Hussien Mohammed Jumaah
CABMLecturer in internal medicine
Mosul College of Medicine
2016
learning-topics
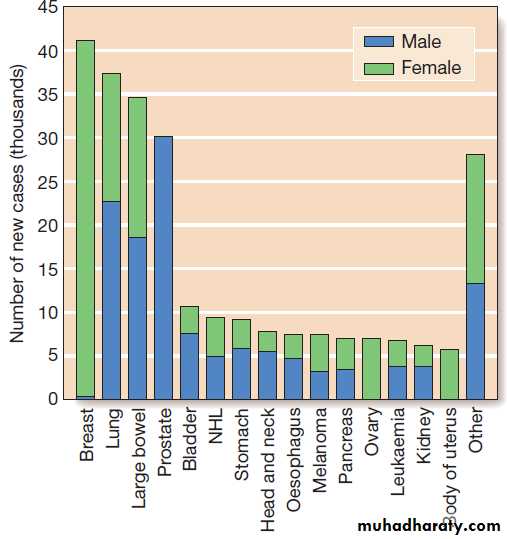
OncologyCancer is a significant global health-care problem, with
an estimated worldwide incidence of 10 million newcases per year, 46% of which are in developed countries.
Mortality is high, with > 7 million deaths per year.
The global costs and socioeconomic impact
are considerable. The most common solid organ malignancies arise in the lung, breast and gastrointestinal
tract , but the most common form worldwide
is skin cancer.
Tobacco is a major factor in the aetiology of 30% of cancers, including lung, nasopharynx, bladder and kidney, and these could be prevented by smoking cessation.
Diet and alcohol contribute to a further 30% of cancers, including those of the stomach, colon, oesophagus, breast
and liver. Lifestyle modification could reduce these
if steps were taken to avoid animal fat and red meat,
reduce alcohol, increase fibre, fresh fruit and vegetable
intake, and avoid obesity.
Infections account for a further 15% of cancers, including those of the cervix, stomach, liver, nasopharynx and bladder, and some of these could be prevented by infection control and vaccination.
The most commonly diagnosed cancers in the UK.
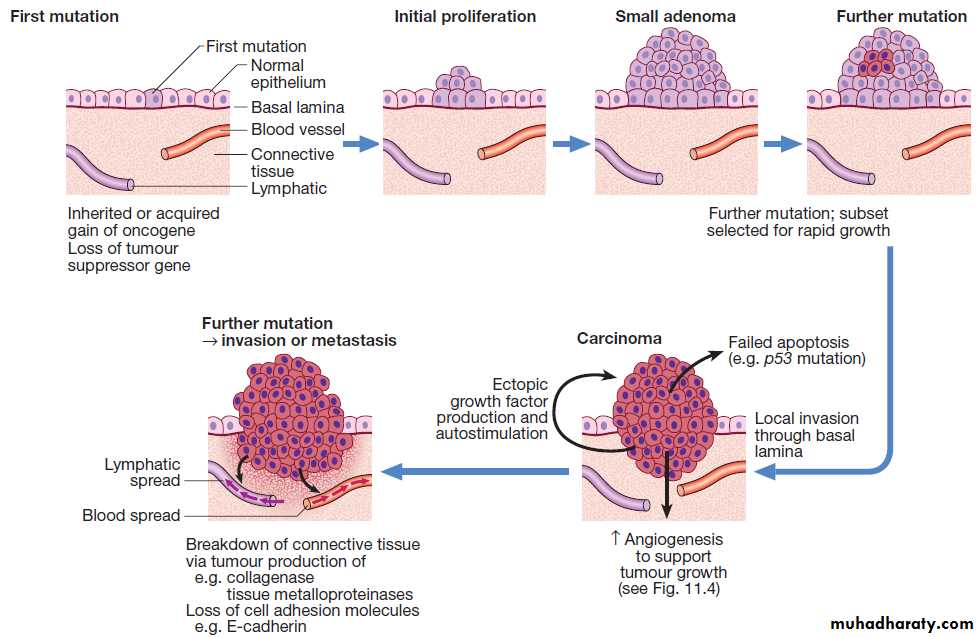
THE TEN HALLMARKS OF CANCERThe formation and growth of cancer constitute a multistep
process, during which sequentially occurring gene
mutations result in the formation of a cancerous cell. For
cells to initiate carcinogenesis, they require key characteristics, referred to as the hallmarks of cancer.
1. Genome instability and mutation
Random genetic mutations occur continuously throughout
all cells of the body and very rarely confer a selective advantage on single cells, allowing overgrowth and
dominance in local tissue environments. Under normal circumstances, cellular DNA repair so effective that almost all spontaneous mutations are corrected without producing phenotypic changes, keeping mutation rates very low.
In cancer cells, the accumulation of mutations can be
accelerated by compromising the surveillance systemsthat normally monitor genomic integrity and force
genetically damaged cells into either senescence or apoptosis. Therefore, they can become more sensitive to mutagenic actions or develop DNA repair mechanism failure.
2. Resisting cell death
There are three principal mechanisms through which
cell death occurs in healthy tissues:
• Apoptosis is programmed cell death and is frequently found at markedly reduced rates in cancers, particularly those of high grade or those resistant to treatment.
The cellular apoptotic system sense intrinsic and extrinsic pro-apoptotic signals and initiate a cascade of proteolysis, nuclear fragmentation, chromosomal condensation,and shrinking of the cell with loss of intercellular contact, cellular fragmentation and the formation of apoptotic bodies that are phagocytosed by neighbouring cells. The most important regulator of apoptosis is the TP53 tumour suppressor gene, often described as the ‘guardian of the genome’, as it is able to induce apoptosis in response to sufficient levels of genomic damage. The largest initiator of apoptosis via TP53 is cellular injury,
particularly due to DNA damage from chemotherapy, oxidative damage and ultraviolet (UV) radiation.
• Autophagy is a catabolic process during which cellular constituents are degraded by lysosomal machinery within the cell. It is an important physiological mechanism, which usually occurs at low levels in cells but can be induced in response to environmental stresses, particularly radiotherapy and cytotoxic chemotherapy, which induce elevated levels of autophagy that are cytoprotective for
malignant cells, thus impeding rather than perpetuating the killing actions of these stress situations. Severely stressed cancer cells have been shown to shrink via autophagy to a state of reversible dormancy.
• Necrosis is the premature death of cells and is
characterised by the release of cellular contents intothe local tissue microenvironment, in marked contrast to apoptosis, where cells are disassembled in a step-by-step fashion and the resulting cellular fragments phagocytosed. Necrotic cell death results in the recruitment of inflammatory immune cells, promotion of angiogenesis, cellular proliferation and tissue invasion.
Necrotic cells also release stimulatory factors, which promote proliferation of neighbouring cells and can promote rather than inhibit carcinogenesis.
3. Sustaining proliferative signalling
Cancer cells can sustain proliferation beyond whatwould be expected for normal cells; this is typically due
to growth factors, which are able to bind to cell surface -bound receptors that activate an intracellular tyrosine kinase-mediated signalling cascade, leading to changes in gene expression and promoting cellular proliferation.
The cell cycle
The cell cycle is comprised of four ordered, strictly regulated phases referred to as G1 (gap 1), S (DNA synthesis), G2 (gap 2) and M (mitosis) (Fig.). Normal cells grown in culture will stop proliferating and enter a
quiescent state called G0 once they become confluent
or are deprived of serum or growth factors.
Mitogenic signals promote progression through G1 to S phase, utilising phosphorylation of the retinoblastoma gene product (pRb). Following DNA synthesis, there is a second gap phase (G2) prior to mitosis (M), allowing cells to repair errors that have occurred during DNA replication and thus preventing propagation of these errors to daughter cells.
Although the duration of individual phases may vary,
depending on cell and tissue type, most adult cells are
in a G0 state at any one time.
Cell cycle regulation
The cell cycle is orchestrated by a number of molecular
mechanisms, most importantly by cyclins and cyclin-dependent kinases (CDKs). Cyclins bind to CDKs, and
are regulated by both activating and inactivating phosphorylation, with two main checkpoints at G1/S and
G2/M transition. The genes that inhibit progression play
an important part in tumour prevention and are referred
to as tumour suppressor genes (e.g. TP53, TP21, TP16 genes). The products of these genes deactivate the cyclin–CDK complexes and halt the cell cycle. The complexity of cell cycle control is susceptible to dysregulation, which may produce a malignant phenotype.
The cell cycle and sites of action of chemotherapeutic agents.
Stimulation of the cell cycleMany cancer cells produce growth factors, which drive
their own proliferation by a positive feedback known as
autocrine stimulation. Examples include transforming
growth factor-alpha (TGF-α) and platelet-derived
growth factor (PDGF). Other cancer cells express growth
factor receptors at increased levels due to gene amplification or express abnormal receptors that are permanently activated. This results in abnormal cell growth in response to physiological growth factor stimulation or
even in the absence of growth factor stimulation (ligandindependent signalling).
The epidermal growth factor receptor (EGFR) is often over-expressed in lung and gastrointestinal tumours and the HER2/neu receptor is frequently over-expressed in breast cancer. Both receptors activate the Ras–Raf–MAP kinase pathway, causing cell proliferation.
4. Evading growth suppressors
In healthy tissues, cell-to-cell contact in dense cell populations acts as an inhibitory factor on proliferation.
This contact inhibition is typically absent in many cancer cell populations. Growth-inhibitory factors can modulate the cell cycle regulators and produce activation of the CDK inhibitors, causing inhibition of the CDKs. Mutations within inhibitory proteins are common in cancer.
5. Enabling replicative immortality
For cancer cells to evolve into macroscopic tumours, they need to acquire the ability for unlimited proliferation.Telomeric DNA sequences, which protect and stabilise
chromosomal ends, play a central role in conferring this limitless replicative potential. During replication of normal cells, telomeres shorten progressively as small fragments of telomeric DNA are lost with successive cycles of replication. This shortening process is thought to represent a mitotic clock and eventually prevents the cell from dividing further. Telomerase, a specialized polymerase enzyme, adds nucleotides to telomeres, allowing continued cell division and thus preventing premature arrest of cellular replication.
The telomerase enzyme is almost absent in normal cells but is expressed at significant levels in many human cancers.
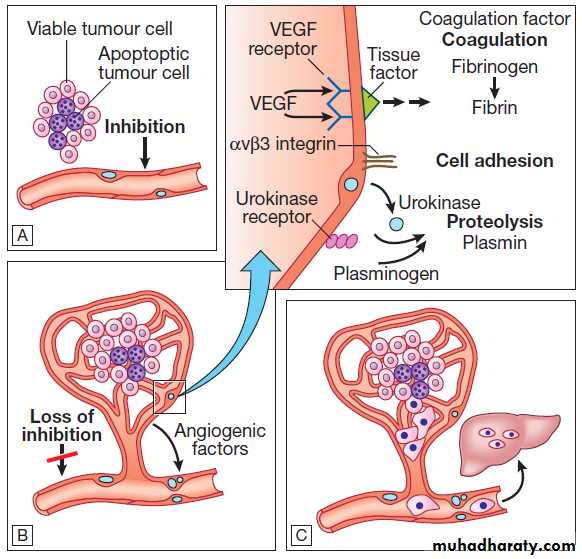
6. Inducing angiogenesis
All cancers require a functional vascular network toensure continued growth and will be unable to grow
beyond 1 mm3 without stimulating the development of
a vascular supply. Tumours require sustenance in the
form of nutrients and oxygen, as well as an ability to
evacuate metabolic waste products and carbon dioxide.
This entails the development of new blood vessels,
which is termed angiogenesis (Figs).
Angiogenesis is dependent on the production of
Angiogenic growth factors, of which vascular endothelial
growth factor (VEGF) and platelet-derived endothelial
growth factor (PDGF) are the best characterised.
During tumour progression, an angiogenic switch is
activated and remains on, causing normally quiescent
vasculature to sprout new vessels continually that help
sustain expanding tumour growth.
A number of cells can contribute to the maintenance of a functional tumour vasculature and therefore sustain
angiogenesis. These include pericytes and a variety of
bone marrow-derived cells such as macrophages, neutrophils, mast cells and myeloid progenitors.
Oncogenesis. The multistep origin of cancer, showing events implicated in cancer initiation, progression, invasion and metastasis.
Angiogenesis, invasion and metastasis.
A For any cancer to grow beyond 1 mm3 it must evoke a blood supply.B New vessel formation results from the release of angiogenic
factors by the tumour cells and loss of inhibition of the
endothelial cells.
