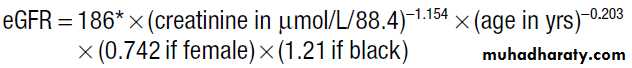Hussien Mohammed Jumaah
CABMLecturer in internal medicine
Mosul College of Medicine
2016
learning-topics
Kidney andurinary tract disease
Each kidney 11–14 cm in length in healthy adults; located retroperitoneally on either side of the aorta and IVC between the 12th thoracic and 3rd lumbar vertebra .The right is a few centimetres lower because the liver above. Both rise and descend several centimetres with respiration. The kidneys have a rich blood supply ,receive 20–25% of cardiac output , play a central role in excretion of metabolic breakdown products, including ammonia, urea and creatinine from protein, and uric acid from nucleic acids, drugs and toxins. They also regulate fluid and electrolyte balance. This is achieved by making large volumes of an ultrafiltrate of plasma (120 mL/min, 170 L/day) at the glomerulus, and selectively reabsorbing components of this ultrafiltrate at points along the nephron.
They regulate acid–base homeostasis, calcium and phosphate homeostasis, vitamin D metabolism and production of red blood cells. They are important in regulating blood pressure.
Renin is secreted from the juxtaglomerular apparatus in response to reduced afferent arteriolar pressure, stimulation of sympathetic nerves, and changes in sodium content of fluid in the distal tubule at the macula densa, and is the first step in the generation of angiotensin II and aldosterone release, which in turn regulate systemic vasoconstriction and extracellular volume. The efferent arteriole, leading from the glomerulus, supplies the distal nephron and medulla in a ‘portal’ circulation .
Under normal circumstances, more than 99% of the 170 litres of glomerular filtrate that is produced each day is reabsorbed in the tubules. The remainder passes through the collecting ducts ,drains into the renal pelvis and ureters.
Healthy kidney contain approximately 1 million nephrons.
The glomerulus comprises a tightly packed loop of capillaries supplied by an afferent arteriole and drained by an efferent arteriole. It is surrounded by a cup-shaped extension of the proximal tubule termed Bowman’s capsule, comprised of epithelial cells.
Blood that enters the glomerulus undergoes ultrafiltration
across the glomerular basement membrane (GBM),
which is formed by fusion of the basement membranes
of tubular epithelial and vascular endothelial cells .
The glomerular capillary endothelial cells contain pores (fenestrae), through which circulating molecules can pass to reach the underlying GBM.
Glomerular epithelial cells (podocytes) have multiple
long foot processes which interdigitate with those of the
adjacent epithelial cells . Mesangial cells lie in
the central region of the glomerulus. They have contractile
properties similar to those of vascular smooth muscle
cells but also have macrophage-like properties. Under normal circumstances, the glomerulus is impermeable to proteins the size of albumin (67 kDa) while proteins of 20 kDa or smaller and positively charged are filtered
freely. Very little lipid is filtered by the glomerulus.
Filtration pressure at glomerulus is normally maintained at a constant level, in the face of wide variations in systemic blood pressure and cardiac output, by alterations in muscle tone within the afferent and efferent arterioles. This is known as autoregulation. When there is a reduction in renal perfusion pressure, renin is released by juxtaglomerular apparatus, cleaves angiotensinogen to release angiotensin I, further cleaved by angiotensin-converting enzyme (ACE) to produce angiotensin II . This restores glomerular perfusion pressure by vasoconstriction of the efferent arterioles and by inducing systemic vasoconstriction to increase blood pressure and thus renal perfusion pressure. In the longer term, angiotensin II increases plasma volume by stimulating aldosterone, which enhances sodium reabsorption by the renal tubules .
Renal tubules, loop of Henle and collecting ducts
The proximal renal tubule, loop of Henle, distal renaltubule and collecting ducts are responsible for reabsorption of water, electrolytes and other solutes, as well as regulating acid–base balance .They also play a key role in regulating calcium homeostasis by converting 25-hydroxyvitamin D to the active metabolite 1,25- dihydroxyvitamin D . Fibroblast-like cells that lie in the interstitium of the renal cortex are responsible for production of erythropoietin, which in turn is required for production of red blood cells. A naemia and hypoxia increase production of erythropoietin, whereas polycythaemia and hyperoxia inhibit it.
The bladder : Sympathetic nerves arising from T10– L2 cause relaxation of the detrusor muscle and contraction of the bladder neck (α-adrenoceptors), thereby preventing release of urine from the bladder. Conversely, parasympathetic nerves arising from S2–4 stimulate detrusor contraction, promoting micturition. Afferent sensory impulses pass to the cerebral cortex, from where reflex-increased sphincter tone and suppression of detrusor contraction inhibit micturition until it is appropriate.
At approximately 75% bladder capacity, there is a desire to void. Voluntary control is now exerted over the desire to void, which disappears temporarily. These actions are coordinated by the pontine micturition centre.
The prostate gland
The prostate gland is situated at the base of the bladder,surrounding the proximal urethra. Exocrine glands within the prostate produce fluid, which comprises about 20% of the volume of ejaculated seminal fluid and is rich in zinc and proteolytic enzymes. The remainder of the ejaculate is formed in the seminal vesicles and bulbo-urethral glands, with spermatozoa arising from the testes. Smooth muscle fibres within the prostate, under sympathetic control, play a role in controlling urine flow through the bulbar urethra, and also contract at orgasm to move seminal fluid through ejaculatory ducts into the bulbar urethra (emission). Contraction of the bulbocavernosus muscle (via a spinal muscle reflex) then ejaculates the semen out of the urethra.
The penis
Blood flow into the corpus cavernosum of the penis iscontrolled by sympathetic nerves from the thoracolumbar
plexus, which maintain smooth muscle contraction.
In response to afferent input from the glans penis and
from higher centres, pelvic splanchnic parasympathetic
nerves actively relax the cavernosal smooth muscle via
neurotransmitters such as nitric oxide, acetylcholine,
vasoactive intestinal polypeptide (VIP) and prostacyclin,
with consequent dilatation of the lacunar space. At the same time, draining venules are compressed, trapping blood in the lacunar space with consequent elevation of pressure and erection (tumescence) of the penis.
INVESTIGATION OF RENAL AND URINARY TRACT Glomerular filtration rate (GFR)
120 ± 25 mL/ min/1.73 m2. measured directly by injecting and measuring the clearance of compounds such as inulin or radiolabelled ethylenediaminetetracetic acid, which are completely filtered and not secreted or reabsorbed . Instead, GFR is usually indirectly by measuring serum creatinine, which is produced by muscle at a constant rate, is almost completely filtered, and is not reabsorbed. Although creatinine is secreted to a small degree by the proximal tubule, this is only usually significant in terms of GFR estimation in severe renal impairment. MDRD equation, accepted standard for assessing estimated GFR (eGFR).A potentially more accurate assessment of GFR can
be obtained by collection of a 24-hour urine sample andrelating serum to urinary creatinine excretion .
Limitations of eGFR
• least reliable at extremes of body composition (malnourished, amputees)
• Creatinine level must be stable over days; eGFR is not valid in assessing acute kidney injury
• In the elderly, who constitute the majority of those with low eGFR, there is controversy about categorising people as having CKD on the basis of eGFR alone, particularly at stage 3A, since there is little evidence of adverse outcomes when eGFR is > 50 unless there is also proteinuria
• eGFR is not valid in under-18s or during pregnancy
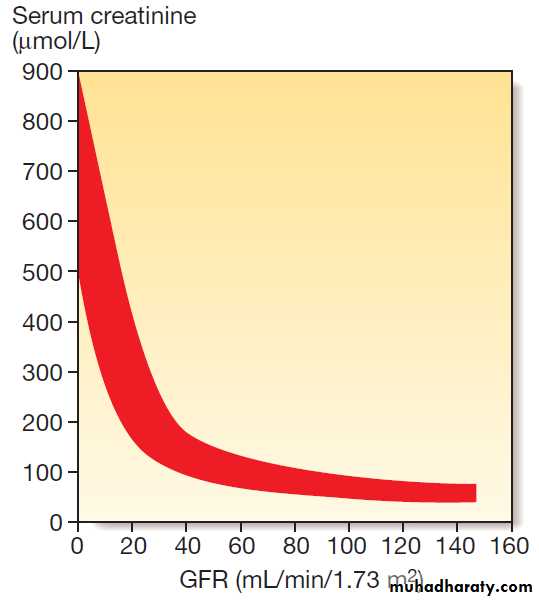
Serum creatinine and the glomerular filtration rate
(GFR). The inverse reciprocal relationship between GFR and serum creatinine is shown for a group of patients with renal disease. The red band indicates the range of values obtained. Note that some individuals have a GFR as low as 30–40 mL/min without serum creatinine rising outof the reference range.
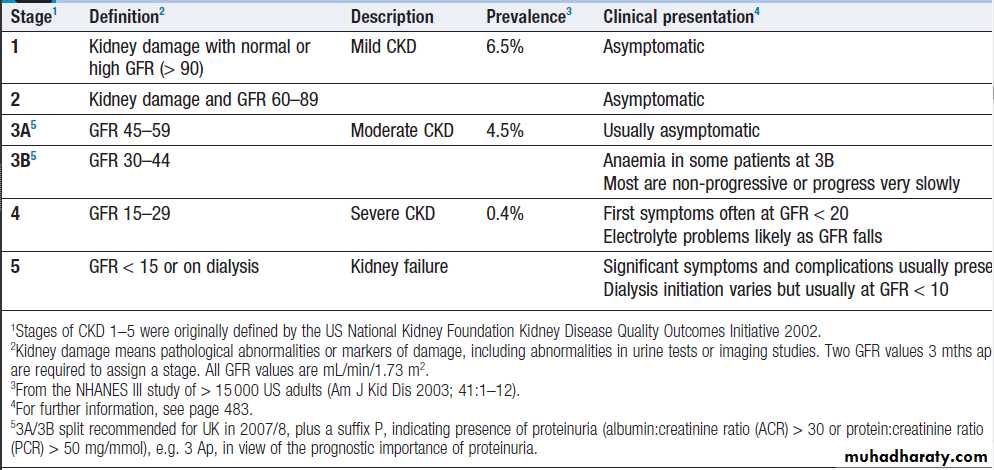
Stages of chronic kidney disease (CKD)
Urinalysis
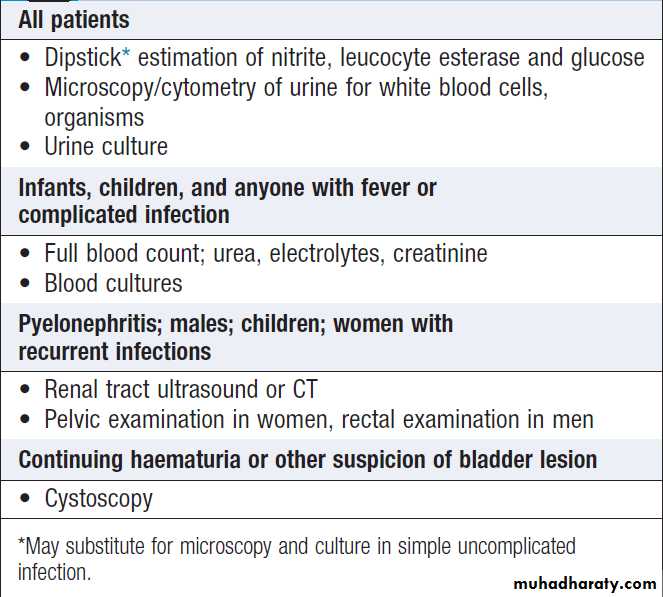
Screening for blood, protein, glucose, ketones, nitrates and leucocytes , pH and osmolality ,can be achieved by dipstick testing .Urine microscopy can detect erythrocytes, dysmorphic erythrocytes, which suggest nephritis; red cell casts, indicative of glomerular disease; and crystals. It should be noted that calcium oxalate and urate crystals can be found in normal urine that has been left to stand. White cell casts , strongly suggestive of pyelonephritis.
Urine pH ,assessment of renal tubular acidosis . 24-hour Urine collection to measure calcium, oxalate and urate, in patients with recurrent renal stone disease . Proteinuria quantified by protein/ creatinine ratio on spot urine. Dynamic tests of tubular function, concentrating ability
Blood tests
HaematologyA normochromic normocytic anaemia is common in
CKD ,due deficiency of erythropoietin and bone marrow suppression secondary to retained toxins.Other causes of anaemia include iron deficiency from urinary tract bleeding, and haemolytic anaemia secondary to disorders such as haemolytic uraemic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP).
Neutrophilia and raised ESR in vasculitis or sepsis; lymphopenia and raised ESR in systemic lupus erythematosus (SLE); fragmented red cells in HUS and TTP.
Biochemistry
Serum levels of creatinine may be raised, reflecting reduced GFR although serum creatinine values can remain within the reference range in patients with reduced muscle mass, even when the GFR has fallen by more than 50%. Serum levels of urea are often increased in kidney disease but this analyte has limited value as a measure of GFR since levels increase with protein intake, following gastrointestinal haemorrhage and in catabolic states.Conversely, urea levels may be reduced liver failure and in malnourished, independently of renal function. Serum calcium tends to be reduced and phosphate increased in CKD, in association with high parathyroid hormone (PTH) levels caused by reduced production of 1,25(OH)2D by the kidney (secondary hyperparathyroidism).
In some patients, this may be accompanied by raised serum alkaline phosphatase levels, which are indicative of renal osteodystrophy. Raised glucose and HbA1c levels in diabetes mellitus and raised levels of C-reactive protein (CRP) in sepsis and vasculitis.
Immunology
Antinuclear antibodies, antibodies to extractable nuclear
antigens and anti-double-stranded DNA antibodies may
be detected in patients with renal disease secondary to
SLE . Antineutrophil cytoplasmic antibodies (ANCA) may be detected in patients with glomerulonephritis secondary to systemic vasculitis, as may antibodies to GBM in patients with Goodpasture’s syndrome and low levels of complement in SLE, systemic vasculitis and HUS.
Imaging
Ultrasound
A valuable non-invasive technique that is indicated to assess renal size and to investigate patients who are suspected of having obstruction of the urinary tract or renal tumours, cysts or stones, to provide images of the prostate gland and bladder, and to estimate the completeness of emptying in patients with suspected bladder outflow obstruction. May show increased density of the renal cortex with loss of distinction between cortex
and medulla, which is characteristic of CKD.
Doppler can be used to study blood flow in extrarenal
and larger intrarenal vessels. High peak velocities can also occur in severe renal artery stenosis.
Computed tomography urography (CTU)
Used to evaluate cysts and mass, gives more information than IVU but entails a larger radiation dose. Contrast enhancement is particularly useful for differentiating benign from malignant lesions . CT without contrast gives clear definition of retroperitoneal anatomy regardless of obesity. Non-contrast CT of kidneys, ureters and bladder (CTKUB) is the method of choice for demonstrating stones.Computed tomography and angiography
computed tomography, following an injection of contrast medium, to obtain images of the renal vasculature. It produces high-quality images of the main renal vessels. Drawbacks include the fact that relatively large doses of contrast medium are required, which can cause renal dysfunction, and that the radiation dose is significant .
Magnetic resonance imaging (MRI)
offers excellent resolution . It is very useful for local staging of prostate, bladder and penile cancers. MR angiography alternative to CT-angiography for imaging renal vessels.Renal arteriography
The main indication is to investigate renal artery stenosis or haemorrhage. Can often be combined with therapeutic balloon dilatation or stenting of the renal artery and can be used to occlude bleeding vessels and arteriovenous fistulae by the insertion of thin platinum wires (coils).
Intravenous urography (IVU)
Involves taking serial plain X-rays immediately before and after an intravenous injection of contrast medium. It has largely been replaced by ultrasound, CTKUB and CTU .
The disadvantages are the need for an injection, dependence on adequate renal function, and exposure to irradiation and contrast medium .
Pyelography
Direct injection of contrast medium into the collecting system from above or below. Antegrade pyelography
requires the insertion of a fine needle into the
pelvicalyceal system under ultrasound or radiographic
control. This approach is much more difficult and hazardous
in a non-obstructed kidney. In the presence of obstruction, percutaneous nephrostomy drainage can be established, and often stents can be passed through any obstruction. Retrograde pyelography can be performed by inserting catheters into the ureteric orifices at cystoscopy.
Radionuclide studies
These are functional studies requiring the injection of
gamma ray-emitting radiopharmaceuticals that are
taken up and excreted by the kidney, a process that can
be monitored by an external gamma camera, can provide valuable information about the perfusion of each kidney but is not a reliable for identifying renal artery stenosis. Renal biopsy
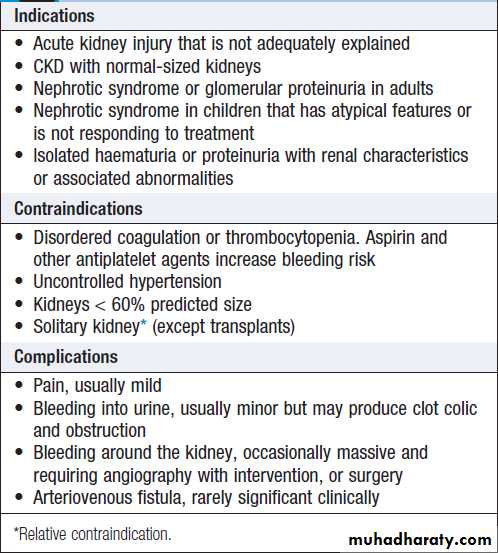
Used to establish the nature and extent of renal disease in order to judge the prognosis and need for treatment . Performed transcutaneously with ultrasound or contrast radiography guidance to ensure accurate needle placement into a renal pole. Light microscopy, electron microscopy and immunohistological assessment of the specimen may all be required.
Renal biopsy
PRESENTING PROBLEMS IN RENAL AND URINARY TRACT DISEASEDysuria
Dysuria refers to painful urination, often described as
burning, scalding or stinging, and commonly accompanied
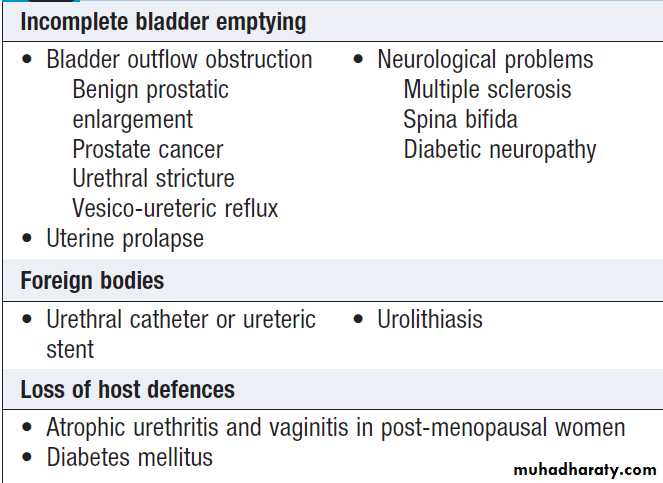
by suprapubic pain. It is often associated with frequency of micturition and a feeling of incomplete emptying of the bladder. By far the most common cause is urinary tract infection.
Other diagnoses in patients with dysuria include sexually transmitted infections and bladder stones .
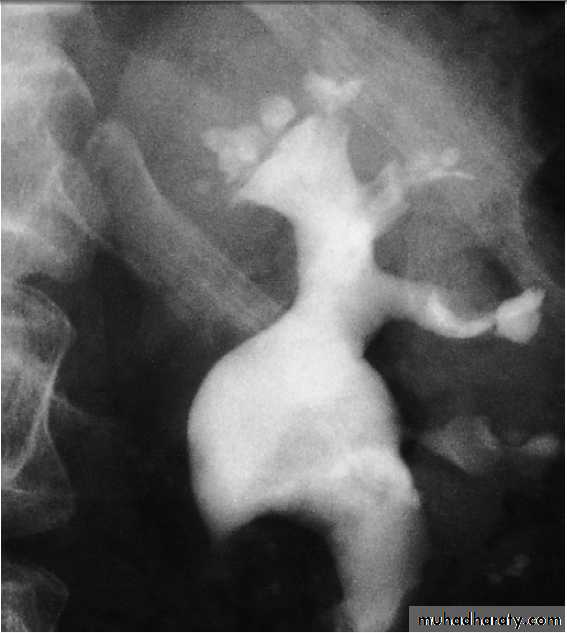
Loin pain
Loin pain is often caused by musculoskeletal disease butcan be a manifestation of renal tract disease; in the latter
case, it may arise from renal stones, ureteric stones, renal
tumours, acute pyelonephritis and urinary tract obstruction.
Acute loin pain radiating anteriorly and often to
the groin is termed renal colic. When combined with
haematuria, this is typical of ureteric obstruction due to
calculi . When loin pain is precipitated by a large
fluid intake (Dietl’s crisis), upper urinary tract obstruction
caused by a congenital abnormality of the pelviureteric
junction (PUJ) is often responsible.
Oliguria/anuria
Oliguria is present when < 300 mL urine is passed per day, whereas anuria exist when < 50 mL urine is passed per Oliguria and anuria may be caused by a reduction in urine production, as in pre-renal acute kidney injury. Obstruction of the renal tract can also produce oliguria and anuria, but to do so, obstruction must be complete and occur distal to the bladder neck, be bilateral, or be unilateral on the side of a single functioning kidney. The presence of pain that is exacerbated by a fluid load suggests an acute obstruction of the renal tract .
Bladder neck obstruction is associated with lower midline abdominal discomfort due to bladder dilatation, ureteric obstruction presents as loin pain radiating to the groin.
