Page 1 of 18
lec-5
Medicine
د
.
حسن
Diabetes mellitus
Is a heterogeneous group of metabolic diseases that are characterized by chronic
hyperglycemia and disturbances in carbohydrate, lipid, and protein metabolism resulting
from defects in insulin secretion and/or insulin action, which can leads to serious
complications.
Insulin is an anabolic hormone with profound effects on the metabolism of carbohydrate,
fat and protein.
Insulin is secreted from pancreatic β cells into the portal circulation, with a brisk increase
in response to a rise in blood glucose.
Endocrine function of pancreas
A(α) cells produce glucagon; Glucagon is a catabolic hormone. It mobilizes glucose, fatty
acids and amino acids from stores into the blood.
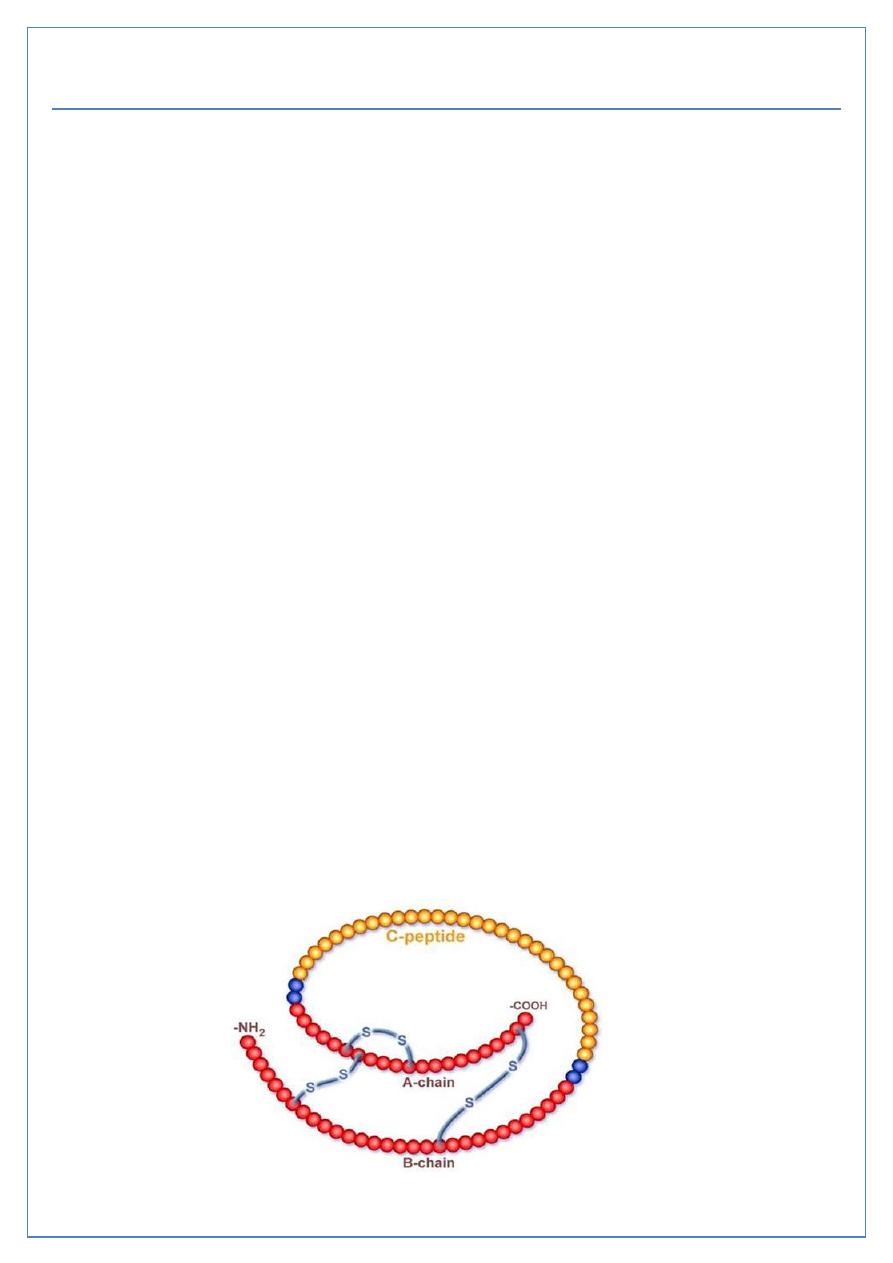
B(β) cells produce insulin; Insulin is an anabolic hormone, that increases the storage of
glucose, fatty acids and amino acids in cells and tissues. Composed of 2 polypeptide chains
linked by disulphide bridges. Secreted as proinsulin and transformed to active form, insulin,
by cleaveage of C peptide by protease enzymes.
D(δ) cells produce somatostatin; Somatostatin may regulate, locally, the secretion of the
other pancreatic hormones.
PP cells produce pancreatic polypeptide; still uncertain although the hormone may
influence gastrointestinal function and promote intra-islet homeostasis.
Page 2 of 18
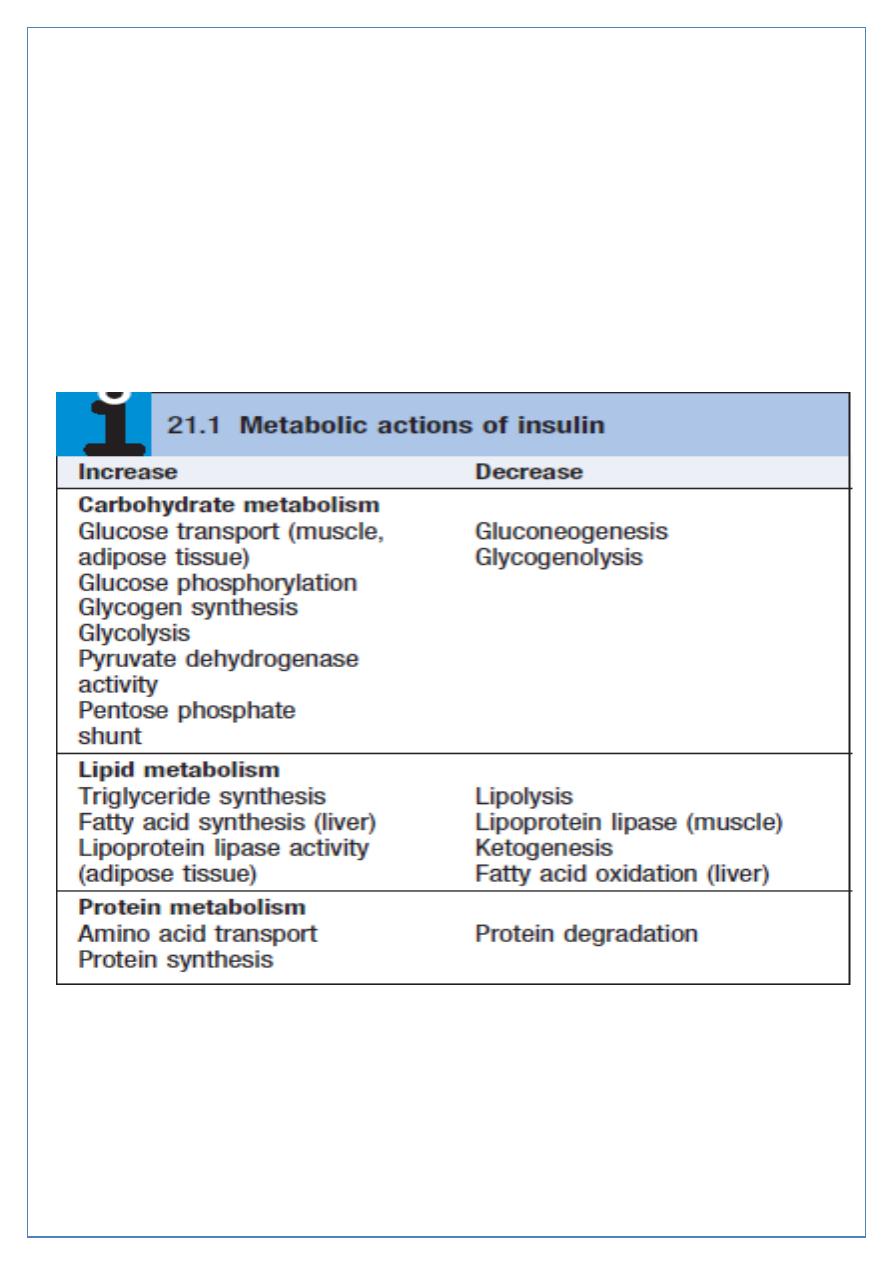
Some characteristics of normal insulin secretion are shown in the table (next slide). Insulin
lowers blood glucose by suppressing hepatic glucose production and stimulating glucose
uptake in skeletal muscle and fat, mediated by the glucose transporter, GLUT 4.
