Hussien Mohammed Jumaah
CABMLecturer in internal medicine
Mosul College of Medicine
2016
learning-topics
PoisoningCOMPREHENSIVE EVALUATION OF THE POISONED PATIENT
Taking a history in poisoning
• What toxin(s) have been taken and how much?• What time were they taken and by what route?
• Has alcohol or any drug of misuse been taken as well?
• Obtain details from witnesses of the circumstances of the
overdose (e.g. family, friends ambulance personnel)
• Ask the general practitioner for background and details of prescribed medication
• Assess suicide risk (full psychiatric evaluation when patient has physically recovered)
• Assess capacity to make decisions about accepting or
refusing treatment
• Establish past medical history, drug history and allergies, social and family history
• Record all information carefully
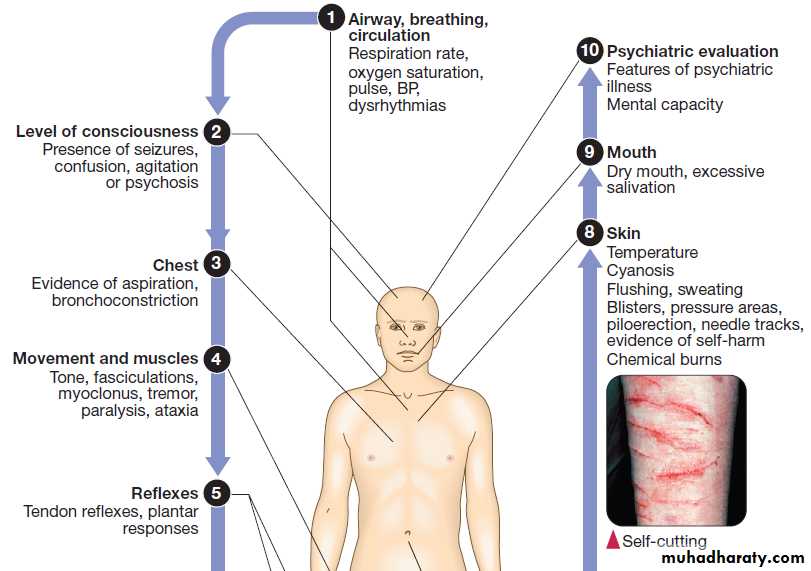
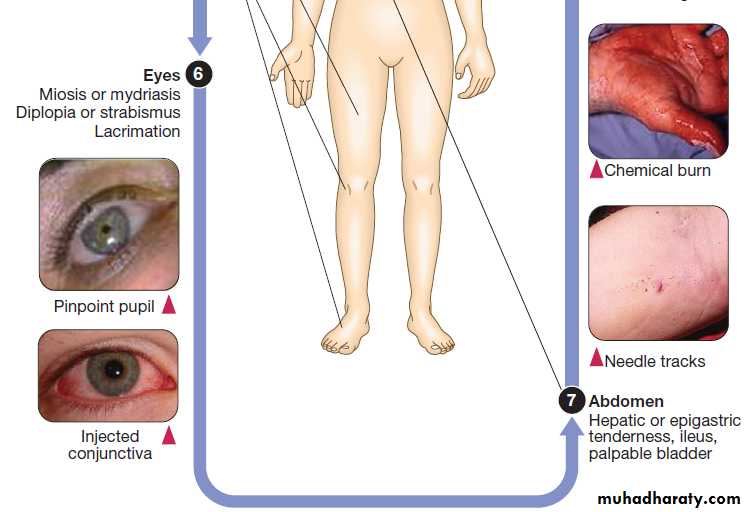
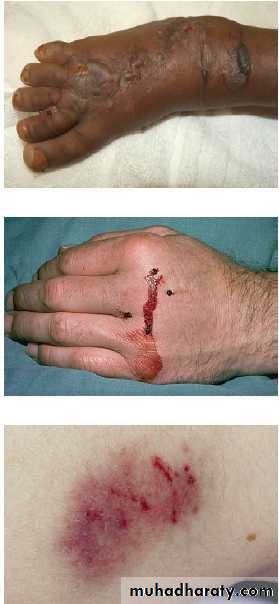
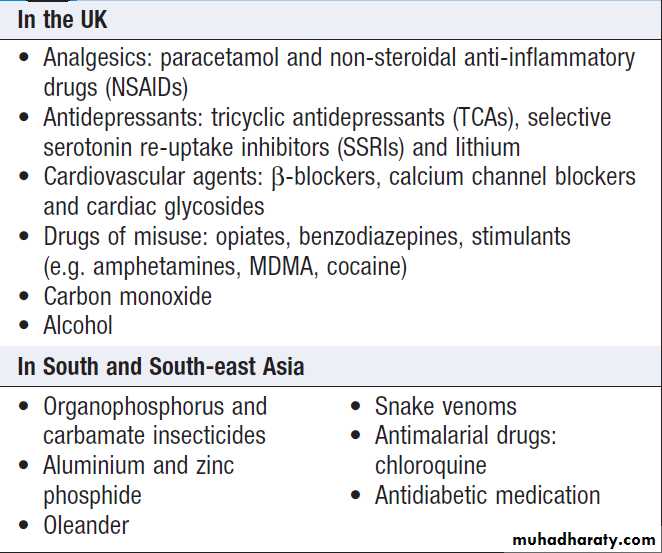
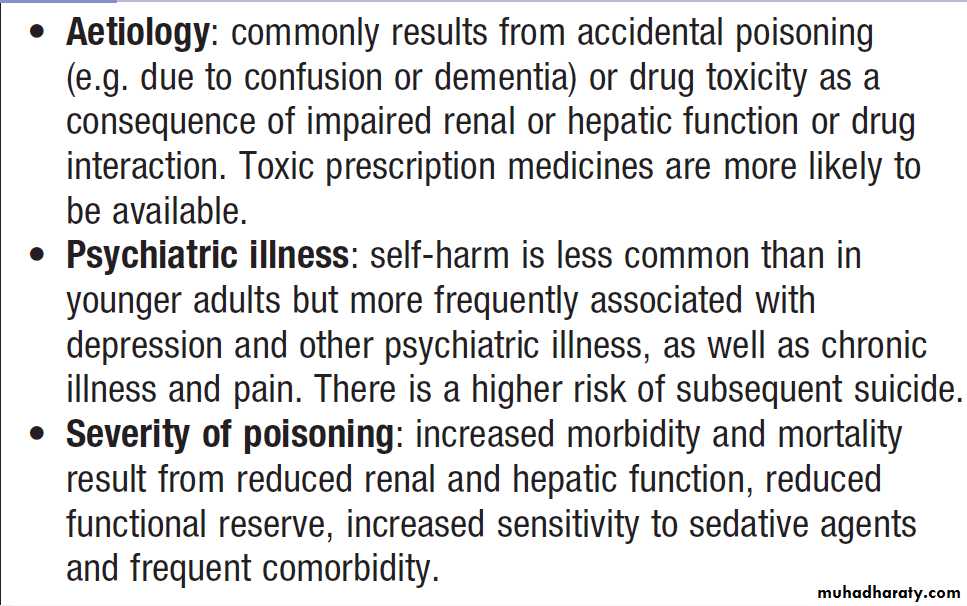
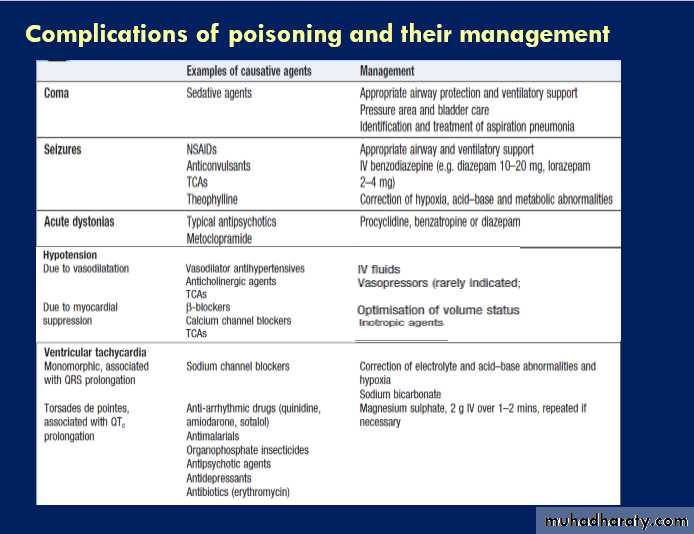
Clinical signs of poisoning by pharmaceutical agents and drugs of misuse.
Bites showing puncture marks, blistering,
bruising and bleeding.Taking a history in envenoming
• When was the patient exposed to a bite/sting?• Was the organism causing it seen and what did it look like (size, colour)?
• What were the circumstances (on land, in water etc.)?
• Was there more than one bite/ sting?
• What first aid was used, when and for how long?
• What symptoms has the patient had (local and systemic)?
• Are there symptoms suggesting systemic envenoming (paralysis, myolysis, coagulopathy etc.)?
• Past medical history and medications?
• Past exposure to antivenom/ venom and allergies?
Acute poisoning is common, accounting for about 1% of
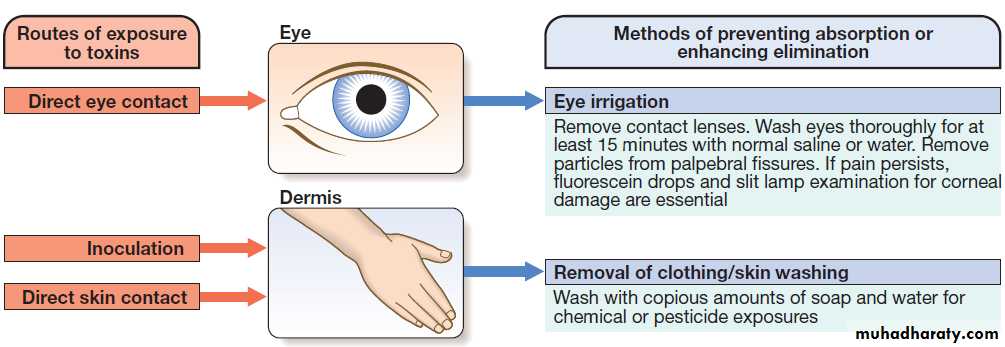
hospital admissions in the UK. In developed countries, the most frequent cause is intentional in the context of self-harm and usually involves ‘over-the-counter’ medicines. Accidental poisoning is also common, especially in children and the elderly (Box). Toxicity also may occur as a result of alcohol or recreational substance use, or following occupational or environmental exposure. Poisoning is a major cause of death in young adults, but most deaths occur before patients reach medical attention, and mortality is much <1% in those admitted to hospital. In developing countries, the frequency of self-harm is more difficult to estimate. Household and agricultural products, such as pesticides and herbicides, are common sources of poisoning and are associated with a much higher case fatality.Important substances involved in poisoning
Poisoning in old age
GENERAL APPROACH TO THE POISONED PATIENTA general approach is shown above.
Triage and resuscitation
Patients who are seriously poisoned must be identified
early so that appropriate management is not delayed.
Triage involves:
• immediate assessment of vital signs
• identifying the poison(s) involved and obtaining
adequate information about them
• identifying patients at risk of further attempts at
self-harm and removing any remaining hazards. Those with possible external contamination with chemical or environmental toxins should undergo appropriate
decontamination .Critically ill patients must be resuscitated.
The Glasgow Coma Scale (GCS) is commonly
employed to assess conscious level, although it has not
been specifically validated in poisoned patients. The
AVPU (alert/verbal/painful/unresponsive) scale is also
a rapid and simple method. An electrocardiogram (ECG)
should be performed and cardiac monitoring instituted
in all patients with cardiovascular features or where
exposure to potentially cardiotoxic substances is suspected.
Patients who may need antidotes should be
weighed when this is feasible, so that appropriate
weight-related doses can be prescribed.
Substances that are unlikely to be toxic in humans
should be identified so that inappropriate admission
and intervention are avoided.
Substances of very low toxicity
Methods of external decontamination.
