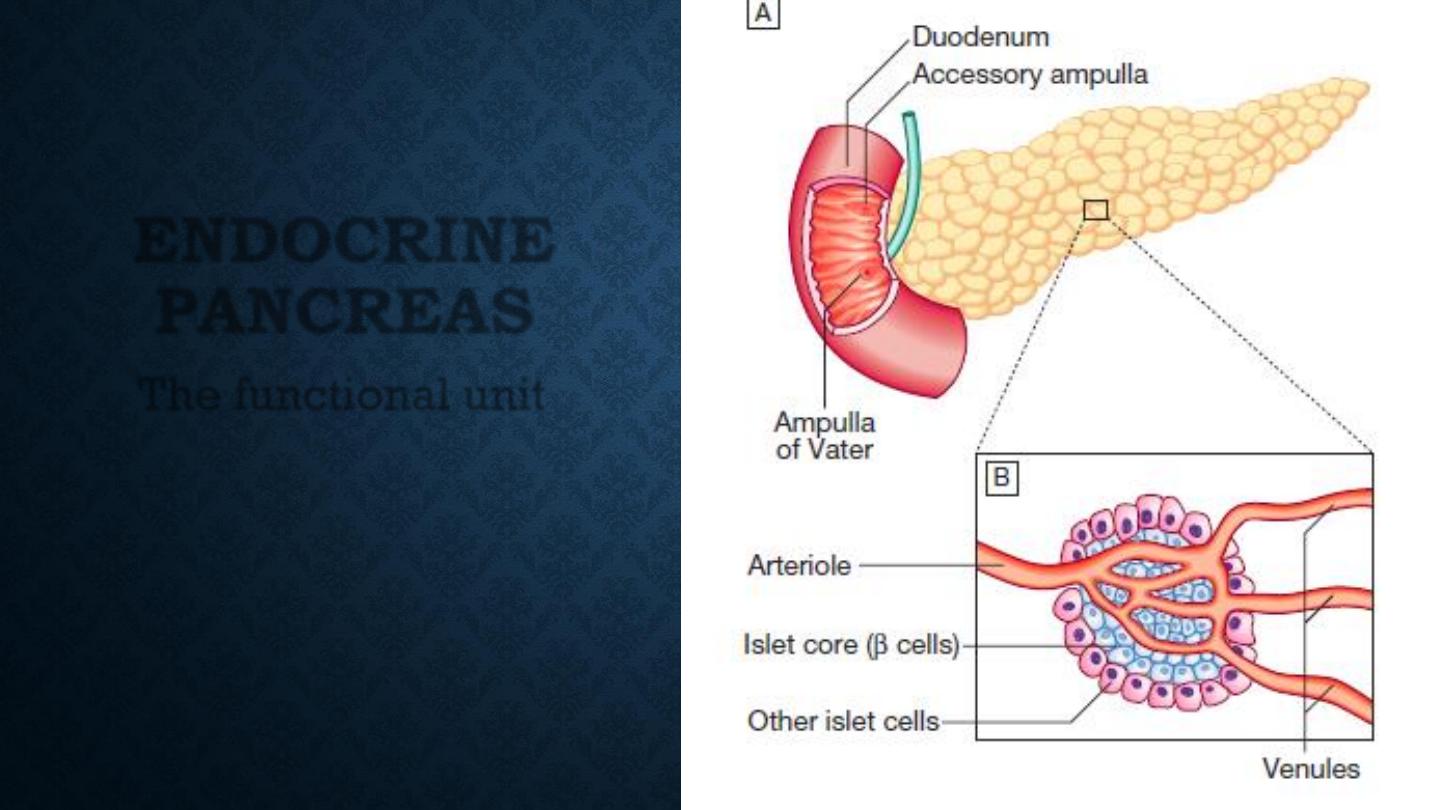
ENDOCRINE
PANCREAS
The functional unit
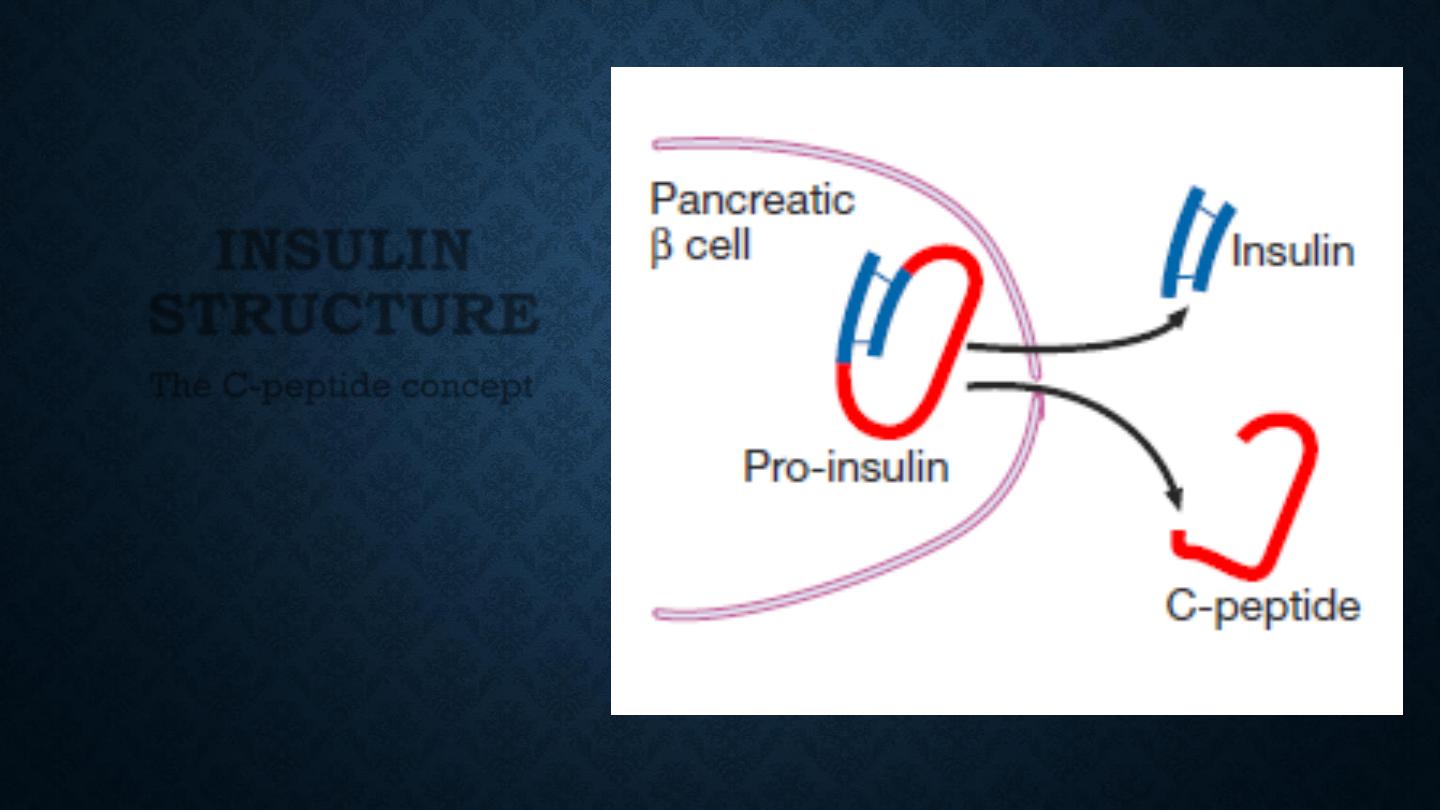
INSULIN
STRUCTURE
The C-peptide concept
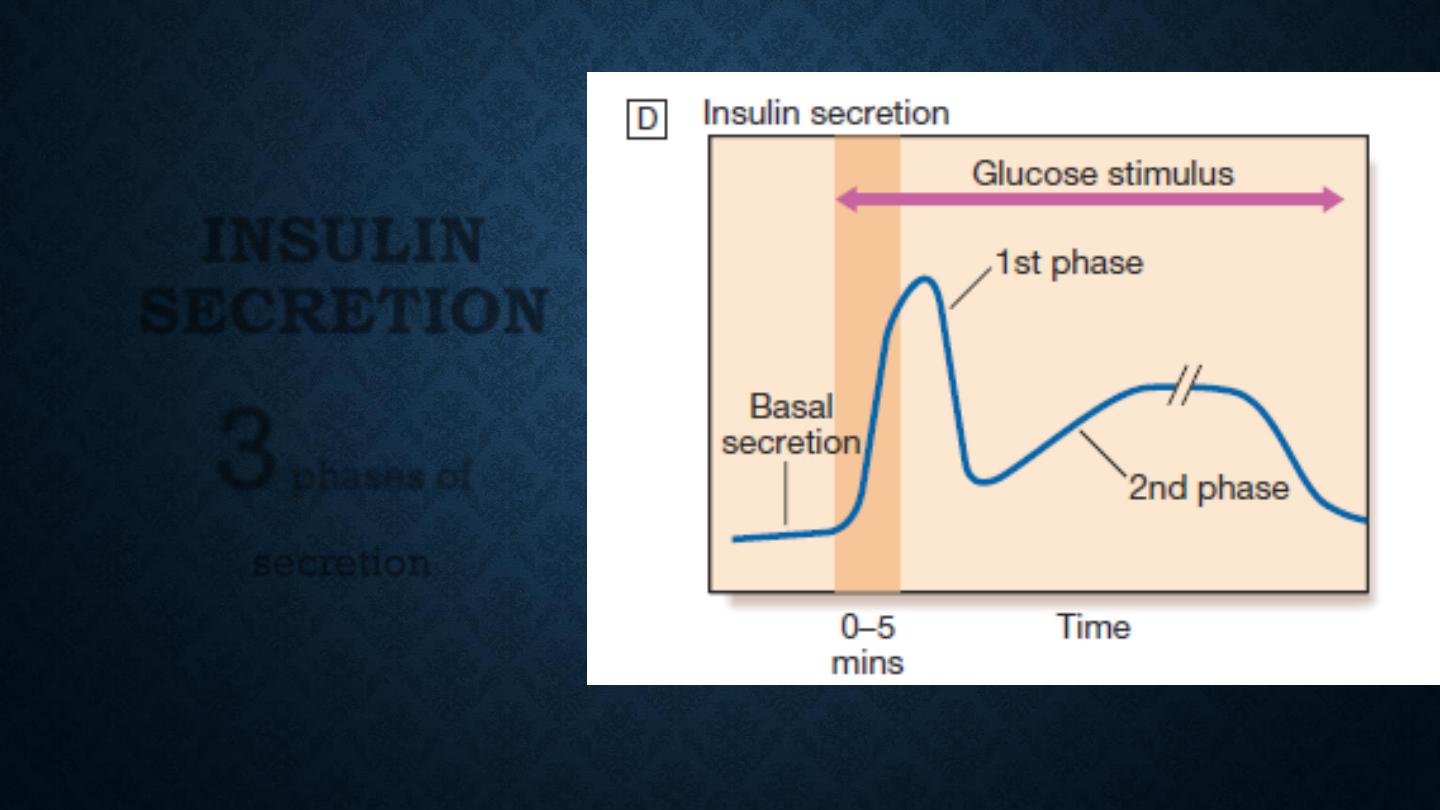
INSULIN
SECRETION
3
phases of
secretion
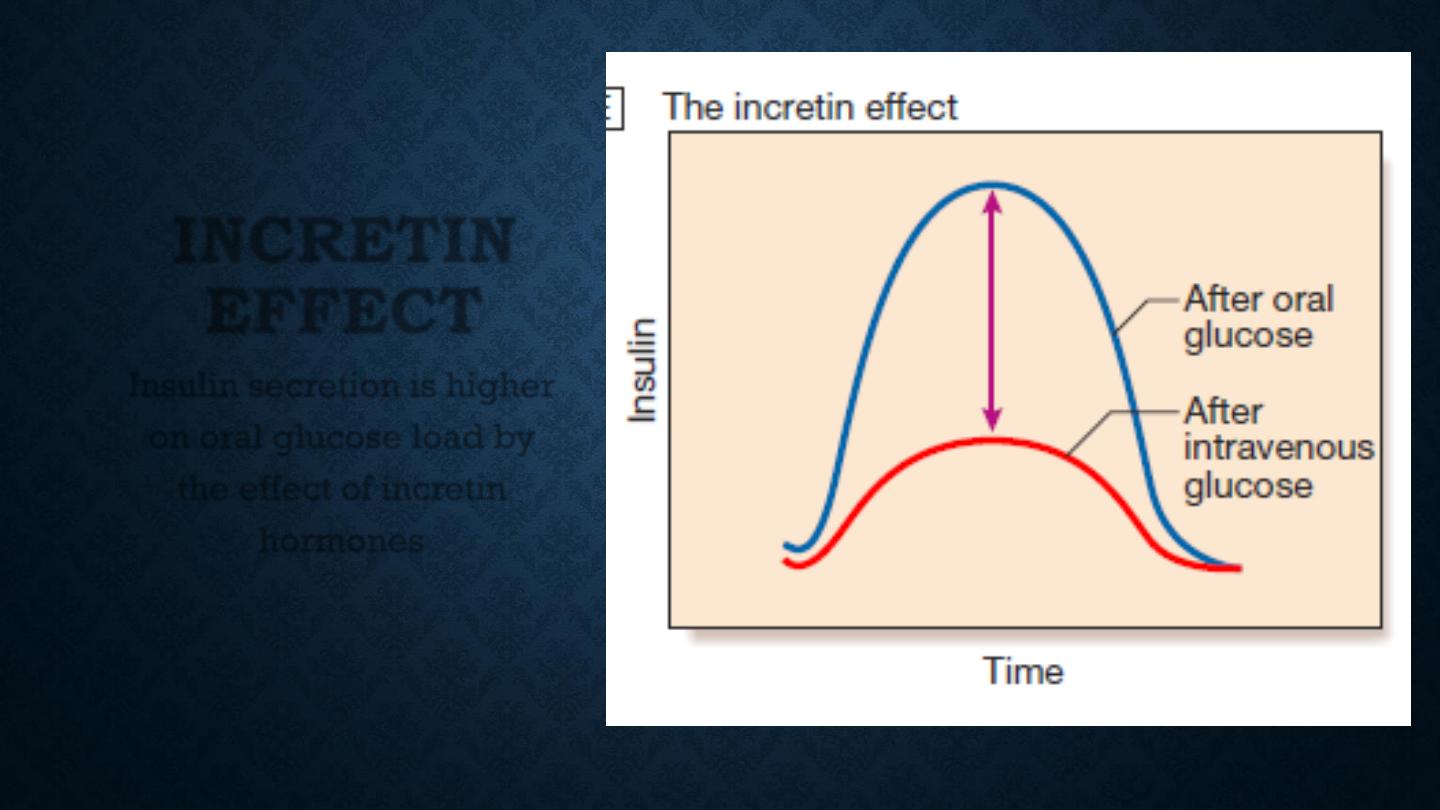
INCRETIN
EFFECT
Insulin secretion is higher
on oral glucose load by
the effect of incretin
hormones
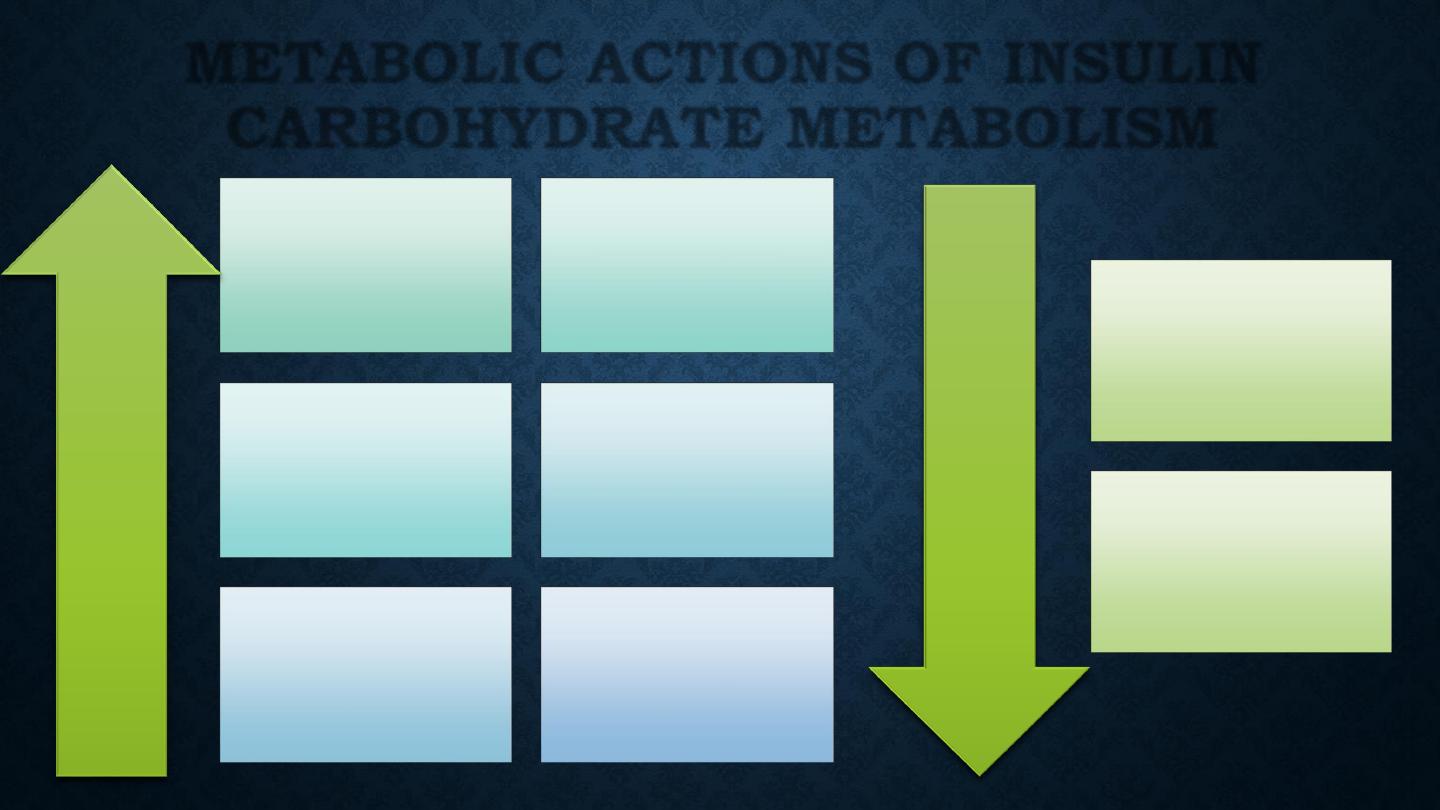
Glucose
transport
(muscle, adipose
tissue)
Glucose
phosphorylation
Glycogen
synthesis
