Hussien Mohammed Jumaah
CABMLecturer in internal medicine
Mosul College of Medicine
2016
learning-topics
Stroke diseaseCLINICAL EXAMINATION IN STROKE DISEASE
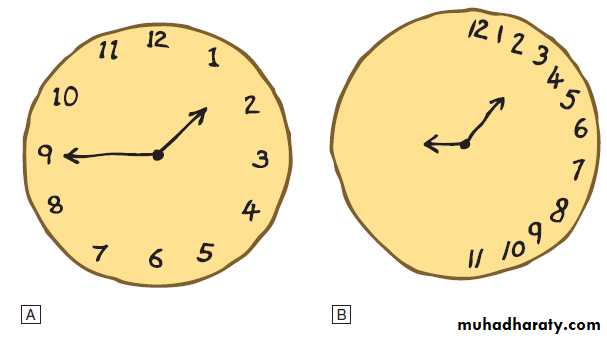
Clock drawing test
A Image drawn by doctor.B Image drawn by patient with left-sided neglect.
Cerebrovascular disease is the third most common cause
of death in high-income countries after cancers andischaemic heart disease, and the most common cause of
severe physical disability. It includes a range of disorders
of the central nervous system . Stroke is the most common clinical manifestation of cerebrovascular disease, and results in episodes of brain dysfunction due to focal ischaemia or haemorrhage.
Subarachnoid haemorrhage (SAH) and cerebral venous
thrombosis (CVT) will be discussed separately, since
their pathophysiology, clinical manifestations and management are distinct from those of stroke.
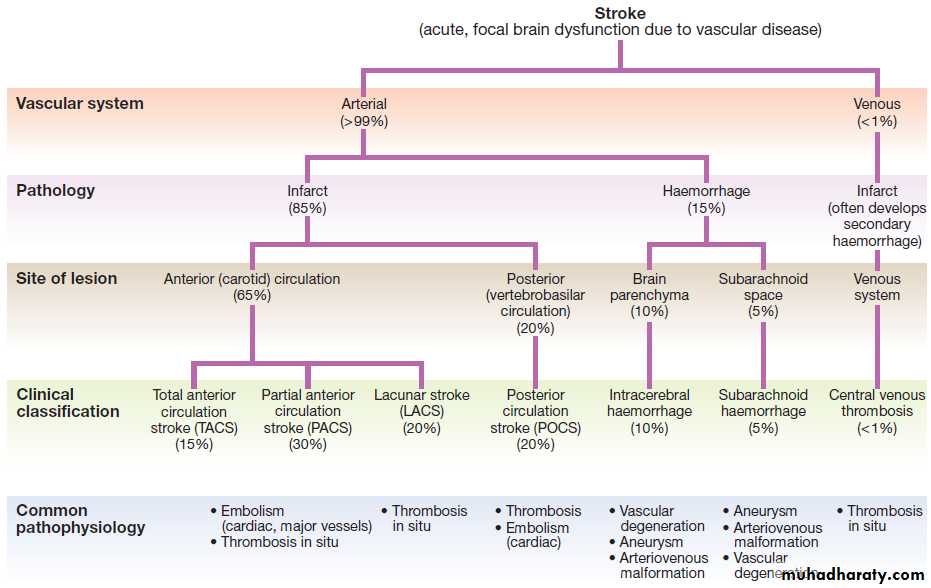
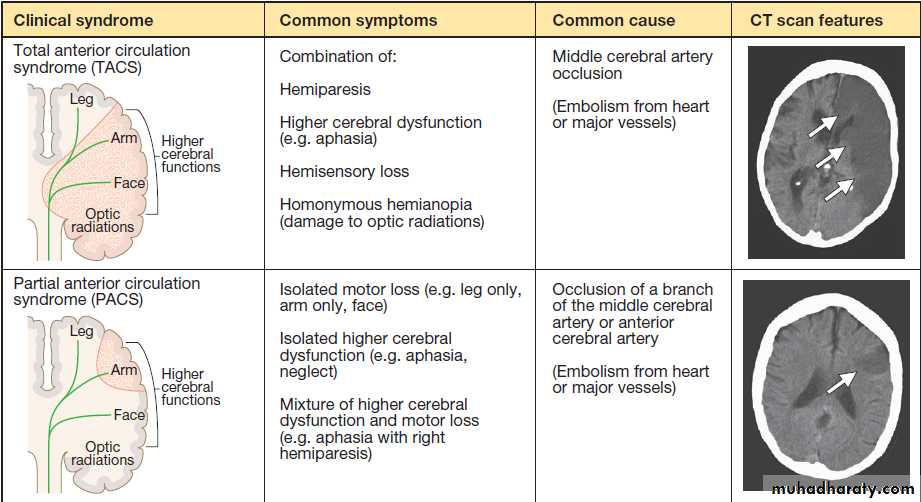
A classification of stroke disease.
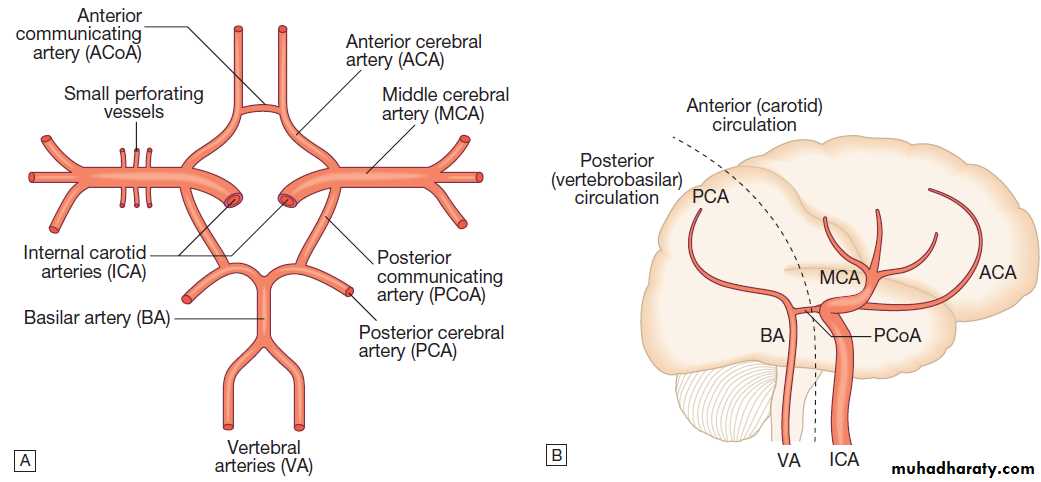
FUNCTIONAL ANATOMY AND PHYSIOLOGYThe main arterial supply of the brain comes from the
internal carotid arteries, which supply the anterior brain,
and the vertebral and basilar arteries (vertebrobasilar system), which provide the posterior circulation. The
anterior and middle cerebral arteries supply the frontal
and parietal lobes, while the posterior cerebral artery
supplies the occipital lobe. The vertebral and basilar arteries perfuse the brain stem, mid-brain and cerebellum .
Communicating arteries provide connections between the anterior and posterior circulations and between left and right hemispheres, creating protective anastomotic connections that form the circle of Willis.
In health, regulatory mechanisms maintain a constant cerebral blood flow across a wide range of arterial blood pressures to meet the high resting metabolic activity of brain tissue; cerebral blood vessels dilate when systemic blood pressure is lowered and constrict when it is raised. This autoregulatory mechanism can be disrupted after stroke.
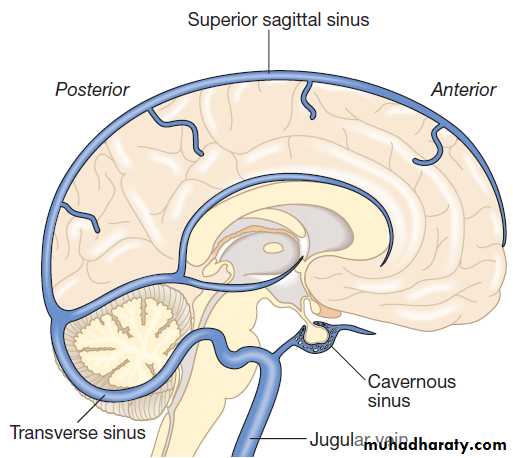
The venous collecting system is formed by a collection of sinuses over the surface of the brain, which drain into the jugular veins
Arterial circulation of the brain.
A Horizontal view. B Lateral view.Venous circulation of the brain.
INVESTIGATIONSA range of investigations may be required to answer
specific questions about brain structure and function
and about the function of the vascular system.
