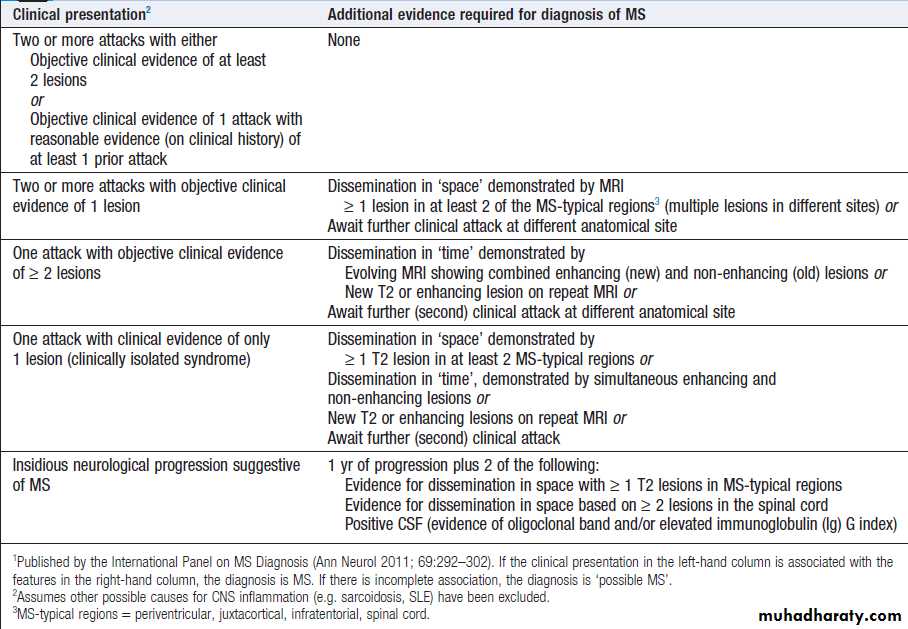
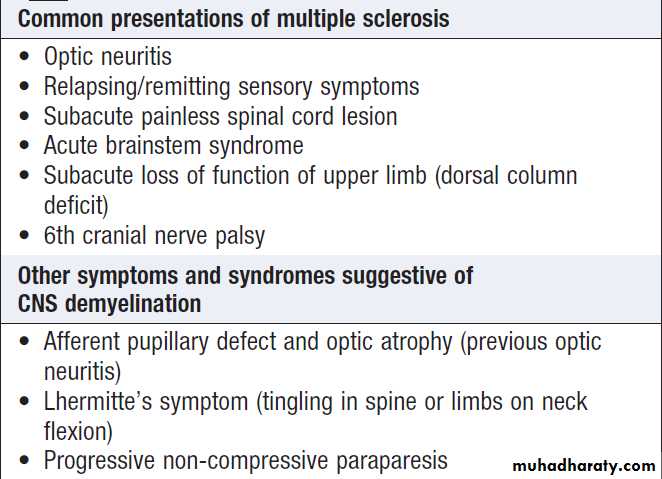
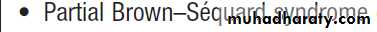Hussien Mohammed Jumaah
CABMLecturer in internal medicine
Mosul College of Medicine
2016
learning-topics
Neurological diseaseCLINICAL EXAMINATION OF THE NERVOUS SYSTEM
4 Examination of cranial nerves
Neurology has long been misperceived as a specialty
in which intricate clinical examination and numerousinvestigations are required to diagnose obscure and
untreatable conditions. In fact, it requires careful historytaking with a lesser contribution from targeted examination and considered investigation. The development of specific, effective treatments has made accurate diagnosis essential.
The brain, spinal cord and peripheral nerves combine
to allow us to perceive and react to the external world,
while maintaining a stable internal environment.
Nervous system disorders are common, accounting for
around 10% of the UK’s general practice consultations,
20% of acute medical admissions, and most chronic
physical disability. While pathological and anatomical localisation is important, skill is required to identify
those neurological symptoms not associated with neurological disease. Initially, it is important to exclude conditions that constitute neurological emergencies . The history should provide a hypothesis for the site and nature of the potential pathology, which a focused examination may refine and inform what further investigation would be useful. A discussion with the patient about possible interventions and rehabilitation may then take place.
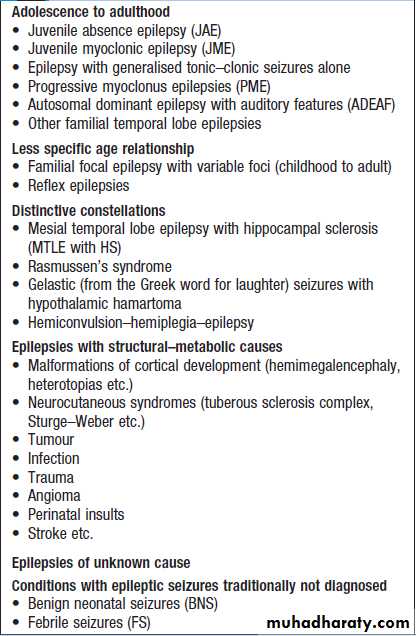
Neurological emergencies
• Status epilepticus• Stroke (if thrombolysis available)
• Guillain–Barré syndrome
• Myasthenia gravis (bulbar and/or respiratory)
• Spinal cord compression
• Subarachnoid haemorrhage
• Neuroleptic malignant syndrome
FUNCTIONAL ANATOMY AND PHYSIOLOGY
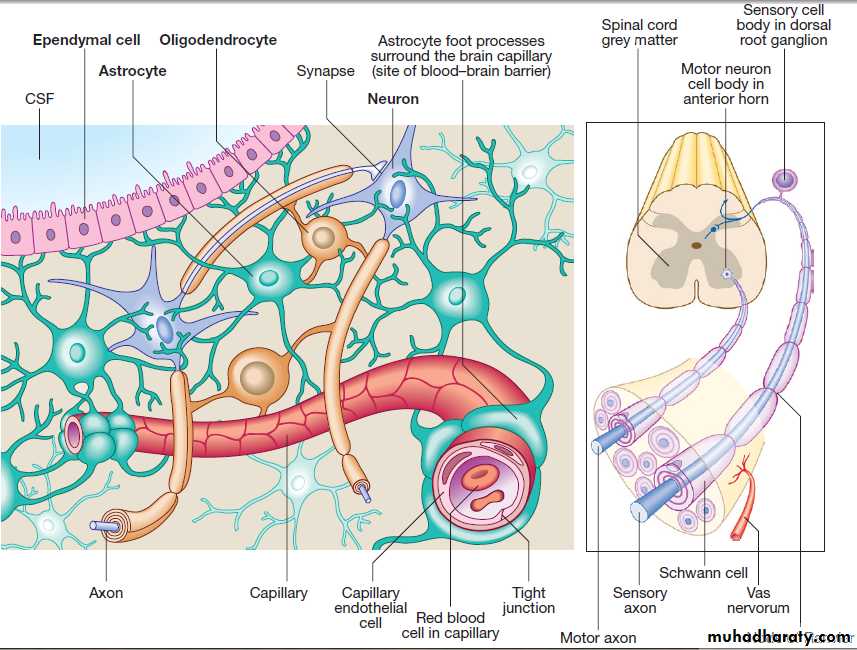
Cells of the nervous systemThe nervous system comprises billions of connections between billions of specialised cells, supplied by a complex network of specialised blood vessels. In addition to neurons, there are three types of glial cells. Astrocytes form the structural framework for neurons and control their biochemical environment. Astrocyte foot processes
are intimately associated with blood vessels, forming the
blood–brain barrier .Oligodendrocytes are responsible for the formation and maintenance of the myelin sheath, which surrounds axons and is essential for the rapid transmission of action potentials. Microglial cells derive from monocytes/ macrophages and play a role in fighting infection and removing damaged cells. Peripheral neurons have axons invested in myelin made by Schwann cells. Ependymal cells line the cerebral ventricles.
Cells of the nervous system
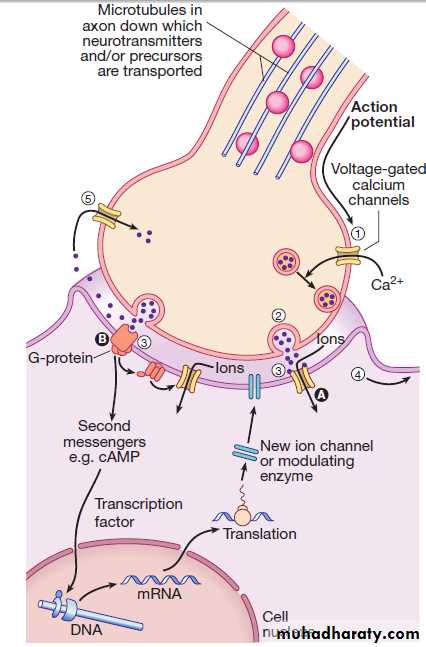
Generation and transmission of the nervous impulseEach neuron receives input by synaptic transmission from dendrites (branched projections of other neurons), which may sum to produce output in the form of an action potential. This is conducted down axons, with synaptic transmission to other neurons or, in the motor system, to muscle cells. Synaptic transmission involves the release of neurotransmitters that modulate the function of the target cell by interacting with structures on the cell surface, including ion channels and other cell surface receptors .
At least 20 different neurotransmitters are known to act at different sites in the nervous system, and all are potentially amenable to pharmacological manipulation.
Neurotransmission and neurotransmitters.
(1) An action potential arriving at the nerve terminal depolarises the membrane and this opens voltage-gated calcium channels. (2) Entry of calcium causes the fusion of synaptic vesicles containing neurotransmitters with the pre-synaptic membrane and release of the neurotransmitter across the synaptic cleft.(3) The neurotransmitter binds to receptors on the post-synaptic membrane either (A) to open ligand-gated ion channels which, by allowing ion entry, depolarise the membrane and initiate an action potential (4), or (B) to bind to metabotrophic receptors that activate an effector enzyme (e.g. adenylyl cyclase) and thus modulate gene transcription via the intracellular second messenger system, leading to changes in synthesis of ion channels or modulating enzymes. (5) Neurotransmitters are taken up at the pre-synaptic membrane and/or metabolised.
(cAMP = cyclic adenosine monophosphate; DNA = deoxyribonucleic acid; mRNA = messenger ribonucleic acid)
The major anatomical components of the nervous
system.Functional anatomy of the nervous system
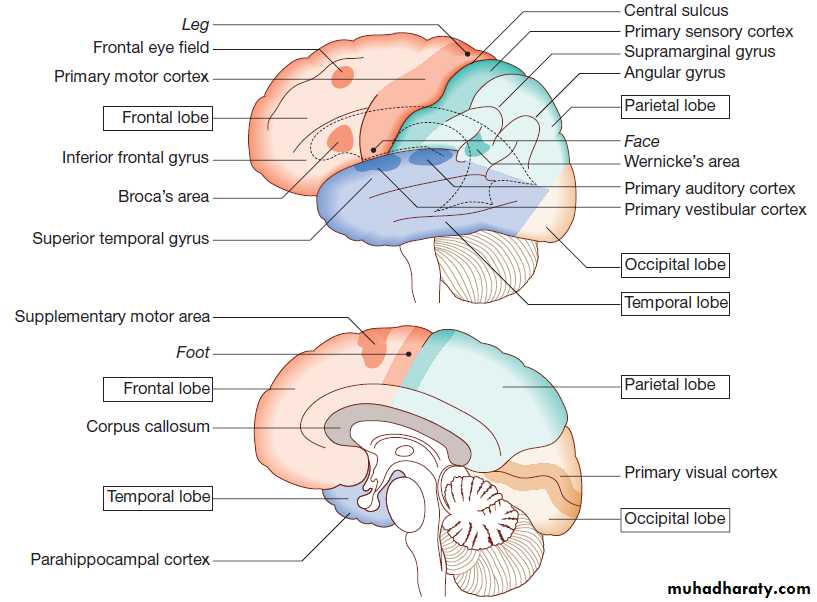
Cerebral hemispheres
The cerebral hemispheres coordinate the highest level
of nervous function, the anterior half dealing with executive
(‘doing’) functions and the posterior half constructing
a perception of the environment. Cerebral dominance aligns limb dominance with language function:
in right-handed individuals the left hemisphere is
almost always dominant, while around half of lefthanders
have a dominant right hemisphere.
The frontal lobes are concerned with executive function,
movement, behaviour and planning. In addition to
the primary and supplementary motor cortex, there are
specialised areas for the control of eye movements,
speech (Broca’s area) and micturition.
The parietal lobes integrate sensory perception. The primary sensory cortex lies in the post-central gyrus of the parietal lobe. Much of the remainder is devoted to ‘association’ cortex, which processes and interprets input from the various sensory modalities. The supramarginal and angular gyri of the dominant parietal lobe form part of the language area . Close to these are regions dealing with numerical function. The nondominant parietal lobe is concerned with spatial awareness and orientation.
The temporal lobes contain the primary auditory
cortex and primary vestibular cortex. On the inner
medial sides lie the olfactory cortex and the parahippocampal cortex, which is involved in memory
function.
The temporal lobes also contain much of the limbic system, including the hippocampus and the amygdala, which are involved in memory and emotional processing.
The dominant lobe also participates in language functions, particularly verbal comprehension (Wernicke’s area).
Musical processing occurs across both temporal lobes, rhythm on the dominant side and melody/pitch on the non-dominant.
The occipital lobes are responsible for visual interpretation. The contralateral visual hemifield is represented in each primary visual cortex, with surrounding areas processing specific visual submodalities such as colour, movement or depth, and the analysis of more complex visual patterns such as faces.
Deep to the grey matter in the cortices, and the white
matter (composed of neuronal axons), are collections ofcells known as the basal ganglia that are concerned with
motor control; the thalamus, which is responsible for
the level of attention to sensory perception; the limbic
system, concerned with emotion and memory; and the
hypothalamus, responsible for homeostasis, such as
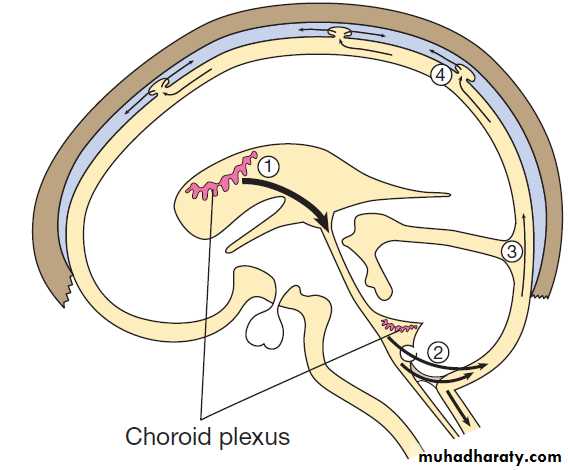
temperature and appetite control. The cerebral ventricles
contain CSF , which cushions the brain during cranial movement. CSF is formed in the lateral ventricles and protects and nourishes the CNS. The CSF flows from third to fourth ventricles and through foramina in the brainstem to dissipate over the surface of the CNS, eventually being reabsorbed into the cerebral venous system.
The anatomy of the cerebral cortex
Cortical lobar functions
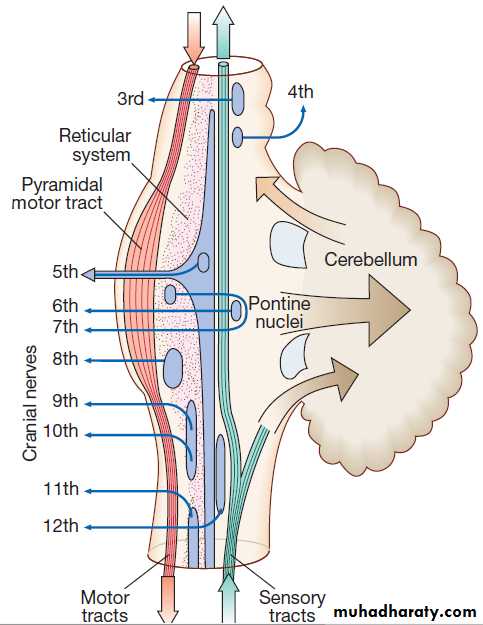
The brainstemIn addition to containing all the sensory and motor pathways entering and leaving the hemispheres, the brainstem houses the nuclei and projections of the cranial
nerves, and other important collections of neurons in the reticular formation . Cranial nerve nuclei provide motor control to muscles of the head (including the face and eyes) and coordinate sensory input from the special sense organs and the face, nose, mouth, larynx and pharynx. They also relay autonomic messages,including pupillary, salivary and lacrimal functions.The reticular formation is predominantly involved in the control of conjugate eye movements,
the maintenance of balance, cardiorespiratory control and the maintenance of arousal.
Anatomy of the brainstem.
The spinal cordIs the route for virtually all communication between the extracranial structures and the CNS.
Afferent and efferent fibres are grouped in discrete
bundles but collections of cells in the grey matter are responsible for lower-order motor reflexes and the primary processing of sensory information, including pain.
Sensory peripheral nervous system
The sensory cell bodies of peripheral nerves are situated just outside the spinal cord, in the dorsal root ganglia , whilst the distal ends of their neurons utilise various specialised endings for the conversion of external stimuli into action potentials. Sensory nerves consist of a combination of large, fast, myelinated axons (which carry information about joint position sense and commands to muscles) and smaller, slower, unmyelinated axons (which carry information about pain and temperature, as well as autonomic function).
Motor peripheral nervous system
The anterior horns of the spinal cord comprise lower motor cell bodies. To increase conduction speed, peripheral motor nerve axons are wrapped in myelin produced by Schwann cells. Motor neurons release acetylcholine across the neuromuscular junction, which initiates muscle contraction.
The autonomic system
The autonomic system regulates the cardiovascular andrespiratory systems, the smooth muscle of the gastrointestinal tract, and many exocrine and endocrine
glands throughout the body. The autonomic system is
controlled centrally by diffuse modulatory systems in
the brainstem, limbic system, hypothalamus and frontal
lobes, which are concerned with arousal and background
behavioural responses to threat. Autonomic output divides functionally and pharmacologically into two divisions:
the parasympathetic and sympathetic systems.
The motor system
A programme of movement formulated by the premotorcortex is converted into a series of signals in the
motor cortex that are transmitted to the spinal cord in
the pyramidal tract . This passes through the
internal capsule and the ventral brainstem before decussating in the medulla to enter the lateral columns of the spinal cord. The pyramidal tract ‘upper motor neurons’
synapse with the anterior horn cells of the spinal cord
grey matter, which form the lower motor neurons.
The motor system consists of a hierarchy of controls that maintain body posture and muscle tone.
Muscle spindles sense lengthening of the muscle; they provide the afferent side of the stretch reflex and initiate a monosynaptic reflex leading to protective or reactive muscle contraction.
Inputs from the brainstem are largely inhibitory. Polysynaptic connections in the spinal cord grey matter control more complex reflex actions of flexion and extension of the limbs that form the basic building blocks of coordinated actions, but complete control requires input from the extrapyramidal system and the cerebellum.
The motor system. Neurons from the motor cortex descend
as the pyramidal tract in the internal capsule and cerebral peduncle to the ventral brainstem, where most cross low in the medulla (A). In the spinalcord the upper motor neurons form the corticospinal tract in the lateral column before synapsing with the lower motor neurons in the anterior horns. The activity in the motor cortex is modulated by influences from the basal ganglia and cerebellum. Pathways descending from these structures
control posture and balance (B).
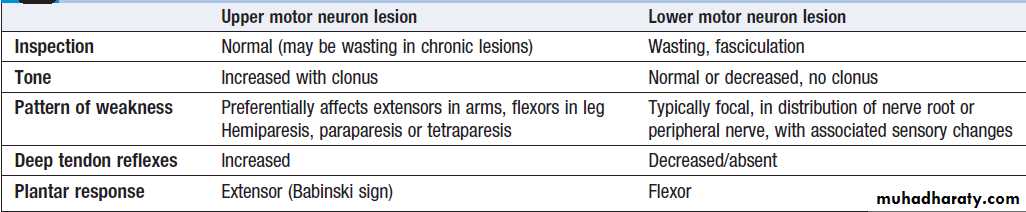
Lower motor neurons
Lower motor neurons in the anterior horn of the spinal cord innervate a group of muscle fibres termed a ‘motor unit’. Loss of lower motor neurons causes loss of contraction within this unit, resulting in weakness and reduced muscle tone. Subsequently, denervated muscle fibres atrophy, causing muscle wasting, and depolarise spontaneously, causing ‘fibrillations’. With the passage of time, neighbouring intact neurons sprout to providere-innervation, but the neuromuscular junctions of the
enlarged motor units are unstable and depolarise spontaneously causing fasciculations (imply chronic partial denervation with re-innervation).
Upper motor neurons
Upper motor neurons have both inhibitory and excitatoryinfluence on the function of anterior horn motor
neurons. Lesions affecting the upper motor neuron
result in increased tone, most evident in the strongest
muscle groups (i.e. the extensors of the lower limbs and
the flexors of the upper limbs). The weakness of upper
motor neuron lesions is conversely more pronounced in
the opposing muscle groups. Loss of inhibition will also
lead to brisk reflexes and enhanced reflex patterns of
movement, such as flexion withdrawal to noxious
stimuli and spasms of extension.
The increased tone is more apparent during rapid stretching (‘spastic catch’), but may suddenly give way with sustained tension (the ‘clasp-knife’ phenomenon).
More primitive reflexes are also released, manifest as extensor plantar responses.
Spasticity may not be present until some weeks after the
onset of an upper motor neuron lesion. Chronic spasticity
in a patient with a spinal cord lesion may also be
exacerbated by increased sensory input – for example,
from a pressure sore or urinary tract infection.
The extrapyramidal system
Circuits between the basal ganglia and the motor cortexconstitute the extrapyramidal system, which controls
muscle tone, body posture and the initiation of movement.
Lesions of the extrapyramidal system produce an increase in tone that, unlike spasticity, is continuous throughout the range of movement at any speed of stretch (‘lead pipe’ rigidity). Involuntary movements are also a feature of extrapyramidal lesions and tremor in combination with rigidity produces typical ‘cogwheel’ rigidity. Extrapyramidal lesions also cause slowed and clumsy movements (bradykinesia), which characteristically reduce in size with repetition, as well as postural instability, which can precipitate falls.
The cerebellum
The cerebellum fine-tunes and coordinates movementsinitiated by the motor cortex. It also participates in the
planning and learning of skilled movements through
reciprocal connections with the thalamus and cortex,
and in articulation of speech. A lesion in a cerebellar
hemisphere causes lack of coordination on the same side
of the body, impairs the smoothness of eye movements, causing nystagmus and renders speech dysarthric. In the limbs, the initial movement is normal, but as the target is approached, the accuracy of the movement deteriorates, producing an ‘intention tremor’. The distances of targets are misjudged (dysmetria), resulting in ‘past-pointing’.
The ability to produce rapid, accurate, regularly alternating movements is also impaired (dysdiadochokinesis).
The central vermis of the cerebellum is concerned with the coordination of gait and posture.
Disorders of this therefore produce a characteristic ataxic gait .
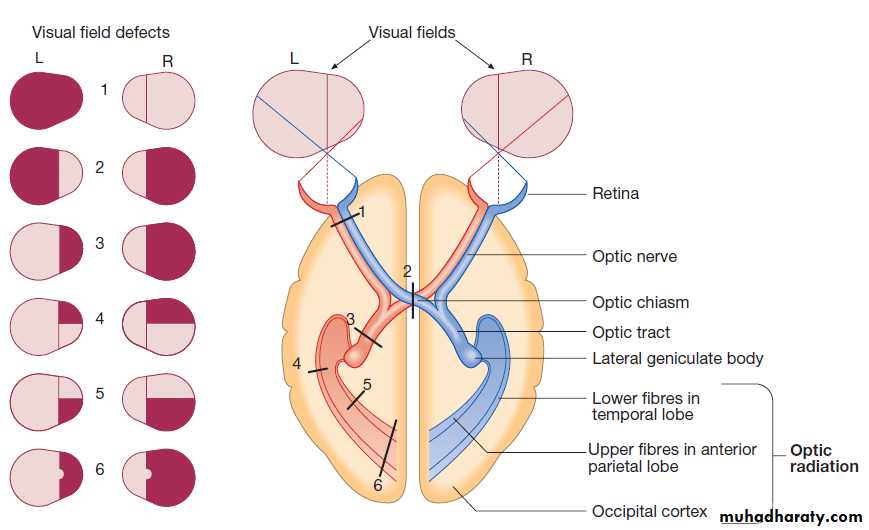
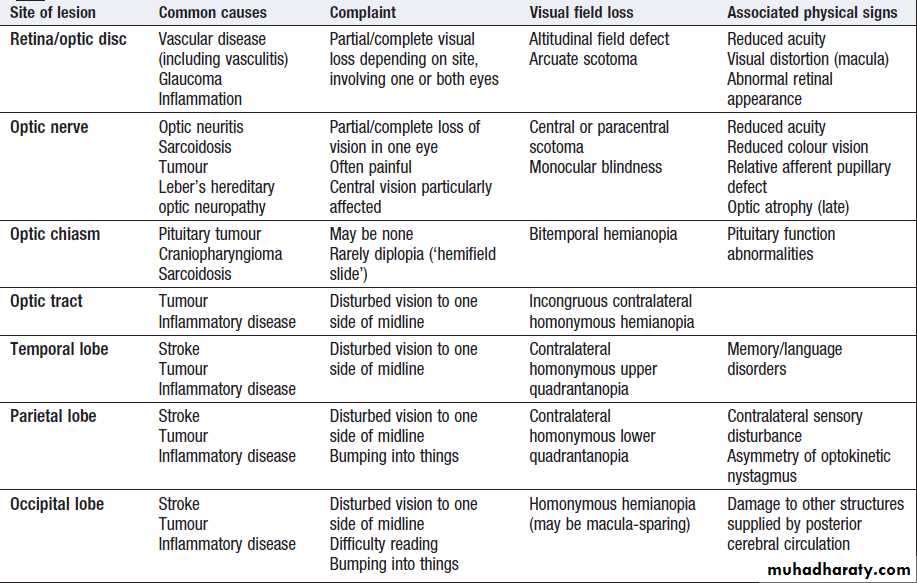
Vision
Fibres from ganglion cells in the retina pass to the optic disc and then backwards through the lamina cribrosa to the optic nerve. Nasal optic nerve fibres (subserving the temporal visual field) cross at the chiasm, but temporal fibres do not.Hence, fibres in each optic tract and further posteriorly carry representation of contralateral visual space.
From the lateral geniculate nucleus, lower fibres pass through the temporal lobes on their way to the primary visual area in the occipital cortex, while the upper fibres pass through the parietal lobe. Normally, the eyes move conjugately (in unison), though horizontal convergence allows visual fusion of objects at different distances.
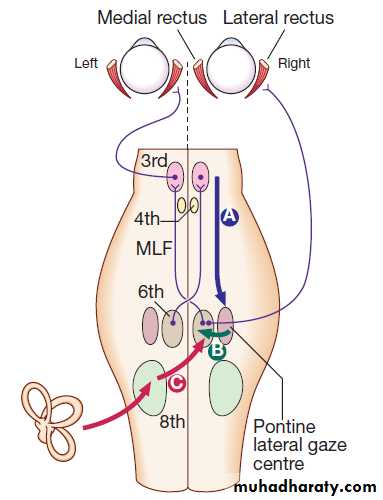
The control of eye movements begins in the cerebral hemispheres, particularly within the frontal eye fields, and the pathway then descends to the brainstem with input from the visual cortex, superior colliculus and cerebellum. Horizontal and vertical gaze centres in the pons and mid-brain, respectively, coordinate output to the ocular motor nerve nuclei (3, 4 and 6), which are connected to each other by the medial longitudinal fasciculus (MLF). The MLF is particularly important in coordinating horizontal movements of the eyes. The extraocular muscles are then supplied by the oculomotor (3rd), trochlear (4th) and abducens (6th) cranial nerves.
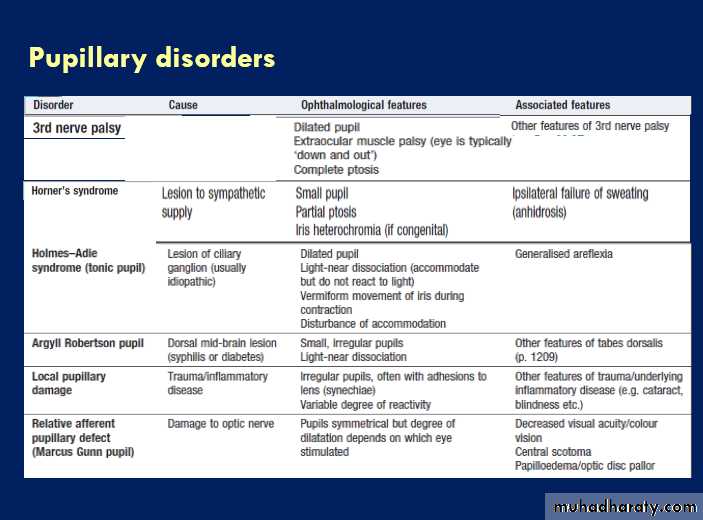
The pupillary response to light is due to a combination
of parasympathetic and sympathetic activity. Parasympathetic fibres originate in the Edinger–Westphalsubnucleus of the 3rd nerve, and pass with the 3rd nerve
to synapse in the ciliary ganglion before supplying the
constrictor pupillae of the iris.
Sympathetic fibres originate in the hypothalamus, pass down the brainstem and cervical spinal cord to emerge at T1, return up to the eye in association with the internal carotid artery, and supply the dilator pupillae.
Visual pathways and visual field defects. Schematic representation of eyes and brain in transverse section.
Control of conjugate eye movements.
Downward projections pass from the cortex to the pontine lateral gaze centre (A).The pontine gaze centre projects to the 6th cranial nerve nucleus (B), which innervates the ipsilateral lateral rectus and
projects to the contralateral 3rd nerve nucleus (and hence medial rectus) via the medial longitudinal fasciculus (MLF).
Tonic inputs from the vestibular apparatus (C) project to the contralateral 6th nerve nucleus via the vestibular nuclei.
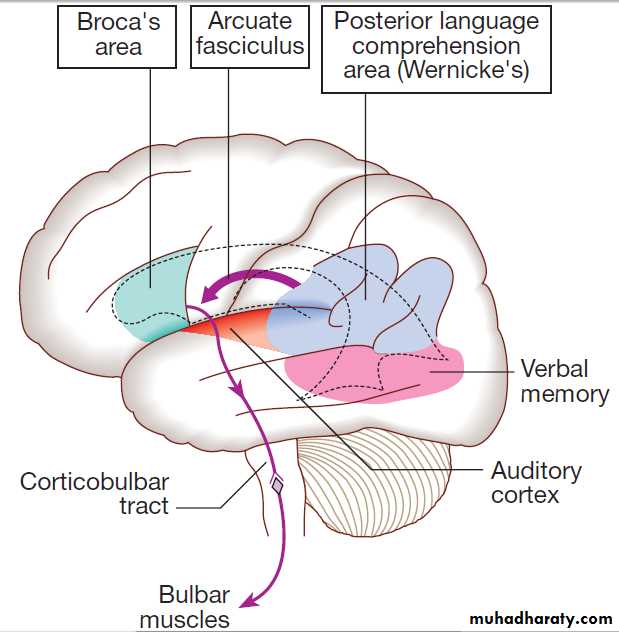
Speech
Much of the cerebral cortex is involved in the process offorming and interpreting communicating sounds, especially
in the dominant hemisphere . The temporal speech comprehension region is referred to as Wernicke’s area . Other parts of the temporal lobe contribute to verbal memory, where lexicons of meaningful words are ‘stored’. Parts of the non-dominant parietal lobe appear to contribute to nonverbal aspects of language in recognising meaningful intonation patterns (prosody).
The frontal language area is in the posterior end of
the dominant inferior frontal gyrus known as Broca’sarea. This receives input from the temporal and parietal
lobes via the arcuate fasciculus. The motor commands
generated in Broca’s area pass to the cranial nerve nuclei
in the pons and medulla, as well as to the anterior horn
cells in the spinal cord. Nerve impulses to the lips,
tongue, palate, pharynx, larynx and respiratory muscles
result in the series of ordered sounds recognised as
speech. The cerebellum also plays an important role in
coordinating speech, and lesions of the cerebellum lead
to dysarthria, problem lies in motor articulation of speech.
Areas of the cerebral cortex involved in the generation
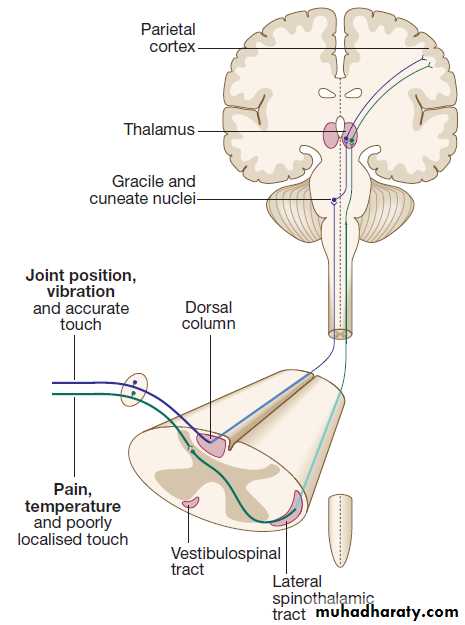
of spoken language.The somatosensory system
Sensory information from the limbs ascends the nervoussystem in two anatomically discrete systems .
Fibres from proprioceptive organs and those mediating
well-localised touch (including vibration) enter the
spinal cord at the posterior horn and pass without synapsing into the ipsilateral posterior columns. Neural
fibres conveying pain and temperature sensory information (nociceptive neurons) synapse with second-order neurons that cross the midline in the spinal cord before ascending in the contralateral anterolateral spinothalamic tract to the brainstem.
The second-order neurons of the dorsal column sensory system cross the midline in the upper medulla to ascend through the brainstem.
Here they lie just medial to the (already crossed) spinothalamic pathway. Brainstem lesions can therefore cause sensory loss affecting all modalities of the contralateral side of the body. Both the dorsal
column and spinothalamic tracts end in the thalamus,
relaying from there to the parietal cortex.
The main somatic sensory pathways.
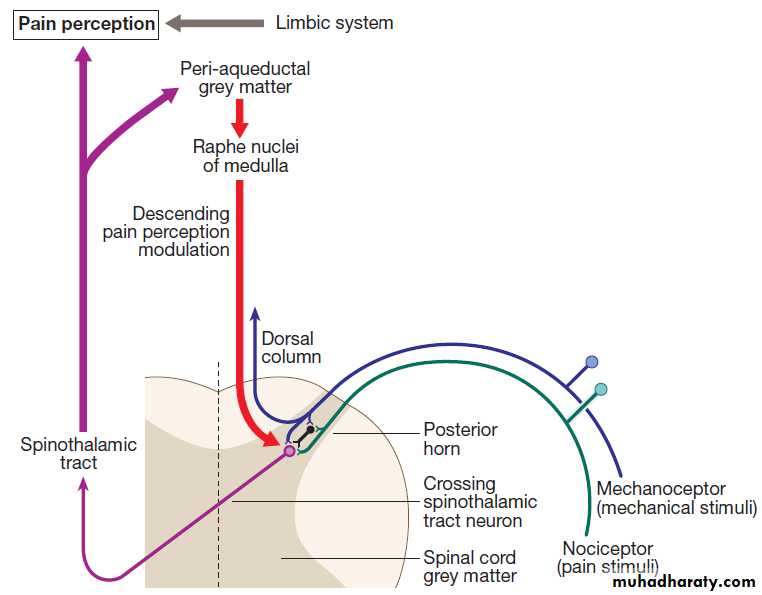
Pain
The nociceptor neurons release neurotransmitters (suchas substance P), in addition to excitatory transmitters,
which influence the excitability of the spinothalamic neurons.Activity in the posterior horn neurons is modulated
by fibres descending from the peri-aqueductal
grey matter of the mid-brain and raphe nuclei of the
medulla. Neurons of this ‘descending analgesia system’
are activated by endogenous opiate (endorphin) peptides.
The spinal cord’s posterior horn is therefore much more than a relay station in pain transmission; its complexity allows it to ‘gate’ and modulate painful sensation before it ascends in the spinothalamic tract. In the diencephalon, the perception of pain is further influenced by the rich interconnections of the thalamus with the limbic system.
The pain perception system.