C The loss of cellular adhesion and disruption of the extracellular matrix allow cells to extravasate into the
blood stream and metastasise to distant sites.
7. Activating invasion and metastasis
Invasion and metastasis are complex processes involving
multiple discrete steps; it begins with local tissue
invasion, followed by infiltration of nearby blood and
lymphatic vessels by cancer cells. Malignant cells are
eventually transported through haematogenous and
lymphatic spread to distant sites within the body, where
they form micrometastases that will eventually grow
into macroscopic metastatic lesions (see Fig.).
Cadherin-1 (CDH1) is a calcium-dependent cell–cell
adhesion glycoprotein that facilitates assembly of organised cell sheets in tissues, and increased expression is
recognised as an antagonist of invasion and metastasis.
In situ tumours usually retain cadherin-1 production,
whereas loss of cadherin-1 production due to downregulation or occasional mutational inactivation ofCDH1 has been observed in human cancers, supporting
the theory that CDH1 plays a key role in suppression of
invasion and metastasis.
Mesenchymal stem cells in tumour stroma have been found to secrete CCL5, a protein chemokine that helps recruit leucocytes into inflammatory sites.
8. Reprogramming energy metabolism
Under aerobic conditions, oxidative phosphorylationfunctions as the main metabolic pathway for energy
production; cells process glucose, first to pyruvate via glycolysis and thereafter to carbon dioxide in the
mitochondria. Whilst under anaerobic conditions, glycolysis
is favoured to produce adenosine triphosphate
(ATP). Cancer cells can reprogramme their glucose
metabolism to limit energy production to glycolysis,
even in the presence of oxygen. This has been termed
‘aerobic glycolysis’. Up-regulation of glucose transporters,
such as GLUT1, is the main mechanism through
which aerobic glycolysis is achieved.
One explanation may be that the increased production of glycolytic intermediates can be fed into various biosynthetic pathways, including those that generate the nucleosides and amino acids, necessary for the production of new cells.
9. Tumour-promoting inflammation
Almost all tumours show infiltration with immune
cells on pathological investigation and historically this
finding was thought to represent an attempt of the
immune system to eradicate the cancer. It is now clear that tumour-associated inflammatory responses promote tumour formation and cancer progression.
Other bioactive molecules, such as growth factors and
pro-angiogenic factors, may be released by inflammatoryimmune cells into the surrounding tumour microenvironment.
In particular, the release of reactive oxygen species, which are actively mutagenic, will accelerate the genetic evolution of surrounding cancer cells, enhancing
growth and contributing to cancer progression.
10. Evading immune destruction
The immune system operates as a significant barrier totumour formation and progression, and the ability to
escape from immunity is a hallmark of cancer development. Cancer cells continuously shed surface antigens into the circulatory system, prompting an immune response that includes cytotoxic T cell, natural killer cell and macrophage production. The immune system is thought to provide continuous surveillance, with resultant elimination of cells that undergo malignant transformation. However, deficiencies in the development or function of CD8+ cytotoxic T lymphocytes, CD4+ Th1 helper T cells, or natural killer cells can each lead to a demonstrable increase in cancer incidence. Also, highly immunogenic
cancer cells may evade immune destruction by
disabling components of the immune system. This is
done through recruitment of inflammatory cells, includingregulatory T cells and myeloid-derived suppressor
cells, both actively immunosuppressive against the
actions of cytotoxic lymphocytes (see Fig).
Cancers develop and progress when there is loss of
recognition by the immune system, lack of susceptibility
due to escape from immune cell action and induction of
immune dysfunction, often via inflammatory mediators.
ENVIRONMENTAL AND GENETIC DETERMINANTS OF CANCER
The majority of cancers do not have a single cause but
rather are the result of a complex interaction between
genetic factors and exposure to environmental carcinogens.
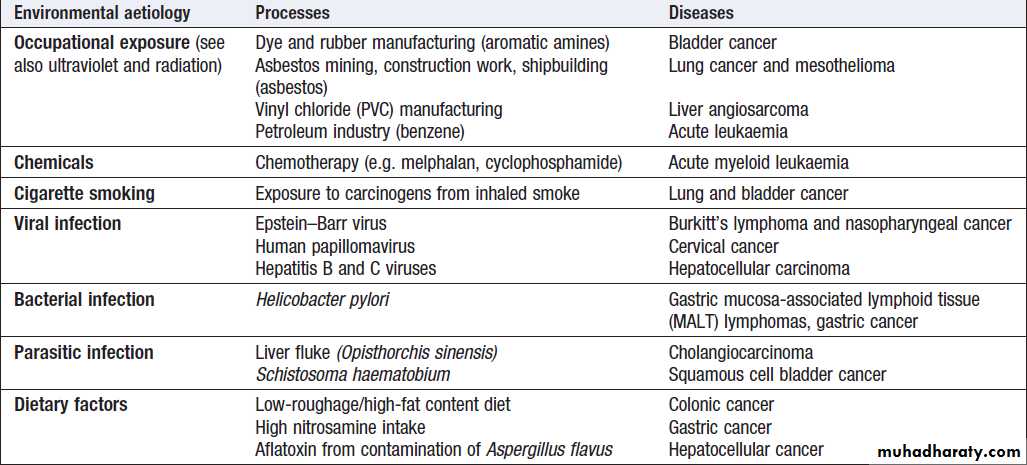
Environmental factors
Environmental triggers for cancer have mainly been
identified through epidemiological studies that examine
patterns of distribution of cancers in patients in whom
age, sex, presence of other illnesses, social class, geography and so on differ.
Sometimes, these give strong pointers to the molecular or cellular causes of the disease, such as the association between aflatoxin production within contaminated food supplies and hepatocellular carcinomas.
However, for many solid cancers, such as breast and colorectal, there is evidence of a multifactorial
pathogenesis, even when there is a principal environmental
cause (Box).
Smoking is now established beyond all doubt as
a major cause of lung cancer, but there are obviously
additional predisposing factors since not all smokers
develop cancer. Similarly, most carcinomas of the
cervix are related to infection with human papillomavirus
(HPV subtypes 16 and 18). For carcinomas of the
bowel and breast, there is strong evidence of an environmental component.
For example, the risk of breast cancer in women of Far Eastern origin remains relatively low when they first migrate to a country with a Western lifestyle, but rises in subsequent generations to approach that of the resident population of the host country.
The precise environmental factor that causes this change is
unclear, but may include diet (higher intake of saturated fat and/or dairy products), reproductive patterns (later
onset of first pregnancy) and lifestyle (increased use of
artificial light and shift in diurnal rhythm).
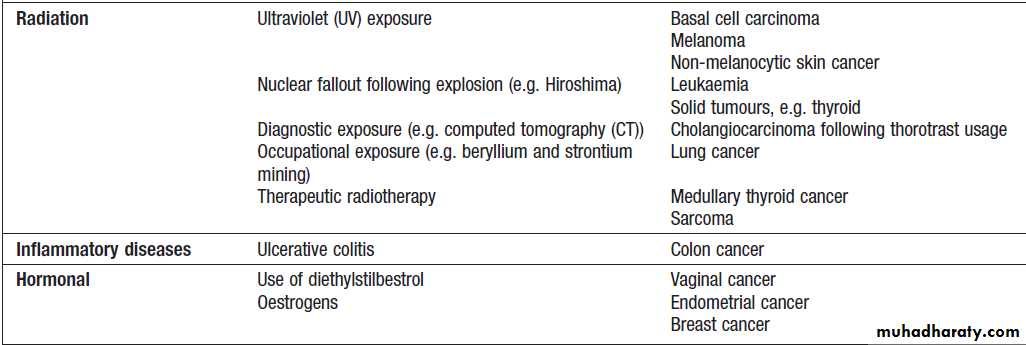
Environmental factors that predispose to cancer
Environmental factors that predispose to cancer – cont’dGenetic factors
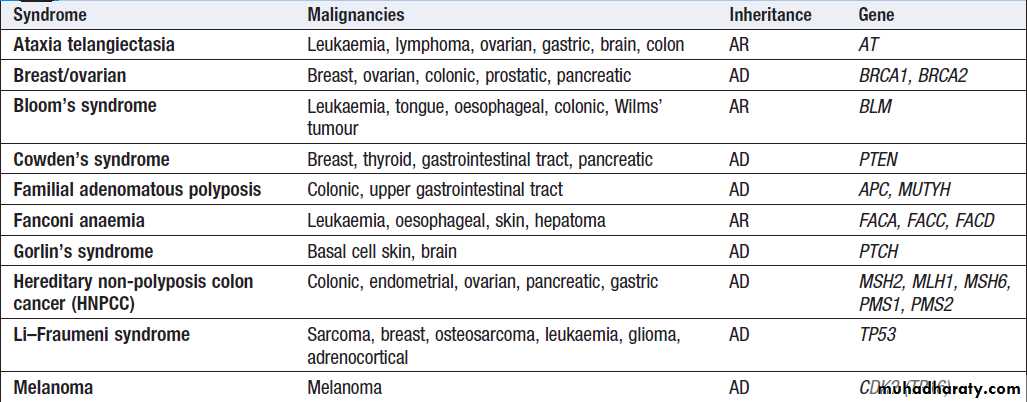
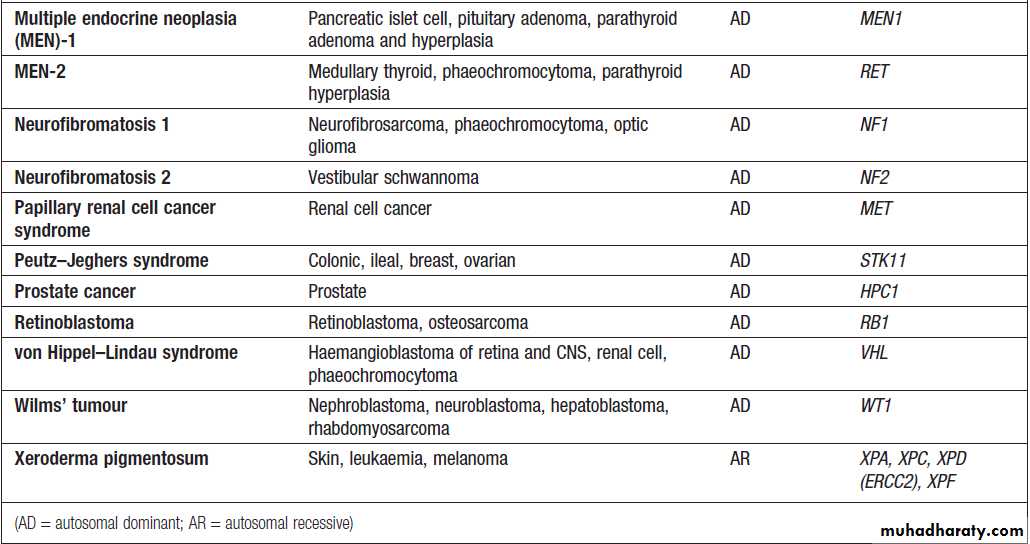
A number of inherited cancer syndromes are recognisedthat account for 5–10% of all cancers (Box), in general
they result from inherited mutations in genes that regulate cell growth, cell death and apoptosis. Examples
include the BRCA1, BRCA2 and AT (ataxia telangiectasia)
genes that cause breast and some other cancers, the
FAP gene that causes bowel cancer, and the Rb gene that
causes retinoblastoma.
Although carriers of these gene mutations have a greatly elevated risk of cancer, none has 100% penetrance and additional modulating factors, both genetic and environmental, are likely to be operative.
Inherited cancer predisposition syndromes
Inherited cancer predisposition syndromes– cont’d
INVESTIGATIONSWhen a patient is suspected of having cancer, a full
history should be taken; specific questions should be
included as to potential risk factors such as smoking and
occupational exposures. A thorough clinical examination
is also essential to identify sites of metastases, and
to discover any other conditions that may have a bearing
on the management plan. In order to make a diagnosis
and to plan the most appropriate management, information is needed on:
• the type of tumour
• the extent of disease, as assessed by staging
investigations
• the patient’s general condition and any comorbidity.
