
Hussien Mohammed Jumaah
CABM
Lecturer in internal medicine
Mosul College of Medicine
2016
learning-topics
CARDIOVASCULAR SYSTEM

In the UK, one-third of men and one-quarter of women
will
die
as a result of ischaemic heart disease(IHD).
In 20% of adults, a patent foramen ovale is Found. The
atria and ventricles are separated by the
annulus
fibrosus
, which forms the skeleton for the atrioventricular
(AV) valves and which
electrically insulates
the atria from
the ventricles.
The right ventricle (RV) is roughly triangular in shape The
RV sits anterior to, and to the right of, the left ventricle
(LV). The LV is more conical in shape and in cross-section is
nearly circular.
The LV myocardium is normally around 10 mm thick
(c.f. RV of 2–3 mm) because
it pumps blood at a higher pressure.
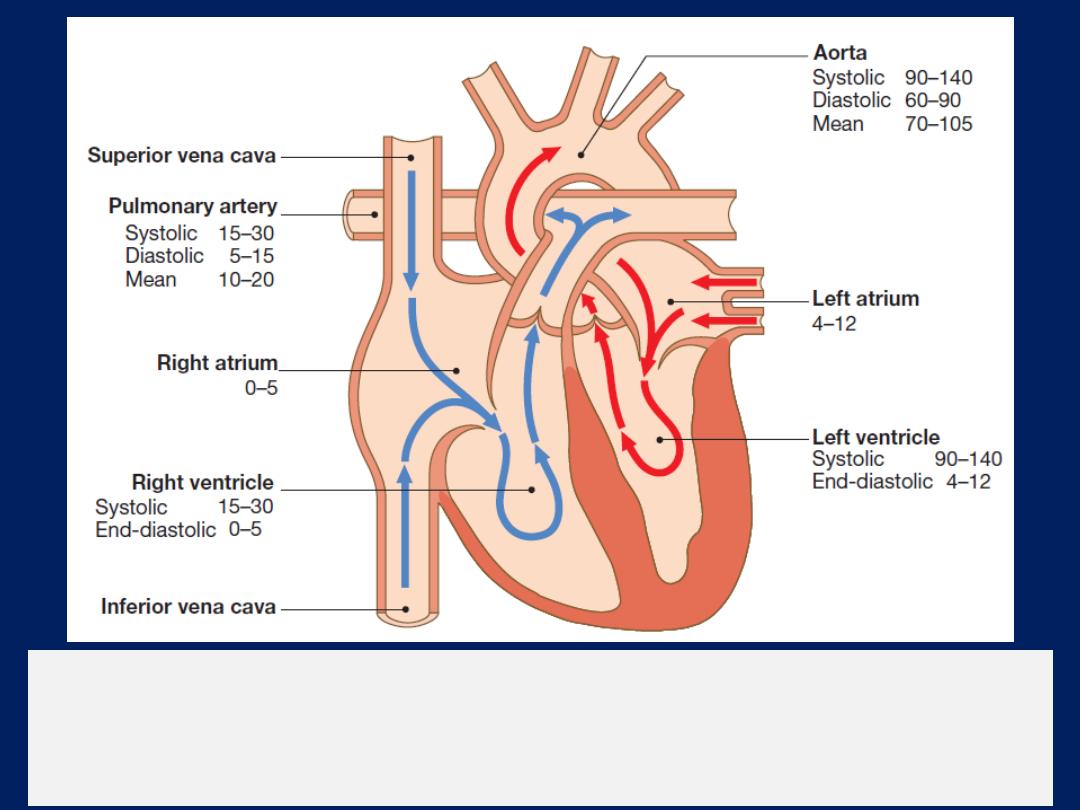
Direction of blood flow through the heart.
The blue arrows show deoxygenated
blood moving through the right heart to the lungs. The red
arrows show oxygenated blood moving from the lungs to the systemic circulation.
The normal pressures are shown for each chamber in mmHg.
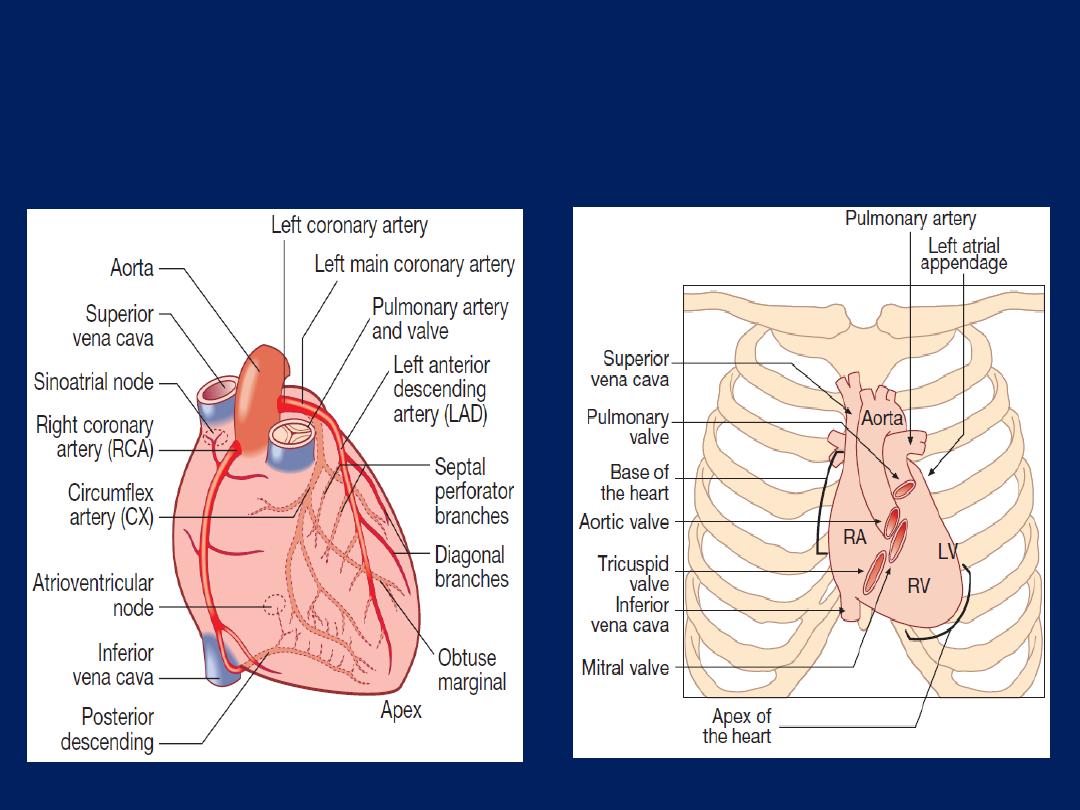
Surface anatomy of
the heart.
The coronary arteries.
anterior view.

The coronary circulation
The left main and right coronary arteries arise from the
left and right sinuses of the aortic root, distal to the aortic
valve . Within 2.5 cm of its origin, the left main
coronary artery divides into the left anterior descending
artery (LAD), which runs in the anterior interventricular
groove, and the left circumflex artery (CX), which runs
posteriorly in the atrioventricular groove.
The LAD gives branches to supply the anterior part of the
septum , the anterior, lateral and apical walls of the LV.
The CX supply the lateral, posterior and inferior segments
of the LV.
The right coronary artery (RCA) runs in the right
atrioventricular groove, supply the RA, RV and
inferoposterior aspects of the LV.

The posterior descending artery runs in the posterior
interventricular groove and supplies the inferior part of
the interventricular septum. This vessel is a branch of the
RCA in approximately 90% of people (
dominant right
system) and is supplied by the CX in the remainder
(
dominant left system
). The RCA supplies the sinoatrial
(SA) node in about 60% and the AV node in about 90%.
Abrupt occlusions in the RCA, result in infarction of the
inferior part of the LV and often the RV. Occlusion of the
left main coronary artery is usually fatal. The venous
system follows the coronary arteries but drains into the
coronary sinus in the atrioventricular groove, and then to
the RA. The lymphatic system drains into vessels that
travel with the coronary vessels and then into thoracic duct.

Conducting system of the heart
The SA node is situated at the junction of SVC and RA . It
comprises specialized atrial cells that depolarise at a rate
influenced by the autonomic nervous system and by
circulating catecholamines.During normal (sinus) rhythm,
wave propagates through both atria via atrial myocytes to
the AV node conduct relatively slowly producing a
necessary time delay between atrial and ventricular
contraction. The His–Purkinje system extending from the
AV node into right and left bundle branches passing along
the septum and into the respective ventricles, the anterior
and posterior fascicles of the left bundle, and the smaller
Purkinje fibres that ramify through the myocardium.
His–Purkinje conduct very rapidly and
allow
near-
simultaneous depolarisation of the entire myocardium.
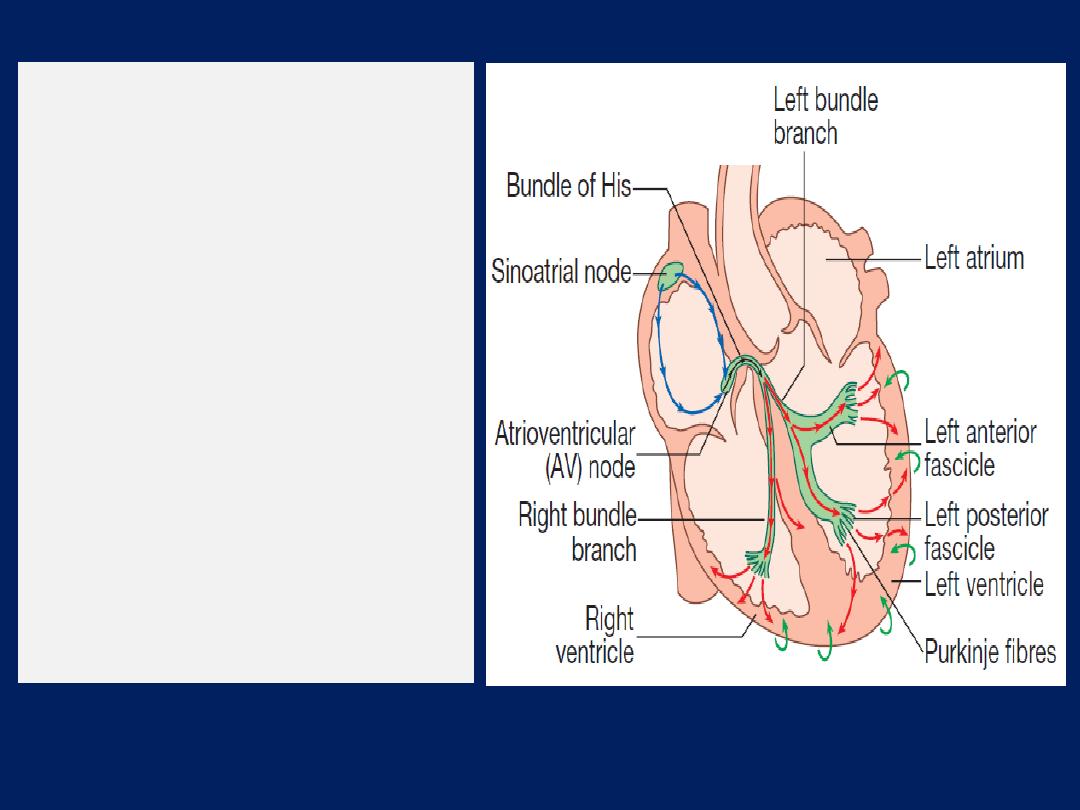
The cardiac conduction
system.
Depolarisation starts
in the sinoatrial node and
spreads through the atria
(blue arrows), and then
through the atrioventricular
node (black arrows).
Depolarisation then spreads
through the bundle of His and
the bundle branches to reach
the ventricular muscle (red
arrows).
Repolarisation
spreads from epicardium
to
endocardium (green arrows).

Nerve supply of the heart
The heart is innervated by
sympathetic
(from the cervical
sympathetic chain , supply muscle fibres in the atria ,
ventricles and the electrical conducting system, β
1
-
adrenoceptors mediate positive inotropic and chronotropic
effects, whereas β
2
-adrenoceptors predominate in vascular
smooth muscle and mediate vasodilatation),
and parasympathetic
fibres . Parasympathetic pre-
ganglionic fibres and sensory fibres reach the heart through
the vagus nerves. Cholinergic nerves supply the AV and SA
nodes via muscarinic (M2) receptors.
Under resting conditions, vagal inhibitory activity
predominates and the heart rate is slow.
Adrenergic stimulation, associated with exercise, emotional
stress, fever and so on, causes the heart rate to increase.

Myocardial contraction
Myocardial cells (myocytes) branches and interdigitates
with adjacent cells.
The basic unit of contraction is the sarcomere . Actin
filaments are attached at right angles to the Z-lines and
interdigitate with thicker parallel myosin filaments.
The cross-links between actin and myosin molecules
contain myofibrillar adenosine triphosphatase (ATPase),
which breaks down adenosine triphosphate (ATP) to
provide the energy for contraction .
The extent to which the sarcomere can shorten determines
stroke volume of the ventricle. The force of cardiac muscle
contraction, or inotropic state, is regulated by the influx
of calcium ions through ‘slow calcium channels’.

Cardiac output
is the product of stroke volume and heart
rate. Stroke volume is the volume of blood ejected in each
cardiac cycle, and is dependent upon end-diastolic volume
and pressure (preload), myocardial contractility and
systolic aortic pressure (afterload).
Starling’s Law of the heart
:
Stretch of cardiac muscle (from increased end-diastolic
volume) causes an increase in the force of contraction,
producing a greater stroke volume
The contractile state of the myocardium is controlled
by neuro-endocrine factors, such as adrenaline and can be
influenced by inotropic drugs and their antagonists.

Blood flow
Blood passes from the heart through the large central
elastic arteries into muscular arteries before encountering
the resistance vessels, and ultimately the capillary bed,
where there is exchange of nutrients, oxygen and waste
products of metabolism.
The central arteries,
such as the
aorta, are predominantly composed of elastic tissue with
little or no vascular smooth muscle cells. When blood is
ejected, the compliant aorta expands to accommodate the
volume of blood before the elastic recoil sustains blood
pressure (BP) and flow following cessation of cardiac
contraction.
This‘Windkessel effect’
(Windkessel when loosely
translated from German to English means 'air chamber‘)
prevents
excessive rises in systolic BP whilst sustaining diastolic BP,
thereby maintaining coronary perfusion.

Windkessel vessels e.g. aorta, common carotid, subclavian,
and pulmonary arteries and their larger branches).
Since the rate of blood entering these elastic arteries
exceeds that leaving them due to the peripheral resistance
there is a net storage of blood during systole which
discharges during diastole. The pulsatile flow of blood
during systole converted into continuous flow due to elastic
recoiling of the vessel during diastole and maintaining
coronary perfusion. These benefits are lost with
progressive arterial stiffening , a feature of ageing and
advanced renal disease as the arteriosclerosis, lead to
fragmentation and loss of elastin and the elastic arteries
become less compliant, The reduction in the Windkessel
effect results in increased pulse pressure and elevated
systolic pressure for a given stroke volume.

Passing down the arterial tree, vascular smooth
muscle cells progressively play a greater role until the
resistance arterioles are encountered. Although all
vessels contribute, the resistance vessels (diameter
50–200 μm) provide the greatest contribution to systemic
vascular resistance, with small changes in radius
having a marked influence on blood flow; resistance
is proportional to the fourth power of the radius
(Poiseuille’s Law).
The tone of these resistance vessels is tightly regulated by
humoral, neuronal and mechanical factors.

Coronary blood vessels receive sympathetic and parasympathetic
innervation.
Stimulation
of α- adrenoceptors causes vasoconstriction;
stimulation
of
β
2
-adrenoceptors causes vasodilatation; the predominant
effect of sympathetic stimulation in coronaries is vasodilatation.
Parasympathetic stimulation (muscarinic) also causes dilatation of
normal coronaries.
As a result
of vascular regulation, an atheromatous narrowing in a
coronary artery does not limit flow, even during exercise, until the
cross-sectional area of the vessel is reduced by at least 70%.
In addition
, systemic and locally released vasoactive substances
influence tone; vasoconstrictors include noradrenaline (norepinephrine),
angiotensin II and endothelin-1, whereas adenosine, bradykinin,
prostaglandins and nitric oxide are vasodilators.
Resistance to blood flow rises with
viscosity
and is mainly influenced
by red cell concentration (haematocrit).

Endothelial function
The endothelium
plays a vital role in
the control of vascular
homeostasis.
It synthesises
and releases many vasoactive
mediators that cause vasodilatation, including nitric oxide,
prostacyclin and vasoconstriction, including endothelin-1
and angiotensin II. A balance exists contributes to the
maintenance and regulation of vascular tone and BP.
Damage to the endothelium may disrupt this balance and
lead to vascular dysfunction, tissue ischaemia and
hypertension.
The endothelium also has a major influence on key
regulatory steps in the
recruitment
of inflammatory cells
and on the
formation and dissolution
of thrombus.

The endothelium also stores and
releases von
Willebrand
factor, which promotes thrombus formation by linking
platelet adhesion to denuded surfaces.
In contrast, once
intravascular thrombus forms, tissue
plasminogen activator is rapidly released from adynamic
storage pool within the endothelium to induce fibrinolysis
and thrombus dissolution.
These processes are critically involved in the development
and progression of atherosclerosis, and endothelial
function and injury are seen as central to the pathogenesis
of many cardiovascular disease states.

Effects of respiration
There is a fall in intrathoracic pressure during inspiration
that tends to promote venous flow into the chest, producing
an increase in the flow of blood through the right heart.
However, a substantial volume of blood is sequestered in
the chest as the lungs expand; the increase in the
capacitance of the pulmonary vascular bed usually exceeds
any increase in the output of the right heart and therefore
there is a reduction in the flow of blood into the left heart
during inspiration. In contrast, expiration is accompanied by
a fall in venous return to the right heart, a reduction in the
output of the right heart, a rise in the venous return to the
left heart (as blood is squeezed out of the lungs) and an
increase in the output of the left heart .
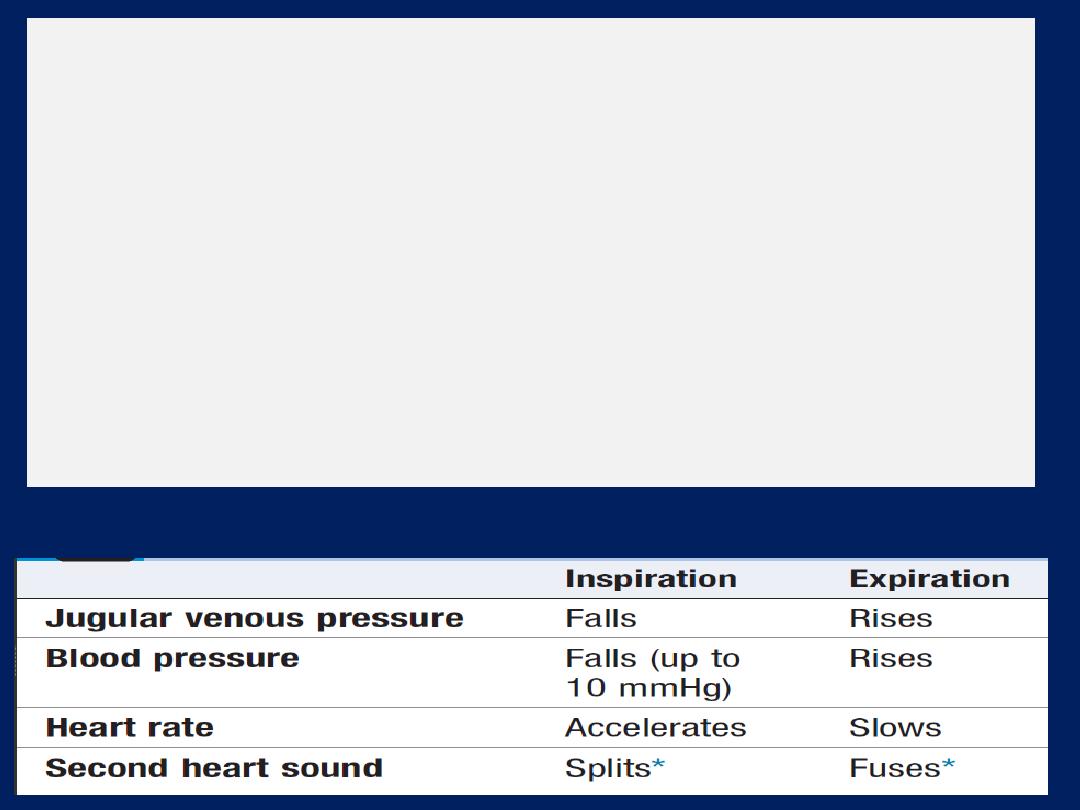
Pulsus paradoxus
exaggerated fall in BP during inspiration, characteristic
of cardiac tamponade and severe airways obstruction. In
airways obstruction, due to accentuation of the change in
intrathoracic pressure with respiration. In tamponade,
compression of the right heart prevents the normal
increase in flow through the right heart on inspiration,
exaggerates the usual drop in venous return to the left
heart and produces a marked fall in BP
(> 10 mmHg fall).
Haemodynamic effects of respiration

INVESTIGATION OF CARDIOVASCULAR DISEASE
Basic tests, such as electrocardiography, chest X-ray and
echocardiography, can be performed in an outpatient
clinic or at the bedside.
Procedures such as cardiac catheterisation, radionuclide
imaging, computed tomography (CT) and magnetic
resonance imaging (MRI) require specialised facilities.
ECG, Leads V1 and V2 lie over the RV, V3 and V4 over
the interventricular septum, V5 and V6 over the LV .
The Depolarisation of the interventricular septum occurs
first and moves from left to right; this generates a small
initial negative deflection in lead V6 (Q wave) and an
initial positive deflection in lead V1 (R wave).
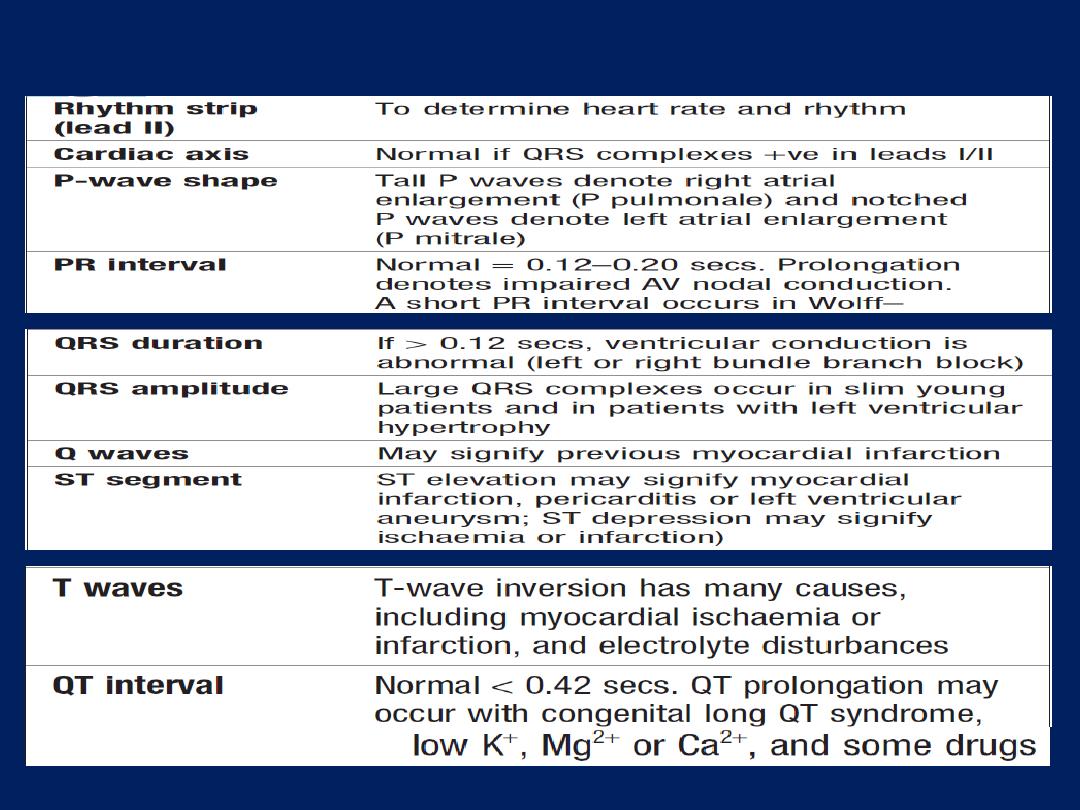
How to read a 12–lead ECG

Exercise (stress) ECG
Used to detect myocardial ischaemia during physical stress
and is helpful in the diagnosis of coronary artery disease
(CAD). A 12-lead ECG is recorded during exercise on a
treadmill or bicycle ergometer. The limb electrodes are
placed on the shoulders and hips rather than the wrists
and ankles. The Bruce Protocol is the most commonly used.
BP is recorded and symptoms assessed throughout the test.
A test is ‘
positive
’ if anginal pain occurs, BP falls or fails to
increase, or ST shifts of >1 mm . It is an unreliable
screening tool because, in low-risk (e.g. asymptomatic
young or middle-aged women), an abnormal response is
more likely to represent a false positive. Stress testing is
contraindicated in the presence of ACS , decompensated
heart failure and severe hypertension.
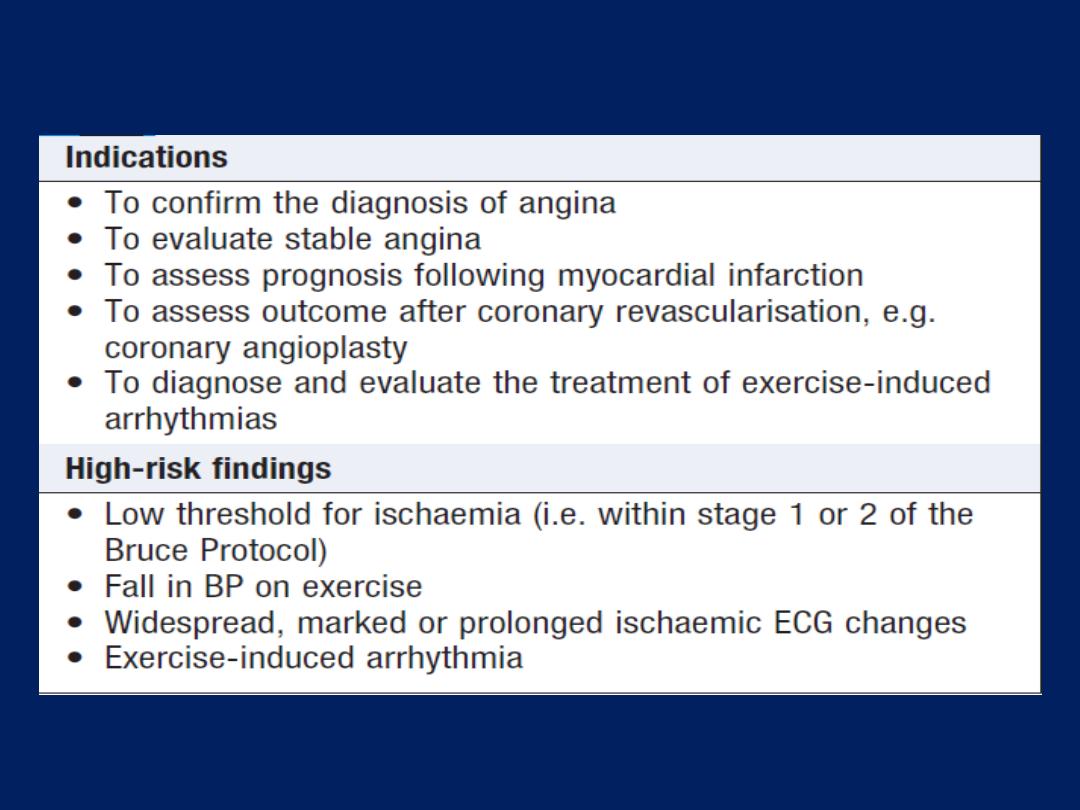
Exercise testing

Ambulatory ECG
Continuous (ambulatory) ECG recordings
using a portable
recorder , record between 1 - 7 days. Principally used in the
investigation of suspected arrhythmia, such as those with
intermittent palpitation, dizziness or syncope, can also be
used to assess rate control in atrial fibrillation, sometimes
used to detect transient myocardial ischaemia. With some
devices, the recording can be transmitted to hospital via
telephone.
Implantable ‘loop recorders’
resemble leadless pacemaker ,
implanted subcutaneously, have a lifespan of 1–3 years and
are used to investigate patients with infrequent but
potentially serious symptoms, such as syncope.

Cardiac biomarkers
Brain natriuretic peptide (BNP)
This is
a 32-amino acid peptide and is
secreted by the LV along
with an inactive
76-amino acid N-terminal fragment
(NT- proBNP).
The latter is diagnostically more useful, as it has a longer
half-life, elevated in LV systolic dysfunction,
aid
the
diagnosis , prognosis and response to therapy of failure.
Troponin I and troponin T
are cardiac muscle proteins
released during myocyte damage, and represent the
cornerstone of the diagnosis of acute MI. However, modern
assays are extremely sensitive and can detect very low
levels of myocardial damage, such as : pulmonary embolus,
septic shock and pulmonary oedema. The diagnosis of MI
therefore relies on the clinical presentation.

Chest X-ray
‘ Cardiomegaly’
is the term used to describe an enlarged
cardiac silhouette where the ‘cardiothoracic ratio’ is >0.5.
(comparing the maximum width of the cardiac outline with the maximum internal
transverse diameter of the thoracic cavity).
It can be caused by chamber dilatation, especially left
ventricular dilatation, or by a pericardial effusion.
Artefactual
cardiomegaly may be due to a mediastinal
mass or pectus excavatum and cannot be reliably
assessed from an AP film. Cardiomegaly is not a sensitive
indicator of left ventricular systolic dysfunction since the
cardiothoracic ratio is normal in many affected patients
(false-negative) and also lacks specificity with many
patients with apparent cardiomegaly having normal
echocardiograms (false-positive).

Left atrial dilatation
results in prominence of the left atrial
appendage, creating the appearance of a straight left
heart border, a double cardiac shadow to the right of the
sternum, and widening of the angle of the carina as the
left main bronchus is pushed upwards .
• Right atrial enlargement
projects from the right heart
border towards the right lower lung field.
• Left ventricular dilatation
causes prominence of the left
heart border and enlargement of the cardiac silhouette.
• Left ventricular hypertrophy
produces rounding of the
left heart border.
• Right ventricular dilatation
increases heart size, displaces
the apex upwards and straightens the left heart border.
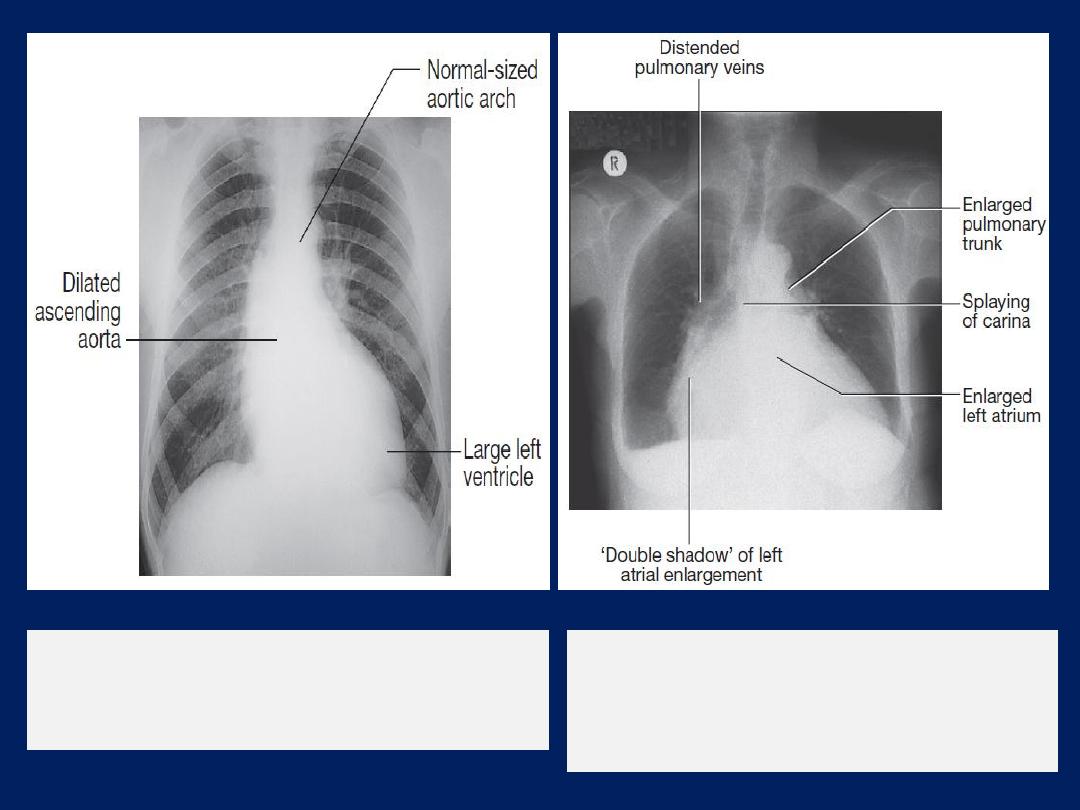
Chest X-ray
of a patient with mitral
stenosis and regurgitation indicating
enlargement of the LA and prominence of
the pulmonary artery trunk.
Chest X-ray
of a patient with aortic
regurgitation, left ventricular enlargement
and dilatation of the ascending aorta.
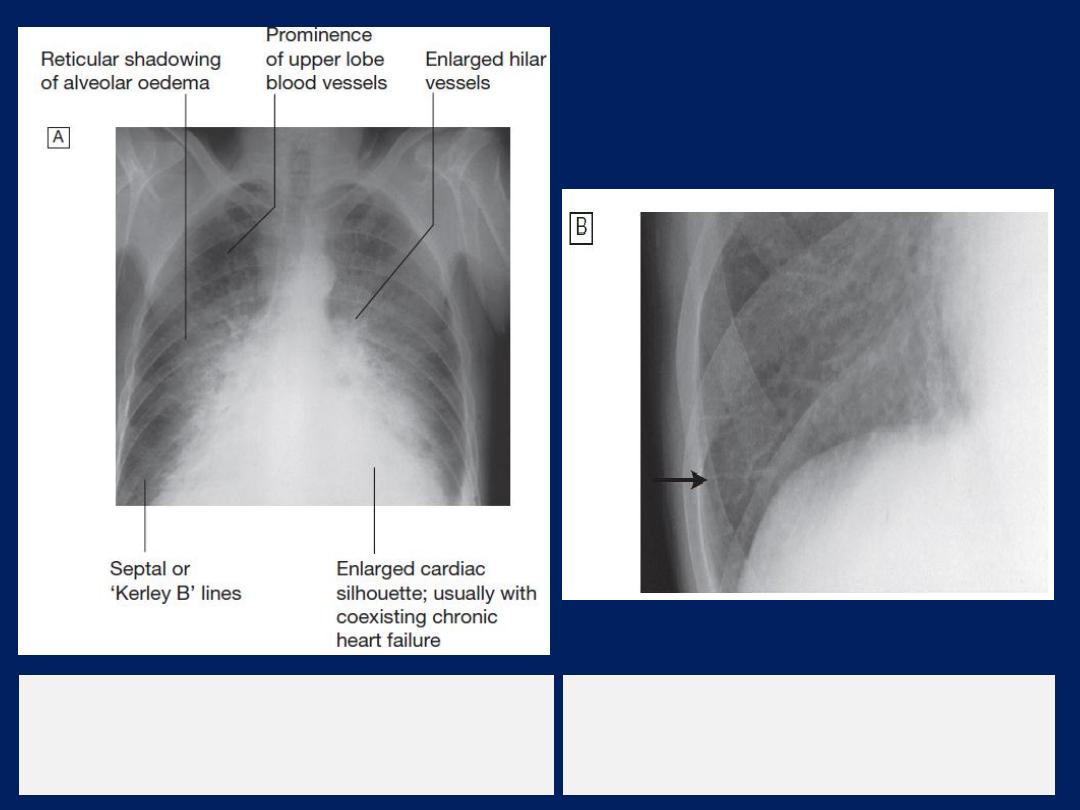
Radiological features of heart failure.
A
Chest X-ray of a patient with pulmonary
oedema.
Radiological features of heart failure.
B
Enlargement of lung base showing
septal or ‘Kerley B’ lines (arrow).

Echocardiography (echo)
This permits the rapid assessment of cardiac structure and
function ,wall thickness and ejection fraction.
Doppler echocardiography
This depends on the Doppler principle that sound waves
reflected from moving objects, such as RBC. The speed and
direction of blood, can be detected in the heart chambers
and great vessels. Can be used to detect valvular
regurgitation, where the direction of blood flow is
reversed, and to detect high pressure gradients associated
with stenosed valves. For example, the normal
resting
systolic flow velocity
across the
aortic
valve is 1 m/sec; in
the presence of aortic stenosis, this is increased as blood
accelerates through the narrow orifice.
In severe
stenosis,
the peak aortic velocity may be increased to 5 m/sec .

An estimate of the
pressure gradient
across a valve or
lesion is given by the modified Bernoulli equation:
Advanced techniques
include three-dimensional echo,
intravascular US (defines vessel wall abnormalities and
guides coronary intervention), intracardiac US (provides
high-resolution images) and tissue Doppler imaging
(quantifies myocardial contractility and diastolic function).
Bernoulli equation
Common indications for echocardiography

Transoesophageal echocardiography (TOE)
overweight or has obstructive airways disease, structures
that are difficult to visualise in transthoracic views, such as
the left atrial appendage, pulmonary veins, thoracic
aorta and interatrial septum, TOE uses an endoscope-like
ultrasound probe which is passed into the oesophagus
under light sedation and positioned behind the LA. This
produces high-resolution images, which makes the technique
particularly valuable for investigating patients with
prosthetic (especially mitral) valve dysfunction, atrial
septal defect, aortic dissection, infective endocarditis
(vegetations that are too small to be detected by
transthoracic echo) and systemic embolism (intracardiac
thrombus or masses).

Stress echocardiography
Is used to investigate patients with CAD who are
unsuitable for exercise stress testing, such as those with
mobility problems or pre-existing bundle branch block.
A two-dimensional echo is performed before and after
infusion of a moderate to high dose of an inotrope,
such as dobutamine.
Myocardial segments with poor perfusion become ischaemic
and contract poorly under stress, showing as a wall motion
abnormality on the scan. Low-dose dobutamine can induce
contraction in ‘hibernating’ myocardium; such patients may
benefit from bypass surgery or PCI .

Computed tomographic imaging
Imaging chambers, great vessels, pericardium, and
mediastinal structures and masses.
Contrast scans
are
very useful for imaging aortic dissection , pulmonary
arteries and branches in suspected pulmonary embolism .
Some centres use it for quantification of coronary artery
calcification, which may serve as an index of CV risk.
However, modern
multidetector
scanning allows non-
invasive coronary angio with a resolution approaching that
of conventional angio and at a lower radiation.
CT coronary angiography
is particularly useful in the
initial assessment of chest pain and a low or intermediate
likelihood of disease, since its negative predictive value is
very high. Modern volume scanners are also able to assess
myocardial perfusion, often at the same sitting.

Magnetic resonance imaging (MRI)
MRI requires no ionizing radiation ,used to image the heart,
lungs and mediastinal structures. It provides better
differentiation of
soft tissue
structures than CT but is poor
at demonstrating calcification. It needs to be ‘gated’ to the
ECG, allowing the scanner to produce moving images of the
throughout the cardiac cycle. Very useful for imaging the
aorta, including suspected
dissection
. It is also useful for
detecting
infiltrative
conditions affecting the heart.
Quantification of blood flow across
regurgitant or stenotic
valves. It is also possible to analyse regional wall motion in
patients with suspected CAD or cardiomyopathy.
The RV
is difficult to assess using echo because of its
retrosternal position but is readily visualised with MRI.

Cardiac catheterisation
This involves passage of catheter via a vein or artery into the heart
under X-ray guidance, which allows the measurement of
pressure
and
oxygen
saturation in the cardiac chambers and great vessels, and the
performance of
angiograms
by injecting contrast media into a
chamber or blood vessel.
Left heart catheterisation
is a day case procedure and is
relatively safe, with serious complications occurring in < 1 in 1000
cases. Usually via the radial artery, to allow catheterisation of the
aorta (
aortography
defines the size of the aortic root and thoracic
aorta, and can help quantify aortic regurgitation).
Left
ventriculography
to determine the size and function of the LV and to
demonstrate mitral regurgitation.
Coronary angiography
in which the left and right coronary arteries
are selectively cannulated and imaged, providing information about
the extent and severity of coronary stenoses, thrombus and
calcification and permits planning of PCI and CABG .

Right heart catheterisation
is used to assess right
heart and pulmonary artery pressures, and to detect
intracardiac shunts by measuring oxygen saturations. For
example, a step up in oxygen saturation from 65% in the
RA to 80% in the pulmonary artery is indicative of a large
left-to-right
shunt
that might be due to a VSD .
Cardiac output
can also be measured using thermodilution
techniques.
Left atrial pressure
can be measured directly
by puncturing the interatrial septum from the RA. For most
purposes, however, a satisfactory approximation can be
obtained by ‘wedging’ Swan– Ganz balloon catheter , to
monitor ‘wedge’ in a branch of the pulmonary artery as a
guide to left filling pressure in critically ill patients .
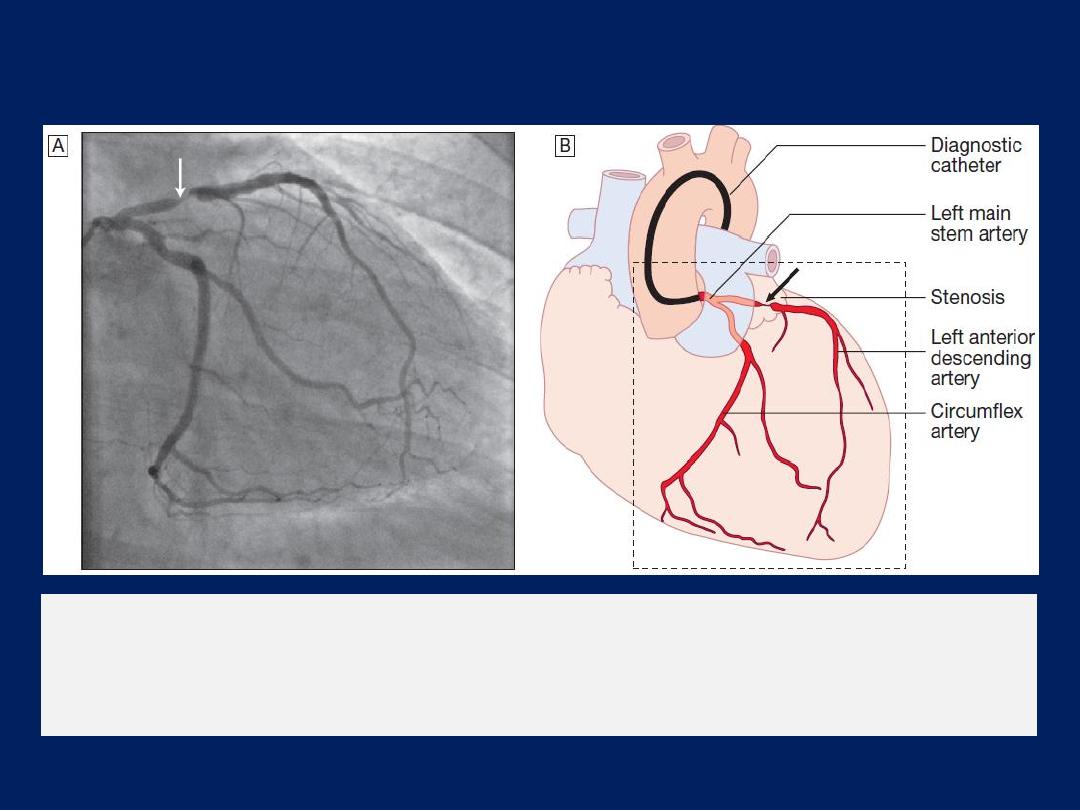
The left anterior descending and circumflex
coronary arteries with
a stenosis in the left anterior descending vessel.
A
Coronary artery
angiogram.
B
Schematic of the vessels and branches

Electrophysiology study (EPS)
Patients with known or suspected arrhythmia investigated
by percutaneous placement of electrode catheters into the
heart via the femoral and neck veins.
EPS performed to evaluate patients for catheter ablation,
normally done during the same procedure.
Radionuclide imaging
Non-invasive gamma-emitting radionuclides study cardiac
function .
Two techniques are available,
although their use is
declining due to the availability of equivalent or superior
imaging that have lower or no exposure to radiation.

Blood pool imaging
The isotope is injected IV. A gamma camera detects the
amount of radiation-emitting blood in the heart at different
phases of the cardiac cycle, permitting the calculation of
ventricular
ejection fractions
and assessment
size and
shape
of the cardiac chambers.
Myocardial perfusion imaging
A dministration of an IV radioactive isotope, such as
99
technetium
tetrofosmin to obtain scintiscans of the
myocardium at rest and during stress . More sophisticated
quantitative information is obtained with positron emission
tomography
(PET),
which can also be used to assess
myocardial metabolism, but this available in a few centres

PRESENTING PROBLEMS IN CARDIOVASCULAR DISEASE
A close relationship
between symptoms and exercise is the
hallmark of heart disease.
The New York Heart Association
(NYHA)
functional classification is used
to grade disability .
Pain that occurs after rather than during exertion is usually
musculoskeletal or psychological in origin.
The pain of MI typically takes several minutes or longer to
develop; similarly, angina builds up gradually .
The pain of :
aortic dissection, pneumothorax , or massive pulmonary
embolism is usually very sudden or instantaneous in onset.
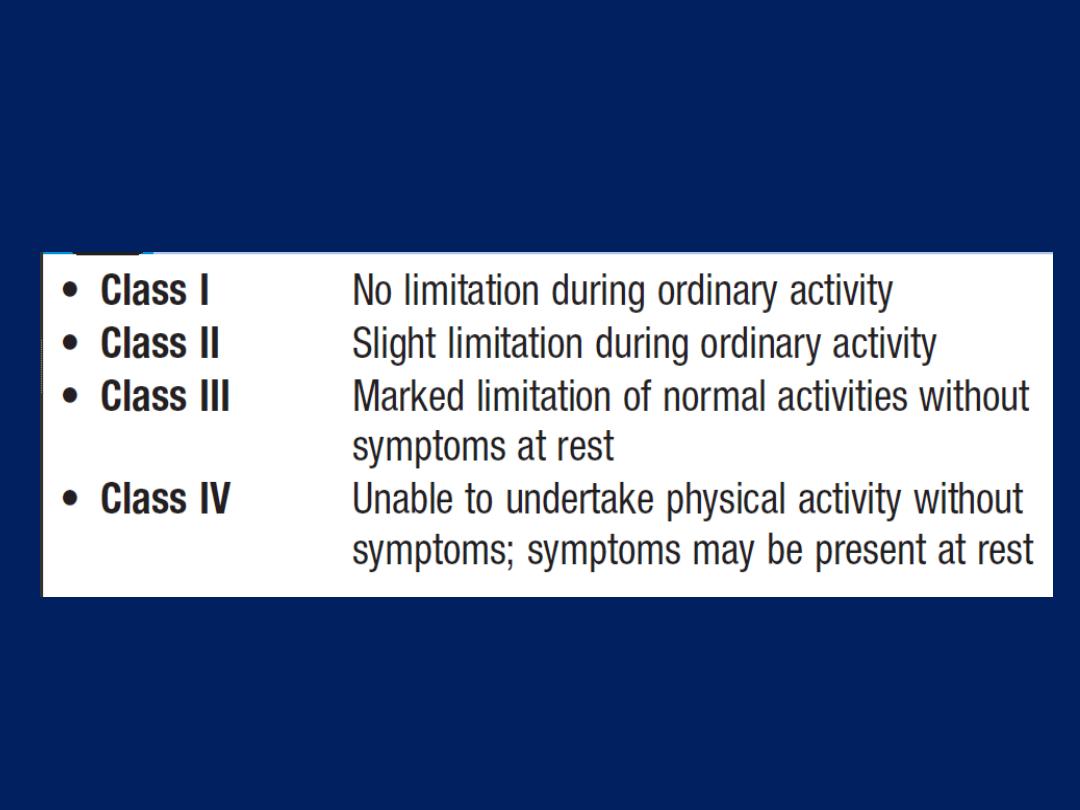
New York Heart Association (NYHA)
functional classification
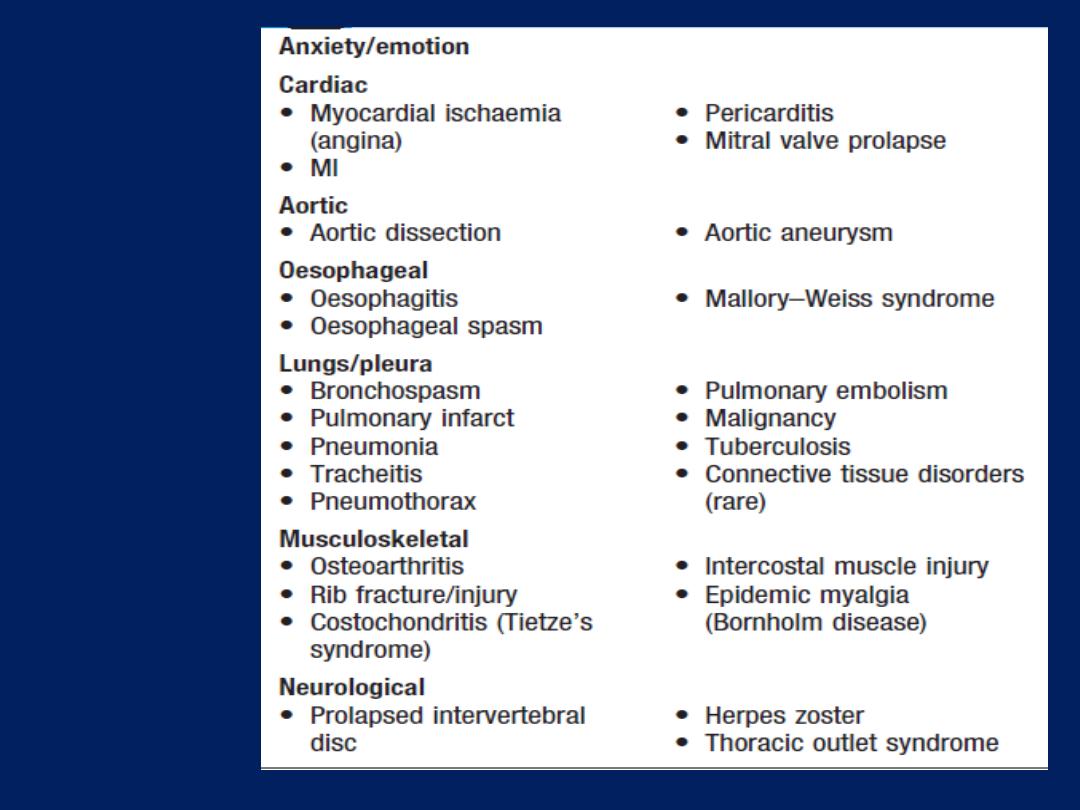
Common
causes of
chest pain

Chest pain
Characteristics of cardiac pain
• Site.
Cardiac pain is typically located in the centre
of the chest because of the derivation of the nerve
supply to the heart and mediastinum.
• Radiation
.
Ischaemic cardiac pain may radiate to the
neck, jaw, and upper or even lower arms. Occasionally,
experienced only at the sites of radiation or in the back.
Pain situated over the left anterior chest and radiating
laterally is unlikely to be due to ischaemia and may
have many causes, including pleural or lung disorders,
musculoskeletal problems and anxiety.

• Character.
Cardiac pain is typically dull, constricting,
choking or ‘heavy’, squeezing, crushing, burning or aching
but not sharp, stabbing, pricking, can be described as
breathlessness. Patients often emphasise that it is a
discomfort rather than a pain. They typically use
characteristic hand gestures (e.g. open hand or clenched
fist) when describing ischaemic pain .
• Provocation
.
Anginal pain occurs
during (not after)
exertion, promptly relieved (<5 minutes) by rest, may be
precipitated or exacerbated by emotion , exertion, large
meal or in a cold wind. In crescendo or unstable angina,
similar pain may be precipitated by minimal exertion or
at rest. The increase in venous return or preload induced
by lying down may also be sufficient to provoke pain in
vulnerable patients (decubitus angina).

The pain of MI
may be preceded by a period of stable or
unstable angina but often occurs de novo.
Pleural or pericardial pain
described as a ‘sharp’ or
‘catching’ sensation , exacerbated by breathing, coughing
or movement. Pain associated with a specific movement
(bending,stretching,turning) is likely to be
musculoskeletal
.
• Onset
.
The pain of MI typically takes several minutes or
longer to develop; similarly, angina builds up gradually in
proportion to the intensity of exertion.
The pain of aortic dissection, massive pulmonary
embolism or pneumothorax is usually very sudden or
instantaneous in onset.
Pain that occurs after rather than during exertion is usually
musculoskeletal or psychological in origin.

• Associated features.
The pain of MI, massive pulmonary
embolism or aortic dissection is often accompanied by
autonomic disturbance, including sweating, nausea and
vomiting.
Breathlessness, due to pulmonary congestion arising from
transient ischaemic left ventricular dysfunction, is often a
prominent and occasionally the dominant feature of MI or
angina (
angina equivalent
). Breathlessness
may also accompany any of the respiratory causes
of chest pain and can be associated with cough, wheeze or
other respiratory symptoms. Gastrointestinal disorders, such
as gastrooesophageal reflux, peptic ulceration or biliary
colic, may present with chest pain but effort-related
‘indigestion’ is usually due to heart disease.

Myocarditis and pericarditis
Pain is characteristically felt retrosternally, to the left of
the sternum, or in the left or right shoulder, and typically
varies in intensity with movement and the phase of
respiration. The pain is described as ‘sharp’ and may
‘catch’the patient during inspiration, coughing or lying
flat; there may be a history of a prodromal viral illness.
Mitral valve prolapse
Sharp left-sided chest pain that is suggestive of a
Musculoskeletal problem may be a feature of prolapse .
Aortic dissection
This pain is severe, sharp and tearing, is often felt in or
penetrating through to the back, typically very abrupt in
onset .The pain follows the path of the dissection.

Oesophageal pain
This can mimic the pain of angina very closely, is sometimes
precipitated by exercise and may be relieved by
nitrates. However, it is usually possible to elicit a history
relating chest pain to supine posture or eating, drinking
or oesophageal reflux. It often radiates to the
interscapular region and dysphagia may be present.
Bronchospasm
Patients with reversible airways obstruction, such as
asthma, may describe exertional chest tightness that is
relieved by rest. This may be difficult to distinguish from
ischaemic chest tightness. Bronchospasm may be associated
with wheeze, atopy and cough .

Musculoskeletal chest pain
This is a common problem that is very variable in site
and intensity but does not usually fall into any of the
patterns described above. The pain may vary with
posture or movement of the upper body and is sometimes
accompanied by local tenderness over a rib or costal
cartilage. There are numerous causes, including arthritis,
costochondritis, intercostal muscle injury and Coxsackie viral
infection (epidemic myalgia or Bornholm disease). Many
minor soft tissue injuries are related to everyday activities,
such as driving, manual work and sport.

Initial evaluation of suspected cardiac pain
A careful history is crucial in determining whether pain
is cardiac or not. Although the physical findings and
subsequent investigations may help to confirm the
diagnosis, they are of more value in determining the
nature and extent of any underlying heart disease,
the risk of a serious adverse event, and management.
Stable angina
Effort-related chest pain is the hallmark of angina
pectoris or ‘choking in the chest’ . The reproducibility,
predictability and relationship to physical exertion (and
emotion) of the chest pain are the most important features.
The duration of symptoms should be noted because patients
with recent-onset angina are at greater risk than those with
long-standing and unchanged symptoms.

Physical examination is often normal but may reveal
evidence of risk factors (e.g. xanthoma, left ventricular
dysfunction (e.g. dyskinetic apex beat, gallop rhythm),
manifestations of arterial disease (e.g. bruits, signs of
peripheral vascular disease) and conditions that
exacerbate angina (e.g. anaemia, thyroid disease).
Stable angina is usually a symptom of CAD but may be a
manifestation of other forms of heart disease, aortic valve
disease and hypertrophic cardiomyopathy.
Investigations
A full blood count, fasting blood glucose, lipids, thyroid
tests and ECG. Exercise testing may confirm the diagnosis
and also identify high-risk patients .CT angiography is very
useful to exclude the presence CAD where doubt exists.
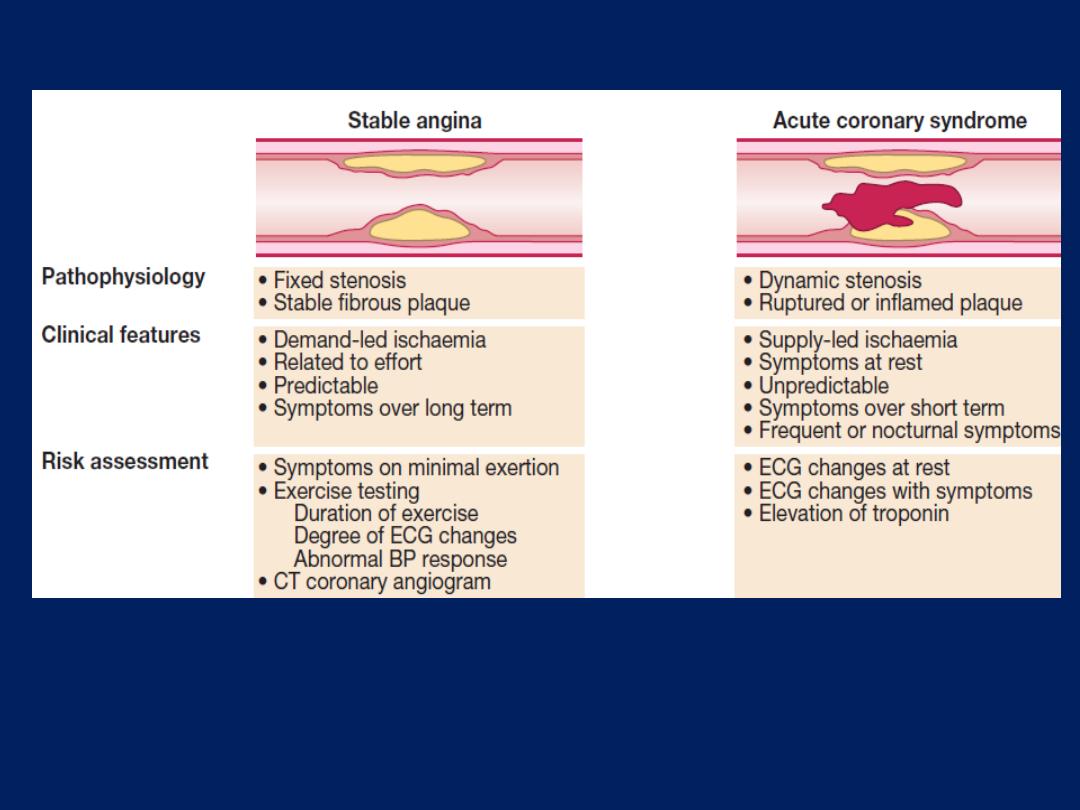
Pathophysiology, clinical features and risk assessment of
patients with stable or unstable coronary heart disease.

Acute coronary syndromes (ACS)
Prolonged
,
severe
chest pain may be due to unstable
angina (recent-onset limiting, rapidly worsening or
crescendo angina, and angina at rest) or acute MI; known
collectively as the ACS. Although there may be a history of
chronic stable angina, an episode is often the first
presentation of CAD . Patients presenting with symptoms
consistent with an ACS require
urgent
evaluation because
there is a high risk of avoidable complications, such as
sudden death and MI. Signs of haemodynamic
compromise (hypotension, pulmonary oedema), ECG
(The most
useful method of initial triage )
ST changes and biochemical markers
of cardiac damage, such as CK, myoglobin , elevated
troponin I or T,
are
powerful indicators of short-term risk.

Repeated ECG are valuable, particularly during an
episode of pain . Plasma troponin should be measured at
presentation and, if normal, repeated 6–12 hours after
the onset.
If the
pain has not recurred, troponin not
elevated and no new ECG changes, the patient may be
discharged from hospital.
Physical examination
may reveal signs of important
comorbidity, such as peripheral or cerebrovascular
disease, autonomic disturbance (pallor or sweating) and
complications (arrhythmia or heart failure).
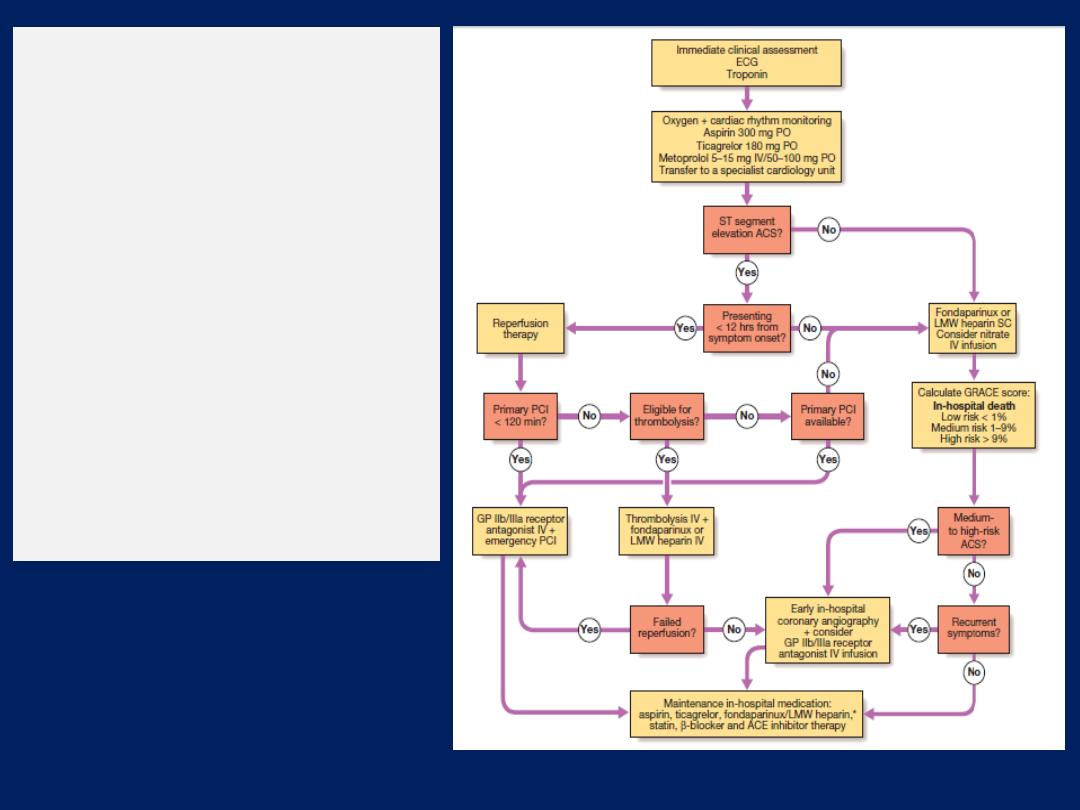
Summary of treatment for
acute coronary syndrome
(ACS). *Not required
following PCI. Amended
from SIGN 93. For details
of the GRACE score, (ACE =
angiotensin-converting
enzyme; GP =
glycoprotein; LMW = low
molecular weight;
PCI = percutaneous
coronary intervention).

Breathlessness (dyspnoea)
Dyspnoea of cardiac origin may vary in severity from an
uncomfortable awareness of breathing to a frightening
sensation of ‘fighting for breath’. The sensation of
dyspnoea originates in the cerebral cortex.
There are several causes of cardiac dyspnoea:
acute left heart failure,
chronic heart failure,
arrhythmia and
angina equivalent .

Breathlessness (dyspnoea)
Acute left heart failure
May be triggered by a major event, such as MI, in a
previously healthy heart, or by a relatively minor event,
such as atrial fibrillation, in a diseased heart. An increase
in the LV-diastolic pressure causes the pressure in the LA,
pulmonary veins and pulmonary capillaries to rise.
When
the hydrostatic pressure of the pulmonary capillaries
exceeds the oncotic pressure of plasma (25–30 mmHg),
fluid moves from the capillaries into alveoli. This stimulates
respiration through a series of autonomic reflexes,
producing rapid shallow respiration.
Congestion of the bronchial mucosa may cause
wheeze
(cardiac asthma).

Breathlessness (dyspnoea) – cont’d
Acute pulmonary oedema
is a terrifying experience
because of the sensation of ‘fighting for breath’. Sitting
upright or standing may provide some relief by helping
to reduce congestion at the apices of the lungs.
The patient may be unable to speak and is typically
distressed, agitated, sweaty and pale.
Respiration is rapid, with recruitment of accessory muscles,
coughing and wheezing. Sputum may be profuse, frothy
and blood-streaked or pink.
Extensive crepitations and rhonchi are usually audible
and there may also be signs of right heart failure.

Breathlessness– cont’d Chronic heart failure
Is the most common cardiac cause of chronic dyspnoea.
Symptoms may first present on moderate exertion, such as
walking up a steep hill, and may be described as a
difficulty in ‘catching my breath’. As failure progresses,
the dyspnoea is provoked by less exertion and, ultimately,
the patient may be breathless walking from room to room,
washing, dressing or trying to hold a conversation.
• Orthopnoea.
Lying down increases the venous return to
the heart and provokes breathlessness. Patients may prop
themselves up with pillows to prevent this.
• Paroxysmal nocturnal dyspnoea.
In severe heart failure,
fluid shifts from the interstitial tissues into the circulation within 1–2
hours of lying down. Pulmonary oedema supervenes, causing the
patient to wake and sit upright, profoundly breathless.

Cheyne–Stokes respiration.
This cyclical pattern of
respiration is due to impaired responsiveness of the
respiratory centre to carbon dioxide and occurs in severe
left ventricular failure. The pattern of slowly diminishing
respiration, leading to apnoea, followed by progressively
increasing respiration and hyperventilation, may be
accompanied by asensation of breathlessness and panic
during the period of hyperventilation. The Cheyne–Stokes
cycle length is a function of the circulation time.
The condition can also occur in diffuse cerebral
atherosclerosis, stroke or head injury, and may be
exaggerated by sleep, barbiturates and opiates.

Breathlessness – cont’d
Arrhythmia
Usually only does so if the heart is structurally abnormal,
such as with the onset of atrial fibrillation in mitral stenosis.
Angina equivalent :
Breathlessness is a common feature of
angina. Patients will sometimes describe chest tightness as
‘breathlessness’.
However, myocardial ischaemia may also induce true
breathlessness by provoking transient left V dysfunction or
heart failure.
When
breathlessness is the dominant or sole feature of
myocardial ischaemia, it is
known as ‘angina equivalent’.
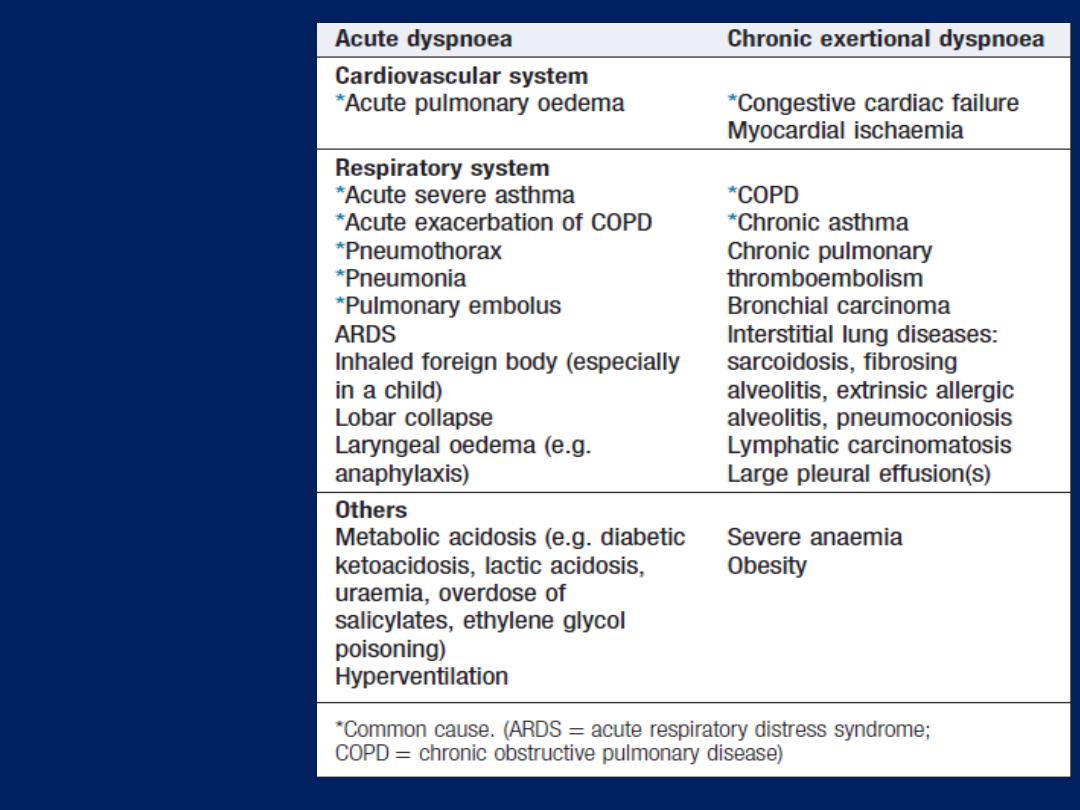
Some causes
of dyspnoea

Acute circulatory failure (cardiogenic shock)
‘Shock’ is used to describe the clinical syndrome that
develops when there is critical impairment of tissue
perfusion due to some form of acute circulatory failure.
There are numerous causes of shock.
Myocardial infarction
Shock in acute MI is due to left ventricular dysfunction
in more than 70% of cases. However, it may also be due
to infarction of the RV and a variety of mechanical
complications, including tamponade (due to infarction and
rupture of the free wall), an acquired VSD (due to
infarction and rupture of the septum) and
acute mitral regurgitation (due to infarction or rupture
of the papillary muscles).

Severe myocardial systolic dysfunction causes a fall
in cardiac output, BP and coronary perfusion pressure.
Diastolic dysfunction causes a rise in left ventricular
end-diastolic pressure, pulmonary congestion and
oedema, leading to hypoxaemia that worsens myocardial
ischaemia. This is further exacerbated by peripheral
vasoconstriction.
These factors combine to create the
‘downward spiral’ of cardiogenic shock.
Hypotension,
oliguria, confusion and cold, clammy peripheries are the
manifestations of a low cardiac output, whereas
breathlessness, hypoxaemia, cyanosis and inspiratory
crackles at the lung bases are typical features of
pulmonary oedema. A chest X-ray may reveal signs of
pulmonary congestion when clinical examination is normal.
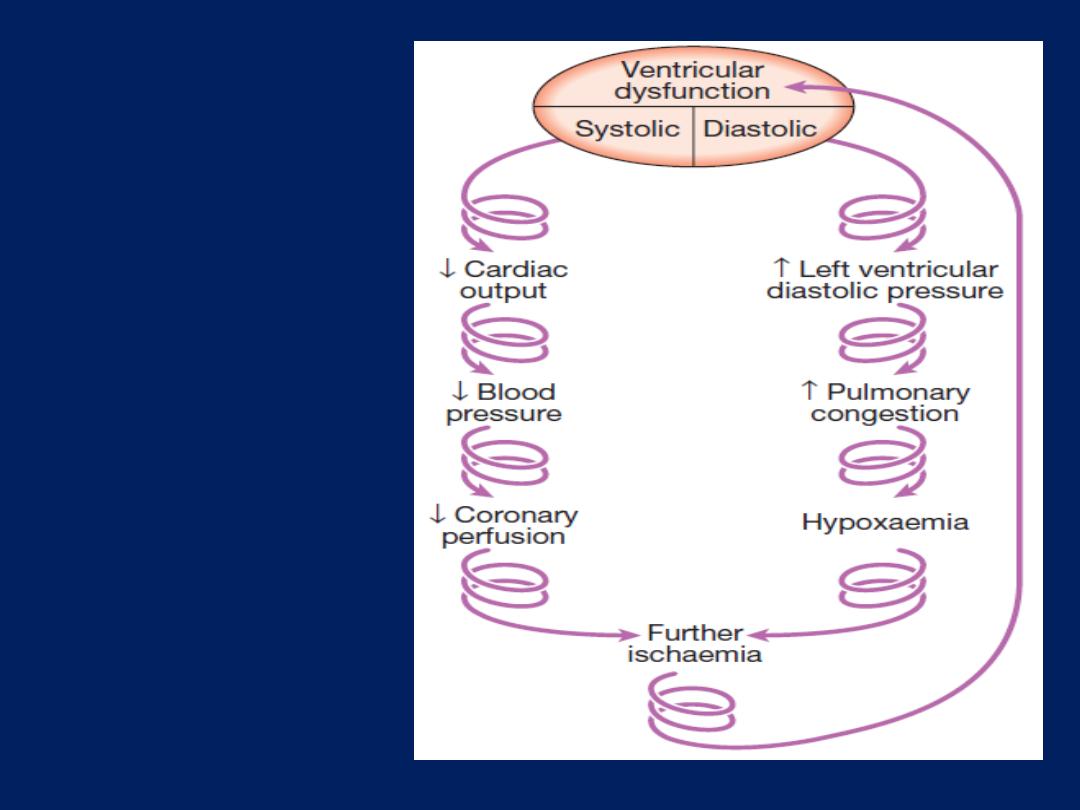
The downward
spiral of
cardiogenic
shock.

If necessary, a Swan– Ganz catheter can be used to
measure the pulmonary artery wedge pressure and to
guide fluid replacement.
The findings can be used to categorise patients with acute
MI into four haemodynamic subsets. Those with
cardiogenic shock should be considered for immediate
coronary revascularisation.
The viable myocardium surrounding a fresh infarct
may contract poorly for a few days and then recover.
This phenomenon is known as
myocardial stunning
and means that acute heart failure should be treated
intensively because overall cardiac function may
subsequently improve.

Acute circulatory failure (cardiogenic shock) – cont’d
Acute massive pulmonary embolism
This may complicate leg or pelvic vein thrombosis and
usually presents with sudden collapse. Bedside echo may
demonstrate a small, underfilled, vigorous LV with a dilated
RV; it is sometimes possible to see thrombus in the right
ventricular outflow tract or main pulmonary artery.
CT pulmonary angio usually provides a definitive diagnosis.
Cardiac tamponade
This is due to a collection of fluid or blood in the pericardial
sac, compressing the heart; the effusion may be small <100
mL. Sudden deterioration may be due to bleeding into the
pericardial space. Tamponade may complicate any form
of pericarditis but can be caused by malignant disease.

Other causes
include trauma and rupture of the free wall
of the myocardium following MI. An ECG may show
features of the underlying disease, such as pericarditis or
acute MI. When there is a large pericardial effusion, the
ECG complexes are small and there may be electrical
alternans: a changing axis with alternate beats caused by
the heart swinging from side to side in the pericardial fluid.
A chest X-ray shows an enlarged globular heart but can
look normal.
Echo is the best way of confirming the diagnosis and helps
to identify the optimum site for aspiration of the fluid.
Prompt recognition of tamponade is important because the
patient usually responds dramatically to percutaneous
pericardiocentesis or surgical drainage.

Clinical features of pericardial tamponade

Acute circulatory failure (cardiogenic shock) – cont’d
Valvular heart disease
Acute left ventricular failure and shock may be due to
the sudden onset of aortic, mitral regurgitation or prosthetic
valve dysfunction . Murmurs are often unimpressive because
there is usually a tachycardia and a low cardiac output.
Transthoracic echo will establish the diagnosis in most cases;
however, transoesophageal echo is sometimes required,
especially in prosthetic mitral valves. Patients with acute
valve failure usually require cardiac surgery.
Aortic dissection may lead to shock by causing aortic
regurgitation, coronary dissection, tamponade .
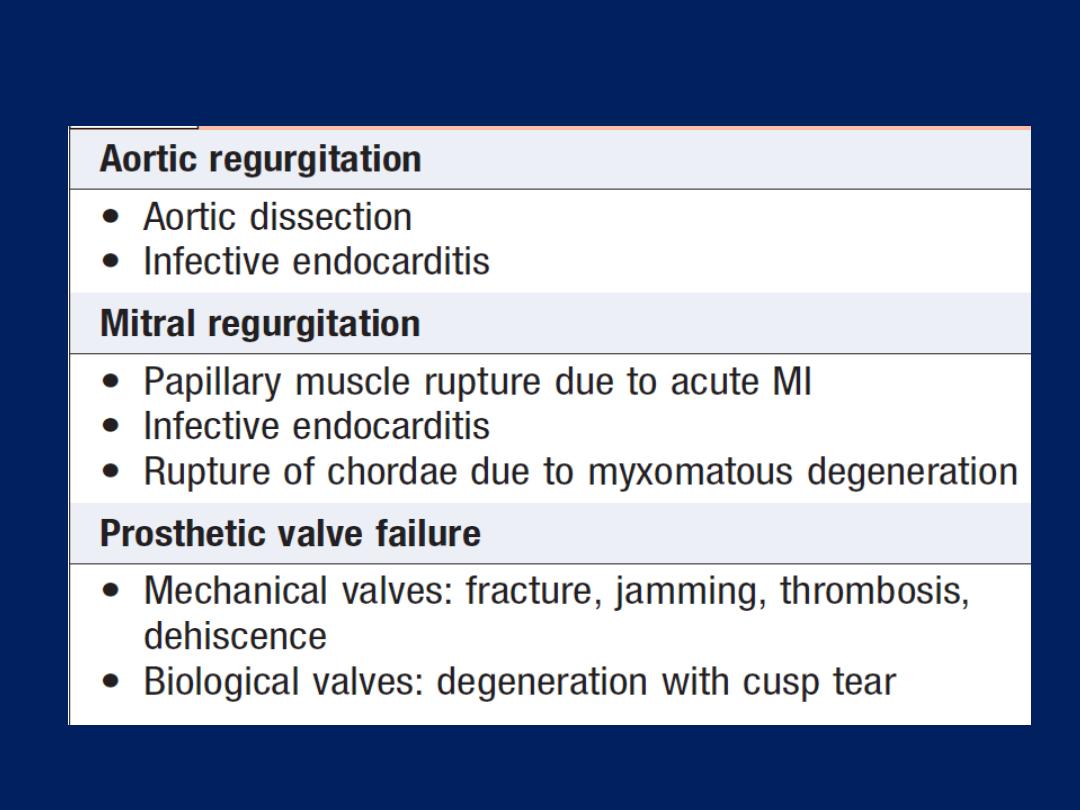
Causes of acute valve failure

Heart failure
Heart failure describes the clinical syndrome that develops
when the heart cannot maintain adequate output, or can
do so only at the expense of elevated ventricular filling
pressure. In mild to moderate forms of heart failure,
cardiac output is normal at rest and only becomes
impaired when the metabolic demand increases during
exercise or some other form of stress. In practice, heart
failure may be diagnosed when a patient with significant
heart disease develops the signs or symptoms of a low
cardiac output, pulmonary congestion or systemic
venous congestion. Almost all forms of heart disease can
lead to heart failure.
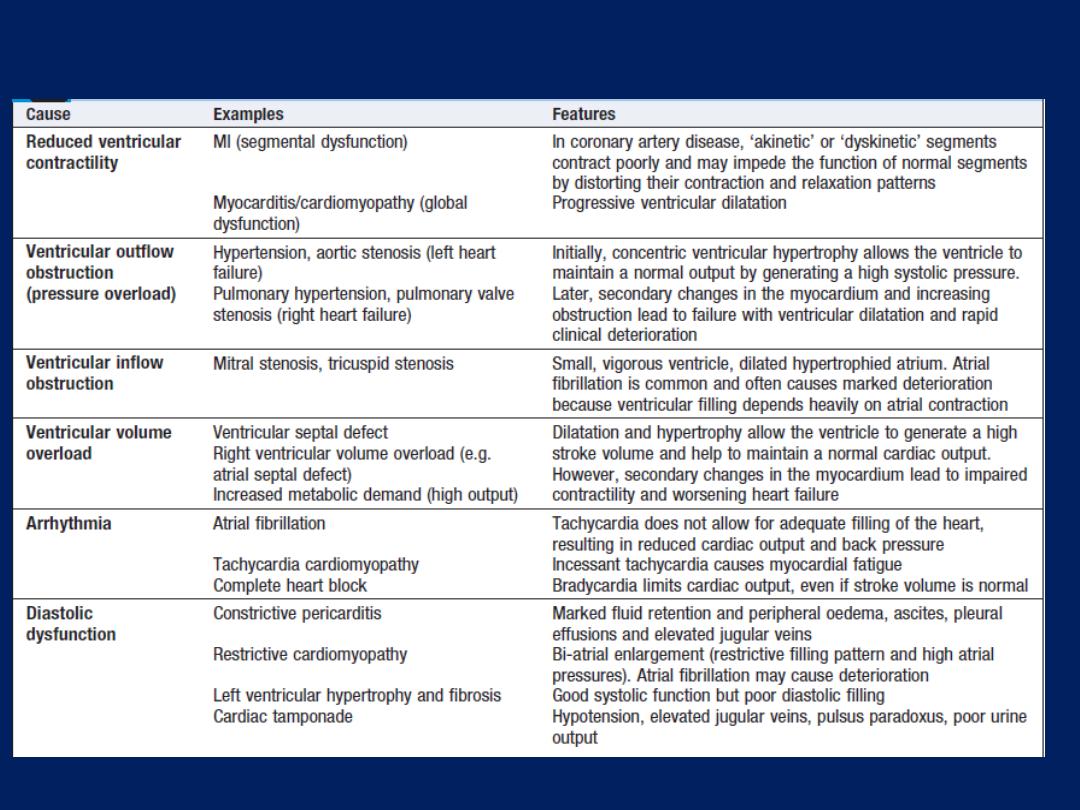
Mechanisms of heart failure

Mechanisms of heart failure
Cause
Reduced ventricular contractility
In coronary artery disease,
‘akinetic’ or ‘dyskinetic’ segments contract poorly and may impede the
function of normal segments by distorting their contraction and
relaxation patterns.
Myocarditis/cardiomyopathy
Global dysfunction and progressive ventricular dilatation.
Ventricular
outflow
obstruction (pressure overload)
a. Hypertension, aortic stenosis (left heart failure)
b. Pulmonary hypertension, pulmonary valve stenosis (right failure).
Initially, concentric ventricular hypertrophy allows the ventricle to
maintain a normal output by generating a high systolic pressure.
Later, secondary changes in the myocardium and increasing
obstruction lead to failure,ventricular dilatation and rapid deterioration

Mechanisms of heart failure – cont’d
Cause
Ventricular
inflow
obstruction
a. Mitral stenosis
b. tricuspid stenosis
Small, vigorous ventricle, dilated hypertrophied atrium. Atrial
fibrillation is common ,
often causes marked deterioration because ventricular filling
depends on atrial contraction.
Ventricular volume overload
a. Ventricular septal defect.
b. Right ventricular volume overload (e.g. atrial septal defect).
c.
Increased metabolic demand (high output).
Dilatation and hypertrophy allow the ventricle to generate a high
stroke volume and help to maintain a normal cardiac output.
However, secondary changes in the myocardium lead to impaired
contractility and worsening heart failure

Mechanisms of heart failure – cont’d
Cause
Arrhythmia
a. Atrial fibrillation :
Tachycardia does not allow for adequate filling
of the heart, resulting in reduced output and back pressure.
b. Tachycardia cardiomyopathy :
Incessant tachycardia causes
myocardial fatigue
c. Complete heart block :
Bradycardia limits cardiac output, even if
stroke volume is normal
Diastolic dysfunction
a. Constrictive pericarditis :
Marked fluid retention , peripheral
oedema, ascites, pleural effusions and elevated jugular veins
b. Restrictive cardiomyopathy :
Bi-atrial enlargement (restrictive
filling pattern and high atrial pressures). AF cause deterioration
c. Left ventricular hypertrophy and fibrosis,Cardiac tamponade :
Good systolic function but poor diastolic filling.Hypotension,
elevated jugular veins, pulsus paradoxus, poor urine output.

The most common aetiology is CAD and MI. Untreated
heart failure carries a poor prognosis; approximately
50% with severe failure due to left V- dysfunction will die
within 2 years, because of pump failure or malignant V-
arrhythmias.
Pathophysiology
Cardiac output is determined by preload (volume and
pressure of blood in the ventricles at the end of diastole),
afterload (volume and pressure of blood in the ventricles
during systole) and myocardial contractility; this is the
basis of
Starling’s Law.

The fall in cardiac output can occur because of impaired
systolic contraction, impaired diastolic relaxation, or both.
This activates counterregulatory neurohumoral mechanisms
that, in normal physiological circumstances, would support
cardiac function but, in the setting of impaired ventricular
function, can lead to a deleterious increase in both afterload
and preload . A vicious circle may be established because
any additional fall in cardiac output will cause further
neurohumoral activation and increasing peripheral vascular
resistance. Stimulation of the renin–angiotensin–aldosterone
system leads to vasoconstriction, sodium and water
retention, and sympathetic nervous system activation.
This is mediated by angiotensin II, a potent constrictor
of arterioles, in both the kidney and the systemic circulation.

Activation of the sympathetic nervous system may
initially sustain cardiac output through increased
myocardial contractility (inotropy) and heart rate
(chronotropy).
Prolonged sympathetic stimulation
also
causes
negative effects, including cardiac myocyte
apoptosis, hypertrophy and focal myocardial necrosis.
Sympathetic stimulation
also causes
peripheral
vasoconstriction
and
arrhythmias. Sodium and water
retention is promoted by the release of aldosterone,
endothelin-1
(a potent vasoconstrictor peptide with marked effects on the renal
vasculature)
and antidiuretic hormone (ADH).
Natriuretic
peptides
are released from the atria in response to atrial
stretch, and act as physiological antagonists to the fluid-
conserving effect of aldosterone.