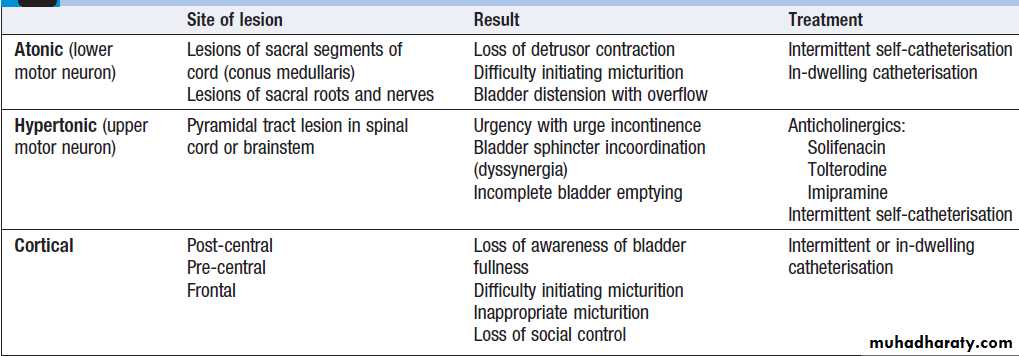
Sphincter controlThe sympathetic supply to the bladder leaves from
T11–L2 to synapse in the inferior hypogastric plexus, parasympathetic supply leaves from S2–4. In addition, a somatic supply to the external (voluntary) sphincter arises from S2–4, travelling via the pudendal nerves. Storage of urine is maintained by inhibiting parasympathetic activity , thus relaxing the detrusor muscle. Continence is by simultaneous sympathetic and somatic (pudendal nerve) mediated tonic contraction of the urethral sphincters. Voiding is usually under conscious control, and triggered by relaxation of tonic inhibition on the pontine micturition centre from higher centres, leading to relaxation of the pelvic floor muscles and external and internal urethral sphincters, along with parasympathetic-mediated detrusor contraction.
Personality and mood
Any process affecting brain function will have some effect on mood and affect.
Sleep
Sleep is controlled by the reticular activating system in the upper brainstem and diencephalon. It is composed of different stages that can be visualised on EEG. As drowsiness occurs, normal EEG background alpha rhythm disappears and activity becomes dominated by deepening slow-wave activity.
As sleep deepens and dreaming begins, the limbs become flaccid, movements are ‘blocked’ and EEG signs of rapid eye movements (REM) are superimposed on the slow wave.
REM sleep persists for a short spell before another slow-wave spell starts, the cycle repeating several times throughout the night.
REM periods become longer as the sleep period progresses.
REM sleep seems to be the most important part of the sleep cycle for refreshing cognitive processes, and REM sleep deprivation causes tiredness, irritability and impaired
judgement.
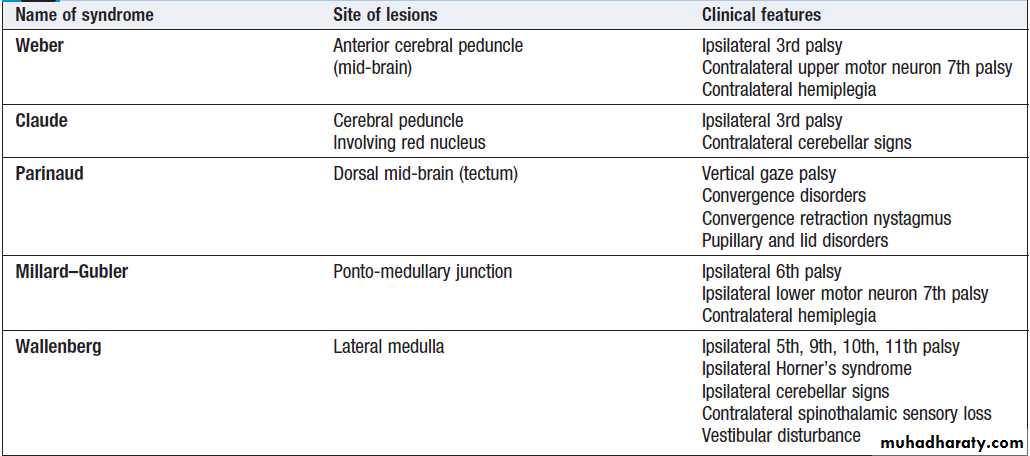
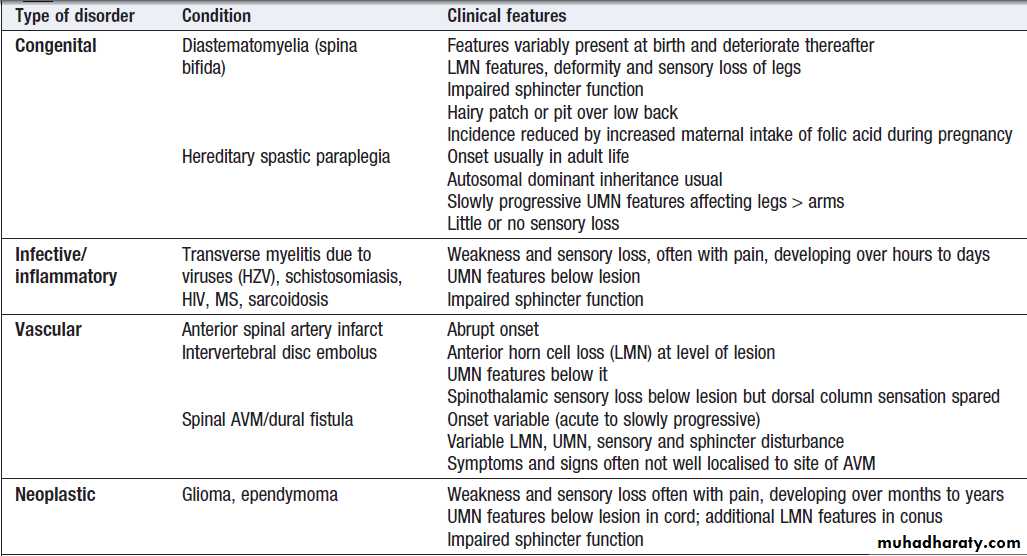
Localising lesions in the brainstem
Given the density of tracts and nuclei in the brainstem , detailed localisation may be possible on the basis of history and examination alone, to be confirmed or refuted by investigation. Brainstem lesions typically present with symptoms due to cranial nerve, cerebellar and upper motor neuron dysfunction and are most commonly caused by vascular disease. Since the anatomy of the brainstem is very precisely organised, it is usually possible to localise the site of a lesion on the basis of careful history and examination. For example, in a patient presenting with sudden onset of UMN features affecting the right face, arm and leg with a left 3rd nerve palsy, the lesion will be in the left cerebral peduncle in the brainstem and the pathology is likely to have been a small stroke, as the onset was sudden. This known as Weber’s syndrome.Major focal brainstem syndromes
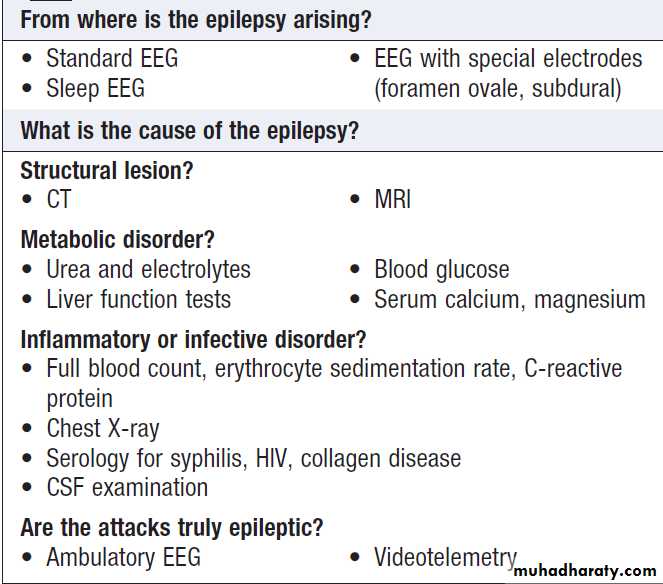
INVESTIGATION OF NEUROLOGICAL DISEASEExperienced clinicians will make around 90% of neurological diagnoses on history alone, with a lesser
contribution from examination and investigation. As
investigations become more complex and more easily
available, it is tempting to adopt a ‘scan first, think later’
approach to neurology. The frequency of ‘false-positive’
results, the wide range of normality and the unnecessary
expense, inconvenience and worry caused to patients
should encourage a more thoughtful approach. Investigation include assessment of structure (imaging)
and function (neurophysiology). Neurophysiological
testing has become so complex that it constitutes a separate specialty focusing on EE evoked potentials, nerve conduction studies and electromyography.
Neuroimaging
Various techniques are available, including X-rays (plain X-rays, CT, CT angiography, myelography and angiography),
MRI, MR angiography (MRA)) and ultrasound (Doppler imaging of blood vessels). It is now possible to use imaging techniques to assess CNS function. Single photon emission clinical tomography (SPECT) scanning can use the lipid-soluble properties of radioactive tracers to mark cerebral blood flow at the time of injection. This can be useful in investigating dementia or epilepsy and by examining dopamine activity to assess the function of the basal ganglia in patients with possible parkinsonism.
Imaging techniques for the nervous system
Head and orbitPlain skull X-rays restricted to the diagnosis of fractures and sinus disease. CT will show bone and calcification well, and collections of blood. It will also detect abnormalities of the brain and ventricles, such as atrophy, tumours, cysts, abscesses, vascular lesions and hydrocephalus. Diagnostic yield is often improved by the use of IV contrast and
thinner slicing using spiral CT. Surrounding bone structures
render posterior fossa CT images less useful, and CT is less sensitive to white matter changes than MRI.
MRI resolution is unaffected by bone and so is more
useful in the investigation of posterior fossa disease. Its sensitivity to cortical and white matter changes makes it effective in picking up inflammatory conditions such as multiple sclerosis, and in investigating epilepsy.
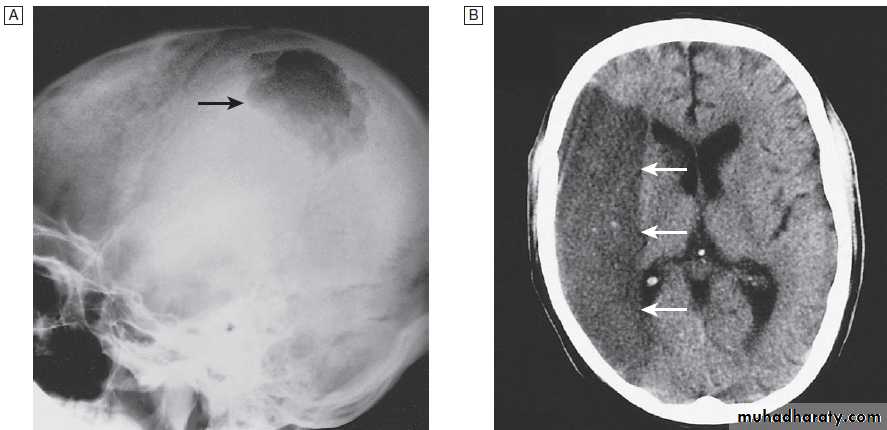
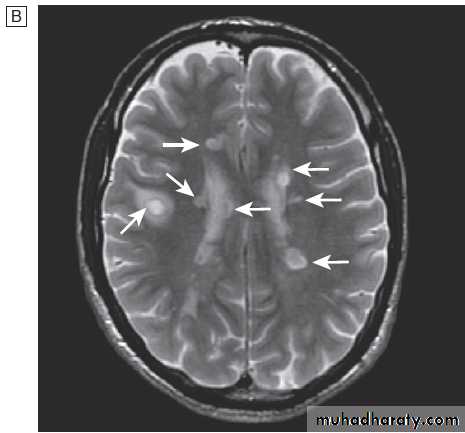
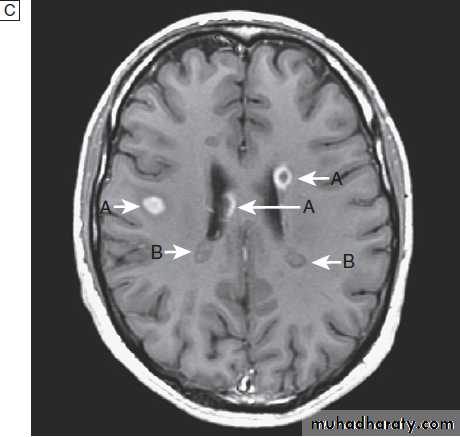
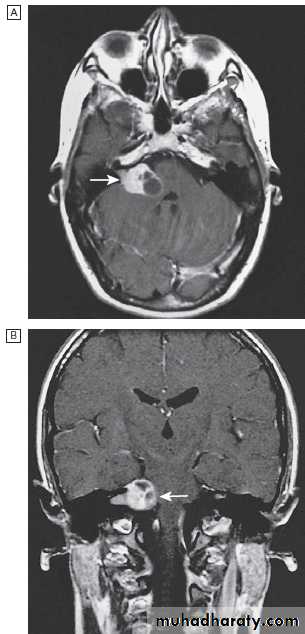
Fig. Different techniques of imaging the head and brain. A Skull X-ray showing lytic skull lesion (eosinophilic granuloma – arrow). B CT
showing complete middle cerebral artery infarct (arrows). C MRI showing widespread areas of high signal in multiple sclerosis (arrows). D SPECT after
caudate infarct showing relative hypoperfusion of overlying right cerebral cortex (arrows).
Cervical, thoracic and lumbar spine
Plain X-rays are useful in the investigation of trauma toVertebrae. MRI has transformed the investigation of these areas, since it can give information not only about the vertebrae and intervertebral discs, but also about their effects on the spinal cord and nerve roots.
Myelography is an invasive technique involving injection of contrast into the lumbar theca. While the outline of the nerve roots and spinal cord provides information about abnormal structure, the accuracy and wide availability of MRI have reduced the need for this.
Myelography may still be used for technical reasons or where MRI is unavailable, contraindicated, or precluded by the patient’s claustrophobia.
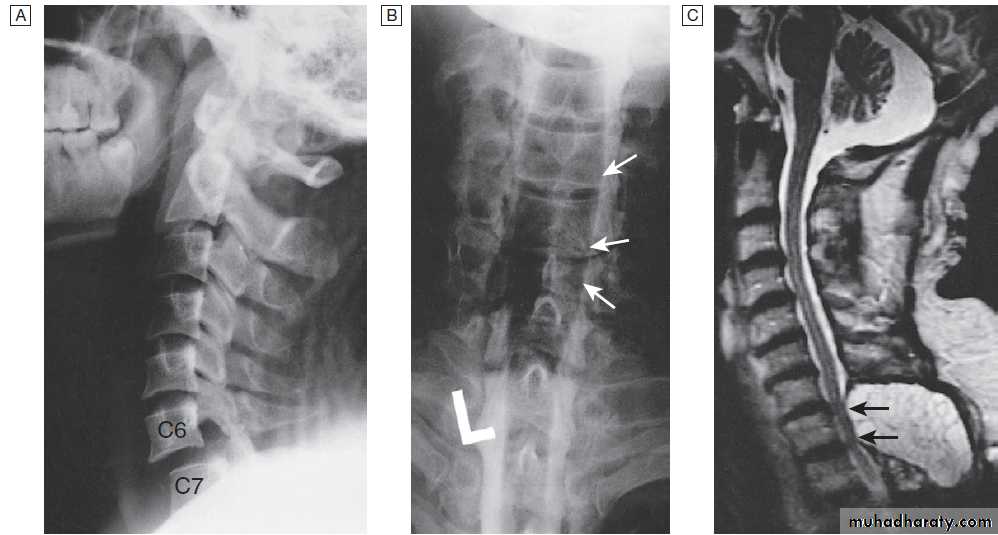
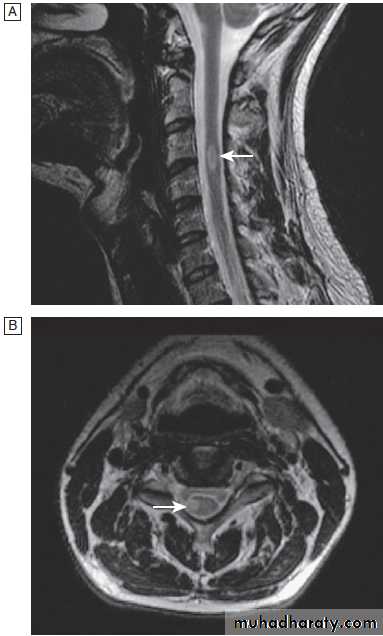
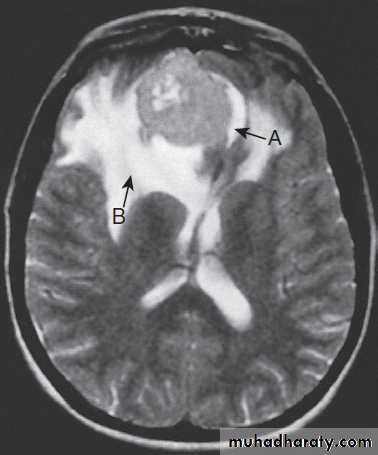
Different techniques of imaging the cervical spine. A Lateral X-ray showing bilateral C6/7 facet dislocation. B Myelogram showing
widening of cervical cord due to astrocytoma (arrows). C MRI showing posterior epidural compression from adenocarcinomatous metastasis to the posterior arch of T1 (arrows).
Neurophysiological testing
Electroencephalography (EEG)The EEG is used to detect electrical activity arising in the cerebral cortex. The EEG involves placing electrodes on the scalp to record the amplitude and frequency of the resulting waveforms. With closed eyes, the normal background activity is 8–13 Hz (alpha rhythm), most prominent occipitally and suppressed on eye opening.
Other frequency seen over different parts of the brain in different circumstances are beta (faster than 13/s), theta (4–8/s) and delta (slower than 4/s). In recent years, digital technology has allowed longer, cleaner EEG recordings’. The development of intracranial recording allows more sensitive monitoring via surgically placed electrodes in and around lesions to help increase the efficacy and improve the safety of epilepsy surgery.
Abnormalities in the EEG. Examples fast frequencies (beta) seen with sedating drugs such as benzodiazepines, or marked focal slowing noted over a structural lesion such as a tumour or an infarct. Since sleep induces marked changes in cerebral activity, EEG can be useful in characterising those conditions where sleep patterns are disturbed. In paroxysmal disorders such as epilepsy, EEG is useful when it captures activity during one of the events in question. Over 50% of patients with proven epilepsy will have a normal ‘routine’ EEG, and, conversely, the presence of epileptiform features does not of itself make a diagnosis Up to 5% of some normal populations may demonstrate epileptiform discharges on EEG which prevent its use as a screening test for epilepsy.
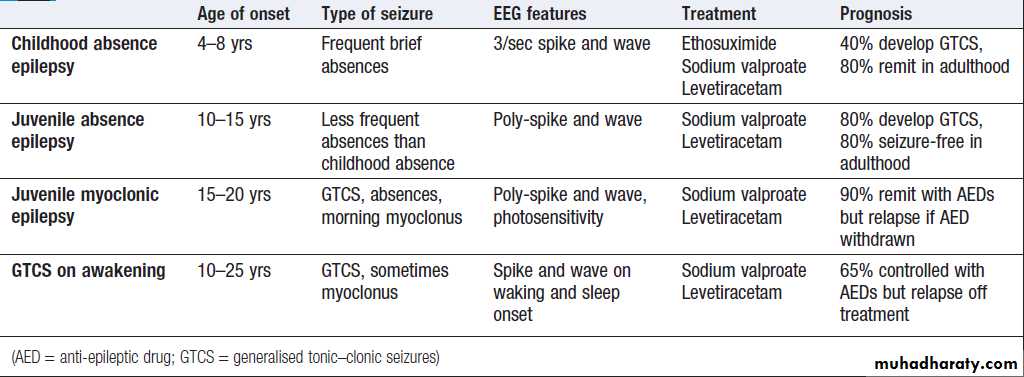
The EEG in epilepsy is predominantly used in its classification and prognosis, and in some patients to localise the seat of epileptiform discharges when surgery is being considered. Techniques such as hyperventilation or
photic stimulation can be used to increase the yield of
epileptiform changes, particularly in the generalised epilepsy syndromes. While some argue that it is possible to detect ‘spikes’ and ‘sharp waves’ to lend support to a clinical diagnosis, these are non-specific and therefore not diagnostic. During an epileptic seizure, highvoltage disturbances of background activity (‘discharges’) will often be noted. These may be generalised, as in the 3-Hz ‘spike and wave’ of childhood absence epilepsy, or more focal, as in localisation-related epilepsies.
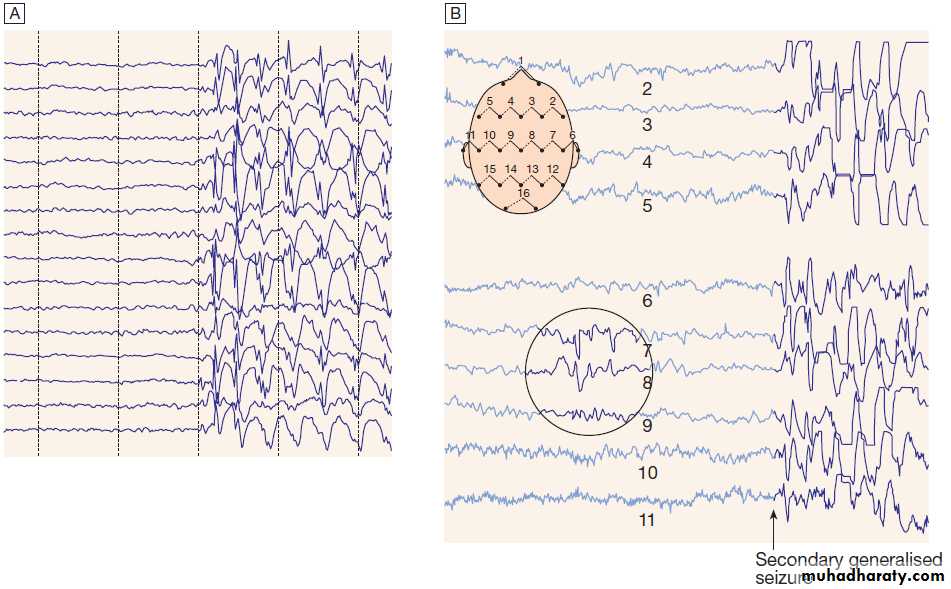
EEGs in epilepsy.
A Generalised epileptic discharge, as seen in epilepsy syndromes such as childhood absence or juvenile myoclonic epilepsy.B Focal sharp waves over the right parietal region (circled), with spread of discharge to cause a generalised tonic–clonic seizure.
Nerve conduction studies (NCS)
Electrical stimulation of a nerve causes an impulseto travel both efferently and afferently along the underlying axons. NCS make use of this, recording action potentials as they pass along peripheral nerves and (with motor nerves) as they pass into the muscle belly. Digital recording has enhanced sensitivity and reproducibility of these tiny potentials.
By measuring the time taken to traverse a known distance, it is possible to calculate nerve conduction velocities (NCVs). Healthy nerves at room temperature will conduct at a speed of 40–50 m/s . Significant slowing of conduction velocity, suggests impaired saltatory conduction due to peripheral nerve demyelination.
Such changes in NCS may be diffuse (as in a hereditary demyelinating peripheral neuropathy), focal (as in pressure palsies) or multifocal (e.g. Guillain–Barré syndrome, mononeuritis multiplex). The information gained can allow the disease responsible for peripheral nerve dysfunction to be better deduced. Repetitive nerve stimulation (RNS) at 3–15/s provides consistent CMAPs in healthy muscle. In myasthenia gravis ,where there is partial blockage of
acetylcholine receptors, however, there is a diagnostic
fall (decrement) in CMAP amplitude.
In contrast, an increasing CMAP with high-frequency RNS is seen in Lambert–Eaton myasthenic syndrome .
Motor nerve conduction tests.
Electrodes (R) on the muscle (abductor pollicis here) record the compound muscle action potential (CMAP) after stimulation at the median nerve at the wrist (S1) and from the elbow (S2). The velocity from elbow to wrist can be determined if the distance between the two stimulating electrodes (d) is known. A prolonged L1 (L = latency) would be caused by dysfunction distally in the median nerve (e.g. in carpal tunnel syndrome).A prolonged L2 is caused by slow nerve conduction (as in
demyelinating neuropathy). The F wave is a small delayed response that appears when the electrical signal travels backwards to the anterior horn cell, sparking a second action potential in a minority of fibres (see text). (NCV = nerve conduction velocity)
Electromyography (EMG)
Is usually performed with NCS, and involves needle recording of muscle electrical potential during rest and contraction. At rest, muscle is electrically silent but loss of nerve supply causes muscle membrane to become unstable, manifest as fibrillations, positive sharp waves (‘spontaneous activity’) or fasciculations.During muscle contraction, motor unit action potentials are recorded. Axonal loss or destruction will result in fewer motor units. Resultant sprouting of remaining units will lead to increasing size of each individual unit on EMG. Myopathy, in contrast, will cause muscle fibre splitting, which will result in a large number of smaller units on EMG.
Other abnormal activity, such as myotonic discharges, may signify abnormal ion channel conduction, as in myotonic dystrophy or myotonia congenita. Specialised single fibre EMG (SFEMG) can be used to investigate neuromuscular junction transmission. Measuring ‘jitter’ and ‘blocking’ can identify the effect of antibodies in reducing the action of acetylcholine on the receptor.
Evoked potentials (EP).
The cortical response to visual, auditory or electrical
stimulation can be measured on an EEG as EP. Assessing
the latency (the time delay) and amplitude can give
information about the integrity of the relevant pathway. In practice, visual evoked potentials (VEPs) are most commonly used to help differentiate CNS demyelination from small-vessel white-matter changes .
Magnetic stimulation
Central conduction times can also be measured usingelectromagnetic induction of action potentials in the
cortex or spinal cord by the local application of specialized coils.
Routine blood tests
Many systemic conditions that can affect the nervous
system can be identified by simple blood tests.
Nutritional deficiencies, metabolic disturbances, inflammatory conditions, or infections may all present or be
associated with neurological symptoms, and basic blood
tests (full blood count, ESR, C-reactive protein, biochemical screening) may provide clues.
Immunological tests
Recent developments have seen a host of new immunemediated conditions emerge in clinical neurology, with effects ranging from muscle and neuromuscular junction
disturbance (causing weakness and muscle pain) to specific
neuronal ion channels (causing cognitive decline,
epilepsy and psychiatric changes).
The last decade has seen the identification of many causative antibodies , and it is likely that further conditions will turn out to have an immune basis.
Genetic testing
An increasing number of inherited neurological conditionscan now be diagnosed by DNA analysis .
These include diseases caused by increased numbers
of trinucleotide repeats, such as Huntington’s disease
, myotonic dystrophy and some types
of spinocerebellar ataxia . Mitochondrial DNA
can also be sequenced to diagnose relevant disorders.
Lumbar puncture
Lumbar puncture (LP) is the technique used to obtaina CSF sample and provides an indirect measure of
intracranial pressure. After local anaesthetic injection, a
needle is inserted between lumbar spinous processes (usually between L3 and L4) through the dura and into
the spinal canal. Intracranial pressure can be deduced (if
patients are lying on their side) and CSF removed for
analysis. CSF pressure measurement is important in the
diagnosis and monitoring of idiopathic intracranial
hypertension . In this condition, the LP itself is therapeutic.
CSF is normally clear and colourless, and the tests
that are usually performed include a naked eye examination of the CSF and centrifugation to determine the
colour of the supernatant (yellow, or xanthochromic,
some hours after subarachnoid haemorrhage .
Routine analysis will involve a cell count, as well as
assay of glucose and protein.
CSF assessment is important in investigating infections
(meningitis or encephalitis), subarachnoid haemorrhage
and inflammatory conditions (multiple sclerosis,
sarcoidosis and cerebral lupus).
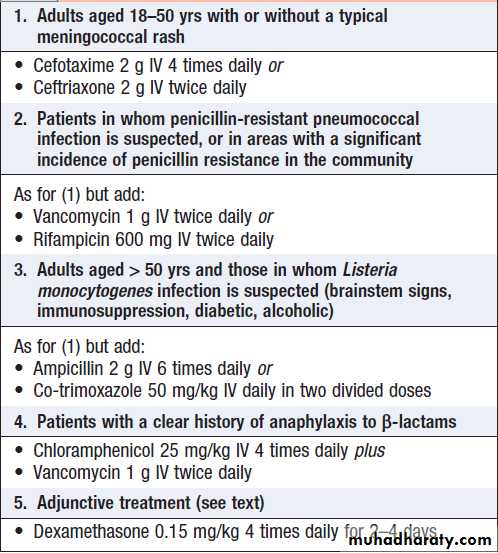
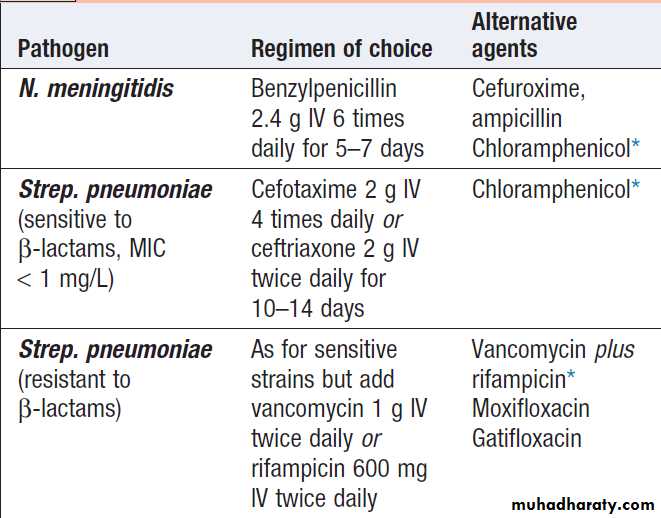
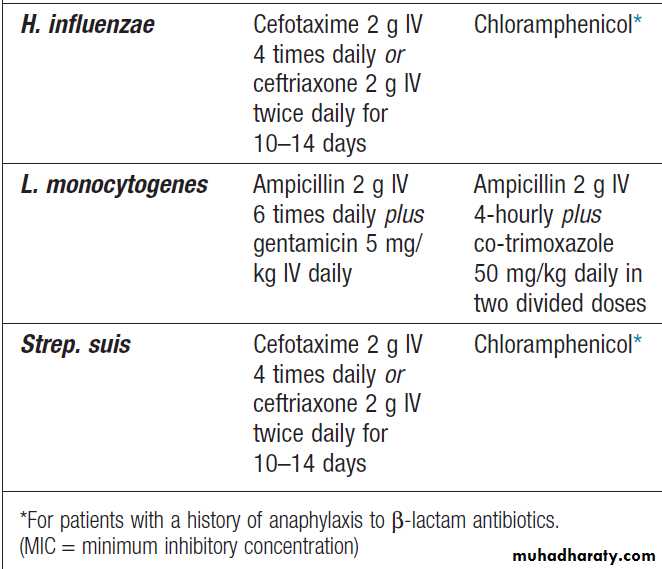
Normal values and abnormalities found in specific conditions are shown in Box.
More sophisticated analysis allows measurement of
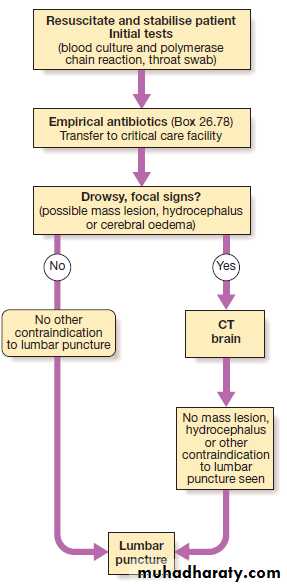
antibody formation solely within the CNS (oligoclonalbands), genetic analysis (e.g. PCR for herpes simplex or tuberculosis), immunological tests (paraneoplastic antibodies) and cytology (to detect malignant cells). If there is a cranial space-occupying lesion causing raised intracranial pressure, LP presents a theoretical risk of downward shift of intracerebral contents, a potentially fatal process known as coning . Consequently, LP is contraindicated if there is any clinical suggestion of raised intracranial pressure (papilloedema), depressed level of consciousness, or focal neurological signs suggesting a cerebral lesion, until imaging (by CT or MRI) has excluded a space occupying lesion or hydrocephalus.
When there is a risk of local haemorrhage (thrombocytopenia, disseminated intravascular coagulation or anticoagulant treatment), then caution should be exercised or specific measures should be taken. LP can be safely performed in patients on antiplatelet drugs or low-dose heparin, but may be unsafe in patients who are fully anticoagulated due to the increased risk of epidural haematoma.
About 30% of LPs are followed by a postural headache,
due to reduced CSF pressure. The frequency of
headache can be reduced by using smaller or atraumatic
needles. Other rarer complications involve transient
radicular pain, and pain over the lumbar region during
the procedure. Aseptic technique renders secondary
infections such as meningitis extremely rare.
How to interpret CSF results
BiopsyBiopsies of nervous tissue (peripheral nerve, muscle,
meninges or brain) are occasionally required for
diagnosis. Histological examination can help identify underlying causes, such as vasculitides or infiltrative disorders like amyloid. Nerve biopsy should not
be undertaken lightly since there is an appreciable morbidity; it should be reserved for cases where the diagnosis is in doubt after routine investigations and where it will influence management. Muscle biopsy is performed more frequently and is indicated for the differentiation of myositis and myopathies. These conditions can usually be distinguished by histological examination, and enzyme histochemistry can be useful when mitochondrial diseases and storage diseases are suspected.
The quadriceps muscle is most commonly biopsied. Although pain and infection can follow the procedure, these are less of a problem than after nerve biopsy.
Brain biopsy is required when imaging fails to clarify
the nature of intracerebral lesions: for example, in unexplained degenerative diseases such as unusual cases of dementia and in patients with brain tumour. Most biopsies are performed stereotactically through a burr-hole
in the skull, which lowers complication rates.
Nevertheless, haemorrhage, infection and death still occur and brain biopsy should only be considered if a diagnosis is otherwise elusive.
PRESENTING PROBLEMS IN NEUROLOGICAL DISEASE
History-taking allows doctor and patient to get to know one another – many neurological diseases follow chronic paths, and this may be the first of many such consultations. It also allows the clinician to obtain information about thepatient’s affect, cognition and psychiatric state.
It is important to be clear about what patients mean
by certain words. Patients may find it difficult to
describe symptoms – for instance, weakness may be
called ‘numbness’, while there are many possible interpretations of ‘dizziness’. These must be clarified; even in emergency situations, a clear, accurate history is the
foundation of any management plan.
While the story should come primarily from the patient, input from eye-witnesses and family members is crucial if the patient is unable to provide details or if there has been loss of consciousness.
The aim of the history is to answer two key issues:
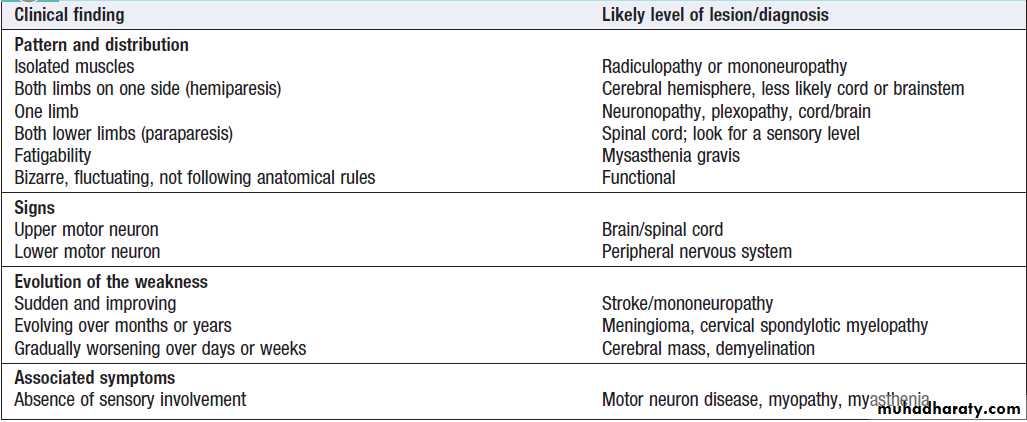
where is the lesion and what is the lesion ?