Polyuria
Defined as a urine volume in excess of 3 L/ day.Nocturia
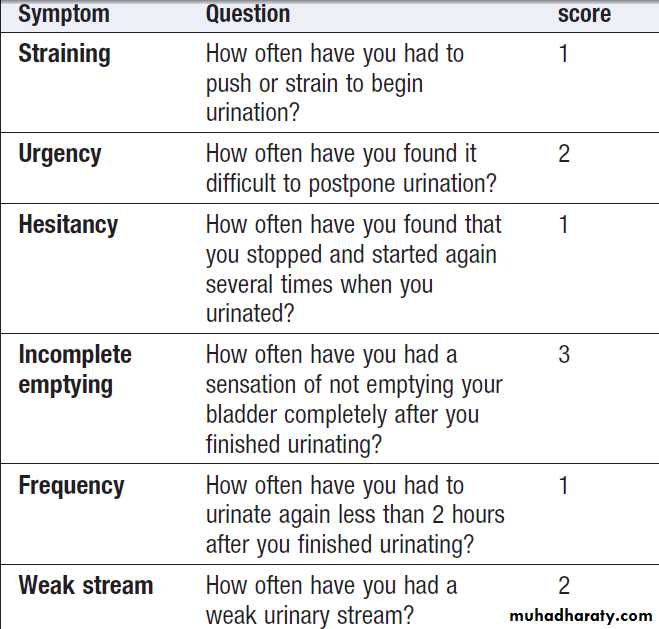
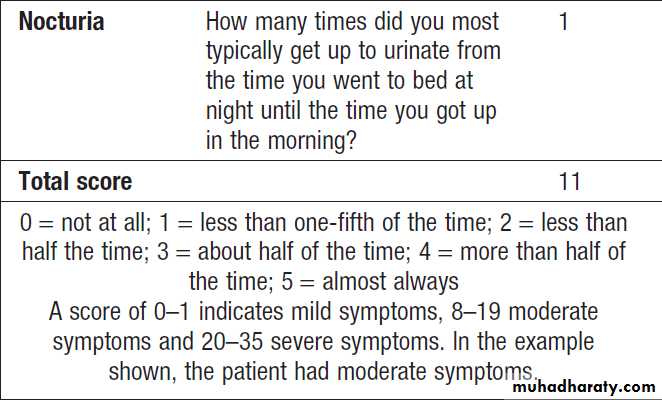
Defined as waking up at night to void urine. It may be a consequence of polyuria , increased fluid intake or diuretic use in the late evening. Nocturia also occurs in CKD, and in prostatic enlargement when it is associated with poor stream, hesitancy, incomplete bladder emptying, terminal dribbling and urinary frequency due to partial urethral obstruction . Nocturia may also occur in sleep disturbance without functional abnormalities.
Frequency
Micturition more often than a patient’s expectations. It may be a consequence of polyuria, in patients with dysuria and prostatic diseases, when the urine volume is low.
Causes of polyuria
Urinary incontinenceInvoluntary leakage of urine. It may occur in patients with a normal urinary tract, as the result of dementia or poor mobility, or transiently during an acute illness or hospitalisation, especially in older people. Diuretics, alcohol and caffeine may worsen incontinence.
Stress incontinence
This occurs because passive bladder pressure exceeds
the urethral pressure, due to either poor pelvic floor
support or a weak urethral sphincter. Is very common in women and seen most frequently following childbirth.
It is rare in men and usually follows surgery to the
prostate. The presentation is with incontinence during coughing, sneezing or exertion.
Urge incontinence
This usually occurs because of detrusor overactivity,
which produces an increased bladder pressure that
overcomes the urethral sphincter. Is usually idiopathic, neurological such as spina bifida or multiple sclerosis .
It is also seen in men with lower urinary tract obstruction.
Continual incontinence
This is suggestive of a fistula, usually between the
bladder and vagina (vesicovaginal), or the ureter and vagina (ureterovaginal). It is most common following
gynaecological surgery ,gynaecological malignancy or post-radiotherapy. Continual incontinence may also
be seen in infants with congenital ectopic ureters. Occasionally, stress incontinence is so severe that the patient leaks continuously.
Overflow incontinence
This occurs with chronically overdistended bladder. It is most commonly seen with benign prostatic enlargement or bladder neck obstruction , or failure of the detrusor muscle (atonic bladder). The latter may be idiopathic but more commonly is the result of damage to the pelvic nerves, either from surgery (commonly, hysterectomy or rectal excision), trauma or infection, or from compression of the cauda equina by disc prolapse, trauma or tumour.Post-micturition dribble
This is very common in men, even in young. It is due to a small amount of urine becoming trapped in the U-bend of the bulbar urethra, which leaks out when the patient moves, pronounced with a urethral diverticulum or urethral stricture.
Incontinence in old age
Investigations
Urinalysis, culture, Ultrasound. Urine flow rates and full urodynamic assessment by cystometrography. An IVU should be performed in patients with continual incontinence. MRI is indicated when a urethral diverticulum is suspected.
Management
stress incontinence respond physiotherapy. The mainstay of treatment for urge incontinence is bladder retraining, which involves teaching patients to hold more urine voluntarily in their bladder, assisted by anticholinergic medication. Surgery may be required in patients who have severe incontinence despite conservative treatment.
The treatment of fistula, bladder obstruction is surgical. Incontinence secondary to neurological diseases
can be treated by intermittent self-catheterisation.
Erectile dysfunction
Vascular, neuropathic and psychological causes are mostcommon. If the patient has erections on wakening,
vascular and neuropathic causes are much less likely
and a psychological cause should be suspected.
Investigations
Glucose, prolactin, testosterone, LH and FSH. Nocturnal
tumescence monitoring (using a plethysmograph placed
around the shaft of the penis overnight) to establish
whether blood supply and nerve function are sufficient
to allow erections to occur during sleep; intracavernosal
injection of PG E1 to test the adequacy of blood supply; internal pudendal artery angiography; and tests of autonomic and peripheral sensory nerve conduction.
Causes of erectile dysfunction
ManagementFirst-line therapy is oral phosphodiesterase type 5 inhibitors, such as sildenafil, which elevate cGMP levels in the corpus cavernosum, causing vasodilatation and penile erection. Co-administration of these drugs with trinitrate, is contraindicated because of the risk of severe hypotension , intracavernosal injection or urethral administration of prostaglandin E1; vacuum devices which achieve an erection that is maintained by a tourniquet around the base of the penis; and prosthetic implants, either of a fixed rod or an inflatable reservoir.
Psychotherapy involving the patient and sexual partner
may be helpful. peripheral neuropathy and vascular disease is difficult to treat. If hypogonadism
is detected, it should be managed .
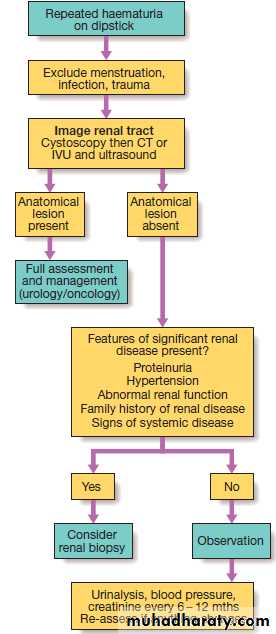
Haematuria
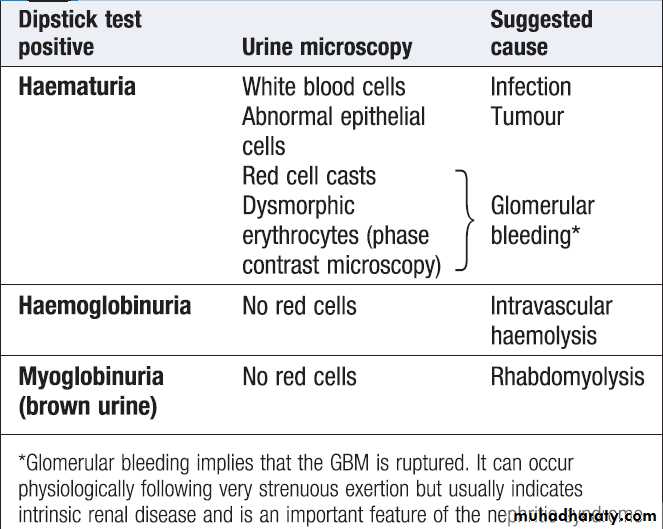
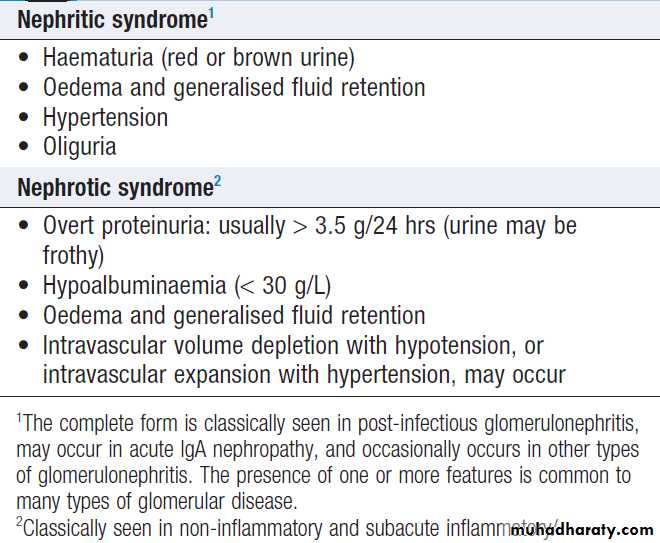
Healthy individuals may have up to 12 500 cells/mL, but the presence of macroscopic haematuria or on dipstick testing (15 000–20 000 cells/mL) is indicative of significant bleeding. First, it is important to confirm the presence of blood cells in the urine by microscopy , since dipstick tests can also be positive in the presence of free haemoglobin or myoglobin. Microscopy confirms the diagnosis ,also helpful in establishing the cause . Positive tests may also occur during menstruation, infection or strenuous exercise. In GN, one or more other features of the ‘nephritic syndrome’ may be present . Other causes are infection and stones. If haematuria occurs with proteinuria , renal biopsy may be indicated.Management should be directed at the underlying cause.
Interpretation of dipstick-positive
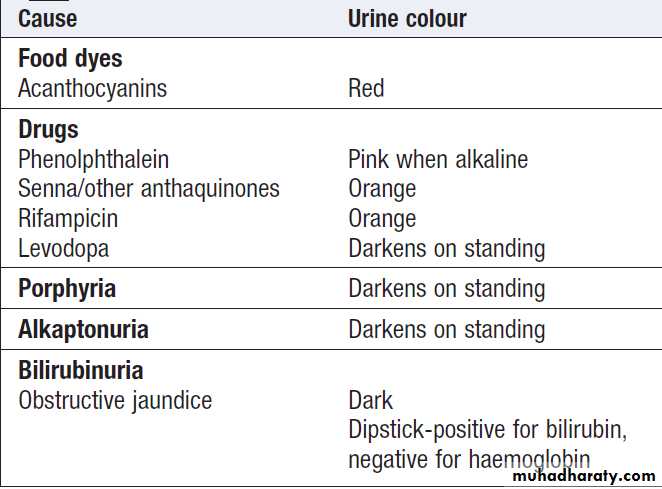
haematuriaDipstick-negative dark urine
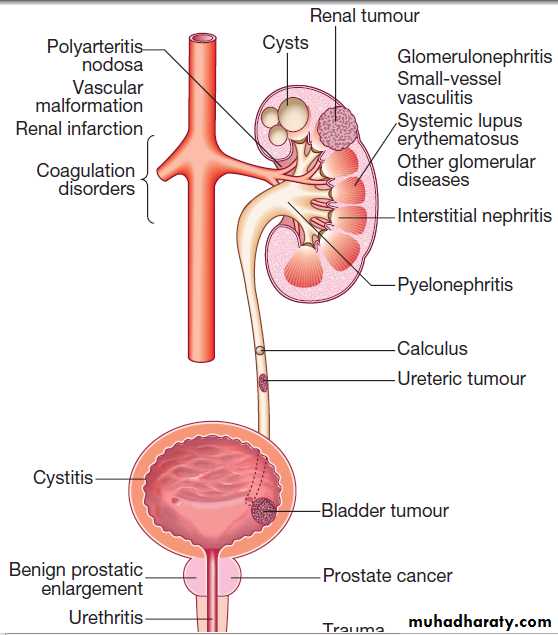
Causes of haematuria
Investigation of haematuria.
Nephritic and nephrotic syndromesProteinuria and nephrotic syndrome
Whilst moderate amounts of low-molecular-weightprotein pass through the healthy GBM, these proteins
are normally reabsorbed by receptors on tubular cells.
In healthy individuals, less than 150 mg of protein is
excreted in the urine each day. A proportion of this is Tamm–Horsfall protein, secreted by the renal tubules.
The presence of larger amounts of protein is usually
indicative of significant renal disease .
Clinical assessment
Proteinuria is usually asymptomatic and is often picked
up by urinalysis.
Transient proteinuria can occur after vigorous exercise, during fever, in heart failure and urinary tract infection.
Orthostatic proteinuria may arise in the absence of renal disease. This occurs only during the day, in association with an upright posture, and the first morning sample is negative. Typically, less than 1 g protein per day is excreted. Orthostatic proteinuria is regarded as a benign disorder which does not require treatment.
Large amounts of protein make urine froth easily and this may be noticed by the patient.
Very large amounts can cause nephrotic syndrome,
which presents with oedema.
In adults, this predominantly affects the lower limbs but extends to the genitalia and lower abdomen
as it becomes more severe. The upper limbs and face
may be more affected on waking in the morning.
In children, ascites occurs early and oedema is often seen only in the face. Blood volume may be normal, reduced or increased. Renal sodium retention is an early and universal feature . Microalbuminuria refers to the urinary excretion of small amounts of albumin. The consistent presence of albumin in the urine is abnormal and is clinically important in identifying the very early stages of glomerular disease, as occurs in conditions like diabetic nephropathy .
Persistent microalbuminuria has also been associated with an increased risk of atherosclerosis and cardiovascular mortality but neither the mechanism of proteinuria nor an explanation for this association has been established.
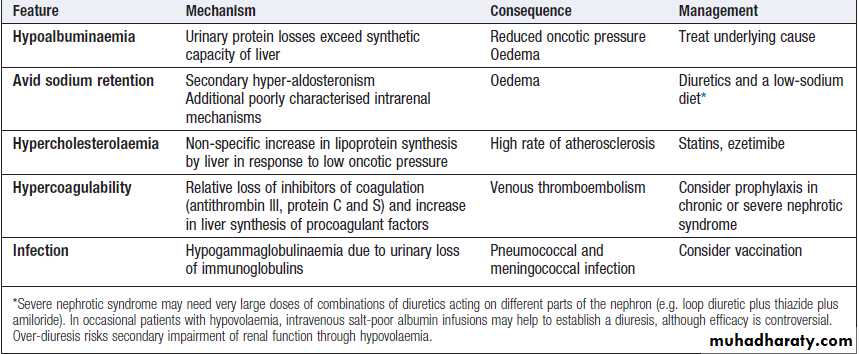
Consequences of the nephrotic syndrome and their management
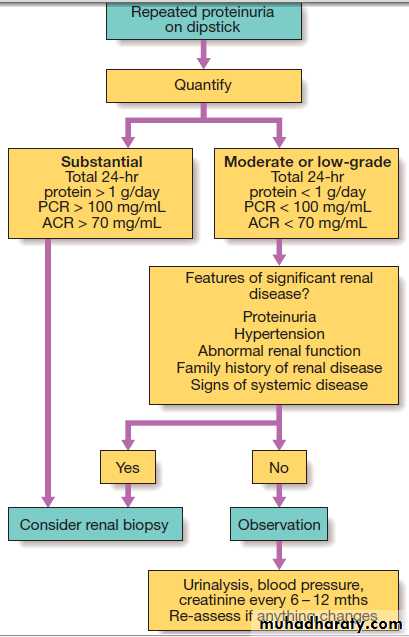
InvestigationsSince quantification by 24-hour urine collection is often inaccurate, the protein : creatinine ratio (PCR) in a spot sample of urine is preferred. It is possible to measure albumin : creatinine ratio (ACR). LMW proteins such as β2-macroglobulin may appear in the urine in >150 mg/day, Large amounts of small proteins in the urine suggest renal tubular damage (tubular proteinuria), rarely exceeds 1.5–2 g/24 hours . When >2 g protein per day is being excreted, glomerular disease is likely and renal biopsy is usually required. A notable exception is in children, when nephrotic syndrome are most commonly caused by minimal change GN and renal biopsy is not usually required unless the patient fails to respond to high-dose corticosteroid therapy.
Free immunoglobulin light chains are filtered freely at the glomerulus and can be identified as ‘Bence Jones protein’ in fresh urine samples.
Bence Jones protein is poorly identified by dipstick tests
and so specific immunodetection methods are required.
This may occur in AL amyloidosis (and in B-cell disorders, but is particularly important as a marker for
myeloma .
Management
Management of proteinuria should be directed at the
underlying cause. This may involve immunosuppressive
therapy in glomerulonephritis and supportive management
for nephrotic syndrome .
Investigation of proteinuria. (ACR = albumin : creatinine
ratio; PCR = protein : creatinine ratio.)Oedema
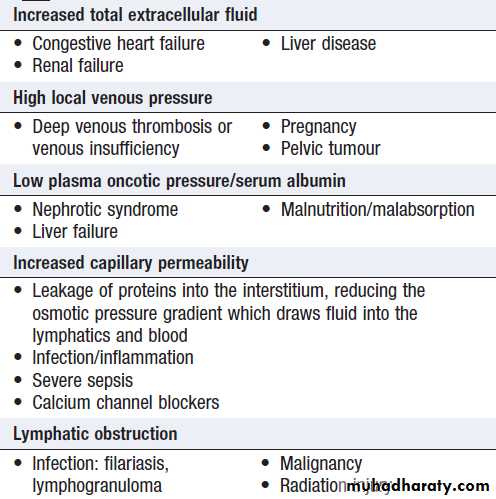
Oedema is caused by an excessive accumulation offluid within the interstitial space. Clinically, detected by persistence of an indentation in tissue following pressure on the affected area (pitting oedema).
Non-pitting oedema is typical of lymphatic obstruction
and may also occur as the result of excessive matrix
deposition in tissues – for example, in hypothyroidism
or scleroderma . Pitting oedema tends to accumulate in the ankles during the day and improves overnight as the interstitial fluid is reabsorbed.
Clinical assessment
The ankles and lower parts of the leg are typicallyaffected first but oedema can be restricted to the sacrum
in bed-bound patients. With increasing severity, oedema
spreads to affect the upper parts of the legs, the genitalia
and abdomen. Ascites is common and often an earlier
feature in children or young adults, and in liver disease.
Pleural effusions are common and can be a feature of
generalised oedema. Facial oedema on waking is common. Features of intravascular volume depletion (tachycardia, postural hypotension) may occur when oedema is due to decreased oncotic pressure or increased capillary permeability. If oedema is localised – for example, to one ankle but not the other, then venous thrombosis, inflammation or lymphatic disease should be suspected.
Investigations
The cause of oedema is usually apparent from the
history and examination of the cardiovascular system
and abdomen. Blood should be taken for measurement
of urea and electrolytes and serum albumin, and the
urine tested for protein. Further imaging of the liver,
heart or kidneys may be indicated, based on history and
clinical examination. Where ascites or pleural effusions
occur in isolation, aspiration of fluid with measurement
of protein and glucose, and microscopy for cells, will
usually help to clarify the diagnosis in differentiating a
transudate (typical of oedema) from an exudate (more
suggestive of local pathology .
Causes of oedema
Management
Mild oedema usually responds to a thiazide or a lowdose of a loop diuretic, such as furosemide or bumetanide.
In nephrotic syndrome, renal failure and severe
cardiac failure, very large doses of diuretics, sometimes
in combination, may be required to achieve a negative
sodium and fluid balance. In resistant cases, restriction
of sodium intake and fluid intake may be required. Diuretics are not helpful in the treatment of oedema caused by venous or lymphatic obstruction or by increased capillary permeability. Specific causes of oedema, such as venous thrombosis, should be treated.
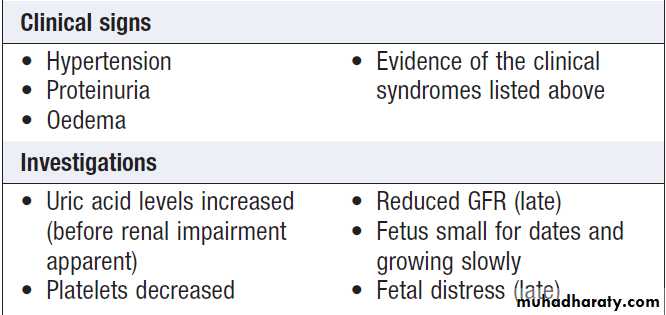
Hypertension
Hypertension is a very common feature of renal disease.Additionally, the presence of hypertension identifies a
population at risk of developing CKD and current recommendations are that patients on antihypertensive
medication should have renal function checked annually.
Control of hypertension is very important in patients with renal impairment because of its close relationship with further decline of renal function and because of the exaggerated cardiovascular risk associated with CKD.
