IMMUNOLOGY
Dr. Ali Abdul-Rahman YounisRHEUMATOLOGIST(FIBMS)
FUNCTIONAL ANATOMY AND PHYSIOLOGY OF THE IMMUNE SYSTEM
The immune system consists of an intricately linked network of cells, proteins and lymphoid organs that are strategically placed to ensure maximal protection against infection.Immune defences are normally categorised into the innate immune response, which provides immediate protection against an invading pathogen, and the adaptive or acquired immune response, which takes more time to develop but confers exquisite specificity and longlasting protection.
The innate immune system
Innate defences against infection include anatomical barriers, phagocytic cells, soluble molecules, such as complement and acute phase proteins, and natural killer cells.The innate immune system recognises generic microbial structures present on nonmammalian tissue and can be mobilised within minutes.
A specific stimulus will elicit essentially identical responses in different individuals (in contrast with antibody and Tcell responses, which vary greatly between individuals).
Constitutive barriers to infection
SkinThe tightly packed, highly keratinised cells of the skin constantly undergo renewal , which physically limits colonisation by microorganisms.
Microbial growth is inhibited by physiological factors, such as low pH and low oxygen tension, and sebaceous glands secrete hydrophobic oils that further repel water and microorganisms.
Sweat also contains lysozyme, an enzyme that destroys the structural integrity of bacterial cell walls; ammonia, which has antibacterial properties; and several antimicrobial peptides such as defensins.
Constitutive barriers to infection
The mucous membranes of the respiratory, gastrointestinal and genitourinary tract provide a constitutive barrier to infection.
Secreted mucus acts to trap invading pathogens, and immunoglobulin A (IgA) prevents bacteria and viruses attaching to and penetrating epithelial cells.
Lysozyme and antimicrobial peptides within mucosal membranes can directly kill invading pathogens.
Within the respiratory tract, cilia directly trap pathogens and contribute to removal of mucus, assisted by physical manœuvres, such as sneezing and coughing.
In the gastrointestinal tract, hydrochloric acid and salivary amylase chemically destroy bacteria, while normal peristalsis and induced vomiting or diarrhoea assist clearance of invading organisms.
Phagocytes
Phagocytes (‘eating cells’) are specialised cells which ingest and kill microorganisms, scavenge cellular and infectious debris, and produce inflammatory molecules which regulate other components of the immune system.They include neutrophils, monocytes and macrophages, and are particularly important for defence against bacterial and fungal infections.
Phagocytes express a wide range of surface receptors that allow them to identify microorganisms.
Phagocytes
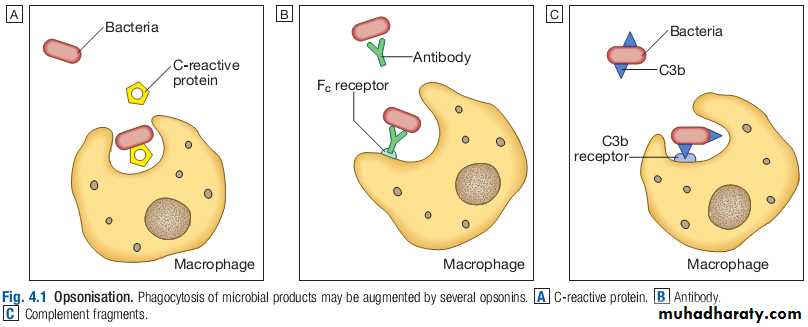
While phagocytes can recognise microorganisms through pattern recognition receptors alone, engulfment of microorganisms is greatly enhanced by opsonisation.Opsonins include acute phase proteins such as Creactive protein (CRP), antibodies and complement.
They bind both to the pathogen and to phagocyte receptors, acting as a bridge between the two to facilitate phagocytosis.
Neutrophils
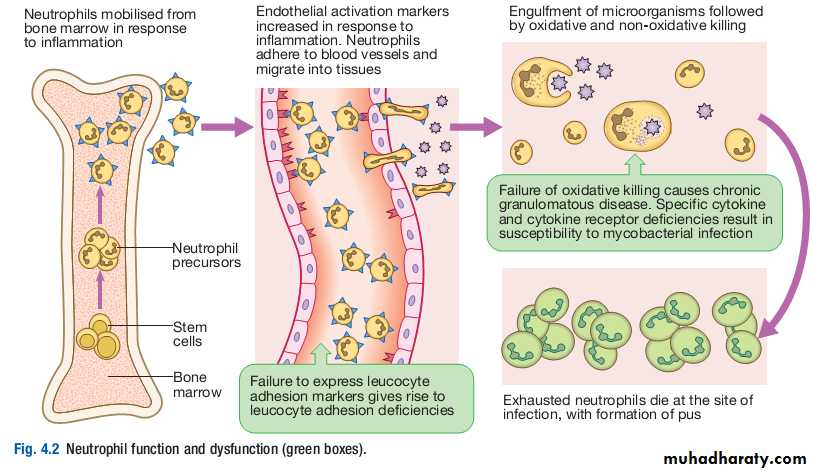
Neutrophils, also known as polymorphonuclear leucocytes, are derived from the bone marrow. They are shortlived cells with a halflife of 6 hours in the blood stream.
Their functions are to kill microorganisms directly, facilitate the rapid transit of cells through tissues, and nonspecifically amplify the immune response.
This is mediated by enzymes contained in granules which also provide an intracellular milieu for the killing and degradation of microorganisms.
