Defects of carbohydrate digestion and absorption:
A. Lactase deficiency = lactose intolerance:1. Definition:
a) This is a deficiency of lactase enzyme which digest lactose into
glucose and galactose.
b) It may be:
1- Congenital: which occurs very soon after birth (rare).
2- Acquired: which occurs later on in life (common).
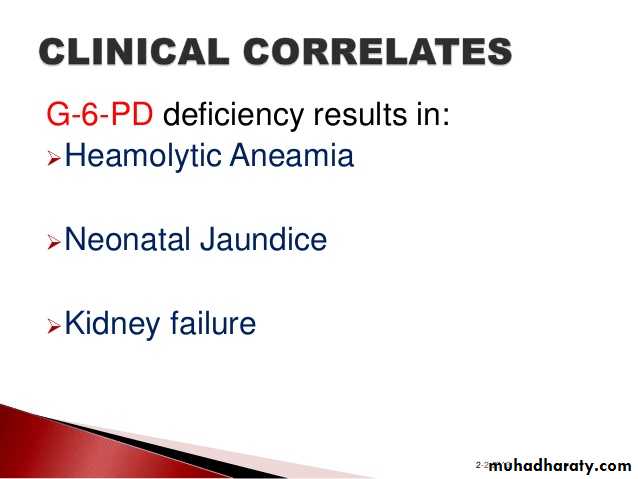
2. Effect: The presence of lactose in intestine causes:
a) Increased osmotic pressure: So water will be drawn from the tissue
(causing dehydration) into the large intestine (causing diarrhea).
b) Increased fermentation of lactose by bacteria: Intestinal bacteria
ferment lactose with subsequent production of CO2 gas. This causes
distention and abdominal cramps.
c) Treatment: Treatment of this disorder is simply by removing lactose
(milk) from diet.
B. Sucrose deficiency:
A rare condition, showing the signs and symptoms of lactase deficiency. It
occurs early in childhood.
C. Monosaccharide malabsorption:
This is a congenital condition in which glucose and galactose are absorbed
only slowly due to defect in the carrier mechanism. Because fructose is not
absorbed by the carrier system, its absorption is normal.
Fate of absorbed sugars:
Monosaccharides (glucose, galactose and fructose) resulting from carbohydrate digestion are absorbed and undergo the following:
A. Uptake by tissues (liver):
After absorption the liver takes up sugars, where galactose and fructose are converted into glucose.
B. Glucose utilization by tissues:
Glucose may undergo one of the following fate:
1. Oxidation: through
a) Major pathways (glycolysis and Krebs' cycle) for production of energy.
b) Hexose monophosphate pathway: for production of ribose, deoxyribose
and NADPH + H+
c) Uronic acid pathway, for production of glucuronic acid, which is used in
detoxication and enters in the formation of mucopolysaccharide.
2. Storage: in the form of:
a) Glycogen: glycogenesis.
b) Fat: lipogenesis.
3. Conversion: to substances of biological importance:
a) Ribose, deoxyribose RNA and DNA.
b) Lactose milk.
c) Glucosamine, galactosamine mucopolysaccharides.
d) Glucoronic acid mucopolysaccharides.
e) Fructose in semen.
Main Classes of Metabolic Reactions
Oxidation-Reduction Reactions
Reactions that make or break carbon - carbon bonds
Internal rearrangements, isomerizations, eliminations.
Group transfer reactions. Free radical reactions
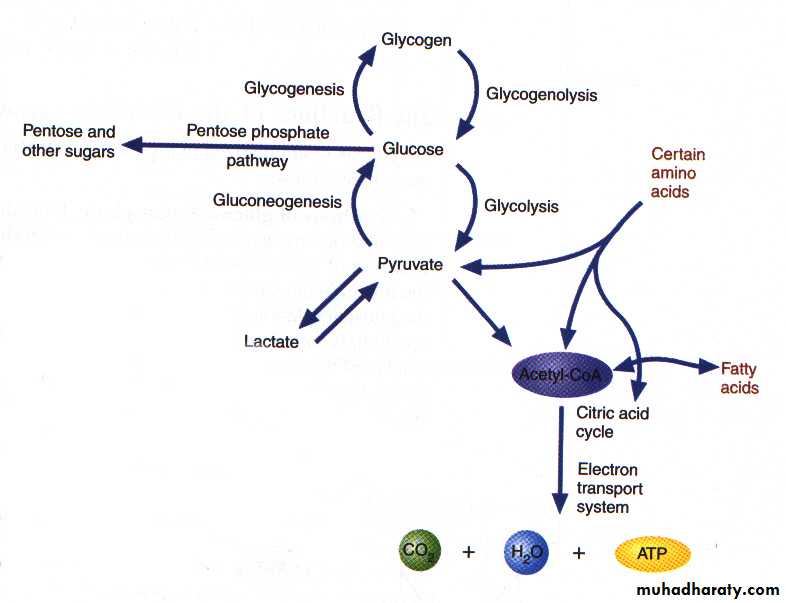
Carbohydrate metabolism
Carbohydrate metabolism denotes the various biochemical processes responsible for the formation, breakdown and interconversion of carbohydrates in living organisms. CHO metabolism in mammalian cells can be classified into:
1- Glycolysis: Oxidation of glucose to pyruvate (aerobic state) or lactate (anaerobic state).
2- Krebs cycle: After oxidation of pyruvate to acetyl CoA, acetyl CoA enters the Krebs cycle for the aim of production of ATP.
3- Hexose monophosphate shunt: Enables cells to produce ribose-5-phosphate and NADPH.
4- Glycogenesis: Synthesis of glycogen from glucose, when glucose levels are high.