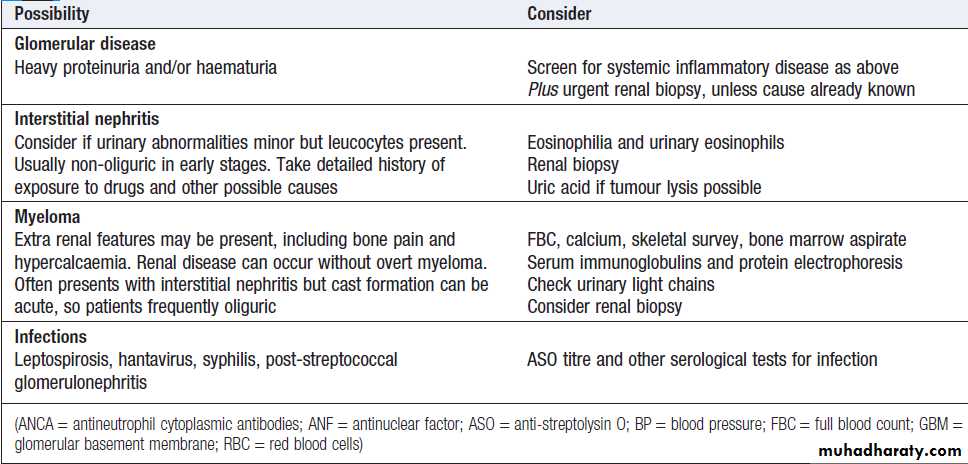
ACUTE KIDNEY INJURY (AKI)
AKI also referred to as acute renal failure, is a suddenand often reversible loss of renal function, which develops
over days or weeks and is usually accompanied by
a reduction in urine volume. Approximately 7% of all
hospitalised patients and 20% of acutely ill patients
develop signs of AKI. In AKI associated with serious infection and multiple organ failure, mortality is 50–70%. Pathophysiology
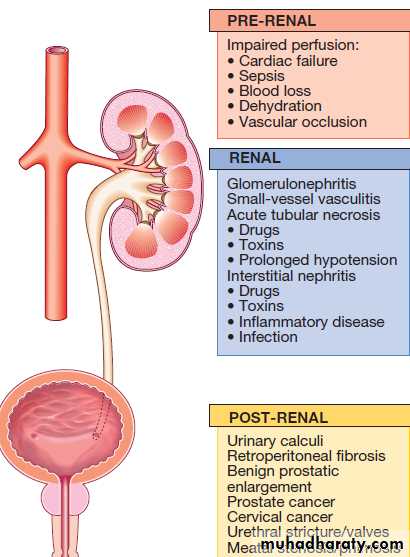
Three subtypes: ‘prerenal’,when perfusion to the kidney is reduced; ‘renal’,when the primary insult affects the kidney itself; and‘post-renal’, when there is obstruction to urine flow at any point from the tubule to the urethra .In
pre-renal AKI, the kidney becomes damaged as the
result of hypoperfusion, leading to acute tubular necrosis.
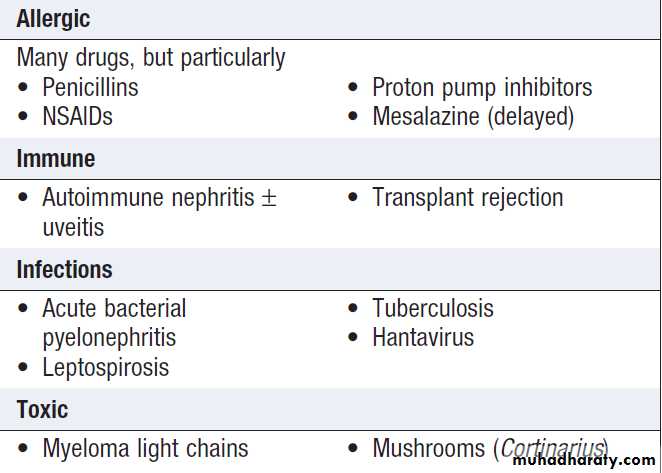
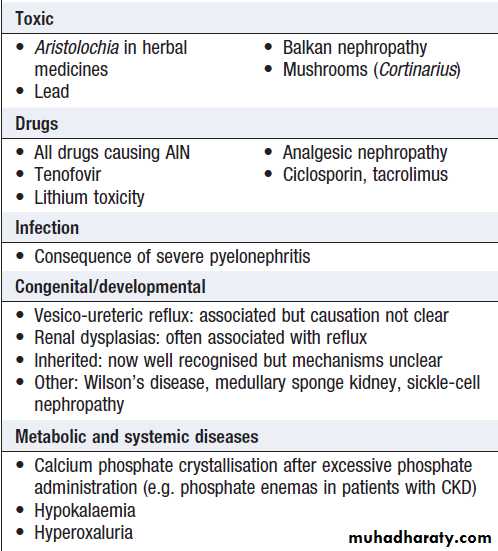
Causes of acute kidney injury
Clinical featuresall emergency admissions should have renal function, blood pressure, temperature and pulse checked on arrival and assessment for the likelihood of developing AKI. If a patient is found to have a high serum creatinine, it is important to establish whether this is an acute or acute-on-chronic or a sign of CKD. Anaemia is common in AKI and may occur as the result of blood loss, haemolysis or decreased erythropoiesis. In established AKI, there is an increased risk of bleeding and GI haemorrhage due to the uraemia. Patients with AKI need to be assessed quickly to determine the likely underlying cause. Various criteria have been proposed to classify AKI, guide treatment and provide information regarding prognosis. The most commonly used are the KDIGO and RIFLE criteria.
• Investigation of patients with established acute kidney injury
• Urea , creatinine• Compare to previous results. Chronically abnormal in CKD
• Electrolytes
• If potassium > 6 mmol/L, treat urgently
• Calcium and
• phosphate
• Low calcium and high phosphate may indicate CKD , Calcium low in
• rhabdomyolysis: measure CK, Hypercalcaemia in myeloma
• Albumin
• Low albumin in nephrotic syndrome
• Full blood count
• Clotting screen
• Anaemia may indicate CKD or myeloma , Anaemia and fragmented
• RBC on blood film with raised LDH in thrombotic microangiopathy
• Low platelets and abnormal coagulation in DIC, including in sepsis:
• take blood cultures
• C-reactive protein
• ESR is misleading in renal failure , High CRP may indicate sepsis or inflammatory disease
• Urinalysis
• Marked haematuria suggests GN , tumour of renal tract or
• bleeding disorder . Heavy proteinuria suggests glomerular disease:
• measure PCR or ACR
• Urine microscopy
• Casts or dysmorphic RBC suggest GN .WBC suggest infection
• /interstitial nephritis, Crystals in drug-induced or uric acid
• nephropathy
• Renal ultrasound
• Hydronephrosis ± enlarged bladder in UT obstruction: consider PSA.
• Small kidneys (CKD). Asymmetric kidneys in renovascular or
• developmental disease: consider renal artery imaging
Cultures , blood, urine, sputum.
Chest X-ray Pulmonary oedema .
Globular heart in pericardial (uraemic) effusion: perform echocardiogram.
Fibrotic change in systemic inflammatory disease with lung and kidney involvement: request pulmonary function and high-resolution CT
Serology HIV and hepatitis serology is urgent if dialysis is needed
ECG If patient is > 40 yrs or has electrolyte abnormalities or risk of cardiac disease
Investigation of patients with established acute kidney injury - cont'd
• Clinical features and investigations of specific causes of acute kidney injury
Possibility• consider
Vascular occlusion
Aorta, or renal artery to single kidney;
Pointers include missing pulses, anuria
Urgent arteriography
Doppler ultrasound
Malignant hypertension
BP very high; RBC fragments on blood
film and haemolysis
Examine optic fundi
Check previous BP readings
Scleroderma
Sclerodactyly, other features.
Autoantibodies to extractable nuclear antigens. Imaging
Systemic inflammatory disease
Multi-organ involvement, rash and
evidence of glomerular disease
Differential diagnosis includes infection,
especially endocarditis or TB.
Complement, ANCA, ANF, anti-GBM antibodies,
cryoglobulins and biopsy
Cultures, echocardiogram
Clinical features and investigations of specific causes of acute kidney injury – cont’d
• Criteria for acute kidney injury
• KDIGO (Kidney disease: improving global outcomes)• RIFLE(Risk, Injury, failure,
• loss, end-stage)
• Stage 1:
• serum creatinine increase > 1.5-1.9-fold, urine production of < 0.5 mL/kg/ hr for 6-12 hrs
• = Risk
• Stage 2:
• serum creatinine increase > 2.0-2.9-fold, urine production of < 0.5 mL/kg/ hr for ≥12 hrs
• = Injury
• Stage 3:
• serum creatinine increase> 3.0-fold, urine
• production of < 0.3 mL/kg/hr for≥24 hrs or
• absolute anuria for ≥12 hrs, or absolute serum
• creatinine > 354 µ mol /L with an acute rise of
• > 44 µ mol /L
• = Failure
• Loss: persistent AKI, or complete
• loss of kidney function for > 4 wks
• End-stage renal disease: need for RRT
• for > 3 mths
Pre-renal AKI
Patients are typically hypotensive and tachycardic with signs of poor peripheral perfusion, such as delayed capillary return. Postural hypotension (a fall in blood pressure of > 20/10 mmHg from lying to standing) are valuable signs of early hypovolaemia. The cause of the hypotension is usually obvious clinically, but concealed blood loss can occur into the gastrointestinal tract, following trauma (particularly fractures of the pelvis or femur), and into the pregnant uterus.Large volumes of intravascular fluid may also be lost into tissues after crush injuries or burns, and in severe inflammatory skin diseases or sepsis.
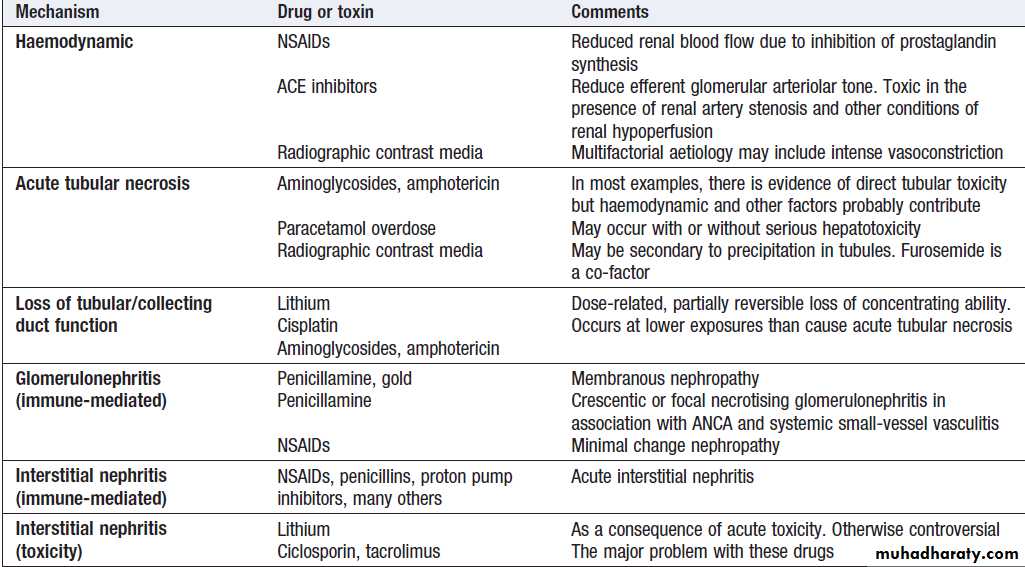
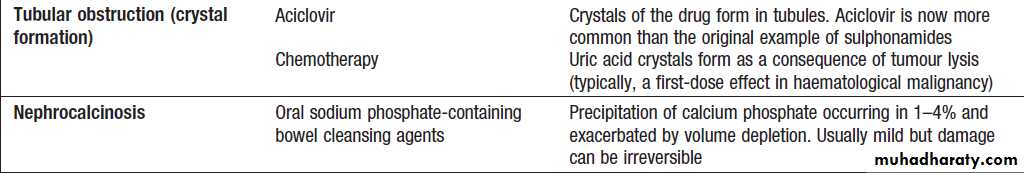
Renal AKI
Patients with glomerulonephritis usually demonstratesignificant haematuria and proteinuria and may have clinical manifestations of an underlying disease. Immunological screen, a renal biopsy is usually required. Drug-induced (like gentamicin, omeprazole, cisplatin and amphotericin B) acute interstitial nephritis is harder to spot but should be suspected in a previously well patient if there is an acute deterioration of renal function coinciding with introduction of a drug treatment.
Post-renal AKI
Patients should be examined for evidence of bladder enlargement and should also undergo imaging with ultrasound to detect evidence of obstruction above the level of the bladder.
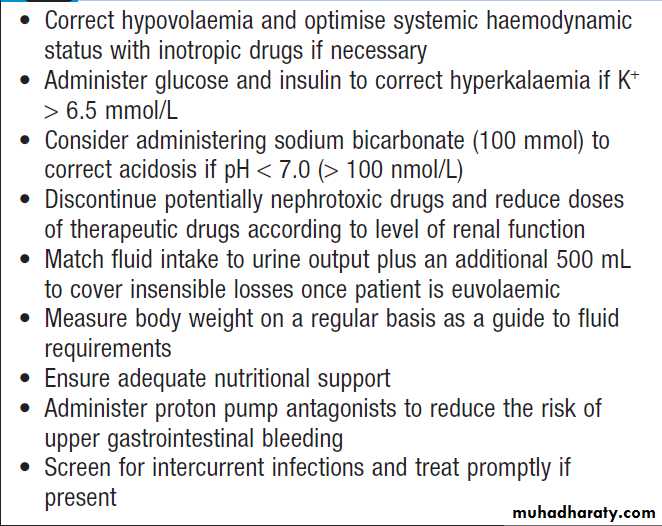
Management of acute kidney injury
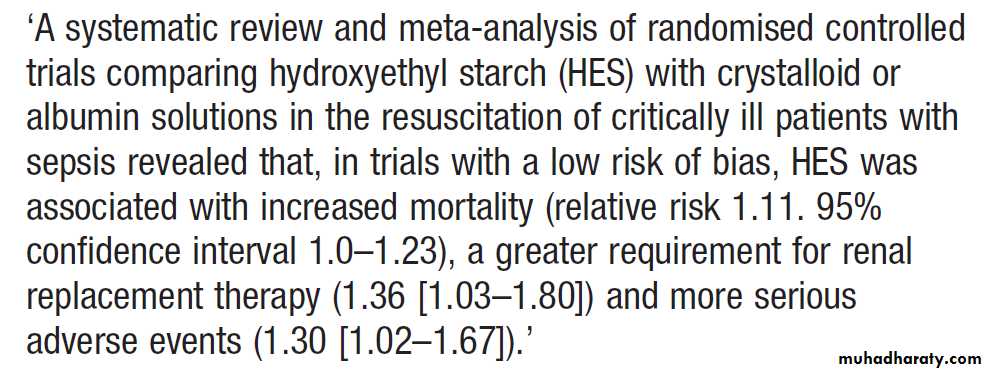
Critically ill patients may require inotropic drugs to restore an effective blood pressure but clinical trials do not support a specific role for low-dose dopamine .Colloid resuscitation fluids in the critically ill
Hyperkalaemia and acidosis
If serum K+ is > 6.5 mmol/L, should be treated immediately to prevent life-threatening arrhythmias.Metabolic acidosis develops unless prevented by loss of
hydrogen ions through vomiting or aspiration of gastric
contents. Restoration of blood volume will correct acidosis by restoring kidney function. Infusions of sodium bicarbonate (50 mL of 8.4%) may also be used, if acidosis is severe, to reduce life-threatening hyperkalaemia. Cardiopulmonary complications
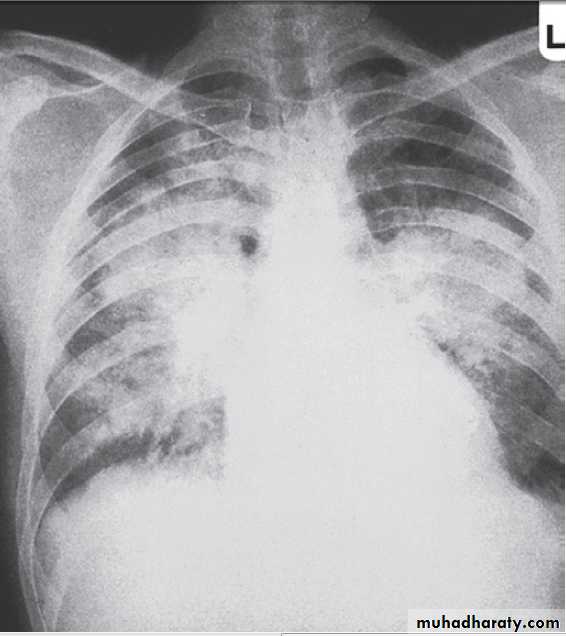
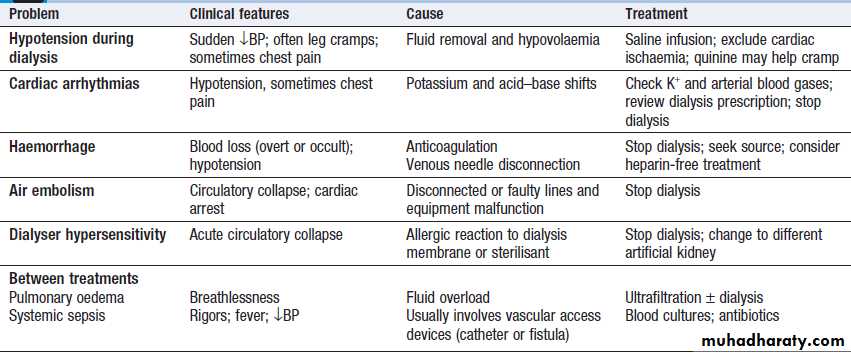
Pulmonary oedema may result from the administration of excessive amounts of fluids relative to urine output and because of increased pulmonary capillary permeability. Dialysis may be required to remove excess fluid.
Temporary respiratory support may also be necessary
with CPAP or IPPV. Once initial resuscitation has been performed, fluid intake should be matched to urine output plus 500 mL to cover insensible losses, unless diarrhoea present, in which case additional fluids might be required.Electrolyte disturbances
Dilutional hyponatraemia, may occur if the patient has continued to drink freely despite oliguria or has received inappropriate amounts of intravenous dextrose. They can be avoided by paying careful attention to fluid balance. Modest hypocalcaemia is common but rarely requires treatment.
Serum phosphate levels are usually high but may fall to dangerously low levels in patients on daily or continuous dialysis or haemofiltration, necessitating replacement.
Pulmonary oedema in acute kidney injury. The appearances are indistinguishable from left ventricular failure but the heart size is usually normal. Blood pressure is often high.
Dietary measures
Adequate nutritional support should be ensured and it
is important to give sufficient amounts of energy and
adequate amounts of protein; high protein intake should
be avoided. This is particularly important in patients
with sepsis and burns who are hypercatabolic.
Infection
AKI are at substantial risk of Intercurrent infection because humoral and cellular immune mechanisms are depressed. Regular clinical examination, supplemented by microbiological investigation where appropriate, is required to diagnose infection. If infection is discovered, it should be treated promptly .
Medications
Patients with drug-induced ATN or drug-induced AIN should have the offending drug withdrawn. NSAIDs and ACE inhibitors, should be discontinued, as they may prolong AKI. H2-receptor antagonists should be given to prevent GI bleeding. Other drug treatments should be reviewed and the doses adjusted if necessary, to take account of renal function. Stop non-essential drug.Immunosuppression , plasma infusion and exchange may be required for GN .
Renal tract obstruction
In post-renal AKI, the obstruction should be relieved as
soon as possible. This may involve catheterisation in
urethral obstruction, or correction of ureteric obstruction
with a ureteric stent or percutaneous nephrostomy.
Renal replacement therapy
Conservative management can be successful in AKIwith meticulous attention to fluid balance, electrolytes and nutrition, but renal replacement therapy (RRT) may
be required in patients who are not showing signs of
recovery with these measures. Typically, the decision to
start RRT is driven by hyperkalaemia, fluid overload or
acidosis. Severe uraemia with pericarditis and neurological signs (uraemic encephalopathy) is uncommon in AKI but, when present, is a strong indication for RRT.
No specific cut-off values for serum urea or creatinine
have been identified at which RRT should be commenced,
and clinical trials of earlier versus later RRT in unselected patients with AKI have not shown differences in outcome.
Furthermore, RRT can be a risky intervention in patients with comorbidity, since it requires the placement of large intravenous catheters that may become infected and can also represent a major haemodynamic challenge in unstable patients. Accordingly, the decision to institute RRT should be made on an individual basis, taking account of the potential risks and benefits, comorbidity and other aspects of the patient’s care, including an assessment of whether early or delayed recovery is likely. The two main options for RRT in AKI are haemodialysis and high-volume haemofiltration, or the hybrid approach of haemodiafiltration.
Peritoneal dialysis is also an option if haemodialysis is
not available .
Recovery from AKI
This is usually heralded by a gradual return of urineoutput and a steady improvement in plasma biochemistry.
During recovery, there is often a diuretic phase for several days before returning to normal. This may be due in part to tubular damage and to temporary loss of the medullary concentration gradient. This gradient plays a key role in concentrating urine in the collecting duct. After a few days, urine volume falls to normal as the concentrating mechanism and tubular reabsorption are restored. During the recovery phase of AKI, it may be necessary to provide supplements of sodium chloride, sodium bicarbonate, potassium chloride and sometimes phosphate temporarily, to compensate for increased urinary losses.
Acute kidney injury in old age
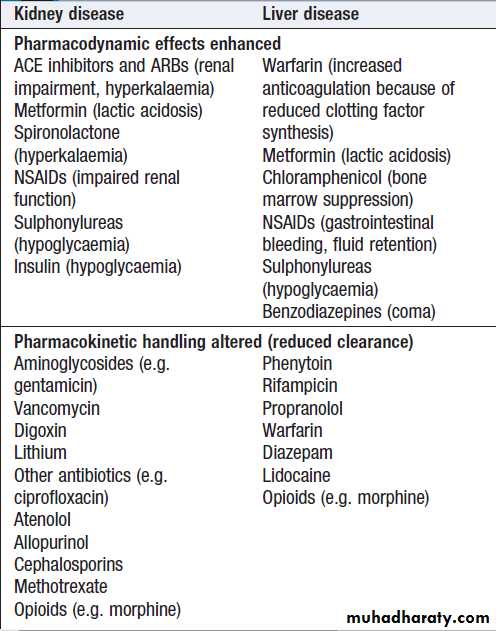
Physiological change: nephrons decline in number with age and average GFR falls progressively.Creatinine: as muscle mass falls with age, less creatinine is produced each day. Serum creatinine can be misleading as a guide to renal function.