The two main types of granule are primary or azurophil granules, and the more numerous secondary or specific granules. Primary granules contain myeloperoxidase and other enzymes important for intracellular killing and digestion of ingested microbes.
Secondary granules are smaller and contain lysozyme, collagenase and lactoferrin, which can be released into the extracellular space. Granule staining becomes more intense in response to infection (‘toxic granulation’), reflecting increased enzyme production.
Monocytes and macrophages
Monocytes are the precursors of tissue macrophages. They are produced in the bone marrow and constitute about 5% of leucocytes in the circulation.From the blood stream, they migrate to peripheral tissues, where they differentiate into tissue macrophages and reside for long periods.
Specialised populations of tissue macrophages include Kupffer cells in the liver, alveolar macrophages in the lung, mesangial cells in the kidney, and microglial cells in the brain.
Macrophages are capable of phagocytosis and killing of microorganisms but also play an important role in the amplification and regulation of the inflammatory response .
They are particularly important in tissue surveillance, monitoring their immediate surroundings for signs of tissue damage or invading organisms.
Dendritic cells
Dendritic cells are specialised antigenpresenting cells which are prevalent in tissues in contact with the external environment, such as the skin and mucosa. They can also be found in an immature state in the blood.They sample the environment for foreign particles, and once activated, carry microbial antigens to regional lymph nodes, where they interact with T cells and B cells to initiate and shape the adaptive immune response
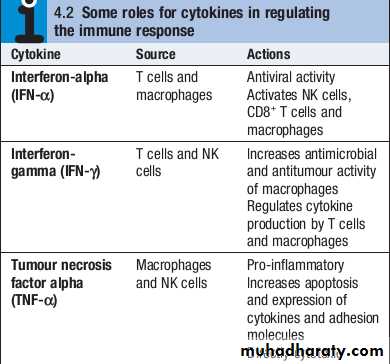
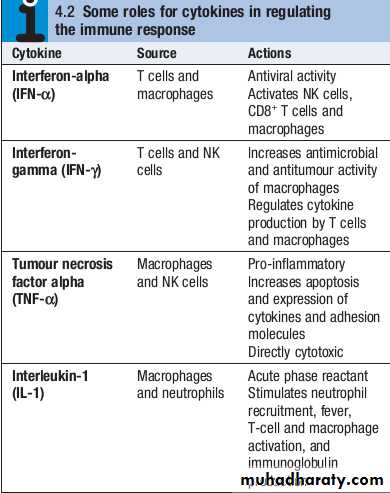
Cytokines
Cytokines are small soluble proteins that act as multipurpose chemical messengers.
They are produced by cells involved in immune responses and by stromal tissue.
More than 100 cytokines have been described, with overlapping, complex roles in intercellular communication.
Their clinical importance is demonstrated by the efficacy of ‘biological’ therapies that target specific cytokines.
Complement
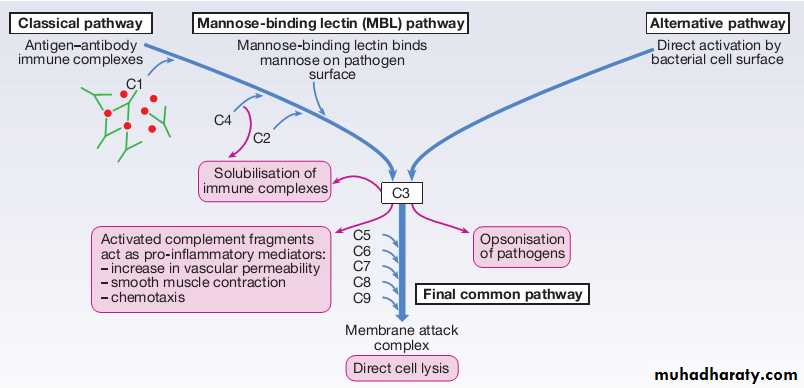
The complement system is a group of more than 20 tightly regulated, functionally linked proteins that act to promote inflammation and eliminate invading pathogens.Complement proteins are produced in the liver and are present in the circulation as inactive molecules.
When triggered, they enzymatically activate other proteins in a rapidly amplified biological cascade analogous to the coagulation cascade.
There are three mechanisms by which the complement cascade may be triggered.
Complement
The alternative pathwayis triggered directly by binding of C3 to bacterial cell wall components, such as lipopolysaccharide of Gramnegative bacteria and teichoic acid of Grampositive bacteria.The classical pathwayis initiated when two or more IgM or IgG antibody molecules bind to antigen, forming immune complexes. The associated conformational change exposes binding sites on the antibodies for C1. C1 is a multiheaded molecule which can bind up to six antibody molecules. Once two or more ‘heads’ of a C1 molecule are bound to antibody, the classical cascade is triggered.
The lectin pathwayis activated by the direct binding of mannosebinding lectin to microbial cell surface carbohydrates. This mimics the binding of C1 to immune complexes and directly stimulates the classical pathway.
Complement
Activation of complement by any of these pathways results in activation of C3. This, in turn, activates the final common pathway, in which the complement proteins C5–C9 assemble to form the membrane attack complex. This can puncture target cell walls, leading to osmotic cell lysis.This step is particularly important in the defence against encapsulated bacteria, such as Neisseriaspp. and Haemophilus influenzae.
Complement fragments generated by activation of the cascade can also act as opsonins, rendering microorganisms more susceptible to phagocytosis by macrophages and neutrophils .