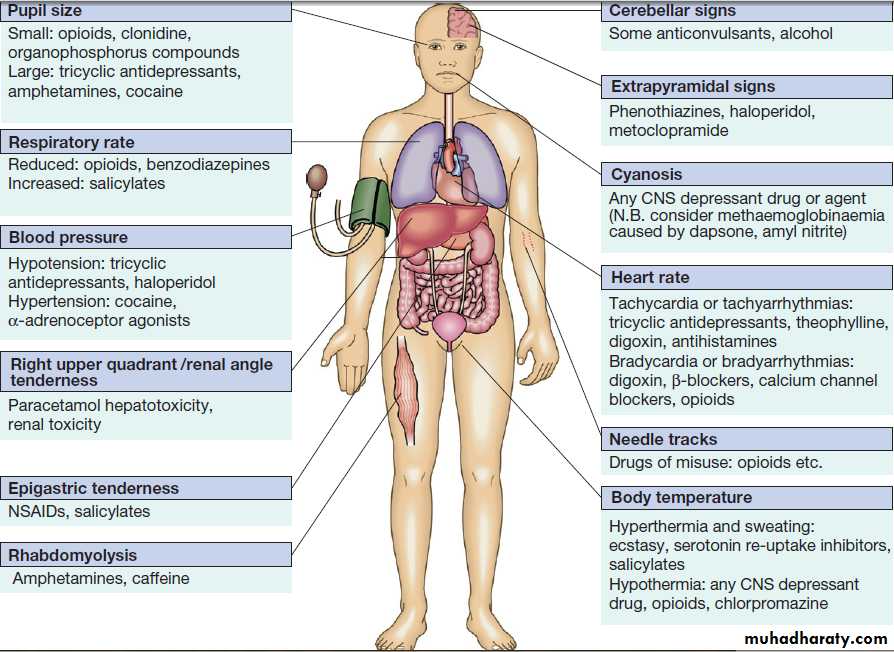
Clinical assessment and investigationsHistory and examination described above.Occasionally, patients may be unaware or confused about what they have taken, or may exaggerate (or less commonly underestimate) the size of the overdose, but rarely mislead medical staff deliberately. In regions of the world where self-poisoning is illegal, patients may be reticent about giving a history. Toxic causes of abnormal physical signs are shown above.The patient may have a cluster of clinical
features (‘toxidrome’) suggestive of poisoning with a
particular drug type, e.g. anticholinergic, serotoninergic,
stimulant, sedative, opioid or cholinergic feature clusters.
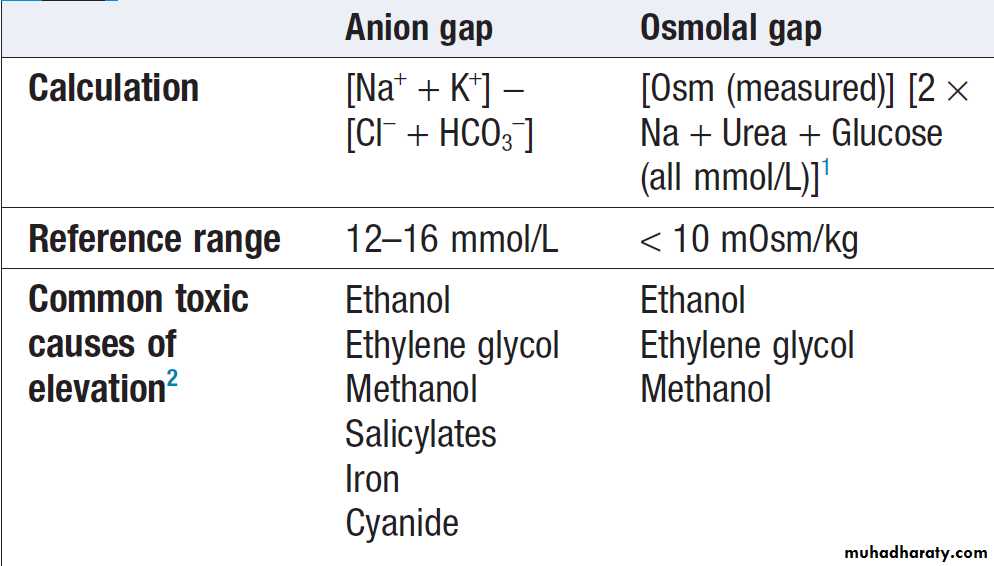
Poisoning is a common cause of coma, especially in younger people, but it is important to exclude other potential causes unless the aetiology is certain. Urea, electrolytes and creatinine should be measured in all patients with suspected systemic poisoning. Arterial blood gases should be checked in those with significant respiratory or circulatory compromise, or when poisoning with substances likely to affect acid–base status is suspected .Calculation of anion and osmolar gaps may help to inform diagnosis and management . For a limited number of specific substances, management may be facilitated by measurement of the amount of toxin in the blood . Qualitative urine screens
for potential toxins, including near-patient testing kits,
have a limited clinical role.
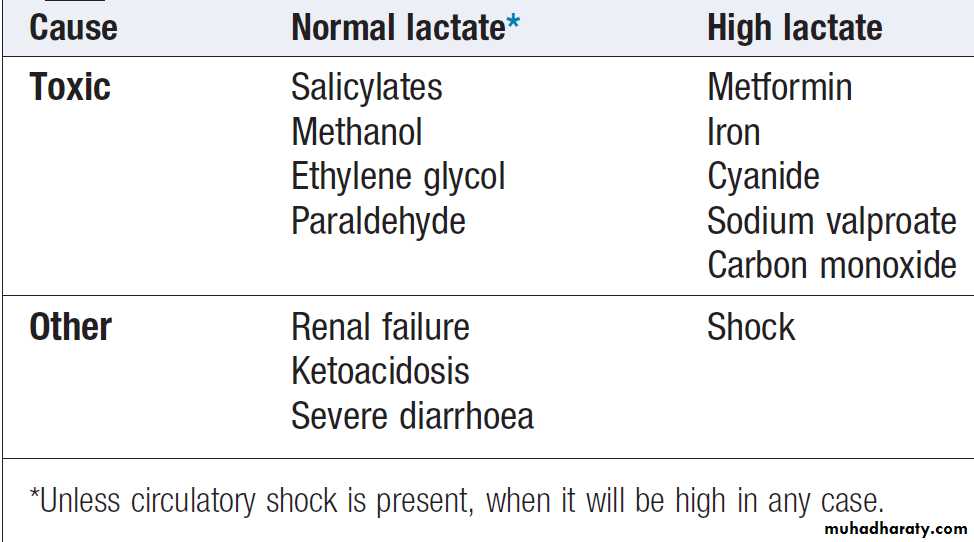
Causes of acidosis in the poisoned patient
Anion and osmolal gaps in poisoning
Laboratory analysis in poisoning
Organophosphates• Plasma cholinesterase is reduced more rapidly but is less specific than red cell cholinesterase
• Antidote use should not be delayed pending results
Carboxyhaemoglobin
• > 20% indicates significant carbon monoxide exposure
Digoxin
• Therapeutic range usually 1–2 ng/mL (1.28–2.46 mmol/L)
• Concentrations > 4 ng/mL (5.12 mmol/L) usually associated
with toxicity, especially with chronic poisoning
Ethanol
• Toxicity at concentrations > 1.8 g/L
Iron
• Take sample ≥ 4 hrs after overdose or if clinical signs of toxicity
• Concentrations > 5 mg/L suggest severe toxicity
Lithium
• Take sample ≥ 6 hrs after overdose or if clinical signs oftoxicity
• Usual therapeutic range 0.4–1.0 mmol/L
Methaemoglobin
• Poisoning with nitrites, benzocaine, dapsone, chloroquine and
aniline dyes is associated with methaemoglobinaemia
• Concentrations > 20% may require treatment with
methylthioninium chloride (methylene blue)
Paracetamol
• Take sample ≥ 4 hrs after overdose
• Use nomogram to determine need for antidotal treatment
Salicylate
• Take sample ≥ 2 hrs (symptomatic patients) or 4 hrs
(asymptomatic patients) after overdose
• Concentrations > 500 mg/L suggest serious toxicity
• Repeat after 2 hrs if severe toxicity is suspected
Theophylline
• Take sample ≥ 4 hrs after overdose or if clinical signs of
toxicity
• Repeat after 2 hrs if severe toxicity is suspected
• Concentrations > 60 mg/L suggest severe toxicity
Psychiatric assessment
All patients presenting with deliberate drug overdoseshould undergo psychiatric evaluation .This should take place once the patient has recovered from any features of poisoning.
General management
Patients presenting with eye/skin contamination should
undergo local decontamination procedures .
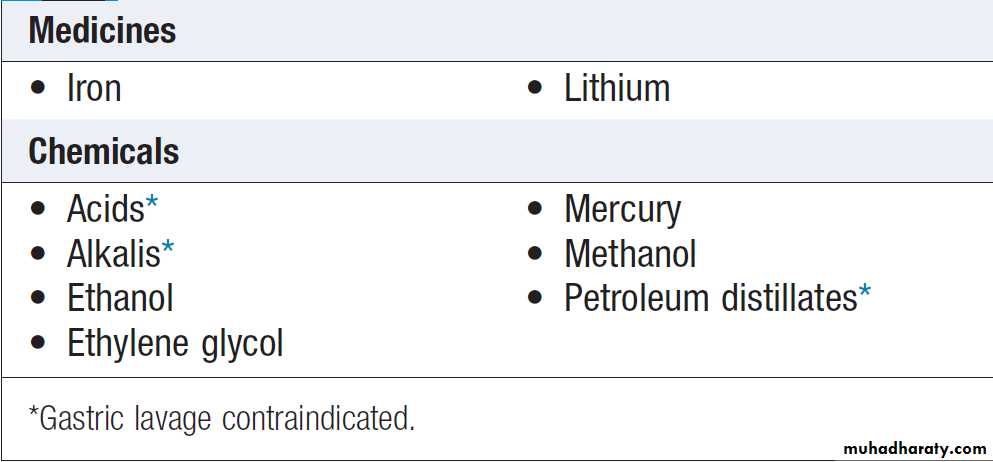
Gastrointestinal decontamination
Patients who have ingested potentially life-threatening
quantities of toxins may be considered for gastrointestinal
decontamination if poisoning has been recent (Box ).
Induction of emesis using ipecacuanha is no longer
recommended.
Activated charcoal
Given orally as slurry, activated charcoal absorbs toxins
in the bowel as a result of its large surface area. If given
sufficiently early, it can prevent absorption of an important
proportion of the ingested dose of toxin. Efficacy
decreases with time and current guidelines do not advocate
use more than 1 hour after overdose in most circumstances.
However, use after a longer interval may be reasonable when a delayed-release preparation has been taken
or when gastric emptying may be delayed.
Some toxins do not bind to activated charcoal so it will not affect their absorption.
In patients with impaired swallowing or a reduced level of consciousness, activated charcoal, even via a nasogastric tube, carries a risk of aspiration pneumonitis, which can be reduced (but not eliminated) by protecting the airway with a cuffed endotracheal tube.
Multiple doses of oral activated charcoal (50 g 6 times
daily in an adult) may enhance the elimination of some
drugs at any time after poisoning and are recommended
for serious poisoning with some substances .
This interrupts enterohepatic circulation or reduces the concentration of free drug in the gut lumen, to the extent that drug diffuses from the blood back into the bowel to be absorbed on to the charcoal: so-called ‘gastrointestinal dialysis’.
A laxative is generally given with the charcoal to reduce the risk of constipation or intestinal obstruction by charcoal ‘briquette’ formation in the gut lumen.
Evidence suggests that single or multiple doses of
activated charcoal do not improve clinical outcomes
after poisoning with pesticides or oleander.
Gastric aspiration and lavage
Gastric aspiration and/or lavage is very infrequently
indicated in acute poisoning, as it is no more effective
than activated charcoal and complications are common,
especially aspiration.
Use may be justified for lifethreatening overdoses of some substances that are not absorbed by activated charcoal.
Use of gastric decontamination methods
Substances poorly adsorbed by activated charcoal
Whole bowel irrigationThis is occasionally indicated to enhance the elimination
of ingested packets of illicit drugs or slow-release tablets
such as iron and lithium that are not absorbed by activated charcoal. It involves the administration of large
quantities of osmotically balanced polyethylene glycol
and electrolyte solution (1–2 L/hr for an adult), usually
by a nasogastric tube, until the rectal effluent is clear.
Contraindications include inadequate airway protection,
haemodynamic instability, gastrointestinal haemorrhage,
obstruction or ileus. Whole bowel irrigation
may precipitate nausea and vomiting, abdominal pain
and electrolyte disturbances.
Urinary alkalinisation
Urinary excretion of weak acids and bases is affected by
urinary pH, which changes the extent to which they are
ionised. Highly ionised molecules pass poorly through
lipid membranes and therefore little tubular reabsorption
occurs and urinary excretion is increased. If the
urine is alkalinised (pH > 7.5) by the administration of
sodium bicarbonate (e.g. 1.5 L of 1.26% sodium bicarbonate over 2 hrs), weak acids (e.g. salicylates, methotrexate and the herbicides 2,4-dichlorophenoxyacetic acid and mecoprop) are highly ionised, resulting in enhanced urinary excretion.
Urinary alkalinisation is currently recommended for
patients with clinically significant salicylate poisoningwhen the criteria for haemodialysis are not met (see
below). It is also sometimes used for poisoning with
methotrexate.
Complications include alkalaemia, hypokalaemia and occasionally alkalotic tetany.
Hypocalcaemia may occur but is rare.