Renal tubular function: declines with age, leading to loss of urinary concentrating ability.
Drugs: increased drug prescription in older people (diuretics, ACEI and NSAIDs) may contribute to risk of AKI.
Causes: infection, renal vascular disease, prostatic obstruction, hypovolaemia and severe cardiac dysfunction .
Mortality:
rises with age, primarily because of comorbid conditions.
CHRONIC KIDNEY DISEASE (CKD)
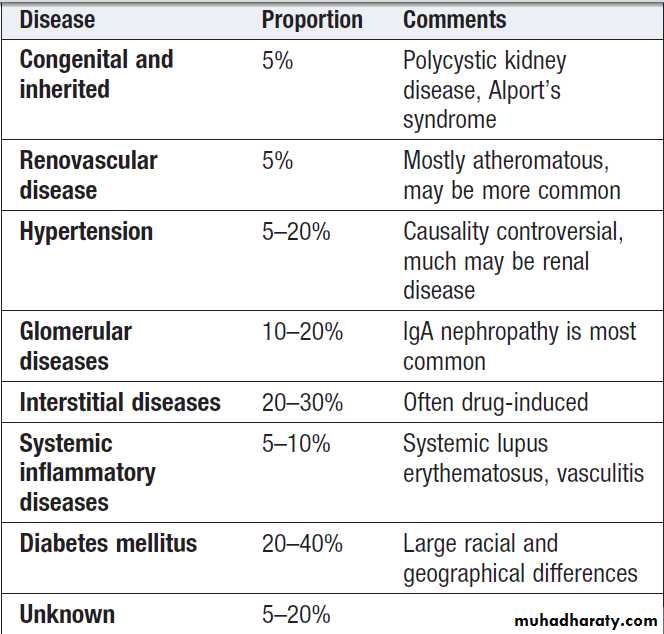
Previously termed chronic renal failure, irreversible deterioration in renal function which usually develops over a period of years . Initially, it is manifest only as a biochemical abnormality but, eventually, loss of the excretory, metabolic and endocrine functions of the kidney leads to the clinical symptoms and signs of renal failure, collectively referred to as uraemia. When death is likely without RRT (stage 5), it is called end-stage renal disease or failure (ESRD or ESRF). The great majority of stages 1–3 CKD never develop ESRD, which is fortunate. Many at a late stage have bilateral small kidneys and renal biopsy is rarely undertaken since it is more risky, less likely to provide a diagnosis because of the severity of damage, and unlikely to alter management.Common causes of end-stage renal failure
Clinical featuresThe typical presentation is with a raised urea and creatinine found during routine blood tests, frequently accompanied by hypertension, proteinuria or anaemia.
Most patients with slowly progressive disease are
asymptomatic until GFR falls < 30 mL/min/1.73 m2
(stage 4 or 5). An early symptom is nocturia, due to the loss of concentrating ability and increased osmotic load per nephron, but this is nonspecific. When GFR falls <15–20 mL/min/1.73 m2, symptoms and signs are common and can affect almost all body systems .
They typically include tiredness or breathlessness, which may, in part, be related to renal anaemia, pruritus, anorexia, weight loss, nausea and vomiting.
With further deterioration in renal function, patients may suffer hiccups, experience unusually deep respiration related to metabolic acidosis (Kussmaul’s respiration), and develop muscular twitching, fits, drowsiness and coma.
Immune dysfunction
Cellular and humoral immunity is impaired in advanced
CKD and there is increased susceptibility to infections,
the second most common cause of death in dialysis
patients, after cardiovascular disease.
Many infections are associated with access devices but some are common infections, such as pneumonia.
Haematological
There is an increased bleeding tendency in advancedCKD, manifests as cutaneous ecchymoses and mucosal bleeds. Platelet function is impaired and bleeding
time prolonged. Adequate dialysis partially corrects
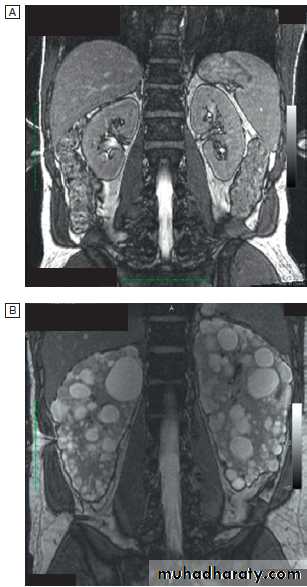
the bleeding tendency, but these patients are at significantly increased risk of complications from all anticoagulants, including those that are required during dialysis. Anaemia is common and is due in part to reduced erythropoietin production. Haemoglobin can be as low as 50–70 g/L in CKD stage 5, although it is often less severe or absent in patients with polycystic kidney disease.
Causes of anaemia in chronic kidney disease
Electrolyte abnormalitiesCKD often develop electrolyte abnormalities , metabolic acidosis , fluid retention, sometimes leading to episodic pulmonary oedema. This is particularly associated with renal artery stenosis. Conversely, some patients with tubulo-interstitial disease can develop ‘salt-wasting’ disease and may require a high sodium and water intake, including supplements of sodium salts, to prevent fluid depletion and worsening of renal function.
Although it is usually asymptomatic, it may be associated with increased tissue catabolism and decreased protein synthesis, and may exacerbate bone disease and the rate of decline in renal function.
Endocrine function
Loss of libido at least in part, to hypogonadism as a consequence of Hyperprolactinaemia. The half-life of insulin is prolonged due to reduced tubular metabolismand there is also insulin resistance. Because of this, insulin requirements are unpredictable in diabetic.
Neurological and muscle function
Generalised myopathy may occur due to a combination
of poor nutrition, hyperparathyroidism, vitamin D deficiency and disorders of electrolyte metabolism. Muscle
cramps are common. The ‘restless leg syndrome’, patient’s legs are jumpy during the night, may be troublesome.
Both sensory and motor neuropathy can arise, presenting as paraesthesia and foot drop, respectively, they can often improve once dialysis is established.
Cardiovascular disease
The risk is substantially increased in stage 3 or worse (GFR< 60 mL/min/1.73 m2) and those with microalbuminuria. LVH may occur, secondary to hypertension, and may account for the increased risk of sudden death (dysrhythmias). Pericarditis may complicate untreated or inadequately treated ESRD and cause pericardial tamponade or constrictive pericarditis. Medial vascular calcification due, in part, to the high serum phosphate levels which are present in stage 3b. Hyperphosphataemia may also contribute to the itching that arises in advanced CKD and probably contributes to the increased risk of CVD. Reflecting this fact, serum fibroblast growth factor 23 (FGF23) levels (increase in response to serum phosphate) are an independent predictor of mortality in CKD.
Metabolic bone disease
Disturbances of calcium and phosphate metabolism are
almost universal in advanced CKD, and various types
of metabolic bone disease may also occur, including
osteitis fibrosa cystica, osteomalacia and osteoporosis .
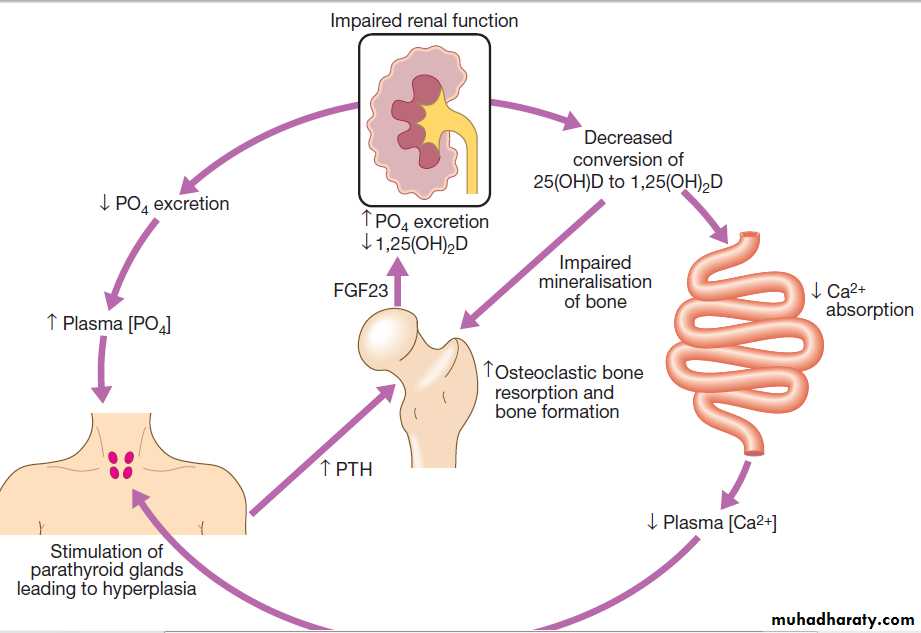
There is impaired conversion of 25-hydroxyvitamin D to its active metabolite, 1,25-dihydroxyvitamin D, due in part to renal tubular cell damage and elevated FGF23 levels. The reduced 1,25-dihydroxyvitamin D levels impair intestinal absorption of calcium, thereby causing hypocalcaemia, which leads in turn to increased PTH production by the parathyroid glands. Serum phosphate levels also start to rise because of the reduction in GFR, leads to increased production of the hormone FGF23 from osteocytes .
The FGF23 promotes phosphate excretion, thereby compensating in part for the reduced glomerular filtration of phosphate. This homeostatic response eventually fails, and hyperphosphataemia develops. The raised phosphate complex with calcium in the extracellular space, causing ectopic calcification in blood vessels and other tissues. Patients commonly develop parathyroid gland hypertrophy secondary and in some, tertiary hyperparathyroidism supervenes, due to autonomous production of PTH; this presents with hypercalcaemia.
In osteitis fibrosa cystica, there is increased bone turnover due to the high levels of PTH, whereas low bone turnover
(adynamic bone disease) may be observed in patients
over-treated with vitamin D metabolites. Osteomalacia can occur with over-treatment of hyperphosphataemia .
Pathogenesis of renal osteodystrophy. Low 1,25(OH)2D levels cause calcium malabsorption and this, combined with high phosphate
levels, causes hypocalcaemia, which increases PTH production by the parathyroid glands. The raised level of PTH increases osteoclastic bone resorption
and bone formation. Although production of FGF23 from osteocytes also increases, promoting phosphate excretion, this is insufficient to prevent
hyperphosphataemia in advanced CKD.
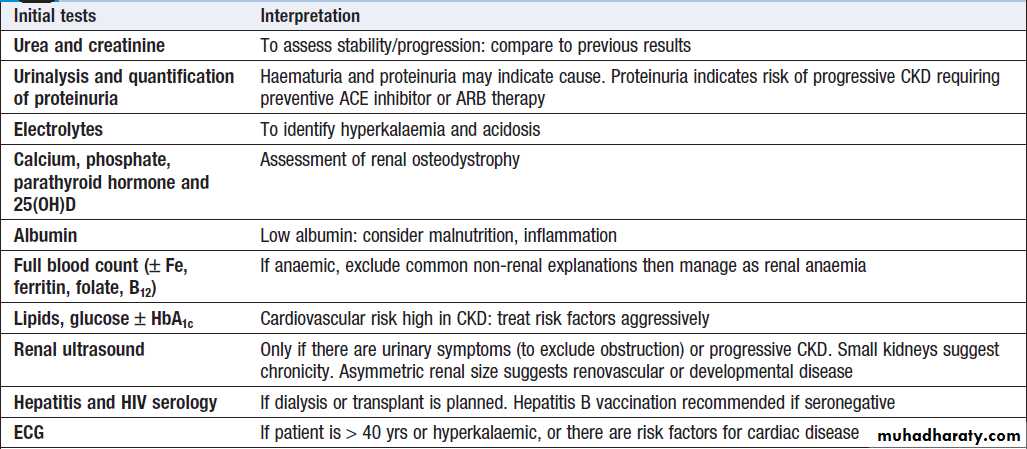
Investigations
Their main aims are:• to identify the underlying cause where possible,
since this may influence the treatment
• to identify reversible factors that may worsen renal
function, such as hypertension, urinary tract obstruction, nephrotoxic drugs, and salt and water depletion
• to screen for complications of CKD, such as
anaemia and renal osteodystrophy
• to screen for cardiovascular risk factors.
Suggested investigations in chronic kidney disease
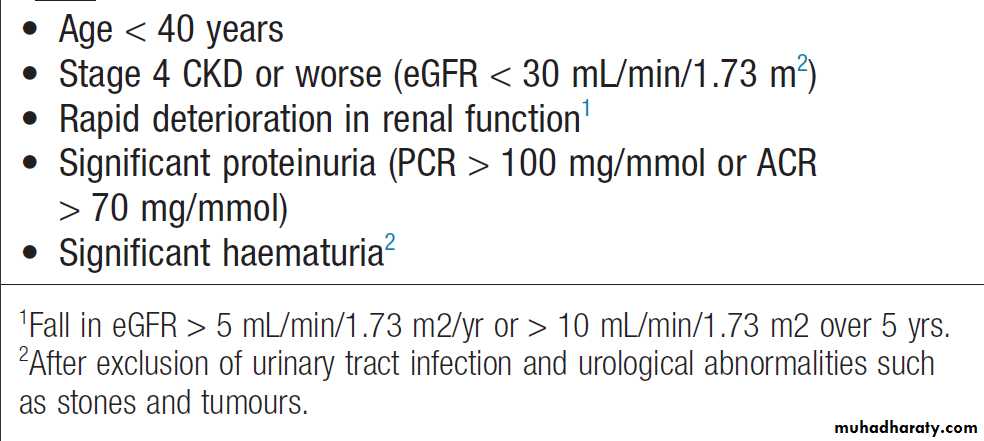
Criteria for referral of chronic kidney disease patients to a nephrologist
ManagementThe aims of management in CKD are to prevent or
slow further renal damage; to limit the adverse effects of renal impairment on the skeleton and on haematopoiesis; to treat risk factors for CVD; and to prepare for RRT .
Antihypertensive therapy
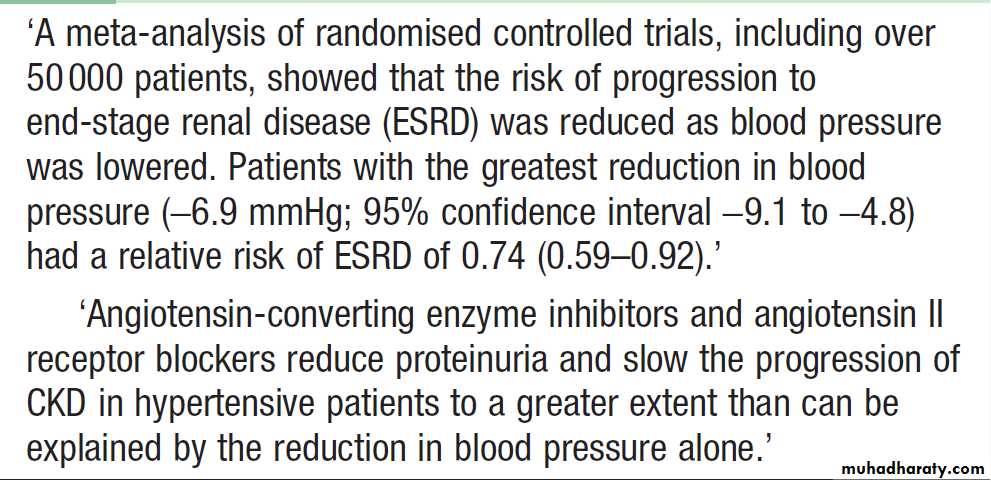
Lowering of blood pressure slows the rate at which renal
function declines, independently of the agent
used and has additional benefits in lowering the risk of hypertensive heart failure, stroke and peripheral vascular disease, as well as reducing proteinuria . Various targets have been suggested, such as 130/80 mmHg for uncomplicated, and 125/75 mmHg for CKD complicated by significant proteinuria of more than 1 g/day (PCR > 100 mg/mmol or ACR > 70 mg/mmol).
Lowering blood pressure in chronic kidney disease
Reduction of proteinuria
There is a clear relationship between the degree of proteinuria and the rate of progression of renal disease, and strong evidence that reducing proteinuria reduces the
risk of progression. ACE inhibitors and angiotensin II receptor blockers (ARBs) reduce proteinuria and retard the progression of CKD . These effects are partly due to the
reduction in blood pressure but there is evidence for a
specific beneficial effect in patients with proteinuria
(PCR > 50 mg/mmol or ACR > 30 mg/mmol) and those
with incipient or overt diabetic nephropathy. In addition, ACE inhibitors have been shown to reduce the risk of cardiovascular events and all-cause mortality in CKD.
Treatment with ACE inhibitors and ARBs may be
accompanied by an immediate reduction in GFR whentreatment is initiated, due to a reduction in glomerular
perfusion pressure. Treatment can be continued so long as the reduction in GFR is less than 20% and is not progressive. Accordingly ACE inhibitors and/or ARBs
should be prescribed to all patients with diabetic nephropathy and those with proteinuria, irrespective of
whether or not hypertension is present, providing that
hyperkalaemia does not occur.
Dietary and lifestyle interventions
There is experimental evidence that restricting dietaryprotein can reduce progression of CKD in animal models
but the results are less clear-cut in humans. All patients
with stage 4 and 5 CKD should be given dietetic advice
aimed at preventing excessive consumption of protein,
ensuring adequate calorific intake and limiting potassium
and phosphate intake. Severe protein restriction is
not recommended. All patients should be advised to
stop smoking, since there is evidence that this slows the
decline in renal function in addition to reducing cardiovascular risk. Exercise and weight loss may also reduce proteinuria and have beneficial effects on cardiovascular risk profile.
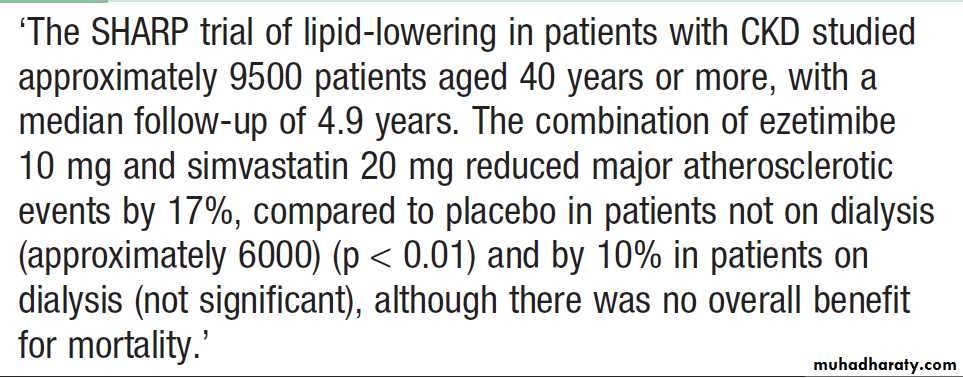
Lipid-lowering therapy
Hypercholesterolaemia is almost universal in patients
with significant proteinuria, and increased triglyceride
levels are also common in patients with CKD. Lipid
lowering has been shown to reduce vascular events in
non-dialysis CKD patients . There is some evidence that control of dyslipidaemia with statins may slow the rate of progression of renal disease.
Effects of lipid-lowering therapy in CKD
Treatment of anaemiaAnaemia is common iwith a GFR < 30 mL/min/1.73 m2 and. Recombinant human erythropoietin is effective in correcting the anaemia. Erythropoietin treatment does not influence mortality, however, and correcting haemoglobin to normal levels may carry some extra risk, including hypertension and thrombosis (including thrombosis of the arteriovenous fistulae). The target 100 and 120 g/L.
Erythropoietin is less effective in the presence of iron deficiency, active inflammation or malignancy, and with aluminium overload, which may occur in dialysis.
Maintaining fluid and electrolyte balance
Patients with evidence of fluid retention should have
dietary sodium intake limited to about 100 mmol/day,
loop diuretics may be required to treat fluid overload.
If hyperkalaemia occurs, drug therapy should be reviewed, to reduce or stop potassium-sparing diuretics, ACE inhibitors and ARBs. Correction of acidosis may be helpful, and limiting potassium intake to 70 mmol/day may be necessary. Potassium-binding resins, such as calcium resonium, may be useful in the short term.
The plasma bicarbonate should be maintained
above 22 mmol/L by giving sodium bicarbonate
supplements (starting dose of 1 g 3 times daily, increasing as required).
If the increased sodium intake induces hypertension or oedema, calcium carbonate (up to 3 g daily) may be used as an alternative, since this has the advantage of also binding dietary phosphate.
Renal bone disease
Treatment should be initiated with active vitamin D
metabolites (either 1-α-hydroxyvitamin D or 1,25-
dihydroxyvitamin D) in patients who are found to have
hypocalcaemia or serum PTH levels more than twice
the upper limit of normal. The dose should be adjusted
to try to reduce PTH levels to between 2 and 4 times
the upper limit of normal to avoid over-suppression of
bone turnover and adynamic bone disease, but care
must be exercised in order to avoid hypercalcaemia.
Hyperphosphataemia should be treated by dietary
restriction of foods with high phosphate content (milk,
cheese, eggs and protein-rich foods) and by the use of
phosphate-binding drugs.
Various drugs are available, including calcium carbonate, aluminium hydroxide, lanthanum carbonate and polymer-based phosphate binders such as sevelamer. The aim is to maintain serum phosphate values at 1.8 mmol/L (5.6 mg/dL) or below if possible, but many of these drugs are difficult to take and compliance may be a problem.
Parathyroidectomy may be required for the treatment
of tertiary hyperparathyroidism. An alternative is to
employ calcimimetic agents, such as cinacalcet, which
bind to the calcium-sensing receptor and reduce PTH
secretion. They have a place if parathyroidectomy is
unsuccessful or not possible.