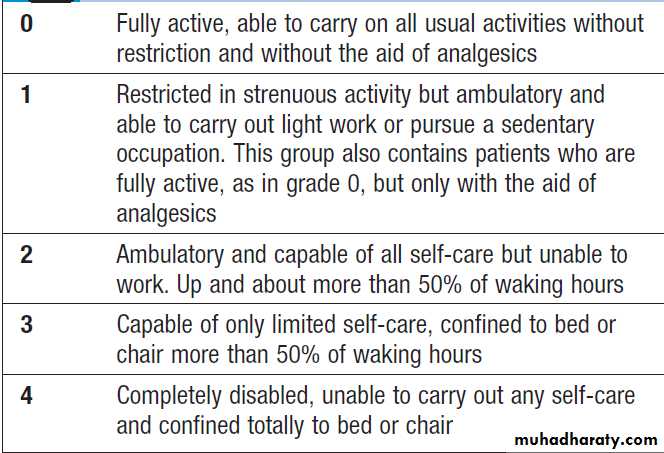
The overall fitness of a patient is often assessed by the
Eastern Cooperative Oncology Group (ECOG) performance scale (Box). The outcome for patients with a
performance status of 3 or 4 is worse in almost all malignancies than for those with a status of 0–2, and this has a strong influence on the approach to treatment in the
individual patient.
The process of staging determines the extent of the
tumour; it entails clinical examination, imaging and in
some cases surgery, to establish the extent of disease
involvement.
Therapeutic decisions and prognostic predictions can be made using the evidence base for the disease.
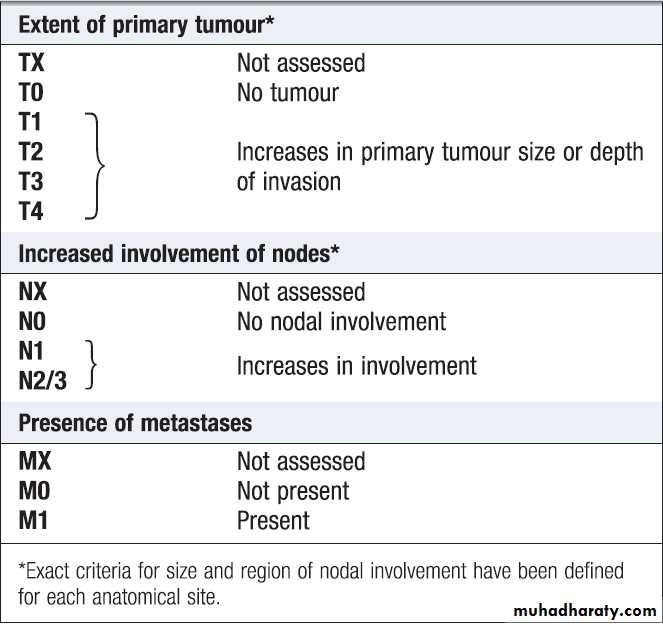
One of the most commonly used systems is the
T (tumour), N (regional lymph nodes), M (metastatic sites) approach of the International Union against Cancer (UICC).For some tumours, such as colon cancer, the Dukes system is used rather than the UICC classification.
Histology
Histological analysis of a biopsy or resected specimen is
pivotal in clinching the diagnosis and in deciding on the
best form of management.
Light microscopy
Examination of tumour samples by light microscopyremains the core method of cancer diagnosis and, in
cases where the primary site is unclear, may also give
clues to the origin of the tumour:
• Signet-ring cells favour a gastric primary.
• Presence of melanin favours melanoma.
• Mucin is common in gut/lung/breast/endometrial
cancers, but particularly common in ovarian cancer
and rare in renal cell or thyroid cancers.
• Psammoma bodies are a feature of ovarian cancer
(mucin +) and thyroid cancer (mucin −).
Immunohistochemistry (IHC)
IHC staining for tumour markers can provide useful diagnostic information and can help with treatment decisions. Commonly used
examples of IHC in clinical practice include:
• Oestrogen (ER) and progesterone (PR) receptors.
Positive results indicate that the tumour may be sensitive to hormonal manipulation.
• Alpha-fetoprotein (AFP) and human chorionic gonadotrophin (hCG) ± placental alkaline phosphatase (PLAP). These favour germ-cell tumours.
• Prostate-specific antigen (PSA) and prostatic acid phosphatase (PAP). These favour prostate cancer.
• Carcinoembryonic antigen (CEA), cytokeratin and epithelial membrane antigen (EMA). These favour carcinomas.
• HER2 receptor. Breast cancers that have high levels of expression of HER2 indicate that the tumour may respond to trastuzumab (herceptin), an antibody directed against the HER2 receptor.
The pattern of immunoglobulin, T-cell receptor and
cluster designation (CD) antigen expression on thesurface is also helpful in the diagnosis and classification
of lymphomas. This can be achieved by IHC staining of
biopsy samples or flow cytometry.
Electron microscopy
Electron microscopy (EM) can sometimes be of diagnosticvalue. Examples include the visualisation of melanosomes
in amelanotic melanoma and dense core granules
in neuro-endocrine tumours. EM may also help to distinguish
adenocarcinoma from mesothelioma, as the
ultrastructural properties of these two diseases are different (mesothelioma appears to have long, narrow,
branching microvilli while adenocarcinomas appear to
have short, stubby microvilli). EM is also useful for differentiating spindle-cell tumours (sarcomas, melanomas, squamous cell cancers) from small round-cell tumours, again due to their ultra-structural differences.
Cytogenetic analysis
Some tumours demonstrate typical chromosomal
changes that help in diagnosis. The utilisation of fluorescent
in situ hybridisation (FISH) techniques can be
useful in Ewing’s sarcoma and peripheral neuroectodermal
tumours where there is a translocation between
chromosome 11 and 22–t(11; 22)(q24; q12). In some
cases, gene amplification can also be detected via FISH
(e.g. determining over-expression of HER2/neu).
Imaging
Imaging plays a critical role in oncology, not only inlocating the primary tumour, but also in staging the
disease, usually more than one modality is required.
Radiography
Plain radiographs remain part of the initial workup, but
have a limited role in defining disease extent and have
been superseded by more sophisticated techniques. Ultrasound
Useful in characterising lesions within the liver, kidney, pancreas and reproductive organs. It can be used for guiding biopsies of tumours in breast and
liver. Endoscopic ultrasound is helpful in staging upper
gastrointestinal and pancreatic cancers.
Computerised tomography
Computerised tomography (CT) is a key investigation
in cancer patients and is particularly useful in imaging
the thorax and abdomen. With some modern scanners it
is possible to visualise the bowel, and sometimes detection
of colorectal adenomas and cancer is feasible.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) has a high resolution
and because of this is the preferred technique for brain
imaging. It is also used to image structures within the
pelvis and is widely employed for staging of rectal, cervical and prostate cancers.
Positron emission tomography
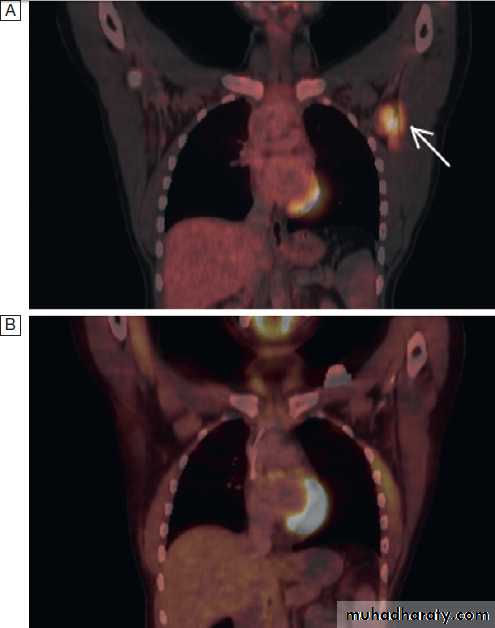
Positron emission tomography (PET) visualises metabolicactivity of tumour cells and is widely used, often
in combination with CT (PET-CT), to evaluate patients with various cancers, including lung cancer and lymphoma
(Fig.). It can accurately assess the severity
and spread of cancer by detecting tumour metabolic
activity following injection of small amounts of radioactive
tracers such as fluorodeoxyglucose (FDG). In
addition to having a role in diagnosis, PET can also be
used in some patients to assess treatment response.
Biochemical markers
Many tumours produce substances called tumour
markers, which can be used in diagnosis and surveillance.
Some are useful in population screening, diagnosis,
prognostication, treatment monitoring, detection of
relapse and imaging of metastasis. Unfortunately, most
tumour markers are not sufficiently sensitive or specific
to be used in isolation and need to be interpreted in the
context of the other clinical features. However, some can
be used for antibody-directed therapy or imaging, where
they have a greater role in diagnosis.
Eastern Cooperative Oncology Group (ECOG)
performance status scaleTNM classification
PET-CT images.
A There is a neoplastic lesion in the leftaxilla, evidenced by the increased uptake of FDG traces.
B Imaging after
chemotherapy, demonstrating that the abnormal uptake has disappeared
and Indicating a response to treatment.
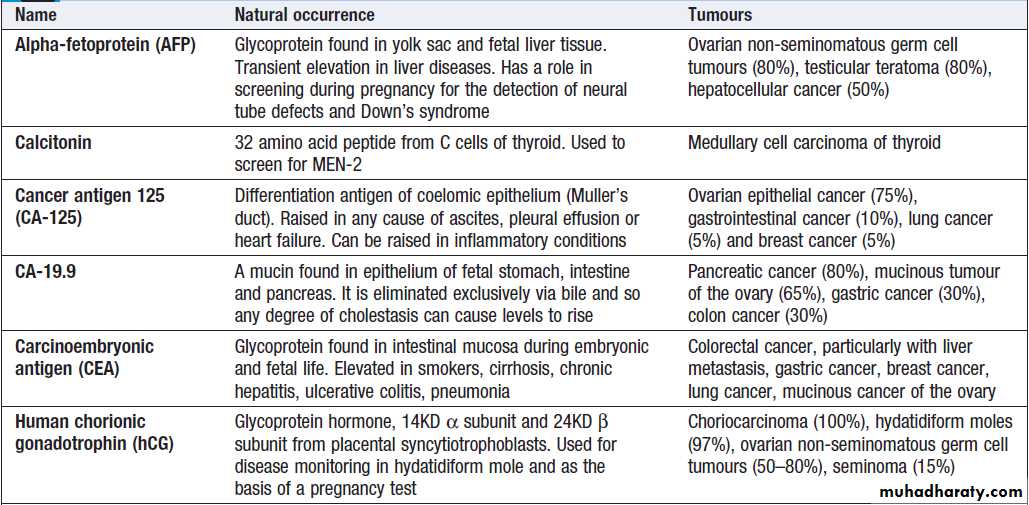
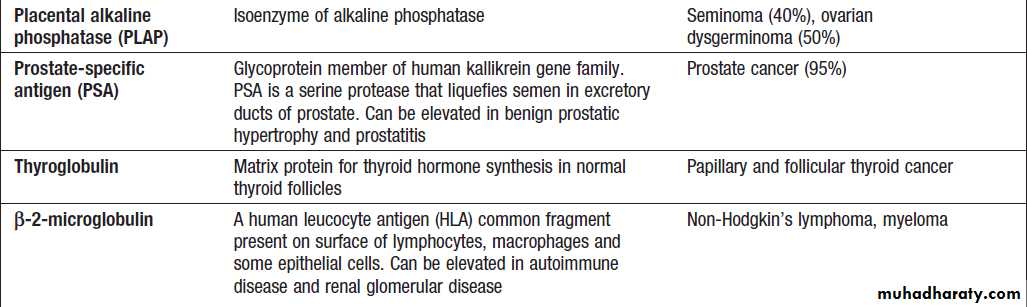
Commonly used serum tumour markers
Commonly used serum tumour markers – cont’d
PRESENTING PROBLEMS IN ONCOLOGY
In the early stages of cancer development, the numberof malignant cells is small and the patient is usually
asymptomatic. With tumour progression, localised signs
or symptoms develop due to mass effects and/or invasion of local tissues.
With further progression, symptoms may occur at distant sites as a result of metastatic disease or from non-metastatic manifestations due to production of biologically active hormones by the tumour or as the result of an immune response to the tumour.