After MI,
cardiac contractility is impaired and neurohumoral
activation causes hypertrophy of non-infarcted segments,
with thinning, dilatation and expansion of the infarcted
segment
(remodelling).
This leads to further deterioration in ventricular function
and worsening heart failure.
Pulmonary and peripheral oedema occurs because of
high left and right atrial pressures, respectively; this
is compounded by sodium and water retention, caused
by impairment of renal perfusion and by secondary
hyperaldosteronism.
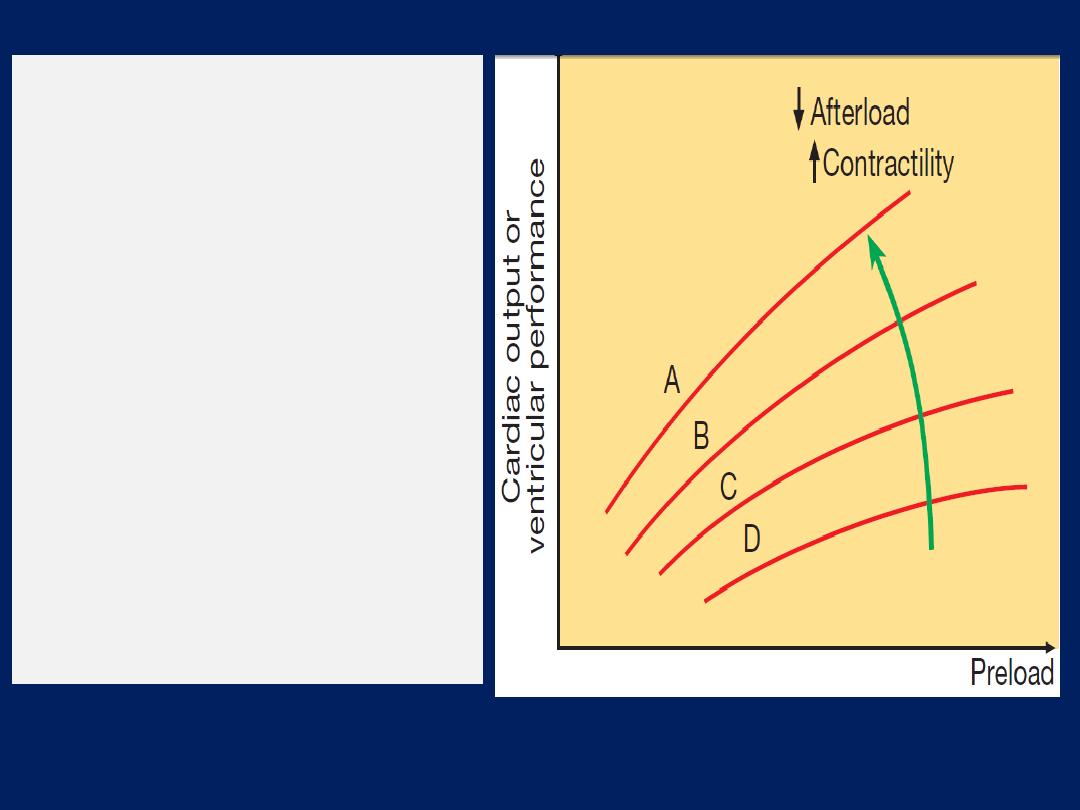
Starling’s Law
.
Normal (A), mild (B),
moderate (C) and severe (D) heart
failure. Ventricular performance is
related to the degree of
myocardial
stretching.
An increase in
preload (end-diastolic volume,
end-diastolic pressure, filling
pressure or atrial pressure) will
therefore enhance function; however,
overstretching
causes marked
deterioration.
In heart failure, the curve moves to
the right and becomes flatter.
An increase
in myocardial
contractility
or a reduction
in
afterload will shift the curve upwards
and to the left (
green arrow).
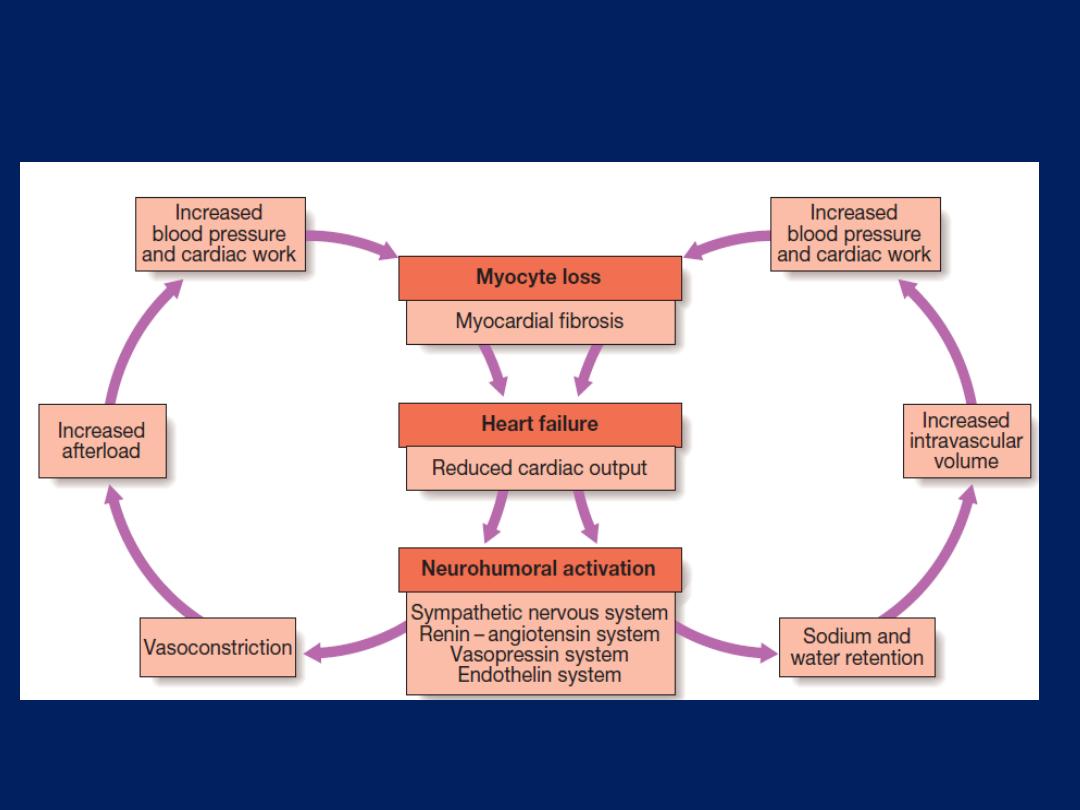
Neurohumoral activation and compensatory mechanisms in heart
failure. There is a vicious circle in progressive heart failure.

Types of heart failure
Left, right and biventricular heart failure
Left-sided heart failure.
There is a reduction in left
ventricular output
increase in left atrial and
pulmonary venous pressure. An acute increase in left
atrial pressure causes pulmonary congestion or
pulmonary oedema; a more gradual increase in left atrial
pressure, as occurs with mitral stenosis, leads to reflex
pulmonary vasoconstriction, which protects the patient
from pulmonary oedema. This increases pulmonary
vascular resistance and causes pulmonary hypertension,
which can, in turn, impair right ventricular function.

Right-sided heart failure.
There is a reduction in right
ventricular output
increase in right atrial
and systemic venous pressure.
Causes of
isolated
right heart failure include chronic lung
disease (cor pulmonale), pulmonary embolism and
pulmonary valvular stenosis.
Biventricular heart failure.
May develop because the
disease process, such as dilated cardiomyopathy or
ischaemic heart disease, affects both ventricles or
because disease of the left heart leads to chronic
elevation of the left atrial pressure, pulmonary
hypertension and right heart failure.

Diastolic and systolic dysfunction
Heart failure may develop as a result of impaired
myocardial contraction (systolic dysfunction) but can also
be due to poor ventricular filling and high filling pressures
stemming from abnormal ventricular relaxation (diastolic
dysfunction). The latter is caused by a stiff, noncompliant
ventricle and is commonly found in patients with left
ventricular hypertrophy.
Systolic and diastolic dysfunction often coexist, particularly
in patients with CAD .
High-output failure
A large arteriovenous shunt, beri-beri, severe anaemia or
thyrotoxicosis can occasionally cause heart
failure due to an excessively high cardiac output.

Acute and chronic heart failure
Heart failure may develop suddenly, as in MI, or gradually,
as in progressive valvular heart disease. The term
‘compensated heart failure’ is sometimes used to describe
the condition of those with impaired cardiac function, in
whom adaptive changes have prevented the development
of overt heart failure. A minor event, such as an
intercurrent infection or development of atrial fibrillation,
may precipitate overt or acute heart failure .Acute left
heart failure occurs, either de novo or as an acute
decompensated episode, on a background of chronic heart
failure: so-called acute-on- chronic heart failure.
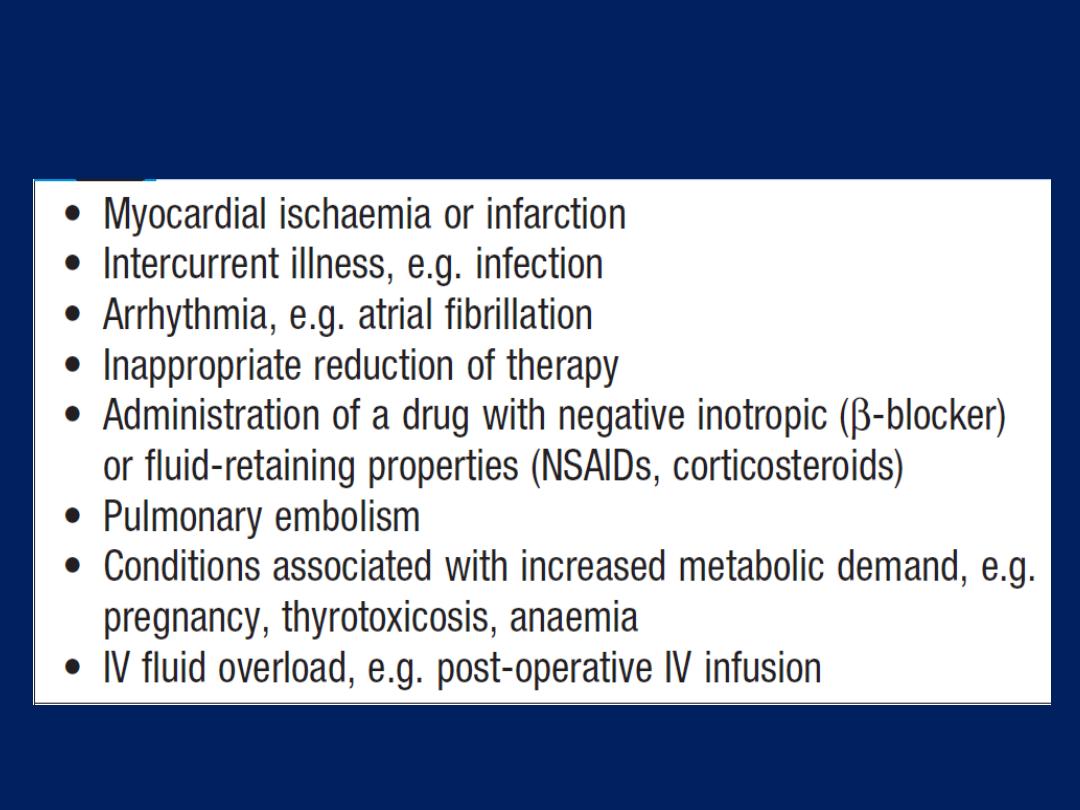
Factors that may precipitate or aggravate
heart failure in pre-existing heart disease

Clinical assessment
Acute left heart failure
Acute de novo left ventricular failure presents with
a sudden onset of dyspnoea at rest that rapidly
progresses to acute respiratory distress, orthopnoea and
prostration. The precipitant, such as acute MI, is often
apparent from the history. The patient appears agitated,
pale and clammy. The peripheries are cool to the touch and
the pulse is rapid. Inappropriate bradycardia or excessive
tachycardia should be identified promptly, as this may be
the precipitant for the acute episode of heart failure.
The BP is usually high because of sympathetic nervous
system activation, but may be normal or low if the patient is
in cardiogenic shock.

JVP is usually elevated, particularly with associated fluid
overload or right heart failure. In acute de novo heart
failure,
there has been no time for
ventricular dilatation
and the apex is not displaced. A ‘gallop’ rhythm, with a
third heart sound, is heard quite early in the development
of acute left sided heart failure.
A new systolic murmur
may signify
acute mitral regurgitation or ventricular septal
rupture. Auscultatory findings in pulmonary oedema are
crepitations at the lung bases, or throughout the lungs if
pulmonary oedema is severe. Expiratory wheeze often
accompanies this. Acute-on-chronic heart failure will have
additional features of long-standing heart failure ,
precipitants, respiratory tract infection or inappropriate
cessation of diuretic medication, should be identified.

Chronic heart failure
Commonly follow a relapsing and remitting course. Low
cardiac output causes fatigue, listlessness , poor effort
tolerance; the peripheries are cold and the BP is low. To
maintain perfusion of vital organs, blood flow is diverted
away from skeletal muscle and this may contribute to
fatigue and weakness. Poor renal perfusion leads to
oliguria and uraemia. Pulmonary oedema due to left heart
failure presents with inspiratory crepitations over the lung
bases. In contrast, right heart failure produces a high JVP
with hepatic congestion and dependent peripheral
oedema. In ambulant, the oedema affects the ankles,
whereas, in bed-bound patients, it collects around the
thighs and sacrum. Ascites or pleural effusion may occur .

Chronic heart failure is sometimes associated with
marked weight loss (cardiac cachexia), caused by
a combination of :
anorexia and impaired absorption due to
gastrointestinal congestion,
poor tissue perfusion due to a low cardiac output,
and skeletal muscle atrophy due to immobility.
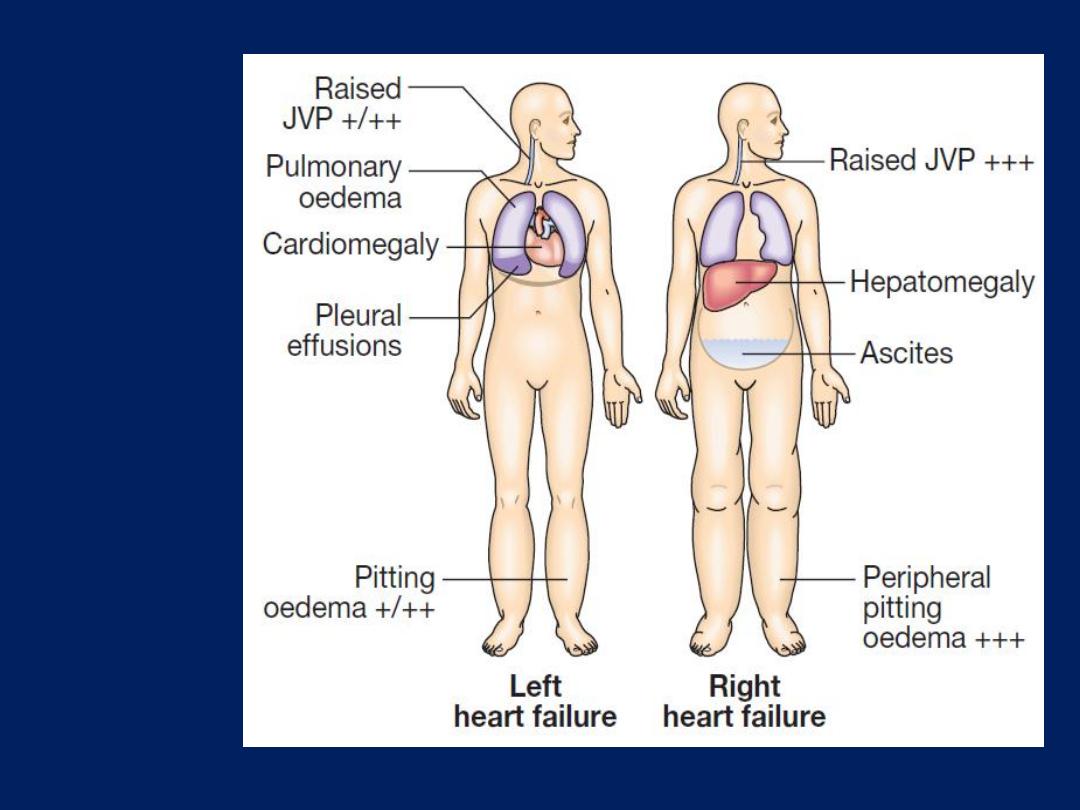
Clinical
features of
left and
right heart
failure.
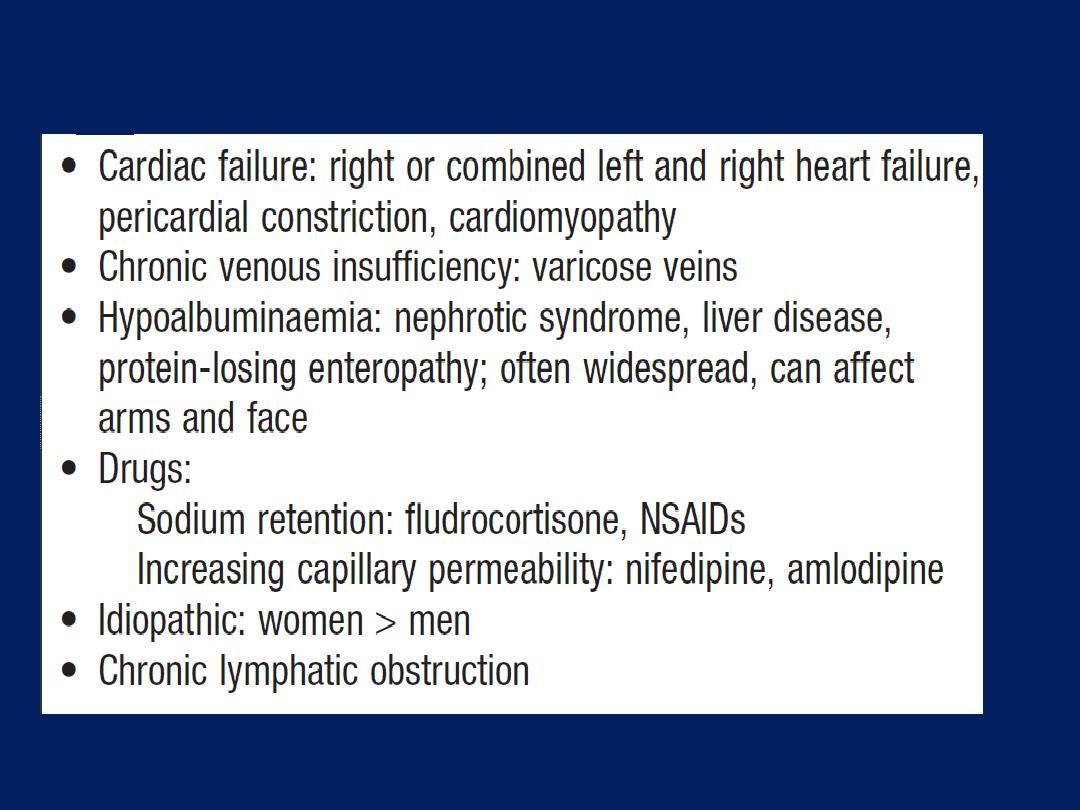
Differential diagnosis of peripheral oedema

Complications of heart failure
In advanced heart failure, the following may occur:
• Renal failure
is caused by poor renal perfusion due
to low cardiac output and may be exacerbated by
diuretic, ACE inhibitors and angiotensin receptor blockers
• Hypokalaemia
may be the result of treatment with
potassium-losing diuretics or hyperaldosteronism caused by
activation of the renin–angiotensin system and impaired
aldosterone metabolism due to hepatic congestion.
• Hyperkalaemia
may be due to the effects of drugs
in particular the combination of ACE inhibitors or ARB
mineralocorticoid receptor antagonists. These effects are
amplified if there is renal dysfunction.

• Hyponatraemia
is a feature of severe failure and is a
poor prognostic sign. It may be caused by diuretic,
inappropriate water retention due to high ADH secretion.
• Impaired liver function
is caused by hepatic venous
congestion, which frequently cause mild jaundice and
abnormal liver function tests; reduced synthesis of clotting
factors can make anticoagulant control difficult.
• Thromboembolism
.
VTE may occur due to the effects
of a low cardiac output and enforced immobility.
Systemic emboli occur in patients with atrial fibrillation or
flutter, or with intracardiac thrombus complicating conditions
such as mitral stenosis, MI or left ventricular aneurysm.

• Atrial and ventricular arrhythmias
are very common
and may be related to electrolyte changes (e.g.
hypokalaemia, hypomagnesaemia), the underlying
cardiac disease, and the pro-arrhythmic effects of
sympathetic activation. Atrial fibrillation occurs in
approximately 20% of patients with heart failure and
causes further impairment of cardiac function.
Sudden death occurs in up to 50% with heart failure and
is often due to a ventricular arrhythmia. Frequent
ventricular ectopic beats and runs of non-sustained
ventricular tachycardia are common findings and
are associated with an adverse prognosis.

Investigations
Serum urea, creatinine and electrolytes, haemoglobin,
thyroid function, ECG and chest X-ray. Brain natriuretic
peptide (BNP) is elevated in heart failure and is a marker
of risk; it is useful in the investigation of patients with
breathlessness or peripheral oedema.
Echocardiography is very useful and should be considered
in all patients with heart failure in order to:
• determine the aetiology
• detect unsuspected valvular disease, such as mitral
stenosis
• identify patients who will benefit from long-term
drug therapy, e.g. ACE inhibitors .

Chest X-ray
High pulmonary venous pressure in left-sided heart
failure first shows on the chest X-ray . as an abnormal
distension of the upper lobe pulmonary veins
(with the patient in the erect position). The vascularity
of the lung fields becomes more prominent, and the
right and left pulmonary arteries dilate. Subsequently,
interstitial oedema causes thickened interlobular septa
and dilated lymphatics. These are evident as horizontal
lines in the costophrenic angles (
septal or ‘Kerley B’
lines). More advanced changes due to alveolar oedema
cause a hazy opacification spreading from the hilar
regions, and pleural effusions.
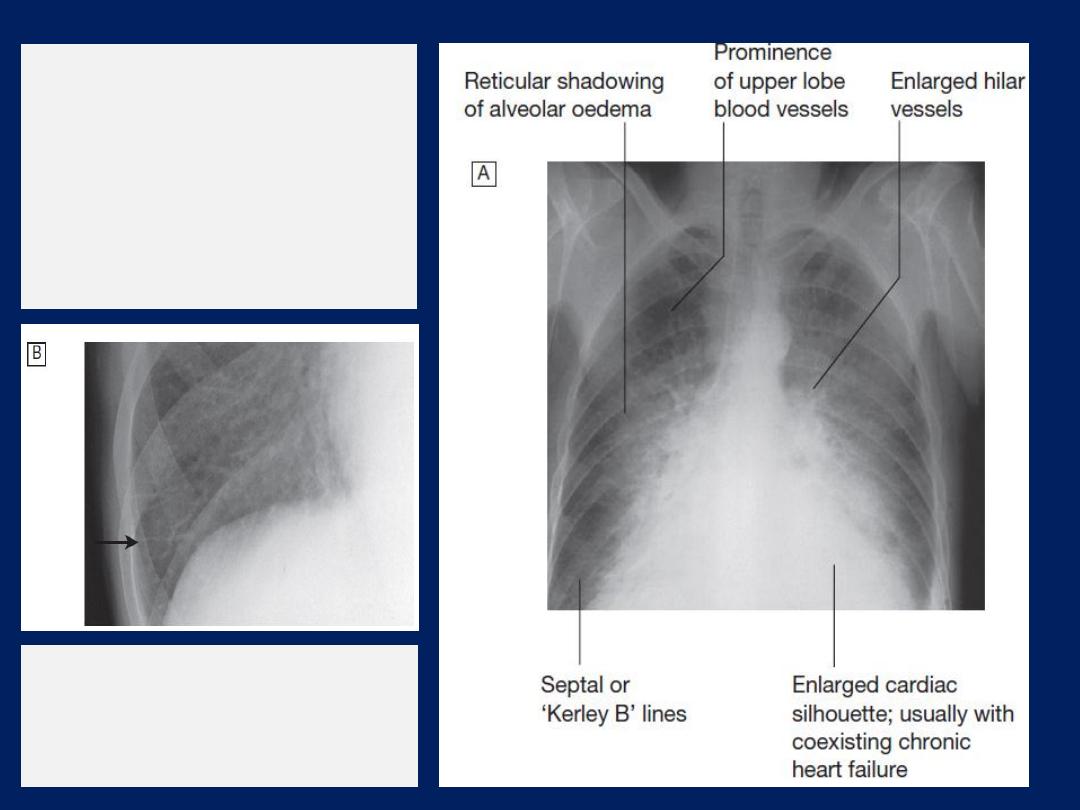
Radiological features
of heart failure.
A
Chest X-ray of
a patient with
pulmonary oedema.
B
Enlargement of lung
base showing septal or
‘Kerley B’ lines (arrow).

Management of acute pulmonary oedema
This is an acute medical emergency:
• Sit the patient up to reduce pulmonary congestion.
• Give oxygen (high-flow, high-concentration).
Non-invasive positive pressure ventilation
(continuous positive
airways pressure (CPAP) of 5–10 mmHg)
by a tight-fitting facemask
results in a more rapid clinical improvement.
• Administer nitrates, IV glyceryl trinitrate
(10–200 μg/min or
buccal glyceryl trinitrate 2–5 mg, titrated upwards every 10 minutes),
until clinical
improvement occurs or systolic BP falls to < 110 mmHg.
• Administer a loop diuretic, furosemide
(50–100 mg IV).
The patient should initially be kept rested, with continuous
monitoring of rhythm, BP and pulse oximetry.

• Intravenous opiates must be used sparingly in distressed
patients, as they may cause respiratory depression and
exacerbation of hypoxaemia and hypercapnia.
•
If these measures prove ineffective,
inotropic agents
may be required to augment cardiac output, particularly
in hypotensive patients. Insertion of an intra-aortic
balloon pump may be beneficial in patients with acute
cardiogenic pulmonary oedema and shock.

Management of chronic heart failure
1. General measures
Education of patients. Some may need to weigh themselves
daily, as a measure of fluid load, and adjust their diuretic.
Treat the underlying cause (e.g. CAD ) is important.
2. Drug therapy
Cardiac function can be improved by increasing
contractility, optimising preload or decreasing afterload.
Drugs that reduce preload are appropriate in patients with
high end-diastolic filling pressures and evidence of
pulmonary or systemic venous congestion.
Those that reduce afterload or increase myocardial
contractility are more useful in patients with signs and
symptoms of a low cardiac output.

Diuretic therapy
In heart failure, diuretics produce an increase in urinary
sodium and water excretion , reduces preload and
improves pulmonary and systemic venous congestion. It may
also reduce afterload, leading to a fall in ventricular wall
tension and increased cardiac efficiency.
Although a fall in preload tends to reduce cardiac output,
the ‘Starling curve’ in heart failure is flat, so there may be
a substantial and beneficial fall in filling pressure with little
change in cardiac output . Nevertheless, excessive diuretic
may cause an undesirable fall in cardiac output, especially
in patients with a marked diastolic component. This leads to
hypotension, lethargy and renal failure.

In some patients with severe chronic heart failure,
oedema may persist, despite oral loop diuretic therapy.
In such patients, an intravenous infusion of furosemide
(5–10 mg/ hr) may initiate a diuresis. Combining a loop
with a thiazide diuretic (e.g.
bendroflumethiazide 5 mg daily
) may
prove effective, but this can cause an excessive diuresis.
Mineralocorticoid receptor antagonists,
such as
spironolactone and eplerenone, are of particular benefit
in patients with heart failure with severe left ventricular
systolic dysfunction. They may cause hyperkalaemia,
particularly when used with an ACE inhibitor.
They
improve longterm clinical outcome in
patients with
severe heart failure or heart failure following acute MI.

Angiotensin-converting enzyme (ACE) inhibition therapy
ACE inhibition therapy
interrupts the vicious circle of
neurohumoral activation that is characteristic of moderate
and severe heart failure by preventing the conversion of
angiotensin I to angiotensin II, thereby preventing peripheral
vasoconstriction, activation of the sympathetic nervous
system ,
and
salt and water retention due to aldosterone
release.
These drugs
also prevent
the undesirable activation of the
renin–angiotensin system caused by diuretic . In moderate
and severe heart failure, ACE inhibitors can produce a
substantial improvement in effort tolerance and in mortality.

Beta-adrenoceptor blocker therapy
Counteract the deleterious effects of enhanced
sympathetic stimulation and reduces arrhythmias and
sudden death. When initiated in standard doses, they may
precipitate acute-on-chronic heart failure, but when given
in small incremental doses (e.g. bisoprolol started at a
dose of 1.25 mg daily, and increased gradually over a
12-week period to a target maintenance dose of 10 mg
daily), they can increase ejection fraction, improve
symptoms, reduce hospitalisation and reduce mortality in
patients with chronic heart failure . Betablockers are
more
effective at
reducing mortality than ACE inhibitors
:
relative risk reduction of 33% versus 20%, respectively.

They can also improve outcome and prevent the onset of
overt heart failure in patients with poor residual left
ventricular function following MI.
ACE inhibitors can cause symptomatic hypotension
and impairment of renal function, especially in patients
with bilateral RAS or preexisting renal disease. An increase
in serum potassium concentration. Short-acting ACE
inhibitors can cause marked falls in BP, particularly in the
elderly or when started in the presence of hypotension,
hypovolaemia or hyponatraemia. In stable patients without
hypotension (systolic BP > 100 mmHg), ACE inhibitors can
usually be safely started.

However, in other patients, it is usually advisable to
withhold diuretics for 24 hours before starting treatment
with a small dose of a long-acting agent, preferably given
at night.
Renal function and serum potassium must be monitored and
should be checked 1–2 weeks after starting therapy.
Angiotensin receptor blocker therapy
Act by blocking the action of angiotensin II on the heart,
peripheral vasculature and kidney. They produce beneficial
haemodynamic changes that are similar to the effects of
ACE inhibitors but are generally better tolerated. They have
comparable effects on mortality and are a useful
alternative for patients who cannot tolerate ACE inhibitors.

ARBs are normally used as an alternative to ACE
inhibitors, but the two can be combined in patients with
resistant or recurrent heart failure.
Vasodilator therapy
These drugs are valuable in chronic heart failure,
when
ACE inhibitor or ARB drugs are contraindicated (e.g. in
severe renal failure). Venodilators, such as nitrates,
reduce preload, and arterial dilators, such as
hydralazine, reduce afterload .Their use is limited by
pharmacological tolerance and hypotension.

Ivabradine
Ivabradine inhibit the
I
f
inward current in the SA node,
resulting in reduction of heart rate.
It reduces hospital admission and mortality rates in
patients with heart failure due to moderate or severe left
ventricular systolic impairment.
In trials, its effects were most marked in patients with a
relatively high heart rate (over 77/min), so ivabradine is
best suited to patients who cannot take β-blockers or in
whom the heart rate remains high despite β-blockade.
It is ineffective in patients in atrial fibrillation.

Digoxin
Digoxin can be used to provide rate control in
patients with heart failure and atrial fibrillation. In
patients with severe heart failure (NYHA class III–IV ) ,
digoxin reduces the likelihood of hospitalisation for heart
failure, although it has no effect on long-term survival.
Amiodarone
This is a potent anti-arrhythmic drug that has little negative
inotropic effect and may be valuable in patients with poor
left ventricular function.
It is only effective in the treatment of symptomatic
arrhythmias, and
should not
be used as a preventative
agent in asymptomatic patients.

3. Implantable cardiac defibrillators
and
resynchronisation therapy
Patients with symptomatic ventricular arrhythmias and
heart failure have a very poor prognosis. Irrespective of
their response to anti-arrhythmic therapy, all should be
considered for implantation because it
improves survival .
In patients
with marked
intraventricular conduction delay,
prolonged depolarisation may lead to uncoordinated left
ventricular contraction. When this is associated with severe
symptomatic heart failure,
cardiac resynchronisation
should be considered. Here, both the LV and RV are paced
simultaneously to generate a more coordinated left
ventricular contraction and improve cardiac output. This is
associated with improved
symptoms and survival.

4. Coronary revascularisation
Coronary artery bypass surgery or percutaneous
coronary intervention may improve function in
areas of the myocardium that are ‘hibernating’
because of inadequate blood supply, and can be
used to treat carefully selected patients with heart
failure and coronary artery disease.
If necessary, ‘
hibernating
’ myocardium can be
identified by stress echocardiography and
specialized nuclear or MR imaging.

5. Heart transplantation
Cardiac transplantation is an established and successful
treatment for patients with
intractable
heart failure.
CAD and dilated cardiomyopathy are the most common
indications.
The introduction of ciclosporin for immunosuppression has
improved survival, which is around 80% at 1 year. The use
of transplantation is limited by the efficacy of modern
drug and device therapies, as well as the availability of
donor hearts, so it is generally reserved for young patients
with severe symptoms despite optimal therapy.

Conventional heart transplantation is contraindicated
in patients with pulmonary vascular disease due
to long-standing left heart failure, complex congenital
heart disease (e.g. Eisenmenger’s syndrome) or primary
pulmonary hypertension because the RV of the donor
heart may fail in the face of high pulmonary vascular
resistance.
However,
heart–lung transplantation can be
successful in patients with Eisenmenger’s syndrome.
Lung transplantation has been used for primary
pulmonary hypertension.
Although
cardiac transplantation usually produces a
dramatic improvement in the recipient’s quality of life,
serious complications may occur:

Complications
• Rejection
. In spite of routine therapy with ciclosporin A,
azathioprine and corticosteroids, episodes of rejection are
common and may present with heart failure, arrhythmias;
cardiac biopsy is often used to confirm the diagnosis before
starting treatment with high-dose corticosteroids.
• Accelerated atherosclerosis
.
Recurrent heart failure is
often due to progressive atherosclerosis in the coronary
arteries of the donor heart.
Angina is rare because
the
heart has been denervated.
• Infection.
Opportunistic infection with organisms such as
cytomegalovirus or Aspergillus remains a major cause of
death in transplant recipients.

6. Ventricular assist devices (VADs)
• as a bridge to cardiac transplantation
• potential long-term therapy
• short-term restoration therapy following a potentially
reversible insult, e.g. viral myocarditis.VADs assist cardiac
output by using pulsatile pump that, in some cases, is
implantable and portable. They withdraw blood through
cannulae inserted in the atria or apex and pump it into the
pulmonary artery or aorta. They unload the ventricles and
to provide support to the pulmonary and systemic
circulations. Their application is limited by high
complication rates (haemorrhage, systemic embolism,
infection, neurological and renal sequelae), although some
improvements in survival and quality of life have been
demonstrated in patients with severe heart failure.
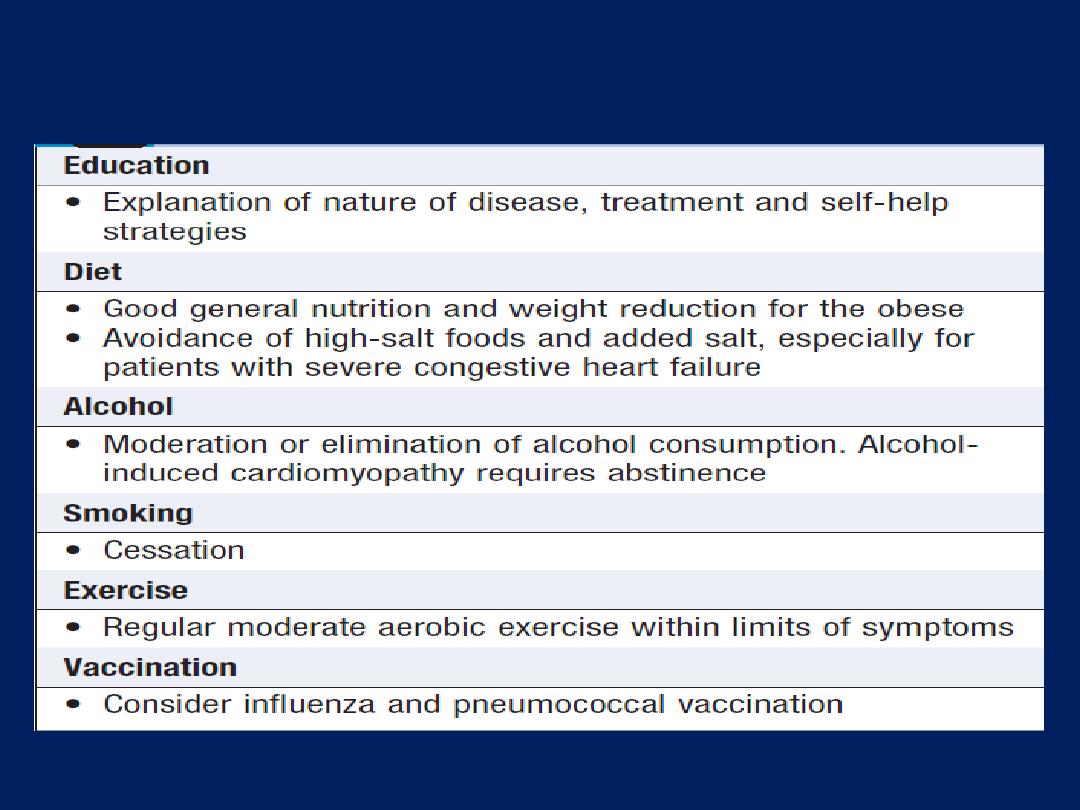
General measures for the management of heart failure
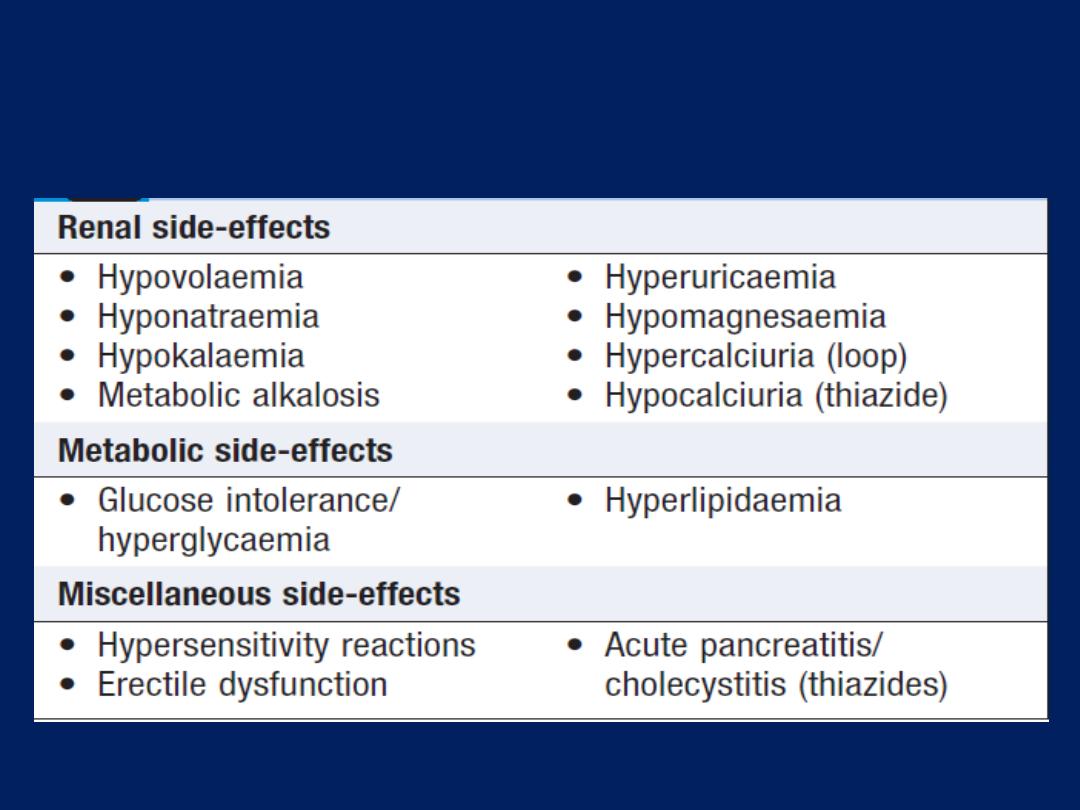
Adverse effects of loop-acting and thiazide
diuretics
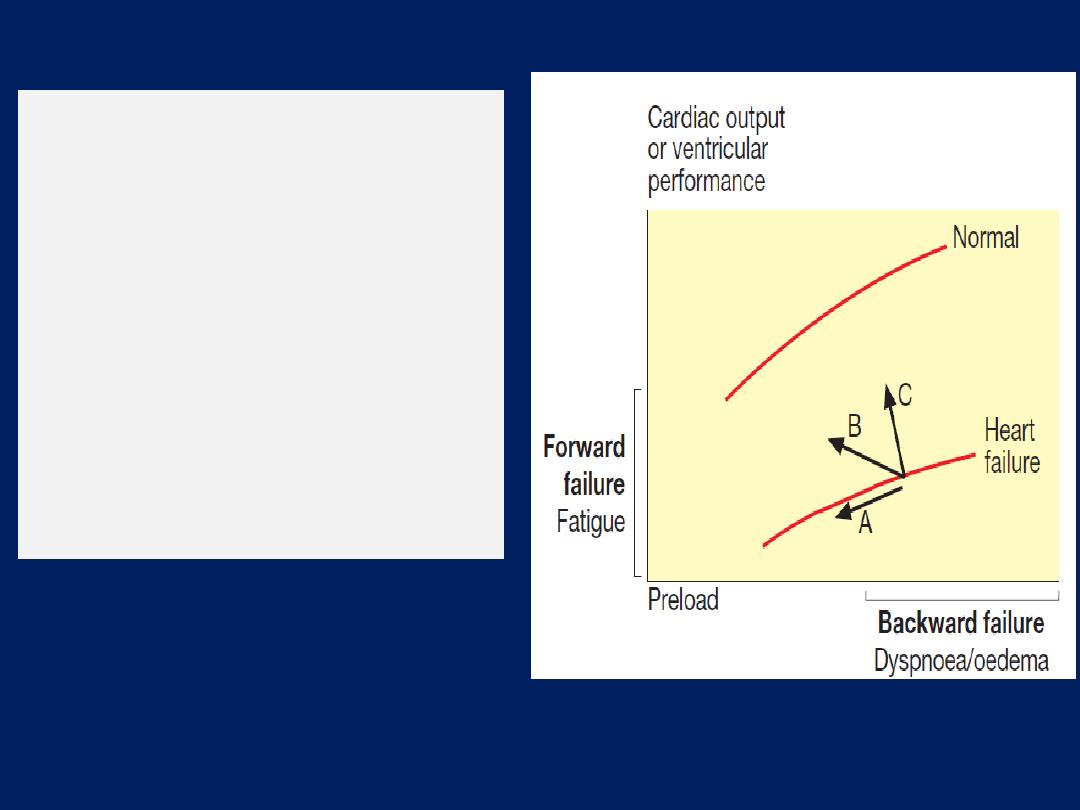
The effect of treatment on
ventricular performance
curves in heart failure.
(A)
Diuretics and
venodilators
(B)
Angiotensin converting
enzyme (ACE) inhibitors
and mixed vasodilators
(C)
Positive inotropic agents
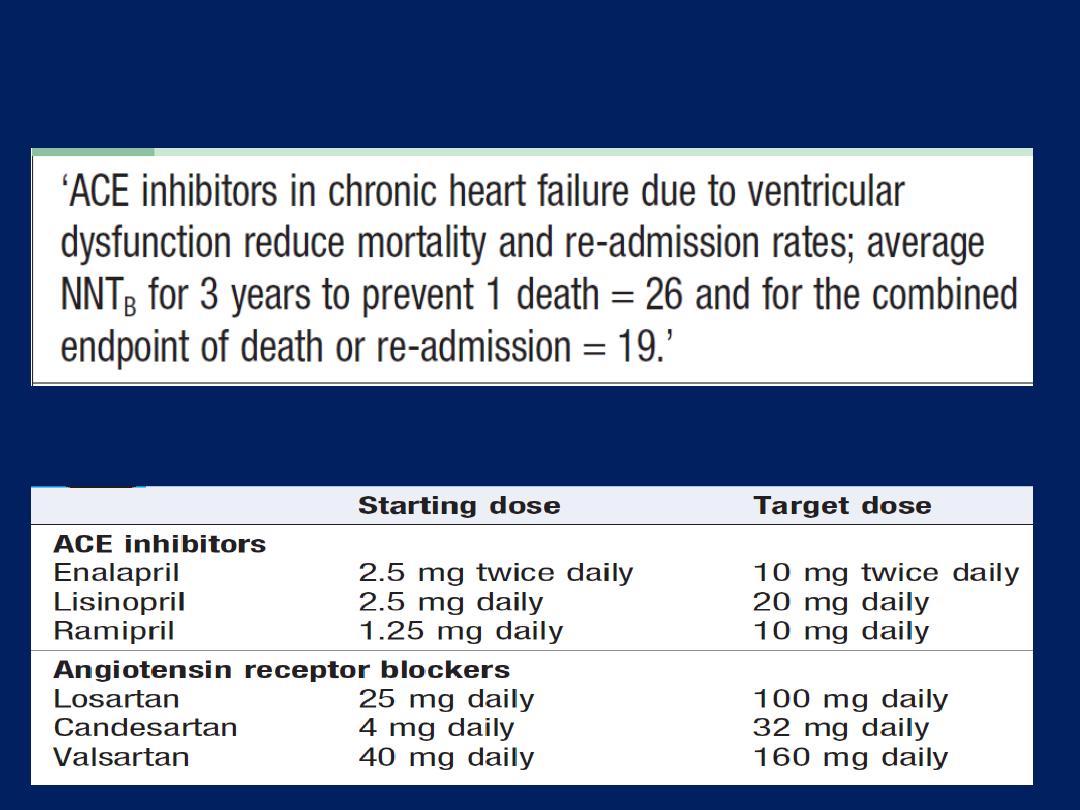
ACE inhibitors and treatment of chronic heart failure
ACE inhibitor and ARB dosages in heart failure
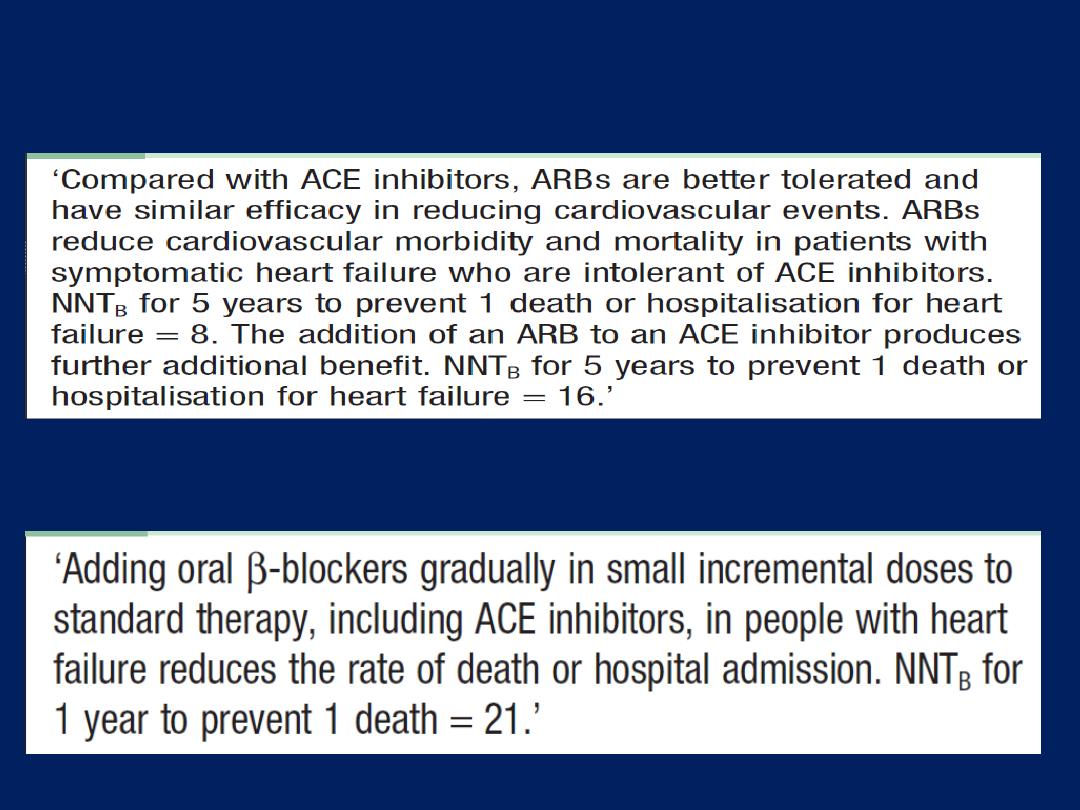
Angiotensin receptor blockers and chronic heart failure
Beta-blockers and treatment of chronic heart failure
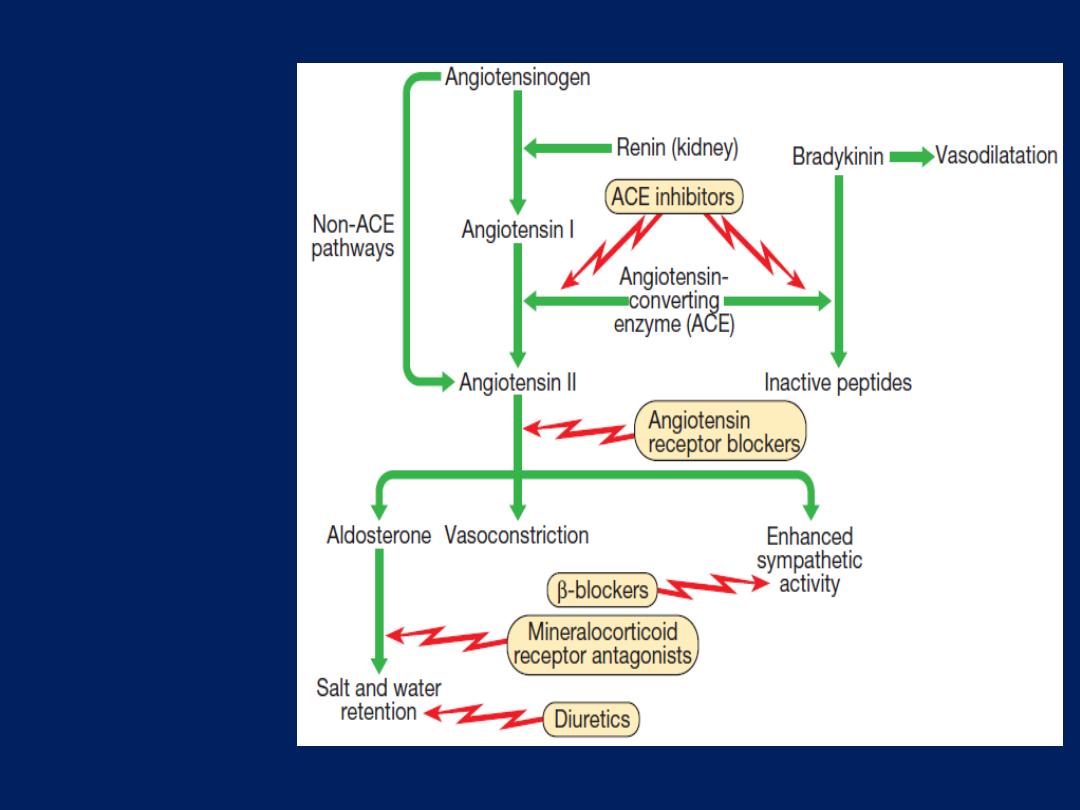
Neurohumor
al activation
and sites of
action of
drugs used
in
the treatment
of heart
failure.
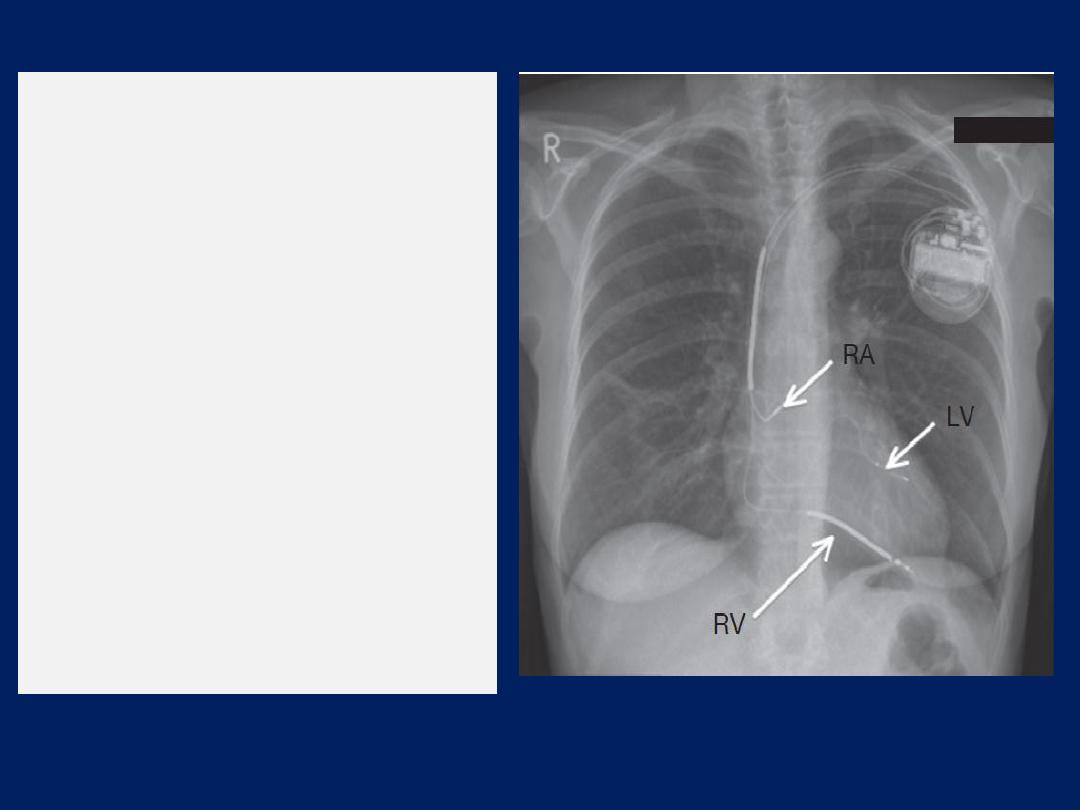
Chest X-ray of a biventricular
pacemaker and defibrillator
(cardiac resynchronisation
therapy).
The right ventricular lead (RV)
is in position in the ventricular
apex and is
used for
pacing
and defibrillation.
The left ventricular lead (LV) is
placed via the coronary sinus,
and the right atrial lead (RA) is
placed in the right atrial
appendage;
both are used
for
pacing only.
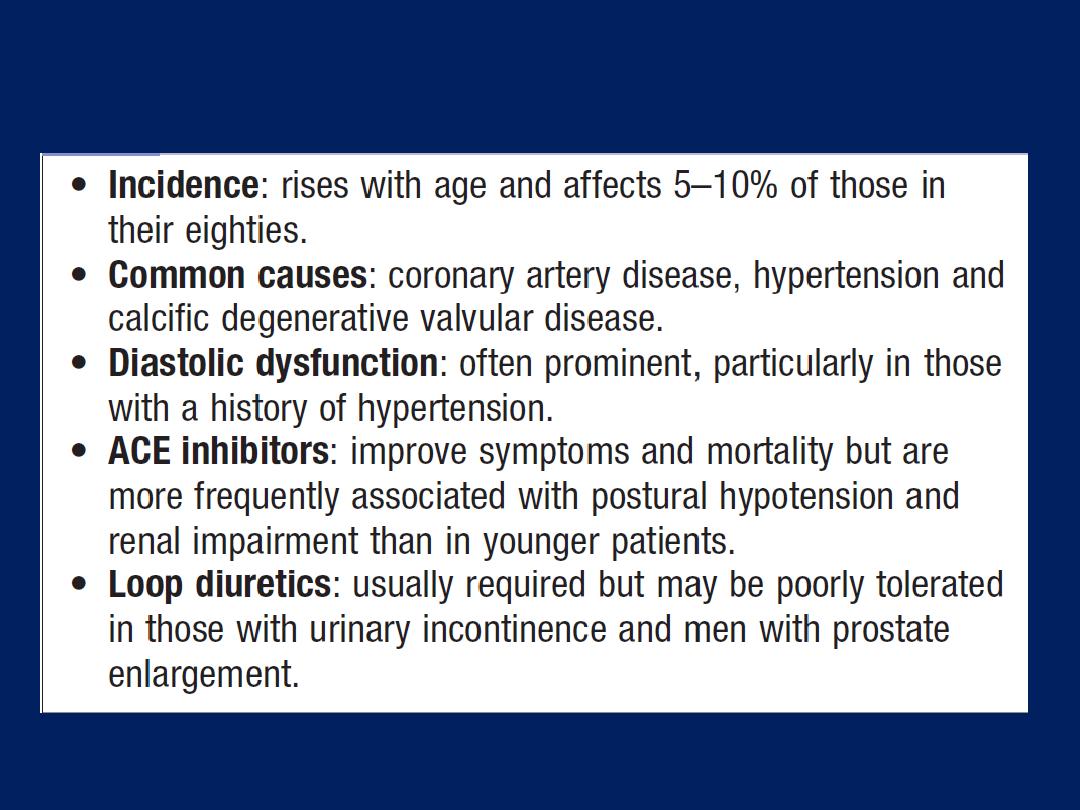
Congestive cardiac failure in old age

Syncope and presyncope
The term ‘syncope’ refers to sudden loss of consciousness
due to reduced cerebral perfusion.
Presyncope
refers to lightheadedness in which the individual thinks
he or she may black out. Syncope affects around 20% of
the population at some time and accounts for more than
5% of hospital admissions. Dizziness and presyncope are
very common in old age . Symptoms are disabling,
undermine confidence and independence, and can affect
an individual’s ability to work or to drive.

There are three principal mechanisms that underlie
recurrent presyncope or syncope:
• cardiac syncope
due to mechanical cardiac dysfunction
or arrhythmia
• neurocardiogenic syncope,
abnormal autonomic reflex
causes bradycardia and/or hypotension
• postural hypotension,
in which physiological
peripheral vasoconstriction on standing is impaired,
lead to hypotension.
Loss of consciousness can also be caused by non-cardiac
pathology, such as
epilepsy, cerebrovascular ischaemia or hypoglycaemia .
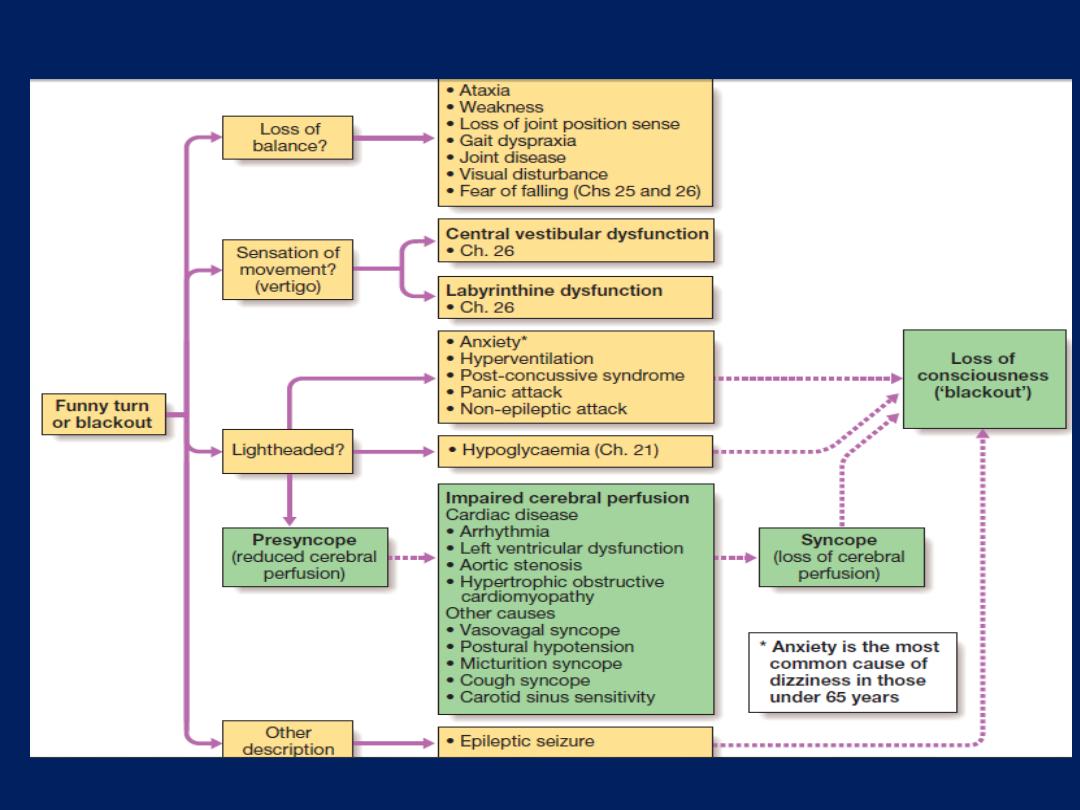
The differential diagnosis of syncope and presyncope.

Differential diagnosis
History-taking, from the patient or a witness, is the key
to establishing a diagnosis. Attention should be paid to
potential triggers (e.g. medication, exertion, posture), the
victim’s appearance (e.g. colour, seizure activity), the
duration of the episode and the speed of recovery .
Cardiac syncope is usually sudden but can be
associated with premonitory lightheadedness, palpitation
or chest discomfort. The blackout is usually brief and
recovery rapid.
Neurocardiogenic syncope will often be associated with
a situational trigger, and the patient may experience
flushing, nausea and malaise for several minutes
afterwards.

Patients with seizures do not exhibit pallor, may have
abnormal movements, usually take more than 5 minutes
to recover and are often confused. A history of rotational
vertigo is suggestive of a labyrinthine or vestibular
disorder .The pattern and description of the patient’s
symptoms should indicate the probable mechanism and
help to determine subsequent investigations.
Postural hypotension is normally obvious from the
history, with presyncope or, less commonly, syncope,
occurring within a few seconds of standing.
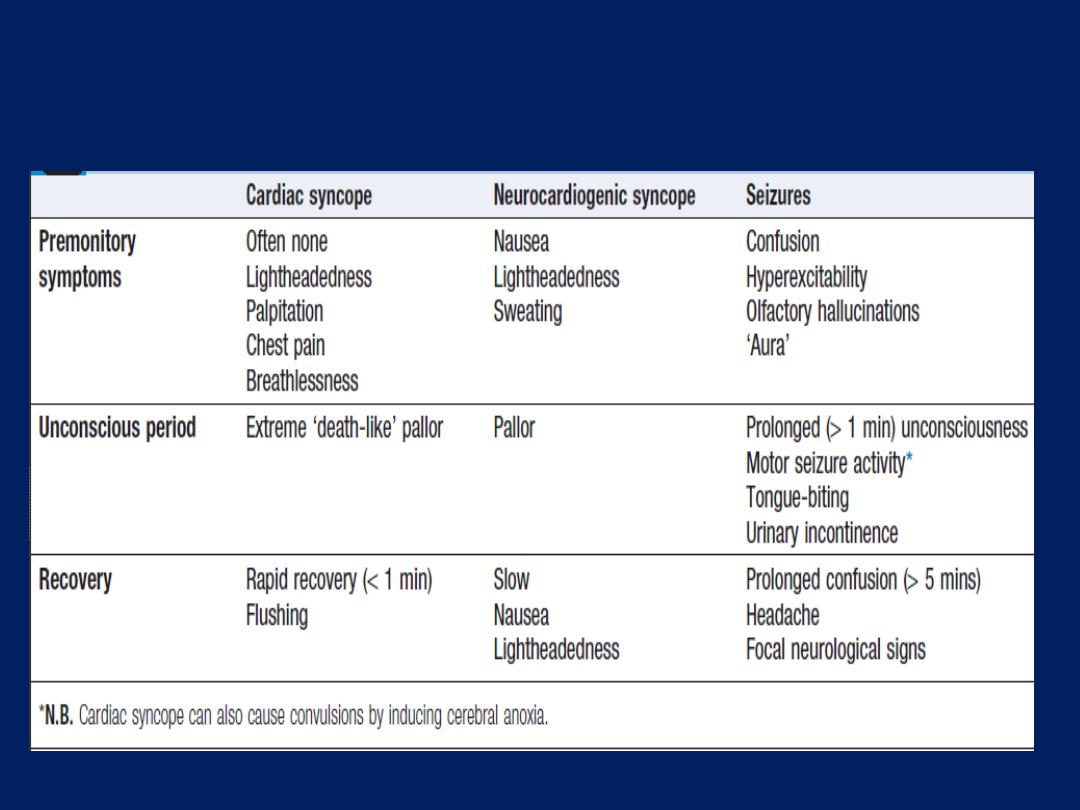
Typical features of cardiac syncope, vasovagal
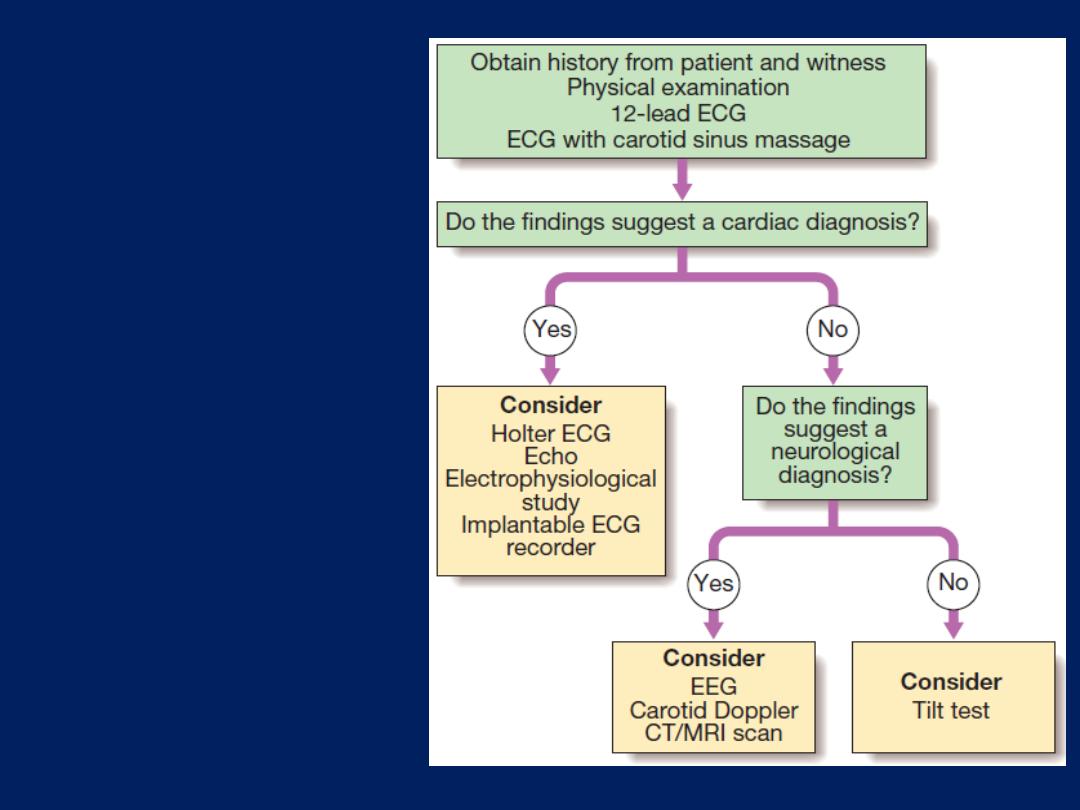
syncope and seizures
A simple guide to
the investigation
and diagnosis of
recurrent
presyncope and
syncope.

1. Cardiac syncope
Arrhythmia
Lightheadedness may occur with many arrhythmias, but
blackouts (Stokes–Adams attacks) are usually due to
profound
bradycardia
or malignant ventricular
tachyarrhythmias
. ECG may show evidence of conducting
system disease (e.g. sinus bradycardia, AV block, BB block)
which would predispose to bradycardia, but the key to
establishing a diagnosis is to obtain an ECG recording
while symptoms are present. Ambulatory ECG recordings
are helpful only if symptoms occur several times per week.
an implantable ECG recorder can be sited subcutaneously
over the upper left chest. Stored ECGs can be accessed by
the implanting centre, using a telemetry device

Structural heart disease
Severe aortic stenosis and hypertrophic obstructive
cardiomyopathy can lead to lightheadedness or syncope
on exertion. This is caused by profound hypotension due
to a fall in cardiac output, or failure to increase output
during exertion, coupled with exercise-induced peripheral
vasodilatation.
Severe coronary artery disease can produce the same
symptoms because of ischaemic left ventricular
dysfunction.
Exertional arrhythmias also occur in these patients.

2. Neurocardiogenic syncope
Bradycardia and/or hypotension occur because of a
series of abnormal autonomic reflexes.
The two main conditions are hypersensitive carotid sinus
syndrome and malignant vasovagal syncope.
Situational syncope
This is the collective name given to some variants of
neurocardiogenic syncope that occur in the presence of
identifiable triggers (e.g. cough, micturition syncope).
Vasovagal syncope
This is normally triggered by a reduction in venous
return due to prolonged standing, excessive heat or a
large meal. It is mediated by the Bezold– Jarisch reflex,

Bezold–Jarisch reflex, a combination of sympathetic
activation, and reduced venous return due to an impaired
vasoconstrictor response to standing, leads to vigorous
contraction of relatively under-filled ventricles. This
stimulates ventricular mechanoreceptors, producing
parasympathetic (vagal) activation and sympathetic
withdrawal, causing bradycardia, vasodilatation or both.
Head-up
tilt-table
testing is a provocation test used to
establish the diagnosis, and involves positioning the patient
supine on a padded table that is then tilted to an angle of
60–70° for up to 45 minutes, while the ECG and BP
responses are monitored.
A positive test is characterised
by bradycardia (cardio-
inhibitory response) and/or hypotension (vasodepressor
response) associated with typical symptoms.

Initial management involves lifestyle modification
(salt supplementation and avoiding prolonged standing,
dehydration or missing meals). In resistant cases, drug
therapy can be tried, although efficacy is inconsistent in
clinical trials. Fludrocortisone (causes sodium and water
retention and expands plasma volume), disopyramide
(a vagolytic agent) or midodrine (a vasoconstrictor
α- adrenoceptor agonist) may be helpful. Beta-blockers
(inhibit the initial sympathetic activation) are seldom
effective and are rarely used. In patients with resistant
vasovagal syncope in which bradycardia is the
predominant response, a dual-chamber pacemaker can be
useful. Patients with a urinary sodium excretion of less than
170 mmol/day may respond to salt loading.

Hypersensitive carotid sinus syndrome (HCSS)
Causes presyncope or syncope because of reflex
bradycardia and vasodilatation.
Carotid baroreceptors
are involved in BP regulation and
are activated by increased BP, resulting in a vagal
discharge that causes a compensatory drop in BP. In HCSS,
the baroreceptor is sensitive to external pressure (e.g.
during neck movement or if a tight collar is worn), so that
pressure over the carotid artery causes an inappropriate
and intense vagal discharge.
The diagnosis can be established by monitoring the ECG
and BP during carotid sinus pressure.
This manoeuvre
should not
be attempted in patients with
a carotid bruit or with a history of cerebrovascular disease
because of the risk of embolic stroke.

A positive cardio-inhibitory response is defined as a
sinus pause of 3 seconds
or more;
A positive vasodepressor response is defined as
a fall
in
systolic
BP of
> 50 mmHg.
Carotid sinus pressure will produce positive findings in
about 10% of elderly individuals but less than 25% of
these experience spontaneous syncope.
Symptoms should not therefore be attributed to HCSS
unless they are reproduced by carotid sinus pressure.
Dual-chamber pacemaker implantation usually prevents
syncope in patients with the more common cardio-
inhibitory response.

Syncope and presyncope– cont’d
3. Postural hypotension
This is caused by
a failure of normal postural
compensatory mechanisms. Relative hypovolaemia (often
due to excessive diuretic therapy), sympathetic
degeneration (diabetes mellitus, Parkinson’s disease,
ageing) and drug therapy (vasodilators, antidepressants)
can all cause or aggravate the problem.
Treatment
is often ineffective; however, withdrawing
unnecessary medication and advising the patient to wear
graduated elastic stockings and to get up slowly may be
helpful. Fludrocortisone which can expand blood volume
through sodium and water retention, may be of value.

Palpitation
Is a very common and sometimes frightening symptom.
Patients use the term to describe many sensations, including
erratic, fast, slow or forceful heart beat, or even chest
pain or breathlessness. Initial evaluation should
concentrate on determining its likely mechanism, and
whether or not there is significant underlying heart disease.
A detailed description of the sensation is essential and
patients should be asked to describe their symptoms clearly,
or to demonstrate the sensation of rhythm by tapping with
their hand.
The diagnosis should be confirmed by an ECG recording
during an episode using an ambulatory ECG monitor or a
patient-activated ECG recorder.

Recurrent but short-lived bouts of an irregular heart
beat are usually due to atrial or ventricular extrasystoles
(ectopic beats). Some patients will describe the
experience as a ‘flip’ or a ‘jolt’ in the chest, while others
report dropped or missed beats , more frequent during
periods of stress or debility; they can be triggered by
alcohol or nicotine.
These may also reflect a hyperdynamic circulation, such
as anaemia, pregnancy and thyrotoxicosis, and can occur
in some forms of valve disease (e.g. aortic regurgitation).
Discrete bouts of a very rapid (>120/min) heart beat
are more likely to be due to a paroxysmal
tachyarrhythmia (SVT or VT).

In contrast, episodes of atrial fibrillation typically present
with irregular and usually rapid palpitation.
Palpitation is usually benign and, even if the patient’s
symptoms are due to an arrhythmia, the outlook is good if
there is no underlying structural heart disease.
Most cases are due to an awareness of the normal heart
beat, a sinus tachycardia or benign extrasystoles, in which
case an explanation and reassurance may be all that is
required.
Palpitation associated with
presyncope or
syncope may
reflect
more serious structural or electrical
disease and should be investigated without delay.

How to evaluate palpitation
• Is the palpitation continuous or intermittent?
• Is the heart beat regular or irregular?
• What is the approximate heart rate?
• Do symptoms occur in discrete attacks?
Is the onset abrupt ? How do attacks terminate?
• Are there any associated symptoms?
Chest pain, lightheadedness, polyuria (feature of SVT )
• Are there any precipitating factors, e.g. exercise,
alcohol?
• Is there a history of structural heart disease, e.g.
coronary artery disease, valvular heart disease?
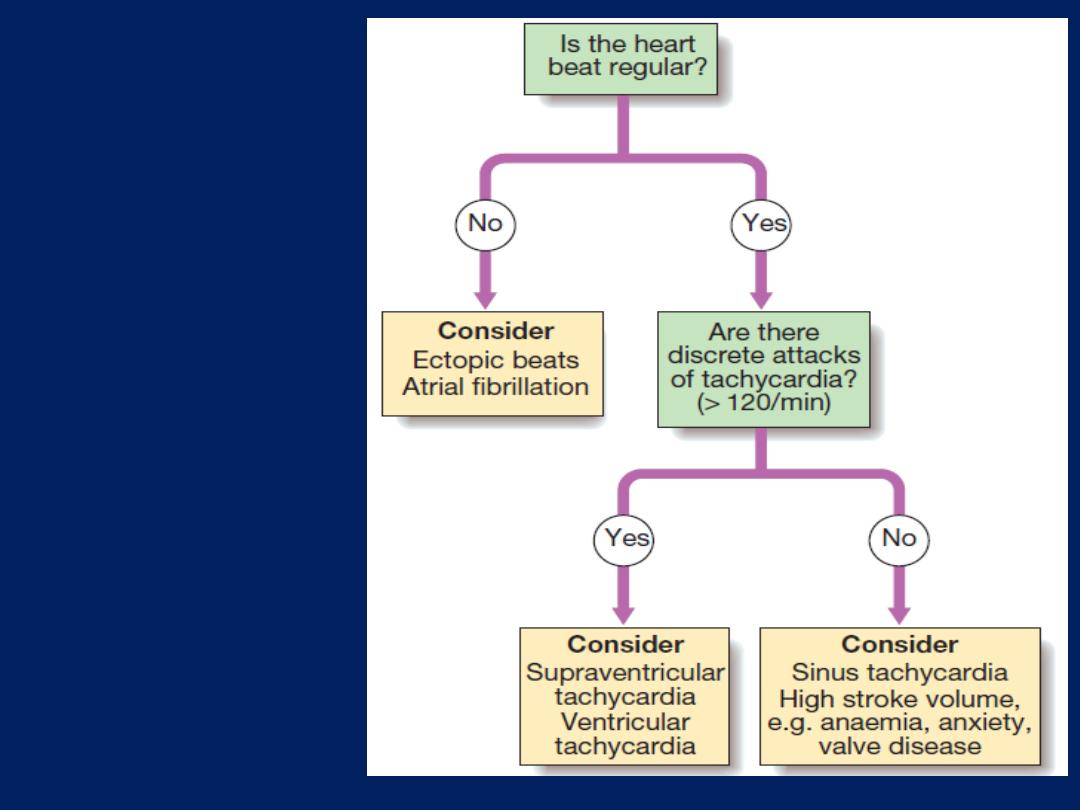
A simple
approach to
the diagnosis
of palpitation.

Cardiac arrest and sudden cardiac death
Cardiac arrest describes the sudden and complete
loss
of cardiac output due to
Asystole,
Ventricular
tachycardia or fibrillation, or
loss of mechanical
cardiac contraction (pulseless electrical
activity). The clinical diagnosis is based on the victim being
unconscious and pulseless; breathing may take some time
to stop completely after cardiac arrest. Death is virtually
inevitable, unless effective treatment is given promptly,
accounts for 25–30% of deaths from CVD.
Coronary artery disease(CAD) is the most common
condition leading to cardiac arrest. Ventricular fibrillation
or tachycardia is common in the first few hours of MI and
many victims die before medical help is sought.

Up to one-third of people developing MI die before
reaching hospital, emphasising the importance of educating
the public to recognise symptoms and to seek medical help
quickly. Acute myocardial ischaemia (in the absence of
infarction) can also cause these arrhythmias, although less
commonly. Patients with a history of previous MI may be at
risk of sudden arrhythmic death, especially if there is
extensive left ventricular scarring and impairment, or if
there is ongoing myocardial ischaemia. In these patients,
the risk is
reduced by
the treatment of heart failure with
β-blockers and ACE inhibitors, and by coronary
revascularisation. For some, the risk of sudden death is
reduced by the implantation of a cardiac defibrillator.

Aetiology of cardiac arrest
Cardiac arrest may be caused by ventricular fibrillation,
pulseless ventricular tachycardia, asystole or pulseless
electrical activity.
Ventricular fibrillation and pulseless ventricular
tachycardia
These are the most common and most easily treatable
cardiac arrest rhythms. Ventricular fibrillation produces
rapid, ineffective, uncoordinated movement of the
ventricles, which therefore produces no pulse. The ECG
shows
rapid, bizarre and irregular
ventricular complexes.
Ventricular tachycardia can cause cardiac arrest if the
ventricular rate is so rapid that effective mechanical
contraction and relaxation cannot occur, especially in the
presence of severe left ventricular impairment.

Defibrillation will restore
cardiac output in more
than 80%, if delivered immediately. However, the chances
of survival fall by at least 10% with each minute’s delay,
and by more if basic life support is not given; thus provision
of these is the key to survival.
Asystole
This occurs when there is
no electrical activity
within the
ventricles and is usually due to failure of the conducting
tissue or massive ventricular damage complicating MI.
A precordial
thump
, external cardiac massage, or
administration of intravenous
atropine
or
adrenaline
may
restore cardiac activity.
When due to conducting tissue failure, permanent
pacemaker
implantation will be required if survive.
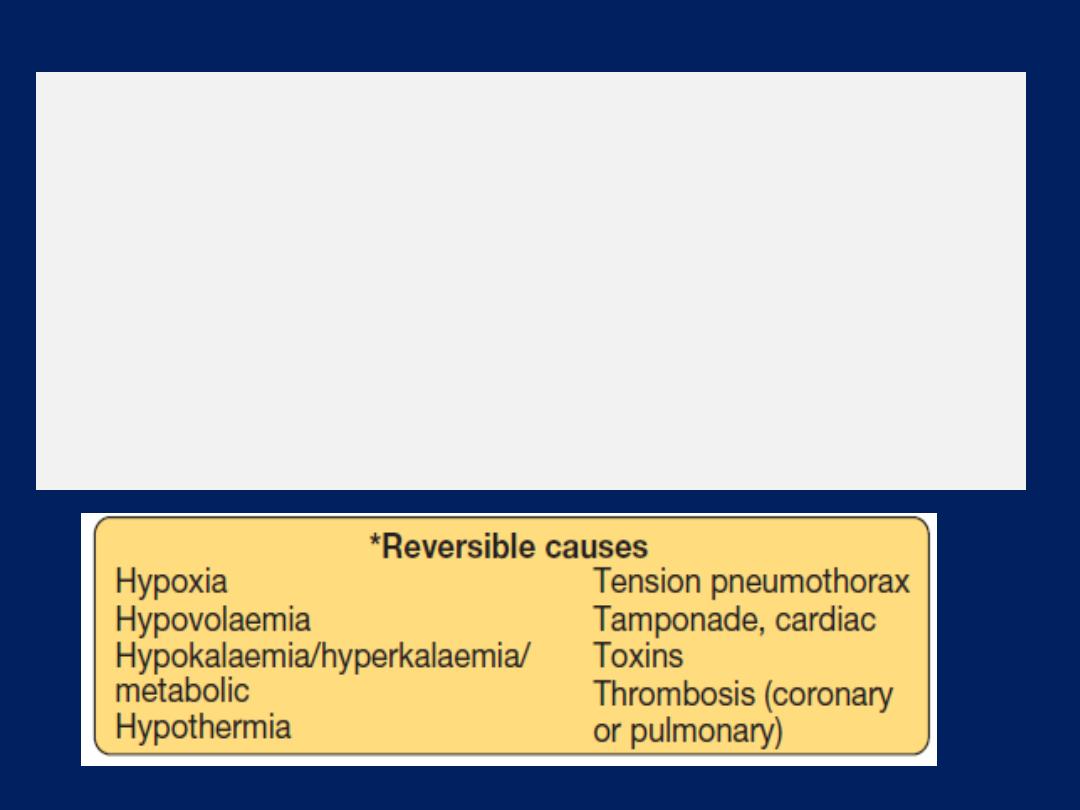
Pulseless electrical activity
This occurs when there is no effective cardiac output
despite the presence of
organised electrical activity.
It
may be caused by reversible conditions, such as
hypovolaemia, cardiac tamponade or tension
pneumothorax , but is often due to a catastrophic event,
such as cardiac rupture or massive pulmonary
embolism, therefore carries an extremely poor prognosis.
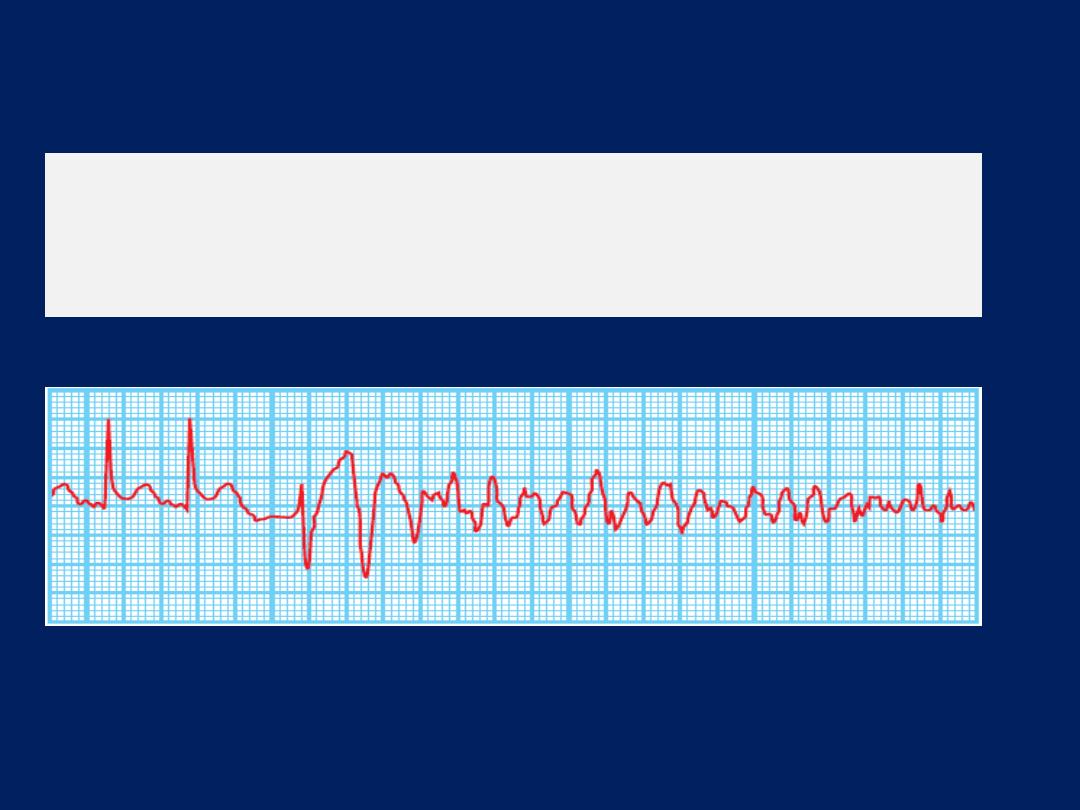
Ventricular fibrillation.
A bizarre chaotic rhythm, initiated in this case by two
ventricular ectopic beats in rapid succession.