Insulin stimulates lipogenesis and inhibits lipolysis, so preventing fat catabolism.
Partial oxidation of FFAs in the liver provides energy to drive gluconeogenesis and also
produces ketone bodies (acetoacetate, which can be reduced to 3-hydroxybutyrate or
decarboxylated to acetone) which are generated in hepatocyte mitochondria.
Page 3 of 18
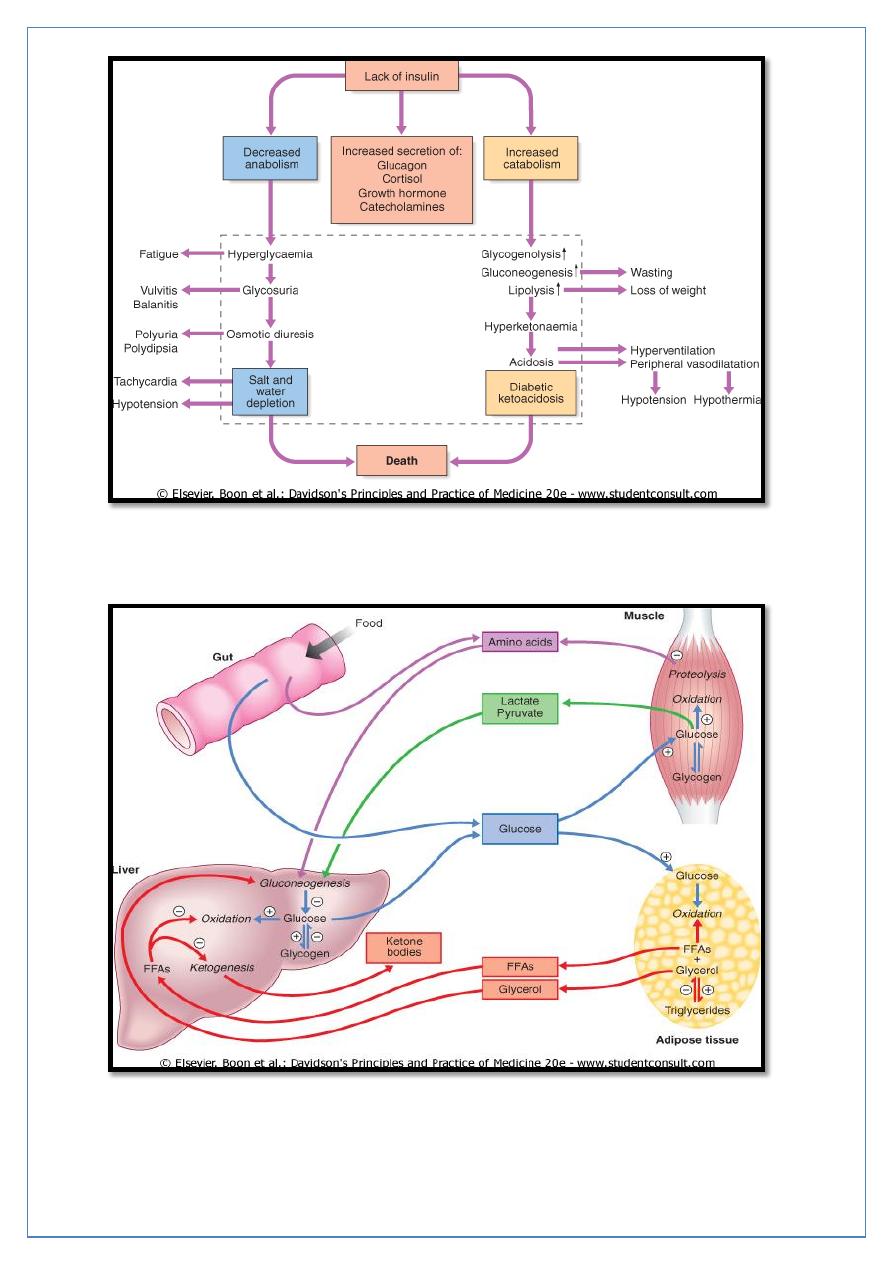
Insulin deficiency can lead to hyperglycemia and many metabolic derangements which
occur mainly in type 1 DM that can leads to death if not treated correctly.
Major metabolic pathways of fuel metabolism and the actions of insulin
Page 4 of 18
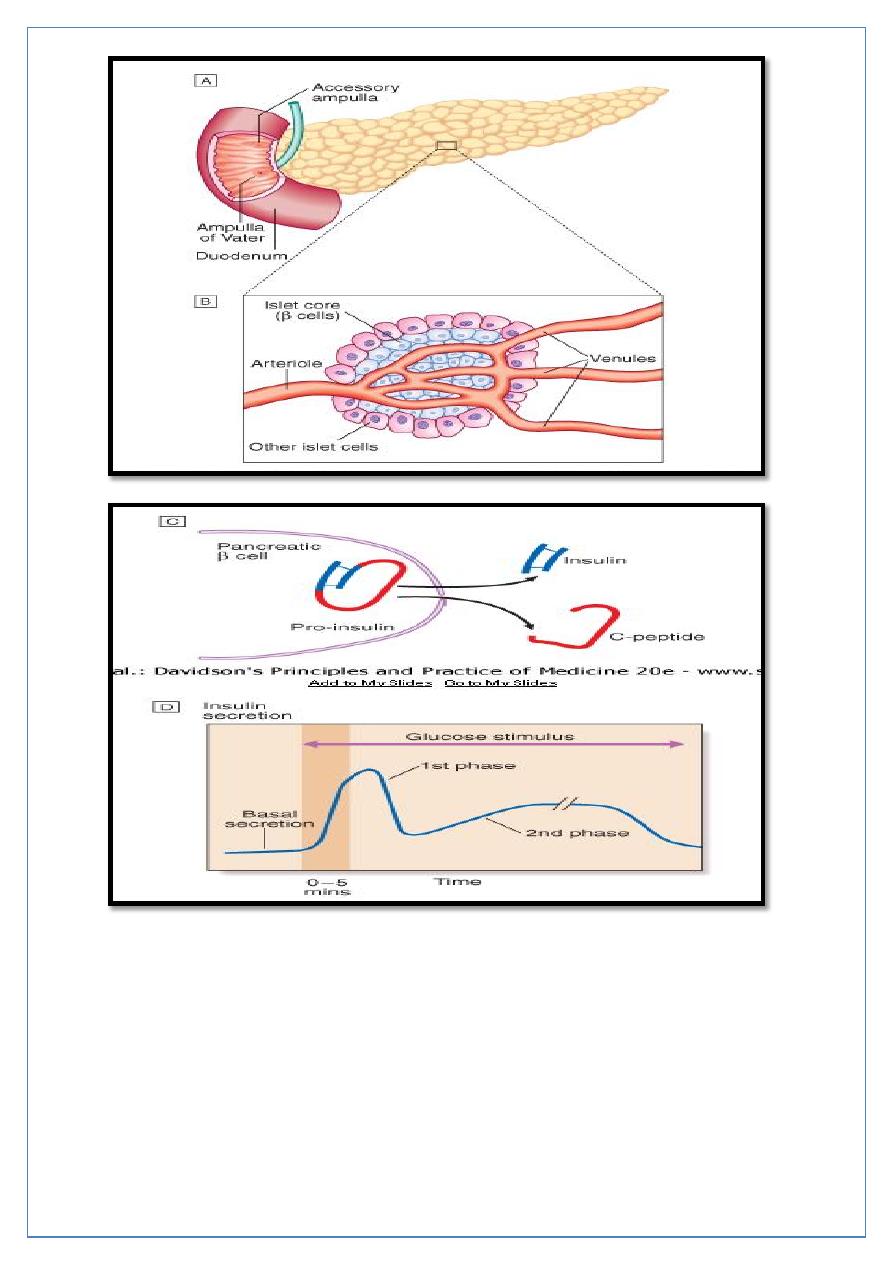
A- The normal adult pancreas contains about 1 million islets which are scattered
throughout the exocrine parenchyma. Histology is shown in
B- The core of each islet consists of β cells that produce insulin, and is surrounded
by a cortex of endocrine cells that produce other hormones including glucagon (α
cells), somatostatin (δ cells).