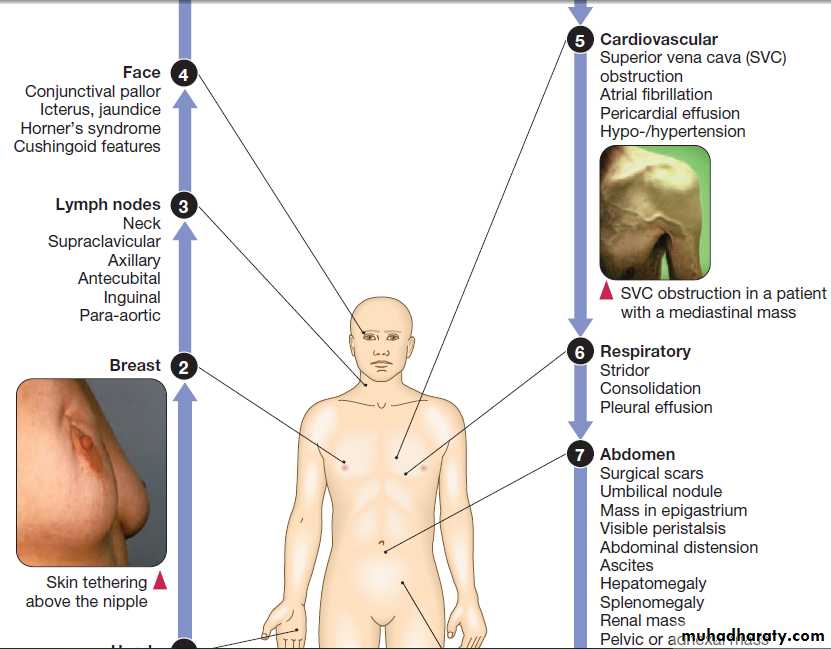
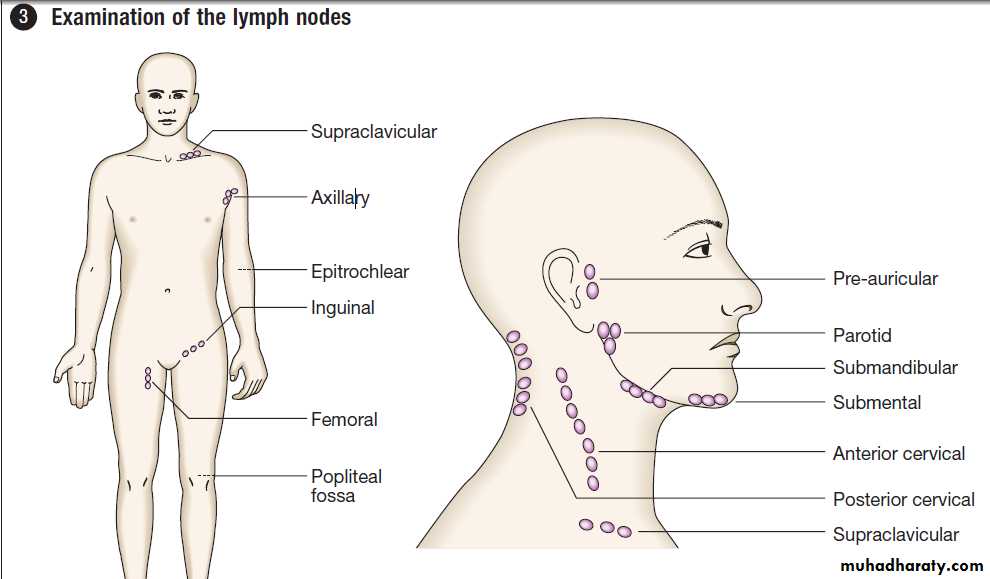
Palpable mass
A palpable mass detected by the patient or physicianmay be the first sign of cancer. Primary tumours of the
thyroid, breast, testis and skin are often detected in this
way, whereas palpable lymph nodes in the neck, groin or axilla may indicate secondary spread of tumour.
Hepatomegaly may be the first sign of primary liver
cancer or tumour metastasis, whereas skin cancer may
present as an enlarging or changing pigmented lesion.
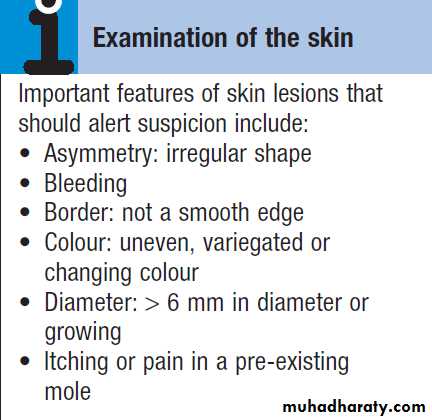
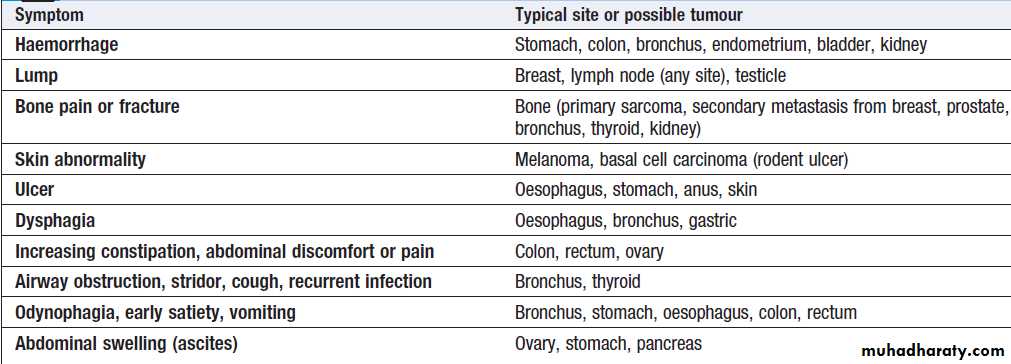
Local features of malignant disease
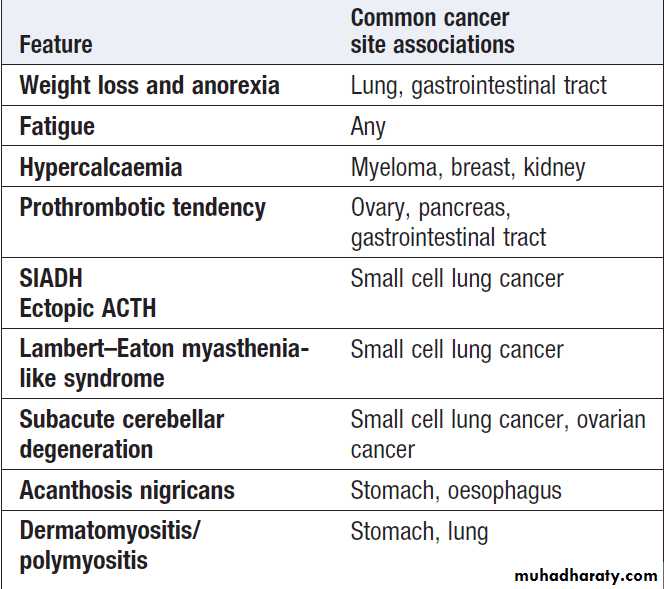
Non-metastatic manifestations of
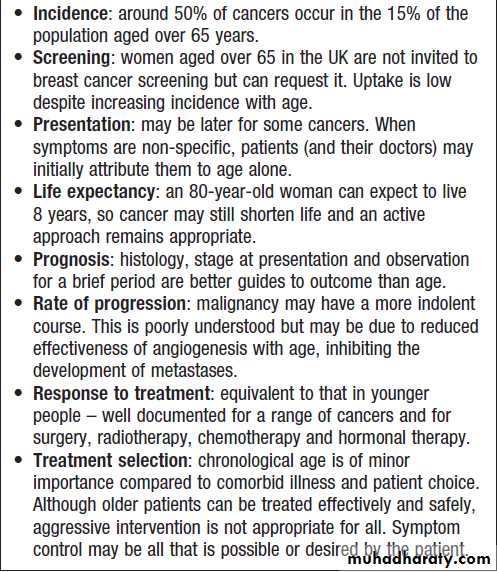
malignant diseaseCancer in old age
Weight loss and feverUnintentional weight loss is a characteristic feature of
advanced cancer, but can be due to other causes such as thyrotoxicosis, chronic inflammatory disease and
chronic infective disorders. Fever can occur in any
cancer secondary to infection, but may be a primary
feature in Hodgkin’s disease, lymphoma, leukaemia,
renal cancer and liver cancer. The presence of unexplained weight loss or fever warrants investigation to exclude the presence of occult malignancy.
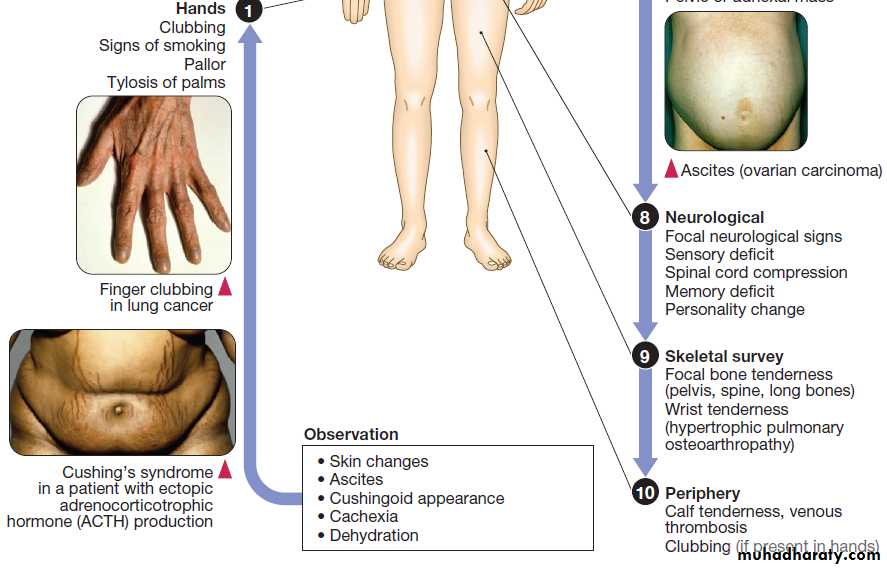
Finger clubbing
Is a characteristic feature of lung cancer,and especially non-small cell lung cancer, although benign causes are recognised. It is often part of the wider process of hypertrophic osteoarthropathy in which there
is periosteal new bone formation and arthritis due to
increased levels of prostaglandin E.
The diagnosis is primarily clinical, but X-rays show periosteal reaction and an isotope bone scan shows increased tracer update in the affected digits.
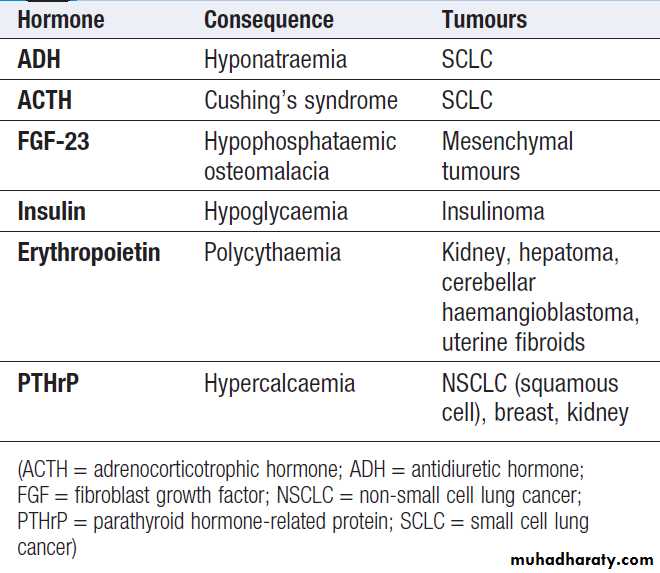
Ectopic hormone production
Including insulin, ACTH, ADH, fibroblast growth factor (FGF) 23, erythropoietin and parathyroid hormone-related protein (PTHrP).
Ectopic hormone production by tumours
Neurological paraneoplastic syndromesThese form a group of conditions associated with cancer
thought to be due to an immunological response to the
tumour that results in damage to the nervous system or
muscle. The cancers most commonly implicated are those of the lung (small cell and non-small cell), pancreas,
breast, prostate, ovary and lymphoma.
• Peripheral neuropathy results from axonal degeneration or demyelination.
• Encephalomyelitis can present with diverse symptoms, depending on which region of the brain is involved. Lumbar puncture shows raised protein in the cerebrospinal fluid (CSF) and a pleocytosis, predominantly that of lymphocytes. In some centres, flow cytometry of the CSF is also used to detect carcinomatous cells. MRI shows meningeal enhancement, particularly at the level of the brain stem, and anti-Hu antibodies may be detectable inserum. Encephalomyelitis is due to perivascular
inflammation and selective neuronal degeneration.
Most cases are caused by small cell lung cancer (75%).
• Cerebellar degeneration may be the presenting feature
of an underlying malignancy and presents with rapid onset of cerebellar ataxia. Diagnosis is by MRI or CT, which may show cerebellar atrophy. Patients with these neurological paraneoplastic syndromes may be found to have circulating anti-Yo, Tr and Hu antibodies, but these are not completely specific and negative results do not exclude the diagnosis.