Glycolysis
Pyruvate
dehydrogenase
Activity
Pentose
phosphate shunt
Incr
ease
Decr
ease
Gluconeogenesis
Glycogenolysis
METABOLIC ACTIONS OF INSULIN
CARBOHYDRATE METABOLISM
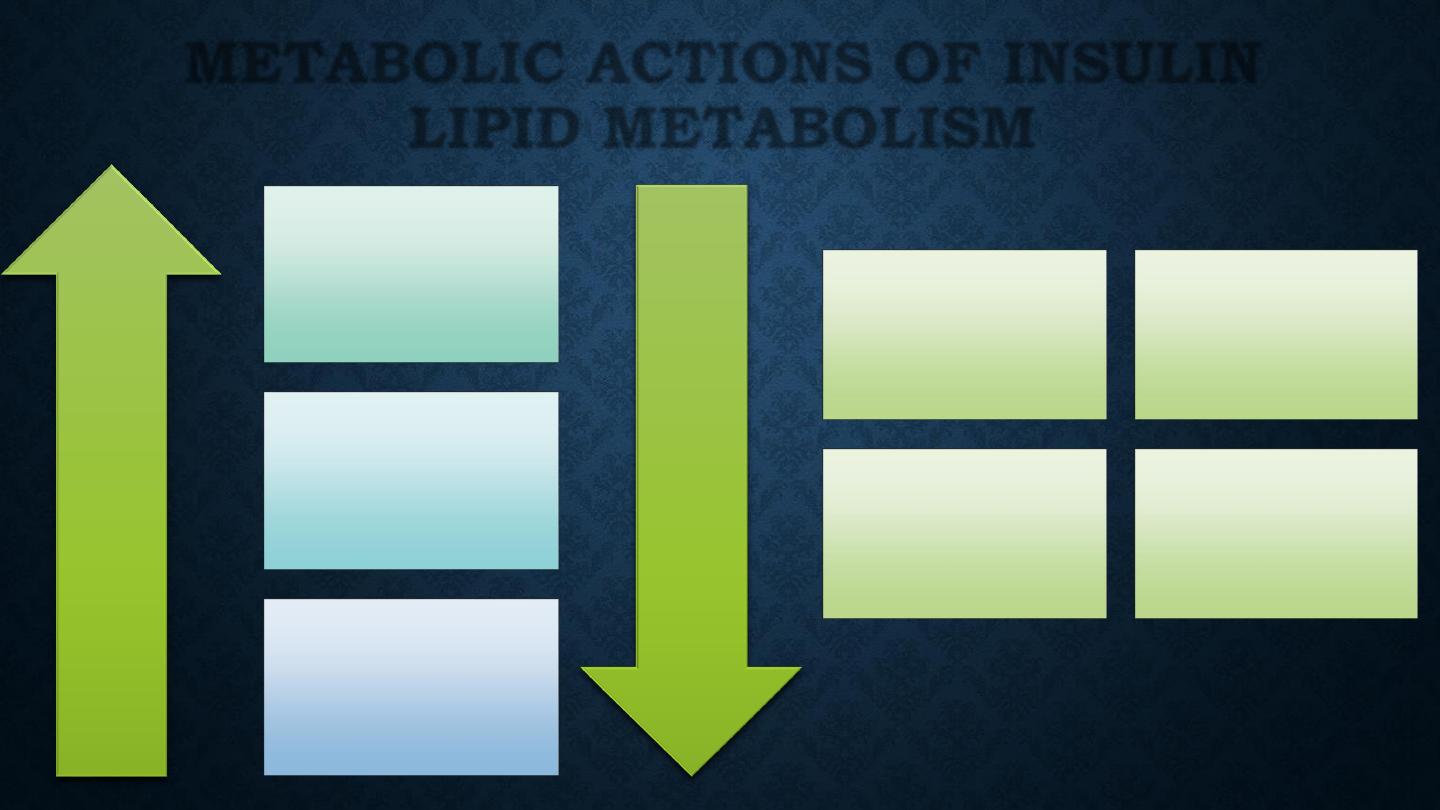
Triglyceride
synthesis
Fatty acid
synthesis (liver)
Lipoprotein
lipase activity
(adipose tissue)
Incr
ease
Decr
ease
Lipolysis
Lipoprotein
lipase
(muscle)
Ketogenesis
Fatty acid
oxidation
(liver)
METABOLIC ACTIONS OF INSULIN
LIPID METABOLISM
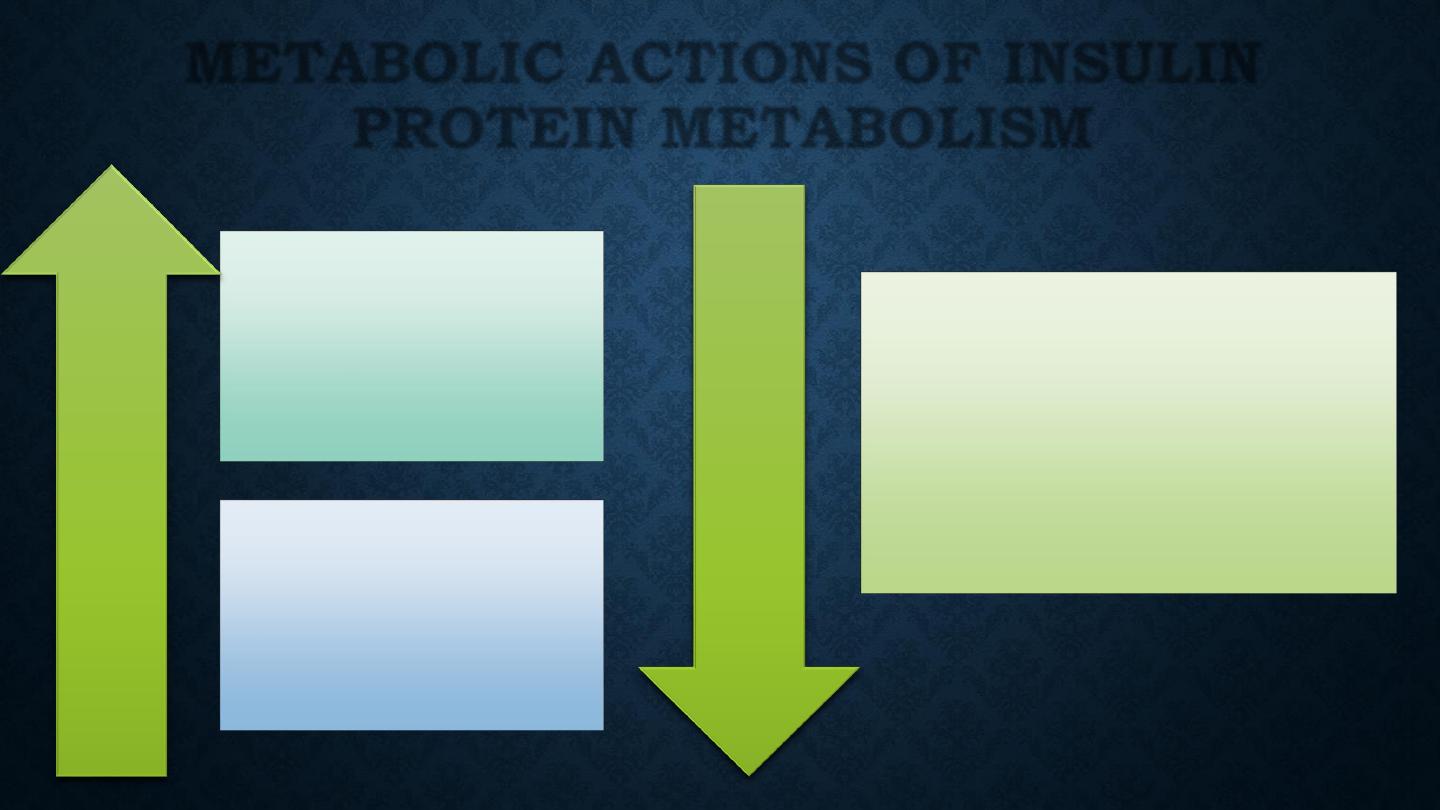
Amino acid