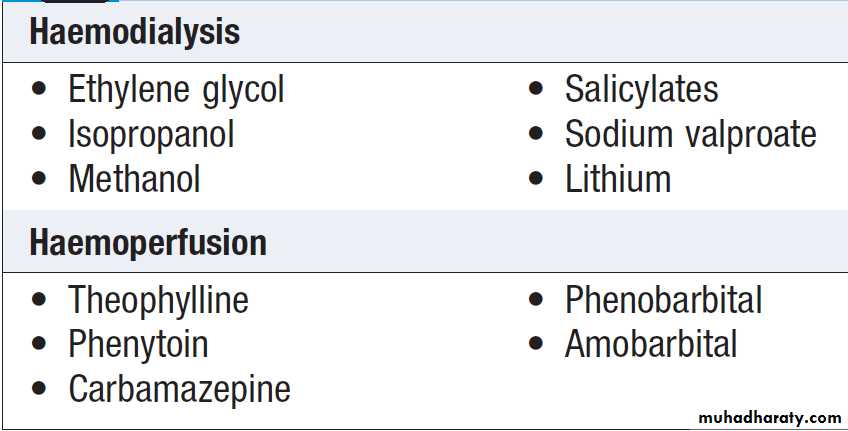
Haemodialysis and haemoperfusion
These techniques can enhance the elimination of poisonsthat have a small volume of distribution and a long half-life
after overdose, and are appropriate when poisoning
is sufficiently severe to justify invasive elimination
methods. The toxin must be small enough to cross the
dialysis membrane (haemodialysis) or must bind to activated charcoal (haemoperfusion) . Haemodialysis
may also correct acid–base and metabolic disturbances
associated with poisoning.
Poisons effectively eliminated by haemodialysis or haemoperfusion
Lipid emulsion therapyLipid emulsion therapy, or ‘lipid rescue’, is being used
increasingly for the management of poisoning with
lipid-soluble agents, such as local anaesthetics, tricyclic
antidepressants, calcium channel blockers and lipid-soluble
β-blockers such as propranolol. It involves intravenous infusion of 20% lipid emulsion (e.g. Intralipid
®) at an initial dose of 1.5 mL/kg, followed by a
continued infusion of 0.25 mL/kg/min until there is
clinical improvement. It is thought that lipid-soluble
toxins partition into the intravenous lipid, reducing
target tissue concentrations.
The elevated myocardial concentration of free fatty acid induced by Intralipid administration may also have beneficial effects on myocardial metabolism and performance by counteracting the inhibition of myocardial fatty acid oxidation produced by local anaesthetics and some other cardiotoxins.
This reverses cardiac depression by enabling increased
ATP synthesis and energy production. Animal studies
have suggested efficacy and case reports of use in human
poisoning have also been encouraging, with recovery of
circulatory collapse reported in cases where other treatment modalities have been unsuccessful. No controlled
trials of this technique have been performed, however,
and as a result, its efficacy remains uncertain.
Supportive care
For most poisons, antidotes and methods to accelerate
elimination are inappropriate, unavailable or incompletely
effective. Outcome is dependent on appropriate
nursing and supportive care, and on treatment of complications.
Patients should be monitored carefully
until the effects of any toxins have dissipated.
Antidotes
Antidotes are available for some poisons and work by avariety of mechanisms: for example, by specific antagonism
(isoprenaline for β-blockers), chelation (desferrioxamine
for iron) or reduction (methylene blue for dapsone).
The use of some antidotes is described in the
management of specific poisons below.
POISONING BY SPECIFIC PHARMACEUTICAL AGENTS
AnalgesicsParacetamol
Paracetamol (acetaminophen) is the drug most commonly
used in overdose in the UK. Toxicity results from
formation of an intermediate reactive metabolite that
binds covalently to cellular proteins, causing cell death.
This results in hepatic and occasionally renal failure. In
therapeutic doses, the toxic intermediate metabolite is
detoxified in reactions requiring glutathione, but in
overdose, glutathione reserves become exhausted.
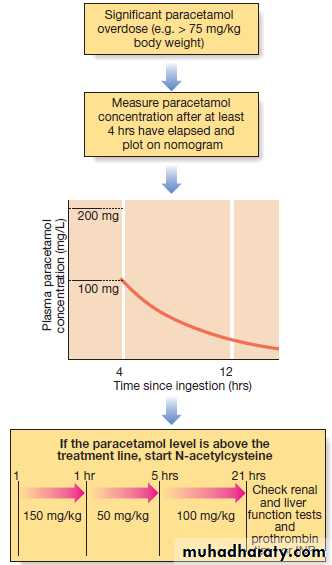
Management
Management is summarised in Figure . Activated charcoal may be used in patients presenting within 1 hour. Antidotes for paracetamol act by replenishing hepatic glutathione and should be administered to all patients with paracetamol concentrations above the ‘treatment line’ provided on paracetamol poisoning nomograms. Acetylcysteine given IV (orally in some countries) is highly efficacious if administered within 8 hours of the overdose. However, since efficacy declines thereafter, administration should not be delayed in patients presenting after 8 hours to await a paracetamol blood concentration result. The antidote can be stopped if the paracetamol concentration is shown to be below the nomogram treatment line.
The most important adverse effect of acetylcysteine
is related to dose-related histamine release, the ‘anaphylactoid’ reaction, which causes itching and urticaria, and in occasional severe cases, bronchospasm and hypotension. Most cases can be managed by temporary discontinuation of acetylcysteine and administration of an antihistamine.An alternative antidote is methionine 2.5 g orally (adult dose) every 4 hours to a total of 4 doses, but less effective, especially after delayed presentation.
If a patient presents >15 hours after ingestion, liver function tests, prothrombin time (or INR), renal function tests and a venous bicarbonate should be measured, the antidote started, and a poisons information centre or local liver unit contacted for advice if results are abnormal.
An arterial blood gas sample should be taken in patients
with severe liver function abnormalities; metabolic acidosis
indicates severe poisoning. Liver transplantation should be considered in individuals who develop life-threatening liver failure due to paracetamol poisoning.
If multiple ingestions of paracetamol have taken place over several hours or days (i.e. a staggered overdose),
acetylcysteine may be indicated. Recommended
thresholds for treatment vary between countries.
The management of a paracetamol overdose.
Salicylates (aspirin)Clinical features
Salicylate overdose commonly causes nausea, vomiting,
sweating, tinnitus and deafness. Direct stimulation of
the respiratory centre produces hyperventilation and respiratory alkalosis. Peripheral vasodilatation with bounding pulses and profuse sweating occurs in moderately
severe poisoning. Serious salicylate poisoning is associated
with metabolic acidosis, hypoprothrombinaemia,
hyperglycaemia, hyperpyrexia, renal failure, pulmonary
oedema, shock and cerebral oedema. Agitation, confusion,
coma and fits may occur, especially in children.
Toxicity is enhanced by acidosis, which increases salicylate
transfer across the blood–brain barrier.
Management
Activated charcoal should be administered if the patient
presents within 1 hour. Multiple doses of activated charcoal
may enhance salicylate elimination but currently
are not routinely recommended.
The plasma salicylate concentration should be measured
at least 2 (in symptomatic patients) or 4 hours
(asymptomatic patients) after overdose and repeated
in suspected serious poisoning, since concentrations
may continue to rise some hours after overdose. In
adults, concentrations above 500 mg/L and 700 mg/L
suggest serious and life-threatening poisoning respectively,
although clinical status is more important than
the salicylate concentration in assessing severity.
Dehydration should be corrected carefully, as there is
a risk of pulmonary oedema, and metabolic acidosisshould be identified and treated with intravenous
sodium bicarbonate (8.4%), once plasma potassium has
been corrected. Urinary alkalinisation is indicated for
adults with salicylate concentrations above 500 mg/L.
Haemodialysis is very effective at removing salicylate
and correcting acid–base and fluid balance
abnormalities, and should be considered when serum
concentrations are above 700 mg/L in adults with severe
toxic features, or when there is renal failure, pulmonary
oedema, coma, convulsions or refractory acidosis.
Non-steroidal anti-inflammatory drugs
Clinical features
Overdose of most NSAIDs usually causes little more than minor abdominal discomfort, vomiting and/or diarrhoea,
but convulsions can occur occasionally, especially with
mefenamic acid. Coma, prolonged seizures, apnoea,
liver dysfunction and renal failure can occur after but are rare. Features of toxicity are unlikely to develop in patients who are asymptomatic >6 hours after overdose.
Management
Electrolytes, liver function tests and a full blood count
should be checked in all but the most trivial cases. Activated
charcoal may be given if the patient presents sufficiently
early. Symptomatic treatment for nausea and
gastrointestinal irritation may be necessary.
Antidepressants
Tricyclic antidepressants (TCAs)are used frequently in overdose and carry a high morbidity and mortality relating to their sodium channel-blocking, anticholinergic and α-adrenoceptor-blocking effects.
Clinical features
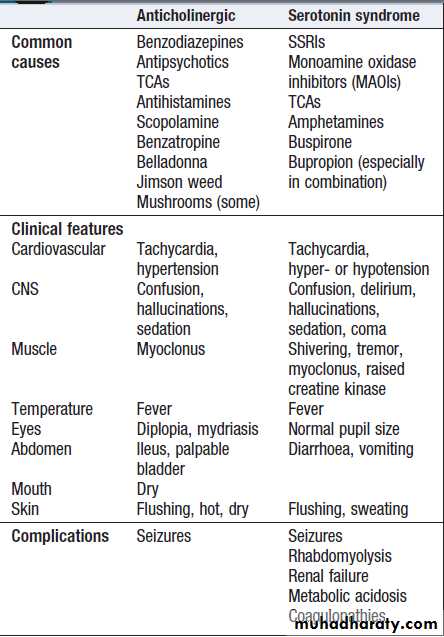
Anticholinergic effects are common .Life-threatening
complications are frequent, including convulsions,
coma, arrhythmias (ventricular tachycardia,
ventricular fibrillation and, less commonly, heart block)
and hypotension, which results from inappropriate
vasodilatation or impaired myocardial contractility.
Serious complications appear to be more common with
dosulepin and amitriptyline.
Anticholinergic and serotonergic
feature clustersManagement
Activated charcoal should be administered if the patientpresents within 1 hour. All patients with possible TCA
overdose should have a 12-lead ECG and ongoing
cardiac monitoring for at least 6 hours. Prolongation
of the QRS interval (especially if > 0.16 s) indicates
severe sodium channel blockade and is associated with
an increased risk of arrhythmia . QT interval
prolongation may also occur. Arterial blood gases
should be measured in suspected severe poisoning.
In patients with arrhythmias, significant QRS or QT
prolongation or acidosis, intravenous sodium bicarbonate(50 mL of 8.4% solution) should be administered
and repeated to correct pH. The correction of the acidosis
and the sodium loading that result is often associated
with rapid improvement in ECG features and arrhythmias.
Hypoxia and electrolyte abnormalities should
also be corrected. Anti-arrhythmic drugs should only
be given on specialist advice. Prolonged convulsions
should be treated with intravenous benzodiazepines.
There is anecdotal evidence of benefit from lipid emulsion therapy in severe intractable poisoning.
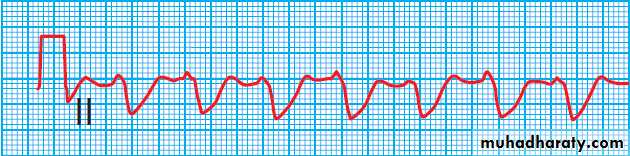
ECG in severe tricyclic antidepressant poisoning. This rhythm strip shows a broad QRS complex due to impaired conduction.
Selective serotonin and noradrenaline re-uptake inhibitors
Selective serotonin re-uptake inhibitors (SSRIs) are agroup of antidepressants that include fluoxetine, paroxetine,
fluvoxamine, sertraline, citalopram and escitalopram.
They are increasingly used to treat depression,
partly because they are less toxic in overdose than TCAs.
A related group of compounds termed serotonin–
noradrenaline reuptake inhibitors (SNRIs), such as venlafaxine and duloxetine, are also in common use and are sometimes taken in overdose.
Clinical features and management
Overdose of SSRIs may produce nausea and vomiting,tremor, insomnia and sinus tachycardia. Agitation,
drowsiness and convulsions occur infrequently and may
be delayed for several hours after ingestion. Occasionally,
features of serotonin syndrome may develop , especially if SSRIs are taken in combination or with other serotonergic agents. Cardiac arrhythmias occur infrequently and most patients require supportive care only.
The toxic effects of SNRIs are similar but tachycardia, hypertension or hypotension and ECG changes (QRS and QT prolongation) may be more prominent and hypoglycaemia can also occur.
Lithium
Severe lithium toxicity is uncommon after intentional
overdose and is more often encountered in patients
taking therapeutic doses as the result of interactions
with drugs such as diuretics or NSAIDs that can cause
dehydration or renal impairment, or because an excessive
dose has been prescribed. Severe toxicity is more
common with acute overdose in patients taking chronic
therapy (‘acute on chronic’ poisoning).
Clinical features
Nausea, diarrhoea, polyuria, dizziness ,tremor ,muscular weakness, drowsiness, confusion, myoclonus, fasciculations, choreoathetosis and renal failure. Coma, convulsions, ataxia, cardiac dysrhythmias, blood pressure disturbances and renal failure may all occur in severe poisoning.
Management
Activated charcoal is ineffective. Gastric lavage is of theoretical benefit if used early after overdose, but lithium tablets are likely to remain intact in the stomach and may be too large for aspiration via a lavage tube.Some advocate whole bowel irrigation after substantialoverdose but efficacy is unknown. Lithium concentrations should be measured immediately in symptomatic patients or after at least 6 hours in asymptomatic patients following acute overdose. Adequate hydration should be maintained with intravenous fluids.