• Retinopathy is a rare and presents with blurred vision, episodic visual loss and impaired colour vision. If left untreated, it may lead to blindness. The diagnosis should be suspected if the electroretinogram is abnormal and anti-retinal antibodies are detected.
• Lambert–Eaton syndrome (LEMS) is due to underlying cancer in about 60% of cases. It presents with proximal muscle weakness that improves on exercise and is caused by the development of antibodies to pre-synaptic calcium channels .
The diagnosis is made by electromyelogram (EMG),
which shows a low-amplitude compound muscle action potential that enhances to near normal following exercise.
• Dermatomyositis or polymyositis may be the first
presentation of some cancers.
Cutaneous manifestations of cancer
Many cancers can present with skin manifestations thatare not due to metastases:
• Pruritus may be a presenting feature of lymphoma,
leukaemia and CNS tumours.
• Acanthosis nigricans may precede cancers by many
years and is particularly associated with gastric cancer.
• Vitiligo may be associated with malignant melanoma, and is possibly due to an immune response to melanocytes.
• Pemphigus may occur in lymphoma, Kaposi’s
sarcoma and thymic tumours.
• Dermatitis herpetiformis associated with coeliac disease
may precede tumour development by many years,
and is associated with gastrointestinal lymphoma.
EMERGENCY COMPLICATIONS OF CANCER
Spinal cord compression
Spinal cord compression complicates 5% of cancers and
is most common in myeloma, prostate, breast and lung
cancers that involve bone. Cord compression often
results from posterior extension of a vertebral body
mass but intrathecal spinal cord metastases can cause
similar signs and symptoms.
Clinical features
The earliest sign is back pain, particularly on coughingand lying flat. Subsequently, sensory changes develop
in dermatomes below the level of compression and
motor weakness distal to the block occurs. Finally,
sphincter disturbance, causing urinary retention and
bowel incontinence, is observed. Involvement of the
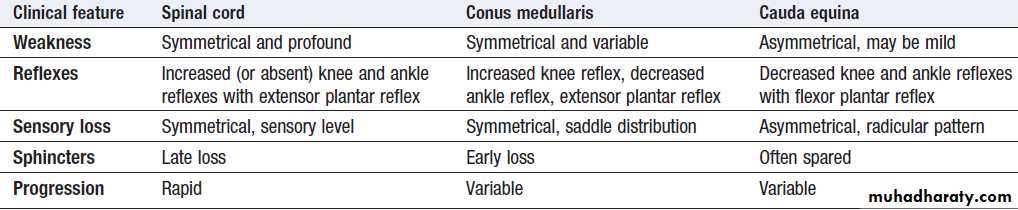
lumbar spine may cause conus medullaris or cauda
equina compression (Box). Physical examination
reveals findings consistent with an upper motor neuron
lesion, but lower motor neuron findings may predominate
early on or in cases of nerve root compression.
Comparison of features of neurological deficit
ManagementSpinal cord compression is a medical emergency and
should be treated with analgesia and high-dose steroid
therapy (Box). Neurosurgical treatment produces
superior outcome and survival compared to radiotherapy
alone. Radiotherapy is used for the remaining patients
and selected tumour types when the cancer is likely to
be radiosensitive. The prognosis varies considerably,
depending on tumour type, but the degree of neurological
dysfunction at presentation is the strongest predictor
of outcome. Ambulation can be preserved in more than 80% of patients who are ambulatory at presentation, but neurological function is seldom regained in patients with established deficits such as paraplegia.
Management of suspected spinal cord compression
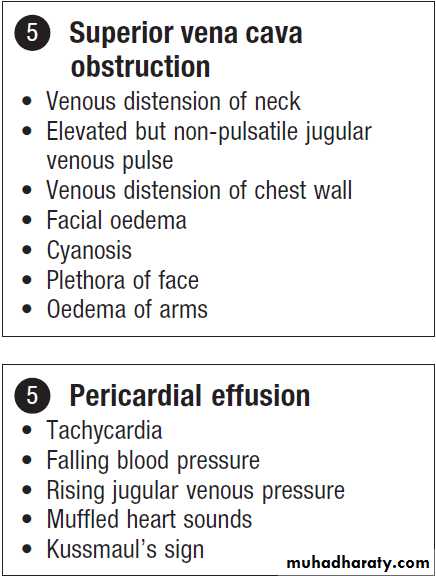
Superior vena cava obstruction
Superior vena cava obstruction (SVCO) is a common
complication of cancer that can occur through extrinsic
compression or intravascular blockage. The most
common causes of extrinsic compression are lung
cancer, lymphoma and metastatic tumours. Patients
with cancer can also develop SVCO due to intravascular
blockage in association with a central catheter or
thrombophilia secondary to the tumour.
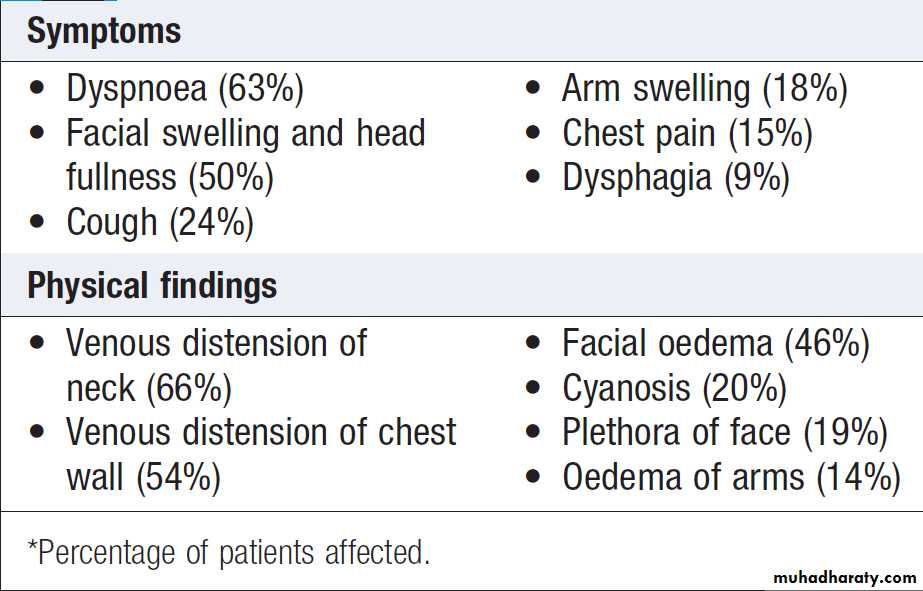
Clinical features
The typical presentation is with oedema of the arms andface, distended neck and arm veins and dusky skin coloration over the chest, arms and face. Collaterals may
develop over a period of weeks and the flow of blood in
the collaterals helps to confirm the diagnosis. Headache
secondary to cerebral oedema arising from the backflow
pressure may also occur and tends to be aggravated by
bending forward, stooping or lying down. The severity
of symptoms is related to the rate of obstruction and the
development of a venous collateral circulation.
Common symptoms and physical findings in superior vena cava obstruction*
Investigations and managementThe investigation of choice is CT of the thorax since
it can clinch the diagnosis and distinguish between
extra- and intravascular causes. A biopsy should be
obtained when the tumour type is unknown. CT of
the brain may be indicated if cerebral oedema is
suspected. Tumours that are exquisitely sensitive to chemotherapy, such as germ cell tumours and lymphoma,
can be treated with chemotherapy alone, but for most other tumours mediastinal radiotherapy is required. This relieves symptoms within 2 weeks in 50–90% of patients.
In most centres, stenting is now increasingly favoured over radiotherapy, as it produces rapid results and can be repeated with reasonable effectiveness.
This technique is particularly useful when dealing with tumours that are relatively chemoor radio-resistant, such as non-small cell lung cancer or carcinoma of unknown primary. Where possible, these measures should be followed by treatment of the primary tumour, as long-term outcome is strongly dependent on the prognosis of the underlying cancer.
Hypercalcaemia
Hypercalcaemia is the most common metabolic disorderin patients with cancer and has a prevalence of 15–20
cases per 100 000 persons. The incidence is highest in
myeloma and breast cancer (approximately 40%), intermediate in non-small cell lung cancer, and uncommon
in colon, prostate and small cell lung carcinomas.
It is most commonly due to over-production of PTHrP,
which binds to the PTH receptor and elevates serum
calcium by stimulating osteoclastic bone resorption and
increasing renal tubular reabsorption of calcium.
Clinical features
The symptoms of hypercalcaemia are often non-specific
and may mimic those of the underlying malignancy.
They include drowsiness, confusion, nausea and vomiting,
constipation, polyuria, polydipsia and dehydration.
Investigations and management
The diagnosis is made by measuring serum total calcium
and adjusting for albumin. It is especially important to
correct for albumin in cancer because hypoalbuminaemia
is common and total calcium values underestimate
the level of ionised calcium. The principles of
management are outlined in Box. Patients should initially be treated with intravenous 0.9% saline to improve renal function and increase urinary calcium excretion. This alone often results in clinical improvement.
Concurrently, intravenous bisphosphonates should be given to inhibit bone resorption.
Calcitonin acts rapidly to increase calcium excretionand to reduce bone resorption and combined with fluid and bisphosphonate therapy for the first 24–48 hours in patients with life-threatening hypercalcaemia.
Bisphosphonates will usually reduce the serum calcium levels to normal within 5 days, but if not, treatment can be repeated. The duration of action is up to 4 weeks and repeated therapy can be given at 3–4-weekly intervals as an outpatient. Hypercalcaemia is frequently a sign of tumour progression and the patient requires further investigation to establish disease status and review of the anti-cancer therapy.
Medical management of severe hypercalcaemia
Neutropenic fever
Neutropenia is a common complication of malignancy.It is usually secondary to chemotherapy but may occur
with radiotherapy if large amounts of bone marrow are
irradiated; it may also be a component of pancytopenia
due to malignant infiltration of the bone marrow. Neutropenic fever is defined as a pyrexia of 38°C for over1 hour in a patient with a neutrophil count < 1.0 × 109/L. The risk of sepsis is related to the severity and duration of neutropenia and the presence of other risk factors such as intravenous or bladder catheters. Neutropenic fever is an emergency in cancer patients as, if left untreated, it can result in septicaemia with a high mortality rate.
Clinical features
The typical presentation is with high fever. Examinationis usually unhelpful in defining a primary source of the
infection. Hypotension is an adverse prognostic feature
and may progress to systemic circulatory and organ failure.
Investigations and management
blood cultures (both peripheral and from central lines),
urine culture, chest X-ray, and swabs for culture (throat,
central line, wound). High-dose IV Antibiotics should then be commenced, pending the results of cultures. Typical first-line empirical therapy consists of an anti-pseudomonal β-lactam (ceftazidime, cefotaxim or meropenem), or a combination of an aminoglycoside and a broad-spectrum penicillin with anti-pseudomonal activity (gentamicin and piperacillin).
Metronidazole should be added if anaerobic infection is suspected, and flucloxacillin or vancomycin or teicoplanin where Gram-positive infection is suspected (for example, in patients with central lines). If there is no response after 36–48 hours, treatment with amphotericin B or voriconazole should be considered to cover fungal infection.
Antibiotics should be adjusted according to culture results, though these are often negative. Other supportive therapy, including intravenous fluids, inotrope therapy, ventilation or haemofiltration, may be required.
METASTATIC DISEASE
Metastatic disease is the major cause of death in cancer
patients and the principal cause of morbidity. For the
majority, the aim of treatment is palliative, but treatment
of a solitary metastasis can occasionally be curative.
Brain metastases
Brain metastases occur in 10–30% of adults and 6–10%
of children with cancer. Most involve the brain parenchyma but can also affect the cranial nerves, the blood vessels and other intracranial structures.
In cases of solitary metastasis to the brain, the use
of surgery and adjuvant radiotherapy has been shown
to increase survival.
In these cases, median survival without treatment is approximately 1 month. Steroids can increase survival to 2–3 months and whole-brain radiotherapy improves survival to 3–6 months, but the true efficacy of these interventions has not been proven adequately in a randomised trial setting.
Patients with brain metastases as the only manifestation
of an undetected primary tumour have a more
favourable prognosis, with an overall median survival
of 13.4 months. Tumour type also influences prognosis;
breast cancer patients have a better prognosis than those
with other types of tumour, and those with colorectal
carcinoma tend to have a poorer prognosis.
Primary tumour sites that metastasise to brain
Clinical featuresPresentation is with headaches (40–50%), focal neurological dysfunction (20–40%), cognitive dysfunction
(35%), seizures (10–20%) and papilloedema (< 10%).
