
Essentials of
Clinical Periodontology
and
Periodontics
System requirement:
•
Windows XP or above
•
Power DVD player (Software)
•
Windows media player version 10.0 or above
•
Quick time player version 6.5 or above
Accompanying DVD ROM is playable only in Computer and not in DVD player.
Kindly wait for few seconds for DVD to autorun. If it does not autorun then please do the following:
•
Click on my computer
•
Click the drive labelled JAYPEE and after opening the drive, kindly double click the file Jaypee

DVD Content
Procedure on
Live Periodontal Surgery
Essentials of
Clinical Periodontology
and
Periodontics
Shantipriya Reddy
BDS MDS (Periodontia)
Professor
Dr Syamala Reddy Dental College and Hospital
Bengaluru, Karnataka, India
SECOND
EDITION
JAYPEE BROTHERS MEDICAL PUBLISHERS (P) LTD
• New Delhi • Ahmedabad • Bengaluru • Chennai • Hyderabad • Kochi • Kolkata • Lucknow • Mumbai • Nagpur

Published by
Jitendar P Vij
Jaypee Brothers Medical Publishers (P) Ltd
B-3 EMCA House, 23/23B Ansari Road, Daryaganj
New Delhi
110 002, India
Phones: +91-11-23272143, +91-11-23272703, +91-11-23282021, +91-11-23245672
Rel: +91-11-32558559, Fax: +91-11-23276490, +91-11-23245683
e-mail: jaypee@jaypeebrothers.com
Visit our website: www.jaypeebrothers.com
Branches
•
2/B, Akruti Society, Jodhpur Gam Road Satellite
Ahmedabad
380 015, Phones: +91-79-26926233, Rel: +91-79-32988717
Fax: +91-79-26927094, e-mail: ahmedabad@jaypeebrother.com
•
202 Batavia Chambers, 8 Kumara Krupa Road, Kumara Park East
Bengaluru
560 001, Phones: +91-80-22285971, +91-80-22382956, +91-80-22372664
Rel: +91-80-32714073, Fax: +91-80-22281761, e-mail: bangalore@jaypeebrothers.com
•
282 IIIrd Floor, Khaleel Shirazi Estate, Fountain Plaza, Pantheon Road
Chennai
600 008, Phones: +91-44-28193265, +91-44-28194897, Rel: +91-44-32972089
Fax: +91-44-28193231, e-mail: chennai@jaypeebrothers.com
•
4-2-1067/1-3, 1st Floor, Balaji Building, Ramkote Cross Road
Hyderabad
500 095, Phones: +91-40-66610020, +91-40-24758498, Rel:+91-40-32940929
Fax:+91-40-24758499, e-mail: hyderabad@jaypeebrother.com
•
No. 41/3098, B & B1, Kuruvi Building, St. Vincent Road
Kochi
682 018, Kerala, Phones: +91-484-4036109, +91-484-2395739, +91-484-2395740
e-mail: kochi@jaypeebrothers.com
•
1-A Indian Mirror Street, Wellington Square
Kolkata
700 013, Phones: +91-33-22651926, +91-33-22276404, +91-33-22276415
Rel: +91-33-32901926, Fax: +91-33-22656075, e-mail: kolkata@jaypeebrothers.com
•
Lekhraj Market-III, B-2, Sector-4, Faizabad Road, Indira Nagar
Lucknow
226 016, Phones: +91-0522-3040554, e-mail: lucknow@jaypeebrothers.com
•
106 Amit Industrial Estate, 61 Dr SS Rao Road, Near MGM Hospital, Parel
Mumbai
400 012, Phones: +91-22-24124863, +91-22-24104532, Rel: +91-22-32926896
Fax: +91-22-24160828, e-mail: mumbai@jaypeebrothers.com
•
“KAMALPUSHPA” 38, Reshimbag, Opp. Mohota Science College, Umred Road
Nagpur
440 009 (MS), Phone: Rel: +91-712-3245220, Fax: +91-712-2704275
e-mail: nagpur@jaypeebrothers.com
Essentials of Clinical Periodontology and Periodontics
© 2008, Shantipriya Reddy
All rights reserved. No part of this publication and DVD ROM should be reproduced, stored in a retrieval system, or transmitted in any
form or by any means: electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of the
author and the publisher.
This book has been published in good faith that the materials provided by author is original. Every effort is made to ensure
accuracy of material, but the publisher, printer and author will not be held responsible for any inadvertent error(s). In case of any
dispute, all legal matters are to be settled under Delhi jurisdiction only.
First Edition: 2006
Second Edition:
2008
ISBN 81-8448-148-9
Typeset at JPBMP typesetting unit
Printed at Ajanta Offset

Dedicated to
My late grandfather, who had believed in my abilities, it is because of
whom I chose this profession.
My grandmother, for her constant support.
My father and mother, for always being there.
My uncle for his continuous encouragement.
My husband for his unique mentorship.
My daughter and son for being so understanding

Any book requires the help and assistance of others in order to be completed successfully. In this regard, I would
like to extend special thanks to all those who had helped to accomplish this goal. First and foremost I would
like to thank my student Dr Deepti Sinha for drawing those excellent diagrams inserted in this book. Special
thanks must go to all my colleagues, postgraduate students, (Drs) Jeeth Rai, Keshav, Satish, Shabeer and Anil,
Mamatha and Sumaira, who have helped me to prepare the manuscript. I would like to extend my special thanks
to Dr Anil Kumar who helped me to collect the photographs used in this book. I am very grateful to Dr Prasad
MGS and Dr Sabna Kakarala for being so generous with their clinical photographs which helped to enhance
the overall excellence of this edition. Dr Divakaranand and Dr Rudrakshi’s helped in typing the manuscript of
this edition is greatly appreciated. My heartfelt thanks to Dr Srinivas for helping me in writing the chapter on
Oral Malodor. I would also like to thank Dr Ashwath (Smart desq navigators) for helping me with animation
photographs of various suturing techniques. The excellent co-operation of the publisher is also greatly acknowledged.
Acknowledgements

Preface to the Second Edition
I am extremely happy to present the second edition of Essentials of Clinical Periodontology and Periodontics.
Three new chapters including glossary of periodontal terminology has been included in this edition. Chapters
on Host Modulation and Oral Malodor are written with same clarity and simplicity, that has been religiously
followed in the previous edition. More photographs have been included to make the student understand the
subject better.
I thank all the fellow colleagues and students who have supported me with useful suggestions while revising
the first edition. I hope to receive the same support and suggestions for the second edition also.
Shantipriya Reddy

This basic text when conceived in the year 2003 has been written in an attempt to make our understanding
of periodontal disease accessible to the undergraduate students, general practitioners and dental hygienists. The
Essentials of Clinical Periodontology and Periodontics is a learning textbook intended to serve the needs of several
groups of dental care professionals and is written with credibility and readability maintained at every level.
Undergraduate students especially will find it useful in integrating the concepts they have been taught in a more
elaborate way. Clarity and simplicity in language has been my objective while writing this book. The organization
of the chapters and the key points with review questions at the end of every chapter serve as a programmed-
guide for the reader.
This text can be of help for the academicians to re-think the modes of presenting information and also as
a model to test whether the students have grasped the concepts they have been taught and are able to use
them in a practical manner.
All the efforts have been made to make the text as accurate as possible and the information provided in
this text was in accordance with the standards accepted at the time of publication. In order to attain these goals,
suggestions as well as critiques from many students and clinicians have been received and utilized.
Much of the style in this textbook is compact with adequate bibliographies. The reader is advised to use them
to gain greater depth of knowledge. The way of periodontist is hard and this book will reflect the difficulty of
that path. I hope that this textbook will fulfill all the requirements and expectations of the students and practitioners
as this is a special branch of our profession. The field of periodontics remains a work in progress.
Shantipriya Reddy
Preface to the First Edition

HISTORICAL BACKGROUND OF PERIODONTOLOGY
Various forms of gingival and periodontal diseases have affected the human race since the dawn of history. In
the earlier historical records almost all the writings have information regarding the diseases affecting oral cavity
and majority of it is about periodontal diseases.
Early Civilizations
Summerians of 3,000 BC first practiced oral hygiene. Babylonians and Assyrians, who also have suffered from
periodontal diseases, have treated themselves using gingival massage combined with various herbal medications.
Research on embalmed bodies of the ancient Egyptians pointed out that periodontal disease was the most common
of all the diseases. Medical writings of the time Ebers Papyrus had many references to gingival diseases and also
contain various prescriptions for strengthening the teeth and gums.
The medical works of ancient India, Susruta Samhita and Charaka Samhita describe severe periodontal disease
with loose teeth and purulent discharge from the gingiva and the treatment advised was to use a stick that is
bitter for cleaning teeth.
Periodontal disease was also discussed in Ancient Chinese books. The oldest book written in 2,500 BC describes
various conditions affecting oral cavity. Gingival inflammation, periodontal abscesses and gingival ulcerations are
described in detail. They were among the earliest people to use the toothbrush to clean the teeth.
Middle Ages
The systematic therapeutic approach was not developed until the middle ages. This was a period of golden age
of Arabic science and medicine. Avicenna and Albucasis made a refined, novel approach to surgical work. Albucasis
had a clear understanding of calculus as etiology of periodontal disease and described the technique of removing
it. He had also developed a set of scalers for removing calculus. He also wrote in detail on other treatment
procedures like extraction of teeth, splinting loose teeth with gold wire, etc.
18th Century
Modern dentistry was developed in 18th century. Pierre Fauchard in 1678 who is rightly considered as Father
of Modern Dentistry designed periodontal instruments and described the technique in detail. His book “The
Surgeon Dentist” published in 1728 presented all aspects of dental practice (i.e. restorative dentistry, prosthodontics,
oral surgery, periodontics and orthodontics). Fauchard wrote in that, confections and sweets destroy the teeth
by sticking to the surfaces producing an acid. John Hunter (1728-93) known as an anatomist, surgeon and
pathologist of 18th century wrote a book entitled “The Natural History of the Human Teeth” describing the
anatomy of the teeth and their supporting structures with clear illustrations.
Prolog
Thomas Berdmore (1740-85) known as “Dentist to His Majesty” published the Treatise in the disorders and
deformities of the teeth and gums. He not only offered detailed descriptions of instrumentation but also stressed
on prevention.
19th Century
A German born dentist, Leonard Korecker in his paper Philadelphia Journal of Medicine and Physical Sciences
mentioned the need for oral hygiene by the patient, to be performed in the morning and after every meal using
an astringent powder and a toothbrush.
Levi Spear Parmly was considered the Father of Oral Hygiene and the Inventor of Dental Floss. The name
“Pyorrhea Alveolaris” was used to describe periodontal disease. John W Riggs was the first individual to limit
his practice to periodontics and was considered the first specialist in this field. Periodontitis was known as “Riggs
disease.” Several major developments took place in the second half of the 19th century starting the era called
modern medicine.
The first was the discovery of anesthesia and second scientific breakthrough was made by Louis Pasteur who
established the Germ Theory of Disease. The third scientific finding was the discovery of radiographs by Wilhelm
Roentgen.
Also the late 19th century has witnessed a proper understanding of the pathogenesis of periodontal disease
based on histopathologic studies. GV Black in 1899 gave the term gelatinous microbic plaque and described
its relationship to caries. Xenophon recognized ANUG in 4th century BC Salomon Robicsek developed a surgical
technique called gingivectomy.
20th Century
Early 20th century witnessed major changes in the treatment of periodontal disease. Gottlieb published extensive
microscopic studies of periodontal diseases in humans. It was realized that, removal of calculus and other deposits
was not enough. In addition, removal of periodontal pockets was necessary to control the disease.
Leonard Widman and Newman described flap surgery for the removal of periodontal pockets. Removal of
bone was considered essential at that time.
After World War II, the focus was on periodontal research and this led to a better understanding to the pathological,
microbiological and immunological aspects of periodontal disease.
The first workshop in periodontology was conducted in 1951. It was realized at that time, scientific methods
should be introduced in the periodontal research. Subsequent workshops conducted in periodontics has witnessed
significant scientific contributions in the field of periodontics.
JOURNALS OF PERIODONTOLOGY
The various journals of periodontology available are:
• The Journal of Periodontology by Robert. J.Genco.
• Journal of Periodontal Research by Isao Ishikawa, Jorgen Slots, Maurizio Tonetti
• Journal of Clinical Periodontology by Jan Lindhe.
• Periodontology 2000 by Jorgen Slots.
• International Journal of Periodontics and Restorative Dentistry in India by Myron Nevins.
• Journal of Indian Society of Periodontology.
xiv
Essentials of Clinical Periodontology and Periodontics
Contents
Part I: The Normal Periodontium
Chapter 1: Anatomy and Development of the Structures
of Periodontium, 3
Introduction, 3
External Anatomic Features, 3
Development of Periodontium, 4
Early Development of Cementum, 4
Later Development of Cementum, 5
Development of Junctional Epithelium, 6
Chapter 2: Biology of Periodontal Tissues, 8
Introduction, 8
The Gingiva, 8
Macroscopic Features, 8
Marginal Gingiva, 8
Gingival Sulcus, 9
Attached Gingiva, 9
Interdental Gingiva, 9
Microscopic Features, 10
Morphologic Characteristics of the Different Areas of
Gingival Epithelium, 11
Cells of the Basal Layer, 11
Stratum Spinosum, 11
Stratum Granulosum, 12
Stratum Corneum, 12
Oral Sulcular Epithelium, 12
Junctional Epithelium, 12
Epithelium-Connective Tissue Interface, 13
Supra-alveolar Connective Tissue, 14
Cells, 14
Fibers, 14
Principal Fibers, 15
Secondary Fibers, 15
Blood Supply, Lymphatics and Nerves, 16
Periodontal Ligament, 16
Definition, 16
Structure, 16
Synthetic Cells, 17
Resorptive Cells, 17
Epithelial Cell Rests of Malassez, 17
Extracellular Components, 18
Fibers, 18
Principal Fibers, 18
Ground Substance, 19
Development of Principal Fibers, 19
Structure Present in the Connective Tissue, 20
Functions of Periodontal Ligament, 20
Clinical Considerations, 21
Alveolar Bone, 22
Definition, 22
Parts of Alveolar Bone, 22
Composition of Alveolar Bone, 22
Osseous Topography, 23
Fenestrations and Dehiscences, 23
Blood Supply to the Bone, 24
Clinical Considerations, 25
Cementum, 25
Definition, 25
Classification, 25
Functions, 26
Composition, 26
Cementoenamel Junction, 26
Cemental Resorption and Repair, 27
Chapter 3: Periodontal Structures in Aging Humans, 30
General Effects of Aging, 30
Skin, 30
Bone, 30
Age Changes in the Periodontium, 30
Oral Mucosa, 30
Periodontal Ligament, 31
Alveolar Bone and Cementum, 31
Bacterial Plaque and Immune Response, 31
Effects of Aging on the Progression of Periodontal Diseases, 31
Effects of Treatment on the Aging Individuals, 31
xvi
Essentials of Clinical Periodontology and Periodontics
Part II: Classification and Epidemiology of
Periodontal Diseases
Chapter 4: Classification Systems of Periodontal
Diseases, 35
Need for Classification, 35
Current Classification Systems of Periodontal Diseases,35
World Workshop in Clinical Periodontics (1988), 35
World Workshop in Clinical Periodontics (1989), 36
Genco (1990), 36
Ranney (1993), 36
European Workshop on Periodontology (1993), 36
AAP 1999 (International Workshop of Periodontal
Disease), 37
Synopsis of Types and Characteristics of Gingival
Diseases, 38
Synopsis of Types and Characteristics of Periodontal
Diseases, 39
Chapter 5:
Epidemiology of Gingival and Periodontal
Diseases, 41
Definition, 41
Types of Epidemiologic Research, 41
Descriptive Study, 41
Analytical Study, 42
Experimental Epidemiology, 42
Index, 42
Definition, 42
Purposes and Uses of Index, 42
Characteristics of Index, 43
Various Indices Used to Assess Periodontal Problems, 43
To Assess Gingival Inflammation, 44
Papillary Marginal Attachment (PMA), 44
Gingivitis Component of Periodontal Disease, 44
Gingival Index by Loe H and Sillness J (1963), 44
Indices of Gingival Bleeding, 45
Sulcular Bleeding Index, 45
Papillary Bleeding Index, 45
Bleeding Points Index, 45
Interdental Bleeding Index, 45
Gingival Bleeding Index, 45
Indices Used to Measure Periodontal Destruction, 46
Russell’s Periodontal Index, 46
Periodontal Disease Index, 46
Extent and Severity Index, 47
Radiographic Approach, 47
The Periodontitis Severity Index, 47
Indices Used to Measure Plaque Accumulation, 47
Plaque Component of Periodontal Disease
Index, 47
Simplified Oral Hygiene Index, 48
Turesky-Gilmore-Glickman, 49
Plaque Index, 49
Modified Navy Plaque Index, 49
Plaque-free Score, 50
Papillary Bleeding on Probing, 50
Indices Used to Measure Calculus, 51
Calculus Component of Simplified Oral Hygiene
Index, 51
Probe Method of Calculus Assessment, 51
Calculus Surface Index, 51
Marginal Line Calculus Index, 51
Indices Used to Assess Treatment Needs, 51
Gingival Periodontal Index, 51
Community Periodontal Index of Treatment Needs,
52
Periodontal Treatment Need System, 52
Part III: Etiopathogenesis
Chapter 6:
Periodontal Microbiology (Dental Plaque), 57
Definition, 57
Dental Plaque as a Biofilm, 57
Types of Dental Plaque, 57
Supragingival Plaque, 58
Subgingival Plaque, 58
Tooth-associated Subgingival Plaque, 58
Epithelium-associated Subgingival Plaque, 58
Connective Tissue-associated Plaque, 59
Composition of Dental Plaque, 59
Formation/Development of Dental Plaque, 59
Structural and Microscopic Properties of Plaque, 61
Clinical Significance of Plaque, 62
Microbial Specificity of Periodontal Diseases, 62
Non-specific Plaque Hypothesis, 62
Specific Plaque Hypothesis, 62
What Makes Plaque Pathogenic, 63
Microorganisms Associated with Periodontal Diseases, 63
Chapter 7: Calculus and Other Etiological Factors, 66
Calculus, 66
Definition, 66
Types, 66
Supragingival Calculus, 66
Subgingival Calculus, 66
Structure, 67
Composition, 67
Difference Between Supragingival and Subgingival
Calculus, 68
Formation of Calculus, 68
Pathogenic Potential of Calculus in Periodontal
Diseases, 69
Other Contributing Etiological Factors Including Food
Impaction, 69
Iatrogenic Factors, 69
Faulty Restorations, 69
Margins of Restorations, 69
Contour of Restorations, 69
Occlusion, 70

xvii
Contents
Material, 70
Design of Removable Partial Dentures, 70
Periodontal Problems Associated with Orthodontic
Therapy, 71
Food Impaction, 71
Unreplaced Missing Teeth, 71
Malocclusion, 72
Habits, 72
Chapter 8: Host Response: Basic Concepts, 76
Introduction, 76
Role of Saliva in the Host Defence, 76
Gingival Epithelium, 77
Gingival Crevicular Fluid, 77
Complement, 77
Inflammatory Cell Response, 78
Neutrophils, 78
Neutrophil Disorder Associated with Periodontal Disease,
80
Periodontal Diseases Associated with Neutrophil
Disorders, 80
Functions of Macrophages, 81
Immunological Mechanism, 82
Anaphylactic Reactions, 83
Cytotoxic Reactions, 84
Arthus Reactions, 84
Delayed Hypersensitivity, 84
Chapter 9: Trauma from Occlusion, 87
Physiologic Adaptive Capacity of the Periodontium to
Occlusal Forces, 87
Trauma from Occlusion, 87
Definition and Terminology, 87
Types, 88
Signs and Symptoms, 88
Histologic Changes, 89
Injury, 89
Repair, 89
Adaptive Remodeling, 89
Reversibility of Traumatic Lesion, 90
Effect of Increased Occlusal Forces on Pulp, 90
Role of the Trauma from Occlusion in the Progression of
Periodontal Disease, 90
Pathologic Tooth Migration, 92
Chapter 10: Role of Systemic Diseases in the Etiology
of Periodontal Diseases, 95
Introduction, 95
Dietary and Nutritional Aspects of Periodontal Disease,
95
Consistency of Diet, 95
Protein Deficiency, 96
Vitamin D Deficiency, 96
Effects of Hematological Disorders on Periodontium, 97
White Blood Cell Disorders, 97
Neutropenias, 97
Leukemia, 98
Thrombocytopenic Purpura, 99
Disorders of WBC Function, 99
Red Blood Cell Disorders, 99
Anemia, 99
Metabolic and Endocrine Disorders, 100
Diabetes mellitus, 100
Thyroid Gland, 101
Pituitary Gland, 101
Parathyroid Glands, 101
Gonads, 102
Cardiovascular Diseases, 103
Arteriosclerosis, 103
Antibody Deficiency Disorders, 103
AIDS, 103
HIV, 103
Other Systemic Diseases, 104
Bismuth Intoxication, 104
Lead Intoxication, 104
Mercury Intoxication, 104
Psychosomatic Disorders, 104
Chapter 11: Oral Malodor, 106
Introduction, 106
Classification of Halitosis, 106
Pseudo-halitosis, 106
Halitophobia, 107
Etiology, 107
Causes for Physiologic Halitosis, 107
Causes for Pathologic Halitosis, 107
Diagnosis of Halitosis, 107
Clinical Examination, 107
Measurement of Oral Malodor, 107
Organoleptic Method, 107
Gas Chromatography, 108
Halimeters, 108
BANA, 108
Chemiluminescence, 108
Treatment and Management of Oral Malodor, 108
Summary, 109
Chapter 12: Pathogenesis of Periodontal Diseases, 110
Introduction, 110
Role of Bacterial Invasion, 110
Role of Exotoxins, 110
Role of Cell Constituents, 111
Role of Enzymes, 111
Evasion of Host Responses, 111
Host Derived Bone Resorbing Agents, 112
Cytokines, 113
Prostaglandins, 113

Chapter 13: Periodontal Medicine, 115
Introduction, 115
Era of Focal Infection, 115
Periodontal Disease and Coronary Heart Disease/
Atherosclerosis, 116
Effect of Periodontal Infection, 117
Ischemic Heart Disease, 117
Thrombogenesis, 117
Periodontal Infection and Stroke, 118
Periodontal Disease and Diabetes Mellitus, 118
Role of Periodontitis in Pregnancy Outcome, 119
Periodontal Disease and Chronic Obstructive Pulmonary
Disease, 119
Periodontal Disease and Acute Respiratory Infection, 120
Periodontal Medicine in Clinical Practice, 120
Chapter 14: Smoking and Periodontal Diseases, 122
Effect of Smoking on the Prevalence and Severity of
Periodontal Disease, 122
Effect of Smoking on the Etiology and Pathogenesis of
Periodontal Disease, 122
Microbiology, 122
Immunology, 122
Physiology, 123
Effect of Smoking on the Response to Periodontal
Therapy, 123
Effect of Smoking Cessation, 123
Chapter 15: Host Modulation in Periodontal Therapy,125
Introduction, 125
Regulation of Immune and Inflammatory Responses, 125
Excessive Production of MMP’s, 126
Role of MMP’s in Human Periodontal Diseases, 126
Production of Arachidonic Acid and Metabolites, 126
Regulation of Bone Metabolism, 127
Part IV: Periodontal Pathology
SECTION 1: GINGIVAL DISEASES
Chapter 16: Defence Mechanisms of the Gingiva, 131
Defence Mechanisms, 131
Non-specific Mechanisms, 131
Bacterial Balance, 131
Surface Integrity, 131
Surface Fluid and Enzyme, 131
Phagocytosis, 131
Inflammatory Reaction, 132
Specific Protective Mechanism, 132
Sulcular Fluid, 132
Anatomy of Gingival Crevice, 132
Significance of Gingival Sulcus and Fluid, 133
Significance of Gingival Vasculature and
Crevicular Fluid, 133
Permeability of Sulcular and Junctional Epithelia,
133
Methods of Collection, 134
Absorbing Paper Strips, 134
Micropipettes, 134
Gingival Washings, 135
Other Methods, 135
Composition of GCF, 135
Clinical Signficance, 138
Drugs in Gingival Fluid, 138
Influence in Mechanical Stimuli, 138
Periodontal Therapy and Gingival Fluid, 139
Chapter 17: Gingival Inflammation, 140
Introduction, 140
Initial Lesion, 140
Early Lesion, 141
Established Lesion, 141
Advanced Lesion, 142
Chapter 18: Clinical Features of Gingivitis, 143
Types of Gingivitis, 143
Depending on the Course and Duration, 143
Depending on the Distribution, 143
Clinical Findings, 143
Gingival Bleeding on Probing, 144
Color Change, 147
Change in Consistency, 147
Change in Size, 147
Surface Texture, 148
Position, 148
Recession, 148
Classification and Etiology, 148
Clinical Significance, 150
Chapter 19: Gingival Enlargements, 151
Introduction, 151
Classification, 151
Inflammatory Enlargement, 152
Acute Inflammatory Enlargement, 152
Chronic Inflammatory Enlargement, 153
Non-inflammatory Enlargement, 154
Phenytoin-induced Gingival Hyperplasia, 155
Idiopathic Gingival Fibromatosis, 157
Combined Enlargement, 157
Enlargement Associated with Systemic Disease or
Condition, 159
Conditioned Enlargement, 159
Enlargement in Pregnancy, 159
Enlargement in Puberty, 160
Vitamin C Deficiency, 160
Plasma Cell Gingivitis, 161
Granuloma Pyogenicum, 161
Systemic Disease Causing Gingival Enlargement, 161
Granulomatous Diseases, 163
xviii
Essentials of Clinical Periodontology and Periodontics

Neoplastic Enlargement, 165
Benign Tumors, 165
Malignant Tumors, 165
False Enlargement, 165
Underlying Osseous Lesions, 166
Underlying Dental Tissues, 166
Chapter 20: Acute Gingival Infections, 167
Classification of Various Acute Gingival Lesions, 167
Acute Necrotizing Ulcerative Gingivitis, 168
Terminology, 168
Clinical Features, 168
Intraoral/Extraoral Signs and Symptoms, 168
Clinical Course, 169
Etiology, 169
Histopathology, Diagnosis and Treatment, 170
Acute Herpectic Gingivostomatitis, 172
Clinical Features, 172
Etiology, 172
History, 173
Histopathology, 173
Diagnosis, 173
Differential Diagnosis, 173
Treatment, 174
Pericoronitis, 174
Definition, 174
Types, 174
Clinical Features, 174
Complications, 175
Treatment, 175
Chapter 21: Periodontal Diseases in Children and
Young Adolescents, 177
Introduction, 177
Anatomic Considerations in Children, 177
Gingiva, 177
Cementum, 177
Periodontal Ligament and Alveolar Bone, 178
Histopathology of Gingivitis in Children, 178
Microbiology of Periodontal Diseases in Children, 178
Classification of Periodontal Diseases in Children, 178
Gingival Lesions, 178
Acute Herpetic Gingivostomatitis, 178
Candidiasis, 180
Gingivitis Artefacta, 181
Localized Gingival Recession, 181
Types of Periodontitis, 181
Prepubertal Periodontitis, 181
Juvenile Periodontitis, 181
Periodontitis Associated with Syndromes, 183
Papillon-Lefévre Syndrome, 183
Ehler-Danlos Syndrome, 183
Down’s Syndrome, 183
Neutropenias, 183
Chédiak-Higashi Syndrome 183
Hypophosphatasia, 183
Chapter 22: Desquamative Gingivitis, 185
Introduction, 185
Diagnosis, 185
Clinical Features, 185
Mild Form, 185
Moderate Form, 186
Severe Form, 186
Histopathology, 186
Therapy, 186
Local Treatment, 186
Systemic Treatment, 188
Diseases Clinically Presenting as Desquamative
Gingivitis, 188
Lichen Planus, 188
Cicatricial Pemphigoid, 189
Bullous Pemphigoid, 189
Linear IgA Disease, 190
Dermatitis Herpetiformis, 190
Pemphigus Vulgaris, 190
Drug Reaction or Eruptions, 191
SECTION 2: PERIODONTAL DISEASES
Chapter 23: The Periodontal Pocket, 192
Introduction, 192
Definition, 192
Classification of Pockets, 192
Clinical Features, 193
Signs and Symptoms, 193
Pathogenesis, 194
Histopathology, 197
Changes in the Soft Tissue Wall, 197
Microtopography of the Gingival Wall of the Pocket, 197
Periodontal Pocket as Healing Lesions, 197
Pocket Contents, 198
Changes in Root Surface Wall, 198
Periodontal Disease Activity, 198
Relation of Loss of Attachment and Bone Loss to Pocket
Depth, 198
Periodontal Cyst, 198
Differences Between Suprabony and Infrabony Pocket,
200
Determination of Pocket Depth, 200
Treatment of Periodontal Pocket, 200
Chapter 24: Bone Loss and Patterns of Bone
Destruction, 202
Introduction, 202
Normal Anatomy of Alveolar Bone, 202
Mechanism of Bone Formation and Bone Destruction, 202
Local Factors, 203
Gingival Inflammation, 203
Trauma from Occlusion, 204
Systemic Factors, 204
Pharmacological Agents and Bone Resorption,
204
xix
Contents
Radius of Action, 204
Rate of Bone Loss, 204
Periods of Destruction, 204
Factors Determining Bone Morphology in Periodontal
Disease, 205
Bone Destruction Patterns in Periodontal Disease, 205
Angular Defects, 205
Osseous Craters, 206
Bulbous Bony Contours, 206
Reversed Architecture, 206
Ledges, 207
Furcation Involvements, 207
Prevalence and Distribution of Bone Defects, 207
Chapter 25: Chronic Periodontitis, 209
Introduction, 209
Definition, 209
Diagnostic Criteria, 209
Clinical Features, 209
Microbiological Features, 210
Radiographic Features, 210
Types Based on Disease Distribution and Severity, 210
Nature of Disease Progression, 211
Risk Factors for Disease, 211
General Concept for Etiology, 212
Chapter 26: Aggressive Periodontitis, 213
Introduction, 213
Localized Aggressive Periodontitis, 213
Historical Background, 213
Clinical Features, 214
Radiographic Findings, 215
Histopathologic Features, 215
Bacteriology, 215
Immunology, 216
Treatment, 216
Generalized Aggressive Periodontitis, 216
Clinical Characteristics, 216
Radiographic Findings, 217
Risk Factors, 217
Microbiologic Factors, 217
Immunologic Factors, 217
Genetic Factors, 217
Environmental Factors, 217
Chapter 27: Necrotizing Ulcerative Periodontitis,
Refractory Periodontitis and Periodontitis as a
Manifestation of Systemic Disease, 218
Necrotizing Ulcerative Periodontitis (NUP), 218
Non-AIDS type, 218
AIDS associated, 219
Refractory Periodontitis, 219
Etiology, 219
Clinical Features, 219
Treatment, 220
Periodontitis as a Manifestation of Systemic Disease, 220
Papillon-Lefévre Syndrome, 220
Chédiak-Higashi Syndrome, 221
Down Syndrome, 221
Hypophosphatasia, 221
Neutropenia, 221
Leukocyte Adhesion Deficiency, 221
Chapter 28: AIDS and the Periodontium, 223
HIV Opportunistic Infections, 223
Classification of Periodontal Diseases Associated with
HIV Infection, 223
Linear Gingivitis, 224
Necrotizing Ulcerative Gingivitis, 224
Necrotizing Ulcerative Periodontitis, 224
Necrotizing Stomatitis, 224
CDC Surveillance Care Classification, 224
Most Common Oral and Periodontal Manifestations of HIV
Infection, 225
Oral Hairy Leukoplakia, 225
Oral Candidiasis, 225
Kaposi’s Sarcoma, 225
Bacillary Angiomatosis, 225
Oral Hyperpigmentation, 225
Atypical Ulcers and Delayed Healing, 225
Management, 225
Part V: Treatment of Periodontal Diseases
SECTION 1: DIAGNOSIS, PROGNOSIS AND
TREATMENT PLAN
Chapter 29: Diagnosis of Periodontal Diseases, 229
Periodontal Diagnosis, 229
Principles of Diagnosis, 229
Clinical Diagnosis, 229
Stages of Diagnosis, 229
Diagnosis with Detailed Case History Recording, 230
Case History Proforma, 232
Chapter 30: Determination of Prognosis, 237
Definition of Prognosis, 237
Difference Between Prognosis and Risk, 237
Determination of Prognosis, 237
Factors for Determination of Prognosis, 238
Overall Clinical Factors, 238
Systemic/Environmental Factors, 239
Local Factors, 240
Prosthetic/restorative Factors, 241
Relationship Between Diagnosis and Prognosis, 241
Re-evaluation of Prognosis After Phase I Therapy, 243
Essentials of Clinical Periodontology and Periodontics
xx

Chapter 31: Related Risk Factors Associated with
Periodontal Diseases, 244
Definition, 244
Risk Factors, 244
Risk Determinants, 245
Risk Indicators, 245
Risk Markers/Predictors, 245
Clinical Risk Assessment, 246
Demographic Data, 246
Medical History, 246
Dental History, 246
Clinical Examination, 246
Chapter 32: Various Aids Including Advanced
Diagnostic Techniques, 247
Aids used in Clinical Diagnosis, 247
Periodontal Probes, 247
Conventional Probes, 247
PSR, 248
Aids used in Radiographic Diagnosis, 249
Orthopantomograph, 249
Xeroradiography, 249
Advanced Radiographic Techniques, 249
Iodine-125, 249
Photodensitometric Analysis, 250
Digital Radiography, 250
Subtraction Radiography, 250
CADIA, 250
Nuclear Medicine Bone Scan, 250
Aids in Microbiological Diagnosis, 250
Identification of Bacteria, 250
Speciation Techniques, 251
DNA Probes, 252
Aids in Immunological Diagnosis, 252
Immunofluorescence, 252
Latex Agglutination, 252
ELISA, 253
Flow Cytometry, 253
Biochemical Diagnosis, 253
Prostaglandins, 253
Collagenase, 253
Other Diagnostic Aids, 253
BANA Test, 253
FSEIA, 254
PCR, 254
Chapter 33: Treatment Plan, 256
Introduction, 256
Sequence of Therapeutic Procedures, 256
Emergency Phase, 256
Etiotropic Phase, 256
Surgical Phase, 256
Restorative Phase, 256
Preferred Sequence of Periodontal Therapy, 257
Chapter 34: Rationale for Periodontal Treatment, 258
Objectives of Periodontal Therapy, 258
Factors which Affect Healing, 259
Local Factors, 259
Systemic Factors, 259
Healing After Periodontal Therapy, 259
Regeneration, 259
Repair, 259
New Attachment, 259
Reattachment, 259
Chapter 35: Periodontal Instrumentarium, 262
Periodontal Instruments, 262
Classification of Periodontal Instruments, 262
Diagnostic Instruments, 262
Periodontal Probes, 263
Explorers, 264
Scaling and Root Planing, and Curettage Instruments,
262
Sickle Scalers, 264
Curettes, 265
Ultrasonic Instruments, 267
Dental Endoscope, 267
Cleansing and Polishing Instruments, 267
Surgical Instruments, 268
SECTION 2: PERIODONTAL THERAPY
(A) Non-Surgical Therapy
Chapter 36: Principles of Periodontal Instrumentation
including Scaling and Root Planing, 269
Introduction, 269
Accessibility, 269
Clinician Position, 269
Patient Position, 269
Visibility, Illumination and Retraction, 270
Condition of Instruments, 271
Maintaining a Clean Field, 271
Instrument Stabilization, 271
Instrument Grasp, 271
Finger Rest, 272
Instrument Activation, 273
Adaptation, 274
Angulation, 274
Lateral Pressure, 274
Strokes, 274
Stroke Direction, 275
Principles of Scaling and Root Planing, 275
Root Planing, 275
Supragingival Scaling Technique, 276
Subgingival Scaling, 276
Ultrasonic Instruments, 277
Aerosol Production, 278
xxi
Contents

Chapter 37: Plaque Control, 280
Definition, 280
Goals of Plaque Control Measures, 280
Rationale, 280
Basic Approaches for Plaque Control, 280
Mechanical Plaque Control, 280
Toothbrush, 281
Brushing Techniques, 282
Bass Method, 282
Modified Bass Method, 283
Stillman’s Method, 283
Modified Stillman’s Method, 283
Charter’s Method, 283
Roll Method/Fones Technique, 283
Powered Toothbrushes, 284
Dentifrices, 284
Interdental Cleaning Aids, 285
Other Aids, 286
Chemical Plaque Control, 286
Ideal Properties of a Mouthwash, 287
Classification of Antimicrobial Agents, 287
Bisbiguanides, 288
Disclosing Agents, 289
Section B: Surgical Therapy
Chapter 38: Principles of Periodontal Surgery, 291
Indications, 291
Contraindications, 291
General Principles of Surgery, 291
Patient Preparation, 292
Premedication, 292
Tissue Management, 292
Suturing Material, 292
Suturing Techniques, 293
Periodontal Dressing, 293
Instructions for the Patient After Surgery, 299
Complications During Surgery, 300
Syncope, 300
Hemorrhage, 300
Complications in the First Postoperative Week, 300
Hospital Periodontal Surgery, 301
Chapter 39: Gingival Curettage, 303
Definition, 303
Types, 303
Rationale, 303
Indications, 304
Procedure, 304
Basic Technique, 304
ENAP, 304
Ultrasonic Curettage, 305
Caustic Drugs, 305
Healing After Scaling and Curettage, 305
Clinical Appearance After Scaling and Curettage, 305
Chapter 40: Gingivectomy, 307
Introduction, 307
Definitions, 307
Pre-requisites, 307
Indications, 307
Contraindications, 308
Types of Gingivectomy, 308
Surgical Gingivectomy, 308
Procedure for Gingivoplasty, 310
Healing After Surgical Gingivectomy, 310
Electrosurgery, 310
Laser Gingivectomy, 312
Gingivectomy by Chemosurgery, 312
Chapter 41: Periodontal Flap, 314
Introduction, 314
Indications/Objectives of Flap Surgery, 314
Definition, 315
Classification, 315
Incisions, 316
Flap Techniques for Pocket Therapy, 320
Modified Widman Flap, 320
Undisplaced Flap, 325
Palatal Flap, 326
Apically-displaced Flap, 328
Distal Molar Surgery, 328
Healing After Flap Surgery, 329
Chapter 42: Osseous Surgery, 330
Definition, 330
Rationale, 330
Terminology, 330
Types of Osseous Surgery, 331
Resective Osseous Surgery, 331
Indications, 331
Contraindications, 331
Methods of Osseous Surgery, 332
Instruments Used, 332
Technique, 332
Vertical Grooving, 332
Radicular Blending, 332
Flattening of Interproximal Bone, 332
Gradualizing Marginal Bone, 332
Healing After Resective Osseous Surgery, 333
Reconstructive Osseous Surgery, 333
Evaluation of New Attachment, 333
Clinical Method, 333
Radiographic Methods, 333
Surgical Re-entry, 333
Histological Methods, 333
Nongraft-associated New Attachment, 334
Removal of Junctional and Pocket Epithelium, 334
Prevention of Epithelial Migration, 334
GTR, 334
Root Biomodification, 336
xxii
Essentials of Clinical Periodontology and Periodontics

Graft-associated New Attachment, 336
Terminology, 336
Ideal Requirements of a Bone Graft Material, 337
Autografts, 337
Allografts, 338
Xenografts, 338
Alloplasts, 338
Chapter 43: Mucogingival Surgery, 341
Definition, 341
Mucogingival Problems, 341
Objectives, Indications and Contraindications of
Mucogingival Surgery, 341
Techniques to Increase the Width of Attached Gingiva, 342
Free Soft Tissue Autograft, 342
Classic Technique, 342
Variant Techniques, 344
Accordian Technique, 346
Strip Technique, 346
Connective Tissue Technique, 346
Combination Techniques, 346
Healing of the Graft, 346
Apically-Displaced Flap, 346
Other Techniques, 348
Procedures for Root Coverage, 348
Indications, 348
Classification, 349
Conventional Procedures, 351
Laterally Displaced Flap, 352
Double Papilla Flap, 353
Coronally-Repositional Flap, 353
Semilunar Flap, 357
Subepithelial Connective Tissue Graft, 358
Modifications, 358
Envelope Technique, 358
Langer’s Technique, 360
Pouch and Tunnel Technique, 360
Guided Tissue Regeneration Technique for Root
Coverage, 360
Operations for Removal of Frena, 362
Frenectomy, 362
Frenotomy, 362
Technique, 362
Chapter 44: Furcation Involvement and Its
Management, 365
Definition, 365
Etiology, 365
Primary Etiologic Factor, 365
Anatomic Considerations, 365
Classification, 367
Clinical Features, 368
Prognosis, 368
Treatment, 369
Traditional Treatment, 369
Reconstructive/Regenerative Treatment, 369
Resective Treatment, 370
Definition, 370
Indications and Contraindications, 370
Chapter 45: Pulpoperiodontal Problems, 373
Introduction, 373
Pathways of Communication Between Pulp and
Periodontium, 373
Developmental Origin, 373
Pathological Origin, 373
Iatrogenic Origin, 373
Effects of Pulpal Disease on the Periodontium, 373
Effects of Periodontitis on the Pulp, 374
Classification of Endo-Perio Lesions, 375
Microbiological Findings of Endo-Perio Lesions, 375
Diagnosis and Treatment of Endo-Perio Lesions, 375
Chapter 46: Splints in Periodontal Therapy, 378
Definition, 378
Objectives of Splinting, 378
Classifications of Splints, 379
Various Commonly Used Splints, 379
Principles of Splinting, 379
Indications and Contraindications of Splinting, 379
Advantages and Disadvantages of Splinting, 380
Chapter 47: Dental Implants: Periodontal
Considerations, 381
Introduction, 381
Terminology, 381
Historical Background, 382
Biological Considerations of Implants, 382
Soft Tissue Implant Interface, 382
Bone Implant Interface, 383
Biomaterials Used for Implants, 383
Classification of Implants, 383
Classification of Implant Systems, 384
Treatment Planning, 384
Clinical Assessment, 384
Absolute Requirements, 384
Indications, 384
Absolute Contraindications, 385
Intraoral Contraindications, 385
Radiographs, 385
Other Radiographic Procedures, 385
Surgical Procedures, 385
One-stage: Endosseous Implant Surgery, 385
Two-stage: Endosseous Implant Surgery, 386
Healing Following Implant Surgery, 386
Early Phase, 386
Late Stage, 386
Peri-implant Complications, 387
Types of Peri-implant Disease, 387
xxiii
Contents

Clinical Features, 387
Diagnosis, 387
Management, 387
Maintenance, 388
Chapter 48: Maintenace Phase (Supportive Periodontal
Treatment), 390
Importance of Maintenance Phase, 390
Rationale for Supportive Periodontal Therapy, 390
Causes for Recurrence of Periodontal Disease, 390
Objectives of Maintenace Phase, 391
Parts of Maintenance Phase, 391
Part-I: Examination, 391
Part-II: Treatment, 391
Part-III: Schedule Next Procedure, 391
Determination of Maintenance Recall Intervals, 392
Management of Particular Type of Recall Patients, 392
Chapter 49: Occlusal Evaluation and Therapy in the
Management of Periodontal Disease, 394
Terminology, 394
Clinical Evaluation of Occlusion, 394
Temporomandibular Disorders, 394
Intraoral Evaluation, 395
Management of Trauma from Occlusion, 395
Occlusal Therapy, 395
Chapter 50: The Role of Orthodontics as an Adjunct
to Periodontal Therapy, 396
Introduction, 396
Rationale for Orthodontic Treatment in Periodontal
Therapy, 396
Reducing Plaque Retention, 396
Improving Gingival and Osseous Form, 396
Improving Esthetics, 397
Indications and Contraindications of Orthodontic Therapy,
397
Timing of Orthodontic Procedures in Periodontal
Treatment, 397
Iatrogenic Effects Associated with Orthodontic Treatment,
397
Response of Periodontal Ligament to Orthodontic Forces,
398
Chapter 51: Periodontal: Restorative Inter-relationship,
400
Margins of Restorations, 400
Restorative Margins Encroaching on the Biologic Width,
400
Crown Contour, 401
Hypersensitivity to Dental Materials, 401
Proximal Contact and Embrasure, 401
Pontic Design, 401
Chapter 52: Drugs Used in Periodontal Therapy, 403
Introduction, 403
Classification of Various Drugs Used in Periodontal
Therapy, 403
Depending on Antimicrobial Efficacy and
Substantivity, 403
Chemicals Used for Supragingival Plaque Control,
404
Phenols, 404
Quaternary Ammonium Compounds, 404
Antibiotics Used in Periodontal Therapy, 404
Systemic Administration of Antibiotics, 404
Local Administration of Antibiotics, 405
Tetracyclines, 405
Metronidazole, 406
Penicillins, 407
Non-steroidal Anti-inflammatory Drugs (NSAIDs), 407
Mechanism of Action of NSAID, 407
Local Administration of Antibiotics and Antimicrobial
Agents, 407
Classification of Controlled-Release, 408
Methods of Delivery of Chemotherapeutic Agents, 408
Keyes Technique, 408
Root Biomodification, 408
Home Irrigation Devices, 409
Conclusion, 409
53. Questionnaire for Clinical Case Discussion, 411
Glossary, 439
Index, 465
Essentials of Clinical Periodontology and Periodontics
xxiv



1
Anatomy and Development
of the Structures of
Periodontium
❒
❒
❒
❒
❒ EXTERNAL ANATOMIC FEATURES
❒
❒
❒
❒
❒ DEVELOPMENT OF PERIODONTIUM
• Early Development of Cementum
• Later Development of Cementum
• Development of Junctional Epithelium
INTRODUCTION
The term periodontium arises from the Greek word “Peri”
meaning around and “odont” meaning tooth, thus it
can be simply defined as the “tissues investing and
supporting the teeth”. The periodontium is composed
of the following tissues namely alveolar bone, root
cementum, periodontal ligament (supporting tissues) and
gingiva (investing tissue).
The various diseases of the periodontium are
collectively termed as Periodontal diseases. Their
treatment is referred to as Periodontal therapy. The
clinical science that deals with the periodontium in health
and disease is called Periodontology. The branch of
dentistry concerned with prevention and treatment of
periodontal disease is termed Periodontics or Periodontia.
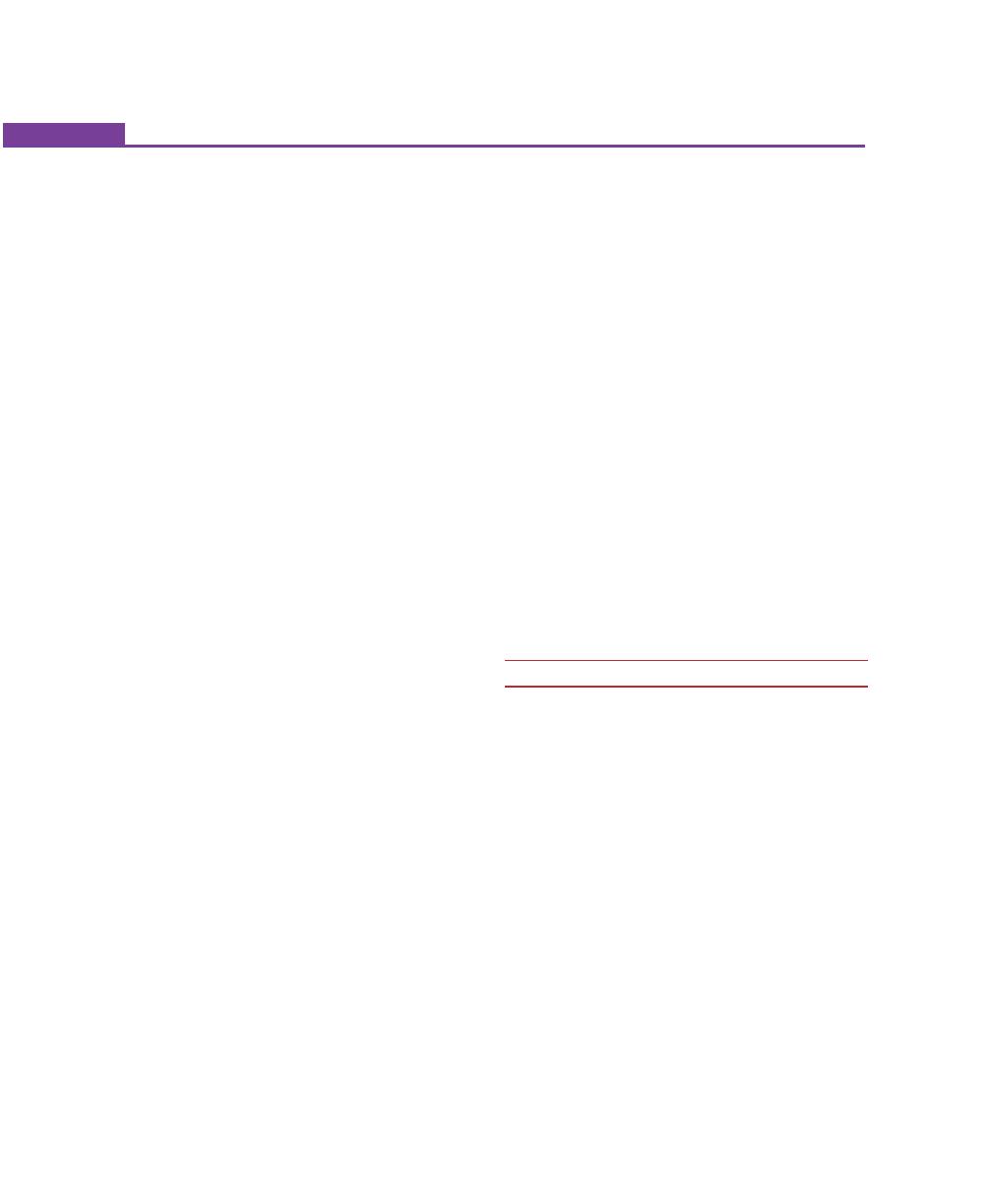
EXTERNAL ANATOMIC FEATURES
The oral mucosa consists of three zones:
1. Masticatory mucosa: It includes the gingiva and the
covering of the hard palate.
2. Specialized mucosa: It covers the dorsum of the
tongue.
3. Lining mucosa: Is the oral mucous membrane that
lines the oral cavity. Among all the structures of the
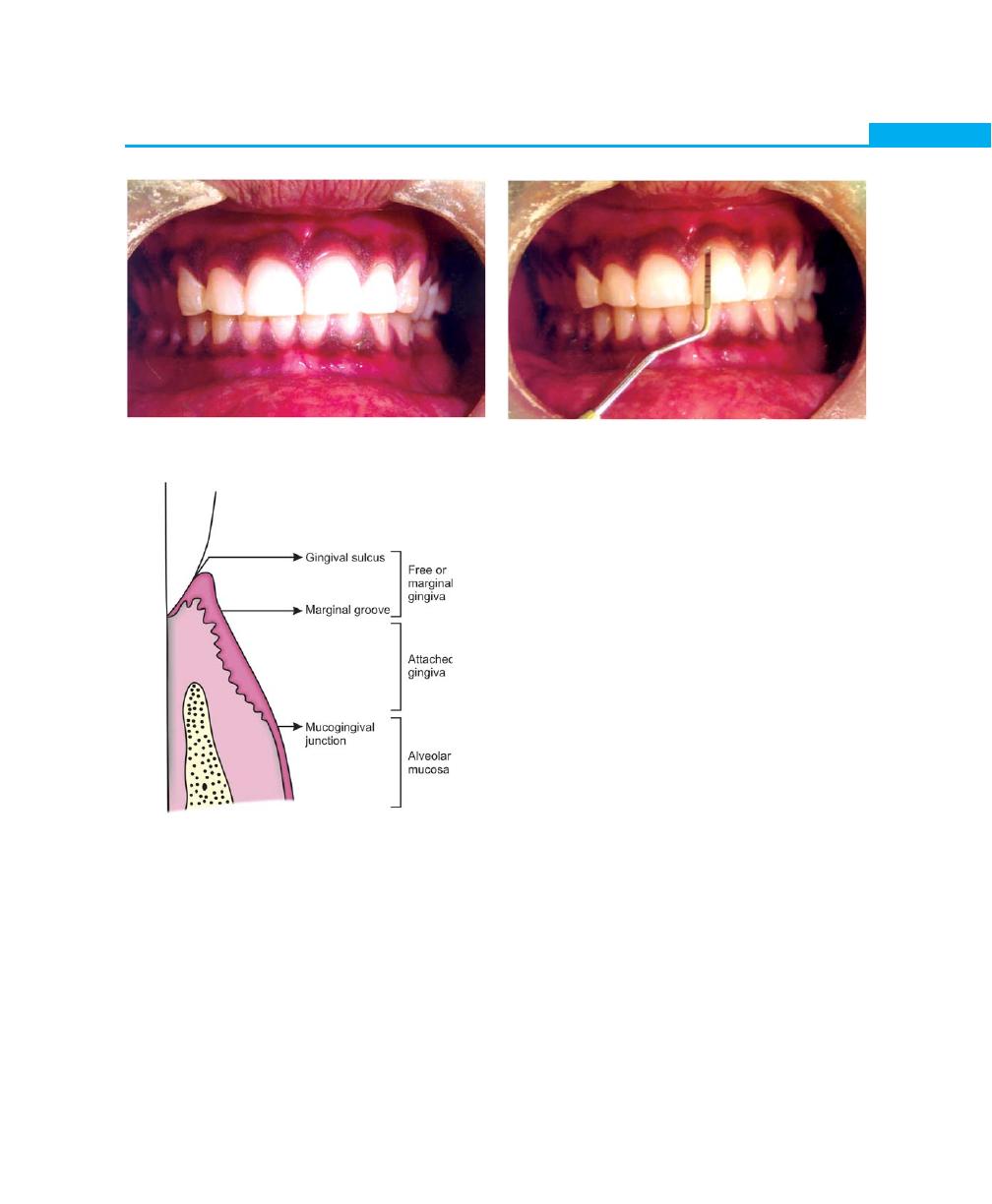
periodontium, only the gingiva is visible clinically. The
gingiva is divided anatomically into free or marginal,
attached and interdental gingiva. The border or
groove between marginal and attached gingiva is
called as a free gingival groove, a shallow depression
on the faciogingival surface that roughly corresponds
to the base of the gingival sulcus. The junction
between the attached gingiva and alveolar mucosa
is called as mucogingival line or junction (Fig. 1.1).
The normal gingiva is pink in color (salmon coral
pink) and accumulation of melanin pigmentation is
normal. The surface of the gingiva exhibits an orange
peel-like appearance referred to as stippling. In health,
the gingiva completely fills the embrasure spaces between
the teeth and is known as the interdental gingiva. In the
posterior teeth, where the contact areas between the
teeth are usually broad, the interdental gingiva consists
of two papillae, facial and lingual which are connected
by the col. The significance of col is that, it is made up
4
Essentials of Clinical Periodontology and Periodontics
Fig. 1.1:
Surface characteristics of the clinically-
normal gingiva
Fig. 1.2:
Root sheath of Hertwig
Fig. 1.3:
Proliferation of root sheath and further dentine
formation in an apical direction
of non-keratinized epithelium and hence represents the
most frequent site for initiation of disease process.
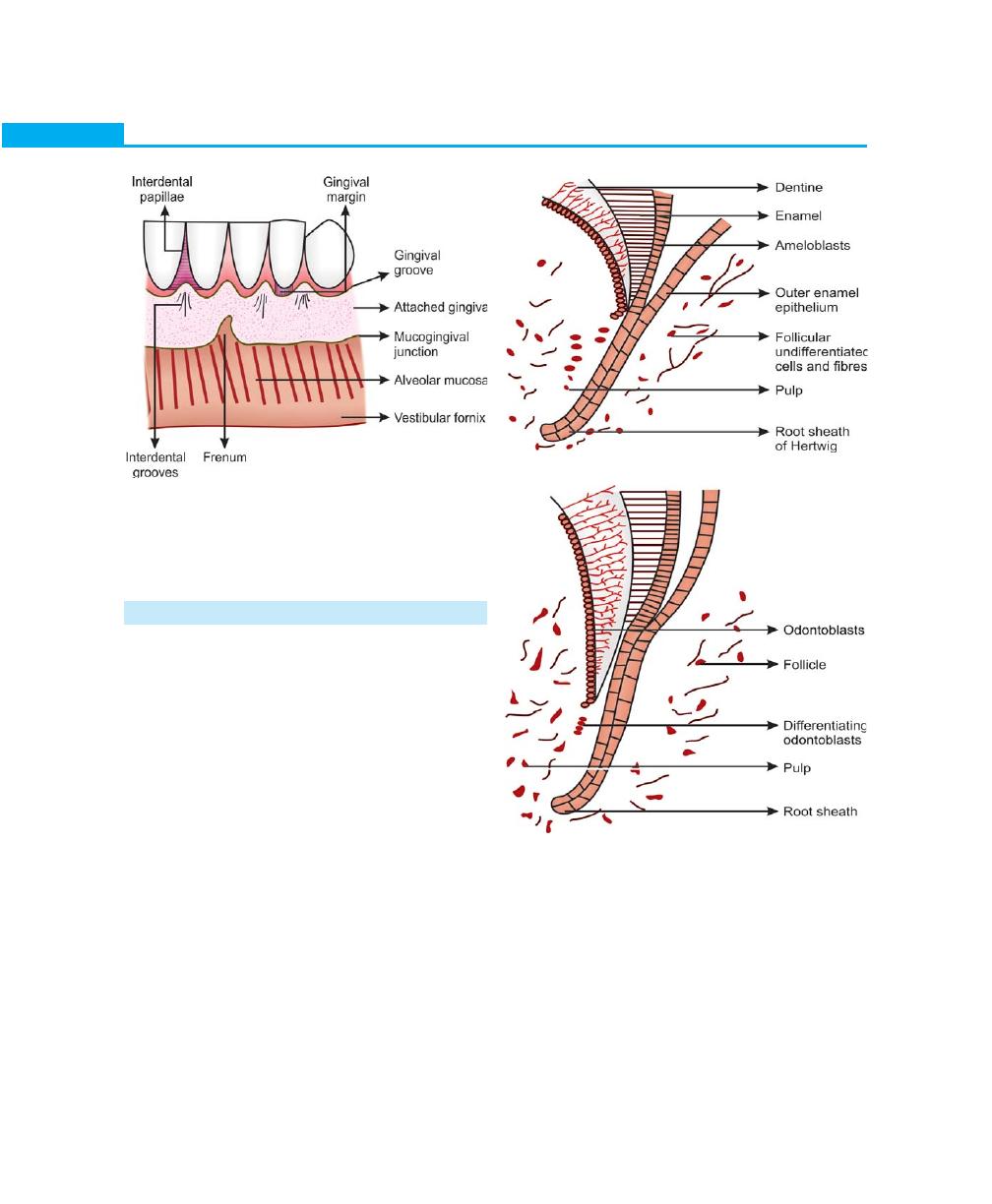
DEVELOPMENT OF PERIODONTIUM
To understand the development of periodontal tissues
one has to have a clear understanding of the root
formation. Development of cementum and roots of the
teeth starts once the formation of enamel is completed.
The outer and inner epithelia together form the epithelial
root sheath of Hertwig, which is responsible for deter-
mining the shape of the root.
Early Development of Cementum
The outer and inner epithelial layers become continuous
(without stratum intermedium or stellate reticulum) in
the area of the future cementoenamel junction and form
a two-layered sheath, which grows into the underlying
mesenchyme. The apical portion of the root sheath
remains constant whereas the coronal portion, which
is associated with dentin and cementum formation moves
in the direction of the oral cavity. The root sheath bends
horizontally at the level of future cementoenamel junction
forming the epithelial diaphragm, following which the
cervical opening becomes smaller (Figs 1.2 and 1.3).
Once the crown formation is complete the cells of
the inner enamel epithelium loose their ability to form
enamel and is called reduced enamel epithelium. They
retain the ability to induce perimesenchymal cells to
differentiate into odontoblasts and to proceed with the
formation of predentin and dentin.
After the dentin formation is completed, certain
changes occur in the root sheath. Recent studies have
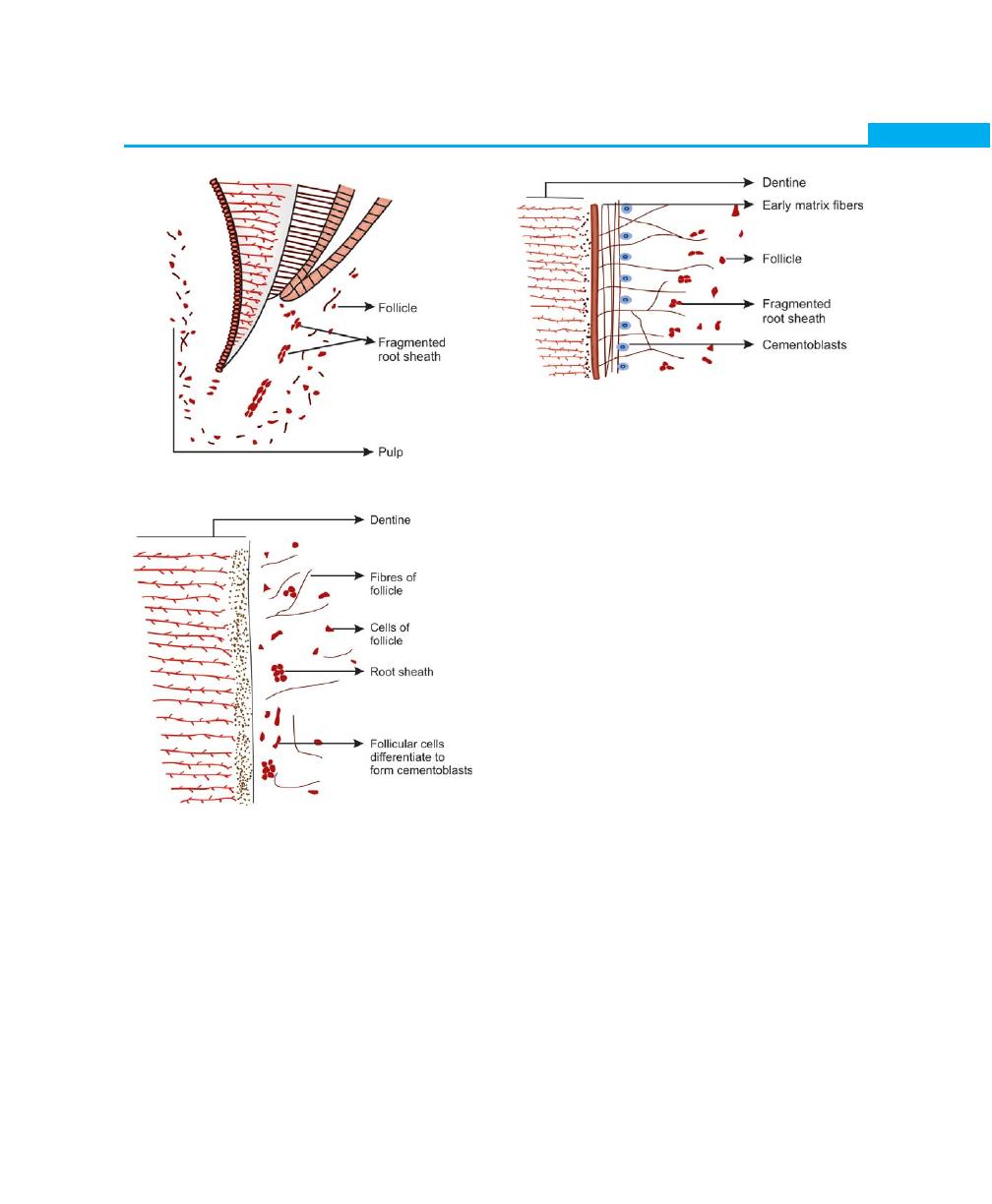
5
Anatomy and Development of the Structures of Periodontium
Fig. 1.4:
Fragmentation of root sheath
Fig. 1.5:
Follicular cells and fibres contact dentine surface
Fig. 1.6:
Early deposition of matrix
shown that, the epithelial cells of the root sheath produce
a layer on the root dentin, which is 10 /xm thick, has
a hyaline appearance and contains fine granules and
fibrils. This layer is called the hyaline layer of Hopewell
Smith or intermediate cementum. The epithelial cells of
the root sheath secrete enamel proteins such as
amehgenin or enameloids. The root sheath at this stage
becomes discontinuous and enables the surrounding
follicular mesenchyme to come in contact with the
amelogenin. These follicular cells then differentiate into
cementoblasts and deposit the organic matrix of
cementum on the root surface (Figs 1.4 and 1.5).
Later Development of Cementum
Cementoblasts are cuboidal cells that are arranged on
the outer surface of the hyaline layer. These cells are
responsible for the deposition of the organic matrix of
cementum, which consists of proteoglycan ground
substance, intrinsic collagen fibers and is followed by
subsequent mineralization of the organic matrix (Fig.
1.6).
Mineralization starts with the formation of a thin layer
called cementoid. Mineral salts are derived from the tissue
fluid containing calcium and phosphate ions and are
deposited as hydroxyapatite crystals.
The disintegrated Hertwigs root sheath slowly moves
away from the root surface and remain in the periodontal
ligament as epithelial cell rests of Malassez. The
periodontal ligament forms from the dental follicle soon
after root formation begins. Before a tooth erupts, fibers
from the follicle are incorporated in the cementum and
they lie parallel to the root surface. Once the tooth erupts,
the fibers are arranged in an oblique manner and are
regarded as the precursor of the periodontal ligament
fibers. As the cementum continues to increase in thickness,
more fibers become incorporated into the cementum
and eventually called as Sharpey’s fibers, when
periodontal ligament becomes established.
Alveolar bone forms around the periodontal ligament.
With continuous bone deposition the periodontal
6
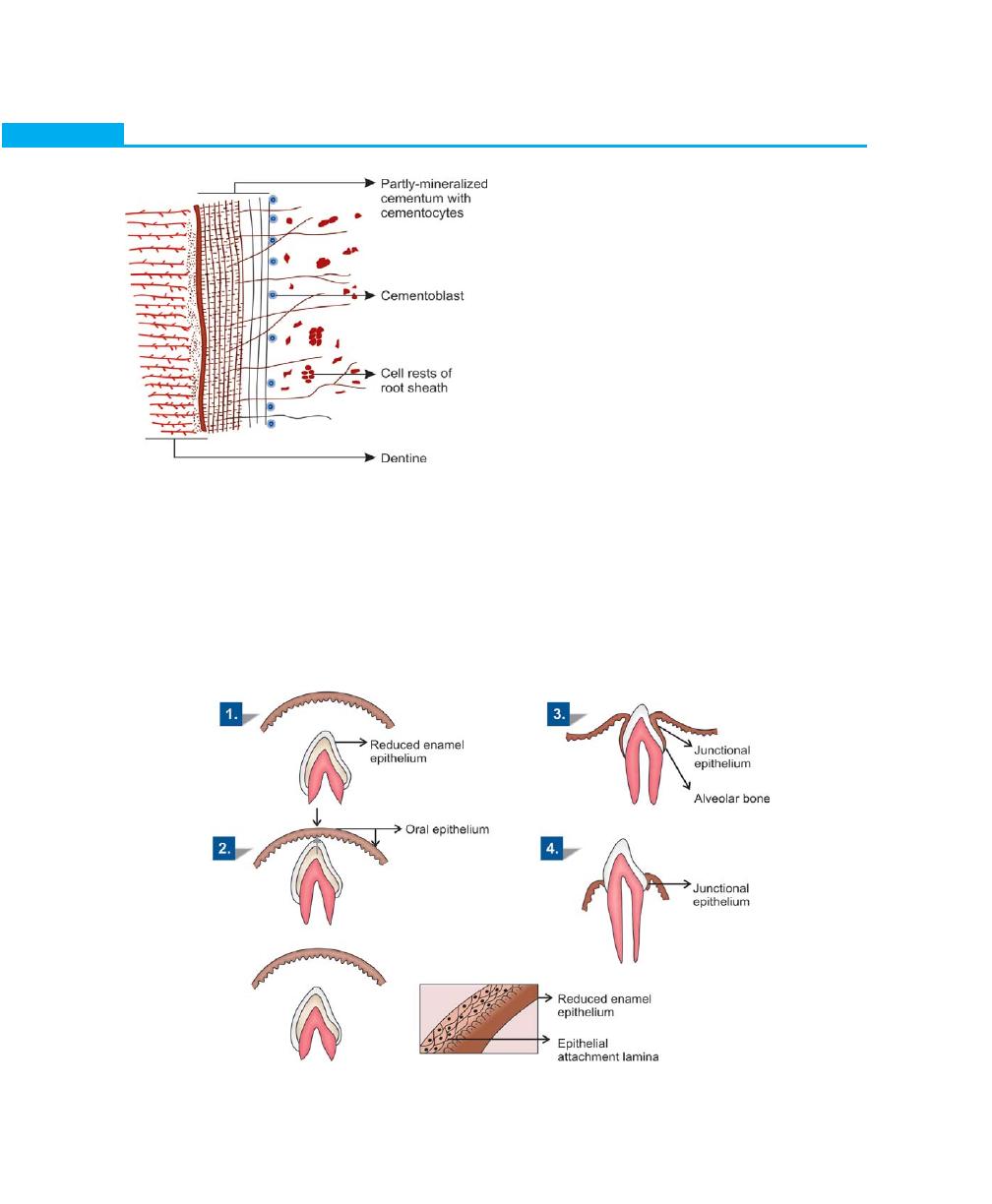
Essentials of Clinical Periodontology and Periodontics
the surface of the enamel called primary enamel cuticle.
The inner enamel epithelium after laying down enamel
reduces to a few layers of flat cuboidal cells, which is
then called as reduced enamel epithelium. It covers the
entire enamel surface extending till the cemento-enamel
junction. During eruption, the tip of the tooth approaches
the oral mucosa leading to fusion of the reduced enamel
epithelium with the oral epithelium. As the crown
emerges into the oral cavity the former ameloblasts that
-are in contact with the enamel get transformed into
Junctional epithelium. Coronally the Junctional
epithelium is continuous with the oral epithelium. As the
tooth erupts, the reduced enamel epithelium grows
shorter gradually. A shallow groove, the gingival
sulcus may develop between the gingiva and the tooth
surface.
Hence, the ameloblasts pass through two phases, in
one they form enamel and in the other phase they help
in formation of primary epithelial attachment or
Junctional epithelium. When the Junctional epithelium
forms from the ameloblasts it is called primary epithelial
attachment. Junctional epithelium that forms after surgical
therapy takes its origin from the basal cells of oral
ligament space gradually becomes narrower. The alveolar
process develops during the eruption of the teeth and
cells responsible for bone formation are osteoblasts
(Fig. 1.7).
Development of Junctional Epithelium (Fig. 1.8)
When the enamel formation is complete the ameloblasts
become shorter, and they leave a thin membrane on
Fig. 1.7:
Advanced formation of cementum
Fig. 1.8:
Development of junctional epithelium
7
Anatomy and Development of the Structures of Periodontium
epithelium instead of ameloblasts. This is called secondary
epithelial attachment.
KEY POINTS TO NOTE
1. The periodontium is composed of alveolar bone, root
cementum, periodontal ligament (supporting tissue) and
gingiva (investing tissue).
2. The gingiva is the only structure of the periodontium that
is clinically visible and anatomically it can be divided into
marginal, attached and interdental gingiva.
3. The junction between the marginal and attached gingiva is
called free gingival groove, where as the junction between
the attached gingiva and alveolar mucosa is called as
mucogingival junction.
4. Once the dentin formation is completed, the root sheath
becomes discontinuous and allows the surrounding
mesenchyme to come in contact with the products of the
epithelial cells of the root sheath i.e. amelogenin. These
follicular cells then differentiate into cementoblasts,
fibroblasts and osteoblasts by which cementum, periodontal
ligament fibers and alveolar bone are deposited.
5. Fusion of the oral epithelium along with the reduced enamel
epithelium gives rise to the Junctional epithelium or epithelial
attachment.
REVIEW QUESTIONS
1. Define periodontology and periodontics.
2. What are the parts of periodontium?
3. Describe the development of structures of
periodontium.
BIBLIOGRAPHY
1. BG Jansen Van Rensburg. Oral Biology, Chapter 8.
Quintessence Publishing Co Ltd, 1995; 301-7.
2. BRR Varma, RP Nayak. Current Concepts in Periodontics,
Chapter 2. Arya Publishing, 2002; 4-8.
3. S.N.Bhasker. Orbans, Oral Histology and Embryology,
10th edition, CBSP Publishers and Distributers, New Delhi,
41-4.
4. Tencate. Oral Histology, Development, Structure and
Function, 3rd edition, Jaypee Bros. 228–43.
8
Essentials of Clinical Periodontology and Periodontics
INTRODUCTION
Periodontium is the functional unit of tissues supporting
the tooth including gingiva, the periodontal ligament,
the cementum and the alveolar process. The tooth and
periodontium are together called as the dentoperiodontal
unit. The main support of the tooth is provided by the
periodontal ligament, which connects the cementum of
the root to the alveolar bone or tooth socket, into which
the root fits. The main function of the gingiva is to protect
the surrounding tissues from the oral environment.
THE GINGIVA
Macroscopic Features
The gingiva is that part of the oral mucosa (masticatory
mucosa) that covers the alveolar process of the jaws and
surrounds the necks of the teeth. Anatomically the gingiva
is divided into—marginal, attached and interdental
gingiva (Fig. 2.1).
Marginal Gingiva or Free Gingiva or
Unattached Gingiva
It is defined as the terminal edge or border of the gingiva
surrounding the teeth in a collar-like fashion. In some
cases it is demarcated apically by a shallow linear
depression called the “free gingival groove”. Though the
❒
❒
❒
❒
❒ THE GINGIVA
• Macroscopic Features
• Microscopic Features
❒
❒
❒
❒
❒ TOOTH-SUPPORTING STRUCTURES
❒
❒
❒
❒
❒ PERIODONTAL LIGAMENT
• Definition
• Structure
• Cellular Composition
• Extracellular Components
• Development of Principal Fibers of
Periodontal Ligament
• Structures Present in the Connective
Tissue
• Functions of Periodontal Ligament
• Clinical Considerations
❒
❒
❒
❒
❒ ALVEOLAR BONE
• Definition
• Parts of Alveolar Bone
• Composition of Alveolar Bone
• Osseous Topography
• Blood Supply to the Bone
• Clinical Considerations
❒
❒
❒
❒
❒ CEMENTUM
• Definition
• Classification
• Functions
• Composition
• Thickness of Cementum
• Cementoenamel Junction
• Cemental Resorption and Repair
2
Biology of
Periodontal Tissues
9
Biology of Periodontal Tissues
Fig. 2.1:
Normal gingiva in health
marginal gingiva is well-adapted to the tooth surface,
it is not attached to it (Fig. 2.2).
Gingival Sulcus
It is defined as the space or shallow crevice between the
tooth and the free gingiva, which extends apical to the
junctional epithelium. It is V-shaped and barely permits
the entrance of a periodontal probe. Under normal or
ideal conditions it is about 0 mm (seen only in germ
free animals). The so-called probing depth of a clinically-
Fig. 2.2:
Anatomic land marks of gingiva
Fig. 2.3:
Sulcus depth in healthy gingiva
normal gingival sulcus in humans is 2 to 3 mm
(Fig. 2.3).
Attached Gingiva
It is defined as that part of the gingiva that is firm, resilient
and tightly-bound to the underlying periosteum of the
alveolar bone. On the facial aspect it extends upto the
loose and movable alveolar mucosa, from which it is
demarcated by the mucogingival junction. The width of
attached gingiva is the distance between the mucogingival
junction and the projection on the external surface of
the bottom of the gingival sulcus or the periodontal
pocket.
It varies in different areas of the mouth, greater in
the maxilla than mandible, least width in the mandibular
first premolar area, greatest width in the maxillary incisor
region. The width of attached gingiva increases with age
and in supraerupted teeth.
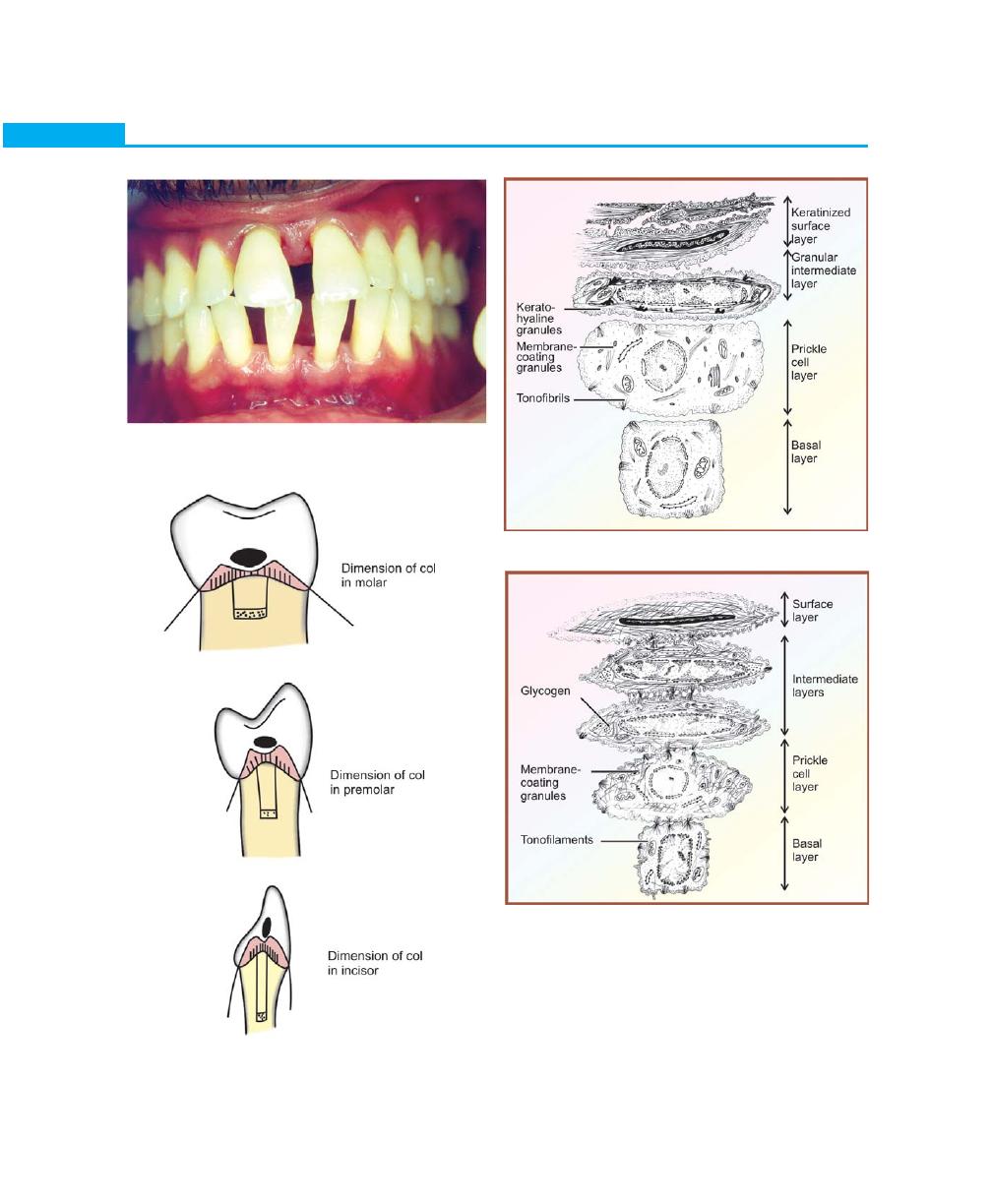
Interdental Gingiva
Usually occupies the gingival embrasure. There are three
parts of interdental gingiva, facial papilla, lingual papilla
and col, which is a valley-like depression that connects
the facial and lingual papilla. The lateral borders and
tips of the interdental papilla are formed by continuation
of marginal gingiva and the intervening portion by the
attached gingiva. In the presence of diastema the
interdental papilla will be absent (Figs 2.4 and 2.5).
10
Essentials of Clinical Periodontology and Periodontics
Fig. 2.5:
‘COL’ in various types of contacts
Fig. 2.6:
Orthokeratinized epithelium
Fig. 2.7:
Non-keratinized epithelium
Fig. 2.4:
Absence of interdental papilla in
diastema cases
Microscopic Features
The gingiva consists of a central core of connective tissue
covered by stratified squamous epithelium. Three types
of epithelium exists in the gingiva.
1. The oral or outer epithelium (Keratinized epithelium)
(Fig. 2.6).

11
Biology of Periodontal Tissues
2. The sulcular epithelium
3. The junctional epithelium (non-keratinized epithe-
lium) (Fig. 2.7).
In the keratinized epithelium, the principal cell type
is the keratinocyte, which can synthesize keratin. The
process of keratinization involves a sequence of bioche-
mical and morphological events that occur in a cell as
it migrates from the basal layer towards the cell surface.
The non-keratinized epithelium contains clear cells,
which include Melanocytes, Langerhans cells, Merkel cells
and Lymphocytes.
The oral epithelium has the following cell layers:
1. Basal layer (Stratum basale or Stratum germinativum).
2. Spinous layer (Stratum spinosum).
3. Granular layer (Stratum granulosum).
4. Keratinized cell layer (Stratum corneum).
There are three distinct differences between the oral
sulcular epithelium, oral epithelium and the junctional
epithelium:
a. The size of the cells in the junctional epithelium is,
relative to the tissue volume, larger than in the oral
sulcular epithelium.
b. The intercellular space in the junctional epithelium
is, comparatively wider than in the oral epithelium.
c. Granular layer, which is seen in the oral epithelium,
is absent in sulcular and junctional epithelium.
Morphologic Characteristics of the Different
Areas of Gingival Epithelium
Oral or outer epithelium: It covers the crest and outer
surface of the marginal gingiva and the surface of the
attached gingiva. It is keratinized or parakeratinized or
combination of both. Keratinization varies in different
areas in the following order: palate (most keratinized),
gingiva, ventral aspect of the tongue and cheek (least
keratinized). The keratinized epithelium of the gingiva
consists of four layers, namely stratum basale, stratum
spinosum, stratum granulosum and stratum corneum.
The cells of the basal layer are either cylindrical
or cuboidal and are in contact with the basement
membrane. The basal cells possess the ability to divide,
it is in the basal layer that the epithelium is renewed
and therefore this is also called as stratum germinativum.
When two daughter cells have been formed by cell
division an adjacent “older” basal cell is pushed into the
spinous cell layer and starts as a keratinocyte, to traverse
the epithelium. It takes approximately one month
for a keratinocyte to reach the outer epithelial surface
where it becomes desquamated from the stratum
corneum.
The basal cells are separated from the connective
tissue by a basement membrane. In light microscopy
this membrane appears as a zone approximately 1 µm
wide and reacts positively to a PAS stain (periodic acid
Schiff stain), which indicates the presence of carbo-
hydrates (glycoproteins) in the basement membrane. In
electron micrograph, immediately beneath the basal cell,
an approximately 400 Å wide electrolucent zone can
be seen which is called lamina lucida. Beneath the lamina
lucida an electron dense zone – lamina densa is observed.
From the lamina densa so-called anchoring fibrils project
in fan-shaped fashion into the connective tissue. The
epithelial cells facing the lamina lucida contain a number
of electron dense, thicker zones called hemidesmosomes.
The hemidesmosomes are involved in the attachment
of the epithelium to the underlying basement
membrane.
Stratum spinosum consists of large cells with short
cytoplasmic processes resembling spines. Since they are
arranged at regular intervals they give the cells a prickled
appearance. The cells are attached to one another by
numerous desmosomes (pairs of hemidesmosomes),
which are located between the cytoplasmic processes of
adjacent cells.
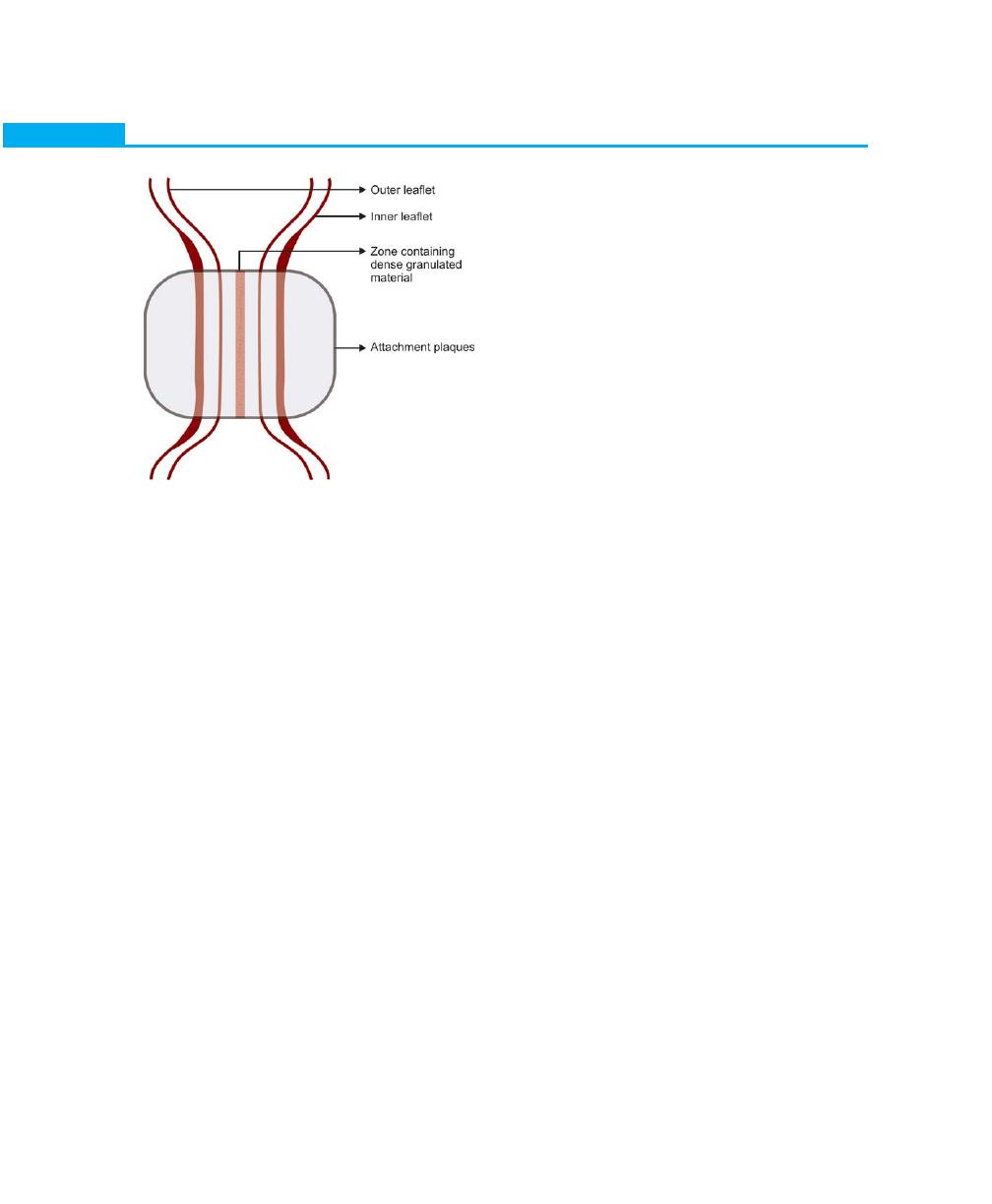
Composition of a desmosome: It consists of two adjoining
hemidesmosomes separated by a zone containing
electron dense granulated material (GM). In addition,
outer and inner leaflets of the cell (OL, IL) and the
attachment plaque (AP), represent granular and fibrillar
material in the cytoplasm (GM) (Fig. 2.8).
12
Essentials of Clinical Periodontology and Periodontics
Stratum granulosum: Electron dense keratohyalin
bodies begin to occur, these granules are believed to
be related to the synthesis of keratin. Here, there is
conversion of cells to “acellular” structure bordered by
a cell membrane indicating keratinization of the cytoplasm
of the keratinocyte.
Stratum corneum: The cytoplasms of the cells in this
layer are filled with keratin and the entire nucleus is lost.
But in parakeratinized epithelia, the cells contain remnants
of nuclei.
Keratinization is considered as a process of diffe-
rentiation rather than degeneration. The keratinocytes
while traversing from the basal layer to the epithelial
surface undergoes certain changes.
a. From the basal layer to the granular layer both the
number of tonofilaments in the cytoplasm and the
number of desmosomes increase significantly.
b. In contrast, the number of organelles such as
mitochondria, lamellae of rough endoplasmic
reticulum and Golgi apparatus decrease.
Oral sulcular epithelium: The soft tissue wall of the
gingival sulcus is lined coronally with sulcular epithelium,
extending from the gingival margin to the junctional
epithelium. It is made up of basal and prickle cell layer.
The sulcular epithelium resembles the oral/gingival
epithelium in all respects with the exception that it does
not become fully keratinized. Although it contains
keratinocytes they do not undergo keratinization.
However, the surface cells are flattened and exhibit a
tendency towards partial keratinization in response to
physical stimulation. Normally, there are no regular
retepegs in sulcular epithelium but they form during the
inflammation of the lateral wall.
Junctional epithelium: Denotes the tissue that joins to
the tooth on one side and to the oral sulcular epithelium
and connective tissue on the other. It forms the base
of the sulcus. It has been examined in detail by several
investigators, many hypothesis have been put forward
to explain the mode of attachment of the epithelium
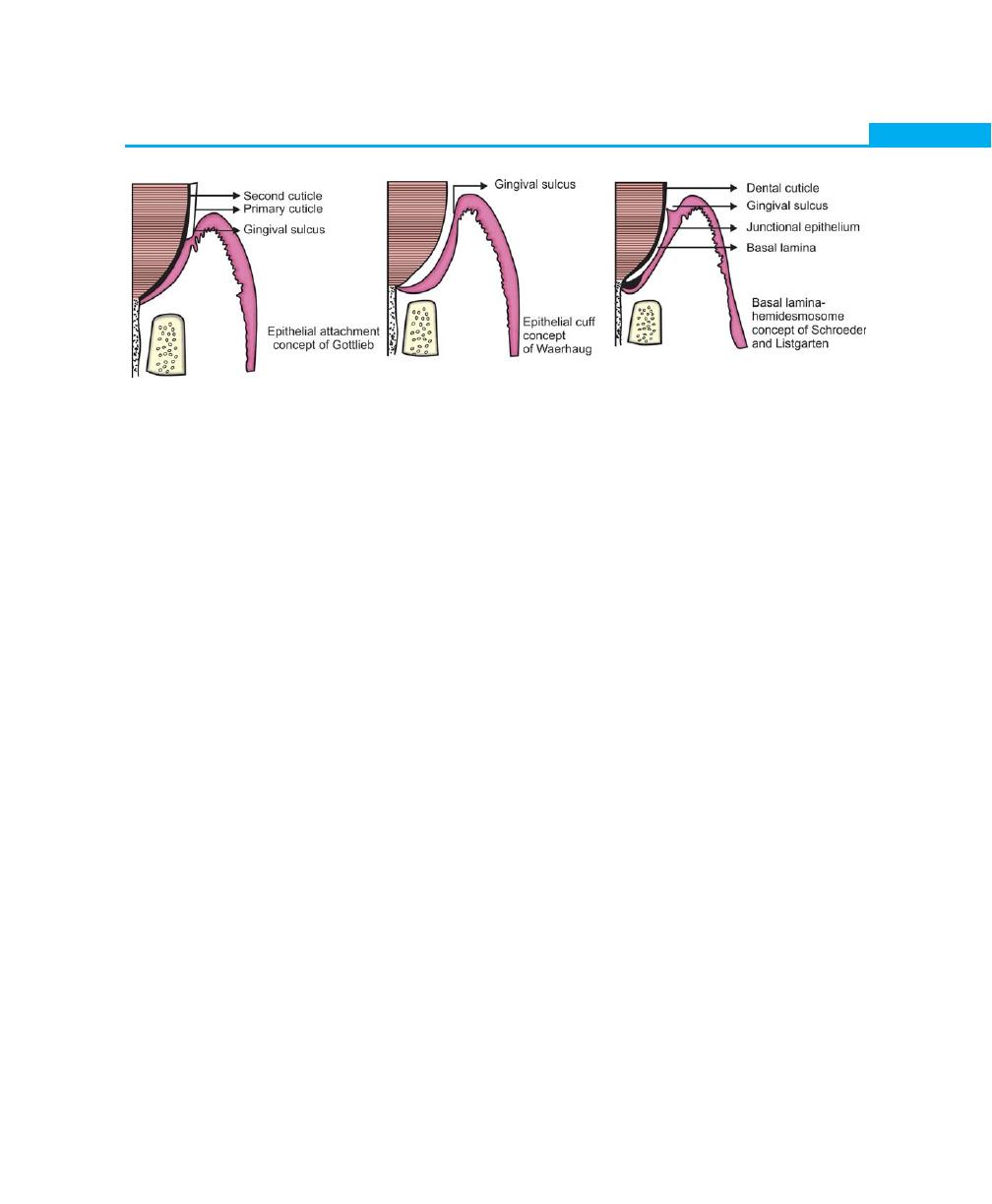
to the tooth surface.Prior to the Gottlieb concept, it was
believed generally, that the gingival soft tissues were closely
opposed, but not organically united to the surface of
the enamel. This concept was based on the clinical finding
that the gingiva could be easily deflected from the tooth
surface during instrumentation. However, experimental
and clinical observations have led Gottlieb to the concept
that the soft tissues of the gingiva are organically united
to the enamel surface. He termed it as “epithelial
attachment”. Although it was accepted generally, the
concept did not explain how exactly junctional epithelium
attaches to the root surface (Fig. 2.9).
Waerhaugh in 1952, based on his observations
presented the concept of the “epithelial cuff, he concluded
that the gingival tissues are closely apposed, but not
organically united to the tooth surface. In 1962 Stern
showed that the attachment to the tooth surface is
through hemidesmosomes. This was supported by
extensive studies conducted by Schroeder and
Listgarten, they revealed that there is a structural
continuity between the tooth surface and epithelium.
These studies by Schroeder and Listgarten states that
the epithelium-tooth interface was observed to be similar
to the epithelium-connective tissue interface and that is
Fig. 2.8:
Composition of a desmosome
13
Biology of Periodontal Tissues
Fig. 2.9:
Concepts of epithelial attachment
by hemidesmosome and basal lamina. A basal lamina
is always interposed between epithelial cells and crown
or root surface, and the epithelial cells are united to the
basal lamina by hemidesmosomes.
The junctional epithelium is attached to the tooth
surface by internal basal lamina and to the gingival
connective tissue by an external basal lamina. The internal
basal lamina consists of a lamina densa (adjacent to the
enamel) and a lamina lucida to which hemidesmosomes
are attached. Various studies have also shown that
junctional epithelial cells are involved in the production
of laminin and play a key role in the adhesion
mechanism. The attachment of the junctional epithelium
to the tooth surface is reinforced by the gingival fibers.
Hence the junctional epithelium and gingival fibers are
considered as a functional unit, referred to as the
dentogingival unit.
General structural features of junctional epithelium: It
consists of a collar like band of stratified squamous non
keratinizing epithelium. Thickness varies from three or
four layers in early life and increases with age upto 15
to 20 layers at the base of the gingival sulcus, and only
1 or 2 cells at the most apical portion. The length of
the junctional epithelium ranges from 0.25 to 1.35 mm.
The cells are arranged into basal and suprabasal layers
and they do not have granular layer or cornified layers.
They exhibit unusual cytologic features and differ
significantly from other oral epithelia. Three zones in
junctional epithelium have been described, apical,
coronal and middle. Apical is for germination, middle
is for adhesion and coronal is permeable.
The basal cells are cuboidal or in some cases, flattened.
They contain slightly more rough endoplasmic reticulum
and lysosomal content. The content of mitochondria of
the cells as they migrate toward and along the tooth
surface decreases. Cells of the suprabasal layer especially
those adjacent to the tooth surface exhibit complex
microvillus formation and interdigitation. Junctional
epithelial cells, especially those near the base of the
gingival sulcus, appear to have phagocytic capacity.
The structural features of junctional epithelium
indicate that it is highly permeable. Leukocytes and
lymphocytes within junctional epithelium are seen even
in clinically healthy gingiva. The densities of desmosomes
that interconnect the cells are considerably less than that
of oral epithelia and represents wider intercellular
junctions.
Epithelium–Connective Tissue Interface
Histological sections have demonstrated that, the
retepegs of epithelial cells project deeply into the
connective tissue leading to the formation of a series of
inter- connecting epithelial ridges. Basement membrane
seems to form a continuous sheet that connects the
epithelium and connective tissue. Electron microscope
reveals a faintly fibrillar structure, called as the basal
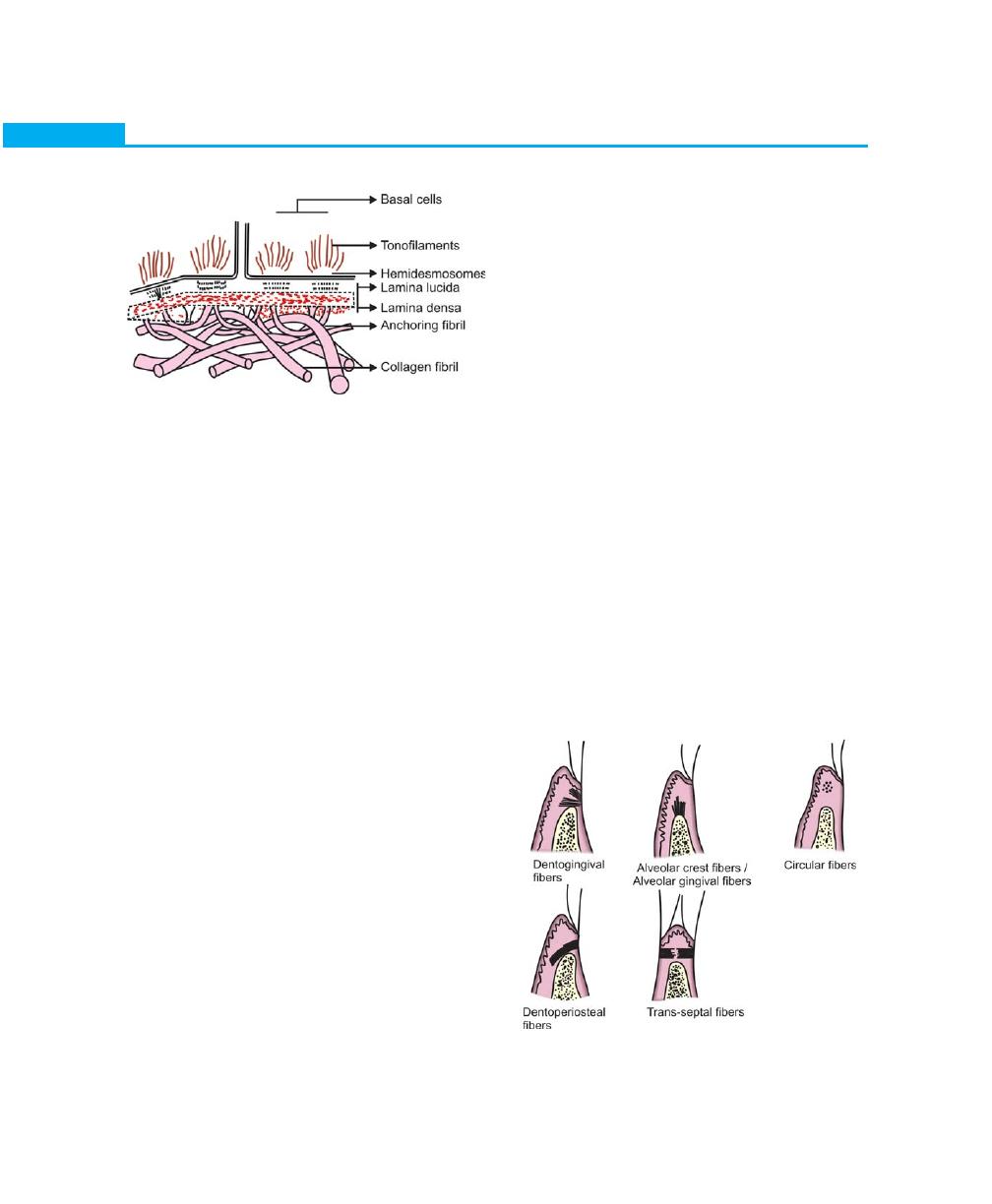
14
Essentials of Clinical Periodontology and Periodontics
Fig. 2.11:
The principal group of fibers
lamina, which is a part of the basement membrane. This
structure has lamina lucida adjacent to the basal epithelial
cells and lamina densa towards connective tissue. Basal
lamina is produced by adjacent epithelial cells and is made
up of collagenous proteins and proteoglycans binded
together into a totally-insoluble complex. It also contains
laminin and fibronectin. Fibrils measuring 20 to 40 nm
in diameter seem to extend from the basal cells through
the basal lamina and into the lamina propria of the
connective tissue. These structures called as anchoring
fibrils are supposed to bind the basal cells, basal lamina
and connective tissue together (Fig. 2.10).
Supra-alveolar Connective Tissue
The connective tissue supporting the oral epithelium is
termed as the lamina propria and for descriptive purpose
it can be divided into two layers:
a. The superficial papillary layer—associated with the
epithelial ridges.
b. Deeper reticular layer—that lies between the papillary
layer and the underlying structures.
The term reticular here means net-like and refers to the
arrangement of collagen fibers. In the papillary layer,
the collagen fibers are thin and loosely-arranged and
many capillary loops are present. The reticular layer is
dominated by collagen arranged in thick bundles.
The lamina propria consists of cells, fibers, blood
vessels embedded in amorphous ground substances.
I. Cells: Different types of cells present are:
a. Fibroblast
b. Mast cells
c. Macrophages
d. Inflammatory cells.
II. Fibers: The connective tissue fibers are produced by
fibroblasts and can be divided into:
a. Collagen fibers
b. Reticulin fibers
c. Oxytalan fibers
d. Elastin fibers.
Collagen type I form the bulk of the lamina propria
and provide the tensile strength to the gingival tissues.
Type II collagen is seen in the basement membrane.
The functions of gingival fibers are the following:
a. It braces the marginal gingiva firmly against the tooth.
b. It helps to withstand the forces exerted by mastication.
c. It unites the free gingiva to the root cementum and
the adjacent attached gingiva.
The arrangement of gingival fibers is described as
principal group of five bundles and secondary group
of minor fibers consisting of six sets (Fig. 2.11)
Fig. 2.10:
Structure of the junction between epithelium
and connective tissue
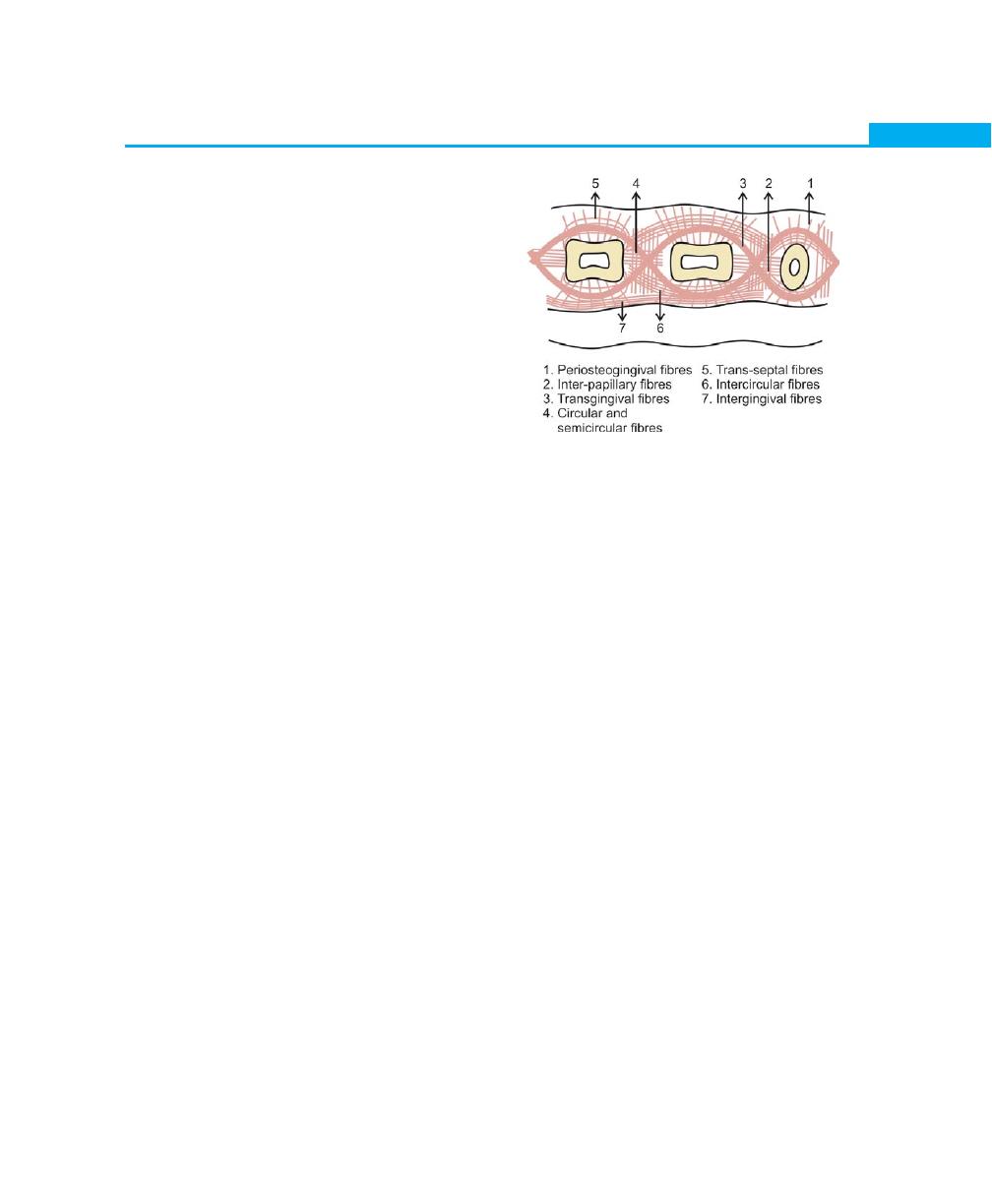
15
Biology of Periodontal Tissues
The principal group fibers are:
1. Dentogingival fibers: They project from the
cementum in a fan-like conformation towards the
crest and outer surface of the marginal gingiva. They
provide support to the gingiva by attaching it to the
tooth.
2. Alveolar gingival fibers: They extend from the
periosteum of the alveolar crest coronally into the
lamina propria. Their function is to attach the gingiva
to the alveolar bone.
3. Dentoperiosteal fibers: They arise from the
cementum near the cementoenamel junction and
insert into the periosteum of the alveolar bone and
protect the periodontal ligament.
4. Circular fibers: They surround the tooth in a cuff or
ring like fashion and course through the connective
tissue of the marginal and attached gingiva.
5. Trans-septal fibers: They are located interproximally,
they extend from cementum of one tooth to the
cementum of the neighbouring tooth. Their function
is to protect the interproximal bone and maintain
tooth-to-tooth contact.
Fibers of the secondary group (Fig. 2.12) are:
1. Periosteogingival fibers: They extend from the
periosteum of the alveolar bone to the attached
gingiva. They help to attach the gingiva to the alveolar
bone.
2. Interpapillary fibers: They are seen in the interdental
gingiva extending in a faciolingual direction and
support the gingival papilla.
3. Transgingival fibers: These are seen in and around
the teeth with in the attached gingiva. They maintain
the alignment of teeth in the arch.
4. Intercircular fibers: They extend from the cementum
on distal surface of a tooth splaying buccally and
lingually around the next tooth and are inserted on
the mesial surface.
5. Intergingival fibers: They are seen within the attached
gingiva adjacent to the basement membrane
extending mesiodistally. They provide support and
contour for the attached gingiva.
6. Semicircular fibers: They extend from the mesial
surface of a tooth to the distal surface of same tooth
in a half circle.
7. Oxytalan fibers: They are present in all connective
tissue structures of the periodontium. The function
of these fibers is yet unknown.
8. Elastin fibers: Elastin fibers are only present in
connective tissue of the gingiva and periodontal
ligament. They are also seen in the connective tissue
of alveolar mucosa in large numbers.
Extracellular matrix/ground substance: It is produced by
fibroblasts, followed by mast cells and other components
derived from the blood. The matrix is the medium in
which the connective tissue cells are embedded and is
essential for the maintenance of the normal function of
the connective tissue. Thus, the transportation of water,
electrolytes, nutrients, metabolites etc. to and from the
individual connective tissue cells occurs with in the matrix.
The main constituents of connective tissue matrix
are protein polysaccharide matrix. These complexes are
normally differentiated into proteoglycans and glyco-
proteins. The proteoglycans contains glycosaminogly-
cans, e.g. chondroitin sulfate, heparin sulfate, hyaluronic
acid etc. The proteoglycans act as a molecular filter and
Fig. 2.12:
Secondary group of fibers
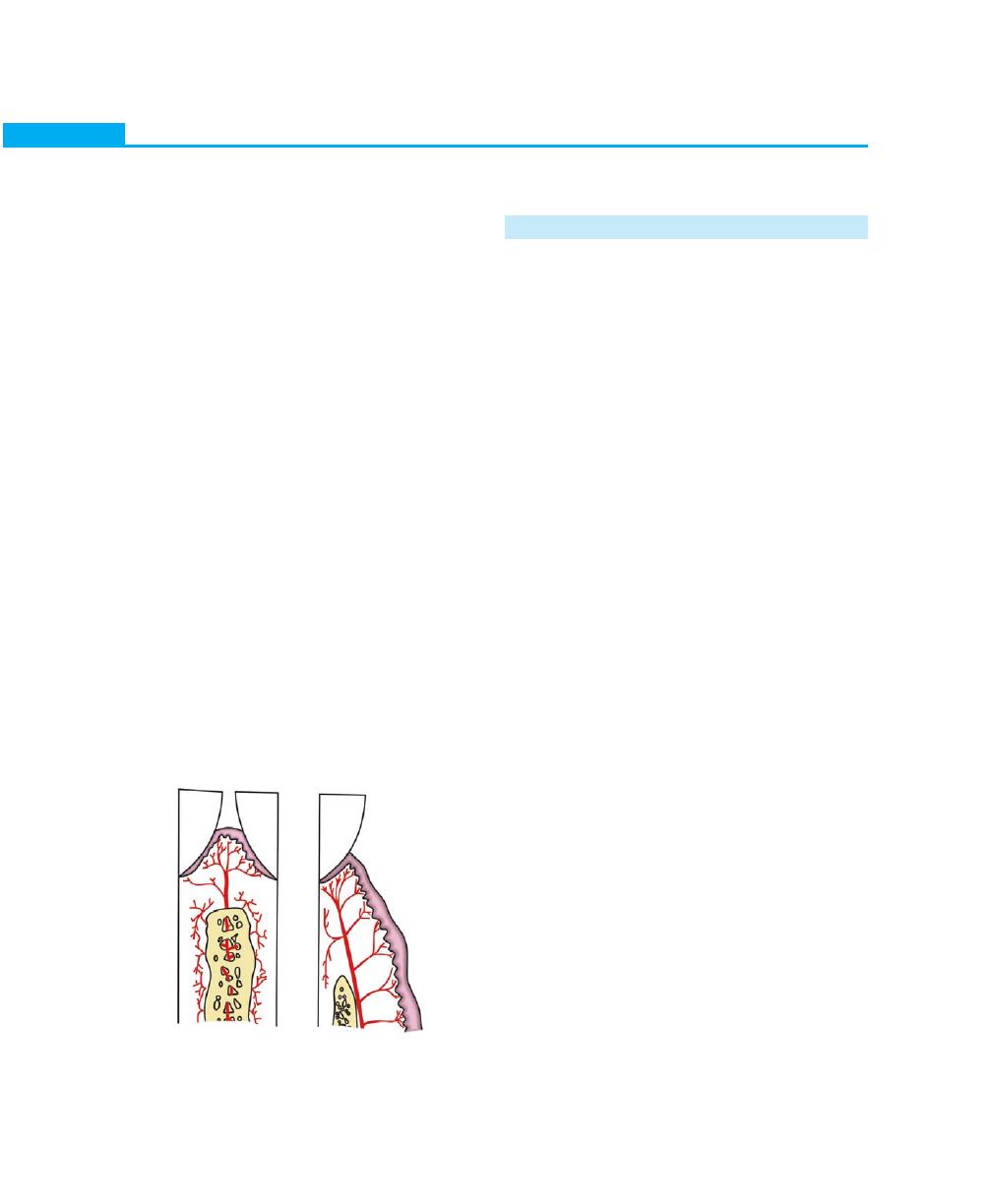
16
Essentials of Clinical Periodontology and Periodontics
in addition, play an important role in the regulation of
cell migration in the tissue. Due to their structure and
hydration, the macromolecules exert resistance and
hydration, towards deformation. Hence, when gingiva
is suppressed, the macromolecule become deformed,
when the pressure is eliminated the macromolecules
regain their original form. Thus, the macromolecules are
of importance for the resilience of the gingiva.
Blood Supply, Lymphatics and Nerves
Three major sources of blood supply to the gingiva (Fig.
2.13) has been described:
1. Supraperiosteal arterioles: Overlying the alveolar
bone along the facial and lingual surfaces, sends
branches to the surrounding tissue.
2. Vessels of the periodontal ligament: They extend into
the gingiva and anastamose with the capillaries in
the sulcus area.
3. Arterioles emerging from the crest of the interdental
septa.
Lymphatic drainage of the gingiva brings in the
lymphatics of the connective tissue papillae. It progresses
to the periosteum of the alveolar process and then to
regional lymph nodes (mainly submaxillary group).
Nerve supply to gingiva is derived from fibers arising
from nerves in the periodontal ligament and from the
labial, buccal and palatal nerves.
TOOTH-SUPPORTING STRUCTURES
PERIODONTAL LIGAMENT
Definition
Tooth-supporting structures include, the periodontal
ligament, cementum and alveolar bone. The periodontal
ligament is a connective tissue structure that surrounds
the root and connects it with the bone. In the past,
periodontal ligament has been described by many terms.
Among these are desmodont, gomphosis, pericemen-
tum, alveolodental ligament and periodontal membrane.
Since it is a soft connective tissue providing continuity
between two mineralized connective tissues, the term
periodontal ligament appears to be more appropriate.
In the coronal direction, the periodontal ligament is
continuous with the lamina propria of the gingiva and
communicates with the marrow spaces of the alveolar
bone through Volkmann’s canals.
Structure
The periodontal ligament space has the shape of an
hourglass and is narrowest at the mid-root level.
The width of periodontal ligament is approximately
0.25 mm ± 50 percent.
Cellular Composition
Cells of periodontal ligament are categorized as:
1. Synthetic cells
a. Osteoblasts
b. Fibroblasts
c. Cementoblasts
2. Resorptive cells
a. Osteoclasts
b. Cementoclasts
c. Fibroblasts
3. Progenitor cells
4. Other epithelial cells
a. Epithelial cell rests of Malassez
5. Connective tissue cells
a. Mast cells and macrophages.
Fig. 2.13:
Blood supply to gingiva. Arterioles penetrating the
interdental bone (left), supra periosteal arterioles (right)

17
Biology of Periodontal Tissues
Characteristics of a Synthetic Cell
1. Should be actively-synthesizing ribosomes
2. Increase in the complement of rough endoplasmic
reticulum and Golgi apparatus
3. Large open faced or vesicular nucleus containing
prominent nucleoli.
Osteoblasts: Covers the periodontal surface of the
alveolar bone. Alveolar bone contains endosteum and
a periosteum. A periosteum contains at least two distinct
layers, cambium layer or cellular layer and a fibrous layer.
A cellular layer is present on the periodontal surface of
the alveolar bone.
Fibroblasts: It is the most prominent connective tissue
cell (65% of total cell population). Periodontal ligament
fibroblasts are phenotypically different from gingival
fibroblasts. They consist of subtypes with distinct
phenotypes and found to synthesize higher quantities
of chondroitin sulfates and lesser quantities of heparan
sulfate and hyaluranan sulfate. The main function of the
fibroblasts is the production of various types of fibers
and it is also instrumental in the synthesis of the
connective tissue matrix. The fibroblast is a stellate or
spindle-shaped cell which produce
• Collagen fibers
• Reticulin fibers
• Oxytalan fibers
• Elastin fibers
Various stages in the production of collagen fibers
are as follows:
The first molecule released by fibroblasts is tropo-
collagen which contains three polypeptide chains
interwined to form a helix. Tropocollagen molecules are
aggregated longitudinally to form protofibrils, which are
subsequently laterally-arranged in parallel to form colla-
gen fibrils. Collagen fibers are bundles of collagen fibrils.
Cementoblasts are seen lining the cementum.
Resorptive Cells
Osteoclasts: These are the cells that resorb the bone and
tend to be large and multinucleated. The precursor cells
of the osteoclasts are circulating monocytes. The
characteristic features of osteoclasts are the plasma
membrane of the cell lying adjacent to the bone that
has been actively undergoing resorption is raised in
characteristic folds and is termed as ruffled or striated
border.
Fibroblasts: It must be made clear that the fibroblasts
may be capable of both synthesis and resorption. The
fibroblasts responsible for resorption contain fragments
of collagen that appear to be undergoing digestion. The
presence of these cells indicates resorption of fibers
occurring during either disease or physiological turnover
or remodeling of periodontal ligament.
Cementoclasts: The main observation in this is that
cementum is not remodelled in the fashion of alveolar
bone and periodontal ligament, but that it undergoes
continual deposition during life. However resorption of
cementum occurs in certain circumstances and in these
instance cementoclasts are located in Howship’s lacunae.
Progenitor cells: Little is known about these cells. It is
believed that, generally, as and when the need arises,
the daughter cell after the division differentiates into the
functional type of connective tissue cells.
Epithelial cell rests of Malassez: They are found close
to cementum. These cells are first described by Malassez
in 1884 and are remnants of the epithelium of Hertwig’s
epithelial root sheath. The epithelial cell rests persists as
a network, strands, island or tubule like structures near
and parallel to the surface of the root.
Electron microscope observation shows that the
epithelial cell rests exhibits tonofilaments and they are
attached to one another by desmosomes. The physiologic
role of these cells is not known. When certain pathologic
conditions are present, cells of the epithelial rests can
undergo rapid proliferation and can produce a variety
of cysts and tumours of the jaws.
Mast cells: These are relatively small, round or oval cell
having a diameter of almost 12 to 15µm. The cells contain
numerous cytoplasmic granules with small or round
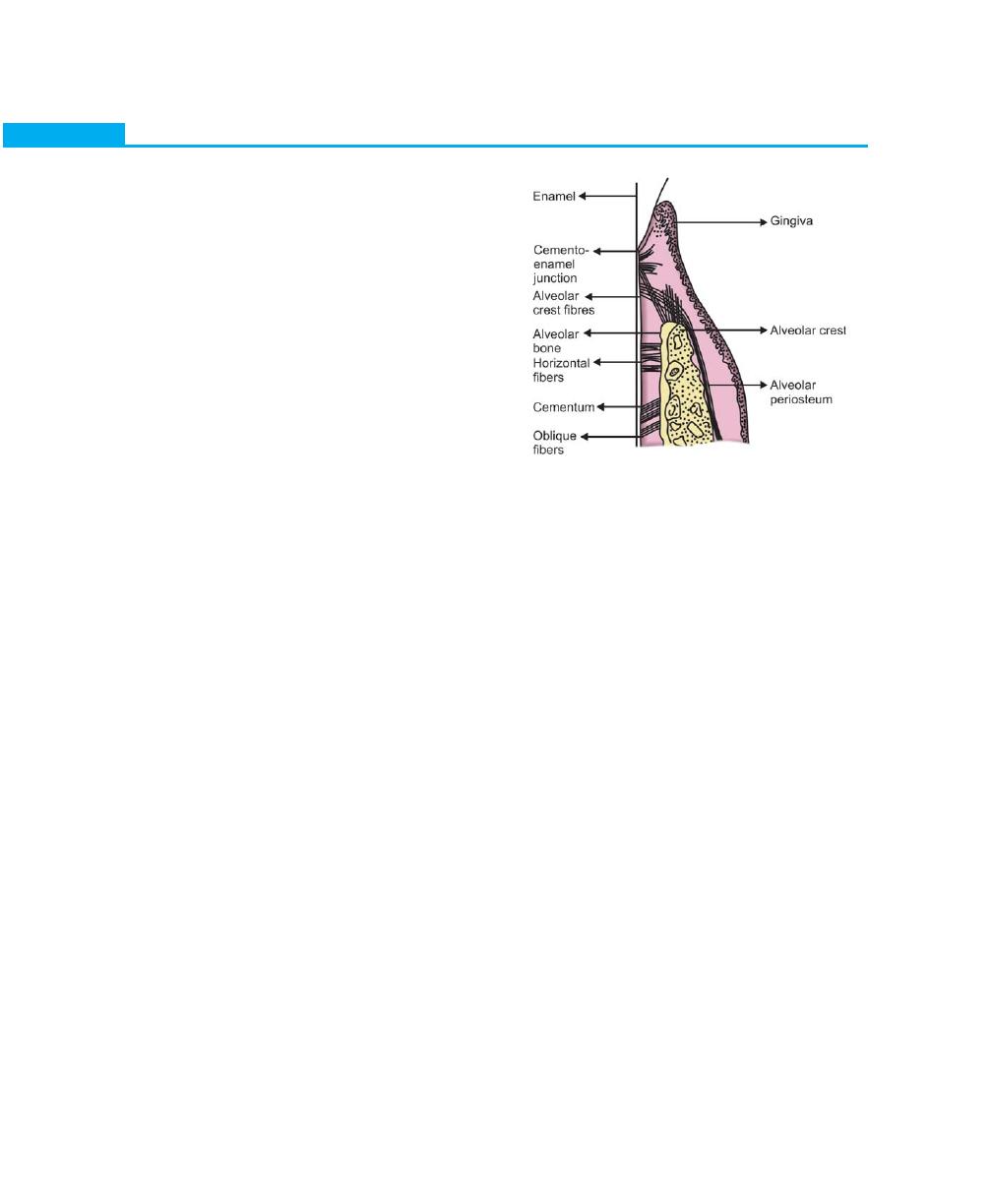
18
Essentials of Clinical Periodontology and Periodontics
Fig. 2.14:
Principal fiber groups in periodontal ligament
nucleus. The granules have been shown to contain
heparin and histamine. The physiologic role of heparin
in mast cells does not appear to be clear. Where as mast
cell histamine plays a role in the inflammatory reaction
and they have been shown to degranulate in response
to antigen-antibody formation on their surface.
Macrophages may also be present in the ligament. They
are capable of phagocytosis.
Extracellular Components
1. Fibers:
• Collagen
• Oxytalin
2. Ground substance:
• Proteoglycans
• Glycoproteins
Periodontal fibers: The most important elements of the
periodontal ligament are the principal fibers. These fibers
are collagenous in nature and are arranged in bundles,
they follow a wavy course. The terminal portion of these
principal fibers that insert into the cementum and bone
are termed Sharpey’s fibers. Collagen is a specific, high
molecular weight protein to which a small number of
amino acids are attached, the most important of which
are glycine, proline and hydoxyproline. Collagen is
synthesised by fibroblasts, chondroblasts, osteoblasts,
odontoblasts and other cells. Several types of collagen
have been demonstrated. The principal fibers are
composed primarily of Type I collagen, where as reticular
fibers are made up of Type III collagen. Type IV collagen
is seen in the basal lamina.
The principal fibers of periodontal ligament (Fig. 2.14)
are arranged in six groups that develop sequentially in
the developing root. They are:
• Trans-septal group
• Alveolar crest, horizontal, oblique, apical and inter-
radicular fibers.
Trans-septal group: They may be considered to belong
to the gingiva because they do not have osseous
attachment.
Alveolocrestal group: They extend obliquely from the
cementum just beneath the junctional epithelium to the
alveolar crest. Their function is to retain tooth in socket,
resist lateral tooth movement and protect deeper
periodontal ligament structure.
Horizontal group: Extend from cementum to the alveolar
bone at right angles to the long axis of the tooth.
Oblique group: They are the largest group in the
periodontal ligament; extend coronally in an oblique
direction from the cementum to the bone. They resist
axially directed forces.
Apical group: They originate from cementum of root
apex, splaying apically and laterally into the bone of the
alveolar fundus. Their main function is it prevents tooth
tipping; resists luxation, protects blood, lymph and nerve
supply to the tooth.
Inter-radicular fibers: Extends from cementum of
bifurcation areas, splaying from apical into furcal bone.
It resists luxation and also tipping and torquing.
Secondary fibers of periodontal ligament: In addition
to the principal fiber groups, periodontal ligament
contains other well-formed fiber bundles, that inter-
digitate at right angles or splay around and between the
regular fiber bundles. These fibers are associated with
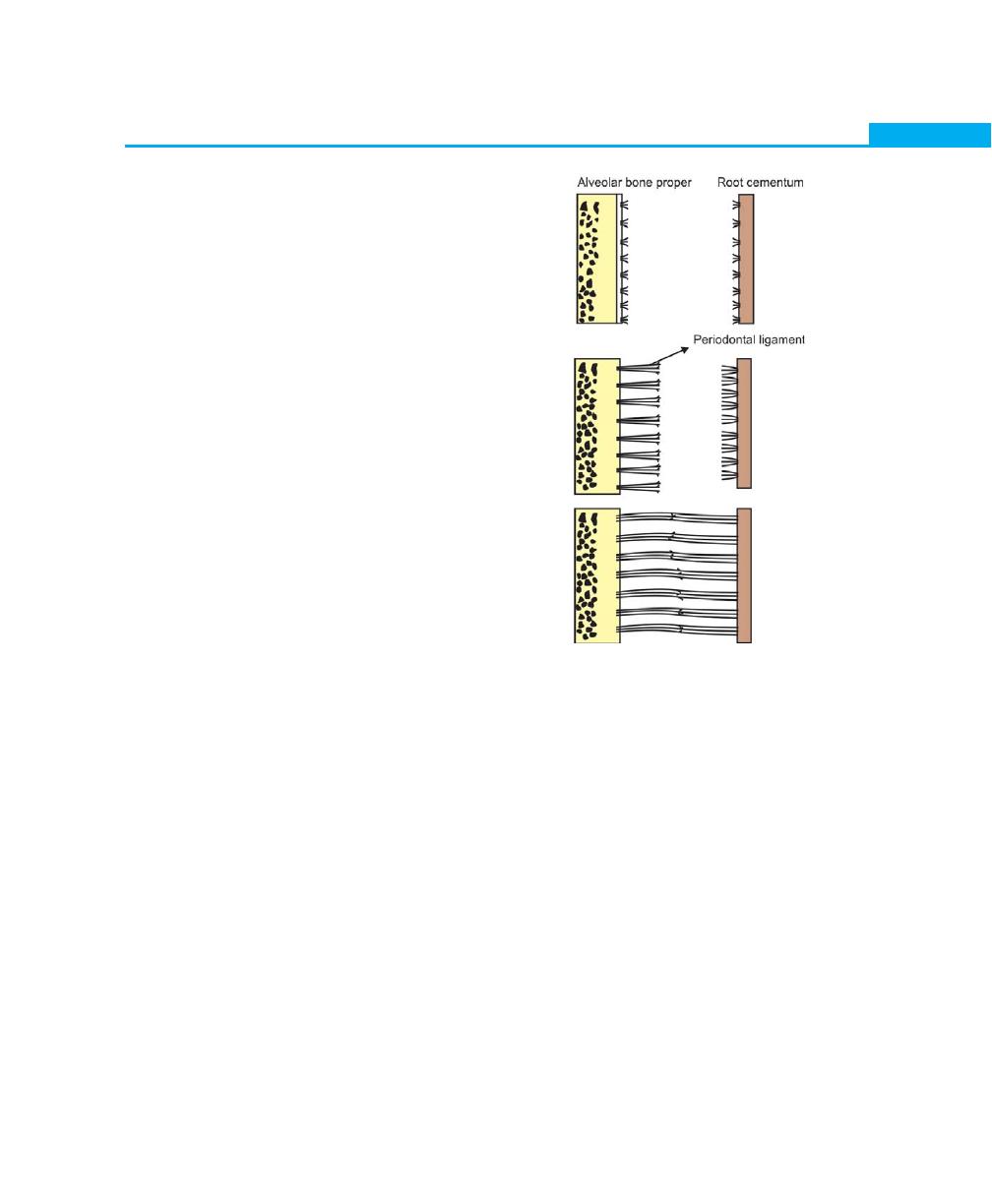
19
Biology of Periodontal Tissues
blood vessels and nerves of the periodontal ligament.
Although periodontal ligament does not contain mature
elastin, two immature forms have been described,
the so-called oxytalan fibers and eluanin. It was sug-
gested that, they provide elastic properties to periodontal
ligament. There are also reticulate fibers, which are fine,
immature collagen fibers with a lattice like arrangement.
In addition to the above fiber types, small collagen
fibers arranged in all directions, forming a plexus have
also been reported. They are closely-associated with
principal fibers and are termed as the indifferent fiber
plexus.
Ground substance: The space between cells, fibers, blood
vessels and nerves in the periodontal space is occupied
by ground substance. The ground substance is made
up of two major groups of substances. Glyco-
saminoglycans such as hyaluronic acid, proteoglycans
and glycoprotiens such as fibronectin and laminin. It also
has high water content (70%).
Development of principal fibers of periodontal ligament
(Fig. 2.15): The principal fibers develop in conjunction
with the eruption of the tooth. The fibroblasts surround-
ing the developing root produce collagen fibers. These
fibers are seen in the periodontal space without a specific
orientation. As and when the tooth erupts, the orientation
of the fibers alters.
1. First small, fine brush-like fibrils are seen arising from
the root cementum and projecting into the perio-
dontal ligament space.
2. Similar fibers are seen on the surface of the bone
but only in thin, small numbers.
3. Later on, the number and thickness of fibers
originating from the bone increase and elongate. They
radiate towards the loose connective tissue in the mid-
portion of the periodontal ligament.
4. The fibers originating from the cementum also
increase in length and thickness and fuses with the
fibers originating from the alveolar bone in the
periodontal ligament space.
5. They mature progressively towards the root apex as
the eruption progresses. When the tooth, following
eruption, reaches contact in occlusion and starts to
function, the principal fibers become organized in
bundles and run continuously from bone to cemen-
tum.
For long it was believed that this middle portion where
the splicing of fibers from cementum and bone takes
place,it forms the intermediate plexus. These plexus were
thought to play a significant role in orientation and
adjustment of fibers during eruption and functional
movement of teeth. But recent investigations have
revealed that, in humans these plexus disappears
once the fusion of cemental and osseous fibers are
completed.
Fig. 2.15:
Development of periodontal ligament fibers
(principal)
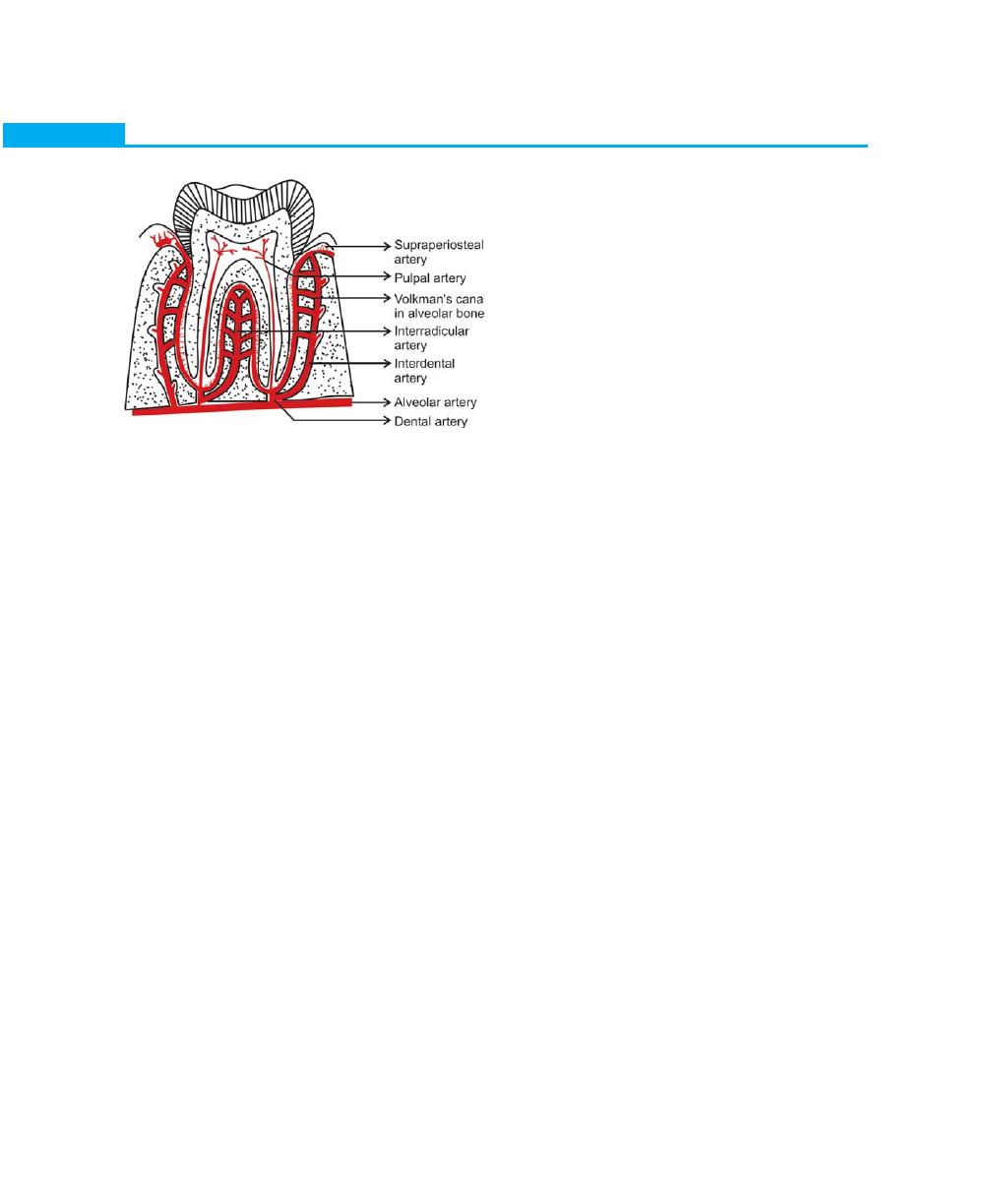
20
Essentials of Clinical Periodontology and Periodontics
Structures Present in the Connective Tissue
1. Blood vessels
2. Lymphatics
3. Nerve innervation
4. Cementicles
Blood vessels: Periodontal ligament is supplied by
branches derived from three sources dental, inter-
radicular and interdental arteries (Fig. 2.16).
1. Dental artery before it enters apical foramen gives
off branches to the periodontal ligament which also
supplies the pulp.
2. The inter-radicular artery gives off branches in the
alveolar process that supplies the periodontal ligament
through the cribriform plate.
3. Interdental artery emerges from the crest of the
alveolar bone and supplies the coronal part of the
periodontal ligament.
Lymphatic vessels are seen to follow the path of blood
vessels in the periodontal ligament.
Nerve supply: In an un-erupted tooth, the developing
periodontal ligament is supplied by fine, unmyelinated
nerve fibers. Whether this persists after the tooth is
erupted is not known. Periodontal ligament is mainly
supplied by the dental branches of the alveolar nerve
through the apical perforations of the tooth socket or
from the cribriform plate. Many studies have proved
that periodontal ligament is richly supplied by
mechanoreceptors whose cell bodies are located in the
trigeminal ganglion. These receptors provide sense of
touch, pressure, pain, and proprioception during
mastication.
Cementicles are calcified masses adherent to or
detached from the root surface. They may be developed
from calcified epithelial rests, calcified Sharpey’s fibers,
and calcified thrombosed vessels within the periodontal
ligament.
Functions of Periodontal Ligament
The following functions of periodontal ligament have
been explained:
1. Physical
2. Formative and remodeling
3. Nutritional and sensory function
Physical Function
The physical functions of the periodontal ligament are:
a. Provides soft tissue “casing” in order to protect the
vessels and nerves from injury due to mechanical
forces.
b. Transmit the occlusal forces to the bone. Depending
on the type of force applied, axial force when applied
causes stretching of oblique fibers of periodontal
ligament. Transmission of this tensional force to the
alveolar bone encourages bone formation rather than
bone resorption. But when horizontal or tipping force
is applied the tooth rotates around the axis, at first
the tooth movement is within the confines of the
periodontal ligament. When a greater force is applied,
displacement of facial and lingual plates may occur.
The axis of rotation, in single rooted teeth is located
in the area between the apical and middle third of
the root. In multirooted teeth, the axis of rotation
is located at the furcation area.
c. Attaches the teeth to the bone
d. Maintains the gingival tissues in their proper
relationship to the teeth.
e. Shock absorption resists the impact of occlusal forces.
Fig. 2.16:
Periodontal blood supply

21
Biology of Periodontal Tissues
Two theories have been explained for the mechanism
of tooth support.
A. Tensional theory
B. Viscoelastic theory
A. Tensional theory: According to it the principal fibers
of periodontal ligament plays a major role in
supporting the tooth and transmitting forces to the
bone. When forces are applied to the tooth, principal
fibers unfold and straighten and then transmit the
forces to the alveolar bone, causing elastic defor-
mation of the socket.
B. Viscoelastic theory is based on the fact that, the fluid
movement largely controls the displacement of the
tooth, with fibers playing a secondary role. When
the forces are transmitted to the tooth, the extra-
cellular fluid is pushed from the periodontal ligament
into the marrow spaces through the cribriform plate.
After the depletion of the tissue fluids, the bundle
fibers absorb the shock and tighten. This leads to
blood vessel stenosis → arterial back pressure →
ballooning of the vessels →Tissue replenishes with
fluids.
Formative and Remodeling Function
Cells of the periodontal ligament have the capacity to
control the synthesis and resorption of the cementum,
ligament and alveolar bone. Periodontal ligament
undergoes constant remodeling; old cells and fibers are
broken down and replaced by new ones.
Nutritional and Sensory Function
Since periodontal ligament has a rich vascular supply
it provides nutrition to the cementum, bone and gingiva.
Periodontal ligament is supplied by nerve fibers that can
transmit sensation of touch, pressure and pain to higher
centers. The nerve bundle follows the course of blood
vessel and enters the periodontal ligament from
periapical area through channels from the alveolar bone.
These bundles divide into single myelinated fibers, which
later on lose their myelin sheath and end in one of the
four types of neural termination.
• Free endings, carry pain sensations
• Ruffini like mechanoreceptors located in the apical
area
• Meissners corpuscles are also mechano receptors
located primarily in the mid-root region.
• Spindle like pressure and vibration endings, located
mainly in the apex.
Pain sensation is transmitted by small diameter nerves,
temperature by intermediate type; pressure by large
myelinated fibers.
Clinical Considerations
The primary role of periodontal ligament is to support
the tooth in the bony socket. Its thickness varies in
individuals and in different teeth in the same person.
Periodontal ligament is shaped like an hourglass and is
narrowest in the middle region of the root and thus seems
to be the fulcrum of physiological movement. In
connection with the physiological mesial migration of
the teeth, the periodontal ligament is thinner on the mesial
root surface than on the distal surface.
Due to acute trauma to the periodontal ligament or
in accidental blows, many pathological changes will be
produced, such as fracture or resorption of cementum
and alveolar bone. Hence there will be loss of alveolar
bone and widening of periodontal ligament, which results
in the tooth becoming loose. When the trauma is
removed repair usually will take place.
Orthodontic Tooth Movement
Depends on the resorption and formation of both bone
and periodontal ligament. These activities can be
stimulated by properly regulated pressure and tension.
If the movement of the tooth is within physiological limits,
the compression of the periodontal ligament on pressure
side results in bone resorption, where as on the tension
side bone apposition is seen. Application of the large
forces results in the necrosis of periodontal ligament and
alveolar bone.
If gingivitis is not controlled or treated it will invariably
extend to the periodontal ligament and bone and
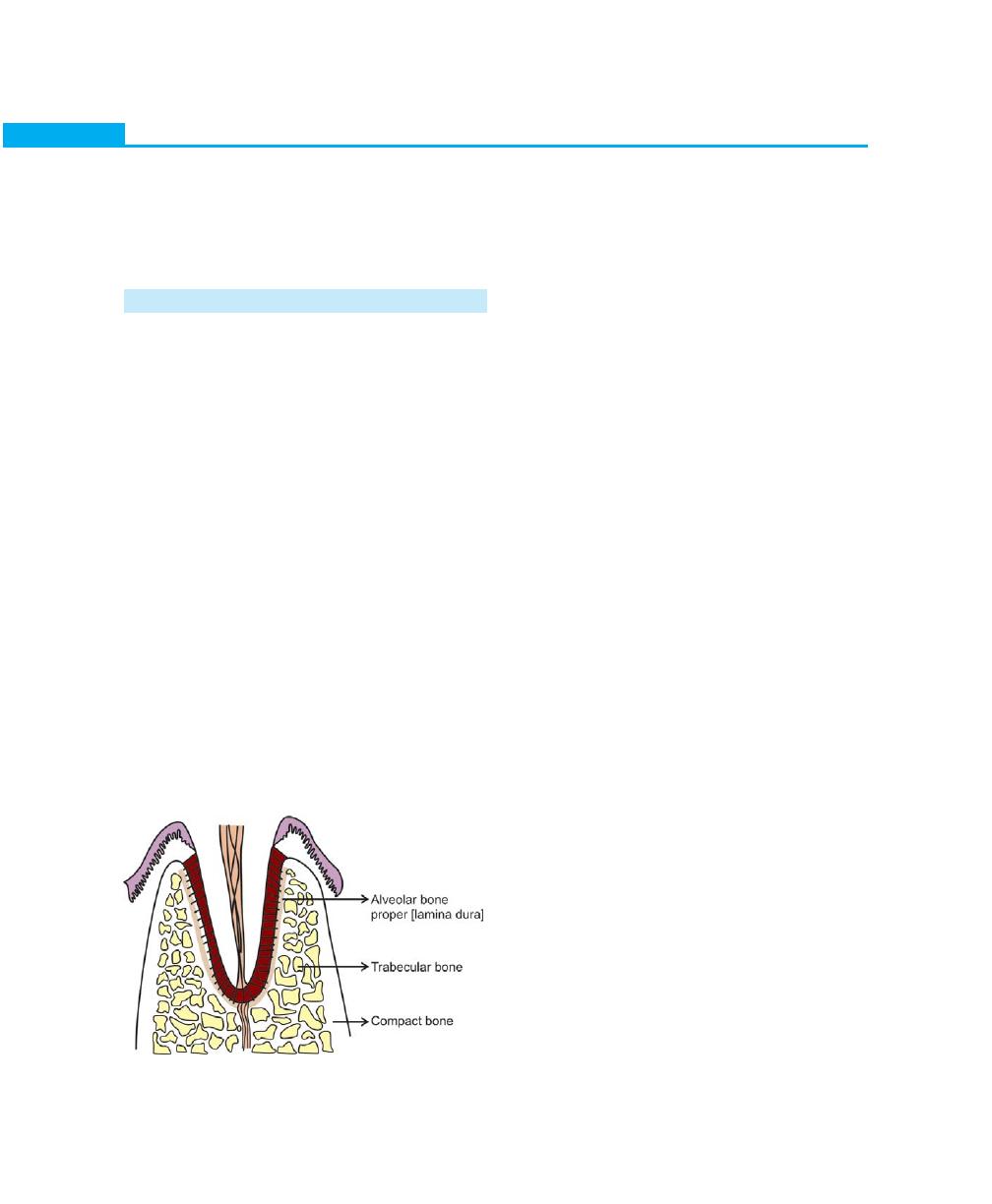
22
Essentials of Clinical Periodontology and Periodontics
Fig. 2.17:
Alveolar bone structure
produces the destruction of the same. Once they are
destroyed it is difficult for them to regenerate and
therefore the diseases of periodontal ligament are often
irreversible.
ALVEOLAR BONE
Definition
It is that portion of the maxilla and mandible that forms
and supports the tooth socket (alveoli). It is formed when
the tooth erupts, in order to provide osseous attachment
to the forming periodontal ligament and gradually
disappears after the tooth is lost.
Parts of Alveolar Bone (Fig. 2.17)
Alveolar bone consists of the following:
1. Inner and outer cortical plate.
2. The bone lining the socket.
3. An interior portion of cancellous bone.
The cortical plates consist of compact bone, in
which the lamellae are often arranged circumferentially
around blood vessels forming Haversian systems, which
are the internal mechanisms that bring a vascular supply
to bones that are too thick to be supplied only by surface
vessels. The cortical plates and the bone lining the socket
meet at the alveolar crest, usually 2 mm below the
cementoenamel junction.
The bone lining the socket is also compact bone and
can be known as any of the following:
1. Bundle bone, since bundles of Sharpey’s fibers from
the periodontal ligament are embedded in it
2. The cribriform plate, because it is perforated by
numerous vascular channels
3. Alveolar bone proper, as it provides direct bony
support for the teeth
4. Lamina dura, which is radiographically seen as a
dense plate.
Cancellous bone: It consists of narrow irregular bony
trabeculae, which, by branching and uniting forms a
network of spaces between the trabeculae. There is a
considerable variation in the proportions of compact to
cancellous bone in different regions of the jaws and in
different tooth surfaces. For example: In relation to lower
anterior teeth i.e. lower incisors, there is a thin bone
that consists of an outer cortical plate and the bone lining
the socket with no intervening cancellous bone. In
contrast, the buccal and interdental bone of molars is
relatively thick and cancellous bone may predominate.
As the diameter of the tooth root gradually decreases
in an apical direction, there is a corresponding increase
in the thickness of alveolar bone with increase in
cancellous bone being present. These variations are
important to note because they influence the pattern
and progression of bone loss in destructive forms of
periodontal diseases.
Roentgenogram permits the classification of trabecular
patterns of the alveolar process into two main types:
• In type I, the interdental and inter-radicular
trabeculae are regular, horizontal and are arranged
in a ladder-like pattern. (Common in mandible)
• In type II, irregularly arranged numerous interdental
and inter-radicular trabeculae are seen most com-
monly in maxilla.
Composition of Alveolar Bone
It has two basic constituents:
a. The cells consist of osteoblasts, osteoclasts, and
osteocytes.
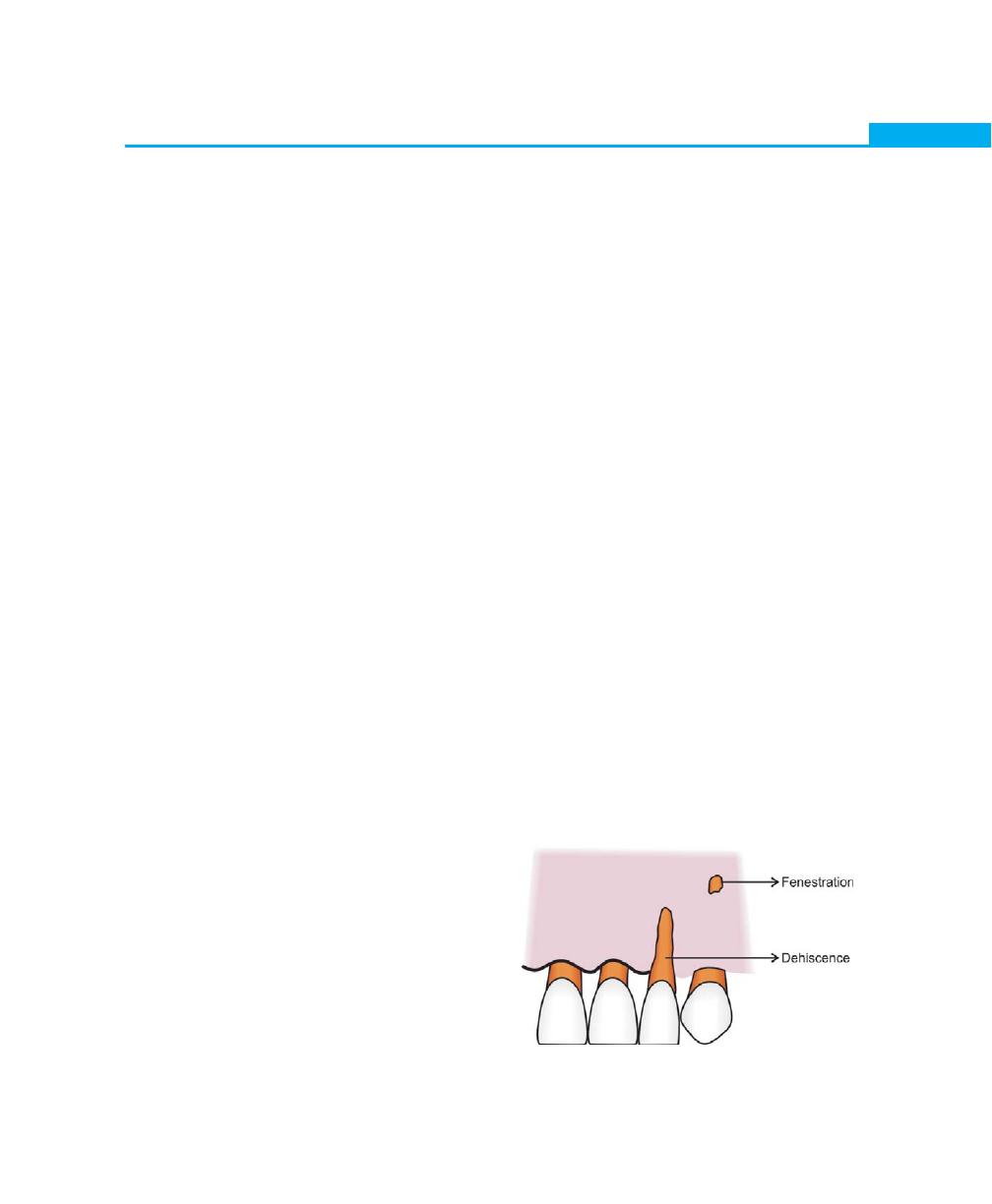
23
Biology of Periodontal Tissues
b. Extra-cellular matrix consists of 65 percent inorganic
and 35 percent organic matter.
The inorganic component is composed of minerals
such as calcium, phosphate along with hydroxyl,
carbonate, citrate and trace amounts of other ions, such
as sodium, magnesium and fluorine. The minerals are
in the form of hydroxyapatite crystals.
The organic matrix consists of 90 percent of type I
collagen, with small amounts of non-collagenous proteins
such as osteocalcin, osteonectin, bone morphogenetic
proteins, proteoglycans, and glycoproteins.
Cellular Components
a. Osteoblasts: They are cuboidal cells with well-
developed rough endoplasmic reticulum, a large
Golgi apparatus, and secretory vesicles. It synthesizes
bone matrix (osteoid), type I collagen and regulate
its mineralization. Each osteoblast carries out a cycle
of matrix synthesis after which it is either buried as
an osteocyte or remains on the surface as a resting
or inactive osteoblast. Osteoblasts are derived from
the progenitor cells at sites of bone formation. These
progenitor cells belong to the mesenchymal cell
family. In contrast, osteoclasts originate from blood
– borne monocytes.
b. Osteoclasts: They are large multinucleated giant cells.
They originate from hematopoietic tissue and are
formed by the fusion of mononuclear cells of
asynchronous populations. Generally they are found
in bay like depressions in the bone called Howship’s
lacunae. The part of the cell in contact with bone
shows a convoluted surface and a ruffled border
from which hydrolytic enzymes are believed to be
secreted. The ruffled border is surrounded by a clear
zone, which contains cytoplasm exclusively and is
believed to be associated with binding of cells to the
bone surface and isolation of areas of resorptive
activity.
c. Osteocytes: Alveolar bone is formed during foetal
growth by intra membranous ossification and consists
of a calcified matrix with osteocytes enclosed within
a space called lacunae. The osteocytes extend
processes into canaliculi that radiate from the lacunae.
The main function of these canaliculi is to bring
oxygen and nutrients to the osteocytes through the
blood and remove metabolic waste products.
The main cells responsible for bone resorption are
osteoclasts, on rare occasions bone resorption by
osteocytes has been reported which is called as “osteocytic
osteolysis”.
Osseous Topography
The anatomy of the alveolar bone varies from patient
to patient. Normally it conforms to the root prominence,
with intervening depressions that taper towards the
margin. The factors that affect the height and thickness
of the facial and lingual bony plates are alignment of
the teeth, angulation of the root to the bone and the
occlusal forces.
When the teeth are in a labial version, the margin
of the bone is located more apically and is thinned to
a knife-edge as compared to the teeth in proper
alignment. When teeth are in a lingual version, the facial
bony plate is thicker than normal and the margin is blunt,
rounded and horizontal rather than arcuate.
Fenestrations and Dehiscences (Fig. 2.18)
Fenestrations are isolated areas in which the root surface
is covered only by the periosteum and gingiva, but in
these situations, the marginal bone remains intact. When
Fig. 2.18:
Fenestration and dehiscence

24
Essentials of Clinical Periodontology and Periodontics
the marginal bone is also denuded, the defect is called
dehiscence.
These defects are seen more often on the facial bone
than on the lingual, and are more common in anterior
teeth than on the posterior. The etiologies of these defects
are not clear; some of the predisposing factors are root
prominence, malposition, and teeth in labial version with
thin bony plates. The diagnosis of these defects is important
as it may affect the outcome of the surgical treatment.
Periosteum and Endosteum
The tissue covering the outer surface of bone is termed
as periosteum, where as the tissue lining the internal bone
cavities is called endosteum. The periosteum consists of
two layers, the inner layer, next to the bone surface,
consists of bone cells that have the potential to
differentiate into osteoblasts and an outer layer which
is more fibrous containing blood vessels and nerves.
The endosteum is composed of a single layer of
osteoprogenitor cells and a small amount of connective
tissue.
Remodeling and Resorption
During both embryonic bone development and the
entire pre adult period of human growth, bone is being
formed very rapidly, primarily on the periosteal surface.
Simultaneously, bone is being destroyed along the
endosteal surface and at focal points along the periosteal
surface (bone modeling).
Bone growth occurs by apposition of an organic
matrix that is deposited by osteoblasts. Although the
alveolar bone tissue is constantly changing in its internal
organization, it retains approximately the same form from
childhood through adult life. Haversian systems (osteons)
are the internal mechanisms that bring vascular supply
to bones too thick to be supplied only by surface vessels.
Bone deposition by osteoblasts is balanced by resorption
brought about by osteoclasts during tissue remodeling
and repair.
Osteoblasts, lay down non mineralised bone matrix
called osteoid, while new osteoid is being deposited, the
older osteoid becomes mineralized. Bone resorption is
a complex process and appears as eroded bone surfaces
namely Howship’s lacunae. It has been suggested that
bone resorption at any site is a chemotactic
phenomenon. This is, initiated by the release of some
chemotactic factors like interleukin-1 and 6, which
attract the monocytes to the target site. Osteoblasts release
Leukaemia inhibiting factor (LIF), which coalesce
monocytes to form multi-nucleated osteoclasts, which
then resorb bone.
During bone resorption three processes occur:
a. Decalcification
b. Degradation of matrix
c. Transport of soluble factors to the extra cellular fluid
Since calcified matrix is resistant to proteases of all
kinds, bone must first be decalcified. This is achieved
at the ruffled border of the osteoclasts by secretion of
some acids such as citric acid and lactic acid, which chelate
bone, the low pH leads to increase in solubility of
hydroxyapatite. The next step is degradation of matrix,
takes place by collagenase enzymes and other proteases
like cathepsin-B. Collagenolysis occurs outside the
osteoclast. Finally, the break down products of bone are
transported to the extracellular fluids and to the blood
vascular system.
Tencate described the following sequence of events
during bone resorption:
a. Attachment of osteoclasts to the mineralized bone
surface.
b. Creation of a sealed acidic environment, which
demineralises the bone and exposes the organic
matrix.
c. Degradation of exposed organic matrix by the action
of enzymes such as acid phosphatase and cathepsine.
d. Sequestering of mineral ions and amino acids within
the osteoclasts.
Blood Supply to the Bone
The vessels enter the interdental septa through nutrient
canals together with veins, nerves, and lymphatics. The
alveolar arteries sends off branches through periodontal
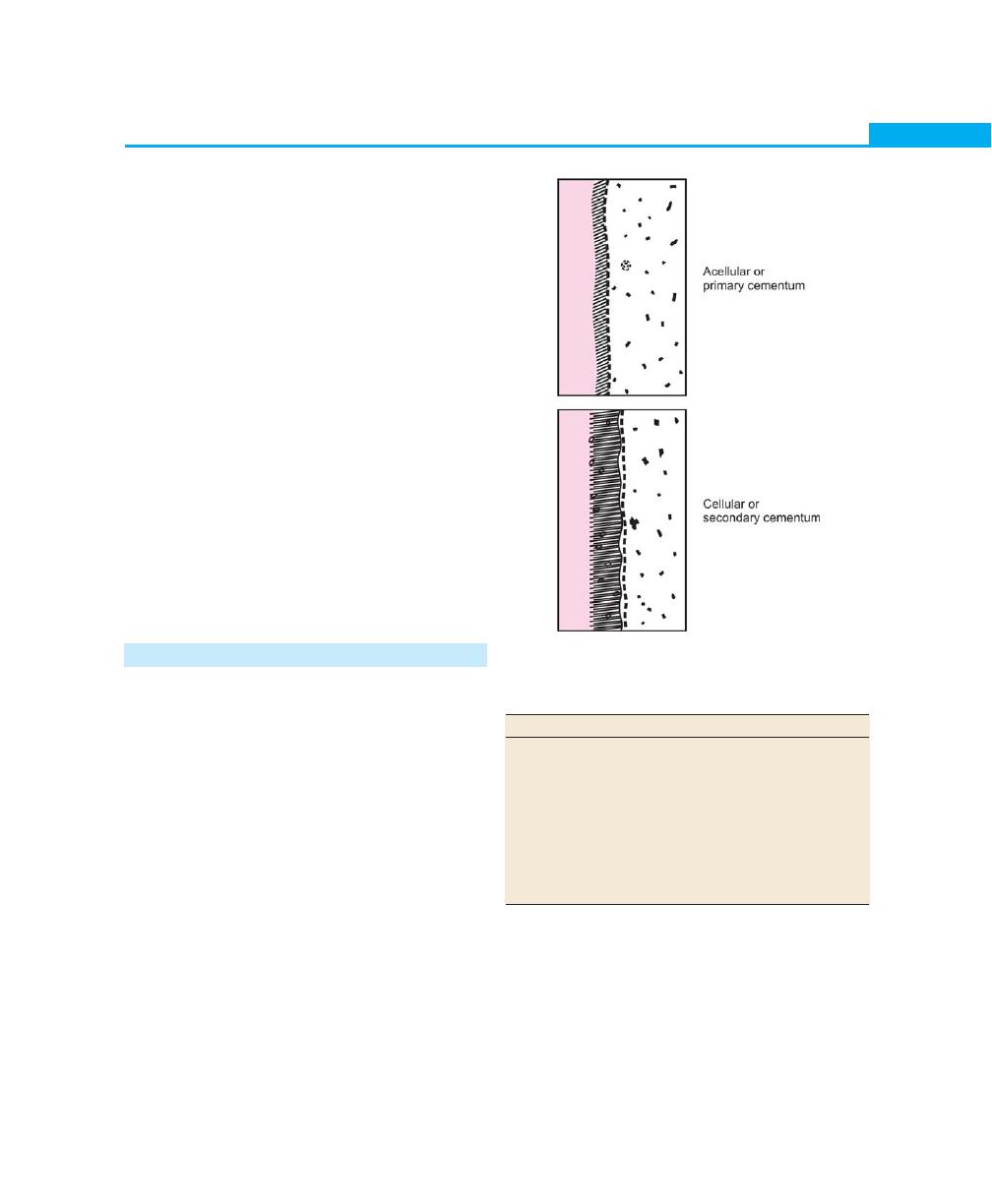
25
Biology of Periodontal Tissues
ligament and enter the marrow spaces through the
perforations in the cribriform plate.
Clinical Considerations
Bone although one of the hardest tissue of human body,
is biologically a highly plastic tissue. Bone is resorbed
on the side of pressure and apposed on the side of
tension. It has been shown that on the pressure side
there is an increase in the level of cyclic adenosine
monophosphate (cAMP) in cells, which may play a role
in bone resorption.
The most frequent and harmful change in the alveolar
process is that which is associated with periodontal
diseases. The bone resorption caused by periodontal
diseases is usually symmetrical, occurs in episodic manner,
and is both of the horizontal and vertical type. Once
lost, this bone is very difficult to regenerate. Regeneration
of just a few millimetres of bone that has been lost is
the greatest challenge to the periodontists across the
world.
CEMENTUM
Definition
Cementum is a calcified avascular mesenchymal tissue
that forms the outer covering of the anatomic root. It
provides anchorage mainly to the principal fibers of
periodontal ligament. Two sources of collagen fibers can
be found in the cementum:
1. Sharpey’s (extrinsic) fibers which are formed by the
fibroblasts.
2. Fibers belonging to the cementum matrix per se
(intrinsic) produced by cementoblasts.
Two types of cementum were described earlier:
a. Acellular cementum/primary cementum (Fig. 2.19)
b. Cellular cementum/secondary cementum.
The differences between the two are given in Table
2.1.
Classification
Depending on location, morphology and histological
Fig. 2.19:
Acellular/cellular cementum
Table 2.1: Differences between acellular and
cellular cementum
Acellular cementum
Cellular cementum
a. Forms during root
Forms after the eruption
formation
of the tooth and in
response to functional
demands
b. Does not contain any cells
Contains cementocytes
c. Seen at the coronal
Seen at a more apical
portion of root
portion of root
d. Formation is slow
Deposition is more rapid
e. Arrangement of collagen
Collagen fibers are
fibers are more organized
irregularly arranged
appearance, Shroeder and Page have classified
cementum as:
a. Acellular afibrillar cementum (AAC): It contains only
the mineralized ground substance. It does not contain
collagen fibers nor does it exhibit entrapped
cementocytes. It is a product of cementoblasts and
is found almost exclusively on the enamel near the

26
Essentials of Clinical Periodontology and Periodontics
cementoenamel junction with a thickness of 1 to
15 µm.
b. Acellular extrinsic fiber cementum (AEFC): By
definition it is composed primarily of Sharpey’s fibers
of periodontal ligament but does not contain
cementocytes. Developmentally they come to occupy
the coronal one half of the root surface. Its thickness
is between 30 and 230 µm.
c. Cellular mixed stratified cementum (CMSC): It
harbours both intrinsic (cementoblasts derived) and
extrinsic (fibroblast derived) fibers and may contain
cells. In humans it is seen in the apical third of the
roots, apices and furcation areas. Its thickness varies
from 100 to 1000 µm.
d. Cellular intrinsic fiber cementum (CIFC): It contains
only intrinsic fibers secreted by cementoblasts and
not by the periodontal ligament fibroblasts. In
humans it fills the resorption lacunae.
e. Intermediate cementum (or) the hyaline layer of
Hope Well Smith: It is an ill-defined zone extending
from pre-cementoenamel junction to the apical
1/3rd of the root. It appears to contain cellular
remnants of Hertwigs Sheath embedded in calcified
ground substance. The significance of this layer is that,
it contains enamel like proteins, which helps in
attachment of cementum to dentin. It has been
observed by many that, when this layer is removed
during root planing procedure, the resultant
reparative cementum that is formed will not be
attached firmly on the dentin.
Functions
a. Primary function of cementum is to provide
anchorage to the tooth in its alveolus. This is achieved
through the collagen fiber bundles of the periodontal
ligament, whose ends are embedded in cementum.
b. Cementum also plays an important role in
maintaining occlusal relationships, whenever the
incisal and occlusal surfaces are abraded due to
attrition, the tooth supra erupts in order to
compensate for the loss and deposition of new
cementum occurs at the apical root area.
Composition
The cementum is composed of both inorganic (46%)
and organic matter. The organic matrix is chiefly
composed of 90 percent Type I collagen, 5 percent Type
III collagen and non-collagenous proteins like enamel
proteins, adhesion molecules like tenacin and fibronectin,
glycosaminoglycans like chondroitin sulfate, dermatan
sulfate and heparan sulfate which constitute the
remaining organic matrix.
Thickness of Cementum
Formation of cementum is a continuous process,
the formative rate of which varies throughout life.
It is most rapid at the apical regions. At the coronal half
the thickness varies from 16 to 60 µm (almost the
thickness of hair) and at the apical third it varies from
150 to 200 µm. It is thicker in the distal surfaces as
compared to the mesial surfaces and this can be
explained by functional stimulation following mesial
migration.
Hypercementosis or cemental hyperplasia is a
prominent thickening of the cementum. It can be
localized or generalized. It may appear as a generalized
thickening of the cementum, with nodular enlargement
at the apex or as spike like projections (cemental spikes).
The etiology of hypercementosis is not very well
understood. The spike like projections could be as a result
of excessive tension from orthodontic appliance or
occlusal forces. The generalized type may be associated
with a variety of situations like, teeth without antagonists,
in teeth with chronic pulpal and periapical infections.
Hypercementosis of the entire dentition may be seen
in patients with Paget’s disease.
Cementoenamel Junction (Fig. 2.20)
At the cementoenamel junction three types of
relationships may exist. In about 60 to 65 percent of
cases the cementum overlaps the enamel, in about 30
percent of cases end-to-end relationship of enamel and
cementum is seen and in 5 to 10 percent the cementum
and enamel fail to meet.
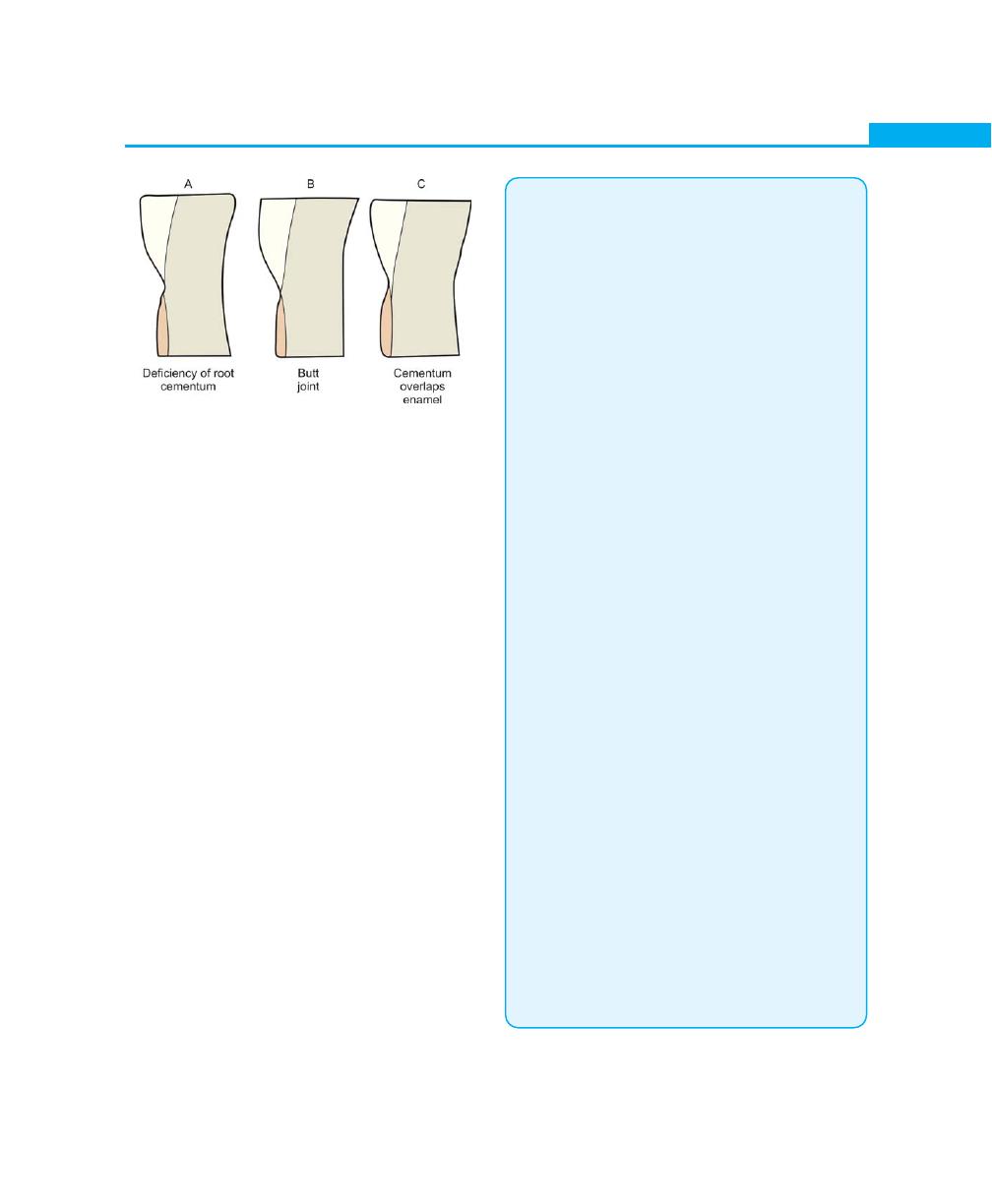
27
Biology of Periodontal Tissues
Cemental Resorption and Repair
Cemental resorption may be caused by local, systemic
or idiopathic factors. Local conditions that contribute to
cemental resorption are, trauma from occlusion,
orthodontic tooth movement, pressure from erupting
teeth, cysts and tumors, teeth without functional
antagonist, periapical disease and periodontal disease.
Systemic conditions that may predispose to cemental
resorption are calcium deficiency, hypothyroidism and
Paget’s disease.
The resorptive process may not necessarily be a
continuous process; it may alternate with periods of
repair and deposition, which can be demarcated by
formation of reversal line. Remodelling of cementum
requires the presence of viable connective tissue and
can occur even in non-vital teeth.
Cementum is not exposed to the oral environment
because it is covered by alveolar bone and gingiva. In
cases of gingival recession and as a consequence of loss
of attachment in pocket formation, cementum can
become exposed to the oral environment. Once
exposed, organic substances, inorganic ions and bacteria
penetrate the sufficiently permeable cementum. Caries
of the cementum may also develop.
KEY POINTS TO NOTE
The Gingiva
1. The gingiva is that part of the oral mucosa that covers the
alveolar process of the jaws and surrounds the necks of
the teeth.
2. Anatomically it is divided into marginal, attached and
interdental gingiva.
3. Microscopically, the gingival epithelium is divided into
keratinized i.e., oral epithelium and non-keratinized i.e.,
junctional and sulcular epithelium.
4. The keratinized epithelium of the gingiva consists of four
layers, namely stratum basale, stratum spinosum, stratum
granulosum and stratum corneum.
5. The granular layer, which is seen in the oral epithelium
is absent in sulcular and junctional epithelium.
6. Junctional epithelium is attached to the tooth surface by
hemidesmosomes and basal lamina.
7. Lamina propria/gingival connective tissue consists of cells,
fibers, and blood vessels, embedded in the extracellular
matrix.
Tooth-supporting Structures
Periodontal Ligament
8. Periodontal ligament is a connective tissue structure that
surrounds the root and connects it with the bone.
9. It consists of cells and extra-cellular substances.
10. Cellular components are categorized as synthetic cells,
resorptive cells, progenitor cells, epithelial cells and
connective tissue cells.
11. Extracellular matrix is composed of fibers like collagen,
oxytalin and ground substance with proteoglycans and
glycoproteins.
12. The principal fibers of periodontal ligament are arranged
in six groups i.e. transseptal group, alveolar crest group,
horizontal, oblique, apical and inter-radicular group.
13. In the connective tissue other structures like blood vessels,
nerves, and lymphatics are also present.
14. Functions of periodontal ligament are explained under,
physical function, formative and remodelling function,
nutritive and sensory function.
15. Finally any trauma to the periodontal ligament can result
in the loss of alveolar bone and widening of ligament but
once the trauma is removed repair usually takes place.
16. When the inflammation extends from the gingiva to the
periodontal ligament, destruction of periodontal structures
will result, which is difficult to regenerate, hence the disease
of periodontal ligament are often irreversible.
Fig. 2.20:
Configuration of cementoenamel junction
28
Essentials of Clinical Periodontology and Periodontics
Alveolar bone
17. Alveolar bone is that portion of the maxilla and mandible
that forms and supports the tooth sockets.
18. It consists of inner and outer cortical plate, the bone lining
the socket and an interior portion of cancellous bone.
19. It has two basic constituents (a) cells like osteoblasts,
osteoclasts, and osteocytes, (b) extracellular matrix made
up of 65 percent inorganic and 35 percent organic.
Inorganic is composed of calcium, phosphate along with
some trace elements. Organic matrix consists of 90 percent
Type I collagen with small amounts of non-collagenous
proteins such as osteonectin, osteocalcin, bone
morphogenetic proteins etc.
20. Fenestrations are isolated areas in which the root surface
is covered only by periosteum and gingiva, but the
marginal bone remains intact. When marginal bone is also
involved, the defect is called dehiscence.
21. Bone resorption occurs by three processes—(a) decalcifi-
cation, (b) degradation of matrix and (c) transport of
soluble factors to the extracellular fluid.
Cementum
22. Cementum is a calcified avascular mesenchymal tissue that
forms the outer covering of the anatomic root.
23. Acellular cementum forms during root formation and is
seen at the coronal portion of the root, whereas cellular
cementum forms after eruption of the tooth and is seen
apically on the root.
24. The cementum consists of 46 percent inorganic matter and
the rest 90 percent organic being Type I collagen and the
remaining consists of non-collagenous proteins.
25. Three types of relationships of cementum may exist at the
cementoenamel junction. In 60 to 65 percent of cases
cementum overlaps the enamel, in 30 percent edge-to-
edge butt joint exists and in 5 to 10 percent the cementum
and enamel do not meet.
REVIEW QUESTIONS
The Gingiva
1. Describe the microscopic and macroscopic features
of gingiva.
2. Discuss the development, structure and mode of
attachment of junctional epithelium.
3. Describe the blood supply to the gingiva.
4. Describe various gingival fiber groups with
illustrations.
Tooth-Supporting Structures
Periodontal Ligament
5. Describe the development, structure and
composition of periodontal ligament.
6. Enumerate various principle fibers of periodontal
ligament with the help of illustrations.
7. Discuss in detail the functions of periodontal
ligament.
Alveolar Bone
8. Discuss the alveolar bone with reference to the
following:
I. Structure, composition and parts of alveolar bone
II. Fenestration and dehiscence
III. Resorption and remodeling.
Cementum
9. Discuss cementum with reference to the following:
I. Structure and composition of cementum
II. Cementoenamel junction
III. Hypercementosis
IV. Classification.
BIBLIOGRAPHY
The Gingiva
1. AR Tencate. Oral Histology, Development, Structure and
Function. 5th edition, Mosby Publication.
2. Jan Lindhe. Clinical Periodontology and Implant Dentistry.
4th edition 2003 Blackwell Munksgaard Publication.
3. Jansen Van Rensburg. Oral biology. Quintessence
Publishing Co, Inc 1995.
4. Newman, Takei, Fermin A Carranza. Clinical periodon-
tology, 9th edition 2002, W B Saunders Co.
5. Thomas M Hassell. Tissues and cells of the periodontium.
Periodontol 2000, 1993;3.
Tooth-Supporting Structures
Periodontal Ligament
6. Jan Lindhe. Clinical Periodontology and Implant
Dentistry, IV edition, Blackwell Munksgaard publication,
2003.
7. Newman, Takei, Fermin A Carranza. Clinical periodon-
tology, IX edition, WB Saunders Co, 2002.
8. Thomas M Hassell. Tissues and cells of the periodontium,
Periodontol 2000; vol 3, 1993.

29
Biology of Periodontal Tissues
Alveolar Bone
9. AR Tencate. Oral histology, Development, Structure and
Funtion, V edition, Mosby Publication.
10. Grant, Stern, Listgarten. Periodontics, VI edition, Mosby
Publication, 1998.
11. J.D Manson, B Meley. Outline of Periodontics III edition,
British Library Cataloguing in Publication Data, 1995.
12. Jan Lindhe. Clinical Periodontology and Implant Dentistry,
IV edition, Blackwell Munksgaard Publication, 2003.
13. Newman, Takei, Fermin A Carranza. Clinical Periodon-
tology, IX edition, WB Saunders Co, 2002.
Cementum
14. AR Tencate. Oral Histology, Development, Structure and
Function, IIIrd edition, St. Louis: C.V. Mosby Company,
1989.
15. BG Jansen Van Rensburg. Oral Biology, Quintenssence
Books, Chicago, 1995.
16. Bhaskar S N. Orbans, Oral Histology and Embryology, 11th
edition, St. Louis CV Mosby company.
17. Newman, Takei, Fermin A. Carranza. Clinical Periodon-
tology, IX edition, W.B.Saunders and Co, 2002.
18. Schroeder H F. Oral sructural Bology, Thieme Medical
Publishers, Inc. New York, 1991.
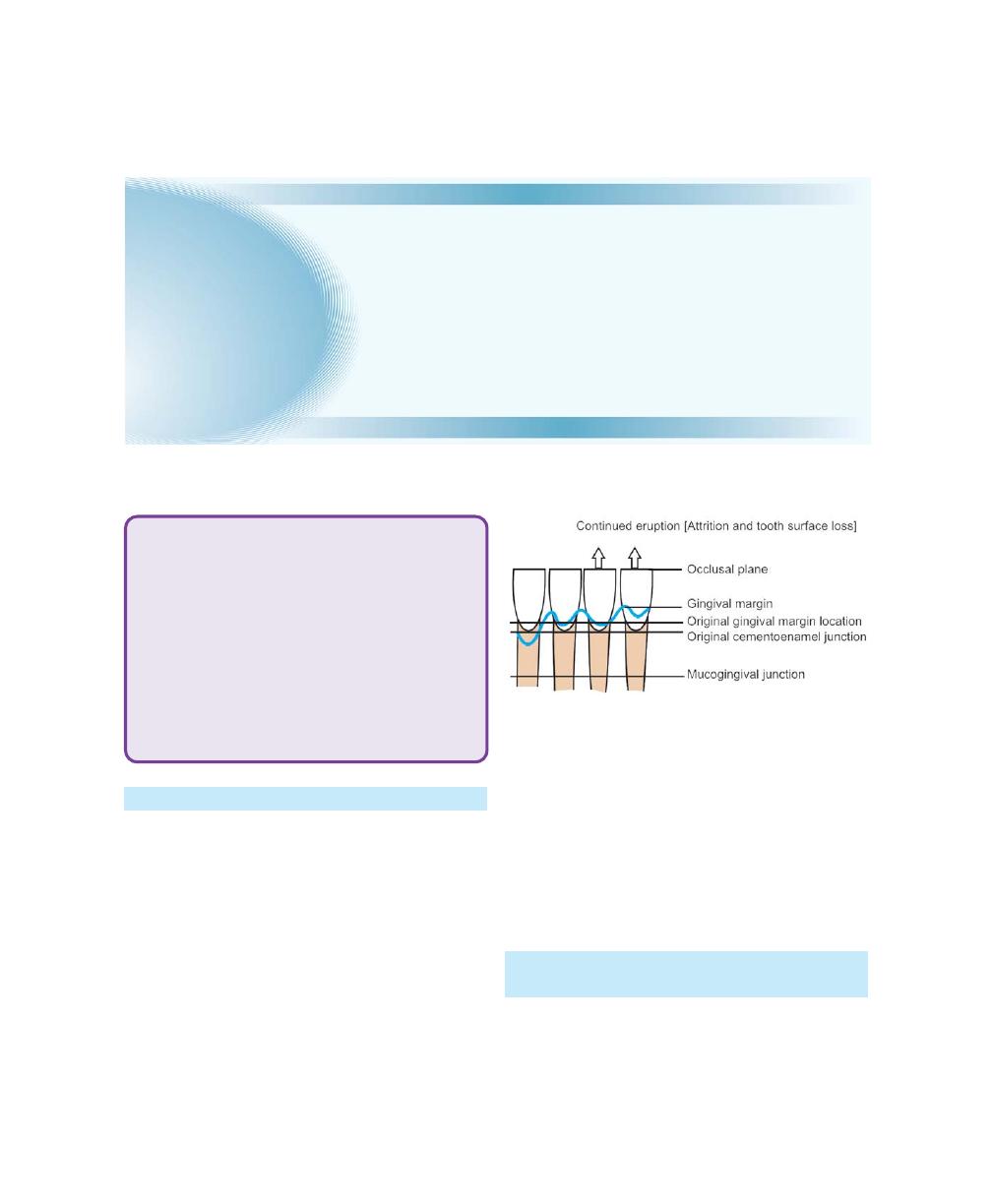
30
Essentials of Clinical Periodontology and Periodontics
GENERAL EFFECTS OF AGING
In the Skin
a. The dermis and epidermis are thinned
b. Keratinization is diminished
c. Blood supply is diminished
d. Degeneration of nerve endings occur
e. Capillaries appear to become more fragile which may
result in haemangiomas after minor traumas
f. Tissue elasticity is decreased.
Bone
a. Undergoes osteoporosis with aging
b. The bone is rarified, trabeculae are reduced in
number and cortical plates are thinned
c. Vascularity is reduced and lacunar resorption is more
prominent
d. Increased susceptibility to fracture
e. With age water content of bone is reduced, the
mineral crystals are increased in size and collagen
fibers are thickened.
AGE CHANGES IN THE PERIODONTIUM
(Fig. 3.1)
Gingiva and Other Areas of the Oral Mucosa
1. Decreased keratinization.
Fig. 3.1:
Aging of the periodontium
❒ GENERAL EFFECTS OF AGING
❒ AGE CHANGES IN THE PERIODONTIUM
• Gingiva and Other Areas of the Oral
Mucosa
• Periodontal Ligament and Age Changes in
the Periodontium
• Changes in the Alveolar Bone and
Cementum
• Bacterial Plaque and Immune Response
❒ EFFECTS OF AGING ON THE PROGRES-
SION OF PERIODONTAL DISEASES
❒ EFFECTS OF TREATMENT ON THE AGING
INDIVIDUALS
3
Periodontal Structures in
Aging Humans
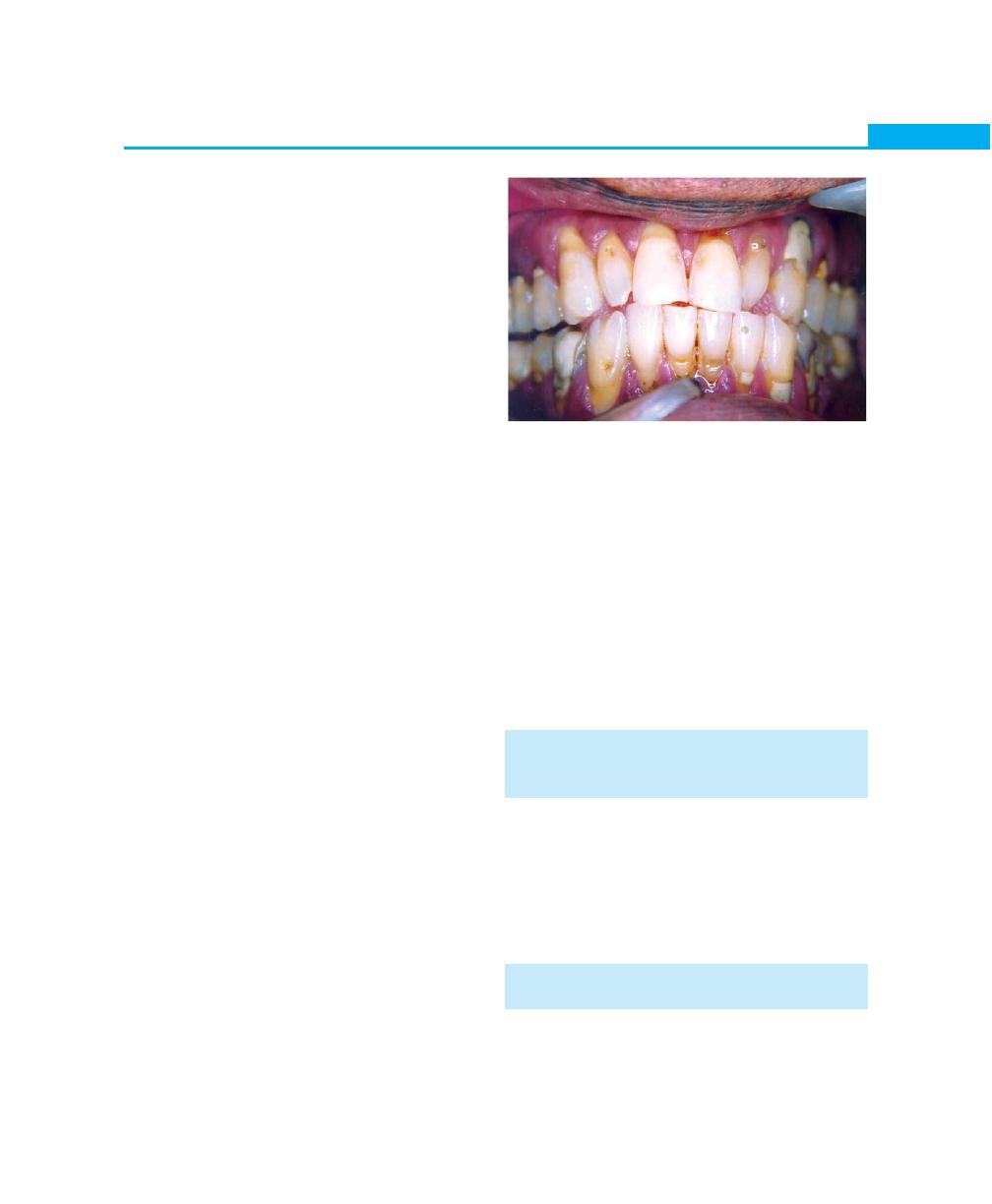
31
Periodontal Structures in Aging Humans
2. Reduced or unchanged amount of stippling.
3. Decreased connective tissue cellularity.
4. Decreased oxygen consumption—which may reflect
upon the metabolic activity.
5. Increased width of attached gingiva.
6. Greater amounts of intercellular substances.
7. Atrophy of the connective tissue with loss of elasticity.
8. Increase in the number of mast cells.
Periodontal Ligament and Age Changes
in the Periodontium
1. Decrease in vascularity.
2. Decrease in mitotic activity.
3. Decrease in fibroblasts.
4. Collagen fibers and mucopolysaccharides are
decreased.
5. Increase in elastic fibers and arteriosclerotic changes
are seen.
6. Both increase and decrease in the width of the
periodontal ligament is seen. The periodontal ligament
width is increased as a result of less number of teeth
supporting the entire functional load and decrease
in its width is associated with reduced strength of the
masticatory musculature and continuous deposition
of cementum and bone.
Changes in the Alveolar Bone and Cementum
1. Osteoporosis.
2. Decreased vascularity.
3. Reduction in metabolic rate and healing capacity.
4. Resorption activity is increased and the rate of bone
formation is decreased.
5. Continuous deposition of cementum occurs with age
and greater irregularity in the surface of both the
cementum and alveolar bone facing the periodontal
ligament is seen.
Bacterial Plaque and Immune Response
(Fig. 3.2)
1. Plaque accumulation has been suggested to increase
with age.
2. Some studies have shown a qualitative change in the
subgingival flora, it has been speculated that a shift
occurs in certain periodontal pathogens with age,
including increase in number of enteric rods,
Porphyromonas gingivalis and decreased role for
Actinobacillus actinomycetemcomitans. Some-age
related changes have been shown to affect the host
response, but there is no evidence to show that these
changes correlate with periodontitis in elderly
patients.
EFFECTS OF AGING ON THE
PROGRESSION OF PERIODONTAL
DISEASES
The conclusions drawn from the various studies are
strikingly-consistent and show that, age has either no
effect or provides a small and clinically insignificant
increased risk of loss of periodontal support. Therefore
age has been suggested to be not a true risk factor but
a background or an associated factor for periodontitis.
EFFECTS OF TREATMENT ON THE
AGING INDIVIDUALS
The few studies that have been done so far have clearly
demonstrated that inspite of certain changes in the
periodontium with aging, no differences in response to
Fig. 3.2:
Periodontal changes associated
with aging

32
Essentials of Clinical Periodontology and Periodontics
nonsurgical or surgical treatment have been shown for
periodontitis.
REVIEW QUESTIONS
1. Describe the age changes in the periodontium.
2. Role of aging on the progression of periodontal
diseases.
BIBLIOGRAPHY
1. JD Manson, B Meley. Outline of Periodontics, Third
Edition 1995, British Library Cataloguing in Publication
Data.
2. Newman, Takei, Fermin A, Carranza. Clinical
Periodontology, Ninth edition, 2002 W.B. Saunders.


NEED FOR CLASSIFICATION
• For the purpose of diagnosis, prognosis and treatment
planning.
• To understand the etiology, pathology of the diseases
of the periodontium.
• For logical, systematic separation and organization of
knowledge about disease.
• Facts can be filed for future references.
• Helps to communicate among clinicians, researchers,
educators, students, epidemiologists and public health
workers.
CURRENT CLASSIFICATION SYSTEMS OF
PERIODONTAL DISEASES
World Workshop in Clinical Periodontics
(1988)
Gingivitis
• Childhood gingivitis
• Chronic (adult) gingivitis
• Acute necrotizing ulcerative gingivitis
Periodontitis
• Adult Periodontitis:
Possible sub-groups:
– High risk
– Normal risk
– Refractory periodontitis
• Early Onset Periodontitis:
– Localized juvenile periodontitis
– Rapidly progressive periodontitis
– Pre-pubertal periodontitis
* Localized
* Generalized
– Periodontitis associated with systemic
diseases
❒
❒
❒
❒
❒ NEED FOR CLASSIFICATION
❒
❒
❒
❒
❒ CURRENT CLASSIFICATION SYSTEMS
OF PERIODONTAL DISEASES
• Gingivitis
• Periodontitis
❒
❒
❒
❒
❒ SYNOPSIS OF TYPES AND
CHARACTERISTICS OF GINGIVAL
DISEASES
❒
❒
❒
❒
❒ SYNOPSIS OF TYPES AND
CHARACTERISTICS OF PERIODONTAL
DISEASES
4
Classification Systems of
Periodontal Diseases

36
Essentials of Clinical Periodontology and Periodontics
World Workshop in Clinical Periodontics (1989)
I. Adult periodontitis.
II. Early onset periodontitis.
• Prepubertal
– Generalized or localized
• Juvenile
– Generalized or localized
• Rapidly progressive periodontitis
III. Periodontitis associated with systemic diseases
• Down’s Syndrome
• Diabetes – Type I
• Papillon – Lefévre syndrome
• AIDS and other diseases
IV. Necrotizing ulcerative periodontitis
V. Refractory periodontitis
Genco (1990)
• Periodontitis in adults.
• Periodontitis in juveniles
– Localized form
– Generalized form
• Periodontitis with systemic involvement
– Primary neutrophil disorders
– Secondary or associated neutrophil impairment.
– Other systemic diseases
• Miscellaneous conditions
Ranney (1993)
Gingivitis
• Gingivitis, plaque bacterial
• Non-aggravated
• Systemically-aggravated by sex hormones, drugs,
systemic diseases
• Necrotizing ulcerative gingivitis
• Systemic determinants unknown
• Related to HIV
• Gingivitis, non-plaque
• Associated with skin diseases, allergic, infections.
Periodontitis
• Adult periodontitis
– Non-aggravated
– Systematically-aggravated (neutropenias, leuke-
mias, lazy leukocyte syndrome, AIDS, diabetes
mellitus, Crohn’s disease, Addison’s disease).
• Early onset periodontitis:
– Localized early onset periodontitis
– Neutrophil abnormality
– Generalized early onset periodontitis, neutrophil
abnormality, immunodeficient
• Early onset periodontitis related to systemic disease:
– Leukocyte adhesion deficiency, hypophospha-
tasia, Papillon- Lefevre syndrome, neutropenias,
leukemias, Chediak- Higashi syndrome, AIDS,
diabetes mellitus type-I, trisomy-21, histiocytosis
X, Ehlers Danlos syndrome (type VIII)
• Early onset periodontitis, systemic determinants
unknown
• Necrotizing ulcerative periodontitis
– Systemic determinants unknown
– Related to HIV
– Related to nutrition
• Periodontal abscess
European Workshop on Periodontology
(1993)
• Adult periodontitis
• Early onset periodontitis
• Necrotizing periodontitis
All the above-mentioned classification systems have
been widely used by clinicians and research scientists
throughout the world, unfortunately they have been many
shortcomings including:
1. Considerable overlap in disease categories.
2. Absence of a gingival disease component
3. Inappropriate emphasis on age of onset of disease
and rates of progression.
4. Inadequate or unclear classification criteria.
Hence, the need for a revised classification system
for periodontal diseases was emphasized and in 1999,
the international workshop had considered designing a
new classification system.