Convulsions should be treated as in Box .In patients with features suggesting severe toxicity associated with high lithium concentrations (e.g. > 4.0 mmol/L after chronic or ‘acute on chronic’ poisoning, or > 7.5 mmol/L after acute poisoning), haemodialysis should be considered.
Lithium concentrations are reduced substantially during dialysis but rebound increases occur after discontinuation, and multiple sessions may be required.
Cardiovascular medications Beta-adrenoceptor blockers
These have negative inotropic and chronotropic effects.Some have additional properties that may increase toxicity,
such as blockade of sodium channels with propranolol,
acebutolol and carvedilol, and blockade of
potassium channels with sotalol.
Clinical features
The major features of toxicity are bradycardia and hypotension. Heart block, pulmonary oedema and cardiogenic shock occur in severe poisoning. Beta-blockers
with sodium channel-blocking effects may cause seizures,
confusion and coma, while sotalol may be associated
with repolarisation abnormalities (including QTc
prolongation) and torsades de pointes.
Management
Intravenous fluids may reverse hypotension but care
is required to avoid pulmonary oedema. Bradycardia
and hypotension may respond to high doses of atropine
(up to 3 mg in an adult). The adrenoceptor agonist isoprenaline may also be effective but high doses are
often needed. Glucagon (5–10 mg over 10 mins, then
1–5 mg/hr by infusion), which counteracts the effect
of β-blockers by stimulating intracellular production of
cyclic adenosine monophosphate (cAMP), is now more
commonly used. In severe cases, ‘hyperinsulinaemia
euglycaemic therapy’ has been used, as described under
calcium channel blockers. Lipid emulsion therapy may
have a role in severe poisoning with lipid-soluble agents
such as propranolol, carvedilol and oxprenolol.
Calcium channel blockers
Calcium channel blockers are highly toxic in overdosebecause of their inhibitory effects on L-type calcium
channels. Dihydropyridines, affect vascular smooth muscle in particular, resulting in vasodilatation, whereas diltiazem and verapamil, have predominantly cardiac effects, including bradycardia and reduced myocardial contractility. Clinical features
The usual presentation is with hypotension due to
vasodilatation or myocardial depression. Bradycardias
and heart block may also occur, especially with verapamil
and diltiazem. Gastrointestinal disturbances,
confusion, metabolic acidosis, hyperglycaemia and
hyperkalaemia may also be present.
Management
Hypotension should be corrected with IV fluids, taking care to avoid pulmonary oedema. Persistent hypotension may respond to IV calcium gluconate (10 mg IV over 5 mins, repeated as required). Isoprenaline and glucagon may also be useful.
Successful use of IV insulin with glucose (10–20% dextrose with insulin initially at 0.5–2.0 U/kg/hr, increasing to 5–10 U/kg/hr according to clinical response), so-called ‘hyperinsulinaemia euglycaemic therapy’, has been reported in patients unresponsive to other strategies.The mechanism of action remains to be fully elucidated, but in shock state, myocardial metabolism switches from use of free fatty acids to glucose. C C blocker poisoning is also associated with hypoinsulinaemia and insulin resistance, impeding glucose uptake by myocytes.
High doses of insulin inhibit lipolysis and increase glucose uptake and the efficiency of glucose utilisation.
Cardiac pacing may be needed for severe unresponsive bradycardias or heart block.
Lipid emulsion therapy has been used in severe
poisoning with apparent benefit, although evidence is
largely anecdotal.
Digoxin and oleander
Poisoning with digoxin is usually accidental, arisingfrom prescription of an excessive dose, impairment of
renal function or drug interactions.
Clinical features
Characteristic cardiac effects of toxicity are tachyarrhythmias (atrial or ventricular) and bradycardias, with or without atrioventricular block. Ventricular bigeminy is common and atrial tachycardia with evidence of atrioventricular block is highly suggestive of the diagnosis. Severe poisoning is associated with hyperkalaemia. Non-cardiac features include confusion, headache, nausea, vomiting, diarrhoea and (rarely) altered colour vision.
Management
Activated charcoal is commonly administered to patientspresenting within 1 hour of ingestion of an acute overdose,
although evidence of benefit is lacking. Urea, electrolytes
and creatinine should be measured, ECG performed and cardiac monitoring instituted. Hypoxia, hypokalaemia (sometimes associated with concurrent diuretic use), hypomagnesaemia and acidosis increase the risk of arrhythmias and should be corrected. Significant bradycardias may respond to atropine, although temporary pacing is sometimes needed. Ventricular arrhythmias may respond to IV magnesium.If available, digoxin-specific antibody fragments should be administered when there are severe ventricular arrhythmias or unresponsive bradycardias.
Antimalarials
Chloroquine
Chloroquine is highly toxic in overdose and quantities
of 5 g or more of chloroquine base are likely to be fatal
in an adult.
Clinical features
Features of toxicity occur within 1 hour of ingestion and include nausea, vomiting, agitation, drowsiness, hypokalaemia, acidosis, headaches and blurred vision.
Coma, convulsions and hypotension may occur in severe
poisoning. ECG changes indicating conduction and
repolarisation delay (prolonged QRS and QTc intervals)
occur and are associated with VT (including torsades de pointes), ventricular fibrillation and sudden death.
Management
Activated charcoal should be given to all patients presenting within 1 hour of ingestion of chloroquine inamounts greater than 15 mg/kg. Cardiac rhythm should
be monitored and dysrhythmias managed . The arterial pH should be corrected, but hypokalaemia is thought to have a protective effect and should not be corrected in the first 8 hours after poisoning. High-dose diazepam (2 mg/kg body weight IV over 30 mins followed by an infusion of 2 mg/kg/hr)
has been suggested to have a protective effect, especially
if given in the early stages of severe chloroquine poisoning,
but evidence is limited as yet. One controlled trial
did not show beneficial effects on the ECG. Diazepam
therapy requires intubation and mechanical ventilation
to avoid pulmonary aspiration.
Quinine
Quinine salts are widely used for treating malaria and
leg cramps. Deaths have been reported with ingestion of
as little as 1.5 g in an adult and 900 mg in a child.
Clinical features
Features of toxicity include nausea, vomiting, tremor,
tinnitus and deafness. Hypotension, haemolysis, renal
failure, ataxia, convulsions and coma are features of
serious poisoning. Conduction and repolarisation delay
results in prolonged QRS and QTc intervals on the ECG,
and VT (including torsades de pointes), ventricular fibrillation and sudden death may occur.
Quinine-induced retinal photoreceptor cell toxicity
may result in blurred vision and impaired colourperception. This usually develops a few hours after
overdose and progresses to constriction of the visual
field, scotoma and complete blindness associated with
pupillary dilatation and unresponsiveness to light. Fundoscopy may show retinal artery spasm, disc pallor and
retinal oedema. Although visual loss can be permanent,
some degree of recovery, especially of central vision,
often occurs over several weeks.
Management
Multiple-dose activated charcoal should be commenced
in patients who have taken quinine in amounts greater
than 15 mg/kg. Gastric lavage should be considered in
patients who have taken a substantial overdose who
present within 1 hour. All patients should have a 12-lead
ECG and cardiac monitoring, and their urea, electrolytes
and glucose checked. Dysrhythmias, hypotension,
seizures and coma should be managed as outlined in
Box . There are no effective treatments for the visual effects
of quinine. Stellate ganglion block and retrobulbar or
intravenous injections of vasodilators such as nitrates
were previously used but are ineffective, as are haemodialysis and haemoperfusion.
Iron
Overdose with iron can cause severe and sometimesfatal poisoning. The toxicity of individual iron preparations
is related to their elemental iron content.
Clinical features
Early clinical features include gastrointestinal disturbance
with the passage of grey or black stools. Hyperglycaemia
and leucocytosis may occur. Haematemesis,
rectal bleeding, drowsiness, convulsions, coma, metabolic
acidosis and cardiovascular collapse may occur in
severe poisoning. Early symptoms may improve or even resolve within 6–12 hours, but hepatocellular necrosis may develop 12–24 hours after overdose and occasionally progresses to hepatic failure. Gastrointestinal strictures are late complications of iron poisoning.
Management
Gastric lavage may be considered in patients presenting
within 1 hour of life-threatening overdose but efficacy
has not been established. Activated charcoal is ineffective
since iron is not bound. Serum iron concentration should
be measured .The antidote desferrioxamine
chelates iron and should be administered immediately
in patients with severe features, without waiting
for serum iron concentrations to be available. Symptomatic
patients with high serum iron concentrations (e.g.
> 5 mg/L) should also receive desferrioxamine. Desferrioxamine may cause hypotension, allergic reactions and occasionally pulmonary oedema. Otherwise, treatment
is supportive and directed at complications.
Antipsychotic drugs
Often prescribed for patients at high risk of self-harm or suicide, and are commonly encountered in overdose.Clinical features
Drowsiness, tachycardia and hypotension. Anticholinergic features and acute dystonias, such as oculogyric crisis, torticollis and trismus, may occur after overdose with typical antipsychotics like haloperidol or chlorpromazine. QT interval prolongation and torsades de pointes can occur with typical antipsychotics such as thioridazine and haloperidol, and atypical antipsychotics like quetiapine, amisulpride and ziprasidone. Convulsions may occur with both groups.
Management
Activated charcoal may be of benefit if given within 1 hour of overdose. Cardiac monitoring. Management is largely supportive, with treatment directed at complications.
Antidiabetic agents
Antidiabetic agents commonly causing toxicity in overdoseinclude sulphonylureas such as chlorpropamide,
glibenclamide, gliclazide, glipizide and tolbutamide; biguanides like metformin and phenformin; and insulins.
Overdose may also be encountered with some of
the newer antidiabetic drugs, such as thiazolidinediones
(pioglitazone), meglinides (nateglinide, repaglinide) and
dipeptidyl peptidase (DPP)-IV inhibitors (sitagliptin).
Clinical features
Sulphonylureas, meglitinides and parenteral insulin
cause hypoglycaemia when taken in overdose, although
insulin is non-toxic if ingested by mouth. The duration
of hypoglycaemia depends on the half-life or release
characteristics of the preparation and may be prolonged
over several days with long-acting agents such as chlorpropamide, insulin zinc suspension or insulin glargine.
Features of hypoglycaemia include nausea, agitation,
sweating, aggression and behavioural disturbances, confusion, tachycardia, hypothermia, drowsiness, coma or
convulsions . Permanent neurological damage can
occur if hypoglycaemia is prolonged.
Hypoglycaemia can be diagnosed using bedside glucose strips but venous blood should also be sent for laboratory confirmation.
Metformin is uncommonly associated with hypoglycaemia.
Its major toxic effect in overdose is lactic acidosis,
which can have a high mortality, and is particularly
common in older patients and those with renal or hepatic
impairment, or when ethanol is co-ingested. Other features
of metformin overdose are nausea and vomiting,
diarrhoea, abdominal pain, drowsiness, coma, hypotension
and cardiovascular collapse.
There is limited experience of overdose involving
thiazolidinediones and DPP-IV inhibitors, but significant
hypoglycaemia is unlikely.
Management
Activated charcoal should be considered for all patients
who present within 1 hour of ingestion of a substantial
overdose of an oral hypoglycaemic agent. Venous blood
glucose and urea and electrolytes should be measured
and tests repeated regularly. Hypoglycaemia should be
corrected using oral or intravenous glucose (50 mL of
50% dextrose); an infusion of 10–20% dextrose may be
required to prevent recurrence.
Intramuscular glucagon can be used as an alternative, especially if intravenous access is unavailable.
Failure to regain consciousness within a few minutes of normalisation of the blood glucose can indicate that a central nervous system (CNS) depressant has also been ingested, the hypoglycaemia has been prolonged, or that the coma has another cause (e.g. cerebral haemorrhage or oedema).
Arterial blood gases should be taken after metformin
overdose to assess the extent of acidosis. If present,
plasma lactate should be measured and acidosis should
be corrected with intravenous sodium bicarbonate
(250 mL 1.26% solution or 50 mL 8.4% solution, repeated
as necessary). In severe cases, haemodialysis or haemodiafiltration is used.
DRUGS OF MISUSE
CannabisCannabis is derived from the dried leaves and flowers
of Cannabis sativa. When it is smoked, the onset of effect
occurs within 10–30 minutes, whereas after ingestion the onset is 1–3 hours later. The duration of effect is
4–8 hours. Cannabis produces euphoria, perceptual
alterations and conjunctival injection, followed by
enhanced appetite, relaxation and occasionally hypertension, tachycardia, slurred speech and ataxia.
High doses may produce anxiety, confusion, hallucinations and psychosis .
Psychological dependence is common but tolerance and withdrawal symptoms are unusual.
Long-term use is thought to increase the lifetime risk of
developing schizophrenia. Ingestion or smoking of cannabis rarely results in serious poisoning and supportive
treatment is all that is required.