Complement fragments are chemotactic agents, promoting leucocyte trafficking to sites of inflammation.
Some fragments act as anaphylotoxins, binding to complement receptors on mast cells and triggering release of histamine, which increases vascular permeability.
The products of complement activation also help to target immune complexes to antigenpresenting cells, providing a link between the innate and the acquired immune systems.
Mast cells and basophils
Mast cells and basophils are bone marrowderived cells which play a central role in allergic disorders. Mast cells reside predominantly in tissues exposed to the external environment, such as the skin and gut, while basophils are located in the circulation and are recruited into tissues in response to inflammation.Both contain large cytoplasmic granules which contain preformed vasoactive substances such as histamine.
Mast cells and basophils express IgE receptors on their cell surface.
On encounter with specific antigen, the cell is triggered to release preformed mediators and synthesise additional mediators, including leukotrienes, prostaglandins and cytokines.
These trigger an inflammatory cascade which increases local blood flow and vascular permeability, stimulates smooth muscle contraction, and increases secretion at mucosal surfaces.
Natural killer cells
Natural killer (NK) cells are large granular lymphocytes which play a major role in defence against tumours and viruses.They exhibit features of both the adaptive and innate immune systems: they are morphologically similar to lymphocytes and recognise similar ligands, but they are not antigenspecific and cannot generate immunological memory.
NK cells express a variety of cell surface receptors. Some recognise stress signals, while others recognise the absence of human leucocyte antigen (HLA) molecules on cell surfaces.
NK cells can also be activated by binding of antigen–antibody complexes to surface receptors. This physically links the NK cell to its target in a manner analogous to opsonisation, and is known as antibodydependent cellular cytotoxicity (ADCC)
Natural killer cells
Activated NK cells can kill their targets in various ways.
Poreforming proteins, such as perforin, induce direct cell lysis, while granzymes are proteolytic enzymes which stimulate apoptosis.
In addition, NK cells produce a variety of cytokines, such as tumour necrosis factor (TNF)αand interferonγ(IFNγ), which have direct antiviral and antitumour effects
The adaptive immune system
If the innate immune system fails to provide effective protection against an invading pathogen, the adaptive immune system is mobilised.This has three key characteristics:
• It has exquisite specificity and is able to discriminate between very small differences in molecular structure.
• It is highly adaptive and can respond to an unlimited number of molecules.
• It possesses immunological memory, such that subsequent encounters with a particular antigen produce a more effective immune response than the first encounter.
There are two major arms of the adaptive immune response: humoral immunity involves antibodies produced by B lymphocytes; cellular immunity is mediated by T lymphocytes, which release cytokines and kill immune targets.
These interact closely with each other and with the innate immune system, to maximise the effectiveness of the response.
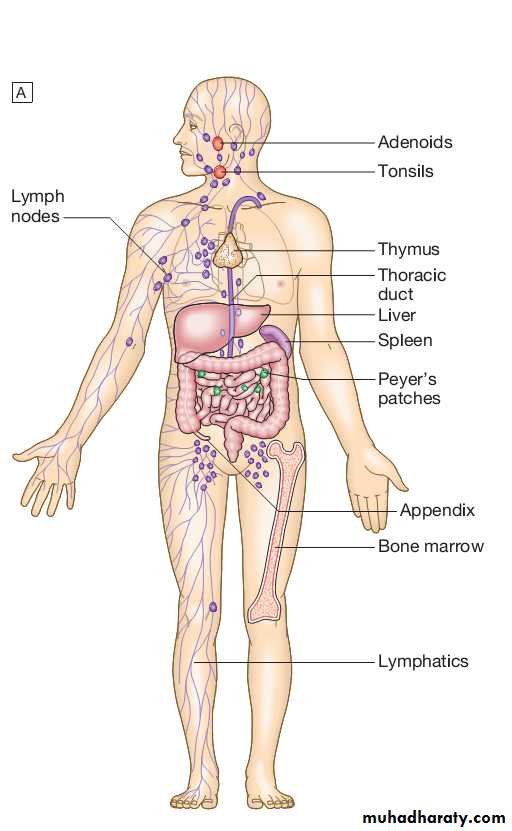
Lymphoid organs
Primary lymphoid organs.The primary lymphoid organs are involved in lymphocyte development.
They include the bone marrow, where both T and B lymphocytes are derived from haematopoietic stem cells and where B lymphocytes also mature, and the thymus, where T lymphocytes mature.
Secondary lymphoid organs.
After maturation, lymphocytes migrate to the secondary lymphoid organs.
These include the spleen, lymph nodes and mucosaassociated lymphoid tissue.
These organs trap and concentrate foreign substances, and are the major sites of interaction between naïve lymphocytes and microorganisms.
The thymus
The thymus is a bilobed structure organised into cortical and medullary areas.
The cortex is densely populated with immature T cells, which migrate to the medulla to undergo selection and maturation.
The thymus is most active in the fetal and neonatal period, and involutes after puberty.