RENAL REPLACEMENT THERAPY (RRT)
May be required on a temporary basis in patients with AKI or on a permanent basis for those with CKD secondary to the many different types of renal disease. Since the advent of long-term RRT in the 1960s, the numbers of patients with ESRD who are kept alive have increased considerably. Following initiation of dialysis in the UK, the survival is 84% at 1 year and 50% after 5 years. Comorbid conditions, such as diabetes mellitus and generalised vascular disease, also have a strong influence on mortality.
The aim of RRT is to replace the excretory functions , to maintain normal electrolyte and fluid balance. Various options available, haemodialysis, haemofiltration, haemodiafiltration, peritoneal dialysis and renal transplantation.
Preparing for renal replacement therapy
It is crucial that patients who are known to have progressive CKD are prepared well in advance for the institution of RRT. It is often possible to predict when RRT will be required from serial measurements of serum creatinine . Such patients, preparations for the initiation of RRT should be started at least 12 months before the predicted start date. Since there is no evidence that early initiation of RRT improves outcome, the overall aim is to commence RRT by the time symptoms of CKD have started to appear but before serious complications have occurred.Preparation for RRT involves providing the patient with psychological and social support for both them and their relatives from the renal multidisciplinary team, discussing the various choices of treatment.
For those that decide to go ahead with RRT, there are further choices between haemodialysis and peritoneal dialysis between hospital and home treatment, and on referral for renal transplantation. Conservative treatment
In older patients with multiple comorbidities, conservative
treatment of stage 5 CKD, aimed at limiting the adverse symptomatic effects of ESRD without commencing RRT, is increasingly viewed as a positive choice . Current evidence suggests that survival without dialysis can be similar or only slightly shorter than who undergo RRT, but they avoid the hospitalisation and interventions. Many of these enjoy a good quality of life for several years. It is also appropriate to discontinue dialysis, with the consent of the patient, and offer conservative therapy and palliative care when quality of life on dialysis is inadequate.
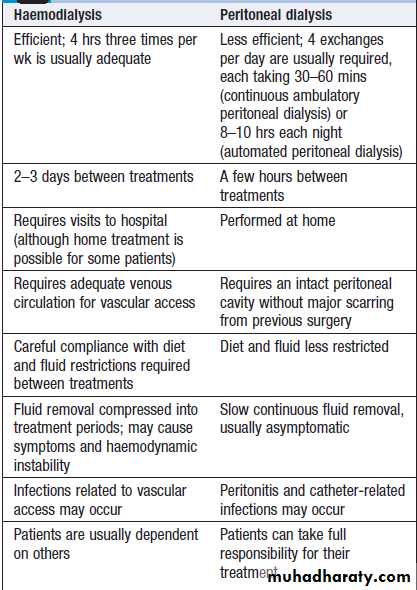
Comparison of haemodialysis and
peritoneal dialysisHaemodialysis
Is the most common form of RRT in ESRD and is also used in AKI. It involves gaining access to the circulation, either through an arteriovenous fistula, a central venous catheter or an arteriovenous shunt, such as a Scribner shunt. The patient’s blood is pumped through a haemodialyser, which allows bidirectional diffusion of solutes between blood and the dialysate across a semipermeable membrane down a concentration gradient . Haemodialysis offers the best rate of small solute clearance in AKI, but should be started gradually because of the risk of confusion and convulsions due to cerebral oedema (dialysis disequilibrium). Typically, 1–2 hours is prescribed initially but, subsequently, can be treated by 4–5 hours of on alternate daysDuring dialysis, it is standard practice to anticoagulate patients with heparin but the dose may be reduced if there is a bleeding risk. For short dialyses and in patients with abnormal clotting, it may be possible to avoid anticoagulation altogether. In AKI, dialysis is performed through a large-bore, dual-lumen catheter inserted into the femoral or internal jugular vein. Subclavian lines are avoided where possible, as thromboses will compromise the ability to form a functioning fistula in the arm if the patient fails to recover and needs chronic dialysis.
In CKD, vascular access is gained by formation of an arteriovenous fistula, in the forearm, up to a year before dialysis. After 4–6 weeks, increased pressure transmitted from the artery to the vein leading from the fistula causes
distension and thickening of the vessel wall (arterialisation).
Large-bore needles can then be inserted into the
vein to provide access for each haemodialysis treatment.Preservation of arm veins is thus very important in patients with progressive renal disease who may require haemodialysis in the future. If this access is not possible, venous or Gortex grafts may be fashioned or plastic catheters in central veins can be used for short-term access. These may be tunneled under the skin to reduce infection risk. All patients must be screened in advance for hepatitis B, hepatitis C and HIV, and vaccinated
against hepatitis B if they are not immune. All dialysis
units should have segregation facilities for hepatitis
B-positive patients, given its easy transmissibility.
Patients with hepatitis C and HIV are less infectious and
can be treated satisfactorily using machine segregationand standard infection control measures. Haemodialysis is usually carried out for 3–5 hours three times weekly, either at home or in an outpatient unit. The intensity and frequency of dialysis should be adjusted to achieve a reduction in urea during dialysis (urea reduction ratio) of over 65%. Most patients notice an improvement in symptoms during the first 6 weeks of treatment. Plasma urea and creatinine are lowered by each treatment but do not return to normal. More frequent dialysis and nocturnal dialysis can achieve better fluid balance and electrolyte control than standard dialysis and, in particular, better control of serum phosphate levels.
Problems with haemodialysis
HaemofiltrationPrincipally used in the treatment of AKI. Water and solutes are filtered from blood across a porous semipermeable membrane under a pressure gradient. Haemofiltration may be either intermittent or continuous, and typically 1–2 litres of filtrate is replaced per hour (equivalent to a GFR of 15–30 mL/min); higher rates of filtration may be of benefit in patients with sepsis and multi-organ failure.
Haemodiafiltration
This technique combines haemodialysis with approximately
20–30 litres of ultrafiltration (with replacement of filtrate) over a 3–5-hour treatment.
It is sometimes used in the treatment of AKI but is increasingly favoured in the treatment of CKD.
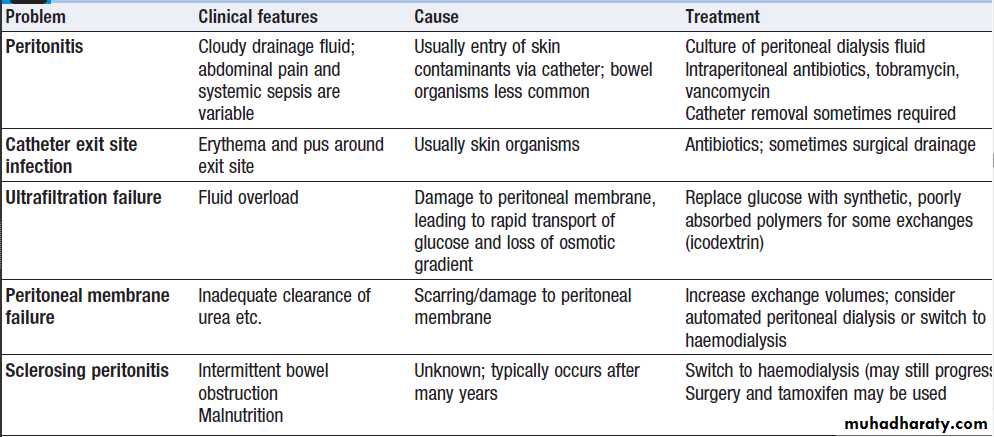
Peritoneal dialysis
Is principally used in the treatment of CKD. It requires the insertion of a permanent Silastic catheter into the peritoneal cavity .Two types are in common use. In continuous ambulatory peritoneal dialysis (CAPD), about 2 litres of sterile, isotonic dialysis fluid are introduced and left in place for approximately 4–6 hours. Metabolic waste products diffuse from peritoneal capillaries into the dialysis fluid down a concentration gradient. The fluid is then drained and fresh dialysis fluid introduced, in a continuous four-times-daily cycle.
The inflow fluid is rendered hyperosmolar by the addition of glucose or glucose polymer; this results in net removal of fluid from the patient during each cycle, due to diffusion of water from the blood through the peritoneal membrane down an osmotic gradient (ultrafiltration).
The patient is mobile and able to undertake normal daily activities. Automated peritoneal dialysis (APD) is similar to CAPD but uses a mechanical device to perform the fluid exchanges during the night, leaving the patient free, or with only a single exchange to perform, during the day.
CAPD is particularly useful in children, as a first
treatment in adults with residual renal function, and as
a treatment for elderly patients with cardiovascular
instability. The long-term use of peritoneal dialysis may
be limited by episodes of bacterial peritonitis and
damage to the peritoneal membrane, including sclerosing
peritonitis, but some patients have been treated successfully for more than 10 years.
Renal replacement therapy in old age
Problems with continuous ambulatory peritoneal dialysis
Renal transplantation
Renal transplantation offers the best chance of long-termsurvival in ESRD, and is the most cost-effective treatment.
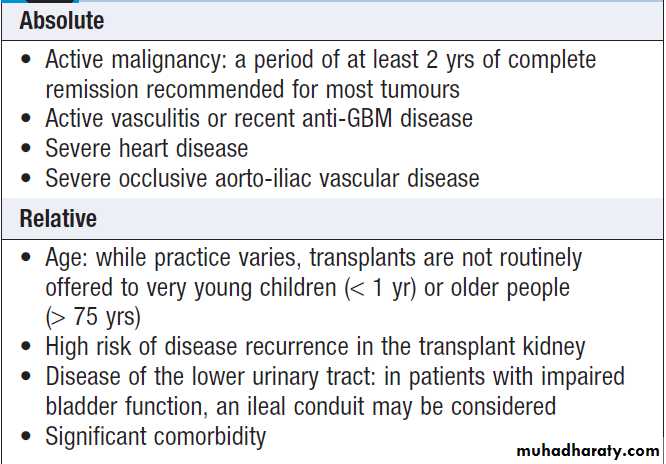
Transplantation can restore normal kidney function and correct all the metabolic abnormalities of CKD but requires long-term immunosuppression with its attendant risks . All patients with ESRD should be considered for transplantation, unless there are contraindications.
Kidney grafts may be taken from a cadaver after brain death or circulatory death or from a living donor . Compatibility of ABO blood group between donor and recipient is usually required and the degree of matching for major histocompatibility (MHC) antigens, particularly HLADR, influences the incidence of rejection.
Immediately prior to transplantation, tests should be performed for antibodies against HLA antigens and for antibodies that can bind to lymphocytes of the donor . Positive tests predict early rejection. Although some ABO- and HLA-incompatible transplants are now possible, this
involves appropriate preparation with pre-transplant
plasma exchange and/or immunosuppression, so that
recipient antibodies to the donor’s tissue are reduced to
acceptably low levels. This option is generally only available for living donor transplants because of the preparation required. During the transplant operation, the kidney is placed in the pelvis; the donor vessels are usually anastomosed to the recipient’s internal iliac artery and vein, the donor ureter anastomosed to the bladder .
The failed kidneys are usually left in place.
Peri-operative problems include:
• Fluid balance. Careful matching of input to output isrequired. Patients can be very polyuric in the initial
period after transplantation.
• Primary graft non-function.Causes include hypovolaemia, preservation injury/acute tubular necrosis during storage and transfer, other pre-existing renal damage, hyperacute rejection, vascular occlusion and urinary tract obstruction.
• Sepsis. In addition to risks of sepsis associated with
any operation, there is an increased risk due to the
uraemia and immunosuppression. Once the graft begins to function, near-normal biochemistry is usually achieved within days to weeks.
The median eGFR of patients in the UK receiving a deceased donor transplant at 1 year is 51 mL/min/1.73 m2.
Life-long immunosuppressive therapy is required to prevent rejection but needs to be more intensive in the early post-transplantation period, when the risk is highest. A common regimen is triple therapy with prednisolone; ciclosporin or tacrolimus; and azathioprine or mycophenolate mofetil. Sirolimus (rapamycin) is an alternative that can be introduced later. Antibodies to deplete or modulate specific lymphocyte populations are increasingly used; targeting the lymphocyte interleukin (IL)-2 receptor is particularly
effective for preventing rejection. Acute rejection is usually treated, by short courses of methylprednisolone 500 mg IV on 3 consecutive days. Other therapies, such as antilymphocyte antibodies, IV immunoglobulin and plasma exchange, can be used for episodes of acute rejection that do not respond to high dose corticosteroids.
Complications of immunosuppression include infections
and malignancy .Approximately 50% of white patients develop skin malignancy by 15 years after transplantation.
The prognosis after kidney transplantation is good.
Recent UK statistics for transplants from cadaver donors
indicate 96% patient survival and 93% graft survival at
1 year, and 88% patient survival and 84% graft survival
at 5 years. Even better figures are obtained with living
donor transplantation (91% graft survival at 5 years). Advances in immunosuppression have greatly improved
results from using genetically unrelated donors .
Contraindications to renal transplantation
RENAL VASCULAR DISEASESDiseases which affect renal blood vessels may cause
renal ischaemia, leading to acute or CKD or secondary hypertension.
Renal artery stenosis (RAS)
Arelatively uncommon disorder, which presents clinically with hypertension, occur in about 2% of unselected patients
with hypertension but may affect up to 4% of older
patients with hypertension who have evidence of
atherosclerotic disease elsewhere. Most cases are caused by atherosclerosis but fibromuscular dysplasia of the vessel wall may be responsible in young. Rare causes : vasculitis (Takayasu’s arteritis and polyarteritis nodosa) ,
thromboembolism and aneurysms of the renal artery.
Pathophysiology
RAS results in a reduction in renal perfusion pressure, which activates the renin–angiotensin system, leading to increased circulating levels of angiotensin II. This provokes vasoconstriction and increases aldosterone production by the adrenal, causing sodium retention by the renal tubules. Significant reduction of renal blood flow occurs when there is >70% narrowing of the artery . The characteristic lesion is an ostial stenosis. As the stenosis becomes more severe, renal ischaemia leads to shrinkage of the affected kidney and may cause renal failure (ischaemic nephropathy). In young, fibromuscular dysplasia is a more likely cause. This is an uncommon of unknown cause. It is characterised by hypertrophy of the media (medial fibroplasia), which narrows the artery but rarely leads to total occlusion.
85% of patients will not develop progressive renal impairment. Unfortunately, methods of predicting which patients are at risk of progression or who will respond to treatment are still imperfect.
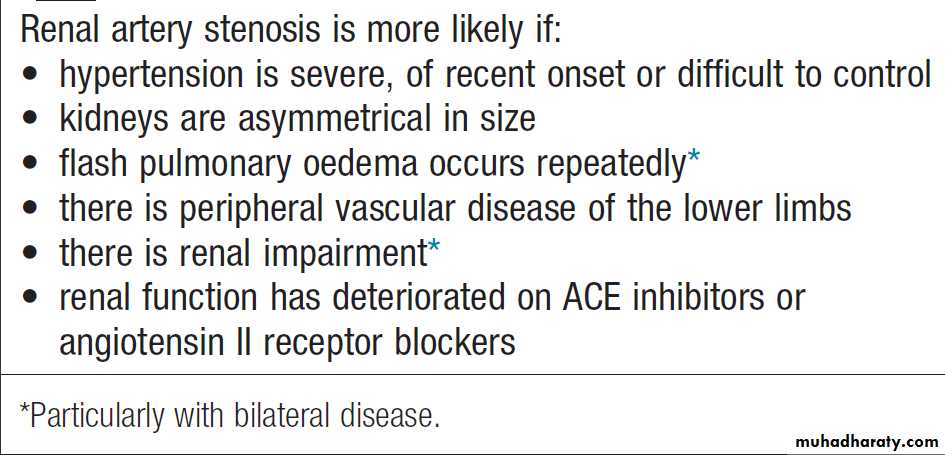
Clinical features
RAS can present in various ways ,including hypertension, renal failure (with bilateral disease), a deterioration in renal function when ACE inhibitors or ARBs are used, or acute pulmonary oedema. Although many patients experience a slight drop in GFR when commencing these drugs, an increase in serum creatinine of 25% or more raises the possibility of renal artery stenosis. Acute pulmonary oedema is particularly characteristic of bilateral renovascular disease.
Presentation and clinical features of renal artery stenosis
InvestigationsWhen appropriate, imaging of the renal vasculature
with either CT or MR angiography should be performed to confirm the diagnosis .
Biochemical testing may reveal impaired renal
function and an elevated plasma renin activity, sometimes
with hypokalaemia due to hyperaldosteronism.
Ultrasound may also reveal a discrepancy in size
between the two kidneys. While these investigations provide supportive information, they are insufficiently
sensitive or specific to be of value in diagnosis of renovascular disease in hypertensive patients.
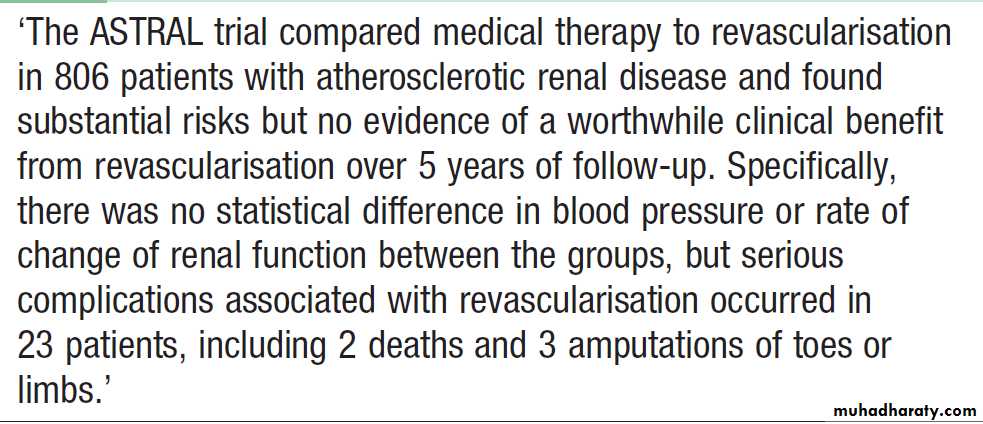
Management
The first-line management in patients with renal artery
stenosis is medical therapy with antihypertensive drugs, supplemented, where appropriate, by statins and low-dose aspirin in those with atherosclerotic disease.
Interventions to correct the vessel narrowing should be considered in young patients (age < 40) suspected of having renal artery stenosis; those in whom blood pressure cannot easily be controlled with antihypertensive; those who have a history of ‘flash’ pulmonary oedema or accelerated phase (malignant) hypertension; and those in whom renal function is deteriorating.
The most commonly used technique is angioplasty.
The best results are obtained in non-atheromatousfibromuscular dysplasia, where correction of the stenosis
has a high chance of success in improving blood
pressure and protecting renal function. Angioplasty and
stenting can sometimes be successful in atherosclerotic.
The risks of angioplasty and stenting include renal artery occlusion, renal infarction, and atheroemboli from manipulations in a severely diseased aorta.
Small vessel disease distal to the stenosis may preclude substantial functional recovery.
Stenting for renal artery stenosis
Microvascular disorders associated with
acute renal damage
Haemolytic uraemic syndrome (HUS)
Characterised by thrombotic microangiopathy that causes damage to endothelial cells of the microcirculation. This is accompanied by swelling of the endothelial cells, increasedplatelet adherence and intravascular thrombosis. There
is a marked reduction in the platelet count and anaemia,
with features of intravascular haemolysis , such as a raised unconjugated bilirubin level, raised levels of LDH and decreased circulating levels of haptoglobin. The most common cause of classical HUS is infection associated with organisms that produce enterotoxins called verotoxins: so-called diarrhea-positive HUS (D+HUS).
The organisms most commonly implicated in (D+HUS)
are enterohaemorrhagic Escherichia coli .
Management
Supportive care, optimizing fluid and electrolyte balance, transfusion and RRT if necessary. Infusion of fresh frozen plasma can be helpful (presumably by replacing complement components), as can plasma exchange (presumably by removing pathogenic autoantibodies). Recently, impressive results have been reported with the anti-C5 monoclonal antibody, eculizumab, which binds to C5, thereby preventing activation of the terminal complement cascade.Thrombotic thrombocytopenic purpura (TTP)
Like HUS, TTP is characterised by thrombotic microangiopathy, which causes damage to endothelial cells of the microcirculation.This leads to swelling of the endothelial cells, increased platelet adherence and intravascular thrombosis.
In contradistinction to HUS, the brain is more commonly
affected in TTP and involvement of the kidney is
usually less prominent. TTP is an autoimmune disorder
caused by antibodies against ADAMTS-13, which is involved in regulating platelet aggregation.
Disseminated intravascular coagulation
Occur as a complication of a range of illnesses and can be accompanied by multi-organ failure and renal failure.
Systemic sclerosis
Renal involvement is a serious complication of SS, which is more likely to occur in diffuse cutaneous systemic sclerosis (DCSS) than in limited (LCSS). The renal lesion is caused by intimal cell proliferation and luminal narrowing of intrarenal arteries and arterioles. There is intense intrarenal vasospasm and plasma renin activity is markedly elevated. Renal involvement usually presents clinically with severe hypertension, microangiopathic features and progressive oliguric renal failure (scleroderma renal crisis).
Use of ACE inhibitors to control the hypertension
has improved the 1-year survival from 20% to 75% but
about 50% of patients continue to require RRT.