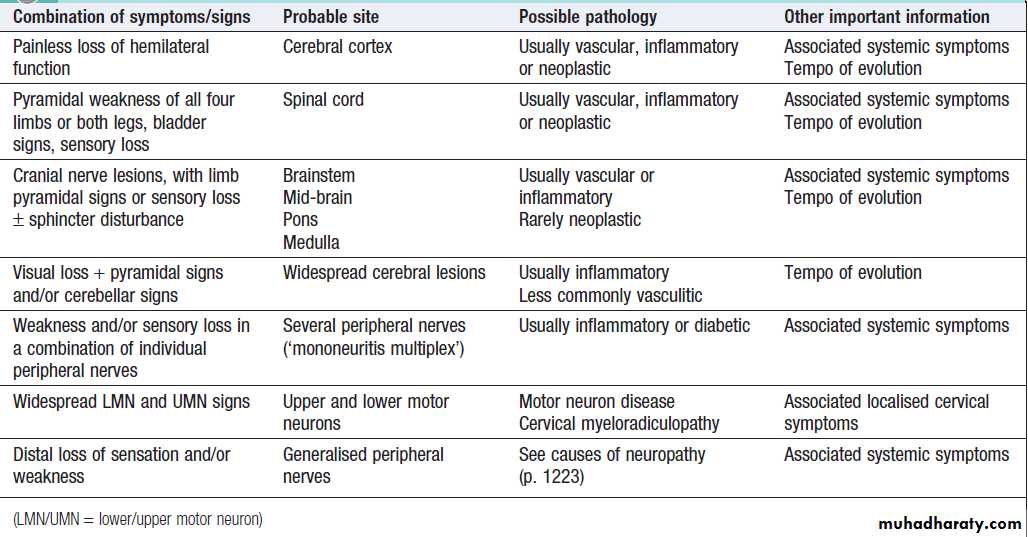
Some common combinations of symptoms may suggest particular locations for a lesion .
Enquiry about handedness is important; lateralisation of the dominant hand helps designate the dominant hemisphere, which in turn may help to localise any pathologies, or to plan rehabilitation or treatment strategies in asymmetrical disorders such as stroke or Parkinson’s disease.
Epidemiology must be borne in mind; how likely is
it that this particular patient has any specific conditionunder consideration? For example, a 20-year-old with
right-sided headache and tenderness will not have temporal arteritis, but this is an important possibility if such
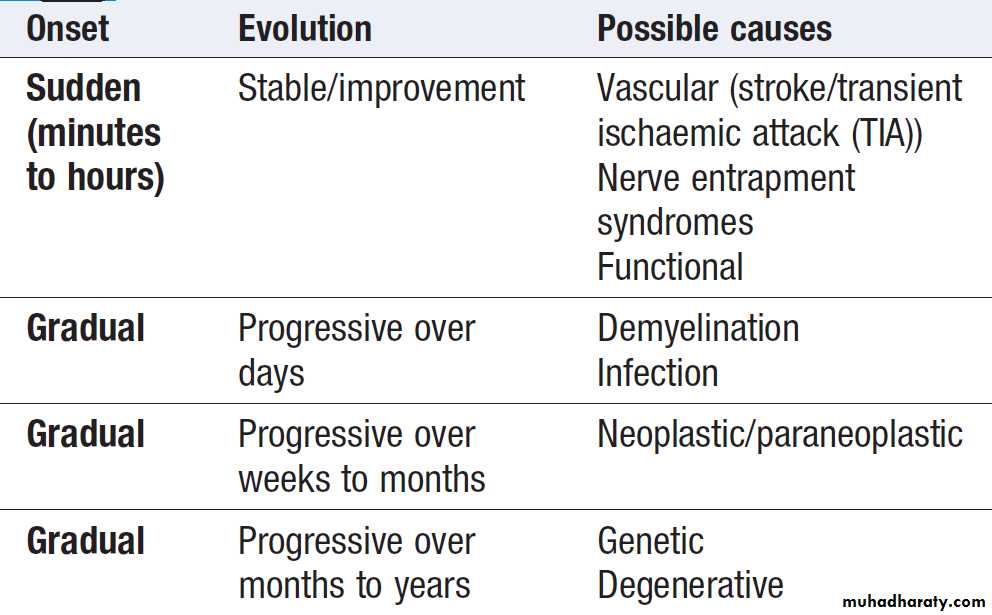
symptoms present in a 78-year-old female. Determining the evolution and speed of onset and progression of a disease is important . For example, if right-hand weakness occurred overnight, it would suggest a stroke in an older person or an acute entrapment neuropathy in a younger one. Evolution over several days, however, might make demyelination (multiple sclerosis) a possible diagnosis, or perhaps a subdural haematoma if the weakness was preceded by a head injury in an older person taking warfarin.
Progression over weeks might bring an intracranial mass
lesion or motor neuron disease into the differential. Slowprogression over a year or so, with difficulty in using the
hand, could suggest a degenerative process such as
Parkinson’s disease. The impact on day-to-day activities,
such as walking, climbing stairs and carrying out fine
hand movements, should also be established in order to
gauge the level of associated disability.
Estimates of the frequency and duration of specific
events are essential when taking details of a paroxysmal
disorder such as migraine and epilepsy.
How to take
a neurological historyThe key diagnostic questions
How to ‘localise’ neurological disease
The evolution of symptoms
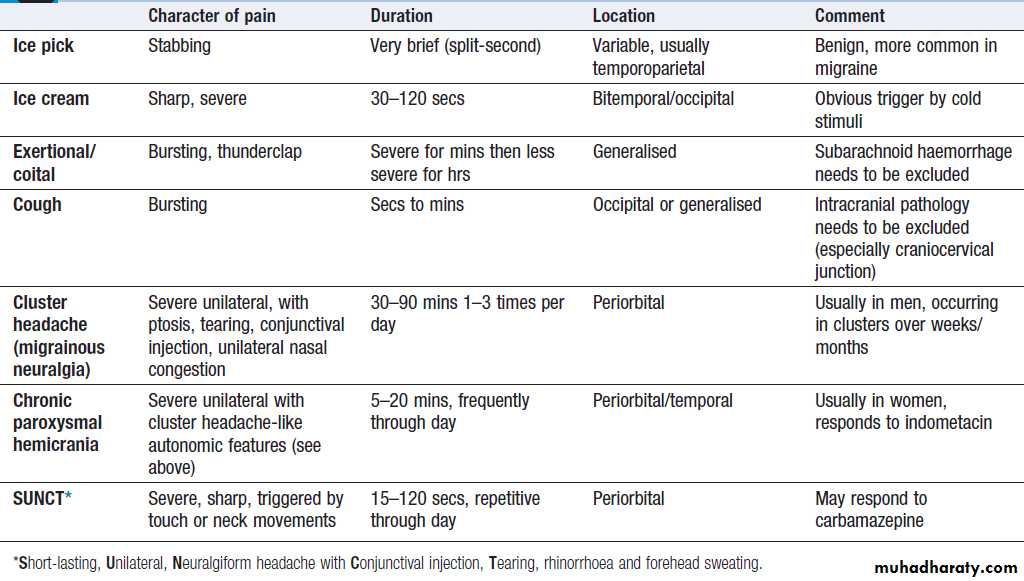
Headache and facial pain
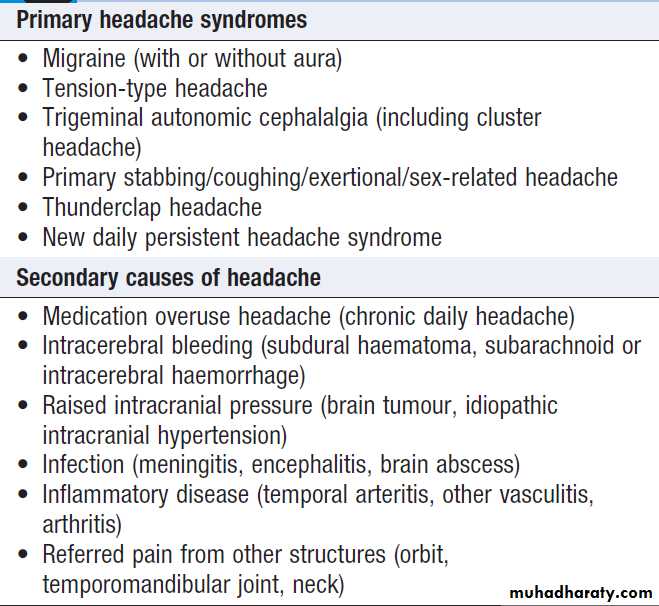
Headache is common and causes considerable worry,
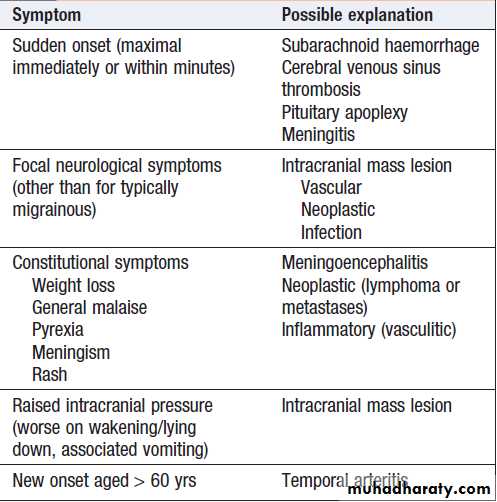
but rarely represents sinister disease. The tempo of evolution of headache is critical; sudden-onset headache, maximal immediately, is always a ‘red flag’ and should prompt rapid assessment in hospital for possible subarachnoid hemorrhage or other sinister causes, even though only 10–25% of patients harbor serious pathology. Clues to other possible causes (e.g. rash in meningitis) should be sought . Headache that evolves over hours to days is much less likely to be sinister. It is important to establish whether the headache comes and goes, with periods of no headache in between (usually migraine), or whether it is present all or almost all the time.
Associated symptoms, such as preceding visual symptoms, nausea/vomiting or photophobia/ phonophobia, may support a diagnosis of migraine, but others, such as progressive focal symptoms or constitutional upset like weight loss or fever, may suggest a more sinister cause (e.g. cancer or meningitis). The behaviour of the patient during headache is often instructive; migraine patients typically retire to bed to sleep in a dark room, whereas cluster headache often induces agitated and restless behaviour. Headache duration may indicate a diagnosis; headaches
that have been present for months or years are
almost never sinister (although paradoxically worry
patients), whereas new-onset headache, especially in the
elderly, is more of a concern.
In a patient over 60 years with head pain localised to one or both temples, temporal arteritis should be considered, especially if temporal pulses are absent and/or the arteries are enlarged and tender. Most outpatients with headache will have migraine (intermittent, lasting a few hours, associated with migrainous symptoms) or chronic daily headache syndrome (often present for months to years, without associated symptoms and refractory to analgesia). These are easy to recognise but patients are often very worried about their symptoms, so it is important to elicit such concerns and to explain why sinister disease is unlikely and investigation is rarely required. Sinusitis, ‘eye strain’, food allergies and uncomplicated hypertension are almost never the explanation for persistent headache.
Primary and secondary headache syndromes
‘Red flag’ symptoms in headache
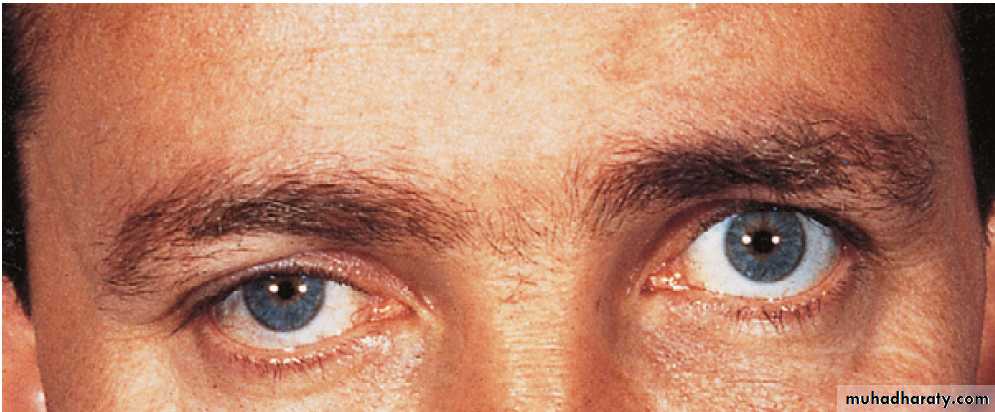
Ocular painAssuming ocular disease (acute glaucoma) has been excluded, ocular pain may be due to trigeminal autonomic cephalalgias (TACs) or, rarely, inflammatory or infiltrative lesions at the apex of the orbit or the cavernous sinus, when 3rd, 4th, 5th or 6th cranial nerve involvement is usually evident.
Facial pain
Can be due to dental or TM joint problems. Acute sinusitis is apparent from other features of sinus congestion/infection
and may cause localised pain over the affected sinus. Facial pain is not uncommon in migraine but some syndromes can present solely with facial pain. The most common neurological causes are trigeminal neuralgia, herpes zoster (shingles) and post-herpetic neuralgia, all characterised by their extreme severity.
In trigeminal neuralgia, the patient describes bouts of brief (seconds), lancinating pain (‘electric shocks’), most frequently felt in the second and third divisions of the
nerve and often triggered by talking or chewing.
Shingles most commonly affects the first (ophthalmic)
division of the trigeminal nerve, and pain usually precedes
the rash. Post-herpetic neuralgia may follow, typically
a continuous burning pain throughout the affected
territory, with marked sensitivity to light touch (allodynia)
and resistance to treatment.
Destructive lesions of the trigeminal nerve usually cause numbness rather than pain.
Persistent idiopathic facial pain is most frequently
seen in middle-aged women.
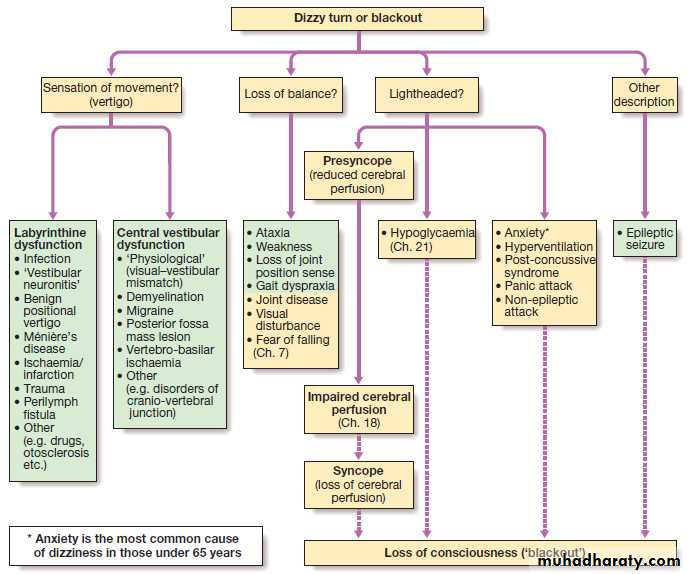
Dizziness, blackouts and ‘funny turns’
Episodic lost or altered consciousness is a frequentsymptom in primary care and hospital practice. Some
patients use ‘blackout’ to describe a purely visual
symptom, rather than loss of consciousness. Some
individuals may understand ‘dizziness’ to mean an
abnormal perception of movement (vertigo), but others
will mean a feeling of faintness, and yet others unsteadiness .
The clinician thus needs to elucidate the exact nature of the symptoms that the patient experiences . Careful history with corroboration will usually establish whether there has been full consciousness, altered consciousness, vertigo, transient amnesia or something else.
A diagnostic approach to the patient with dizziness, funny turns or blackouts. (Neurological causes are shown in green.)
Dizziness in old age
Loss of consciousness (LOC)Consciousness may be defined as an awareness of the
environment and ability to respond to it. LOC, other than in sleep, suggests a global dysfunction of the brain. This most commonly occurs because of temporary loss of blood supply to the whole brain (syncope or faint). Alternatively, LOC can occur due to a sudden electrical dysfunction of the brain, as occurs during a seizure. Whilst most episodes of transient LOC are due to syncope or seizure, psychogenic blackouts (also known as non-epileptic seizure or pseudoseizure) need to be considered. No amount of investigation can replace a clear history in these circumstances. The subjective experience is important, but objective description from an eye-witness is equally helpful.
How to differentiate seizures from syncope
SyncopeTypically, syncope is preceded by a brief feeling of lightheadedness.
Neurocardiogenic syncope is more likely on standing, and may be provoked by pain or emotion.
There may be darkening of vision, ringing in the ears, symptoms of hyperventilation, distal tingling, feelings of nausea, clamminess or sweating.
The LOC is gradual and brief, and the patient recovers quickly without confusion as long as he or she has assumed a horizontal position. There is often some brief stiffening
and limb twitching, which requires differentiation from
seizure-like movements.
It is rare for syncope to cause injury or to cause amnesia after regaining awareness.
During a syncopal attack, incontinence of urine can
occur. Tongue-biting is less common in syncope and, if
present, usually involves little trauma.
Cardiac syncope caused by a sudden drop in
cardiac output, may be provoked by exertion in those
with severe aortic stenosis, ischaemia or hypertrophic
obstructive cardiomyopathy, or without warning in
patients with cardiac arrhythmia.
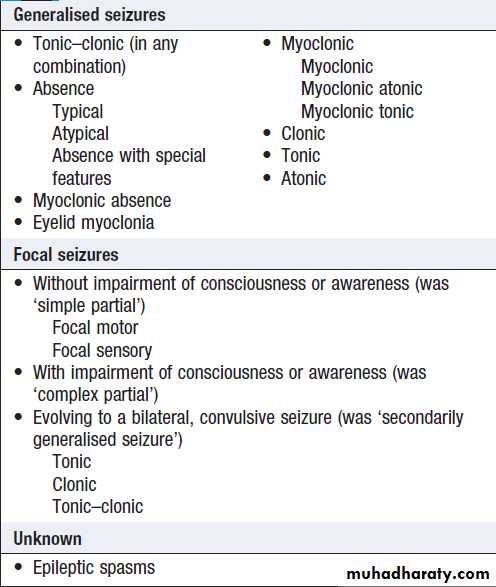
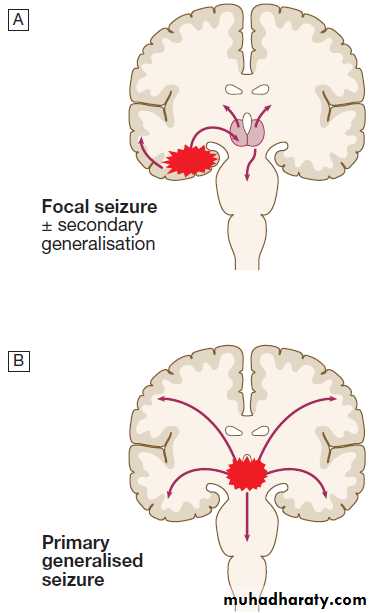
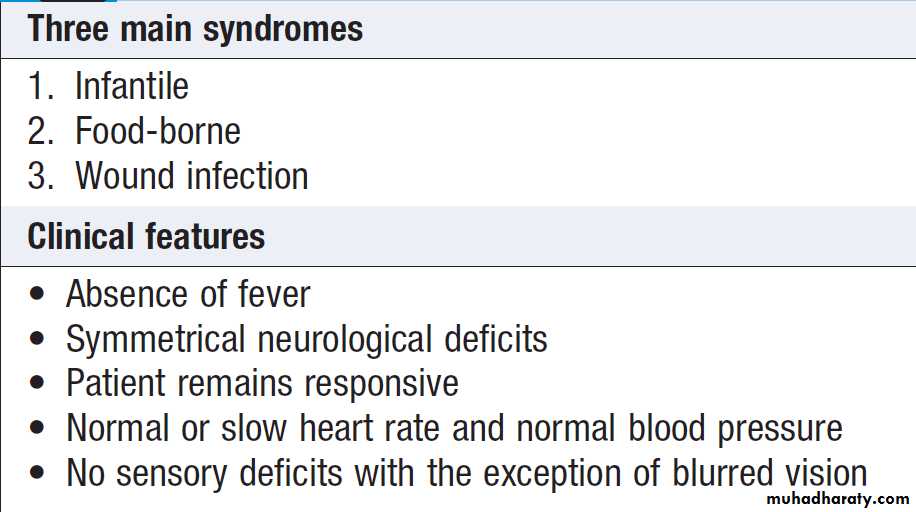
Seizures
The diagnosis of generalised tonic–clonic seizures, inwhich there is loss of consciousness is easy but lack of eyewitness accounts can leave uncertainty. Less dramatic seizures, such as absences or some focal seizures which cause alteration of consciousness without the patient falling to the ground, may merely be experienced as ‘lost time’.
Non-epileptic, psychogenic, pseudoseizures, psychogenic non-epileptic seizures . Around 10% of patients referred to a first seizure clinic will have LOC resulting from psychological reactions to circumstances or traumatic life events. Clinical pointers to include specific emotional triggers, partially retained awareness, dramatic movements, very prolonged duration (up to hours ).
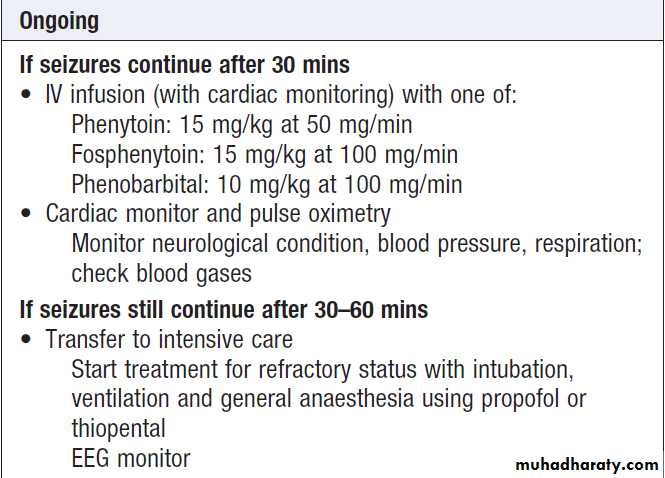
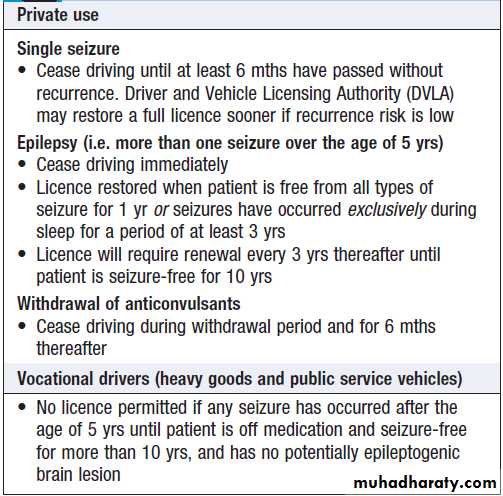
Status epilepticus
Status epilepticus is seizure activity not resolving spontaneously, or recurrent seizure with no recovery of consciousness in between. Persisting seizure activity has arecognised mortality and is a medical emergency.
Cyanosis, pyrexia, acidosis and sweating may occur, and complications include aspiration, hypotension,
cardiac arrhythmias and renal or hepatic failure.
In patients with pre-existing epilepsy, the most likely
cause is a fall in anti-epileptic drug levels. In de novo
status epilepticus, it is essential to exclude precipitants
such as infection (meningitis, encephalitis), neoplasia
and metabolic derangement (hypoglycaemia, hyponatraemia, hypocalcaemia).
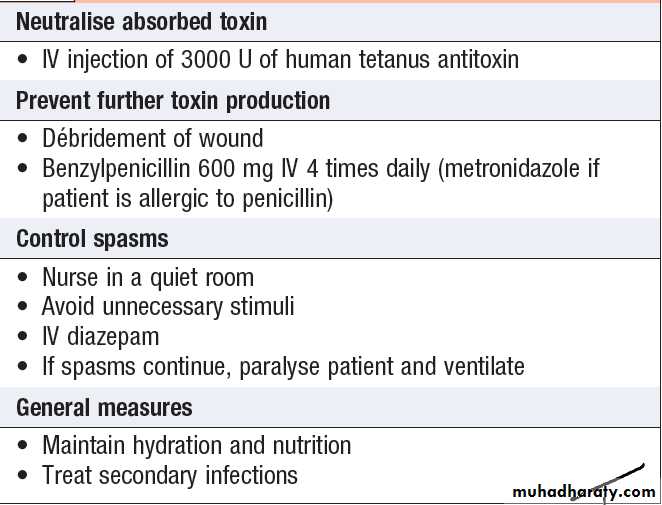
Management of status epilepticus
Management of status epilepticus'cont'd
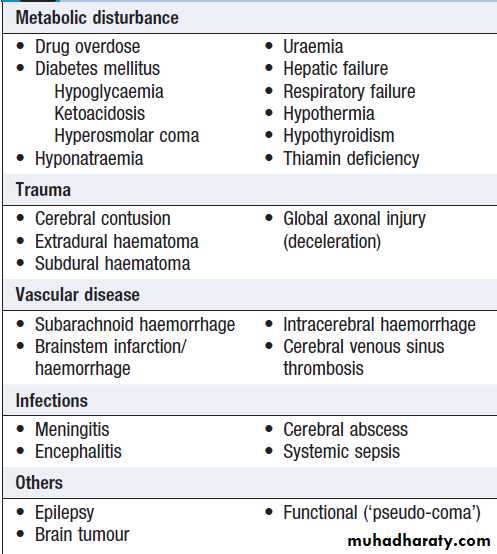
Coma
Conscious level should be measured using the GlasgowComa Scale (GCS). Although developed for use in head injury, GCS is widely used in medical coma, but disorders that affect language or limb function (e.g. left hemisphere stroke, locked-in syndrome) may reduce its usefulness. There are many causes of coma including neurological (structural or non-structural brain disease) or non-neurological (e.g. type II respiratory failure). The mode of onset of coma and any precipitating event is crucial to establishing the cause, and should be obtained from family or other witnesses .
Failure to obtain an adequate history for patients in coma is a common cause of diagnostic delay.
Once the patient is stable from a cardiorespiratory perspective, examination should include accurate assessment of conscious level (see below) and thorough
general medical examination, looking for clues such as
needle tracks indicating drug abuse, rashes, fever and
focal signs of infection, including neck stiffness or evidence
of head injury.
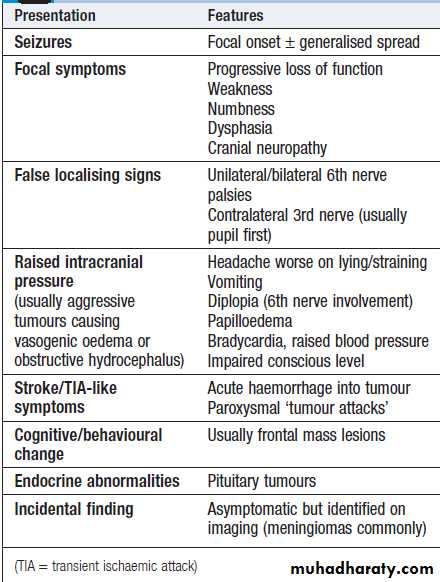
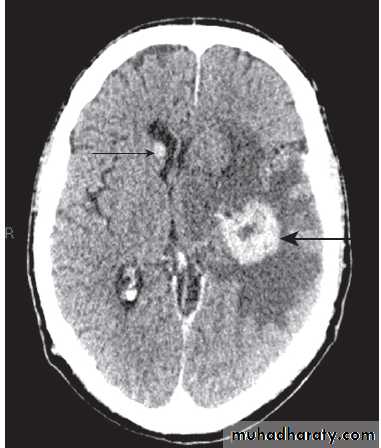
Focal neurological signs may suggest a structural explanation (stroke or tumour) or may be falsely localising .
Causes of coma
Glasgow Coma Scale
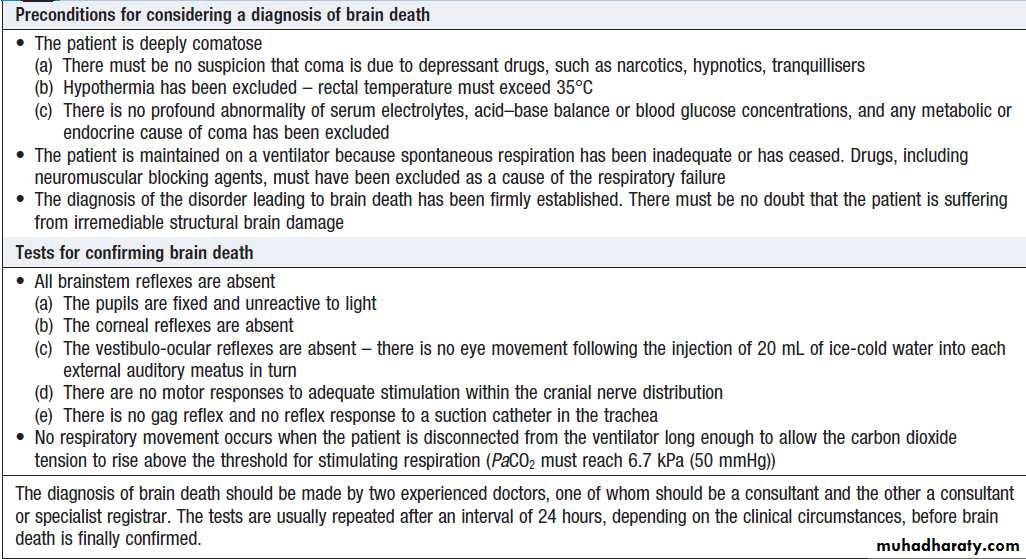
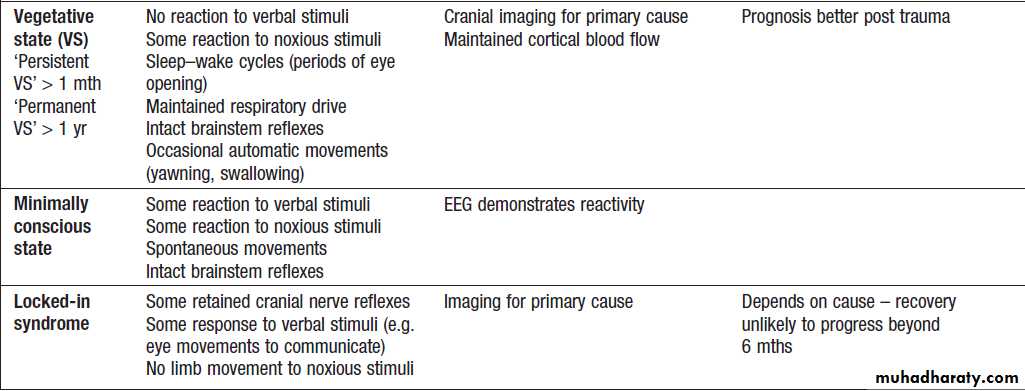
Brain death and minimally conscious statesComa is loss of consciousness related to loss of brain
function. Brain death is a state in which cortical and
brainstem function is irreversibly lost. Diagnostic criteria vary between countries if satisfied. Diagnosing brain death is complex and should only be done by a clinician with appropriate expertise, as clinical differentiation from reduced consciousness can be challenging .
The ‘locked-in’ syndrome, Patient is paralysed except for eye movements, requires preserved hemisphere function (and thus consciousness), but a strategically placed lesion in the ventral pons (usually infarction) causes complete paralysis.
The vegetative state implies some retention of brainstem function and minimal cortical function, with loss of awareness of the environment.
UK criteria for the diagnosis of brain death
Classification of brain death and reduced conscious states
DeliriumDelirium is a common result of cortical impairment and
is more common in old age. It manifests as a disturbance
of arousal with global impairment of mental function
causing drowsiness with disorientation, perceptual
errors and muddled thinking. Fluctuation is typical and
confusion is often worse at night.
Emotional disturbance (anxiety, irritability or depression) and psychomotor changes (agitation, restlessness or retardation) are common.
Amnesia
Memory disturbance is a common symptom. In the
absence of significant functional impairment (e.g. inability
to work, dyspraxias, loss of daily function), many will prove to have benign memory dysfunction related to age, mood. Temporary loss of memory may be due to a transient
toxic confusional state, the post-ictal period after seizure,
or transient global amnesia.
Transient global amnesia
This predominantly affects middle-aged with an abrupt, discrete loss of anterograde memory function lasting up to a few hours. During the episode, patients are unable to record new memories, results in repetitive questioning, the hallmark of this condition. Consciousness is preserved and patients may perform even complex motor acts normally.
During the attack there is retrograde amnesia for the events of the past few days, weeks or years. After 4–6 hours, memory function and behaviour return to normal but the patient has persistent, complete amnesia for the duration of the attack itself. A vascular aetiology is unlikely and amnesia may be due to a benign process similar to migraine. The patient has no physical signs and, if imaging is normal and no seizure markers are present, then the patient can be reassured.
Persistent amnesia
Patients with persistent memory disturbance must have
serious neurological disease excluded. Symptoms corroborated by relatives or colleagues are likely to be more significant than those noted by the patient only.
Disturbance of episodic or working memory (previously
called ‘short-term memory’) must be distinguished fromsemantic memory (memory for concept-based knowledge
unrelated to specific experiences). Episodic memory is selectively impaired in Korsakoff’s syndrome (often secondary to alcohol) or bilateral temporal lobe damage. Progressive deterioration over months suggests an underlying dementia, but it is important to perform a full medical assessment to detect any underlying medical problem.
It is important to identify and treat depression
in patients with memory loss. Depression may present as a ‘pseudo-dementia’, with concentration and memory impairment as dominant features, and this is often reversible with antidepressant medication.
Weakness
The pattern and evolution of weakness and the clinical signs provide clues to the site and nature of the lesion.
It is important to establish whether the patient has loss of power rather than reduced sensation or fatigue. Pain may restrict movement and thus mimic weakness. Paradoxically, sensory neglect may leave patients unaware of even severe weakness. Patients with parkinsonism may complain of weakness; extrapyramidal signs of rigidity (cogwheel or lead pipe) and bradykinesia should be evident, and a resting tremor, usually asymmetrical, may provide a further clue . Simple observation of the patient walking into the consulting room may be diagnostic.
On examination, the signs are often variable (e.g. the patient can walk but appears to have no leg movement when assessed on the couch), and strength may appear to ‘give way’, with the patient able to achieve full power for brief bursts. This does not occur in disease.
Hoover’s sign is useful to confirm functional weakness,
and relies on the normal phenomenon of simultaneous
hip extension when the contralateral hip flexes. In functional weakness, one may see hip extension weakness (rare in organic disease), which then returns to full strength on testing contralateral hip flexion. This sign may be demonstrated to the patient in a nonconfrontational manner, to show that the potential limb power is intact.
Distinguishing signs in upper versus LMN syndromes
How to assess weakness
Patterns of motor loss according to the anatomical site of the lesion.
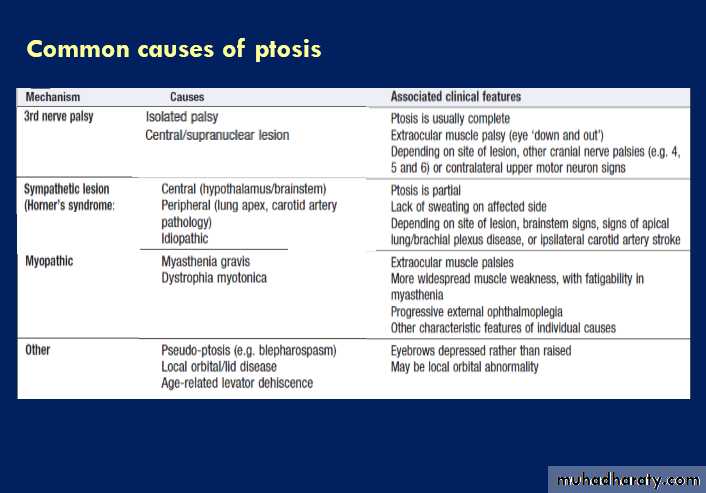
Facial weakness
Facial nerve palsy (Bell’s palsy)
One of the most common causes of facial weakness is
Bell’s palsy, a LMN of the 7th (facial) nerve, affecting all ages and both sexes. The lesion is within the facial canal. Symptoms usually develop subacutely over a few hours, with pain around the ear preceding the unilateral facial weakness. Patients often describe the face as ‘numb’, but there is no objective sensory loss (except to taste if the chorda tympani is involved). Hyperacusis may occur if the
nerve to stapedius is involved, and there may be diminished salivation and tear secretion. Examination reveals an ipsilateral LMN facial nerve palsy. Vesicles in the ear or palate may indicate primary HZ infection .
Steroids improve recovery rates if started within 72 hours of onset but antiviral drugs are not effective.Taping the eye shut overnight helps prevent exposure keratitis and corneal abrasion. About 80% recover spontaneously within 12 weeks. Plastic surgery may be considered for the minority left with facial disfigurement after 12 months. Recurrence is unusual ,should prompt further investigation.