37
Classification Systems of Periodontal Diseases
Classification of Periodontal
Disease and Condition
By AAP 1999 (International Workshop for Classification
of Periodontal Disease).
Gingival Disease (Table 4.1)
A. Dental plaque–induced gingival diseases: These
diseases may occur on a periodontium with no
attachment loss or on one with attachment loss that
is stable and not progressing.
1. Gingivitis associated with dental plaque only:
a. Without local contributing factors.
b. With local contributing factors
2. Gingival diseases modified by systemic factors:
a. Associated with endocrine system:
i. Puberty-associated gingivitis
ii. Menstrual cycle—associated gingivitis
iii. Pregnancy-associated
– Gingivitis
– Pyogenic granuloma
iv. Diabetes mellitus – associated gingivitis
b. Associated with blood dyscrasias:
i. Leukemia-associated gingivitis
ii. Others
3. Gingival diseases modified by medications:
a. Drug-influenced gingival diseases
i. Drug-influenced gingival enlargements.
ii. Drug-influenced gingivitis
– Oral contraceptive- associated gingivitis
– Others
4. Gingival diseases modified by malnutrition:
a. Ascorbic acid deficiency gingivitis
b. Others
B. Nonplaque-induced gingival lesions:
1. Gingival diseases of specific bacterial origin.
a. Neisseria gonorrhoeae
b. Treponema pallidium
c. Streptococcal species
d. Others
2. Gingival diseases of viral origin
a. Herpes-virus infections
• Primary herpetic gingivostomatitis
• Recurrent oral herpes
• Varicella zoster
b. Others
3. Gingival diseases of fungal origin
a. Candida species infections; generalized gingival
candidiasis
b. Linear gingival erythema
c. Histoplasmosis
d. Others.
4. Gingival lesions of genetic origin
a. Hereditary gingival fibromatosis
b. Others
5. Gingival manifestations of systemic conditions
a. Mucocutaneous lesions:
i. Lichen planus
ii. Pemphigoid
iii. Pemphigus vulgaris
iv. Erythema multiforme
v. Lupus erythematosus
vi. Drug induced
vii. Others
b. Allergic reactions:
i. Dental restorative materials
– Mercury
– Nickel
– Acrylic
– Others
ii. Reactions attributable to:
– Toothpaste’s or dentifrices
– Mouth rinses or mouthwashes
– Chewing gum additives
– Foods and additives
iii. Others
6. Traumatic lesions (factitious, iatrogenic, or
accidental)
a. Chemical injury
b. Physical injury
c. Thermal injury
7. Foreign body reactions
8. Not otherwise specified (NOS).
38
Essentials of Clinical Periodontology and Periodontics
Table 4.1: Synopsis of types and characteristics of gingival diseases
Types of gingival disease Causes
Signs and symptoms
Treatment
Gingivitis
Bacterial plaque, local
Gingival redness and
Debridement, plaque control,
plaque—retention factors
swelling, bleeding, does
correct plaque retentive
not cause loss of clinical
factors, supportive
attachment
periodontal therapy
Acute necrotizing
Bacterial plaque, may be
Pain, gingival redness,
Debridement, plaque control,
gingivitis
associated with AIDS at any age
swelling, bleeding, necrosis
antimicrobial rinse, supportive
of interproximal papilla
periodontal therapy
Desquamative
Skin diseases, lichen planus,
Gingival redness, epithelial
Gentle plaque control,
gingival disease
pemphigus and cicatrical
denudation, pain with
palliative and symptomatic
pemphigoid
trauma as on eating and
therapy, supportive
brushing
periodontal therapy
Gingivitis associated
Manifestation of systemic
Dependent on
Treatment of systemic disease,
with systemic
diseases in gingiva
systemic disease
atraumatic plaque control,
diseases
antimicrobial rinse, supportive
periodontal therapy
Gingivitis associated
Bacterial plaque, local
Gingival redness and
Debridement and plaque
with pregnancy
plaque, retentive factors,
swelling, bleeding,
control, supportive
hormonal influence
pyogenic granuloma
periodontal therapy, possile
excision of pyogenic granuloma
Drug-induced
Calcium channel blocking
Gingival enlargement
Debridement and plaque
gingival enlargement
drugs, phenytoin, cyclosporine
control, surgical excision, use of
alternative medications,
supportive periodontal therapy
Allergic reaction
Local allergens
Gingival redness and
Identification and elimination
swelling
of allergic agent
Herpetic
Herpes type I virus
Pain, vesicle formation,
Palliative and symptomatic
gingivostomatitis
ulceration
therapy, antiviral medication
Gingival disease of
Neiserria gonorrhoeae,
Varies according to
Identification and elimination
specific bacteria or
Treponema pallidum,
infectious agent
or control of infectious
fungal origin
streptococcal species,
agent, appropriate
Candida, histoplasmosis
chemotherapy
Chronic Periodontitis (Table 4.2)
a. Localized
— Less than 30 percent of sites
involved or,
b. Generalized — More than 30 percent of sites
involved.
c. Slight
— 1 to 2 mm clinical attachment
loss.
d. Moderate
— 3 to 4 mm clinical attachment loss
and,
e. Severe
— More than 5 mm clinical attachment
loss.
Aggressive Periodontitis
a. Localized–slight, moderate or severe
b. Generalized.
39
Classification Systems of Periodontal Diseases
Table 4.2: Synopsis of types and characteristics of periodontal diseases
Types of periodontal
Causes
Signs and symptoms
Treatment
disease
Chronic periodontitis
Bacterial plaque, smoking,
Overall slow progression
Plaque control, smoking
local plaque retentive factors
with generalized periodontal
cessation, scaling and
such as dental calculus and
pockets, bone and clinical
root planing, correction of
faulty restorations
attachment loss, may be
local plaque retentive factors
generalized or localized
antimicrobial chemotherapy,
periodontal surgery, supportive
periodontal therapy
Aggressive
Bacterial plaque, superinfection
Severe and rapid periodontal Specific, antimicrobial therapy
periodontitis
with specific periodontal bacteria,
destruction possibly followed
based on microbial analysis,
possible impaired host response,
by periods of remission, may
smoking cessation,
smoking
be generalized or localized
debridement, possible
periodontal surgery,
supportive periodontal therapy
Refractory periodontitis
Bacterial plaque, superinfection,
Progression of disease
Specific, antimicrobial,
of any type
with specific, periodontal
despite good conventional
therapy based on microbial
bacteria, possible impaired host
therapy and supportive
analysis, smoking cessation
response, smoking
periodontal therapy
debridement, possible perio-
dontal surgery, supportive
periodontal therapy
Periodontitis as a
Associated with disorders of the
Generalized and localized
Treatment of systemic disease,
manifestation of
blood or blood forming organs
forms of severe destruction
atraumatic plaque control,
systemic diseases
such as neutropenia, leukemia
of bone and connective
anti-microbial rinse,
or genetic disorders
tissue tooth support
supportive periodontal therapy
Juvenile periodontitis:
Probably major autosomal
Localized juvenile,
Scaling and root planing,
localized and
gene effect and infection with
periodontitis: typically loss
specific antimicrobial therapy
generalized
Actinobacillus
of bone support of first
based on microbial analysis,
actinomycetemcomitans
molars and incisors.
possible regenerative surgery,
Generalized juvenile
supportive periodontal therapy
periodontitis: generalized
loss of support throughout
dentition
Periodontitis
May be of endodontic or
Periodontal pocket
If primarily of endodontic
associated with
periodontal origin
extending to area of
origin, endodontic therapy
endodontic lesions
endodontic lesion
alone, if primarily of
periodontal origin, endodontic
and periodontal therapy or
extraction may be necessary
Periodontal abscess
Subgingival bacteria
Painful, acute swelling of
Nonsurgical or surgical
periodontal tissues associated debridement, antibiotic
with deep periodontal pocket
therapy, regeneration of
lost periodontal support is often
a possibility
Acute necrotizing
Immunocompromised,
Pain, rapid loss of bone
Debridement, atraumatic
periodontitis
may be associated
and tooth support associated
plaque control, analgesic
with HIV
with gingival and
medication, antimicrobial rinse,
bony necrosis
supportive periodontal therapy
40
Essentials of Clinical Periodontology and Periodontics
Periodontitis as a Manifestation of
Systemic Diseases
a. Associated with hematological disorders:
i. Acquired neutropenia
ii. Leukemias
iii. Others
b. Associated with genetic disorders:
i. Familial and cyclic neutropenia
ii. Down’s syndrome
iii. Leukocyte adhesion deficiency syndrome
iv. Papillon-Lefévre syndrome
v. Chediak-Higashi syndrome
vi. Histiocytosis syndrome
vii. Glycogen storage disease
viii. Infantile genetic agranulocytosis
ix. Cohen syndrome
x. Ehlers-Danlos syndrome (Type IV and VIII)
xi. Hypophosphatasia
xii. Others
Necrotizing Periodontal Diseases
a. Necrotizing ulcerative gingivitis
b. Necrotizing ulcerative periodontitis.
Abscesses of the Periodontium
a. Gingival abscess
b. Periodontal abscess
c. Pericoronal abscess
Periodontitis Associated with Endodontic Lesions
a. Combined periodontic—endodontic lesions.
Developmental or Acquired Deformities
and Conditions
a. Localized tooth-related factors that modify or pre-
dispose to plaque induced gingival diseases/
periodontitis.
i. Tooth anatomic factors
ii. Dental restorations/appliances
iii. Root fractures
iv. Cervical root resorption and cemental tear
b. Mucogingival deformities and conditions around
teeth:
i. Gingival/soft tissue recession, facial or lingual
surfaces, interproximal (papillary)
ii. Lack of keratinized gingiva
iii. Decreased vestibular depth
iv. Aberrant frenum/muscle position
v. Gingival excess:.
– Pseudopocket
– Inconsistent gingival margin
– Excessive gingival display
– Gingival enlargement
vi. Abnormal color
c. Mucogingival deformities and conditions on eden-
tulous ridges:
i. Vertical and/or horizontal ridge deficiency.
ii. Lack of gingival/keratinized tissue
iii. Gingival/soft tissue enlargement
iv. Aberrant frenum/muscle position
v. Decreased vestibular depth
vi. Abnormal color
d. Occlusal trauma:
i. Primary occlusal trauma
ii. Secondary occlusal trauma
BIBLIOGRAPHY
1. Carranza Jr. Newman, Clinical Periodontology. W.B.
Saunders Company, Ninth Edition.
2. Gary C. Armitage, Development of a classification system
for periodontal diseases and conditions. Ann. Periodontol,
1999;4:1-6.
3. Ranney RR. Classification of periodontal diseases.
Periodontology 2000, 1993:2-13.
4. Robert J, Genco. Development of classification systems for
periodontal diseases and condition. Annals of
Periodontology December, 1999;4(1).
41
Epidemiology of Gingival and Periodontal Diseases
EPIDEMIOLOGY
Definition
Study of health and disease in populations and how the
states are influenced by heredity, biology, physical and
social environmental ways of living.
Types of Epidemiologic Research
a. Descriptive studies.
b. Analytical studies.
c. Experimental epidemiology.
Descriptive Studies
These are used to observe and document the occurrence,
progression and distribution of a disease or condition
in populations, in relation to host and environmental
factors (when, where and who).
Measurement of disease: Incidence can be obtained from
“Longitudinal studies” and prevalence from “cross-
sectional” studies.
a. Incidence rate: This is defined as the number of new
cases occurring in a defined population during a
specific period of time.
b. Prevalence rate: This refers to all current cases (new
and old) existing at a given point in time or over
a period of time in a given population.
Two types are described:
a. Point prevalence.
b. Period prevalence.
Longitudinal Studies
The observations are repeated in the same population
over a prolonged period of time by means of follow-
up examinations.
Uses
i. To study the natural history of disease and its future
outcome.
ii. For identifying the risk factors of disease.
iii. For finding out the incidence rate or rate of
occurrence of new cases.
❒
❒
❒
❒
❒ DEFINITION, TYPES AND AIMS OF
EPIDEMIOLOGY
❒
❒
❒
❒
❒ DEFINITION, USES AND CHARACTERIS-
TICS OF AN INDEX
❒
❒
❒
❒
❒ VARIOUS INDICES USED TO STUDY
PERIODONTAL PROBLEMS
• To Assess Gingival Inflammation
• To Measure Periodontal Destruction
• To Measure Plaque Accumulation
• To Measure Calculus
• To Assess Treatment Needs
5
Epidemiology of Gingival
and Periodontal Diseases
42
Essentials of Clinical Periodontology and Periodontics
Disadvantages
They are difficult to organize and time consuming as
compared to cross-sectional studies
Cross-sectional Studies
It is the simplest form of an observation study. It is based
on a single examination of a cross-section of population
at one point of time. (It is also known as prevalence
study).
Uses
i. It is more useful for chronic (long-term diseases)
as compared to acute (short-term) diseases.
ii. It gives information about the distribution of a
disease in a population rather than its etiology.
Analytical Studies
Includes two distinct types:
a. Case control study
b. Cohort study
Case control or retrospective study: The study precedes
backwards from effect to cause.
Cohort study: It is a prospective study (incidence study
or longitudinal study), which is usually undertaken to
obtain, additional evidence to refute and support the
existence of an association between the suspected cause
and disease.
Experimental Epidemiology
It is used to test hypothesis further by introducing a
preventive or therapeutic agent and comparing the
outcome in the test subjects with concurrent observations
in control groups.
Aims of Epidemiology
Three main aims have been proposed:
a. To describe the distribution and size of disease
problems in human populations.
b. To identify the etiological factors in the pathogenesis
of disease.
c. To provide the data essential for the planning,
implementation and evaluation of services for the
prevention, control and treatment of disease.
INDEX
Definition
These are numerical values describing the relative
status of the population on a graduated scale with
definite upper and lower limits, which are designed
to permit and facilitate comparisons with other
populations and are classified by the same criteria and
methods.
Purposes and Uses of an Index
For Individual Patients
An index can,
i. Provide individual assessment to help a patient to
recognize an oral problem.
ii. Reveal the degree of effectiveness of present oral
hygiene practices.
iii. Motivate the person in preventive and professional
care for the elimination and control of oral
disease.
iv. Evaluate the success of an individual and
professional treatment over a period of time by
comparing index scores.
In Research
An index is used to,
i. Determine the baseline data before the
experimental factors are introduced.
ii. Measure the effectiveness of specific agents for
the prevention control and treatment of oral
conditions.
iii. Measures the effectiveness of mechanical devices
for personal care, such as, toothbrushes, interdental
cleaning devices or water irrigators.

43
Epidemiology of Gingival and Periodontal Diseases
In Community Health
An index
i. Can show the prevalence and trends of incidence,
of a particular condition occurring within a given
population.
ii. It provides a baseline data to show the existing
dental health practices.
iii. It assesses the needs of a community and compares
the effects of a community program and evaluates
the results.
Characteristics of an Index
1. It should be simple to use and accurate.
2. It should require minimal equipment and expenses.
3. It should have clear- cut criteria, which are readily
understandable.
4. It should be as free as possible from subjective
interpretation.
5. It should be reproducible by the same examiner or
different examiners.
6. Be amenable to statistical analysis, have validity and
reliability.
7. Not require an excessive amount of time to complete.
8. Not cause patient discomfort or be otherwise
unacceptable to a patient.
Indices used to Assess the following
Periodontal Problems
1. The degree of inflammation of the gingival tissues.
2. The degree of periodontal destruction.
3. The amount of plaque accumulated.
4. The amount of calculus present.
5. In addition, indices are developed to assess the
treatment needs.
Indices used to Assess Gingival Inflammation
a. PMA index by Schour and Massler.
b. Gingivitis component of the periodontal disease.
c. Gingival index by Loe and Sillness.
d. Indices of gingival bleeding.
i. Sulcus bleeding index (SBI) of Muhlemann and
Mazor.
ii. Papillary bleeding index by Muhlemann.
iii. Bleeding points index by Lennox and Kopczy K
iv. Interdental bleeding index by Caton and Polson.
v. Gingival bleeding index by Ainamo and Bay.
Indices used to Measure
Periodontal Destruction
a. Russell’s periodontal index.
b. Periodontal disease index by Ramfjord.
c. Extent and severity index by Carlos and co-workers.
d. Radiographic approaches to measure bone loss.
i. GBI—Gingival bone count index by Dunning and
Leach.
ii. PSI—Periodontal severity index by Adams and
Nystrom.
Indices used to Measure Plaque Accumulation
a. Plaque component of periodontal disease index by
Ramfjord.
b. Simplified oral hygiene index by Greene and
Vermillion.
c. Turskey-Gillmore-Glickman modification of the
Quigley-Hein plaque index.
d. Plaque index by Sillness and Loe.
e. Modified navy plaque index.
f. Patient hygiene performance index by Podshadley
and Haley.
g. Plaque weight.
h. Plaque free score by Grant, Stern and Everett.
i. Plaque control record by O’leary.
Indices used to Measure Calculus
a. Calculus component of simplified oral hygiene index
by Greene and Vermillion.
b. Calculus component of the periodontal disease index
by Ramfjord.
c. Probe method of calculus assessment by Volpe and
associates
44
Essentials of Clinical Periodontology and Periodontics
d. Calculus surface index by Ennever and co-workers
e. Marginal line calculus index by Muhlemann and
Villa
Indices used to Assess Treatment Needs
a. Gingival periodontal index.
b. Community periodontal index of treatment needs
by Ainamo and associates.
c. PTNS—Periodontal treatment need system by Belini
HT.
INDICES USED TO ASSESS
GINGIVAL INFLAMMATION
Papillary Marginal Attachment (PMA) Index by
Schour and Massler (1944)
The basic philosophy used in the development of the
PMA index was very similar to the DMF index, i.e. the
number of gingival units affected were counted rather
than the severity of the inflammation. A gingival unit
is divided into three component parts:
i. Papillary gingiva (P)
ii. Marginal gingiva (M)
iii. Attached gingiva (A)
The presence or absence of inflammation on each
gingival unit is recorded as 1 or 0 respectively. The P,
M, A numerical values for all the teeth are added
separately and then added together to express the PMA
index score per person. The developers of this index
eventually added a severity component for assessing
gingivitis, the papillary units (P) were scored on a scale
of 0 to 5, and the marginal (M) and attached gingiva
were scored on a scale of 0 to 3.
Gingivitis Component of the
Periodontal Disease
The periodontal disease index (PDI) is similar to PI, in
that; both are used to measure the presence and severity
of periodontal disease. The PDI does so by combining
the assessments of gingivitis and gingival sulcus depth
on six selected teeth (# 3, 9, 12, 19, 25 and 28). This
group of teeth frequently referred to as the Ramfjord
teeth, have been tested as reliable indicators for the
various regions of the oral cavity. Calculus and plaque
are also examined to assist in formulating a
comprehensive assessment of periodontal status.
A numerical score for the gingival status component
of the PDI is obtained by adding the values for all the
gingival units and dividing it by the number of teeth
present. This index has been used in epidemiological
surveys, longitudinal studies and clinical trials.
Gingival Index by Loe H and Sillness J (1963)
This index was solely developed for the purpose of
assessing the severity of gingivitis and its location in four
possible areas.
Method
The severity of gingivitis is scored on all surfaces of all
teeth, or selected teeth, or on selected surfaces of all
teeth, or, selected teeth. The tissues surrounding each
tooth are divided into four gingival scoring units:
• Distal facial papillae,
• Facial margin,
• Mesial facial papillae,
• Entire lingual gingival margin.
A blunt instrument is used for recording the scores
based on the following criteria:
0 — No inflammation
1 — Mild inflammation, no bleeding elicited on probing
2 — Moderate inflammation, bleeding on probing
3 — Severe inflammation
The scores around each tooth are added and divided
by four to arrive at the score for that particular tooth.
Total all the teeth scores and divide it by the number
of teeth. This provides the gingival index score per
person. The numerical values are correlated as follows:
0.1 to 1.0 — Mild gingivitis
1.1 to 2.0 — Moderate gingivitis
2.1 to 3.0 — Severe gingivitis

45
Epidemiology of Gingival and Periodontal Diseases
Indices of Gingival Bleeding
Sulcular bleeding Index by
Muhlemann and Son (1971)
The purpose of the index is to locate areas of the gingival
sulcus that bleed upon gentle probing and thus recognize
and record the presence of early inflammatory gingival
disease. Four gingival units are scored systemically for
each tooth: the labial and lingual marginal gingiva (M
units) and the mesial and distal papillary gingiva (P units).
The probe is held parallel with the long axis of the tooth
and 30 seconds after probing; scoring is done based on
the criteria, which ranges from 0 to 5. Each of the four
gingival units is scored 0 to 5. Scores for the four units
are added and divided by four. Adding the scores of
the undivided teeth and dividing them by the number
of teeth can determine the sulcus-bleeding index.
Scoring criteria:
0 — Normal appearing gingiva, no bleeding upon
probing
1 — No color or contour changes, but bleeding on
probing
2 — Bleeding on probing, color change (reddening),
no edema and contour changes
3 — Bleeding on probing, color change, mild
inflammatory edema
4 — Bleeding on probing, color change, severe
inflammatory edema.
5 — Spontaneous bleeding on probing, color change,
very severe inflammatory edema with or without
ulceration.
Papillary Bleeding Index by Muhlemann HR (1977)
It is based on bleeding elicited, following gentle probing
of the interdental papilla. A blunt periodontal probe is
carefully inserted into the gingival sulcus at the base of
the interdental papilla on the mesial aspects, and then
moved coronally to the papilla tip. This is repeated on
the distal aspect of the same papilla. The intensity of
any bleeding thus provoked will be recorded on a scale
of 0 to 4.
Scoring criteria:
0 — No bleeding
1 — A single discrete bleeding point appears
2 — Several isolated bleeding points or a single fine
line of blood appears
3 — The interdental triangle fills with blood shortly after
probing
4 — Profuse bleeding occurs after probing, blood flows
immediately into the marginal sulcus
Bleeding Points Index by Lennox and
Kopczy K
The index was developed to assess a patient’s oral hygiene
performance. It determines the presence or absence of
gingival bleeding interproximally and on the facial and
lingual surfaces of each tooth. A periodontal probe is
drawn horizontally through the gingival crevice of a
gradient, and the gingiva is examined for bleeding after
30 seconds.
Interdental Bleeding Index by
Caton and Polson
The index utilizes a triangle-shaped toothpick made of
soft, pliable wood to stimulate the interproximal
gingival tissue. The interproximal cleaner is inserted
horizontally between the teeth from the facial surface,
depressing the interproximal papillae by up to 2 mm.
The wooden cleaner is inserted and removed four times,
and the presence or absence of bleeding within 15 seconds
is noted. The score is determined by dividing the number
of bleeding sites by the number of sites evaluated.
Gingival Bleeding Index by Ainamo and Bay
The index was developed as an easy and suitable way
for the practitioner to assess a patient’s progress in plaque
control. The presence or absence of gingival bleeding
is determined by gentle probing of the gingival crevice
with a periodontal probe. The appearance of the bleeding
within 10 seconds indicates a positive score, which is
expressed as a percentage of the total number of gingival
margins examined.
46
Essentials of Clinical Periodontology and Periodontics
INDICES USED TO MEASURE
PERIODONTAL DESTRUCTION
Russell’s Periodontal Index by Russell AL (1956)
The index was intended to estimate deeper periodontal
disease by measuring the presence or absence of the
gingival inflammation and its severity, pocket formation
and masticatory function.
All the teeth present are examined. All of the gingival
tissues surrounding each tooth are assessed for gingival
inflammation and periodontal involvement. Russell
choses the scoring values (0, 1, 2, 6, 8) in order to relate
the stages of the disease in an epidemiological survey
to the clinical conditions observed.
Sum of individual scores
PI score per person = —————————————
Number of teeth present
Since only a mouth mirror and no calibrated probe
or radiographs is used while performing the PI
examination, the results tend to under estimate the true
level of periodontal disease. The number of periodontal
pockets without obvious supragingival calculus is also
underestimated in the periodontal index.
Scoring Criteria
Score Criteria and scoring
Additional X-ray criteria
for field studies
followed in the clinical test
0
Negative: There is neither
Radiographic appearance
overt inflammation in the
is essentially normal
investing tissues nor loss
of function due to des-
truction of supporting
tissues.
1
Mild Gingivitis: There is
an overt area of inflam-
mation in the free gingiva,
but this area does not
circumscribe the tooth
2
Gingivitis: Inflammation
completely circumscribing
the tooth, but there is no
apparent break in the
epithelial attachment.
4
Used when radiographs
There is early notch-like
are available
resorption of alveolar
crest
6
Gingivitis with pocket
There is horizontal bone
formation: The epithelial
loss involving the entire
attachment has been
alveolar crest, up to half
broken and there is a
of the length of the tooth
pocket. There is no
root.
interference with normal
masticatory functions, the
tooth is firm and has not
drifted. There is hori-
zontal bone loss involving
the entire alveolar crest,
up to half of the length
of the tooth root.
8
Advanced destruction with
There is advanced bone
loss of masticatory func-
loss, involving more than
tion: The tooth may be
one-half of the length of
loose, may have drifted,
tooth root, infrabony
may sound dull on per-
defects, widening of
cussion and may be
periodontal ligament,
depressible in socket.
root resorption.
Clinical Conditions and Periodontal Scores
Clinical conditions
Group PI scores
Stage of disease
Clinically-normal
0 to 0.2
supportive tissues
Simple gingivitis
0.3 to 0.9
Beginning of des-
0.7 to 1.9
Reversible
tructive–periodontal
disease
Established destructive
1.6 to 5.0
Irreversible
periodontal disease
Terminal disease
3.8 to 8.0
Irreversible
Uses
i. Epidemiological surveys.
ii. More data can be assembled using periodontal
index.
iii. Used in the National Health Survey (NHS).
Periodontal Disease Index by
Sigurd P Ramfjord (1959)
This index is a clinician’s modification of the Russell’s
periodontal index for epidemiological surveys of
periodontal disease. Emphasis is placed on the recording
Contd...
Contd...
Score Criteria and scoring
Additional X-ray criteria
for field studies
followed in the clinical test

47
Epidemiology of Gingival and Periodontal Diseases
of the attachment level of the periodontal disease relative
to the cementoenamel junction.
Only six selected teeth are scored for assessment of
the periodontal status of the oral cavity, which are 1,
6, 21, 24, 36, 41, 44. The first step is scoring of the
gingival status. The gingiva around the teeth is dried
superficially by gently dabbing it with absorbing cotton.
Changes in color, consistency, contour; evidence of
ulceration of gingiva is evaluated by a periodontal probe.
The next step is recording of the crevice depth related
to a cementoenamel junction. For this purpose a
University of Michigan 0 probe is used. The end of probe
should be placed against the enamel surface coronally
to the margin of the gingiva and minimal force is applied
in an apical direction maintaining tooth contact. The
crevicular measurements are recorded in the following
manner. Distance from free gingival margin to the
bottom of the gingival crevice or pocket on the buccal
and mesial aspect of the each tooth. The buccal measure-
ments should be made at the middle of the buccal
surfaces and the mesial measurement should be made
at the buccal aspect of the interproximal contact area.
The scoring criteria ranges from 0 to 6. The PDI score
for the individual can be obtained by adding the scores
for each tooth examined and then, dividing by the
number of teeth examined. The PDI score will range
from 0 to 6.
Scoring Criteria
0 — Absence of inflammation
1 — Mild to moderate inflammatory gingival changes
not extending all around the tooth
2 — Mild to moderately severe gingivitis extending all
around the tooth
3 — Severe gingivitis, characterized by marked
redness, tendency to bleed and ulceration
4 — Gingival crevice in any of the four measured areas
(mesial, distal, buccal, lingual), extending apically
to the CEJ, but not more than 3 mm
5 — Gingival crevice in any of the four measured areas
extending apically, 3-6 mm from the CEJ
6 — Gingival crevice in any of the four measured areas
extending apically more than 6 mm from the CEJ
Extent and Severity Index by
Carlos and Coworkers
In this newer model, periodontal disease is viewed as
a chronic process with intermittent periods of activity
and remission that affects individual teeth and sites around
the teeth at different rates within the same mouth. It
uses, a periodontal probe (NIDR probe) to determine
attachment levels. The ESI score is a bivariate statistic.
It expresses the percentage of sites that exhibit disease.
ESI is based on probe measurements (at the mesiobuccal,
interproximal and midbuccal locations on all teeth
excluding molars and at the mesiobuccal interproximal
and midbuccal of the mesial root of molars) at 14 sites
in half of the maxillary arch and at 14 sites in the
contralateral mandibular arch.Attachment level
measurements are made using the Ramfjord criteria.
Radiographic Approaches to
Measure Bone Loss
Gingival Bone Count Index by Dunning and Leach
This index records the gingival condition on a scale of
0 to 3 and the level of the crest of the alveolar bone.
The Periodontitis Severity Index
by Adams and Nystrom
The index assesses the presence or absence of
periodontitis as the product of clinical inflammation and
interproximal bone loss determined radiographically using
a modified schei rule.
INDICES USED TO MEASURE
PLAQUE ACCUMULATION
Plaque Component of Periodontal
Disease Index by Ramfjord
The index is used on the six teeth selected by Ramfjord
(teeth number 3, 9, 12, 19, 25 and 28) after staining
with Bismarck brown solution. The criteria is to measure

48
Essentials of Clinical Periodontology and Periodontics
the presence and extent of plaque on a scale of 0 to
3, looking specifically at all interproximal facial and lingual
surfaces of the index teeth. The scoring criteria is as
follows:
0 — No plaque present.
1 — Plaque present on some but not all interproximal,
buccal and lingual surfaces of the tooth.
2 — Plaque present on all interproximal buccal and
lingual surfaces, but covering less than one half
of the surfaces.
3 — Plaque extending over all interproximal, buccal
and lingual surfaces, and covering more than one
half of these surfaces.
Only fully-erupted teeth are scored and missing teeth
should not be substituted.
Total score
Plaque score of an individual = —————————
Number of teeth
examined
Uses
Suitable for:
a. Longitudinal studies of periodontal disease.
b. Epidemiological surveys.
c. Clinical trials of preventive or therapeutic agents.
Simplified Oral Hygiene Index by
Greene and Vermillion (1964)
The OHI-S measures the surface area of the tooth that
is covered by debris and calculus. It consists of two
components:
a. Debris index-simplified (DI-S).
b. Calculus index-simplified (CI-S).
Scoring Criteria for D1-S
0 — No debris or stain present.
1 — Soft debris covering not more than one-third of
the tooth surface or the presence of extrinsic stains
without other debris, regardless of surface area
covered.
2 — Soft debris, covering more than one-third but not
more than two- thirds of the exposed tooth
surface.
3 — Soft debris, covering more than two-thirds of the
exposed tooth surface.
Scoring Criteria for CI-S
0 — No calculus present.
1 — Supragingival calculus covering not more than
one-third of the exposed tooth surface.
2 — Supragingival calculus covering more than one-
third, but not more than two-thirds of the exposed
tooth surface or the presence of the individual
flecks of subgingival calculus around the cervical
portion of the tooth or both.
3 — Supragingival calculus covering more than two-
thirds of the exposed tooth surface or a continuous
heavy band of subgingival calculus around the
cervical portion of the tooth, or both.
Method
Each component is assessed on a scale of 0 to 3. Only
a mouth mirror and a Shepherd’s Crook or sickle-type
dental explorer, and no disclosing agent are used for
examination. The six tooth surfaces examined are No
3, 8, 14, 24 (Facial surface) and No 19, 30 (Lingual
surfaces). Each tooth surface is divided horizontally into
gingival, middle and incisal thirds. For the DI-S, a dental
explorer is placed on the incisal third and moved towards
the gingival third and scores are awarded according to
the criteria. The DI-S score per person is obtained by
totaling the debris score per the tooth surface and dividing
it by the number of surfaces examined. The CI-S
assessment is performed by gently placing a dental
explorer into the distal gingival crevice and drawing it
subgingivally from the distal contact area to the mesial
contact area. Scoring is done according to the criteria.
The CI-S score per person is obtained by totaling the
calculus scores per tooth surface and dividing it by the
number of surfaces examined. The OHI-S score per

49
Epidemiology of Gingival and Periodontal Diseases
person is the total of DI-S and CI-S scores per person.
The clinical levels of oral cleanliness for debris that can
be associated with groups (DI-S, CI-S) scores are as
follows:
Good — 0.0 to 0.6
Fair
— 0.7 to 1.8
Poor — 1.9 to 3.0
The clinical levels of oral hygiene that can be
associated with group OH1-S scores are as follows:
Good — 0.0 to 1.2
Fair
— 1.3 to 3.0
Poor — 3.1 to 6.0
Turesky-Gilmore-Glickman Modification of the
Quigley Hein Plaque Index (1970)
Plaque is assessed on the facial and lingual surfaces of
all of the teeth after using a disclosing agent. A plaque
score per person is obtained by totaling all the plaque
scores and dividing it by the number of surfaces
examined. This system of scoring plaque is relatively easy
to use because of the objective definitions of each
numerical score. The strength of this plaque index is its
application to longitudinal studies and clinical trials of
prevention and therapeutic agents.
Scoring Criteria
0 — No plaque
1 — Separate flecks of plaque at the cervical margin
of the tooth
2 — A thin, continuous band of plaque (upto 1 mm)
at the cervical margin
3 — A band of plaque wider than 1 mm but covering
less than one-third of the crown
4 — Plaque covering atleast one-third but less than
two-thirds of the crown
5 — Plaque covering two-thirds or more of the
crown.
Plaque Index by Sillness and Loe (1964)
It is unique among the indices used for assessment of
plaque because it ignores the coronal extent of plaque
on the tooth surface area and assesses only the thickness
of plaque at the gingival area of the tooth.
Method
The evaluation or scoring is done on the entire dentition
or on selected teeth. The surfaces examined are the four
gingival areas of the tooth i.e. the distofacial, facial,
mesiofacial and lingual surfaces. A mouth mirror, light
source, a dental explorer and air-drying of the teeth and
gingiva are used in the scoring of this index. Only plaque
of the cervical third of the tooth is evaluated with no
attention to plaque that has extended to the middle or
incisal thirds.
Scoring Criteria
0 — No plaque in the gingival area
1 — A film of plaque adhering to the free gingival
margin and adjacent area of the tooth. The plaque
may be recognized only by running a probe across
the tooth surface
2 — Moderate accumulation of soft deposits within the
gingival pocket and on the gingival margin and/
or adjacent tooth surface that can be seen by
naked eye
3 — Abundance of soft matter within the gingival
pocket and/or on the gingival margin and adjacent
tooth surface.
The score for the area is obtained by totaling the
four scores per tooth and dividing it by four. The plaque
index score for the person is obtained by adding the
plaque index scores per tooth and dividing by the
number of teeth examined.
Modified Navy Plaque Index
Advantage
It is of value in assessing health education programs and
the ability of the individuals to perform oral hygiene
practices. A variation of the modified navy plaque index
is the DMPI (Distal mesial plaque index), which places
more emphasis on the gingival, interproximal areas of
a tooth.
50
Essentials of Clinical Periodontology and Periodontics
Procedure
Each tooth surface is divided into gingival, middle and
incisal third. Gingival 3rd is divided into two halves
(horizontally) both the gingival halves are again divided
longitudinally into distal, middle and mesial thirds. The
middle 3rd is divided into distal and mesial halves. The
incisal 3rd is not subdivided, thereby emphasizing more
of gingival two-thirds of the tooth.
Total scores per
tooth surface
Modified navy plaque index = ——————————
Number of
surfaces examined
Patient Hygiene Performance Index (PHP)
by Podshadley and Hadley
Advantages
i. It was the first index developed for the sole purpose
of assessing an individual’s performance in
removing debris after tooth brushing instruction.
ii. It is easy to use and can be performed.
iii. For individual patient education.
Selection of Teeth and Surfaces
Six surfaces of the six teeth (3, 8, 18, 19, 24 and 30)
are selected.
It records presence or absence of debris as 1 or 0
respectively.
Total debris score
PHP =
No of teeth scored
Suggested Nominal Scale
Rating
Excellent — score
Good
— 0.1 to 1.7
Fair
— 1.8 to 3.4
Poor
— 3.5 to 5.0
Plaque Weight
1. Sand-blasted standardized mylar foils attached to the
lingual surface of lower anterior teeth are weighed
on removal after a set time.
2. Removal of plaque directly from the tooth and
subsequent weighing is less precise but simpler than
the foil techniques.
Plaque-free Score by Grant, Stern and Everett
Purpose
To determine the location, number and percentage of
plaque-free surfaces for individual motivation and
instruction.
Selection of Teeth and Surfaces
All erupted teeth are included; four surfaces are recorded
for each tooth (facial, lingual, mesial and distal).
Procedure
Apply disclosing agent and examine each tooth surface
for evidence of plaque and record the surfaces in red
color.
Papillary Bleeding on Probing
Total the number of small circles marked for bleeding.
For a person with 32 teeth there are 30 interdental areas.
The mesial or distal of tooth adjacent to an edentulous
area is probed and counted.
Calculations
Plaque-free score = Total the number of teeth present
and total the number of surfaces with plaque
Number of
plaque-free scores
Plaque-free score =
× 100
Number of
available surfaces
Plaque Control Record by O’Leary
Similar to plaque-free score by Grant, Stern and
Everett.
Interpretations
Although 0 percent is ideal, less than 10 percent has
been suggested as a guideline in periodontal therapy.
After initial therapy, when a patient reached 10 percent

51
Epidemiology of Gingival and Periodontal Diseases
level of plaque control necessary periodontal and
restorative procedures are initiated. In comparison, a
similar evaluation using a plaque-free score record would
mean a goal of over 90 percent or better plaque-free
score before the surgical phase of treatment should be
undertaken.
INDICES USED TO MEASURE CALCULUS
Calculus Component of the Simplified Oral
Hygiene Index by Greene and Vermillion—
Discussed in Plaque Index
Calculus Component of the Periodontal Disease
Index by Ramjford
Advantages
1. It has a high degree of examiner reproducibility.
2. It can be performed quickly and has the best
application in epidemiological surveys and
longitudinal studies.
Procedure
Six teeth are examined (3, 9, 12, 19, 25 and 28).
Four surfaces are recorded (facial, lingual, mesial, distal)
with the help of an explorer and score ranges from
0 to 3.
1. Supragingival plaque extending not more than 1mm
2. Moderate amount of supragingival and subgingival
calculus
3. Abundance of supragingival and subgingival calculus
Total number
of scores
Calculus index for an individual =
Number of teeth
Probe Method of Calculus Assessment by
Volpe and Associates
Advantages and Disadvantages
It has been shown to possess a high degree of
interexaminer and intra–examiner reproducibility.
However, excessive training under a experienced
investigator is required to master it.
Purpose
It is used for longitudinal studies.
Method
The lingual surfaces of six mandibular teeth are measured
in millimeter divisions with the help of graduated
periodontal probe.
Calculus Surface Index by
Ennever and Coworkers
Objective
It is to determine rapidly whether a specific agent has
any effect on reducing or preventing supragingival or
subgingival calculus.
Four mandibular incisors are examined for the
presence or absence by visual and tactile exami-
nation.
Scoring Criteria
1. Calculus not exceeding 0.5 mm in width or thickness
2. Calculus not exceeding 1 mm
3. Calculus exceeding more than I mm in width and
thickness
Marginal Line Calculus Index
by Muhlemann and Villa
Purpose
To assess the supragingival calculus along the margins
of the gingiva. It only scores the supragingival calculus
formed in the cervical area along the marginal gingiva
on the lingual side of the four mandibular incisors.
INDICES USED TO ASSESS
TREATMENT NEEDS
Gingival Periodontal Index
It assesses three components of periodontal disease:
gingival status, periodontal status, and collectively materia
alba, calculus and overhanging restorations. The maxillary
and mandibular arches are each divided into three
52
Essentials of Clinical Periodontology and Periodontics
segments: the six anterior teeth, the left posterior teeth,
and the right posterior teeth. The primary objective in
using this index is to determine the tooth or surrounding
tissues with the severest condition within each of the six
segments. Each segment is assessed for each of the three
components of periodontal disease described previously.
The specific criteria for the gingival status components
of the GPI are as follows:
0 — Tissue tightly adapted to the teeth, firm consistency
with physiologic architecture.
1 — Slight to moderate inflammation, as indicated by
changes in color and consistency, involving one
or more teeth in the same segment but not
completely surrounding any one tooth.
2 — The above changes occur either singly or
combined completely encircling one or more teeth
in a segment.
3 — Marked inflammation, as indicated by loss of
surface continuity (ulceration), spontaneous
hemorrhage, loss of faciolingual continuity or any
interdental papilla, marked deviation from normal
contour, recession and clefts.
The area with the highest score determines the gingival
score for the entire segment, and the gingival status for
the oral cavity is obtained by dividing the sum of the
gingival scores by the number of segments.
Community Periodontal Index of Treatment
Needs by Ainamo and Associates
Procedure
The periodontal treatment needs are recorded for
sextants. Third molars are not included except when they
are functioning in place of second molars. The treatment
need in a sextant is recorded only when 2 or more teeth
are present and are not indicated for extraction. If only
one functioning tooth is remaining in the maxilla, the
jaw would be recorded as one sextant. Missing sextants
are indicated with a diagonal line through the appropriate
box. The index teeth to be examined are:
WHO Numbering
American Equivalent
17,16
11
26,27
2,3
8
14,15
47,46
31
36,37
31,30
25
18,19
The worst findings from these teeth surfaces are
recorded. WHO probe is used.
Criteria
Periodontal status:
a. Healthy periodontium
b. Bleeding observed directly or by using mouth mirror.
c. Calculus felt during probing, but entire blunt area
of the probe is visible.
d. Pocket 4 or 5 mm
e. Pocket more than 6 mm
Treatment needs:
0 No treatment neede
1 Oral hygiene needs improvement
2 Oral hygiene improvement and professional scaling
3 Oral hygiene improvement, professional scaling and
complex treatment.
Advantages
The value of CPITN is that it permits rapid examination
of a population to determine periodontal treatment
needs.
Disadvantages
1. A great deal of useful information is lost when only
the worst score per sextant is recorded.
2. CPITN underestimates the pockets greater than 6 mm
in older age groups and overestimates the need for
scaling in younger age groups.
Periodontal Treatment Need System by Bellini HT
It attempts to place individuals into one of the four classes
based on treatment procedures relative to time
requirements. It considers the presence or absence of
53
Epidemiology of Gingival and Periodontal Diseases
Table 5.1: Criteria for periodontal treatment
need system
PTNS
Unit
Plaque Calculus Inflam- Pocket
classification
and/or mation
depth
overhangs
Class-0
Mouth
No
No
No
No
Class-A
Mouth
Yes
No
Yes
<5 mm
Class-B
Quadrant
Yes
Yes
Yes
<5 mm
Class-C
Quadrant
Yes
Yes
Yes
>5 mm
gingivitis and plaque, and the presence of pockets 5 mm
or deeper in each quadrant of mouth.
Criteria for periodontal treatment need system is
described in Table 5.1.
BIBLIOGRAPHY
1. Esther M Wilkins. Clinical Practice of the Dental Hygienist, 8th
Edition. Wolters Kluwer Company.
2. Newman, Takei, Fermin A. Carranza: Clinical Periodontology,
Ninth Edition (2002), W.B. Saunders.
3. Soben Peter: Essentials of Preventive and Community
dentistry, First Edition, Arya (MEDI), Publishing House.
‘


DEFINITION
Dental Plaque
Dental Plaque is an adherent intercellular matrix
consisting primarily of proliferating microorganisms, along
with a scattering of epithelial cells, leukocytes and
macrophages.
Plaque
Plaque can also be defined as the soft deposits that form
the biofilm adhering to the tooth surface or other hard
surfaces in the oral cavity, including removable and fixed
restorations. Dental Plaque is a host-associated biofilm.
Biofilms
Biofilms are defined as “Matrix—enclosed bacterial
populations adherent to each other and or/to surface
or interfaces (by Costerton). According to the recent data
(Widerer and Charaklis 1989) Biofilm is defined as the
relatively undefinable microbial community associated
with a tooth surface or any other hard, non-shedding
material.
DENTAL PLAQUE AS A BIOFILM
Structurally dental plaque is now considered to be a
biofilm of complex and dynamic microbial community.
It contains areas of high and low bacterial biomass
interlaced with aqueous channels of different size, which
are the nutrient channels for bacterial colonization. The
intercellular matrix forms a hydrated gel in which bacteria
can survive and proliferate. Hence, biofilm adheres firmly
to the tooth surface and is resistant to mechanical
removal, as well as antibiotics.
TYPES OF DENTAL PLAQUE
Based on its relationship to the gingival margin, plaque
❒
❒
❒
❒
❒ DEFINITION
❒
❒
❒
❒
❒ DENTAL PLAQUE AS A BIOFILM
❒
❒
❒
❒
❒ TYPES OF DENTAL PLAQUE
❒
❒
❒
❒
❒ COMPOSITION OF DENTAL PLAQUE
❒
❒
❒
❒
❒ FORMATION/DEVELOPMENT OF DENTAL
PLAQUE
❒
❒
❒
❒
❒ STRUCTURAL AND MICROSCOPIC
PROPERTIES OF PLAQUE
❒
❒
❒
❒
❒ CLINICAL SIGNIFICANCE OF A PLAQUE
❒
❒
❒
❒
❒ MICROBIAL SPECIFICITY OF
PERIODONTAL DISEASES
❒
❒
❒
❒
❒ WHAT MAKES PLAQUE PATHOGENIC
❒
❒
❒
❒
❒ MICROORGANISMS ASSOCIATED WITH
PERIODONTAL DISEASES
❒
❒
❒
❒
❒ BACTERIA ASSOCIATED WITH
PERIODONTAL HEALTH AND DISEASE
6
Periodontal Microbiology
(Dental Plaque)
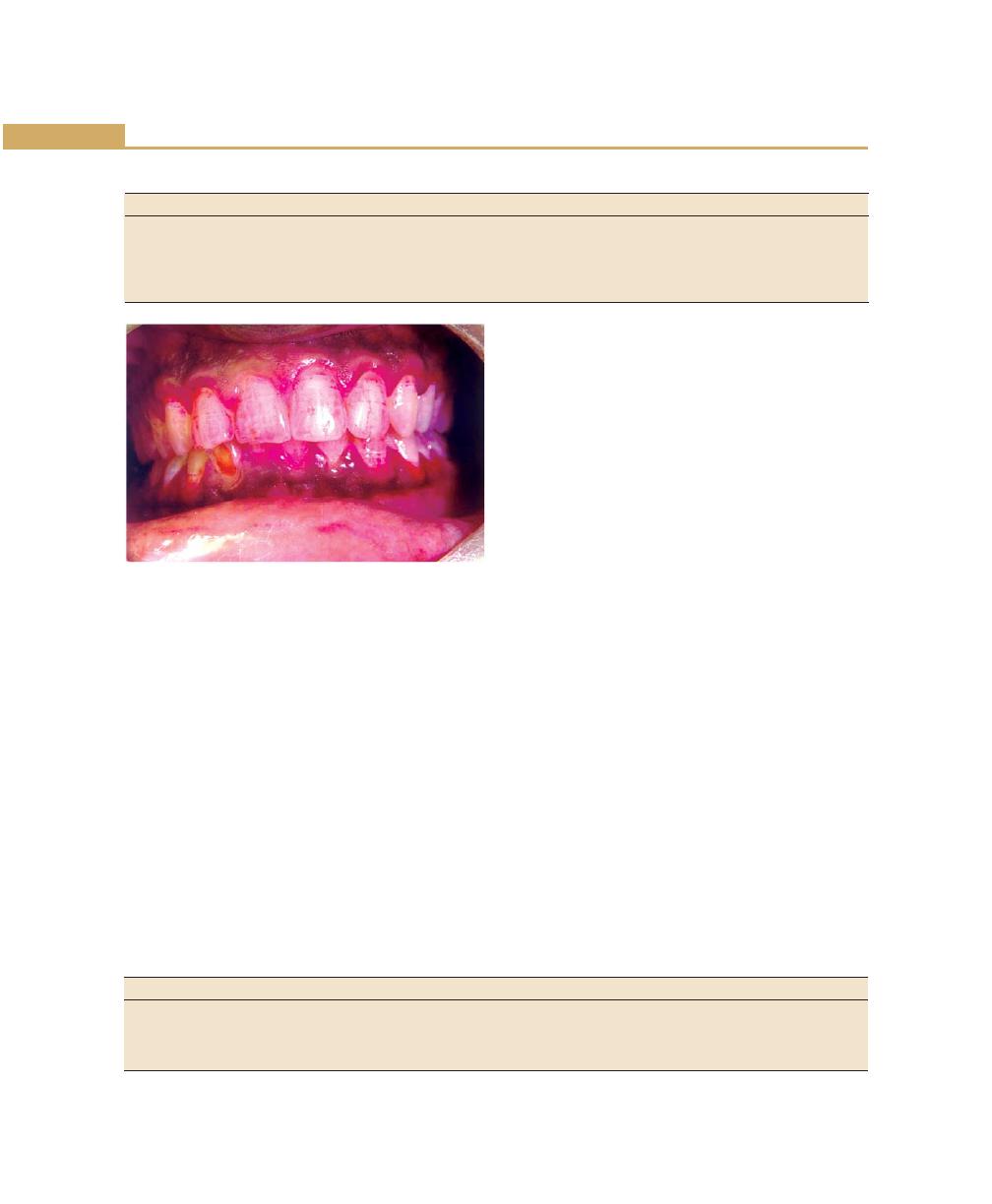
58
Essentials of Clinical Periodontology and Periodontics
Table 6.1: Differences between supragingival and subgingival plaque
Supragingival plaque
Subgingival plaque
1. Matrix
50% Matrix
Little or no matrix
2. Flora
Mostly Gram-positive
Mostly Gram-negative
3. Motile bacteria
Few
Common
4. Anaerobic/ Aerobic
Aerobic unless thick
Highly anaerobic areas present
5. Metabolism
Predominantly carbohydrates
Predominantly proteins
Fig. 6.1:
Supragingival plaque
is differentiated into two categories, supragingival and
subgingival plaque (Table 6.1).
Supragingival plaque is further differentiated into:
Coronal plaque, which is in contact with only the
tooth surface, and Marginal plaque, which is associated
with the tooth surface at the gingival margin.
Subgingival plaque can be further differentiated into:
• Attached plaque
• Unattached subgingival plaque
• Attached plaque can be tooth, epithelium and/or
connective tissue associated.
Supragingival Plaque (Fig. 6.1)
It can be detected clinically only after it has reached a
certain thickness. Small amounts of plaque can be
visualized by using disclosing agents. The color varies
from grey to yellowish-grey to yellow. The rate of
formation and location of plaque vary among individuals
and is influenced by diet, age, salivary factors, oral
hygiene, tooth alignment, systemic diseases and host
factors.
Subgingival Plaque (Figs 6.2 and 6.3)
It is usually thin, contained within the gingival sulci or
periodontal pocket and thus cannot be detected by direct
observation. Its presence can be identified only by
running the end of a probe around gingival margin (Table
6.2).
Tooth-associated Subgingival Plaque
The structure is similar to the supragingival plaque. The
flora is dominated by Gram-positive cocci, rods,
filamentous bacteria and some/few Gram-negative cocci
and rods. This flora is associated with calculus formation,
root caries and root resorption.
Epithelium-associated Subgingival Plaque
This type of plaque is loosely adherent because it lacks
the interbacterial matrix and is in direct association with
the gingival epithelium, extending from the gingival margin
to the junctional epithelium. This plaque predominantly
contains Gram-negative rods and cocci, as well as a large
number of flagellated bacteria and Spirochetes.
Table 6.2: Characteristics of subgingival plaque
Sl. No.
Tooth-associated subgingival plaque
Epithelium-associated plaque
1.
Gram-positive bacteria predominates
Gram- positive and Gram-negative bacteria
2.
Does not extend to junctional epithelium
Extends to junctional epithelium
3.
May penetrate cementum
May penetrate epithelium and connective tissue
4.
Associated with calculus formation and root caries
Associated with gingivitis and periodontitis
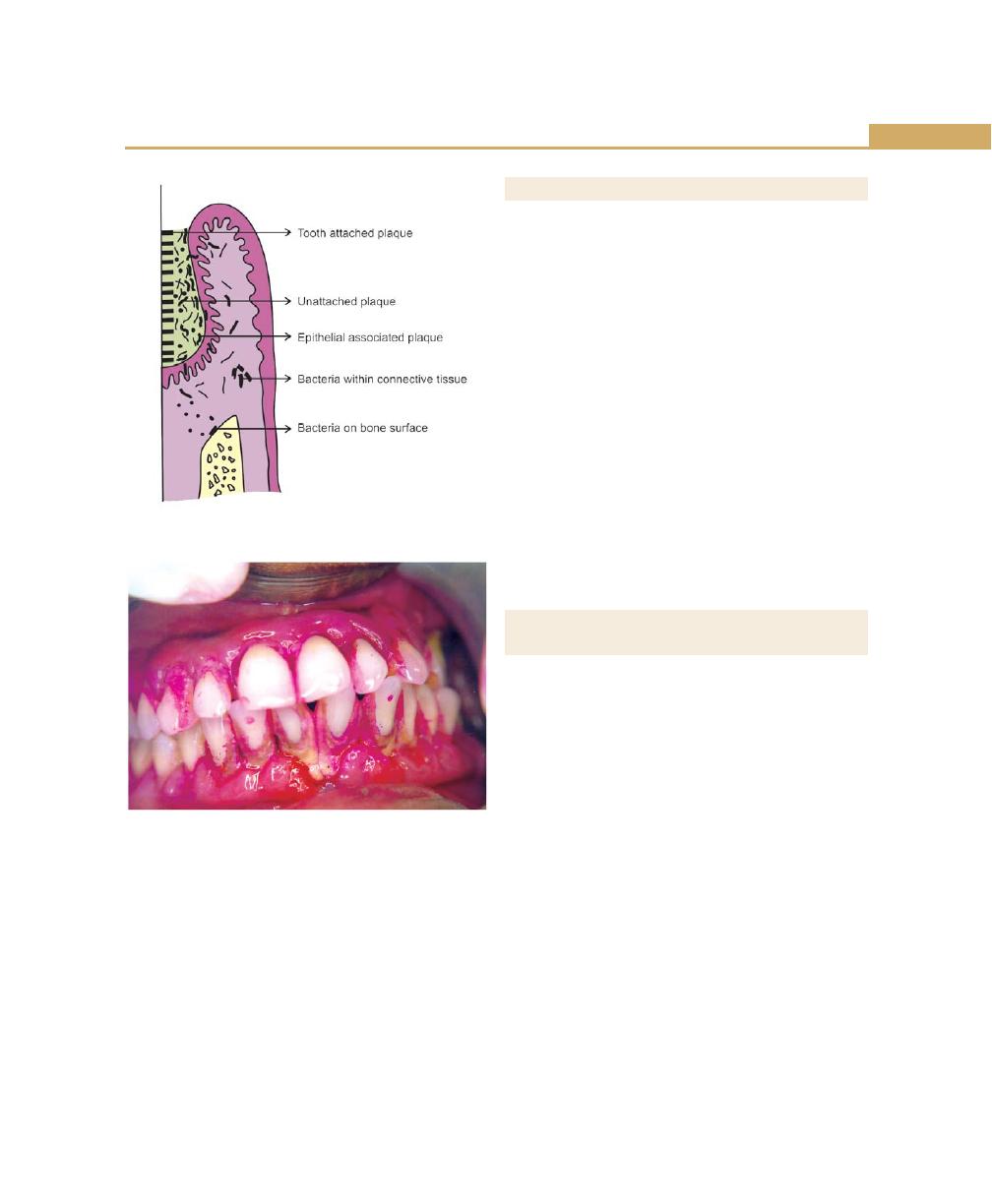
59
Periodontal Microbiology
Fig. 6.2:
Subgingival plaque bacteria associated with
tooth surface and periodontal tissues
Fig. 6.3:
Subgingival plaque and calculus
Connective Tissue-associated Plaque
It is usually demonstrated in ANUG and localized juvenile
periodontitis patients. The clinical significance is unclear.
The unattached plaque can be seen anywhere. Thus,
the tooth-associated subgingival plaque is most important
in calculus formation, root caries and slowly progressive
periodontal destruction, whereas unattached bacterial
component is associated with rapid periodontal
destruction.
COMPOSITION OF DENTAL PLAQUE
Bacteria + Intercellular matrix = Dental plaque
Bacteria make up approximately 70 to 80 percent
of total material.One mg of dental plaque is estimated
to contain 250 million bacteria. Other than bacteria,
mycoplasma, fungi, protozoa and viruses may be present.
The material among the bacteria in dental plaque is termed
as inter microbial/cellular matrix. It contains organic and
inorganic portions. The organic matrix is composed of
protein—polysaccharide complex produced by micro-
organisms.Carbohydrates in the form of levans (fructans)
provides mainly energy while glucans (dextran) provide
not only energy, but also act as the organic skeleton of
plaque. Other carbohydrates are galactose and rhamnose.
Glycoproteins provide the protein component and small
amounts of lipids are also present. Inorganic components
include, primarily calcium, phosphorus with small amounts
of magnesium, potassium and sodium.
FORMATION/DEVELOPMENT OF
DENTAL PLAQUE
Pellicle is the initial organic structure that forms on the
surfaces of the teeth and artificial prosthesis. The first
stage in pellicle formation involves adsorption of salivary
proteins to apatite surfaces. This results from the
electrostatic ionic interaction between hydroxyapatite
surface which has negatively-charged phosphate groups
that interacts with opposite charged groups in the salivary
macromolecules. The mean pellicle thickness varies from
100 nm at 2 hours to 500 to 1,000 nm.
The transition from pellicle to dental plaque is
extremely rapid. The first components include mainly
cocci with small number of epithelial cells and PMNL’s,
they form a monolayer within a few hours, and the
attached bacteria proliferate and form small colonies of
cocci. With time other types of microorganisms proliferate
and form different microcolonies.
Hence, in dental plaque development, two adhesion
processes are required. First, bacteria must adhere to
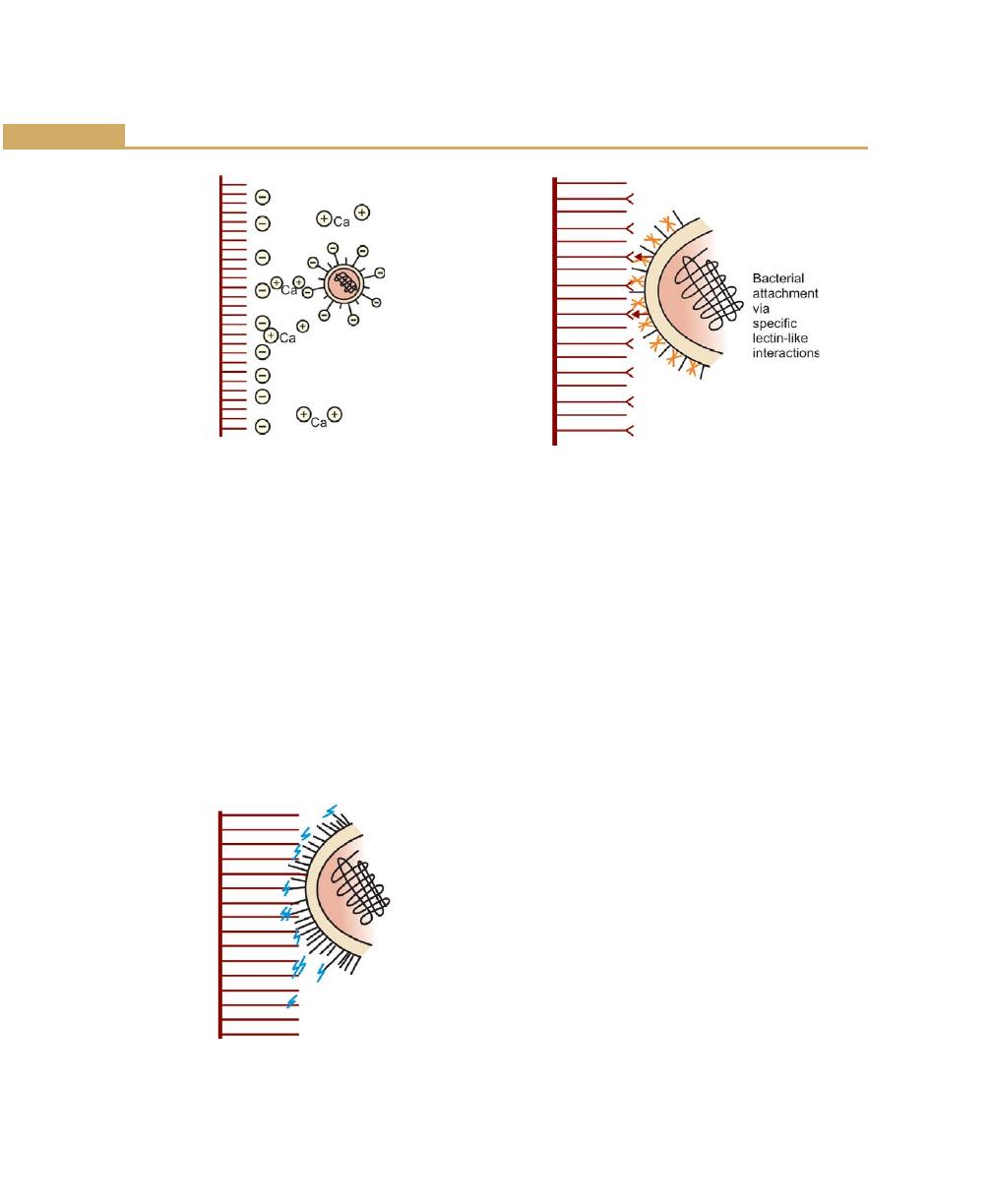
60
Essentials of Clinical Periodontology and Periodontics
the pellicle surface and become sufficiently attached to
withstand oral cleansing forces. Second, they must grow
and adhere to each other to allow plaque accumulation.
Bacterial Adherence
During initial adherence, interactions occur mainly
between specific bacteria and the pellicle. They are:
Bacterial Attachment via Electrostatic Interactions
Oral bacteria bear an overall net negative charge,
negatively-charged components of the bacterial surface
and negatively-charged components of pellicle become
linked by cations such as calcium (Fig. 6.4).
Bacterial Attachment via Hydrophobic Interactions
These interactions are based on the close structural fit
between molecules on the pellicle and bacterial surfaces.
The nature of the hydrophobicity of the cell is not clearly
known. The contributing factor might be (LTA)
lipoteichoic acid, which may provide a long hydrophobic
area (Fig. 6.5).
Bacterial Attachment via Specific Lectin-like
Substances (Fig. 6.6)
Lectins in the bacterial surfaces recognize specific
carbohydrate structure in the pellicle and become linked.
Adhesion and attachment (Fig. 6.7) occurs between:
• Bacteria and clean tooth surface
• Bacteria and pellicle
• Bacteria and same species
• Bacteria and different species
• Bacteria and matrix
Next step in plaque formation is:
Growth and Accumulation of Bacteria
Once the bacteria is adhered to the pellicle, subsequent
growth leads to bacterial accumulation and increased
plaque mass. Dental plaque growth (Fig. 6.8) depends on:
a. Growth via adhesion of new bacteria
b. Growth via multiplication of attached bacteria
Fig. 6.4:
Bacterial attachment via electrostatic
interactions
Fig. 6.5:
Bacterial attachment via
hydrophobic interactions
Fig. 6.6:
Bacterial attachment via specific lectin-like
interactions
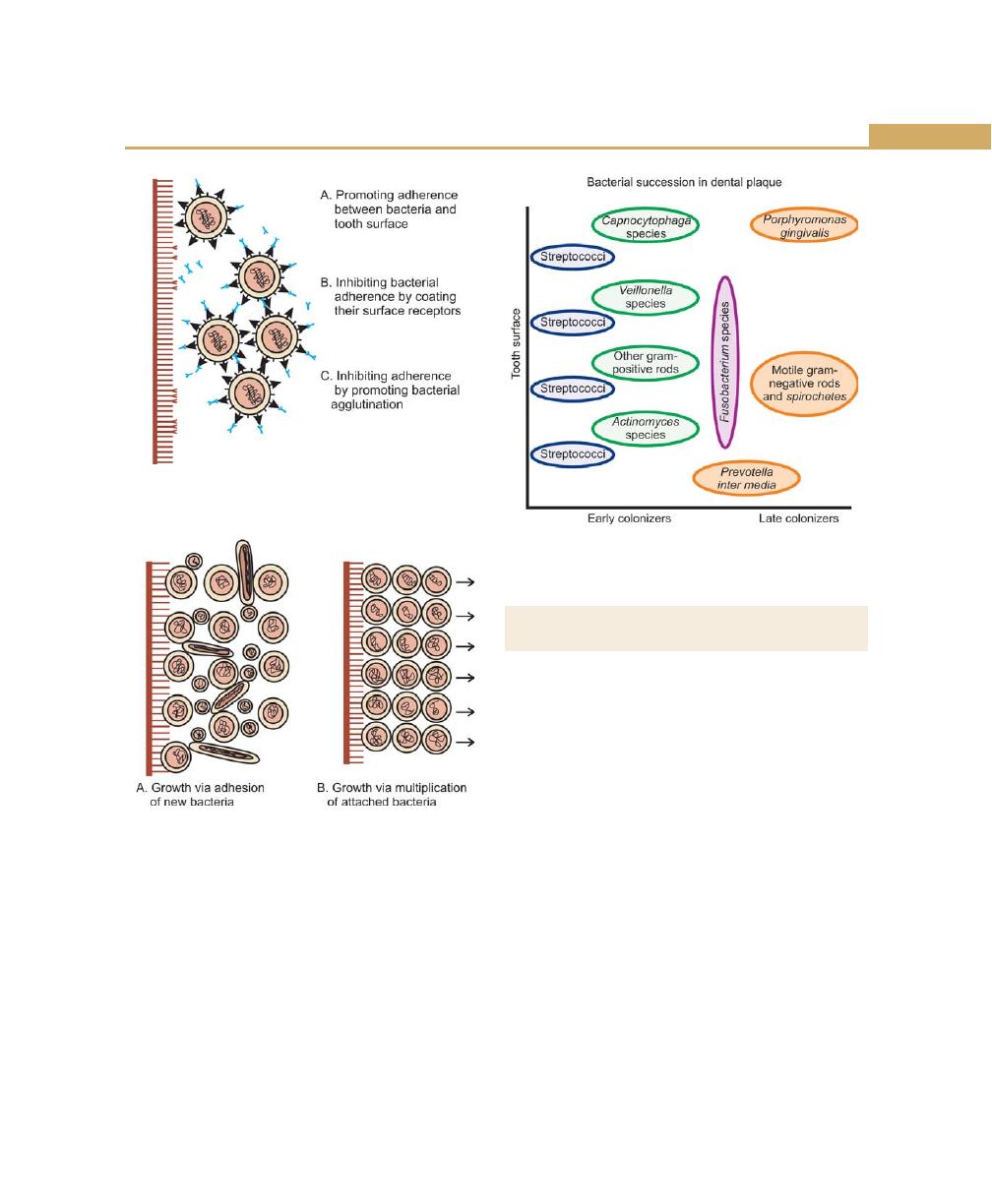
61
Periodontal Microbiology
Fig. 6.7:
Role of saliva in the development and
maturation of dental plaque
Fig. 6.8:
Growth of dental plaque
Fig. 6.9:
Bacterial succession in dental plaque
The initial bacteria that colonize the pellicle surface
are mostly Gram-positive facultative microorganisms such
as Actinomyces viscosus and Streptococcus sanguis, as
the plaque matures, secondary colonization of Prevotella
intermedia, Capnocytophaga, Porphyromonas gingivalis
takes place. This ability of bacteria to adhere to different
species and genera of micro-organisms is known as co-
aggregation (Fig. 6.9).
STRUCTURAL AND MICROSCOPIC
PROPERTIES OF PLAQUE
Supragingival Plaque
It is usually adherent to the tooth surface. It contains
gram-positive cocci and gram-negative rods and
filaments. The morphologic arrangement of the flora in
supragingival plaque described as “corncob” formations,
characterized by central core consisting of rod-shaped
bacterial cells, e.g. Fusobacterium nucleatum and coccal
cells, e.g. streptococci which attaches along the surface
of the rod-shaped cell.
The subgingival plaque differs from supragingival
plaque, in that it contains many large filaments with
flagella and is rich in Spirochetes. Tooth-associated plaque
is similar to supragingival plaque; whereas tissue-
associated plaque is covered with flagellated bacteria
without a well-defined extracellular matrix and numerous
bristle—brush formations. This arrangement is also called
as “test tube-brush” formation characterized by large
62
Essentials of Clinical Periodontology and Periodontics
filaments that forms the long axis; and short filaments
or Gram-negative rods embedded in a amorphous
matrix (Fig. 6.10).
CLINICAL SIGNIFICANCE OF PLAQUE
The microbial aggregations on the tooth surface if
prevented from maturing may become compatible with
gingival health. Supragingival plaque if allowed to grow
and mature, may induce gingivitis and can lead to the
formation of a microenvironment that permits the deve-
lopment of subgingival plaque. Therefore, supragingival
plaque strongly influences the growth, accumulation and
pathologic potential of sub gingival plaque, especially
in the early stages of gingivitis and periodontitis.
Subgingival Plaque
In association with the presence of supragingival plaque,
there are inflammatory changes that modify the
anatomic relationships of the gingival margin and tooth
surface. This result in enlarged gingiva, which increases
the space for bacterial colonization and also protects
bacteria from normal cleansing mechanisms. They derive
nutrients from gingival crevicular fluid. Many of these
microorganisms lack the adherence ability and utilizes
supragingival plaque bacteria as a means of colonization
of the subgingival area.
Electron microscopic studies have demonstrated the
existence of an organic material called cuticle between
the root surface and subgingival plaque. It is covered
by a dense layer of microorganisms and is believed to
be a remnant or secretory product of the junctional
epithelial cells.
MICROBIAL SPECIFICITY OF
PERIODONTAL DISEASES
Walter Loesche proposed the non-specific and specific
plaque hypothesis in 1976. The non-specific plaque
hypothesis states that it is the total bulk of plaque, which
determines the pathogenicity rather than the individual
species within it. In other words all plaque is equally
pathogenic. According to this, when only small amounts
of plaque are present, the products released by this gets
neutralized by the host. Similarly, large amounts of plaque
would produce large amounts of noxious products, which
would overwhelm the host’s defenses. However, several
authors have contradicted this concept. First, many
patients have considerable amounts of plaque and
calculus as well as gingivitis, but only a minority suffer
from destructive periodontal disease even then in only
few sites. This paradox might be explained by specific
plaque hypothesis, which states that destructive
periodontal disease is a result of specific microbial
pathogens in plaque. Thus although the amount of
plaque present correlates well with disease severity it
correlates poorly in individual patients.
But it is the non-specific plaque hypothesis, which
forms the basis for virtually all the current modalities for
treatment, and prevention, which relies on the principle
of reducing plaque scores to a minimum. Thus, although
the non-specific plaque hypothesis has been discarded
in favor of the specific plaque hypothesis, much clinical
treatment is still based on the non-specific plaque
hypothesis.
Specific Plaque Hypothesis
It states that, not all plaque is pathogenic and its
pathogenicity depends on the presence of certain specific
Fig. 6.10:
Mature plaque diagram

63
Periodontal Microbiology
microbial pathogens in plaque. This is based on the fact
that, the specific microorganisms responsible for
periodontal diseases release certain damaging factors that
mediates the destruction of the host tissue. This concept
was accepted easily due to the recognition of
Actinobacillus actinomycetemcomitans as a possible
pathogen responsible for localized juvenile periodontitis.
WHAT MAKES PLAQUE PATHOGENIC?
The following are the possible pathogenic mechanisms
by which the plaque microorganisms can cause
periodontal disease.
a. Physical nature of plaque
b. Invasion of tissues by bacteria
c. Release of toxic and inflammatory substances
d. Role of bacterial specificity
MICROORGANISMS ASSOCIATED WITH
PERIODONTAL DISEASES
It is well accepted that a plaque bacteria is the primary
etiologic agent in periodontal disease. Bacteria are seen
in the oral cavity from birth to death. It is estimated that
about 400 different species are capable of colonizing in
the mouth. Counts in subgingival sites range from about
10
3
in healthy sulci to greater than 10
8
in deep periodontal
pockets. It has not been possible to identify and study
all the organisms present in the bacterial plaque, of nearly
400 species; only 30 of them are considered to be
periodontopathic. Koch’s postulates, generally used to
identify the periodontopathogenecity of a micro-
organism, are not applicable in periodontal disease, as
more than one organism is involved in periodontal
diseases. Hence, Socransky (1977) had proposed the
following criteria for identifying the possible causative
organisms in periodontal diseases:
1. The number of etiologic organisms in the diseased
site must be increased and conversely the number
of organisms must be reduced or absent in healthy
sites.
2. If the etiologic organism is eliminated or suppressed
the disease should stop.
3. Presence of specific antibodies to those micro-
organisms.
4. Presence of virulence factors associated with certain
microorganisms, e.g. toxins, enzymes, etc.).
5. In vitro or animal experiments should be able to
demonstrate the human disease process.
There are many speculations regarding the pathogens
responsible for periodontitis whether they could be
exogenous or components of indigenous flora. To explain
this controversy, two theories have been proposed.
According to the first theory, periodontopathic
organisms are a part of indigenous flora and they tend
to overgrow during the disease progression. The second
proposal is that, they are not components of indegenous
oral flora, but are of exogenous pathogens derived from
outside sources. This concept hints that, the quantity of
plaque is not necessary for disease onset. Instead, the
site should be contaminated with specific periodonto-
pathogens.
Recent reports have demonstrated a possibility of viral
etiology in periodontitis and implicated viruses are Epstein
Barr virus, human cytomegalovirus and mixed herpes
viral infections. Viral infection can contribute to
periodontitis by altering the functions of neutrophils,
macrophages and lymphocytes which inturn promotes
the overgrowth of periodontopathic organisms in the
subgingival flora. The other possibility is that, the viral
infection can destroy the oral epithelial cells thus
disrupting the barrier function of the periodontium.
Bacteria Associated with Periodontal
Health and Disease
See Table 6.3.
Health
• Actinomyces (viscosus and naeslundii)
• Streptococcus (S. mitis and S. sangius)
• Veillonella parvula, small amounts of Gram-negative
species are also found.

64
Essentials of Clinical Periodontology and Periodontics
Chronic Gingivitis
Gram-positive (56%), Gram-negative (44%) organisms
are found. Predominant Gram-positive species include,
S. sangius, S. mitis, S. oralis, A. viscosus, A. naeslundii,
Peptostreptococcus micros.
Gram-negative organisms are:
• Fusobacterium nulceatum
• Prevotella intermedia
• Veillonella parvula as well as Haemophilus,
Capnocytophaga and Campylobacter species
Pregnancy-associated gingivitis
• Prevotella intermedia
Acute necrotizing ulcerative gingivitis
• Spirochetes
• Prevotella intermedia
Adult periodontitis
• Porphyromonas gingivalis
• Bacteroides forsythus
• Prevotella intermedia
• Campylobacter rectus
• Eikenella corrodens
• Fusobacterium nucleatum
• Actinobacillus actinomycetemcomitans
• Peptostreptococcus micros
• Treponema, and
• Eubacterium species.
Viruses such as:
• EBV-1 (Ebstein-Barr virus)
• HCMV (Human cytomegalovirus)
Table 6.3: Reclassification of periodontal bacteria
Previous status
Current status
Wolinella recta
Campylobacter rectus (C. rectus)
Bacteroides gingivalis
Porphyromonas gingivalis (P. gingivalis)
Bacteroides intermedius
Prevotella intermedia (P. intermedia)
Bacteroides melaninogenicus
Prevotella melaninogenica (P. melaninogenica)
Localized juvenile periodontitis
• Actinobacillus actinomycetemcomitans
• Porphyromonas gingivalis
• Eikenella corrodens
• Campylobacter rectus
• Fusobacterium nucleatum
• Bacteroides capillus
• Eubacterium brachy
• Capnocytophaga
• Herpes virus.
Generalized juvenile periodontitis
• Actinobacillus actinomycetemcomitans
• Porphyromonas gingivalis
• Prevotella intermedia
• Capnocytophaga
• Eikenella corrodens
• Neisseria
Refractory periodontitis
• Actinobacillus actinomycetemcomitans
• Bacteroides forsythus
• Porphyromonas gingivalis
• Prevotella intermedia
• Wolinella recta
Abscesses of the periodontium
•
Fusobacterium nucleatum
•
Prevotella intermedia
•
Peptostreptococcus micros
•
Bacteroides forsythus
•
Porphyromonas gingivalis
65
Periodontal Microbiology
KEY POINTS TO NOTE
1. Dental plaque is defined as an adherent intercellular matrix,
composed primarily of proliferating microorganisms, along
with a scattering of epithelial cells, leukocytes and
macrophages.
2. Structurally dental plaque is now considered to be a biofilm
of complex and dynamic microbial community.
3. Based on its relationship to the gingival margin, plaque is
differentiated into two categories, supragingival and
subgingival plaque.
4. Subgingival plaque can be, tooth-associated, epithelium-
associated, connective tissue-associated and unattached
plaque.
5. Dental plaque is mainly composed of bacteria and
intercellular matrix.
6. In the formation of dental plaque first step is pellicle
formation followed by bacterial adherence and growth and
accumulation of bacteria.
REVIEW QUESTIONS
1. Define plaque and describe the steps in formation
of plaque.
2. What are the types and composition of dental plaque?
3. What is specific and non-specific plaque hypothesis?
4. Describe the role of plaque in periodontal disease.
5. What is the clinical significance of plaque?
6. What are differences between supra and subgingival
plaque?
BIBLIOGRAPHY
1. Jan Lindhe. Clinical Periodontology and Implant Dentistry.
Fourth Edition 2003, Blackwell Munksgaard Publication.
2. Jorgen slots and Martin A Taubman. Contemporary oral
microbiology and immunology.
3. Newman, Nissengaard. Oral microbiology and immunology,
II edition, WB Saunders and company.
4. Max A, Listgarten. The structure of dental plaque. Periodontol
2000;5:1994.
5. Sigmund S, Socransky, Anne D. Haffajee. Dental biofilms:
difficult therapeutic targets. Periodentol 2000;28:2002.
6. Sigmund, Socransky, Anne D. Haffajee. Evidence of bacterial
etiology, a historical perspective. Periodontol 2000;5:1994.
7. WEC Moore, Lillian VH Moore. The bacteria of periodontal
diseases. Periodontol 2000;5:1994.
66
Essentials of Clinical Periodontology and Periodontics
CALCULUS
Definition
Dental calculus is an adherent, calcified or calcifying mass
that forms on the surfaces of teeth and dental appliances.
It is covered on its external surface by vital, tightly
adherent, nonmineralized plaque.
Types
Depending upon the position of calculus in relation to
the marginal gingiva it is classified as:
1. Supragingival calculus.
2. Subgingival calculus.
Supragingival Calculus
It is the tightly adherent calcified deposit that forms on
the clinical crowns of the teeth above the free gingival
margin. Hence it is clinically-visible. It is also called as
salivary calculus because it forms from the saliva.
Subgingival Calculus
As the name implies, it is that calcified deposits that is
formed on the root surfaces below the free marginal
gingiva. It is believed to be formed from the gingival
Fig. 7.1:
Radiograph illustrating subgingival deposits in
lower anterior and posterior teeth
❒
❒
❒
❒
❒ CALCULUS
• Definition
• Types
• Structure
• Composition
• Differences between Supra and
Subgingival Calculus
❒
❒
❒
❒
❒ OTHER CONTRIBUTING ETIOLOGICAL
FACTORS INCLUDING FOOD IMPACTION
7
Calculus and other
Etiological Factors
67
Calculus and other Etiological Factors
exudate and hence called serumal calculus (Figs 7.1 and
7.2).
Structure
The deposits of supragingival calculus are usually whitish-
yellow in color and can get stained by tobacco or food
pigments, consistency is hard and clay-like. Since they
derive the mineral salts from salivary secretions, they
are most abundant on the lingual surfaces of lower
anterior teeth, opposite Wharton’s duct and Bartholin’s
duct and buccal aspects of maxillary molars opposite
the Stenson’s duct (Fig. 7.3).
Subgingival calculus is usually dark-brown or
greenish-black in color and the deposits are firmly
attached to the tooth surface. Since they are hard and
firm, it cannot be removed easily. Unlike supragingival
calculus, subgingival calculus can be found on any root
surface with a periodontal pocket. Morphologically, it
can appear in different forms, most commonly ring-like
or ledge-like formations and crusty, spiny or nodular
deposits. Less frequently it can be seen as finger-like and
fern-like formations (Fig. 7.4).
Composition
It consists of inorganic and organic components (Tables
7.1 and 7.2).
Trace amounts of zinc, strontium, bromine, copper,
manganese, gold and aluminium are also seen. Atleast,
Fig. 7.2:
Subgingival deposits on the root surface of
extracted lower anterior tooth
Fig. 7.3:
Supragingival calculus on the lingual surfaces of
lower anteriors
Fig. 7.4:
Radiograph illustrating subgingival calculus in
the interproximal areas
Table 7.1: Inorganic components
Component
Dry weight (in percent)
Inorganic
70-90
Calcium
27-29
Phosphorus
16-18
Carbonate
2-3
Sodium
1.5-2.5
Magnesium
0.6-0.8
Fluoride
0.003-0.04
Crystal forms
Hydroxyapatite
58
Magnesium whitlockite
21
Octacalcium phosphate
12
Brushite
9
68
Essentials of Clinical Periodontology and Periodontics
two-thirds of inorganic component is crystalline in
structure. The main crystal forms are: Hydroxyapatite,
Magnesium whitlockite, Octacalcium phosphate and
Brushite.
Differences between Supragingival and
Subgingival Calculus
See Table 7.3.
Attachment to the Tooth Surface
Four types of attachment of calculus to tooth surface
have been reported.
1. Attachment by means of an organic pellicle.
2. Mechanical interlocking into surface irregularities such
as resorption lacunae and caries.
3. Penetration of calculus bacteria into cementum.
4. Close adaptation of calculus under surface depre-
ssions to the gently sloping mounds of the unaltered
cementum surface.
Calculus when embedded deeply in cementum may
appear similar in morphology and thus has been termed
as calculocementum.
Formation of Calculus
Calculus is nothing but, dental plaque that has undergone
mineralization. Calculus is formed by the precipitation
of mineral salts, which can start between 1st and 14th
day of plaque formation. In two days plaque can be
50 percent mineralized and 60 to 90 percent gets
mineralized in 12 days. Calcification starts in separate
foci on the inner surface of the plaque. These foci of
mineralization gradually increase in size and coalesce to
form a solid mass of calculus.
Calculus formation continues until it reaches
maximum levels in about 10 weeks and 6 months, after
which there is a decline in its formation, due to
mechanical wear from food and from the lips, cheeks
and tongue. This decline is referred to as reversal
phenomenon.
Theories of Calculus Formation
It can be explained mainly under two categories:
1. Precipitation of minerals can occur from a local rise
in the degree of saturation of calcium and phosphate
ions, this is explained in,
a. Booster mechanism: According to this theory,
precipitation of calcium phosphate salts results
from a local rise in the pH of the saliva. Factors
such as loss of carbon dioxide and production
of ammonia could lead to rise in pH.
Other ways by which the precipitation of
calcium phosphate salts can occur are:
b. Colloidal proteins in saliva bind to calcium and
phosphate ions thus producing a super-saturated
solution. When saliva stagnates in the oral cavity,
colloids settle and result in the precipitation of
calcium and phosphorous salts.
c. Phosphatase liberated from dental plaque,
desquamated epithelial cells, or bacteria
Table 7.2: Organic components
Component
Dry weight (in percent)
Mixture of protein, polysaccharide
1.9-9.1
complexes, desquamated epithelial
cells, leukocytes and various
micro-organisms. Carbohydrate
(consists of glucose, galactose,
rhamnose, mannose)
Proteins
5.9-8.2
Lipids
0.2
Table 7.3: Differences between supra and
subgingival calculus
Supragingival
Subgingival
Location—above the gingival
Deposits present below the
margin
margins of the gingiva
Color—white, yellow in color
Brown or greenish-black
Source—derived from
Formed from gingival
salivary secretions
exudate
Composition—more brushite
Conversely less brushite and
and octacalcium phosphate
octacalcium phosphate and
less magnesium whitlockite
more magnesium whitlockite
Salivary proteins are present
They are absent
Sodium content is lesser
Sodium content increases
with the depth of the pocket

69
Calculus and other Etiological Factors
precipitate calcium phosphate by hydrolyzing
organic phosphates in saliva, thus increasing the
concentration of free phosphate ions.
2. Another concept that has been most widely held is
“Epitactic Concept”. (heterogenous nucleation).
According to this, seeding agents induce small foci
of calcification. These foci enlarge and coalesce to
form calculus. Hence more appropriately called as
heterogenous nucleation. The seeding agents in
calculus is not clearly known, but suspected agents
could be, intercellular matrix of plaque, carbohydrate
protein complexes and plaque bacteria.
3. Inhibition theory: This theory considers the possibility
of calcification occurring only at specific sites because,
there exists an inhibiting mechanism at non-calcifying
sites. Where ever calcification occurs, the inhibitor
is either altered or removed. One such inhibiting
agents could be pyrophosphate which prevents the
initial nucleus from growing, by possibly ‘poisoning’
the growth centers of the crystal.
Pathogenic Potential of Calculus in
Periodontal Diseases
Before 1960s the belief was that calculus was the principle
etiologic factor in periodontal diseases. However, the
current view is that the initial damage to the gingival
margin in the periodontal disease is due to the pathogenic
effects of microorganisms in plaque. However, the effect
could get more pronounced by calculus accumulation
because it further provides retention of more plaque
microorganisms.
Hence, there is no doubt that, the mineralized
deposits can—
a. Bring the bacterial deposits more closely to the
supporting structures.
b. Interfere with the local self cleansing defense
mechanisms.
c. And also enable the patients to perform proper oral
hygiene methods.
OTHER CONTRIBUTING ETIOLOGICAL
FACTORS INCLUDING FOOD IMPACTION
Iatrogenic Factors
Faults in the dental restorations and prosthesis referred
to as iatrogenic factors are common causes of gingival
inflammation and periodontal destruction.
Faulty Restorations
Six characteristics of restorations are important from
periodontal point of view.
a. Margins of restorations
b. Contours and overhanging dental restorations
c. Occlusion
d. Materials
e. Design of removable partial dentures
f. Restorative procedures themselves
Biologic width is defined as the dimension of the soft
tissue, which is attached to the portion of the tooth
coronal to the crest of the alveolar bone. The biologic
width is commonly stated to be 2.04 mm, which
represents the sum of epithelial and connective tissue
measurements. Hence encroachment of the biologic
width frequently leads to gingival inflammation, clinical
loss of attachment and bone loss.
a. Margins of restorations: Subgingival restorations can
contribute to periodontal diseases by,
i. Providing ideal locations for the accumulation
of plaque.
ii. Changing the ecological balance of the gingiva
to one that favors the growth of the disease
associated organisms, at the expense of the
health-associated organisms.
iii. It was also demonstrated by Waerhaug et al
(1978) that subgingival restorations are plaque
retentive areas that are inaccessible to scaling
instruments, hence greater chance of severe
gingivitis and deeper pockets (Fig. 7.5).
b. Contour of restorations/artificial crowns: Over-
contoured or improperly-contoured restorations tend
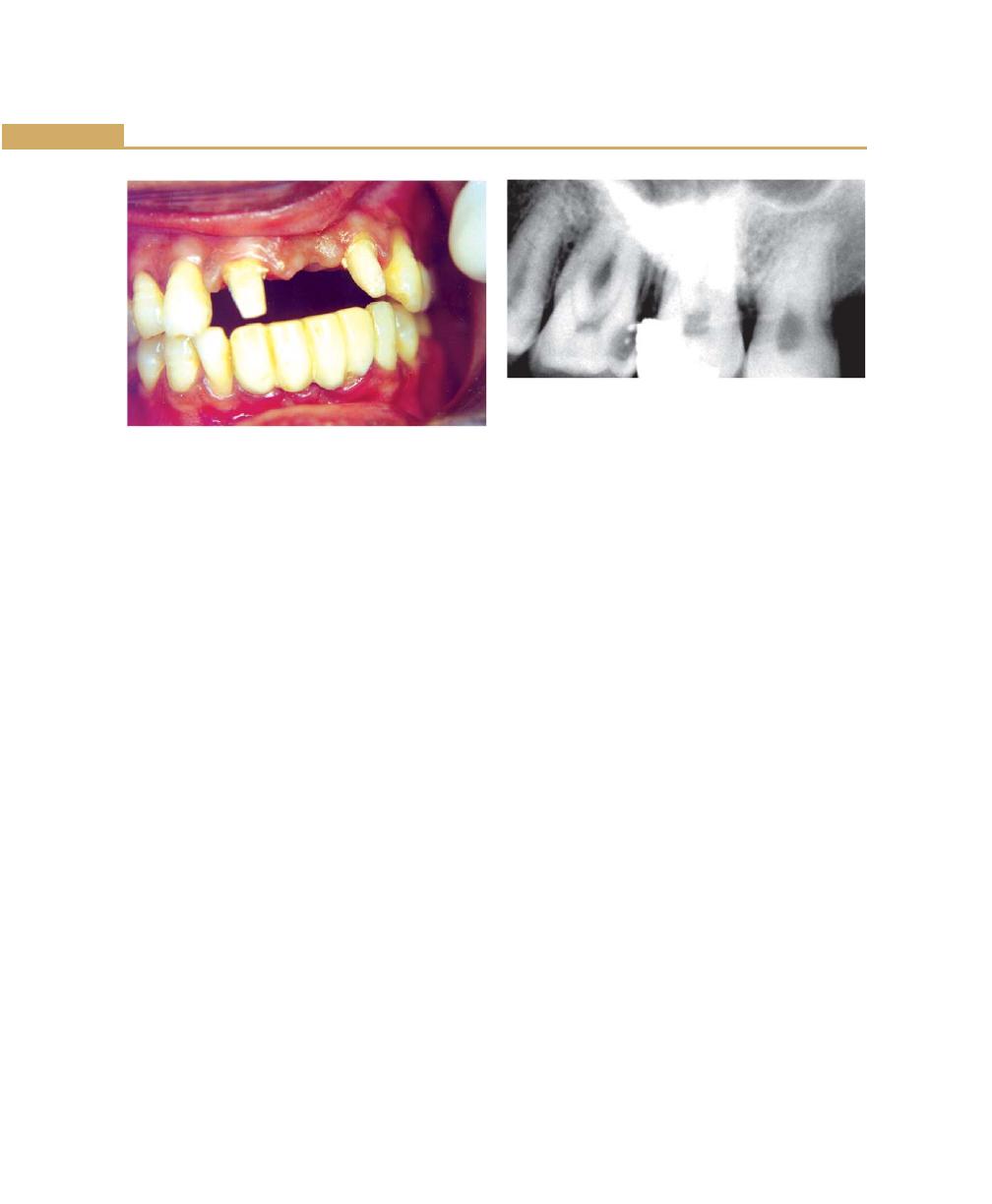
70
Essentials of Clinical Periodontology and Periodontics
to accumulate plaque and possibly prevent the self-
cleansing mechanisms of the adjacent cheek, lips and
tongue. Proper contours of the artificial crown are
necessary for maintaining gingival health. In a review
of periodontal prosthetic interactions, Becker and
Kaldahl (1981) opined that buccal and lingual crown
contours should be ‘flat’, not fat, usually <0.5 mm
wider than the CEJ and that the furcation areas,
should be “fluted or barrelled out” to accommodate
oral hygiene in these areas.
Overhanging dental restorations has been
considered to be a contributing factor to gingivitis
and possible periodontal attachment loss. Many
authors have demonstrated radiographic bone loss
adjacent to posterior teeth with overhanging
restorations. The placement of the subgingival
overhangs result in the changes in the associated
microflora to that of one resembling the flora seen
in chronic periodontitis. Hence overhangs not only
increase plaque mass but also increase the specific
periodontal pathogens in the plaque (Fig. 7.6).
c. Occlusion: Poorly-constructed restoration will cause
occlusal disharmonies that may be injurious to the
supporting normal periodontal tissues. This type of
tissue injury is called “primary trauma from the
occlusion”. Some of the examples are:
i. Insertion of a “high filling”.
ii. Insertion of a prosthesis, replacement that
creates excessive forces on abutment or
antagonistic teeth.
iii. The drifting movement or extrusion of the teeth
into spaces created by unreplaced missing teeth
or,
iv. The orthodontic movement of teeth into
functionally unacceptable positions.
d. Materials: In general, restorative materials are not by
themselves injurious to the periodontal tissues. It is
the rough, unpolished surfaces that favor plaque
accumulation and contributes to periodontal diseases.
Compared to all restorative materials that are
available to the clinician, glass ionomer restorations
and porcelain seems to retain less plaque and thus
are more acceptable from periodontal point of view.
Fluoride constantly leaking from the glass ionomer
cement prevents the attachment of the bacteria to
the pellicle and it also interferes with the metabolism
and growth of bacteria, where as highly-polished
surfaces of porcelain inhibits plaque formation and
permits its rapid removal too.
e. Design of removable partial dentures: Several studies
have shown that after the insertion of partial dentures,
there is an increase in the mobility of the abutment
teeth within gingival inflammation and periodontal
pocket formation. This is due to the increased plaque
accumulation. The presence of removable partial
dentures not only induces quantitative changes in
Fig. 7.6:
Radiographic illustration of overhanging
amalgam restoration
Fig. 7.5:
Margins of restoration in relation to lower
anterior teeth

71
Calculus and other Etiological Factors
plaque, but also qualitative changes by promoting
the development of most pathogenic bacteria. Hence
from periodontal point of view fixed prosthesis is
more acceptable than the removable one.
f. Restorative procedures themselves can also cause
destruction of periodontium. The use of the rubber
dam clamps, copper bands, matrix bands and discs
in such a manner as to lacerate the gingiva, results
in the varying degrees of inflammation.
Periodontal Problems Associated with
Orthodontic Therapy
Orthodontic therapy may affect the periodontium by:
a. Favoring plaque retention and also modifying the
gingival ecosystem.
b. Directly injuring the gingiva as a result of over
extended bands which may also lead to forceful
detachment of gingiva from tooth causing gingival
recession.
c. Creating excessive and/or unfavorable forces on the
supporting tooth structures causes necrosis of the
periodontal ligament and adjacent alveolar bone,
excessive forces also increase the risk of apical root
resorption (Fig. 7.7).
Food Impaction
It is the forceful wedging of the food into the
periodontium by occlusal forces. Cusps that tend to
forcibly wedge food interproximally are known as
“Plunger cusps”.
According to Hirschfeld food impaction can occur
in the following conditions:
a. Uneven occlusal wear: It can lead to food impaction
because deflection of food away from the proximal
areas does not occur.
b. Loss of proximal contact: This is one of the most
common cause for food impaction. It may be due
to, periodontal disease, non-replaced missing teeth,
proximal caries and abnormal biting habits.
c. Congenital morphologic abnormalities of teeth.
d. Improperly-constructed restorations.
e. Lateral food impaction: In addition to food impaction
caused by occlusal forces, lateral pressure from the
lips, cheeks, tongue may force food interproximally.
This usually occurs when the gingival embrasure is
enlarged by periodontitis or by recession.
The following signs and symptoms may occur in the
association with food impaction:
i. Feeling of pressure and urge to dig the material
from between the teeth.
ii. Vague pain that radiates deep in the jaws.
iii. Gingival inflammation with bleeding and a foul
taste in the involved area.
iv. Gingival recession.
v. Periodontal abscess formation.
vi. Varying degrees of inflammatory involvement of
periodontal ligament, sensitivity to percussion.
vii. Destruction of the alveolar bone.
viii. Root caries.
Unreplaced Missing Teeth
Failure to replace extracted teeth initiates a series of
changes that produce various degrees of periodontal
diseases. The pattern of changes that may follow, failure
to replace missing first molars is characteristic (Fig. 7.8).
In extreme cases it consists of the following:
a. The second and third molars tilt, resulting in a decrease
in the vertical dimension.
b. The pre-molars move distally and the mandibular
incisors tilt or drift lingually.
Fig. 7.7:
Periodontal changes associated with orthodontic
therapy
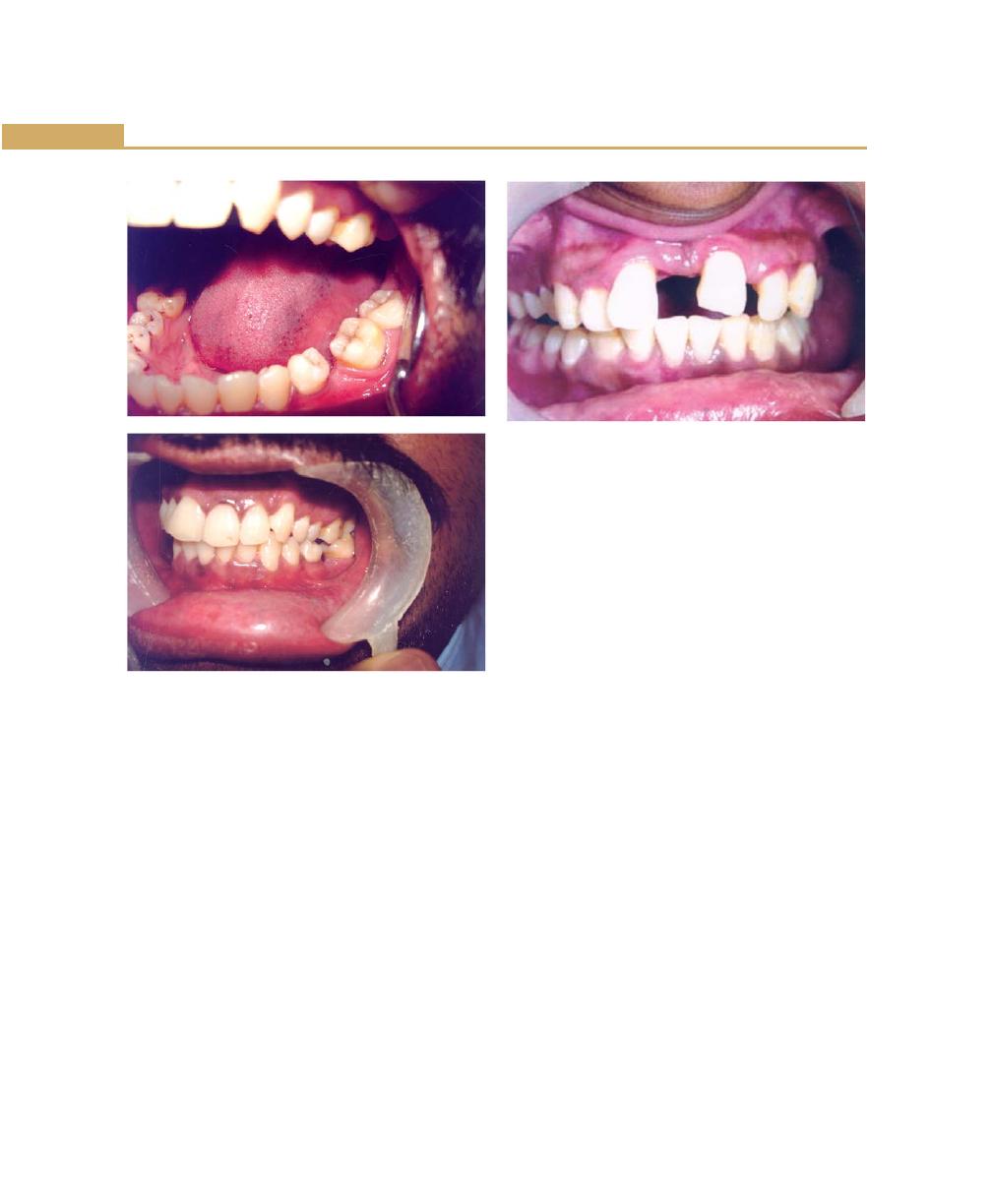
72
Essentials of Clinical Periodontology and Periodontics
c. The anterior overbite is increased. The mandibular
incisors strike the maxillary incisors near the gingiva
or traumatize the gingiva.
d. The maxillary incisors are pushed labially and laterally.
e. The anterior teeth extrude because the incisal
opposition has largely-disappeared.
f. Diastema is created by the separation of the anterior
teeth.
Loss of proximal contact relationships lead to,
Food impaction
↓
Gingival inflammation
↓
Pocket formation
↓
Followed by bone loss and tooth mobility.
Extraction of impacted third molars: Numerous clinical
studies have reported that the extraction of third molars
often results in the creation of vertical defects distal to
the second molars and appears to occur more often in
individuals older than 25 years than in those younger
than 25.
Malocclusion
Depending on its nature, malocclusion exerts varied
effect on the etiology of gingivitis and periodontal diseases
(Fig. 7.9).
a. Irregular alignment of teeth: Makes plaque control
difficult.
b. Spacing between teeth: Same sequelae following loss
of proximal contact can occur.
c. Facially-displaced teeth: Can lead to gingival
recession.
d. Occlusal disharmony: Results in injury to periodontium.
e. Deep bite: Inflammation of palatal mucosa.
f. Open bite: Leads to accumulation of plaque and
periodontal atrophy.
Habits
Sorren has classified habits of significance in the etiology
of periodontal diseases as follows:
a. Neurosis: Such as lip biting and cheek biting which
would lead to extra functional positioning of the
mandible, others include tongue thrusting, fingernail
biting and occlusal neurosis.
Figs 7.8A and B:
Sequelae of unreplaced
missing first molar
Fig. 7.9:
Gingival changes associated with malocclusion
A
B
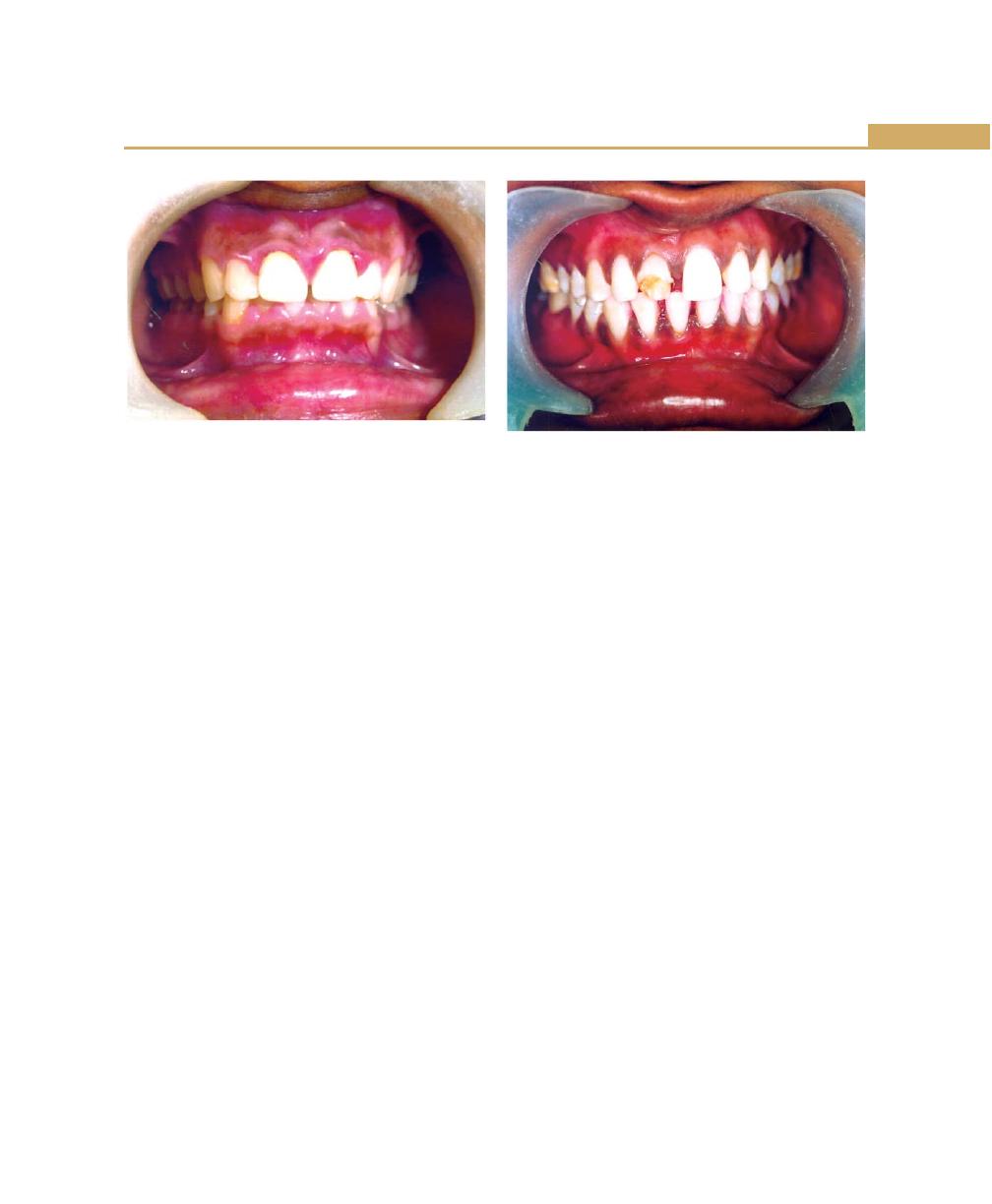
73
Calculus and other Etiological Factors
Fig. 7.10:
Gingival changes associated with mouth
breathing
b. Occupational habits: Such as holding of nails in the
mouth, e.g. carpenters, cobblers, etc.
c. Miscellaneous habits: Such as pipe or cigarette
smoking, tobacco chewing, incorrect methods of tooth
brushing, mouth breathing and thumb sucking.
I. Mouth breathing: Gingivitis is often associated with
mouth breathing. The gingival changes include
erythema, edema, enlargement and a diffuse shiny
appearance on the exposed areas (Fig. 7.10).
II. Tongue thrusting: It is the persistent, forceful wedging
of the tongue against the teeth. Instead of the dorsum
of the tongue being placed against the palate with
the tip behind the maxillary teeth during swallowing,
the tongue is thrust forward against the anterior teeth.
Tongue thrusting causes excessive lateral pressure,
which may be traumatic to the periodontium. It also
causes spreading and tilting of the anterior teeth,
which tilt and also spread laterally.
↓
Numerous secondary sequelae may develop from
tongue thrusting. They include, change in the
direction of the functional forces so that lateral
pressure against the crowns is increased.
↓
Also interferes with food excursion and favors the
accumulation of the food debris at the gingival
margin.
Fig. 7.11:
Anterior overbite, pathologic migration
associated with tongue thrusting
Tongue thrusting is an important contributing factor
in the pathologic tooth migration (Fig. 7.11).
III. Use of tobacco: The following oral changes may occur
in the smokers:
1. Brownish, tar-like deposits and discoloration of tooth
structure. (Due to the nicotine and its major
metabolite, cotinine are deposited on the root
surfaces).
2. Diffuse greyish discoloration and leukoplakia of the
gingiva may occur.
3. “Smokers palate” (nicotinic stomatitis), characterized
by prominent mucous glands with inflammation of
the orifices and a diffuse erythema or by a wrinkled,
“cobble stone” surface, may occur.
4. Predisposition to acute necrotizing ulcerative gingivitis
5. Delayed post-surgical healing.
6. Marked increase in gingival crevicular fluid flow.
7. More severe gingivitis and periodontitis have been
reported in smokers.
Special type of gingivitis, termed “gingivitis toxica”
characterized by destruction of the gingiva and
alveolar bone has been attributed to the chewing of
tobacco (Fig. 7.12).
8. Oral polymorphonuclear cells from smokers show
reduced ability to phagocytose particles.

74
Essentials of Clinical Periodontology and Periodontics
IV. Toothbrush trauma: Acute or chronic gingival changes.
Acute changes are:
1. Sloughing of the epithelial surface occurs along with
denudation of the underlying connective tissue to
form a painful gingival bruise.
2. Punctate lesions are produced by the penetration of
the gingiva by the toothbrush bristles.
3. Painful vesicle formation in the traumatized areas is
also seen.
4. Diffuse erythema and denudation of the attached
gingiva throughout the mouth may be the striking
sequelae of the overzealous brushing.
The acute gingival changes noted can commonly
occur when the patient uses a new brush.
5. Tooth bristles forcibly embedded and retained in the
gingiva are a common cause of the acute gingival
abscess.
Chronic toothbrush trauma results in:
1. Gingival recession with denudation of root surface
2. Often the gingival margin is enlarged and appears
to be “piled up”, as if it were molded in conformity
with the strokes of the toothbrush.
Chemical irritation: Acute gingival inflammation may be
caused by chemical irritation. The gingival changes range
from simple erythema to painful vesicle formation and
ulceration. The substances include, strong mouthwashes,
dentifrices or denture materials, application of aspirin
tablet to alleviate toothache and injudicious use of
escharotic drugs.
Radiation: Patients with cancer of the oral cavity and
adjacent regions who are treated with radiations initially
develop erythema and desquamation of the oral mucosa
including gingiva, which leads to ulcerations, infections
and suppuration. The bone also undergoes degeneration.
Radiation also includes atrophy of the salivary glands
leading to xerostomia and changes in the oral flora
predisposing to dental caries.
Occlusal neurosis or parafunctional habits: They are
bruxism and clenching.
Bruxism: It is the clenching/grinding of the teeth when
the individual is not chewing or swallowing.
Clenching: It is the closure of the jaws under vertical
pressure.
Bruxism may lead to fracture of the teeth or dental
restorations, tooth wear or uncosmetic muscle
hypertrophy.
Two types of bruxism have been reported Nocturnal
Bruxism/Nonstress bruxists and diurnal bruxism/stress
bruxists.
Clinical features of bruxism: Clinically you can diagnose
bruxism by the presence of facet patterns. Sleep studies
have shown that bruxism occurring during REM (rapid
eye movement) sleep may be the most damaging. No
association has been shown between bruxism and
periodontitis or gingival inflammation.
Treatment of bruxism: Occlusal adjustment is contra-
indicated.
Maxillary stabilization appliance is advised, which is the
most effective means of treating Bruxism. The main aim
of this appliance is to protect the tooth surface and to
dissipate forces built up in the musculoskeletal system
through the bruxism. This is more ideal to treat nocturnal
bruxism than for correcting daytime clenching habits.
It results in an immediate reduction in the masseter and
temporalis muscle activity levels. The appliance should
Fig. 7.12:
Tobacco stains on the lingual surface of the
lower anterior teeth
75
Calculus and other Etiological Factors
be readjusted in 2 to 4 weeks and thereafter over longer
intervals bruxofacets should be observed in the follow
up visits and the surface should be burnished with a
smooth, pumice impregnated rubber wheel.
KEY POINTS TO NOTE
Calculus
1. Dental calculus is an adherent, calcified or calcifying mass
that forms on the surface of the teeth and dental
appliances.
2. Two types of calculus, supragingival and subgingival
calculus is seen.
3. It is made up of organic and inorganic constituents.
4. Four types of attachments of calculus to the tooth surface
has been reported.
5. Calculus formation takes place by the precipitation of
mineral salts.
6. Theories that are explained in calculus formation are:
a. Booster mechanism.
b. Epitactic or heterogenous nucleation
c. Inhibition theory
7. Finally, although a positive correlation exists between
calculus and periodontal disease, it is plaque that shows
a greater correlation with periodontal disease.
Other Contributing Etiological Factors including
Food Impaction
8. Faults in the dental restorations and prosthesis are referred
to as iatrogenic factors.
9. From the periodontal point of view, 6 characteristics of
restorations are important:
a. Margins of restorations.
b. Contours and overhanging dental restorations.
c. Occlusion.
d. Materials.
e. Design of removable partial dentures.
f. Restorative procedures themselves.
10. Orthodontic therapy may affect the periodontium by,
directly injuring the gingiva due to over extension of
bands, favoring the plaque retention and changing
gingival ecosystem and creating unfavorable forces on the
supporting tooth structures.
11. Food impaction is defined as the forceful wedging of the
food into the periodontium.
12. Bruxism is the clenching or grinding of the teeth when
the individual is not chewing or swallowing. Clenching
is the closure of the jaws under vertical pressure.
REVIEW QUESTIONS
Calculus
1. Define dental calculus. Describe the types and theories
of formation of dental calculus.
2. What is the composition of dental calculus?
3. Differences between supragingival and subgingival
calculus.
Other Contributing Etiological Factors
including Food Impaction
4. What are the effects of overhanging restorations on
periodontium?
5. What are the causes for the food impaction?
6. What is the sequelae following unreplaced missing
teeth?
7. What are the effects of smoking on periodontium?
8. What are para functional habits?
BIBLIOGRAPHY
1. Genco. Contemporary Periodontics. CV Mosby Company
Publication 1990.
2. Grant, Stern, Listgarten. Periodontics. Mosby Publications,
6th edn 1988.
3. JD Manson, Bmeley. Outline of Periodontics. 3rd edn. British
Library Cataloguing in Publication Data 1995.
4. Kenneth S, Kornman, Harold Loe. The role of local factors
in the etiology and periodontal diseases. Periodontol
2000;2:1993.
5. Kourkenta, Walsh, Pavis. The effect of porcelain laminate
veneers on gingival health and bacterial plaque characteristics,
Journal of Clinical Periodontol 1994;21:638-40.
6. Newman, Takei, Fermin A. Carranza. Clinical Periodontology.
WB Saunders Co, Ninth Edition 2002.
7. Robert G Keim. Esthetics in clinical orthodontic periodontic
interaction. Periodontology 2000;27:2001.
8. Robert I Sachs: Restorative dentistry and periodontium.
Dental Clinics of North America 1985;29:266-78.
9. Robert J Genco. Contemporary Periodontics. CV Mosby
Company Publications 1990.
76
Essentials of Clinical Periodontology and Periodontics
INTRODUCTION
Health is not a static condition. It is a dynamic state in
which the living and functioning organism or tissue
remains in balance with a constantly changing
environment. This constant process of readjustment to
maintain a functional integrity is known as “Homeostasis”.
It is a well known fact that bacteria constitute an important
part of environment and all the external surfaces in
nature including living tissues are covered by bacteria,
the skin, the gut and oral mucosa are of no exceptions.
When different forms of life exist together there is
competition for existence, hence various mechanisms
have evolved to help one form to protect it from another
called “Host defence mechanisms”.
The tissues of the periodontium are exposed to various
environmental factors in the oral cavity. Over 300 species
of bacteria have been isolated in the oral cavity. The
periodontal tissues remain in a state of partnership
(symbiosis) with most of the bacteria and only under
certain circumstances do we suffer from their attack
because, host defence system strikes a balance between
the two.
The host responds to the attack of bacteria and its
toxins at various levels.
ROLE OF SALIVA IN THE HOST DEFENCE
1. A vehicle for swallowing bacteria.
2. Inhibition of attachment of bacteria.
3. Bactericidal action by the peroxidase system.
4. Bactericidal action by lysozyme, lactoferrin and other
factors.
Salivary Peroxidase System
SCN
–
+
H
2
O
2
→
HOSCN
(Thiocyanate (Generated
(Peroxidase
Hypothio-
from salivary by salivary
enzyme)
cyanous
glands)
glands, bacteria,
acid, kills the
neutrophils, etc.)
bacteria)
Peroxidase is synthesized by salivary gland acini and
secreted into the saliva, where it becomes bound to
bacteria and thiocyanate is secreted into saliva by the
❒
❒
❒
❒
❒ ROLE OF SALIVA IN THE HOST
DEFENCE
❒
❒
❒
❒
❒ GINGIVAL EPITHELIUM
❒
❒
❒
❒
❒ GINGIVAL CREVICULAR FLUID
❒
❒
❒
❒
❒ COMPLEMENT
❒
❒
❒
❒
❒ INFLAMMATORY CELL RESPONSE
❒
❒
❒
❒
❒ IMMUNOLOGICAL MECHANISMS
❒
❒
❒
❒
❒ IMMUNOLOGY OF PERIODONTAL
DISEASE
8
Host Response:
Basic Concepts
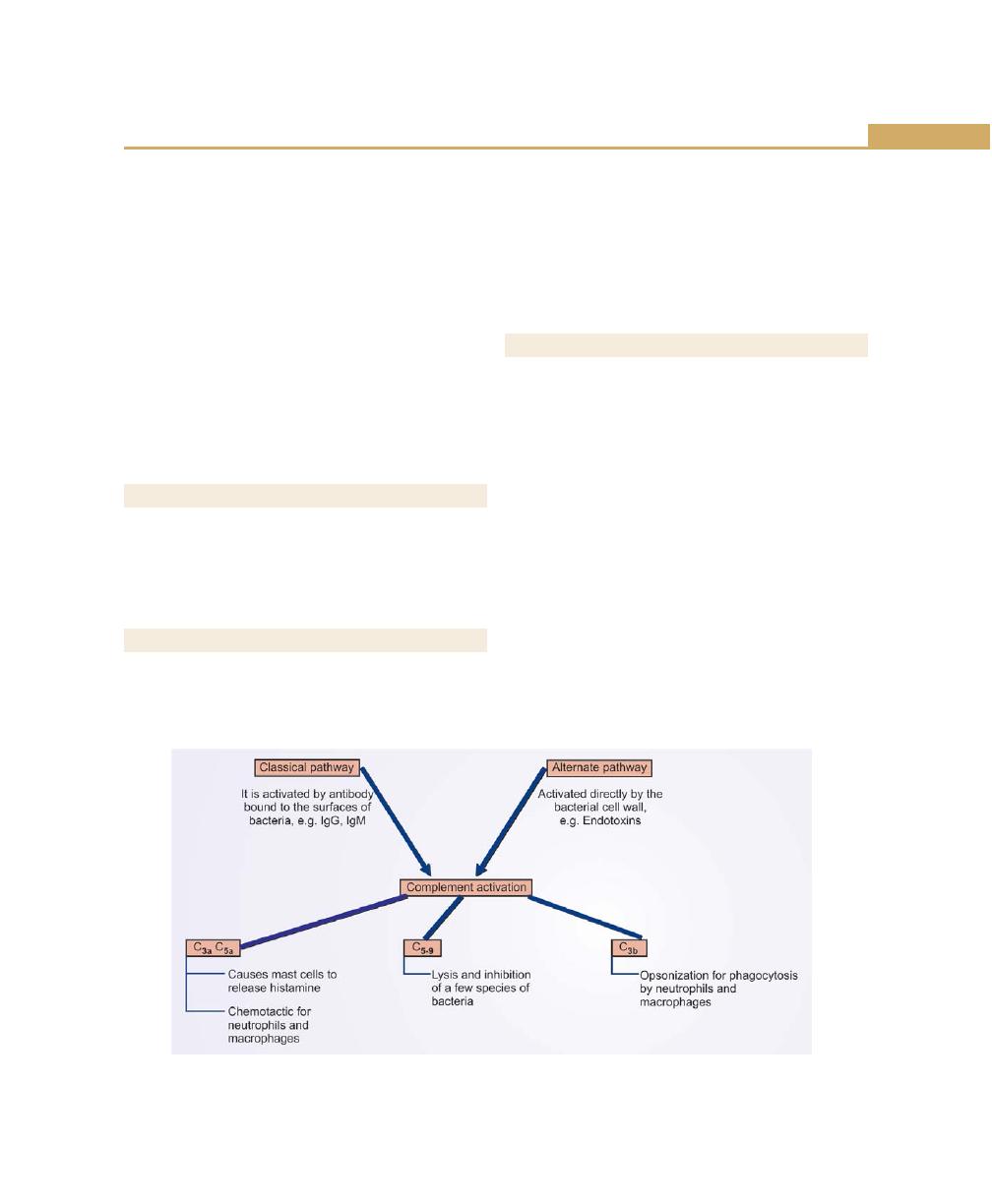
77
Host Response: Basic Concepts
ductal cells. Hydrogen peroxide is constantly secreted
in low concentration by bacteria, neutrophils and other
host cells and is used by peroxidase to oxidize the
thiocyanate to hypothiocyanous acid, which kills bacteria.
Lactoferrin: It is secreted by serous salivary gland, which
binds iron, an important growth factor or requirement
for many micro-organisms. This action is bacteriostatic
rather than bactericidal.
Lysozyme: It is an antimicrobial enzyme in the saliva
secreted mainly by mucous salivary glands and it degrades
mucopeptides in the cell wall of gram-positive bacteria,
weakening the wall and causing lysis.
GINGIVAL EPITHELIUM
Gingival epithelium has three functions:
1. Epithelial cells are tightly attached to each other.
2. Keratinization to resist trauma.
3. Presence of permeability barriers.
GINGIVAL CREVICULAR FLUID
Gingival crevicular fluid functions are:
1. Washing non-adherent bacteria and their products
out of the crevice.
2. Reducing the diffusion of plaque products into the
tissues.
3. It also carries a steady supply of inflammatory
mediators, protease inhibitors and host defence
agents such as complement and antibody, into the
crevice.
COMPLEMENT (Fig. 8.1)
The functions of complement are:
a. Chemotaxis cellular activation: Complement products
released in this reaction attracts phagocytes to the
site of infection, e.g. C
3a
and C
5a
.
b. Opsonization: Once they arrive at the site of infection
the complement components coat the bacterial
surface and allow the phagocytes to recognize the
bacteria and there by facilitating the bacterial
phagocytosis, e.g. C
3b
.
c. Cytolysis: Damage to the plasma membranes of the
cells can lead to lysis of the cell, e.g. C
1
-C
9
.
The complement system comprises of nine major
complement proteins which circulate in an inactive form
and which, like the clotting system, are activated in an
enzyme cascade.
C
1
-C
9
causes cytolytic and cytotoxic damage to the cell.
Fig. 8.1:
Complement activation

78
Essentials of Clinical Periodontology and Periodontics
Classical Pathway
The sequence is C
1
, C
4
, C
2
, C
3
, C
5
, C
6
, C
7
, C
8
, C
9
.
Alternate Pathway
It is activated by antibodies of immunoglobulins and also
endotoxins .They can intiate the third component of the
complement without starting from the beginning of the
cascade.
The sequence is C
3
, C
5
, C
6
, C
7
, C
8
, C
9
.
THE INFLAMMATORY CELL RESPONSE
It involves emigration of neutrophils, macrophages and
lymphocytes from the blood vessels into the tissues.
Neutrophils and macrophages perform inflammatory
functions whereas macrophages and lymphocytes
perform immunological functions.
Neutrophils
They are the initial leukocytes seen in the gingiva. They
exit the circulation and migrate into the junctional
epithelium and gingival crevice, where they provide the
first cellular host mechanism to control periodontopathic
bacteria.
Functions of Neutrophils
Emigration and chemotaxis:
Leukocytes normally travel along the center of the lumen
of the blood vessel, but in inflamed tissues the blood
flow is slowed by fluid exudation and they adhere more
readily to endothelial cells, the mechanism is called
“rolling” and “margination”. When the neutrophils
migrate across the endothelium it is called “diapedesis”
and “interendothelial transmigration”.
Hence there are two phases of leukocyte endothelium
adherence.
a. The selectin-dependent phase (primarily in rolling and
margination).
b. The integrin-dependent phase (primarily in diape-
desis).
The selectin-dependent phase: The various selectins
are:
1. L-selectin—Expressed on the surface of the leukocyte.
2. P-selectin—Stored in the granules of endothelial cells
(Weibel-Palade bodies).
3. E-selectin—Expressed by endothelial cells.
P-selectin and E-selectin: They both strengthen the
binding between the leukocyte and the endothelial cell
and increase the number of leukocyte “rolling”.
The integrin-dependent phase: (Leukocyte B
2
-integrins):
The three leukocyte integrins are sequestered in the
specific granules of leukocytes,
• LFA-1—leukocyte function-associated antigen-1
• CD
11a
/ CD
18
(or)
Mac-1 / CD
11b
/ CD
18
• CD
11c
/
CD
18
These leukocyte B
2
-integrins act as the molecular
mediators of binding to the endothelial cells and their
binding affinity can be increased or decreased as the
leukocyte traverses the postcapillary venule.
Chemotaxis: It is the directed movement of a cell along
a chemical gradient. The neutrophils are attracted by
chemical signals from multiple sources, e.g. chemotaxins
which include compounds such as complement fragments
(C
5a
) (Fig. 8.2).
LTB
4
: It is secreted by mast cells, neutrophils macro-
phages, and also bacterial products including LPS and
factors released by damaged tissues.
Chemotaxis requires phagocyte, which possess
specific chemotaxin receptors, and the most well-studied
chemotaxin receptor is the receptor for formylmethionyl
peptides, known as the FPR (Formylmethionyl Peptide
Receptor).
Stages in neutrophil chemotaxis (Fig. 8.3)
Stage I: Soluble products diffuse forming a concentration
gradient.
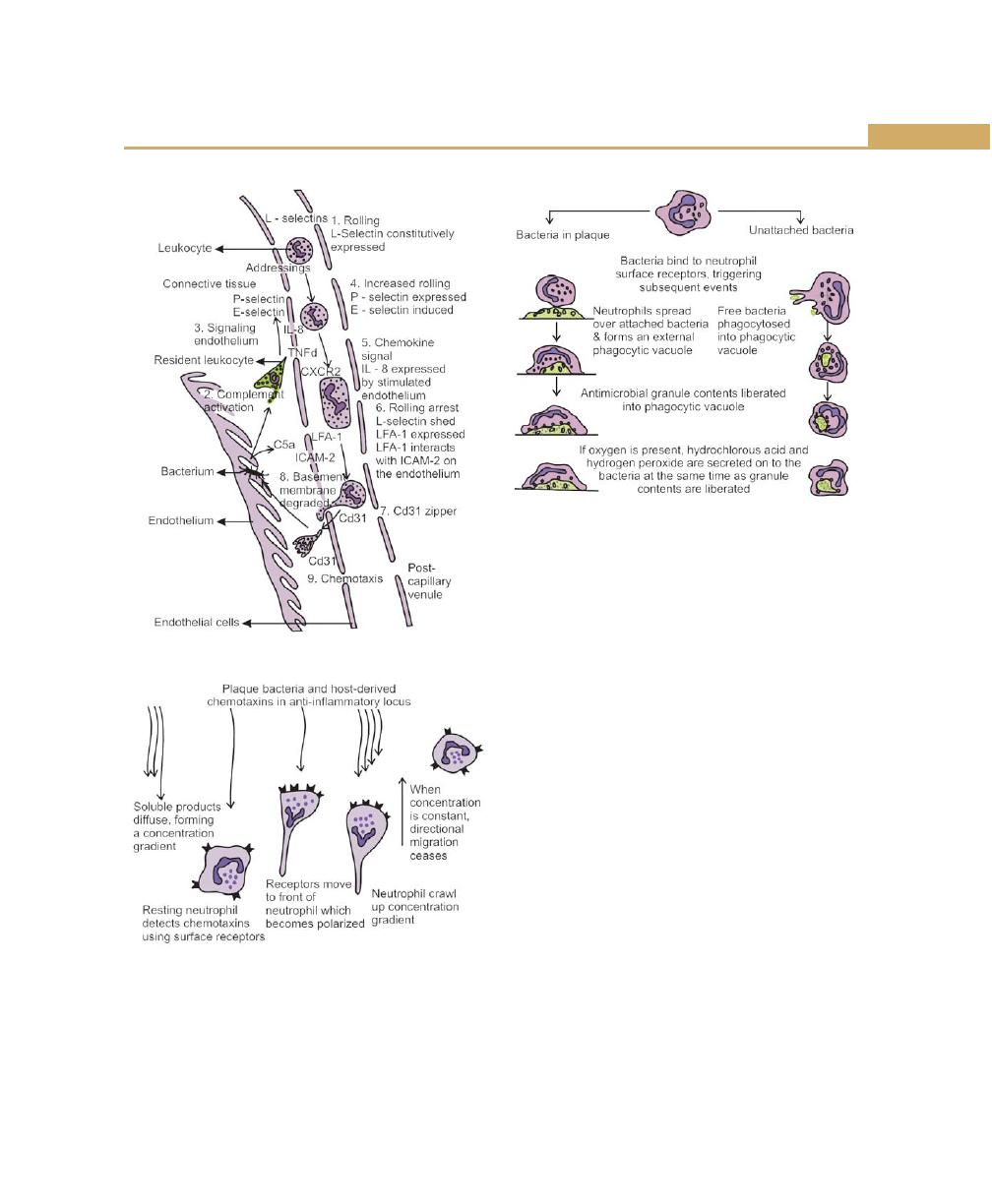
79
Host Response: Basic Concepts
Fig. 8.2:
Chemotaxis and migration
Fig. 8.3:
Stages in neutrophil chemotaxis
Fig. 8.4:
Phagocytosis
Stage IV: Neutrophils crawl upto the concentration
gradient.
Stage V: When concentration is constant, directional
migration ceases.
II Phagocytosis (Fig. 8.4)
Once they arrive at the site of inflammation, the
phagocytes have to recognize the infectious agent. This
can be enhanced if the organism has been coated by
C
3b
. They may then attach to micro-organism via their
non-specific cell surface receptors. After attachment the
phagocytes proceed to engulf the micro-organism by
extending pseudopodia around it. Once inside,
lysozymes fuse with the phagosome /phagocytic vacuole
to form a phagolysozyme and the infectious agent is
killed by a battery of microbiocidal mechanisms.
The main stages of bacterial killing by phagocytes:
For efficient phagocytosis, the particle should be coated
with one or more host serum proteins. This process is
called “opsonization” (meaning “to prepare for eating).
The two principal types of serum proteins referred to
as opsonins are IgG and C
3b
. There are receptors of these
two proteins on the phagocytes. Hence the opsonization
could be either complement-dependent, antibody-
Stage II: Resting neutrophils detect chemotaxins using
surface receptors.
Stage III: Receptors move to front of neutrophils, which
becomes polarized.
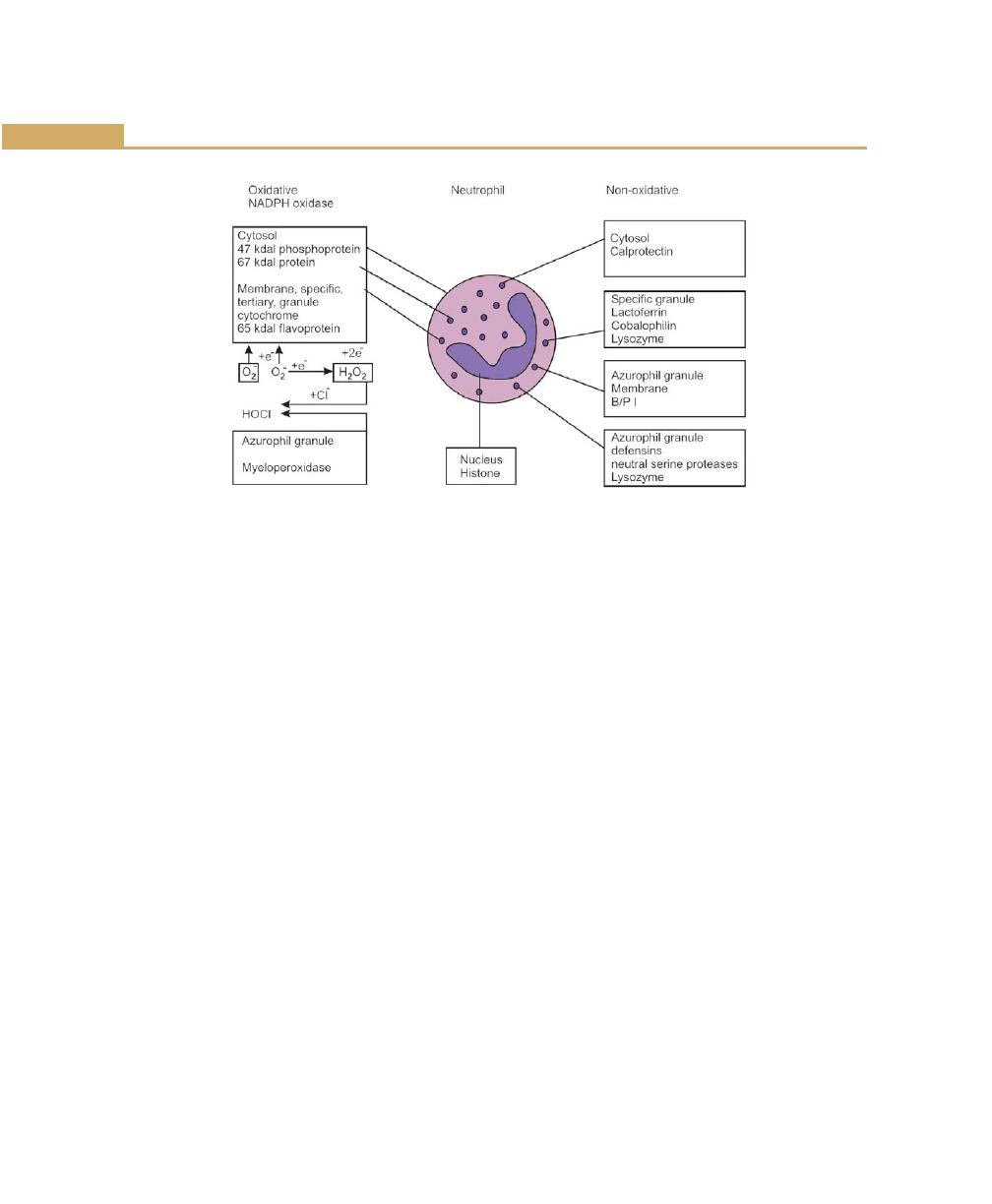
80
Essentials of Clinical Periodontology and Periodontics
Fig. 8.5:
Neutrophil: Oxidative and non-oxidative mechanisms for controlling microbes
dependent and the combination of both. Once the
organism has been internalised, lysozymes fuse with the
phagosome to form a phagolysozyme. Various killing
mechanisms are activated, they are (Fig. 8.5):
A. Non-oxygen-dependent killing mechanisms.
B. Oxygen-dependent killing mechanisms.
Oxidative mechanism: Stimulation of phagocytic cells
leads to an increase in cellular consumption of molecular
oxygen, a process termed as the “respiratory burst”. This
is associated with the generation of various oxygen
metabolites, which are injurious to many species of micro-
organisms. The majority of the oxygen consumed by
the phagocyte is converted directly to superoxide anion
(O
2
¯) through the action of a membrane—bound NADPH
oxidase. Superoxide radicals inturn may undergo
conversion to hydrogen peroxide (H
2
O
2
) either
spontaneously or via superoxide dismutase and
contribute significantly to microbicidal activity of the
phagocytic cells. Additional oxidants, e.g. hypochlorous
acid and toxic aldehydes are generated as a consequence
of the interaction between hydrogen peroxide and
azurophil enzyme (MPO). Hence the oxygen-dependent
bactericidal activity is further divided into:
• Myeloperoxidase—dependent
• Myeloperoxidase—independent.
Non-oxidative mechanisms: It appears to be based
on the various components of the cell. Neutrophils
contain three types of granules:
1. Primary granules/azurophilic granules: Myeloperoxi-
dase, lysozyme, acid phosphatase, and acid
hydrolases.
2. Secondary/specific granules: Lactoferrin, lysozyme,
azurocidin.
3. Tertiary granules: Alkaline phosphatase, collagenase,
and gelatinase.
Neutrophil Disorders Associated with
Periodontal Diseases
1. Diabetes mellitus.
2. Papillon Lefevre syndrome.
3. Down’s-syndrome.
4. Chediak-Higashi syndrome.
5. Drug-induced agranulocytosis.
6. Cyclic neutropenia.
Periodontal Diseases Associated with
Neutrophil Disorders
• Acute necrotizing ulcerative gingivitis (ANUG).
• Localized juvenile periodontitis (LJP).
• Prepubertal periodontitis (PPP).
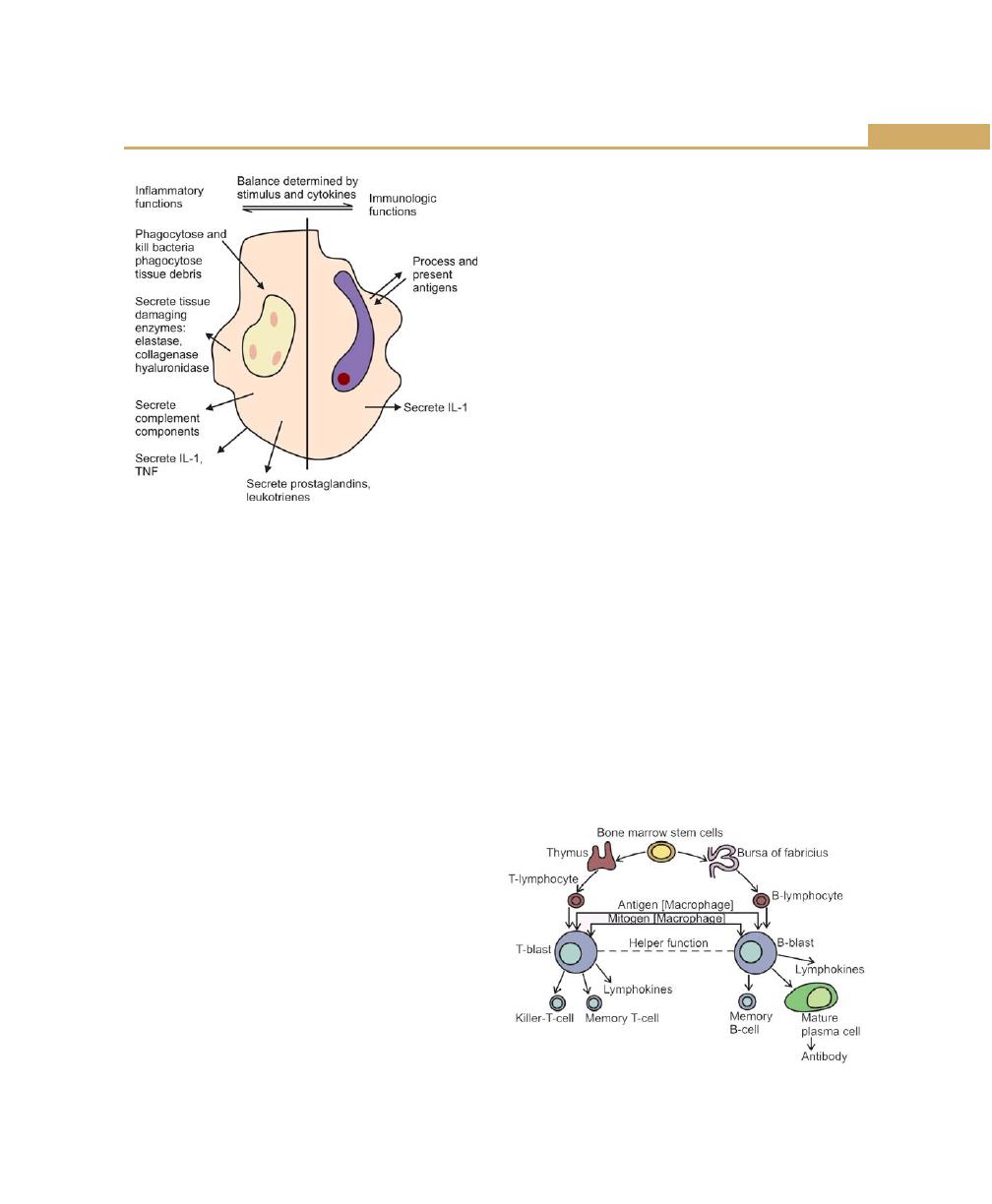
81
Host Response: Basic Concepts
• Rapidly progressive periodontitis (RPP).
• Refractory periodontitis (RP).
Functions of Macrophages in the Gingiva,
Crevice, and Pocket
Macrophages develop from blood monocytes, which
emigrate into the tissues from the blood and are triggered
to develop into mature macrophages by cytokines, other
inflammatory mediators, bacterial products such as
endotoxins.
Functions of macrophages (Fig. 8.6) includes:
1. Phagocytose and kill bacteria.
2. Remove damaged host tissue during inflammation
3. And also trap and present antigens to lymphocytes
for induction of immune responses.
Because these functions unite inflammation and
immunity the macrophages play an important role in
all bacterial infections.
Most of the above mentioned functions are carried
out through the secretion of inflammatory mediators,
including cytokines, prostaglandins, leukotrienes and
complement components. Particularly important is the
secretion of the cytokines and although other cells such
as fibroblasts, endothelial cells and keratinocytes also
secrete cytokines, macrophages secrete the greatest
quantities. The most significant cytokine in inflammation
is interlenkin-1 (IL-1), which is a key mediator both in
inflammation and immunity-induced by bacteria. Tumor
necrosis factor (TNF) is also produced by macrophages.
Both TNF and IL-1 have similar functions.
They increase inflammation by, releasing histamine
from mast cells, attracting neutrophils and more
macrophages into the tissues and by causing many other
cells to release prostaglandins.
In summary, macrophages, like neutrophils are
required for the effective host response, but can mediate
a small amount of bystander damage. The damage could
be caused by direct effect that is by secreting enzymes
and toxins (similar to neutrophils) and indirectly by
secretion of cytokines. In excess amounts IL-1 and TNF
can have several damaging effects such as stimulation
of bone resorption and tissue fibrosis.
Other cells such as mast cells, fibroblasts, endothelial
cells, plasma cells and epithelial cells are also seen in
gingival connective tissue during inflammatory response.
Lymphocytes (Fig. 8.7): Three types of cells are included,
1. T-lymphocytes or T-cells: Derived from the thymus
and play a role in cell-mediated immunity.
2. B-lymphocytes or B-cells: Derived from liver, spleen
and bone marrow. They are precursors for plasma
cells and play a role in humoral immunity.
3. Natural killer (NK) cells.
Fig. 8.6:
Functions of macrophage in periodontal tissues
Fig. 8.7:
Derivation and response of B and T lymphocytes
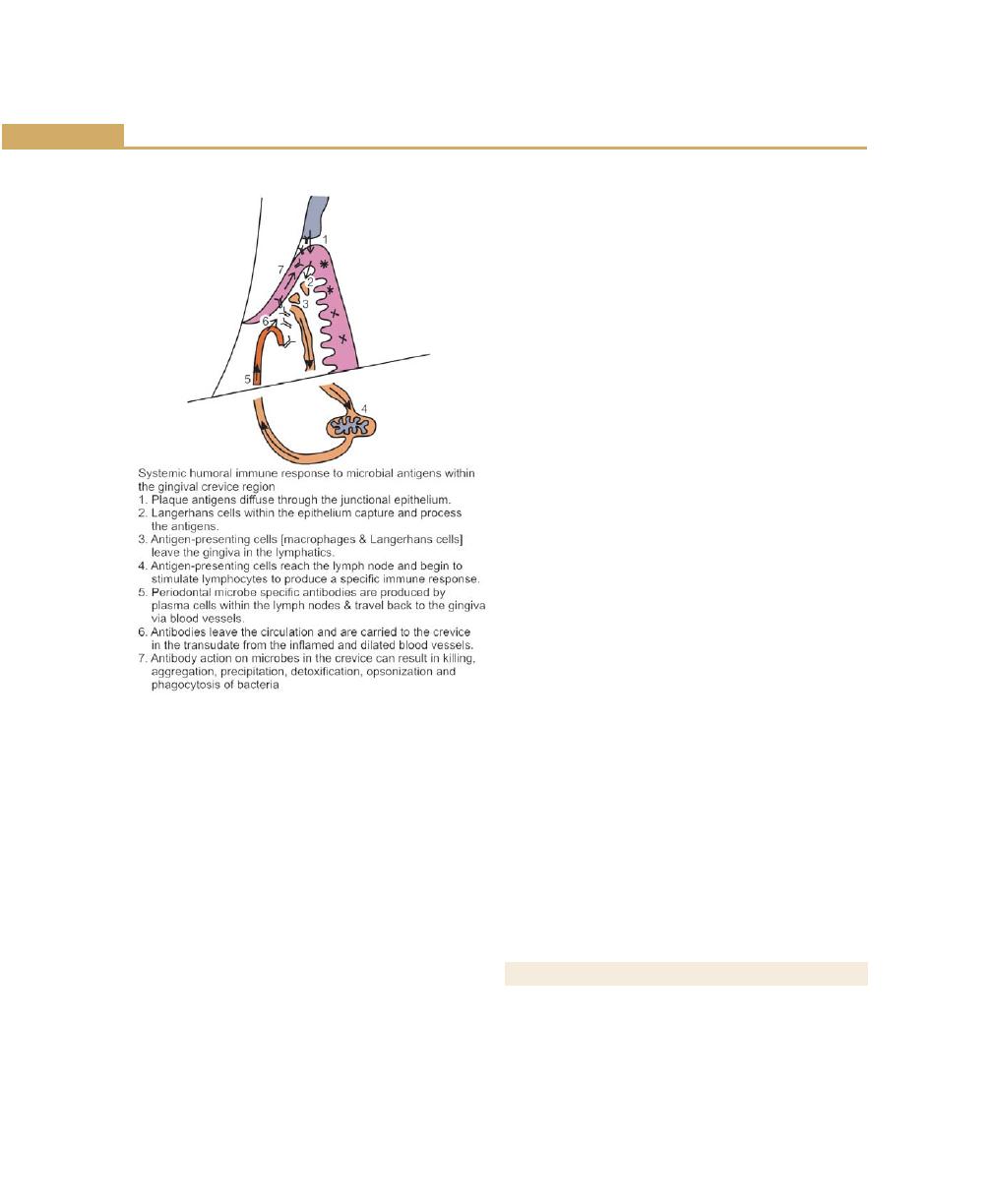
82
Essentials of Clinical Periodontology and Periodontics
Antigens, which pass into the tissues, are carried to the
local lymph nodes, probably by macrophages, where
they are presented to lymphocytes, which circulate
continually through the nodes and tissues. The
lymphocytes, which recognize each individual antigen,
are activated, undergo clonal expansion and differentiate
into plasma cells, which secrete antibody under the
control of helper and suppressor T-lymphocytes. Most
of the lymphocytes and plasma cells remain in the lymph
nodes and secrete antibodies into the blood stream. The
antibody predominantly is IgG, which can opsonize and
activate the complement. Small amounts of IgM is also
seen which is more of an activator of the complement
and less of an effective opsonin. These antibodies pass
into the gingival inflammatory exudates and then out
into the gingival crevice in the crevicular fluid.
Possible mechanisms of action of antibodies in
periodontitis:
I. Binding to bacteria, Thus:
a. Opsonizing for phagocytosis
b. Activating neutrophil enzyme secretion
c. Coating bacteria and inhibiting attachment
d. Activating complement and thus enhancing
opsonization
e. Directly inhibiting bacterial metabolism.
II. Binding to soluble factors; Thus:
a. Neutralizing toxins.
b. Inhibiting enzymes.
The cell-mediated response in periodontal diseases: It
is so called because it involves contact between cytotoxic
T-cells and the target to be destroyed. These reactions
are effective against persistent antigens, which are
resistant to degradation, and the cells infected with viruses
and tumor cells (Fig. 8.9).
IMMUNOLOGICAL MECHANISMS (FIG. 8.10)
They are stimulus – specific and differentiate between
individual pathogenic species and sometimes-individual
strains. Micro-organisms and their products are
recognized as being different from the host because they
Fig. 8.8:
Systemic humoral immune response
Different types of T-cells include:
Helper inducer T-cells (TH-cells) or CD
4
: They aid in the
cellular response of the B-cells to differentiate into plasma
cells and produce antibodies.
Suppressor-cytotoxic T-cells (TS-cells) or CD
8
: Stimulates
cytotoxic and microbicidal activity of the immune cells.
Subdivided into TH
1
TH
2
TH
0
.
• TH cells release IL-2 and IFN (interferons)
• TS cells release interleukin: IL-4 and IL-5
• In adult periodontitis TH cells increase and TS cells
decrease with increased gingival inflammation.
The humoral response to plaque (Fig. 8.8): Plaque
bacteria and their soluble products such as enzymes and
toxins carry out activation of the humoral response.
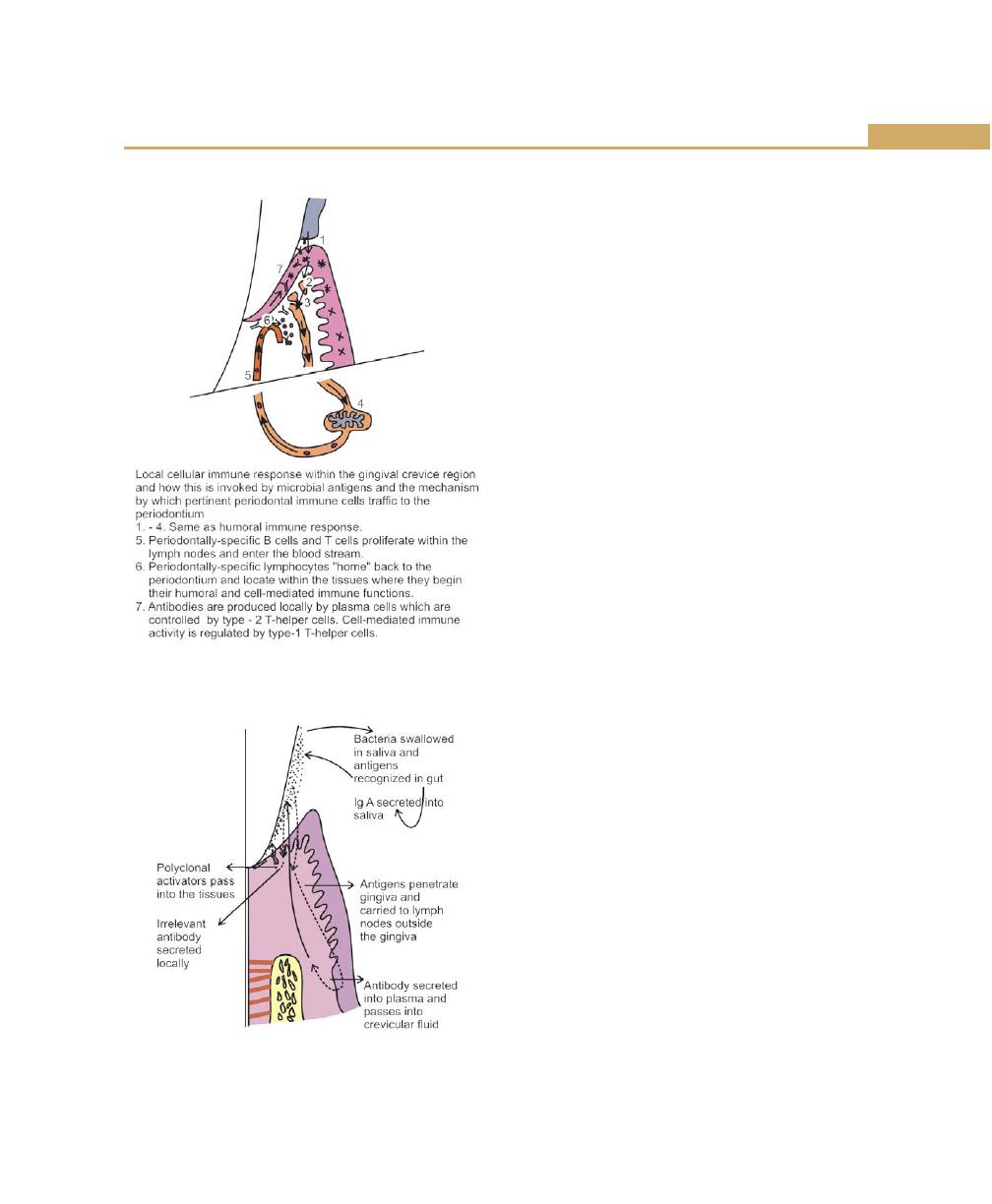
83
Host Response: Basic Concepts
Fig. 8.9:
Local cellular immune response
Fig. 8.10:
Antibody production by salivary and humoral
immune systems and by polyclonal B cell activation
contain structures, which are not found in the human
body. These so called antigens (antibody –generating)
are first recognized by the lymphocytes and each
lymphocyte is capable of recognizing only one foreign
antigen and when it comes in contact with that antigen
it is triggered to divide several times and therefore with
in few days there are many more cells with the same
specificity. This amplification process is known as clonal
expansion. This will result in the production of larger
pool of cells, which are differentiated to protect the host
either by humoral or cell-mediated mechanisms.
Humoral responses are carried out by lymphocytes,
which differentiate into plasma cells and secrete antibody
directed against the original antigen. Cell mediated
responses, in contrast, do not require antibody, but
depend on the clonal expansion to provide large number
of lymphocytes, which destroy targets directly. This direct
effect of an immune reaction against a foreign antigen
results in significant tissue damage is referred to as
hypersensitivity reaction.
Type I or Anaphylactic Reactions
Two variations in the anaphylactic hypersensitivity may
occur, depending on the route of the administration of
antigen. If injected locally into the skin, the reaction is
called cutaneous anaphylaxis.
If the antigen is injected intravenously it is called
systemic or generalized anaphylaxis. The basic
mechanisms of both types are similar (Fig. 8.11).
Mechanisms of Anaphylactic Hypersensitivity
Anaphylaxis occurs when two Ig E antibodies that are
fixed to a mast cell or basophil react with the antigen
through the Fab portion of the antibodies. This antibody-
antigen reaction causes the release of the pharma-
cologically-active substances from the sensitized-cells.
These mediators released by the human mast cells
include:
a. Histamine—Increased capillary permeability.
b. Alpha 2-macroglobulins—Collagenase activation.
c. SRS-A—Smooth muscle contraction.
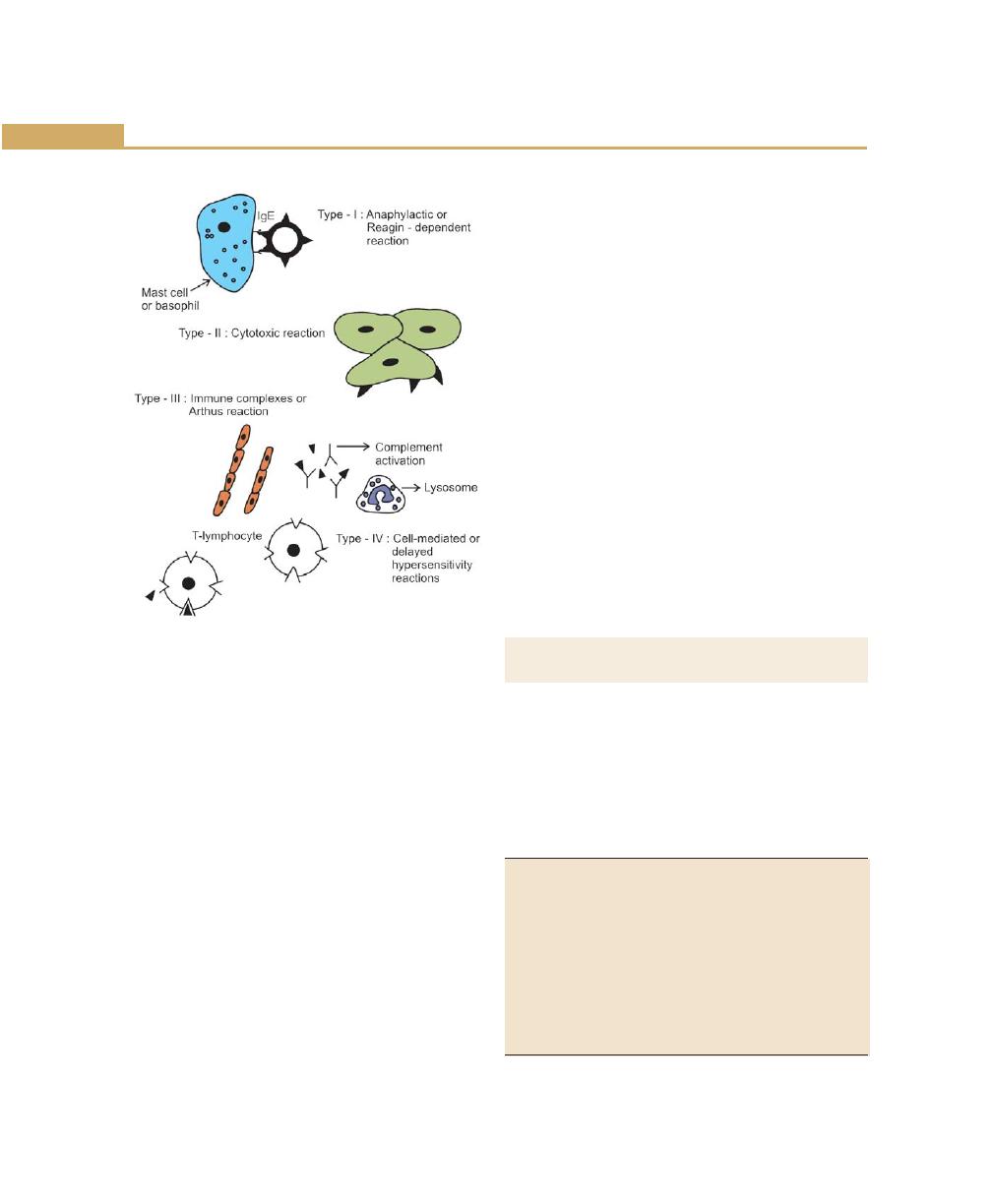
84
Essentials of Clinical Periodontology and Periodontics
To date evidence suggests an important role for the
cytotoxic reactions in the gingivitis and periodontitis.
Type III: Immune Complex or Arthus Reactions
When high levels of antigen are present and persist
without being eliminated antigen-antibody (IgG or IgM)
complexes precipitate in and around small blood vessels
and with subsequent complement activation cause tissue
damage at the site of the local reaction. Inflammation,
haemorrhage and necrosis may occur. Tissue damage
appears to be due to the release of lysozymal enzymes
from various cells such as neutrophils, mast cells, etc.
This reaction is referred to as immune complex or Arthus
reaction.
Type IV: Cell-Mediated or Delayed
Hypersensitivity
Cellular immunity does not include circulating antibodies
but is based on the interaction of the antigens with the
surface of T-Lymphocytes.
IMMUNOLOGY OF PERIODONTAL DISEASE
(Fig. 8.12)
Immune responses may be both beneficial and
detrimental. Several components of the immune system
are active in periodontal disease. These host variables
may influence bacterial colonization, bacterial invasion,
tissue destruction, healing and fibrosis (Tables 8.1 and
8.2).
Fig. 8.11:
Immunologic mechanism of tissue damage
d. Bradykinin—Increased permeability same as SRS-A.
(Slow release substance of anaphylaxis).
Type II: Cytotoxic Reactions
In cytotoxic type, antibodies react directly with antigens
tightly bound to cells. A cytotoxic reaction involving these
cells will result in hemolysis. Cytotoxic antibodies are of
IgG or IgM class. In addition to inducing cell lysis, cytotoxic
antibodies may cause tissue damage by increasing the
synthesis and release of lysozymal enzymes by cells
(PMNs). The tissue in the vicinity of these enzymes will
then be damaged.
Cytotoxic reactions are seen in the autoimmune
diseases where antibodies react with body’s own tissue
components. For example, this occurs in pemphigus where
antibodies react with cell membranes and pemphigoid
antibodies react with the epithelial basement membrane.
Table 8.1: Influence of host responses on
periodontal diseases
• Bacterial colonization: Subgingivally, antibody, complement
in crevicular fluid inhibits adherence and co-aggregation of
bacteria and potentially reduces their numbers by lysis.
• Bacterial invasion: Antibody-complement-mediated lysis
reduces bacterial counts. Neutrophil-mediated lysis also
reduces bacterial counts.
• Tissue destruction: Antibody-mediated hypersensitivity, cell-
mediated immune responses, activation of tissue factors such
as collagenase.
• Healing and fibrosis: Lymphocytes and macrophage
produced chemotactic factor for fibroblasts, fibroblast-
activating factors.
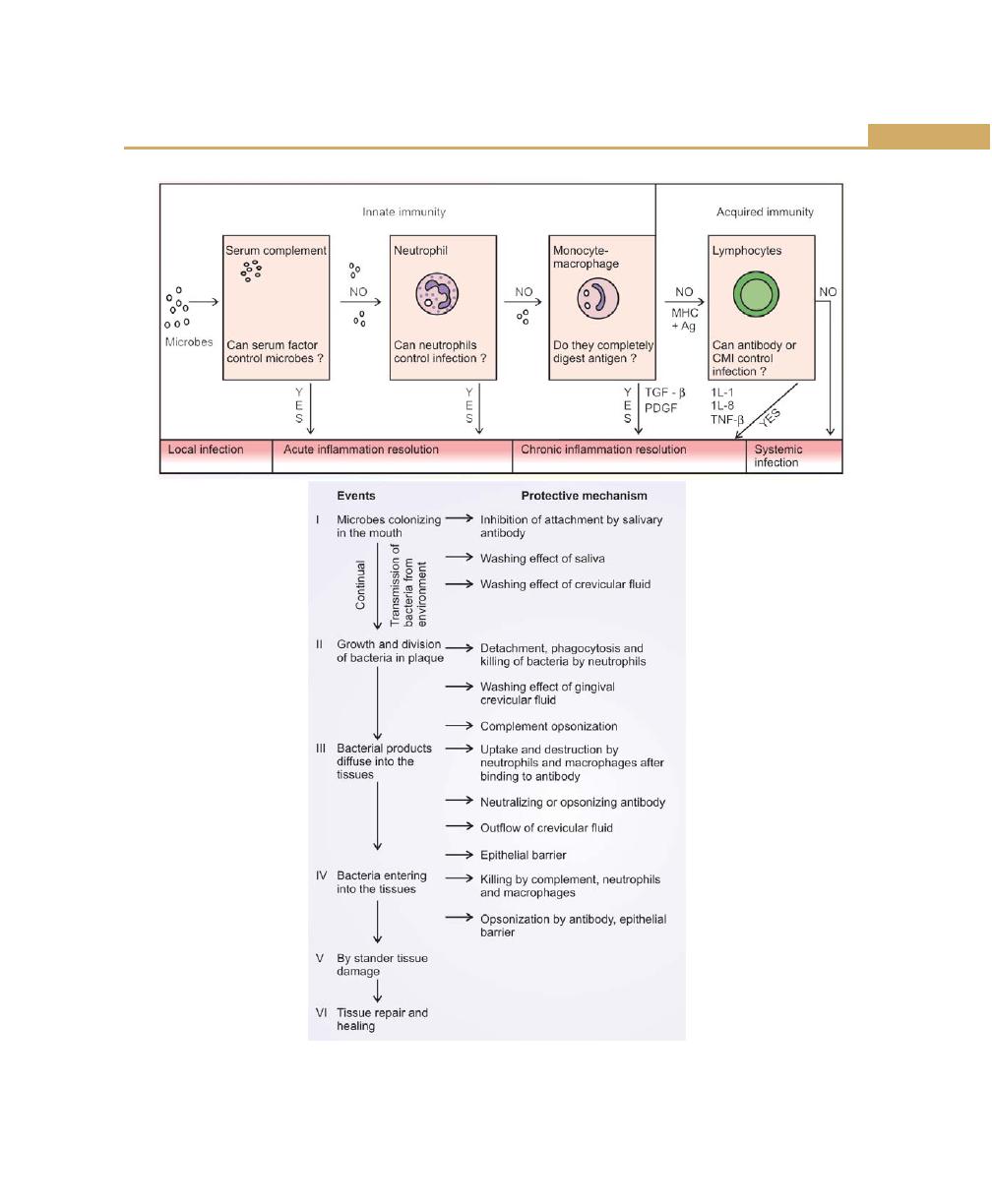
85
Host Response: Basic Concepts
Fig. 8.12:
Host defence against local and systemic infections of periodontal origin
86
Essentials of Clinical Periodontology and Periodontics
Table 8.2: Significant immune findings in periodontal diseases
Disease
Immune response
ANUG
• PMN chemotactic defects
• Elevated antibody titres to Prevotella intermedia and intermediate-sized spirochetes
Pregnancy gingivitis
• No significant findings reported
Adult periodontitis
• Elevated antibody titres to the Porphyromonas gingivalis and other pathogens
• Presence of immune complexes in tissues
• Cell-mediated immunity to gingival bacteria
Juvenile Periodontitis:
LJP
• Polymorphonuclear leukocytes chemotactic defect and depressed phagocytosis
• Elevated antibody levels to Actinobacillus actinomycetemcomitans
• Defect in GP
110
receptors
GJP
• PMN chemotactic defect and depressed phagocytosis
• Elevated antibody levels to P. gingivalis
Prepubertal periodontitis
• PMN and monocyte chemotactic defects
Rapidly-progressing periodontitis
• Suppressed or enhanced PMN, monocyte chemotaxis
• Elevated antibody levels to several Gram-negative bacteria
Refractory periodontitis
• Reduced PMN chemotaxis
KEY POINTS TO NOTE
1. Host defence is established at different levels.
2. Saliva acts as a vehicle for swallowing bacteria, inhibits the
attachment of bacteria, and kills the bacteria by the
peroxidase system, lysozyme and lactoferrin.
3. Functions of gingival epithelium are structural arrangement
of epithelial cells, prevents bacterial entry, keratinization,
resists trauma and also has a permeability barrier.
4. Complement functions are cellular activation, opsonization
by C
3b
and cytolysis (C
1
-C
9
).
5. The complement activation can be classical pathway with
the sequence of C
1
, C
4
, C
2
, C
3
, C
5
, C
6
, C
7
, C
8
, C
9
, alternative
pathway C
3
, C
5
, C
6
, C
7
, C
8
, C
9
.
6. Various killing mechanisms of neutrophils are, non-oxygen-
dependent mechanisms and oxidative mechanisms.
7. Macrophages phagocytose and kill bacteria, removes
damaged host tissue during inflammation and also traps and
presents the antigens to lymphocytes for induction of
immune responses.
8. There are three types of lymphocytes, T-lymphocytes, B-
lymphocytes and natural killer (NK) cells.
REVIEW QUESTIONS
1. What are the function of saliva and gingival crevicular
fluids ?
2. Role of neutrophils in periodontal disease.
3. Describe the complement activation and functions
of antibodies.
BIBLIOGRAPHY
1. Erica Gemmell, Gregory J Seymour, Modulation of immune
response to periodontol bacteria. Current Opinion in
Periodontol 1994:18–34.
2. Genco. Contemporary Periodontics. CV Mosby Company
Publication, 1990.
3. JD Manson, B Meley. Outline of Periodontics, British library
Cataloguing in Publication data, Third edition (1995).
4. Jeffrey L, Ebersole, Martin A Taubman. The Protective nature
of host responses in periodontal disease. Periodontol 2000;
5:1994.
5. Newman, Takei, Fermin. A. Carranza, Clinical Periodontology,
9th edn, WB Sounders Co., 2002.
6. Robert J Genco, Host response in periodontal disease: Current
concepts. Jr of Periodontol 1992;63(4).
7. Sigmund S. Socransky, Anne D Haffajee, Bacterial etiology
of destructive periodontal disease: current concepts Jr. of
periodontal April 1992 (Supply Copy), Vol 63 No.4.
8. Thomas E Van Dyke, Jaywanth Vaikuntan. Neutrophil
function and dysfunction in periodontal disease. Current
Opinion in periodontol 1994:19–28.
9. Williams, Francis J Hughey. Pathology of Periodontal Disease
1992 Oxford Medical Publications.
87
Trauma from Occlusion
PHYSIOLOGIC ADAPTIVE CAPACITY OF THE
PERIODONTIUM TO OCCLUSAL FORCES
One must appreciate the dynamics of the periodontium
to accomodate the forces exerted on the crown, which
is called as adaptive capacity. This varies in different
persons and in the same person at different times. This
is mainly explained by four factors which mainly influences
the effect of occlusal forces on the periodontium.
a. Magnitude (the amount): When it is increased the
periodontium responds (a) with a thickening of the
periodontal ligament, (b) an increase in the number
and width of periodontal ligament fibers and an (c)
increase in the density of the alveolar bone.
b. Direction: Changes in the direction causes a
reorientation of the stresses and strains within the
periodontium (lateral or horizontal forces, torque or
rotational forces are more likely to injure the
periodontium).
c. Duration: Constant pressure on the bone is more
injurious than intermittent forces.
d. Frequency: The more frequent the application of an
intermittent force, the more injurious to the
periodontium.
TRAUMA FROM OCCLUSION (TFO)
Definition and Terminology
According to Orban and Glickman et al (1968): Trauma
from occlusion is defined as, when occlusal forces exceed
the adaptive capacity of the periodontal tissues, the tissue
injury results. This resultant injury is termed as trauma
from occlusion.
WHO in 1978 defined trauma from occlusion as
“damage in the periodontium caused by, stress on the
teeth produced directly or indirectly by the teeth of the
opposing jaw”.
❒
❒
❒
❒
❒ PHYSIOLOGICAL ADAPTIVE CAPACITY
OF THE PERIODONTIUM TO OCCLUSAL
FORCES
❒
❒
❒
❒
❒ TRAUMA FROM OCCLUSION
• Definition and Terminology
• Types
• Signs and Symptoms
• Histologic Changes
• Other Properties
* Effect of Insufficient Occlusal Force
* Reversibility of Traumatic Lesion
* Effect of Increased Forces on Pulp
❒
❒
❒
❒
❒ ROLE OF THE TRAUMA FROM
OCCLUSION IN THE PROGRESSION OF
PERIODONTAL DISEASE
❒
❒
❒
❒
❒ PATHOLOGIC TOOTH MIGRATION
❒
❒
❒
❒
❒ OTHER CAUSES
9
Trauma from Occlusion

88
Essentials of Clinical Periodontology and Periodontics
Other terms often used are, traumatizing occlusion,
occlusal trauma, occlusal overload, periodontal
traumatism, occlusal disharmony, functional imbalance
and occlusal dystrophy. One must note that trauma from
occlusion refers to the tissue injury, not the occlusal force.
An occlusion that produces such an injury is called as
traumatic occlusion.
Types
i. Depending on the onset and duration.
ii. Depending on the cause:
a. Due to the alterations in the occlusal forces.
b. Reduced capacity of the periodontium.
iii. Depending on the onset and duration:
a. Acute trauma from occlusion (TFO).
b. Chronic trauma from occlusion (TFO).
Acute trauma from occlusion: Results from the
abrupt changes in the occlusal forces, such as
that produced by biting on a hard object, in
addition, could also be due to iatrogenic factors
(faulty restorations/ prosthetic appliance).
Chronic trauma from occlusion: As a result of the
gradual changes produced in the periodontium
due to the tooth wear, drifting movement, extru-
sion of the teeth combined with parafunctional
habits such as bruxism and clenching.
iv. Depending on the cause (Table 9.1):
Changes produced by primary trauma from
occlusion are usually reversible, may be because,
the supracrestal gingival fibers are not affected
and thus prevents the apical migration of junctional
epithelium.
In summary,
1. The criterion that determines whether an occlusion
is traumatic is whether it produces periodontal injury,
not how the teeth occlude.
2. Any occlusion that produces periodontal injury is
considered traumatic.
3. Malocclusion is not necessary to produce trauma.
Signs and Symptoms
1. Clinical and,
2. Radiographic changes.
Clinical Signs and Symptoms
a. In acute situations: Excessive tooth pain, tenderness
on percussion, increased tooth mobility (hyper-
mobility) is seen. In severe cases periodontal abscess
formation and cemental tears can be seen. Others
such as presence of infrabony pockets, furcation
involvement, attrition, pathologic migration may also
be present.
b. Fremitus test is positive.
c. Radiographic changes
i. Increase in the width of the periodontal ligament
space often with thickening of the lamina dura
along the lateral borders of the root, apical and
bifurcation areas.
ii. “Vertical” rather than horizontal destruction of the
interdental septum.
iii. Radiolucency and condensation of the alveolar
bone.
iv. Root resorption.
Table 9.1: Types of TFO: Depending on the cause
Primary trauma from occlusion
Secondary trauma from occlusion
It is a tissue injury, which is elicited around
It is related to situations in which occlusal forces cause
a tooth with normal height of periodontium.
injury in a periodontium of reduced height. For example:
For example: Insertion of high fillings, insertion
Periodontitis.
of the prosthetic replacement, orthodontic
movement in functionally-unacceptable positions.

89
Trauma from Occlusion
Table 9.2: Histologic changes in periodontal tissues after injury
a. Slightly-excessive pressure
Slightly-excessive tension
Changes are as follows:
1. Widening of the periodontal ligament.
1. Elongation of periodontal ligament fibers.
2. Resorption of alveolar bone called as
2. Apposition of alveolar bone.
direct bone resorption.
3. The number of blood vessels are
3. Blood vessels are enlarged and less.
increased but the size is reduced.
b. Greater pressure
Greater/severe tension
The changes in the tissues are as follows:
1. Causes widening of the periodontal ligament,
1. Compression of fibres producing areas
thrombosis, haemorrhage, tearing of periodontal ligament.
of the hyalinization
2. Injury to the cells like fibroblasts and
2. Resorption of alveolar bone.
other connective tissue cells leading to
necrosis of areas of the ligament.
3. Changes in the blood vessels-breaking
of vessel wall.
4. Increased resorption of alveolar bone.
5. Resorption of tooth surface.
c. Pressure severe enough to force the root against bone causes necrosis of periodontal ligament and bone. The bone
is resorbed from viable periodontal ligament adjacent to necrotic areas and from marrow spaces, a process called
undermining resorption or indirect bone resorption takes place. The furcation is the most susceptible area to injury
due to excessive occlusal forces.
Histologic Changes
The response of tissues to increased occlusal forces is
explained under three stages.
• Stage 1: Injury
• Stage 2: Repair
• Stage 3: Adaptive remodeling of the periodontium.
Stage 1: Injury
When a tooth is exposed to excessive occlusal forces,
the periodontal tissues are unable to withstand and hence
they distribute, while maintaining the stability of the tooth.
This may lead to certain well-defined reactions in the
periodontal ligament and alveolar bone, eventually
resulting in adaptation of the periodontal structures to
altered functional demand. When the tooth is subjected
to horizontal forces the tooth rotates or tilts in the direction
of force. This tilting results in the pressure and tension
zones, within the marginal and apical parts of the
periodontium. Depending on the types of forces there
can be many histologic changes (Table 9.2).
Stage 2: Repair
TFO stimulates increased reparative activity.
When bone is resorbed by excessive occlusal forces,
the body attempts to reinforce the thinned-bony
trabeculae with new bone. This attempt to compensate
for lost bone is called buttressing bone formation which
is an important feature of reparative process associated
with trauma from occlusion (also occurs during
inflammation or tumors).
Buttressing bone formation can occur within the jaw,
called central buttressing and on the bone surface, called
as peripheral buttressing. It usually occurs on the facial
and lingual plates of the alveolar bone, if it produces
a shelf-like thickening of alveolar bone it is referred as
lipping.
Stage 3: Adaptive Remodeling of the
Periodontium
If the repair process cannot keep pace with the destruction
caused by occlusion, the periodontium may get
90
Essentials of Clinical Periodontology and Periodontics
remodelled inorder to maintain the structural relationship.
This may result in thickened periodontal ligament, angular
defects in the bone with no pocket formation, loose teeth
and increased vascularization.
In summary, histometric changes shown during these
three stages are:
1. The injury phase shows an increase in areas of
resorption and a decrease in bone formation.
2. The repair phase shows increase in areas of bone
formation and decreased resorption.
3. Remodeling phase: return of normal resorption and
formation.
The animal experiments conducted to study the effect
of traumatic occlusion on the periodontium had raised
a lot of criticism .In these studies the teeth were subjected
to a unilateral horizontal force and the changes were
observed. However, it has been suggested that in
humans, the occlusal forces act alternatively in one and
then in the opposite direction, which is termed as jiggling
forces. It is important to note the difference in the forces
because, unilateral horizontal force creates pressure and
tension zones in the coronal and apical parts of the
periodontium, where as with jiggling forces pressure and
tension zones occur on both sides of the jiggled tooth.
Moreover, in humans the tooth rotates around a fulcrum
which in single rooted teeth is located at the junction
between the apical-third and the middle-third of the root.
In multirooted teeth the fulcrum is located at the furcation
area.
In the response to increased occlusal forces, the
periodontal ligament gradually increases in width on both
the sides of the tooth. This is associated with, (a)
Inflammatory changes in the ligament tissue, (b) Active
bone resorption. (c) Progressive mobility of the teeth.
When the effect of the forces applied has been compen-
sated by the increased width of the periodontal ligament
space, the ligament no longer shows the above mentioned
signs, except that the tooth remains hypermobile, but
the mobility is no longer of the progressive type.
Other Properties
Effect of Insufficient Occlusal Force
It may also be injurious to periodontal tissues which
results in the thinning of the periodontal, ligament,
atrophy of the fibres, osteoporosis of alveolar bone and
reduction in alveolar bone height. Hypofunction can
result from an open bite relationship, absence of
functional antagonists, or unilateral chewing habits.
Reversibility of Traumatic Lesion
Trauma from occlusion is reversible. When the injurious
force is removed, the repair occurs. The presence of inflam-
mation in the periodontium as a result of plaque accumu-
lation may impair the reversibility of traumatic lesions.
Effect of Increased Occlusal Forces on Pulp
The effects on the pulp have not been established.
ROLE OF THE TRAUMA FROM
OCCLUSION IN THE PROGRESSION OF
PERIODONTAL DISEASE
The various studies conducted on animals and humans
have shown changes produced by the pressure and
tension sides of the tooth, with an increase in the width
of the periodontal ligament and increased tooth mobility.
None of these methods have caused gingival inflam-
mation or pocket formation. This was explained by:
Glickman’s concept (1965, 1967). He claimed that
the pathway of the spread of plaque associated gingival
lesion can be changed if the forces of an abnormal
magnitude are acting on teeth harbouring subgingival
plaque. He has explained that, teeth which are nontrau-
matized exhibit suprabony pockets and horizontal bone
loss, where as teeth with trauma exhibit angular bony
defects and infrabony pockets.
According to him the periodontal structures are
divided into two zones:
1. The zone of irritation and
2. The zone of co-destruction.
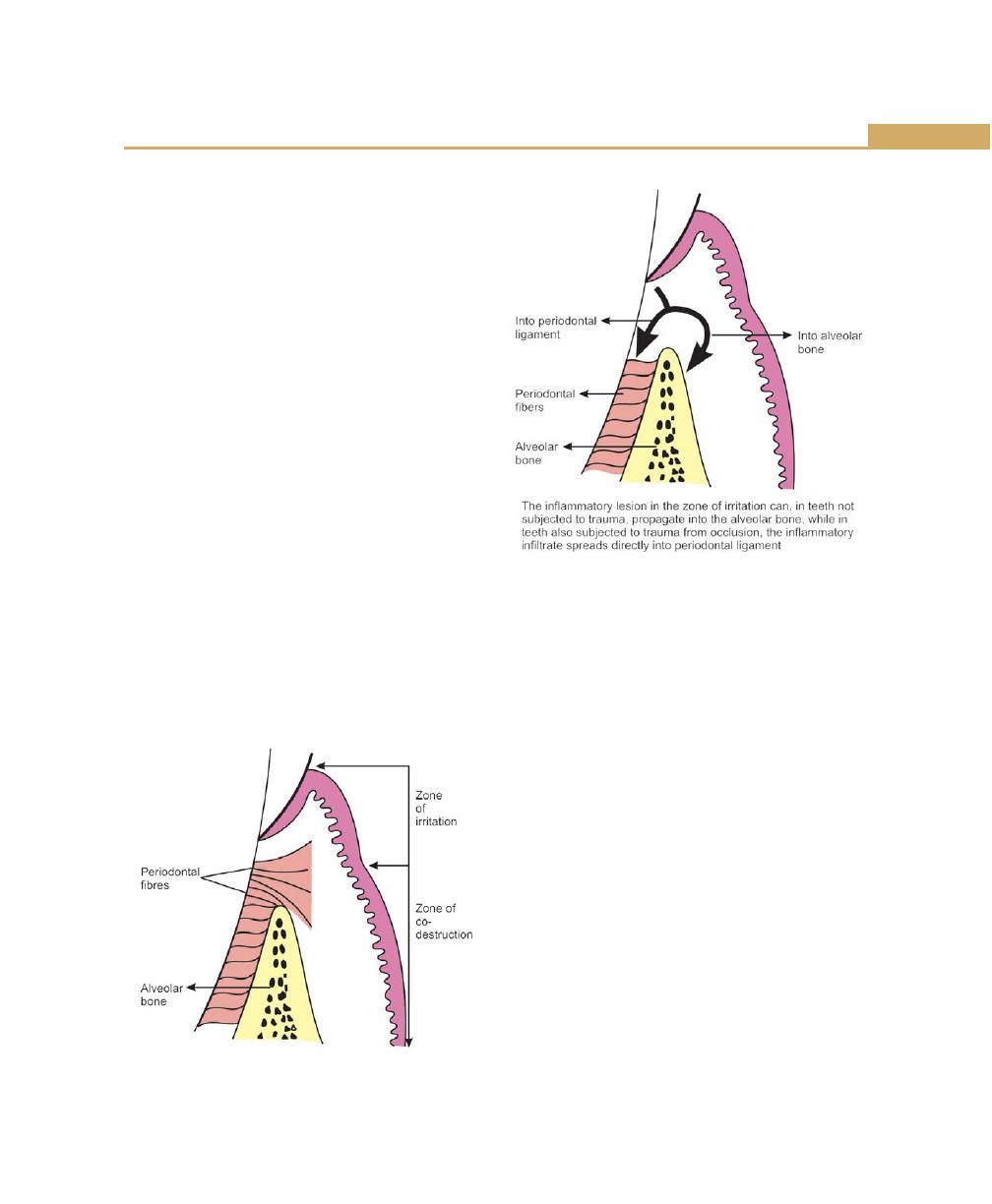
91
Trauma from Occlusion
The zone of irritation includes the marginal and
interdental gingiva, which is affected only by microbial
plaque. In a plaque associated lesion, at a “nontrau-
matized” tooth, the inflammation spreads in an apical
direction, first involving the alveolar bone and later the
periodontal ligament area. Hence there is an even
(horizontal) bone loss.
The zone of co-destruction includes the periodontal
ligament, the root cementum and the alveolar bone,
which are coronally demarcated by the transseptal and
the dentoalveolar collagen fibers. The tissue in this region
becomes the seat of a lesion caused by the trauma from
occlusion. Here the spread of inflammation is from the
zone of irritation directly down into the periodontal
ligament and hence angular bony defects with infrabony
pockets are seen (Figs 9.1 and 9.2).
The summary of this concept is that trauma from
occlusion is a co-destructive factor of importance
especially in situations where angular defects combined
with infra bony pockets are found in one or several teeth.
Waerhaug’s concept: From his similar studies he
concluded that angular defects and infrabony pockets
occur often at periodontal sites of teeth not affected by
trauma from occlusion. In other words, he refuted the
hypothesis that trauma from occlusion played a role in
the spread of a gingival lesion into the zone of co-
destruction. The loss of periodontium, according to
Waerhaug was as a result of inflammatory lesions
associated with subgingival plaque. He concluded that
angular defects occur when the subgingival plaque of
one tooth has reached a more apical level than the
microbiota on the neighbouring tooth, and when the
volume of the alveolar bone surrounding the roots is
comparatively large. This was also supported by Prichard
(1965) and Manson (1976).
In conclusion, four possibilities can occur when a tooth
with gingival inflammation is exposed to trauma.
1. Trauma from occlusion may alter the pathway of
extension of gingival inflammation to the underlying
tissues. Inflammation may proceed to the periodontal
ligament rather than to the alveolar bone and the
resulting bone loss would be angular with infrabony
pockets.
2. It may favour the environment for the formation and
attachment of plaque and calculus and may be
responsible for development of deeper lesions.
Fig. 9.1:
Zone of irritation and zone of co-destruction
Fig. 9.2:
Pathway of inflammatory process
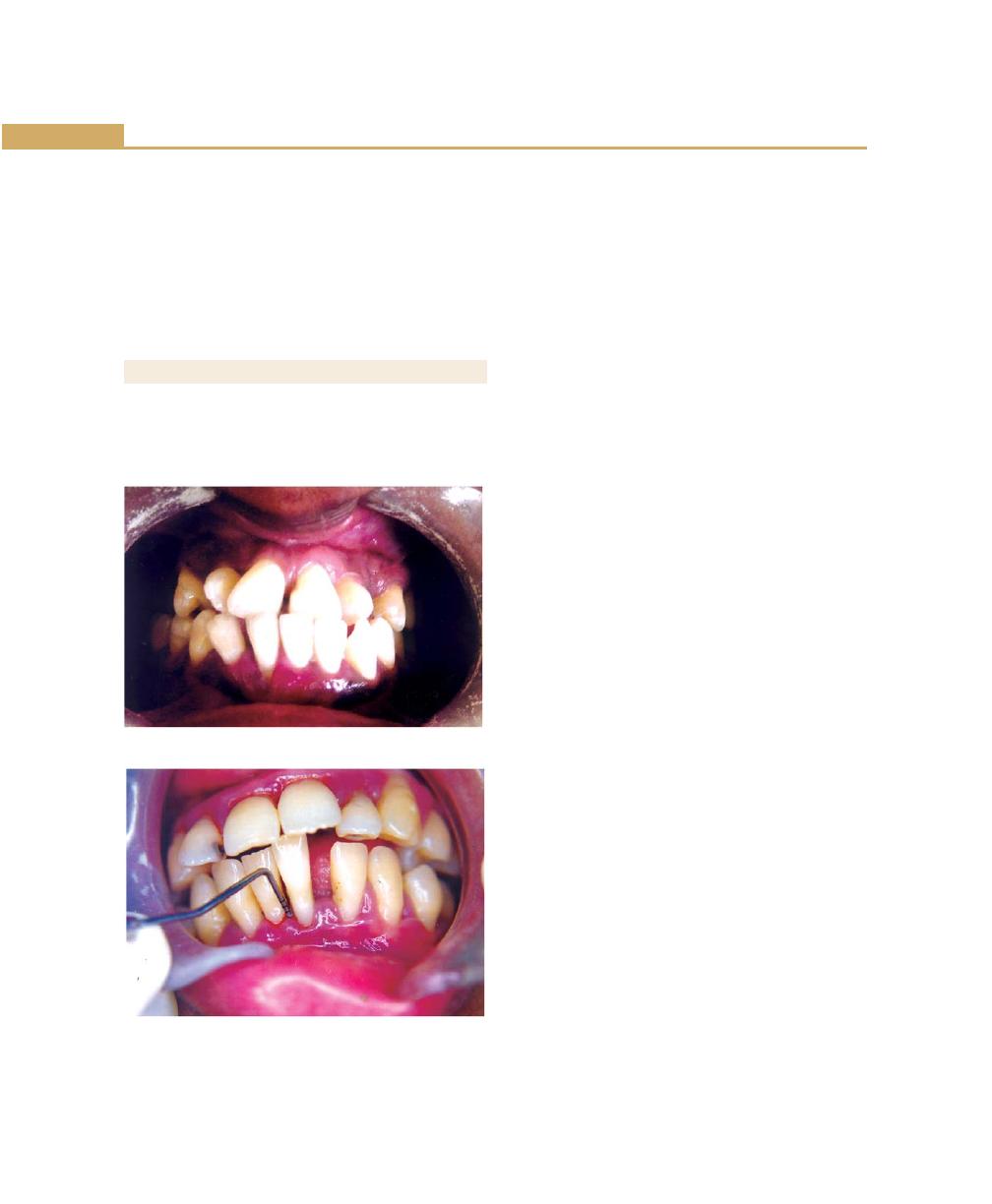
92
Essentials of Clinical Periodontology and Periodontics
3. Supragingival plaque can become subgingival if the
tooth is tilted orthodontically or migrates into an
edentulous area, resulting in the transformation of
a suprabony pocket into an infra bony pocket.
4. Increased tooth mobility associated with trauma to
the periodontium may have a pumping effect on
plaque metabolites increasing their diffusion.
PATHOLOGIC TOOTH MIGRATION
Refers to tooth displacement that results when the balance
among the factors that maintain physiologic tooth
position is disturbed by periodontal disease.
Pathologic migration occurs most frequently in the
anterior region, but posterior teeth may also be affected.
The teeth may move in any direction and the migration
is usually accompanied by mobility and rotation.
Pathologic migration in occlusal or incisal direction is called
as “extrusion” (Figs 9.3 and 9.4).
Pathogenesis
Two major factors play a role in maintaining the normal
position of the teeth.
1. The health and normal height of the periodontium.
2. The forces exerted on the teeth.
The Health and Normal Height of the
Periodontium
A tooth with weakened periodontal support is unable
to withstand the forces and moves away from the
opposing force. It is important to understand that the
abnormality in pathologic migration rests with the
weakened periodontium. The force itself need not be
abnormal. Forces that are acceptable to an intact
periodontium become injurious when periodontal
support is reduced. Pathologic migration may continue
even after a tooth no longer contacts its antagonist.
Changes in the Forces Exerted on the Teeth
Changes in the forces may occur as a result of (a)
unreplaced missing teeth, (b) Failure to replace first
molars, or (c) other causes. These forces do not have
to be abnormal to cause pathologic migration, if the
periodontium is sufficiently weakened.
a. Unreplaced missing teeth: This leads to drifting of
teeth into the spaces created by unreplaced missing
teeth. Drifting differs from pathologic migration, in
that it does not result from destruction of the
periodontal tissues. However, it usually creates
conditions that leads to periodontal diseases and thus
the initial tooth movement is aggrevated by loss of
periodontal support.
Fig. 9.3:
Pathologic migration
Fig. 9.4:
Pathologic migration associated with tongue
thrusting
93
Trauma from Occlusion
b. Failure to replace first molars: It consists of the following:
1. The second and third molars tilt resulting in
decrease in vertical dimension.
2. The premolars move distally and the mandibular
incisors tilt or drift lingually.
3. Anterior overbite is increased.
4. The maxillary incisors are pushed labially and
laterally.
5. The anterior teeth extrude due to disappearance
of incisal apposition.
6. Diastema is created by the separation of the
anterior teeth.
OTHER CAUSES
1. Pressure from the tongue: It may either have a direct
efffect, that is, it may cause drifting of teeth in the
absence of periodontal disease or may contribute to
pathologic migration of the teeth with reduced
periodontal support.
2. Pressure from the granulation tissue of periodontal
pocket: It has also been listed as a contributing factor
to pathologic migration. Usually tooth may return
to their original position after pockets are treated,
but if the destruction of the periodontium is more
severe on one side of a tooth rather than the other,
the healing tissue may pull the tooth in the direction
of lesser destruction.
3. To summarize, trauma from occulsion does not have
any:
a. Effect on the supracrestal gingival tissue.
b. Human and animal studies have shown neither
unilateral nor jiggling force can result in pocket
formation.
c. Bone resorption and increased mobility in the
absence of the pockets can be associated with
trauma from occlusion.
d. In cases of teeth affected by periodontal disease,
trauma from occlusion may aggravate the rate
of progression of the disease and can also act as
a co-factor in tissue destruction.
KEY POINTS TO NOTE
1. When occlusal forces exceed the adaptive capacity of the
periodontal tissues, tissue injury results. This resultant injury
is termed as “trauma from occlusion”.
2. Depending on the onset of duration trauma from the
occlusion (TFO) may be divided into, acute trauma from
occlusion, chronic trauma from occlusion. Depending on
the cause, primary trauma from occlusion, secondary trauma
from occlusion.
3. Primary trauma from occlusion is a tissue injury, which is
elicited around a tooth with a normal height of periodontium
whereas secondary trauma from occlusion is related to
situations in which occlusal forces cause injury in a
periodontium of reduced height.
4. Role of trauma from occlusion in the progression of
periodontal disease is explained by two concepts, (a)
Glickman’s concept supports trauma from occlusion that is
a co-destructive factor of importance, especially in situations
where angular defects combined with infrabony pockets are
found in one or several teeth where as Waerhaug’s concept
refutes this hypothesis and proposes that, angular defects
and infrabony pockets occur often at sites not affected by
trauma from occlusion. He proceeds to explain, that, angular
defects occur, when the subgingival plaque of one tooth
reaches a more apical level than the plaque on the
neighbouring tooth and also when the volume of the
alveolar bone surrounding the roots is comparatively large.
5. Pathologic tooth migration refers to tooth displacement that
results when the balance among the factors that maintain
physiologic tooth position is disturbed by periodontal
diseases.
REVIEW QUESTIONS
1. Define trauma from the occlusion. Describe the clinical
and radiographic changes associated with trauma
from occlusion.
2. What is the role of trauma from occlusion in the
progression of periodontal diseases ?
3. What are the causes for pathologic tooth migration ?
4. Differences between primary trauma from occlusion
and secondary trauma from occlusion.
BIBLIOGRAPHY
1. Burgett FG. Trauma from occlusion, periodontal concerns.
Dental Clinics of North America 1995;39(2): 301.