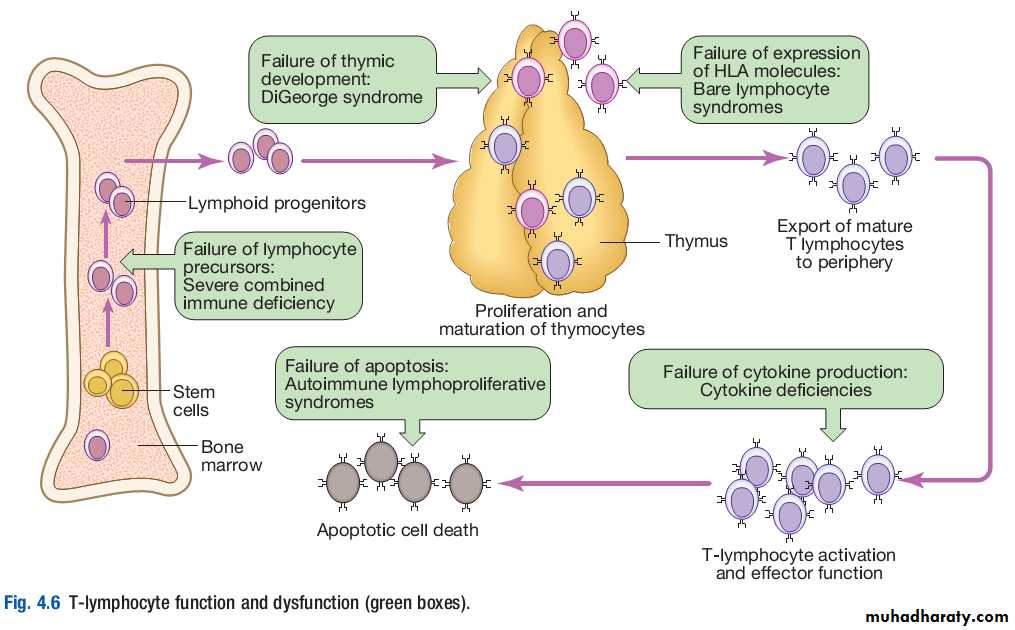
Failure of thymic development is associated with profound Tcell immune deficiency , but surgical removal of the thymus in childhood (usually in the context of major cardiac surgery) is not associated with significant immune dysfunction.
The spleen
The spleen is the largest of the secondary lymphoid organs.It is highly effective at filtering blood and is an important site of phagocytosis of senescent erythrocytes, bacteria, immune complexes and other debris.
It is also a major site of antibody synthesis.
It is particularly important for defence against encapsulated bacteria, and asplenic individuals are at risk of overwhelming Streptococcus pneumoniae and H. influenzae infection.
Lymph nodes and mucosa-associated lymphoid tissue
Lymph nodes are positioned to maximise exposure to lymph draining from sites of external contact.Their structure is highly organised.
More diffuse unencapsulated lymphoid cells and follicles are also present on mucosal surfaces: for example, in Peyer’s patches in the small intestine.
Lymphatics
Lymphoid tissues are physically connected by a network of lymphatics, which has three major functions: it provides access to lymph nodes, returns interstitial fluid to the venous system, and transports fat from the small intestine to the blood stream.The lymphatics begin as blindending capillaries, which come together to form lymphatic ducts. These enter and then leave regional lymph nodes as afferent and efferent ducts respectively.
They eventually coalesce and drain into the thoracic duct and thence into the left subclavian vein.
Lymphatics may be either deep or superficial, and, in general, follow the distribution of major blood vessels.
Humoral immunity
B lymphocytesThese specialised cells arise in the bone marrow. Mature B lymphocytes (also known as B cells) are found in bone marrow, lymphoid tissue, spleen and, to a lesser extent, the blood stream.
They express a unique immunoglobulin receptor on their cell surface (the Bcell receptor), which binds to soluble antigen.
Encounters with antigen usually occur within lymph nodes, where, if provided with appropriate signals from nearby T lymphocytes, stimulated antigenspecific B cells respond by proliferating rapidly in a process known as clonal expansion.
This is accompanied by a highly complex series of genetic rearrangements, which generates Bcell populations that express receptors with greater affinity for antigen than the original.
These cells differentiate into either long-lived memory cells, which reside in the lymph nodes, or plasma cells, which produce antibody.
Immunoglobulins
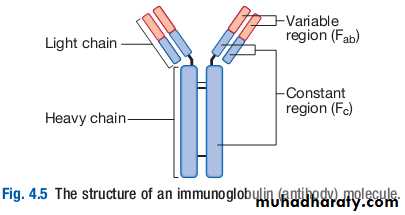
Immunoglobulins (Ig) are soluble proteins made up of two heavy and two light chains.The heavy chain determines the antibody class or isotype, i.e. IgG, IgA, IgM, IgE or IgD. Subclasses of IgG and IgA also occur.
The antigen is recognised by the antigenbinding regions (Fab) of both heavy and light chains, while the consequences of antibodybinding are determined by the constant region of the heavy chain (Fc).
Antibodies can initiate a number of different actions. They facilitate phagocytosis by acting as opsonins , and can also facilitate cell killing by cytotoxic cells (ADCC ). Binding of antibodies to antigen can trigger activation of the classical complement pathway. In addition, antibodies may act directly to neutralise the biological activity of toxins. This is a particularly important feature of IgA antibodies, which act predominantly at mucosal surfaces.
Immunoglobulins
Immunoglobulins
The humoral immune response is characterised by immunological memory: that is, the antibody response to successive exposures to antigen is qualitatively and quantitatively different from that on first exposure.When a previously unstimulated (naïve) B lymphocyte is activated by antigen, the first antibody to be produced is IgM, which appears in the serum after 5–10 days. Depending on additional stimuli provided by T lymphocytes, other antibody classes (IgG, IgA and IgE) are produced 1–2 weeks later.