Aberrant re-innervation may occur during recovery, producing unwanted facial movements, such as eye closure when the mouth is moved (synkinesis), or ‘crocodile tears’ (tearing during salivation). Unlike Bell’s palsy, lesions with an UMN origin partly spare the upper face. Cortical
lesions may cause a facial weakness either in isolation
or with associated hemiparesis and speech difficulties.
Sensory disturbance
Patients often find sensory symptoms difficultto describe, and sensory examination is difficult for
both doctor and patient. While neurological disease
can cause sensory symptoms, systemic disorders can
also be responsible. Tingling in both hands and around
the mouth can occur as the result of hyperventilation
or hypocalcaemia .
Numbness and paraesthesia
The history may give the best clues to localisation and
pathology. Certain common patterns are recognised:
in migraine, the aura may consist of spreading tingling
or paraesthesia, followed by numbness evolving over
20–30 minutes over one half of the body.
Sensory loss caused by a stroke or TIA occurs much more rapidly and is typically negative (numbness) rather than positive (tingling). Rarely, unpleasant paraesthesia of sensory epilepsy spreads within seconds. The sensory alteration of inflammatory spinal cord lesions often ascends from one or both lower limbs to a distinct level on the trunk over hours to days.
Psychogenic sensory change can occur as a manifestation of anxiety or as part of a conversion disorder . In such cases, the distribution usually does not conform to a known anatomical pattern nor fit with any organic disease. Care must be taken in diagnosing non-organic sensory problems.
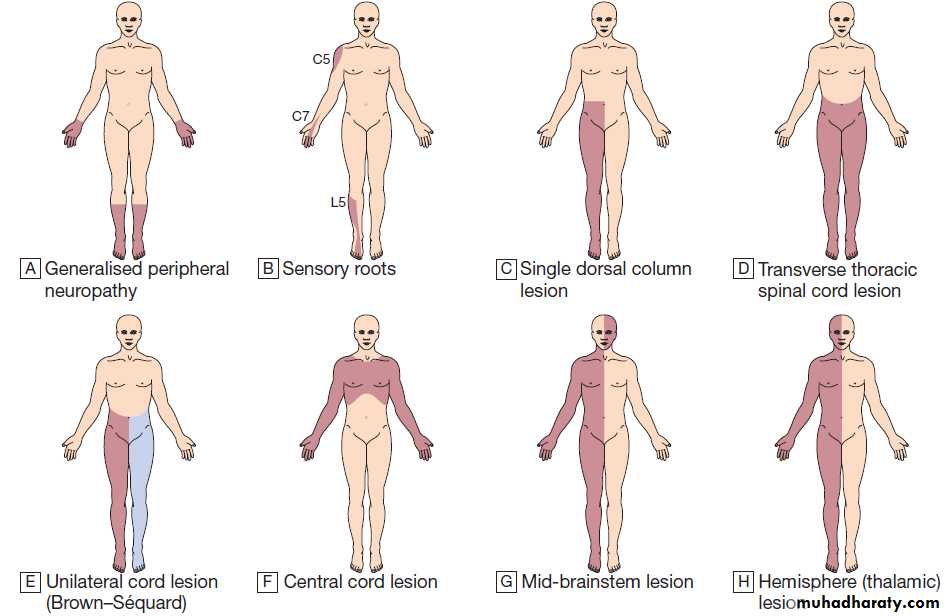
Patterns of sensory loss. A Generalised peripheral neuropathy. B Sensory roots: some common examples. C Single dorsal column lesion (proprioception and some touch loss). D Transverse thoracic spinal cord lesion. E Unilateral cord lesion (Brown–Sequard): ipsilateral dorsal
column (and motor) deficit and contralateral spinothalamic deficit. F Central cord lesion: ‘cape’ distribution of spinothalamic loss. G Mid-brainstem lesion: ipsilateral facial sensory loss and contralateral loss on body below the vertex. H Hemisphere (thalamic) lesion: contralateral loss on one side of face and body.
Sensory loss in peripheral nerve lesions
Here the symptoms are usually of sensory loss and paraesthesia. Single nerve lesions cause disturbance in thesensory distribution of the nerve, whereas in diffuse
neuropathies the longest neurons are affected first,
giving a characteristic ‘glove and stocking’ distribution.
If smaller nerve fibres are preferentially affected (e.g. in
diabetic neuropathy), temperature and pin-prick (pain)
are reduced, whilst vibration sense and proprioception
(modalities served by the larger, well-myelinated,
sensory nerves) may be spared. In contrast, vibration
and proprioception are particularly affected if the neuropathy is demyelinating in character , producing symptoms of tightness and swelling with impairment
of proprioception and vibration sensation.
Sensory loss in nerve root lesions
Typically present with pain, either within the spine or limb plexuses.It is often felt in myotome rather than dermatome.
Sensory loss in spinal cord lesions
Transverse lesions of the spinal cord produce loss of all
sensory modalities below that segmental level, although
the clinical level may only be manifest 2–3 segments
lower than the anatomical site of the lesion. Very often,
there is a band of paraesthesia or hyperaesthesia at the
top of the area of sensory loss. Clinical examination may
reveal dissociated sensory loss, i.e. different patterns in
the spinothalamic and dorsal columnar pathways.
If the transverse lesion is vascular due to anterior spinal artery thrombosis, the spinothalamic pathways may be affected while the posterior one-third of the spinal cord (the dorsal column modalities) may be spared.
Lesions damaging one side of the spinal cord will produce loss of spinothalamic modalities (pain and temperature) on the opposite side, and of dorsal column modalities (joint position and vibration sense) on the same side of the body – the Brown–Séquard syndrome.
Lesions in the centre of the cord such as Syringomyelia spare the dorsal columns but involve the spinothalamic fibres crossing the cord from both sides over the the lesion. There is no sensory loss in segments above and below the lesion; this is described as ‘suspended’ sensory loss.
There is sometimes reflex loss at the level of the lesion if afferent fibres of the reflex arc are affected.
An isolated lesion of the dorsal columns is not uncommon in multiple sclerosis. This produces a characteristic unpleasant, tight feeling over the limb(s) involved and, while there is no loss of pin-prick or temperature sensation, the associated loss of proprioception may severely limit function of the affected limb(s).
Sensory loss in brainstem lesions
Can be associated with sensory loss, but the distribution depends on the site of the lesion. A lesion limited to the trigeminal nucleus or its sensory projections will cause ipsilateral facial sensory disturbance. For example, pain resembling trigeminal neuralgia can be seen in MS.
Sensory loss in hemispheric lesions
The temporal, parietal and occipital lobes receive sensory
information regarding the various modalities of touch,
vision, hearing and balance .
The initial points of entry into the cortex are the respective
primary cortical areas (see Fig.).
Damage to any of these primary areas will result in reduction or loss of the ability to perceive that particular modality:
‘negative’ symptomatology. Abnormal excitation of
these areas can result in a false perception (‘positive’
symptoms), the most common of which is migrainous
visual aura (flashing lights or teichopsiae).
Cortical lesions are more likely to cause a mixed
motor and sensory loss. Substantial lesions of the parietalcortex (as in large strokes) can cause severe loss of
proprioception and may even abolish conscious awareness
of the existence of the affected limb(s). The resulting
loss of function in the limb may be impossible to distinguish
from paralysis. Pathways are so tightly packed in
the thalamus that even small lacunar strokes can cause
isolated contralateral hemisensory loss.
Neuropathic pain
Neuropathic pain is a positive neurological symptom
caused by dysfunction of the pain perception apparatus,
in contrast to nociceptive pain, which is secondary to
pathological processes such as inflammation. Neuropathic
pain has distinctive features and typically provokes
a very unpleasant, persistent, burning sensation.
There is often increased sensitivity to touch, so that light
brushing of the affected area causes exquisite pain (allodynia). Painful stimuli are felt as though they arise from
a larger area than that touched, and spontaneous bursts
of pain may also occur. Pain may be elicited by other
modalities (allodynia) and is considerably affected by
emotional influences.
The most common causes of neuropathic pain are diabetic neuropathies, trigeminal and post-herpetic neuralgias, and trauma to a peripheral nerve.
Treatment of these syndromes can be difficult.
Drugs that modulate various parts of the nociceptive
system, such as gabapentin, carbamazepine or tricyclic
antidepressants, may help. Localised treatment (topical
treatment or nerve blocks) sometimes succeeds but may
increase the sensory deficit and worsen the situation.
Electrical stimulation has occasionally proved successful.
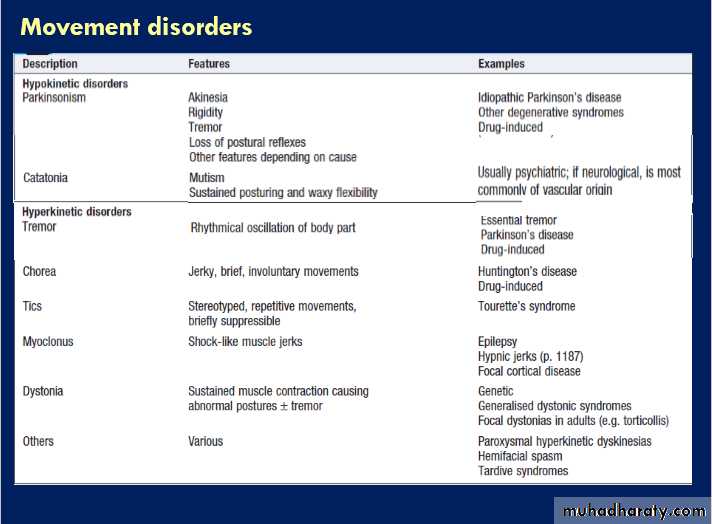
Abnormal movements
Disorders of movement lead to either extra, unwanted
movement (hyperkinetic disorders) or too little movement
(hypokinetic disorders) . In either case, the lesion often localises to the basal ganglia, although some tremors are related to cerebellar or brainstem disturbance.
Functional movement disorders are common,
and may mimic all of the organic syndromes below. The
most important hypokinetic disorder is Parkinson’s
disease .
Parkinsonism is a clinical description of a collection of symptoms, including tremor, bradykinesia and rigidity.
Causes and characteristics of tremors
TremorTremor is caused by alternating agonist/antagonist
muscle contractions and produces a rhythmical oscillation.
In the assessment of tremor, the position, body part affected, frequency and amplitude should be considered, as these provide diagnostic clues .
Other hyperkinetic syndromes
Non-rhythmic involuntary movements include chorea,
athetosis, ballism, dystonia, myoclonus and tics. They
are categorised by clinical appearance, and coexistence
and overlap are common, such as in choreoathetosis.
• Chorea: jerky, brief, purposeless involuntary
movements, appearing as fidgety movements affecting different areas; they suggest disease in the caudate nucleus (as in Huntington’s disease ) and are a common complication of prolonged levodopa treatment for Parkinson’s disease.
• Athetosis: slower, writhing movement of the limbs,
often combined with chorea and having similar causes.
• Ballism: a more dramatic form of chorea, causing
often-violent flinging movements of one limb(monoballism) or one side of the body (hemiballism). The lesion localises to the contralateral subthalamic nucleus and the most common cause is stroke.
• Dystonia: sustained involuntary muscle contraction
that causes abnormal postures or movement. It may be generalised (usually in childhood-onset genetic syndromes) or, more commonly, focal/segmental (such as in torticollis, when the head is twisted repeatedly to one side). Some dystonias only occur with specific tasks, such as writer’s cramp or other occupational ‘cramps’. Dystonic tremor is associated, and is asymmetrical and of large amplitude.
• Myoclonus: brief, isolated, random jerks of muscle
groups. This is physiological at the onset of sleep(hypnic jerks). Similarly, a myoclonic jerk is a component of the normal startle response, which may be exaggerated in some rare (mostly genetic) disorders. Myoclonus may occur in disorders of the cerebral cortex, such as some forms of epilepsy.
Alternatively, myoclonus can arise from subcortical
structures or, more rarely, from segments of the spinal cord.
• Tics: stereotyped repetitive movements, such as
blinking, winking, head shaking or shoulder shrugging. Unlike dyskinesias, the patient may be able to suppress them, although only for a short time. Isolated tics are common in childhood and usually disappear. Tourette’s syndrome is defined by the presence of multiple motor and vocal tics that may evolve over time; it is frequently
associated with psychiatric disease, including
obsessive compulsions, depression, self-harm or attention deficit disorder. Tics may also occur in Huntington’s and Wilson’s diseases, or after streptococcal infection.
Causes of chorea
Abnormal perceptionThe parietal lobes are involved in the higher processing
and integration of the primary sensory information. This
takes place in areas referred to as ‘association’ cortex, damage to which gives rise to sensory (including visual)
inattention, disorders of spatial perception, and disruption
of spatially orientated behaviour, leading to apraxia.
Apraxia is the inability to perform complex, organised
activity in the presence of normal basic motor, sensory
and cerebellar function (after weakness, numbness anda taxia have been excluded as causes). Examples of complex motor activities include dressing. Other abnormalities that can result from damage to the association cortex involve difficulty reading (dyslexia) or writing (dysgraphia), or the inability to recognise familiar objects (agnosia).
Cortical lobar functions
Altered balance and vertigoBalance is a complicated dynamic process that requires
ongoing modification of both axial and limb muscles to
compensate for the effects of gravity and alterations in
body position in order to prevent a person from falling. This requires input from a variety of sensory modalities (visual, vestibular and proprioceptive), processing by the cerebellum and brainstem, and output via a number of descending pathways (e.g. vestibulospinal, rubrospinal and reticulospinal tracts). Disorders of balance can therefore arise from any part of this process. Disordered input (loss of vision, vestibular disorders or lack of joint position sense), processing (damage to vestibular nuclei or cerebellum) or motor function (spinal cord lesions, leg weakness of any cause) can all impair balance.
For example, loss of joint position sense or cerebellar function may result in unsteadiness, while damage to the vestibular nuclei or labyrinth may result in an illusion of movement such as vertigo . Since vision can often compensate for lack of joint position sense, patients with peripheral neuropathies or dorsal column loss will often find their problem more noticeable in the dark. Sensory abnormalities may be manifest as altered visual acuities or visual fields, possibly with abnormalities on fundoscopy, altered eye movements (including nystagmus), impaired vestibular function or lack of joint position sense. Disturbance of cerebellar function may be manifest as nystagmus, dysarthria or ataxia, or difficulty with gait (unsteadiness or inability to perform tandem gait).
Vertigo
Vertigo is defined as an abnormal perception of movement of the environment or self, and occurs because of conflicting visual, proprioceptive and vestibular information about a person’s position in space. Vertigo commonly arises from imbalance of vestibular input and is within the experience of most people, since this is the ‘dizziness’ that occurs after someone has spunround vigorously and then stops. Bilateral labyrinthine dysfunction often causes some unsteadiness. Labyrinthine vertigo usually lasts days at a time, though it may recur, whilst vertigo arising from central (brainstem) disorders is often persistent and accompanied by other brainstem signs. Benign paroxysmal positional vertigo lasts a few seconds on head movement.
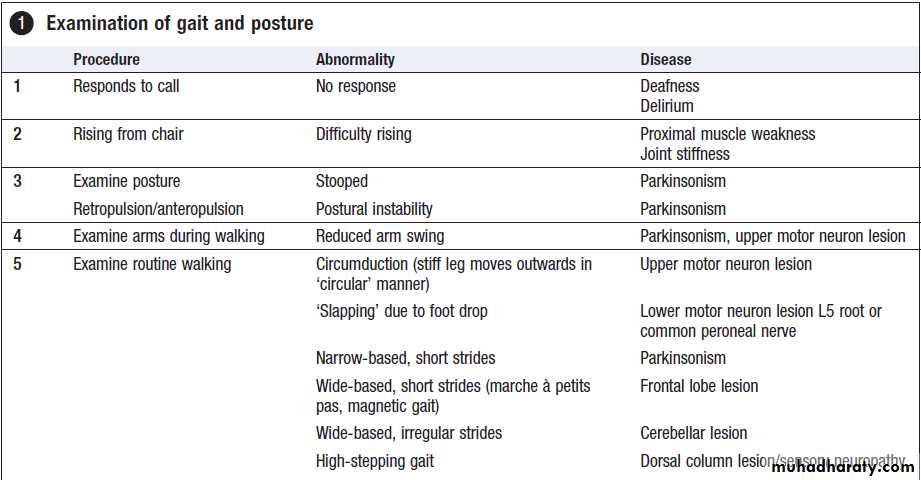
Abnormal gait
Many neurological disorders can affect gait. Observingpatients as they walk into the consulting room can be
very informative.
Neurogenic gait disorders need to be distinguished from those due to skeletal abnormalities, usually characterised by pain producing an antalgic gait, or limp. Gait alteration incompatible with any anatomical or physiological deficit may be due to functional disorders.
Pyramidal gait
Upper motor neuron lesions cause characteristic extension
of the affected leg. The resultant tendency for the
toes to strike the ground on walking requires the leg
to swing outwards at the hip (circumduction).
Nevertheless, a shoe on the affected side worn down at the toes may provide evidence of this type of gait. In hemiplegia, the asymmetry between affected and normal
sides is obvious on walking, but in paraparesis both
lower limbs swing slowly from the hips in extension and
are dragged stiffly over the ground ‘walking in mud’.
Foot drop
In normal walking, the heel is the first part of the foot to
hit the ground. A LMN lesion affecting the leg will cause weakness of ankle dorsiflexion, resulting in a less controlled descent of the foot, which makes a slapping noise as it hits the ground. In severe cases, the foot will have to be lifted higher at the knee to allow room for the inadequately dorsiflexed foot to swing through, resulting in a high-stepping gait.
Myopathic gait
During walking, alternating transfer of the body’sweight through each leg requires adequate hip abduction.
In proximal muscle weakness, usually caused by muscle disease, the hips are not properly fixed by these muscles and trunk movements are exaggerated, producing
a rolling or waddling gait.
Ataxic gait
lesions in the cerebellum, vestibular apparatus or peripheral nerves. Patients with lesions of the central portion of the cerebellum (vermis) walk with broad-based gait ‘as if drunk’. Acute vestibular disturbances walk similarly but the accompanying vertigo. Inability to walk heel to toe may be the only sign of cerebellar dysfunction.
Proprioceptive defects can also cause an ataxic gait. The impairment of joint position sense makes walking unreliable, especially in poor light. The feet tend to be placed on the ground with greater emphasis, to enhance proprioceptive input, resulting in a ‘stamping’ gait.
Apraxic gait
In an apraxic gait, power, cerebellar function and proprioception are normal. The patient may be able to carry out complex motor tasks (e.g. bicycling) while recumbent and yet cannot formulate the motor act of walking. In this higher cerebral dysfunction, the feet appear stuck to the floor and the patient cannot walk. Gait apraxia is a sign of diffuse bilateral hemisphere disease (such as normal pressure hydrocephalus) or diffuse frontal lobe disease.
Extrapyramidal gait
The rigidity and bradykinesia of basal ganglia dysfunctionlead to a stooped posture and characteristic
gait difficulties, with problems initiating walking and
controlling the pace of the gait. Patients may become
stuck whilst trying to start walking or when walking
through doorways (‘freezing’). The centre of gravity will
be moved forwards to aid propulsion, which, with poor
axial control, can lead to an accelerating pace of shuffling
and difficulty stopping. This produces the festinant
gait: initial stuttering steps that quickly increase in frequency while decreasing in length.
Abnormal speech and language
Disruption of sound output (dysarthria) or language
disturbance (dysphasia). Dysphonia (reduction in the
sound/volume) is usually due to mechanical laryngeal
disruption, whereas dysarthria is more typically neurological in origin. Dysphasia is always neurological
and localises to the dominant cerebral hemisphere
(usually left, regardless of handedness).
Dysphonia
Hoarse or whispered speech. The most common cause is laryngitis, but dysphonia can also result from a lesion of the 10th cranial nerve or disease of the vocal cords. Parkinsonism may cause hypophonia with marked reduction
in speech volume, often in association with dysarthria.
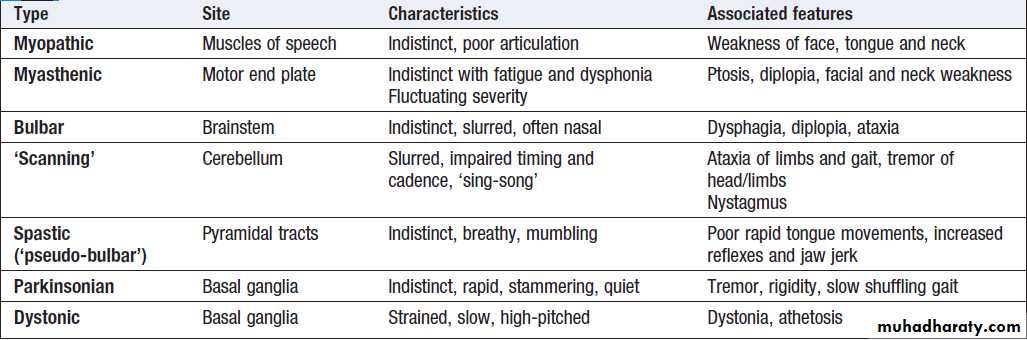
Dysarthria
Dysarthria is characterised by poorly articulated orslurred speech and can occur in association with lesions
of the cerebellum, brainstem and lower cranial nerves,
as well as in myasthenia or myopathic disease. Language
function is not affected. The quality of the speech
tends to differ depending on the cause, but it can be very difficult to distinguish the different types clinically (Box ).
Causes of dysarthria
Dysphasia
Dysphasia (or aphasia) is a disorder of the languagecontent of speech. It can occur with lesions over a
wide area of the dominant hemisphere . Dysphasia
may be categorised according to whether the speech output is fluent or non-fluent. Fluent aphasias, also called receptive aphasias, are impairments related mostly to the input or reception of language, with difficulties either in auditory verbal comprehension or in the repetition of words, phrases or sentences spoken by others. Speech is easy and fluent, but there are difficulties related to the output of language as well, such as paraphasia (either substitution of similar-sounding non-words, or incorrect words) and neologisms (non-existent words).
Examples include Wernicke’s aphasia (which localises to the superior posterior temporal lobe), transcortical sensory aphasia, conduction aphasia and anomic aphasia.
Non-fluent aphasias, also called expressive aphasias,
are difficulties in articulating, but in most cases
there is relatively good auditory verbal comprehension.
Examples include Broca’s aphasia (pathologies in the inferior frontal region), transcortical motor aphasia and global aphasia. ‘Pure’ aphasias are selective impairments in reading,
writing or the recognition of words.
For example, a person is able to read but not write, or is able to write but not read. Examples include pure alexia, agraphia and pure word deafness.
Classification of cortical speech problems.
(1) Wernicke’s aphasia: fluent dysphasia with poor comprehension and poor repetition.(2) Conduction aphasia: fluent aphasia with good comprehension and poor repetition.
(3) Broca’s aphasia: non-fluent aphasia with good comprehension and poor repetition.
(4) Transcortical sensory aphasia: fluent aphasia with poor comprehension and good repetition.
(5) Transcortical motor aphasia: non-fluent aphasia with good
comprehension and good repetition. Large lesions affecting all of regions 1–5 cause global aphasia.
Disturbance of smell
Symptomatic olfactory loss almost always is due to local
causes (nasal obstruction), follows head injury or is idiopathic.
Hyposmia may occur early in Parkinson’s disease. Frontal lobe lesions are a rare cause. Positive olfactory symptoms may arise from Alzheimer’s disease or epilepsy.
Visual disturbance and ocular abnormalities
Disturbances of vision may be due to primary oculardisease or to disorders of the central connections and
visual cortex. Visual symptoms are usually negative
(loss of vision) but sometimes are positive, most commonly in migraine. Eye movements may be disturbed, giving rise to double vision (diplopia) or blurred vision.
Visual loss
Visual loss can occur as the result of lesions in any areas
between the retina and the visual cortex. Patterns of visual field loss are explained by the anatomy of the
visual pathways . Visual symptoms affecting one eye only are liable to be due to lesions anterior to the optic chiasm.
Transient visual loss is quite common and sudden onset
visual loss lasting less than 15 minutes is likely tohave a vascular origin. It may be difficult to know
whether the visual loss was monocular (carotid circulation)
or binocular (vertebrobasilar circulation), and it can
help to ask if the patient tried closing each eye in turn
to see whether the symptom affected one eye or both.
Visual field testing is an important part of the examination,
either at the bedside or formally with perimetry.
Field defects become more symmetrical (congruous), the
closer the lesion comes to the visual cortex.
Migrainous visual symptoms are very common and,
when associated with typical headache and other
migraine features, rarely pose a diagnostic challenge.
However, they may occur in isolation, making distinction
from TIA difficult, but TIAs typically cause negative
(transient blindness) symptoms, whereas migraine
causes positive phenomena (see below).
TIAs often last for a shorter time (a few minutes), compared to the 10–60-minute duration of migraine aura, and will have an abrupt onset and end, unlike the gradual evolution of a migraine aura.
Clinical manifestations of visual field loss
Positive visual phenomenaThe most common cause is migraine; patients may
describe silvery zigzag lines (fortification spectra) or
flashing coloured lights (teichopsia), usually preceding the headache. Simple flashes of light (phosphenes) may
indicate damage to the retina (e.g. detachment) or to
the primary visual cortex.
Formed visual hallucinations may be caused by drugs, or may be due to epilepsy or ‘release phenomena’ in a blind visual field (Charles Bonnet’s syndrome).
Double vision
Subtle double vision (diplopia) may be reported as
blurred rather than double vision and most commonly
arises from misalignment of the eyes. Monocular diplopia
is rare and indicates ocular disease, while binocular
diplopia suggests a probable neurological cause.
Closing either eye in turn will abort binocular diplopia. Once the presence of binocular diplopia is confirmed, it should be established whether the diplopia is maximal in any particular direction of gaze, whether the images are
separated horizontally or vertically, and whether there
are any associated symptoms or signs, such as ptosis or
pupillary disturbance.
Binocular diplopia occurs when eye movement is
impaired, so that the image is not projected to the samepoints on the two retinae. It may result from central
disorders or from disturbance of the ocular motor
nerves, muscles or the neuromuscular junction.
The pattern of double vision, along with any associated features, usually allows inference of which nerves/muscles are affected, whilst the mode of onset and other features (e.g. fatigability in myasthenia) provide further clues about the cause.
Common causes of damage to cranial nerves
3, 4 and 6
Nystagmus
Nystagmus describes a repetitive to-and-fro movementof the eyes. Usually slow drifts are the primary
abnormal movement, each followed by fast (corrective)
phases. The direction of the fast phase is usually designated as the direction of the nystagmus because it is easier to see. Nystagmus may be horizontal, vertical or torsional, and usually involves both eyes synchronously. It may be a physiological phenomenon in response to sustained vestibular stimulation or movement of the visual world (optokinetic nystagmus). There are many causes of pathological nystagmus, the most common sites of lesions being the vestibular system, brainstem and cerebellum.
In vestibular lesions, cause the eyes to drift towards the side of the lesion. This elicits recurrent compensatory fast movements away from the side of the lesion, manifest as unidirectional horizontal nystagmus.
The nystagmus of peripheral labyrinthine lesions is accompanied by vertigo and usually by nausea, vomiting and unsteadiness, but as the CNS habituates, the nystagmus
disappears (fatigues) quite quickly.
Central vestibular nystagmus is more persistent.
The brainstem and the cerebellum are involved in
maintaining eccentric positions of gaze. Lesions will
therefore allow the eyes to drift back in towards primary
position, producing nystagmus with fast component
beats in the direction of gaze (gaze-evoked nystagmus).
This is the most common type of ‘central’ nystagmus; it
is most commonly bidirectional and not usually accompanied by vertigo. Brainstem disease may also cause vertical nystagmus.Unilateral cerebellar lesions may result in gazeevoked
nystagmus when looking in the direction of the lesion, where the fast phases are directed towards the side of the lesion. Cerebellar hemisphere lesions also cause ‘ocular dysmetria’, an overshoot of targetdirected, fast eye movements (saccades) resembling ‘past-pointing’ in limbs. Nystagmus also occurs as a consequence of drug toxicity and nutritional deficiency (e.g. thiamin). Nystagmus may occur as a congenital phenomenon, in which case both phases are equal and ‘pendular’ rather than having alternating fast and slow components.
Ptosis
drooping of the eyelids (ptosis)Abnormal pupillary responses
Abnormal pupillary responses may arise from lesions atseveral points between the retina and brainstem. Lesions
of the oculomotor nerve, ciliary ganglion and sympathetic
supply produce characteristic ipsilateral disorders
of pupillary function.
‘Afferent’ defects result from damage to an optic nerve, impairing the direct response of a pupil to light, although leaving the consensual response from stimulation of the normal eye intact.
Structural damage to the iris itself can also result in
pupillary abnormalities.
Right-sided Horner’s syndrome due to paravertebral
metastasis at T1.There is ipsilateral partial ptosis and a small pupil.
Papilloedema
There are several causes of swelling of the optic disc, butthe term ‘papilloedema’ is reserved for swelling secondary to raised intracranial pressure, when obstructed axoplasmic flow from retinal ganglion cells results in swollen nerve fibres, which in turn cause capillary and venous congestion, producing papilloedema. The earliest sign is the cessation of venous pulsation seen at the disc, progression causing the disc margins to become
red (hyperaemic). Disc margins become indistinct and
haemorrhages may occur in the retina . Lack
of papilloedema never excludes raised intracranial pressure. Other causes of optic disc swelling are listed in Box .Some normal variations of disc appearance (e.g. optic nerve drusen) can mimic disc swelling.
Common causes of optic disc swelling
Mechanism of optic disc oedema (papilloedema). A Normal. B Disc oedema (e.g. due to cerebral tumour). C Fundus photographof the left eye showing optic disc oedema with a small haemorrhage on the nasal side of the disc.
Optic atrophy
Loss of nerve fibres causes the optic disc to appear pale,as the choroid becomes visible .
A pale disc (optic atrophy) follows optic nerve damage, and causes include previous optic neuritis or ischaemic damage, long-standing papilloedema, optic nerve compression, trauma and degenerative conditions (e.g. Friedreich’s ataxia).
Fundus photograph of the left eye of a patient with
familial optic atrophy. Note marked pallor of optic disc.Hearing disturbance
Each cochlear organ has bilateral cortical representation,so unilateral hearing loss is a result of peripheral organ
damage. Bilateral hearing dysfunction is usual, and is
most commonly due to age-related degeneration or
noise damage, although infection and drugs (particularly
diuretics and aminoglycoside antibiotics) can be a
primary cause. Prominent deafness may suggest a mitochondrial disorder
Bulbar symptoms – dysphagia and dysarthria
Swallowing involving the coordinated action of lips, tongue, soft palate, pharynx and larynx, which are innervated by cranial nerves 7, 9, 10, 11 and 12. Neurological mechanisms are vulnerable to damage at different points, resulting in dysphagia that is usually accompanied by dysarthria. Tempo is again crucial: acute onset of dysphagia may occur as a result of brainstem stroke or a rapidly developing neuropathy, such as Guillain–Barré syndrome or diphtheria. Intermittent fatigable muscle weakness (including dysphagia) would suggest myasthenia gravis. Dysphagia developing over weeks or months may be seen in motor neuron disease, polymyositis, basal meningitis. More slowly developing dysphagia suggests a myopathy or possibly a brainstem or skull-base tumour.
Pathologies affecting (9, 10, 11 and 12) frequently manifest bilaterally, producing dysphagia and dysarthria. The term ‘bulbar palsy’ is used to describe lower motor neuron lesions, either within the medulla or outside the brainstem. The tongue is wasted and fasciculating, and palatal movement is reduced.
Upper motor neuron innervation of swallowing is
bilateral, so persistent dysphagia is unusual with a unilateral upper motor lesion (the exception being in the
acute stages of, for example, a hemispheric stroke).
Widespread lesions above the medulla will cause upper
motor neuron bulbar paralysis, known as ‘pseudobulbar
palsy’. Here the tongue is small and contracted, and
moves slowly; the jaw jerk is brisk.
Causes of pseudobulbar and bulbar palsy
Pseudobulbar BulbarBulbar
Genetic• –
• Kennedy’s disease (X-linked bulbospinal neuronopathy
Vascular• Bilateral hemisphere (lacunar) infarction
• Medullary infarction
Degenerative
Motor neuron disease
Motor neuron disease
Syringobulbia
Inflammatory
/infective
Multiple sclerosis
Cerebral vasculitis
Myasthenia
Guillain–Barré syndrome Poliomyelitis ,,Lyme
Vasculitis
Neoplastic
High brainstem tumours
Brainstem glioma
Malignant meningitis
Bladder, bowel and sexual disturbance
Whilst isolated disturbances of bladder, bowel andsexual function are rarely the sole presenting features
of neurological disease, they are common complications
of many chronic disorders such as multiple sclerosis,
stroke and dementia, and are frequently found post
head injury.
Bladder dysfunction
In the absence of conscious control (e.g. in coma or
dementia), distension of the bladder to near-capacity evokes reflex detrusor contraction (analogous to the
muscle stretch reflex), and reciprocal changes in sympathetic activation and relaxation of the distal sphincter result in coordinated bladder emptying.
Damage to the lower motor neuron pathways (the
pelvic and pudendal nerves) produces a flaccid bladder
and sphincter with overflow incontinence, often accompanied by loss of pudendal sensation. Such damage may be due to disease of the conus medullaris or sacral nerve roots, either within the dura (as in inflammatory or carcinomatous meningitis) or as they pass through the
sacrum (trauma or malignancy), or due to damage to the
nerves themselves in the pelvis (infection, haematoma,
trauma or malignancy).
Damage to the pons or spinal cord results in an ‘upper motor neuron’ pattern of bladder dysfunction due to uncontrolled over-activity of the parasympathetic supply.
The bladder is small and highly sensitive to being stretched. This results in frequency, urgency and
urge incontinence. Loss of the coordinating control of
the pontine micturition centre will also result in the phenomenon of detrusor–sphincter dyssynergia, in which
detrusor contraction and sphincter relaxation are not
coordinated; the spastic bladder will often try to empty
against a closed sphincter. This manifests as both
urgency and an inability to pass urine, which is distressing
and painful. The resultant incomplete bladder
emptying predisposes to urinary infection, and the
prolonged high bladder pressure may result in renal
failure; post-micturition bladder ultrasound may
confirm incomplete bladder emptying.
More severe lesions of the spinal cord, as in spinal cord compression or trauma, can result in painless urinary retention, as bladder sensation, normally carried in the lateral spinothalamic tracts, will be cut off.
Damage to the frontal lobes gives rise to loss of awareness of bladder fullness and consequent incontinence.Coexisting cognitive impairment may result in inappropriate micturition. These features are seen typically in hydrocephalus, frontal tumours, dementia and bifrontal
subdural haematomas.
Remember that most bladder problems are not neurological unless there are overt neurological signs.
Neurogenic bladder: clinical features and treatment
Rectal dysfunctionThe rectum has an excitatory cholinergic input from the
parasympathetic sacral outflow, and inhibitory sympathetic
supply similar to the bladder. Continence depends
largely on skeletal muscle contraction in the puborectalis
and pelvic floor muscles supplied by the pudendal
nerves, as well as the internal and external anal sphincters.
Damage to the autonomic components usually
causes constipation (a common early symptom in
Parkinson’s disease) but diabetic neuropathy can be
associated with diarrhoea. Lesions affecting the conus
medullaris, the somatic S2–4 roots and the pudendal
nerves may cause faecal incontinence.
Erectile failure and ejaculatory failure
These related functions are under autonomic control via
the pelvic nerves (parasympathetic, S2–4) and hypogastric
nerves (sympathetic, L1–2). Descending influences
from the cerebrum are important for erection, but it can
occur as a reflex phenomenon in response to genital
stimulation. Erection is largely parasympathetic, and
may be impaired by a number of drugs, including
anticholinergic, antihypertensive and antidepressant
agents. Sympathetic activity is important for ejaculation,
and may be inhibited by α-adrenoceptor antagonists.
Personality change
While this is often due to psychiatric illness, neurologicalconditions that alter the function of the frontal
lobes can cause personality change and mood disorder .
Personality change due to a frontal lobe disorder may occur as the result of structural damage due to stroke, trauma, tumour or hydrocephalus. The nature of any change may help localise the lesion.
Patients with lesions of the dorsolateral pre-frontal
cortex develop a dysexecutive syndrome, which involvesdifficulties with speech, motor planning and organisation.