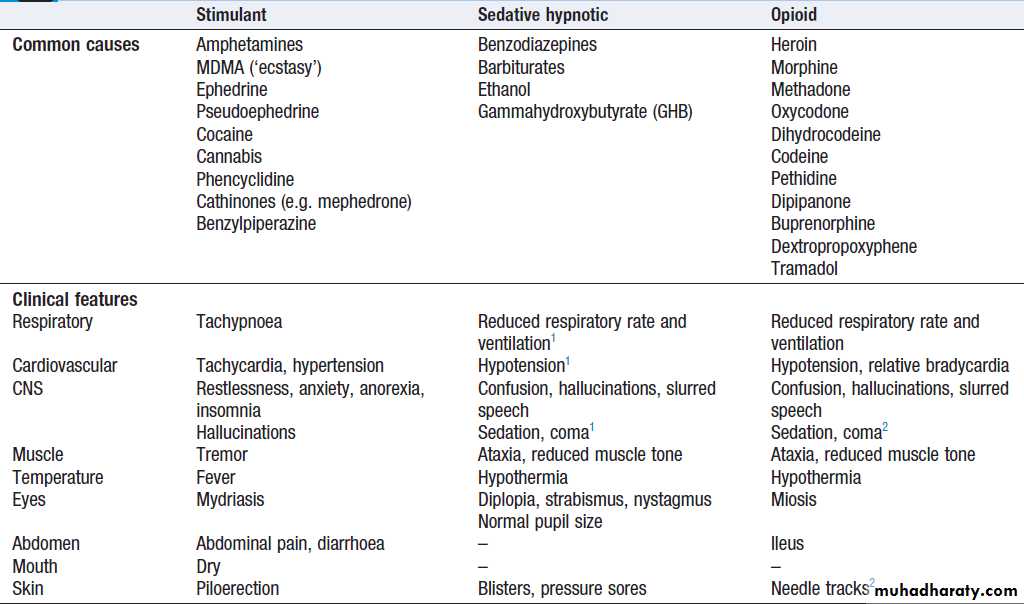
Stimulant, sedative and opioid feature clusters
Stimulant, sedative and opioid feature clusters – cont’d
BenzodiazepinesBenzodiazepines may be prescribed or used illicitly.
They are of low toxicity when taken alone in overdose
but can enhance CNS depression when taken with other
sedative agents, including alcohol. They may also cause
significant toxicity in the elderly and those with chronic
lung or neuromuscular disease.
Clinical features
Clinical features of toxicity include drowsiness, ataxia
and confusion .
Respiratory depression and hypotension may occur with severe poisoning in susceptible groups, especially after intravenous administration of short-acting agents.
Management
Activated charcoal may be useful after ingestion in susceptible patients or after mixed overdose, if given within
1 hour. Conscious level, respiratory rate and oxygen
saturation should be monitored for at least 6 hours after
substantial overdose.
The specific benzodiazepine antagonist flumazenil
increases conscious level in patients with overdose but
carries a risk of seizures, and is contraindicated in
patients co-ingesting proconvulsant agents such as
TCAs and in those with a history of seizures.
Stimulants and entactogens
This group includes amphetamines, ecstasy, cathinonessuch as mephedrone, piperazines and cocaine. These
are sympathomimetic and serotonergic amines; as a
result, they have clinical features of poisoning that overlap
Cocaine
Cocaine is available as a water-soluble hydrochloride
salt suitable for nasal inhalation (‘snorting’), or as an
insoluble free base (‘crack’ cocaine) that, unlike the
hydrochloride salt, vaporises at high temperature and
can be smoked, giving a more rapid and intense effect. Cocaine hydrochloride is usually purchased as a white crystalline powder, and crack cocaine in ‘rocks’.
Clinical features
Effects appear rapidly after inhalation and especially
after smoking. Sympathomimetic stimulant effects are
common . Serious complications usually occur within 3 hours of use and include coronary artery spasm, which may result in myocardial ischaemia or infarction, even in patients with normal coronary arteries.
This may lead to hypotension, cyanosis and
ventricular arrhythmias. Cocaine toxicity should be considered in young adults who present with ischaemic
chest pain. Hyperpyrexia may be associated with rhabdomyolysis, acute renal failure and disseminated intravascular coagulation.
Management
All patients should be observed with ECG monitoringfor a minimum of 4 hours. A 12-lead ECG should be
performed. Abnormalities are common, including ST
segment elevation, which may occur even in the absence
of myocardial infarction. Troponin T estimations are
the most sensitive and specific markers of myocardial
damage. Benzodiazepines and intravenous nitrates are
useful for managing patients with chest pain or hypertension, but β-blockers are best avoided because of the risk of unopposed α-adrenoceptor stimulation. Coronary
angiography should be considered in patients with
myocardial infarction or acute coronary syndromes. Acidosis
should be corrected. Physical cooling measures
may be required for hyperthermia.
Amphetamines and cathinones
These include amphetamine sulphate (‘speed’),
methylamphetamine (‘crystal meth’), 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) and mephedrone. Tolerance is common, leading regular users to
seek progressively higher doses.
Clinical features
Toxic features usually appear within a few minutes
of use and last 4–6 hours, or substantially longer after
a large overdose. Sympathomimetic stimulant and serotonergic effects are common .
A proportion of ecstasy users develop hyponatraemia as
a result of excessive water drinking and inappropriate
antidiuretic hormone secretion. Muscle rigidity, pain
and bruxism (clenching of the jaw) may occur.
Hyperpyrexia, rhabdomyolysis, metabolic acidosis, acute renal failure, disseminated intravascular coagulation, hepatocellular necrosis, acute respiratory distress syndrome
(ARDS) and cardiovascular collapse have all been
described following MDMA use but are rare. Cerebral
infarction and haemorrhage have been reported, especially after intravenous amphetamine use.
Management
Management is supportive and directed at complications.
Gammahydroxybutyrate and gamma butyrolactone
Gamma hydroxybuterate (GHB) and gamma butyrolactone(GBL) are sedative agents with psychedelic and
body-building effects. They are easily manufactured
from commonly available industrial chemicals, including
1,4 butanediol, which is metabolised to GHB in vivo
and has similar effects after ingestion. GHB solution is
drunk by users, who titrate the dose until the desired
effects are achieved.
Clinical features
Toxic features are those of a sedative hypnotic). Nausea, diarrhoea, vertigo, tremor, myoclonus, extrapyramidal signs, euphoria, bradycardia, convulsions, metabolic acidosis, hypokalaemia and hyperglycaemia may also occur. As the drug may be produced in batches and shared amongst a number of individuals, several patients may present with coma at the same time. The sedative effects are potentiated by other CNS depressants, such as alcohol, benzodiazepines, opioids and neuroleptics. Coma usually
resolves spontaneously and abruptly within a few hours
but may occasionally persist for several days. Dependence
may develop in regular users, who may experience severe prolonged withdrawal effects if use is discontinued abruptly.
Management
Activated charcoal is recommended within 1 hour foringestion of GHB in amounts greater than 20 mg/kg.
Urea, electrolytes and glucose should be measured in all
but the most trivial of cases. All patients should be
observed for a minimum of 2 hours, with monitoring of
blood pressure, heart rate, respiratory rate and oxygenation.
Patients who remain symptomatic should be
observed in hospital until symptoms resolve, but require
supportive care only. Withdrawal may require treatment
with high doses of benzodiazepines.
d-Lysergic acid diethyamide
d-Lysergic acid diethylamide (LSD) is a synthetic hallucinogen usually ingested as small squares of impregnated absorbent paper (often printed with a distinctive design) or as ‘microdots’. The drug causes perceptual effects, such as heightened visual awareness of colours, distortion of images, and hallucinations that may bepleasurable or terrifying (‘bad trip’) and associated with
panic, confusion, agitation or aggression. Dilated pupils,
hypertension, pyrexia and metabolic acidosis may occur
and psychosis may sometimes last several days.
Patients with psychotic reactions or CNS depression
should be observed in hospital, preferably in a quiet,
dimly lit room to minimise external stimulation. Where
sedation is required, diazepam is the drug of choice.
Antipsychotics should be avoided if possible, as they
may precipitate cardiovascular collapse or convulsions.
Opioids
Commonly encountered opioids are shown in Box. Toxicity may result from misuse of illicit drugs such as heroin or after overdose of medicinal opiates such as dextropropoxyphene.Intravenous use of heroin or morphine gives a rapid, intensely pleasurable experience, often accompanied by heightened sexual arousal. Physical dependence occurs within a few weeks of regular high-dose injection; as a result, the dose is escalated and the user’s life becomes increasingly centred on obtaining and taking the drug.
Withdrawal, which can start within 12 hours, presents with intense craving, rhinorrhoea, lacrimation, yawning, perspiration, shivering, piloerection, vomiting, diarrhoea and abdominal cramps. Examination reveals tachycardia, hypertension, mydriasis and facial flushing.
Accidental overdose with prescribed strong opioid
preparations is common, especially in the elderly.
Clinical features
These are shown in Box. Needle tracks may be visible in intravenous drug misusers and drug-related paraphernalia may be found amongst their possessions. Severe poisoning results in respiratory depression, hypotension, non-cardiogenic pulmonary oedema and hypothermia, leading to respiratory arrest or aspiration of gastric contents. Dextropropoxyphene (the opioid component of co-proxamol) may cause cardiac conduction effects, particularly QRS prolongation, ventricular arrhythmias and heart block, and has been withdrawn in the UK and other countries. Methadone may cause QTc prolongation and torsades de pointes. Symptoms can be prolonged for up to 48 hours after use of long-acting agents such as methadone, dextropropoxyphene and oxycodone.Management
The airway should be cleared and, if necessary, respiratory support and oxygen given. Oxygen saturation monitoring and measurement of arterial blood gases should be performed.Prompt use of the specific opioid antagonist naloxone (0.4–2 mg IV in an adult, repeated if necessary)
may obviate the need for intubation, although excessive
doses may precipitate acute withdrawal in chronic opiate
users. An infusion may be required in some cases because
the half-life of the antidote is short compared to that of
most opiates, especially those with prolonged elimination.
Patients must be monitored for at least 6 hours after
the last naloxone dose. Other complications of naloxone
therapy include fits and ventricular arrhythmias,
although these are rare. Box describes the management of coma, fits and hypotension.
Noncardiogenic pulmonary oedema does not usually respond to diuretic therapy, and continuous positive airways pressure (CPAP) or positive end-expiratory pressure (PEEP) ventilatory support may be required.
Body packers and body stuffers
Body packers (‘mules’) attempt to smuggle illicit drugs(usually cocaine, heroin or amphetamines) by ingesting
multiple small packages wrapped in several layers of
clingfilm or in condoms. Body stuffers are those who have ingested unpackaged or poorly wrapped substances,
often to avoid arrest. Both groups are at risk of
severe toxicity if the packages rupture. This is more
likely for body stuffers, who may start to develop symptoms
of poisoning within 8 hours of ingestion.
The risk of poisoning depends on the quality of the wrapping, and the amount and type of drug ingested. Cocaine, for example, presents a much higher risk than heroin because of its high toxicity and lack of a specific antidote.
Patients suspected of body packing or stuffing should
be admitted for observation. A careful history taken in
private is important, but for obvious reasons patients
may withhold details of the drugs involved. The mouth,
rectum and vagina should be examined as possible sites for concealed drugs. A urine toxicology screen performed at intervals may provide evidence of leakage, although positive results may reflect earlier recreational drug use.
Packages may be visible on plain abdominal
films but this is not always the case, and ultrasound
and computed tomography (CT) are more sensitive
methods of visualisation. One of these (preferably
CT) should be performed in all suspected body packers.
Antimotility agents are often used by body packers
to prevent premature passage of packages, so it can take
a number of days for packages to pass spontaneously;
during this period the carrier is at risk from package
rupture. Whole bowel irrigation is commonly used to
accelerate passage and is continued until all packages
have passed. Surgery may be required for mechanical
bowel obstruction or when evolving clinical features
suggest package rupture, especially with cocaine.
CHEMICALS AND PESTICIDES
Carbon monoxideCarbon monoxide (CO) is a colourless, odourless gas
produced by faulty appliances burning organic fuels. It
is also present in vehicle exhaust fumes and sometimes
in smoke from house fires.
It causes toxicity by binding with haemoglobin and cytochrome oxidase, which reduces tissue oxygen delivery and inhibits cellular respiration.
It is a common cause of death by poisoning and
most patients who die of CO poisoning do so before
reaching hospital.
Clinical features
Early clinical features of acute severe carbon monoxide
poisoning include headache, nausea, irritability, weakness
and tachypnoea. Because these are non-specific, the
correct diagnosis will not be obvious if the exposure is
occult, such as from a faulty domestic appliance. Subsequently, ataxia, nystagmus, drowsiness and hyperreflexia may develop, progressing to coma, convulsions, hypotension, respiratory depression, cardiovascular collapse and death.
Myocardial ischaemia may occur and results in arrhythmias or myocardial infarction.
Cerebral oedema is common and rhabdomyolysis may lead to myoglobinuria and renal failure. In those who recover from acute toxicity, longer-term neuropsychiatric effects are common, such as personality change, memory loss and concentration impairment. Extrapyramidal effects,
urinary or faecal incontinence, and gait disturbance may
also occur. Poisoning during pregnancy may cause fetal
hypoxia and intrauterine death.