Coronary artery disease (85%)
• Myocardial ischaemia
• Acute MI
• Prior MI with myocardial scarring
Structural heart disease (10%)
• Aortic stenosis
• Hypertrophic cardiomyopathy
• Dilated cardiomyopathy
• Arrhythmogenic right ventricular dysplasia
• Congenital heart disease
No structural heart disease (5%)
• Long QT syndrome
• Brugada syndrome
• Wolff–Parkinson–White syndrome
• Adverse drug reactions (torsades de pointes)
• Severe electrolyte abnormalities
Causes of sudden arrhythmic death

Management of cardiac arrest
The Chain of Survival
Refers to the sequence of events that is necessary to
maximise the chances of surviving, basic life support is
administered by a trained individual, the emergency
medical services respond promptly, and defibrillation is
achieved within a few minutes. In recent years, public
access defibrillation has been introduced in places of high
population density, particularly where traffic congestion
railway stations, airports and sports stadia. Designated
individuals can respond to a cardiac arrest using basic
life support and an automated external defibrillator.
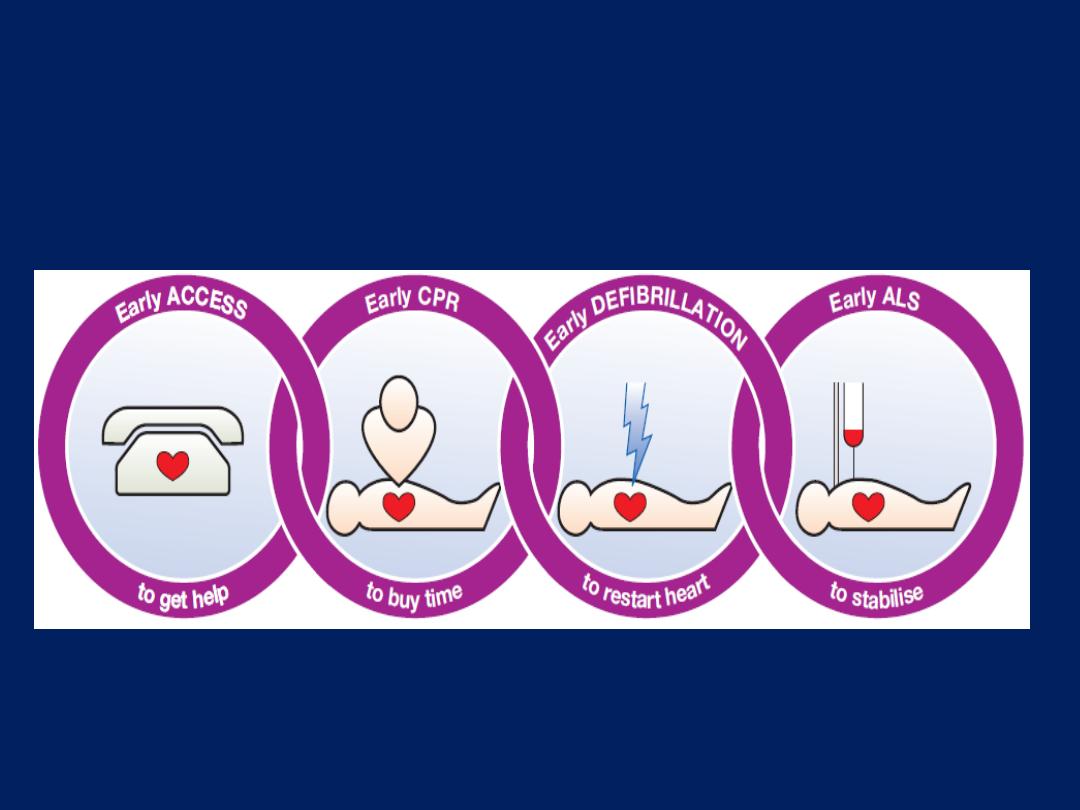
The Chain of Survival in cardiac arrest.
(ALS = advanced life support;
CPR = cardiopulmonary resuscitation

Basic life support (BLS)
BLS encompasses manoeuvres that aim to maintain a low
level of circulation until more definitive treatment with
advanced life support can be given. The ABCDE approach
to management of the collapsed patient should be
followed: prompt assessment and restoration of the Airway,
maintenance of ventilation using rescue Breathing (‘mouth-
to-mouth’ breathing), maintenance of the Circulation using
chest compressions; Disability, in resuscitated patients, refers
to assessment of neurological status, and Exposure entails
removal of clothes to enable defibrillation, auscultation of
the chest, and assessment for a rash caused by anaphylaxis,
injuries or so on .Chest compression-only (‘hands-only’)
CPR is easier for members of the public to learn and
administer, now advocated in public education campaigns.
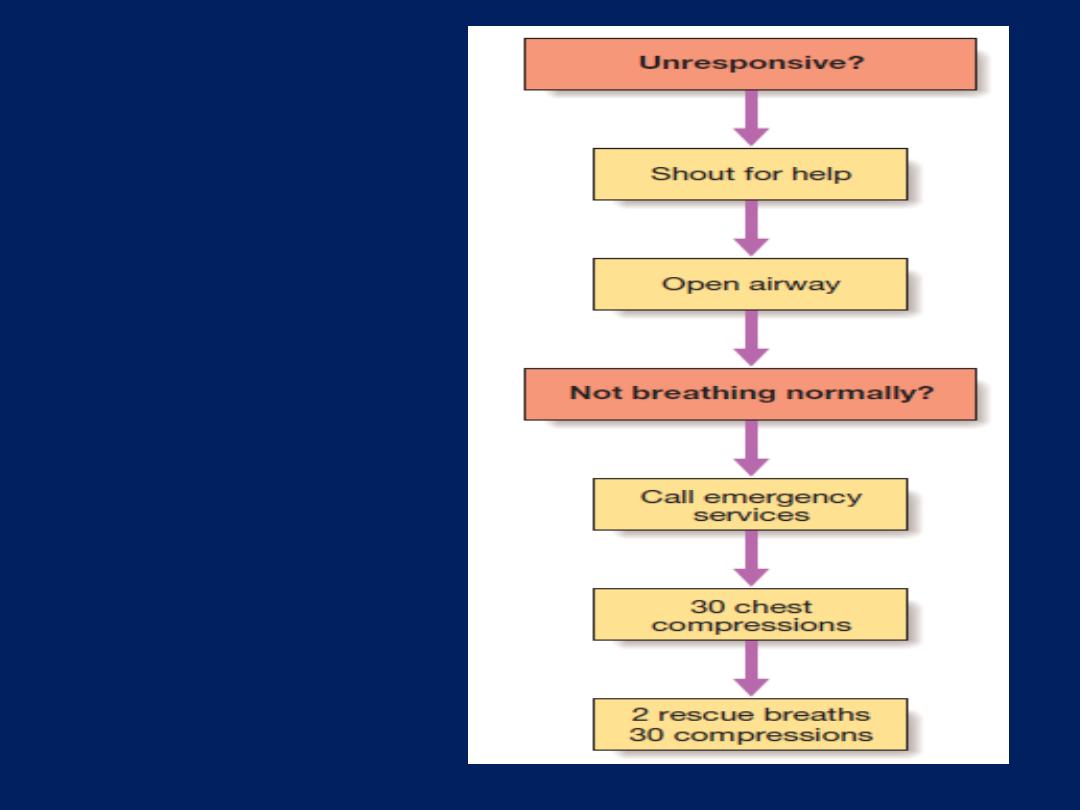
Algorithm for adult
basic life support.
From Resuscitation
Council (UK)
guidelines

Advanced life support (ALS)
ALS aims to restore normal cardiac rhythm by
defibrillation when the cause of arrest is tachyarrhythmia,
or to restore cardiac output by correcting other reversible
causes. ALS can also involve administration of intravenous
drugs to support the circulation, and endotracheal
intubation. If cardiac arrest is witnessed, a precordial
thump may sometimes convert ventricular fibrillation or
tachycardia to normal rhythm. Ventricular fibrillation or
pulseless ventricular tachycardia is treated with immediate
defibrillation.
Defibrillation is more likely to be effective if a
biphasic shock defibrillator is used, where the polarity
of the shock is reversed midway through its delivery.

Defibrillation is usually administered using a 150-Joule
biphasic shock, and CPR resumed immediately for
2 minutes without attempting to confirm restoration
of a pulse, because restoration of mechanical cardiac
output rarely occurs immediately after successful
defibrillation. If, after 2 minutes, a pulse is not restored,
a further biphasic shock of 150–200 joules is given.
Thereafter, additional biphasic shocks of
150–200 joules
are
given every 2 minutes after each cycle of
cardiopulmonary resuscitation (CPR). During resuscitation,
adrenaline
(1 mg IV)
should be given every 3–5 minutes and
consideration given to the use of IVamiodarone, especially
if ventricular fibrillation or tachycardia re-initiates after
successful defibrillation.

If asystole cannot be confidently diagnosed, the patient
should be treated for VF and defibrillated.
If an electrical rhythm is observed that would be
expected to produce a cardiac output, ‘pulseless
electrical activity’ is present. Pulseless electrical
activity is treated by continuing CPR and adrenaline
administration whilst seeking reversible causes.
Asystole
is treated similarly, with the additional support
of atropine and sometimes external or transvenous
pacing in an attempt to generate an electrical rhythm.
There are many potentially reversible causes of
cardiac arrest and the main causes can be easily
remembered as a list of four Hs and four Ts.
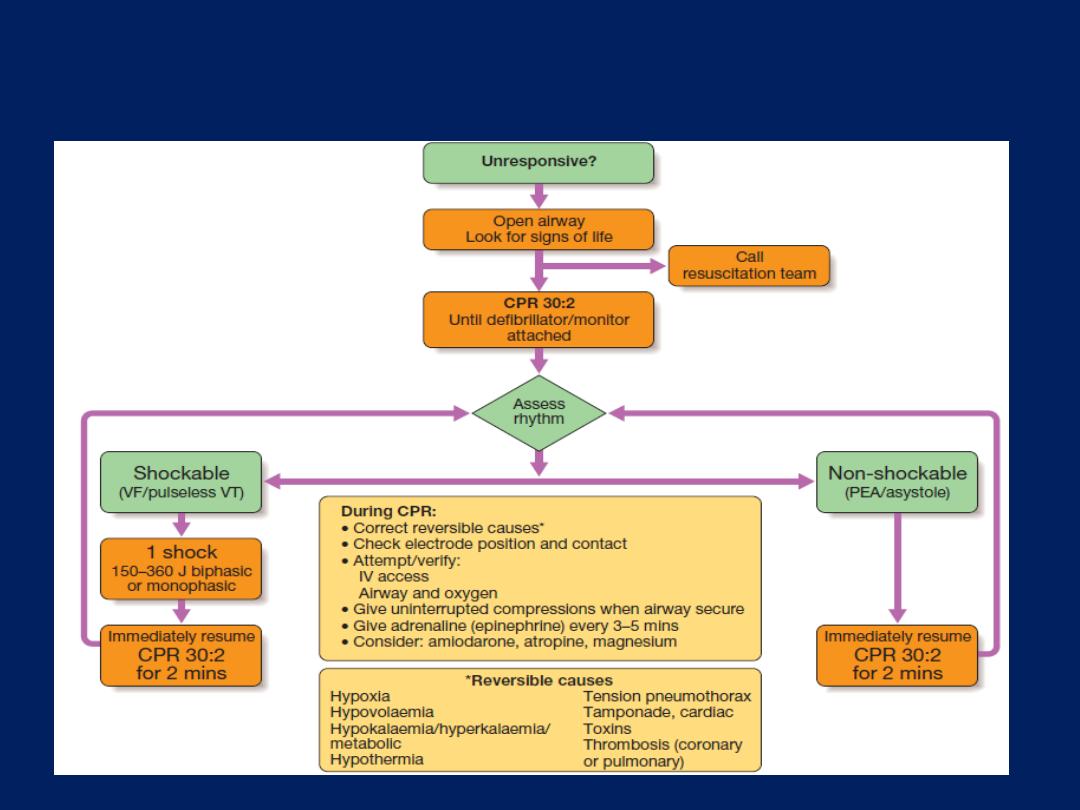
Algorithm for adult advanced life support. (CPR = cardiopulmonary
resuscitation: PEA = pulseless electrical activity; VF = ventricular
fibrillation; VT = pulseless ventricular tachycardia)

Survivors of cardiac arrest
Patients
who survive
a cardiac arrest caused by acute
MI need no specific treatment beyond that given to
those recovering from an uncomplicated infarct, since their
prognosis is similar .
Those with reversible
causes, such
as exercise-induced ischaemia or aortic stenosis, should
have the underlying cause treated if possible.
Survivors of
ventricular tachycardia or ventricular
fibrillation arrest in whom no reversible cause can be
identified may be at risk of another episode, and should
be considered for an implantable cardiac defibrillator
and anti-arrhythmic drug therapy.
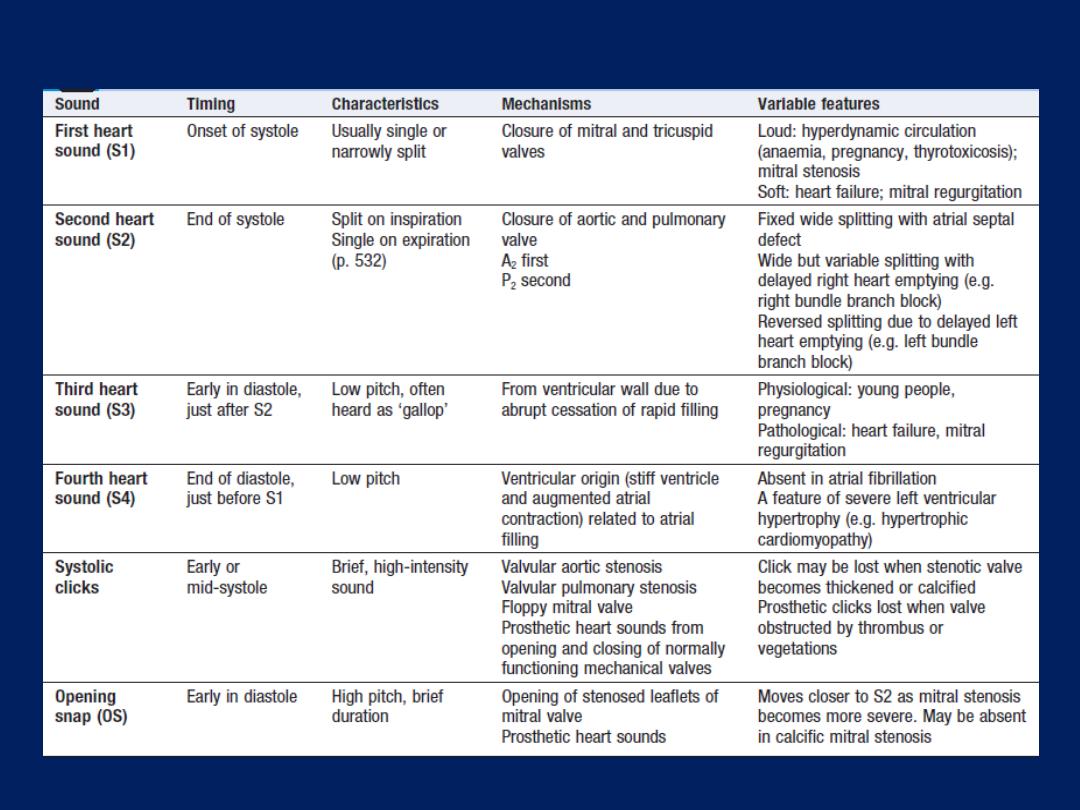
Normal and abnormal heart sounds

Opening snap (OS)
Early in diastole, high pitch, brief duration .
1.Opening of stenosed leaflets of mitral valve .
2. Prosthetic heart sounds.
Systolic clicks
Early or mid-systole. Brief, high-intensity.
Valvular aortic stenosis.Valvular pulmonary stenosis
Floppy mitral valve. Prosthetic heart sounds from opening and closing
of normally functioning mechanical valves.
High pitch murmur indicates high-velocity flow.
The position of a murmur in relation to the cardiac cycle is crucial
and should be assessed by timing it with the heart sounds, carotid
pulse and apex beat
Mitral, tricuspid R and VSD are the only causes of a pansystolic
murmur.
Late systolic murmurs may occur in mitral valve prolapse, if the mitral
regurgitation is confined to late systole, and HOCM, if dynamic
obstruction occurs late in systole.
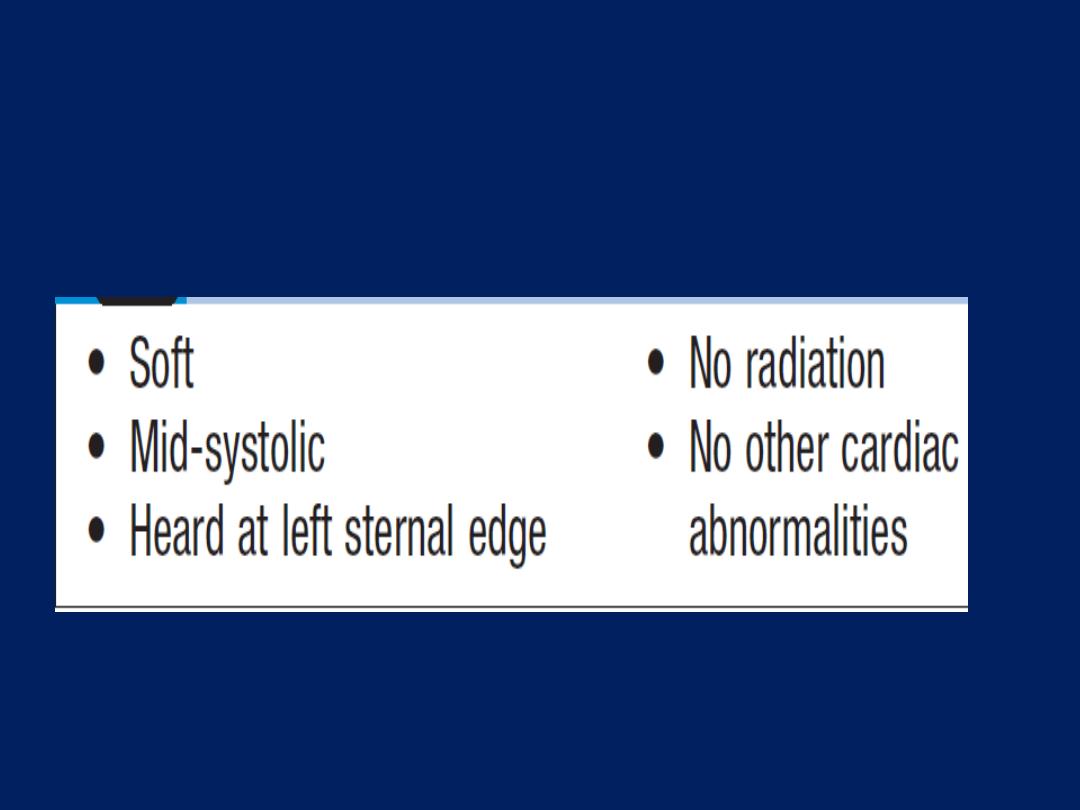
Features of a benign or innocent
heart murmur

Auscultatory evaluation of a heart murmur
Timing, intensity, location, radiation and quality are all
useful clues to the origin and nature of a heart murmur .
Radiation of a murmur is determined by the direction of
turbulent blood flow and is only detectable when there is
a high-velocity jet, such as in mitral regurgitation (radiation
from apex to axilla) or aortic stenosis (radiation from base
to neck). Similarly, the pitch and quality of the sound can
help to distinguish the murmur, such as the ‘blowing’ murmur
of mitral regurgitation or the ‘rasping’ murmur of aortic
stenosis.
The position of a murmur
in relation to the cardiac cycle is
crucial and should be assessed by timing it with the heart
sounds, carotid pulse and apex beat.
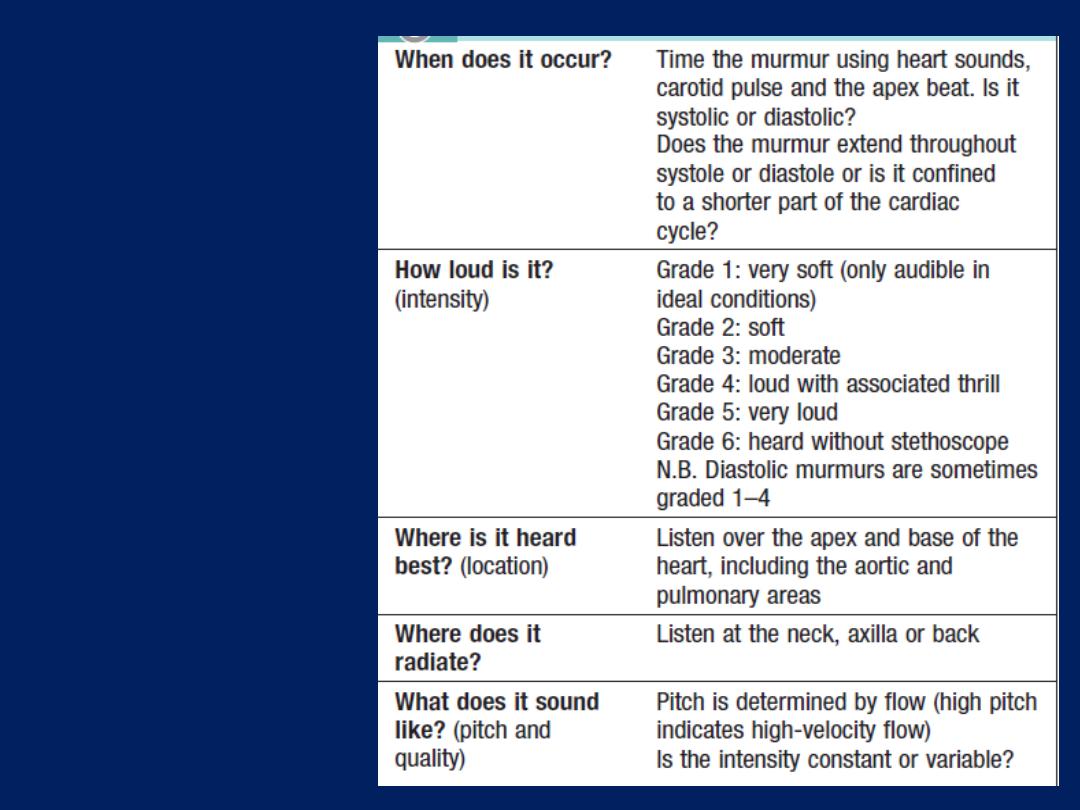
How to assess
a heart
murmur

Systolic murmurs
Ejection systolic murmurs are associated with ventricular
outflow tract obstruction and occur in mid-systole with a
crescendo–decrescendo pattern, reflecting the changing
velocity of blood flow . Pansystolic murmurs maintain a
constant intensity and extend from the first to the second
heart sound, sometimes obscuring it. They occur when blood
leaks from a ventricle into a low-pressure chamber at an
even or constant velocity. Mitral, tricuspid regurgitation
and VSD are the only causes of a pansystolic murmur. Late
systolic murmurs are unusual but may occur in mitral valve
prolapse, if the MR is confined to late systole, and
hypertrophic obstructive cardiomyopathy, if dynamic
obstruction occurs late in systole.
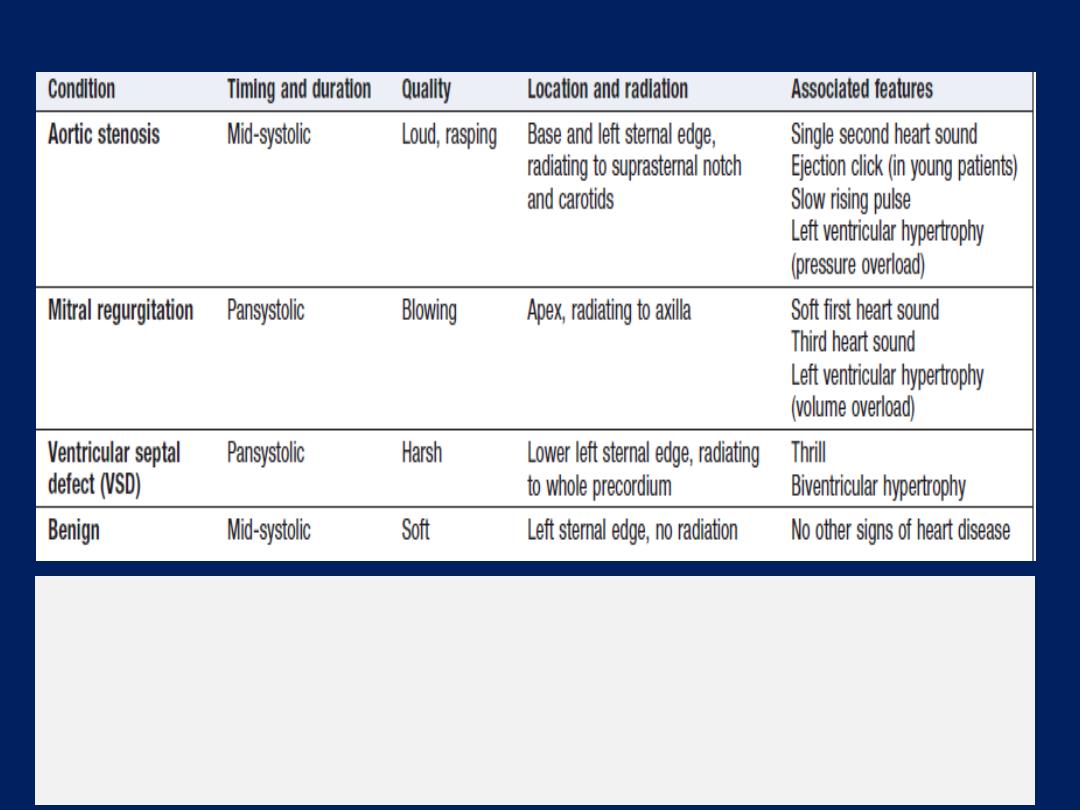
Features of some common systolic murmurs
Continuous murmurs
These result from a combination of systolic and diastolic flow (e.g.
persistent ductus arteriosus), and must be distinguished from
extracardiac noises such as bruits from arterial shunts, venous hums
(high rates of venous flow in children) and pericardial friction rubs.

Diastolic murmurs
These are due to accelerated or turbulent flow across the
mitral or tricuspid valves. They are low-pitched noises
that are often difficult to hear and should be evaluated
with the bell. A mid-diastolic murmur may be due to MS
(located at the apex and axilla), tricuspid stenosis (located
at the left sternal edge), increased flow across the mitral
valve (e.g. the to-and-fro murmur of severe MR ) or
increased flow across the tricuspid valve (e.g.a large ASD).
Early diastolic murmurs have a soft, blowing quality with
adecrescendo pattern and should be evaluated with the
diaphragm of the stethoscope. They are due to
regurgitation across the aortic or pulmonary valves and
are best heard at the left sternal edge, with the patient
sitting forwards in held expiration.

DISORDERS OF HEART RATE, RHYTHM
AND CONDUCTION
The sinus node acts as a
pacemaker
and its intrinsic
rate is regulated by the
autonomic
nervous system; vagal
activity decreases the heart rate, and sympathetic activity
increases it via cardiac sympathetic nerves and circulating
catecholamines. If the sinus rate becomes unduly slow,
another, more distal part of the conducting system may
assume the role of pacemaker. This is known as an
escape rhythm
and
may arise in
the
AV
node or
His
bundle (junctional rhythm)
or
the
ventricles
(idioventricular rhythm). A cardiac
arrhythmia is a disturbance of the electrical rhythm of the
heart. A heart rate of > 100/min is called a tachycardia,
and a heart rate of l< 60/min is called a bradycardia.

There are three main mechanisms of tachycardia:
• Increased automaticity.
repeated spontaneous
depolarisation of an ectopic focus.
• Re-entry.
initiated by an ectopic and sustained by a re-
entry circuit . Most tachyarrhythmias are due to re-entry.
• Triggered activity.
This can cause ventricular arrhythmias
in patients with CAD , a form of secondary depolarisation
arising from an incompletely repolarised cell membrane
Bradycardia may be due to:
• Reduced automaticity, e.g. sinus bradycardia.
• Blocked or abnormally slow conduction, e.g. AV block.
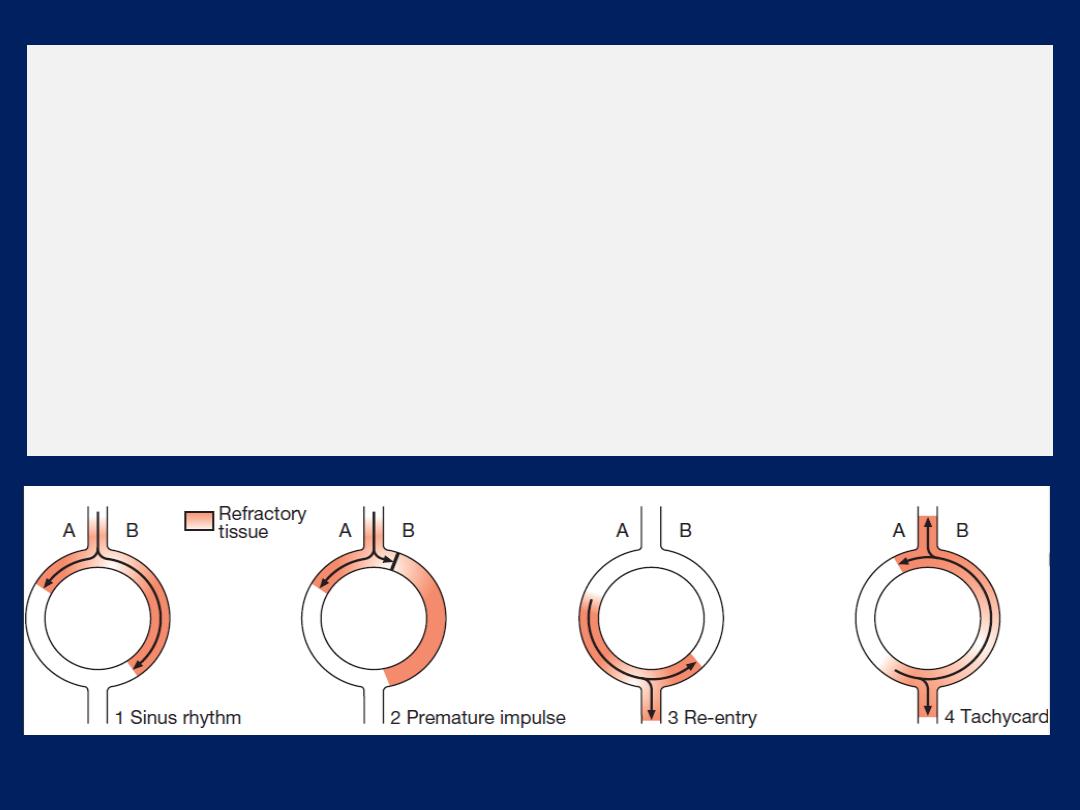
The mechanism of re-entry.
Re-entry can occur when there are two alternative
pathways with different conducting properties (e.g. the AV node and an accessory
pathway, or an area of normal and an area of ischaemic tissue). Here, pathway A
conducts slowly and recovers quickly, while pathway B conducts rapidly and
recovers slowly. (1) In sinus rhythm, each impulse passes down both pathways before
entering a common distal pathway. (2) As the pathways recover at different rates, a
premature impulse may find pathway A open and B closed. (3) Pathway B may
recover while the premature impulse is travelling selectively down pathway A. The
impulse can then travel retrogradely up pathway B, setting up a closed loop or re-
entry circuit. (4) This may initiate a tachycardia that continues until the circuit is
interrupted by a change in conduction rates or electrical depolarisation.

An arrhythmia may be
‘
supraventricular
’ (sinus, atrial or
junctional) or
ventricular
in origin. Supraventricular rhythms
usually produce narrow QRS complexes because the
ventricles are depolarised in their normal sequence.
Occasionally, however, a supraventricular rhythm can
produce broad or wide QRS complexes due to coexisting
bundle branch block or the presence of an additional
atrioventricular connection (accessory pathway).
Bradycardias cause symptoms that
reflect low cardiac
output: fatigue, lightheadedness and syncope.
Tachycardias
cause rapid palpitation, dizziness, chest
discomfort or breathlessness. Extreme tachycardias can
cause syncope.
Extreme
bradycardias or tachycardias can precipitate
sudden death or cardiac
arrest
.

Sinus arrhythmia
The sinus rate increases during inspiration and slows during
expiration is a consequence of normal parasympathetic
activity and can be pronounced in children. Absence of this
normal variation with breathing or with changes in posture
may be a feature of autonomic neuropathy .
Sinus bradycardia
A sinus rate of <60/min may occur in healthy people at rest
and is a common finding in athletes. Asymptomatic sinus
bradycardia requires no treatment.
Symptomatic
acute
sinus bradycardia usually responds to IV atropine 0.6–1.2
mg. Recurrent or persistent symptomatic sinus bradycardia
should be considered for pacemaker implantation.

Sinus tachycardia
A sinus rate of >100/min, due to an increase in
sympathetic activity associated with exercise, emotion,
pregnancy or pathology. Young adults can produce a
rapid sinus rate, up to 200/min, during intense exercise.
Sinoatrial disease (sick sinus syndrome)
The underlying pathology involves fibrosis, degenerative
changes or ischaemia of the SA node. The condition is
characterised by a variety of arrhythmias and may
present with palpitation, dizzy spells or syncope, due to
intermittent tachycardia, bradycardia, or pauses with no
atrial or ventricular activity (SA block or sinus arrest) .
A permanent pacemaker may benefit patients with
troublesome symptoms due to spontaneous bradycardias
Causes of sinus bradycardia and tachycardia
Common features of sinoatrial disease
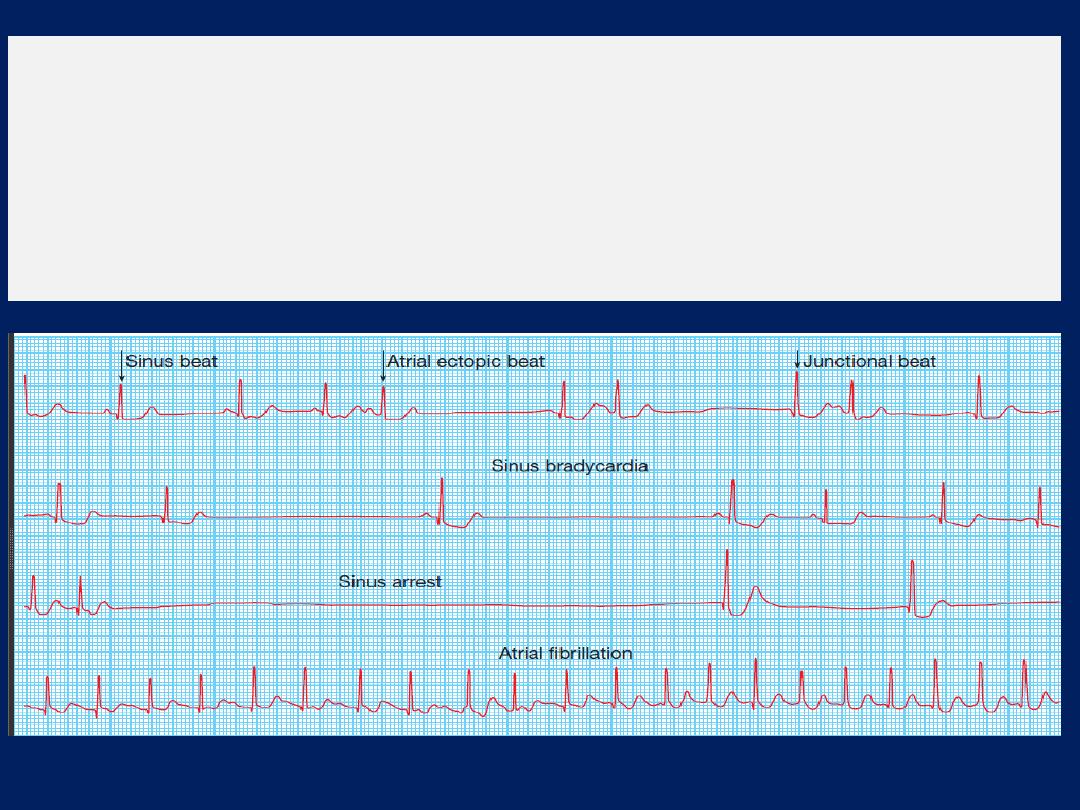
Sinoatrial disease (sick sinus syndrome).
A continuous
rhythm strip from a 24-hour ECG tape recording illustrating
periods of sinus rhythm, atrial ectopics, junctional beats,
sinus bradycardia, sinus arrest and paroxysmal atrial
fibrillation.

Atrial tachyarrhythmias
Atrial ectopic beats (extrasystoles, premature beats)
These usually cause no symptoms but can give the
sensation of a missed beat or an abnormally strong
beat. The ECG shows a premature but otherwise
normal QRS complex; if visible, the preceding P wave
has a different morphology because the atria activate
from an abnormal site.
In most cases, these are of no consequence, although
very frequent atrial ectopic beats may herald the onset
of atrial fibrillation. Treatment is rarely necessary but β-
blockers can be used if symptoms are intrusive.
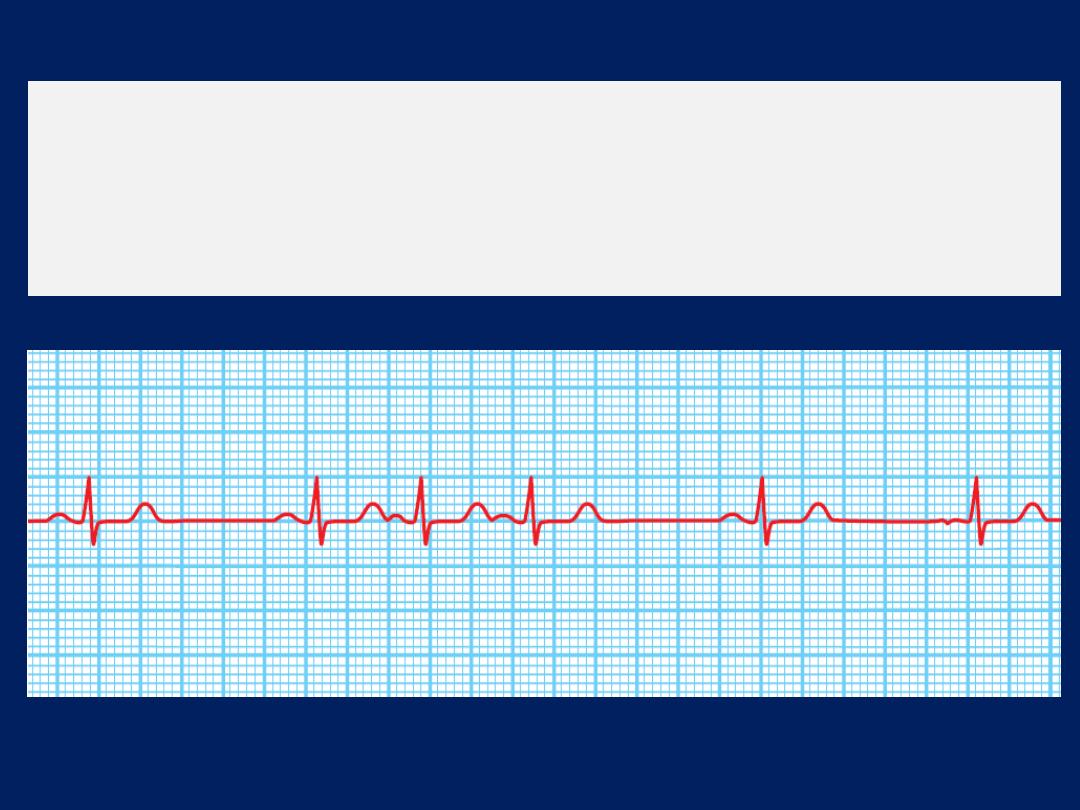
Atrial ectopic beats.
The first, second and fifth complexes
are normal sinus beats. The third, fourth and sixth complexes
are atrial ectopic beats with identical QRS complexes and
abnormal
(sometimes barely visible)
P waves.

Atrial tachycardia
May be a manifestation of increased atrial automaticity,
sinoatrial disease or digoxin toxicity.
It produces a narrow-complex tachycardia with abnormal
P-wave morphology, sometimes associated with AV
block if the atrial rate is rapid. It may respond to
β-blockers, which reduce automaticity
, or class I or III
anti-arrhythmic drugs. The ventricular response in rapid
atrial tachycardias may be controlled by AV node-
blocking drugs.
Catheter ablation can be used to target the ectopic site
and should be offered as an alternative to anti-arrhythmic
drugs in patients with recurrent atrial tachycardia.

Atrial flutter
characterised by a large (macro) re-entry circuit, usually
within the right atrium encircling the tricuspid annulus. The
atrial rate is approximately 300/min, usually associated
with 2 : 1, 3 : 1 or 4 : 1 AV block (with corresponding rates
of 150, 100 or 75/min). Rarely, in young, every beat is
conducted, producing a rate of 300/min and, potentially,
haemodynamic compromise. The ECG shows saw-toothed
flutter waves .When there is regular 2 : 1 AV block, it may
be difficult to identify flutter waves that are buried in QRS
complexes and T waves. Atrial flutter
should
always be
suspected when there is anarrow-complex tachycardia of
150/min
. Carotid sinus pressure or IV adenosine may
help to establish the diagnosis by temporarily increasing the
degree of AV block and revealing flutter waves .
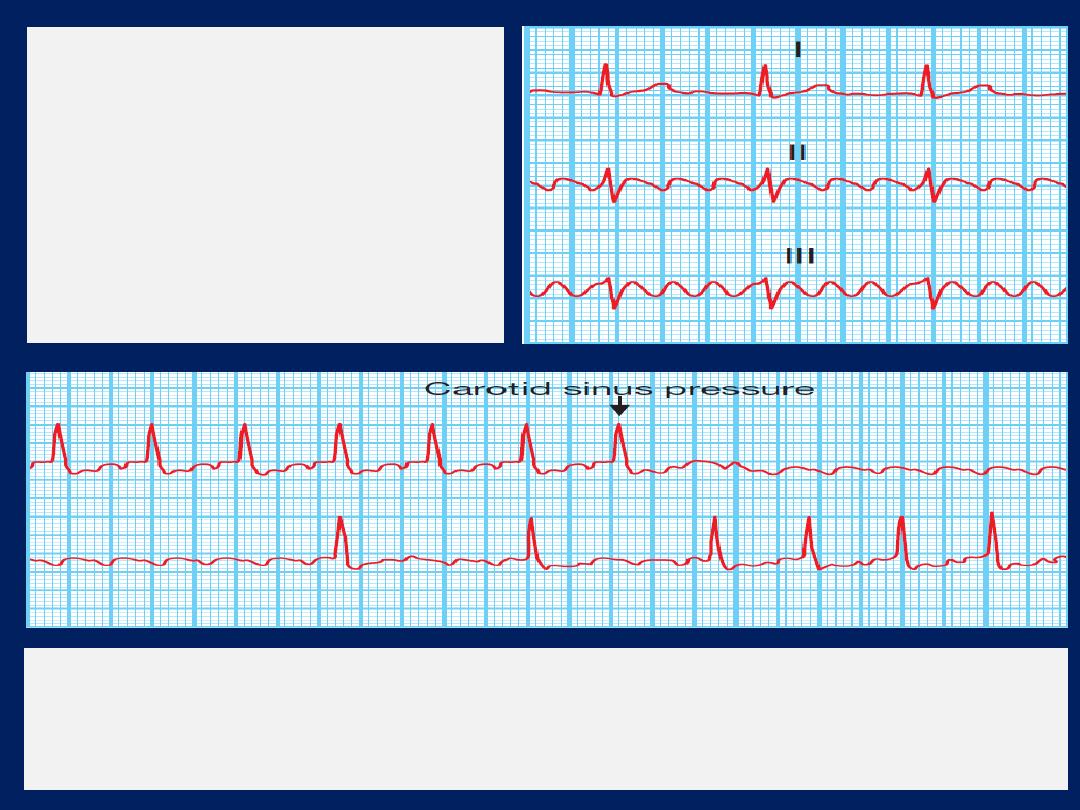
Atrial flutter
Simultaneous recording
showing atrial flutter
with 3 : 1 AV block; flutter
waves are only visible in
leads II and III.
Carotid sinus pressure
in atrial flutter: continuous trace. The diagnosis
of atrial flutter with 2 : 1 block was established when carotid sinus
pressure produced temporary AV block, revealing the flutter waves.

Management
Digoxin, ββ or verapamil can control the ventricular rate .
However,
in many cases, it may be
preferable
to try to
restore sinus rhythm by direct current (DC) cardioversion
or
by using IV amiodarone. ββ or amiodarone can also be
used to prevent recurrent episodes of flutter.
Although
flecainide can also be used for acute treatment
or prophylaxis, it should be avoided because there is a risk
of slowing the flutter circuit and facilitating 1 : 1 AV nodal
conduction. This can cause a paradoxical tachycardia and
haemodynamic compromise. If used, it should always be
prescribed along with an AV node-blocking drug, such as a
β-blocker.
Catheter ablation
offers a 90% chance of
complete cure and is the treatment of choice for patients
with persistent symptoms.

Atrial fibrillation
Is the most common sustained cardiac arrhythmia. The
prevalence rises with age, affecting 1% of those aged 60–
64 years, increasing to 9% of those aged over 80 years.
AF is a complex arrhythmia characterised by abnormal
automatic firing and the presence of multiple interacting
re-entry circuits looping around the atria. Episodes of
fibrillation are initiated by rapid bursts of ectopic beats
arising from conducting tissue in the pulmonary veins or
from diseased atrial tissue. AF becomes sustained because
of re-entrant conduction within the atria or sometimes
because of continuous ectopic firing. Re-entry is more
likely to occur in atria that are enlarged, or conduction is
slow (as is the case in many forms of heart disease).
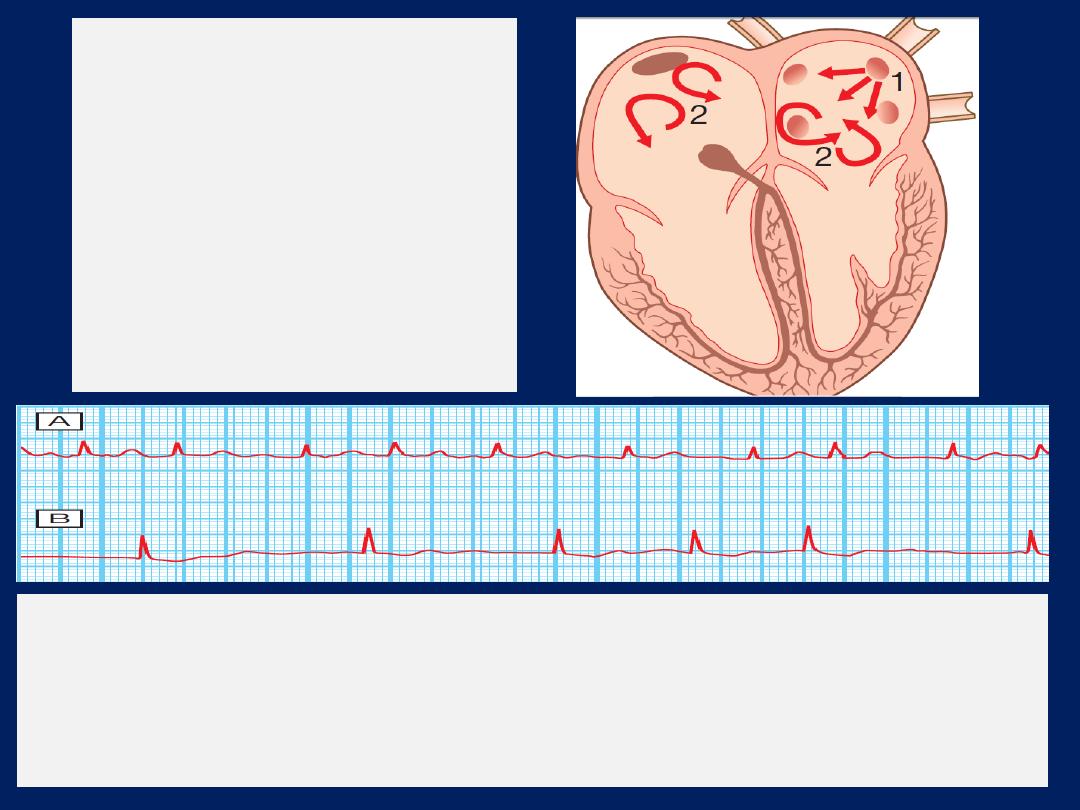
Mechanisms initiating atrial
fibrillation.
(1)
Ectopic beats, often arising
from the pulmonary veins, trigger
atrial fibrillation.
(2)
Re-entry within the atria
maintains atrial fibrillation, with
multiple
interacting re-entry circuits
operating simultaneously.
Two examples of atrial fibrillation.
The QRS complexes are irregular and there
are no P waves.
A
There is usually a fast ventricular rate, e.g. between 120 and
160/min, at the onset of atrial fibrillation.
B
In chronic atrial fibrillation, the
ventricular rate may be much slower, due to the effects of medication and AV
nodal fatigue.

During episodes of AF, the atria beat rapidly but in an
uncoordinated and ineffective manner. The ventricles
are activated irregularly at a rate determined by
conduction through the AV node. This produces the
characteristic ‘irregularly irregular’ pulse.
AF can be classified as
paroxysmal
(intermittent
episodes which self-terminate within 7 days),
persistent
(prolonged episodes that can be terminated by electrical
or chemical cardioversion) or
permanent.
Unfortunately
for many patients, paroxysmal AF will become permanent
as the underlying disease process progresses.
Electrophysiological changes occur in the atria within a
few hours of the onset of AF that tend to maintain
fibrillation:
electrical remodelling
.

When AF persists for a period of months, structural
remodelling occurs, with atrial fibrosis and dilatation that
further predispose to AF. Thus early treatment will prevent
re-initiation of the arrhythmia. AF may be the first
manifestation of many forms of heart disease ,
particularly those that are associated with enlargement or
dilatation of the atria.
Alcohol excess, hyperthyroidism and chronic lung disease are
also common causes of AF, although multiple
aetiological factors often coexist, such as the combination
of alcohol, hypertension and CAD. About 50% of all
patients with paroxysmal AF and 20% of patients with
persistent or permanent AF have structurally normal
hearts; this is known as
‘lone atrial fibrillation’.

AF can cause palpitation, breathlessness and fatigue. In
patients with poor ventricular function or valve disease, it
may precipitate or aggravate cardiac failure.
A fall in BP may cause lightheadedness, and chest pain
may occur with underlying CAD . In older patients, AF
may not be associated with a rapid ventricular rate and
is thus often asymptomatic, in which case it is usually
discovered as a result of a routine examination or ECG.
AF is associated with significant morbidity and a
twofold increase in mortality (mainly because of its
association with other underlying heart disease). By far
the most disabling consequence is its association with
stroke and systemic embolism.
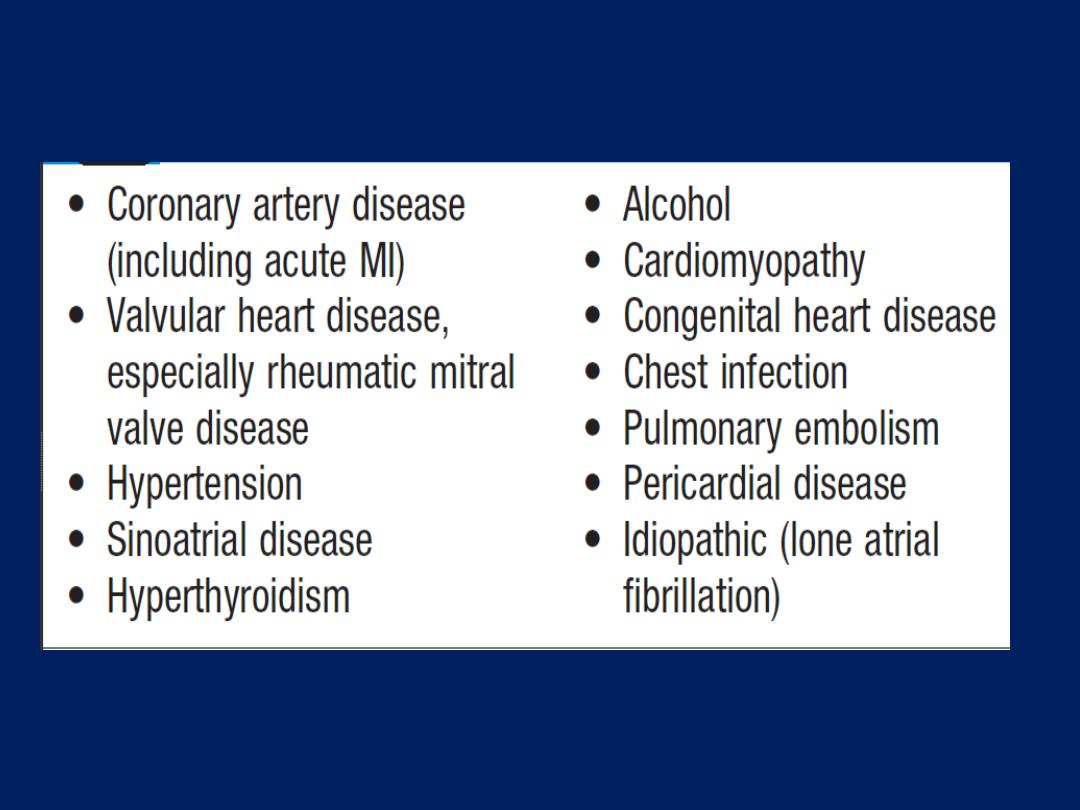
Common causes of atrial fibrillation

Management
Assessment includes a full history, physical examination,
ECG, echo and thyroid function tests. Additional
investigations may be needed to determine the nature and
extent of any underlying heart disease. When AF
complicates an acute illness (e.g. chest infection, pulmonary
embolism), effective treatment of the primary disorder will
often restore sinus rhythm.
Otherwise, the main objectives are restoration of sinus
rhythm (when possible), prevention of recurrent AF,
optimisation of the heart rate during periods of AF,
reduction of the risk of thromboembolism, and treatment
of underlying cardiac disease.

Paroxysmal atrial fibrillation
Occasional attacks that are well tolerated do not
necessarily require treatment.
Beta-blockers
are normally
used as first-line therapy if symptoms are troublesome,
and are
particularly useful for
treating patients with AF
associated with CAD , hypertension and cardiac failure.
Beta-blockers reduce the ectopic firing that normally
initiates AF. Class Ic drugs, such as
propafenone or
flecainide,
are also effective at preventing episodes
but
should not be given to patients with CAD or left ventricular
dysfunction.
Flecainide is usually prescribed along
with a rate limiting
β-blocker because it occasionally precipitates atrial flutter.

Class III drugs can also be used;
amiodarone is the
most effective agent for preventing
AF
but its side-
effects
restrict its use when other measures fail.
Dronedarone is an effective alternative, but is
contraindicated in heart failure or significant left
ventricular impairment.
Digoxin and verapamil are not effective drugs for
preventing paroxysms of AF, although they do limit the
heart rate when AF occurs by blocking the AV node.
In patients with AF in whom anti-arrhythmic drug
therapy is ineffective or causes side-effects, catheter
ablation can be considered. Ablation is used to
disconnect the pulmonary veins from the LA
electrically, preventing ectopic triggering of AF.

In addition, lines of conduction block can be created within
the atria to prevent re-entry.
Ablation prevents AF in approximately 75% of patients
with prior drug-resistant episodes, although a repeat
procedure is sometimes required before this is achieved.
Ablation
is an attractive treatment for patients in whom
drugs are ineffective or poorly tolerated but associated
with a risk of cardiac tamponade, stroke.
Persistent and permanent atrial fibrillation
There are two options
• rhythm control:
to restore and maintain sinus rhythm
• rate control:
accepting that AF will be permanent
and using treatments to control the ventricular rate
and to prevent embolic complications.

Rhythm control.
particularly appropriate for troublesome
symptoms and if there is a modifiable or treatable cause.
Electrical
cardioversion is initially successful in 75% but
relapse is frequent
(25–50% at 1 month and 70–90% at 1 year)
.
Attempts to restore and maintain sinus rhythm are most
successful if AF has been present for < 3 months, the patient
is young and no important structural heart disease.
Immediate cardioversion, after IV heparin, is appropriate
if AF has been present for < 48 hours.
In stable
patients with no history of structural disease, IV
flecainide
(2 mg/kg over 30 mins, maximum dose 150 mg)
can be used
for pharmacological cardioversion and will restore sinus
rhythm in 75% of patients within 8 hours.
In patients
with structural or ischaemic heart disease,
IV amiodarone can be given.

In other situations,
DC cardioversion should be deferred
until the patient has been established on warfarin, with an
INR of >2.0 for a minimum of 4 weeks, and any problems,
such as hypertension or alcohol excess, eliminated.
Anticoagulation should be maintained for at least 3 months
following successful cardioversion. If AF recurs, further
cardioversion may be appropriate but consideration should
be given to pretreatment with amiodarone to reduce the
risk of recurrence. Catheter ablation is sometimes used to
help restore and maintain sinus rhythm in resistant cases, but
is a less effective treatment than for paroxysmal AF.

Rate control.
If sinus rhythm cannot be restored,Digoxin,
ββ and rate-limiting CA (verapamil or diltiazem) reduce
ventricular rate by increasing the degree of AV block.
This alone may produce a striking improvement,
particularly in patients with mitral stenosis.
β-blockers and rate-limiting calcium antagonists are more
effective than digoxin at controlling the heart rate during
exercise and have additional benefits in patients with
hypertension or structural heart disease. Combination
therapy (e.g. digoxin and atenolol) is often advisable.
In patients with permanent atrial fibrillation and poor rate control,
in whom drugs are ineffective or are not tolerated, rate control by:
(i)
Implantation of a permanent pacemaker, followed by
(ii) ablation of the AV node to induce complete AV block and
bradycardia, thus allowing the pacemaker to assume
control
of
the
heart rate.
‘pace and ablate’ strategy.

Prevention of thromboembolism
Stasis of blood in the LA and may lead to thrombus
formation in the left atrial appendage. This predisposes
to stroke and other forms of systemic embolism. Several
large randomised trials have shown that treatment with
adjusted-dose warfarin
(target INR 2.0–3.0)
reduces the risk
of stroke by about two-thirds, at the cost of an annual
risk of bleeding of 1–1.5%, whereas treatment with
aspirin reduces the risk of stroke by only one-fifth, is
associated with significant bleeding risk. Warfarin is thus
indicated for patients with AF who have specific risk
factors for stroke.
Stroke prevention guidelines
do not distinguish between
those with paroxysmal, persistent and permanent AF.

An assessment of the risk
using the (
CHA
2
DS
2
-VASc
)
scoring system of embolism helps to
define
the possible
benefits of antithrombotic which must be balanced against
its potential hazards. Left atrial size is of limited value in
predicting stroke risk.
The direct thrombin inhibitor, dabigatran
, is the first novel
oral anticoagulant shown to be as effective and safe as
warfarin. No monitoring is required and there are few
drug interactions but there are
no
current antidotes. For
all anticoagulant, comorbid conditions, such as peptic ulcer,
uncontrolled hypertension, alcohol misuse, frequent falls,
poor drug compliance and drug interactions, are all relative
contraindications.
Young patients < 65 years with no
evidence
of structural heart disease have a very low risk
and may not require anticoagulation.
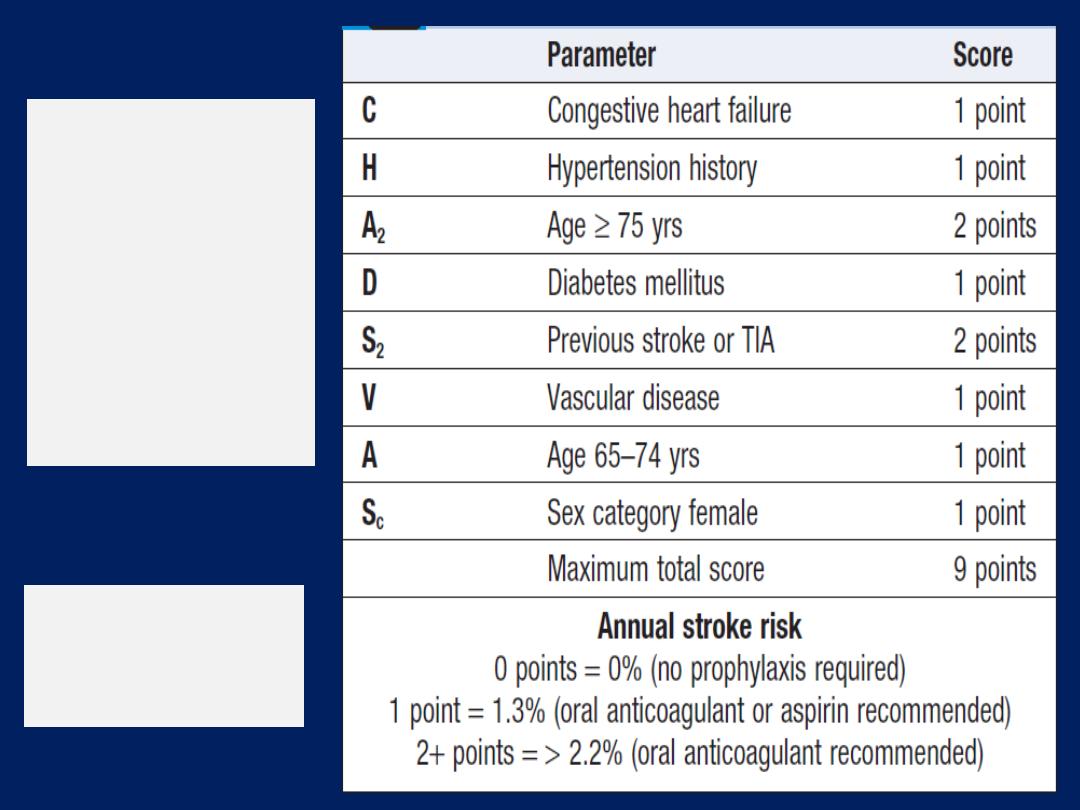
CHA
2
DS
2
-VAS
c
stroke risk
scoring system
for
non-
valvular
atrial
fibrillation
Vascular disease =
Prior MI, peripheral
arterial disease, or
aortic plaque
Anticoagulation in atrial fibrillation
Atrial fibrillation in old age

Atrial fibrillation in old age 'cont'd
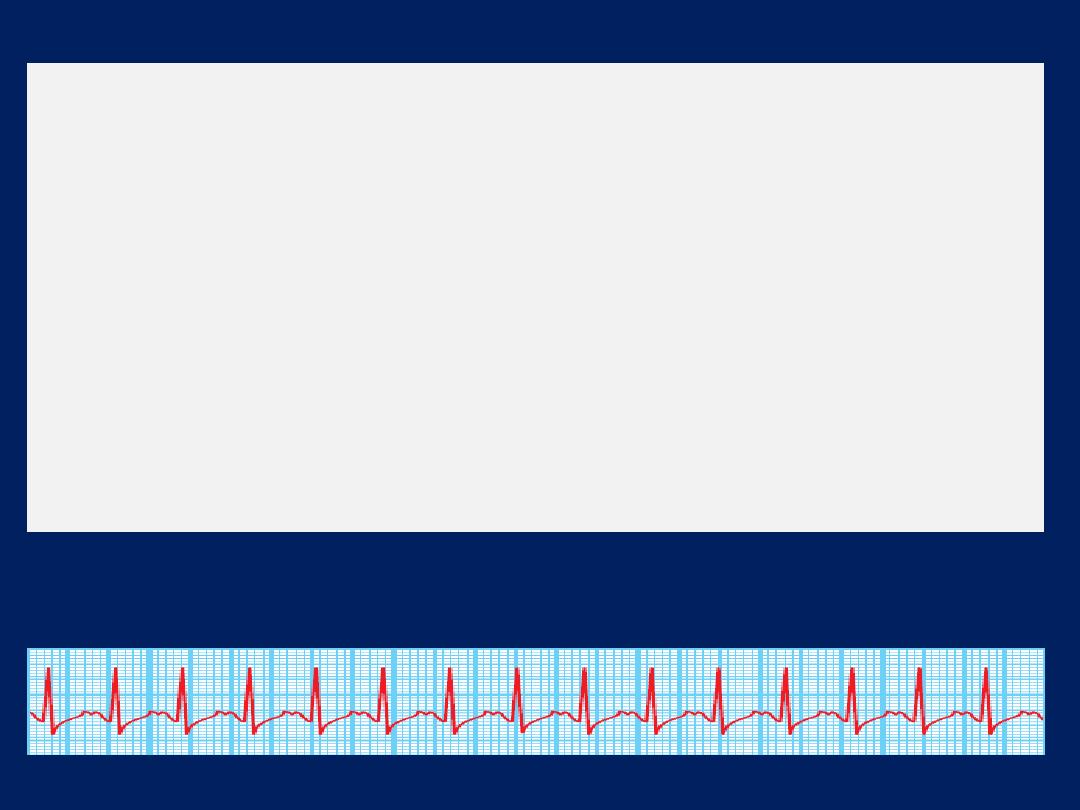
‘Supraventricular’ tachycardias (SVT)
The term SVT is commonly used to describe regular
tachycardias that have a similar appearance on ECG.
These are usually associated with a narrow QRS complex
and are characterised by a re-entry circuit or automatic
focus involving the atria.
The term SVT is misleading, as, in many cases, the
ventricles also form part of the re-entry circuit, such as in
patients with Atrioventricular re-entrant tachycardia.
SVT. the rate is 180/min ,the QRS complexes are normal.

Atrioventricular nodal re-entrant tachycardia (AVNRT)
AVNRT is due to re-entry in a circuit involving the AV node
and its two right atrial input pathways: a superior ‘fast’
pathway and an inferior ‘slow’ pathway. This produces a
regular tachycardia with a rate of 120–240/min. It tends
to occur in the absence of structural heart disease and
episodes may last from a few seconds to many hours. The
patient is usually aware of a rapid, very forceful, regular
heart beat and may experience chest discomfort,
lightheadedness or breathlessness. Polyuria, mainly due to
the release of atrial natriuretic peptide, is sometimes a
feature. The ECG usually shows a tachycardia with normal
QRS complexes but occasionally there may be rate-
dependent bundle branch block.
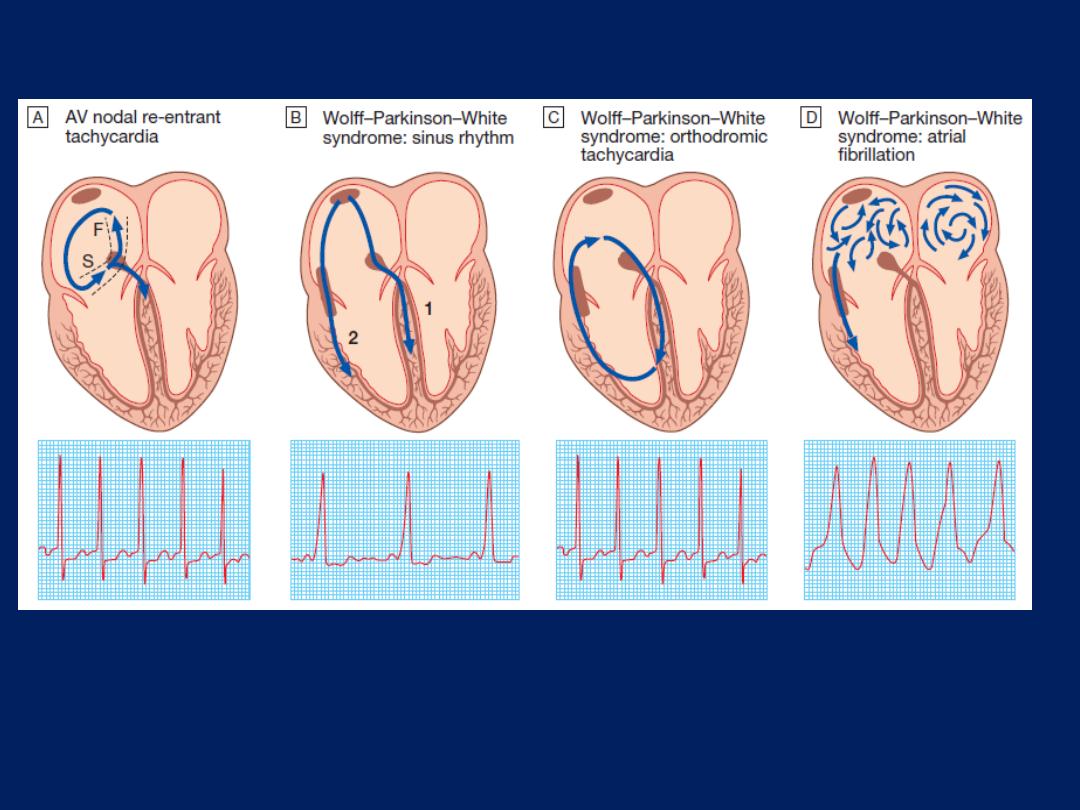

A The mechanism of AVNRT occurs via two right atrial AV nodal input pathways: the slow
(S) and fast (F) pathways. Antegrade conduction occurs via the slow pathway; the
wavefront enters the AV node and passes into the ventricles, at the same time re-entering
the atria via the fast pathway. In WPW syndrome, there is a strip of accessory conducting
tissue that allows electricity to bypass the AV node and spread from the atria to the
ventricles rapidly and without delay. When the ventricles are depolarised through the AV
node, the ECG is normal, but when the ventricles are depolarized through the accessory
conducting tissue, the ECG shows a very short PR interval and a broad QRS complex.
B In sinus rhythm, the ventricles are depolarised through (1) the AV node and (2) the
accessory pathway, producing an ECG with a short PR interval and broadened QRS
complexes; the characteristic slurring of the upstroke of the QRS complex is known as a
delta wave. The degree of pre-excitation (the proportion of activation passing down the
accessory pathway) and ECG appearances may vary a lot, and at times the ECG can
look normal.
C Orthodromic tachycardia. This is the most common form of tachycardia in WPW. The
re-entry circuit passes antegradely through the AV node and retrogradely through the
accessory pathway. The ventricles are therefore depolarised in the normal way,
producing a narrow-complex tachycardia that is indistinguishable from other forms of SVT.
D Atrial fibrillation. the ventricles are largely depolarised through the accessory
pathway, producing an irregular broad-complex tachycardia

Management
Treatment is
not always
necessary. However, an episode
may be terminated by
carotid sinus
pressure or by the
Valsalva
manoeuvre.
Adenosine
(3–12 mg rapidly IV in
incremental doses until tachycardia stops)
or
verapamil
(5 mg IV over 1 min)
will restore sinus rhythm in most cases. IV
ββ
or
flecainide
can also be used.
In rare cases,
when there is
haemodynamic
compromise, should be terminated by
DC
cardioversion . In patients with
recurrent SVT,
catheter
ablation
is the most effective and will permanently prevent
SVT in more than 90% of cases. Alternatively, prophylaxis
with oral ββ, verapamil or flecainide may be used but
commits predominantly young patients to long-term drug
therapy and can create difficulty in female, as these
normally avoided during pregnancy.

Wolff–Parkinson–White syndrome and atrioventricular
re-entrant tachycardia
Here, an abnormal band of conducting tissue connects
the atria and ventricles. This ‘accessory pathway’
comprises rapidly conducting fibres which resemble
Purkinje tissue, in that they conduct very rapidly and are
rich in sodium channels. In around
half
of cases, this
pathway only conducts in the
retrograde
direction (from
ventricles to atria) and thus does
not alter
the appearance
of the ECG in sinus rhythm. This is known as
a concealed
accessory
pathway. In
the rest
, the pathway also conducts
antegradely
(from atria to ventricles) so AV conduction in
sinus rhythm is mediated via
both
the AV node and the
accessory pathway,
distorting
the QRS complex.

Premature ventricular activation via the pathway shortens
the PR interval and produces a ‘slurred’ initial deflection of
the QRS complex, called a delta wave .This is known as
a manifest accessory
pathway. As the AV node and
accessory pathway have different conduction speeds and
refractory periods, a re-entry circuit can develop, causing
tachycardia , when associated with symptoms, the
condition is known as Wolff–Parkinson–White syndrome.
The ECG during this
tachycardia
is almost indistinguishable
from that of AVNRT .
Carotid sinus pressure or
intravenous
adenosine can terminate
the tachycardia.
If atrial fibrillation occurs, it may produce a dangerously
rapid ventricular rate because the accessory pathway
lacks the rate-limiting properties of the AV node .

This is known as
pre-excited atrial fibrillation
and may
cause collapse, syncope and even death. It should be
treated as an
emergency
, usually
with DC
cardioversion.
Catheter ablation
is first-line treatment
in symptomatic
patients and is
nearly always curative.
Alternatively, prophylactic anti-arrhythmic drugs, such as
flecainide or propafenone , can be used to slow
conduction in, and prolong the refractory period of, the
accessory pathway. Long-term drug therapy is not the
preferred treatment for most patients and amiodarone
should not be used, as its side-effect profile cannot be
justified and ablation is safer and more effective.
Digoxin and verapamil shorten
the refractory period of
the accessory pathway and
should not be used.

Ventricular tachyarrhythmias
Ventricular ectopic beats (extrasystoles, premature beats)
The complexes of ventricular ectopic beats (VEBs) are
premature, broad and bizarre because the ventricles are
activated sequentially rather than simultaneously. The
complexes may be unifocal
(identical beats arising from a single ectopic
focus)
or multifocal
(varying morphology with multiple foci).
‘Couplet’ and
‘triplet’ two or three successive ectopic beats.
Ventricular ‘bigeminy’.A run of alternating sinus and ectopic.
Ectopic produce a low stroke volume because LV
contraction occurs before filling is complete. The pulse is
irregular, with weak or missed beats . Patients are usually
asymptomatic but may complain of an irregular heart beat,
missed beats or abnormally strong beats (due to the
increased output of the post-ectopic sinus beat).

The significance of VEBs depends on the presence or
absence of underlying heart disease.
Ventricular ectopic beats in otherwise healthy subjects
Ectopic beats in patients with otherwise normal hearts
are more prominent at rest and disappear with exercise.
Treatment is not necessary, unless the patient is highly
symptomatic, in which case β-blockers or, in some
situations, catheter ablation can be used. VEBs are
sometimes a manifestation of heart disease, such as CAD
or cardiomyopathy. There is no evidence that anti-
arrhythmic therapy improves prognosis but the discovery
of very frequent VEBs should prompt investigations,
looking for structural heart disease.

Ventricular ectopic beats associated with heart disease
Frequent VEBs often occur during acute MI but need no
treatment. Persistent, frequent (over 10/ hr) VEBs in
patients who have survived the acute phase of MI indicate
a poorer long-term outcome.
Other than β-blockers,
anti-arrhythmic drugs do not improve and may even
worsen prognosis.
Effective treatment of the heart failure may suppress the
ectopic beats.
VEBs are also a feature of digoxin toxicity, and may
occur as ‘escape beats’ in patients with bradycardia.
Treatment is that of the underlying condition.
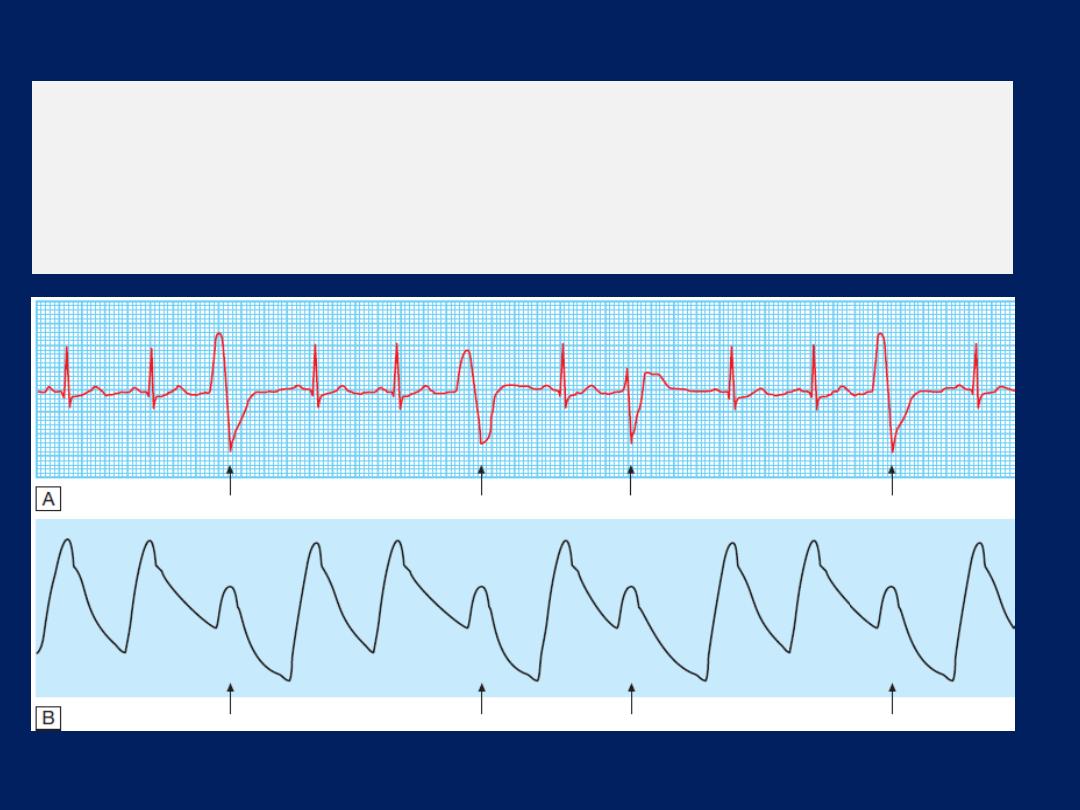
Ventricular ectopic beats.
A There are broad, bizarre QRS complexes (arrows)
with no preceding P wave in between normal sinus beats. Their configuration
varies, so these are multifocal ectopics. B A simultaneous arterial pressure trace
is shown. The ectopic beats result in a weaker pulse (arrows), which may be
perceived as a ‘dropped beat’.

Ventricular tachycardia (VT)
VT occurs most commonly in the settings of acute MI, chronic
CAD , and cardiomyopathy. It occurs when there is
extensive ventricular disease, such as impaired left
ventricular function or a left ventricular aneurysm. In these
settings, VT may cause haemodynamic compromise or
degenerate into ventricular fibrillation. It is caused by
abnormal automaticity or triggered activity in ischaemic
tissue, or by re-entry within scarred ventricular tissue.
Patients may complain of palpitation or symptoms of
low cardiac output, e.g. dizziness, dyspnoea or syncope.
The ECG shows tachycardia and broad, abnormal QRS
complexes with a rate of more than 120/min .

Features more in keeping with ventricular tachycardia
• History of MI
• AV dissociation (pathognomonic)
• Capture/fusion beats (pathognomonic)
• Extreme left axis deviation
• Very broad QRS complexes (> 140 ms)
• No response to carotid sinus massage or IV adenosine
VT may be difficult to distinguish from SVT with
bundle branch block
or
pre-excitation (WPW syndrome).
A 12-lead ECG or electrophysiology study may help
establish the diagnosis.
When there
is doubt, it is safer to
manage the problem as VT.

Management
Prompt action to
restore
sinus rhythm is required and should
usually be
followed
by prophylactic therapy. Synchronised
DC cardioversion is the treatment of choice if systolic BP is
< 90 mmHg. If the arrhythmia is well tolerated,
intravenous amiodarone may be given as a bolus,
followed by a continuous infusion . Intravenous lidocaine
can be used but may depress left ventricular function,
causing hypotension or acute heart failure. Hypokalaemia,
hypomagnesaemia, acidosis and hypoxaemia should be
corrected. Beta-blockers are effective at preventing VT by
reducing ventricular automaticity. Amiodarone can be
added if additional control is needed.

Class Ic anti-arrhythmic drugs
such as
propafenone or
flecainide
should not
be used for prevention of VT in
patients with CAD or heart failure because they depress
myocardial function and can be pro-arrhythmic (increase
the likelihood of a dangerous arrhythmia). In patients at
high risk of arrhythmic death (e.g. those with poor left
ventricular function, or where VT is associated with
haemodynamic compromise), the use of an implantable
cardiac defibrillator is recommended . Rarely, surgery
(e.g. aneurysm resection) or catheter ablation can be
used to interrupt the arrhythmia focus or circuit in patients
with VT associated with a myocardial infarct scar.

Patients recovering from MI sometimes
have periods
of
idioventricular rhythm (‘slow’ VT)
at a rate only slightly
above the preceding sinus rate and below 120/min. These
episodes often reflect reperfusion of the infarct territory
and may be a good sign. They are usually self-limiting
and asymptomatic, and do not require treatment.
Other forms of sustained VT will require treatment,
often
as an emergency.
VT occasionally occurs in patients with otherwise healthy
hearts
(‘normal heart VT’),
usually because of abnormal
automaticity in the right ventricular outflow tract or one of
the fascicles of the left bundle branch. The prognosis is
good and
catheter ablation can be curative.
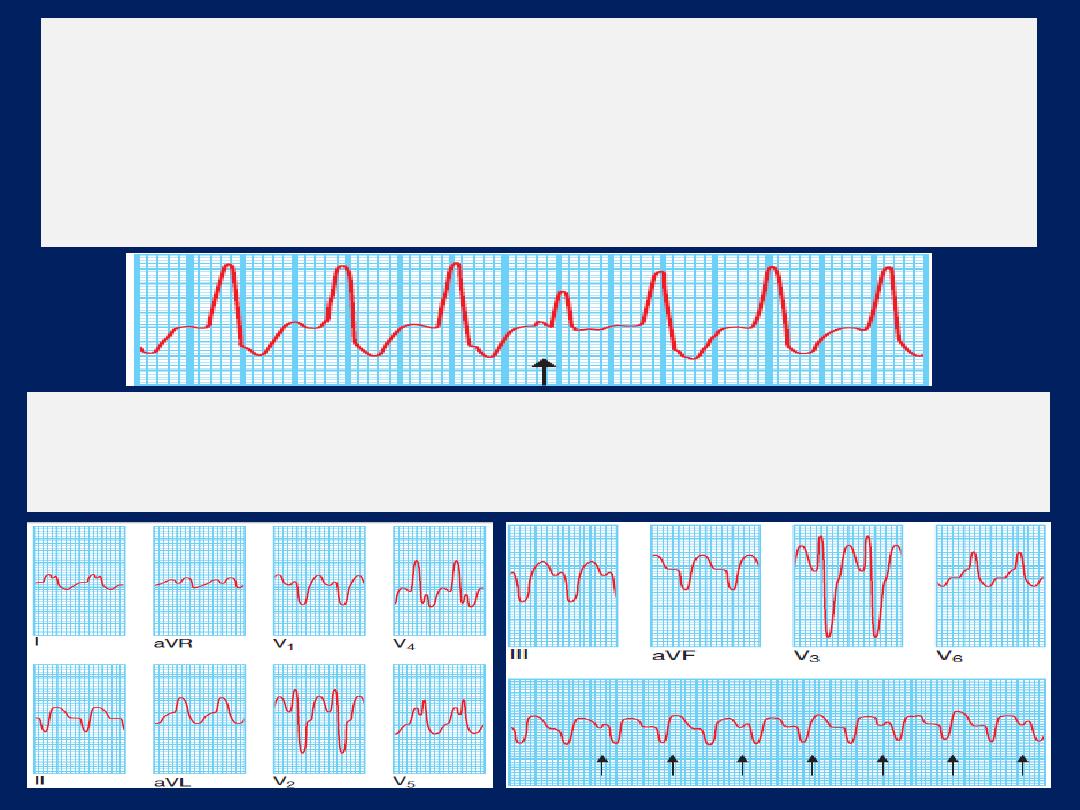
Ventricular tachycardia:
There are typically very broad QRS complexes and
marked left axis deviation. There is also AV dissociation; some P waves are visible
and others are buried in the QRS complexes (arrows).
Ventricular tachycardia:
There is independent atrial and ventricular activity.
Occasionally, a P wave is conducted to the ventricles through the AV node,
producing
a normal sinus beat in the
middle of the tachycardia
(a capture
beat);
more commonly, however, the conducted impulse fuses with an impulse
from the tachycardia
(a fusion beat).
This can only occur when there is AV
dissociation and is therefore
diagnostic
of ventricular tachycardia.
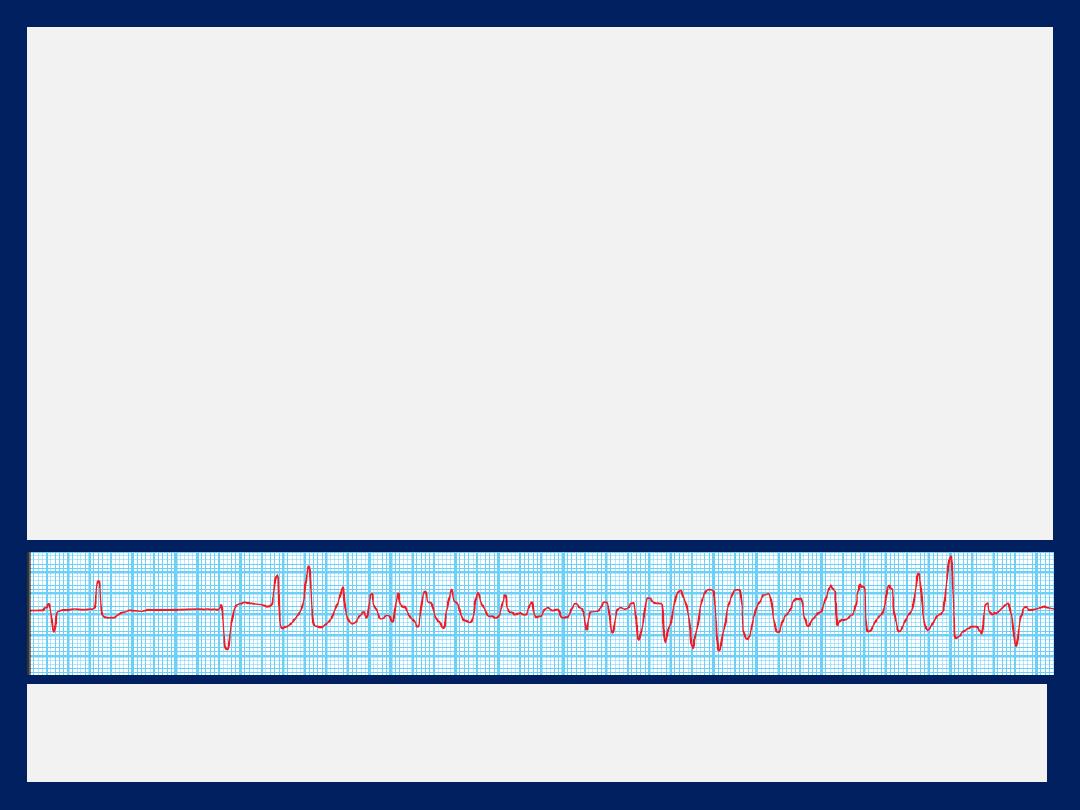
Torsades de pointes (ventricular tachycardia)
This form of polymorphic VT is a complication of
prolonged ventricular repolarisation
(prolonged QT interval).
The
ECG shows rapid irregular complexes that oscillate from an
upright to an inverted position and seem to twist around the
baseline as the mean QRS axis changes .The arrhythmia is
usually non-sustained and repetitive, but may degenerate
into ventricular fibrillation. During periods of sinus rhythm,
the ECG will usually show a prolonged QT interval
(> 0.43 s
in men, > 0.45 s in women when corrected to a heart rate of 60/min).
Torsades de pointes.
A bradycardia with a long QT interval is
followed by polymorphic VT that is triggered by an R on T ectopic.

The arrhythmia is more common in women and is often
triggered by medications and hypokalaemia. The
congenital long QT syndromes are a family of genetic
disorders that are characterised by mutations in genes
that code for cardiac sodium or potassium channels.
Adrenergic stimulation (e.g. exercise) is a common trigger
in long QT type 1, and a sudden noise (e.g. an alarm
clock) may trigger arrhythmias in long QT type 2.
Arrhythmias are more common during sleep in type 3.
Treatment
should be directed at the underlying cause.
IV magnesium (8 mmol over 15 mins, then 72 mmol over
24 hrs) should be given in all cases. Atrial pacing will
usually suppress the arrhythmia through rate-dependent
shortening of the QT interval.