Neuroimaging
CT scanning is the mainstay of stroke imaging. It allows the rapid identification of intracerebral bleeding and stroke ‘mimics’ (i.e. pathologies other than stroke that have similar presentations), such as tumours. MRI is used when there is diagnostic uncertainty or delayed presentation, and when more information on brain structure and function is required.
Contraindications to MRI include cardiac pacemakers and
claustrophobia on entering the scanner.
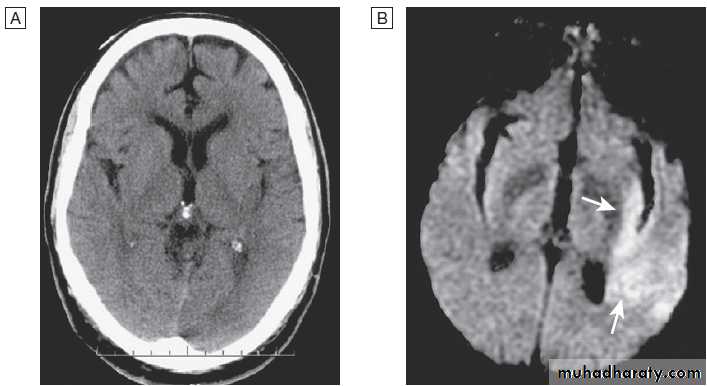
Acute stroke seen on CT scan with corresponding MRI appearance.
A CT may show no evidence of early infarction.B Corresponding image seen on MRI diffusion weighted imaging (DWI) with changes of infarction in middle cerebral artery (MCA) territory (arrows).
Vascular imaging
Various techniques are used to obtain images of extracranial and intracranial blood vessels . The
least invasive is ultrasound (Doppler or duplex scanning),
which is used to image the carotid and the vertebral
arteries in the neck. In skilled hands, reliable
information can be provided about the degree of arterial
stenosis and the presence of ulcerated plaques. Blood
flow in the intracerebral vessels can be examined using
transcranial Doppler. While the anatomical resolution is
limited, it is improving and many centres no longer
require formal angiography before proceeding to carotid
endarterectomy (see below).
Blood flow can also be detected by specialised sequences in MR angiography (MRA) but the anatomical resolution is still not comparable to that of intra-arterial angiography, which outlines blood vessels by the injection of radio-opaque contrast intravenously or intra-arterially. The X-ray
images obtained can be enhanced by the use of computer-assisted digital subtraction or spiral CT. Because of the significant risk of complications, intra-arterial contrast angiography is reserved for patients in whom non-invasive methods have provided a contradictory picture or incomplete information, or in whom it is necessary
to image the intracranial circulation in detail: for
example, to delineate a saccular aneurysm, an arteriovenous malformation or vasculitis.
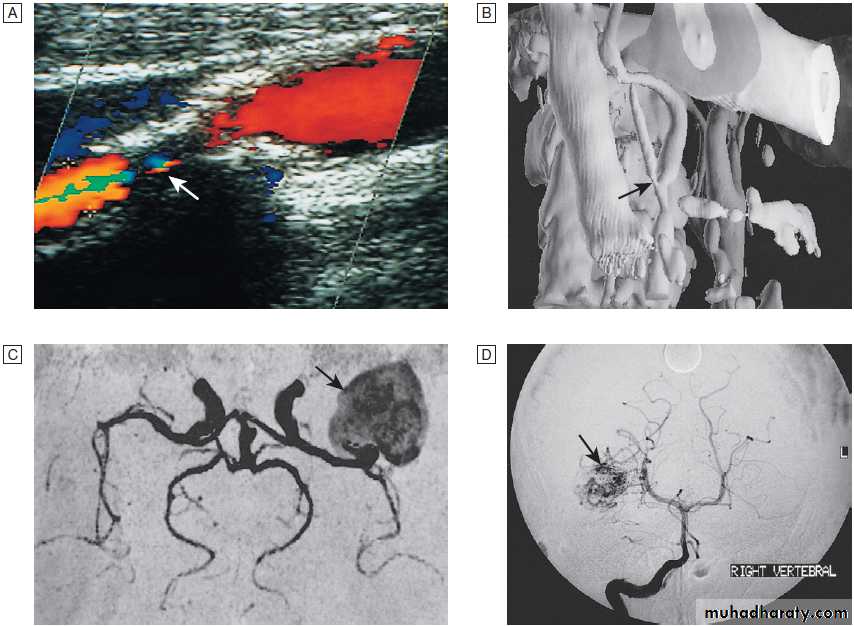
Fig. Different techniques of imaging blood vessels.
A Doppler scan showing 80% stenosis of the internal carotid artery (arrow).B Three-dimensional reconstruction of CT angiogram showing stenosis at the carotid bifurcation (arrow).
C MR angiogram showing giant aneurysm at
the middle cerebral artery bifurcation (arrow).
D Intra-arterial angiography showing arteriovenous malformation (arrow).
Blood tests
These help identify underlying causes of cerebrovasculardisease: for example, blood glucose (diabetes mellitus),
triglycerides and cholesterol (hyperlipidaemia) or
full blood count (polycythaemia) in stroke. Erythrocyte
sedimentation rate (ESR) and immunological tests, such
as measurement of antineutrophil cytoplasmic antibodies
(ANCA) may be required when vasculitis
is suspected. Genetic testing for rarer inherited
conditions, such as CADASIL (cerebral autosomal dominant
arteriopathy with subcortical infarcts and leucoencephalopathy), may be indicated.
Lumbar puncture
Lumbar puncture is largely reserved for theinvestigation of SAH.
Cardiovascular investigations
Electrocardiography and echocardiography may reveal abnormalities that may cause cardiac embolism in stroke.
PRESENTING PROBLEMS
Most vascular lesions develop suddenly within a matter
of minutes or hours, and so should be considered in the
differential diagnosis of patients with any acute neurological presentation.
Weakness
Unilateral weakness is the classical presentation of stroke and, much more rarely, of cerebral venous thrombosis.
The weakness is sudden, progresses rapidly and
follows a hemiplegic pattern .
There is rarely any associated abnormal movement. Reflexes are initially reduced but then become increased
with a spastic pattern of increased tone. Upper motor neuron weakness of the face (7th cranial nerve) is often present.
Speech disturbance
Dysphasia and dysarthria are the most common presentations of disturbed speech in stroke . Dysphasiaindicates damage to the dominant frontal or parietal
lobe , while dysarthria is a nonlocalising feature reflecting weakness or incoordination of the face, pharynx, lips, tongue or palate.
Visual deficit
Visual loss in stroke can be due to unilateral opticischaemia (called amaurosis fugax if transient), caused
by disturbance of blood flow in the internal carotid
artery and ophthalmic artery, which leads to monocular
blindness. Ischaemic damage to the occipital cortex or
post-chiasmic nerve tracts results in a contralateral
hemianopia .
Visuo-spatial dysfunction
Damage to the non-dominant cortex often results in contralateral visuo-spatial dysfunction, such as sensory or
visual neglect and apraxia (inability to perform complex
tasks despite normal motor, sensory and cerebellar function. This is sometimes misdiagnosed as acute confusion.
Ataxia
Stroke causing damage to the cerebellum and its connections can present as an acute ataxia and there may be associated brainstem features such as diplopia and vertigo .The differential diagnosis includes vestibular disorders .
Headache
Sudden severe headache is the cardinal symptom of
SAH but also occurs in intracerebral haemorrhage.
Although some degree of headache is common in acute
ischaemic stroke, it is rarely a dominant feature .
Headache also occurs in cerebral venous disease.
Seizure
Seizure is unusual in acute stroke but may be generalisedor focal in cerebral venous disease.
Coma
Coma is an uncommon feature of stroke, though it may
occur with a brainstem event. If it is present in the first
24 hours, it usually indicates a subarachnoid or intracerebral haemorrhage .
STROKE
Stroke is a common medical emergency. The incidencerises steeply with age, and in many lower- and middle income countries it is rising in association with less
healthy lifestyles.
About one-fifth of patients with an acute stroke die within a month of the event and at least half of those who survive are left with physical disability.
Pathophysiology
Of the 180–300 patients per 100 000 population presenting annually with a stroke, 85% sustain a cerebral infarction due to inadequate blood flow to part of the brain, and most of the remainder have an intracerebral haemorrhage .
Cerebral infarction
Cerebral infarction is mostly caused by thromboembolic
disease secondary to atherosclerosis in the major extracranial arteries (carotid artery and aortic arch). About 20% of infarctions are due to embolism from the heart, and a further 20% are due to thrombosis in situ caused by intrinsic disease of small perforating vessels (lenticulostriate arteries), producing so-called lacunar infarctions.