C- Pro-insulin in the pancreatic β cell is cleaved to release insulin and equimolar
amounts of inert C-peptide (connecting peptide). Measurement of C-peptide can be
used to assess endogenous insulin secretory capacity.

Page 5 of 18
D- An acute first phase of insulin secretion occurs in response to an elevated blood
glucose, followed by a sustained second phase.
AETIOLOGICAL CLASSIFICATION OF DIABETES MELLITUS
Type 1 diabetes Immune-mediated , Idiopathic
Type 2 diabetes
Other specific types
• Genetic defects of β-cell function (MODY)
• Pancreatic disease (e.g. pancreatitis, pancreatectomy, neoplastic disease, cystic
fibrosis, haemochromatosis)
• Excess endogenous production of hormonal antagonists to insulin (e.g. growth
hormone-acromegaly; glucocorticoids-Cushing's syndrome; glucagon-glucagonoma;
catecholamines- phaeochromocytoma; thyroid hormones-thyrotoxicosis)
• Drug-induced (e.g. corticosteroids, thiazide diuretics, phenytoin)
• Viral infections (e.g. congenital rubella, mumps, Coxsackie virus B)
• Uncommon forms of immune-mediated diabetes (LADA)
• Associated with genetic syndromes (e.g. Down's syndrome; Klinefelter's syndrome;
Turner's syndrome; DIDMOAD (Wolfram's syndrome)-diabetes insipidus, diabetes
mellitus, optic atrophy, nerve deafness; Friedreich's ataxia; myotonic dystrophy)
• Gestational diabetes
AETIOLOGY AND PATHOGENESIS OF DIABETES
In both of the common types of diabetes, environmental factors interact with genetic
susceptibility.
TYPE 1 DIABETES:
Is a slowly progressive T cell-mediated autoimmune disease, destruction of the insulin-
secreting cells in the pancreatic islets takes place over many years. Hyperglycaemia
accompanied by the classical symptoms of diabetes occurs only when 70-90% of β cells
have been destroyed.
Type 1 diabetes is associated with other autoimmune disorders, including thyroid disease,
coeliac disease, Addison's disease, pernicious anaemia and vitiligo.
Page 6 of 18
Environmental factors;
• Viral infection cause autoimmune damage to β-cells, including mumps, Coxsackie B4,
retroviruses, rubella (in utero), cytomegalovirus and Epstein-Barr virus.
• Bovine serum albumin (BSA), a major constituent of cow's milk.
• Various nitrosamines
• Stress
• TYPE 2 DIABETES:
• More complex; is a combination of resistance to the actions of insulin in liver and muscle
together with impaired pancreatic β-cell function leading to 'relative' insulin deficiency.
• Coexisted with 'metabolic syndrome‘.
• Pathological changes; is deposition of amyloid.
• LADA: Latent autoimmune diabetes of adult, is form of type 1 DM occur in middle aged
patients.
• MODY: Maturity onset diabetes of young, is a rare autosomal form of type 2 DM (less
than 5% of cases of DM) affecting young people with positive family history.
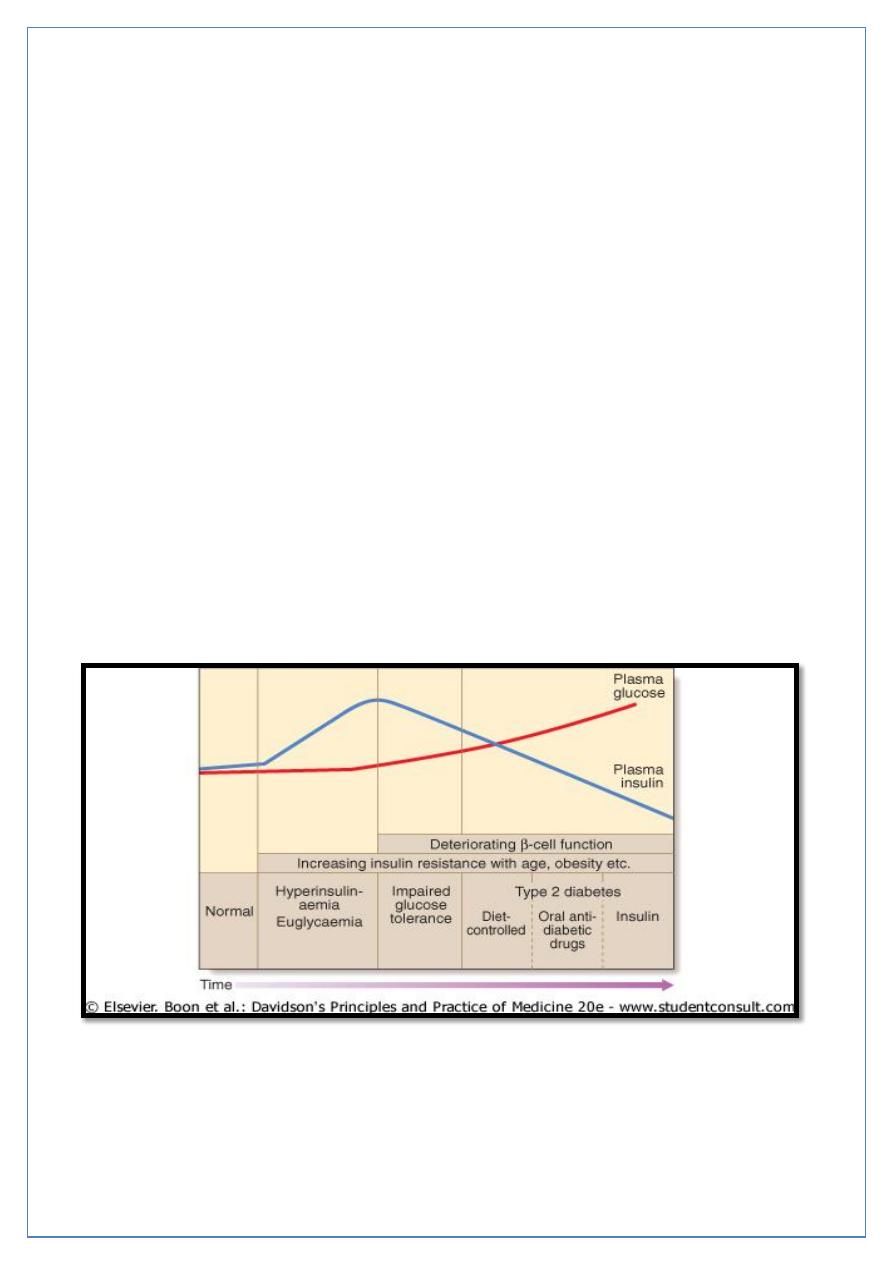
In the early stage of the disorder the response to progressive insulin resistance is an
increase in insulin secretion by the pancreatic cells, causing hyperinsulinaemia. Eventually
the β cells are unable to compensate adequately and blood glucose rises, producing
hyperglycaemia. With further β-cell failure (type 2 diabetes) glycaemic control deteriorates
and treatment requirements escalate.