Management
Patients should be removed from exposure as soon aspossible and resuscitated as necessary. Oxygen should
be administered in as high a concentration as possible
via a tightly fitting facemask, as this reduces the half-life
of carboxyhaemoglobin from 4–6 hours to about
40 minutes.
Measurement of carboxyhaemoglobin is useful for confirming exposure but levels do not correlate well with the severity of poisoning, partly because concentrations fall rapidly after removal of the patient from exposure, especially if supplemental oxygen has been given.
An ECG should be performed in all patients with
acute poisoning, especially those with pre-existing heart
disease. Arterial blood gas analysis should be checked
in those with serious poisoning. Oxygen saturation readings
by pulse oximetry are misleading since both carboxyhaemoglobin and oxyhaemoglobin are measured.
Excessive intravenous fluid administration should be
avoided, particularly in the elderly, because of the risk
of pulmonary and cerebral oedema.
Convulsions should be controlled with diazepam.
Hyperbaric oxygen therapy is controversial. At 2.5
atmospheres it reduces the half-life of carboxyhaemoglobinto about 20 minutes and increases the amount
of dissolved oxygen by a factor of 10, but systematic
reviews have shown no improvement in clinical outcomes.
The logistical difficulties of transporting sick
patients to hyperbaric chambers and managing them
therein should not be underestimated.
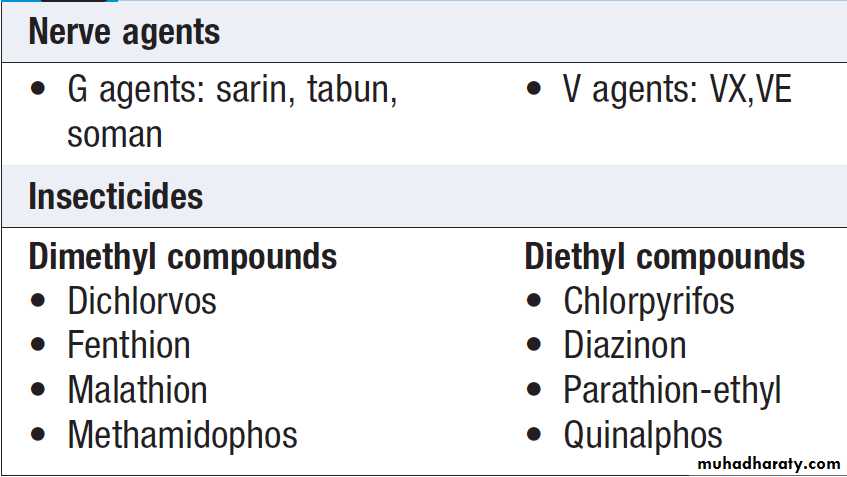
Organophosphorus (OP) insecticides and nerve agents
OP are widely used as pesticides, especially in developingcountries. The case fatality rate following deliberate
ingestion of OP pesticides in developing countries in
Asia is 5–20%. Nerve agents developed for chemical warfare are derived from OP insecticides but are much more toxic. They are commonly classified as G (originally synthesized in Germany) or V (‘venomous’) agents.
The ‘G’ agents, such as tabun, sarin and soman, are volatile, absorbed by inhalation or via the skin, and dissipate rapidly after use. ‘V’ agents, such as VX, are contact poisons unless aerosolised, and contaminate ground for weeks or months. The toxicology and management of nerve agent and pesticide poisoning are similar.
Organophosphorus compounds
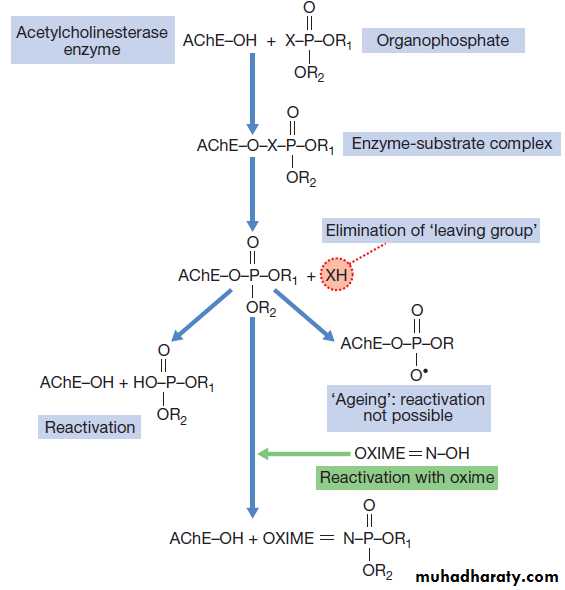
Mechanism of toxicityOP compounds phosphonylate the active site of acetylcholinesterase (AChE), inactivating the enzyme and
leading to the accumulation of acetylcholine (ACh) in
cholinergic synapses . Spontaneous hydrolysis of the OP–enzyme complex allows reactivation of the enzyme. However, loss of a chemical group from the
OP–enzyme complex prevents further enzyme reactivation,
a process termed ‘ageing’. After ageing has taken
place, new enzyme needs to be synthesised before function
can be restored. The rate of ageing is an important
determinant of toxicity and is more rapid with dimethyl
compounds (3.7 hours) than diethyl (31 hours), and
especially rapid after exposure to nerve agents (soman
in particular), which cause ageing within minutes.
Mechanism of toxicity of
organophosphorus compounds and treatment with
Oxime
Clinical features and management
OP poisoning causes an acute cholinergic phase, whichmay occasionally be followed by the intermediate syndrome or organophosphate-induced delayed polyneuropathy (OPIDN).
The onset, severity and duration of poisoning depend on the route of exposure and agent involved.
Cholinergic features may be prolonged over
several weeks with some lipid-soluble agents.
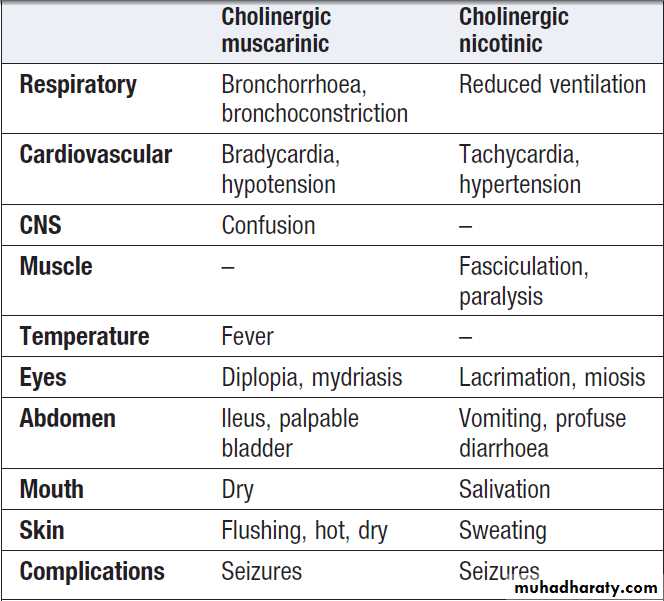
Acute cholinergic syndrome
The acute cholinergic syndrome usually starts within afew minutes of exposure. Nicotinic or muscarinic features
may be present . Vomiting and profuse diarrhoea are typical following oral ingestion. Bronchoconstriction,
bronchorrhoea and salivation may cause
severe respiratory compromise. Miosis is characteristic
and the presence of muscle fasciculations strongly suggests
the diagnosis, although this feature is often absent,
even in serious poisoning. Subsequently, generalised flaccid paralysis which can affect respiratory and ocular muscles and result in respiratory failure. Ataxia, coma and convulsions may occur.
In severe poisoning, cardiac repolarisation abnormalities
and torsades de pointes may occur. Other early
complications of OP poisoning include extrapyramidal
features, pancreatitis, hepatic dysfunction and pyrexia.
Management
The airway should be cleared of excessive secretions,
breathing and circulation assessed, high-flow oxygen administered and intravenous access obtained. In the
event of external contamination, further exposure should
be prevented, contaminated clothing and contact lenses
removed, the skin washed with soap and water, and the
eyes irrigated.
Gastric lavage or activated charcoal may be considered if the patient presents within 1 hour of ingestion.
Convulsions should be treated as described in
Box . The ECG, oxygen saturation, blood gases, temperature, urea and electrolytes, amylase andglucose should be monitored closely.
Early use of sufficient doses of atropine is potentially
life-saving in patients with severe toxicity. Atropine
reverses ACh-induced bronchospasm, bronchorrhoea,
bradycardia and hypotension. When the diagnosis is
uncertain, a marked increase in heart rate associated
with skin flushing after a 1 mg intravenous dose makes
OP poisoning unlikely. In OP poisoning, atropine should
be administered in doses of 0.6–2 mg IV, repeated every
10–25 mins until secretions are controlled, the skin is dry
and there is a sinus tachycardia.
Large doses may be needed but excessive doses may cause anticholinergic effects . In patients requiring atropine, an oxime such as pralidoxime chloride (or obidoxime), if available, should also be administered, as this may reverse or prevent muscle weakness, convulsions or coma, especially if given rapidly after exposure. The pralidoxime dose for an adult is 2 g IV over 4 mins, repeated 4–6 times daily.
Oximes work by reactivating AChE that has not undergone
‘ageing’ and are therefore less effective with dimethyl
compounds and nerve agents, especially soman.
Oximes may provoke hypotension, especially if administered rapidly.
Ventilatory support should be instituted before
the patient develops respiratory failure. Benzodiazepines may be used to reduce agitation and fasciculations,treat convulsions and sedate patients during mechanical ventilation.
Exposure is confirmed by measurement of plasma
(butyrylcholinesterase) or red blood cell cholinesterase
activity. These correlate poorly with the severity of clinical
features, although values are usually less than 10%
in severe poisoning, 20–50% in moderate poisoning and
> 50% in subclinical poisoning.
The acute cholinergic phase usually lasts 48–72 hours,
with most patients requiring intensive cardiorespiratory
support and monitoring.
Cholinergic features in poisoning*
The intermediate syndromeAbout 20% of patients with OP poisoning develop weakness that spreads rapidly from the ocular muscles to
those of the head and neck, proximal limbs and the
muscles of respiration, resulting in ventilatory failure.
This ‘intermediate syndrome’ (IMS) generally develops
quite rapidly between 1 and 4 days after exposure, often
after resolution of the acute cholinergic syndrome, and
may last 2–3 weeks. There is no specific treatment but
supportive care, including maintenance of airway and
ventilation, should be provided if necessary.
Organophosphate-induced delayed polyneuropathy
Organophosphate-induced delayed polyneuropathy(OPIDN) is a rare complication that usually occurs
2–3 weeks after acute exposure. It is a mixed sensory/
motor polyneuropathy, especially affecting long myelinated neurons, and appears to result from inhibition of
enzymes other than AChE. It is a feature of poisoning
with some OPs such as trichlorocresylphosphate, but is
less common with nerve agents,. Early clinical features
are muscle cramps followed by numbness and paraesthesiae, proceeding to flaccid paralysis of the lower and subsequently the upper limbs.
Paralysis of the lower limbs is associated with foot drop and a high-stepping gait, progressing to paraplegia. Paralysis of the arms leads to wrist drop. Sensory loss may also be present but is variable. Initially, tendon reflexes are reduced or lost but mild spasticity may develop later.
There is no specific therapy for OPIDN. Regular
physiotherapy may limit deformity caused by muscle-wasting.
Recovery is often incomplete and may be limited to the hands and feet, although substantial functional recovery after 1–2 years may occur, especially in younger patients.
Carbamate insecticides
Carbamate insecticides inhibit a number of tissue esterases,including AChE. The mechanism of action, clinical
features and management are similar to those of OP
compounds. However, clinical features tend to be less
severe and of shorter duration, because the carbamate–
AChE complex dissociates quickly, with a half-life of
30–40 minutes, and does not undergo ageing. Also, carbamates penetrate the CNS poorly. OPIDN and IMS are
not common features of carbamate poisoning. Pancreatitis
has been reported as a sequel, and deaths have
occurred. Atropine may be given intravenously in frequent
small doses (0.6–2.0 mg IV for an adult) until signs of
atropinisation develop. Diazepam may be used to relieve
anxiety. The use of oximes is unnecessary.