5- Glycogenolysis: Degradation of glycogen to glucose when glucose in short supply.
6- Gluconeogenesis: Formation of glucose from noncarbohydrate sources.
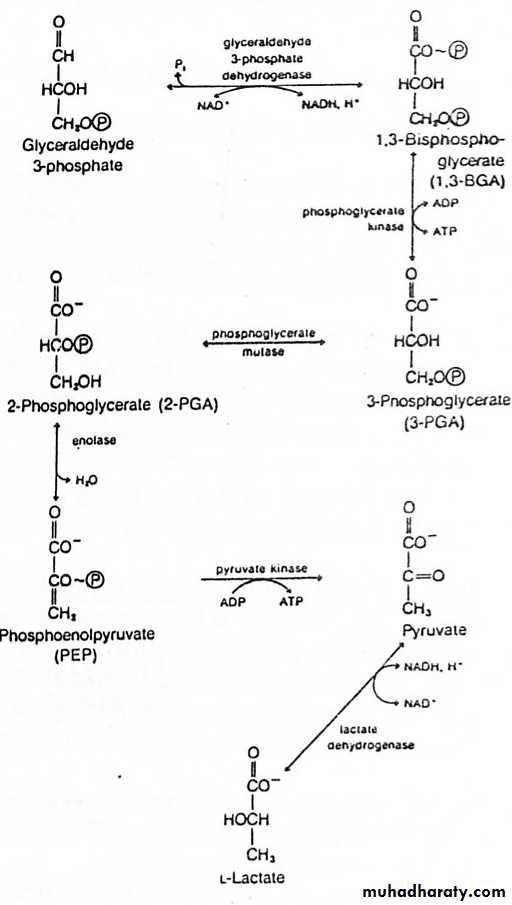
Glycolysis (Embden-Meyerhof Pathway) 1-[glycolysis: from the Greek glyk-, sweet, and lysis, splitting]
A. Definition:
1. Glycolysis means oxidation of glucose to give pyruvate (in the
presence of oxygen) or lactate (in the absence of oxygen).
B. Site:
cytoplasm of all tissue cells, but it is of physiological importance in:
1. Tissues with no mitochondria: mature RBCs, cornea and lens.
2. Tissues with few mitochondria: Testis, leucocytes, medulla of the
kidney, retina, skin and gastrointestinal tract.
3. Tissues undergo frequent oxygen lack: skeletal muscles especially
during exercise.
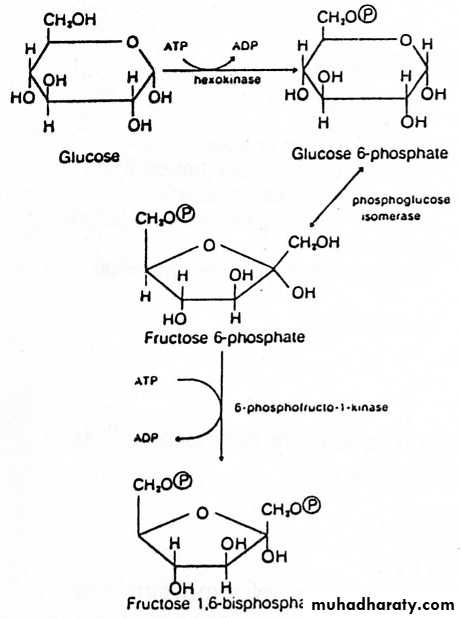
Glycolysis (10 reactions in 3 stages, all in cytoplasm)
1) Priming stage: D-Glucose + 2ATP D-fructose 1,6-biphosphate + 2ADP + 2) Splitting stage : D-Fructose 1,6-biphosphate 2 D-Glyceraldehyde 3-phosphate
3) Oxidoreduction – Phosphorylation stage:
2 D-Glyceraldehyde 3-phosphate + 4ADP + 2Pi + 2H+ 2Lactate + 4ATP
Sum:
Glucose + 2ADP + 2Pi ----- 2 Lactate + 2ATP + 2H2O (Anaerobic)Glucose + 2ADP + 2Pi + 2NAD+ ---- 2 pyruvate + 2ATP + 2NADH + 2H+ + 2H2O (Aerobic)
1) Priming Stage
1- Glucose (and other hexoses) are phosphorylated immediately upon entry into the cell. Phosphorylation prevents transport of glucose out of the cell and increases the reactivity of oxygen in the resulting phosphate ester.2- Several isoenzymes of hexokinase with different Km values for glucose are located in different tissues. Brain hexokinase has a particularly low Km for glucose.
3- The major enzyme for phosphorylating glucose in liver is glucokinase.
Steps catalyzed by hexokinase & PFK-1 are irreversible. 4-
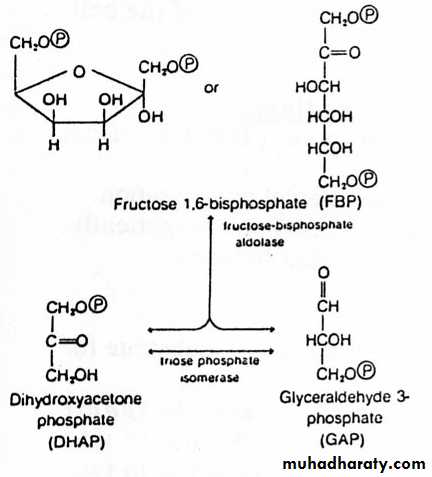
2) Splitting Stage
The reaction catalysed by aldolase is the reverse of aldol condensation.1- Although the cleavage of F1,6BP is energetically unfavourable, rapid removal of the product drives the reaction forward.
2- Of the two products of the aldolase reaction, only GAP (or G3P) serves as a substrate for the next reaction in glycolysis.
