Complications of diabetes mellitus
TUCOMInternal Medicine
4th year
Dr. Hasan.I.Sultan
Complications of diabetes mellitus
A- ACUTE COMPLICATIONS• Hypoglycemia
• Diabetic Ketoacidosis
• Non-ketotic Hyperosmolar Diabetic Syndrome or Coma
• Lactic Acidosis
B- CHRONIC COMPLICATIONS
1- Microvascular;
Nephropathy
Retinopathy
Neuropathy
2- Macrovascular;
Coronary artery disease
Cerebrovascular disease
Peripheral arterial disease
ACUTE COMPLICATIONS
HYPOGLYCEMIA:
Definition: Hypoglycemia is a clinical syndrome of diverse causes in which low levels of serum glucose can eventually lead to neuroglycopenia. Which documented by Whipple's triad: signs and symptoms of hypoglycemia, in the presence of a low plasma glucose concentration <45 mg/dL (2.5 mmol/L), that are relieved by restoration of plasma glucose to normal concentrations.
The lower limit of the fasting plasma glucose concentration is normally approximately 70 mg/dL (3.9 mmol/L) but low plasma glucose values without signs and symptoms, or vice versa, do not constitute clinical hypoglycemia.
As plasma glucose levels decline just below the physiologic range, glucose counterregulatory (plasma glucose–raising) hormones are released, among these:
• Reduce insulin secretion
• Increase glucagon secretion
• Increase epinephrine secretion
• Increase cortisol and growth hormone secretion
Glucagon is the most important in the acute response to hypoglycemia.
In patients with type 1 diabetes of more than 5 years duration, the glucagon response is lost. After 10 years the epinephrine response will be lost.
Causes of hypoglycemia
• Hypoglycemia in diabetic patients; it is a result of treatment and not a manifestation of the disease itself.• Hypoglycemia in non-diabetic patients; it is called 'spontaneous' hypoglycemia, which have different causes and require different investigations.
Causes of hypoglycaemia in diabetes;
Missed, delayed or inadequate mealUnexpected or unusual exercise
Alcohol
Errors in oral hypoglycaemic agent or insulin dose
Lipohypertrophy at injection sites
Gastroparesis due to autonomic neuropathy
Malabsorption, e.g. coeliac disease
Unrecognised other endocrine disorder, e.g. Addison's disease
Factitious (patient induced)
Breastfeeding by diabetic mother
Causes of spontaneous hypoglycaemia
SIGNS AND SYMPTOMS OF HYPOGLYCEMIA
NeuroglycopenicConfusion
Drowsiness
Speech difficulty
Inability to concentrate
Incoordination
Non-specific
Nausea
Tiredness
Headache
Autonomic
Sweating
Trembling
Pounding heart
Hunger
Anxiety
Severe hypoglycaemia;
defined as any episode requiring the assistance of another person for recovery, can result in serious morbidity and has a recognized mortality of 2-4% in insulin-treated patients.Diagnosis of hypoglycemia:
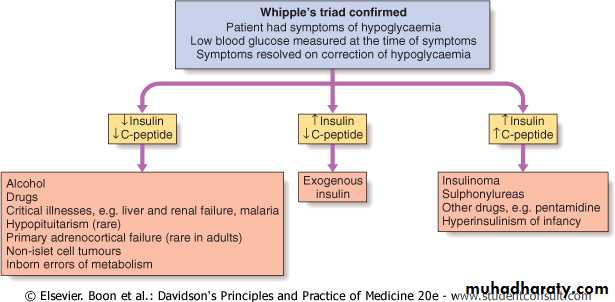
• Clinical suspicion: in patients with typical symptoms, confusion, altered level of consciousness, seizure, or in a clinical setting in which hypoglycemia is known to occur.• Documentation of Whipple's triad.
• Blood sample: to confirm hypoglycaemia and for later measurement of insulin, C-peptide, proinsulin and circulating oral hypoglycemic agents.