IV isoprenaline is a reasonable alternative to pacing but
should be avoided in patients with the congenital long QT
syndromes. Beta-blockers are effective at preventing
syncope in patients with congenital long QT syndrome,
those with extreme QT interval prolongation
(> 500 ms) should be considered for implantation of a
defibrillator. Left stellate ganglion block may be of value.
The Brugada syndrome
is a related genetic disorder that
may present with polymorphic
VT
or sudden
death
.
It is characterised by a defect in
sodium
channel function
and an abnormal ECG (RBBB and ST elevation in V1 and
V2 but not usually prolongation of the QT interval).
The only known treatment is an
implantable
defibrillator.
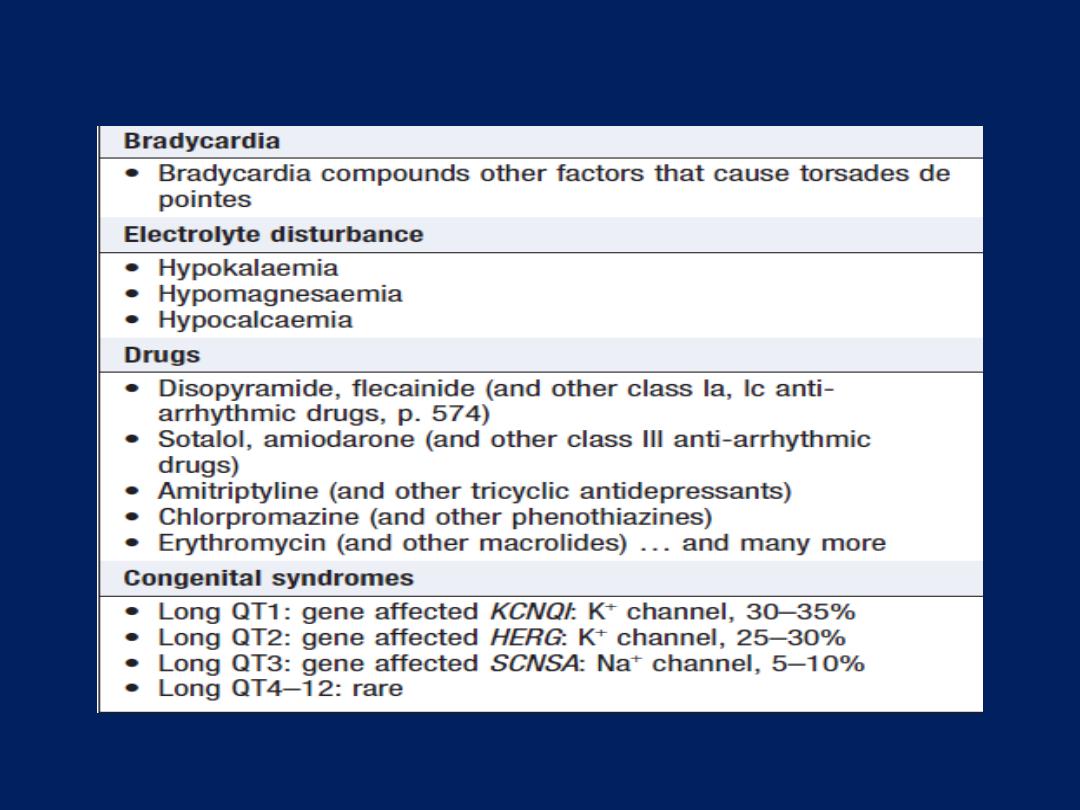
Causes of long QT interval and torsades de pointes

Atrioventricular and bundle branch block
First-degree atrioventricular block
AV conduction is delayed and so the PR interval is
prolonged (> 0.20 s). It rarely causes symptoms.
Second-degree atrioventricular block
Wenckebach phenomenon , Mobitz type I block , there is
progressive lengthening of successive PR intervals,
culminating in a dropped beat. The cycle then repeats itself,
is usually due to impaired conduction in the
AV node
itself.
The phenomenon may be physiological and is sometimes
observed at rest or during sleep in athletic with high vagal
tone. In Mobitz type II, the PR interval of the conducted
impulses remains constant but some P waves are not
conducted. This is usually caused by disease of the
His–
Purkinje system
and carries a risk of asystole.
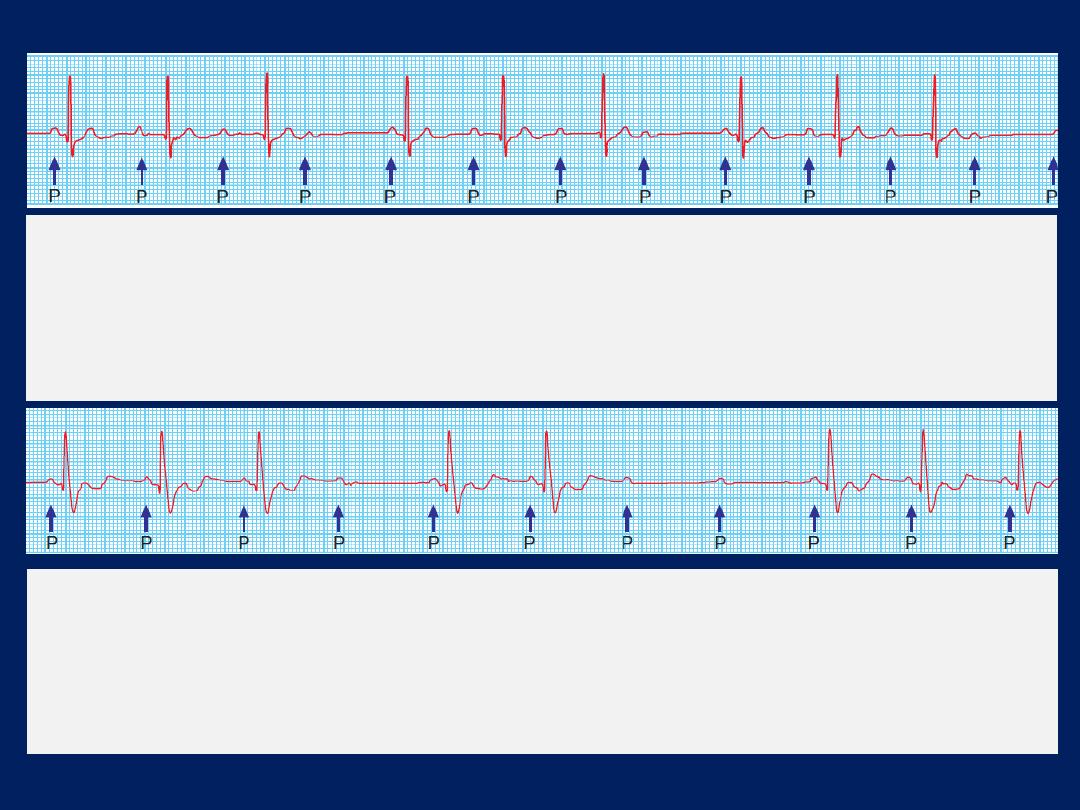
Second-degree AV block
(Mobitz type I – Wenckebach’s
phenomenon). The PR interval progressively increases until a P wave
is not conducted. The cycle repeats itself. In this example, conduction is
at a ratio of 4 : 3, leading to groupings of three V complexes.
Second-degree AV block
(Mobitz type II). The PR interval of
conducted beats is normal but some P waves are not conducted.
The constant PR interval distinguishes this from Wenckebach’s
phenomenon.
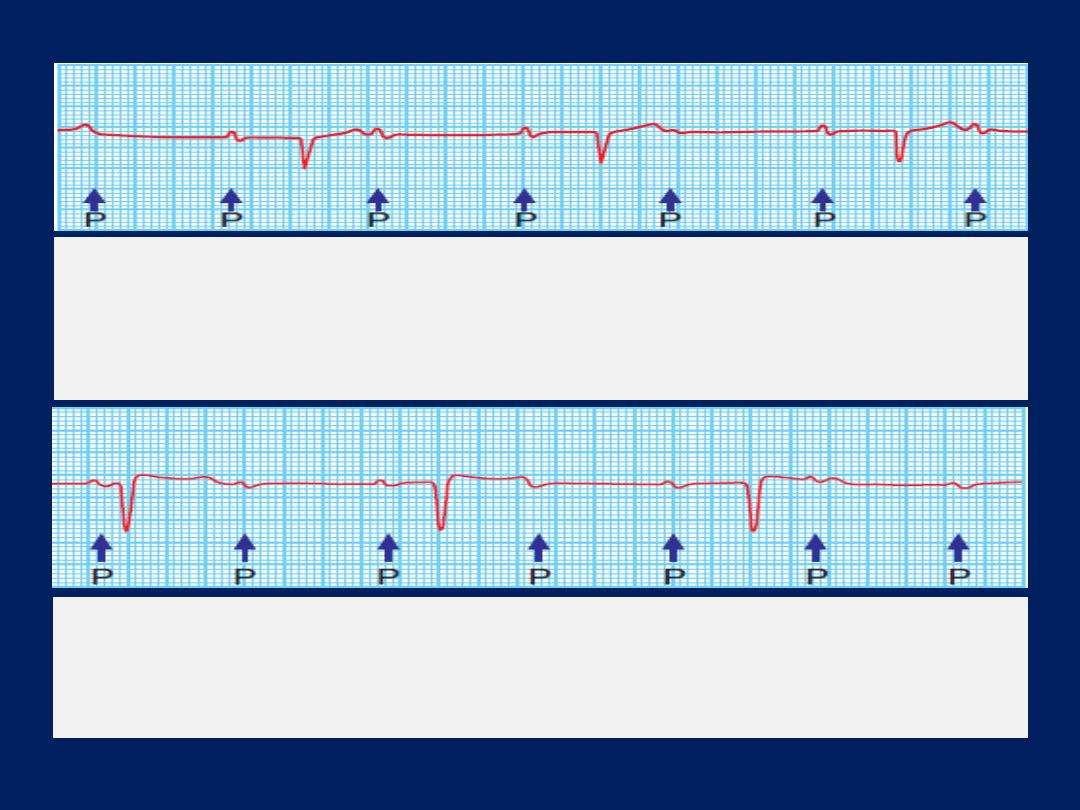
Second-degree AV block with fixed
2 : 1 block.
Alternate P waves are not conducted. This may be due
to Mobitz type I or II block.
Complete (third-degree) AV block.
There is complete
dissociation of atrial and ventricular complexes. The atrial rate is
80/min and the ventricular rate is 38/min.

Third-degree (complete) atrioventricular block
When AV conduction fails completely, the atria and
ventricles beat independently (AV dissociation).
Ventricular activity is maintained by an escape rhythm
arising in the AV node or bundle of His (narrow QRS )
or the distal Purkinje tissues (broad QRS complexes).
Distal escape rhythms tend to be slower and less reliable.
Complete AV block produces a slow(25–50/min), regular
pulse that, except in the case of congenital complete AV
block, does not vary with exercise. There is usually a
compensatory increase in stroke volume, producing a large-
volume pulse. Cannon waves may be visible in the neck
and the intensity of the first heart sound varies due to the
loss of AV synchrony.

Aetiology of complete AV block
Congenital
Acquired
• Idiopathic fibrosis
• MI/ ischaemia
• Inflammation
Acute (e.g. aortic root abscess in infective endocarditis)
Chronic (e.g. sarcoidosis, Chagas’ disease,)
• Trauma (e.g. cardiac surgery)
• Drugs (e.g. digoxin,
β-blocker)

Stokes–Adams attacks
Episodes
of ventricular
asystole
and recurrent
syncope
may
complicate
, Mobitz type II , complete heart block or
sinoatrial disease. A typical episode is characterised by
sudden loss of consciousness that occurs without warning
and results in collapse. A brief anoxic seizure (due to
cerebral ischaemia) may occur if there is prolonged
asystole.
There is pallor and a death-like appearance during the
attack, but when the heart starts beating again, there is
a characteristic flush. Unlike in epilepsy, recovery is
rapid. Sinoatrial disease and neurocardiogenic syncope
may cause similar symptoms.

Management
Atrioventricular block complicating MI
Acute inferior MI is often complicated by transient AV
block because the right coronary artery (RCA) supplies
the AV node. There is usually a reliable escape rhythm
and, if the patient remains well, no treatment is
required. Symptomatic second- or third-degree AV block
may respond to atropine
(0.6 mg IV, repeated as necessary)
or, if this fails, a temporary pacemaker. In most cases,
the AV block will resolve within 7–10 days. Second- or
third-degree AV heart block complicating acute anterior
MI indicates extensive ventricular damage involving
both bundle branches and carries a poor prognosis.
Asystole may ensue and a temporary pacemaker should
be inserted promptly.

If the patient presents with asystole, IV atropine (3 mg) or IV
isoprenaline (2 mg in 500 mL 5% dextrose, infused at 10–
60 mL/ hr) may help to maintain the circulation until a
temporary pacing electrode can be inserted.
Chronic atrioventricular block
Patients with symptomatic bradyarrhythmias associated
with AV block should receive a permanent pacemaker.
Asymptomatic first-degree or Wenckebach does not
require treatment. A permanent pacemaker is indicated in
patients with asymptomatic Mobitz type II second- or
third-degree AV block because of the risk of asystole and
sudden death. Pacing improves prognosis.

Bundle branch block and hemiblock
Conduction block in the right or left bundle branch can
occur as a result of many pathologies, including ischaemic
or hypertensive heart disease or cardiomyopathies.
RBBB can occur in healthy people but LBBB often signifies
important underlying heart disease. The left bundle
branch divides into an anterior and a posterior fascicle.
Damage to the conducting tissue at this point (hemiblock)
does not broaden the QRS complex but alters the mean
direction of ventricular depolarisation, causing left axis
deviation in left anterior hemiblock and right axis
deviation in left posterior hemiblock .
The combination of right branch block and left anterior
or posterior hemiblock is known as bifascicular block.
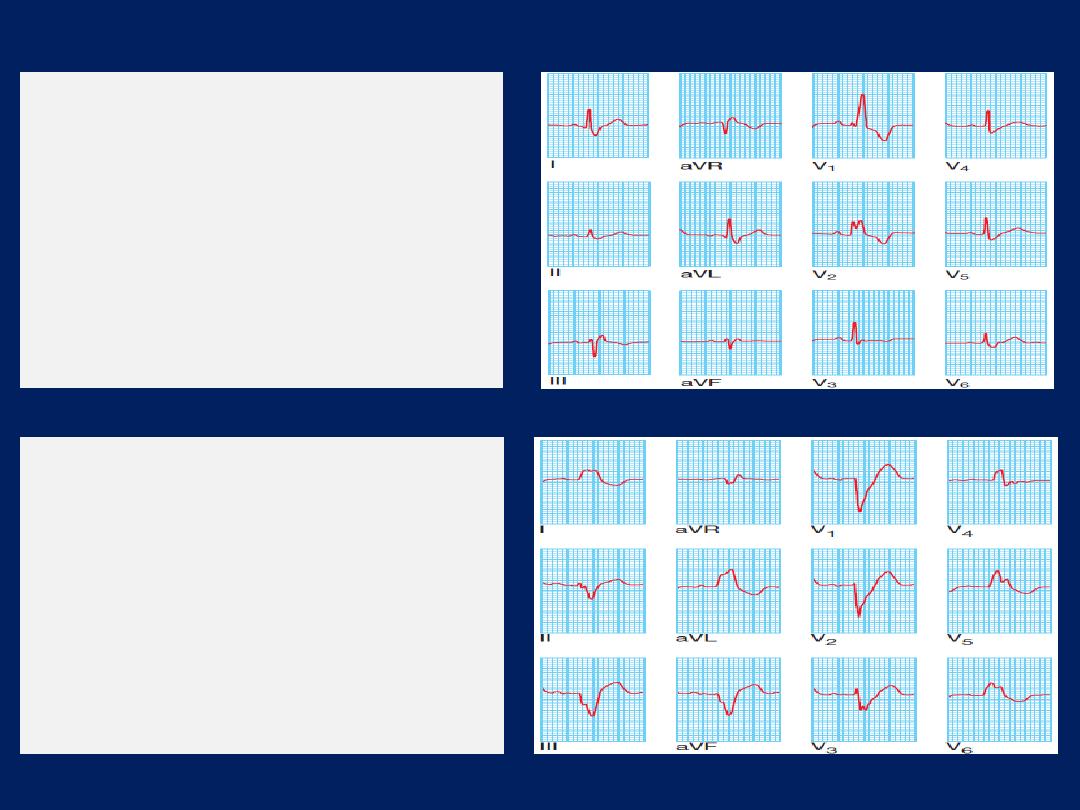
Right bundle branch
block.
Note the wide QRS
complexes with ‘M’-shaped
configuration in leads V,
and V2 and a wide S
wave in lead I.
Left bundle branch block.
Note the wide QRS
complexes with loss of the
Q wave or septal vector in
lead I and ‘M’-shaped
QRS in V5 and V6.
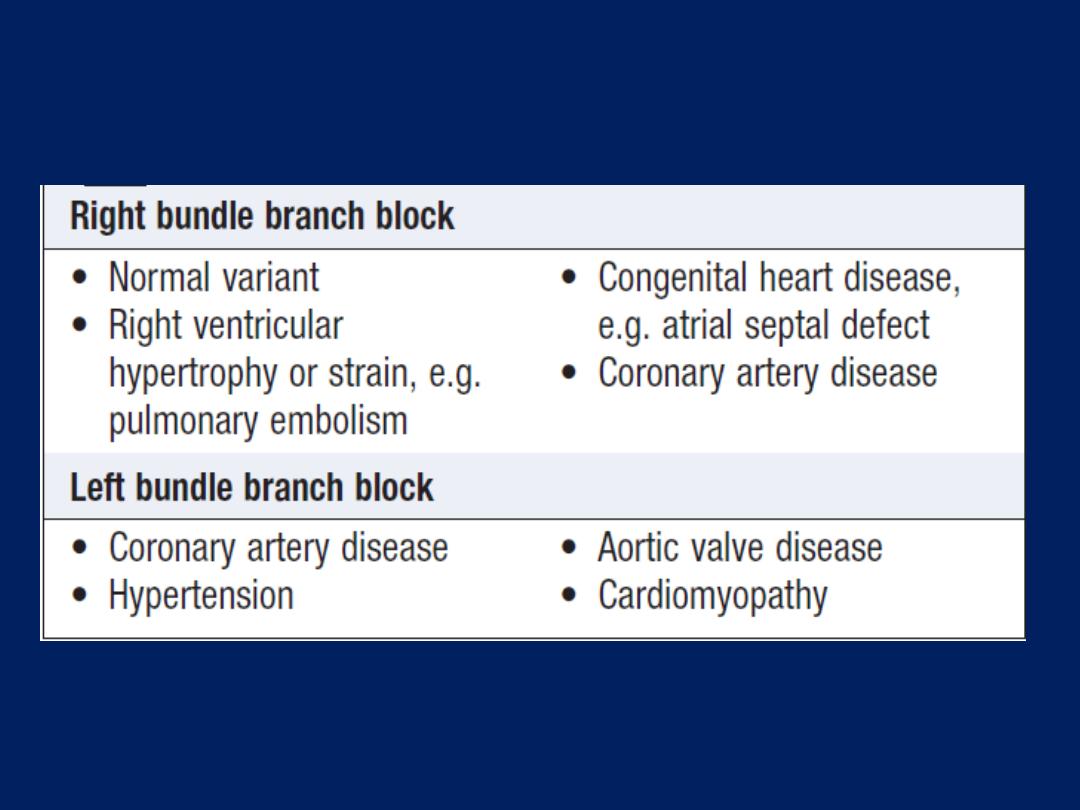
Common causes of bundle branch block

Anti-arrhythmic drug therapy
Classification
Anti-arrhythmic drugs may be classified according to
their mode or site of action .
The Vaughan-Williams classification is a simple system
but is convenient for describing the main mode of action
of anti-arrhythmic drugs that should be used following
guiding principles.
Anti-arrhythmic drugs can also be more accurately
categorised by referring to the cardiac ion channels and
receptors on which they act.
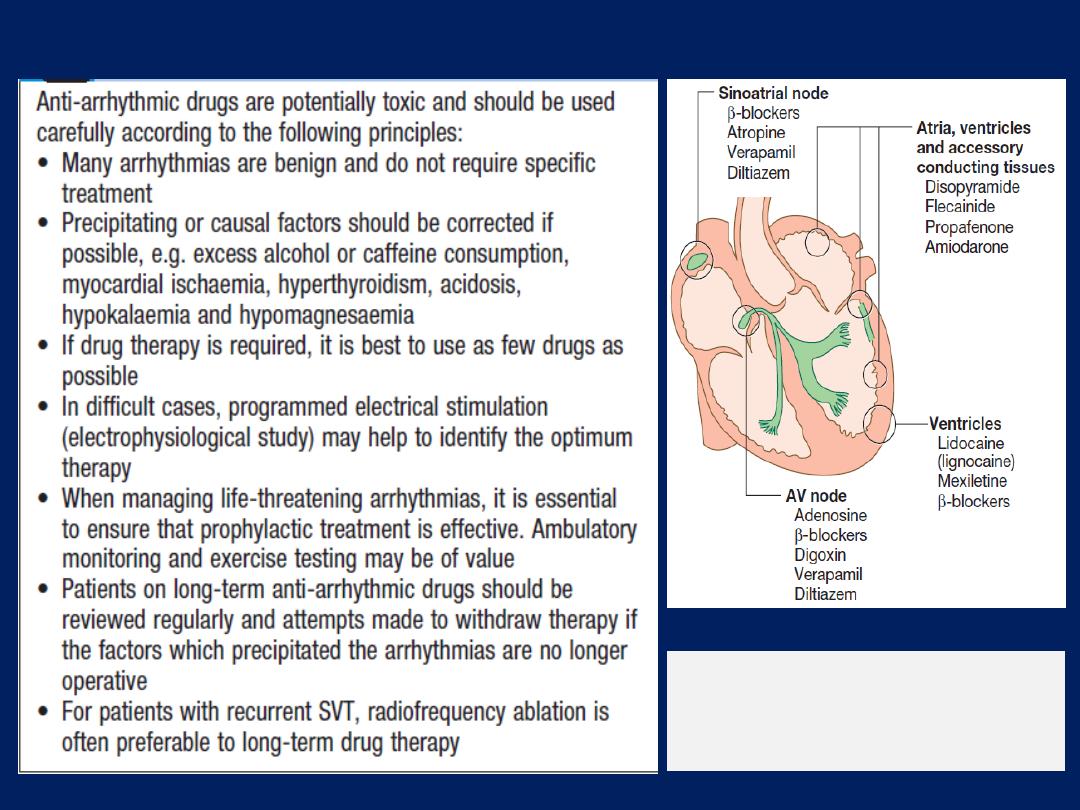
Anti-arrhythmic drugs: principles of use
Classification of
anti-arrhythmic
drugs by site
of action.
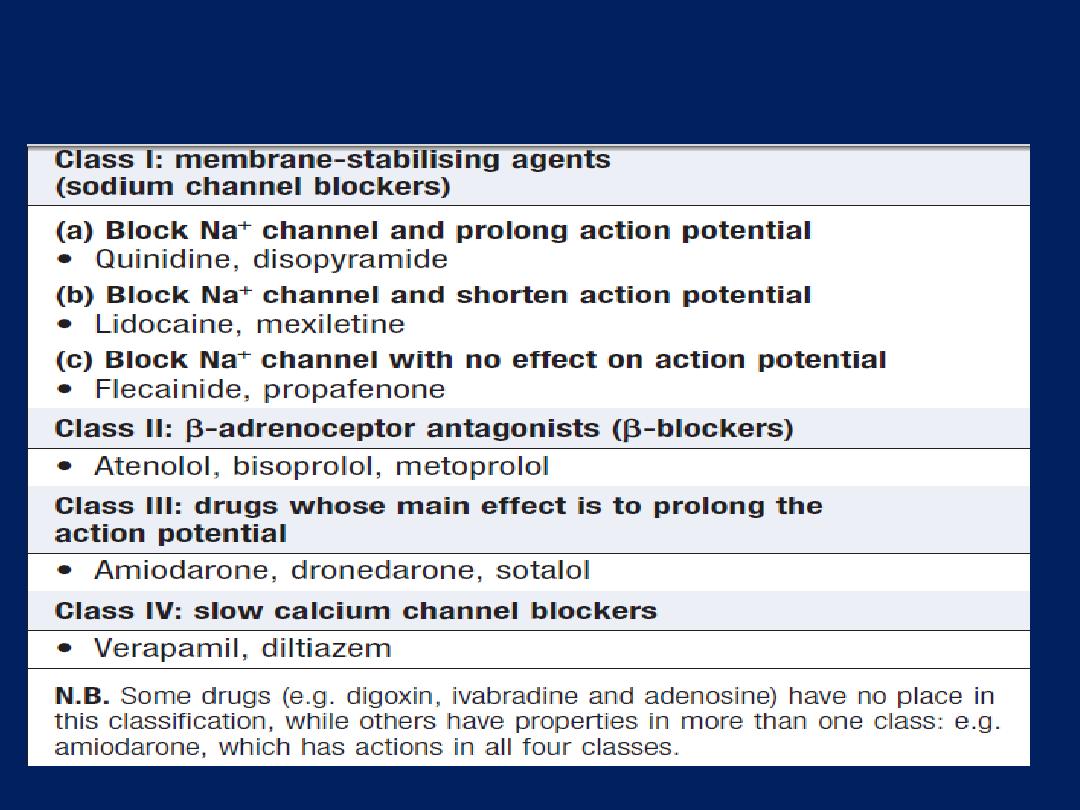
Classification of anti-arrhythmic drugs by effect on the
intracellular action potential
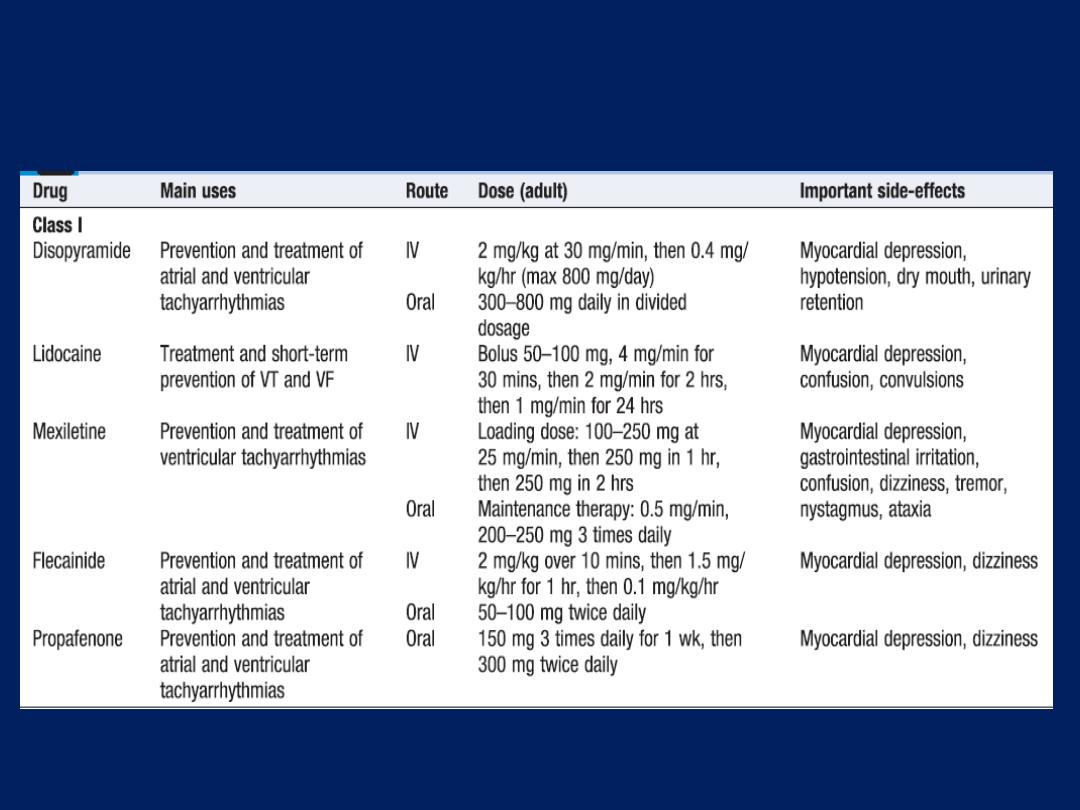
The main uses, dosages and side-effects of the most
widely used anti-arrhythmic drugs
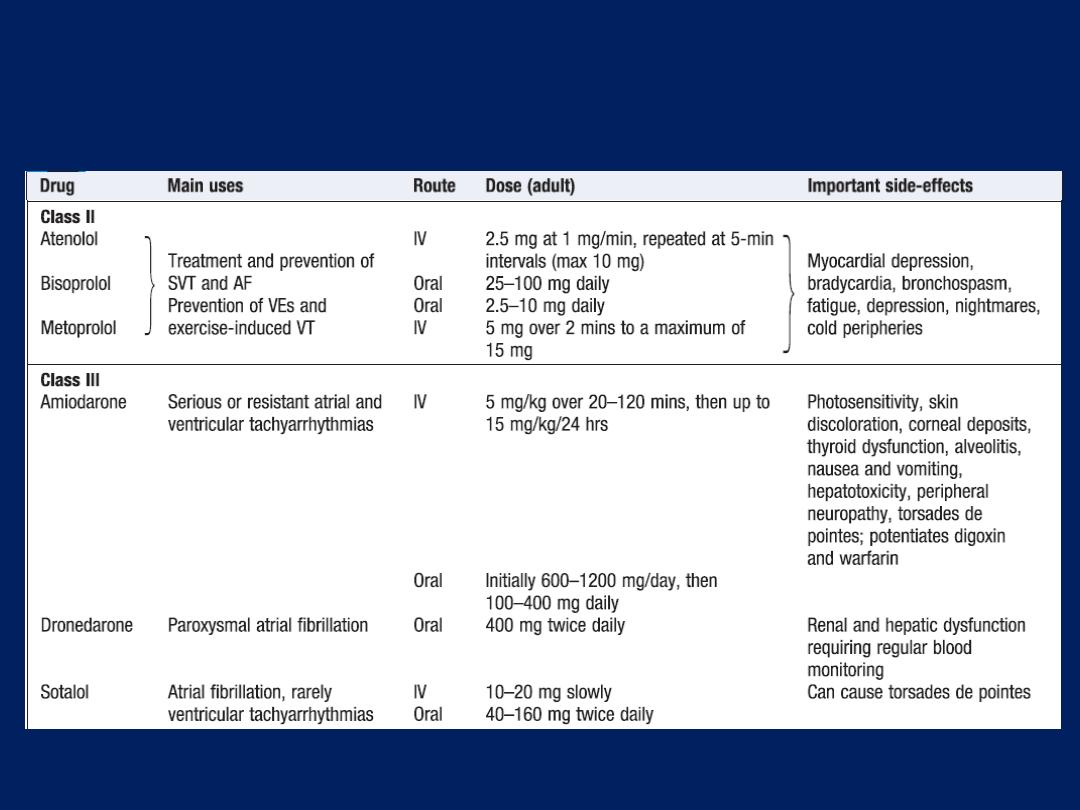
The main uses, dosages and side-effects of the most
widely used anti-arrhythmic drugs'cont'd
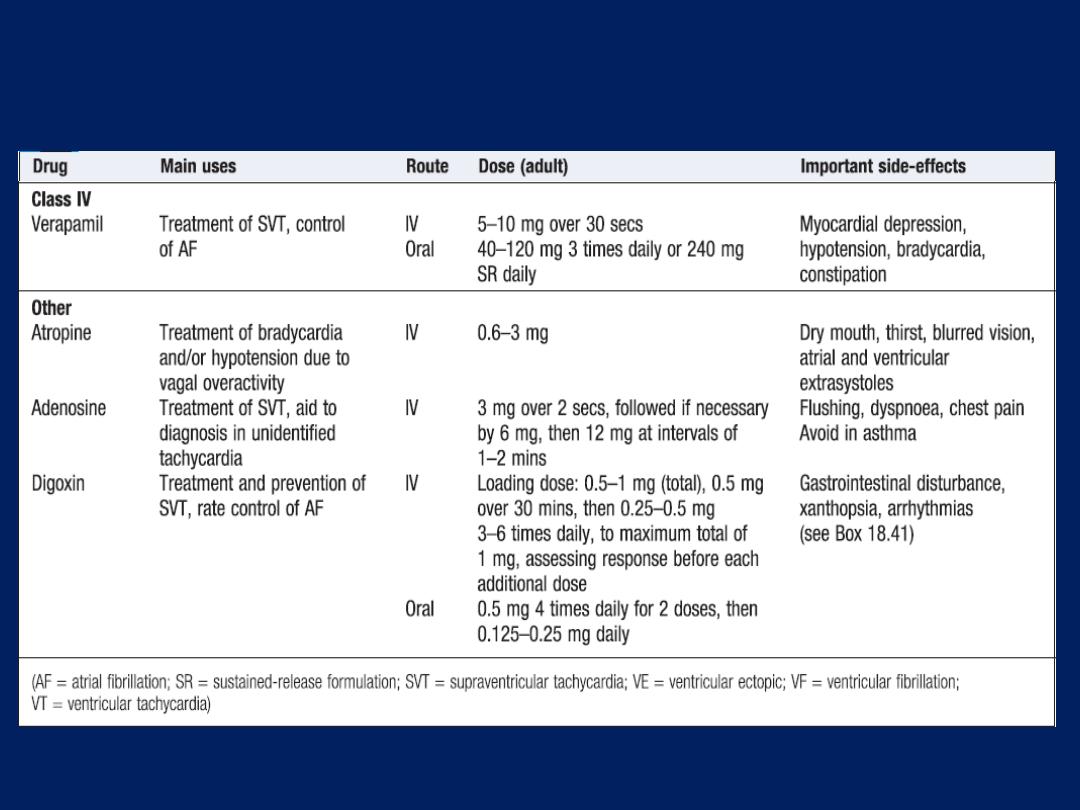
The main uses, dosages and side-effects of the most
widely used anti-arrhythmic drugs'cont'd

Class I drugs
Act principally by suppressing excitability and slowing
conduction in atrial or ventricular muscle. They block
sodium channels . These drugs should generally be
avoided in patients with heart failure because they
depress myocardial function, and class Ia and Ic drugs are
often pro-arrhythmic.
Class Ia drugs
Prolong action potential duration and increase refractory
period. They are used to prevent atrial and V. arrhythmias.
Disopyramide. Causes anticholinergic side-effects, such as
urinary retention, and can precipitate glaucoma. It can
depress myocardium and should be avoided in H.failure.
Quinidine. Now rarely used, as it increases mortality
and causes gastrointestinal upset.

Class Ib drugs
Shorten action potential and refractory period. They act
predominantly in ventricular myocardium and so are used to
treat or prevent ventricular tachycardia and fibrillation.
Lidocaine. Must be given IV and has a very short half-life.
Mexiletine. Given IV or orally, has many side-effects .
Class Ic drugs
These affect the slope of the action potential without
altering its duration or refractory period. They are
used mainly for prophylaxis of atrial fibrillation but
are effective in prophylaxis and treatment of SVT and VT.
They are useful for WPW syndrome because they block
conduction in accessory pathways.
They
should not be used
as oral prophylaxis in patients
with previous MI because of pro-arrhythmia.

Flecainide
Intravenous infusion may be used for pharmacological
cardioversion of atrial fibrillation of less than 24 hours’
duration. It should be prescribed along with an AV node-
blocking drug, such as a β-blocker, to prevent pro-
arrhythmia. Effective for prevention of atrial fibrillation
Propafenone. has some ββ (class II) properties.
interactions with digoxin, warfarin and cimetidine.
Class II drugs
This group comprises β-blockers. These agents reduce the
rate of SA node depolarisation and cause relative block
in the AV node, making them useful for rate control in
atrial flutter and fibrillation, can be used to prevent SVT
and VT . They reduce risk of arrhythmic death in patients
with coronary artery disease and heart failure.

‘Non-selective’ β-blockers. Act on both β
1
and β
2
receptors. Beta
2
blockade causes side-effects, such as
bronchospasm and peripheral vasoconstriction.
Propranolol ,nadolol and carvedilol are non-selective and
is subject to extensive first-pass metabolism in the liver.
‘
Cardioselective’ β-blockers. Act mainly on myocardial
β1 receptors and are relatively well tolerated. Bisoprolol
and metoprolol are examples.
Sotalol. A racemic mixture of two isomers with nonselective
β-blocker (mainly l- sotalol) and class III (mainly
d- sotalol) activity. It may cause torsades de pointes.

Class III drugs
Prolonging the plateau phase of the action potential, thus
lengthening the refractory Period, are very effective at
preventing atrial and ventricular tachyarrhythmias. They
prolong QT and can predispose to torsades de pointes.
Amiodarone.
The principal drug, although disopyramide
and sotalol have class III activity. Amiodarone is a complex
drug that also has class I, II and IV activity. It is probably
the most effective drug
currently
available for
controlling
paroxysmal atrial fibrillation. It is also used
to
prevent
episodes of recurrent VT, particularly in
patients with poor left ventricular function or those with
implantable defibrillators (to
prevent
unnecessary DC
shocks). Has a very long tissue half-life (25–110 days).

The drug’s effects may last for weeks or months after
treatment has been stopped.
Side-effects are common (up to one-third of patients),
numerous and potentially serious. Drug interactions are
also common .
Dronedarone.
A related drug that has a short tissue
half-life and fewer side-effects. It has recently been
shown to be effective at preventing episodes of atrial
flutter and fibrillation.
It is
contraindicated in
patients with permanent atrial
fibrillation, or if there is heart failure or left ventricular
impairment, because it increases mortality.
Regular liver function test monitoring is required.

Class IV drugs
These
block
the ‘slow calcium channel’, which is
important for
impulse
generation and conduction in
atrial and nodal tissue, although it is also present in
ventricular muscle. Their main indications are prevention
of SVT (by blocking the AV node) and rate control in
patients with atrial fibrillation.
Verapamil.
The most widely used drug in this class.
Intravenous verapamil may cause profound bradycardia
or hypotension, and should not be used in conjunction
with β-blockers.
Diltiazem.
Has similar properties.

Other anti-arrhythmic drugs
Atropine sulphate
(0.6 mg IV, repeated if necessary to a maximum of
3 mg).
Increases the sinus rate and SA and AV conduction,
and is the treatment of choice for severe bradycardia or
hypotension due to vagal overactivity. It is used for initial
management of symptomatic bradyarrhythmias
complicating inferior MI, and in cardiac arrest due to
asystole.
Adenosine.
Must be given IV. It produces transient AV
block lasting a few seconds. It is used to terminate SVT
when the AV node is part of the re-entry circuit, or to
help establish the diagnosis in difficult arrhythmias, such
as atrial flutter with 2 : 1 AV block or broad-complex
tachycardia .
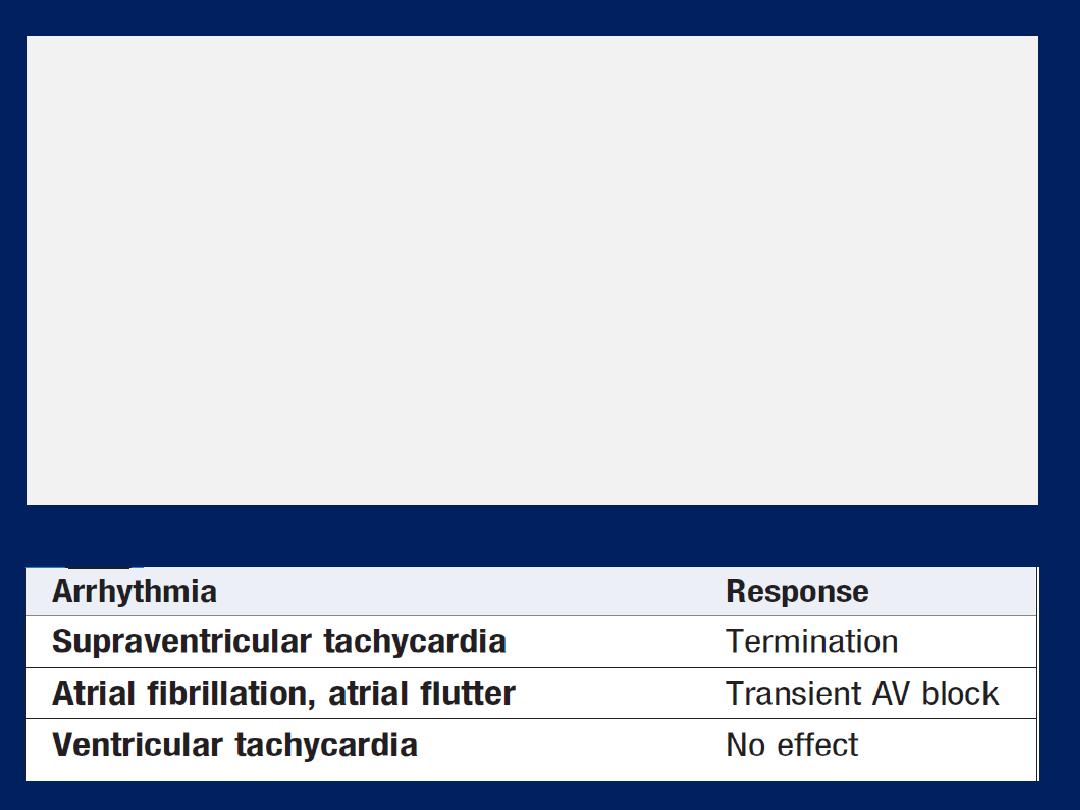
Adenosine is given as an intravenous bolus, initially 3 mg
over 2 seconds . If there is no response after 1–2 minutes,
6 mg should be given; if necessary, after another 1–2
minutes, the maximum dose of 12 mg. Patients
should be
warned to expect
short-lived and sometimes distressing
flushing, breathlessness and chest pain. Adenosine can
cause bronchospasm and should be avoided in asthmatics;
its effects are greatly potentiated by dipyridamole and
inhibited by xanthines.
Response to intravenous adenosine

Digoxin.
A purified glycoside from foxglove, Digitalis
lanata, which slows conduction and prolongs the refractory
period in the AV node. This helps to control the ventricular
rate in atrial fibrillation and may interrupt SVT involving
the AV node. It shortens refractory periods and enhances
excitability and conduction in other parts of the heart
(including accessory pathways). It may therefore
increase atrial and ventricular ectopic activity and can
lead to atrial and ventricular tachyarrhythmias.
Excreted by the kidneys, maintenance dose should be
reduced in children, olde and those with renal impairment.
It has a long tissue half-life (36 hours), so that effects
may persist for several days. Measurement of plasma
concentration helps identify digoxin toxicity .
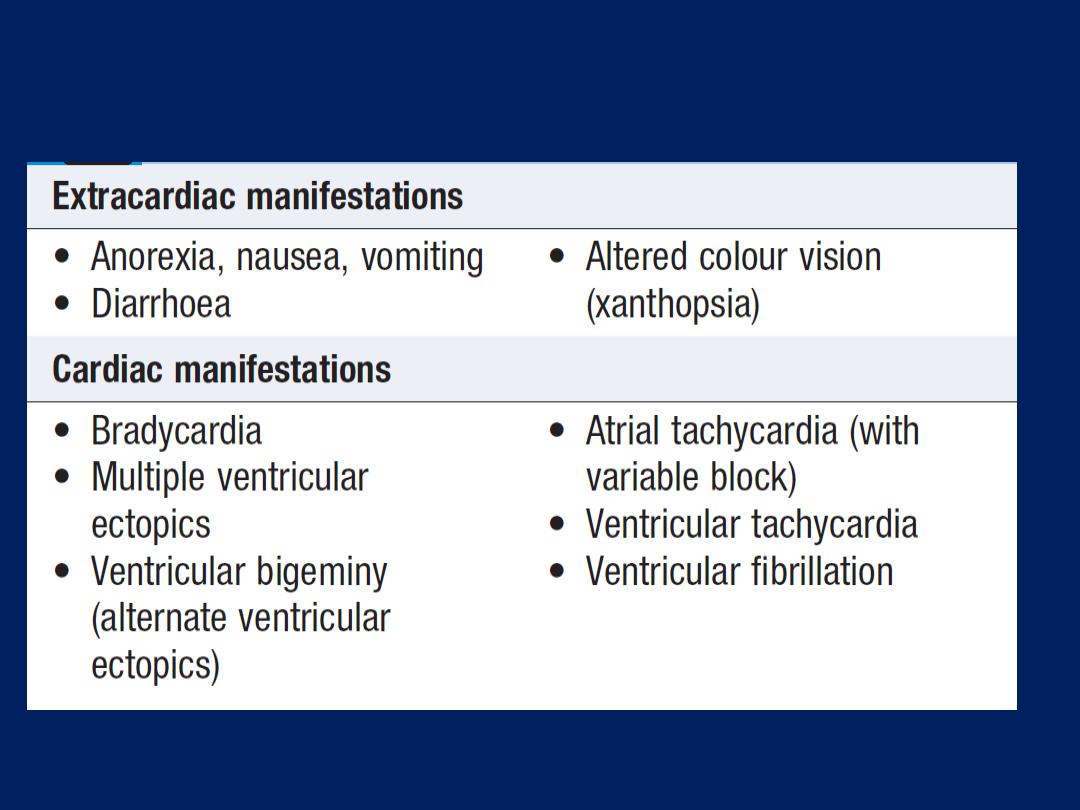
Digoxin toxicity

There are five main classes in the Singh Vaughan Williams
classification of antiarrhythmic agents:
Class I interfere
with the (Na
+
) channel. block inward sodium current
Ia Quinidine , procainamide, disopyramide
Ib Lidocaine, phenytoin , mexiletine , tocainide
Ic flecainide, propafenone (also ββ activity).
Class II
are anti-sympathetic NS agents.
ββ , propranolol, esmolol , timolol , metoprolol, bisoprolol.
Class III
agents affect (K
+
) efflux. prolong action potential duration;
Amiodarone and dronedarone ,sotalol, (also ββ) Ibutilide,dofetilide
Class IV
agents affect calcium channels and the AV node .
verapamil ,diltiazem , effective at SA and AV nodes; reduce rate and
conduction.
Class V
agents work by other or unknown mechanisms.
Adenosine, digoxin ,decreases conduction of impulses through the AV node and
increases vagal activity, MG sulfate used for torsade de pointes
Amiodarone , dronedarone grouped in class III, also have class I, II, IV properties.
Common Nonarrhythmic Toxicity of Most Frequently Used Antiarrhythmic Agents
Drug
Common Nonarrhythmic Toxicity
Amiodarone
Tremor, peripheral neuropathy, pulmonary inflammation, hypo-
and hyperthyroidism, photosensitivity
Adenosine
Cough, flushing
Digoxin
Anorexia, nausea, vomiting, visual changes
Disopyramide
Anticholinergic effects, decreased myocardial contractility
Dofetilide
Nausea
Dronedarone
Gastointestinal intolerance, exacerbation of heart failure
Flecainide
Dizziness, nausea, headache, decreased myocardial contractility
Ibutilide
Nausea
Lidocaine
Dizziness, confusion, delirium, seizures, coma
Mexiletine
Ataxia, tremor, gait disturbances, rash, nausea
Moricizine
Mood changes, tremor, loss of mental clarity, nausea
Procainamide
Lupus erythematosus-like syndrome (more common in slow
acetylators), anorexia, nausea, neutropenia
Propafenone
Taste disturbance, dyspepsia, nausea, vomiting
Quinidine
Diarrhea, nausea, vomiting, cinchonism, thrombocytopenia
Sotalol
Hypotension, bronchospasm

Electrical cardioversion
The heart can be completely depolarised by passing a sufficiently
large electrical current through it from an external source with a
synchronized shock, usually under general anaesthesia. This will
interrupt any arrhythmia and produce a brief period of asystole that
is usually followed by the resumption of sinus rhythm.
The shock is delivered immediately after detection of the R wave
because,
if it is
applied during ventricular repolarization (
on the T
wave
), it may provoke ventricular fibrillation. High-energy shocks
may cause chest wall pain so, if there is no urgency, it is appropriate
to begin with a lower-amplitude shock (e.g.
50 joules
), going on to
larger if necessary. Patients with atrial fibrillation or flutter of >48
hours’ duration are at risk of left atrial appendage thrombus, and
thus systemic embolism after cardioversion.
In such cases, cardioversion should be delayed until effective
anticoagulation has been given for at least 4 weeks.

Defibrillation
Defibrillators deliver a DC, high-energy, short-duration
shock via two large electrodes or paddles coated with
conducting jelly or a gel pad, positioned over the upper
right sternal edge and the apex.
Modern units deliver a
biphasic
shock, during which the
shock polarity is reversed mid-shock. This reduces the
total shock energy required to depolarise the heart.
Defibrillators is the delivery of an
unsynchronised
shock
during a cardiac arrest caused by ventricular fibrillation.
The precise timing of the discharge
is not
important in this
situation. In ventricular fibrillation and other emergencies,
the energy of the first and second shocks should be
150
joules and thereafter up to 200 joules; there is no need for
an anaesthetic, as the patient is unconscious.

Catheter ablation therapy
is the treatment of choice for
SVT or atrial flutter, symptomatic WPW
(first-line treatment and is
nearly always curative)
. This is effective at reducing atrial
fibrillation in 70–80% of young with structurally normal
hearts, and tends to be reserved for drug-resistant atrial
fibrillation. It is a useful for some with V. arrhythmias,
AVNRT and AVre-entrant (accessory pathway) T, curative in
> 90% of cases. (via
radiofrequency
current) or freezing
(
cryoablation
). The
ablation at two sites:
the ostia of the
pulmonary veins, from which ectopic beats may trigger
paroxysms of arrhythmia, and in the LA itself.
The procedure takes approximately1–4 hours and does not require
GA .The patient may experience some discomfort during the
ablation. Serious complications are rare (< 1%) include inadvertent
CHB requiring pacemaker implantation, and cardiac tamponade.

Temporary pacemakers
Involves delivery of an electrical impulse into the heart to
initiate tissue depolarisation and cardiac contraction. This is
achieved by inserting a bipolar pacing electrode via the
internal jugular, subclavian or femoral vein and positioning
it at the apex of the RV, using fluoroscopic imaging. The
electrode is connected to an external pacemaker with an
adjustable energy output and pacing rate. The ECG of
right ventricular pacing is characterised by regular broad
QRS complexes with a left bundle branch block pattern.
Each complex is immediately preceded by a‘pacing spike’.
Nearly all pulse generators are used in the ‘demand’
mode, so that the pacemaker will only operate if the heart
rate falls below a preset level.

Temporary pacing may be
indicated in
the management
of transient AV block and other arrhythmias complicating
acute MI or cardiac surgery, to maintain the rhythm in
other situations of reversible bradycardia (i.e. due to
metabolic disturbance or drug overdose),
or as a bridge
to permanent pacing.
Complications include
pneumothorax, brachial plexus or subclavian artery
injury, local infection or septicaemia (usually Staphy-
aureus), and pericarditis. Failure of the system may be
due to lead displacement or a progressive increase in the
threshold (exit block) caused by tissue oedema.
Complications increase with time and so a temporary
pacing system should ideally
not be used for >7 days.
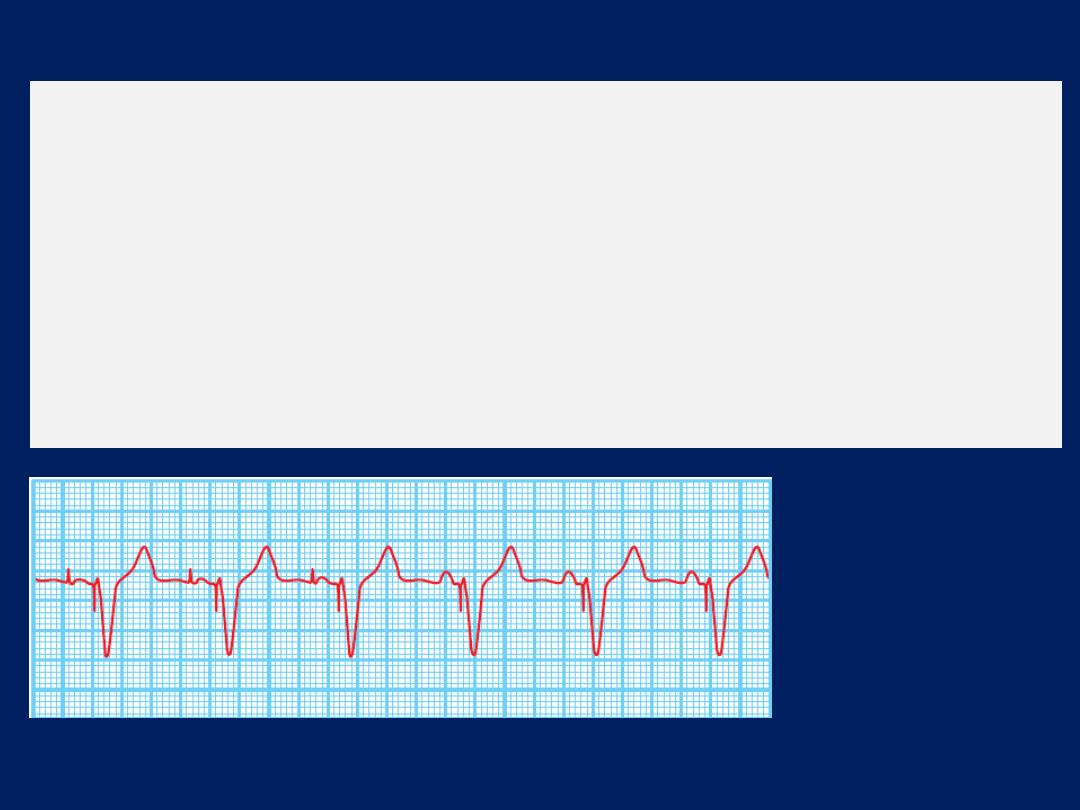
Dual-chamber pacing.
The first three beats show atrial and
ventricular pacing with narrow pacing spikes in front of
each P wave and QRS complex.
The last beats show spontaneous P waves with a different
morphology and no pacing spike; the pacemaker senses or
tracks these P waves and maintains AV synchrony by pacing
the ventricle after an appropriate interval.

Permanent pacemakers
Small, flat, metal devices implanted under the skin, usually
in the pectoral area. They contain a battery, a pulse
generator, and programmable electronics that allow
adjustment of pacing and memory functions. Pacing
electrodes (leads) can be placed via the subclavian or
cephalic veins into the RV (usually at the apex), the right
atrial appendage or,
to maintain AV synchrony
, both.
Permanent pacemakers are programmed using an
external programmer via a wireless telemetry system.
Pacing rate, output, timing and other parameters can be
adjusted. This allows the device to be set to the optimum
settings to suit the patient’s needs. Pacemakers store useful
diagnostic data about the patient’s heart rate

Single-chamber atrial
pacing is used in sinoatrial disease
without AV block (acts as an external sinus node).
Single-chamber ventricular
pacing is used in patients with
continuous atrial fibrillation and bradycardia.
Dual-chamber pacing
is most often used in second- or
third-degree AV block; here, the atrial electrode is used to
detect spontaneous atrial activity and trigger ventricular
pacing , thereby preserving AV synchrony and allowing the
ventricular rate to increase, together with the sinus node
rate, during exercise and other forms of stress.
Dual-chamber advantages over ventricular pacing; include
superior haemodynamics, a better effort tolerance, a
lower prevalence of atrial arrhythmias and
avoidance
of
‘pacemaker syndrome’
(a fall in BP and dizziness
precipitated by loss of AV synchrony).

A code is used to signify the pacing mode
. For example,
a system that
paces
the
atrium
, senses the atrium and is
inhibited if it senses spontaneous activity is designated
AAI.
Most dual-chamber
pacemakers are programmed to a
mode termed
DDD;
in this case, ventricular pacing is
triggered by a sensed sinus P wave and inhibited by a
sensed spontaneous QRS complex. A fourth letter,
‘R’, is
added if the pacemaker has a rate response function (e.g.
AAIR = atrial demand pacemaker with rate response
function). Rate-responsive pacemakers are used in patients
with chronotropic incompetence, who are unable to
increase their heart rate during exercise.

These devices have a sensor that triggers an increase in
heart rate in response to movement or increased
respiratory rate.
The sensitivity of the sensor is programmable, as is the
maximum paced heart rate.
Early complications of permanent pacing include
pneumothorax, cardiac tamponade, infection and lead
displacement.
Late complications include infection (which usually
necessitates removing the pacing system), erosion of the
generator or lead, chronic pain related to the implant
site, and lead fracture due to mechanical fatigue.
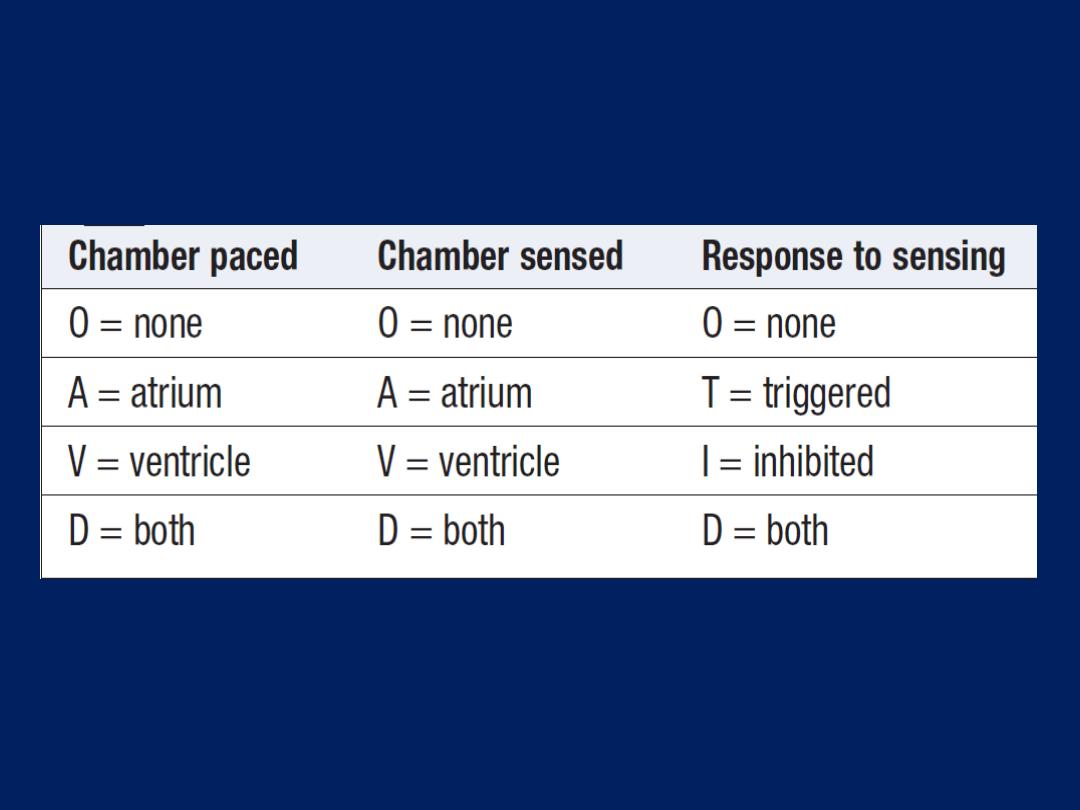
International generic pacemaker code

Implantable cardiac defibrillators (ICDs)
ICDs can also detect and terminate life-threatening
ventricular tachyarrhythmias.
ICDs treat ventricular tachyarrhythmias
using overdrive
pacing
,
cardioversion
or
defibrillation. They are
implanted in a similar manner to pacemakers and carry
a similar risk of complications. In addition,patients can be
prone to psychological problems and anxiety, particularly if
they have experienced repeated shocks from their device.
The evidence-based indications
for ICD implantation
can be divided into ‘secondary prevention’ indications,
when patients have already had a potentially life-
threatening ventricular arrhythmia, and ‘primary
prevention’ indications, when patients are considered to be
at significant future risk of arrhythmic death.
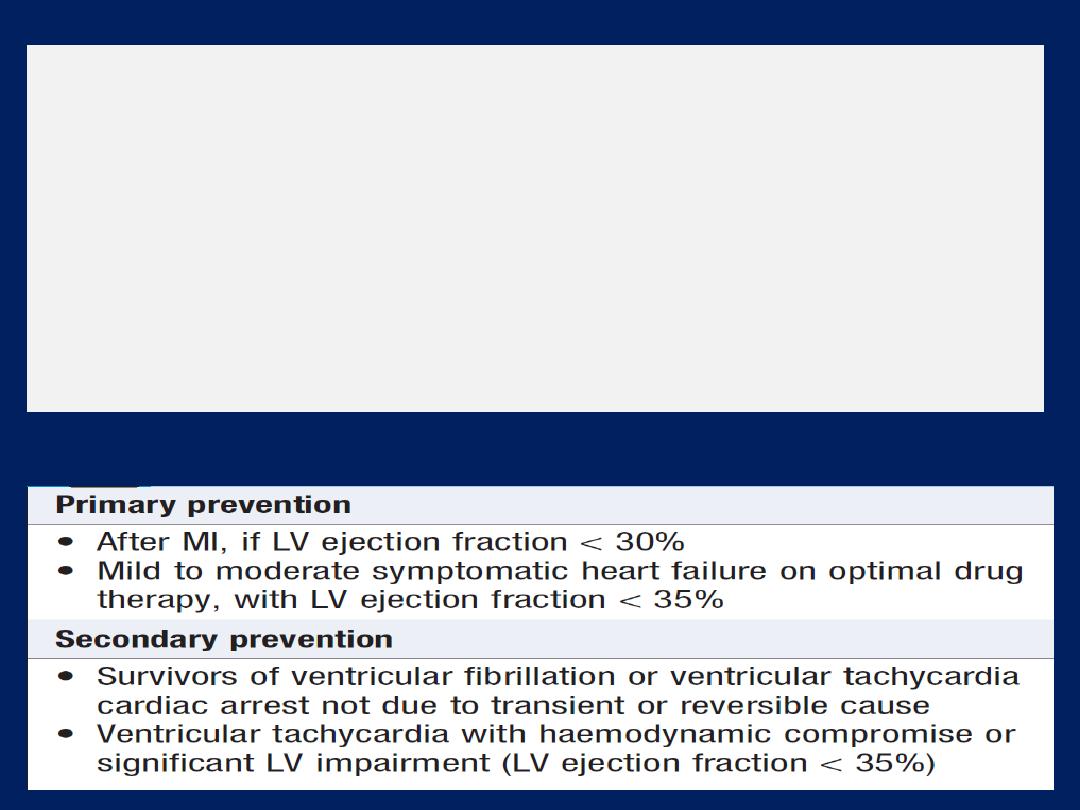
ICDs may be used prophylactically in selected patients
with inherited conditions associated with a high risk of
sudden cardiac death, such as long QT syndrome ,
hypertrophic cardiomyopathy and arrhythmogenic right
ventricular dysplasia . ICD treatment is expensive and so
the indications for which the devices are routinely
implanted depend on the health-care resources available.
Key indications for ICD therapy

Cardiac resynchronisation therapy (CRT)
A treatment for selected patients with heart failure, in whom
cardiac function is further impaired by the presence of
LBBB.This conduction defect is associated with left
ventricular dys-synchrony
(poorly coordinated left ventricular contraction)
and can aggravate heart failure. CRT systems have an
additional lead that is placed via the coronary sinus into one
of the veins on the epicardial surface of the LV .
Simultaneous septal and left ventricular epicardial pacing
resynchronises LV contraction. It improve effort tolerance,
reduce symptoms and are more effective in sinus rhythm
than in those with atrial fibrillation. Most devices are also
defibrillators (CRT-D) because many are predisposed to
ventricular arrhythmias. CRTpacemakers (CRT-P) are used in
patients with relatively low risk of these arrhythmias.

ATHEROSCLEROSIS
Atherosclerosis can affect any artery in the body.
Pathophysiology
Atherosclerosis is a progressive inflammatory disorder
of the arterial wall characterised by focal lipid rich
deposits of atheroma that remain clinically silent
until they become large enough to impair tissue perfusion,
or until ulceration and disruption of the lesion result in
thrombotic occlusion or distal embolisation of the vessel.
Atherosclerosis begins early in life. Abnormalities of arterial
function have been detected among high-risk children and
adolescents, such as cigarette smokers and those with
familial hyperlipidaemia or hypertension.

Early atherosclerosis
Fatty streaks tend to occur at sites of altered arterial
shear stress, such as bifurcations, associated with
abnormal endothelial function. Inflammatory cells,
predominantly monocytes, bind to receptors expressed by
endothelial cells, migrate into the intima, take up oxidised
LDL particles and become lipid-laden macrophages or
foam
cells. Extracellular lipid pools appear in the intimal
space when foam cells die and release their contents.
In response to
cytokines and growth factors produced by
activated macrophages, smooth muscle cells migrate from
the media into the intima, change from a contractile to a
repair phenotype , lipid core will be covered by
smooth muscle cells and matrix, producing a stable
atherosclerotic plaque.
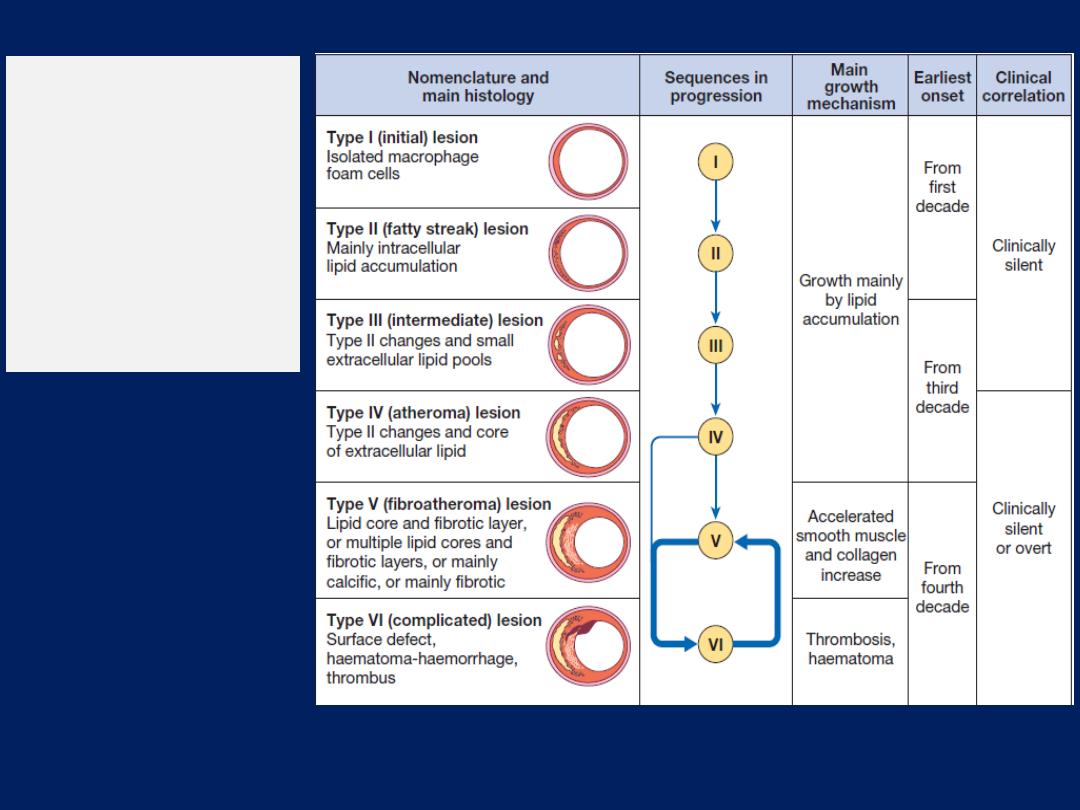
The six stages
of
atherosclerosis.
American Heart
Association
classification.

Advanced atherosclerosis
In an established
atherosclerotic plaque, macrophages
mediate inflammation and smooth muscle cells promote
repair.
If
inflammation predominates, the plaque becomes
active or unstable and may be complicated by ulceration
and thrombosis.
Cytokines,
such as interleukin-1, tumour
necrosis factor-alpha, interferon-gamma, platelet-derived
growth factors, are released by activated macrophages;
they cause the intimal smooth muscle cells overlying the
plaque to become senescent and collagen cross-struts within
the plaque to degrade. This results in
thinning
of the
protective fibrous cap, making the lesion vulnerable to
mechanical stress that ultimately causes
erosion, fissuring or rupture of the plaque surface .

Any breach
in the plaque will expose its contents to blood
and will trigger platelet aggregation and thrombosis that
extend into the atheromatous plaque and the lumen, this
may cause partial or complete obstruction. The number and
complexity of plaques increase with age and with risk
factors.
‘Vulnerable’ plaques
are characterised by a lipid-
rich core, a thin fibrocellular cap, speckled calcification
and an increase in inflammatory cells that release specific
enzymes to degrade matrix proteins. In contrast,
stable plaques
are typified by a small lipid pool, a thick
fibrous cap, heavy calcification and collagenous cross-struts.
Fissuring or rupture tends to occur at sites of maximal
mechanical stressand may be triggered by a surge in BP, as
during exercise or emotional stress.

Surprisingly,
the majority of plaque events are subclinical
and heal spontaneously, although this may allow
thrombus to be incorporated into the lesion, producing
plaque growth and further obstruction to flow.
Atherosclerosis may induce complex changes in the
media
that lead to arterial
remodelling
. Some arterial
segments may slowly constrict (negative remodelling), whilst
others may gradually enlarge (positive remodelling).
These changes are important because they may
amplify or minimise the degree to which atheroma
encroaches into the arterial lumen.

Risk factors
The relative importance of many risk factors have been
defined in animal, epidemiological studies and clinical
interventional trials. Key factors have emerged but do not
explain all the risk, and unknown factors may account for
up to 40% of the variation in risk from one person to the
next.The impact of
genetic risk
is illustrated by twin
studies; a monozygotic twin of an affected individual has
an eightfold increased risk and a dizygotic twin a fourfold
increased risk of dying from CAD , compared to the
general population.
The effect of risk factors is multiplicative rather
than
additive. It is important to distinguish between relative
risk (the proportional increase in risk) and
absolute risk
(the
actual chance
of an event).

Thus, a man of 35 years with a plasma cholesterol of
270 mg/ dL, who smokes 40 cigarettes a day, is relatively
much more likely to die from CAD within the next decade
than a non-smoking woman of the same age with a normal
cholesterol, but the absolute likelihood of his dying during
this time is still small (high relative risk, low absolute risk).
• Age and sex.
the most powerful independent
risk factor. Pre-menopausal women have lower rates of
disease than men, although this sex difference disappears
after the menopause. However, hormone replacement
therapy has no role in the primary or secondary prevention
of CAD, and isolated oestrogen therapy may cause an
increased cardiovascular event rate.

• Family history
. Atherosclerotic vascular disease often
runs in families, due to a combination of shared genetic,
environmental and lifestyle factors. The most common
inherited risk characteristics (hypertension, hyperlipidaemia,
diabetes mellitus) are polygenic. A ‘positive’ family history
is present when clinical problems in first-degree relatives
occur at relatively young age, such as < 50 years for men
and below 55 years for women.
• Smoking.
This is probably the most important avoidable
cause of atherosclerotic vascular disease. There is a strong,
consistent and dose-linked relationship between cigarette
smoking and CAD, especially in younger (< 70 years).
• Hypertension.
The incidence of atherosclerosis increases as
BP rises, and this excess risk is related to both systolic and
diastolic BP, as well as pulse pressure.

Antihypertensive therapy reduces cardiovascular mortality,
stroke and heart failure.
• Hypercholesterolaemia
.Risk rises with increasing serum
cholesterol concentrations. Lowering serum total and LDL
cholesterol concentrations reduces the risk of cardiovascular
events, death, MI, stroke and coronary revascularisation.
• Diabetes mellitus.
This is a potent risk factor for all forms
of atherosclerosis and is often associated with diffuse
disease that is difficult to treat. Insulin resistance
(normal
glucose homeostasis with high levels of insulin)
is associated with
obesity and physical inactivity, and is a risk factor for CAD .
• Haemostatic factors.
Platelet activation and high plasma
fibrinogen concentrations are associated with an increased
risk of coronary thrombosis. Antiphospholipid antibodies
are associated with recurrent arterial thromboses .

• Physical activity.
Physical inactivity roughly doubles the
risk of CAD and is a major risk factor for stroke. Regular
exercise (brisk walking, cycling or swimming for 20 minutes
two or three times a week) has a protective effect that may
be related to increased serum high-density lipoprotein
(HDL) cholesterol concentrations, lower BP, and collateral
vessel development.
• Obesity
, particularly if central or truncal, is an
independent risk factor, although it is often associated with
other adverse factors, such as hypertension, diabetes
mellitus and physical inactivity.
• Alcohol.
associated with reduced rates of coronary
artery disease. Excess alcohol consumption is associated
with hypertension and cerebrovascular disease.

• Other dietary factors.
Diets deficient in fresh fruit,
vegetables and polyunsaturated fatty acids are associated
with an increased risk of CVD. The introduction of a
Mediterranean-style diet reduces CV events. However,
dietary supplements, such as vitamin C and E, beta-
carotene, folate and fish oils, do not reduce cardiovascular
events and, in some cases, have been associated with harm.
• Personality.
Certain personality traits are associated
with an increased risk of coronary disease. Nevertheless,
there is little or no evidence to support the popular belief
that stress is a major cause of CAD.
• Social deprivation.
The impact of established risk factors
is amplified in patients who are socially deprived and
current guidelines recommend that treatment thresholds
should be lowered for them.

Two complementary strategies can be used to prevent
atherosclerosis in apparently healthy but at-risk
individuals:
population
and
targeted
strategies.
The population strategy
aims to modify the risk factors of
the whole population through diet and lifestyle advice, on
the basis that even a small reduction in smoking or average
cholesterol, or modification of exercise and diet will
produce worthwhile benefits . Some risk factors for
atheroma, such as obesity and smoking, are also associated
with a higher risk of other diseases and should be actively
discouraged through public health measures. Legislation
restricting smoking in public places is associated with
reductions in rates of MI.

The targeted strategy
aims to identify and treat
high-risk individuals, who usually have a combination
of risk factors and can be identified by using composite
scoring systems . It is important to consider the absolute
risk of atheromatous CVD before contemplating specific
antihypertensive or lipid-lowering therapy because this will
help to determine whether the possible benefits of
intervention are likely to outweigh the expense,
inconvenience and possible side-effects.
Population advice to prevent CAD

Secondary prevention
Energetic correction of modifiable risk factors, particularly
smoking, hypertension and hypercholesterolaemia, is
particularly important. All patients with CAD should be
given statin therapy,irrespective of their serum cholesterol .
BP treated to a target of 140/85 mmHg or lower .
Aspirin and ACE inhibitors are of benefit in patients with
evidence of vascular disease. Betablockers benefit patients
with a history of MI or heart failure.
Patients who have just survived an MI or undergone
bypass surgery are usually keen to help themselves and
may be particularly receptive to lifestyle advice, such as
dietary modification and smoking cessation.
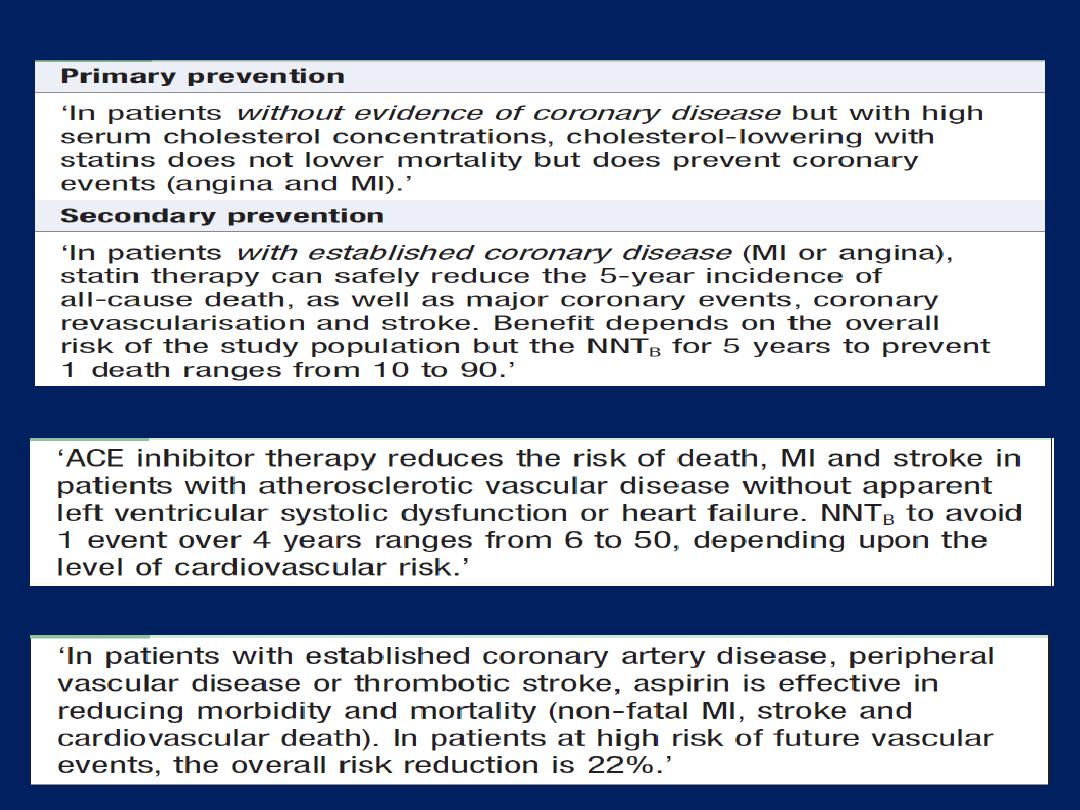
Use of statins in prevention of atherosclerotic disease
ACE I and secondary prevention of atherosclerotic disease
Aspirin and secondary prevention in atherosclerotic vascular disease

CORONARY ARTERY DISEASE (CAD)
CAD is the most common form of heart disease and the
single most important cause of premature death in
Europe, Russia, North and South America. Disease of the
coronary arteries is almost always due to atheroma and its
complications, particularly thrombosis . Occasionally, the
coronary arteries are involved in aortitis, polyarteritis and
other connective tissue disorders.
Stable angina
Angina pectoris is the symptom complex caused by
transient myocardial ischaemia. It may occur whenever
there is an imbalance between myocardial oxygen
supply and demand . Coronary atheroma is by far the
most common cause, although it may be a manifestation
of other heart diseases, aortic valve disease, HOCM.

Clinical features
Stable angina is characterised by central chest pain,
discomfort or breathlessness that is precipitated by
exertion or other forms of stress, and is promptly relieved
by rest. Some patients find the discomfort comes when
they start walking, and that later it does not return
despite greater effort (‘warm-up angina’).
Physical examination is frequently unremarkable but
should include a careful search for evidence of valve
disease (particularly aortic), important risk factors (e.g.
hypertension, diabetes mellitus), left ventricular
dysfunction (cardiomegaly, gallop rhythm), other
manifestations of arterial disease (carotid bruits,
peripheral vascular disease) and unrelated conditions
that may exacerbate angina (anaemia, thyrotoxicosis).
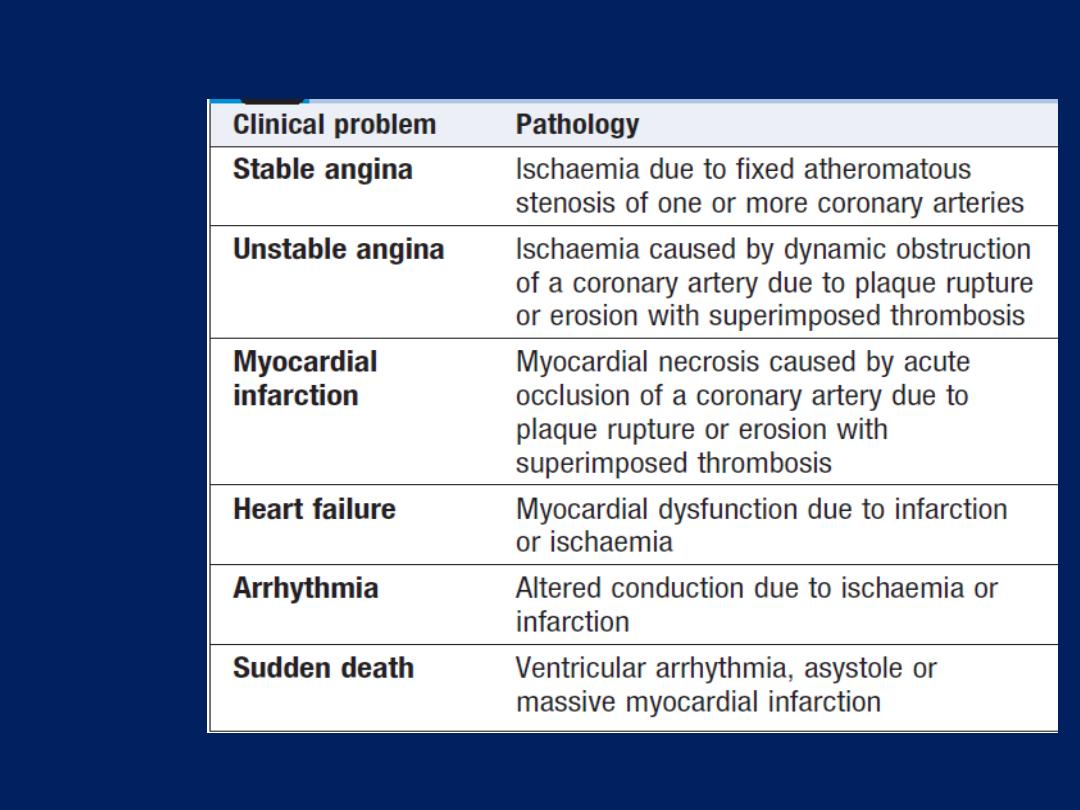
Coronary artery disease: clinical manifestations and
pathology
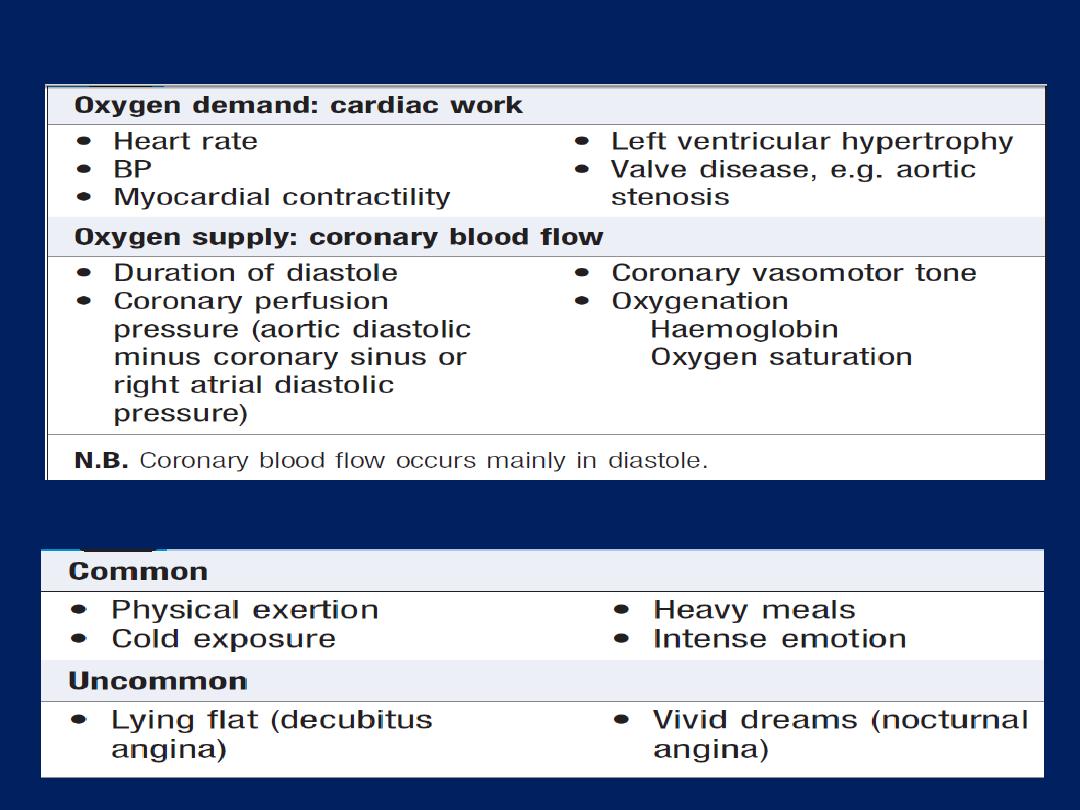
Factors influencing myocardial oxygen supply and demand
Activities precipitating angina

Investigations
Resting ECG
The ECG may be normal or show evidence of previous MI.
The most convincing ECG evidence of myocardial
ischaemia is the demonstration of reversible ST segment
depression or elevation, with or without T-wave inversion,
at the time the patient is experiencing symptoms
(spontaneous or induced by exercise testing).
Exercise ECG
An exercise tolerance test (ETT) is usually performed
using a standard treadmill or bicycle ergometer protocol
while monitoring the patient’s ECG, BP and general
condition. Planar or down-sloping ST segment depression
of 1 mm or more is indicative of ischaemia. Up-sloping ST
depression is less specific and often occurs in normal.

Exercise testing is also a useful means of assessing the
severity of coronary disease and identifying high-risk
individuals . Exercise testing is not infallible and may
produce false-positive results in the presence of digoxin
therapy, left ventricular hypertrophy, bundle branch block
or WPW syndrome.
The predictive accuracy of exercise testing is lower in
women than in men. The test should be classed
as inconclusive (rather than negative) if the patient
cannot achieve an adequate level of exercise because of
locomotor or other non-cardiac problems.
Forms of exercise-induced ST depression.
A
Planar ST depression is usually indicative of myocardial
ischaemia.
B
Downsloping depression also usually indicates
myocardial ischaemia.
C
Up-sloping depression may be a normal finding.
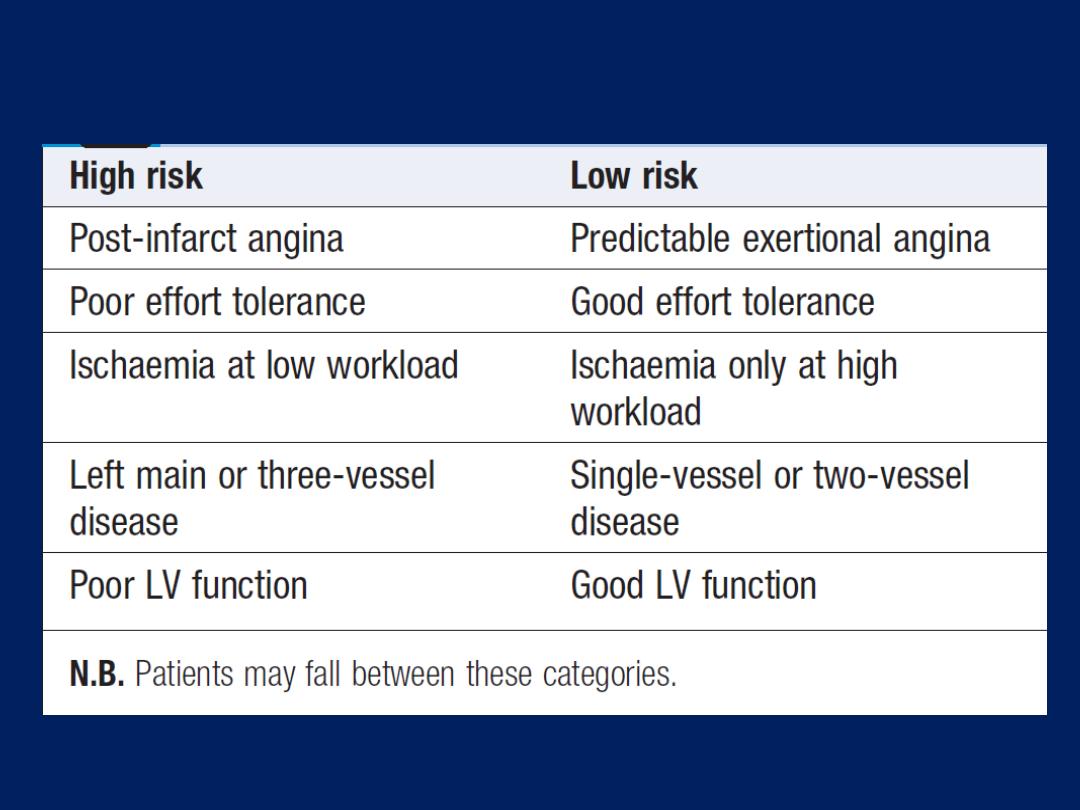
Risk stratification in stable angina
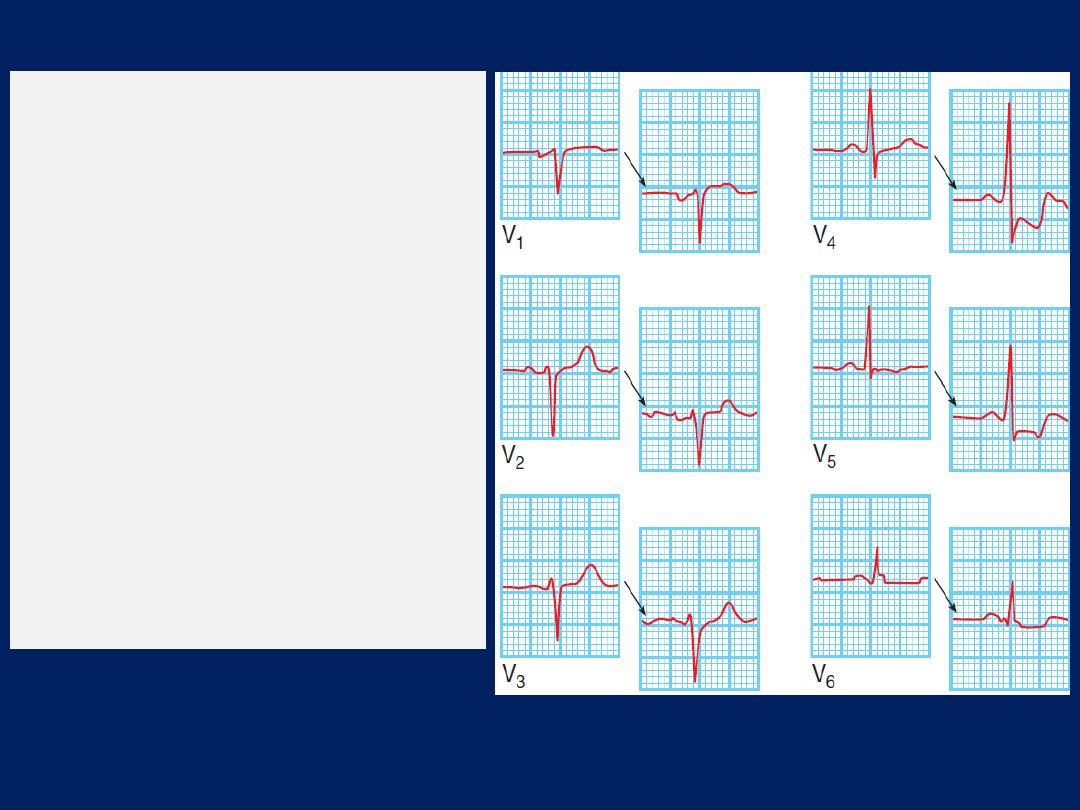
A positive exercise test
(chest
leads only). The resting
12-lead ECG shows some minor
T-wave changes in the
inferolateral leads but is
otherwise normal.
After 3 minutes’ exercise on a
treadmill, there is marked
planar ST depression in leads
V4 and V5 (right offset).
Subsequent coronary
angiography revealed critical
three-vessel CAD .