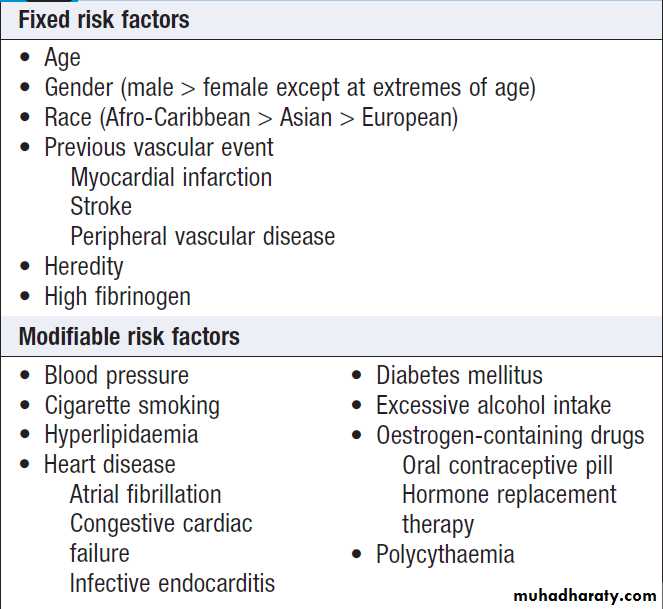
The risk factors for ischaemic stroke reflect the risk
factors for the underlying vascular disease . About 5% are due to rare causes, including vasculitis , endocarditis and cerebral venous disease (see below).
Cerebral infarction takes some hours to complete, even though the patient’s deficit may be maximal shortly after the vascular occlusion. After the occlusion of a cerebral artery, infarction may be forestalled by the opening of anastomotic channels from other arterial territories that restore perfusion to its territory.
Similarly, reduction in perfusion pressure leads
to compensatory homeostatic changes to maintain tissue
oxygenation . These compensatory changes can sometimes prevent occlusion of even a carotid artery from having any clinically apparent effect.
However, if and when these homeostatic mechanisms
fail, the process of ischaemia starts, and ultimatelyleads to infarction unless the vascular supply is restored.
As the cerebral blood flow declines, different neuronal
functions fail at various thresholds . Once blood flow falls below the threshold for the maintenance of electrical activity, neurological deficit develops. At this level of blood flow, the neurons are still viable; if the blood flow increases again, function returns and the patient will have had a transient ischaemic attack (TIA).
However, if the blood flow falls further, a level is reached
at which irreversible cell death starts.
Hypoxia leads to an inadequate supply of adenosine triphosphate (ATP), which leads to failure of membrane pumps, thereby allowing influx of sodium and water into the cell (cytotoxic oedema) and the release of the excitatory neurotransmitter glutamate into the extracellular fluid.
Glutamate opens membrane channels, allowing the
influx of calcium and more sodium into the neurons.
Calcium entering the neurons activates intracellular
enzymes that complete the destructive process.
The release of inflammatory mediators by microglia and
astrocytes causes death of all cell types in the area of
maximum ischaemia. The infarction process is worsened
by the anaerobic production of lactic acid and
consequent fall in tissue pH. There have been attempts
to develop neuroprotective drugs to slow down the
processes leading to irreversible cell death but so far
these have proved disappointing.
The final outcome of the occlusion of a cerebral blood
vessel therefore depends upon the competence of the
circulatory homeostatic mechanisms, the metabolic
demand, and the severity and duration of the reduction
in blood flow.
Higher brain temperature, as occurs in fever, and higher blood sugar have both been associated with a greater volume of infarction for a given reduction in cerebral blood flow. Subsequent restoration of blood flow may cause haemorrhage into the infarcted area
(‘haemorrhagic transformation’). This is particularly
likely in patients given antithrombotic or thrombolytic
drugs, and in patients with larger infarcts.
Radiologically, a cerebral infarct can be seen as a
lesion that comprises a mixture of dead brain tissue
that is already undergoing autolysis, and tissue that is
ischaemic and swollen but recoverable (the ‘ischaemic
penumbra’).
The infarct swells with time and is at its maximal size a couple of days after stroke onset.
At this stage, it may be big enough to exert mass effect both clinically and radiologically; sometimes, decompressive craniectomy is required (see below). After a few weeks, the oedema subsides and the infarcted area is replaced by a sharply defined fluid-filled cavity.
Risk factors for stroke
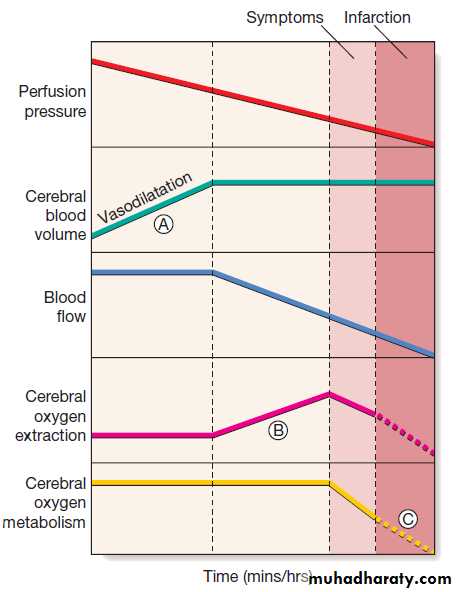
Homeostatic responses to falling perfusion pressure in the brain following arterial occlusion. Vasodilatation initially maintains cerebral blood flow (A), but after maximal vasodilatation further falls in perfusion pressure lead to a decline in blood flow. An increase in tissue
oxygen extraction, however, maintains the cerebral metabolic rate for oxygen (B). Still further falls in perfusion, and therefore blood flow, cannot be compensated; cerebral oxygen availability falls and symptoms appear, then infarction (C).
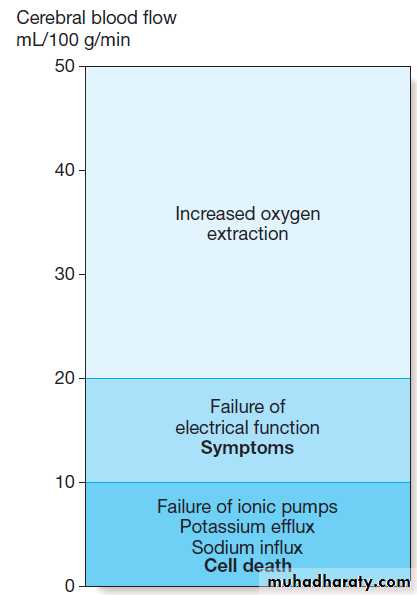
Thresholds of cerebral ischaemia. Symptoms of cerebral ischaemia appear when the blood flow has fallen to less than half of normal and energy supply is insufficient to sustain neuronal electrical function. Full recovery can occur if this level of flow is returned to normal but not if it is sustained.
Further blood flow reduction below the next threshold causes failure of cell ionic pumps and starts the ischaemic cascade, leading to cell death.
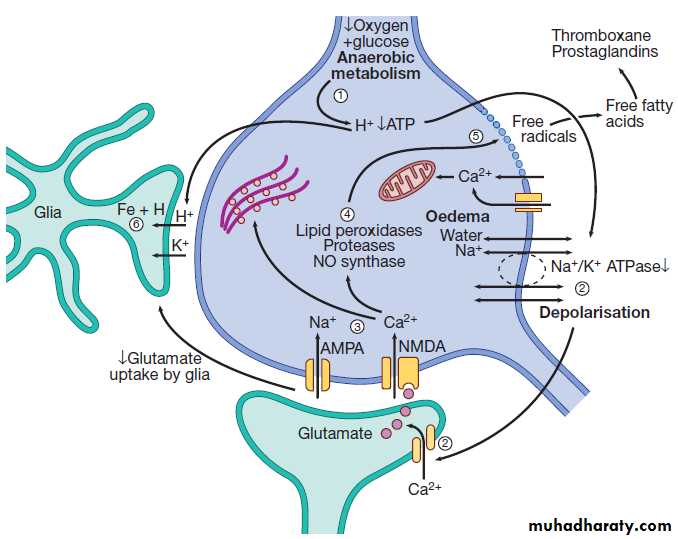
Fig. The process of neuronal ischaemia and infarction.