Investigations and management
The diagnosis can be confirmed by CT or contrast-enhanced MRI. Treatment options include high-dose
steroids (dexamethasone 4 mg 4 times daily) for tumour-associated oedema, anticonvulsants for seizures, whole-brain radiotherapy, and chemotherapy. Surgery may be considered for single sites of disease and can be curative; stereotactic radiotherapy may also be considered for solitary site involvement where surgery is not possible.
Lung metastases
These are common in breast cancer, colon cancer andtumours of the head and neck. Solitary lesions require investigation, as single metastases can be difficult to distinguish from a primary lung tumour.
Patients with two or more pulmonary nodules can be
assumed to have metastases. The approach to treatment
depends on the extent of disease in the lung and elsewhere. For solitary lesions, surgery should be considered with a generous wedge resection. Radiotherapy, chemotherapy or endocrine therapy can be used as systemic treatment and is dependent on the underlying primary cancer diagnosis.
Liver metastases
Can represent the sole component of disease for many withcolorectal cancer, ocular melanoma, neuro-endocrine
tumours. The most common clinical presentations are with right upper quadrant pain due to stretching of the liver capsule, jaundice, deranged liver function tests or an abnormality detected on imaging. In selected cases, resection of the metastasis can be contemplated. In colorectal cancer, successful resection of a metastasis improves 5-year survival from 3% to 30–40%. Other techniques, such as chemoembolisation or radiofrequency ablation, can also be used, provided the number and size of metastases remain small. If these are not feasible, symptoms may respond to systemic chemotherapy.
Bone metastases
Bone is the third most common organ involved by
metastasis, after lung and liver. Bone metastases are a
major clinical problem in myeloma and breast or prostate cancers, but other tumours that commonly metastasise to bone include those of the kidney and thyroid.
Clinical features
The main presentations are with pain, pathological fractures and spinal cord compression . The pain
tends to be progressive and worst at night, and may be
partially relieved by activity, but subsequently becomes
more constant and exacerbated by movement.
Most pathological fractures occur in metastatic breast (53%); kidney (11%), lung (8%), thyroid (5%), lymphoma (5%) and prostate (3%).
Investigations and management
The most sensitive way of detecting bone metastases isby isotope bone scan. This can have false-positive results
in healing bone, particularly as a flare response following
treatment and false-negative results occur in multiple
myeloma due to suppression of osteoblast activity.
Therefore plain X-ray films are preferred for any sites of
bone pain, as lytic lesions may not be detected by a bone
scan. In patients with a single lesion, it is especially
important to perform a biopsy to obtain a tissue diagnosis,
since primary bone tumours may look very similar
to metastases on X-ray.
The main goals of management are:
• pain relief
• preservation and restoration of function
• skeletal stabilisation
• local tumour control (e.g. relief of tumour
impingement on normal structure).
Surgical intervention may be warranted where there
is evidence of skeletal instability (e.g. anterior or posterior spinal column fracture) or an impending fracture
(e.g. large lytic lesion on a weight-bearing bone
with more than 50% cortical involvement).
Intravenous bisphosphonates (pamidronate, zoledronic acid or ibandronate) are widely used for bone metastases and are effective at improving pain and in reducing further skeletal related events, such as fractures and hypercalcaemia (Box).
In certain types of cancer, such as breast and prostate, hormonal therapy may be effective. Radiotherapy, in the form of external beam therapy or systemic radionucleotides (strontium treatment), can also
be useful for these patients. In some settings (e.g. breast
carcinoma), chemotherapy may also be used in the management of bony metastases.
Use of bisphosphonates in bony metastases
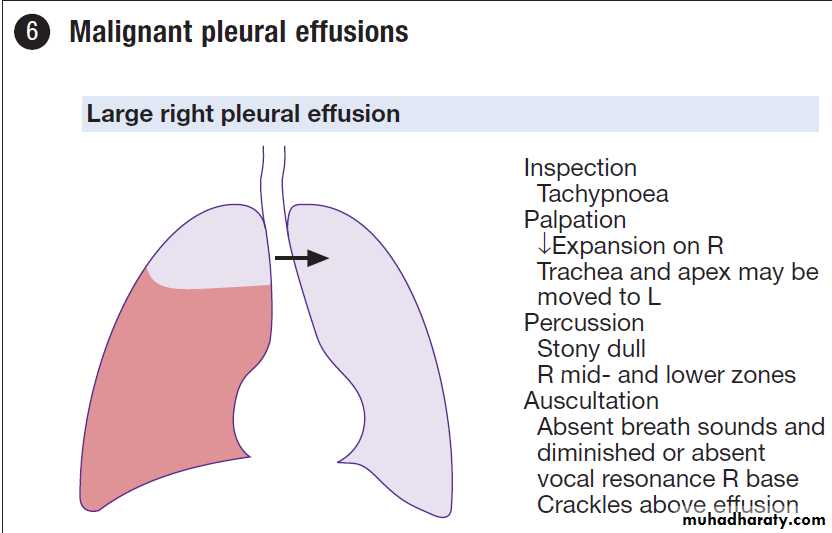
Malignant pleural effusion
This is a common complication of cancer and 40% of all
pleural effusions are due to malignancy. The most
common causes are lung and breast cancers, and the
presence of an effusion indicates advanced and incurable
disease. The presentation may be with dyspnoea, cough or chest discomfort, which can be dull or pleuritic in nature. Investigations and management
Pleural aspirate is the key investigation and may show
the presence of malignant cells. Malignant effusions are
commonly blood-stained and are exudates with a raised
fluid to serum lactate dehydrogenase (LDH) ratio (> 0.6)
and a raised fluid to serum protein ratio (> 0.5).
Treatment should focus on palliation of symptoms.
Aspiration alone may be an appropriate treatmentin frail patients with a limited life expectancy .Those who present with malignant pleural effusion as the initial manifestation of breast cancer, small cell lung cancer, germ cell tumours or lymphoma should have the fluid aspirated and should be given systemic chemotherapy to try to treat disease in the pleural space. Treatment options for patients with recurrent pleural effusion include pleurodesis, pleurectomy and Pleuroperitoneal shunt.
Ideally, pleurodesis should be attempted once effusions recur after initial drainage.
How to aspirate a malignant
pleural effusionTHERAPEUTICS IN ONCOLOGY
Anti-cancer therapy may be either curative or palliative.
• Palliative chemotherapy is the most common treatment and is primarily used to treat patients with metastasis. The goal is an improvement in symptoms with a focus on improving quality of life, and any survival increments are secondary. As a result, the treatment should be well tolerated and should aim to minimise adverse effects.
• Adjuvant chemotherapy is given after an initial
intervention that is designed to cyto-reduce the tumour bulk and remove all macroscopic disease. Chemotherapy is then given with the intention of eradicating the micrometastatic disease that remains.
• Neoadjuvant chemotherapy or primary medical
therapy is where chemotherapy is administered firstbefore a planned cyto-reductive procedure. This can
result in a reduced requirement for surgery,
increase the likelihood of successful debulking,
reduce the duration of hospitalisation and improve
the fitness of the patient prior to interval debulking.
• Chemoprevention is the use of pharmacological
agents to prevent cancer developing in patients
identified as being at particular risk. Therefore the
agents used aim to modify risk and, as such, should
not have significant adverse effects.
Surgical treatment
Surgery has a pivotal role in the management ofcancer. There are three main situations in which it is
necessary.
Biopsy
In the vast majority of cases, a histological or cytological
diagnosis of cancer is necessary, and tissue will also
provide important information such as tumour type and
differentiation, to assist subsequent management. Cytology
can be obtained with fine needle aspiration but a
biopsy is usually preferred. This can be a core biopsy,
an image-guided biopsy or an excision biopsy.
Excision
The main curative management of most solid cancers is
surgical excision. In early, localised cases of colorectal, breast and lung cancer, cure rates are high with surgery.
There are some cancers for which surgery is one of two or more options for primary management, and the role of the multidisciplinary team is to recommend appropriate treatment for a specific patient.
Examples include prostate and transitional cell carcinoma
of the bladder, in which radiotherapy and surgery
may be equally effective.
Palliation
Surgical procedures are often the quickest and mosteffective way of palliating symptoms. Examples include
the treatment of faecal incontinence with a defunctioning
colostomy; fixation of pathological fractures and
decompression of spinal cord compression; and the
treatment of fungating skin lesions by ‘toilet’ surgery. A
more specialist role for surgery is in resection of residual
masses after chemotherapy and, in very selected cases,
resection of metastases.
Systemic chemotherapy
Chemotherapeutic drugs are classified by their mode ofaction. They have the greatest activity in proliferating
cells and this provides the rationale for their use in the
treatment of cancer.
Chemotherapeutic agents are not specific for cancer cells, however, and the side-effects of treatment are a result of their anti-proliferative actions in normal tissues such as the bone marrow, skin and gut.
Combination therapy
In order to overcome drug resistance and to limit the
side-effects of different drugs, chemotherapy is most
commonly given as a combination of agents. Combinations
usually include drugs from different classes, with
the aim of targeting several pathways and gaining
maximum therapeutic effect. Drugs are conventionally
given by intravenous injection every 3–4 weeks, allowing
enough time for the patient to recover from shortterm
toxic effects before the next dose. Between four and
eight such cycles of treatment are usually given in total.
More recently, other strategies have been developed.
For example, 5-fluorouracil (5-FU), which has a very short
half-life, has increased efficacy when given by continuousintravenous infusion, using a semi-permanent
in-dwelling intravenous catheter. However, the use of
such catheters is not without risk and the potential of
oral 5-FU is now being explored, using precursors such
as capecitabine. Schedules of administration at weekly
or 2-weekly intervals have also found their place in the
management of both solid and haematological malignancies.
Each tumour type has specific regimens that are
used at various stages of the disease.
Mode of administration
Most drugs have to be given intravenously, and many
are vesicant or locally irritant if there is an extravasation.
Chemotherapy should be administered into a vein in
which the infusion is free-flowing to minimise the risk
of extravasation. A few patients require central venous catheters due to the nature of their treatment or poor
vascular access. Patients who receive chemotherapy
through a peripheral line must be carefully observed,
and the chemotherapy stopped at the first sign of any
extravasation. Chemotherapy is potentially dangerous
to the person giving the therapy, because cytotoxics are
carcinogenic and teratogenic.
In view of this, policies must be in place for the use of gloves and aprons and for the safe disposal of syringes containing cytotoxics.
Other oral chemotherapeutic agents have been developed
over the past 30 years, although not many have
replaced their intravenous counterparts.
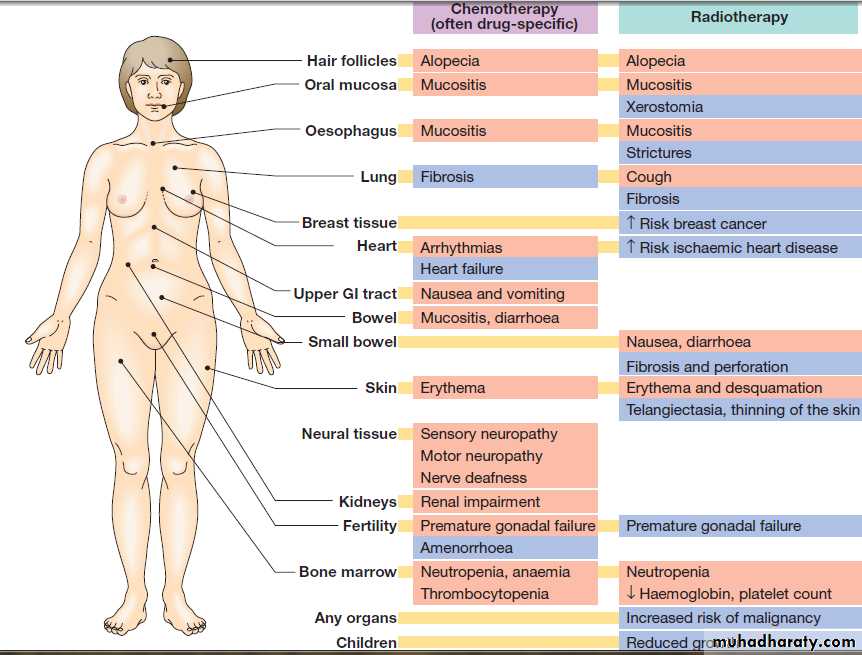
Adverse effects
Most cytotoxics have a narrow therapeutic windowor index and can have significant adverse effects, as
shown in Figure. Considerable supportive therapy
is often required to enable patients to tolerate therapy
and achieve benefit. Nausea and vomiting are common,
but with modern antiemetics, regimens such as the
combination of dexamethasone and highly selective
5-hydroxytryptamine (5-HT3) receptor antagonists like
ondansetron, most patients now receive chemotherapy
without any significant problems. Myelosuppression is
common to almost all cytotoxics.