Other forms of stress testing
• Myocardial perfusion scanning.
This may be helpful in
the evaluation of patients with an equivocal or
uninterpretable exercise test and those who are unable to
exercise .It entails obtaining scintiscans of the myocardium
at rest and during stress (either exercise testing or
pharmacological stress, such as a controlled infusion of
dobutamine), after the administration of an IV radioactive
isotope, such as
99
technetium tetrofosmin. Thallium and
tetrofosmin are taken up by viable perfused myocardium. A
perfusion defect present during stress but not at rest
provides evidence of reversible myocardial ischaemia ,
whereas a persistent perfusion defect seen during both
phases of the study is usually indicative of previous MI.
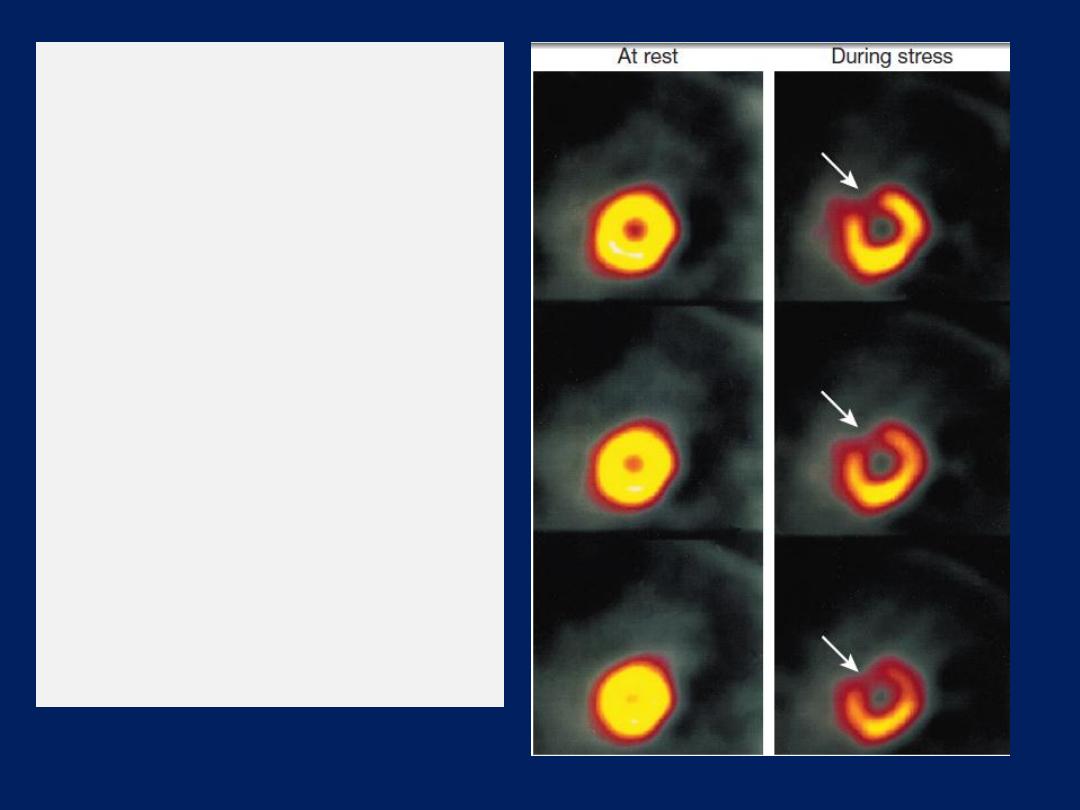
A myocardial perfusion scan
showing reversible anterior
myocardial ischaemia. The
images are cross-sectional
tomograms of the LV. The
resting scans (left) show even
uptake of the
99
technetium-
labelled tetrofosmin and look
like doughnuts. During stress
(e.g. a dobutamine infusion),
there is reduced uptake of
technetium, particularly along
the anterior wall (arrows), and
the scans look like
crescents (right).

• Stress echocardiography.
This is an alternative to
myocardial perfusion scanning and can achieve similar
accuracy. It uses transthoracic echocardiography to
identify ischaemic (do not contract at rest or during stress) .
• Coronary arteriography
This provides detailed anatomical information about
the extent and nature of CAD and is usually performed
with a view to CABG surgery or PCI . In some patients,
diagnostic coronary angiography may be indicated when
non-invasive tests have failed to establish the cause of
atypical chest pain, performed under local anaesthesia
and requires specialized radiological equipment, cardiac
monitoring and an experienced operating team.
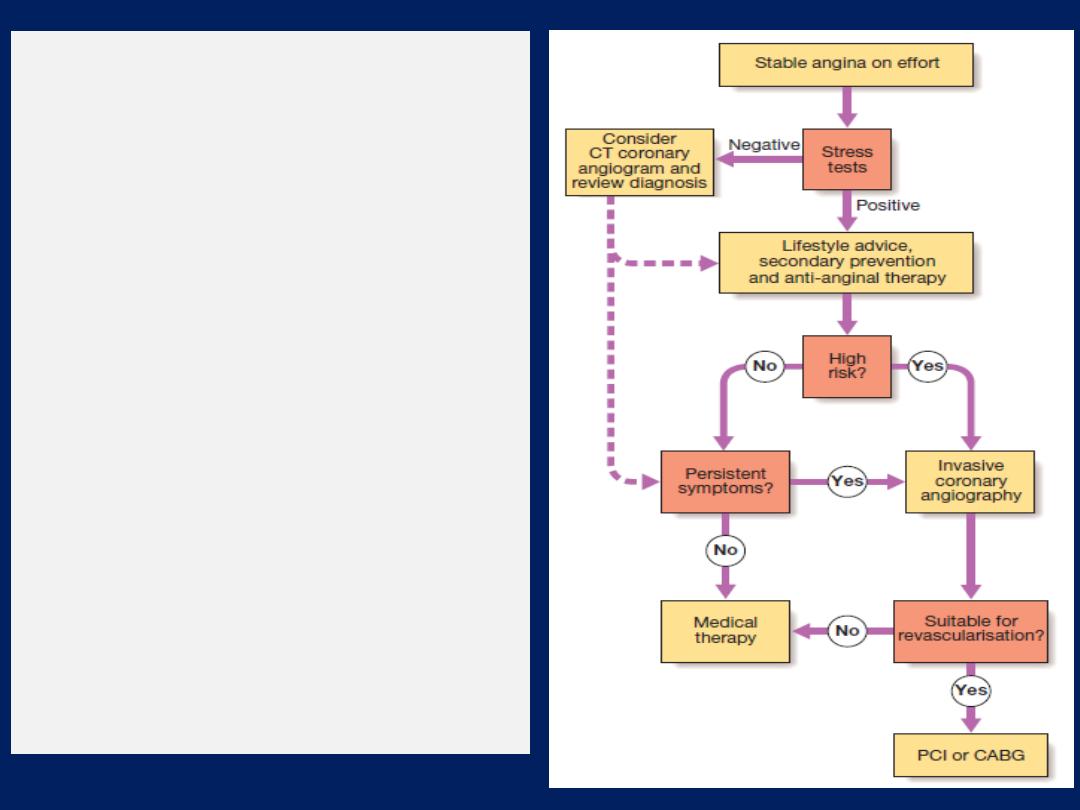
A scheme for the
investigation and treatment
of stable angina.
The
selection of percutaneous
coronary intervention (PCI) or
coronary artery bypass
grafting (CABG) depends
upon patient choice, coronary
artery anatomy and extent
of coronary artery disease.
In general, left main stem and
three-vessel coronary artery
disease should be treated by
CABG surgery.

Management:
general measures
• a careful assessment of severity of arterial disease
• the identification and control of risk factors
• the use of measures to control symptoms
• the identification of high-risk patients for treatment
to improve life expectancy.
Symptoms alone are a poor guide to the extent.
Stress testing is therefore advisable in all patients who
are potential candidates for revascularisation.
Management should start with a careful explanation of
the problem and a discussion of the potential lifestyle
and medical interventions that may relieve symptoms
and improve prognosis .

Advice to patients with stable angina

Anxiety and misconceptions often contribute to
disability; for example, some patients avoid all forms of
exertion because they believe that each attack of angina
is a ‘mini heart attack’ that results in permanent damage.
Effective management of these psychological factors can
make a huge difference to the patient’s quality of life.
Antiplatelet therapy
Low-dose (75 mg) aspirin reduces the risk of adverse
events such as MI and should be prescribed for all
patients with CAD indefinitely .
Clopidogrel (75 mg daily) is an equally effective that
can be prescribed if aspirin causes troublesome
dyspepsia or other side-effects.

Anti-anginal drug treatment
Five groups
of drug are used to help relieve or prevent
the symptoms of angina: nitrates, ββ, calcium antagonists,
potassium channel activators and an
I
f
channel antagonist.
Nitrates
Act directly on vascular smooth muscle to produce
venous and arteriolar dilatation. Their beneficial effects
are due to a reduction in myocardial oxygen demand
(lower pre and afterload) and an increase in myocardial
oxygen supply (coronary vasodilatation). Sublingual
glyceryl trinitrate (GTN), administered from a metered-
dose aerosol
(400 μg per spray)
or as a tablet
(300 or 500 μg),
relieve
an attack of angina in 2–3 minutes. Side-effects include
headache, symptomatic hypotension and, rarely, syncope.

Patients should be
encouraged
to use the drug
prophylactically before taking exercise that is liable to
provoke symptoms. Sublingual GTN has a short duration
of action , however, a variety of alternative nitrate
preparations can provide a more prolonged therapeutic
effect.
GTN
can be given transcutaneously as a patch
(5–10
mg daily)
, or as a slow-release buccal tablet
(1–5 mg 4 times daily).
GTN undergoes extensive first-pass metabolism in the liver
and is ineffective when swallowed. Other nitrates, such as
isosorbide dinitrate
(10–20 mg 3 times daily)
and isosorbide
mononitrate
(20–60 mg once or twice daily),
can be given by mouth.
Headache is common but tends to diminish if the patient
perseveres with the treatment.

Continuous nitrate therapy can cause pharmacological
tolerance. This can be avoided by
a 6–8-hour nitrate-free
period,
best achieved at night when the patient is inactive.
If nocturnal angina is a predominant symptom, long acting
nitrates can be given at the end of the day.
Beta-blockers
These lower myocardial oxygen demand by reducing
heart rate, BP and myocardial contractility, but they may
provoke bronchospasm in patients with asthma. In theory,
non-selective ββ may aggravate coronary vasospasm by
blocking the coronary artery β
2
- adrenoceptors and so a
once-daily cardioselective preparation is used (e.g. slow-
release metoprolol 50–200 mg, bisoprolol 5–15 mg daily).

Beta-blockers should not be withdrawn abruptly as
rebound effects may precipitate dangerous arrhythmias,
worsening angina or MI:
the ββ withdrawal syndrome.
Calcium channel antagonists
These drugs inhibit the slow inward current caused by
the entry of extracellular calcium through the cell
membrane of excitable cells, particularly cardiac and
arteriolar smooth muscle, and lower myocardial oxygen
demand by reducing BP and myocardial contractility.
Dihydropyridine calcium antagonists, such as nifedipine
and nicardipine, often cause a reflex tachycardia.
This may be counterproductive and it is best to use
them in combination with a ββ.

In contrast, verapamil and diltiazem are particularly
suitable for patients who are not receiving a β-blocker
(e.g. those with airways obstruction) because they slow
SA node firing, inhibit conduction through the AV node
and tend to cause a bradycardia. They reduce
myocardial contractility and can aggravate or precipitate
heart failure. Other unwanted effects include peripheral
oedema, flushing, headache and dizziness .
Potassium channel activators
These have arterial and venous dilating properties but
do not exhibit the tolerance seen with nitrates.
Nicorandil
(10–30 mg twice daily orally)
is the only drug in this
class currently available for clinical use.
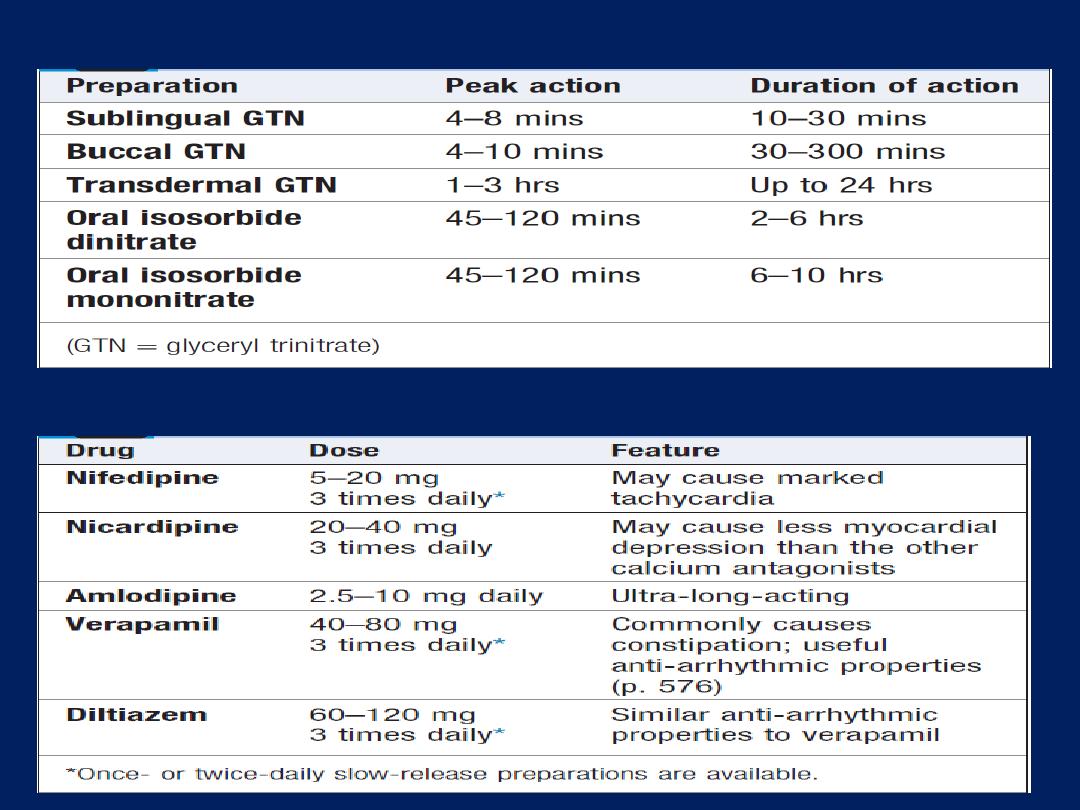
Duration of action of some nitrate preparations
Calcium channel antagonists used for the treatment of angina

I
f
channel antagonist
Ivabradine
. It induces bradycardia by modulating ion
channels in the sinus node. In contrast to β-blockers and
rate-limiting calcium antagonists, it does not have other
cardiovascular effects. It appears to be safe to use in
patients with heart failure.
Although each of these anti-anginal drugs
is superior to
placebo, there is little evidence that one group is more
effective than another. It is conventional to start with low-
dose aspirin, a statin, sublingual GTN and a ββ, and then
add a CC antagonist or a long-acting nitrate later, if
needed. The goal is the control of angina with minimum
side-effects and the simplest drug regimen.

There is little evidence that multiple anti-anginal drugs is
of benefit, and revascularisation should be considered if
an appropriate combination of two or more drugs fails to
achieve an acceptable symptomatic response.
Invasive treatment
Percutaneous coronary intervention (PCI)
PCI is performed by passing a fine guidewire across a
coronary stenosis under radiographic control and using it
to position a balloon, which is then inflated to dilate the
stenosis . A coronary stent is a piece of coated metallic
‘scaffolding’ that can be deployed on a balloon and used
to maximise and maintain dilatation of a stenosed vessel.
The routine use of stents in appropriate vessels reduces
both acute complications and the incidence of clinically
important re-stenosis .

PCI provides an effective symptomatic treatment
but
definitive evidence
that it improves survival in patients
with chronic stable angina is
lacking.
It is mainly used in single- or two-vessel disease. Stenoses in
bypass grafts can be dilated, as well as those in the native
coronary arteries. Coronary surgery is usually the
preferred option in patients with three-vessel or left main
stem disease, although recent trials have demonstrated
that PCI is also feasible in such patients.
The main acute complications of PCI
are occlusion of the
target vessel or a side branch by thrombus or a loose flap
of intima (coronary artery dissection), and consequent
myocardial damage. This occurs in about 2–5% of
procedures and can often be corrected by deploying a
stent; however, emergency CABG is sometimes required.

Minor myocardial damage, as indicated by elevation of
sensitive intracellular markers , occurs in up to 10% .
The main long-term complication
of PCI is re-stenosis , in
up to one-third of cases. This is due to a combination of
elastic recoil and smooth muscle proliferation (neo-intimal
hyperplasia) and tends to occur within 3 months.
Stenting
substantially reduces
the risk of re-stenosis, probably
because it allows the operator to achieve more complete
dilatation in the first place. Drug-eluting stents reduce this
risk even further by allowing an antiproliferative drug,
e.g. sirolimus or paclitaxel, to elute slowly from the coating
and prevent neo-intimal hyperplasia and in-stent re-
stenosis. There is an increased risk of late drug-stent
thrombosis, although the absolute risk is small(< 0.5%).

Recurrent angina
(affecting up to 15–20% of patients
receiving an intracoronary stent at 6 months) may require
further PCI or bypass grafting.
The risk of complications
and the likely success of the
procedure are closely related to the morphology of the
stenoses, the experience of the operator and the presence
of important comorbidity, e.g. diabetes, peripheral arterial
disease. A good outcome is less likely if the target lesion
is complex, long, eccentric or calcified, lies on a bend or
within a tortuous vessel, involves a branch or contains acute
thrombus.
In combination
with aspirin and heparin,
adjunctive therapy with potent platelet inhibitors, such as
clopidogrel or glycoprotein IIb/ IIIa receptor antagonists,
improves the outcome of PCI, with lower short- and
long-term rates of death and MI.
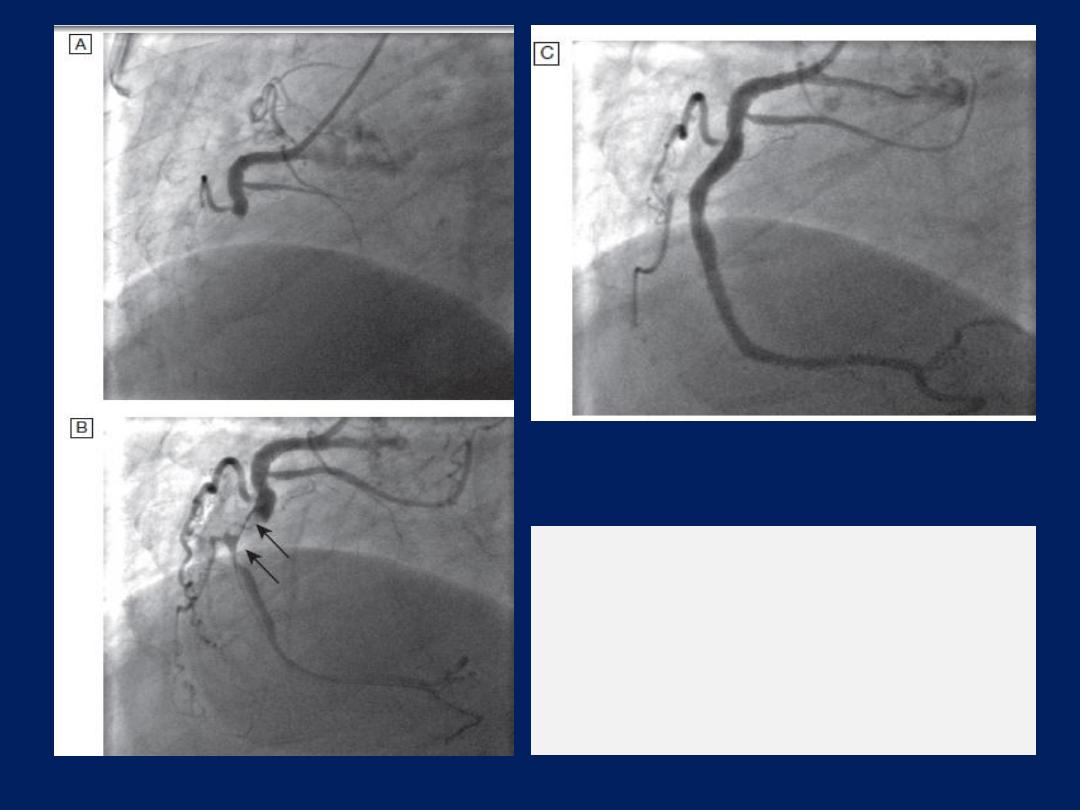
Primary PCI.
A Acute right coronary artery occlusion.
B Initial angioplasty demonstrates a
large thrombus filling defect (arrows).
C Complete restoration of normal flow
following intracoronary stenting.

Angioplasty and intracoronary stents in angina
PCI vs medical therapy in stable angina

Coronary artery bypass grafting
The internal mammary arteries, radial arteries or
reversed segments of the patient’s own saphenous vein
can be used to bypass coronary artery stenoses .This
usually involves major surgery under cardiopulmonary
bypass but, in some cases, grafts can be applied to the
beating heart: ‘off-pump’ surgery. The operative
mortality is approximately 1.5% but risks are higher in
elderly patients, those with poor left ventricular
function and those with significant comorbidity, such
as renal failure.
Approximately 90% of patients are free of angina 1
year after CABG surgery, but fewer than 60% of
patients are asymptomatic after 5 or more years.

Early postoperative angina
is usually due to graft failure
arising from technical problems during the operation,
or poor ‘run-off’ due to disease in the distal native
coronary vessels. Late recurrence of angina may be due
to progressive disease in the native coronary arteries or
graft degeneration.
Fewer than 50% of vein grafts are patent 10 years
after surgery. However, arterial grafts have a much
better long-term patency rate, with more than 80% of
internal mammary artery grafts patent at 10 years.

This has led many surgeons to consider total arterial
revascularisation during CABG surgery. Aspirin
(75–150 mg daily) and clopidogrel (75 mg daily) both
improve graft patency, and one or other should be
prescribed indefinitely, if well tolerated. Intensive lipid
lowering therapy slows the progression of disease in the
native coronary arteries and bypass grafts, and reduces
clinical cardiovascular events. There is substantial excess
cardiovascular morbidity and mortality in patients who
continue to smoke after bypass grafting. Persistent
smokers are twice as likely to die in the 10 years
following surgery than those who give up at surgery.

CABG improves survival in
symptomatic patients with left
main stem stenosis or three-vessel coronary disease (i.e.
involving LAD, CX and right coronary arteries) or two-vessel
disease involving the proximal LAD coronary artery.
Improvement in survival is most marked in those with
impaired left ventricular function or positive stress testing
prior to surgery and in those who have undergone left
internal mammary artery grafting.
Neurological complications are common, with a
1–5% risk of peri-operative stroke. Between 30% and
80% of patients develop short-term cognitive impairment
that typically resolves within 6 months. There are
also reports of long-term cognitive decline that may be
evident in more than 30% of patients at 5 years.

Coronary artery bypass grafting for stable angina
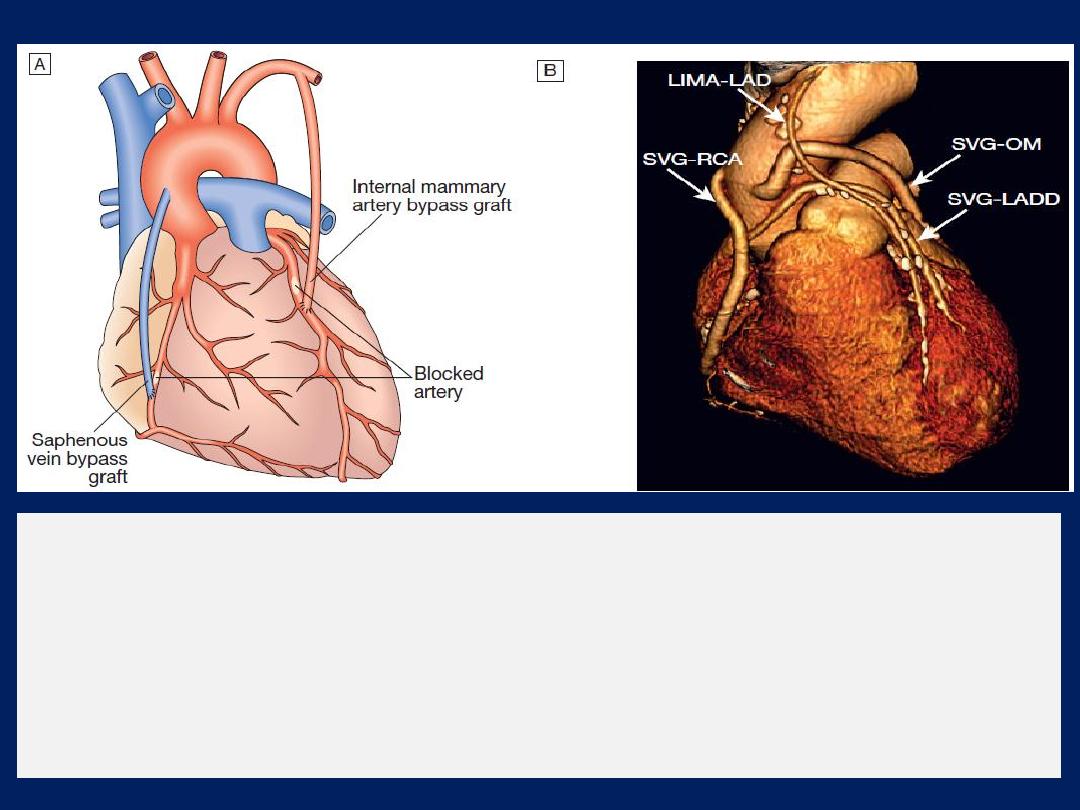
Coronary artery bypass graft surgery.
A
Narrowed or stenosed arteries are bypassed using saphenous vein grafts
connected to the aorta: or by utilising the internal mammary artery.
B
Three dimensional reconstruction of multidetector CT of the heart. The image shows
the patent saphenous vein grafts (SVG) to the right coronary artery (RCA), obtuse
marginal branch (OM) and diagonal branch (LADD), and left internal mammary
artery graft (LIMA) to the left anterior descending (LAD) coronary artery.
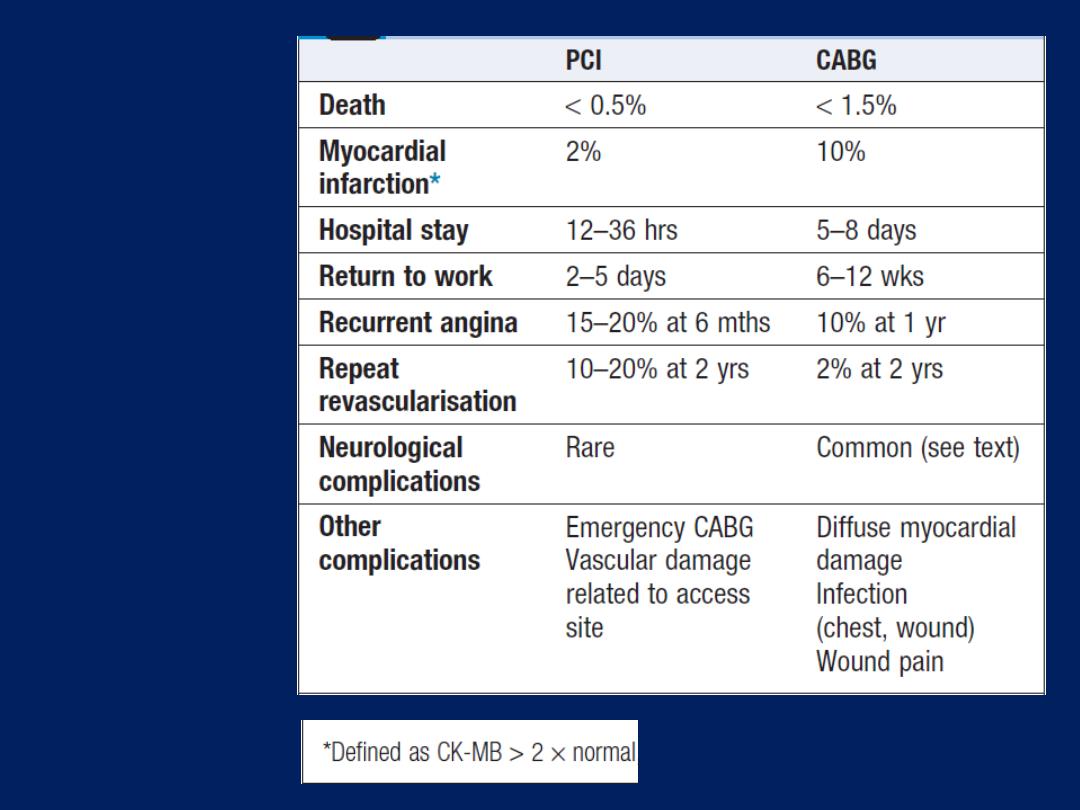
Comparison
of PCI and
CABG

Comparison of PCI and CABG surgery in
stable angina

Prognosis
Symptoms are a poor guide to prognosis;
nevertheless, the 5-year mortality of patients with
severe angina (NYHA class III or IV) is nearly
double that of patients with mild symptoms.
Exercise testing and other forms of stress testing
are much more powerful predictors of mortality;
for example, in one study, the 4-year mortality of
patients with stable angina and a negative
exercise test was 1%, compared to more than
20% in those with a strongly positive test.

In general, the prognosis
of coronary artery disease
is related to the number of diseased vessels and the
degree of left ventricular dysfunction. A patient with
single-vessel disease and good left ventricular function
has an excellent outlook (5-year survival > 90%),
whereas a patient with severe left ventricular
dysfunction
and extensive
three-vessel
disease has a
poor prognosis (5-year survival < 30%) without
revascularisation.
Spontaneous symptomatic improvement due to the
development of collateral vessels is common.

Angina with normal coronary arteries
Approximately
10%
of patients who report stable angina
on effort will have angiographically normal coronary
arteries. Many of these patients are women and it is
important to review the original diagnosis and explore
other potential causes.
Coronary artery spasm
Vasospasm in coronary arteries may coexist with atheroma,
especially in unstable angina; in <1% of cases, vasospasm
may occur without angiographically detectable atheroma.
This is sometimes known as variant angina, and may be
accompanied by spontaneous and transient ST elevation on
the ECG (Prinzmetal’s angina). Calcium channel
antagonists, nitrates and other coronary vasodilators are
the most useful therapeutic agents but may be ineffective.

Syndrome X
The constellation of typical angina on effort, objective
evidence of myocardial ischaemia on stress testing, and
angiographically normal coronary arteries is sometimes
known as syndrome X.
This disorder is poorly understood but carries a good
prognosis and may respond to treatment with anti-
anginal therapy.

Acute coronary syndrome (ACS )
Is a term that encompasses both unstable angina and
myocardial infarction (MI). It is characterised by new-onset
or rapidly worsening angina (crescendo angina), angina
on minimal exertion or angina at rest in the absence of
myocardial damage. In contrast, MI occurs when symptoms
occur at rest and there is evidence of myocardial necrosis,
as demonstrated by an elevation in cardiac troponin or
creatine kinase-MB isoenzyme .
ACS may present as a new phenomenon or against a
background of chronic stable angina. The culprit lesion is
usually a complex ulcerated or fissured atheromatous
plaque with adherent platelet-rich thrombus and local
coronary artery spasm .

This is a
dynamic process
whereby the degree of
obstruction
may either increase, leading to complete
vessel occlusion, or regress due to the effects of platelet
disaggregation and endogenous fibrinolysis. In acute MI,
occlusive thrombus is almost always present at the site of
rupture or erosion of an atheromatous plaque.
Without treatment, the infarct-related artery remains
permanently occluded in 20–30% of patients. The process
of infarction progresses over several hours and most
patients present when it is still possible to salvage
myocardium and improve outcome.

Clinical features
Pain is the cardinal symptom of an ACS but breathlessness,
vomiting and collapse are common features .The pain
occurs in the same sites as angina but is usually more
severe and lasts longer; it is often described as a tightness,
heaviness or constriction in the chest. In acute MI, the pain
can be excruciating, and the patient’s expression and
pallor may vividly convey the seriousness of the situation.
Painless or ‘silent’ MI is
particularly common in older
patients or those with diabetes mellitus. If syncope
occurs, it is usually due to an arrhythmia or profound
hypotension. Vomiting and sinus bradycardia are often
due to vagal stimulation and are particularly common
in patients with inferior MI.

Nausea and vomiting may also be caused or aggravated
by opiates given for pain relief. Sometimes infarction
occurs in the absence of physical signs. Sudden death,
from ventricular fibrillation or asystole, may occur
immediately and often within the first hour. If the patient
survives the critical stage, the liability to dangerous
arrhythmias remains, but diminishes as each hour goes by.
It is vital that patients know not to delay calling for help if
symptoms occur. The development of cardiac failure
reflects the extent of ischaemia and is the major cause of
death in those who survive the first few hours.

Diagnosis and risk stratification
The differential diagnosis is wide and includes most
causes of central chest pain or collapse .
The assessment of acute chest pain depends heavily on
an analysis of the character of the pain and its associated
features, evaluation of the ECG, and serial measurements
of biochemical markers of cardiac damage, such
as troponin I and T. A 12-lead ECG is mandatory and
defines the initial triage, management and treatment.
Patients with ST-segment elevation or new bundle
branch block require emergency reperfusion therapy .In
patients with ACS without ST-segment elevation, the ECG
may show transient or persistent ST–T wave changes,
including ST depression and T-wave inversion.

Approximately 12% of patients will die within 1 month and
a fifth within 6 months of the index event.
The risk markers
that are indicative of an adverse prognosis
include
recurrent ischaemia, extensive ECG changes at rest or
during pain, the release of biochemical markers (creatine
kinase or troponin), arrhythmias, recurrent ischaemia and
haemodynamic complications (e.g. hypotension, mitral
regurgitation) during episodes of ischaemia. Risk
stratification is important because it guides the use of more
complex pharmacological and interventional treatment.
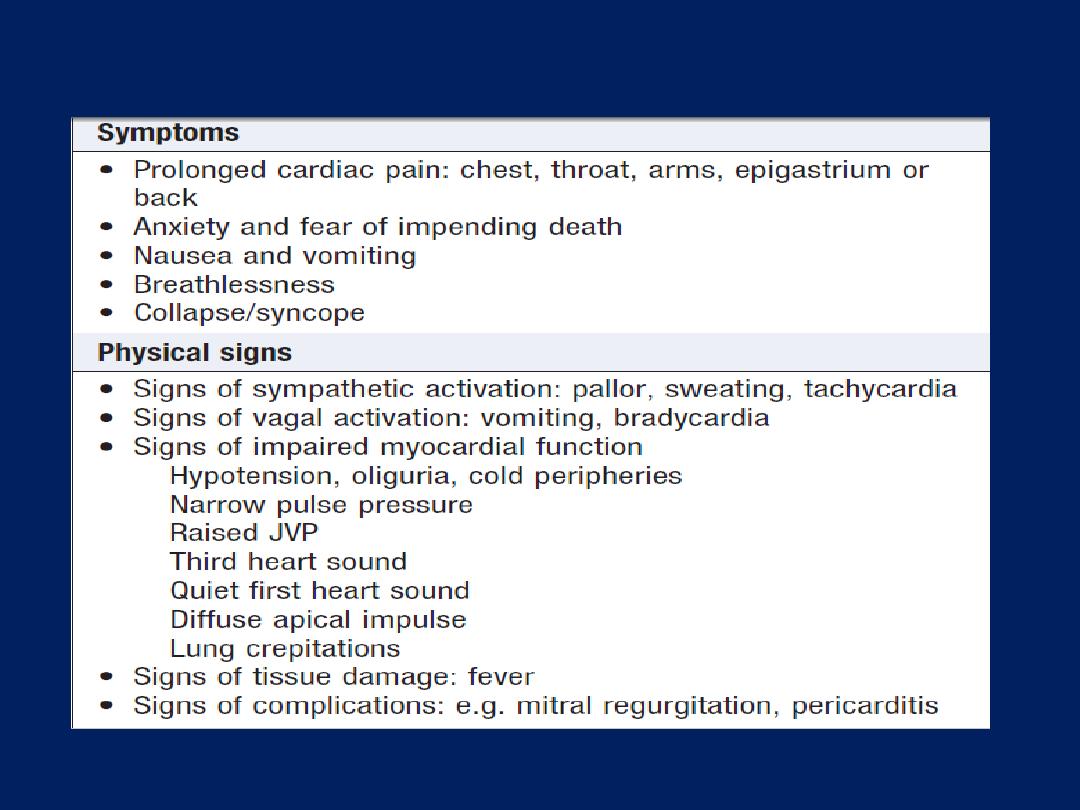
Clinical features of acute coronary syndromes
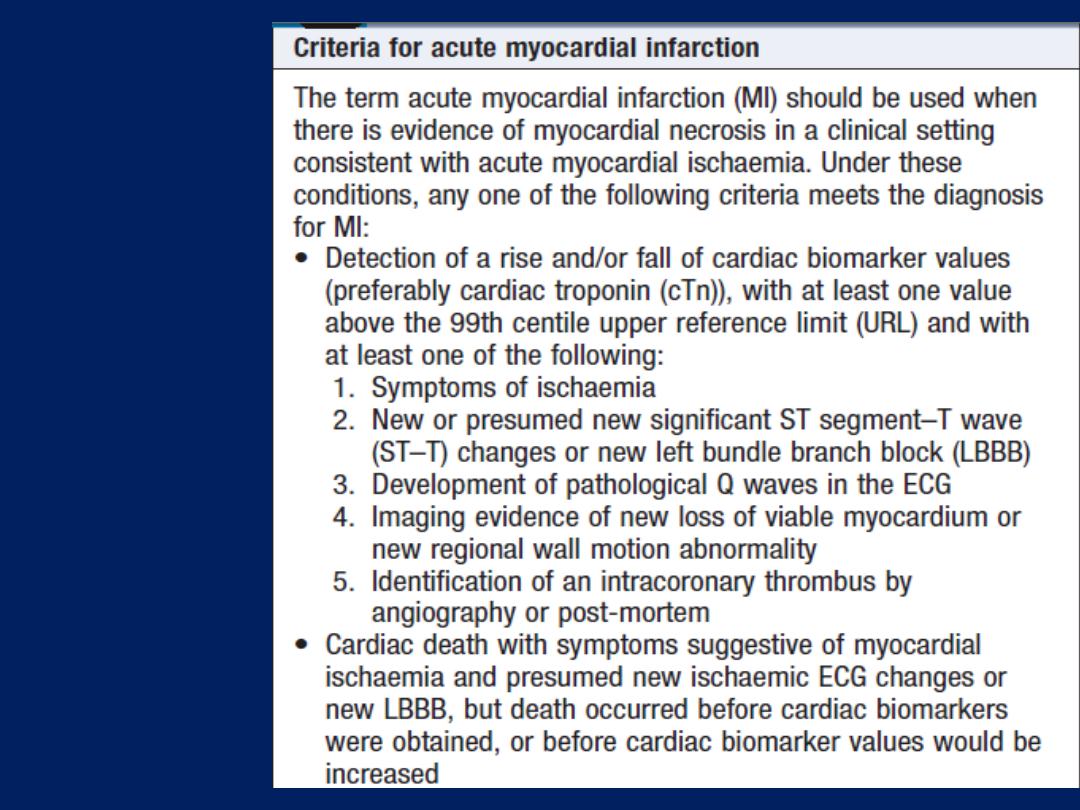
Universal
definition
of
myocardial
infarction
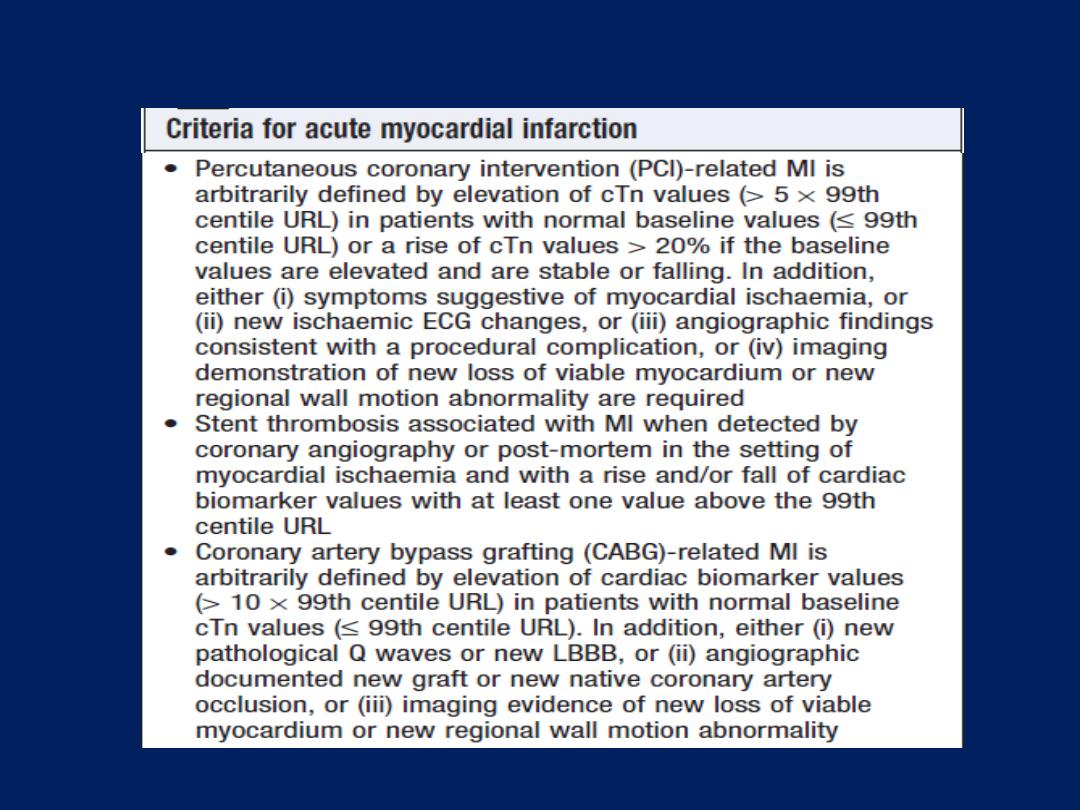
Universal definition of myocardial infarction 'cont'd
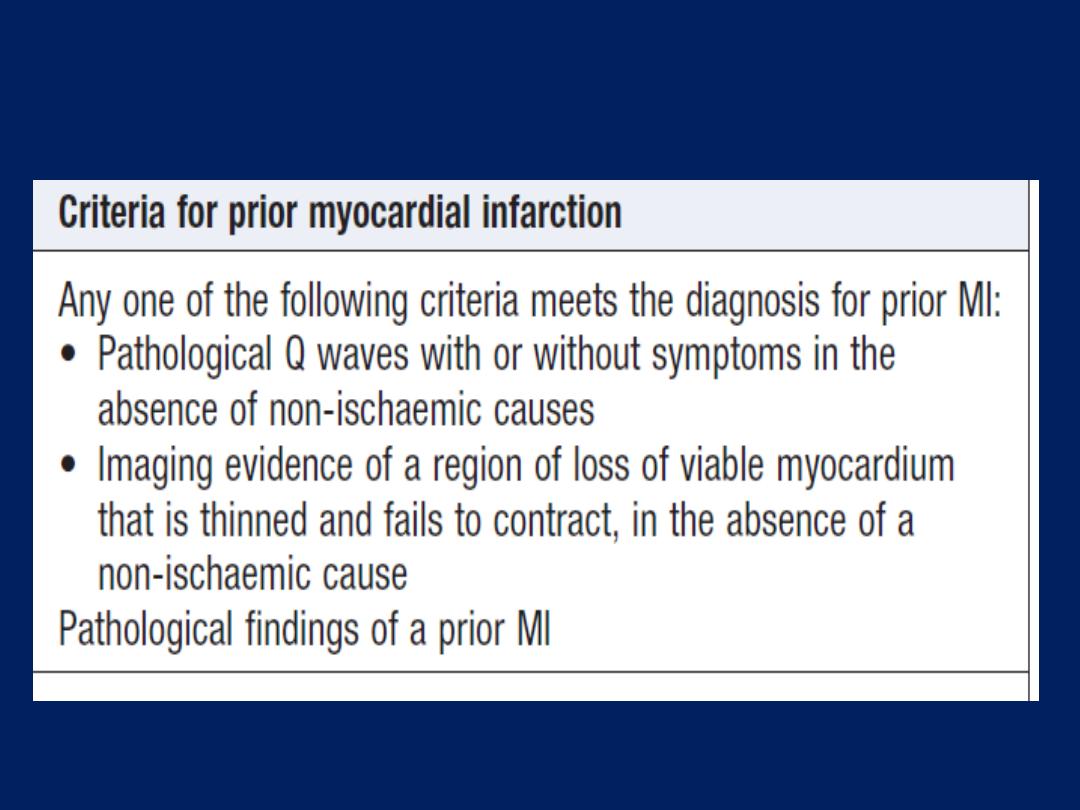
Universal definition of myocardial infarction'cont'd
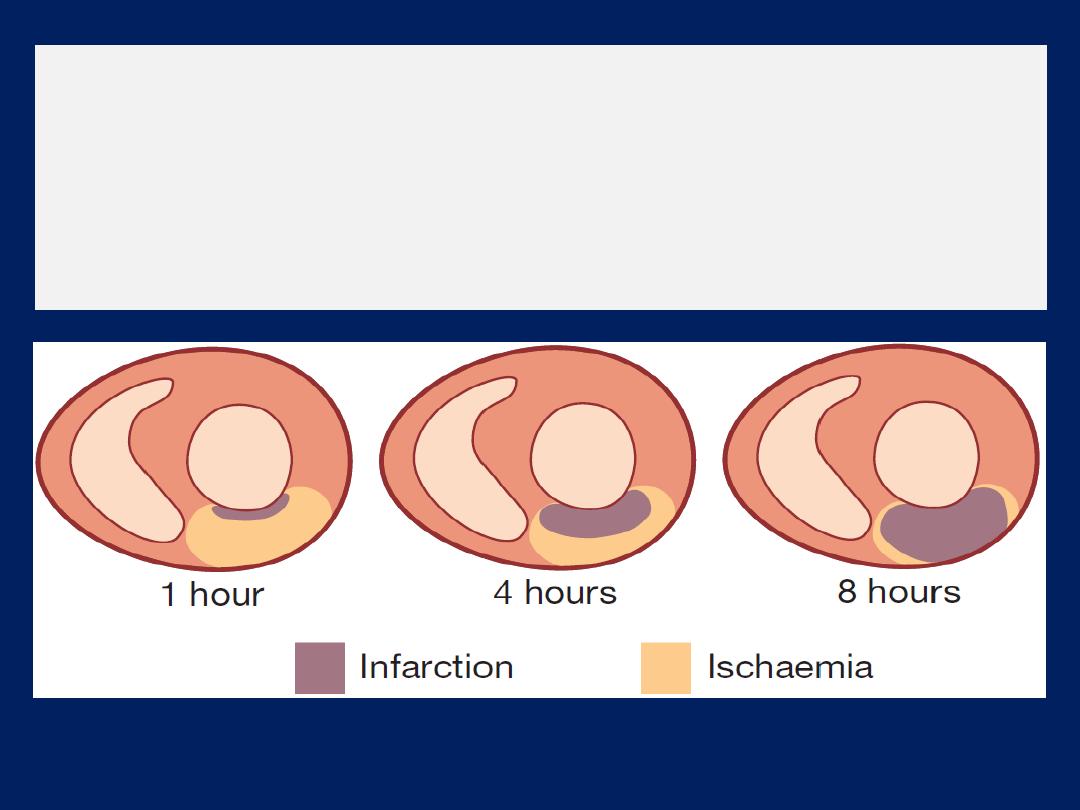
The time course of MI.
The relative proportion of ischaemic, infarcting and
infarcted tissue slowly changes over a period of 12 hours.
In the early stages of MI, a significant proportion of the
myocardium in jeopardy is potentially salvageable.

Investigations :
Electrocardiography
The ECG is central to confirming the diagnosis but may
be difficult to interpret if there is BBB or previous MI.
Repeated ECGs are important, especially where the
diagnosis is uncertain or the patient has recurrent or
persistent symptoms. The earliest ECG change is usually ST-
segment deviation. With proximal occlusion of a major
coronary artery, ST-segment elevation (or new bundle
branch block) is seen initially, with later diminution in the size
of the R wave and, in transmural (full-thickness) infarction,
development of a Q wave.
Subsequently, the T wave becomes inverted because of a
change in ventricular repolarisation; this change persists
after the ST segment has returned to normal.

In non-ST segment elevation ACS, there is partial
occlusion of a major vessel or complete occlusion of a
minor vessel, causing unstable angina or partial-thickness
(subendocardial) MI. This is usually associated with ST-
segment depression and T-wave changes.
Anteroseptal infarction, abnormalities are found in one or more
leads from V1 to V4, while anterolateral infarction produces changes
from V4 to V6, in aVL and in lead I. Inferior infarction is best shown
in leads II, III and aVF, while, at the same time, leads I, aVL and the
anterior chest leads may show ‘reciprocal’ changes of ST depression .
Infarction of the posteriorwall of the LV does not cause ST elevation
or Q waves in the standard leads, but can be diagnosed by the
presence of reciprocal changes (ST depression and a tall R wave in
leads V1–V4). Some infarctions (especially inferior) also involve the
RV. This may be identified by recording from additional leads
placed over the right precordium.

Plasma cardiac biomarkers
In unstable angina, there is no detectable rise in cardiac
biomarkers or enzymes, and the initial diagnosis is made
from the clinical history and ECG only. In contrast, MI
causes a rise in the plasma concentration of enzymes and
proteins that are normally concentrated within cardiac
cells. These biochemical markers are creatine kinase
(CK), a more sensitive and cardio-specific isoform of this
enzyme (CK-MB), and the cardio-specific proteins,
troponins T and I .
Admission and serial (usually daily) estimations are
helpful because it is the change in plasma concentrations
of these markers that confirms the diagnosis of MI .

CK starts to rise at 4–6 hours, peaks at about 12 hours
and falls to normal within 48–72 hours. CK is also
present in skeletal muscle, and a modest rise in CK (but
not CK-MB) may sometimes be due to an intramuscular
injection, vigorous physical exercise or, particularly in
older people, a fall. Defibrillation causes significant
release of CK but not CK-MB or troponins.
The most sensitive markers
of myocardial cell damage
are the cardiac troponins T and I, which are released
within 4–6 hours and remain elevated for up to 2 weeks.
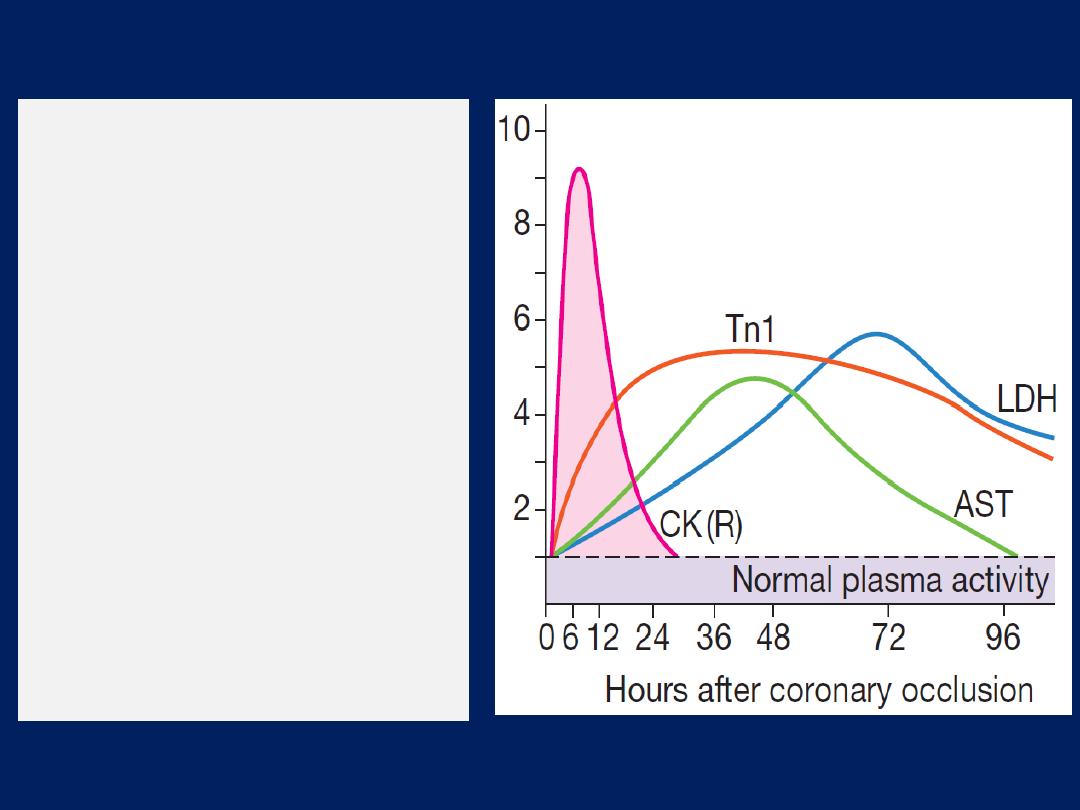
Changes in plasma cardiac
biomarker
concentrations
after MI. Creatine kinase (CK)
and troponin I (Tn I) are the
first to rise, followed by
aspartate aminotransferase
(AST) and then lactate
hydroxybutyrate)
dehydrogenase (LDH). In
patients treated with
reperfusion therapy, a rapid
rise in plasma creatine kinase
(curve CK (R)) occurs, due
to a washout effect.
The serial evolution of ECG changes in
transmural MI.
A
Normal ECG complex.
B
Acute ST elevation (‘the current of
injury’).
C
Progressive loss of the R wave, developing
Q wave, resolution of the ST elevation and
terminal T-wave inversion.
D
Deep Q wave and T-wave inversion.
E
Old or established infarct pattern; the Q
wave tends to persist but the T-wave
changes become less marked. The rate of
evolution is very variable but, in general,
stage B appears within minutes, stage C
within hours, stage D within days and
stage E after several weeks or months.
Recent anterior non-ST elevation
(subendocardial) MI. deep symmetrical
T-wave inversion, together with a
reduction in the height of the R wave
in leads V1, V2, V3 and V4.
Acute transmural anterior MI.
There is
ST elevation in leads I, aVL, V2, V3,
V4, V5 and V6, and there are Q
waves in leads V3, V4 and V5.
Anterior infarcts with prominent
changes in leads
V2, V3 and V4
are
sometimes called ‘anteroseptal’
infarcts, as opposed to anterolateral’
infarcts, found in
V4, V5 and V6
.
Acute transmural inferolateral
MI
.
This ECG was
recorded from a patient who
had developed severe chest
pain 4 hours earlier. There is ST
elevation in the inferior leads II,
III and aVF and the lateral
leads V4, V5 and V6. There is
also ‘reciprocal’ ST depression
in leads aVL and V2.

Other blood tests
A leucocytosis is usual, reaching a peak on the first day.
The erythrocyte sedimentation rate (ESR) and C-reactive
protein (CRP) are also elevated.
Chest X-ray
This may demonstrate pulmonary oedema that is not
evident on clinical examination .
The heart size is often normal but there may be
cardiomegaly due to pre-existing myocardial damage.
Echocardiography
This is useful for assessing ventricular function and for
detecting important complications, such as mural thrombus,
cardiac rupture, ventricular septal defect, mitral
regurgitation and pericardial effusion.

Immediate management: the first 12 hours
Patients should be admitted urgently to hospital because
there is a significant risk of death or recurrent
myocardial ischaemia during the early unstable phase,
and appropriate medical therapy can reduce the
incidence of these by at least 60%. The essentials of the
immediate in-hospital management of acute coronary
syndrome are shown in previous Figure.
Patients are usually managed in a dedicated cardiac unit,
where the necessary expertise, monitoring and
resuscitation facilities can be concentrated. If there are
no complications, the patient can be mobilised from the
second day and discharged after 3–5 days.

Analgesia
Adequate analgesia is essential, to relieve distress
and to lower adrenergic drive and thereby reduce
vascular resistance, BP, infarct size and susceptibility to
ventricular arrhythmias. Intravenous opiates
(initially, morphine
sulphate 5–10 mg or diamorphine 2.5–5 mg)
and antiemetics (initially,
metoclopramide 10 mg) should be administered, and
titrated by giving repeated small aliquots until the patient
is comfortable.
Intramuscular injections should be avoided because the
clinical effect may be delayed by poor skeletal muscle
perfusion, and a painful haematoma may form following
thrombolytic or antithrombotic therapy.

Antithrombotic therapy
Antiplatelet therapy
In patients with ACS , oral administration of 75–325 mg
aspirin daily improves survival, with a 25% relative risk
reduction in mortality. The first tablet (300 mg) should be
given orally within the first12 hours and therapy should be
continued indefinitely if there are no side-effects. In
combination with aspirin, the early (within 12 hours) use of
clopidogrel
(600 mg, followed by 150 mg daily for 1 week and 75 mg daily thereafter)
confers a further reduction in ischaemic events . In patients
with an ACS, with or without ST-segment elevation,
ticagrelor
(180 mg, followed by 90 mg twice daily)
is more effective than
clopidogrel in reducing vascular death, MI or stroke, and all-
cause death, without affecting overall major bleeding risk.

Glycoprotein IIb/ IIIa receptor antagonists, such as
tirofiban and abciximab, block the final common
pathway of platelet aggregation and are potent inhibitors
of platelet-rich thrombus formation. They are of particular
benefit in patients with ACS who undergo PCI , those with
recurrent ischaemia and those at particularly high risk,
such as patients with diabetes mellitus or an elevated
troponin concentration.
Anticoagulants
Reduces the risk of thromboembolic complications, and
prevents re-infarction in the absence of reperfusion
therapy or after successful thrombolysis. Anticoagulation can
be achieved using unfractionated heparin, fractioned (low-
molecular weight) heparin or a pentasaccharide.
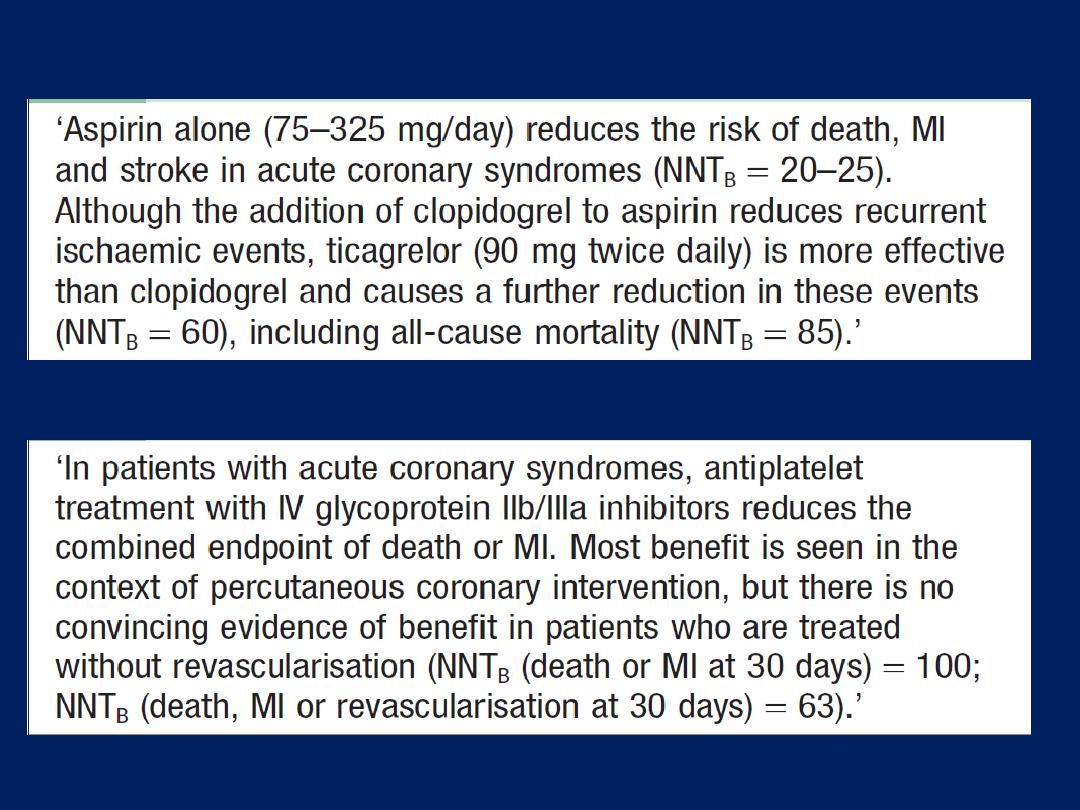
Oral antiplatelet agents in ACS
IV glycoprotein IIb/IIIa inhibitors in ACS

Anticoagulation in acute coronary syndromes

Comparative clinical trials suggest that the pentasaccharides
(
subcutaneous fondaparinux
2.5 mg daily) have the best
safety and efficacy profile, with low-molecular-weight
heparin (subcutaneous enoxaparin 1 mg/kg twice daily)
being a reasonable alternative. Anticoagulation should be
continued for 8 days or until discharge from hospital or
coronary revascularisation.
A period of treatment with warfarin should be considered
if there is persistent atrial fibrillation or evidence
of extensive anterior infarction, or if echocardiography
shows mobile mural thrombus, because these patients
are at increased risk of systemic thromboembolism.

Anti-anginal therapy
Sublingual glyceryl trinitrate
(300–500 μg)
is a valuable
first-aid measure in unstable angina or threatened
infarction, and intravenous nitrates (glyceryl trinitrate
0.6–1.2 mg/ hr or isosorbide dinitrate 1–2 mg/ hr) are
useful for the treatment of left ventricular failure and the
relief of recurrent or persistent ischaemic pain.
Intravenous β-blockers (e.g. atenolol 5–10 mg or
metoprolol 5–15 mg given over 5 mins) relieve pain, reduce
arrhythmias and improve short-term mortality in patients
who present within 12 hours of the onset of symptoms .
However, they should be avoided if there is heart failure
(pulmonary oedema), hypotension (systolic BP < 105 mmHg)
or bradycardia (heart rate < 65/min).

A dihydropyridine calcium channel antagonist (e.g.
nifedipine or amlodipine) can be added to the β-blocker
if there is persistent chest discomfort but may cause
tachycardia if used alone. Because of their rate-limiting
action, verapamil and diltiazem are the calcium channel
antagonists of choice if a β-blocker is contraindicated.

Non-ST segment elevation acute coronary syndrome
Immediate emergency reperfusion therapy has no
demonstrable benefit in patients with non-ST segment
elevation MI and thrombolytic therapy may be harmful.
Selected
medium- to high-risk patients do benefit from
in-hospital coronary angiography and coronary
revascularization but this does not need to take place in the
first 12 hours.
high-risk features such as elevated cardiac enzymes, ST-
segment depression, Continued or recurrent angina,
hemodynamic instability , hypotension, arrhythmia, or heart
failure, sustained VT, diabetes, prior PCI, or bypass are
recommended to undergo early PCI (<48 hours).

ST segment elevation acute coronary syndrome
Immediate reperfusion therapy restores coronary artery
patency, preserves left ventricular function and improves
survival. Successful therapy is associated with pain
relief, resolution of acute ST elevation and, sometimes,
transient arrhythmias (e.g. idioventricular rhythm).
Primary percutaneous coronary intervention (PCI). This
is the treatment of choice for ST segment elevation MI.
Outcomes are best when it is used in combination with
glycoprotein IIb/IIIa receptor antagonists and
intracoronary stent implantation. In comparison to
thrombolytic therapy, it is associated with a greater
reduction in the risk of death, recurrent MI or stroke .

The universal use of primary PCI has been limited by
availability of the necessary resources to provide this
highly specialised emergency service.
Thus, intravenous thrombolytic therapy remains
the first-line reperfusion treatment in many hospitals,
especially those in rural or remote areas.
When primary PCI cannot
be achieved
within 2 hours of
diagnosis,
thrombolytic therapy should be administered.
Primary PCI in acute ST segment elevation MI

Thrombolysis.
The appropriate use of thrombolytic
therapy can reduce hospital mortality by 25–50% and
this survival advantage is maintained for at least 10 years .
The benefit is greatest in those patients who receive
treatment within the first few hours:
‘minutes mean muscle’.
Alteplase (human tissue plasminogen activator, or tPA) is a
genetically engineered drug that is given over 90 minutes
(bolus dose of 15 mg, followed by 0.75 mg/ kg body
weight but not exceeding 50 mg, over 30 mins, and then 0.5
mg/kg BW but not exceeding 35 mg, over 60 mins). Its use
is associated with better survival rates than other
thrombolytic agents, such as streptokinase, but carries a
slightly higher risk of intracerebral bleeding (10 per 1000
increased survival, but 1 per 1000 more non-fatal stroke).

Analogues of tPA, such as tenecteplase (TNK) and reteplase
(rPA), have a longer plasma half-life than alteplase and
can be given as an intravenous bolus. TNK is as effective as
alteplase at reducing death and MI, whilst conferring
similar intracerebral bleeding risks.
However, other bleeding and transfusion risks are lower and
the practical advantages of bolus administration provide
opportunities for prompt treatment in the emergency
department or in the pre-hospital setting. rPA is
administered as a double bolus and also produces a similar
outcome to that achieved with alteplase, although
some of the bleeding risks appear slightly higher.

An overview of all large randomised trials confirms that
thrombolytic therapy reduces short-term mortality in
patients with MI if given
within 12 hours
of the onset of
symptoms and the ECG shows
bundle branch block
or
characteristic
ST segment elevation of more than 1 mm in
the limb leads or 2 mm in the chest.
Thrombolysis
appears to be of little net benefit and may
be
harmful in
those who present
more than 12
hours after
the onset of symptoms and in those with a
normal ECG or
ST depression.
In patients with ST elevation or BBB, the
absolute benefit of thrombolysis plus aspirin is
approximately 50 lives saved per 1000 patients treated within 6 hours, and 40
lives saved per 1000 treated between 7 and 12 hours after the onset of symptoms.
The benefit is
greatest
if given
within the first 2 hours.

The major hazard of thrombolytic therapy is bleeding.
Cerebral haemorrhage causes 4 extra strokes per
1000 patients treated, and the incidence of other major
bleeds is between 0.5% and 1%. Accordingly, the
treatment should be withheld if there is a significant risk
of serious bleeding .
For some patients, thrombolytic therapy is contraindicated
or fails to achieve coronary arterial reperfusion.
Early emergency PCI may then be considered,
particularly where there is evidence of cardiogenic shock.
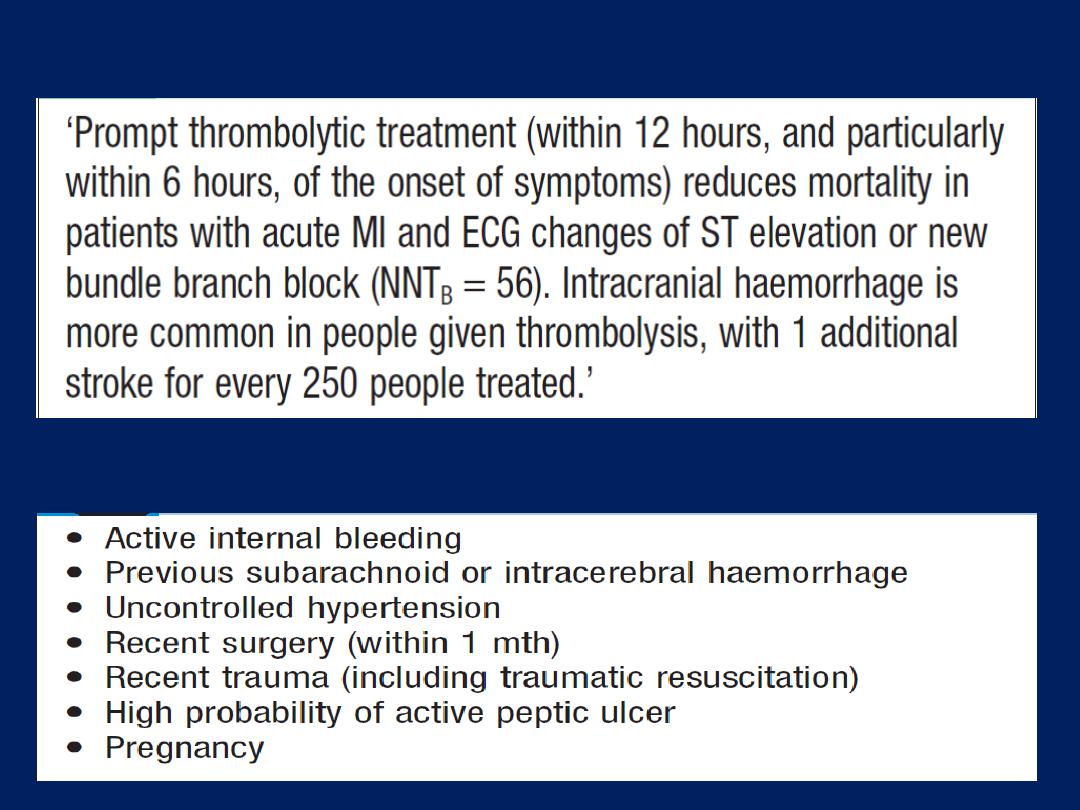
Thrombolytic treatment in acute STEMI
Relative contraindications to thrombolytic
therapy: potential candidates for primary PCI

Complications of acute coronary syndrome
Complications are seen in all forms of acute coronary
syndrome, although the frequency and extent vary with
the severity of ischaemia and infarction. Major
mechanical and structural complications are seen only
with significant, often transmural, MI.
Arrhythmias
Many patients with acute coronary syndrome have
some form of arrhythmia .In the majority of cases this is
transient and of no haemodynamic or prognostic
importance. Pain relief, rest and the correction of
hypokalaemia may help prevent arrhythmias.

Ventricular fibrillation
This occurs in 5–10% of patients who reach hospital
and is thought to be the major cause of death in those
who die before receiving medical attention. Prompt
defibrillation restores sinus rhythm and is life-saving.
The prognosis of patients with early ventricular fibrillation
(within the first 48 hours) who are successfully and
promptly resuscitated is identical to that of patients
who do not suffer ventricular fibrillation.
Atrial fibrillation
This is common but frequently transient, and usually does
not require emergency treatment. However, if it causes a
rapid ventricular rate with hypotension or circulatory
collapse, prompt cardioversion by immediate synchronised
DC shock is essential.

In other situations, digoxin or a β-blocker is usually the
treatment of choice. Atrial fibrillation (due to acute atrial
stretch) is often a feature of impending or overt left
ventricular failure, and therapy may be ineffective if
heart failure is not recognised and treated appropriately.
Anticoagulation is required if atrial fibrillation persists.
Bradycardia
This does not usually require treatment, but if there is
hypotension or haemodynamic deterioration, atropine
(0.6–1.2 mg IV) may be given. AV block complicating
inferior infarction is usually temporary and often
resolves following reperfusion therapy. If there is clinical
deterioration due to second-degree or complete AV
block, a temporary pacemaker should be considered.
Common arrhythmias in ACS

AV block complicating anterior infarction is more serious
because asystole may suddenly supervene; a prophylactic
temporary pacemaker should be inserted .
Ischaemia
Patients who develop recurrent angina at rest or on
minimal exertion following an acute coronary syndrome are
at high risk and should be considered for prompt
coronary angiography with a view to revascularisation.
Patients with dynamic ECG changes and ongoing pain
should be treated with intravenous glycoprotein IIb/IIIa
receptor antagonists.
Patients with resistant pain or marked haemodynamic
changes should be considered for intra-aortic balloon
counterpulsation and emergency coronary revascularisation.

Post-infarct angina occurs in up to 50% of patients
treated with thrombolysis. Most patients have a residual
stenosis in the infarct-related vessel, despite successful
thrombolysis, and this may cause angina if there is still
viable myocardium downstream. For this reason, all
patients who have received successful thrombolysis
should be considered for early (within the first 6–24
hours) coronary angiography with a view to coronary
revascularisation.
Acute circulatory failure
Acute circulatory failure usually reflects extensive
myocardial damage and indicates a bad prognosis. All
the other complications of MI are more likely to occur
when acute heart failure is present.

Pericarditis
Common on the second and third days. The patient may
recognise that a different pain developed, even though it
is at the same site, and that it is positional and tends to be
worse or sometimes only present on inspiration. A
pericardial rub may be audible.
Opiate
analgesia should
be used. NSAIDs and steroidal drugs may increase the
risk of aneurysm formation and myocardial rupture in the
early recovery period, and so should be avoided.
The post-MI syndrome (Dressler’s syndrome)
characterized by fever, pericarditis and pleurisy, and is
probably due to autoimmunity. Occur a few weeks or
months after the infarct and often subside after a few
days; prolonged or severe may require high-dose aspirin,
NSAIDs or even corticosteroids.

Mechanical complications
Part of the necrotic muscle in a fresh infarct may tear or
rupture, with devastating consequences:
• Rupture of the papillary muscle
can cause acute
pulmonary oedema and shock due to the sudden
onset of severe mitral regurgitation, which presents
with a pansystolic murmur and third heart sound.
In the presence of severe regurgitation, the murmur
may be quiet or absent. The diagnosis is confirmed
by echocardiography and emergency valve
replacement may be necessary. Lesser degrees of
mitral regurgitation due to papillary muscle
dysfunction are common and may be transient.

• Rupture of the interventricular septum
causes left-to
right shunting through a ventricular septal defect. This
usually presents with sudden haemodynamic
deterioration accompanied by a new loud pansystolic
murmur radiating to the right sternal border, but may be
difficult to distinguish from acute mitral regurgitation.
However, patients with an acquired VSD tend to develop
right heart failure rather than pulmonary oedema.
Doppler and right heart catheterisation will confirm the
diagnosis. Without prompt surgery, is usually fatal.
• Rupture of the ventricle
may lead to cardiac
tamponade and is usually fatal , although it may rarely
be possible to support a patient with an incomplete
rupture until emergency surgery is performed.

Embolism
Thrombus often forms on the endocardial surface of
freshly infarcted myocardium. This can lead to systemic
embolism and occasionally causes a stroke or ischaemic
limb. Venous thrombosis and pulmonary embolism may
occur but have become less common with the use of
prophylactic anticoagulants and early mobilisation.
Impaired ventricular function, remodeling and ventricular
aneurysm
Acute transmural MI is often followed by thinning and
stretching of the infarcted segment (infarct expansion).
This leads to an increase in wall stress with progressive
dilatation and hypertrophy of the remaining ventricle
(ventricular remodelling). As the ventricle dilates, it becomes
less efficient and heart failure may supervene.

Infarct expansion occurs over a few days and weeks but
ventricular remodelling can take years. ACE inhibitor
therapy reduces late ventricular remodelling and can
prevent the onset of heart failure.
A left ventricular aneurysm develops in approximately
10% of patients with MI and is particularly common when
there is persistent occlusion of the infarct related vessel.
Heart failure, ventricular arrhythmias, mural thrombus
and systemic embolism are all recognised complications
of aneurysm formation.
Other features include a
paradoxical impulse on the chest wall, persistent ST
elevation on the ECG, and sometimes an unusual bulge from
the cardiac silhouette on the chest X-ray. Echo is diagnostic.
Surgical removal of a left ventricular aneurysm carries a
high morbidity and mortality but is sometimes necessary.
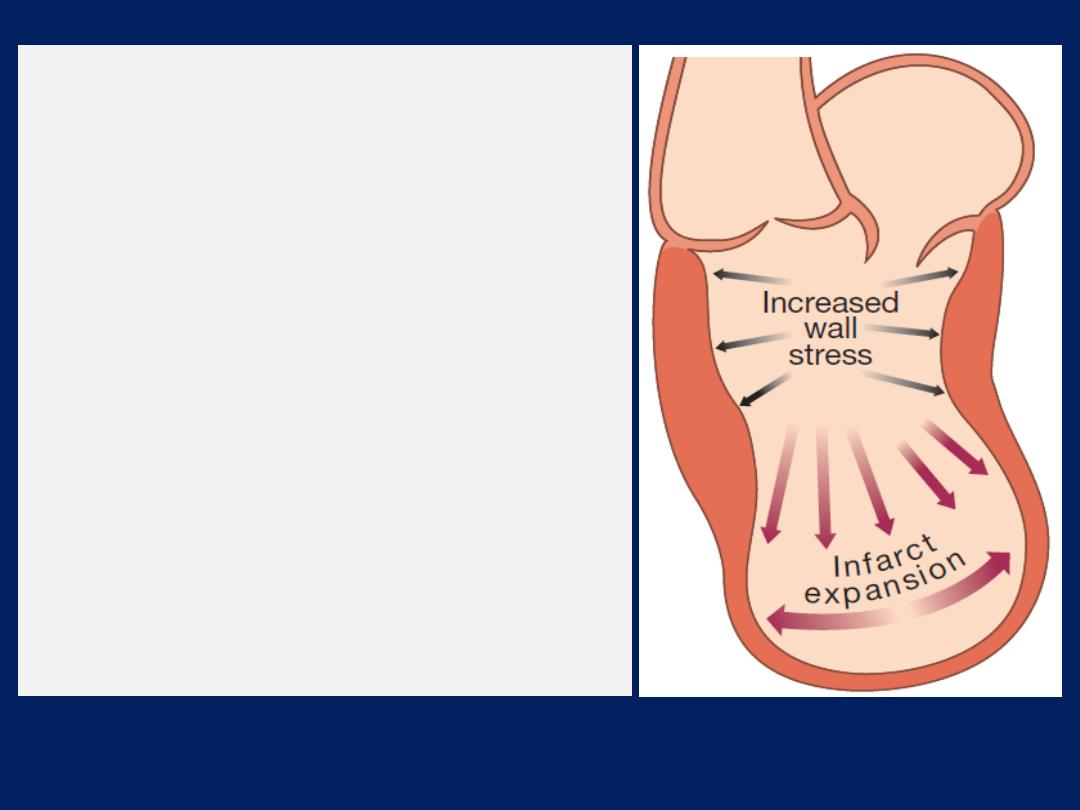
Infarct expansion and
ventricular remodelling.
Full-thickness Ml causes
thinning and stretching of the
infarcted segment (infarct
expansion), which leads to
increased wall stress with
progressive dilatation and
hypertrophy of the
remaining ventricle
(ventricular remodelling).
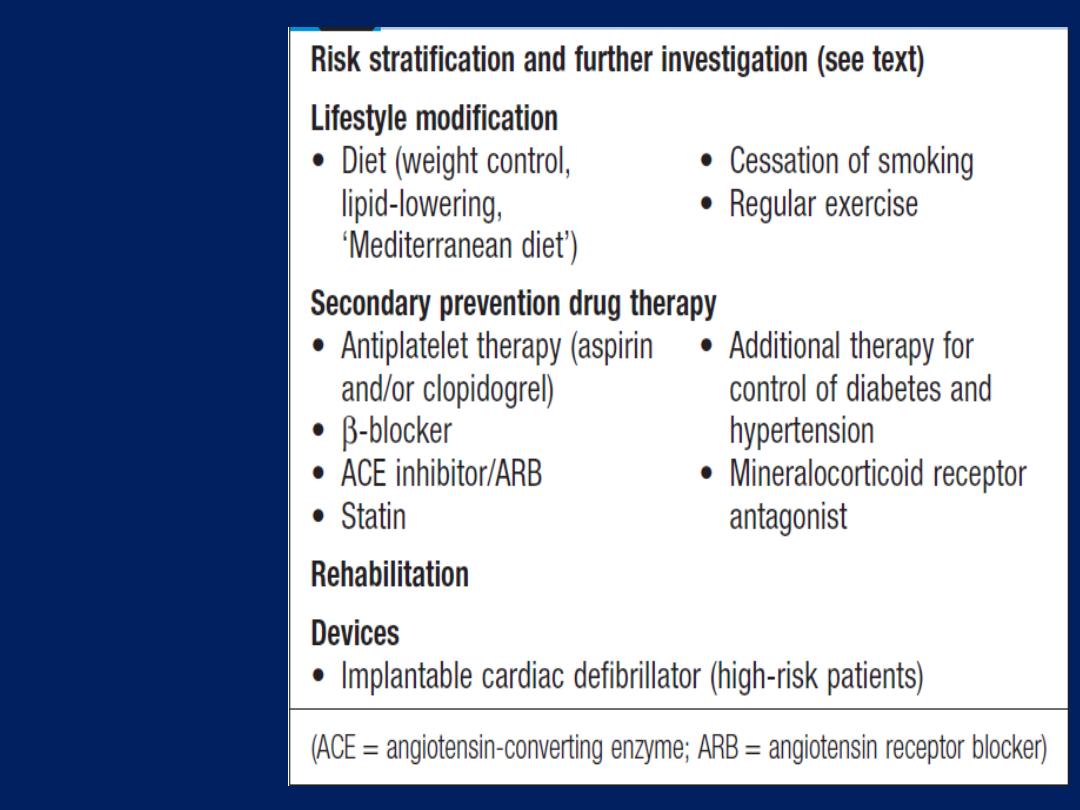
Late
management
of Ml

Later in-hospital management
Risk stratification and further investigation
The GRACE score is a simple method of calculating early
mortality that can help guide which patients should be
selected for intensive therapy, and coronary angiography.
Left ventricular function
The degree of left ventricular dysfunction can be crudely
assessed from physical findings (tachycardia, third heart
sound, crackles at the lung bases, elevated venous pressure
and so on), ECG changes and chest X-ray (size of the
heart and presence of pulmonary oedema). Echo.
The prognosis of patients survived an ACS is related to the
extent of residual myocardial ischaemia, the degree of
damage and the presence of ventricular arrhythmias.

Ischaemia
Patients with early ischaemia following an ACS should
undergo coronary angio with a view to revascularisation.
Low-risk patients without spontaneous ischaemia should
undergo an exercise tolerance test approximately 4
weeks after the ACS . This will help to identify those
individuals with residual myocardial ischaemia who require
further investigation. If the exercise test is negative and the
patient has a good effort tolerance, the outlook is good,
with a 1–4% chance of an adverse event in the next 12
months. In contrast, patients with residual ischaemia in the
form of chest pain or ECG changes at low exercise levels
are at high risk, with a 15–25% chance of suffering a
further ischaemic event in the next 12 months.

Arrhythmias
The presence of ventricular arrhythmias during the
convalescent phase may be a marker of poor ventricular
function and may herald sudden death. Although empirical
anti-arrhythmic treatment is of no value and is even
hazardous, selected patients may benefit from
electrophysiological testing and specific anti-arrhythmic
therapy (including implantable cardiac defibrillators).
Recurrent ventricular arrhythmias are sometimes
manifestations of myocardial ischaemia or impaired left
ventricular function and may respond to appropriate
treatment directed at the underlying problem.

Lifestyle and risk factor modification
Smoking
The 5-year mortality of patients who continue to smoke
cigarettes is double that of those who quit smoking. Giving
up smoking is the single most effective contribution a patient
can make to his or her future.
Hyperlipidaemia
The importance of lowering serum cholesterol following
ACS has been demonstrated in large-scale randomised
trials. Lipids should be measured within 24 hours of
presentation
because
there is often a transient fall in
cholesterol in the 3 months following infarction.

HMG CoA reductase enzyme inhibitors (‘statins) can
produce marked reductions in total (and LDL) cholesterol
and reduce the subsequent risk of death, re-infarction,
stroke and the need for revascularisation. Irrespective
of serum cholesterol concentrations, all patients should
receive statin therapy after ACS, but those with serum LDL
cholesterol concentrations above 3.2 mmol/L (~120 mg/
dL) benefit from more intensive therapy, such as
atorvastatin 80 mg daily.
Other risk factors
Maintaining an ideal body weight, eating a
Mediterranean-style diet, taking regular exercise, and
achieving good control of hypertension and diabetes
mellitus may all improve the long-term outlook.

Mobilisation and rehabilitation
The necrotic muscle of an acute myocardial infarct takes
4–6 weeks to be replaced with fibrous tissue and it is
conventional to restrict physical activities during this
period. When there are no complications, the patient can
mobilise on the second day, return home in 3–5 days
and gradually increase activity, with the aim of returning
to work in 4–6 weeks.
The majority of patients may resume driving after 4–6
weeks, although, in most countries, vocational driving
licence holders (e.g. heavy goods and public service
vehicles) require special assessment.

Emotional problems, such as denial, anxiety and
depression, are common and must be addressed. Many
patients are severely and even permanently incapacitated
as a result of the psychological effects of ACS rather than
the physical ones, and all benefit from thoughtful
explanation, counselling and reassurance at every stage of
the illness. Many patients mistakenly believe that ‘stress’
was the cause of their heart attack and may restrict their
activity inappropriately.The patient’s spouse or partner will
also require emotional support, information and counselling.
Formal rehabilitation programmes, based on graded
exercise protocols with individual and group counselling, are
often very successful and, in some cases, have been shown to
improve the long-term outcome.