(1) Reduction of blood flow reduces supply of oxygen and henceATP. H+ is produced by anaerobic metabolism of available glucose. (2) Energy-dependent membrane ionic pumps fail, leading to cytotoxic oedema and membrane depolarisation, allowing calcium entry and releasing glutamate.
(3) Calcium enters cells via glutamate-gated channels and
(4) activates destructive intracellular enzymes (5), destroying intracellular organelles and cell membrane, with release of
free radicals. Free fatty acid release activates pro-coagulant pathways that exacerbate local ischaemia.
(6) Glial cells take up H+, can no longer take up extracellular glutamate and also suffer cell death, leading to liquefactive necrosis of whole arterial territory.
Intracerebral haemorrhage
Intracerebral haemorrhage causes about 10% of acutestroke events but is more common in low-income
countries. It usually results from rupture of a blood
vessel within the brain parenchyma but may also occur
in a patient with an SAH (see below) if the artery ruptures
into the brain substance as well as into the subarachnoid space. Haemorrhage frequently occurs into an area of brain infarction and, if the volume of haemorrhage is large, it may be difficult to distinguish from primary intracerebral haemorrhage both clinically and radiologically .The risk factors and underlying causes of intracerebral haemorrhage are listed in Box .
The explosive entry of blood into the brain parenchyma causes immediate cessation of function in that area as neurons are structurally disrupted and white-matter fibre tracts are split apart. The haemorrhage itself may expand over the first minutes or hours, or it may be associated with a rim of cerebral oedema, which, along with the haematoma, acts like a mass lesion to cause progression of the neurological deficit. If big enough, this can cause shift of the intracranial contents, producing transtentorial coning and sometimes rapid death . If the patient survives, the haematoma is gradually absorbed, leaving a haemosiderin-lined slit in the brain parenchyma.
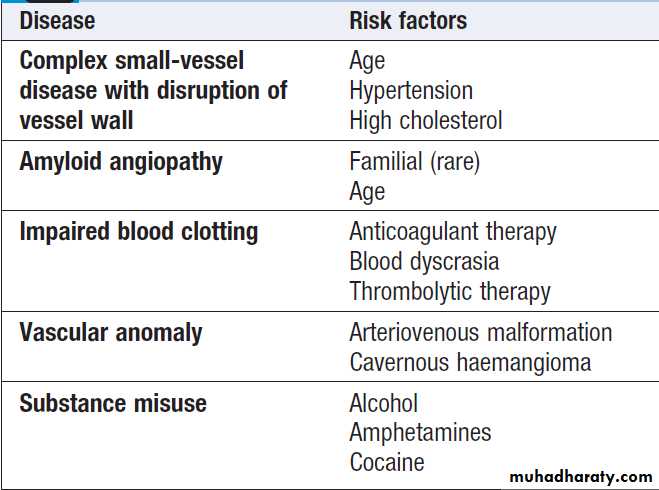
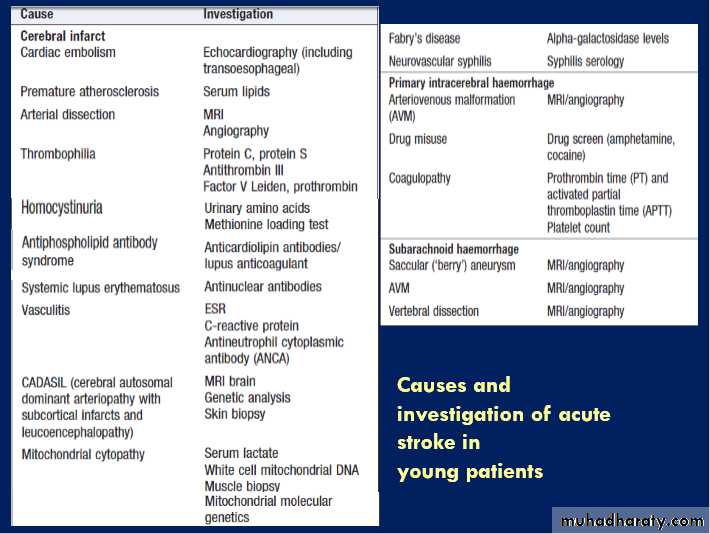
Causes of intracerebral haemorrhage and
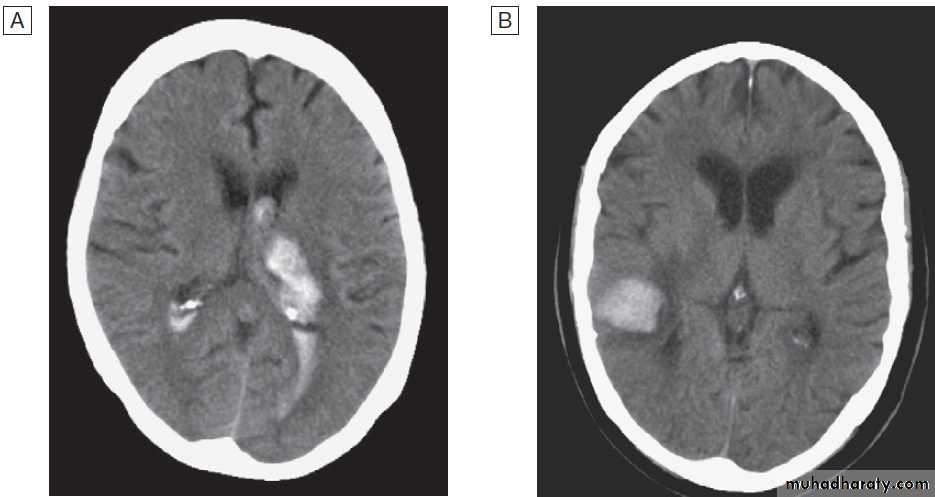
associated risk factorsCT scans showing intracerebral haemorrhage.
A Basal ganglia haemorrhage with intraventricular extension.B Small cortical haemorrhage.
Clinical features
Acute stroke and TIA are characterised by a rapid-onset,focal deficit of brain function. The typical presentation
occurs over minutes, affects an identifiable area of the
brain and is ‘negative’ in character (i.e. abrupt loss of
function without positive features such as abnormal
movement). Provided that there is a clear history of this,
the chance of a brain lesion being anything other than
vascular is 5% or less . If symptoms progress over hours or days, other diagnoses must be excluded.
Confusion and memory or balance disturbance are
more often due to stroke mimics. Transient symptoms,
such as syncope, amnesia, confusion and dizziness,
do not reflect focal cerebral dysfunction, but are often mistakenly attributed to TIA
Public health campaigns to raise awareness of the emergency nature of stroke exploit the fact that
weakness of the face or arm, or disturbance of speech is
the commonest presentation.
The clinical presentation of stroke depends upon
which arterial territory is involved and the size of the
lesion . These will both have a bearing on management, such as suitability for carotid endarterectomy.
The neurological deficit can be identified from the
patient’s history and (if it is persistent) the neurological
examination. The presence of a unilateral motor deficit,
a higher cerebral function deficit such as aphasia or
neglect, or a visual field defect usually places the lesion
in the cerebral hemisphere.
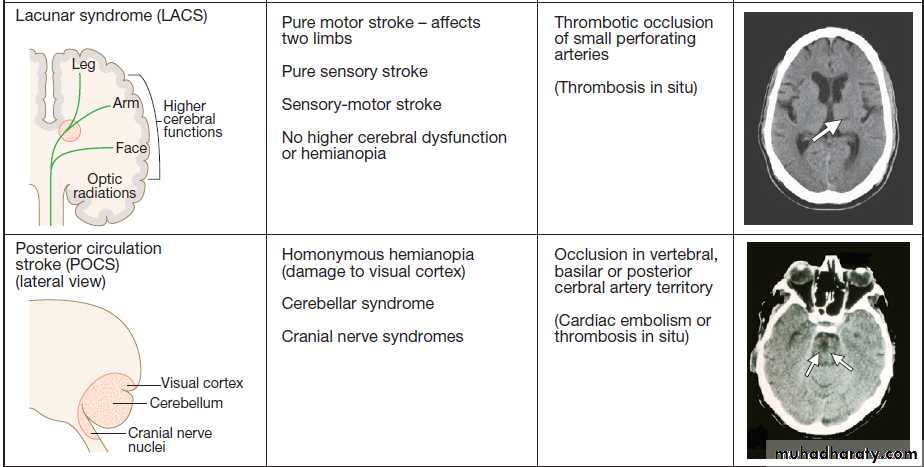
Ataxia, diplopia, vertigo and/or bilateral weakness usually indicate a lesion in the brainstem or cerebellum. Different combinations of these deficits define several stroke syndromes which reflect the site and size of the lesion and may provide clues to underlying pathology.