transport
Protein
synthesis
Incr
ease
Decr
ease
Protein
degradation
METABOLIC ACTIONS OF INSULIN
PROTEIN METABOLISM

PATHOGENESIS OF
DIABETES

•
In both of the common types of diabetes, environmental
factors interact with genetic susceptibility to determine
which people develop the clinical syndrome, and the timing
of its onset.
•
However, the underlying genes, precipitating environmental
factors and pathophysiology differ substantially between
type 1 and type 2 diabetes.

TYPE 1 DIABETES
PATHOLOGY
•
Type 1 diabetes is a T cell-mediated autoimmune disease
involving destruction of the insulin-secreting β cells in the
pancreatic islets.
•
Progressive loss of β cell function takes place over a prolonged
period (months to years), but marked hyperglycaemia,
accompanied by the classical symptoms of diabetes, occurs only
when 80–90% of the functional capacity of β cells has been lost.
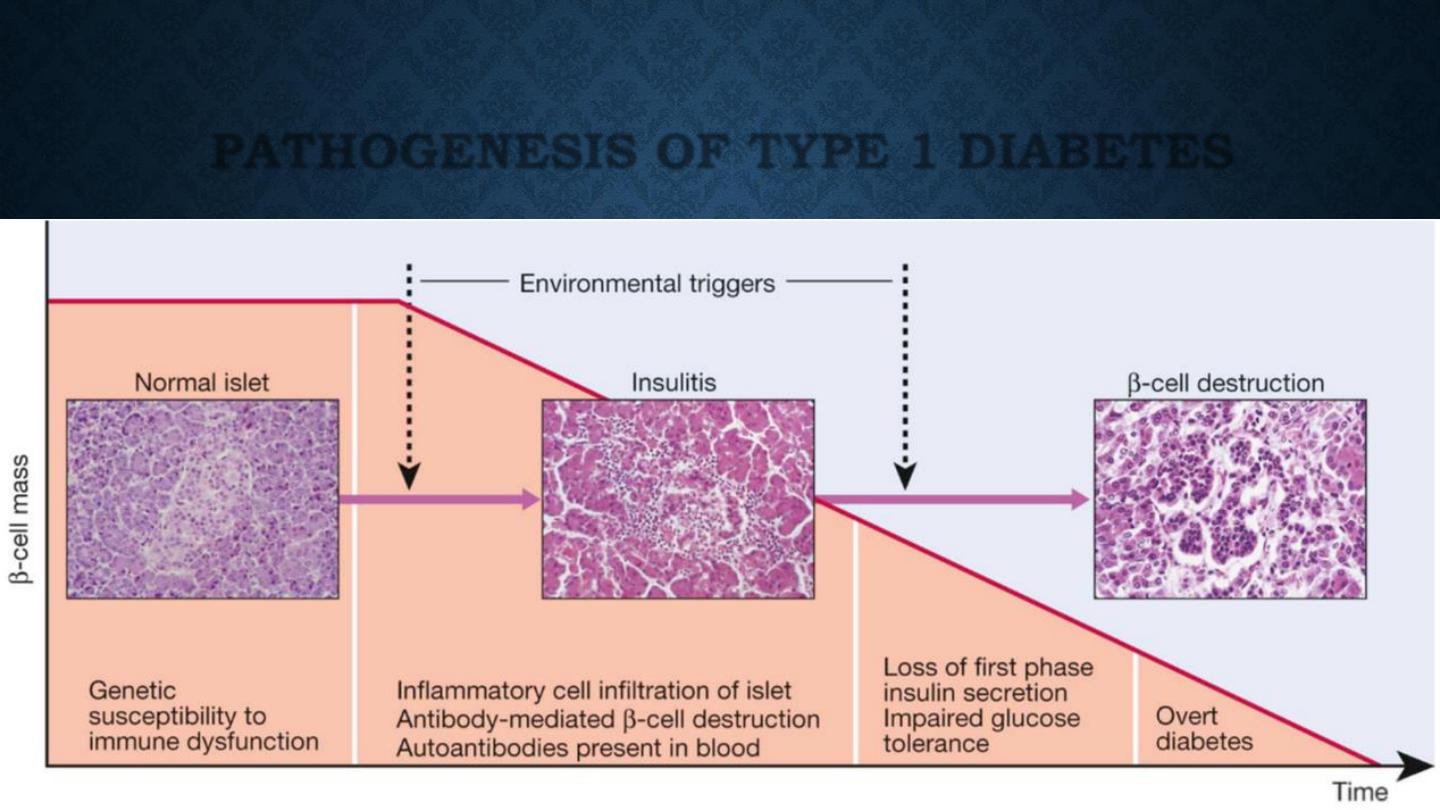
PATHOGENESIS OF TYPE 1 DIABETES

METABOLIC DISTURBANCES IN TYPE 1
DIABETES
•