If, some time later, a memory B cell is reexposed to antigen, the lag time between antigen exposure and the production of antibody is decreased (to 2–3 days), the amount of antibody produced is increased, and the response is dominated by IgG antibodies of high affinity.
In contrast o the initial antibody response, secondary antibody responses do not require additional input from T lymphocytes. This allows the rapid generation of highly specific responses on pathogen reexposure.
Cellular immunity
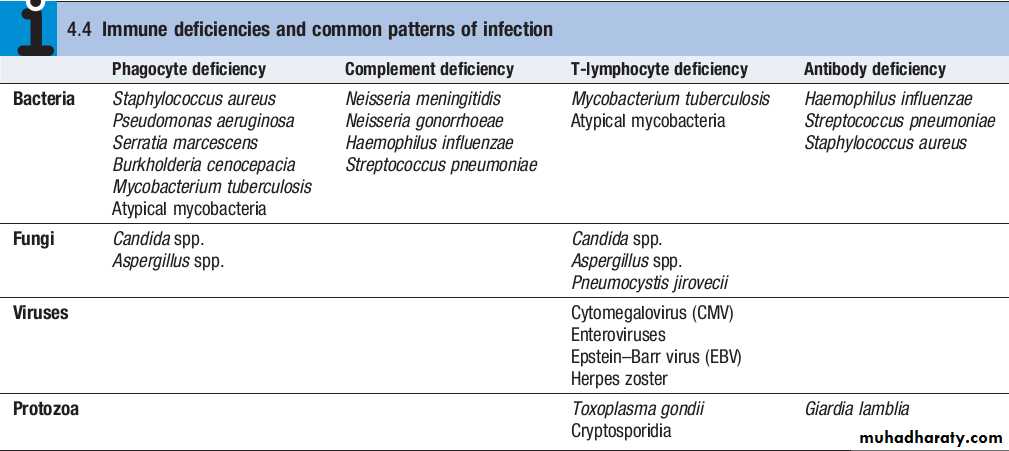
T lymphocytes (also known as T cells) mediate cellular immunity and are important for defence against viruses, fungi and intracellular bacteria.They also play an important immunoregulatory role, orchestrating and regulating the responses of other components of the immune system.
Tlymphocyte precursors arise in bone marrow and are exported to the thymus while still immature.
Within the thymus, each cell expresses a Tcell receptor with a unique specificity. These cells undergo a process of stringent selection to ensure that autoreactive T cells are deleted.
Mature T lymphocytes leave the thymus and expand to populate other organs of the immune system. It has been estimated that an individual possesses 107 –109 Tcell clones, each with a unique Tcell receptor, ensuring at least partial coverage for any antigen encountered.
Cellular immunity
T cells respond to protein antigens, but they cannot recognise these in their native form. Instead, intact protein must be processed into component peptides which bind to a structural framework on the cell surface known as HLA (human leucocyte antigen). This process is known as antigen processing and presentation, and it is the peptide/HLA complex which is recognised by individual T cells.While all nucleated cells have the capacity to process and present antigens, specialised antigenpresenting cells include dendritic cells, macrophages and B lymphocytes.
T lymphocytes can be segregated into two subgroups on the basis of function and recognition of HLA molecules. These are designated CD4+and CD8+T cells, according to the ‘cluster of differentiation’ (CD) antigen expressed on their cell surface.
Cellular immunity
CD8+T cells recognise antigenic peptides in association with HLA class I molecules (HLAA, HLAB, HLAC). They kill infected cells directly through the production of poreforming molecules such as perforin, or by triggering apoptosis of the target cell, and are particularly important in defence against viral infection.
CD4+T cells recognise peptides presented on HLA class II molecules (HLADR, HLADP and HLADQ) and have mainly immunoregulatory functions. They produce cytokines and provide costimulatory signals that support the activation of CD8+T lymphocytes and assist the production of antibody by B cells.
Cellular immunity
CD4+lymphocytes can be further subdivided into subsets on the basis of the cytokines they produce:• Th1 cells produce IL2, IFNγand TNFα, and support the development of delayed type hypersensitivity responses .
• Th2 cells typically secrete IL4, IL5 and IL10, and promote allergic responses .
• A further subset of specialised CD4+lymphocytes known as regulatory cells are important in immune regulation of other cells and the prevention of autoimmune disease.
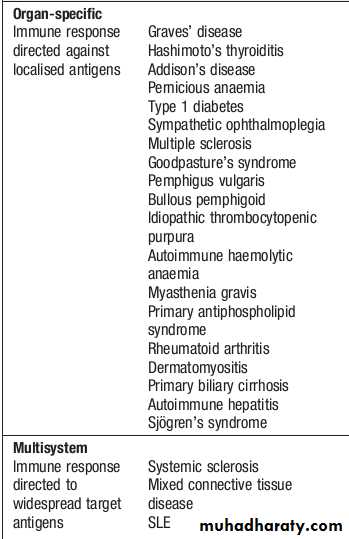
AUTOIMMUNE DISEASE
Autoimmunity can be defined as the presence of immune responses against self tissue.This may be a harmless phenomenon, identified only by the presence of low titre autoantibodies or autoreactive T cells.
However, if these responses cause significant organ damage, this results in autoimmune diseases, which are a major cause of chronic morbidity and disability, affecting up to 1 in 30 adults at some time .