Reduced conscious level usually indicates a large volume
lesion in the cerebral hemisphere but may result
from a lesion in the brainstem or complications such as
obstructive hydrocephalus, hypoxia or severe systemic
infection. The combination of severe headache and vomiting at the onset of the focal deficit is suggestive of
intracerebral haemorrhage.
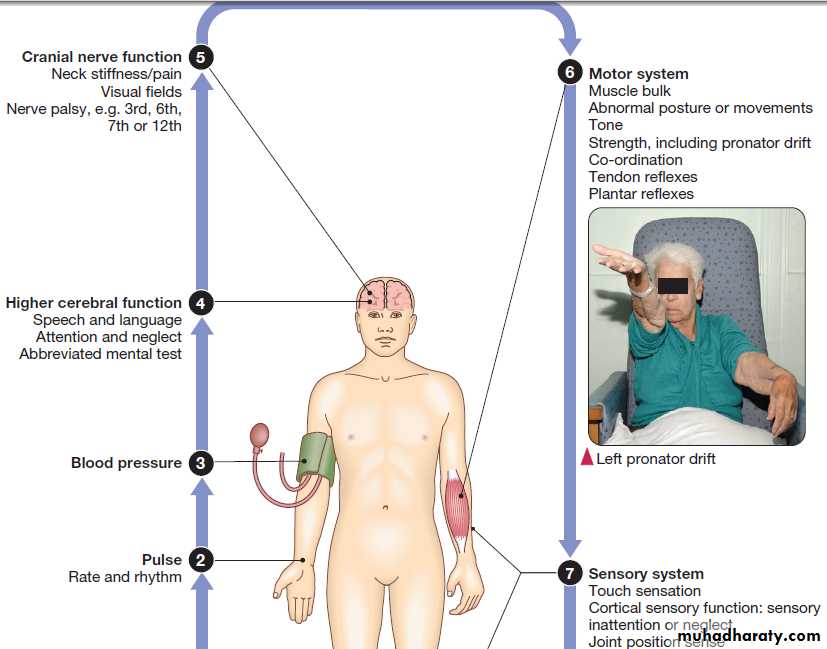
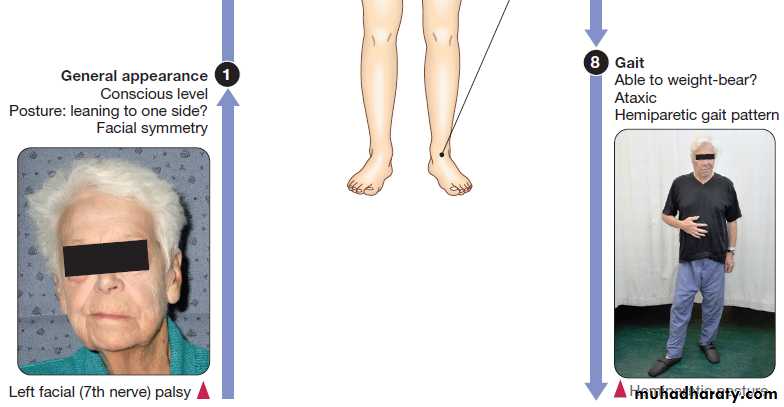
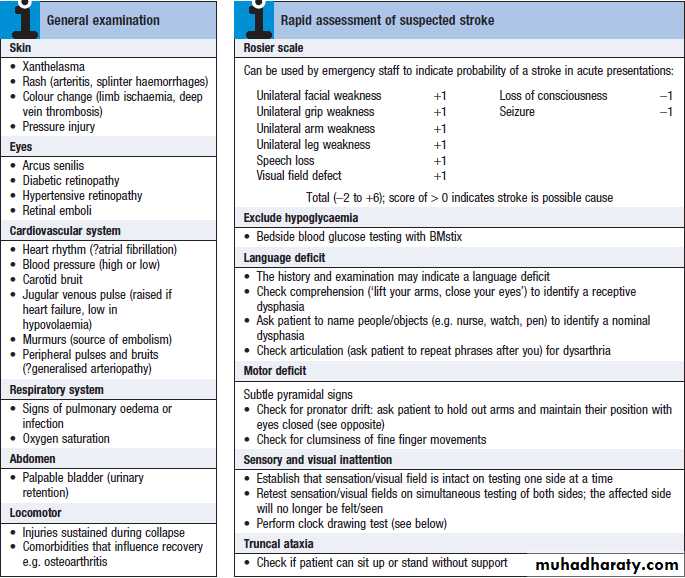
General examination may provide clues to the cause,
and identify important comorbidities and complications. Several terms have been used to classify strokes, often
based on the duration and evolution of symptoms.
• Transient ischaemic attack (TIA) describes a stroke in
which symptoms resolve within 24 hours – an arbitrary cutoff that has little value in practice, apart from perhaps indicating that underlying cerebral haemorrhage or extensive cerebral infarction is extremely unlikely. The term TIA traditionally also includes patients with amaurosis fugax, usually due to a vascular occlusion in the retina.
• Stroke describes those events in which symptoms
last > 24 hours. The differential diagnosis
of patients with symptoms lasting a few minutes or
hours is similar to those with persisting symptoms .
• Progressing stroke (or stroke in evolution) describes a
stroke in which the focal neurological deficit worsens after the patient first presents. Such worsening may be due to increasing volume of infarction, haemorrhagic transformation or increasing cerebral oedema.• Completed stroke describes a stroke in which the focal
deficit persists and is not progressing.
When assessing a patient within hours of symptom
onset, it is not possible to distinguish stroke from TIA
unless symptoms have already resolved.
Differential diagnosis of stroke and TIA
‘Structural’ stroke mimics• Primary cerebral tumours
• Metastatic cerebral tumours
• Subdural haematoma
• Cerebral abscess
• Peripheral nerve lesions (vascular or compressive)
• Demyelination
‘Functional’ stroke mimics
• Todd’s paresis (after epileptic seizure)
• Hypoglycaemia
• Migrainous aura (with or without headache)
• Focal seizures
• Ménière’s disease or other vestibular disorder
• Conversion disorder
• Encephalitis
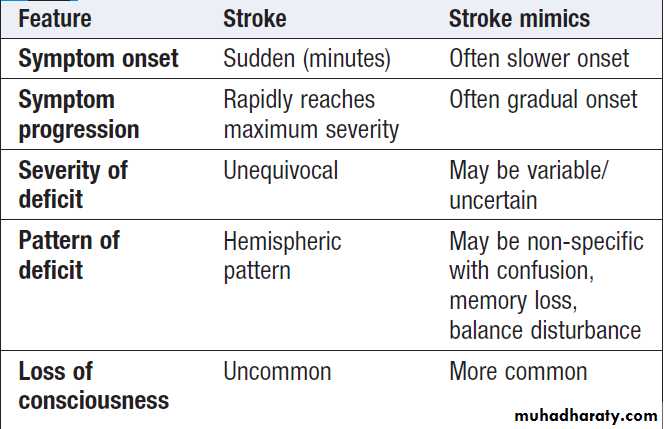
Characteristic features of stroke and
non-stroke syndromes (‘stroke mimics’)Fig. Clinical and radiological features of the stroke syndromes. The top three diagrams show coronal sections of the brain, and the bottom one shows a sagittal section. The anatomical locations of cerebral functions are shown with the nerve tracts in green. A motor (or sensory) deficit (shown by the red shaded areas) can occur with damage to the relevant cortex (PACS), nerve tracts (LACS) or both (TACS). The corresponding CT scans show horizontal slices at the level of the lesion, highlighted by the arrows.
Investigation of a patient with
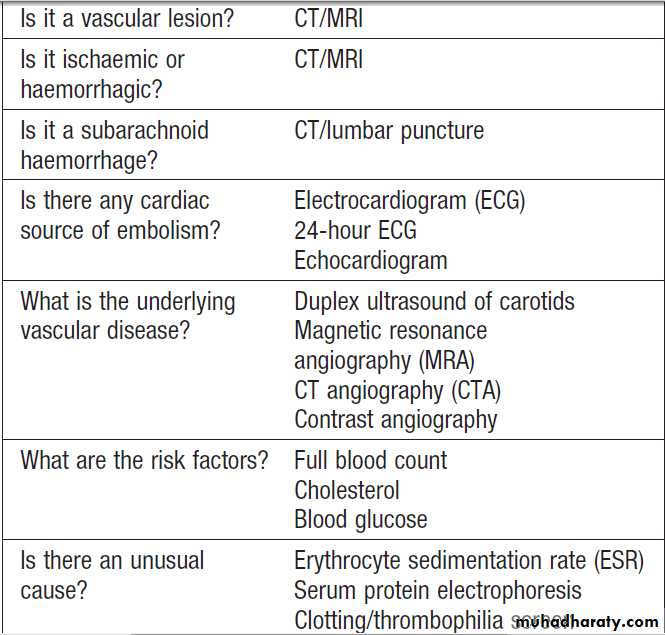
an acute strokeInvestigations
Investigation of acute stroke aims to confirm the vascularnature of the lesion, distinguish cerebral infarction
from haemorrhage and identify the underlying vascular
disease and risk factors .