Note: Hypoglycaemia + high insulin + high C-peptide concentrations = insulinoma or sulphonylurea ingestion
Condition
Plasma Glucose (mg/dL)Insulin (μU/mL)
C-Peptide (nmol/L)
Proinsulin (pmol/L)
Plasma Sulfonylurea Level (nmol/L)
Normal
≥45
<6
<0.2
<5
-
Insulinoma
≤45
≥6
≥0.2
≥5
-
Exogenous insulin
≤45
≥6
<0.2
<5
-
Sulfonylurea agents
≤45
≥6
≥0.2
≥5
>0.2
Interpretation of blood results in hypoglycemic patient
Treatment of hypoglycemia:
If the patient is unable to swallow, intravenous glucose (30-50 ml of 20-50% dextrose) or glucagon as 1 mg by intramuscular injection (but this is ineffective in patients with depleted glycogen reserves such as liver disease).
As soon as the patient is able to swallow, glucose should be given orally. Full recovery may not occur immediately and reversal of cognitive impairment may not be complete until 60 minutes after normoglycaemia is restored.
glucose gel given orally
The possibility of recurrence should be anticipated in a patient using a long- or intermediate-acting insulin or a long-acting sulphonylurea and to prevent this a 10% dextrose infusion, titrated to the patient's blood glucose, may be necessary.Causes of failure to regain consciousness after blood glucose is restored to normal:
• Cerebral oedema
• Alcohol intoxication
• Post-ictal state
• Cerebral haemorrhage
DIABETIC KETOACIDOSIS
Ketoacidosis is a major medical emergency and remains a serious cause of morbidity, principally in people with type 1 diabetes.The cardinal biochemical features of DKA are:
• Hyperglycemia: plasma glucose levels greater than 250 mg/dL.
• Ketosis: moderate to severe ketonemia and moderate ketonuria (2+ to 3+ by the nitroprusside method)
• Acidosis: pH less than or equal to 7.3 and/or bicarbonate less than or equal to 15 mEq/L
CLINICAL FEATURES OF DIABETIC KETOACIDOSIS
Symptoms
Polyuria
Thirst
Weight loss
Weakness
Nausea
Vomiting
Leg cramps
Blurred vision
Abdominal pain
Signs
Dehydration
Hypotension (postural or supine)
Cold extremities
Peripheral cyanosis
Tachycardia
Air hunger (Kussmaul breathing)
Smell of acetone
Hypothermia
Confusion, drowsiness
Coma
Dehydration
Investigations:The following are important but should not delay the institution of intravenous fluid and insulin replacement:
• Blood glucose, urea and electrolytes,, plasma bicarbonate
• Arterial blood gases to assess the severity of acidosis.
• Urinalysis for ketones (a semi-quantitative guide to the plasma concentration of acetoacetate and acetone)
• ECG
• Infection screen: full blood count, blood and urine culture, C-reactive protein, chest X-ray.
Treatment of DKA:
The principal components of treatment are:• Fluid replacement
• Administration of short-acting (soluble) insulin
• Potassium replacement
• Administration of antibiotics if infection is present
Fluid replacement:
0.9% saline (NaCl) i.v.1 litre over 30 minutes
1 litre over 1 hr
1 litre over 2 hrs
1 litre over next 2- 4 hrs
When blood glucose < 15 mmol/l (270 mg/dl)
Switch to 5% dextrose, 1 litre 8-hourly
If still dehydrated, continue 0.9% saline and add 5% dextrose 1 litre per 12 hrs
Typical requirement is 6 litres in first 24 hrs but avoid fluid overload in elderly patients
Subsequent fluid requirement should be based on clinical response including urine output
Insulin:
50 units soluble insulin in 50 ml 0.9% saline i.v. via infusion pump
6 units/hr initially
3 units/hr when blood glucose is < 15 mmol/l (270 mg/dl)
2 units/hr if blood glucose declines to < 10 mmol/l (180 mg/dl)
Intramuscular injection (loading dose of 10-20 U followed by 5 U hourly) If an I V infusion is not possible. Or, alternatively, a fast-acting insulin analogue can be given hourly by subcutaneous injection.