Those with orbitofrontal lesions of the frontal lobes,
in contrast, become disinhibited, displaying grandiosity
or irresponsible behaviour. Memory is substantially
intact but frontal release signs may emerge, such as a
grasp reflex, palmomental response or pout. Proximity
to the olfactory bulb and tracts means that inferior
frontal lobe tumours may be associated with anosmia.
Disturbance to the cortical areas responsible for
speech or memory can result in changes that may be
interpreted as changes in personality.
Sleep disturbance
Disturbances of sleep are common and are not usually
due to neurological disease. Patients may complain of
insomnia (difficulty sleeping), excessive daytime sleepiness,
disturbed behaviour during night-time sleep, parasomnia
(sleep walking and talking, or night terrors) or
disturbing subjective experiences during sleep and/or
its onset (nightmares, hypnagogic hallucinations, sleep
paralysis). A careful history (from the bed-partner as
well as the patient) usually allows specific causes of
sleep disturbance to be identified .
Psychiatric disorders
Care is needed in their identification, as effective management will help the underlying neurological illness. FUNCTIONAL SYMPTOMSMany patients presenting with neurological symptoms
do not have a defined neurological disease and are best
described as having functional symptoms .Some of these are psychogenic (or conversion) disorders. Such patients often have unexplained symptoms affecting multiple systems and a long list of consultations and negative tests from other medical specialties when they present.
Considering the possibility of a functional origin may save the patient further unnecessary, invasive and inconvenient investigation and anxiety.
Weakness and sensory symptoms predominate among functional symptoms, but pain or loss of consciousness
can occur. Associated symptoms, such as tiredness, lethargy, poor concentration, bowel upset (irritable bowel syndrome) and gynaecological complaints, are common.
A functional origin of neurological problems should always be considered, as it can allow for more rapid diagnosis and avoid unnecessarily painful or hazardous investigation.
Clinical features suggestive of functional disorder
HEADACHE SYNDROMESHeadaches may be classified as primary or secondary,
depending on the underlying cause .Secondary headache may be due to structural, infective, inflammatory or vascular conditions. The primary headache syndromes are described here.
Tension-type headache
This is the most common type of headache and is
experienced to some degree by the majority of the
population.
Pathophysiology
Tension headache is incompletely understood. Emotions
and anxiety are common precipitants and there is sometimes an associated depressive illness.
Anxiety about the headache itself may lead to continuation of symptoms, and patients often become convinced of a serious underlying condition. Muscular spasms may worsen this in some patients.
Clinical features
The pain of tension headache is usually characterised
as ‘dull’, ‘tight’ or like a ‘pressure’, and there may be
a sensation of a band round the head or pressure at
the vertex. It is of constant character and generalised,
but often radiates forwards from the occipital region.
In contrast to migraine, the pain can remain unabated
for weeks or months without interruption, although
the severity may vary, and there is no associated vomiting
or photophobia.
Activities are usually continued throughout, and the pain may be less noticeable when the patient is occupied.
The pain is usually less severe in the early part of the day, becoming more troublesome as the day goes on. Tenderness may be present over the skull vault or in the occiput but is easily distinguished from the triggered pains of trigeminal neuralgia and the exquisite tenderness of temporal arteritis. Analgesics may be taken with chronic regularity despite little effect, and may serve to perpetuate the symptoms (see ‘Medication overuse headache’ below).
Management
Most benefit is given by providing a careful assessment,followed by discussion of likely precipitants and reassurance that the prognosis is good. Excessive use of
analgesia, particularly those containing codeine, may
maintain and exacerbate the headache (analgesic headache). Physiotherapy (with muscle relaxation and stress management) may help and low-dose amitriptyline
can provide benefit. There is evidence that patients benefit from a perception that their problem has been taken seriously and rigorously assessed. Investigation may contribute to such reassurance, especially if concerns
about an underlying lesion are strong, but patients should understand the purpose and likely outcome of such imaging.
Migraine
Migraine usually appears before middle age; it affectsabout 20% of females and 6% of males at some point in
life. Some patients assume that migraine is a term
encompassing any severe headache but it has a characteristic presentation, discussed below.
Pathophysiology
The cause of migraine is unknown but there is increasing
evidence that the aura (see below) is due to dysfunction
of ion channels causing a spreading front of cortical depolarisation (excitation) followed by hyperpolarisation
(depression of activity). This process (the ‘spreading depression of Leao’’) spreads over the cortexat a rate of about 3 mm/minute, corresponding to the aura’s symptomatic spread.
The headache phase is associated with vasodilatation of extracranial vessels and may be relayed by hypothalamic activity. Activation of the trigeminovascular system is probably important. A genetic contribution is implied by frequently positive family history, and similar phenomena
occurring in disorders such as CADASIL .
The female preponderance and the frequency of migraine
attacks at certain points in the menstrual cycle also suggest hormonal influences.
Oestrogen-containing oral contraception sometimes exacerbates migraine, and increases the small risk of stroke in patients who suffer from migraine with aura.
Doctors and patients often over-estimate the role of dietary precipitants such as cheese, chocolate or red wine. When psychological factors contribute, the migraine attack often occurs after a period of stress, being more likely on Friday evening at the end of the working week or at the beginning of a holiday.
Clinical features
Some patients report a prodrome of malaise, irritabilityor behavioural change for some hours or days. Around
20% of patients experience an aura, and are said to have
migraine with aura (previously known as classical
migraine). The aura is most often visual, consisting of
fortification spectra, which are shimmering, silvery
zigzag lines that march across the visual fields for up to
40 minutes, sometimes leaving a trail of temporary
visual field loss (scotoma). In some there is a sensory
aura of tingling followed by numbness, spreading
over 20–30 minutes, from one part of the body to
another. Dominant hemisphere involvement may also
cause transient speech disturbance.
The 80% of patients with characteristic headache but no ‘aura’ are said to have migraine without aura (previously called ‘common’ migraine).
Migraine headache is usually severe and throbbing,
with photophobia, phonophobia and vomiting lasting
from 4 to 72 hours. Movement makes the pain worse,
and patients prefer to lie in a quiet, dark room.
In a smaller number of patients, the symptoms of the
aura do not resolve, leaving more permanent neurological
disturbance. This persistent migrainous aura may occur with or without evidence of brain infarction.
Management
Avoidance of identified triggers or exacerbating factors(such as the combined contraceptive pill) may prevent
attacks. Treatment of an acute attack consists of simple
analgesia with aspirin, paracetamol or non-steroidal
anti-inflammatory agents. Nausea may require an
antiemetic such as metoclopramide or domperidone.
Severe attacks can be aborted by one of the increasing
number of ‘triptans’ (e.g. sumatriptan), which are potent
5-hydroxytryptamine (5-HT) agonists. These can be
administered orally, by subcutaneous injection or by
nasal spray. Caution is needed with ergotamine preparations since they may lead to dependence.
Overuse of any analgesia, including triptans, may contribute to associated medication overuse headache.
If attacks are frequent (more than 3–4 per month),
prophylaxis should be considered. Many drugs can be
used, but the most frequently used are vasoactive drugs
(calcium channel blockers and β-adrenoceptor antagonists
(β-blockers)), antidepressants (amitriptyline,
dosulepin) and anti-epileptic drugs (valproate, topiramate).
Women with aura should avoid oestrogen treatment for either oral contraception or hormone replacement, although the increased risk of ischaemic stroke is minimal.
Medication overuse headache (MOH)
With increasing availability of over-the-counter medication,headache syndromes perpetuated by analgesia intake are becoming much more common. MOH can complicate any other especially associated with migraine and tension headache. The medications that are the most common culprits are compound analgesia (particularly codeine and other opiate-containing preparations) and triptans.
Management is by withdrawal of the responsible analgesics; patients should be warned that the initial effect will be to exacerbate the headache. Migraine prophylactics may be helpful in reducing the rebound headaches. In severe cases, hospital admission with or
without a course of corticosteroids may be helpful.
Cluster headache
Cluster headaches (also known as migrainous neuralgia)are much less common than migraine. There is a 5 : 1
male predominance and onset is usually in the third
decade.
Pathophysiology
The cause is unknown, but this type of headache differs
from migraine in its character, lack of genetic predisposition, lack of provoking dietary factors, opposing
gender imbalance and different drug effect. Functional
imaging studies have suggested abnormal hypothalamic
activity. Patients are more often smokers with a higher
than average alcohol consumption.
Clinical features
Cluster headache is strikingly periodic, featuring runs of
identical headaches beginning at the same hour for
weeks at a time (the eponymous ‘cluster’). Patients may
experience either one or several attacks within a 24-hour
period. Cluster headache causes severe, unilateral periorbital pain with autonomic features, such as unilateral
lacrimation, nasal congestion and conjunctival injection
(occasionally with the other features of Horner’s syndrome).
The pain, though severe, is characteristically brief (30–90 minutes). In contrast to the behaviour of those with migraine, patients are often highly agitated during the headache phase. The cluster period is typically a few weeks, followed by remission for months to years, but a small proportion do not experience remission.
Management
Acute attacks can usually be halted by subcutaneousinjections of sumatriptan or by inhalation of 100%
oxygen. The brevity of the attack probably prevents
other migraine therapies from being effective. Migraine
prophylaxis is often ineffective too but attacks can be
prevented in some patients by sodium valproate, verapamil, methysergide or short courses of oral corticosteroids.
Patients with severe debilitating clusters can be helped with lithium therapy, although this requires monitoring .
Trigeminal neuralgia
This is characterised by unilateral lancinating facialpain, most commonly involving the second and/or third
divisions of the trigeminal nerve territory, usually in
patients over the age of 50 years.
Pathophysiology
Trigeminal neuralgia is thought to be caused by an
irritative lesion involving the trigeminal root zone, in
some cases an aberrant loop of artery. Other compressive lesions, usually benign, are occasionally found.
Trigeminal neuralgia associated with multiple sclerosis result from a plaque of demyelination in the brainstem.
Clinical features
The pain is repetitive, severe and very brief. It may be
triggered by touch, a cold wind or eating. Physical signs
are usually absent, although the spasms may make the
patient wince and sit silently (tic douloureux). Similar
symptoms may occur in multiple sclerosis or with other
brainstem lesions, in which case there may be associated
sensory changes in the trigeminal nerve territory or elsewhere.
There is a tendency for the condition to remit and
relapse over many years.
Management
The pain usually responds at least partially to carbamazepine.It is wise to start with a low dose and increase gradually, according to effect. In patients who cannot tolerate carbamazepine, gabapentin, pregabalin, amitriptyline or steroids may be effective. The possibility
of surgical treatment should be entertained, especially
where response is incomplete in younger patients.
Decompression of the vascular loop encroaching on the trigeminal root is said to have a 90% success rate.
Otherwise, localised injection of alcohol or phenol into
a peripheral branch of the nerve may be effective.
Headaches associated with specific activities
These usually affect men in their thirties and forties.Patients develop a sudden, severe headache with exertion,
including sexual activity. There is usually no vomiting
and no neck stiffness, and the headache lasts less
than 10–15 minutes, though a less severe dullness may
persist for some hours. Subarachnoid haemorrhage
needs to be excluded by CT and/or CSF examination
after a first event. The pathogenesis of these headaches is unknown. Although frightening, attacks are usually brief and patients may only need reassurance and simple analgesia for the residual headache.
The syndrome may recur, and prevention may be
necessary with propranolol or indometacin.
Benign paroxysmal headaches
EPILEPSYA seizure can be defined as the occurrence of signs and/
or symptoms due to abnormal, excessive or synchronous neuronal activity in the brain. ‘Epilepsy’ is the tendency
to have unprovoked seizures. The lifetime risk of seizure
is about 5%, although incidence is highest at the extremes
of age. Whilst the prevalence of active epilepsy in European countries is about 0.5%, the figure in developing
countries may be higher because of parasitic illnesses
such as cysticercosis
Historical terms such as ‘grand mal’ (implying tonic–
clonic seizures) and ‘petit mal’ (intended by its originatorsto mean ‘absence seizures’ but commonly used to
describe ‘anything other than grand mal’) have been
superseded. Subsequent revisions, including terms such
as ‘complex partial’ and ‘simple partial’, have been
imprecise and confusing, carrying little information
about underlying pathology, treatment or prognosis.
The modern equivalents for these terms will be given
below, but it is preferable to adhere to the 2010 iteration
of the International League Against Epilepsy’s classification .
Classification of seizures
(2010 ILAE Classification)Pathophysiology
To function normally, the brain must achieve an ongoingbalance between excitation and inhibition, in order
to remain responsive to the environment without continued
unrestrained spontaneous activity. The inhibitory
transmitter gamma-aminobutyric acid (GABA) is
particularly important, acting on ion channels to enhance
chloride inflow and reduce the chances of action potential
formation.
Excitatory amino acids (glutamate and aspartate) allow influx of sodium and calcium, producing the opposite effect.
It is likely that many seizures result from an imbalance between this excitation and inhibition.
In vivo, epileptic cortex shows repetitive discharges involving large groups of neurons.
Seizures may be related to a localised disturbance in the cortex, becoming manifest in the first instance as focal seizures. Any disturbance of cortical architecture and function can precipitate this, whether focal infection, tumour, hamartoma or trauma-related scarring. If focal seizures remain localised, the symptoms experienceddepend on which cortical area is affected.
If areas in the temporal lobes become involved, then awareness of the environment becomes impaired but without associated tonic–clonic movements. When both hemispheres are involved, either at onset or after spread, the seizure becomes generalised
In seizures that are generalised at onset, the abnormal
activity probably originates in the central mechanisms
controlling cortical activation and spreads rapidly. Such epilepsies constitute around 30% of all epilepsy, and are likely to reflect widespread disturbance of structure or function. Animal models have revealed mutations in genes for ion channels and receptors that cause seizures. In humans, many generalised epilepsies will have a genetic basis, and these almost always become apparent before the age of 35. Seizure activity is usually apparent on EEG as spike and wave discharges .
Other generalised seizure activity may involve merely
brief loss of awareness (absence seizures), single jerks
(myoclonus) or loss of tone (atonic seizures).
Clinical features
Seizure type and epilepsy typeTo classify seizure type, the clinician should ask first
whether there is a focal onset, and second whether the
seizures conform to one of the recognised patterns. Epilepsy that starts in patients beyond their mid-thirties will almost invariably reflect a focal cerebral event. Where activity remains focal, this will be obvious.
Even when generalised tonic–clonic seizures occur, seizures beginning in one cortical area will cause positive neurological symptoms and signs corresponding to the normal function of that area.
Occipital onset will cause visual changes (lights and blobs of colour), temporal lobe onset will cause false recognition (déjàvu), sensory strip involvement will cause sensory alteration (burning, tingling), and motor strip involvement will cause jerking.
Patients can experience more than one type of seizure attack, and it is important to document each attack type and the patient’s age at its onset, along with its frequency, duration and typical features.
Any triggers should be identified .
The pathophysiological classification of seizures.
A A focal seizure originates from a paroxysmal discharge in a focal
area of the cerebral cortex (often the temporal lobe); the seizure may
subsequently spread to the rest of the brain (secondary generalisation) via
diencephalic activating pathways.
B In primary generalised seizures the
abnormal electrical discharges originate from the diencephalic activating system and spread simultaneously to all areas of the cortex.
Trigger factors for seizures
Focal seizuresThe classification of focal seizures is shown in Box.
They are caused by localised cortical activity with
retained awareness. The localisation of such symptoms
is described above. A spreading pattern of seizure may
occur, the abnormal sensation spreading much faster
(in seconds) than a migrainous focal sensory attack.
Awareness may become impaired if spread occurs to the temporal lobes (previously ‘complex partial seizure’). Patients stop and stare blankly, often blinking repetitively, making smacking movements of their lips or displaying other automatisms, such as picking at their clothes.
After a few minutes, consciousness returns but the patient may be muddled and feel drowsy for a period of up to an hour. The age of onset, preceding aura, longer duration and post-ictal symptoms usually make these easy to differentiate from childhood absence seizures .
Seizures arising from the anterior parts of the
frontal lobe may produce bizarre behaviour patterns,
including limb posturing, sleep walking, or even frenetic
ill-directed motor activity with incoherent screaming.
Video EEG may be necessary to differentiate these from
psychogenic attacks (which are more common), but
abruptness of onset, stereotyped nature, relative brevity
and nocturnal preponderance may indicate the frontal
onset. Causes of focal seizures are given in Box .
Focal structural lesions
Genetic• Tuberous sclerosis • Autosomal dominant frontal lobe epilepsy
• Autosomal dominant partial with auditory features (ADPEAF)
• von Hippel–Lindau disease • Neurofibromatosis
• Cerebral migration abnormalities
Infantile hemiplegia
Dysembryonic
• Cortical dysgenesis • Sturge–Weber syndrome
Mesial temporal sclerosis (associated with febrile convulsions)
Cerebrovascular disease
• Intracerebral haemorrhage • Cerebral infarction • Arteriovenous malformation • Cavernous haemangioma
Causes of focal seizures
Causes of focal seizures'cont'd
Generalised seizuresTonic–clonic seizures. An initial ‘aura’ may be experienced by the patient, depending on the cortical area
from which the seizure originates (as above). The
patient then becomes rigid (tonic) and unconscious,
falling heavily if standing (‘like a log’) and risking facial
injury. During this phase, breathing stops and central
cyanosis may occur. As cortical discharges reduce in
frequency, the limbs produce jerking (clonic) movements
for a variable time. Afterwards, there is a flaccid
state of deep coma, which can persist for some minutes.
The patient may be confused, disorientated and/or
amnesic after regaining consciousness.
During the attack, urinary incontinence and tongue-biting may occur. A severely bitten, bleeding tongue after an
attack of loss of consciousness is pathognomonic of a
generalised seizure. Subsequently, the patient usually
feels unwell and sleepy, with headache and myalgia.
Witnesses are usually frightened by the event, often
believe the person to be dying, and may struggle to give
a clear account of the episode. Some may not describe
the tonic or clonic phase, and may not mention cyanosis
or tongue-biting. In less typical episodes, post-ictal confusion, or sequelae such as headache or myalgia, may be the main pointers to the diagnosis. Causes of generalised tonic–clonic seizures are listed in Box
Causes of generalised tonic– clonic seizures
Generalisation from focal seizuresGenetic
• Inborn errors of metabolism • Storage diseases • Phakomatoses (e.g. tuberous sclerosis )
Cerebral birth injury
Hydrocephalus
Cerebral anoxia
Drugs
• Antibiotics: penicillin, isoniazid, metronidazole • Antimalarials: chloroquine, mefloquine • Ciclosporin • Cardiac anti-arrhythmics: lidocaine, disopyramide • Psychotropic agents: phenothiazines, tricyclic antidepressants, lithium • Amphetamines (withdrawal)
Alcohol (especially withdrawal)
Toxins• Organophosphates (sarin) • Heavy metals (lead, tin)
Metabolic disease
• Hypocalcaemia • Hyponatraemia • Hypomagnesaemia • Hypoglycaemia • Renal failure • Liver failure
Infective
• Post-infectious encephalopathy • Meningitis
Inflammatory
• Multiple sclerosis (uncommon) • SLE
Diffuse degenerative diseases
• Alzheimer’s disease (uncommonly) • Creutzfeldt–Jakob disease
Causes of generalised tonic– clonic seizures'cont'd
Absence seizures.
Absence seizures (previously ‘petit mal’) always start in childhood.. They can occur so frequently (20–30 times a day) that they are mistaken for daydreaming or poor concentration in school.
Myoclonic seizures.
Are typically brief, jerking movements, predominating in the arms. In epilepsy, they are more marked in the morning or on awakening from sleep, and tend to be provoked by fatigue, alcohol or sleep deprivation.
Atonic seizures.
These are seizures involving brief loss of muscle tone, usually resulting in heavy falls with or without loss of consciousness. They only occur in the context of epilepsy syndromes that involve other forms of seizure.
Tonic seizures. These are associated with a generalised
increase in tone and an associated loss of awareness.They are usually seen as part of an epilepsy syndrome
and are unlikely to be isolated.
Clonic seizures. Clonic seizures are similar to tonic–
clonic seizures. The clinical manifestations are similar
but without a preceding tonic phase.
Seizures of uncertain generalised or focal nature
Epileptic spasms. While these are highlighted in the
classification system, they are unusual in adult practice
and occur mainly in infancy. They signify widespread cortical disturbance and take the form of marked contractions of the axial musculature, lasting a fraction
of a second but recurring in clusters of 5–50, often on awakening.
Epilepsy syndromes
Many patients with epilepsy fall into specific patterns,depending on seizure type(s), age of onset and treatment
responsiveness: the so-called electroclinical syndromes .
It is anticipated that genetic testing will ultimately demonstrate similarities in molecular pathophysiology.
Box highlights the more common epilepsy syndromes,
which are largely of early onset and are sensitive
to sleep deprivation, hyperventilation, alcohol and photic stimulation. Epilepsies that do not fit into any of
these diagnostic categories can be delineated first on the
basis of the presence or absence of a known structural
or metabolic condition (presumed cause), and then on
the basis of the primary mode of seizure onset (generalised versus focal).
Electroclinical epilepsy syndromes
Common epilepsy syndromes
InvestigationsSingle seizure
All patients with transient loss of consciousness should
have a 12-lead ECG. Where seizure is suspected or definite, patients should have imaging with either CT or
MRI, although the yield is low unless focal signs are
present. EEG may help to assess prognosis once a firm
diagnosis has been made. The recurrence rate after a first
seizure is approximately 40%, and most recurrent attacks
occur within a month or two of the first. Further seizures
are less likely if an identified trigger can be avoided .
Other investigations for infective, toxic and metabolic
causes may be appropriate. An EEG performed
immediately after a seizure may be more helpful
in showing focal features than if performed after a delay.
Investigation of epilepsy
EpilepsyThe same investigations are required in a patient with
suspected epilepsy . Where more than one seizure has occurred, an EEG may help to establish the type of epilepsy and guide therapy. As imaging becomes
more sensitive, focal changes are picked up more often.
Investigations should be revisited if the epilepsy is
intractable to treatment. Inter-ictal EEG is abnormal in only about 50% of patients with recurrent seizures, so it cannot be used to exclude epilepsy. The sensitivity can be increased to about 85% by prolonging recording time and including a period of natural or drug-induced sleep, but this does not replace a well-taken history.
Ambulatory EEG recording or video EEG monitoring may help with differentiation of epilepsy from other attack disorders if these are sufficiently frequent.
Indications for imaging are summarised in Box.
Imaging cannot establish a diagnosis of epilepsy but
identifies any structural cause. It is not required if a confident diagnosis of a recognised epilepsy syndrome
(e.g. juvenile myoclonic epilepsy) can be made. While
CT will exclude a major structural cause of epilepsy,
MRI is required to demonstrate subtle changes such as
hippocampal sclerosis, which may direct or inform
surgical intervention.
Indications for brain imaging in epilepsy
ManagementIt is important to explain the nature and cause of seizures
to patients and their relatives, and to instruct relatives
in the first aid management of seizures (Box).
Many people with epilepsy feel stigmatised and may
become unnecessarily isolated from work and social life.
It should be emphasised that epilepsy is a common disorder
that affects 0.5–1% of the population, and that full
control of seizures can be expected in approximately
70% of patients (Box).
How to administer first aid for seizures
Immediate care
Little can or needs to be done for a person during a
major seizure except for first aid and common-sense
manoeuvres to limit damage or secondary complications .
Advice should be given that on no account should anything be inserted into the patient’s mouth.
Lifestyle advice
Patients should be advised to avoid activities wherethey might place themselves or others at risk if they
have a seizure. This applies at work, at home and
at leisure. At home, only shallow baths (or showers)
should be taken. Prolonged cycle journeys should be
discouraged until reasonable freedom from seizures
has been achieved.
Activities requiring prolonged proximity to water (swimming, fishing or boating) should always be carried out in the company of someone who is aware of the risks and the potential need for rescue measures.
Driving regulations vary between countries, and the patient should be made aware of these. Certain occupations, such as firefighter or airline pilot, are not open to anyone who has a previous or active diagnosis of epilepsy; further information is available from epilepsy support organisations.
The recognised mortality of epilepsy should be discussed
at around the time of diagnosis. This should be
done with care and sensitivity, and with the aim of motivating the patient to adapt habits and lifestyle to optimize epilepsy control.
UK driving regulations
Anticonvulsant therapyAnticonvulsant drug treatment ( or anti-epileptic drugs(AEDs )) should be considered after more than one unprovoked seizure.
The decision to start treatment should be shared with the patient, to enhance compliance.
A wide range of drugs is available. These agents either increase inhibitory neurotransmission in the brain or alter neuronal sodium channels to prevent abnormally rapid transmission of impulses.
In the majority of patients, full control is achieved with a single drug. Dose regimens should be kept as simple as possible. Guidelines are listed in Box .
For seizures of focal onset, one large study suggests that lamotrigine is the best-tolerated monotherapy, which, alongside its favourable side-effect profile and relative lack of pharmacokinetic interactions, makes it a good first-line drug, although caution must be exercised with oral contraceptive use. Unclassified or specific syndromes respond best to valproate.
Problems in pregnancy mean that sodium valproate
should not be used in women of reproductive age unless
the benefits outweigh the risks. The first choice should
be an established first-line drug with more recently introduced drugs as second choice.
Guidelines for anticonvulsant therapy
Guidelines for choice of anti-epileptic drug
Monitoring therapySome practitioners confuse epilepsy care with serum
level monitoring. The newer drugs have much more
predictable pharmacokinetics than the older ones, and
the only indication for measuring serum levels is if there
is doubt that the patient is taking the medication. Blood
levels need to be interpreted carefully, and dose changes
made to treat the patient rather than to bring a serum
level into the ‘therapeutic range’.
Some centres advocate serum level monitoring during pregnancy (notably with lamotrigine) but the evidence of benefit for this is not strong.
Epilepsy surgery
Some patients with drug-resistant epilepsy benefit fromsurgical resection of epileptogenic brain tissue. Less
invasive treatments, including vagal nerve stimulation
or deep brain stimulation, may also be helpful in some
patients. All those who continue to experience seizures
despite appropriate drug treatment should be considered
for surgical treatment. Planning such interventions
will require intensive specialist assessment and investigation
to identify the site of seizure onset and the dispensability
of any targets for resection, i.e. whether the
area of brain involved is necessary for a critical function
such as vision or motor function.
Withdrawing anticonvulsant therapy
Withdrawal of medication may be considered after a
patient has been seizure-free for more than 2 years.
Childhood-onset epilepsy, particularly classical absence
seizures, carries the best prognosis for successful drug
withdrawal. Other epilepsy syndromes, such as juvenile
myoclonic epilepsy, have a marked tendency to recur
after drug withdrawal.
Seizures that begin in adult life, particularly those
with partial features, are also likely to recur, especially
if there is an identified structural lesion. Overall, the
recurrence rate after drug withdrawal depends on the
individual’s epilepsy history.
An individualised estimate may be gained from the SIGN guideline tables .
Patients should be advised of the risks of recurrence,to allow them to decide whether or not they wish to
withdraw. If undertaken, withdrawal should be done
slowly, reducing the drug dose gradually over weeks
or months. Withdrawal may necessitate precautions
around driving or occupation .
Contraception
Some AEDs induce hepatic enzymes that metabolise
synthetic hormones, increasing the risk of contraceptive
failure. This is most marked with carbamazepine,
phenytoin and barbiturates, but clinically significant
effects can be seen with lamotrigine and topiramate.
If the AED cannot be changed, this can be overcome by
giving higher-dose preparations of the oral contraceptive.
Sodium valproate and levetiracetam have no interaction
with hormonal contraception.
Pregnancy and reproduction
Epilepsy presents specific management problems duringpregnancy . There is usually great concern
about teratogenesis associated with AEDs. It is important
to recognise that the background risk of severe fetal malformation in the population is around 2–3%. The
modern AED most associated with teratogenesis is
sodium valproate, which, at high dose, increases the risk
to around 6–7%. Long-term observational studies show
that most of the commonly used AEDs can be given
safely in pregnancy.
Pre-conception treatment with folic acid (5 mg daily),
along with use of the smallest effective doses of as few
AEDs as possible, may reduce the risk of fetal abnormalities. The risks of abrupt AED withdrawal to the mother should be stressed. Seizures may become more frequent during pregnancy, particularly if pharmacokinetic changes decrease serum levels of AEDs . Menstrual irregularities and reduced fertility are more common in women with epilepsy, and are also increased by sodium valproate. Patients with epilepsy are at greater risk of osteoporosis, almost independently of the drug used. Some centres advocate vitamin D supplementation in any patient with epilepsy, but the higher female risk of osteoporosis makes this most important in women.
Epilepsy in pregnancy
Epilepsy in adolescence
Epilepsy in old agePrognosis
The outcome of newly diagnosed epilepsy is generallygood. Overall, generalised seizures are more readily
controlled than focal seizures. The presence of a structural
lesion reduces the chances of freedom from seizures.
The overall prognosis for epilepsy is shown in Box .
Epilepsy: outcome after 20 years
Status epilepticus
While generalised status epilepticus is most easilyrecognised, non-convulsive status may be less dramatic
and less easily diagnosed. It may cause only altered awareness, confusion or wandering with automatisms.
In an intensive care unit setting, EEG monitoring is essential to ensure that diagnosis and treatment are optimised.
Non-epileptic attack disorder
Patients may present with attacks that resemble epileptic seizures but which are caused by psychological phenomena and have no abnormal epileptic discharges. Such attacks may be prolonged, sometimes mimicking status epilepticus.
Non-epileptic attack disorder (NEAD) may be accompanied by dramatic flailing of the limbs and arching of the back, with side-to-side head movements.
Cyanosis and severe biting of the tongue are rare,
but urinary incontinence can occur. Distress and crying are common following non-epileptic attacks. The distinction
between epileptic attacks originating in the frontal lobes and non-epileptic attacks may be especially difficult, and may require videotelemetry with prolonged EEG. Non-epileptic attacks are three times more common in women and have been linked with a history of past or ongoing life trauma.They are not necessarily associated with formal psychiatric illness. Prevention requires psychotherapeutic interventions rather than drug therapy .
VESTIBULAR DISORDERS
Vertigo is the typical symptom caused by vestibulardysfunction, and most patients with vertigo have acute
vestibular failure, benign paroxysmal positional vertigo
or Ménière’s disease. Central (brain) causes of vertigo
are rare by comparison, with the exception of migraine .
Acute vestibular failure
Although commonly called ‘labyrinthitis’ or ‘vestibular
neuronitis’, acute vestibular failure is a more accurate
term, as most cases are idiopathic. It usually presents as
isolated severe vertigo with vomiting and unsteadiness.
It begins abruptly, often on waking, and many patients
are initially bed-bound.
The vertigo settles within a few days, though head movement may continue to provoke transient symptoms (positional vertigo) for some time. During the acute attack, nystagmus will be present for a few days.
Cinnarizine, prochlorperazine or betahistine provides
symptomatic relief but should not be used longterm
as this may delay recovery. A small proportion of
patients fail to recover fully, and complain of ongoing imbalance and dysequilibrium rather than vertigo; vestibular rehabilitation by a physiotherapist may help.
Benign paroxysmal positional vertigo
Benign paroxysmal positional vertigo (BPPV) is due tothe presence of otolithic debris from the saccule or
utricle affecting the free flow of endolymph in the
semicircular canals (cupulolithiasis). It may follow
minor head injury but typically is spontaneous. The
history is diagnostic, with transient (seconds) vertigo
precipitated by movement (typically, rolling over in
bed or getting into or out of bed). Although benign,
and usually self-limiting after a few weeks or months,
patients are often very alarmed by the symptoms. The
diagnosis can be confirmed by the ‘Hallpike manoeuvre’
to demonstrate positional nystagmus .
Treatment
comprises explanation and reassurance, along with positioning procedures designed to return otolithicdebris from the semicircular canal to saccule or utricle
(such as the Epley manoeuvre) and/or to re-educate the
brain to cope with the inappropriate signals from the
labyrinth (such as Cawthorne–Cooksey exercises: see
‘Further information’).
The Hallpike manoeuvre
The Hallpike manoeuvre for diagnosis of benign paroxysmal positional vertigo (BPPV). Patients are asked to keep their eyesopen and look at the examiner as their head is swung briskly backwards through 120° to overhang the edge of the couch.
A Perform first with the right ear down.
B Perform next with the left ear down. The examiner looks for nystagmus (usually accompanied by vertigo). In BPPV, the nystagmus
typically occurs in A or B only and is torsional, the fast phase beating towards the lower ear. Its onset is usually delayed a few seconds, and it lasts 10–20 seconds. As the patient is returned to the upright position, transient nystagmus may occur in the opposite direction. Both nystagmus and vertigo typically decrease (fatigue) on repeat testing.
Ménière’s disease
This is due to an abnormality of the endolymph thatcauses episodes of vertigo accompanied by tinnitus and
fullness in the ear, each attack typically lasting a few
hours. Over the years, patients may develop progressive
deafness (typically low-tone on audiometry). Examination
is typically normal in between attacks. The diagnosis
is clinical, supported by abnormal audiometry.
Ménière’s disease is idiopathic, but a similar syndrome
may be caused by middle ear trauma or infection. Management
includes a low-salt diet, vestibular sedatives for
acute attacks, and occasionally surgery.
DISORDERS OF SLEEP
Sleep disturbances include too much sleep (hypersomnolence or excessive daytime sleepiness), insufficient or poor-quality sleep (insomnia), and abnormal behavior during sleep (parasomnias). Insomnia is usually caused by psychological or psychiatric disorders, shift work and other environmental causes, pain and so on. Many symptoms and disorders may affect sleep and sleep quality (e.g. pain, depression/ anxiety, parkinsonism).
Excessive daytime sleepiness (hypersomnolence)
The most common causes are impaired sleep due to lifestyle issues or sleep-disordered breathing . Sleepiness may be measured using the Epworth Sleepiness Score . Most causes will be identified by a detailed history from the patient and their bed partner.
Causes of hypersomnolence
NarcolepsyThis has a prevalence of about 1 in 2000, with peak
onset in adolescence and early middle age. The key
symptom is sudden, irresistible ‘sleep attacks’, often in inappropriate circumstances such as whilst eating or
talking. Other characteristic features help distinguish
this from excessive daytime sleepiness. Symptoms may be due to loss of hypocretin-secreting hypothalamic neurons. Diagnosis requires sleep study with sleep latency testing (demonstrating rapid onset of REM sleep).
Treatment is with sodium oxybate, modafinil, dexamfetamine or methylphenidate. Cataplexy may respond to sodium oxybate, clomipramine or venlafaxine.
Narcolepsy symptoms
Parasomnias
Parasomnias are abnormal motor behaviours that occuraround sleep. They may arise in either REM or non-REM
sleep, with characteristic features and timing. Non-REM
parasomnias tend to occur early in sleep. Parasomnias
should be distinguished from other motor disturbances
(such as periodic limb movements, hypnic jerks or sleep
talking) and sleep-onset epileptic seizures . History from a sleeping partner or other witness is essential.
Restless legs syndrome (RLS)
RLS is common, with a prevalence of up to 10%, but many patients never seek medical attention. It is characterised by unpleasant leg (and sometimes arm) sensations that are eased by movement (motor restlessness); the diagnosis is clinical (Box).It has a strong familial tendency and can present with
daytime somnolence due to poor sleep. It is usually idiopathic, but may be associated with haematinic deficiency, pregnancy, peripheral neuropathy, Parkinson’s
disease or uraemia. It should be distinguished from akathisia, the daytime motor restlessness that is an
adverse effect of anti-psychotic drugs.
Treatment, if required, is with dopaminergic drugs (dopamine agonists or levodopa) or benzodiazepines.
Diagnostic criteria for restless legs syndrome
Periodic limb movements in sleep
Unlike RLS, period limb movements in sleep (PLMS)only occurs during sleep and causes repetitive flexion
movements of the limbs, usually in the early (non-REM)
stages of sleep. Although patients are unaware of the
symptoms, they may disturb sleep quality and often
disturb partners. The pathological significance of PLMS
is uncertain and it often occurs in normal health. There
is an overlap with RLS. Treatment is most successful
with clonazepam or dopaminergic drugs.
NEURO-INFLAMMATORY DISEASES
Multiple sclerosis (MS)MS is an important cause of longterm disability in adults, especially in the UK, where the prevalence is about 120 per 100 000. The incidence is higher in Northern Europeans, and the disease is about twice as common in females.
Pathophysiology
There is evidence that both genetic and environmental
factors play a causative role. The prevalence of MS is low
near the equator and increases in the temperate zones of
both hemispheres. Most importantly, people retain the risk of developing the disease in the zone in which they grew up, indicating that environmental exposures during growth and development are important.