Patients with type 1 diabetes present when progressive β-
cell destruction has crossed a threshold at which adequate
insulin secretion and normal blood glucose levels can no
longer be sustained.
•
Above a certain level, high glucose levels may be toxic to the
remaining β cells, so that profound insulin deficiency rapidly
ensues, causing the metabolic sequelae shown in figure in
the next slide.
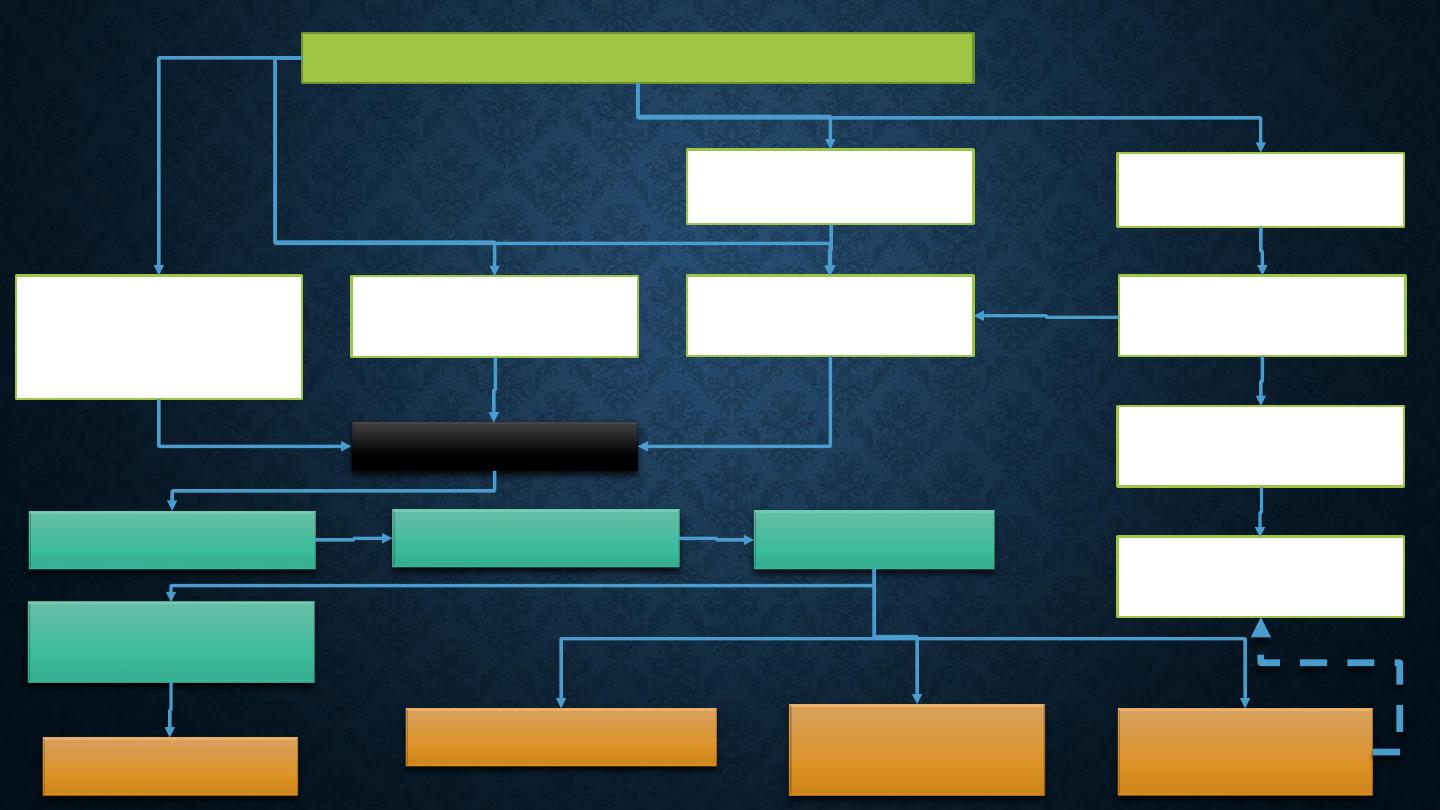
Insufficient insulin
Increase FFA &
Glycerol to liver
Increase
proteolysis
Increase lipolysis
Increase
gluconeogenesis
Increase
glycogenolysis
Decreased glucose
uptake &
Utilization
Increase
ketogenesis
Metabolic acidosis
Hyperglycemia
Glycosuria
Osmotic diuresis
Dehydration
Secondary
hyperldosteronism
K
+
deficiency
Hyperosmolarity
Impaired renal
function
Increased
lactate

•
Hyperglycaemia leads to glycosuria and dehydration, causing fatigue,
polyuria, nocturia, thirst and polydipsia, susceptibility to urinary and genital
tract infections, and later tachycardia and hypotension.