Immunological tolerance
Autoimmunity results from the failure of immunological tolerance, the process by which the immune system recognises and accepts self tissue.
There are a number of mechanisms of immune tolerance.
Central tolerance occurs during lymphocyte development, when T and B lymphocytes that recognise self antigens are eliminated before they differentiate into fully immunocompetent cells. This process is most active in fetal life, but continues throughout life as immature lymphocytes are generated.
Immunological tolerance
Inevitably some autoreactive cells evade deletion and reach the peripheral tissues, where they are controlled by peripheral tolerance mechanisms. These include suppression of autoreactive cells by regulatory T cells, generation of functional hyporesponsiveness (‘anergy’) in lymphocytes which encounter antigen in the absence of the costimulatory signals that accompany inflammation, and T cell death by apoptosis.Autoimmune diseases develop when selfreactive lymphocytes escape from these tolerance mechanisms and become activated.
Factors predisposing to autoimmune disease
Autoimmune diseases are much more common in women than in men, for reasons which remain unclear.Most autoimmune diseases have multiple genetic determinants. Many are associated with variation at specific HLA loci, reflecting the importance of HLA genes in shaping lymphocyte responses.
Other important susceptibility genes include those determining cytokine activity, costimulation and cell death.
Several acquired factors can trigger autoimmunity in genetically predisposed individuals, including infection, cigarette smoking and hormone levels. The most widely studied of these is infection, as occurs in acute rheumatic fever following streptococcal infection or reactive arthritis following bacterial infection.
Factors predisposing to autoimmune disease
A number of mechanisms have been postulated, such as crossreactivity between the infectious pathogen and self antigens (molecular mimicry), and release of sequestered antigens, which are not usually visible to the immune system, from damaged tissue.Alternatively, infection may result in the production of inflammatory cytokines, which overwhelm the normal control mechanisms that prevent bystander damage.
Occasionally, the development of autoimmune disease is a sideeffect of drug treatment. For example, the metabolic products of the anaesthetic agent halothane bind to liver enzymes, resulting in a structurally novel protein. This is recognised as a new (foreign) antigen by the immune system, and the autoantibodies and activated T cells directed against it may cause hepatic necrosis.
Classification of autoimmune diseases
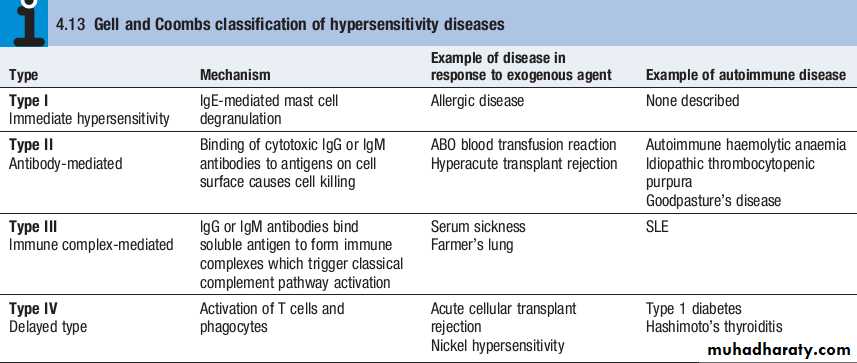
The spectrum of autoimmune diseases is broad. They can be classified by organ involvement or by the predominant mechanism responsible for tissue damage.The Gell and Coombs classification of hypersensitivity is the most widely used, and distinguishes four types of immune response which result in bystander tissue damage.
• Type I hypersensitivityis relevant in allergy but is not associated with autoimmune disease.
• In type II hypersensitivity, injury is localised to a single tissue or organ and is mediated by specific autoantibodies.
• Type III hypersensitivityis a generalised reaction resulting from immune complex deposition which initiates activation of the classical complement cascade, as well as recruitment and activation of phagocytes and CD4+lymphocytes. The site of immune complex deposition is determined by the relative amount of antibody, size of the immune complexes, nature of the antigen and local haemodynamics. Generalised deposition of immune complexes gives rise to systemic diseases such as SLE.
• In type IV hypersensitivity, activated T cells and macrophages mediate phagocytosis and tissue damage.
ALLERGY
Allergic diseases are a common and increasing cause of illness, affecting between 15% and 20% of the population at some time.They comprise a range of disorders from mild to lifethreatening, and affect many organs.
Atopy is the tendency to produce an exaggerated IgE immune response to otherwise harmless environmental substances, while an allergic disease can be defined as the clinical manifestation of this inappropriate IgE immune response.
Pathology of allergy
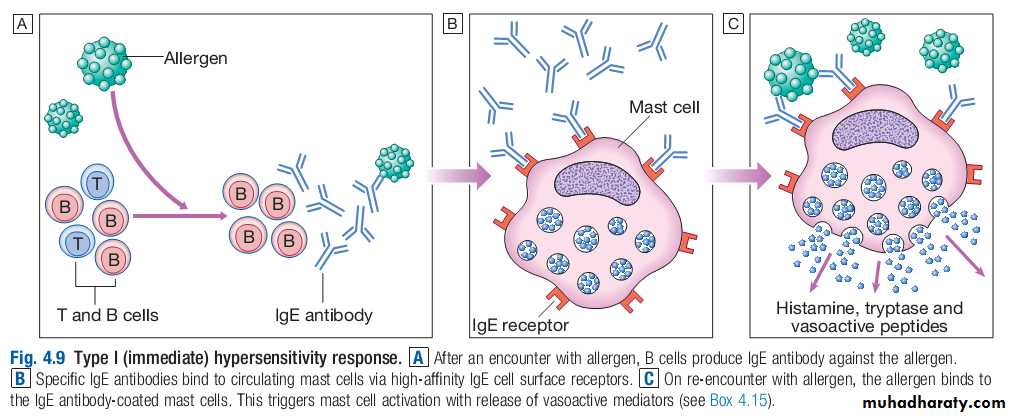
Normally, the immune system does not make detectable responses to the many environmental substances to which it is exposed daily.However, in an allergic reaction, initial exposure to an otherwise harmless exogenous substance (known as an allergen) triggers the production of specific IgE antibodies by activated B cells.