Prevalence also correlates with environmental factors, such as sunlight exposure, vitamin D and exposure to Epstein– Barr virus (EBV). Genetic factors are also relevant; the risk of familial recurrence in MS is 15%, with highest risk in first-degree relatives. Monozygotic twins have a concordance rate of 30%. The genes that predispose to MS are incompletely defined but inheritance appears to be polygenic, with influences from genes for human leucocyte antigen (HLA) typing, interleukin receptors.
An immune hypothesis is supported by increased levels of activated T lymphocytes in the CSF and increased immunoglobulin synthesis within the CNS.
Initial CNS inflammation in MS involves entry of activated T lymphocytes across the blood–brain barrier.
These recognise myelin-derived antigens on the surface
of the nervous system’s antigen-presenting cells, themicroglia, and undergo clonal proliferation. The resulting
inflammatory cascade releases cytokines and initiates
destruction of the oligodendrocyte–myelin unit by
macrophages. Histologically, is a plaque of inflammatory demyelination, most commonly in the periventricular regions of the brain, the optic nerves, and the subpial regions of the spinal cord . Much of the initial acute clinical deficit is caused by the effect of inflammatory cytokines on transmission of the nervous impulse rather than structural disruption of the myelin, and may explain the rapid recovery of some deficits and probably the acute benefit from corticosteroids.
In the long term, accumulating myelin loss reduces the efficiency of impulse propagation or causes complete conduction block, contributing to sustained impairment of CNS functions.
Inflammatory mediators released during the acute attack (particularly nitric oxide) probably also initiate axonal damage, which is a feature of the latter stages of the disease. In established MS there is progressive axonal loss, probably due to the successive damage from acute attacks and the subsequent loss of neurotrophic factors from oligodendrocytes.
This axonal loss may account for the phase of the disease characterised by progressive and persistent disability .
Fig. Multiple sclerosis. A Photomicrograph from demyelinating plaque, showing perivascular cuffing of blood vessel by lymphocytes.
B Brain MRI in multiple sclerosis. Multiple high-signal lesions (arrows) seen particularly in the paraventricular region on T2 image. C In T1 image with gadolinium enhancement, recent lesions (A arrows) show enhancement, suggesting active inflammation (enhancement persists for
4 weeks); older lesions (B arrows) show no enhancement but low signal, suggesting gliosis.
The progression of disability in fulminant, relapsing–
remitting and progressive multiple sclerosis.Clinical features
A diagnosis of MS requires the demonstration of otherwiseunexplained CNS lesions separated in time and
space .
The peak age of onset of MS is the fourth decade; onset before puberty or after the age of 60 years is rare. Where there is widespread inflammation,
this usually leads to widespread symptoms and/
or signs. Symptoms and signs of MS usually come on
over days or weeks, resolving over weeks or months.
Rarely, a more rapid stroke-like presentation may occur.
Around 80% of patients have a relapsing and remitting
clinical course of episodic dysfunction of the CNS withvariable intervening recovery. Of the remaining 20%,
most follow a slowly progressive clinical course, while
a tiny minority have a fulminant variety leading to
early death . Frequent relapses with incomplete recovery indicate a poor prognosis for the patient. Some milder cases have an interval of years or even decades between attacks, while in others (particularly if optic neuritis is the initial manifestation) there is no recurrence of disease. In some individuals, a phase of secondary progression, caused by secondary axonal degeneration, supersedes the phase of relapse and remission.
There are a number of clinical symptoms and syndromes
suggestive of MS, occurring either at presentation
or during the course of the illness . The physical signs are determined by the anatomical site of demyelination. Combined spinal cord and brainstem signs are common, although evidence of previous optic neuritis may be found in the form of an afferent pupillary deficit. Significant intellectual impairment only supervenes late, when loss of frontal functions and impairment of memory are common.
The prognosis for patients with MS is difficult to predict. About 15% of patients have a single attack of demyelination and do not suffer further events, whilst those with relapsing and remitting MS experience, on average, 1–2 events every 2 years.
Approximately 5% of patients die within 5 years of disease onset, whilst others have a benign outcome.
Prognosis is good for patients with optic neuritis and only sensory relapses. Overall, about one-third of patients are disabled to the point of needing help with walking after 10 years, and this proportion rises to about one-half after 15 years. It would appear likely (though this is as yet unproven) that disease-modifying drugs will have an effect on future prognostication.
The Macdonald criteria for the diagnosis of multiple sclerosis (2011)1
Clinical features of multiple sclerosisInvestigations
There is no single diagnostic test that is definitive forMS, and the results of investigation need to be combined
with the clinical picture in order to make a diagnosis .
Other conditions should be excluded, and investigations should provide support for the diagnosis,
with evidence for a chronic widespread inflammatory
condition. Following the first clinical event,
investigations may help prognosis by confirming the
disseminated nature of the disease. MRI is the most
sensitive technique for imaging lesions in brain and
spinal cord and in excluding other causes that have provoked the neurological deficit.
However, the MRI appearances in MS may be confused with those of small-vessel disease or cerebral vasculitis, and these diagnoses should be considered and excluded. Visual evoked potentials can detect clinically silent lesions in up to 70%, but auditory and Somatosensory evoked potentials are seldom of diagnostic value.
The CSF may show a lymphocytic pleocytosis in the
acute phase and oligoclonal bands of IgG in 70–90% of
between attacks. Oligoclonal bands are not specific for MS and only denote intrathecal inflammation. These can appear in other disorders, which should be excluded by examination and other investigations. It is important to exclude other treatable conditions, such as infection, vitamin B12 deficiency and spinal cord compression.
Investigations in a patient suspected of
having multiple sclerosisMultiple sclerosis: demyelinating lesion in cervical
spinal cord, high-signal T2 images (arrow).A Sagittal plane.
B Axial plane.
Management
The management of MS involves four different strands:treatment of the acute episode, prevention of future
relapses, treatment of complications and management of
the patient’s disability.
The acute episode
In a function-threatening exacerbation of MS, pulses of
high-dose steroid, given either intravenously or orally
over 3–5 days, will shorten the duration of the acute
episode . Prolonged administration of steroids
does not alter the long-term outcome and causes
severe adverse effects and should therefore be avoided.
Pulses of steroids can be given up to three times in a year
but use should be restricted to those individuals with
significant function-threatening deficits. Prophylaxis to
prevent the occurrence of steroid-induced osteoporosis
should be considered in patients requiring multiple
courses of corticosteroids.
Disease-modifying treatment
Despite its immune basis, the administration of long-term
steroids, standard chemotherapies and immunoglobulin
is not practical or helpful in established MS. This motivated the search for innovative immuno-active treatments.
In relapsing and remitting MS, an increasing number
of beta-interferons have been used to reduce relapserates and improve long-term outlook. Their pharmacological properties tend to vary, but there has been no good trial evidence clinically separating the licensed compounds. Subcutaneous or intramuscular interferon-beta reduces the number of relapses by some 30%, with a small effect on long-term disability. Longterm
effects are usually local and development of antibodies
should be sought; their development should preclude further long-term treatment.
Other treatments are shown in Box.
Glatiramer acetate is a parenterally administered
oligopeptide that may act as a competitor antigen,but in addition it increases numbers of regulatory
CD4 T cells in relapsing–remitting MS, thereby helping
to reduce relapse rates. Unlike other immunomodulatory
treatments used for MS, glatiramer antibodies
do not block function and actually appear to enhance
efficacy. Mitoxantrone was initially developed as a chemotherapeutic agent and has been shown to decrease the relapse rate in MS, as well as lowering the number of
MRI lesions and reducing disability with long-term use.
Its similarity to doxorubicin is reflected in its adverse
effects, the most notable of which is cardiotoxicity.
Natalizumab is a monoclonal antibody to an antigen
expressed on activated T cells and monocytes, and has
been associated with a dramatic fall in the number of
lesions evident on MRI, with a corresponding decrease
in the number of relapses and reduction in axonal
damage. Rarely, neutralising antibodies block its efficacy
and indicate that the drug should be withdrawn.
Progressive multifocal leucoencephalopathy (PML) has
been seen in some natalizumab-treated patients. Occasional cases of CNS lymphoma and toxoplasmosis may be related to drug use.
Other newer immunomodulatory treatments have
been developed, including fingolimod, which is derivedfrom the ascomycete metabolite ISP-1 (myriocin) and
can be given orally. Further studies are needed to predict
which immune treatment should be tried in which
patients.
Special diets, including a gluten-free diet or linoleic acid supplements, and hyperbaric oxygen therapy are popular with patients but their efficacy has not yet been proven.
Treatment of symptoms, complications and disability
Treatments for the complications of MS are summarisedin Box. It is important to provide patients with a careful explanation of the nature of the disease and its outcome. When and if disability occurs, patients and their relatives need appropriate support. Specialist nurses working in a multidisciplinary team of healthcare professionals are of great value in managing the chronic phase of the disease. Periods of physiotherapy and occupational therapy may improve functional capacity in those patients who become disabled, and guidance can be provided on the provision of aids at home, reducing handicap.
Care of the bladder is particularly important. Urgency and frequency can be treated pharmacologically , but this may
lead to a degree of retention with an attendant risk of
infection. Retention can be managed initially by intermittent urinary catheterisation (performed by the
patient, if possible), but an in-dwelling catheter may
become necessary.
Sexual dysfunction is a frequent source of distress. Sildenafil or tadalafil helps impotence in men, and skilled counselling and prosthetic aids are often beneficial.
Corticosteroids in multiple sclerosis
Disease-modifying therapies in multiple sclerosis
Disease-modifying treatments in multiple sclerosis
Treatment of complications inmultiple sclerosis
Care of the bladder
Multiple sclerosis in pregnancy
Acute disseminated encephalomyelitisThis is an acute monophasic demyelinating condition in
which there are areas of perivenous demyelination widely disseminated throughout the brain and spinal
cord. The illness may apparently arise spontaneously
but often occurs a week or so after a viral infection,
especially measles and chickenpox, or following vaccination, suggesting that it is immunologically mediated .
Clinical features
Headache, vomiting, pyrexia, confusion and meningism
may be presenting features, often with focal or multifocal
brain and spinal cord signs. Seizures or coma may occur. A minority of patients who recover have further episodes.
Investigations
MRI shows multiple high-signal areas in a patternsimilar to that of MS, although often with large confluent
areas of abnormality. The CSF may be normal or show
an increase in protein and lymphocytes (occasionally
over 100 × 106 cells/L); oligoclonal bands may be found
in the acute episode but, unlike in MS, will not persist
beyond clinical recovery. The clinical picture may be
very similar to a first relapse of MS.
Management
The disease may be fatal in the acute stages but is otherwise self-limiting. Treatment with high-dose intravenous
methylprednisolone, using the same regimen as
for a relapse of MS, is recommended.
Transverse myelitis
Acute, usually monophasic, demyelinating disorder affecting the spinal cord. It is usually thought to be post-infectious in origin. It occurs at any age and presents with a subacute paraparesis with a sensory level, accompanied by severe pain in the neck or back at the onset.
MRI should distinguish this from an external lesion affecting the spinal cord.
CSF examination shows cellular pleocytosis, often with polymorphs. Oligoclonal bands are usually absent.
Treatment is with high-dose IV methylprednisolone.
The outcome is variable: one-third have static deficit, one-third go on to develop MS and onethird recover with no subsequent relapse.
Prognostic features predicting multiple sclerosis after transverse myelitis
Neuromyelitis opticaNeuromyelitis optica (also known as Devic’s disease) is
the occurrence of transverse myelitis and bilateral optic
neuritis. The disease has been recognised for many
years, particularly in Asia. The majority of cases are
associated with an antibody to a neuronal membrane
channel, aquaporin 4. If changes are seen on brain MRI
(this is variable), they are typically high-signal lesions
restricted to periventricular regions. Spinal MRI scans
show lesions that are typically longer than three spinal segments (unlike the shorter lesions of MS). Clinical deficits tend to recover less well than in MS, and the disease may be more aggressive with more frequent relapses. Treatment with older immunosuppressive agents, such as steroids, azathioprine or cyclophosphamide, and/or plasmapheresis seems to be more effective than in MS.
PARANEOPLASTIC NEUROLOGICAL DISORDERS
Neurological disease may occur with systemic malignant
tumours in the absence of cerebral metastases.
It is now recognised that, in the majority of these
cases, antigen production in the body of the tumour
leads to development of antibodies to parts of the
CNS. These syndromes are particularly associated with small-cell carcinoma of lung, ovarian tumours, and lymphomas. Autoantibodies are found in the serum and/or CSF, and biopsy will show a lymphocytic infiltrate of the neural tissue affected.
Paraneoplastic disorders of the central nervous system
Paraneoplastic disorders of the peripheral nervous systemClinical presentations
These are summarised in Boxes. In most instances, the neurological condition progresses quite rapidly over a few months, preceding the malignant disease in around half of cases. The paraneoplastic disorders of the peripheral nervous system particularly affect the synaptic cleft . Investigations and managementThe presence of characteristic autoantibodies in the
context of a suspicious clinical picture may be diagnostic.
The causative tumour may be very small and therefore
CT of the chest or abdomen or PET scanning may
be necessary to find it. These investigations should only
be pursued when paraneoplastic disease has been
proven, rather than when it is suspected.
The CSF often shows an increased protein and lymphocyte count with oligoclonal bands.
Treatment is directed at the primary tumour. Occasionally, successful therapy of the tumour is associated with improvement of the paraneoplastic syndrome. Some improvement may occur following administration of intravenous immunoglobulin .
NEURODEGENERATIVE DISEASES
Whilst MS is the most common cause of disabilityin young people in the UK,
vascular and neurodegenerative diseases are increasingly important in later life. The neurodegenerative diseases are united in having a pathological process that leads to specific neuronal death, causing relentlessly progressive symptoms that increase in incidence in older age.
The precise causes are not yet known. Alzheimer’s disease and Parkinson’s disease are the most common.
Movement disorders
They may be genetic or acquired, and the most important is Parkinson’s disease.Idiopathic Parkinson’s disease
Parkinsonism is a clinical syndrome characterised primarily
by bradykinesia ,with associated increased tone (rigidity), tremor and loss of postural reflexes. There are many causes but the most common is Parkinson’s disease (PD). Age has a critical influence on incidence and prevalence, the latter rising to 300–500/100 000 after 80 years of age.
Average age of onset is about 60 years, and fewer than 5% of patients present under the age of 40. Genetic factors are increasingly recognised and several single genes causing parkinsonism have been identified.
Having a first-degree relative with PD confers a 2–3 times increased risk of developing PD. It is a progressive and incurable condition, with a variable prognosis.
Whilst motor symptoms are the most common
presenting features, non-motor symptoms (particularly
cognitive impairment, depression and anxiety) become
increasingly common as the disease progresses, and significantly reduce quality of life.
Pathophysiology
Although mutations in several genes have been identified in a few cases, in most patients the cause remains unknown. The discovery that methyl-phenyltetrahydropyridine (MPTP) caused severe parkinsonism in young drug users suggested that PD might be due to an environmental toxin.
Causes of parkinsonism
The pathological hallmarks of PDare depletion of the pigmented dopaminergic neurons in the substantia nigra and the presence of α-synuclein and other protein inclusions in nigral cells (Lewy bodies).
It is thought that environmental or genetic factors
alter the α-synuclein protein, rendering it toxic and
leading to Lewy body formation within the nigral cells.
Lewy bodies are also found in the basal ganglia, brainstem and cortex, and increase with disease progression.
PD is recognised as a synucleinopathy alongside multiple
system atrophy and dementia with Lewy bodies. The
loss of dopaminergic neurotransmission is responsible
for many of the clinical features.
Clinical features
Non-motor symptoms, including reduction in senseof smell (hyposmia), constipation and REM sleep
behavioural disturbance (RBD), may precede the development of typical motor features by many years, but
patients rarely present at this stage. The motor symptoms
are almost always initially asymmetrical. The hallmark
is bradykinesia, leading to classic symptoms such
as increasingly small handwriting, difficulty with tying
shoelaces or buttoning, and difficulty rolling over in bed.
Tremor is an early feature but may not be apparent in at
least 20% of people with PD. It is typically a unilateral
rest tremor affecting limbs, jaw and chin, but not the head.
In some patients, tremor remains the dominant
symptom for many years. Rigidity causes stiffness
and a flexed posture. Although postural righting reflexes
are impaired early on in the disease, falls tend not to
occur until later. As the disease advances, speech
becomes softer and indistinct. There are a number of
abnormalities on neurological examination (Box).
Although the features are initially unilateral, gradual
bilateral involvement evolves with time.
Cognition is spared in early disease; if impaired, it should trigger consideration of alternative diagnoses, such as dementia with Lewy bodies.
Physical signs in Parkinson’s disease
Non-motor symptomsSome non-motor symptoms (NMS) precede the onset of
more typical symptoms by many years (see above).
Depression or anxiety may also be presenting features
of PD. For most patients, however, NMS become increasingly common and disabling as PD progresses. Cognitive impairment, including dementia, is the symptom
most likely to impair quality of life for patients and
their carers. Other distressing NMS include other neuropsychiatric features (anxiety, depression, apathy, hallucinosis/psychosis), sleep disturbance and hypersomnolence, fatigue, pain, sphincter disturbance and constipation, sexual problems (erectile failure, loss of
libido or hypersexuality) and drooling.
Investigations
The diagnosis is clinical. Structural imaging (CT or MRI)
is usually normal for age and thus rarely helpful, although it may support a suspected vascular cause.
Functional dopaminergic imaging (SPECT or PET) is
abnormal, even at early stages , but does not
differentiate between the different forms of degenerative
parkinsonism and so is not specific for PD. In younger patients, specific investigations may be appropriate (e.g. exclusion of Huntington’s and Wilson’s diseases).
Some patients with family histories may wish to consider genetic testing, although the role of genetic counselling is uncertain at present.
Imaging in Parkinson’s disease.
A SPECT scan in Parkinson’s disease showing reduced dopamine activity in the basal ganglia.B Normal.
Parkinson’s disease
High power (× 400) of substantia nigra of a patient with Parkinson’s disease showing classical Lewy body (haematoxylin and eosin).Management of Parkinson’s disease
ManagementDrug therapy
Drug treatment for PD remains symptomatic rather than
curative, and there is no evidence that any of them are neuroprotective. Levodopa (LD) remains the most effective, other include dopamine agonists, anticholinergics, monoamine oxidase inhibitors (MAOI)-B and catechol-O-methyl-transferase (COMT), and amantadine. Debate continues about when to start treatment, and with which drug. Most specialists recommend initiating treatment when symptoms are impacting on everyday life; whether it is best to start with LD, a dopamine agonist or MAOI-B remains unclear. Many motor symptoms, such as tremor, freezing, falling, head-drop and abnormal flexion, are quite resistant to treatment.
In the UK, rivastigmine is licensed for use in PD-associated dementia, although its effect is modest at best. Many other NMS are resistant.
Levodopa. LD is the precursor to dopamine. When
administered orally, more than 90% is decarboxylated to
dopamine peripherally in the GIT and blood vessels, and only a small proportion reaches the brain. This peripheral conversion is responsible for the high frequency of adverse effects, and to avoid this, LD is combined with a dopa decarboxylase inhibitor (DDI); the inhibitor does not cross the blood–brain barrier, thus avoiding unwanted decarboxylation-blocking in the brain. Two DDIs, carbidopa and benserazide, are available as combination with LD, as Sinemet® and Madopar®, respectively.
LD is most effective at relieving the akinesia and
rigidity; tremor response is often less satisfactory, and ithas no effect on many motor (posture, freezing) and
non-motor symptoms. Failure of akinesia/rigidity to
respond to LD 1000 mg/day should prompt reconsideration of the diagnosis. Although controlled-release versions of LD exist, these are usually best reserved for use overnight, as their variable bioavailability makes them difficult to use throughout the day. Madopar® is also available as a dispersible tablet for more rapid-onset effect.
Adverse effects include postural hypotension, nausea and vomiting, which may be offset by domperidone. LD may exacerbate or trigger hallucinations, and abnormal LD-seeking behaviour may occur uncommonly (dopamine dysregulation syndrome) in which the patient takes excessive doses of LD.
As PD progresses, the response to LD becomes less
predictable in many patients, leading to motor fluctuations. This end-of-dose deterioration is due to progressive loss of dopamine storage capacity by dwindling numbers of striatonigral neurons. LD-induced involuntary movements (dyskinesia) may occur as a peak-dose phenomenon or as a biphasic phenomenon (occurring
during both the build-up and wearing-off phases). More complex fluctuations present as sudden, unpredictable changes in response, in which periods of parkinsonism
(‘off’ phases) alternate with improved mobility but with dyskinesias (‘on’ phases). Motor complication management is difficult; wearing-off effects may respond to increased dose or frequency of LD or addition of a COMT inhibitor .
More complex fluctuations may be improved by the addition of dopamine agonists (including continuous infusion of apomorphine), intrajejunal LD via a percutaneous endoscopic jejunostomy, or deep brain stimulator implantation.
Dopamine receptor agonists. Originally introduced in
the hope of delaying the initiation of LD, and thus delaying
motor complications, several dopamine agonists are
available, and may be delivered orally, transdermally or
subcutaneously . The ergot-derived agonists are no longer recommended because of rare but serious fibrotic effects. With the exception of apomorphine, all the agonists are less powerful than LD in relieving parkinsonism, have more adverse effects (nausea, vomiting, confusion and hallucinations) and are more expensive.
Their role in the management of PD (monotherapy or adjunctive) remains uncertain, and evidence is accruing to suggest that their usefulness as initial monotherapy is short-lasting.
MAOI-B inhibitors. Monoamine oxidase type B facilitates
breakdown of excess dopamine in the synapse. Two inhibitors are used in PD: selegiline and rasagiline.
The effects of both are modest, although usually well
tolerated. Neither is neuroprotective, despite initial hopes.
COMT inhibitors. Catechol-O-methyl-transferase (along
with dopa decarboxylase) is involved in peripheral
breakdown of LD. Two inhibitors are available: entacapone
and tolcapone (which also inhibits central COMT).
Entacapone has a modest effect and is most useful for early wearing-off. It is available either as a single tablet taken with each LD/DDI dose, or as a combination tablet with LD and DDI. The more potent tolcapone is less used because of rare but serious hepatotoxicity.
Amantadine. This has a mild, usually short-lived effect
on bradykinesia and is rarely used unless patients are
unable to tolerate other drugs. It is more commonly
employed as a treatment for LD-induced dyskinesias,
although again benefit is modest and short-lived.
Adverse effects include livedo reticularis, peripheral
oedema, confusion and other anticholinergic effects.
Anticholinergic drugs. These were the main treatment for PD prior to the introduction of LD. Their role now is limited by lack of efficacy (apart from an effect on tremor sometimes) and adverse effects, including dry mouth, blurred vision, constipation, urinary retention, confusion and hallucinosis. Several anticholinergics are available, including trihexyphenidyl (benzhexol) and orphenadrine.
Surgery
Destructive neurosurgery was commonly used before
the introduction of LD. In the last 20 years, stereotactic
surgery has emerged and most commonly involves
deep brain stimulation (DBS), rather than the destructive
approach of previous eras. Various targets have been identified, including the thalamus (though this is only effective for tremor), globus pallidus and subthalamic nucleus.
DBS is usually reserved for refractory tremor or motor fluctuations, and careful patient selection is vital to success. Intracranial delivery of fetal grafts or specific growth factors remains experimental.
Physiotherapy, occupational therapy and speech therapy
Patients at all stages of PD benefit from physiotherapy,
which helps reduce rigidity and corrects abnormal
posture. Occupational therapists can provide equipment
to help overcome functional limitations. Speech therapy can help dysarthria and dysphonia, and advice may also
be provided to those with dysphagia on the use of
altered-texture diets. As with many complex neurological
disorders, patients with PD should ideally be managed by a multidisciplinary team, including PD specialist nurses.
Dopamine agonists*
Other parkinsonian syndromesCerebrovascular disease and drug-induced parkinsonism
are the most common alternative causes of parkinsonism.
There are several degenerative conditions that cause parkinsonism, including multiple system atrophy, progressive supranuclear palsy and corticobasal degeneration.
They typically have a more rapid progression than PD and tend to be resistant to treatment with LD.
They are defined pathologically and identification during life is difficult. There are other conditions that may rarely manifest as parkinsonism, including Huntington’s and Wilson’s diseases.
Wilson’s disease
This is an autosomal recessive disorder causing a defectof copper metabolism . It is a treatable cause of various movement disorders, including tremor, dystonia, parkinsonism and ataxia; psychiatric symptoms may also occur. Wilson’s should always be excluded in younger patients presenting with any movement disorder.
Huntington’s disease (HD)
Autosomal dominant disorder, usually presenting in adults but occasionally children. It is due to expansion of a trinucleotide CAG repeat in the Huntingtin gene on chromosome 4 . The disease frequently demonstrates the phenomenon of anticipation, in which there is a younger age at onset as the disease is passed through generations, due to progressive expansion of the repeat.
Clinical features
HD typically presents with a progressive behaviouraldisturbance, abnormal movements (usually chorea), and
cognitive impairment leading to dementia. Onset under
18 years is rare, but patients may then present with
parkinsonism rather than chorea (the ‘Westphal variant’).
There is always a family history.
Investigations and management
The diagnosis is confirmed by genetic testing; presymptomatic testing for other family members is available but must be preceded by appropriate counselling. Brain imaging may show caudate atrophy but is
not a reliable test. There are a number of HD mimics.
Management is symptomatic. The chorea may respond to neuroleptics such as risperidone or sulpiride, or other drugs such as tetrabenazine. Depression and anxiety are common, and may be helped by medication.
Ataxias
The ataxias are a heterogeneous group of inherited and
acquired disorders, presenting either with pure ataxia
or in association with other neurological and nonneurological features. The differential is wide (Boxes)
, and diagnosis is guided by age of onset,
evolution and clinical features. A significant proportion
of cases remain idiopathic despite investigation.
The hereditary ataxias are a group of inherited disorders
in which degenerative changes occur to varyingextents in the cerebellum, brainstem, pyramidal tracts,
spinocerebellar tracts, and optic and peripheral nerves,
and which influence the clinical manifestations.
Onset ranges from infancy to adulthood, with recessive, sex-linked or dominant inheritance .
Whilst the genetic abnormality has been identified for some, allowing diagnostic testing, this is not currently the case for many of the hereditary ataxias
Causes of acquired ataxia
Inherited ataxias
Tremor disorders
Tremor is a feature of many disorders, but the most important clinical syndromes are PD, essential tremor, drug-induced tremors and functional (psychogenic) tremors.Essential tremor
This has a prevalence of about 300/100 000 and may display a dominant pattern of inheritance, although no genes have thus far been identified. It may present at any age with a bilateral arm tremor (8–10 Hz), rarely at rest but typical with movement. The head and voice may be involved. The tremor improves in about 50% of patients with small amounts of alcohol. There are no specific tests, and it should be distinguished from other tremor syndromes, including dystonic tremor. Betablockers and primidone are sometimes helpful, and DBS of the thalamus is an effective treatment for severe cases.
Drug-induced tremor (usually postural)
DystoniaDystonia is characterised by a focal increase in tone
affecting muscles in the limbs or trunk. It may be a
feature of a number of neurological conditions (PD,
Wilson’s disease), or occur secondary to brain damage
(trauma, stroke) or drugs (tardive syndromes). Dystonia
also occurs as a primary disorder. In childhood onset,
the cause is usually genetic and dystonia is generalised
but adult onset is usually focal; examples include a
twisted neck (torticollis), repetitive blinking (blepharospasm) or tremor. Task-specific symptoms (e.g. writer’s cramp, musician’s dystonia) are often dystonic. Treatment is difficult but botulinum toxin injections or DBS
may be useful.
Hemifacial spasm
This usually presents after middle age with intermittent
twitching around one eye, spreading ipsilaterally to
other facial muscles. The spasms are exacerbated by
talking, eating and stress. Hemifacial spasm is usually
idiopathic (similarly to trigeminal neuralgia, it has been
suggested that it is due to an aberrant arterial loop irritating the 7th nerve just outside the pons), but may be
symptomatic and secondary to structural lesions or MS.
Drug treatment is not effective but injections of botulinum
toxin into affected muscles help, although these usually have to be repeated every 3 months or so. In refractory cases, microvascular decompression may be considered.
Motor neuron disease (MND)
Is a neurodegenerative condition caused by loss of upper and lower motor neurons in the spinal cord, cranial nerve nuclei and motor cortex. Annual incidence is about 2/100 000, with a prevalence of about 7/100 000. Most cases are sporadic but between 5 and 10% of cases are familial. Abnormalities in the superoxide dismutase (SOD1) gene account for about 20% of such cases, and recently an expanded repeat sequence in the C9ORF72 gene on chromosome 9 has been associated with MND and frontotemporal dementia. The most common form of MND is amyotrophic sclerosis (ALS), and many use the terms MND and ALS interchangeably.ALS is characterised by a combination of upper and lower motor neuron signs; there are rarer, pure lower (progressive muscular atrophy) or upper (progressive lateral sclerosis) motor neuron variants of MND.
The average age of onset is 65, with 10% presenting before 45 years.
Clinical features
MND typically presents focally, either with limb onset (e.g. foot drop) or with bulbar symptoms; respiratory onset is rare but respiratory failure is a common terminal event. Sensory, autonomic and visual symptoms do not occur, although cramp is common .Examination reveals a combination of lower and upper motor neuron signs (e.g. brisk reflexes in wasted, fasciculating muscles) without sensory involvement .Cognitive impairment is under-recognised in MND: up to 50% will have a mainly executive impairment on formal testing, and around 10% develop a frontotemporal dementia (FTD). About 10% of patients presenting with FTD will develop ALS within a few years of dementia onset. MND is relentlessly progressive and up to 50% die within 2 years of diagnosis.Clinical features of motor neuron disease
Patterns of involvement in motor neuron disease
InvestigationsClinical features are often typical but alternative diagnoses should be excluded. Exclusion of treatable causes, such as immune-mediated multifocal motor neuropathy with conduction and cervical myeloradiculopathy, is essential. Blood tests are usually
normal, other than a mildly raised creatine kinase.
Sensory and motor nerve conduction studies are normal
but there may be reduction in amplitude of motor action
potentials due to axonal loss. Electromyography
Will usually confirm the typical features of widespread
denervation and re-innervation. Spinal fluid analysis is
not usually necessary. DNA testing may become more
important as genetic factors become clearer.
Management
Patients should be managed within a multidisciplinaryservice, including physiotherapists, speech and occupational
therapists, dietitians, ventilatory and feeding
support, and palliative care teams, with neurological
and respiratory input. Riluzole is licensed for ALS but
has only a modest effect . Non-invasive ventilatory
support and/or feeding by percutaneous gastrostomy
may improve quality of life in selected patients.
Rapid access to palliative care teams is essential for
patients as they enter the terminal stages of MND.
Effective treatments for amyotrophic lateral sclerosis/motor neuron disease
Spinal muscular atrophyThis is a group of genetically determined disorders
affecting spinal and cranial lower motor neurons, characterised by proximal and distal wasting, fasciculation
and weakness of muscles. Involvement is usually symmetrical
but occasional localised forms occur. With the
exception of the infantile form, progression is slow and
the prognosis better than for MND.
INFECTIONS OF THE NERVOUS SYSTEM
The clinical features of nervous system infectionsdepend on the location of the infection (the meninges
or the parenchyma of the brain and spinal cord), the
causative organism (virus, bacterium, fungus or parasite),
and whether the infection is acute or chronic. The major infections of the nervous system are listed in
Box. The frequency of these varies geographically.
Infections of the nervous system
Infections of the nervous system'cont'dMeningitis
Acute infection of the meninges presents with a characteristic combination of pyrexia, headache and meningism. Meningism consists of headache, photophobia and stiffness of the neck, often accompanied by other signs of meningeal irritation, including Kernig’s sign (extension at the knee with the hip joint flexed causes spasm in the hamstring muscles) and Brudzinski’s sign (passive flexion of the neck causes flexion of the hips and knees). Meningism is not specific to meningitis and can occur in patients with subarachnoid haemorrhage. The severity of clinical features varies with the causative organism, as does the presence of other features such as a rash. Abnormalities in the CSF are important in distinguishing the cause of meningitis. Causes of meningitis are listed in Box
Causes of meningitis
Causes of meningitis'cont'd
Viral meningitisViruses are the most common cause of meningitis,
usually resulting in a benign and self-limiting illness
requiring no specific therapy. It is much less serious than bacterial meningitis unless there is associated
encephalitis (which is rare). A number of viruses can
cause meningitis , the most common being enteroviruses. Where specific immunisation is not employed, the mumps virus is a common cause.
Clinical features
Viral meningitis occurs mainly in children or young
adults, with acute onset of headache and irritability and
the rapid development of meningism. The headache is
usually the most severe feature. There may be a high
pyrexia but focal neurological signs are rare.
Investigations
The diagnosis is made by lumbar puncture. The CSF
usually contains an excess of lymphocytes but glucose
and protein levels are commonly normal; the protein
level may be raised. It is extremely important to verify
that the patient has not received antibiotics (for whatever
cause) prior to the lumbar puncture, as this picture can also be found in partially treated bacterial meningitis.
Management
There is no specific treatment and the condition isusually benign and self-limiting. The patient should be
treated symptomatically in a quiet environment. Recovery
usually occurs within days, although a lymphocytic
pleocytosis may persist in the CSF. Meningitis may also
occur as a complication of a systemic viral infection such
as mumps, measles, infectious mononucleosis, herpes
zoster and hepatitis. Whatever the virus, complete
recovery without specific therapy is the rule.
Bacterial meningitis
Many bacteria can cause meningitis. Bacterial meningitis is usually part of a bacteraemic illness, although direct spread from an adjacent focus of infection in the ear, skull fracture or sinus can be causative. Antibiotics have rendered this less common but mortality and morbidity remain significant. The meningococcus and other common causes of meningitis are normal commensals of the upper respiratory tract. New and potentially pathogenic strains are acquired by the air-borne route but close contact is necessary. Epidemics of meningococcal meningitis occur particularly in cramped living conditions or where the climate is hot and dry. The organism invades through the nasopharynx, producing septicaemia and meningitis.
Bacterial causes of meningitis
PathophysiologyThe meningococcus (Neisseria meningitidis) is now the
most common cause of bacterial meningitis in Western
Europe after Streptococcus pneumoniae, whilst in the
USA Haemophilus influenzae remains common.
The infection stimulates an immune response, causing the pia–arachnoid membrane to become congested and infiltrated with inflammatory cells. Pus then forms in layers,
which may later organise to form adhesions. These may obstruct the free flow of CSF, leading to hydrocephalus, or they may damage the cranial nerves at the base of the brain. Hearing loss is a frequent complication.
The CSF pressure rises rapidly, the protein content increases, and there is a cellular reaction. An obliterative endarteritis of the leptomeningeal arteries passing through the meningeal exudate may produce secondary cerebral infarction.Pneumococcal meningitis is often associated with a very purulent CSF and a high mortality.
Clinical features
Headache, drowsiness, fever and neck stiffness are the
usual presenting features. In severe bacterial meningitis
the patient may be comatose and later there may be focal
signs. Ninety percent with meningococcal meningitis will have two of the following:
fever, neck stiffness, altered consciousness and rash.
When accompanied by septicaemia, it may present very
rapidly, abrupt onset of obtundation (cerebral oedema).
Chronic meningococcaemia is a rare condition in which the patient can be unwell for weeks or even months with recurrent fever, sweating, joint pains and transient rash.
It usually occurs in the middle-aged and elderly, and in those who have previously had a splenectomy. In pneumococcal and Haemophilus infections there may be an associated otitis media.
Pneumococcal meningitis may be associated with pneumonia and occurs especially in older patients and alcoholics, as well as those without functioning spleens.
Listeria monocytogenes is an increasing cause of meningitis
and rhombencephalitis (brainstem encephalitis) in the immunosuppressed, people with diabetes, alcoholics and pregnant women .It can also cause meningitis in neonates.
Complications of meningococcal septicaemia
InvestigationsLumbar puncture is mandatory unless there are contraindications.
If the patient is drowsy and has focal neurological signs or seizures, is immunosuppressed, has undergone recent neurosurgery or has suffered a head injury, it is wise to obtain a CT to exclude a mass lesion (such as a cerebral abscess) before lumbar puncture because of the risk of coning. This should not, however, delay treatment of a presumptive meningitis.
If lumbar puncture is deferred or omitted, it is essential
to take blood cultures and to start empirical treatment .