This not only limits the dose of drug, but also can cause life-threatening complications.
The risk of neutropenia can be reduced with the use of specific growth factors that accelerate the re-population of myeloid precursor cells. The most commonly employed is granulocyte–colony-stimulating factor (G–CSF), which is widely used in conjunction with chemotherapy regimens that induce a high rate of neutropenia.
More recently, it has also been used to ‘accelerate’ the administration of chemotherapy, enabling standard doses to be given at shorter intervals where the rate-limiting factor has been the time taken for the peripheral neutrophil count to recover.
Accelerated chemotherapy regimens have now been demonstrated to offer therapeutic advantages in small cell lung cancer, lymphoma and possibly breast cancer.
Adverse effects of chemotherapy and radiotherapy. Acute effects are shown in pink and late effects in blue.
Radiation therapy
Radiation therapy (radiotherapy) involves treating thecancer with ionising radiation; for certain localised
cancers it may be curative. Ionising radiation can be
delivered by radiation emitted from the decay of radioactive isotopes or by high-energy radiation beams,
usually X-rays. Three methods are usually employed:
• Teletherapy: application from a distance by a linear
accelerator.
• Brachytherapy: direct application of a radioactive source on to or into a tumour. This allows the delivery of a very high, localised dose of radiation and is integral to the management of localised cancers of the head and neck and cancer of the cervix and endometrium.
• Intravenous injection of a radioisotope: such as 131iodine for cancer of the thyroid and 89strontium for the
treatment of bone metastases from prostate cancer.
The majority of treatments are now delivered by
linear accelerators; these produce electron or X-ray
beams of high energy that are used to target tumour
tissue. Whatever the method of delivery, the biological
effect of ionising radiation is to cause lethal and
sublethal damage to DNA. Since normal tissues are
also radiosensitive, treatment has to be designed to
maximise exposure of the tumour and minimise exposure
of normal tissues. This is now possible with modern
imaging techniques such as CT and MRI, which allow
better visualisation of normal and tumour tissue.
In addition, techniques such as conformal radiotherapy,
where shaped rather than conventional square or rectangular beams are used, allow much more precise targeting of therapy to the tumour, and reduce the volume of normal tissue irradiated by up to 40% compared to nonconformal techniques.
Biological differences between normal and tumour
tissues are also used to obtain therapeutic gain. Fundamental to this is fractionation, which entails delivering
the radiation as a number of small doses on a daily basis.
This allows normal cells to recover from irradiation
damage but recovery occurs to a lesser degree in malignant cells.
Fractionation regimens vary from centre to centre but radical treatments given with curative intent are often delivered in 20–30 fractions given daily, 5 days
a week over 4–6 weeks. Radiotherapy can also be
extremely useful for the alleviation of symptoms, and
for palliative treatments such as this a smaller number
of fractions (1–5) is usually adequate.
Both normal and malignant tissues vary widely in their sensitivity to radiotherapy. Germ cell tumours and lymphomas are extremely radiosensitive and relatively low doses are adequate for cure, but most cancers require doses close to or beyond that which can be tolerated by adjacent normal structures.
Normal tissue also varies in its radiosensitivity, the central nervous system, small bowel and lung being amongst the most sensitive.
The side-effects of radiotherapy (see Fig.) depend
on the normal tissues treated, their radiosensitivity and
the dose delivered.
Adverse effects
An acute inflammatory reaction commonly occurs towards the end of most radical treatments and is localised to the area treated. For example, skin reactions are common with breast or chest wall radiotherapy, and proctitis and cystitis with treatment to the bladder or prostate.
These acute reactions settle over a period of a
few weeks after treatment, assuming normal tissue tolerance has not been exceeded.
Late effects of radiotherapy develop 6 weeks or more after treatment and occur in 5–10% of patients.
Examples include brachial nerve damage and subcutaneous fibrosis after breast cancer treatment, and shrinkage and fibrosis of the bladder after treatment for bladder cancer.
There is a risk of inducing cancer after radiotherapy, which varies depending on the site treated and whether the patient has had other treatment such as chemotherapy.
Hormone therapy
Is most commonly used in the treatment of breast cancer and prostate cancer. Breast tumours that are positive for expression of the oestrogen receptor (ER) respond well to anti-oestrogen therapy, and assessment of ER status is now standard in the diagnosis of breast cancer. Several drugs are now available that reduce oestrogen levels or block the effects of oestrogen on the receptor.When targeted appropriately, adjuvant hormone therapy reduces the risk of relapse and death at least as much as chemotherapy, and in advanced cases can induce stable disease and remissions that may last months to years, with acceptable toxicity. Hormonal manipulation may be effective in other cancers.
In prostate cancer, hormonal therapy (e.g. luteinising hormone releasing hormone (LHRH) analogues such as goserelin and/or anti-androgens such as bicalutamide) aimed at reducing androgen levels can provide good long-term control of advanced disease, but there is no convincing evidence that it is an effective therapy following potentially curative surgery.
Progestogens are active in the treatment of endometrial
and breast cancer. In the metastatic setting, progestogen
use (e.g. megestrol acetate) is associated with response
rates of 20–40% in endometrial cancer. In breast cancer,
progestogens are used in patients whose disease has
progressed with conventional anti-oestrogen therapy.
Their exact mechanism in this setting is not fully understood.
Immunotherapy
A profound stimulus to the patient’s immune system can sometimes alter the natural history of a malignancy,and the discovery of interferons was the impetus for
much research. Although solid tumours show little benefit, interferons are active in melanoma and lymphoma, and there is evidence that they are beneficial as adjuvants (after surgery and chemotherapy respectively) to delay recurrence. Whether interferon induced stimulation of the immune system is capable of eradicating microscopic disease remains unproven. More powerful immune responses can be achieved with potent agents like interleukin-2 (IL-2), but the accompanying systemic toxicity is a problem still to be overcome.
The most striking example of successful immunotherapy is that with rituximab, an antibody against the common B-cell antigen CD20.
It increases complete response rates and improves survival in diffuse large cell non-Hodgkin’s lymphoma when combined with chemotherapy, and is also effective in palliating advanced follicular non-Hodgkin’s lymphoma.
Biological therapies
Advances in knowledge about the molecular basis of cancer have resulted in the development of a new generation of treatments to block the signalling pathwaysresponsible for the growth of specific tumours. This has
created the potential to target cancer cells more selectively, with reduced toxicity to normal tissues. Some examples are discussed below, but in the years to come
many more such agents will come into clinical use, with
the potential to revolutionise our approach to some cancers.
Gefitinib/erlotinib
These agents inhibit the activity of the epidermalgrowth factor receptor, which is over-expressed in many
solid tumours. However, the drugs’ activity does not
depend on the amount of receptor over-expression but
on factors such as gene copy number and mutation status.
Imatinib
Imatinib was developed to inhibit the BCR-ABL gene
product, tyrosine kinase, that is responsible for chronic
myeloid leukaemia , and it does this extremely
effectively. It is also active in gastrointestinal stromal
tumour (GIST), a type of sarcoma that has overexpression
of another cell surface tyrosine kinase, c-kit. This agent has good tolerability and is particularly useful in GIST, where conventional chemotherapy is less effective.
Bevacizumab
This is a humanised monoclonal antibody that inhibits
vascular endothelial growth factor A (VEGF-A), a key
stimulant of angiogenesis in tumours. This has activity
in colorectal, lung, breast, renal and ovarian cancers,
although the licence was subsequently revoked for
breast cancer; while bevacizumab slows the rate of progression of metastatic breast cancer, it had little impact
on survival or improved quality of life.
Trastuzumab
Trastuzumab (herceptin) targets the HER2 receptor, anoncogene that is over-expressed in around one-third of
breast cancers and in a number of other solid tumours.
It is effective as a single-agent therapy, but also improves
survival in patients with advanced breast cancer when
used in conjunction with chemotherapy.
Unfortunately, trastuzumab can induce cardiac failure by an unknownbiological mechanism, especially in combination with doxorubicin.
SPECIFIC CANCERS
Breast cancerGlobally, the incidence of breast cancer is only second
to that of lung cancer and the disease represents the
leading cause of cancer-related deaths among women.
Invasive ductal carcinoma with or without ductal carcinoma
in situ (DCIS) is the most common histology,
accounting for 70%, whilst invasive lobular carcinoma accounts for most of the remaining cases.
DCIS constitutes 20% of breast cancers detected by mammography screening.
It is multifocal in one-third of women and has a high risk of becoming invasive (10% at 5 years following excision only). Pure DCIS does not cause lymph node metastases, although these are found in 2% of cases where nodes are examined, owing to undetected invasive cancer. Lobular carcinoma in situ (LCIS) is a predisposing risk factor for developing cancer in either breast (7% at 10 years).
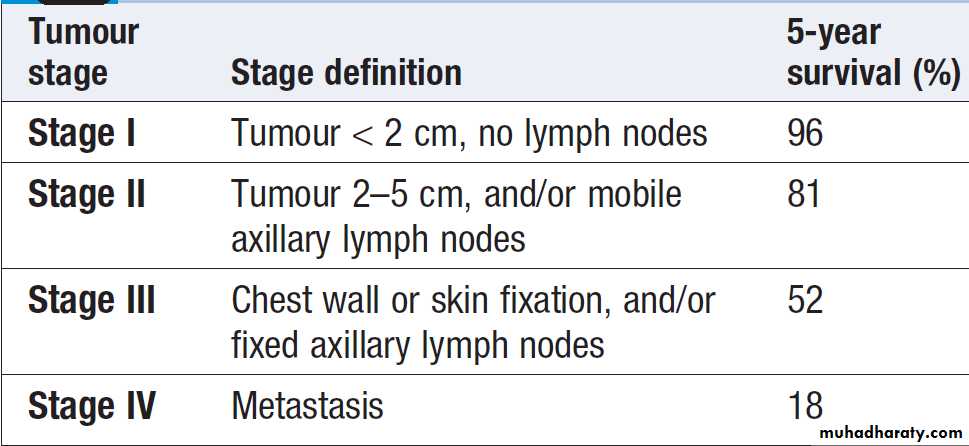
Five-year survival rates for breast cancer by stage
PathogenesisBoth genetic and hormonal factors play a role; about
5–10% of breast cancers are hereditary and occur in
patients with mutations of BRCA1, BRCA2, AT or TP53
genes. Prolonged oestrogen exposure associated with
early menarche, late menopause and use of hormone
replacement therapy (HRT) has been associated with an
increased risk. Other risk factors include obesity, alcohol
intake, nulliparity and late first pregnancy.
There is no definite evidence linking use of the contraceptive pill to breast cancer.
Clinical features
Breast cancer usually presents as a result of mammographic screening or as a palpable mass with nipple discharge in 10% and pain in 7% of patients. Lesscommon presentations include inflammatory carcinoma
with diffuse induration of the skin of the breast, and this
confers an adverse prognosis. Around 40% of patients
will have axillary nodal disease, with likelihood correlating
with increasing size of the primary tumour.
Distant metastases are infrequently present at diagnosis
and the most common sites of spread are: bone (70%),
lung (60%), liver (55%), pleura (40%), adrenals (35%),
skin (30%) and brain (10–20%).
Investigations
Following clinical examination, patients should have
imaging with mammography or ultrasound evaluation
and a biopsy using fine needle aspiration for cytology
or core biopsy for histology. Histological assessment
should be carried out to assess tumour type and to determine oestrogen and progesterone receptor (ER/PR)
status and HER2 status.
If distant spread is suspected, CT of the thorax and abdomen and an isotope bone scan are required.
Management
Surgery is the mainstay of treatment for most patients,and this can range from a lumpectomy, where only the
tumour is removed, to mastectomy, where the whole
breast is removed. Lymph node sampling is performed
at the time of surgery.
Adjuvant radiotherapy is given to reduce the risk of local recurrence to 4–6%.
Adjuvant hormonal therapy improves disease-free and overall survival in pre- and post-menopausal patients who have tumours that express ER.
Patients at low risk, with tumours that are small and ER-positive, only require adjuvant hormonal therapy with tamoxifen.
Patients with tumours that are ER-positive and who are premenopausal should receive an LHRH analogue. Aromatase inhibitors also have benefit in this setting and are still under investigation.