Risk factor analysis
Initial investigation includes a range of simple blood
tests to detect common vascular risk factors and markers
of rarer causes, an ECG and brain imaging. Where there
is uncertainty about the nature of the stroke, further
investigations are indicated.
This especially applies to younger patients, who are less likely to have atherosclerotic disease .
Neuroimaging
Brain imaging with either CT or MRI should be performedin all patients with acute stroke. Exceptions are
where results would not influence management, such as
in the advanced stage of a terminal illness. CT remains
the most practical and widely available method of
imaging the brain. It will usually exclude non-stroke
lesions, including subdural haematomas and brain
tumours, and will demonstrate intracerebral haemorrhage
within minutes of stroke onset . However, especially within the first few hours after symptom onset, CT changes in cerebral infarction may be completely absent or only very subtle. Changes often develop over time , but small
cerebral infarcts may never show up on CT scans.
For most purposes, a CT scan performed within 24 hours is adequate but there are certain circumstances in which
an immediate CT scan is essential. Even in the absence of changes suggesting infarction, abnormal perfusion of brain tissue can be imaged with CT after injection of contrast media (i.e. perfusion scanning).
This can be useful in guiding immediate treatment of
ischaemic stroke.
MRI is not as widely available as CT and scanning
times are longer. However, MRI diffusion weighted
imaging (DWI) can detect ischaemia earlier than CT, and
other MRI sequences can also be used to demonstrate
abnormal perfusion .
MRI is more sensitive than CT in detecting strokes affecting the brainstem and cerebellum, and unlike CT, can reliably distinguish haemorrhagic from ischaemic stroke even several weeks after the onset.
CT and MRI may reveal clues as to the nature of the arterial lesion. For example, there may be a small, deep lacunar infarct indicating small-vessel disease, or a more peripheral infarct suggesting an extracranial source of embolism . In a haemorrhagic lesion, the location might indicate the presence of an underlying vascular malformation, saccular aneurysm or amyloid angiopathy.
Vascular imaging
Many ischaemic strokes are caused by atheroscleroticthromboembolic disease of the major extracranial
vessels. Detection of extracranial vascular disease can
help establish why the patient has had an ischaemic
stroke and, in highly selected patients, may lead on to
specific treatments, including carotid endarterectomy to
reduce the risk of further stroke (see below). The presence
or absence of a carotid bruit is not a reliable indicator of the degree of carotid stenosis. Extracranial arterial disease can be non-invasively identified with duplex ultrasound, MRA or CT angiography, or occasionally intra-arterial contrast radiography as above.
Cardiac investigations
Approximately 20% of ischaemic strokes are due toembolism from the heart. The most common causes are
atrial fibrillation, prosthetic heart valves, other valvular
abnormalities and recent myocardial infarction. These
may be identified by clinical examination and ECG, but
a transthoracic or transoesophageal echocardiogram is
also required to confirm the presence of a clinically
apparent cardiac source or to identify an unsuspected
source such as endocarditis, atrial myxoma, intracardiac
thrombus or patent foramen ovale. Such findings may
lead on to specific cardiac treatment.
Indications for emergency CT/MRI in
acute strokeManagement
Management is aimed at minimising the volume of brainthat is irreversibly damaged, preventing complications
reducing the patient’s disability and handicap
through rehabilitation, and reducing the risk of
recurrent stroke or other vascular events. With TIA there
is no brain damage and disability, so the priority is to
reduce the risk of further vascular events.
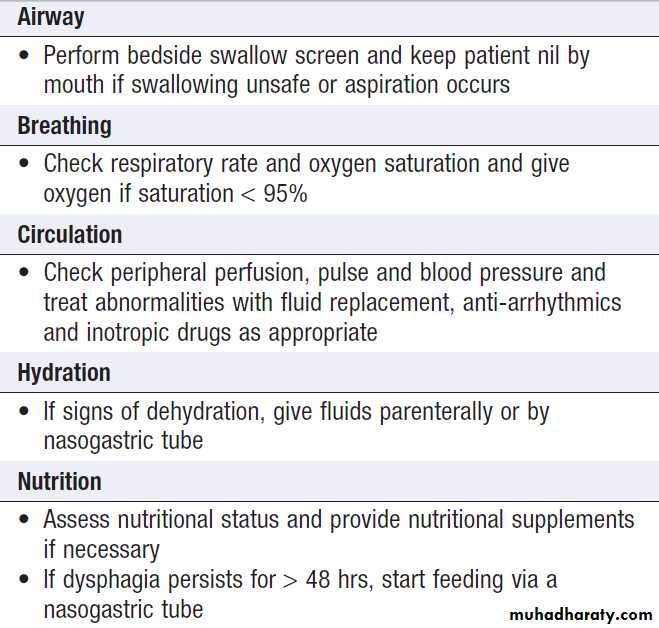
Supportive care
Early admission of patients to a specialised stroke unitfacilitates coordinated care from a specialised multidisciplinary team and has been shown to reduce
both mortality and residual disability amongst survivors.
Consideration of a patient’s rehabilitation needs should commence at the same time as acute medical management. Dysphagia is common and can be detected by an early bedside test of swallowing. This allows hydration, feeding and medication to be given safely, if necessary by nasogastric tube or intravenously. In the acute phase, a checklist may be useful to ensure that all the factors that might influence outcome have been addressed.
The patient’s neurological deficits may worsen during
the first few hours or days after their onset. This is most
common in those with lacunar infarcts but may occur in
other patients, due to extension of the area of infarction,
haemorrhage transformation, or the development of
oedema with consequent mass effect. It is important
to distinguish these patients from those who are deteriorating as a result of complications such as hypoxia, sepsis, epileptic seizures or metabolic abnormalities that
may be reversed more easily.
Patients with cerebellar haematomas or infarcts with mass effect may develop obstructive hydrocephalus and some will benefit from insertion of a ventricular drain and/or decompressive surgery .
Some patients with large haematomas or infarction with massive oedema in the cerebral hemispheres may benefit from anti-oedema agents, such as mannitol or artificial ventilation. Surgical decompression to reduce intracranial pressure should be considered in appropriate patients.
Thrombolysis
Intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA) increases the risk of haemorrhagic transformation of the cerebral infarct with
potentially fatal results.
However, if it is given within 4.5 hours of symptom onset to carefully selected patients, the haemorrhagic risk is offset by an improvement in overall outcome . The earlier treatment is given, the greater the benefit.