•
Unrestrained lipolysis and proteolysis result in weight loss.
•
Ketoacidosis occurs when generation of ketone bodies exceeds the capacity
for their metabolism.
•
Elevated blood H
+
ions drive K
+
out of the intracellular compartment, while
secondary hyperaldosteronism encourages urinary loss of K
+
.
•
Thus patients usually present with a short history (typically a few weeks) of
hyperglycaemic symptoms (thirst, polyuria, nocturia and fatigue), infections
and weight loss, and may have developed ketoacidosis.

TYPE 2 DIABETES
PATHOLOGY
•
Type 2 diabetes is a diagnosis of exclusion, i.e. it is made
when type 1 diabetes and other types of diabetes are ruled
out, and is highly heterogeneous.
•
The natural history of typical type 2 diabetes is shown in
following figure.
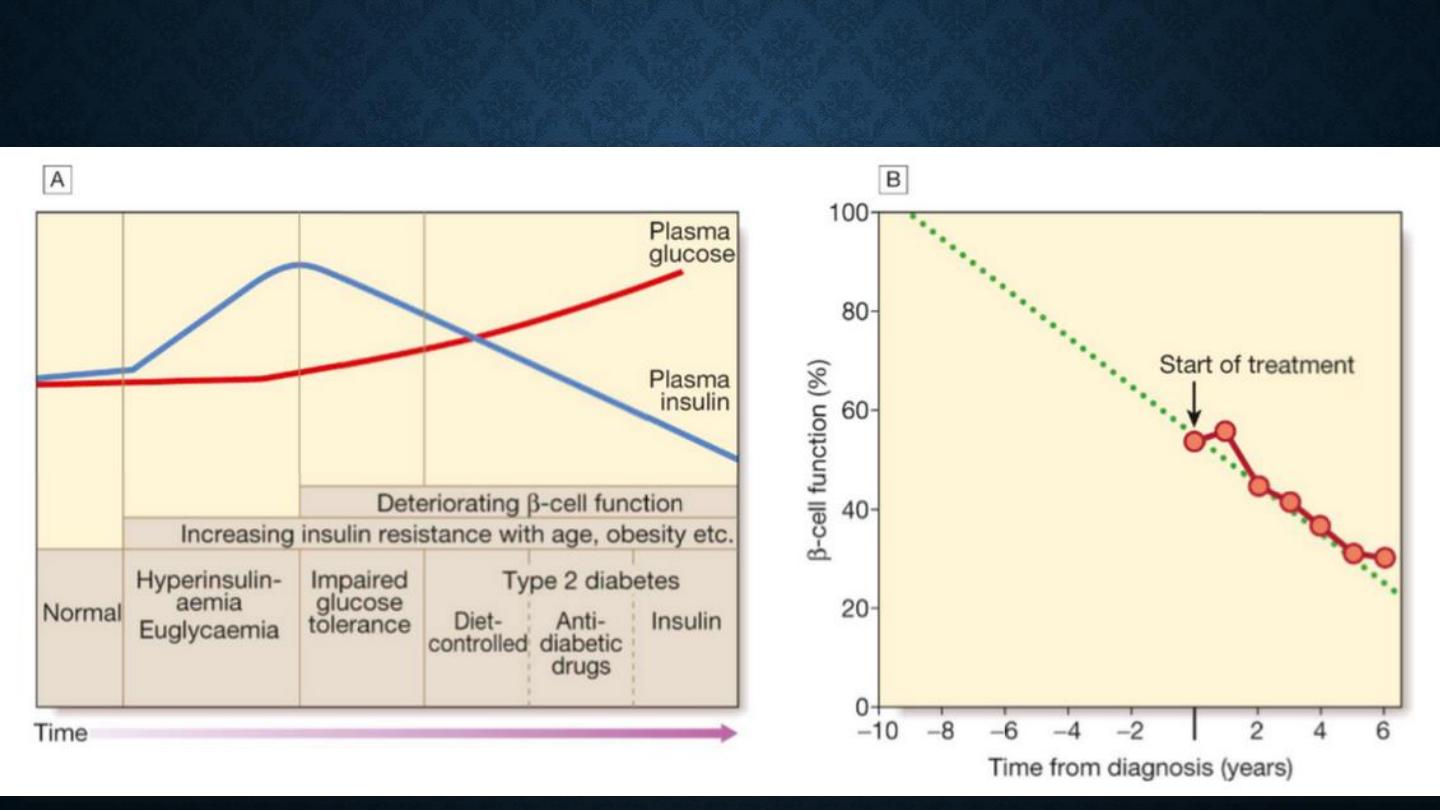

•
Initially, insulin resistance leads to elevated insulin secretion in order to maintain
normal blood glucose levels.
•
However, in susceptible individuals, the pancreatic β cells are unable to sustain
the increased demand for insulin and a slowly progressive insulin deficiency
develops.
•
Some patients develop diabetes at a young age, usually driven by insulin
resistance due to obesity and ethnicity; others, particularly the elderly, develop
diabetes despite being non-obese and may have more pronounced β-cell failure.
•
The key feature is a ‘relative’ insulin deficiency, such that there is insufficient
insulin production to overcome the resistance to insulin action.
•
This contrasts with type 1 diabetes, in which there is rapid loss of insulin
production and an absolute deficiency, resulting in ketoacidosis and death if the
insulin is not replaced.