Secondary prevention drug therapy
Aspirin and clopidogrel
Low-dose aspirin therapy reduces the risk of further
infarction and other vascular events by approximately
25% and should be continued indefinitely if there are no
unwanted effects. Clopidogrel should be given in
combination with aspirin for at least 3 months. If patients
intolerant of long-term aspirin, clopidogrel is alternative.
Beta-blockers
Continuous treatment with an oral β-blocker reduces
long-term mortality by approximately 25% among the
survivors of acute MI . Unfortunately, a minority of patients
do not tolerate β-blockers because of bradycardia, AV
block, hypotension or asthma.
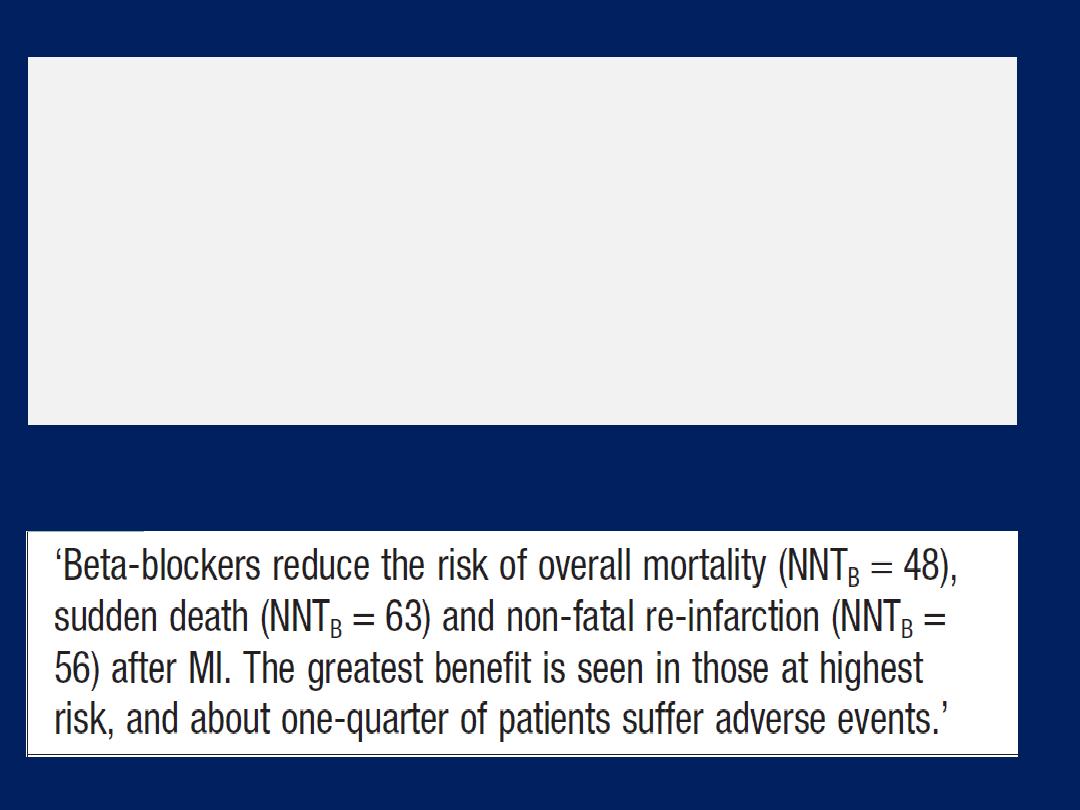
Patients with heart failure, irreversible chronic
obstructive pulmonary disease or peripheral vascular
disease derive similar, if not greater secondary
preventative benefits from β-blocker therapy if they can
tolerate it, so it should be tried.
The secondary preventative role of β-blockers in patients
with unstable angina is unknown.
Beta-blockers in secondary prevention after MI

ACE inhibitors
Several clinical trials have shown that long-term treatment
with an ACE inhibitor (e.g. enalapril 10 mg twice
daily or ramipril 2.5–5 mg twice daily)
can counteract
ventricular remodelling
, prevent the onset of heart
failure, improve survival, reduce recurrent MI and avoid
rehospitalisation.
The benefits are greatest in those with overt heart failure
(clinical or radiological) but extend to patients with
asymptomatic left ventricular dysfunction and those with
preserved left ventricular function.
They should therefore be considered in all patients with
acute coronary syndrome.

Caution must
be exercised in hypovolaemic or
hypotensive patients because the introduction of an ACE
inhibitor may exacerbate hypotension and impair coronary
perfusion. In patients intolerant of ACE inhibitors,
angiotensin receptor blockers (e.g. valsartan 40–160 mg
twice daily or candesartan 4–16 mg daily) are
alternatives and are better tolerated.
Patients with acute MI and left ventricular dysfunction
(ejection fraction < 35%) and either pulmonary oedema
or diabetes mellitus further benefit from additional
mineralocorticoid receptor antagonism
(e.g. eplerenone 25–50 mg daily).

Coronary revascularisation
Most low-risk patients stabilise with aspirin, clopidogrel,
anticoagulation and anti- anginal therapy, and can
be rapidly mobilised. In the absence of recurrent
symptoms, low-risk patients do not benefit from routine
coronary angiography. Coronary angiography should be
considered with a view to revascularisation in all
patients at moderate or high risk, including those who
fail to settle on medical therapy, those with extensive
ECG changes, those with an elevated plasma troponin
and those with severe pre-existing stable angina. This
often reveals disease that is amenable to PCI or urgent
CABG. In these cases, coronary revascularisation is
associated with short- and long-term benefits, including
reductions in MI and death.

Device therapy
Implantable cardiac defibrillators are of benefit in
preventing sudden cardiac death in patients who have
severe left ventricular impairment (ejection fraction
≤ 30%) after MI .
Prognosis
In almost one-quarter of all cases of MI, death occurs
within a few minutes without medical care. Half the
deaths occur within 24 hours of the onset of symptoms and
about 40% of all affected patients die within the first
month. The prognosis of those who survive to reach hospital
is much better, with a 28-day survival of more than 85%.
Patients with unstable angina have a mortality of
approximately half that of those patients with MI.

Early death is usually due to an arrhythmia and is
independent of the extent of MI. However, late outcomes
are determined by the extent of myocardial damage, and
unfavourable features include poor left
ventricular function, AV block and persistent ventricular
arrhythmias. The prognosis is worse for anterior than
for inferior infarcts. Bundle branch block and high cardiac
marker levels both indicate extensive myocardial
damage. Old age, depression and social isolation are
also associated with a higher mortality.
Of those who survive an acute attack,
more than 80%
live for a further year, about 75% for 5 years, 50% for
10 years and 25% for 20 years.

Cardiac risk of non-cardiac surgery
Non-cardiac surgery, particularly major vascular,
abdominal or thoracic surgery, can precipitate serious
peri-operative cardiac complications, such as MI and
death, in patients with coronary artery and other forms
of heart disease. Careful pre-operative cardiac
assessment may help to determine the balance of benefit
versus risk on an individual basis, and identify measures
that minimise the operative risk .
A hypercoagulable state is part of the normal
physiological response to surgery, and may promote
coronary thrombosis leading to an acute coronary
syndrome in the early post-operative period.

Patients with a history of recent PCI or acute coronary
syndrome are at greatest risk and, whenever possible,
elective non-cardiac surgery should be
avoided for 3
months
after such an event.
Antiplatelet agents, statins and β-blockers reduce the
risk of peri-operative MI in patients with coronary
artery disease and, where possible, should be prescribed
throughout the peri-operative period.
Careful attention to fluid balance during and after
surgery is particularly important in patients with
impaired left ventricular function and valvular heart
disease because antidiuretic hormone is released as part
of the normal physiological response to surgery and, in
these circumstances, the overzealous administration of
intravenous fluids can easily precipitate heart failure.

Patients with severe valvular heart disease, particularly
aortic stenosis and mitral stenosis, are also at increased
risk because they may not be able to increase their
cardiac output in response to the stress of surgery.
Atrial fibrillation may be triggered by hypoxia,
myocardial ischaemia or heart failure, and is a common
postoperative complication in patients with pre-existing
heart disease.
It usually terminates spontaneously when the precipitating
factors have been eliminated, but digoxin or β-blockers
can be prescribed to control the heart rate.

Major risk factors for cardiac complications of
non-cardiac surgery
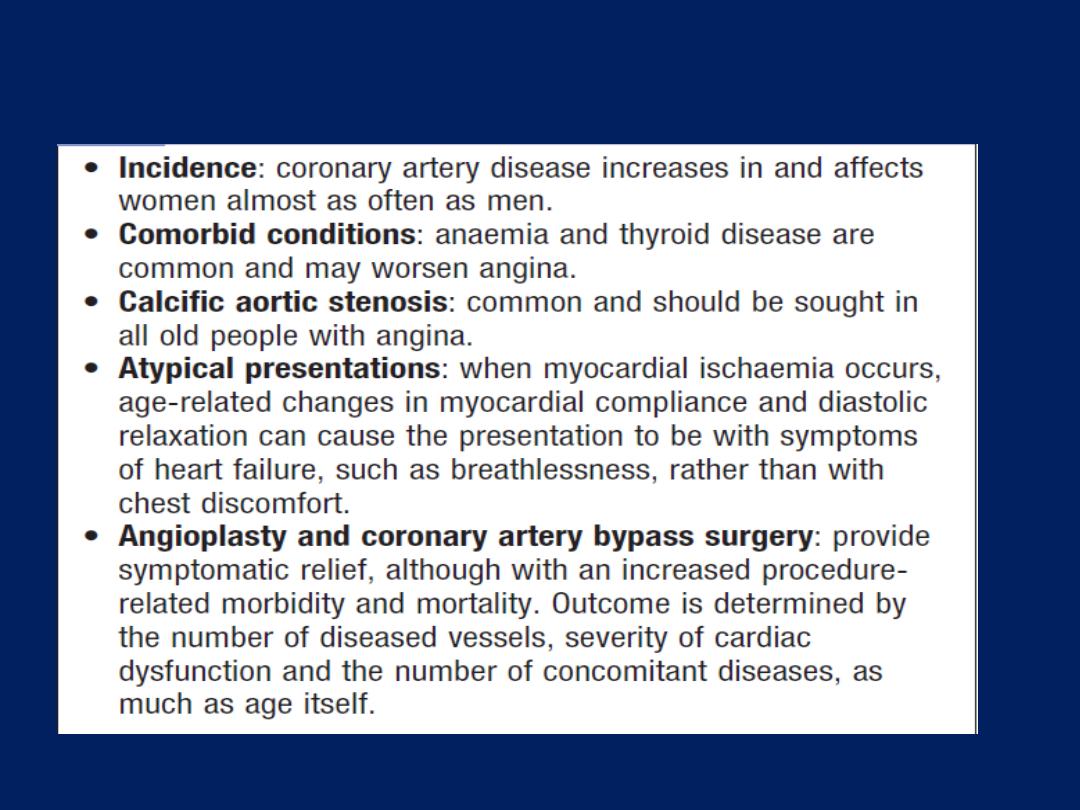
Angina in old age
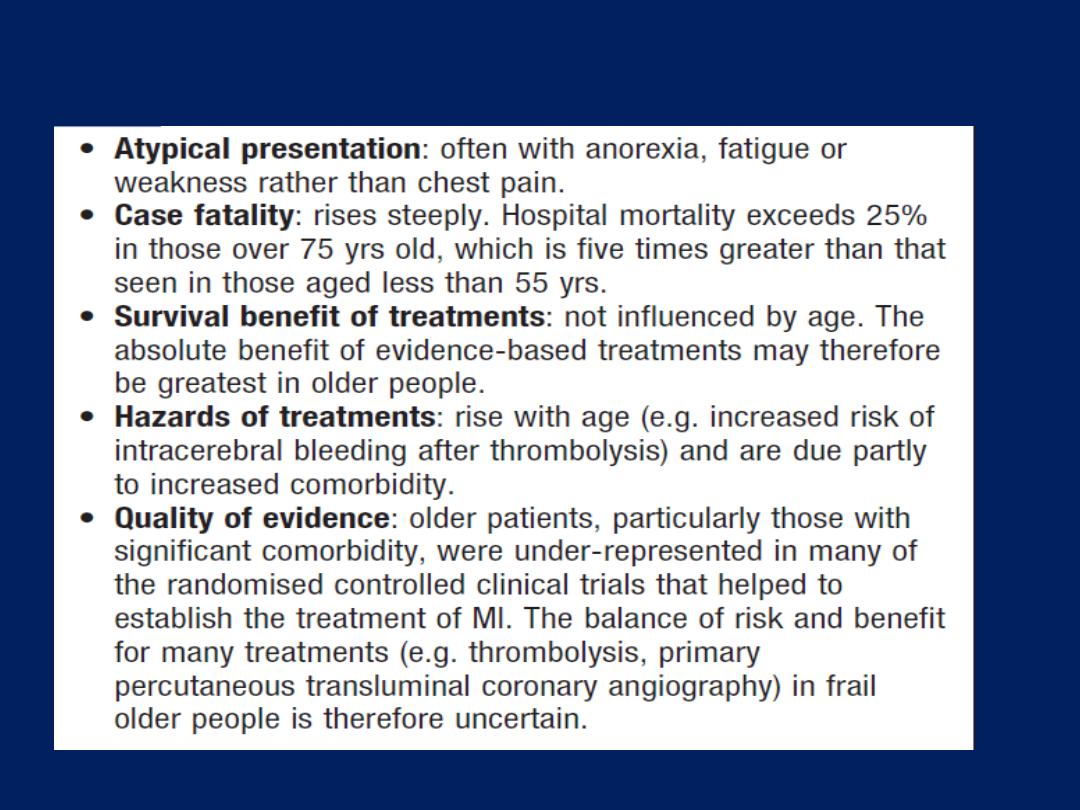
Myocardial infarction in old age

VASCULAR DISEASE
Peripheral arterial disease (PAD)
In developed countries, almost all PAD is due to
atherosclerosis and so shares common risk factors with
coronary artery disease: namely, smoking, diabetes
mellitus, hyperlipidaemia and hypertension. As with CAD,
plaque rupture is responsible for the most serious
manifestations of PAD, and not infrequently occurs in a
plaque that hitherto has been asymptomatic.
Approximately 20% of middle-aged
(55–75 years)
people in
the UK have PAD but only one-quarter of them will have
symptoms. The clinical manifestations depend upon the
anatomical site, the presence or absence of a collateral
supply, the speed of onset and the mechanism of injury.
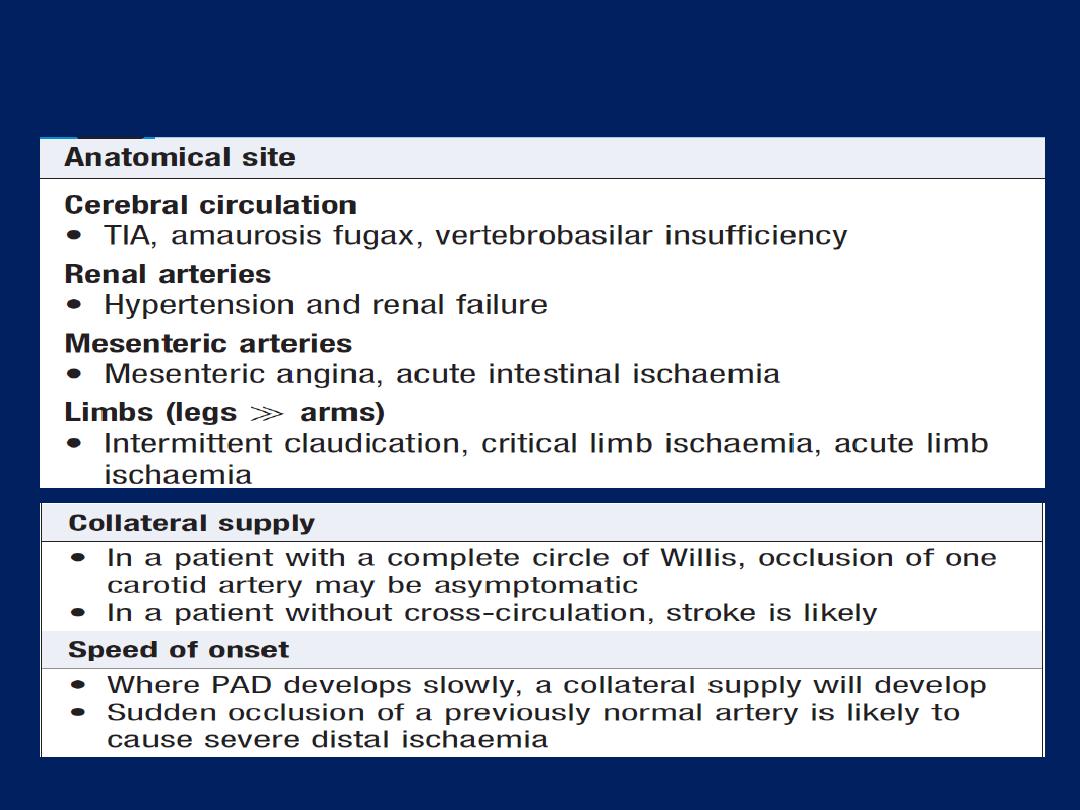
Factors influencing the clinical manifestations of
peripheral arterial disease
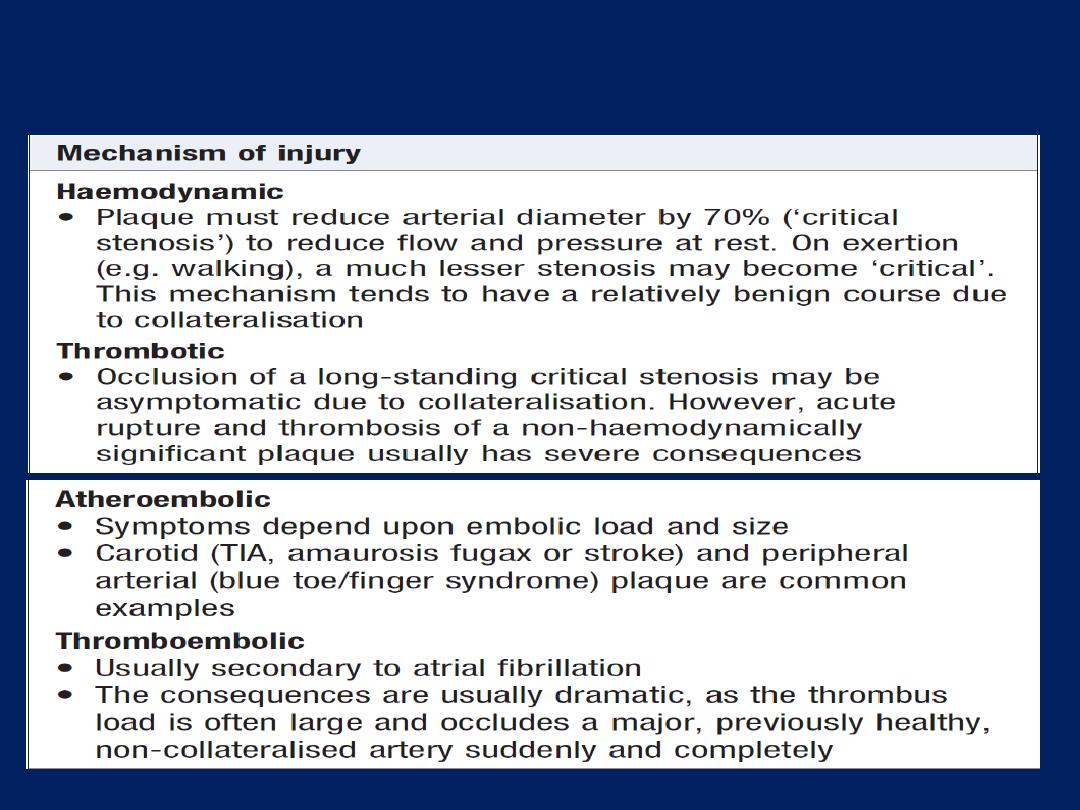
Factors influencing the clinical manifestations of
peripheral arterial disease 'cont'd

Chronic lower limb arterial disease
PAD affects the
leg eight times
more often than the arm.
The lower limb arterial tree comprises the aorto-iliac
(‘inflow’), femoro-popliteal and infra-popliteal (‘outflow’)
segments. Lower limb ischaemia presents as two distinct
clinical entities:
intermittent claudication(IC)and critical limb ischaemia(CLI).
The presence and severity of ischaemia can be
determined by clinical examination and measurement of
the ankle–brachial pressure index (ABPI), which is the
ratio between the (highest systolic) ankle and brachial
blood pressures. In health, the ABPI is over 1.0, in
IC typically 0.5–0.9 and in
CLI usually below 0.5.

Clinical features of chronic lower limb ischaemia

Intermittent claudication (IC)
This term describes ischaemic
pain affecting the muscles
of the leg upon
walking
. The pain is usually felt in the
calf
because the disease most commonly affects the
superficial femoral artery. However, the pain may be felt
in the thigh or buttock if the iliac arteries are involved.
Typically, the pain comes on after a reasonably constant
‘claudication distance’ and rapidly subsides on stopping
walking. Resumption of walking leads to a return of the
pain. Most patients describe a cyclical pattern of
exacerbation and resolution due to the progression of
disease and the subsequent development of collaterals.
5% of middle-aged men report IC. Provided patients
comply with
‘best medical therapy’(BMT)
1–2% per year,
amputation and/or revascularisation are required.

However, the annual mortality rate exceeds 5%,
2–3 times higher than in an equivalent non- claudicant .
This is because IC is nearly always found in association
with widespread atherosclerosis, so that most claudicants
succumb to MI or stroke. The mainstay of treatment is
BMT, including (preferably supervised)
exercise
therapy.
The peripheral
vasodilator
, cilostazol, has been shown to
improve walking distance. Intervention with angioplasty,
stenting, endarterectomy or bypass is usually only
considered after BMT has been given at least 6 months to
effect symptomatic improvement, and then only in patients
who are severely disabled or whose livelihood is
threatened by their disability.

Best medical therapy (BMT) for peripheral
arterial disease*

Critical limb ischaemia
This is defined as rest (night) pain, requiring opiate
analgesia,
and/or
tissue loss (ulceration or gangrene),
present for > 2 weeks, in the presence of an ankle BP of
< 50 mmHg . Rest pain only, with ankle pressures above
50 mmHg, is known as subcritical limb ischaemia (SCLI). The
term severe limb ischaemia (SLI) is used to describe both
CLI and SCLI.
Whereas IC is usually due to single-segment plaque, SLI
is always due to multilevel disease.
Many patients with SLI have not previously sought
medical advice for IC, principally because they have
other comorbidity that prevents them from walking to a
point where claudication pain might develop.
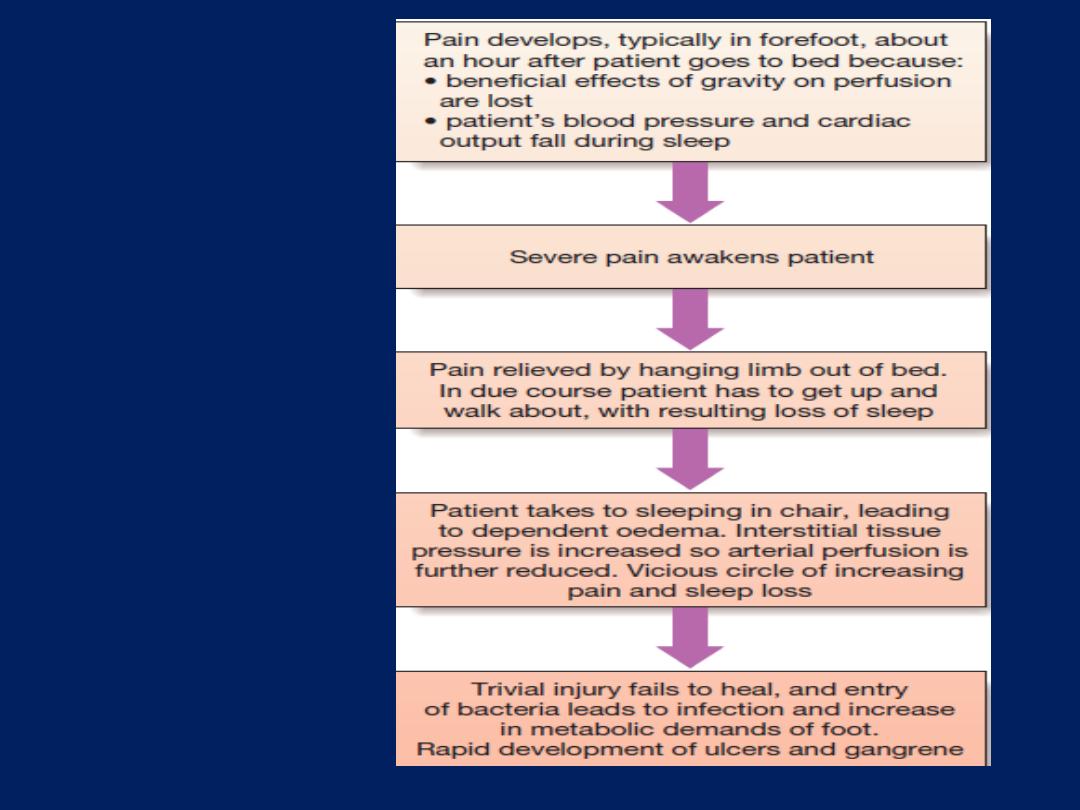
Progressive night
pain and the
development of
tissue loss.

In contrast to
patients with IC, those with SLI are at high
risk of losing their limb, and sometimes their life, in a
matter of weeks or months without surgical bypass or
endovascular revascularisation by angioplasty or stenting.
Treatment is difficult, however, because patients
have extensive and severe (often bilateral) end-stage
disease, are usually elderly and nearly always have
significant multisystem comorbidity. Imaging is performed
using duplex ultrasonography, MRI or CT with intravenous
injection of contrast agents. Intra-arterial digital
subtraction angiography (IA-DSA) is usually reserved
for those undergoing endovascular revascularisation.

Diabetic vascular disease
Approximately 5–10% of patients with PAD have
diabetes but this proportion increases to 30–40% in those
with SLI. Diabetes does not cause obstructive
microangiopathy at the capillary level, as previously
thought, and so is not a contraindication to lower limb
revascularisation. Nevertheless, the ‘diabetic foot’ does
pose a number of particular problems. If the blood
supply is adequate, then dead tissue can be excised in
the expectation that healing will occur, provided infection
is controlled and the foot is protected from pressure.
However, if significant ischaemia is also present, the
priority is to revascularise the foot if possible. Sadly,
many diabetic patients present late with extensive tissue
loss, which accounts for the high amputation rate.
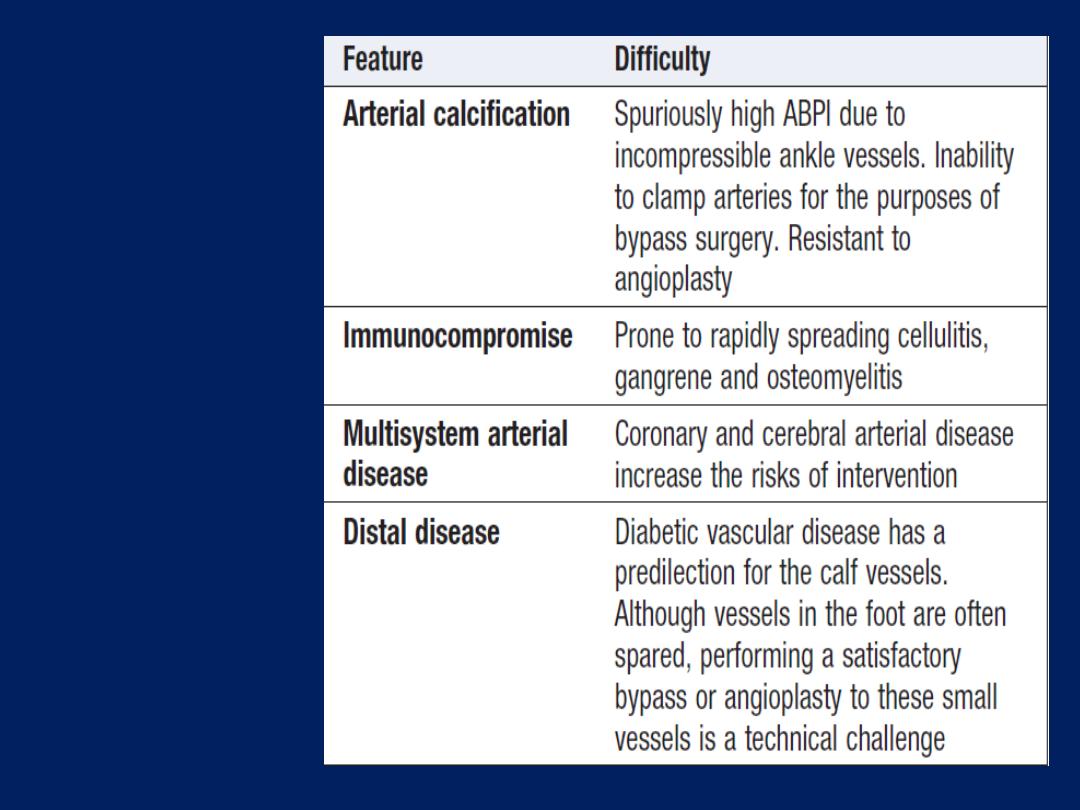
Diabetic
vascular
disease:
The
‘diabetic foot’
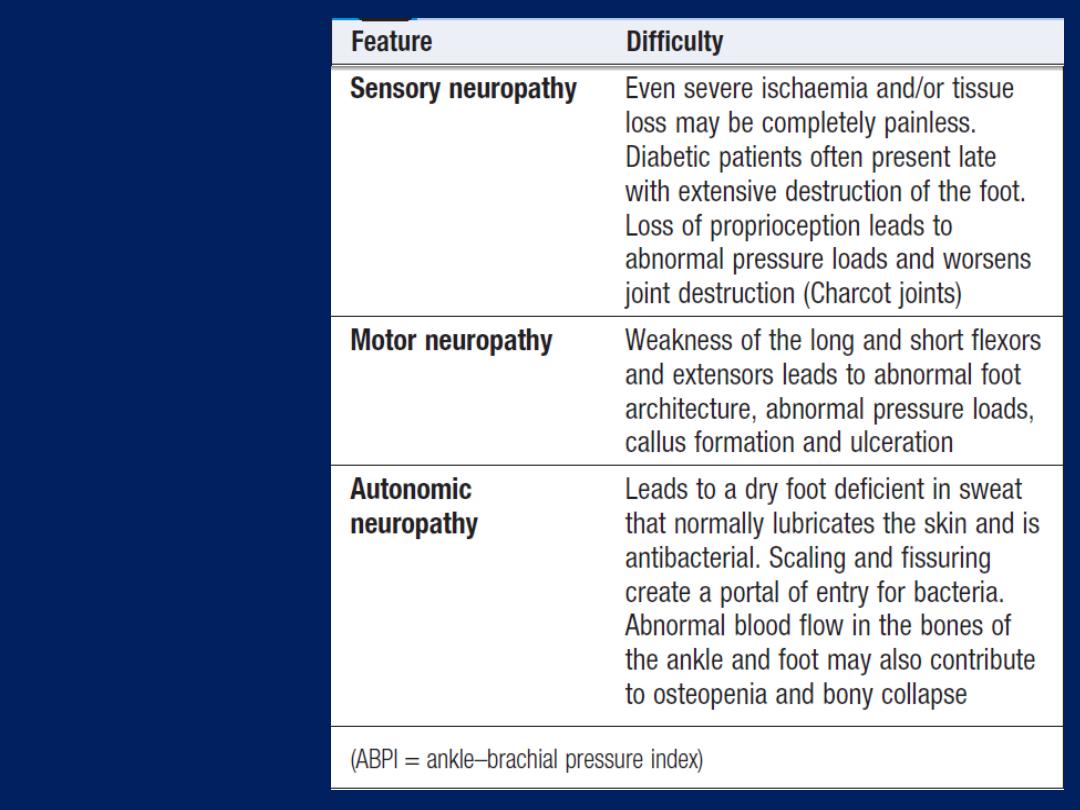
Diabetic
vascular
disease:
the ‘diabetic
foot’ 'cont'd

Buerger’s disease (thromboangiitis obliterans)
This is an inflammatory obliterative arterial disease that
is distinct from atherosclerosis and usually presents in
young (20–30 years) male smokers. It is most common
in those from the Mediterranean and North Africa. It
characteristically affects distal arteries, giving rise to
claudication in the feet or rest pain in the fingers or toes.
Wrist and ankle pulses are absent
but
brachial and
popliteal pulses are present. Disease also affects the veins,
giving rise to superficial thrombophlebitis.
It often remits if
the patient stops smoking; sympathectomy
and prostaglandin infusions may be helpful.
Major limb amputation is the most frequent outcome if
patients continue to smoke.

Chronic upper limb arterial disease
In the arm, the subclavian artery is the most common
site of disease, which may manifest as:
• Arm claudication (rare).
• Atheroembolism
(blue finger syndrome). Small emboli
lodge in digital arteries and may be confused with
Raynaud’s , but in this case, the symptoms are unilateral.
Failure to diagnosis may eventually lead to amputation.
• Subclavian steal.
When the arm is used, blood is ‘stolen’
from the brain via the vertebral artery. This leads to
vertebro-basilar ischaemia, which is characterised by
dizziness, cortical blindness and/ or collapse. Where
possible, subclavian artery disease is treated by means of
angioplasty and stenting, as surgery (e.g. carotid–
subclavian bypass) can be difficult.

Raynaud’s phenomenon and Raynaud’s disease
Cold (and emotional) stimuli may trigger vasospasm,
leading to the characteristic sequence of digital pallor
due to vasospasm, cyanosis due to deoxygenated blood,
and rubor due to reactive hyperaemia.
Primary Raynaud’s phenomenon (or disease)
This affects 5–10% of young women aged 15–30 years in
temperate climates and may be familial. It does not
progress to ulceration or infarction, and significant pain
is unusual. The underlying cause is unclear. No investigation
is necessary. The patient should be reassured and advised
to avoid exposure to cold. Long-acting nifedipine may be
helpful but sympathectomy is not indicated.

Secondary Raynaud’s phenomenon (or syndrome)
This occurs in older people in association with connective
tissue disease (most commonly systemic sclerosis
or CREST syndrome), vibration-induced injury
(from the use of power tools) and thoracic outlet obstruction
(e.g. cervical rib). Unlike primary disease, it is often
associated with fixed obstruction of the digital arteries,
fingertip ulceration, and necrosis and pain. The fingers
must be protected from cold and trauma, infection
requires treatment with antibiotics, and surgery should
be avoided if possible. Vasoactive drugs have no clear
benefit. Sympathectomy helps for a year or two.
Prostacyclin infusions are sometimes beneficial.

Acute limb ischaemia
This is most frequently caused by acute thrombotic
occlusion, thromboembolism, and trauma. Apart from
paralysis (inability to wiggle toes/fingers) and paraesthesia
(loss of light touch over the dorsum of the foot/hand), the
so-called ‘Ps of acute ischaemia’ are non-specific for
ischaemia and/or inconsistently related to its severity.
Pain on squeezing the calf indicates muscle infarction and
impending irreversible ischaemia.
All patients with suspected acutely ischaemic limbs
must be discussed immediately with a vascular surgeon;
a few hours can make the difference between death/
amputation and complete recovery of limb function.

If there are no contraindications (acute aortic dissection
or trauma, particularly head injury), an IV bolus of
heparin
(3000–5000 U)
should be administered to limit
propagation of thrombus and protect the collateral
circulation. Acute limb ischaemia due to thrombosis in situ
can usually be treated medically in the first instance with
IV heparin
(activated partial thromboplastin time (APTT) 2.0–3.0),
antiplatelet agents, high-dose statins, IV fluids to avoid
dehydration, correction of anaemia, oxygen and
sometimes prostaglandins, such as iloprost. Careful
monitoring is required. Embolism will normally result in
extensive tissue necrosis within 6 hours unless the limb is
revascularised. The indications for thrombolysis, if any,
remain controversial. Irreversible ischaemia mandates
early amputation or palliative care.
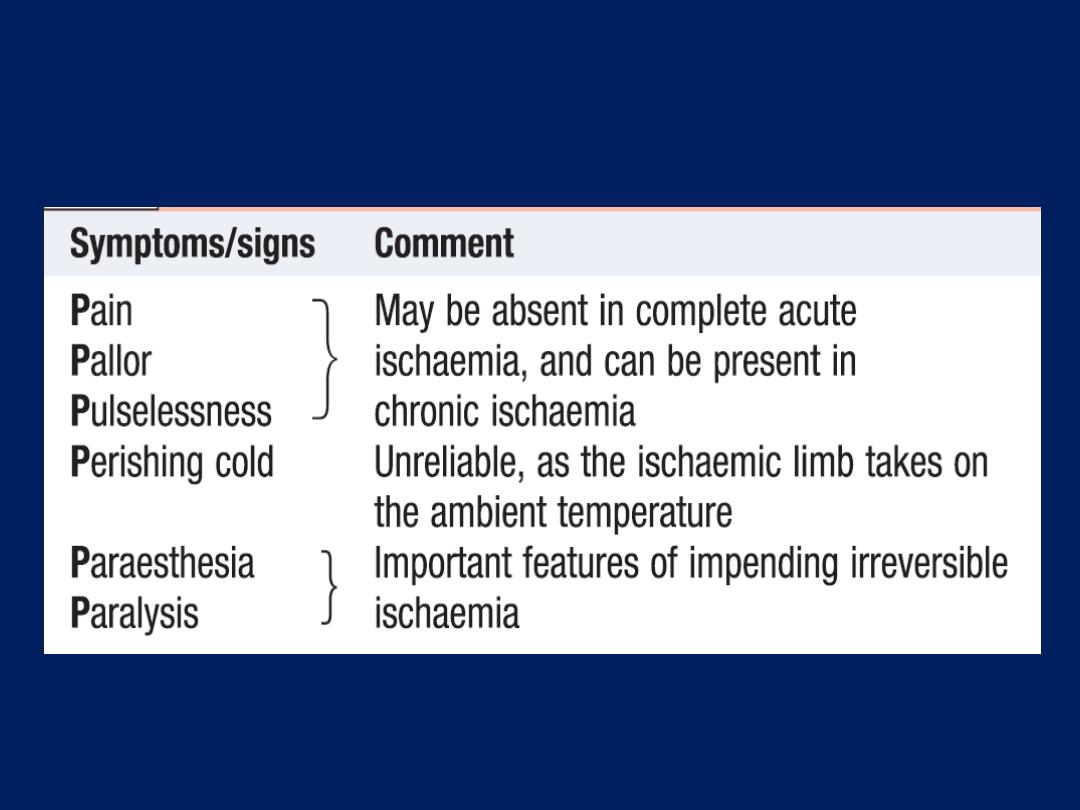
Symptoms and signs of acute limb ischaemia
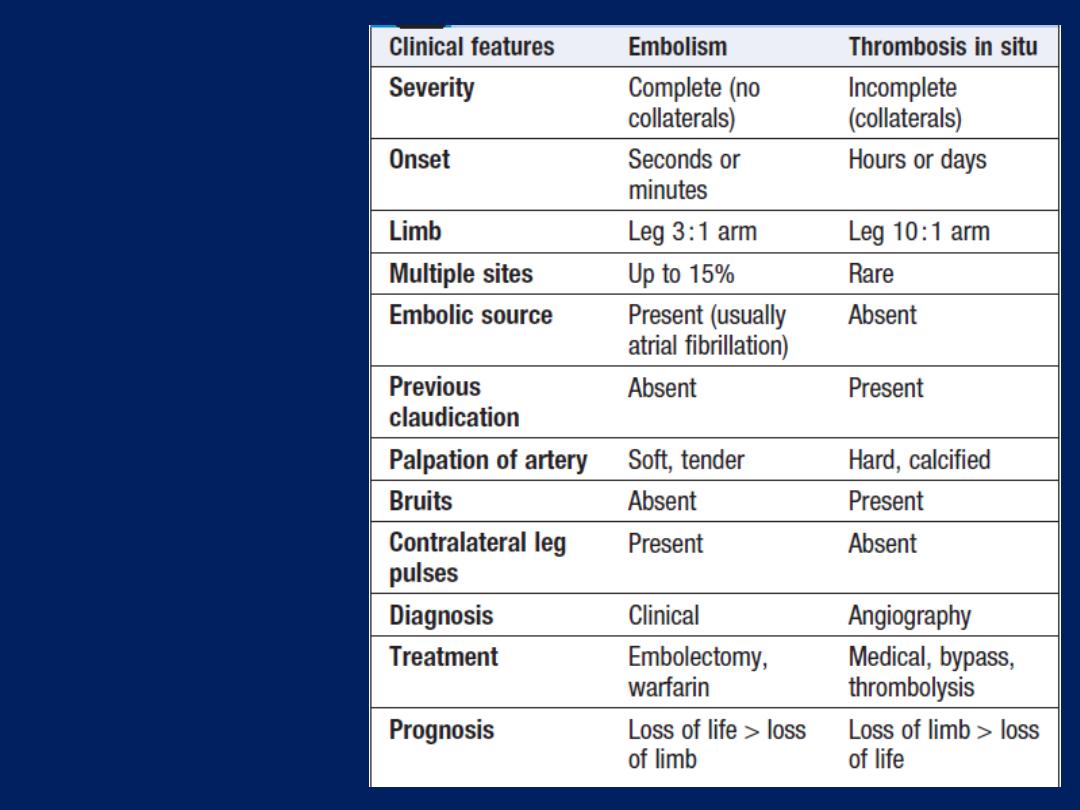
Acute limb
ischaemia:
distinguishing
features of
embolism and
thrombosis in situ
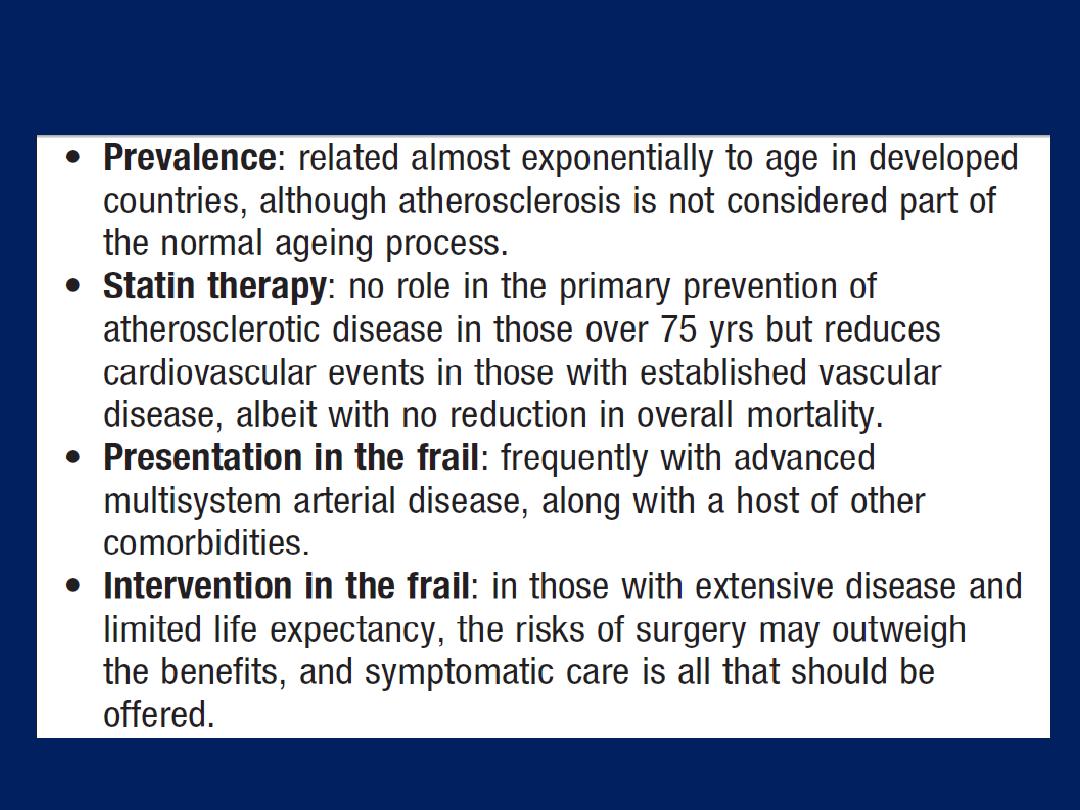
Atherosclerotic vascular disease in old age

Diseases of the aorta
Aneurysm, dissection and aortitis are the main pathologies.
Aortic aneurysm
This is an abnormal dilatation of the aortic lumen; a true
aneurysm involves all the layers of the wall, whereas a
false aneurysm does not.
Aetiology and types of aneurysm
Non-specific aneurysms
Why some patients develop occlusive vascular disease,
some develop aneurysmal vascular disease and some
develop both in response to atherosclerosis risk factors
remains unclear. Unlike occlusive disease, aneurysmal
disease tends to run in families and genetic factors are
undoubtedly important.
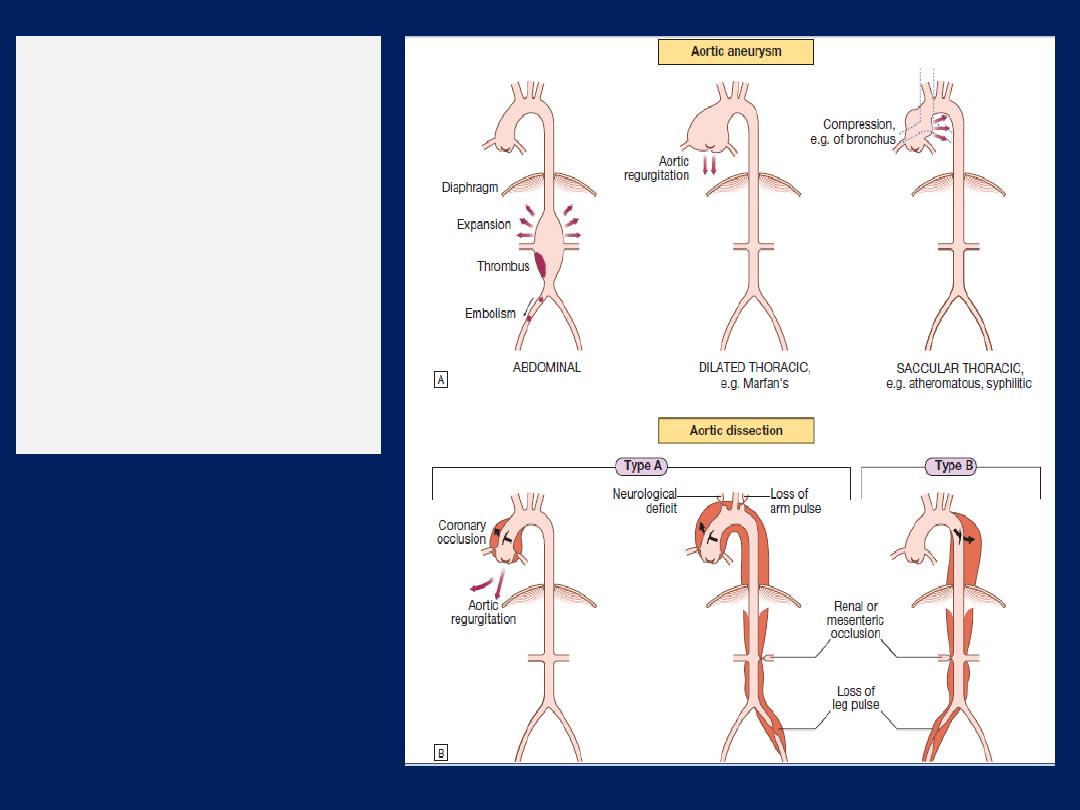
Types of aortic
disease and their
complications.
A
Types of aortic
aneurysm.
B
Types of aortic
dissection

The most common site for ‘nonspecific’ aneurysm
formation is the infrarenal abdominal aorta. The
suprarenal abdominal aorta and a variable length of the
descending thoracic aorta may be affected in 10–20% of
patients but the ascending aorta is usually spared.
Marfan’s syndrome
This disorder of connective tissue is inherited as an
autosomal dominant trait and is caused by mutations in
the fibrillin gene on chromosome 15. Affected systems
include the skeleton (arachnodactyly, joint hypermobility,
scoliosis, chest deformity and high arched palate),
the eyes (dislocation of the lens) and the cardiovascular
system (aortic disease and mitral regurgitation).

Weakening of the aortic media leads to aortic root
dilatation, regurgitation and dissection .Pregnancy is
particularly hazardous. Chest X-ray, echocardiography, MRI
or CT may detect aortic dilatation at an early stage and
can be used to monitor the disease. Treatment with β-
blockers reduces the rate of aortic dilatation and the risk of
rupture. Elective replacement of the ascending aorta may
be considered in patients with evidence of progressive
aortic dilatation but carries a mortality of 5–10%.
Aortitis
Syphilis
is a rare cause of aortitis that characteristically
produces saccular aneurysms of the ascending aorta
containing calcification. Other rare conditions associated
with aortitis include
Takayasu’s
disease .
Reiter’s
syndrome ,
giant
cell arteritis and
ankylosing
spondylitis .

Thoracic aortic aneurysms
These may produce chest pain, aortic regurgitation,
compressive symptoms such as stridor (trachea, bronchus)
and hoarseness (recurrent laryngeal nerve), and superior
vena cava syndrome . If the erode into adjacent structures,
e.g. aorto-oesophageal fistula, massive bleeding occurs.
Abdominal aortic aneurysms
Abdominal aortic aneurysms (AAAs) are present in 5%
of men aged over 60 years and 80% are confined to the
infrarenal segment. Men are affected three times more
commonly than women. AAAs can present in a number
of ways (Box). The usual age at presentation is
65–75 years for elective presentations and 75–85 years
for emergency presentations.

Population screening and prevention of
ruptured abdominal aortic aneurysm

Abdominal aortic aneurysm: common presentations

Ultrasound is the best way of establishing the diagnosis
and of following up patients with asymptomatic aneurysms
that are not yet large enough to warrant surgical repair.
CT provides more accurate information about the size and
extent of the aneurysm, the surrounding structures and
whether there is any other intra-abdominal pathology. It is
the standard pre-operative investigation but is not suitable
for surveillance because of cost and radiation dose.
Management.
Until an asymptomatic AAA has reached
a maximum of 5.5 cm in diameter, the risks of surgery
generally outweigh the risks of rupture (Box). All
symptomatic AAAs should be considered for repair, not
only to rid the patient of symptoms but also because
pain often predates rupture. Distal embolisation is a
strong indication for repair,

Distal embolisation is a strong indication for repair,
regardless of size, because otherwise limb loss is
common. Most patients with a ruptured AAA do not
survive to reach hospital, but if they do and surgery is
thought to be appropriate, there must be no delay in
getting them to the operating theatre to clamp the aorta.
Open AAA repair has been the treatment of choice in
both the elective and the emergency settings, and entails
replacing the aneurysmal segment with a prosthetic
(usually Dacron) graft. The 30-day mortality for this
procedure is approximately 5–8% for elective
asymptomatic AAA, 10–20% for emergency
symptomatic AAA and 50% for ruptured AAA.

However, patients who survive the operation to leave
hospital have a longterm survival which approaches that
of the normal population.
Increasingly, endovascular aneurysm repair
(EVAR),
using a
stent-graft
introduced via the femoral arteries in
the groin, is replacing open surgery. It is costeffective
and likely to become the treatment of choice for
Infrarenal AAA. It is possible to treat many suprarenal
and thoraco-abdominal aneurysms by EVAR too.
In the UK, a national screening programme for men
over 65 years of age has been introduced using
ultrasound scanning. For every 10 000 men scanned, 65
ruptures are prevented and 52 lives saved.

Aortic dissection
A breach in the integrity of the aortic wall allows
arterial blood to enter the media, which is then split into
two layers, creating a ‘false lumen’ alongside the
existing or ‘true lumen’ .The aortic valve may be
damaged and the branches of the aorta may be
compromised.
Typically, the false lumen eventually re-enters the true
lumen, creating a double-barrelled aorta, but it
may also rupture into the left pleural space or
pericardium with fatal consequences.
The primary event is often a spontaneous or iatrogenic
tear in the intima of the aorta; multiple tears or
entry points are common.

Other dissections are triggered by primary haemorrhage
in the media of the aorta, which then ruptures through the
intima into the true lumen. This form of spontaneous
bleeding from the vasa vasorum is sometimes confined to
the aortic wall, when it may present as a painful
intramural haematoma.
Aortic disease and hypertension are the most important
aetiological factors but other conditions may also be
implicated .
Chronic dissections may lead to aneurysmal dilatation
of the aorta, and thoracic aneurysms may be
complicated by dissection. It can therefore be difficult to
identify the primary pathology.

The peak incidence is in the sixth and seventh decades
but dissection can occur in younger patients, usually in
association with Marfan’s syndrome, pregnancy or
trauma; men are twice as frequently affected as women.
Aortic dissection is classified anatomically and for
management purposes into type A and type B, involving
or sparing the ascending aorta, respectively.
Type A dissections account for two-thirds of cases and
frequently also extend into the descending aorta.
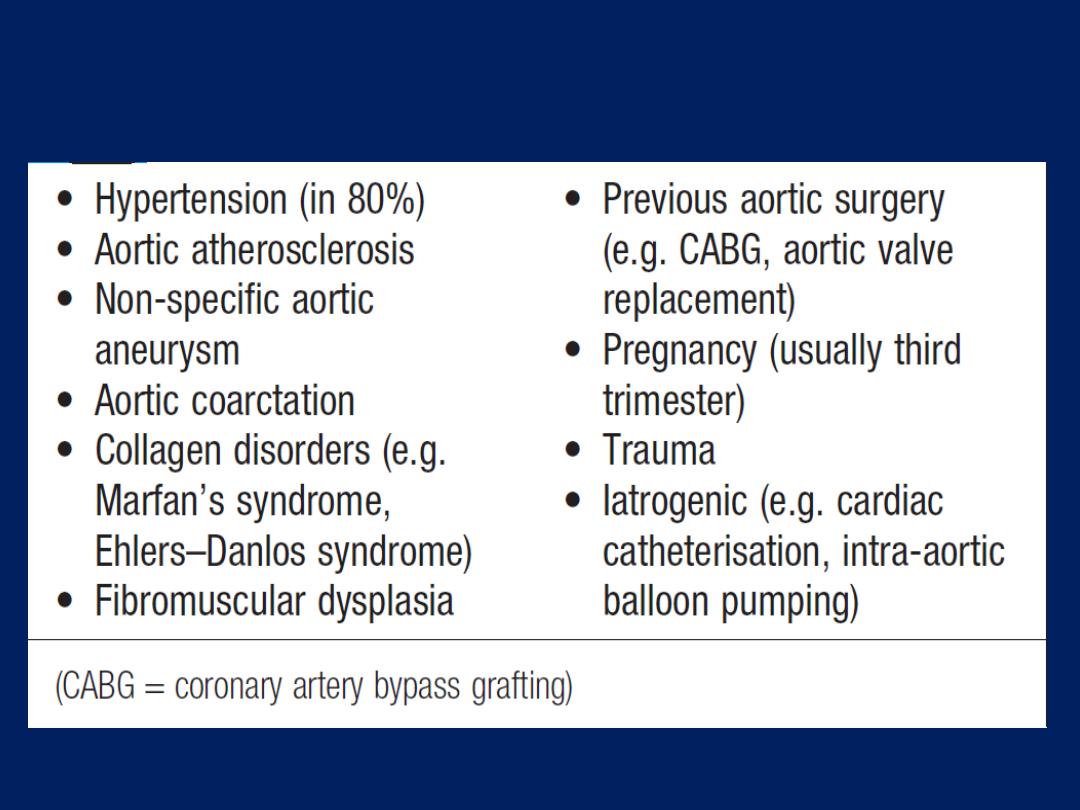
Factors that may predispose to aortic dissection

Clinical features
Involvement of the ascending aorta typically gives rise
to anterior chest pain, and involvement of the
descending aorta to intrascapular pain. The pain is
typically described as ‘tearing’ and very abrupt in onset;
collapse is common.
Unless there is major haemorrhage, the patient is
invariably hypertensive. There may be asymmetry of the
brachial, carotid or femoral pulses and signs of aortic
regurgitation. Occlusion of aortic branches
may cause MI (coronary), stroke (carotid) paraplegia
(spinal), mesenteric infarction with an acute abdomen
(coeliac and superior mesenteric), renal failure (renal)
and acute limb (usually leg) ischaemia.

Investigations
The chest X-ray characteristically shows broadening of
the upper mediastinum and distortion of the aortic
‘knuckle’, but these findings are variable and are absent
in 10% of cases. A left-sided pleural effusion is common.
The ECG may show left ventricular hypertrophy in patients
with hypertension, or rarely changes of acute MI (usually
inferior). Doppler echocardiography may show aortic
regurgitation, a dilated aortic root and, occasionally, the
flap of the dissection. Transoesophageal echo is
particularly helpful because transthoracic echo can only
provide images of the first 3–4 cm of the ascending aorta
(Fig.). CT and MRI angiography (Figs) are both highly
specific and sensitive.

Management
The early mortality of acute dissection is approximately
1–5% per hour and so treatment is urgently required.
Initial management comprises pain control and
antihypertensive treatment. Type A dissections require
emergency surgery to replace the ascending aorta. Type
B aneurysms are treated medically unless there is actual
or impending external rupture, or vital organ (gut, kidneys)
or limb ischaemia, as the morbidity and mortality
associated with surgery are very high.
The aim of medical management is to maintain a mean
arterial pressure (MAP) of 60–75 mmHg to reduce the
force of the ejection of blood from the LV.

First-line therapy is with β-blockers; the additional α-
blocking properties of labetalol make it especially
useful.
Rate-limiting calcium channel blockers, such as verapamil
or diltiazem, are used if β-blockers are contraindicated.
Sodium nitroprusside may be considered if these fail to
control BP adequately.
Percutaneous or minimal access endoluminal repair
is sometimes possible and involves either ‘fenestrating’
(perforating) the intimal flap so that blood can return
from the false to the true lumen (so decompressing the
former), or implanting a stent graft placed from the
femoral artery .
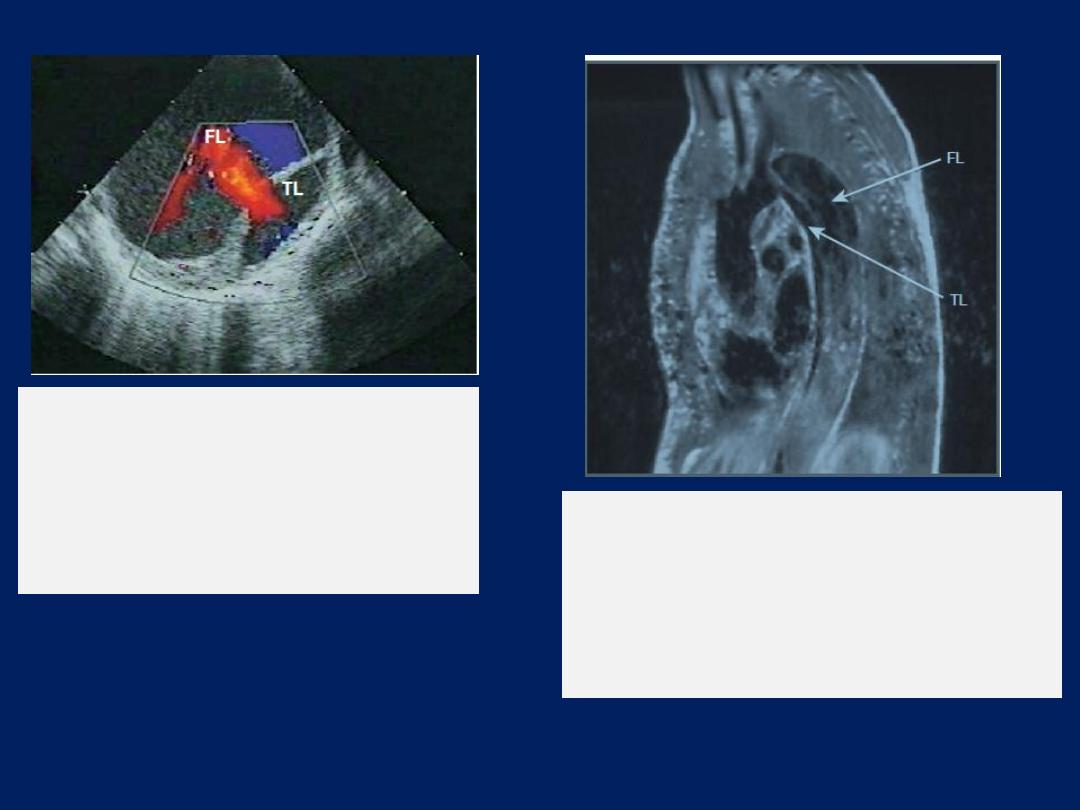
Echocardiograms from a patient with a
chronic aortic
dissection. Colour flow Doppler shows
flow from the larger false lumen (FL) into
the true lumen (TL), characteristic of
chronic disease.
Sagittal view of an MRI scan from a patient
with longstanding aortic dissection,
illustrating a biluminal aorta. There
is sluggish flow in the false lumen (FL),
accounting for its grey appearance.
(TL = true lumen)

Hypertension
Systemic BP rises with age, and the incidence of
cardiovascular disease(CVD) (particularly stroke and
coronary artery disease) is closely related to average BP at
all ages, even when BP readings are within the so-called
‘normal range’. The cardiovascular risks associated with BP
depend upon the combination of risk factors in an individual,
such as age, gender, weight, physical activity, smoking,
family history, serum cholesterol, diabetes mellitus and
pre-existing vascular disease.
Thus a practical definition
of hypertension is ‘the level of BP at which the benefits
of treatment outweigh the costs and hazards’.
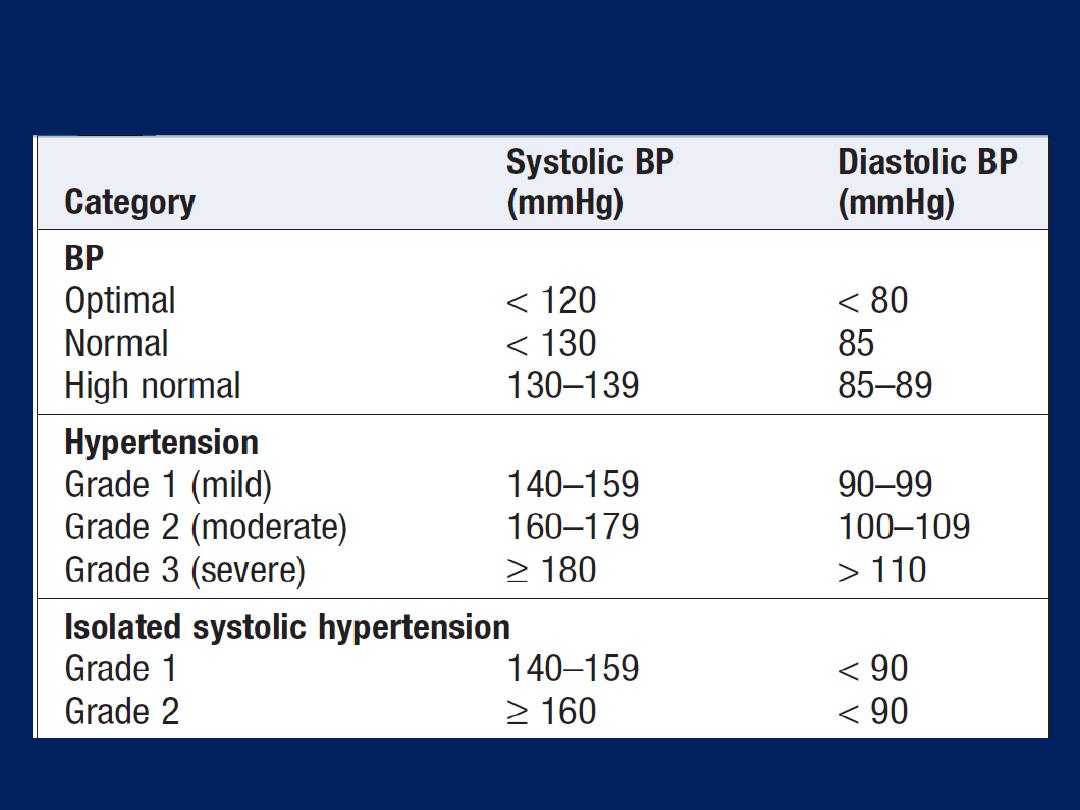
Definition of hypertension

Aetiology
In >95% of cases, a specific underlying cause cannot be
found. Such patients are said to have essential
hypertension. Many factors may contribute, including
renal dysfunction, peripheral resistance vessel tone,
endothelial dysfunction, autonomic tone, insulin resistance
and neurohumoral factors. Hypertension is more common
in some ethnic groups,
particularly African Americans and Japanese
,
and 40–60% is explained by genetic factors.
Important environmental factors include a high salt
intake, alcohol, obesity, lack of exercise and impaired
intrauterine growth. There is little evidence that ‘stress’
causes hypertension. In about 5% of cases, hypertension
can be shown to be a consequence of a specific disease
leading to sodium retention and/or vasoconstriction.
Causes of secondary hypertension
Alcohol
Obesity
Pregnancy (pre- clampsia)
Renal disease
• Parenchymal renal
disease, particularly
glomerulonephritis
• Renal vascular disease
• Polycystic kidney disease
Drugs
• e.g. Oral contraceptives
containing oestrogens,
anabolic steroids,
corticosteroids, NSAIDs,
carbenoxolone,
sympathomimetic agents
Coarctation of the aorta
Endocrine disease
• Phaeochromocytoma
• Cushing’s syndrome
• Primary hyperaldosteronism
(Conn’s syndrome)
• Glucocorticoid-suppressible
hyperaldosteronism
• Hyperparathyroidism
• Acromegaly
• Primary hypothyroidism
• Thyrotoxicosis
• Congenital adrenal
hyperplasia due to
11-
β-hydroxylase or
17-
α-hydroxylase
deficiency
• Liddle’s syndrome
• 11-β-hydroxysteroid
dehydrogenase
deficiency
Causes of secondary hypertension'cont'd

Approach to newly diagnosed hypertension
Hypertension is predominantly an asymptomatic
condition and the diagnosis is usually made at routine
examination or when a complication arises.
A BP check is advisable every 5 years in adults.
The objectives of the initial evaluation are
:
• to obtain accurate, representative BP measurements
• to identify contributory factors and any underlying
cause (secondary hypertension)
• to assess other risk factors and cardiovascular risk
• to detect any complications (target organ damage)
• to identify comorbidity that may influence the
choice of antihypertensive therapy.
These goals are attained by a careful history, clinical
examination and some simple investigations.

Measurement of blood pressure
A decision to embark upon antihypertensive therapy
effectively commits life-long treatment, so BP readings must
be as accurate as possible. Measurements should be made
to the nearest 2 mmHg, in the sitting position with the arm
supported, and repeated after 5 minutes’ rest if the first
recording is high . To avoid spuriously high readings in
obese subjects, the cuff should contain a bladder that
encompasses at least two-thirds of the arm circumference.
A series of automated
ambulatory
BP measurements
obtained over 24 hours or longer provides a better
profile than a limited number of clinic readings and
correlates more closely with evidence of target organ
damage than casual BP measurements.

Because ambulatory BP readings are systematically
lower (approximately 12/7 mmHg) than clinic
measurements. The average ambulatory daytime
(not 24-hour or night-time) BP should be used to guide
management decisions.
Patients can measure their own BP at home using a
range of commercially available semi-automatic devices.
The value of such measurements is less well established
and is dependent on the environment and timing of the
readings measured.
Home or ambulatory BP
measurements are
particularly helpful in
patients with
unusually labile BP, those with refractory hypertension,
those who may have symptomatic hypotension, and
those in whom white coat hypertension is suspected.

How to measure blood pressure

History
Family history, lifestyle (exercise, salt intake, smoking
habit) and other risk factors should be recorded. A
careful history will identify those patients with drug- or
alcohol-induced hypertension and may elicit the symptoms
of other causes of secondary hypertension, such as
phaeochromocytoma (paroxysmal headache, palpitation
and sweating) or complications such as coronary
artery disease (e.g. angina, breathlessness).

Examination
Radio-femoral delay (coarctation), enlarged kidneys (PKD),
abdominal bruits (RAS) and the characteristic facies and
habitus of Cushing’s syndrome . Examination may also
reveal features of important risk factors, such as central
obesity and hyperlipidaemia (tendon xanthomas and so
on). Most abnormal signs are due to the complications of
hypertension. Non-specific findings may include left
ventricular hypertrophy (apical heave), accentuation of the
aortic component of the second heart sound, and a fourth
heart sound.
The optic fundi are often abnormal and there may be
evidence of generalised atheroma or specific complications,
such as aortic aneurysm or peripheral vascular disease.

Target organ damage
Blood vessels
larger arteries
(> 1 mm in diameter),
the internal elastic lamina is
thickened, smooth muscle is hypertrophied and fibrous
tissue is deposited. The vessels dilate and become tortuous,
and become less compliant. In smaller arteries
(< 1 mm),
hyaline arteriosclerosis occurs in the wall, the lumen narrows
and aneurysms may develop. Widespread atheroma may
lead to coronary and cerebrovascular disease, particularly
if risk factors (smoking, hyperlipidaemia, diabetes) are
present. These structural changes often perpetuate and
aggravate hypertension by increasing peripheral vascular
resistance and reducing renal blood flow, thereby
activating the RAA axis . Hypertension is a major risk factor
for aortic aneurysm and dissection.

Central nervous system
Stroke is a common complication of hypertension and
may be due to cerebral haemorrhage or infarction.
Carotid atheroma and TIAs are more common in
hypertensive patients. Subarachnoid haemorrhage is also
associated with hypertension.
Hypertensive encephalopathy is a rare condition
characterised by high BP and neurological symptoms,
including transient disturbances of speech or vision,
paraesthesiae, disorientation, fits and loss of
consciousness. Papilloedema is common. A CT scan of the
brain often shows haemorrhage in and around the basal
ganglia; however, the neurological deficit is usually
reversible if the hypertension is properly controlled.

Retina
The optic fundi reveal a gradation of changes linked to
the severity of hypertension; fundoscopy can, therefore,
provide an indication of the arteriolar damage occurring
elsewhere .
‘Cotton wool’ exudates are associated with retinal
ischaemia or infarction, and fade in a few weeks. ‘Hard’
exudates (small, white, dense deposits of lipid) and
microaneurysms (‘dot’ haemorrhages) are more
characteristic of diabetic retinopathy. Hypertension is also
associated with central retinal vein thrombosis .
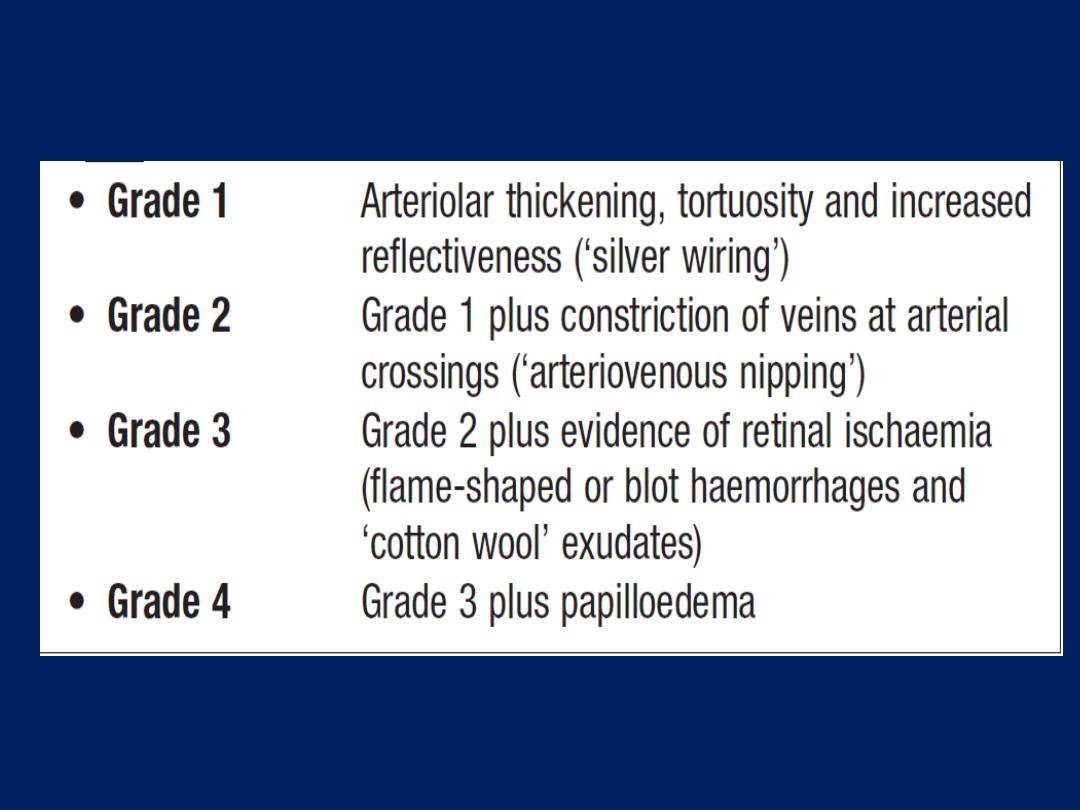
Hypertensive retinopathy

Heart
The excess cardiac mortality and morbidity are largely
due to a higher incidence of CAD . High BP places a
pressure load on the heart and may lead to LVH with a
forceful apex beat and fourth heart sound. ECG or echo
evidence of LVH is highly predictive of complications and
therefore particularly useful in risk assessment.
Atrial fibrillation is common and may be due to diastolic
dysfunction caused by LVH or the effects of CAD. Severe
hypertension can cause left ventricular failure in the
absence of CAD , particularly when renal function, and
therefore sodium excretion, are impaired.
Kidneys
Long-standing hypertension may cause proteinuria and
renal failure by damaging the renal vasculature.

‘Malignant’ or ‘accelerated’ phase hypertension
This rare condition may complicate hypertension of any
aetiology and is characterised by accelerated
microvascular damage with necrosis in the walls of small
arteries and arterioles (‘fibrinoid necrosis’) and by
intravascular thrombosis. The diagnosis is based on
evidence of high BP and rapidly progressive end organ
damage, such as retinopathy (grade 3 or 4), renal
dysfunction (especially proteinuria) and/or hypertensive
encephalopathy (see above). Left ventricular failure may
occur and, if this is untreated, death occurs within months.

Investigations
All hypertensive patients should undergo a limited number
of investigations .Additional investigations are appropriate
in selected patients .
Management
Quantification of cardiovascular risk .The sole objective of
antihypertensive therapy is to reduce the incidence of
adverse cardiovascular events, particularly CAD, stroke and
heart failure. Randomised trials have demonstrated that
antihypertensive therapy can reduce the incidence of
stroke and, to a lesser extent, CAD. The relative benefits
(approximately 30% reduction in risk of stroke and 20%
reduction in risk of CAD) are similar in all patient groups, so
the absolute benefit of treatment (total number of events
prevented) is greatest in those at highest risk.

For example, to extrapolate from the Medical Research
Council (MRC) Mild Hypertension Trial (1985), 566
young patients would have to be treated with
bendroflumethiazide for 1 year to prevent 1 stroke, while
in the MRC trial of antihypertensive treatment in the
elderly (1992), 1 stroke was prevented for every 286
patients treated for 1 year.
A formal estimate of absolute cardiovascular risk,
which takes account of all the relevant risk factors, may
help to determine whether the likely benefits of therapy
will outweigh its costs and hazards. A variety of risk
algorithms are available for this purpose.

Most of the excess morbidity and mortality
associated with hypertension is attributable to
coronary artery disease and many treatment
guidelines are therefore based on estimates of the
10-year coronary artery disease risk.
Total cardiovascular risk can be estimated
by multiplying coronary artery disease risk by 4/3
(i.e. if coronary artery disease risk is 30%,
cardiovascular risk is 40%).
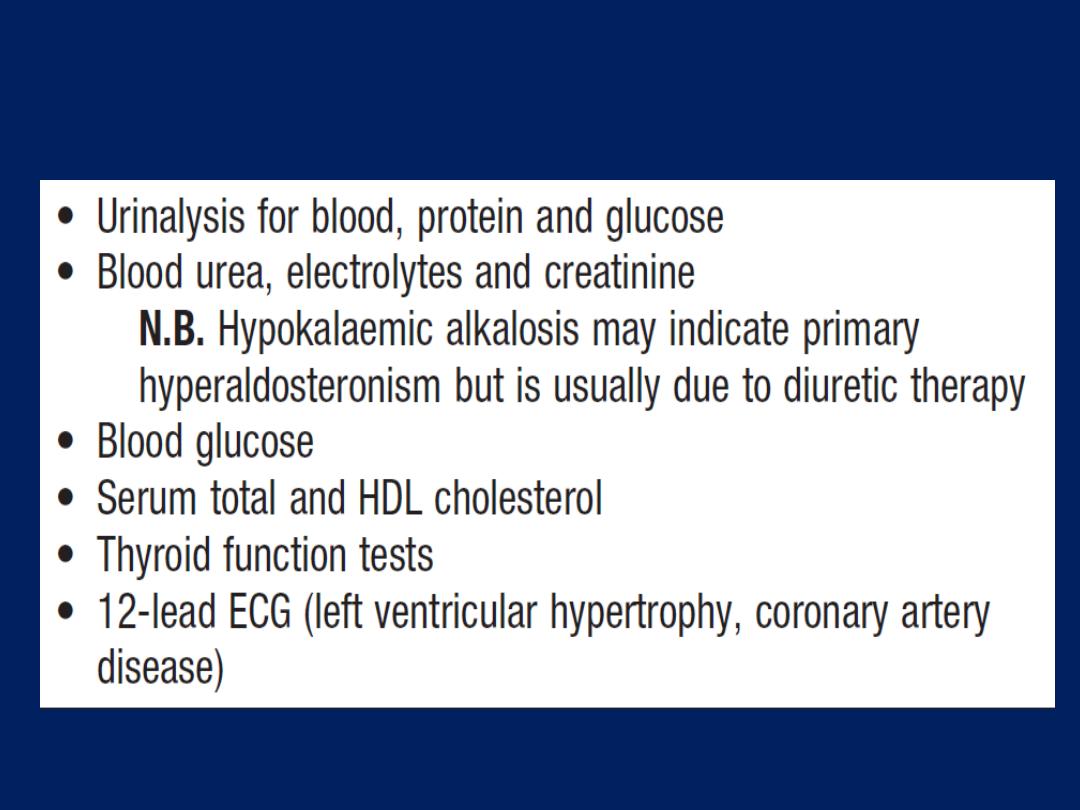
Hypertension: investigation of all patients

Hypertension: investigation of selected patients
• CX-ray: cardiomegaly, heart failure, Coarctation
• Ambulatory BP recording: to assess borderline or ‘white
coat’ hypertension
• Echocardiogram: to detect or quantify LVH
• Renal ultrasound: to detect possible renal disease
• Renal angiography: to detect or confirm presence of RAS
• Urinary catecholamines: phaeochromocytoma
• Urinary cortisol and dexamethasone suppression test: to
detect possible Cushing’s syndrome
• Plasma renin activity and aldosterone: to detect
possible primary aldosteronism

Threshold for intervention
Systolic BP and diastolic BP are both powerful predictors
of cardiovascular risk. The British Hypertension
Society management guidelines therefore utilise both
readings, and treatment should be initiated if they
exceed the given threshold .
Patients with diabetes or CVD are at particularly high risk
and the threshold for initiating antihypertensive therapy is
therefore lower (≥ 140/90 mmHg) in these patient
groups. The thresholds for treatment in the elderly are
the same as for younger patients .
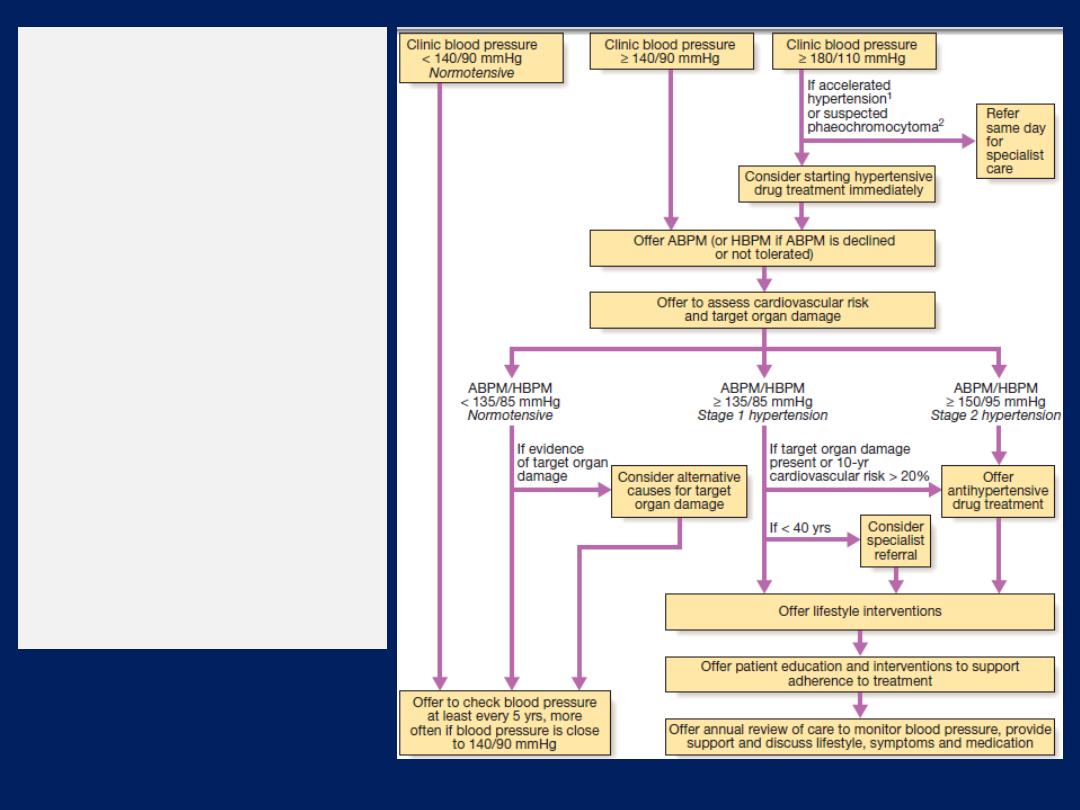
Management of
hypertension
:
British
Hypertension Society
guidelines.
1
Signs of
papilloedema or retinal
haemorrhage.
2
Labile or
postural hypotension,
headache, palpitations,
pallor and diaphoresis
(ABPM = ambulatory
blood pressure
monitoring; HBPM =
home blood pressure
monitoring).

Treatment targets
The optimum BP for reduction of major cardiovascular
events has been found to be 139/83 mmHg, and even
lower in patients with diabetes mellitus. Moreover,
reducing BP below this level causes no harm. Primary care
strategies have been devised to improve screening and
detection of hypertension that, in the past, remained
undetected in up to half of affected. Application of new
guidelines should help establish patients on appropriate
treatment, and allow step-up if lifestyle modification and
first-line drug therapy fail to control patients’ BP. Patients
taking antihypertensive therapy require follow-up at 3-
monthly intervals to monitor BP, minimize side-effects and
reinforce lifestyle advice.
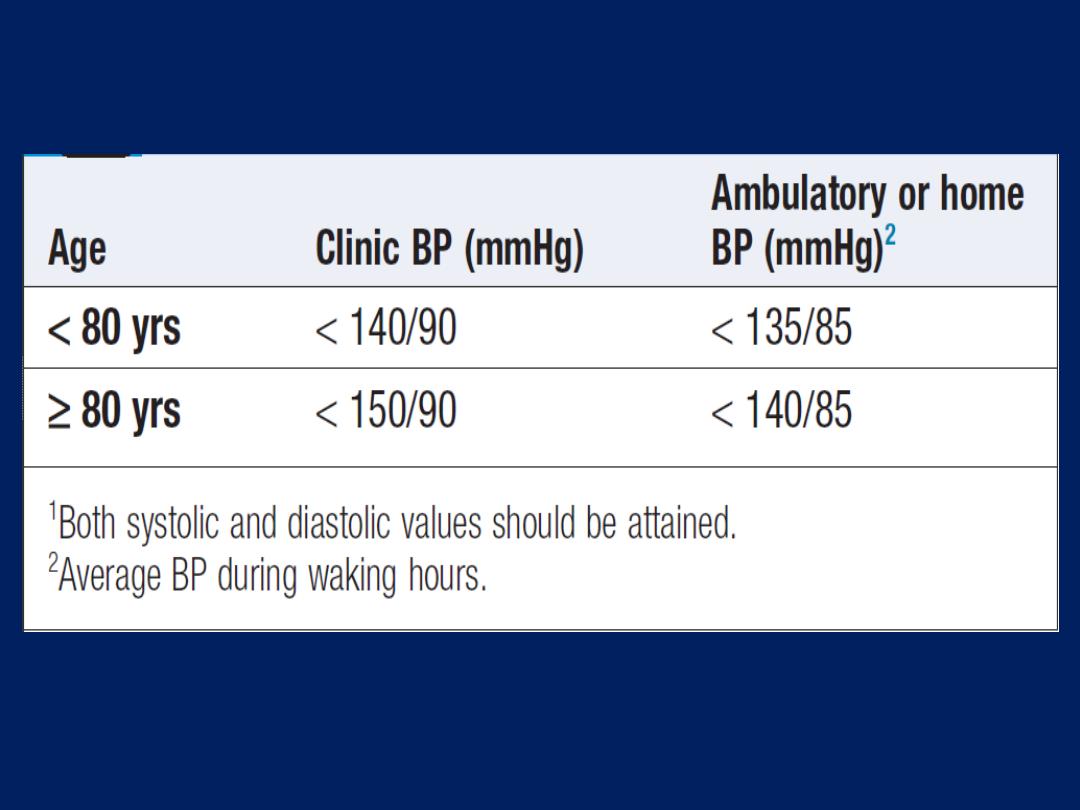
Optimal target blood pressures1

Non-drug therapy
Appropriate lifestyle measures may obviate the need for
drug therapy in patients with borderline hypertension,
reduce the dose and/or the number of drugs required
in patients with established hypertension, and directly
reduce cardiovascular risk.
Correcting obesity, reducing alcohol intake, restricting
salt intake, taking regular physical exercise and
increasing consumption of fruit and vegetables can all
lower BP. Moreover, quitting smoking, eating oily fish
and adopting a diet that is low in saturated fat may
produce further reductions in cardiovascular risk.

Antihypertensive drugs
Thiazide and other diuretics. The mechanism of action
of these drugs is incompletely understood and it may
take up to a month for the maximum effect to be
observed. An appropriate daily dose is 2.5 mg
bendroflumethiazide or 0.5 mg cyclopenthiazide. More
potent loop diuretics, such as furosemide (40 mg daily) or
bumetanide (1 mg daily), have few advantages over
thiazides in the treatment of hypertension, unless there is
substantial renal impairment or they are used in
conjunction with an ACE inhibitor.