94
Essentials of Clinical Periodontology and Periodontics
2. Elizabeth A Pawlak, Philip M Hoag. Essentials of Periodontics.
Mosby, Jaypee Brothers.
3. Gher ME. Changing concepts. The effect of occlusion on
periodontics. Dental Clinics of North America 1998;
42(2):285.
4. Jan lindhe. Clinical periodontology and Implant dentistry, IV
edition 2003. BlackWell Munksgaurd Publication.
5. JD Manson, BM Eley. Outline of periodontics, III edition, KM
Varghese Company.
6. Newman, Takei, Fermin A Carranza. Clinical periodontology,
9th edn, WB Saunders Co., 2002.
7. Robert J Genco, Henry M Goldman, D Walter Cohen.
Contemporary periodontics, CV Mosby Company, 1990.
95
Role of Systemic Diseases in the Etiology of Periodontal Diseases
INTRODUCTION
It is a well established fact that the primary etiological
agent in periodontal disease is bacterial plaque. The toxins
and enzymes produced by the bacterial plaque elicit
inflammatory and immunologic changes in the
periodontal tissues at both cellular and molecular levels.
These responses can be affected by a variety of systemic
factors that can alter the response of the tissue to plaque.
Further more, certain systemic disorders can have a direct
effect on the periodontal tissues and these represent the
periodontal manifestations of systemic diseases. In general
these diseases do not initiate chronic destructive
periodontitis but may accelerate its progression and
increase tissue destruction.
DIETARY AND NUTRITIONAL ASPECTS OF
PERIODONTAL DISEASE
The vitality of periodontal tissues, in both health and
disease depends strongly on the essential nutrients. The
epithelium of the dentogingival junction and the under-
lying connective tissues are the most dynamic tissues.
The majority of opinions and research findings point
to the following:
1. Nutritional deficiencies produce changes in the oral
cavity.
2. There are no nutritional deficiencies that by them-
selves cause gingivitis or periodontal pockets.
The Consistency of Diet
From the viewpoint of promoting and maintaining
gingival and periodontal health it is often stated that a
firm and fibrous diet is more beneficial than an intake
of soft and more loosely-textured food. Softer diets tend
to produce greater deposits of plaque and an increase
in plaque can be noticed, when the soft diet especially
contains high proportion of sucrose. Diets that are
predominantly fibrous are considered advantageous as
they posses the ability to impart a natural cleansing action
to the teeth and the periodontium.
❒
❒
❒
❒
❒ DIETARY AND NUTRITIONAL ASPECTS
OF PERIODONTAL DISEASE
❒
❒
❒
❒
❒ EFFECTS OF HEMATOLOGICAL
DISORDERS ON PERIODONTIUM
❒
❒
❒
❒
❒ METABOLIC AND ENDOCRINE
DISORDERS
❒
❒
❒
❒
❒ CARDIOVASCULAR DISORDERS
❒
❒
❒
❒
❒ ANTIBODY DEFICIENCY DISORDERS
❒
❒
❒
❒
❒ OTHER SYSTEMIC DISEASES
❒
❒
❒
❒
❒ PSYCHOSOMATIC DISORDERS
10
Role of Systemic Diseases
in the Etiology of
Periodontal Diseases

96
Essentials of Clinical Periodontology and Periodontics
A coarse diet, requires vigorous mastication and the
plaque that forms approximately tends to be towards
the cleansable buccal and lingual surfaces of the teeth.
However coarse and granular diets can predispose to
a direct traumatic injury to the supporting tissues.
Protein Deficiency and Periodontal
Disease
Proteins are constituents of the organic matrices of all
the dental tissues including the alveolar bone. The
integrity of the periodontal ligament is also dependent
upon proteins (amino acid). Studies have indicated that
on deprivation of proteins, extreme pathologic changes
occur and there is marked degeneration of periodontal
support.
Vitamins and Periodontal Disease
Vitamins are essential, biologically-active constituents of
a diet which cannot be replaced by other dietary
components.
Vitamin C
Its deficiency in humans results in scurvy, a disease
characterized by hemorrhagic diathesis and retardation
of wound healing.
Clinical manifestations
1. Increased susceptibility to infections.
2. Impaired wound healing.
3. Bleeding and swollen gums.
4. Mobile teeth.
Histopathological features
1. Defective formation and maintenance of collagen.
2. Retardation or cessation of osteoid formation and
impaired osteoblastic function.
3. Increased capillary permeability.
4. Susceptibility to traumatic hemorrhage.
5. Hyporeactivity of contractile elements of the peri-
pheral blood vessels.
6. Sluggishness of blood flow.
Possible Etiologic Relationships between
Ascorbic Acid and Periodontal Disease
1. Low levels of Ascorbic acid influences the metabolism
of collagen within the periodontium, thereby affecting
the ability of the tissue to regenerate and repair by
itself.
2. It interferes with bone formation leading to the loss
of the alveolar bone.
3. Increases the permeability of oral mucosa to tritiated
endotoxin and inulin.
4. Increased levels of Ascorbic acid enhances both the
chemotactic and migratory action of leukocytes
without influencing phagocytic activity.
5. Optimal level of Ascorbic acid is required to maintain
the integrity of the periodontal microvasculature as
well as the vascular response to bacterial irritation
and wound healing.
6. Depletion of vitamin C may interfere with the ecologic
equilibrium of bacteria in plaque and increases its
pathogenicity.
Periodontal Features of Scurvy
The oral symptoms are that of chronic gingivitis which
can involve the free gingiva, attached gingiva and alveolar
mucosa. In severe cases the gingiva becomes brilliant-
red, tender and grossly swollen. The spongy tissues are
extremely hyperemic and bleed spontaneously. In long
standing cases the tissues attain a dark blue or purple
hue. Alveolar bone resorption with increased tooth
mobility has also been reported.
Vitamin D Deficiency
Vitamin D is essential for the absorption of calcium from
the gastrointestinal tract and the maintenance of calcium-
phosphorus balance.
Radiographically, there is a generalized partial to
complete disappearance of the lamina dura and reduced
density of supporting bone, loss of trabeculae, increased
radiolucency of the trabecular interstices and increased
prominence of the remaining trabeculae.
97
Role of Systemic Diseases in the Etiology of Periodontal Diseases
Vitamin E
Evidence suggests that vitamin E acts as a antioxidant
and plays an important role in maintaining the stability
of cell membranes and protecting blood cells against
hemolysis.
The possible role is based upon its ability to interfere
with the production of prostaglandins.
Vitamin A
It is essential for normal functions of the retina, for growth,
differentiation and maintenance of epithelial tissues and
for bone growth and embryonic development.
Vitamin B-Complex
Oral disease is rarely due to a deficiency in just one
component of the B-complex group. Oral changes
common to—Vitamin B-complex deficiencies are
gingivitis, glossitis, glossodynia, angular cheilitis and
inflammation of the entire oral mucosa.
EFFECTS OF HEMATOLOGICAL
DISORDERS ON PERIODONTIUM
Disorders of the blood and blood forming tissue can
have a profound effect on the periodontal tissues and
their response to bacterial plaque. The WBC disorders
have the most pronounced effect on the periodontal
tissues. Disorders of hemostasis can be classified according
to the underlying defect. There can be a defect in the
vascular constriction, platelet adhesion and aggregation,
coagulation and fibrinolysis.
White Blood Cell Disorders
The WBC’s disorders that affect the periodontium can
be categorized as either a disorder of numbers or defect
in function.
Neutropenias
a. Cyclic neutropenia.
b. Chronic benign neutropenia of childhood.
c. Benign familial neutropenia.
d. Severe familial neutropenia.
e. Chronic idiopathic neutropenia.
Cyclic Neutropenia
It is characterized by a cyclic depression of the PMN count
in peripheral blood. The cyclic intervals are usually
between 19 and 21 days. Clinical problems include
pyrexia, oral ulceration and skin infections.
Periodontal manifestations include oral ulceration,
inflamed gingiva, rapid periodontal breakdown, and
alveolar bone loss. Bone loss is most obvious around
the lower incisors and first permanent molars.
Treatment: Plaque control, supportive measures like
antiseptic mouth wash, antimicrobial therapy has been
proposed.
Chronic Benign Neutropenia of Childhood
The onset is usually between 6 to 20 months of age
and in most patients, the condition is self-limiting.
The main periodontal feature is a bright-red,
hyperplastic, edematous gingiva confined to the width
of attached gingiva. The gingival tissues exhibit bleeding
on probing and show areas of desquamation, varying
degrees of gingival recession and pocketing are seen.
Treatment: Appropriate antimicrobial agent should
be prescribed.
Benign Familial Neutropenia
It is transmitted as an autosomal dominant trait. The
periodontal manifestations include hyperplastic gingivitis
exhibiting edematous and bright-red appearance. There
is marked bone loss around the first molars. The gingival
tissues bleed profusely on probing.
Treatment: Plaque control and use of antimicrobial
mouth washes.
Chronic Idiopathic Neutropenia
There is a persistent neutropenia from birth and is not
cyclical. Clinical symptoms include persistent recurrent
infections throughout the patient’s life.
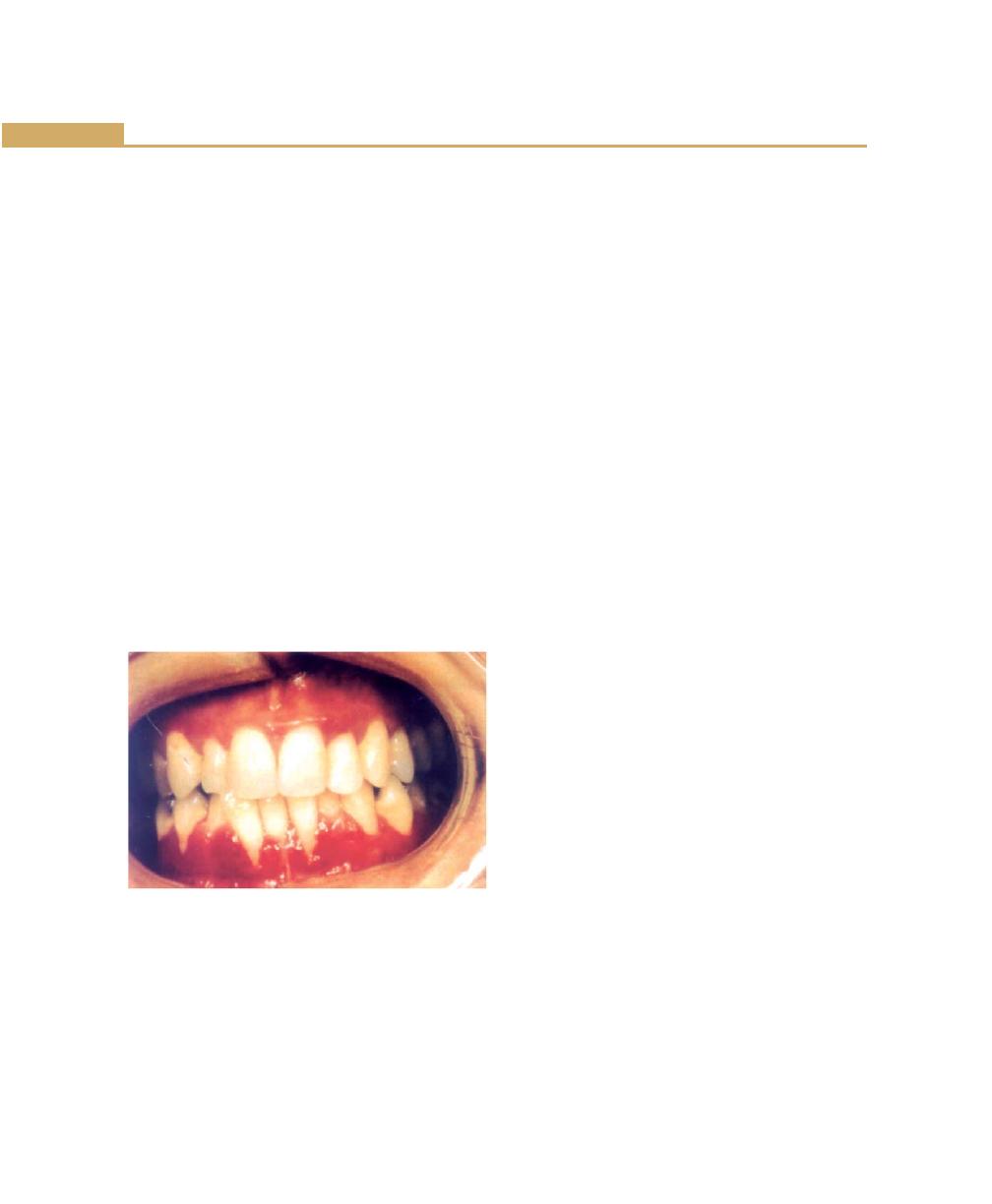
98
Essentials of Clinical Periodontology and Periodontics
Periodontal manifestations include persistent severe
gingivitis. The gingiva is cherry-red edematous and
hypertrophic with occasional desquamation.
Treatment: Strict oral hygiene programme, scaling
and regular prophylaxis. Antiseptic irrigation and
antibiotic prophylaxis are advisable before tissue
manipulation.
Leukemia
It is a malignant disease caused by proliferation of WBC
forming tissue, especially those in bone marrow. Acute
leukemia is more frequent in people under 20 years of
age. Chronic leukemia’s occur in people over 40 years
of age.
Periodontal Manifestations
The major manifestations being gingival enlargement,
gingival bleeding and periodontal infections. The
incidence and severity of these problems varies according
to the type and nature of leukemia (Fig. 10.1).
b. Gingival bleeding is a common oral manifestation of
acute leukemia. The bleeding is secondary to
thrombocytopenia that accompanies leukemia.
c. Infections of the periodontal tissues secondary to
leukemia can be of two types, either an exacerbation
of an existing periodontal disease or an increased
susceptibility of the periodontium to fungal, viral or
bacterial infections.
Treatment Plan for Leukemic Patients
1. Refer the patient for medical evaluation and
treatment.
2. Prior to chemotherapy, a complete periodontal plan
should be developed.
a. Monitor hematologic laboratory values.
b. Administer suitable antibiotics before any
periodontal treatment.
c. Periodontal treatment consist of scaling and root
planing, twice daily rinsing with 0.12 percent
chlorhexidine gluconate is recommended. If there
is irregular bleeding time, careful debridement
with cotton pellets soaked in 3 percent hydrogen
peroxide is performed.
3. During the acute phases of leukemia:
a. Cleanse the area with 3 percent hydrogen
peroxide (H
2
O
2
) or 0.12 percent chlorhexidine.
b. Carefully explore the area and remove any
etiologic local factors.
c. Re-cleanse the area with 3 percent H
2
O
2
.
d. Place a cotton pellet soaked in thrombin against
the bleeding point.
e. Cover with gauze and apply pressure for 15 to
20 minutes.
f. Acute gingival or periodontal abscesses are treated
by systemic antibiotics, gentle incision and
drainage or by treating with 3 percent H
2
O
2
/0.12
percent chlorhexidine gluconate.
g. Oral ulcerations should be treated with antibiotics
and bland mouth rinses.
Fig. 10.1: Gingival changes associated with leukemia
a. Gingival enlargement is primarily due to a massive
leukemic cell infiltration into the gingival connective
tissue. The enlarged gingiva will hinder mechanical
plaque removal; hence there will be an inflammatory
component enhancing this enlargement.
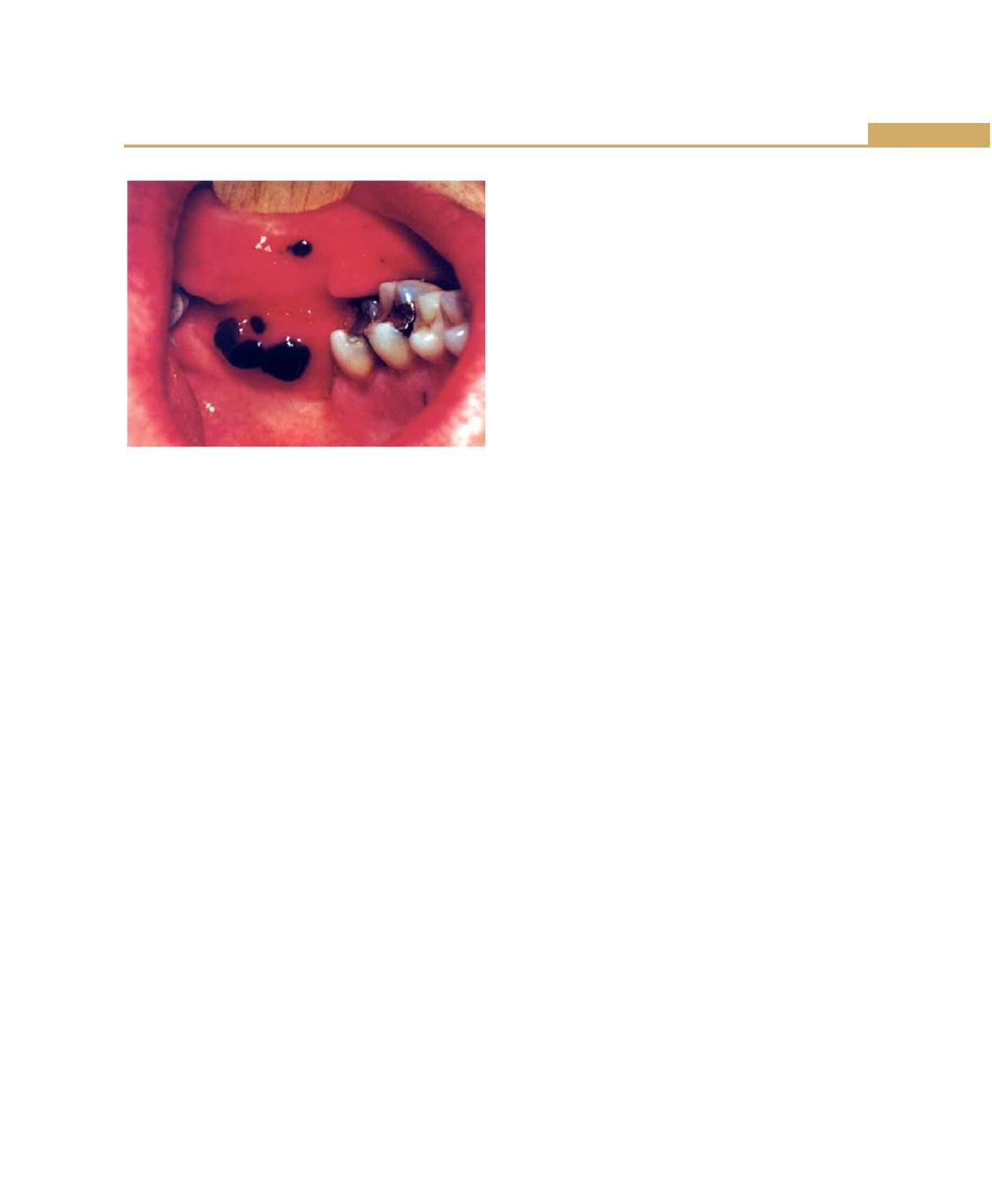
99
Role of Systemic Diseases in the Etiology of Periodontal Diseases
4. In patients with chronic leukemia scaling and root
planing can be performed but periodontal surgery
should be avoided. Plaque control and frequent recall
visits should receive particular attention.
Thrombocytopenic Purpura
It is characterized by a low platelet count, a prolonged
clot retraction and bleeding time, and a normal or slightly-
prolonged clotting time.
Clinical manifestations include spontaneous bleeding
into skin or from mucous membranes. Petechiae and
hemorrhagic vesicles occur in the oral cavity. Gingiva
is swollen, soft and friable. Bleeding occurs spontaneously
(Fig. 10.2).
Treatment
1. Physician referral for a definitive diagnosis.
2. Oral hygiene instructions.
3. Prophylactic treatment of potential abscesses.
4. No surgical procedures are indicated unless platelet
count is at least 80,000 cells/mm
3
.
5. Scaling and root planing may be carefully performed
at low platelet levels.
If surgery is indicated, it should be as atraumatic as
possible, stents or thrombin-soaked cotton pellets placed
interproximally, gentle hydrogen peroxide mouth washes
and close postsurgical follow-up is recommended.
Disorders of WBC Function
Chédiak-Higashi Syndrome
It is a rare familial and often fatal disease which is
transmitted as an autosomal recessive trait. PMNL’s
from patients with this syndrome show defective
migration, defective chemotaxis, failure of post-
phagocytic degranulation and diminished intracellular
bactericidal capacity.
Severe gingival inflammation appears to be a
common finding in Chédiak-Higashi syndrome. The
nature of the inflammatory changes may be plaque
induced, secondary to infection or related to the
underlying PMNL’s defect.
Lazy Leukocyte Syndrome
The feature of the syndrome is a defect in leukocyte
chemotaxis and random mobility. Marked gingivitis has
also been described.
Chronic Granulomatous Disease
A genetically-transmitted disorder characterized by the
inability of phagocytic cells to destroy certain infecting
micro-organisms.
Periodontal manifestations include marked, diffuse,
gingivitis with an accompanying ulceration of buccal
mucosa.
Red Blood Cell Disorders
There are many types of red blood cell disorders but
only a few appear to have any effect on the periodontal
tissues.
Aplastic Anemia
It is a bone marrow disorder characterized by a reduction
in hematopoietic tissue, bone marrow is replaced with
fat and pancytopenia. Bleeding from the gingival margins
appears to be a feature in these cases.
Fig. 10.2: Thrombocytopenic purpura with petechiae and
hemorrhagic vesicles in the lining mucosa
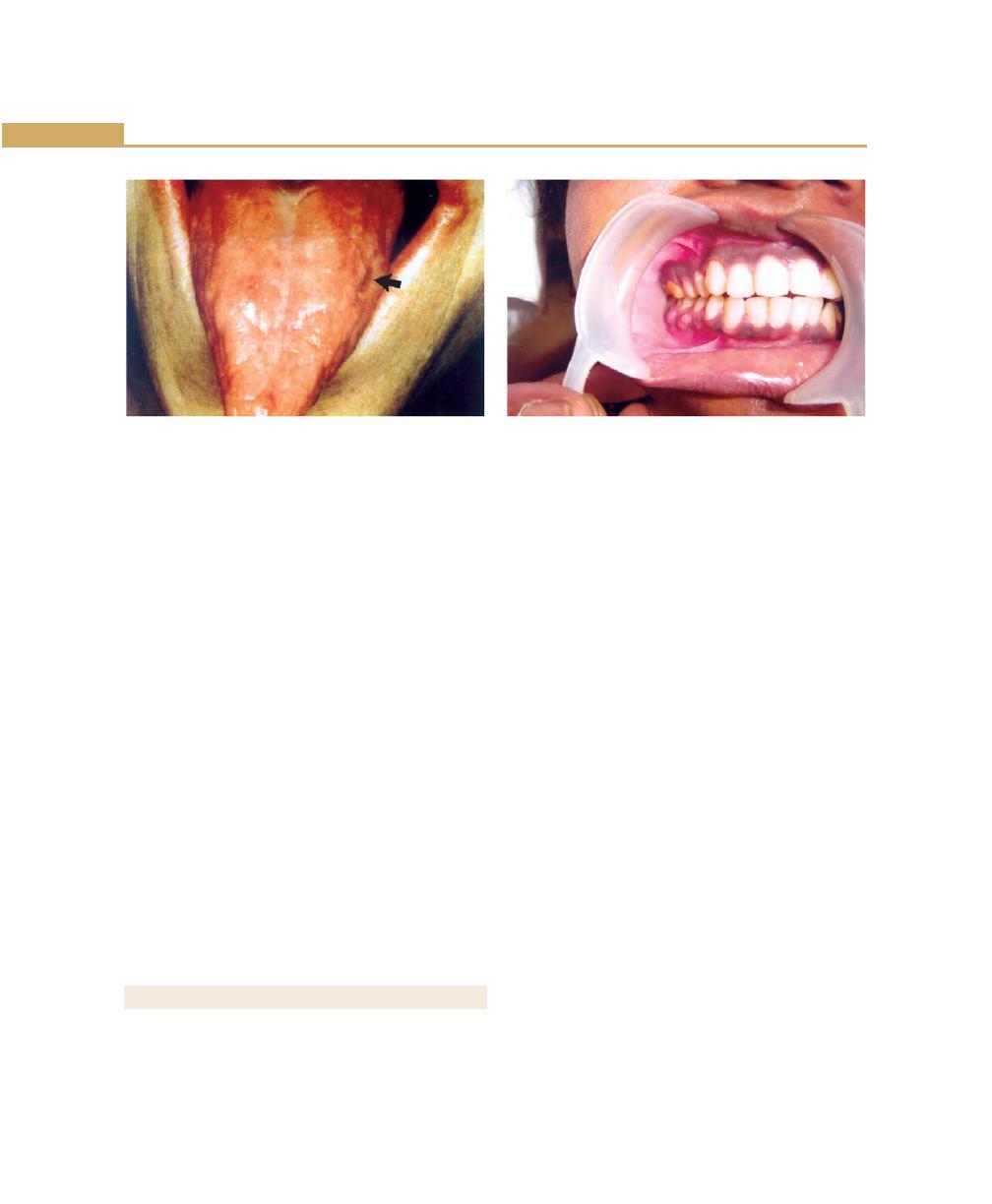
100
Essentials of Clinical Periodontology and Periodontics
periodontium. The sex hormone can alter the response
of periodontal tissues to plaque. Disorders of the pituitary,
thyroid and adrenal glands have little direct effect on
the periodontal structures or in altering the host response
to bacterial plaque.
Diabetes Mellitus and Periodontal Disease
Studies suggested that diabetic patient is more suscep-
tible to periodontal breakdown, which is characterized
by extensive bone loss, increased tooth mobility,
widening of periodontal ligament, suppuration and
abscess formation (Fig. 10.4).
Pathogenesis
There are several underlying factors that accompany
diabetes mellitus which may account for the apparent
increased prevalence of periodontal disease in this
condition. These factors can be considered under the
following headings.
1. Vascular changes: Changes include thickening and
hyalinization of vascular walls, PAS-positive, diastase-
resistant thickening of capillary basement membranes,
swelling and occasional proliferation of the endothelial
cells, and splitting of capillary basement membrane.
Diabetic-induced changes in the capillary basement
membrane may have an inhibitory effect on the
transport of oxygen, white blood cells, immune
factors and waste products all of which could affect
tissue repair and regeneration.
Fig. 10.3: Pernicious anemia
Fanconi’s Anemia
This is a rare type of aplastic anemia characterized by
a familial bone marrow hypoplasia that becomes
manifested in the first decade of life. The periodontal
manifestations being loss of several teeth, severe bone
loss with pocketing in excess of 10 mm. The gingiva will
be bluish-red, bleed on probing, and shows suppuration
on gentle pressure.
Sickle Cell Anemia
In this condition, the red blood cells undergoes sickling
when subjected to hypoxia. Hence patients with sickle
cell anemias are susceptible to infections. In some patients
with sickle cell anemia, periodontal disease may provide
a sufficient inflammatory response to precipitate a sickling
crisis.
Acatalasia
It is caused by a lack of the enzyme catalase in many cells,
especially the red blood cells and leukocytes. It causes
hypoxia and necrosis of the gingival tissues. Severe
periodontal destruction and gingival necrosis are seen.
METABOLIC AND ENDOCRINE DISORDERS
The endocrine glands produce hormones that control
metabolism and maintain hemostasis. Diabetes mellitus
is the main endocrine disorder that affects the
Fig. 10.4: Diabetic patient—multiple periodontal abscess

101
Role of Systemic Diseases in the Etiology of Periodontal Diseases
2. PMNL’s function: Impairment of PMN function is a
feature of diabetes mellitus. Disorders include
reduced phagocytosis and intracellular killing,
impaired adherence and impaired chemotactic
response. Suggested causes include inhibition of the
glycolytic pathway with the PMNL’s, abnormal cyclic
nucleotide metabolism, which disrupts the
organization of microtubules and microfilaments, or
a reduction in leukocyte membrane receptors.
3. Biochemistry of crevicular fluid: Alterations in the
constituents and flow rate of crevicular fluid have
been shown to be associated with diabetes. Cyclic
AMP levels seems to be reduced in the diabetes group
when compared with control.
4. Changes in plaque microflora: Studies have indicated
that proteolytic activity has not been altered but
hyaluronidase activity is lower in plaque from
diabetes.
Treatment
a. Periodontal treatment in patient with uncontrolled-
diabetes is contraindicated.
b. If suspected to be a diabetic, following procedures
should be performed
1. Consult the patients physician
2. Analyze laboratory tests, fasting blood glucose,
post-prandial blood glucose, glycated, hemoglobin,
glucose tolerance test (GTT), urinary glucose.
3. If there is periodontal condition that requires
immediate care, prophylactic antibiotics should
be given.
4. If patient is a ‘brittle’ diabetic, optimal periodontal
health is a necessity. Glucose levels should be
continuously monitored and periodontal treat-
ment should be performed when the disease is
in a well-controlled state. Prophylactic antibiotics
should be started 2 days preoperatively, Penicillin
is the drug of first choice.
Guidelines
1. Clinician should make certain that the prescribed
insulin has been taken followed by a meal. Morning
appointments are ideal, after breakfast because of
optimal insulin levels.
2. After any surgical procedures, postoperative insulin
dose should be altered.
3. Tissues should be handled as atraumatically and as
minimally (less than 2 hours) as possible. For anxious
patient’s preoperative sedation is required,
epinephrine concentration should not be greater than
1:1,00,000.
4. Diet recommendation should be made.
5. Antibiotic prophylaxis is recommended for extensive
therapy.
6. Recall appointments and fastidious home oral care
should be stressed.
Thyroid Gland
Hypothyroidism leads to cretinism in children and
myxedema in adults. There are no notable periodontal
changes.
Treatment
1. Patients with thyrotoxicosis and those with inadequate
medical management should not receive periodontal
therapy until the condition is stabilized.
2. Medications such as epinephrine, atropine and other
pressor amines should be given with caution.
3. Hypothyroid patients require careful administration
of sedatives and narcotics because of their diminished
ability to tolerate drugs.
Pituitary Gland
Hyperpituitarism causes enlarged lips; localized areas of
hyper-pigmentation are seen along nasolabial folds. It
is also associated with food impaction and hyper-
cementosis is seen.
Hypopituitarism leads to crowding and malposition
of teeth.
Parathyroid Glands
Parathyroid hypersecretion produces generalized
demineralization of the skeleton. Oral changes include
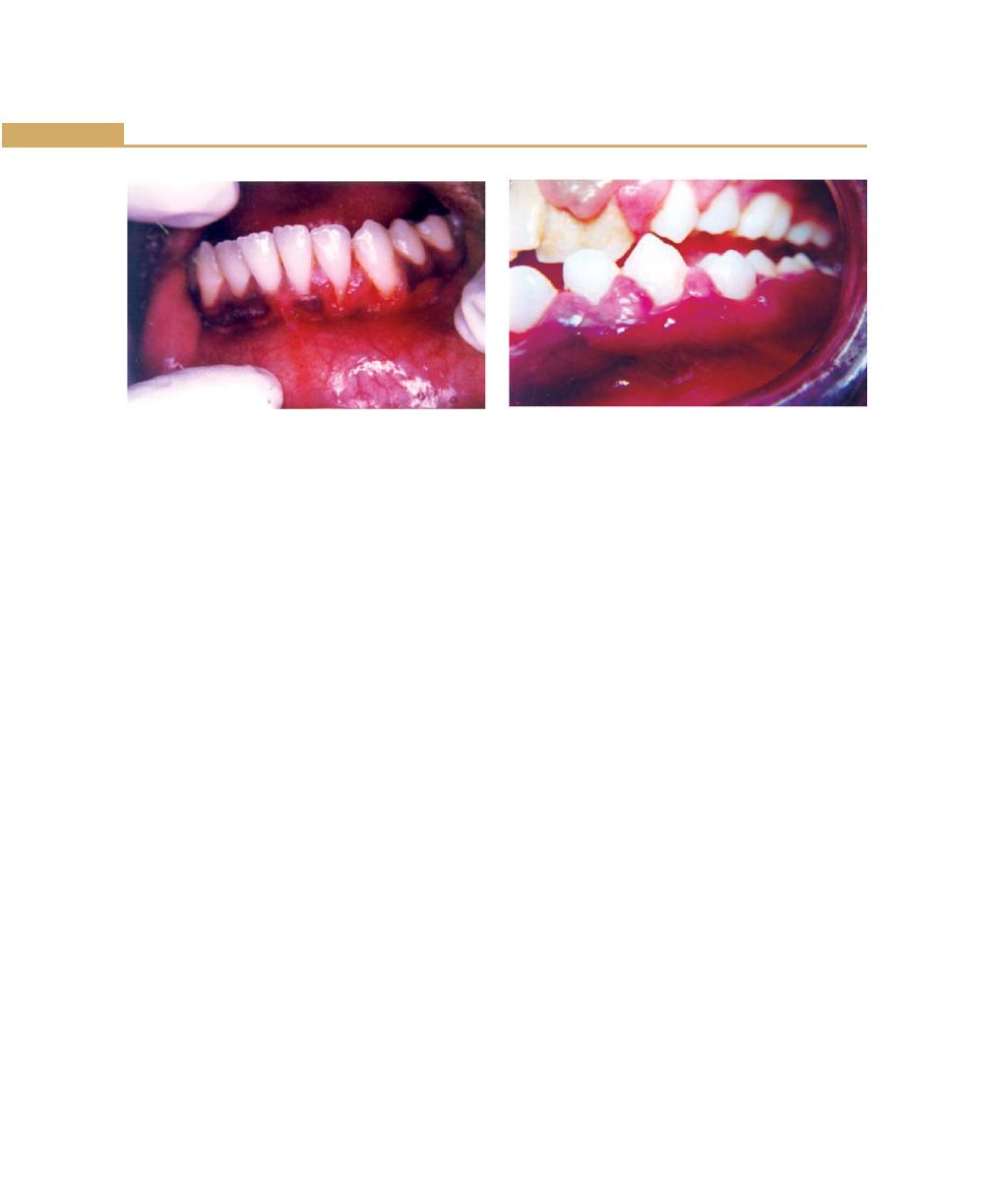
102
Essentials of Clinical Periodontology and Periodontics
malocclusion and tooth mobility, radiographic evidence
of alveolar osteoporosis, widening of the periodontal
space and absence of lamina dura.
Treatment: Routine periodontal therapy must be
instituted but the dental practitioner must be attuned
to the oral and dental changes that occur.
Gonads
There are several types of gingival diseases in which
modification of the sex hormones is considered to be
either an initiating or complicating factor; gingival
alterations are associated with physiologic hormonal
changes with a predominant marked hemorrhagic
tendency.
Gingiva in Puberty (Fig. 10.5)
Pronounced inflammation, bluish-red discoloration,
edema and enlarged gingiva may be seen.
Treatment: It is treated by scaling and curettage,
removal of all sources of irritation and plaque control.
In severe cases, surgical removal of enlarged tissue may
be required.
Gingival Changes Associated with Menstrual Cycle
There is increased prevalence of gingivitis, bleeding
gingiva. Exudation from inflamed gingiva is also increased,
but the crevicular fluid is not affected. The salivary
bacterial count is increased. No active treatment is
required.
Gingival Diseases in Pregnancy (Fig. 10.6)
Pregnancy accentuates the gingival response to plaque.
The severity of gingivitis is increased during pregnancy
beginning, in the second or third month. It becomes
more severe by the eight-month and decreases during
ninth month.
Clinical features
1. Pronounced base of bleeding.
2. Gingiva is bright-red to bluish-red.
3. Marginal and interdental gingiva is edematous, pits
on pressure and sometime presents raspberry like
appearance.
4. It has been suggested that during pregnancy there
is depression of maternal T-lymphocyte response.
5. Aggravation of gingivitis has been attributed
principally to increased levels of progesterone which
produces dilatation and tortuosity of the gingival
microvasculature, circulatory stasis and increased
susceptibility to mechanical irritation.
6. Increased crevicular fluid flow, pocket depth and
mobility are also seen.
Treatment: Requires elimination of all local irritants
that are responsible for precipitating gingival changes.
Fig. 10.5: Gingivitis in puberty with edema, discoloration
and gingival enlargement
Fig. 10.6: Gingiva in pregnancy showing edema,
discoloration and enlargement

103
Role of Systemic Diseases in the Etiology of Periodontal Diseases
Marginal and interdental gingival inflammations and
enlargement are treated with scaling and root planing.
Treatment of tumor-like gingival enlargements
consists of surgical excision, scaling and planing of tooth
surfaces. In pregnancy emphasis should be on:
• Preventing gingival disease before it occurs.
• Treating existing gingival disease before it becomes
worse.
Menopausal Gingivostomatitis
It occurs during menopause or in the postmenopausal
period. Clinical manifestations include dry, shiny oral
mucosa, dry burning sensation of oral mucosa, abnormal
taste sensation described as salty, peppery or sour.
CARDIOVASCULAR DISEASES
Arteriosclerosis
In aged individuals arteriosclerotic changes in the
blood vessels are characterized by, initial thickening,
narrowing of lumen, thickening of media, and
hyalinization of media and adventitia, with or without
calcification are common.
Congenital Heart Disease
In cases of tetralogy of fallot, oral changes include a
purplish-red discoloration of the lips and gingiva and
sometimes severe marginal gingivitis and periodontal
destruction. The tongue appears coated, fissured and
edematous and there is extreme reddening of the
fungiform and filliform papillae.
ANTIBODY DEFICIENCY DISORDERS
Acquired Immunodeficiency Syndrome
It is caused by a persistent HIV virus and is characterized
by destruction of lymphocytes, rendering the patient
susceptible to opportunistic infections including
destructive periodontal lesions.
Clinical Manifestations
HIV gingivitis: Persistent, linear, easily bleeding,
erythematous gingivitis has been described. Linear
gingivitis lesions may be localized or generalized in nature.
The erythematous gingivitis may be limited to marginal
tissue, or extend into attached gingiva in a punctuate
or a diffuse erythema or extend into alveolar mucosa.
A severely destructive, acutely painful necrotizing
ulcerative stomatitis has been reported.
HIV periodontitis: NUP (Necrotizing ulcerative
periodontitis) is characterized by soft tissue necrosis and
rapid periodontal destruction that results in marked inter-
proximal bone loss. It is severely painful at onset.
Treatment: Recommended management for linear
gingival erythema is as follows:
a. Instruct the patient to perform meticulous oral
hygiene.
b. Scale and polish affected areas and perform
subgingival irrigation with chlorhexidine.
c. Prescribe chlorhexidine gluconate mouth rinse
d. Reevaluation and frequent recall visits
e. Systemic antibiotics such as metronidazole or
amoxicillin should be prescribed for patients with
moderate to severe tissue destruction. Use of
prophylactic antifungal medication should be
considered.
In summary, treatment for necrotizing ulcerative
stomatitis includes prescription of an antibiotic such as
metronidazole or amoxicillin and use of an antimicrobial
mouth rinse.
Treatment for necrotizing ulcerative periodontitis
includes local debridement, scaling and root planing,
irrigation with Betadine solution and establishment of
meticulous oral hygiene, including home use of
antimicrobial rinses. In severe NUP antibiotic therapy
is a must (metronidazole 400 mg thrice daily for 5 to
7 days).
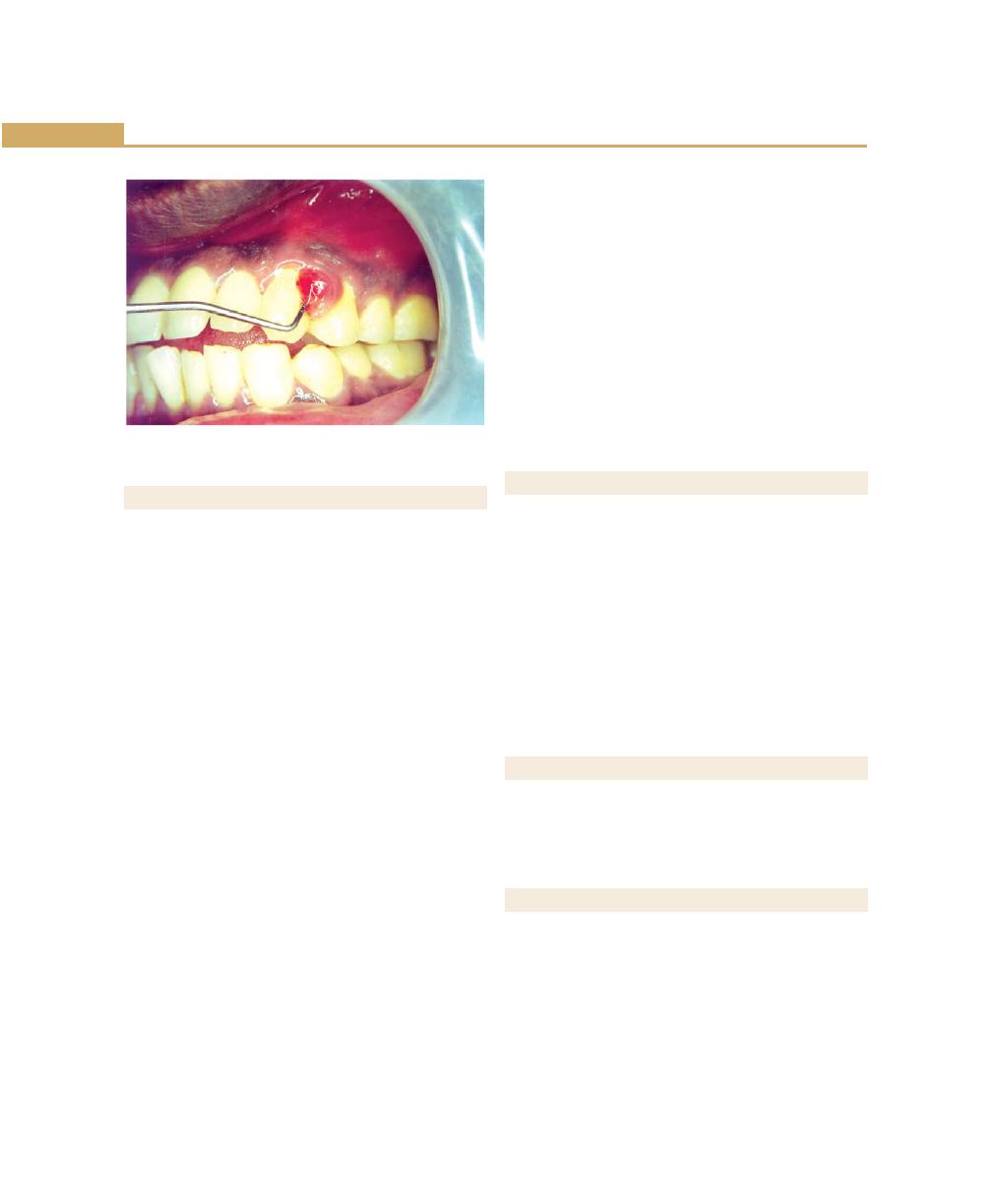
104
Essentials of Clinical Periodontology and Periodontics
OTHER SYSTEMIC DISEASES
Ingestion of metals such as mercury, lead, bismuth may
result in oral manifestations owing to their intoxication
or absorption without evidence of toxicity.
Bismuth Intoxication
Chronic bismuth intoxication is characterized by
gastrointestinal disturbances, nausea, vomiting and
jaundice as well as by an ulcerative gingivostomatitis.
Generally pigmentation is accompanied by a metallic
taste and a burning sensation of the oral mucosa. The
tongue may be sore and inflamed. Utricaria,
exanthematous eruptions of different types, bullous and
purpuric lesions are among the dermatologic lesions that
are attributed to bismuth intoxication.
It usually appears as a narrow, bluish-black discolo-
ration of the gingival margin in areas of pre-existent
gingival inflammation.
Lead Intoxication
Lead is slowly absorbed and toxic symptoms are not
particularly definitive when they do occur. Among the
oral signs are increased salivation, coated-tongue,
peculiar sweetish taste, gingival pigmentation and
ulceration. The pigmentation of gingiva is linear
(Burtonian line), steel gray and associated with local
irritation.
Mercury Intoxication
Gingival pigmentation in linear form results from the
deposits of mercuric sulfide. The chemical also acts as
an irritant which accentuates the pre-existing inflam-
mation and commonly leads to notable ulceration of
the gingiva and adjacent mucosa and destruction of
underlying bone.
Other Chemicals
Phosphorus, Arsenic, chromium, may cause necrosis
of the alveolar bone with loosening and exfoliation of
teeth.
PSYCHOSOMATIC DISORDERS
There are two ways by which psychosomatic disorders
may be induced in the oral cavity, through the
development of habits injurious to the periodontium and
by the direct effect of the autonomous nervous system
on the physiologic tissue balance.
However, under the conditions of mental and
emotional stress, the mouth may subconsciously become
an outlet for the gratification of basic drives in the adult.
Gratification may be derived from neurotic habits, which
are potentially injurious to the periodontium.
REVIEW QUESTIONS
1. Describe the influence of systemic diseases on
periodontium.
2. Role of diabetes in periodontal disease.
BIBLIOGRAPHY
1. Behivaz Yalda, Steven Offenbacher, John G Collins. Diabetes
as a modifier of periodontal disease expression. Periodontol
2000;6:1994.
2. Carlos Mendieta, Charles M Recve. Periodontal manifes-
tations of systemic disease and management of patients
with systemic disease, Current Opinion Periodontol
1993;111-28.
3. Newman, Takaê, Fermin A. Carranza’s Clinical
Periodontology, 9th edn, WB Saunders Co., 2002.
Fig. 10.7: Gingiva in pregnancy with localized gingival
enlargement

105
Role of Systemic Diseases in the Etiology of Periodontal Diseases
4. Raul I Garcia, Michelle M Henshaw, Elizabeth A Krall.
Relationship between periodontal disease and systemic
health, Periodontol 2000;25:2001.
5. Robert J Genco and Harald Loe. The role of systemic
conditions and disorders in periodontal disease. Periodontol
2000;2:1993.
6. Robin A Seymour, Peter A Heasman, Ian DM Macgregor.
Drugs, Diseases and the Periodontium, Oxford University
Press Publication, 1992.
7. Salmon Amar, Kang Mun Chung. Influence of hormonal
variations on the periodontium on woman. Periodontol 2000;
6:1994.
106
Essentials of Clinical Periodontology and Periodontics
INTRODUCTION
Halitosis is a term used to describe noticeably unpleasant
odor exhaled in breathing. It is a general term used to
describe the unpleasant breath regardless of its sources,
oral or non-oral. Whereas, oral malodor is the term
especially used to describe the odor from the oral cavity.
Halitosis which is in synonym with breath malodor, foul
breath and Fetor oris or simply bad breath affects a large
proportion of population which may cause a significant
social or psychological handicap to those suffering from
it. This common disease has been ignored for too long
by periodontologists even though the most common
cause is related to the micro biota of the subgingival
areas and the related tongue coating. The intensity of
the bad breath differs during the day, (which may be
due to the stress or fasting), eating certain foods (such
as garlic, onions, meat, fish and cheese), smoking and
alcohol consumption. Because the mouth is dry and
inactive during the night, the odor is usually worse upon
awakening (morning breath). Bad breath may also be
persistent (chronic bad breath) which is a more serious
condition, affecting at least 25% of population in varying
degrees.
CLASSIFICATION OF HALITOSIS
Genuine Halitosis
• Physiologic halitosis
• Pathologic halitosis
• Oral
• Extraoral
Is an obvious malodor, with intensity beyond socially
acceptable level is perceived.
Pseudo-halitosis
Obvious malodor is not perceived by others, although
the patient stubbornly complains of its existence,
❒
❒
❒
❒
❒ INTRODUCTION
❒
❒
❒
❒
❒ CLASSIFICATION OF HALITOSIS
❒
❒
❒
❒
❒ ETIOLOGY
• Causes for Physiologic Halitosis
• Causes for Pathologic Halitosis
❒
❒
❒
❒
❒ DIAGNOSIS OF HALITOSIS
• History
• Clinical Examination
• Intraoral
• Periodontal Examination
• Measurement of Oral Malodor
❒
❒
❒
❒
❒ TREATMENT AND MANAGEMENT OF ORAL
MALODOR
❒
❒
❒
❒
❒ SUMMARY
11
Oral Malodor
107
Oral Malodor
condition can be improved by counselling and simple
oral hygiene measures.
Halitophobia
After treatment for genuine halitosis or pseudo-halitosis,
the patient persists in believing that he/she has halitosis.
ETIOLOGY
At least 90% of all malodor originates from the oral
cavity, whereas, the remaining 10% has systemic or
normal causes. Oral malodor is commonly the result of
microbial putrefaction of food debris, cells, saliva and
blood within the oral cavity. In particular, proteolysis of
proteins to peptides and amino acids takes place. The
resultant substrates with free thiol groups such as
cystein and reduced glutathionine, rises to Volatile
Sulfur Compounds (VSC’s), which are malodour
substances. The most common physiological and
pathological causes of halitosis are discussed below:
Causes for Physiologic Halitosis
a. Mouth breathing.
b. Medications.
c. Aging and poor dental hygiene.
d. Fasting/starvation.
e. Tobacco.
f. Foods (onion, garlic, etc.,) and alcohol.
Causes for Pathologic Halitosis
Oral and other contributing factors such as:
a. Periodontal infection: Odor from subgingival dental
biofilm. Specific diseases like Acute Necrotizing
Ulcerative Gingivitis and Pericoronitis.
b. Tongue coating harbors microorganisms.
c. Stomatitis, Xerostomia.
d. Faulty restorations retaining food and bacteria.
e. Unclean dentures.
f. Oral pathologic lesions like oral cancers, candidiasis.
g. Parotitis, cleft palate.
h. Aphthous ulcers, dental abscesses.
Systemic and Extra-oral factors include:
a. Nasal infections like Rhinitis, Sinusitis, Tumors and
foreign bodies.
b. Diseases of Gastrointestinal tract (GIT) like Hiatus
hernia, carcinomas, GERD (Gastroesophagal Reflux
Disorder).
c. Pulmonary infections like Bronchitis, Pneumonia,
Tuberculosis, and Carcinomas.
d. Certain hormonal changes that occur during
Ovulation, Menstruation, Pregnancy and Menopause.
e. Systemic diseases like Diabetes Mellitus, Hepatic
failure, Renal failure, Uremia, Blood dyscrasias,
Rheumatologic diseases, Dehydration and Fever,
Cirrhosis of liver.
DIAGNOSIS OF HALITOSIS
a. Review of Medical, Dental and Personal
history.
b. Clinical examination:
i. Intraoral examination
i. Tongue coating
ii. Evidence of mouth breathing.
iii. Xerostomia: Dry mucosa.
iv. Other oral causes.
ii. Complete Periodontal examination
i. General personal care, state of oral hygiene.
ii. Probing for attachment levels, probing
depths (Periodontal status).
iii. Evidence of neglect; past history of dental
hygiene care.
c. Measurement of oral malodor: Patients should
be instructed not to eat, chew, rinse or smoke for
at least two hours before examination. Patients who
are on antibiotics should be seen 2 weeks after
discontinuation of medicines. The tests used to detect
halitosis are as follows:
i. Subjective organoleptic method: This has
been used as a bench mark for oral malodor
measurement.

108
Essentials of Clinical Periodontology and Periodontics
ii. Gas chromatography: In order to assess oral
malodor objectively, a portable industrial
monitor has been developed. These machines
are specifically designed to digitally measure
molecular levels of the three major Volatile
Sulfur Compounds (VSC) in a sample of mouth
air (hydrogen sulfide, methyl mercaptan
and dimethyl sulfide). It is accurate in
measuring the sulfur components of the breath
and produces visual results in graph form via
computer interface.
iii. Halimeters: These machines measure the level
of sulfide gas found in a persons breath. But
it has certain drawbacks in clinical applications,
some of the common sulfides such as mercaptan
are not easily recorded and can be misrep-
resented in test results. The Halimeter is also
very sensitive to alcohol, so one should avoid
drinking alcohol or using alcohol-containing
mouthwashes for at least 12 hours prior to being
tested.
iv. BANA Test (Benzoyl-d, L-arginine-naphthy-
lamide): Some of the bacteria like P. gingivalis,
T. denticola and B. forsythus produce waste
products that are quite odiferous and as a result
contribute in causing bad breath. These bacteria
in question have the characteristic of being able
to produce an enzyme that degrades the com-
pound benzoyl-d, L-arginine-naphthylamide.
When a sample of patients saliva that contains
these bacteria is placed within the BANA testing
compound they cause it to breakdown. As a
result of this degradation the test compound
changes its color indicating a positive reaction.
v. Chemiluminescence: This test involves mixing
a sample containing sulfur compound (VSC’s)
with the mercury compound and the resultant
reaction causes fluorescence. This test is highly
sensitive as it can measure even the low levels
of sulfur compounds in the sample, which is
in contradiction to testing with a halimeter.
TREATMENT AND MANAGEMENT OF
ORAL MALODOR
Treatment of oral malodor is a step-by-step problem
solving procedure. Before commencing the treatment
a clinician must determine the source of malodor. The
simplest way to distinguish oral from non-oral origin is
to compare the smell from mouth and nose. If the origin
is nasal or due to any other medical etiology they must
be referred to a concerned specialist. The odor gene-
rating from the mouth often requires dental treatment.
There are no standard and accepted protocols for the
treatment of oral malodor, however, the possible
protocols contains the basic elements including standard
dental and periodontal treatment.
For genuine halitosis with oral causes the treatment is
as follows:
a. Reduction of anaerobic load by improving oral
hygiene and periodontal health through basic dental
care, if necessary incorporate advanced hygiene
methods including oral irrigation and sonic or
ultrasonic tooth brushes.
b. If oral malodor persists in spite of adequate con-
ventional oral hygiene, tongue brushing should be
advised.
c. Chemical reduction of oral microbial load includes
rinsing or gargling with an effective mouthwash. One
way to treat oral malodor associated with periodontitis
is to combine regular periodontal treatment and a
chlorhexidine mouth rinse. However, their long-term
effect remains to be determined.
d. Another treatment strategy for oral malodor is
conversion of volatile sulfur compounds by using
various metal ions. Zinc (Zn++) is an ion which
bonds to the twice negatively charged sulfur radicals
to reduce the expression of VSC’s. Halita™ is a new
solution containing 0.05% Chlorhexidine, 0.05%
Cetyl pyridium chloride (CPC) and 0.14% zinc lactate
with no alcohol has been more efficient than 0.2%
Chlorhexidine formulation in reducing the VSC levels.
The special effect of Halita may result from the VSC
109
Oral Malodor
conversion ability of zinc, besides its antimicrobial
action.
SUMMARY
Halitosis affects a large proportion of population and
may cause a significant social or psychological handicap
to those who are suffering from it. Oral malodor can
also reveal important diseases, if it can be analyzed
accurately. VSC in periodontal pockets might be used
as a predictor of periodontal diseases and also to monitor
therapy sites. However, current available tools/tests are
not applicable for achieving these goals due to lack of
accuracy and objectivity.
The dental research community has long since ignored
the subject of oral malodor. Recently, along with the
growing public and media interest in oral malodor, dental
professionals are becoming more aware of this problem.
Let us hope that future research will overcome the
problems related to diagnosing and treating oral malodor
effectively.
KEY POINTS TO NOTE
1. Halitosis is a term used to describe noticeably unpleasant
odor exhaled in breathing.
2. Halitosis is classified into genuine halitosis, pseudo-halitosis
and halitophobia.
3. Most commonly halitosis is as a result of problems
originating from the oral cavity (90%). The remaining 10%
is from systemic and non-oral origin.
4. Measurement of oral malodor is done by various methods
like, organoleptic method, Gas chromatography, Halimeters
and BANA tests.
5. Treatment of oral malodor is a step-by-step problem solving
procedure. It involves a complete recording of history,
periodontal examination, and oral hygiene maintenance.
6. If oral malodor persists, chemical reduction of oral microbial
load should be incorporated.
REVIEW QUESTIONS
1. Describe the causes for oral malodor.
2. Enumerate various methods of treating oral malodor.
BIBLIOGRAPHY
1. Donaldson AC. Clinical examination of subjects with
halitosis. Oral Diseases 2007;13: 63-70.
2. Hannah Ben-Aryeh,Gershon Horowitz, Dan Nir, and Don
Laufer. Halitosis: An Interdisciplinary approach. Am
Journal of Otolaryngology 1998;19:8-11.
3. Nadanovsky p, Carvalho LBM, Ponce de Leon A. Oral
Malodor and its association with age and sex in a general
population in Brazil. Oral Diseases 2007;13:105-109.
4. Yaegaki K, Coil JM. Examination, Classification, and
treatment of halitosis; clinical perspectives. J Can Dent
Assoc 2000;66:257-261.
110
Essentials of Clinical Periodontology and Periodontics
INTRODUCTION
Gingivitis and periodontitis are caused by bacteria that
colonize the gingival crevice and attach to the tooth
surface. The pathogenic potential of the bacteria within
the plaque varies from gingiva site-to-site. Small amounts
of plaque can be tolerated without causing periodontal
disease, may be because of the host defence.
When bacteria and its products in the plaque increase
beyond the threshold level of the host, then the balance
shifts from health to disease. The mechanisms by which
subgingival bacteria contribute to the pathogenesis of
the periodontal disease are different. The pathogenesis
of periodontal disease can be caused by subgingival
bacteria alone or indirectly by evasion of immunologic
host response. Direct effect can be by bacterial invasion,
production of exotoxins, role of cell constituents, and
production of various enzymes. The direct exposure or
effect can be on the fibroblasts, epithelial cells, endothelial
cells and inflammatory cells.
Junctional epithelium: It is the tissue most directly
challenged by the pathogenic plaque bacteria. The
microbial mass releases large quantities of metabolites
like butyric acid and propionic acids which are toxic to
periodontal tissues. The keratinocytes respond to these
bacterial products by releasing cytokines and proinflam-
matory mediators. Interleukin (IL) and prostaglandin E
2
(PGE
2
) and matrix metalloproteinases are released from
the junctional epithelial cells. Neutrophils are present in
the junctional epithelium as a part of host defence system.
Activation of complement plays an important role in the
earliest host response in the gingival crevice.
ROLE OF BACTERIAL INVASION
The properties that enable the bacterium to cause a
disease are termed virulence factors, hence entry of
bacterium itself (invasion) or of bacterial products into
the periodontal tissues may be essential in the disease
process.
The gingival sulcus and periodontal pockets are
bathed in gingival crevicular fluid. Bacterial species that
colonize this region must attach to the available surfaces
to avoid the displacement by the fluid flow. The surfaces
❒
❒
❒
❒
❒ ROLE OF BACTERIAL INVASION
❒
❒
❒
❒
❒ ROLE OF EXOTOXINS
❒
❒
❒
❒
❒ ROLE OF CELL CONSTITUENTS
❒
❒
❒
❒
❒ ROLE OF ENZYMES
❒
❒
❒
❒
❒ EVASION OF HOST RESPONSES
❒
❒
❒
❒
❒ THE HOST DERIVED BONE
RESORBING AGENTS
❒
❒
❒
❒
❒ ROLE OF CYTOKINES
12
Pathogenesis of
Periodontal Diseases

111
Pathogenesis of Periodontal Diseases
available for attachment include the tooth or the root,
the tissues and pre-existing plaque mass.
Bacteria that initially colonize the periodontal
environment most likely attach to the pellicle or saliva-
coated tooth surface, e.g. adherence of A. viscosus
through fimbriae on the bacterial surface to a proline-
rich protein found on the saliva-coated tooth surfaces.
Bacterial attachment to the pre-existing plaque is
studied by examining the adherence between different
bacterial strains (co-aggregation), e.g. A. viscosus with
S. sanguis. These are important in the colonization of
the periodontal environment. The presence of bacteria
in patients with ANUG was studied by Listgarten (1965).
Micro-organisms were recognized in the gingival
connective tissue and in proximity to alveolar bone.
Bacteria may also enter through ulceration in the pocket
epithelium. In the Localized Juvenile Periodontitis (LJP),
A. actinomycetemcomitans have been observed in
gingival connective tissue. The presence of this organism
within the tissues appears to make the disease more
resistant to treatment and requires the use of antibiotics.
ROLE OF EXOTOXINS
A. actinomycetemcomitans produces an exotoxin
referred to as leucotoxin because of its toxic effect on
the human polymorphonuclear neutrophils (PMN’s). The
production of leucotoxin may enable A. actinomycetem-
comitans to evade the host defence.
ROLE OF CELL CONSTITUENTS
Cell constituents of both Gram-positive and Gram-
negative bacteria include endotoxins, bacterial surface
components and capsular components.
Endotoxin or lipo-oligosaccharide (LOS) previously
termed as lipopolysaccharide (LPS) is found in the outer
membrane of all Gram- negative bacteria. They are toxic
substances affecting the tissues directly and through
activation of the host responses. Its role in periodontal
disease is, their ability to produce leukopenia, activate
factor XII, activate the complement (C) system by the
alternative pathway, lead to localized Schwartzman
phenomena, with tissue necrosis occurring after two or
more exposure to the endotoxin, may also have cytotoxic
effects on the cells such as fibroblasts and induce bone
resorption. These endotoxins can penetrate gingival
epithelium. Bacteria can also produce fatty and organic
acids such as butyric acid and propionic acids, amines,
volatile sulphur compounds, indole, ammonia and
glycans.
Peptidoglycan: A cell wall component may affect a variety
of host responses including complement activation,
immunosuppressive activity and stimulation of the
reticuloendothelial system. It helps in the bone resorption
and in stimulating macrophages to produce prostaglandin
and collagenases.
ROLE OF ENZYMES
The bacterial enzymes that are capable of contributing
to the disease process include collagenases, hyaluro-
nidase, gelatinase, aminopeptidase, phospholipases and
alkaline and acid phosphatases.
Destruction of periodontal tissues is by degradation of
collagen. P. gingivalis and some strains of A. actinomy-
cetemcomitans have been found to produce collagenase.
Bacterial hyaluronidase is capable of altering gingival
permeability by allowing the apical proliferation of the
junctional epithelium along the root surfaces. Hyaluro-
nidase is found in high concentrations in periodontal
pocket.
EVASION OF HOST RESPONSES
(Tables 12.1 to 12.5)
Bacterial factors can also indirectly compromise the host
tissues. These factors influence both the cellular and
humoral immune responses.
For example,
1. Inhibition of PMN’s by leukotoxin chemotactic
inhibitors, decreased phagocytosis.
2. Immunoglobulins are inactivated by proteases.
3. Lymphocyte alterations
112
Essentials of Clinical Periodontology and Periodontics
Table 12.1: Influence of host response
on periodontal disease
Aspect of
Host factors
disease
1. Bacterial
Subgingivally, antibody, complement in
colonization
crevicular fluids inhibits adherence and
co-aggregation of bacteria and potentially
reduces their numbers by lysis.
2. Bacterial
Antibody-complement
invasion
1. Mediated lysis reduces bacterial counts.
2. Neutrophils as a consequence of chemo-
taxis, phagocytosis and lysis reduces
bacterial counts
3. Tissue
By antibody-mediated hypersensitivity,
destruction
cell-mediated immune responses. Activation
of tissue destruction factors such as colla-
genase.
4. Healing and
Lymphocytes and macrophages produce
fibrosis
chemotactic factors for fibroblasts, fibroblast
activating factors.
Table 12.2: Impact of micro-organisms
on inflammation and immunity
Microbes and products
Effects
1. Microorganisms
• Activate complement
• Activate neutrophils and
macrophages.
• Are antigenic.
2. Most peptides and
• Chemotactic for neutrophils
proteins secreted by
and macrophages.
microorganisms
• Are antigenic.
3. Enzymes
• Damage host cells.
• Degrade connective tissue
matrix.
• Activate and degrade
complement.
• Degrade antibody.
• Are antigenic.
4. Lipopolysaccharide
• Activate complement.
• Damage host cells and
alveolar bone resorption.
• Is antigenic.
5. Polysaccharide plaque
• Polyclonal B-cell activator.
matrix and bacterial
• Are antigenic.
capsule
6. Other toxins, acids,
• Damage host cells.
reducing agents
• Are antigenic.
Table 12.3: Mechanism which results in
changes in epithelium
Mechanism
Effects
1. Bacterial toxins, e.g.
• Cytotoxic to keratinocytes
endotoxins
• Disruption of normal
epithelial turnover and
differentiation
2. Bacterial enzymes
• Damage to keratinocytes
3. Release of enzymes
• Damage of keratinocytes
from neutrophils
4. Complement activation
• Cell damage
5. Production of TNF
• Decreased → keratinocyte
and interferon G by
proliferation
activated T-lymphocytes
6. Production of IL-1 by
• Increased → keratinocyte
macrophages
proliferation
Table 12.4: Mechanisms which results in
changes in connective tissue
Mechanisms
Effects
Bacterial toxins, e.g.
• Toxic to fibroblasts
endotoxins
• Decrease in collagen
production
Bacterial enzymes, e.g.
• Degradation of extracellular
collagenase, hyaluronidase
matrix components.
Release of enzymes from
• Damage to extracellular
neutrophils
matrix components.
Complement activation
• Damage to fibroblasts
• Decrease in collagen
production
Production of IL-1 and
• Increased secretion of colla-
TNF
genase and proliferations
by fibroblasts
Production of TGF and
• Stimulation of fibroblast
PDGF (Platelet derived
chemotoxin proliferation,
growth factors)
matrix synthesis and
attempts at repair.
Products of tissue damage
• Stimulation of fibroblast cells
4. Endotoxicity
5. Catalase reaction.
THE HOST DERIVED BONE
RESORBING AGENTS INCLUDE
Cytokines
• IL-1
• TNF

113
Pathogenesis of Periodontal Diseases
Important Cytokines Associated
with Periodontal Disease
1. IL-1: It is produced predominantly by macrophages
and lymphocytes. Fibroblasts, platelets and
keratinocytes and endothelial cells also release IL-1.
It upregulates adhesion molecules on endothelial
cells, lymphocytes, neutrophils and monocytes. It
activates T and B lymphocytes and promotes
antibody production. IL-1α and IL-1β are potent
stimulators of connective tissue destructions. It triggers
the release of large quantities of prostaglandins E2
from fibroblasts and monocytes and stimulates
secretion of matrix metalloproteinases.
2. IL-2: Monocytes and T lymphocytes produce IL-2.
It stimulates T cells and enhances clonal expansion
of beta cells into plasma cells.
3. IL-4, IL-5 and IL-10: They are produced by TH
2
cells
and help in the activation of beta cells into plasma
cells and down regulate monocytic response.
4. IL-6: It is released by lymphocytes, fibroblasts and
monocytes. Lipopolysaccharide, IL-1 and tumor
necrosis factor alpha upregulates the secretion of the
IL-6. It is believed to be responsible for conversion
of blood monocytes into osteoclasts.
5. IL-8: It is secreted by monocytes, keratinocytes and
fibroblasts. It is a strong chemoattractant of PMNL’s
at low concentrations.
6. TNF: Tumor necrosis factor are produced by macro-
phages and release lymphotoxins. They stimulate the
proliferation of osteoclast precursor cells and also
activate the mature osteoclasts to resorb bone. It
augments leukocyte chemotaxis, degranulation,
adherence to endothelial cells and its ability to kill
bacteria.
7. Prostaglandin E2: Sources are macrophages and
fibroblasts. IL-1 induces its production. It is a potent
mediator of osteoclastic resorption. Matrix metallopro-
teinases (MMP’s) are a family of enzymes capable
of degrading connective tissue matrix. These enzymes
Table 12.5: Pathogenic immune reactions
Types
Description
Definition
Examples
Type-I
Anaphylactic Antigen reacts with
Urticaria, hay
cells sensitized by
fever, asthma,
IgE antibodies and
food allergies.
release mediators.
Type-II
Cytotoxic
Antibody reacts
Transfusion
with cell-associated
reaction auto-
antigen usually,
immune hemo-
but not always,
lytic anemia
kills cells with help
of complement or
phagocytic cells.
Type-III Arthus
Antibody reacts
Serum sickness,
reaction
with antigen in
Arthus reaction
tissue spaces or
blood stream to
cause vasculitis,
requires
complement.
Type-IV Delayed
Lymphocyte reacts
Tuberculin
hyper-
with antigen. This
reaction, con-
sensitivity
reaction is medi-
act dermatitis,
ated by lympho-
allograft rejec-
cytes, macrophages tion.
or their products.
• TGF-β
• IL-6
Other Inflammatory Mediators
• Prostaglandins
• Leukotrienes
Role of Cytokines
Meaning “cell protein” is used for molecules which
transmit information or signals from one cell to another.
Interleukins, growth factors, chemokines and interferon
belong to the family of cytokines. They act on fibroblasts,
macrophages, keratinocytes and PMNL’s to release matrix
metalloproteinases (MMP’s) that degrade connective
tissue matrix. However, most findings show that tissue
destruction that occurs in periodontal disease is mainly
due to host response to the bacteria and their products.
114
Essentials of Clinical Periodontology and Periodontics
are secreted in the latent form by fibroblasts,
macrophages, keratinocytes and PMNL’s, e.g.
collagenase, gelatinase, stromalysin, matrilysin. IL-1
play an important role in upregulation of MMP’s.
The leukotrienes, prostaglandins and related mole-
cules are short range hormones that are produced
by many cells; they exert their effect locally and are
destroyed rapidly and spontaneously.
Prostaglandins and leukotrienes have been detected
in biologically-active concentrations in the inflammatory
exudates, leukotrienes C4, D4, and E4 known as slow
reacting substances of anaphylaxis (SRS-A) are released
from mast cells and basophils.
REVIEW QUESTIONS
1. Describe the role of plaque bacteria in the pathoge-
nesis of periodontal disease.
2. What are endotoxins?
3. What are cytokines?
BIBLIOGRAPHY
1. Denis F Kinane. Causation and pathogenesis of
periodontal disease. Periodontol 2000;25:2001.
2. Genco. Contemporary Periodontics. CV Mosby Company
Publication, 1990.
3. Jan Lindhe. Clinical Periodontology and Implant Dentistry
4th edn, Blackwell Munksgaard Publication, 2003.
4. Michael S Reddy, Harjoice K Jeffcoat. Periodontal disease
progression. Current Opinion in Periodontology 1993;
111: 128.
5. Saul Schluger. Periodontal diseases basic phenomena,
clinical management and occlusal and restorative inter-
relationships. 2nd edn, Lea and Iebiger publication.
6. William B Clark Harald Loe. Mechanisms of initiation and
progression of periodontal disease. Periodontol 2000;2:
1993.
115
Periodontal Medicine
INTRODUCTION
Advances in science and technology over the last century
have greatly expanded our knowledge of the patho-
genesis of periodontal diseases. It is clear that certain
systemic conditions may affect the initiation and
progression of gingivitis and periodontitis.
The oral cavity is continuously challenged by
opportunistic infections on one hand and the oral
complications of systemic diseases and disorders occur
on the other hand, thus an association between oral
infections and systemic diseases has been suspected for
centuries. The effect of oral health on the rest of the
human body was proposed by the Assyrian’s in the
seventh century B.C. In the 18th century a Pennsylvania
physician name Benjamin Rush quoted that arthritis could
be treated in some people after they had extracted the
infected teeth. But, over the past decade, growing
scientific evidence suggests an exquisite association
between oral infection (e.g. viruses, bacteria, yeasts) and
systemic diseases (e.g. atherosclerosis, cardiovascular
diseases, cerebrovascular diseases, premature low-birth
infants and pulmonary diseases). Thus the emerging
evidence has shed light on the converse relationship
between systemic health and oral health, i.e. the potential
effects of periodontal disease on a wide range of organ
systems. Thus, this chapter examines the emerging new
evidence collected since the early 1990’s implicating
periodontal infection as a risk factor for several systemic
conditions.
ERA OF FOCAL INFECTION
At the beginning of the twentieth century, medicine and
dentistry were searching for reasons to explain why
people became afflicted with a wide range of systemic
diseases. Medicine at that time had very little insight into
what caused diseases such as arthritis, pneumonia and
❒
❒
❒
❒
❒ ERA OF FOCAL INFECTION
❒
❒
❒
❒
❒ PERIODONTAL DISEASES AND
CORONARY HEART DISEASE/
ATHEROSCLEROSIS
❒
❒
❒
❒
❒ EFFECT OF PERIODONTAL INFECTION
❒
❒
❒
❒
❒ PERIODONTAL DISEASE AND DIABETES
MELLITUS
❒
❒
❒
❒
❒ ROLE OF PERIODONTITIS IN
PREGNANCY OUTCOME
❒
❒
❒
❒
❒ PERIODONTAL DISEASE AND CHRONIC
OBSTRUCTIVE PULMONARY DISEASE
❒
❒
❒
❒
❒ PERIODONTAL DISEASE AND ACUTE
RESPIRATORY INFECTION
❒
❒
❒
❒
❒ PERIODONTAL MEDICINE IN CLINICAL
PRACTICE
13
Periodontal Medicine

116
Essentials of Clinical Periodontology and Periodontics
pancreatitis, to name a few. Then, through the writings
and lectures of principally two individuals, W.D. Miller
and William Hunter, the concept that oral bacteria and
infection were the likely cause of most of a person’s
systemic illness suddenly became very popular. For the
next 40 years infections, especially those originating in
the mouth caused most of the man’s suffering and illness.
This era, which came to be known as the ‘era of focal
infection’ can be attributed primarily to a microbiologist
in Philadelphia, Willoughby D. Miller and a London
Physician, William Hunter.
‘Focal infection’ implied that there was a nidus of
infection somewhere in the body, such as periodontitis,
which via the blood stream could affect distant sites and
organs. Throughout the 1920’s and 1930’s, dentists and
physicians believed that bacteria on the teeth and the
resultant infectious diseases such as caries, gingivitis and
periodontitis that followed were a ‘focus of infection’ that
led to a wide variety of systemic problems. It became
popular during this period to extract teeth as a means
of ridding the body of oral bacteria and preventing and
/ or treating diseases affecting the joints as well as diseases
of the heart, liver, kidneys and pancreas.
Hunter believed that teeth were liable to septic
infection primarily due to their structure and their
relationship to the alveolar bone. He stated that ‘the
degree of systemic effect produced by oral sepsis
depended on the virulence of the oral infection and
degree of resistance of the individual’. He also felt that
oral organisms has specific actions on different tissues
and that these organisms acted by producing toxins,
resulting in low grade ‘sub-infections’, which produced
systemic effect over prolonged periods.
However, by 1940, medicine and dentistry were
realizing that there was much more to explain a patient’s
general systemic condition than bacteria in his or her
mouth. Dentists and physicians realized that (1) extracting
a person’s teeth did not necessarily make the person
better or make their disease go away. (2) People with
very healthy mouths and no obvious oral infection
developed systemic diseases and (3) people who had
no teeth and thus no apparent oral infection still
developed systemic diseases.
By 1950, medicine was making great strides in
discovering the true etiologies and dentistry was making
great strides in the prevention as well as the treatment
of caries and periodontal disease and so the era of ‘focal
infection’ as a primary cause of systemic diseases finally
came to an end.
However, it was not until the last decade of the
twentieth century that dentistry and medicine again
began to examine the relationship of oral infections as
a risk for systemic disease and now there is a careful
new look at periodontitis as a possible risk for systemic
disease using discrete scientific levels of evidence.
PERIODONTAL DISEASE AND CORONARY
HEART DISEASE / ATHEROSCLEROSIS
CHD and CHD-related events are a major cause of
death. Myocardial infarction has been associated with
acute systemic bacterial and viral infections and infarction
is sometimes preceded by influenza like symptoms.
Is it possible that oral infections is similarly related to
myocardial infarction?
Traditional risk factors such as smoking, dislipidemia,
hypertension and diabetes mellitus do not explain
the presence of coronary atherosclerosis in a large
number of patients. Localized infection resulting in a
chronic inflammatory reaction has been suggested as a
mechanism underlying coronary heart disease in these
individuals.
In cross-sectional studies of patients with acute
myocardial infarction or confirmed CHD compared with
age and gender matched control patients, myocardial
infarction patients had significantly worse dental health
than did controls. This association between poor dental
health and myocardial infarction was independent of
known risk factors for heart disease such as age, cholestrol
level, hypertension, diabetes and smoking, because
artherosclerosis is a major determinant of CHD-related
events, dental health has also been related to coronary
atheromatosus.

117
Periodontal Medicine
Mattila and colleagues performed oral radiographic
examinations and diagnostic coronary angiography
examinations on men with CHD. There was a significant
correlation between the severity of dental disease and
degree of coronary atheromatosus. This relationship
remained significant after accounting for other known
risk factors for coronary artery disease. Cross-sectional
studies, thus suggest a link between oral health and
coronary heart disease.
However, such studies cannot determine causality in
this relationship. Rather, dental diseases may be indicators
of general health practices for example, periodontal
disease and CHD are both related to lifestyle and share
numerous risk factors such as smoking, diabetes and low
socioeconomic status. Bacterial infections have significant
effects on endothelial cells, blood coagulation, lipid
metabolism and monocyte macrophages. The research
of Mattila and colleagues showed that dental infections
were the only factors, other than classic and well-
recognized coronary risk factors, that were associated
independently with the severity of coronary arterio-
sclerosis.
This study and others in which periodontal condition
is known to have preceded the CHD-related events,
support the concept that periodontal disease is a risk
factor for CHD, independent of other classic risk factors.
EFFECT OF PERIODONTAL INFECTION
Periodontal infection may affect the onset or progression
of atherosclerosis and coronary heart disease through
certain mechanisms. Periodontitis and atherosclerosis
both have complex etiologic factors, combining genetic
and environmental influences. The diseases share many
risk factors and have distinct similarities in basic
pathogenic mechanisms.
Ischemic Heart Diseases
Ischemic heart disease is associated with the process of
atherogenesis and thrombogenesis. Increased viscosity
of blood may promote major ischemic heart disease and
stroke by increasing the thrombus formation.
Increased plasma fibrinogen is a recognized risk factor
for cardiovascular events and peripheral vascular disease.
Also elevated white blood cell count and coagulation
factor VIII has been associated with risk of ischemic heart
disease (Fig. 13.1).
Thrombogenesis
Platelet aggregation plays a major role in thrombogenesis,
and most cases of acute myocardial infarction are
precipitated by thromboembolisms. Oral organisms may
be involved in coronary thrombogenesis since platelets
selectively bind some strains of Streptococcus sangius,
a common component of supragingival plaque and
Porphyromonas gingivalis, a pathogen closely associated
with periodontitis.
Fig. 13.1:
Factors affecting blood viscosity in health
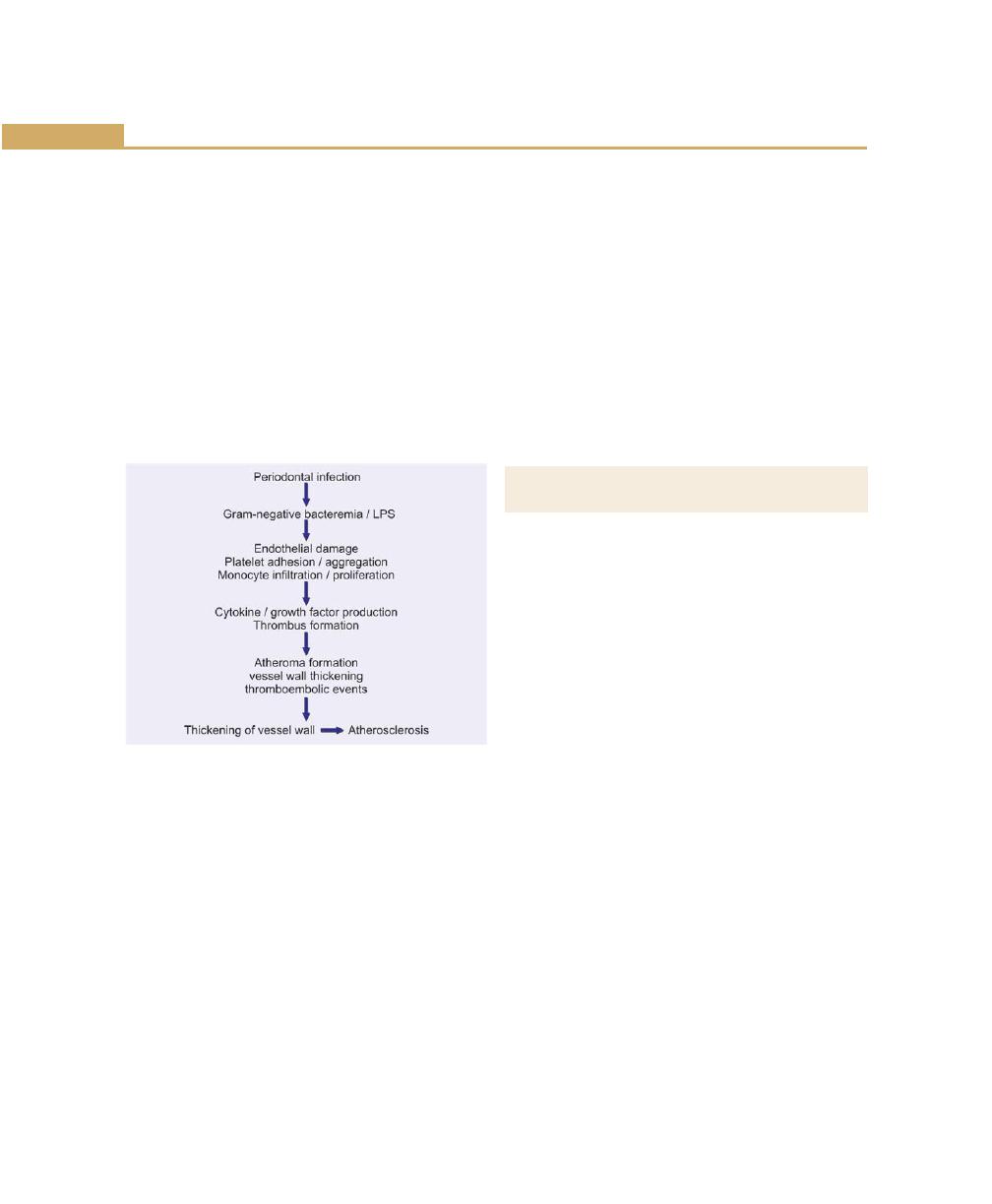
118
Essentials of Clinical Periodontology and Periodontics
Daily activity: Routine daily activity such as mastication
and oral hygiene procedures results in frequent
bacteremia with oral organisms. The exposure time to
bacteremias from routine daily chewing and tooth
brushing is much greater than from dental procedures.
Periodontal disease may predispose the patient to an
increased incidence of bacteremias.
It has been estimated that about 8 percent of all cases
of infective endocarditis are associated with periodontal
or dental diseases without any preceding dental
procedure.
Atherosclerosis (Fig. 13.2)
Fig. 13.2:
Atherosclerosis
Periodontal Infection and Stroke
Ischemic cerebral infarction or stroke is often preceded
by systemic bacterial or viral infections. In one study
patients with cerebral ischemia were five times more likely
to have had a systemic infection within 1 week before
the ischemic event than were non-ischemic control
subjects. Recent infection was a significant risk factor for
cerebral ischemia and was independent of other known
risk factors such as hypertension, history of a previous
stroke, diabetes, smoking and coronary heart disease.
In case-control studies, poor dental health was a
significant risk factor for cerebrovascular ischemia. In
one study bleeding on probing, suppuration, subgingival
calculus and number of periodontal and periapical lesions
were significantly greater in male stroke patients than
in controls.
Overall 25 percent of all stroke patients had significant
dental infections compared with only 2.5 percent of
controls. This study supports an association between
poor oral health and stroke in men under age 50. In
another study men and women’s of age 50 and older
who had a stroke had significantly more severe
periodontitis and more periapical lesions as compared
to the non-stroke control subjects.
PERIODONTAL DISEASE AND
DIABETES MELLITUS
An understanding of effects of other infections is useful
in determining the mechanisms by which the periodontal
infection influences glycemia. Acute bacterial and viral
infections have shown to increase insulin resistance and
aggravate glycemic control. This occurs in individuals with
and without diabetes. Systemic infection increases tissue
resistance to insulin, preventing glucose from entering
target cells causing elevated blood glucose level and
requiring increased pancreatic insulin production to
maintain normoglycemia.
It is possible that chronic gram-negative periodontal
infection may also result in increased insulin resistance
and poor glycemic control.
In patients with periodontitis, persistent systemic
challenge with periodontopathic bacteria and their
products may act in a way similar to well recognized
systemic infection. This mechanism would explain the
worsening of glycemic control associated with severe
periodontitis. Periodontal treatment designed to decrease
the bacterial insult and reduce inflammation might restore
insulin sensitivity overtime, resulting in improved
metabolic control. The improved glycemic control seen
in several studies of periodontal therapy would support
such a hypothesis.
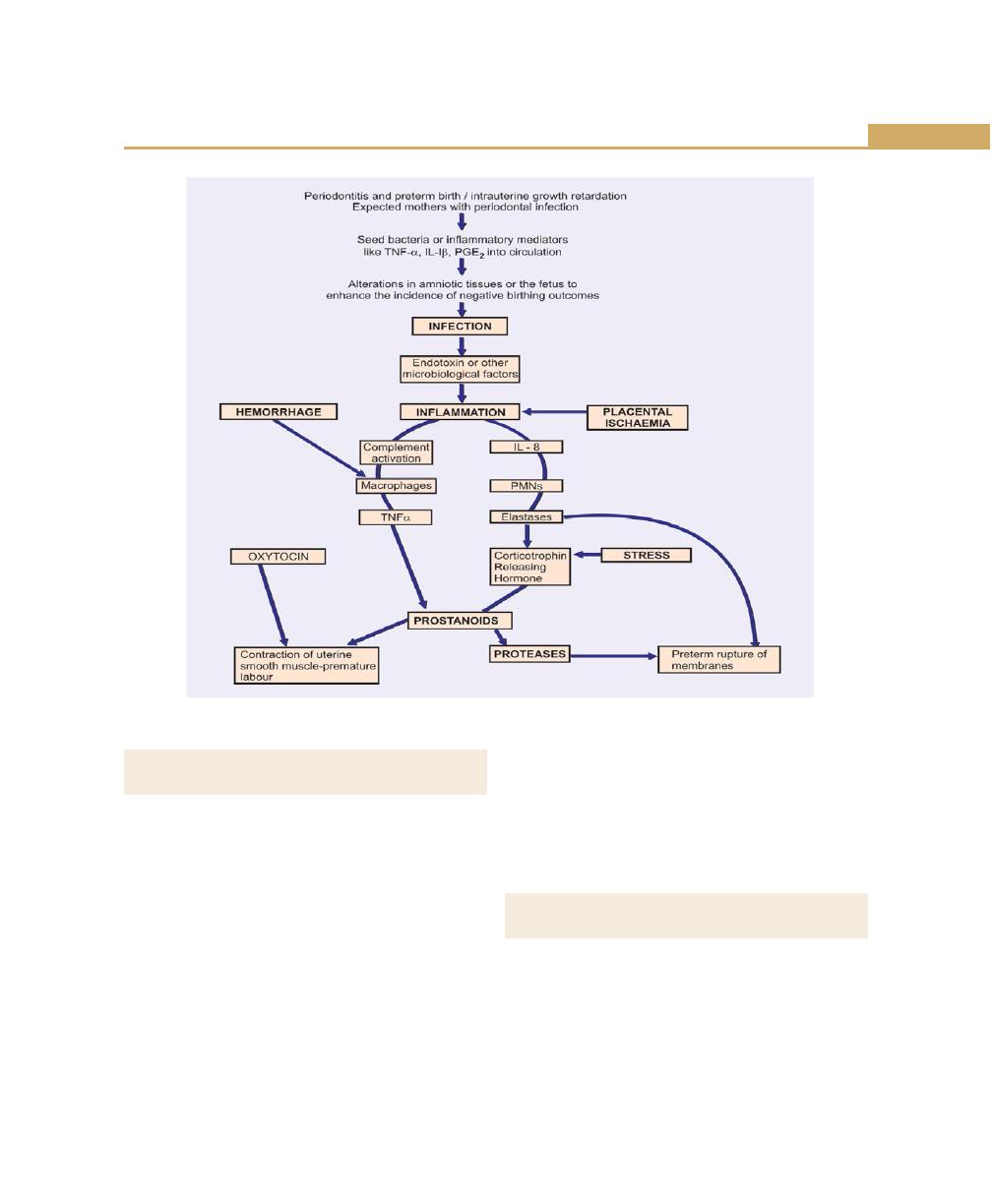
119
Periodontal Medicine
ROLE OF PERIODONTITIS IN
PREGNANCY OUTCOME
Periodontitis is a remote Gram-negative infection that
may play a role in low birth weight (Fig. 13.3).
Periodontopathic organisms and their products may
have wide range effects, most likely mediated through
stimulation of host cytokine production in target tissues.
Animal studies suggest that remote reservoirs of gram-
negative organisms and their products may have a
negative impact on pregnancy. Porphyromonas gingivalis
implanted in subcutaneous chambers during gestation
caused significant increase in fetal death and a decrease
in fetal birth weight for those that remained viable, when
compared with control animals that were not innoculated.
There was a significant increase in TNF-α and PGE
2
levels.
There was a significant correlation between TNF-α and
PGE
2
levels, as well as fetal death and growth retardation.
These data suggest that a remote, non-disseminated
infection with Porphyromonas gingivalis may result in
abnormal pregnancy outcome in this model.
PERIODONTAL DISEASE AND CHRONIC
OBSTRUCTIVE PULMONARY DISEASE
Chronic obstructive pulmonary disease is a disease state
characterized by airflow obstruction due to chronic
bronchitis or emphysema. Bronchial mucous glands
enlarge and an inflammatory process occurs in which
neutrophils and mononuclear inflammatory cells
accumulate within the lung tissue.
Fig. 13.3:
Periodontitis and preterm birth / intrauterine growth retardation
120
Essentials of Clinical Periodontology and Periodontics
Chronic obstructive pulmonary disease shares similar
pathogenic mechanism with periodontal disease. In both
diseases, a host inflammatory response is mounted in
response to chronic challenge by bacteria in periodontal
disease and by factors such as cigarette smoke in chronic
obstructive pulmonary disease. The resulting neutrophil
influx leads to release of oxidative and hydrolytic enzymes
that cause tissue destruction directly.
In analyzing data from a longitudinal study of more
than 1100 men alveolar bone loss was associated with
the risk of chronic obstructive pulmonary disease. The
increase in the risk was independent of age, smoking
status and other known risk factors for chronic
obstructive pulmonary disease. Individuals with poor oral
hygiene have also been found to be at an increased risk
for chronic respiratory diseases such as bronchitis and
emphysema. These associations remain to be confirmed
by further research.
PERIODONTAL DISEASE AND ACUTE
RESPIRATORY INFECTION
Pneumonia is an infection of lungs caused by bacteria
virus, fungi or mycoplasma and is broadly categorized
as either
• Community acquired, or
• Hospital acquired
Community acquired bacterial pneumonia is caused
primarily by inhalation of infectious aerosols or by
aspiration of oropharyngeal organisms. S.pneumonia
and Haemophilus influenzae are most common, although
numerous other species may occur, including anaerobic
bacteria. To date, no associations have been found
between oral hygiene or periodontal disease and the
risk for acute respiratory conditions such as pneumonia
in community dwelling individuals.
Hospital acquired (nosocomial) bacterial pneumonia
has very high morbidity and mortality rate. The incidence
of nosocomial pneumonia is highest in severely ill patients
such as those in intensive care units or on ventilatory
support.
Hospital–acquired pneumonia is usually caused by
aspiration of oropharyngeal contents. Oropharyngeal
colonization with potential respiratory pathogens (PRPs)
increase during hospitalizations and the longer the
hospital stay the greater the prevalence of PRPs. PRPs
are found predominantly in the gastrointestinal tract and
may be passed through oesophageal reflux into the
oropharynx where they colonize. Subsequent aspirations
may lead to pneumonia.
The PRPs may also originate in the oral cavity, with
dental plaque serving as a reservoir of these organisms.
Poor oral hygiene is common in the hospital and nursing
home settings, especially in severely ill patients. PRPs are
commonly isolated from supragingival plaque and buccal
mucosa of patients in intensive care units than in
outpatient setting. Thus, organisms that are not routinely
found in dental plaque become plaque colonizers after
prolonged hospitalizations. Subgingival plaque may also
harbor PRPs and putative periodontal pathogens have
been associated with nosocomial pneumonia. Further-
more anaerobic organisms from periodontal pockets may
serve as the primary innoculum for suppurative
respiratory disease such as pulmonary abscesses that
have significant morbidity and mortality. Although
considerable circumstantial evidence suggests that
periodontal pathogens may cause acute nosocomial
pulmonary infection, currently no published studies
specifically demonstrate an increased risk of such
infections in patients with periodontal disease.
PERIODONTAL MEDICINE IN
CLINICAL PRACTICE
The concept of periodontal diseases as localized entities
affecting only the teeth and supporting apparatus is over-
simplified and needs to be revised. Rather than being
confined to periodontium, periodontal diseases may have
wide-ranging systemic effects. In most persons these
effects may be relatively inconsequential or at least not
clinically evident. However in susceptible individuals,
periodontal infection may act as an independent risk
121
Periodontal Medicine
factor for systemic disease and may be involved in the
basic pathogenic mechanism of these conditions.
Furthermore, periodontal infection may exacerbate
existing systemic disorders.
Proper use of knowledge of relationship between
periodontal disease and systemic health requires the
dental professional to expand his or her horizons, to
step back from the technically demanding aspects of
dental art, and to recognize the oral cavity as one of
the many interrelated organ systems. Patient education
in this regard is also very important.
KEY POINTS TO NOTE
1. Periodontal infection may affect the onset or progression of
atherosclerosis and coronary heart disease through certain
mechanisms.
2. Altered microbiota associated with deep periodontal pockets
→ bacterial translocations / or systemic inflammation →
endothelial and altered vascular response → cytokines and
acute phase proteins atherosclerotic changes → coronary
artery disease (MI).
3. Studies conducted by Offenbacher has shown that women
with severe periodontitis are 7.5 times more likely than
women without periodontal disease to have an infant with
preterm low-birth weight.
REVIEW QUESTION
1. Describe the role of periodontal disease as a risk factor
for systemic diseases (Periodontal Medicine).
BIBLIOGRAPHY
1. Carrazas Clinical Periodontology. 9th edn, Newman, Takie,
Fermin A Carranza, WB Saunders Co., 2002.
2. Frank A. Scanntrapillo. Relationships between periodontal
disease and respiratory diseases. Periodontal Medicine, B.C.
Decker, 2000.
3. George W. Taylor, Brian A. Burt, Mark P. Becker, Robert J Genco.
Severe periodontitis and risk for poor glycemic control in
patients with non-insulin diabetes mellitus.
4. James Beck, Raul Garcia, Gerardottei. Periodontal disease
and cardiovascular disease. J. Periodontol No 67, Oct
1996.
5. Offenbacher S, Katz V, Fertik G, Collins J. Periodontal infection
as a possible risk factor for preterm low birth weight.
J Periodontol 1996; 67: 1103-13.
6. Thoden Van Velzen, Abraham Inpigin and Moorer. Plaque
and systemic disease: A reappraisal of the focal infection
concept. J Clin Periodontol 1984; 11: 209-20.
122
Essentials of Clinical Periodontology and Periodontics
EFFECT OF SMOKING ON THE
PREVALENCE AND SEVERITY OF
PERIODONTAL DISEASE
See Table 14.1.
EFFECT OF SMOKING ON THE
ETIOLOGY AND PATHOGENESIS
OF PERIODONTAL DISEASE
Microbiology
• Smokers had higher level of Bacteroides forsythus.
• Smokers do not respond to mechanical therapy and
this is associated with increased level of B.forsythus,
❒
❒
❒
❒
❒ EFFECT OF SMOKING ON THE
PREVALENCE AND SEVERITY OF
PERIODONTAL DISEASE
• Gingivitis
• Periodontitis
❒
❒
❒
❒
❒ EFFECT OF SMOKING ON THE
ETIOLOGY AND PATHOGENESIS OF
PERIODONTAL DISEASE
• Microbiology
• Immunology
• Physiology
❒
❒
❒
❒
❒ EFFECT OF SMOKING ON THE
RESPONSE TO PERIODONTAL THERAPY
❒
❒
❒
❒
❒ EFFECT OF SMOKING CESSATION
Table 14.1: Impact of smoking on periodontal diseases
Periodontal disease
Impact of smoking
Gingivitis
• Decreased gingival inflammation and
bleeding on probing.
Periodontitis
• Increased prevalence and severity of
periodontal destruction.
• Increased pocket depth, attachment
loss and bone loss.
• Increased rate of periodontal destruc-
tion.
• Increased prevalence of severe perio-
dontitis.
• Increased tooth loss.
• Increased prevalence with increased
number of cigarettes smoked per day.
• Decreased prevalence and severity
with smoking cessation.
A. actinomycetemcomitans and P. gingivalis remaining
in the pocket after therapy when compared to
nonsmokers.
• Eikenella nodatum, Fusobacterium nucleatum, S.
vincentii, P. gingivalis, P. intermedia, Peptostrepto-
coccus micros, Prevotella nigrescens, B. forsythus
were significantly more prevalent in current smokers
than in nonsmokers and former smokers.
Immunology
• Altered neutrophil chemotaxis, phagocytosis and
oxidative burst.
• Increased TNF-α, and PGE
2
in GCF.
14
Smoking and
Periodontal Diseases
123
Smoking and Periodontal Diseases
• Increased production of PGE
2
by monocyte in
response to lipopolysaccharides (LPS).
• IgG
2
level is reduced suggesting reduced protection
against periodontal infection.
• Nicotine, a major component of tobacco adversely
affect fibroblast function.
• Nicotine suppresses osteoblast proliferation while
stimulating alkaline phosphatase activity.
• Tobacco products alters normal reparative and
regeneration potential of periodontium.
Physiology
• Clinical signs of inflammation are less pronounced,
due to alteration in the inflammatory response in
smokers or due to alteration in vascular response of
gingival tissues.
• Increased gingival blood vessels with increased
inflammation.
• Decreased GCF flow and bleeding on probing with
increased inflammation.
• Decreased subgingival temperature.
• Increased time needed to recover from local anes-
thesia.
EFFECT OF SMOKING ON THE
RESPONSE TO PERIODONTAL THERAPY
See Table 14.2.
EFFECT OF SMOKING CESSATION
• Gingiva of treated current smokers exhibit minimal
redness and bleeding while brushing presumably
because of immunosupression or vascular effect of
smoking.
• Several weeks following smoking cessation, gingival
inflammation and bleeding on brushing occurs
because of smoking cessation, gingiva loses its thick
fibrotic appearance and assumes normal anatomy.
Therefore smoking status should be considered in
diagnosis, prognosis and treatment planning of
periodontitis patients.
Table 14.2: Effect of smoking on periodontal therapies
Therapy
Effect of smoking
Non-surgical
• Decreased clinical response to scaling
and root planing
• Decreased reduction in pocket depth
• Decreased gain in clinical attachment
level.
• Decreased negative impact of smoking
with increased level of plaque control.
Surgery and
implants
• Decreased pocket depth reduction post
surgery.
• Increased deterioration of furcation
postsurgery.
• Decreased gain in clinical attachment
level, decreased bone fill, increased
recession and increased membrane
exposure following guided tissue rege-
neration (GTR).
• Decreased pocket depth reduction after
DFDBA allograft.
• Decreased pocket depth reduction and
gain in clinical attachment level after
open flap debridement.
• Conflicting data on the impact of
smoking on implant success.
• Smoking cessation should be
recommended prior to implant.
Maintenance
• Increased pocket depth during main-
tenance.
• Decreased gain in clinical attachment
level.
Recurrent
(refractory) disease • Increased recurrent/refractory disease in
smokers.
• Increased need for retreatment in
smokers.
• Increased need for antibiotics in
smokers to control the negative effect of
periodontal infection on surgical
outcome.
• Increased tooth loss in smokers after
surgical therapy.
KEY POINTS TO NOTE
1. In smokers there is decreased gingival inflammation and
bleeding on probing.But, increased prevalence and severity
of periodontal destruction.
2. There is increased levels of periodontopathic organisms
namely, B. forsythus, P. gingivalis, A. actinomycetemcomitans
and others.

124
Essentials of Clinical Periodontology and Periodontics
3. Increased production of inflammatory mediators.
4. Fibroblast function is diminished. It also alters normal
reparative and regeneration potential of periodontium.
5. In general, there is increased need for retreatment in smokers.
REVIEW QUESTION
1. What are the effects of smoking on periodontal
disease?
BIBLIOGRAPHY
1. Ah MBK, Johnson GK, Kaldahl WB et. al. The effect of smoking
on the response to periodontol therapy. Journal of Clinical
Periodontol 1994; 21: 91.
2. Bergstrom J, Eliassons, Dock J. A 10-year prospective study
of tobacco smoking and periodontol health. Journal of
Periodontol 2000; 71: 1338.
3. Haber J, Wattler J, Crowley H et. al. Evidence of cigarette
smoking as a major risk factor for periodontitis. Journal of
Periodontol 1993; 64: 16.
4. Newman, Takei, Fermin. A Carranza. Clinical Periodontogy
by W.B Saunders Co.
5. Robin A. Seymour, Peter A. Heasman. Drugs, Diseases and
Periodontium. Oxford University Press Publication (1992).
6. Tonneti MS. Cigarette smoking and periodontal diseases,
etiology and management of disease. Annals of Periodontol
1998; 3: 88.
125
Host Modulation in Periodontal Therapy
INTRODUCTION
SPECIFIC ASPECTS OF DISEASE
PATHOGENESIS AS POTENTIAL
TARGETS FOR HOST MODULATION
Considering the fact that, periodontal disease is as a result
of host interaction between the plaque biofilm and host
responses, the area of research is mainly directed at
altering an individual’s reaction to the bacterial challenge.
Hence, various host modulatory therapies (HMT) have
been developed or proposed to block the pathways
responsible for periodontal tissue breakdown. In context
to this, specific aspects of disease pathogenesis that have
been investigated for modulation include,
a. Regulation of immune & inflammatory responses.
b. Excessive production of matrix metalloproteinases.
c. Arachidonic acid metabolism.
d. Bone metabolism.
❒
❒
❒
❒
❒ INTRODUCTION
❒
❒
❒
❒
❒ REGULATION OF IMMUNE AND
INFLAMMATORY RESPONSES
❒
❒
❒
❒
❒ EXCESSIVE PRODUCTION OF MATRIX
METALLOPROTEINASES (MMP’s)
❒
❒
❒
❒
❒ PRODUCTION OF ARACHIDONIC ACID
METABOLITES
❒
❒
❒
❒
❒ REGULATION OF BONE METABOLISM
Currently one systemically administered agent is
available for modulation of host i.e., sub-antimicrobial
dose doxycycline marketed as Periostat™ by collagens
pharmaceuticals.
REGULATION OF IMMUNE AND
INFLAMMATORY RESPONSES
Currently, it is believed that, small groups of periodon-
topathic microorganisms within the plaque biofilm are
often associated with disease initiation and progression.
Some of the strongly implicated organisms include
Porphyromonas gingivalis, Actinobacillus actinomyce-
temcomitans and Bacteroides forsythus. The disease
initiation occurs by microbial challenge consisting of
antigens, lipopolysaccharides (LPS) and other virulence
factors, stimulates host responses thereby resulting in
disease either limited to gingiva (gingivitis) or progresses
to periodontitis.
Activation of host has both protective & destructive
aspects. Protective aspects of host response include
recruitment of neutrophils, production of protective
antibodies and possibly the release of various anti-
inflammatory cytokines including TGF-β (transforming
growth factor-β and interleukins (IL-4, IL-10 and IL-
12). On the other hand perpetuation of the host response
15
Host Modulation in
Periodontal Therapy
126
Essentials of Clinical Periodontology and Periodontics
due to persistent bacterial onslaught may disrupt the
homeostatic mechanism and results in release of
mediators including pro-inflammatory cytokines, (e.g.,
IL-1, IL-6, TNF-α), matrix metalloproteinases (proteases)
and prostaglandin E
2
(PGE
2
), which can promote
extracellular matrix destruction in the gingiva to stimulate
bone resorption. However, there are other set of
cytokines, which can suppress the action of pro-
inflammatory cytokines, they include IL-4, IL-10, IL-11
and TGF-β. When administered for therapeutic
purposes, these antagonists can reduce inflammation.
However, there are certain unresolved issues regarding
the cytokine modulation therapy like identifying an ideal
method to maintain or inhibit cytokines for long periods
and also understanding of the systemic implications
associated with altering cytokine levels on tissue
homeostasis.
Therefore, additional animal & human studies are
required to apply it routinely in the treatment of perio-
dontitis.
EXCESSIVE PRODUCTION OF MATRIX
METALLOPROTEINASES (MMP’S)
MMP’s are a family of zinc and calcium dependent
endopeptidases secreted or released by a variety of
infiltrating cells like neutrophils, macrophages and
resident cells like fibroblasts, epithelial cells, osteoblasts
and osteoclasts found in the periodontium.
The major functions of MMP’s are to degrade the
constituents of the extracellular matrix like laminin,
collagen, fibronectin etc.
Role of MMP’s in Human Periodontal Diseases
One hypothesis regarding periodontal disease
pathogenesis is that host cells stimulated directly or
indirectly by components of the plaque biofilm secrete
MMP’s, which are associated with altered connective
tissue remodeling and alveolar bone resorption.
Although, several periodontal pathogens (e.g., P.
gingivalis and A.actinomycetem comitans) produce
MMP’s including collagenase. It is however believed that
endogenous MMP’s are not the bacterial proteinases
primarily responsible for tissue destruction. This further
emphasizes the role of host modulatory approaches in
periodontal therapy.
Several synthetic MMP inhibitors are being studied
in clinical trials. One such most extensively studied
inhibitor are the family of tetracycline antibiotics, which
can inhibit host derived MMP’s by mechanisms
independent of their antimicrobial properties. The
development of host modulating therapy (HMT), utilizing
tetracyclines primarily involves the use of a reduced dose
of doxycycline (Periostat™ 20 mg b.i.d). These doses
reportedly do not exhibit antimicrobial effects, but can
effectively lower MMP levels. This reduced dose has been
referred to as subantimicrobial dose doxycycline (SDD).
In addition to use of SDD in host modulating therapy,
10 different chemically modified tetracyclines (CMT’s)
have been developed, 9 of which inhibits MMP’s and
do not possess antimicrobial properties. In animal models
CMT’s have been reported to reduce the progression
of experimentally induced periodontitis, however
inhibition of human periodontitis is still not established.
PRODUCTION OF ARACHIDONIC ACID
METABOLITES
Another destructive pathway in the pathogenesis of
periodontal disease is synthesis and release of
prostaglandins and other arachidonic acid metabolites
within periodontal tissues. Whenever there is tissue
damage due to bacterial and host factors the
phospholipids in the plasma membrane of cells becomes
available to phospholipase A
2
, thereby results in
production of free arachidonic acid. Arachidonic acid
can be metabolized via the cyclooxygenase or
lipoxygenase pathway. The final products of the cyclo-
oxygenase pathway include prostaglandins, prostacycline
and thromboxane, whereas the end result of the
lipoxygenase pathway include leukotrienes and other
hydroxyeicosate traenoic acids. Elevated levels of PGE
2
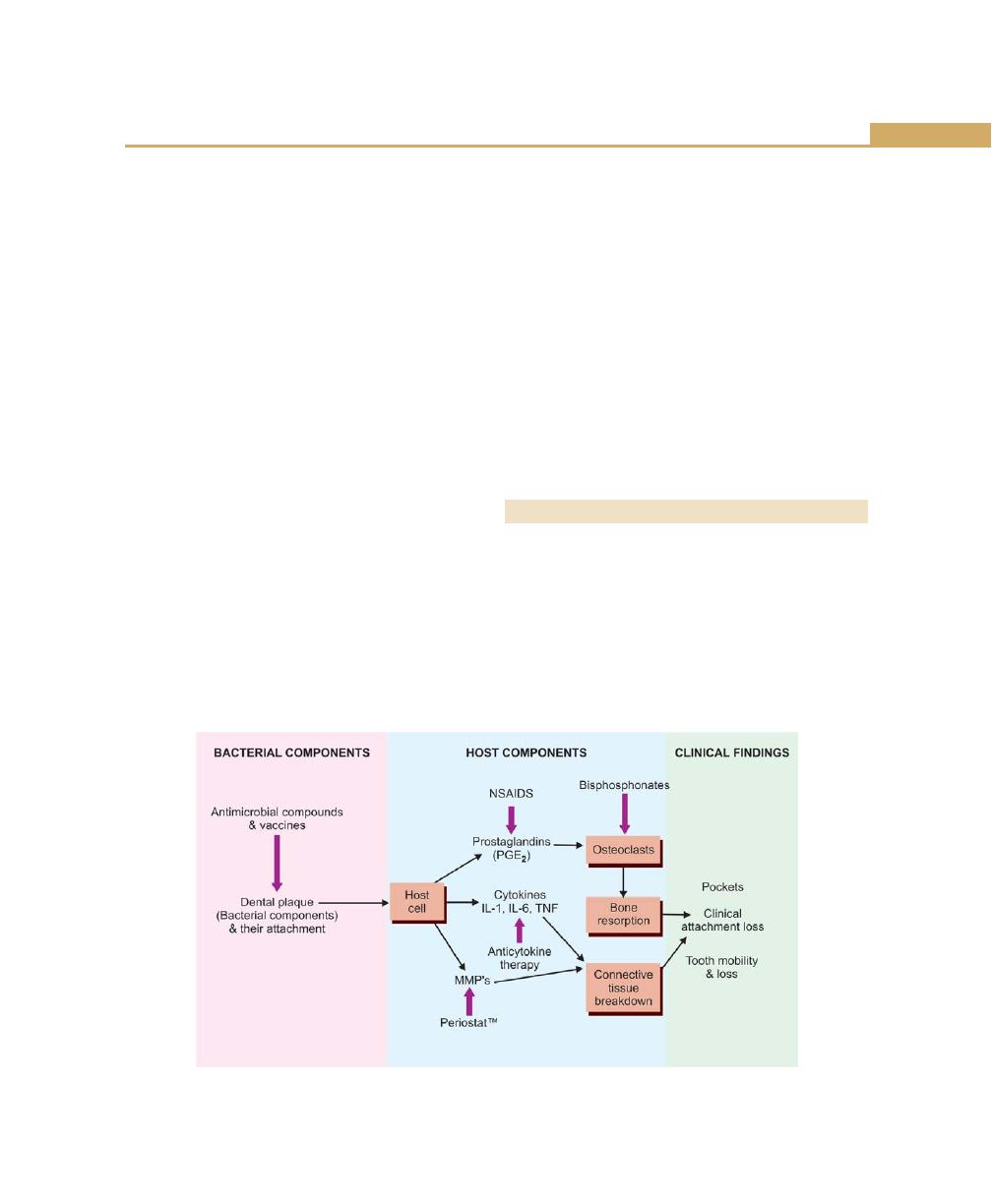
127
Host Modulation in Periodontal Therapy
and other arachidonic acid metabolites have been
reported in crevicular fluid in patients with gingivitis and
periodontitis. Hence, one proposed approach to
modulate host response is inhibition of enzymes
responsible for the release of these destructive products.
Various in vitro studies have been conducted to assess
the Non-steroidal anti-inflammatory drugs (NSAID’s) as
inhibitors of bone resorption. Multiple NSAID’s including
indomethacin, flurbiprofen, ibuprofen, naproxen,
meclofenamic acid and piroxicam have demonstrated
their ability to reduce gingivitis and progression of
periodontitis in animal models. Ketoprofen, an NSAID
which can inhibit both cyclooxygenase and lipoxygenase
pathways, has recently received a lot of attention. In
humans, most of these NSAID’s have shown significant
reduction in bone loss, however disease progression
returned upon the withdrawal of the agent. Hence topical
administration of NSAID’s has been considered as an
alternative method to deliver these agents. Various drugs
that have been evaluated for topical administration
include, ketorolac tromethamine rinse and S-ketaprofen
dentifrice. However, further studies are required to
determine the efficacy of these agents to provide clinically
significant improvements, when used as adjuncts to
scaling and root planing. Other agents that have been
tried for topical use are lipoxins, which are a series of
oxygenated arachidonic acid derivatives functioning as
endogenous inflammatory mediators. In animal models,
these lipoxins at a metabolically stable state blocked the
neutrophil infiltration induced by P. gingivalis and also
reduced PGE
2
levels. However, additional studies are
required to demonstrate the role of lipoxins in the
pathogenesis of periodontitis. Recently, a compound that
has received interest as both antibacterial and anti-
inflammatory agent is triclosan. Dentifrice containing
sodium fluoride, triclosan and a co-polymer has been
tested. However, additional studies are required to
examine the effect of this combination of drugs on
periodontitis.
REGULATION OF BONE METABOLISM
Various drugs have been tried to inhibit the activity of
osteoclasts. A new class of drugs used to manage
osteoporosis, which may also have beneficial effects on
the periodontium, are the bisphosphonates. These
compounds through the mechanism of chelation of
cations seem to inhibit MMP activity thereby inhibiting
osteoclastic activity.
One of these drugs, alendronate has been evaluated
in ligature induced periodontitis models. Although it
Fig. 15.1:
Pathological events of periodontal diseases with various host modulatory approaches
128
Essentials of Clinical Periodontology and Periodontics
inhibited loss of bone density in animal models, the
human trials have shown minimal effects on clinical
parameters. Further studies are required to evaluate the
effectiveness of these drugs in the treatment of perio-
dontal diseases.
KEY POINTS TO NOTE
1. The current paradigm, for the etiology & pathogenesis of
periodontitis includes the involvement of periodontal
pathogens and destructive host responses.
2. To prevent the disease initiation and progression, mechanical
and antimicrobial pharmaceutical agents have been used
successfully.
3. For the long term clinical management of periodontitis a
novel adjunctive therapies such as host modulation has
been introduced.
4. The use of HMT (Host Modulation Therapy) as an adjunct
may be particularly useful in susceptible, high risk patients
(e.g., smoking, diabetes, genetic predisposition) FDA has
recently approved sub anti-microbial dose doxycycline
(Periostat™) for systemic administration as an adjunct to
scaling and root planing in the treatment of chronic
periodontitis.
5. The future holds much hope for application of HMT not
only for managing the periodontitis patients, but also for
the practice of periodontal medicine.
REVIEW QUESTIONS
1. Enumerate various host modulating agents in
periodontal therapy.
BIBLIOGRAPHY
1. Genco RJ. Host responses in periodontal diseases: Current
Concepts, J Periodontol 1992; 63: 338.
2. Golub LM, Suomalainen K, Sorsa T. Host modulation with
tetracyclines and their chemically modified analogues, Curr
Opin Dent 1992; 2: 80.
3. Offenbacher S. Periodontal diseases: pathogenesis, Ann
Periodontol 1996; 1: 821.
4. Page RC, Kornman KS. The pathogenesis of human
periodontitis: an introduction, Periodontol 2000 1997, 14:
9.
5. Salvi GE, Lawrence HP, Offenbacher S, Beck JD. Influence
of risk factors on the pathogenesis of periodontitis,
Periodontol 2000 1997; 14: 173.


DEFENCE MECHANISMS
A number of mechanisms operate to protect the body
from attack by foreign bodies and toxins, including infec-
tions by bacteria. These mechanisms can be classified as:
1. Non-specific mechanisms.
2. Mechanisms specific to invading foreign proteins
called antigens which stimulate the immune system.
Non-specific Protective Mechanisms
Bacterial Balance
The mouth as a whole and various zones in the mouth,
including what has been called the ‘crevicular domain’
can be viewed as an ecosystem in which a balance exist
between different species of microorganisms, their flora
and their tissues.
Surface Integrity
The surface integrity of skin and mucous membrane
barrier, including the gingiva, is maintained by the
persistent renewal of the epithelium from its base and
desquamation of the surface layers. These two activities
are balanced and this helps in maintaining a constant
thickness of the epithelium. The efficiency of the surface
barrier is enhanced by keratinization and parakeratini-
zation. The junctional epithelium, although semi-
permeable, has a very high rate of cell turnover.
Surface Fluid and Enzymes
All vital surfaces are washed by fluids, which are capable
of attacking foreign materials, e.g. gastric acid, lysozyme,
saliva. Saliva bathes the oral mucosa and contains
antibacterial substances. The gingival fluid exudates flow
through the junctional epithelium into the gingival crevice
and this fluid contains phagocytic leukocytes and enzymes
(Table 16.1).
Phagocytosis
Certain cells in the blood stream and in the tissues are
capable of engulfing and digesting foreign materials. The
Section 1: GINGIVAL DISEASES
❒
❒
❒
❒
❒ DEFENCE MECHANISMS
• Non-specific Protective Mechanisms
• Specific Protective Mechanisms
❒
❒
❒
❒
❒ SULCULAR FLUID
• Anatomy of Gingival Crevice or Sulcus
• Significance of Gingival Sulcus and Fluid
• Significance of Gingival Vasculature
and Crevicular Fluid
• Permeability of Sulcular and Junctional
Epithelia
• Methods of Collection
• Composition
• Clinical Significance
16
Defence Mechanisms
of the Gingiva
132
Essentials of Clinical Periodontology and Periodontics
metabolism and stimulates production of toxic
metabolites.
The Inflammatory Reaction
The inflammatory reaction is stimulated by tissue injury
and infection and leads to changes in the local
microcirculation, which produces hyperemia, increased
vascular permeability and the formation of a fluid and
cellular exudate.
Specific Protective Mechanism
Animals (vertebrae) have a developed surveillance and
attack system called the immune system. This can protect
the body from bacteria, viruses and even cancer cells.
The system has three characteristics:
1. It can distinguish between itself and the enemy;
thereby it does not attack parts that it recognizes as
self. Sometimes it may go wrong and thereby results
in certain disease known as the autoimmune diseases.
2. The defences contain elements specific against any
given antigen.
3. The system has a memory. The specific immune
mechanism has two basic components.
a. Humoral immunity
Both arise from stem cells
b. Cell-mediated
in the bone marrow
immunity
SULCULAR FLUID
Anatomy of Gingival Crevice or Sulcus
The gingival sulcus is the shallow crevice or space around
the tooth, bounded by the surface of the tooth on one
side and the epithelium lining the free margin of the
gingiva, on the other.
Sections of the marginal region of clinically healthy
gingiva will show the presence of three types of epithelia,
the oral or keratinized epithelium covering the gingival
connective tissue, in continuation with the oral sulcular
epithelium, which is not keratinized. It forms the soft
tissue wall of the gingival sulcus and the junctional
epithelium is in continuation with the oral sulcular
Table 16.1: Functions of saliva
Functions
Salivary
Probable mechanism
components
Lubrication
Glycoproteins
Coating similar to
mucoids
gastric mucin
Physical
Glycoproteins
Coating similar to
protection
mucoids
gastric mucin
Cleansing
Physical flow
Clearance of debris
and bacteria
Buffering
Bicarbonate and
Antacids
phosphate
Tooth integrity
Minerals glyco-
Maturation, reminer-
maintenance
protein pellicle
alization mechanical
protection
IgA
Control of bacterial
colonization
Antibacterial
Lysozyme
Breaks bacterial cell
action
walls
Lactoperoxidase
Oxidation of suscep-
tible bacteria
most important phagocytic cells are polymorphonuclear
neutrophils and the macrophages.
Macrophages are basically monocytes, which when
moved into the tissues mature and become
macrophages, unlike polymorphonuclear neutrophils
which have the capacity to undergo several divisions
within the tissues, which progressively increase in number.
While polymorphonuclear neutrophils are the main line
of defence in acute infections, monocytes are more
important in long-term chronic infections.
Phagocytosis is aided by a battery of nine related
proteins known as ‘complement’ which are activated by
two systems, namely classical and alternative pathway.
In the process of activation of the complement system,
fragments are generated by cleavage of C
3
, C
4
and C
5
.
These fragments are termed as C
3a
, C
4a
and C
5a
, these
take part in the defense mechanism, in the tissue fluids
and are referred to as anaphylotoxins, since they can
induce smooth muscle contraction, increase permeability
of blood vessels and cause the release of histamine from
mast cells and basophils.
C
5a
, in addition, is chemotactic for neutrophils and
monocytes. It augments cell adherence and causes
degranulation of these cells, it also enhances arachidonic
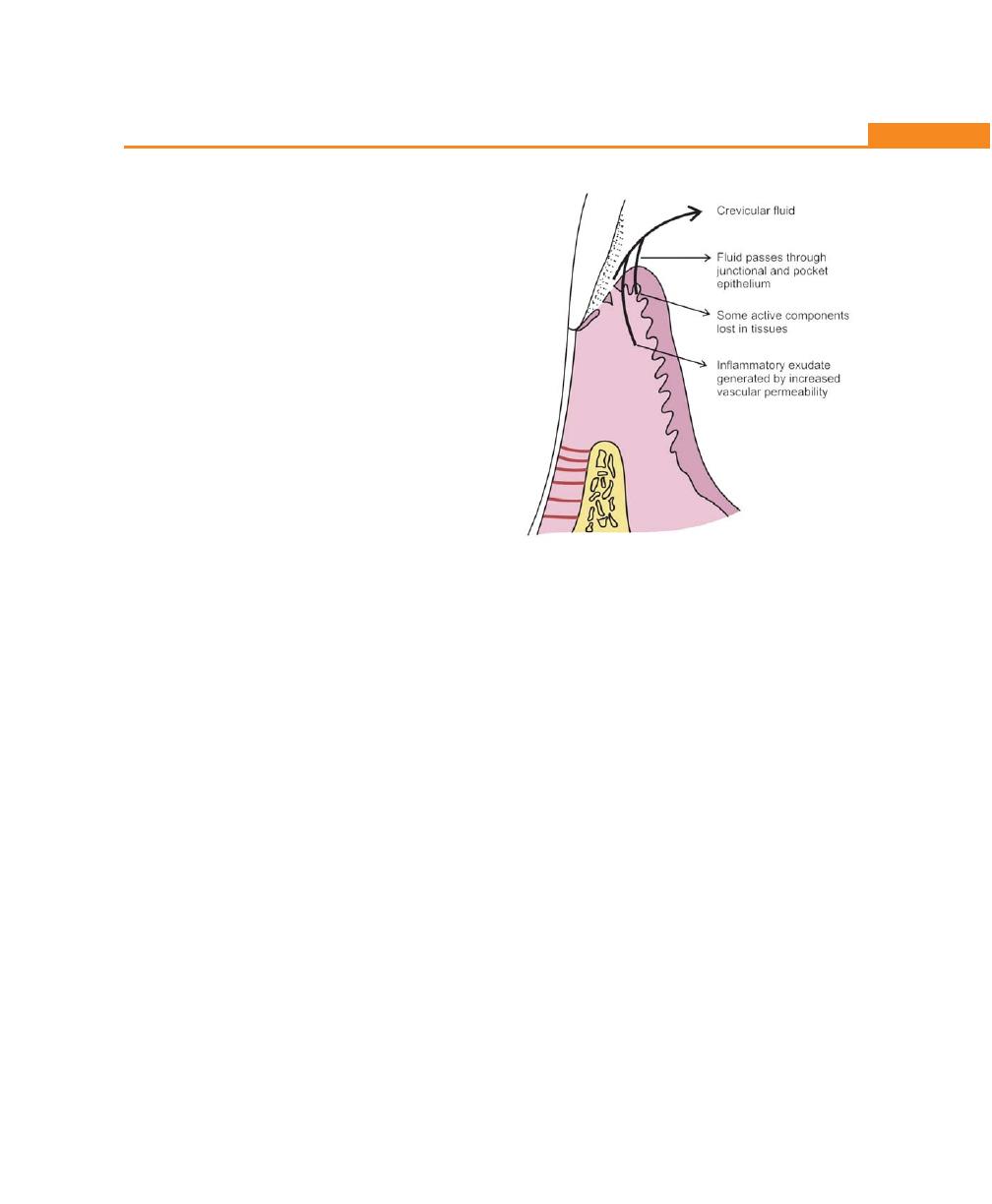
133
Defence Mechanisms of the Gingiva
epithelium. It is formed by a few strata of cells, with a
long flat basal layer and a very small desquamating
surface that forms the base of the gingival sulcus.
Normally, the depth of the gingival sulcus is zero,
however 1.5 to 3.0 mm of the so called probing depth of
the gingival sulcus may be considered normal. When the
probing depth exceeds more than 3 mm a new type of
epithelium called the “pocket epithelium” appears. It is
characterized by irregular ridges, ulceration, discontinuous
basal layer and it is not attached to the tooth. Below this
epithelium a typical junctional epithelium is present;
forming a short epithelial attachment, the designation of
gingival pocket can be used. Finally, in the presence of
periodontitis, pocket depth greater than 3 to 5 mm is seen,
active bone resorption takes place, bacterial colonies are
found situated very deep along the cementum surface.
Significance of Gingival Sulcus and Fluid
Brill and Krasse have clearly demonstrated that
parenterally administered tracer material could be
removed from the gingival sulcus by using 4 mm wide
filter paper strips intracrevicularly. Brill reported various
types of tissue damage using the same technique. Now
new methods of collecting crevicular fluid are available
which do not cause any irritation, for example
extracrevicular technique and a simple procedure of
drying a chronically inflamed gingiva by a blast of air.
Significance of Gingival Vasculature and
Crevicular Fluid (Fig. 16.1)
The blood supply to marginal gingiva is by the vessels
of both the periodontal ligament and oral mucosa, each
consisting of arterioles and venules. In the presence of
inflammation, the width and length of capillary and post-
capillary venules increases, this results in twisting and
looping of the vessels underlying sulcular and junctional
epithelium. The vessels immediately below the sulcular
epithelium and junctional epithelium are damaged in
a flat layer. Since these epithelia do not possess ridges
projecting into the connective tissue, their vasculature
network is located in a very superficial position.
The significance of such an arrangement in the
mechanism of the production of gingival fluid was clearly
demonstrated by Egelberg, who has demonstrated that
the production of crevicular fluid is primarily related to
an increase in the permeability of the vessels underlying
junctional and sulcular epithelium.
Permeability of Sulcular and
Junctional Epithelia
The main pathway for the transport of substances across
the junctional and sulcular epithelia seems to be the
intercellular spaces which form, 18 percent of the total
volume of the junctional epithelium and 12 percent of
that of the outer sulcular epithelium. Barriers to the
passage of substances through junctional and sulcular
epithelium are represented by the intercellular junctions
and especially by the basement membrane.
In the presence of inflammation, enlargement of
intercellular space of both junctional epithelium and
sulcular epithelium along with partial destruction of the
basal membrane results in the inward passage of foreign
substances.
Fig. 16.1:
The generation of crevicular fluid

134
Essentials of Clinical Periodontology and Periodontics
Variety of enzymes such as hyaluronidase and
collagenase has the ability to alter the permeability
properties of the junctional epithelium and the sulcular
epithelium. They have the ability to penetrate even the
intact junctional and sulcular epithelia. Nutritional
deficiencies such as Ascorbic acid might also alter sulcular
permeability and allow the passage of substances from
the gingival sulcus into gingival connective tissue, like
histamine, leucine, thymidine, phenytoin, peroxidase,
albumin, dextran, carbon particles, endotoxins and
others.
One can conclude that the epithelia covering the
gingival sulcus, represents a relative barrier to the
penetration of foreign material from the sulcus into the
connective tissue. It is conceivable that plaque compo-
nents, even of relatively high molecular weight could
pass within the gingival connective tissue, when allowed,
to accumulate in the sulcus.
Methods of Collection
It is usually made from the anterior teeth (least
contamination). Mainly three techniques are available:
a. Absorbing paper strips
b. Sampling by means of micropipettes
c. Gingival washings
d. Other methods.
Absorbing Paper Strips
Two techniques are followed:
• Intracrevicular: The end of the paper strip is gently
inserted into the pocket until minimum resistance is
felt.
• Extracrevicular: The strip is placed at the entrance
of the gingival crevice. This technique has
subsequently never been used.
Evaluation of Amount of Fluid Collected
a. Appreciation by direct viewing and staining, was
proposed by Egelberg and Attstrom. The strip was
stained with an alcoholic solution of ninhydrin at
concentration of 0.2 percent (gives blue or purple
color). The stained area can then be measured with
a transparent scale, calipers or calibrated magnifying
glass.
b. By weighing the strip: The strip is weighed before
collection of the sample within a sealed micro-
centrifugation plastic tube and is also weighed
immediately after the collection of the sample.
c. Use of periotron
®
: This is the latest and standard
method for measuring gingival fluid absorbed on
paper strips. It was developed by Harco Electronics.
HAR 600 is an electronic device whose functioning
units are a pair of upper and lower counter parts
which can be opened and closed in order to insert
or remove the strip of filter paper. A moistened strip
of paper when inserted between the two jaws will
give a reading on the screen. HAR-6000 is the latest
technique which was found to be sensitive in detecting
small volumes of fluids as compared to the former
two models.
Advantages:
• It is a simple procedure. It can be viewed directly.
• Quantitative assessment of the fluid can be obtained.
• It seems to be compatible with subsequent chemical
analysis.
• By using periotron, evaporation is kept to a minimum.
Disadvantages:
• Contamination can occur. In case of evaporation of
sample, it has to be repeated many times. It is not
very reliable (Ninhydrin technique).
• Dislocation of the paper strip thereby disturbing the
integrity of the marginal tissues.
• When periotron
®
is used, daily check on the reading
accuracy should be performed; care should be taken
to insert the paper strips into the machine in a
standardized position for correct reading.
Sampling by Means of Micropipettes (Fig. 16.2)
Krasse and Egelberg were the first to utilize capillary
tubing. This micropipette permits absorption by capilla-
rity. Capillary tubes of standardized length and diameter
135
Defence Mechanisms of the Gingiva
are placed in the pockets, their content is centrifuged
and analysed.
Disadvantages
• The collection of fluid is difficult because the viscosity
of the fluid makes aspiration through a pipette very
difficult.
• Finally the recovery of the sample can also be very
demanding.
Gingival Washings
There are two techniques that are available for the study
of gingival fluid components.
First method: This was proposed by Tokamoli and
Oppenheim; is based on the individual acrylic appliances.
Advantages
• Useful for longitudinal studies.
• Concentrations of various enzymes and the number
of cells at the marginal area could be followed by
this technique (polymorphonuclear leucocytes and
epithelial cells).
• It permits collection of gingival fluid without disturbing
the integrity of the marginal tissue.
• Contamination is least by this technique.
Disadvantages
• It is a complex procedure.
• It represents a dilution of crevicular fluid.
Second method: This was proposed by Skapski and
Lehner. The procedure involves the ejection and re-
aspiration of a known amount of the solution into a given
interdental crevice.
Advantages
• It has an advantage of being useful for cases of
clinically normal gingiva.
• It is useful for studying the number and functional
state of cells and bacteria from the crevicular area.
• Total and differential leukocyte counts can be
obtained.
Disadvantages
• The technique does not permit absolute quantitative
assessments, as the dilution factor cannot be
determined.
Other Methods
• Plastic strips
• Platinum loops
The strips are placed along the long axis of the tooth
or inserted into the sulcus and pressure is applied.
Volume of gingival fluid: The volume is calculated by
using an isotope dilution method. The mean gingival
fluid volume in spaces from molar teeth ranged from
0.43 to 1.56 µl. In anterior teeth the volume was between
0.24 to 0.43 µl/tooth.
Challa Combe calculated an amount of 0.5 to
2.4 µl of fluid/day.
Composition of Gingival Crevicular Fluid
(Table 16.2)
A. Cellular elements
1. Epithelial cells
2. Leukocytes
3. Bacteria
B. Electrolytes
1. Sodium
2. Potassium
3. Calcium
Fig. 16.2:
Collection of GCF by micropipettes

136
Essentials of Clinical Periodontology and Periodontics
Table 16.2: Composition of gingival crevicular fluid
Source
Action
Correlation with disease activity
A Cellular elements
1. Epithelial cells
Junctional and sulcular
Positive correlation is found
epithelium
2. Leukocytes
Dentogingival vessels
Phagocytosis and killing
Positive correlation only in some
of micro-organisms
instances
3. Bacteria
Oral cavity
Poor correlation between bacterial
count in gingival fluid and perio-
dontal parameters
B Electrolyte
1. Sodium
Plasma and extracellular
• Genesis of plaque
Shows positive correlation significantly
fluid
• Precipitation of proteins
increases in the presence of inflammation
2. Potassium
Plasma and
• Precipitation of mucoprotein
Positive correlation between
extracellular
along enamel surface
potassium concentration and the
fluid
average pocket depth
3. Calcium
Plasma and extracellular fluid
Positive correlation
C. Organic compounds
1. Carbohydrates
Extracellular fluid
Glucose concentration is increased in
(glucose, hexo-
inflamed tissues hexosamine and
samine and
hexuronic acid has no correlation with
hexuronic acid)
variation in gingival inflammation
2. Proteins
a. Immunoglobulins
Plasma or synthesized locally
Immune function
Positive correlation
b. Complement
Blood
1. Control of inflammatory reaction
2. Elimination of antigen
3. Activation of cells
4. Preparation of microbes and
foreign particles for phago-
cytosis
5. Play a role in immune
response
3. Lipids
Serum
D. Metabolic and
bacterial products
1. Lactic acid
Breakdown product of tissue
Positive correlation to both the clinical
degree of inflammation and intensity
of gingival fluid flow
2. Hydroxyproline
Breakdown product of collagen
In presence of inflammation the
correlation tends to increase one month
after surgery and return to base line
level postoperatively
3. Prostaglandins
• Synthesized by most mam-
• Vasodilation
It is positively correlated with disease
malian cells.
• Bone resorption
activity
• Are component of inflam-
• Inhibition of collagen
matory reaction
synthesis
4. Urea
Break down products of
Elevates the pH of supragin-
Urea concentration in gingival fluid
bacteria
gival plaque in presence of
decreases when gingival inflammation
gingivitis and periodontitis
increases
due to production of ammonia
by micro-organisms
5. Endotoxins
These are lipopolysaccharides
Highly toxic to gingival tissue
Positively correlated with the presence
of cell wall of Gram-negative
of varying degree of periodontal
bacteria
inflammation
Contd...

137
Defence Mechanisms of the Gingiva
6. Cytotoxic sub-
Bacteria
Highly toxic metabolite
Positively correlated with gingival
stances (like H
2
S)
(cytotoxic effect)
inflammation
7. Antibacterial
Saliva
Prevents growth of bacteria
factors
E. Enzyme and enzyme
inhibitors
1. Acid phosphatase
PMNL’s and desquamating
• Associated with connective
Negative correlation was found
epithelial cells
tissue catabolism
between the intercellular concentration
• Attacks teichoic acid one of
of acid phosphatase and both the flow
the component of bacterial
of gingival fluid and the percentage
cell wall.
of bone loss.
2. Alkaline phos-
In gingival sulcus it is found
Play a role in calcification
Positively correlated with pocket
phatase
in PMNL’s
depth
3. Pyrophosphatase
• Plaque
Play a role in calculus formation
Positive correlation
• Bacteria
4. β-glucoronidase
• It is a hydrolase found in
Used as lysosomal marker
Positive correlation between the
azurophilic granules of
concentration of β-glucoronidase and
PMNL’s
the flow of gingival fluid and depth
• Bacterial plaque
of periodontal pocket
• Macrophages, fibroblast,
endothelial cells
5. Lysozyme
PMNL’s
• Bactericidal properties and
Positively correlated with the severe
also some detrimental effect
periodontal destruction
upon epithelial cells
• Lytic effect of connective
tissue thereby contributing to
formation of pocket
6. Hyaluronidase
Serum
• Widening of intercellular
Significantly increases in presence of
spaces in the junctional
inflammation
epithelium
7. Mammalian
proteases
a. Cathepsin-D
Lysosomal enzyme seen in
Attacks various component of
Its concentration is positively corre-
human mononuclear leukocytes
epithelium and connective tissue
lated with periodontal destruction
b. Elastase
Azurophil granules of PMNL’s
• Active upon elastin, proteo-
Positively correlated with disease
glycans, hemoglobin, fibrino-
progression
gen and collagen
• Widening of epithelial
intercellular spaces, partial
destruction of basal mem-
brane and loss of collagen
c. Cathepsin-G
Serine endopeptidase contained
• Hydrolyzes hemoglobin,
Positive correlation
in azurophil granules of PMN’s
fibrinogen, casein, collagen
and proteoglycans
d. Plasminogen acti-
Blood
• Fibrinolysis
Concentration increases as severity of
vator (Streptoki-
• Plays a role in inflammation
periodontitis increases
nase, urokinase)
• Essential for wound healing
e. Collagenase
Specific granules of PMN’s
• Collagenolytic activity
Higher concentration in chronically-
inflamed gingiva
f. Bacterial proteases Bacteria
• Tissue damage
Positive correlation
g. Serum proteinase
Plasma
• Modulates the activity of
Positive correlation
inhibitor (α
2
)
proteases in the tissue
macroglobulin and
α
1
antitrypsin)
8. Lactic dehydro-
Bacteria
• Catalyzes the reversible
No significant correlation between total
genase
reduction of pyruvate to
activity of lactic dehydrogenase in
lactate
gingival fluid and any of the clinical
parameters
Contd...
Source
Action
Correlation with disease activity

138
Essentials of Clinical Periodontology and Periodontics
C. Organic compounds
1. Carbohydrates
2. Proteins
• Immunoglobulins
• Complement components
3. Lipids
D. Metabolic and bacterial products
1. Lactic acid
2. Hydroxyproline
3. Prostaglandins
4. Urea
5. Endotoxins
6. Cytotoxic substances
7. Antibacterial factors
E. Enzyme and enzyme inhibitors
1. Acid phosphatase
2. Alkaline phosphatase
3. Pyrophosphatase
4. β-glucoronidase
5. Lysozyme
6. Hyaluronidase
7. Proteolytic enzymes
• Mammalian proteinases
• Bacterial proteinases
• Serum proteinase inhibitors
8. Lactic dehydrogenase
Clinical Significance
General Health and Gingival Fluid
A. Gingival fluid flow and sex hormones: Three groups
of females are studied.
First group–during menstruations: There is increase
in the gingival fluid flow because of the sex hormones
(estrogen and progesterone) cause increase in the
gingival vascular permeability.
Second group–females on birth control pills: There is
significant increase in the amount of exudate recorded.
Third group–females during pregnancy: The gingival
exudates reached maximum values during the last
trimester and decreased to minimum after delivery.
B. Gingival fluid in diabetic patients: The gingiva of diabetic
patients significantly showed higher incidence of
vascular modifications, which could be an increase
in the width of the basal membrane of capillaries,
small arteries and venules resulting in higher produc-
tion of gingival fluid. The exudates collected from
the diabetic patients showed significantly more levels
of glucose than that collected from healthy individuals.
Drugs in Gingival Fluid
Since the gingival fluid seems to be a characteristic feature
of gingival inflammation one could reasonably expect
that when suitable drugs are given to a patient it can
be carried from the general circulation to the gingival
sulcus or pocket by flow of the fluid.
Bader and Goldhaber (1966) were able to show that
intravenous administered tetracycline in dogs rapidly
emerges within the sulcus. It seems likely that a major
pathway of entrance into oral cavity of systemically
administered tetracycline is in the gingival sulcus. The
concentration of drug seems to be five times higher in
samples of gingival fluid as compared to the concen-
trations in serum; other drugs that have been detected
in human gingival crevicular fluid are minocycline,
erythromycin, clindamycin and metronidazole.
Influence of Mechanical Stimuli
Mechanical stimulation of the marginal gingiva, such as
massage by means of a round instrument, causes a
significant increase in the permeability of the blood vessels
located below the junctional and sulcular epithelia. The
sensitivity of gingival vasculature has led several investi-
gators to find out whether certain usual powerful
mechanical stimuli such as chewing or occlusal overload
could influence the rate of gingival fluid production.
The effect of chewing was investigated by Brill (1959)
in a group of 15 nurses aged 18 to 22 years, showing
clinically healthy gingival margins, each subject chewed
a piece of paraffin for 10 minutes and samples of gingival
fluid was recovered by the Brill technique. The amount
of gingival fluid was shown to increase significantly under
the influence of chewing. Even the minor stimulus in
139
Defence Mechanisms of the Gingiva
the form of intrasulcular placement of paper strips
increases the production of fluid.
Smoking: Smoking produces an immediate but transient
increase in gingival crevicular fluid flow.
Periodontal Therapy and Gingival Fluid
Measurements of gingival fluid flow have been per-
formed before and after different types of periodontal
therapy.
Oral prophylaxis: It causes a decrease in the fluid flow
one week after oral prophylaxis and then slowly returned
to pre treatment values.
After surgical procedure: One week after gingivectomy
there was a striking increase in the gingival fluid flow
due to the increased inflammatory cells in the smear from
the sulci. This increase was probably the result of the
inflammatory reaction from the gingival trauma with the
restoration of gingival integrity, a gradual drop in fluid
flow occurred and the scores for the gingival fluid flow
reached to minimum values five weeks after gingivectomy.
KEY POINTS TO NOTE
1. The structure and function of the gingival marginal area are
now known with more precision. In a perfectly sound
histologically normal gingiva, a few polymorphonuclear
neutrophils can be seen migrating through the junctional
epithelium while very little or no gingival fluid can be
collected.
2. In a clinically, healthy gingiva a small area of infiltrated
connective tissue can be seen and a very little fluid can be
collected in the absence of irritation. In the beginning, the
fluid seems to contain a low concentration of proteins and
could represent interstitial liquid generated locally by an
osmotic gradient as a result of an increased permeability of
gingival venules; it may progress to a classical inflammatory
exudate, containing higher amounts of total protein.
3. The junctional and sulcular epithelia are permeable to a
variety of substances. Two methods have been proposed
for the collection of material from the gingival sulcus,
absorbant paper strips, capillaries, gingival washings and
plastic strips or platinum loops are used. The first method,
absorbant paper strips are most utilized, even for the
quantitative investigations on the composition of gingival
fluid. Approximate amount of fluid projected into the oral
cavity is 0.5 to 2.4 ml/day.
4. It has been shown, that the absolute number of leukocytes
increases with the intensity of the inflammatory process.
More than 90 percent of the leukocytes are polymorpho-
nuclear neutrophils and most of them possess the capacity
to phagocytosis.
5. Various concentrations of ions in the gingival fluid were
demonstrated. The sodium potassium ratio of the fluid was
proved to be positively correlated with the severity of
periodontal destruction. The general organic composition
of gingival fluid seems to be similar to that of serum. Various
immunoglobulins and complement components were also
demonstrated. Furthermore, the fluid contains metabolic
products which are normally not found in serum or found
only in minute concentrations.
6. In preliminary investigations, the pH of fluid was found to
be around 7.54 to 7.89. The fluid contains a variety of
enzymes, produced both by the cells of the host or by the
bacteria.
7. The concentration of lysosomal enzymes in the fluid seems
to increase in case of gingivitis and periodontitis, indicating
a possible role of the enzymes in the pathogenesis of the
lesions. It was also proved that, systemic administration of
antibiotics such as tetracycline is found in a higher
concentration in human gingival fluid as compared to serum.
8. In conclusion, one can say that the origin, the composition
and the clinical significance of gingival fluid are now known
with more precision and have significantly helped in the
understanding of the pathogenesis of periodontal disease.
REVIEW QUESTIONS
1. Describe the functions and significance of gingival
crevicular fluid.
2. Describe the methods of collection of gingival
crevicular fluid.
3. What is the composition of gingival crevicular fluid?
4. Describe the various defence mechanisms of gingiva.
BIBLIOGRAPHY
1. Cimasoni G. Monographs in oral science crevicular fluid
updated 1992.
2. Lamster IB, Celenti R, Ebersole J. The relationship of serum
IgG antibody titers to periodontal pathogens to indicators of
the host response in gingival crevicular fluid. J. Clin
Periodontol 1990; 17: 419.
3. Mc Laughlin WS, Lovat FM, Macgregor IDM et al. The
immediate effects of smoking on gingival fluid flow. J Clin
Periodontol 1993; 20: 448.
4. Newman, Takei, Carranza. Clinical Periodontology. Ninth
edition, W.B Saunders. 2003.
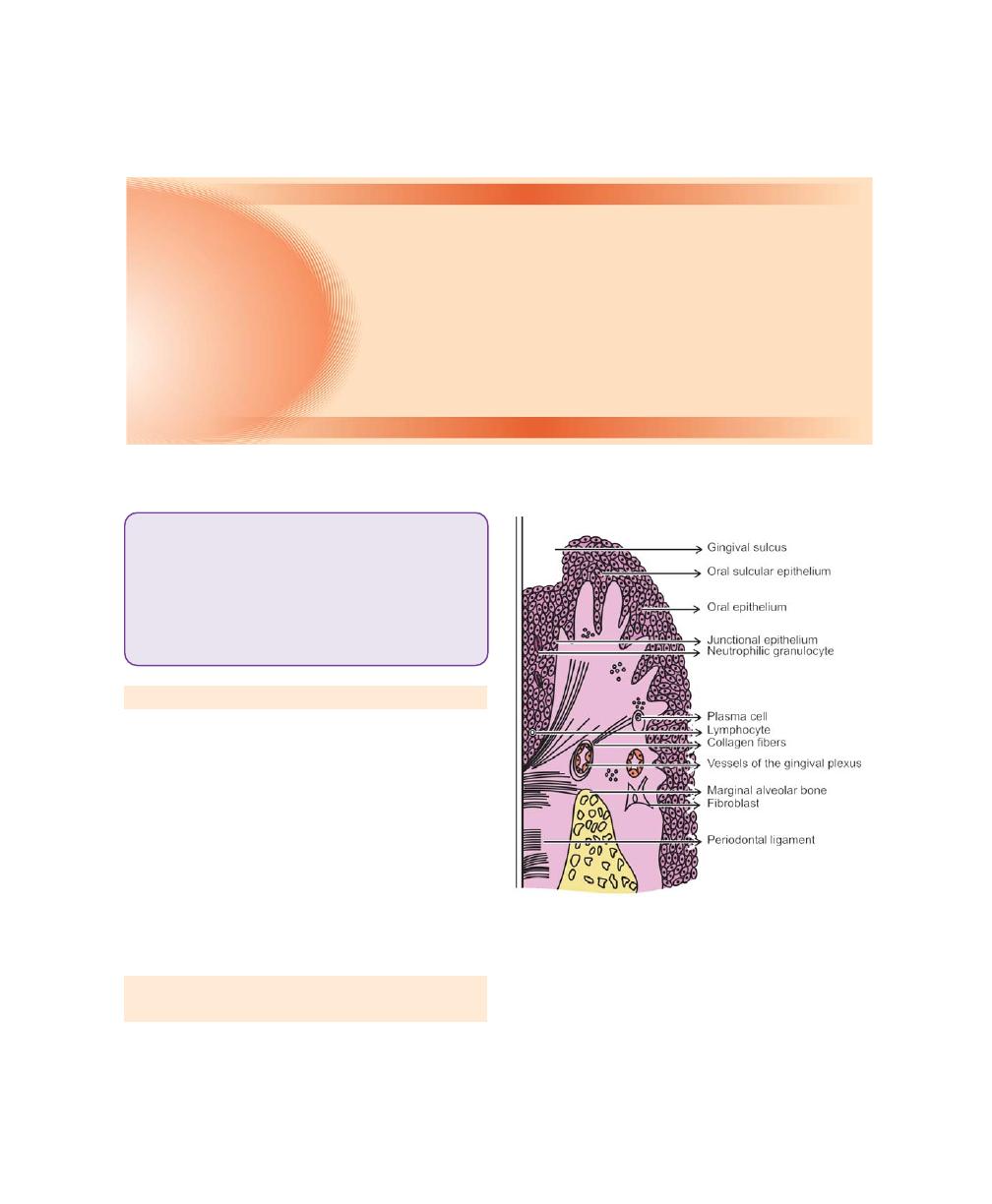
140
Essentials of Clinical Periodontology and Periodontics
INTRODUCTION
Inflammation of gingiva is termed as gingivitis. The
plaque microorganisms can exert its effect on perio-
dontium by releasing certain products (e.g. collagenase,
hyaluronidase, protease, chondroitin sulfatase) which can
cause damage to the epithelial and connective tissue
constituents. The intercellular spaces between the
junctional epithelial cells are destroyed and may permit
the bacterial products or bacteria themselves to gain access
into the connective tissue (Fig. 17.1).
The sequence of events during the development of
gingivitis can occur in four different stages.
STAGE I GINGIVITIS: THE INITIAL
LESION (Fig. 17.2)
Clinically, no visible changes are seen except presence
of exudation of fluid from the gingival sulcus, hence this
condition is called subclinical gingivitis.
Fig. 17.1:
Normal marginal gingiva
The following features are observed in stage I
gingivitis:
1. Classic vasculitis of vessels subjacent to the junctional
epithelium.
2. Exudation of fluid from gingival sulcus.
3. Changes in the coronal most portion of the junctional
epithelium.
❒
❒
❒
❒
❒ STAGE I GINGIVITIS: THE INITIAL
LESION
❒
❒
❒
❒
❒ STAGE II GINGIVITIS: THE EARLY
LESION
❒
❒
❒
❒
❒ STAGE III GINGIVITIS: THE
ESTABLISHED LESION
❒
❒
❒
❒
❒ STAGE IV GINGIVITIS: THE
ADVANCED LESION
17
Gingival Inflammation
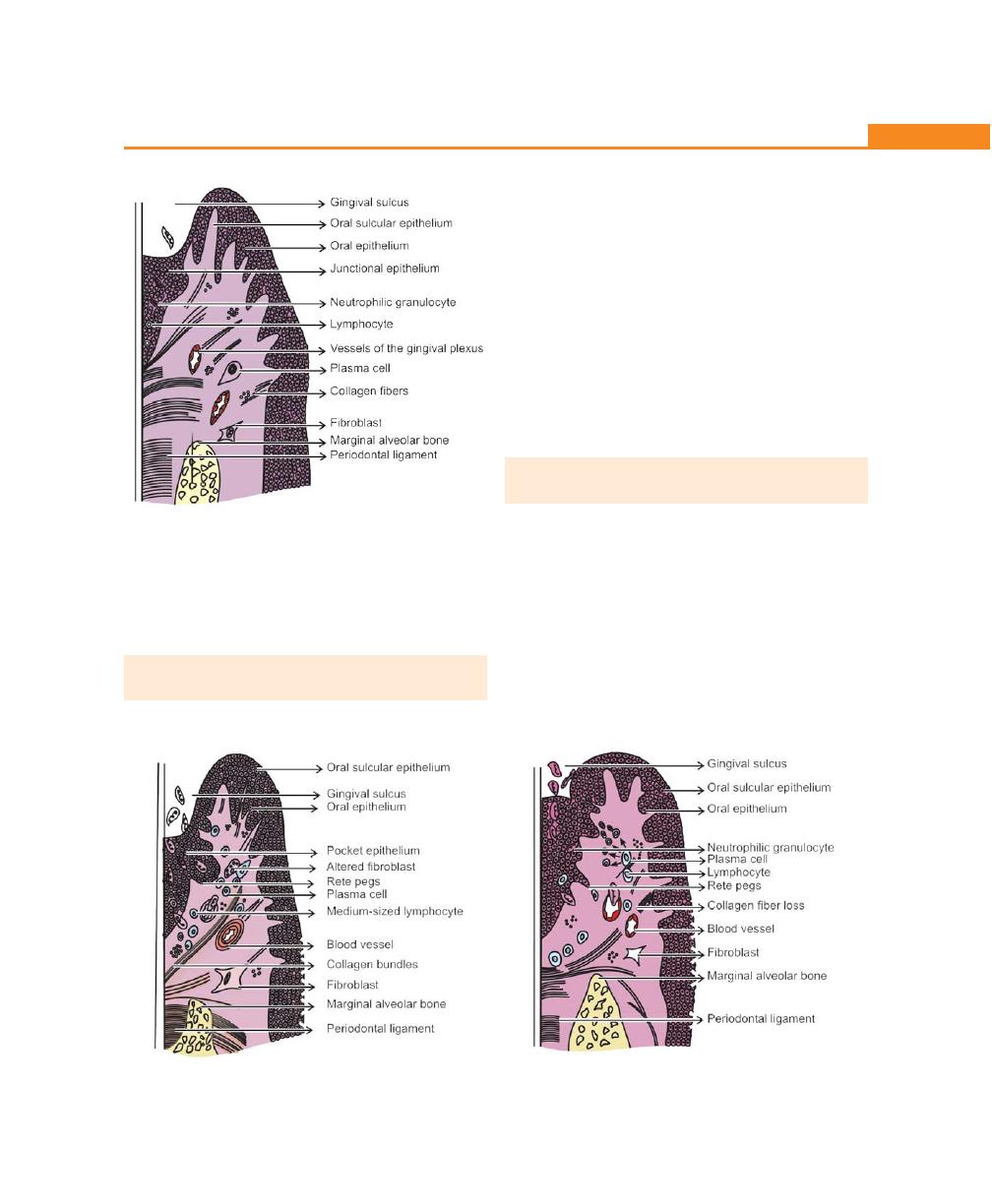
141
Gingival Inflammation
Fig. 17.2:
Initial lesion
4. Increased migration of the leukocytes into the
junctional epithelium and gingival sulcus.
5. Presence of serum proteins.
6. Loss of perivascular collagen.
STAGE II GINGIVITIS: THE EARLY LESION
(Fig. 17.3)
Clinically, erythematous gingiva and bleeding on probing
Fig. 17.3:
Early lesion
may be evident. Microscopic features of the early lesion
include:
1. All the changes seen in the initial lesion continue to
intensify.
2. The junctional epithelium may begin to show the
development of rete pegs or ridges.
3. Accumulation of lymphocytes (majority of them are
T cells) beneath the junctional epithelium.
4. Further loss of collagen fibre network supporting the
marginal gingiva.
5. Fibroblasts show cytotoxic alteration with a decreased
capacity for collagen production.
STAGE III GINGIVITIS: THE ESTABLISHED
LESION (Fig. 17.4)
Clinical changes include:
a. A bluish hue on the reddened gingiva due to impaired
venous return.
b. The gingiva appears to be moderately to severely
inflammed.
Microscopically
1. Predominant inflammatory cell types are plasma cells,
which invades epithelium and also deep into the
Fig. 17.4:
Established lesion

142
Essentials of Clinical Periodontology and Periodontics
connective tissue, around the blood vessels and
between the bundles of collagen fibres.
2. Proliferation, apical migration and lateral extension
of the junctional epithelium is seen. Early pocket
formation may or may not be present.
3. Further collagen destruction and continuing loss of
connective tissue substance seen in the early lesion.
4. The following enzyme levels are said to be elevated
in chronically inflammed gingiva, acid and alkaline
phosphatase, β-glucuronidase, aminopeptidase and
others.
STAGE IV GINGIVITIS: THE ADVANCED
LESION
The advanced lesion is also known as phase of advanced
periodontal breakdown.
The following clinical and microscopic features are
seen:
1. Persistence of features described in the established
lesion.
2. Extension of the lesion into the alveolar bone and
periodontal ligament leading to significant amount
of bone loss.
3. Continued loss of collagen.
4. Formation of periodontal pockets.
5. Conversion of bone marrow into fibrous tissue.
6. Presence of almost all the types of inflammatory cells.
Progression from Health to Periodontitis
Clinically
Histologically
Pristine condition
→
Health
↓
↓
Clinically healthy
→
Initial lesion
↓
↓
Early gingivitis
→
Early lesion
↓
↓
Chronic periodontitis
→
Advanced lesion
BIBLIOGRAPHY
1. Brecx MC. Histophysiology and histopathology of the gingiva.
J West Soc Periodontal 1991;39:33.
2. Brecx MC, Lehman B, Siegmart CM et al. Observations of the
initial stages of healing following human experimental
gingivitis. A clinical and morphological study. J Clin
Periodontal 1988;15:123.
3. Saul Schluger. Periodontal diseases, basic phenomena,
chemical management and oclusal and restorative
interrealtionshipes, 2nd edn, Lea and Febiger, 1990.
143
Clinical Features of Gingivitis
TYPES OF GINGIVITIS
• Depending on course and duration.
• Depending on distribution.
Depending on the Course and Duration
• Acute gingivitis is of sudden onset and short duration
and can be painful.
• Subacute is a less severe phase of acute condition.
• Recurrent gingivitis reappears either after treatment
or disappears spontaneously.
• Chronic gingivitis is slow in onset, of long duration,
usually painless and the most commonly occurring
gingival condition.
Depending on the Distribution
If the condition is involving a single tooth or group of
teeth it is called localized gingivitis, while generalized
gingivitis involves entire mouth.
According to distribution, gingivitis could be marginal,
papillary or diffuse. If the inflammation is limited to the
marginal gingiva, the condition is termed as marginal
gingivitis. In papillary gingivitis the inflammation is limited
to the interdental papilla. When the inflammation
spreads to attached gingiva also, it is termed as diffuse
gingivitis, i.e. involving marginal, papilla and attached
gingiva. Papillary, marginal and diffuse gingivitis can
occur as localized or generalized conditions (Figs 18.1
to 18.5).
CLINICAL FINDINGS
While examining the gingiva clinically, one must adapt
a systematic approach. Close attention should be given
to any tissue alterations, because they contribute to
diagnosis. The gingiva is examined for the following
characteristics, color, contour, consistency, size, position,
severity of bleeding, surface texture. (Summary of these
features are discussed in detail in the Table 18.1).
❒
❒
❒
❒
❒ TYPES OF GINGIVITIS
• Depending on Course and Duration
• Depending on the Distribution
❒
❒
❒
❒
❒ CLINICAL FINDINGS
• Gingival Bleeding on Probing
• Color Changes in the Gingiva
• Changes in Consistency and Size of
Gingiva
• Surface Texture
• Changes in the Position of Gingiva
• Changes in Gingival Contour
18
Clinical Features of
Gingivitis
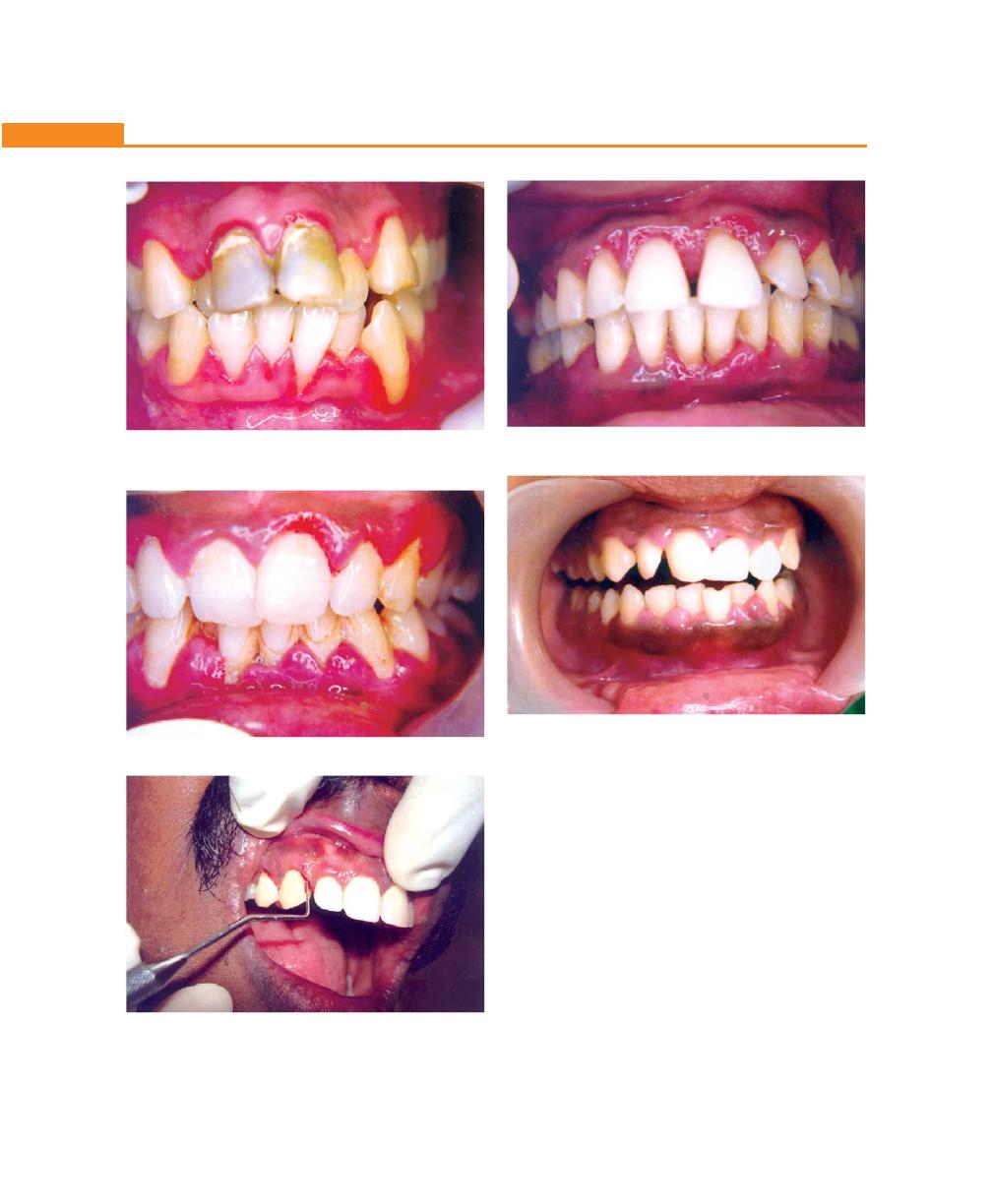
144
Essentials of Clinical Periodontology and Periodontics
Fig. 18.2:
Papillary gingivitis
Fig. 18.3:
Bleeding on probing
Fig. 18.4:
Gingivitis—inflammatory type
Fig. 18.5:
Gingivitis—fibrotic type
Gingival Bleeding on Probing
1. Significance of gingival bleeding.
2. Etiological factors responsible for gingival bleeding.
3. Associated microscopic changes.
Significance of Gingival Bleeding on Probing
a. It is one of the earliest visual signs of inflammation.
b. It can appear earlier than color changes or any other
visual signs of inflammation.
c. It also provides an additional advantage, by being
a more objective sign that requires less subjective
estimation by the examiner.
Fig. 18.1:
Chronic gingivitis. The marginal and interdental
gingiva are smooth edematous and discolored

145
Clinical Features of Gingivitis
Table 18.1: Gingiva in health and disease
Gingival
In health
Factors
In disease
Factors
features
Responsible
responsible
Clinical changes
Disease conditions
1. Color
Coral
– Vascular
Color change may be
Chronic
• Vascular prolifera-
Pink
supply
• Marginal
gingivitis
tion (Erythematous)
– Thickness
• Diffuse
• Reduction of kera-
and degree of
• Diffuse or patch like
tinization owing to
keratinization
epithelium compres-
of epithelium
Varying shades of red,
Chronic gingivitis
sion by inflamed
– Presence of pigment
reddish blue, deep blue.
tissues
containing cells
Color changes from
Acute gingivitis
bright red erythema
• A.N.U.G./H.I.V.
(Erythematous)
– Shiny slate gray
gingivitis
• Venous stasis
– Dull whitish gray
• Herpetic
(Bluish red)
gingivostomatitis
• Tissue necrosis
• Chemical irritation
(Bluish red)
Black line following the
Bismuth, arsenic and
Perivascular precipita-
contour of margin.
mercury pigmentation
tion of metallic sulfides
in subepithelial connec-
Bluish red or deep blue
Lead
tive tissue, only in areas
linear pigmentation
pigmentation
of inflammation due to
(Burtonian line)
increased permeability
of irritated blood vessel.
Violet marginal line.
Silver pigmentation
Systemic diseases
causing pigmentation
2. Contour Marginal gin-
• Shape of the tooth
Marginal gingiva becomes
Chronic gingivitis
Inflammatory changes
giva: Scalloped
and thus alignment
rolled or rounded Interden-
and knife edged
in the arch
tal papilla becomes blunt
and flat
Interdental
• Location and size of
Punched out and crater
ANUG
papilla:
proximal contact
like depressions at the
Anterior:
• Dimensions of facial
crest of inter dental
pyramidal
and lingual gingival
papilla extending to the
Posterior:
embrasures
marginal gingiva
tent shaped
Irregularly-shaped
Chronic desquamative
denuded appearance
gingivitis
Exaggerated scalloping
Ginginval recession
Apostrophe-shaped
Stillman’s cleft
Described by Stillman as
indentations extending
a result of trauma from
from and into the gingival
occlusion and by Box as
margins for varying dis-
a Pathological pocket.
tances on the facial
Enlargement of inter-
surface
dental papilla with no
Life saver like enlargement
Mc Call’s festoon
enlargement of marginal
of the marginal gingiva
gingiva (Pseudocleft)
(Canine and premolar
facial region)
3. Con-
Firm and
Collagenous nature of
• Soggy puffiness that
• Chronic gingivitis
• Infiltration by fluids
sistency resilient
lamina propria and its
pits on pressure
and cells of inflam-
(except free
contiguity with the
matory exudate
margins)
mucoperiosteum of
• Marked softness and
• Exudative
• Degeneration of
alveolar bone. Cellular
friability
connective tissue and
and fluid content of
epithelium
tissue.
• Firm leathery
• Fibrotic
• Fibrosis and epithe-
lium proliferation
Contd...

146
Essentials of Clinical Periodontology and Periodontics
• Diffuse puffiness and
• Diffuse edema of
softening
acute inflammatory
origin
• Sloughing: grayish flake
• Necrosis with pseudo
like particle of debris
Acute gingivitis
membrane formation
• Vesicle formation
• Intercellular and
intracellular edema.
4. Size
Normal
Sum total of the bulk
Increased
Gingival enlargement
Increase in fibers and
of cellular and inter-
decrease in cells – Non-
cellular elements and
inflammatory type.
their vascular supply
Increase in cells and
decrease in fibers –
Inflammatory type.
5. Surface Stippling is
• Due to attachment of
Loss of stippling
Gingivitis
Due to destruction of
texture
present
the gingival fibers to
• Smooth and shiny
• Exudative chronic
gingival fibres as a
(viewed by
the underlying bone.
gingivitis
result of inflammation
drying)
• Firm and nodular
• Fibrotic chronic
gingivitis
• Microscopically by
• Peeling of surface
• Chronic desqua-
alternate rounded
mative gingivitis
protuberance and
• Leathery texture
• Hyperkeratosis
depressions in the
• Minutely nodular
• Non inflammatory
gingival surface.
surface
gingival hyperplasia
(Papillary layer of
connective tissue
projects into the
elevations)
6. Position 1 mm above
• Position of tooth in
• Apically placed
Gingival recession
• Tooth brush trauma
the cemento
the arch
enamel
• Root bone angle
• Coronally placed
Pseudo pockets
• Gingival inflamma-
junction
• Mesio distal cur-
tion
vature of tooth
• High frenum attach-
surface
ment
• Tooth malposition
• Friction from soft
tissue
7. Bleeding
Intact sulcular epithe-
Present
Dilation and engorge-
on
lium and normal
• Chronic recurrent,
• Chronic gingivitis
ment of capillaries and
probing
capillaries
spontaneous bleeding
• A.N.U.G.,
thinning or ulceration of
or bleeding on slight
Systemic diseases
sulcular epithelium
provocation
Contd...
Gingival
In health
Factors
In disease
Factors
features
Responsible
responsible
Clinical changes
Disease conditions
d. Gingival bleeding on probing also helps us to
determine whether the lesion is in an active or inactive
state. In inactive lesion there will be little or no
bleeding on probing, whereas active lesions bleed
more readily on probing.
e. The severity and ease with which bleeding can be
provoked indicates the intensity of the inflammation.
Etiological Factors Responsible for Gingival
Bleeding on Probing
These are divided into:
a. Local factors: Those factors that result in acute
bleeding.
b. Systemic factors: Those factors that cause chronic
bleeding.

147
Clinical Features of Gingivitis
Acute bleeding is caused due to:
1. Tooth brush trauma,
2. Impactation of sharp pieces of hard food,
3. Gingival burns from hot foods or chemicals,
4. In conditions such as acute necrotizing ulcerative
gingivitis (ANUG).
Chronic bleeding: The most common causes are:
1. Chronic inflammation due to the presence of plaque
and calculus
2. Mechanical trauma e.g., from tooth brushing, tooth
picks or food impaction.
3. Biting into solid foods such as apples.
Systemic factors: include various systemic diseases such
as:
1. Hemorrhagic diseases including, vitamin C deficiency,
vitamin K deficiency, platelet disorders such as
thrombocytopenic purpura, other coagulation defects
such as hemophilia, leukemia and others.
2. Bleeding could also be as a result of excessive
administration of drugs such as salicylates and
anticoagulants such as dicumarol and heparin.
Microscopic Changes Associated with Gingival
Bleeding on Probing
In plaque-induced gingival inflammation, the following
histological changes are seen:
a. In the epithelium: Thinning and micro-ulcerations of
the sulcular epithelium is seen. Thinning of the
epithelium could be due to the toxic substances
released by plaque bacteria which destroys the inter-
cellular junctions. Micro-ulcerations are as a result of
bacteria trying to gain entry into the connective tissue
by breaking through the epithelium or in response
to inflammation, the neutrophils from the connective
tissue cross the epithelial barrier to reach the site of
infection, in doing so they cause ulceration in the
epithelium or combination of both.
b. In the connective tissue: Dilation and engorgement
of the capillaries takes place. Since the capillaries are
engorged and closer to the surface which is already
thinned and less protective, stimuli that are otherwise
innocuous can cause rupture of the capillaries which
may result in gingival bleeding.
Color Changes in the Gingiva
Color of the gingiva is an important clinical sign of gingival
diseases. Normally gingiva appears to be “coral pink”.
The factors that are responsible for this are tissue
vascularity,degree of keratinization and thickness of the
epithelium. Generally color of the gingiva may change
to red, to bluish-red, to pale-pink. When there is
increased vascularity or reduced epithelial keratinization,
the gingiva becomes more red. The color becomes pale
when vascularization is reduced or epithelial keratinization
increases. Venous stasis gives a bluish hue to the gingiva.
Detailed description of the changes in color in health
and disease is discussed in Table 18.1.
Changes in the color may start from the interdental
papilla and later spread to marginal and attached gingiva.
Systemically absorbed heavy metals may also cause
gingival pigmentation, e.g. bismuth, arsenic, mercury,
lead and silver. Abnormal melanin pigmentation of the
gingiva may be observed in conditions like Addison’s
disease, Peutz-Jeghers syndrome, Albright’s syndrome
and von-Recklinghausen’s disease.
Changes in the Consistency of Gingiva
Normal gingiva exhibits a firm and resilient consistency.
Factors that are responsible are cellular and fluid content
and collagenous nature of lamina propria. In disease
conditions, it can be soggy and edematous or firm and
leathery in consistency.
Changes in the Size of the Gingiva
Normal size depends on the sum of the bulk of cellular
and intercellular elements and their vascular supply. In
disease; the size is increased which can be termed as
gingival enlargement. The factors responsible for this are
increase in fibers and decrease in cells as in non
inflammatory type. Where as in inflammatory type there
will be increase in cells and decrease in fibers.
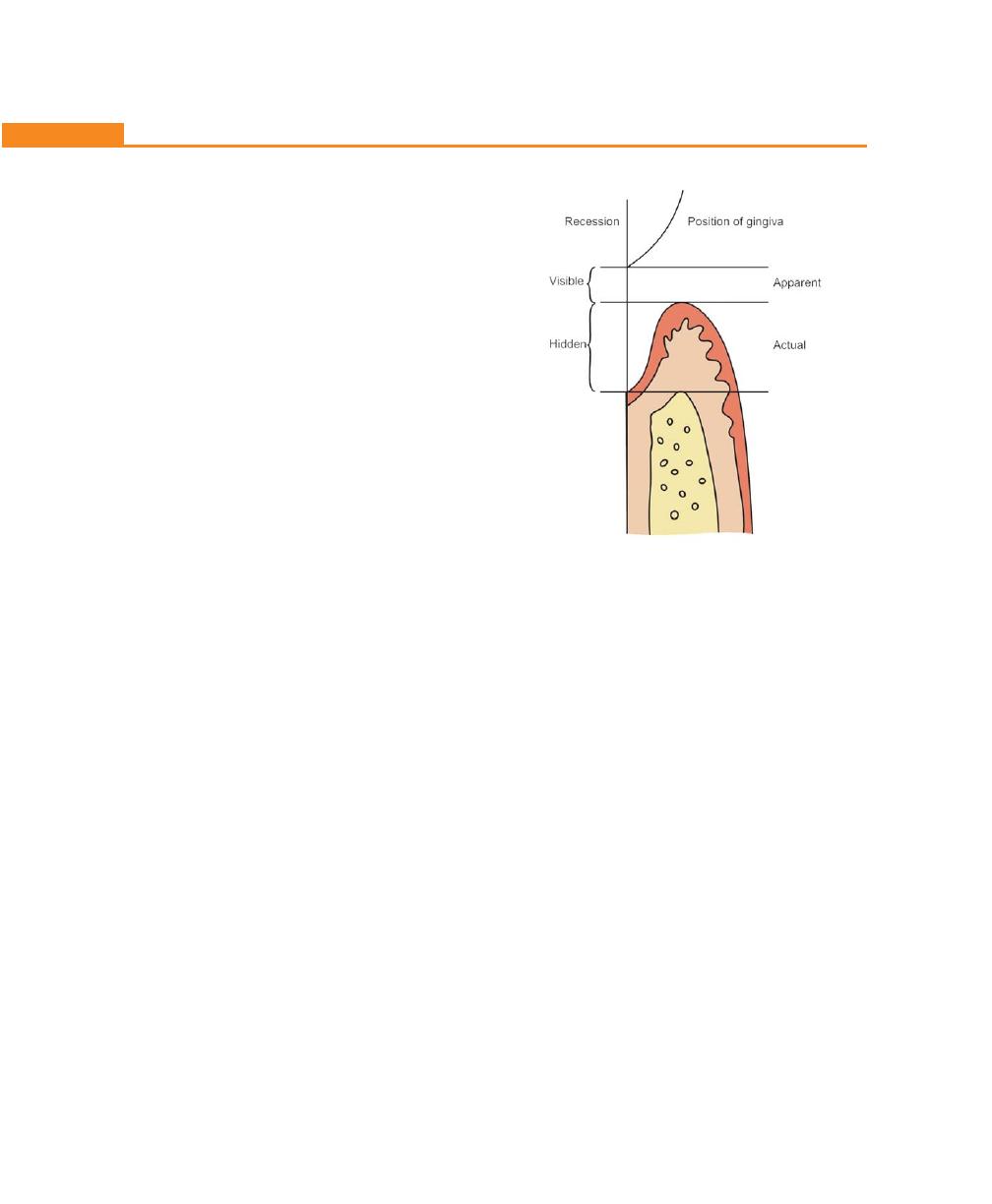
148
Essentials of Clinical Periodontology and Periodontics
Surface Texture
Under normal conditions, gingiva appears to be stippled
(orange peel appearance) due to attachment of gingival
fibers to the underlying bone. Microscopically, alternate
rounded protuberance and depressions in the gingival
layer may give rise to stippled appearance. Stippling is
absent in disease conditions, hence the gingiva may
appear smooth and shiny.
Changes in the Position of Gingiva
Normally the gingiva is attached to the tooth at the
cemento-enamel junction. In disease, the position can
be shifted either coronally (pseudopocket) or apical to
the cemento-enamel junction (gingival recession).
Definition
Gingival recession is defined as the exposure of the root
surface by an apical shift in the position of the gingiva.
Types
There are two types of recession i.e., visible, which is
clinically observable and hidden, which is covered by
gingiva and can only be measured with probe. Gingival
recession may also be localized or generalized.
Position of the gingiva can be actual or apparent (Fig.
18.6). Actual position is the level of epithelial attachment
on the tooth i.e., from the cemento-enamel junction to
the probable depth of the pocket, whereas apparent
position is the level of crest of the gingival margin i.e.,
from the cemento-enamel junction to the gingival margin.
Classification of Gingival Recession
Two classification systems are available:
I. According to Sullivan and Atkins—Shallow-narrow,
shallow-wide, deep-narrow and deep-wide.
II. According to P.D. Miller’s—Class I, Class II, Class III
and Class IV.
Class I: Marginal tissue recession that does not extend
to the mucogingival junction. There is no loss of bone
or soft tissue in the interdental area. This can be narrow
or wide.
Class II: Marginal tissue recession that extends to or
beyond the mucogingival junction. There is no loss of
bone or soft tissue in the interdental area. This can be
narrow or wide.
Class III: Marginal tissue recession that extends to or
beyond the mucogingival junction. In addition, there is
loss of bone and/or soft tissue in the interdental area
or there is malpositioning of the tooth.
Class IV: Marginal tissue recession that extends to or
beyond the mucogingival junction with severe loss of
bone and soft tissue interdentally and/or severe
malpositioning of the tooth.
• Prognosis of class I and II is good to excellent
• Class III–only partial coverage can be expected
• Class IV–Poor prognosis.
Etiology of Gingival Recession
(Figs 18.7 to 18.11)
Plaque-induced gingival inflammation is the primary
etiological factor responsible for gingival recession; next
most common cause is faulty tooth brushing. Other
Fig. 18.6:
Apparent and actual positions of gingiva
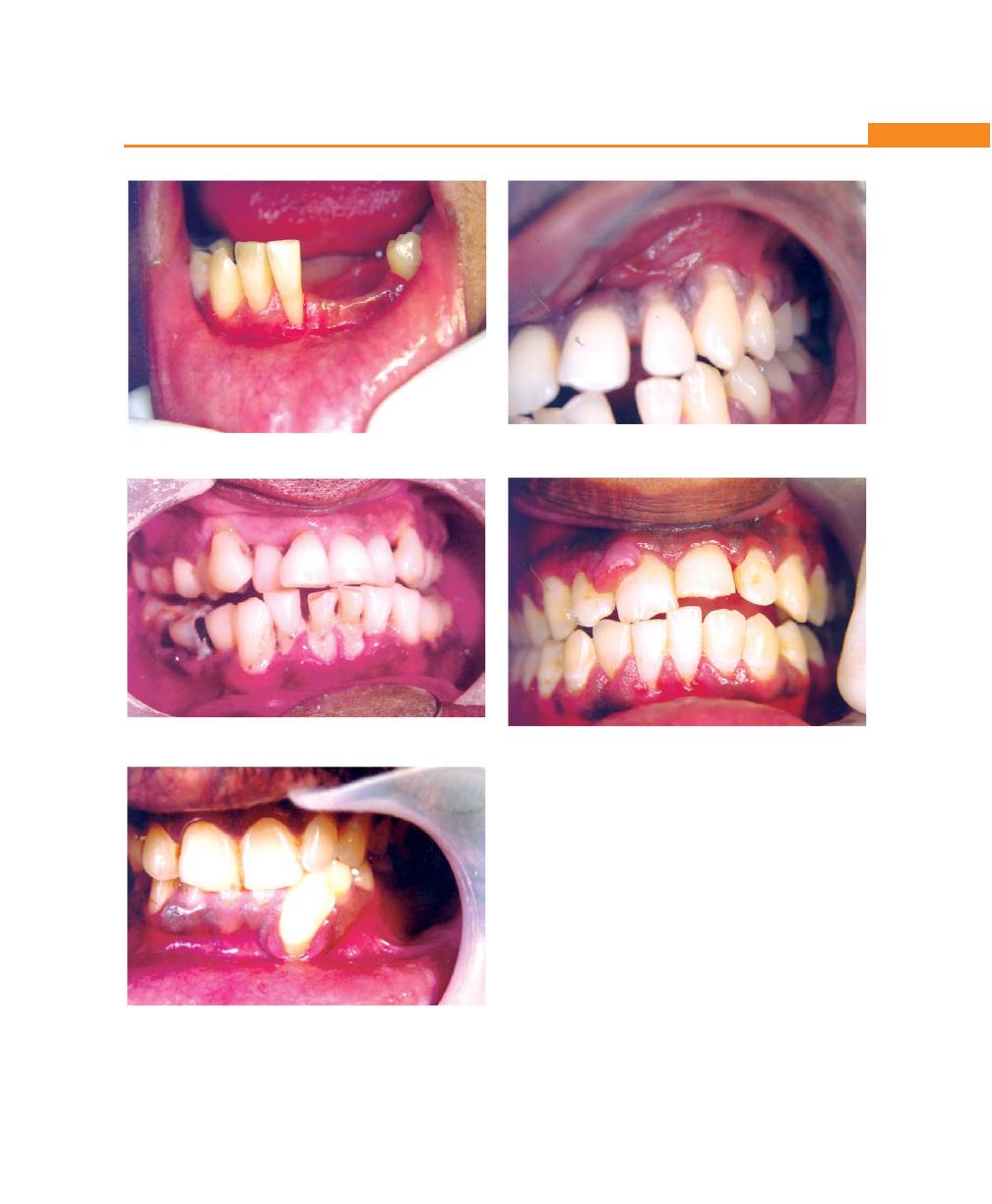
149
Clinical Features of Gingivitis
Fig. 18.7:
Gingival recession due to inflammation
Fig. 18.8:
Gingival recession associated with periodontitis
Fig. 18.9:
Gingival recession associated with tooth
malpositioning
Fig. 18.10:
Gingival recession associated with faulty tooth
brushing
Fig. 18.11:
Stillman’s cleft in the lower gingiva
secondary/contributing factors of gingival recession are
broadly categorized (for convenience) as:
a. Anatomic factors
b. Habits
c. Iatrogenic factors
d. Physiologic factors
a. Anatomic factors include:
1. Tooth malposition or position of the tooth in the
arch. When a tooth is labially placed, the perio-
dontium on the labial aspect will be invariably
thin. When this is exposed to any kind of trauma
or frictional forces, gingival recession results.
150
Essentials of Clinical Periodontology and Periodontics
2. Presence of dehiscence and fenestrations.
3. Gingival ablation from soft tissues like cheek, lips,
etc.
4. Root-bone angle and mesiodistal curvature of the
tooth surface. In rotated and facially-displaced
teeth, bony plates are either thinned or shortened
and recession results from repeated trauma to the
thin periodontal tissues.
b. Habits: Faulty tooth brushing or brushing with hard
bristles may lead to gingival recession. Recently it has
been noted that there may be a positive relationship
between smoking and recession. But the exact
mechanism is not reported.
c. Iatrogenic factors: Primary trauma from occlusion has
been reported to cause gingival recession. Ortho-
dontic movement in a labial direction and improper
restorations can lead to gingival recession.
d. Physiologic factors: Gingival recession was thought
to be a physiologic process related to aging. However,
this idea was discarded because there was no
convincing evidence for a physiologic shift of the
gingival attachment.
Clinical Significance of Gingival Recession
1. The exposed root surface may be extremely sensitive
2. Hyperemia of the pulp may result due to gingival
recession
3. Interproximal recession creates oral hygiene problems
thereby resulting in plaque accumulation.
4. Finally it is aesthetically unacceptable.
Changes in Gingival Contour
Normally, marginal gingiva is scalloped and knife edged,
whereas interdental papilla in the anterior region is
pyramidal and posteriorly tent-shaped. The factors that
maintain normal contour are, shape of the teeth and
its alignment in the arch, location and size of the proximal
contact and dimensions of facial and lingual gingival
embrasures. In diseased conditions, the marginal gingiva
may become rounded or rolled whereas interdental
papilla can become blunt and flat. The various diseased
conditions related to gingival contour is given in
Table 18.1.
KEY POINTS TO NOTE
1. Inflammation of the gingiva is termed as gingivitis.
2. Gingivitis can be classified depending on its course and
duration; and its distribution.
3. Gingiva can be examined for its color, contour, consistency,
size, position, surface texture and gingival bleeding on
probing.
4. Causes for gingival bleeding on probing could be local
factors and systemic factors.
5. Normal color of gingiva is “coral pink”. In disease it can
change to red, bluish-red or pale-pink.
6. Normally, consistency of gingiva is firm and resilient. In
diseased conditions it can exhibit soft and edematous or
firm and leathery consistency.
7. Contour is scalloped and knife edged, interdental papilla
in the anterior region is pyramidal and posteriorly tent-
shaped. In disease, it can become rounded or rolled, where
as interdental papilla can become blunt and flat.
8. Normal stippled gingiva may appear smooth and shiny. In
diseased conditions, destruction of gingival fibers is
responsible for loss of stippling.
9. Position of the gingiva can be actual or apparent. When
position is shifted apically exposing the root surface, it is
called as gingival recession.
REVIEW QUESTIONS
1. Describe in detail gingiva in health and disease.
2. What are the causes of gingival bleeding of probing?
3. Write about definition, types and etiology of gingival
recession.
BIBLIOGRAPHY
1. David M. Williams, Francis J. Hugley. Pathology of Periodontal
Diseases. Oxford Medical Publishers 1992.
2. Newman, Takei, Fermin A. Carranza. Clinical Periodontology.
Ninth Edition 2002; W.B. Saunders Co.
151
Gingival Enlargements
INTRODUCTION
Current clinical descriptive terminology used to describe
increase in size of the gingiva is, gingival enlargement
and gingival overgrowth.
CLASSIFICATION
A. According to etiologic factors and pathologic
changes, gingival enlargements could be listed out
as:
I. Inflammatory enlargement
a. Chronic
b. Acute
II. Drug-induced enlargement
III. Enlargements associated with systemic diseases
a. Conditioned enlargement
1. Pregnancy
2. Puberty
3. Vitamin C deficiency
4. Plasma cell gingivitis
5. Non-specific conditioned enlargement
(Granuloma pyogenicum)
b. Systemic diseases causing gingival enlargements
1. Leukemia
2. Granulomatous diseases
❒
❒
❒
❒
❒ CLASSIFICATION OF GINGIVAL
ENLARGEMENTS
❒
❒
❒
❒
❒ INFLAMMATORY ENLARGEMENT
• Acute Inflammatory enlargement
• Chronic Inflammatory enlargement
❒
❒
❒
❒
❒ NON-INFLAMMATORY GINGIVAL
ENLARGEMENT
• Phenytoin-induced Gingival Hyperplasia
• Idiopathic Gingival Fibromatosis
• Combined Enlargement
❒
❒
❒
❒
❒ ENLARGEMENT ASSOCIATED WITH
SYSTEMIC DISEASE OR CONDITION
• Conditioned Enlargement
• Enlargement in Pregnancy
• Enlargement in Puberty
• Vitamin C Deficiency
• Plasma Cell Gingivitis
• Non-specific Conditioned Enlargement
(Granuloma Pyogenicum)
• Systemic Disease Causing Gingival
Enlargement
• Granulomatous Diseases
❒
❒
❒
❒
❒ NEOPLASTIC ENLARGEMENT (GINGIVAL
TUMOR)
• Benign Tumors
• Malignant Tumors
❒
❒
❒
❒
❒ FALSE ENLARGEMENT
• Underlying Osseous Lesions
• Underlying Dental Tissues
19
Gingival Enlargements

152
Essentials of Clinical Periodontology and Periodontics
Table 19.1: Inflammatory enlargement
Chronic Inflammatory Enlargement
Acute Inflammatory Enlargement
Etiology
Prolonged exposure to plaque and the
From bacteria carried deep into the tissues.
factors that favor plaque retention
When a foreign object like a tooth brush or a
lobster shell fragment is forcefully embedded into
the gingiva.
Location and Distribution Generally papillary or marginal gingiva.
Localized: Marginal or papillary, e.g. gingival/
May be localized or generalized.
periodontal abscess
Clinical Features
Life preserver like bulge around the
Painful, rapidly-expanding lesion of sudden onset.
involved tooth. Occasionally, occurs as
Within 24 to 48 hours, it becomes fluctuant and
a discrete mass which is sessile or
pointed with a surface orifice through which
pedunculated. Sometimes painful
purulent exudate comes out.
ulceration in the fluid between marginal
and adjacent gingiva.
Histopathology
Preponderance of inflammatory cells
Gingival abscess consists of a purulent focus in the
and fluid with vascular engorgement,
connective tissue surrounded by a diffuse
capillary formation and degenerative
infiltration of polymorphonuclear neutrophils,
changes.
edematous tissue and vascular engorgement.
IV. Neoplastic enlargement (Gingival tumors)
a. Benign tumors
b. Malignant tumors
V. False enlargement
B. According to location and distribution, gingival
enlargement can be classified as follows:
Localized
: Gingival enlargement limited to one or
more (group of) teeth.
Generalized : Entire mouth, the gingiva is enlarged
Marginal
: Limited to the marginal gingiva
Papillary
: Confined to the interdental papilla
Diffuse
: Involves all the parts of the gingiva, i.e.
marginal, attached and interdental
gingiva
Discrete
: Isolated sessile or pedunculated tumor-
like enlargement
According to the Degree of Gingival Enlargement
Grade 0 : No sign of gingival enlargement
Grade I : Enlargement confined to the interdental
papilla
Grade II : Enlargement involves papilla and marginal
gingiva
Grade III : Enlargement covers three quarters or more
of the crown
INFLAMMATORY ENLARGEMENT (Table 19.1)
It can be of two types:
a. Acute inflammatory enlargement
b. Chronic inflammatory enlargement
Acute Inflammatory Enlargement (Table 19.2)
Gingival abscess is a localized, painful, rapidly expanding
lesion, that is usually sudden in onset.
Signs and Symptoms
a. It is a rapidly expanding lesion, which is usually limited
to marginal gingiva or interdental papilla
b. It appears as a red swelling with smooth shiny surface
which is painful and the associated teeth are sensitive
to percussion.
c. The lesion becomes fluctuant and pointed with a
surface orifice from which purulent exudate may be
expressed. If it is allowed to progress the lesion may
rupture spontaneously.
Etiology
It occurs as a result of bacteria, being carried deep into
the tissues when foreign substances such as toothbrush
bristle or fragments of food substance are forcefully
embedded into the gingiva.