3- To prevent the loss of the other three-carbon unit, triose phosphate isomerase catalyses the interconversion of DHAP & G3P. Because of this reaction, the original molecule of glucose has now been converted to two molecules of G3P.
3) Oxidoreduction – Phosphorylation Stage
1- G-3-P dehydrogenase is a tetramer, each subunit contains 1 binding site for G3P & another for NAD+ (NAD+ is permanently bound to the enzyme).2- G3P 1,3-BPG 3PG and PEP Pyruvate are examples of “substrate–level” phosphorylation.
3- 3-PG 2-PG is mediated by an intermediate [2,3-BPG]. Most cells have low amounts of 2,3-BPG except in RBCs, which act as allosteric modifier of Hb-O2 binding.
4- PEP Pyruvate is an “irreversible reaction” due to free energy loss associated with tautomerization of the enol to the more stable keto form.
Differences between Glucokinase & Hexokinase
HexokinaseGlucokinase
Present in all tissues
Liver only
Low Km for glucose (<0.1 mM)
Higher Km for glucose
Strongly inhibited by G6P
Not inhibited by G6P
Non-inducible enzyme, not affected by diabetes or insulin
Inducible,synthesis induced by insulin & repressed in diabetes
Level of enzyme is not affected by fasting or high CHO diet
Depends on glucose concentration
Act on glucose, fructose and galactose
Glucose only
Differences between aerobic and anaerobic glycolysis:
AnaerobicAerobic
Lactate
Pyruvate
1. End product
2 ATP
6 or 8 ATP
2 .energy
Through Lactate formation
Through respiration chain in mitochondria
3. Regeneration of NAD+
Not available as lactate is cytoplasmic substrate
Available and 2 Pyruvate can oxidize to give 30 ATP
4. Availability to TCA in mitochondria
Substrate level phosphorylation:
This means phosphorylation of ADP to ATP at the reaction itself .inglycolysis there are 2 examples:
- 1.3 Bisphosphoglycerate + ADP 3 Phosphoglycerate + ATP
- Phospho-enol pyruvate + ADP Enolpyruvate + ATP
I. Special features of glycolysis in RBCs:
1. Mature RBCs contain no mitochondria, thus:
a) They depend only upon glycolysis for energy production (=2 ATP).
b) Lactate is always the end product.
2. Glucose uptake by RBCs is independent on insulin hormone.
3. Reduction of met-hemoglobin: Glycolysis produces NADH+H+, which
used for reduction of met-hemoglobin in red cells.
Biological importance (functions) of glycolysis:
1. Energy production:
a) anaerobic glycolysis gives 2 ATP.
b) aerobic glycolysis gives 8 ATP.
2. Oxygenation of tissues:
Through formation of 2,3 bisphosphoglycerate, which decreases the
affinity of Hemoglobin to O2.
3. Provides important intermediates:
a) Dihydroxyacetone phosphate: can give glycerol-3phosphate, which is
used for synthesis of triacylglycerols and phospholipids (lipogenesis).
b) 3 Phosphoglycerate: which can be used for synthesis of amino acid
serine.
c) Pyruvate: which can be used in synthesis of amino acid alanine.
4. Aerobic glycolysis provides the mitochondria with pyruvate, which gives
acetyl CoA Krebs' cycle.
Energy production of glycolysis:
Net energy
ATP utilized
ATP produced
2 ATP
2ATP
From glucose to glucose -6-p.
From fructose -6-p to fructose 1,6 p.
4 ATP
(Substrate level phosphorylation) 2ATP from 1,3 DPG.
2ATP from phosphoenol pyruvate
In absence of oxygen (anaerobic glycolysis)
6 ATP
Or
8 ATP
2ATP
-From glucose to glucose -6-p.
From fructose -6-p to fructose 1,6 p.
4 ATP
(substrate level phosphorylation)
2ATP from 1,3 BPG.
2ATP from phosphoenol pyruvate.
In presence of oxygen (aerobic glycolysis)
+ 4ATP or 6ATP
(from oxidation of 2 NADH + H in mitochondria).Importance of lactate production in anerobic glycolysis:
1. In absence of oxygen, lactate is the end product of glycolysis:
2. In absence of oxygen, NADH + H+ is not oxidized by the
respiratory chain.
3. The conversion of pyruvate to lactate is the mechanism for
regeneration of NAD+.
4. This helps continuity of glycolysis, as the generated NAD+ will be
used once more for oxidation of another glucose molecule.
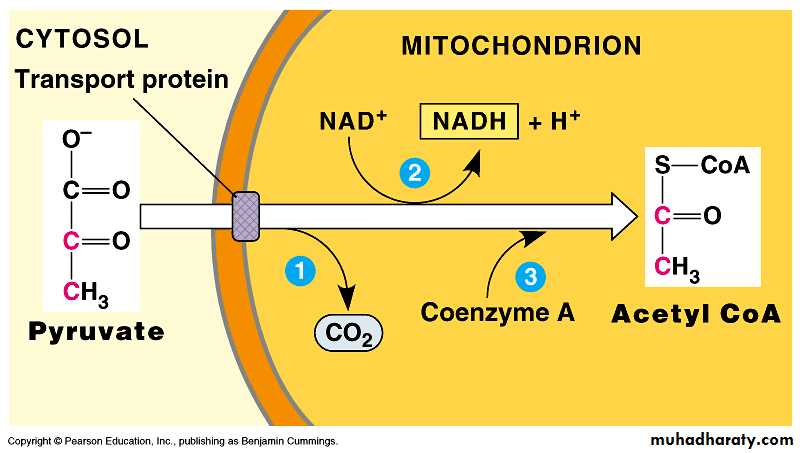
As pyruvate enters the mitochondrion, a multienzyme complex modifies pyruvate to acetyl CoA which enters the Krebs cycle in the matrix.