Check blood glucose hourly initially, if no reduction in first hour, rate of insulin infusion should be increased
Aim for fall in blood glucose of 3-6 mmol/l (∼55-110 mg/dl) per hour. If blood glucose does not fall within 1 hour of commencing treatment, the dose of insulin should be increased until a satisfactory response is obtained.
Potassium: None in first litre of i.v fluid unless < 3.0 mmol/l
If plasma potassium < 3.5 mmol/l, give 40 mmol added potassiumGive in 1 litre of fluid
Avoid infusion rate of > 20 mmol/hr
If plasma potassium is 3.5- 5.0 mmol/l, give 20 mmol added potassium
If plasma potassium is > 5.0 mmol/l, or patient is anuric, give no added potassium
Bicarbonate: Its use is controversial. It can be used in severely acidotic patient (pH < 7.0) . Complete correction of the acidosis should not be attempted.
Antibiotics: Infections must be carefully sought and vigorously treated since it may not be possible to abolish ketosis until they are controlled.
COMPLICATIONS OF DIABETIC KETOACIDOSIS
• Cerebral oedema; may be caused by very rapid reduction of blood glucose, use of hypotonic fluids and/or bicarbonateHigh mortalityTreat with mannitol, oxygen• Acute respiratory distress syndrome
• Thromboembolism
• Disseminated intravascular coagulation (rare)
• Acute circulatory failure
NON-KETOTIC HYPEROSMOLAR DIABETIC COMA
This condition is characterised by;
• Severe hyperglycaemia (> 50 mmol/l (900 mg/dl))
• Without significant hyperketonaemia or acidosis.
• Severe dehydration and pre-renal azotemia are common.
• hyperosmolality (>350 mosmol/L)
• Plasma osmolality = 2 (Na+) + 2 (K+) + Glucose + Urea (all in mmol/L). The normal value is 280-300 mmol/L
• It usually affects elderly patients, with type 2 DM, with a several-week history of polyuria, weight loss, and diminished oral intake that lead to confusion, lethargy, or coma. Physical examination reflects profound dehydration, hypotension, tachycardia, and altered mental status.
• Mortality is high (40%).
Treatment differs from ketoacidosis in two main respects;
Firstly, these patients are usually relatively sensitive to insulin and approximately half the dose of insulin recommended for the treatment of ketoacidosis should usually be employed (3 units/hr).Secondly, the plasma osmolality is high, which may be depressed conscious level if it (> 340 mmol/kg). The patient should be given 0.45% saline until the osmolality approaches normal, when isotonic (0.9%) saline should be substituted.
Thromboembolic complications are common, and prophylactic subcutaneous low molecular weight heparin is recommended.
LACTIC ACIDOSIS
In coma due to lactic acidosis the patient is likely to be taking metformin for type 2 diabetes.patient is very ill and overbreathing but not as profoundly dehydrated.
The patient's breath does not smell of acetone, ketonuria is absent and the plasma bicarbonate and pH are markedly reduced (pH < 7.2).
The diagnosis is confirmed by a high (usually > 5.0 mmol/l) concentration of lactic acid in the blood
Treatment:
Intravenous sodium bicarbonate sufficient to raise the arterial pH to above 7.2, along with insulin and glucose.
Sodium dichloroacetate may be given to lower blood lactate.
The mortality in this condition is > 50%.
Microvascular Chronic Complications
DIABETIC NEPHROPATHY;
Is an important cause of morbidity and mortality, and is now among the most common causes of end-stage renal failure (ESRF) in developed countries.
About 20 to 30% of diabetic patients develop nephropathy, and the incidence increases with duration of diabetes.
Microalbuminuria; (30 to 300 mg/24 hours) is an important indicator of risk of developing diabetic nephropathy after 10 years of type 1 diabetes and it increase the risk of macrovascular disease in patients with type 2 diabetes.