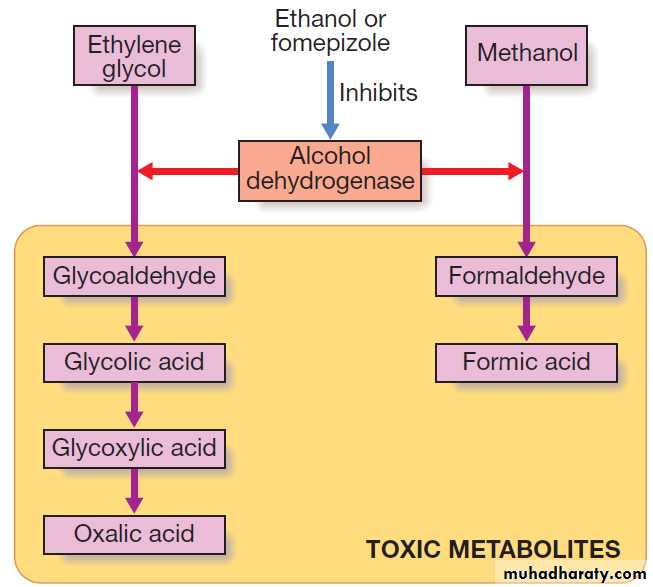
Methanol and ethylene glycol
Ethylene glycol (1,2-ethanediol) is found in antifreeze,brake fluids and, in lower concentrations, windscreen
washes. Methanol is present in some antifreeze products
and commercially available industrial solvents, and in
low concentrations in some screen washes and methylated
spirits. It may also be an adulterant of illicitly produced
alcohol. Both are rapidly absorbed after ingestion.
Although methanol and ethylene glycol are not of high
intrinsic toxicity, they are converted via alcohol dehydrogenase to toxic metabolites that are largely responsible for their clinical effects.
Metabolism of methanol and ethylene glycol.
Clinical featuresEarly features with either methanol or ethylene glycol
include ataxia, drowsiness, dysarthria and nystagmus,
often associated with vomiting. As the toxic metabolites
are formed, metabolic acidosis, tachypnoea, coma and
seizures may develop.
Toxic effects of ethylene glycol include ophthalmoplegia,
cranial nerve palsies, hyporeflexia and myoclonus.
Renal pain and acute tubular necrosis occur
because of precipitation of calcium oxalate in the
kidneys. Hypocalcaemia, hypomagnesaemia and hyperkalaemia are common.
Features of methanol poisoning include headache,
confusion and vertigo. Visual impairment and photophobiadevelop, associated with optic disc and retinal
oedema and impaired pupil reflexes. Blindness may be
permanent, although some recovery may occur over
several months. Pancreatitis and abnormal liver function
have also been reported.
Management
Urea, electrolytes, chloride, bicarbonate, glucose,
calcium, magnesium, albumin and plasma osmolarity
and arterial blood gases should be measured in all patients with suspected methanol or ethylene glycol toxicity. The osmolal and anion gaps should be calculated.
Initially, poisoning is associated with an increased osmolar gap, but as toxic metabolites are produced, an increased anion gap associated with metabolic acidosis will develop. The diagnosis can be confirmed by measurement of ethylene glycol or methanol concentrations, but assays are not widely available.
An antidote, either ethanol or fomepizole, should be
administered to all patients with suspected significantexposure while awaiting the results of laboratory investigations.
These block alcohol dehydrogenase and delay
the formation of toxic metabolites until the parent
drug is eliminated in the urine or by dialysis. The
antidote should be continued until ethylene glycol or
methanol concentrations are undetectable. Metabolic
acidosis should be corrected with sodium bicarbonate
(e.g. 250 mL of 1.26% solution, repeated as necessary).
Convulsions should be treated with an intravenous
benzodiazepine.
In ethylene glycol poisoning, hypocalcaemia should only be corrected if there are severe ECG features or seizures occur, since this may increase calcium oxalate crystal formation.
Haemodialysis or haemodiafiltration should be used
in severe poisoning, especially if renal failure is present
or there is visual loss in the context of methanol poisoning.
It should be continued until acute toxic features are
no longer present and ethylene glycol or methanol concentrations are no longer detectable.
Aluminium and zinc phosphide
These rodenticides and fumigants are a common means
of self-poisoning in northern India. The mortality rate
for aluminium phosphide ingestion has been estimated
at 60%; zinc phosphide ingestion appears less toxic, at
about 2%. When ingested, both compounds react with
gastric acid to form phosphine, a potent pulmonary and
gastrointestinal toxicant. Clinical features include severe
gastrointestinal disturbances, chest tightness, cough and
breathlessness progressing to ARDS and respiratory
failure, tremor, paraesthesiae, convulsions, coma, tachycardia, metabolic acidosis, electrolyte disturbances,
hypoglycaemia, myocarditis, liver and renal failure, and
leucopenia. Just a few tablets can be fatal.
Detection of phosphine in the exhaled air or stomach
aspirate using either a silver nitrate-impregnated stripor specific phosphine detector tube is diagnostic, but gas
chromatography provides the most sensitive indicator.
Treatment is supportive and directed at correcting electrolyte abnormalities and treating complications; there
is no specific antidote. Early gastric lavage is sometimes
used, often with vegetable oil to reduce the release of
toxic phosphine, but the benefit is uncertain.
ENVIRONMENTAL POISONING AND ILLNESS
Arsenism
Chronic arsenic exposure from drinking water has been reported in many countries, especially India, Bangladesh, Nepal, Thailand, Taiwan, China, Mexico and South America, where a large proportion of the drinking water (ground water) has a high arsenic content, placing large population groups at risk. The WHO guideline value for arsenic content in tube well water is 10 μg/L.
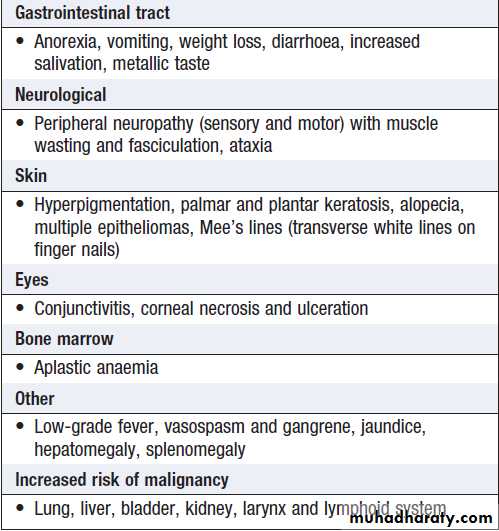
Health effects associated with chronic exposure to
arsenic in drinking water are shown in Box.
In exposed individuals, high concentrations of arsenic are
present in bone, hair and nails. Specific treatments areof no benefit in chronic arsenic toxicity and recovery
from the peripheral neuropathy may never be complete.
The emphasis should be on the prevention of exposure
to arsenic in drinking water.
Clinical features of chronic
arsenic poisoningFluorosis
Fluoride poisoning can result either from exposure toexcessive quantities of fluoride (> 10 ppm) in drinking
water or from industrial exposure to fluoride dust and
consumption of brick teas. Clinical features include
yellow staining and pitting of permanent teeth, osteosclerosis, soft tissue calcification, deformities (e.g.
kyphosis) and joint ankylosis.
Changes in the bones of the thoracic cage may lead to rigidity that causes dyspnoea on exertion. Very high doses of fluoride may cause abdominal pain, nausea, vomiting, seizures and muscle spasm. In calcium-deficient children, the toxic effects of fluoride manifest even at marginally high exposures to Fluoride.
In endemic areas, such as Jordan, Turkey, Chile,
India, Bangladesh, China and Tibet, fluorosis is a major
public health problem. The maximum impact is seen in
communities engaged in physically strenuous agricultural
or industrial activities. Dental fluorosis is endemic
in East Africa and some West African countries.
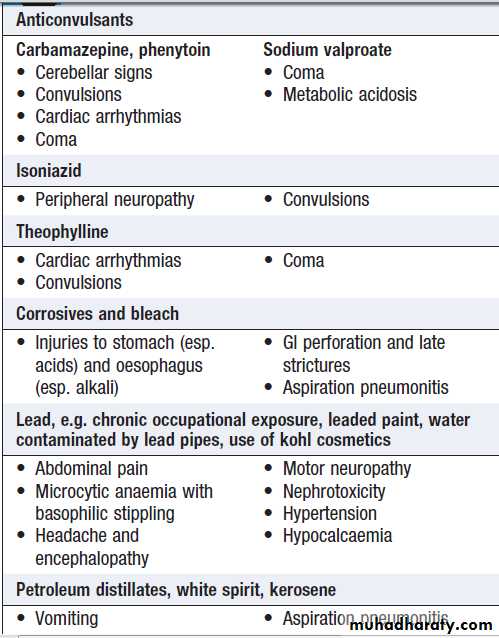
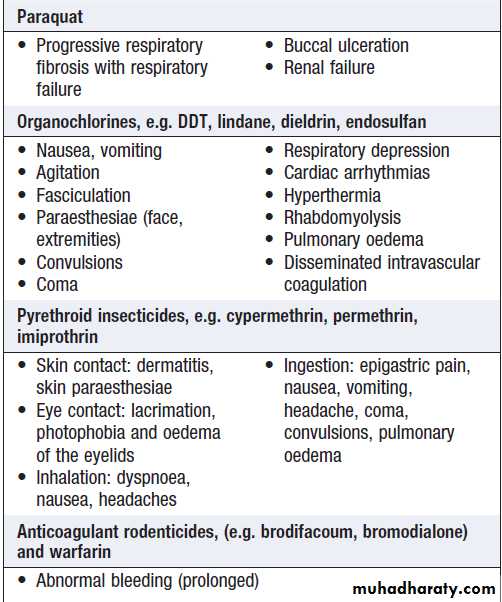
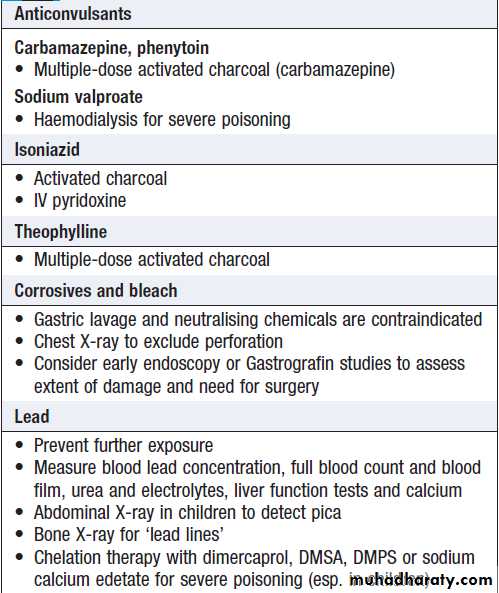
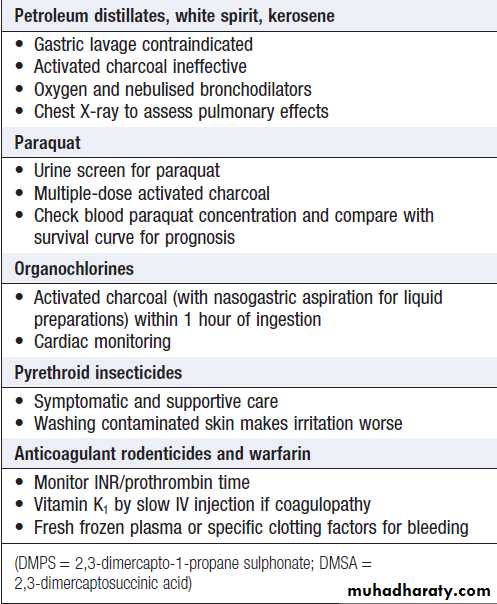
SUBSTANCES LESS COMMONLY TAKEN IN OVERDOSE
Boxes give an overview of the clinical features and management for some substances that are less often encountered in overdose.Clinical features associated with substances
taken less commonly in overdoseClinical features associated with substances
taken less commonly in overdose – cont’dSpecific management of poisoning by
substances taken less commonly in overdoseSpecific management of poisoning by
substances taken less commonly in overdose – cont’dENVENOMING
Envenoming occurs when a venomous animal injectssufficient venom by a bite or a sting into a prey item or
perceived predator to cause deleterious local and/or
systemic effects. Venomous animals generally use their
venom to acquire and in some cases predigest prey,
with defensive use a secondary function. Accidental
encounters between venomous animals and humans are frequent, particularly in the rural tropics, where millions
of cases of venomous bites and stings occur annually.
Globally, an increasing number of exotic venomous
animals are kept privately, so cases of envenoming may
present to hospitals where doctors have insufficient
knowledge to manage potentially complex presentations.
Doctors everywhere should thus be aware of the
basic principles of management of envenoming and how
to seek expert support.
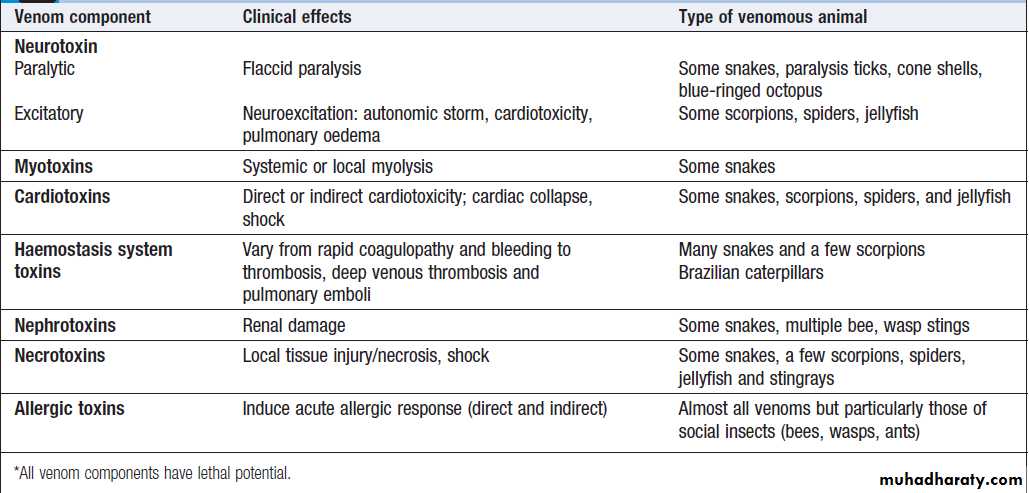
Venom
Venom is a complex mixture of diverse components,often with several separate toxins that can cause adverse
effects in humans, and each potentially capable of
multiple effects . Venom is produced at considerable
metabolic cost, so is used sparingly; thus only
some bites/stings by venomous animals result in significant
envenoming, the remainder being ‘dry bites’.