Lumbar puncture will help differentiate the causative organism: in bacterial meningitis the CSF is cloudy (turbid) due to the presence of many neutrophils (often > 1000 × 106 cells/L), the protein content is significantly
elevated and the glucose reduced. Gram film and
culture may allow identification of the organism. Blood
cultures may be positive. PCR techniques can be used
on both blood and CSF to identify bacterial DNA. These
methods are useful in detecting meningococcal infection
and in typing the organism.
The investigation of meningitis
ManagementThere is an untreated mortality rate of around 80%, so
action must be swift. If bacterial meningitis is suspected,
the patient should be given parenteral benzylpenicillin
immediately (intravenous is preferable) and prompt admission should be arranged. The only contraindication is a history of penicillin anaphylaxis. Recommended empirical therapy before the cause of meningitis is known is given in Box, and the preferred Antibiotic when the organism is known after CSF examination is stipulated in Box .
Adjunctive
corticosteroid therapy is useful in both children and
adults in developed countries where the incidence
of penicillin resistance is low.
But corticosteroid role in settings where there are high rates of resistance or in under-developed countries where there are high rates of untreated HIV is unclear.
In meningococcal disease, mortality is doubled if the
patient presents with features of septicaemia rather than
meningitis. Individuals likely to require intensive care
facilities and expertise include those with cardiac,
respiratory or renal involvement, and those with CNS
depression prejudicing the airway. Early endotracheal
intubation and mechanical ventilation protect the airway
and may prevent the development of the ARDS.
Adverse prognostic features include hypotensive shock, a rapidly developing rash, a haemorrhagic diathesis, multisystem failure and age over 60 years.
Treatment of pyogenic meningitis of unknown cause
Chemotherapy of bacterial meningitis when the cause is known
Chemotherapy of bacterial meningitis when the cause is known'cont'd
Adjunctive dexamethasone for bacterial meningitis
Prevention of meningococcal infectionClose contacts of patients with meningococcal infection
(Box) should be given 2 days of oral rifampicin. In adults, a single dose of ciprofloxacin is an alternative.
If not treated with ceftriaxone, the index case should
be given similar treatment to clear infection from the
nasopharynx before hospital discharge. Vaccines are
available for most meningococcal subgroups but not
group B, which is among the most common serogroup
isolated in many countries.
Chemoprophylaxis following
meningococcal exposureTuberculous meningitis
Is now uncommon in developed countries in previously healthy individuals, although it is still seen in those born in endemic areas and in the immunocompromised. It remains common in developing countries and is seen frequently as a secondary infection in patients with the AIDS.Pathophysiology
Tuberculous meningitis most commonly occurs shortly after a primary infection in childhood or as part of miliary tuberculosis .The usual local source of infection is a caseous focus in the meninges or brain substance adjacent to the CSF pathway. The brain is covered by a greenish, gelatinous exudate, especially around the base, and numerous scattered tubercles are found on the meninges.
Clinical features
The clinical features and staging criteria are listed in Box .Onset is much slower than in other bacterial meningitis
– over 2–8 weeks. If untreated, it is fatal in a few
weeks but complete recovery is usual if treatment is
started at stage I .When treatment is initiated later, the rate of death or serious neurological deficit may be as high as 30%.
Clinical features and staging of
tuberculous meningitis
Investigations
Lumbar puncture should be performed if the diagnosisis suspected. The CSF is under increased pressure. It is usually clear but, when allowed to stand, a fine clot (‘spider web’) may form. The fluid contains up to 500 × 106 cells/L, predominantly lymphocytes, but can contain neutrophils. There is a rise in protein and a marked fall in glucose. The tubercle bacillus may be detected in a smear of the centrifuged deposit from the CSF but a negative result does not exclude the diagnosis. The CSF should be cultured but, as this result will not be known for up to 6 weeks, treatment must be started without waiting for confirmation. Brain imaging may show hydrocephalus, brisk meningeal enhancement on enhanced CT or MRI, and/or an intracranial tuberculoma.
Management
As soon as the diagnosis is made or strongly suspected,chemotherapy should be started using one of the regimens
that include pyrazinamide.
The use of corticosteroids in addition to anti-tuberculous
therapy has been controversial. Recent evidence suggests
that it improves mortality, especially if given early,
but not focal neurological damage. Surgical ventricular
drainage may be needed if obstructive hydrocephalus
develops. Skilled nursing is essential during the acute
phase of the illness, and adequate hydration and nutrition
must be maintained.
Other forms of meningitis
Fungal meningitis (especially cryptococcosis)usually occurs in patients who are immunosuppressed
and is a recognised complication of HIV infection .The CSF findings are similar to those of tuberculous
meningitis, but the diagnosis can be confirmed
by microscopy or specific serological tests.
In some areas, meningitis may be caused by spirochaetes
(leptospirosis, Lyme disease and syphilis), rickettsiae (typhus fever) or protozoa (amoebiasis).
Meningitis can also be due to non-infective pathologies.
This is seen in recurrent aseptic meningitis due to
SLE, Behçet’s disease or sarcoidosis, as well as a condition
of previously unknown origin known as Mollaret’s
syndrome, in which the recurrent meningitis is associated
with epithelioid cells in the spinal fluid (‘Mollaret’
cells). Recent evidence suggests that this condition may
be due to human herpes virus type 2, and is therefore
infective after all. Meningitis can also be seen due to
direct invasion of the meninges by neoplastic cells
(‘malignant meningitis’).
Parenchymal viral infections
Infection of the substance of the nervous system will produce symptoms of focal dysfunction (deficits and/or seizures) with general signs of infection, dependingon the acuteness of the infection and the type of organism.
Viral encephalitis
A range of viruses can cause encephalitis but only a
minority of patients have a history of recent viral infection.
In Europe, the most serious cause of viral encephalitis
is herpes simplex, which probably reaches the brain via the olfactory nerves. Varicella zoster is also an important cause. The development of effective therapy for some forms of encephalitis has increased the importance of clinical diagnosis and virological examination of the CSF.
In some parts of the world, viruses transmitted by mosquitoes and ticks (arboviruses) are an important cause of encephalitis. The epidemiology of some of these infections is changing. Japanese encephalitis
has spread relentlessly across Asia to Australia, and there have been outbreaks of West Nile encephalitis in Romania, Israel and New York. HIV may cause encephalitis with a subacute or chronic presentation, but occasionally has an acute presentation with seroconversion.
Pathophysiology
The infection provokes an inflammatory response thatinvolves the cortex, white matter, basal ganglia and
brainstem. The distribution of lesions varies with the
type of virus. For example, in herpes simplex encephalitis,
the temporal lobes are usually primarily affected,
whereas cytomegalovirus can involve the areas adjacent
to the ventricles (ventriculitis). Inclusion bodies may be present in the neurons and glial cells and there is an infiltration of polymorphonuclear cells in the perivascular space. There is neuronal degeneration and diffuse glial proliferation, often associated with cerebral oedema.
Clinical features
Viral encephalitis presents with acute onset of headache,fever, focal neurological signs (aphasia and/or hemiplegia, visual field defects) and seizures. Disturbance
of consciousness ranging from drowsiness to deep
coma supervenes early and may advance dramatically.
Meningism occurs in many patients. Rabies presents a
distinct clinical picture and is described below.
Investigations
Imaging by CT scan may show low-density lesions inthe temporal lobes but MRI is more sensitive in detecting
early abnormalities. Lumbar puncture should be performed
once imaging has excluded a mass lesion. The CSF usually contains excess lymphocytes but polymorphonuclear
cells may predominate in the early stages. The CSF may be normal in up to 10% of cases. Some viruses, including the West Nile virus, may cause a sustained neutrophilic CSF. The protein content may be elevated but the glucose is normal. The EEG is usually abnormal in the early stages, especially in herpes simplex encephalitis, with characteristic periodic slow-wave activity in the temporal lobes. Virological investigations of the CSF, including PCR for viral DNA, may reveal the
causative organism but treatment initiation should not await this.
Management
Optimum treatment for herpes simplex encephalitis (acyclovir 10 mg/kg IV 3 times daily for 2–3 weeks) has
reduced mortality from 70% to around 10%. This should
be given early to all patients suspected of suffering from
viral encephalitis.
Some survivors will have residual epilepsy or cognitive
impairment.
Anticonvulsant treatment may be needed and raised intracranial pressure may indicate the need for dexamethasone.
Rabies
Rabies is caused by a rhabdovirus that infects the centralnervous tissue and salivary glands of a wide range of
mammals, and is usually conveyed by saliva through
bites or licks on abrasions or on intact mucous membranes.
Humans are most frequently infected from dogs
and bats. In Europe, the maintenance host is the fox. The
incubation period varies in humans from a minimum of
9 days to many months but is usually between 4 and
8 weeks. Severe bites, especially if on the head or neck,
are associated with shorter incubation periods. Human
rabies is a rare disease, even in endemic areas. However,
because it is usually fatal, major efforts are directed at
limiting its spread.
Clinical features
At the onset there may be fever, and paraesthesia at the
site of the bite. A prodromal period of 1–10 days, during which the patient becomes increasingly anxious, leads
to the characteristic ‘hydrophobia’. Although the patient
is thirsty, attempts at drinking provoke violent contractions
of the diaphragm and other inspiratory muscles.
Delusions and hallucinations may develop, accompanied
by spitting, biting and mania, with lucid intervals
in which the patient is markedly anxious. Cranial nerve
lesions develop and terminal hyperpyrexia is common.
Death ensues, usually within a week of the onset.
Investigations
During life, the diagnosis is usually made on clinicalgrounds but rapid immunofluorescent techniques can detect antigen in corneal impression smears or skin biopsies.
Management
Established disease
Only a few patients with established rabies have survived.
All received some post-exposure prophylaxis and needed intensive care facilities to control cardiac and respiratory failure. Otherwise, only palliative treatment is possible once symptoms have appeared. The patient should be heavily sedated with diazepam, supplemented by chlorpromazine if needed. Nutrition and fluids should be given intravenously or through a gastrostomy.
Pre-exposure prophylaxis
Pre-exposure prophylaxis is required by those whohandle potentially infected animals professionally,
those who work with rabies virus in laboratories and
those who live at special risk in rabies-endemic areas.
Protection is afforded by intradermal injections of
human diploid cell strain vaccine, or two IM injections given 4 weeks apart, followed by yearly boosters.
Post-exposure prophylaxis
The wounds should be thoroughly cleaned, preferably
with a quaternary ammonium detergent or soap;
damaged tissues should be excised and the wound left
unsutured. Rabies can usually be prevented if treatment
is started within a day or two of biting. Delayed treatment
may still be of value. For maximum protection,
hyperimmune serum and vaccine are required.
The safest antirabies antiserum is human rabies
immunoglobulin. The dose is 20 U/kg body weight; half
is infiltrated around the bite and half is given IM at a different site from the vaccine. Hyperimmune animal serum may be used but hypersensitivity reactions, including anaphylaxis, are common.
The safest vaccine, free of complications, is human
diploid cell strain vaccine; 1.0 mL is given intramuscularlyon days 0, 3, 7, 14, 30 and 90. In developing
countries, where human rabies globulin may not be
obtainable, 0.1 mL of vaccine may be given intradermally
into eight sites on day 1, with single boosters on
days 7 and 28. Where human products are not available
and when risk of rabies is slight (licks on the skin, or
minor bites of covered arms or legs), it may be justifiable
to delay starting treatment while observing the biting
animal or awaiting examination of its brain, rather than
use the older vaccine.
Poliomyelitis
Pathophysiology
Disease is caused by one of three polioviruses, which
constitutes a subgroup of the enteroviruses. Poliomyelitis
has become much less common in developed countries
following the widespread use of oral vaccines but
is still a problem in the developing world, especially
parts of Africa. Infection usually occurs through the
nasopharynx.
The virus causes a lymphocytic meningitis and infects
the grey matter of the spinal cord, brainstem and cortex.
There is a particular propensity to damage anterior horn
cells, especially in the lumbar segments.
Clinical features
The incubation period is 7–14 days. Many patientsrecover fully after the initial phase of a few days of mild
fever and headache. In other individuals, after a week
of well-being, there is a recurrence of pyrexia, headache
and meningism. Weakness may start later in one muscle
group and can progress to widespread paresis. Respiratory failure may supervene if intercostal muscles are paralysed or the medullary motor nuclei are involved.
Epidemics vary widely in terms of the incidence of nonparalytic cases and in mortality rate.
Death occurs from respiratory paralysis.
Muscle weakness is maximal at the end of the first week and gradual recovery may then take place over several months. Muscles showing no signs of recovery after a month will probably not regain useful function.
Second attacks are very rare but occasionally patients show late deterioration in muscle bulk and power many years after the initial infection
(this is termed the ‘post-polio syndrome’).
Poliomyelitis. Possible consequences of infection
InvestigationsThe CSF shows a lymphocytic pleocytosis, a rise in
protein and a normal sugar content. Poliomyelitis virus
may be cultured from CSF and stool.
Management
Established disease : early stages, bed rest is imperative because exercise appears to worsen the paralysis or precipitate it. At the onset of respiratory difficulties, a tracheostomy and ventilation are required. Subsequent treatment is by physiotherapy and orthopaedic measures.
Prophylaxis : Prevention of poliomyelitis is by immunisation with live (Sabin) vaccine. In developed countries where
polio is now very rare, the live vaccine has been replaced
by the killed vaccine in childhood immunization schedules.
Herpes zoster (shingles)
Herpes zoster is the result of reactivation of the varicellazoster virus that has lain dormant in a nerve root ganglion
following chickenpox earlier in life. Reactivation
may be spontaneous (as usually occurs in the middle-aged
or elderly) or due to immunosuppression (as in
patients with diabetes, malignant disease or AIDS).
Subacute sclerosing panencephalitis
This is a rare, chronic, progressive and eventually fatal complication of measles, presumably a result of an inability of the nervous system to eradicate the virus. It occurs in children and adolescents, usually many years after the primary virus infection. There is generalised neurological deterioration and onset is insidious, with intellectual deterioration, apathy and clumsiness, followed by myoclonic jerks, rigidity and dementia. The CSF may show a mild lymphocytic pleocytosis and the EEG demonstrates characteristic periodic bursts of triphasic waves.
Although there is persistent measlesspecific IgG in serum and CSF, antiviral therapy is ineffective and death ensues within a few years.
Progressive multifocal leucoencephalopathy
This was originally described as a rare complication of lymphoma, leukaemia or carcinomatosis, but has become more frequent as a feature of AIDS . It is an infection of oligodendrocytes by human polyomavirus JC, which causes widespread demyelination of the white matter of the cerebral hemispheres. Clinical signs include dementia, hemiparesis and aphasia, which progress rapidly, usually leading to death within weeks or months. Areas of low density in the white matter are seen on CT but MRI is more sensitive, showing diffuse high signal in the cerebral white matter on T2-weighted images. The only treatment available is to restore the immune response (by treating AIDS or any other cause of immunosuppression).Parenchymal bacterial infections
Cerebral abscessBacteria may enter the cerebral substance through penetrating injury, by direct spread from paranasal sinuses or the middle ear, or secondary to septicaemia. The site of abscess formation and the likely causative organism are both related to the source of infection .
Initial infection leads to local suppuration followed by
loculation of pus within a surrounding wall of gliosis,
which in a chronic abscess may form a tough capsule.
Haematogenous spread may lead to multiple abscesses.
Aetiology and treatment of bacterial cerebral abscess
Clinical featuresMay present acutely with fever, headache, meningism and drowsiness, but more commonly presents over days or weeks as a cerebral mass lesion. Seizures, raised
intracranial pressure (IP) and focal hemisphere signs occur
alone or in combination. Distinction from a cerebral
tumour may be impossible on clinical grounds.
Investigations
LP is potentially hazardous in the presence of raised IP and CT should always precede it. CT reveals single or multiple lowdensity areas, which show ring enhancement with contrast and surrounding cerebral oedema . There may be an elevated WBC and ESR. The possibility of cerebral toxoplasmosis or tuberculous disease
secondary to HIV infection should always be considered.
Right temporal cerebral abscess (arrows), with
surrounding oedema and midline shift to the left.A Unenhanced CT image.
B Contrast-enhanced CT image.
Management and prognosis
Antimicrobial therapy is indicated once the diagnosis ismade. The likely source of infection should guide the
choice of antibiotic . In neurosurgical patients, the addition of vancomycin should be considered.
Surgical drainage by burr-hole aspiration or excision
may be necessary, especially where the presence of
a capsule may lead to a persistent focus of infection. Epilepsy frequently develops and is often resistant to
treatment.
Despite advances in therapy, the mortality rate
remains at 10–20% and this may partly relate to delay in
diagnosis and initiation of treatment.
Subdural empyema
This is a rare complication of frontal sinusitis, osteomyelitis of the skull vault or middle ear disease. A collection of pus in the subdural space spreads over the surface of the hemisphere, causing underlying cortical oedema or thrombophlebitis. Patients present with severe pain in the face or head and pyrexia, often with a history of preceding paranasal sinus or ear infection.
The patient then becomes drowsy, with seizures and focal signs such as a progressive hemiparesis.
The diagnosis rests on a strong clinical suspicion in patients with a local focus of infection. Careful assessment with contrast-enhanced CT or MRI may show a subdural collection with underlying cerebral oedema.
Management requires aspiration of pus via a burr-hole and appropriate parenteral antibiotics. Any local source of infection must be treated to prevent re-infection.
Spinal epidural abscess
The characteristic clinical features are pain in a rootdistribution and progressive transverse spinal cord
syndrome with paraparesis, sensory impairment and
sphincter dysfunction. Features of the primary focus of
infection may be less obvious and thus can be overlooked.
The resurgence of resistant staphylococcal infection
and intravenous drug misuse has contributed to a
recent marked rise in incidence. X-ray changes occur late if present, so MRI or myelography should precede urgent neurosurgical intervention. Decompressive laminectomy with draining of the abscess relieves the pressure on the dura. Organisms may be grown from the pus or blood. Surgery, together ith appropriate antibiotics, may prevent complete and irreversible paraplegia.
Lyme disease
This can cause numerous neurological problems, includingpolyradiculopathy, meningitis, encephalitis and
mononeuritis multiplex .
Neurosyphilis
Neurosyphilis may present as an acute or chronic
process and may involve the meninges, blood vessels
and/or parenchyma of the brain and spinal cord. The
decade to 2008 saw a ten-fold increase in the incidence
of syphilis, mostly as a result of misguided relaxation
of safe sex measures with the advent of effective antiretroviral treatments for AIDS. Parallel increases in
neurosyphilis are inevitable.
The clinical manifestations are diverse and early diagnosis and treatment remain important.
Clinical features
The clinical and pathological features of the three most
common presentations are summarised in Box .
Neurological examination reveals signs indicative of
the anatomical localisation of lesions. Delusions of grandeur suggest general paresis of the insane, but more
commonly there is simply progressive dementia.
Small and irregular pupils that react to convergence but not light, as described by Argyll Robertson , may accompany any neurosyphilitic syndrome, but most commonly tabes dorsalis.
Clinical and pathological features of
neurosyphilisInvestigations
Routine screening for syphilis is warranted in manyneurological patients. Serological tests are positive in the serum in most patients, but CSF examination is essential if neurological involvement is suspected.
Active disease is suggested by an elevated cell count,
usually lymphocytic, and the protein content may be
elevated to 0.5–1.0 g/L with an increased gamma globulin
fraction.
Serological tests in the CSF are usually positive, but progressive disease can occur with negative CSF serology.
Management
The injection of procaine benzylpenicillin (procaine penicillin) and probenecid for 17 days is essential in the
treatment of neurosyphilis of all types . Further
courses of penicillin must be given if symptoms are not
relieved, if the condition continues to advance or if the
CSF continues to show signs of active disease. The cell
count returns to normal within 3 months of completion
of treatment, but the elevated protein takes longer to
subside and some serological tests may never revert to
normal. Evidence of clinical progression at any time is
an indication for renewed treatment.
Diseases caused by bacterial toxins
TetanusThis disease results from infection with Clostridium
tetani, a commensal in the gut of humans and domestic
animals that is found in soil. Infection enters the body
through wounds, which may be trivial. It is rare in the UK, occurring mostly in gardeners and farmers, but a
recent increase has been seen in intravenous drug misusers.
By contrast, the disease is common in many developing
countries, where dust contains spores derived
from animal and human excreta. Unhygienic practices
soon after birth may lead to infection of the umbilical
stump or site of circumcision, causing tetanus neonatorum.
Tetanus is still one of the major killers of adults,
children and neonates in developing countries, where
the mortality rate can be nearly 100% in the newborn
and around 40% in others.
In circumstances unfavourable to the growth of the
organism, spores are formed and these may remain
dormant for years in the soil. Spores germinate and
bacilli multiply only in the anaerobic conditions that
occur in areas of tissue necrosis or if the oxygen tension
is lowered by the presence of other organisms, particularly
if aerobic. The bacilli remain localised but produce
an exotoxin with an affinity for motor nerve endings and
motor nerve cells.
The anterior horn cells are affected after the exotoxin
has passed into the blood stream and their involvementresults in rigidity and convulsions. Symptoms first
appear from 2 days to several weeks after injury – the
shorter the incubation period, the more severe the attack
and the worse the prognosis.
Clinical features
By far the most important early symptom is trismus –
spasm of the masseter muscles, which causes difficulty
in opening the mouth and in masticating; hence the
name ‘lockjaw’. Lockjaw in tetanus is painless, unlike
the spasm of the masseters due to dental abscess, septic
throat or other causes. Conditions that can mimic tetanus
include hysteria and phenothiazine overdosage, or overdose in IV drug misusers. In tetanus, the tonic rigidity spreads to involve the muscles of the face, neck and trunk. Contraction of the frontalis and the muscles at the angles of the mouth leads to the so-called ‘risus sardonicus’. There is rigidity of the muscles at the neck and trunk of varying degree.
The back is usually slightly arched (‘opisthotonus’) and
there is a board-like abdominal wall.In the more severe cases, violent spasms lasting for a
few seconds to 3–4 minutes occur spontaneously, or may
be induced by stimuli such as movement or noise. These
convulsions are painful, exhausting and of very serious
significance, especially if they appear soon after the
onset of symptoms. They gradually increase in frequency
and severity for about 1 week and the patient may die from exhaustion, asphyxia or aspiration pneumonia. In less severe illness, convulsions may not commence
until a week or so after the first sign of rigidity,
and in very mild infections they may never appear.
Autonomic involvement may cause cardiovascular complications, such as hypertension.
Rarely, the only manifestation of the disease may be ‘local tetanus’ – stiffness or spasm of the muscles near the infected wound – and the prognosis is good if treatment is commenced at this stage.
Investigations
The diagnosis is made on clinical grounds. It is rarely
possible to isolate the infecting organism from the original
locus of entry.
Management
Established disease
Management of established disease should begin as
soon as possible, as shown in Box .
Treatment of tetanus
PreventionTetanus can be prevented by immunisation and prompt
treatment of contaminated wounds by débridement and
antibiotics. In patients with a contaminated wound, the
immediate danger of tetanus can be greatly reduced by
the injection of 1200 mg of penicillin followed by a 7-day
course of oral penicillin. For those allergic to penicillin,
erythromycin should be used. When the risk of tetanus
is judged to be present, an intramuscular injection of
250 U of human tetanus antitoxin should be given, along
with toxoid which should be repeated 1 month and
6 months later. For those already immunised, only a
booster dose of toxoid is required.
Botulism
Botulism is caused by the neurotoxins of Clostridium
botulinum, which are extremely potent and cause disease
after ingestion of even picogram amounts. Its classical
form is an acute onset of bilateral cranial neuropathies
associated with symmetric descending weakness.
Anaerobic conditions are necessary for the organism’s
growth. It may contaminate and thrive in many
foodstuffs, where sealing and preserving provide the
requisite conditions. Contaminated honey has been
implicated in infant botulism, in which the organism
colonises the gastrointestinal tract. Wound botulism is a
growing problem in injection drug-users.
The toxin causes predominantly bulbar and ocular
palsies (difficulty in swallowing, blurred or doublevision, ptosis), progressing to limb weakness and respiratory paralysis. Criteria for the clinical diagnosis are
shown in Box .
Management includes assisted ventilation and
general supportive measures until the toxin eventually
dissociates from nerve endings 6–8 weeks following
ingestion. A polyvalent antitoxin is available for postexposure prophylaxis and for the treatment of suspected botulism.
It specifically neutralises toxin types A, B and E and is not effective against infantile botulism (in which active growth of the organism allows continued toxin production).
The US Centers for Disease Control (CDC) definition of botulism
Transmissible spongiform encephalopathies (TSEs)Include a number of veterinary and medical conditions
that are characterised by the histopathological triad of
cortical spongiform change, neuronal cell loss and gliosis. Associated with these changes, there is deposition of amyloid made up of an altered form of a normally
occurring protein, the prion protein. The precise nature of the infective agent is not yet clear but almost certainly involves the cascade formation of an abnormal prion protein. TSEs may also occur spontaneously or as an inherited disorder. Diseases affecting animals include bovine and feline spongiform encephalopathies (BSE and FSE). These diseases achieved media prominence in the 1990s, when a form of Creutzfeldt– Jakob disease emerged that was associated with prion protein ingestion.
Creutzfeldt–Jakob disease
Creutzfeldt–Jakob disease (CJD) is the best-characterisedhuman TSE. Some 10% of cases arise due to a mutation
in the gene coding for the prion protein. The sporadic
form is the most common, occurring in middle-aged
to elderly patients. Clinical features usually involve
a rapidly progressive dementia, with myoclonus and
a characteristic EEG pattern (repetitive slow-wave
complexes), although a number of other features, such
as visual disturbance or ataxia, may also be seen. These
are particularly common in CJD transmitted by inoculation
(e.g. by infected dura mater grafts). Death occurs
after a mean of 4–6 months. There is no effective
treatment.
INTRACRANIAL MASS LESIONS AND RAISED INTRACRANIAL PRESSURE
Many different types of mass lesion may arise within the
intracranial cavity . In developing countries, tuberculoma and other infections are frequent causes, but in the West, intracranial haemorrhage and brain tumours are more common. The clinical features depend on the site of the mass, its nature and its rate of expansion.
Raised intracranial pressure
Raised intracranial pressure (RIP) may be caused by mass lesions, cerebral oedema, obstruction to CSF circulation causing hydrocephalus, impaired CSF absorption and cerebral venous obstruction .
• Intracranial haemorrhage (traumatic or spontaneous)
Extradural haematoma . Subdural haematomaIntracerebral haemorrhage
• Cerebral tumour (particularly posterior fossa lesions or high-grade gliomas)
• Infective
Cerebral abscess .Tuberculoma .Cysticercosis
Hydatid cyst
• Colloid cyst (in ventricles)
Common causes of raised intracranial pressure
Common causes of raisedintracranial pressure
'cont'd
Clinical features of intracranial
mass lesionsClinical features
In adults, intracranial pressure is less than 10–15 mmHg.The features of RIP are listed in Box above . The speed of
pressure increase influences presentation. If slow, compensatory mechanisms may occur, including alteration
in the volume of fluid in CSF spaces and venous sinuses,
which minimise symptoms. Rapid pressure increase (as
in aggressive tumours) does not permit these compensatory
mechanisms to occur, leading to early symptoms,
including sudden death. Papilloedema is not always
present, either because the pressure rise has been too
rapid or because of anatomical anomalies of the meningeal sheath of the optic nerve.
A false localising sign is one in which the pathology
is remote from the site of the expected lesion; in RIP, the6th cranial nerve (unilateral or bilateral) is most commonly
affected, but the 3rd, 5th and 7th nerves may also
be involved. Sixth nerve palsies are thought to be due
either to stretching of the long slender nerve or to
compression against the petrous temporal bone ridge. Trans-tentorial herniation of the uncus may compress
the ipsilateral 3rd nerve and usually involves the pupillary
fibres first, causing a dilated pupil; however, a false
localising contralateral 3rd nerve palsy may also occur,
perhaps due to extrinsic compression by the tentorial
margin. Vomiting, coma, bradycardia and arterial
hypertension are later features of RIP.
The rise in intracranial pressure from a mass lesion
may cause displacement of the brain. Downward displacement of the medial temporal lobe (uncus) through
the tentorium due to a large hemisphere mass may cause
‘temporal coning’ .
This may stretch the 3rd and/or 6th cranial nerves, or cause pressure on the contralateral cerebral peduncle (causing ipsilateral upper motor neuron signs), and is usually accompanied by progressive coma. Downward movement of the cerebellar tonsils through the foramen magnum may compress the medulla – ‘tonsillar coning’ . This may result in brainstem haemorrhage and/or acute obstruction of the CSF pathways. As coning progresses, coma and death occur unless rapidly treated.
Cerebral tumour displacing medial temporal lobe and
causing pressure on the mid-brain and 3rd cranial nerve.Tonsillar cone
Downward displacement of the cerebellartonsils below the level of the foramen magnum.
Management
Primary management of RIP should be targeted atrelieving the cause (e.g. surgical decompression of mass
lesion, steroids to reduce vasogenic oedema or shunt
procedure to relieve hydrocephalus). Supportive treatment
includes maintenance of fluid balance, blood pressure
control, head elevation, and use of diuretics such as
mannitol. Intensive care support may be needed .
Brain tumours
Primary brain tumours are a heterogeneous collection of
neoplasms arising from the brain tissue or meninges,
and vary from benign to highly malignant. Primary
malignant brain tumours are rare, accounting
for 1% of all adult tumours but a higher proportion
in children. The most common benign brain tumour is a
meningioma. Primary brain tumours do not metastasise
due to the absence of lymphatic drainage in the brain.
There are rare pathological subtypes, however, such as
medulloblastoma, which do have a propensity to metastasise; the reasons for this are not clear. Most cerebral
tumours are sporadic but may be associated with genetic
syndromes such as neurofibromatosis or tuberous sclerosis.
Brain tumours are not classified by the usual TNM
system but by the WHO grading I–IV; this is basedon histology (e.g. nuclear pleomorphism, presence of
mitoses and presence of necrosis), with grade I being the
most benign and grade IV the most malignant. Gliomas
account for 60% of brain tumours, with the aggressive
glioblastoma multiforme (WHO grade IV) being the
most common glioma, followed by meningiomas (20%)
and pituitary tumours (10%). Although the lower-grade
gliomas (I and II) may be very indolent, with prognosis
measured in terms of many years, these tumours may
transform to higher-grade disease at any time, with a
resultant sharp decline in life expectancy.
Most malignant brain tumours are due to metastases,
with intracranial metastases complicating about 20%
of extracranial malignancies. The rate is higher with primaries in the bronchus, breast and gastrointestinal
tract . Metastases usually occur in the white
matter of the cerebral or cerebellar hemispheres, but
there are diffuse leptomeningeal types.
Primary brain tumours
Contrast-enhanced CT head showing a large
metastasis within the left hemisphere (large arrow). There is surrounding cerebral oedema, and a smaller metastasis (small arrow) within the wall of the right lateral ventricle. The primary lesion was a lung carcinoma.Clinical features
The presentation is variable and usually influenced bythe rate of growth. High-grade disease (WHO grade III
and IV) tends to present with a short 4–6-week history of
mass effect (headache, nausea secondary to RIP), while
more indolent tumours can present with slowly progressive
focal neurological deficits, depending on their location
generalised or focal seizures are common. Headache, if present, is usually accompanied by focal deficits or seizures, and isolated stable headache is almost never due to intracranial tumour. The size of the primary tumour is of far less prognostic significance than its location within the brain. Tumours within the brainstem will result in early neurological deficits, while those in the frontal region may
be quite large before symptoms occur.
Investigations
Diagnosis is by neuroimaging and pathological grading following biopsy or resection where this is possible.
The more malignant tumours are more likely to demonstrate contrast enhancement on imaging.
Management
Brain tumours are treated with a combination of surgery,
radiotherapy and chemotherapy, depending on the type
of tumour and the patient. Advancing age is the most
powerful negative prognostic factor in CNS tumours, so
best supportive care (including steroid therapy) may be
most appropriate in older patients with metastases or high-grade disease. Treatment may not always be indicated in indolent (low-grade, WHO I or II) disease, and watchful waiting after surgery is often appropriate in such situations.
Dexamethasone given orally (or intravenously where
RIP is acutely or severely raised) may reduce thevasogenic oedema typically associated with metastases
and high-grade gliomas. Prolactin- or growth hormone-secreting pituitary adenomas may respond well to treatment with dopamine agonists (such as bromocriptine, cabergoline or quinagolide); in this situation, imaging and hormone levels may be all that is required to establish a formal diagnosis, precluding the need for surgery.
Surgical
The mainstay of primary treatment is surgery, either
resective (full or partial debulking) or biopsy, depending
on the site and likely radiological diagnosis.
MRI of an acoustic neuroma (arrows) in the posterior
fossa compressing the brainstem.A Axial image.
B Coronal image.
MRI showing a meningioma in the frontal lobe
(arrow A) with associated oedema (arrow B).Clearly, if a tumour occurs in an area of brain that is highly
important for normal function (e.g. motor strip), thenbiopsy may be the only safe surgical intervention but, in
general, maximal safe resection is the optimal surgical
management. Meningiomas and acoustic neuromas
offer the best prospects for complete removal
and thus cure. Some meningiomas can recur, however,
particularly those of the sphenoid ridge when partial
excision is often all that is possible. Thereafter, postoperative surveillance may be required, as radiotherapy is effective at preventing further growth of residual tumour. Pituitary adenomas may be removed by a transsphenoidal route, avoiding the need for a craniotomy.
Unfortunately, gliomas, which account for the majority
of brain tumours, cannot be completely excised, since
infiltration spreads well beyond the apparent radiological
boundaries of the intracranial mass. Recurrence
is therefore the rule, even if the mass of the tumour
is apparently removed completely; partial excision
(‘debulking’) may be useful in alleviating symptoms
caused by RIP, but although there is increasing evidence
that the degree of surgical excision may have a positive
influence on survival, this has not yet been demonstrated
in a randomised study.
Radiotherapy and chemotherapy
In the majority of primary CNS tumours, radiationand chemotherapy are used to control disease and
extend survival rather than for cure. Meningioma and
pituitary adenoma offer the best chance of life-long
remission. The gliomas are incurable; high-grade, WHO
IV disease still carries a median survival of just over a
year. In this situation, patient and family should always
be involved in decisions regarding treatment. The diagnosis, and often the symptoms, are devastating, and
support from palliative care and social work is crucial at
an early stage. In WHO grade III disease, prognosis is
a little better (2–4 years) and in rarer, more indolent
tumours, very prolonged survival is possible.
Advances have been made recently in terms of therapeutic
outcome. Standard care for WHO grade IV glioblastoma
multiforme is now combination radiotherapy
with oral temozolomide chemotherapy; although this
improves median survival of the population from only
12 to 14.5 months, up to 25% of patients survive for
more than 2 years (compared to approximately 10% with
radiotherapy alone). Ten percent will survive more than
5 years with temozolomide (virtually unheard of with
radiotherapy alone). Benefits are more likely in well-debulked patients who are younger and fitter. Implantation of chemotherapy gives a small survival benefit.
Understanding of the molecular biology of brain
tumours has allowed the use of biomarkers to guidetherapy and prognostic discussions. In patients with
methylation of the promoter region of the MGMT
(methyl guanine methyl transferase) gene (about 30% of
the population), 2-year survival is almost 50%. MGMT
reduces the cytotoxicity of temozolomide, and this
mutation also reduces the enzyme’s activity, rendering
the tumour more sensitive to chemotherapy. In grade II
and III gliomas, the presence of the loss of heterozygocity
(LOH) 1p19q chromosomal abnormality confers
chemosensitivity and thus improves prognosis. The
presence of a rare mutation in the IDH-1 (isocitrate
dehydrogenase) gene confers a very favourable prognosis
in patients with glioblastoma.
There is a small group of highly malignant grade IV
tumours that can be cured with aggressive therapy.
Patients with medulloblastomas have a good chance of
long-term survival with maximal surgery followed by
irradiation of the whole brain and spine; younger
patients may also benefit from concomitant and adjuvant
chemotherapy. Older patients do not tolerate this,
however.
Once tumours relapse, chemotherapy response rates
are low and survival is short in high-grade disease. In
the more uncommon low-grade tumours, repeated
courses of chemotherapy can result in much more prolonged survival.
In metastatic disease, radiotherapy offers a modest
improvement in survival but with costs in terms ofquality of life; treatment therefore needs careful discussion
with the patient. Benefits may be superior in breast
cancer but there is little to separate other pathologies.