153
Gingival Enlargements
Table 19.2: Acute inflammatory enlargement
Gingival abscess
Periodontal abscess
Periapical abscess
Pericoronal abscess
Location
Localized swelling
Usually affects the
Usually seen near the
Seen near the incompletely
affecting the
deeper periodontal
root apex, i.e. in the
erupted teeth
marginal and
structures including
mucogingival junction
interdental gingiva
deep pockets,
and alveolar mucosa
furcations and vertical
osseous defects and
located beyond the
mucogingival junction
Etiology
Impactation of
Periodontal pocket
Due to dental caries
Plaque induced inflammation
foreign objects in
related to destruction
involving the pulp and its
of the pericoronal flap. i.e.
previously healthy
by periodontitis
extension into the
pericoronitis
sites
periapical area
Associated
Gingiva appears to
• Associated with a
• Mostly deep carious
• Gingiva overlying the
clinical
be red, swollen and
periodontal pocket
involvement of tooth
partially erupted or
findings
extremely painful
which may be either
which is non-vital.
unerupted tooth.
and sometimes
suprabony or
• Pocket may or may
• Appears to be red,
impacted foreign
infrabony.
not be present.
erythematous, swollen
object may still be
• Tooth elevation and
Mobility is absent.
and extremely painful.
embedded into the
mobility may be seen • Tooth is tender on
• Swelling may interfere
gingiva
• Tooth is tender on
percussion
with occlusion.
lateral percussion.
• Pain cannot be
• Flap can be separated
• Pain is localized and
localized
from the tooth with
patient can identify
• May be associated
severe food impaction.
the offending tooth.
with sinus tract.
• Affected tooth may
be vital or sometimes
non-vital
• May be associated
with a fistula.
Radiographic No bone loss is
Bone loss is seen. Radio- No bone loss.
Impacted tooth
features
evident
lucency along the lateral Periapical radiolucency
aspect of the root.
seen.
Histopathology
It consists of a purulent focus surrounded by a diffuse
infiltration of polymorphonuclear leukocytes, edematous
tissue and vascular engorgement. The surface epithelium
is ulcerated and shows varying degrees of intra and
extracellular edema, invaded by leukocytes.
Periodontal (lateral abscess) also produce enlarge-
ment of the gingiva, but they also involve the supporting
periodontal tissues.
Treatment of Gingival Abscess
• If the cause of the abscess is still present it should
be removed.
• Drainage can be established by warm salt water
mouthwashes used every 2 hours.
• If the lesion persists it can be curetted under local
anesthesia or incised, if it is pointing.
• If it is persistent and severe systemic antibiotic may
be prescribed.
• Any residual pocketing can be removed by
subgingival curettage or localized gingivectomy.
Chronic Inflammatory Enlargement (Fig. 19.1)
Types are:
a. Localized
b. Generalized
c. Discrete (Tumor-like)
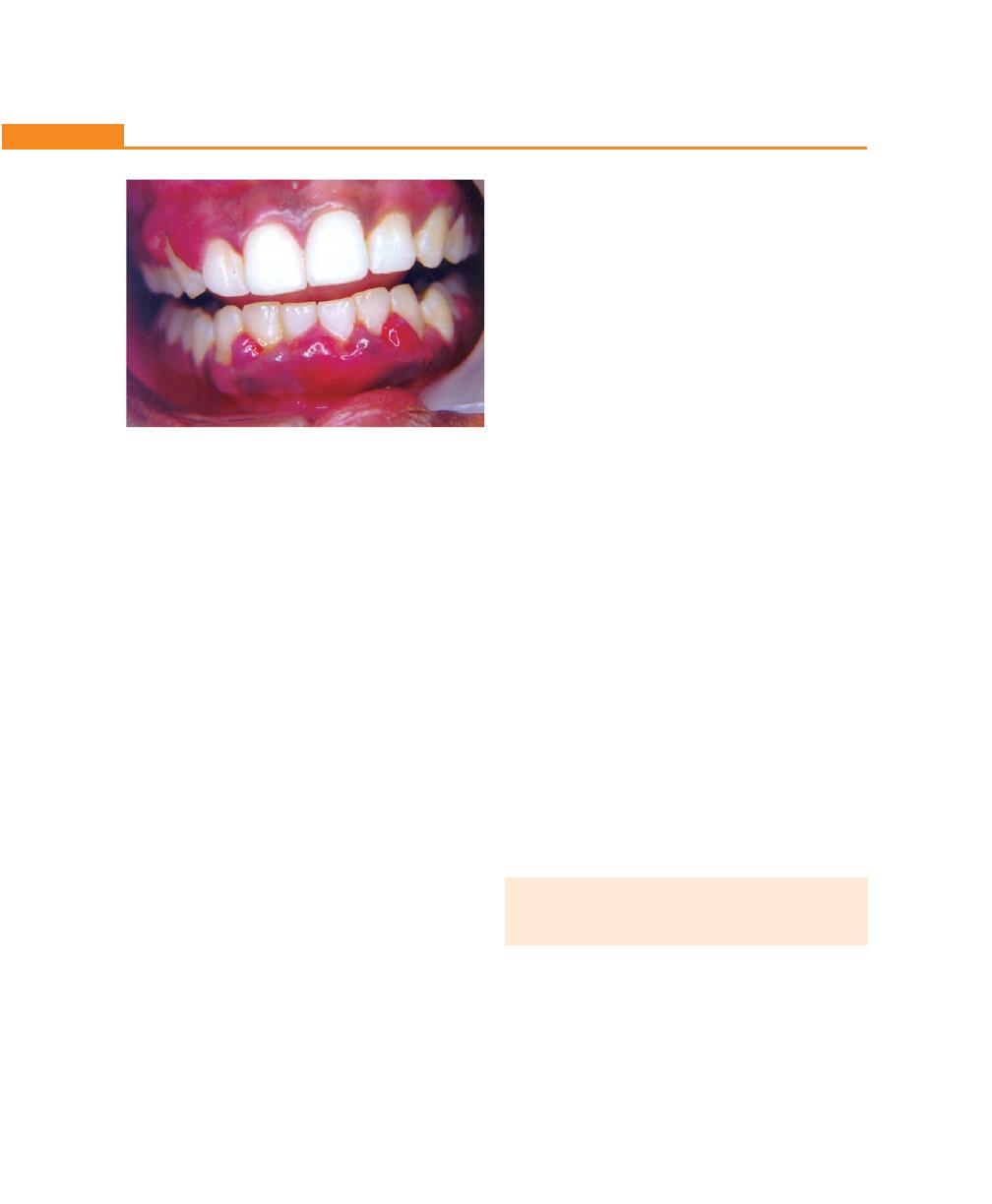
154
Essentials of Clinical Periodontology and Periodontics
Localized/Generalized
• It originates as a slight ballooning of the interdental
papilla or marginal gingiva.
• In the early stages, it produces a lifesaver-like bulge
around the involved tooth and this bulge increases
until it covers part of the crown.
• It progresses slowly and painlessly unless it is
complicated by acute infection or trauma.
Discrete/Tumor-like
Occasionally they may occur as a discrete sessile or
pedunculated mass resembling a tumour. It may occur
in the interproximal/marginal/attached gingiva.
Histopathology
• Microscopically it can exhibit either exudative/
proliferative features depending on the clinical lesion.
• The lesions are clinically deep red or bluish-red, are
soft and friable with a smooth-shiny surface and bleed
easily.
• They will have preponderance of inflammatory
cells and fluid with vascular engorgement, new
capillary formation and associated degenerative
changes.
• Lesions that are relatively firm, resilient and pink will
have a greater fibrotic component with an abundance
of fibroblasts and collagen fibers.
Etiology
It is caused by prolonged local irritation. Following are
the typical etiological factors; poor oral hygiene,
abnormal relationships of adjacent teeth and opposing
teeth, lack of tooth function, cervical cavities, overhanging
margins of dental restorations, food impaction, irritation
from clasps or saddle areas of removable prosthesis, nasal
obstructions, habits such as mouth breathing and tongue
thrusting.
In mouth breathers:
• Gingivitis and gingival enlargement are often seen.
• The gingiva appears to be red and edematous with
a diffuse shiny surface at the exposed area.
• Maxillary anterior region is the common site and their
effects are generally attributed to irritation from
surface dehydration.
Treatment
a. Scaling and curettage: If the size of the enlargement
does not interfere with the complete removal of depo-
sits, the enlargement caused due to inflammation is
treated by scaling and curettage.
b. Surgical removal: It is indicated for two reasons,
i. In enlargement with significant fibrotic component
that does not undergo shrinkage following scaling
and curettage.
ii. If the size of the enlargement interferes with the
access to the root surface deposits.
Surgical techniques include:
a. Gingivectomy technique: The incision should be
atleast 1 to 2 mm coronal to the mucogingival line.
b. Flap operation.
NON-INFLAMMATORY GINGIVAL
ENLARGEMENT (FIBROTIC GINGIVAL
ENLARGEMENT) (Table 19.3)
• This is produced by factors other than local irritation.
• This condition is uncommon and most of the cases
occur due to drugs such as phenytoin, cyclosporin,
nifedipine.
• In few cases, dilitiazam, verapamil and sodium
valproate can also result in fibrotic enlargement.
Fig. 19.1:
Chronic inflammatory gingival enlargement
localized to the lower anterior region
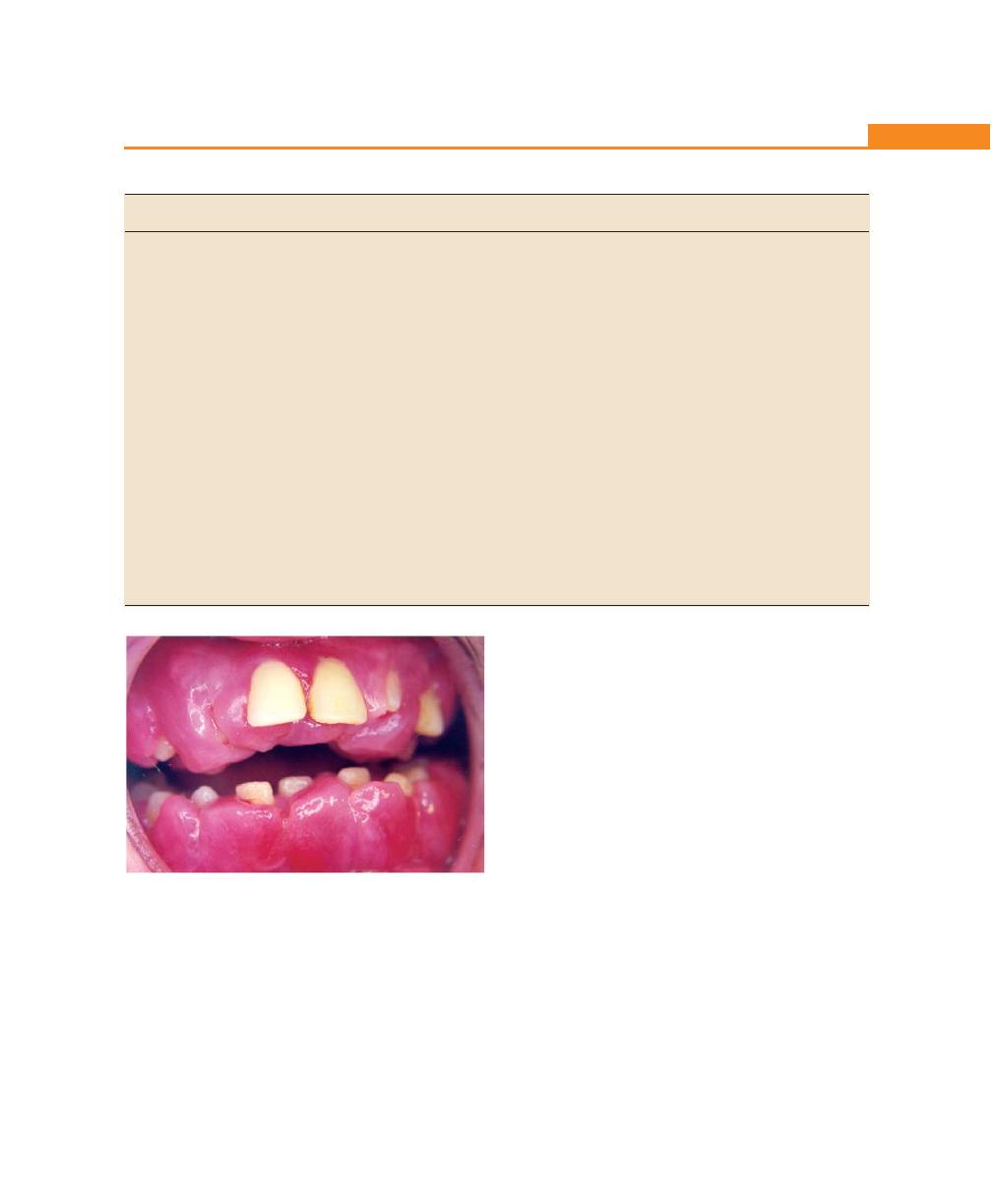
155
Gingival Enlargements
Table 19.3: Non-inflammatory gingival enlargement
Drug-induced fibrotic enlargement
Idiopathic gingival enlargement
(Phenytoin, cyclosporin, nifedipine)
Etiology
Long-term therapy of the respective drug
Unknown, possible etiology may be hereditary
Location
Marginal and papillary, generalized
Diffuse enlargement and generalized
Clinical features • Bead-like enlargement of facial and lingual
• Facial and lingual surface of maxillary and
gingival margins
mandibular teeth are affected but involvement
• Massive tissue folds covering the crowns
limited to either jaw
of teeth interfering with occlusion
• Enlarged gingiva is pink in color, firm and leathery
• Appears to project from beneath the
in consistency and has a characteristic pebbled
gingival margin
surface
• Does not occur in edentulous spaces
• Enlargement projects into the oral vestibule
• More severe in maxillary and mandibular
and jaw appears distorted
anterior region
• It may occur in mouths with little or no
plaque and may be absent in mouths with
abundant deposits
Histopathology
• Hyperplasia of connective tissue and
Increase in the amount of connective tissue and
epithelium
consists of densely-arranged collagen bundles and
• Abundance of amorphous ground substance
numerous fibroblasts
• Fibroblast to collagen ratio is equal to that
of normal gingiva
• The connective tissue appears highly vascularized
in cyclosporine-induced enlargement
Fig. 19.2:
Phenytoin-induced gingival
enlargement
Clinical Features
• The overgrowth of the gingiva usually becomes
apparent in the first three months after phenytoin
dosage and is most rapid in the first year.
• Clinically it starts as a painless, bead-like enlargement
of facial and lingual gingival margins and interdental
papillae.
• As the condition progresses, the marginal and papillary
enlargement unite and develop into a massive tissue
fold covering a considerable portion of the crown
and may interfere with the occlusion.
• When uncomplicated by inflammation, the lesion is
mulberry-shaped, firm, pale-pink and resilient with
a minutely lobulated surface and no tendency to bleed.
• The enlargement characteristically appears to project
from beneath the gingival margin, from which it is
separated by a linear groove.
• The hyperplasia is usually generalized throughout the
mouth, but is more severe in maxillary and
mandibular anterior region.
Phenytoin-induced Gingival Hyperplasia
(Fig. 19.2)
Phenytoin is an anticonvulsant drug widely used in the
control of epilepsy and other convulsive disorders. It
is also used in trigeminal and glossopharyngeal
neuralgias.
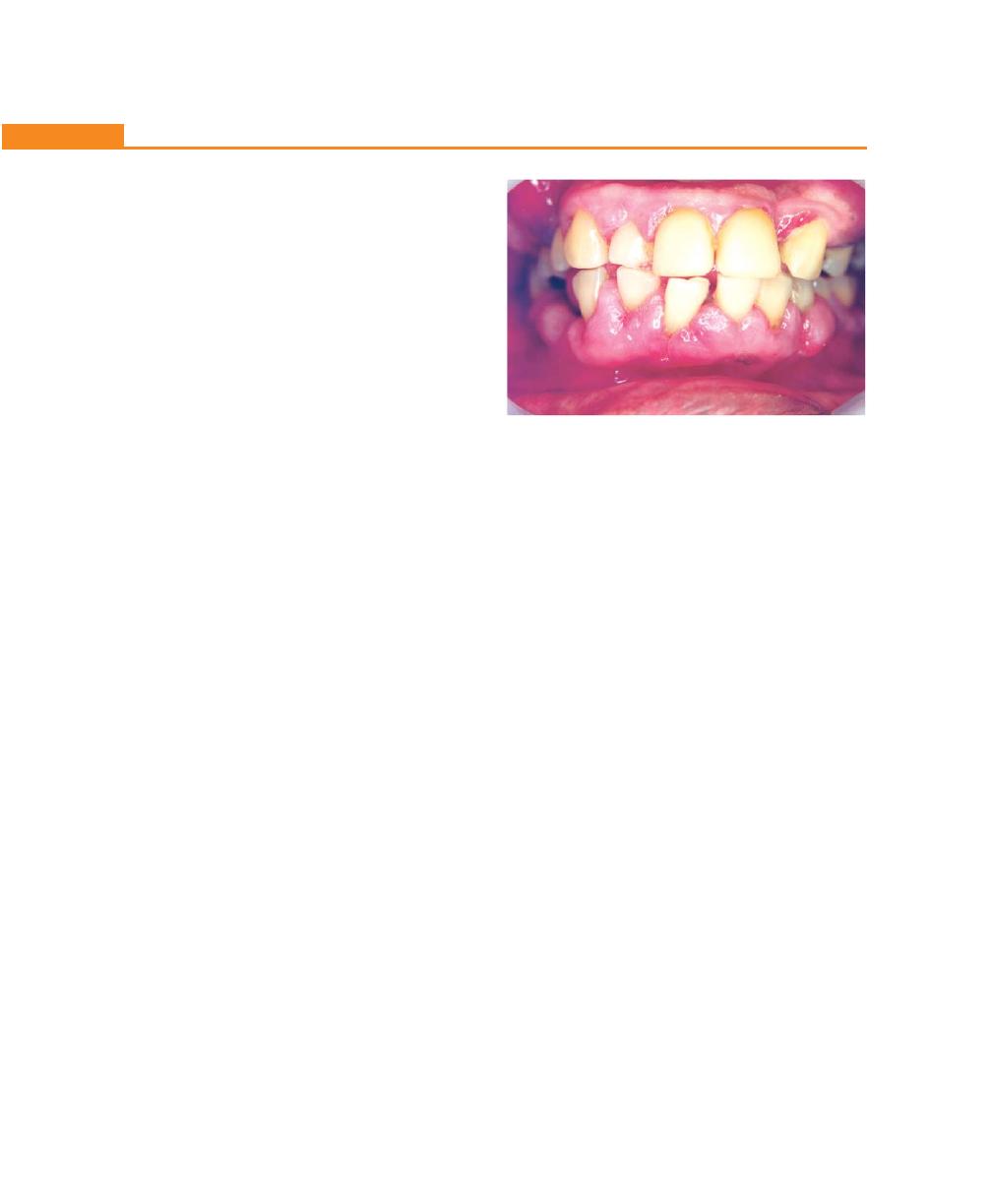
156
Essentials of Clinical Periodontology and Periodontics
• It occurs in areas in which teeth are present, but
hyperplasia of the mucosa in the edentulous mouth
has been reported, but is rare.
• The presence of the enlargement will result in a
secondary inflammatory process that complicates
gingival hyperplasia caused by the drug.
• Secondary inflammatory changes produce red or
bluish-red discoloration and result in an increased
tendency towards bleeding.
Histopathology
Several changes have been observed in both epithelium
and connective tissue.
• The epithelium shows varying degree of acanthosis,
with elongated, thin rete pegs/ridges that tend to
divide at their ends.
• This can give rise to increased incidence of epithelial
pearls.
• The degree of inflammation will determine the
presence and extent of polymorphonuclear neutro-
phils in the gingival epithelium.
• The main change in the lamina propria is prolifera-
tion of fibroblasts and increase in the collagen
production.
Pathogenesis
There are many theories as to why phenytoin causes
gingival overgrowth. The most convincing at present is
the direct effect of drug or metabolites on the gingival
tissue.
Major metabolite of phenytoin is 5-(parahydroxy
phenyl) –5–phenyl hydantoin (5-P-HPPH).
It is suggested that there are different sub-population
of fibroblasts in gingival tissue, some of which synthesize
large amount of protein and collagen (high activity
fibroblasts) and others which are only capable of low
protein synthesis (low activity fibroblasts).
“Hassell” suggested that high activity fibroblasts
become sensitive to phenytoin, with subsequent increase
in collagen production.
Cyclosporine (Fig. 19.3)
• It is a fairly potent immunosuppressive agent used
to prevent organ transplant rejection and to treat
several diseases of autoimmune origin.
• It appears to be selectively and reversibly inhibit
T helper cells, which play a role in cellular and humoral
immune responses.
• In 30 percent of the cases, gingival growth was
recorded.
• Clinically and microscopically the gingival hyperplasia
induced by cyclosporine is similar to that, induced
by phenytoin.
Nifedipine
• It is a calcium channel blocker that induces direct
dilatation of coronary arteries and arterioles,
improving oxygen supply to heart muscle.
• It also reduces hypertension by dilating the peripheral
vasculature.
• Gingival over growth occurs in about 20 percent of
cases.
Treatment of Drug-associated Gingival
Enlargement
Three different types of drugs are associated with gingival
enlargement, namely anti-convulsants, calcium channel
blockers and the immunosuppresants like cyclosporine.
Treatment options are:
Fig. 19.3:
Gingival enlargement associated with
cyclosporine therapy
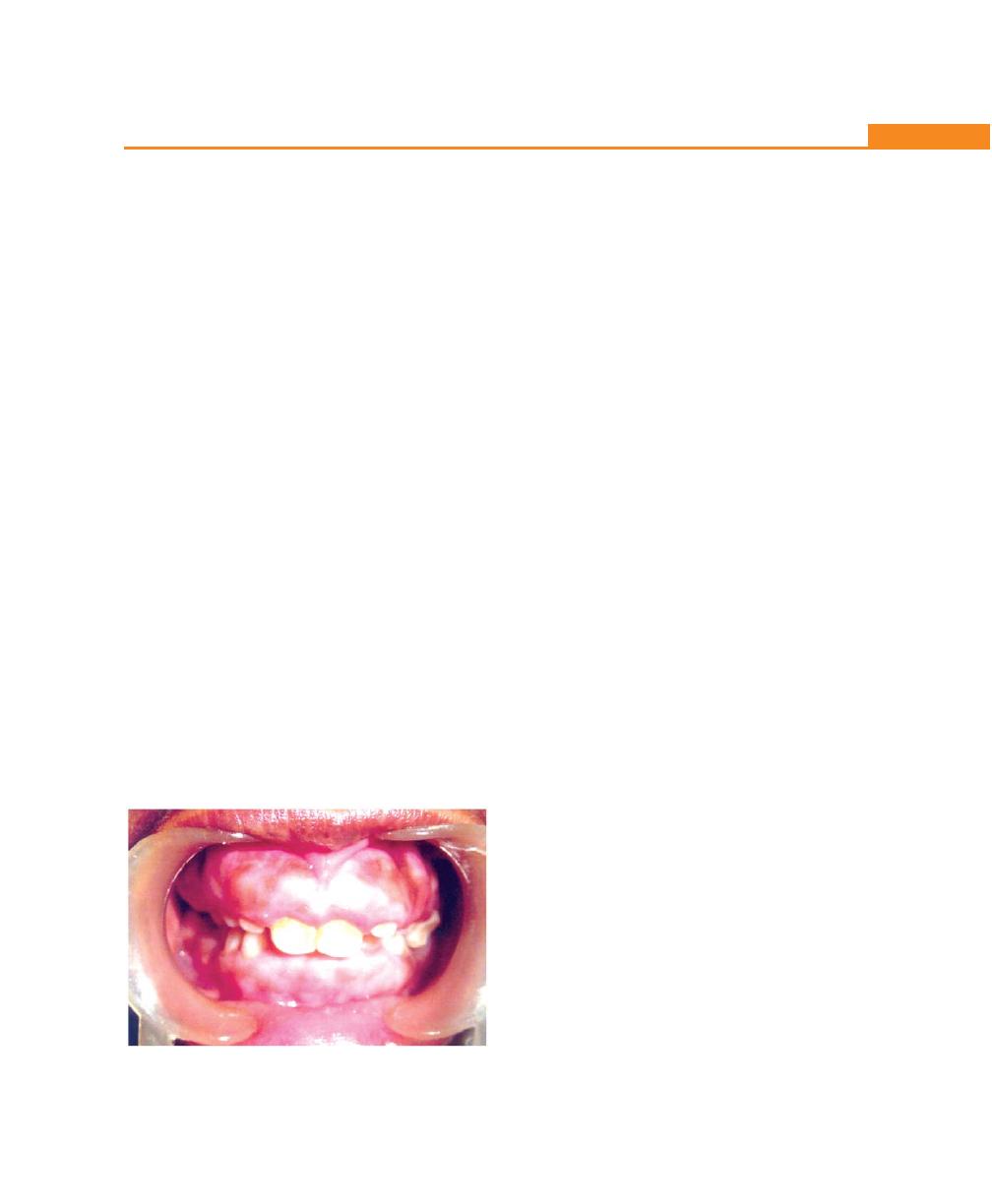
157
Gingival Enlargements
Clinical Features
• The enlargement affects the attached gingiva as well
as gingival margins and interdental papillae (diffuse).
• The gingiva is firm, pink and leathery in consistency
and has a characteristic pebbled surface.
• In severe cases, the teeth are almost completely
covered and the gingival enlargement projects into
the oral vestibule.
• The jaws appear distorted because of bulbous
enlargement of the gingiva. Secondary inflammatory
changes are common at the gingival margin.
Histopathology
• The surface epithelium is thickened and acanthotic
with elongated rete pegs.
• There is an increase in the amount of connective
tissue, which is relatively avascular and consists of
densely-arranged collagen fiber bundles and
numerous fibroblasts.
Etiology
It is unknown. Some cases have hereditary basis. But
the exact mechanism is not well understood.
• Gingival hyperplasia has been described in “tuberous
sclerosis”, which is an inherited condition characterized
by a triad of epilepsy, mental deficiency and cutaneous
angiofibromas.
Combined Enlargement
It results when gingival hyperplasia is complicated by
secondary inflammatory changes. These changes occur
when gingival hyperplasia produces conditions favorable
for the accumulation of plaque and interferes with
effective oral hygiene measures.
It consists of two components:
a. Primary or basic hyperplasia of connective tissue and
epithelium, the origin of which is unrelated to
inflammation.
b. Secondary complicating inflammatory component.
Fig. 19.4:
Idiopathic hyperplastic gingival enlargement
First Step
• Oral hygiene reinforcement, chlorhexidine gluconate
rinses, scaling and root planing.
• Possible drug substitution. When it is attempted it is
necessary to allow at least a period of 6 to 12 months
between the discontinuation of the offending
drug and the possible resolution of gingival
enlargement.
• Professional recalls.
Second Step
If enlargement persists even after following the above
mentioned approaches, surgical therapy is indicated.
There are two surgical options available based on the
features it presents:
• Small areas of enlargement with no attachment loss
or bone loss and has good keratinized tissue,
gingivectomy is the technique of choice.
• Large areas of enlargement with presence of osseous
defects and limited keratinized gingiva, periodontal
flap surgery may be indicated.
Idiopathic Gingival Fibromatosis (Fig. 19.4)
Other designated terms include, gingivomatosis,
elephantiasis gingivae, diffuse fibroma, idiopathic
fibromatosis, hereditary gingival hyperplasia and
congenital familial fibromatosis.
158
Essentials of Clinical Periodontology and Periodontics
T
able 19.4:
Enlargement-associated with systemic disease/conditions
Marginal
Puberty
Vitamin C
Plasma cell
Non-specific condi-
Le
uk
emia
Pregnancy
deficiency
gingivitis
tioned enlargement
Pregnancy tumor
(Atypical
(Granuloma
gingivitis)
pyogenicum)
Etiology
•
Altered tissue metabolism
Altered endocrine
Deficiency of
Allergic in origin
Trauma
Malignant neoplasms
(increased estrogen and
disturbance with
vitamin C
possibly to
of
leukocyte
progesterone)
local factors
chewing gums,
precursors
•
Pregnancy accentuates
dentifrices or
the
response to local
various
dietary
irritants
components
Location and
•
Marginal
•
Pregnancy tumor
Marginal and
Marginal gingiva
Marginal and
Localized, discrete
Incidence:
distribution
•
Incidence—
1.8-5% (after
interdental facial
is
involved
attached
•
Acute monocytic
10-70%
third month of
gingiva is enlarged
gingiva
66.7%
Generalized.
pregnancy)
•
Acute myelocytic
More
•
La
bial aspect of
monocytic 18.7%
prominent
anterior teeth
•
Diffuse or marginal
interproximally
•
Localized or generalized
Clinical
Gingiva is
•
Discrete,
•
Prominent bulbous
Gingiva is bluish-
•
Red,
friable,•
Discrete
spherical,
Bluish-red, sponge-
features
bright-red or
mushroom-like,
interproximal
red, soft, friable
so
m
et
im
es
tumor like mass
like and friable,
magenta
spherical mass
papillae, facial
ha
s smooth, shiny
granular
with a peduncu-
bleeds persistently
in color
, soft
flattened,
gi
ng
iv
a i
s e
nl
ar
ge
d,
surface. Tissue is
and bleeds
lated attachment
on
slight provoca-
and friable and
spherical mass
lingual
gingiva
spongy
, hyper
-
easily
to
a
fl
attened,
tion
or spontane-
has a smooth
with sessile or
is unaltered.
emic and bleeds
•
Located on
keloid-like enlar-
ously
. Gingival
shiny surface.
pedunculated base
•
Featur
es ar
e
similar
spontaneously
oral aspect
gement having a
necrosis and pseu-
It has a tendency
that protrude from
to chronic inflam-
•
Surface necrosis
of attached
broad base
domembrane
to bleed
gingival margin
matory
gingival
with pseudo-
gingiva
•
Bright-red
or
formation is seen
spontaneously
or
interproximal
disease
membrane
purple and can
space
formation
be
friable or firm.
•
Generally dusky-
Surface ulceration
red or magenta,
and purulent
has smooth,
exudation
shiny surface with numerous deep-red pin point markings, painless, consis- tency varies from soft and friable to semifirm
Histopathology
Keratinized and
Endothelial proli-
Chronic inflammation
Epithelium
•
Oral epi-
•
Mass
of
granu-
•
Chronic infla-
stratified
feration with
with prominent edema
undergoes
thelium is
lation tissue with
mmation with
squamous
capillary formation
and associated
thinning and
parakerati-
chronic inflam-
mature leuko-
epithelium,
and associated
degenerative changes
shows spongiosis
nized
matory infiltrate
cytes and
which is
inflammation
and may show
•
Ultrastruc-
and endothelial
connective
thickened with
sever
e atrophy
.
turally
proliferation
tissue infiltrated
prominent rete
Connective tissue
shows
•
Surface ulceration
with immature
pegs. Connective
sh
ow
s p
oo
rly
-
signs
of
and exudation are
and prolifera-
tissue shows
formed collagen
damage to
common
ting leukocytes
numerous newly
fibres and many
spinous and
•
Isolated areas of
formed and
thin-walled and
basal layer
ac
ut
e
necrotizing
engorged
leaking blood
•
Connective
inflammation with
capillaries
vessels
tissue
pseudomembra-
with edema
shows
neous meshwork
and leukocyte
dense infil-
of
fibrin, necrotic
infiltration
trate of
epithelial cells,
pl
as
m
a c
el
ls
polymorphonuclear leukocytes, bacteria

159
Gingival Enlargements
Treatment
Gingivectomy/gingivoplasty is indicated.
ENLARGEMENT ASSOCIATED WITH
SYSTEMIC DISEASE OR CONDITION
(Table 19.4)
The systemic diseases/condition can affect the
periodontium by two different mechanisms:
a. Magnification of an existing inflammation initiated by
dental plaque. These groups of diseases include some
of the hormonal conditions (e.g. pregnancy and
puberty), nutritional diseases such as vitamin C
deficiency. Both belong to conditioned enlargement
and some cases in which systemic influence is not
identified—Conditioned enlargement.
b. Manifestation of systemic disease, independently of
the inflammatory status of gingiva. This includes
systemic disease causing gingival enlargement and
neoplastic enlargement.
Conditioned Enlargement
• Hormonal (Pregnancy, puberty)
• Nutritional (Vitamin C deficiency)
• Allergic
Local irritation is necessary for the initiation of this
type of enlargement. Conditioned enlargement occurs
when the systemic condition of the patient exaggerates
the usual gingival response to dental plaque.
Enlargement in Pregnancy
Marginal Enlargement
• Results from the aggravation of previous
inflammation and does not occur without the clinical
evidence of local irritation.
• Pregnancy does not cause the condition. The altered
tissue metabolism in pregnancy accentuates the
response to local irritation.
Clinical features
• The enlargement is usually generalized and tends to
be more prominent interproximally than on the facial
and lingual surface.
• The enlarged gingiva is bright-red or magenta, soft
and friable and has a smooth, shiny surface.
• Bleeding occurs spontaneously or on slight provo-
cation.
Tumor-like Gingival Enlargement or
Pregnancy Tumor
It is not a neoplasm but an inflammatory response to
local irritation and is modified by the patient’s condition.
It usually appears after the first trimester but may also
occur earlier.
Clinical features
• The lesion appears as a discrete mushroom-like
flattened spherical mass that protrude from the
interdental papilla or the gingival margin and is
attached by a sessile or pedunculated base.
• It tends to expand laterally and pressure from the
tongue and cheek increases its flattened appearance.
• Color—dusky red or magenta with smooth glistening
surface that frequently exhibits numerous deep red,
pin-point markings.
• Consistency—semifirm, but may have varying
degrees of softness and friability.
• It is usually painless, unless complicated by either
accumulation of debris under its margin or
interference with occlusion—in which case, painful
ulceration may occur.
Histopathology
• Both marginal, tumor-like enlargement consists of a
central mass of connective tissue, the periphery of
which is outlined with stratified squamous epithelium.
• The connective tissue consists of numerous, engorged
capillaries and between the capillary network is a
fibrous stroma with varying degree of edema and
leukocyte infiltration.
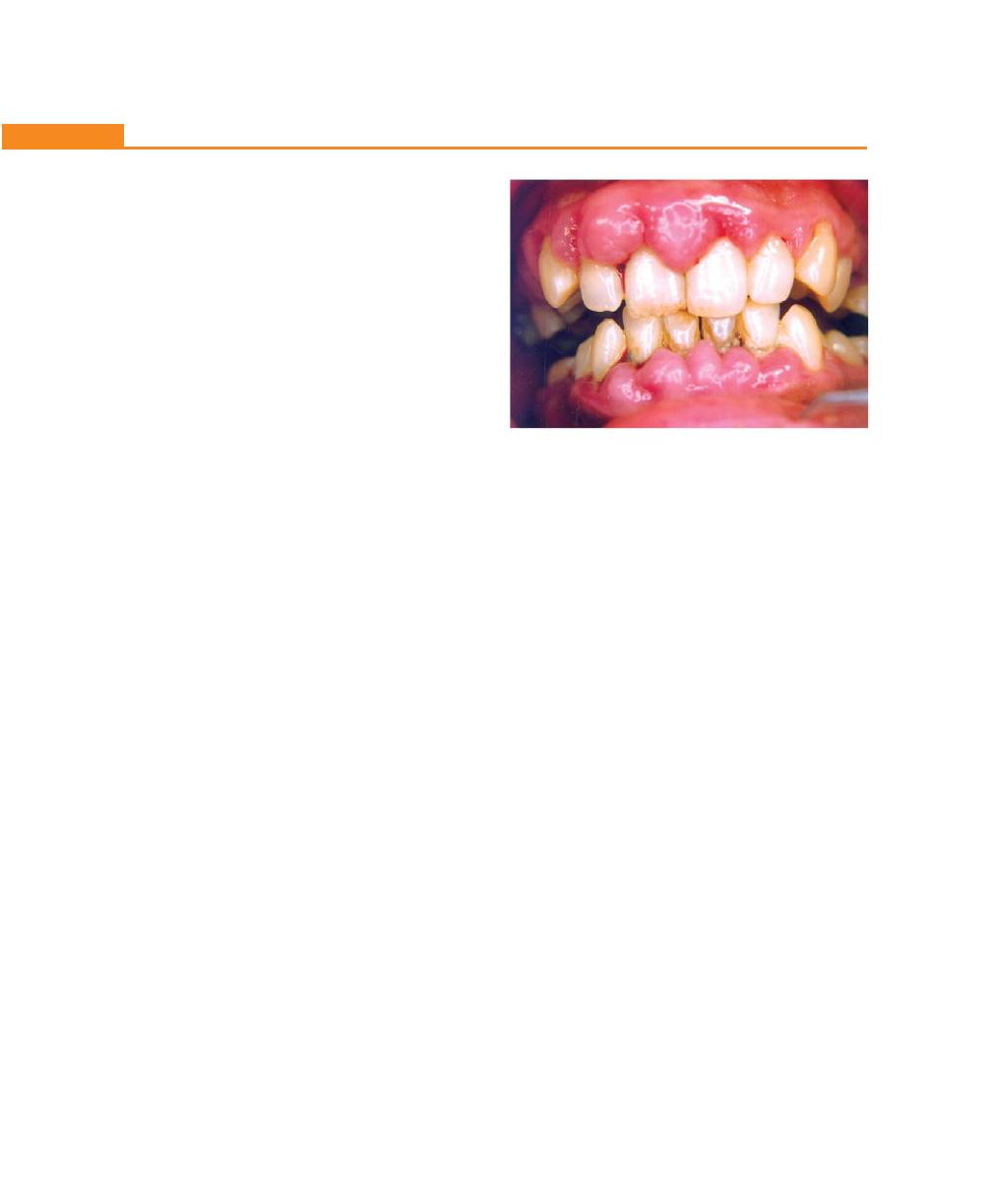
160
Essentials of Clinical Periodontology and Periodontics
• The epithelium is thickened with varying degree of
extra and intracellular edema. The epithelium also
exhibits prominent rete pegs.
• Gingival enlargement in pregnancy is also termed as
angiogranuloma in order to avoid implication of
neoplasm. (Implicated in terms such as pregnancy
tumors or fibrohemangioma).
Treatment
• The aim of the periodontal therapy for pregnant patient
is to minimize the potential exaggerated inflammatory
response related to hormonal alteration.
• Meticulous plaque control, scaling and root planing,
polishing should be the only non-emergent
periodontal procedures performed.
• The second trimester is the safest time in which
treatment may be performed. However long stressful
appointment and periodontal surgical procedures
should be postponed until postpartum.
• One must be aware of a condition, ‘supine hypotensive
syndrome’ that occurs during the third trimester which
is characterized by a decreased blood pressure,
syncope and loss of consciousness. In view of this,
the appointments should be kept short and the patient
should be allowed to change the position frequently.
• Fully reclining position should be avoided as far as
possible.
• Medication and radiographs should not be prescribed.
• In case of marginal and interdental enlargement,
scaling and curettage can be performed.
• In case of a tumor-like enlargement, surgical excision
is required which if possible should be postponed
until postpartum. During pregnancy the lesion should
be removed surgically only when it interferes with
mastication and causes severe disfigurement and if
the patient willingly wants to get it removed.
Enlargement in Puberty (Fig. 19.5)
Clinical Features
The enlargement is seen in both marginal and interdental
papilla and is characterized by prominent bulbous
interproximal papilla.
• Frequently only the facial gingiva is affected, because
mechanical action of the tongue prevents accumu-
lation of food on the lingual surfaces.
• Gingival enlargement during puberty has all the
clinical features associated with chronic inflammatory
gingival disease. It is the degree of enlargement and
tendency to develop massive recurrence in the
presence of relatively little local irritation that
distinguishes pubertal gingival enlargement from
uncomplicated chronic inflammatory gingival
enlargement.
• Eleven to seventeen years of age showed a high
prevalence of gingival enlargement. The gingival
microbiology of children between the ages of 11 to
14 and their association with clinical parameters has
implicated Capnocytophaga species in the initiation
of pubertal gingivitis.
Histopathology
Same as chronic inflammatory enlargement.
Treatment
Scaling, curettage and oral hygiene instructions. Surgical
removal may be performed in severe cases.
Vitamin C Deficiency
Acute vitamin C deficiency does not itself cause gingival
inflammation but it causes hemorrhage, collagen
Fig. 19.5:
Conditioned gingival enlargement in puberty
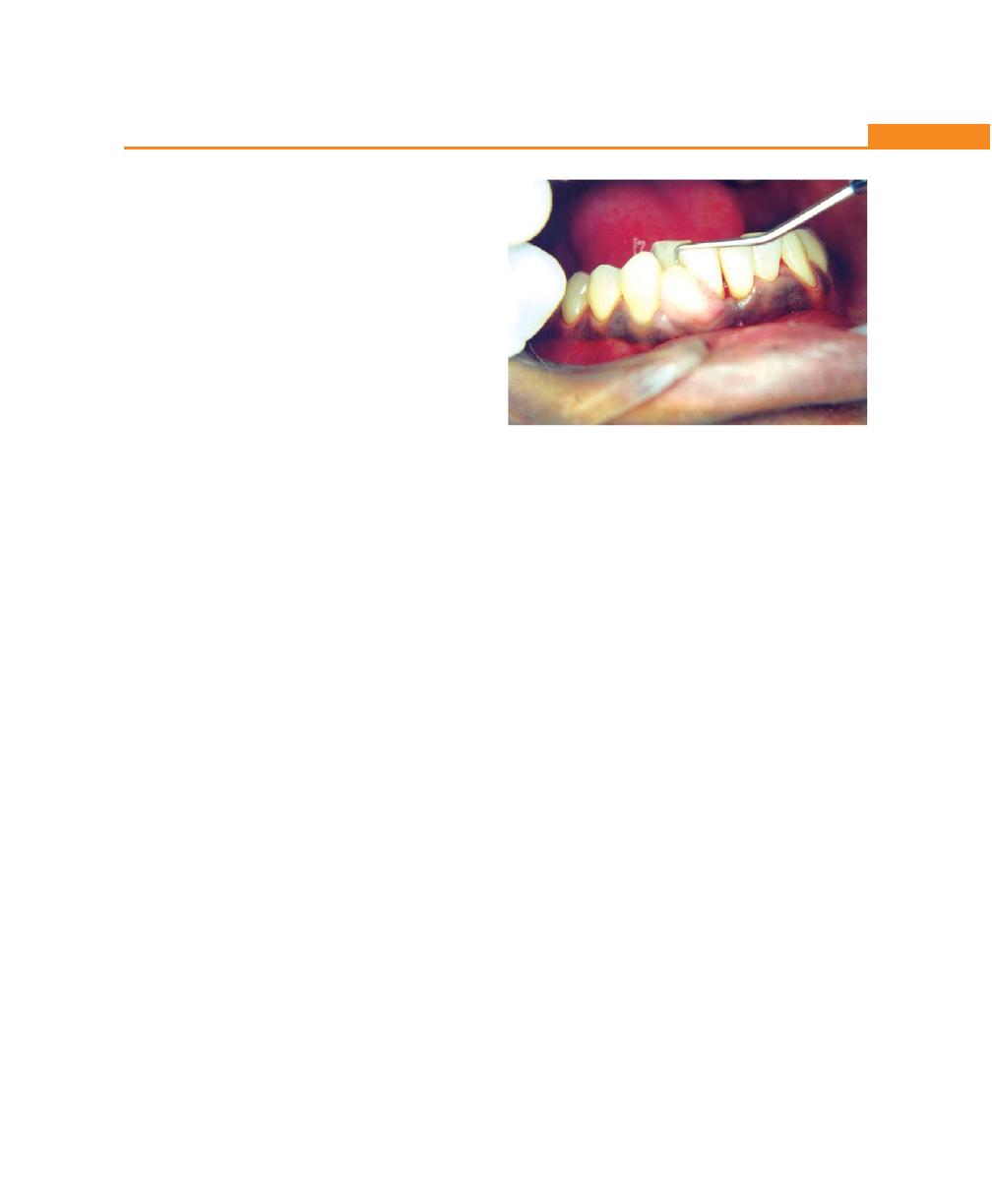
161
Gingival Enlargements
degeneration and edema of gingival connective tissue.
These changes modify the response of the gingiva to
plaque.
Clinical Features
Gingival enlargement is marginal and is bluish-red, soft,
friable and has smooth, shiny surface. Hemorrhage
occurring either spontaneously or on slight provocation
and surface necrosis with pseudomembrane formation
are common features.
Histopathology
Areas of hemorrhage with engorged capillaries and marked
diffuse edema, collagen degeneration and scarcity of
collagen fibers and fibroblasts are striking features.
Plasma Cell Gingivitis
• It is also referred to as atypical and plasma cell
gingivostomatitis and frequently consists of a mild-
marginal gingival enlargement that extends to
attached gingiva.
• Clinically, gingiva appears red, friable and bleeds
easily. An associated cheilitis and glossitis have been
reported.
• It is thought to be allergic in origin, possibly related
to the components of chewing gum or dentrifices.
• Microscopically the connective tissue contains a dense
infiltrate of plasma cells, that also extends to oral
epithelium.
Non-Specific Conditioned Enlargement
(Granuloma Pyogenicum) (Fig. 19.6)
It is a tumor-like gingival enlargement that is considered
to be an exaggerated conditioned response to minor
trauma. The exact nature of the systemic conditioning
factor has not yet been identified.
Clinical Features
• The lesion varies from a discrete, spherical tumor-
like mass with a pedunculated attachment to a
flattened, keloid-like enlargement with a broad base.
• It is a bright-red or purple and either friable or firm,
depending on its duration. In majority of the cases
it presents with surface ulceration and purulent
exudation.
• The lesion tends to involute spontaneously to become
a fibro-epithelial papilloma or persists relatively
unchanged for years.
Histopathology
It appears as a mass of granulation tissue with chronic
inflammatory cellular infiltration. Surface ulceration and
exudation are common features.
Treatment
Consists of removal of the lesion along with the
elimination of local irritating factors.
Systemic Disease Causing Gingival Enlargement
Leukemia
• The enlargement may be diffuse or marginal, localized
or generalized. It may appear as an oversized
extension of the marginal gingiva or a discrete tumor-
like interproximal mass.
• The gingiva appears as a bluish-red with a shiny
surface.
• The consistency is moderately firm but there is a
tendency towards friability and hemorrhage occurring
either spontaneously or on slight provocation.
Fig. 19.6:
Pyogenic granuloma in a young women
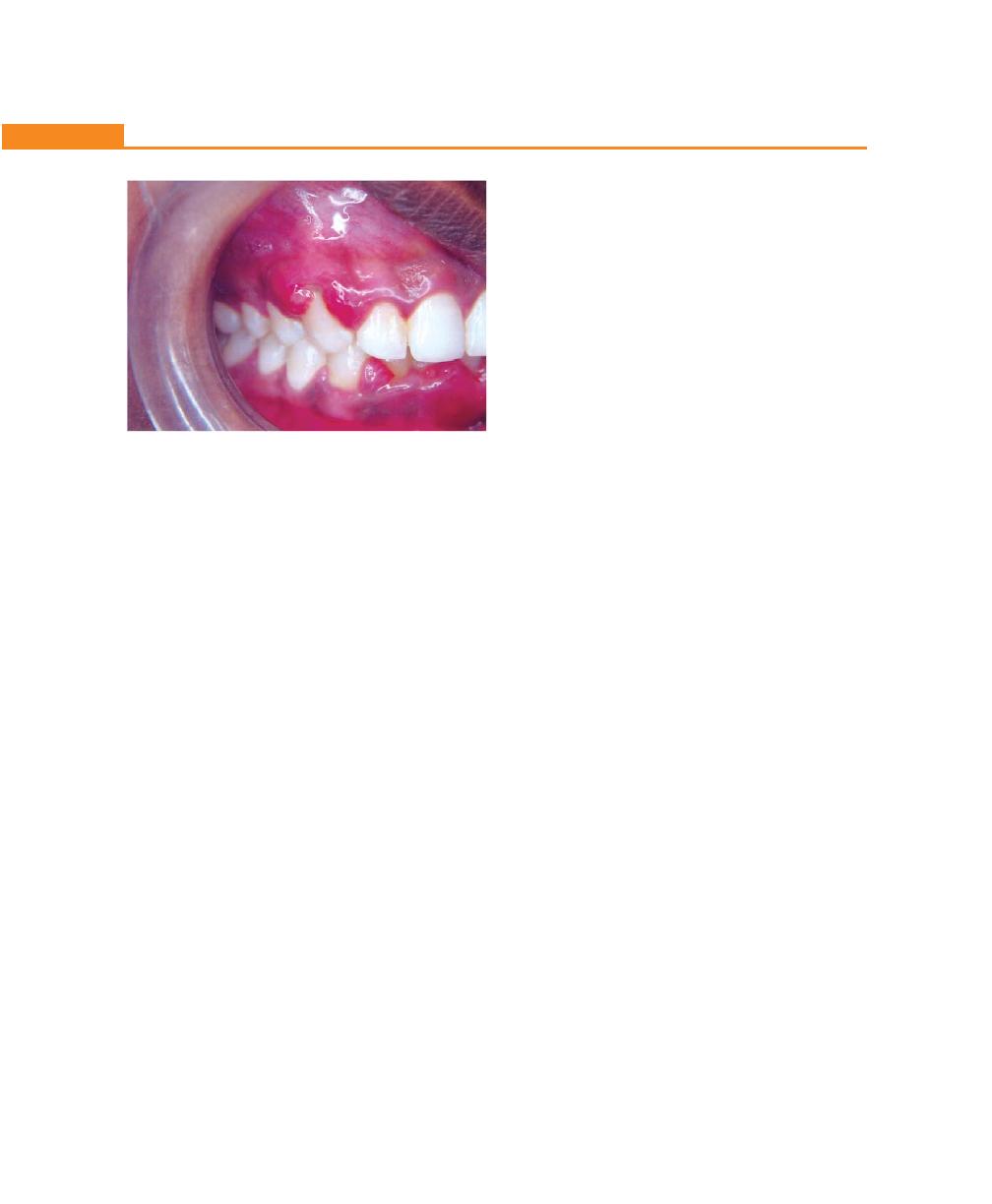
162
Essentials of Clinical Periodontology and Periodontics
• ANUG may sometimes be seen.
• True leukemic enlargement occurs commonly in
acute leukemia but may also be seen in sub-acute
leukemia. It seldom occurs in chronic leukemia (Fig.
19.7).
Histopathology (Fig. 19.7)
It shows varying degrees of chronic inflammation with
mature leukocytes and areas of connective tissue
infiltrated with a dense mass of immature and
proliferating leukocytes the specific nature of which varies
with type of leukemia.
Isolated surface areas of acute necrotizing
inflammation with a pseudomembranous meshwork of
fibrin, necrotic epithelial cells, polymorphonuclear
neutrophils and bacteria are frequently seen.
Treatment
• After the acute symptoms subside, attention is directed
to correct the gingival enlargement. The rationale of
treatment is to remove the local deposits and
to control inflammatory component of the enlarge-
ment.
• The enlargement is treated by scaling and curettage
which is carried out in stages under topical anesthesia.
Oral hygiene procedures are extremely important in
these cases.
• Antibiotics are administered systemically the evening
before and for 24 hours after each treatment to
reduce the risk of infection.
In leukemic patients the following instructions should be
followed (in general):
1. Refer the patient to the physician for medical
evaluation and treatment. For this close co-operation
with the physician is required.
2. Prior to chemotherapy, a complete periodontal
treatment plan should be prepared with the physician,
because once the chemotherapy is started the patient
will become immunosuppressed, thus increasing the
risk of secondary infection.
The treatment plan for these patients is:
a. Monitor hematological lab values daily (bleeding time,
clotting time, partial thromboplastin time and platelet
count).
b. Administer antibiotics prior to any periodontal
therapy.
c. Extract all hopeless, non-maintainable or potentially
infectious teeth, atleast 10 days prior to initiation of
chemotherapy.
d. Thorough periodontal debridement (scaling and root
planing) is done and oral hygiene instructions are
given. If there is an irregular bleeding time, careful
debridement with cotton pellets soaked in hydrogen
peroxide may be performed around the neck of the
teeth.
During acute phases of leukemia, patients should receive
only emergency periodontal care.
A. If there is a persistent gingival bleeding: It should be
treated as follows:
i. Cleanse the area with 3 percent hydrogen
peroxide.
ii. Carefully explore the area and remove any etiologic
local factors making sure to avoid gingival injury.
iii. Recleanse the area with 3 percent hydrogen
peroxide.
Fig. 19.7:
Leukemic gingival enlargement

163
Gingival Enlargements
iv. Place a cotton pellet soaked in thrombin against
the bleeding point.
v. Cover with a gauze and apply pressure for 15 to
20 minutes.
vi. If oozing persists after the removal of the gauze
and pressure, replace the cotton pellet saturated
with 3 percent H
2
O
2
firmly, then place a perio-
dontal dressing over the area for 24 hours.
B. ANUG: Follow routine treatment for ANUG.
C. Acute gingival or periodontal abscess: They are usually
associated with regional lymphadenopathy and
systemic complications.
Treatment: It is as follows:
i. Systemic antibiotics.
ii. Gentle incision and drainage.
iii. Cleanse the area with cotton pellets saturated with
3 percent hydrogen peroxide.
iv. Apply topical pressure with gauze for 15 to 20
minutes.
D. Oral ulceration: It is treated with antibiotics and bland
mouth rinses—topical rinses such as viscous xylocaine
or promethazine hydrochloride syrup may be
prescribed. Topical protective ointments and sharp
irritational areas or appliances should be removed.
Oral moniliasis is common in leukemic patient and
can be treated with nystatin suspensions.
In Chronic Leukemia
Scaling and root planing can be performed without
complication, but an effort should be made to avoid
periodontal surgery.
Before every procedure: Bleeding time should be
measured. If any changes are seen, postpone the
appointment and refer the patient to physician.
Plaque control and frequent recall intervals should
be given attention particularly.
Granulomatous Diseases (Table 19.5)
a. Wegener’s Granulomatosis
• It is a rare disease characterized by a granulomatous
necrotizing lesion of the respiratory tract, including
nasal and oral defects.
• The initial manifestation of Wegener’s granulomatosis
may involve the orofacial region and include oral
mucosal ulceration, gingival enlargement, abnormal
tooth mobility, exfoliation of teeth and delayed
healing response.
• Clinically the enlargement appears reddish-purple in
color and bleeds easily on stimulation. The etiology
of Wegener’s ganulomatosis is unknown, but the
condition is considered as an immunologically
mediated tissue injury.
• At one time the usual outcome was death from
kidney failure but more recently the use of immuno-
suppressive drugs has produced prolonged remission.
Table 19.5: Granulomatous disease
Wegener’s Granulomatosis
Sarcoidosis
(Multisystem Granuloma)
Etiology
Immunologically-mediated tissue injury
Unknown, but may be impaired cell-mediated
immunity
Location and Distribution Papillary enlargement
Non-specific
Clinical features
Shows oral mucosal ulceration, reddish
Red, smooth enlargement
purple gingival enlargements, bleeds on
stimulation, abnormal tooth mobility,
exfoliation of teeth, and delayed healing
response
Histopathology
Chronic inflammation with giant cells
Sarcoid granuloma consists of whorls of epithelioid
and micro-abscesses covered by thin
cells and multi-nucleated Langerhans giant cells with
acanthotic epithelium
peripheral mono-nuclear cells. Caseation and necrosis
do not occur
164
Essentials of Clinical Periodontology and Periodontics
T
able 19.6:
Benign tumors of gingiva
Fibroma
Papilloma
Peripheral giant
Central giant
Leukoplakia
Gingival cyst
cell granuloma
cell granuloma
Etiology
Reaction to trauma
Mostly due to
Local injury
History of injury
Tobacco
, chronic
•
Remnants of
or chronic irritation
papilloma virus
irr
ita
tion, alcohol,
dental lamina,
Some unknown
syphilis, vitamin
enamel organ,
deficiency
, hor
-
epithelial islands of
mones, candidiasis
periodontal membrane
•
Traumatic implantation of epithelium
Location and
Localized, diffuse
Localized, diffuse
Interdental or from
Mandible is more
Buccal mucosa,
Mandibular bicus-
distribution
and discrete
and discrete
gingival margin.
commonly involved
commissures,
pid, cuspid incisor
Fr
equently on labial
than maxilla.
alveolar mucosa,
area
surface
More common in
tongue, lips, hard
anterior
segment
and soft palate,
and does not
floor of the mouth
uncommonly cross
and gingiva
midline
Clinical features
Slowly growing
Hard, wart-like
Smooth, regularly
A
rise
w
ith
in t
he
ja
w
Varies from
•
Involves marginal
spherical mass that
protuberance
from
outlined
masses to
and
produces
grayish-white,
or
attached gingiva
tends to be firm
gingival surface
irregularly-shaped
cavitation. No pain
flattened scaly lesion
•
O
ccurs in mandi-
and nodular
, but
multilobulated
but slight discomfort.
to a
thick irregularly-
bular canine or
may be soft and
protuberance with
Slight to moderate
shaped keratinous
premolar area.
vascular
. Usually
surface indentation.
bulging of jaw due
plaque.
•
Painless and causes
pedunculated
Sessile or peduncu-
to
expansion of
erosion of bone
lated, painless. Firm
cortical plate is
seen.
with
expansion.
or spongy and color varies from pink to deep red or purplish blue. Ulcerations are sometimes seen.
Histopathology
Consists of bundles
It
consists of many
It has numerous foci
•
It
consists of loose
Thickening of
epi-
Cyst cavity lined
of
interlacing
collagen
long thin, finger-
of multinuclear giant
fibrillar
connective
thelium with hyper-
by
thin flattened
fibers interspersed
like projections
cells
and hemosiderin
tissue stroma with
keratosis,
acanthosis
epithelium with or
with varying number
extending above
particles and chronic
many proliferating
and some degree of
without localized
of fibroblasts and
the surface of
inflammatory infiltrate
fibroblasts
an
d
small
dyskeratosis, infla-
areas
of thickening.
small blood vessels.
mucosa, each made
in
the
connective
tissue.
capillaries.
mmatory infiltrate
The surface is
up of
stratified
Bone
formation is
•
Collagen
fibres
show
of underlying
covered by stratified
squamous epithelium
occasionally seen.
whorled appearance.
connective tissues.
squamous epithelium.
and containing a
Overlying
epithelium
•
Multinucleated giant
Areas of calcification
thin
central connec-
is
hyperplastic with
cells are prominent.
are also seen.
tive
tissue
core.
ulceration at the base.
Foci of e
xtra vascu-
lated blood and osteoid are seen.
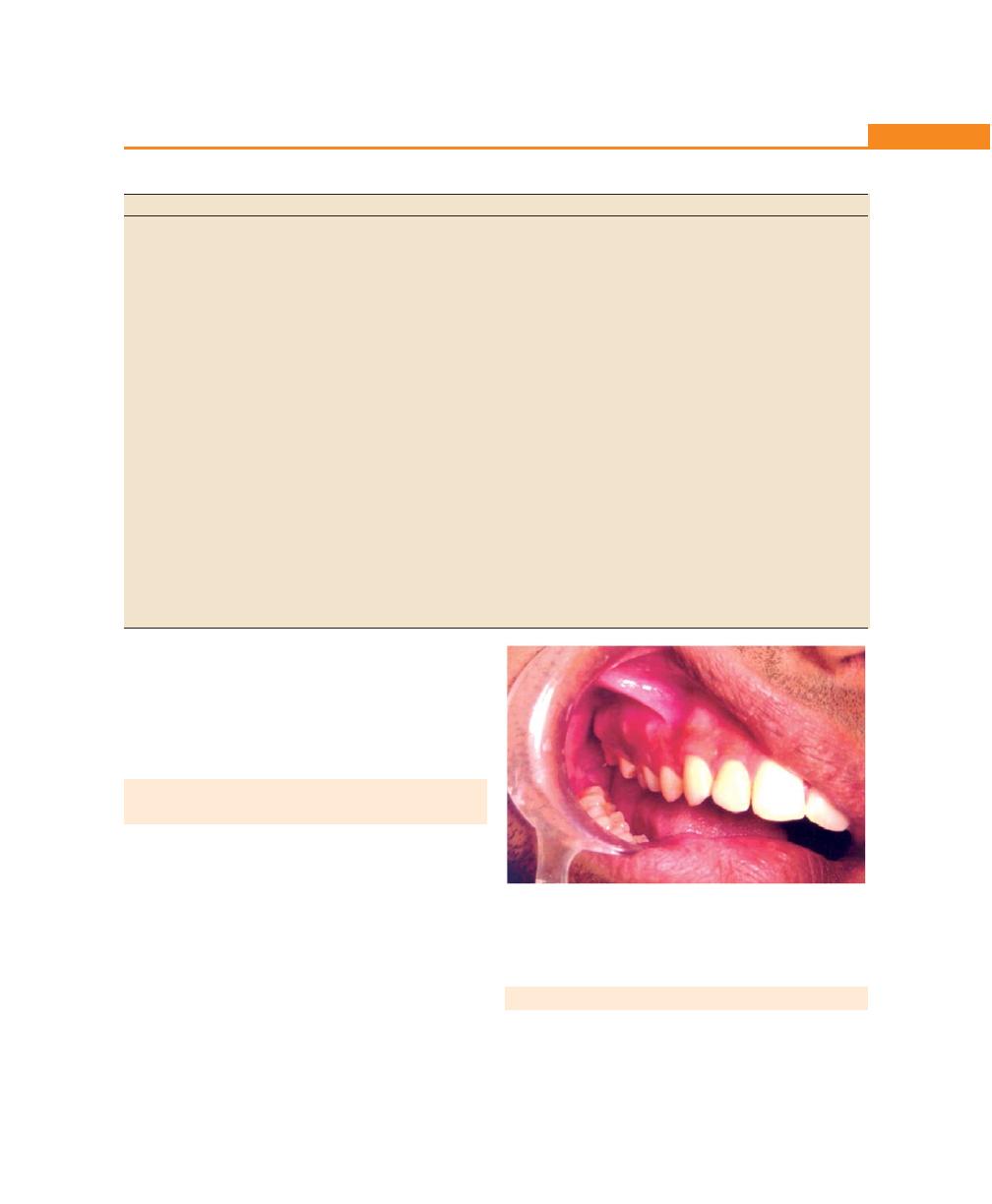
165
Gingival Enlargements
Table 19.7: Malignant tumors of the gingiva
Squamous cell carcinoma
Malignant melanoma
Etiology
Chronic irritation, tobacco, alcohol, syphilis,
Neoplasms of epidermal melanocytes.
nutritional deficiency.
Sunlight exposure is possible etiological
factors in cutaneous melanoma.
Location and distribution
Mandibular gingiva is more commonly
Palate, maxillary gingiva and alveolar ridge.
involved than the maxillary gingiva.
Attached gingiva is more frequently
involved than free gingiva.
Clinical Features
• Exophytic or ulcerative which appears
Deeply-pigmented area at times ulcerated
as flat, erosive lesion.
and hemorrhagic which increases in size.
• Locally invasive to underlying bone
or adjacent mucosa.
• It may or may not be painful.
• Attached gingiva is more frequently
involved than free gingiva.
• Metastasis is common.
Histopathology
Well-differentiated lesion which consists
• Radial growth phase of superficial
of sheets and nests of cells with origin
spreading melanoma is characterized
from squamous epithelium. Nuclei of
by presence of large, epitheloid
neoplastic cells are hyperchromatic.
melanocytes distributed in pagetoid
Mitotic figures are atypical. Individual
manner. Vertical growth phase is
cell keratinization or epithelial pearls
characterized by proliferation of
are present. Shows rapidly dividing
malignant epitheloid melanocytes
malignant cells. Resembles less to cells
into connective tissue. Macrophages
of origin.
and melanophages are present. Melanin
pigment is scanty.
b. Sarcoidosis
It is a granulomatous disease of unknown etiology. It
starts in individuals in their twenties and thirties and can
involve almost any organ, including the gingiva, where
a red, smooth enlargement may occur.
NEOPLASTIC ENLARGEMENT
(GINGIVAL TUMOR) (Table 19.6)
Benign Tumors of the Gingiva (Table 19.6)
• Fibroma
• Papilloma
• Peripheral giant cell granuloma
• Central giant cell granuloma
• Leukoplakia
• Gingival cyst
Malignant Tumors of the Gingiva (Table 19.7)
• Carcinoma
• Malignant melanoma
Fig. 19.8:
False enlargement in the right upper posterior region
• Sarcoma most commonly Kaposi’s sarcoma
• Metastasis
FALSE ENLARGEMENT (Fig. 19.8)
These are not true enlargements of gingival tissues but
may appear as a result of increase in size of the underlying
osseous or dental tissues.
166
Essentials of Clinical Periodontology and Periodontics
Underlying Osseous Lesions
Enlargement of the gingiva due to enlargement of
underling bone occurs most commonly in tori and
exostosis but it can also occur in Paget’s disease, fibrous
dysplasia, cherubism, central giant cell granuloma,
osteoma, osteosarcoma. The gingiva usually presents
with no abnormal clinical features except the massive
increase in size in the specific area.
Underlying Dental Tissues
During the various stages of eruption of primary dentition,
the gingiva may show bulbous, marginal distortion caused
by the superimposition of the bulk of the gingiva on
the normal prominence of the enamel in the gingival
half of the crown. This enlargement has been termed
as developmental enlargement.
In strict sense, this type of enlargement is physiologic.
However, it may be complicated by marginal inflammation
in which case alleviation of the marginal inflammation
is sufficient rather than resection of the enlargement.
KEY POINTS TO NOTE
1. Gingival enlargements can be classified according to,
etiologic factors and pathologic changes, location and
distribution and the degree of gingival enlargement.
2. Inflammatory enlargement can be of two types acute type,
e.g. gingival abscess and chronic type of enlargements.
3. Three categories of drugs have been identified to be
associated with gingival enlargements, anti-convulsants,
calcium channel blockers and immuno-suppressants.
4. The drug-induced enlargements are differentiated from
idiopathic enlargement, in the latter the enlargement involves
all parts of the gingiva, hence called diffuse enlargement.
5. Treatment options for drug induced enlargements are, drug
substitution and surgical therapy including gingivectomy or
flap surgery.
6. Enlargements associated with systemic diseases can be:
a. Magnification of an existing inflammation caused by
dental plaque discussed as “conditioned enlargement”
which includes some hormonal conditions (e.g.
pregnancy and puberty), nutritional diseases such as
vitamin C deficiency and some cases in which the
systemic influence is not identified (non-specific
conditioned enlargement).
b. Second category includes, manifestation of the systemic
disease not dependent on the inflammatory status of the
gingiva discussed as “systemic diseases causing gingival
diseases” and “neoplastic enlargement” (Gingival
tumors).
REVIEW QUESTIONS
1. Classify gingival enlargement. Discuss in detail clinical
features, pathogenesis and treatment of drug-induced
gingival enlargement.
2. What are the differences between gingival and
periodontal abscess?
3. What is granuloma pyogenicum?
BIBLIOGRAPHY
1. Newman, Takei, Fermin A, Carranza. Clinical Periodontology,
9th edn, WB Saunders, 2002.
2. Robin A Seymeur, Peter A Heasman. Drugs, Diseases and
the Periodontium, Oxford University Press Publication 1992.
3. Thomas M. Hassell, A. Paul Burtner, Donald McNeal. Oral
problems and genetic aspects of individuals with Epilepsy.
Periodontal 2000;6:1994.
167
Acute Gingival Infections
CLASSIFICATION OF VARIOUS
ACUTE GINGIVAL LESIONS
According to Manson
a. Traumatic lesions of gingiva:
• Physical injury
• Chemical injury
b. Viral infections:
• Acute herpetic gingivostomatitis
• Herpangina
• Hand, foot and mouth diseases
• Measles
• Herpes varicella/zoster virus infections
• Glandular fever
c. Bacterial infections:
• Acute necrotizing ulcerative gingivitis
• Tuberculosis
• Syphilis
d. Fungal diseases:
• Candidiasis
e. Gingival abscess
f. Apthous ulceration
g. Erythema multiforme
h. Drug allergy and contact hypersensitivity
❒
❒
❒
❒
❒ CLASSIFICATION OF VARIOUS ACUTE
GINGIVAL LESIONS
❒
❒
❒
❒
❒ ACUTE NECROTIZING ULCERATIVE
GINGIVITIS
• Terminology
• Clinical Features
• Intraoral/Extraoral Signs and
Symptoms
• Clinical Course
• Etiology
• Histopathology, Diagnosis and
Treatment
❒
❒
❒
❒
❒ ACUTE HERPETIC
GINGIVOSTOMATITIS
• Clinical Features
• Etiology
• History
• Histopathology
• Diagnosis
• Differential Diagnosis
• Treatment
❒
❒
❒
❒
❒ PERICORONITIS
• Definition
• Types
• Clinical Features
• Complications
• Treatment
20
Acute Gingival
Infections
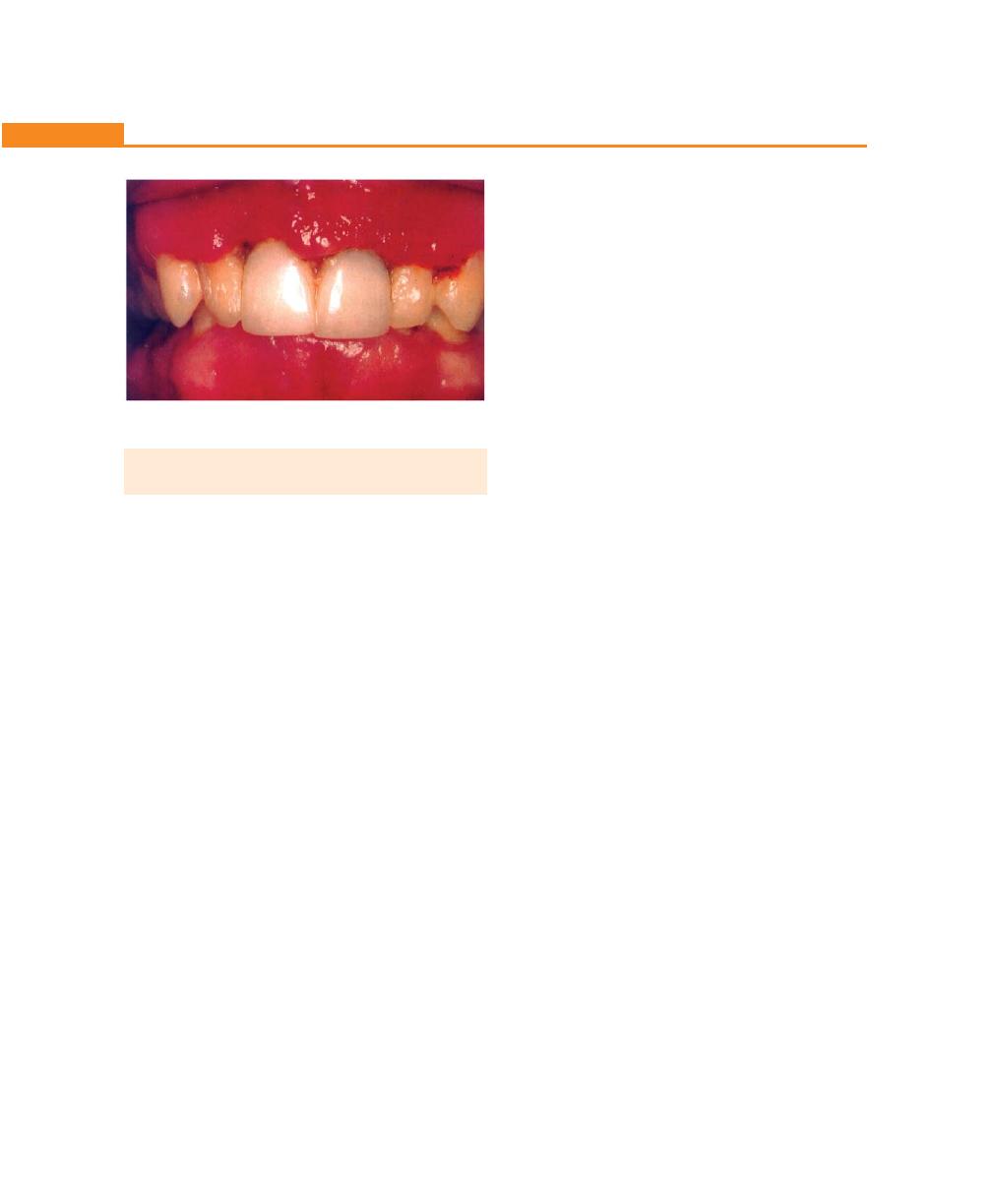
168
Essentials of Clinical Periodontology and Periodontics
ACUTE NECROTIZING ULCERATIVE
GINGIVITIS (ANUG)
Terminology
Acute necrotizing ulcerative gingivitis (ANUG) is an
inflammatory, destructive disease of the gingiva, which
presents characteristic signs and symptoms (Fig. 20.1).
Other terms used to describe this condition are:
• Vincent’s infection
• Trench mouth
• Acute ulceromembranous gingivitis and others.
NUG (necrotizing ulcerative gingivitis) can cause
destruction of the supporting structures. When it involves
the bone it causes bone loss, the condition is referred
as necrotizing ulcerative periodontitis (NUP). Necrotizing
ulcerative gingivitis can occur in acute, subacute and
recurrent forms.
Clinical Features (Fig. 20.1)
Necrotizing ulcerative gingivitis is characterized by sudden
onset, sometimes may be followed by an episode of
debilitating diseases or acute respiratory tract infections.
Long hours of working without adequate rest and
psychologic stress are also frequent features in the history
of necrotizing ulcerative gingivitis.
Intraoral Signs and Symptoms
a. Oral signs: Lesions are characterized by punched out,
crater-like depressions at the crest of the interdental
papillae, subsequently involving marginal gingiva and
rarely attached gingiva.
b. These craters are covered by grayish pseudo-
membranous slough, which is demarcated from the
remaining of the mucosa by a pronounced linear
erythema.
c. The ulcerations of necrotizing ulcerative gingivitis could
be of two types, lateral ulceration and necrosis, deep
ulceration and necrosis. This is related to the fact that
the gingival tissues are supplied by two main sources
of blood supply. Supraperiosteal vessels supplying
the attached gingiva, lateral margins and lateral parts
of the papillae, while intracellular vessels supplying
to the col and central portions of the papillae.
Lateral ulceration, which is (less common) involving
primarily the buccal wall of the papillae, margins and
possibly the attached gingiva, occurs in the
distribution of the lateral blood supply. Deep
ulceration involves necrosis of the tissues of the
embrasure giving rise to typical truncated papillae,
occurs in the distribution of the intra-alveolar vessels
(more common).
d. Other signs include gingival hemorrhage or
pronounced bleeding on the slightest stimulation.
e. Fetid odour and increased salivation.
Oral Symptoms
1. The lesions are extremely sensitive to touch.
2. Complains of a constant radiating, gnawing pain that
is intensified by eating spicy or hot foods and chewing.
3. There is a metallic foul taste, and the patient is
conscious of an excessive amount of “pasty saliva”.
Extraoral Signs and Symptoms
In mild to moderate stages of the disease local
lymphadenopathy and a slight elevation in temperature
are common features. In severe, cases marked systemic
complications such as high fever, increased pulse rate,
leukocytosis, loss of appetite and general lassitude are
common. Systemic reactions could be more severe in
children. In rare cases, severe sequelae such as the
Fig. 20.1:
ANUG (Punched out interdental papilla
between two central incisors)

169
Acute Gingival Infections
following may occur, noma or gangrenous stomatitis,
fusospirochetal meningitis, peritonitis, toxemia, and fatal
brain abscess.
Clinical Course
It is indefinite, if left untreated, it may lead to destruction
of the periodontium, and denudation of roots
(necrotizing ulcerative periodontitis), combined with
severe toxic systemic complications.
Horning and Cohen have described the following
stages in the progression of necrotizing ulcerative gingivitis
(NUG) (Table 20.1).
Etiology
Role of Bacteria
Plaut and Vincent introduced the concept that acute
necrotizing ulcerative gingivitis is caused by a specific
bacteria namely, a fusiform bacillus and a spirochetal
organisms. More recently Loesche and colleagues descri-
bed a constant and a variable flora associated with ANUG.
Constant flora is composed of fusospirochetal
organisms and also Bacteroides intermedius. The variable
flora consists of a heterogenous array of bacterial types.
These bacteriologic findings have been supported by
immunologic data, increased IgG and IgM antibody titers
to spirochetes (intermediate sized upto 90%) and
Prevotella intermedia has been demonstrated. Electron
microscopic studies have demonstrated three types of
spirochetes, small, intermediate sized (maximum number
upto 90%) and large spirochetes. The specific cause of
acute necrotizing ulcerative gingivitis has not been
established. The common opinion is that it is produced
by a complex of bacterial organisms but requires
underlying tissue changes to facilitate the pathogenic
activity of the bacteria.
Local Predisposing Factors
Most important predisposing factors are:-
i. Pre-existing gingivitis
ii. Injury to the gingiva
iii. Smoking
Deep periodontal pockets, pericoronal flaps are
particularly vulnerable areas for the occurrence of the
disease because they offer a favourable environment
for the proliferation of the fusospirochetes and hence
they are called as incubation zones.
Areas of the gingiva traumatized by opposing teeth
in malocclusion, such as the palatal surface behind the
maxillary incisors and the labial gingival surface of the
mandibular incisors are frequent sites of acute necrotizing
ulcerative gingivitis.
Many investigators have reported a positive
correlation between smoking and acute necrotizing
ulcerative gingivitis. The possible reasons are:
• Direct toxic effect of tobacco on the gingiva.
• Vascular or other changes induced by nicotine or other
substances.
• Smoking and acute necrotizing ulcerative gingivitis
are both reflections of stress.
Table 20.1: Stages in the progression of NUG
Stages Involvement of the Lesion
% of Cases
Clinical Conditions
1.
Necrosis of the tip of the interdental papilla
93%
NUG Necrotizing ulcerative gingivitis
2.
Necrosis of the entire papilla
19%
NUG or NUP Necrotizing ulcerative
gingivitis or necrotizing ulcerative
periodontitis
3.
Necrosis extending to the gingival margin
21%
Necrotizing ulcerative periodontitis
4.
Necrosis extending also to the attached gingiva
1%
Necrotizing ulcerative periodontitis
5.
Necrosis extending into buccal or labial mucosa
6%
Necrotizing stomatitis
6.
Necrosis exposing alveolar bone
1%
Necrotizing stomatitis
7
Necrosis perforating skin of cheek
0%
Noma

170
Essentials of Clinical Periodontology and Periodontics
Systemic Predisposing Factors
Nutritional Deficiency: A poor diet has been reported
as a predisposing factor in NUG. Nutritional deficiencies
such as vitamin B and vitamin C accentuate the response
of gingival tissues produced by increased pathogenic
flora. Several researchers have found an increase in the
fusospirochetal flora in patients with nutritionally-deficient
diets.
Debilitating Diseases: May predispose to the development
of acute necrotizing ulcerative gingivitis. Such systemic
disturbances are metallic intoxication, severe
gastrointestinal disorders, blood dyscrasias such as
anemia, leukemia and acquired immunodeficiency
syndrome.
Psychosomatic Factors: Appears to be important in the
etiology of acute necrotizing ulcerative gingivitis. The
mechanisms whereby psychological factors create or
predispose to gingival damage have not been established,
but an alteration in digital and gingival capillary responses
suggestive of increased autonomous nervous activity has
been demonstrated. Cohen and coworkers have
suggested that a psychiatric disturbance may lead to
activation of the hypothalamic pituitary adrenal axis. This
results in elevation of serum and urine cortisol levels,
which is associated with a depression of lymphocyte and
polymorphonuclear leukocytes function that may
predispose to acute necrotizing ulcerative gingivitis.
Histopathology
Microscopically, the lesions involve both epithelium and
underlying connective tissue. The surface epithelium is
destroyed and is replaced by a pseudomembranous
mesh work of fibrin, necrotic epithelial cells, polymorpho-
nuclear neutrophils and various types of micro-
organisms. This is the zone that appears clinically as the
surface pseudomembrane. The underlying connective
tissue is markedly hyperemic, with numerous engorged
capillaries and dense infiltration of polymorphonuclear
neutrophils. This acutely-inflamed hyperemic zone
appears clinically as the surface pseudomembrane.
Relationship of Bacteria to the
Characteristic Lesions
Listgarten and colleagues described four zones namely:
1. Zone I—Bacterial zone: It is the most superficial zone,
consists of varied bacteria, including a few spirochetes
of the small, medium-sized and large types.
2. Zone II—Neutrophil-rich zone: Contains numerous
leukocytes predominantly neutrophils with bacteria
including spirochetes of various types.
3. Zone III—Necrotic zone: Consists of a dead tissue
cells, remnants of connective tissue fragments, and
numerous spirochetes.
4. Zone IV—Zone of spirochetal infiltration: Consists
of a well preserved tissue infiltrated with spirochetes of
intermediate and large-sized without other organisms.
Diagnosis
Diagnosis is based on clinical findings. A bacterial smear
may be used to corroborate the clinical diagnosis, but it
is not necessary nor definitive because the bacterial picture
is not appreciably different from the other conditions.
Differential diagnosis include:
a. Gonococcal stomatitis
b. Agranulocytosis
c. Vincent’s angina
d. Desquamative gingivitis
e. Acute necrotizing ulcerative gingivitis in leukemia
f. Acute necrotizing ulcerative gingivitis in AIDS
g. Streptococcal gingivostomatitis.
Treatment
1. Non-ambulatory patient: With symptoms of genera-
lized systemic complications.
2. Ambulatory patient: With no serious systemic comp-
lications.
Treatment for Non-ambulatory Patients
Day 1:
a. Local treatment limited to gently removing the
necrotic pseudomembrane with a pellet of cotton
saturated with hydrogen peroxide (H
2
O
2
).

171
Acute Gingival Infections
b. Advised bed rest and rinse the mouth every 2 hours
with a diluted 3 percent hydrogen peroxide (H
2
O
2
).
c. Systemic antibiotics like penicillin or metronidazole
can be prescribed.
Day 2:
a. If condition is improved, proceed to the treatment
described for ambulatory patients. If there is no
improvement at the end of the 24 hours, a bedside
visit should be made. The treatment again includes
gently swab the area with hydrogen peroxide,
instructions of the previous day are repeated.
Day 3:
a. Most cases, the condition will be improved, start the
treatment for ambulatory patients.
Treatment for Ambulatory Patients
First visit:
A topical anesthetic is applied and after 2 or 3 minutes
the areas are gently swabbed with a cotton pellet to
remove pseudomembrane and non-attached surface
debris. After the area is cleansed with warm water the
superficial calculus is removed with ultrasonic scalers.
Patients with moderate or severe necrotizing ulcerative
gingivitis and local lymphadenopathy, are placed on
antibiotic regime of penicillin 500 mg thrice daily, for
penicillin-sensitive patients erythromycin or metroni-
dazole 200 mg or 400 mg twice daily for seven days.
Sub-gingival scaling and curettage are contraindicated
at this time because of possibility of extending the
infection to deeper tissues.
Instructions to the patient:
1. Avoid smoking and alcohol.
2. Rinse with 3 percent hydrogen peroxide and warm
water for every two hours.
3. Confine toothbrushing to the removal of surface
debris with a bland dentifrice, use of interdental aids
and chlorhexidine mouth rinse are recommended.
Second visit:
Scalers and curettes are added to the instrumentarium,
shrinkage of the gingiva may expose previously covered
calculus which is gently removed. Same instructions are
reinforced.
Third visit:
Scaling and root planing are repeated, plaque control
instructions are given. Hydrogen peroxide rinses are
discontinued.
Fourth visit:
Oral hygiene instructions are reinforced and thorough
scaling and root planing are performed.
Fifth visit:
Appointments are fixed for treatment of chronic
gingivitis, periodontal pockets and pericoronal flaps, and
for the elimination of all local irritants. Patient is placed
on maintenance programme.
Further Treatment Considerations
1. Gingivoplasty.
2. Role of drugs (escharotic drugs and silver nitrate,
hydrogen peroxide, sodium perborate).
Table 20.2: Distinction between necrotizing ulcerative gingivitis and primary herpetic gingivostomatitis
Necrotizing ulcerative gingivitis (NUG)
Primary herpetic gingivostomatitis
Etiology:Host bacterial interaction, mostly fusospirochetes
Specific viral etiology
Necrotizing condition
Diffuse erythema and vesicular eruptions
Punched out crater-like lesions affecting marginal gingiva.
Vesicles rupture leaving slightly depressed oval and
The lesions are covered with pseudomembranous slough,
spherical ulcer. Diffuse involvement of gingiva, may include
other oral tissues are rarely affected.
buccal mucosa and lips
Uncommon in children
Occurs more frequently in children
No definite duration
Duration of 7 to 10 days
Not contagious
Contagious
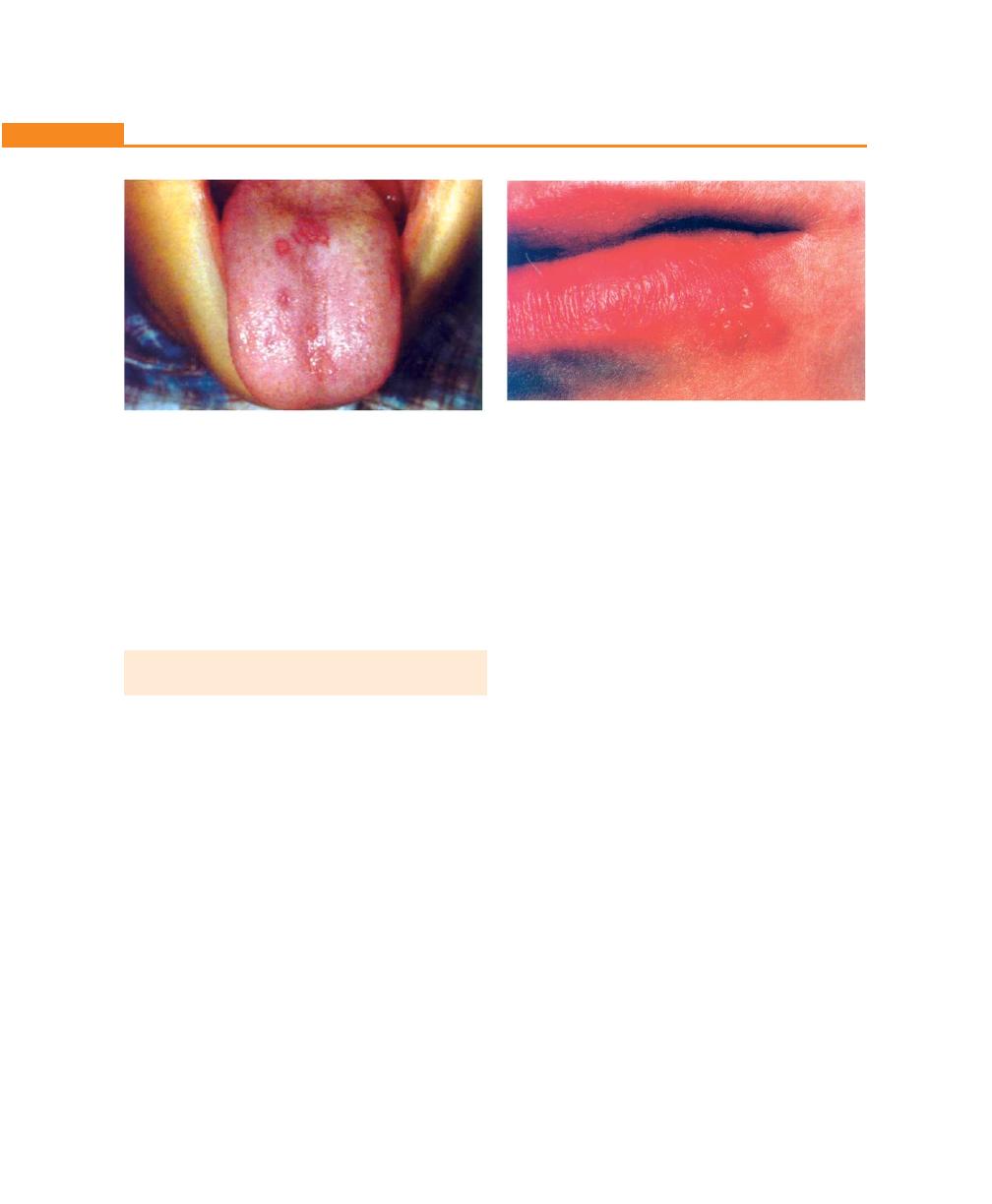
172
Essentials of Clinical Periodontology and Periodontics
3. Systemic antibiotics—only in patients with toxic
systemic complications.
4. Supportive systemic treatment—copious fluid
consumption and administration of analgesics and
adequate bed rest.
5. Nutritional supplements—vitamin B/C supple-
ments.
ACUTE HERPETIC GINGIVOSTOMATITIS
(AHG) (Fig. 20.2)
It is a viral infection of the oral mucous membrane caused
by HSV (Herpes simplex virus). It occurs most frequently
in infants and children younger than 6 years of age but
is also seen in adults.
Clinical Features
Oral Signs
1. It appears as a diffuse, shiny erythematous, involve-
ment of the gingiva and the adjacent oral mucosa
with varying degrees of edema and gingival bleeding.
2. In its initial stage it may appear as discrete, spherical,
gray vesicles dispersed in different areas, e.g. labial
and buccal mucosa, soft palate, pharynx and tongue.
After approximately 24 hours the vesicles rupture
and form painful small ulcers with a red, elevated,
halo-like margins and a depressed yellow and grayish
white central portion.
3. Diffuse, edematous, erythematous enlargement of
the gingiva with a tendency towards bleeding is seen.
4. The course of the disease is 7 to 10 days. It may
appear in a localized form following operative
procedures in the oral cavity and by placement of
cotton rolls or by vigorous application of digital
pressure.
Oral Symptoms
1. Generalized soreness of the oral cavity which
interferes with eating and drinking.
2. The ruptured vesicles are sensitive to touch, thermal
changes and foods.
Extraoral and Systemic Signs and Symptoms
Involvement of the lips, face (Herpes labialis, “cold sore”)
with vesicles and surface scale formation may accompany
the intra oral disease, cervical adenitis, fever as high as
101 to 105°F and generalized malaise are common (Fig.
20.3).
Etiology
Acute herpetic gingivostomatitis is caused by HSV
(Herpes simplex virus) with a size of approximately 100
to 200 µm. When fully formed it consists of a core
containing genetic material (DNA) surrounded by a
capsid. The capsid is made up of a hexagonal particle
called capsomers. The capsid in turn is surrounded by
Fig. 20.3:
Herpetic vesicles (
Herpes labialis)
Fig. 20.2:
Vesicles on the tongue in primary herpetic
gingivostomatitis

173
Acute Gingival Infections
a membrane or envelope. The fully grown virion
penetrates the cell membrane, once inside, the virion
loses, its coating and later it gains entrance into the
nucleus. In the nucleus the DNA of the virus converts
the host nucleus and codes the host DNA for the
production of material necessary for viral replication.
Herpes viruses have been classified on the basis of
morphologic findings into five types in humans:
1. Herpes virus type 1—oropharyngeal lesions responsi-
ble for acute herpetic gingivo stomatitis (HGS) and
cold sores.
2. Herpes virus type 2—genital lesion
3. Cytomegalovirus—Hodgkin’s lymphomas
4. Varicella zoster virus—responsible for chicken pox and
shingles
5. Epstein-Barr virus—responsible for infectious mono-
nucleosis and Burkitt’s lymphoma.
History
The condition frequently occurs after an episode of febrile
diseases such as pneumonia, meningitis, influenza and
typhoid. It also tends to occur during periods of anxiety,
strain or exhaustion.
The location of virus is in the gasserian ganglion. The
virus may descend to the lip through the trigeminal nerve,
which may explain why the location of the blister on
the lip is usually seen. Resistance to recurrent attacks
may depend on antibody, complement and leukocytes.
Histopathology
Vesicles rupture to form a discrete ulceration, which
appears to have a central portion of acute inflammation
characterized by ulceration and varying degrees of
purulent exudate, surrounded by a zone rich in engorged
blood vessels.
The microscopic picture of the vesicle is characterized
by extra- and intracellular edema and degeneration of
the epithelial cells. The cell cytoplasm appears to be
liquified and clear. Later the nucleus also degenerates.
The vesicle formation results from fragmentation of the
degenerated epithelial cells. Occasionally, round
eosinophilic inclusion bodies are found in the nuclei of
epithelial cells. These inclusion bodies may be a colony
of virus particles, degenerated protoplasmic remnants
of the affected cell, or a combination of both, called
Lipschutz’s bodies. Connective tissue is infiltrated by
plasma cells. Smear obtained is Tzanks smear and the
stain used is Giemsa’s stain.
Diagnosis
It is usually established from the patients history and the
clinical findings. For confirmatory tests the material may
be obtained from the lesion and submitted to the
laboratory.
1. Direct smear: The material is obtained from the base
of the lesion and smeared and stained. The finding
of multinucleated cells with swelling, ballooning and
degeneration is adequate for diagnosis.
2. Inoculation of the virus from a suspected site, to tissue
culture.
3. Serological studies: Its role is still uncertain.
Differential Diagnosis
1. Acute necrotizing ulcerative gingivitis
2. Erythema multiforme
3. Steven’s-Johnson syndrome
4. Lichen planus
5. Desquamative gingivitis
6. Apthous stomatitis (canker sores)
Aphthous Stomatitis
It is characterized by the appearance of discrete spherical
vesicles that rupture after 1 or 2 days and form depressed
spherical ulcers. The ulcers consist of a saucer like red
or grayish red central portion and an elevated rim at
the periphery.
The lesions may occur anywhere in the oral cavity,
the mucobuccal fold, the floor of the mouth being the
most common sites. It’s a painful lesion and may occur
as a single lesion or as lesions scattered throughout the
mouth. The duration of each lesion is 7 to 10 days.
Aphthous stomatitis may occur in the following forms:
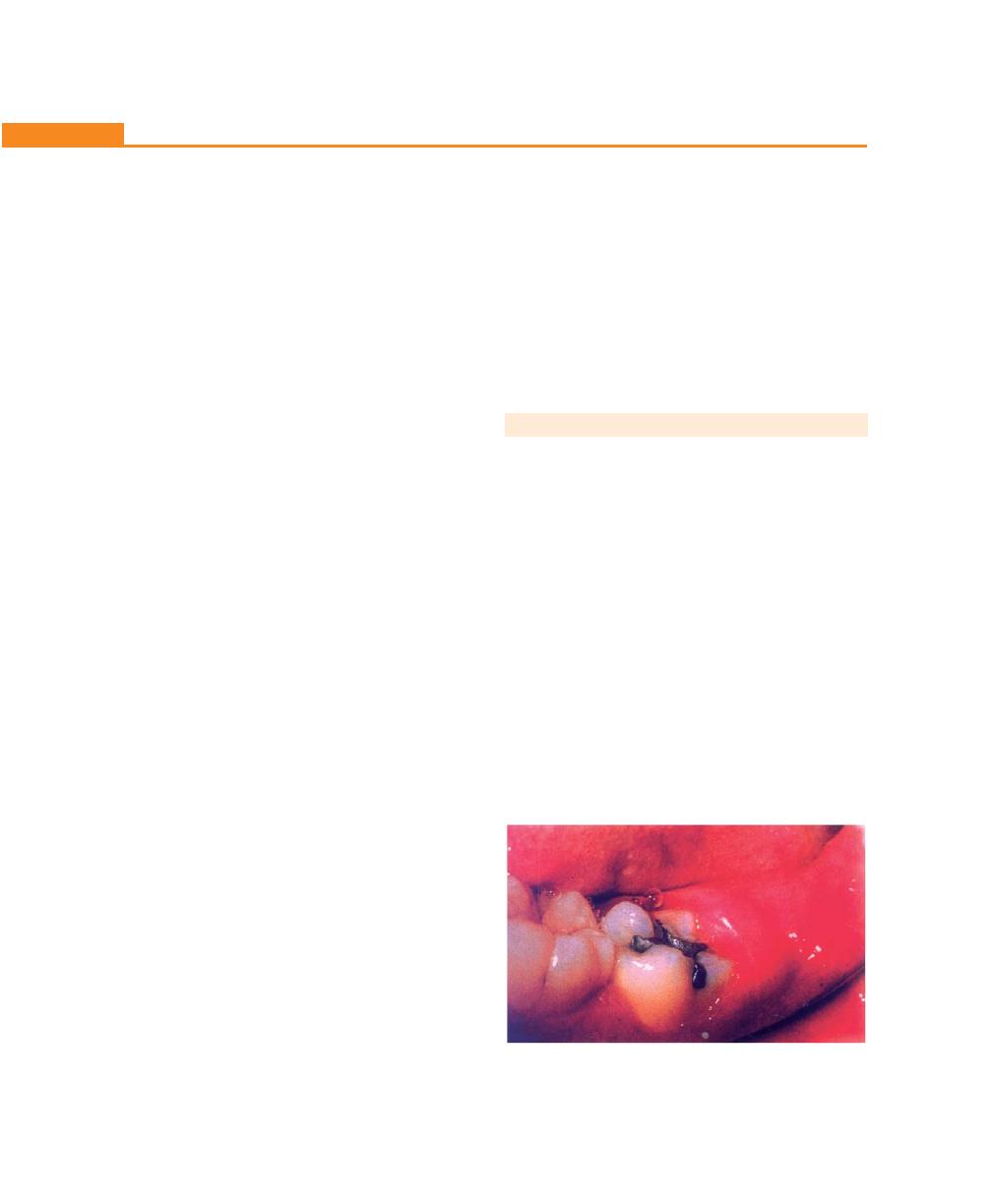
174
Essentials of Clinical Periodontology and Periodontics
Occasional aphthae: Are single lesions that occurs
occasionally, at intervals that vary from months to years.
Acute aphthae: There is an acute episode of aphthous
ulcer, which may persist for weeks; such acute episodes
are often seen in children and adults with acute
gastrointestinal disorders.
Chronic recurrent aphthae: It is a condition in which
one or more oral lesions are always present. It is a
recurrent condition with involvement spanning a period
of many years.
Etiology
It is unknown. Herpes simplex virus was suspected to
be the cause but antibody and tissue culture studies
discourage this opinion. Predisposing factors include
hormonal disturbances, allergic phenomena, gastro-
intestinal disorders and psychosomatic factors.
Acute herpetic gingivostomatitis (AHG)—It is associated
with diffuse erythematous involvement of gingiva and
acute toxic systemic symptoms.
Transmissible: Denotes a capacity for the maintenance
of an infectious agent in successive passages through a
susceptible animal host.
Communicable: Signifies a capacity for the maintenance
of infection by natural modes of spread such as direct
contact through drinking water, food, utensils, etc.
Evidence show that ANUG is a transmissible disease where-
as acute herpetic gingivostomatitis is a contagious one.
Treatment
Various medications have been used in the treatment
of this condition including:
a. Local applications: Using 8 percent zinc chloride,
Talbot’s iodine, phenol, riboflavin, thiamine, etc.
Chlortetracycline (aureomycin) has been
successfully used as a mouthwash applied topically
in a 3 percent ointment or administered systemically
in the form of 250 mg capsules
b. Palliative treatment: Makes the patient comfortable
until the disease runs its course (7-10 days).
Fig. 20.4:
Pericoronitis–third molar partially-covered by
infected flap
• Plaque, food debris and superficial calculus are
removed to reduce gingival inflammation.
• Relief in pain is obtained with diclonine hydro-
chloride—a topical anesthetic mouth wash, which is
available in a 0.5 percent solution that maybe diluted
1:1 with water.
c. Supportive treatment: Copious fluid intake and
systemic antibiotic therapy for management of toxic
systemic complications. For relief of pain, systemically
administered aspirin is usually sufficient.
PERICORONITIS (Fig. 20.4)
Definition
It is an acute infection which refers to inflammation of
gingiva and surrounding soft tissues of an incompletely
erupted tooth. It occurs most frequently in the
mandibular third molar area.
Types
Acute, sub-acute or chronic.
Clinical Features
Signs and Symptoms
Include markedly red, edematous suppurating lesion that
is extremely tender with radiating pain to the ear, throat
and floor of the mouth.
The patient is extremely uncomfortable because of
the foul taste and inability to close the jaws. In addition
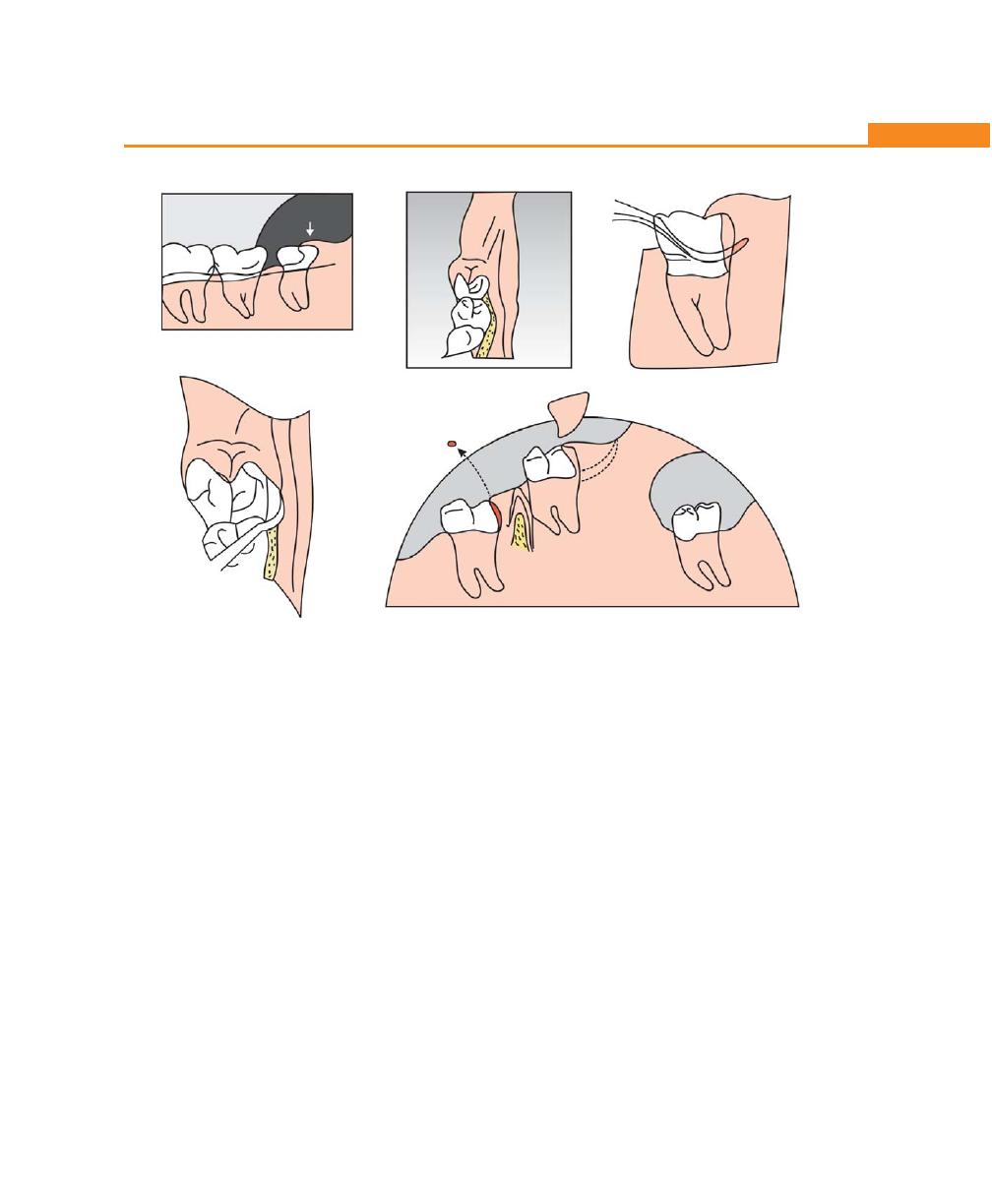
175
Acute Gingival Infections
Figs 20.5A to E:
Treatment of pericoronitis. (A,B) Pericolonal flap covering partially erupted lower IIIrd molar, (C,D) With
the help of a scalor flap is reflected to cleanse the underlying debris, (E) A wedge-shaped incision is made to section
the tissue
to the pain, swelling of the cheek in the region of the
angle of the jaw is seen.
Acute Pericoronitis
It is identified by varying degrees of involvement of
pericoronal flap as well as with systemic complications.
An influx of inflammatory fluid and cellular exudates
results in an increase in bulk of the flap which interferes
with complete closure of the jaws. The flap is traumatized
by contact with the opposing jaw and inflammatory
involvement is aggravated.
Lymphadenitis is a common finding; the patient may
also have toxic systemic complications such as fever,
leukocytosis and malaise.
Complications
• The involvement may become localized, in the form
of pericoronal abscess.
• If it occurs in a partly-erupted vital tooth it may give
rise to cyst formation.
• It may spread posteriorly into the oropharyngeal
area and medially into the base of the tongue, making
it difficult for the patient to swallow.
• Depending on the severity there is involvement of
the submaxillary, cervical, deep cervical and retro-
pharyngeal lymph node.
• Peritonsillar abscess formation, cellulitis and Ludwig’s
angina are infrequent but nevertheless potential
sequelae of acute pericoronitis.
Treatment (Fig. 20.5)
The treatment of pericoronitis depends on:
• Severity of the inflammation.
• The systemic complications and,
• The advisability of, retaining the involved tooth.
A
B
C
D
E
176
Essentials of Clinical Periodontology and Periodontics
First Visit
1. The area is gently flushed with warm water to remove
superficial debris and exudate followed by application
of topical anesthetic agent.
2. The flap is reflected with a scaler and the underlying
debris is also removed and the area is flushed with
warm water.
3. Instructions to the patient include hourly rinses
with a solution of a tea spoonful of salt in a glass
of warm water, rest, copious fluid intake and
administration of systemic antibiotics, if toxic
symptoms are present.
4. If the gingival flap is swollen and fluctuant an
anteroposterior incision to establish drainage is made
with a No. 15 bard parker blade, followed by insertion
of 1/4th inch gauze wick.
In the next visit, determination is made as to whether
the tooth is to be retained or extracted. This decision
is governed by the likelihood of further eruption into
a good functional position.
If it is decided to retain the tooth, the necessary
surgical procedures are performed using a periodontal
knife or electro-surgery. Under anesthesia, a wedge-
shaped incision is made to section a tissue that includes
the gingival flap with the tissue distal to the involved
tooth as well.
After the tissue is removed, a periodontal pack is
placed.
KEY POINTS TO NOTE
1. Acute gingival lesions are classified as acute necrotizing
ulcerative gingivitis (ANUG), primary herpetic gingivosto-
matitis, and pericoronitis.
2. ANUG is an inflammatory, destructive disease of the gingiva,
which presents with characteristic signs and symptoms.
3. Oral lesions are characterized by punched-out, crater-like
depressions at the crest of the interdental papillae, subse-
quently involving marginal gingiva and rarely attached
gingiva.
4. These craters are covered by greyish pseudomembranous
slough.
5. Patients with ANUG complain of a constant radiating,
gnawing pain and metallic taste with excessive amount of
pasty saliva.
6. Etiological factors responsible for ANUG are divided into:
• Role of bacteria (fusospirochetal complex)
• Local predisposing factors like preexisting gingivitis,
injury to the gingiva and smoking.
• Systemic predisposing factors like nutritional deficiency,
debilitating diseases and psychosomatic factors
7. Primary herpetic gingivostomatitis is a viral infection
characterized by diffuse erythema and vesicular eruptions.
8. Pericoronitis is an acute infection, which refers to
inflammation of the gingiva and the surrounding soft tissues
of an incompletely erupted tooth. (Mostly in mandibular III
molar area).
REVIEW QUESTIONS
1. Classify acute gingival lesions; discuss the etiology,
clinical features and treatment for acute necrotizing
ulcerative gingivitis.
2. Differentiate between acute necrotizing ulcerative
gingivitis and acute herpetic gingivostomatitis.
3. Pericoronitis—definition, clinical features and treat-
ment.
BIBLIOGRAPHY
1. Carranza, Newman. Clinical Periodontology. 8th
edition, WB
Saunders.
2. JD Manson, Bmeley. Outline of Periodontitis. 3rd edn, British
Library Cataloging in Publication Data, 1995.
3. Loesche WJ, Syed SA, Langhorn BE. The bacteriology of acute
necrotizing ulcerative gingivitis. J Periodontol 1982;53:223.
4. Yoji Murayama, Hidemi Kurihara, Thomas E. Van Dyke. Acute
necrotizing ulcerative gingivitis, risk factors involving host
defense mechanisms. Periodontol 2000;6:1994.
177
Periodontal Diseases in Children and Young Adolescents
INTRODUCTION
Periodontal disease consists of a group of infections
affecting the gingiva and the supporting structures:
cementum, periodontal ligament and alveolar bone.
Gingival and periodontal changes in children could be
due to the plaque and certain systemic diseases, which
have a direct effect on the periodontium.
While the periodontal diseases are generally
considered to affect the adults, children and adolescents
can also manifest certain periodontal disorders, for
example, prepubertal and juvenile periodontitis. This
group of patients will benefit from an early periodontal
evaluation and any necessary treatment.
The gingiva and periodontium of children differ in
some respects from those of adults. Hence periodontal
diseases in children can be studied from anatomical,
microbiological and cellular aspects.
ANATOMIC CONSIDERATIONS IN
CHILDREN
Changes in Gingiva
1. The gingival tissues are more reddish due to a thinner
epithelium, a lesser degree of cornification, and a
greater vascularity.
2. The gingiva lacks the stippling, due to shorter and
flatter papillae from the lamina propria.
3. The gingival margins appear to be rounded, rolled
due to hyperemia and edema that follows eruption.
4. Greater sulcular depth, due to relative ease of gingival
retraction.
5. The gingiva appears to be flabbier due to the lower
density of the connective tissue in the lamina propria.
Cementum
Changes include:
The cementum in the children is thinner and less dense
and shows a tendency to hyperplasia of cementum apical
to the epithelial attachments.
❒
❒
❒
❒
❒ ANATOMIC CONSIDERATIONS IN
CHILDREN
• Changes in Gingiva, Cementum,
Periodontal Ligament and Alveolar
Bone
❒
❒
❒
❒
❒ HISTOPATHOLOGY OF GINGIVITIS IN
CHILDREN
❒
❒
❒
❒
❒ MICROBIOLOGY OF PERIODONTAL
DISEASES IN CHILDREN
❒
❒
❒
❒
❒ CLASSIFICATION OF PERIODONTAL
DISEASES IN CHILDREN
• Gingival Lesions
• Types of Periodontitis
21
Periodontal Diseases in
Children and Young
Adolescents

178
Essentials of Clinical Periodontology and Periodontics
Periodontal Ligament
Changes in the periodontal ligament include:
The periodontal ligament in children is wider, has
fewer and less dense fibers per unit area. It also has
increased hydration with a greater blood and lymph
supplies than in adults.
Alveolar Bone
Changes in the alveolar bone include:
1. In children, the lamina dura is thinner, fewer
trabeculae and larger marrow spaces. There is also
a smaller amount of calcification, greater blood and
lymph supply and the crest of the alveolar bone
appears flatter.
2. The contact points between the deciduous teeth
are not as tight as those between the permanent
dentition.
HISTOPATHOLOGY OF
GINGIVITIS IN CHILDREN
1. Adult tissues show a greater density of plasma cells
whereas in children, there were seen seven times as
many lymphocytes as plasma cells.
2. Increased vascularity is seen.
MICROBIOLOGY OF PERIODONTAL
DISEASES IN CHILDREN
Experimental gingivitis in children and the adults has
shown in Table 21.1.
Table 21.1: Microbial species in children’s and
adult plaques
Species in greater numbers
Species in adult plaques
in children’s plaque
• Leptotrichia species
• Fusobacterium
• Capnocytophaga
• Eubacterium
• Selenomonas species
• Bacteroides species
CLASSIFICATION OF PERIODONTAL
DISEASES IN CHILDREN
Gingival Lesions
1. Acute gingivitis
• Herpetic gingivostomatitis
• Necrotizing-ulcerative gingivitis
• Candidiasis
2. Chronic marginal gingivitis
• Plaque induced
• Puberty gingivitis
3. Factitious gingivitis
4. Localized gingival recession
Periodontal Lesions
Early Onset Periodontitis
• Prepubertal
– Localized
– Generalized
• Juvenile
– Localized
– Generalized
Periodontitis Associated with
Systemic Disease
• Papillon-Lefévre syndrome
• Ehler-Danlos syndrome
• Hypophosphatasia
• Chediak-Higashi syndrome
• Leukocyte adhesion deficiency
• Neutropenia
• Down’s syndrome.
Gingival Lesions
Acute Herpetic Gingivostomatitis
It is a viral infection of the oral mucosa caused by herpes
simplex virus. It occurs most frequently in infants and
children younger than 6 years of age.

179
Periodontal Diseases in Children and Young Adolescents
Clinical Features
I. Intraoral signs and symptoms
II. Extraoral signs and symptoms
I. Intraoral signs include:
1. Gingiva appears to be diffuse red, erythematous, with
varying degree of edema and gingival bleeding.
2. In the initial stage, it appears to be discrete, spherical
grey vesicles involving labial and buccal mucosa, soft
palate, pharynx and tongue, approximately after 24
hours the vesicles rupture leaving painful ulcers.
3. The ulcers appear to be red, elevated with halo-like
margins and a depressed yellowish or grayish-white
central portion.
4. The course of the disease is 7 to 10 days.
Oral symptoms
1. Generalized soreness of the oral cavity, which
interferes with the eating and drinking.
2. The ruptured vesicles are sensitive to touch, thermal
changes and food.
II. Extraoral and systemic signs and symptoms
1. Involvement of the lips and face (Herpes labialis, “cold
sore”)
2. Cervical adenitis, fever is as high as 101 to 105ºF
and generalized malaise are common.
Treatment
a. Local application—using Talbot’s iodine, zinc chloride
80 percent, riboflavin, thiamine, have been used.
Chlortetracycline (Aureomycin) has been used
successfully.
b. Palliative treatment—food debris and superficial
debris is removed, relief of pain is obtained with 0.5
percent dyclonine hydrochloride mouthwash which
has a topical anesthetic effect.
c. Supportive treatment—copious fluid intake, for relief
of pain systemically administered asprin is usually
sufficient.
Acute Necrotizing Ulcerative Gingivitis (ANUG)
Affects both children and adults, and is characterized by
sudden onset. Sometimes following an episode of
debilitating disease of acute respiratory tract infections.
Clinical features
• Intraoral signs and symptoms
• Extraoral signs and symptoms.
Oral signs
1. Two types of ulcerations are seen, lateral ulceration,
deep ulceration and necrosis. It has been suggested
that these patterns of necrosis are related to the fact
that major blood supply to the two areas of gingiva
is different.The gingival tissues gets blood supply from
two main sources, i.e. supraperiosteal and intra-
alveolar vessels.
2. Lateral ulceration is characterized by involvement of
buccal wall of the papillae, margins and possibly the
attached gingiva occurs in the distribution of lateral
blood supply (less common). Deep ulceration
involving primarily necrosis of the tissues of the
embrasure, giving rise to the typical truncated
papillae occurs in the distribution of the intra alveolar
vessels (comparatively more common).
3. These craters are caused by gray pseudomem-
braneous slough demarcated from the remiander of
the gingival mucosa by a pronounced linear
erythema.
4. Other signs include—gingival hemorrhage or pro-
nounced bleeding on slightest provocation.
Oral symptoms
1. The lesions are extremely sensitive to touch.
2. Constant radiating, gnawing pain that is intensified
by eating spicy or hot food and chewing.
3. Metallic foul taste and the patient is conscious of an
excessive amount of “pasty saliva”.
Extraoral and systemic signs and symptoms:
In mild to moderate stages—local lymphadenopathy and
slight elevation in temperature is seen.
In severe cases—high fever, increased pulse rate,
leucocytosis, loss of appetite is seen.

180
Essentials of Clinical Periodontology and Periodontics
In very rare cases, severe sequlae may follow:
• Noma or gangrenous stomatitis.
• Fusospirochetal meningitis.
• Toxemia and fatal brain abscess.
Etiology
a. Bacteriology consists of constant flora composed of
fuso-spirochetal organisms, variable flora consists of
spirochetes of varying sizes and Bacteroides
intermedius.
b. Predisposing factors—local and systemic.
Local predisposing factors include:
• Pre-existing gingivitis, e.g. incubation zones.
• Injury to the gingiva, e.g. malocclusion
• Smoking.
a. Direct toxic effect of tobacco
b. Vascular or other changes induced by nicotine or
other substances
c. As a reflection of stress.
Systemic predisposing factors include:
• Nutritional deficiency.
• Debilitating diseases.
• Psychosomatic factors.
Treatment
Should follow an orderly sequence.
First visit: Treatment is confined to the acutely involved
areas, after applying topical anesthesia. The areas are
gently swabbed to remove the pseudomembrane.
Superficial calculus is removed with ultrasonic scaler. The
patient is instructed to rinse the mouth every 2 hours
with a glassful of an equal mixture of warm water and
3 percent hydrogen peroxide (chlorhexidine rinses are
also recommended). In the presence of systemic
symptoms penicillin or erythromycin or metronidazole
is prescribed.
Second visit: Scaling is performed (After 1 to 2 days).
Third visit: After 1 to 2 days of second visit. Scaling and
root planing are repeated with plaque control
instructions, hydrogen peroxide rinses are discontinued,
but chlorhexidine rinses can be maintained for 2 to 3
weeks.
Candidiasis: Acute Candidiasis
(Moniliasis, Thrush)
It is the most common mycotic infection of the oral
mucosa caused by Candida albicans. It is seen in three
types of individuals; debilitated or immunosuppressed
adults, infants and adults who have been on antibiotic
therapy for sometimes.
Oral lesions are described as four clinical types:
a. Pseudomembranous type—white curd-like plaques.
b. Atrophic type—usually seen on the dorsum of the
tongue with erythema and papillary atrophy.
c. Hyperplastic type—hyperkeratosis of the epithelium
with white plaques.
d. Epidermal and perioral type—scaling patches at the
corner of the lips.
Treatment
Current treatment is the use of the antimycotic agent
like co-trimoxazole in the form of oral troches every 3
hours (for a total of 6 per day) for 7 to 10 days.
Chronic Candidiasis
It is a rare type of C. albicans infections resulting in a
granulomatous lesion that begins in infancy or early
childhood and may persists for several years.
Chronic Marginal Gingivitis
It is the most common type of gingival disease in
childhood. Gingival color changes and swelling appear
to be more common expressions of gingivitis in children
than a bleeding and increased pocket depth.
Etiology
Plaque and calculus are most common causes. In children
certain conditions predispose the gingivitis they include:
a. Gingivitis associated with tooth eruption is called as
eruption gingivitis.

181
Periodontal Diseases in Children and Young Adolescents
b. Partially-exfoliated, loose deciduous teeth frequently
cause gingivitis.
c. Gingivitis occurs more frequently around malposed
teeth.
d. Gingivitis is increased in children with excessive
overbite and overjet, mouth breathing and nasal
obstruction.
e. A higher prevalence and severity of gingivitis and
gingival enlargement is found in the circum pubertal
period. This form of gingivitis has been termed as
pubertal gingivitis. There may be a gingival enlarge-
ment as a result of hormonal changes that magnify
tissue response to the local irritants.
These inflammatory changes may persist during the
time the primary tooth is in the mouth and with exfo-
liation may become more severe. These inflammatory
lesions are usually non-destructive and do not progress
to attachment loss. The treatment is plaque control
instruction and debridement. The accumulation of plaque
in children is probably more rapid than in adults, but
the host response is generally not as intense. Calculus,
in contrast, is found less frequently in children than in
adults, but gradually increases as the child enters the
teenage years.
Factitious Gingivitis (Gingivitis Artefacta)
It is of two types:
• Major form.
• Minor form.
Minor form results from the rubbing or picking the
gingiva with finger nail (habitual). Major form is more
severe and involves deeper peridontal tissues
(psychological causes).
Localized Gingival Recession
It may be seen around individual teeth or groups of
teeth. The recession may be seen in the presence or
absence of inflamed gingiva depending on the local
irritants. In children the position of the tooth in the arch
is the most important cause, e.g. labially-positioned, tilted
or rotated teeth and anterior open bite, the recession
may be transitional phase in tooth eruption and may
correct itself or it may be necessary to realign the tooth
orthodontically.
Types of Periodontitis
Prepubertal Periodontitis
Prepubertal periodontitis occurs in localized and
generalized forms.
Localized Prepubertal Periodontitis
Clinical features
• The age of onset is approximately 4 years.
• Plaque levels are usually low.
• Alveolar bone loss is rapid.
• Defect in neutrophil or monocyte functions has been
reported.
Generalized Prepubertal Periodontitis
• Entire width of attached gingiva appears to be fiery-
red.
• Gingival hyperplasia, cleft formation and recession.
• Rapid destruction of the alveolar bone.
• Systemic involvement like recurrent bacterial infe-
ctions.
• Defects in polymorphonuclear leukocytes and mono-
cytes.
Juvenile Periodontitis
Definition was given by Baer, who described it, “as a
disease of the periodontium occurring in an otherwise
healthy adolescents, which is characterized by a rapid
loss of alveolar bone around more than one tooth of
the permanent dentition”. It exists in two forms:
a. Localized
b. Generalized.
Clinical features of localized juvenile periodontitis (LJP)
1. Age and sex distribution—between 11-15 years some
studies show predilection for female patients.

182
Essentials of Clinical Periodontology and Periodontics
2. Distribution of lesions—three types of involvement
is seen:
a. First molar and/or incisors.
b. First molar and/or incisors with additional teeth
(not exceeding 14 teeth).
c. Generalized involvement.
3. The most striking feature is lack of clinical infla-
mmation, despite the presence of deep periodontal
pockets.
4. Small amounts of plaque is seen which rarely
mineralized to become calculus.
5. Most common initial symptoms are mobility,
migration of the incisors and first molars. Classically,
a distolabial migration of the maxillary incisors with
diastema formation occurs.
For LJP, classic distribution is the involvement of first
molars and incisors with least distribution in the cuspid,
premolar area. The reasons could be:
1. Production of opsonizing antibodies against Aa
(Actinobacillus actinomycetem comitans).
2. Bacteria antagonistic to Aa may develop thereby
decreasing the number of colonization sites.
3. Aa may lose its leukotoxin producing ability for
unknown reasons.
Localization of the lesions could also be due to the
defect in cementum formation [hypoplastic/aplastic
cementum].
Pathogenesis of LJP is related to the interplay of several
factors. These include the specific microbiology of
subgingival plaque, defects in cementum, hereditary
factors, impaired PMN functions and disorders of the
immune system.
Microbiology of LJP: Two types of bacteria are
considered to be pathogenic in LJP
• Actinobacillus actinomycetem comitans
• Capnocytophaga
Virulence factors produced by A. actinomycetemco-
mitans are as follows:
a. Leukotoxin—destroys polymorphonuclear leukocytes
(PMN’s) and macrophages.
b. Endotoxin—activates host cells to secrete inflam-
matory mediators (PG’s, IL1β, TNFα).
c. Bacteriocin—may inhibit the growth of beneficial
species.
d. Immunosuppressive factors—may inhibit IgG and
IgM production.
e. Collagenase—causes degradation of collagen.
f. Chemotactic inhibition factors—may inhibit neutro-
phil chemotaxis.
Radiographic findings:
1. Vertical/angular bone loss around, the first molars
and incisors in an otherwise healthy teenagers is a
diagnostic sign of classic Juvenile periodontitis (J.P.)
“Arc-shaped” loss of alveolar bone extending from
the distal surface of the 2nd premolar to the mesial
surface of the 2nd molar is seen.
2. Bilateral symmetrical patterns of bone loss is seen
[“mirror-image”pattern].
Immunologic findings:
A large proportion of patients (70%) with LJP have a
defect in PMN chemotaxis, which is cell-associated and
depressed PMN phagocytosis, which is serum-associated.
PMN show reduced cell surface receptors to the synthetic
polypeptide chemotactic factors. N-formylmethionyl
phenylalanine (FMLP) and the complement factor C
5a
,
also have reduced amounts of surface glycoproteins GP-
110 which is important for chemotactic response.
Clinical features of GJP: The age of diagnosis is between
20 and 30 years. Severe generalized bone loss is the
characteristic feature. This may be restricted to upper
or lower arch. Patients of GJP often show good plaque
control and the extent of bone loss does not
commensurate with the level of oral hygiene.
Treatment: In the past, the prognosis for juvenile
periodontitis was considered to be poor.
Current therapy: Systemic tetracycline hydrochloride
250 mg q.i.d. for atleast 1 week should be given in
conjunction with the local mechanical therapy. Several

183
Periodontal Diseases in Children and Young Adolescents
reports have shown excellent bone fill in cases of LJP
treated with tetracycline, flap surgery and placement of
grafts.
In refractory cases, tetracycline resistant Aa has been
suspected. In such cases a combination of amoxicillin
and metronidazole has been suggested.
Periodontitis Associated with Syndromes
Papillon-Lefévre syndrome
a. It is characterized by hyperkeratotic skin lesions and
severe destruction of the periodontium.
b. These changes may appear before the age of 4 years.
c. Characteristic skin lesions are hyperkeratosis of
localized areas on palms and soles, knees and elbows.
d. Periodontal involvement includes, early inflammatory
changes that lead to bone loss and exfoliation of
teeth. Primary teeth are lost by 5 or 6 years of age.
The permanent dentition erupts normally but within
few years the permanent teeth are also lost.
Ehler-Danlos syndrome: It is an inherited disorder
affecting the connective tissues, the defect is in collagen
molecular biology, but the nature of the defect is
unknown. The syndrome is named after two clinicians,
who described excessive joint mobility, skin
hyperextensibility, easy bruising and peculiar scarring,
which occur after skin wounds.
Oral and periodontal manifestations: The oral mucosa,
gingival tissues, teeth and temporomandibular joints can
all be affected by Ehlers-Danlos syndrome.
Oral mucosa:
• It is often fragile and susceptible to bruising.
• Post-extraction hemorrhage can be a problem, due
to fragility of blood vessels and defects in the
supporting connective tissues.
Gingival tissues: These are often fragile and bleed readily
on toothbrushing. Some forms of Ehler-Danlos syndrome
(type VII) are reported to have advanced periodontal
destruction.
Teeth: Teeth in Ehler-Danlos syndrome are fragile and
fracture easily.
TMJ: Subluxation of temporomandibular joints have
been reported.
Treatment
1. A thorough preventive program should be followed.
2. Due to the fragility of oral mucosa and gingiva, the
periodontal therapy in Ehlers-Danlos syndrome
should be as atraumatic as possible.
Down’s syndrome (Mongolism, trisomy 21)
It is a congenital disease caused by a chromosomal
abnormality and characterized by mental deficiency and
growth retardation.
The oral findings include presence of plaque, calculus,
other local irritants, e.g. diastema, crowding of teeth,
high frenum attachment and malocclusion.
Periodontal disease in Down’s syndrome: include
the formation of deep periodontal pockets associated
with plaque accumulation and moderate gingivitis,
usually generalized but more severe in the lower anterior
region (may be due to high frenal attachment). Acute
necrotizing lesions are also common.
Two factors have been proposed to explain high
prevalence and increased severity of periodontal
destruction in Down’s syndrome.
1. Reduced resistance to infections because of poor
circulation (especially peripheral).
2. Defect in T-cell maturation and polymorphonuclear
leukocyte chemotaxis.
Neutropenias: Destructive generalized periodontal
lesions have been described in children with
neutropenia.
Chédiak-Higashi syndrome (C-H syndrome): This is a
rare syndrome characterized by recurrent bacterial
infections. It exhibits oral ulcerations and rapidly
destructive periodontitis.
Hypophosphatasia: This is a rare familial skeletal disease
characterized by rickets, poor cranial formation,
premature loss of primary dentition—particularly incisors.
Patients have low levels of serum alkaline phosphatases.
184
Essentials of Clinical Periodontology and Periodontics
Teeth are lost with no clinical evidences of gingival
inflammation and show reduced cementum formation.
Acute and subacute Leukemia (Malignant Neoplasias of
WBC Precursors): Accomplished by severe periodontal
destruction.
Leukocyte Adhesion Deficiency: These cases are rare and
begin during, or immediately after eruption of the primary
teeth. Extreme acute inflammation and proliferation of
gingival tissues with rapid bone loss are found. Profound
defects in peripheral blood neutrophils and monocytes
are seen, hence they are absent in gingival tissues.
Patients with leukocyte adhesion disease also have
frequent respiratory tract infection and sometimes otitis
media.
KEY POINTS TO NOTE
1. Various anatomic variations in the gingiva, periodontal
ligament, cementum and alveolar bone may predispose to
the disease of the periodontium.
2. Most common lesions in children are gingival lesions
including acute gingivitis, chronic marginal gingivitis
facititious gingivitis and localized gingival recession.
3. Periodontal lesions include, early onset periodontitis,
prepubertal and juvenile periodontitis, periodontitis
associated with systemic diseases.
4. Most of the signs of periodontitis are seen during
adolescence, with primary prevention one may help to
reduce the tooth loss.
5. Further investigations should be carried out whenever there
is bleeding after gentle probing in the presence of relatively
healthy gingiva, so that early onset periodontal lesions and
prepubertal conditions can be ruled out.
BIBLIOGRAPHY
1. Baer PN. The case for the periodontitis as a clinical entity.
J of Periodontol 1971; 42:516-19.
2. Boer PN, Benjamin SD. Periodontal disease in children and
adolescents. Philadelphia;JB, 1974.
3. Bradely RE. Periodontal lesions in children: their recognition
and treatment. Dent Clin North Am 1961; 5:671-85.
4. Carranza. Newman Clinical Periodontology, 8th edition.
5. Cohen B. Morphological factors in the pathogenesis of
periodontal disease. Br Dent J 1959;107:31-9.
6. Davies RM, Smith RG, Porter SR. Destructive forms of
periodontal disease in adolescent and young adults. Br Dent
J 1985;158:429-36.
7. Longhurst P, Johnson NW, Hopps RM. Differences in
lymphocytes and plasma cell densities in inflamed gingiva
from adults and young children. J Periodontol 1977;48:
707-10.
8. Machtei EE, Zubery Y, Bimstein E, Becker A. Anterior open
bite and gingival recession in children and adolescents. Int
Dent J 1990;40:369.
9. More WEC et al. Bacteriology of experimental gingivitis in
children. Infection and Immunity 1984;46:1-6.
10. Page RC et al. Rapidly progressive periodontitis, a distinct
clinical condition. J Periodontol 1983;54:197-209.
11. Page RC, Schroeder HE. Periodontitis in man and other
animals. A comparative review. Karger, Basle, 1982.
12. Ruber MP, Frankel SNM, Wallace S. The histopathology of
periodontal disease in children. J Periodontol 1971;42:473-
84.
13. Schwartz, Lamster, Fine. Clinical Guide to Periodontics, WB
Saunders Company, 1995.
14. Waite IM, Furniss JS. Periodontal disease in children, a review.
Journal of Paediatric Dentistry 1987;3:59-67.
15. Zapplers. Periodontal Disease in Children 1948;27:333-
40.
185
Desquamative Gingivitis
INTRODUCTION
The term chronic desquamative gingivitis was coined by
Prinz in 1932 to describe a peculiar condition charac-
terized by intense erythema, desquamation and
ulceration of the free and attached gingiva. In 1960,
Mc Carthy and colleagues suggested that desquamative
gingivitis was not a specific disease entity, but a gingival
response associated with a variety of conditions.
Desquamative gingivitis is a generic term that may
represent a variety of specific disease processes, but the
clinical picture is indisputable. There may be threads or
tags of loose necrotic epithelium, as the name suggests.
Desquamative gingivitis involves not only marginal
gingiva, as do most cases of gingivitis, but it also peels
the attached gingiva often in a band-like fashion. The
clinical description of desquamative gingivitis represents
gingival manifestations of a variety of diseases such as
lichen planus, cicatricial pemphigoid, pemphigus vulgaris,
bullous pemphigoid, adult linear IgA dermatosis,
dermatitis herpetiformis and drug reactions.
DIAGNOSIS
The success of any given therapeutic approach resides
on the establishment of an accurate final diagnosis. The
following approach helps us to elucidate the disease
triggering desquamative gingivitis (Fig. 22.1).
CLINICAL FEATURES
The clinical features vary in severity as mild, moderate
and severe forms.
Mild Form
There is diffuse erythema of the marginal, interdental
and attached gingiva. It is usually painless and occurs
most frequently in females between 17 and 23 years
of age.
❒
❒
❒
❒
❒ DIAGNOSIS
❒
❒
❒
❒
❒ CLINICAL FEATURES
❒
❒
❒
❒
❒ HISTOPATHOLOGY
❒
❒
❒
❒
❒ THERAPY
❒
❒
❒
❒
❒ DISEASES CLINICALLY PRESENTING AS
DESQUAMATIVE GINGIVITIS
• Lichen Planus
• Cicatricial Pemphigoid
• Bullous Pemphigoid
• Linear IgA Disease
• Dermatitis Herpetiformis
• Pemphigus Vulgaris
• Drug Reaction or Eruptions
22
Desquamative
Gingivitis
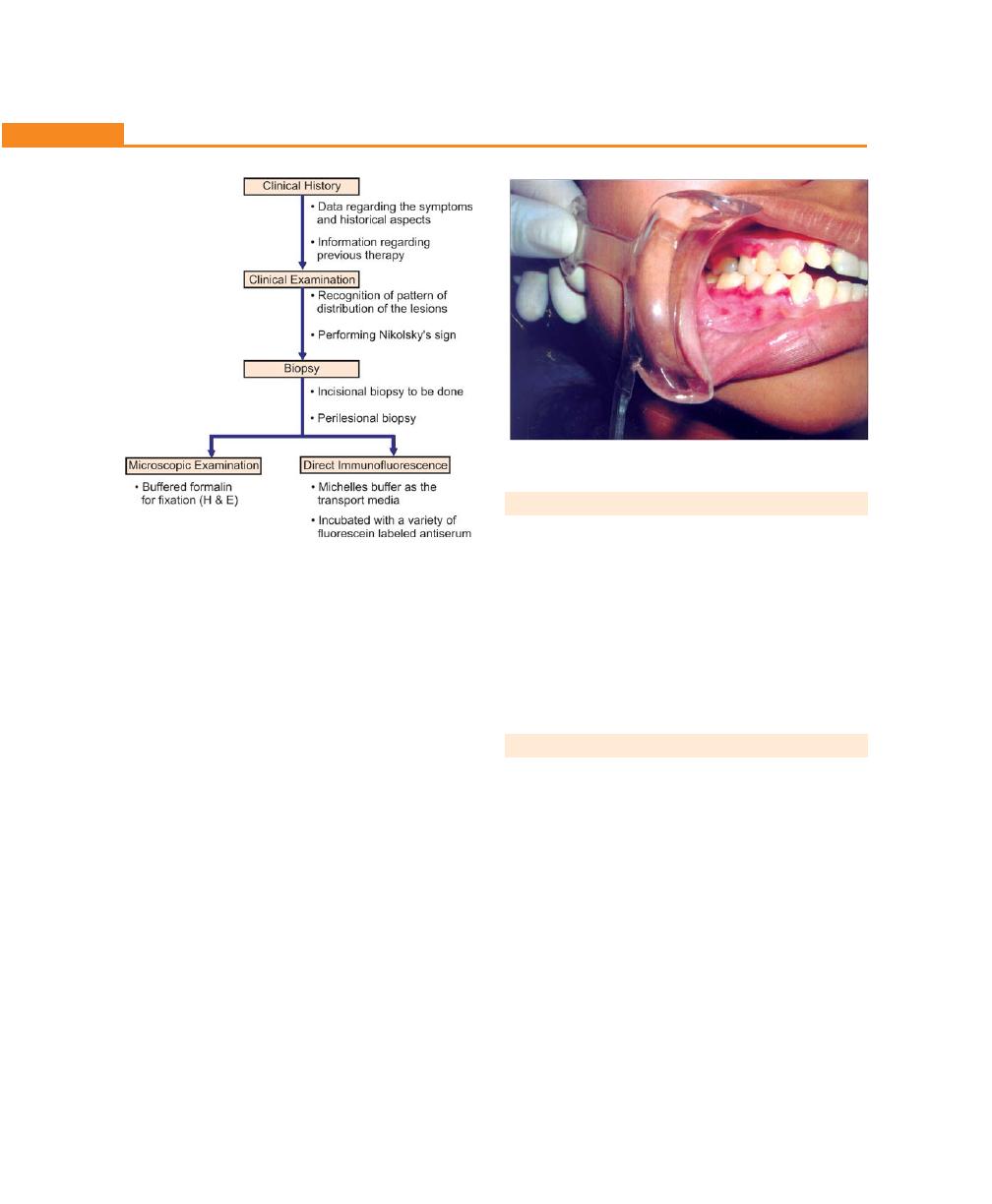
186
Essentials of Clinical Periodontology and Periodontics
HISTOPATHOLOGY
Microscopically, desquamative gingivitis often appears
as bullous lesions or lichenoid lesions, occasionally
there will be a thin, atrophic epithelium with little or no
keratin at the surface and a dense, diffuse infiltration
of chronic inflammatory cells in the underlying
connective tissue. Histochemical and ultrastructural
studies revealed separation of collagen fibrils and a
decrease in the number of anchoring fibrils.
THERAPY
The therapy must be based on an understanding of the
basic disease process causing the gingival reaction. It can
be of two phases:
a. Local treatment
b. Systemic treatment
Local Treatment
• Oral hygiene instructions (Soft toothbrush).
• Oxidizing mouthwashes (H
2
O
2
3% diluted).
• Topical corticosteroid ointments or cream-like
triamcinolone 0.1 percent, flucocinamide 0.05
percent, desonide 0.05 percent.
Fig. 22.2:
A case of chronic desquamative gingivitis
Fig. 22.1:
An approach to diagnosing
desquamative gingivitis
Moderate Form
Patchy distribution of bright-red and gray areas involving
marginal and attached gingiva. The surface is smooth
and shiny, normal resilient gingiva becomes soft,
edematous and massaging of gingiva results in peeling
off the epithelium. Usually seen in the age group of 30
to 40 years. Patient complains of burning sensation. The
labial surface is more frequently involved.
Severe Form
This form is characterized by scattered irregularly-shaped
areas in which the gingiva is denuded and strikingly red
in appearance. The gingiva seems to be speckled and
the surface epithelium seem shredded, friable and can
be peeled off in small patches. The mucous membrane
other than gingiva is smooth and shiny and may present
fissuring in the cheek adjacent to the line of occlusion.
The condition is painful. There is a constant, dry, burning
sensation throughout the oral cavity (Fig. 22.2).

187
Desquamative Gingivitis
T
able 22.1:
Diseases clinically presenting as desquamative gingivitis
Lichen
Cicatricial
Bullous
Pemphigus
Dermatitis
Linear IgA
planus
pemphigoid
pemphigoid
vulgaris
herpe
tiformis
disease
Clinical
•
Bilateral white
•
Multiple painful
•
Skin disease
•
Multiple painful
•
Skin disease with
•
Vesicles, painful
features
striae
ulcers
with infrequent
ulcers
preceded
rare oral
ulcerations
•
Purple pruritic
•
Preceded by
oral lesions
by
bullae
involvement
•
Erosive gingivitis
pappule
bullae
•
Ulcers preceded
•
Middle age
•
Vesicles and
•
Seen in middle
•
Positive
by
bullae
•
Positive Nikolsky
’s
pustules
ag
e
Nikolsky’s sign
•
No scarring
sign
•
Exacerbation
•
Buccal mucosa
•
Middle aged
•
Seen in elderly
•
Progressive
and remission
commonly
or elderly women
persons
disease
•
Young and
affected
•
May effect
middle aged
mucous
membranes of
oral cavity
,
eyes, genitalia.
Histopathologic
•
Hyperkeratosis
•
Subepithelial
•
Subepithelial
•
Intraepithelial
•
Collection of
•
Separation of
features
•
Hydropic
clefting with
clefting with
clefting above
ne
utrophil
s,
basement
degeneration of
epithelial
epithelial
the basal cell
eosinophils and
membrane
the basal layer
separation
separation
layer
fibrin in
•
Saw toothed
from lamina
from lamina
•
“T
ombstone”
connective
rete pegs
propria leaving
propria
appearance
tissue papillae
•
Colloid bodies
an
intact basal
leaving an
of basal cells
present
la
ye
r
intact basal
•
Acantholysis
•
Dense infiltration
layer
present
of T
-lymphocytes
Diagnosis
•
B
y
clinical
•
Based on
•
Sub-epidermal
•
Based on
•
C
ha
racteristic
•
Linear deposits
appearance
clinical features
blister on
clinical features
histopathology
of
I
gA
a
t t
he
•
Fibrillar deposits
•
Linear deposits
histologic
•
Intercellular
and
demonstration
basement
of fibrin at the
of C3 with or
examination
deposits in
of
specific IgA
membrane zone
dermal-epidermal
without IgG
•
Linear deposits
epithelium,
immunofluorescence
junction
at
basement
of C3 with or
IgG in all cases
membrane
without IgG at
•
C3 in most
basement
cases
membrane
Tr
eatment
•
Topical or
•
Topical or
•
Systemic
Systemic steroids,
•
Dapsone,
•
Sulfones or
systemic
systemic steroids
steroids/
occassionally
sulfoxane,
corticosteroids
steroids
immunosup-
immunosuppressive
sulfapyridine
•
Retinoids may
pressive drugs
drugs for their
be helpful
steroid sparing
•
Follow
-up
properties
examination
necessary
188
Essentials of Clinical Periodontology and Periodontics
Systemic Treatment
• Systemic corticosteroids in moderate doses.
• Prednisolone can be used in a daily or every-other-
day dose of 30 to 40 mg and gradually-reduced to
a daily maintenance dose of 5 to 10 mg.
DISEASES CLINICALLY PRESENTING AS
DESQUAMATIVE GINGIVITIS
It is listed in Table 22.1.
LICHEN PLANUS
It is a relatively common, chronic dermatosis charac-
terized by the presence of cutaneous violaceous
papules that may coalesce to form plaques. Majority of
the patients with oral lichen planus are middle aged (Fig.
22.3).
Clinical Features
Oral Lesions
The most common are the reticular and erosive subtypes.
The typical reticular lesions are asymptomatic, bilateral
and consist of interlacing white lines on the posterior
region of the buccal mucosa (the lateral border and the
dorsum of tongue, hard palate, alveolar ridge and
gingiva are also affected).
Gingival Lesions
Upto 10 percent of patients with oral lichen planus have
lesions restricted to the gingival tissue, that may occur
in one or more forms.
1. Keratotic lesions: Raised white lesions may present
as groups of individual plaques, linear or reticulate
lesions.
2. Erosive lesions: These extensive erythematous areas
with a patchy distribution may present as focal or
diffuse hemorrhagic areas.
3. Vesicular or bullous lesions: These raised fluid filled
lesions are short lived on gingiva resulting in an
ulceration.
4. Atropic lesions: Atrophy of the gingival tissues results
in erythema confined to gingiva.
Histopathology
Microscopic criteria include hyperkeratosis, basal layer
vacuolization, with apoptic keratinocytes and a lympho-
phagocytic infiltrate at the epithelial-connective tissue
surface. There is an increase in number of Langerhans’
cells. Discrete eosinophilic ovoid bodies representing the
apoptic keratinocytes in the basal zone. Direct immuno-
fluorescence demonstrates the presence of fibrinogen
in the basement membrane.
Differential Diagnosis
• Lichenoid drug reaction •Squamous cell carcinoma
• Lupus erythematosis
•Contact hypersensitivity
• Cheek biting
• Cicatricial pemphigoid
• Candidiasis (Fig. 22.4)
• Leukoplakia (Fig. 22.5)
Treatment and Prognosis
Corticosteroids are the single most effective group of
drugs. Topical application and local injection of steroids
have also been successful. Addition of antifungal
therapy typically enhances clinical results. The erosive,
bullous and ulcerative lesions are treated with high
potency topical steroid such as 0.05 percent flucoci-
Fig. 22.3:
A case of lichen planus
189
Desquamative Gingivitis
namide ointment. Intralesional injection of triamcinolone
acetonide (10 to 20 mg) has also been used succesfully.
Other treatment modalities are retinoids, hydroxy-
chloroquine, cyclosporine and free gingival grafts.
CICATRICIAL PEMPHIGOID
It is characterized by subepidermal blister formation with
subsequent scarring of mucosal surfaces. While the oral
and ocular mucosa are most often involved, other
mucosal surfaces may also be affected.
Clinical Features
It tends to affect women more than men. The oral mucosal
presentation ranges from erosion or desquamation of
attached gingival tissues to large areas of vesiculobullous
eruptions. Bullae are rarely seen because the blisters are
fragile and short lived. Lesions are chronic and may heal
with scarring. Gingival lesions appear as patchy red zones.
Concomitant ulcers may be seen on marginal and attached
gingiva. With chronicity, pain typically diminishes in
intensity. Nikolsky’s sign may also be seen.
Histopathology
It is a subepithelial or sub-basal clefting disorder. There
is no evidence of acantholysis. Lamina propria is
infiltrated by lymphocytes.
Differential Diagnosis
• Pemphigus vulgaris
• Atrophic lichen planus
• Discoid lupus erythematosus
• Contact allergy.
Treatment and Prognosis
Topical or systemic corticosteroids are typically used.
Prednisolone is used for moderate to severe disease
because of its side effects which may outweigh benefits,
highly potent topical steroids such as clobetasol,
betamethasone dipropionate, fluocinoxide are used for
gingival diseases, a custom made flexible mouth guard
may be used. Rinsing with chlorhexidine is often a useful
adjunct. In severe cases immunosuppressive agents
(azathioprine, cyclophosphamide, cyclosporine) may be
occassionally added to the prednisolone regimen. But
it often provides disappointing results, very high dose
may be required to achieve any significant results.
BULLOUS PEMPHIGOID
It is the most common blistering disorder often seen in
elderly individuals usually presents with urticaria-like
lesions.
Clinical Features
It is seen primarily in the elders, with the peak evidence
in the seventh and eighth decades. Lesions character-
Fig. 22.4:
A case of candidiasis
Fig. 22.5:
A case of leukoplakia

190
Essentials of Clinical Periodontology and Periodontics
istically appear in skin, although concomitant vesiculo-
bullous lesions may occur. Pruritus may be associated with
skin lesions. Bullae and erosions may be noted. Other areas
include soft palate, mucosa and floor of the mouth.
Histopathology
Bullae are subepithelial in bullous pemphigoid and
appear similar to those in cicatricial pemphigoid.
Ultrastructurally the basement membrane is cleaved at
the level of lamina lucida.
Treatment
Systemic corticosteroids are generally used to control
this disease. Non-steroidal immunosuppressive agents
may also affect control of the disease process as well
as reduce the side effects. Antibiotics (tetracycline and
erythromycin) and niacinamide have provided some
clinical success.
LINEAR IgA DISEASE
It is an uncommon mucocutaneous disorder with pre-
dilection in women. Clinically it represents as a pruritic
vesiculobullous rash, characteristic plaque or crops with
an annular presentation surrounded by a peripheral rim
of blisters.
Oral Lesions
It consists of vesicles, painful ulcerations or erosions. The
hard and soft palates are commonly affected. Rarely oral
lesions may be the only manifestation before the
presentation of cutaneous lesions.
Histopathology
Separation at the basement membrane is seen.
Differential Diagnosis
• Erosive lichen planus
• Chronic ulcerative stomatitis
• Pemphigus vulgaris
• Bullous pemphigoid
• Lupus erythematosus.
Treatment
The primary treatment comprises combination of sulfone
and dapsones. Small amounts of prednisone (10 to 30
mg) can be added. Alternatively tetracycline combined
with nicotinamide is used.
DERMATITIS HERPETIFORMIS
It is a skin eruption. The cause is unknown, but most
patients have an associated gluten-sensitive enteropathy.
Clinical Features
It is a chronic disease typically seen in young and middle
aged adults. Periods of exacerbation and remission
characterize the disease. Lesions are usually symmetrical
in their distribution, aggregated but are often individually
disposed. In the oral cavity vesicles and bullae are
evanescent. Subsequent to rupture, superficial non-
specific ulcers have a fibrinous base with erythematous
margins. Lesions may involve both keratinized and non-
keratinized mucosae.
Histopathology
Collections of neutrophils, eosinophils and fibrin are seen
at the papillary tips of dermis. Subsequent exudation
at this location contributes to epidermal separation. A
lymphophagocytic infiltrate is seen in perivascular spaces.
Treatment
It is generally treated with dapsone, sulfoxone and
sulfapyridine. Because patients often have an associated
enteropathy, a gluten-free diet may also be a part of
the therapeutic regimen.
PEMPHIGUS VULGARIS
It is a mucocutaneous disease characterized by intra-
epithelial blister formation (Fig. 22.6).
Clinical Features
Skin lesions present as ulcers preceded by bullae.
Presentation of the lesions may initially be as fluid-filled

191
Desquamative Gingivitis
bullae. Bullae rapidly rupture leaving a collapsed roof.
This grayish membrane is easily removed with a guaze
sponge, leaving a red, painful ulcerated base, Nikolsky’s
sign is seen. The incidence is equal in both sexes.
Histopathology
It represents the prototypical suprabasal or intraepithelial
clefting morphology. It is the acantholytic lesion that
features squamous epithelial cells lying free within the
bulla or vesicle cavity. Tzanck cells are seen, characterized
by nuclear enlargement and hyperchromatosis
subsequent to formation of the suprabasal cleft, the intact
basal layer remains attached to lamina propria.
Differential Diagnosis
• Bullous and cicatricial pemphigoid
• Erythema multiforme
• Bullous lichen planus
• Dermatitis herpetiformis.
Treatment
Disease control may be achieved with intermediate dose
of steroid. For more severely affected patients, high-dose
of corticosteroid is used followed by a combined drug
approach that includes alternate day prednisolone plus
immunosuppressive agent such as azathioprine,
methotrexate or cyclophosphamide.
DRUG REACTION OR ERUPTIONS
Drugs can act as an allergen either alone or in combina-
tion, sensitizing the tissues and then resulting in allergic
reaction of skin and oral cavity. Stomatitis medicamentosa
is characterized by eruptions in the oral cavity resulting
from sensitivity to drugs that have been taken by mouth
or parenterally. The local use of medicaments in the
mouth is referred to as stomatitis venenata or contact
stomatitis, examples are, aspirin burn or stomatitis due
to topical penicillin. Clinically, drug eruption in the oral
cavity could be:
• Vesicular or bullous—most commonly seen
• Pigmented or nonpigmented macular lesions
Erosions, followed by deep ulceration with purpuric
lesions, may also occur in different parts of oral cavity
with gingiva most commonly affected. Some of the
compounds that may cause contact allergy in the gingiva
are, mercurial compounds, examples are, amalgam,
tartar control tooth pastes, example pyrophosphatas,
cinnamon compounds can result in intense erythema
of gingival tissue (plasma cell gingivitis). Elimination of
the offending agent usually leads to resolution of the
lesions with in a week.
KEY POINTS TO NOTE
1. Chronic desquamative gingivitis is characterized by intense
redness and desquamation of the surface epithelium of
marginal and attached gingiva.
2. Chronic desquamative gingivitis is now believed to be the
oral manifestation of dermatosis like lichen planus, mucous
membrane pemphigoid, bullous pemphigoid or pemphigus,
drug reaction and pemphigus vulgaris.
REVIEW QUESTION
1. Classify desquamative lesions. Describe the types,
histopathology and treatment of lichen planus.
BIBLIOGRAPHY
1. Newman, Fermin Carranza. Clinical Periodontology. Ninth
edition (2002), WB Saunders Co.
2. Robin A. Seymour, Peter A. Heasman, Ian DM Macgregs.
Drugs, Diseases and the Periodontium, Oxford University
Press.
Fig. 22.6:
A case of pemphigus
192
Essentials of Clinical Periodontology and Periodontics
INTRODUCTION
A sulcus depth up to 3 mm is considered to be normal,
provided the patient can maintain oral hygiene. If it is
increased beyond 3 mm it is called as a pocket. The
cause for this is mainly extension of inflammation leading
to pathologic deepening of the gingival sulcus, and, this
marks the transition from gingivitis to periodontitis.
Periodontitis is always preceded by gingivitis, but not all
gingivitis progresses to periodontitis.
DEFINITION
“Pocket can be defined as deepening of the gingival
sulcus.” If this happens due to coronal migration of the
marginal gingiva it is called as gingival or pseudo-pocket.
Deepening due to apical migration of the junctional
epithelium is referred to as “true pocket.”
CLASSIFICATION OF POCKETS (Fig. 23.1)
1. Depending upon its morphology
a. Gingival/false/relative pocket.
b. Periodontal/absolute/true pocket.
c. Combined pocket.
2. Depending upon its relationship to crestal bone
periodontal pockets are further classified as:
a. Suprabony/supracrestal/supra-alveolar pocket.
b. Infrabony/intrabony/subcrestal/intra-alveolar
pocket.
3. Depending upon the number of surfaces involved:
a. Simple pocket—involving one tooth surface.
b. Compound pocket—involving two or more tooth
surfaces.
Section 2: PERIODONTAL DISEASES
❒
❒
❒
❒
❒ DEFINITION AND CLASSIFICATION
❒
❒
❒
❒
❒ CLINICAL FEATURES
• Signs and Symptoms
❒
❒
❒
❒
❒ PATHOGENESIS OF POCKET
FORMATION
❒
❒
❒
❒
❒ HISTOPATHOLOGY
• Changes in the Soft Tissue Wall of the
• Microtopography of the Gingival Wall
of the Pocket
• Periodontal Pocket as Healing Lesions
• Pocket Contents
• Changes in Root Surface Wall
• Periodontal Disease Activity
❒
❒
❒
❒
❒ RELATION OF LOSS OF ATTACHMENT
AND BONE LOSS TO POCKET DEPTH
❒
❒
❒
❒
❒ DIFFERENCES BETWEEN SUPRABONY
AND INFRABONY POCKETS
❒
❒
❒
❒
❒ PERIODONTAL CYST
❒
❒
❒
❒
❒ DETERMINATION OF POCKET DEPTH
❒
❒
❒
❒
❒ TREATMENT OF PERIODONTAL POCKETS
23
The Periodontal Pocket
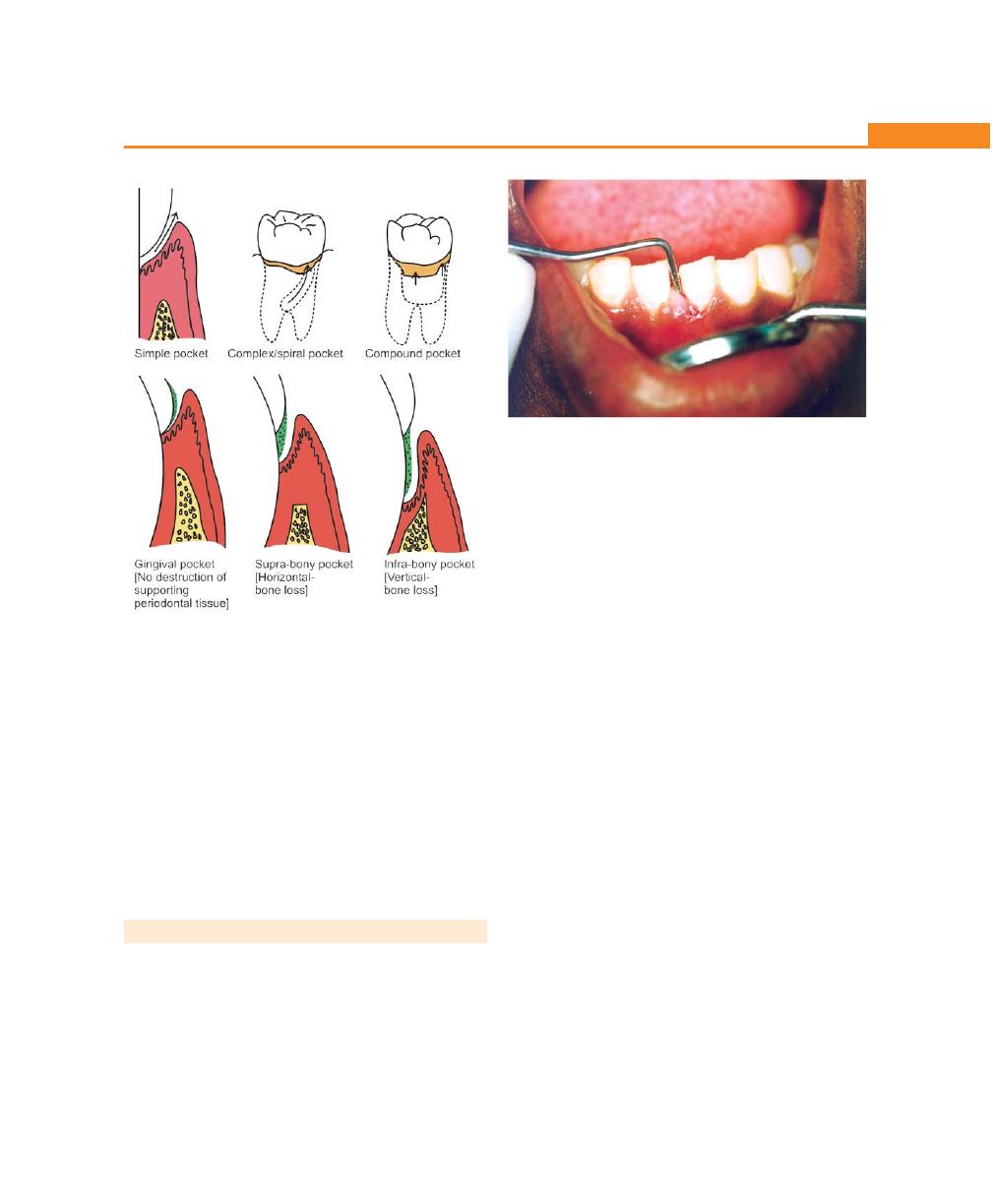
193
The Periodontal Pocket
c. Complex pocket—where the base of the pocket
is not in direct communication with the gingival
margin. It is also known as spiral pocket.
4. Depending upon the nature of the soft tissue wall
of the pocket
a. Edematous pocket.
b. Fibrotic pocket.
5. Depending upon the disease activity
a. Active pocket.
b. Inactive pocket.
CLINICAL FEATURES
Clinical Signs
1. Enlarged, bluish-red marginal gingiva with a ‘rolled’
edge separated from the tooth surface.
2. A bluish-red vertical zone extending from the gingival
margin to the alveolar mucosa.
3. A break in the faciolingual continuity of the interdental
gingiva.
4. Shiny, discolored and puffy gingiva associated with
exposed root surfaces.
5. Gingival bleeding, purulent exudate from the gingival
margin.
6. Mobility, extrusion and migration of teeth.
7. The development of diastema where none had existed
previously.
Symptoms
a. Localized pain or a sensation of pressure in the gingiva
after eating, which gradually diminishes.
b. A foul taste in localized areas.
c. A tendency to suck material from the interproximal
spaces.
d. Radiating pain “deep in the bone.”
e. A “gnawing’ feeling or feeling of itching in the
gums.
f. The urge to dig a pointed instrument into the
gums and relief is obtained from the resultant
bleeding.
g. Patient complains that food “sticks between the teeth”
or that the teeth “feel loose” or a preference to “eat
on the other side.”
h. Sensitivity to heat and cold; toothache in the absence
of caries (Fig. 23.2).
Fig. 23.2:
Probing of a pocket
Fig. 23.1:
Types of pockets
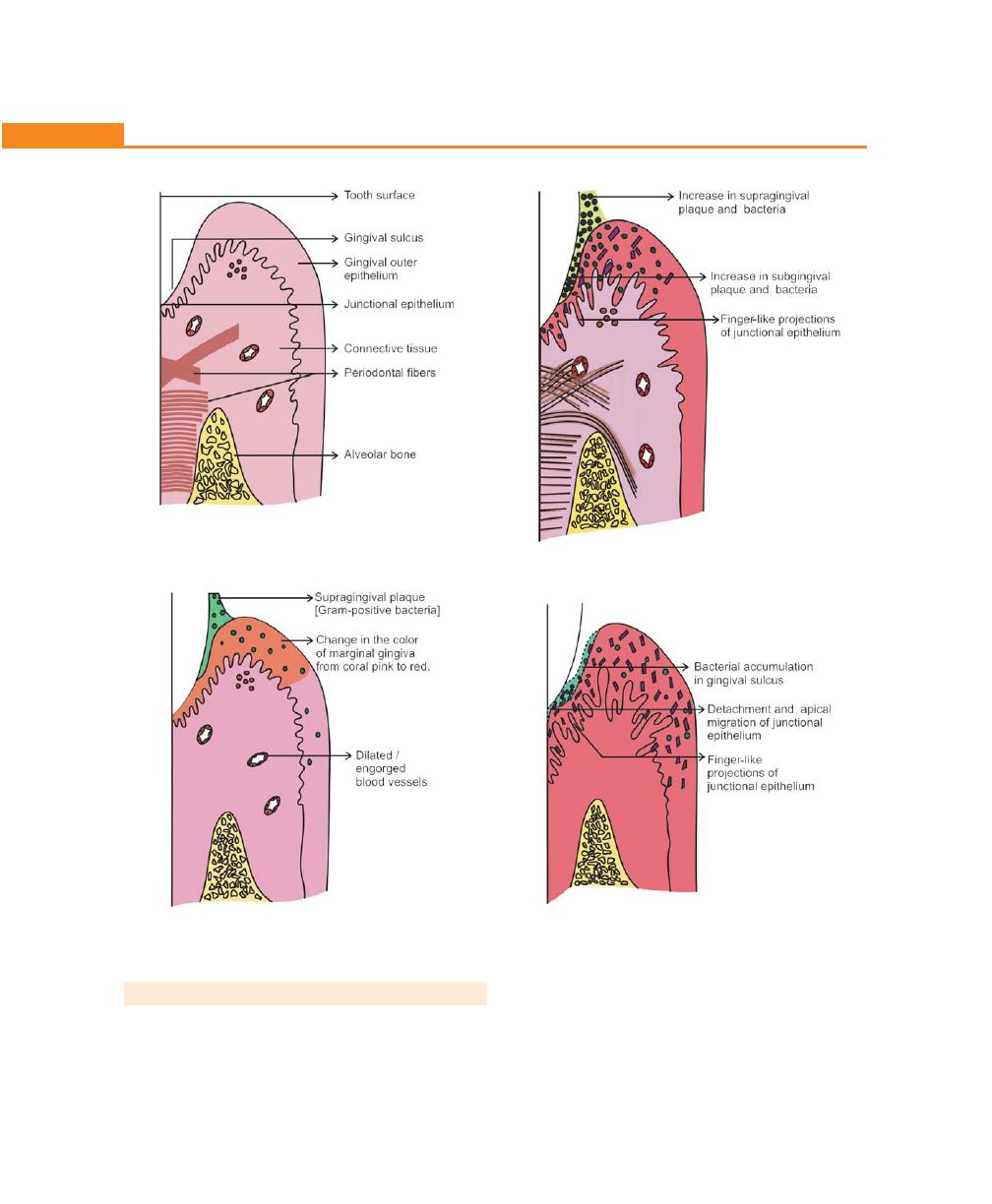
194
Essentials of Clinical Periodontology and Periodontics
Fig. 23.3A:
Schematic illustration of normal gingiva
Fig. 23.3B:
Accumulation of supragingival plaque
Fig. 23.3C:
Extension of supragingival plaque into the
gingival sulcus
Fig. 23.3D:
Detachment and apical migration of junctional
epithelium
PATHOGENESIS (Figs 23.3A to G)
The first event in pocket formation is the laying down
of a lawn of gram-positive bacteria on the supragingival
tooth surface and its extension into the gingival sulcus.
The periodontal pockets are caused by micro-
organisms and their products, which produce pathologic
changes that lead to the deepening of the gingival
sulcus.
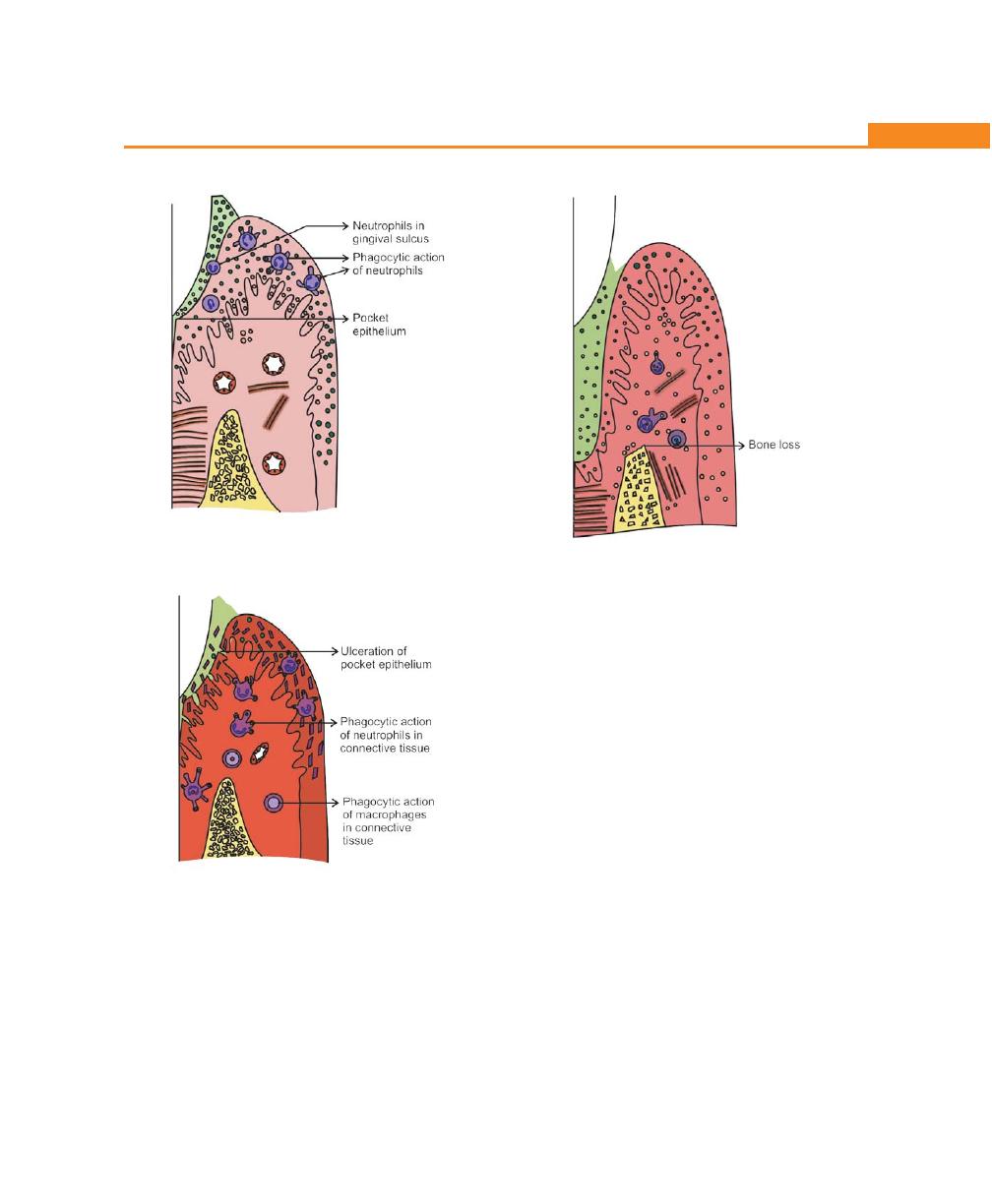
195
The Periodontal Pocket
Fig. 23.3E:
Phagocytic action of neutrophils
Fig. 23.3F:
Ulceration of pocket epithelium
Fig. 23.3G:
Periodontal pocket is established
As a result of inflammation, the following changes
are seen in the junctional epithelium.
1. The junctional epithelium proliferates along the root
in the form of finger-like projections.
2. The coronal portion of the junctional epithelium
detaches from the root as the apical portion migrates.
The epithelial cell detachment can take place due to:
a. Enzymes released by bacteria
b. Physical force exerted by rapidly growing bacteria
c. Bacteria may also interfere with growth and
synthetic activities of junctional epithelial cells and
also with the normal maintenance of the
attachment lamina.
d. Exudate associated with the advancing bacteria
may also be important.
Thus the sulcus base shifts apically and this will be
replaced by pocket epithelium.
3. Under normal conditions a constant stream of
neutrophils emigrate from the vessels of the gingival
plexus through the junctional epithelium into the
gingival sulcus and oral cavity. Most bacteria produce
substances that chemotactically attract neutrophils.
A chemical gradient of chemotactic agents seems to
exist across normal, intact junctional epithelium and
connective tissue. Neutrophils leaving the blood
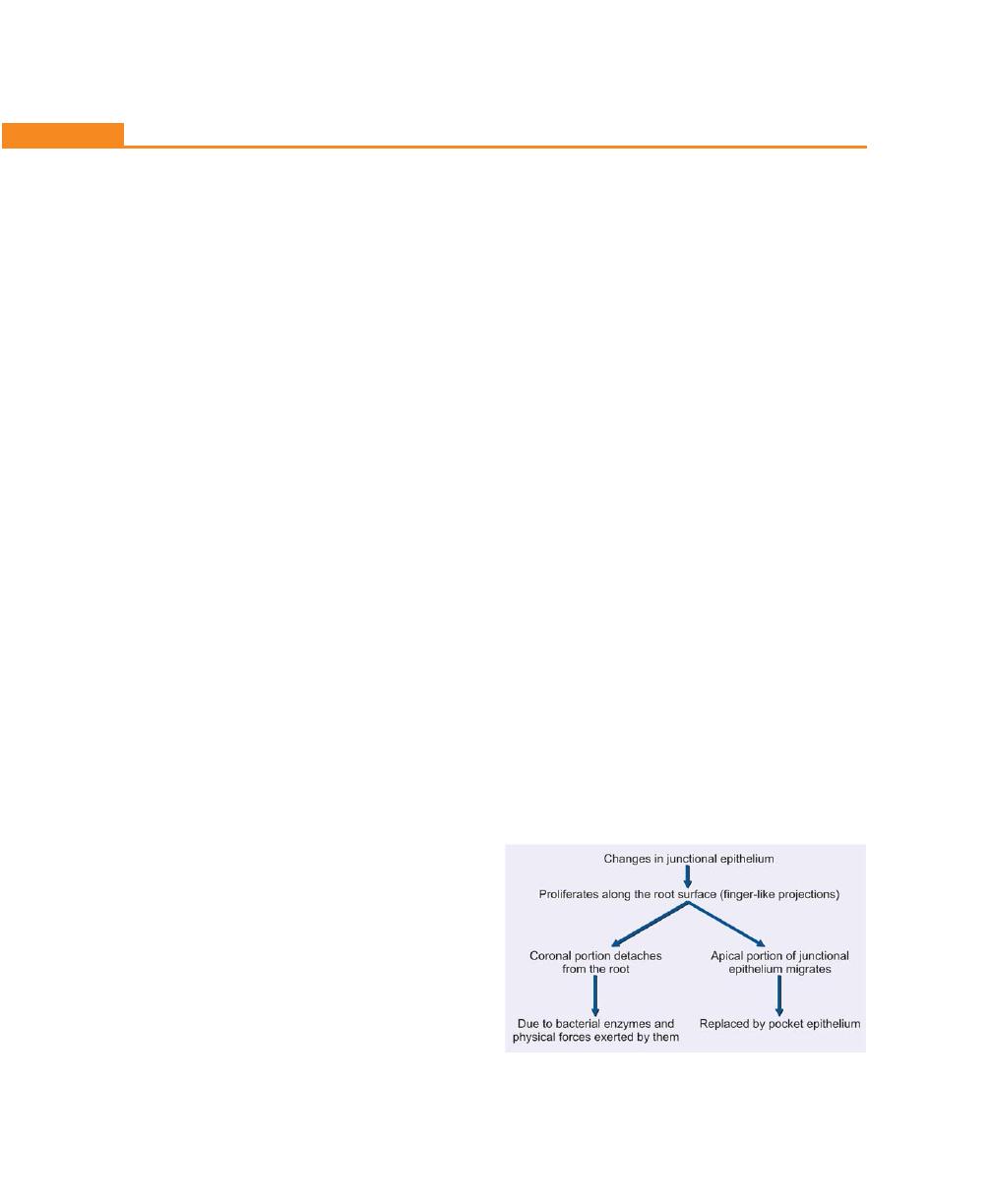
196
Essentials of Clinical Periodontology and Periodontics
vessels are guided by this gradient towards the gingival
margin and into the gingival sulcus. Under normal
conditions, the transmigrating cells leave no trace of
their passage and cause no damage. These
neutrophils are the primary and first line of defence
around the teeth; the epithelial barrier is the
second.
4. Extension of plaque subgingivally causes an increase
in the number of transmigrating neutrophils, which
may be due to the increased concentration of
chemotactic factors and other inflammation induced
substances derived from the bacteria. These
substances cause vasculitis. Neutrophils adhere to the
endothelial lining and migrate into the connective
tissue, but they still do not accumulate there. Instead
they rapidly pass through the junctional or pocket
epithelium to form a thick layer that covers the surface
of the subgingival plaque. Upon arrival at the plaque
surface, the neutrophils are viable partly, but not
completely functional. Their role is to limit further
extension and spread of bacteria by phagocytosis and
killing. This may be inhibited by lipopolysaccharides
and leukotoxin. A constant battle occurs at the
neutrophil plaque interface. Usually the normal
neutrophil activities are sufficient to limit the extension
of plaque, however increasing growth rate of bacteria,
swamps the neutrophil system and permits tissue
destruction to occur.
5. Assuming the pocket formation proceeds either
because of aggressive growth and action of bacteria
or because of ever increasing number of neutrophils
that transmigrate in constant streams through the
junctional epithelium and pocket epithelium causes
open communication between the pocket and
connective tissue (by disrupting the epithelial
barrier.)
Ulceration of this sort is the second major event
in pocket formation. Once the epithelial barrier is
breached, the gradient of chemotactic agents released
by the pocket bacteria may be disrupted. As a result
the neutrophils no longer have a guidance systems,
to direct them from the vessels through tissues and
into the pocket; they remain in the connective tissue
moving randomly. Also because of the rupture of
the epithelial barrier, the connective tissue become
flooded with the bacterial substances, and bacteria
may enter the connective tissue.
6. The neutrophils now encounter these substances
within the connective tissue rather than outside in
the pocket. The neutrophils become activated and
undertake phagocytosis with resultant release of
lysosomal enzymes, collagenases and other substances
(PGE
2
) that cause extensive tissue damage.
As soon as the bacterial substances have entered
the connective tissue many systems other than the
neutrophils are activated like macrophages
lymphocytes and complement system.
7. When the epithelial barrier is re-established the
chemotactic gradient is formed again and the
destructive process subsides. If this barrier is not re-
established, tissue destruction continues and alveolar
bone is resorbed. A periodontal pocket is now
established.
Summary of Pathogenesis
First Event
Colonization of Gram-positive bacteria supragingivally
and its extension into the gingival sulcus and conversion
of Gram-positive aerobes to Gram-negative anaerobes.
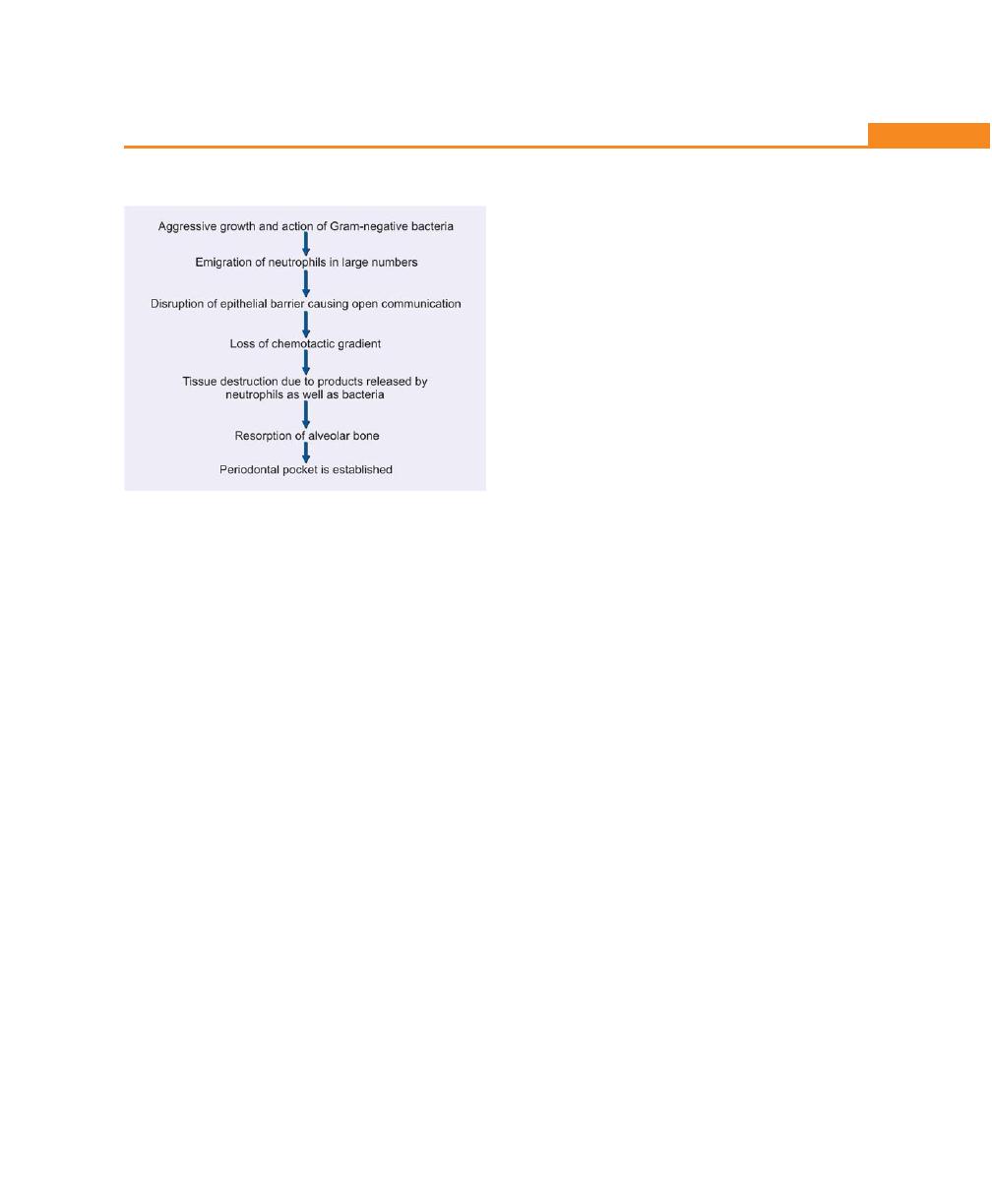
197
The Periodontal Pocket
Second Event
Histopathology
During the initial stages of pocket formation the changes
that takes place are described in stages I, II, III gingivitis.
Once the pocket is formed the following microscopic
features are present.
Changes in the Soft Tissue Wall
• The blood vessels are engorged and dilated.
• The connective tissue is edematous and densely
infiltrated with plasma cells (80%), lymphocytes
and a scattering of polymorphonuclear leukocytes
(PMNLs).
• Special note should be made of the fact that
extension of the junctional epithelium along the
root requires the presence of healthy epithelial
cells, because of this it is reasonable to assume
that the degenerative changes occur along the
lateral walls of the pocket.
The changes are as follows:
1. The epithelium along the lateral wall of the pocket
presents striking proliferative and degenerative
changes.
2. The epithelial projection extends deep into the
connective tissue and also extends further apically
than the junctional epithelium.
3. The epithelium is infiltrated with leukocytes and other
inflammatory cells.
4. Degeneration and necrosis of the epithelium leading
to ulceration of the epithelium and exposure of the
underlying connective tissue.
5. Bacterial invasion along the lateral and apical areas
of the pocket. Some bacteria traverse the basement
lamina and invade the subepithelial connective tissue.
The following morphotypes of bacteria have been
identified, filamentous rods and coccoid organisms with
predominant Gram-negative bacteria. Increased num-
bers of Langerhans’ cells were found in the epithelium.
Microtopography of the Gingival Wall of the Pocket
Under scanning electron microscope the following areas
have been noted:
1. Areas of relative quiescence.
2. Areas of bacterial accumulation—mainly cocci, rods,
filamentous rods with a few spirochetes.
3. Area of emergence of leukocytes.
4. Area of leukocyte—bacterial interaction.
5. Areas of intense epithelial desquamation.
6. Areas of ulceration with exposed connective tissue.
7. Areas of hemorrhage with numerous erythrocytes.
The transition could be postulated as follows:
Bacteria accumulate in the previously quiescent
areas, triggering the emergence of leukocytes and the
leukocyte-bacterial interaction. This leads to intense
epithelial desquamation and finally to ulceration and
hemorrhage.
Periodontal Pocket as a Healing Lesion
It is characterized by interplay of destructive and
constructive tissue changes.
The destructive changes are characterized by fluid
and cellular inflammatory exudates and by the associated
degenerative changes initiated by the plaque bacteria.
The constructive changes consist of the formation
of blood vessels in an effort to repair the tissue damage
caused by inflammation. The balance between
destructive and constructive changes determines clinical
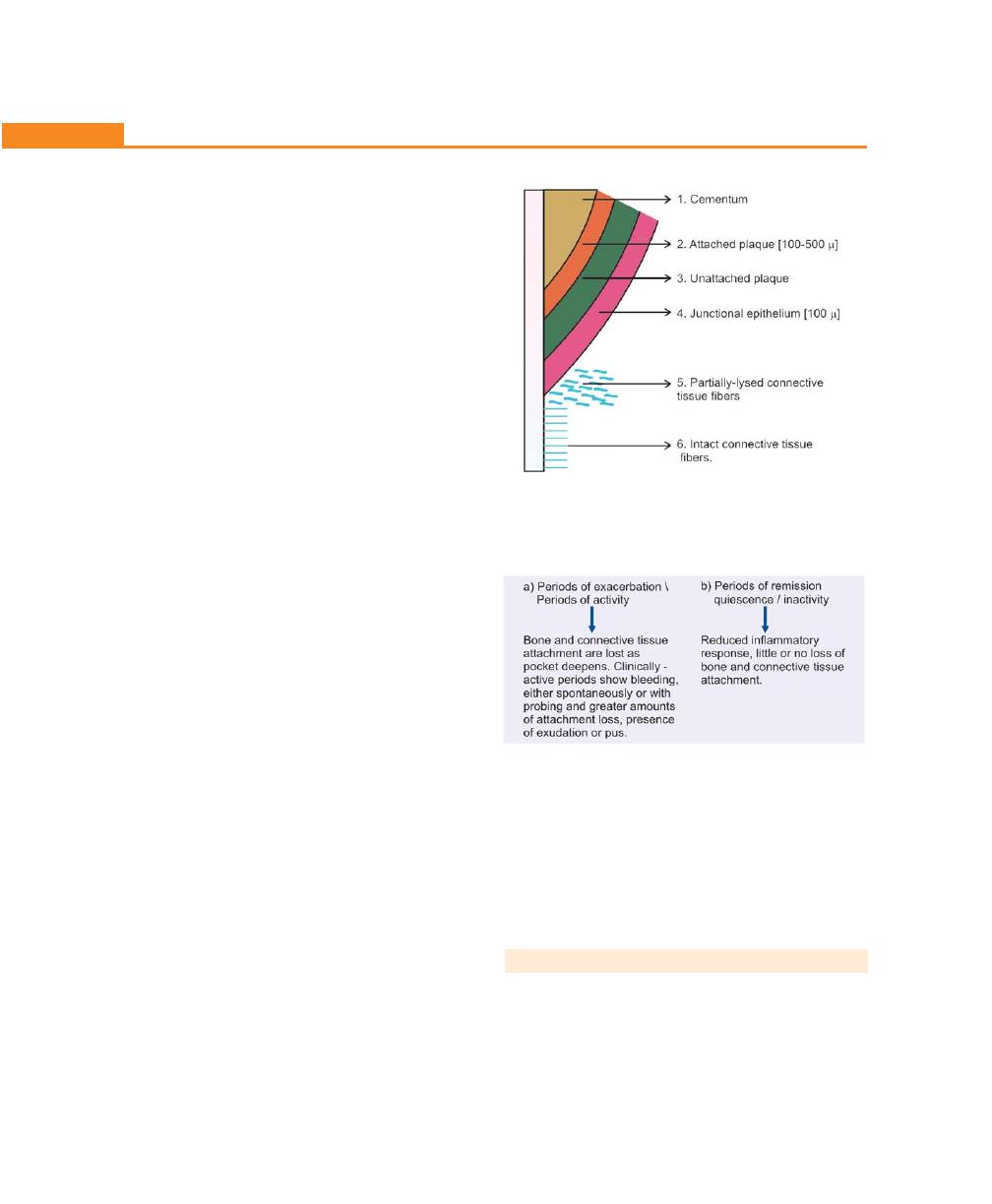
198
Essentials of Clinical Periodontology and Periodontics
features such as color, consistency and surface texture
of the pocket wall.
Pocket Contents
It consists of debris principally containing micro-organisms
and their products, (like enzymes, endotoxins and other
metabolic products) dental plaque, gingival fluid, food
remnants, salivary mucin, desquamated epithelial cells
and leukocytes. If purulent exudate is present, it consists
of living, degenerated and necrotic leukocytes (PMNL’s),
living and dead bacteria, serum and a scanty amount
of fibrin. Pus formation is a common feature in
periodontal disease but it is only a secondary sign.
Changes in the Root Surface Wall
1. Structural changes:
a. Presence of pathologic granules
b. Areas of increased mineralization
c. Areas of demineralization/root caries
2. Chemical changes: The mineral content of exposed
cementum is increased. The following minerals are
increased in diseased root surfaces: calcium,
magnesium, phosphate, fluoride and others. Hence
a highly increased resistant calcified layer to decay
is formed. This can also be harmful if the adsorbed
products are toxic.
3. Cytotoxic changes: Bacterial penetration into the
cementum can be found as deep as cemento-dentinal
junction. In addition, bacterial products such as
endotoxins have also been detected.
The following zones can be found in the base of a
periodontal pocket (Fig. 23.4):
1. Cementum covered by calculus where all the changes
described earlier takes place.
2. Attached plaque which covers calculus (100 to 500 μ)
3. The zone of unattached plaque.
4. The zone where the junctional epithelium is attached
to the tooth. This zone, which is normally more than
500 µ is usually reduced to less than 100 µ in
periodontal pocket.
5. Apical to the junctional epithelium there may be a
zone of semidestroyed connective tissue fibers.
Periodontal Disease Activity
Relation of Loss of Attachment and Bone
Loss to Pocket Depth (Fig. 23.5)
Pocket of same depth may be associated with different
degree of attachment loss. Pocket of different depth may
be associated with same amount of attachment loss.
Area between the base of the pocket and the alveolar
bone is always constant. The radius of action of the plaque
bacteria is 0.5 mm to 2.7 mm.
PERIODONTAL CYST
• It is an uncommon lesion that produces localized
destruction of the periodontal tissues along the lateral
root surface.
• Most common in the mandibular canine—premolar
area.
Fig. 23.4:
Zones in the base of the periodontal pocket
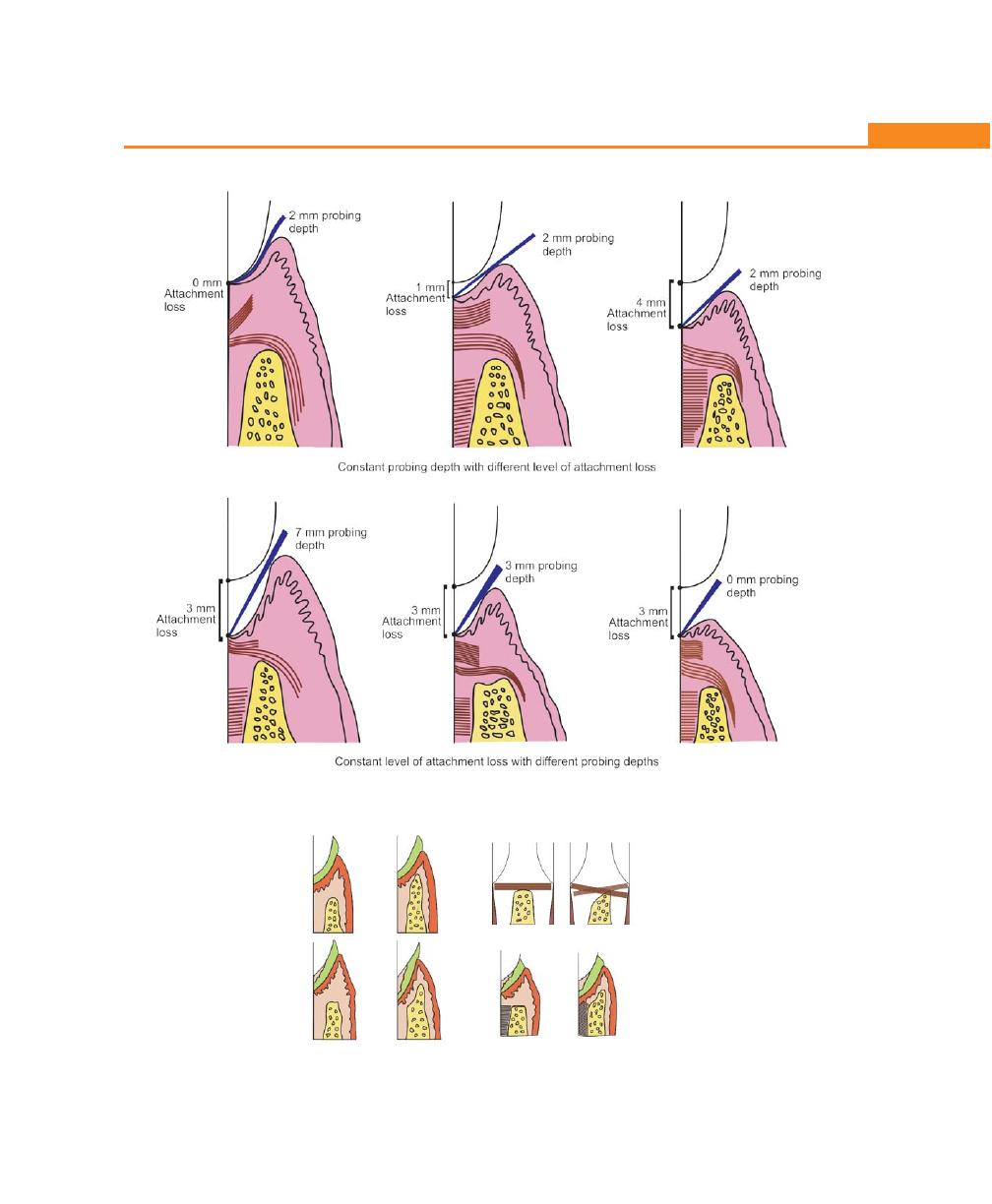
199
The Periodontal Pocket
Fig. 23.5:
Relation of loss of attachment
Fig. 23.6:
Differences between suprabony and infrabony
pockets
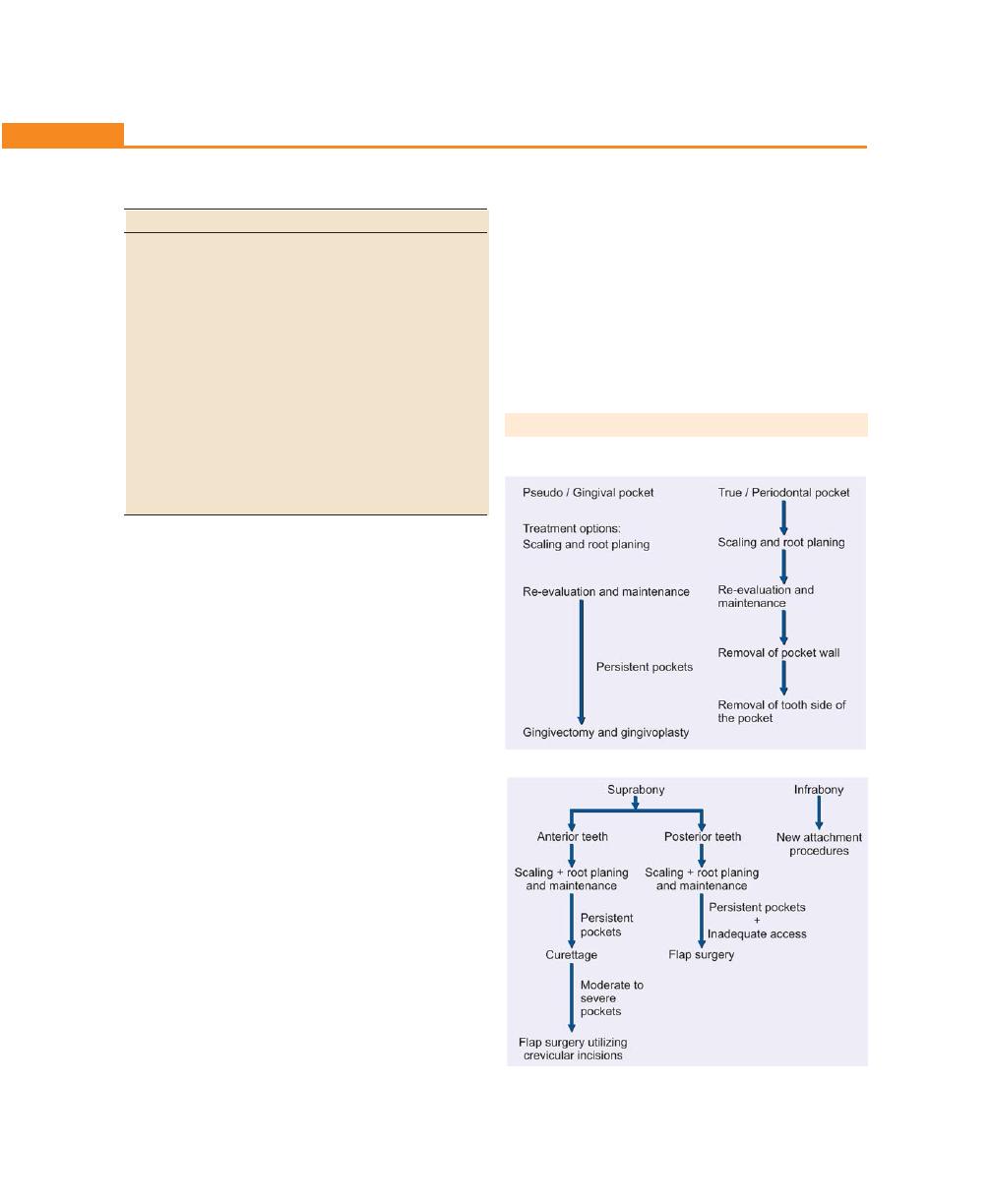
200
Essentials of Clinical Periodontology and Periodontics
The following possible etiologies have been
suggested:
• Odontogenic cyst caused by proliferation of the
epithelial rests of Malassez.
• Lateral dentigerous cyst (retained in the jaw after
tooth eruption).
• Primordial cyst of supernumerary tooth germ.
• Stimulation of epithelial rests of the periodontal
ligament by infection from a periodontal abscess or
from the pulp through an accessory canal.
Usually asymptomatic but may present as a localized
tender swelling. Radiographically seen at the site of the
root as a radiolucent area bordered by a radiopaque
line (can not be differentiated radiographically from
periodontal abscess).
Determination of Pocket Depth
• Probing depth measurement.
• Clinical detection of attachment loss.
• Clinical detection of suprabony and infrabony
pockets.
Clinically, probing depth measurement is recorded
from the crest of the marginal gingiva to the probable depth
of the pocket. Whereas attachment level is measured from
cementoenamel junction to the probable depth of the
pocket. To differentiate between pseudo and true pocket,
attachment level measurement should be considered.
Clinical detection of attachment level is done by first
identifying the cemento-enamel junction (CEJ) with the
probe tip (since junctional epithelium is attached at the
CEJ), if the probe passes beyond the point of CEJ then
it is considered to be a true pocket.
While probing, soft tissue resistance on lateral pressure
indicates suprabony pocket and hard tissue resistance
indicates infrabony pocket.
TREATMENT OF PERIODONTAL POCKET
I. Treatment of pocket depends on the type of pocket
II. Treatment of suprabony and infrabony pockets
Table 23.1: Differences between suprabony and infrabony
pocket (Fig. 23.6)
Suprabony pockets
Infrabony pockets
a. The base of the pocket
a. The base of the pocket is
is coronal to the crest
apical to the crest of the
of alveolar bone.
alveolar bone.
b. The pattern of bone
b. The pattern of bone des-
destruction is horizontal
truction is vertical/angular
c. Interproximally, the
c. Interproximally, the tran-
transeptal fibers are
septal fibers are arranged
arranged horizontally.
in an oblique pattern, ex-
d. On the facial and lingual
tending from cementum
surfaces, the periodontal
below the pocket over to
ligament fibers follow
the cementum of the
normal horizontal-oblique
adjacent tooth.
course between the tooth
d. On facial and lingual sur-
and bone.
faces the periodontal
ligament fibers follow the
angular pattern of the
adjacent bone.
201
The Periodontal Pocket
III. Treatment of pockets can also be classified under three
main headings.
a. New attachment techniques: It offers ideal result by
reuniting the gingiva to the tooth at a position coronal
to the base of pre-existing pocket. Here all the
structures of lost periodontium are restored. Following
are the techniques for new attachment,
a. Non-graft associated new attachment procedures.
b. Graft associated new attachment procedures.
c. Combined techniques.
b. Removal of pocket wall by,
1. Retraction or shrinkage, e.g. scaling and root
planing.
2. Surgical removal by gingivectomy or by means
of an undisplaced flap.
3. Apical displacement of pocket wall by apically
displaced flap.
c. Removal of the tooth side of the pocket, by tooth
extraction or partial tooth extraction such as
hemisection or root resection.
KEY POINTS TO NOTE
1. Pockets are defined as the pathologic deepening of the
gingival sulcus.
2. They can be pseudo or true pockets; simple, compound or
complex pockets; suprabony or infrabony pockets.
3. Microscopically, in a periodontal pocket certain changes are
observed in both the soft tissue wall and the root surface.
4. Clinically, true pocket can be identified by recording the
attachment level (which is measured from the cemento-
enamel junction to the probable depth of the pocket).
5. Pockets can be treated by a) new attachment techniques, b)
removal of the pocket wall, c) removal of the tooth side of
the pocket.
6. Only infrabony pockets can be treated successfully by various
regenerative procedures.
REVIEW QUESTIONS
1. Define, classify periodontal pockets, and describe the
pathogenesis and treatment of a periodontal pocket.
2. Describe the various pocket elimination procedures.
3. Describe the histopathology of a periodontal pocket.
4. What are the root surface changes associated with
a periodontal pocket?
5. Differentiate between suprabony and infrabony
pockets.
BIBLIOGRAPHY
1. Bonakdar MPS, Barber PM, Newman HN. The vasculature
in chronic adult periodontitis: A qualitative and quantitative
study. J. Periodontol 1997; 68:50.
2. Boshardt DP, Selvig KA. Dental cementum: the dynamic tissue
covering the root. Periodontol 2000, 1997; 13: 41.
3. Krayer JW, Rees TD. Histologic observation on the topography
of human periodontal pocket viewed in transverse step serial
sections. J.Periodontol 1993; 64: 585.
4. Newman, Takei; Carranza. Clinical periodontology, ninth
edition W.B. Saunders.
5. Takada T, Donath K. The mechanism of pocket formation. A
light microscopic study of undecalcified human transverse
step serial sections. J. Periodontol; 1988; 59: 215.
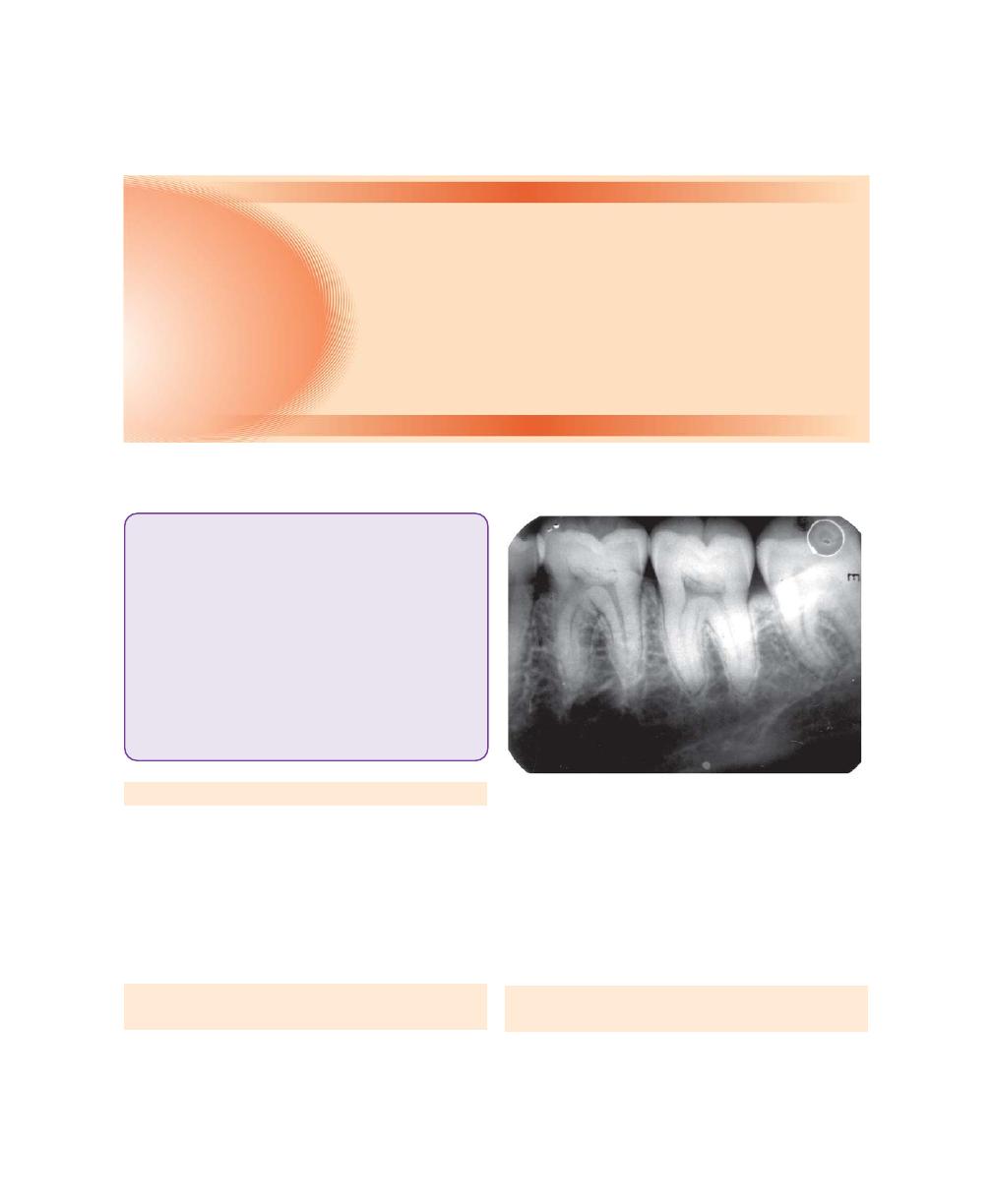
202
Essentials of Clinical Periodontology and Periodontics
INTRODUCTION
Osseous defects are those defects, which are formed
as a result of destruction of alveolar bone due to perio-
dontal disease. The normal height of alveolar bone is
at cemento-enamel junction and this height is maintained
by physiologic equilibrium between bone formation by
osteoblasts and bone loss by osteoclasts, which in turn
are regulated by local and systemic influences.
NORMAL ANATOMY OF ALVEOLAR BONE
(Fig. 24.1)
Alveolar bone is that part of jaw bone that surrounds
and supports the teeth. It has facial and lingual cortical
plate or compact bone between which cancellous bone
is sandwiched.
It is partially tooth-dependent and hence it is resorbed
once the tooth is extracted. The shape, size, thickness
varies in different regions of the same mouth. The margins
of the alveolar crest run parallel to the cementoenamel
junction at a remarkably constant distance of 1 to 2 mm.
MECHANISM OF BONE FORMATION AND
BONE DESTRUCTION
Osteoblasts are the primary cells responsible for the
synthesis of the bone matrix, which subsequently
Fig. 24.1:
Normal anatomy of alveolar bone
❒
❒
❒
❒
❒ NORMAL ANATOMY OF ALVEOLAR BONE
❒
❒
❒
❒
❒ MECHANISM OF BONE FORMATION
AND BONE DESTRUCTION
• Local Factors
• Systemic Factors
❒
❒
❒
❒
❒ FACTORS DETERMINING BONE
MORPHOLOGY IN PERIODONTAL
DISEASE
❒
❒
❒
❒
❒ BONE DESTRUCTION PATTERNS IN
PERIODONTAL DISEASE
❒
❒
❒
❒
❒ PREVALENCE AND DISTRIBUTION OF
BONE DEFECTS IN MODERATE ADULT
PERIODONTITIS
24
Bone Loss and Patterns
of Bone Destruction

203
Bone Loss and Patterns of Bone Destruction
undergoes calcification. Initially, uncalcified matrix, called
osteoid, is formed and this is mineralized as a result of
deposition of crystals of hydroxyapatite.
Bone destruction in periodontal disease is caused by
local factors and systemic factors.
Bone destruction in periodontal disease is not a
process of bone necrosis. It involves the activity of the
live cells along the viable bone. Tissue necrosis and pus
if present are seen in the soft tissue walls of the
periodontal pocket but not along the resorbing margins
of the underlying bone.
Local Factors
Local factors could be:
a. Chronic gingival inflammation
b. Trauma from occlusion
c. Combination of both
Role of Chronic Gingival Inflammation
It is the most common cause for bone destruction in
periodontal disease. It is believed that inflammation
spreads from the gingiva into the deeper tissues along
two pathways (This marks the transition from gingivitis
to periodontitis).
The transition from gingivitis to periodontitis is asso-
ciated with changes in the composition of bacterial plaque
or resistance of the host. The lesion presents with most
pathogenic bacteria, inflammatory cell infiltrate, the lesion
becoming more progressive and destructive with the
conversion of T-lymphocyte to B-lymphocytic lesion.
Pathway of spread of inflammation could be,
Interproximally:
a. From the gingiva
↓
Bone
↓
Periodontal ligament
b. From the gingiva
↓ (Less common; seen in trauma from occlusion)
Periodontal ligament
Facially and lingually:
a. From the gingiva along the outer periosteum
↓
Into the bone
b. From the gingiva
↓
Into the periodontal ligament
When the inflammation reaches the bone by
extension from the gingiva, it spreads into marrow spaces
and it replaces it with a leukocytic and fluid exudate,
new blood vessels and proliferating fibroblasts.
Multinuclear osteoclasts and mononuclear phagocytes
are increased in number and bone surfaces are lined
with cone-like resorption lacunae. In the marrow spaces,
resorption proceeds within, causing first thinning of
the surrounding bony trabeculae and enlargement of
marrow spaces, followed by destruction of bone and
reduction in bone height. Around the resorption areas,
normally fatty bone marrow is partially or totally replaced
by a fibrous type of marrow.
In summary, the changes in the bone could be as follows:
Gingival inflammation
↓
Marrow spaces
↓
Replaced by leukocytes and fluid exudates,
new blood vessels and proliferating fibroblasts
↓
Increase in osteoclasts and mononuclear cells
↓
Thinning of bone trabeculae and enlargement
of the marrow spaces
↓
Destruction of the bone and reduction in bone height
↓
Replacement of fatty bone marrow with the fibrous
type (around the resorption areas)
The following are the possible pathways by which
bone destruction is caused by extension of gingival
inflammation (by Hausmann):
1. Direct action of plaque products on bone progenitor
cells to release osteoclasts.