Page 7 of 18
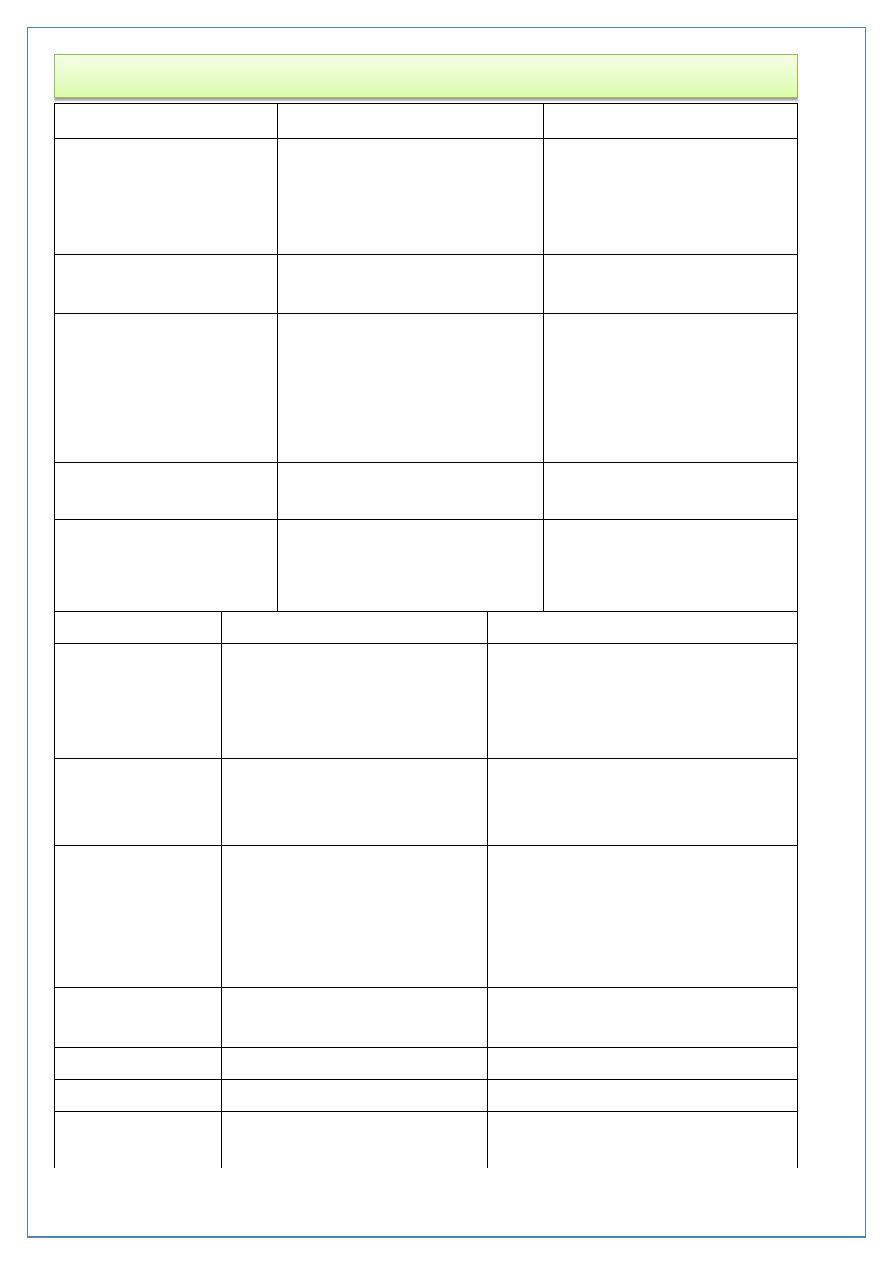
Comparison of the Two Types of Diabetes Mellitus
Type 1
Type2
Previous terminology
Insulin-dependent diabetes
mellitus (IDDM), type I,
juvenile-onset diabetes
Non-insulin-dependent
diabetes mellitus, type II,
adult-onset diabetes
Age of onset
Usually < 30yr
Usually > 40 yr
Genetic predisposition
Moderate; environmental
factors required for
expression; 35-50%
concordance in
monozygotic twins
Strong; 60-90%
concordance in
monozygotic twins
Family history
Uncommon
Common
Human leukocyte
antigen associations
Linkage to DQA and DQB,
influenced by DRB
None known
Type 1
Type 2
Other associations
Autoimmune; Graves' disease,
Hashimoto's thyroiditis,
vitiligo, Addison's disease,
pernicious anemia
Metabolic syndrome
Precipitating and
risk factors
Largely unknown; microbial,
chemical, dietary, other
Age, obesity (central), sedentary
lifestyle, previous gestational
diabetes.
Findings at
diagnosis
85-90% of patients have one
or more autoantibodies like;
islet cell antibodies (ICA) and
glutamic acide decarboxylase
(GAD) antibodies.