ACE inhibitors. ACE inhibitors (e.g. enalapril 20 mg
daily, ramipril 5–10 mg daily or lisinopril 10–40 mg
daily) inhibit the conversion of angiotensin I to angiotensin
II and are usually well tolerated. They should
be used with particular care in patients with impaired
renal function or renal artery stenosis because they can
reduce the filtration pressure in the glomeruli and
precipitate renal failure.
Electrolytes and creatinine
should be checked
before and 1–2 weeks after
commencing therapy. Side-effects include first-dose
hypotension, cough, rash, hyperkalaemia and renal
dysfunction. Angiotensin receptor blockers. Angiotensin
receptor blockers (e.g. irbesartan 150–300 mg daily,

Valsartan 40–160 mg daily) block the angiotensin II type I
receptor and have similar effects to ACE inhibitors; however,
they do not cause cough and are better tolerated.
Calcium channel antagonists. The dihydropyridines
(e.g. amlodipine 5–10 mg daily, nifedipine 30–90 mg
daily) are effective and usually well-tolerated that are
particularly useful in older people. Side-effects include
flushing, palpitations and fluid retention.
The rate-limiting calcium channel antagonists
(e.g. diltiazem 200–300 mg daily, verapamil 240 mg
daily) can be useful when hypertension coexists with
angina but they may cause bradycardia. The main side
effect of verapamil is constipation.

Beta-blockers. These are
no longer used as first-line
antihypertensive therapy,
except in
patients with
another indication for the drug (e.g. angina). Metoprolol
(100–200 mg daily), atenolol (50–100 mg daily) and
bisoprolol (5–10 mg daily) preferentially block cardiac
β
1
-adrenoceptors, as opposed to the β
2
-adrenoceptors that
mediate vasodilatation and bronchodilatation.
Labetalol and carvedilol. Labetalol (200 mg–2.4 g daily
in divided doses) and carvedilol (6.25–25 mg twice
daily) are combined β- and α- adrenoceptor antagonists
which are sometimes more effective than pure β-blockers.
Labetalol can be used as an infusion in malignant phase
hypertension (see below).

Other drugs. A variety of vasodilators may be used.
These include the α
1
-adrenoceptor antagonists
(α-blockers), such as prazosin (0.5–20 mg daily in
divided doses), indoramin (25–100 mg twice daily) and
doxazosin (1–16 mg daily), and drugs that act directly
on vascular smooth muscle, such as hydralazine
(25–100 mg twice daily) and minoxidil (10–50 mg
daily).
Side-effects include first-dose
and
postural hypotension,
headache, tachycardia and fluid retention.
Minoxidil also causes increased facial hair and is
therefore unsuitable for female patients.

Choice of antihypertensive drug
Trials that have compared thiazides, calcium antagonists,
ACEI and ARB have not shown consistent differences in
outcome, efficacy, side-effects or quality of life. Beta-
blockers, which previously featured as first-line therapy in
guidelines, have a weaker evidence base . The choice is
initially dictated by the age and ethnic background,
although cost and convenience will influence the exact drug
used. Response to initial therapy and side-effects guides
subsequent treatment. Comorbid conditions also have an
influence on initial selection ,for example, a β-blocker might
be the most appropriate treatment for a patient with
angina. Thiazide diuretics and dihydropyridine calcium
channel antagonists are the most suitable for older people.

Although some patients can be treated with a single
antihypertensive drug,
a combination
of drugs is often
required to achieve optimal BP control . Combination
therapy may be desirable for other reasons; for
example, low-dose therapy with two drugs may produce
fewer unwanted effects than treatment with the
maximum dose of a single drug.
Some drug combinations have complementary or synergistic
actions; for example, thiazides increase activity of the renin–
angiotensin system, while ACE inhibitors block it.
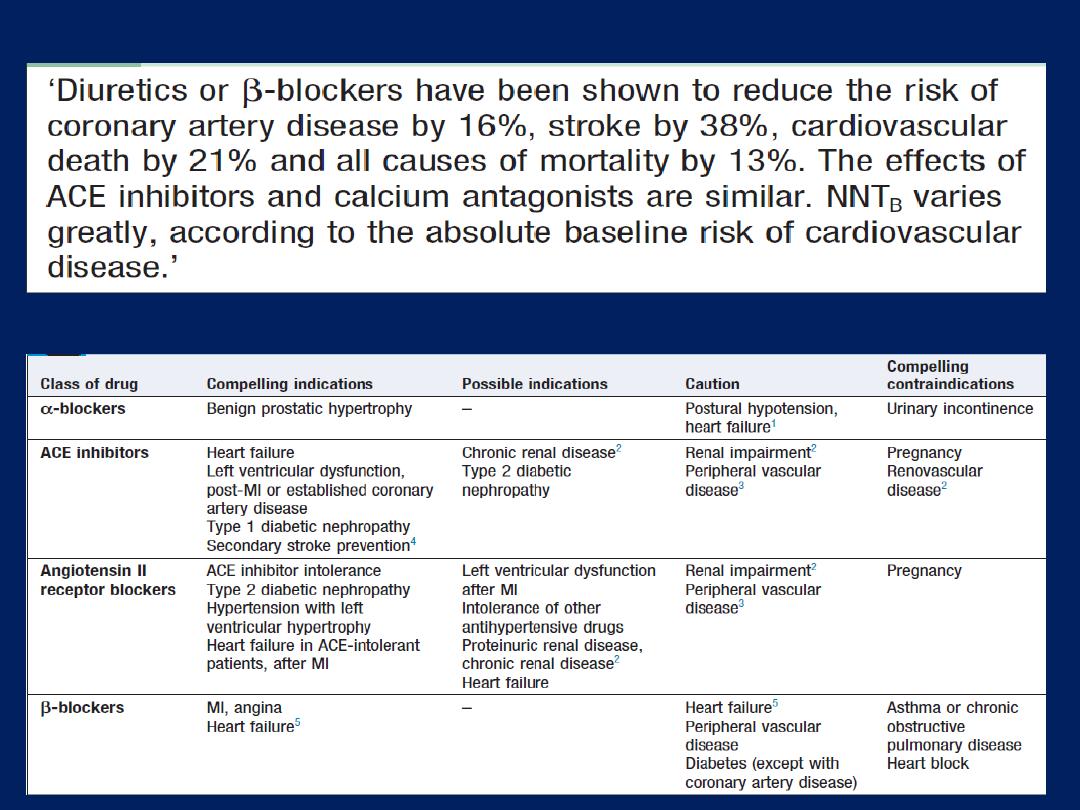
Benefit of antihypertensive drug therapy
The influence of comorbidity on the choice of antihypertensive
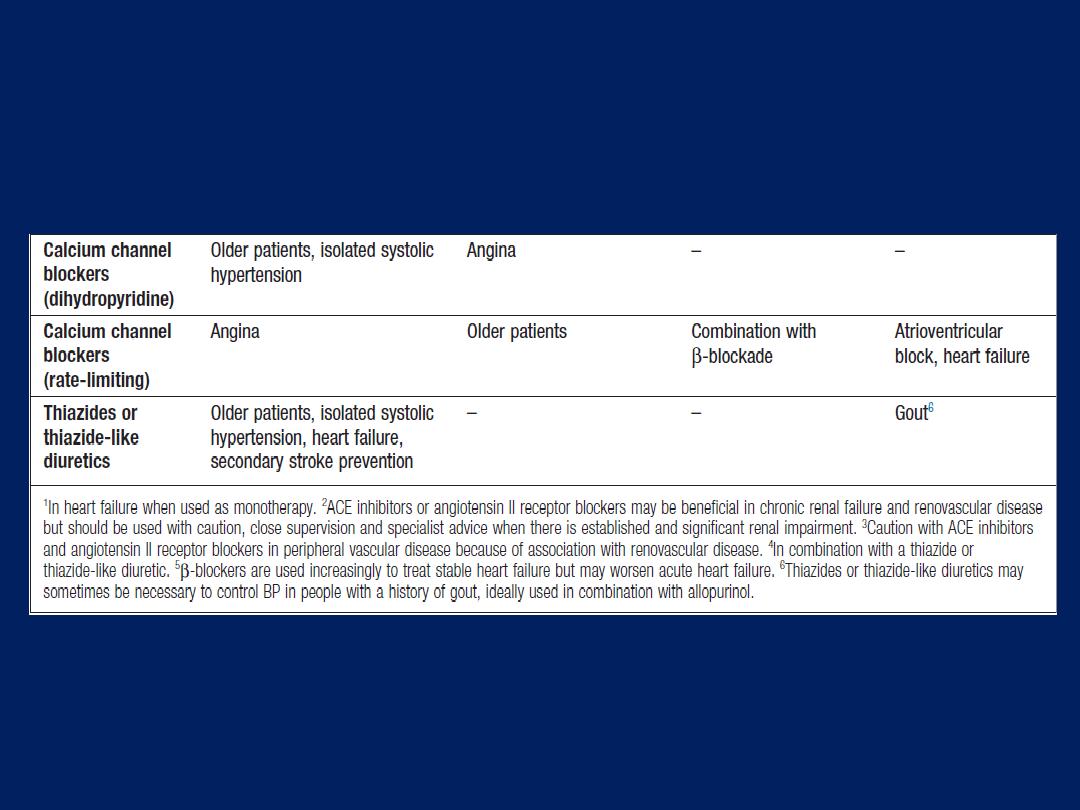
The influence of comorbidity on the choice of
antihypertensive drug therapy'cont'd
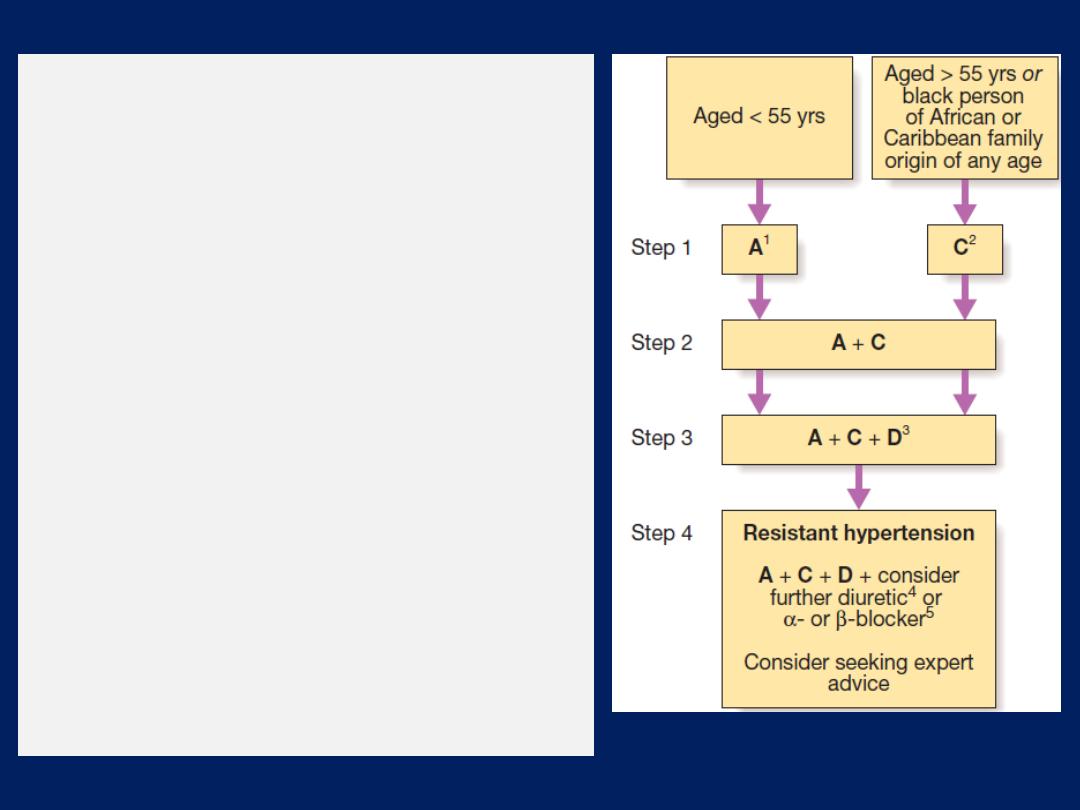
Antihypertensive drug combinations.
Black
patients are those of African or Caribbean
descent, and not mixed-race, Asian or
Chinese patients.
1
A
= ACE inhibitor or
consider angiotensin II receptor blocker (ARB);
2
C
= calcium channel blocker (CCB); but
consider a thiazide-like diuretic if a CCB is not
tolerated or the person has oedema, evidence
of heart failure or a high risk of heart failure.
3
D
= thiazide-type diuretic.
4
Consider a low
dose of spironolactone or higher doses of a
thiazide-like diuretic.
At the time of publication by NICE (August
2011), spironolactone did not have a UK
marketing authorisation for this indication.
Informed consent should be obtained and
documented.
5
Consider an α- or β-blocker if
further diuretic therapy is not tolerated, or is
contraindicated or ineffective.

Emergency treatment of accelerated or malignant HBP
In accelerated phase hypertension, lowering BP too
quickly may compromise tissue perfusion (due to altered
autoregulation) and can cause cerebral damage, including
occipital blindness, and precipitate coronary or renal
insufficiency. Even in the presence of cardiac failure or
hypertensive encephalopathy, a controlled reduction
to a level of about 150/90 mmHg over a period of
24–48 hours is ideal.
In most
patients,
it is possible to
avoid parenteral therapy and bring BP under control with
bed rest and oral drug therapy. IV or IM labetalol
(2 mg/min
to a maximum of 200 mg),
IV glyceryl trinitrate
(0.6–1.2 mg/ hr),
IM
hydralazine (
5 or 10 mg aliquots repeated at half hourly intervals)
and IV
sodium nitroprusside
(0.3–1.0 μg/kg body weight/min)
are all
effective but require careful supervision.

Refractory hypertension
The common causes of treatment failure in hypertension
are non-adherence to drug, inadequate therapy, and
failure to recognise an underlying cause, such as renal
artery stenosis or phaeochromocytoma; of these , the first
is by far the most prevalent. Simple treatment regimens,
attempts to improve rapport with.
Adjuvant drug therapy
• Aspirin. a powerful means of reducing cardiovascular
risk but may cause bleeding, particularly intracerebral
haemorrhage, in a small number of patients. The benefits
outweigh the risks in hypertensive patients aged 50 years
or over who have well controlled BP and either target
organ damage, diabetes or a 10-year CAD risk 15% (or
10-year cardiovascular disease risk of at least 20%).

• Statins. Treating hyperlipidaemia can produce a
substantial reduction in cardiovascular risk. These
drugs are strongly indicated in patients who have
established vascular disease, or hypertension with
a high (at least 20% in 10 years) risk of
developing cardiovascular disease .
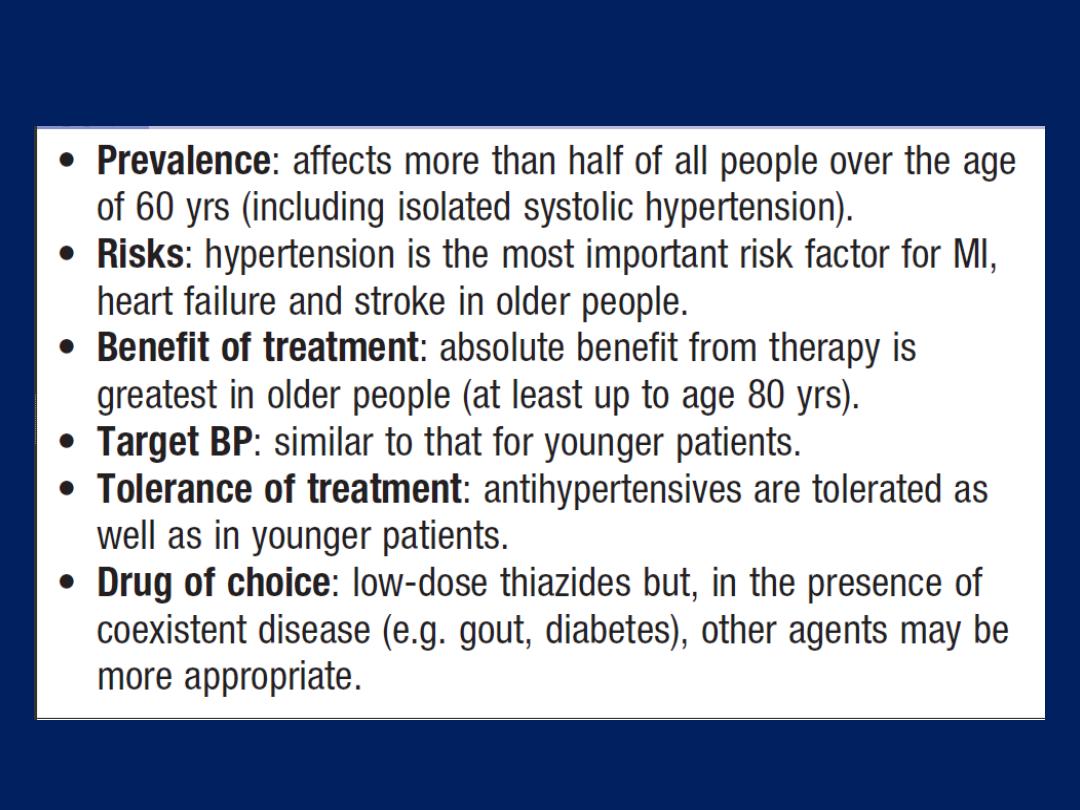
Hypertension in old age

DISEASES OF THE HEART VALVES
A diseased valve may be narrowed or may fail to close
adequately, and thus permit regurgitation of blood.
‘Incompetence’ is a less precise term for regurgitation or
reflux, and should be avoided. Doppler echo is the most
useful technique for assessing valvular heart disease.
Patients with valvular heart disease are susceptible to
bacterial endocarditis, which can be prevented by good
dental hygiene.
The routine use of antibiotic prophylaxis e.g. dental
extraction, is no longer recommended.
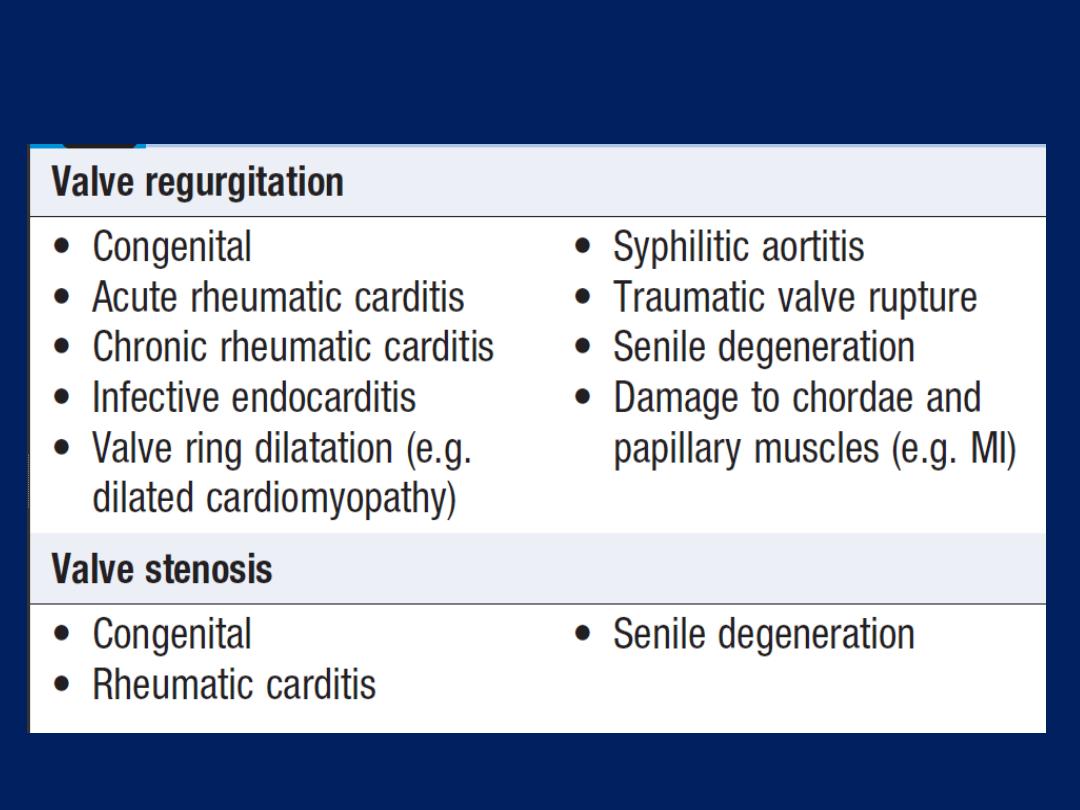
Principal causes of valve disease

Rheumatic heart disease
Acute rheumatic fever
Usually affects children (5 - 15 years) or young adults.
The condition is triggered by an immune-mediated delayed
response to infection with specific strains of group A
streptococci, which have antigens that may cross-react with
cardiac myosin and sarcolemmal membrane protein.
Antibodies produced against the streptococcal antigens
cause inflammation in the endocardium, myocardium and
pericardium, as well as the joints and skin. Histologically,
Aschoff nodules
are pathognomonic and occur only in
the heart.They are composed of multinucleated giant cells
surrounded by macrophages and T lymphocytes, and not
seen until the
subacute or chronic phases of rheumatic carditis.
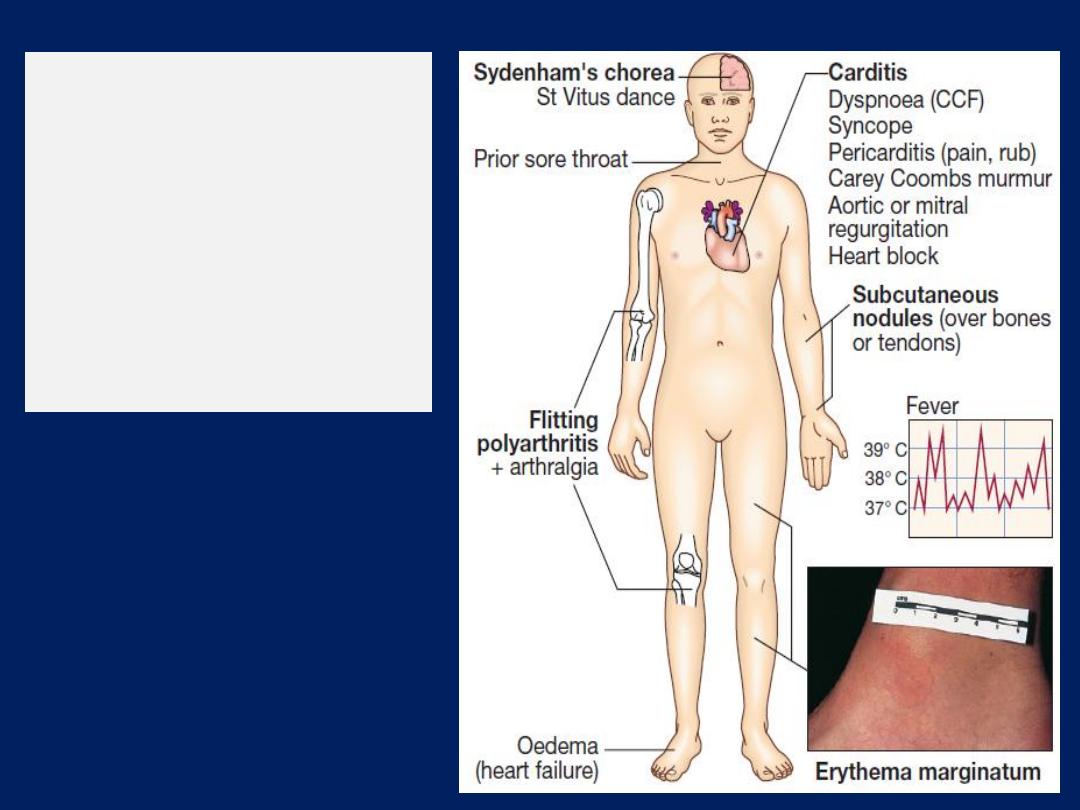
Clinical features of
rheumatic fever.
Bold labels indicate
Jones major criteria
(CCF = congestive
cardiac failure).
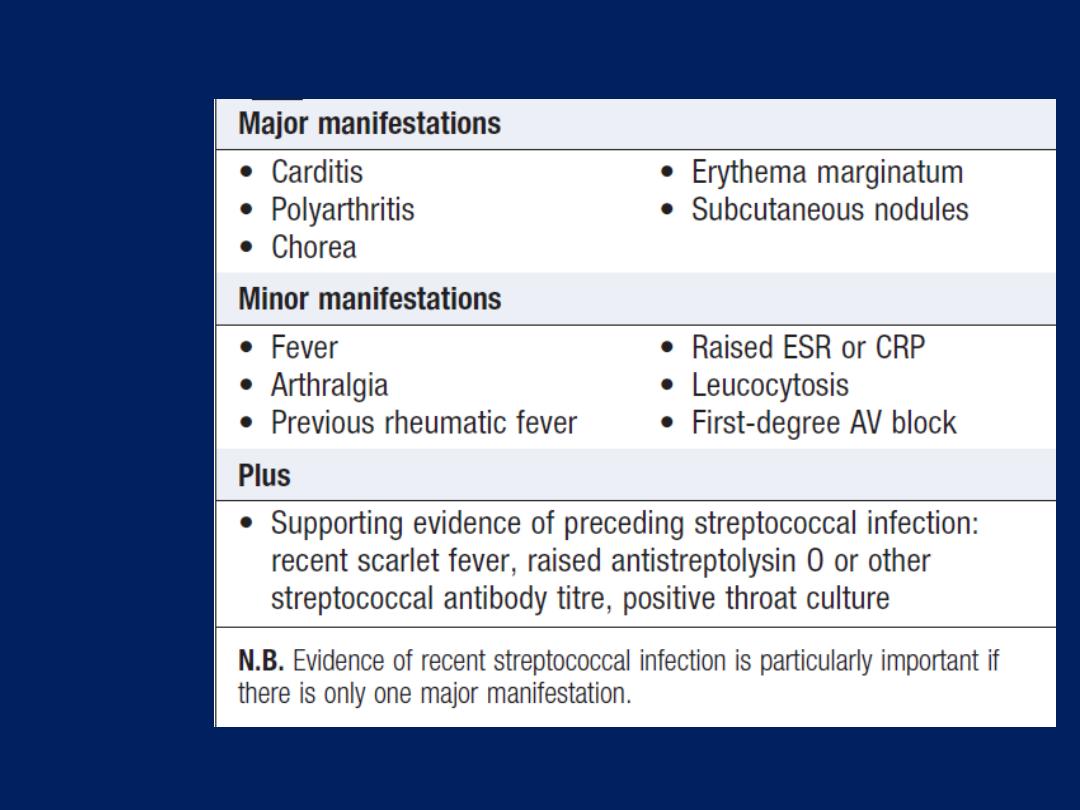
Jones
criteria for
the
diagnosis
of
rheumatic
fever

Clinical features
Acute rheumatic fever is a multisystem disorder that
usually presents with fever, anorexia, lethargy and joint
pain, 2–3 weeks after an episode of streptococcal
pharyngitis. There may, however, be no history of sore
throat. Arthritis occurs in approximately 75% of patients.
Other features include rashes, carditis and neurological
changes .The diagnosis, made using the
revised Jones
criteria
is based upon two or more major manifestations,
or
one major and two or more minor manifestations,
along
with evidence of preceding streptococcal infection.
Only about 25% of patients will have a positive culture for
group A streptococcus at the time of diagnosis because
there is a latent period between infection and presentation.

Serological evidence of recent infection with a raised
antistreptolysin O (ASO) antibody titre is helpful.
A presumptive diagnosis
of acute rheumatic fever can
be made without evidence of preceding streptococcal
infection in cases of isolated chorea or pancarditis, if
other causes for these have been excluded.
Carditis
A ‘pancarditis’ . It may manifest as breathlessness (due to
heart failure or pericardial effusion), palpitations or chest
pain (usually due to pericarditis or pancarditis).
Other features include tachycardia, cardiac enlargement
and new or changed murmurs.
A soft systolic murmur due to mitral R is very common.

A soft mid-diastolic murmur (
Carey Coombs murmur
) is
typically due to valvulitis, with nodules forming on the
mitral valve leaflets.
Aortic regurgitation occurs in 50% of cases but the
tricuspid and pulmonary valves are rarely involved.
Pericarditis may cause chest pain, a pericardial friction
rub and precordial tenderness.
Cardiac failure may be due to myocardial dysfunction or
valvular regurgitation.
ECG changes commonly include STT changes. Conduction
defects sometimes occur and may cause syncope.

Arthritis
This is the most common major manifestation and occurs
early when streptococcal antibody titres are high.
An acute painful asymmetric and migratory
inflammation of the large joints typically affects the
knees, ankles, elbows and wrists.
The joints are involved in quick succession and are usually
red, swollen and tender for between a day and 4 weeks.
The pain characteristically
responds to aspirin; if not, the diagnosis is in doubt.

Skin lesions
Erythema marginatum
occurs in less than 5% of patients. The lesions start as red
macules that fade in the centre but remain red at the
edges, and occur mainly on the trunk and proximal
extremities but not the face. The resulting red rings or
‘margins’ may coalesce or overlap .
Subcutaneous nodules
occur in 5–7% of patients. They are small (0.5–2.0 cm),
firm and painless, and are best felt over extensor surfaces
of bone or tendons. They typically appear > 3 weeks after
the onset of other manifestations and therefore help to
confirm rather than make the diagnosis.
Other rare
manifestations pleurisy, effusion and pneumonia.

Sydenham’s chorea (St Vitus dance)
This is a late neurological manifestation that appears at
least 3 months after the episode of acute rheumatic
fever, when all the other signs may have disappeared. It
occurs in up to one-third of cases and is more common in
females. Emotional lability may be the first feature and
is typically followed by purposeless, involuntary,
choreiform movements of the hands, feet or face. Speech
may be explosive and halting. Spontaneous recovery
usually occurs within a few months. Approximately
one-quarter of affected
patients will go on to develop
chronic rheumatic valve disease.

Investigations
The
ESR
and
CRP
are useful for monitoring
progress of the disease . Positive throat swab
cultures
are obtained in only
10–25
% of cases.
ASO
titres are normal in one-fifth of adult cases of
rheumatic fever and most cases of chorea.
Echo
typically shows mitral regurgitation with
dilatation of the mitral annulus and prolapse of the
anterior mitral leaflet, and may also show aortic
regurgitation and pericardial effusion.
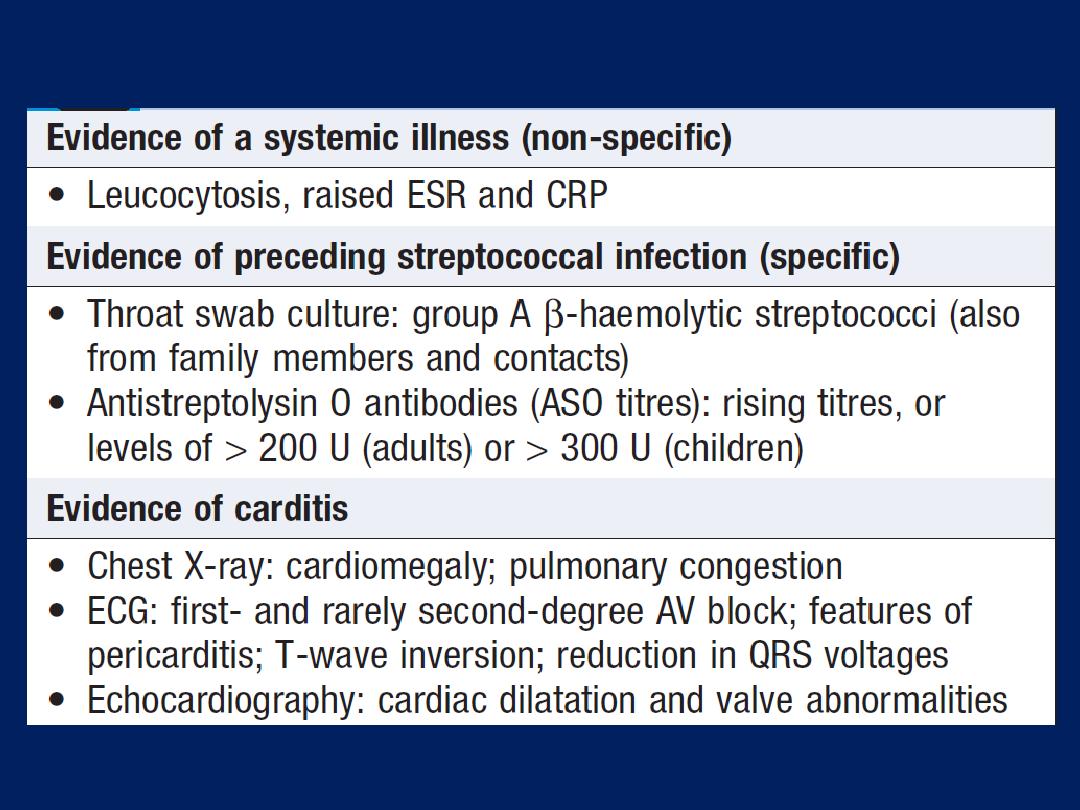
Investigations in acute rheumatic fever

Management of the acute attack
A single
dose of benzyl penicillin (1.2 million U IM) or
oral phenoxymethylpenicillin (250 mg 4 times daily for
10 days) should be given on diagnosis to eliminate any
residual streptococcal infection. If the patient is penicillin-
allergic, erythromycin or a cephalosporin can be used.
Treatment is then directed towards limiting cardiac damage
and relieving symptoms.
Bed rest and supportive therapy
Bed rest is important, as it lessens joint pain and reduces
cardiac workload. The duration guided by symptoms, along
with temperature, leucocyte count and ESR, and should be
continued until these have settled.

Patients can then return
to normal physical activity
but
strenuous exercise should be avoided in those who have
had carditis.
Cardiac failure should be treated as necessary.
Some patients, particularly
those in early adolescence,
develop a fulminant form of the disease with severe
mitral regurgitation and, sometimes, concomitant aortic
regurgitation. If heart failure in these cases does not
respond to medical treatment, valve replacement may be
necessary and is often associated with a dramatic decline
in rheumatic activity. AV block is seldom progressive and
pacemaker insertion rarely needed.

Aspirin
This usually relieves the symptoms of arthritis rapidly
and a response within 24 hours helps confirm the
diagnosis.
A reasonable starting dose is 60 mg/kg BW/day ,
divided into six doses.
In adults, 100 mg/ kg per day may be needed up to the
limits of tolerance or a maximum of 8 g per day.
Mild toxicity
includes nausea, tinnitus and deafness;
vomiting,
tachypnoea and acidosis are more serious.
Aspirin should be continued until the ESR has fallen, and
then gradually tailed off.

Corticosteroids
These produce more rapid symptomatic relief than
aspirin and are indicated in cases with carditis or severe
arthritis. There is no evidence that long-term steroids
are beneficial. Prednisolone (1.0–2.0 mg/kg per day in
divided doses) should be continued until the ESR is
normal, and then tailed off.
Secondary prevention
Patients are susceptible to further attacks of rheumatic
fever if another streptococcal infection occurs, and longterm
prophylaxis with penicillin should be given as benzathine
penicillin (1.2 million U IM monthly), if compliance is in
doubt, or oral phenoxymethylpenicillin
(250 mg twice daily).

Sulfadiazine or erythromycin may be used if the patient is
allergic to penicillin; sulphonamides prevent infection but
are not effective in the eradication of group A
streptococci. Further attacks of rheumatic fever are
unusual after the age of 21, when treatment may be
stopped. However, it should be extended if an attack has
occurred in the last 5 years, or if the patient lives in an
area of high prevalence or has an occupation (e.g.
teaching) with high exposure to streptococcal infection. In
those with residual heart disease
, prophylaxis should
continue until 10 years after the last episode or 40 years
of age, whichever is later.
Long-term antibiotic prophylaxis prevents another attack
of acute rheumatic fever but does not protect against
infective endocarditis.

Chronic rheumatic heart disease
Chronic valvular heart disease develops in at least
half
of those affected by rheumatic
fever with carditis.
Two
thirds of cases occur in women. Some episodes of rheumatic
fever pass unrecognised and it is only possible to elicit a
history of rheumatic fever or chorea in about half of all
patients with chronic rheumatic heart disease.
The mitral valve is affected in more than 90% of cases;
the aortic valve is the next most frequently involved,
followed by the tricuspid and then the pulmonary valve.
Isolated mitral stenosis accounts for about 25% of all
cases, and an additional 40% have mixed mitral stenosis
and regurgitation. Valve disease may be symptomatic
during fulminant forms of acute rheumatic fever but
may remain asymptomatic for many years.

Pathology
The main pathological process in chronic rheumatic
heart disease is progressive fibrosis. The heart valves are
predominantly affected but involvement of the pericardium
and myocardium may contribute to heart failure and
conduction disorders. Fusion of the mitral commissures
and shortening of the chordae tendineae may lead to
mitral stenosis with or without regurgitation. Similar changes
in the aortic and tricuspid valves produce distortion and
rigidity of the cusps, leading to stenosis and regurgitation.
Once a valve has been damaged, the altered
haemodynamic stresses perpetuate and extend the
damage, even in the absence of
a continuing rheumatic process.

Mitral valve disease
Mitral stenosis
Aetiology and pathophysiology
Mitral stenosis is almost always rheumatic in origin,
although in older people it can be caused by heavy
calcification of the mitral valve apparatus. There is also
a rare form of congenital mitral stenosis.
In rheumatic mitral stenosis, the mitral valve orifice is
slowly diminished by progressive fibrosis, calcification of
the valve leaflets, and fusion of the cusps and subvalvular
apparatus. The blood flow from LA to LV is restricted and
left atrial pressure rises, leading to pulmonary venous
congestion and breathlessness.

There is dilatation and hypertrophy of the LA, and left
ventricular filling becomes more dependent on left
atrial contraction. Any increase in heart rate shortens
diastole when the mitral valve is open and produces a
further rise in left atrial pressure. Situations that demand
an increase in cardiac output also increase left atrial
pressure, so exercise and pregnancy are poorly tolerated.
The mitral valve orifice is normally about 5 cm
2
in
diastole and may be reduced to 1 cm
2
in severe mitral
stenosis.
Patients usually remain asymptomatic until the stenosis is
<2 cm
2
. Reduced lung compliance, due to chronic
pulmonary venous congestion, contributes to breathlessness,
and a low cardiac output may cause fatigue.

Atrial fibrillation due to progressive dilatation of
the LA is very common. Its onset often precipitates
pulmonary oedema because the accompanying
tachycardia and loss of atrial contraction lead to marked
haemodynamic deterioration with a rapid rise in left
atrial pressure.
In contrast,
a more gradual rise in left
atrial pressure tends to cause an increase in pulmonary
vascular resistance, which leads to pulmonary
hypertension that may protect the patient from pulmonary
oedema. Pulmonary hypertension leads to right ventricular
hypertrophy and dilatation, tricuspid regurgitation
and right heart failure. Fewer than 20% of patients remain
in sinus rhythm; many of these have a small fibrotic LA and
severe pulmonary hypertension.
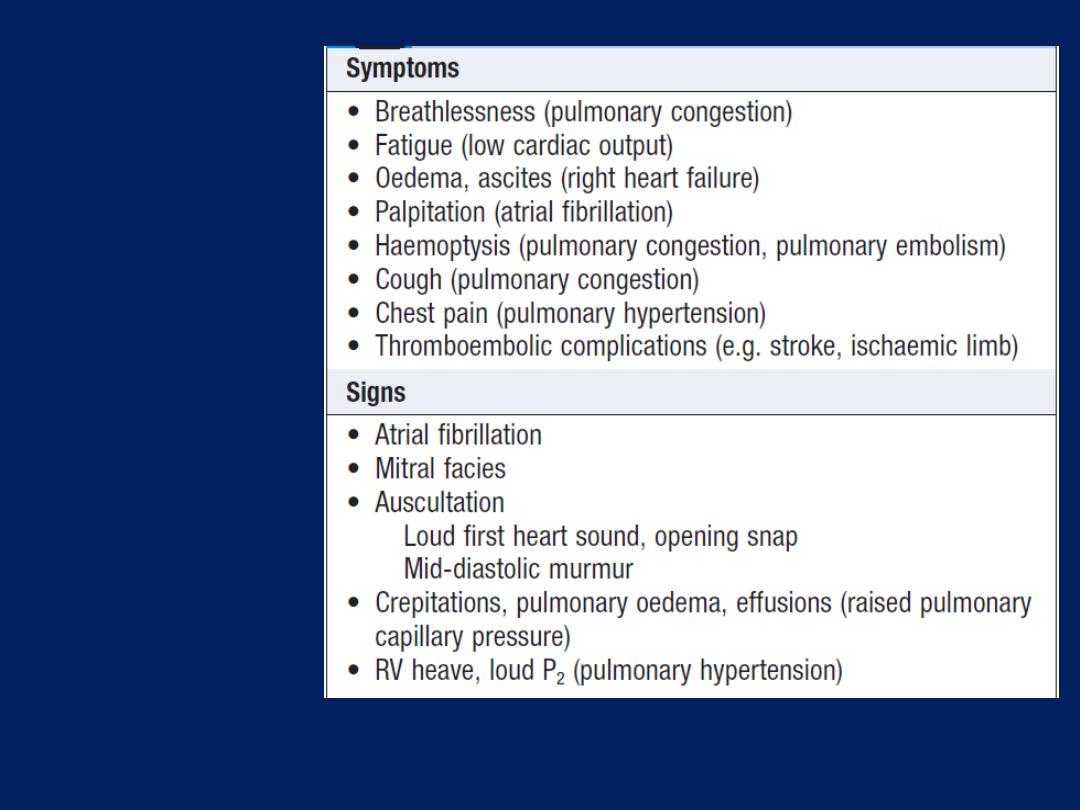
Clinical
features (and
their causes) in
mitral stenosis

Clinical features
Effort-related dyspnoea is usually the dominant symptom .
Exercise tolerance typically diminishes very slowly over
many years. Eventually, symptoms occur at rest. Acute
pulmonary oedema or pulmonary hypertension can lead
to haemoptysis. All patients, and particularly those with
atrial fibrillation, are at risk from left atrial thrombosis and
systemic thromboembolism. The physical signs of MS are
often found before symptoms develop. The forces that
open and close the mitral valve increase as left atrial
pressure rises. S1 is therefore loud and can be palpable
(tapping apex beat). An opening snap may be audible
and moves closer to the S2 as the stenosis becomes more
severe and left atrial pressure rises.

However, the first heart sound and opening snap may be
inaudible if the valve is heavily calcified. Turbulent flow
produces the characteristic low-pitched mid-diastolic
murmur and sometimes a thrill. The murmur is accentuated
by exercise and during atrial systole (pre-systolic
accentuation).
Coexisting mitral regurgitation causes a pansystolic
murmur that radiates towards the axilla.
Pulmonary hypertension may ultimately lead to right
ventricular hypertrophy and dilatation with secondary
tricuspid regurgitation, which causes a systolic murmur
and giant ‘v waves’ in the venous pulse.
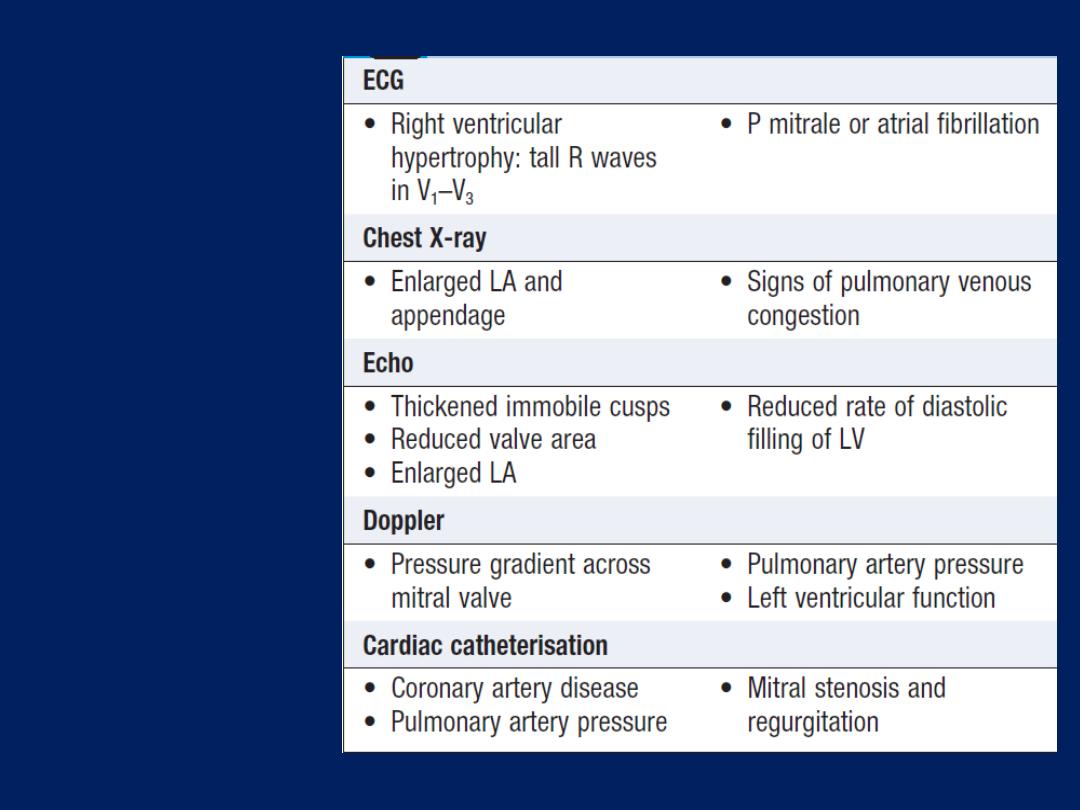
Investigations
in mitral
stenosis

Investigations
The ECG may show either atrial fibrillation or bifid P
waves (P mitrale) associated with left atrial hypertrophy .
A typical chest X-ray is shown in Figure previously.
Doppler provides the definitive evaluation of MS .
Cardiac cath is used to assess coexisting conditions.
Management
Patients with minor symptoms should be treated
medically, consists of anticoagulation, ventricular rate
control (digoxin, ββ or rate-limiting calcium antagonists) in
atrial fibrillation, and diuretic therapy to control pulmonary
congestion. Intervention by balloon valvuloplasty,
valvotomy or replacement should be considered
if the patient remains symptomatic despite medical
treatment or if pulmonary hypertension develops.

Mitral balloon valvuloplasty and valve replacement
Valvuloplasty is the treatment of choice if specific criteria
are fulfilled . Patients should be followed up at 1–2-yearly
intervals because re-stenosis may occur. Surgical closed or
open mitral valvotomy is an acceptable alternative.
Valve replacement is indicated if there is substantial
mitral reflux or if the valve is rigid and calcified.
Criteria for mitral valvuloplasty

Mitral regurgitation
Aetiology and pathophysiology
Rheumatic disease is the principal cause in countries
where rheumatic fever is common, but elsewhere,, other
causes are more important. Mitral regurgitation may also
follow mitral valvotomy or valvuloplasty.
Chronic mitral regurgitation causes gradual dilatation
of the LA with little increase in pressure and therefore
relatively few symptoms. Nevertheless, the LV dilates
slowly and the left ventricular diastolic and left atrial
pressures gradually increase as a result of chronic volume
overload of the LV. In contrast, acute MR causes a rapid rise
in left atrial pressure (because left atrial compliance is
normal) and marked symptomatic deterioration.
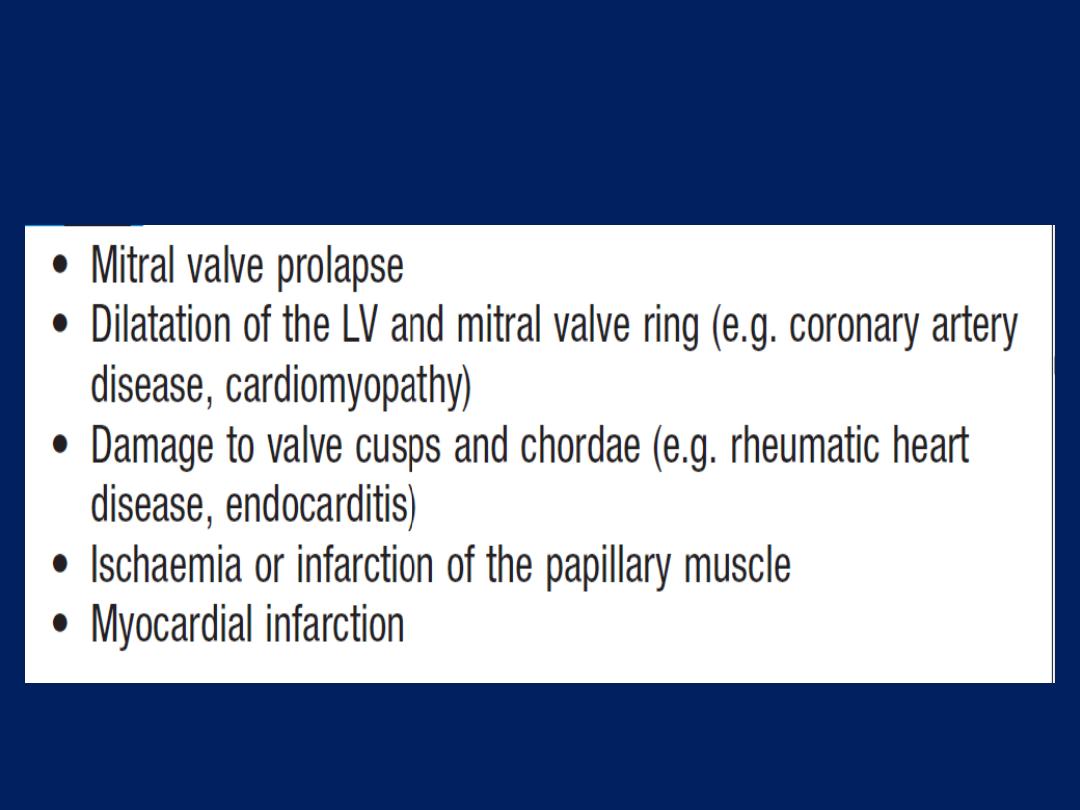
Causes of mitral regurgitation

Mitral valve prolapse
This is also known as ‘floppy’ mitral valve and is one of
the more common causes of mild mitral regurgitation.
It is caused by congenital anomalies or degenerative
myxomatous changes, and is sometimes a feature of
connective tissue disorders such as Marfan’s syndrome .
In its mildest forms, the valve remains competent but
bulges back into the atrium during systole, causing a
mid-systolic click but no murmur.
In the presence of a
regurgitant
valve, the click is followed by a late systolic
murmur, which lengthens as the regurgitation becomes
more severe. A click is not always audible and the physical
signs may vary with both posture and respiration.

Progressive elongation of the chordae tendineae leads to
increasing mitral regurgitation, and if chordal rupture
occurs, regurgitation suddenly becomes severe. This is
rare before the fifth or sixth decade of life.
Mitral valve prolapse is associated with a variety of
typically benign arrhythmias, atypical chest pain and a
very small risk of embolic stroke or TIA.
Nevertheless, the overall long-term prognosis is good.
Other causes of mitral regurgitation
Dilatation of the LV distorts the geometry of these and may
cause mitral regurgitation. Dilated cardiomyopathy and
heart failure from coronary artery disease are common
causes of so-called ‘functional’ mitral regurgitation.
Endocarditis is an important cause of acute regurgitation.

Clinical features
Symptoms depend on how suddenly the regurgitation
develops . Chronic mitral regurgitation produces
a symptom complex that is similar to that of mitral
stenosis, but
sudden-onset
mitral regurgitation usually
presents with acute pulmonary
oedema
.
The regurgitant jet causes an apical systolic murmur
which radiates into the axilla and may be accompanied
by a thrill.
Increased forward flow through the mitral valve causes
a loud third heart sound and even a short mid-diastolic
murmur.
The apex beat feels active and rocking due to left
ventricular volume overload and is usually displaced to
the left as a result of left ventricular dilatation.
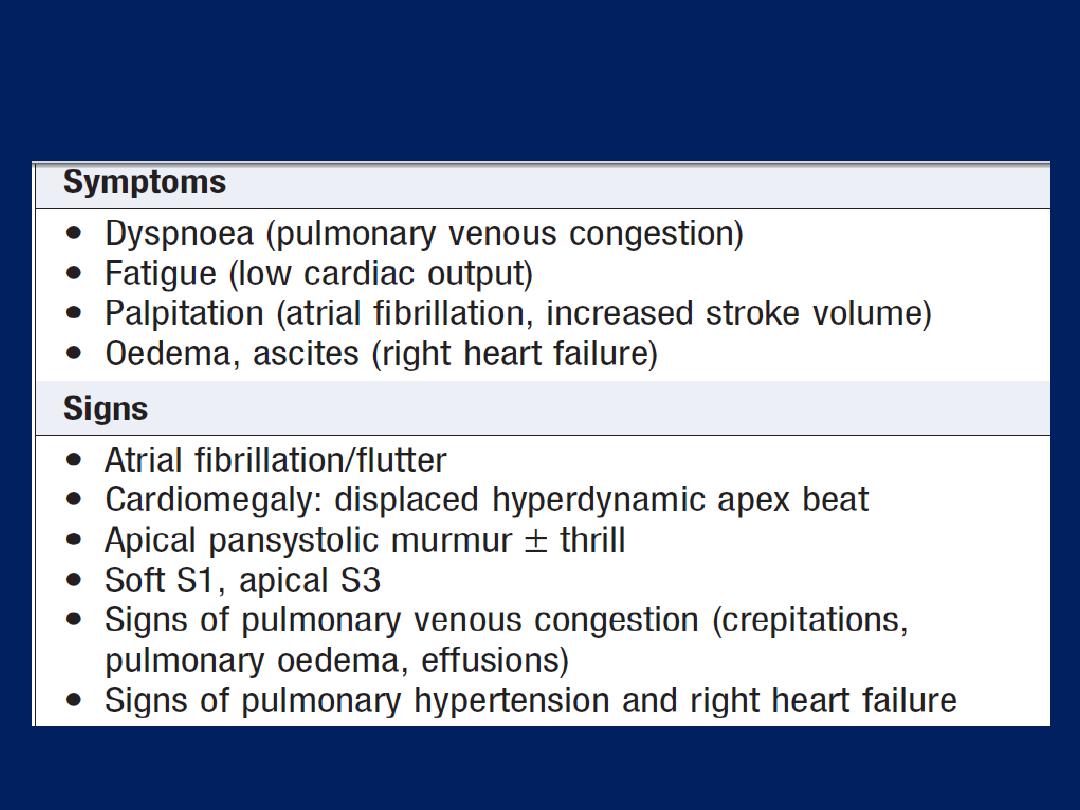
Clinical features (and their causes) in mitral
regurgitation

Investigations
Atrial fibrillation is common, as a consequence of atrial
dilatation. At cardiac catheterisation , the severity of mitral
regurgitation can be assessed by left ventriculography and
by the size of the
v
(systolic) waves in the left atrial or
pulmonary artery wedge pressure trace.
Management
MR of moderate severity can be treated medically . In all
patients, high afterload may worsen the degree of
regurgitation, and hypertension should be treated with
vasodilators, such as ACE inhibitors. Patients should be
reviewed at regular intervals, progressive cardiomegaly or
echo evidence of deteriorating left ventricular function are
indications for mitral valve replacement or repair.

Mitral valve repair is used to treat valve prolapse and
offers many advantages when compared to replacement,
such that it is now advocated for severe regurgitation,
even in asymptomatic, because results are excellent and
early repair prevents irreversible LVdamage. MR often
accompanies the ventricular dilatation and dysfunction that
are concomitants of CAD . If such patients are to undergo
CABG , it is common practice to repair the valve and
restore mitral valve function by inserting an annuloplasty
ring to overcome annular dilatation.
It can be difficult,
however, to determine whether V-dilatation or the MR that
is the predominant problem. If dilatation is the underlying
cause, then valve repair or replacement may actually
worsen ventricular function, as the ventricle can no longer
empty into the low-pressure LA.
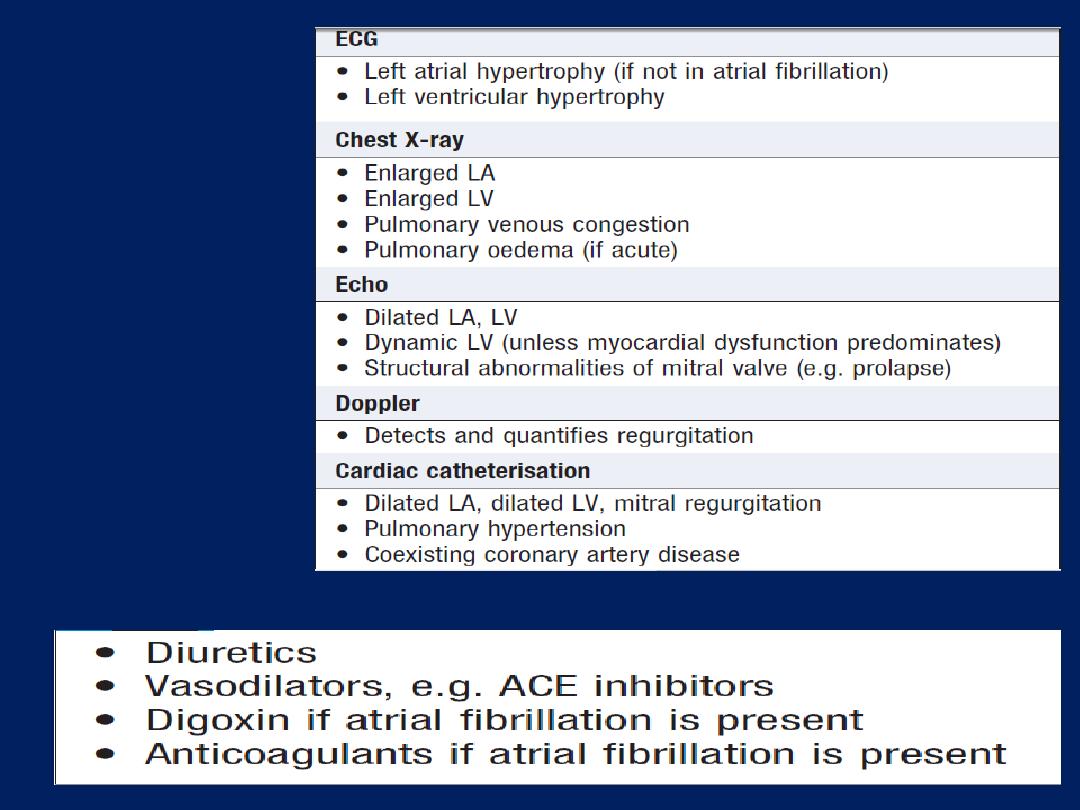
Investigations
in mitral
regurgitation
Medical management of mitral regurgitation

Aortic valve disease
Aortic stenosis
Aetiology and pathophysiology
The likely aetiology depends on the age of the patient.
In congenital aortic stenosis, obstruction is present from
birth or becomes apparent in infancy. With bicuspid
aortic valves, obstruction may take years to develop as
the valve becomes fibrotic and calcified. The aortic valve
is the second most frequently affected by rheumatic
fever and, commonly, both the aortic and mitral valves
are involved.
In older people
, a structurally normal
tricuspid aortic valve may be affected by fibrosis and
calcification, in a process that is histologically similar to
that of atherosclerosis affecting the arterial wall.

Haemodynamically significant stenosis develops slowly,
typically occurring
at 30–60 years
in those with rheumatic,
50–60
in bicuspid valves and
70–90 years
with degenerative
calcific disease. Cardiac output is initially maintained at the
cost of a steadily increasing pressure gradient across the
valve. The LV becomes increasingly hypertrophied and
coronary blood flow may then be inadequate; patients
may therefore develop angina, even in the absence of
concomitant coronary disease. The fixed outflow obstruction
limits the increase in cardiac output required on exercise.
Eventually, the LV can no longer overcome the obstruction
and pulmonary oedema supervenes.
In contrast
to MS , which
tends to progress very slowly, aortic typically remain asymptomatic
for many years
but
deteriorate rapidly when symptoms develop, and
death usually ensues within 3–5 years of these.
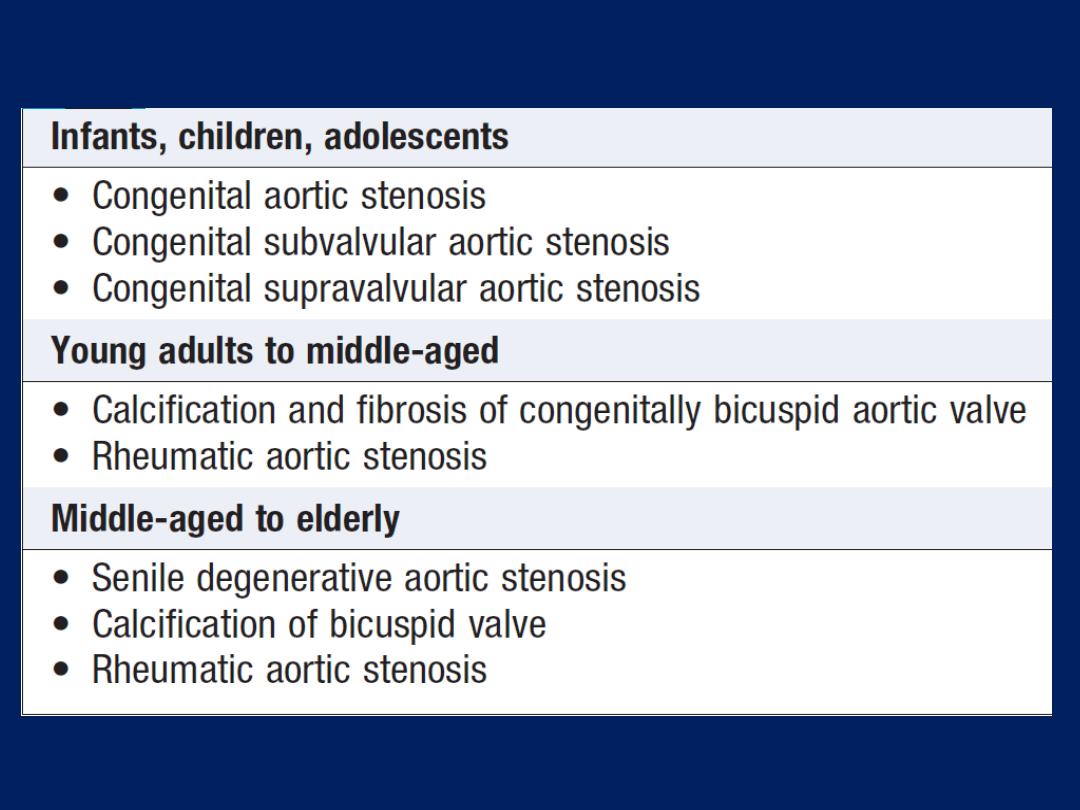
Causes of aortic stenosis

Clinical features
AS is commonly picked up in asymptomatic at routine
clinical examination but the three
cardinal symptoms
are
angina, breathlessness and syncope .
Angina
arises
because of the increased demands of the hypertrophied
LV working against the high-pressure outflow tract
obstruction, leading to a mismatch between oxygen
demand and supply, but may also be due to coexisting
CAD , especially in old age, when it affects over
50% of patients.
Exertional breathlessness
suggests
cardiac decompensation as a consequence of the excessive
pressure overload placed on the LV.
Syncope
usually occurs on exertion when cardiac output
fails to rise to meet demand, leading to a fall in BP.

A harsh ejection systolic murmur radiates to the neck,
with a soft S2, particularly in those with calcific valves.
The murmur is often likened to a saw cutting wood and
may have a musical quality like the ‘mew’ of a
seagull.The severity may be difficult to gauge clinically,
as older patients with a non-compliant ‘stiff’ arterial
system may have an apparently normal carotid upstroke
in the presence of severe stenosis.
Milder degrees of
stenosis may be difficult to
distinguish from aortic
sclerosis, in which the valve is thickened or calcified but
not obstructed.
A careful examination should be made for other valve
lesions, particularly in rheumatic heart disease, when
there is frequently concomitant mitral valve disease.
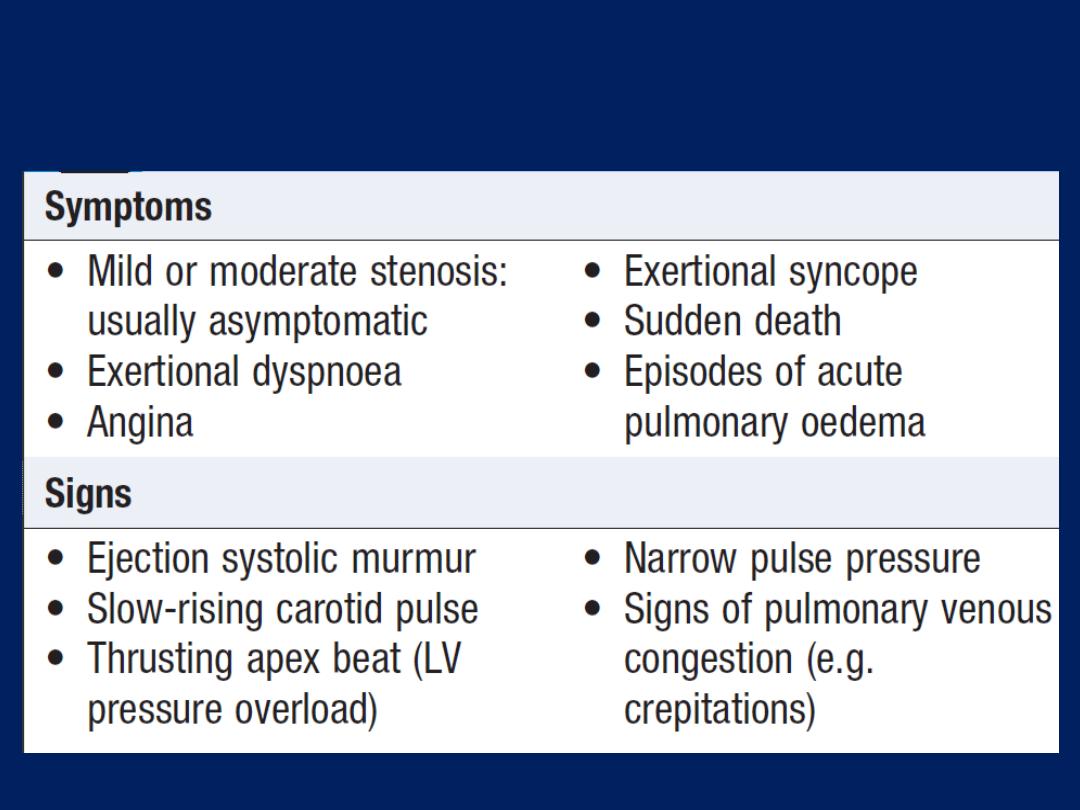
Clinical features of aortic stenosis

Investigations
In advanced cases, ECG features of hypertrophy and
down-sloping ST segments and T inversion (‘strain pattern’).
Nevertheless, especially in old, the ECG can be normal, despite severe stenosis.
Echo demonstrates restricted opening and Doppler
assessment permits calculation of the systolic gradient
across the valve. Aortic valve area calculated from
Doppler is a more accurate assessment of severity. CT and
MRI are useful in assessing the degree of valve calcification
and stenosis, respectively, but are rarely necessary.
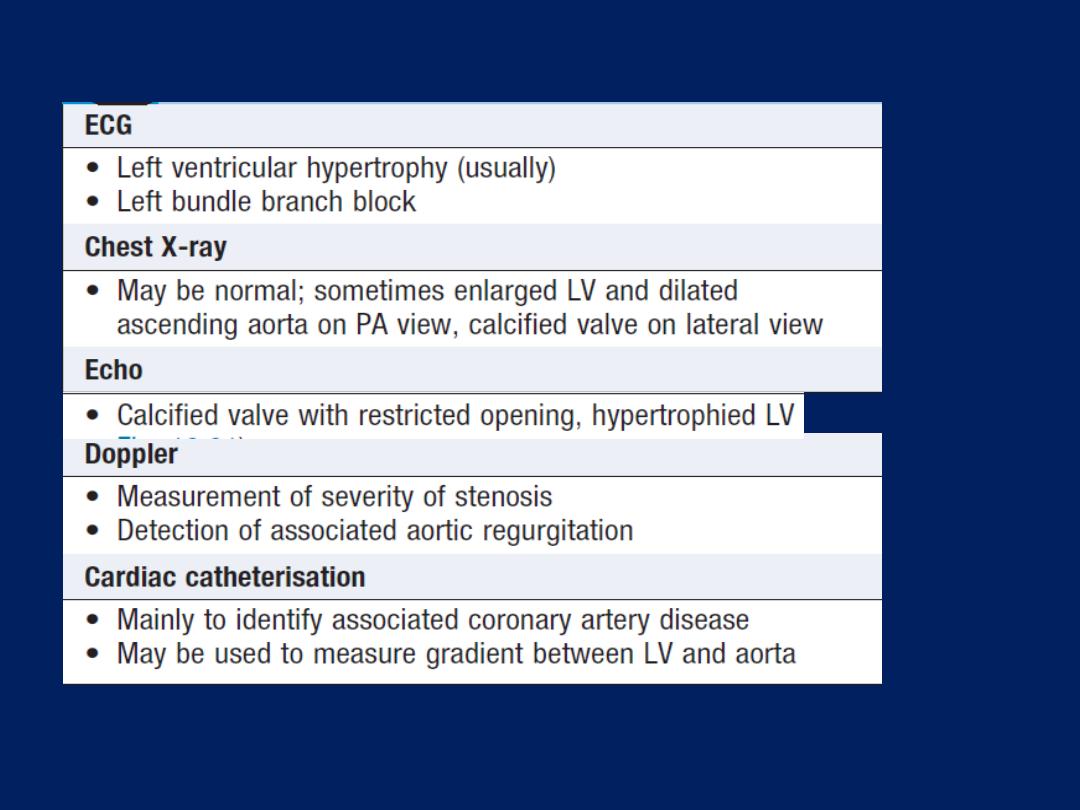
Investigations in aortic stenosis

Management
Irrespective of the severity ,asymptomatic have a good
immediate prognosis and conservative management is
appropriate, should be kept under review, as the
development of symptoms has a poor prognosis and
is an indication for prompt surgery. In practice, patients
with moderate or severe stenosis are evaluated every
1–2 years.Patients with symptomatic severe AS should
have prompt replacement. Some with severe AS deny
symptoms, could be due to a sedentary lifestyle, a careful
exercise test may reveal symptoms on modest exertion.
Aortic balloon valvuloplasty is useful in congenital AS
but is of no value in old with calcific stenosis.
Anticoagulants for have atrial fibrillation or those who
have had replacement with a mechanical prosthesis.

Aortic stenosis in old age

Aortic regurgitation
Aetiology and pathophysiology
This condition is due to disease of the cusps or dilatation of
the aortic root . The stroke volume of the LV may eventually
be doubled or trebled, and the major arteries are then
conspicuously pulsatile. As the disease progresses, LV
diastolic pressure rises and breathlessness develops.
Clinical features
Until the onset of breathlessness, the only symptom may
be an awareness of the heart beat , particularly when
lying on the left, results from the increased stroke volume.
Paroxysmal nocturnal dyspnoea is sometimes the first
symptom, and peripheral oedema or angina may occur.
The characteristic murmur is best heard to the left of the
sternum during held expiration , a thrill is rare.

A systolic murmur due to the increased stroke volume is
common and does not necessarily indicate stenosis. The
regurgitant jet causes fluttering of the mitral valve and, if
severe, causes partial closure of the anterior mitral leaflet,
leading to functional MS and a soft mid-diastolic (
Austin
Flint
) murmur.
In acute severe regurgitation (e.g. perforation of aortic
cusp in endocarditis), there may be no time for
compensatory LVH and dilatation to develop and the
features of heart failure may predominate and the
classical signs may be masked by tachycardia and an
abrupt rise in left ventricular end-diastolic pressure; thus,
the pulse pressure may be near normal and the diastolic
murmur may be short or absent.

Investigations
Regurgitation is detected by Doppler. In acute cases, the
rapid rise in LVdiastolic pressure may cause premature
mitral valve closure. catheterisation and aortography help
in assessing the severity, and dilatation of the aorta and
the presence of coexisting CAD. MRI is useful in assessing
the degree and extent of aortic dilatation
.
Management
Treatment for underlying conditions, such as endocarditis
or syphilis. Replacement is indicated if it causes
symptoms, may need to be combined with aortic root
replacement and CABS. chronic regurgitation can remain
asymptomatic for many years because compensatory
ventricular dilatation and hypertrophy occur, but should be
advised to report the development of any symptoms.

Asymptomatic should also be followed up annually with
echo for evidence of increasing ventricular size. If this
occurs or if the end-systolic dimension increases to 55 mm
or more, then valve replacement should be undertaken.
Systolic BP should be controlled with vasodilating
drugs, such as nifedipine or ACE inhibitors. There is
conflicting evidence regarding the need for aortic valve
replacement in asymptomatic patients with severe aortic
regurgitation. When aortic root dilatation is the cause of
aortic regurgitation (e.g. Marfan’s syndrome), aortic root
replacement is usually necessary.
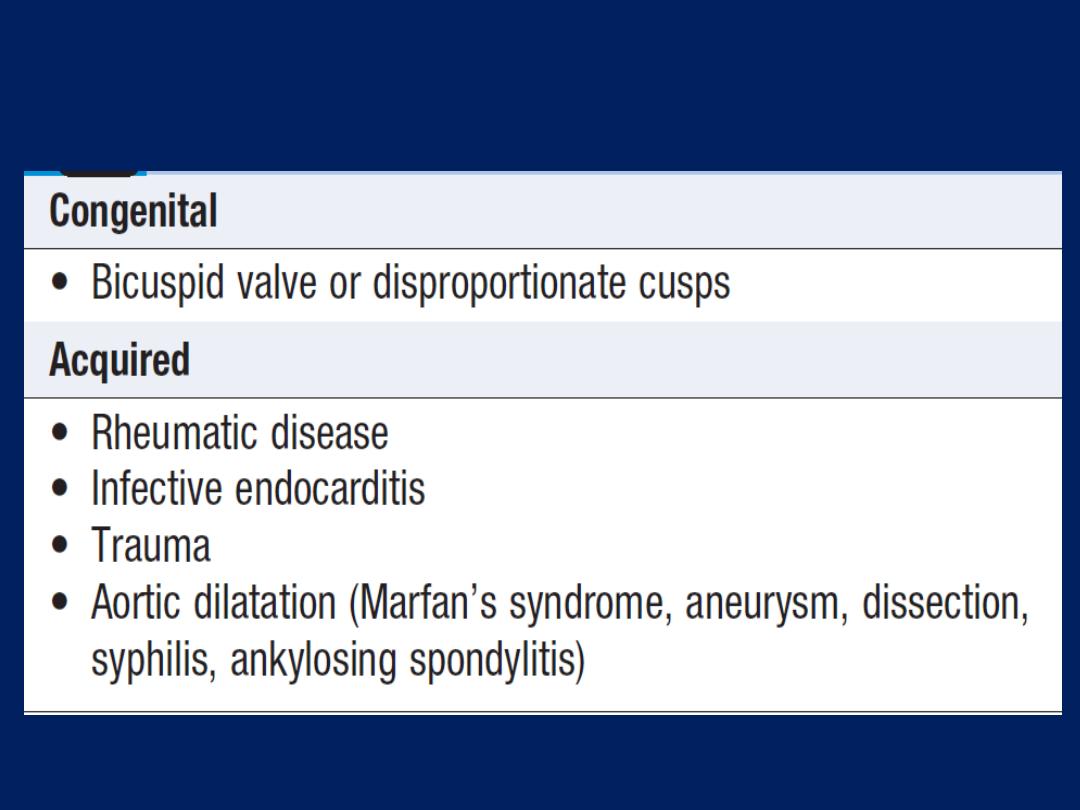
Causes of aortic regurgitation
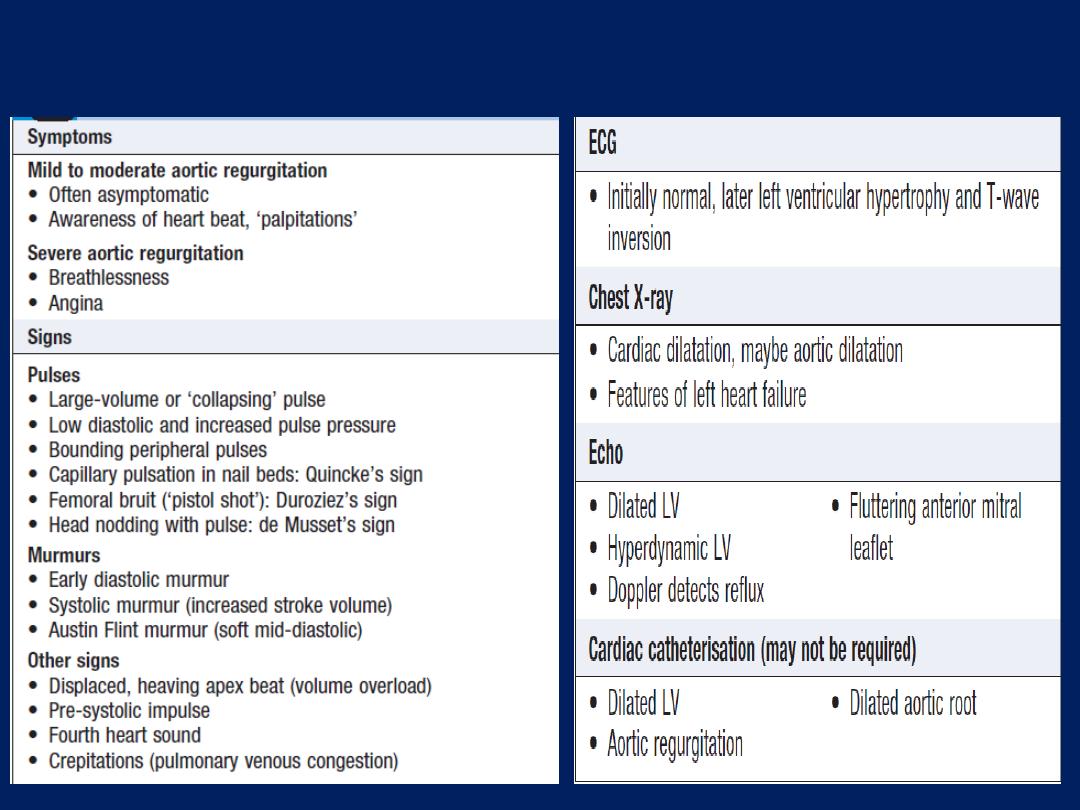
Investigations
Clinical features of AR

Tricuspid valve disease
Tricuspid stenosis
Usually rheumatic in origin. Tricuspid disease occurs in
< 5% of patients with rheumatic heart disease and then
nearly always in association with mitral and aortic valve
disease. Tricuspid stenosis and regurgitation are features
of the carcinoid syndrome .
Clinical features and investigations
Although the symptoms of mitral and aortic disease
predominate, tricuspid stenosis may cause symptoms of
right heart failure, a raised JVP with a prominent a wave,
and a slow y descent due to the loss of normal rapid right
ventricular filling . There is also a mid-diastolic murmur,
best heard at the lower left or right sternal edge.

This is generally higher-pitched than the murmur of mitral
stenosis and is increased by inspiration, hepatomegaly
with pre-systolic pulsation (large a wave), ascites and
peripheral oedema. On Doppler, the valve has similar
appearances to those of rheumatic mitral stenosis.
Management
In patients who require surgery to other valves, either
the tricuspid valve is replaced or valvotomy is performed
at surgery. Balloon valvuloplasty can be used to
treat rare cases of isolated tricuspid stenosis.

Tricuspid regurgitation
Aetiology, clinical features and investigations
TR is common, and is most frequently ‘functional’ as a result
of right ventricular dilatation.Symptoms are usually non-
specific, with tiredness related to reduced forward flow,
and oedema and hepatic enlargement due to venous
congestion. The most prominent sign is a ‘giant’ v wave in
the jugular venous pulse , pansystolic murmur at the left
sternal edge and a
pulsatile liver
. Echo may reveal
dilatation of the RV.
Ebstein’s anomaly is a congenital abnormality in which the
tricuspid valve is displaced towards the right ventricular
apex, with consequent enlargement of the RA. It is
commonly associated with tricuspid regurgitation.

• Rheumatic heart disease
• Endocarditis, particularly in injection drug-users
• Ebstein’s congenital anomaly
• Right ventricular dilatation due to chronic left heart
failure (‘functional tricuspid regurgitation’)
• Right ventricular infarction
• Pulmonary hypertension (e.g. cor pulmonale)
Causes of tricuspid regurgitation

Management
Tricuspid regurgitation due to right ventricular dilatation
often improves when the cause of right ventricular
overload is corrected, with diuretic and vasodilator
treatment of congestive cardiac failure. Patients with a
normal pulmonary artery pressure tolerate isolated
tricuspid reflux well, and valves damaged by endocarditis
do not usually need to be replaced. Patients undergoing
mitral valve replacement, who have tricuspid regurgitation
due to marked dilatation of the tricuspid annulus,
benefit from valve repair with an annuloplasty ring to
bring the leaflets closer together. Those with rheumatic
damage may require tricuspid valve replacement.

Pulmonary valve disease
Pulmonary stenosis
This can occur in the carcinoid but is usually congenital, in
which case it may be isolated or associated with other
abnormalities, such as Fallot’s tetralogy .
The principal finding is ejection systolic murmur, loudest
at the left upper sternum and radiating towards the left
shoulder. There may be a thrill, best felt when the patient
leans forward and breathes out. The murmur is often
preceded by an ejection sound (click). Delay in right
ventricular ejection may cause wide splitting of S2. Severe
PS is characterised by a loud harsh murmur, an inaudible
pulmonary closure sound (P2), an increased right
ventricular heave, prominent a waves in the jugular pulse

ECG evidence of right ventricular hypertrophy, and post-
stenotic dilatation in the pulmonary artery on the chest X-
ray. Doppler is the definitive investigation.
Mild to moderate isolated pulmonary stenosis is relatively
common and does not usually progress or require
treatment. Severe pulmonary stenosis (resting gradient
> 50 mmHg with a normal cardiac output) is treated by
percutaneous pulmonary balloon valvuloplasty or, if
this is not available, by surgical valvotomy. Long-term
results are very good. Post-operative pulmonary
regurgitation is common but benign.

Pulmonary regurgitation
This is rare in isolation and is usually associated with
pulmonary artery dilatation due to pulmonary
hypertension.
It may complicate mitral stenosis, producing an
early diastolic decrescendo murmur at the left sternal
edge that is difficult to distinguish from aortic
regurgitation (Graham Steell murmur). The pulmonary
hypertension may be secondary to other disease of the
left side of the heart, primary pulmonary vascular
disease or Eisenmenger’s syndrome .
Trivial pulmonary regurgitation is a frequent finding in
normal individuals and has no clinical significance.

Infective endocarditis
This is caused by microbial infection of a heart valve (native
or prosthetic), the lining of a cardiac chamber or blood
vessel, or a congenital anomaly (e.g. septal defect).
The causative organism is usually a bacterium, but may be
a rickettsia, chlamydia or fungus.
Pathophysiology
Infective endocarditis typically occurs at sites of preexisting
endocardial damage, but infection with particularly virulent
or aggressive organisms (e.g. Staphylococcus aureus) can
cause endocarditis in a previously normal heart;
staphylococcal endocarditis of the tricuspid valve
is a common complication of intravenous drug misuse.

Many acquired and congenital cardiac lesions are
vulnerable to endocarditis, particularly areas of
endocardial damage caused by a high-pressure jet of
blood, such as VSD , mitral and aortic regurgitation, many
of which are haemodynamically insignificant. In contrast,
the risk of endocarditis at the site of haemodynamically
important low-pressure lesions, such as a large ASD , is
minimal.
Infection tends to occur at sites of endothelial damage
because they attract deposits of platelets and fibrin that
are vulnerable to colonisation by blood-borne organisms.
The avascular valve tissue and presence of fibrin
and platelet aggregates help to protect proliferating
organisms from host defence mechanisms.

When the infection is established,
vegetations composed
of
organisms, fibrin and platelets grow and may become
large enough to cause obstruction or embolism. Adjacent
tissues are destroyed and abscesses may form. Valve
regurgitation may develop or increase if the affected
valve is damaged by tissue distortion, cusp perforation
or disruption of chordae.
Extracardiac manifestations, such as vasculitis and skin
lesions, are due to emboli or immune complex deposition.
Mycotic aneurysms may develop in arteries at the site of
infected emboli.
At autopsy, infarction of the spleen and kidneys and,
sometimes, an immune glomerulonephritis are found.

Microbiology
Over three-quarters
of cases are caused by
streptococci
or
staphylococci
. The viridans group of streptococci
(Streptococcus mitis, Strep. sanguis) are commensals in the
upper respiratory tract that may enter the blood stream
on chewing or teeth-brushing, or at the time of dental
treatment, and are common causes of subacute
endocarditis.
Other organisms,
including Enterococcus faecalis, E.
faecium and Strep. bovis, may enter the blood from the
bowel or urinary tract. Strep. milleri and Strep. bovis
endocarditis is associated with large-bowel neoplasms.

Staph. aureus has now
overtaken streptococci as the most
common cause of acute endocarditis. It originates from
skin infections, abscesses or vascular access sites (e.g.
intravenous and central lines), or from intravenous
drug use. It is highly virulent and invasive, usually
producing florid vegetations, rapid valve destruction
and abscess formation.
Other causes
of acute endocarditis include Strep.
pneumoniae and Strep. pyogenes.
Post-operative
endocarditis after cardiac surgery may
affect native or prosthetic heart valves or other prosthetic
materials. The most common organism is a coagulase-
negative staphylococcus (Staph. epidermidis), a normal
skin commensal.

In Q fever
endocarditis due to Coxiella burnetii, the patient often has
a history of contact with farm animals.
The aortic valve is usually affected and there may also be hepatitis,
pneumonia and purpura. Life-long antibiotic therapy may be
required. Gram-negative bacteria of the so-called HACEK group
(Haemophilus spp., Actinobacillus actinomycetemcomitans,
Cardiobacterium hominis, Eikenella spp. And Kingella kingae) are slow-
growing, fastidious organisms that are only revealed after prolonged
culture and may be resistant to penicillin.
Brucella
is associated with a
history of contact with goats or cattle and often affects the
aortic
valve.
Yeasts and fungi
(Candida, Aspergillus) may attack previously
normal or prosthetic valves, particularly in
immunocompromised
patients or those with indwelling intravenous lines. Abscesses and
emboli are common, therapy is difficult (surgery is often required)
and mortality is high.
Concomitant bacterial infection may be present.

Incidence
The incidence of infective endocarditis in community based
studies
ranges from 5 to 15 cases per 100 000 per annum.
In a large
British study, the underlying condition was
rheumatic
heart
disease in 24%,
congenital
heart disease in 19%, and
other cardiac
abnormalities (e.g. calcified aortic valve,
floppy mitral valve) in 25%. The remaining 32% were not
thought to have a pre-existing cardiac abnormality.
Clinical features
Endocarditis can take either an
acute or a more insidious
‘subacute’ form, clinical pattern is
influenced
not only by
the organism, but also by the site of infection, prior
antibiotic and presence of a valve or shunt prosthesis. The
subacute form may abruptly develop life-threatening
complications, such as valve disruption or emboli.