204
Essentials of Clinical Periodontology and Periodontics
2. Plaque products act directly on bone, destroying it
through a non-cellular mechanism.
3. Plaque products stimulate gingival cells, to release
mediators which inturn induces progenitor cells to
differentiate into osteoclasts.
4. Stimulates gingival cells to release agents that destroy
bone by direct chemical action without osteoclasts.
5. Plaque products act as co-factors in bone resorption.
It has been hypothesized that two cell types are
responsible for bone resorption,
1. Osteoclast: Removes the mineral portion of bone.
2. Mononuclear cells: Plays a role in organic matrix
degradation.
Both are found near the resorbing bone.
Bone Destruction Caused by Trauma
from Occlusion Alone
Trauma from occlusion in the absence of inflammation
can cause the following changes:
• Increased compression and tension of periodontal
ligament.
• Increased osteoclasis of alveolar bone and necrosis
of periodontal ligament.
These changes are reversible, if offending forces are
removed. However persistent trauma from occlusion
results in funnel-shaped bony defects.
Systemic Factors
Local and systemic factors regulate the physiologic
equilibrium of bone. When there is generalized tendency
towards bone resorption, bone loss is initiated by a local
inflammatory process that may be magnified. This
systemic influence on the response of alveolar bone has
been termed as the bone factor concept in periodontal
diseases. In recent years, a lot of studies focussed on
the possible relationship between periodontal bone loss
and osteoporosis,. Osteoporosis is a physiologic condition
of postmenopausal women, resulting in loss of bone
mineral content and microstructural bone changes.
Periodontal bone loss may also occur in generalized
skeletal disturbances (e.g. hyperparathyroidism, leukemia
etc) by mechanism that may not be related to the usual
periodontal bone destruction.
Pharmacological Agents and
Bone Resorption
These include prostaglandins and their precursors and
osteoclast activating factors, all of which are present in
inflamed gingiva. Complement can also induce bone
resorption by enhancing the synthesis of prostaglandins.
Prostaglandins are synthesized by fatty acid precursors
such as arachidonic acid and is controlled by cyclo-
oxygenase pathway. Flurbiprofen (NSAID) is a potent
inhibitor of cyclo-oxygenase pathway of arachidonic acid
metabolism which retards the rate of bone loss.
Radius of Action
Some of the authors suggested that, locally produced
bone resorption factors may have to be present in the
proximity of the bone surface to be able to exert their
action. On the basis of Waerhaug’s measurements, it
was postulated that there is a range of effectiveness of
about 1.5 to 2.5 mm within which bacterial plaque can
induce bone loss, beyond 2.5 mm there is no effect.
Interproximal angular defects can appear only in spaces
wider than 2.5 mm because narrower spaces are
destroyed completely, Large defects far exceeding
2.5 mm can be seen in certain conditions, like localized
juvenile periodontitis and Papillon-Lefevre syndrome,
may be caused by the presence of bacteria within the
tissues.
Rate of Bone Loss
Loe and associates found the rate of bone loss on an
average to be about 0.2 mm a year for facial surfaces
and about 0.3 mm a year for proximal surfaces, when
periodontal disease is allowed to progress untreated.
Periods of Destruction
Periodontal destruction occurs in an episodic, intermittent
pattern characterized by periods of activity and exacer-
bation followed by periods of remission and quiescence.
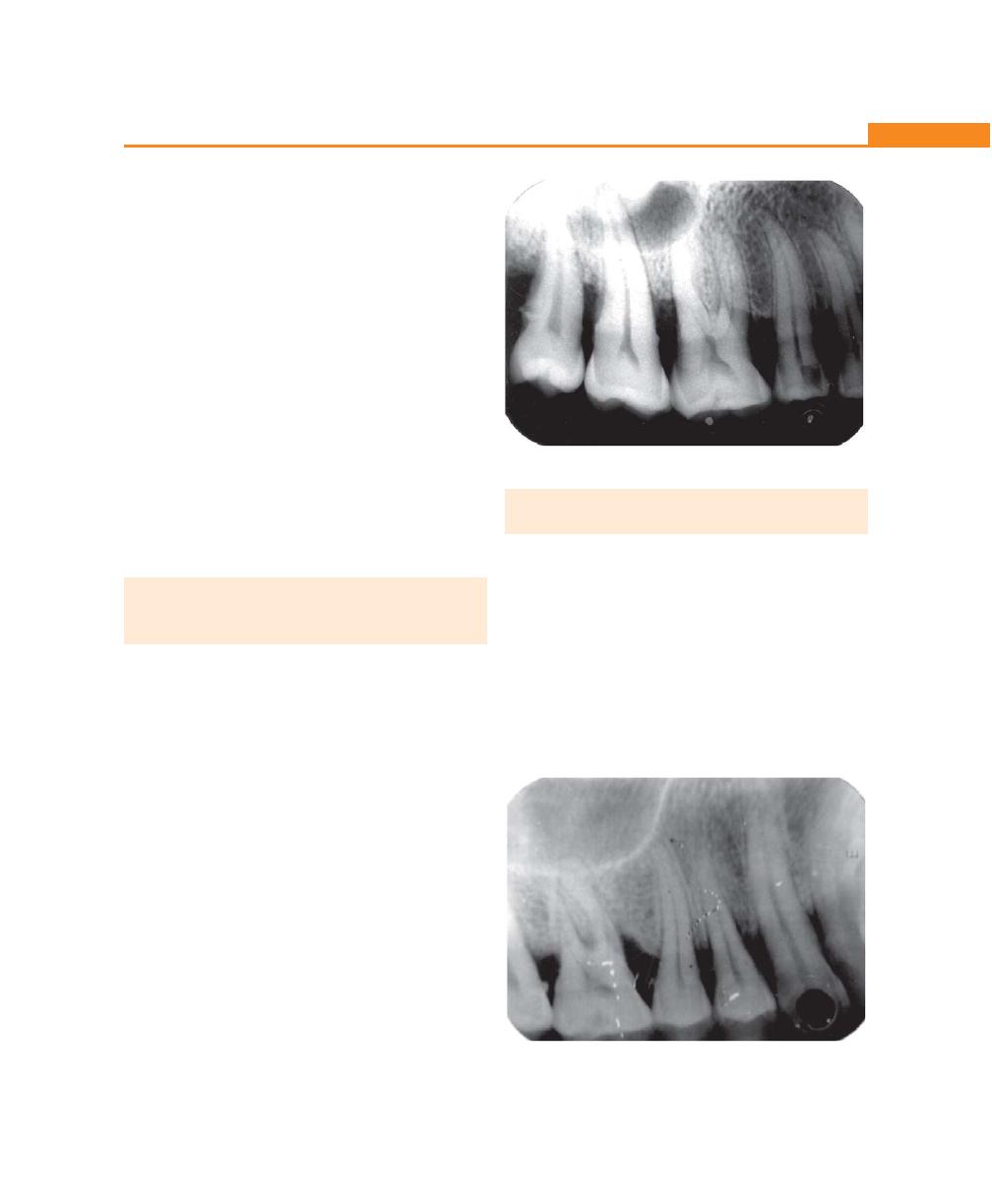
205
Bone Loss and Patterns of Bone Destruction
The destruction results in loss of collagen and alveolar
bone and deepening of periodontal pocket.
The reasons for the onset of destructive patterns are
not totally understood but the following theories have
been offered:
1. Bursts of activity is associated with subgingival
ulceration and an acute inflammatory reaction
resulting in loss of alveolar bone.
2. Bursts of activity coincides with a predominantly T-
lymphocyte lesion to one with predominance of B-
lymphocyte plasma cell infiltrate.
3. Periods of exacerbation is associated with an increase
in loose, unattached motile, Gram-negative,
anaerobic pocket flora and period of remission
coincides with the formation of a dense, unattached,
non-motile, Gram-positive flora.
4. Presence of antibodies.
FACTORS DETERMINING BONE
MORPHOLOGY IN PERIODONTAL
DISEASE
Normal Variation in Alveolar Bone
It can affect the osseous contours produced by
periodontal disease. The anatomic features that
substantially affect the bone destructive pattern in
periodontal disease include the following:
• Thickness, width and crestal angulation of the
interdental septa
• Thickness of facial and lingual alveolar plates
• The presence of fenestrations and dehiscences
• Increased thickness of alveolar bone margins to
accommodate functional demands.
• The alignment of the teeth, root trunk anatomy.
For example, angular bone defects cannot form
in thin facial and lingual alveolar plates, which have
little or no cancellous bone between the outer and
inner cortical layers. In such an instance the entire crest
of the bone is destroyed and the height of bone is
reduced.
BONE DESTRUCTION PATTERNS IN
PERIODONTAL DISEASE
Horizontal Bone Loss
It is the most common pattern of bone loss in periodontal
disease. The bone is reduced in height but the bone
margins remain roughly perpendicular to the tooth
surface (Fig. 24.2).
Vertical or Angular Defects (Figs 24.3 and 24.4)
They are those that occur in an oblique direction, leaving
a hollowed out trough in the bone alongside the root,
the base of the defect is located apical to the surrounding
Fig. 24.2:
Radiographic illustration of horizontal bone loss
Fig. 24.3:
Angular defect in relation to upper first molar
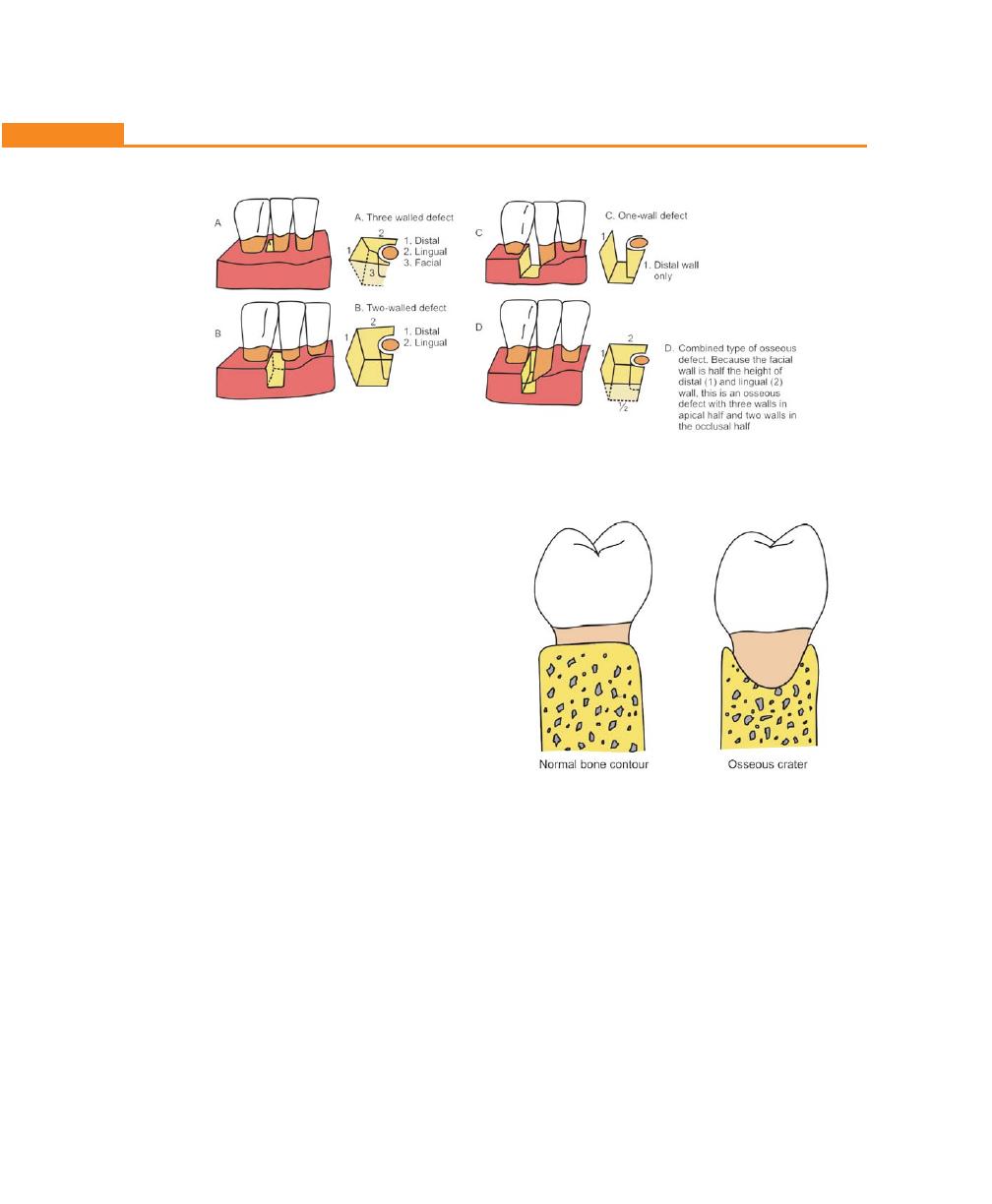
206
Essentials of Clinical Periodontology and Periodontics
Fig. 24.4:
Types of angular defects
bone. In most situations angular defects are accompanied
by infrabony pockets.
Angular defects are classified on the basis of number
of walls present as:
• One-walled or hemiseptal defect—One wall is present
• Two-walled defect—Two walls are present
• Three-walled or intrabony defect—Three walls are
present (more common on mesial surfaces of upper
and lower molars)
• Combined osseous defect—The number of walls in
the apical portion of the defect are greater than that
in its occlusal portion. Radiographs may help upto
some extent to locate vertical defects, but the best
would be surgical exposure of the defect.
Osseous Craters (Fig. 24.5)
They are concavities in the crest of the interdental bone
confined with in the facial and lingual walls. It is found
to make up two-thirds of all mandibular defects, can
be diagnosed by transgingival probing.
The following reasons have been suggested for the
high frequency of interdental craters.
• Interdental areas are more prone to the accumulation
of plaque and are more difficult to clean
• The normal flat or even concave faciolingual shape
of the interdental septum in lower molars may favor
crater formation
Fig. 24.5:
Diagrammatic representation of an osseous crater
in a faciolingual section between two lower molars
• Vascular patterns from the gingiva to center of the
crest may provide a pathway for inflammation.
Bulbous Bony Contours (Fig. 24.6)
They are bony enlargements caused by exostoses,
adaptation to function or buttressing bone formation.
They are found more frequently in the maxilla than
mandible.
Reversed Architecture
These defects are produced by loss of interdental bone,
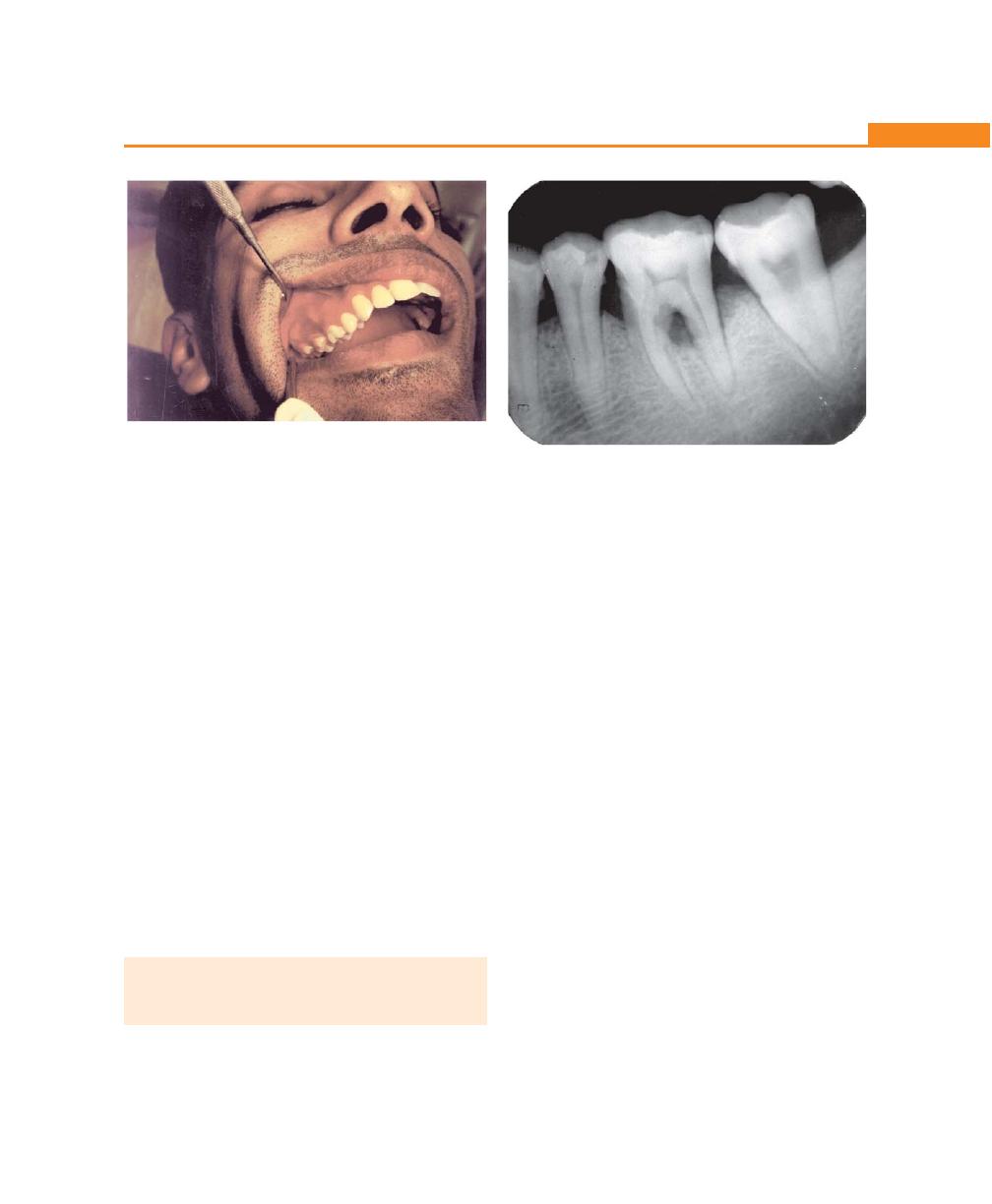
207
Bone Loss and Patterns of Bone Destruction
including the facial and lingual plates without concomitant
loss of radicular bone, thereby reversing the normal
architecture (more common in maxilla).
Ledges
They are plateau-like bone margins caused by resorption
of thickened bony plates.
Furcation Involvements (Fig. 24.7)
It refers to the invasion of the bifurcation and trifurcation
of multirooted teeth by periodontal disease. The
mandibular first molars are most common sites and least
common are maxillary premolars.
The role of trauma from occlusion in the etiology
of furcation involvement is controversial; others include
presence of enamel projections into the furcation,
presence of accessory pulpal canals. Diagnosis is made
by careful probing with Naber’s probe and radiograph
of this area is helpful, but can be obscured by various
factors like, angulation of the beam and radio-opacity
of adjacent structures.
PREVALENCE AND DISTRIBUTION OF
BONE DEFECTS IN MODERATE ADULT
PERIODONTITIS
Different classifications of bone defects have been
proposed:
I. Goldman and Cohen (1958)
According to morphology of bone defects, can be
classified as:
• One-walled defect
• Two-walled defect
• Three-walled defect
• Combined defect
II. Glickman (1964) classified bony defects as:
1. Osseous/interdental craters
2. Hemiseptal defects
3. Infrabony defects
4. Bulbous bone contours (more seen in maxillae
and are enlargement of bone due to exostoses,
buttressing bone formations).
5. Inconsistent margins and ledges (plateau like bony
margins)
6. Reversed architecture.
III. Prichard (1967) expanded this classification and
included furcation involvement, anatomic aberrations
of the alveolar process, i.e. thick marginal ledges,
exostoses and tori, dehiscence and fenestrations.
There is a high prevalence of bony defects in
posterior segments (due to thicker bone). Thin bone
leads to horizontal defects. In the posterior segments,
Fig. 24.6:
Bony exostosis in the buccal aspect
Fig. 24.7:
Furcation involvement in relation to mandibular
first and second molar (Grade-II)
208
Essentials of Clinical Periodontology and Periodontics
percentage of osseous defects are more in the
mandible. Interdental crater is the most common
defect in the molars and hemisepta presents lower
proportion.
KEY POINTS TO NOTE
1. Osseous defects are those defects which are formed as a
result of destruction of alveolar bone due to periodontal
disease.
2. Osteoblasts are the primary cells responsible for the
synthesis of bone matrix, which later on undergoes
calcification.
3. Bone destruction in periodontal disease is caused by
osteoclasts and mononuclear cells and is mediated by local
and systemic factors.
4. Local factors, that are responsible for bone destruction
are:
a. Chronic gingival inflammation
b. Trauma from occlusion
c. Combination of both.
5. Bone destruction patterns in periodontal disease are:
a. Horizontal bone loss.
b. Vertical or angular defects.
c. Osseous craters.
d. Bulbous bony contours.
e. Reversed architecture.
f. Ledges.
g. Furcation involvement.
REVIEW QUESTIONS
1. Describe the mechanism of bone destruction in
periodontal disease.
2. Describe the various patterns of bone loss in
periodontal disease.
BIBLIOGRAPHY
1. Elizabeth A. Pawlak Philip M. Hoag. Essentials of periodontics
third edition; Mosby, Jaypee Brothers.
2. Jeffcoat MT, Lewis CE, Reddy MS, et al. Post-menopausal bone
loss and its relationship to oral bone loss. Periodontol 2000,
2000; 23: 94.
3. Manson JD. Bone morphology and bone loss in periodontal
disease. J. Clin Periodontol 1976; 3: 14.
4. JD Manson, BM Eley. Outline of periodontics, third edition,
KM Varghese Company.
5. Moskow BS, Polson AM. Histological studies on the extension
of the inflammatory infiltrate in human periodontitis. J Clin
Periodontol 1991; 18: 534.
6. Newman, Takei, Caranza. Clinical periodontology, ninth
edition; W.B. Saunders.
7. Papapanou PN, Tonetti MS. Diagnosis and epidemiology of
periodontal osseous lesions. W.B. Saunders: Periodontol
2000; 2000; 22: 8.
8. Schroeder HE. Discussion: Pathogenesis of periodontitis J.
Clin Periodontol 1980; 13: 426.
9. Schwartz Z, Goultschin J, Dean DD, et al. Mechanisms of
alveolar bone destruction in periodontitis. Periodontol 2000,
1997; 14: 158.
209
Chronic Periodontitis
INTRODUCTION
Chronic periodontitis was previously known as “adult
periodontitis” or “slowly progressive periodontitis”.
DEFINITION
Chronic periodontitis occurs as a result of extension of
inflammation from the gingiva into the deeper
periodontal tissues. It has recently been defined as “an
infectious disease resulting in inflammation within the
supporting tissues of the teeth, progressive attachment
loss and bone loss.”
DIAGNOSTIC CRITERIA FOR CHRONIC
PERIODONTITIS (Fig. 25.1)
Clinical Features
1. Age of onset is usually 30 to 35 years.
2. The disease is usually generalized, although some
areas are more deeply involved than the other
areas.
3. No consistent pattern of distribution of lesion is
seen, except that they are usually not isolated to
one or two sites.
Fig. 25.1:
Chronic periodontitis in a 45-year-old male
❒
❒
❒
❒
❒ DEFINITION
❒
❒
❒
❒
❒ DIAGNOSTIC CRITERIA
• Clinical Features
• Microbiological Features
• Radiographic Features
❒
❒
❒
❒
❒ TYPES BASED ON DISEASE
DISTRIBUTION AND SEVERITY
❒
❒
❒
❒
❒ NATURE OF DISEASE PROGRESSION
❒
❒
❒
❒
❒ RISK FACTORS FOR DISEASE
❒
❒
❒
❒
❒ GENERAL CONCEPT FOR ETIOLOGY
25
Chronic Periodontitis

210
Essentials of Clinical Periodontology and Periodontics
4. Highly acute inflammatory sites are not seen,
mostly gingiva appear to be slight to moderately
swollen and color may range from pale-red to
magenta.
5. Loss of stippling, blunt or rolled gingival margins
and flattened or cratered papillae may be seen.
6. Spontaneous bleeding and inflammation related
exudate from the pockets may also be found.
7. When the pocket occludes it may result in abscess
formation.
8. Pocket depths are variable and both suprabony
and infrabony pockets can be found.
9. Conditions that enhance plaque accumulation like
open interdental contacts, defective restorative
margins and malposed teeth may be frequently
seen.
10. The amount of microbial deposits are consistent
with severity of the disease.
11. Tooth mobility is seen in advanced cases.
12. No serum neutrophil / monocyte abnormalities
are seen.
Microbiological Features
Causative organisms of chronic periodontitis are:
• Porphyromonas gingivalis (P. gingivalis)
• Prevotella intermedia (P. intermedia)
• Capnocytophaga
• A.actinomycetem comitans (A.a)
• Eikenella corrodens (E. corrodens)
• Campylobacter rectus (C. rectus)
Radiographic Features (Fig. 25.2)
Pattern of bone loss observed in chronic periodontitis
may be vertical or horizontal. When attachment loss and
bone loss on one tooth surface is greater than that on
an adjacent surface is referred to as vertical bone loss
and is usually associated with angular bony defects and
infrabony pockets. When attachment loss and bone loss
occur at a uniform rate on majority of tooth surfaces
it is called horizontal bone loss and is generally associated
with suprabony pockets.
TYPES
Types are based on the following:
Disease Distribution
Localized periodontitis: Periodontitis is considered locali-
zed when less than 30 percent of the sites assessed in the
oral cavity demonstrate attachment loss and bone loss.
Generalized periodontitis: It is considered generalized
when more than 30 percent of the sites assessed in the
oral cavity demonstrate attachment loss and bone loss.
Disease Severity
Slight (mild) Periodontitis
1. Periodontal destruction is generally considered slight
when there is not more than 1 to 2 mm of clinical
loss of attachment.
2. Usually generalized involvement.
3. Minimal furcation invasions with little or no mobility
are observed.
4. Bone loss is minimal (less than 20% of total attach-
ment).
Moderate Periodontitis
It is considered moderate when there is:
1. 3 to 4 mm of clinical attachment loss.
Fig. 25.2:
Radiograph showing generalized bone loss
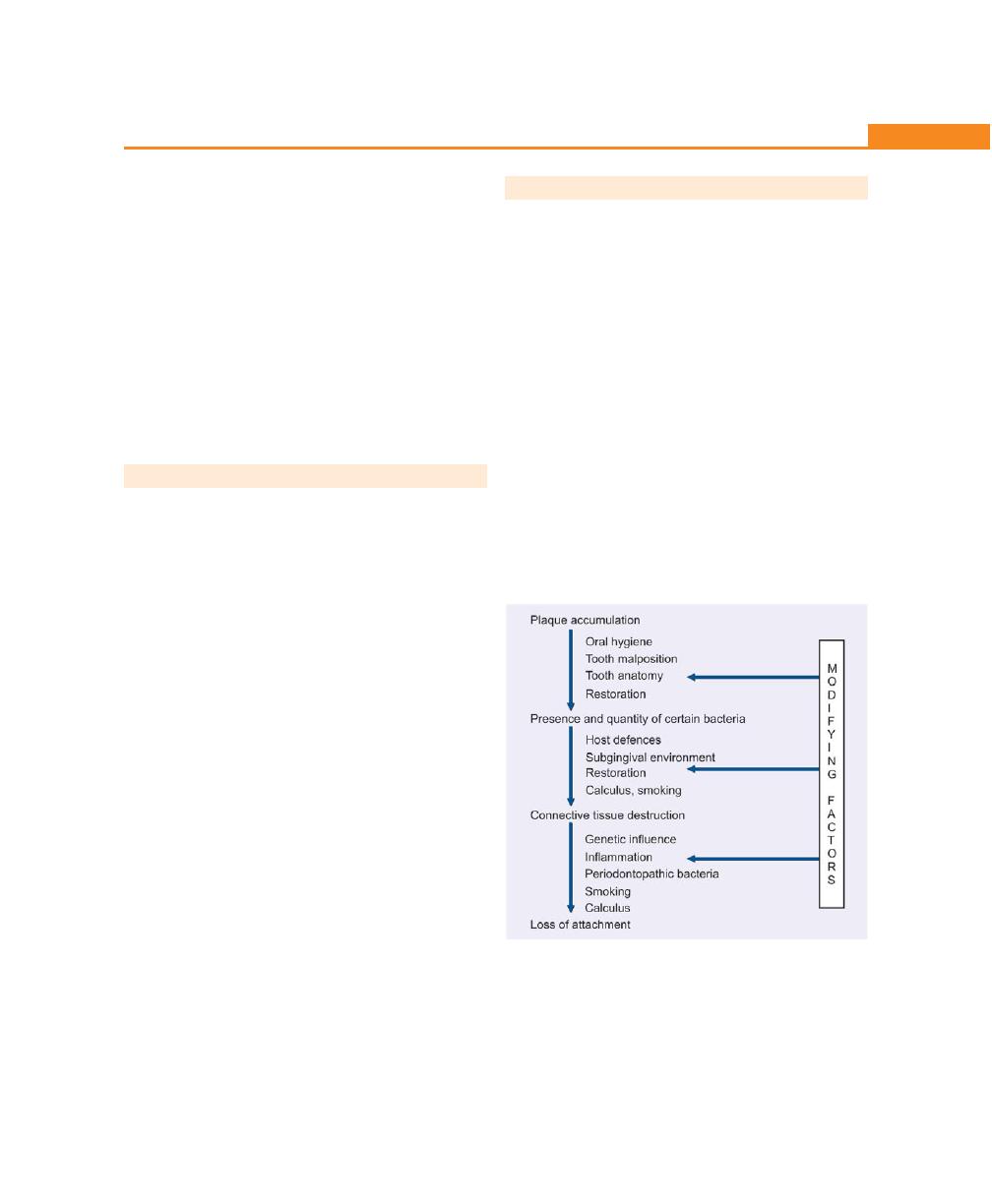
211
Chronic Periodontitis
2. Early to moderate furcation involvement with slight
to moderate tooth mobility.
3. Bone loss up to 40 percent of the total periodontal
attachment on the tooth.
Severe Periodontitis
1. When attachment loss is 7 mm or more, the condition
is severe.
2. Furcation involvement up to grade III.
3. Excessive tooth mobility.
4. Bone loss more than 40 percent—both horizontal
and angular bony defects are observed.
NATURE OF DISEASE PROGRESSION
The rate of disease progression in chronic periodontitis
is slow, but sometimes may be modified by systemic or
other underlying factors. Though onset can occur at
anytime, because of its slow progression it usually becomes
clinically significant in the mid 30s or later. Several models
have been proposed to describe the rate of disease
progression.
1. Continuous paradigm.
2. Random burst theory.
3. Asynchronous multiple burst hypothesis.
Continuous Paradigm implies slow, continuous and
progressive destruction of periodontium. This type of
progression has been reported in longitudinal studies,
not responsive to treatment.
The Random Burst Theory proposes that the progression
of disease occurs at short periods of active destruction,
which are followed by periods of remission that randomly
occur with respect to time and site in an individual. An
example for this is chronic adult periodontitis.
In Asynchronous Multiple Burst Model, the tissue
destruction occurs at a definite period of time in one’s
life, then it passes into a state of remission as in juvenile
periodontitis.
RISK FACTORS FOR DISEASE
a. Local factors: These include plaque and plaque
retentive factors. Plaque attached to the tooth and
gingival surfaces at the dentogingival junction is
considered to be the primary etiologic factor in chronic
periodontitis. P. gingivals, B. forythus and Treponema
denticola are frequently associated with chronic
periodontitis. Plaque retentive factors are those that
facilitate plaque accumulation or prevent the removal
of plaque by routine oral hygiene procedures. They
play an important role in the development of chronic
periodontitis because they allow plaque micro-
organisms to be in close proximity to periodontal
tissues. Some of these factors include, calculus,
subgingival and/or overhanging margins of restora-
tions, deep carious lesions that extend subgingivally,
crowded or malaligned teeth and root surface
irregularities (Fig. 25.3).
Fig. 25.3:
Local factors in chronic periodontitis
b. Systemic factors: The role of systemic factors in
periodontal diseases can influence the host response
and increase the rate of progression of periodontal
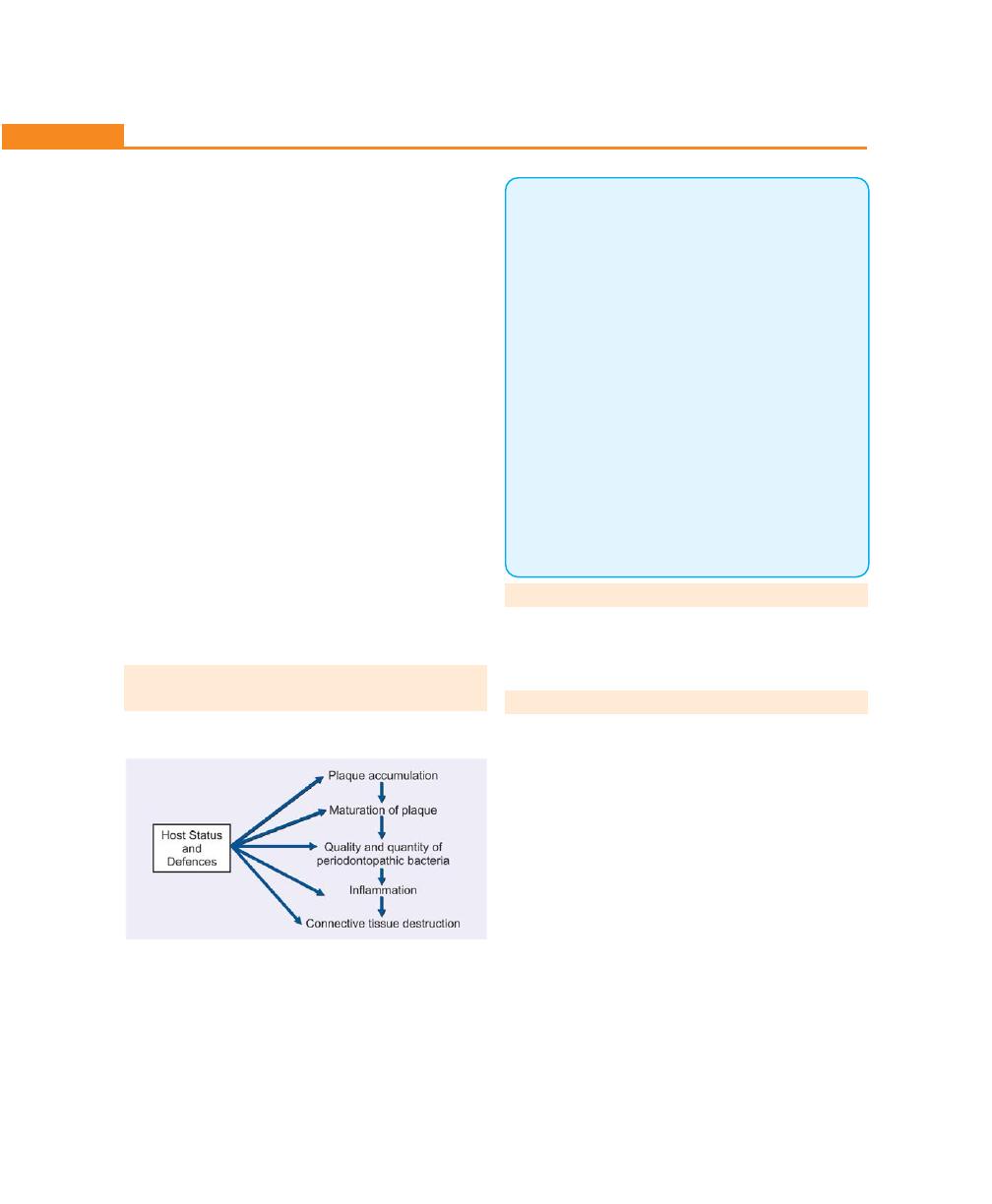
212
Essentials of Clinical Periodontology and Periodontics
disease. Diabetes mostly type II, a non-insulin
dependent diabetes mellitus is considered to be one
of the most important systemic condition that can
increase the extent and severity of periodontal
disease.
c. Environmental or behavioral factors: Smoking is one
such factor that presently is receiving a lot of attention.
It has been shown that when combined with plaque
it induces periodontitis. It can result in greater
attachment loss, bone loss, furcation involvement and
deeper pockets. There is abundant evidence
suggesting that emotional stress may also influence
the extent and severity of chronic periodontitis.
d. Genetic factors: Genetic basis for periodontal disease
is based on the recent studies that have demonstrated
periodontal destruction among the family members
and different generations within a family. Although
no clear determinants have been described for
chronic periodontitis, a genetic predisposition may
be observed in aggressive periodontal breakdown in
response to accumulation of plaque and calculus.
GENERAL CONCEPT FOR ETIOLOGY OF
CHRONIC PERIODONTITIS
KEY POINTS TO NOTE
1. Chronic periodontitis is recently been defined as an
“infectious disease resulting in inflammation within the
supporting tissues of the teeth, which is characterized by
progressive attachment loss and bone loss.”
2. Depending on the disease distribution it is classified as,
localized periodontitis, i.e. less than 30 percent of the sites
showing attachment loss and bone loss. It is generalized
when more than 30 percent of sites demonstrate attachment
loss and bone loss.
3. Depending on the disease severity it can be described as
mild, moderate and severe forms.
4. Three models have been proposed to describe the rate of
disease progression:
a. Continuous Paradigm,
b. Random Burst Theory,
c. Asynchronous Multiple Burst Hypothesis.
5. Various risk factors for chronic periodontitits are:
1. Local factors like plaque and plaque retentive factors.
2. Systemic factors—systemic diseases like diabetes.
3. Environmental factors like smoking.
4. Genetic factors—no clear determinants.
REVIEW QUESTIONS
1. Describe the types, etiology and clinical features of
chronic periodontitis.
2. What are risk factors for chronic periodontitis?
BIBLIOGRAPHY
1. Fleming TF. Periodontitis. Ann Priodontol 1999; 4: 32.
2. Kornman KS, di Giouine FS. Genetic variations on cytokine
expression: A risk factor for severity of adult periodontitis. Ann
periodontol 1998; 3: 327.
3. Newman, Takei, Carranza. Clinical periodontology, 9th
edition, WB Saunders.
4. Saul schluger. Periodontol diseases, 2nd edition, Lea and
Febiger, 1999.
5. Socransky SS, Haffajee AD, Goodson JM et al. New concepts
of destructive periodontal disease. J Clin Periodontol 1984;
11: 21.
6. William B Clark, Harold Loe. Mechanisms of initiation and
progression of periodontal disease. Periodontol 2000; 2:
1993.
213
Aggressive Periodontitis
INTRODUCTION
Aggressive periodontitis is characterized by the rapid loss
of attachment and bone loss occurring in an otherwise
clinically healthy patient with the amount of microbial
deposits inconsistent with disease severity and familial
aggregation of diseased individuals. Aggressive
periodontitis was formerly classified as early onset
periodontitis, i.e. localized juvenile periodontitis (LJP)
has been changed to localized aggressive periodontitis,
generalized aggressive periodontitis was previously
classified as generalized juvenile periodontitis (GJP) and
rapidly progressive periodontitis (RPP).
LOCALIZED AGGRESSIVE PERIODONTITIS/
LOCALIZED JUVENILE PERIODONTITIS
Historical Background
• In 1923, Gottilieb reported a case as diffuse atrophy
of alveolar bone characterized by the loss of collagen
fibers in the periodontal ligament and loss of alveolar
bone
• In 1928 Gottilieb attributed this condition to the
inhibition of cementum formation and termed the
disease as cementopathia
• In 1938 Wannenmacher described incisor, first molar
involvement and called the disease as periodontitis
marginalis progressiva. Unlike others he considered
this as an inflammatory disease
• In 1940, Thoma and Goldman used the term
paradontosis and reported that the initial abnormality
was located in the alveolar bone rather than in the
cementum
• In 1942, Orban and Weinmann introduced the term
periodontosis and on the basis of autopsy case, des-
cribed three stages in the development of the disease.
Stage 1: Involves the degeneration of principle fibers
of the periodontal ligament, which induces cessation of
cementum formation and resorption of alveolar bone.
❒
❒
❒
❒
❒ LOCALIZED AGGRESSIVE
PERIODONTITIS
• Historical Background
• Clinical Features
• Radiographic Findings
• Histopathologic Features
• Bacteriology
• Immunology
• Treatment
❒
❒
❒
❒
❒ GENERALIZED AGGRESSIVE
PERIODONTITIS
• Clinical Characteristics
• Radiographic Findings
• Risk Factors
26
Aggressive Periodontitis
214
Essentials of Clinical Periodontology and Periodontics
In this stage tooth migration occurs without detectable
inflammatory involvement.
Stage 2: The lack of periodontal fibers results in the rapid
proliferation of the junctional epithelium along the root
and earliest signs of inflammation appear.
Stage 3: It is characterized by progressive inflammation
and the development of deep, infrabony periodontal
pockets.
Most of these studies considered ‘periodontosis’ as
a degenerative disease caused by unknown systemic
factors. Other investigations denied the existence of a
degenerative type of periodontal disease and attributed
the changes observed to trauma from occlusion.
In 1996, the World Workshop concluded that
periodontosis as a degenerative entity was unsubstan-
tiated and the term should be eliminated from
periodontal nomenclature.
The term juvenile periodontitis was introduced by
Chaput and colleagues in 1967 and by Butler in 1969.
In 1971, Baer defined it as ‘a disease of periodontium
occurring in an otherwise healthy adolescent which is
characterized by a rapid loss of alveolar bone, about
more than one tooth of the permanent dentition. The
amount of destruction is not commensurate with the
amounts of local irritants.’
A more recent definition by Genco et al in 1986
describes localized juvenile periodontitis as a disease
occurring in otherwise healthy individuals under the age
of 30 years with destructive periodontitis localized to the
first permanent molars and incisors not involving more
than two other teeth.
Generalized juvenile periodontitis is “defined as
destructive periodontitis in individuals under the age of
30 years affecting more than fourteen teeth, i.e.
generalized to an arch or an entire dentition.”
Clinical Features
Age and Sex Distribution
Affects both the sexes and is seen mostly between
puberty and 20 years of age. Some studies show
predilection to female patients.
Distribution of Lesions
Three areas of localization of bone loss have been
described:
1. First molar and/or incisors.
2. First molar and/or incisors + additional teeth (not
exceeding 14 teeth).
3. Generalized involvement.
For localized juvenile periodontitis classic distribution
is in the first molars and incisors with least destruction
in the cuspid, premolar area.
Limitations of destruction to certain teeth could be
for the following reasons:
1. Production of opsonizing antibodies against A.
actinomycetemcomitans called “burn-out”
phenomenon.
2. Bacteria antagonistic to A. actinomycetemcomitans
may develop thereby decreasing the number of
colonization sites.
3. A. actinomycetemcomitans may loose its leukotoxin
producing ability for unknown reasons.
4. Localization of the lesions could also be due to the
defect in cementum formation (hypoplastic/aplastic
cementum).
Clinical Findings (Fig. 26.1)
1. The most striking feature is lack of clinical inflammation
despite the presence of deep periodontal pockets.
Fig. 26.1:
Generalized aggressive periodontitis in a
30-year-old patient

215
Aggressive Periodontitis
2. There is a small amount of plaque, which forms a
thin film on the tooth and rarely mineralizes to
become calculus.
3. Most common initial symptoms are mobility and
migration of first molars and incisors. Classically, a
distolabial migration of the maxillary incisors with
diastema formation occurs, lower incisors rarely
migrate compared to upper incisors, all changes
followed by sequelae of migration are seen.
4. As the disease progresses other symptoms like root
surface sensitivity, deep dull radiating pain,
periodontal abscess formation and regional lymph
node enlargement may occur.
Radiographic Findings (Fig. 26.2)
• Vertical or angular bone loss around the first molars
and incisors in an otherwise healthy teenagers is a
diagnostic sign of classic juvenile periodontitis. The
pattern appears to be, “Arc-shaped loss of alveolar
bone extending from distal surface of 2nd premolar
to mesial surface of 2nd molar.”
• Frequently, bilaterally symmetrical patterns of bone
loss occurs, called as “mirror image pattern.”
Pathogenesis of aggressive periodontitis is due to an
interplay of several factors these include, the specific
microbiology of subgingival plaque, defects in
cementum, hereditary factors, impaired PMNs function
and disorders of the immune system.
Histopathology/microscopic Features
These are the same as those seen during pocket
formation.
• Like ulcerated pocket epithelium
• Accumulation of various inflammatory cells in the
connective tissue mainly leukocytes, plasma cells and
small number of lymphocytes and macrophages.
• Electron microscopic studies of juvenile periodontitis
revealed bacterial invasion of connective tissue that
reaches the bone surface.
• The flora involves A. actinomycetemcomitans,
Capnocytophaga sputigena and others.
Bacteriology
Two types of bacteria are considered to be pathogens
in localized aggressive periodontitis — A. actinomycetem-
comitans and Capnocytophaga. A. actinomycetemco-
mitans is a short, facultatively anaerobic, non-motile
Gram-negative rod.
Virulence factors associated with A. actinomycetem-
comitans are:
Factors
Significance
Leukotoxin
Destroys polymorphonuclear leukocytes
and macrophages
Endotoxin
Activates host cells to secrete inflammatory
mediators (prostaglandins, interleukin’s 1
and 3, tumor necrosis factor-α)
Bacteriocin
May inhibit IgG and IgM production
Collagenase
Causes degradation of collagen
Chemotactic
May inhibit neutrophil chemotaxis
inhibition
factors
Fig. 26.2:
Radiograph showing progressive bone loss
216
Essentials of Clinical Periodontology and Periodontics
Immunology
Immune defects that have been implicated in the patho-
genesis of localized aggressive periodontitis are functional
defects of polymorphonuclear leukocytes/monocytes
thereby it impairs the chemotactic attraction of PMNLs
(polymorphonuclear leukocytes) to the site of infection.
Treatment
Prognosis is no more considered as poor for patients
with aggressive periodontitis. The following treatment
has been tried in the past with varying results:
1. Extraction: Extraction of involved teeth especially first
molars results in uneventful healing.
Transplantation of developing third molars into
the sockets of previously extracted 1st molars has
been tried but with limited success.
2. Standard periodontal therapy: Includes scaling, root
planing, curettage, flap surgery with/without bone
grafts, root amputation, hemisection, occlusal
adjustment and strict plaque control has been tried.
However response is unpredictable and frequent
maintenance visits are a must.
3. Antibiotic therapy: Several authors reported
successful results using antibiotics as adjuncts to
standard therapy.
• Genco and coworkers reported scaling and root
planing and tetracycline 250 mg qid for 14 days every
8 weeks.
• Several other investigations have also noticed excellent
bone fill in cases of localized juvenile periodontitis
treated with tetracycline, flap surgery and placement
of grafts.
Current Approach to Therapy
• In almost all cases systemic tetracycline hydrochloride
250 mg qid for atleast a week should be given in
conjunction with local mechanical therapy. If surgery
is indicated systemic antibiotics are advised with
patient instructed to begin taking the antibiotic
approximately 1 hour before surgery
• Doxycycline 100 mg/day may also be used
• Chlorhexidine rinses should be prescribed
• In refractory cases, tetracycline resistant Actinobacillus
species have been suspected. In such cases a
combination of amoxicillin and metronidazole has
been suggested.
GENERALIZED AGGRESSIVE
PERIODONTITIS
Previously classified as Generalized Juvenile Periodontitis
(GJP) and Rapidly Progressive Periodontitis (RPP).
Generalized aggressive periodontitis is usually
characterized by, ‘generalized interproximal attachment
loss affecting atleast three permanent teeth other than
first molars and incisors.’ Patients with generalized
aggressive form may exhibit minimal amounts of
microbial plaque associated with the affected teeth, i.e.
quantitatively, the amount of plaque seems to be
inconsistent with the amount of periodontal destruction,
qualitatively most pathogenic organisms may be
associated, e.g. Porphyromonas gingivalis, A.
actinomycetemcomitans, and Bacteroids forsythus.
Clinical Characteristics
a. Age and sex distribution: It affects persons between
puberty and 35 years (but may be older) no sex
discrimination is seen
b. Distribution of lesion: No specific pattern is observed,
all or most of the teeth are affected.
c. Two types of gingival responses: May be seen in
generalized aggressive periodontitis. One is severe,
acutely inflamed tissue which is often proliferating,
ulcerated and fiery red, spontaneous bleeding and
suppuration are commonly seen. In the other cases,
the gingival tissue may appear pink and free of
inflammation but deep pockets can be demonstrated
by probing.
d. Some of the patients may have systemic
manifestations such as weight loss, mental depression
and general malaise.
217
Aggressive Periodontitis
Radiographic Findings
No definite pattern of distribution occurs but, the radio-
graphic picture can range from severe bone loss associated
with the minimal number of teeth, to advanced bone
loss affecting the majority of teeth in the dentition.
Risk Factors for Aggressive Forms of
Periodontitis
Microbiologic Factors
A. actinomycetemcomitans has been implicated as the
primary pathogen associated with this disease.
Microscopically, the lesions of localized aggressive
periodontitis have revealed bacterial invasion of
connective tissue that reaches the bone surface. These
invading bacteria have been identified as A. actino-
mycetemcomitans, Capnocytophaga sputigena,
Mycoplasma sub-species and Spirochetes.
Immunologic Factors
Some of the immune defects that have been implicated
in the pathogenesis of localized aggressive periodontitis
are:
a. Approximately 75 percent of patients with localized
aggressive periodontitis (LAP) have dysfunctional
neutrophils, which are seen as decrease in the
chemotactic response to several chemotactic
agents, including the complement component C5a,
N-formyl-methionyl leucylphenylalanine (FMLP) and
leukotriene B4. The defect is also associated with a
40 percent deficiency in glycoprotein, GP110 ,on
the neutrophil surface.
b. Patients with LAP demonstrate a strong antibody
response to A. actinomycetemcomitans which
explains the limitation of the infection. In LAP the
dominant serum antibody is IgG2 type which is
specific to antigens of A. actinomycetemcomitans.
c. In generalized form of aggressive periodontitis diverse
microbial patterns including organisms associated with
chronic periodontitis have been implicated. Host
response is often characterized by defects in either
neutrophils or monocytes.
Genetic Factors
Some of the above mentioned immunologic defects
seen in aggressive periodontitis may have a genetic basis,
i.e. familial clustering of neutrophil abnormalities may
be seen. It has been suggested by some authors that,
a major gene plays a role in aggressive periodontal
disease, which could be transmitted through an autosomal
dominant mode of inheritance.
Environmental Factors
Smoking is one of the factors that can influence the
extent of destruction seen in young patients. Especially,
smokers with generalized aggressive periodontitis exhibit
more number of teeth affected by loss of clinical
attachment than non-smokers with generalized
aggressive periodontitis.
KEY POINTS TO NOTE
Juvenile Periodontitis
It is defined as “ a disease of periodontium occurring in an
otherwise healthy adolescent which is characterized by a rapid
loss of alveolar bone, about more than one tooth of the
permanent dentition. The amount of destruction is not
commensurate with the amount of local irritants.
1. Previously classified as generalized juvenile periodontitis
(GJP) and rapidly progressive periodontitis (RPP).
2. Usually affects individuals under the age of 30, but older
patients may also be affected.
3. They usually produce a poor antibody response to the
pathogens present.
4. Two types of gingival responses may be seen, one is a
severe form, which is characterized by acute inflammatory
changes in the gingival tissue. Other cases, the gingival tissue
may appear pink, free of inflammation but in the presence
of deep pockets.
5. There is no specific pattern for distribution of lesions, radio-
graphically again no definite pattern of distribution seen.
BIBLIOGRAPHY
1. Jan Lindhe. Clinical Periodontology and Implant Dentistry,
4th edition, Blackwell Munksgaard Publication, 2003.
2. Lang N, Bartold PM, Cullinan M et al. Consensus report:
Aggressive periodontitis. Ann periodontol 1999; 4: 53.
3. Tonnetti MS, Mombelli A. Early onset periodontitis. Ann
periodontol 1999; 4: 39.
218
Essentials of Clinical Periodontology and Periodontics
NECROTIZING ULCERATIVE
PERIODONTITIS (NUP)
Necrotizing ulcerative periodontitis occurs as a result
of extension of necrotizing ulcerative gingivitis into
the periodontal structures, leading to loss of attachment
and bone loss. There are two types of necrotizing
ulcerative periodontitis described, based on its relation-
ship to acquired immunodeficiency syndrome (AIDS):
Non-AIDS type necrotizing ulcerative periodontitis and
AIDS-associated necrotizing ulcerative periodontitis.
Non-AIDS Type Necrotizing
Ulcerative Periodontitis
Clinical Features
Since necrotizing ulcerative periodontitis occurs after
repeated attacks of necrotizing ulcerative gingivitis (NUG)
all the characteristic clinical features of necrotizing
ulcerative gingivitis are seen, i.e.
i. Ulceration and necrosis of gingival margin, which
gets covered by a pseudo-membranous slough.
ii. The ulcerated margins are surrounded by an
erythematous halo.
iii. The lesions are extremely painful and bleed
spontaneously.
iv. Localized lymphadenopathy, fever and malaise.
These lesions, especially in long-standing cases, can
extend to the deeper periodontal structures resulting
in deep, crater-like osseous lesions especially in
interdental areas. Such cases are identified as necro-
tizing ulcerative periodontitis (NUP), most striking
feature of this condition is absence of deep conven-
tional pockets associated with deep interdental
osseous craters. This is because the necrotizing and
ulcerative properties of the gingival lesion destroys
the marginal epithelium, resulting in total destruction
of the marginal tissue leading to recession.
❒
❒
❒
❒
❒ NECROTIZING ULCERATIVE
PERIODONTITIS
• Non-AIDS type
• AIDS associated
❒
❒
❒
❒
❒ REFRACTORY PERIODONTITIS
• Etiology
• Clinical Features
• Treatment
❒
❒
❒
❒
❒ PERIODONTITIS AS A MANIFESTATION
OF SYSTEMIC DISEASE
• Papillon-Lefevre Syndrome
• Chediak-Higashi Syndrome
• Down Syndrome
• Hypophosphatasia
• Neutropenia
• Leukocyte Adhesion Deficiency
27
Necrotizing Ulcerative
Periodontitis, Refractory
Periodontitis and Periodontitis as
a Manifestation of Systemic
Disease
219
Necrotizing Ulcerative Periodontitis
AIDS-Associated Ulcerative Periodontitis
(Discussed in Detail Elsewhere in this book)
Gingival and periodontal lesions of HIV positive patients
appear to have similar findings that are seen in non-
AIDS-associated necrotizing ulcerative periodontitis
patients, in addition, they may exhibit certain
complications such as:
1. Large areas of soft tissue necrosis with exposure of
bone and sequestration of bone.
2. Sometimes these lesions may extend onto the buccal
vestibule or the palate and become necrotizing
stomatitis.
REFRACTORY PERIODONTITIS
According to American Academy of Periodontology,
refractory periodontitis has been defined as “those cases
which do not respond to any treatment provided,
whatever the thoroughness or frequency”. It should be
differentiated from the cases of recurrent periodontitis,
in which, after remission of the disease, recurrence
follows due to inadequate plaque control either by the
patient or clinician. Hence recurrent periodontitis, which
is recurrence of the disease due to incomplete treatment
should be differentiated from refractory cases whose
reasons for not responding to adequate treatment, in
certain cases, is still not clearly understood (Table 27.1).
Etiology
Risk Factors Responsible for Refractory Cases
a. Abnormal host response.
b. Resistant strains of pathogenic periodontal microflora.
c. Failure to eliminate plaque retentive factors, such as
furcation involvement, irregular root surface, palato-
gingival groove, etc. which may in turn interfere with
complete plaque removal.
d. On the other hand smoking and systemic diseases
may result in generalized lesions, which may not
respond favorably to treatment.
Specific microorganisms have been identified in lesions
of refractory periodontitis. Haffajee et al (1988) reported
Table 27.1: Distinction between recurrent and
refractory periodontitis
Disease
Recurrent periodontitis
Refractory
periodontitis
Definition
Sites are successfully
Sites do not respond
treated but disease
to conventional
returns, may refer to
therapy; usually
site/patients
refers to patients but
may refer to sites.
Phase of
May be because of
May be because of
therapy
inadequate therapy
inadequate therapy
during maintenance/
during active treat-
no maintenance
ment/other factors.
Etiology
May be because of
May be because of
re-infection, with
infection with tissue
microbes that were
invasive microbes
suppressed but not
that cannot be elimi-
eliminated. Reinfec-
nated with conven-
tion with eliminated
tional therapy or
organisms or new
because of immuno-
bacteria.
incompetence
Immune
Immunocompetent
May not be immuno-
system
competent
Antibiotic
Not usually needed
Usually needed
therapy
three major microbial complexes in refractory perio-
dontitis cases.
1. B. forsythus, F. nucleatum and C. rectus.
2. S. intermedius, P. gingivalis and P. micros.
3. S. intermedius and F. nucleatum.
Clinical Features
In the classification proposed by the American Academy
of Periodontology (1999), the term refractory
periodontitis has been removed as a single entity due
to the diversity of clinical conditions and treatments under
which periodontal therapy fails to arrest the progression
of periodontitis. Therefore the participants of the
workshop have concluded that, rather than a single
disease entity, the ‘refractory’ designation could be
applied to all forms of periodontitis, e.g. refractory chronic
periodontitis, refractory aggressive periodontitis and
others.
Hence, refractory periodontitis in no way differs from
other forms of periodontitis. Magnusson et al (1991)
noticed no changes in the amount of plaque in relation

220
Essentials of Clinical Periodontology and Periodontics
to the sites that have been treated, however, the sites
that showed attachment loss had also showed persistent
bleeding on probing. The refractory cases can be iden-
tified either by presenting new areas of attachment loss
or progressive attachment loss in already treated sites.
Treatment
Antimicrobial therapy along with mechanical
debridement has been found to be effective in reducing
the microbial population at the refractory sites.
Antibiotic therapy aims to reinforce mechanical
periodontal therapy and also helps the host defence
system by killing subgingival pathogens that remain after
conventional mechanical periodontal therapy. Many
antibiotics have been used to treat the refractory sites
(Table 27.2).
Table 27.2: Drugs used to treat refractory sites
Drug
Dosage
Tetracycline hydrochloride
250 mg of q.i.d.
Amoxicillin
250 mg + 125 mg thrice daily
Clavulanate potassium
for 2 weeks
(Augmentin)
R
Clindamycin hydrochloride
150 mg of q.i.d. for 1 week
Combination of the above
drugs, i.e. metronidazole/
amoxicillin, metronidazole
doxycycline are also used
In addition to this, intrasulcular irrigation with 10
percent povidone iodine solution and chlorhexidine
solution have also been used successfully.
In cases of localized lesions, local drug delivery system
has been tried successfully. The advantages of this are
smaller doses and minimal side effects. These local
therapies are available in the form of gels, fibers or chips.
Another approach in treating refractory periodontitis
is through modulation of host by sub-antimicrobial doses
of doxycycline or non-steroidal anti-inflammatory drugs
(NSAIDs) in conjunction with conventional therapy. The
sub-antimicrobial or low dose doxycycline helps to
prevent the periodontal destruction by controlling the
production of collagen and gelatinase, whereas
flurbiprofen, indomethacin and naproxen may reduce
the production of inflammatory mediators during chronic
periodontal disease.
PERIODONTITIS AS A MANIFESTATION
OF SYSTEMIC DISEASE
Severe periodontitis has been observed in patients who
exhibit either defective numbers of neutrophils or
defective neutrophil function. Some of the conditions
associated with defective neutrophils are:
a. Papillon-Lefévre syndrome.
b. Chédiak-Higashi syndrome.
c. Down’s syndrome.
d. Hypophosphatasia.
e. Neutropenia.
f. Leukocyte adhesion deficiency.
Papillon-Lefévre Syndrome (Fig. 27.1)
1. This is characterized by hyperkeratotic skin lesions
and severe destruction of the periodontium.
Fig. 27.1:
A case of Papillon-Lefévre syndrome
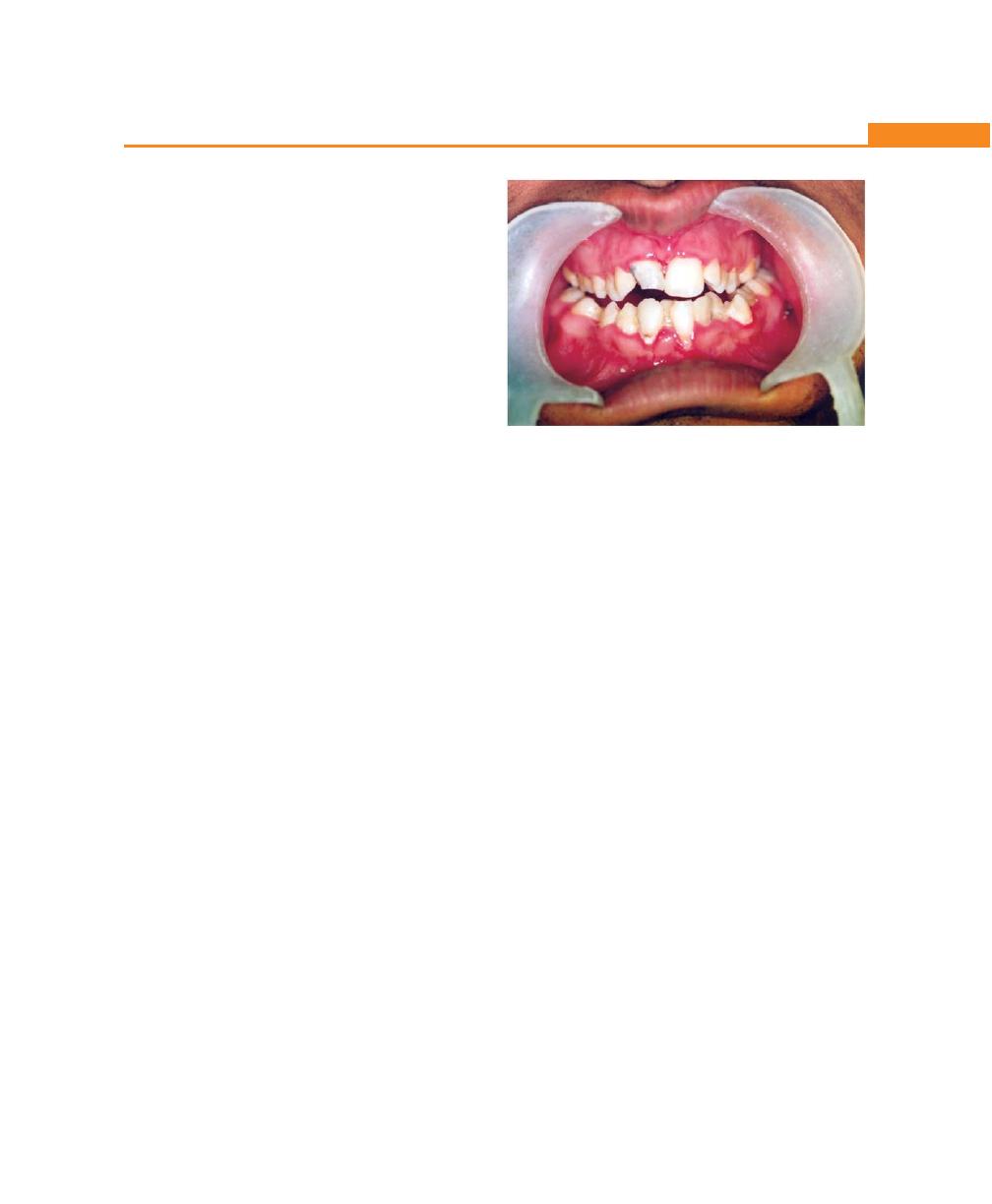
221
Necrotizing Ulcerative Periodontitis
2. These changes may appear before the age of 4
years.
3. Skin lesions are—hyperkeratosis of localized areas on
palms, soles, knees and elbows.
4. Periodontal involvement is early inflammatory
changes that lead to bone loss and exfoliation of
teeth. Primary teeth are lost by 5 or 6 years of age.
The permanent dentition erupts normally but within
few years the permanent teeth are also lost.
Chédiak-Higashi Syndrome
(C-H Syndrome)
This is a rare syndrome characterized by recurrent
bacterial infections. It exhibits oral ulceration and rapidly
destructive periodontitis. Chédiak- Higashi syndrome is
a hereditary disease with defects in both neutrophils and
monocytes.
Down Syndrome (Mongolism, Trisomy 21)
This is a congenital disease caused by a chromosomal
abnormality and is characterized by mental deficiency
and growth retardation.
The oral findings include presence of plaque, calculus,
and other local factors, like diastema, crowding of teeth,
high frenal attachment and malocclusion.
Periodontitis in Down syndrome include the formation
of deep periodontal pocket associated with plaque
accumulation and moderate gingivitis, usually genera-
lized but more severe in the lower anterior region; marked
recession is also seen in this region (may be due to high
frenal attachment). Acute necrotizing lesions are also
common (Fig. 27.2).
Two factors have been proposed to explain high
prevalence and increased severity of periodontal
destruction in Down syndrome:
1. Reduced resistance to infections because of poor
circulation (especially peripheral).
2. Defect in T-cell maturation and polymorphonuclear
leukocyte chemotaxis
Hypophosphatasia
This is a rare familial skeletal disease characterized by
rickets, poor cranial formation, premature loss of primary
dentition particularly incisors. Patients have low level of
serum alkaline phosphatase. Teeth are lost with no clinical
evidence of gingival inflammation and show reduced
cementum formation.
Neutropenia
It is a condition where the circulating neutrophils are
reduced, i.e. less than 1,500 per ml. Destructive
generalized periodontal lesions have been described in
children with neutropenia. Neutropenia could be inherent
or acquired. Certain drugs and infections can lead to
reduction in number of neutrophils.
Leukocyte Adhesion Deficiency
These cases are rare and begin during or immediately
after eruption of the primary teeth. Extreme acute
inflammation and proliferation of gingival tissues
accompanied by rapid bone loss is found, profound
defects in peripheral blood neutrophils and monocytes
are seen. Hence they are absent in gingival tissues.
Patients with LAD (Leukocyte adhesion deficieny) also
have frequent respiratory tract infections and sometimes
otitis media.
Fig. 27.2:
Gingival changes associated with Down syndrome
222
Essentials of Clinical Periodontology and Periodontics
KEY POINTS TO NOTE
1. When ulcerative gingivitis extends deeper into the
periodontal structures, it results in loss of attachment and
bone loss. This condition is termed as necrotizing ulcerative
periodontitis (NUP).
2. It can be of two types, based on its relationship to acquired
immunodeficiency syndrome (AIDS). Non-AIDS type NUP
and AIDS-associated NUP.
3. A non-AIDS type occurs as a result of repeated attacks of
NUG (Necrotizing ulcerative gingivitis).
4. AIDS-associated NUP (Necrotizing ulcerative periodontitis)
shares most of the features of non-AIDS type, but for, larger
areas of soft tissue necrosis with exposure of bone and
sequestration of bone fragments.
5. Refractory periodontitis is defined as, “those cases which
do not respond to any treatment, provided, whatever the
thoroughness or frequency.” It should be differentiated from
recurrent periodontitis cases, in which, after the remission
of the disease, recurrence follows due to inadequate plaque
control either by the patient or the clinician.
6. According to the classification proposed by AAP (American
Academy of Periodontology, 1999) the term refractory
periodontitis has been removed as a single entity.
7. Many systemic conditions associated with defective number
or function of neutrophils exhibit severe periodontitis. Some
of the conditions are, Papillon-Lefèvre syndrome, Chediak-
Higashi syndrome, Down syndrome, hypophosphatasia,
neutropenia and leukocyte adhesion deficiency.
REVIEW QUESTION
1. Describe the features of necrotizing ulcerative
periodontitis.
BIBLIOGRAPHY
1. Bullon P, Pascual A, Fernandez-Novoa MC, et al. Late
onset Paillon-Lefèvre syndrome. J Clin Periodontol 1993; 20:
662.
2. Colombo AP, Haffajee AD, Dewhirst FE, et al. Clinical and
microbiological features of refractory periodontitis subjects.
J Clin. Periodontol 1998; 25: 169.
3. Ciclon P, Crawford L, Grimm WD. Early onset periodontitis
associated with Down’s syndrome—Clinical interventional
study. Ann. Periodontol 1998; 3: 370.
4. Denis F Kinane. Periodontitis modified by systemic factors.
Ann Periodontol 1999; 4: 1 December 1999.
5. Fardalo, Drangsholt E, Olsen I. Palmar plantar keratosis and
unusual periodontal findings. Observations from a family of
4 members. J Clin Periodontol 1998; 25: 181.
6. Haffajee AD, Socransky SS, Dzink JL, et al. Clinical,
microbiological and immunological features of subjects with
refractory periodontal disease. J. Clin. Periodontol 1988; 15:
390.
7. Izumi Y, Sugiyama S, Shinozuka O, et al. Defective neutrophil
chemotaxis in Down’s syndrome patients and its relationship
to periodontal disease. J. Periodontol 1989; 60: 238.
8. Magnusson I, Marks RG, Clark WB, et al. Clinical, micro-
biological and immunological characteristics of subjects
with “refractory” periodontitis. J Clin Periodontol 1991;18:
291.
9. Newman, Takei, Carranza. Clinical periodontology, 9th
edition, WB Saunders.
10. Walker CB, Gordan JM, Magnusson I, et al. A role of antibiotics
in the treatment of refractory periodontitis. J Periodontol 1993;
64: 772.
223
AIDS and the Periodontium
HIV OPPORTUNISTIC INFECTIONS
Most of the opportunistic infections seen in HIV-positive
patients are caused by, protozoan, fungal, or viral
pathogens. This is because the effective immune defence
for these pathogens is the cell-mediated response which
is impaired by HIV infection. Of the various types of
oral lesions found in the HIV-positive population, the
most destructive and problematic are those of bacterial
origin. Bacterial infections seen in HIV-positive patients
include diseases caused by encapsulated or enteric
bacteria such as Campylobacter, Klebsiella, Salmonella
and Streptococcus.
CLASSIFICATION OF PERIODONTAL
DISEASES ASSOCIATED WITH HIV INFECTION
Four distinct disease types are seen,
1. HIV-associated gingivitis (HIV-G): A distinctive linear
inflammation is seen around the gingival margin with
possible punctate erythema extending throughout the
width of the attached gingiva that may occur in the
presence of excellent oral hygiene.
2. HIV-associated periodontitis (HIV-P): Characterized
by rapid loss of attachment, connective tissue
destruction and deep bone pain.
3. HIV-necrotizing gingivitis (HIV-NG).
4. Necrotizing stomatitis (NS): In which spontaneous
sequestration of interdental bone along with extensive
soft tissue necrosis occurs.
Since it was felt that the prefix ‘HIV’ was over-
descriptive and caused potential, ethical and legal
problems with confidentiality, the new classification has
dropped the term HIV from individual disease titles.
1. HIV-G has been changed to linear gingivitis.
2. HIV necrotizing gingivitis has been changed to
necrotizing ulcerative gingivitis (NUG).
3. HIV-P has been changed to necrotizing ulcerative
periodontitis (NUP).
4. Necrotizing stomatitis.
❒
❒
❒
❒
❒ HIV OPPORTUNISTIC INFECTIONS
❒
❒
❒
❒
❒ CLASSIFICATION OF PERIODONTAL
DISEASES ASSOCIATED WITH HIV
INFECTION
• Linear Gingivitis
• Necrotizing Ulcerative Gingivitis
• Necrotizing Ulcerative Periodontitis
• Necrotizing Stomatitis
• CDC Surveillance Care Classification
❒
❒
❒
❒
❒ MOST COMMON ORAL AND
PERIODONTAL MANIFESTATIONS OF
HIV INFECTION
❒
❒
❒
❒
❒ MANAGEMENT
28
AIDS and the
Periodontium
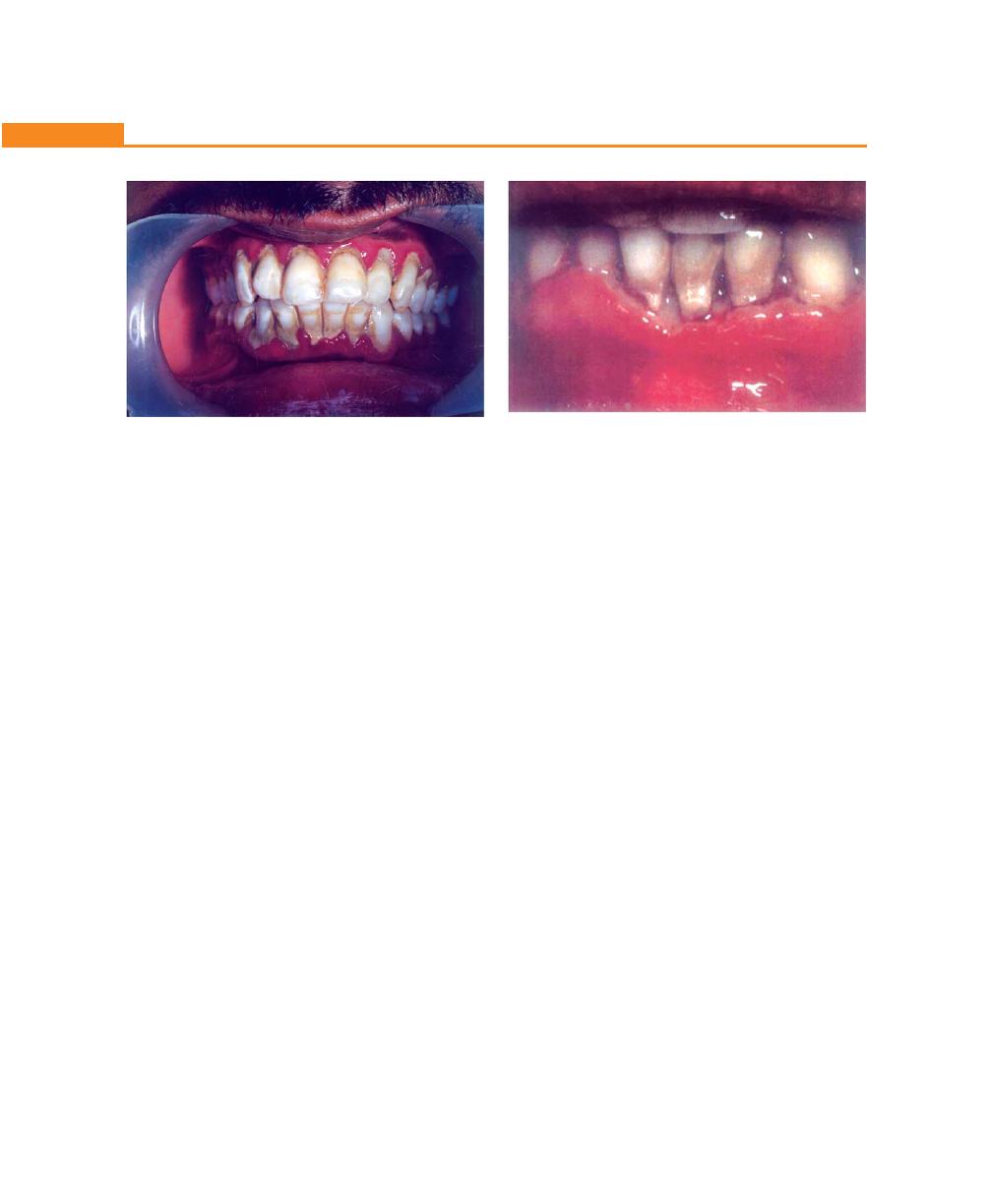
224
Essentials of Clinical Periodontology and Periodontics
Linear Gingivitis (Fig. 28.1)
It is characterized by:
• Marginal linear erythema across the attached gingiva
generally involving all the teeth
• Punctate lesions appear to coalesce giving the entire
gingiva a bright-red appearance
• Spontaneous bleeding or bleeding on probing
• The amount of supragingival plaque is not propor-
tional to the amount of erythema
• No ulceration, no loss of attachment is seen
• Does not respond to the removal of plaque by intensive
scaling, root planing and plaque control measures.
Necrotizing Ulcerative Gingivitis (NUG)
• Sudden onset, bleeding on toothbrushing.
• Pain and characteristic halitosis.
• The gingiva appears fiery-red and swollen and yellow
to grayish necrosis is observed on the tip of the
interdental papilla and margins of the gingiva.
• Mostly anterior gingiva is affected and normally limited
to the soft tissue of the periodontium.
Necrotizing Ulcerative Periodontitis (Fig. 28.2)
• Severe pain, localized soft tissue necrosis,ulceration
and interproximal cratering
• Not associated with deep pocket formation but instead
there is a loss of crestal bone coinciding with soft tissue
destruction
• Rapid horizontal bone loss in the absence of severe
gingival inflammation has been reported
• Tooth mobility is a common feature
• Associated with severe immune suppression with
CD
4+
cell count below 200 cells/mm
3
.
Necrotizing Stomatitis
• Extensive soft tissue and bony necrosis with
sequestration.
• It resembles noma and cancrum oris and represents
the most severe form of periodontal infection seen
in association with HIV.
All of the above mentioned conditions may occur
in isolation or in combination in any one patient. A
common feature of these periodontal diseases which
distinguishes them from conventional periodontal
conditions are, a lack of response to the removal of plaque
and to the patients maintenance of good oral hygiene.
CDC Surveillance Care Classification (1993)
AIDS patients have also been grouped as follows:
Category A: Includes patients with acute symptoms or
asymptomatic diseases, along with individuals with
persistent generalized lymphadenopathy, with or without
malaise, fatigue or low grade fever.
Category B: Patients have symptomatic conditions such
as oropharyngeal or vulvovaginal candidiasis,
Fig. 28.1:
Marginal gingival erythema and early necrosis
of the interdental papilla
Fig. 28.2:
A case of necrotizing ulcerative periodontitis

225
AIDS and the Periodontium
• Erythematous candidiasis
• The hyperplastic candidiasis
• Angular cheilitis
3. Kaposi’s sarcoma: Multifocal, vascular neoplasm
manifest as nodules, papules or non-elevated macules
that are usually brown, blue or purple in color.
4. Bacillary angiomatosis: It is an infectious vascular,
proliferative disease. It appears as red, purple or blue
edematous soft tissue lesions that may cause
destruction of periodontal ligament and bone.
5. Oral hyperpigmentation
6. Atypical ulcers and delayed healing.
MANAGEMENT
Step I: Thorough medical and dental history (should be
kept confidentially).
Step II: Periodontal therapy
Treatment of Linear Gingival Erythema and
Necrotizing Ulcerative Gingivitis
• Medical history and appropriate medical consulta-
tion.
• Scaling of affected areas and oral hygiene instructions.
• Intrasulcular irrigation using 10 percent povidone
iodine.
• 0.12 percent chlorhexidine mouth-rinse twice
daily.
• Antifungal agents like nystatin oral suspension and
clotrimazole.
Disadvantage of oral antifungal agents are, lack of patients
compliance because of strong, sweet flavor and also high
sucrose content in them preventing its use for long term
(risk of rampant tooth decay).
Hence systemic antifungal agents are recommended.
Ketoconazole (may cause liver toxicity), fluconazole–
200 mg tablets once and twice daily (most preferred).
• Follow-up one day and one week post-initial therapy.
• Recall every 4 weeks until periodontal condition is
stable, then every 3 to 6 months.
Oral Lesions Associated with HIV Infection
Group I
Group II
Group III
Oral lesions
Lesions less com-
Lesions seen in HIV
strongly asso-
monly associated
infection
ciated with HIV
with HIV infection
infection
1. Candidiasis
1. Salivary gland
1. Recurrent apthous
2. Hairy leuko-
diseases
stomatitis
plakia
2. Melanotic hyper- 2. Osteomyelitis
3. Non-Hodgkin’s
pigmentation
3. Sinusitis
lymphoma
3. Viral infection
4. Fungal lesions
4. Kaposi’s
4. Bacterial infec-
other than candi-
sarcoma
tions
diasis
5. Periodontal
5. Necrotizing sto-
5. Cytomegalovirus
Diseases
matitis
infection
• LGE: Linear
6. Bacterial infections
gingival
7. Exacerbation of
erythema
apical periodontitis
• NUG:
Necrotizing
ulcerative
gingivitis
• NUP:
Necrotizing
ulcerative
periodontitis
herpes zoster, oral hairy leukoplakia, idiopathic
thrombocyto-penia or constitutional symptoms of fever,
diarrhoea and weight loss.
Category C: Are those with outright AIDS as manifested
by life-threatening conditions identified by C
D4
+T
4
lymphocyte levels of less than 200 cells/mm
3
.
MOST COMMON ORAL AND
PERIODONTAL MANIFESTATIONS OF
HIV INFECTION
1. Oral hairy leukoplakia
• Found on lateral borders of tongue
• Caused by human papilloma virus
• Keratotic, asymptomatic area with vertical striations
giving a corrugated appearance
• When dried appears hairy and does not rub off
2. Oral candidiasis manifested as
• Pseudomembranous (thrush) candidiasis
226
Essentials of Clinical Periodontology and Periodontics
Treatment of Necrotizing Ulcerative Periodontitis
• Medical history
• Scaling of affected areas under local anesthesia
• Remove necrotic bone and soft tissue
• Perform 10 percent povidone iodine irrigation
• Oral hygiene instructions
• 0.12 percent Chlorhexidine mouth-rinse
• Systemic analgesics
• Consider systemic antibiotic such as metronidazole
• Antifungal agents
• Follow-up (1 day to 4 weeks, 1 to 6 months)
Treatment of Necrotizing Stomatitis
Same steps followed + protect lesion with mouth guard
if possible.
KEY POINTS TO NOTE
1. Four distinct types of periodontal diseases associated with
HIV infection are:
a. HIV-associated gingivitis (HIV-G)
b. HIV-associated periodontitis (HIV-P)
c. Necrotizing stomatitis (NS)
d. HIV-necrotizing gingivitis (HIV-NG)
2. Oral lesions associated with HIV infection could be classified
under
Group I: Oral lesions strongly associated with HIV infection.
Group II: Lesions less commonly associated with HIV
infection.
Group III: Lesions seen in HIV infection.
3. Most common oral and periodontal manifestations of HIV
infections are:
a. Oral hairy leukoplakia
b. Oral candidiasis
c. Kaposi’s sarcoma
d. Bacillary angiomatosis
e. Oral hyperpigmentation
f. Atypical ulcers and delayed wound healing
REVIEW QUESTION
1. What are the various periodontal manifestations of
HIV infection?
BIBLIOGRAPHY
1. Jan Lindhe. Clinical periodontology and implant dentistry.
4th edn, Blackwell Munksgaard Publication, 2003.
2. Mark I Ryder. Periodontal considerations in the patients with
HIV. Curropin Periodontol 1993;111-28.
3. Newman, Takei, Fermin A Carranza. Clinical periodontology.
9th edn, WB Saunders Co., 2002.
4. Patrica A, Murray. Periodontal diseases in patients infected
by human immunodeficiency virus. Periodontol 2000;6:
1994.
5. Robert J. Genco, Henry M. Goldman. Contemporary
Periodontics. CV Mosby Company Publication 1990.
6. Robinson P. Periodontal disease and HIV infection. J Clin
Periodontol 1992; 19: 609.
7. Young SCH, Stewart GJ, Cooper DA et al. Progression of
periodontal disease in HIV seropositive patients. J
Periodontol 1993; 64(7): 651.


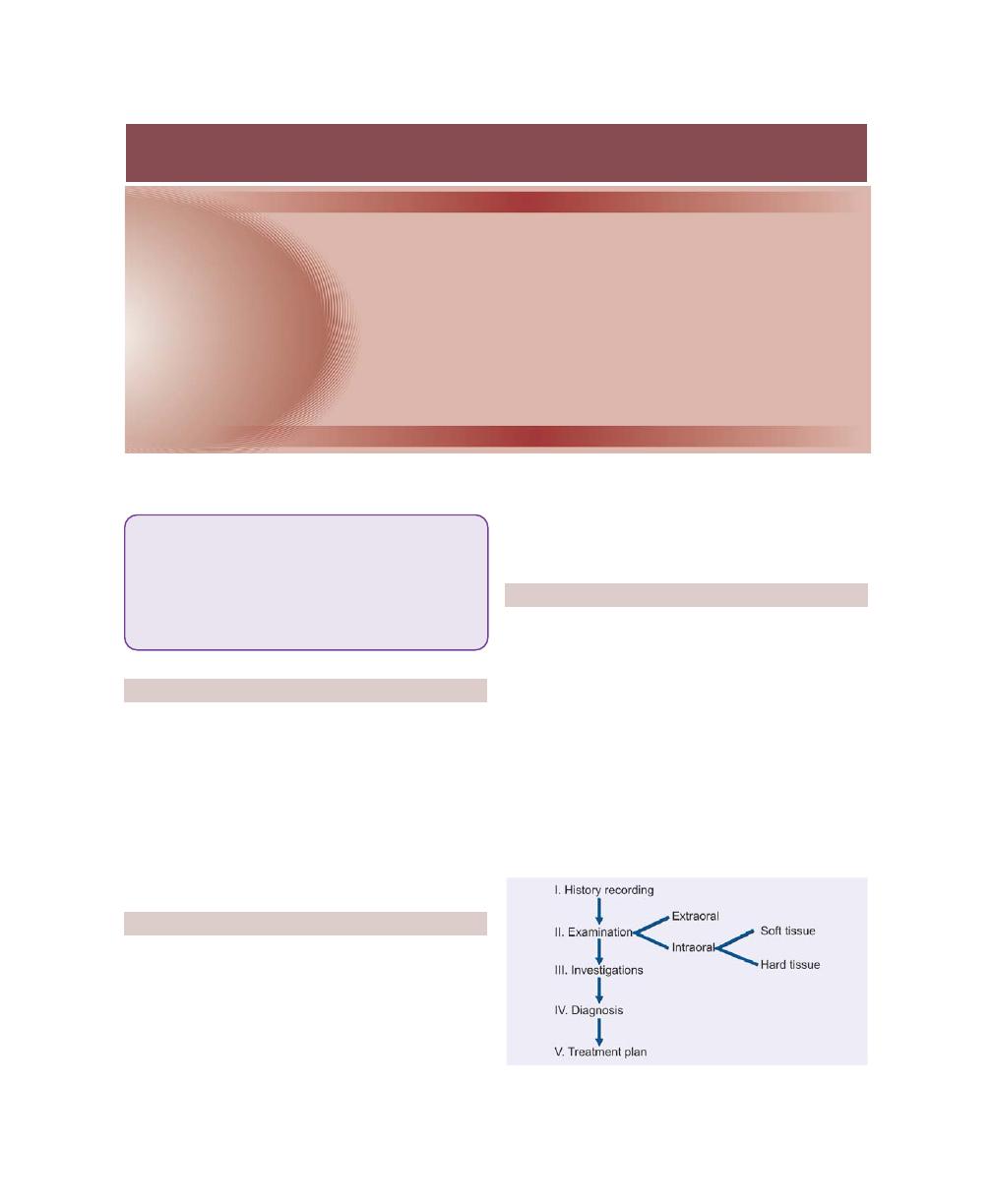
PERIODONTAL DIAGNOSIS
Proper periodontal diagnosis is essential for successful
treatment. Hence diagnosis should first determine
whether disease is present; then identify its type, extent,
distribution and severity and finally provide an
understanding of the underlying pathologic processes
and their cause. Hence, diagnosis may be defined as,
identifying disease from an evaluation of the history, signs
and symptoms, laboratory tests and procedures.
PRINCIPLES OF DIAGNOSIS
This includes:
Sensitivity—refers to the ability of a test or observation
to detect the disease whenever it is present.
Specificity—refers to the ability of a test or observation
to clearly differentiate one disease from another.
Predictive value—refers to the probability of the test
results.
CLINICAL DIAGNOSIS
Periodontal diagnosis is mainly based on careful
analysis of the case history and recording clinical
findings with the help of various aids. One must remember
that the importance should be given to the patient who
has the disease and not simply the disease itself. The
clinical findings are recorded in a systematic manner,
when pieced together should provide a meaningful
explanation.
Key Stages of Periodontal Diagnosis
Section 1: DIAGNOSIS, PROGNOSIS AND TREATMENT PLAN
❒
❒
❒
❒
❒ PERIODONTAL DIAGNOSIS
❒
❒
❒
❒
❒ PRINCIPLES OF DIAGNOSIS
❒
❒
❒
❒
❒ CLINICAL DIAGNOSIS
• Key Stages of Periodontal Diagnosis
• Diagnosis with Detailed Case History
Recording
❒
❒
❒
❒
❒ CASE HISTORY PROFORMA
29
Diagnosis of
Periodontal Diseases
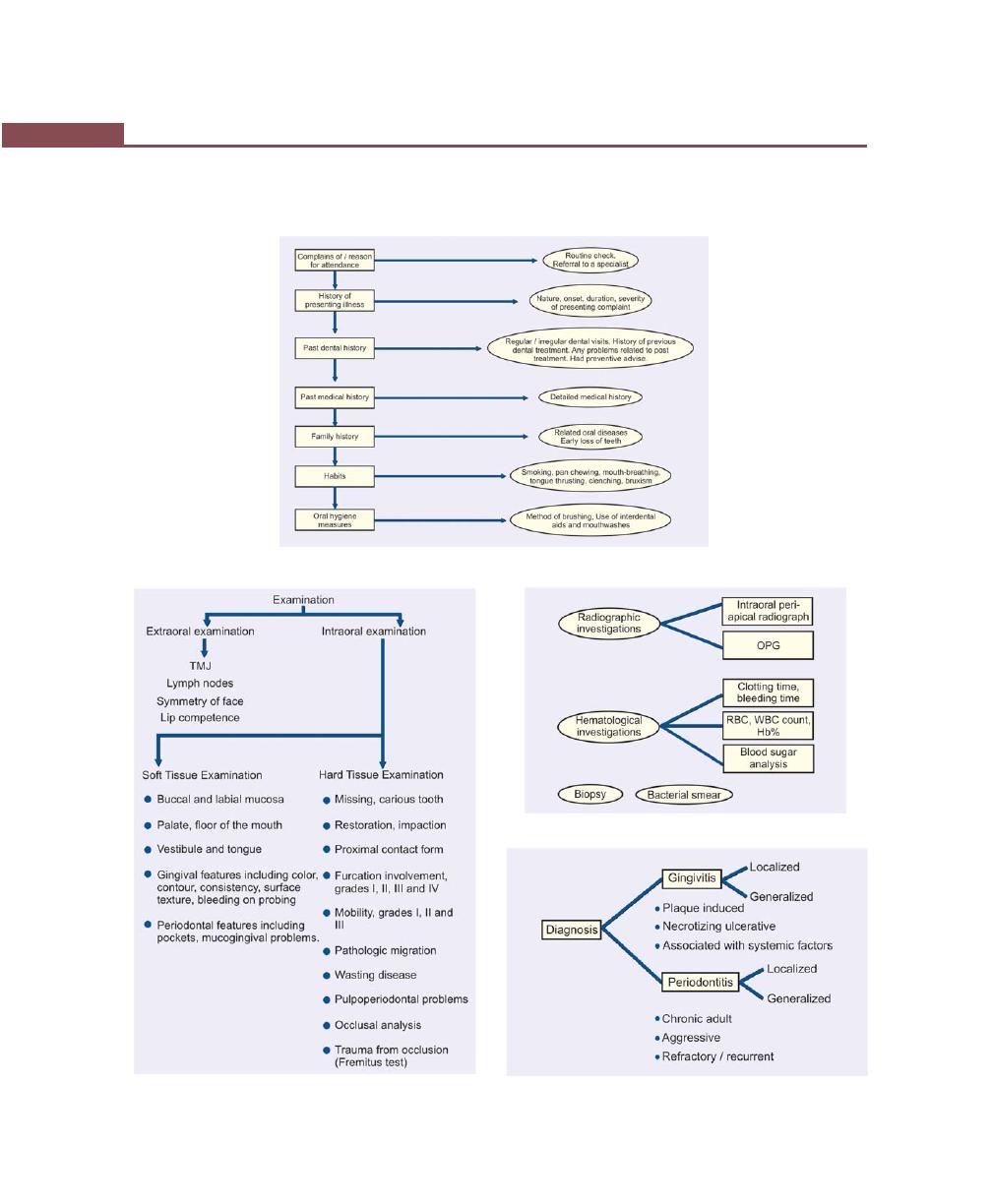
230
Essentials of Clinical Periodontology and Periodontics
Clinical Diagnosis with Detailed Case History Recording
History Recording
Examination
Investigations
Diagnosis
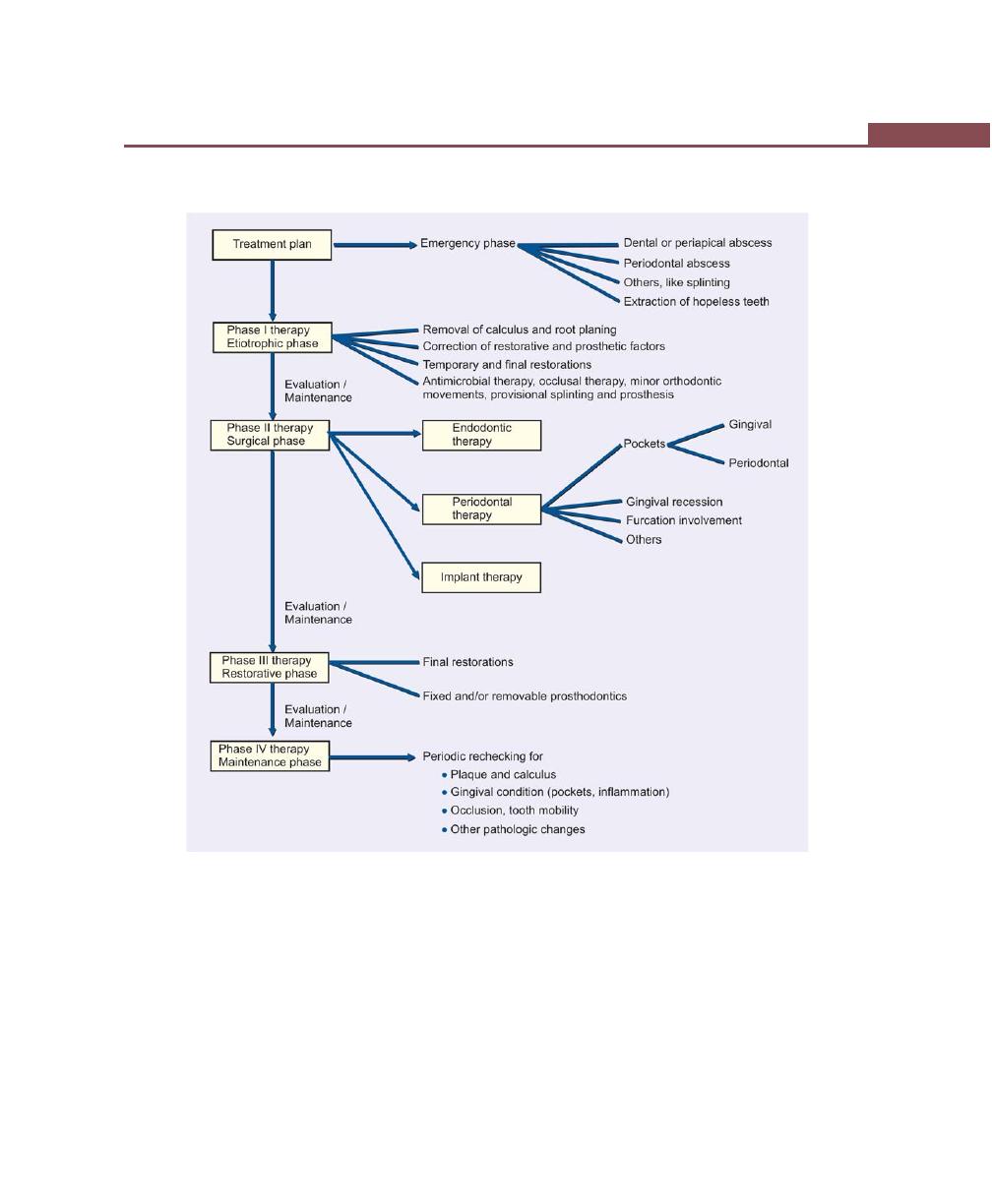
231
Diagnosis of Periodontal Diseases
Treatment Plan

232
Essentials of Clinical Periodontology and Periodontics
CASE HISTORY PROFORMA
Name:
O.P. No:
Sex:
Age:
Address:
Occupation:
Date:
Chief Complaint
History of present illness:
Past dental history:
Medical history:
Family history:
Drug allergy:
Habits:
Clenching
[ ]
Tongue-thrusting
[ ]
Mouth-breathing
[ ]
Pan-chewing
[ ]
Bruxism
[ ]
Cigarette/pipe-smoking
[ ]
Chronic biting of cheek/tongue
[ ]
Tobacco chewing
[ ]
Oral Hygiene Methods
1. Toothbrushing:
Age of brush:
Type of brush:
Methods:
Horizontal
[ ]
Vertical [ ]
Circular
[ ]
Frequency of brushing:
Estimate of time spent:
2. Dental floss
[ ]
3. Tooth picks
[ ]
4. Interproximal brushes
[ ]
5. Mouthwashes
[ ]
6. Others specify
[ ]
Clinical Examination
Extraoral
Symmetry of face:

233
Diagnosis of Periodontal Diseases
T.M.J:
Tenderness:
Clicking:
Jaw deviation:
Lymph nodes:
Submental:
Submandibular:
Cervical:
Intraoral
Lip seal:
Halitosis:
Soft Tissue:
Buccal/labial mucosa:
Palate:
Floor the mouth:
Lips:
Vestibule:
Tongue:
Hard Tissue (Teeth):
Missing teeth:
Carious teeth:
Restored teeth:
Loss of proximal contact:
Crowding of teeth:
Wasting disease:
Attrition:
Abrasion:
Erosion:
Fillings/restorations
Tender on percussion
Pulp/periapical problem:
Pathologic migration
Occlusal Analysis:
Angles classification:
Over-bite and over jet:
Open-bite:
Premature contact:
Cross-bite:
Plunger cusps:
Edge to edge bite:
Prematurities:

234
Essentials of Clinical Periodontology and Periodontics
Gingival Status
Upper right posterior
Upper anterior
Upper left posterior
Color
Contour
Size
Consistency
Stippling
Position
Bleeding on
probing
Exudation
Lower right posterior
Lower anterior
Lower left posterior
Color
Contour
Size
Consistency
Stippling
Position
Bleeding on
probing
Exudation
Investigations
Radiological Investigations (IOPA/OPG)
Horizontal bone loss:
Vertical bone loss:
Furcation involvement:
Endodontic treatment/overhanging restorations:
Periapical pathology
Impacted/supernumerary/embedded teeth:
Caries:
Crown/root-ratio:
Special Investigations
Biopsy:
Bacterial smear:
Other Investigations
Diagnosis
Prognosis
Individual:
Good [ ]
Fair
[ ]
Poor [ ]
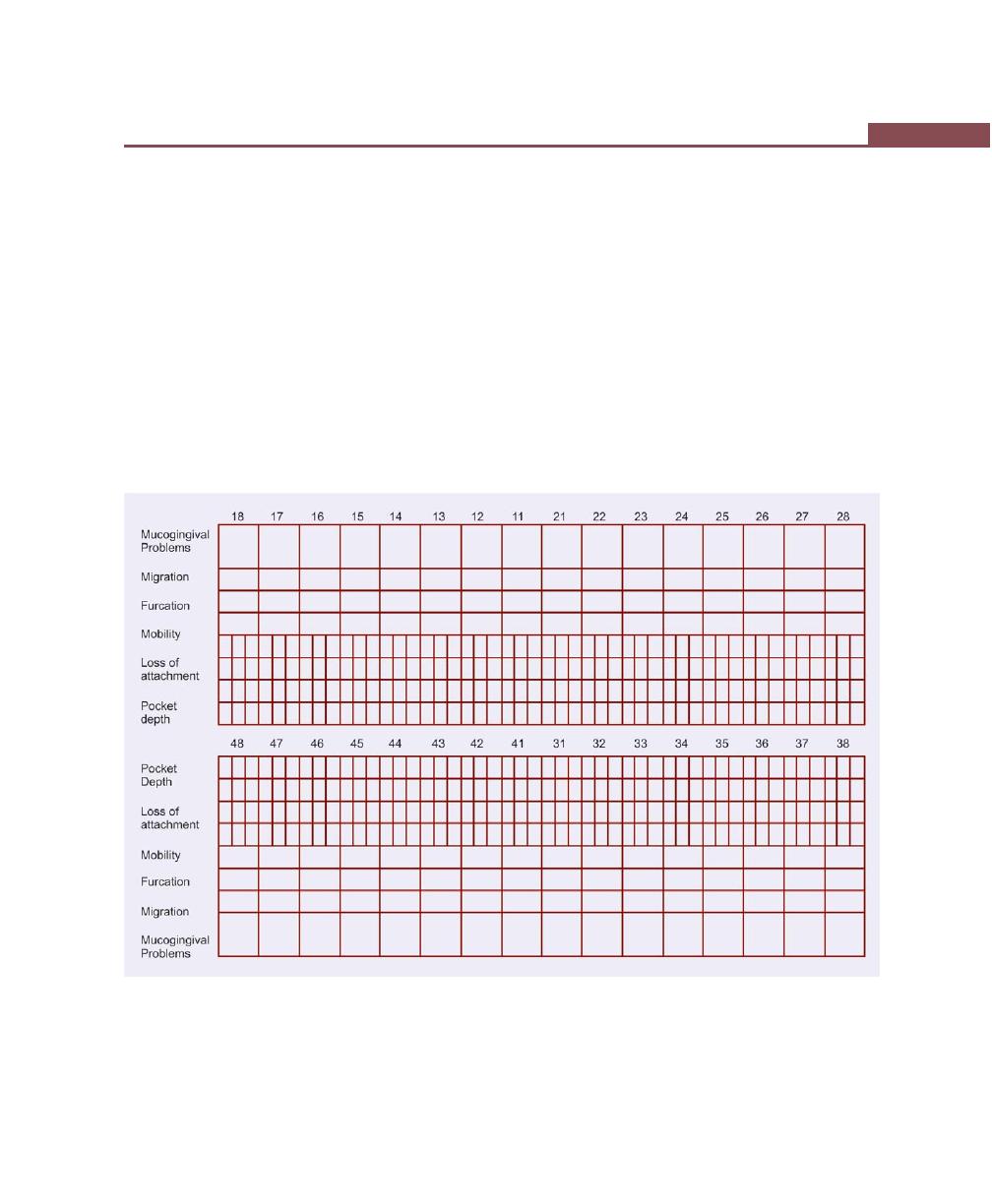
235
Diagnosis of Periodontal Diseases
Overall:
Good [ ]
Fair
[ ]
Poor [ ]
Treatment Plan
Preliminary Phase
Phase I therapy:
Phase II therapy (Surgical phase):
Phase III therapy (Restorative phase):
Phase IV therapy (Maintenance phase):
Surgical Assessment
236
Essentials of Clinical Periodontology and Periodontics
BIBLIOGRAPHY
1. Gary C Armitage. Periodontal Diseases: Diagnosis. Ann
Periodontol 1996;1:37-215.
2. Greenstein G. The role of bleeding upon probing in the
diagnosis of periodontal disease. A literature reivew. J
Periodontol 1984;55:684.
3. Saul Schluger. Periodontal diseases, basic phenomena,
clinical management and occlusal and restorative
interrelationshpis. 2nd edition, Lea and Febiger publication.
4. Thomas G Wilson, Kenneth S Korman, Michael G Newman.
Advances in Periodontics, QB Publishing Company, 1992.
237
Determination of Prognosis
DEFINITION OF PROGNOSIS
It is a prediction of the probable course, duration and
outcome of a disease based on a general knowledge
of the pathogenesis of the disease and the presence of
risk factors for the disease.
It is established after the diagnosis is made and before
the treatment plan is established. Prognosis is often
confused with the term risk. The differences between
prognosis and risk are given in Table 30.1.
There are certain situations where risk factors and
prognostic factors are similar, for example, patients with
diabetes and smokers are more at risk for developing
periodontal diseases, and once they acquire it, they are
considered to have a poor prognosis.
❒
❒
❒
❒
❒ DEFINITION, DIFFERENCES BETWEEN
PROGNOSIS AND RISK
❒
❒
❒
❒
❒ DETERMINATION OF A PROGNOSIS
❒
❒
❒
❒
❒ FACTORS FOR DETERMINATION OF
PROGNOSIS
• Overall Clinical Factors
• Systemic/Environmental Factors
• Local Factors
❒
❒
❒
❒
❒ RELATIONSHIP BETWEEN DIAGNOSIS
AND PROGNOSIS
❒
❒
❒
❒
❒ RE-EVALUATION OF PROGNOSIS AFTER
PHASE I THERAPY
Table 30.1: Differences between prognosis and risk
Prognosis
Risk
i. It is the prediction of the
i. Deals with the likelihood
duration, course and the
that an individual will
termination of disease and
get a disease in a
its response to treatment
specified period.
ii. Prognostic factors are chara-
ii. Risk factors are those
cteristics that predict the
characteristics of an
outcome of disease once the
individual that put
disease is present.
them at increased risk
for getting a disease.
DETERMINATION OF PROGNOSIS
It is based on the factors to be considered while deter-
mining the prognosis. Prognosis may be:
i. Excellent prognosis: No bone loss, excellent
gingival condition, good patient cooperation, no
systemic/environmental factors.
ii. Good prognosis: One or more of the following:
adequate remaining bone support, possibilities to
control etiologic factors and establish a main-
tainable dentition, adequate patient co-operation,
no systemic/ environmental factors or if present
are well-controlled.
iii. Fair prognosis: One or more of the following: less
than adequate remaining bone support, some
tooth mobility, grade I furcation involvement,
30
Determination of
Prognosis
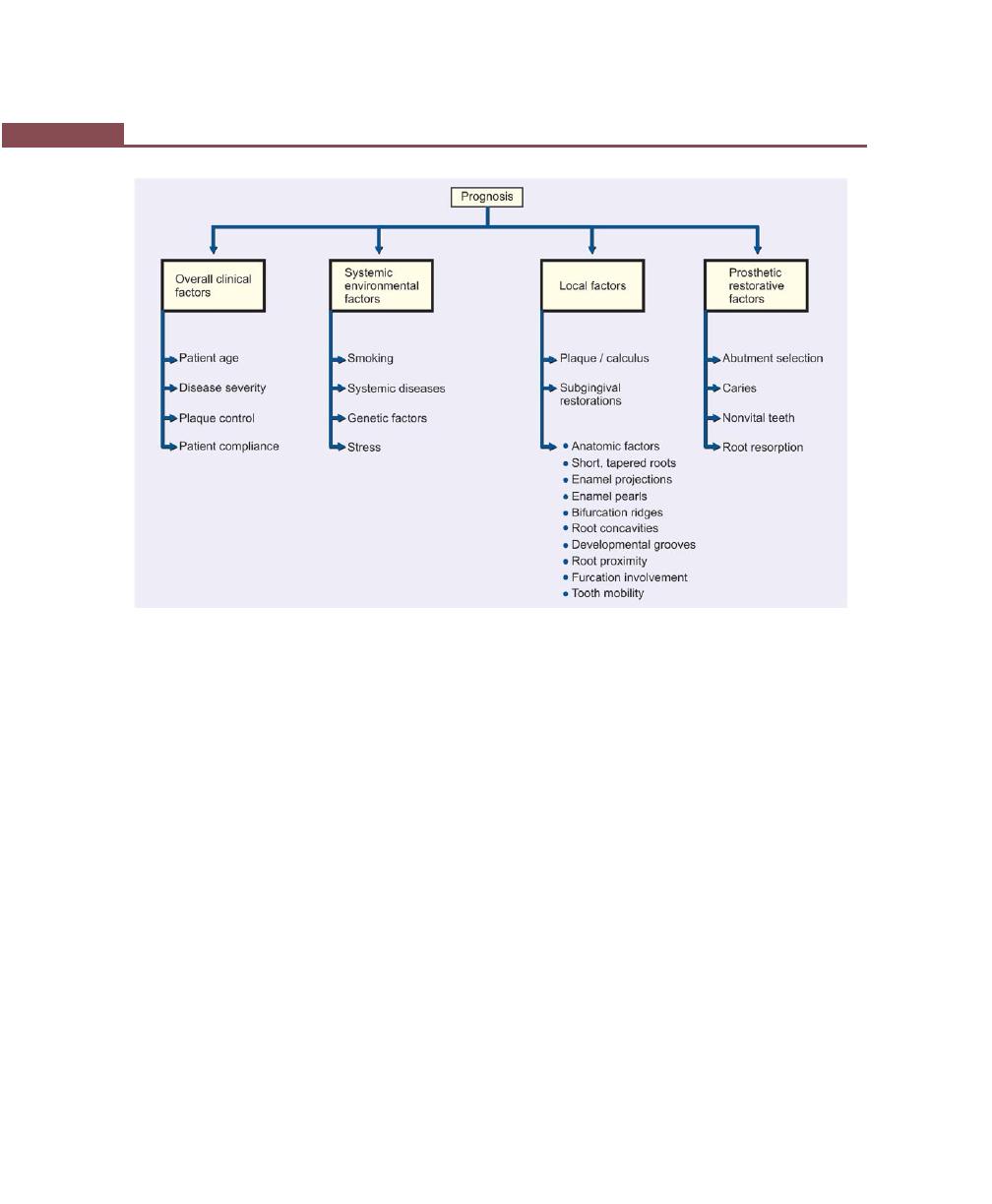
238
Essentials of Clinical Periodontology and Periodontics
Fig. 30.1:
Factors for determination of prognosis
adequate maintenance, acceptable patient co-
operation, presence of limited systemic/environ-
mental factors.
iv. Poor prognosis: One or more of the following:
moderate to advanced bone loss, tooth mobility,
grade I and II furcation involvements, doubtful
patient co-operation, difficult to maintain areas,
presence of systemic/environmental factors.
v. Questionable prognosis: One or more of the
following: advanced bone loss, grade II and III
furcation involvements, tooth mobility, inaccessible
areas, systemic/environmental factors.
vi. Hopeless prognosis: One or more of the following:
advanced bone loss, non-maintainable areas,
extraction indicated, presence of uncontrolled
systemic/environmental factors.
It is advisable to establish a provisional prognosis
until phase I therapy is completed and evaluated. The
provisional prognosis allows initiating treatment of teeth
having a doubtful outlook. The re-evaluation phase
allows to assess the tissue response to scaling, oral hygiene
and root planing and also the use of chemotherapeutic
agents where indicated.
Factors for Determination of Prognosis (Fig. 30.1)
i. Overall clinical factors.
ii. Systemic/Environmental factors
iii. Local factors.
iv. Prosthetic/restorative factors.
Overall Clinical Factors
a. Patient age: In two patients with comparable levels
of remaining connective tissue attachment and
alveolar bone, the prognosis is better in the older
of the two. In younger patient, the prognosis is not
as good because of the increased periodontal
destruction in a shorter time frame. The cause may
be aggressive type of periodontitis or the progression
of disease may be increased due to systemic diseases
or smoking.

239
Determination of Prognosis
b. Disease severity: It is determined by recording the
patients past history of periodontal disease for this
the following variables should be carefully recorded.
i. Pocket depth
ii. Level of attachment.
iii. Degree of bone loss.
iv Type of bony defect.
These can be determined by clinical and
radiographic evaluation.
i. Pocket depth: This is not necessarily related
to bone loss. A tooth with deep pockets and
little attachment and bone loss has a better
prognosis than one with shallow pockets and
severe attachment and bone loss.
ii. Level of attachment: Reveals the approximate
extent of root surface that is devoid of perio-
dontal ligament; the radiographic examination
reveals the amount of root surface still invested
in bone. Prognosis is adversely affected if the
base of the pocket is close to the root apex.
The presence of apical disease as a result of
endodontic involvement also worsens the
prognosis.
iii. Degree of bone loss: The prognosis can also
be related to the height of the remaining bone.
The height of the remaining bone is usually
somewhere in between making bone level
assessment alone insufficient for determining
the overall prognosis.
iv. Type of bony defect: The prognosis for
horizontal bone loss depends upon the height
of the existing bone. The prognosis for angular,
intrabony defects depends upon the contour
of the existing bone and the number of osseous
walls. The chance to regenerate bone in
vertical bony defect is excellent as compared
to horizontal bony defects.
When greater bone loss has occurred on
one surface of a tooth, the bone height on
the less involved surfaces should be considered
when determining the prognosis. Because
greater the height of bone in relation to other
surfaces, the center of rotation of the tooth
will be nearer the crown which results in
favourable distribution of forces to the perio-
dontium and less tooth mobility. No heroic
attempts should be made to retain hopelessly
involved tooth because it may indirectly affect
the health of adjacent teeth.
c. Plaque control: Plaque is the primary etiological factor
for periodontal disease. Therefore effective removal
of plaque is important for the success of periodontal
therapy and to the prognosis.
d. Patient compliance and co-operation: The prognosis
for patients with gingival and periodontal disease is
dependent on patient’s attitude; desire to retain the
natural teeth, willingness and ability to maintain good
oral hygiene. If the patient is unwilling or unable to
perform adequate plaque control and receive periodic
maintenance checkups, the treatments to be
considered are:
1. Refuse to accept the patient for treatment.
2. Extract teeth that have a hopeless or poor
prognosis and perform scaling and root planing
on the remaining teeth.
Systemic/Environmental Factors
a. Smoking: It is the most important environmental risk
factor affecting the development and progression of
periodontal disease. There is a direct relationship
between smoking and the prevalence and incidence
of periodontitis. It even affects the healing potential
of the periodontal tissues. Therefore the prognosis
in patients who smoke and have slight to moderate
periodontitis is generally fair to poor. But with the
cessation of smoking the prognosis may be upgraded
from fair to good in case of slight to moderate perio-
dontitis and poor to fair in case of severe periodontitis.
b. Systemic disease/condition: Studies have shown that
patients with type I and type II diabetes have increased
severity of periodontitis than in those without diabetes.
Prognosis in these cases is dependent on patient

240
Essentials of Clinical Periodontology and Periodontics
compliance relative to both their medical and dental
status well-controlled diabetics.
In patients with uncontrolled diabetics the
prognosis is questionable when surgical periodontal
treatment is required. In well-controlled diabetic
patients with mild to moderate periodontitis, who
comply well to recommended instructions respond
well and hence have good prognosis. Similarly,
patients with incapacitating conditions and other
systemic disorders can affect the progression of disease
hence correction of systemic problem may improve
the prognosis. Newer automated oral hygiene devices
like electric tooth brushes may help patients with
Parkinson’s disease to maintain oral hygiene and
thereby improve their prognosis.
c. Genetic factors: Genetic polymorphisms in the
interleukin –1 (IL-1) genes, resulting in increased
production in IL1–β, have been associated with a
significant increase in risk for severe, generalized,
chronic periodontitis. Genetic factors also appear to
influence serum IgG2 antibody titers and the
expression of Fc-yRII receptors on the neutrophil,
both of which may be significant in aggressive
periodontitis. Other genetic disorders such as
leukocyte adhesion deficiency type I can influence
neutrophil function, creating an additional risk factor
for aggressive periodontitis.
Detection of genetic variations that are linked with
periodontal disease can potentially influence the
prognosis in several ways. First, early detection of
patients at risk due to genetic factors, can lead to
early implementation of preventive and treatment
measures. Second, identification of genetic risk factors
during the course of treatment can influence
treatment recommendations such as the use of
adjunctive antibiotic therapy. Finally, identification
of young individuals who are identified as being at
risk because of the familial aggregation seen in
aggressive periodontitis can lead to the development
of early intervention strategies. Early diagnosis,
intervention and/or alterations in the treatment
regimen may lead to an improved prognosis for the
patient.
d. Stress: Physical and emotional stress as well as
substance abuse alter the patients ability to respond
to periodontal treatment.
Local Factors
a. Plaque/calculus: It is the most important local factor
in periodontal diseases. In most cases having a good
prognosis is dependent on the ability of the patient
and the clinician to remove these etiologic factors.
b. Subgingival restorations: Contribute to increased
plaque accumulation, increased inflammation and
increased bone loss. Overhangs can have negative
impact on the periodontium. The amount of
destruction is mainly dependent upon the size of these
discrepancies and the amount of time they have been
present. In general, a tooth with a discrepancy in its
subgingival margins has a poorer prognosis than a
tooth with well-contoured, supragingival margins.
c. Anatomic variations/factors: Anatomic factor that
predispose the periodontium to disease include short,
tapered roots with large crowns, cervical enamel
projections (CEP) and enamel pearls, intermediate
bifurcation ridges, root concavities and developmental
grooves, root proximity, furcation involvement and
tooth mobility.
Prognosis is poor for teeth with short, tapered-
roots and relatively large crowns because of
disproportionate crown to root ratio and reduced
root surface area available for periodontal support.
The periodontium may be injured by occlusal forces.
Cervical enamel projections (CEP) are flat, ectopic
extensions of enamel that extend beyond the normal
contours of the cemento-enamel junction. They may
also extend into furcations, most likely found on
buccal surfaces of maxillary second molars. Enamel
pearls are larger, round deposits of enamel that can
be found in furcations or other areas of root surface.
They are less frequent than CEP, the presence of these
enamel pearls on the root surface interferes with the

241
Determination of Prognosis
attachment apparatus and may prevent regenerative
procedures from achieving their maximum potential.
Therefore it has a negative effect on the prognosis
of individual teeth.
Scaling and root planing is a fundamental
procedure in periodontal therapy. Anatomic factors
that decrease the efficiency of this procedure have
a negative impact on prognosis. Root concavities
exposed through loss of attachment can vary from
shallow flutings to deep depressions. These concavities
increase the attachment area and produce a root
shape that may be more resistant to torquing forces,
and also create areas that can be difficult for dentist
and patients to clean.
Other anatomic factors that present accessibility
problems include developmental grooves, root proxi-
mity and furcation involvements. The presence of
any of these can worsen the prognosis. The develop-
mental grooves, which initiates on enamel can extend
to a significant distance on the root surface. It provides
plaque-retentive areas that are difficult to clean by
the instrument. Root proximity result in interproximal
areas that are difficult for the clinician and patient
to access. Furcation areas are also difficult to access.
d. Tooth mobility: The principal causes are loss of alveolar
bone, inflammatory changes in the periodontal
ligament and trauma from occlusion. Tooth mobility
caused by inflammation and trauma from occlusion
may be correctable but tooth mobility caused by loss
of alveolar bone is not likely to be corrected. The
stabilization of tooth mobility through the use of
splinting may have a beneficial impact on the overall
and individual tooth prognosis.
Prosthetic/Restorative Factors
The overall prognosis requires general consideration of
bone levels and attachment levels to establish whether
teeth can be saved for functional and aesthetic purposes
or to serve as abutments for prosthesis. When few teeth
remain, the prosthodontic need become more important
and sometimes periodontally treatable teeth may have
to be extracted if they are not compatible with the design
of the prosthesis. Teeth that serve as abutments are
subjected to increased functional demands. A tooth that
has undergone endodontic treatment post is more likely
to fracture when serving as a distal abutment supporting
a distal removable partial denture. Special oral hygiene
measures should be instituted in these areas.
Caries, non-vital teeth and root resorption: Teeth with
extensive caries should be adequately restored and
endodontic therapy should be considered before under-
taking periodontal treatment. Extensive idiopathic root
resorption or root resorption as a result of orthodontic
therapy, jeopardises the stability of teeth and adversely
affects the response to periodontal treatment. The
periodontal prognosis of treated non-vital teeth is not
different from that of vital teeth. New attachment can
occur to cementum of both non-vital and vital teeth.
RELATIONSHIP BETWEEN DIAGNOSIS
AND PROGNOSIS
Factors such as patients age, severity of disease, genetic
susceptibility and presence of systemic disease are all
important criteria in the diagnosis of the condition. These
are also important in developing a prognosis (Table 30.2).
Prognosis for Patients with Gingival Disease
Dental Plaque-induced Gingival Disease
a. Gingivitis associated with dental plaque only: The
prognosis for patients with gingivitis associated with
only dental plaque is good, provided all local irritants
are eliminated i.e. other local factors contributing to
Table 30.2: Distinction between condition and prognosis
Condition
Prognosis
i. Dermatological disorders
It is linked to manage-
like lichen planus, pemphi-
ment of the associated
goid, pemphigus vulgaris,
dermatologic conditions.
erythema multiforme, lupus
erythematosis
ii. Allergic, toxic and foreign
body reactions, Mechanical
and thermal trauma.
242
Essentials of Clinical Periodontology and Periodontics
plaque retention are eliminated, gingival contours
conducive for the preservation of health are attained
and the patient co-operates by maintaining good oral
hygiene.
b. Plaque-induced gingival diseases modified by systemic
factors: The systemic factors which influence the
inflammatory response to bacterial plaque include
endocrine related changes associated with puberty,
menstruation, pregnancy and diabetes and the
presence of blood dyscrasias, prognosis for these
patients depends on not only control of bacterial plaque
but also on control or correction of the systemic factors.
c. Plaque-induced gingival diseases modified by
medications: Drug-influenced gingival enlargement
is often seen with phenytoin, cyclosporin, nifedipine
and oral contraceptive-associated gingivitis. Plaque
control alone does not prevent the development of
the lesions and surgical intervention is usually necessary
to correct the alterations in gingival contour. Continued
use of the drug causes recurrences of the enlargement
even following surgical intervention. Therefore
prognosis is dependant on whether the patient’s
systemic problem can be treated with an alternative
medication. In oral contraceptive-associated gingivitis,
frank signs of gingival inflammation can be seen in
the presence of relatively little plaque. Prognosis in
these patients is dependant not only on the control
of dental plaque, but also on the likelihood of
continued use of the oral contraceptive.
d. Gingival diseases modified by malnutrition: Most of
the clinical studies have disproved the importance
of malnutrition in developing gingival disease.
Prognosis in these cases may be dependant on the
severity and duration of deficiency and on the
likelihood of reversing the deficiency through dietary
supplementation.
Non-plaque-induced Gingival Lesion
It can be seen in patients with a variety of bacterial,
fungal and viral infections. Prognosis is dependant
on elimination of the source of infectious agent.
Prognosis for Patients with Periodontitis
See Table 30.3.
Table 30.3: Distinction between chronic and
aggressive periodontitis
Chronic periodontitis
Aggressive periodontitis
i. Slowly progressive
i. Rapidly progressive
disease associated with
disease with minimal/no
local environmental
local factors with incre-
factors.
ased level of Aa and P
gingivalis.
ii. Can be localized or
ii. Can be localized or
generalized.
generalized
iii. In case of not advanced
iii. Two common features are
attachment loss prognosis
observed.
is generally good. But
a. Rapid attachment loss
inflammation has to be
and bone destruction in
controlled through good
an otherwise clinically
oral hygiene and removal
healthy person.
of local plaque-retentive
b. A familial aggregation.
factors.
iv. In cases of severe disease
iv. Localized type, which
with furcations involve-
occurs around the age
ment and increasing
of puberty and is loca-
clinical mobility/who are
lized to first molars and
non-compliant with oral
incisors, if diagnosed
hygiene, prognosis is
early can be treated con-
down graded from fair
servatively with oral
to poor.
hygiene instructions and
systemic antibiotic thera-
py. Resulting in excellent
prognosis. In advanced
cases, the prognosis is
still good if the lesions
are treated with debri-
dement, local and syste-
mic antibiotics and rege-
rative therapy.
v. In generalized type, also
seen in young patients
with generalized inter-
proximal attachment loss
and poor antibody res-
ponse. Secondarily aggra-
vated by cigarette smok-
ing does not respond well
to conventional periodo-
ntal therapy therefore
prognosis is often fair,
poor or questionable and
the use of systemic anti-
biotics should be consi-
dered.
243
Determination of Prognosis
Periodontitis as a Manifestation
of Systemic Disease
It can be divided into two categories:
i. Associated with hematological disorders such as
leukemia and acquired neutropenias
ii. Associated with genetic disorders such as familial
and cyclic neutropenia, Down’s syndrome,
hypophosphatasia, Papillon-Lefévre syndrome.
In both these cases prognosis may be fair to
poor and is mainly dependant on the treatment
of systemic disease.
Necrotizing Periodontal Disease
Necrotizing periodontal disease can be divided into
necrotic diseases that affect the gingival tissues [NUG]
and necrotic diseases that affect deeper tissues of the
periodontium, resulting in loss of connective tissue
attachment and alveolar bone [NUP]. In these [NUG]
cases the predisposing factor is bacterial plaque. The
disease is further complicated by both presence of
secondary factors such as acute psychological stress,
tobacco smoking and poor nutrition, all of which can
contribute to immunosuppression. With the control of
both the bacterial plaque and the secondary factors, the
prognosis is good.
In necrotizing ulcerative periodontitis cases the
treatment is not only reducing local and secondary factors
but also in dealing with the systemic problem. Prognosis
depends on management of disease.
RE-EVALUATION OF PROGNOSIS
AFTER PHASE I THERAPY
A frank reduction in pocket depth and inflammation after
phase I therapy indicates favourable response to
treatment and is suggestive of a better prognosis and
in vice versa cases the overall prognosis may be
unfavourable.
KEY POINTS TO NOTE
1. Prognosis is defined as the prediction of the probable
course, duration and outcome of a disease based on a
general knowledge of the pathogenesis of the disease and
the presence of risk factors for the disease.
2. Prognosis is established after the diagnosis and before the
treatment plan.
3. Prognosis may be excellent, good, fair, poor, questionable,
and hopeless.
4. Prognosis can be divided into, overall prognosis and
individual tooth prognosis.
5. Factors that are to be considered while determining the
prognosis are:
a. Overall clinical factors like age of the patient, disease
severity, systemic restorative and environmental factors
and local factors, patient compliance and prosthetic
possibilities.
b. Factors influencing individual tooth prognosis are,
plaque/calculus, subgingival restorations, caries, non-
vital teeth and root resorption.
REVIEW QUESTIONS
1. Define prognosis and describe the factors influencing
overall prognosis.
2. Describe the factors to be considered for determining
prognosis.
BIBLIOGRAPHY
1. Grant, Stern. Listgarten Periodontics. 6th edn. Mosby
publication, 1988.
2. JD Manson and BM. Eley Outline of Periodontics. 3rd edn,
British Library Cataloguing in Publication Data, 1995.
3. MC Guire MK. Prognosis versus actual outcome: a long term
survey of two related periodontal patients under maintenance
care. J Periodontol 1991:62:51.
4. MC Guire MK, Nunn ME. Prognosis versus actual outcome
III. The effectiveness of clinical parameters in accurately
predicting tooth survival. J Periodontol 1996; 67:666.
5. MC Guire MK, Nunn ME. Prognosis versus actual outcome
IV. The effectiveness of clinical parameters and IL-1 genotype
in accurately predicting prognosis and tooth survival.
J Periodontol 1999; 70-49.
6. Newman, Takei, Fermin A Carranza. Clinical Periodontology.
Ninth edition, 2002, W.B.Saunders Co.
244
Essentials of Clinical Periodontology and Periodontics
DEFINITION
Risk is the probability that an individual will get a specific
disease in a given period which may vary from one
individual to other. Risk assessment involves identifying
elements that either may predispose a patient to
developing periodontal disease or may influence
progression of disease that already exists.
RISK FACTORS FOR PERIODONTAL DISEASE
Risk factors may be environmental, behavioural or
biologic factors that when present increases the likelihood
that an individual will get the disease. Following are the
risk factors:
1. Tobacco smoking: A direct relationship exists between
smoking and prevalence of periodontal disease.
Smoking has a negative impact on response to
therapy.
2. Diabetes: Diabetes is a risk factor for periodontitis.
Prevalence and severity is higher in diabetes than in
those without diabetes.
3. Pathogenic bacteria and microbial tooth deposits:
• Quantity of plaque present on teeth is not of major
importance. Composition or quality of the plaque
biofilm is of importance.
• In terms of plaque, three specific bacteria have
been identified as etiologic agents:
a. Actinobacillus actinomycetemcomitans
b. Porphyromonas gingivalis
c. Bacteroides forsythus
Anatomic Factors
Such as furcations, root concavities, developmental
grooves, cervical enamel projections, enamel pearls and
bifurcation ridges. All predispose to periodontitis, as they
harbour bacterial plaque and present a challenge to
clinician during instrumentation.
Presence of calculus: It serves as a reservoir for bacterial
plaque.
❒
❒
❒
❒
❒ DEFINITION
❒
❒
❒
❒
❒ RISK FACTORS
❒
❒
❒
❒
❒ RISK DETERMINANTS/BACKGROUND
CHARACTERISTICS
❒
❒
❒
❒
❒ RISK INDICATORS
❒
❒
❒
❒
❒ RISK MARKERS/PREDICTORS
❒
❒
❒
❒
❒ CLINICAL RISK ASSESSMENT
• Demographic Data
• Medical History
• Dental History
• Clinical Examination
31
Related Risk Factors
Associated with
Periodontal Diseases

245
Related Risk Factors Associated with Periodontal Diseases
RISK DETERMINANTS/BACKGROUND
CHARACTERISTICS FOR PERIODONTAL
DISEASE
It is defined as those risk factors that can not be
modified.
1. Genetic factors: Genetic factors influence clinical
measures of gingivitis probing pocket depth,
attachment loss and interproximal bone height.
• A specific interleukin-1 (IL-1) genotype has been
associated with severe chronic periodontitis.
• Immunologic alterations such as neutrophil
abnormalities are under genetic control.
• Genetics plays a role in regulating the titre of
protective IgG
2
antibody response to A. actino-
mycetemcomitans in patients with aggressive
periodontitis.
2. Age:
• Both the prevalence and severity of periodontal
disease increases with age.
• Attachment loss and bone loss seen in elderly
individuals is a result of prolonged exposure to
other risk factors over a longer period of time.
• Changes related to old age such as intake of
medications, decreased immune functions and
altered nutritional status may increase susceptibility
to periodontitis.
3. Gender:
• Males have more attachment loss than females
and have a poorer oral hygiene therefore more
males are prone to periodontal diseases.
4. Socio-economic status:
• Poor oral health is seen in lower socio-economic
status. This can be attributed to:
1. Decreased dental awareness
2. Decreased dental visits.
5. Stress: Incidence of ANUG increases during stressful
situations.
• Emotional stress may interfere with normal
immune function.
• There is an apparent link between psychosocial
factors and risk behavior such as smoking, poor
oral hygiene and chronic periodontitis.
• Individuals with financial strain, distress, depression
and inadequate coping mechanisms have more
loss of attachment.
RISK INDICATORS FOR
PERIODONTAL DISEASE
Risk indicators are probable or putative risk factors that
have been identified in cross-sectional studies but have
not been confirmed through longitudinal studies.
Following are the risk indicators:
1. HIV / acquired immunodeficiency syndrome: It has
been hypothesized that immune dysfunction asso-
ciated with high HIV infection and AIDS increases
the susceptibility to periodontal disease, though the
evidence is not conclusive.
2. Osteoporosis: Osteoporosis does not by itself initiate
periodontitis, there is reduced bone mass in
osteoporosis, this may enhance the progression of
periodontal disease.
3. Infrequent dental visits: Some studies have shown
increased risk for severe periodontitis in patients who
have not visited dentist for 3 or more years (although
age factor also plays a role).
RISK MARKERS/PREDICTORS
These are associated with increased risk for disease, but
do not cause the disease. These factors also are identified
in cross-sectional and longitudinal studies. A risk factor
that can be used to predict the future course of disease,
is known as a risk marker.
1. Previous history of periodontal disease.
2. Bleeding on probing: Bleeding on probing along with
increased pocket depth may serve as an excellent
predictor for future loss of attachment.
246
Essentials of Clinical Periodontology and Periodontics
CLINICAL RISK ASSESSMENT FOR
PERIODONTAL DISEASE
It is done by careful evaluation of the following:
Demographic Data
Age:
• Duration of exposure to the risk elements.
• Postmenopausal women
• Evidence of aggressive disease
Gender:
• Males
• Frequency of care and preventive practices.
Socio-economic status:
• Dental awareness
• Frequency of care
Medical History
The following conditions either predispose or make the
patient more susceptible to periodontitis.
• Diabetes
• Tobacco smoking
• HIV/AIDS
• Osteoporosis
• Stress
Dental History
• Genetic predisposition to aggressive disease.
• Previous history of periodontal disease.
• Frequency of dental care.
Clinical Examination
• Plaque accumulation
• Calculus
• Bleeding on probing
• Extent of loss of attachment
• Tooth examination
– Plaque retentive areas
– Anatomic factors
• Restorative factors
• Once a risk patient is identified and a diagnosis is
made, treatment plan may be modified accordingly.
KEY POINTS TO NOTE
1. Risk factors may be environmental, behavioural or biologic
in nature that when present increases the likelihood of a
disease, e.g. smoking, diabetes, presence of pathogenic
bacteria.
2. Risk determinants are those risk factors that cannot be
modified, e.g. age, gender, socio-economic status, stress.
3. Risk indicators are probable or putative risk factors that have
been identified in cross-sectional studies but not confirmed
through longitudinal studies, e.g. AIDS, osteoporosis,
infrequent dental visits.
4. A risk factor that can be used to predict the future course
of disease, is known a risk marker, e.g. previous history of
periodontitis, bleeding on probing.
5. In conclusion, risk assessment may have a role at two levels,
one involving identification of factors that may predispose
to developing periodontal disease secondly, may influence
the progression of already existing disease.
REVIEW QUESTIONS
1. Define risk, risk factors, risk indicators, risk markers
and risk determinants.
2. Enumerate various risk factors for periodontal
disease.
BIBLIOGRAPHY
1. Blieden TM. Tooth related issues. Ann Periodontol
1999;4:91-6.
2. Carranza, Newman, Takei. Clinical Periodontology, 9th edn,
WB Saunders Co., 2002.
3. Robert J Genco. Current view of risk factors for periodontal
diseases. J. of Periodontol 1996;67:935-45.
247
Various Aids Including Advanced Diagnostic Techniques
c. Measurement of temperature by pressure-sensitive
probes.
d. Mouth odors—Olfactometer
e. Tooth mobility—Mobilometer/Periodontometer
f. PSR (Periodontal screening and recording).
Periodontal Probes
Uses of Probes
1. Periodontal probes are used to measure the depth.
2. Quantification of bacterial plaque and gingival
inflammation.
3. Determination of mucogingival relationship.
4. Measurement of gingival recession.
5. Location of calculus.
6. Identification of tooth irregularities.
7. Identification of tissue characteristics.
8. Determination of bleeding tendency.
9. Evaluation of bone support in the furcation areas
of bifurcated and trifurcated teeth.
Types of Conventional Periodontal Probes (Fig.
32.1)
1. Marquis color coded probe: Calibrations are in 3 mm
sections
2. The UNC–15 probe: 15 mm long and markings are
at each mm and color coding at the 5th, 10th and
❒
❒
❒
❒
❒ A
IDS
USED IN CLINICAL DIAGNOSIS
• Periodontal Probes
• Conventional Probes
• PSR
❒
❒
❒
❒
❒ A
IDS
USED IN RADIOGRAPHIC DIAGNOSIS
• Orthopantomograph
• Xeroradiography
• Advanced Radiographic Techniques
❒
❒
❒
❒
❒ A
IDS
IN MICROBIOLOGICAL DIAGNOSIS
• Identification of Bacteria
• Speciation Techniques
❒
❒
❒
❒
❒ A
IDS
IN IMMUNOLOGICAL DIAGNOSIS
• Immunofluorescence
• Latex Agglutination
• ELISA
• Flow Cytometry
❒
❒
❒
❒
❒ BIOCHEMICAL DIAGNOSIS
• Prostaglandins
• Collagenase
❒
❒
❒
❒
❒ OTHER DIAGNOSTIC AIDS
• BANA Test
• FSEIA
• PCR
A
IDS
USED IN CLINICAL DIAGNOSIS
a. Millimeter probe for gingival bleeding.
b. Measurement of gingival crevicular fluid flow with
the help of a filter paper. Newer method is by use
of a periotron 6000.
32
Various Aids Including
Advanced Diagnostic
Techniques

248
Essentials of Clinical Periodontology and Periodontics
15 mm.
3. The University of Michigan 0 probe with Williams
markings (at 1, 2, 3, 5, 7, 8, 9) 4 and 6 missing.
4. The Michigan ‘0’ probe with markings at 3, 6, and
8 mm.
5. The WHO probe, which has a 0.5 mm ball at the
tip and millimeter markings at 3.5, 8.5 and 11.5 mm
and color coding from 3.5 to 5.5. mm.
6. Furcation areas can best be evaluated with the
curved, blunt Naber’s probe.
Periodontal probes may be divided into:
First generation probes are conventional, hand held
probes, e.g. conventional periodontal probes.
Second generation probes are pressure-sensitive probes.
It has been shown that, with forces upto 30 grams the
probe tip remains within junctional epithelium and forces
upto 50 grams are necessary to diagnose osseous defects.
This probe did solve many of the problems of the
conventional probes, but lacked tactile sensitivity.
Third generation probes are computerized probes. Gibbs
et al designed Florida probe. Other examples are—Foster
Miller probe, Toronto automated probes,which can detect
the cementoenamel junction.
Limitations of Conventional Probes
1. Probing depth obtained with periodontal probe does
not coincide with the histological pocket depth,
because the probe normally penetrates the coronal
level of the junctional epithelium.
2. Another limitation is related to the reproducibility,
which has been correlated with the variation in the
probing force.
Other factors that are likely to influence clinical
measurement of attachment level include intra and
interexaminer reliability, patient discomfort, accuracy
of probe markings and anatomical variations in tooth
contours or position.
Limitations of all Automated Controlled Force Probes
1. Reduced tactile sense of the operator.
2. Increased patient discomfort.
3. Presence or absence of inflammation often produced
inaccurate measurement.
PSR (Periodontal Screening and Recording)
It is designed for easier and faster screening and recording
of the periodontal status of a patient or a group of
population. It uses a specially designed probe that has
a 0.5 mm ball tip and is color-coded from 3.5 to 5.5
mm.
The patient’s mouth is divided into six sextants
maxillary right quadrant, left quadrant and anteriors,
mandibular left and right quadrants and anteriors, and
at least six points around each tooth is examined.
The deepest finding is recorded in each sextant,
according to the following code.
Code 0: In the deepest sulcus of the sextant, the probes
coloured band remains completely visible, gingival tissue
is healthy and does not bleed on gentle probing. No
calculus or defective margins are found. These patients
require only appropriate preventive care.
Code 1: The colored band remains completely visible
in the deepest sulcus of the sextant. No calculus or
defective margins are found but some bleeding after
gentle probing is found. Treatment for these patients
include subgingival plaque removal and appropriate oral
hygiene instructions.
Code 2: The probe’s colored band is still completely
Fig. 32.1:
Florida probe

249
Various Aids Including Advanced Diagnostic Techniques
visible, but there is bleeding on probing. Supragingival
or subgingival calculus and/or defective margins are
found. Treatment includes plaque and calculus removal,
correction of plaque retentive margins of restorations
and oral hygiene instructions.
Code 3: The colored band is partially-submerged. This
indicates the need for a comprehensive periodontal
examination and charting of the affected sextant to
determine the necessary treatment plan. If two or more
sextants score code 3, a comprehensive full mouth
examination and charting is indicated.
Code 4: The colored band completely disappears in the
pocket, indicating a depth greater than 5.5 millimeters.
In this case a comprehensive full-mouth periodontal
examination, charting and treatment planning are
needed.
Code*: When any of the abnormalities are seen, an
asterisk (*) is entered, in addition to the code number,
(for example furcation involvement, tooth mobility,
mucogingival problem or gingival recession extending
to the colored band of the probe).
A
IDS
USED IN RADIOGRAPHIC DIAGNOSIS
Radiographs are used to obtain a visual image of the
bone support around a tooth or dental implant. The
radiographic image is the result of X- ray passing through
the area of interest and exposing the silver halide emulsion
on the radiographic film.
The most commonly used radiographs in periodontal
diagnosis are transmission radiographs. Transmission
radiographs, including periapical and bite-wing films are
used to detect the amount of bone loss in any type of
periodontitis. They should be used with less exposure
to avoid any burn-out effect.
Though there are many advantages of radiographs
there is also equal number of disadvantages:
1. Thirty to sixty percent of the mineral content of the
bone must be lost to visualize the change in the
radiographic image, hence though very specific, lacks
sensitivity.
2. Actual damage is more extensive than radiographs.
3. Radiographs are a two-dimensional representation
of a three-dimensional anatomy.
Techniques are available to minimize this source of
distortion. First a long cone should be used (because
parallel rays will minimize distortion).
Secondly, the use of parallel positioning devices helps
to standardize the relationship between film, object and
X-ray source.
Disadvantage: Only a limited view of the osseous crest
is available. Hence the use of extended cone projection
instruments are recommended.
Bite-wing: It is an often forgotten radiograph in
periodontal diagnosis.
Orthopantamograph
When compared with I.O.P.A. radiography, they have
a tendency to under estimate minor bone changes, and
also, magnification, unsharpness; distortion of image may
be seen.
Xeroradiography
It does not involve wet chemical processing or the use
of a dark room. Instead of X-ray film, xeroradiography
uses a uniformly charged selenium plate held in a light
tight cassette. Exposure to X- irradiation and adequate
processing produces a real image on opaque paper,
which is viewed by reflected light.
Advantages: Less expensive, edge enhancement
Advanced Radiographic Techniques
Techniques have been developed to enhance the ability
to “see” small changes overtime in the bone. They are:
Iodine–125 Absorptiometry
A non-radiographic method to analyze the periodontal
bone mass changes. It is based on the absorption by
bone of a low energy gamma beam, originating from
a radioactive source of 125–1, this method has shown
to measure bone changes with a high degree of accuracy
250
Essentials of Clinical Periodontology and Periodontics
and precision.
Disadvantage: Technical considerations limit the use of
this system on posterior sites. To overcome this,
photodensitometric analysis has been developed.
Photodensitometric Analysis
A beam of light is passed onto the radiographic film and
the image is shown on an aluminium scale and then
it transforms the density readings into millimeter of
aluminium equivalents. It is mainly developed to evaluate
bone resorption especially in furcation areas. This
technique mainly enables the clinician to detect the
variations in the bone density that cannot be detected
by visual inspection.
Digital Radiography
This is useful in detecting small changes in hard tissues
that occur between examinations. The purpose of digital
subtraction radiography is to remove all unchanging
structures from a set of two films and to display only
the area of changes in periodontal defects.
Subtraction Radiography
Two radiographs are taken and the changes are noted
depending on their gray levels.
Digital Subtraction Radiography
Digitization is done before subtraction, i.e. serial
radiographs are converted into digital images. These
images are superimposed and are used on a video
screen. Light areas indicate bone gain and dark areas
indicate bone loss.
CADIA (Computer Assisted Densitometric
Image Analysis)
In this technique, parallelization errors can also be
corrected and values of difference are shown between
two X-rays. A video camera measures the light transmitted
through a radiograph and the signals from the camera
are converted into gray levels. The images can be stored
in the computer.
Computerized Tomography
Unlike conventional radiography, which is a two-
dimensional representation of a three-dimensional object.
Computed tomography gives an exact picture of the
bone levels in coronal, axial and sagittal plane by which
all the osseous defects can be visualized accurately.
Nuclear Medicine Bone Scan
This involves the detection of changes in bone meta-
bolism—hence can detect the earliest stage of bone loss.
A bone seeking radiopharmaceutical diphosphonate
compound is injected intravenously and after a waiting
period the uptake by the bone is measured by the
semiconductor probe radiation detector. This technique
has the ability to detect bone changes before structural
alterations occur.
A
IDS
IN MICROBIOLOGICAL DIAGNOSIS
It is based on the concept of bacterial specificity. These
microbiological tests may have the potential not only
to diagnose various forms of periodontal diseases but
also to determine the sites which are at a higher risk
of undergoing active destruction.
Predictive treatment model: It is a combination of clinical
and microbiological parameters to predictably
recommend specific therapy.
Microbiology and Disease Progression
Because bacteria are the causative agents in periodontal
diseases it makes sense to look for specific bacteria as
indicators of disease activity.
Identification of Bacteria
1. Direct examination—Microscopy
• Light
• Dark field
2. Culture and sensitivity assay
i. Culture techniques

251
Various Aids Including Advanced Diagnostic Techniques
• Aerobic
• Anaerobic
ii. Speciation techniques
• GLC (Gas liquid chromatography)
• DNA homology
Direct microscopy: Specimens are viewed directly
under the light. They are of two types.
a. Light microscopy: Under this stained or unstained
specimens can be read.
Gram’s staining: Differentiates Gram-positive and
Gram-negative organisms. Gram-positive appears
violet. Gram-negative appears pink under the
microscope. This may be important because it
differentiates between health and disease.
b. Dark field and phase contrast microscopy: Fresh,
unstained samples are examined. It uses a special
condenser in which the light rays are either reflected
or refracted off bacterial cell surface. So the outline
of the bacterium is dark against the light background
in phase contrast microscopy and light against a dark
background in dark-field microscopy.
Advantages of direct microscopy: It is quick, easy and
inexpensive means of screening a microbial sample
for major morphotypes.
Disadvantages
• Inability to identify species.
• Specimens have to be examined as soon as
they are collected from the patients.
Culture methods: These are used for cultivation and
identification of organisms, then to determine its
susceptibility or resistance to various antimicrobial agents.
Types of specimens are:
• Blood samples.
• Mucosal surfaces.
• Periodontal pockets.
Subgingival plaque sampling methods (Fig. 32.2) are:
• Nickel-plated curettes
• Scalers
• Paper points
• Irrigation
• Surgical excision
For the identification of anaerobes from the clinical
material many artificial media and culture techniques
are available.
Different kinds of media
Supportive media: Only allows growth of non-fastidious
organisms.
Enriched media: Encourages the growth of organisms.
Non-selective media: Permits the growth of most oral
micro-organisms without specific inhibitory agents.
Selective media: Contains dyes, antibiotics that are
inhibitory to all organisms except those being sought.
Different culture techniques:
a. Jar technique: Removes air /oxygen with in the jar
and replaced by oxygen-free gas containing 80 to
90 percent nitrogen, 5 to 10 percent hydrogen and
5 to 10 percent carbon dioxide.
b. PRAS: Pre-reduced anaerobically sterilized roll tubes
contain a medium which is boiled to remove the
dissolved air and is then flushed with oxygen-free
gas.
c. Anaerobic chamber techniques.
d. Enzyme reduction technique: It contains certain
enzymes which can sweep the oxygen out.
Speciation techniques:
Fig. 32.2:
Microbial sampling method
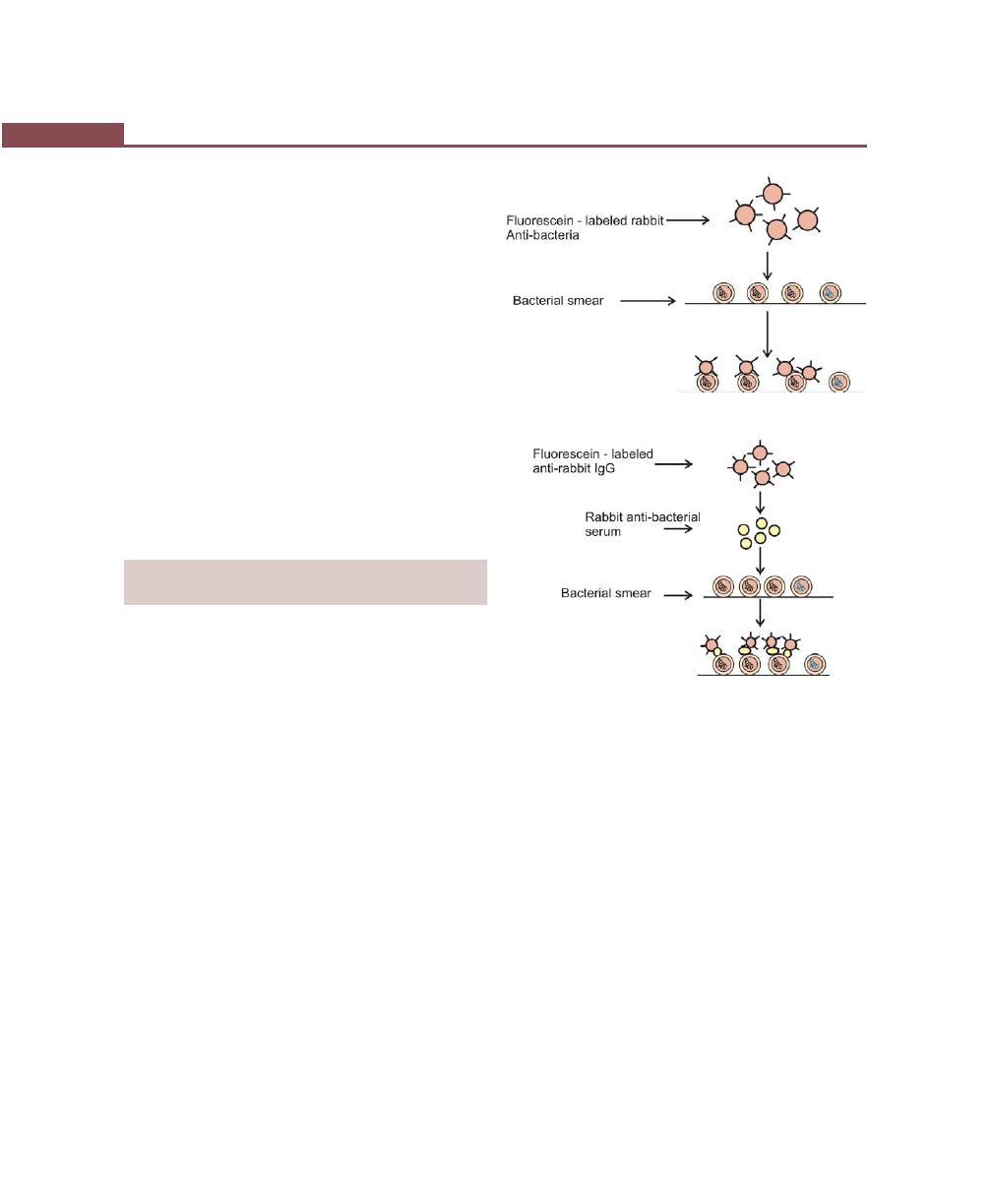
252
Essentials of Clinical Periodontology and Periodontics
a. GLC (Gas Liquid Chromatography): In which
various metabolic products of anaerobes are studied
which are unique enough to serve as markers for
identification.
b. DNA probes in the identification of periodontal
pathogens: It is based on the ability of DNA to
hybridize or bind to the complementary strands of
DNA having the exact base sequence.
Procedure: The plaque is first denatured to obtain single
strain bacterial DNA and then incubated on a membrane
such as nitrocellulose. The specific labelled DNA probe
is incubated on the membrane to allow hybridization
and then washed off. The plaque sample contains
complementary DNA; hybridization of the two single
strains takes place, which can be visualized via the label
of the probe.
METHODS/A
IDS
IN IMMUNOLOGICAL
DIAGNOSIS
Immunofluorescence
This method permits the identification of specific bacteria
in bacterial smears.
Antiserum: A serum that contains antibody or antibodies,
it may be obtained from an animal that has been
immunized either by injecting the antigen into the body
or by infecting with microorganisms containing the
antigen.
Direct Immunofluorescence (Fig. 32.3)
Antiserum to a microorganism is conjugated to
fluorescein. The conjugate is incubated on a clinical smear
containing the microorganisms and then washed off. The
antigen antibody reactions take place and organism is
visualized by its fluorescent outline, when observed under
a fluorescent microscope, if the microorganism is not
present it appears dark with no fluorescence.
Indirect Immunofluorescence (Fig. 32.4)
It is a two step procedure. Antiserum to the micro-
Fig. 32.3:
Direct immunofluorescence
Fig. 32.4:
Indirect immunofluorescence
organism is incubated on the clinical smear and washed
off, then a conjugate of a fluorescent dye and an
antiserum to the first antisera are incubated and then
washed off.
It not only identifies but also quantifies the percentage
of the pathogens in the latex smear.
Others are:
• Enzyme-linked immunosorbant assay (ELISA).
• Flow cytometry.
Latex Agglutination (Fig. 32.5)
It is based on the binding of protein to latex. Latex
beads are coated with species specific antibody
and when these beads come in contact with the
microbial cell surface antigens cross-linking occurs and
its clumping/ agglutination is made visible within 2 to
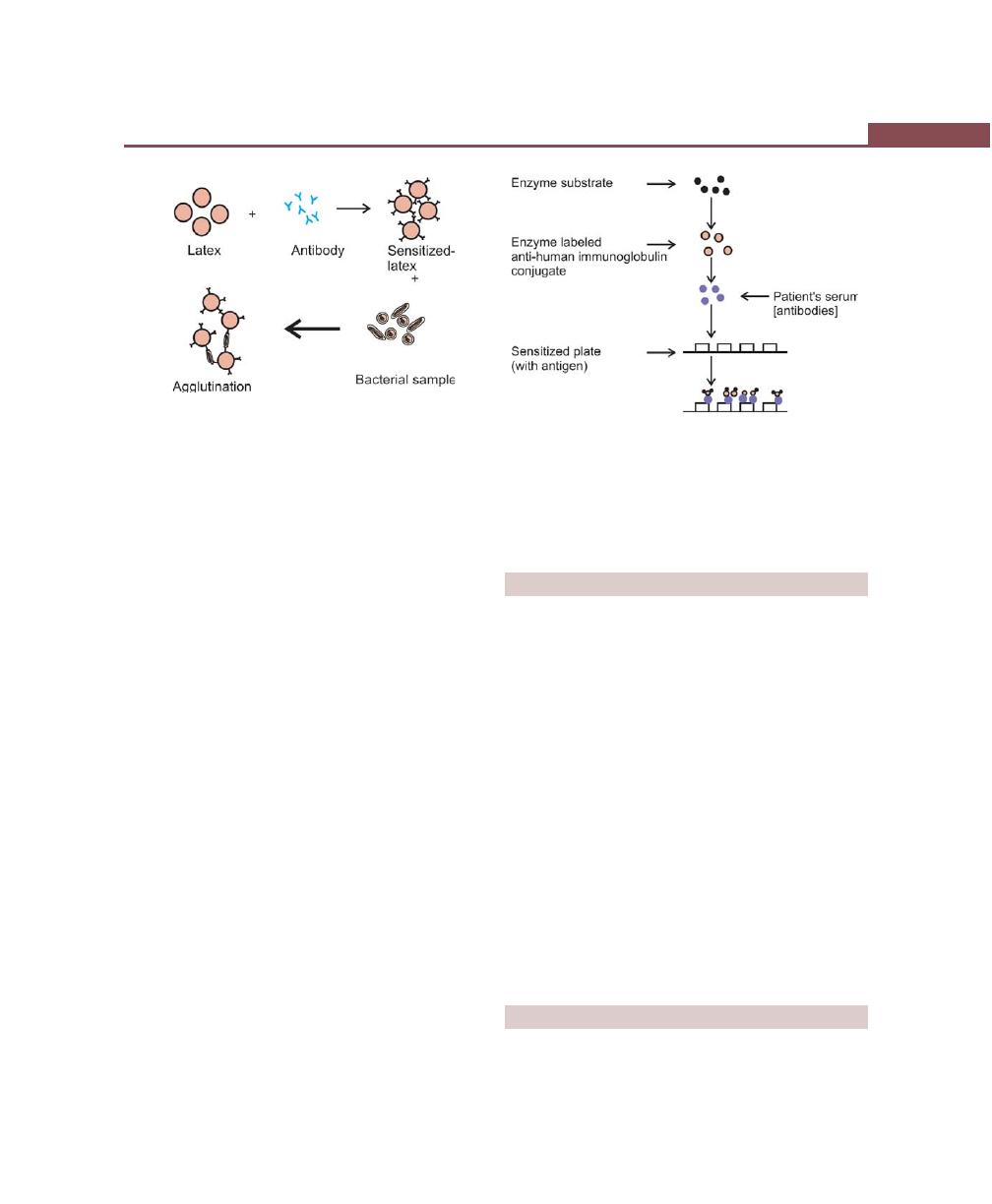
253
Various Aids Including Advanced Diagnostic Techniques
5 minutes.
Enzyme-linked Immunosorbant Assay (ELISA)
(Fig. 32.6)
In this bacterial antigens are incubated in a well, on a
plastic plate to allow coating by the material. After
washing to remove the free antigen, the plates are ready
for tests. Samples containing suspected antibodies and
controls are then incubated in separate wells to allow
antibodies bind the antigen on the surface of the wells.
After washing to remove unbound serum components,
antisera to the antibody is conjugated to either alkaline
phosphatase or horseradish peroxidase then incubated
in the wells.
A positive reaction is visualized by addition of a
chromogen which changes from a colorless to colored
solution.
Flow Cytometry
This is for rapid identification of oral bacteria. This
involves labelling bacterial cells from a patient plaque
sample with both species specific antibody and a second
fluorescein conjugated antibody. The suspension is then
introduced into the flow cytometer, which separates the
bacterial cells into an almost single cell suspension by
means of a laminar flow through a narrow tube. After
incubation, the cells are passed through a focussed laser
beam. The cells then scatter the light at low and wide
angles, and the fluorescent emission can be measured
by appropriate detectors.
BIOCHEMICAL DIAGNOSIS
By-products of the cells (PMNL’s), complement cleavage,
e.g. C
3
, C
4
in gingival crevicular fluid are studied.
Prostaglandins
Levels of prostaglandin E
2
are studied which can
differentiate between gingivitis and periodontitis, e.g.
aggressive forms showed higher levels than chronic
periodontitis. Active sites exhibited five fold increase in
PGE
2
levels than inactive sites.
PGE
2
levels are studied by RIA (Radio-isotope assay).
Collagenase
It showed positive correlation with disease activity. It is
studied by sodium dodecylsulphate polyacrylamide gel
electrophoresis (PAGE) in this they studied breakdown
products resulting from incubation of collagen with
gingival crevicular fluid.
OTHER DIAGNOSTIC AIDS
a. BANA test
b. FSEIA
Fig. 32.5:
Principle of latex agglutination test
Fig. 32.6:
Principle of ELISA assay
254
Essentials of Clinical Periodontology and Periodontics
c. PCR (Polymerase chain reaction)
N-benzoyl–DL–arginine 2–naphthylamide (BANA)
Can identify:
• B. forsythus
Have a common trypsin–like
• P. gingivalis
enzyme, which hydrolyzes
• Treponema denticola the colorless substrate.
• Capnocytophaga
N-benzoyl-DL-arginine-2-naphthylamide—when
hydrolysis takes place it releases the chromphore
betanaphthylamide, which turns orange-red when a drop
of fast garnet is added to the solution .
Filter Separation Enzyme Immunoassay
(FSEIA)
It can identify A. actinomycetemcomitans, P. intermedia
P. gingivalis.
The clinician mixes the plaque sample taken with a
paper point with this reagent to produce a colored
reaction which may be positive or negative, It requires
10 to 15 min of the office time.
PCR (Polymerase Chain Reaction)
History
Kary Mullis had just conceived a simple method of
producing virtually unlimited copies of a specific DNA
sequence in a test tube and introduced to the scientific
community at a conference in Oct 1985.
DNA Hybridization
The chemistry of PCR, as with much of molecular biology
depends on the complementary’s of DNA bases.
Mechanism of Action of PCR
1. The PCR is a test tube system for DNA replication
that allows a “target” DNA sequence to be selectively
amplified, or enriched, several million fold in just a
few hours.
2. It involves a series of enzyme-mediated reactions
whose end result is a copy of the entire genome.
3. PCR uses just one indispensable enzyme DNA poly-
merase to amplify a specific fraction of a genome.
4. DNA acts as “Priming site” for the attachment of DNA
polymerase, two different primer sequences are used
to bracket the target region to be amplified one primer
is complementary to one DNA strand at the beginning
of the target region and second primer is
complementary to a sequence on the opposite DNA
strand at the target region.
Advantages
a. It is a quick, reliable method for detecting all manner
of mutations associated with genetic diseases from
insertions to deletions and to point mutations.
b. Used for detection of tiny amounts of human
immunodeficiency virus and numerous genetic
anomalies.
KEY POINTS TO NOTE
1. Probes are commonly used to detect and measure the
pockets.
2. Periodontal probes can be divided into first generation,
second generation and third generation probes.
3. First generation probes are conventional probes which are
calibrated with or without color coding. Where as second
and third generation probes are pressure-sensitive, and also
third generations probes can automatically detect the CEJ
with computerized data capture.
4. Radiographs are used to obtain a visual image of the bone
support around the teeth or implants. The most commonly
used radiographs are, intraoral periapical and bite wing
X-rays.
5. Various advanced radiographic techniques include, photo-
densitometric analysis, digital radiography, computerized
tomography and nuclear medicine bone scan.
6. Identification of bacteria can be done by, direct examination
using light microscopy, phase contrast and dark field
microscopy.
7. Methods used in immunological diagnosis include,
immunofluorescence, latex agglutination, ELISA and flow
cytometry.
BIBLIOGRAPHY
1. Beck JD. Issues in assessment of diagnostic tests and risk for
periodontal diseases. Periodontol 2000, 1995; 7:100.

255
Various Aids Including Advanced Diagnostic Techniques
2. Bragger U, pasquali L, Rylander H et al. Computer assisted
densitometric image analysis in periodontal radiography. A
methodological study. J Clin Periodontol 1998; 15; 27.
3. Clark WB, Yang MCK, Magnusson I. Measuring clinical
attachment : Reproducibility and relative measurements with
an electronic probe. J Periodontol 1992; 63: 831.
4. Jeffcoat MK. Diagnosing periodontal disease : New tool to
solve old problems. J Am Dent Assoc 1991; 122: 54.
5. Jeffcoat MK. Radiographic methods for the detection of
progressive alveolar bone loss. J Periodontol 1992; 63: 367.
6. Newman, Takei, Fermin. A. Carranza. Clinical periodontology,
9th edn, WB Saunders and Co, 2002.
7. Roy E. Mintzer, John P. Derdivanis. Automated periodontal
probing and recording. Curr. Opin Periodontol 1993;60-67.
8. Socransky SS, Haffajee AD, Cuginity et al. Microbial
complexes in subgingival plaque. J Clin Periodontol 1998;
25:134.
9. Thomas G Wilson Jr. The current status of determining
periodontal prognosis. Curr Opin Periodontol 1993;67-74.
10. Thomas G Wilson, Kenneth S Kornman. Advances in
Periodontics. Quintessence publishing Co, 1992.
256
Essentials of Clinical Periodontology and Periodontics
INTRODUCTION
The aim of the treatment plan is, total treatment i.e.,
co-ordination of all treatment procedures for the purpose
of creating a well-functioning dentition in a healthy
periodontal environment. Treatment plan is the blue
print for the management of a case and establishment
of periodontal health. Treatment procedures should be
performed in a systematic sequence and should be
planned well in advance.
SEQUENCE OF THERAPEUTIC
PROCEDURES
Preliminary Phase or Emergency Phase
Treatment of emergencies.
• Dental or periapical abscess.
• Periodontal abscess.
Extraction of hopeless teeth and provisional replace-
ment if needed.
Phase I Therapy (Etiotropic Phase)
• Plaque control.
• Diet control.
• Removal of calculus and root planing.
• Correction of restorative and prosthetic irritational
factors.
• Excavation of caries and restorations (Temporary or
final).
• Antimicrobial therapy.
• Occlusal therapy.
• Minor orthodontic movement
• Provisional splinting
Evaluation of Response to Phase I
Rechecking:
• Pocket depth and gingival inflammation
• Plaque and calculus, caries
Phase II Therapy (Surgical Phase)
• Periodontal-surgery including placement of implants
• Root canal treatment.
Phase III Therapy (Restorative Phase)
• Final restorations.
❒
❒
❒
❒
❒ SEQUENCE OF THERAPEUTIC
PROCEDURES
❒
❒
❒
❒
❒ PREFERRED SEQUENCE OF
PERIODONTAL THERAPY
33
Treatment Plan
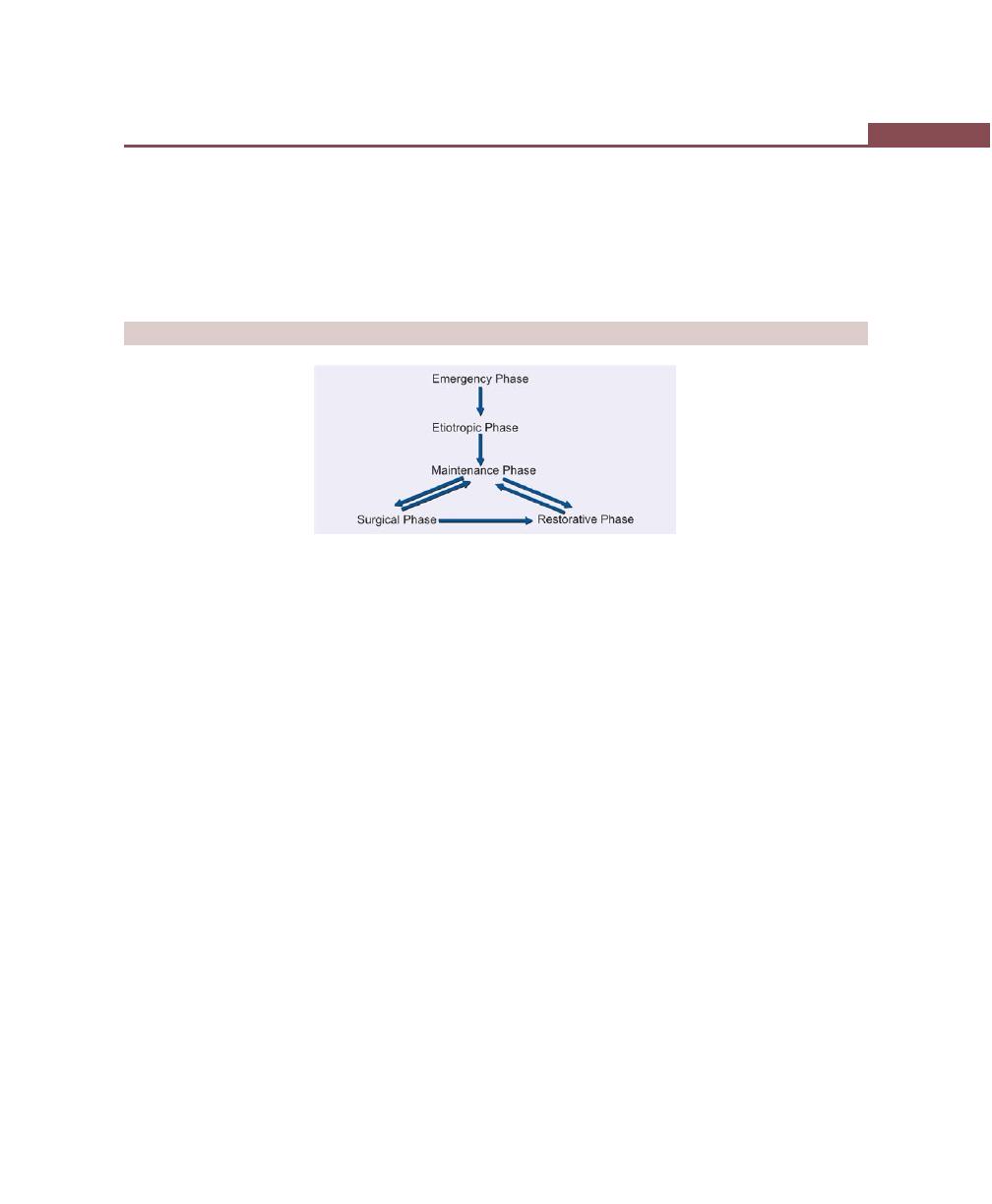
257
Treatment Plan
• Fixed and removable prosthodontics.
• Evaluation of response to restorative procedures.
• Periodontal examination.
Phase IV Therapy (Maintenance Phase)
• Periodic recall visits.
• Checking for plaque and calculus.
• Gingival condition (Pockets, inflammation).
• Occlusion, tooth mobility and other pathologic
changes.
PREFERRED SEQUENCE OF PERIODONTAL THERAPY
258
Essentials of Clinical Periodontology and Periodontics
OBJECTIVES OF PERIODONTAL THERAPY
If properly performed, periodontal treatment can
accomplish the following:
• Eliminate pain.
• Eliminate gingival inflammation.
• Eliminate gingival bleeding.
• Eliminate infection.
• Reduces periodontal pockets and mobility of the
teeth.
• Stops pus formation.
• Arrests the destruction of soft tissue and bone.
• Establish optimal occlusal function.
• Restores tissue destroyed by disease.
• Re-establish the physiologic gingival contour.
• Prevent the recurrence of disease.
• Reduces tooth loss.
The treatment of periodontal disease is based on the
fact that it is caused by bacterial plaque. Hence, the
removal of plaque and all factors that favour its
accumulation is therefore the primary consideration in
local therapy. Systemic therapy may be used as an adjunct
to local measures especially if it is indicated in localized
juvenile periodontitis and rapidly progressing perio-
dontitis cases. Here the systemic antibiotics are used to
completely eliminate the bacteria that invade gingival
tissues. The accumulation of plaque can be favoured
by a variety of local factors such as calculus, overhanging
margins of restorations, food impaction. Hence the
primary consideration in local therapy should be removal
of plaque and all the factors that favour its accumulation.
Systemic therapy may be employed as an adjunct to
local measures and for specific purposes such as systemic
complications from acute infections, post-treatment
bacteremia, control of systemic diseases that aggravate
the patient’s general periodontal condition. Evidence has
shown that some ‘Non-steroidal Anti-inflammatory
Drugs’ such as flurbiprofen and ibuprofen can slow down
the development of experimental gingivitis and the studies
have shown that it can also inhibit alveolar bone loss
in periodontitis. Another drug that has been shown to
reduce bone loss associated with periodontitis, in
experimental animals, is Alendronate a biphosphonate
❒
❒
❒
❒
❒ OBJECTIVES OF PERIODONTAL
THERAPY
❒
❒
❒
❒
❒ FACTORS WHICH AFFECT HEALING
• Local Factors
• Systemic Factors
❒
❒
❒
❒
❒ HEALING AFTER PERIODONTAL
THERAPY
• Regeneration
• Repair
• Reattachment
34
Rationale for
Periodontal Treatment
259
Rationale for Periodontal Treatment
which is also used to treat Paget’s disease and other
metabolic diseases. Although some of the systemic
conditions associated with periodontal diseases are
treated primarily by other than local measures, local
therapy is indicated to reduce or prevent the progression
of periodontal disease.
FACTORS WHICH AFFECT HEALING
As elsewhere in the body, healing is affected by local
and systemic factors.
Local Factors
Healing is delayed by contamination of micro-organisms,
irritation from plaque, food debris, necrotic tissue
remnants and trauma from occlusion. Excessive tissue
manipulation during treatment, trauma to the tissues
can delay healing. In addition repetitive treatment
procedures which affect the orderly cellular activity in
the healing process, topically applied cortisone and
ionizing radiation can retard healing. Healing is improved
by a local increase in temperature, debridement,
immobilization of the healing area and pressure on the
wound.
Systemic Factors
Healing is delayed in:
• Older patients (Because of atherosclerotic vascular
changes which results in reduced blood circulation).
• Generalized infections especially in patients with
diabetes and other debilitating diseases.
• By insufficient food intake, vitamin C deficiency,
deficiency of proteins and other nutrients.
• Increased levels of hormones such as cortisone hinder
repair by depressing the inflammatory reaction or
inhibiting the growth of fibroblasts, the production
of collagen and the formation of endothelial cells.
• Systemic stress, thyroidectomy, testosterone,
adrenocorticotropic hormone and large doses of
estrogen suppresses the formation of granulation tissue
and retard healing.
HEALING AFTER PERIODONTAL THERAPY
(Figs 34.1 and 34.2)
Regeneration, repair and new attachment are the aspects
of healing that have a special bearing on the outcome
of periodontal treatment.
a. Regeneration: It is the biologic process by which the
architecture and function of lost tissues are completely
restored by formation of new periodontal ligament,
alveolar bone and cementum.
b. Repair: It is the healing of tissues without completely
restoring the lost tissues.
c. New attachment: This is the reunion of connective
tissue with a root surface that has been pathologically-
exposed.
d. Reattachment: This is the reunion of connective tissue
and a root surface that have been separated by incision
or injury.
Figs 34.1A and B:
Healing after periodontal therapy
A
B
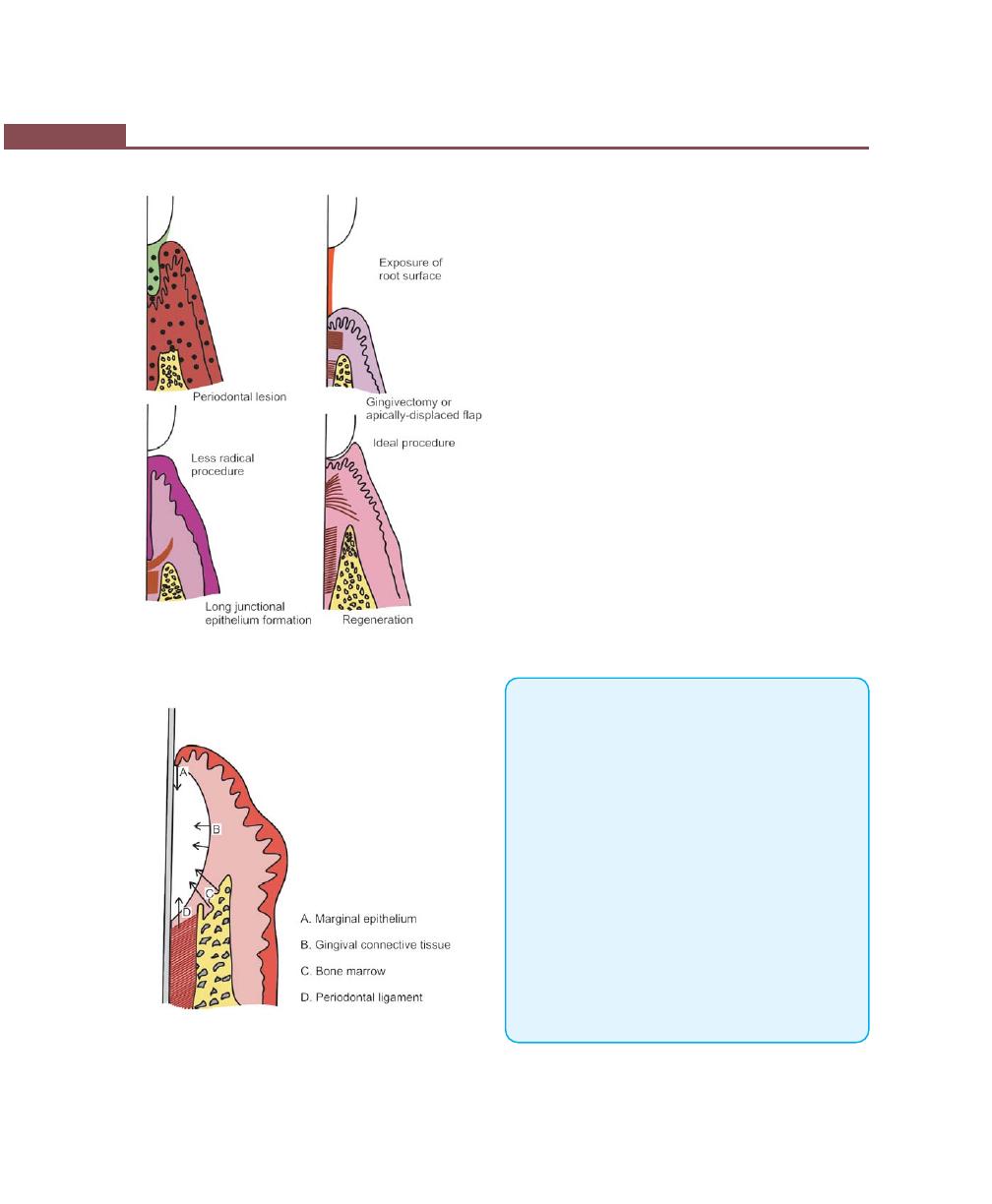
260
Essentials of Clinical Periodontology and Periodontics
Fig. 34.2:
Outcome of periodontal therapy
Fig. 34.3:
Sources of cells that populate the pocket area
During the healing stages of periodontal pockets, the
area is invaded by cells from four different sources: oral
epithelium, gingival connective tissue, bone and
periodontal ligament (Fig. 34.3).
The final outcome of periodontal pocket healing
depends on the sequence of events during the healing
stages. If the epithelium proliferates along the tooth
surface before the cells from other tissues reach the area,
the result will be a long junctional epithelium. If the cells
from connective tissue populate, the result will be fibers
parallel to the tooth surface and remodelling of bone
and no attachment to the cementum. If bone cells arrive
first, root resorption and ankylosis may occur. Finally
when only cells from the periodontal ligament proliferate
coronally, there is new formation of cementum and
periodontal ligament. Hence, Melcher pointed out that
the regeneration of the periodontal ligament is the key
to new attachment because it provides continuity
between the alveolar bone and the cementum and also
because it contains cells that can synthesize and remodel
the three connective tissues of the supporting structures
of periodontium.
KEY POINTS TO NOTE
1. If periodontal treatment is properly performed it can restore
the normal health of the periodontal tissues.
2. The periodontal treatment consists of both local and systemic
therapy.
3. The primary objective of local therapy is removal of plaque
and all those factors that may favour its accumulation.
4. Systemic therapy is used as an adjunct to local therapy and
is mainly indicated in localized and generalized aggressive
periodontitis.
5. Healing is affected by local and systemic factors, under local
factors, those factors that can delay the healing are, excessive
tissue manipulation, unnecessary trauma to the tissue,
presence of foreign bodies etc. Healing is improved mainly
by good debridement and proper immobilization of the
wound.
6. Systemic conditions that may have an effect on healing are,
infections like diabetes, and other debilitating diseases,
malnutrition, increased levels of hormones, systemic
stress.
261
Rationale for Periodontal Treatment
7. During healing, the area may be invaded by cells from four
different sources:
a. Oral epithelium—results in long junctional epithelium.
b. Gingival connective tissue results in fibres parallel to root
surface.
c. Bone cells—root resorption and ankylosis
d. Only cells from periodontal ligament—results in new
attachment.
REVIEW QUESTIONS
1. Describe factors that can affect the healing following
periodontal treatment.
2. Define regeneration, repair, new attachment and
reattachment.
BIBLIOGRAPHY
1. CAG McCulloch. Basic considerations in periodontal
wound healing to achieve regeneration. Periodontol
2000;1:1993.
2. Jack G Caton, Gary Greenstein. Factors related to periodontal
regeneration. Periodontol 2000;1:1993.
3. Salomon Amar, Kong Mun Chung. Clinical implications of
cellular biologic advances in periodontal regeneration. Curr.
Opin Periodontol 1994;187-93.
4. Takashi Takata. Oral wound healing concepts in periodonto-
logy. Curr. Opin Periodontol 1994; 187-93.
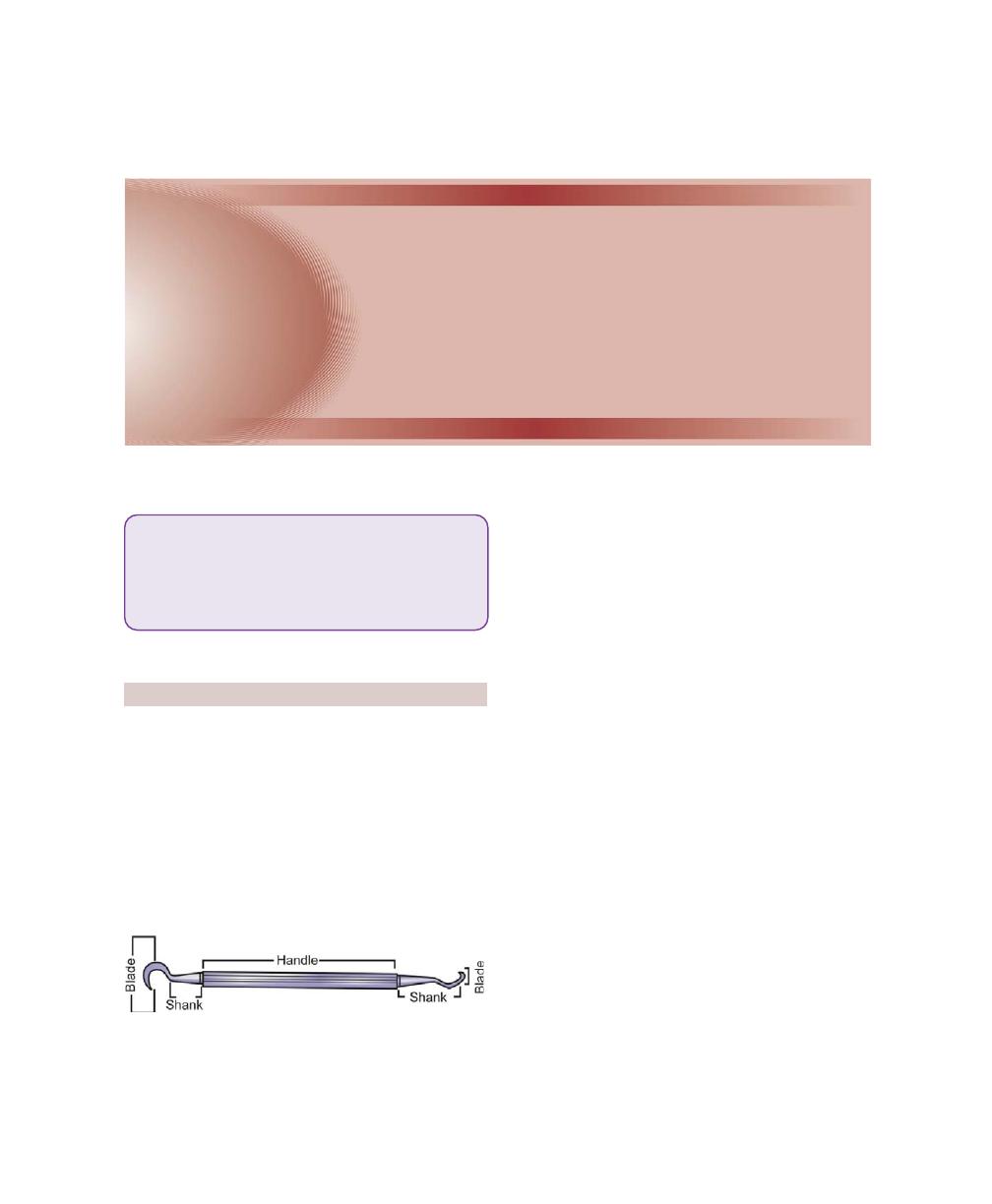
262
Essentials of Clinical Periodontology and Periodontics
PERIODONTAL INSTRUMENTS
Periodontal instruments are designed for specific
purposes, such as removing calculus, planing root
surfaces, curetting the gingival wall or removing diseased
tissue.
Periodontal instrument (Fig. 35.1) is composed of:
a. Blade
b. Shank
c. Handle
Classification of Periodontal Instruments
Diagnostic Instruments
• Periodontal probes are used to locate, measure mark
pockets.
• Explorers are used to locate calculus deposits and
caries.
Scaling and Root Planing, and Curettage
Instruments
Scaling and root planing instruments are classified as
follows:
a. For supragingival scaling:
• Sickle scalers, cumine universal scaler, posterior
Jacquette scaler, Morse scaler, surface scaler,
cingulum scaler.
b. For subgingival scaling:
• Hoe scaler, chisel and file scalers are used to
remove tenacious subgingival deposits.
• Curettes are used to plane the root surfaces by
removing altered cementum and also, for scraping
the soft tissue wall of the pocket.
c. Sonic and ultrasonic instruments.
The Periodontal Endoscope
Used to visualize deep pockets and furcations during
scaling and root planing.
Fig. 35.1:
Parts of an instrument
❒
❒
❒
❒
❒ PERIODONTAL INSTRUMENTS
• Diagnostic
• Surgical
• Scaling and Curettage
• Ultrasonic and Sonic
• Cleansing and Polishing Instruments
35
Periodontal
Instrumentarium
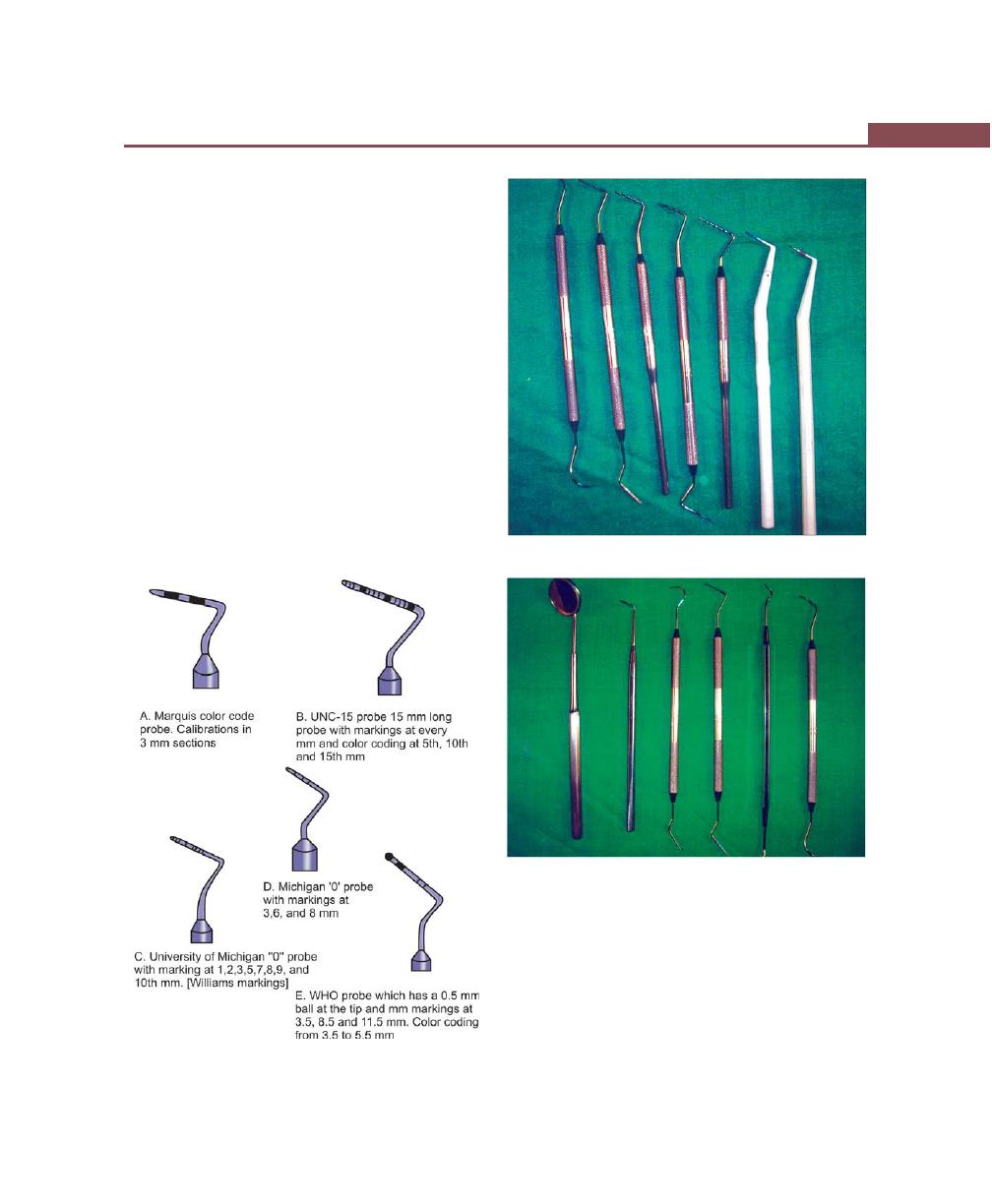
263
Periodontal Instrumentarium
Cleansing and Polishing Instruments
• Rubber cups, brushes, dental tapes
• Air-powder abrasive system.
Surgical Instruments
Excisional and incisional instruments, surgical curettes
and sickles, periosteal elevators, surgical chisels, Hoes
files, scissors and nippers.
Periodontal Probes
A typical probe is a tapered rod-like instrument calibrated
in millimeters with a blunt, rounded tip. Periodontal
probes are used to measure the depth of the pocket
and to determine their configuration.
When measuring a pocket, the probe is inserted with
a firm gentle pressure to the base of the pocket. The
shank should be aligned with the long axis of the tooth
Fig. 35.2:
Different types of probes
Fig. 35.3:
Types of periodontal probes
Fig. 35.4:
Diagnostic instruments
surface to be probed. Furcation areas can be best
evaluated with the curved, blunt Naber’s probe (Figs
35.2 to 35.4).
Types of periodontal probes
• Color-coded
• Noncolor-coded
a. The Marquis color-coded probe: The calibrations are
in 3 millimeter sections.
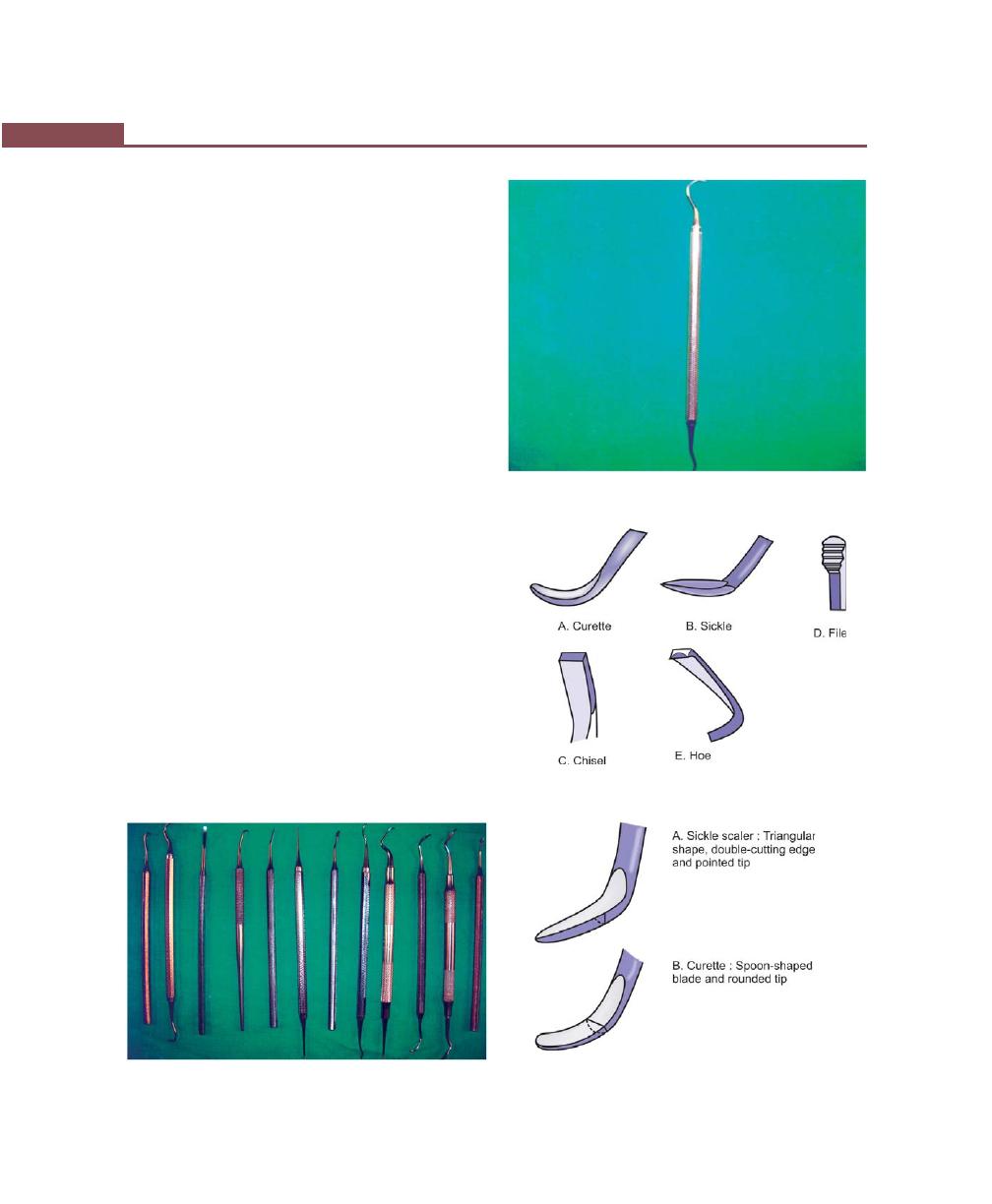
264
Essentials of Clinical Periodontology and Periodontics
b. The UNC-15 probe: It is a 15 mm long probe with
millimeter markings at each millimeter and color
coding at the 5th, 10th, and 15th mm.
c. Williams probe: Has both color and non-color coding
with markings at 1,2,3,5,7,8,9 and 10 mm.
d. The Michigan “O” probe with Williams marking: At
1, 2, 3,5,7,8,9,10 mm (4 and 6 are missing)
e. The Michigan “O” probe with markings: At 3, 6,
and 8 mm.
f. The WHO probe: It has a 0.5 mm ball at the tip and
millimeter marking at 3.5, 8.5 and 11.5 mm and
color coding from 3.5 to 5.5 mm
Explorers
They are used to locate subgingival deposits in various
areas, and to check the smoothness of the root surfaces
after root planing. Explorers are designed with different
shapes and angles for a variety of use.
Scaling and Curettage Instruments
(Figs 35.5 to 35.8)
Sickle scalers: Sickle scalers have a flat surface and two
cutting edges that converge in a sharply-pointed tip. The
arch-shape of the instrument makes the tip so strong
that it will not break off during use. They appear
triangular in cross-section. The sickle scaler is inserted
under ledges of calculus no more than 1 mm below the
gingival sulcus. It is used with a pull stroke.
The Morse sickle has a very small, miniature blade;
it is useful in the mandibular, anterior area where there
Fig. 35.5:
Scaling instruments
Fig. 35.6:
Sickle scaler
Fig. 35.7:
Basic scaling instruments
Fig. 35.8:
Basic characteristics of scalers and curettes
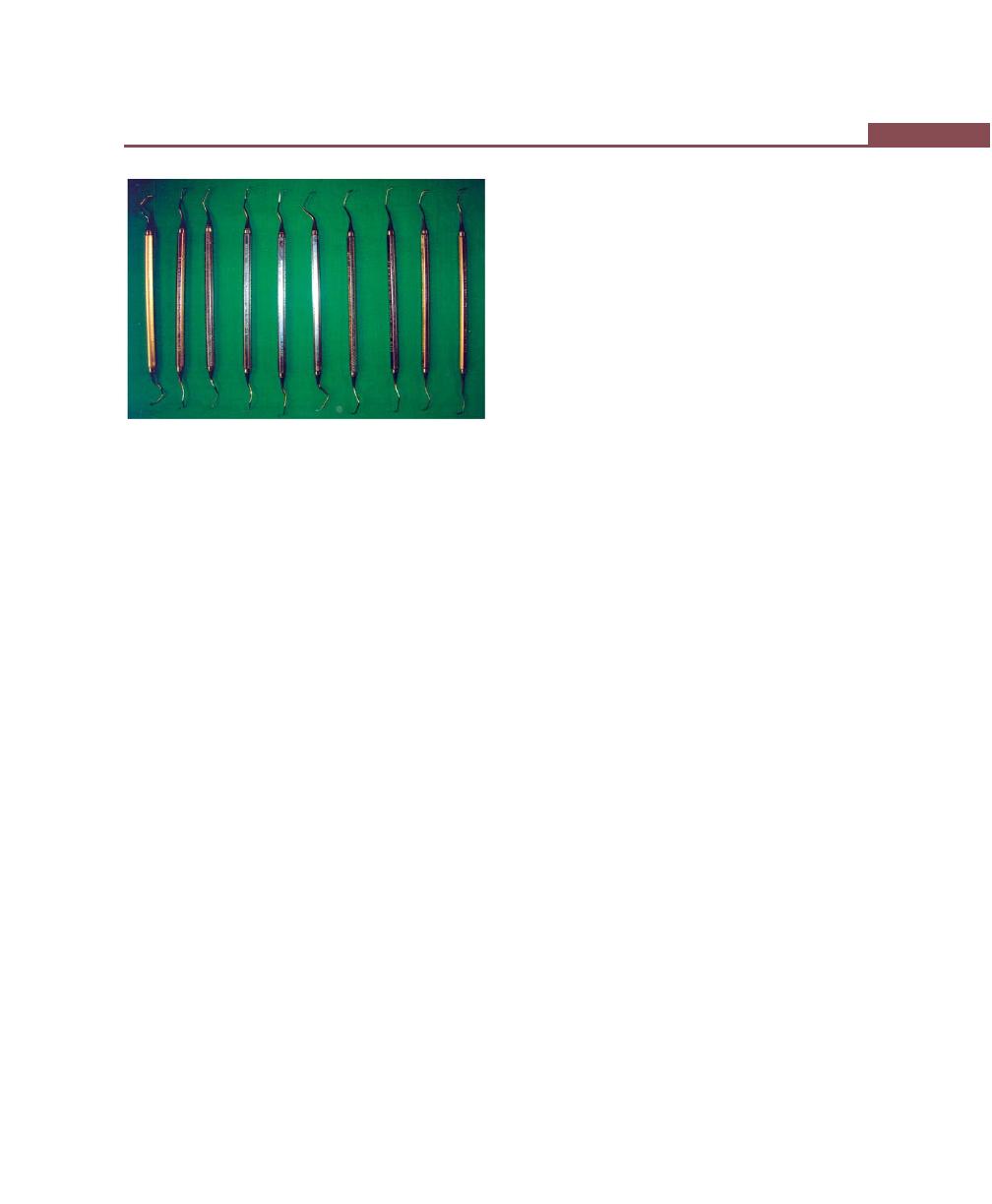
265
Periodontal Instrumentarium
is narrow, interproximal space. Sickles with straight shanks
are designed for use on anterior teeth and premolars.
Sickle scalers with contra-angled shanks adapt to posterior
teeth.
Curettes (Figs 35.9 to 35.11): The curette is the
instrument of choice for removing deep subgingival
calculus, altered cementum, for root planing and for
removing the soft tissue lining the periodontal pocket.
Curette can be adapted to provide good access
to deep pockets, with minimal soft tissue trauma.
There are cutting edges on both sides of the blade.
Both single and double-ended curette may be obtained
depending upon the preference of the operator.
The curved blade and rounded toe of the curette allows
the blade to adapt better to the root surface. In cross-
section the blade appears to be semicircular with a convex
base.
There are two basic types of curettes.
A. Universal
B. Area-specific
Universal curettes: Universal curettes have cutting edges
that may be inserted in most areas of the dentition by
altering and adapting the finger rest, fulcrum and hand
position of the operator.
The face of the blade of every universal curette is
at a 90 degree angle to the lower shank, when seen
in cross section from the tip.
Example of universal curettes: Barnhart curettes #
1-2 and 5-6 and Columbia curettes # 13-14, 2R-2L
and 4R-4L.
Area-specific curettes:
Gracey curettes: They are area-specific curettes, designed
and angled to adapt to specific anatomic areas of the
dentition. These curettes and their modifications are
probably the best instruments for subgingival scaling and
root planing because they provide the best adaptation
to complex root anatomy.
The term offset blade is used to describe Gracey
curettes, because they are angled approximately 60–70
degrees from the lower shank. This unique angulation
allows the blade to be inserted in a precise position,
necessary for subgingival scaling and root planing,
provided that the lower shank is parallel to the long axis
of the tooth surface being scaled.
Double-ended Gracey curettes are paired in the
following manner:
Gracey # 1-2 and 3-4 : for anterior teeth
Gracey # 5-6
: for anterior teeth and
premolars
Gracey # 7-8 and 9-10 : posterior teeth; facial
and lingual
Gracey # 11-12
: posterior teeth; mesial
Gracey # 13-14
: posterior teeth; distal
Recent additions to Gracey set are:
Gracey # 15-16
:
#15-16 is a modification of
and 17-18
#11-12; # 17-18 is a
modification of # 13-14. It
has a shank elongated by
3 mm.
Distinction between Gracey and universal curettes is
given in Table 35.1.
Extended Shank Curettes or After Five Curettes
Hu Friedy After Five Curettes are modifications of the
standard Gracey curette design. The shank is 3 mm longer
allowing extension into deeper periodontal pockets of
5 mm or more, other features include a thinned-blade
Fig. 35.9:
Types of curettes
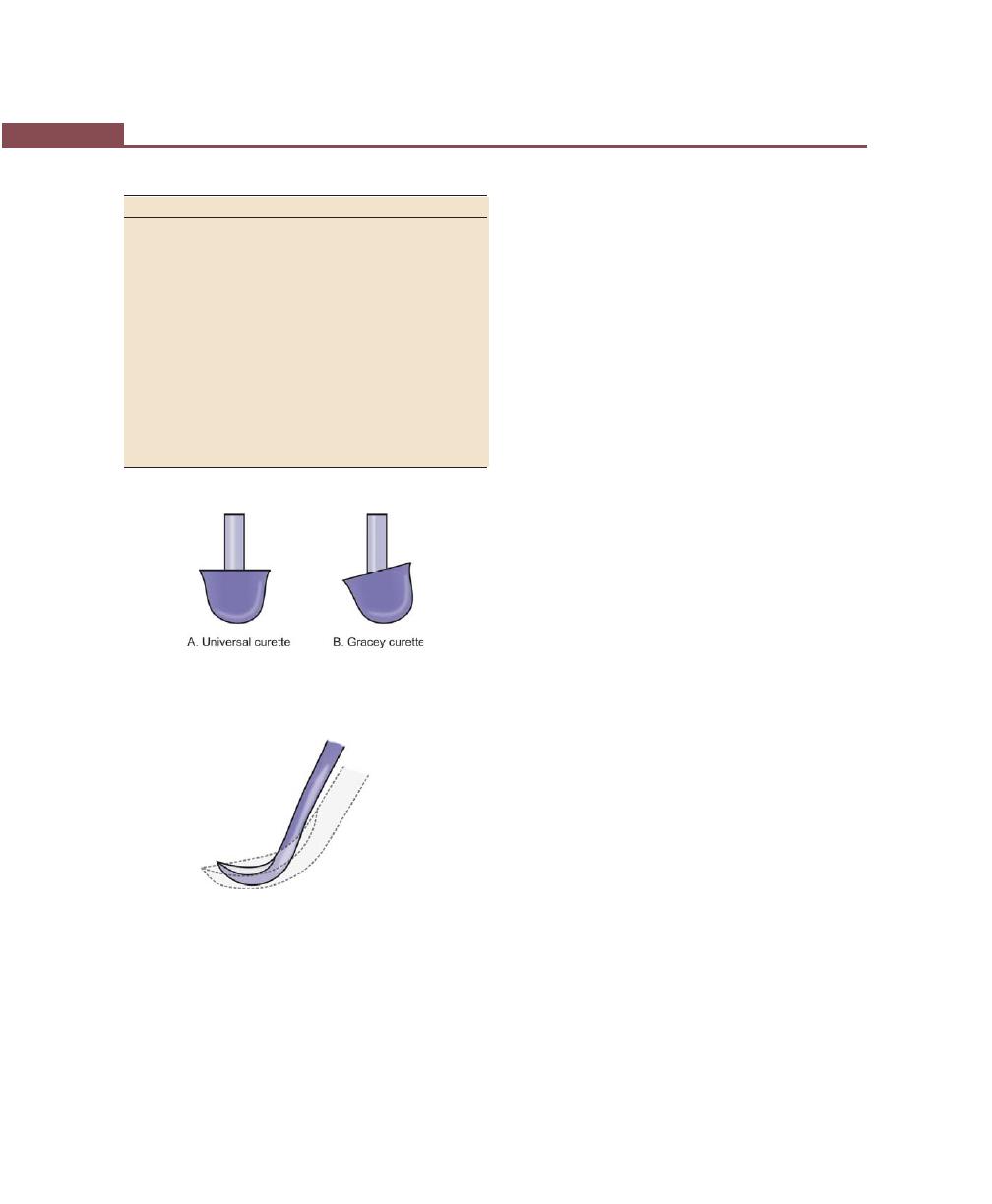
266
Essentials of Clinical Periodontology and Periodontics
Mini-bladed curettes: They are modifications of After Five
Curettes The shorter blade allows easier insertion and
adaptation in deep, narrow pockets, furcations, develop-
mental grooves, line angles, and deep, tight, facial, lingual,
or palatal pockets. As with the After Fives, the Mini Fives
are available in all standard Gracey number except for
the # 9-10.
Gracey curvettes: They are another set of four mini-
bladed curettes.
Sub-0 and # 1-2—anterior and premolars
# 11-12—posterior mesial surfaces.
# 13-14—posterior distal surfaces.
The blade length of these instruments is 50 percent,
shorter than that of conventional Gracey curette and
the blade is curved slightly upward. This curvature allows
the curettes to adapt, more closely to the tooth surfaces.
Langer and mini Langer curettes: This set of 3 curettes
combines the shank design of the standard Gracey #
5-6, 11-12, and 13-14 curettes with a universal blade
honed at 90 degrees rather than the offset blade of the
Gracey curette. Hence these curettes offer a blend of
both Gracey and universal curette and can be adapted
both on the mesial and distal surfaces without changing
instruments.
Schwartz periotreivers: They are a set of two double-
ended, highly-magnetized instruments designed for the
retrieval of broken instrument tips from the periodontal
pocket.
Plastic instruments for implants: It is imperative that plastic
rather than metal instruments be used, to avoid scarring
and permanent damage to the implants.
Hoe scalers: They are used for scaling ledges or rings
of calculus. The blade is bent at a 99-degree angle; the
cutting edge is beveled at 45 degrees. The Hoe scalers
are used in the following manner:-
a. The blade is inserted to the base of the periodontal
pocket, so that it makes a two point contact with the
tooth. This stabilizes the instrument and prevents
nicking of the tooth.
Table 35.1: Distinction between Gracey and universal curettes
Gracey curette
Universal curette
Area of use
Set of many curet-
One curette designed
tes designed for
for all areas and sur-
specific areas and
faces
surfaces.
Cutting edge
One cutting edge
Both cutting edges
used, work is done used, work is done
with the outer
with outer or inner
edge only
edge
Curvature
Curved in 2 planes Curved in one plane
blade curves up
blades curves up and
and to the side
not to the side
Blade angle
Offset blade, face
Not offset, face of
of blade beveled
blade beveled at 90
at 60 degrees to
degrees to the shank.
the shank.
Fig. 35.10:
Universal and Gracey curette. Note: offset
blade angulation of Gracey curette
Fig. 35.11:
Gracey curette blade. Note that the Gracey curette
is 50 percent shorter and the tip of the blade is turned
upwards as compared to a standard Gracey curette blade
for smoother subgingival insertion or reduced tissue
distention with a large diameter, tapered shank.
All the standard Gracey numbers except # 9-10 are
available in the After Five series.