BASIC PATHOPHYSIOLOGY IN TYPE 2 DM
•
Insulin resistance
•
Obesity
•
Genetic predisposition
•
Environmental factors

AETIOLOGICAL CLASSIFICATION OF
DIABETES MELLITUS
•
Type 1 diabetes
•
Immune-mediated
•
Idiopathic
•
Type 2 diabetes
•
Gestational diabetes
•
Other specific types

AETIOLOGICAL CLASSIFICATION OF
DIABETES MELLITUS
•
Other specific types
•
Genetic defects of β-cell function
•
Genetic defects of insulin action (e.g. leprechaunism,
lipodystrophies)
•
Pancreatic disease (e.g. pancreatitis, pancreatectomy,
neoplastic disease, cystic fibrosis, haemochromatosis,
fibrocalculous pancreatopathy)

AETIOLOGICAL CLASSIFICATION OF
DIABETES MELLITUS
•
Other specific types
•
Excess endogenous production of hormonal antagonists to
insulin, e.g.
•
Growth hormone – acromegaly
•
Glucocorticoids – Cushing’s syndrome
•
Glucagon – glucagonoma
•
Catecholamines – phaeochromocytoma
•
Thyroid hormones – thyrotoxicosis

AETIOLOGICAL CLASSIFICATION OF
DIABETES MELLITUS
•
Other specific types
•
Drug-induced (e.g. corticosteroids, thiazide diuretics, phenytoin)
•
Uncommon forms of immune-mediated diabetes (e.g. IPEX
(immunodysregulation polyendocrinopathy X) syndrome)
•
Associated with genetic syndromes (e.g. Down’s syndrome; Klinefelter’s
syndrome; Turner’s syndrome; DIDMOAD (Wolfram’s syndrome) –
diabetes insipidus, diabetes mellitus, optic atrophy, nerve deafness;
Friedreich’s ataxia; myotonic dystrophy)
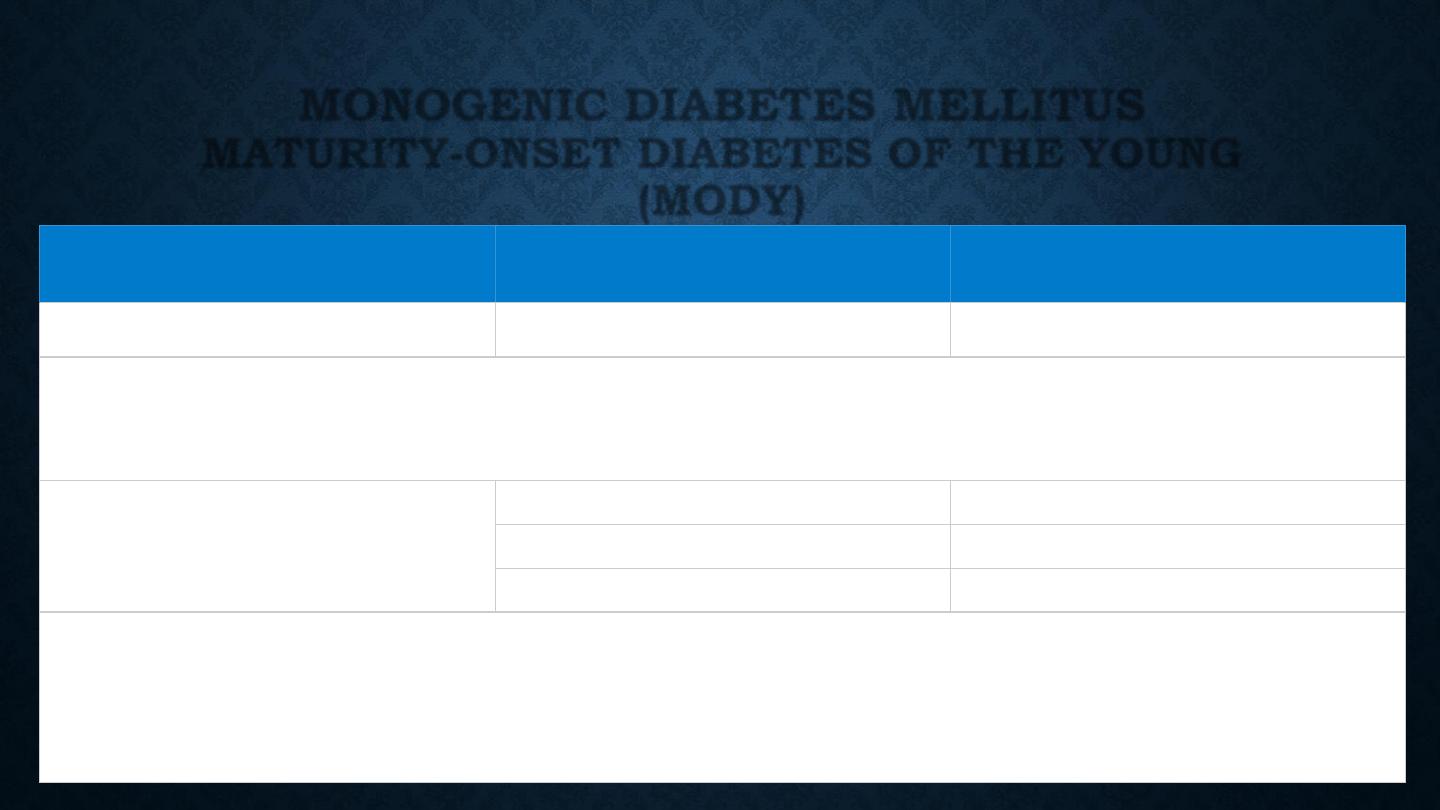
MONOGENIC DIABETES MELLITUS
MATURITY-ONSET DIABETES OF THE YOUNG
(MODY)
Functional defect
Main type
*
Gene mutated
*
β-cell glucose sensing
MODY2
GCK
The set point for basal insulin release is altered, causing a high fasting glucose, but sufficient insulin
is released after meals. As a result, the HbA
1c
is often normal and microvascular complications are
rare. Treatment is rarely required
β-cell transcriptional regulation
MODY3
HNF-1α
MODY5
HNF-1β
MODY1
HNF-4α
Diabetes develops during adolescence/early adulthood and can be managed with diet and tablets for
many years, but ultimately, insulin treatment is required. The HNF-1
α and 4α forms respond
particularly well to sulphonylurea drugs. All types are associated with microvascular complications.
HNF-1
β mutations also cause renal cysts and renal failure