These IgE antibodies bind to the surface of mast cells via highaffinity IgE receptors, a step that is not itself associated with clinical sequelae.
However, upon reexposure, the allergen binds to membranebound IgE which activates the mast cells, releasing a variety of mediators .
This type I hypersensitivity reaction is the basis of the symptoms of allergic reactions, which range from sneezing and rhinorrhoea to anaphylaxis
Susceptibility to allergic diseases
The incidence of allergic diseases is increasing. This trend is largely unexplained but one widely held theory is the ‘hygiene hypothesis’.
This proposes that infections in early life are critically important in maturation of the immune response and bias the immune system against the development of allergies.
It is suggested that the high prevalence of allergic disease is the penalty for the decreased exposure to infection that has resulted from improvements in sanitation and health care.
A number of factors predispose to allergic diseases, the strongest of which is a family history. A wide array of genetic determinants of disease susceptibility have been identified, including genes controlling innate immune responses, cytokine production, IgE levels and the ability of the epithelial barrier to protect against environmental agents.
Contributory environmental factors include bacterial and viral infection, pollutants and cigarette smoke.
Anaphylaxis
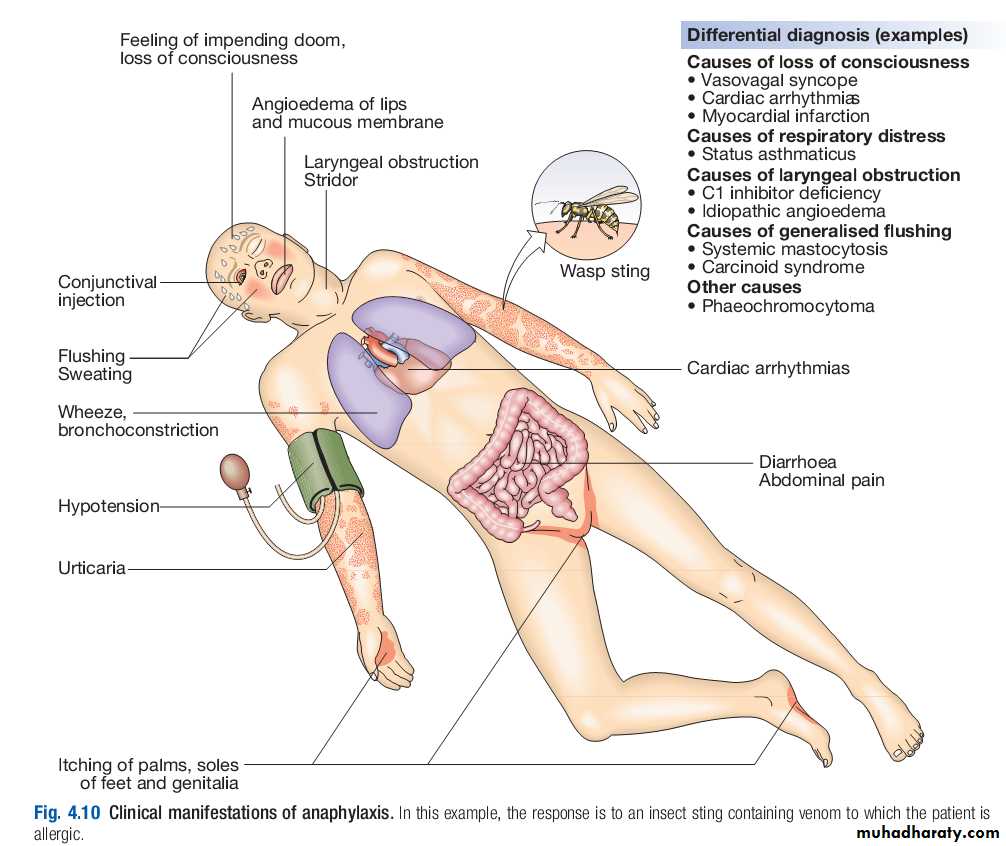
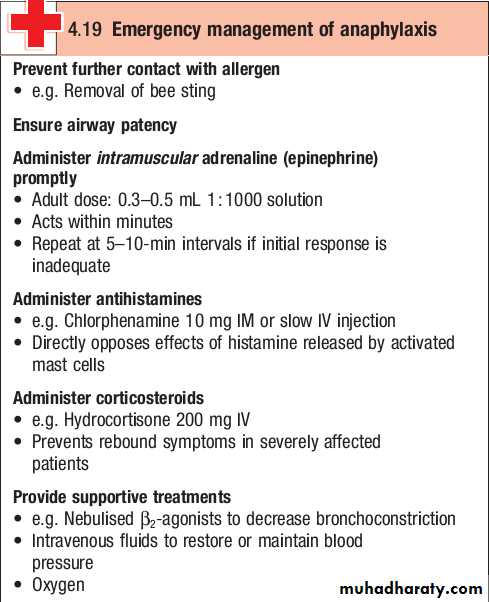
Anaphylaxis is a potentially lifethreatening, systemic allergic reaction caused by the release of histamine and other vasoactive mediators from mast cells.The risk of death is increased in patients with preexisting asthma, particularly if this is poorly controlled, and when treatment with adrenaline (epinephrine) is delayed.