267
Periodontal Instrumentarium
b. The instrument is activated with a firm pull stroke
towards the crown, with every effort being made to
preserve the two point contact with the tooth.
Mc. Calls Hoe scalers # 3, 4,5,6,7 and 8 are a set
of six Hoe scalers designed to provide access to all
the tooth surfaces
Files: They have a series of blades on a base. Their
primary function is to fracture or crush tenacious calculus.
Files can easily gouge and roughen root surfaces
when used improperly. Therefore they are not suitable
for fine scaling and root planing. They are sometimes
used for removing overhanging margins of dental
restorations.
Chisel scalers: Usually used in the proximal surfaces of
anterior teeth (too closely spaced). It is a double-ended
instrument with a curved shank at one end and a straight
shank at the other. The instrument is activated with a
push motion.
Ultrasonic and Sonic Instruments (Fig. 35.12)
Used for removing plaque, scaling, curetting and
removing stains
Two types of ultrasonic units are:
• Magnetostrictive: Vibration of the tip is elliptical; hence
all the sides can be used.
• Piezo-electric: Pattern of vibration of the tip is linear;
only two sides of the tip are active.
Ultrasonic vibrations range from 20,000 to 45,000
cycles/second. They operate in a wet field and have
attached water outlets.
Dental Endoscope
It is introduced for use subgingivally, in the diagnosis,
treatment of periodontal diseases. Produced by dental
view. Inc and called as the perioscopy system.
• It consists of re-usable fiber optic endoscope, over
which there is a sterile sheath. The fiber optic
endoscope fits onto the periodontal probes and
ultrasonic instruments that have been designed to
accept it.
• The sheath delivers water for irrigation that flushes
the pocket while the endoscope is in use, and it keeps
the field clear.
• This device allows clear visualization, subgingivally,
in deep pockets and in furcations. It enables the
operator to detect the presence and location of
subgingival deposits and guides the operator in their
thorough removal.
• Using this device it is possible to achieve levels of
root debridement and cleanliness that are much more
difficult to produce without it.
The EVA system: They are most efficient and least
traumatic instruments, for correcting overhanging or
over contoured proximal alloy and resin restorations.
Cleansing and Polishing Instruments
Rubber cups: They consist of a rubber shell with or
without configuration in the hollow interiors.
They are used in the hand piece with special
prophylaxis angle. A good cleansing and polishing paste
that contains fluoride should be used.
Bristle brushes: Available in wheel and cup shapes, used
in hand piece with a polishing paste
Dental tape: It is used with a polishing paste and is used
for polishing proximal surfaces that are inaccessible to
other polishing instruments.
Fig. 35.12:
Ultrasonic scaler (EMS scaler)
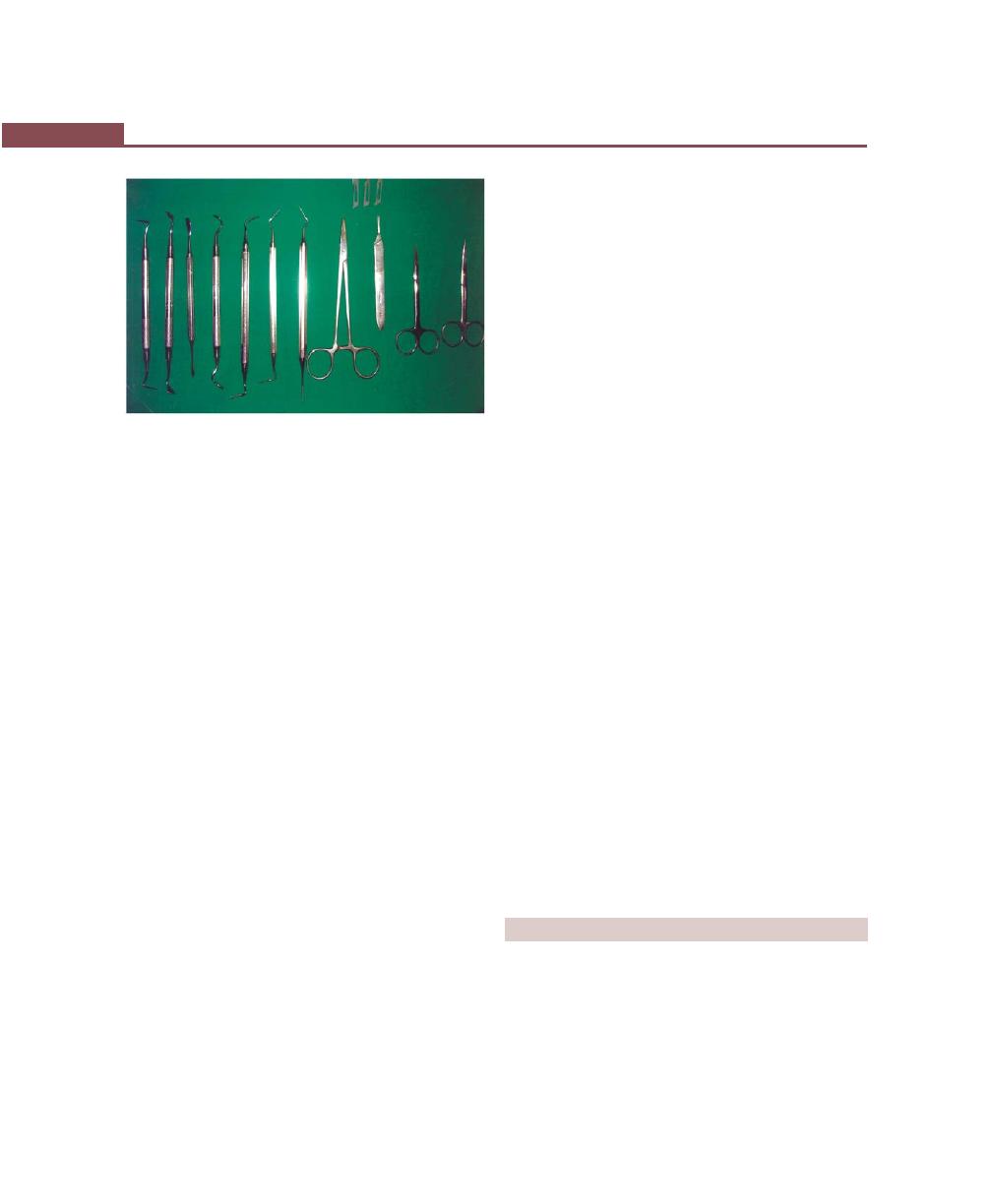
268
Essentials of Clinical Periodontology and Periodontics
Air powder polishing: A specially designed hand piece
that delivers air powdered slurry of warm water and
sodium bicarbonate, this instrument is called prophy-
jet. Effective for the removal of extrinsic stains and soft
deposits.
Disadvantages: Tooth substance can be lost, damage to
gingival tissue is transient and insignificant clinically, but
amalgam restorations, composite resins and cements can
be roughened
Contraindications: Patients with medical histories of
respiratory illness, hypertension, and patients on
medications affecting the electrolyte balance are
contraindicated.
Surgical Instruments (Fig. 35.13)
1. Excisional and incisional instruments:
• Periodontal knives (Gingivectomy knives): Example
Kirkland knife.
• Interdental knives: Example, Orban knife #1-2,
Merrifield knife #1,2,3 and 4
• Surgical blades: Example, # 12D, 15 and 15C.
• Electrosurgery (Radiosurgery) techniques and
instrumentation:
– Electrosection used for incisions, excisions and
tissue planing.
– Electrocoagulation, coagulation or hemorrhage
control
– Electrofulguration not in general use in dentistry
– Electrodessication not in general use in dentistry
2. Surgical curettes and sickles: Required for the removal
of granulation tissue, fibrous interdental tissue, and
tenacious subgingival deposits.
Examples:
• Kramer curettes # 1, 2, 3 and Langer curettes.
• Kirkland surgical instruments.
• Ball scaler # B
2
-B
3
.
3. Periosteal elevators: Necessary to reflect and move
the flap after the incision has been made for flap surgery.
Example: Goldman Fox #14.
4. Surgical chisels and hoes: They are used during
periodontal surgery for removing and reshaping bone.
Chisels are used with a push stroke whereas surgical hoes
are used with a pull stroke.
Example:
• Ochsenbein #1-2, chisel.
• Rhodes chisel.
5. Surgical files: They are used primarily to smoothen
rough, bony, ledges and to remove all areas of necrotic
bone.
Example: Schluger and Sugarman files.
6. Scissors and nippers: Used for removing tabs of tissue
during gingivectomy, trimming the margins of flaps,
enlarging incisions in periodontal abscesses and removing
muscle attachments in mucogingival surgery
Example: Goldman – Fox # 16 scissors.
7. Needle holders: They are used to suture the flap at
the desired position.
Example: Castroviejo needle holder.
BIBLIOGRAPHY
1. Jill, S Nield-Gehrig. Fundamentals of Periodontal
Instrumentation, 4th edn, Lippincott Williams and Wilkins,
2000.
2. Newman Takei, Fermin A Carranza. Clinical Periodontology,
9th edn, WB Saunders, 2002.
3. Wilkins EM. Clinical Practice of the Dental Hygienist, 7th edn,
Baltimore, Williams and Wilkins, 1994.
Fig. 35.13:
Surgical instruments
269
Principles of Periodontal Instrumentation including Scaling and Root Planing
INTRODUCTION
Effective instrumentation is governed by a number of
general principles that are common to all periodontal
instruments. Proper position of the patient and the
operator, illumination and retraction for optimal visibility
and sharp instruments are the fundamental pre-requisites.
ACCESSIBILITY: (POSITIONING OF
PATIENT AND OPERATOR)
It facilitates thoroughness of instrumentation. The
position of the patient and operator should provide
maximal accessibility to the area of operation. Inadequate
accessibility impedes thorough instrumentation,
prematurely tires the operator, and diminishes his or her
effectiveness.
Neutral Seated Position for the Clinician
• Forearm parallel to the floor.
• Weight evenly balanced.
• Thighs parallel to the floor.
• Hip angle of 90 degrees
• Seat height positioned low enough so that the heels
of your feet touch the floor.
• When working from clock positions 9-12:00, spread
feet apart so that your legs and the chair base form
a tripod which creates a stable position.
• Avoid positioning your legs under the back of the
patient’s chair.
• Back straight and the head erect.
Patient’s Position
The patient should be in a supine position and placed
in such a way that the mouth is close to the resting elbow
of the clinician.
Body: The patient’s heels should be slightly higher than
the tip of his or her nose. The back of the chair should
be nearly parallel to the floor for maxillary treatment
areas. The chair back may be raised slightly for
mandibular treatment areas.
Section 2: PERIODONTAL THERAPY:
(A) NON-SURGICAL THERAPY
❒
❒
❒
❒
❒ POSITIONING OF PATIENT AND
OPERATOR
❒
❒
❒
❒
❒ VISIBILITY, ILLUMINATION AND
RETRACTION
❒
❒
❒
❒
❒ CONDITION OF INSTRUMENTS
❒
❒
❒
❒
❒ MAINTAINING A CLEAN FIELD
❒
❒
❒
❒
❒ INSTRUMENT STABILIZATION
❒
❒
❒
❒
❒ INSTRUMENT ACTIVATION
❒
❒
❒
❒
❒ PRINCIPLES OF SCALING AND ROOT
PLANING
❒
❒
❒
❒
❒ ULTRASONIC INSTRUMENTS
❒
❒
❒
❒
❒ AEROSOL PRODUCTION
36
Principles of Periodontal
Instrumentation including
Scaling and Root Planing
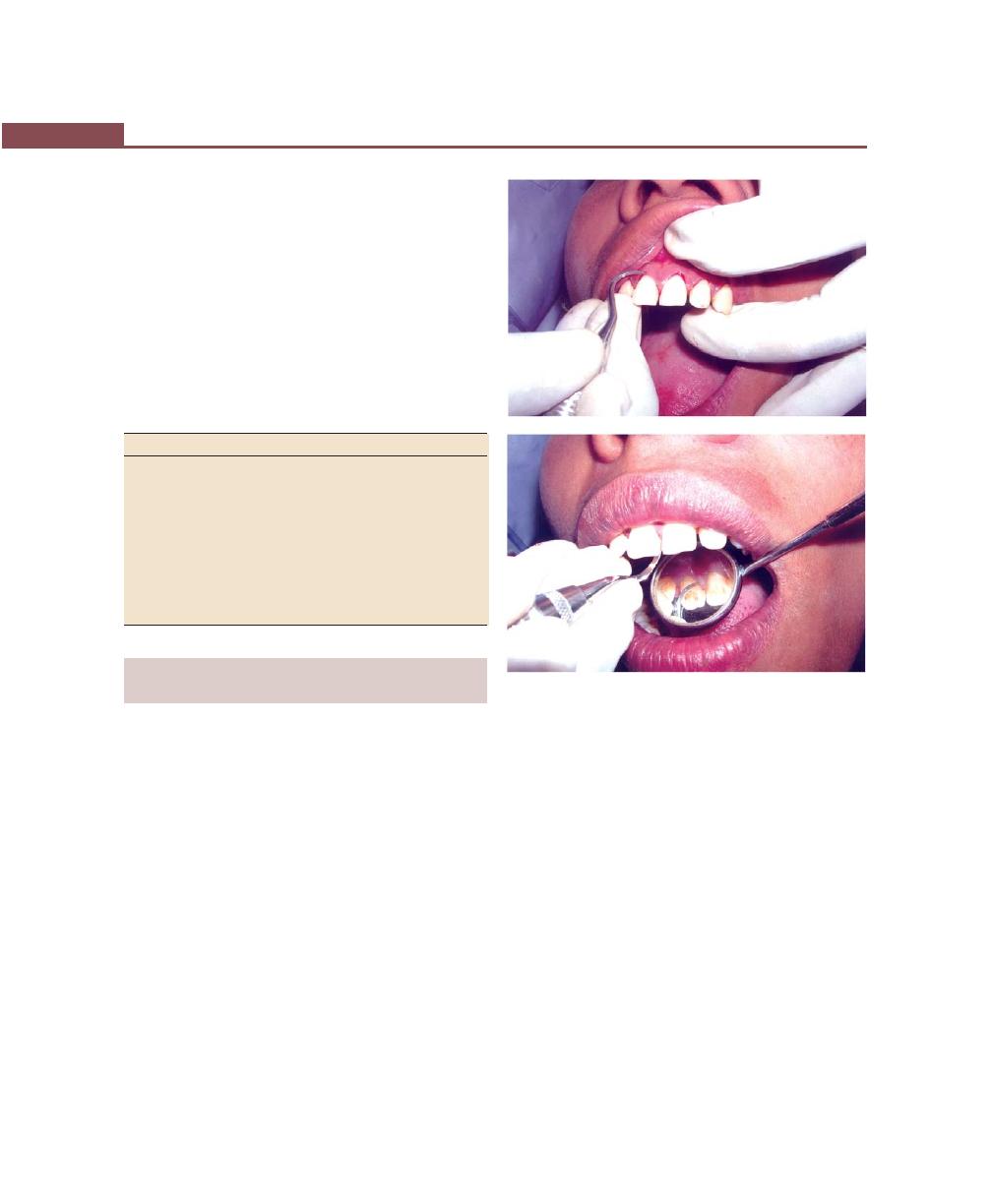
270
Essentials of Clinical Periodontology and Periodontics
Head: The foremost of the patient’s head should be even
with the upper edge of the head rest.
• For mandibular areas—chin down position.
• Maxillary areas—chin up position.
Head rest: If the head rest is adjustable, it should be
raised or lowered, so that the patient’s neck and head
are aligned with the torso.
There are four basic clinical positions for the right-
handed and left-handed clinician (Table 36.1).
Table 36.1: Clinical positions for right and
left-handed clinicians
Right-handed clinician
Left-handed clinician
1. 7 o’ clock position to the
1. 5 o’ clock position, to the
front of the patient’s head.
front of the patient’s head
2. 9 o’ clock position to the
2. 3 o’ clock position, to the
side of the patient’s head.
side of the patient’s head.
3. 10 to 11 o’ clock, to the
3. 2 to 10 o’ clock position,
back of the patient’s head
to the back of the patient’s
head.
4. 12 o’ clock position, direc- 4. 12 o’ clock position, direc-
tly behind the patient’s
tly behind the patient’s
head.
head.
VISIBILITY, ILLUMINATION
AND RETRACTION (Figs 36.1A and B)
Whenever possible, direct vision with direct illumination
from the dental light is most desirable. If this is not possible,
indirect vision may be obtained by using a mouth mirror
to reflect light where it is needed. Indirect vision and
indirect illumination are often used simultaneously.
Dental Mirror
It is a hand instrument which has a reflecting mirrored
surface used to view tooth surfaces that cannot be seen
by direct vision.
Various Types of Mirror Surfaces
• Front surface
– Produces clean clear image with no distortion
– Good image quality
– Easily scratchable
• Concave surface
– Image is magnified.
– Distortion of the image.
• Plane (Flat surface)
– Produces double image.
– Double image is distracting.
Various uses of a Dental Mirror
1. Indirect vision.
2. Retraction.
3. Indirect illumination.
4. Transillumination.
Transillumination: When transilluminating a tooth, the
mirror is used to reflect light through the tooth surface.
The transilluminated-tooth almost will appear to glow.
Figs 36.1A and B:
Direct and indirect vision
A
B

271
Principles of Periodontal Instrumentation including Scaling and Root Planing
It is effective only with anterior teeth because they are
thin enough to allow the light to pass through them.
Procedure:
Step 1 : Position yourself in 12’O clock position
Step 2 : Using a modified pen grasp, hold the mirror in
the non-dominant hand. Bring the arm up and
over the patients, face. Gently rest your ring
finger on the side of the patient’s lip or cheek.
Step 3 : Hold the dental mirror behind the central
incisors so that the reflecting surface is parallel
to the lingual surface. Position the unit light
so that the light beam shines on the dental
mirror at a 90 degree angle to the mirrors
reflecting surfaces.
Step 4 : Properly-positioned light and the mirror will
result in glow.
Retraction: It provides visibility, accessibility and illumi-
nation. The following methods are effective for retraction:
1. Use of the mirror to deflect the cheek while the fingers
of the non-operating hand retract the lips and protect
the angle of the mouth from irritation by the mirror
handle.
2. Use of the mirror alone to retract the lips and cheek.
3. Use of fingers of the non-operating hand to retract
the lips.
4. Use of the mirror to retract the tongue
5. Combination of the preceding methods.
While retracting, care should be taken to avoid
irritation to the angles of the mouth.
CONDITION OF INSTRUMENTS
(SHARPNESS)
Prior to any instrumentation, all instruments should be
inspected to make sure that they are clean, sterile and
in good condition. The working ends of pointed or
bladed instruments must be sharp to be effective.
Advantages of Sharpness
1. Easier calculus removal.
2. Improved stroke control.
3. Reduced number of strokes.
4. Increased patient comfort.
5. Reduced clinician fatigue.
Ideally, it is best to sharpen your instruments after
autoclaving and then re-autoclave them prior to patient
treatment. Dull instruments may lead to incomplete
calculus removal and unnecessary trauma because of
excess force applied.
MAINTAINING A CLEAN FIELD
Despite good visibility, illumination and retraction,
instrumentation can be hampered, if the operative field
is obscured by saliva, blood and debris. Adequate suction
is essential and can be achieved with a saliva ejector or,
an aspirator.
Blood and debris can be removed from the operative
field with suction and by wiping or blotting with gauze
squares. The operative field should also be flushed
occasionally with water. Compressed air and gauze
square can be used to facilitate visual inspection of tooth
surfaces just below the gingival margin during
instrumentation. Retractable tissue can also be deflected
away from the tooth by gently packing the edge of gauze
square into the pocket with the back of a curette.
INSTRUMENT STABILIZATION
Stability of the instrument and the hand is the primary
requisite for controlled-instrumentation, stability and
control is essential for effective instrumentation and to
avoid injury to the patient or clinician. The two factors
that provide stability are, instrument grasp and finger
rest (Table 36.2).
Instrument Grasp (Figs 36.2 and 36.3)
A proper grasp is essential for precise control of
movements made during periodontal instrumentation.
The most effective and stable grasp for all periodontal
instruments is the modified pen grasp. This grasp allows
precise control of the working end, permits a wide range
of movements and facilitates good tactile conduction.
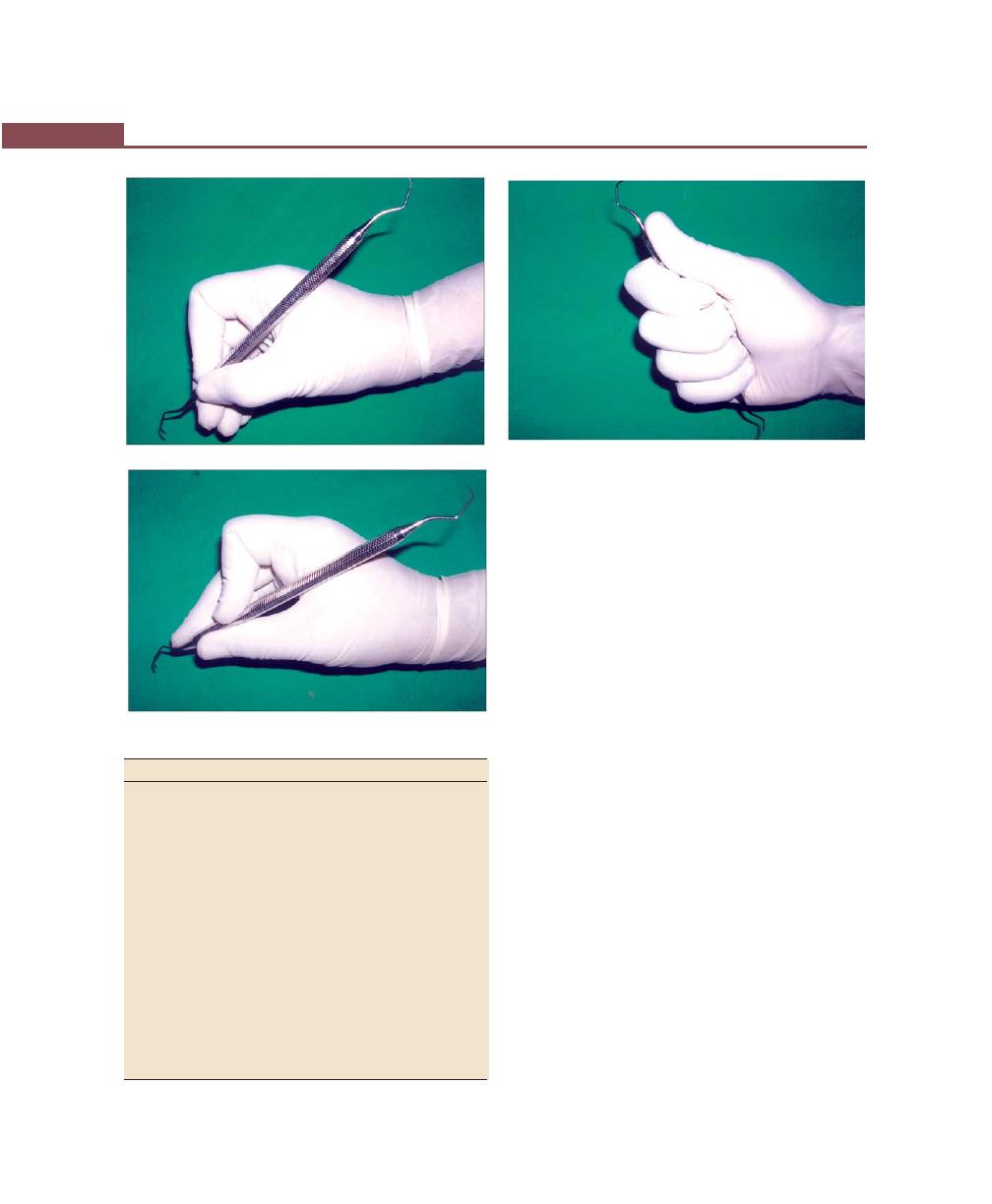
272
Essentials of Clinical Periodontology and Periodontics
The palm and thumb grasp is useful for stabilizing
instruments during sharpening and for manipulating air
and water syringes (Fig. 36.4).
Finger Rest
The finger rest serves to stabilize the hand and the
instrument by providing a firm fulcrum, as movements
are made to activate the instrument. A good finger rest
prevents injury and laceration of the gingival and
surrounding tissues. The ring finger is preferred by most
clinicians for the finger rest. Maximal control is achieved
when the middle finger is kept between the instrument
shank and the fourth finger. This built-up fulcrum is an
integral part of the wrist-forearm action that activates
the powerful working stroke for calculus removal.
Finger rests may be generally classified as intraoral
finger rests or extraoral fulcrums.
Standard Intraoral Finger Rest
The finger rests on a stable tooth surface immediately
adjacent to the working area.
Advantages:
• Provides the most stable, secured support for the
hand.
• Provides leverage and power for instrumentation.
• Provides excellent tactile transfer to the fingers.
• Permits precise stroke control.
Fig. 36.2:
Pen grasp
Fig. 36.3:
Modified pen grasp
Fig. 36.4:
Palm and thumb grasp
Table 36.2: Correct finger placement
Digit
Recommended position and function
Thumb and
The finger pads rest opposite to each other at
index
or near the junction of the handle and the shank.
They do not overlap and a tiny space exists
between them. The instrument is held in a rela-
xed manner. The index finger and thumb curve
outward from the handle in a C-shape. The main
function of these digits is to hold the instrument.
Middle
One side of the finger pad rests lightly on the
instrument shank. The other side of the finger pad
rests against the ring finger. It helps to guide the
working end and also feel the vibration.
Ring
Finger tip balances firmly on the tooth to support
the weight of the hand and instrument. The finger
is held straight and upright to act as a strong
support beam for the hand.
Little
It should be held in a relaxed manner with no
function.

273
Principles of Periodontal Instrumentation including Scaling and Root Planing
• Allows forceful stroke pressure with the least amount
of stress to the hand and fingers.
• Decreases the likelihood of injury to the patient.
Disadvantages:
• May not be practical for use in edentulous areas.
• May be difficult to obtain parallelism of the lower
shank to the tooth surface for accessing deep pockets.
Advanced Intraoral Finger Rests
a. Modified intraoral fulcrum: It is achieved by combi-
ning an altered modified pen grasp with a standard
intraoral fulcrum. It is useful while instrumenting the
maxillary teeth. It alters the point of contact between
the middle and ring fingers in the grasp.
Advantages:
• Provides good stable support for the clinician’s
hand.
• Provides leverage, strength and good stroke
control.
• Provides good tactile sensitivity to clinician’s finger.
• Improves access to deep pockets on maxillary teeth
and facilitates parallelism of lower shank to
proximal root surfaces.
Disadvantages:
• Requires more muscle control.
b. Piggy-backed fulcrum: The middle finger rests on top
of the ring finger.
Advantages
• Improved access to mandibular posterior aspects
away from the clinician.
• Enhances the whole hand working together as
a unit.
Disadvantages
• In patients with limited opening it cannot be used.
c. Cross-arch fulcrum: It is accomplished by resting the
ring finger on a tooth on the opposite side of the
arch from the teeth being instrumented.
Advantage
• Allows improved access to the lingual aspect of
mandibular posterior teeth.
Disadvantage
• Decreases tactile sensitivity and makes strokes
difficult.
d. Opposite arch fulcrum: It is accomplished by resting
the ring finger on the opposite arch.
Advantage
• Facilitates access to deep pockets.
Disadvantages
• Decreases tactile information
• Uncomfortable for patients with TMJ prob-
lems.
e. Finger-on-finger fulcrum: It is accomplished by resting
the ring finger on the index finger.
Advantages
• Provides stable rest to fulcrum finger
• Improves access to deep pockets.
Disadvantages
• Non-dominant hand cannot be used for
retraction or to hold the mirror.
f. Basic extra-oral fulcrums: They are essential for
effective instrumentation of some aspects of maxillary
posterior teeth.
I. Knuckle-rest technique or palm up technique: The
clinician rests the Knuckle against the patients chin
or cheek.
II. Chin-cup technique or palm down technique: The
clinician cups the patients chin with the palm of
the hand.
Advantages
• Facilitates instrumentation of the proximal root
surfaces of maxillary molars.
Disadvantages
• Least effective of all fulcrum techniques.
• Stroke control is more difficult and decreases
tactile information.
INSTRUMENT ACTIVATION
• Adaptation
• Angulation
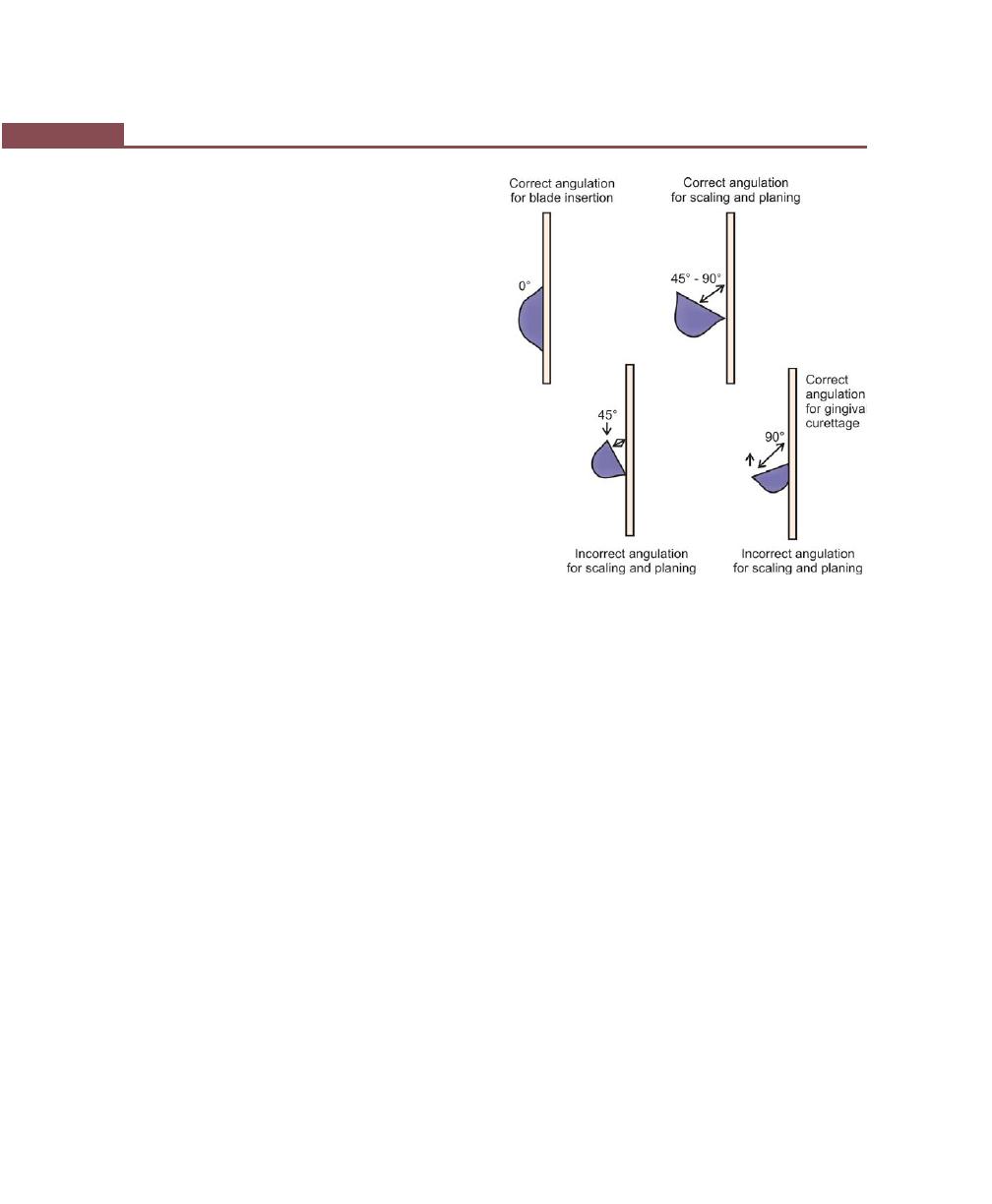
274
Essentials of Clinical Periodontology and Periodontics
• Lateral pressure
• Strokes
Adaptation
It refers to the manner in which the working end of a
periodontal instrument is placed against the surface of
a tooth. The object of adaptation is to make the working
end of the instrument conform to the contour of the
tooth surface.
The cutting edge has three imaginary sections:
1. Leading third—used more often during instrumen-
tation.
2. Middle third.
3. Heel third.
Precise adaptation must be maintained with all
instruments to avoid trauma to the soft tissues and root
surfaces and to ensure maximum effectiveness of
instrumentation. Bladed instruments such as curette and
sharp pointed instruments such as explorers are more
difficult to adapt.
Angulation (Fig. 36.5)
It refers to the angle between the face of a bladed
instrument and the tooth surface.
1. For insertion beneath the gingival margin, the face
to tooth surface angulation should be an angle
between 0 to 40 degrees.
2. For calculus removal, angulation should be between
45 to 90 degrees. The exact blade angulation
depends on the amount and nature of calculus, the
procedure being performed and condition of tissue
during scaling or root planing, with angulation of less
than 45 degrees, the cutting edge will slide over the
calculus smoothening or burnishing it. When gingival
curettage is indicated, angulation greater than 90
degrees is deliberately established.
Lateral Pressure
It refers to the pressure created when force is applied
against the surface of a tooth with the cutting edge of
a bladed instrument. Exact amount of pressure depends
upon the procedure performed. It may be firm, moderate
or light when insufficient lateral pressure is applied rough
ledges or lumps may be shaved to thin, smooth sheets
of burnished calculus.
Repeated application of excessively heavy strokes will
nick or gouge the root surface. The careful application
of varied and controlled amounts of lateral pressure
during instrumentation is an integral part of effective
scaling and root planing techniques.
Strokes (Fig. 36.6)
There are four types of strokes:
1. Placement stroke.
2. Exploratory stroke or assessment stroke.
3. Scaling stroke.
4. Root planing stroke.
The placement stroke is used to position the working
end of an instrument apical to a calculus deposit or at
the base of a sulcus or pocket. Characteristics of strokes
are described in Table 36.3.
Fig. 36.5:
Blade angulations
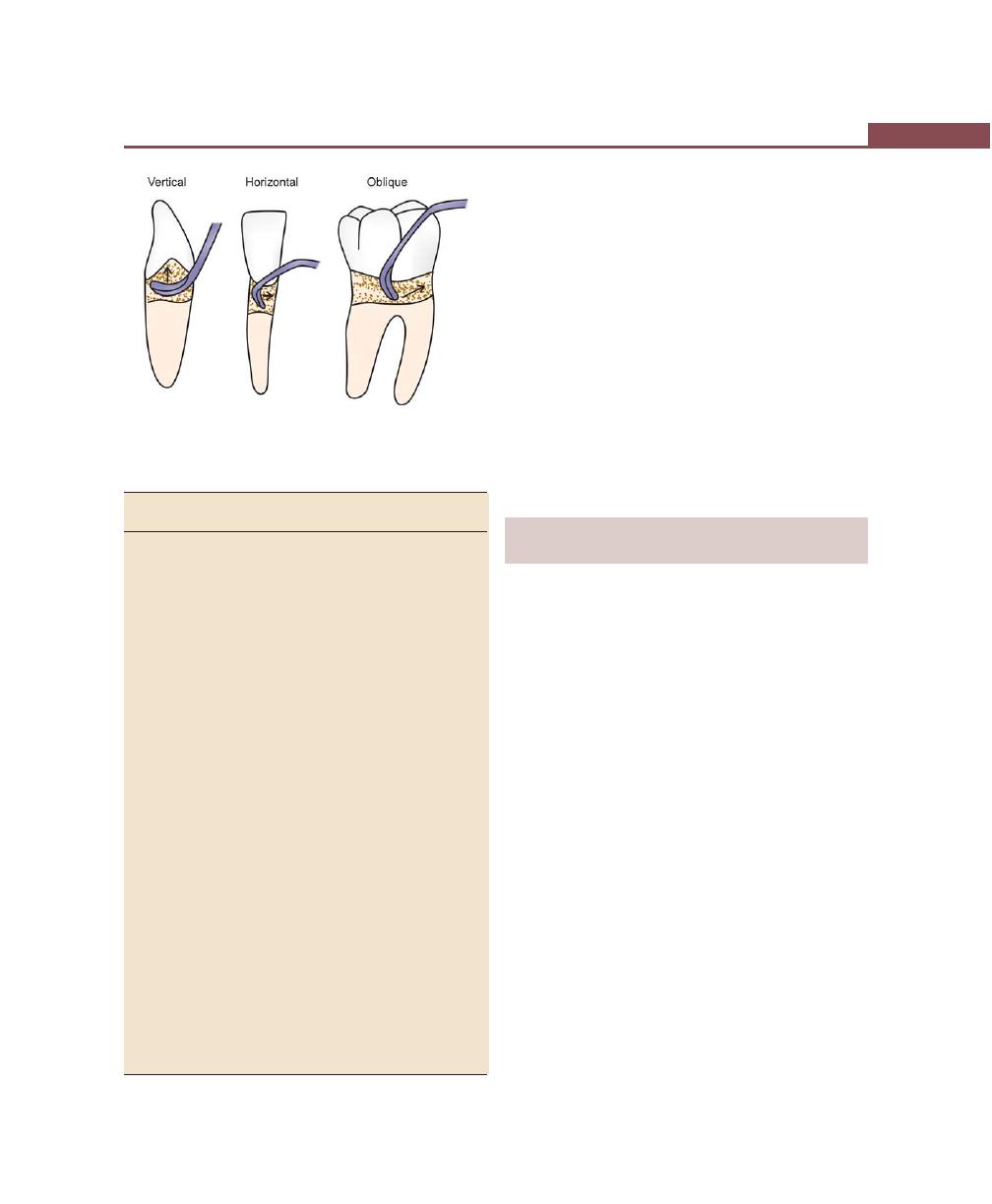
275
Principles of Periodontal Instrumentation including Scaling and Root Planing
Table 36.3: Characteristics of strokes
Assessment
Calculus removal/ Root planing
stroke
scaling stroke
stroke
Purpose Assess tooth–
Remove calculus
Remove resi-
anatomy.
deposits.
dual calculus,
Level of
bacterial pla-
attachment.
que and by-
Detect cal-
products.
culus and
other plaque
retentive
factors.
Used
Probes/explo-
Sickle scalers,
Curettes
with
rers, curettes
curettes, files
Insertion 0 to 40 degrees 0 to 40 degrees
0 to 40
degrees
Working 50 to 70
70 to 80 degrees
60 to 70
angula-
degrees
degrees
tion
Lateral
Contacts tooth
Moderate to firm
Light to
pressure surface, but no scraping
moderate
pressure
applied
Character Fluid stroke
Powerful strokes
Lighter
of moderate
short in length
strokes of
length
moderate
length
Direction Vertical,
Vertical, oblique,
Vertical,
oblique,
horizontal
oblique,
horizontal
horizontal
Number Many, covering Limited, to area
Many, cover-
entire root
where needed
ing entire
surface
root surface
Stroke Direction
Instrument strokes are initiated using a pull stroke in a
coronal direction away from the junctional epithelium.
Pull strokes may be made in vertical, oblique or
horizontal directions.
Vertical strokes
:
Facial, lingual, proximal
surfaces of anterior teeth
mesial and distal surfaces
of posterior teeth.
Oblique strokes
:
Facial and lingual surfaces
of anterior and posterior
teeth.
Horizontal strokes or :
Line angles of posterior
circumferential strokes
teeth, furcation areas.
PRINCIPLES OF SCALING AND
ROOT PLANING
Scaling
This is the process by which plaque and calculus are
removed from both supragingival and subgingival tooth
surfaces.
Root Planing
This is the process by which residual embedded calculus
and portions of cementum are removed from the roots
to produce a smooth, hard, clean surface.
The prime objective of scaling and root planing is to
restore gingival health by completely removing the tooth
surface elements that provoke gingival inflammation.
Principles of Curettes
• Universal
• Gracey
Universal curettes: The working ends of the universal
curettes are designed in pairs so that all surfaces of the
teeth can be treated with one-double ended instrument
or a matched pairs of single- ended instruments.
In any given quadrant, one end of universal curette
will adapt to the mesial surface and the other end to
Fig. 36.6:
Basic stroke directions

276
Essentials of Clinical Periodontology and Periodontics
the distal surface. The end that adapts to the mesial
surface of the facial aspects also adapts to the distal surface
on the lingual aspect and vice versa. When adapting the
universal curette blade, as much of the cutting edge
should be in contact with the tooth surface. Although
the entire cutting edge should contact the tooth, pressure
should be concentrated on the lower-third of blade
during scaling stroke. During root planing stroke,
however, lateral pressure should be distributed evenly
along the cutting edge.
In the posterior teeth, a single working end can be
used to treat both mesial and distal surfaces by using
both of its cutting edges.
Gracey curettes:
1. Determine the cutting edge by visually inspecting the
blade and confirmed by lightly adapting the chosen
cutting edge to the tooth with the lower shank parallel
to the surface of the tooth.
2. Make sure the lower shank is parallel to the surface
to be instrumented.
3. When using intraoral finger rests, keep the fourth
and middle fingers together in a built-up fulcrum for
maximum control and wrist-arm action.
4. Use extraoral fulcrum or mandibular finger rests for
optimal angulation when working on the maxillary
posterior teeth.
5. Concentrate on using the cutting edge (lower) for
calculus removal.
6. Allow the wrist and forearm to carry the burden of
the stroke, rather than flexing the fingers.
7. Roll the handle slightly between the thumb and
fingers to keep the blade adapted as the working
end is advanced around line angles and into
concavities.
8. Module lateral pressure from firm to moderate to
light depending on the nature of calculus.
Supragingival Scaling Technique
Supragingival calculus is less tenacious and less calcified
than subgingival calculus. Scaling strokes are not
confirmed by surrounding tissues.
Sickles, curettes and ultrasonic and sonic instruments
are most commonly used for the removal of supragingival
calculus. Hoes and chisels are less frequently used. To
perform supragingival scaling, the sickle is held with
modified pen grasp and a firm finger rest is established
on the teeth adjacent to the working area. The blade
is adapted at an angulation of slightly less than 90 degrees
to the surface being scaled. The cutting edge should
engage the apical margin of supragingival calculus while
short, powerful, overlapping scaling strokes are activated
coronally in a vertical or oblique direction.
Subgingival Scaling and Root Planing Technique
Subgingival calculus is usually harder than supra-gingival
calculus and is often locked into root irregularities, making
it more tenacious.
The direction and length of the strokes are limited
by adjacent pocket wall. The curette is preferred by most
clinicians for subgingival scaling and root planing because
of the advantages afforded by its design. Hoes, files and
ultrasonic instruments are also used for subgingival
scaling of heavy calculus, but are more hazardous than
the curette in terms of trauma to the root surface and
the surrounding tissues.
The curette is held with a modified pen grasp and
a stable finger rest is established. The correct cutting edge
is slightly adapted to the tooth, with the lower shank
kept parallel to the tooth surface. The working angulation
is established and calculus is removed by a series of
controlled overlapping, short powerful strokes primarily
utilizing wrist arm motion. Longer, lighter root planing
strokes are then activated with less lateral pressure until
the root surface is completely smooth and hard. Scaling
and root planing strokes should be confirmed to the
position of the tooth where calculus or altered cementum
is found. This zone is known as instrumentation zone.
Evaluation of scaling and root planing: Although
smoothness is the criteria by which scaling and root
planing are evaluated. The ultimate evaluation is based
on tissue response. Clinical evaluation should not
be conducted earlier than 2 weeks postoperatively.

277
Principles of Periodontal Instrumentation including Scaling and Root Planing
Re-epithelialization of wound created during instrumen-
tation takes 1 to 2 weeks. Any gingival bleeding on
probing noted after this interval is due to persistent
inflammation produced by residual deposits. Positive
clinical changes after instrumentation often continues for
weeks or months. So longer period of evaluation is
indicated deciding whether to intervene to further
instrumentation or surgery.
ULTRASONIC INSTRUMENTS
They use a water-cooled instrument tip, vibrating at high
frequency, to remove supragingival and subgingival
calculus deposits from the tooth and bacterial plaque
from periodontal pocket. The two categories of
mechanized instruments are ultrasonic and sonic hand
piece. Ultrasonic units are comprised of electric generator,
a hand piece and interchangeable instrument tip.
Ultrasonic devices work by converting electrical current
to mechanical energy in the form of high frequency
vibration of instrument tip. They operate at frequencies
18,000 to 50,000 cycles/sec. Two types of ultrasonic units
are magnetostrictive and piezoelectric units.
Ultrasonic instrument tip must be cooled by fluid to
prevent overheating of the vibrating instrument tip. They
have been shown to be as effective as hand instruments
in subgingival calculus removal, removal of attached and
unattached subgingival plaque, removal of toxins from
root surfaces, and in reduction and maintenance of
pocket depth.
The water lavage has three benefits on the treatment
site.
• Flushing action–flushes calculus, blood, bacteria,
plaque from treatment site.
• Cavitation.
• Acoustic streaming.
As the water exits from instrument tip, it forms a spray
of tiny bubbles that collapses and releases shock waves
in a process known as cavitation. It causes lysis of bacterial
cell wall.
The continuous stream of water produces tremendous
pressure within the confined space of periodontal pocket.
This effect is called acoustic streaming. Bacteria, Gram-
negative rods are sensitive to acoustic streaming.
Equipment and Armamentarium for Ultrasonic
and Sonic Instrumentation
Unit selection:
When purchasing equipment,
look for a unit with adjustable
power and a selection of varied
instrument tip designs.
Instrument selection:
Larger, stronger tip should be
used for heavy calculus removal,
thin tip should be used on light
deposits, de-plaquing, and
endotoxin removal.
Infection control:
Ultrasonic and sonic instru-
ments produce a high level of
aerosol contamination in dental
operatory. Use of barriers,
surface disinfectants, protective
clothing and laminar airflow
system are recommended.
Aerosol production is redu-
ced by proper patient position
(Supine, head turned), pretreat-
ment rinse with antimicrobial
solution, cupping of cheeks or
lips for water containment, and
use of high volume suction tip.
Personal protection:
Clinician should always use
recommended personal protec-
tion equipment, like gown is
high neck and long sleeves, hair
covering, mask, protective
eyewear, face shield, and gloves.
Patients should rinse with
antimicrobial solution prior to
start of ultrasonic or sonic
procedures.
Patient protection:
Personal protection gear for the
patient includes; plastic drape,
towel or bib, protective eye
wear, and hair covering cap.

278
Essentials of Clinical Periodontology and Periodontics
Water control:
Use a disposable high-volume
evacuation tip for suction, water
control reduces aerosol produc-
tion, improves the visibility of
the treatment area, and increa-
ses patient comfort.
Power level:
Thin tips may damage the root
surface if used on high power
setting. All tip designs should be
used at lowest frequency power
setting, the high setting should
be avoided.
Water:
The water spray around the
instrument tip should create a
light mist or halo effect with no
excess dripping of water.
Insufficient water can result in
trauma to pulp.
Patient chair position: Position the patient in normal
supine position. This position
reduces aerosol production,
and gagging, facilitates proper
instrumentation.
Patient head position: The head should be turned to
a side. This causes the water to
pool in the cheek minimizing
aerosol production.
Adaptation:
Adapt the side of last several
millimeters of the tip to tooth
surface. Length of the tip should
be parallel to the long axis of
tooth. Direct contact of tip to
tooth surface should be avoided.
Stroke pressure:
Light strokes
Stroke pattern:
Strokes overlap one another in
a sweeping or erasing type
motion. Multidirectional strokes
are used.
Stroke technique:
Keep the tip moving at all times.
To remove heavier deposits,
keep the tip in constant motion
while making light strokes in
sweeping motion, back and
forth, over the teeth.
Advantages of Ultrasonic and
Sonic Instruments
1. Design of modified tip: Modified tips are significantly
smaller than hand-activated curettes. Thin tips are
easier to insert and easier to adapt to root surface
concavities and furcation.
• Entire length of tip is active
• Tips have no cutting edge to cut or tear the tissue,
so less tissue damage.
• Removes less cementum so more conservative
approach to subgingival debridement.
2. Water lavage:
• Flushes calculus, debris and plaque.
• Removes blood and debris allowing better vision.
• Endotoxin removal.
• Antimicrobial effect.
3. Technique for use: These are used with a light grasp
and pressure which is less fatiguing to the clinician
4. Overhangs can be removed easily.
AEROSOL PRODUCTION
Dental procedures produce airborne particles called
aerosols, into surrounding environment. These aerosols
contain micro-organisms, blood, saliva, and oral debris.
Micro-organisms can survive upto 24 hours. Hence
patient should rinse with antimicrobial solution prior to
treatment.
Laminar airflow system, which filters the organisms
in air, is recommended.
Contraindications
Use of ultrasonic instruments is contraindicated in
patients with,
1. Cardiac pacemaker.
2. Communicable disease.
3. Respiratory disease or difficulty in breathing.
4. With compromised gag reflex, or difficulty in
swallowing.
279
Principles of Periodontal Instrumentation including Scaling and Root Planing
5. With porcelain crown, titanium dental implants,
composite resin-restoration, demineralized enamel
surface, or dentinal hypersensitivity.
6. In children: Vibrations may damage growing tissue.
Newly-erupted and primary teeth have large pulp
chamber that are more susceptible to heat generated
by instruments.
KEY POINTS TO NOTE
1. Periodontal instruments are classified as:
a. Diagnostic instruments–Probes, explorers.
b. Scaling instruments.
• Supragingival scalers.
• Subgingival scalers.
• Ultrasonic scalers.
• Supragingival scalers:
– Sickle scalers.
– Surface scalers.
– Jacquette scalers.
• Subgingival scalers:
– Hoe scalers.
– Chisel scalers.
– Root scalers.
• Ultrasonic scalers:
c. Root planing and curetting instruments
• Gracey curettes.
• Universal curettes.
d. Cleansing and polishing instruments
• Rubber cups, bristle brushes.
• Dental tape, air powder polishing.
e. Surgical instruments
2. Fundamental pre-requisites for effective instrumentation are
governed by a number of general principles like, proper
position of the patient and the operator, illumination and
retraction for optimal visibility and sharp instruments.
3. Stabilization of the instrument is very essential to avoid any
injury to the patient or clinician.
4. There are four types of strokes used in periodontal
instrumentation, placement stroke, exploratory stroke or
assessment stroke, scaling stroke and root planing stroke.
5. Scaling is the process by which plaque and calculus are
removed from both supragingival and subgingival tooth
surfaces where as root planing is the process by which
residual embedded calculus and portions of cementum are
removed from the roots to produce a smooth, hard, clean
surface.
6. The objective of scaling and root planing is to restore gingival
health by completely removing all the tooth surface elements
that can provoke gingival inflammation.
REVIEW QUESTIONS
1. How do you classify periodontal instrumentarium?
2. What are the various types of probes?
3. Describe the characteristics of a sickle scaler.
4. How are curettes different from scalers?
5. What are the modifications in curettes?
6. Name some differences in Universal and Gracey
curettes.
7. What are the principles of periodontal instrumen-
tation?
8. Define scaling and root planing.
9. What are the different types of instrument grasps
and finger rests?
10. What are the different types of strokes used in
periodontal instrumentation?
BIBLIOGRAPHY
1. Drisco CL. Scaling and root planing without over
instrumentation : Hand versus power-driven scalers. Curr
opin periodontol 1993; 78.
2. Drisko CL, Cochran DL etal. Position paper sonic and
ultrasonic scalers in periodontics. J. Periodontol 2000;
71(ll):1792.
3. Jill and Nield Gegrig. Fundamentals of Periodontal
Instrumentation, 4th edn, Lippincott Williams and Wilkins,
2000.
4. Newman, Takei, Fermin A Carranza. Clinical periodontology,
9th edn, WB Saunders and Co, 2002.
5. Wilkins EM. Clinical Practice of the Dental Hygienist, 7th
edition Baltimore, Williams and Wilkins 1994.
280
Essentials of Clinical Periodontology and Periodontics
DEFINITION OF PLAQUE CONTROL
It is the removal of microbial plaque and the prevention
of its accumulation on the teeth and adjacent gingival
surfaces.
GOALS OF PLAQUE CONTROL MEASURES
1. Plaque control retards the formation of calculus.
2. Removal of plaque leads to resolution of gingival
inflammation.
3. Good plaque control facilitates return to and
preservation of oral health.
RATIONALE
Controlling periodontal disease by regular plaque
removal is based on the fact that if supragingival plaque
is left undisturbed, it will become subgingival with the
potential to become colonized by pathogenic bacteria.
BASIC APPROACHES FOR PLAQUE CONTROL
There are two basic approaches for plaque control:
1. Mechanical:
• Individual
• Professional—for subgingival plaque control, e.g.
scaling and root planing.
2. Chemical:
• Individual
• Professional.
Mechanical Plaque Control
Individual mechanical plaque control is achieved by:
I. Toothbrush: Manual or powered
II. Interdental aids:
• Dental floss:
– Unwaxed
– Waxed
• Triangular tooth picks:
– Hand held
– Proxa-pic
❒
❒
❒
❒
❒ DEFINITION
❒
❒
❒
❒
❒ GOALS OF PLAQUE CONTROL MEASURES
❒
❒
❒
❒
❒ RATIONALE
❒
❒
❒
❒
❒ BASIC APPROACHES FOR PLAQUE
CONTROL
• Mechanical
• Chemical
❒
❒
❒
❒
❒ MECHANICAL PLAQUE CONTROL
• Manual Toothbrushes
• Powered Toothbrushes
• Interdental Aids
• Others
❒
❒
❒
❒
❒ CHEMICAL PLAQUE CONTROL
• Ideal Requirements of Mouthwash
• Classification of Antimicrobial Agents
37
Plaque Control

281
Plaque Control
• Brushes: Proxa brush, bottle brushes
• Yarns, gauze strips, pipe cleaners.
III. Others:
• Rubber tip stimulator
• Water irrigators.
Toothbrushes (Fig. 37.1)
The following aspects are discussed:
a. Historical background.
b. ADA specification of a toothbrush.
c. Design of the toothbrush.
d. Brushing techniques.
e. Instruction in toothbrushing.
a. Historical background
i. Initially aromatic plants like chewing twigs were
used and Miswak from arrack trees were used.
ii. Then Chinese invented the modern toothbrush
in the year 1600.
iii. In 1,780, William Addis designed a toothbrush
with bone handle and hog bristles.
iv. In 1,900 celluloid (plastic) brushes with bone
handle were introduced.
v. World War II—Nylon bristles were introduced.
b. ADA specifications of a toothbrush: The head of the
brush should be:
i. 1 inch to 1
1
/
4
inches long.
ii. 2 – 4 rows of bristles.
iii.
5
/
16
inch to
3
/
8
inches wide.
iv. 5 – 12 tufts per row.
v. 80 – 86 bristles per tuft.
c. Design of the toothbrush: A toothbrush consists of
handle, shank and head. It has bristles which when
bunched together are called tufts. The extreme end of
the head is toe and that, close to the handle is the heel.
• Size—Large, medium and small.
• Lateral profile—Flat, convex, concave and scalloped.
Bristles: Two types of bristles are available, nylon
(synthetic) and natural (hog). Nylon bristles are preferred.
Natural bristles are more susceptible to breakage and
fraying, contamination with bacteria is high.
Hardness: Depends on material, diameter and length.
• Nylon bristles are more flexible.
• Soft: 0.007 inches to 0.009 inches (No 7, 8, 9)
• Medium: 0.010 inches to 0.012 inches (No. 10, 11,
12)
• Hard: 0.013 inches to 0.014 inches (No. 13, 14)
• Extra hard: 0.015 inches (No. 15)
If the bristles are soft, they should be set close. If
they are hard they should be more widely spaced.
Handle design:
i. Straight
ii. Angulation in the shank.
iii. Indentation of handle for a better grip.
• Frequency of brushing—Every 12 hours.
• Frequency of change of brush—Every 3 months.
• Length of brushing time—Initially 10-20 minutes is
required until the patient becomes more precise. Later
3-5 minutes may suffice.
d. Brushing motions in brushing techniques:
i. Horizontal:
• Reciprocating (Scrub)
ii. Vibratory:
• Bass method
• Stillman’s method
• Charter’s method
Fig. 37.1:
Different types of toothbrushes

282
Essentials of Clinical Periodontology and Periodontics
iii. Vertical sweeping:
• Rolling stroke
• Modified bass
• Modified Stillman’s
• Leonard
• Smithbell (Physiological technique)
iv. Rotary–Fones.
BRUSHING TECHNIQUES
The Bass method:
Also called as intrasulcular method.
Technique:
1. Place the head of a soft brush parallel to the occlusal
plane, with the brush head covering three teeth,
beginning at the most distal tooth in the arch.
2. Place the bristles at the gingival margin, establishing
an angle of 45 degrees to the long axis of the teeth.
3. Exert gentle vibratory pressure, using short back and
forth motions without dislodging the tips of the bristles.
This forces the bristle ends into the sulci, as well as
into interproximal embrassures and should produce
perceptible blanching of the gingiva.
4. Complete 20 strokes in the same position.
5. Lift the brush, move it anteriorly and repeat the
process for next three teeth. To help reach the lingual
surfaces of the anterior teeth, if the brush seems too
large, insert the brush vertically. Press the heel of the
brush into the gingival sulci and proximal surfaces
at a 45° angle to the long axis of the teeth. Activate
the brush with 20 short vibratory strokes.
To reach occlusal surfaces, press the bristles firmly
into the pits and fissures. Activate the brush into 20 short
back and forth strokes, advancing section by section
until all posterior teeth in all quadrants are cleaned.
To reach distal surfaces of the last tooth in the
arch, open the mouth wide and vibrate the tip of
the brush against that surface, 20 times for each tooth.
Advantages
1. Short back and forth motion is easy to master.
2. Cleaning action is concentrated on the cervical and
interproximal portions of the teeth, where most of
the dental plaque detrimental to the gingivae is located.
Errors: As described by Perry and Schmid
1. Placement of bristles on the attached gingiva, rather
than into the gingival sulcus. When the brush is
activated, the gingival margin and tooth surfaces are
neglected; whereas the attached gingiva and alveolar
mucosa are traumatized.
2. Bristles are pressed against the teeth rather than
directed into the gingival sulci. Activating the brush
cleans the facial surfaces but misses the interproximal
surfaces and surfaces along the gingival margin.
3. Relaxing the arm owing to tiredness so that the brush
is allowed to slide down, creating an angle between
the occlusal plane and the long axis of the brush.
This prevents the main bulk of bristles from adequately
penetrating interproximally and into the gingival
sulci.
Fig. 37.2:
Modified Bass method
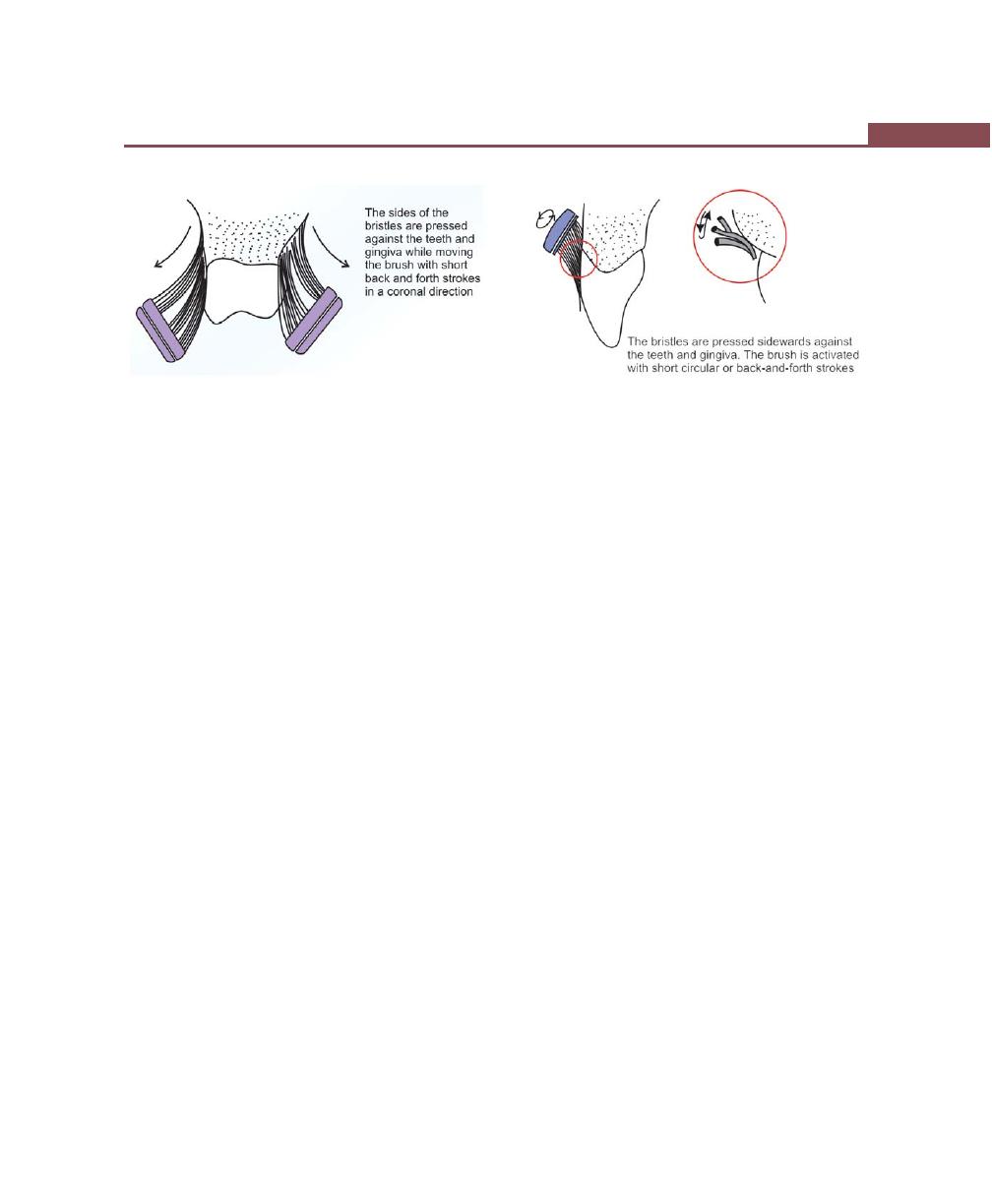
283
Plaque Control
Modified Bass method (Fig. 37.2)
The first part of the modified Bass method is identical
to the Bass method. The modification consists of sweeping
the bristles downwards over the tooth surface occlusally
after completing the vibratory motion in the gingival
sulci.
Stillman’s method (Fig. 37.3)
With Stillman’s technique the bristle ends are placed at
a 45° angle, with the bristles placed partly on the gingiva
and partly on the cervical portion of the teeth.
Once the bristles are in place pressure is applied to
blanch the gingiva and a gentle but firm vibratory rotary
motion is applied to the brush with the bristles remaining
in the same position.
Advantage: It removes soft deposits from cervical areas.
Modified Stillman’s technique:
1. The soft or medium, multitufted brush should be
placed with the bristle ends resting partly on the
cervical portion of the teeth and partly on the adjacent
gingiva, pointing in an apical position, directed at an
oblique angle to the long axis of the teeth.
2. Pressure is applied laterally against the gingival margin
to produce a perceptible blanching.
3. The brush is activated with 20 short back and forth
strokes and is simultaneously moved in a coronal
direction along the attached gingiva, the gingival
margin and tooth surface. This process is repeated
on all the teeth.
To reach lingual surfaces of the maxillary and
mandibular incisors, the handle of the brush is held
in a vertical position, engaging heel of the brush. With
this technique the sides rather than the ends of the
bristles are used, penetration of the bristles into
gingival sulci is avoided.
Advantages: The modified Stillman method may be
recommended for cleaning areas with progressing
gingival recession and root exposure to prevent abrasive
tissue destruction.
The Charter’s method (Fig. 37.4)
1. A soft or medium, multi tufted brush is placed on
the tooth with bristles pointing towards the crown
at a 45° angle to long axis of the teeth.
2. The sides of bristles are flexed against the gingiva,
and the back and forth vibratory motion is used to
massage the gingiva.
Advantages: This method is effective particularly in cases
with receded interdental papillae, it is suitable for gentle
plaque removal and gingival massage, when using soft
brush, and this technique can be recommended for
temporary cleaning in areas of healing wounds after
periodontal surgery.
Roll method/Fones technique:
Technique: The bristles are first directed apically and then
swept in an occlusal direction with a rolling motion.
Advantages: It is popular because it is very easy to learn.
Fig. 37.3:
Modified Stillman method
Fig. 37.4:
Charter’s method
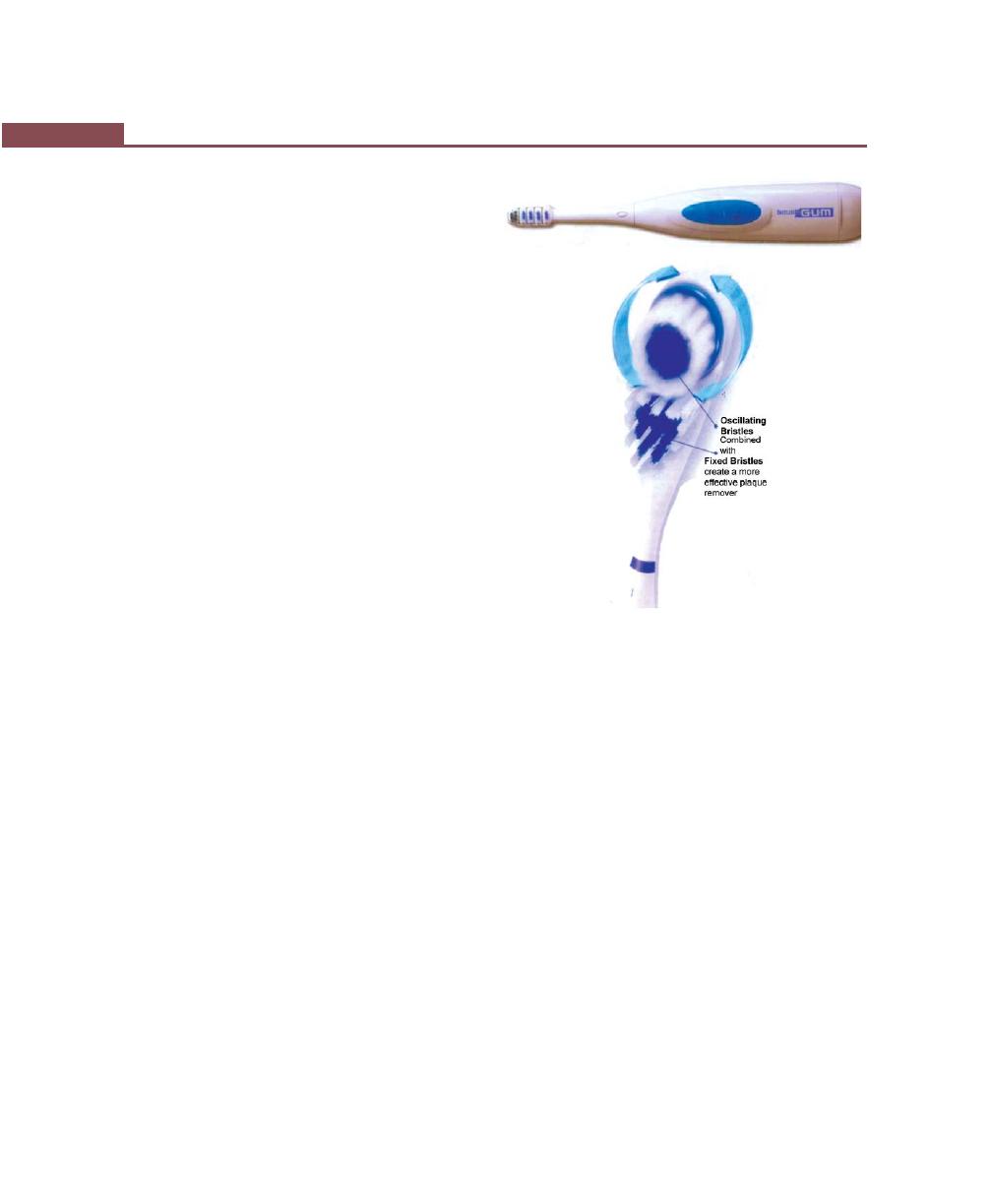
284
Essentials of Clinical Periodontology and Periodontics
Disadvantage: Rolled-gingival margins prevent removal
of plaque from sulcus area.
Scrub technique:
• It is the simplest method.
• Consists of merely placing the bristles next to the teeth
and moving them back and forth or scrubbing.
Advantage:
It is easy to master.
Disadvantage:
Poor plaque removal causes cervical abrasion and gingival
recession.
e. Instructions in brushing:
i. It should be started on the last/terminal teeth.
ii. Three teeth are covered at a time.
iii. 20 strokes are used in each area.
iv. Overzealous brushing can lead to gingival
recession, wedge-shaped defects in the cervical
areas of root surfaces and painful ulceration of
the gingiva. This type of brushing technique
should be identified and discouraged.
v. To maintain cleaning efficacy toothbrushes must
be replaced as soon as the bristles begin to fray.
Powered Toothbrushes (Fig. 37.5)
They were introduced in 1939. Various types of motions
used in powered toothbrushes are:
1. Reciprocal or back and forth.
2. Circular.
3. Elliptical or combination.
Powered toothbrushes are recommended for:
1. Individuals lacking fine motor skills.
2. Small children or handicapped or hospitalized
patients who need to have their teeth cleaned by
someone else.
3. Patients with orthodontic appliances.
4. Patients who prefer them.
Conclusion: In conclusion, no specific toothbrush can
be singled out as clearly superior for the routine removal
of microbial deposits from the teeth. It differs greatly
among individuals and should be recommended after
considering factors such as the morphology of the
dentition, periodontal health and manual dexterity.
Dentifrices
• These are the aids for cleaning and polishing of teeth
surfaces.
• They are used in the form of powders, pastes and
gels.
Composition of tooth paste:
1. Abrasives such as 20 to 40 percent CaCO
3
, Ca
3
(PO
4
)
2
both of which react with fluoride. Now,
silicon oxides, Al
2
O
3
, granular polyvinylchloride
are used.
2. Humectants: 20 to 40 percent; maintains moisture,
e.g. glycerine, sorbitol, mannitol, propylene glycol.
3. Preservatives: Such as benzoic acid.
4. Thickening agents: Synthetic sodium carboxy-
methyl cellulose is used.
Figs 37.5A and B:
Powered toothbrush
A
B
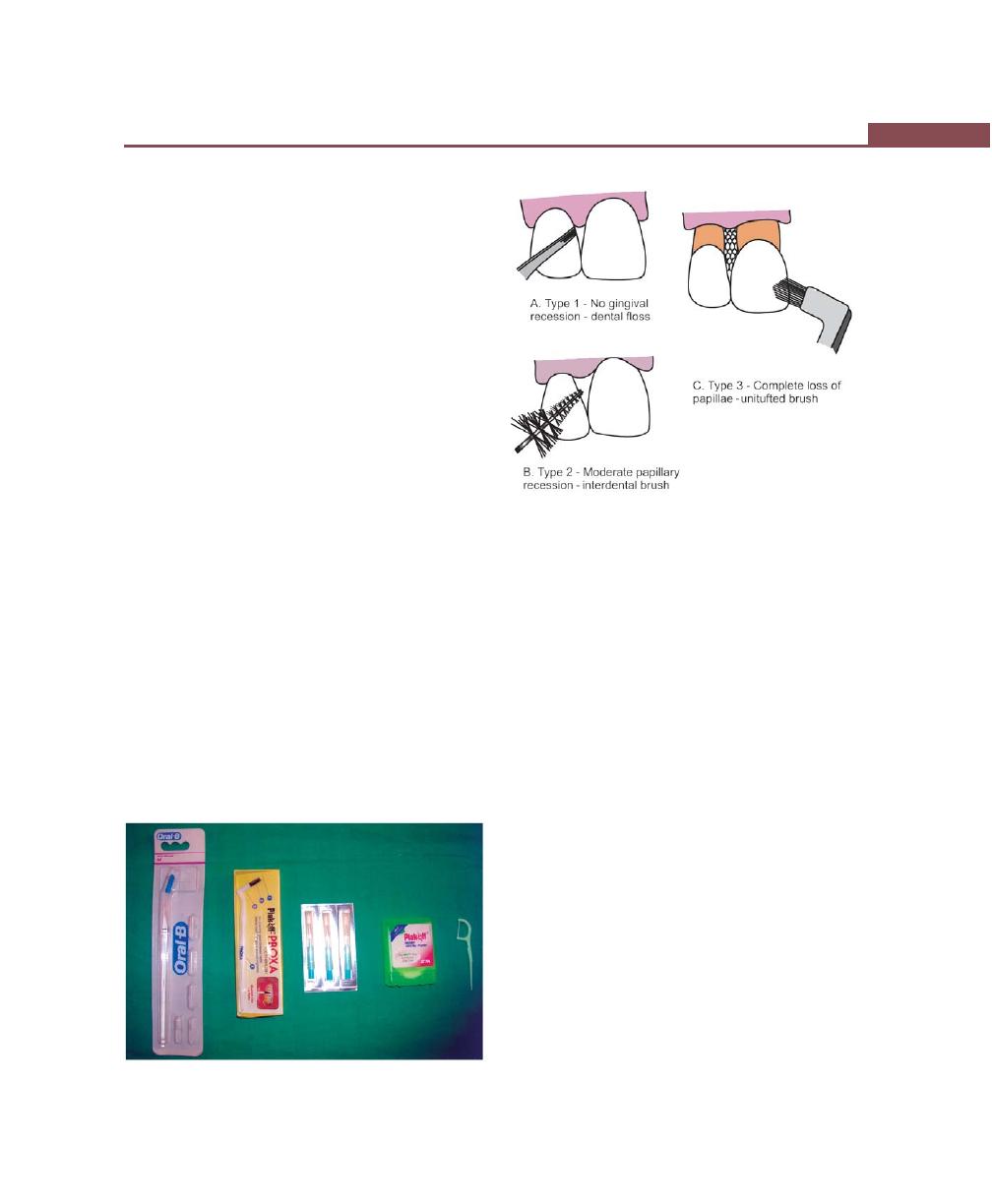
285
Plaque Control
5. Water: 20 to 40 percent.
6. Foaming agents: 1 to 2 percent soap/detergent,
e.g. sodium lauryl sulphate.
7. Flavoring and sweetening agents:
• Two percent essential oils and synthetic flavors,
e.g. mint and others.
• Sweetening agents such as saccharine, sorbitol,
mannitol.
8. Desensitising agents: Upto to 2 percent strontium
salts, sodium fluoride, formalin, potassium nitrate
and others.
9. Coloring and preservatives: <1 percent
10. Anticaries agents: Like sodium monofluoro-
phosphate, sodium fluoride, formalin. Dentifrices
containing pyrophosphates or zinc compounds
have reportedly shown 10 to 50 percent reduction
in calculus. They are thought to produce this
effect by the fact that they are absorbed on to
hydroxyapatite crystals thus inhibiting the growth
of larger organized crystals.
Interdental Cleaning Aids (Fig. 37.6)
Toothbrush regardless of the brushing method used does
not completely remove interdental plaque either in
individuals with healthy periodontal support or in
periodontally-involved patients, with open embrasures.
Interdental plaque removal is of importance because
most of the pathogenic organisms originate in the
interproximal areas. Among the numerous aids available,
dental floss and interdental cleaners such as wooden or
plastic tips and interdental brushes are most commonly
recommended.
Factors determining the selection of interdental aids are
the type of embrasures (Fig. 37.7)
• Type 1: The interdental papilla fills up the embra-
sure. Dental floss is advised
• Type 2: Moderate papillary recession is seen in such
situations, miniature interdental brushes
and wood tips are recommended.
• Type 3: Where there is complete loss of papilla and
interdental gingiva is tightly bound to
underlying bone (seen in diastema). Unitu-
fted brushes are recommended.
Dental floss:
It is the most commonly recommended method of
removing plaque from interdental areas.
Types of dental floss:
1. Twisted or non-twisted
2. Bonded or non-bonded
3. Waxed or unwaxed
4. Thick or thin.
Fig. 37.6:
Interdental aids
Fig. 37.7:
Types of embrasures with interdental
cleansing aids

286
Essentials of Clinical Periodontology and Periodontics
Factors determining the choice of dental floss:
a. Tightness of tooth contacts.
b. Roughness of proximal surfaces.
c. The patient’s manual dexterity.
The floss must contact the proximal surface from
line angle to line angle to clean effectively. It should also
cover the entire proximal surface, not just slipped apical
to the contact area.
Technique:
The floss should be at least 12 to 18 inches long. It is
wrapped around the fingers or the ends may be tied
together in a loop.
After stretching the floss between thumb and
forefinger, pass it gently through each contact area in
a back and forth motion.
Once the floss is apical to the contact area, move
it up along the tooth till the contact area and down into
the sulcus again, this is repeated several times and the
same is repeated on the proximal surfaces of other
teeth.
Using floss holder: Indicated in patients with low
manual dexterity and for handicapped and hospitalized
patients in cleaning their teeth. Ideally, floss holder should
possess forks that are rigid enough to hold the floss firmly
and simple mounting mechanism, but the disadvantages
of floss holders are more time consuming and they must
be rethreaded whenever the floss becomes soiled or
frayed.
Interdental brushes:
They are cone-shaped or cylindrical brushes made of
bristles mounted on a handle. Two types are available:
a. Single tufted brushes.
b. Small conical brushes—They are mainly useful to
clean large, irregular concave tooth surfaces adjacent
to wide interdental spaces.
Technique: They are inserted interproximally and are
activated with short back and forth strokes in between
the teeth. They are most useful in furcation areas, isolated
gingival recession and on the lingual surfaces of
mandibular molars and premolars.
Wooden tips: Wooden tips are either with or without
handle. Soft triangular wooden toothpicks such as Stim-
U – Dent are placed in the interdental space in such
a way that the base of the triangle rests on the gingiva
and the sides are in contact with the proximal tooth
surfaces and it is moved in and out of the embrasure
removing soft deposits from the teeth and also
mechanically stimulating the papillary gingiva but its
usefulness is limited to the facial surfaces in the anterior
region of the mouth.
Wooden toothpicks can be attached to a handle.
Example Perio – Aid and can be used on the facial and
lingual surfaces throughout the mouth.
Other Aids
Gingival massage: Can be performed with a toothbrush,
rubber tip stimulator or interdental cleaning devices. It
produces epithelial thickening, increased keratinization
and increased mitotic activity in the epithelium and
connective tissue. It is questionable whether the above
mentioned factors can provide substantial protection
against microorganisms or not. But these methods also
provide plaque control hence the plaque removal effect
is far more important to periodontal health.
Oral Irrigation devices: They are of several types, one
can use water faucet to irrigate between and around
the teeth. The water pressure is steady and is controlled
by turning the faucet handle. Others use an intermittent
water jet. Oral irrigators clean non-adherent bacteria and
debris from the oral cavity. It has been shown to disrupt
and detoxify subgingival plaque and can be useful in
delivering antimicrobial agents into periodontal pockets
(subgingival irrigation). Currently, there are two types
of irrigator tips useful for subgingival irrigation. One is
the cannula type tip recommended for office use and
other is a soft rubber tip for patient’s use at home.
Chemical Plaque Control
Mechanical plaque removal remains to be a primary
preventive method to control dental diseases and it
should not be replaced by chemical plaque control.

287
Plaque Control
However, chemical plaque control can be used as an
adjunct to effectively control gingival inflammation and
prevent the recurrence or progression of periodontal
disease. Chemical methods are very effective during
phase I therapy, for patients with recurrent problems,
ineffective plaque control for any reason and for use
after periodontal or oral surgery.
The ADA Council on Dental Therapeutics has adopted
a program for acceptance of plaque control agents. The
agents must be evaluated in placebo controlled clinical
trials of 6 months or longer and demonstrate significantly
improved gingival health compared with controls. To date,
only two agents have been accepted by ADA for
treatment of gingivitis: chlorhexidine digluconate mouth
wash and essential oil mouth rinse.
Ideal Properties of a Mouthwash
It should,
1. Eliminate pathogenic micro-organisms only.
2. Prevent development of resistant bacteria.
3. Exhibit substantivity.
4. Be safe to oral tissues at the recommended
concentration.
5. Significantly reduce plaque formation and gingivitis
6. Inhibit calcification of plaque to calculus.
7. Not stain and alter taste.
8. Not have adverse effects on teeth or dental
materials.
9. Be easy to use.
10. Be inexpensive.
Classification of Antimicrobial Agents
Depending on antimicrobial efficacy and substantivity.
First Generation Agents: Reduces plaque scores by 20
to 50 percent, efficacy is limited by their poor retention
in the oral cavity. Hence, used 4 to 6 times daily (poor
substantivity)
Examples: Antibiotics, quaternary ammonium
compounds, phenols and sanguinarine.
Second Generation Agents: These are retained
longer in the oral cavity or tissues and slow release
property provides overall reduction in plaque score by
70 to 90 percent; used 1 to 2 times daily (higher
substantivity).
Example: Bisbiguanides.
Third Generation Agents: It should be effective against
specific periodontopathic organisms. Yet to be developed
clinically.
Chemicals Used for Supragingival Plaque
Control [Addy’s classification]
A. Antibiotics
• Penicillin
• Vancomycin
• Kanamycin
• Erythromycin
• Spiramycin
• Metronidazole
B. Enzymes
• Mucinase
• Protease
• Lipase
• Amylase
• Elastase
• Lactoperoxidase
• Hypothiocynase
• Mutanase
C. Quaternary ammonium compounds
• Cetylpyridinium chloride
• Benzethonium chloride
• Benzalkonium chloride
• Domiphen bromide
D. Bisbiguanides
• Chlorhexidine
• Alexidine
• Octenidine/Bispyridines
E. Metallic salts
• Copper
• Tin
• Zinc
F. Herbal extracts
• Sanguinarine

288
Essentials of Clinical Periodontology and Periodontics
G. Fluorides
• Strontium Fluoride
H. Oxygenating agents
• Hydrogen peroxide
I. Phenolic compounds
• Thymol
• Menthol
• Eucalyptol
J. Other antiseptics
• Iodine
• Povidone iodine
• Sodium hypochlorite
• Hexetidine
• Triclosan
A chemical approach to therapy can be used for
either of the following purposes:
1. Prevention or chemoprophylaxis
2. Treatment or chemotherapy
Based on these purposes, antimicrobials are divided
into two groups:
1. Preventive agents—which affect development of
supragingival plaque.
2. Therapeutic agents—which are directed against
subgingival plaque.
The chemotherapeutic agents would be either:
1. Non-specific: Affecting all plaque bacteria uniformly
and leading to a quantitative reduction in plaque.
2. Specific: Acting as a ‘Silver bullet’ to quantitatively
reduce only the periodontopathic plaque bacteria.
Bisbiguanides (Chlorhexidine)
Indications and uses
1. Used as an adjunct to mechanical oral hygiene in
initial periodontal therapy.
2. During postsurgical period—immediately after pack.
removal, complete plaque control can be achieved
without extensive use of proximal cleaning aids
(which are painful to use and may delay healing).
3. Improves healing after routine oral surgical proce-
dures and in the post-operative management of
immediate denture construction.
4. Patients wearing fixed orthodontic appliances or
intermaxillary fixation devices will benefit from a daily
rinse with a 0.2 percent chlorhexidine solution (also
available in 0.12% concentration).
5. For handicapped patients whose plaque control and
gingival status are often very poor.
6. Chlorhexidine will help to control plaque accumula-
tion in patients with drug-induced gingival over-
growth.
7. In medically-compromised patients who suffer from
recurrent generalized oral infections.
8. It can also be advised in patients with local, oral
infections such as denture induced stomatitis, apthous
ulceration, dry socket and acute ulcerative gingivitis.
9. Finally, it can be used as a prophylactic rinse in the
prevention of post-extraction bacteremia, dry sockets
and to reduce the bacterial content of the aerosol
sprays during the ultrasonic scaling.
Disadvantages/unwanted effects
1. Extrinsic staining of teeth.
2. Painful, desquamative lesions on the oral mucosa may
be associated with burning sensation.
3. Impaired taste sensation.
4. Parotid swelling is rare—due to mechanical
obstruction of parotid duct.
Mechanism of action: It has a broad spectrum of
antibacterial activity. In general, gram-positive bacteria
are more susceptible as compared to gram-negative
bacteria. In relatively high concentrations, Chlorhexidine
is bactericidal but low concentrations may be
bacteriostatic to susceptible bacteria.
The cationic molecules of chlorhexidine bind readily
to the oppositely charged cell wall and interfere with the
membrane transport initiating a leakage of low molecular
weight substances. In high concentrations chlorhexidine
penetrates the cell and causes precipitation of the
cytoplasm. This explains the bactericidal action of
Bisbiguanides in general.
When 10 ml of 0.2 percent chlorhexidine solution
is used as a mouthwash for one minute, 30 percent of
the drug is retained in the mouth due to its property

289
Plaque Control
of substantivity (i.e. the bound drug is released slowly
over a period of 8 to 12 hours). No ill effects have been
reported following small quantities of chlorhexidine being
swallowed since it is poorly absorbed from gastro intestinal
tract and is excreted in faeces. Long term human trials
have shown that, 0.12 percent chlorhexidine is equally
effective in plaque inhibition when used as a mouth rinse
(twice daily) however; chemical plaque control cannot
be used as a supplement to scaling and root planing
(monotherapy) as it is not so effective in controlling total
plaque inhibition. Commercially available products in
the concentration of 0.2 percent are Rexidine, Hexidine,
Clohex; in 0.12 percent, as Periogaurd.
Essential oil rinse: Contains thymol, eucalyptol, menthol
and methylsalicylate. It has been proved to be effective
in reducing plaque and gingival scores. Commercially
available as Listerine
®
(Discussed in detail in chapter 50).
Disclosing agents (Fig. 37.8): Offers following
advantages:
• Discloses plaque.
• Permits patients to evaluate their performance at
home.
Various disclosing agents are:
1. Erythrosine dye:
• FDC No.3
2. Two-tone dye:
• FDC No.3—Red
• No.3—Green
It can differentiate older to newer plaque; old plaque
stains deep violet and new plaque pale violet.
3. Plaque lite system: Available as wafers/tablets or
solutions which are swished around the mouth &
excess dye is removed by rinsing the mouth.
KEY POINTS TO NOTE
1. Plaque control is defined as the removal of microbial plaque
and the prevention of its accumulation on the teeth and
adjacent gingival surfaces.
2. There are two basic approaches for plaque control,
i. Mechanical by means of tooth brush, and inter-dental
aids,
ii. Chemical by means of various chemical agents like
chlorhexidine, phenols, quaternary ammonium com-
pounds.
3. The chemical agents are very effective when used along with
the mechanical means. However, the plaque inhibiting effect
is not as striking when used as a monotherapy.
4. To date, mechanical plaque control seems to be the gold
standard in preventing dental diseases. However, chemicals
can be used as an adjunct to mechanical therapy.
5. For the treatment of gingivitis only two agents have been
accepted by ADA, chlorhexidine mouth wash and essential
oil mouth wash.
REVIEW QUESTIONS
1. What are the ideal requirements of a toothbrush?
2. Describe the various brushing techniques.
3. What is the composition of a toothpaste?
4. What are interdental aids? What are its selection
criteria?
5. Classify the chemicals used for plaque control.
6. What is the composition of disclosing agents and add
a note on its uses?
7. What are the ideal properties of a mouthwash?
8. Write in detail on chlorhexidine gluconate.
Fig. 37.8:
Disclosing solution
290
Essentials of Clinical Periodontology and Periodontics
BIBLIOGRAPHY
1. Christon V, Timmerman MF, Van der Veldenll et al.
Comparison of different approaches of interdental oral
hygiene: Interdental brushes versus dental floss.
J Periodontol 1998;69:759.
2. Heasman PA, McCracken GI. Powered toothbrushes: A review
of clinical trials. J Clin Periodontol 1999;26:407.
3. Jan Lindhe. Clinical Periodontology and Implant Dentistry,
fourth edition (2003), Blackwell Munksgaard Publication.
4. Jepson S. The role of manual toothbrushes in effective plaque
control; advantages and limitation. Proceedings of the
European Workshop on Mechanical Plaque Control Chicago,
Quintessence, 1998.
5. Karen Baker. Mouthrinses in the prevention and treatment
of periodontal disease. Curr Opin Periodontol 1993;11-128.
6. Newman, Takei, Fermin A Carranza. Clinical Periodontology,
ninth edition, (2002), WB Saunders.
7. Robin A Seymour, Peter A. Heasman. Drugs, Diseases
and the Periodontium Oxford University Press Publication,
1992.
8. Wilkins EM. Oral disease control: Toothbrushes and
Toothbrushing in: Clinical Practice of the Dental Hygienist,
sixth edition, Philadelphia 1992.
291
Principles of Periodontal Surgery
INDICATIONS OF PERIODONTAL
SURGERY
1. Areas with irregular bony contours, deep craters and
others requiring a surgical approach.
2. Deep pockets where complete removal of root irritants
is not possible, especially in inaccessible areas like
molars and premolar areas.
3. In cases of Grade II and III furcation involve-
ment, where apart from removing local irritants,
necessary root resection or hemisection can be
considered.
4. Infrabony pockets in non-accessible areas which are
not responsive to non surgical methods.
5. Persistent inflammation in areas with moderate and
deep pockets.
6. Correction of mucogingival problems.
CONTRAINDICATIONS OF
PERIODONTAL SURGERY
These may be oral or systemic.
1. In patients of advanced age where teeth may last
for life without resorting to radical treatment
(Procedures indicated in a person of 60 years of age
may not be justified in someone of 70 years of age).
2. Patients with systemic diseases such as cardiovascular
disease, malignancy, liver diseases, blood disorders,
uncontrolled-diabetes, consultation with the patient’s
physician is essential.
3. Where thorough subgingival scaling and good home
care will remove or control the lesion.
4. Where patient motivation is inadequate.
5. In the presence of infection.
6. Where the prognosis is so poor that tooth loss is
inevitable.
GENERAL PRINCIPLES OF SURGERY
a. Preparation of the patient
b. The general conditions that are common to all
periodontal surgical techniques and,
c. Complications that may occur during or after
surgery.
Section 2: (B) SURGICAL THERAPY
❒
❒
❒
❒
❒ INDICATIONS
❒
❒
❒
❒
❒ CONTRAINDICATION
❒
❒
❒
❒
❒ GENERAL PRINCIPLES
• Preparation of the Patient
• General Conditions
❒
❒
❒
❒
❒ COMPLICATIONS DURING SURGERY
❒
❒
❒
❒
❒ HOSPITAL PERIODONTAL SURGERY
38
Principles of
Periodontal Surgery
292
Essentials of Clinical Periodontology and Periodontics
PREPARATION OF THE PATIENT
Almost every patient has to undergo the initial or
preparatory phase of therapy. (scaling + root planing
and removal of etiotropic elements) because it:
I. Eliminates some lesions completely.
II. Renders the tissues more firm and consistent, thus
facilitating more accurate and delicate surgery
and,
III. Acquaints the patients with the office and with
the operator and assistants, thereby reducing the
patient’s apprehension and fear. The re-evaluation
phase consists of re-probing and re-examining all
the findings that previously indicated the need
for the surgical procedure. Persistence of these
findings will confirm the indication for surgery.
The number and dates of the surgical procedures,
the outcome and the postoperative care that is
needed are all decided before hand. Informed
consent should be taken from the patient after
explaining the details of surgical procedures, both
verbally and in writing.
General Conditions that are
Common to All Procedures
Premedication
For normal patients their use is not clearly demonstrated.
The prophylactic use of antibiotics has been advocated
for both medically-compromised patients as well as
patients undergoing bone-grafting procedures.
Emergency equipment should be readily available at all
the times.
All the measures should be taken to prevent the
transmission of infections. These include the use of
disposable gloves, surgical masks and protective eye wear.
All surfaces that may be contaminated with blood or
saliva and cannot be sterilized, must be covered with
aluminium foil/plastic wrap. Ultrasonic scaling is
contraindicated in patients with infectious diseases, as
it generates aerosols and special care should be taken
while using it (Pre-procedural mouth rinsing).
Sedation and Anesthesia
In order to prevent pain during the surgery, the entire
area to be treated should be thoroughly anesthetized
by means of a regional block and local infiltration. Patients
who are apprehensive and neurotic may require special
management with agents like sedatives and anti-anxiety
drugs.
Tissue Management
1. Operate gently and carefully: In addition to being
most considerate to the patient, tissue manipulation
should be gentle because it produces excessive tissue
injury; causes postoperative discomfort and delays
healing.
2. Observe the patient at all times.
3. Be certain the instruments are sharp: Dull instruments
will cause unnecessary trauma because of excess force
usually applied to compensate for their ineffective-
ness.
Suturing
Suturing materials are classified as either non-absorbable
or absorbable.
Non-absorbable
• Natural: example—braided-silk
• Synthetic: example—Dacron-coated and impregna-
ted with teflon.
Absorbable
• Natural: example—Surgical gut.
• Synthetic: example—Polyglycolic acid derivatives like
vicryl.
Goals of suturing:
1. Maintains hemostasis
2. Permits healing by primary intention
3. Reduces postoperative pain
4. Permits proper flap position
5. Prevents bone exposure resulting in delayed healing
and unnecessary resorption.
Parts of surgical needles:
1. Eye.
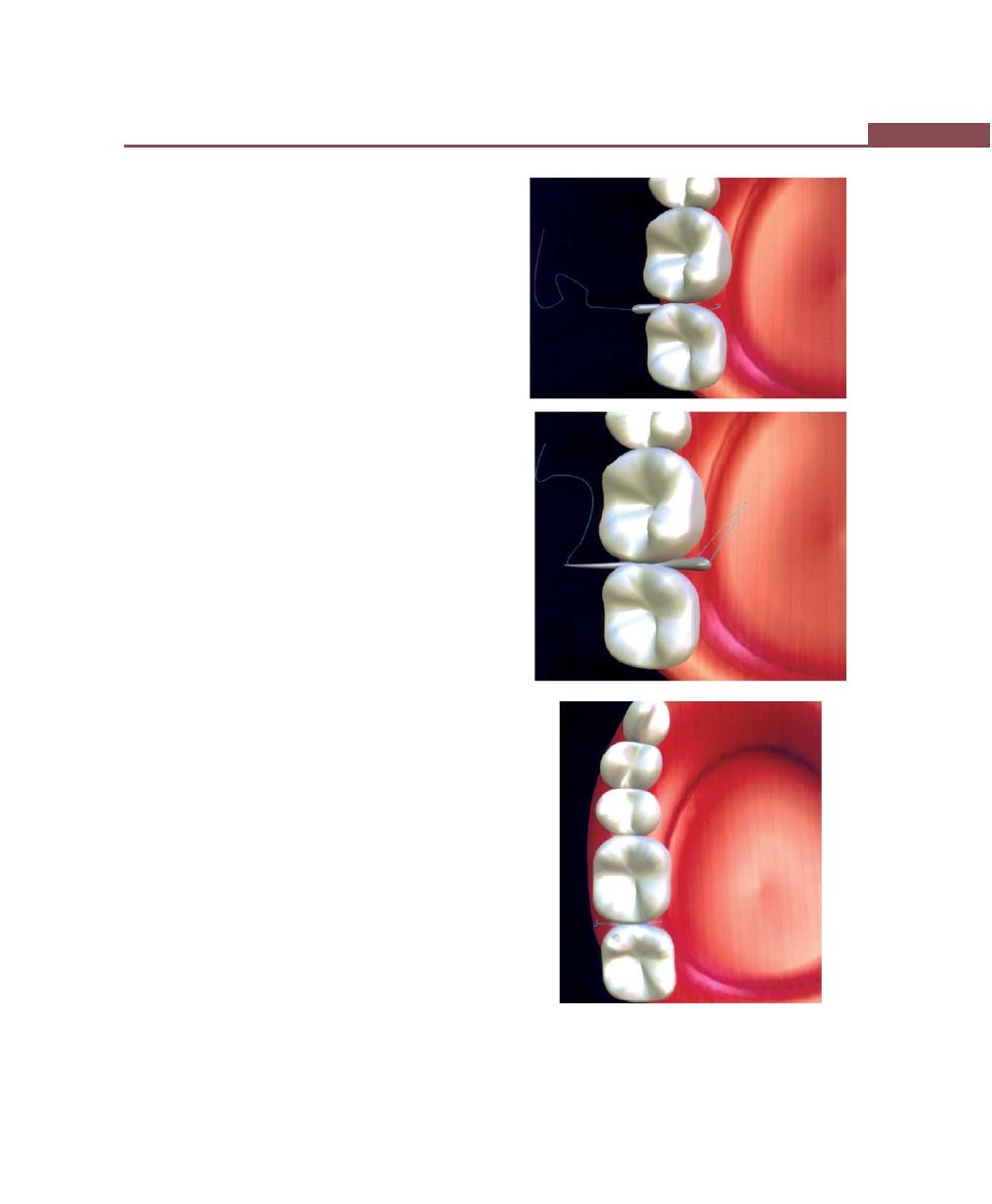
293
Principles of Periodontal Surgery
2. Body: Widest point of the needle and is referred to
as the “grasping area”.
3. Point: or the tip can be conventional or reverse
cutting.
Knots and Knot typing: Have three components:
1. The loop created by the knot.
2. The knot itself.
3. Ears—cut ends of the suture.
Suturing techniques and materials: One of the
cardinal rules in suturing is to avoid placing excessive
tension on the tissues being sutured to the extent of
inducing blanching. Such tension will result in nec-
rosis of sutured area and subsequent loss of suture
entirely.
Suturing Techniques (Figs 38.1 to 38.8)
Interrupted Suture
a. Direct or loop suture
b. Figure eight
c. Horizontal mattress
d. Vertical mattress
e. Distal wedge or Anchor suture
f. Periosteal suturing (Fig 36.6)
Continuous Suture
a. Papillary sling
b. Horizontal mattress
c. Vertical mattress
Periodontal dressing
Various commercially-available periodontal dressings
are:
a. Coe pak
b. Kirkland periopak
c. Peridres
d. Periocare
e. Periodontal pack
f. Perioputty
g. Zone periodontal pak.
C
Figs 38.1A to C:
Direct or loop technique. (A) The needle
penetrates from the outer surface of the first flap and engages
the inner surface of the opposite flap. (B) The suture is
brought back to the initial side. (C) Where the knot is tied
A
B
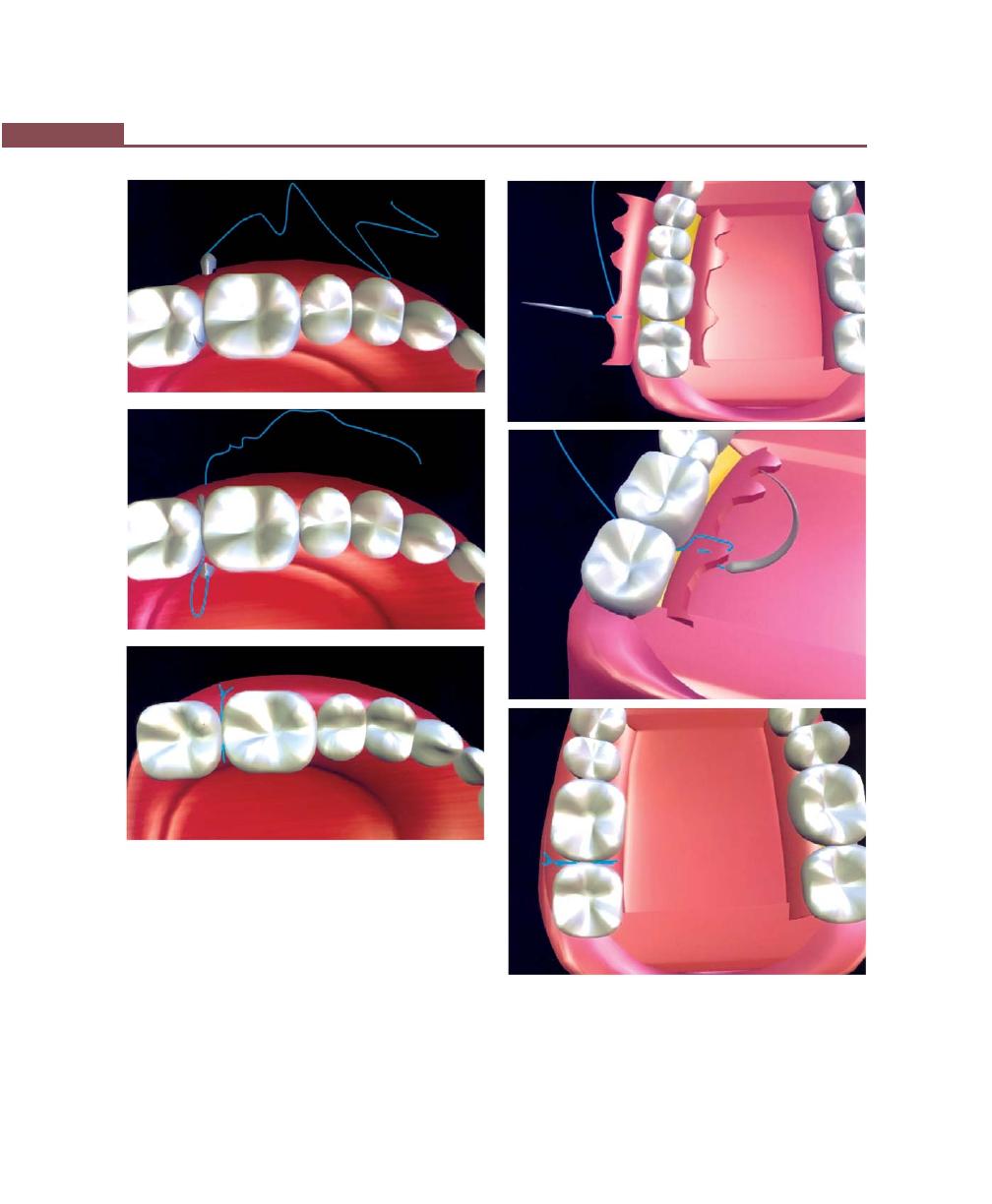
294
Essentials of Clinical Periodontology and Periodontics
A
B
Figs 38.2A to C:
Interrupted figure—eight suture. (A) The
needle penetrates the outer surface of the buccal flap.
(B) And then the outer surface of the opposite flap. (C) The
suture is brought to the first flap and the knot is tied
C
Advantages of Periodontal Packs/Dressings are
1. It minimizes the likelihood of postoperative infection
and hemorrhage.
2. Facilitates healing by preventing surface trauma during
mastication.
3. Protects against pain induced by contact of the wound
with food or with tongue during mastication.
A
B
C
Figs 38.3A to C:
Vertical mattress: Interrupted. (A) The needle
is inserted approximately 7 mm apical to the tip of the papilla,
emerging again from the epithelialized surface of the flap
2 to 3 mm from the tip of the papilla. (B) The needle is brought
back through the embrassure, where the technique is again
repeated lingually or palatally. (C) The knot is tied buccally
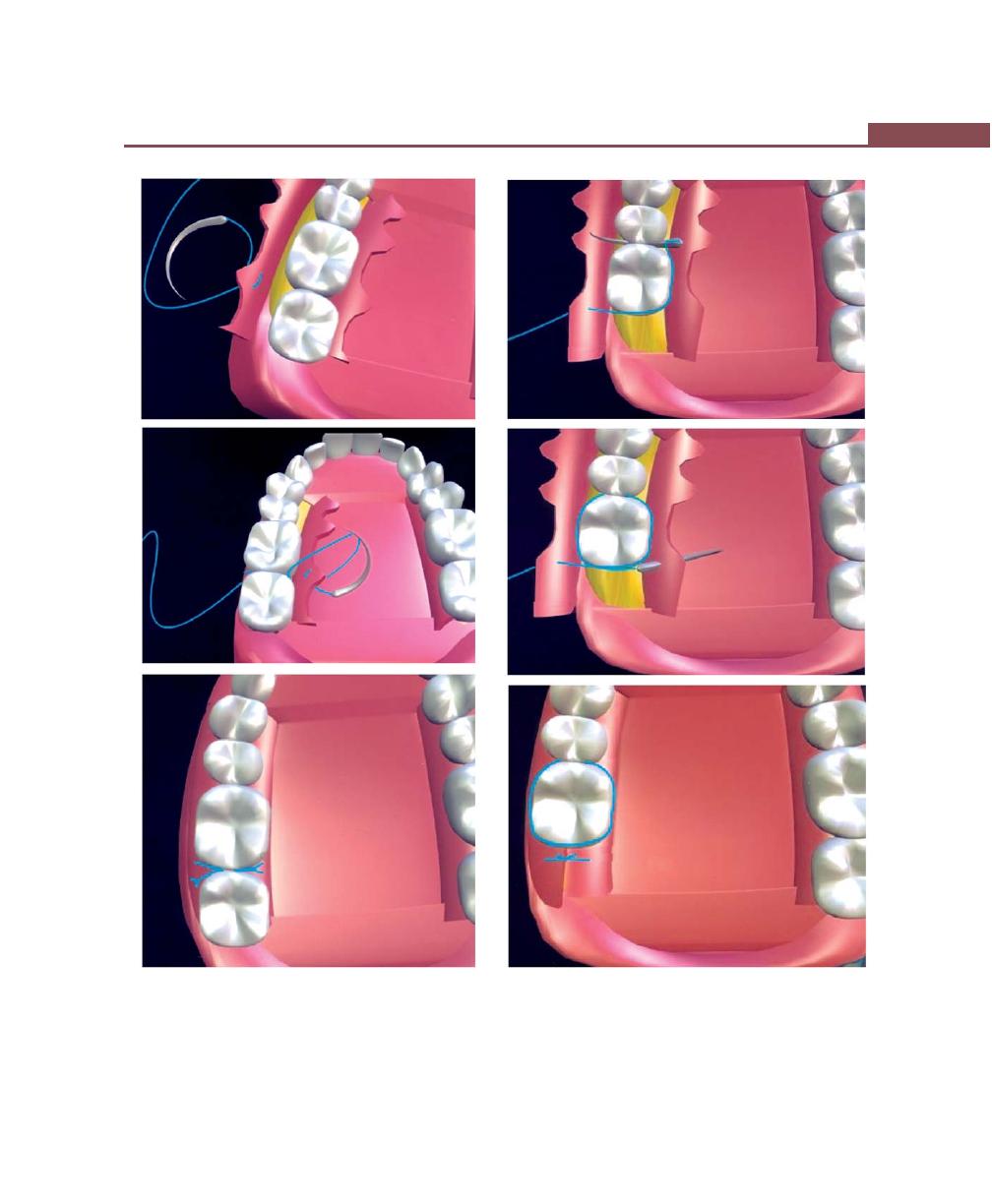
295
Principles of Periodontal Surgery
Figs 38.4A to C:
Horizontal mattress—interrupted. (A) The
needle is inserted 7 to 8 mm apical to one side of the midline
of the papilla, that is at the mesiobuccal line angle emerging
again 4 to 5 mm through the epithelialized surface on the
distobuccal line angle. (B) The needle is passed through the
embrassure and the same maneuver is duplicated again on
the lingual or palatal surface. (C) Then the knot is tied buccally
A
B
C
Figs 38.5A to C:
Distal wedge technique or anchor suture.
(A) The needle is placed at the line angle area of the facial
surface, suture is anchored around the tooth. (B) Passed
through the inner surface of the opposite flap. (C) The knot
is tied
A
B
C
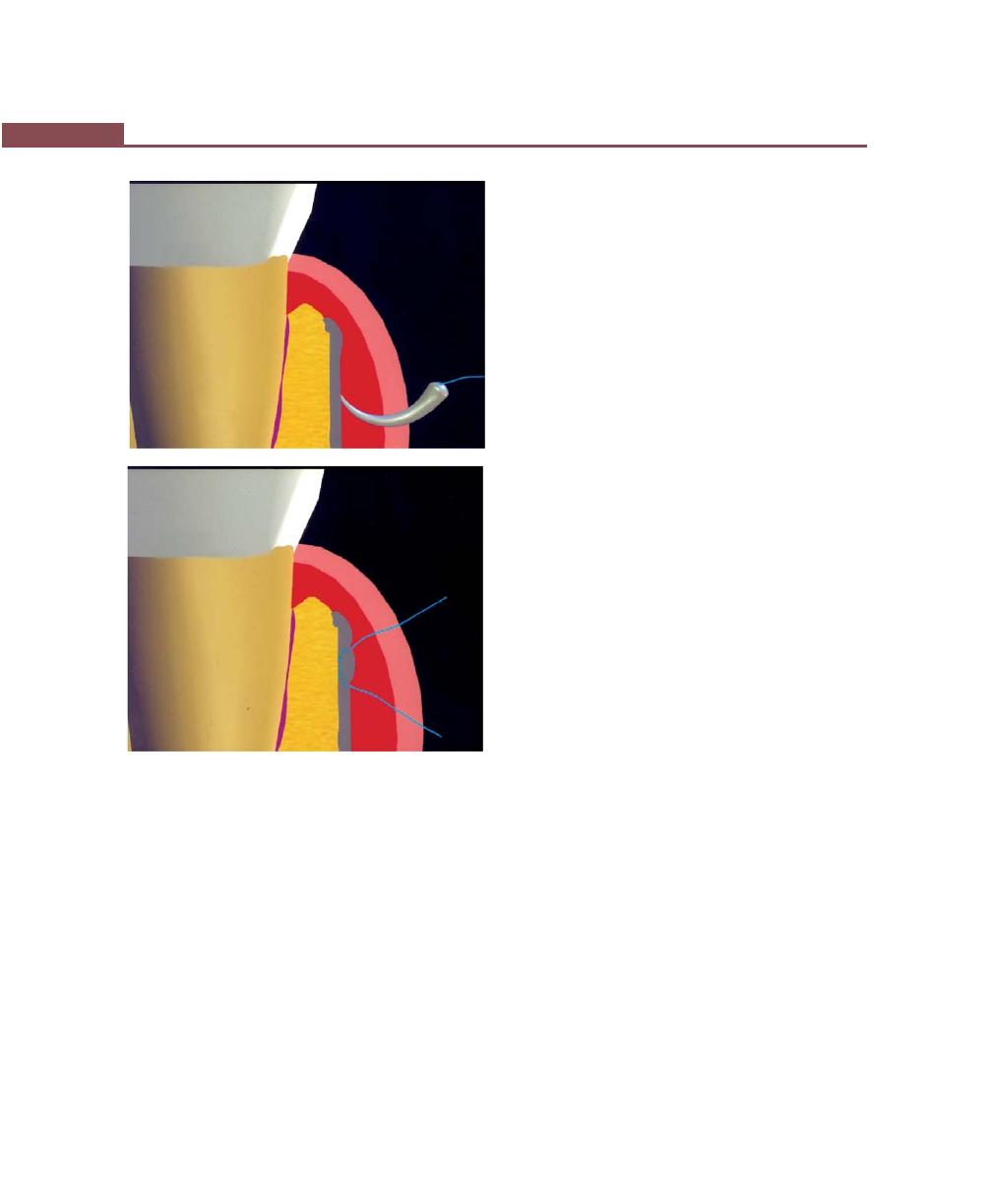
296
Essentials of Clinical Periodontology and Periodontics
Fig. 38.6:
Periosteal suturing involves five steps. (1) Needle
penetration where needle point is perpendicular to bone. (2)
Rotation of the needle point, (3) Glide, where needle point
glides against the bone. (4) Rotation, (5) Exits out of the
periosteum and bone
A
B
Types of Packs
• Zinc oxide eugenol packs.
• Non-eugenol packs.
Zinc oxide eugenol packs: Example—Wondre-pak
developed by Ward. It is based on the reaction of zinc
oxide and eugenol.
Composition: Apart from zinc oxide and eugenol, it has
zinc acetate as an accelerator, asbestos used as a binder
and filler, and tannic acid. However, asbestos can induce
lung disease and tannic acid may lead to liver damage.
Hence, both the substances have been eliminated. They
are supplied in a liquid and powder form that is mixed
prior to use. Eugenol may produce allergic reaction that
may render the area erythematous combined with a
burning sensation in some patients.
Non-eugenol packs: Example-Coe Pak. It is based on
the reaction between a metallic oxide and fatty acids.
It is supplied in two tubes.
Composition: One of the tubes contains zinc oxide, oil
for plasticity, a gum for cohesiveness and lorothiodol—
a fungicide. The other tube contains liquid coconut fatty
acids thickened with rosin and chlorothymol—a
bacteriostatic agent. Other non-eugenol packs include
cyanoacrylates and methacrylic gels. Retention of packs
is obtained by mechanical interlocking in the interdental
spaces and by joining lingual and facial portions of the
pack.
Preparation and Application of
Periodontal Dressing (Figs 38.9 to 38.11)
Coe pak (Non-eugenol) is prepared by mixing equal
lengths of pastes from the tubes supplied, i.e. accelerator
and base until it has a uniform color. The paste is then
placed in a cup of water at room temperature for 2 to
3 minutes, when the pack loses its tackiness it is ready
to be placed on the surgical site.
The pack is then rolled into two strips approximately
to the length of the treated area and placed on buccal
surface from mesial-distal end and the remainder can
be placed the same way on the lingual/palatal surfaces.
The strips are joined at the distal end by hooking it around
the distal most tooth as well as interproximally by applying
gentle pressure (with the help of a probe) to join facial
and lingual surfaces of the pack. Any overextension onto
uninvolved area should be avoided. It is usually kept
on for 1 week after surgery.
Findings at pack removal
a. If gingivectomy has been performed, the cut surface
is covered with a meshwork of new epithelium. If
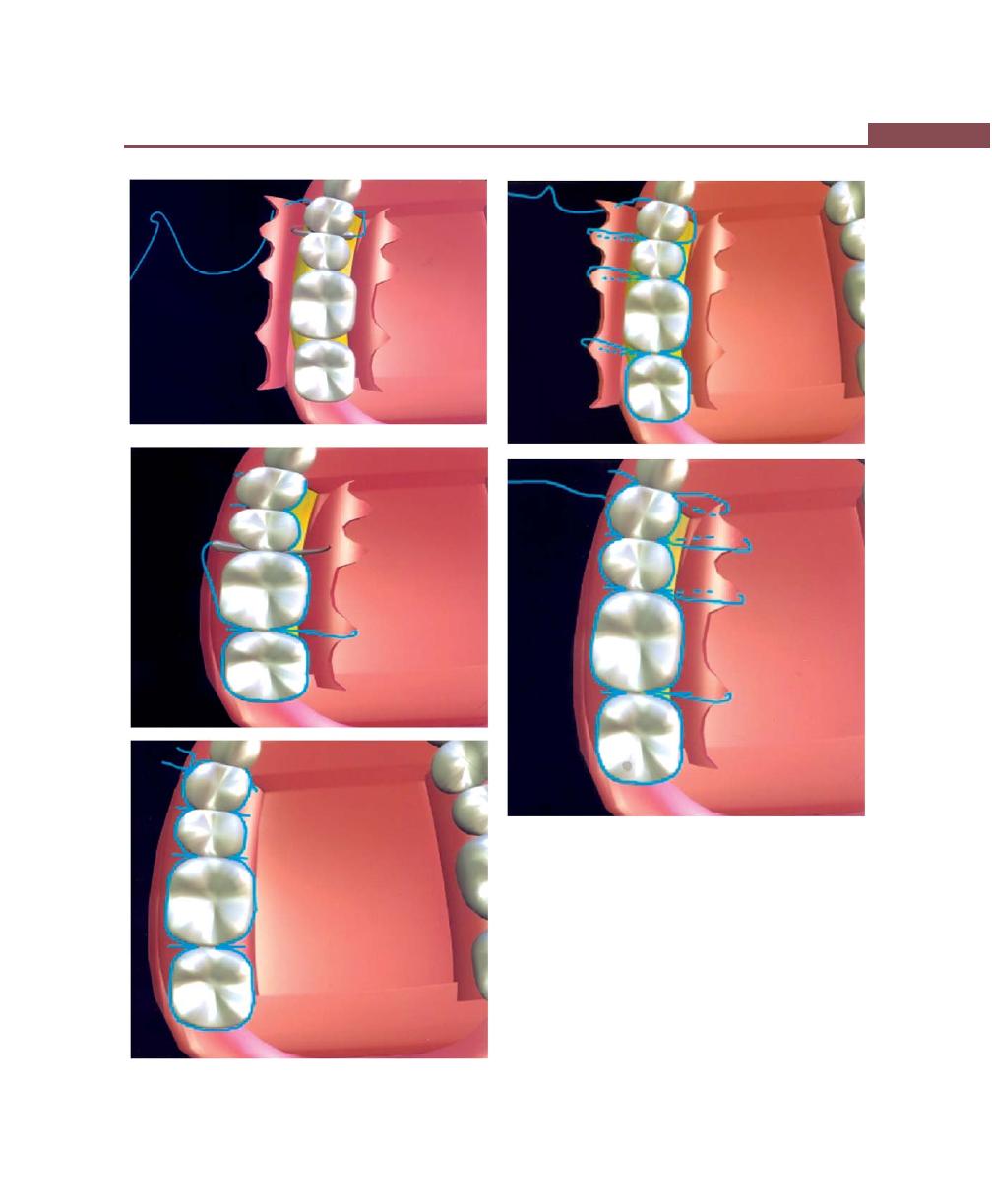
297
Principles of Periodontal Surgery
A
B
C
E
Figs 38.7A to E:
Independent sling suture. (A) The needle
is inserted on the facial papilla, a sling is placed on the
palatal surface. (B) The facial papillae were sutured together.
(C) When the last tooth is reached, the suture is anchored
around it. (D) The palatal flaps are sutured together in a
similar fashion. (E) Final knot is placed
D
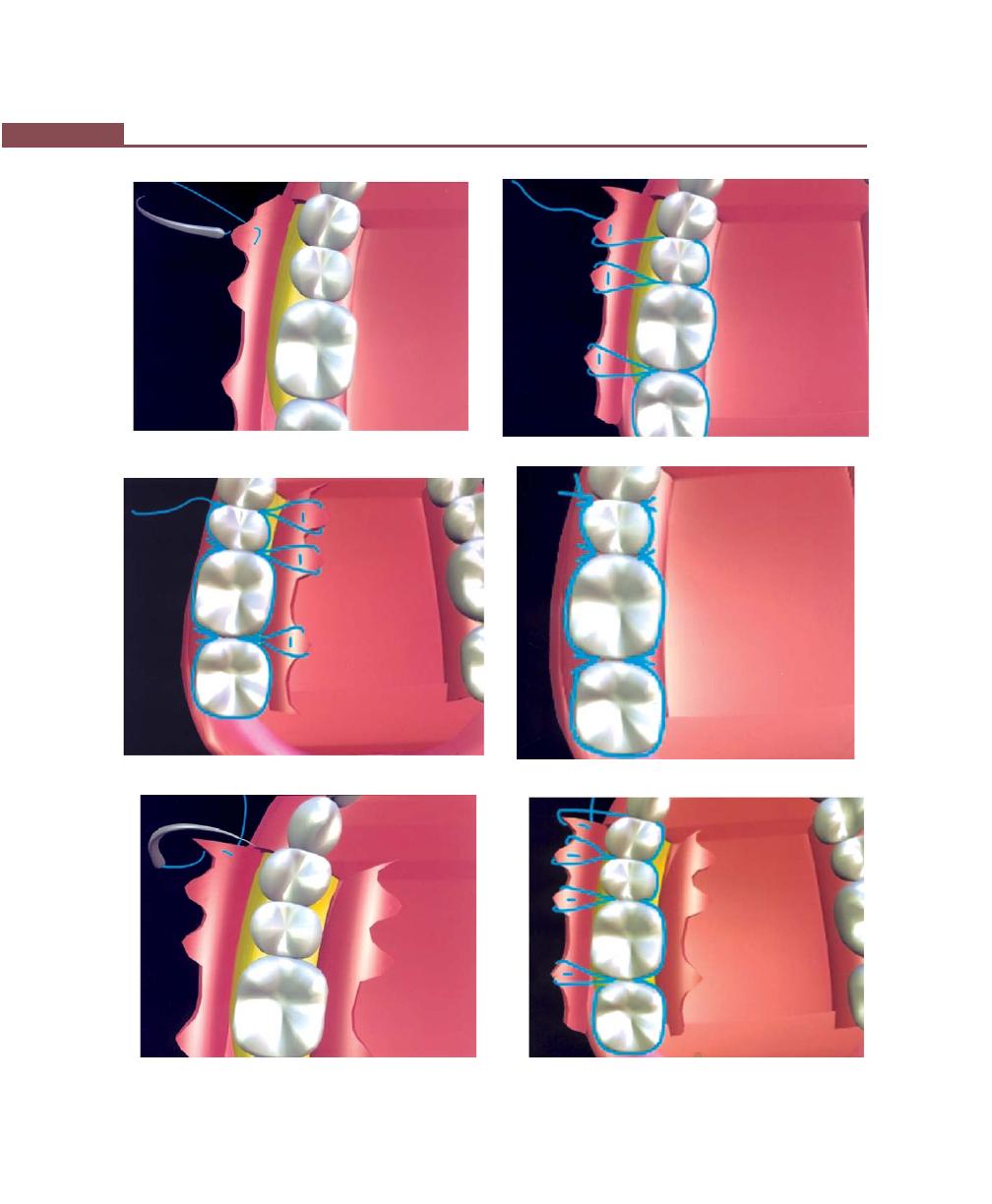
298
Essentials of Clinical Periodontology and Periodontics
Fig. 38.8A
Fig. 38.8B
Fig. 38.8C
Fig. 38.8D
Fig. 38.8F
Fig. 38.8E
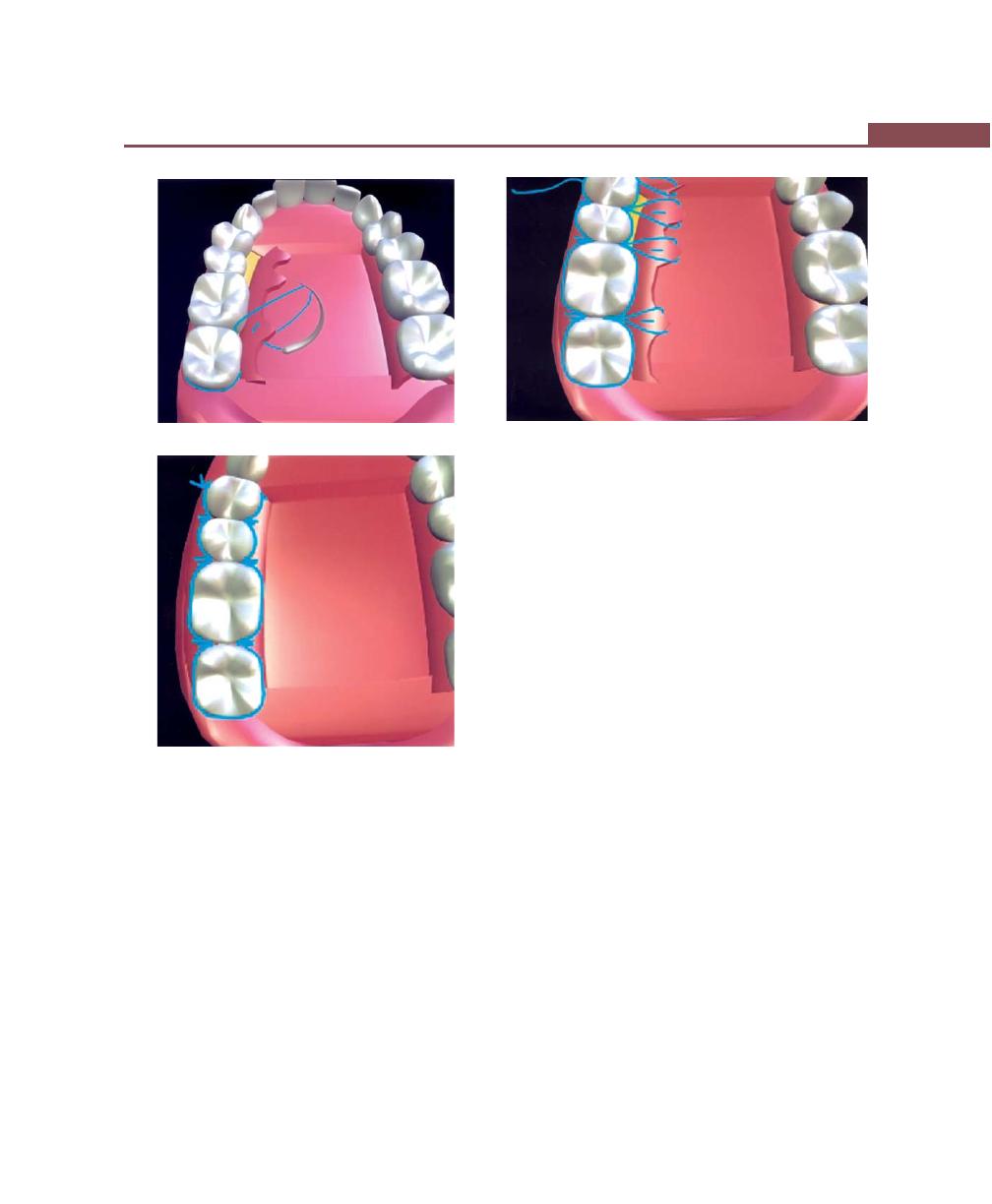
299
Principles of Periodontal Surgery
calculus has not been removed completely, red bead-
like protuberances of granulation tissue will persist,
which should be removed with a curette.
b. After a flap operation, the incision areas are epithe-
lialized but may bleed readily when probed, hence
pockets should not be probed.
c. The facial and lingual mucosa may be covered with
a grayish-yellow or white granular layer of food debris
that has seeped under the pack and can be easily
removed with a moist cotton pellet.
Instructions for the Patient after Surgery
1. Patients should take the advised medication.
Fig. 38.8G
Fig. 38.8H
Figs 38.8A to I:
Continuous vertical/horizontal mattress
sutures: Technique is identical to independent papillary sling
except that vertical or horizontal mattress sutures are
substituted for the simple papillary sling
2. The pack should remain in place until it is removed
after one-week.
3. For the first three hours after the operation, avoid
hot foods to permit the pack to harden, try to chew
on the non-operated side of the mouth. Avoid citrus
juices and spiced-food because it causes pain and
burning.
4. Do not smoke.
5. Do not brush over the pack.
6. During the first day apply ice.
7. Follow your daily activities, but avoid excessive
exertion of any type.
8. Swelling is not unusual.
Fig. 38.8I
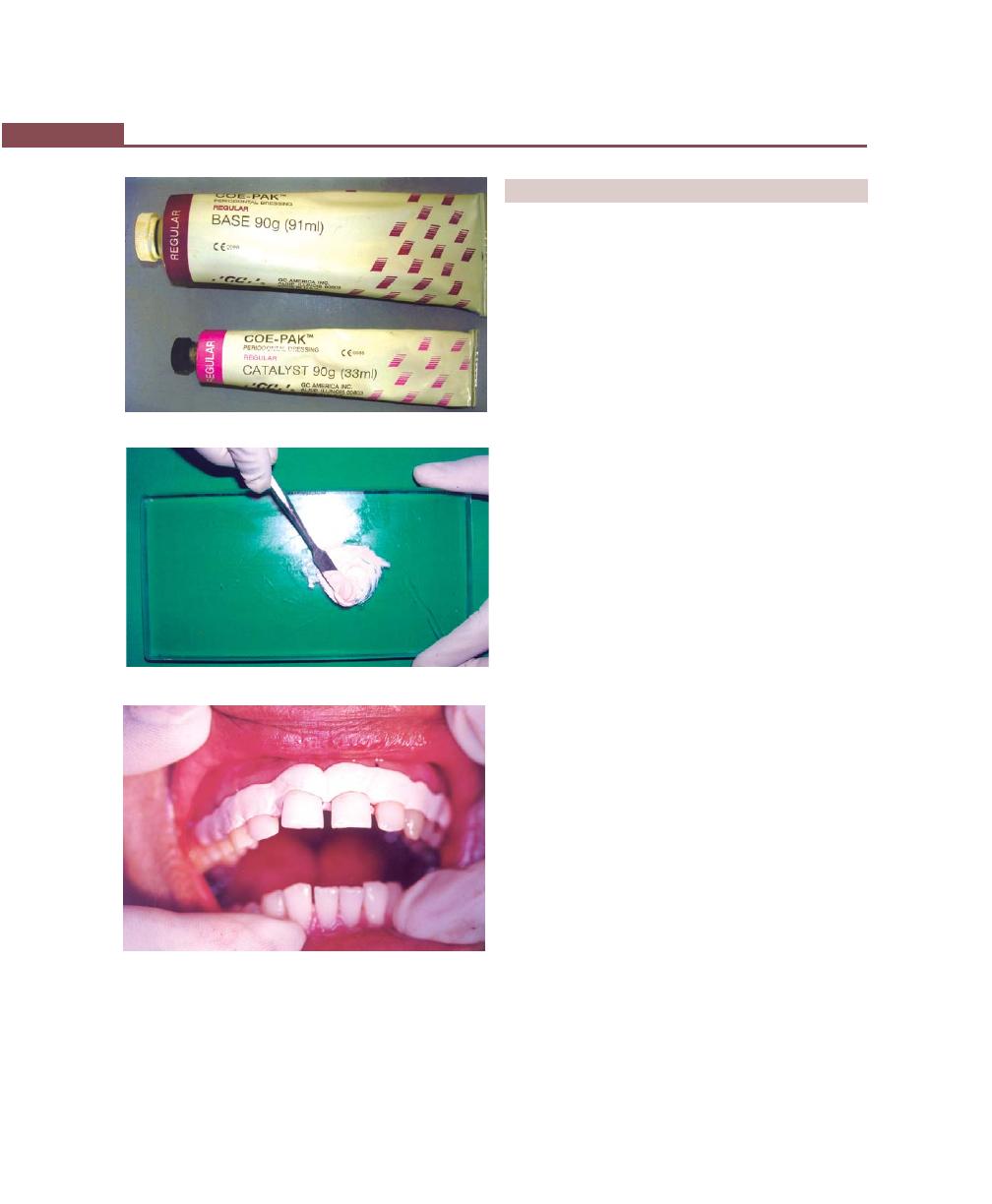
300
Essentials of Clinical Periodontology and Periodontics
Fig. 38.9:
Periodontal pack
Fig. 38.10:
Mixing
Fig. 38.11:
Application of Coe pak
9. There may be an occasional tinge of blood in the
saliva for the first 4 to 5 hours after the operation,
beyond this if there is bleeding, report to the doctor
immediately.
COMPLICATIONS DURING SURGERY
1. Syncope or transient loss of consciousness owing to
a reduction in cerebral blood flow. The most common
cause is fear and anxiety. It is usually preceded by
a feeling of weakness which is followed by pallor,
sweating, coldness of the extremities, dizziness and
slowing of the pulse.
Management: Patient should be placed in a
supine position with legs elevated, tight clothes should
be loosened and an open airway ensured, adminis-
tration of oxygen is also useful. History of previous
syncopal attacks during dental appointments should
be explored before treatment is begun.
2. Hemorrhage: Periodontal surgery produces profuse
bleeding in its initial incisional steps. However after
the granulation tissue is removed, bleeding will
disappear or reduce considerably. Excessive hemor-
rhage after the initial steps may be due to lacerated
capillaries and arterioles or damage to larger vessels
due to surgical invasion of anatomic areas.
Treatment: Pressure pack, cotton pellet dipped in
ferric subsulphate powder. Thrombin, hastens the
process of blood clotting, oxidized cellulose and Gel
foam are most commonly used to control the
hemorrhage.
Complications during the
First Postoperative Week
1. Persistent bleeding after surgery: The pack is removed,
the bleeding points are located and the bleeding is
stopped with pressure, sutures, electrosurgery or
electrocautery. After the bleeding is stopped the pack
is replaced.
2. Sensitivity to percussion may be due to extension
of inflammation into periodontal ligament. Gradual
diminution of severity is a favorable sign. The pack
should be removed and the gingiva should be
checked for localized areas of infection or irritation.
The particles of calculus that may have been
overlooked should be removed. Relieving the

301
Principles of Periodontal Surgery
occlusion is usually helpful. Sensitivity to percussion
may also be caused by excess pack which interferes
with occlusion. Removal of excess should correct the
condition.
3. Swelling: Within the first two postoperative days
patient reports a soft, painless swelling of the cheek
in the area of operation. There may be lymph node
enlargement and temperature may be slightly
elevated. This type of involvement is due to localized
inflammatory reaction to operative procedures. If the
swelling persists and associated with increased pain.
Antibiotics like amoxicillin, 500 mg every 8 hours for
1 week should be prescribed.
4. Feeling of weakness: Patients may experience a
“washed out”, weakened feeling for about 24 hours
after the surgery. This represents a systemic reaction
to a transient bacteremia-induced by operative
procedure. It can be prevented by prescribing
prophylactic antibiotics.
5. Postoperative pain: Periodontal surgery performed
following basic principles should produce only minor
pain and discomfort. In a study the results revealed
mucogingival procedures results in maximum
discomfort and followed by osseous surgery than any
other plastic gingival surgeries.
Common sources of postoperative pain are:
a. Over-extension of pack beyond mucogingival
junction.
b. Extensive and excessively prolonged exposure and
dryness of bone can also induce severe pain.
c. When severe postoperative pain is present, the
patient should be treated on an emergency basis.
The wound should be examined (under local
anesthesia). This type of pain is related to infection
accompanied by localized lymphadenopathy and
a slight elevation in temperature.
Treatment Antibiotics and analgesics should be
prescribed.
6. Sensitive Roots/Root hypersensitivity: May occur
spontaneously when the root becomes exposed as
a result of gingival recession or pocket formation or
it may appear after scaling and root planing and
surgical procedures because the cementum at
cementoenamel junction is extremely thin and is
removed during the above procedures.
Mechanism: Transmission of stimuli from the surface
of dentin to the nerve endings is located in the dental
pulp, which may occur through the odontoblastic
process or owing to a hydrodynamic mechanism.
Example: by displacement of fluid. Treatment for root
sensitivity include, use of various desensitizing agents
like, Strontium chloride, potassium nitrate and sodium
citrate available in the form of pastes and are used
by the patient.
Agents used in Dental Office are:
• Cavity varnishes
• Anti-inflammatory agents
• Various agents are used to partially-obturate dentinal
tubules.
Examples:
• Silver nitrate
• Zinc chloride
• Formalin
• Calcium compounds
• Sodium fluoride
• Stannous fluoride
• Strontium chloride and,
• Potassium oxalate
Other procedures like iontophoresis, restorative resins
and dentin bonding agents have been used.
HOSPITAL PERIODONTAL SURGERY
Usually, periodontal surgery is performed in dental clinics,
either sextant or quadrant wise at weekly or longer
intervals. Under certain circumstances, the full mouth
periodontal surgery may have to be done in a hospital
operating room under general anesthesia. Indications
for this, include:
a. To control and manage the apprehensive patient.
302
Essentials of Clinical Periodontology and Periodontics
b. Convenience for individuals who cannot endure
multiple visits to complete surgical treatment.
c. Patient protection—some patients who are suffering
from systemic conditions that are not severe enough
to contraindicate surgery but at the same time require
special precautions that best provided in a hospital
setting. Example: patients with cardiovascular disease,
abnormal bleeding tendencies, prolonged steroid
therapy and others.
One must clearly understand that the purpose of
hospitalization is to protect patients by anticipating
their special needs, but not to perform surgery when
it is contraindicated by the patient’s general condition.
KEY POINTS TO NOTE
1. Prior to any surgical procedure, every patient must undergo
the initial phase of therapy including scaling and root planing
and reevaluation phase.
2. Periodontal surgery must be performed painlessly, to achieve
this; effective local anesthesia should be administered.
3. Proper tissue handling is most important, as it can cause
postoperative patient discomfort and delayed wound
healing.
4. Excessive hemorrhage following surgery should be of great
concern to the operator and should be handled carefully.
Hemostasis may be achieved with various hemostatic agents
such as gelatin sponge, oxidized cellulose and others.
5. The periodontal dressings that are placed on the surgical
area have no curative properties; but they help in protecting
the tissue and thereby assist in healing.
6. After the pack placement, written and verbal instructions
should be given to the patient before he or she leaves the
operatory.
REVIEW QUESTIONS
1. Indications and contraindications of periodontal
surgery.
2. Describe various suturing techniques in periodontics.
3. Advantages, disadvantages and types of periodontal
packs.
4. Treatment for dentinal hypersensitivity.
BIBLIOGRAPHY
1. Curro FA. Tooth hypersensitivity. Dent Clin North Am 1990;
34(3):403.
2. Nevins, James T, Helloing. Periodontal therapy clinical
approaches and evidence of success volume 1, Quintessence
Publishing Co, Inc. 1998.
3. Newman, Takei, Fermin A Carranza. Clinical Periodontology,
9th edn, WB Saunders Co., 2002.
4. Trowbridge HO, Silver DR. A review of current approaches
to in-office management of tooth hypersensitivity. Dent Clin
North Am 1990;34:583.
303
Gingival Curettage
DEFINITION
The term curettage is used in periodontics to mean the
scraping of gingival wall of a periodontal pocket to
separate diseased soft tissue.
Whereas scaling refers to removal of deposits from
tooth/root surface and root planing means smoothening
the root to remove infected and necrotic tooth surface.
TYPES
I . Gingival curettage: Consists of removal of inflamed
soft tissue lateral to pocket wall (Fig. 39.1).
a. Subgingival curettage: It is a procedure that is per-
formed apical to epithelial attachment (Fig. 39.2).
b. Inadvertant curettage: Curettage that is done
unintentionally during scaling and root planing.
II. Surgical curettage, chemical curettage, ultrasonic
curettage.
RATIONALE
The main accomplishment of curettage is the removal
of chronically-inflamed granulation tissue that forms
in the lateral wall of the periodontal pocket. This tissue
apart from having its usual components like fibroblastic
and angioblastic proliferations, also contains areas of
chronic inflammation, pieces of dislodged calculus and
bacterial colonies (Justification to curettage is more so
from the fact that this granulation tissue which is lined
Fig. 39.1:
Gingival curettage with a curette
❒
❒
❒
❒
❒ DEFINITION AND TYPES
❒
❒
❒
❒
❒ RATIONALE
❒
❒
❒
❒
❒ INDICATIONS
❒
❒
❒
❒
❒ PROCEDURE
❒
❒
❒
❒
❒ HEALING AFTER SCALING AND
CURETTAGE
❒
❒
❒
❒
❒ CLINICAL APPEARANCE AFTER
SCALING AND CURETTAGE
39
Gingival Curettage
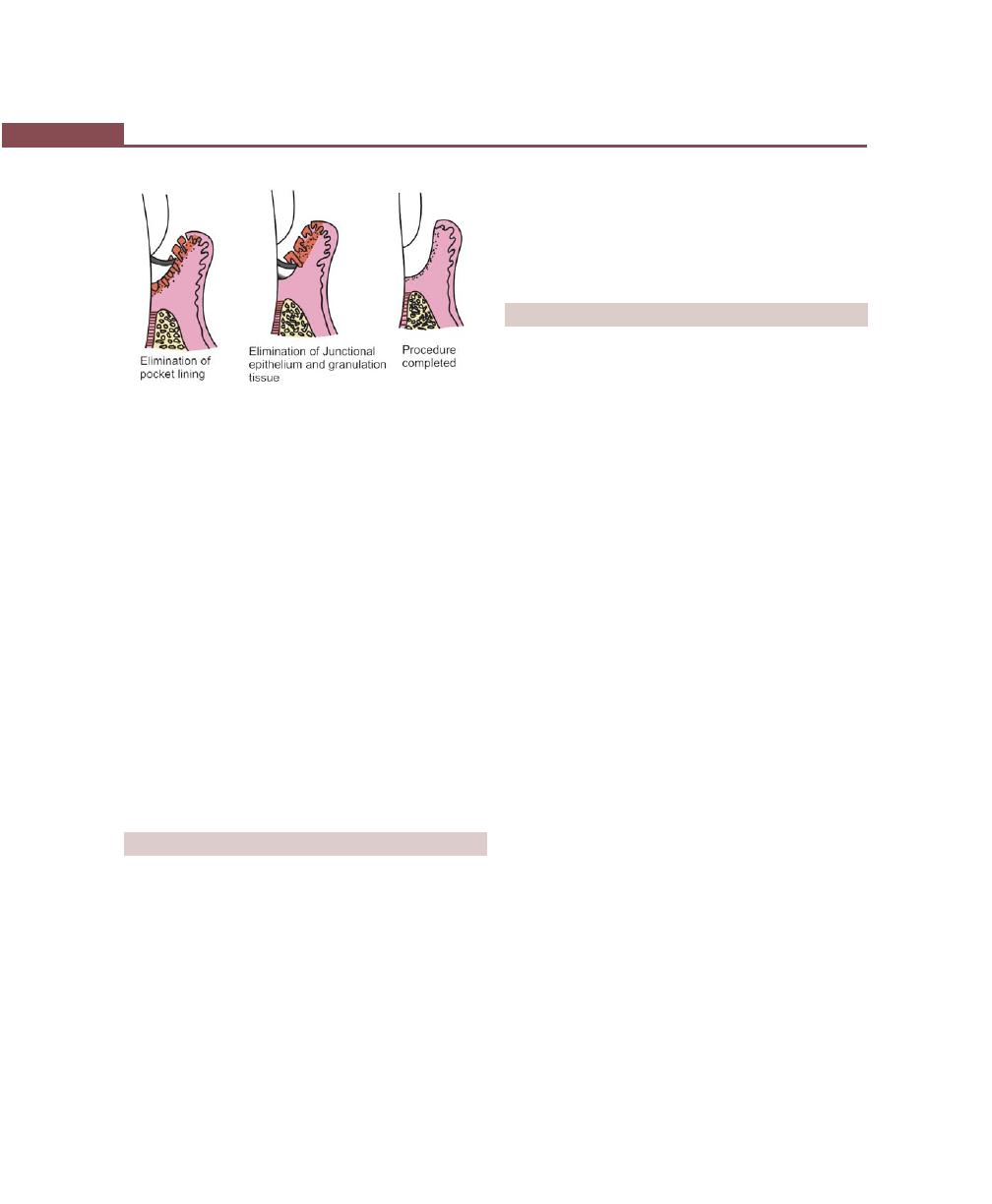
304
Essentials of Clinical Periodontology and Periodontics
by epithelium may hamper or act as a barrier for the
attachment of new fibers).
The dilemma now is that, is it justified to do curettage,
just to eliminate the inflamed granulation tissue? Because
when the root is thoroughly planed, the major source
of bacteria disappears and the pathologic changes in the
periodontal pocket disappears without any need for
curettage. Due to this existing granulation tissue also
disappears, if any bacteria is present, is destroyed by
defence mechanism due to their less number.
On the other hand curettage may also eliminate all
or most of epithelium that lines the pocket wall and
underlying junctional epithelium, though there are
differing opinions regarding this, the purpose of curettage
is still valid particularly in presurgical phase where there
is persistant gingival inflammation even after repeated
scaling and root planing.
INDICATIONS
1. Can be performed as a part of new attachment in
moderately deep infrabony pockets located in accessi-
ble areas where a type of “closed surgery” is advised.
2. Can be done as a non-definite procedure to reduce
inflammation prior to pocket elimination procedures
like flap surgeries.
3. It can also be performed in patients where extensive
surgical procedures are contraindicated like aging,
systemic complications, etc. where the treatment is
compromised and prognosis is impaired.
4. Curettage is frequently performed on recall visits as
a method of maintenance treatment for areas of
recurrent inflammation and pocket depth, particularly
where pocket reduction surgery has previously been
performed.
PROCEDURE
• Basic technique.
• Other techniques.
Curettage as such does not eliminate local factors
like plaque and calculus, therefore it should always be
followed by scaling and root planing procedures.
Basic Technique
After adequate local anesthesia, the correct curette is
selected and adapted in such a way that the cutting edge
is against the tissues.The instrument is inserted so as to
engage the inner lining of the pocket wall and is carried
along the soft tissue wall usually in a horizontal stroke.
The pocket wall may be supported by gentle finger
pressure on external surface.
In subgingival curettage, the tissue attached between
the bottom of pocket and the alveolar crest is removed
with a scooping motion of the curette to the tooth
surface. The area is flushed to remove debris. If necessary
sometimes sutures and a pack may be indicated.
Other Techniques
ENAP (Excisional New Attachment Procedure)
(Fig. 39.3)
It was developed by United States Naval Corps. It is a
definitive subgingival curettage procedure performed
with a knife.
The technique is:
1. After adequate local anesthesia, an internal bevel
incision is made from margin of free gingiva apically
below the base of pocket, it is carried all around the
tooth surface, attempting to retain as much interdental
tissue as possible.
2. The excised tissue is then removed with a curette
and the root surface is planed to a smooth hard
consistency.
Fig. 39.2:
Subgingival curettage
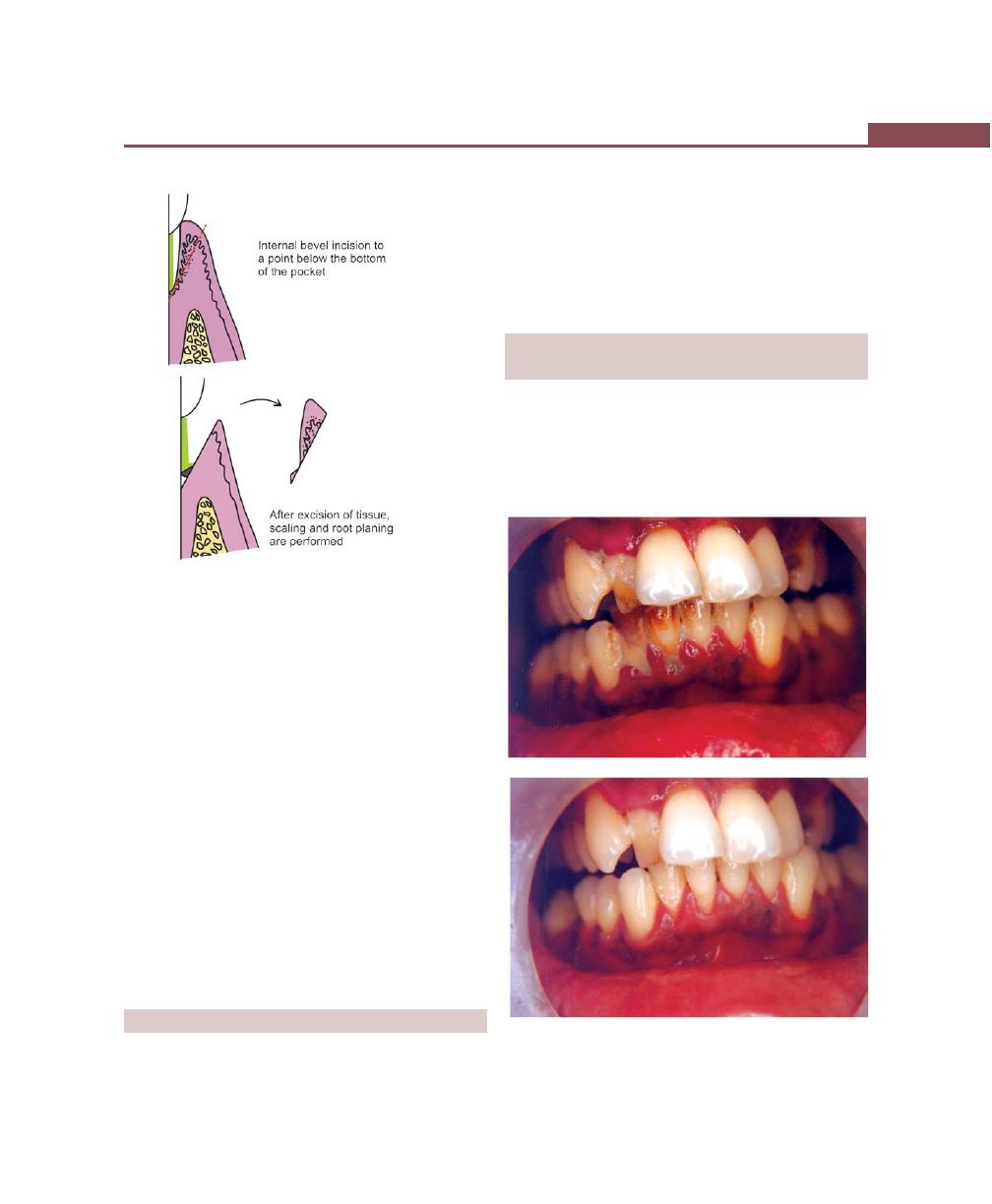
305
Gingival Curettage
3. Approximate wound edges if necessary, place sutures
and a periodontal-dressing.
Ultrasonic Curettage
Ultrasonic scalers are used for ultrasonic curettage, here
the ultrasonic vibrations disrupt tissue continuity, and
the epithelium is lifted off. It also alters the morphologic
features of fibroblast nuclei. This method has proved
to be as effective as the manual method but results in
decreased inflammation and less removal of connective
tissue.
Caustic Drugs
Drugs such as sodium sulfide, Antiformin and phenol
have been used to induce chemical curettage of the
lateral wall of the pocket. Disadvantage is the extent
of tissue destruction with these drugs cannot be controlled.
HEALING AFTER SCALING AND CURETTAGE
Immediately after curettage, a blood clot fills the pocket
area, hemorrhage is also present in tissues with dilated
capillaries and increase in polymorphonuclear leukocytes
appear on wound surface. Rapid proliferation of
granulation tissue occurs shortly thereafter with a decrease
in the number of blood vessel. Restoration and
epithelialization of sulcus takes place in 2 to 7 days.
CLINICAL APPEARANCE AFTER SCALING
AND CURETTAGE (Figs 39.4A and B)
Immediately after curettage, the gingiva appears
hemorrhagic and bright-red. After one week, the gingiva
appears reduced in height with apical shift. The redness
is slightly reduced. After two weeks, with proper oral
hygiene the gingiva comes back to normal.
Fig. 39.3:
Excisional new attachment procedure (ENAP)
Figs 39.4A and B:
Clinical appearance before and after
curettage
A
B
306
Essentials of Clinical Periodontology and Periodontics
KEY POINTS TO NOTE
1. Curettage is the scraping of the gingival wall of a periodontal
pocket to separate the diseased soft tissue.
2. There are gingival, subgingival and inadvertant curettage
which should be differentiated.
3. Indications for curettage are very limited and can be used
after scaling and root planing.
4. Curettage can also be performed with the help of chemicals
and ultrasonic scalers.
REVIEW QUESTIONS
1. What are the indications of gingival curettage?
2. What is ENAP?
BIBLIOGRAPHY
1. JD Manson, BM Eley. Outline of Periodontics. 3rd edn, British
Library Cataloguing in Publication Data, 1995.
2. Myron Newins, James T Mellonig. Periodontal Therapy
Clinical Approaches and Evidence of Success Vol 1,
Quintessence Publishing Co, Inc, 1998.
3. Newman, Takei, Fermin A Carranza. Clinical Periodontology,
9th edn, WB Saunders, 2002.
307
Gingivectomy
INTRODUCTION
The term gingivectomy was coined by Pickerill in 1912
where in all the periodontal tissues including the bone
should be removed to achieve healing. It was later
modified by Kronfeld (1935) and Orban in about 1940
who introduced modern uses of gingivectomy.
DEFINITIONS
• Gingivectomy is the excision of the soft tissue wall
of the pocket (It’s objective is the elimination of
pockets).
• Gingivoplasty is the recontouring of gingiva that has
lost its physiologic form rather than elimination of
pockets.
These two procedures are performed together
although they may be considered separately for teaching
purposes.
PRE-REQUISITES
The basic pre-requisites for gingivectomy are as follows:
1. There should be adequate zone of attached gingiva
so that excision of part of it will still leave a functionally
adequate zone.
2. The underlying alveolar bone must be in normal or
nearly normal form. If there is bone loss it should
be of horizontal in nature.
3. There should be no infrabony defects or pockets.
INDICATIONS
If the above mentioned pre-requisites are met, gingivec-
tomy may be used to do the following.
1. Eliminate supra-alveolar pockets and pseudo-
pockets.
2. Remove fibrous or edematous enlargements of the
gingiva.
3. Transform rolled or blunted margins to physiologic
form.
4. Create more esthetic form in cases in which exposure
of the anatomic crown has not fully occurred.
5. Create bilateral symmetry (where the gingival margin
of one incisor has receded somewhat more than that
of the adjacent incisor).
❒
❒
❒
❒
❒ DEFINITIONS
❒
❒
❒
❒
❒ PRE-REQUISITES
❒
❒
❒
❒
❒ INDICATIONS
❒
❒
❒
❒
❒ CONTRAINDICATIONS
❒
❒
❒
❒
❒ TYPES AND TECHNIQUES
❒
❒
❒
❒
❒ ADVANTAGES AND DISADVANTAGES
40
Gingivectomy
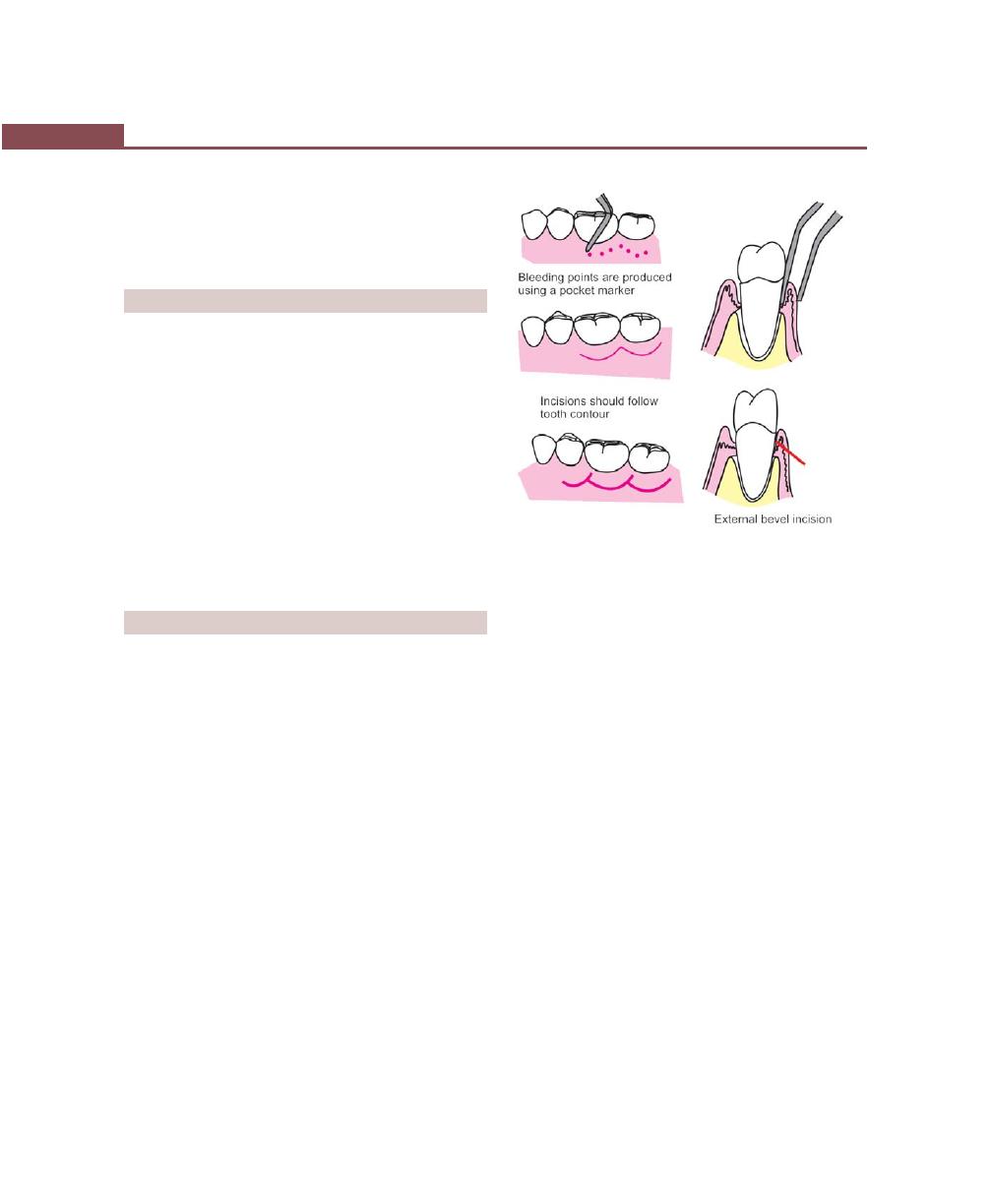
308
Essentials of Clinical Periodontology and Periodontics
6. Expose additional clinical crown to gain added reten-
tion for restorative procedures (access to subgingival
areas, etc.).
7. Correct gingival craters.
CONTRAINDICATIONS
Gingivectomy and gingivoplasty are not indicated in the
following situations:
1. In the presence of thick alveolar edges, interdental
craters or bizarre crestal bone form.
2. When infrabony pockets are present.
3. If pockets extends till/below the mucogingival junction.
4. Inadequate oral hygiene maintenance by the patients
5. Un-cooperative patients.
6. Medically-compromised patients.
7. Dentinal hypersensitivity before the surgical proce-
dure (requires considerable preparation of the patient
mentally and is not exactly a contraindication).
TYPES OF GINGIVECTOMY
1. Surgical gingivectomy.
2. Gingivectomy by electrosurgery.
3. Laser gingivectomy.
4. Gingivectomy by chemosurgery.
Surgical Gingivectomy (Figs 40.1 to 40.3)
Technique
Armamentarium.
1. Mouth mirror, probe.
2. Pocket markers, Kirkland and Orban interdental
gingivectomy knives.
3. Surgical blade, Bard Parker handle.
4. Surgical curettes, Gracey curettes, tissue forceps,
scissors.
5. Periodontal dressing.
Surgical Procedure
Step 1: The pockets on each surface are explored with
a periodontal probe and marked with the pocket marker.
Insert the probing beak to the bottom of the pocket and
mark the depth with the puncturing beak. Mark the
bleeding points in all the areas with probing depth.
Step 2: Periodontal-knives are used for incisions on the
facial and lingual surfaces as auxiliary instruments, Bard
Parker blades No. 11 and 12 and scissors are then used.
The incision is started apical to the points marking the
course of the pockets and is directed coronally to a point
between the base of the pocket and the crest of the
bone. Exposure of bone is undesirable but if it occurs
healing may not present a problem if the area is
adequately covered by the periodontal-pack.
Discontinuous or continuous incisions may be used.
The incision should be beveled at approximately 45
degrees to the tooth surface and should recreate as far
as possible the normal festooned pattern of gingiva ,
failure to do this may lead to the formation of fibrous
plateau that takes more time to heal and develop a
physiologic contour than is ordinarily required.
Step 3: Remove the excised pocket wall. Clean the area
and closely examine the root surface for any deposits.
Fig. 40.1:
Steps in gingivectomy
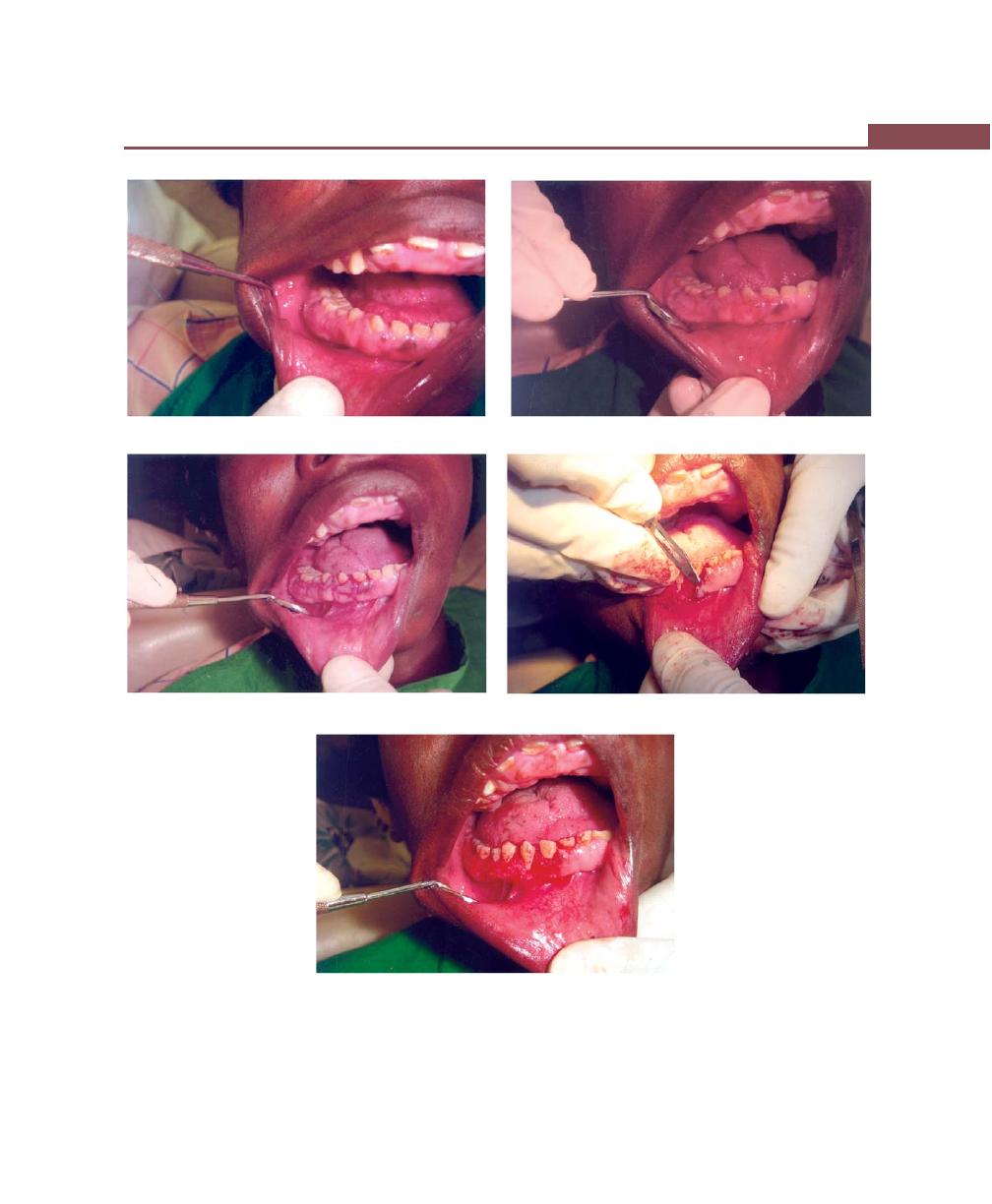
309
Gingivectomy
A
B
C
D
E
Figs 40.2A to E:
Clinical steps in gingivectomy. (A) Preoperative view, (B) Bleeding points, (C) External bevel incision,
(D) Excision using blade, (E) Immediately after gingivectomy
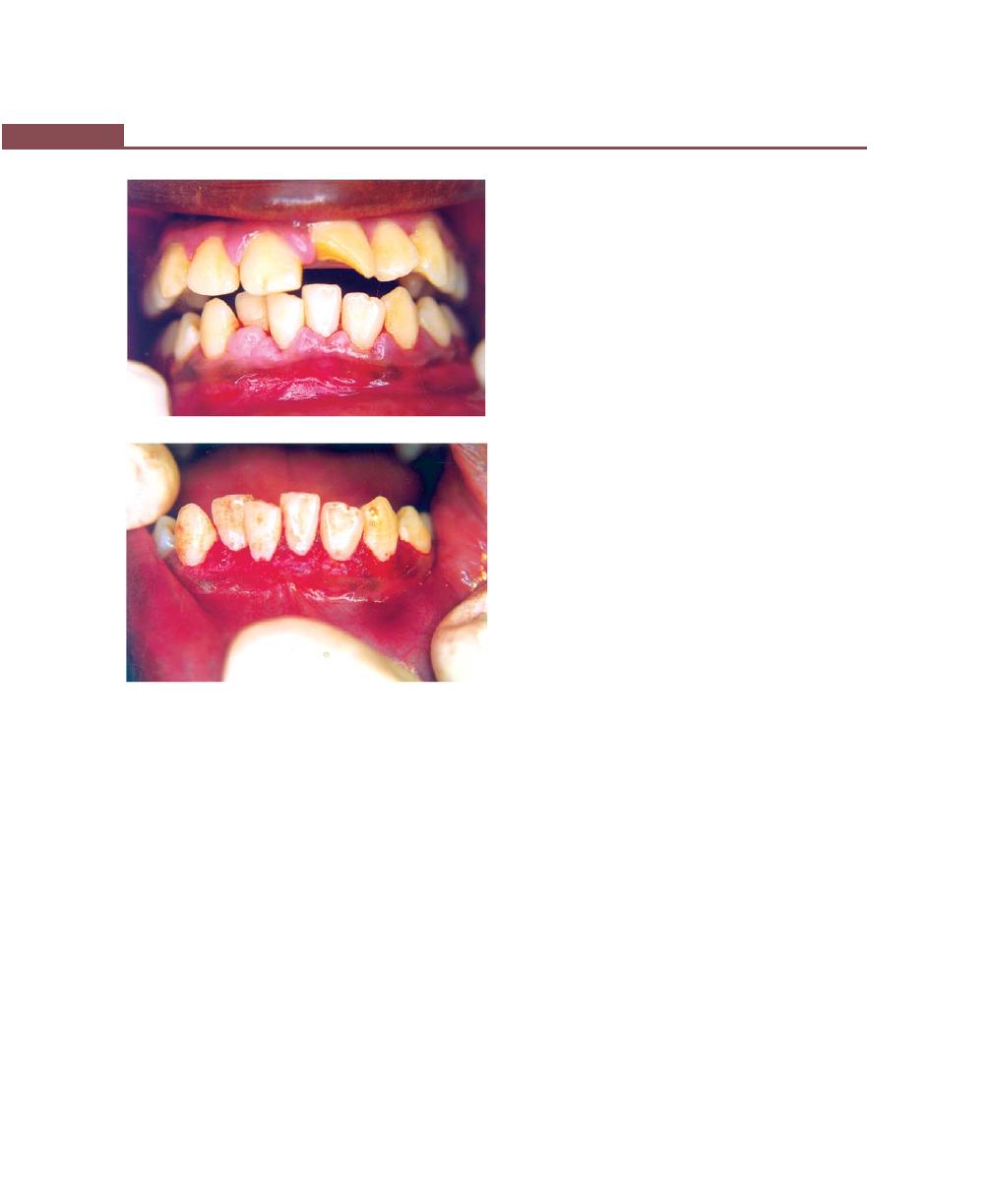
310
Essentials of Clinical Periodontology and Periodontics
Step 4: Carefully curette out the granulation tissue and
remove any calculus and necrotic cementum so as to
leave a clean and smooth root surface.
Step 5: Cover the area with surgical pack.
Procedure for Gingivoplasty
Instruments used are periodontal-knife, scalpel, diamond
stones or electrodes. It consists of following steps:
a. Tapering the gingival margins.
b. Scalloped marginal outline.
c. Thinning of the attached gingiva and creating vertical
interdental grooves.
d. Shaping the interdental papillae to provide sluice ways
for the passage of food.
Healing after Surgical Gingivectomy
Basically healing is by secondary intention:
a. The initial response is the formation of a protective
surface clot.
b. The clot is then replaced by granulation tissue.
c. Within 24 hours, there is an increase in new
connective tissue cells mainly angioblasts and by third
day numerous fibroblasts are located in this area.
The highly vascular granulation tissues grow coronally,
creating a new free gingival margin and sulcus.
d. Capillaries derived from blood vessels of periodontal
ligament migrate into the granulation tissue, and with
in two weeks they connect with gingival vessels.
Surface epithelialization is generally complete after
5 to 14 days. Initially, keratinization is less than what
it was prior to surgery. Complete epithelialization takes
about 1 month.
Electrosurgery (Surgical Diathermy)
Uses high frequency current of 1.5 to 7.5 million cycles
per second.
There are three classes of electrodes used:
1. Single wire electrodes for incising and excising.
2. Loop electrodes for planing tissues.
3. Heavy bulkier electrodes for coagulation proce-
dures.
Four types of electrosurgical techniques are
available:
a. Electrosection: Three procedures are performed
incising, excising and planing.
b. Electrocoagulation: Used to prevent hemorrhage.
c. Electrofulguration: Uses high voltage current. It has
limited application in dentistry.
d. Electrodesiccation: Uses dehydrating current and least
used, as it is a dangerous technique.
Here the active electrode is inserted into the tissue
and the tissue surrounding the electrode is mass
coagulated in situ. This procedure is useful only in
dermatological and cancer surgeries.
G
F
Figs 40.2F and G:
Clinical appearance after gingivectomy
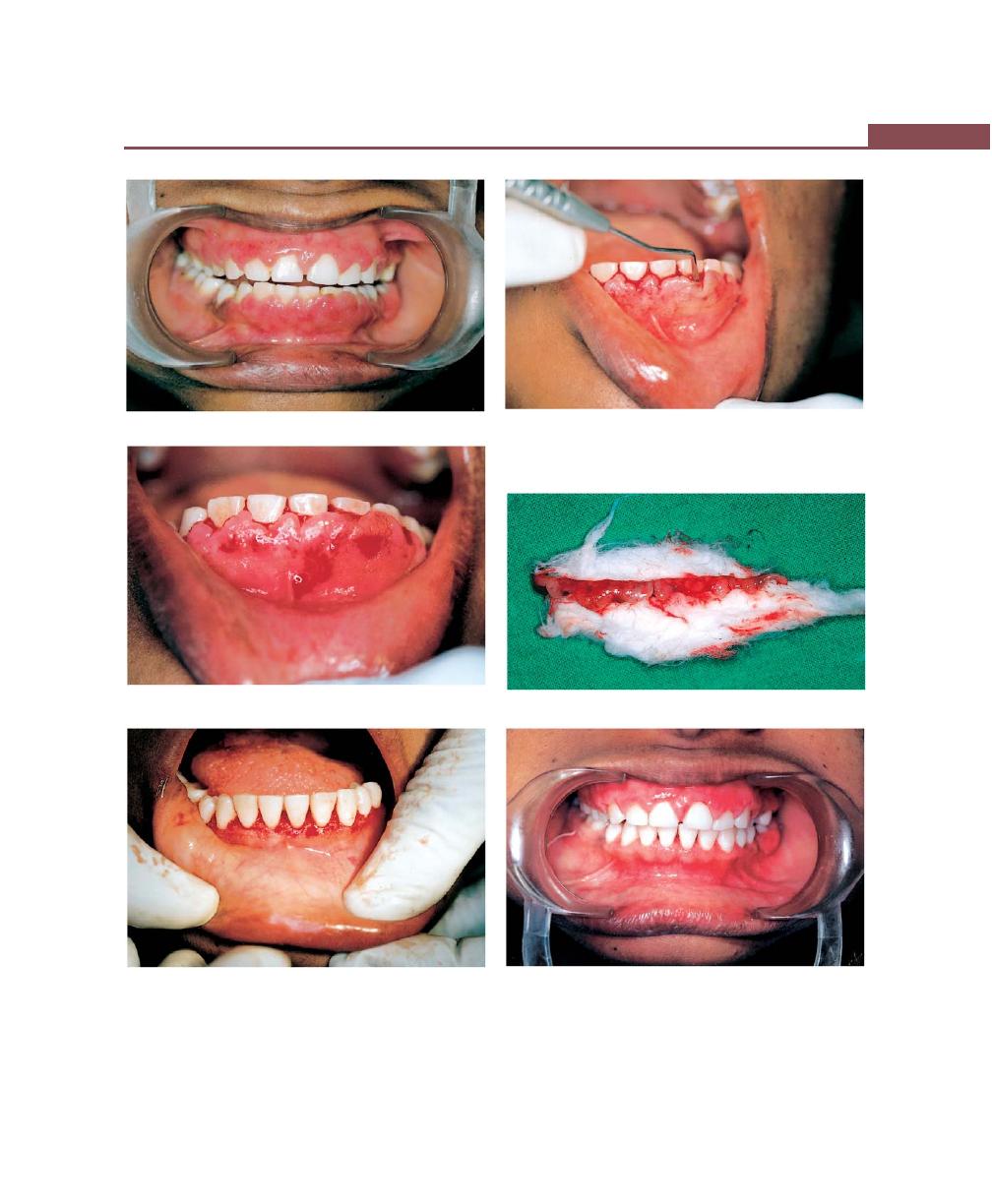
311
Gingivectomy
A
B
C
D
E
F
Figs 40.3A to F

312
Essentials of Clinical Periodontology and Periodontics
G
Figs 40.3A to G: Case II Gingivectomy. (A) Preoperative view.
(B) Probing of pocket depth. (C) Pocket marking. (D) Strip of
gingival tissue after gingivectomy. (E) Gingival contouring
(immediately after gingivectomy). (F) After healing—facial
view. (G) Lingual view
Gingivectomy by Electrosurgery
Advantages
Permits adequate contouring of the tissues and controls
hemorrhage.
Disadvantages
1. Cannot be used in patients with poorly shielded
cardiac pacemaker.
2. Causes unpleasant odor.
3. If it touches the bone irreparable damage may result.
4. Heat generated by this may cause tissue damage and
areas of cemental necrosis.
Indications
1. Removal of gingival enlargements.
2. Gingivoplasty.
3. Relocation of frenum and muscle attachments.
4. Incision of periodontal abscesses and pericoronal
abscess.
Technique
a. For gingivoplasty: Needle electrodes and diamond
shaped electrodes are used for festooning. In all
reshaping procedures electrodes are activated and
moved in a concise “Shaving” motion (Cutting and
Coagulating current is used).
b. For abscess drainage: Incisions can be made with the
needle electrode.
c. For haemostasis: Ball electrode is used.
d. For relocation of frenum and muscle attachment:
Loop electrode is used.
Healing after Electrosurgery
Some investigators report no significant differences but
others however have reported delayed healing, greater
reduction in gingival height and more bone injury after
electro surgery.
Laser Gingivectomy
Most commonly used lasers are carbon dioxide and
Nd:YAG lasers. They are used for excision of gingival
over growth. Their use in periodontal surgery is not
supported by research.
Gingivectomy by Chemosurgery
Chemicals used are 5 percent Para formaldehyde or
potassium hydroxide to remove gingiva.
Disadvantage
1. Their depth of action cannot be controlled hence it
may also injure normal tissues.
2. Gingival remodelling is not possible.
3. Healing is delayed.
KEY POINTS TO NOTE
1. Excision of soft tissue wall of the pocket is called Gingivec-
tomy.
2. Gingivectomy may be performed by means of scalpels,
electrodes, laser beams or chemicals.
3. In surgical Gingivectomy, the incision should be bevelled
at approximately 45 degrees to the tooth surface and should
recreate normal festooned pattern of the gingiva.
4. Gingivectomy should be differentiated from gingivoplasty
in that it is performed mainly to eliminate the pocket and
also includes reshaping where as the latter is performed for
reshaping the gingiva to create normal physiological contour.
5. Basic healing after Gingivectomy is by secondary intention.
6. The main advantage of laser and Gingivectomy by
electrosurgery is contouring of the tissue.