Adjuvant chemotherapy is considered for patients at higher risk of recurrence. Factors that increase the risk of recurrence include a tumour of >1 cm, tumour that is ER-negative or the presence of involved axillary lymph nodes. Such patients should be offered adjuvant chemotherapy, which improves disease-free and overall survival . Data support the use of adjuvant trastuzumab, a humanized monoclonal antibody to HER2, in addition to standard chemotherapy for early HER2-positive cancer.
Metastatic disease management includes radiotherapy
to palliate painful bone metastases and second-line
endocrine therapy with aromatase inhibitors, which
inhibit peripheral oestrogen production in adrenal and
adipose tissues. Advanced ER-negative disease may be
treated with combination chemotherapy.
Use of adjuvant chemotherapy and endocrine therapy in early breast cancer
Ovarian cancerOvarian cancer is the most common gynaecological
tumour in Western countries. Most ovarian cancers are
epithelial in origin (90%), and up to 7% of women with
ovarian cancer have a positive family history. Patients
often present late in ovarian cancer with vague abdominal
discomfort, low back pain, bloating, altered bowel
habit and weight loss. Occasionally, peritoneal deposits
are palpable as an omental ‘cake’ and nodules in the
umbilicus (Sister Mary Joseph nodules).
Pathogenesis
Genetic and environmental factors play a role. The
risk of ovarian cancer is increased in patients with
BRCA1 or BRCA2 mutations, and Lynch type II families
(a subtype of hereditary non-polyposis colon cancer
(HNPCC)) have ovarian, endometrial, colorectal and
gastric tumours due to mutations of mismatch repair
enzymes. Advanced age, nulliparity, ovarian stimulation
and Caucasian descent all increase the risk of
ovarian cancer, whilst suppressed ovulation appears to protect, so
pregnancy, prolonged breastfeeding and the
contraceptive pill have all been shown to reduce the risk.
Investigations
Initial workup for patients with suspected ovarianmalignancy includes imaging in the form of ultrasound
and CT. Serum levels of the tumour marker CA-125
are often measured. Surgery plays a key role in the
diagnosis, staging and treatment of ovarian cancer, and
in early cases, palpation of viscera, intraoperative
washing and biopsies are generally performed to define
disease extent.
Management
In early disease, surgery followed by adjuvant chemotherapy with carboplatin, or carboplatin plus paclitaxel, is the treatment of choice. Surgery should include
removal of the tumour along with total hysterectomy,
bilateral salpingo-oophorectomy, and omentectomy.
Even in advanced disease, surgery is undertaken to
debulk the tumour and is followed by adjuvant chemotherapy, typically using carboplatin and paclitaxel.
Bevacizumab is indicated for patients with high-grade
tumours that are suboptimally debulked or those with
a more aggressive biological pattern. Monitoring for
relapse is achieved through a combination of serum
CA-125 and clinical examination with CT imaging for
those with suspected relapse.
Second-line chemotherapy is aimed at improving symptoms and should not be used for CA-125 elevation only in the absence of symptoms.
Treatments can include further platinum/ paclitaxel combination, liposomal doxorubicin or topotecan.
These regimens are associated with a response
rate of 10–40%.
The best responses are observed in patients with a treatment-free interval of more than 12 months.
Endometrial cancer
Accounts for 4% of all female malignancies, producing a 1 in 73 lifetime risk. The majority of patients are post-menopausal, with a peak incidence at 50–60 years of age. Mortality from endometrial cancer is currently falling. The most common presentation is with post-menopausal bleeding, which often results in detection of the disease before distant spread has occurred.Pathogenesis
Oestrogen plays an important role in the pathogenesis
and factors that increase the duration of oestrogen exposure, such as nulliparity, early menarche, late menopause and unopposed HRT, increase the risk. Endometrial cancer is 10 times more common in obese, and this is thought to be due to elevated levels of oestrogens.
Investigations
The diagnosis is confirmed by endometrial biopsy.
Management
Surgery is the treatment of choice and is also used
for staging. A hysterectomy and bilateral salpingo oophorectomy are performed with peritoneal cytology and, in some cases, lymph node dissection. Where the tumour extends beyond the inner 50% of the myometrium, involves the cervix and local lymph nodes, or there is lymphovascular space invasion, adjuvant pelvic radiotherapy is recommended. Chemotherapy and hormonal therapies have not demonstrated a sufficient
survival advantage to be recommended for routine use in the adjuvant setting but have a role in recurrent disease.
Cervical cancer
This is the second most common gynaecological tumourworldwide. The incidence is decreasing in developed
countries but continues to rise in developing nations.
Cervical cancer is the leading cause of death from gynaecological cancer. The most common presentation is with an abnormal smear test, but with locally advanced
disease the presentation is with vaginal bleeding, discomfort, discharge or symptoms attributable to involvement of adjacent structures, such as bladder, or rectal or pelvic wall. Occasionally, patients present with distant metastases to bone and lung.
Pathogenesis
There is a strong association between cervical cancerand sexual activity that includes sex at a young age and multiple sexual partners. Infection with HPV has an
important causal role, and this has underpinned the introduction of programmes to immunise teenagers
against HPV in an effort to prevent the later development of cervical cancer.
Investigations
Diagnosis is made by smear or cone biopsy. Dilatationand curettage is also used diagnostically, with cystoscopy
and flexible sigmoidoscopy if there are symptoms
referable to the bladder, colon or rectum. In contrast to
other gynaecological malignancies, cervical cancer is a
clinically staged disease. MRI is often used to characterise
the primary tumour. A routine chest X-ray should be
obtained to help rule out pulmonary metastasis. CT of
the abdomen and pelvis is performed to look for metastasis in the liver and lymph nodes and to exclude
hydronephrosis and hydroureter.
Management
This depends on the stage of disease. Pre-malignant
disease can be treated with laser ablation or diathermy,
whereas in microinvasive disease a large loop excision
of the transformation zone (LLETZ) or a simple hysterectomy
is employed. Invasive but localised disease
requires radical surgery, while chemotherapy and radiotherapy, including brachytherapy, may be given as
primary treatment, especially in patients with adverse
prognostic features such as bulky or locally advanced
disease, or lymph node or parametrium invasion. In
metastatic disease, cisplatin-based chemotherapy may
be beneficial in improving symptoms but does not
improve survival significantly.
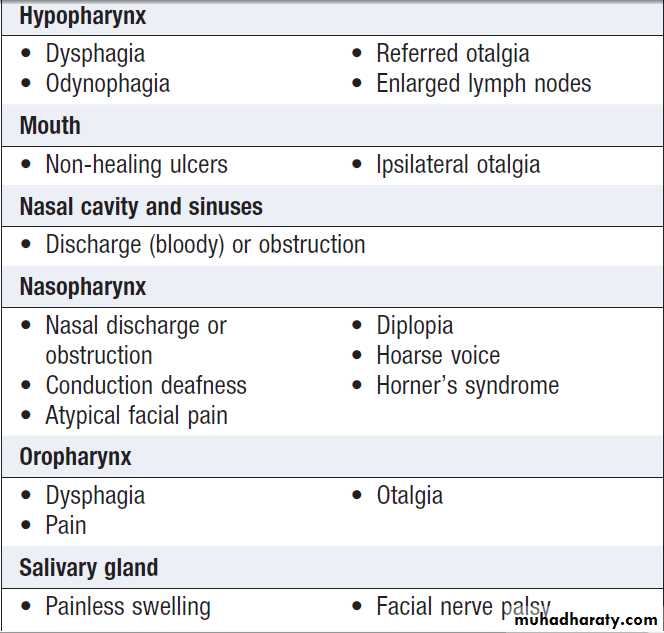
Head and neck tumours
Are typically squamous tumours arise in the nasopharynx, hypopharynx and larynx. They are most common in elderly males, but now occur with increasing frequency in a younger cohort, as well as in women. The rising incidence of oropharyngeal cancers, especially in the developed world,is thought to be secondary to HPV infection. Presentation
depends on the location of the primary tumour
and the extent of disease. For example, early laryngeal cancers may present with hoarseness, while more extensive local disease may present with pain due to invasion of local structures or with a lump in the neck.
Patients who present late often have pulmonary symptoms,
as this is the most common site of distant metastases.
Common presenting features by location in
head and neck cancerPathogenesis
The tumours are strongly associated with a historyof smoking and excess alcohol intake, but other recognised risk factors include Epstein–Barr virus for nasopharyngeal cancer and HPV infection for oropharyngeal tumours.
Investigations
Careful inspection of the primary site is required as part
of the staging process, and most patients will require
endoscopic evaluation and examination under anaesthesia.
Tissue biopsies should be taken from the most accessible
site. CT of the primary site and the thorax is the investigation of choice for visualising the tumour, while
MRI may be useful in certain cases.
Management
Generally speaking, the majority of patients with earlyor locally advanced disease are treated with curative
intent. In localised disease where there is no involvement
of the lymph nodes, long-term remission can
be achieved in up to 90% of patients with surgery or
radiotherapy. The choice of surgery versus radiotherapy
often depends on patient preference, as surgical treatment
can be mutilating with an adverse cosmetic
outcome. Patients with lymph node involvement or
metastasis are treated with a combination of surgery
and radiotherapy (often with chemotherapy as a radiosensitising agent – proven agents include cisplatin or
cetuximab), and this produces long-term remission in
approximately 60–70% of patients.
Recurrent or metastatic tumour may be palliated with further surgery or radiotherapy to aid local control, and systemic chemotherapy has a response rate of around 20–30%. Second malignancies are common (3% per year) following successful treatment for primary disease, and all patients should be encouraged to give up smoking and drinking alcohol to lower their risk.
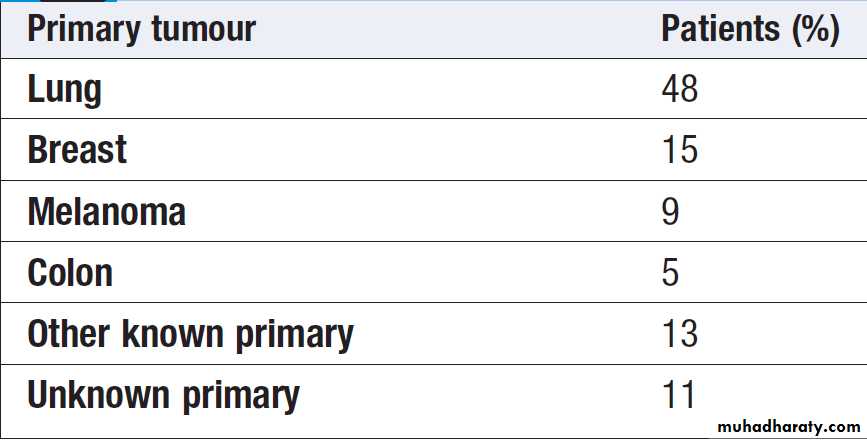
Carcinoma of unknown origin
Some patients are found to have evidence of metastaticdisease at their initial presentation prior to diagnosis of
a primary site. In many cases, a subsequent biopsy
reveals adenocarcinoma but the primary site is not
always clear.
Investigations
In this situation, there is a temptation to investigate the
patient endlessly in order to determine the original primary site. However, there is a compromise between exhaustive investigation and obtaining sufficient information to plan appropriate management. For all patients, histological examination of an accessible site of metastasis is required.
The architecture of the tissue can assist the pathologist in determining the likely primary site, and therefore it is better to perform a biopsy rather than fine needle aspiration. The greater volume of tissue also permits the use of immunohistochemistry.
Extensive imaging to search for the primary is rarely indicated; a careful history to identify symptoms and risk factors (including familial) will often permit a judicious choice of imaging.
Management
Management of the patient will depend on that person’scircumstances, as well as on the site(s) involved and the
likely primary sites. The overriding principle is to ensure
that a curable diagnosis has not been overlooked. For
example, lung metastases from a testicular teratoma do
not preclude cure; nor do one or two liver metastases
from a colorectal cancer. Early discussion with an oncologist
within a multidisciplinary team is essential and avoids unnecessary investigation; for example, a single hCG-based pregnancy test in a young man with lung metastases might confirm the presence of a teratoma and allow rapid administration of potentially curative chemotherapy.
Treatment should not necessarily wait for a
definitive diagnosis; appropriate analgesia, radiotherapy and surgical palliation can all be helpful. Some patients remain free of cancer for some years after resection of a single metastasis of an adenocarcinoma of
unknown primary, justifying this approach in selected
patients.
In those with no obvious primary, systemic chemotherapy
may achieve some reduction in tumour burden and alleviation of symptoms, but long-term survival is rare.