A carboxyl group is removed as CO2.
A pair of electrons is transferred from the remaining two-carbon fragment to NAD+ to form NADH.
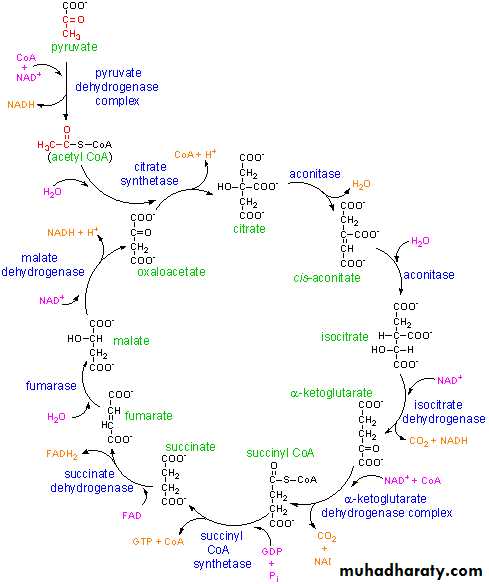
2- Krebs cycle:
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle: is a series of chemical reactions used by all aerobic organisms to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate, (ATP.) In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.
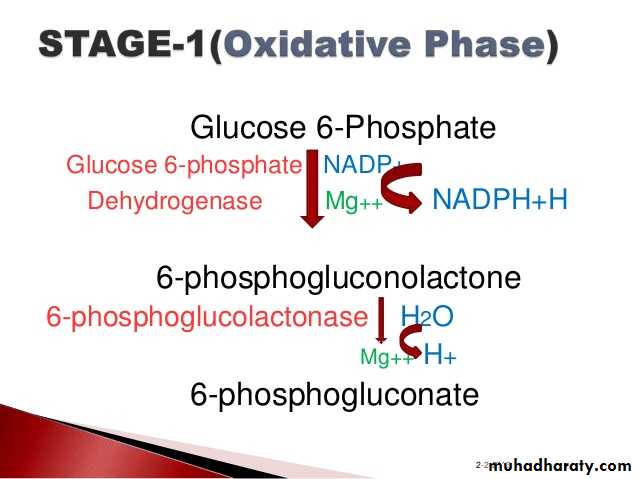
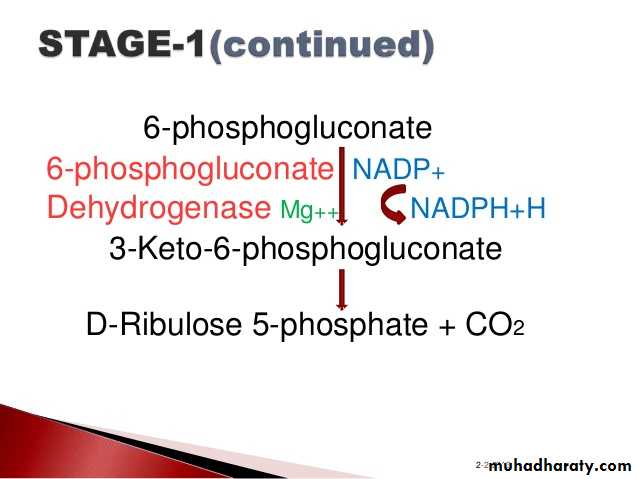
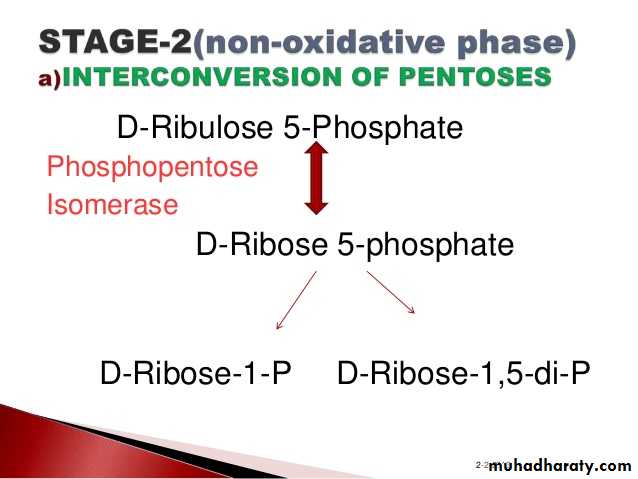
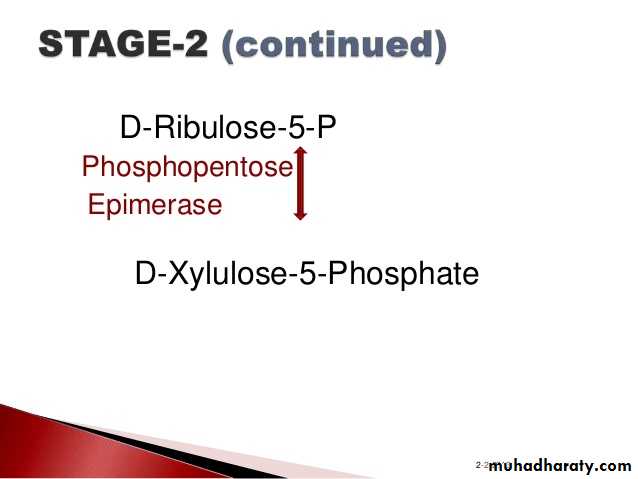
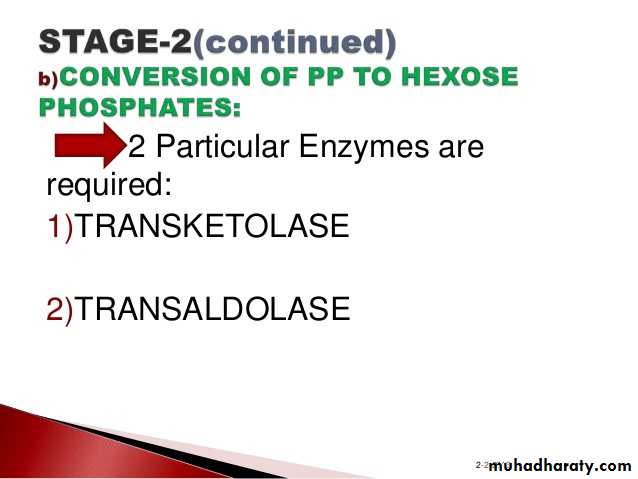
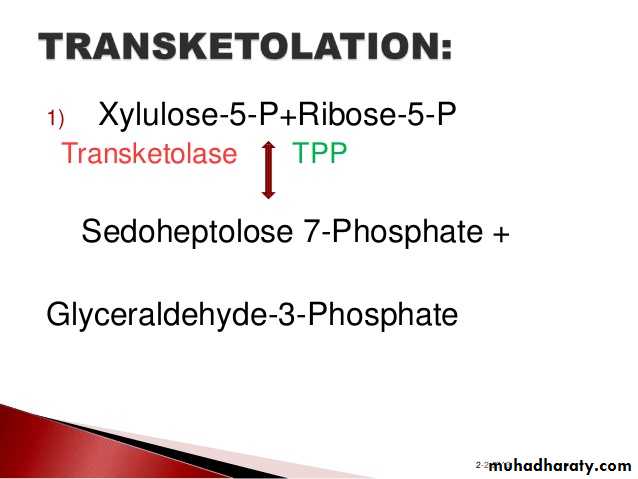
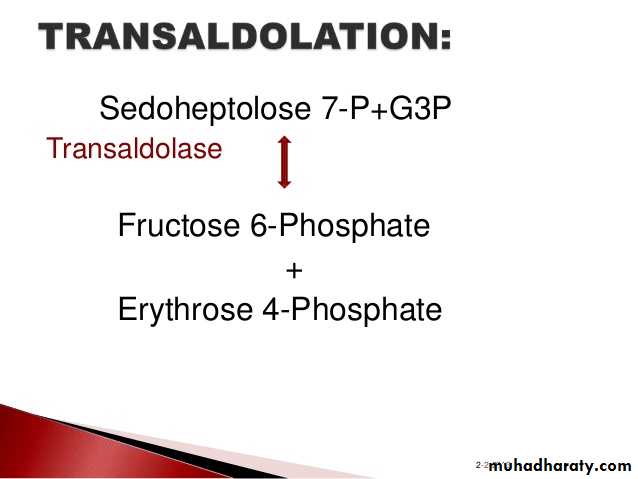
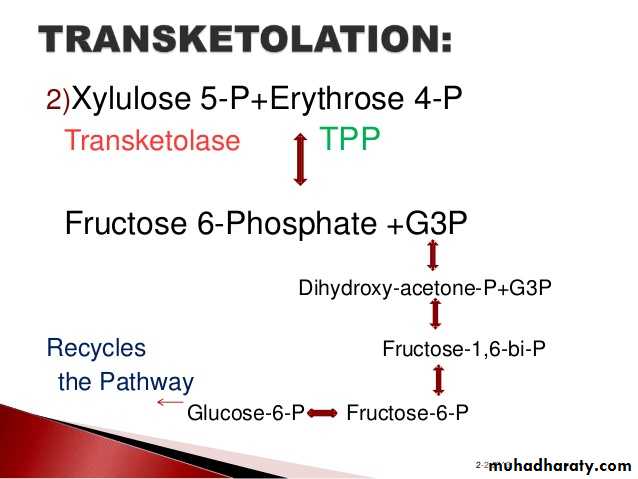
3- Hexose monophosphate shunt:
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (5-carbon sugars) as well as Ribose 5-phosphate, the last one a precursor for the synthesis of nucleotides. While it does involve oxidation of glucose, its primary role is anabolic rather than catabolic.
There are two distinct phases in the pathway.
The first is the oxidative phase, in which NADPH is generated, and the second is the non-oxidative synthesis of 5-carbon sugars. For most organisms, the pentose phosphate pathway takes place in the cytosol; in plants, most steps take place in plastids. The reactions of this pathway are mostly enzyme - catalyzed in modern cells, and are catalyzed by metal ions, particularly ferrous ions (Fe(II)).4- Glycogenesis:
Glycogen FunctionIn liver – The synthesis and breakdown of glycogen is regulated to maintain blood glucose levels.
Glycogenesis:
In muscle - The synthesis and breakdown of glycogen is regulated to meet the energy requirements of the muscle cell.