313
Gingivectomy
REVIEW QUESTIONS
1. Define Gingivectomy and describe the step by step
procedure of surgical Gingivectomy.
2. Describe various Gingivectomy techniques.
3. Describe the indications and contraindications of
Gingivectomy.
4. Describe healing after Gingivectomy.
BIBLIOGRAPHY
1. Gottsegen R, Ammons WF Jr. Research in lasers in
periodontics. Position paper. Chicago, American Academy
of Periodontology, May 1992.
2. Jan Lindhe. Clinical Periodontology and Implant Dentistry,
4th edn, Blackwell Munksgaard Publication, 2003.
3. Newman, Takei, Fermin A Carranza. Clinical Periodontology,
9th edn,WB Saunders and Co, 2002.
314
Essentials of Clinical Periodontology and Periodontics
INTRODUCTION
Although in a strict sense all the instrumental therapy
can be considered surgical (as by Webster’s definition
of surgery), those techniques mentioned here are
considered as surgical procedures because it involves
intentional severing of tissues.
Hence the definition of surgery is,“the art, practice or
work of treating diseases, injuries or deformities by manual
operation or instrumental application.”
Periodontal surgery (open) is defined as intentional
severing or incising of gingival tissue with the purpose
of controlling or eliminating periodontal disease.
Therefore scaling and root planing (blind or closed) are
not included.
When should one consider for surgical pocket
therapy ?
1. For opening up the pocket area in order to remove
all the irritants from the tooth surface.
2. For elimination or reduction of the periodontal
pocket.
The successful periodontal therapy is based on total
elimination of plaque, calculus and diseased cementum
from the tooth surface. Whenever there is a pocket
present, the plaque control becomes difficult and also
deeper the pocket the more difficult the access because,
the surface area to be scaled is increased, presence of
root surface irregularities and also furcation involvement
also creates various problems.
All the above mentioned problems can be reduced
by either resecting or displacing the soft tissue wall of
the pocket. Gingivectomy and the flap surgery are
the treatment procedures to attain this result (Fig. 41.1).
INDICATIONS/OBJECTIVES OF
FLAP SURGERY
1. Gain access for root debridement.
2. Reduction or elimination of pocket depth, so that
patient can maintain the root surfaces free of
plaque.
❒
❒
❒
❒
❒ INDICATIONS
❒
❒
❒
❒
❒ DEFINITION
❒
❒
❒
❒
❒ CLASSIFICATION
❒
❒
❒
❒
❒ INCISIONS
❒
❒
❒
❒
❒ FLAP TECHNIQUES FOR POCKET
THERAPY
• Modified Widman Flap
• Undisplaced Flap
• Palatal Flap
• Apically-displaced Flap
• Distal Molar Surgery
❒
❒
❒
❒
❒ HEALING AFTER FLAP SURGERY
41
Periodontal Flap
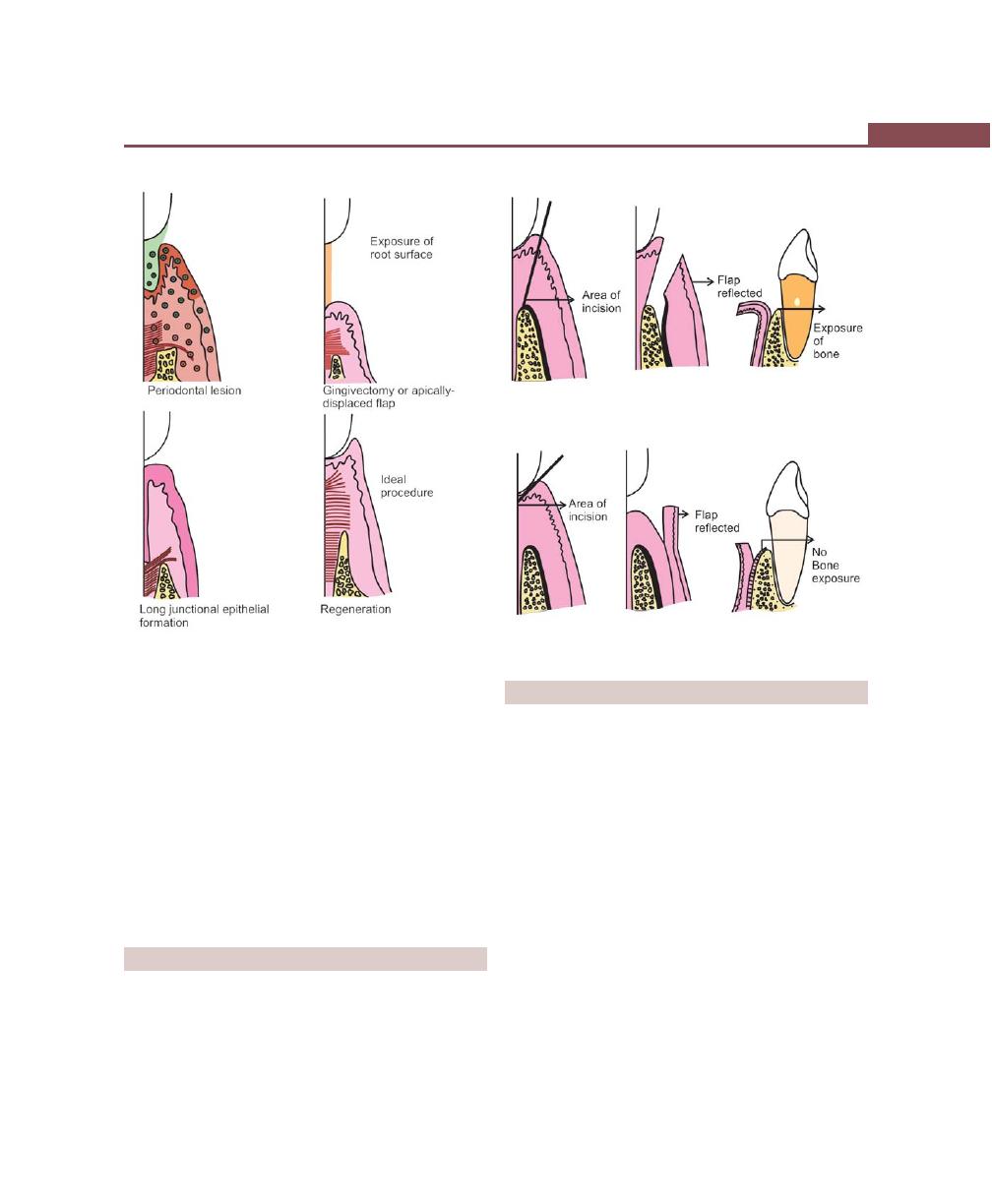
315
Periodontal Flap
3. Reshaping soft and hard tissues to attain a harmonious
topography (physiologic architecture).
4. Regeneration of alveolar bone, periodontal ligament
and cementum.
Today the flap has become the basic surgical
procedure of periodontal therapy. It not only provides
access to underlying tissues, but also permits the
surgeon to perform a variety of regenerative proce-
dures.
DEFINITION
A periodontal flap is a section of gingiva and/or mucosa
surgically-elevated from the underlying tissues to
provide visibility of and access to the bone and root
surface.
Fig. 41.1:
Outcome of periodontal therapy
Fig. 41.2:
Mucoperiosteal / full thickness flap
Fig. 41.3:
Partial / split thickness flap
CLASSIFICATION
According to the Thickness of the Flap/Bone
Exposure after Flap Reflection
The flaps are classified as:
a. Full thickness/mucoperiosteal flap—All the soft tissues
including the periosteum is elevated (Fig. 41.2).
b. Partial thickness/mucosal flap/split thickness flap—
Reflection of only the epithelium and a layer of
underlying connective tissue, the bone is covered by
a layer of connective tissue including periosteum (Fig.
41.3).
Indications
I. Full thickness
: If osseous surgery is
contemplated.
II. Partial thickness
: For displacing flaps in the
presence of dehiscence and
fenestrations.
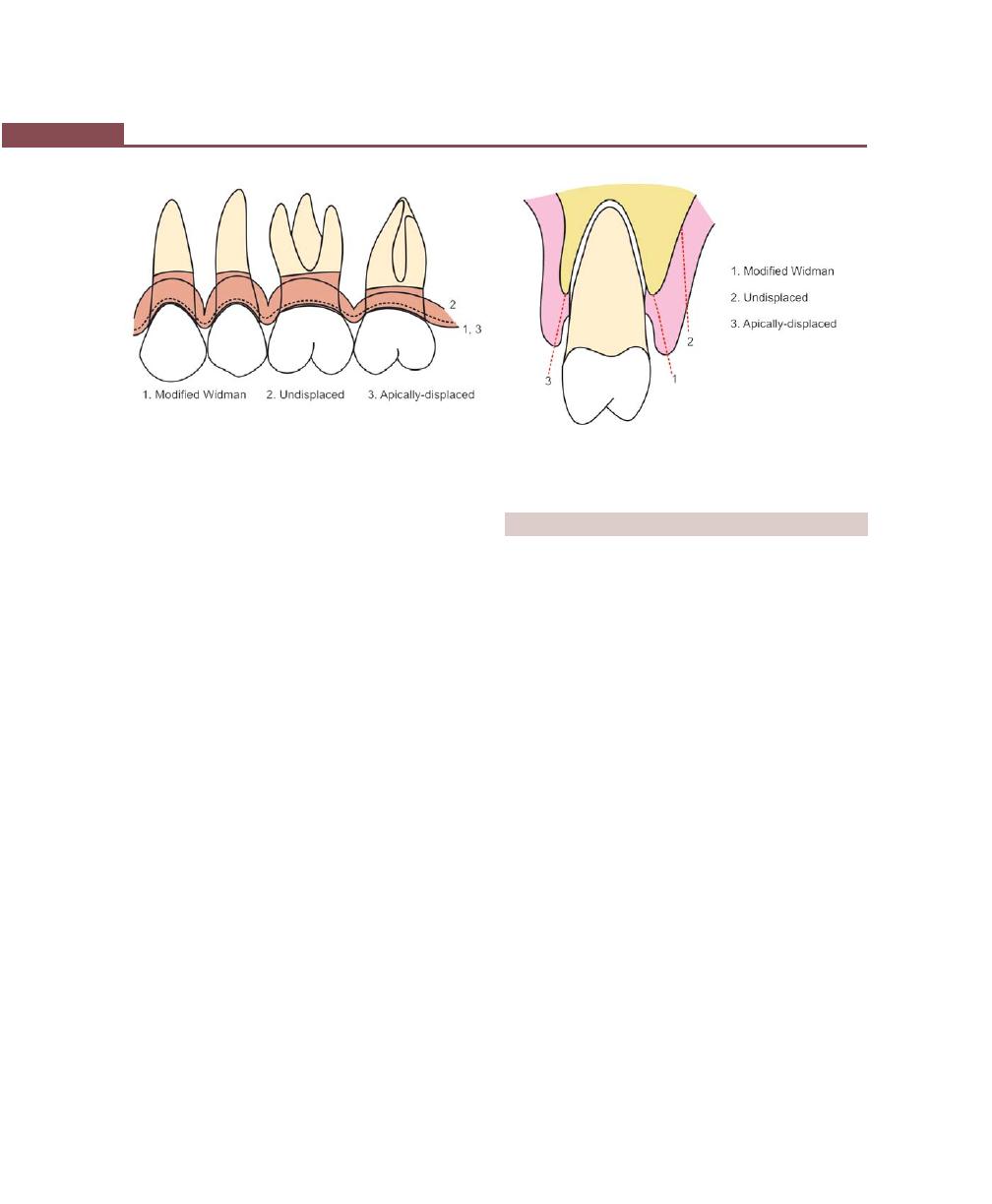
316
Essentials of Clinical Periodontology and Periodontics
According to the Placement of
Flap after Surgery
a. Nondisplaced flap—The flap is returned and sutured
back in its original position.
b. Displaced flaps—The flap is repositioned coronal,
apical or lateral to its original position. However,
palatal flaps cannot be displaced due to the absence
of unattached gingiva.
According to Design of the
Flap/Management of the Papilla
The flaps can be:
a. Conventional flaps—Splitting the papilla into a facial
half and lingual/palatal half. For example, modified
Widman flap, undisplaced flap, apically-displaced flap
(Fig. 41.4).
Indications:
i. When the interdental areas are too narrow to
permit the preservation of flap.
ii. When there is a need for displacing flaps.
b. Papilla preservation flaps—Entire papilla is incor-
porated into one of the flaps.
Indications:
i. Where esthetics is of concern.
ii. Where bone regeneration techniques are
attempted.
INCISIONS
For Conventional Flap:
1. Horizontal Incision:
• Internal bevel incision (Fig. 41.5)
• Crevicular incision
• Interdental incision
2. Vertical incision
• Oblique releasing incision
For Papilla Preservation Flap:
Crevicular incision with no incisions across the interdental
papilla is given.
Horizontal Incisions (Figs 41.6A and B)
Internal bevel incision: It starts at a distance (1 to 2 mm)
from the gingival margin and is aimed at the bone crest.
It is a basic incision to most of the flap procedures.
It accomplishes three important objectives:
1. Removes pocket lining.
2. Conserves relatively uninvolved outer surface of the
gingiva.
3. It produces a sharp, thin flap margin for adaptation
to tooth—bone junction.
Fig. 41.4:
Scallopings required for the different types of
flaps
Fig. 41.5:
Location of the internal bevel incisions for the
different types of flaps
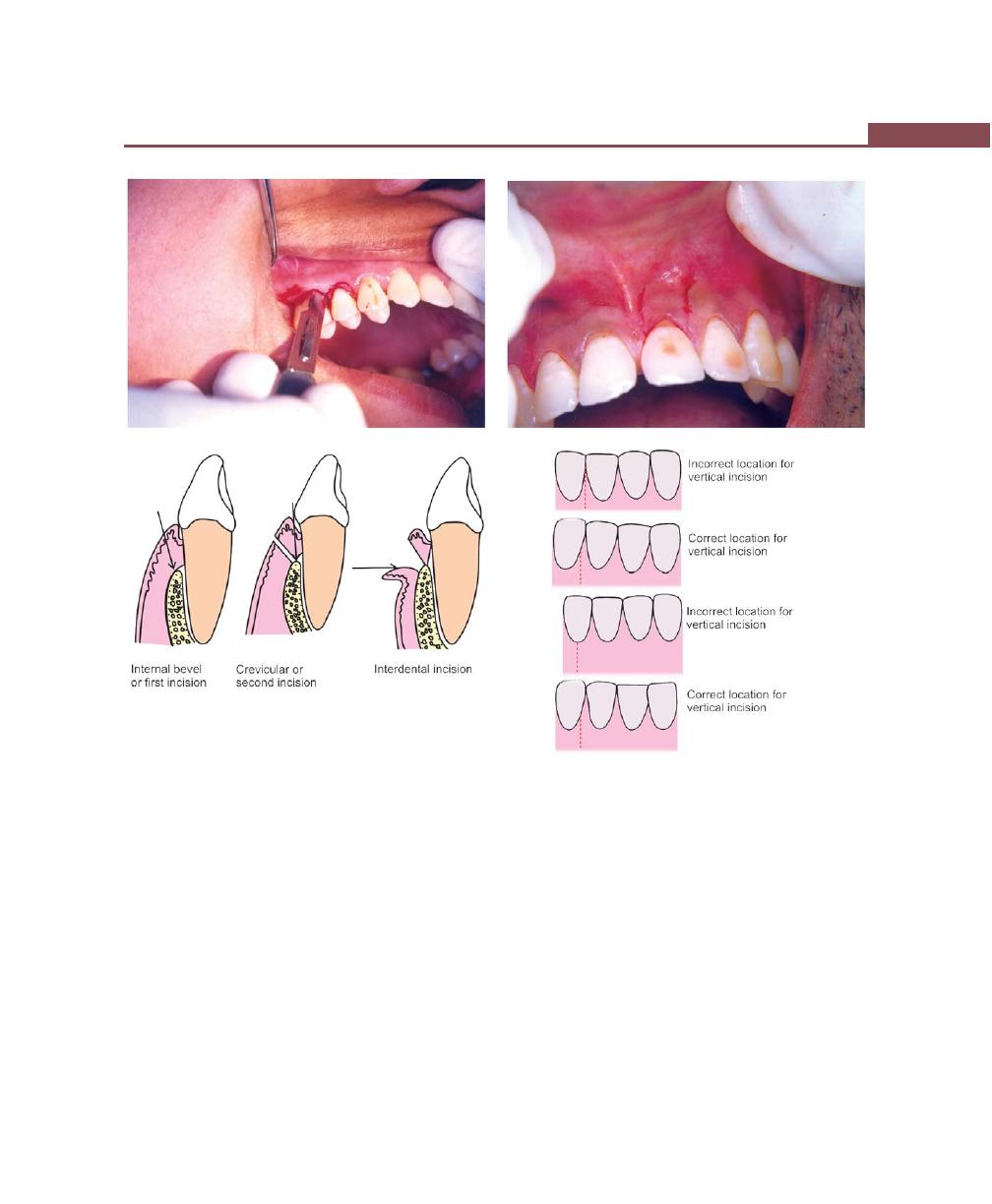
317
Periodontal Flap
Figs 41.6A and B:
Horizontal incisions
It is also known as first incision, reverse bevel incision.
No. 15 and No. 11 surgical scalpels are most commonly
used.
Crevicular Incision
It is also termed as the second incision, is made from the
base of the pocket to the crest of the bone. The incision
is carried around the entire tooth. No. 12B blade is used.
This results in V-shaped wedge of tissue containing
inflammed granulomatous tissue consisting of lateral wall
of the pocket, junctional epithelium and connective tissue
fibres between the base of the pocket and crest of the bone.
After this, a periosteal elevator is used to separate
the flap from the bone. With this access, the surgeon
is able to make the third or interdental incision, to
separate the collar of the gingiva that is left around the
Figs 41.7A and B:
(A) Vertical incisions without involving
interdental papilla. (B) Location of vertical incision
tooth. The orban knife is usually utilized for this incision.
A curette or a large scaler (U15/30) can be used to
remove the gingiva around the tooth. If no vertical
incisions are made, the flap is called an envelope flap.
Vertical Incision (Figs 41.7A and B)
It can be done on one or both the ends of the horizontal
incision. If the flap has to be displaced the incisions at
both ends should be made. Another indication for vertical
incision is in the presence of isolated deep pockets. Vertical
incisions should be made extending beyond mucogin-
gival junction for easy displacement of flap.
A
B
A
B
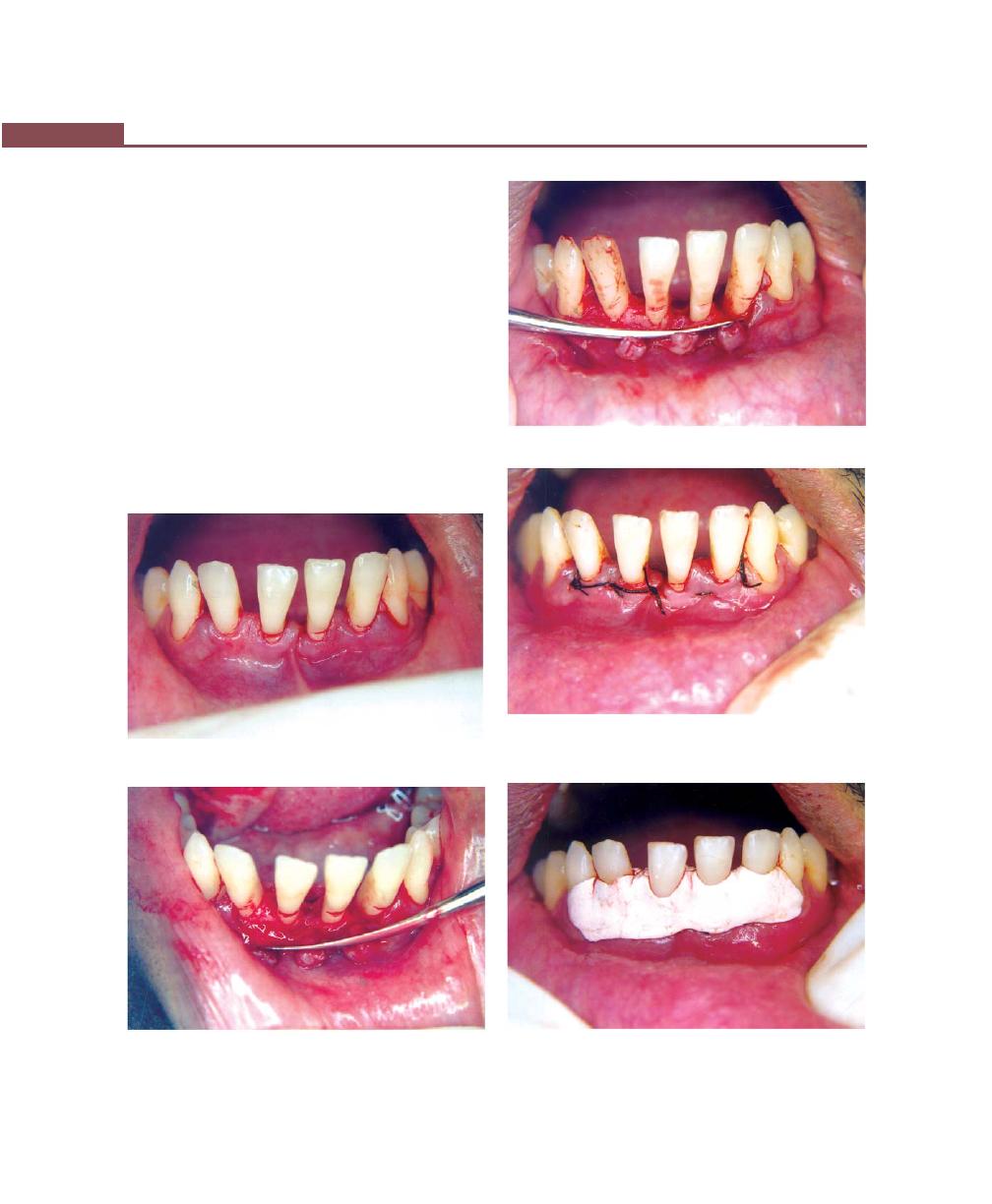
318
Essentials of Clinical Periodontology and Periodontics
In general, vertical incisions are avoided:
a. In lingual or palatal areas.
b. At the center of the interdental papilla or over
the radicular surface.
They are made along the line angles of the tooth,
avoid short flaps with long, apically directed incisions as
it compromises blood supply to the flap.
Papilla Preservation Flap (Figs 41.8 to 41.10)
Step 1:
Crevicular incision is made around each
tooth. No incisions through the interdental
papilla.
Step 2:
Papilla is usually incorporated facially, hence
a semi-lunar incision across the interdental
Fig. 41.8A:
Facial view after sulcular incisions have been
made
Fig. 41.8B:
Flap reflection
Fig. 41.8C:
After curettage and root planing
Fig. 41.8D:
Flap is secured with sutures
Fig. 41.8E:
The surgical site is covered with periodontal
dressing
Figs 41.8A to E:
Papilla preservation flap
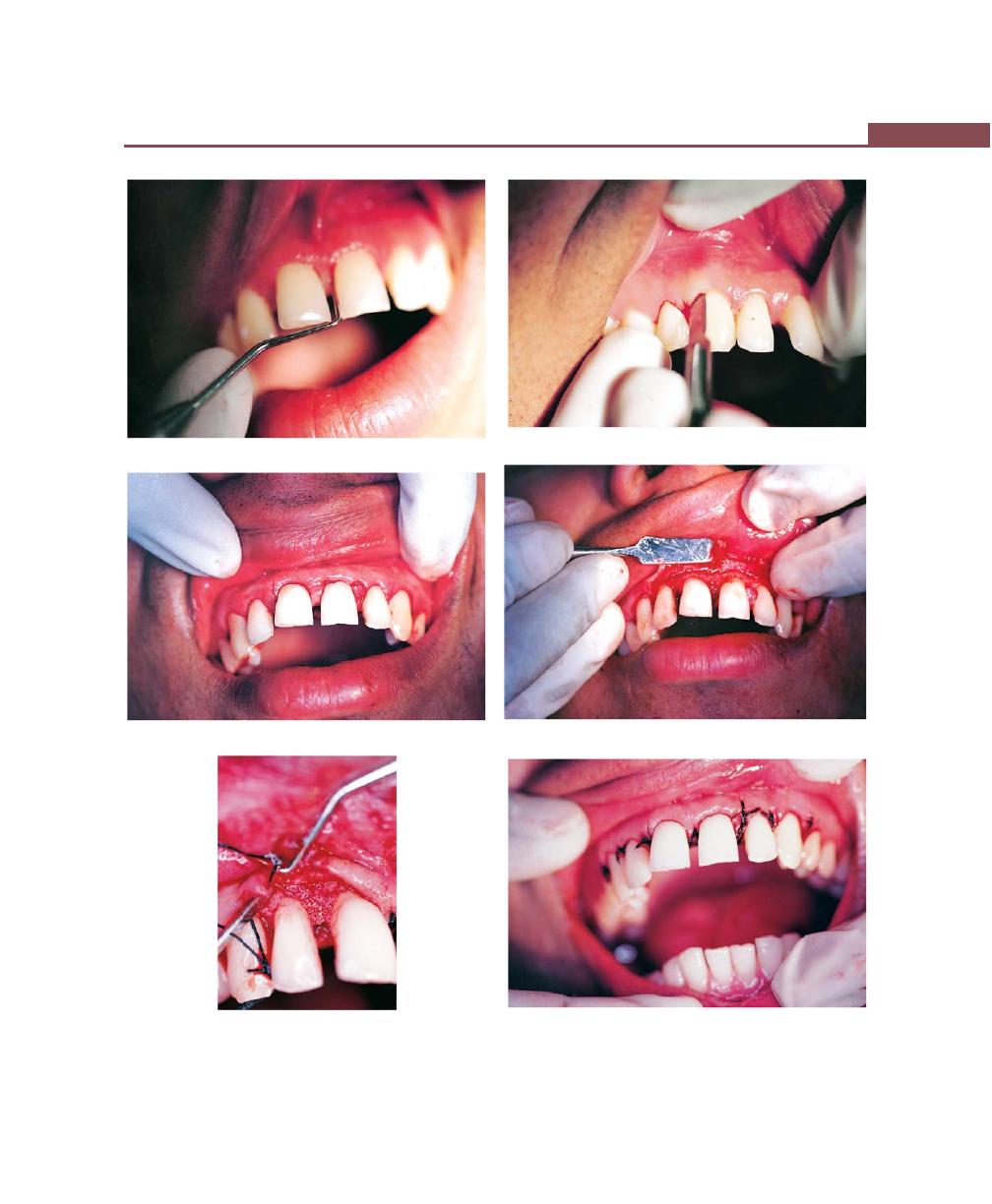
319
Periodontal Flap
Figs 41.9A to F:
Case-2: Papilla preservation flap. (A) Probing depth. (B) Crevicular incision placed. (C) Intrasulcular and
semilunar incisions. (D) Reflection and degranulation. (E) Bone graft placement, (F) Suturing of palatal and buccal flaps
A
B
C
D
F
E
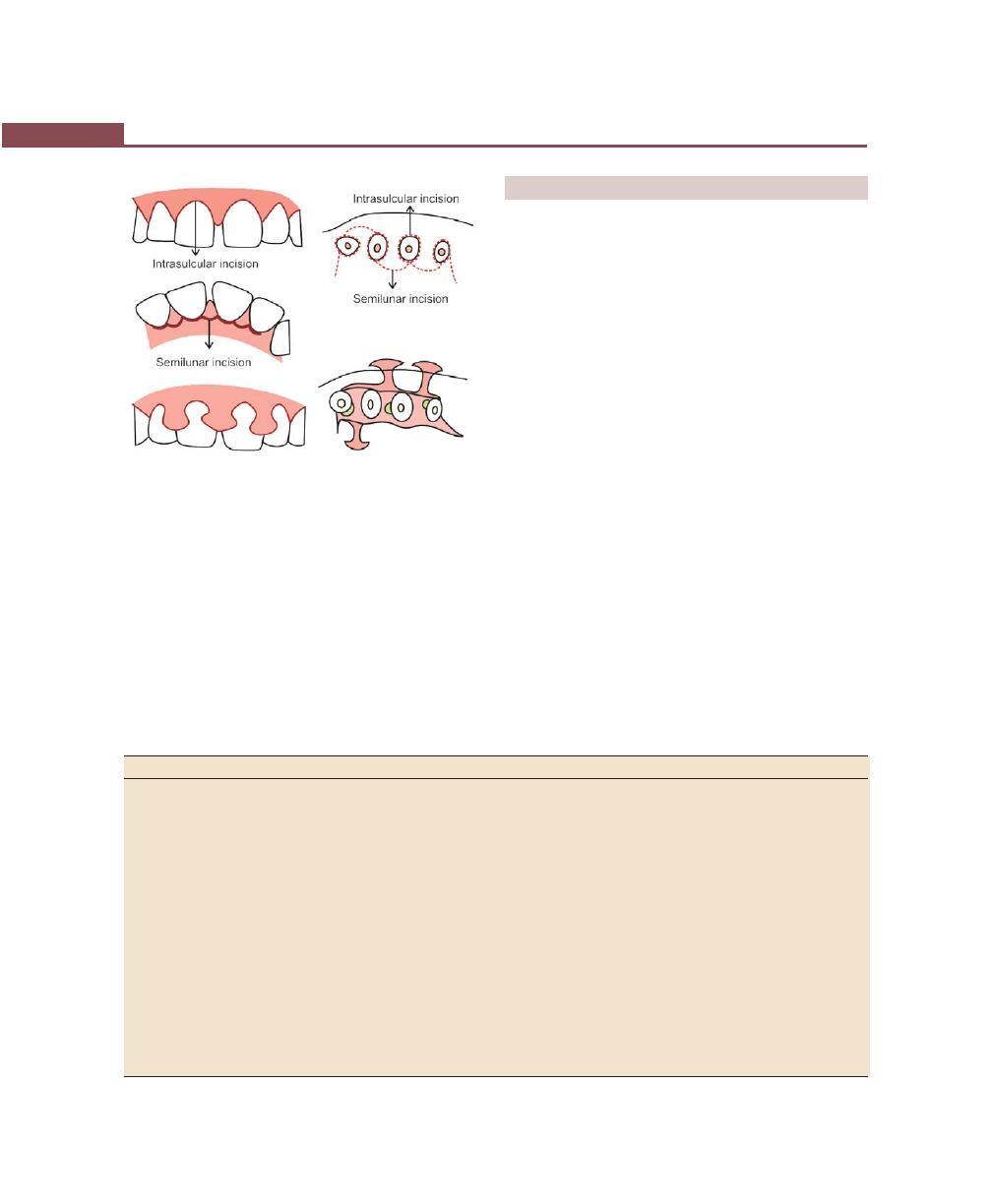
320
Essentials of Clinical Periodontology and Periodontics
FLAP TECHNIQUES FOR POCKET THERAPY
The periodontal flap is one of the most frequently
employed procedure particularly for moderate to deep
pockets in posterior areas. Considering the objectives
it offers, there are three flap techniques available in
current use (Table 41.1). They are:
i. Modified Widman flap.
ii. Undisplaced flap.
iii. Apically-displaced flap.
Modified Widman Flap (Figs 41.11 to 41.13)
Presented in 1974 by Ramfjord and Nissle.
Step 1:
It is an initial, internal bevel incision 0.5 to
1 mm away from the gingival margin, directed
to the alveolar crest. Vertical releasing incisions
are not required (different from Widman
flap).
Step 2:
Gingiva is reflected with a periosteal elevator
Step 3:
A crevicular incision is made.
Step 4:
After the flap is reflected, third incision is made
in the interdental spaces with Orban’s knife
and the gingival collar is removed.
Step 5:
Tissue tags and granulation tissue are removed
with a curette. The root surfaces are checked
and scaled.
Table 41.1: Differences between various flap techniques
Modified Widman flap
Undisplaced flap
Apically-displaced flap
I. Purpose
1. To expose root surfaces for
1. Accessibility for instrumentation.
1. Improves accessibility.
instrumentation.
2. For removal of pocket lining.
2. To remove the pocket wall to
2. It also eliminates the pocket by
(It is not indicated to eliminate/
reduce or eliminate the pocket.
transforming the previously
reduce pocket depth, except for
(An excisional procedure of the
unattached keratinized pocket
the reduction that occurs in
gingiva).
wall into attached tissues.
healing by shrinkage).
(offers dual function).
II. Variations in the design
It does not intend to remove the
Internal bevel incision is started at or
The internal bevel incision should be
pocket wall but eliminate pocket
near a point just coronal to the
placed as close to the tooth as possible
lining. Therefore, internal bevel
projection of the bottom of the pocket
(0.5 to 1 mm) because the purpose of
incision starts close (no more than
on the outer surface of the gingiva.
this technique is to preserve maximum
1 to 2 mm apical) to the gingival
(Only performed when sufficient
amount of keratinized tissue, displace
margin.
attached gingiva is to be left behind).
it apically and transform it into attached
The incision should be scalloped to
gingiva. The flap is positioned approxi-
preserve as much as interdental papilla.
mately at the tooth bone junction.
papilla in the palatal or lingual surface is made,
which is atleast 5 mm from the crest of the
papilla (the deepest curve).
Step 3:
The papilla is dissected from the lingual or
palatal aspect using Orban knife and elevated
intact with the facial flap.
After the desired incisions are made, the flap is
elevated either by blunt dissection for a full thickness
flap or sharp dissection for partial thickness flap or
combination of both for various surgical procedures.
Fig. 41.10:
Papilla preservation flap—incisions
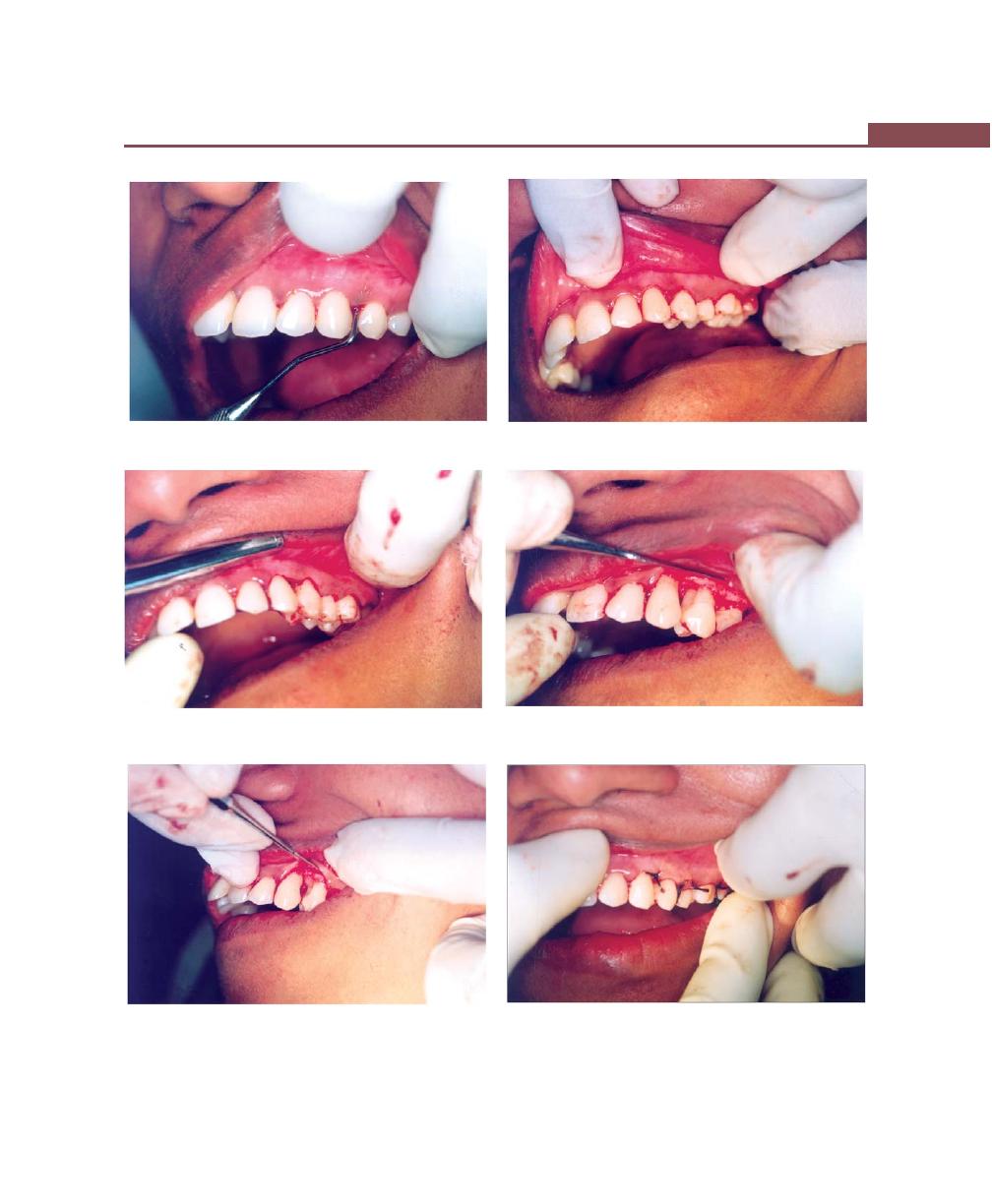
321
Periodontal Flap
D
E
A
B
C
F
Figs 41.11A to F:
Periodontal flap technique (Modified Widman flap). (A) Persistent pockets 2-3 weeks after initial therapy,
(B) Facial incisions (internal bevel, crevicular), (C) Interdental incisions performed, (D) Facial flaps are reflected, (E)
Curettage and root planing performed, (F) Interrupted interdental sutures are placed
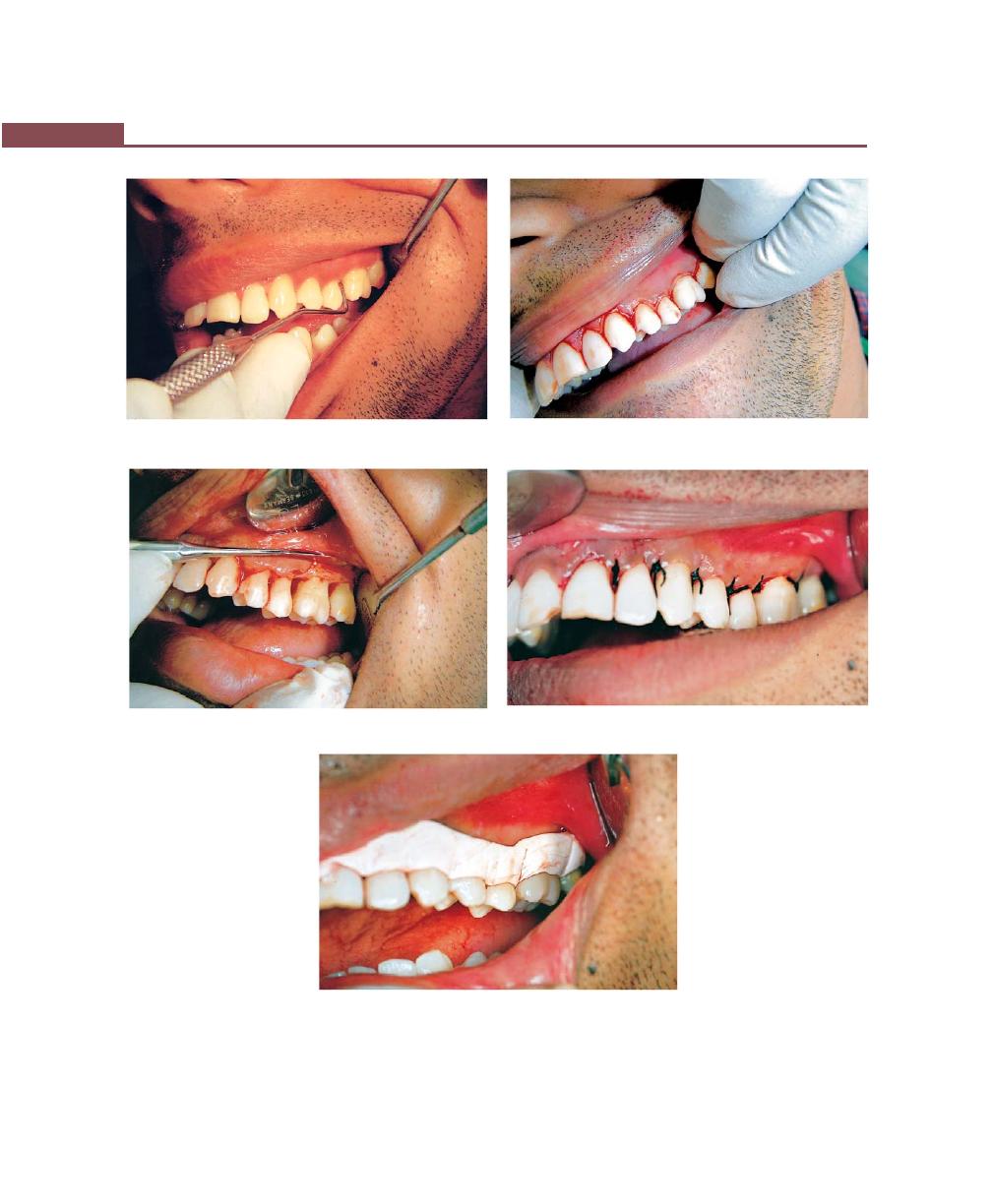
322
Essentials of Clinical Periodontology and Periodontics
Figs 41.12A to E:
Case-2: Modified widman flap procedure. (A) Preoperative view with probing. (B) Internal bevel and
crevicular incision’s are placed. (C) Flap reflection and degranulation. (D) Suturing with direct loop sutures. (E) Pack placed
A
B
C
D
E
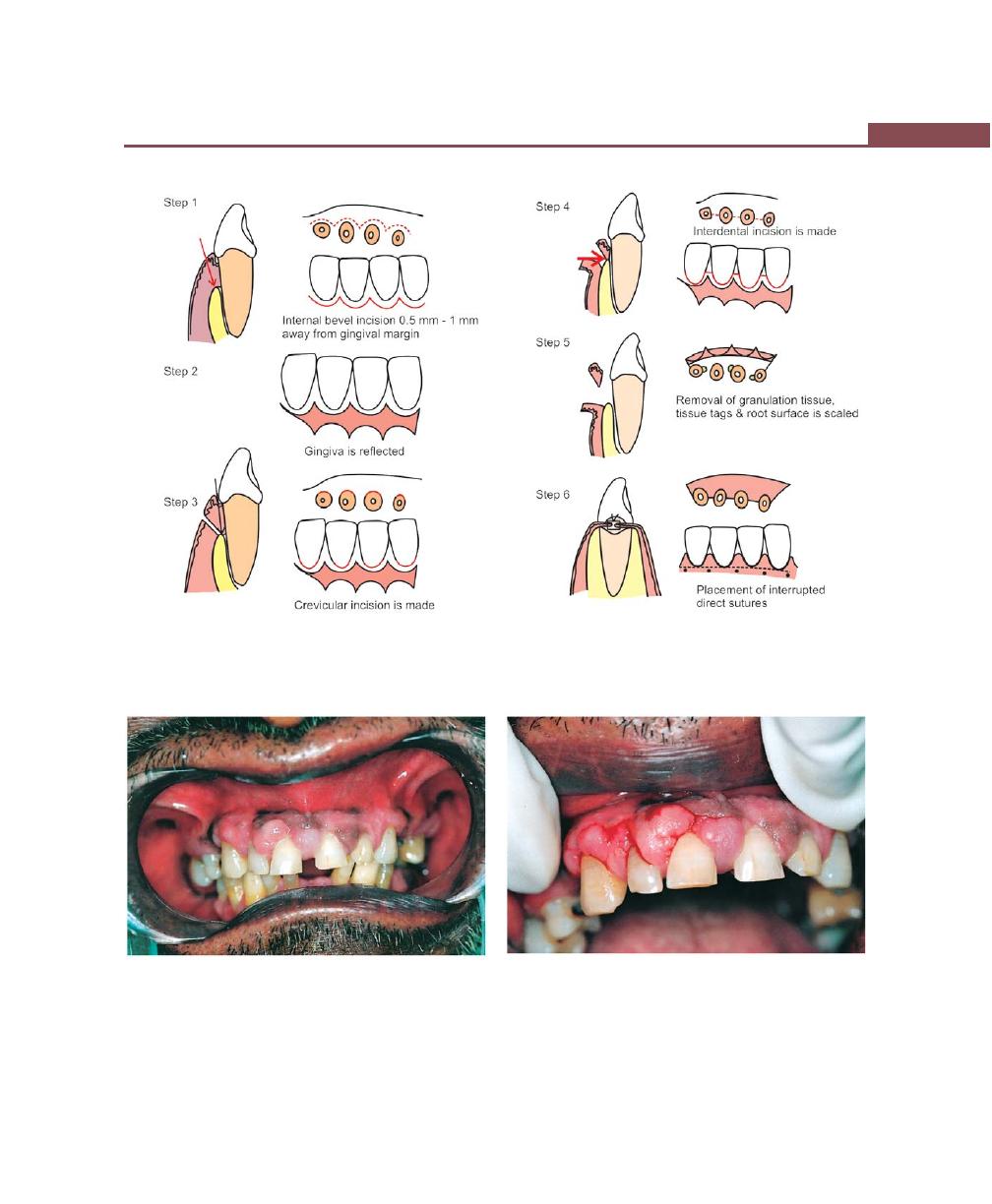
323
Periodontal Flap
Fig. 41.13:
Modified Widman flap—various steps
A
B
Figs 41.14A and B
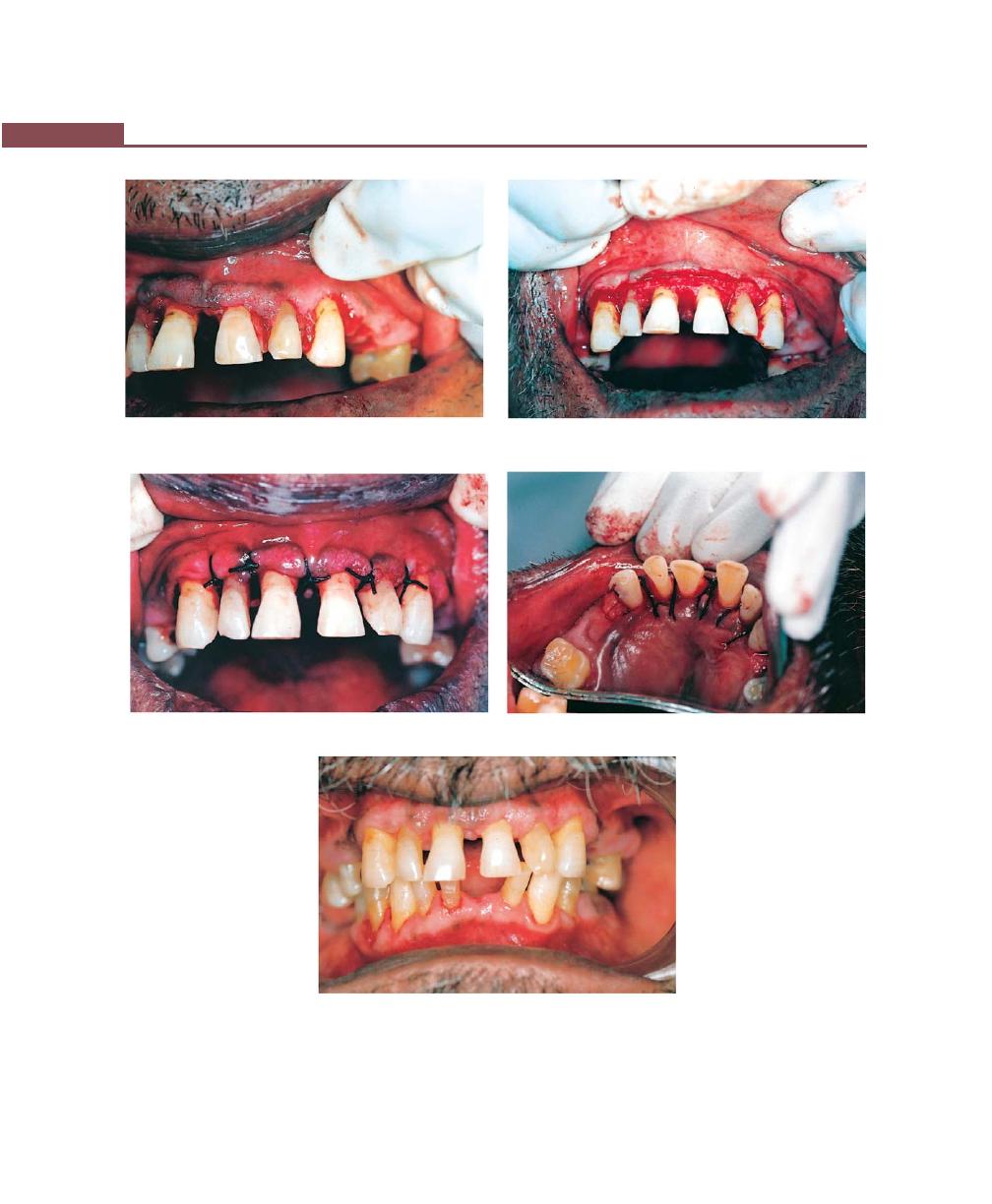
324
Essentials of Clinical Periodontology and Periodontics
C
D
E
F
Figs 41.14A to G:
Undisplaced flap. (A) Preoperative view. (B) Internal bevel incision. (C and D) Reflection and
degranulation. (E) Suturing buccal and palatal flaps. (F) Palatal view after suturing. (G) Facial view after healing
G
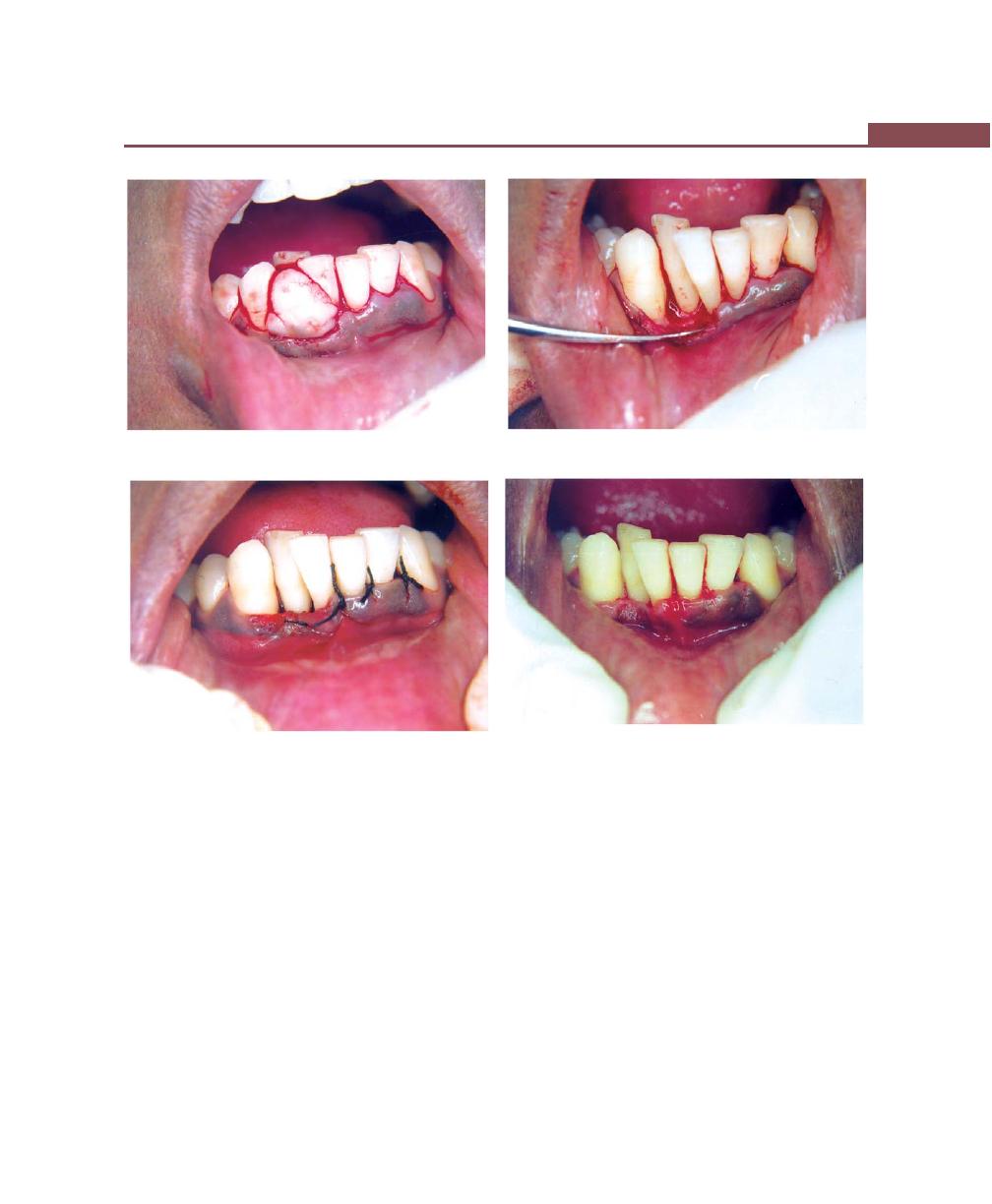
325
Periodontal Flap
A
D
Figs 41.15A to D:
Case-2: Undisplaced flap—step-by-step procedure. Clinical presentation. (A) Incisions placed at the base
of the pocket. (B) Flap reflection. (C) After thorough debridement, sutures are placed. (D) Facial view after healing
Step 6:
Bone architecture is not corrected, good
approximation of flaps is necessary, hence
sometimes flaps may have to be thinned.
Step 7:
Interrupted direct sutures are placed.
Undisplaced Flap (Figs 41.14 to 41.16)
In this procedure the entire soft tissue pocket wall is
removed with the initial incision. Thus, it may be
considered as internal bevel gingivectomy. To perform
this enough attached gingiva should remain after removal
of the pocket wall.
Step 1:
The pockets are measured with the
periodontal probe and a bleeding point is
produced on the outer surface of the gingiva
to mark the base of the pocket. In this
procedure, the final placement of the flap is
determined by first incision.
Step 2:
The initial, internal bevel incision is made
following the scalloping bleeding points made
on the gingiva. This incision is usually carried
to a point apical to the alveolar crest
depending on the thickness of the tissue. The
B
C
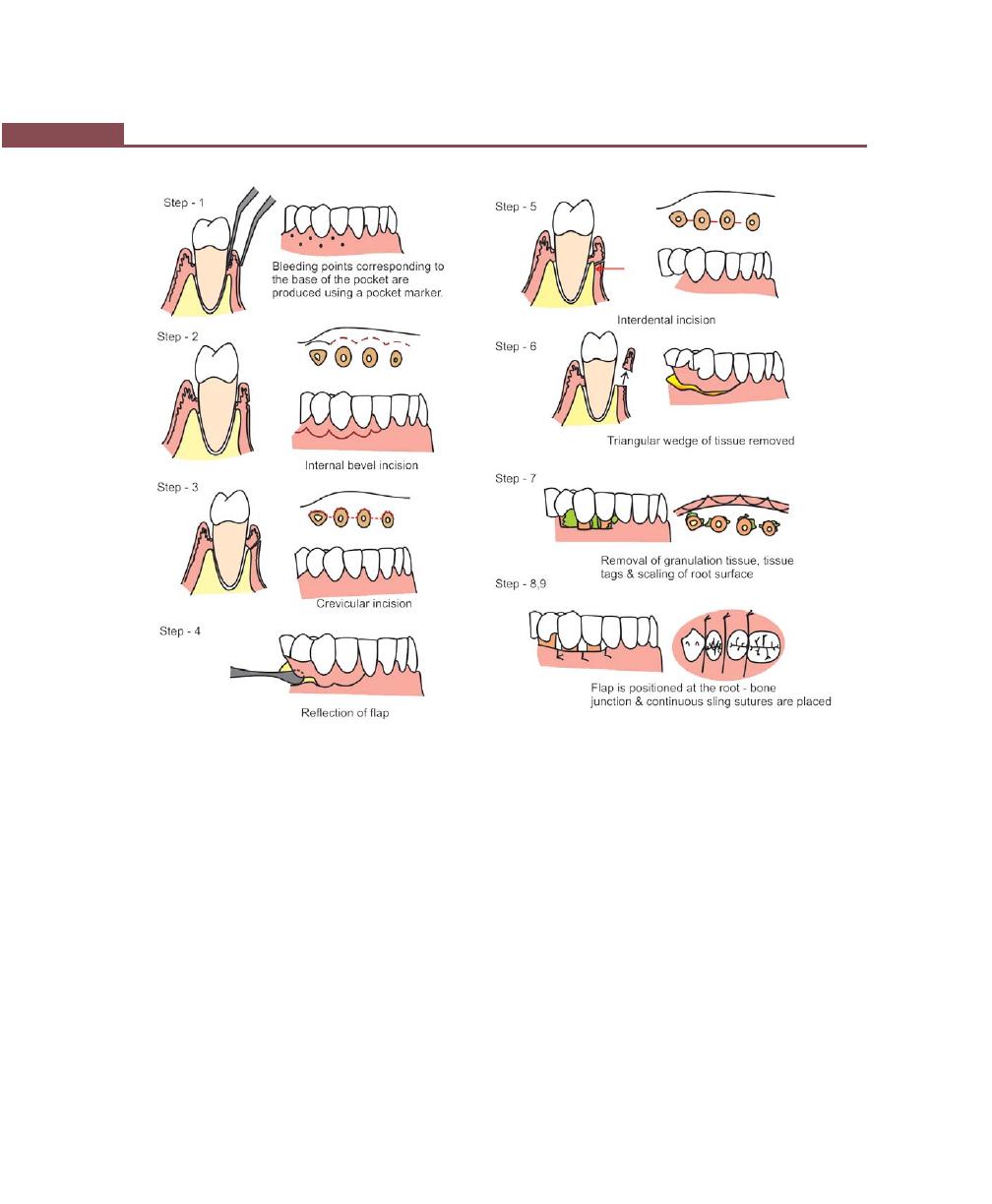
326
Essentials of Clinical Periodontology and Periodontics
Figs 41.16:
Undisplaced flap technique—various steps
thicker the tissue, the more apical will be the
end point. The flap should be thinned with
the initial incision only.
Step 3:
The second or crevicular incision is made from
the bottom of the pocket to the bone.
Step 4:
The flap is then reflected with a periosteal
elevator (blunt dissection).
Step 5:
Interdental incision is made with a knife.
Step 6:
Triangular wedge of tissue is removed with
a curette.
Step 7:
The area is debrided, removing tissue tags and
granulation tissue with sharp curettes. The
roots are scaled.
Step 8:
The flap is then placed back to end at the
root bone junction.
Step 9:
The flaps are sutured together with continuous
sling suture or interrupted sutures.
Palatal Flap (Figs 41.17 and 41.18)
The surgical approach is different here because of the
nature of the palatal tissue which is attached, keratinized
tissue and has no elastic properties associated with other
gingival tissues, hence no displacement and no partial
thickness flaps.
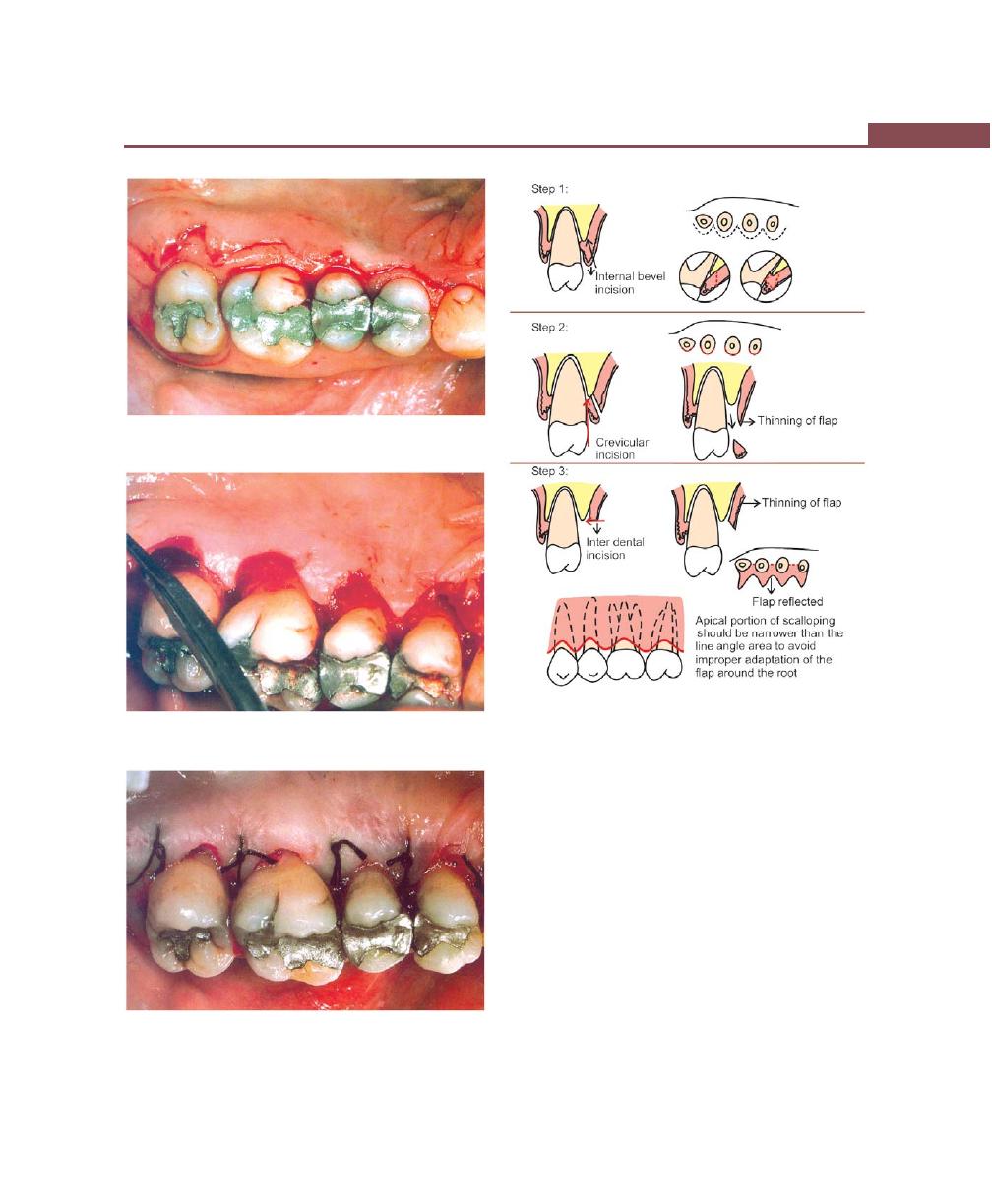
327
Periodontal Flap
Figs 41.17A to C:
Palatal flap
A
B
C
Fig. 41.18:
Palatal flap—step-by-step procedure
Variations in the Techniques include
1. Usual internal bevel incision, followed by crevicular
and interdental incisions, but if the tissue is thick
horizontal gingivectomy incision is made, followed
by an internal bevel incision. Thinning of the flap
should be done prior to reflection of the flap.
2. The apical portion of the scalloping incision should
be narrower than the line angle area, because the
palatal root tapers apically, rounded scallop will result
in improper adaptation of the flap around root.
Basically, one should make sure the flap fits
around the tooth snugly without exposing the bone,
to achieve this proper placement of the incision is
very important.
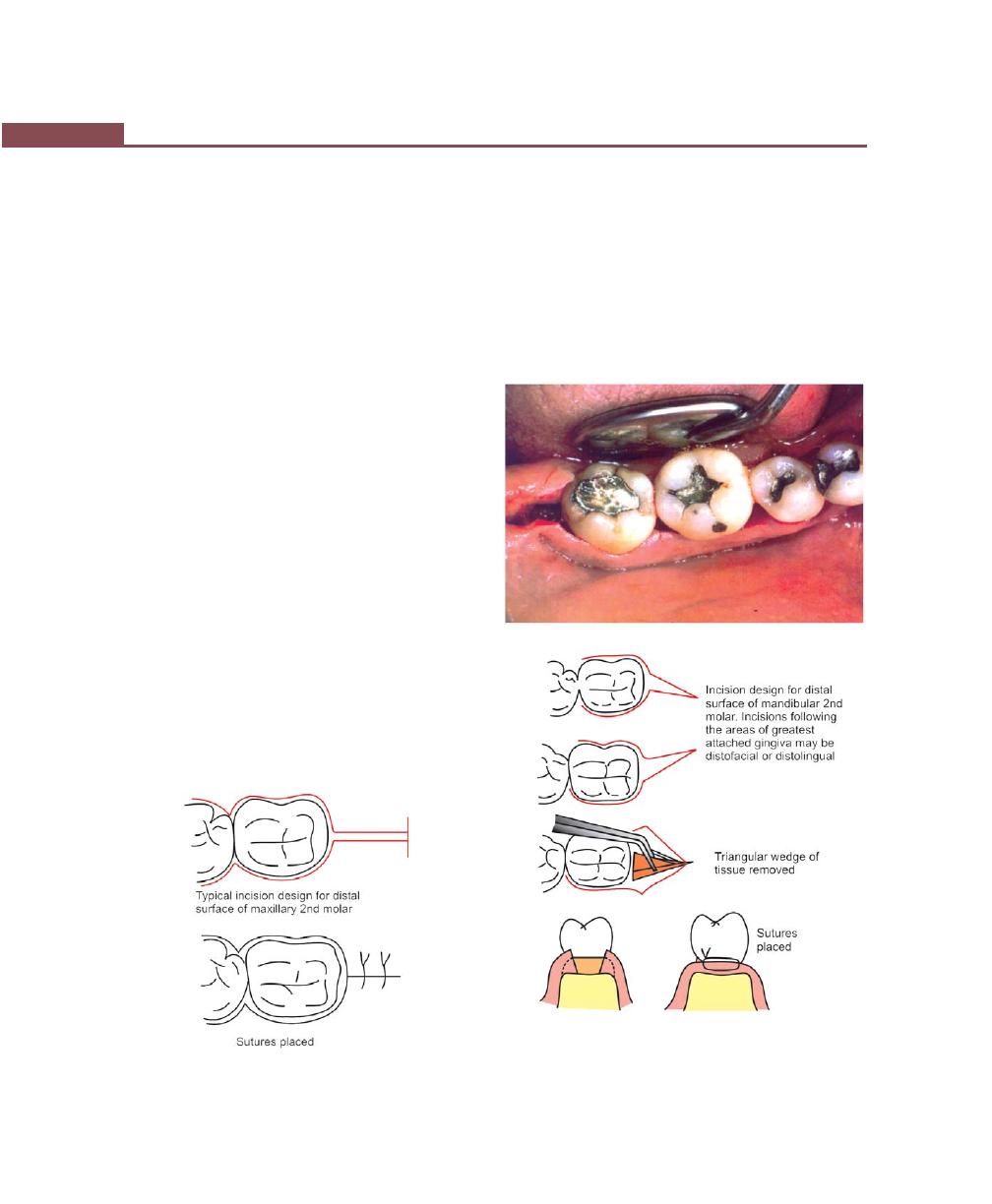
328
Essentials of Clinical Periodontology and Periodontics
Figs 41.20 A and B:
Distal molar surgery for mandibular
molars
(A)
(B)
Apically-displaced Flap
Used for both pocket eradication and/widening the zone
of attached gingiva.
Step 1 : Internal bevel incision is made, 1 mm from
the crest of the gingiva and directed towards
the crest of the bone.
Step 2 : Crevicular incisions are made followed by
initial elevation of flap and then interdental
incision is performed, the wedge of tissue
containing the pocket wall is removed.
Step 3:
Vertical releasing incisions are made extending
beyond the mucogingival junction and flap
is elevated with a periosteal elevator (either
split thickness or full thickness).
Step 4:
Remove all the granulation tissue, root planing
is done and flap is positioned apically at the
tooth bone junction.
Step 5 : Flaps are sutured together.
Distal Molar Surgery
• For maxillary molars
• For mandibular molars.
Technique for maxillary molars (Fig. 41.19): Two parallel
incisions at the distal surface of terminal tooth are made.
The deeper the pocket, the greater will be the distance
between the two parallel incisions. Followed by this a
transversal incision is made so that a long rectangular
piece of tissue is removed. This can be confirmed with
the regular flap in the quadrant being treated. These
incisions can be placed using No. 12 blade, after flap
reflection and curetting the bone surface, the flaps are
sutured together.
Technique for mandibular molars (Figs 41.20A and B):
Two parallel incisions at the retromolar pad area are
Fig. 41.19:
Distal molar surgery for maxillary molars
329
Periodontal Flap
Healing after Full Thickness Flap
Same as above but superficial bone necrosis takes place
in one to three days followed by osteoclastic resorption
at 4 to 6 days and declines thereafter. This results in bone
loss of about 1 mm and greater, if the bone is thin.
KEY POINTS TO NOTE
1. Periodontal flap is a section of gingiva and or mucosa
surgically-elevated from the underlying tissues to provide
visibility of and access to the bone and root surface.
2. Periodontal flaps are classified according to the thickness as,
full thickness and partial thickness flap; and according to
the placement of flap as displaced and undisplaced flap;
according to the design of the flap as conventional flap and
papilla preservation flap.
3. In conventional flap, horizontal incisions and vertical
incisions are given.
4. There are three types of horizontal incisions, internal bevel,
crevicular and interdental incision.
5. Currently there are three types of periodontal flaps available,
Modified Widman flap, Undisplaced flap and Apically-
displaced flap.
6. Main objectives of periodontal flap surgery are gain access
to root surface, reduction or elimination of pocket depth and
attempt regeneration of periodontal ligament, alveolar bone
and cementum.
REVIEW QUESTIONS
1. Define and classify the periodontal flaps. Describe
the step-by-step procedure of modified Widman flap.
2. What are the indications of flap surgery?
3. Describe healing following flap surgery.
BIBLIOGRAPHY
1. Jan Lindhe. Clinical Periodontology and Implant Dentistry,
4th edn, Blackwell Munksguard Publication, 2003.
2. Myron Nevins, James T, Mellning. Periodontal Therapy and
Clinical Approaches and Evidence of Success, Vol. 1,
Quintessence Publishing Co. Inc., 1998.
3. Newman, Takei, Fermin A. Carranza. Clinical Periodontology,
9th edn, WB Saunders Co., 2002.
made. The incisions should follow the areas of greatest
attached gingiva and underlying bone. After the reflection
of the flap and removal of tissue, osseous surgery
may be performed (if necessary) and flaps are sutured
so as to approximate the flap margins closely to each
other.
HEALING AFTER FLAP SURGERY
Immediately after suturing (0 to 24 hours), connection
between the flap and the tooth or bone surface is
established by the blood clot, which consists of a fibrin
reticulum with many polymorphonuclear leukocytes,
erythrocytes, debris from injured cells and capillaries at
the edge of the wound. There are also bacteria and an
exudate or transudate as a result of tissue injury.
One to three days after flap surgery—space between
the flap and the tooth or the bone is thinner and epithelial
cells migrate over the border of the flap, when the flap
is closely-adapted to the alveolar process there is only
a minimal inflammatory response.
One week after flap surgery—an epithelial attachment
to the root has been established by means of hemi-
desmosomes and a basal lamina. The blood clot is
replaced by granulation tissue derived from gingival
connective tissue, bone marrow and the periodontal
ligament.
Two weeks after surgery—collagen fibres begin to appear
parallel to the tooth surface. Union of the flap and the
tooth is still weak (due to immature collagen fibres) but
clinically it appears almost normal.
One month after surgery—a fully-epithelialized gingival
crevice with a well-defined epithelial attachment is
present. Supracrestal fibers begin to adapt a functional
arrangement.
330
Essentials of Clinical Periodontology and Periodontics
DEFINITION
Osseous surgery maybe defined as the procedure by
which changes in the alveolar bone can be accomplished
to rid it of deformities induced by the periodontal disease
or other related factors, such as exostosis and tooth supra-
eruption.
RATIONALE
It is based on the fact that the discrepancies in levels
and shapes of the bone and gingiva predisposes patients
to the recurrence of pocket depth post-surgically. Hence,
the goal of osseous resective therapy is reshaping the
marginal bone to resemble the alveolar process
undamaged by periodontal disease. The technique
usually involves apically-displaced flaps hence the
procedure not only eliminates periodontal pockets but
also improves tissue contour to provide a more easily
maintainable environment.
TERMINOLOGY
1. Osteoplasty—It refers to reshaping the bone without
removing the bone supporting the tooth.
2. Ostectomy—It refers to removal of bone supporting
the tooth.
According to the third edition of AAP’s (American
Academy of Periodontology) Glossary of Periodontal
Terminology.
3. Osseous surgery—It is a periodontal surgery involving
modification of the bony support of the teeth.
4. Osteoplasty—It is defined as reshaping of the alveolar
process to achieve a more physiologic form without
removal of supporting bones.
5. Ostectomy—It is defined as the excision of bone or
portion of a bone in periodontics, ostectomy is
done to correct or reduce deformities caused by
periodontitis and includes removal of the supporting
bone.
❒
❒
❒
❒
❒ DEFINITION
❒
❒
❒
❒
❒ RATIONALE
❒
❒
❒
❒
❒ TERMINOLOGY
❒
❒
❒
❒
❒ TYPES AND TECHNIQUES
❒
❒
❒
❒
❒ RESECTIVE OSSEOUS SURGERY
• Indications
• Contraindications
• Examination Prior to Surgery
• Methods of Osseous Surgery
• Healing after Surgery
❒
❒
❒
❒
❒ RECONSTRUCTIVE OSSEOUS SURGERY
42
Osseous Surgery
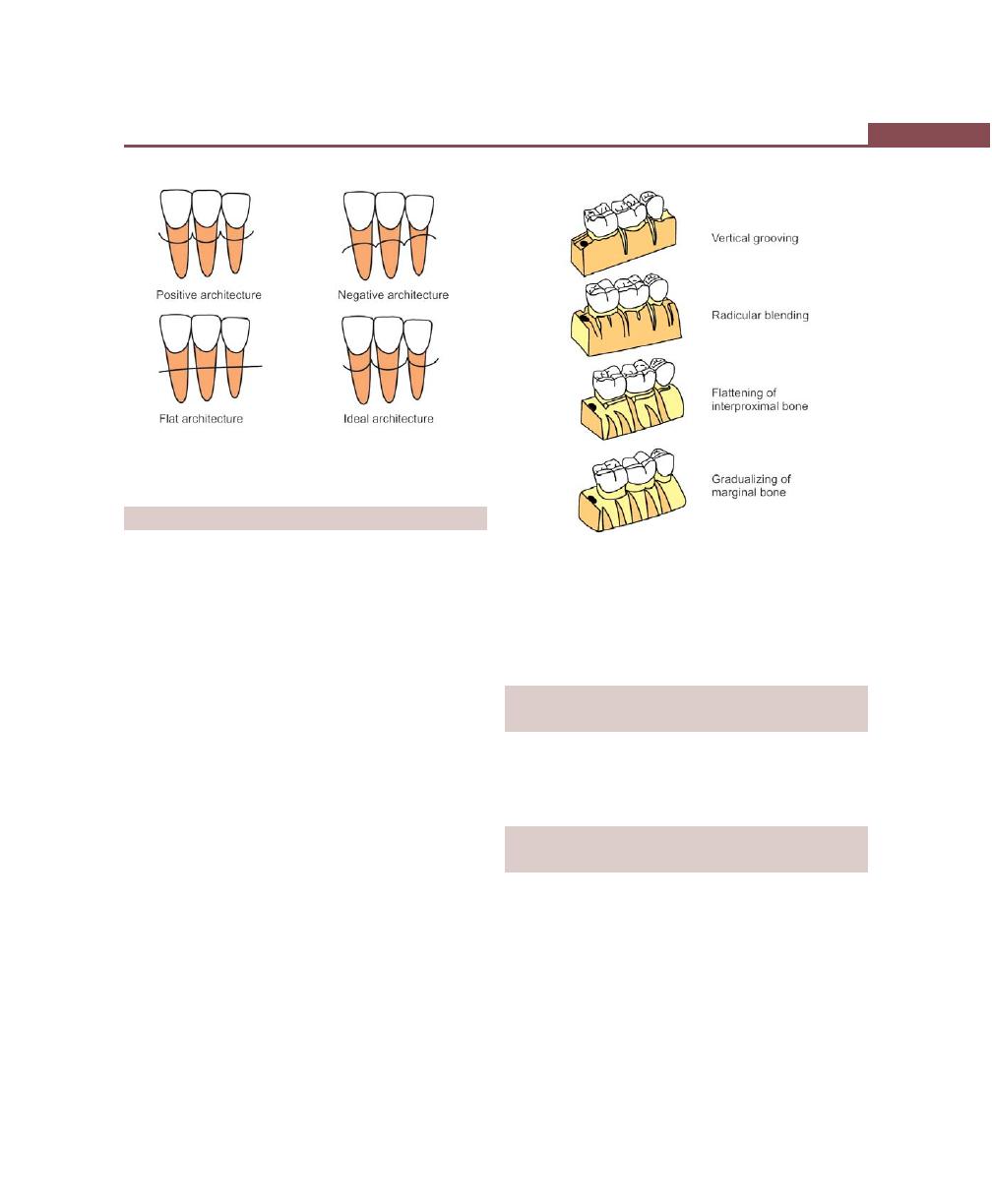
331
Osseous Surgery
TYPES OF OSSEOUS SURGERY
Depending on the relative position of the interdental
bone to radicular bone, osseous surgery is of following
types (Fig. 42.1):
1. Positive architecture—When the radicular bone is
apical to the interdental bone.
2. Negative architecture—If the interdental bone is more
apical than the radicular bone.
3. Flat architecture—It is the reduction of interdental
bone to the same height as radicular bone.
4. Ideal—When the bone is consistently more coronal
on the inter-proximal surface than on the facial and
lingual surfaces.
Depending on the thoroughness of the osseous
reshaping techniques, osseous surgery is of following
types:
1. Definitive osseous reshaping—Implies that further
reshaping would not improve the overall result.
2. Compromise osseous reshaping—It indicates a bone
pattern that cannot be improved without significant
osseous removal, that would be detrimental to the
overall result.
Osseous surgery (Fig. 42.2) can also be:
1. Additive—Directed towards restoring the bone to
original levels.
2. Subtractive—It is designed to restore the form of the
pre-existing alveolar bone to the level existing at the
time of surgery or slightly apical to this level.
INDICATIONS OF RESECTIVE
OSSEOUS SURGERY
1. One-walled angular defects.
2. Thick, bony margins.
3. Shallow crater formations.
CONTRAINDICATIONS OF
RESECTIVE OSSEOUS SURGERY
1. Anatomic factors such as close proximity of the roots
to the maxillary antrum or the ramus.
2. Age.
3. Systemic health.
4. Improper oral hygiene.
5. High caries index.
6. Extreme root sensitivity.
7. Advanced periodontitis.
8. Unacceptable esthetic result.
Fig. 42.1:
Relative position of interdental bone to radicular
bone
Fig. 42.2:
Steps in osseous resective surgery
332
Essentials of Clinical Periodontology and Periodontics
EXAMINATION PRIOR TO
RESECTIVE SURGERY
Clinical examination and probing determines the
presence and the depth of periodontal pockets and also
gives a general sense of the bony topography.
Transgingival probing or sounding under local anesthesia
confirms the extent and configuration of the infrabony
component or furcation defects.
METHODS OF OSSEOUS
RESECTIVE SURGERY
• Instruments used—combination of hand and rotary
instruments are used.
Hand instruments include:
1. Rongeurs—Friedman and Blumenthal.
2. Interproximal files—Schluger and Sugarman.
3. Back action chisels.
4. Oschsenbein chisels.
Rotary instruments include:
1. Carbide round burs.
2. Slow-speed hand piece.
3. Diamond burs.
TECHNIQUE
The following steps are suggested:
1. Vertical grooving.
2. Radicular blending.
3. Flattening of interproximal bone.
4. Gradualizing marginal bone.
Not all the steps are necessary in each case.
Vertical Grooving
It is indicated to reduce the thickness of alveolar housing
and it provides continuity from the interproximal surface
into the radicular surface. It is the first step of the resective
process and is usually performed with rotary instruments
such as, round, carbide or diamond burs.
It is indicated in thick bony margins, shallow crater
formation and is contraindicated in areas with close root
proximity or thin alveolar housing.
Radicular Blending
It is the second step of the osseous reshaping technique.
It is the continuation of the first step and it attempts to
gradualize the bone over the entire radicular surface and
thereby provides a smooth, blended surface for good
flap adaptation.
The indications are the same as in step one. Both
step one and step two are purely osteoplastic procedures.
Apart from thick bony margins and craters, grade-I and
grade II furcation involvements are treated with these
two steps.
Flattening of the Interproximal Bone
It requires removal of very small amount of supporting
bone. It is indicated when interproximal bone levels vary
horizontally, e.g. one wall defects or hemiseptal defects.
This step is also best-utilized in areas where there are
combined defects, i.e. coronally one-walled defect and
apically three-walled defect, so that it is helpful in
obtaining good flap closure and hence improved healing.
But the main limitation is that, it can not be utilized in
advanced defects where removal of inordinate amounts
of bone may be required.
Gradualizing Marginal Bone
The final step in the osseous resective technique, is also
an ostectomy procedure. Bone removal is minimal but
necessary to provide a sound regular base for the gingival
tissue to follow. Failure to do so may result in ‘widow’s
peaks’, allows the tissue to rise to a higher level than
the base of the bone loss in the interdental area. This
may result in selective recession and incomplete pocket
reduction.This ostectomy procedure should be
performed with great care so as to not damage the roots.
Following the osseous resection, the flaps are positioned
back to its original position or apically and sutured with
minimal tension.

333
Osseous Surgery
The osteoblastic activity has been observed even after
1 year postoperatively in humans, hence the initial loss
in bone height gets compensated to some extent by
repair and remodeling.
RECONSTRUCTIVE OSSEOUS SURGERY
Periodontal therapy for treatment of periodontitis involves
the elimination of bacterial plaque. When periodontitis
is resolved, an anatomic defect remains in the
periodontium. This anatomic defect is characterized by
reformation of gingival fibres, substantial reduction in
inflammation, persistent loss of bone and ligament and
the formation of long junctional epithelium.
Thus periodontal therapy involves two primary
components: Elimination of bacterial plaque and elimina-
tion of the anatomic defects produced by periodontitis.
There are two primary approaches to eliminating these
anatomic defects—resective and regenerative, both being
surgical.
Evaluation of New Attachment and
Bone Regeneration
Clinical Methods
Consist of:
1. Probing depth measurements (pre and post-
treatment).
2. Clinical gingival indices.
3. Determination of attachment level, care should be
taken to measure the defect by placing the probe
at the exact same point before and after treatment
and also with the same angulation. Pre and post
operative probing measurements without standar-
dized method may not be reliable.
Radiographic Methods
They also have the following drawbacks. Standardized
techniques for reproducible positioning of the film and
tubing is very difficult. Even with standardization, the
radiograph may not show the entire topography of the
area before or after the treatment. Furthermore, thin
bony trabeculae which was present before treatment may
go undetected because minimal amount of mineralized
tissue must be present to be seen or registered on the
radiograph. Only future studies with subtraction
radiography will enhance the usefulness of radiographic
evaluation.
Surgical Re-entry
This can give the best view of the state of the bone crest
that can be compared with the one taken during the
initial surgical intervention and can also be subjected to
measurements. Even models can be used to appreciate
the results of the therapy (pretreatment and post-
treatment). This method is very useful but has two
shortcomings:
a. It requires a frequently unnecessary second operation.
b. It does not show the type of attachment that exists
(epithelial or connective tissue attachments).
Histological Methods
The type of attachment can only be determined by the
histologic analysis of the tissue block taken from the
healed area. Although this can give us the clear picture
of regeneration of the attachment apparatus, it is not
without problems. They are:
a. The need to remove a tooth with its periodontium
treated successfully limits the volunteers.
HEALING AFTER RESECTIVE
OSSEOUS SURGERY
334
Essentials of Clinical Periodontology and Periodontics
b. Animal models (monkey, dog, pigs) can be used to
clarify some aspects and but one must always
remember the differences that exists when extrapola-
tions to humans are attempted. Mainly the exact
nature of the periodontal disease cannot be
reproduced in animals and also bone defects have
to be experimentally-induced which may lack their
chronicity and self sustaining features. Even if these
defects are allowed to become chronically-infected,
they are never identical because all these types of
lesions have different healing sequences and thereby
provides different types of information.
In addition, the exact location of epithelial attachment
will be lost once you reflect the flap because it will be
reflected beyond the normal periodontium. Hence a
notch should be placed on the root surface either apical
to the calculus (slightly coronal to the attachment) or
crest of the alveolar bone (slightly apical to the
attachment). Base of the calculus is a better landmark
(for which presence of calculus is required).
The following reconstructive surgical techniques have
been proposed.
1. Nongraft-associated new attachment.
2. Graft-associated new attachment .
3. Combination of both.
Nongraft-associated New Attachment
New attachment can be achieved without the use of grafts
in:
a. Meticulously treated three-walled defects, (Infrabony
defect).
b. Perioendodontal abscesses.
c. When the destructive procedure has occurred very
rapidly, for example, after treatment of pockets which
had acute periodontal abscess.
Various techniques of nongraft-associated new
attachment are:
Removal of Junctional and Pocket Epithelium
The methods used to do so include:
i. Curettage—Only 50 percent of junctional epithelium
and pocket epithelium can be removed.
ii. Chemical agents—Mostly used in conjunction with
curettage. The most commonly used drugs are
sodium sulfide, phenol, camphor, sodium hypo-
chlorite and antiformin. The main disadvantage is
that the depth of action can not be controlled.
iii. Ultrasonic methods—It is again not very useful,
because of lack of clinicians tactile sense while
using these methods.
iv. Surgical methods—
• Excisional new attachment procedure with
internal bevel incision (ENAP).
• Gingivectomy procedure.
• Modified Widman flap.
• Coronal displacement of the flap.
All these procedures are discussed elsewhere in this
book.
Prevention of Epithelial Migration
Eliminating junctional and pocket epithelium may not
be sufficient because the epithelium from the excised
margin may rapidly proliferate apically to become
interposed between the healing connective tissue and
cementum.
GTR (Guided Tissue Regeneration)
This concept is based on the assumption that periodontal
ligament cells have the potential for regeneration of the
attachment apparatus of the tooth (Figs 42.3 and 42.4).
Fig. 42.3:
Guided tissue regeneration—adaptation of
barrier membrane on the defect
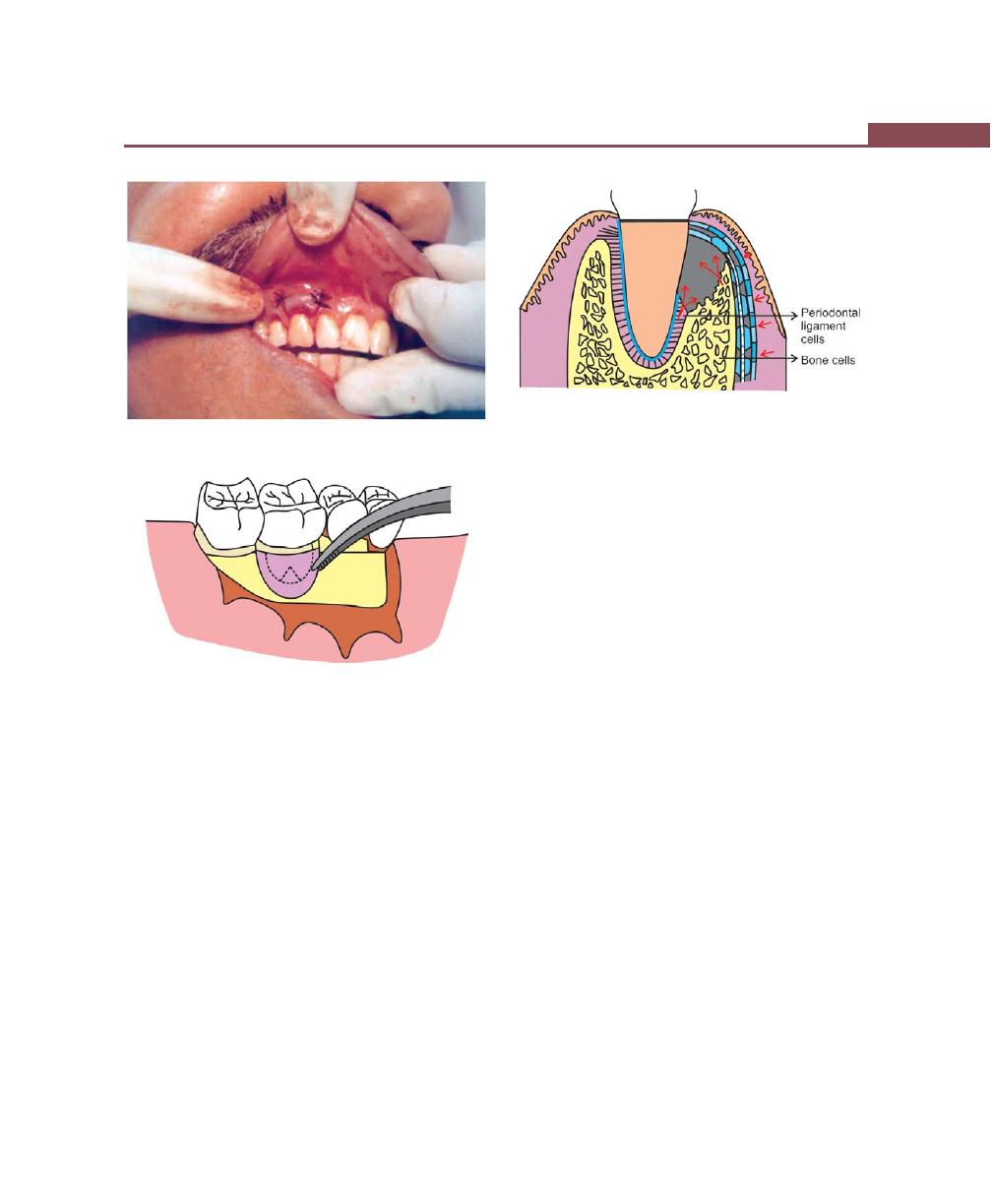
335
Osseous Surgery
Fig. 42.4:
Flap is positioned coronally and sutured
Fig. 42.5:
Membrane adaptation
Two types of membranes have been used (Figs 42.5
and 42.6).
a. Degradable—Collagen, Polylactic acid, Vycril
(polyglactin 910) and Guidor membrane.
b. Nondegradable—They must be removed in three to
six weeks time, e.g. Millipore, Teflon membrane, Gore-
tex periodontal material.
Surgical procedure
Step 1: Raise a full thickness flap utilizing vertical
incisions, extending a minimum of two teeth
anteriorly and one tooth distally, to the tooth
being treated.
Step 2: Debride the osseous defect and plane the root
surfaces.
Step 3: Trim the membrane according to the size of the
area being treated. The membrane should
extend approximately beyond 2 to 3 mm on
all the sides.
Step 4: Suture the membrane around the tooth with
a sling suture.
Step 5: The flap is positioned back to its original position
or slightly coronal to it and is sutured using
interrupted sutures. Make sure the membrane
is covered completely. In case of non-resorbable
membrane, after 5 weeks of the operation, it
must be removed with a gentle tug.
Clot Stabilization, Wound Protection and Space
Creation
The successful results obtained with graft materials, barrier
membranes and coronally-displaced flaps have been
attributed to the fact that all of these protect the wound
and create a space for undisturbed and stable maturation
of the clot. Hence this hypothesis suggests that preser-
vation of the root surface that is, a fibrin clot interface
prevents apical migration of the gingival epithelium and
allows for connective tissue attachment during the early
wound healing period. A lot of research is required to
explore this possibility for example, it requires more
postoperative care, root conditioning to enhance fibrin
clot and connective tissue attachment.
Fig. 42.6:
Membrane barrier in place
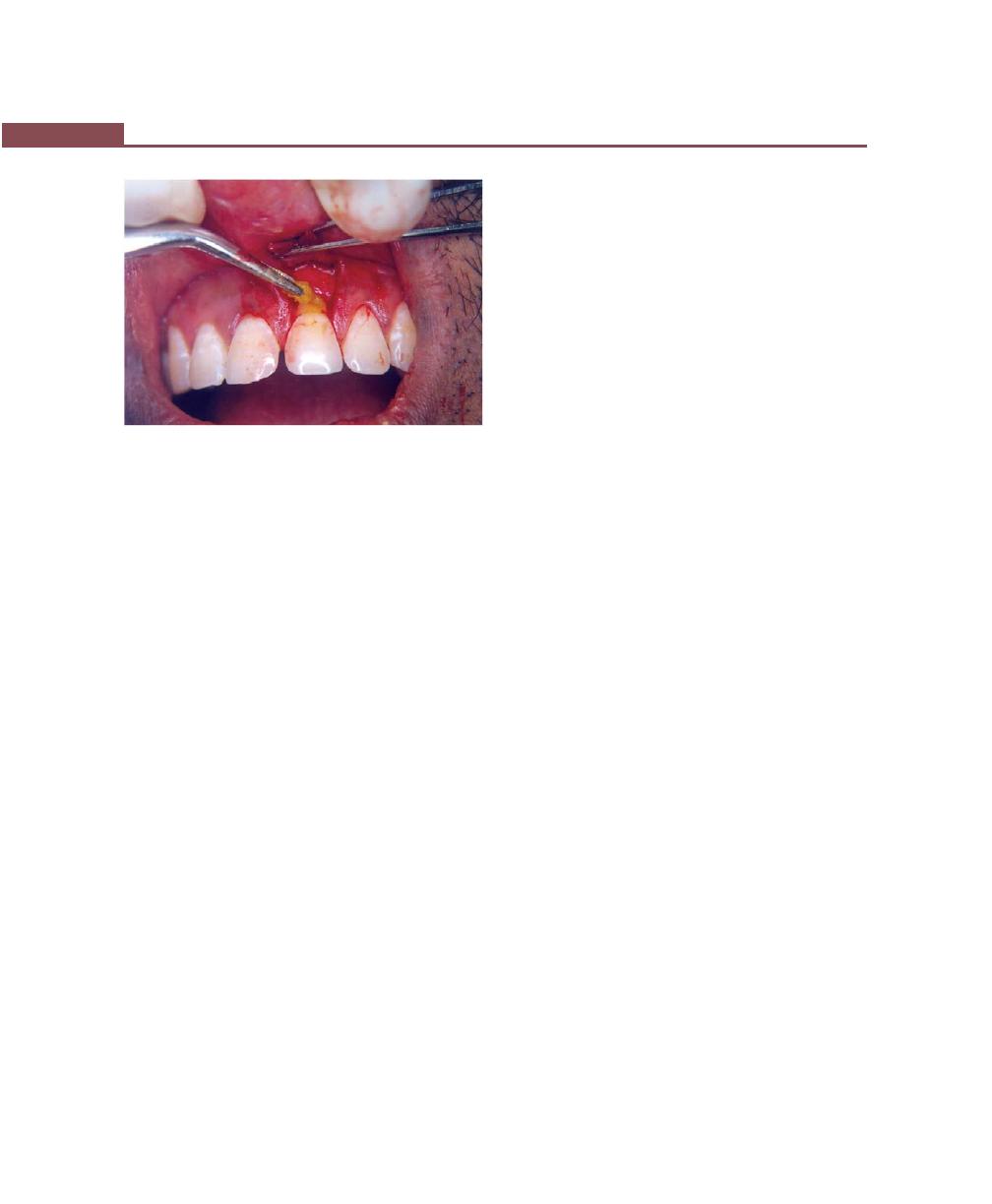
336
Essentials of Clinical Periodontology and Periodontics
Fig. 42.7:
Root biomodification using
tetracycline solution
Preparation of the Root Surface
(Root Biomodification) (Fig. 42.7)
Several substances have been used to condition the
root surface, for attachment of new connective, tissue
fibers. These include citric acid, fibronectin and
tetracycline.
Citric acid: When used with pH1 for two to three minutes
on root surface, after surgical debridement, it produces
a surface demineralization, which inturn induces
cementogenesis and attachment of collagen fibres. The
following actions of citric acid have been reported by
Register and Burdick in 1975.
1. It removes the smear layer and may open dentinal
tubules, thus allowing cementum to form within these
tubules creating the blunderbuss effect and produce
cementum pins. This could be associated with
accelerated cementogenesis.
2. It has also been shown to expose collagen fibres on
the root surface, which may splice with the collagen
fibres of a soft tissue graft or flap (called as collagen
splicing) resulting in collagen adhesion without
cementum formation and accelerated healing.
3. Epithelium does not migrate apically because of the
accelerated healing either by connective tissue
attachment or a collagen adhesion may occur before
epithelium migrates.
4. Finally, citric acid, may demineralize small bits of
residual calculus, disinfect the root surface and aid
in removing endotoxins.
Technique:
a. Raise full thickness flap.
b. Perform thorough root planing.
c. Apply cotton pellets soaked in citric acid pH1 for two
to three minutes.
d. Remove and irrigate root surface profusely with
water.
e. Replace the flap and suture.
Growth factors: They are polypeptide molecules released
by the cells in the inflamed area, that regulates events
in wound healing. These factors are primarily secreted
by macrophages, endothelial cells, fibroblasts and
platelets. They include platelet derived growth factor
(PDGF), insulin-like growth factor (IGF), fibroblast
growth factor (FGF) and TGF (transforming growth factor
alpha and beta ). These can all be used to control events
during periodontal wound healing, e.g. promoting
proliferation of fibroblasts from periodontal ligament
thereby favoring bone formation.
Enamel matrix proteins: This is based on the observations
that amelogenin secreted by Hertwig’s epithelial root
sheath during tooth development can induce acellular
cementum formation which is believed to favor perio-
dontal regeneration, e.g. Emdogain approved by FDA.
Graft-associated New Attachment
Terminology
1. Graft—It is a viable tissue/organ that after removal
from donor site is implanted/transplanted within the
host tissue, which is then repaired, restored and
remodelled.
2. Xenograft or heterograft—The donor of the graft is
from a species different from the host.
3. Allograft or homograft—A tissue transfer between
individuals of the same species but with non identical
genes.

337
Osseous Surgery
4. Autograft—A tissue transfer from one position to a
new position in the same individual
5. Alloplastic graft—A graft of inert synthetic material
which is sometimes called implant material.
6. Osteoinduction—A process by which the graft
material is capable of promoting cementogenesis,
osteogenesis and new periodontal ligament.
7. Osteoconduction—The graft material acts as a passive
matrix, like a trellis or scaffolding for new bone to cover.
8. Contact inhibition—The process by which the graft
material prevents apical proliferation of the epithelium.
9. Guided tissue regeneration—An epithelial exclu-
sionary technique that promotes new connective
tissue attachment without the use of any implant
material.
Ideal Requirements of a Bone Graft Material
An ideal bone graft material should have biologic
acceptability, predictability, clinical feasibility, minimal
postoperative hazards, minimal postoperative sequelae
and good patient acceptance.
To date there is a no single material that fulfills all
the above criteria. Once the material is placed in the
defect it may act in a number of ways. It may have no
effect, act only as a scaffolding material for the host to
lay down new bone, it may actively induce bone
formation or through its own validity it may deposit new
bone in the defect.
All grafting techniques require presurgical scaling,
occlusal adjustment as needed and exposure of defect
with full thickness flap, best-suited is papilla preservation
flap because it provides complete coverage of the
interdental area after suturing. The use of antibiotics after
the procedure is generally recommended.
Intraoral autograft: It is the tissue transfer from one area
to another in the same individual, sources of intraoral
autografts include healing extraction wound, bone from
edentulous ridges, immature bone removed during
osteoplasty and ostectomy, bone removed from a
predetermined site like tori and symphysis (Fig. 42.9).
Osseous coagulum: Rationale of this technique is that
small particles of donor bone are better resorbed and
replaced than the larger particles. This technique uses
small particles of donor bone and hence it provides
additional surface area for the interaction of cellular
and vascular elements. Sources of the implant material
include the lingual ridge on the mandible, exostosis,
tori, edentulous ridges, the bone distal to the terminal
tooth.
In this technique a bur is used in the donor site to
reduce it to small particles which when coated with blood
becomes coagulum and is placed in the defect until there
is considerable excess and the flap is replaced.
Disadvantages:
1. Low predictability.
2. Inability to procure adequate material.
3. Inability to use aspiration for large defects which leads
to poor surgical visibility.
Bone blend: To overcome the above mentioned
problems, bone blend technique has been proposed.
It uses an autoclaved plastic capsule and pestle. Bone
is removed from the predetermined site with chisels or
rongeur forceps, placed in the capsule with a few drops
of saline, and triturated for sixty seconds to a workable
plastic-like mass and is packed into the bony defect.
Intraoral cancellous bone marrow chips: It can be
obtained from:
a. Maxillary tuberosity—It contains good amount of
cancellous bone with foci of red marrow and the bone
is removed with a cutting rongeur.
b. Edentulous areas—The bone is removed with curette.
c. Healing sockets—They are allowed to heal for eight
to twelve weeks and the apical portion is utilized as
donor material.
Bone swaging: This technique requires presence of an
endentulous area adjacent to the defect from which the
bone is pushed into contact with root surface without
fracturing the bone at its base.
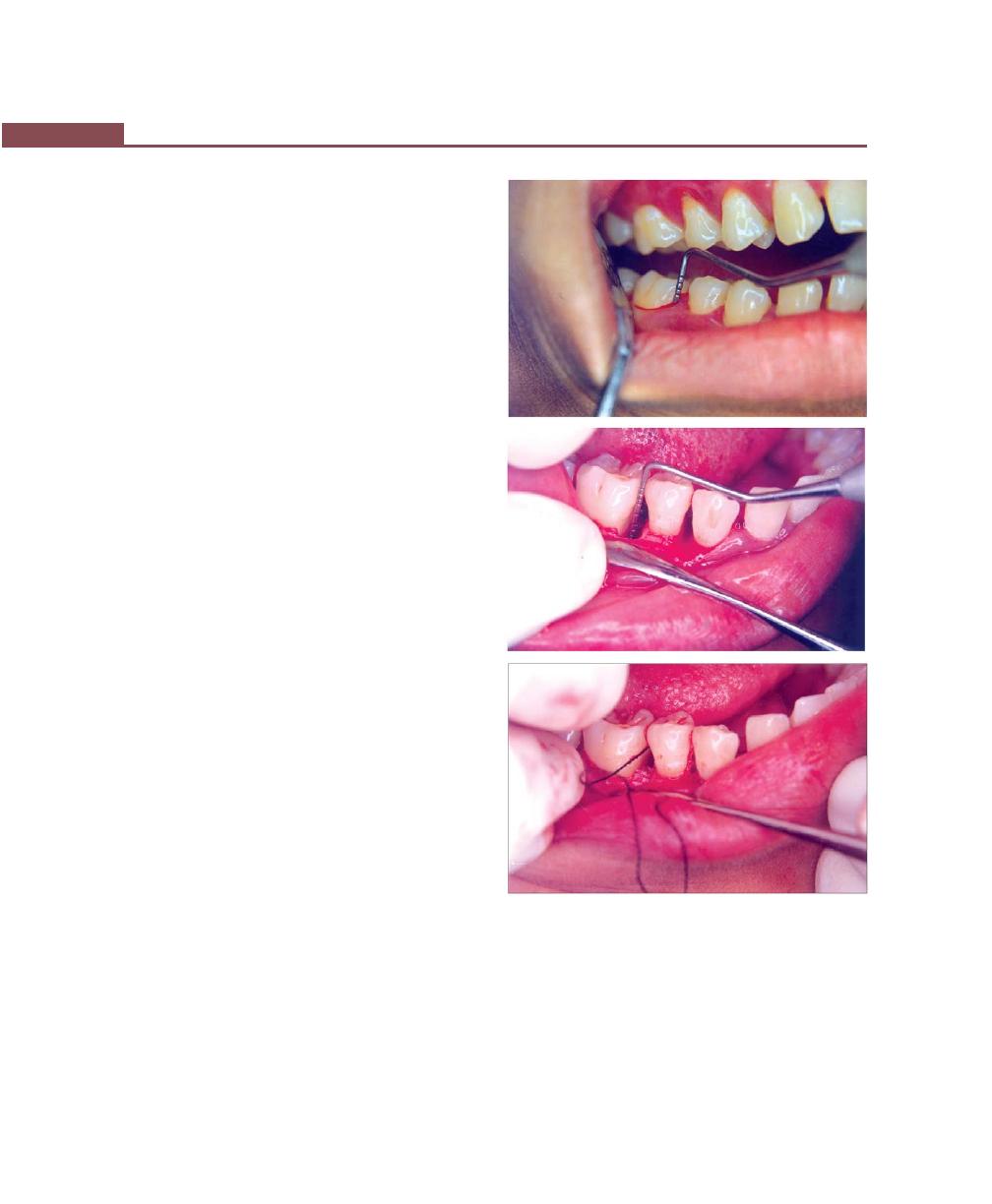
338
Essentials of Clinical Periodontology and Periodontics
Disadvantages:
i. It is technically difficult.
ii. Its usefulness is limited.
Bone from extraoral sites:
Iliac Autografts/Extraoral Hip Marrow: The use of iliac
cancellous bone marrow has shown good results in bony
defects with varying number of walls and furcation
defects. However, there are many disadvantages
associated with it.
i. Additional surgical trauma.
ii. Postoperative morbidity infection, exfoliation,
sequestration.
iii. Root resorption.
iv. Rapid recurrence of the defect.
Hence this technique is no longer used.
Allografts: Allograft or homograft is the tissue transfer
between individuals of the same species, but of non-
identical genetic composition. Since autografts induce
surgical trauma in another part of the patient’s body,
it would be advantageous if a suitable substitute can be
obtained commercially. Bone grafts are commercially
available in tissue banks.
They can be:
1. FDBA (Freeze dried bone allograft)—It is an
osteoconductive material, varying results have been
observed using this material. Average bone fill of
50 percent has been reported.
2. DFDBA (Demineralized freeze dried bone allograft)—
Demineralization process exposes the components
of bone matrix termed as bone morphogenic protein,
e.g. osteogenin, which is a bone inductive protein
isolated from the extracellular matrix of human bones.
Hence this is an osteo inductive material.
Xenografts: They have been shown to cause severe
immunologic reactions because of molecular
divergencies, therefore it is not used any longer. Examples
of xenograft are calf bone, kiel bone,anorganic bone.
Alloplasts/non-bone graft material: Non-bone graft
materials have also been used for restoration of the
A
B
C
Figs 42.8A to C:
Reconstructive periodontics. (A) Preoperative
view. (B) Defect is exposed after flap reflection and
debridement. (C) The defect is filled with hydroxyapatite
crystals
periodontium. Some of them are sclera, dura, cartilage,
plaster of Paris, ceramics, and coral derived materials.
All alloplastic materials have shown more or less similar
results because all of them are osteoconductive in nature.
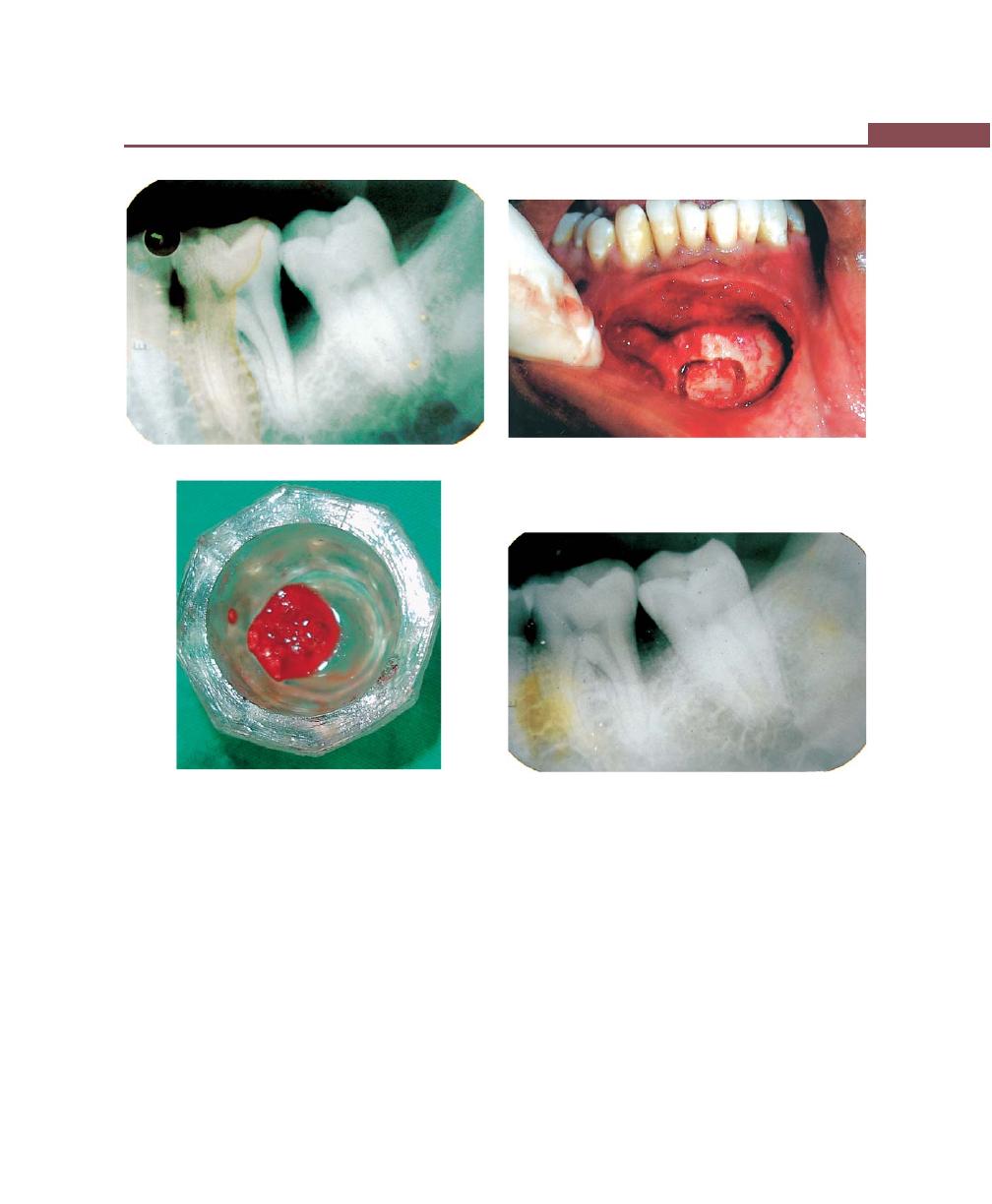
339
Osseous Surgery
Figs 42.9A to D:
Autogenous bone grafting. (A) Preoperative radiograph. (B) Harvesting of bone graft from symphysis
region. (C) Harvested autogenous cancellous bone graft. (D) Postoperative radiographic view (after 3 months)
A
B
C
D
Lot of attention has been given to calcium phosphate
ceramics which are of two types:
a. Hydroxyapatite—nonresorbable (Figs 42.8A to C).
b. Tricalcium phosphate—partially-bioresorbable.
Bioactive glass—Consists of sodium and calcium salts,
phosphates and silicon dioxide with particle size
ranging from 90 to 170 um (perioglas ®) or 300 to
355 um (biogran ®).
Coral-derived materials—Two types of materials are
available, natural coral and coral-derived porous
hydroxyapatite (both are proven to be biocompatible).
Combined techniques—A combination of both graft and
non-graft associated methods have been proposed, e.g.
combination of barrier techniques with bone grafts have
been suggested by many authors.
340
Essentials of Clinical Periodontology and Periodontics
BIBLIOGRAPHY
1. Alan M Polson. Periodontal Regeneration, Current Status and
Directions. Quintessence Publishing Company, Inc.
2. Edwin Rosenberg, Louis F Rose. Biologic and clinical
considerations for autografts and allografts in periodontal
regeneration therapy. Dental Clin North Am 1998;42(3): 467.
3. Gary Greenstein, Jack G Caton. Biodegradable barriers and
guided tissue regeneration. Periodontol 2000, 1993;1:36.
4. Hessam Newzari. Aesthetic osseous surgery in the treatment
of periodontitis: Periodontol 2000, 2001;27.
5. Mary E, Aichelmann Reidy, Raymond A Yukna. Bone
replacement grafts: The bone substitutes. Dent Clin North Am
1998; 42(3):491.
6. Michael A Brunsvold, James T Mellonig. Bone grafts and
periodontal regeneration. Periodontol 2000, 1993; 1:80.
7. Newman, Takei, Fermin A Carranza. Clinical Periodontology,
9th edn, WB Saunders Co., 2002.
8. Raul G Caffess, Carlos R Quinomes. Polypeptide growth
factors and attachment proteins in periodontal wound healing
and regeneration. Periodontol 2000, 1993;1:69.
9. Roxanne A. Lowenguth, Timothy M Blieden. Periodontal
regeneration: root surface demineralization. Periodontol
2000, 1993;1:S4.
10. Raymond A Yukna. Synthetic bone grafts in periodontics.
Periodontol 2000, 1993;1:92.
11. Thorkild Karving, Sture Nyman, Jan Gottlow, Lars Laurell.
Development of the biological concept of guided tissue
regeneration—Animal and human studies. Periodontol
2000, 1993;1:26.
KEY POINTS TO NOTE
1. Osseous surgery can be of resective type and regenerative
types.
2. Under resective surgery the following techniques exist:
a. Vertical grooving.
b. Radicular blending.
c. Flattening of interproximal bone and,
d. Gradualizing marginal bone.
3. Reconstructive surgical techniques are subdivided into—
Nongraft-associated and Graft-associated new attachment
techniques.
4. Nongraft-associated new attachment procedures are:
a. Removal of junctional and pocket epithelium.
b. Prevention of epithelial migration.
c. Clot stabilization, wound protection and space creation.
d. Preparation of the root surface.
5. Graft-associated techniques include grafts like autogenous
bone grafts, allografts, xenografts and alloplasts which have
been suggested and used successfully.
REVIEW QUESTIONS
1. Define osseous surgery, describe the steps in resective
osseous surgery.
2. Define regeneration, repair, new attachment and
reattachment.
3. What is GTR?
4. What is root biomodification?
5. What is osteoconduction and osteoinduction?
6. Classify graft-associated new attachment procedures.
341
Mucogingival Surgery
DEFINITION
Mucogingival surgery was first introduced in the 1950s.
According to the glossary of periodontal terms muco-
gingival surgery refers to “Periodontal surgical procedures
designed to correct defects in the morphology, position
and/or amount of gingiva surrounding the teeth. Recently
it has been suggested that “periodontal plastic surgery”
may be more appropriate and would be defined as
“surgical procedures performed to correct or eliminate
anatomic, developmental or traumatic deformities of the
gingiva or alveolar mucosa”.
Mucogingival surgery consists of “plastic surgical
procedures for the correction of gingiva, mucous
membrane relationships that complicate periodontal
diseases and may interfere with the success of periodontal
treatment” (According to Glickman).
MUCOGINGIVAL PROBLEMS
These are:
i. Pockets extending upto or beyond mucogingival
junction.
ii. Recession causing denudation of root surfaces.
iii. High frenum and muscle attachments.
iv. Inadequate width of attached gingiva.
OBJECTIVES, INDICATIONS AND
CONTRAINDICATIONS OF
MUCOGINGIVAL SURGERY
Objectives
1. Widening the zone of attached gingiva.
2. Coverage of denuded roots.
3. Removal of aberrant frenum.
4. Creation of some vestibular depth when it is lacking.
5. As an adjunct to routine pocket elimination pro-
cedures.
Indications
1. Augmentation of the edentulous ridge.
❒
❒
❒
❒
❒ DEFINITION
❒
❒
❒
❒
❒ MUCOGINGIVAL PROBLEMS
❒
❒
❒
❒
❒ OBJECTIVES, INDICATIONS AND
CONTRAINDICATIONS
❒
❒
❒
❒
❒ TECHNIQUES TO INCREASE WIDTH OF
ATTACHED GINGIVA
• Gingival Extension Operation
• Apical Displacement
• Other Techniques
❒
❒
❒
❒
❒ TECHNIQUES FOR ROOT COVERAGE
• Indications
• Modifications
❒
❒
❒
❒
❒ OPERATIONS FOR REMOVAL OF FRENA
43
Mucogingival Surgery
342
Essentials of Clinical Periodontology and Periodontics
2. Prevention of ridge collapse associated with tooth
extraction.
3. Crown-lengthening.
4. Loss of interdental papilla which presents as esthetic/
or phonetic defect.
Contraindications
They are the same as in any other periodontal surgery.
The treatment procedures that may fall within the
definition of periodontal plastic surgery are:
• Gingival augmentation procedures for correction of
mucosal defects (around implants)
• Root coverage procedures
• Gingival preservation at ectopic tooth eruption
• Removal of aberrant frenulum.
• Ridge augmentation
• Crown-lengthening procedures.
The most commonly performed mucogingival surgical
procedures are discussed in this chapter.
TECHNIQUES TO INCREASE THE WIDTH
OF ATTACHED GINGIVA
Gingival Extension Operation
The width of attached gingiva varies in different
individuals and on different teeth in the same individual.
Attached gingiva is different from the keratinized gingiva.
The width of the attached gingiva is determined by
subtracting the depth of the sulcus or pocket from the
total distance between the crest of the margin to the
mucogingival junction. Originally it was thought that
minimal width of attached gingiva is required for optimal
gingival health to be maintained. However, several studies
have challenged this and concluded that even in the
presence of minimal amounts of attached gingiva the
tissues can be maintained in a normal state.
Various gingival extension procedures are:
Free Soft Tissue Autograft
Advantages
1. High degree of predictability—only with increasing
width of keratinized gingiva.
2. Simplicity.
3. Ability to treat multiple teeth at the same time.
4. This procedure can be performed where there is
inadequate keratinized gingiva adjacent to the
involved area.
Disadvantages:
1. Two operative sites.
2. Compromised blood supply.
3. Greater discomfort.
4. Lack of predictability in attempting root coverage.
Free soft tissue autografts are indicated in the presence
of:
1. An inadequate zone of attached gingiva.
2. Abnormal muscle attachment.
3. Shallow vestibular depth.
4. Gingival recession.
5. Deep pockets to prevent rapid initial down growth
of epithelium.
Procedure
1. Classic technique.
2. Variant techniques.
The Classic Technique (Figs 43.1 and 43.2)
Step 1: Eliminate the pockets—If pockets are present
resect them with a gingivectomy incision and scale and
plane the root surfaces. If there are no pockets present
in the area, the gingival margin is left intact.
Step 2: Preparation of the recipient site—It can be done
by two techniques.
1. First technique—By incising at the existing muco-
gingival junction with a #15 BP blade to a little more
than the desired depth and then blending the incision
on both ends with the existing mucogingival line.
Periosteum should be left covering the bone.
2. Another technique—Consists of outlining the recipient
site with two vertical incisions from the cut gingival
margin into the alveolar mucosa. Extend the incisions
to approximately twice the desired width of the
attached gingiva, allowing for 50 percent contraction
of the graft when healing is complete. A # 15 blade
is inserted along the cut gingival margin and is used
to separate a flap consisting of epithelium and
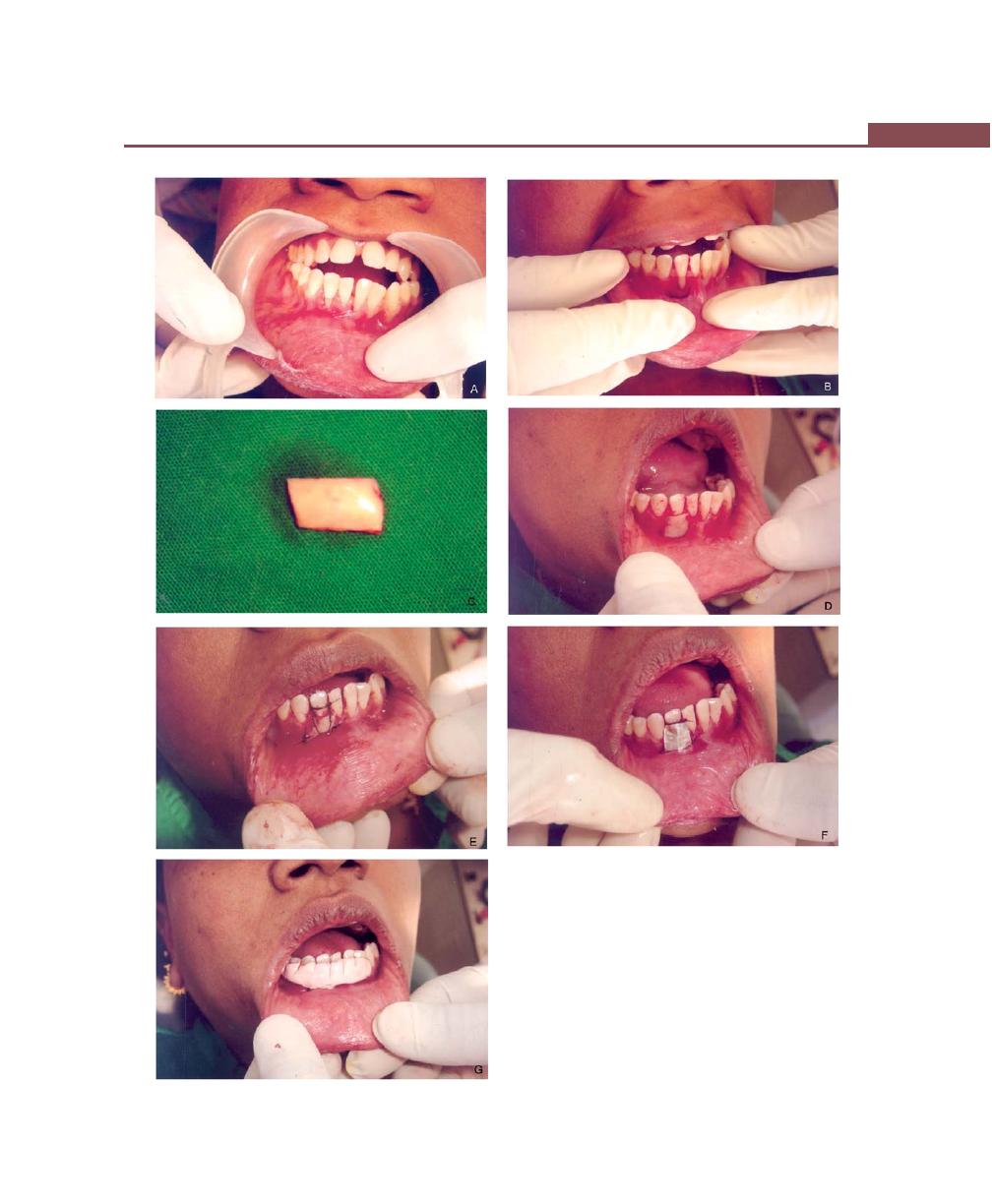
343
Mucogingival Surgery
Figs 43.1A to G:
Free soft tissue autograft. (A) Lack of
attached gingiva in relation to lower right lateral incisor,
(B) Classic technique—Surgical bed preparation, (C) Free
gingival graft procured from the palate, (D) Donor tissue
placed on the surgical bed, (E) The graft is sutured with silk
sutures, (F) Tinfoil covering the graft site, (G) Periodontal
dressing is placed
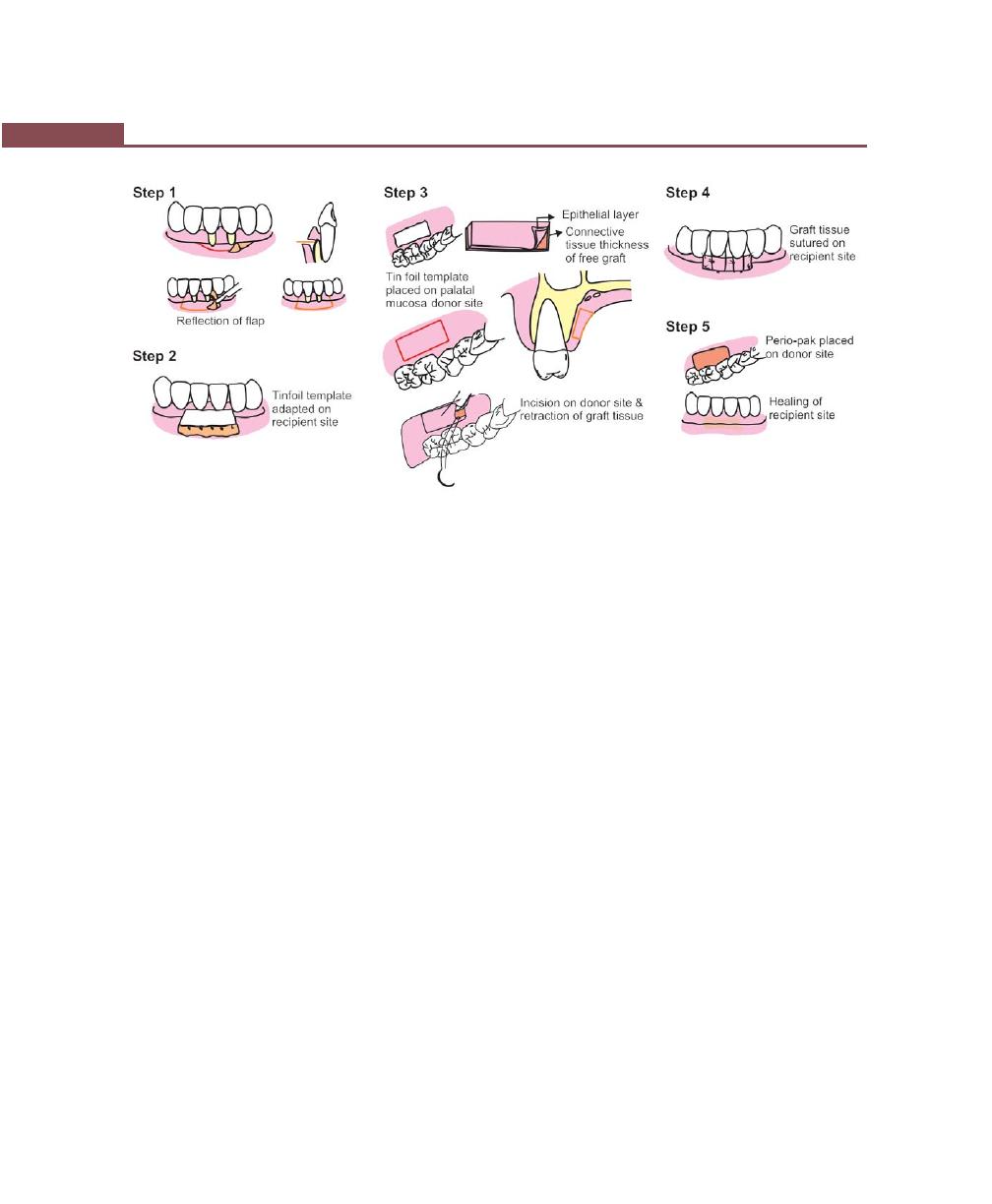
344
Essentials of Clinical Periodontology and Periodontics
Fig. 43.1H:
Various steps in classic technique
connective tissue without disturbing the periosteum.
Suture the flap where the apical portion of the free
gingival graft will be located.
Grafts can also be placed directly on the bone tissue.
The advantages of this variant includes, less post-operative
mobility of the graft, less swelling, better hemostasis—1½
to 2 times less shrinkage, however a healing lag is observed
for the first two weeks. Next make a tinfoil template of the
recipient site, to be used as a pattern for the graft.
Step 3: Obtaining the graft from the donor site.
For classic technique—A partial thickness graft is used,
the sites from which it can be obtained are in order of
preference:
• Attached gingiva
• Masticatory mucosa from an edentulous ridge
• Palatal mucosa.
The graft should consist of epithelium and a thin layer
of underlying connective tissue. Place the template over
the donor site and make a shallow incision around it with
a # 15 blade. Insert the blade to the desired thickness at
one edge of the graft and elevate it by holding with tissue
forceps or placing sutures at the margins of the graft.
Proper thickness is important for the survival of the
graft. It should be thin enough to allow the ready diffusion
of nutritive fluid from the recipient site. If it is too thin
the graft may shrivel and expose the recipient site. If
the graft is too thick, its peripheral layer is jeopardized
because of the excessive tissue that separates it from new
circulation and nutrients. Thick grafts may also leave a
deeper wound at the recipient site and may cause damage
to the palatal arteries. The ideal thickness of a graft is
between 1 to 1.5 mm. After the graft is separated, remove
loose tissue tags from the under surface.
Step 4: Transfer and immobilization of the graft—
Remove the excess clot from the recipient site because
thick clot interferes with vascularization of the graft.
Position the graft and adapt it firmly to the recipient site.
Dead space will retard the vascularization and jeopardize
the graft. Suture the graft at the lateral borders and to
the periosteum to secure it in position. Be sure that the
graft is immobilized because movement interferes with
healing.
Step 5: Protection of the donor site—Cover the donor
site with a periodontal pack for one week and repeat
if necessary. A modified Hawley retainer is useful to cover
the pack on the palate and over the edentulous ridges.
Variant techniques
Four variants to the classic technique are described:
a. Accordian technique.
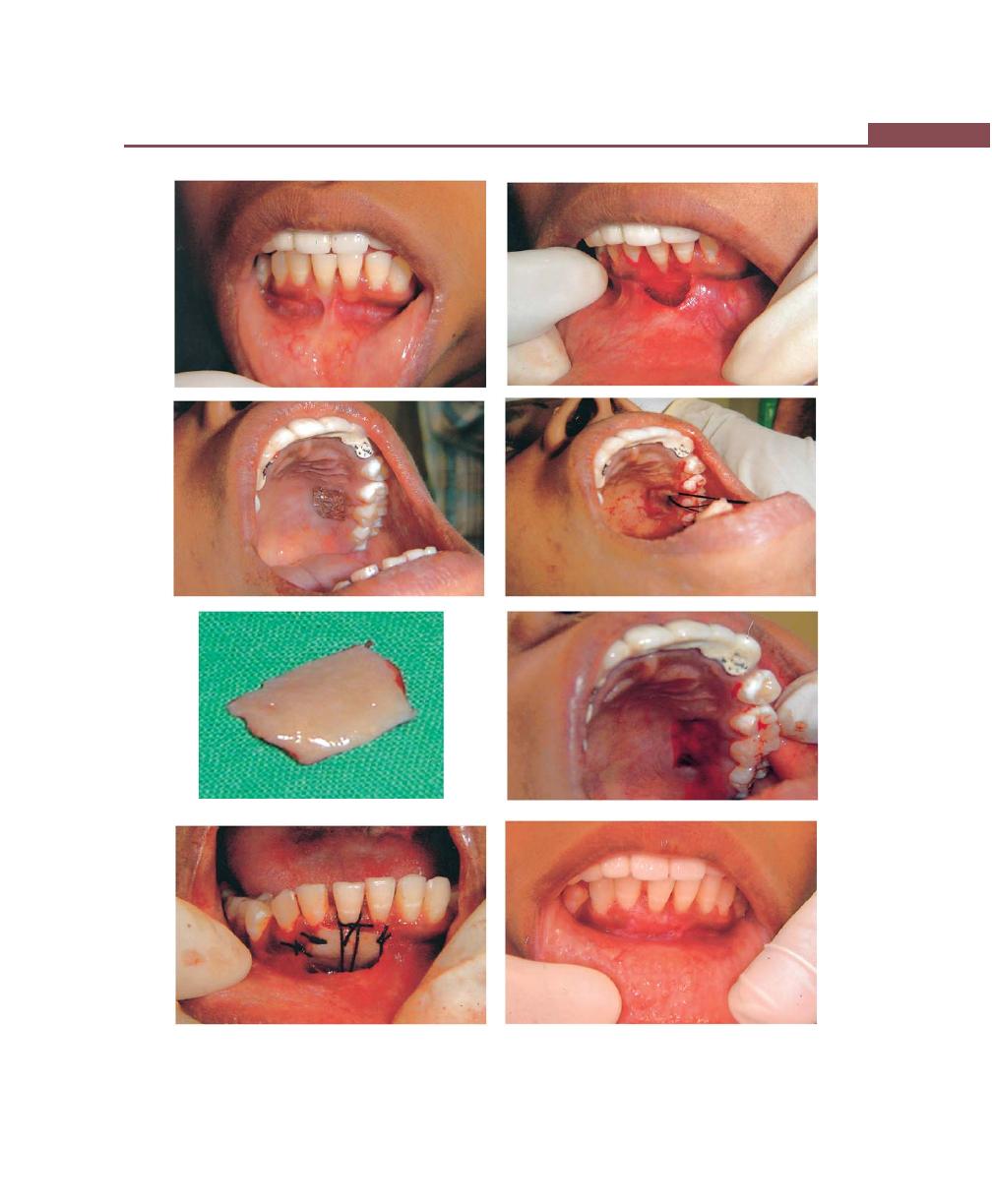
345
Mucogingival Surgery
Figs 43.2A to H:
Case-2: Free soft tissue autograft. (A) Gingival recession with lack of attached gingiva. (B) Surgical bed
preparation. (C) Tin foil template placed on palatal donor site. (D) Harvesting of graft tissue. (E) Harvested donor tissue.
(F) Donor site after harvesting. (G) Graft tissue sutured with graft streching and vertical sutures. (H) Facial view after healing
A
B
C
D
E
F
G
H

346
Essentials of Clinical Periodontology and Periodontics
b. Strip technique.
c. The connective tissue technique.
d. Combination of strip and connective tissue
techniques.
Accordian technique (Figs 43.3A to F)
Accordian technique has been described by Rateitchak
and colleagues. Expansion can be achieved by giving
alternate incisions on the opposite sides of the graft.
Strip technique (Fig. 43.4)
The strip technique by Han and associates consists of
2 or 3 strips of tissue about 1 mm wide and long enough
to cover the entire length of the recipient site. These
strips are placed at the center and base of the recipient
site and sutured from the oral mucosa. The area is then
covered with tinfoil and a surgical pack.
Connective tissue technique
The connective tissue technique was originally described
by Edel. The advantages of this technique include:
a. Donor site—Healing by primary intention is achieved
because the donor material can be obtained from
connective tissue that is present beneath the palatal
flap.
b. Color matching is better.
Combination techniques:
It can be performed as follows:
Remove a strip of tissue about 3 to 4 mm thick from
the palate, place it between two wet tongue depressors,
and slice it longitudinally with a sharp BP blade. Use
a superficial portion that contains epithelium and
connective tissue, and the deeper portion that only
consists of connective tissue.
Healing of the graft (Figs 43.5A and B)
The full thickness graft consists of fat, glandular tissue
as well as the epithelium. It is not easily accepted and
not required in periodontal surgery.
The process of accepting a graft:
I. Plasmatic circulation stage (2-3 Days): Direct
joining of capillaries between the graft and the
recipient bed is seen and capillaries of the recipient
bed invade the graft.
II. Capillary circulation stage (4-5 Days): Capillaries
start functional circulation again. The epithelium
at this stage is almost desquamated and sparse.
III. Organization stage (10-14 Days): Organization is
completed by growth of fibroblasts in the graft
and in the recipient bed. The network of capillaries
become dense and regeneration of epithelium
becomes active to complete the epithelialization
(wound healing is by secondary intention).
Apically-Displaced Flap (Fig. 43.6)
This technique can be used for the combined purposes
of eliminating pockets and widening the zone of attached
gingiva. Depending on the purpose it can be a full
thickness (or) split thickness flap. The partial (split)
thickness flap is generally used to avoid exposure of bone
and the accompanying risks of bone resorption and
aggravation of bone dehiscences and fenestrations. The
full thickness flap is indicated when access to bone is
desired for recontouring purposes.
Procedure
Step I : Internal bevel incision 0.5-1 mm from the
crest of the marginal gingiva is given.
Step II : Crevicular incision and interdental incisions
are placed.
Step III : Vertical incisions extending beyond the
mucogingival junction are made so that the
flap can be displaced easily. Reflect the flap
depending on the purpose (split thickness or
full thickness).
Step IV : Debride the area and place the flap apical to
its original position and suture.
The edge of the flap can be located in any of the
three following positions:
1. Slightly coronal to the crest of the bone—This may
create the risk of recurrent pockets with thick gingival
margins.
2. At the level of the crest—This results in satisfactory
gingival contour.
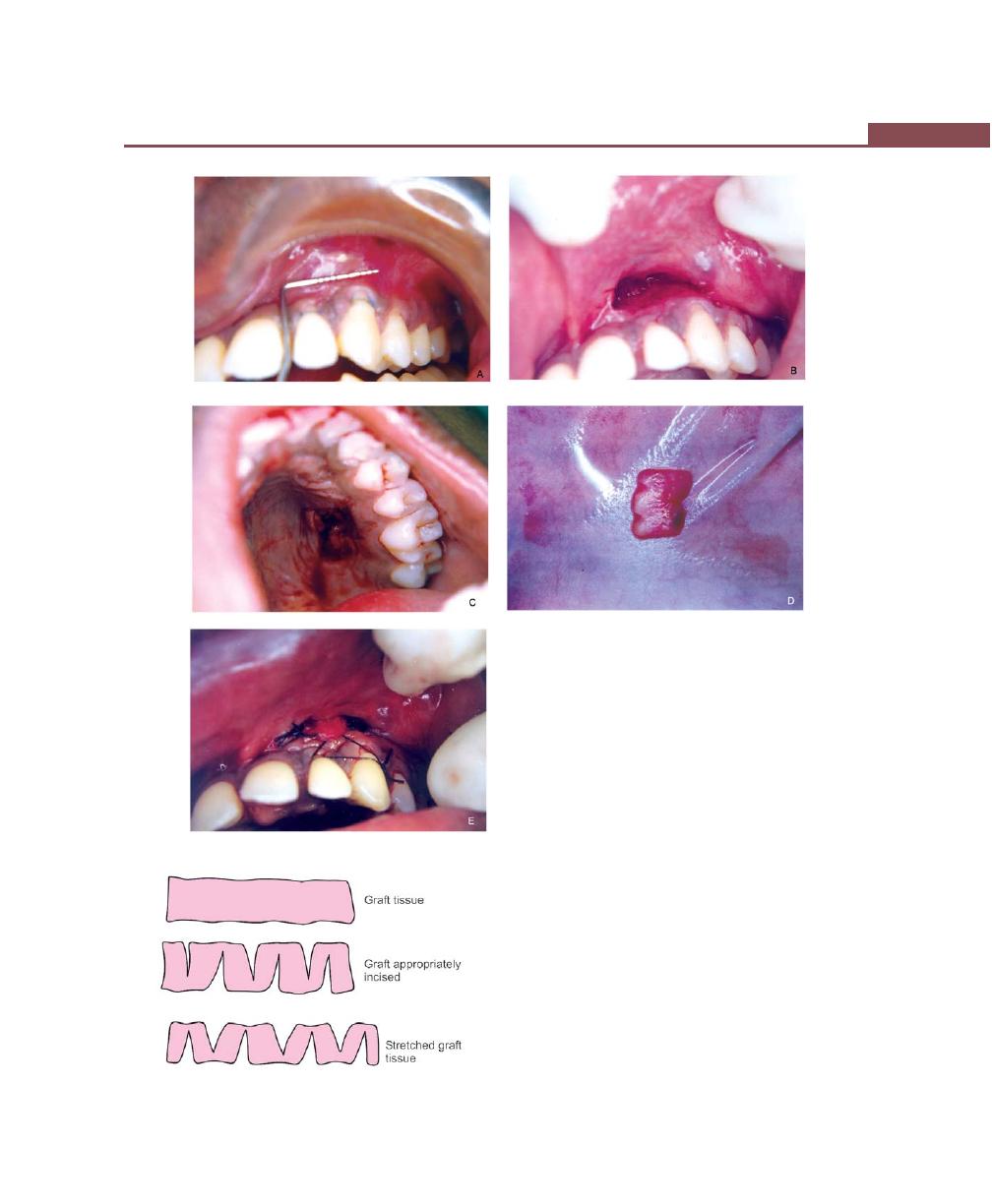
347
Mucogingival Surgery
Figs 43.3A to E:
Free soft tissue autograft (Accordian technique):
(A) Lack of attached gingiva, (B) Recipient bed preparation,
(C) Donor site immediately after the removal of tissue for
grafting, (D) Donor tissue (with alternate incisions), (E) Graft
transferred to recipient site and sutured in place
Fig. 43.3F:
Various steps in Accordian technique
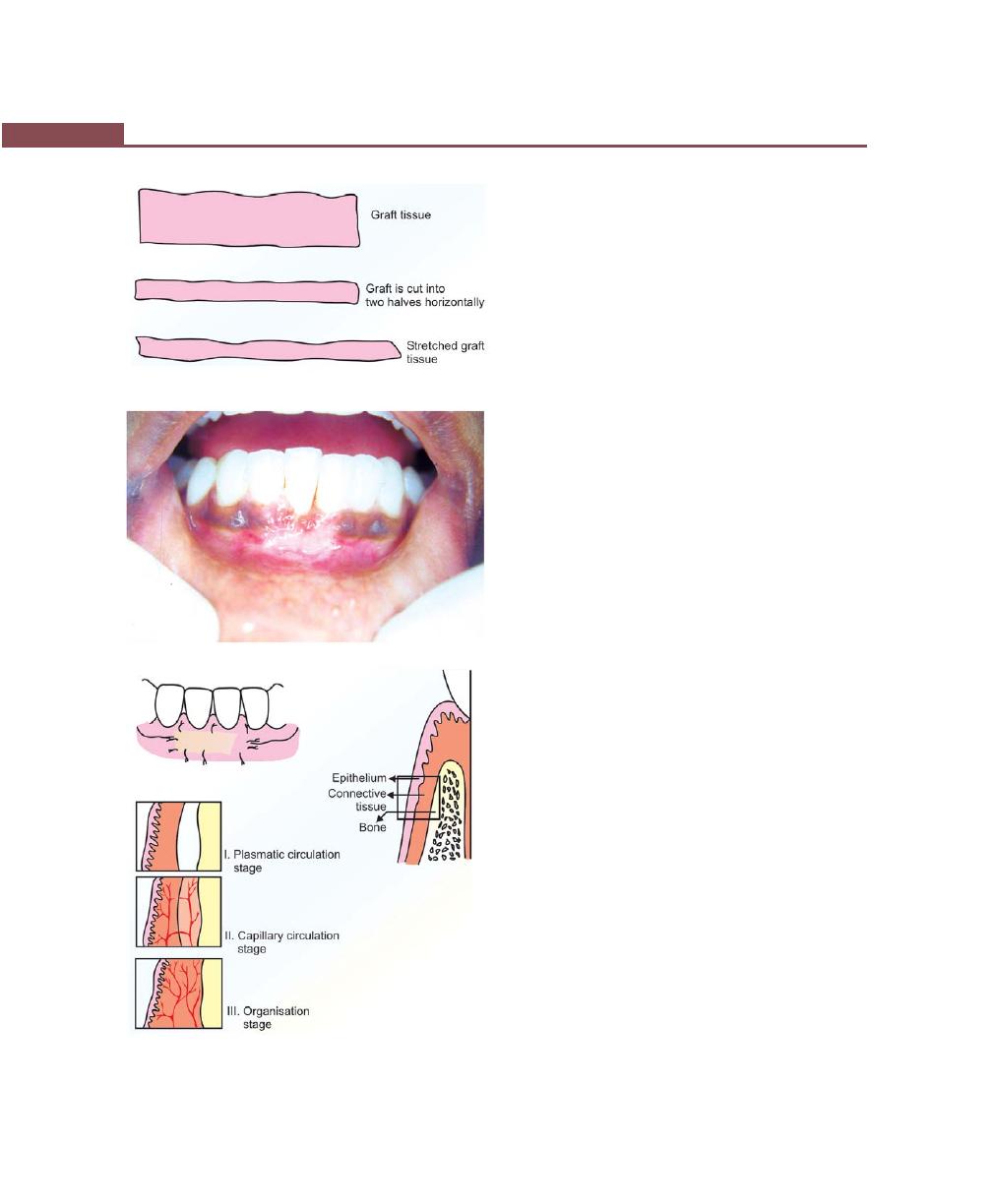
348
Essentials of Clinical Periodontology and Periodontics
Fig. 43.4:
Various steps in strip technique
Fig. 43.5B:
Healing phases of the graft
3. 2 mm short of the crest—This produces the most
desired gingival contour because new tissue will cover
the crest of the bone to produce a firm, tapered
gingival margin. But it also increases the risk of a slight
reduction in bone height.
Other Techniques for Widening the
Zone of Attached Gingiva
Fenestration Operation/Periosteal Separation
It utilizes a partial thickness flap, except in a rectangular
area at the base of the operative field where the
periosteum is removed, exposing the bone. This is the
area of fenestration and its purpose is to create a scar
that is firmly bound to the bone.
The results obtained are not as predictable as those
obtained with the free gingival graft or apically-displaced
flap (Fig. 43.7).
Vestibular Extension Operation
Originally described by Edlan and Mejchar produces
statistically significant widening of attached non-
keratinizing tissue. Currently this technique is of historical
interest only.
Procedure
The operative field is outlined by two vertical incisions
from the junction of the marginal and attached gingiva
to approximately 12 mm from the alveolar margin into
the vestibule. The vertical incisions are joined by
horizontal incision. A mucosal flap is elevated exposing
the periosteum on the bone . The periosteum is separated
from the bone, starting from the line of attachment of
the mucosal flap. The periosteum including muscle
attachments is transported to bone and is sutured to the
inner surface of the periosteum. The periosteum is then
transported to the lip and is, sutured where the initial
horizontal incision was made.
Procedures for Root Coverage
a. Indications for root coverage procedures are:
i. Reduces root sensitivity.
ii. Improves esthetics.
Fig. 43.5A:
Healing of a grafted site
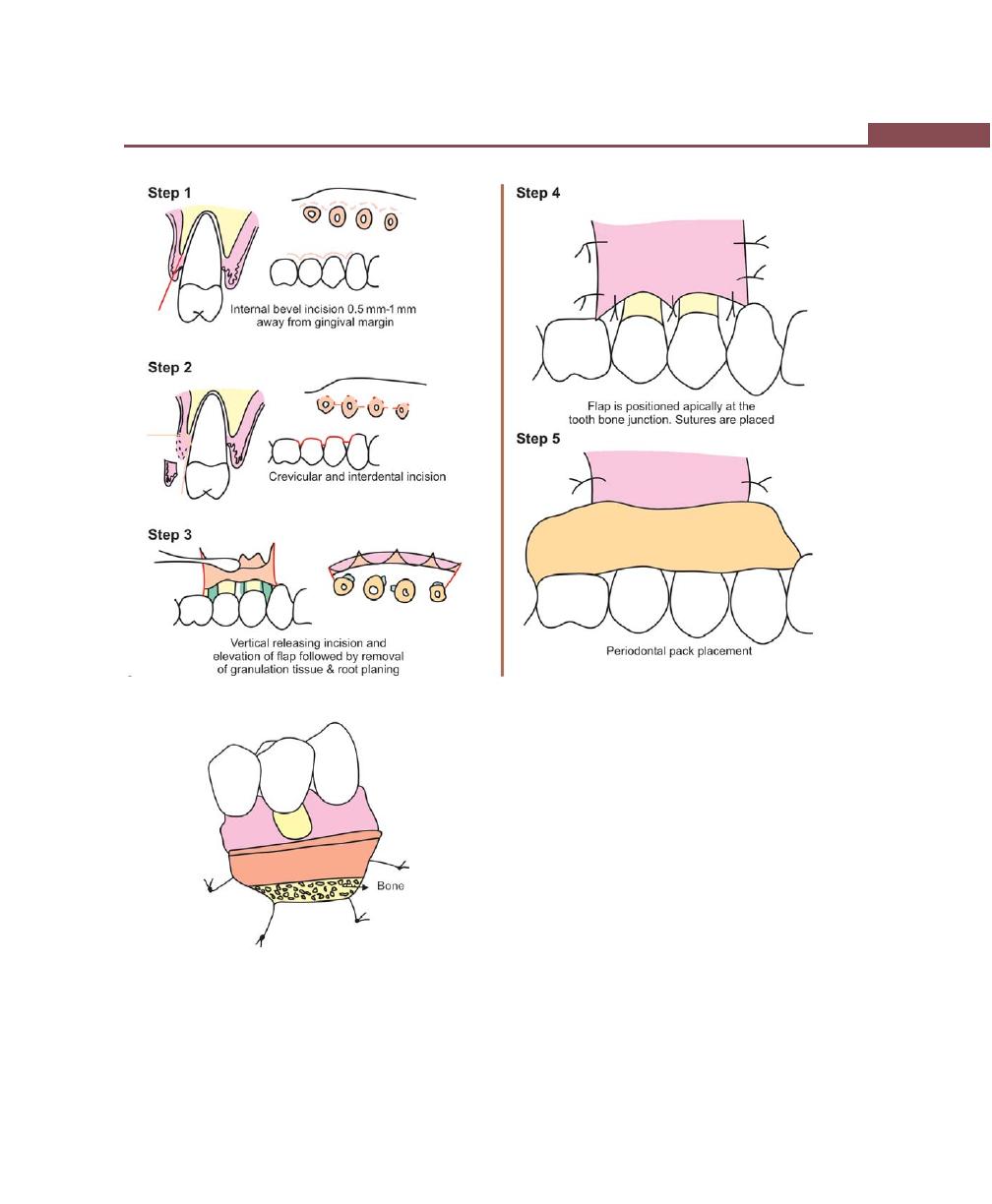
349
Mucogingival Surgery
Fig. 43.6:
Various steps in apically-displaced flap
Fig. 43.7:
Periosteal separation/Fenestration
iii. Manage the defects resulting from root caries
removal and/or cervical abrasions.
iv. Manage mucogingival defect which fail to
respond to altering abusive tooth-brushing
techniques and/or plaque removal.
b. Classification—Several classifications have been
proposed in 1960s, Sullivan and Atkins classified
isolated gingival recession into four types:
i. Shallow-narrow.
ii. Shallow-wide.
iii. Deep-narrow.
iv. Deep-wide.
Miller in (1985) expanded this classification so as to
help the clinician to predict the outcome of the therapy.
The following four classes of recession have been
proposed by Miller (Figs 43.8A to D).
Class I : Marginal tissue recession that does not
extend to the mucogingival junction. There
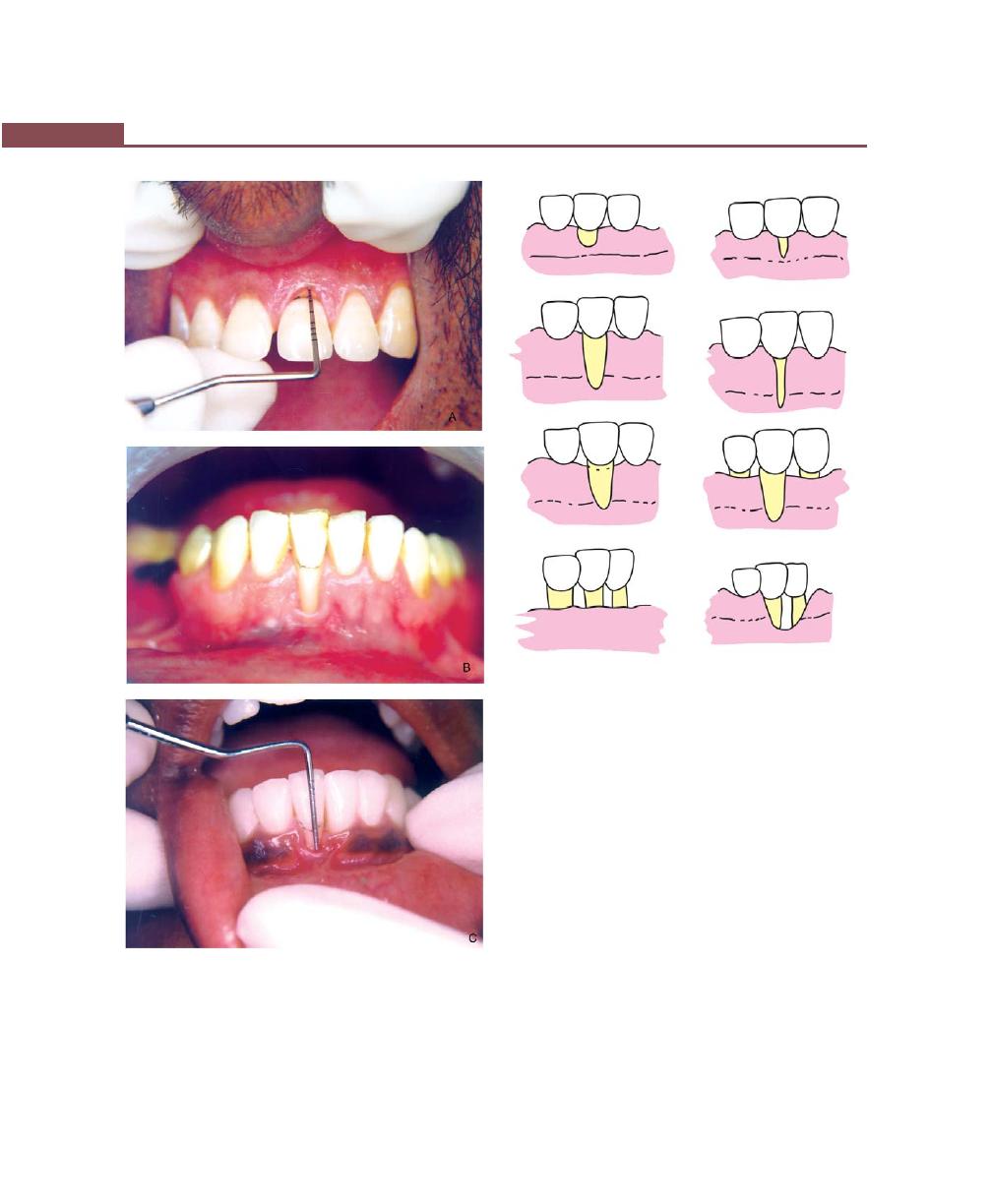
350
Essentials of Clinical Periodontology and Periodontics
Fig. 43.8D:
Class I, II, III and IV recession
is no loss of bone or soft tissue in the
interdental area. This type of recession can
be narrow or wide.
Class II : Marginal tissue recession that extends to or
beyond the mucogingival junction. There is
no loss of bone and soft tissue in the
interdental area. This type of recession may
be wide or narrow.
Class III : Marginal tissue recession that extends to or
beyond the mucogingival junction. In
addition, there is bone and/or soft tissue loss
interdentally or tooth may be malposed.
Class IV : Marginal tissue recession extends to or
beyond the mucogingival junction. With
severe bone and soft tissue loss interdentally
and/or severe tooth malposition.
Type of recession
Prognosis
Class I and II
— good to excellent
Figs 43.8A to C:
Miller’s classification of gingival
recession (Class I, II and III)
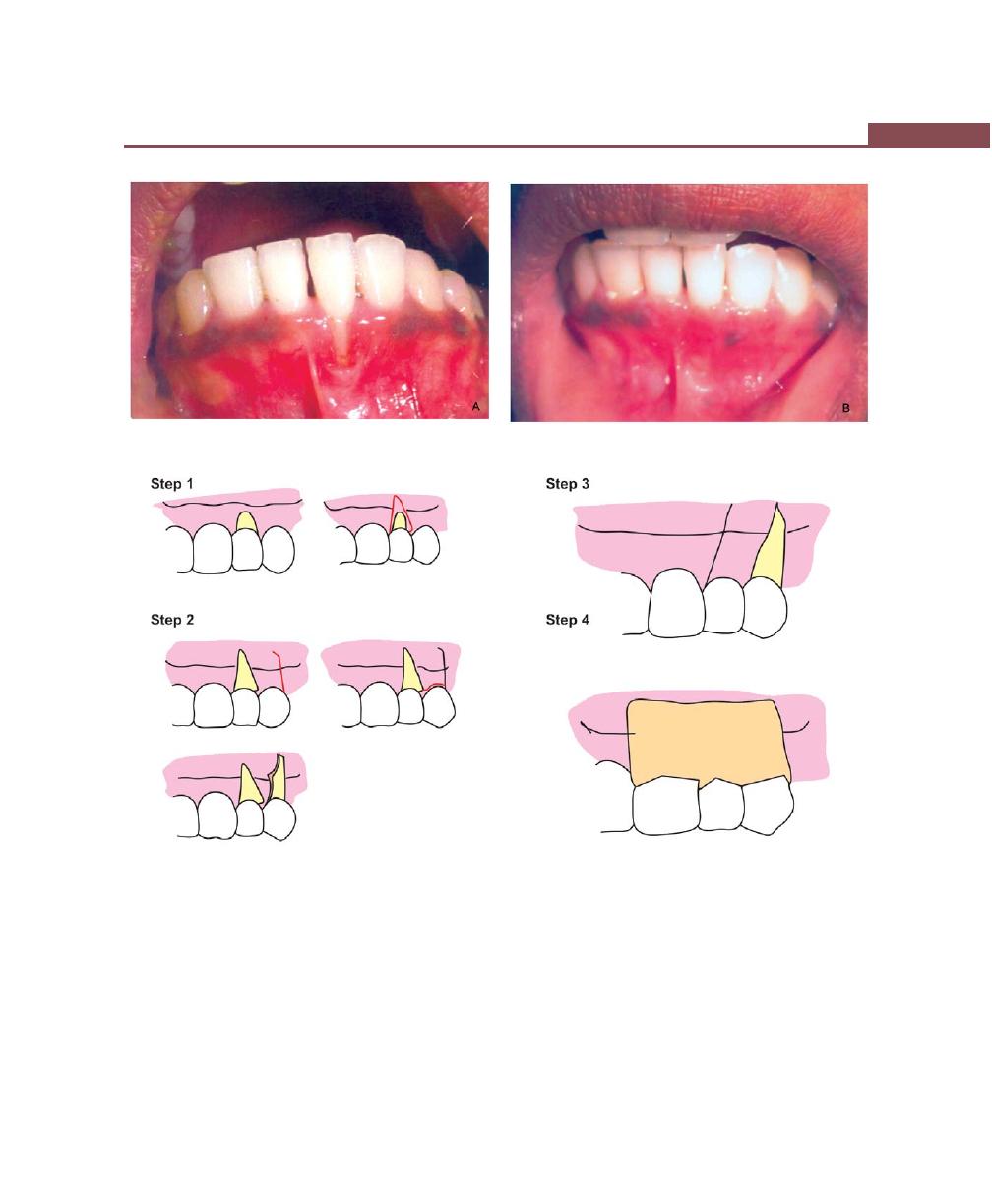
351
Mucogingival Surgery
Figs 43.9A and B:
Laterally-displaced flap. (A) Preoperative view, (B) Postoperative view (after 2 month)
Class III
— only partial coverage can be
expected
Class IV
— very poor.
Procedures for root coverage can be divided into:
1. Conventional procedures.
2. Regenerative procedures.
Conventional procedures:
Depending on the width of the attached gingiva, i.e. if
adequate width is present at the donor site the following
procedures can be selected:
a. Laterally (horizontally)-displaced flap.
b. Double-papilla flap.
c. Coronally-positioned flap
Fig. 43.9C: Various steps in laterally displaced flap (See the text for explanation)

352
Essentials of Clinical Periodontology and Periodontics
If the donor site is associated with inadequate width:
i. Free soft tissue autograft.
ii. Subepithelial connective tissue grafts are available.
Regenerative procedures:
Guided tissue regeneration (GTR) has been proposed.
Pedicle autografts:
a. Laterally (Horizontally)-displaced flap (Figs
43.9A to C)
In 1956 Grupe and Warren developed an original
and unique procedure called the sliding flap operation.
Advantages of laterally-displaced flap:
a. One surgical site.
b. Good vascularity of the pedicle flap.
c. Ability to cover isolated, denuded roots that have
adequate donor tissue laterally.
Disadvantages of laterally-displaced flap:
a. Limited by the amount of adjacent keratinized
attached gingiva.
b. Possibility of recession at the donor site.
c. Dehiscence or fenestration at the donor site.
d. Limited to one or two teeth with gingival recession.
Indications:
a. For covering the isolated denuded root.
b. When there is sufficient width of interdental papilla
in the adjacent teeth.
c. Sufficient vestibular depth.
Contraindications:
a. Presence of deep interproximal pockets.
b. Excessive root prominence.
c. Deep or extensive root abrasion or erosion.
d. Significant loss of interproximal bone height.
The following is the step-by-step procedure for
laterally-displaced flap:
Step I : Preparation of the recipient site
Make an incision, resecting gingival margin
around the exposed roots. This band of
marginal gingiva is removed with a scaler or
curette. The exposed root surface is planed
well. If granulation tissue is present along the
incised edge of the gingiva, it should be
removed carefully with curettes.
Step II : With a # 15 blade a vertical incision is made
extending from marginal gingiva into the
mucogingival junction. A crevicular incision
is then made from the vertical incision to the
defect. A flap is then raised utilizing either
partial thickness or full thickness reflection.
Each of these has its own advantages and
limitations, for e.g. partial thickness flap offers
advantages like rapid healing at the donor
site and reduced risk of facial bone loss.
However, if the gingiva is thin, flap survival
becomes difficult. It may sometimes be
necessary to give a short oblique incision into
the alveolar mucosa at the distal corner of
the flap, pointing more towards the recipient
site. This will enable us to slide the flap laterally
without excess tension at the base.
Step III : Transfer the flap
After the flap is transferred onto the adjacent
root, the flap is sutured to the adjacent gingiva
and alveolar mucosa with interrupted sutures.
Step IV : Protect the flap and donor site
Cover the surgical site with a periodontal pack
and after one week the pack and sutures can
be removed. Postoperatively, antibiotics are
not always necessary in the normal course of
treatment, but analgesics are prescribed to
control pain. The flap may heal by connective
tissue adhesion, or connective tissue
attachment or long junctional epithelium.
Some of the studies have shown better results
with citric acid root conditioning.
Modifications:
Considering the drawbacks of the procedure many
modifications of lateral sliding flap have been proposed.
a. Converging oblique incisions over the recipient site
and a vertical or oblique incision at the distal end

353
Mucogingival Surgery
of the donor site so that the flap which is being
transposed is wider at its base.
b. In the donor site, the marginal periodontium is left
undisturbed in order to reduce the likelihood of
recession and bone resorption.
b. Double papilla flap (Figs 43.10A to G)
First described by Wainberg as the Double Lateral
Repositioned Flap and was refined by Cohen and Ross
as the Double Papilla Flap.
Indications:
1. When the interproximal papillae adjacent to the
mucogingival problem are sufficiently wide.
2. When the attached gingiva on an approximating tooth
is insufficient to allow for a Lateral Pedicle Flap.
3. When periodontal pockets are not present.
Advantages:
1. The risk of loss of alveolar bone is minimized because
the interdental bone is more resistant to loss than
is radicular bone.
2. The papillae usually supply a greater width of attached
gingiva than from the radicular surface of a tooth.
3. The clinical predictability of this procedure is fairly
good.
Disadvantages:
1. Technique sensitive—Having to join together the small
flap in such a way so that they act as a single flap.
c. Coronally-repositioned flap or
coronally-positioned flap (Figs 43.11 and 43.12)
Indications:
• Esthetic coverage of exposed roots.
• For tooth sensitivity owing to gingival recession.
Advantages:
• Treatment of multiple areas of root exposure.
• No need for involvement of adjacent teeth.
• High degree of success.
• Even if the procedure does not work, it does not
increase the existing problem.
Disadvantages:
• There is a need for two surgical procedures if the
zone of keratinized gingiva is inadequate.
This procedure can be performed utilizing two
techniques.
First Technique
Step 1 : Make two apically-divergent vertical releasing
incisions, extending from a point coronal to
the cemento-enamel junction at the mesial
and distal line angles of the tooth and apically
into the lining mucosa.
Step 2 : A split thickness flap is prepared by sharp
dissection at the mesial and distal ends and
is connected with an intracrevicular incision.
Facially, apical to the recession, a full thickness
flap is raised.
Step 3 : Once the flap is reflected the root surfaces
are debrided thoroughly. Some authors have
suggested the use of citric acid with a pH 1.0
for conditioning the root surface.
Step 4 : At the base of the inner surface of the flap,
approximately 3 mm apical to the bone
dehiscense, a horizontal incision is made
through the periosteum, followed by a blunt
dissection into the lining mucosa to release
muscle tension. Now the mucosal graft can
be easily positioned coronally at the level of
cementoenamel junction.
Step 5 : The flap is secured firmly with the help of
interrupted sutures and additional sling sutures
can be placed to maintain the flap in place.
Periodontal dressing is placed to protect the
wound during initial healing.
Modifications:
One of the limitations of this procedure is, it cannot be
performed when there is insufficient width of attached
gingiva. To solve this problem, a two stage surgical
procedure has been proposed. First, a gingival extension
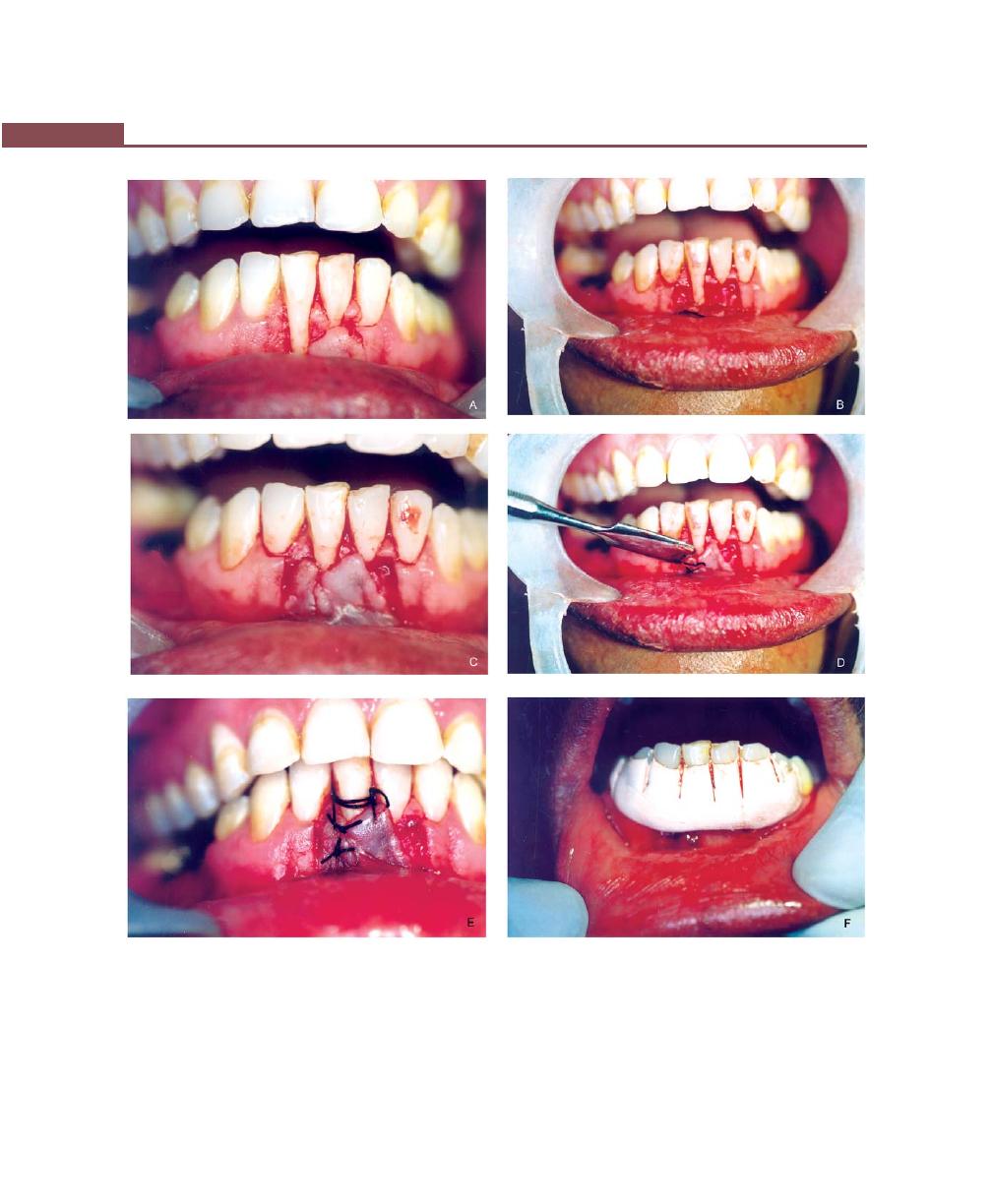
354
Essentials of Clinical Periodontology and Periodontics
Figs 43.10A to F:
Double papilla flap: (A) Recipient site preparation, (B) Two vertical or oblique incision at mesial and distal
end of the defect, (C) Flaps are reflected (partial thickness), (D) The transposed flaps are sutured to obtain a single flap,
(E) Final suturing, (F) The area covered with periodontal dressing
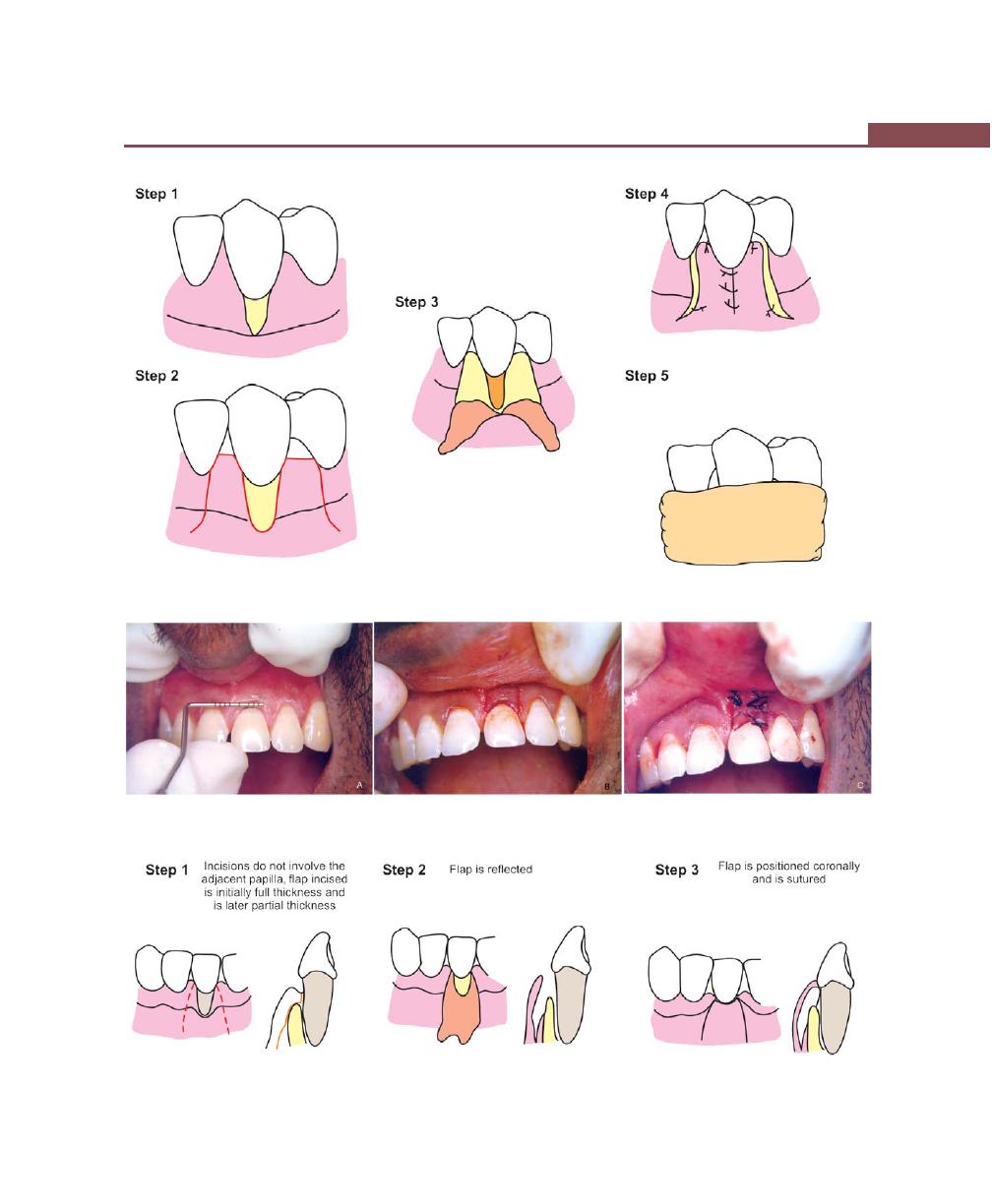
355
Mucogingival Surgery
Fig. 43.10G:
Various steps in double papilla flap (Refer the text for explanation)
Figs 43.11A to C:
Coronally-positioned flap: (A) Measure the width and length of gingival recession (class I), (B) Two
vertical releasing incisions are placed, (C) Flap positioned coronally and sutured
Fig. 43.11D:
Various steps in coronally-positioned flap (Refer the text for explanation)
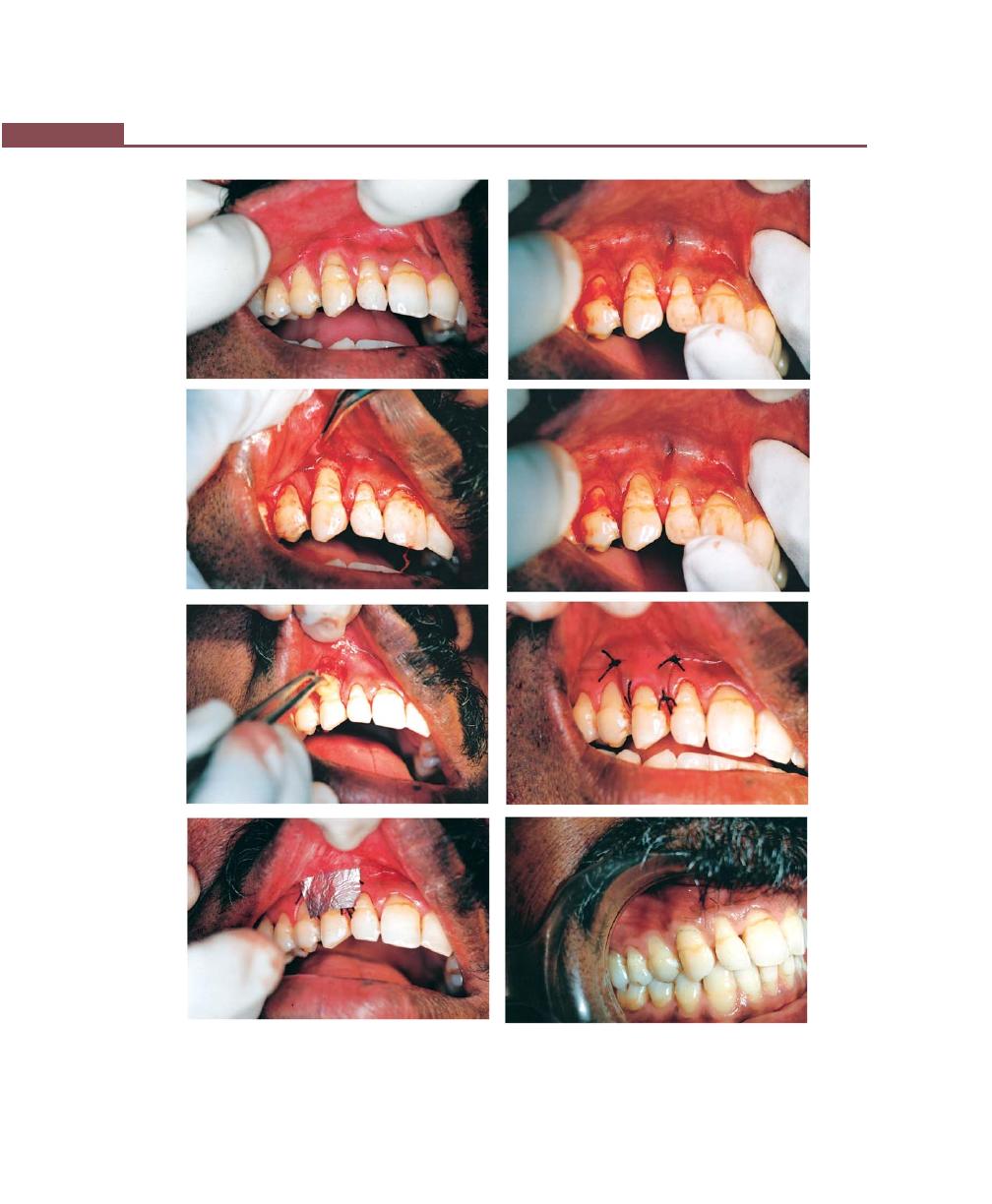
356
Essentials of Clinical Periodontology and Periodontics
Figs 43.12A to H:
Case 2: Coronally repositioned flap procedure. (A) Preoperative view showing class I miller’s recession.
(B) Incision. (C) Reflection of flap. (D) Coronally displaced flap. (E) Root conditioning with tetracycline soaked cotton pellet.
(F) Suturing of coronally displaced flap. (G) Tin foil placed prior to pack placement. (H) Facial view after healing
A
B
C
D
E
F
G
H
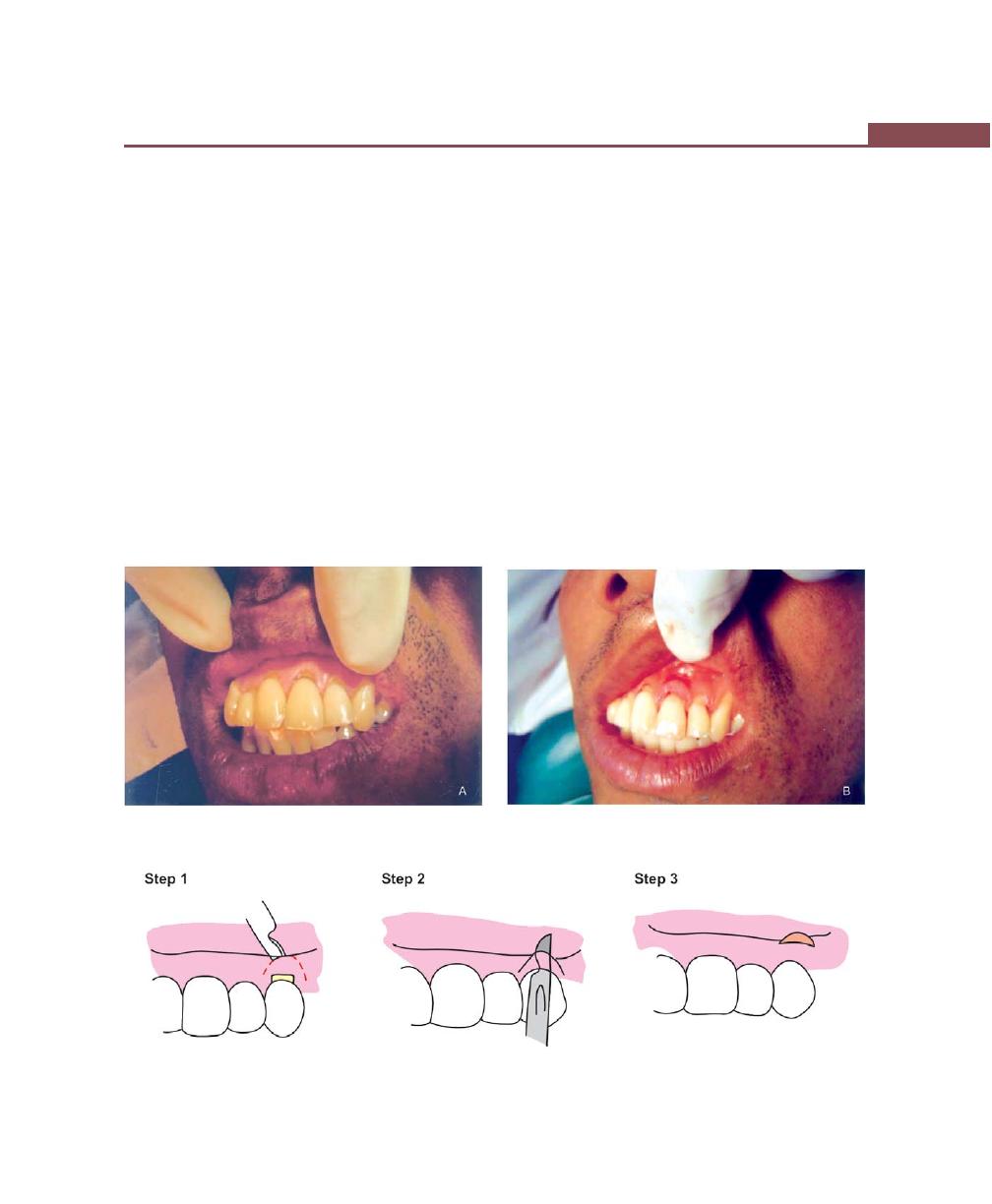
357
Mucogingival Surgery
operation with a free gingival graft is performed. Two
months later a second stage surgery is performed by
coronally repositioning the flap which may include free
soft tissue graft.
Second Technique
Semilunar flap (Figs 43.13A to C)
Indications:
• Areas where gingival recession is only 2 to 3 mm.
Advantages:
• No vestibular shortening ,as occurs with the coronally-
positioned flap.
• No esthetic compromise of interproximal papillae.
• No need for sutures.
Disadvantages:
• Inability to treat large areas of gingival recession.
• The need for a free gingival graft if there is an
underlying dehiscence or fenestration.
Step 1 : A semi-lunar incision is placed, following the
curvature of the gingival recession, make sure
that the incision ends about 2 to 3 mm short
of the tip of the papillae. This is very impor-
tant, as the flap derives all of its blood supply
from the adjacent papillary areas.
Step 2 : Split thickness dissection is performed coro-
nally and this is connected to an intracrevicular
incision.
Step 3 : The tissue will now collapse freely, covering
the denuded root. The flap is then held in
place with a moist gauze for a few minutes.
Suturing is not generally required .Periodontal
dressing may be placed.
Figs 43.13A and B:
Semilunar flap: (A) Class I gingival recession in relation to 21-preoperative view,
(B) Semilunar incision and coronal displacement of flap
Fig. 43.13C:
Various steps in semilunar flap (Refer the text for explanation)

358
Essentials of Clinical Periodontology and Periodontics
Free Gingival Autograft
The classic technique proposed for creating a widened
zone of attached gingiva was described in the earlier
section of this chapter. Miller in 1985 applied this with
few modifications to cover the denuded roots. The
procedure is as follows:
Step 1 : A horizontal right angled incision is made in
the interdental papillae, followed by, vertical
incision at the proximal line angles of the
adjacent teeth. The retracted tissue is excised.
The periosteum should be left intact in the
apical areas. A graft is obtained from the donor
site, transferred to the recipient site and
immobilized. Donor site is protected as
described earlier.
Subepithelial Connective Tissue Graft
(Figs 43.14A to F)
Proposed by Langer and Langer in 1985. In 1994, Bruno
described modification of the original Langer and Langer
technique.
Indications:
• Where esthetics is of prime concern
• For covering multiple denuded roots
• In the absence of sufficient width of attached gingiva
in the adjacent areas.
Advantages:
• High degree of cosmetic enhancement.
• Incurs no additional cost for autogenous donor tissue.
• One step procedure
• Minimal palatal trauma
• Increased graft vascularity.
Disadvantages:
• High degree of technical skills required.
• Complicated suturing.
The technique is as follows:
I. Preparation of recipient site: The initial horizontal
right angle incision is made into the adjacent
interdental papillae at, or slightly coronal to the
cementoenamel junction of the tooth with an exposed
root surface. A butt joint is provided. It is made sure
that the papillary incisions are not more than 1 mm
deep, this is done to preserve the papillary blood
supply. A partial thickness flap is raised without
vertical incisions. The exposed root is meticulously
planed with curettes. At times, it may be desirable
to use finishing burs to reduce the root convexity.
Following root planing, the root is treated with
either citric acid pH 1.0 or tetracycline HCl in a
concentration of 250 mg mixed in 5 ml of sterile
water. The solution is applied to the root surface for
2 to 3 minutes with cotton pellets. Once the root
is treated, the approximate mesio-distal width
necessary for the graft is measured with a periodontal
probe.
II. Excision of the donor tissue (Fig. 43.15): The first
incision is made approximately 2 to 3 mm apical to
the gingival margin, perpendicular to the long axis
of the teeth. The second incision is made parallel to
the long axis of the teeth, 1 to 2 mm apical to the
first incision. A small periosteal elevator is used to
raise a full thickness periosteal connective tissue graft.
Vertical incisions may be necessary at the mesial and
distal extent of the graft to facilitate easy removal of
the connective tissue.
Once the graft is removed, the area is sutured
with 4-0 silk suture material.
III. Grafting to the recipient site: The donor connective
tissue is secured to the papillae with interrupted sutures
and the overlying partial thickness flap is then replaced
over the donor tissue and interrupted sutures are
placed in the mesial and distal papillae. No attempt
is made to cover the donor tissue completely. A
periodontal dressing is placed to cover the surgical
site. The routine post operative care is followed. The
dressing and sutures are removed after seven days
postoperatively.
Modifications:
In the recipient site preparation:
a. Envelope technique (Fig. 43.16)—In this technique,
first eliminate the sulcular epithelium by an internal
bevel incision. Secondly, an ‘envelope’ is prepared
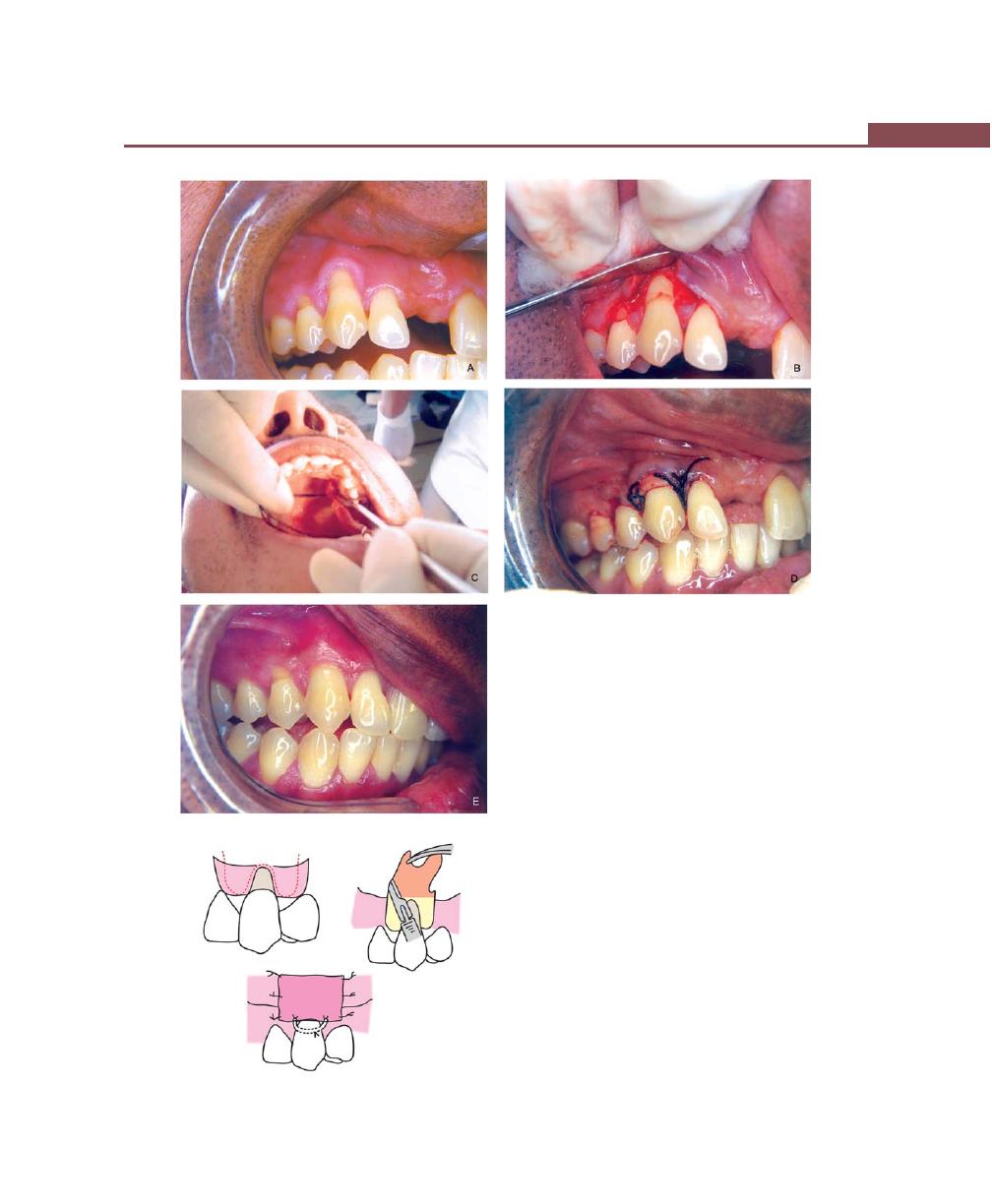
359
Mucogingival Surgery
Figs 43.14A to E:
Subepithelial connective tissue graft: (A) A
recession defect at a maxillary right canine, (B) Recipient site
preparation, (C) Procuring connective tissue graft from the
palate, (D) The graft is placed in the recipient site and secured
in position with interrupted sutures, (E) Shows 3 month
healing result
Fig. 43.14F:
Various steps in free connective tissue graft—
Recipient site preparation (Refer the text for explanation)
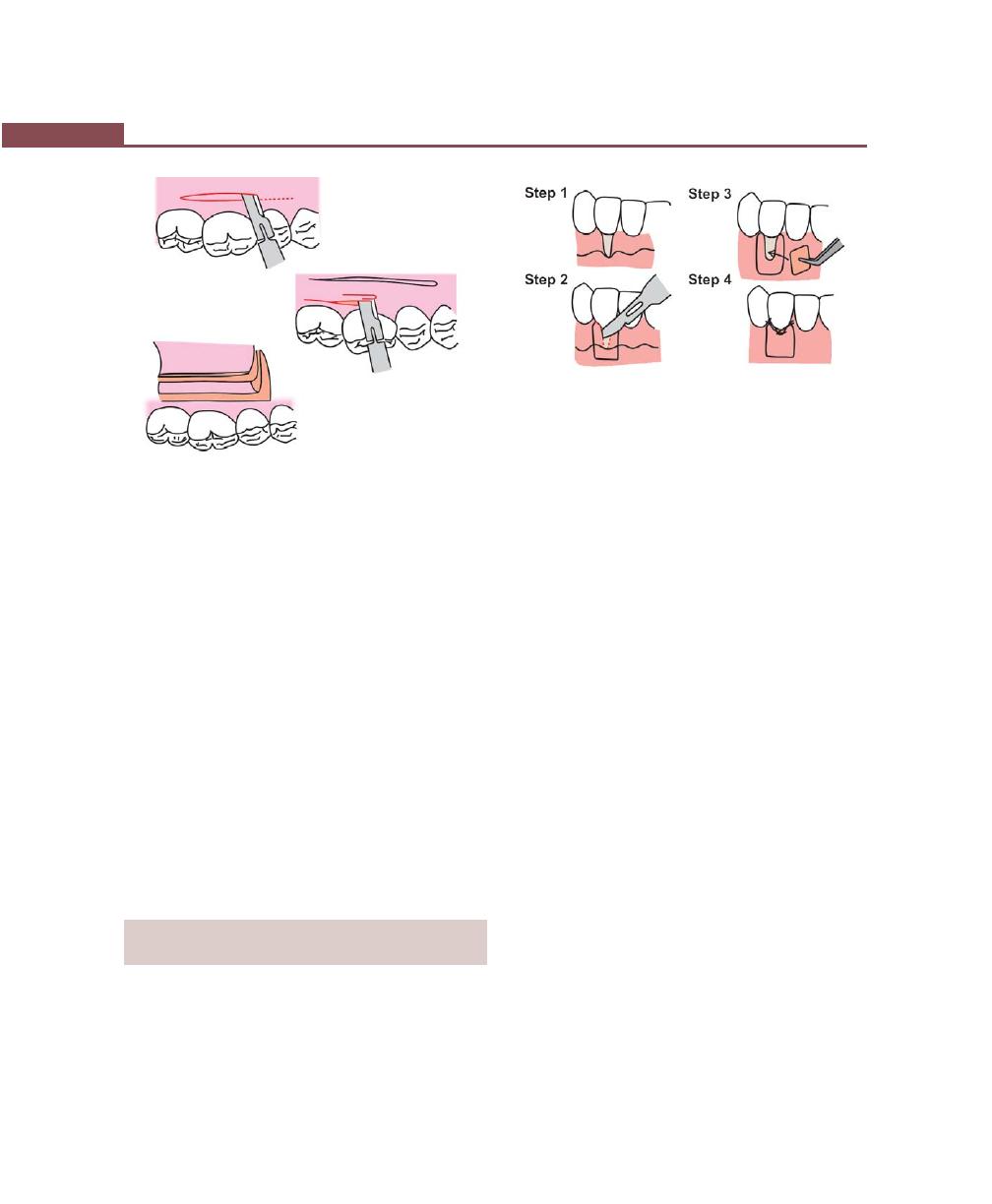
360
Essentials of Clinical Periodontology and Periodontics
apically and laterally to the recession by split
incision.
b. Langer’s technique (Figs 43.17A to D)—In addition
to the horizontal incision, two vertical releasing
incisions 1 to 2 mm away from the gingival margin,
extending one half to one tooth wider mesiodistally
than the area of gingival recession, is given.
c. Pouch and tunnel technique (Fig. 43.18)—In case
of multiple adjacent recessions “envelopes” are pre-
pared on each tooth for receiving connective tissue graft.
Procuring the Graft from the Donor Site
In the palate, a partial thickness flap is raised with two
vertical releasing incisions, followed by this, the connective
tissue is harvested by placing two horizontal and two
vertical incisions to the bone. After the graft removal,
the flap is positioned back and sutured.
GUIDED TISSUE REGENERATION
TECHNIQUE FOR ROOT COVERAGE
Indications
• Esthetic demand.
• Indicated for single tooth with wide, deep localized
recessions.
Fig. 43.15:
Various steps in free connective tissue graft—
Donor site preparation
Fig. 43.16:
Various steps in free connective tissue graft—
envelope technique
• For areas of root sensitivity where oral hygiene is
impaired.
• For repair of recessions associated with failing or
unesthetic class V restorations.
Advantages:
• Techniques does not require a secondary donor
surgical site reducing post operative discomfort.
• New tissue blends evenly with the adjacent tissue,
providing highly esthetic results.
Disadvantages:
• It is sensitive technique.
• Incurence of additional cost of barrier membrane.
The technique consists of the following steps:
Step 1 : A full thickness flap is raised upto the
mucogingival junction, beyond it, atleast 8
mm apical to the mucogingival junction a
partial thickness flap is raised.
Step 2 : The root surface is meticulously planed, and a
Goretex membrane is trimmed according to
the size of the defect and tied to the tooth. Make
sure that it covers atleast 2 mm of marginal
periosteum. In order to prevent the membrane
from collapsing in the defect, some authors
have suggested to pass a suture through the
membrane, that covers the bone and this
suture is knotted on the exterior and tied to
bend the membrane, so that a space is created
between the root and the membrane.
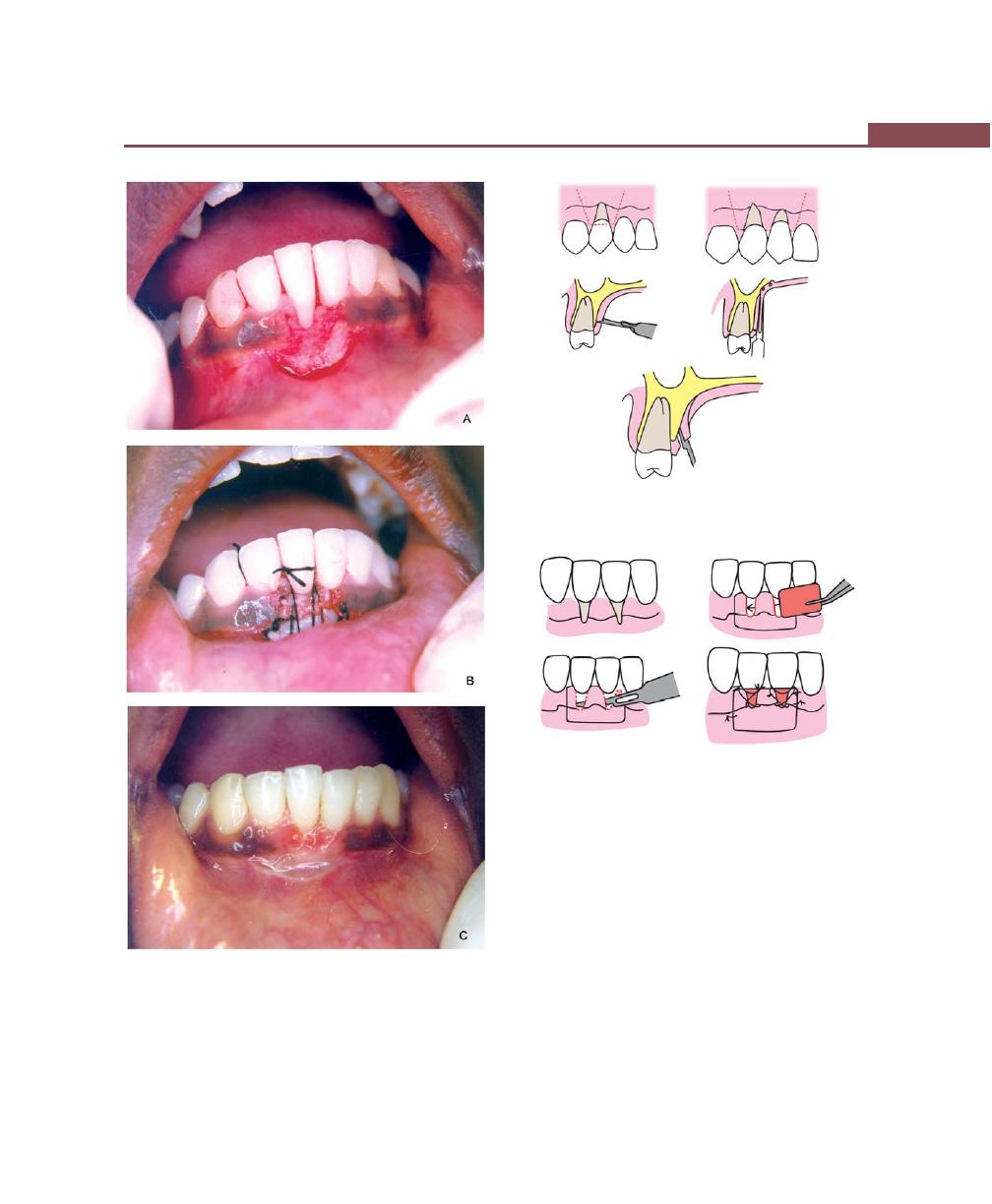
361
Mucogingival Surgery
Figs 43.17A to C:
Subepithelial connective tissue graft
(Langer’s technique). (A) A gingival flap is reflected with two
vertical incisions, (B) A large connective tissue graft is sutured
in place, (C) Two weeks postoperative view
Fig. 43.17D:
Various steps in Langer’s technique
Step 3 : The flap is then positioned coronally and
sutured. Four weeks later, the membrane is
carefully removed without disturbing the
growing tissue.
Modifications
a. Titanium–reinforced membranes are used to create
the space below the membrane.
b. Resorbable membranes have been used to prevent
a second surgery.
Fig. 43.18:
Various steps in pouch and tunnel technique
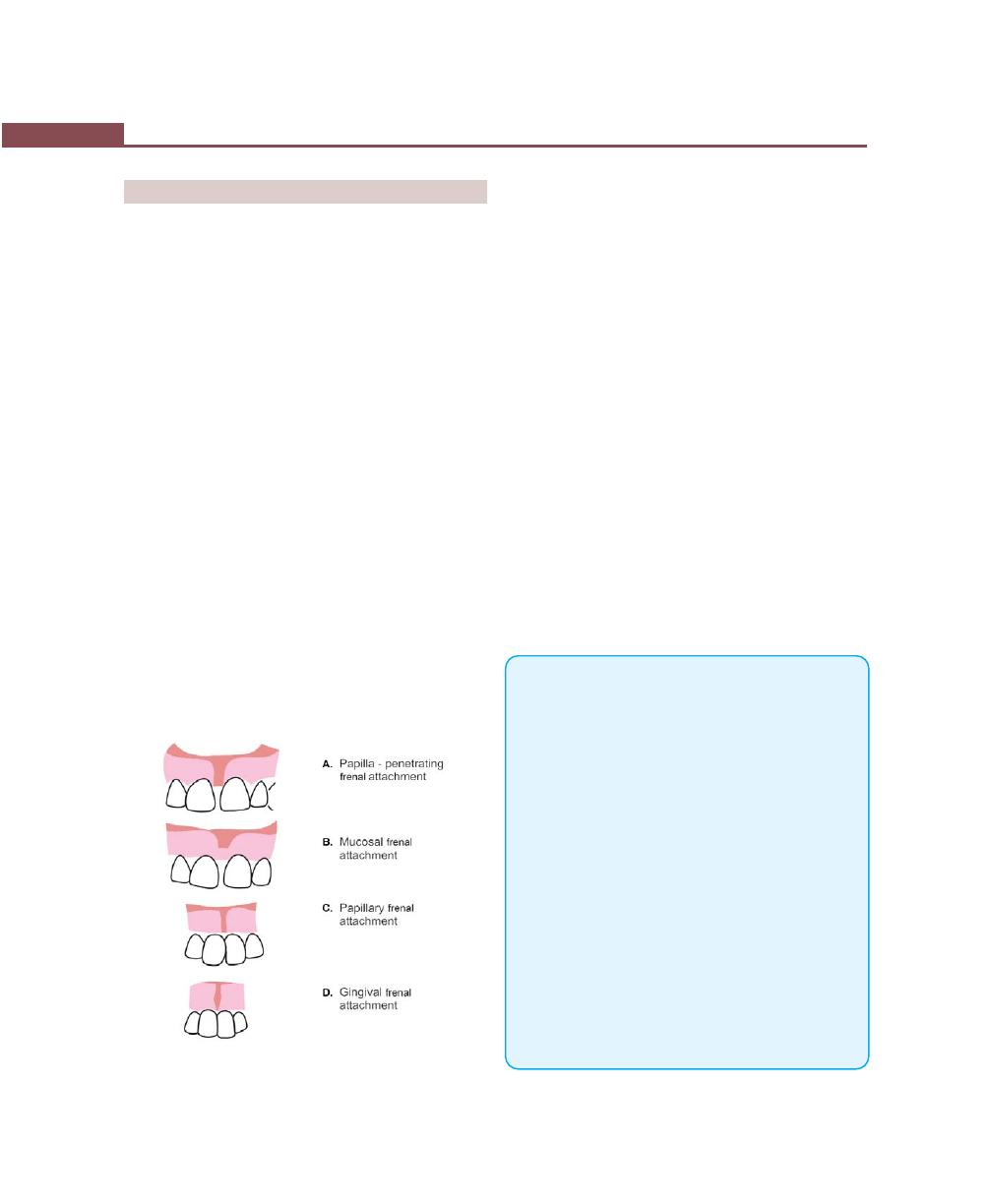
362
Essentials of Clinical Periodontology and Periodontics
OPERATIONS FOR REMOVAL OF FRENA
A frenum is a fold of mucous membrane usually with
enclosed muscle fibers, that attaches the lips and cheeks
to the alveolar mucosa and/or gingiva and underlying
periosteum.
Indications
To prevent—
1. The accumulation of irritants.
2. The deflection of the wall of periodontal pocket which
may aggravate its severity.
3. Its interference with post-treatment healing.
4. Pocket formation.
5. Injury while brushing.
Frenectomy: It is the complete removal of frenum
including its attachment to the bone. It is indicated for,
correction of abnormal diastema.
Frenotomy: It is the incision and relocation of the
frenum to create a zone of attached gingiva between
the gingival margin and the frenum (Suffices for
periodontal problems).
Frenal attachment may be of four types (Fig. 43.19)
a. Papillary—Where the frenum is inserted into the
interdental papilla.
Fig. 43.19:
Types of frenal attachments
b. Mucosal Type—Where the frenum is attached in the
alveolar mucosa.
c. Papillary penetrating types—Where the frenum is
inserted from the facial to palatal papilla.
d. Gingival—Where the frenum is in the attached gingiva.
Techniques for the Removal of the
Frenum (Figs 43.20A to I)
Step 1 : After anesthetizing the area, engage the
frenum with a hemostat.
Step 2 : Incise along the upper surface of the hemo-
stat, simultaneously make a similar incision
along the under surface of the hemostat.
Step 3 : Remove the triangular resected portion of the
frenum along with hemostat. This exposes the
fibrous connective tissue attachment to the
bone.
Step 4 : Make a horizontal incision to dissect and
separate the fibers attached to the bone.
Step 5 : Close the wound by placing interrupted
sutures.
KEY POINTS TO NOTE
1. Mucogingival surgery consists of plastic surgical procedures
for the correction of gingiva mucous membrane
relationships that complicate periodontal diseases and may
interfere with the success of periodontal treatment.
2. Recently, it has been renamed as “Periodontal plastic
surgery”.
3. Most commonly performed mucogingival surgical
procedures are:
• Techniques to increase width of attached gingiva.
• Root coverage procedures
• Operations for removal of frena.
4. Techniques for increasing width of attached gingiva
are:
• Gingival extension operation by free soft tissue autograft.
• Apical displacement of the pocket wall and other
techniques like fenestration operation and vestibular
extension operation.
5. Sullivan and Atkins have classified gingival recession into,
shallow-narrow, shallow-wide, deep-narrow and deep-
wide, PD Miller has modified this into Class-I, Class-II,
Class-III and Class-IV gingival recession.
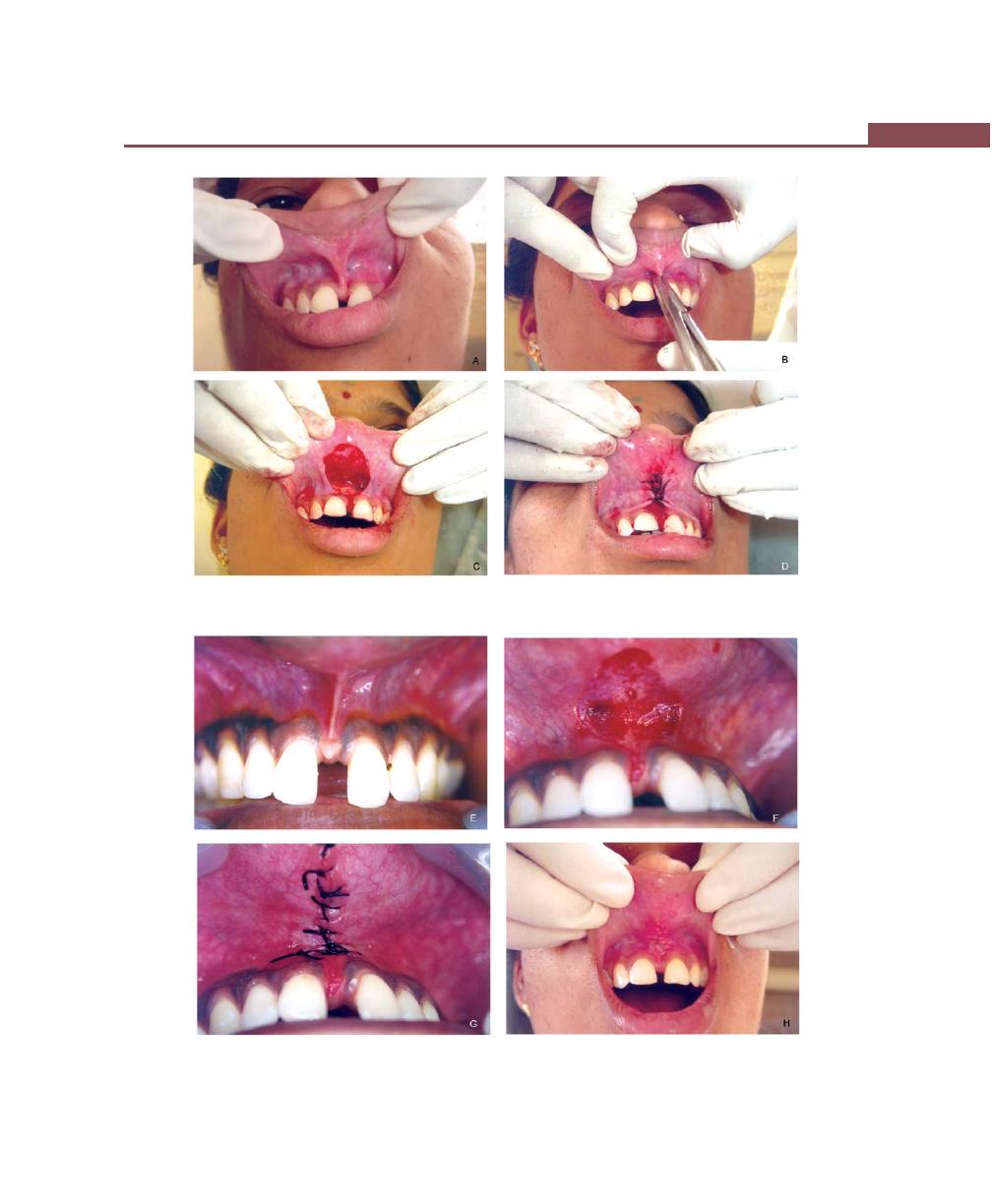
363
Mucogingival Surgery
Figs 43.20A to D:
Frenectomy procedure: (A) A case of high frenal attachment with tension test being
positive, (B) Engage the frenum with a hemostat, (C) After removal of triangular resected portion of
the frenum, (D) The wound is closed with interrupted sutures
Figs 43.20E to H:
Case 2: (E) A case of Frenectomy, (F) Frenum relieved, (G) Sutures placed,
(H) Clinical appearance parmediately after suture removal
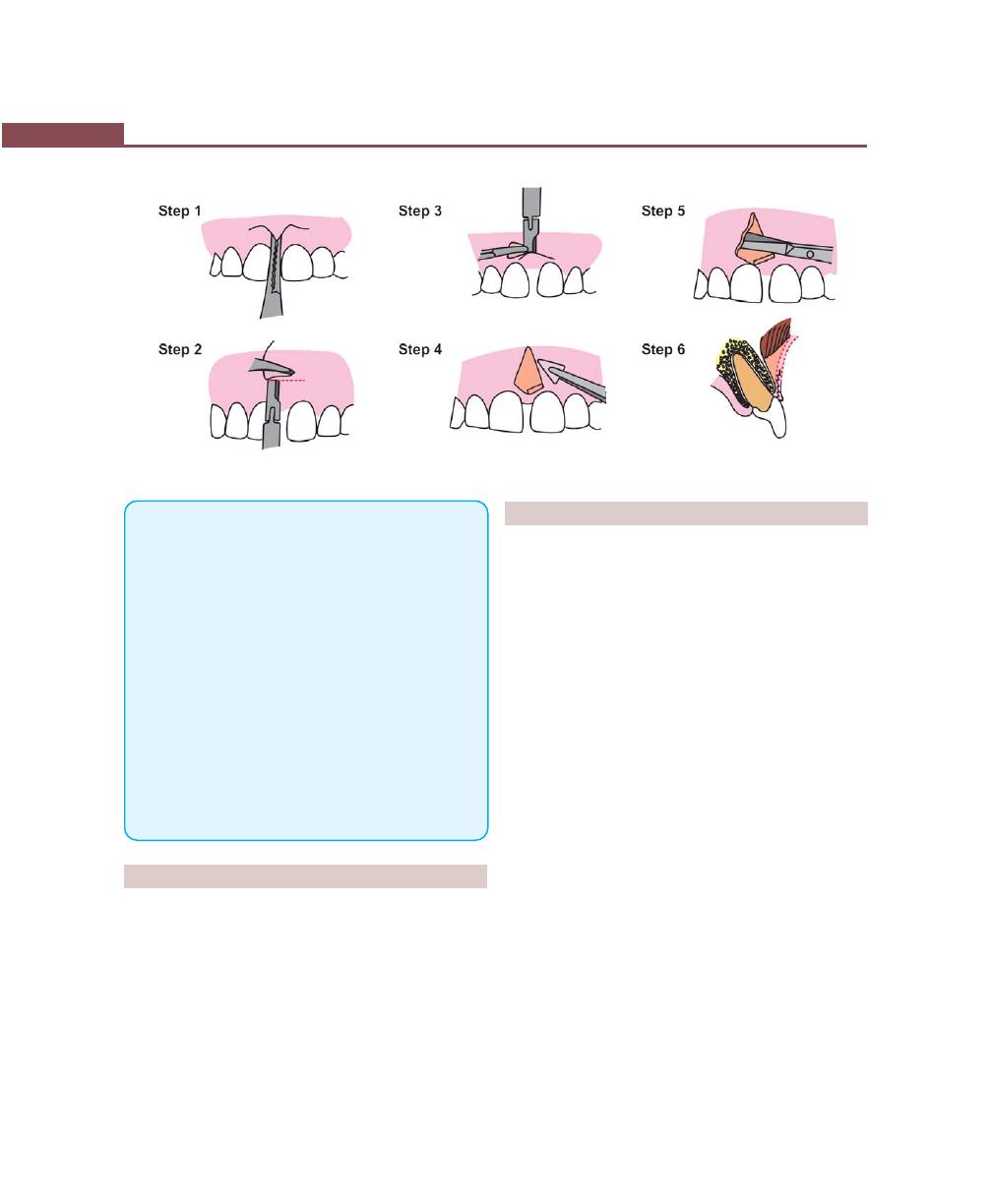
364
Essentials of Clinical Periodontology and Periodontics
Fig. 43.20I:
Various steps in frenectomy (Refer the text for explanation)
6. Procedures for root coverage are divided into:
• Conventional, e.g. pedicle autografts.
• Regenerative procedures.
7. If the width of the attached gingiva is adequate in the donor
site the following conventional procedures can be selected:
• Laterally-displaced flap
• Double papilla flap
• Coronally-positioned flap and Semilunar flap.
8. If the donor site is associated with the inadequate width:
• Free soft tissue autograft, and
• Subepithelial connective tissue grafts are available.
9. Regenerative procedures like guided tissue regeneration
(GTR) using various barrier membranes have been used
successfully to cover the denuded roots.
10. Frenectomy is the complete removal of frenum including
its attachment to the bone. Whereas frenotomy is the
incision and relocation of the frenum to create a zone of
attached gingiva between the gingival margin and the
frenum.
REVIEW QUESTIONS
1. Enumerate various mucogingival problems.
2. Describe the techniques to increase width of attached
gingiva.
3. Define gingival recession. Write about the etiology,
classification and procedures for covering the
denuded root surfaces.
4. Differences between frenectomy and frenotomy.
BIBLIOGRAPHY
1. American Academy of Periodontology. Proceedings of the
World Workshop in Clinical Periodontics. Annals of
Periodontology, Chicago 1996, The Academy.
2. Cortellini P, Clauser C, Pini-Prato GP. Histologic assessment
of new attachment following the treatment of a human buccal
recession by means of a guided tissue regeneration procedure.
J Periodontol 1993; 64: 387.
3. Miller PD Jr. Root coverage using a free soft tissue autograft
following citric acid application. A successful and predictable
procedure in areas of deep wide recession. Int J Periodont
Restor Dent 1985;5:15.
4. Miller PD Jr, Allen EP. The development of the periodontal
plastic surgery. Periodontol 2000. 1996;2-7.
5. Myron Nevins, James T Mellonig. Periodontal therapy clinical
approaches and evidence of success, Volume-1,
Quintessence Publishing Co. Inc. (1998).
6. Newman, Takei, Fermin A. Caranza. Clinical Periodontology,
9th Edition, WB Saunders.
7. Preston D Miller, Jr Periodontal plastic surgery. Curr Opin
Periodontol 1993;111-14.
8. Philippe Bouchard, Jacques Marlot, Alain Borghetti. Decision
making in aesthetics: Root coverage revisited. Periodontol
2000, 2001;27.
9. Pini-Prato G, Tinti-C, Vincenzi-G, et al. Guided tissue
regeneration versus mucogingival surgery in the treatment
of human buccal gingival recession. J Periodontol
1992;63:919.
365
Furcation Involvement and Its Management
DEFINITION
Furcation involvement refers to commonly occurring
conditions in which the bifurcations and trifurcations
of multi-rooted teeth are invaded by the disease
process.
ETIOLOGY
Primary Etiologic Factor
It is bacterial plaque and long-standing inflammation of
periodontal tissues.
The extent of attachment loss in the furcation defect
is related to:
Anatomic Considerations (Fig. 44.1)
1. Root trunk length—It is the portion of the root
between cementoenamel junction and the separation
of the roots (Fig. 44.2).
2. Root separation—It is the portion of the tooth where
adjacent roots forming the furcation are not in contact
with each other and are separated by alveolar bone.
3. The surface of the tooth just coronal to the root sepa-
ration. This area is usually concave, grooved or fluted.
4. The roof of furcation which contains furcation ridges.
These ridges run mesiodistally in lower and bucco-
lingually in upper molars.
The roof of the furcation is concave in about 50
percent of maxillary first molars and is located 4.5 mm
apical to the cemento enamel junction. The roof is 0.5
to 1 mm coronal to the root separation. Access to this
area is extremely difficult for instrumentation of deep
pockets.
Anatomic and clinical characteristics of tooth, bone
and gingiva are of importance for the clinical manage-
ment of furcation lesions.
Tooth
The following features should be considered:
a. Root trunk length—When the root trunk is short, the
furcation will become involved early in the disease
process. When the root trunk is long the furcation
will be invaded later, but will be more difficult for
instrumentation.
❒
❒
❒
❒
❒ DEFINITION
❒
❒
❒
❒
❒ ETIOLOGY
❒
❒
❒
❒
❒ CLASSIFICATION
❒
❒
❒
❒
❒ CLINICAL FEATURES
❒
❒
❒
❒
❒ PROGNOSIS
❒
❒
❒
❒
❒ TREATMENT
❒
❒
❒
❒
❒ ROOT RESECTION AND SEPARATION
44
Furcation Involvement
and Its Management
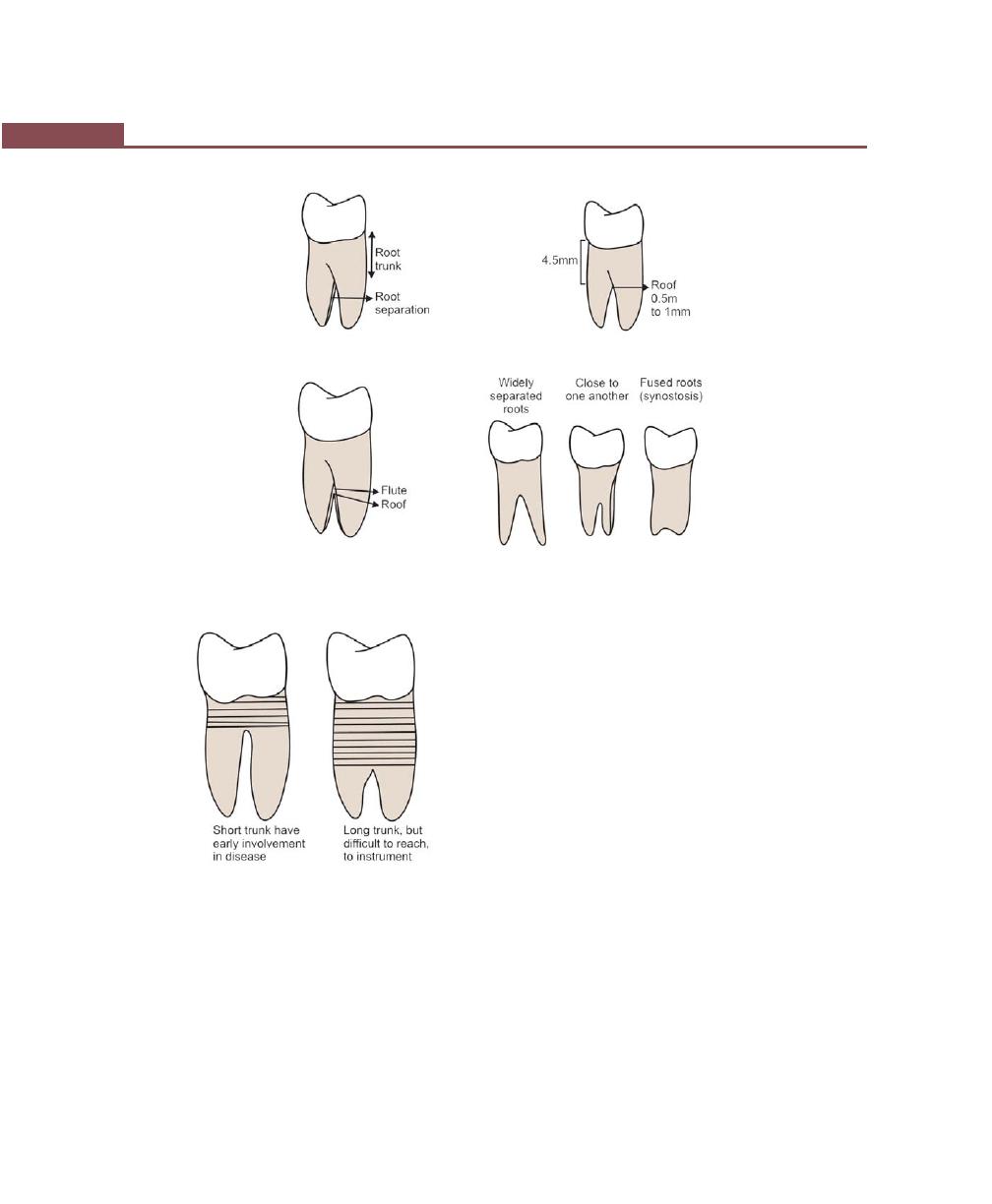
366
Essentials of Clinical Periodontology and Periodontics
Fig. 44.1:
Anatomic considerations
Fig. 44.2:
Relationship between disease involvement and
trunk length
b. Concavity of the inner surface of exposed roots—
All the root surfaces facing the furcation exhibit some
degree of concavity or depression in an occluso-apical
direction. This may make instrumentation for plaque
removal and root planing almost impossible. But
these concavities increases the attachment area of a
tooth and produce a root shape that is resistant to
torque. It is common in mesiobuccal root of maxillary
first molar and mesial root of mandibular first molar.
c. Degree of separation of roots/inter-radicular
dimension—Wide separation of roots improves
access, thereby facilitating instrumentation.
d. Cervical enamel projections—They occur approxi-
mately in 15 percent of molars. They favor plaque
accumulation and must be removed to facilitate
scaling and root planing.
It was classified by Masters and Hoskins in 1964 as:
Grade I: The enamel projection extends from the
cementoenamel junction of the tooth towards the
furcation entrance.
Grade II: The enamel projection approaches the
entrance to the furcation but does not enter the
furcation and hence has no horizontal component.
Grade III: The enamel projection extends horizontally
into the furcation.
e. Anatomy of the furcation—Presence of bifurcation
ridges, presence of accessory canals can complicate
the furcation treatment.