GLOMERULAR DISEASES
Account for a significant proportion of acute and chronic kidney disease. There are many causes of glomerular damage, including immunological injury, inherited such as Alport’s syndrome , metabolic diseases such as diabetes mellitus , and deposition of abnormal proteins such as amyloid in the glomeruli. Most patients do not present acutely and are asymptomatic until abnormalities are detected on routine screening of blood or urine samples.Clinical and laboratory features of glomerular injury
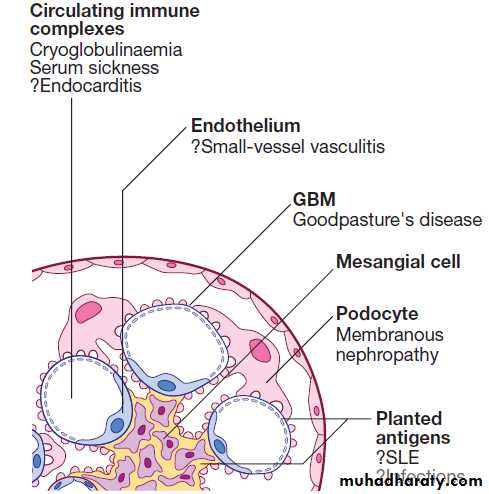
Cells of the glomerulus and targets of immunity and
autoimmunity. Antibodies and antigen–antibody (immune) complexes are described according to their site of deposition: subepithelial, between podocyte and GBM; intramembranous, within the GBM; subendothelial,between endothelial cell and GBM; and mesangial, within the mesangial matrix. (GBM = glomerular basement membrane;
Glomerulonephritis (GN)
literally means ‘inflammation of glomeruli’. The term is used to describe all types of glomerular disease, even though some of these (such as minimal change nephropathy) are not associated with inflammation.Most types are immunologically mediated, deposition of antibody occurs in many but, frequently, the presumed mechanisms involve cellular immunity. Although deposition of circulating immune complexes was previously thought to be a common mechanism, it now seems that most deposits of immunoglobulin are formed
‘in situ’ by antibodies which complex with glomerular antigens, or with other antigens derived from viruses and bacteria that have become deposited in the glomeruli .
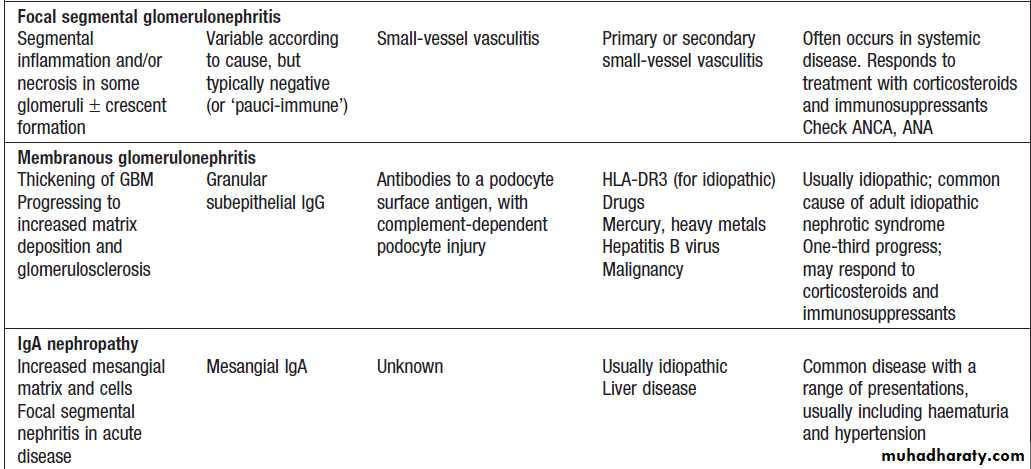
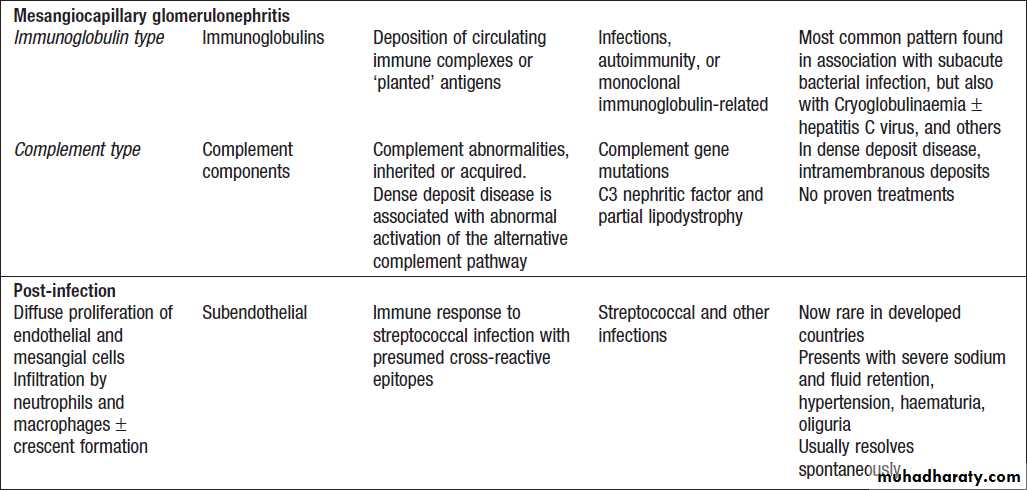
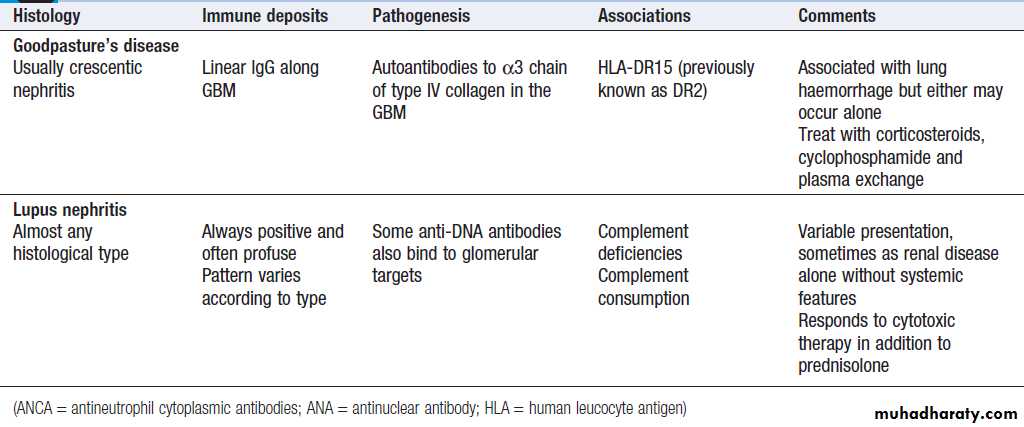
Glomerulonephritis: types, associations and causes
Glomerulonephritis: types, associations and causes'cont'd
Glomerulonephritis: types, associations and causes'cont'd
Glomerulonephritis: types, associations and causes'cont'd
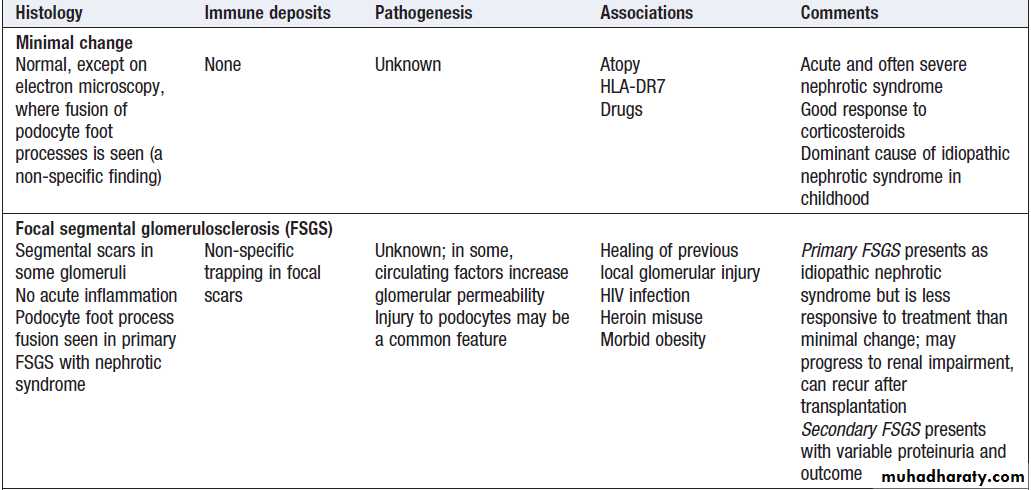
Minimal change nephropathy
Minimal change disease occurs at all ages but accountsfor nephrotic syndrome in most children and about one-quarter of adults. It is caused by reversible dysfunction of podocytes. The presentation is with proteinuria or nephrotic syndrome, which typically remits with high-dose corticosteroid therapy (1 mg/kg prednisolone for 6 weeks). Some patients who respond incompletely or relapse frequently need maintenance corticosteroids, cytotoxic therapy or other agents.
Minimal change disease does not progress to CKD but
can present with problems related to the nephrotic syndrome and complications of treatment.
Focal segmental glomerulosclerosis (FSGS)
can occur in all age groups. In some can have specific causes, such as HIV infection, massive obesity, but in most cases the underlying cause is unknown .Patients with primary FSGS present with massive proteinuria and idiopathic nephrotic syndrome. Histological analysis shows sclerosis affecting segments of the glomeruli, which may also show positive staining for deposits of C3 and IgM on immunofluorescence. Since FSGS is a focal process, abnormal glomeruli may not be seen on renal biopsy if only a few are sampled, leading to an initial diagnosis of minimal change nephropathy. Juxtamedullary glomeruli are more likely to be affected in early disease.Primary FSGS can respond to high-dose corticosteroid
therapy (0.5–2.0 mg/kg/day) but most patients show little or no response. Immunosuppressive drugs, such as ciclosporin, cyclophosphamide and mycophenolate mofetil, have also been used but their efficacy is uncertain. Progression to CKD is common and the disease frequently recurs after renal transplantation.Membranous glomerulonephritis
Also known as membranous nephropathy, is the most common cause of nephrotic syndrome in adults. It is caused by antibodies (autoantibodies)directed at antigen(s) expressed on the surface of podocytes. A proportion of cases are associated with other causes, such as heavy metal poisoning, drugs, infections and tumours but most are idiopathic. Approximately one-third with idiopathic membranous GN undergo spontaneous remission; one-third remain in a nephrotic state, and one-third go on to develop CKD. Short-term high doses of corticosteroids and cyclophosphamide may improve both the nephrotic and the long-term prognosis. However, because of the toxicity of these regimens, many nephrologists reserve such treatment for those with severe or deteriorating renal function.IgA nephropathy
This is one of the most common types GN . Haematuria is the earliest sign and is almost universal, and hypertension is also very common. Proteinuria can also occur but is usually a later feature. In many cases, there is slowly progressive loss of renal function leading to ESRD. A particular hallmark of IgA nephropathy in young adults is the occurrence of acute self-limiting exacerbations, often with gross haematuria, in association with minor respiratory infections. This may be so acute as to resemble acute post-infectious GN , with fluid retention, hypertension and oliguria with dark or red urine.Characteristically, the latency from clinical infection to nephritis is short: a few days or less.
Asymptomatic presentations dominate in older adults, with haematuria, hypertension and loss of GFR. Occasionally, progresses rapidly and crescent formation may be seen. The response to immunosuppressive therapy is usually poor. The management of less acute disease is largely directed towards the control of blood pressure in an attempt to prevent or retard progressive renal disease. There is some evidence for additional benefit from several months of high-dose corticosteroid treatment in high-risk disease, but no strong evidence for other immunosuppressive agents.
Henoch–Schِnlein purpura
This condition most commonly occurs in children but can also be observed in adults, characterised by a systemic vasculitis that often arises in response to an infectious trigger. The presentation is with a characteristic petechial rash typically affecting buttocks and lower legs, and abdominal pain due to the occurrence of vasculitis involving the GIT. The presence of GN is usually indicated by the occurrence of haematuria. When occurs in older children or adults, the GN is usually more prominent and less likely to resolve completely. Renal biopsy shows mesangial IgA deposition and appearances that are indistinguishable from acute IgA nephropathy. Treatment is supportive in nature; the prognosis is good, with spontaneous resolution, but some, particularly adults, progress to develop ESRD.Mesangiocapillary glomerulonephritis
Mesangiocapillary glomerulonephritis (MCGN), alsoknown as membranoproliferative glomerulonephritis
(MPGN), is characterised by an increase in mesangial
cellularity with thickening of glomerular capillary walls
and subendothelial deposition of immune complexes
and/or components of the complement pathway. The
typical presentation is with proteinuria and haematuria.
Several underlying causes have been identified, as summarized in Box. It can be classified into two main
subtypes. The first is characterised by deposition of
immunoglobulins within the glomeruli. This subtype is
associated with chronic infections, autoimmune diseases
and monoclonal gammopathy.
The second is characterised by deposition of complement in the glomeruli and is associated with inherited or acquired abnormalities in the complement pathway. Within this category is so-called ‘dense deposit disease’, which is typified by deposition of electron-dense deposits within the GBM. A third subtype is recognised, in which neither immunoglobulins nor complement are deposited in the glomeruli. This is associated with healing following thrombotic microangiopathies, such as HUS and TTP.
Treatment consists of identifying and treating the
underlying disease, if possible, and the use of immunosuppressive drugs such as mycophenolate mofetil or cyclophosphamide. There is no specific treatment for
MCGN associated with deposition of complement in the
glomeruli or for dense deposit disease.
Infection-related glomerulonephritis
May occur in connection with infections of various types, including subacute bacterial endocarditis. The most common histological pattern in bacterial infection is mesangiocapillary glomerulonephritis, often associated with extensive immunoglobulin deposition in the glomeruli with evidence of complement consumption (low serum C3).
In the developed world, hospital-acquired infections
with various organisms are a common cause of these
syndromes. Worldwide, glomerulonephritis more commonly
follows hepatitis B, hepatitis C, schistosomiasis, leishmaniasis, malaria and other chronic infections. Infection with HIV may be associated with FSGS (see above), particularly in people of African descent.
Causes of glomerulonephritis associated
with low serum complementPost-streptococcal glomerulonephritis
It is more common in children. The latency is about 10 days after a throat infection or longer after skin infection, suggesting an immune mechanism. Sodium retention, hypertension and oedema are particularly pronounced. There is also reduction of GFR, proteinuria, haematuria andreduced urine volume, this gives the urine a red or smoky appearance. Serum concentrations of C3 and C4 are typically reduced, reflecting complement consumption , and evidence of streptococcal infection may be found.
Renal function begins to improve spontaneously within 10–14 days, and management by fluid and sodium restriction with diuretic and hypotensive agents is usually adequate.
The renal lesion in almost all children and many adults seems to resolve completely.
Rapidly progressive glomerulonephritis
(also known as crescentic glomerulonephritis) is characterised by rapid loss of renal function over days to weeks. Renal biopsy shows crescentic lesions, often associated with necrotising lesions ,termed focal segmental (necrotising) glomerulonephritis. It is typically seen in Goodpasture’s (anti-GBM antibodies), and in small-vessel vasculitides. It can also be observed in SLE and occasionally IgA and other nephropathies. Rapid-onset disease may be associated with relatively little proteinuria . Management depends on the underlying cause but corticosteroids and immunosuppressive drugs are often required. Patients with anti-GBM disease should be treated with plasma exchange combined with corticosteroids and immunosuppressants.Inherited glomerular diseases
AIport’s syndrome
X-linked recessive disorder .The accumulation of abnormal collagen results in a progressive degeneration of the GBM .
Affected patients progress from haematuria to ESRD in
their late teens or twenties. Some other basement membranes containing the same collagen isoforms are similarly involved, notably in the cochlea, so that Alport’s syndrome is associated with sensorineural deafness and ocular abnormalities.
ACE inhibitors may slow but not prevent loss of kidney function. Patients with Alport’s syndrome are good candidates for RRT, as they are young and usually
otherwise healthy.
TUBULO-INTERSTITIAL DISEASES
These diseases primarily affect the renal tubules and interstitial components of the renal parenchyma. They are characterised by tubular dysfunction with electrolyte abnormalities, moderate levels of proteinuria and varying degrees of renal impairment. Acute tubular necrosis falls into this category .Acute interstitial nephritis
Acute interstitial nephritis (AIN) is characterised by
acute inflammation affecting the tubulo-interstitium of
the kidney. It is commonly drug-induced but can be
caused by other factors, such as renal toxins, and can
complicate a variety of systemic diseases and infections .
Causes of acute interstitial nephritis
Clinical features
The clinical presentation is typically with renal impairment
but, in some patients with drug-induced AIN , there may be signs of a generalised drug hypersensitivity reaction with fever, rash and eosinophilia. Proteinuria is generally modest (PCR < 100 mg/mmol) and tubular in type . The urine may contain red and white blood cells but is sterile on culture. Eosinophils are present in up to 70% but this is a non-specific finding. Many patients are not oliguric, despite moderately severe renal impairment, and AIN should always be considered in patients with non-oliguric AKI. There may be a rapid deterioration of renal function in some cases of drug-induced AIN, causing the condition to be mistaken for rapidly progressive glomerulonephritis.
Investigations
Renal biopsy is usually required to confirm the diagnosis .This typically shows evidence of intense inflammation, with infiltration of the tubules and interstitium by lymphocytes. And polymorphonuclear leucocytes, Eosinophils may also be observed, especially in drug-induced AIN. The degree of chronic inflammation in a biopsy is a useful predictor of the eventual outcome for renal function.
Management
Some patients with drug-induced AIN recover following
withdrawal of the drug, but high-dose corticosteroids (prednisolone 1 mg/kg/day) accelerate recovery and may prevent long-term scarring.Dialysis is sometimes necessary , usually only short-term. Causes should be treated, if possible.
Chronic interstitial nephritis (CIN)
Characterised by renal dysfunction with fibrosis and infiltration of the renal parenchyma by lymphocytes, plasma cells and macrophages, with tubular damage.Pathophysiology
May follow AIN that does not resolve, or associated with
ingestion of various toxins and drugs, or with metabolic
and chronic inflammatory diseases.
In many no underlying cause can be identified. Toxins that
have been associated with CIN include those contained
within the plant Aristolochia clematitis. The nephropathy is commonly linked with tumours of the collecting system. Ingestion of mushrooms within the Cortinarius genus can cause irreversible renal tubular toxicity.
Acute interstitial nephritis
• Any of the causes of AIN, if persistent Glomerulonephritis
• Varying degrees of interstitial inflammation occur in
association with most types of inflammatory
glomerulonephritis
Immune/inflammatory
• Sarcoidosis
• Sjِgren’s syndrome
• Chronic transplant rejection
• Systemic lupus erythematosus, primary autoimmune
Causes of chronic interstitial nephritis
Causes of chronic interstitial nephritis – cont’d
Clinical featuresMost patients with CIN present in adult life with CKD,
hypertension and small kidneys. Because of tubular dysfunction, electrolyte abnormalities are typically severe,
resulting in hyperkalaemia and acidosis. Urinalysis
abnormalities are non-specific. A minority present with salt-losing nephropathy and impairment of urine-concentrating ability, characterised by hypotension, polyuria and features of sodium and water depletion, which puts them at risk of AKI. Renal tubular acidosis may complicate CIN but is seen most often in myeloma, sarcoidosis, cystinosis, amyloidosis and Sjِgren’s syndrome. Management is supportive, correction of acidosis and hyperkalaemia; replacement of fluid and electrolytes and RRT if irreversible renal damage has occurred.
Reflux nephropathy
This condition, which was previously known as chronic
pyelonephritis, is a specific type of chronic interstitial
nephritis associated with vesicoureteric reflux (VUR) in early life and with the appearance of scars in the kidney. Pathophysiology
Thought to be due to chronic reflux of urine from the bladder into the ureters, in association with recurrent urinary tract infection (UTI) in childhood. Susceptibility to VUR has a genetic component. It can be connected with outflow obstruction, usually caused by urethral valves, but usually occurs with an apparently normal bladder
Clinical features
Usually, are asymptomatic and the patient may present at any age with hypertension , proteinuria or features of CKD. There may be no history of overt UTI.However, symptoms arising from the urinary tract may
be present and include frequency of micturition, dysuria
and aching lumbar pain. VUR may occur in children but
diminishes as the child grows, and usually has disappeared
by adulthood. Urinalysis often shows the presence
of leucocytes and moderate proteinuria (usually
< l g/24 hrs) but these are not invariable. The risk of
renal stone formation is increased.
Investigations
Renal scarring, reduced kidney size can be detected by ultrasound. Radionuclide DMSA scans are more sensitive, and longitudinal imaging by MRI or CT may be useful in assessing progression. Abnormalities may be unilateral or bilateral and of any grade of severity. Radionuclide scan can also be used to demonstrate VUR as an alternative to micturating cystourethrography (MCUG; the bladder is filled with contrast media through a urinary catheter and images are taken during and after micturition).Vesico-ureteric reflux (grade IV) shown by micturating
cystogram. The bladder has been filled with contrast medium through a urinary catheter. After micturition, there was gross VUR into widely distended ureters and pelvicalyceal systems.
Management
Infection, if present, should be treated , if recurrent, it should be prevented with prophylactic therapy. If recurrent pyelonephritis occurs in an abnormal kidney with minimal function, nephrectomy may be indicated. Occasionally, hypertension is cured by the removal of a diseased kidney when the disease is unilateral. As most childhood reflux tends to disappear spontaneously and trials have shown small or no benefits from anti-reflux surgery, such intervention is now less common.Prophylactic antibiotics and vesicoureteric reflux
Papillary necrosisThe renal papillae lie within a hypertonic environment
in the medulla, at the end of the vasa recta. They are susceptible to ischaemic damage because of this and
can undergo necrosis when their vascular supply is impaired as the result of diabetes and sickle-cell disease or long-term ingestion of NSAID. Some are asymptomatic, whereas others present with renal colic and renal impairment as necrosed papillae slough off and cause ureteric obstruction.
Urinalysis may be normal but, more frequently haematuria and sterile pyuria. Significant proteinuria is unusual. The imaging method of choice to make the diagnosis is pyelography.