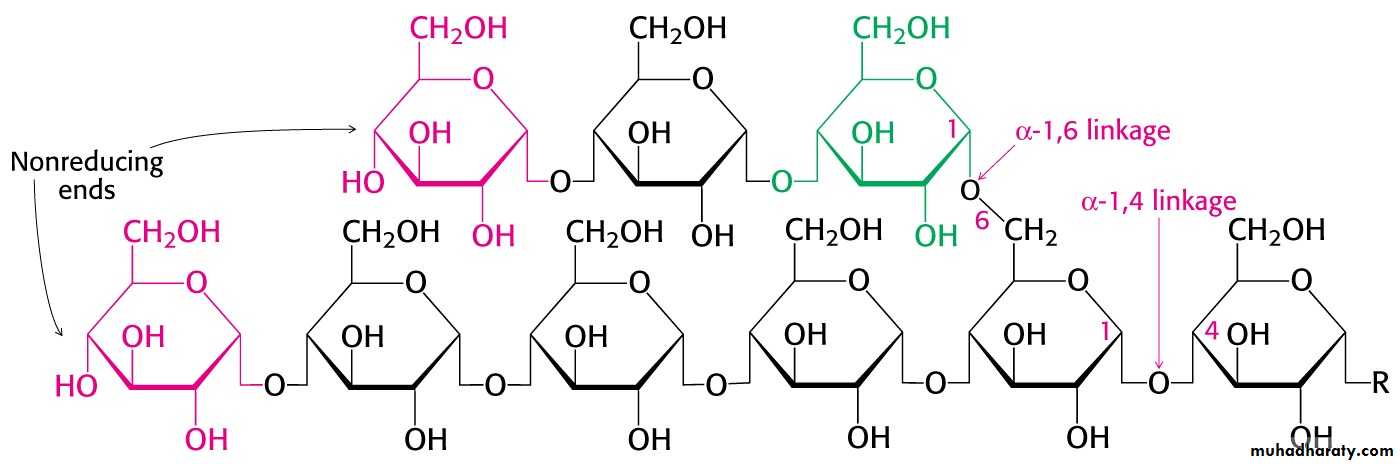
The α-1,4-linkage predominates
Synthesis requires the addition of glucose to the non-reducing ends of glycogen via UDP-glucose.
Phosphorylase is specific for the α-1,4 linkage. Two additional enzymes are required.
1- In eukaryotes the transferase activity and the α-1,6-glucosidase activity are within one bifunctional protein.
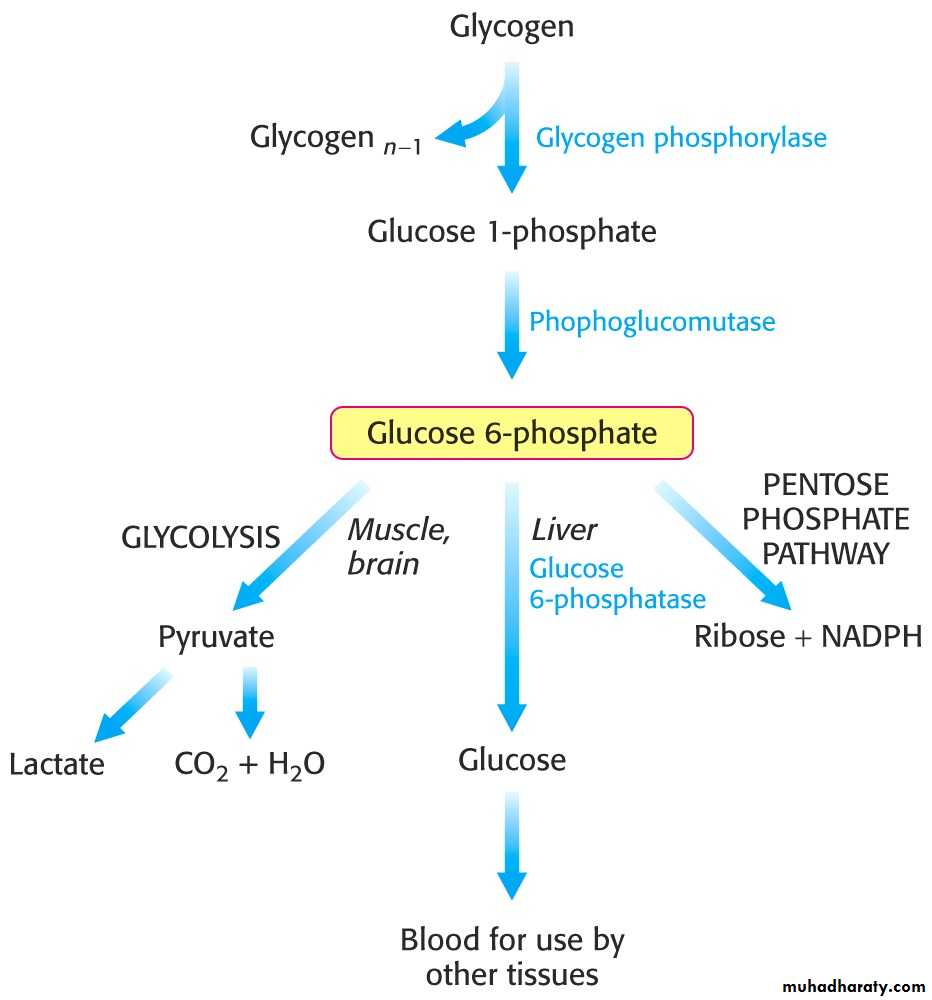
2- The glucose 1-phosphate to converted to glucose 6-phosphate by phosphoglucomutase.
Liver contains glucose 6-phosphatase.
Muscle does not have this enzyme.
WHY?
The liver releases glucose to the blood to be taken up by brain and active muscle. The liver regulates blood glucose levels.
The muscle retains glucose 6-phosphate to be use for energy. Phosphorylated glucose is not transported out of muscle cells.