Possibly complications =25%
(microvascular and macrovascular)
caused by significant hyperglycemia
in the preceding asymptomatic
period
Endogenous
insulin levels
absent
Usually present (relative
deficiency), early hyperinsulinemia
Insulin resistance
absent
present
Prolonged fast
Hyperglycemia, ketoacidosis
Euglycemia
Stress, withdrawal
of insulin
Ketoacidosis
Nonketotic hyperglycemia
Page 8 of 18
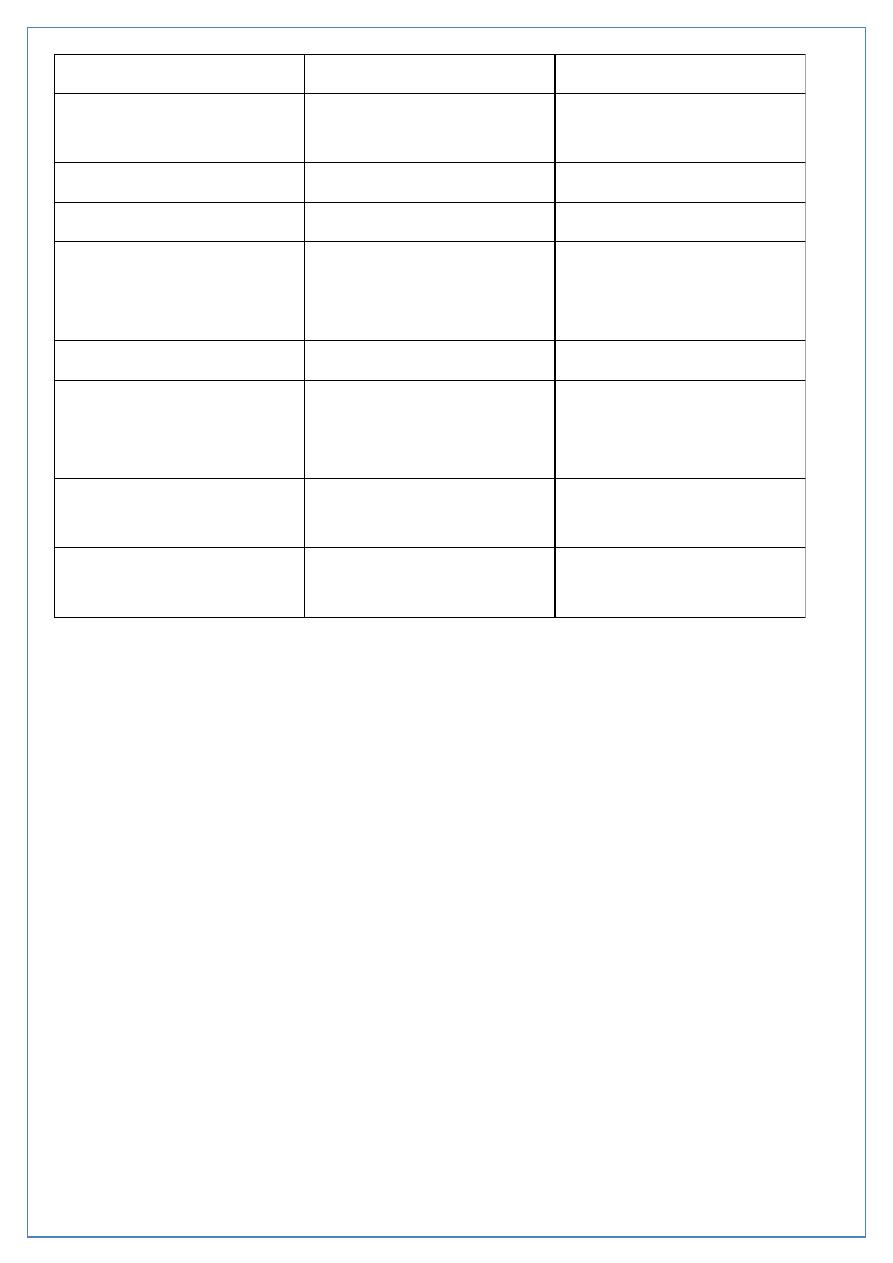
Type 1
Type 2
Duration of symptoms
Weeks
Months to years
Body weight
Low
Obese
Ketonuria
Yes
No
Rapid death without
treatment with insulin
Yes
No
Autoantibodies
Yes
No
Diabetic complications at
diagnosis
No
25%
%
of cases of DM
5- 15%
75- 85%
Other autoimmune disease
Common
Uncommon
SYMPTOMS OF HYPERGLYCAEMIA
• Thirst, dry mouth
• Polyuria
• Nocturia
• Tiredness, fatigue
• Recent change in weight
• Blurring of vision
• Pruritus vulvae, balanitis (genital candidiasis)
• Nausea, headache
• Hyperphagia; predilection for sweet foods
• Mood change, irritability, difficulty in concentrating, apathy

Page 9 of 18
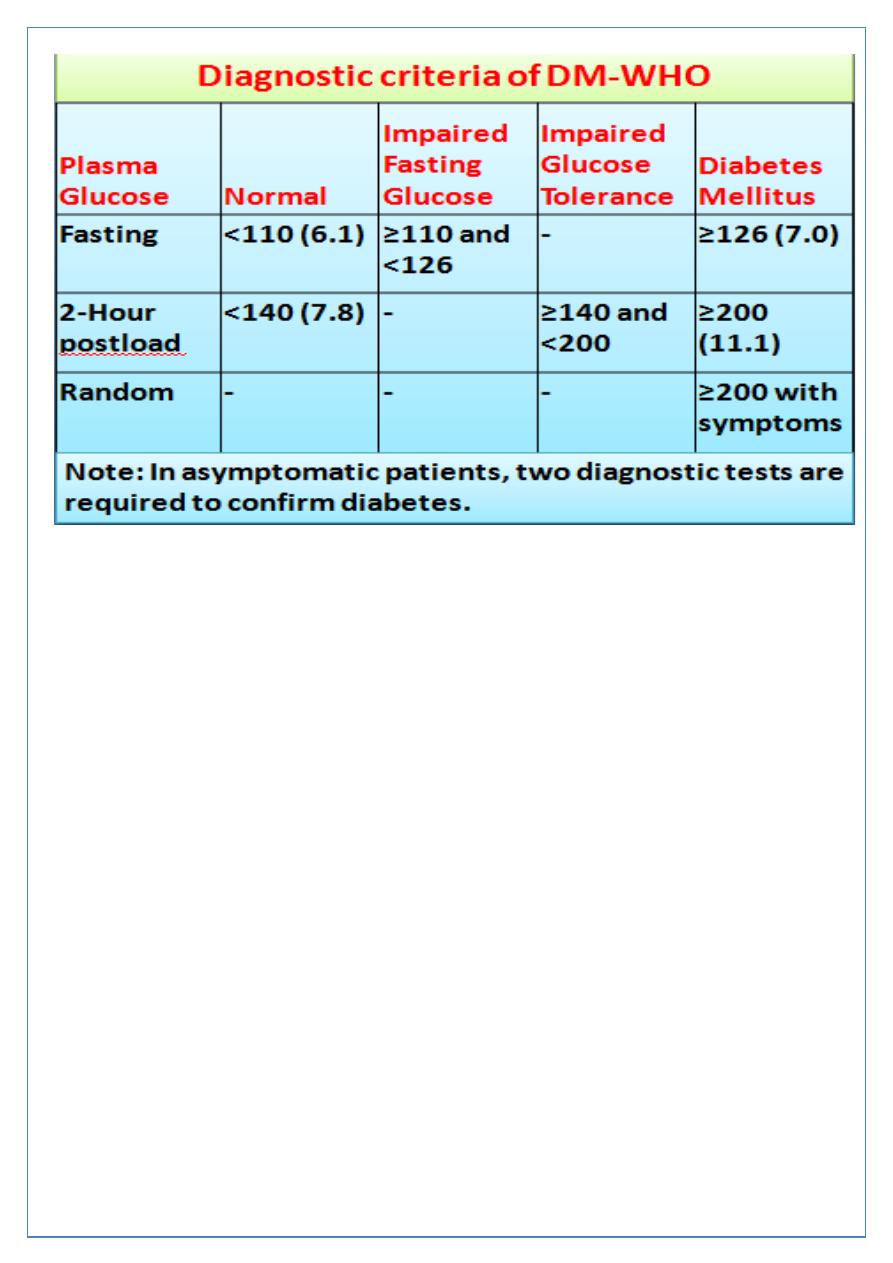
Investigations
1- URINE TESTING:
Glucose; By using sensitive glucose-specific dipsticks 1-2 hours after a meal since this will
detect more cases of diabetes than a fasting specimen.
Ketones; By using dipsticks for ketones.
Ketonuria is not pathognomonic of diabetes but, if associated with glycosuria, the diagnosis
of diabetes is highly likely
.
Protein;
Dipstick testing for albumin. This will detect urinary albumin greater than 300 mg/l.
Smaller amounts of urinary albumin from 30 to 300 mg/24 hours (microalbuminuria) can be
measured by special kit and these provide indicators of the risk of developing diabetic
nephropathy.
2- Blood testing
Glucose; In general, venous plasma values are the most reliable for diagnostic purposes.