Clinical assessment
The severity of a reaction should be assessed; the time between allergen exposure and onset of symptoms provides a guide.
Enquiry should be made about potential triggers; if these are not immediately obvious, a detailed history of the previous 24 hours may be helpful.
The most common triggers are foods, latex, insect venom and drugs.
A history of previous allergic responses to the offending agent is common.
The route of allergen exposure may influence the principal clinical features of a reaction; for example, if an allergen is inhaled, the major symptom is frequently wheezing.
Anaphylactoid reactions
Anaphylactoid reactions result from the nonspecific degranulation of mast cells by drugs, chemicals or other triggers, and do not involve IgE antibodies.The clinical presentations are indistinguishable from anaphylaxis , and in the acute situation discriminating between them is unnecessary.
However, this may be important in identifying precipitating factors and appropriate avoidance measures.
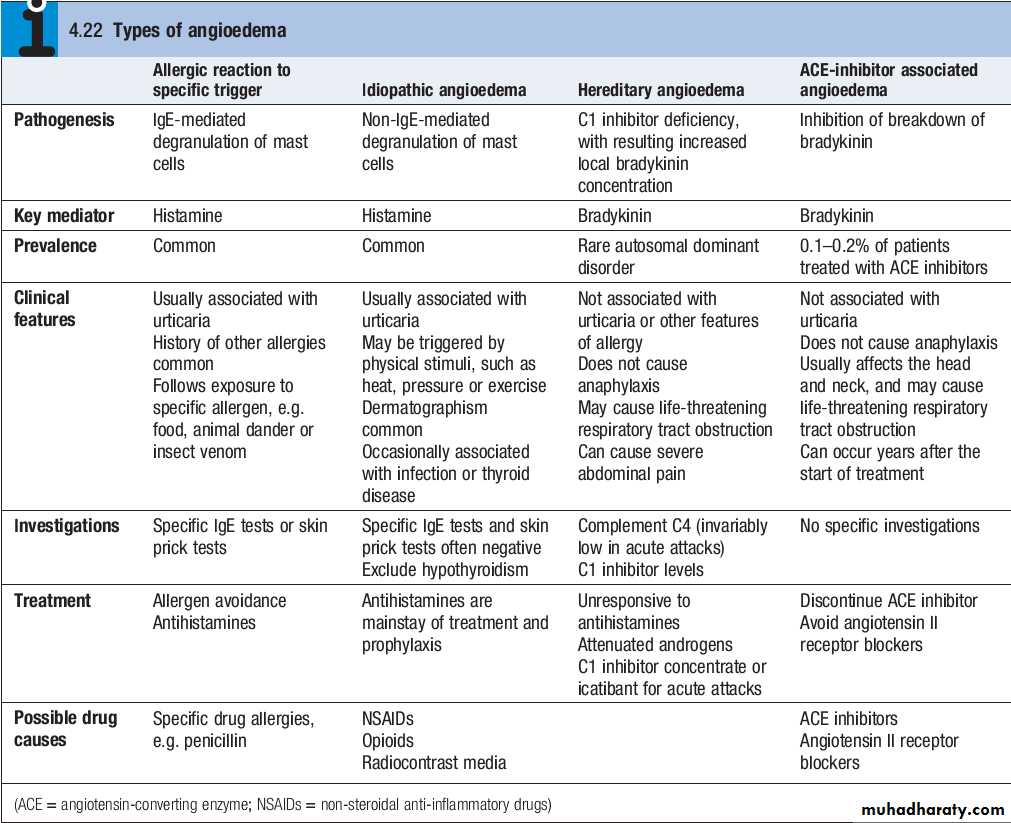
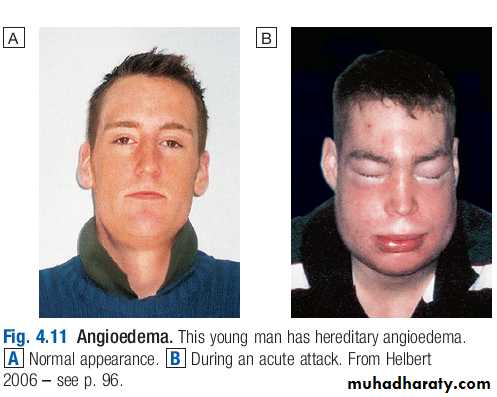
Angioedema
Angioedema is the episodic, localised, nonpitting swelling of submucous or subcutaneous tissues.This most frequently affects the face,extremities and genitalia. Involvement of the larynx or tongue may cause lifethreatening respiratory tract obstruction, and oedema of the intestinal mucosa may cause abdominal pain and distension.
In most cases, the underlying mechanism is degranulation of mast cells. However, angioedema may occasionally be mediated by increased local bradykinin concentration. Differentiating the mechanism of angioedema is important in determining appropriate investigations and treatment.