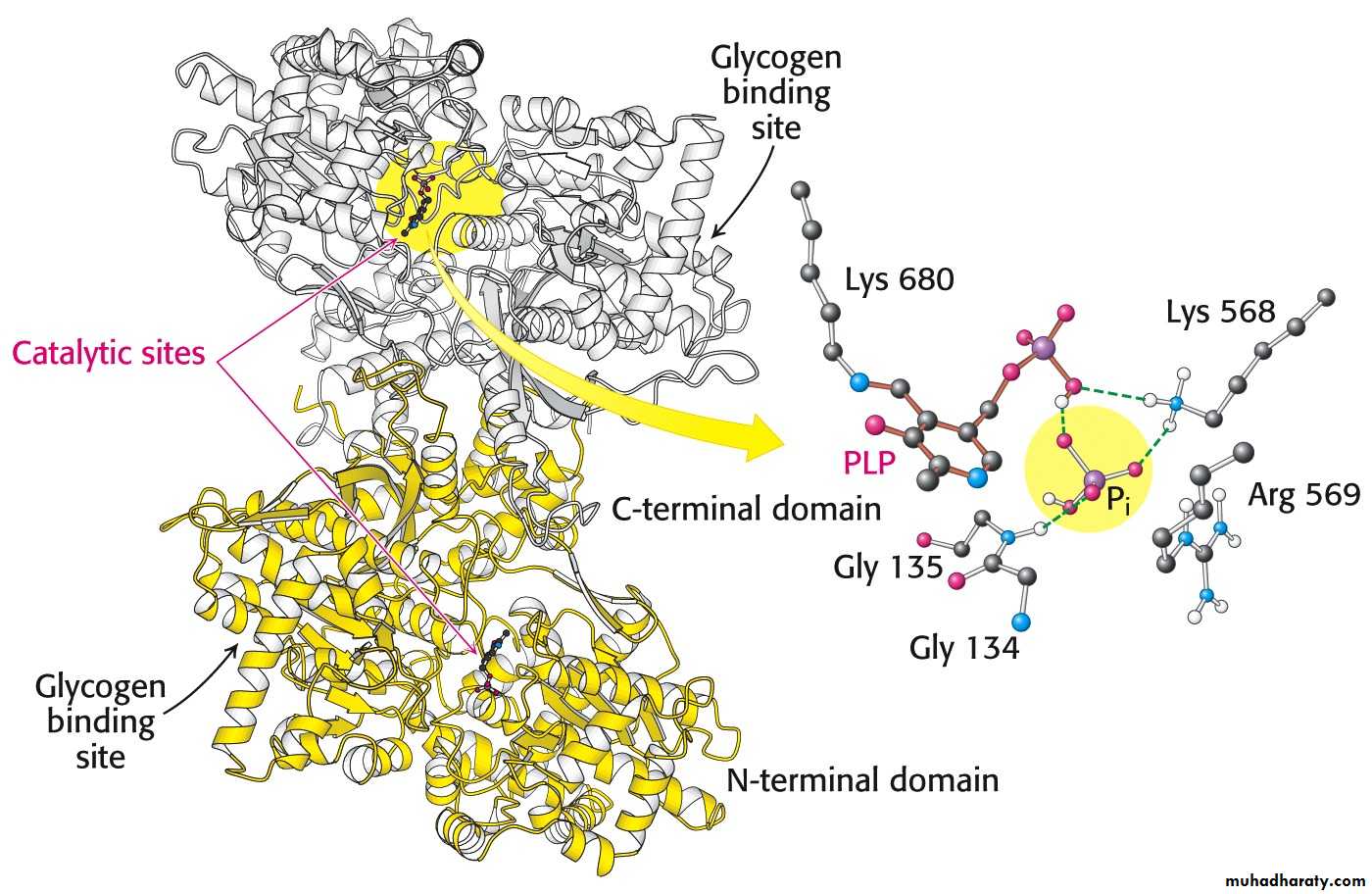
Pyridoxal phosphate is the coenzyme for phosphorylase.
Glycogen phosphorylase uses pyridoxal phosphate (PLP) a derivative of pyridixine (vitamine B6) as a coenzyme.
B6 is required for the mobilization of glucose from glycogen. It is also required for other biochemical reactions such as transamination.
Glycogenesis:
Glycogen is synthesized via uridine diphosphate glucose (UDP – glucose). 1-
2- Synthesis: Glycogenn + UDP-glucose → glucogenn+1 + UDP.
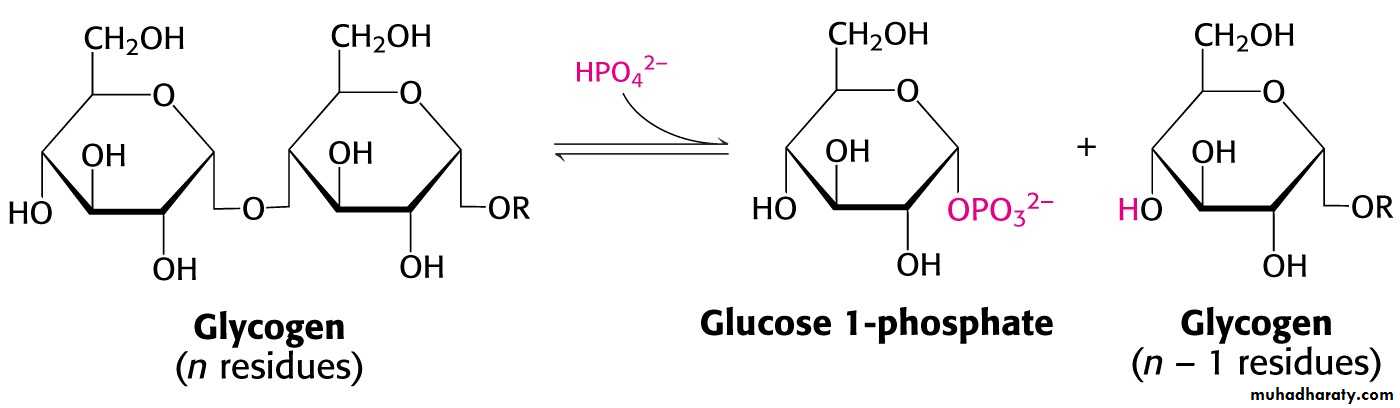
3- Degradation: glucogenn + Pi → Glycogenn-1 + glucose 1-phosphate.