The concept of dry bites is important in understanding
approaches to management.
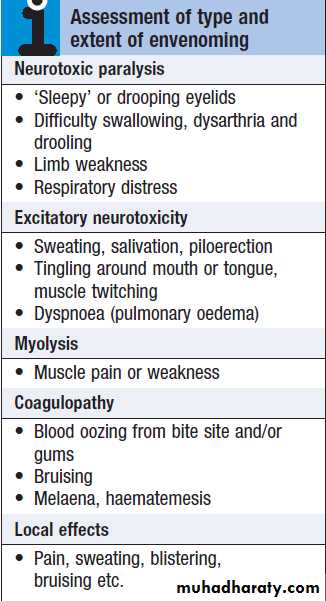
Key venom effects*
Venomous animals
There are many animal groups that contain venomousspecies .The epidemiology estimates shown
reflect the importance of snakes and scorpions as
causes of severe or lethal envenoming. Social insect
stings from bees and wasps may also cause lethal anaphylaxis. Other venomous animals may commonly
envenom humans but cause mostly non-lethal effects.
A few rarely envenom humans, but have a high potential
for severe or lethal envenoming. These include
box jellyfish, cone shells, blue-ringed octopus, paralysis
ticks and Australian funnel web spiders. Within any
given group, particularly snakes, there may be a wide
range of clinical presentations.
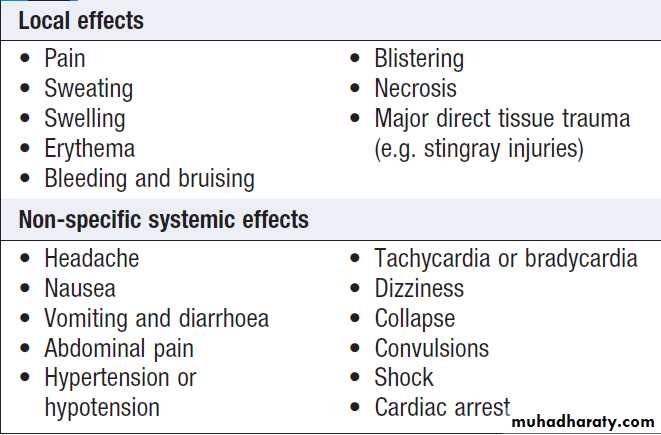
Clinical effects
With the exception of dry bites where no significanteffects occur, venomous bites/stings can result in three
broad classes of effect.
Local effects
These vary from trivial to severe .There may be minimal or no local effects with some snakebites (not even pain), yet lethal systemic envenoming may still be present. For other species, local effects predominate over systemic, and for some species such as snakes, both are important.
General systemic effects
By definition, these are non-specific .Shock is an important complication of major local envenoming by some snake species and, if inadequately treated, can prove lethal, especially in children.
Specific systemic effects
These are important in both diagnosis and treatment.
• Neurotoxic flaccid paralysis can develop very rapidly,
progressing from mild weakness to full respiratory
paralysis in less than 30 minutes (blue-ringed
octopus bite, cone shell sting), or may develop far
more slowly, over hours (some snakes) to days
(paralysis tick). For neurotoxic snakes, the cranial
nerves are usually involved first, with ptosis a
common initial sign . From this, paralysis
may extend to the limbs, with weakness and loss of
deep tendon reflexes, then respiratory paralysis.
• Excitatory neurotoxins cause an ‘autonomic storm’,
with profuse sweating, variable cardiac effects andcardiac failure, sometimes with pulmonary oedema
(notably Australian funnel web spider bite, some
scorpions such as Indian red scorpion). This type of
envenoming can be rapidly fatal (many scorpions,
funnel web spiders), or may cause distressing
symptoms but constitute a lesser risk of death
(widow spiders, banana spiders).
• Myotoxicity can initially be silent, then present with
generalised muscle pain, tenderness, myoglobinuria
and huge rises in serum creatine kinase (CK).
Secondary renal failure can precipitate potentially
lethal hyperkalaemic cardiotoxicity.
• Cardiotoxicity is often secondary, but symptoms and
signs are non-specific in most cases.
• Haemostasis system toxins cause a variety of effects,
depending on the type of toxin, and the specific
features can be diagnostic. Coagulopathy may
present as bruising and bleeding from the bite site,
gums and intravenous sites. Surgical interventions
are high-risk in such cases.
Other venoms cause thrombosis, usually presenting as deep venous thrombosis (DVT), pulmonary embolus or stroke
(particularly Caribbean/Martinique vipers).
• Renal damage in envenoming is mostly secondary,
although some species such as Russell’s vipers can
cause primary renal damage. The presentation is
similar in both cases, with changes in urine output
(polyuria, oliguria or anuria) or rises in creatinine
and urea. In cases with intravascular haemolysis,
secondary renal damage is likely.
The clinical effects of specific animals in different regions of the world are shown in Boxes.
Local and systemic effects of envenoming
ManagementIt is important to determine an accurate diagnosis and
the degree of risk, so that severe and potentially lethal
cases are identified quickly and managed as a priority.
With correct care, even severe cases are treatable, but
delays in initiating effective treatment can severely compromise outcome. Expert advice should thus be sought
at the earliest opportunity.
First aid
Pre-hospital first aid can be critical in major envenoming. It depends on the type of envenoming, butthe key principles are to:
• support vital systems
• delay or prevent the onset of envenoming
• avoid harmful ‘treatments’ such as electric shock,
cut and suck, tourniquets, and cryotherapy in
snakebite.
Many preventable deaths occur prior to hospital
transfer when ineffective cardiorespiratory resuscitation
is given to patients with respiratory paralysis or cardiac
arrest/failure, which can occur due to either primary
envenoming or an anaphylactic reaction.
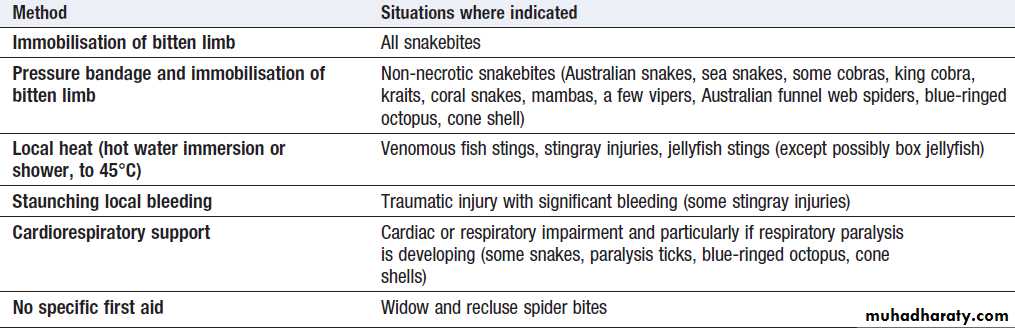
First aid for envenoming
DiagnosisEnvenoming is usually obvious but might not be
on some occasions. Humans may be bitten or stung by
an unseen organism, or may not be aware of a bite or
sting having occurred at all. In such cases the patient
may present with a variety of symptoms but with no
linking history to indicate envenoming. Accordingly,
envenoming should be considered as a possible diagnosis in cases of unexplained paralysis, myotoxicity,
coagulopathy, nephrotoxicity, cardiotoxicity, pulmonary
oedema, necrosis, collapse and convulsions.
History, examination and laboratory findings help
to confirm or exclude a diagnosis of envenoming andto determine its extent. It is also important to obtain
a description of the organism if possible. Multiple
bites or stings are more likely to cause major envenoming.
Ask for specific symptoms and search for specific
signs that may indicate the type and extent of envenoming Specific tests for venom are currently only commercially
available for Australian snakebite but are likely
to be developed for snakebite in other regions.
They are not available for other types of envenoming, where venom concentrations are low. For snakebite, a screen
for envenoming includes full blood count, coagulation
screen, urea and electrolytes, creatinine, CK and ECG.
Lung function tests, peripheral oximetry or arterial
blood gases may be indicated in cases with potential or
established respiratory failure. In areas without access
to routine laboratory tests, the whole-blood clotting
time (using a glass test tube) is a valuable test for
coagulopathy.
A derivative of this, the 20-minute wholeblood clotting test is useful (a few millilitres of venous blood are placed in a glass vessel and checked for clotting at 20 minutes).
If patients state that they have been bitten by a
particular species, ensure this is accurate. Privatekeepers of venomous animals may not have accurate knowledge of what they are keeping, and misidentification
of a snake, scorpion or spider can have dire consequences
if the wrong antivenom is used.
Treatment
Envenoming is managed on two levels, which must bedelivered in tandem:
• supportive management of the organ systems
affected and of the whole patient
• treating the effects with specific treatments/
antidotes (usually antivenom).
For a snakebite by a potentially lethal species such as
Russell’s viper, the patient might have local effects with
oedema, blistering, necrosis, and resultant fluid shifts
causing shock, and at the same time have systemic
effects such as intractable vomiting, coagulopathy,
paralysis and secondary renal failure.
Specific treatment with antivenom will be required to reverse the coagulopathy, and may prevent worsening of the paralysis and reduce the vomiting, but will not greatly
affect the local tissue damage or the renal failure or
shock. The latter will require intravenous fluid therapy,
possibly respiratory support, renal dialysis and local
wound care, possibly including antibiotics.
Each animal will cause a particular pattern
of envenoming, requiring a tailored response.
Pulse, blood pressure, pulse oximetry and
urine output should be monitored in all cases.
Antivenom
This is the most important tool in treating envenoming.It is made by hyperimmunising an animal, usually
horses, to produce antibodies against venom. Once
refined, these bind to venom toxins and render them
inactive or allow their rapid clearance. Antivenom is
only available for certain venomous animals and cannot
reverse all types of envenoming. With a few exceptions,
it should be given intravenously, with adrenaline
(epinephrine) ready in case of anaphylaxis. It should
only be used when clearly indicated, and indications
will vary between animals.
It is critical that the correct antivenom is used at the appropriate dose.
Doses vary widely between antivenoms; those recommended for North American antivenoms are not applicable to those elsewhere.
In some situations (such as the Indian subcontinent), pre-treatment with subcutaneous adrenaline may reduce the chance of anaphylaxis to antivenom.
Antivenom can sometimes reverse post-synaptic
neurotoxic paralysis (α-bungarotoxin-like neurotoxins)
but will not usually reverse established pre-synaptic
paralysis (β-bungarotoxin-like neurotoxins), so should
be given before major paralysis has occurred.
Coagulopathy is best reversed by antivenom, but even after all venom is neutralised, there may be a delay of hours before normal coagulation is restored.
More antivenom should not be given because coagulopathy has failed to normalise fully in the first 1–3 hours (except in very particular circumstances). Thrombocytopenia may persist for days, despite antivenom. The role of antivenom in reversing established myolysis and renal failure is uncertain. Antivenom may help limit local
tissue effects/injury in the bitten limb, but this is quite
variable and time-dependent.
Neuroexcitatory envenoming can respond very well to antivenom (Australian funnel web spider bites; Mexican, South American, Indian scorpion stings), but there is controversy about the effectiveness of antivenom for some species (some North African and Middle Eastern scorpions). The role of antivenom in limiting local venom effects, including necrosis, is also controversial; it is most likely to be effective when given early.
All patients receiving antivenom are at risk of both
early and late adverse reactions, including anaphylaxis
(early; not always IgE-related) and serum sickness (late).
Other treatments
Anticholinesterases are used as an adjunctive treatmentfor post-synaptic paralysis.
Prazosin (an α-adrenoceptor antagonist) is used in
the management of hypertension or pulmonary oedema
in scorpion sting cardiotoxicity, particularly for Indian red scorpion stings, though antivenom is now the preferred
treatment.
Antibiotics are not routinely required for most bites/
stings, though a few animals regularly cause significant
wound infection/abscess, such as some South American
pit vipers and stingrays.
Tetanus is a risk in some bites or stings, such as snakebite, but intramuscular toxoid should not be given until any coagulopathy is reversed.
Mechanical ventilation is vital for established
respiratory paralysis that will not reverse with antivenom,
and may be required for prolonged periods – up
to several months in some cases.
Follow-up
Cases with significant envenoming and those receiving
antivenom should be followed up to ensure that any
complications have resolved and to identify any delayed
envenoming.