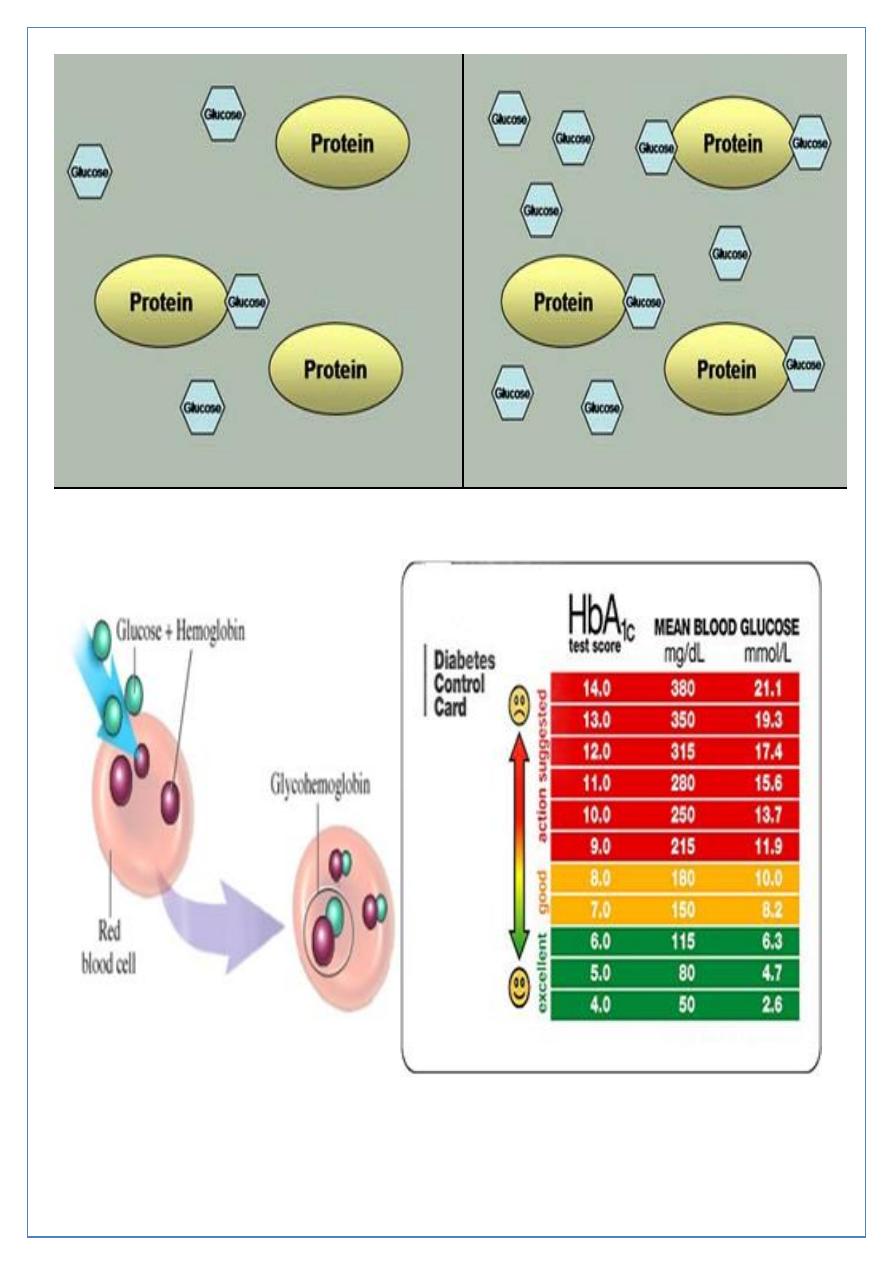
Glycated haemoglobin (HbA
1c
); provides an accurate and objective measure of glycaemic
control over a period of weeks to months but is not sufficiently sensitive to make a
diagnosis
HbA
1c
estimates may be erroneously diminished in anaemia or during pregnancy, and may
be difficult to interpret in uraemia or a haemoglobinopathy.
Glycated serum proteins ('fructosamine') can be measured used in diabetic pregnancy.
Blood lipids;
Serum lipids-total cholesterol, low-density and high-density lipoprotein (LDL and HDL)
cholesterol and triglyceride-is another important index of overall metabolic control.
Oral glucose tolerance test (OGTT)
Done when there is doubt about diagnosis of DM Plasma glucose measured before, and 2
hrs after, 75 g glucose load taken orally.
Page 10 of 18
With normoglycemia, a relatively small
amount of serum protein is glycated.
With persistent hyperglycemia,
increased protein glycation occurs.
Page 11 of 18
Management
1-Educating patients;
• Understand their condition.
• Those requiring insulin need to learn how to measure their dose of insulin accurately
with an insulin syringe or pen device, to give their own injections and to adjust the
dose themselves on the basis of blood glucose values and other factors such as
exercise, illness and episodic hypoglycaemia.
• Familiar with the symptoms of hypoglycaemia .
• Self-assessment of glycaemic control.
• Diet= decrease saturated fat, decrease sugar intake, increase starch, moderate
protein intake.
• Smoking cessation.
• Foot care.
• Preconception advice.
• Regular exercise .
Page 12 of 18
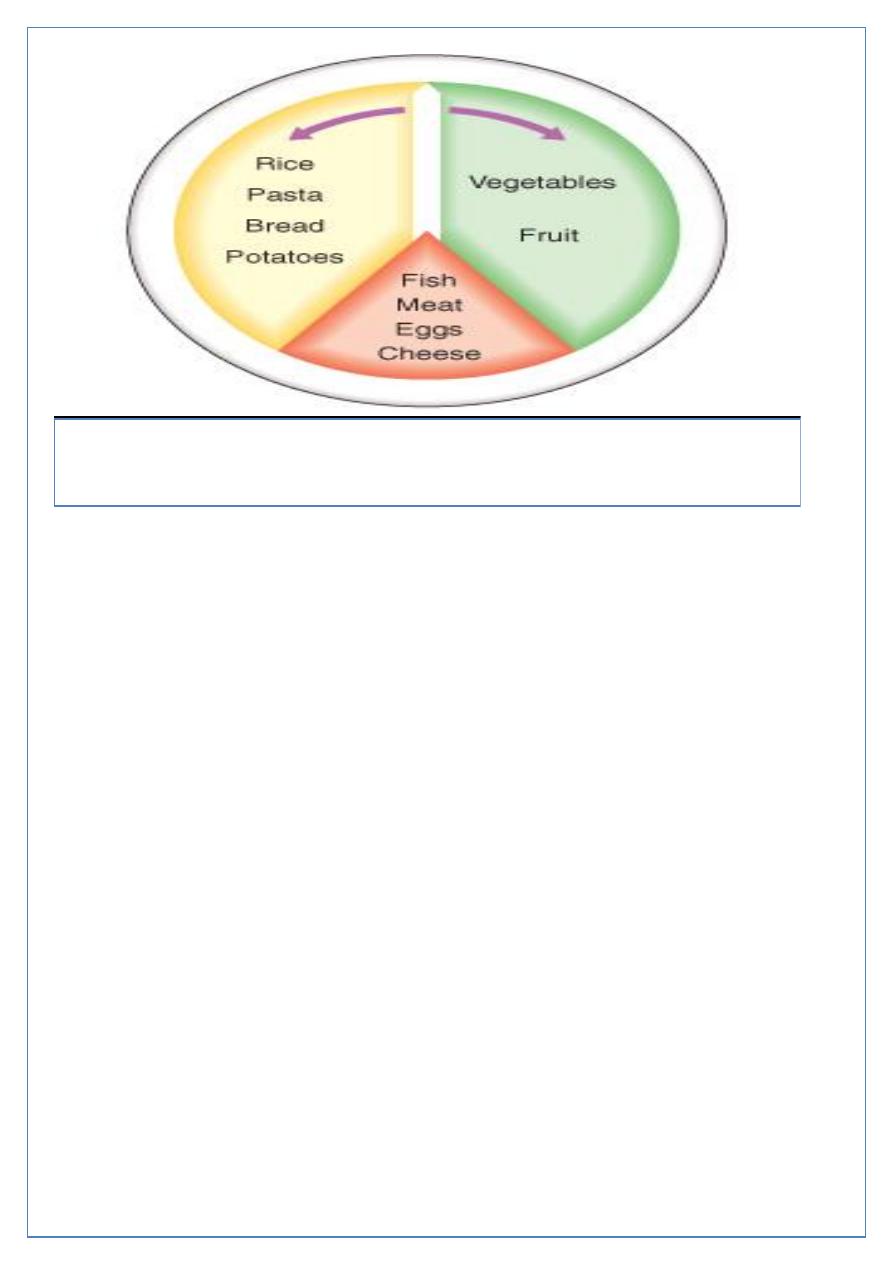
A 'plate model' for meal planning. The plate is divided into three sections. The
smallest section (one-fifth of total area) is for the meat, fish, eggs or cheese, and the
remainder divided in roughly equal proportions between the staple food (rice, pasta,
potatoes, bread etc.) and vegetables or fruit.