Glycogen synthesis and degradation utilize separate pathways.
Glycogen synthesis
Glucose 6-P→ glucose 1-P.
glucose 1-P + UTP→UDP-glucose + PPi.
PPi + H2O→ 2 Pi.
UDP-glucose + glycogenn → glycogenn+1.
UDP + ATP → UTP + ADP.
Glucose 6-P + ATP + glycogenn + H2O →
glycogenn+1 + ADP + 2Pi.
Only one ATP is used to store one glucose residue in glycogen.
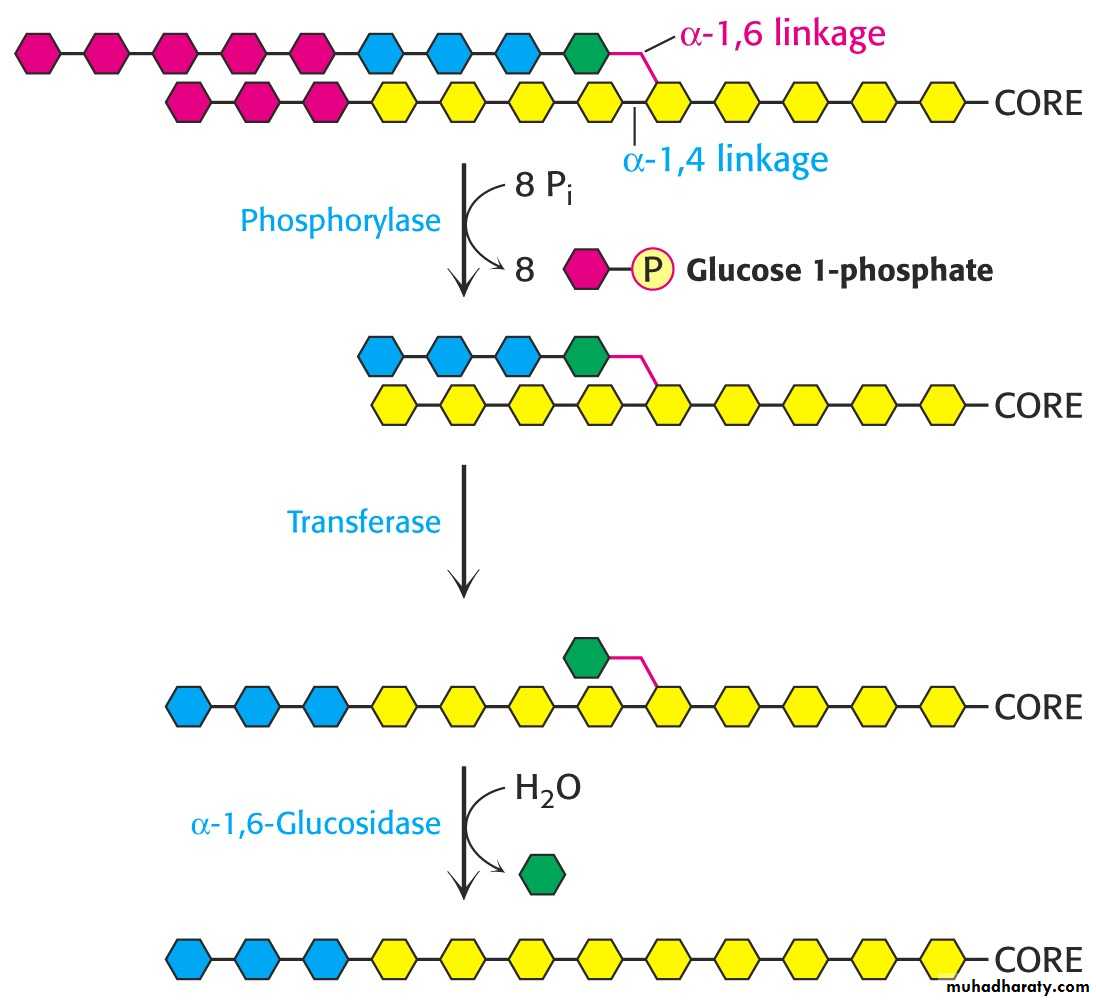
5- Glycogen Mobilization: Glycogenolysis:
1- Glycogenolysis is a catabolic process; the breakdown of glycogen to glucose units.
2- Glycogen is principally stored in the cytosol granules of -
Liver
Muscle
Glycogen Function
1- In liver – The synthesis and breakdown of glycogen is regulated to maintain blood glucose levels.
Glycogenesis:
In muscle - The synthesis and breakdown of glycogen is regulated to meet the energy requirements of the muscle cell.
Glycogen phosphorylase catalyzes the breakdown of glycogen.
Reversibility of glycolysis (Gluconeogenesis): 6-
1. Reversible reaction means that the same enzyme can catalyzes thereaction in both directions.
2. all reactions of glycolysis -except 3- are reversible.
3. The 3 irreversible reactions (those catalyzed by kinase enzymes) can be
reversed by using other enzymes.
Glucose-6-p Glucose
F1, 6 Bisphosphate Fructose-6-p
Pyruvate Phosphoenol pyruvate
4. During fasting, glycolysis is reversed for synthesis of glucose from non-
carbohydrate sources e.g. lactate. This mechanism is called:
gluconeogenesis.