Pathologically: There is thickening of the glomerular basement membrane and glomerulosclerosis which either difusse or nodular (Kimmelstiel–Wilson nodule) which is pathognomonic of diabetic nephropathy.
Risk factors for developing diabetic nephropathy:
Poor control of blood glucose
Long duration of diabetes
Presence of other microvascular complications
Ethnicity (e.g. Asian races, Pima Indians)
Pre-existing hypertension
Family history of diabetic nephropathy
Family history of hypertension
Management
Improved control of blood glucoseTreatment with metformin should be stopped when creatinine is higher than 150 μmol/l (1.7 mg/dl) as the risk of lactic acidosis is increased. Long-acting sulphonylureas should be replaced by short-acting agents to decrease risk of hypoglycemia.
Aggressive reduction of blood pressure
institution of angiotensin-converting enzyme inhibitor (ACE-I) therapy.
ACE inhibitor treatment reduced the progression to established proteinuria regardless of whether blood pressure was elevated or not.
similar benefits from angiotensin II receptor antagonists in patients with type 2 diabetes
Non-dihydropyridine calcium antagonists (diltiazem, verapamil) may be suitable alternatives when ACE inhibitor is contraindicated.
• Aggressive cardiovascular risk factor reduction; with use of ACE inhibitors, statins and aspirin.
• Renal replacement therapy may benefit diabetic patients at an earlier stage than other patients with ESRF.
• Renal transplantation can dramatically improve the life of many.
DIABETIC RETINOPATHY
Diabetic retinopathy is one of the most common causes of blindness in adults between 30 and 65 years of age in developed countries.Pathogenesis; Hyperglycaemia leads to endothelial cell proliferation, resulting in capillary closure and retinal ischemia, that leads to production of vascular endothelial growth factor (VEGF), which stimulate endothelial cell growth (causing new vessel formation).
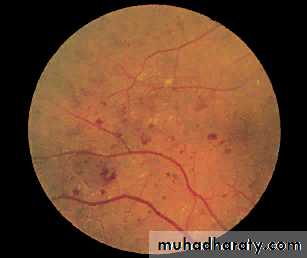
1- Non-proliferative 'background' retinopathy:
Venous dilatationPeripheral Microaneurysms (dots) haemorrhages (Blots) Exudates
Type of diabetic retinopathy
2- Pre-proliferative retinopathy
Clusters/sheets of microaneurysms and small blot haemorrhages and/or large retinal haemorrhagesMultiple cotton wool spots
Macular oedema with Perimacular exudates
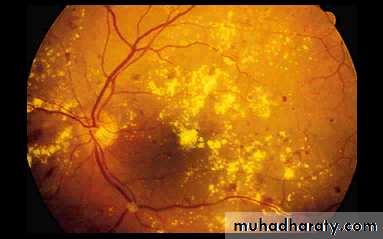
3- Proliferative retinopathy
Pre-retinal haemorrhageNeovascularisation
Fibrosis
Exudative maculopathy
Treatment:
• Good glycaemic and blood pressure control.• Regular screening for retinopathy is essential in all diabetic patients.
• Photocoagulation is used: to destroy areas of retinal ischaemia (since it is thought that this plays a major role in the development of neovascularisation) , to seal leaking microaneurysms and to gliose new vessels directly on the retinal surface.
DIABETIC NEUROPATHY
This is a relatively early and common complication affecting approximately 30% of diabetic patients.
CLASSIFICATION OF DIABETIC NEUROPATHY
A- Somatic
1- Polyneuropathy
Symmetrical, mainly sensory and distal
Asymmetrical, mainly motor and proximal (including amyotrophy)
2- Mononeuropathy (including mononeuritis multiplex)
B- Visceral (autonomic)
Cardiovascular
Gastrointestinal
Genitourinary
Sudomotor
Vasomotor
Pupillary