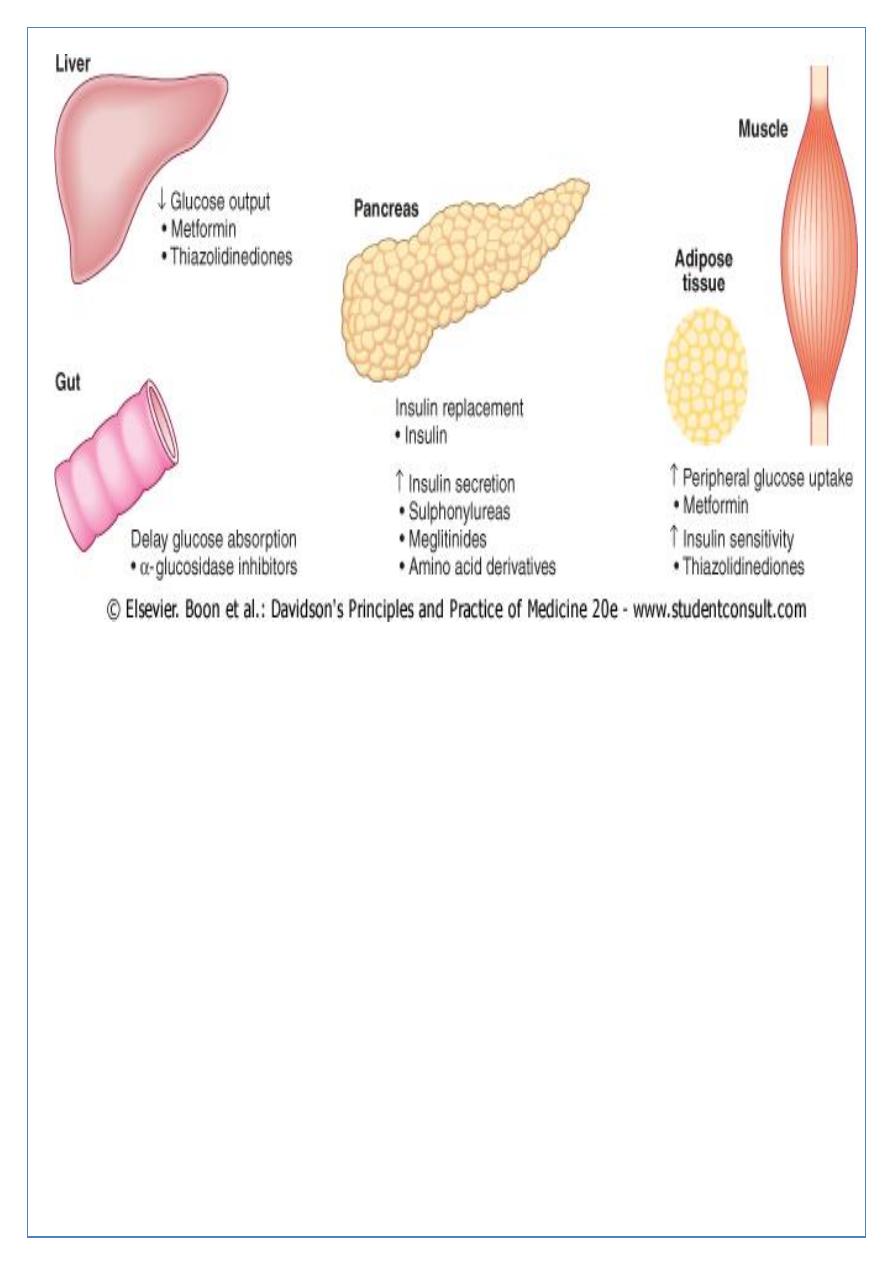
ORAL ANTI-DIABETIC DRUGS
1- SULPHONYLUREAS;
Mechanism of action; The principal effect of sulphonylureas is to stimulate the release of
insulin from the pancreatic β cell.
Indications for use sulphonylureas are valuable in the treatment of non-obese patients
with type 2 diabetes who fail to respond to dietary measures alone.
The first-generation; Tolbutamide, Chlorpropamide.
The second-generation; gliclazide and glipizide cause few side-effects, but glibenclamide is
prone to induce severe hypoglycaemia and should be avoided in the elderly.
Newer long-acting preparations such as glimepiride and a modified-release form of
gliclazide can be administered once daily with no apparent increased risk of hypoglycaemia.
Primary treatment failures; People with type 2 diabetes who fail to respond to initial
treatment with sulphonylureas.
Secondary failure; (i.e. after a period of satisfactory glycaemic control)

Page 13 of 18
2- BIGUANIDES;
Metformin is the only biguanide available.
Mechanism of action; It has no hypoglycaemic effect in non-diabetic individuals, but in
diabetes, insulin sensitivity and peripheral glucose uptake are increased.
Indications for use; metformin is not associated with a rise in body weight and it is
therefore preferred for the obese patient. In addition, It has a synergistic with
sulphonylurea drugs, the two can be combined when either alone has proved inadequate.
The main SE are diarrhea, abdominal cramps, bloating and nausea.
3- ALPHA-GLUCOSIDASE INHIBITORS;
which delay carbohydrate absorption in the gut by selectively inhibiting disaccharidases.
Acarbose or miglitol is available and is taken with each meal. Both lower post-prandial
blood glucose and modestly improve overall glycaemic control. They can be combined with
a sulphonylurea.
The main side-effects are flatulence, abdominal bloating and diarrhoea
4- THIAZOLIDINEDIONES; (glitazones) work by enhancing the actions of endogenous insulin.
For use Rosiglitazone or Pioglitazone are usually prescribed as second-line therapy with
sulphonylureas in patients intolerant of metformin, or as third-line therapy in combination
with sulphonylurea and metformin. However, their use as monotherapy and in combination
with insulin is likely to increase.
side-effects; liver dysfunction, sodium and fluid retention, must be avoided in patients with
cardiac failure.
5- MEGLITINIDES AND AMINO ACID DERIVATIVES; Repaglinide and Nateglinide. These
drugs are called prandial glucose regulators, directly stimulates endogenous insulin
secretion and are taken immediately before food. These drugs are less likely to cause
hypoglycaemia than sulphonylureas.
Page 14 of 18
COMBINED ORAL ANTI-DIABETIC THERAPY AND INSULIN
In diabetic patients who are requiring increasing doses of a sulphonylurea or biguanide,
either alone or in combination with each other or with a thiazolidinedione, the introduction
of a single dose of an intermediate- or long-acting insulin (usually isophane), administered
at bedtime, may improve glycaemic control and delay the development of overt pancreatic
β-cell failure.