Occasional chemosensitive cancers, such as small-cell
lung cancer, may benefit from systemic chemotherapy,
but intracerebral metastases represent a late stage of
disease and have a short prognosis.
Prognosis
The WHO histological grading system is a powerful predictor of prognosis in primary CNS tumours, though it
does not yet take account of individual biomarkers. For
each tumour type and grade, advancing age and deteriorating functional status are the next most important
negative prognostic features. The overall 5-year survival
rate of about 14% in adults masks a wide variation that
depends on tumour type.
Acoustic neuroma
This is a benign tumour of Schwann cells of the 8thcranial nerve, which may arise in isolation or as part of
neurofibromatosis type 2 (see below). When sporadic,
acoustic neuroma occurs after the third decade and is
more frequent in females. The tumour commonly arises
near the nerve’s entry point into the medulla or in the
internal auditory meatus, usually on the vestibular division.
Acoustic neuromas account for 80–90% of tumours
at the cerebellopontine angle(CPA).
Clinical features
Acoustic neuroma typically presents with unilateral progressive hearing loss, sometimes with tinnitus.Vertigo is an unusual symptom, as slow growth allows compensatory brainstem mechanisms to develop. In some
cases, progressive enlargement leads to distortion of
the brainstem and/or cerebellar peduncle, causing
ataxia and/or cerebellar signs in the limbs. Distortion
of the fourth ventricle and cerebral aqueduct may
cause hydrocephalus (see below), which may be the
presenting feature. Facial weakness is unusual at presentation but facial palsy may follow surgical removal of the tumour. The tumour may be identified incidentally
on cranial imaging.
Investigations
MRI is the investigation of choice .
Management
Surgery is the treatment of choice. If the tumour can be
completely removed, the prognosis is excellent, although deafness is a common complication of surgery.
Stereotactic radiosurgery (radiotherapy) may be appropriate for some lesions.
Neurofibromatosis
Encompasses two clinically and genetically separate conditions, with an autosomal dominant pattern of inheritance. The more common neurofibromatosis type 1 (NF1) is caused by mutations in the NF1 gene on chromosome 17, characterised by neurofibromas (benign peripheral nerve sheath tumours) and skin involvement , and may affect numerous systems. NF type 2 (NF2) is caused by mutations of the NF2 gene on chromosome 22, and is characterised by schwannomas (benign peripheral nerve sheath tumours comprising Schwann cells only), with little skin involvement; the clinical manifestations are more restricted to the eye and nervous system (see Box). Malignant change may occur in NF1 neurofibromas but is rare in NF2 schwannomas.Neurofibromatosis types 1 and 2:
clinical featuresA café au lait spot (arrow A) and subcutaneous
nodules (arrows B) on the forearm of a patient withneurofibromatosis type 1.
Von Hippel–Lindau disease
This rare autosomal dominant disease is caused by
mutations of the VHL tumour suppressor gene on chromosome 3. It promotes development of tumours affecting the kidney, adrenal gland, CNS, eye, inner ear,
epididymis and pancreas, which may undergo malignant
change. Benign haemangiomas and haemangioblastomas
affect about 80% of patients, mostly in the
cerebellum and retina.
Paraneoplastic neurological disease
Paraneoplastic neurological syndromes often presentbefore the underlying tumour declares itself and cause
considerable disability.
Hydrocephalus
Hydrocephalus is the excessive accumulation of CSF
within the brain, and may be caused either by increased
CSF production, by reduced absorption, or by
obstruction of the circulation .Symptoms range from none to sudden death, depending upon the speed at which and degree to which hydrocephalus develops. The terms ‘communicating’ and ‘non-communicating’ (also known
as obstructive) hydrocephalus refer to blockage either
outside or within the ventricular system respectively .
Normal pressure hydrocephalus
Normal pressure hydrocephalus (NPH) is a controversialentity, said to involve intermittent rises in CSF pressure,
particularly at night. It is described in old age as
being associated with a triad of gait apraxia, dementia
and urinary incontinence.
Management
Diversion of the CSF by means of a shunt placed between
the ventricular system and the peritoneal cavity or right
atrium may result in rapid relief of symptoms in obstructive
hydrocephalus. The outcome of shunting in NPH is
much less predictable and, until a good response can be
predicted, the management of individual cases will
remain uncertain.
The circulation of cerebrospinal fluid.
(1) CSF is synthesised in the choroid plexus of the ventricles, and flows from the lateral and third ventricles through the aqueduct to the fourth ventricle.(2) At the foramina of Luschka and Magendie it exits the brain, flowing over the hemispheres (3) and down around the spinal cord and roots in the subarachnoid space.
(4) It is then absorbed into the dural venous sinuses via the arachnoid villi.
Causes of hydrocephalus
MRI of hydrocephalus due to aqueduct stenosis.
A Axial T2-weighted image (CSF appears white): note the dilated lateral ventricles.B Sagittal T2-weighted image (CSF appears black): note the
dilated ventricles (top arrow) and narrowed aqueduct (bottom arrow).
Idiopathic intracranial hypertension
This usually occurs in obese young women. The annual incidence is about 3 per 100 000. RIP occurs in the absence of a structural lesion, hydrocephalus or other identifiable cause. The aetiology is uncertain but there may be a defect of CSF reabsorption by the arachnoid villi. It may be associated with a number of drugs, including tetracycline, and with vitamin A and its derivatives.
Clinical features
The usual presentation is with headache, sometimes
accompanied by diplopia and visual disturbance (most
commonly transient obscurations of vision associated
with changes in posture). Clinical examination reveals
papilloedema but little else.
False localising cranial nerve palsies (usually of the 6th nerve) may be present. It is important to record visual fields accurately for future monitoring.
Investigations
Brain imaging is required to exclude a structural or other
(e.g. cerebral venous sinus thrombosis) cause.
The ventricles are typically normal in size or small (‘slit’
ventricles). The diagnosis may be confirmed by lumbar
puncture, which shows raised CSF pressure (usually
> 30 cm CSF) but normal CSF constituents.
Management
Management can be difficult and there is no evidence tosupport any specific treatment. Weight loss in overweight
patients may be helpful but is difficult to achieve.
Acetazolamide or topiramate may help to lower intracranial pressure, the latter perhaps aiding weight loss in some patients. Repeated lumbar puncture is an effective
treatment for headache, but may be technically difficult
in obese patients and is often poorly tolerated. Patients
failing to respond, in whom chronic papilloedema
threatens vision, may require optic nerve sheath fenestration or a lumbo-peritoneal shunt.
Head injury
Diagnosis of head trauma is usually clear – either from
the history or from signs of external trauma to the head.
Brain injury is more likely with skull fracture but can
occur without. Individual cranial nerves may be damaged in fractures of the facial bones or skull base. Intracranial effects can be substantial and take several forms: extradural haematoma (collection of blood between the skull and dura); subdural haematoma (collection of blood between the dura and the surface of the brain); and intracerebral haematoma or diffuse axonal injury.
Whatever pathology occurs, the resultant RIP may lead to coning . Haematomas are identified by CT and management is by surgical
drainage, usually via a burr-hole. Penetrating skull fractures
lead to increased infection risk. Long-term sequelae include headache, cognitive decline and depression, all contributing to significant social, work, personality and family difficulties.
Subdural haematoma may occur spontaneously, particularly in patients on anticoagulants, in old age, and with alcohol misuse. There may or may not be a history of trauma. Patients present with subacute impairment of brain function, both globally (obtundation
and coma) and focally (hemiparesis, seizures). Headache may not be present.
The diagnosis should always be considered in those who present with reduced conscious level.
DISORDERS OF CEREBELLAR FUNCTION
Cerebellar dysfunction can manifest as incoordinationof limb function, gait ataxia , speech or eye
movements. Acute dysfunction may be caused by
alcohol or prescription drugs (especially the sodium
channel-blocking anti-epileptic drugs, phenytoin and
carbamazepine). Inflammatory changes in the cerebellum may cause symptoms in the aftermath of some infections (especially herpes zoster) or as a paraneoplastic phenomenon.
The hereditary spinocerebellar ataxias manifest as progressive ataxias in middle and old age, often with other neurological features that aid specific diagnosis.
DISORDERS OF THE SPINE AND SPINAL CORD
The spinal cord and spinal roots may be affected by
intrinsic disease or by disorders of the surrounding
meninges and bones. The clinical presentation of
these conditions depends on the anatomical level at
which the cord or roots are affected, as well as the
nature of the pathological process involved. It is important
to recognise when the spinal cord is at risk of compression
so that urgent action can be taken.
Cervical spondylosis
Cervical spondylosis is the result of osteoarthritisin the cervical spine. It is characterised by degeneration of the intervertebral discs and osteophyte formation. Such
‘wear and tear’ is extremely common and radiological
changes are frequently found in asymptomatic individuals
over the age of 50. Spondylosis may be associated
with neurological dysfunction. In order of frequency,
the C5/6, C6/7 and C4/5 vertebral levels affect C6, C7
and C5 roots, respectively .
MRI showing cervical cord compression (arrow) in
cervical spondylosis.Cervical radiculopathy
Acute onset of compression of a nerve root occurs whena disc prolapses laterally. More gradual onset may be
due to osteophytic encroachment of the intervertebral
foramina.
Clinical features
The patient complains of pain in the neck that may
radiate in the distribution of the affected nerve root. The
neck is held rigidly and neck movements may exacerbate
pain. Paraesthesia and sensory loss may be found
in the affected segment and there may be lower motor
neuron signs, including weakness, wasting and reflex
impairment
Physical signs in cervical root compression
Investigations
Where there is no trauma, imaging should not becarried out for isolated cervical pain. X-rays offer limited
benefit, except in excluding destructive lesions. MRI is
the investigation of choice in those with radicular symptoms.
Electrophysiological studies rarely add to clinical
examination and have become less important with the
emergence of MRI.
Management
Conservative treatment with analgesics and physiotherapy
results in resolution of symptoms in the great
majority of patients, but a few require surgery in the
form of discectomy or radicular decompression.
Cervical myelopathy
Dorsomedial herniation of a disc and the developmentof transverse bony bars or posterior osteophytes may
result in pressure on the spinal cord or the anterior spinal artery, which supplies the anterior two-thirds of the cord .
Clinical features
The onset is usually insidious and painless, but acute
deterioration may occur after trauma, especially hyperextension injury. Upper motor neuron signs develop
in the limbs, with spasticity of the legs usually appearing
before the arms are involved. Sensory loss in the
upper limbs is common, producing tingling, numbness
and proprioception loss in the hands, with progressive
clumsiness. Sensory manifestations in the legs are much
less common.
Neurological deficit usually progresses gradually and disturbance of micturition is a very late feature.
Investigations
MRI (or rarely myelography) will direct
surgical intervention. MRI also provides information on
the state of the spinal cord at the level of compression.
Management
Surgical procedures, including laminectomy and anterior
discectomy, may arrest progression of disability but
neurological improvement is not the rule. The decision
as to whether surgery should be undertaken may be
difficult. Manual manipulation of the cervical spine is of
no proven benefit and may precipitate acute neurological
deterioration.
Prognosis
The prognosis of cervical myelopathy is variable. Inmany patients, the condition stabilises or even improves
without intervention. If progression results in sphincter
dysfunction or pyramidal signs, surgical decompression
should be considered.
Lumbar spondylosis
This term covers degenerative disc disease and osteoarthritic change in the lumbar spine. Pain in the distribution of the lumbar or sacral roots (‘sciatica’) is almost always due to disc protrusion but can be a feature of other rare but important disorders, including spinal
tumour, malignant disease in the pelvis and tuberculosis
of the vertebral bodies.
Lumbar disc herniation
While acute lumbar disc herniation is often precipitated
by trauma (usually lifting heavy weights while the
spine is flexed), genetic factors may also be important.
The nucleus pulposus may bulge or rupture through the
annulus fibrosus, giving rise to pressure on nerveendings in the spinal ligaments, changes in the vertebral
joints or pressure on nerve roots.
Pathophysiology
The altered mechanics of the lumbar spine result in loss
of lumbar lordosis and there may be spasm of the paraspinal musculature. Root pressure is suggested by limitation of flexion of the hip on the affected side if the
straight leg is raised (Lasègue’s sign). If the third or
fourth lumbar root is involved, Lasègue’s sign may be
negative, but pain in the back may be induced by hyperextension of the hip (femoral nerve stretch test). The
roots most frequently affected are S1, L5 and L4.
Physical signs in lumbar root compression
Clinical featuresThe onset may be sudden or gradual. Alternatively,
repeated episodes of low back pain may precede sciatica
by months or years. Constant aching pain is felt in the
lumbar region and may radiate to the buttock, thigh, calf
and foot. Pain is exacerbated by coughing or straining
but may be relieved by lying flat.
Investigations
Plain X-rays of the lumbar spine are of little value in the
diagnosis of lumbar disc disease, although they may
show other conditions such as malignant infiltration of
a vertebral body. While CT can provide helpful images
of the disc protrusion and/or narrowing of the exit
foramina, MRI is the investigation of choice if available,
since soft tissues are well imaged.
Management
Some 90% of patients with sciatica recover following
conservative treatment with analgesia and early mobilisation; bed rest does not help recovery. The patient
should be instructed in back-strengthening exercises and advised to avoid physical manoeuvres likely to strain the lumbar spine. Injections of local anaesthetic or corticosteroids may be useful adjunctive treatment if symptoms are due to ligamentous injury or joint dysfunction. Surgery may have to be considered if there is no response to conservative treatment or if progressive neurological deficits develop. Central disc prolapse with bilateral symptoms and signs and disturbance of sphincter function requires urgent surgical decompression.
Lumbar canal stenosis
This occurs with a congenitally narrowed lumbar spinalcanal, exacerbated by the degenerative changes that
commonly occur with age.
Pathophysiology
The symptoms of spinal stenosis are thought to be due
to local vascular compromise secondary to the canal stenosis, rendering the nerve roots ischaemic and intolerant
of the increased demand that occurs on exercise.
Clinical features
Patients, who are usually elderly, develop exercise induced
weakness and paraesthesia in the legs
(‘spinal claudication’).
These symptoms progress with continued exertion, often to the point that the patient can no longer walk, but are quickly relieved by a short period of rest.
Physical examination at rest shows preservation
of peripheral pulses with absent ankle reflexes. Weakness
or sensory loss may only be apparent if the patient
is examined immediately after exercise.
Investigations
The investigation of first choice is MRI, but contraindications
(body habitus, metallic implants) may make CT or
myelography necessary.
Management
Lumbar laminectomy may provide relief of symptoms
and recovery of normal exercise tolerance.
Spinal cord compression
Spinal cord compression is one of the more common
neurological emergencies encountered in clinical practice
and the usual causes are listed in Box . A space-occupying lesion within the spinal canal may damage nerve tissue either directly by pressure or indirectly by interfering with blood supply.
Oedema from venous obstruction impairs neuronal function, and ischaemia from arterial obstruction may lead to necrosis of the spinal cord.
The early stages of damage are reversible but severely damaged neurons do not recover; hence the importance of early diagnosis and treatment.
Causes of spinal cord compression
Clinical featuresThe onset of symptoms of spinal cord compression
is usually slow (over weeks) but can be acute as a
result of trauma or metastases, especially if there is associated arterial occlusion.
Pain and sensory symptoms occur early, while weakness
and sphincter dysfunction are usually late manifestations.
The signs vary according to the level of the cord
compression and the structures involved. There may be tenderness to percussion over the spine if there is vertebral
disease and this may be associated with a local kyphosis.
Involvement of the roots at the level of the compression may cause dermatomal sensory impairment and corresponding lower motor signs.
Interruption of fibres in the spinal cord causes sensory loss
and upper motor neuron signs below the level of the lesion, and there is often disturbance of sphincter function.
The distribution of these signs varies with the level of the lesion .
The Brown–Séquard syndrome results if damage is confined to one side of the cord; the findings are explained by the anatomy of the sensory tracts. With compressive lesions, there is usually a band of pain at the level of the lesion in the distribution of the nerve roots subject to compression.
Symptoms of spinal cord compression
Signs of spinal cord compression
InvestigationsPatients with a history of acute or subacute spinal cord
syndrome should be investigated urgently, as listed in
Box. The investigation of choice is MRI , as it can define the extent of compression and associated soft-tissue abnormality. Plain X-rays may show bony destruction and soft-tissue abnormalities.
Routine investigations, including chest X-ray, may
provide evidence of systemic disease. If myelography is
performed, CSF should be taken for analysis; in cases of
complete spinal block, this shows a normal cell count
with a very elevated protein causing yellow discoloration
of the fluid (Froin’s syndrome).
The risk of acute deterioration after myelography in spinal cord compression means that the neurosurgeons should be alerted before it is undertaken. Where a secondary tumour is causing the compression, needle biopsy may be required to establish a tissue diagnosis.
Investigation of acute spinal cord syndrome
Axial MRI of thoracic spine. A neurofibroma (N) is compressing the spinal cord (SC) and emerging in a ‘dumbbell’ fashion through the vertebral foramen into the paraspinal space.
CT myelogram of cervical spine at the level of C2 showing bony erosion of vertebra by a metastasis (arrow).
Management
Treatment and prognosis depend on the nature ofthe underlying lesion. Benign tumours should be surgically
excised, and a good functional recovery can be
expected unless a marked neurological deficit has developed before diagnosis. Extradural compression due to malignancy is the most common cause of spinal cord
compression in developed countries and has a poor
prognosis. Useful function can be regained if treatment,
such as radiotherapy, is initiated within 24 hours of the
onset of severe weakness or sphincter dysfunction; management should involve close cooperation with both
oncologists and neurosurgeons.
Spinal cord compression due to tuberculosis is common in some areas of the world, and may require surgical treatment.
This should be followed by appropriate antituberculous chemotherapy for an extended period.
Traumatic lesions of the vertebral column require specialised neurosurgical treatment.
Intrinsic diseases of the spinal cord
There are many disorders that interfere with spinalcord function due to non-compressive involvement of
the spinal cord itself. A list of these disorders is given in
Box. The symptoms and signs are generally similar
to those that would occur with extrinsic compression
(see Boxes), although a suspended sensory loss (see Fig.) can only occur with intrinsic disease such as syringomyelia. Urinary symptoms usually occur earlier in the course of an intrinsic cord disorder than with compressive disorders.
Investigation of intrinsic disease starts with imaging
to exclude a compressive lesion. MRI provides mostinformation about structural lesions such as diastematomyelia, syringomyelia or intrinsic tumours.
Non-specific signal change may be seen in the spinal
cord in inflammatory or infective conditions and other disorders such as vitamin B12 deficiency.
Lumbar puncture or blood tests may be required
to make a specific diagnosis.
Intrinsic diseases of the spinal cord
Intrinsic diseases of the spinal cord 'cont'd
MRI scan showing syrinx
(arrows A), with herniationof cerebellar tonsils (arrow B).
DISEASES OF PERIPHERAL NERVES
Disorders of the peripheral nervous system are commonand may affect the motor, sensory or autonomic components, either in isolation or combination. The site of
pathology may be nerve root (radiculopathy), nerve
plexus (plexopathy) or nerve (neuropathy). Neuropathies
may present as mononeuropathy (single nerve
affected), multiple mononeuropathies (‘mononeuritis
multiplex’) or a symmetrical polyneuropathy .
Cranial nerves 3–12 share the same tissue characteristics
as peripheral nerves elsewhere and are subject
to the same range of diseases.
Causes of polyneuropathy
Pathophysiology
Damage may occur to the nerve cell body (axon) orthe myelin sheath (Schwann cell), leading to axonal
or demyelinating neuropathies. The distinction is
important, as only demyelinating neuropathies are
usually susceptible to treatment; making the distinction
requires neurophysiology (nerve conduction studies
and electromyography). Neuropathies can occur in association with many systemic diseases, toxins and drugs .
Common causes of axonal and demyelinating chronic polyneuropathies
Axonal• Diabetes mellitus • Alcohol • Uraemia • Cirrhosis • Amyloid • Myxoedema • Acromegaly • Paraneoplastic • Drugs and toxins • Deficiency states • Hereditary • Infection • Idiopathic
Demyelinating
• Chronic inflammatory demyelinating polyradiculoneuropathy
• Multifocal motor neuropathy
• Paraprotein-associated demyelinating neuropathy
• Charcot–Marie–Tooth disease type I and type X
Clinical features
Motor nerve involvement produces features of a lowermotor neuron lesion . Symptoms and signs of
sensory nerve involvement depend on the type of
sensory nerve involved; small-fibre neuropathies
are often painful. Autonomic involvement may
cause postural hypotension, disturbance of sweating,
cardiac rhythm, and gastrointestinal, bladder and sexual
functions; isolated autonomic neuropathies are rare, and
more commonly complicate other neuropathies.
Investigations
The investigations required reflect the wide spectrum of
causes . Neurophysiological tests are key in
discriminating between demyelinating and axonal neuropathies, and in identifying entrapment neuropathies.
Most neuropathies are of the chronic axonal type.
Investigation of peripheral neuropathy
Initial tests• Glucose (fasting)
• Erythrocyte sedimentation
rate, C-reactive protein
• Full blood count
• Urea and electrolytes
• Liver function tests
• Serum protein
electrophoresis
• Vitamin B12, folate
• ANA, ANCA
• Chest X-ray
• HIV testing
If initial tests are negative
• Nerve conduction studies
• Vitamins E and A
• Genetic testing • Lyme serology • Serum ACE
• Serum amyloid
ACE = angiotensin-converting enzyme; ANCA = antineutrophil cytoplasmic
antibody; ANA = antineutrophil antibody
Investigation of peripheral neuropathy 'cont'd
Entrapment neuropathy
Focal compression or entrapment is the usual cause of amononeuropathy. Symptoms and signs of entrapment
neuropathy are listed in Box. Entrapment neuropathies
may affect anyone, but diabetes, excess alcohol
or toxins, or genetic syndromes may be predisposing
causes. Unless axonal loss has occurred, entrapment
neuropathies will recover, provided the primary cause
is removed, either by avoiding the precipitation of activity
or by surgical decompression.
Symptoms and signs in common entrapment neuropathies
Multifocal neuropathyMultifocal neuropathy (mononeuritis multiplex) is characterised by lesions of multiple nerve roots, peripheral
nerves or cranial nerves . Vasculitis is a common cause, either as part of a systemic disease or isolated to the nerves, or it may arise on a background
of a polyneuropathy (e.g. diabetes). Multifocal motor
neuropathy (MMN) with conduction block is a rare
pure motor neuropathy, typically affecting the arms; it
is associated with anti-GM1 antibodies in about 50%,
and responds to intravenous immunoglobulin.
Causes of multifocal mononeuropathy
PolyneuropathyA polyneuropathy is typically associated with a ‘length-dependent’ pattern, occurring in the longest peripheral nerves first and affecting the distal lower limbs before the upper limbs. Sensory symptoms and signs develop in an ascending ‘glove and stocking’ distribution .
In inflammatory demyelinating neuropathies,
the pathology may be more patchy, affecting the
upper rather than lower limbs.
Guillain–Barré syndrome
Guillain–Barré syndrome (GBS) is a heterogeneous
group of immune-mediated conditions with an incidence
of 1–2/100 000/year. In Europe and North
America, the most common variant is an acute inflammatory
demyelinating polyneuropathy (AIDP). Axonal
variants, either motor (acute motor axonal neuropathy,
AMAN) or sensorimotor (acute motor and sensory
axonal neuropathy, AMSAN), are more common in
China and Japan, and account for 10% of GBS in Western
countries (often associated with Campylobacter jejuni).
The hallmark is an acute paralysis evolving over days or
weeks with loss of tendon reflexes. About two-thirds ofthose with AIDP have a prior history of infection, and
an autoimmune response triggered by the preceding
infection causes demyelination.
A number of GBS variants have been described, associated with specific anti-ganglioside antibodies; the best-recognised is Miller Fisher syndrome, which involves anti-GQ1b antibodies.
Clinical features
Distal paraesthesia and pain precede muscle weaknessthat ascends rapidly from lower to upper limbs, and
is more marked proximally than distally. Facial and
bulbar weakness commonly develops, and respiratory
weakness requiring ventilatory support occurs in 20% of
cases. Weakness progresses over a maximum of 4 weeks (usually less). Rapid deterioration to respiratory failure
can develop within hours. Examination shows diffuse
weakness with loss of reflexes. Miller Fisher syndrome
presents with internal and external ophthalmoplegia,
ataxia and areflexia.
Investigations
The CSF protein is raised, but may be normal in the first
10 days. There is usually no increase in CSF white cell
count (> 10 × 106 cells/L suggests an alternative diagnosis).
Electrophysiological changes may emerge after a
week or so, with conduction block and multifocal motor
slowing, sometimes most evident proximally as delayed
F waves . Antibodies to the ganglioside GM1
are found in about 25%, usually the motor axonal form.
Other causes of an acute neuromuscular paralysis
should be excluded (e.g. poliomyelitis, botulism, diphtheria,
spinal cord syndromes or myasthenia), via the
history and examination rather than investigations.
Management
Supportive measures to prevent pressure sores and deepvenous thrombosis are essential. Regular monitoring of
respiratory function (vital capacity) is needed in the
acute phase, as respiratory failure may develop with
little warning. Active treatment with plasma exchange
or intravenous immunoglobulin therapy shortens the
duration of ventilation and improves prognosis. Overall, 80% of patients recover completely within 3–6 months, 4% die and the remainder suffer residual neurological disability, which can be severe. Adverse prognostic features include older age, rapid deterioration to ventilation and evidence of axonal loss on EMG.
Intravenous immunoglobulin and plasma exchange in Guillain– Barré syndrome
Chronic polyneuropathyThe most common axonal and demyelinating causes of
polyneuropathy are shown in Box. A chronic
symmetrical axonal polyneuropathy, evolving over
months or years, is the most common form of chronic
neuropathy. Diabetes mellitus is the most common
cause but in about 25–50% no cause can be found.
Hereditary neuropathy
Charcot–Marie–Tooth disease (CMT) is an umbrellaterm for the inherited neuropathies. This group of syndromes has different clinical and genetic features. The
most common CMT is the autosomal dominantly inherited
CMT type 1, usually caused by duplication of the
PMP-22 gene on chromosome 17. Common signs are
distal wasting (‘inverted champagne bottle’ legs), often
with pes cavus, and predominantly motor involvement.
X-linked and recessively inherited forms of CMT, causing demyelinating or axonal neuropathies, also occur.
Chronic demyelinating polyneuropathy
The acquired include chronic inflammatory demyelinating peripheral neuropathy (CIDP), multifocal motor neuropathy (see above) and paraprotein-associated demyelinating neuropathy. CIDP typically presents with relapsing or
progressive motor and sensory changes, evolving over
more than 8 weeks. It is important to recognise, as it usually responds to corticosteroids, plasma exchange or IV
immunoglobulin. Some 10% of patients with acquired demyelinating polyneuropathy have an abnormal serum paraprotein, sometimes associated with a lymphoproliferative malignancy.
They may also demonstrate positive antibodies to
myelin-associated glycoprotein (anti-MAG antibodies).
Brachial plexopathy
Trauma usually damages either the upper or the lowerparts of the brachial plexus, according to the mechanics
of the injury. The clinical features depend on the anatomical site of the damage .
Lower parts of the brachial plexus are vulnerable to infiltration from breast or apical lung tumours (Pancoast tumour) or damage by therapeutic irradiation; they may also be compressed by a cervical rib or fibrous band between C7 and the first rib at the thoracic outlet. An acute brachial plexopathy of probable inflammatory origin may present with ‘neuralgic amyotrophy’. Severe shoulder pain precedes the appearance of a patchy upper brachial plexus lesion, with motor and/or sensory involvement.
There is no specific treatment and recovery is often incomplete; it may recur in about 25%, and there is a rare autosomal dominant hereditary form. The appearance of vesicles should indicate the alternative diagnosis of motor zoster.
Physical signs in brachial plexus lesions
Lumbosacral plexopathyLesions may be caused by neoplastic infiltration or compression by retroperitoneal haematomas.
A small-vessel vasculopathy can produce a unilateral
or bilateral lumbar plexopathy in association with
type 2 diabetes (‘diabetic amyotrophy’) or idiopathic form. This presents with painful wasting of the quadriceps with weakness of knee extension and absent knee reflex.
Spinal root lesions
Spinal root lesions (radiculopathy) are described above.
Clinical features include muscle weakness and wasting
and dermatomal sensory and reflex loss, which reflect
the pattern of the roots involved. Pain in the muscles
innervated by the affected roots may be prominent.
DISEASES OF THE NEUROMUSCULAR JUNCTION
Myasthenia gravis
This is the most common cause of acutely evolving, fatigable weakness and preferentially affects ocular, facial and bulbar muscles.
Pathophysiology
Myasthenia gravis is an autoimmune disease, most commonly caused by antibodies to acetylcholine receptors
in the post-junctional membrane of the neuromuscular
junction, which are found in around 80% of affected
patients. The resultant blockage of neuromuscular
transmission and complement-mediated inflammatory
response reduces the number of acetylcholine receptors
and damages the end plate .
Other antibodies can produce a similar clinical picture, most notably autoantibodies to muscle-specific kinase (MuSK), which is involved in the regulation and maintenance of acetylcholine receptors.
About 15% of patients (mainly those with late onset)
have a thymoma, most of the remainder displaying
thymic follicular hyperplasia. Myasthenic patients are at
greater risk of associated organ-specific autoimmune
diseases. As with other autoimmune processes, triggers
are not always evident, but some drugs (e.g. penicillamine)
can trigger an antibody-mediated myasthenic syndrome that may persist after drug withdrawal. Other
drugs, especially aminoglycosides and quinolones, may
exacerbate the neuromuscular blockade and should be
avoided in patients with myasthenia.
Myasthenia gravis and Lambert–Eaton myasthenic syndrome (LEMS)
Fig- Myasthenia gravis and Lambert–Eaton myasthenic syndrome (LEMS). In myasthenia there are antibodies to the acetylcholinereceptors on the post-synaptic membrane, which block conduction across the neuromuscular junction (NMJ). Myasthenic symptoms can be transiently improved by inhibition of acetylcholinesterase (e.g. with TensilonR – edrophonium bromide), which normally removes the acetylcholine. A cell-mediated immune response produces simplification of the post-synaptic membrane, further impairing the ‘safety factor’ of neuromuscular conduction. In LEMS, antibodies to the pre-synaptic voltage calcium channels impair release of acetylcholine from the motor nerve ending; calcium is required for the acetylcholine-containing vesicle to fuse with the pre-synaptic membrane for release into the NMJ.
Clinical features
Myasthenia gravis usually presents between the ages of15 and 50 years and there is a female preponderance in
younger patients. In older patients, males are more commonly affected. It tends to run a relapsing and remitting course.
The most evident symptom is fatigable muscle weakness;
movement is initially strong but rapidly weakens
as muscle use continues. Worsening of symptoms
towards the end of the day or following exercise is characteristic.
There are no sensory signs or signs of involvement
of the CNS, although weakness of the oculomotor
muscles may mimic a central eye movement disorder.
The first symptoms are usually intermittent ptosis or
diplopia, but weakness of chewing, swallowing, speakingor limb movement also occurs. Any limb muscle
may be affected, most commonly those of the shoulder
girdle; the patient is unable to undertake tasks above
shoulder level, such as combing the hair, without frequent
rests. Respiratory muscles may be involved and
respiratory failure is an avoidable cause of death. Aspiration may occur if the cough is ineffectual.
Ventilatory support is required where weakness is severe or of abrupt onset.
Investigations
Intravenous injection of the short-acting anticholinesterase,
edrophonium bromide (the Tensilon® test), is
less widely used than before. Improvement in muscle
function occurs within 30 seconds and usually persists
for 2–3 minutes, but the test is not universally specific
or sensitive. Cover with intravenous atropine is necessary
to avoid bradycardia. Planning the assessment
beforehand (e.g. speech or limb movements) allows
some objectivity in gauging the effect.
Repetitive stimulation during nerve conduction
studies may show a characteristic decremental response
if the muscle has been clinically affected.
Management
The goals of treatment are to maximise the activity ofacetylcholine at remaining receptors in the neuromuscular
junctions and to limit or abolish the immunological
attack on motor end plates.
The duration of action of acetylcholine is prolonged
by inhibiting acetylcholinesterase. The most commonly
used anticholinesterase drug is pyridostigmine. Muscarinic
side-effects, including diarrhoea and colic, may
be controlled by propantheline. Overdosage of anticholinesterase drugs may cause a ‘cholinergic crisis’
due to depolarisation block of motor end plates, with
muscle fasciculation, paralysis, pallor, sweating, excessive
salivation and small pupils.
This may be distinguished from severe weakness due to exacerbation of myasthenia (‘myasthenic crisis’) by the clinical features and, if necessary, by the injection of a small dose of edrophonium.
The immunological treatment of myasthenia is outlined
in Box. Thymectomy may improve overall prognosis but awaits clinical trial confirmation.
Prognosis is variable and remissions may occur spontaneously.When myasthenia is entirely ocular, prognosis is excellent and disability slight. Young female patients with generalised disease may benefit from thymectomy, whilst older patients are less likely to have a remission despite treatment. Rapid progression of the disease more than 5 years after onset is uncommon.
Immunological treatment of myasthenia
Other myasthenic syndromesOther rarer conditions can present with muscle weakness
due to impaired transmission across the neuromuscular
junction. The most common of these is the
Lambert–Eaton myasthenic syndrome (LEMS), which
can occur as an inflammatory or paraneoplastic phenomenon.
Antibodies to pre-synaptic voltage-gated calcium channels impair transmitter release.
Patients may have autonomic dysfunction (e.g. dry mouth) in addition to muscle weakness, but the cardinal clinical sign is absence of tendon reflexes, which return after sustained contraction of the relevant muscle.
The condition is associated with underlying malignancy in a high percentage of cases and investigation
must be directed towards identifying any neoplasm.
The condition is diagnosed electrophysiologically by the
presence of post-tetanic potentiation of motor response
to nerve stimulation at a frequency of 20–50/s. Treatment
is with 3,4-diaminopyridine, or pyridostigmine
and immunosuppression.
DISEASES OF MUSCLE
Muscle disease, either hereditary or acquired, is rare.
Most typically, it presents with a proximal symmetrical
weakness. Diagnosis is dependent on recognition of
clinical clues, such as cardiorespiratory involvement,
evolution, family history, exposure to drugs, the presence
of contractures, myotonia and other systemic features,
and on investigation findings, most importantly
EMG and muscle biopsy. Hereditary syndromes include
the muscular dystrophies, muscle channelopathies, metabolic
myopathies (including mitochondrial diseases)
and congenital myopathies.
Muscular dystrophies
These are inherited disorders with progressive muscledestruction, and may be associated with cardiac and/or
respiratory involvement and sometimes non-myopathic
features . Myotonic dystrophy is the most
common, with a prevalence of about 12/100 000.
The muscular dystrophies
Clinical featuresThe pattern of the clinical features is defined by the
specific syndromes. Onset is often in childhood, although
some patients, especially those with myotonic dystrophy,
may present as adults. Wasting and weakness are
usually symmetrical, without fasciculation or sensory
loss, and tendon reflexes are usually preserved until a
late stage. Weakness is usually proximal, except in myotonic dystrophy type 1, when it is distal .
Investigations
The diagnosis can be confirmed by specific moleculargenetic testing, supplemented with EMG and muscle
biopsy if necessary. Creatine kinase is markedly elevated
in the dystrophinopathies (Duchenne and Becker)
but is normal or moderately elevated in the other dystrophies.
Screening for an associated cardiac abnormality
(cardiomyopathy or dysrhythmia) is important.
Management
There is no specific therapy for most of these conditions
but physiotherapy and occupational therapy help
patients cope with their disability. Steroids are used
in Duchenne muscular dystrophy. Treatment of associated
cardiac failure or arrhythmia (with pacemaker
insertion if necessary) may be required; similarly, management of respiratory complications (including nocturnal hypoventilation) can improve quality of life.
Improvements in non-invasive ventilation have led to
significant improvements in survival for patients with Duchenne muscular dystrophy.
Genetic counselling is important.
Inherited metabolic myopathies
There are a large number of rare inherited disorders thatinterfere with the biochemical pathways that maintain
the energy supply (adenosine triphosphate, ATP) to
muscles. These are mostly recessively inherited deficiencies
in the enzymes necessary for glycogen or fatty acid
(β-oxidation) metabolism .
They typically present with muscle weakness and pain.
Inherited disorders of muscle metabolism
Mitochondrial syndromes
Muscle channelopathies
Acquired myopathiesThese include the inflammatory myopathies, or myopathy
associated with a range of metabolic and endocrine
disorders, or drug and toxin exposure .