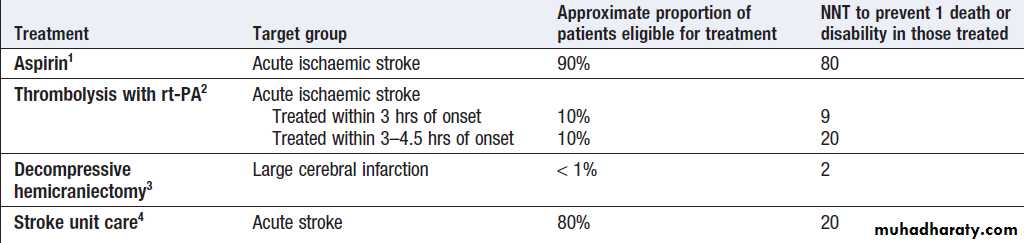
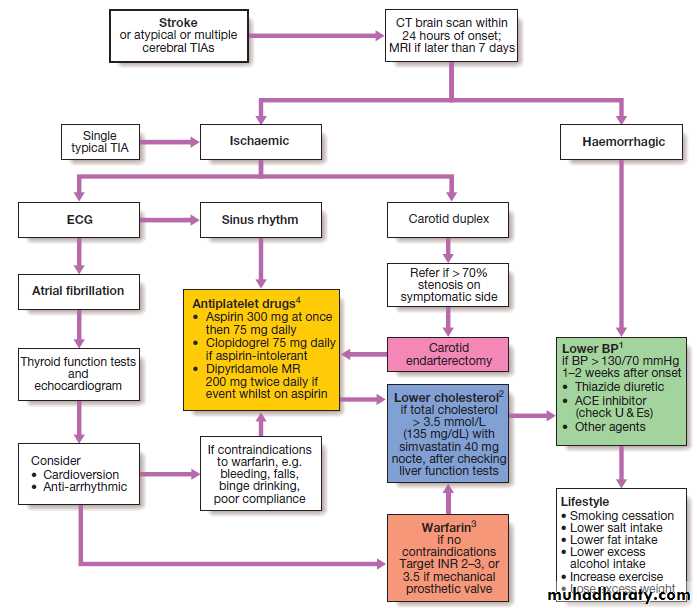
Specialist stroke units
Role of treatments in acute stroke
Management of acute stroke
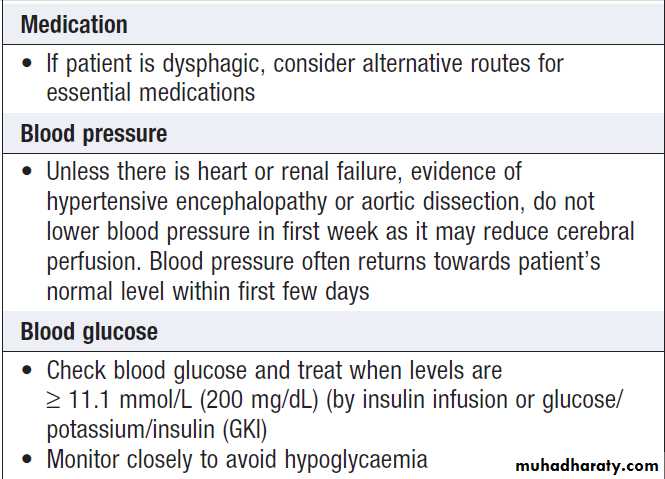
Management of acute stroke'cont'd
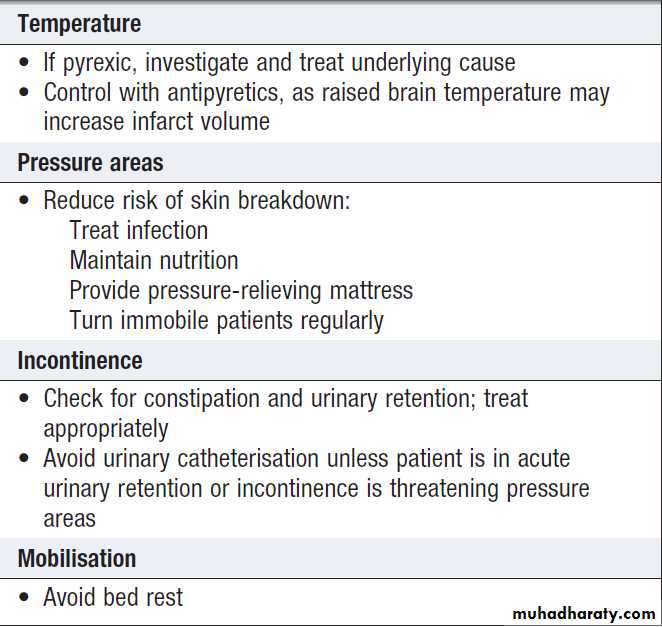
Management of acute stroke'cont'
AspirinIn the absence of contraindications, aspirin (300 mg daily) should be started immediately after an ischaemic
stroke unless rt-PA has been given, in which case it
should be withheld for at least 24 hours. Aspirin reduces the risk of early recurrence and has a small but clinically worthwhile effect on long-term outcome ; it may be given by rectal suppository or by nasogastric tube in dysphagic.
Heparin
Anticoagulation with heparin has been widely used to
treat acute ischaemic stroke in the past. Whilst it reduces
the risk of early ischaemic recurrence and venous
thromboembolism, it increases the risk of both intracranial
and extracranial haemorrhage.
Furthermore, routine use of heparin does not result in better long-term outcomes, and therefore it should not be used in the routine management of acute stroke. It is unclear whether heparin might provide benefit in selected patients, such as those with recent myocardial infarction, arterial dissection or progressing strokes.
Intracranial haemorrhage must be excluded on brain imaging before considering anticoagulation.
Coagulation abnormalities
In those with intracerebral haemorrhage, coagulation
abnormalities should be reversed as quickly as possible
to reduce the likelihood of the haematoma enlarging.
This most commonly arises in those on warfarin therapy.
There is no evidence that clotting factors are useful in
the absence of a clotting defect.
Management of risk factors
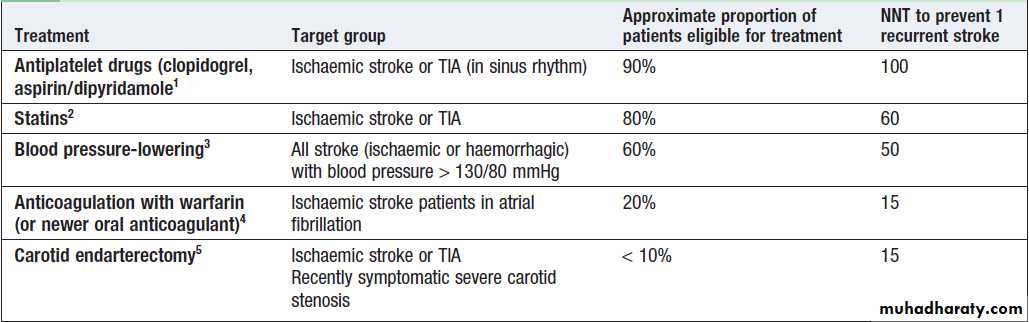
The approaches used are summarised in Figure. The average risk of a further stroke is 5–10% within the first week of a stroke or TIA, perhaps 15% in the first year and 5% per year thereafter. Patients with ischaemic events should be put on long-term antiplatelet drugs and statins to lower cholesterol. For patients in atrial fibrillation, the risk can be reduced by about 60% by using oral anticoagulation to achieve an INR of 2–3. The role of newer oral anticoagulants (such as dabigatran) is currently being investigated. The risk of recurrence after both ischaemic and haemorrhagic strokes can be reduced by blood pressure reduction, even for those with relatively normal blood pressures .Fig. Strategies for secondary prevention of stroke.
(1) Lower BP with caution in patients with postural hypotension, renal impairment or bilateral carotid stenosis. (2) Pravastatin 40 mg can be used as an alternative to simvastatin in patients on warfarin or digoxin. (3) Warfarin and aspirin can be used in combination in patients with prosthetic heart valves.(4) The combination of aspirin and clopidogrel is only indicated in patients with unstable angina who demonstrate ECG or enzyme changes.
Strategies for secondary prevention
Carotid endarterectomy and angioplastyA small proportion of patients with a carotid territory
ischaemic stroke or TIA will have a greater than 50%
stenosis of the carotid artery on the side of the brain
lesion. Such patients have a greater than average risk
of stroke recurrence. For those without major residual
disability, removal of the stenosis has been shown
to reduce the overall risk of recurrence, although the
operation itself carries about a 5% risk of stroke. Surgery is most effective in patients with more severe stenoses (70–99%) and in those in whom it is performed within the first couple of weeks after the TIA or ischaemic stroke.
Carotid angioplasty and stenting are technically feasible but have not been shown to be as effective as endarterectomy for the majority of eligible patients. Endarterectomy of asymptomatic carotid stenosis has been shown to reduce the subsequent risk of stroke, but the small absolute benefit does not justify its routine use.