Subacute endocarditis
This should be suspected when a patient with congenital
or valvular heart disease develops a persistent fever,
complains of unusual tiredness, night sweats or weight
loss, or new signs of valve dysfunction or heart failure.
Less often, it presents as an embolic stroke or peripheral
arterial embolism. Other features include purpura and
petechial haemorrhages in the skin and mucous
membranes, and splinter haemorrhages.
Osler’s nodes are
painful
tender swellings at the fingertips that are
probably the product of
vasculitis
. Digital clubbing is a
late sign. The spleen is frequently palpable; in Coxiella
infections, the spleen and the liver may be enlarged.
Microscopic haematuria is common.

Acute endocarditis
This presents as a severe febrile illness with prominent
and changing heart murmurs and petechiae. Clinical
stigmata of chronic endocarditis are usually absent.
Embolic events are common, and cardiac or renal failure
may develop rapidly. Abscesses may be detected on
echocardiography. Partially treated acute endocarditis
behaves like subacute endocarditis.
Post-operative endocarditis
This may present as an unexplained fever in a patient
who has had heart valve surgery.
The infection usually involves the valve ring and may
resemble subacute or acute endocarditis, depending on
the virulence of the organism.

Morbidity and mortality are high and redo surgery is often
required. The range of organisms is similar to that seen in
native valve disease, but when endocarditis occurs during
the first few weeks after surgery, it is usually due to
infection with a coagulase negative staphylococcus.
A clinical diagnosis can be made on the presence of two
major, one major and three minor, or five minor criteria.
Investigations
Blood culture is crucial because it may identify the infection
and guide antibiotic therapy. Three to six sets of blood
cultures taken prior to therapy and should not wait for
episodes of pyrexia. Aseptic technique is essential and the
risk of contaminants should be minimized by sampling from
different venepuncture sites.

An in-dwelling line should not be used to take cultures.
Aerobic and anaerobic cultures are required. Echo is key
for detecting and following the progress of vegetations, for
assessing valve damage and for detecting abscess
formation. Vegetations as small as
2–4 mm can be detected by transthoracic echo, and even
smaller ones (1–1.5 mm) can be visualised by
transoesophageal echo (TOE). Elevation of the ESR, a
normocytic normochromic anaemia, and leucocytosis are
common.CRP is more reliable than the ESR in monitoring
progress. Proteinuria may occur and microscopic
haematuria is usually present. The ECG may show AV
block . CX-ray may show cardiomegaly and failure.

Management
The case fatality of bacterial endocarditis is approximately
20% and even higher in those with prosthetic endocarditis
and those infected with antibiotic resistant organisms. A
multidisciplinary
approach, with cooperation between the
physician, surgeon and microbiologist, increases the chance
of a successful outcome. Any source of infection should be
removed as soon as possible; for example, a tooth with an
apical abscess should be extracted. Empirical treatment
depends on the mode of presentation, the suspected
organism, and whether the patient has a prosthetic valve or
penicillin allergy. If the presentation is
acute
, flucloxacillin
and gentamicin are recommended, while for a
subacute
benzyl penicillin and gentamicin are preferred.

In those with penicillin allergy, a prosthetic valve or
suspected meticillin-resistant Staph. aureus (MRSA)
infection,
triple therapy
with vancomycin, gentamicin
and oral rifampicin should be considered. Following
identification of the causal organism, determination of
the minimum inhibitory concentration (MIC) for the
organism is essential to guide antibiotic therapy.
A 2-week treatment
regimen may be sufficient for
fully sensitive
strains of Strep. viridans and Strep. bovis,
provided specific conditions are met .
For the empirical
treatment of bacterial endocarditis, penicillin plus
gentamicin is the regimen of choice for most patients;
when
staphylococcal
infection is
suspected
, however,
vancomycin plus gentamicin is recommended.

Cardiac surgery (débridement of infected material
and valve replacement) is advisable in a substantial
proportion of patients, particularly those with Staph. aureus
and fungal infections . Antimicrobial must be started
before surgery.
Prevention
Until recently, antibiotic prophylaxis was routinely
given to people at risk of infective endocarditis undergoing
interventional procedures.
However, as this has
not been proven to be effective and the link between
episodes of infective endocarditis and interventional
procedures has not been demonstrated, antibiotic
prophylaxis is no longer offered routinely for defined
interventional procedures.

Valve replacement surgery
Diseased heart valves can be replaced with mechanical
or biological prostheses. The three most commonly used
types of mechanical prosthesis are the ball and cage,
tilting single disc and tilting bi-leaflet valves. All generate
prosthetic sounds or clicks on auscultation. Pig or
allograft valves mounted on a supporting stent are the
most commonly used biological valves. They generate
normal heart sounds.
All prosthetic valves
used in the
aortic
position
produce a systolic flow murmur.
All
mechanical valves require long-term anticoagulation
because they can cause systemic thromboembolism or may
develop valve thrombosis or obstruction; the prosthetic
clicks may become inaudible if the valve malfunctions.

Biological valves have the advantage of not requiring
anticoagulants to maintain proper function; however,
many undergoing valve replacement surgery, especially
mitral valve replacement, will have atrial
fibrillation
that
requires anticoagulation anyway. Biological valves are less
durable than mechanical valves and may degenerate 7 or
more years after implantation, particularly when used in
the mitral position. They are more durable in the aortic
position and in older patients, so are particularly
appropriate for patients over 65 undergoing aortic valve
replacement. Symptoms or signs of unexplained heart
failure in a patient with a prosthetic heart valve may be
due to valve dysfunction, and urgent assessment is
required. Biological valve dysfunction is usually
associated with a regurgitant murmur.
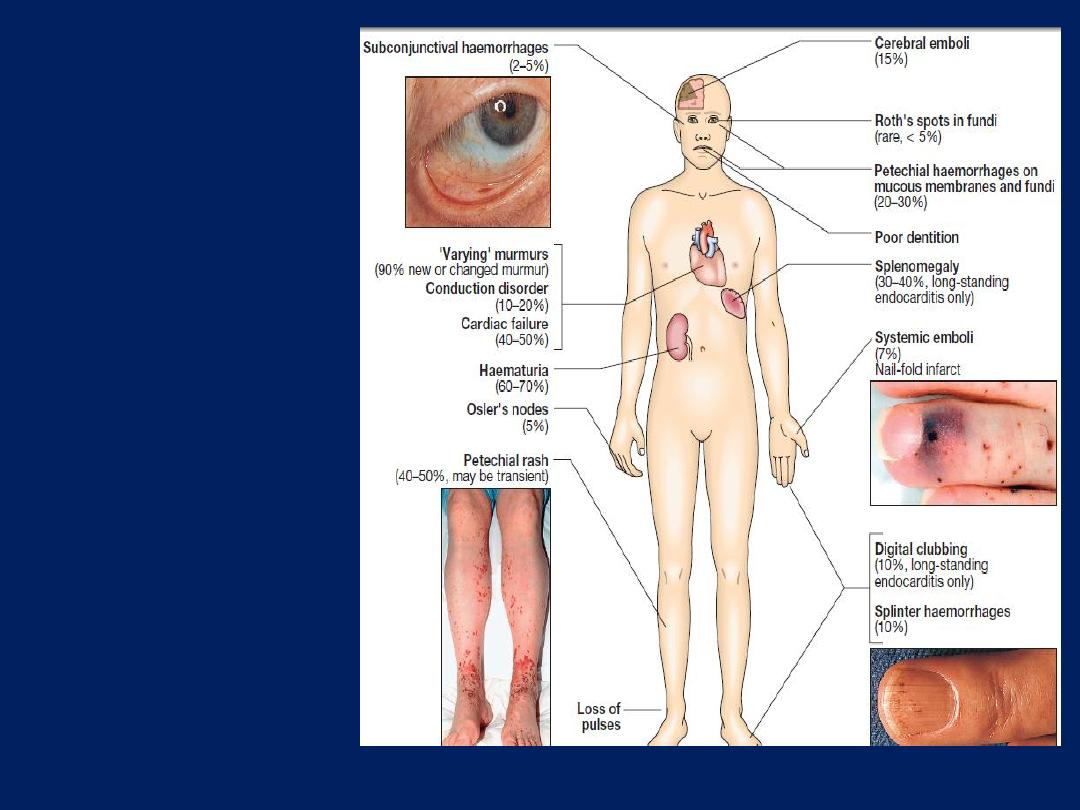
Clinical features
which may be
present in
endocarditis.
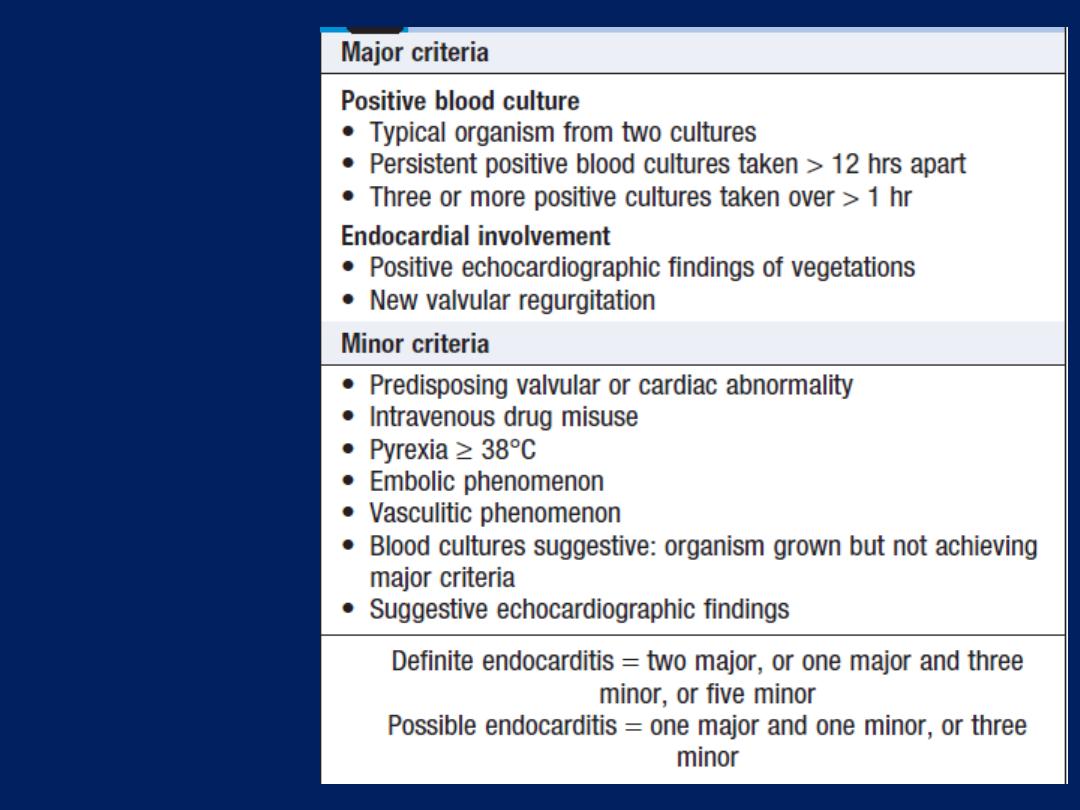
Diagnosis of
infective
endocarditis
(modified
Duke criteria)
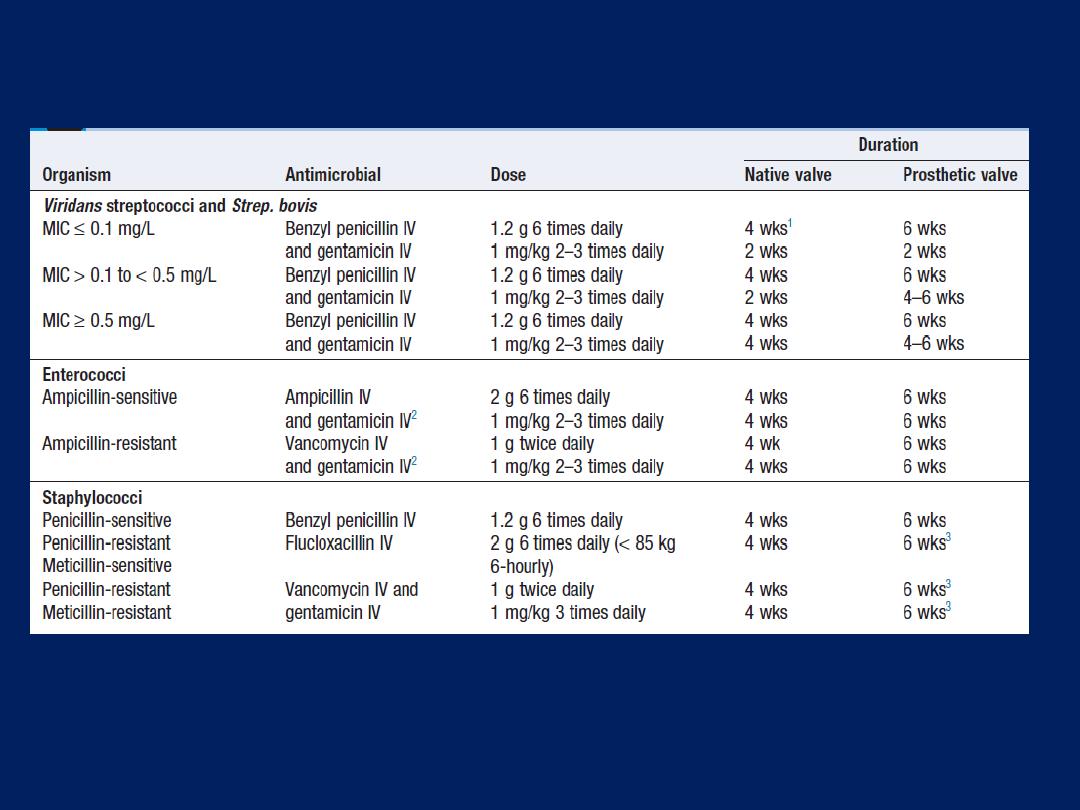
Antimicrobial treatment of common causative
organisms in infective endocarditis
1
Conditions for the short-course treatment of Strep. viridans/ bovis
endocarditis are met, 2 wks of benzyl penicillin.
2
ln high-level
gentamicin resistance, consider streptomycin.
3
Consider additional
rifampicin 300–600 mg twice daily orally for 2 wks. (MIC =
minimum inhibitory concentration)
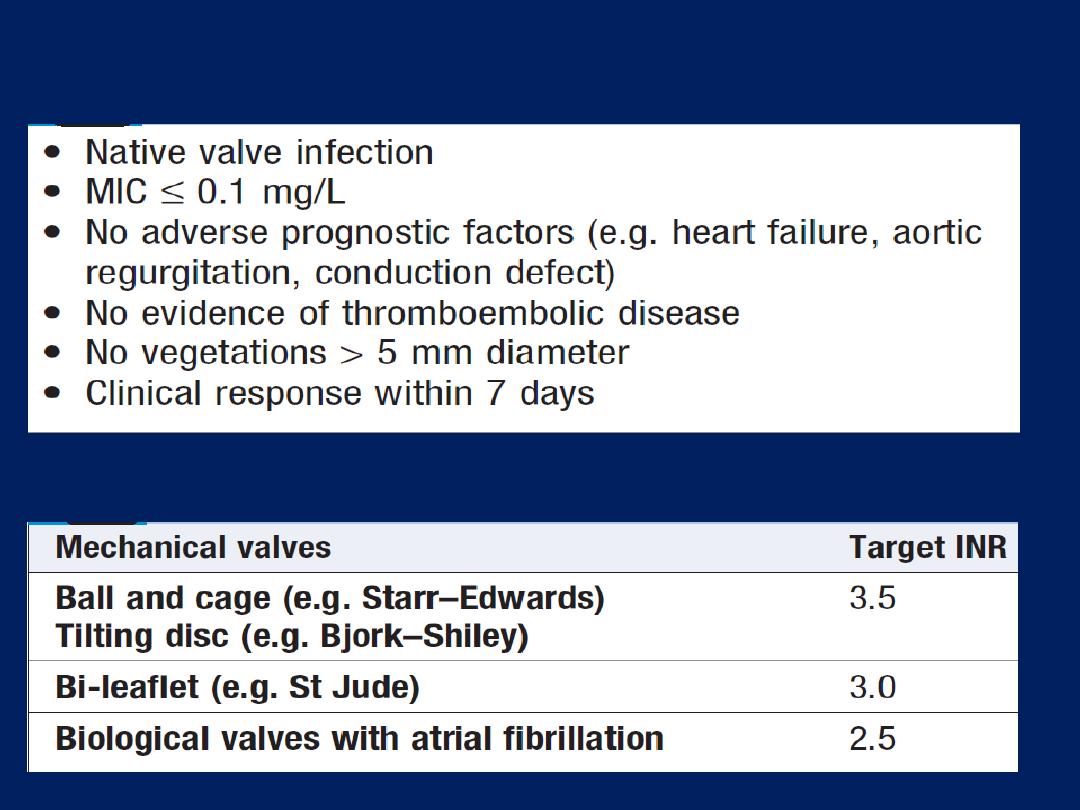
Conditions for the short-course treatment of Strep.
viridans/ bovis endocarditis
Prosthetic heart valves: optimal anticoagulant control
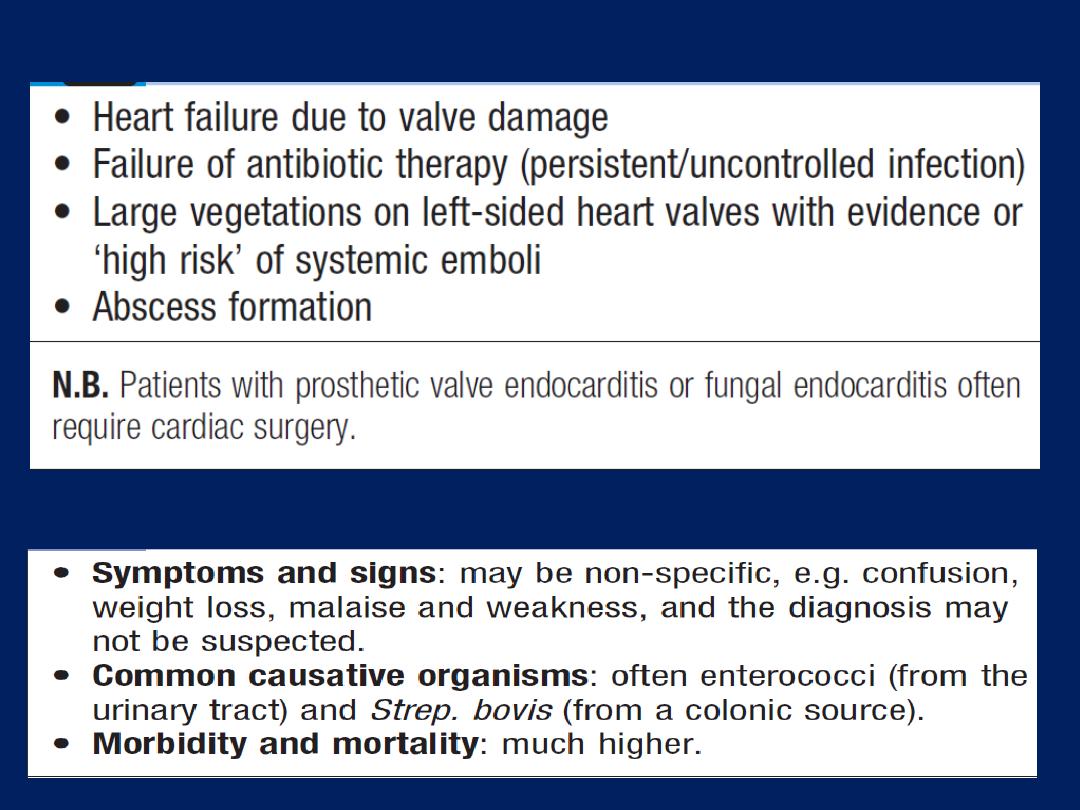
Indications for cardiac surgery in infective endocarditis
Endocarditis in old age
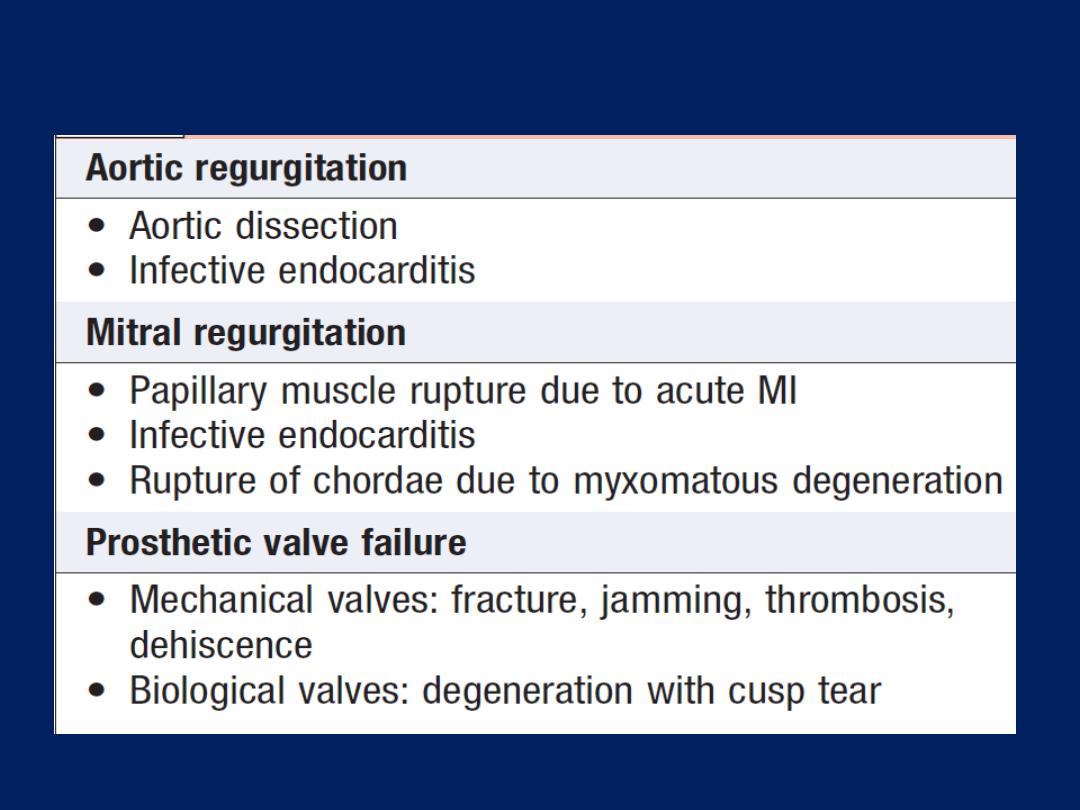
Causes of acute valve failure

CONGENITAL HEART DISEASE
May pass unrecognised and not present until adult life.
Defects that are well tolerated, such ASD , may cause no
symptoms until adult life or may be detected incidentally.
Defects that were previously fatal in childhood can now be
corrected, so that survival to adult life is the norm.
Failure of the aorta to develop at the point of the aortic
isthmus
and where the ductus arteriosus attaches
can lead to
coarctation of the aorta. In fetal development, the heart
develops as a single tube which folds back on itself and
then divides into two separate circulations. Failure of
septation can lead to some forms of atrial and VSD .
Failure of alignment of the great vessels with the ventricles
contributes to transposition of the great arteries, tetralogy
of Fallot and truncus arteriosus.
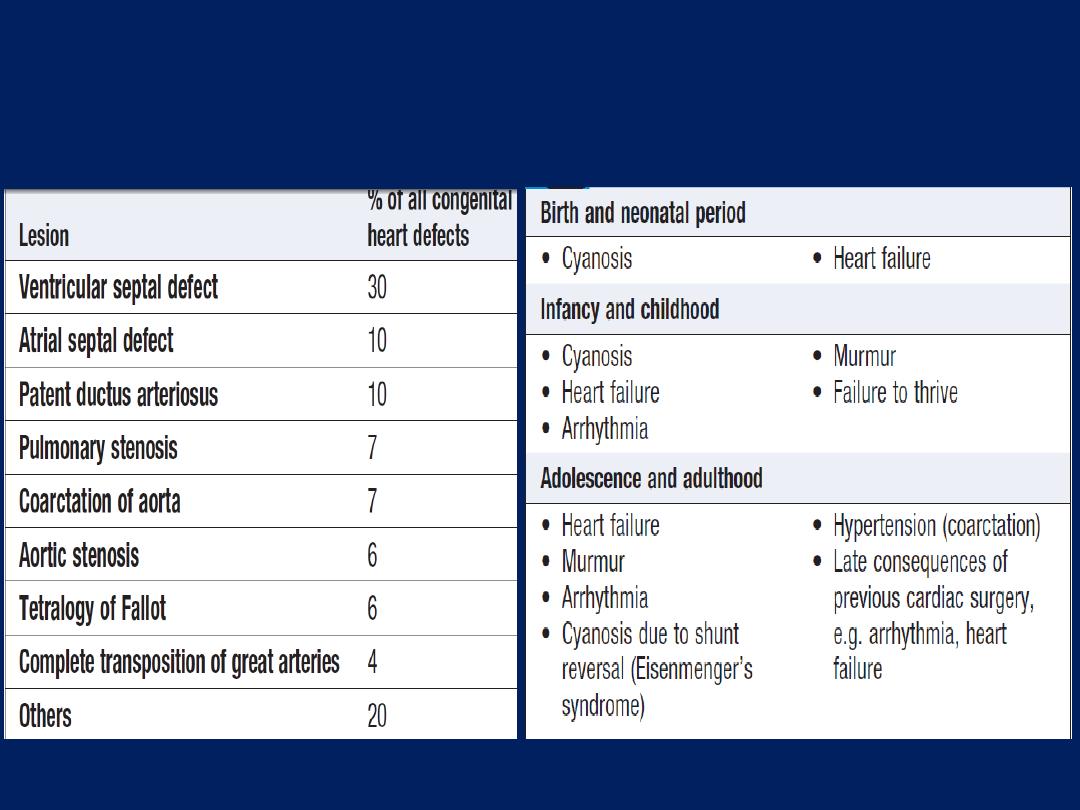
Presentation of congenital
heart disease
Incidence and relative frequency of
congenital cardiac malformations
Changes in the circulation at birth.

Changes in the circulation at birth.
A
In the fetus, oxygenated blood comes
through the umbilical vein where it enters the inferior vena cava via the ductus
venosus (red). The oxygenated blood streams from the RA through the open
foramen ovale to the LA and via the LV into the aorta. Venous blood from the
superior vena cava (blue) crosses under the main blood stream into the RA and
then, partly mixed with oxygenated blood (purple), into the RV and pulmonary
artery. The pulmonary vasculature has a high resistance and so little blood passes
to the lungs; most blood passes through the ductus arteriosus to the descending
aorta. The aortic isthmus is a constriction in the aorta that lies in the aortic arch
before the junction with the ductus arteriosus and limits the flow of oxygen-rich
blood to the descending aorta. This configuration means that less oxygen-rich
blood is supplied to organ systems that take up their function mainly after birth,
e.g. the kidneys and intestinal tract
.
B
At birth, the lungs expand with air and pulmonary vascular resistance falls, so
that blood now flows to the lungs and back to the LA. The left atrial pressure rises
above right atrial pressure and the flap valve of the foramen ovale closes. The
umbilical arteries and the ductus venosus close. In the next few days, the ductus
arteriosus closes under the influence of hormonal changes (particularly
prostaglandins) and the aortic isthmus expands (IVC = inferior vena cava; LA =
left atrium; LV = left ventricle; PA = pulmonary artery; PV = pulmonary vein; RA
= right atrium; RV = right ventricle; SVC = superior vena cava).

Aetiology and incidence
The incidence of haemodynamically significant congenital
cardiac abnormalities is about 0.8% of live births.
Maternal infection or exposure to drugs or toxins may
cause congenital heart disease. Maternal rubella infection
is associated with persistent ductus arteriosus, pulmonary
valvular and/or artery stenosis, and atrial septal defect.
Maternal alcohol misuse is associated with septal defects,
and maternal lupus erythematosus with congenital
complete heart block. Genetic or chromosomal
abnormalities, such as Down’s syndrome, may cause septal
defects, and gene defects have also been identified as
leading to specific abnormalities, such as Marfan’s
syndrome and DiGeorge’s
(deletion in chromosome 22q)
syndrome.

Clinical features
Symptoms may be absent, or the child may be breathless
or fail to attain normal growth and development. Some
defects are not compatible with extrauterine life, or
are so only for a short time. Clinical signs vary. Murmurs,
thrills or signs of cardiomegaly may be present. In
coarctation of the aorta, radiofemoral delay may be
noted and some female patients have the features of
Turner’s syndrome.
Features of other congenital conditions, such as Marfan’s
syndrome or Down’s syndrome, may also be apparent.
Cerebrovascular accidents and cerebral abscesses may
complicate severe cyanotic congenital disease.

Early diagnosis is important because many types of
congenital heart disease are amenable to surgery, but
this opportunity is lost if secondary changes, such as
irreversible pulmonary hypertension, occur.
Central cyanosis and digital clubbing
Central cyanosis of cardiac origin occurs when desaturated
blood enters the systemic circulation without passing through
the lungs (right-to-left shunt).
In the neonate
, the most
common cause is
transposition
of the great arteries (aorta
arises from the RV and the pulmonary artery from the LV in
association with a VSD) . In older children, cyanosis is
usually the consequence of a VSD combined with severe
pulmonary stenosis (tetralogy of Fallot) or with pulmonary
vascular disease (Eisenmenger’s syndrome). Prolonged
cyanosis is associated with finger and toe clubbing .

Growth retardation and learning difficulties
These may occur with large left-to-right shunts at ventricular
or great arterial level, and also with other defects,
especially if they form part of a genetic syndrome. Major
intellectual impairment is uncommon in children with isolated
congenital heart disease; however, minor learning
difficulties can occur and may complicate cardiac surgery if
cerebral perfusion is compromised.
Syncope
In the presence of increased pulmonary vascular resistance
or severe left or right ventricular outflow obstruction,
exercise may provoke syncope as systemic vascular
resistance falls but pulmonary vascular resistance rises,
worsening right-to-left shunting and cerebral oxygenation.
Syncope can also occur because of arrhythmias.

Pulmonary hypertension and Eisenmenger’s syndrome
Persistently raised pulmonary flow (left-to right shunt)
causes increased pulmonary resistance followed by
pulmonary hypertension. Progressive irreversible changes,
including obliteration of distal vessels. Central cyanosis and
digital clubbing develops. The chest X-ray shows enlarged
central pulmonary arteries and peripheral ‘pruning’ of the
pulmonary vessels. The ECG shows right ventricular
hypertrophy. If severe pulmonary hypertension develops,
a left-to-right shunt may reverse, resulting in right-to-left
shunt and marked cyanosis (Eisenmenger’s syndrome),
which may be more apparent in the feet and toes than in
the upper part of the body:
differential cyanosis
. This is
more common with large VSD or PDA than with ASD.

Patients with Eisenmenger’s syndrome are at particular risk
from abrupt changes in afterload that exacerbate right-to-
left shunting, such as vasodilatation, anaesthesia and
pregnancy.
Pregnancy
During pregnancy, there is a 50% increase in plasma
volume, a 40% increase in whole blood volume and a
similar increase in cardiac output, so problems may arise
in women with congenital heart disease . Many with
palliated or untreated disease will tolerate pregnancy well,
however. Pregnancy is particularly hazardous in the
presence of conditions associated with cyanosis or severe
pulmonary hypertension; maternal mortality in patients with
Eisenmenger’s syndrome is more than 50%.
Pregnancy in women with congenital heart disease

Persistent ductus arteriosus
Aetiology
During fetal life, before the lungs begin to function, most
of the blood from the pulmonary artery passes through
the ductus arteriosus into the aorta . Normally, the ductus
closes soon after birth but sometimes fails to do so.
Persistence of the ductus is associated with other
abnormalities and is
more common in females.
Since the pressure in the aorta is higher than that in the
pulmonary artery, there will be a continuous arteriovenous
shunt, the volume of which depends on the size of the
ductus. As much as 50% of the left ventricular output is
recirculated through the lungs, with a consequent increase
in the work of the heart .

Clinical features
With small shunts there may be no symptoms for years
but, when the ductus is large, growth and development
may be retarded. Usually, there is no disability in infancy
but cardiac failure may eventually ensue, dyspnoea
being the first symptom. A continuous ‘machinery’
murmur is heard with late systolic accentuation, maximal
in the second left intercostal space below the clavicle). It is
frequently accompanied by a thrill. Pulses are increased in
volume. A large left-to-right shunt in infancy may cause a
considerable rise in pulmonary artery pressure and
sometimes this leads to progressive pulmonary vascular
damage. Enlargement of the pulmonary artery may be
detected radiologically. The ECG is usually normal.

Persistent ductus with reversed shunting
If pulmonary vascular resistance increases, pulmonary
artery pressure may rise until it equals or exceeds aortic
pressure. The shunt through the defect may then reverse,
causing Eisenmenger’s syndrome. The murmur becomes
quieter, may be confined to systole or may disappear. The
ECG shows evidence of RVH.
Management
A patent ductus is closed at cardiac catheterisation with
an implantable occlusive device. Closure should be
undertaken in infancy if the shunt is significant and
pulmonary resistance not elevated, but this may be
delayed until
later childhood
in those
with smaller
shunts, for whom closure remains advisable to reduce
the risk of endocarditis.

Pharmacological treatment in the neonatal period
When the ductus is structurally intact, a prostaglandin
synthetase inhibitor (indometacin or ibuprofen) may be
used in the first week of life to induce closure.
However, in the presence of a congenital defect with
impaired lung perfusion (e.g. severe pulmonary stenosis
and left-to-right shunt through the ductus), it may be
advisable to improve oxygenation by keeping the ductus
open with prostaglandin treatment.

Coarctation of the aorta
Aetiology
Narrowing of the aorta occurs in the region where the
ductus arteriosus joins the aorta, i.e. at the isthmus just
below the origin of the left subclavian artery.The
condition is
twice as common in males
. It is associated
with other abnormalities, most frequently bicuspid aortic
valve and ‘berry’ aneurysms of the cerebral circulation .
Acquired coarctation is rare but may follow trauma or
occur as a complication of arteritis (Takayasu’s disease).
Clinical features and investigations
Coarctation is an important cause of cardiac failure in the
newborn but symptoms are often absent when it is
detected in older children or adults.

Headaches may occur from hypertension and occasionally
weakness or cramps in the legs may result from
decreased circulation in the lower part of the body. The BP
is raised in the upper body but normal or low in the legs.
The femoral pulses are weak and delayed in comparison
with the radial pulse . A systolic murmur is usually heard
posteriorly, over the coarctation. There may also be an
ejection click and systolic murmur in the aortic area due
to a bicuspid aortic valve.
As a result of the aortic narrowing, collaterals form; they
mainly involve the periscapular, internal mammary and
intercostal arteries, and may result in localised bruits.
MRI is the best imaging method.
The ECG may show evidence of LVH.

The chest X-ray in early childhood is often normal but
later may show changes in the contour of the aorta
(indentation of the
descending
aorta,
‘3 sign’)
and
notching of the under-surfaces
of the ribs from
collaterals.
Management
In untreated cases, death may occur from LV failure,
dissection of the aorta or cerebral haemorrhage.
Correction is advisable in all but the mildest cases. If this
is carried out sufficiently early in childhood, persistent
hypertension can be avoided. Recurrence of stenosis may
occur , may be managed by balloon dilatation and
sometimes stenting. Coexistent bicuspid aortic valve,
which occurs in over 50% of cases, may lead to
progressive aortic stenosis or regurgitation.

Atrial septal defect
Aetiology
Atrial septal defect is one of the most common congenital
heart defects and occurs
twice
as frequently
in females.
Most are ‘ostium secundum’ defects, involving the fossa
ovalis that, in utero, was the foramen vale.
‘Ostium primum’ defects result from a defect in the
atrioventricular septum and are associated with a ‘cleft
mitral valve’ (split anterior leaflet). Since the normal RV is
more compliant than the LV, a large volume of blood shunts
through the defect from the LA to the RA, and then to the
RV and pulmonary arteries . As a result, there is gradual
enlargement of the right side of the heart and of the
pulmonary arteries.

Pulmonary hypertension and shunt reversal sometimes
complicate ASD , but are less common and tend to occur
later in life than with other types of left-to-right shunt.
Clinical features and investigations
Most children are asymptomatic for many years and
often detected at routine clinical examination or CX-ray.
Dyspnoea, chest infections, cardiac failure and
arrhythmias, especially atrial fibrillation, are other
possible manifestations.
The characteristic physical signs
are the result of the
volume overload of the RV:
• wide, fixed splitting
of S2 wide because of delay in
right ventricular ejection (increased stroke volume and
RBBB) and fixed because the defect equalizes left and
right atrial pressures throughout the respiratory cycle

• a systolic flow murmur
over the pulmonary valve.
In children with a large shunt, there may be a diastolic
flow murmur over the tricuspid valve. Unlike a mitral
flow murmur, this is usually high-pitched. Echo can directly
demonstrate the defect and typically shows RV dilatation,
RVH and pulmonary artery dilatation.
The chest X-ray shows enlargement of the heart and the
pulmonary artery, as well as pulmonary plethora. The ECG
shows incomplete RBBB because right ventricular
depolarisation is delayed as a result of dilatation (with a
‘primum’ defect, there is also left axis deviation).
The precise size and location of the defect can be shown
by transoesophageal echocardiography .

Management
Atrial septal defects in which pulmonary flow is
increased 50% above systemic flow (i.e. flow ratio
of 1.5 : 1) are often large enough to be clinically
recognizable and should be closed surgically.
Closure can also be accomplished at cardiac
catheterisation using implantable closure devices .
The long-term prognosis thereafter is excellent,
unless pulmonary hypertension has developed.
Severe pulmonary hypertension and shunt
reversal are both contraindications to surgery.
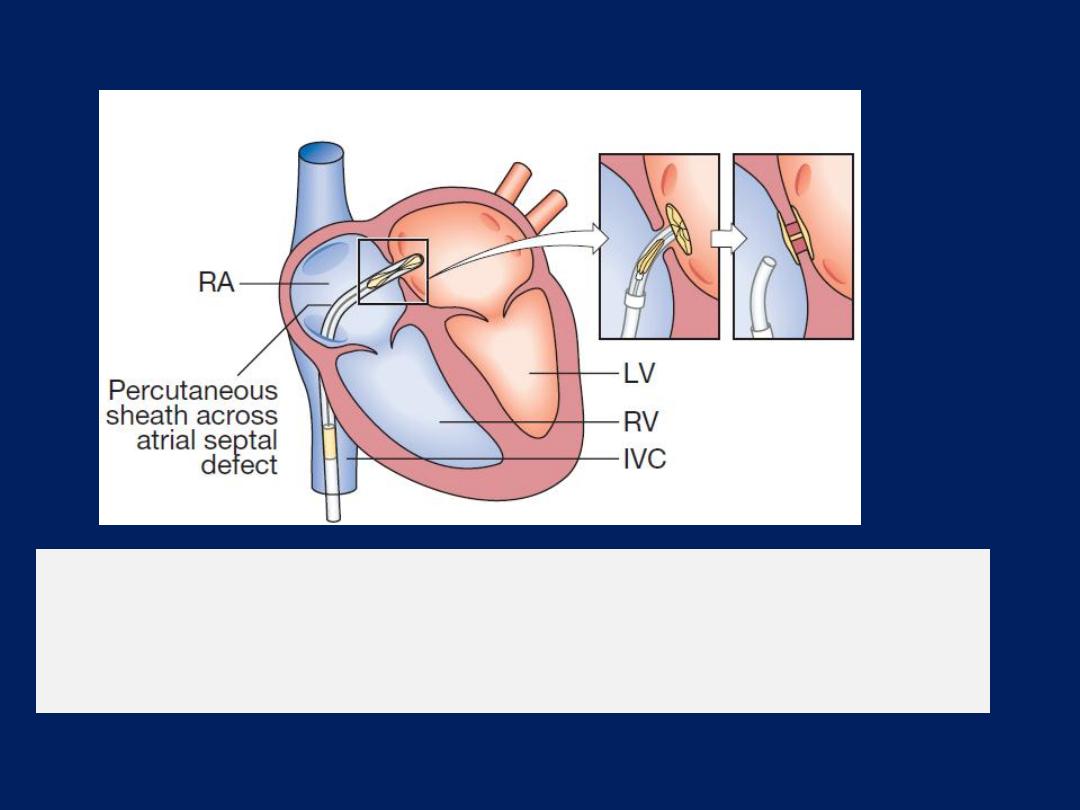
Percutaneous close of atrial septal defect.
The closure
device is delivered across the inter-atrial septum and a
disc deployed on either side to seal the defect.

Ventricular septal defect
Aetiology
Congenital VSD occurs as a result of incomplete septation
of the ventricles. Embryologically, the interventricular
septum has a membranous and a muscular portion, and
the latter is further divided into inflow, trabecular and
outflow portions. Most congenital defects are
‘perimembranous’, i.e. at the junction of the membranous
and muscular portions. VSD are the most common
congenital cardiac defect, occurring once in 500 live births.
The defect may be isolated or part of complex congenital
heart disease.
Acquired ventricular septal defect may result from rupture
as a complication of acute MI or, rarely, from trauma.

Clinical features
Flow from the high-pressure LV to the low-pressure RV
during systole produces a pansystolic murmur, heard best at
the left sternal edge , radiating all over the precordium .
A small defect often produces a loud murmur (maladie de
Roger). Conversely, a large defect produces a softer
murmur, particularly if pressure in the RV is elevated. May
present as cardiac failure
(it is usually absent in the immediate postnatal
period)
in the first 4–6 weeks of life , as a murmur with minor
haemodynamic disturbance in older children, or, rarely, as
Eisenmenger’s syndrome. There is prominent parasternal
pulsation, tachypnoea and indrawing of the lower ribs on
inspiration. CXR shows pulmonary plethora , ECG shows
bilateral VH.
In a proportion
of infants, the murmur gets
quieter or disappears due to
spontaneous closure
.

Management and prognosis
Small VSD require no specific treatment
. Cardiac failure
in infancy is initially treated medically with digoxin and
diuretics.
Persisting failure is an indication for surgical
repair of the defect. Percutaneous closure devices are
under development. Doppler echo helps to predict the
small septal defects that are likely to close spontaneously.
Eisenmenger’s syndrome is avoided by monitoring for
signs of rising pulmonary resistance (ECG and Echo) and
carrying out
surgical repair, when appropriate.
Surgical closure is contraindicated in fully developed
Eisenmenger’s syndrome when heart–lung transplantation
may be the only effective treatment.

Tetralogy of Fallot
The RV outflow obstruction is
most often subvalvular
(infundibular) but may be valvular, supravalvular or a
combination of these . The VSD is usually large and similar
in aperture to the aortic orifice. The combination results in
elevated right ventricular pressure and right-to-left
shunting of cyanotic blood across the VSD.
Aetiology
The embryological cause is abnormal development of
the bulbar septum that separates the ascending aorta
from the pulmonary artery, and which normally aligns
and fuses with the outflow part of the interventricular
septum.
The defect occurs in about 1 in 2000 births and
is the most
common cause of cyanosis in infancy after the first year of life.
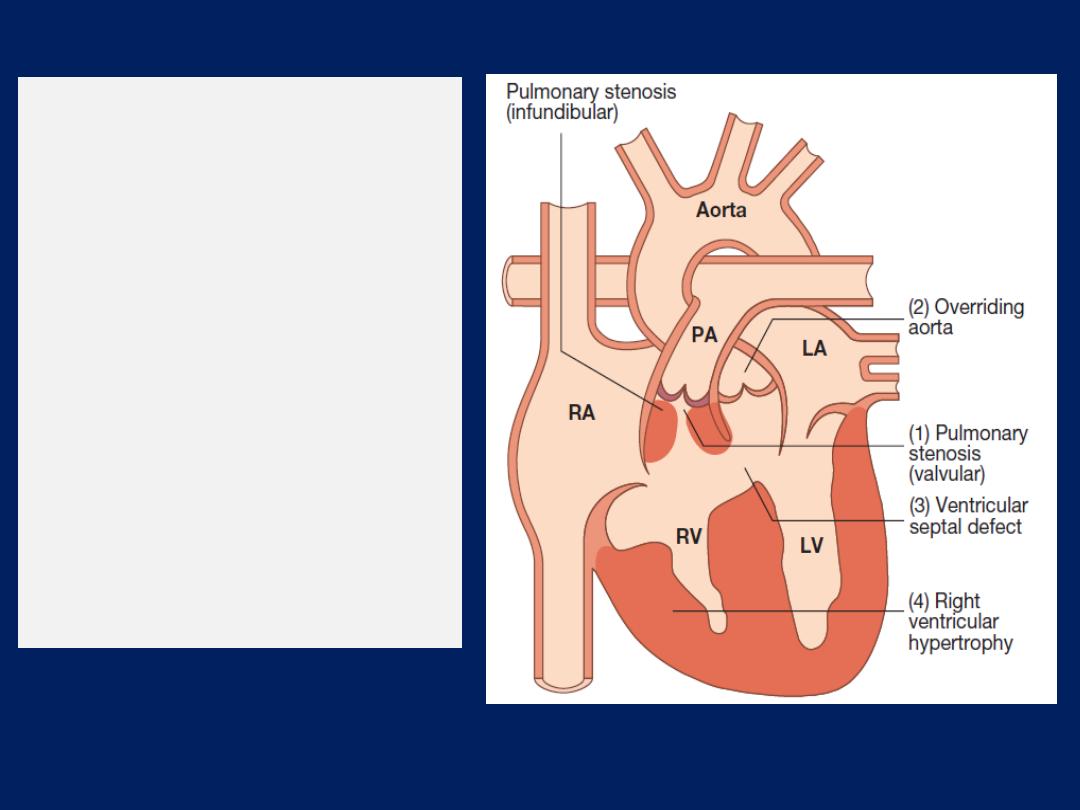
Tetralogy of Fallot.
The
tetralogy comprises
(1) pulmonary
stenosis,
(2) overriding of the
ventricular septal defect
by the aorta,
(3) A ventricular septal
defect and
(4) right ventricular
hypertrophy.

Clinical features
Children are usually cyanosed but this may not be the
case in the neonate because it is only when right
ventricular pressure rises to equal or exceed left
ventricular pressure that a large right-to-left shunt
develops. The subvalvular component of the RV outflow
obstruction is dynamic and may increase suddenly
under adrenergic stimulation. The affected child suddenly
becomes increasingly cyanosed, often after feeding or a
crying attack, and may become apnoeic and unconscious.
These attacks are called
‘Fallot’s spells’.
In older children, Fallot’s spells are uncommon but
cyanosis becomes increasingly apparent, with stunting of
growth, digital clubbing and polycythaemia.

Some children characteristically obtain relief by
squatting after exertion, which increases the afterload
of the left heart and reduces the right-to-left shunting:
Fallot’s sign.
The natural history before the development
of surgical correction was variable but most patients died
in infancy or childhood.
On examination, the most characteristic feature is the
combination of cyanosis with a loud ejection systolic
murmur in the pulmonary area (as for pulmonary
stenosis). However, cyanosis may be absent in the
newborn or in patients with only mild right ventricular
outflow obstruction (‘acyanotic tetralogy of Fallot’).

Investigations and management
The ECG shows right ventricular hypertrophy and the
chest X-ray shows an abnormally small pulmonary
artery and a ‘
boot-shaped’ heart.
Echocardiography is
diagnostic and demonstrates that the aorta is not
continuous with the anterior ventricular septum.
The definitive management is total correction of the
defect by surgical relief of the pulmonary stenosis and
closure of the ventricular septal defect. Primary surgical
correction may be undertaken prior to the age of 5
years. If the pulmonary arteries are too hypoplastic, then
palliation in the form of a Blalock– Taussig shunt may
be performed, with an anastomosis created between the
pulmonary artery and subclavian artery.

This improves pulmonary blood flow and pulmonary
artery development, and may facilitate later definitive
correction.
The prognosis after total correction is good, especially
if the operation is performed in childhood. Follow-up is
needed to identify residual shunting, recurrent pulmonary
stenosis and arrhythmias. An implantable defibrillator is
sometimes recommended in adulthood.
Other causes of cyanotic congenital heart disease
There are other causes of cyanotic congenital heart
disease and echocardiography is usually the definitive
diagnostic procedure, supplemented, if necessary, by
cardiac catheterisation.
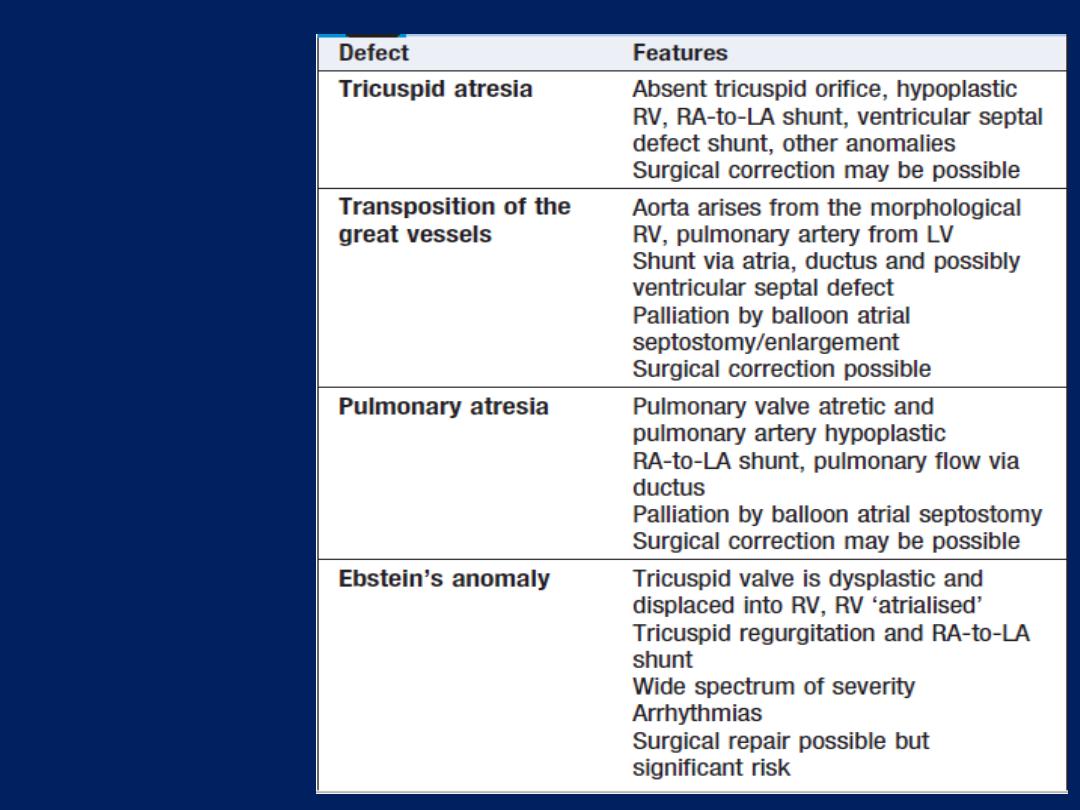
Other causes
of cyanotic
congenital
heart disease

DISEASES OF THE MYOCARDIUM
Although the myocardium is involved in most types
of heart disease, the terms ‘myocarditis’ and
‘cardiomyopathy’ are usually reserved for conditions that
primarily affect the heart muscle.
Myocarditis
This is an acute inflammatory condition that can have
an infectious, toxic or autoimmune aetiology. Myocarditis
can complicate many infections in which inflammation
may be due directly to infection of the myocardium
or the effects of circulating toxins.
Viral infections are the most common causes, such as
Coxsackie and influenza A and B viruses.

Myocarditis may occur several weeks after the initial viral
symptoms and susceptibility is increased by corticosteroid
treatment, immunosuppression, radiation, previous
myocardial damage and exercise. Some bacterial and
protozoal infections may be complicated by myocarditis; for
example, approximately 5% of patients with Lyme
disease (Borrelia burgdorferi) develop myopericarditis, which
is often associated with AV block. Toxic aetiologies include
drugs, which may
directly
injure the myocardium (e.g.
cocaine, lithium and anti-cancer drugs, such as
doxorubicin) or which may cause a
hypersensitivity
reaction and associated myocarditis (e.g. penicillins and
sulphonamides, lead and carbon monoxide).
Occasionally, autoimmune conditions, such as SLE and
rheumatoid arthritis, are associated with myocarditis.

Clinical features and investigations
Myocarditis can be classified by four distinct clinical
presentations:
• Fulminant myocarditis
follows a viral prodrome or
influenza-like illness, and results in severe heart
failure or cardiogenic shock.
• Acute myocarditis
presents over a longer period with
heart failure; it can lead to dilated cardiomyopathy.
• Chronic active myocarditis
is rare and associated with
chronic myocardial inflammation.
• Chronic persistent myocarditis
, characterised by focal
myocardial infiltrates, can cause chest pain and arrhythmia
without necessarily causing ventricular dysfunction.

In myocarditis, ECG changes are common but
nonspecific.
Biochemical markers of myocardial injury (e.g.
troponin I and T, creatine kinase) may be elevated
in the early phases. Echocardiography may reveal
left ventricular dysfunction that is sometimes
regional (due to focal myocarditis), and cardiac
MRI may show diagnostic patterns of myocardial
inflammation or infiltration.
Endomyocardial biopsy is sometimes used to
confirm the diagnosis.

Management and prognosis
In most patients, myocarditis is self-limiting and the
immediate prognosis is good. However, death may
occur due to a ventricular arrhythmia or rapidly
progressive heart failure. Myocarditis has been reported
as a cause of sudden and unexpected death in young
athletes. Some forms may lead to chronic low-grade
myocarditis or dilated cardiomyopathy (see below); for
example, in Chagas’ disease , the patient frequently
recovers from the acute infection but goes on to develop a
chronic dilated cardiomyopathy 10 or 20 years later.
Specific antimicrobial therapy may be used if a causative
organism has been identified; this is rare, however, and in
most cases only supportive therapy is available.

Treatment for cardiac failure or arrhythmias may be
required and patients should be advised to avoid
intense physical exertion because there is some evidence
that this can induce potentially fatal ventricular
arrhythmias.
There is no evidence for any benefit from treatment
with corticosteroids and immunosuppressive agents. Rarely
cardiac transplantation or temporary circulatory support
with a mechanical ventricular assist device is required.
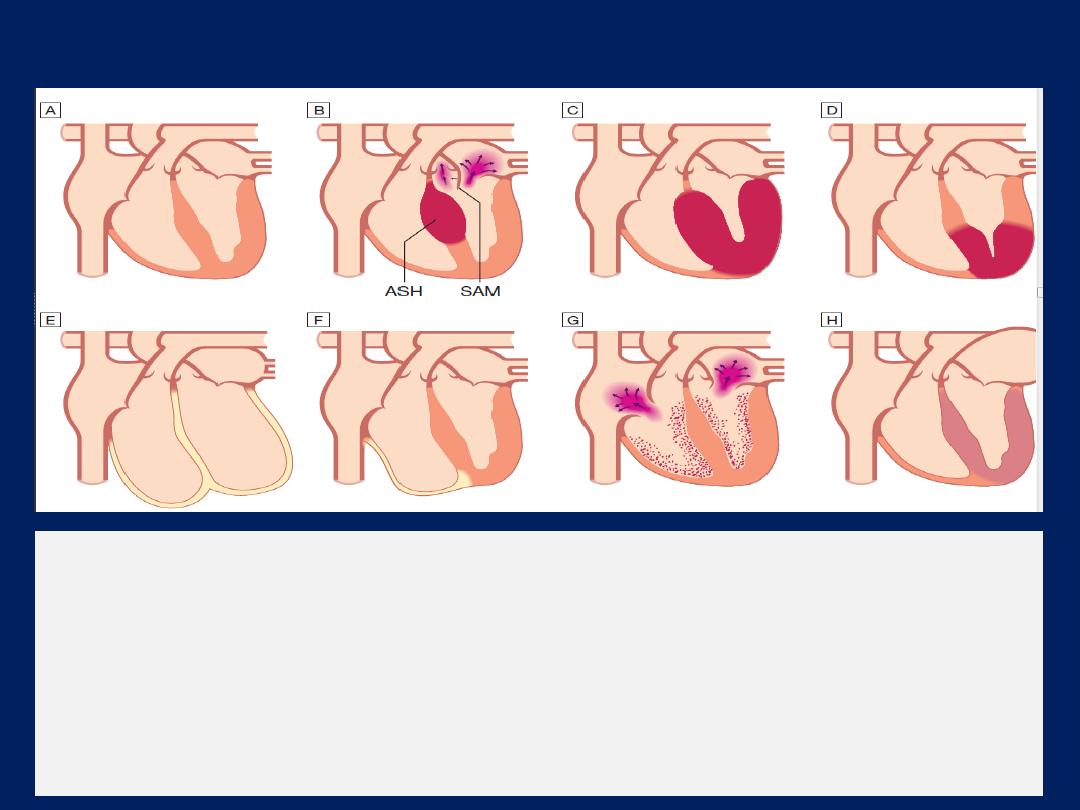
Types of cardiomyopathy
FIG - Types of cardiomyopathy.
A
Normal heart.
B Hypertrophic
cardiomyopathy: asymmetric septal hypertrophy
(ASH) with systolic anterior motion of the mitral valve (SAM), causing mitral reflux
and dynamic left ventricular outflow tract obstruction.
C
Hypertrophic
cardiomyopathy: concentric hypertrophy.
D
Hypertrophic cardiomyopathy: apical
hypertrophy.
E Dilated
cardiomyopathy.
F Arrhythmogenic
right ventricular
cardiomyopathy.
G
Obliterative cardiomyopathy.
H Restrictive
cardiomyopathy.

Cardiomyopathy
Cardiomyopathies are diseases of the myocardium, and
are classified according to their structural and functional
presentation .They can be inherited or have infective,
toxic or idiopathic aetiologies.
Dilated cardiomyopathy
This is characterised by dilatation and impaired
contraction of the LV and often the RV. Left ventricular
mass is increased but wall thickness is normal or reduced .
Dilatation of the valve rings can lead to ‘functional’
mitral and tricuspid incompetence.
Histological changes are variable but include
myofibrillary loss, interstitial fibrosis and T-cell infiltrates.

The differential diagnosis includes ventricular dysfunction
due to CAD , and a diagnosis of dilated cardiomyopathy
should only be made when this has been excluded.
Alcohol may be an important cause in some patients.
At least 25% of cases are inherited as an autosomal
dominant trait. Most of the X-linked inherited skeletal
muscular dystrophies (e.g. Becker and Duchenne) are
associated with cardiomyopathy. Finally, a late
autoimmune reaction to viral myocarditis is thought to be
the cause in a substantial subgroup of patients with dilated
cardiomyopathy; a similar mechanism is thought to be
responsible for the heart muscle disease that occurs in up
to 10% of patients with advanced HIV infection.
Men are affected more than twice as often as women.

Arrhythmia, thromboembolism and sudden death may
occur at any stage; sporadic chest pain is a surprisingly
frequent symptom.
The ECG usually shows non-specific changes but echo and
cardiac MRI are useful in establishing the diagnosis.
Treatment is aimed at controlling the resulting heart failure.
Although some patients remain well for many years, the
prognosis is variable and cardiac transplantation may be
indicated. Patients with dilated cardiomyopathy and
moderate or severe heart failure may be at risk of sudden
arrhythmic death.
This risk is substantially reduced by rigorous medical therapy
with β-blockers and angiotensin receptor antagonists. Some
patients may be considered for implantation of a cardiac
defibrillator and/or cardiac resynchronisation therapy .

Hypertrophic cardiomyopathy
This is the most common form of cardiomyopathy. It is
characterised by inappropriate and elaborate left
ventricular hypertrophy with malalignment of the
myocardial fibres and myocardial fibrosis. The
hypertrophy may be generalised or confined largely to
the interventricular septum (asymmetric septal
hypertrophy) or other regions (e.g. apical hypertrophic
cardiomyopathy).
Heart failure may develop because the stiff noncompliant
ventricles impede diastolic filling.

Septal hypertrophy may also cause dynamic left
ventricular outflow tract obstruction (hypertrophic
obstructive cardiomyopathy, HOCM) and mitral
regurgitation due to abnormal systolic anterior motion of
the anterior mitral valve leaflet.
Effort-related symptoms (angina and breathlessness),
arrhythmia and sudden death are the dominant clinical
presentations.
Hypertrophic cardiomyopathy is a genetic disorder,
usually with autosomal dominant transmission, a high
degree of penetrance and variable expression.

Symptoms and signs are similar to those of aortic
stenosis, except that, in hypertrophic cardiomyopathy,
the character of the arterial pulse is jerky .
The ECG is abnormal and shows features of left
ventricular hypertrophy with a wide variety of often
bizarre abnormalities (e.g. pseudo-infarct pattern, deep
T-wave inversion). Echocardiography is diagnostic,
although the diagnosis may be difficult when another
cause of left ventricular hypertrophy is present (e.g.
physical training – athletes’ heart, hypertension) but the
degree of hypertrophy is greater than expected. Genetic
testing may facilitate diagnosis and, in some cases, is
helpful in screening relatives of affected individuals.

The natural history is variable but clinical deterioration
is often slow. The annual mortality from sudden
death is 2–3% among adults and 4–6% in children and
adolescents .Sudden death typically occurs
during or just after vigorous physical activity; indeed,
hypertrophic cardiomyopathy is the most common
cause of sudden death in young athletes. Ventricular
arrhythmias may be responsible for many of these.
Beta-blockers, rate-limiting calcium antagonists (e.g.
verapamil) and disopyramide can help to relieve symptoms
and sometimes prevent syncopal attacks; however,
there is no pharmacological treatment that is definitely
known to improve prognosis. Arrhythmias are common
and often respond to treatment with amiodarone.

Outflow tract obstruction can be improved by partial
surgical resection (myectomy) or by iatrogenic infarction
of the basal septum (septal ablation) using a catheter
delivered alcohol solution. An implantable cardiac
defibrillator should be considered in patients with clinical
risk factors for sudden death .
Digoxin and vasodilators may increase outflow tract
obstruction and should be avoided.
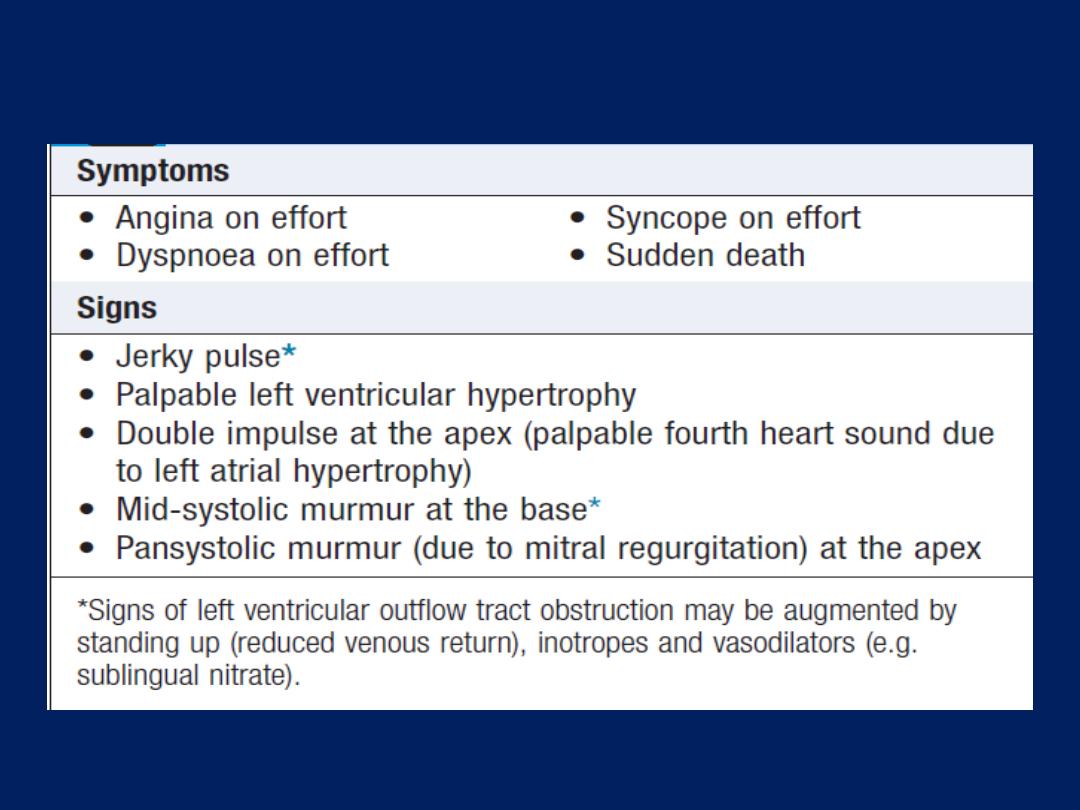
Clinical features of hypertrophic cardiomyopathy

Risk factors for sudden death in hypertrophic
cardiomyopathy

Arrhythmogenic right ventricular cardiomyopathy
In this condition, patches of the right ventricular
myocardium are replaced with fibrous and fatty tissue .It
is inherited as an autosomal dominant trait. The
dominant clinical problems are ventricular arrhythmias,
sudden death and right-sided cardiac failure. The ECG
typically shows a slightly broadened QRS complex and
inverted T waves in the right precordial leads. MRI is a
useful diagnostic tool and is used, along with the 12-lead
ECG and ambulatory ECG monitoring, to screen the first-
degree relatives of affected individuals.
Patients at high risk of sudden death can be offered an
implantable cardiac defibrillator.

Restrictive cardiomyopathy
In this rare condition, ventricular filling is impaired
because the ventricles are ‘stiff’ .This leads to high atrial
pressures with atrial hypertrophy, dilatation and, later,
atrial fibrillation. Amyloidosis is the most common cause
in the UK, although other forms of infiltration (e.g. glycogen
storage diseases), idiopathic perimyocyte fibrosis and a
familial form of restrictive cardiomyopathy do occur.
Diagnosis can be very difficult and requires complex
Doppler echo, CT or MRI, and endomyocardial biopsy.
Treatment
Is symptomatic but the prognosis is usually poor and
transplantation may be indicated.
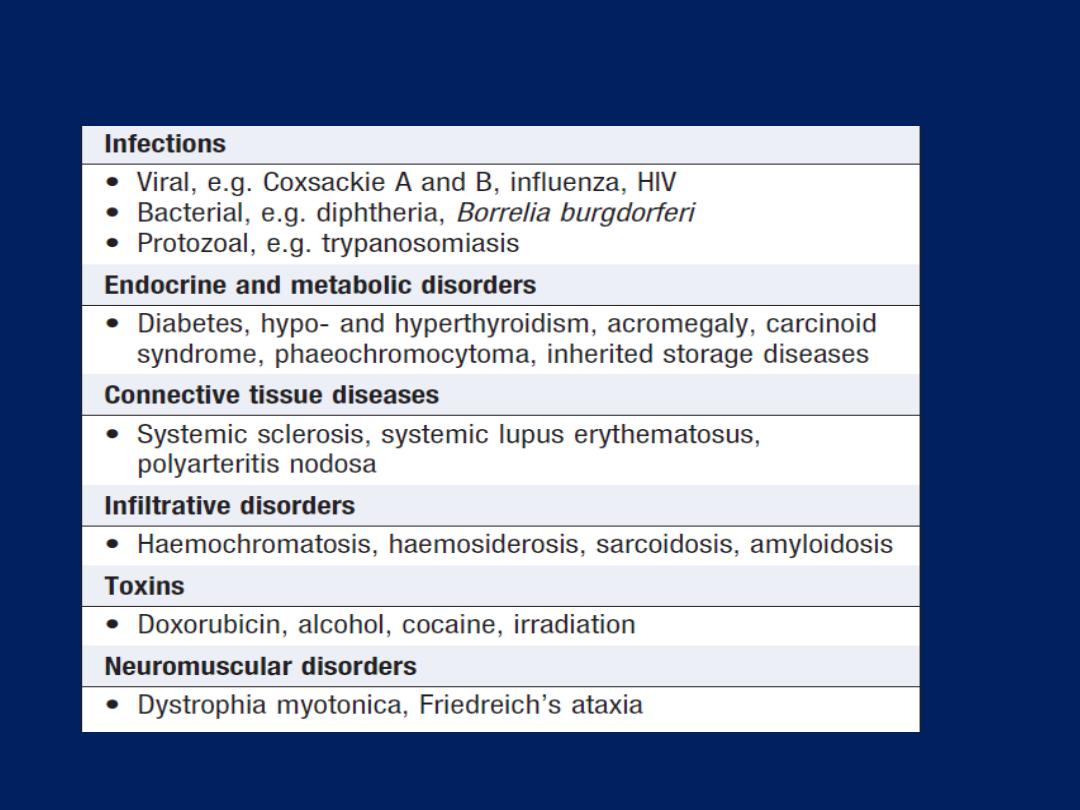
Specific diseases of heart muscle

Cardiac tumours
Primary cardiac tumours are rare (< 0.2% of autopsies)
but the heart and mediastinum may be the sites of
metastases. Most primary tumours are benign (75%)
and, of these, the majority are myxomas.
The remainder are fibromas, lipomas, fibroelastomas and
haemangiomas. Atrial myxoma Myxomas most commonly
arise in the LA as single or multiple polypoid tumours,
attached by a pedicle to the interatrial septum. They are
usually gelatinous but may be solid and even calcified,
with superimposed thrombus.
On examination, the first heart sound is usually loud,
and there may be a murmur of mitral regurgitation with
a variable diastolic sound (tumour ‘plop’) due to
prolapse of the mass through the mitral valve.

The tumour can be detected incidentally on
echocardiography, or following investigation of
pyrexia, syncope, arrhythmias or emboli.
Occasionally, the condition presents with malaise
and features suggestive of a connective tissue
disorder, including a raised ESR.
Treatment is by surgical excision. If the pedicle is
removed, fewer than 5% of tumours recur.

DISEASES OF THE PERICARDIUM
The normal pericardial sac contains about 50 mL
of fluid, similar to lymph, which lubricates the
surface of the heart. The pericardium limits
distension of the heart, contributes to the
haemodynamic interdependence of the
ventricles, and acts as a barrier to infection.
Nevertheless, congenital absence of the
pericardium does not result in significant clinical or
functional limitations.

Acute pericarditis
Aetiology :Pericardial inflammation may be due to a number
of pathologies but sometimes remains unexplained.
Pericarditis and myocarditis often coexist, and all forms of
pericarditis may produce a pericardial effusion that,
depending on the aetiology, may be fibrinous, serous,
haemorrhagic or purulent. A fibrinous exudate may
eventually lead to varying degrees of adhesion formation,
whereas serous pericarditis often produces a large effusion
of turbid, straw-coloured fluid with a high protein content. A
haemorrhagic effusion is often due to malignant disease,
particularly carcinoma of the breast or bronchus, and
lymphoma. Purulent pericarditis is rare and may occur as a
complication of septicaemia, by direct spread from an
intrathoracic infection, or from a penetrating injury.
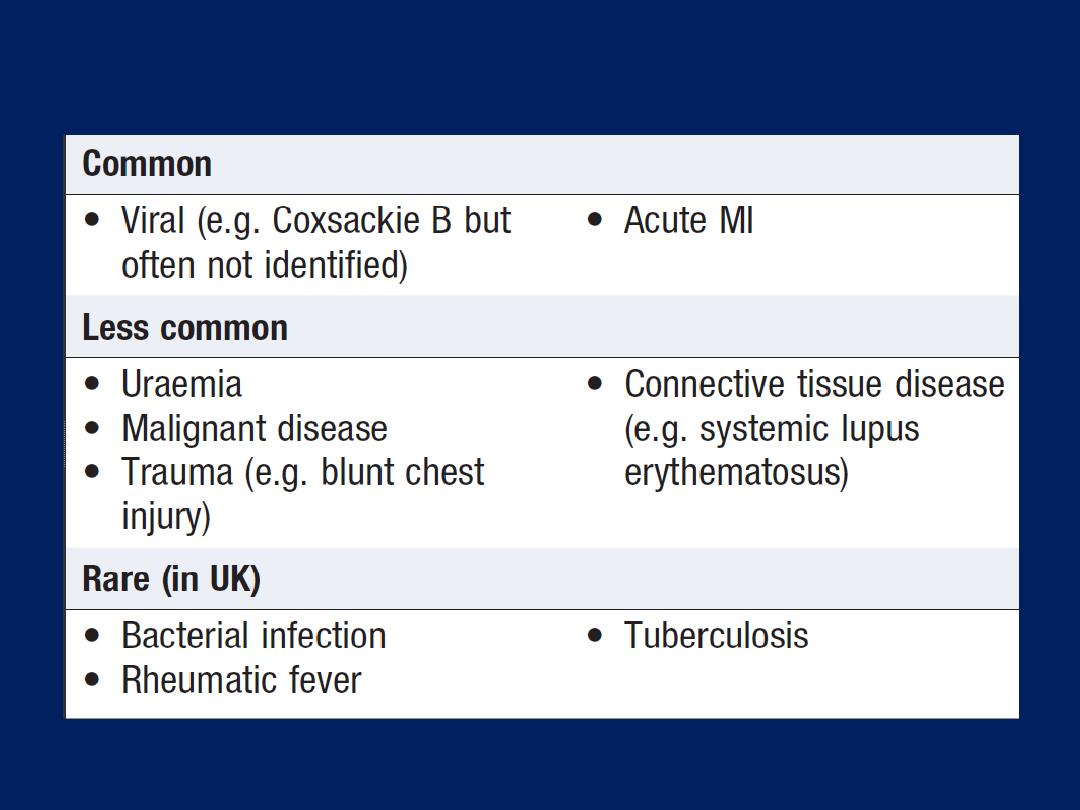
Aetiology of acute pericarditis

Clinical features
The characteristic pain of pericarditis is retrosternal,
radiates to the shoulders and neck, and is typically
aggravated by deep breathing, movement, a change of
position, exercise and swallowing.
A low-grade fever is common.
A pericardial friction rub is a high-pitched
superficial scratching or crunching noise, produced by
movement of the inflamed pericardium, and is
diagnostic of pericarditis; it is usually heard in systole
but may also be audible in diastole and frequently has
a ‘to-and fro’ quality.

Investigations and management
The ECG shows ST elevation with upward concavity
which may be widespread. PR interval depression is a
very specific indicator of acute pericarditis. Later, there
may be T-wave inversion, particularly if there is a degree
of myocarditis. The pain is usually relieved by aspirin
(600 mg 6 times daily) but a more potent anti-
inflammatory agent, such as indometacin (25 mg 3 times
daily), may be required. Colchicine or corticosteroids may
suppress symptoms but there is no evidence that they
accelerate cure. In viral pericarditis, recovery usually
occurs within a few days or weeks but there may be
recurrences (chronic relapsing pericarditis). Purulent
pericarditis requires treatment with antimicrobial therapy,
pericardiocentesis and, if necessary, surgical drainage.
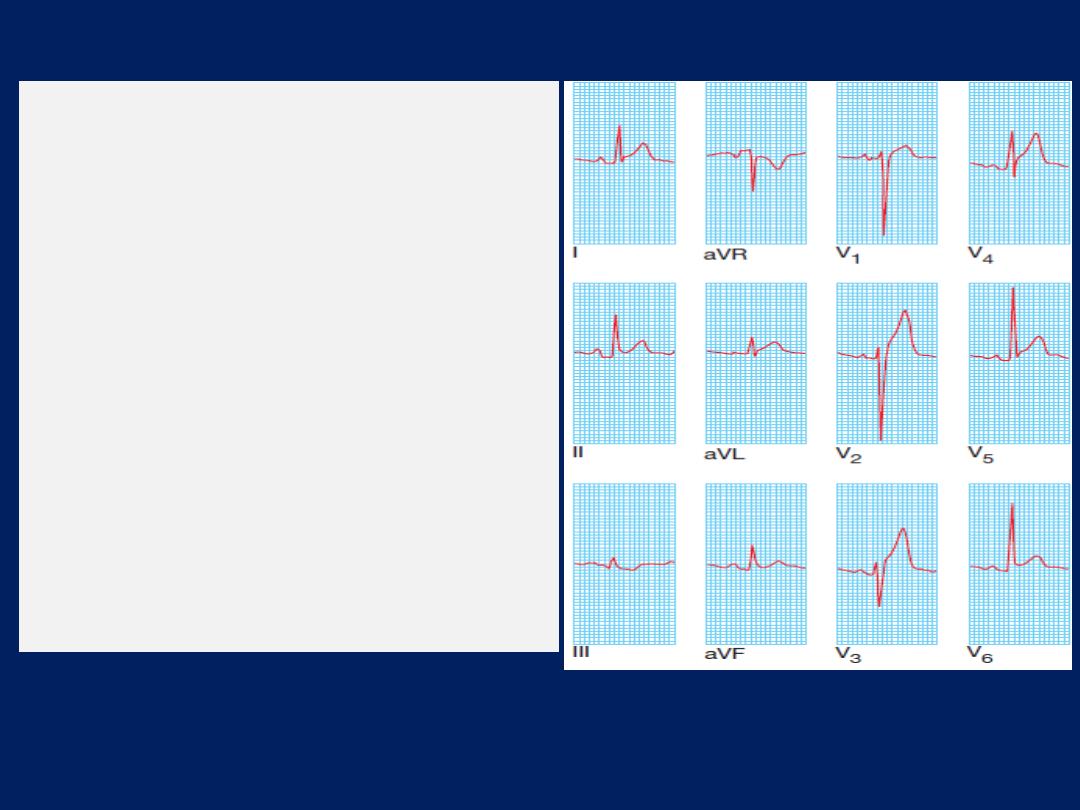
ECG in pericarditis.
Widespread ST elevation
(leads I, II, aVL and V1–V6) is
shown. The upward concave
shape of the ST segments (see
leads II and V6) and the
unusual distribution of
changes (involving anterior
and inferior leads) help to
distinguish pericarditis from
acute MI.

Pericardial effusion
If a pericardial effusion develops, there is sometimes a
sensation of retrosternal oppression. An effusion is
difficult to detect clinically. The heart sounds may become
quieter, although a friction rub is not always abolished.
The QRS voltages on the ECG are often reduced in
the presence of a large effusion. The QRS complexes
may alternate in amplitude due to a to-and-fro motion of
the heart within the fluid-filled pericardial sac (
electrical
alternans
). The chest X-ray may show an increased size
of the cardiac silhouette and, when there is a large
effusion, this has a globular appearance. Echo is the
definitive investigation and is used to monitor the size of
the effusion and its effect on cardiac function.
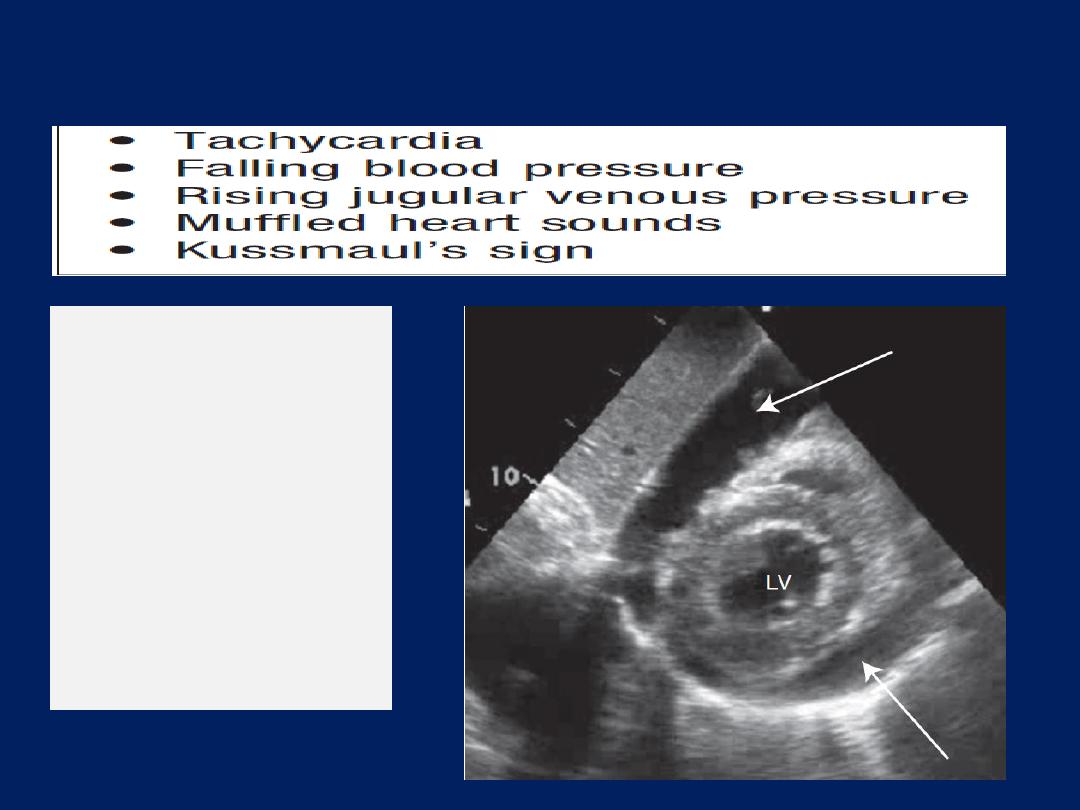
Pericardial effusion
Pericardial effusion:
echocardiogram
(apical view).
Short axis view of the
heart showing a large
circumferential
pericardial
effusion (arrows). (LV
= left ventricle)

Cardiac tamponade
This term is used to describe acute heart failure
due to compression of the heart by a large or
rapidly developing effusion.
Typical physical findings are of a markedly raised
JVP, hypotension, pulsus paradoxus and oliguria.
Atypical presentations may occur when the effusion
is loculated as a result of previous pericarditis or
cardiac surgery.

Clinical features of pericardial tamponade

Pericardial aspiration (pericardiocentesis)
Aspiration of a pericardial effusion is indicated for
diagnostic purposes or for the treatment of cardiac
tamponade.
A needle is inserted under echocardiographic guidance
medial to the cardiac apex or below the xiphoid process,
directed upwards towards the left shoulder. The route of
choice will depend on the experience of the operator, the
shape of the patient and the position of the effusion. A
few millilitres of fluid aspirated through the needle may
be sufficient for diagnostic purposes but pericardial
drainage is needed for symptom relief.

Complications of pericardiocentesis include arrhythmias,
damage to a coronary artery, and bleeding with
exacerbation of tamponade as a result of injury to the RV.
When tamponade is due to cardiac rupture or aortic
dissection, pericardial aspiration may precipitate further
potentially fatal bleeding and, in these situations,
emergency surgery is the treatment of choice.
A viscous, loculated or recurrent effusion may also require
formal surgical drainage.

Tuberculous pericarditis
Tuberculous pericarditis may complicate pulmonary
tuberculosis but may also be the first manifestation of
the infection. In Africa, a tuberculous pericardial effusion
is a common feature of AIDS .
The condition typically presents with chronic malaise,
weight loss and a low-grade fever. An effusion usually
develops and the pericardium may become thick and
unyielding, leading to pericardial constriction or tamponade.
A associated pleural effusion is often present.
The diagnosis may be confirmed by aspiration of the
fluid and direct examination or culture for tubercle bacilli.
Treatment requires specific antituberculous chemotherapy , in
addition, a 3-month course of prednisolone (initial 60 mg a
day, tapering down rapidly) improves outcome.

Chronic constrictive pericarditis
Constrictive pericarditis is due to progressive thickening,
fibrosis and calcification of the pericardium. In effect, the
heart is encased in a solid shell and cannot fill properly. The
calcification may extend into the myocardium, so there may
also be impaired myocardial contraction. The condition
often follows an attack of tuberculous pericarditis but can
also complicate haemopericardium, viral pericarditis,
rheumatoid arthritis and purulent pericarditis. It is often
impossible to identify the original insult.
Clinical features and management
The symptoms and signs of systemic venous congestion
are the
hallmarks
of constrictive pericarditis.
Atrial fibrillation is common and there is often dramatic
ascites and hepatomegaly .

Breathlessness is not
a prominent symptom because the
lungs are seldom congested.
The condition is sometimes overlooked but should be
suspected in any patient with unexplained
right heart
failure and a small heart.
A chest X-ray, which may
show pericardial calcification , and echocardiography
often help to establish the diagnosis. CT scanning is useful
for imaging the pericardial calcification. Constrictive
pericarditis is often difficult to distinguish from restrictive
cardiomyopathy and the final diagnosis may depend on
complex echo–Doppler studies and cardiac cath. Surgical
resection of the diseased pericardium can lead to a
dramatic improvement but carries a high morbidity with
disappointing results in up to 50% of patients.
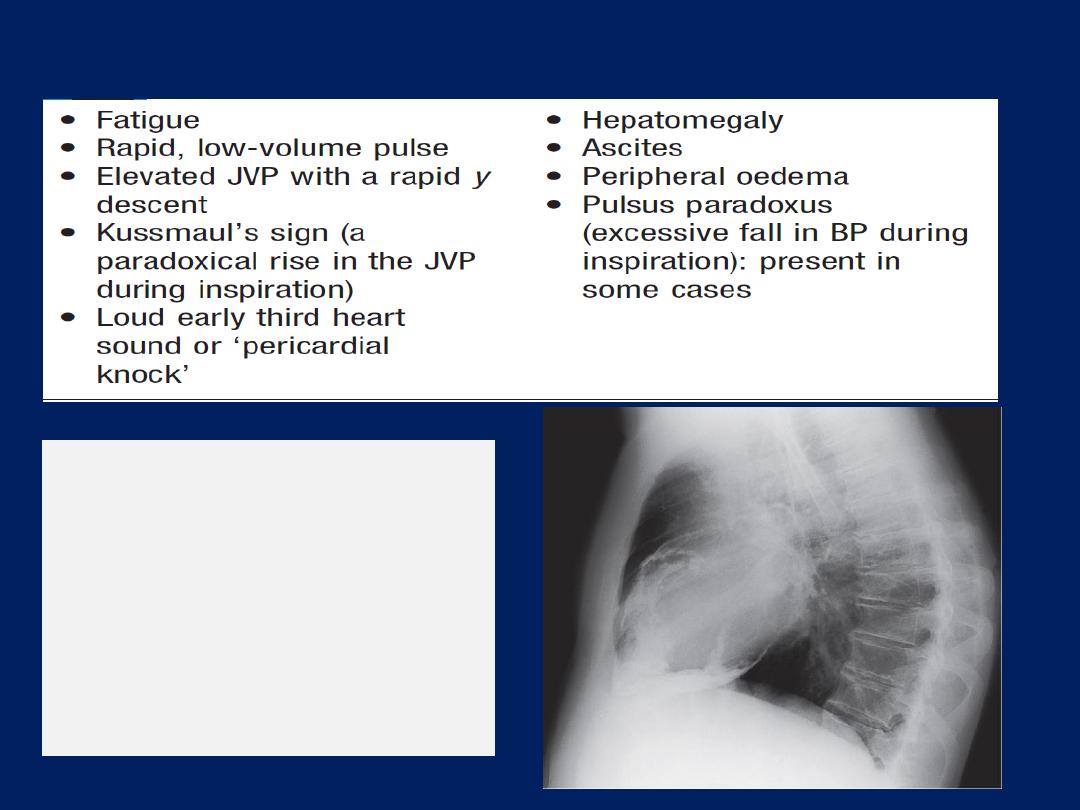
Clinical features of constrictive pericarditis
Lateral chest X-ray
from a
patient with severe heart
failure due to chronic
constrictive pericarditis.
There is heavy
calcification of the
pericardium.