Management is based on relieving obstruction, where present, and withdrawal of the offending drugs
Sickle-cell nephropathy
In the kidney, microvascular occlusion are most pronounced in the medulla, where the vasa recta are the site of sickling because of hypoxia and hypertonicity. Loss of urinary concentrating ability and polyuria are the earliest changes; distal renal tubular acidosis and impaired potassium excretion are typical. Papillary necrosis may also occur . A minority of patients develop ESRD.Response to recombinant erythropoietin is poor due to the haemoglobinopathy. Sickle trait Patients have an increased incidence of unexplained microscopic haematuria.
CYSTIC DISEASES OF THE KIDNEY
It is common to encounter patients with a single renalcyst or even multiple ones as an incidental finding. Usually, these cysts are of no clinical consequence and are asymptomatic, but occasionally they can cause pain or haematuria. In addition, several diseases are recognised as being caused by the formation of multiple renal cysts.
Adult polycystic kidney disease (PKD)
Is a common condition, with a prevalence of approximately 1 : 1000, and is inherited as an autosomal dominant trait. Small cysts lined by tubular epithelium develop from infancy or childhood and enlarge slowly and irregularly. The surrounding normal kidney tissue is compressed and
progressively damaged.
Mutations in the PKD1 gene account for 85% , PKD2 for about 15%. ESRD occurs in approximately 50% with PKD1 mutations, a mean age of onset of 52 years, but in a minority with PKD2 mutations, with a mean age 69 years.
Clinical features
Affected subjects are usually asymptomatic until later life but hypertension usually occurs from the age of 20 onwards. One or both kidneys may be palpable and the surface may feel nodular. About 30% also have hepatic cysts but disturbance of liver function is rare. Sometimes (almost always in women) this causes massive hepatomegaly.
Berry aneurysms are associated feature and about 10% of patients have a subarachnoid haemorrhage. M and A regurgitation is frequent, and colonic diverticula and
abdominal wall hernias may occur.
Adult polycystic kidney disease: common
clinical featuresInvestigations
The diagnosis is usually based on family history, clinicalfindings and ultrasound examination which demonstrates cysts in approximately 95% over the age of 20. It is now possible to make a molecular diagnosis by mutation screening of PDK1 or PDK2 but this is seldom used in routine clinical practice. Screening for intracranial aneurysms is not generally indicated but can be done by MR angiography in families with a history of subarachnoid haemorrhage. The yield of screening is low, however, and the risk–benefit ratio of intervention in asymptomatic aneurysms in this disease is not clear.
MRI images of the kidneys.
A Normal kidneys.B Polycystic kidneys; although the kidney enlargement is extreme, this patient had only slightly reduced GFR.
Management
Good control of blood pressure is important because
cardiovascular morbidity and mortality are so common
in renal disease, but there is no evidence that control of
moderate hypertension retards the development of renal
failure in PKD, in contrast to the evidence for glomerular
diseases. There is some evidence that the vasopressin V2
receptor antagonist, tolvaptan, can slow cyst formation
in some but its place in treatment has yet to be
established. Patients with PKD are usually good candidates for dialysis and transplantation. Sometimes kidneys are so large that one or both have to be removed to make space for a renal transplant. Otherwise, unless they are a
source of pain or infection, they are usually left in situ.
Medullary sponge kidney disease
Cysts confined to papillary collecting ducts. It is not inherited, its cause is unknown. Patients usually present as adults with renal stones. The prognosis is generally good.The diagnosis is made by US or IVU . Contrast medium is fill dilated or cystic tubules, which are sometimes calcified.
Medullary cystic kidney disease
Small cortical cysts , progressive nephron destruction. The childhood variants characterised by nephrogenic diabetes insipidus, often with a family history of similar disease. Sometimes, affected are ‘salt-losing’, which aggravates the degree of renal failure. Renal failure is usual. Sometimes are associated with retinal dystrophies and brain or other abnormalities .The genetic basis of these disorders is emerging.
Medullary sponge kidney. Intravenous urogram showing
contrast medium filling both the collecting system and cavities arising from
collecting ducts, especially within papillae of the upper pole. The cavities have been likened to bunches of grapes. A plain abdominal X-ray may
show calcification in the same regions.
RENAL STONE DISEASE
Renal stone disease is common.Pathophysiology
Urinary calculi consist of aggregates of crystals, usually
containing calcium or phosphate in combination with
small amounts of proteins and glycoproteins. A number of risk factors have been identified (Box). Renal stones vary greatly in size. Staghorn calculi fill the whole renal pelvis and branch into the calyces , they are usually associated with infection and composed largely of struvite.
Deposits of calcium may be present throughout
the renal parenchyma, giving rise to fine calcification
within it (nephrocalcinosis), especially in patients with
renal tubular acidosis, hyperparathyroidism, vitamin D
intoxication and healed renal tuberculosis.
Composition of renal stones
Predisposing factors for kidney stones
Clinical features
The clinical presentation is highly variable. Most patientswith renal stone disease are asymptomatic, whereas
others present with pain, haematuria, UTI or urinary
tract obstruction. A common presentation is with acute loin pain radiating to the anterior abdominal wall,
together with haematuria: a symptom complex termed
renal or ureteric colic. This is most commonly caused by
ureteric obstruction by a calculus but the same symptoms
can occur in association with a sloughed renal
papilla, tumour or blood clot. The patient is suddenly
aware of pain in the loin, which radiates round the flank
to the groin and often into the testis or labium, in the
sensory distribution of the first lumbar nerve.
The pain steadily increases to reach a peak in a few minutes. The patient is restless and generally tries
unsuccessfully to obtain relief by changing position or
pacing the room. There is pallor, sweating and often
vomiting. Frequency, dysuria and haematuria may
occur. The intense pain usually subsides within 2 hours
but may continue unabated for hours or days, usually constant, although slight fluctuations in severity may be seen. Subsequent to an attack, intermittent dull pain in the loin or back may persist for several hours.
Investigations
Patients with symptoms of renal colic should be investigated
to determine whether or not a stone is present, to identify its location and to assess whether it is causing obstruction.
About 90% of stones contain calcium and these can be visualised on plain abdominal X-ray but CTKUB is the gold standard, as 99% are visible using this method. The advantage of CTKUB over IVU is that it is more sensitive and can identify non-radio-opaque stones(uric acid and cysteine). Ultrasound can show stones and dilatation of the renal pelvis and ureter if the stone is obstructing urine flow. The yield of more detailed investigation is low, and hence usually reserved for young patients, those with recurrent or multiple stones, or those with complicated. Chemical analysis of stones is often helpful in defining the underlying cause. Since most stones pass spontaneously through the urinary tract, urine should be sieved for a few days after colic in order to collect the calculus for analysis.
1The most valuable test if a stone can be obtained.
2Only if serum calcium or urinary calcium excretion high.Investigations for renal stones
Management
The immediate treatment is analgesia and antiemetics. Renal colic is often unbearably painful and demands powerful analgesia; diclofenac orally or as a suppository (100 mg) is often very effective, followed by morphine (10–20 mg) or pethidine (100 mg) intramuscularly. Around 90% of stones of < 4 mm diameter pass spontaneously, but only 10% of stones> 6 mm, and these may require endoscopic surgical intervention . Patients with renal stones are at high risk of infection; if surgery is contemplated, it should be covered with appropriate antibiotics. Immediate action is required if infection occurs in the stagnant urine proximal to the stone (pyonephrosis), and in patients with a solitary kidney who develop anuria in association with a stone in the ureter.Stones that do not pass spontaneously may need to be removed surgically or fragmented by extracorporeal shock wave lithotripsy (ESWL).
Measures to prevent further stone formation are
guided by the investigations in Box.
Urate stones can be prevented by allopurinol, but its role in patients with calcium stones and high urate excretion is uncertain. Stones formed in cystinuria can be reduced by penicillamine therapy.
It may also be helpful to attempt to alkalinise the urine
with sodium bicarbonate, as a high pH discourages
urate and cystine stone formation.
*Procedure depends on site of stone (ESWL = extracorporeal
shock wave lithotripsy; PCNL = percutaneous nephrolithotomy)Indications for intervention to remove
stones from the urinary tract
Measures to prevent calcium
stone formationExtracorporeal shock wave lithotripsy (ESWL).
Options for removal of urinary stones. A A patient undergoing extracorporeal shock wave lithotripsy (ESWL). B The procedures that are used for removal of stones in the urinary tract, shown in relation to the site of the stone. Very rarely, open or laparoscopic surgery may be necessary for removal of stones in the upper renal tract, if other methods fail. (PCNL = percutaneous nephrolithotomy)
ISOLATED DEFECTS OF TUBULAR FUNCTION
Renal glycosuria is a benign autosomal recessive defect of tubular reabsorption of glucose, caused by mutations of the sodium/ glucose co-transporter SGLT2. Glucose appears in the urine in the presence of a normal blood glucose concentration.Cystinuria is a rare condition, in which reabsorption of filtered cystine, ornithine, arginine and lysine is defective. It is caused by mutations in the SLC3A1 amino acid transporter gene. The high concentration of cystine in urine leads to cystine stone formation .
Hereditary hypophosphataemic rickets , in which reabsorption of filtered phosphate is reduced.
Nephrogenic diabetes insipidus in which the tubules are resistant to the effects of vasopressin.
Bartter’s and Gitelman’s syndromes, in which there is sodium-wasting and hypokalaemia .
‘Fanconi syndrome’ is used to describe generalised proximal tubular dysfunction, presents with low blood phosphate and uric acid, glycosuria, amino aciduria and proximal renal tubular acidosis. In addition to the causes of interstitial nephritis described above, some congenital metabolic disorders are associated with Fanconi syndrome, notably Wilson’s disease, cystinosis and hereditary fructose intolerance.
Renal tubular acidosis describes the common endpoint of a variety of diseases affecting distal (classical or type 1) or proximal (type 2) renal tubular function.
DISEASES OF THE COLLECTING SYSTEM AND URETERS
Congenital abnormalitiesVarious congenital anomalies of the urinary tract can occur.
Single kidneys
Ureterocele
A ureterocele occurs behind a pin-hole ureteric orifice
when the intramural part of the ureter dilates and bulges
into the bladder. It can become very large and cause
lower urinary tract obstruction. Incision of the pin-hole
opening relieves the obstruction.
Ectopic ureters and duplex kidneys
Ectopic ureters occur with congenital duplication of one
or both kidneys (duplex kidneys).
Obstructive megaureter
In primary obstructive megaureter, there is dilatation of
the ureter in all but its terminal segment without obvious
cause and without vesico-ureteric reflux. Narrowing of
the ureter and re-implantation may be necessary.
Pelvi-ureteric junction obstruction
This causes idiopathic hydronephrosis and results from
a functional obstruction at the junction of the ureter and
renal pelvis. The abnormality is likely to be congenital
and is often bilateral. The common presentation is ill-defined renal pain or ache, exacerbated by drinking large volumes of liquid (Dietl’s crisis). Treatment is surgical excision of the PUJ and re-anastomosis (pyeloplasty), which can now be performed laparoscopically.
Congenital abnormalities of the urinary tract.
Retroperitoneal fibrosisFibrosis encircle and compress the ureter(s), causing obstruction. The fibrosis is most commonly idiopathic, but can represent a reaction to infection, radiation or aortic
aneurysm, or be caused by cancer or a drug reaction –
methysergide, for example. Patients usually present
with symptoms of ureteric obstruction. There is an acute-phase response (high CRP and ESR). Imaging with UVI or CT shows ureteric obstruction .
Idiopathic retroperitoneal fibrosis responds well to corticosteroids and may respond more slowly to tamoxifen, but ureteric stenting is often necessary.
INFECTIONS OF THE URINARY TRACT
In health, bacterial colonisation is confined to the lower
end of the urethra and the remainder of the urinary tract
is sterile . The urinary tract can become infected with various bacteria but the most common is E. coli derived from the gastrointestinal tract.
The most common presenting problem is cystitis with urethritis (generally referred to as urinary tract infection) but this is only part of a spectrum of severity .
The spectrum of presentations of urinary tract infection
• Asymptomatic bacteriuria
• Symptomatic acute urethritis and cystitis
• Acute pyelonephritis
• Acute prostatitis
• Septicaemia (usually Gram-negative bacteria
Urinary tract infection (UTI)
Term used to describe acute urethritis and cystitis caused by a microorganism. The prevalence in women is about 3%. In males, UTI is uncommon, except in the first year in men over 60, complicate bladder outflow obstruction.Pathophysiology
Urine is an excellent culture for bacteria; urothelium of susceptible persons may have more receptors, to which virulent strains of E. coli become adherent. In women, the ascent of organisms into the bladder is easier than in men; the urethra is shorter and the absence of bactericidal prostatic secretions may be relevant. Sexual intercourse may cause minor urethral trauma and transfer bacteria from the perineum into the bladder. Instrumentation of the
bladder may also introduce organisms.
Clinical features
Typical features of cystitis and urethritis include:• abrupt onset of frequency of micturition and urgency
• scalding pain in the urethra during micturition (dysuria)
• suprapubic pain during and after voiding
• intense desire to pass more urine after micturition,
due to spasm of the inflamed bladder wall (strangury)
• urine may appear cloudy and have an unpleasant odour
• microscopic or visible haematuria.
Systemic symptoms are usually slight or absent. However, acute pyelonephritis is suggested by prominent systemic symptoms with fever, rigors, vomiting, hypotension and loin pain, guarding or tenderness. Prostatitis is suggested by perineal or suprapubic pain, pain on ejaculation and prostatic tenderness on rectal examination.
The differential diagnosis of lower urinary tract symptoms includes urethritis due to sexually transmitted disease, notably chlamydia or Reiter’s syndrome . Some patients, usually female, have symptoms suggestive of urethritis and cystitis but no bacteria are cultured from the urine (the ‘urethral syndrome’). Possible explanations include infection with organisms not readily cultured by ordinary methods (such as Chlamydia and certain anaerobes), intermittent or low-count bacteriuria, reaction to toiletries or disinfectants,
symptoms related to sexual intercourse, or
post-menopausal atrophic vaginitis.
The differential diagnosis of acute pyelonephritis
includes pyelonephrosis, acute appendicitis, diverticulitis,
cholecystitis, salpingitis, ruptured ovarian cyst or
ectopic pregnancy.
Investigations
In an otherwise healthy woman with a single lower UTI, urine culture prior to treatment is not mandatory. Investigation is necessary, however,in patients with recurrent infection or after failure of initial treatment, pregnancy, or immunocompromised, those with diabetes or an indwelling catheter, and older people . Most urinary pathogens can reduce nitrate to nitrite, and neutrophils and nitrites can usually be detected in symptomatic infections by urine dipstick tests for leucocyte esterase and nitrite, respectively.The absence of both nitrites and leucocyte esterase in the
urine makes UTI unlikely.
Urine taken by suprapubic aspiration should be sterile, so the presence of any organisms is significant. If the patient has symptoms and there are neutrophils in the urine, a small number of organisms is significant. In asymptomatic patients, more than 105 organisms/mL is usually regarded as significant (asymptomatic bacteriuria). Typical organisms causing UTI in the community include E. coli derived from the GIT (about 75% of infections), Proteus spp., Pseudomonas spp., streptococci and Staphylococcus epidermidis. In hospital, E. coli still predominates, but Klebsiella or streptococci are more common.
Management
Antibiotics are recommended in all cases of proven UTI .For lower urinary tract infection, treatment for 3 days is
the norm and is less likely to induce significant alterations
in bowel flora than more prolonged therapy. Trimethoprim
is the usual choice for initial treatment; however, between 10% and 40% of organisms causing UTI are resistant. Nitrofurantoin, quinolone antibiotics such as ciprofloxacin and norfloxacin, and cefalexin are also generally effective. Co-amoxiclav or amoxicillin should only be used when the organism is known to be sensitive.
Penicillins and cephalosporins are safe to use in pregnancy but trimethoprim, sulphonamides, quinolones and tetracyclines should be avoided.
In more severe infection, antibiotics should be continued
for 7–14 days. Seriously ill patients may require
intravenous therapy with a cephalosporin, quinolone or
gentamicin for a few days , later switching to an oral agent.
A fluid intake of at least 2 L/day is usually recommended,
although this is not based on evidence and may
make symptoms of dysuria worse.
Risk factors for urinary tract infection
Investigation of patients with urinary tract infection
Urinary infection in old age
Antibiotic regimens for UT infection in adults1
Persistent or recurrent UTIIf the causative organism persists on repeat culture
despite treatment, or if there is re-infection with any
organism after an interval, then an underlying cause
is more likely to be present and more detailed investigation is justified .
In women, recurrent infections are common and investigation is only justified if infections are frequent
(three or more per year) or unusually severe. Recurrent UTI, particularly in the presence of an underlying cause, may result in permanent renal damage, whereas uncomplicated infections rarely (if ever) do so (see chronic
reflux nephropathy).
If an underlying cause cannot be treated, suppressive
antibiotic therapy can be used to prevent recurrence and reduce the risk of septicaemia and renal damage. Urine should be cultured at regular intervals; a regime of two or three antibiotics in sequence, rotating every 6 months, is often used in an attempt to reduce the emergence of resistant organisms.Prophylactic measures to be adopted by
women with recurrent urinary infectionsAsymptomatic bacteriuria
This is defined as more than 105 organisms/mL in the
urine of apparently healthy asymptomatic patients.
Approximately 1% of children under the age of one, 1%
of schoolgirls, 0.03% of schoolboys and men, 3% of nonpregnant adult women and 5% of pregnant women have asymptomatic bacteriuria. There is no evidence that this condition causes renal scarring in adults who are not pregnant and have a normal urinary tract and, in general, treatment is not indicated. However, up to 30% will develop symptomatic infection within 1 year.
Treatment is required in infants, pregnant women and those with urinary tract abnormalities.
Catheter-related bacteriuria
In patients with a urethral catheter, bacteriuria increasesthe risk of Gram-negative bacteraemia fivefold. Bacteriuria is common.
Treatment is usually avoided in asymptomatic patients, as this may promote antibiotic resistance.
Careful sterile insertion technique is important, and the catheter should be removed as soon as it is not required.
Acute pyelonephritis
The kidneys are infected in a minority of patients withUTI. Acute renal infection (pyelonephritis) presents
as a classic triad of loin pain, fever and tenderness
over the kidneys. The renal pelvis is inflamed and small
abscesses are often evident in the renal parenchyma .
Renal infection is almost always caused by organisms
ascending from the bladder, and the bacterial profile is
the same as for lower urinary tract infection .
Rarely, bacteraemia may give rise to renal or perinephric
abscesses, most commonly due to staphylococci. Predisposing factors, such as cysts or renal scarring, facilitate infection.
Rarely, acute pyelonephritis is associated with papillary
necrosis. They may cause ureteric obstruction.
Predisposing factors include diabetes mellitus, chronic
urinary obstruction, analgesic nephropathy and sickle cell
disease. A necrotising form of pyelonephritis with
gas formation, ‘emphysematous pyelonephritis’, is occasionally seen in patients with diabetes mellitus. Intravenous rehydration may be needed in severe cases.
If complicated infection is suspected or response to treatment is not prompt, urine should be recultured and
renal tract ultrasound performed to exclude urinary
tract obstruction or a perinephric collection. If obstruction
is present, drainage by a percutaneous nephrostomy
or ureteric stent should be considered.
Tuberculosis
Tuberculosis of the kidney and renal tract is secondaryto tuberculosis elsewhere and is the result of
blood-borne infection. Initially, lesions develop in the
renal cortex; these may ulcerate into the renal pelvis and
involve the ureters, bladder, epididymis, seminal vesicles
and prostate. Calcification in the kidney and stricture
formation in the ureter are typical.
Clinical features may include symptoms of bladder
involvement (frequency, dysuria); haematuria (sometimes
macroscopic); malaise, fever, night sweats, lassitude
and weight loss; loin pain; associated genital
disease; and chronic renal failure as a result of urinary
tract obstruction or destruction of kidney tissue.
Neutrophils are present in the urine but routine urine
culture may be negative (‘sterile pyuria’). Special techniques of microscopy and culture may be required to
identify tubercle bacilli and are most usefully performed
on early morning urine specimens. Bladder involvement
should be assessed by cystoscopy. Radiology of the
urinary tract and a chest X-ray to look for pulmonary
tuberculosis are mandatory. Anti-tuberculous chemotherapy follows standard regimes .
Surgery to relieve urinary tract obstruction or to remove a very severely infected kidney may be required.
BENIGN PROSTATIC ENLARGEMENT
Benign prostatic enlargement (BPE) is extremely common. It has been estimated that about half of all men aged 80 years and over will have lower urinary tract symptoms associated with BPE. Benign prostatic hyperplasia (BPH) is the histological abnormality that underlies BPE.Pathophysiology
The prostate gland increases in volume by 2.4 cm3 per
year on average from 40 years of age. The process begins in the peri-urethral (transitional) zone and involves both glandular and stromal tissue to a variable degree. The cause is unknown, although BPE does not occur in
patients with hypogonadism, suggesting that hormonal
factors may be important.
Clinical features
The primary symptoms of BPE arise because of difficultyin voiding urine due to obstruction of the urethra by the
prostate; they consist of hesitancy, poor urinary flow
and a sensation of incomplete emptying. Other symptoms
include urinary frequency, urgency of micturition
and urge incontinence, although these are not specific
to BPE. Some patients present suddenly with acute
urinary retention, when they are unable to micturate
and develop a painful distended bladder.
This is often precipitated by excessive alcohol intake, constipation and prostatic infection.
Patients may also present with chronic urinary retention.
Here, the bladder slowly distends due to inadequate
emptying over a long period of time. Patients
with chronic retention can also develop acute retention:
so-called acute-on-chronic retention. This condition is
characterised by pain-free bladder distension, which
may result in hydroureter, hydronephrosis and renal
failure (high-pressure chronic retention). On digital
rectal examination, patients with BPE have evidence of
prostatic enlargement with a smooth prostate gland.