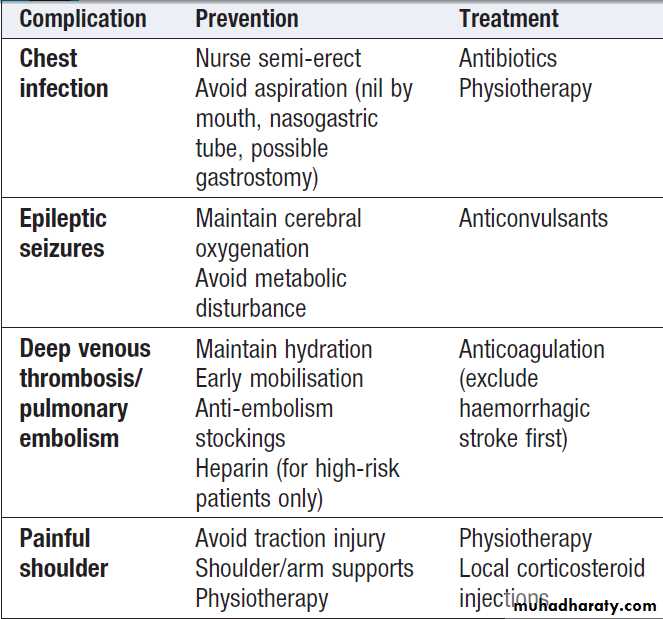
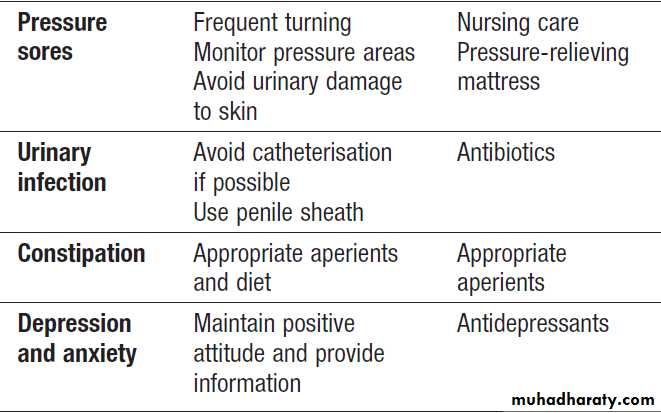
Complications of acute stroke
SUBARACHNOID HAEMORRHAGE (SAH)
SAH is less common than ischaemic stroke or intracerebral haemorrhage and affects about 6/100 000 of the population. Women are affected more commonly than men and the condition usually presents before the age of 65. The immediate mortality of aneurysmal SAH is about 30%;survivors have a recurrence (or rebleed) rate of about
40% in the first 4 weeks and 3% annually thereafter.
Eighty-five percent of SAH are caused by saccular
or ‘berry’ aneurysms arising from the bifurcation
of cerebral arteries , particularly in the region of the circle of Willis. The most common sites are in the anterior communicating artery (30%), posterior communicating artery (25%) or middle cerebral artery (20%).
There is an increased risk in first-degree relatives of those with saccular aneurysms, and in patients with polycystic kidney disease and congenital connective tissue defects such as Ehlers– Danlos syndrome . In about 10% of cases,
SAH are non-aneurysmal haemorrhages (so-called peri-mesencephalic haemorrhages), which have a very characteristic appearance on CT and a benign outcome
in terms of mortality and recurrence.
5% of SAH are due to arteriovenous malformations and vertebral artery dissection.
Clinical features
SAH typically presents with a sudden, severe, ‘thunder-clap’ headache (often occipital), which lasts for hours oreven days, often accompanied by vomiting, raised blood
pressure and neck stiffness or pain. It commonly occurs
on physical exertion, straining and sexual excitement. There may be loss of consciousness at the onset, so SAH
should be considered if a patient is found comatose.
About 1 patient in 8 with a sudden severe headache has
SAH and, in view of this, all who present in this way
require investigation to exclude it .
On examination, the patient is usually distressed and
irritable, with photophobia. There may be neck stiffness
due to subarachnoid blood but this may take some hours
to develop. Focal hemisphere signs, such as hemiparesis
or aphasia, may be present at onset if there is an associated intracerebral haematoma.
A third nerve palsy may be present due to local pressure from an aneurysm of the posterior communicating artery, but this is rare.
Fundoscopy may reveal a subhyaloid haemorrhage, which represents blood tracking along the subarachnoid space around the optic nerve.
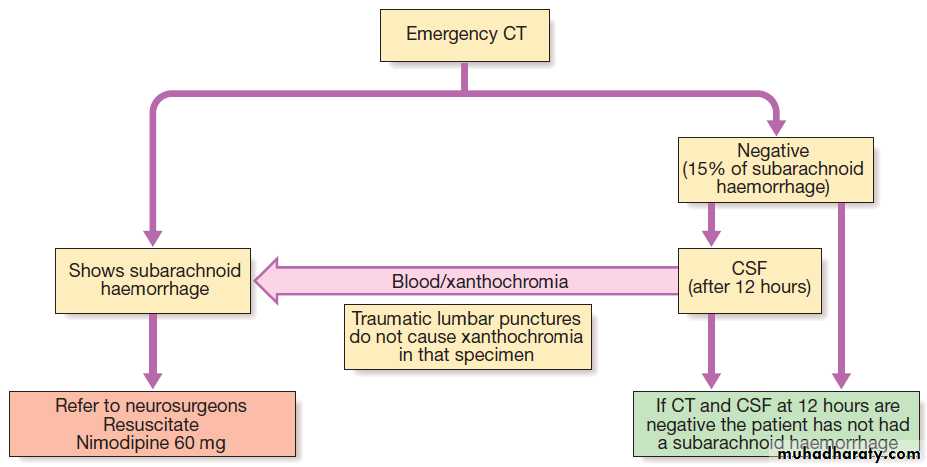
Investigation of subarachnoid haemorrhage.
InvestigationsCT brain scanning and lumbar puncture are required.
The diagnosis of SAH can be made by CT, but a negative
result does not exclude it, since small amounts of blood
in the subarachnoid space cannot be detected by CT. Lumbar puncture should be performed 12 hours after symptom onset if possible, to allow detection
of xanthochromia .
If either of these tests is positive, cerebral angiography is required to determine the optimal approach to prevent recurrent bleeding.
Management
Nimodipine (30–60 mg IV for 5–14 days, followed by
360 mg orally for a further 7 days) is usually given
to prevent delayed ischaemia in the acute phase.
Insertion of platinum coils into an aneurysm (via an endovascular procedure) or surgical clipping of the aneurysm neck reduces the risk of both early and late recurrence.
Coiling is associated with fewer perioperative complications and better outcomes than surgery; where feasible, it is now the procedure of first choice.
Arteriovenous malformations can be managed either by surgical removal, by ligation of the blood vessels that feed or drain the lesion, or by injection of material to occlude the fistula or draining veins.
Treatment may also be required for complications of SAH, which include
obstructive hydrocephalus (that may require drainage
via a shunt),
delayed cerebral ischaemia due to vasospasm
(which may be treated with vasodilators),
hyponatraemia (best managed by fluid restriction) and
systemic complications associated with immobility,
such as chest infection and venous thrombosis.
CEREBRAL VENOUS DISEASE
Thrombosis of the cerebral veins and venous sinuses is much less common than arterial thrombosis. However, it has been recognised with increasing frequency in recent years, as access to non-invasive imaging of the venous sinuses using MR venography has increased.Clinical features
Cerebral venous sinus thrombosis usually presents with
symptoms of raised intracranial pressure, seizures and
focal neurological symptoms. The clinical features vary
according to the sinus involved. Cortical vein thrombosis presents with focal cortical deficits such as aphasia and hemiparesis (depending on the area affected), and epilepsy (focal or generalised). The deficit can increase if spreading thrombophlebitis occurs.
Causes of cerebral venous thrombosis
Predisposing systemic causes
• Dehydration • Thrombophilia
• Pregnancy • Hypotension
• Behçet’s disease • Oral contraceptive use
Local causes
• Paranasal sinusitis • Facial skin infection
• Meningitis, subdural empyema
• Otitis media, mastoiditis
• Skull fracture
• Penetrating head and eye wounds
Clinical features of cerebral venous thrombosis
Cavernous sinus thrombosis• Proptosis, ptosis, headache, external and internal ophthalmoplegia, papilloedema, reduced sensation in trigeminal first division
• Often bilateral, patient ill and febrile
Superior sagittal sinus thrombosis
• Headache, papilloedema, seizures
• May resemble idiopathic intracranial hypertension
• May involve veins of both hemispheres, causing advancing motor and sensory focal deficits
Transverse sinus thrombosis
• Hemiparesis, seizures, papilloedema
• May spread to jugular foramen and involve cranial nerves 9, 10 and 11
Investigations and management
MR venography demonstrates a filling defect in the
affected vessel.
Anticoagulation, initially with heparin followed by
warfarin, is usually beneficial, even in the presence of
venous haemorrhage. In selected patients, the use of
endovascular thrombolysis has been advocated. Management of underlying causes and complications, such as persistently raised intracranial pressure, is also important. About 10% of cerebral venous sinus thrombosis, particularly cavernous sinus thrombosis, is associated with infection (most commonly Staphylococcus aureus), which necessitates antibiotic treatment. Otherwise, the treatment of choice is anticoagulation.