Incretin-based therapies
The incretin effect is the augmentation of insulin secretion seen when a glucose stimulus is
given orally rather than intravenously, and reflects the release of incretin peptides from the
gut. The incretin hormones are primarily glucagon-like peptide 1 (GLP-1) nd gastric
inhibitory polypeptide (GIP).
Page 15 of 18
INSULIN
Manufacture and formulation;
Insulin was discovered in 1921, and obtained from animal sources (bovine and porcine
insulin).
After 1980, the use of recombinant DNA technology has enabled large-scale production of
human insulin.
Recently, rDNA and protein engineering techniques that alter the amino acid sequence of
insulin have been used to produce 'monomeric' analogues of insulin, which are more
rapidly absorbed from the site of injection (e.g. insulin lispro or aspart).
Duration of action (in hours) of insulin preparations
Insulin
Onset
Peak
Duration
Rapid-acting (insulin
analogues-lispro, aspart,
glulisine)
< 0.5
0.5-2.5
3-4.5
Short-acting (soluble
(regular))
0.5-1
1-4
4-8
Intermediate-acting (isophane
(NPH), lente)
1-3
3-8
7-14
Long-acting (bovine
ultralente)
2-4
6-12
12-30
Long-acting (insulin
analogues- glargine, detemir)
1-2
None
18-24
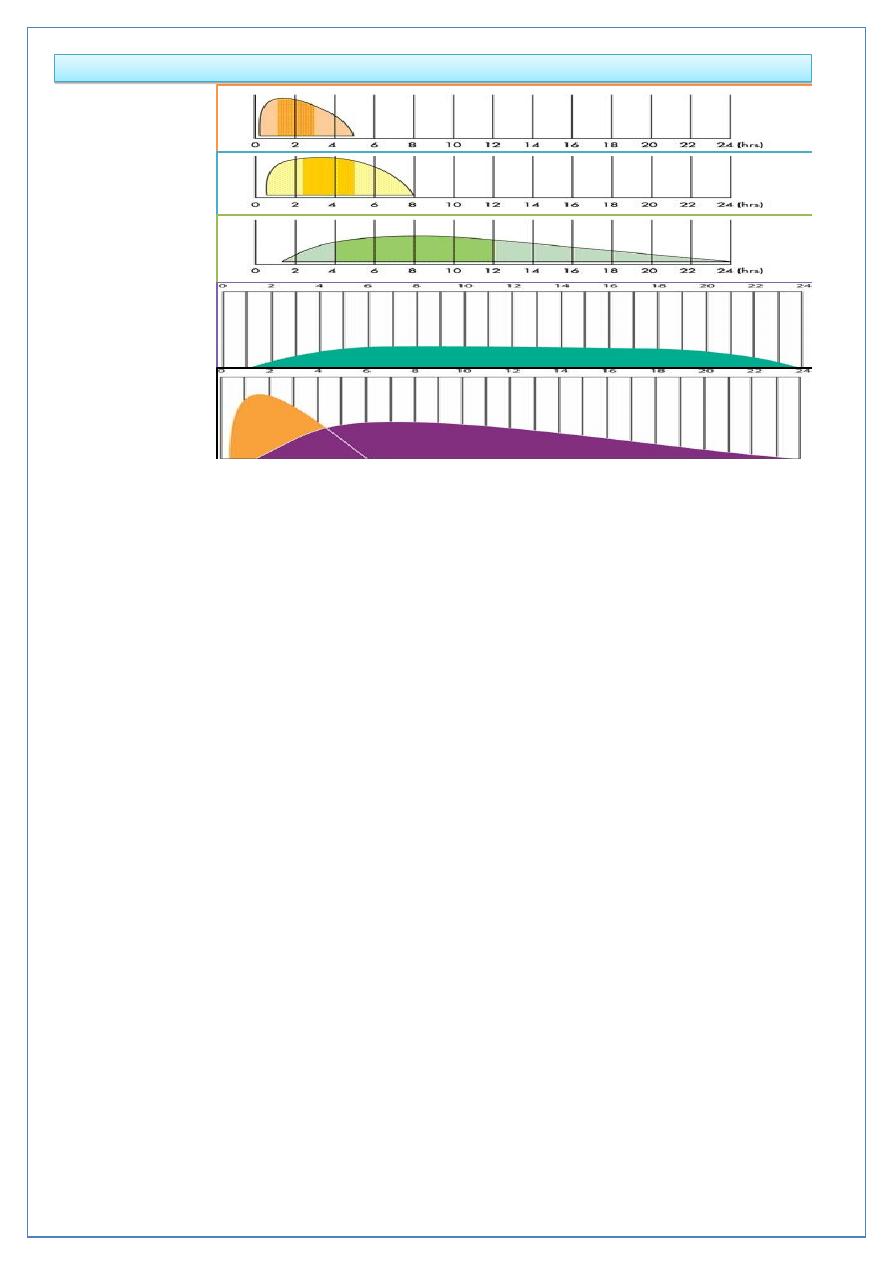
Page 16 of 18
Duration of action (in hours) and types of insulin
Rapid-acting
Short-acting
Intermediate-
acting
Long-acting
insulin
analogues
Mixed insulin
Insulin delivery
The strength of insulin is 100 u/ml.
1-Subcutaneous injection; the most common rout, into the anterior abdominal wall, upper
arms, outer thighs and buttocks. Used in:
• Type 1 DM
• Type 2 DM; in combination with OHA, pregnant woman, or sever illness or major
surgery require hospital admission.
• Gestational DM.
By using a battery-powered portable insulin pump or a disposable plastic syringe with a fine
needle (which can be reused several times), or pen device.
2-Intramuscular injection or I.V. infusion: use just soluble insulin or rapidly acting insulin
analogue, by using infusion pump providing continuous intravenous infusion of insulin. Use
in treatment of DKA or NKHDC.
3-Other rout; intraperitoneal inj. in patient on peritoneal dialysis.

Page 17 of 18
SIDE-EFFECTS OF INSULIN THERAPY
• Hypoglycaemia
• Weight gain
• Peripheral oedema (insulin treatment causes salt and water retention in the short
term)
• Insulin antibodies (animal insulins)
• Local allergy (rare)
• Lipodystrophy at injection sites
Insulin regimens
Twice-daily administration of a short-acting and intermediate-acting insulin (usually soluble
and isophane insulins), given in combination before breakfast and the evening meal, is the
simplest regimen and is still commonly used. Individual requirements vary considerably but
usually two-thirds of the total daily requirement of insulin is given in the morning in a ratio
of 1:2, short: intermediate-acting insulins. The remaining third is given in the evening, and
doses are adjusted according to blood glucose monitoring.
Multiple injection regimens are popular, with short-acting insulin being taken before each
meal, and intermediate-acting insulin being injected at bedtime (basal-bolus regimen). This
type of regimen allows greater freedom of timing of meals and is of value to individuals
with variable day-to-day activities, but snacks may have to be taken between meals to
prevent hypoglycaemia.
Once-daily injections rarely achieve satisfactory glycaemic control and are reserved either
for some elderly patients or for those who retain substantial endogenous insulin secretion
and have a low insulin requirement.
Honey moon period;
The pancreas of patient with type 1 DM may partially recover after the initial diagnosis
resulting in decrease insulin requirement.

Page 18 of 18
Complications of diabetes mellitus
A- ACUTE COMPLICATIONS
1. Hypoglycemia
2. Diabetic Ketoacidosis
3. Hyperosmolar Nonketotic Syndrome (HNKS)
4. Lactic Acidosis
B- CHRONIC COMPLICATIONS
1- Microvascular;
• Retinopathy
• Neuropathy
• Nephropathy
2- Macrovascular;
• Coronary artery disease
• Peripheral arterial disease
Cerebrovascular