Abdominal examination may also reveal evidence
of bladder enlargement in patients with urinary retention.
The International Prostate Symptom Score (IPSS)
The International Prostate Symptom Score (IPSS) 'cont'd
Investigations
The diagnosis of BPE is a clinical one but flow rates canbe accurately measured with a flow meter, and prostate
volume by transrectal ultrasound scan (TRUS). Objective
assessment of obstruction is possible by urodynamics
but this is seldom required. If symptoms or signs,
such as a palpable bladder, nocturnal enuresis, recurrent
urinary tract infections or a history of renal stones, are
present, renal function should be assessed; if it is abnormal, screening should be conducted for evidence of
obstructive nephropathy by ultrasound examination.
Management
Patients who present with acute retention require urgenttreatment and should undergo immediate catheterisation
to relieve the obstruction. Those with mild to moderate
symptoms can be treated by medication (Boxes ).
The first-line treatments are
α1A-adrenoceptor blockers such as tamsulosin, which reduce the tone of smooth muscle cells in the prostate and bladder neck, thereby reducing the obstruction.
The 5α-reductase inhibitors, finasteride and dutasteride, inhibit conversion of testosterone to the more potent dihydrotestosterone in the prostate and so cause the
prostate to shrink.
This class of drugs is indicated in patients with an estimated prostate size of more than 30 g or a prostate specific antigen (PSA) of more than 1.4 μg/L. Patients who fail to respond to a single drug may be treated with a combination of α-blockers and 5α-reductase inhibitors, since this is more efficacious than either agent alone. Symptoms that are resistant to medical therapy require surgical treatment to remove some of the prostate tissue that is causing urethral obstruction.
This is usually achieved by transurethral resection of the prostate (TURP) but enucleation of the prostate by holmium laser is equally effective and has potentially fewer complications. Open surgery is rarely needed, unless the gland is very large.
Treatment for benign prostatic enlargement
Medical management of benign prostatic enlargement
ProstatitisThis condition is due to inflammation of the prostate .
Acute or chronic bacterial prostatitis can be caused by infection with the same bacteria that cause UTI, but can also be ‘nonbacterial’. This is also known as chronic pelvic pain syndrome. Clinical features of prostatitis include frequency, dysuria, painful ejaculation, perineal or groin pain, difficulty passing urine.
The prostate is enlarged and tender. Bacterial prostatitis is confirmed by a positive culture from urine or from urethral discharge obtained after prostatic massage, and the treatment of choice is trimethoprim or a quinolone.
A 4–6-week course is required . Treatment of chronic pelvic pain syndrome is challenging but some respond to a combination of α-blockers, NSAIDs and amitriptyline.
Renal adenocarcinoma
Is by far the most common malignant tumour of the kidney in adults, making up 2.5% of all adult cancers. It is twice as common in males as in females. The peak incidence is between 65 and 75 years of age. The tumour arisesfrom renal tubular cells. Haemorrhage and necrosis give
the cut surface a characteristic mixed golden-yellow and
red appearance . Microscopically, clear cell are the most common (85%), with papillary, chromophobe and collecting duct tumours 15%. With clear cell carcinomas, there is early spread of the tumour into the renal pelvis, causing haematuria, and along the renal vein into the inferior vena cava. Direct invasion is common.
Lymphatic to para-aortic nodes, while blood-borne metastases in the lungs, bone and brain.
Clinical features
In 50% of patients, asymptomatic renal tumours are
identified as an incidental finding during imaging investigations carried out for other reasons. Amongst symptomatic patients, about 60% present with haematuria,
40% with loin pain and 25% with a mass;
about 10% present with a triad
of pain, haematuria and a mass. A remarkable range of systemic effects may be present, including fever, raised ESR, polycythaemia, disorders of coagulation, hypercalcaemia, and abnormalities of plasma proteins and liver function tests.
The patient may present with pyrexia of unknown origin or, rarely, with neuropathy.
Some of these systemic effects are caused by secretion of products by the tumour, such as renin, erythropoietin, PTH-related protein (PTHrP) and gonadotrophins.
The effects disappear when the tumour is removed but may re-appear when metastases develop, and so can be used as markers of tumour activity.
Investigations
US allows differentiation between solid tumour and simple cysts. If the results are suggestive of a tumour, contrast-enhanced CT of the abdomen and chest should be performed for staging . For tumours >3 cm in diameter with no evidence of metastatic spread and when the nature of the lesion is uncertain, US or CT-guided biopsy may be used to avoid nephrectomy for benign disease.
Management
Radical nephrectomy that includes the perirenal fascialenvelope and ipsilateral para-aortic lymph nodes is the
treatment of choice ,commonly performed laparoscopically. Partial nephrectomy is recommended for tumours of 4 cm or less. Patients at high operative risk, who have small tumours, may be treated percutaneously by cryoablation or radiofrequency ablation. Surgery may also play a role in the treatment of solitary metastases, since these can remain single for long periods. It is resistant to most agents. For many years, systemic cytokine, interferon and interleukin-2 was used but in recent years tyrosine kinase inhibitors, sunitinib and pazopanib, and the mammalian target of rapamycin (mTOR) inhibitors, temsirolimus and everolimus , are now the mainstay of therapy.
Urothelial tumours
Tumours arising from the transitional epithelium of therenal tract can affect the renal pelvis, ureter, bladder or
urethra. They are rare under the age of 40, affect males
about 3–4 times as commonly as females. The bladder
is by far the most frequently affected site. Although
almost all tumours are transitional cell carcinomas,
squamous carcinoma may occur in urothelium that has
undergone metaplasia, following chronic inflammation or irritation due to stones or schistosomiasis. Risk factors include cigarette smoking and exposure to carcinogens like aromatic amines, aniline dyes and aldehydes.
Transitional cell carcinoma of the bladder. Stages are
shown from carcinoma in situ (Cis) to invasive tumour progressing beyond the bladder and prostate (T4b).Clinical features
> 80% present with painless, visible haematuria, symptoms of obstruction, and tumours of the bladder present with dysuria or storage symptoms. Physical examination is usually unremarkable, except in very advanced, when bimanual examination may reveal a palpable mass. InvestigationsCystoscopy is mandatory to evaluate the bladder in
cases of haematuria or suspected bladder cancer.
Imaging of the upper urinary tract (CT urogram is the
gold standard but IVU and renal ultrasound are also
acceptable). If evidence of a solid invasive urothelial tumour is found, CT of the abdomen, pelvis and chest should be performed as a staging procedure.
Management
Most bladder tumours are low-grade superficial lesionsthat can be successfully treated endoscopically by
transurethral resection of the tumour. Intravesical chemotherapy with mitomycin C is usually administered
as a one-off treatment post resection to prevent
tumour recurrence, or may be given as a prolonged
course to treat multiple low-grade bladder tumours.
Patients with carcinoma in situ have a high risk of progression to invasive cancer. These patients often respond well to intravesical bacille Calmette–Guérin (BCG) treatment but more radical treatment may also be needed if this is unsuccessful.
The management of invasive bladder tumours involves radical cystectomy with urinary diversion into an incontinent ileal conduit or a continent catheterisable bowel pouch; the latter is usually reserved for patients under the age of 70 years.
Overall, the 5-year survival for patients with muscle-invasive bladder cancer is about 50–70%.
Transitional cell carcinoma of the renal pelvis and
ureter is usually treated by open or laparoscopic nephroureterectomy, but if the tumour is solitary and lowgrade, it can be treated endoscopically.
Prostate cancer
Prostate cancer is the most common malignancy in menin the UK . It rarely occurs before the age of 50 and has
a mean age at presentation of 70 years.
Pathophysiology
Prostate cancers tend to arise within the peripheral zone
of the prostate and almost all are adenocarcinomas.
Metastatic spread to pelvic lymph nodes occurs early
and metastases to bone, mainly the lumbar spine and
pelvis, are common.
Genetic factors are known to play an important role. A family history of prostate cancer greatly increases a man’s chances of developing the disease.
Clinical features
Most patients either are asymptomatic or present withlower urinary tract symptoms indistinguishable from
benign prostatic enlargement. On digital rectal examination (DRE), the prostate may feel nodular and stony-hard, and the median sulcus may be lost, but up to 45% of tumours are impalpable. Symptoms and signs due to metastases are much less common at the initial presentation but may include back pain, weight loss, anaemia and obstruction of the ureters.
Investigations
Measurement of PSA levels in a peripheral blood sample,
together with DRE, is the cornerstone of diagnosis.
Prior to a PSA test, men should be given careful counselling
about the limitations of the test: namely, a normal level
does not exclude prostate cancer; a high value does not
confirm the diagnosis but will open a discussion about
biopsy. The need for radical treatment of localised prostate cancer is still not established; radical treatments
have significant potential morbidity and mortality; and
early identification and treatment of prostate cancer may
save lives. Current evidence suggests that population based screening for prostate cancer with PSA is of limited value, due in part to the fact that over 1000 patients would need to be screened to cure one man of prostate cancer. Individuals suspected of having prostate cancer, based on an elevated PSA and/or abnormal DRE, should undergo transrectal ultrasound-guided prostate biopsies.
Occasionally, a small focus of tumour is found incidentally in patients undergoing transurethral resection of the prostate for benign hyperplasia.
If the diagnosis of prostate cancer is confirmed, staging should be performed by pelvic MRI to assess the presence and extent of local involvement.
An isotope bone scan should be carried out if distant
metastases are suspected (rare if the PSA is below 20 ng/
mL); very high levels of serum PSA (> 100 ng/mL)
almost always indicate distant bone metastases.
Following diagnosis, serial assessment of PSA is useful for monitoring response to treatment and progression.
Management
Tumour confined to the prostate is potentially curableby radical prostatectomy, radical radiotherapy or
brachytherapy (implantation of small radioactive particles into the prostate). These only be considered in patients with >10 years’ life expectancy. Patients who have small foci of tumour do not require specific treatment but should be followed up periodically with PSA, DRE and a schedule of biopsies (active surveillance). Prostatic cancer, like breast cancer, is sensitive to steroid hormones; metastatic cancer is treated by androgen depletion, orchidectomy or androgen-suppressing drugs . Androgen receptor blockers, also prevent tumour cell growth. GnRH analogues, occupy pituitary receptors, preventing them from responding to the GnRH pulses that normally stimulate LH and FSH release.
This initially causes an increase in testosterone before producing a prolonged reduction, and for this reason the initial dose must be covered with an androgen receptor blocker to prevent a tumour flare. Chemotherapy with docetaxel can then be effective and provide a modest (around 3 months) survival advantage. Radiotherapy is useful for localised bone pain but the basis of treatment remains pain control by analgesia .
Provided that patients do not die of another cause, the
10-year survival rate of patients with tumours localised
to the prostate is 95%, but if metastases are present, this falls to 10%.
Life expectancy is not reduced with small foci of tumour.
Hormone manipulation in prostate cancer
Testicular tumoursUncommon. They occur mainly in young men aged between 20 -40 years. They often secrete α-fetoprotein (AFP) and β-human chorionic gonadotrophin (β-hCG), which are useful biochemical markers for diagnosis and prognosis.
Seminoma and teratoma account for 85% of all tumours of the testis. Leydig cell tumours are less common.
Seminomas arise from seminiferous tubules and represent
a relatively low-grade malignancy. Metastases can occur through lymphatic spread and typically involve the lungs. Well-differentiated tumours are the least aggressive, trophoblastic teratoma is highly malignant.
Occasionally, teratoma and seminoma occur together. Leydig cell tumours are small and benign, secrete oestrogens, leading to presentation with gynaecomastia .
Clinical features and investigations
The common presentation is incidental discovery of a
painless testicular lump, although some complain
of a testicular ache. All suspicious scrotal lumps should be imaged by US . Serum levels of AFP and β-hCG are elevated in extensive disease.Oestradiol may be elevated, suppressing LH, FSH and testosterone. Accurate staging is based on CT of the lungs, liver and retroperitoneal area.
Management and prognosis
The primary treatment is surgical orchidectomy. Subsequent treatment depends on the histological type and
stage. Radiotherapy is the treatment of choice for early-stage seminoma. Teratoma confined to the testes may be managed conservatively, but more advanced cancers are treated with chemotherapy, usually the combination of
bleomycin, etoposide and cisplatin. Follow-up is by CT
and assessment of AFP and β-hCG. Retroperitoneal
lymph node dissection is now only performed for residual
or recurrent nodal masses. The 5-year survival rate for patients with seminoma is 90–95%. For teratomas, the 5-year survival varies between 60% and 95%, depending on tumour type, stage and volume.
RENAL INVOLVEMENT IN SYSTEMIC CONDITIONS
Diabetes mellitusDiabetes mellitus is the most common cause of CKD in
developed countries. In patients with diabetes, there is
a steady advance from microalbuminuria to dipstick-positive proteinuria, and a progression to renal failure .
Few patients require renal biopsy to establish the diagnosis.
Management with ACE inhibitors and other hypotensive
agents to slow progression and has been dramatically effective. In some, proteinuria may be eradicated and progression halted, even if renal function is abnormal.
Hepatic–renal disease
Severe hepatic dysfunction may cause a haemodynamicallymediated type of renal failure. Patients with chronic
liver disease are also predisposed to develop AKI (acute tubular necrosis) in response to relatively minor insults,
including bleeding and infection. Such patients are
often difficult to treat by dialysis and have a poor prognosis. Where treatment is justified – for example, if there is a good chance of recovery or of a liver transplant – slow or continuous treatments are less likely to precipitate
or exacerbate hepatic encephalopathy. IgA nephropathy is more common in patients with liver disease.
Pulmonary-renal syndrome
The pulmonary–renal syndrome is a dramatic presentation
with renal and respiratory failure that is not
explained by excess intravascular fluid or by severe
pneumonia; it occurs in Goodpasture’s (anti-GBM)
disease and small-vessel vasculitis (see below). There are
some other uncommon causes of a similar syndrome,
including poisoning with the herbicide paraquat.
Malignant diseases
Direct involvement of the kidneys or other parts of the urinary tract can occur, causing obstructive uropathy, and glomerulonephritis may occur, presumably as the result of an immunological reaction to the tumour. Hypercalcaemia can be caused by parathyroid hormone-related protein (PTHrP) production by tumours, whereas treatment of leukaemia and lymphoma can sometimes be complicated by interstitial nephritic and acute kidney injury due to uric acid release from necrotic tumour cells.Finally, in myeloma and other monoclonal gammopathies, renal impairment can occur as the result of the nephrotoxic effects of Ig- light chains released by the tumour.
Renal effects of malignancies
Systemic vasculitisSmall-vessel vasculitis commonly affects the kidneys, with rapid and profound impairment of glomerular function. Typically, the patient is systemically unwell with an acute phase response, weight loss and arthralgia. In some patients, pulmonary haemorrhage may occur, which can be life-threatening. The most important cause is antineutrophil cytoplasmic antibody (ANCA)-positive vasculitis . Polyangiitis with granulomatosis (Wegener’s granulomatosis)
typically presents with glomerulonephritis and
granulomatous lesions affecting the nasal passages and
lungs. Serological testing for myeloperoxidase (MPO)
and proteinase 3 (PR3) is usually positive but these antibodies are not specific and a biopsy of affected tissue
should be obtained if possible to confirm the diagnosis.
The standard treatment of glomerulonephritis associated
with systemic vasculitis is high-dose glucocorticoids
combined with pulse cyclophosphamide, or mycophenolate mofetil . Recent studies indicate that rituximab , when combined with high-dose steroids, is as effective as oral cyclophosphamide and high-dose steroids in the treatment of ANCA-associated vasculitis .
Plasma exchange can offer additional benefit in patients who are not responding adequately to immunosuppressive therapy. GN secondary to vasculitis may rarely also be seen in rheumatoid arthritis, SLE and cryoglobulinaemia. Medium- to large-vessel vasculitis, such as polyarteritis nodosa), does not cause GN but can cause hypertension, renal infarction or haematuria.
Role of rituximab in ANCA-associated
vasculitisSystemic lupus erythematosus
Subclinical renal involvement, with low-level haematuriaand proteinuria but minimally impaired or normal
renal function, is common in SLE. Usually, this is due to glomerular disease, although interstitial nephritis may also occur, particularly with overlap syndromes such as mixed connective tissue disease and Sjِgren’s syndrome .
Almost any histological pattern of glomerular disease
can be observed in SLE and the clinical presentation
ranges from florid, rapidly progressive glomerulonephritis
to nephrotic syndrome. In severely affected
patients, the most common histological pattern is a proliferative glomerulonephritis with substantial deposits
of immunoglobulins on immunofluorescence.
More recently, it has been shown that the combination of corticosteroids and mycophenolate mofetil is
as effective as corticosteroids and cyclophosphamide, at
least in short-term studies.
Many patients with SLE who develop ESRD go into
remission, possibly because of immunosuppression
related to the ESRD. Patients with ESRD caused by SLE
are usually good candidates for dialysis and transplantation.
Although it may recur in renal allografts, the
immunosuppression required to prevent allograft rejection
usually controls the SLE too.
PREGNANCY AND RENAL DISEASE
Pregnancy has important physiological effects on therenal system. Some diseases are more common in pregnancy, the manifestations of others are modified during pregnancy, and a few diseases, such as pre-eclampsia, are unique to pregnancy.
Physiological adaptations begin in the first few
weeks. Peripheral vascular resistance declines, blood
volume, cardiac output and GFR increase, and there is
usually a reduction in blood pressure and plasma creatinine and urea values in the first trimester.
Baseline blood pressure and urine testing from the first antenatal clinic visit are valuable if problems arise later.
Infections
Pyelonephritis is more common during pregnancy,perhaps because of dilatation of the urinary collecting
system and ureters.
It is important to treat asymptomatic bacteriuria during pregnancy, since this can progress to pyelonephritis, which, in turn, can trigger premature labour .
Treatment of asymptomatic bacteriuria in pregnancy
Glomerular diseases
Proteinuria caused by glomerular disease is usuallyexacerbated, and nephrotic syndrome may develop. This further increases the risk of venous thromboembolism, the leading cause of maternal deaths in developed countries. Pre-eclampsia
Pre-eclampsia is a systemic disorder that occurs in
or near the third trimester of pregnancy (triplets > twins
> singleton). The cause is unknown, although a number
of risk factors have been identified .
Clinical features
Defined by the triad of oedema, proteinuria and hypertension. However, oedema is common in late pregnancy, proteinuria is a late sign and, while hypertension is usually present.
It is important to distinguish pre-eclampsia from preexisting renal disease, since this affects management.
Pre-eclampsia presents progressively, increasing risks to
mother and fetus, which can be reversed almost immediately by early delivery.
In contrast, continuing the pregnancy for as long as possible in patients with preexisting renal disease, may permit delivery of a healthier, more mature baby.
Proteinuria and hypertension in the first trimester of pregnancy suggest pre-existing renal disease.
Management
The only effective management for pre-eclampsia isdelivery. The role of antiplatelet therapy (low-dose
aspirin) remains controversial. Hypertension is a consequence and not the cause of the disorder, and treatment is only justified to lower it from severe and immediately dangerous levels (> 150–160/100–110 mmHg). Treating lower levels has been shown to confer no benefit and exposes the fetus to additional drugs. If life-threatening complications are not present and the baby
is immature, corticosteroids may be given to induce
maturation of fetal lungs, and delivery postponed
while mother and baby are closely observed. Magnesium
sulphate reduces the incidence of eclamptic convulsions.
Acute kidney injury
Maternal AKI may occur in almost any of the preeclamptic
syndromes. Worldwide, a more important
cause of AKI is septic abortion, when the uterus becomes
infected because of retained products of conception or
poor sterility in an often illegally induced abortion.
Renal function is usually recoverable, but AKI in pregnancy
is particularly prone to progress to cortical necrosis,
with incomplete or total failure to recover renal function.
Pre-eclampsia and related diseases in pregnancy
Pre-eclampsia and related diseases
in pregnancy'cont'dAutoimmune diseases
Systemic autoimmune diseases typically are relatively
quiescent during pregnancy but tend to relapse in the
first few weeks and months following delivery. Preexisting
renal disease increases the fetal and maternal
risk involved in pregnancy, to a degree dependent on
the level of renal function, proteinuria and hypertension.
Patients with such diseases who may become pregnant
should be aware of the associated risks. During pregnancy,
therapy should not usually be stopped, but blood
pressure targets may be modified and agents altered to
those of proven safety.
Drug-induced renal disease
The kidney is susceptible to damage by drugs becauseit is the route of excretion of many water-soluble compounds, including drugs and their metabolites. Some
may reach high concentrations in the renal cortex. Others
are concentrated in the medulla. The same applies to certain toxins.Toxic renal damage may occur by a variety of mechanisms. Very commonly, drugs contribute to the development of acute tubular necrosis as one of multiple insults. Numerically, reactions to NSAIDs and ACE
inhibitors are the most important. Haemodynamic renal
impairment, acute tubular necrosis and allergic reactions
are usually reversible if recognised early enough.
Mechanisms and examples of drug-induced renal disease/dysfunction
Mechanisms and examples of drug-induced renal disease/ dysfunction'cont'd
ACE inhibitorsThese abolish the compensatory angiotensin II-mediated
vasoconstriction of the glomerular efferent arteriole that
takes place in order to maintain glomerular perfusion
pressure distal to a renal artery stenosis and in renal
hypoperfusion . Monitoring of renal function before and after initiation of therapy is essential.
NSAIDs
Impairment of renal function may develop since prostaglandins play an important role in regulating renal blood flow. This is particularly likely in patients with heart failure, cirrhosis, sepsis and pre-existing renal impairment. Idiosyncratic immune reactions may occur, causing minimal change nephrotic syndrome and acute interstitial nephritis .
Analgesic nephropathy is now a rare.
Prescribing in renal disease
Many drugs and drug metabolites are excreted by the
kidney and so the presence of renal impairment
(eGFR < 60 L/min)
Alters the required dose and frequency.