367
Furcation Involvement and Its Management
f. Presence of accessory pulpal canals—It is believed
that once the pulp is infected through the accessory
canal, endo-perio communication may result, which
in turn can cause either destruction of inter radicular
periodontium or interfere with the healing response
of either periodontal or endodontic procedures.
Bone
Bone shape in the exposed furcation area has a horizontal
component that determines the grade (I, II and III) of the
involvement and a vertical component that most often
creates a depression in the center of the remaining bone
similar to a crater in an interdental area. The vertical
component can also appear as a vertical or angular loss
towards one of the roots. The latter defect can have one,
two or three osseous walls or can be funnel-shaped around
one root.
Gingiva
The presence of sufficient attached keratinized gingival
tissue and adequate vestibular depth will facilitate the
gingival management of the furcation area.
CLASSIFICATION
Glickman in 1953 (Fig. 44.3) had classified
furcation involvement into:
Grade I: It is the incipient or early lesion. The pocket
is suprabony, involving soft tissue. There is slight bone
loss in the furcation area. No radiographic changes.
Grade II: In grade II cases bone is destroyed on one
or more aspects of the furcation, but a portion of alveolar
bone and periodontal ligament remains intact, permitting
only partial penetration of the probe into the furcation.
The lesion is essentially a cul-de-sac. The radiograph may
or may not reveal the grade II involvement.
Grade III: In this type of furcation involvement, the inter-
radicular bone is completely lost but the facial or lingual
surfaces are occluded by gingival tissues. Therefore, the
furcation opening can not be seen clinically, but it is
essentially a through and through tunnel. If the
radiographs are taken with proper angulation and the
roots are divergent, the lesion will appear as a radiolucent
area between the roots.
Grade IV: As in grade III lesions, the inter-radicular bone
is completely lost but in grade-IV involvement the gingival
tissues recede apically so that the furcation opening is
seen clinically. The radiographic changes are essentially
the same as that of grade-III lesion.
Based on vertical component (Tarnow and
Fletcher) (Fig. 44.4)
Depending on the distance from the base of the defect to
the roof of the furcation, furcations can be classified as:
Subgroup A: Vertical destruction of bone upto one-third
of the inter-radicular height (0-3 mm).
Subgroup B: Vertical destruction of bone upto two-third
of the inter-radicular height (4-7 mm).
Subgroup C: Vertical destruction beyond the apical-third
(7 mm or more).
Figs 44.3A to D:
Glickman’s classification of furcation
involvement. (A) Grade I, (B) Grade II, (C) Grade III,
(D) Grade IV
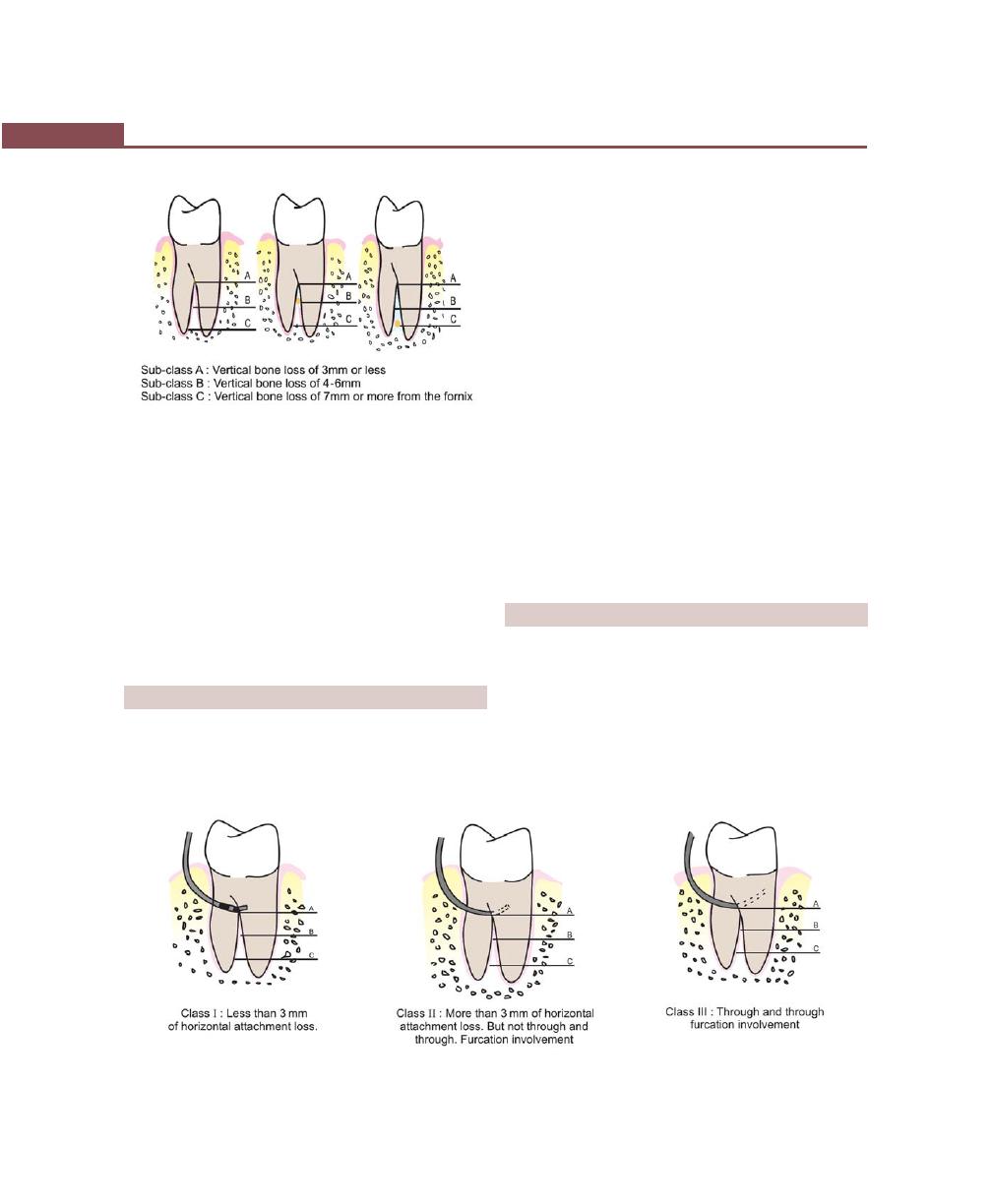
368
Essentials of Clinical Periodontology and Periodontics
Based on horizontal component (Hamp and
Coworkers) (Fig. 44.5)
Furcations can be classified as:
Degree-I : Horizontal bone loss of less than 3 mm.
Degree-II : Horizontal bone loss of more than 3 mm.
Degree-III : Through and through horizontal lesion.
Furcation can be clinically-detected by using Naber’s
probe along with a simultaneous blast of warm air to
facilitate visualization and radiographs also help to detect
the furcation invasions.
CLINICAL FEATURES
Clinically
1. The mandibular first molars are the most common
sites and maxillary premolars are the least common.
2. The denuded furcation may be visible clinically or
covered by the wall of the pocket.
3. Associated with suprabony and infrabony pockets.
4. Periodontal abscess.
5. Root caries and tooth mobility are common.
Microscopically
It is simply a phase in the rootward extension of the
periodontal pocket. In its early stages, there is a widening
of the periodontal space with cellular and inflammatory
fluid exudation, followed by epithelial proliferation into
the furcation area from an adjoining periodontal pocket.
Extension of the inflammation into the bone leads to
resorption and reduction in bone height. The bone
destructive pattern may produce horizontal loss, or there
may be angular osseous defects associated with infrabony
pockets. Plaque, calculus and bacterial debris occupy
the denuded furcation space.
PROGNOSIS
Maxillary first premolars and maxillary as well as
mandibular molars are the teeth with multiple roots.
Furcation involvement will be likely to occur in these
multirooted teeth. Maxillary first premolar often shows
fusion of the roots and the furcation area may be located
very much apically and also the roots of the maxillary
first premolars are placed buccally and palatally with
Fig. 44.4:
Vertical classification of furcation involvement
Fig. 44.5:
Horizontal classification of furcation involvement (Hamp and coworkers)

369
Furcation Involvement and Its Management
furcation opening in a mesiodistal direction. For these
reasons, furcation involvement in maxillary first premolar
has poor prognosis.
In the case of maxillary molars furcations may open
bucally, mesially and distally because of the presence
of the three roots. Since access from proximal areas is
difficult for plaque control, prognosis of furcation
involvement in maxillary molars is not good.
Mandibular molars have two roots, placed mesially
and distally and the furcation opens buccolingually. The
roots are usually divergent especially in mandibular first
molars. As a result prognosis of furcation involvement
in mandibular molar (especially the first molar) is
considered good.
TREATMENT
It is aimed to prevent further attachment loss and
improve the maintenance of furcation area. Two
treatment modalities have been proposed,
1. Traditional treatment procedures.
2. Reconstructive or regenerative treatment.
Factors to be considered when deciding on a mode
of therapy are as follows:
1. Degree of involvement.
2. Crown root ratio.
3. Length of roots.
4. Degree of root separation.
5. Strategic value of the tooth or teeth in question.
6. Root anatomy of the involved tooth.
7. Residual tooth mobility.
8. Endodontic therapy and complications.
9. Ability to eliminate the defect.
10. Periodontal condition of the adjacent teeth.
Traditional Treatment Procedures
They are those which are directed to maintain the state
of the health but do not attempt to regenerate the lost
periodontium. The goal is to prevent the further
progression of the disease and provide an environment
which will help in adequate plaque control.
The procedures are:
Grade I: They are usually associated with suprabony
pockets, hence,
a. Initial preparation or scaling and root planing.
b. Curettage or gingivectomy to expose the furcation
area.
c. Odontoplasty—to reshape the facial groove in order
to prevent plaque accumulation.
Grade II: In shallow grade-II invasions,
a. Osteoplasty with limited ostectomy may be helpful.
b. Odontoplasty can be performed.
In severe grade-II to IV invasions elimination of
furcation by:
a. Root resection or amputation: After periodontal flap
reflection, surgical removal of the root portion of the
affected tooth is most commonly performed in
maxillary first molars.
b. Hemisection or root separation: It is the surgical
removal of the root along with the crown. Most
commonly done in mandibular molars.
c. Bicuspidization/root separation: Splitting of a two-
rooted tooth into two separate portions. Frequently
performed in mandibular molars.
d. Tunnel preparation: It is by transforming the grade
II lesion to grade III and IV for better access, but it
is not performed anymore because of increased
incidence of root caries.
Grade III and grade IV can be treated with root
resection and root separation.
Reconstructive and Regenerative
Treatment Procedures (Figs 44.9A to G)
(Discussed in detail elsewhere in this book)
Grade I: Traditional treatment will do.
Grade II: Various regenerative techniques include
a. Autogenous bone grafting, e.g. osseous coagulum,
bone blend.
b. Allografts, e.g. freeze, dried bone allografts, demine-
ralized freeze-dried bone allografts (FDBA, DFDBA).
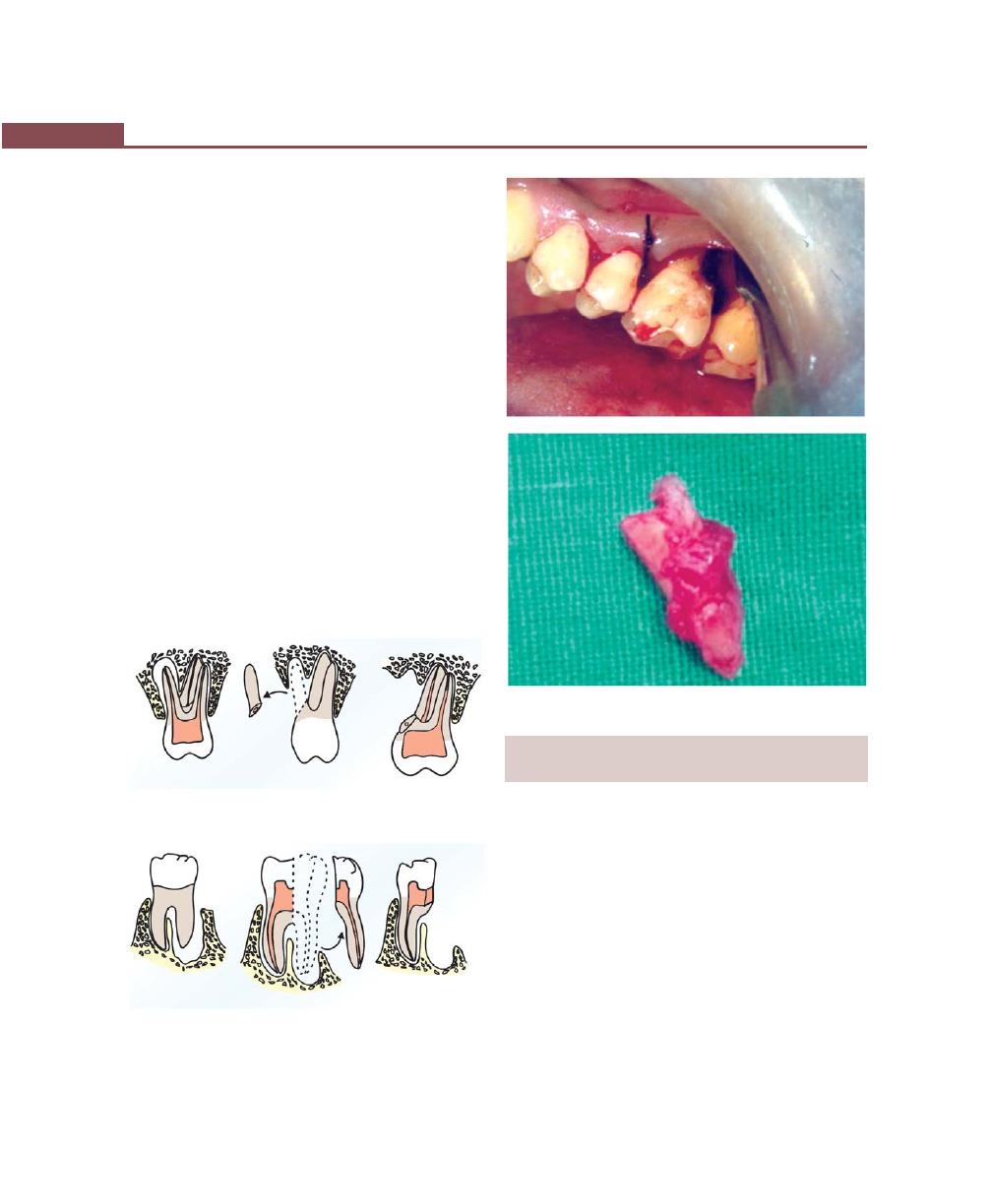
370
Essentials of Clinical Periodontology and Periodontics
c. Alloplasts—hydroxyapatite, tricalcium phosphate.
d. Citric acid root conditioning with coronally positioned
flap.
e. Guided tissue regeneration and combination
techniques.
For grade III and grade IV furcation involvements
the success rate is limited.
Definition of Root Resection, Hemisection and
Root Separation (Figs 44.6 to 42.8)
(According to 1986 glossary of periodontal terms).
Root resection: It is the surgical removal of all or a portion
of the root before or after endodontic treatment.
Hemisection: It is the surgical removal of a root with
the associated part of the crown. It is frequently used
with reference to lower molars.
Root separation/bicuspidization: It is the sectioning of
the root complex and the maintenance of all roots.
Fig. 44.7:
Hemisection
Fig. 44.6:
Root separation/resection
Figs 44.8A and B:
Root separation and resection
INDICATIONS AND CONTRAINDICATIONS
FOR ROOT RESECTION AND SEPARATION
Indications
a. Severe bone loss affecting one or more roots
untreatable with regenerative procedures.
b. Class II or III furcation invasions or involvement.
c. Severe recession or dehiscence of a root.
Contraindications
a. General contraindications like systemic diseases and
poor oral hygiene.
b. Fused roots, unfavorable tissue architecture.
c. Roots that are endodontically-untreatable.
A
B
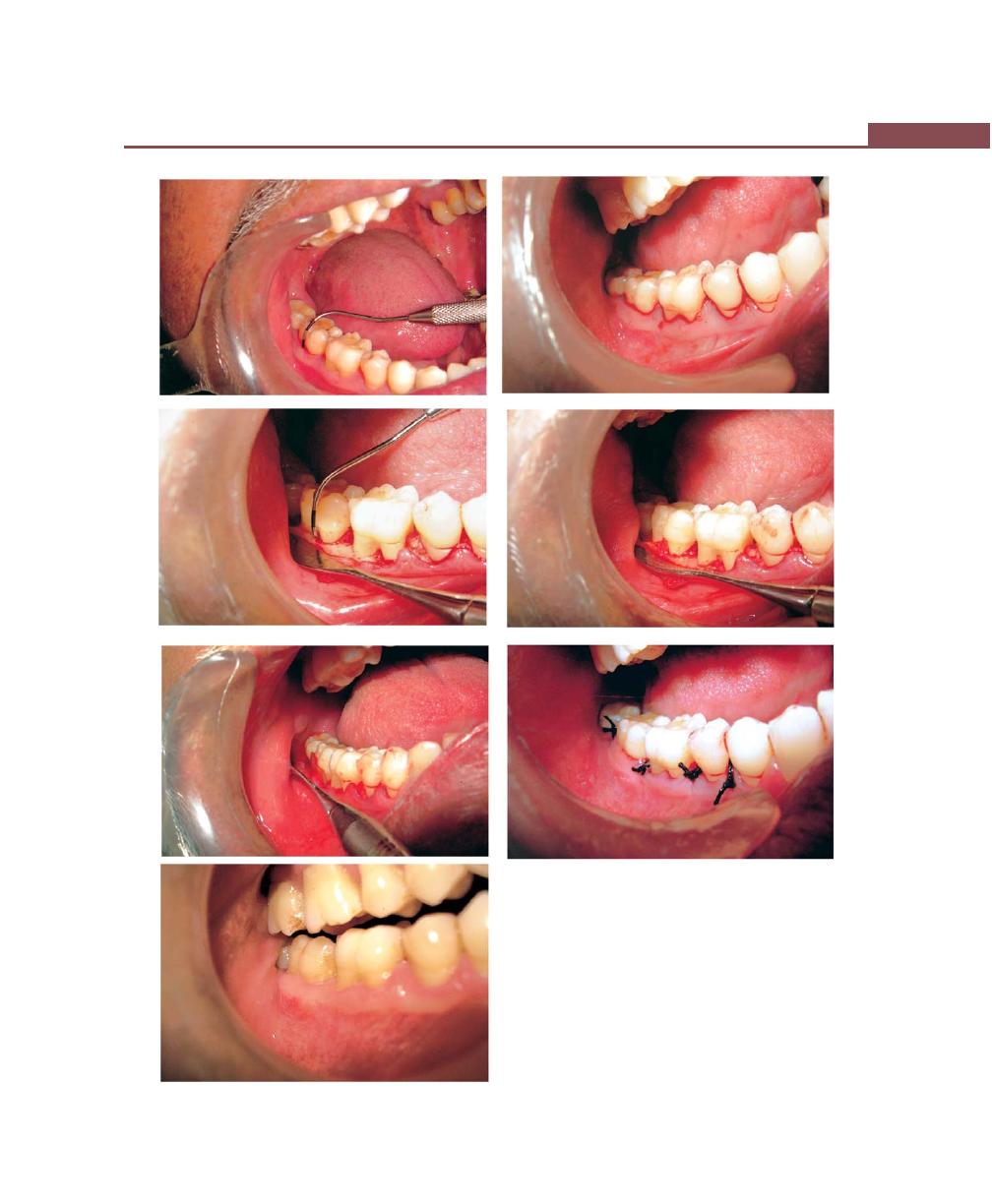
371
Furcation Involvement and Its Management
Figs 44.9A to G:
Furcation involvement. (A) Preoperative,
(B) Incision placement, (C) Reflection of flap, (D) Bone graft
placement, (E) Membrane placement, (F) Suturing, (G) Facial
view after healing
A
B
C
D
E
F
G
372
Essentials of Clinical Periodontology and Periodontics
Three phases of treatment is suggested for root
separation and resection.
a. Endodontic phase—If possible it should be perfor-
med prior to surgery. It offers the following
advantages.
• Better bone recontouring during surgery.
• Allows precise flap closure.
• Easy adaptation for temporary prosthesis.
b. Restorative phase—Construction of a provisional
restoration.
c. Surgical phase—Most commonly distobuccal root of
the maxillary first molar is resected.
The procedure is as follows:
1. After appropriate local anesthesia a full thickness
mucoperiosteal flap is raised.
2. After debridement, resection of the root with
advanced bone loss is carried out. First an oblique
cut/incision is made directed from apical to the contact
point through the tooth to the facial and distal orifices
of the tooth. In case of vital resection, it is advised
to perform a horizontal incision through the root
because an oblique incision can expose a large surface
area of the radicular pulp or pulp chamber which
inturn can lead to more postoperative pain.
3. After sectioning, the root is elevated from its
socket and is removed. If necessary odontoplasty is
performed to remove any furcation deformities .If
any bony defects are present in the adjacent teeth,
then resective or regenerative procedures are
performed.
4. The flaps are then approximated and sutured to
maintain the position.
KEY POINTS TO NOTE
1. Furcation involvement refers to commonly occurring
conditions in which the bifurcation and trifurcation of multi-
rooted teeth are invaded by the disease process.
2. Primary etiologic factor is bacterial plaque, predisposing or
anatomic factors include, aberrant root morphology, cervical
enamel projections or pearls and presence of accessory
canals.
3. Furcation involvement can be classified according to
Glickman as Grade I, Grade II, Grade III and Grade IV.
4. Depending on the distance from the base of the defect to
the roof of the furcation, (vertical component) Tarnow and
Fletcher classified furcation involvement into—subgroup A,
subgroup B, subgroup C.
5. Furcation involvement can be diagnosed by Naber’s probe
and radiographs.
6. Treatment of furcation involvement is divided into:
a. Traditional treatment procedures.
b. Reconstructive or regenerative treatment.
REVIEW QUESTIONS
1. Define and classify furcation involvement. Describe
the treatment for Grade II furcation involvement.
2. What are the indications and contraindications for
root resection/separation?
BIBLIOGRAPHY
1. Alan M. Polson. Periodontal Regeneration, Current Status and
Direction. Quitessence Publishing Company, Inc.
2. Jan Lindhe. Clinical Periodontology and Implant Dentistry.
Fourth edition (2003), Blackwell Munksgaard Publication.
3. Myron Neyin, James T. Mellonig. Periodontal Therapy,
Clinical Appraoches and Evidence of Success. Vol 1,
Quintessence Publishing Co, Inc (1998).
4. Massimode Sanctis, Giovan Paolo Piniprato. Root resection
and root amputation. Curr Opin Periodontol 1993;105.
5. Steven Garette, Gary Bogle. Periodontal regeneration with
bone grafts. Curr Opin Periodontol 1994;187-93.
373
Pulpoperiodontal Problems
INTRODUCTION
Normally, periodontitis is caused by extension of
inflammation from the gingiva into deeper periodontal
tissues. However, periodontitis can also be caused by
pulpal infections that have entered the periodontal
ligament either through the apical foramen or through
the lateral canals. Such a periodontal lesion is termed
as “retrograde periodontitis.” Similarly, “retrograde
pulpitis” can also occur as a result of periodontal disease
and periodontal treatment.
PATHWAYS OF COMMUNICATION
BETWEEN PULP AND PERIODONTIUM
(Fig. 45.1)
It can be classified into three categories:
1. Pathways of developmental origin.
• Apical foramen
• Accessory canals and lateral canals.
• Developmental grooves.
• Enamel projections and pearls at the cervical
portion.
2. Pathways of pathologic origin
• Tooth fracture (vertical).
• Idiopathic resorption can be:
a. Internal: From the pulp to the surface of the
tooth.
b. External: From the external surface of the
root to the pulp.
Both internal and external resorption
produces communication.
• Loss of cementum due to external irritants.
3. Pathways of iatrogenic origin
• Exposure of dentinal tubules following root
planing.
• Accidental lateral perforation during endodontic
procedure.
• Root fracture due to endodontic procedure.
EFFECTS OF PULPAL DISEASE
ON THE PERIODONTIUM
The three major causes of pulpal inflammation are:
❒
❒
❒
❒
❒ PATHWAYS OF COMMUNICATION
BETWEEN PULP AND PERIODONTIUM
❒
❒
❒
❒
❒ EFFECTS OF PULPAL DISEASE ON
PERIODONTIUM
❒
❒
❒
❒
❒ EFFECTS OF PERIODONTITIS ON THE
DENTAL PULP
❒
❒
❒
❒
❒ ENDODONTIC-PERIODONTAL LESIONS
• Classification
• Microbiological Findings
• Diagnosis and Treatments
45
Pulpoperiodontal
Problems
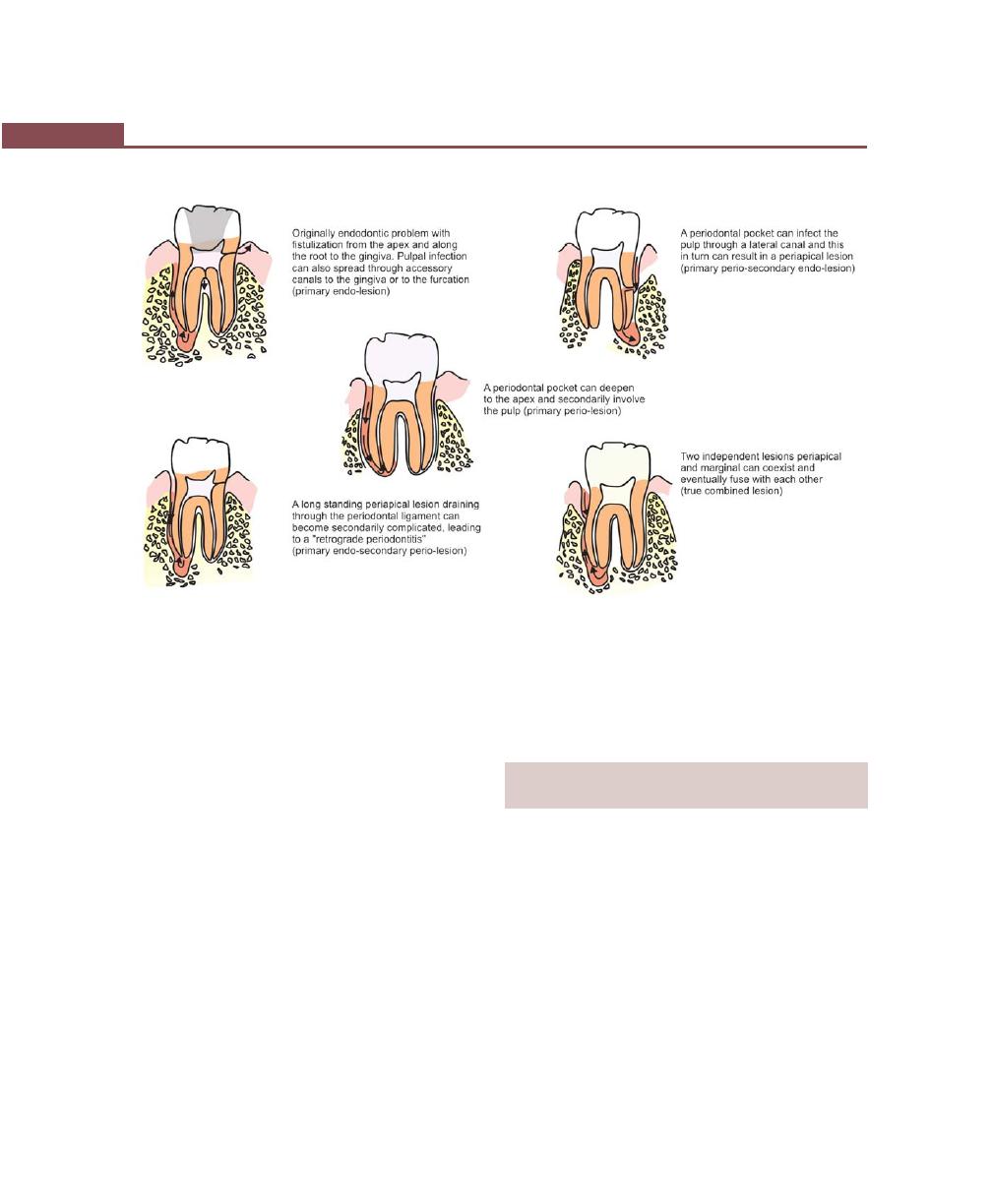
374
Essentials of Clinical Periodontology and Periodontics
Fig. 45.1:
Possible pathways for spread of infection between pulp and periodontal tissues
1. Instrumentation during periodontal, restorative or
prosthetic procedures.
2. Progression of dental caries.
3. Tooth fractures.
Of these dental caries is the most common cause of
pulpal disease. As long as the pulp remains vital, the
inflamed pulpal tissue is unlikely to cause any change
in the periodontium. But acute inflammation of the pulp
increases the intrapulpal pressure and the inflammatory
fluid may be pushed out through the apical foramen
or accessory canals. This results in periapical abscess
which may drain through the periodontal ligament into
the gingival sulcus and a periodontal pocket may form
in due course of the time. Similar lesions may develop
adjacent to accessory or lateral canals.
Accessory canals branch off and run parallel to the
main root canal and are seen in the apical-third of the
roots and furcation areas of multi-rooted teeth, whereas
lateral canals run perpendicular to the root canal and
are seen in greater number in the middle-third of the
root. Passage of infection from the pulp into the furcation
area through the accessory canals has been reported
by many authors.
EFFECTS OF PERIODONTITIS ON
THE DENTAL PULP
Bender and Seltzer (1972) in their studies have reported
that teeth with caries or restorations also suffering from
periodontal disease have more atrophic pulps than teeth
with caries or restorations, but no periodontal disease.
The cause of these atrophic changes (which is observed
radiographically as narrowed canal space) is the disruption
of blood flow through the lateral canals, which leads to
localized areas of coagulation necrosis in the pulp.
Subgingival scaling and root planing may also produce
changes in the pulp, one possible explanation for this
is that blood vessels leading into lateral canals are severed
causing localized areas of pulpal necrosis (Fig. 45.2).
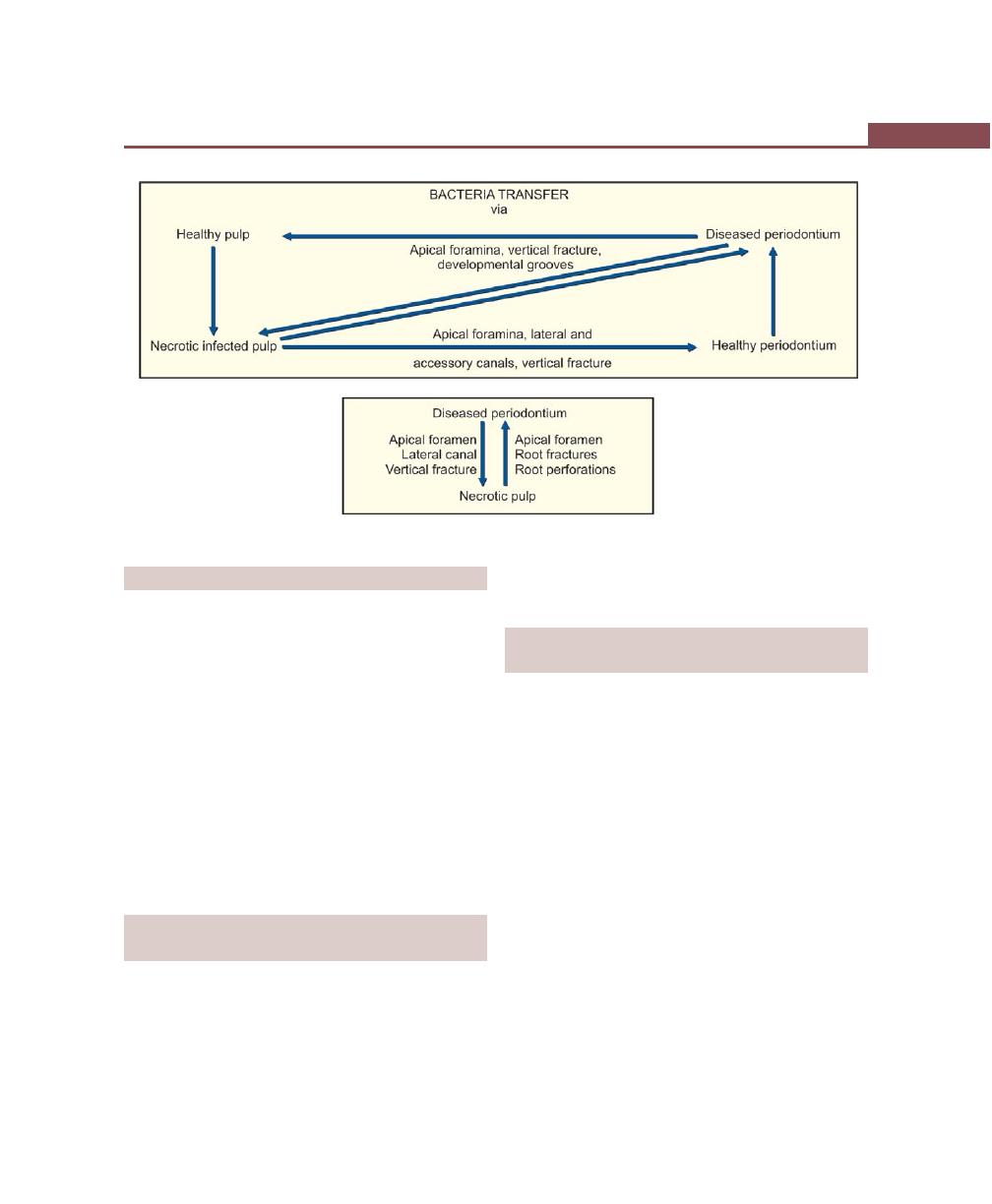
375
Pulpoperiodontal Problems
Fig. 45.2:
Schematic diagram showing possible pathways for spread of infection between pulp and periodontal tissue
CLASSIFICATION OF ENDO-PERIO LESIONS
Pulpoperiodontal lesions have been classified by many
authors based on different criteria. Classification propo-
sed by Simon et al (1972) is most commonly employed.
Based on the etiology, diagnosis, prognosis and
treatment, endo-perio lesions can be classified into five
groups (Table 45.1)
1. Primary endodontic lesion.
2. Primary endodontic with secondary periodontal
lesion.
3. Primary periodontal lesion.
4. Primary periodontal lesion with secondary
endodontic involvement.
5. True combined lesions.
MICROBIOLOGICAL FINDINGS OF
ENDO-PERIO LESIONS
Bacteria play an important role in the pathogenesis of
both pulpal and periodontal disease. There seems to be
significant similarity between the microbiological findings
of root canals and pockets with advanced periodontitis.
B. forsythus, P. gingivalis and T. denticola, Fusobacteria,
Spirochetes, Wolinella and Peptostreptococcus have been
found in endo-perio lesions.
DIAGNOSIS AND TREATMENT OF
ENDO-PERIO LESIONS
When one suspects the possible endodontic periodontal
lesion, the first task is to assess endodontic status of the
tooth in question. Traditional diagnostic aids including,
radiographic analysis with gutta percha tracing, perio-
dontal probing, fiber optic illumination to rule out
whether a fracture exists, more importantly vitality test,
percussion tests should be carried out. Numerous studies
have demonstrated the inaccuracy of vitality testing.
Probably because pulp testing only indicates the neural
response and gives little information about the vascularity
or true vitality of the pulp. Research is currently under
way to improve diagnostic testing through the use of
Doppler devices, pulse oximetry and even magnetic
resonance imaging.
Treatment
Some controversy seems to exist, as to whether
endodontic therapy or periodontal therapy has to be
376
Essentials of Clinical Periodontology and Periodontics
Table 45.1: Classification of endo-perio lesions
Type of lesion
Sequelae
Diagnosis
Treatment
Prognosis
Primary
It is characterized by
Pulp vitality tests
Conventional root canal
It is excellent.
endodontic
necrotic pulp with a chronic are negative.
therapy should be per-
Healing is usually complete
lesion
apical periodontitis and a
formed with multiple
within 3 to 6 months.
draining sinus tract through
appointments.
the periodontal ligament
No root planing
and gingival sulcus.
should be done when
There may not be any
the sinus tract is along
periodontal involvement
the periodontal ligament,
in the other areas. The
because these fibers are
sinus tract can be probed,
important for re-attach-
with gutta percha and
ment to occur.
silver points.
Radiographically, no
crestal bone loss.
Primary
If primary endodontic
Pulp vitality tests
First endodontic therapy
Endodontic component is
endodontic
lesion is not diagnosed
are negative. Perio-
including root canal thera-
excellent but regeneration
lesion with
and treated early, secon-
dontal probing will
py. Periodontal therapy in
of attachment apparatus
secondary
dary periodontal problem
reveal deep perio-
the form of root planing.
is limited by the perio-
periodontal
may result due to accu-
dontal pocket in
dontal prognosis.
involvement
mulation of plaque and
this area.
calculus in the drianage
fistulous tract.
Radiographically, bone
loss may be evident.
Primary
Periodontal pocket which
Pulp is vital, perio-
Periodontal therapy to
It is entirely dependent
periodontal
extends upto the apex of
dontal probing, may
eliminate the pocket is
on the periodontal
lesion
the tooth.
reach the apex of
indicated.
therapy.
Spread of infection from
the involved teeth.
Root canal therapy is not
the pocket into the pulp
The periodontal
usually indicated unless
may occur in these cases.
problems are seen
pulp vitality test results
In the pulp fibrosis
on the other teeth
change. Periodic reevalu-
and calcification is noticed
also.
tion is necessary.
but not necrosis.
Minimal/no pain is
experienced by the
patient.
Primary
Primary periodontal
Similar to those
Both endodontic and
It is dependent on the
periodontal
lesion may involve
used in primary
periodontal therapy.
periodontal therapy,
lesion with
necrosis and patient may
endodontic and
healing response of peri-
endodontic
experience severe pain.
secondary perio-
apical lesion is not predi-
involvement
Pulpal necrosis could be
dontal lesions.
ctable.
as a result of periodontal
Generalized
therapy where the blood
perioodontal
vessels to the pulp are
problem.
severed during perio-
Pulp vitality test
dontal instrumentation.
results can be mixed.
True
These are those lesions
Pulp testing is
Both endodontic and
Questionable prognosis
combined
that are formed when
negative. The tooth
periodontal therapy
lesion
pulpal and periodontal
in question will
including root resec-
pathoses develop inde-
have probing
tion and hemisection
pendently and unite.
depths upto the
is proposed.
These lesions are usually apex of the tooth.
of periodontal origin.
Teeth with vertical
root fractures also
belong to this category.
377
Pulpoperiodontal Problems
performed first, in the management of endo-perio
lesions. Considering many facts it was advised to perform
endodontic therapy prior to periodontal therapy.
KEY POINTS TO NOTE
1. When inflammation from pulp extends into the periodontium
either through the apical foramen or through the lateral
canals destruction of periodontal tissues may occur. This is
termed as ‘retrograde periodontitis’.
2. Pathways of communication between pulp and periodon-
tium could be of :
• Developmental origin.
• Pathologic origin
• Iatrogenic origin.
3. Endo-perio lesions are classified into:
• Primary endodontic lesions
• Primary endodontic with secondary periodontal lesions
• Primary periodontal lesions
• Primary periodontal lesion with secondary endodontic
involvement.
• True combined lesions.
REVIEW QUESTIONS
1. Enumerate various pulpoperiodontal problems.
2. What is retrograde periodontitis?
3. What are the effects of periodontitis on the dental
pulps?
BIBLIOGRAPHY
1. Brian F Paul, Jeffrey W Hutler. The endodontic periodontal
continuum revisited: New-insights into etiology, diagnosis
and treatment. JADA, November, 1997;128.
2. Gerald W Harrington, David R Steiner, William F Ammon Jr.
The periodontal endodontic controvery. Periodontol 2000,
2002;30.
3. Jan Lindhe. Clinical Periodontology and Implant Dentistry,
4th edn, Blackwell Munksgard Publication, 2003.
4. Zehnder M, Gold SI, Hasselgren G. Pathologic interactions
in pulpal and periodontal tissues. J Clin Periodontol 2002;
29: 663-71.
378
Essentials of Clinical Periodontology and Periodontics
DEFINITION
Dental splinting: It is defined as the joining of two or
more teeth into a rigid unit by means of fixed or remova-
ble restorations/devices. Splint by definition is an appliance
used for immobilization of injured or diseased parts.
A periodontal splint: It is an appliance used for
maintaining or stabilizing mobile teeth in their functional
position.
The main objective of splinting is to promote
healing and to increase the patients comfort and function.
There are two schools of thought regarding the use of
splinting.
Critical Aspects
• It creates an environment for plaque accumulation.
• Since functional movement of the tooth within the
socket is not possible it may lead to ankylosis.
Beneficial Aspects
• Since they are splinted to the neighboring healthy
teeth, mobility during mastication is prevented.
• Non-mobile teeth heal faster than mobile teeth.
OBJECTIVES OF SPLINTING
1. Provides rest.
2. For redirection of forces—the forces of occlusion are
redirected in a more axial direction over all the teeth
included in the splint.
3. For redistribution of forces—redistribution ensures
that forces do not exceed the adaptive capacity.
4. To preserve arch integrity—splinting restores proximal
contacts, reducing food impaction and consequent
breakdown.
5. Restoration of functional stability—restores a func-
tional occlusion, stabilizes mobile abutment teeth and
increases masticatory comfort.
6. Psychologic well being—gives the patient comfort
from mobile teeth thereby giving him a sense of well
being.
7. To stabilize mobile teeth during surgical, especially
regenerative therapy.
8. To prevent the eruption of unopposed teeth.
❒
❒
❒
❒
❒ DEFINITION
❒
❒
❒
❒
❒ OBJECTIVES
❒
❒
❒
❒
❒ CLASSIFICATION AND TYPES
❒
❒
❒
❒
❒ PRINCIPLES
❒
❒
❒
❒
❒ INDICATIONS AND
CONTRAINDICATIONS
❒
❒
❒
❒
❒ ADVANTAGES AND DISADVANTAGES
46
Splints in
Periodontal Therapy

379
Splints in Periodontal Therapy
CLASSIFICATION OF SPLINTS
According to the Period of Stabilization
a. Temporary stabilization—worn for less than 6 months,
e.g. Removable/Fixed
b. Provisional stabilization—to be worn for months or
upto several years, e.g. acrylic splints, metal bands.
c. Permanent splints—used indefinitely, e.g. removal/
fixed, intracoronal/extracoronal.
According to the Type of Material
i. Bonded, composite resin button splint.
ii. Braided wire splint.
iii. A-splints.
According to the Location on the Tooth
Intracoronal
• Composite resin with wire
• Inlays
• Nylon wire
Extracoronal
• Tooth-bonded plastic
• Night guard
• Welded-bands
VARIOUS COMMONLY USED SPLINTS
1. Splints for Anterior Teeth
a. Direct bonding system using acid etch techniques
and a light cured resin.
b. Intracoronal wire and acrylic wire resin splint—
It uses preparations of a slot on the lingual aspect
of the tooth, and stabilizing the teeth, using a
stainless steel wire placed in the slots.
2. Splints for Posterior Teeth
a. Intracoronal amalgam wire splints—It uses resin
restoration with wire on the proximal amalgam
restored areas of the tooth.
b. Bite-guard.
c. Rigid occlusal splint.
d. Composite splint.
PRINCIPLES OF SPLINTING
1. Inclusion of sufficient number of healthy teeth: It is
suggested that the healthy teeth included in the splint
should have double the root surface area of the
mobile teeth. Since the posterior teeth are multi-
rooted the number of healthy teeth to be included
in the splint in the posterior segment will be less as
compared to the anterior.
2. Splint around the arch: Muscles of the lips, cheek
and tongue exert some forces on the teeth. Based
on the direction of such forces applied on the teeth,
the dental arch can be divided into two posterior
sextants and an anterior sextant. In the posterior
sextant the tongue pushes the teeth buccally and the
muscles of the cheek counter act it by pushing them
lingually. When the splint is confined to any one
sextant, the splinted teeth tend to tilt lingually or
outwards depending on the muscular forces. Such
a collapse of the splinted sextant can be prevented
by including few teeth from the adjacent sextant. This
is termed splinting around the arch.
3. Coronoplasty may be performed to relieve traumatic
occlusion.
4. The splint should be fabricated in such a way as to
facilitate proper plaque control.
5. Splint should be aesthetically-acceptable and should
not interfere with occlusion.
INDICATIONS AND CONTRAINDICATIONS
OF SPLINTING
Indications
1. It stabilizes moderate to advanced tooth mobility that
can not be reduced by other means and which has
not responded to occlusal adjustment and periodontal
therapy.
2. When it interferes with normal masticatory function.
3. Facilitates scaling and surgical procedures.
4. Stabilizes teeth after orthodontic movement.
5. Stabilizes teeth after acute dental trauma. e.g.
subluxation, avulsion etc.

380
Essentials of Clinical Periodontology and Periodontics
6. In order to prevent tipping and drifting of teeth.
7. Prevent extrusion of unopposed teeth.
Contraindications
1. Moderate to severe tooth mobility in the presence
of periodontal inflammation and/ primary occlusal
trauma.
2. Insufficient number of firm/sufficiently firm teeth to
stabilize mobile teeth.
3. Prior occlusal adjustment has not been done on teeth
with occlusal trauma or occlusal interference.
4. Patient not maintaining oral hygiene.
ADVANTAGES
1. May establish final stability and comfort for patient
with occlusal trauma.
2. Helpful to decrease mobility and accelerate healing
following acute trauma to the teeth.
3. Allows remodelling of alveolar bone and periodontal
ligament for orthodontically, splinted teeth.
4. Helpful in decreasing mobility thereby favoring
regenerative therapy.
5. Distributes occlusal forces over a wider area.
DISADVANTAGES
1. All the splints hamper patient’s self care. Accumu-
lation of plaque at the splinted margins can lead to
further periodontal breakdown in a patient with
already compromised periodontal support.
2. Number of studies have shown that splinting does
not actually reduce tooth mobility (once the splint
is removed).
3. The splint being rigid acts as a lever with uneven
distribution of forces, even if one tooth of the splint
is in traumatic occlusion, it can injure the
periodontium of all the teeth within the splint.
4. Development of caries is an unavoidable risk. It
requires excellent maintenance by the patient.
In conclusion splinting decreases mobility thereby
improving the health of the tooth. It is important to note
that if used incorrectly or not managed properly it may
lead to failure of desired results .
REVIEW QUESTIONS
1. Define and classify periodontal splints.
2. What are the advantages and disadvantages of
periodontal splints?
BIBLIOGRAPHY
1. Ericsson I, Giargia M, Lindhe J, et al. Progression of
periodontal tissue destruction at splinted /non-splinted teeth.
An experimental study in the dog. J Clin Periodontol 1993;
10: 693.
2. Saul Schluger. Periodontal Disease: Basic Phenomena,
Clinical Management and Occlusal and Restorative
Interrelationships, Second Edition, Lea and Febiger
Publication.
3. Sture R Nyman, Niklous P Lang. Tooth mobility and the
biological rationale for splinting teeth. Periodontol 2000,
1994; 4.
381
Dental Implants: Periodontal Considerations
INTRODUCTION
The concept of replacing missing teeth for esthetics and
function have been an elusive goal for more than 1500
years. This has led to the evolution of many materials
and techniques including complete dentures, removable
and fixed partial dentures. To overcome the limitations
of these materials and techniques, dentistry has long
sought a superior method of artificial tooth replacement
through dental implants with a goal of restoring the
normal contour, comfort, esthetics, health and the most
traditional dental disciplines, which include the bone and
soft tissue reconstruction.
TERMINOLOGY
Dental implant is an integral component of the oral
implant complex, which also consists of supportive bone,
interposed keratinized and mucosal oral soft tissues and
prosthetic suprastructure.
A dental implant is a permucosal device that is
biocompatible and biofunctional and is placed on or
within the bone associated with the oral cavity to provide
support for fixed or removable prosthesis.
Oral implantology is the science and discipline
concerned with the diagnosis, design, insertion,
restoration and for management of alloplastic or
autogenous oral structures to restore the loss of contour,
comfort, function, esthetics, speech and/or health of the
partially or completely edentulous patient.
Implant surgery is that part of reconstructive surgery
that is concerned with the placement of endosseous,
subperiosteal and transosseous implants for the
restoration and maintenance of mastication and speech.
Such surgery may also eliminate chronic pain related
to nerve dehiscence, preserving remaining bone structure
and prevent the potential for pathologic fracture.
Osseointegration: Direct structural and functional
connection between ordered living bone and the surface
of the load carrying implant.
❒
❒
❒
❒
❒ TERMINOLOGY
❒
❒
❒
❒
❒ HISTORICAL BACKGROUND
❒
❒
❒
❒
❒ BIOLOGICAL CONSIDERATIONS
• Soft Tissue Implant Interface
• Bone Implant Interface
❒
❒
❒
❒
❒ BIOMATERIALS USED FOR IMPLANTS
❒
❒
❒
❒
❒ CLASSIFICATION OF IMPLANTS
❒
❒
❒
❒
❒ CLASSIFICATION OF IMPLANT SYSTEMS
❒
❒
❒
❒
❒ TREATMENT PLANNING
❒
❒
❒
❒
❒ HEALING FOLLOWING IMPLANT
SURGERY
❒
❒
❒
❒
❒ PERI-IMPLANT COMPLICATIONS AND
DISEASES
47
Dental Implants:
Periodontal Considerations

382
Essentials of Clinical Periodontology and Periodontics
HISTORICAL BACKGROUND
The need to replace missing teeth has haunted for time
immemorial.
According to Marshall (1987) there are five distinct
eras of implant dentistry.
The Ancient Era (Before 1000 AD)
The discovery of 2,000 years old cranium with a cast
iron dental implant in France demonstrated that dental
implantology was one of the oldest existing forms of
dentistry. Ancient Egyptians attempted intraosseous
transplantation of animal teeth. A fine dark flint stone-
shaped like a tooth implanted in a Mayan Skull in Central
America during back to 600 AD.
The Medieval Era (1000 to 1799 AD)
The medieval period of implant dentistry was primarily
concerned with transplantation of teeth with implants
made up of ivory, shells and bone or transplantation
of teeth of one human into other or removing the teeth
from the impoverished people and transplanting them
into the mouths of the affluent.
The Foundation Era (1800 to 1910 AD)
During this period newer materials and methods were
developed like fabricating and inserting gold roots into
freshly extracted socket which were soldered together,
use of gold plate in the treatment of cleft palate, using
teeth made up of porcelain into which a lead coated
platinum post was fixed as an implant. Payne (1898)
gave the first clinical demonstration on implants.
The Modern Era (1910 to 1978 AD)
Implant designs were carried out with the aim of finding
a superior metal which was biocompatible by developing
screw type implants using vitallium, subperiosteal implants
and extending their framework to external oblique region
and introduction of the use of an endosseous implant
with a central post and circumferential extensions.
The Contemporary Era (1978 Till Date)
Implants developed during this era are corevent implant,
screwvent implant, swedevent implant, a hydroxy-
apatite-coated biovent implant; microvent implant,
starvent implant; sterioss, root form endoosseous
implant, step root form design.
Constant research is being conducted in the develop-
ment of newer implant systems with the aim of
developing a perfect implant which will function well
without any failure.
BIOLOGICAL CONSIDERATIONS
OF IMPLANTS
I. Soft tissue implant interface.
II. Bone implant interface.
Soft Tissue Implant Interface
The mucosal tissues around intraosseous implants form
a tightly-adherent band consisting of a dense collagenous
lamina propria covered by stratified squamous keratini-
zing epithelium.
The implant epithelium junction is analogous to the
junctional epithelium around natural teeth; in that the
epithelial cells attach to the titanium implant by means
of hemidesmosomes and basal lamina.
This evidence supports the concept that a viable
biologic seal can exist between the epithelial cells and
the implants.
A sulcus forms around the implant lined with a sulcular
epithelium that is continuous apically with the junctional
epithelium.
Collagen fibers are nonattached and run parallel to
the implant surface, owing to the lack of cementum.
Since endosseous implants are permucosal, the soft
tissue-implant interface should be considered in their
placement and maintenance. This suggests that epithe-
lium adheres to implant surfaces and has similar biological
features to the epithelium tooth interface.

383
Dental Implants: Periodontal Considerations
Bone Implant Interface
The relationship between endosseous implants and bone
involves mechanisms like:
• Fibro-osseous integration
• Osseointegration and
• Bioactive integration.
Fibro-osseous Integration
It is defined as tissue to implant contact by interposition
of a healthy dense collagenous tissue between the
implant and the bone interface. Normally, fibro-osseous
union between the implant surface and adjoining alveolar
bone is not desirable because union formed is a weak
union. The formation of fibro-osseous integration is
attributed to proliferation of connective tissue into the
interface, which hampers the osseous integration process.
Osseointegration
It is defined as a direct structural and functional
connection between ordered living bone and surface of
the load carrying implant.
Bioactive Integration
It is defined as the integration which results by a
physiochemical interaction between collagen of bone and
hydroxyapatite crystals of the implants.
BIOMATERIALS USED FOR IMPLANTS
Metals and Alloys
a. Titanium
100 percent pure Titanium
b. Titanium-Aluminum
Titanium 90 percent,
Vanadium
Aluminum 6 percent,
Vanadium 4 percent
c. Cobalt-Chromium
Cobalt 66 percent +
Chromium 27 percent +
Molybdenum 7 percent
d. Stainless steel
Iron 70 percent +
Chromium 18 percent +
Nickel 12 percent
e. Tantalum
100 percent pure
f. Zirconium
100 percent pure
g. Gold
100 percent pure
h. Platinum
100 percent pure
Inert Ceramics
a. Aluminum oxide (Al
2
O
3
)
• Polycrystalline
• Single crystal.
b. Zirconium oxide zircona.
c. Titanium oxide.
Calcium Phosphate Ceramics
Calcium phosphate.
Bioactive and Biodegradable Ceramics
a. Hydroxyapatite.
b. Tricalcium phosphate.
c. Bioglass.
d. Ceramic.
e. Calcium aluminates.
f. Carbon.
g. Carbon silicon.
h. Polycrystalline glassy carbon.
Polymers
a. Polymethyl methacrylate.
b. Polytetrafluoroethylene.
c. Polyethylene.
d. Polyethylene tetraphthalate.
e. Polypropylene.
f. Polyoxymethylene.
g. Silicone rubber.
h. Polysulfone.
CLASSIFICATION OF IMPLANTS
Implants are classified based on:
1. Shape and form and
2. Surface characteristics.

384
Essentials of Clinical Periodontology and Periodontics
Based on the Shape and Form
1. Endosteal.
2. Subperiosteal.
3. Transosteal.
4. Intramucosal inserts/submucosal implants/subdermal
implants.
5. Endodontic stabilizer.
With regard to shape, it is possible to distinguish between:
a. Post or root form implants—Exhibiting rotation
symmetry.
b. Blade implants—Extension implants.
The post or root implant designs can be of the
following types:
1. Solid tapering types.
2. Solid cylinder type.
3. Pin type.
4. Screw-shaped implant type.
5. Basket design.
6. Hollow cylinder design.
The blade implant designs can be of following types:
1. Conventional blade design.
2. Vented blade design.
Based on surface Characteristics
1. Titanium plasma—sprayed coating.
2. Sand blasting—surface etching.
3. Laser induced surface roughening.
4. Hydroxyapatite coating.
CLASSIFICATION OF IMPLANT SYSTEMS
1. Branemark implant system (Nobel Biocare System).
2. International team for implantology (ITI) system.
3. Implant innovations systems.
4. Astra-dental implant system.
5. IMZ implant system (Interpore IMZ).
6. Corevent system.
7. Sterioss system.
8. Stryker implant system.
9. Endosteal hollow basket system.
TREATMENT PLANNING
Clinical Assessment
Selection of cases for implants is based on the:
I. Age limitations for case selection.
II. Anatomic prerequisites:
1. Resorptive process.
2. Soft tissue situation.
3. Available bone.
4. Mandibular canal.
5. Height of bone.
6. Width of bone.
7. Bone shape (contour).
8. Length of bone.
9. Implant crown relationship.
10. Maxillary sinuses.
The Absolute Requirements for Treating
Implant Patients
1. Have an acceptable patient.
2. Implant made of biocompatible material.
3. Be durable.
4. Have proper surface quality.
5. Have acceptable socket created in bone.
6. Have surgical procedure properly done.
7. Have healing completed with acceptable bone
interface.
8. Have healing period without pathological stress.
9. Have normal implant function without pathological
stress.
Indications for Implant Therapy
A. The edentulous patient:
• Edentulous mandible
• Edentulous maxilla.
B. The partially-edentulous patient:
• Free end edentulous situation
• Multiple missing teeth.
C. Single tooth loss.

385
Dental Implants: Periodontal Considerations
Absolute Contraindications for
Implant Treatment
1. Uncontrolled-diabetes mellitus.
2. Long term immunosuppressant drug therapy.
3. Diseases of connective tissue.
4. Blood dyscrasias and coagulopathies.
5. Regional malignancy.
6. Metastatic disease.
7. Previous radiation to the jaws that might lead to
postsurgical osteoradionecrosis.
8. Alcohol or drug addiction.
9. Severe psychologic disorders.
Intraoral Contraindications
This includes:
1. Unfavourable intermaxillary relationships.
2. Problematic occlusal and functional relationships.
3. Pathologic considerations in alveolar bone, example,
fibro-osseous disease.
4. Pathologic alteration of the oral mucosa, example,
cysts, infections.
5. Xerostomia.
6. Macroglossia.
7. Unrestored teeth—poor oral hygiene.
Radiographs
Radiographs used in dental implants are panoramic
radiographs. However, this technique has certain inherent
problems that have to be taken into consideration like
distortion of spatial relationships. In order to eliminate
the distortion problems panoramic radiographs and their
use of templates with incorporated metal spheres have
been demonstrated.
Other Radiographic Procedures Employed
These are:
1. Periapical dental radiographs.
2. Rast-O-Pan bite blocks.
3. Lateral cephalometric radiograph.
4. Occlusal radiograph.
5. Tomography
6. Computed tomography
Surgical Procedures
The most threaded endosseous implants can be placed
either in one stage (or) two stages.
One-stage: Endosseous Implant Surgery
In this procedure the coronal portion stays exposed
through gingiva during the healing period. For example,
ITI system, TG Implant of 3i system and Life core single–
stage system.
One stage endosseous implant surgery: In this implant
surgery, the implant (or) healing abutment protrudes
about 2 to 3 mm from the bone crest and the flaps are
adapted around the implant. In posterior areas of the
mouth the flap is thinned and sometimes placed apically
to increase the zone of keratinized attached gingiva.
Surgical Technique
Flap design and incisons: The flap design is always a
crestal incision bisecting the existing keratinized tissue.
The soft tissue is not thinned in anterior or other aesthetic
areas of the mouth to prevent the metal collar from
showing, full-thickness flaps are elevated buccally and
lingually.
Placement of the implant: The implant site preparation
to place implants in one stage surgery is identical to
principles of two-stage except, implants or healing
abutment is placed in such a way that head of implant
protrudes about 2 to 3 mm from the bone crest.
Closure of the flap: The keratinized edges of the flap
are tied with independent sutures around the implant,
when keratinized tissue is abundant, scalloping around
the implant provides better flap adaptation.
Advantages and disadvantages of one stage implant
surgery.
Advantages:
a. Mucogingival management around the implant is
easier.
386
Essentials of Clinical Periodontology and Periodontics
b. Patient comfort increases because less surgeries are
involved.
c. Esthetic management is easier in many cases.
Disadvantage:
a. If extensive bone loss occurs at the implant site.
Vertical bone augmentation is necessary, and or bone
quality is poor then two-stage surgical approach is
recommended.
Two-stage: Endosseous Implant Surgery
In the two-stage implant surgical approach, the first stage
ends by suturing the soft tissues over the implant so that
it remains excluded from the oral cavity.
In the mandible, the implants are left undisturbed
for 2 to 3 months, whereas in the maxilla, they remain
covered for approximately 4 to 6 months because of
slower healing due to less dense bone. During this period,
the healing bone makes direct contact with the implant
surface (Osseointegration) and sometimes grows to its
occlusal surface, even covering it.
In second-stage surgery, the buried implant is
uncovered and a titanium abutment is connected to allow
access to the implant from the oral cavity. The restorative
dentist then proceeds with the prosthodontic aspects of
the implant therapy.
HEALING FOLLOWING IMPLANT SURGERY
If the space between an implant and its osseous bed
is narrow, bone formation is comparable to primary
healing after a bone fracture, because no callus forms.
Direct bridging via lamellar bone occurs, at a rate of
about one micrometer/day. Healing of implants with a
wide space around them is comparable to secondary
healing of a bone fracture, as bone formation occurs
via formation of a fibrous and bony callus, at about 50
to 100 micrometer/day. The temporal sequence is woven
bone with subsequent remodeling into lamellar bone.
During preparation of the implant bed, periosteal
intracortical and endosteal blood vessels are damaged.
As a result blood accumulates in peri-implant space, with
a loose attachment of fibrin on the surfaces of both bone
and implant.This hematoma will be remodeled by
proliferating tissue with new capillaries and fibrous
collagen connective tissue in 7 to 14 days. New bone
formation can occur directly in the vicinity of the implant
depending upon the degree of its stability. Implant
instability influences cell differentiation and therefore
also bone formation. So the implant stability is an
absolute requirement for all types of implants with
adequate blood supply.
Bony remodeling of the callus is completed after 4
to 6 weeks, thorough activation of the Haversian system,
numerous resorption canals are formed, and the
remodeling process into lamellar bone begins. These
mineralization processes, which transforms the osteoid
into calcified osseous substance, proceed at about 1
micrometer per day.
Different Phases of Healing
Osseous Healing—Early Phase
Preceded by hemorrahage and formation of a blood
clot, this coagulum consists of fibrin and embedded blood
cells and represents the scaffold for reparative
(granulation) tissue, the coagulum begins to organize
with ingrowth of capillaries and pre-osteoblasts
(centripetal bone growth).
During this early stage, in addition to new bone
formation, the macrophages as well as multinucleated
giant cells appear and recognizes the implant as
foreign body. As bone formation is initiated at the implant
surface, the number of multinucleated giant cells are
reduced.
Osseous Healing—Late Stage
Depending upon the width of the gap between the
implant surface and the osseous bed, direct filling of the
space can occur about 0.2 mm by means of concentric
bony apposition. Wider spaces will usually be filled within
14 days by a network of new woven bone, which will
be remodeled in about 2 months into lamellar bone:
remnants of the early woven bone may persist centrally.

387
Dental Implants: Periodontal Considerations
Direct bony contact with implant surface ranges from
56 to 85 percent with screw-type implants ad 46 to 82
percent with lin kow blade implants. Areas of the implant
surface not covered with bone will manifest adipose cells
without an intervening fibrous layer.
PERI-IMPLANT COMPLICATIONS
Despite the long-term predictability of osseointegrated
implants, biologic, biomechanical, and esthetic compli-
cations can occur in a small percentage of cases.
PERI-IMPLANT DISEASES
Pathologic alterations in the tissues that contact a dental
implant can be placed in the above category.
Types of Peri-implant Diseases
1. Peri-implant mucositis: Inflammatory changes, which
are confined to soft tissue surrounding an implant
is termed as peri-implant mucositis.
2. Peri-implantitis: It is a progressive peri-implant bone
loss in conjunction with soft tissue inflammatory
lesion. Peri-implantitis begins at the coronal portion
of the implant, while the more apical portion of
implant remains osseointegrated. This means that the
implant is not clinically-mobile until late stages when
bone loss had progressed to involve the complete
implant surface.
Clinical Features
• Color changes, bleeding upon gentle probing.
• Pocket formation and radiographic bone destruction.
• Suppuration, calculus build-up and swelling.
• Mobility has been extensively described to detect
early and late failures.
Diagnosis
A number of clinical parameters used to evaluate
periodontal conditions have also been used to assess
peri-implant conditions. These parameters include
evaluation of oral hygiene, peri-implant marginal tissues,
and bone implant interface.
Probing
A successful implant generally allows probe penetration
of approximately 3 to 4 mm and the location of peri-
implant bone level can be expected to be about 1 mm
apical to the position of the probe tip.
Radiographs
Reveal the peri-implant bone status as well as the marginal
bone level. Periapical intraoral radiographs are obtained
instead of OPG (which have lower discrimination power).
Direct imaging may have the potential to replace
conventional radiology.
To diagnose a compromised implant site, soft tissue
measurements using manual or automated probes have
been suggested; careful monitoring of probing depth
and clinical attachment level seems useful in detecting
changes of the peri-implant tissue.
Microbial Monitoring
It is useful in evaluating the peri-implant health condition
and microbial composition of a peri-implantitis site.
Management and Maintenance
Management
Occlusal therapy: When excessive forces are considered
the main etiologic factor for peri-implant bone loss
treatment involves an analysis of fit of the prosthesis
• The number and position of implants.
• Occlusal evaluation.
Change in prosthesis design, improvement of implant
number, position and occlusal equilibration can
contribute to arrest the progression of peri-implant tissue
breakdown.
Anti-infective therapy: The non-surgical treatment of peri-
implantitis involves:
• Local removal of plaque deposits with plastic
instruments and polishing of all accessible surface with
pumice.
388
Essentials of Clinical Periodontology and Periodontics
• Subgingival irrigation of all peri-implant pockets with
0.12 percent chlorhexidine
• Systemic antimicrobial therapy for 10 consecutive
days
• Improved patient compliances with oral hygiene until
a healthy peri-implant site is established.
• Conventional hand and ultrasonic instruments are
not suitable for the preparation and detoxification
of the implant surface.
• Irradiation with soft lasers for elimination of bacteria
associated with peri-implantitis has also shown
promising results in the destruction of bacterial cells.
Surgical techniques for treatment of peri-implantitis: Once
the inflammatory process in the peri-implant tissue is
under control, an attempt may be made to improve or
re-establish osseointegration.
The surgical procedures are modified from techniques
used to treat bone defects around the teeth.
Re-osseointegration: It can be defined as the growth of
new bone in direct contact to the previously contaminated
implant surface without an intervening band of organized
connective tissue.
Maintenance
After surgical intervention, all patients are placed on a
close recall schedule. It is advised to schedule main-
tenance visits at least every 3 months. This allows for
monitoring of plaque levels, soft tissue inflammation, and
changes in the levels of bone.
Oral Hygiene Aids
• Toothbrushes with soft, rounded bristles should be
used because the surfaces of the implants are easily
damaged.
• Toothpaste should be only minimally-abrasive; the
tooth-cleaning procedures should be conducted by
rinsing or brushing with chlorhexidine.
• Gauze strips or superfloss are effective for cleaning
interproximally.
• Irrigators can also be used as adjunctive aids.
KEY POINTS TO NOTE
1. A dental implant is a biologic or alloplastic biomaterial
inserted into soft or hard tissues of the mouth for functional
or cosmetic purposes.
2. Osseointegration is a “Direct structural and functional
connection between ordered living bone and the surface of
the load carrying implant.
3. Soft tissue interface of the implant consists of mucosal tissues
around intraosseous implants which form a tightly adherent
band consisting of dense collagenous lamina propria
covered by stratified squamous keratinizing epithelium.
4. Bone implant interface is the relationship between
endosseous implants and bone which involves mechanisms
like fibro-osseous integration, osseo-integration and
bioactive integration.
5. Based on shape and form implants are classified into—
Endosteal, subperiosteal, transosteal, submucosal implants
and endodontic stabilizer.
6. Based on surface characteristics implants are classified into
Titanium plasma sprayed coating, Sandblasting-surface
etching, laser-induced surface roughening and hydroxy-
apatite coating.
7. Surgical procedure involves:
a. One-stage endosseous implant surgery.
b. Two-stage endosseous implant surgery.
8. Healing involves two phases:
a. Osseous healing—Early phase.
b. Late stage.
9. Pathologic alterations in the tissues that contact a dental
implant are peri-implant diseases, which are peri-implant
mucositis and peri-implantitis.
REVIEW QUESTIONS
1. Describe the biological considerations of implant
therapy.
2. Enumerate various types of implants and implant
systems.
3. What is peri-implantitis?
BIBLIOGRAPHY
1. Belser UC, Buser D, Hess D et al. Aesthetic implant restorations
in partially edentulous patients – a critical appraisal.
Periodontology 2000, 1998; 17: 132.
2. Berglundh T, Lindhe J et al. The topography of the vascular
system in the periodontal and peri-implant tissues in the dog.
J Clin Periodontol 1994; 4: 189.

389
Dental Implants: Periodontal Considerations
3. Berman CL. Osseointegration, complications, prevention,
recognition, treatment. Dent Clin North Am 1989; 33: 635.
4. Buser D, Weber HP, Donath K, et al. Soft tissue reactions to
non-submerged implants. J Periodontol 1990; 61: 597.
5. Ericsson I, Lindhe J. Probing depths at implants and teeth.
J. Clin. Periodontol 1993; 20: 263.
6. Jan Lindhe. Clinical Periodontology and Implant Dentistry,
Fourth edition, Munksgaard, 2003.
7. Michael Norton. Dental implants, Quintessence 1995.
8. Newman, Takei, Carranza. Clinical Periodontology. 9th edn,
WB Saunders 2002.
9. Van Steinberghe D, et al. Survival and success rates with oral
endosseous implants. In Lang NP, Karring T, Lindhe J:
International implant dentistry. Proceedings of 3rd European
Workshop in Periodontology. Berlin, Quintessence
1999.
390
Essentials of Clinical Periodontology and Periodontics
IMPORTANCE OF MAINTENANCE PHASE
Chronic periodontitis, like most other chronic infections
require supervision and maintenance overtime.
Maintenance therapy after active treatment includes not
only the care that patients receive through personal oral
hygiene but also by the recall visits and re-evaluations
done by the dental team. Maintenance therapy is often
supportive in nature hence, it is also known as supportive
periodontal treatment (SPT). In this phase, patients must
be made to understand the purpose of a maintenance
program, and the dentist must emphasize on the fact
that the preservation of the teeth in question are
dependent on it.
RATIONALE FOR SUPPORTIVE
PERIODONTAL THERAPY
Rationale for maintenance phase is to prevent or
minimize the recurrence of periodontal diseases by
controlling factors known to contribute to the disease
process.
The main aim of long-term therapy is to provide
supervised control for the patient in order to maintain
a healthy and functional, natural dentition for life. It is
only with proper maintenance, including early detection
and treatment of recurrent periodontal diseases that such
an objective can be achieved.
CAUSES FOR RECURRENCE OF
PERIODONTAL DISEASE
1. Incomplete subgingival plaque removal.
2. Nature of dentogingival unit.
3. Improper restorations placed after the periodontal
treatment was completed.
4. Failure of the patient to return for periodic recall visits.
5. Presence of some systemic diseases that may affect host
resistance to previously acceptable levels of plaque.
The American Academy of Periodontology position
paper more specifically lists 3 main goals of SPT:
1. To prevent or minimize the recurrence and progression
of periodontal disease in patients who have been
previously treated for gingivitis, periodontitis and for
peri-implantitis.
❒
❒
❒
❒
❒ IMPORTANCE OF MAINTENANCE PHASE
❒
❒
❒
❒
❒ RATIONALE FOR SUPPORTIVE
PERIODONTAL TREATMENT
❒
❒
❒
❒
❒ CAUSES FOR RECURRENCE OF
PERIODONTAL DISEASE
❒
❒
❒
❒
❒ OBJECTIVES OF MAINTENANCE PHASE
❒
❒
❒
❒
❒ DETERMINATION OF MAINTENANCE
RECALL INTERVALS
48
Maintenance Phase
(Supportive Periodontal
Treatment)
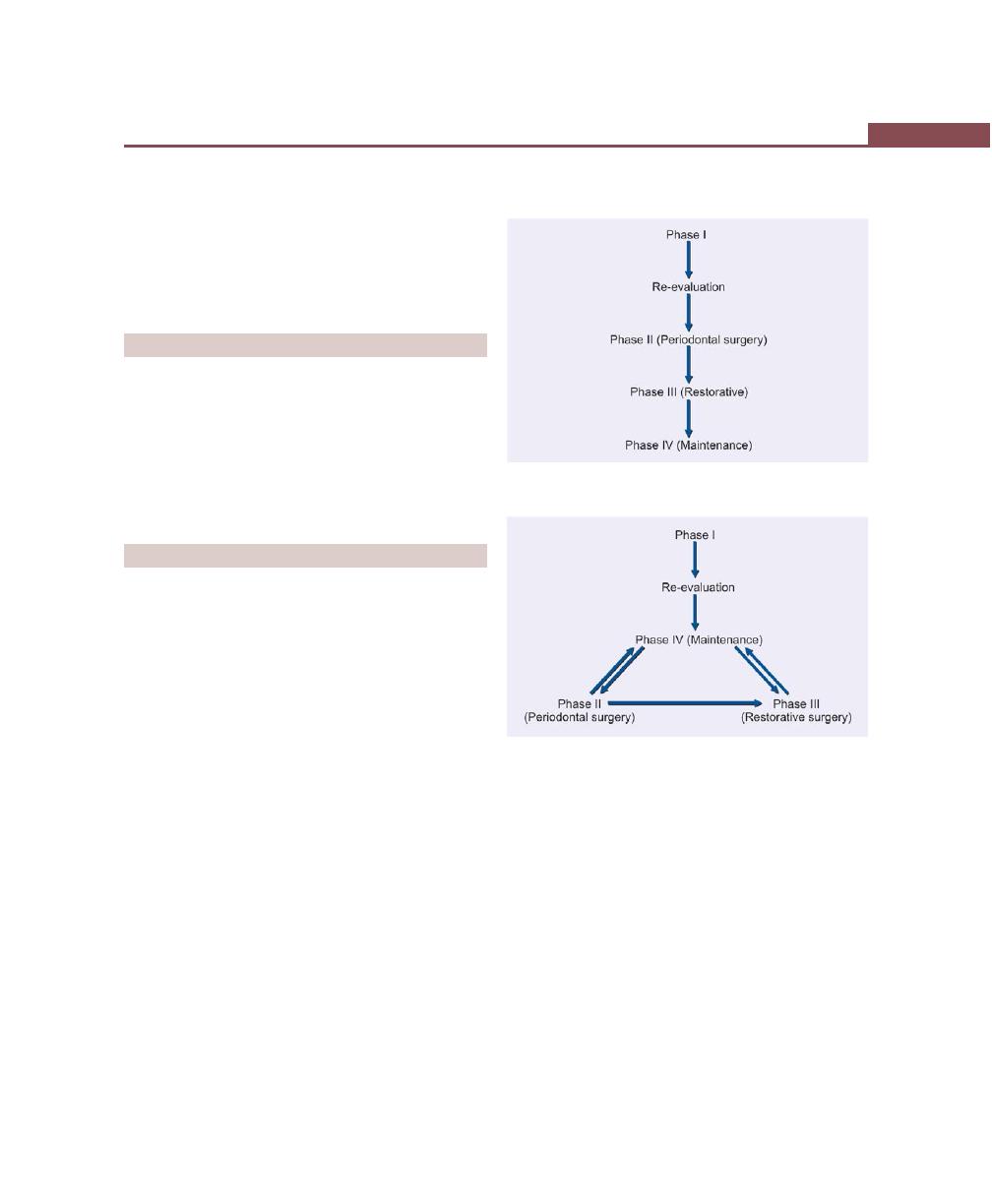
391
Maintenance Phase
2. To prevent or reduce the incidence of tooth loss by
monitoring the dentition and by any prosthetic
replacement of the natural teeth.
3. To increase the probability and treating in a timely
manner, other diseases or conditions found in the
oral cavity.
OBJECTIVES OF MAINTENANCE PHASE
1. Preservation of alveolar bone support (radio-
graphically).
2. Maintenance of stable, clinical attachment level.
3. Reinforcement and re-evaluation of proper home
care.
4. Maintenance of a healthy and functional oral
environment.
PARTS OF MAINTENANCE PHASE
Part–I: Examination
(Approximate time –7 Min.)
• Medical history changes
• Oral pathological examination
• Oral hygiene status
• Gingival changes
• Pocket depth changes
• Mobility changes
• Occlusal changes
• Restorative and prosthetic changes.
Part–II: Treatment
(Approximate time – 35 Min.)
• Oral hygiene reinforcement
• Scaling and polishing
• Chemical irrigation.
Part–III: Schedule Next Procedure
• Schedule next recall visit
• Schedule further periodontal treatment
• Schedule or refer to restorative or prosthetic
treatment.
Sequence of Maintenance Visits
1. Incorrect sequence of periodontal treatment phases
(Flow chart 48.1).
2. Correct sequence of periodontal treatment phases
(Flow chart 48.2).
Schallhorn and Snider (1981) proposed four separate
categories of periodontal maintenance therapy. They are:
1. Preventive maintenance therapy—periodontally-
healthy individuals.
2. Trial maintenance therapy—mild to moderate perio-
dontitis.
3. Compromised maintenance therapy—medically-
compromised patients where active therapy is not
possible.
Flow chart 48.1:
Incorrect sequence of periodontal
treatment phases
Flow chart 48.2:
Correct sequence of periodontal
treatment phases
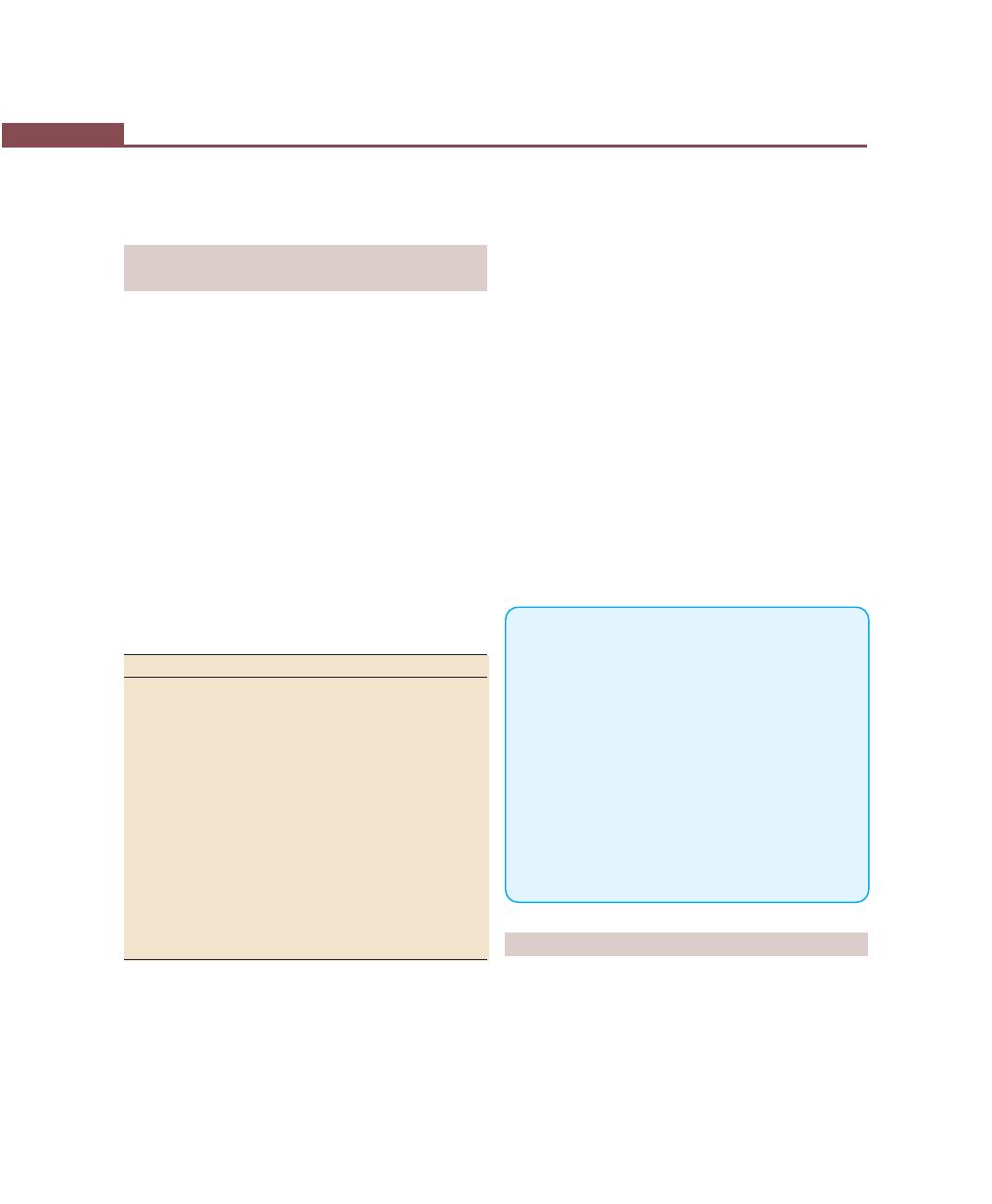
392
Essentials of Clinical Periodontology and Periodontics
4. Post-maintenance treatment therapy—maintenance
for prevention of recurrence of disease.
DETERMINATION OF MAINTENANCE
RECALL INTERVALS
Based on studies of human periodontal treatment, it is
recommended that the patient be seen initially for
recall treatment 2 to 4 weeks following treatment (for
transitional procedures). After 3 or 4 such sessions the
interval can be extended to 3 months, but may be varied
according to the patient’s needs.
Following factors may be considered in determining
the recall intervals:
1. Severity of disease: The more severe the disease, the
more frequently the patient is recalled.
2. Effectiveness of home care: Good home care
decreases the frequency of recall.
3. Degree of control of inflammation achieved: As the
tissue regain the total health, the frequency decreases.
Procedures to be Performed at Recall
Procedure
Evaluation
1. At clinical examination
All findings recorded at the base
line are compared. Evaluations of
complete oral and periodontal
status, occlusal and prosthetic
appliances, etc.
2. Radiographically
Assessment of bone levels. Any
additional findings are recorded.
3. Assessment of disease
By comparing the findings
obtained at base line.
4. Assessment of patients
Comparison with baseline
oral hygiene
data. Behavioral modification if
necessary.
5. Treatment
Removal of any fresh deposits,
occlusal therapy, application of
antimicrobial agents if indicated.
Appointments for future perio-
dontal therapy.
Management of Particular Type of
Recall Patients
1. The patients who will not cooperate in oral hygiene
but will pay for office care: In this case patient
management includes frequent, thorough root
instrumentation.
2. The patient who has refused surgical treatment: The
recall must be shorter for this patient than for patients
who have received surgical therapy. The root
instrumentation phase involves more time and is
difficult to perform properly.
3. The patient who has been hospitalized for several
weeks : If the patients condition permits, periodic
scaling and review of oral hygiene by the hygienist
should be performed in hospital.
4. The patients who has been fully and successfully
treated yet now shows distinct breakdown in localized
areas: A general health review, if this is favorable,
re-treatment of involved areas is done. Flap curettage
and antibiotic therapy should be given. Recall
appointments are scheduled for atleast once in every
3 months.
KEY POINTS TO NOTE
1. Maintenance therapy is often supportive in nature hence it
is also called supportive periodontal treatment (SPT).
2. The main objective of supportive periodontal therapy is to
prevent or minimize the recurrence of periodontal diseases
by controlling factors known to contribute to the disease
process.
3. The interval of recall visits is determined by severity of
disease, effectiveness of patients home care and degree of
control of inflammation achieved.
4. Based on many long-term studies, the interval for
maintenance visits has been recommended. The patient
should be seen initially for recall treatment at 2 to 4 weeks
following treatment. After 3 or 4 such sessions the interval
can be extended to 3 months (can be varied according to
patient needs).
REVIEW QUESTIONS
1. What are the objectives of supportive periodontal
therapy?
2. What is the sequence of maintenance visits?
3. Describe the importance of maintenance therapy in
periodontics.

393
Maintenance Phase
BIBLIOGRAPHY
1. Brady Hancock, Donald H Newell. Preventive strategies and
supportive treatment. Periodontol 2000, 2001; 25.
2. Mendoza AR, NewComb GM, Nixon KC. Compliance with
supportive periodontal therapy. J Periodontol 1991; 62-731.
3. Newman, Takei, Fermin A Carranza. Clinical periodontology,
9th edn, WB Saunder Co., 2002.
4. Novaesab Jr, Novacs AB. Compliance with supportive
periodontal therapy part 1: Risk of non-compliance in the first
5 year period. J Periodontol 1999; 670-79.
394
Essentials of Clinical Periodontology and Periodontics
TERMINOLOGY
Occlusion: It is defined as the functional relationship
between the components of the masticatory system inclu-
ding the teeth, supporting tissues, neuromuscular system,
temporomandibular joints and craniofacial skeleton.
Intercuspal position (ICP): The position of the
mandible when there is maximal intercuspation between
the maxillary and mandibular teeth.
Excursive movement: Any movement of the mandible
away from ICP.
Protrusion: Movement of the mandible anteriorly from
ICP.
Retrusion: Movement of the mandible posteriorly from
ICP.
A physiologic occlusion: It is when no signs of
dysfunction or disease are present and no treatment is
indicated.
A non-physiologic (or traumatic) occlusion: It is
associated with dysfunction or disease due to tissue injury,
and treatment may be indicated.
A therapeutic occlusion: It is the result of specific
interventions designed to treat dysfunction or disease.
CLINICAL EVALUATION OF OCCLUSION
Temporomandibular Disorders (TMD)
Screening Examination
Screening for temporomandibular disorders (TMD)
should be included in all routine dental examinations.
The accepted components of this examination are:
1. Maximal intercuspal opening—recorded in millimeters
2. Opening—closing pathway—deviations if any are
recorded.
3. Auscultation for TMJ sounds—joint sounds can be,
discrete clicks or diffuse grating sounds (crepitus).
4. Palpation for TMJ tenderness—could be mild,
moderate or severe tenderness, recorded with light
bilateral palpation along the lateral aspects of
condyles.
5. Palpation for muscle tenderness—the masseter and
temporalis muscles are examined bilaterally using
moderate finger pressure. The muscle pain may be
mild, moderate or severe type.
❒
❒
❒
❒
❒ TERMINOLOGY
❒
❒
❒
❒
❒ CLINICAL EVALUATION OF OCCLUSION
• TMD Screening Examination
• Intraoral Evaluation
❒
❒
❒
❒
❒ MANAGEMENT OF TRAUMA FROM
OCCLUSION
❒
❒
❒
❒
❒ OCCLUSAL THERAPY
49
Occlusal Evaluation and
Therapy in the
Management of
Periodontal Disease

395
Occlusal Evaluation and Therapy in the Management of Periodontal Disease
Intraoral Evaluation of Occlusion
1. Identification of intercuspal position (ICP) zones of
contact by placing mylar strips between the teeth and
asking the patient to close and open.
2. Guidance in excursive movements.
3. Tooth mobility.
4. Attrition—excessive attrition should be noted.
MANAGEMENT OF TRAUMA
FROM OCCLUSION
When an adequate quantity of periodontal support is
present to withstand the normal forces of occlusion, yet
excessive parafunctional forces exceed the adaptive
capacity of the attachment apparatus, the disease process
is referred to as “primary occlusal trauma”. When the
quantity of the remaining normal attachment apparatus
has been compromised by periodontal disease and
cannot withstand the normal forces of occlusion, the
disease process is referred to as “secondary occlusal
trauma”.
a. In cases of primary occlusal trauma with gingivitis or
periodontitis. The treatment is simple and conser-
vative. First, periodontal therapy is done which
include plaque control, scaling and root planing. If
there is progressive mobility then occlusal therapy
in the form of selective grinding and the use of night
guard may be justified.
b. In cases of secondary occlusal trauma and advanced
periodontitis the treatment is often complicated. It
often requires advanced periodontal therapy,
including root resection, antimicrobial therapy and
regenerative procedures along with adjunctive
orthodontics, occlusal adjustment by selective grin-
ding and splinting for periodontal stabilization is
advocated.
OCCLUSAL THERAPY
Occlusal therapy is performed to establish a stable
functional relationship, favorable to the oral health of
the patient, including the periodontium. Various
procedures to achieve this objective are:
a. Interocclusal appliance therapy.
b. Occlusal adjustment.
c. Restorative procedures.
d. Orthodontic movement and orthognathic surgery.
Restorative procedures and orthodontic tooth
movements in the management of periodontal patient
is covered elsewhere, in this book.
Occlusal adjustment or coronoplasty is the selective
reshaping of occlusal surface with the goal of establishing
a stable, non-traumatic occlusion. This is achieved by
reshaping the crown surfaces and eliminating undesirable
occlusal supra-contacts and the creation of a stable
mandibular position.
Occlusal adjustment had once been the most
commonly employed procedure for treating occlusal
trauma, temporomandibular joint problems and other
associated problems. Since occlusal adjustment is an
invasive, irreversible intervention, it should rarely be con-
sidered. It should never be undertaken as a preventive
measure.
Coronoplasty is generally performed after gingival
inflammation and periodontal pockets have been
eliminated. The author recommends a reference book
for a detailed descripition on the procedure of occlusal
adjustment.
BIBLIOGRAPHY
1. Burgett FG, Ramfjord Sp, Nissle RR, et al. A randomized trial
of occlusal adjustment in the treatment of periodontitis
patients. J. Clin. Periodontol 1992; 19: 381.
2. Gher ME. Changing concepts. The effect of occlusion on
periodontitis. Dent Clin North Am 1998; 2 : 285.
3. Robert S Rosenbaum. The possible effect of periodontal
diseases on occlusal function: Editorial Review : Curr Opin
Periodontol 1993; 163.
4. Sigurd P Ramfjord, Major M Ash. Periodontology and
periodontics. Modern therapy and practice, Euro-America Inc.
USA, 1996.
396
Essentials of Clinical Periodontology and Periodontics
INTRODUCTION
The primary objective of periodontal therapy is to restore
and maintain the health and integrity of the attachment
apparatus of teeth. In adults, the loss of teeth or
periodontal support can result in pathologic tooth
migration involving either a single tooth or a group of
teeth. This may result in the development of a median
diastema or general spacing of the teeth, rotation or
tipping of premolars and molars with the collapse of the
posterior occlusion and decreasing vertical dimension.
Adjunctive orthodontic therapy is necessary to resolve
these problems.
RATIONALE FOR ORTHODONTIC
TREATMENT IN PERIODONTAL THERAPY
Reducing Plaque Retention
Example: Crowded teeth ( arch length deficiency),
mesially-tipped teeth, usually into an edentulous area
creates plaque accumulation sites that are difficult to clean.
In addition, they open the distal contact creating an area
of food impaction. Crowding also creates enlarged contact
surfaces and altered embrasure spaces that are displaced
apically, thereby becoming less accessible to use floss and
other plaque removing devices. In these situations ortho-
dontic treatment can improve the health of the tissues.
Improving Gingival and Osseous Form
There is an interrelation between the position of the tooth,
the shape of the gingiva and bone that surrounds it.
For example, lower first or second molar tilted into an
edentulous mesial space. In these cases there is a narrow
space between its crown and the bone that easily
becomes inflamed and in which case a pocket may
develop. Orthodontic treatment may improve the shape
of the periodontium and reduces the indications for bone
surgery (Figs 50.1A to C).
❒
❒
❒
❒
❒ RATIONALE FOR ORTHODONTIC
TREATMENT IN PERIODONTAL
THERAPY
❒
❒
❒
❒
❒ INDICATIONS AND
CONTRAINDICATIONS OF
ORTHODONTIC THERAPY
❒
❒
❒
❒
❒ TIMING OF ORTHODONTIC
PROCEDURES IN PERIODONTAL
TREATMENT
❒
❒
❒
❒
❒ IATROGENIC EFFECTS ASSOCIATED
WITH ORTHODONTIC TREATMENT
50
The Role of Orthodontics
as an Adjunct to
Periodontal Therapy
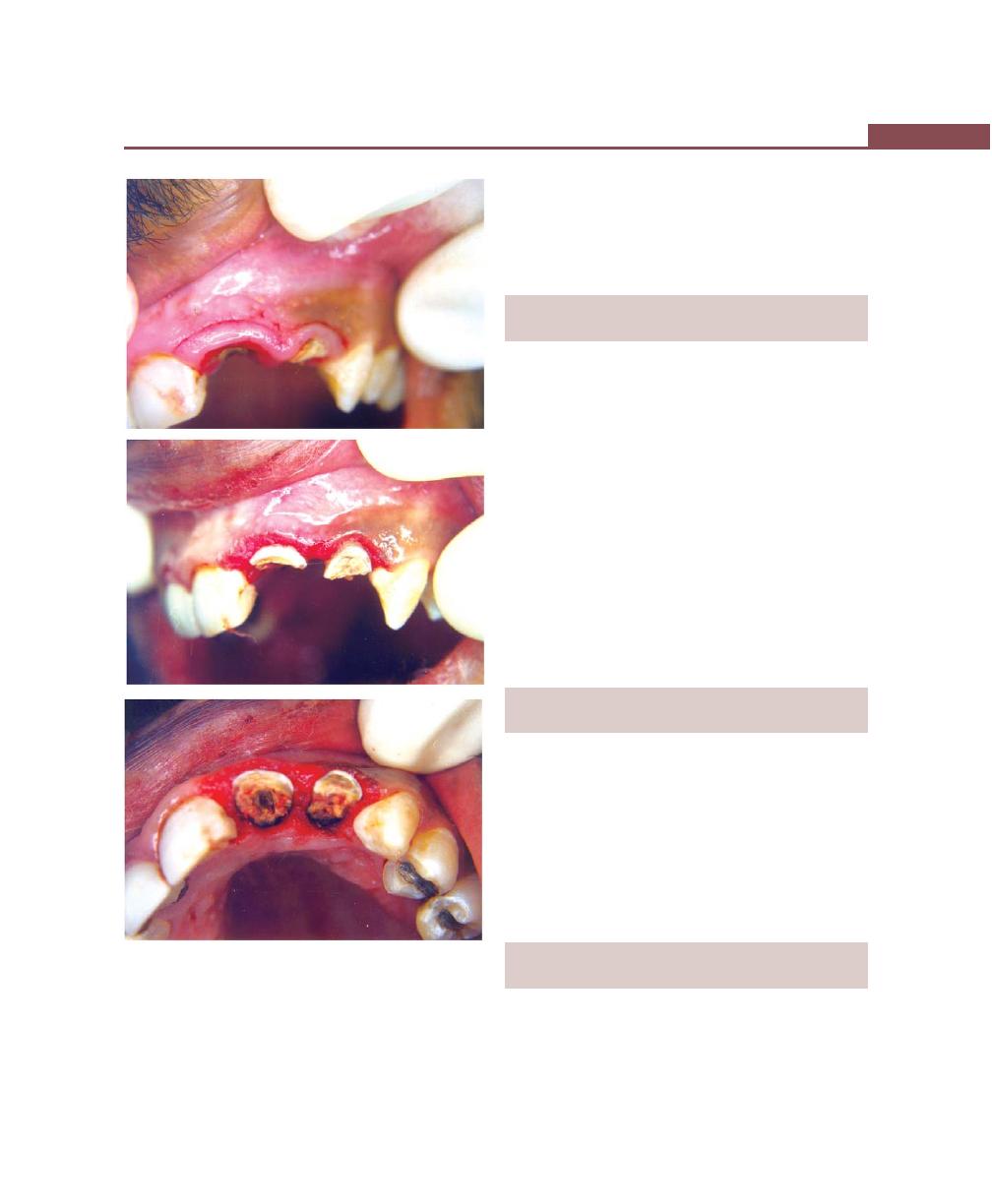
397
The Role of Orthodontics as an Adjunct to Periodontal Therapy
Figs 50.1A to C:
Crown lengthening with internal bevel
gingivectomy
Improving Esthetics
Correction of pathologic tooth migration and diastema
between anterior teeth. Correction of tongue-thrusting
and other habits.
INDICATIONS AND CONTRAINDICATIONS
OF ORTHODONTIC THERAPY
Indications
This includes common problems that can be solved by
minor orthodontic therapy such as crowded teeth, closure
of anterior diastema, mesial tilting of molars and open
contacts.
Contraindications
The only contraindication is the persistence of active
disease inspite of phase-I therapy procedures. The
superimposition of tooth movement on inflamed gingiva
may exacerbate the periodontal problem. This can occur
by shifting the position of plaque subgingivally, increasing
the rate of periodontal attachment loss and altering the
morphology of the bone.
TIMING OF ORTHODONTIC PROCEDURES
IN PERIODONTAL TREATMENT
It is generally recommended that orthodontics be
preceded by periodontal therapy based on the belief
that orthodontics in the presence of inflammation can
lead to rapid and irreversible breakdown of the
periodontium. But any elimination procedures like pocket
or osseous reduction procedures may be postponed until
the end of orthodontic therapy because tooth movement
may modify gingival and osseous morphology.
IATROGENIC EFFECTS ASSOCIATED
WITH ORTHODONTIC TREATMENT
Orthodontic treatment may cause injuries to the teeth
and periodontium but in most of the cases the changes
are reversible and regeneration and repair of the tooth
structures and periodontal tissues can occur. In some
A
B
C
Facilitating Prosthetic Replacements
The uprighting of tilted abutment teeth may be important
for a better contoured crown and this will benefit the
periodontal condition.
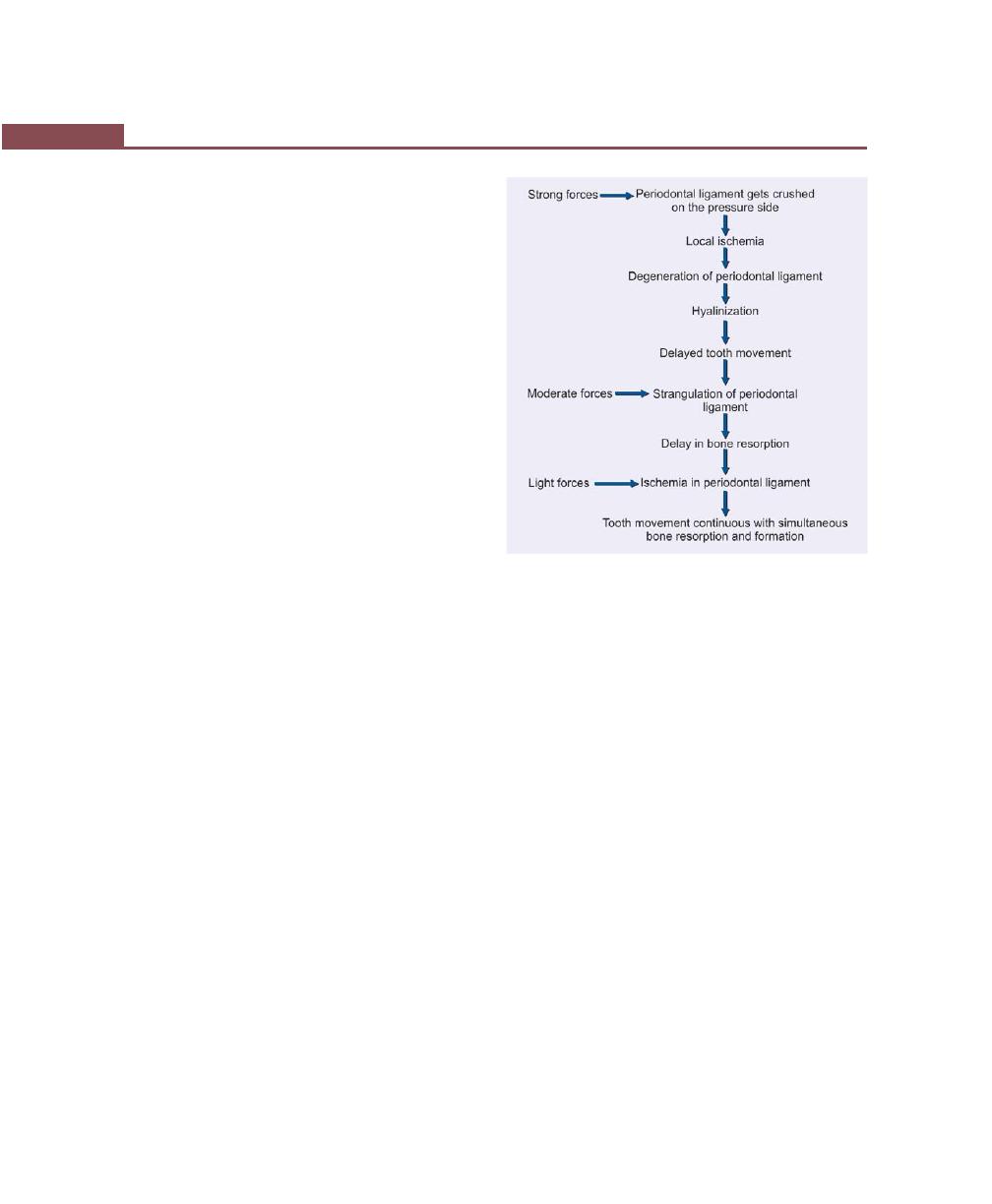
398
Essentials of Clinical Periodontology and Periodontics
cases the changes may get out of control resulting in
irreparable damage. All the precautions should be taken
to avoid this and radiography should be performed at
regular intervals in order to disclose any iatrogenic
effects during the orthodontic treatment. They are as
follows,
Root Resorption
Some amount of root resorption is unavoidable espe-
cially if it is seen at the marginal and middle thirds of
the root, which can be repaired by apposition of cellular
cementum.
Effects of Orthodontic Bands on the
Periodontium
Short-term effects are—Gingivitis and gingival hyper-
plasia, mostly not associated with loss of attachment.
Long-term effects are—Loss of attachment, root
resorption or no effects. Any of these three possibilities
may be seen in adult patients.
Effects of Orthodontics on Dentition with
Normal Height of Attachment Apparatus
Number of experimental studies have demonstrated that
orthodontic forces causes no damage to the supra-
alveolar connective tissue and orthodontic treatment will
therefore not result in periodontal tissue breakdown and
pocket formation.
Dentition with Reduced Height
of Attachment Apparatus
The experimental studies have demonstrated that,
a. In the absence of plaque, orthodontic forces and
tooth movements failed to induce gingivitis ,whereas
in the presence of plaque similar forces cause angular
bone defects associated with attachment loss.
b. Orthodontic forces if kept within biologic limit failed
to cause gingival inflammation even in the regions
with reduced periodontal support ,but is of the non-
inflammatory type.
Response of the Periodontal Ligament
to Orthodontic Forces (Fig. 50.2)
When bone surrounding the tooth is subjected to a force
it responds in the following manner:
1. Resorption occurs where there is pressure and new
bone forms where there is tension.
2. When pressure is applied to the tooth, there is a initial
period of movement for 6 to 8 days as the perio-
dontal ligament is compressed. Compression of
periodontal ligament results in blood supply being
cut off to an area of the periodontal ligament and
this produces an avascular, cell-free zone by a process
termed “hyalinization”. When hyalinization occurs the
tooth stops moving (depending on the forces). The
hyalinized zone is eliminated by periodontal
regeneration that occurs from the reorganization of
the area through resorption by the marrow spaces
(undermining resorption) and adjacent areas of
unaffected periodontal ligament and alveolar
bone. Once the hyalinized zone is removed, tooth
Fig. 50.2:
Response of the periodontal ligament to
orthodontic forces
399
The Role of Orthodontics as an Adjunct to Periodontal Therapy
movement can occur again. Regeneration of perio-
dontal ligament does not occur when inflammation
is present in periodontal tissue. Hence, the inflam-
mation needs to be controlled by periodontal
treatment.
KEY POINTS TO NOTE
1. Adjunctive orthodontic therapy is necessary to resolve
problems like generalized spacing of teeth, rotation or
tipping of teeth, collapse of the posterior occlusion and
decreasing vertical dimension.
2. Basic rationale for orthodontic intervention in periodontal
therapy is to reduce plaque accumulation, improve gingival
and osseous form, facilitate prosthetic replacement and
improve esthetics.
3. The only contraindication for orthodontic treatment is,
persistent inflammation in spite of phase I therapy. In the
presence of active infection, the orthodontic tooth movement
can exacerbate the periodontal problems.
4. Some of the common iatrogenic effects of orthodontic
treatment are, root resorption, gingival inflammation
associated with orthodontic bands, changes in periodontal
ligament and others.
REVIEW QUESTION
1. What are the effects of orthodontic treatment on
periodontium?
BIBLIOGRAPHY
1. Interrelationship between periodontics and adult ortho-
dontics. Jr of. Clin. Periodontol 1998; 25 : 271-77.
2. Jan Lindhe. Clinical Periodontology and Implant Dentistry,
4th Edition, (2003), Blackwell Munkgard Publication.
3. Robert G. Kein. Aesthetics in clinical orthodontic periodontic
interactions. Periodontol 2000; Vol. 27, 2001.
400
Essentials of Clinical Periodontology and Periodontics
INTRODUCTION
It is a well-established fact that the periodontal health
and the restoration of teeth share an intimate and
inseparable interrelationship. One must always
remember that for restorations to survive, long-term
restorative procedures must be performed on a
periodontium free of inflammation and pockets without
any mucogingival involvement, and with the contour
and shape of the periodontium corrected for a good
functional and esthetic restorative result. For the
periodontium to remain healthy, restorations must be
critically prepared so that, they will remain in harmony
with the surrounding periodontal tissues.
MARGINS OF THE RESTORATIONS
A clinician has three options for margin placement:
i. Supragingival
ii. Equigingival (even with the tissue)
iii. Subgingival
Restorations should be chosen not just by keeping
aesthetics in mind but for their favorable periodontal
impact as well.
Supragingival margins have the least impact on the
periodontium. The use of equigingival margins was
thought to retain more plaque thereby interfering with
gingival health, today these concerns are not valid. The
greatest biologic risk occurs when margins are placed
subgingivally. Hence, from periodontal point of view, both
supragingival and equigingival margins are well-tolerated.
In view of the scientific evidence available, restorative
margins should be preferably placed supragingivally.
However in certain situations, where subgingival margins
are unavoidable like carious tooth, tooth fracture or
aesthetic concern, it should be placed not more than
0.5 mm into the sulcus so that, these margins could be
accessible for finishing procedures.
In addition, if the margins are placed too far below
the gingival tissue crest, it violates the gingival attachment
apparatus.
RESTORATIVE MARGIN ENCROACHING
ON THE BIOLOGIC WIDTH (Fig. 51.1)
The soft tissue attachment to the tooth between the base
❒
❒
❒
❒
❒ MARGINS OF RESTORATIONS
❒
❒
❒
❒
❒ RESTORATIVE MARGINS ENCROACHING
ON THE BIOLOGIC WIDTH
❒
❒
❒
❒
❒ CROWN CONTOUR
❒
❒
❒
❒
❒ HYPERSENSITIVITY TO DENTAL
MATERIALS
❒
❒
❒
❒
❒ PROXIMAL CONTACT AND EMBRASURE
❒
❒
❒
❒
❒ PONTIC DESIGN
51
Periodontal: Restorative
Inter-relationship
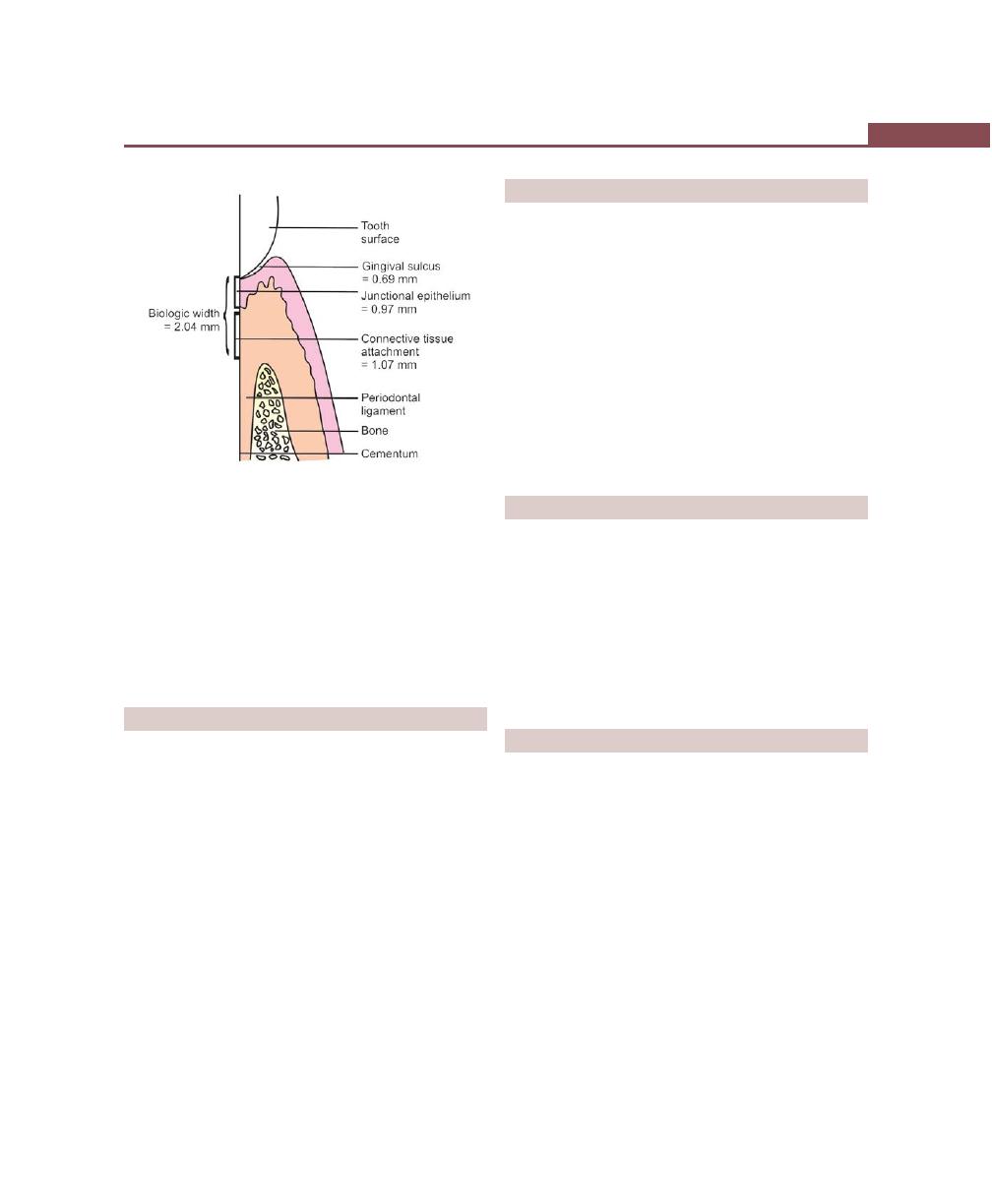
401
Periodontal: Restorative Inter-relationship
of the gingival sulcus and the crest of the alveolar bone
is called the biologic width.
Invasion into this biologic width should be avoided
in order to prevent attachment loss and persistent gingival
inflammation.
Biologic width = Junctional epithelium (0.97mm) +
Connective tissue attachment (1.07mm) = 2.04 mm
CROWN CONTOUR
Restoration contour plays an important role in the
maintenance of periodontal health. An ideal contour
must provide:
i. Access for hygiene.
ii. Fullness to create the desired gingival form.
iii. Esthetically-pleasing tooth contour.
iv. Protection of the marginal gingiva from mechanical
injury during mastication—explained in gingival
protection theory.
Hence, the crown contour should, therefore help in
easy plaque removal, not its retention. Overcontouring
leads to more plaque accumulation with subsequent
gingival inflammation, under contouring of crowns is
therefore considered ideal.
HYPERSENSITIVITY TO DENTAL MATERIALS
Rough dental surfaces favor plaque accumulation and
contribute to periodontal disease. Regardless of the
material used subgingivally, a smooth surface is essential
on all materials. Today, the most commonly used
materials are glass ionomer cements and composite
resins. Though the composite resins have very smooth
surface upon finishing, they do not last very long because,
once it is exposed to the oral environment, its surface
becomes rough. From periodontal point of view glass
ionomer seems to be more acceptable because of its
capability to release fluoride that has the potential to
interfere with adherence of bacteria on the tooth surface.
PROXIMAL CONTACT AND EMBRASURE
It is believed that, the ideal interproximal embrasure
should house the gingival papilla without impinging on
it. Proper proximal contact is essential to prevent food
impaction. The contact point should be placed occlusally
and facially to facilitate access for interproximal plaque
control. The ideal contact should be 2 to 3 mm coronal
to the attachment, which coincides with the depth of
the average interproximal sulcus.
PONTIC DESIGN
Traditionally, four types of pontic designs have been
proposed—Sanitary, ridge lap, modified ridge lap and
oviate pontic designs.
1. Sanitary pontic: Where the tissue surface of the pontic
is 3 mm from the underlying ridge (Fig. 51.2).
2. Ridge lap pontic: Where the tissue surface of the
pontic straddles the ridge much like a saddle. The
entire surface is convex and is very difficult to clean
(Fig. 51.3).
3. Modified ridge lap pontic: The tissue surface on the
facial surface is concave, however, the lingual saddle
has been removed to allow access for oral hygiene
(Fig. 51.4).
Fig. 51.1:
Biologic width
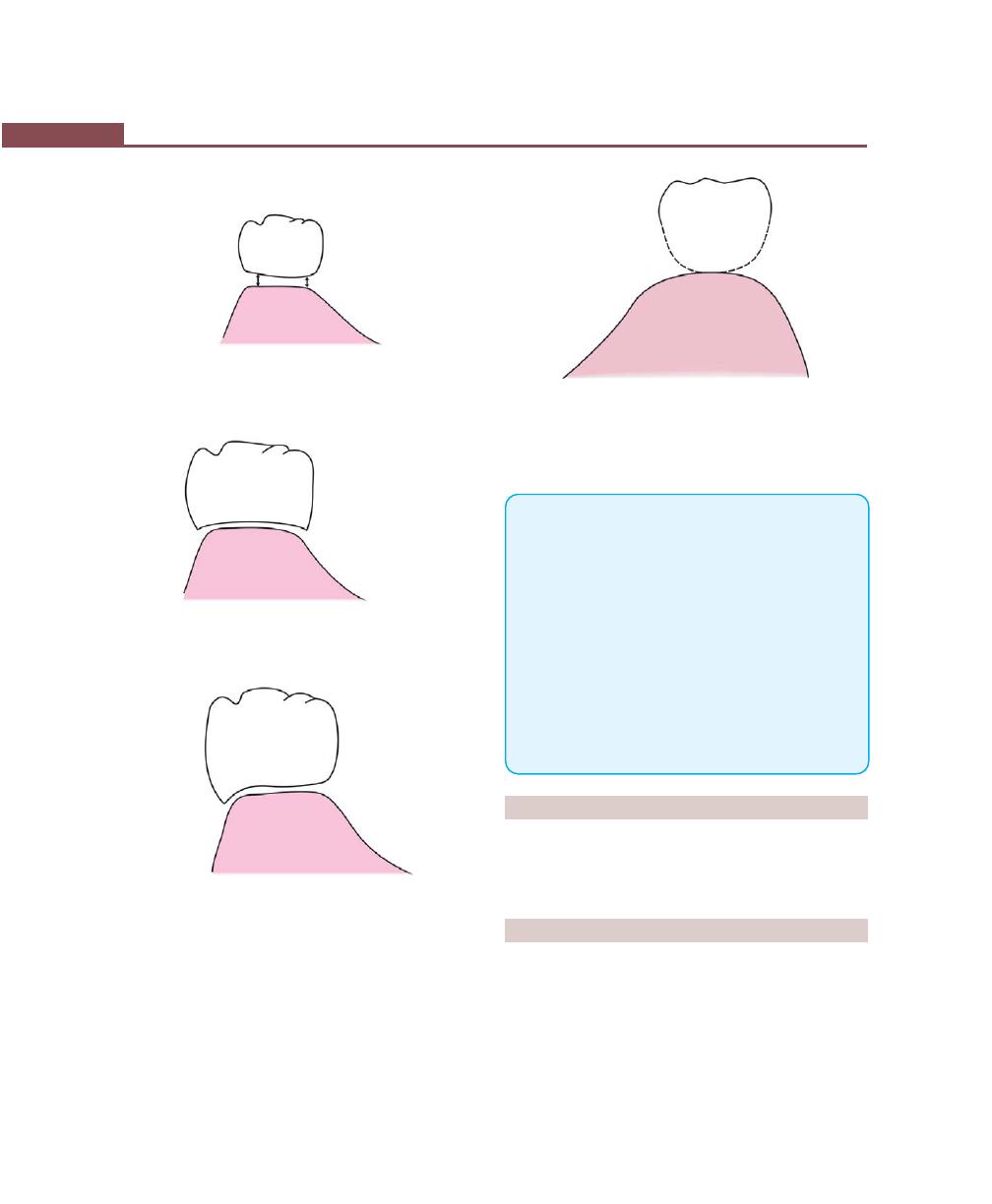
402
Essentials of Clinical Periodontology and Periodontics
Fig. 51.2:
Sanitary pontic
Fig. 51.3:
Ridge lap pontic
Fig. 51.4:
Modified ridge lap pontic
Fig. 51.5:
Oviate pontic
should be avoided or kept minimal so that meticulous
plaque control can be advocated (Fig. 51.5).
KEY POINTS TO NOTE
1. Restorative procedures should be performed on a perio-
dontium free of inflammation and other signs of periodontal
disease.
2. The restorative margins can be placed at supragingival,
equigingival or subgingival locations. Supragingival margin
has the least impact on the periodontium.
3. The soft tissue between the base of the gingival sulcus to
the crest of the alveolar bone is called the ‘biologic width’
which is 2.04 mm.
4. Ideal contour must provide, access for hygiene, fullness to
create the desired gingival form and esthetically-pleasing
tooth contour.
5. Four types of pontic designs have been proposed; Sanitary,
ridge lap, modified ridge lap and oviate pontic designs.
REVIEW QUESTIONS
1. What are the effects of restorative procedure on the
periodontium?
2. What is the biologic width?
BIBLIOGRAPHY
1. Dan Lundgren, Lars Laurell. Biomechanical aspects of fixed
bridge work supported by natural teeth and endosseous
implants. Periodontol 2000, 1994; 4.
2. Myron Nevins. Periodontal considerations in prosthetic
treatment. Curr Opin. Periodontol 1993; 151-54.
3. Niklaus P. Lang, Sture R Nyman. Implant and crown and
bridge therapy in the periodontally compromised patient.
Periodontol 2000, 1994; 4.
4. The oviate pontic: This is the ideal pontic design.
It is created by forming a receptor site in the eden-
tulous ridge with either a diamond bur or electro-
surgery.
Whenever fixed prosthesis is designed to replace
missing teeth, contact between the pontic and mucosa
403
Drugs Used in Periodontal Therapy
INTRODUCTION
It is a well-believed fact that mechanical therapy is
the foundation for periodontal therapy. In the past
only acute necrotizing ulcerative gingivitis was treated
with antibiotic therapy because it was considered as a
fusospirochetal infection. With emerging evidence of
bacterial specificity in relation to aggressive forms of
periodontitis and also the difficulty in suppressing tissue
invaded pathogens with conventional therapy in certain
cases (like juvenile periodontitis) led to the development
of antimicrobial treatment strategies. But total elimination
of the periodontal pathogens with antibiotic therapy alone
may not be possible, unless it is combined with scaling
and root planing.
CLASSIFICATION OF VARIOUS DRUGS
USED IN PERIODONTAL THERAPY
Various drugs used in periodontal therapy can be divided
into:
1. Anti-plaque and anti-calculus agents.
2. Antibiotics in the management of periodontal
disease.
3. Anti-inflammatory drugs.
Depending on Antimicrobial
Efficacy and Substantivity
Depending on antimicrobial efficacy and substantivity
drugs can be classified as:
1. First generation agents: They reduce plaque score
by 20 to 50 percent, efficacy is limited because of
poor substantivity.
Examples: antibiotics, quaternary ammonium
compounds and sanguinarine. It should be used 4
to 6 times daily.
2. Second generation agents: They are retained longer
in the tissues and their slow release property provides
overall reduction in plaque score by 70 to 90 percent
(should be used twice daily).
Example: chlorhexidine, triclosan with either
copolymer or zinc citrate.
❒
❒
❒
❒
❒ CLASSIFICATION OF VARIOUS DRUGS
USED IN PERIODONTAL THERAPY
• Depending on Antimicrobial Efficacy
and Substantivity
• Chemicals Used for Supragingival
Plaque Control
❒
❒
❒
❒
❒ ANTIBIOTICS USED IN PERIODONTAL
THERAPY
❒
❒
❒
❒
❒ NON-STEROIDAL ANTI-INFLAMMATORY
DRUGS
❒
❒
❒
❒
❒ LOCAL ADMINISTRATION OF ANTIBIOTICS
AND ANTIMICROBIAL AGENTS
52
Drugs Used in
Periodontal Therapy

404
Essentials of Clinical Periodontology and Periodontics
3. Third generation agents: It should be effective against
specific periodontopathic organisms. The most
promising agent seems to be Delmopinol which is
a surface active agent. Though it is a weak antimicrobial
agent it can exert its effect by binding to salivary
proteins and thereby alters the cohesive and adhesive
properties of the films formed.
Chemicals used for Supragingival Plaque
Control
It is discussed elsewhere in this book.
Phenols
Mechanism of Action: Most phenols exert a non-specific
antibacterial action which is dependent upon the ability
of the drug in its non-ionized form to penetrate the lipid
component of the cell walls of gram-negative organisms.
The resulting structural damage will affect the permeability
control of microorganisms in addition to several metabolic
processes that are dependent upon enzymes contained
within the cell membranes. Phenolic compounds have
also been shown to exhibit anti-inflammatory properties,
which may result from their ability to inhibit neutrophil
chemotaxis, the generation of neutrophil superoxide ion
and the production of prostaglandin synthetase.
Listerine
TM
is an over- the-counter phenol precipitate
that contains thymol, eucalyptol, menthol, methyl
salicylate, benzoic acid and boric acid.
Quaternary Ammonium Compounds
Quaternary ammonium compounds are cationic
antiseptics and surface active agents. Quaternary
ammonium compounds tend to be more effective against
gram positive than gram-negative organisms. This may
suggest that these compounds are most effective as anti-
plaque agents when used against early developing plaque,
which predominantly contains gram-positive bacteria.
Quaternary Ammonium Compounds include:
i. Benzathonium chloride.
ii. Benzalkonium chloride.
iii. Cetyl pyridium chloride—CPC (Reach
TM
).
iv. Domiphen bromide (Cetrimide).
Mechanism of action: They have some similarities to
chlorhexidine, the molecules possess both hydrophobic,
and hydrophilic groups allowing for ionic and hydro-
phobic interactions. It is assumed that the interaction
with bacteria occurs with cationic binding, to the
phosphate groups in the cell wall teichoic acid in the
Gram-positive bacteria and to the phosphate groups in
the cell wall and to the membrane lipopolysaccharide
of Gram-negative bacteria. The membrane integrity may
subsequently be disrupted by interaction with the
lipophilic portion of the molecule, causing disturbance
of membrane functions and leakage of cytoplasmic
material.
ANTIBIOTICS USED IN
PERIODONTAL THERAPY
Chemotherapeutic agents—refers to the ability of an
active chemical substance to provide a therapeutic clinical
benefit.
Antimicrobial agents are chemotherapeutic agents that
reduce the amount of bacteria present, either by
specifically targeting certain organisms or by non
specifically reducing all bacteria.
Antibiotics are a form of anti-microbial agents produced
by or obtained from micro-organisms, that have the
capacity to kill other micro-organisms or inhibit their
growth.
Chemotherapeutic agents maybe administered
either:
i. Systemically, or
ii. Locally.
Systemic Administration of Antibiotics
It may reduce or eliminate bacteria that cannot be
removed by scaling or root planing, e.g. bacteria in the
tissues/root surfaces.
Other uses of systemic administration of antibiotics
include:
• Decrease in plaque and gingivitis.
• Retards bone loss.

405
Drugs Used in Periodontal Therapy
• Use of antibiotics in conjunction with non-surgical
therapy reduces/eliminates the need for periodontal
surgery.
• It is also useful in cases of aggressive periodontal
diseases that may be resistant to traditional non-
surgical or surgical therapies, e.g. localized juvenile
periodontitis, rapidly progressive periodontitis.
Advantages of Systemic Medication
i. It ensures drug penetration till the base of the
pocket.
ii. Affects tissue invasive organisms.
iii. Takes less time and is inexpensive.
iv. Treats multiple sites simultaneously.
v. In acute conditions like acute necrotizing ulcerative
gingivitis, etc. it is used to decrease active inflam-
mation.
vi. As a premedication for patients with medical prob-
lems requiring prophylactic antibiotic coverage.
Local Administration of Antibiotics
Many chemotherapeutic agents can be delivered locally.
Advantages of Local Drug Administration are:
i. Greater concentrations are achieved with reduced
drug doses.
ii. Systemic side effects are reduced.
iii. Slow releasing devices have the advantage of
releasing antibiotics gradually
iv. Their effect can be directed to specific target area.
Disadvantages
It can induce super-infections or hypersensitivity
reactions.
According to Gibson
An ideal antibiotic for use in prevention and treatment
of periodontal diseases should be:
• Specific to periodontal pathogens.
• Allogenic.
• Non-toxic, substantive.
• Not in general use for treatment of other diseases.
• Inexpensive.
Tetracyclines
Antibacterial Actions
Tetracyclines are a group of antibiotics that are derived
naturally from Streptomyces or derived semi-synthe-
tically. These antibiotics are bacteriostatic and are generally
more effective against gram positive bacteria than gram
negative bacteria. Tetracyclines are very effective in
treating periodontal diseases mainly because of their
concentration in gingival crevicular fluid (GCF) which
is 2 to 10 times more than that in serum. In addition,
many studies have proved that tetracyclines even at low
concentrations are very effective against many periodontal
pathogens.
Tetracycline hydrochloride is also a chelating agent
and chelates Ca,
2+
Mg,
2+
Al,
3+
in the gastrointestinal tract.
These ions, especially calcium are contained in a variety
of food substances. Since the chelate is poorly-absorbed,
it is advisable to take tetracycline either half an hour
before or after food.
Other properties of tetracyclines which are of value
in the management of periodontal diseases are:
a. Tetracyclines and collagenase inhibition (Host-derived
collagenase): These enzymes are derived from a
variety of sources including fibroblasts, epithelial cells,
macrophages and neutrophils. Collagenase derived
from neutrophils are more susceptible to tetracycline
induced inhibition.
b. Tetracyclines and bone resorption: The antiproteolytic
properties together with anticollagenase activity has
resulted in the use of these drugs to stop/inhibit bone
resorption. Tetracyclines inhibit bone resorption
induced by parathyroid hormone, prostaglandin E
series and bacterial endotoxins.
c. Anti-inflammatory actions of tetracyclines: Potential
anti-inflammatory properties include the ability of
tetracyclines to suppress polymorphonuclear
leukocyte activity, in particular, the scavenging action
on reactive oxygen metabolites. Alternatively, the
drugs may block eicosanoid synthesis (PGE
2
) by
inhibiting phospholipase A
2
activity.

406
Essentials of Clinical Periodontology and Periodontics
d. Tetracycline and fibroblast attachment:
• Pretreatment of dentine with tetracyclines
enhances fibroblast attachment and colonization.
Tetracycline can both condition the root surface
and influence the attachment properties of
fibroblasts.
• Tetracyclines can bind to, demineralize and release
from dentine. The substantivity of tetracycline is
proportional to the concentration of the drug
rather than to the time of application.
• The drug also enhances fibronectin binding.
(Hence their use as an adjunct in treating
periodontitis is very well established and their effect
is maximum in patients with localized juvenile
periodontitis and refractory periodontitis).
Adverse Effects of Tetracyclines
a. Gastrointestinal disturbances
• Diarrhea
• Nausea and vomiting
• Severe colitis (rare)
b. Overgrowth of resistant organisms
• Stomatitis
• Vaginitis
• Staphylococcal enterocolitis
c. Photosensitivity
• Skin rashes
• Hypersensitivity reactions.
Significant Drug Interactions
Involving Tetracyclines
Drug
Possible sequelae
Penicillin
Antagonism of bactericidal action
Antacids
Impaired absorption of tetracycline
Insulin
Enhanced hypoglycaemic action of
insulin
Contraceptive pill
Pill failure
Digoxin
Increased serum digoxin concentra-
tion
Warfarin sodium
Enhanced anticoagulant effect
Carbamezapine and
Decreased serum concentration of
phenytoin
doxycycline and minocycline
Contraindications for the
Use of Tetracyclines
a. Pregnancy—staining of deciduous teeth, impaired
bone growth.
b. Breast feeding—staining of developing teeth and
gastrointestinal disturbance in children.
c. Renal impairment—aggravates uremia.
d. Hepatic disease.
e. Systemic lupus erythematosis—exacerbation of
lesions.
Specific Agents and their Dosage
Tetracycline, minocycline and doxycycline are all semi-
synthetic members of the tetracycline group that are
commonly used in periodontal therapy.
Tetracycline—Requires administration of 250 mg four
times a day. It is inexpensive but compliance may be
reduced by taking 4 capsules a day.
Minocycline—Exhibits broad spectrum of antibacterial
activity especially in patients with adult periodontitis.
Dosage—200 mg twice a day for 1 week, side effects
include reversible vertigo.
Doxycycline—Same spectrum of activity as minocycline.
Absorption from gastro-intestinal tract is not altered by
calcium, metal ions or antacids.
Dosage—100 mg twice on the first day followed by 100
mg once a day or 50 mg twice a day for 4 days.
Metronidazole
It is bactericidal to anaerobes and it is believed to disrupt
bacterial DNA synthesis. They are not very effective
against A. actinomycetemcomitans infections but quite
effective against obligate anaerobes such as Porphyro-
monas gingivalis and Prevotella intermedia.
Clinically—It is used to treat acute necrotizing ulcerative
gingivitis, adult periodontitis and rapidly progressive
periodontitis.
Dosage—200 mg four times a day for 1 week/400 mg
three times a day for 1 week.
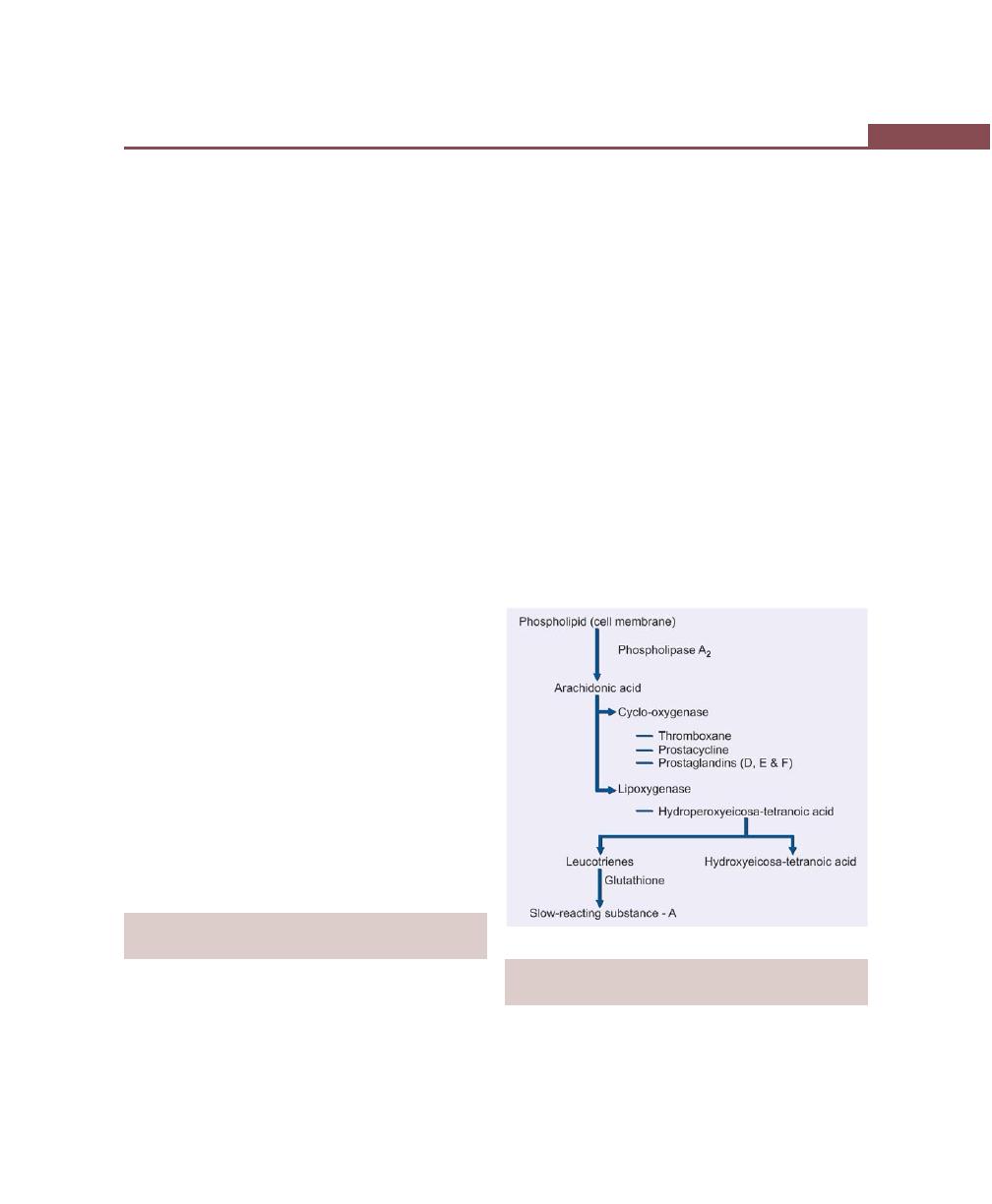
407
Drugs Used in Periodontal Therapy
Side effects—
• It has antabuse effect when alcohol is ingested.
• The symptoms include severe cramps, nausea and
vomiting.
• It inhibits warfarin metabolism.
• Patients on anticoagulant therapy should avoid this
drug because it prolongs prothrombin time.
Penicillins
Pericillins are bactericidal but induce allergic reactions.
Most widely studied antibiotics are amoxicillin and
amoxicillin clavulanate potassium (Augmentin).
Amoxicillin—It exhibits broad spectrum activity and is
useful in patients with refractory or juvenile periodontitis.
Dosage—500 mg three times a day for 1 week.
Augmentin—It is a combination of amoxicillin with
clavulanate potassium. It is useful in patients with
refractory or juvenile periodontitis.
Dosage
• Augmentin 375 mg—amoxicillin 250 mg + clavulanic
acid 125 mg three times a day for 1 week.
• Augmentin 625 mg—amoxicillin 500 mg + clavulanic
acid 125 mg twice a day for 1 week.
Ciprofloxacin—It is active against gram-negative rods but
has minimal effect on Streptococcus species. At present,
ciprofloxacin is the only antibiotic effective against all
the strains of A. actinomycetemcomitans.
Clindamycin—It acts against anaerobic bacteria and is
shown to be effective in patients with refractory
periodontitis. It is associated with pseudomembranous
colitis more often than other antibiotics.
NON-STEROIDAL ANTI-INFLAMMATORY
DRUGS (NSAIDs)
Strong evidence suggests that products of cyclo-
oxygenase pathway (e.g. PGE
2
) maybe important
mediators of pathogenic changes occurring in
periodontium. Hence NSAID may be of therapeutic
value in treating periodontal disease because they
interfere with arachidonic acid metabolism and thereby
inhibit inflammatory process. Various drugs that are
studied include flurbiprofen, ibuprofen, mefenamic acid
and naproxen.
Actions of Flurbiprofen
• Decreased PMNL migration.
• Decreased vascular permeability.
• Decreased platelet aggregation.
Mechanism of Action of NSAID (Fig. 52.1)
After the activation of inflammatory cells in the
periodontium by bacteria, phospholipids in the plasma
membranes of cells are activated by the enzyme
phospholipase A
2
and this leads to release of free
arachidonic acid in the area. Arachidonic acid can then
be metabolized into prostaglandins, thromboxanes and
prostacyclines by cyclo-oxygenase or into leucotrienes,
HETE and SRS-A by lipoxygenase enzyme.
Fig. 52.1:
Mechanism of action of NSAIDs
LOCAL ADMINISTRATION OF ANTIBIOTICS
AND ANTIMICROBIAL AGENTS (Fig. 52.2)
I. Vehicles for local delivery of chemotherapeutic
agents include: Dentifrices, mouth rinses, chewing
gum and slow release devices.
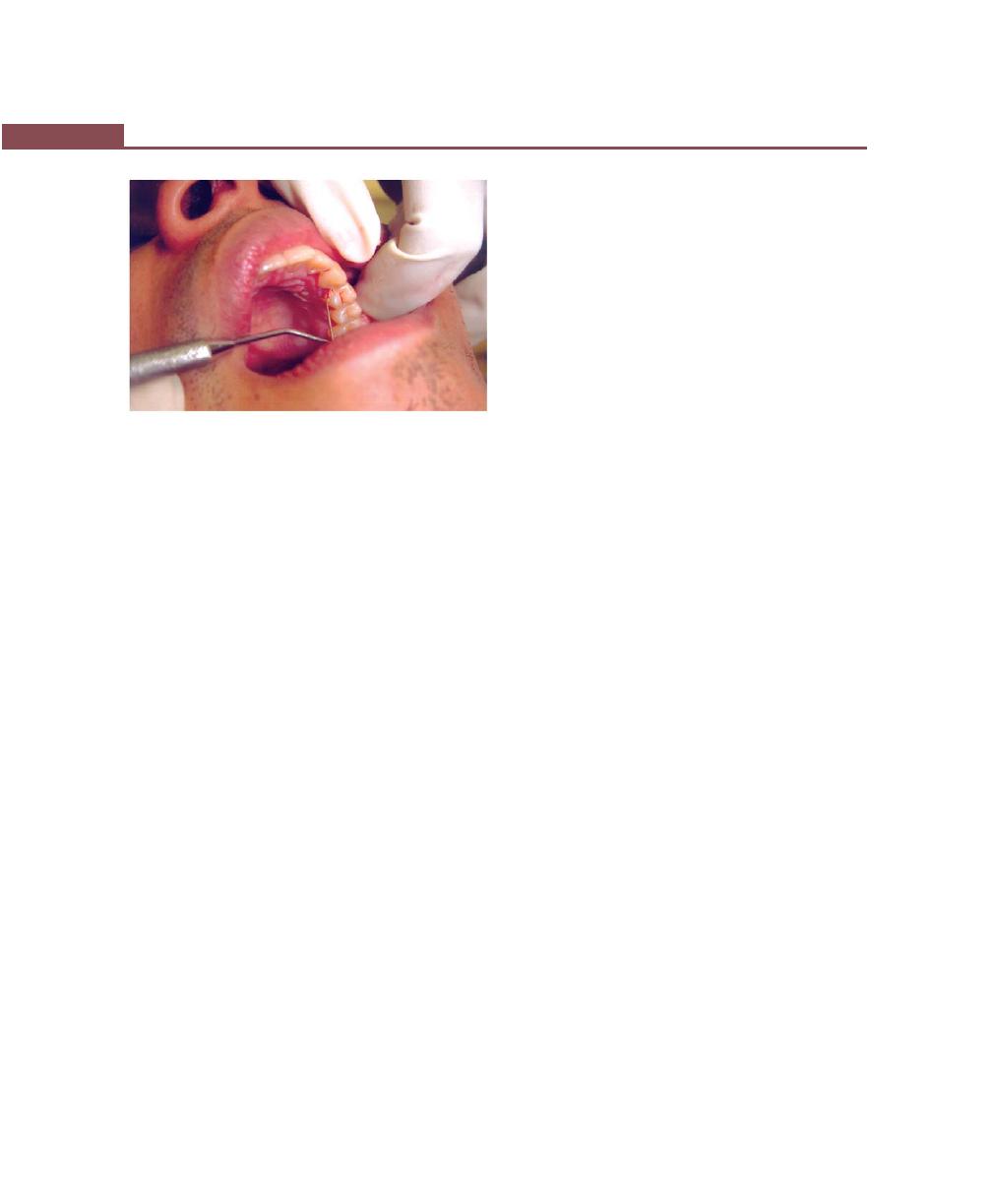
408
Essentials of Clinical Periodontology and Periodontics
II. Methods of delivery of chemotherapeutic agents
include: Root biomodification (direct professional
application to the root surface during surgery),
Keyes technique, irrigation methods include home
and professional drug delivery.
Vehicles for Local Delivery
Dentifrices, mouth rinses and chewing gum are inefficient
delivery systems in periodontics because they fail to direct
drugs into the periodontal pocket. They can be useful
in reducing inflammation associated with gingivitis but
they do not penetrate well into the periodontal pockets.
Slow-release Devices/Controlled-Release
Delivery Systems
They are designed to release chemotherapeutic agents
into the periodontal pocket over an extended period
of time, hence, they can achieve high levels of concen-
tration at sites where they are precisely needed.
Classification of Controlled-Release
Local Delivery Systems
i. Reservoirs without a rate-controlling system, e.g.
hollow fibers, gels and dialysis tubing (effective
only for 24 hours).
ii. Reservoirs with a rate-controlling system, e.g.
polymeric matrices, polymer membranes, mono-
lithic matrices and coated particles (effective for
more than 24 hours).
EVA system (Ethylene vinyl acetate) is based on
polymer technology with tetracycline dispersed within
a solid (monolithic) polymer of EVA. A formulation of
25 percent tetracycline in EVA (Actisite) has been
developed as 0.5 mm nonbiodegradable fiber and has
been approved by the FDA (Food and Drug
Administration).
The use of slow release devices placed in deep
periodontal defects, in conjunction with scaling and root
planing may offer promising results in the management
of such defects.
Methods of Delivery of
Chemotherapeutic Agents
a. Keyes technique.
b. Root biomodification.
c. Irrigation devices.
i. Home irrigation devices
• Supragingival home irrigation devices.
• Subgingival home irrigation devices.
• Marginal home irrigation devices.
ii. Professional subgingival irrigation.
Keyes Technique
Involves application, by tooth brushing, of a slurry of
sodium bicarbonate and hydrogen peroxide for the
control of plaque microorganisms. Various studies have
proved that minimal clinical benefit can be expected from
this technique simply because tooth brushing offers an
ineffective means of delivering medicaments into the
periodontal pocket.
Root Biomodification
Application of various medicaments to root surfaces
during surgical therapy has been evaluated. These agents
include tetracycline, doxycycline, citric acid and
fibronectin. Application of these agents on diseased root
surfaces during surgery may enhance connective tissue
attachment to the roots, although available data are
inconclusive (discussed elsewhere in this book).
Fig. 52.2:
Intrasulcular placement of tetracycline fibers
409
Drugs Used in Periodontal Therapy
Home Irrigation Devices
The advantage of home irrigation device is, it allows the
patient to deliver medicaments into the periodontal
pocket at home on a more frequent basis than in practice
with professional subgingival irrigation. The limiting
factors for these devices are inability to access the depth
of periodontal pocket and manual dexterity of the
patient.
i. Supragingival home irrigation devices: Results in
greater access to periodontal pockets than mouth
rinsing alone. The depth of penetration of
medicament is a maximum penetration of 4 to 5
mm. Therefore, these devices may be useful in
delivering medicaments in cases of gingivitis with
shallow pocket depths, but they are less useful in
delivering medicaments in periodontitis patients
with deeper pockets.
ii. Subgingival home irrigation devices: Generally
includes a blunt ended metal cannula that the
patient inserts into the periodontal pocket. It
increases the depth of penetration of fluid, but
largely depends on the manual dexterity of the
patient and has the potential for injury owing to
the metal tip.
iii. Marginal home irrigation devices: These are a
hybrid of both supragingival and subgingival home
irrigation. It has a jet tip attachment that has been
modified so that it can deliver fluids sub-gingivally.
Home irrigation devices allow penetration of fluid
upto 90 percent in pockets of 6 mm depth and
lesser penetration in pockets with deeper depths.
The irrigation is more effective (supra/subgingival)
if it is pulsed rather than constant.
Professional Subgingival Irrigation
It includes the use of a wide array of powered and
manually operated irrigators. Irrigation using a syringe
with a blunt needle has been used. The most commonly
used solutions are chlorhexidine gluconate, stannous
fluoride, tetracycline, metronidazole and hydrogen
peroxide. Irrigation with medicaments performed in
conjunction with root planing provide little, if any,
additional benefit over root planing alone.
CONCLUSION
In conclusion, all the above mentioned drugs can be
used as adjuncts to periodontal therapy but not as
monotherapy.
Some of the commercially-available local drug
delivery systems are:
1. Actisite
®
—It is a non-resorbable fiber containing
tetracycline.
2. Atridox
®
—It is biodegradable and contains doxy-
cycline.
3. Perio chip
®
—It is a chlorhexidine containing
biodegradable device.
KEY POINTS TO NOTE
1. Mechanical removal of tooth deposits is the foundation of
periodontal therapy. Since there is a possibility of bacterial
invasion of periodontal tissues in cases of aggressive forms
of periodontitis, systemic antibiotic therapy was thought to
be a valuable adjunct to mechanical therapy.
2. Best results can be obtained by antibiotic therapy combined
with scaling and root planing.
3. Tetracyclines are bacteriostatic antibiotics which provides a
broad spectrum of activity against both gram-positive and
gram-negative organisms. They are most commonly used
in the management of periodontal disease because the GCF
(gingival crevicular fluid concentration can be 5-10 times
more than the serum).
4. Apart from anti-microbial activity, it also has other important
properties like collagenase inhibition, anti-inflammatory
action, inhibition of bone resorption, etc. Other drugs that
are commonly used in periodontal therapy are metronida-
zole, penicillins and quinolones.
5. Prolonged antibiotic therapy can cause adverse effects like:
a. Development of resistant organisms
b. Hypersensitivity reaction.
c. Drug interactions.
d. Superinfection.
e. Dependency on patients compliance to take the drug
regularly.
f. Though periodontal disease involves only small area
of the body, due to systemic administration of the drug the
whole body gets unnecessarily loaded with the drug.
6. In order to overcome all the adverse effects of systemic
administration, local drug delivery system was introduced
in periodontics.
410
Essentials of Clinical Periodontology and Periodontics
REVIEW QUESTIONS
1. Classify anti-microbial agents, write in detail on role
of tetracyclines in periodontal therapy.
2. What are the advantages and disadvantages of
systemic antibiotics?
3. Classify local drug delivery systems.
BIBLIOGRAPHY
1. Gibson W. Antibiotics and periodontal disease. A selective
review of the literature. J Am Dent Assoc 1982; 104 : 213.
2. Jan Lindhe. Clinical Periodontology and Implant Dentistry,
4th edn, Blackwell Munkgard Publication, 2003.
3. Jorgen Slots, Miriam Ting. Systemic antibiotics in the treatment
of periodontal diseases. Periodontol 2000, 2002;28.
4. Micahel Rethman and Gary Greenstein. Oral irrigation in the
treatment of periodontal diseases. Curr Opin Peridontol 1994:
187-93.
5. Newman, Takei, Fermin A, Carranza. Clinical Periodontology,
9th edn, WB Saunders and Co., 2002.
6. Robin A. Seymour, Peter A. Heasman. Drugs, Diseases and
the Periodontium. Oxford University Press Publication, 1992.
7. Slots J. Rams TE. Antibiotics and periodontal therapy:
Advantages and disadvantages. J Clin Periodontol 1990;
17:479.
8. Walker CB. Selected anti-microbial agents : Mechanism of
action, side effects and drug interactions. Periodontol 2000,
1996; 10:12.
411
Questionnaire for Clinical Case Discussion
QUESTIONS
1. What is the significance of recording chief complaint,
history of present illness, past dental history, family
history and medical history?
2. What are the effects of bruxism, mouth-breathing,
tongue-thrusting, and cigarette smoking on the
periodontium? How do you diagnose the above
conditions?
3. What are the various methods of plaque control?
4. What are the various brushing techniques and its
advantages and disadvantages?
5. What is the difference between Bass method and
modified Bass method?
6. Describe and demonstrate the modified Bass
method.
7. What are the various interdental aids? Which is
used where? Discuss the criteria for its selection?
8. In which conditions do you see lymph node
pathology?
9. What is the normal color of the gingiva and what
are the factors responsible for maintaining the
normal color of gingiva?
10. What are the conditions and factors responsible
for change in gingival color?
11. Describe normal contour and factors responsible
for it. What changes in contour are seen in
disease?
12. Name some diseases in which gingival contour
are changed.
13. Describe size of gingiva in health and disease.
Name some factors responsible for normal size
and enlarged gingiva.
14. How do you check for normal consistency of the
gingiva? In disease what are the changes that
takes place in consistency?
15. What is the significance of stippling ? How is
normal stippling manifested?
16. What is the normal position of the gingiva?
17. Define gingival recession and describe its etiology,
classification, clinical significance and treatment .
18. What is the significance of bleeding on probing ?
How do you check for gingival bleeding ?
19. What are the etiological factors responsible for
gingival bleeding?
20. What are the microscopic changes associated with
gingival bleeding ?
21. What is the width of attached gingiva ? How do
you measure the width of attached gingiva ?
22. What are the tests done to identify whether the
width of attached gingiva is adequate or
inadequate ?
23. What are the mucogingival problems ?
24. How do you clinically differentiate between true,
pseudo, and combined pocket; supra bony and
infra bony pocket ?
53
Questionnaire for Clinical
Case Discussion
412
Essentials of Clinical Periodontology and Periodontics
25. How do you treat pockets ?
26. Describe the sequelae following unreplaced first
molars.
27. Describe the sequelae following loss of proximal
contact form.
28. How do you diagnose furcation involvement ?
29. Describe the etiology, classification and treatment
of furcation involvement.
30. What are the causes for tooth mobility ? Give the
classification of mobility ?
31. What is pathological migration ? How do you
clinically identify it ?
32. What are the causes for pathological migration ?
33. Define attrition, abrasion and erosion.
34. When is tooth tender on percussion.
35. What is retrograde periodontitis ?
36. How do you diagnose a case of trauma form
occlusion ?
37. What are the types, signs and symptoms of trauma
from occlusion ?
38. What is the difference between mobility and
fremitus ?
39. What are the various indices used for accessing
oral hygiene ?
40. What are the indices for gingival bleeding ?
41. Effects of over-hanging restorations on perio-
dontium.
42. Importance of recording various lab investigations.
43. Effects of improper orthodontic treatment on
periodontium.
44. What are the things you look for in a radiograph ?
45. What are the advantages and disadvantages of
intraoral periapical radiographs ?
46. Justify your diagnosis of the case. If diagnosis is
gingivitis then why ? Or if diagnosis is periodontitis
then why ?
47. Define prognosis. What are factors responsible
for individual and overall tooth prognosis ?
48. Discuss the treatment plan in a sequential order.
QUESTIONS RELATED TO PERIODONTAL
INSTRUMENTS AND TREATMENT
49. Classify periodontal instruments.
50. Give the characteristics of a scaler and curette
with differences.
51. Differences between Universal and Gracey curette.
52. Classify various supragingival and subgingival
scalers.
53. What are the instruments used for root planing ?
54. Define scaling and root planing.
55. What are the principles of periodontal instrumen-
tation ?
56. What are finger rests and grasps ?
57. Different angulations needed for scaling proce-
dures.
58. Recent advances in periodontal instrumentation ?
QUESTIONS AND ANSWERS
Q1. What is the significance of recording chief
complaint, history of present illness, past dental
history, family history and medical history ?
Answer
Chief Complaint: It is the patient’s description of the
symptoms related to the disease for which treatment is
being sought (to be recorded in patient’s own words).
It is often recorded as patient’s desire and when the
patient is questioned about the principal reason for his/
her dental visit, his/her desire is frequently expressed in
the form of cleaning, filling etc. This should not be
recorded as chief complaint. The dentist has to further
derive the actual complaint from the patient with
questions like why he/she desires that particular treatment,
because most of the times in periodontics we deal with
chronic problems, hence they do not actually have any
complaint.
History of present illness: It is the chronological account
of the problem indicated by the chief complaint .It
comprises of the onset, duration, any relieving or
aggravating factors of the illness which best describes
the disease present and helps in determining the diagnosis
and treatment plan. Taking the detailed history of the
illness of the patient enables the operator to be as
confident as possible that the patient will not be
endangered during treatment.

413
Questionnaire for Clinical Case Discussion
Past dental history: It consists of patient’s previous
experience of oral diseases and his treatment for those
diseases. It should acquaint the dentist with the patient’s
previous dental treatment, professional and home care
procedures, frequency and nature of periodontal care,
reason for prior extraction of teeth, history of prior dental
and periodontal disease and bleeding and anesthetic
problem, drug allergies during previous dental
procedures. All these data aid in the development of
diagnosis and prognosis and may have a significant
influence on the plan of treatment.
Family history: Many diseases such as idiopathic gingival
fibromatosis, hemophilia are inherited. Thus family history
should record details of condition existing in other
members of the family.
Medical History:
The objectives are:
1. To determine systemic factors or diseases that will
require special consideration prior to, during or after
periodontal therapy.
2. It aids the clinician in the diagnosis of oral
manifestation of systemic disease and in the detection
of systemic condition that may affect the periodontal
tissue response to local factors or that require special
precaution and modification in treatment procedure.
3. To identify the patients who are taking drugs or
medication that could adversely interact with drugs
prescribed, that would complicate dental therapy or
they may serve as a clue to an underlying systemic
disease, the patient has failed to mention.
4. To provide information for the dentist to modify the
treatment plan for the patient in light of any systemic
diseases or potential drug interaction.
5. To help establish a good patient-doctor relationship.
Q2. What are the effects of bruxism, mouth-
breathing, tongue-thrusting, and cigarette-smoking
on the periodontium ? How do you diagnose the
above conditions?
Answer
Mouth-breathing
It leads to localized gingival inflammation that is usually
confined to the labial gingiva of the maxillary anterior
teeth. The tissue becomes reddened and swollen and
it bleeds easily. The surface of the gingiva is shiny.
Gingival changes associated with mouth-breathing are:
a. Localized gingival inflammation in the maxillary
anterior region.
b. Crowding of teeth with gingivitis.
Diagnosis
It can be made by simple tests:
1. Mirror test: A double side mirror is held between the
nose and the mouth. Fogging on the nasal side of
the mirror indicates nasal-breathing while fogging
towards the oral side indicates oral breathing.
2. Cotton test: A butterfly-shaped piece of cotton is
placed over the upper lip below the nostrils. If the
cotton flutters down it indicates nasal breathing.
3. Water test: The patient is asked to fill his mouth with
water and retain it for a period of time. While nasal
breathers accomplish this with ease, mouth-breathers
find the task difficult.
Tongue-thrusting
It entails persistent, forceful wedging of the tongue against
the teeth, particularly in the anterior region. It causes
excessive lateral pressure, which maybe traumatic to the
periodontium. It also causes spreading and tilting of the
anterior teeth with an open bite anteriorly, posteriorly
or in the premolar area. It causes labial drifting of teeth,
thereby the antagonism between forces that direct the
tooth labially and inward pressure from lip may lead
to tooth mobility. The altered inclination causes
accumulation of food debris. There is loss of proximal
contact, which leads to food impaction. It is also an
important contributing factor in pathologic tooth
migration.
Tongue-thrusting can be diagnosed by its position.
Normally, it is placed against the palate with the tip
behind the maxillary teeth during swallowing. During
tongue-thrusting it is placed behind the mandibular teeth.
It can be diagnosed by altered inclination of the teeth,
spacing between the teeth and marked open bite.

414
Essentials of Clinical Periodontology and Periodontics
Cigarette Smoking
Smoking has a detrimental effect on the progression of
periodontal disease and healing after periodontal therapy.
The following are its effects:
1. Brownish, tar-like deposits and discoloration of tooth
surface.
2. Diffuse grayish discoloration and leukoplakia of the
gingiva may occur.
3. “Smoker’s Palate” characterized by prominent
mucous glands due to inflammation of orifices of
mucous glands and a wrinkled, cobblestone surface
may occur.
4. Post-surgical healing is delayed.
5. Produces an immediate transient but marked increase
in gingival fluid flow, as a result of blood changes
induced by nicotine.
6. More severe gingivitis and periodontitis. More calculus
has been reported in smokers.
7. Factors responsible for increased periodontal
problems in smokers:
a. Increased keratinized cells in gingiva
b. Nicotine metabolites have been found.
c. Oral Polymorphonuclear leukocytes have reduced
phagocytosis
d. Vascular reaction is suppressed in smokers.
Diagnosis can be made by the presence of excessive black
or brown stains on the tooth surface, discoloration of
the gingiva and heavy amount of calculus is also found.
Bruxism
Bruxism is clenching or grinding of the teeth when the
individual is not chewing or swallowing. It has been
estimated that during clenching or grinding the individual
might impose a load of over 20 kg on a tooth over a
period of 2.5 seconds each time. This is far in excess
of normal functional stresses and causes increased flow
within the viscoelastic periodontal ligament and distortion
of alveolar bone. It also causes attrition of teeth.
Clinical features
1. Advanced attrition, presence of wear facets.
2. Increased tooth mobility patterns which are not
commensurate with the amount of attachment loss
or degree of gingival inflammation.
3. Widened periodontal ligament spaces are seen in
radiographs.
4. Hypertonicity of the muscles of mastication.
5. Temporomandibular joint discomfort.
Diagnosis
History and clinical examination in most cases is sufficient
to diagnose bruxism. Occlusal prematurities can be
diagnosed by use of articulating papers. Electromyo-
graphic examination can be carried out to check for
hyperactivity of the muscles of mastication.
Q3. What are the various methods of plaque
control?
Answer
Plaque control is the removal of microbial plaque and
prevention of its formation on teeth. Basically there are
two types of plaque control.
Mechanical Plaque Control
a. Toothbrushes
b. Powered tooth brushes
c. Interdental cleansing aids
• Interdental brushes
• Single tufted brushes
• Dental floss
d. Wooden tips
e. Gum stimulator
f. Oral irrigation devices
g. Dentifrices
Chemical Plaque Control
The following chemicals are available in varying
concentrations:
a. Chlorhexidine gluconate 0.2 percent (Hexidine
®
,
Clohex
®
) and 0.12 percent (Periogard
®
)
b. Phenolic compounds (Listerine
®
) containing,
• Thymol – 0.06 percent
• Eucalyptol – 0.09 percent
• Benzoic acid – 0.15 percent

415
Questionnaire for Clinical Case Discussion
• Menthol – 0.04 percent
• Acetyl salicylate
c. Quaternary ammonium compounds (Cetyl pyridium
chloride) commercially available as Reach
®
.
All the above mentioned chemicals are used only as
adjunct to mechanical plaque control.
Q4. What are the various brushing techniques and
its advantages and disadvantages?
Answer
Many methods of tooth-brushing have been described,
but studies evaluating the effectiveness of the most
common brushing techniques have shown that no one
method is clearly superior. The best method is the one
that suits the individual’s needs and abilities and the
responsibility of the dental professional is to instruct the
patient as to how to perform the task thoroughly.
Various techniques are:
Scrub Technique
Advantages
Very easy to learn and master the technique.
Disadvantages
1. Inefficient plaque removal
2. Causes gingival trauma and recession
3. Causes cervical abrasion.
Roll Technique
Disadvantages
1. Difficult to learn and requires sufficient dexterity
2. Poor plaque removal from the sulcus area.
3. Poor plaque removal from the gingival one-third of
posterior teeth because of the contour of the teeth.
Bass Method
Advantages
1. The short back and forth motion is easy to master
because it requires the same simple movement,
familiar to most patients who were using scrub
technique.
2. It cleans the cervical and interproximal portions of
teeth.
Disadvantages
1. It is time consuming because different areas of teeth
have to be brushed.
2. Patient might miss the gingival sulcus and may place
the brush on the attached gingiva.
3. It only dislodges the plaque in interproximal areas
but does not remove them.
Modified Bass Method
Advantages
1. The short back and forth motion is easy to master
because it requires the same simple movement
familiar to most patients who were using the scrub
techniques.
2. It dislodges and removes plaque from cervical and
interproximal portions of the teeth.
Disadvantages
1. It is time consuming because different areas of teeth
needs to be brushed.
Modified Stillman Method
Advantages
1. The gingiva is mechanically stimulated
2. The gingival third of the tooth is contacted with a
short vibratory motion and plaque is removed
between the gingival margin and the exposed area
of tooth surface. Hence advised only in areas of
gingival recession.
3. The tips of the bristles tend to reach the interproximal
areas to clean and stimulate the interdental papilla
without injury.
Disadvantages
1. Patient might miss the gingiva and cervical areas of
the teeth, thus, leaving behind plaque.
2. Patient might not apply sufficient pressure to produce
blanching of tissue.
Charter’s Technique
Advantages
1. In this method plaque is removed gently, without
much pressure.
416
Essentials of Clinical Periodontology and Periodontics
2. It massages the gingiva and encourages healing,
hence advised only during postsurgical period.
Disadvantages
1. Poor plaque removal when indicated in routine
patients with or without periodontal involvement.
Q5. What is the difference between Bass method
and modified Bass method ?
Answer
Bass method
Modified Bass method
Technique:
The bristles are directed
The modification consists of
apically into the gingival
sweeping the bristles towards
sulcus at 45°
to the long
the occlusal surface after
axis of the tooth and are
completing the vibratory
activated by applying gentle
motion in gingival sulcus.
pressure in an apical direc-
tion and by making short
vibratory strokes.
Efficiency:
It is efficient in removing
It is efficient in removing
dental plaque from the
dental plaque from the gin-
gingival third of the tooth
gival sulcus and interdental
and from shallow gingival
areas.
sulcus
Q6. Describe and demonstrate the modified Bass
method ?
Answer
It is also known as intrasulcus cleansing or intrasulcular
method.
Technique: Here the patient is asked to place the head
of a soft brush parallel with the occlusal plane, beginning
with the most distal tooth in the arch covering 3 to 4
teeth at a time. The patient is then asked to place the
bristles at the gingival margin, creating an angle of 45
degree to the long axis of the teeth and exerting gentle
vibratory pressure, using short back and forth motion,
without dislodging the tips of the bristles. After completing
such 20 strokes in the same position, the head of the
brush is tilted and moved occlusally. This is repeated for
4-5 times. Patient is instructed to continue brushing
around the arch, 3 teeth at a time, both facially and
lingually. The brush is inserted vertically to reach the
lingual surfaces of the anterior teeth. The pits and fissures
of the occlusal surfaces are brushed with 20 short, back
and forth movements.
Advantages:
1. It removes plaque from the sulcus and other areas
of tooth.
2. It aids in gingival massage.
Disadvantage
1. It is hard to master.
Q7. What are the various interdental aids ? Which
is used where ? Discuss the criteria for its selec-
tion.
Answer
Various interdental aids are:
• Dental floss
• Interdental brushes
• Gauze strips
• Unitufted brushes
• Tooth picks
Criteria for selection of these aids depends upon the
type of embrasure:
Type I: Interdental papilla completely fills the embrasure
space. Dental floss can be used.
Type II: Mild loss of interdental papilla due to slight spacing
between the teeth. Miniature bottlebrushes can be
advised.
Type III: No proximal contact between the teeth, like
diastema where there is no interdental papilla. Unitufted
brushes are advised.
Dental floss: It is used for cleaning in narrow gingival
embrasures that are occupied by intact papillae and
bordered by tight contact zones. It is the most effective
dental hygiene aid.
Interdental brushes: It is used in areas of moderate
papillary recession, proximal tooth surfaces adjacent to
open embrasures, orthodontic appliances, fixed pros-
thesis, dental implant and other areas that are hard to
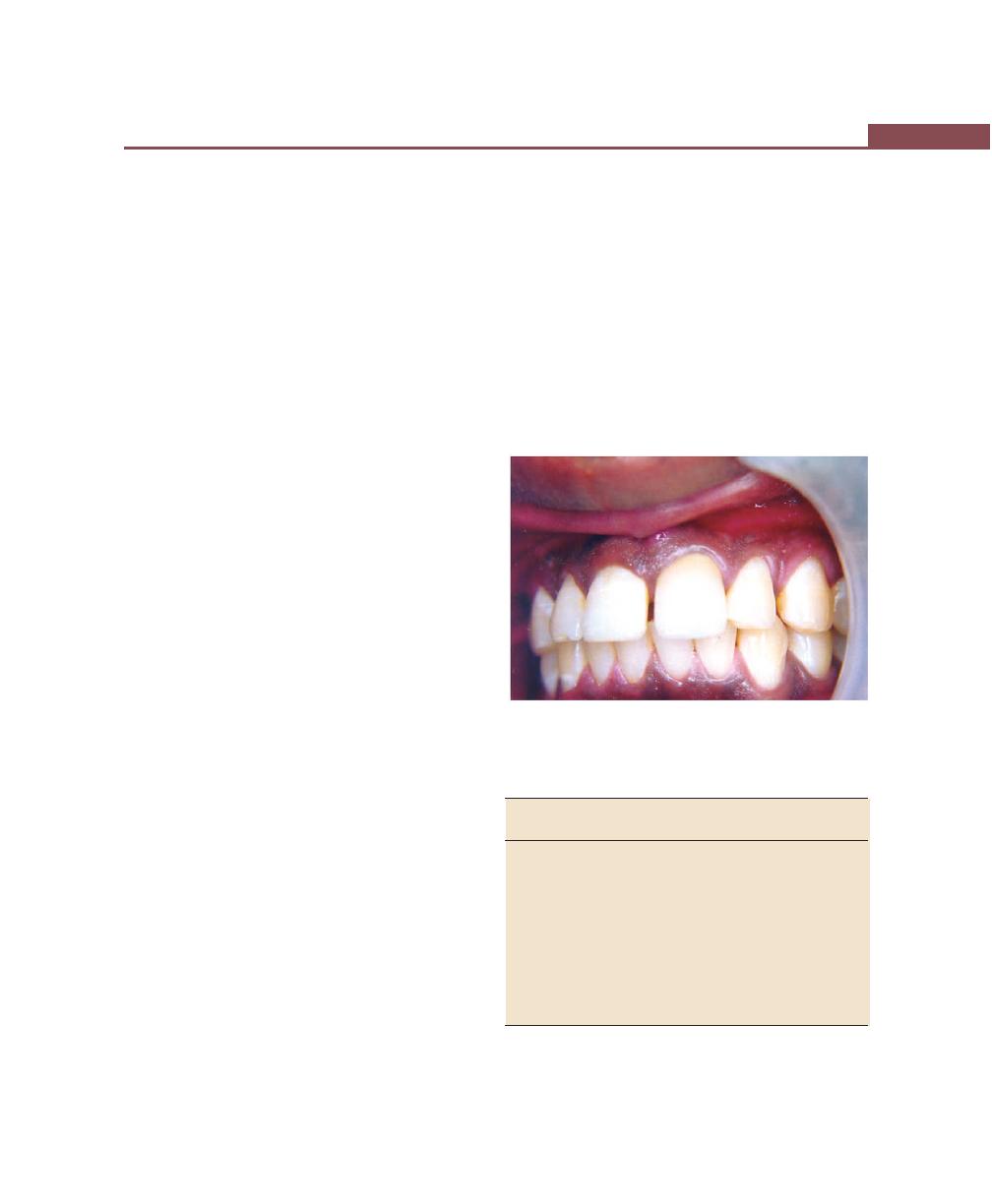
417
Questionnaire for Clinical Case Discussion
reach with regular tooth brush. It is also used in concave
proximal areas and exposed class IV furcation.
Gauze strip: It is used for proximal surface of widely
spaced teeth, for surface of teeth next to edentulous
spaces and fixed appliances.
Unitufted brush: It is used for inaccessible areas such
as lingual surface of the mandibular molars, abutment
teeth, distal surface of the most posterior teeth, crowded
teeth, open interproximal areas and isolated areas of
deep recession. It is also indicated in fixed dental
prosthesis.
Toothpicks: It is used for plaque removal at or just
underneath gingival margin, for concave proximal tooth
surface and for exposed furcation areas.
Q8. In which conditions do you see lymph node
pathology ?
Answer
Various systemic diseases contribute for lymph node
enlargements, which are as follows:
i. Syphilis
ii. Tuberculosis
iii. Acute bacterial infections
iv. Fungal/parasitic infections
v. Malignant tumor metastasis from nasopharynx,
oral cavity, pharynx, thyroid.
vi. Lymphatic diseases such as Hodgkin’s disease and
lymphatic lymphoma
vii. Leukemia’s such as chronic lymphatic, chronic
granulocytic leukemia
viii. Endocrinal disorders such as hyperthyroidism,
hypopituitarism.
Common conditions of oral cavity, which cause
cervical lymphadenopathy are:
i. Acute inflammatory conditions such as perio-
dontal/periapical abscess, acute necrotizing ulcer-
ative gingivitis, acute herpetic gingivostomatitis,
apthous ulcers and others.
ii. Oral manifestations of systemic diseases.
iii. Malignant conditions: Secondary carcinoma such
as epithelioma of tongue, lip, cheek and face.
Q9. What is the normal color of the gingiva and
what are the factors responsible for maintaining
the normal color of gingiva ?
Answer
Normal color of gingiva is coral pink (Fig. 53.1)
Factors responsible are:
• Vascular supply
• Thickness and degree of keratinization of epithelium.
• Presence of pigment containing cells.
Fig. 53.1:
Color of the gingiva in health
Q10. What are the conditions and factors res-
ponsible for change in gingival color (Fig. 53.2) ?
No Disease condition Color change/
Factors
clinical change
responsible
1. Chronic gingivitis
Varying shades
1. Vascular pro-
of red, reddish-
liferation (ery-
blue, deep-blue
thematous)
2. Reduction of
keratinization
owing to epi-
thelial com-
pression by
inflamed tissue
3. Venous stasis
4. Tissue necrosis
Contd...
418
Essentials of Clinical Periodontology and Periodontics
Contd...
2.
Acute gingivitis
Brightish-red
Same as above
a. ANUG/HV
erythema Shiny-
b. Herpetic gin-
slate gray
givostomatitis
Dull-whitish gray
c. Chemical
irritation
3.
Bismuth, arsenic,
Black line follow- Perivascular pre-
mercury pig-
ing the contour
cipitation of
mentation
of marginal
metallic sulfides
gingival
in subepithelial
connective tissue
seen only in areas
of inflammation
due to increased
permeability of
blood vessels
4.
Lead pigmen-
Bluish-red or
Perivascular pre-
tation
deep-blue linear cipitation of
pigmentation
metallic sulfides
(Burtonian line)
5.
Silver pigmen-
Violet marginal
Perivascular pre-
tation
line
cipitation of
metallic sulfides.
The factors responsible for the contour of gingiva
are:
• Shape of teeth and their alignment in the arch.
• Location and size of proximal contact.
• Dimensions of facial and lingual gingival embrasures.
During disease process:
1. The marginal gingiva becomes rolled or rounded and
interdental papilla becomes blunt and flat, e.g. chronic
gingivitis
2. Punched out crater-like depressions at the crest of
interdental papilla extending to the marginal gingiva
is seen in ANUG.
3. Irregularly-shaped denuded appearance of the
gingiva, seen in chronic desquamative gingivitis.
Fig. 53.2:
Color of the gingiva in disease. (Note the
marginal erythema)
Q11. Describe the normal contour and factors
responsible for it. What changes in contour are
seen in disease?
Answer
The normal contour of the marginal gingiva is scalloped
and knife edged, whereas the interdental papilla, in the
anterior region is pyramidal in shape and posteriorly it
is tent-shaped (Figs 53.3 and 53.4).
Fig. 53.3:
Contour of the gingiva in health
Fig. 53.4:
Contour of the gingiva in disease
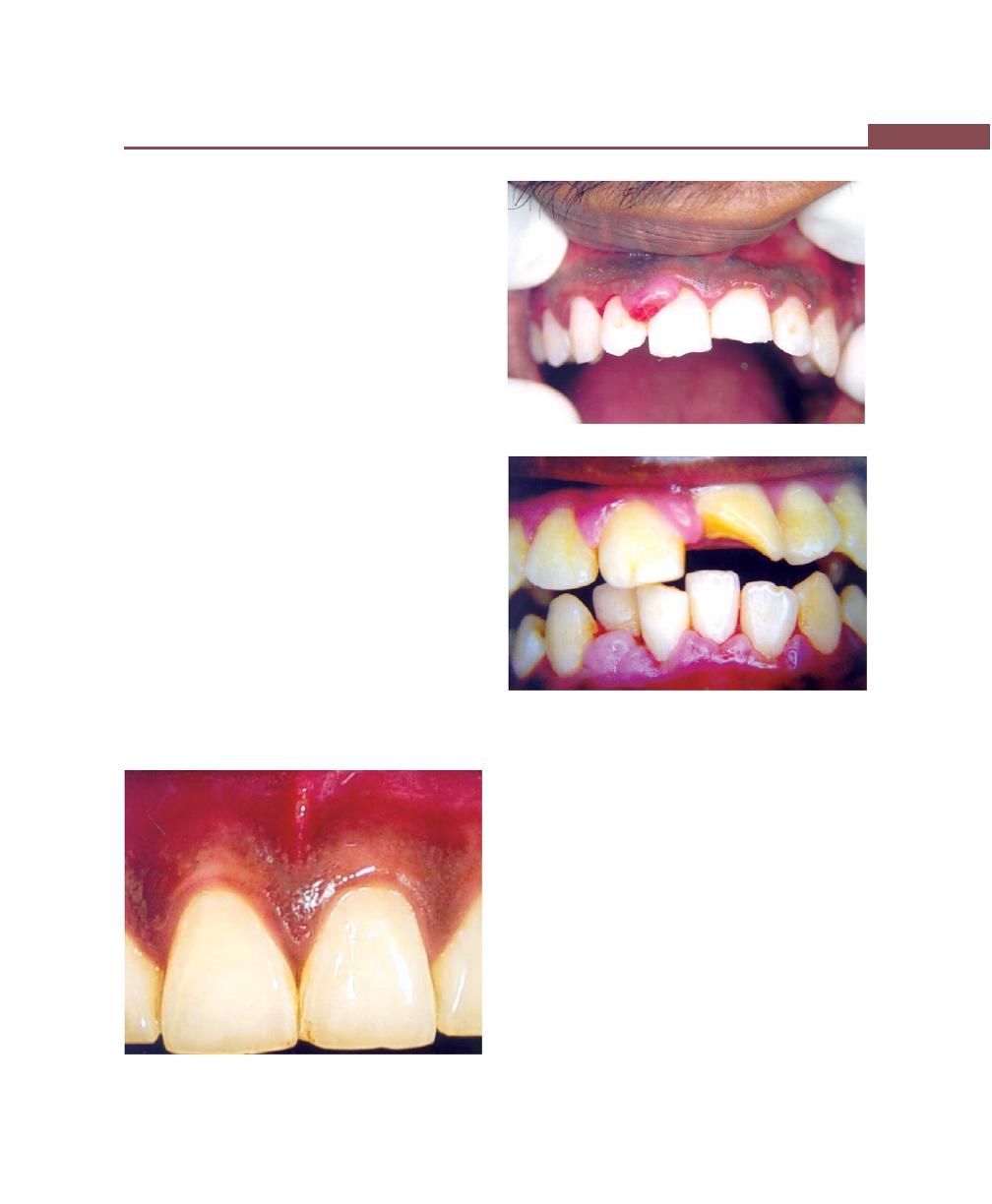
419
Questionnaire for Clinical Case Discussion
4. Exaggerated scalloping of the gingiva as seen in
gingival recession.
5. Apostrophe shaped indentation extending from and
into the gingival margin for varying distances on the
facial surfaces as seen in Stillman’s clefts
6. Life-saver like enlargement on the marginal gingiva,
most commonly on the canine and premolar facial
surface as seen in McCall’s festoons.
Q12. Name some diseases in which gingival
contour is changed.
Answer
Gingival contour is changed in conditions like:
• Gingival recession
• Gingival enlargement
• Acute and chronic gingivitis
• Stillman’s clefts and McCall’s festoons.
Q13. Describe size of gingiva in health and
disease. Name some factors responsible for
normal size and enlarged gingiva.
Answer
Normal
The size of gingiva corresponds to sum of total bulk of
cellular and intercellular elements and their vascular
supply. Alteration in size is a common feature of gingival
disease (Figs 53.5 to 53.7).
Change in disease:
Size of gingiva is enlarged and is called as gingival
enlargement. It maybe inflammatory or noninflamma-
tory. In inflammatory, there is increase in cells and
decrease in fibers. In noninflammatory there is increase
in fibers and decrease in cells.
Hypertrophy constitutes an increase in size of cells
and with such change, an increase in the size of the organ.
Hyperplasia constitutes increase in number of cells
in an organ or tissue there by contributing to an overall
increase in organ size.
Types of gingival enlargement:
i. Inflammatory enlargement
• Chronic
• Acute
Fig. 53.5:
Size of the gingiva in health
Fig. 53.6:
Inflammatory gingival enlargement
Fig. 53.7:
Fibrotic gingival enlargement
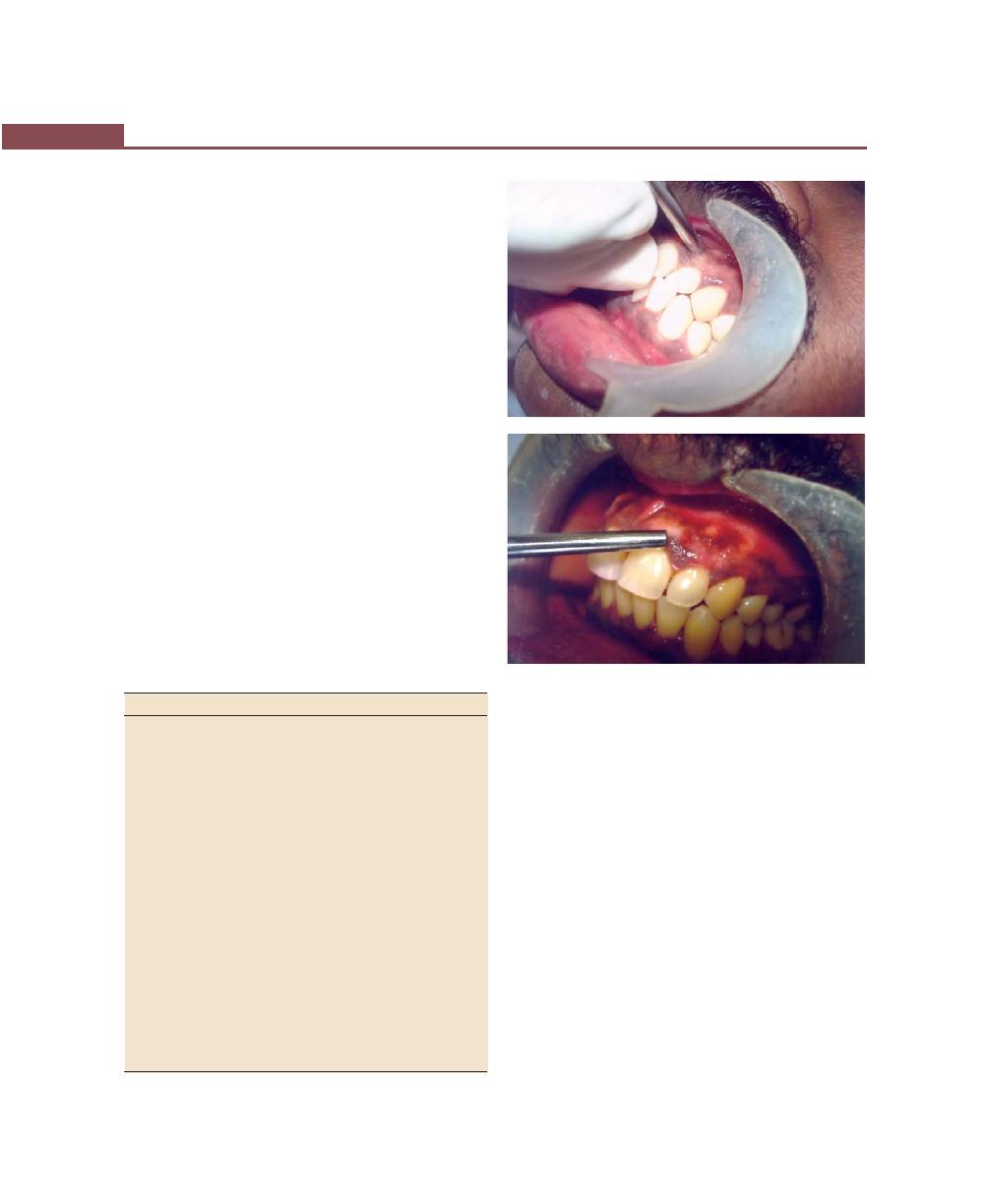
420
Essentials of Clinical Periodontology and Periodontics
ii. Fibrotic enlargement
• Drug-induced
• Idiopathic
iii. Combined enlargement
iv. Enlargement associated with systemic diseases or
conditions
• Pregnancy
• Puberty
• Vitamin c deficiency
• Plasma cell gingivitis
• Nonspecific conditioned enlargement
• Leukemia
• Granulomatous disease
v. Neoplastic enlargement
• Benign
• Malignant
vi. False enlargement
Q14. How do you check for normal consistency
of gingiva? In disease what are the changes that
takes place in consistency?
Answer
Clinical and histopathologic changes in gingival consis-
tency in disease (Figs 53.8A and B).
Clinical changes
Microscopic changes
Chronic gingivitis
1. Soggy puffiness that
Infiltration by fluid and cells of
pits on pressure
inflammatory exudates.
2. Marked softness and
Degeneration of connective tissue
friability with ready
and epithelium, thinning of epi-
fragmentation and
thelium, edema and leukocytic
pinpoint surface areas
invasion.
of redness and
desquamation
3. Firm, leathery consis-
Fibrotic and epithelial proliferation
tency.
associated with long standing
chronic inflammation.
Acute gingivitis
1. Diffuse puffiness and
Diffuse edema, fatty infiltration.
softening
2. Sloughing with grayish
Necrosis with formation of a
flake like particles of
pseudomembrane and degenera-
debris adhering to eroded
ted epithelial cells in a fibrous
surface
mesh work. Inter and intracellular
3. Vesicle formation
edema with degeneration of
nucleus and cytoplasm and rup-
ture of cell wall.
The normal consistency of the gingiva can be checked
by palpation either with a blunt instrument or digital
pressure. Pitting on palpation indicates soft and
edematous gingiva.
Q15. What is the significance of stippling? How
is normal stippling manifested?
Answer
Stippling is a form of adaptive specialization or
reinforcement for function. It is a feature of healthy
gingiva and reduction and loss of stippling is a common
sign of gingival disease (Fig. 53.9).
Stippling is produced by alternate rounded
protruberances and depressions in the gingival surface.
Papillary layer of the connective tissue projects into the
elevations, and the elevated and depressed areas are
Figs 53.8A and B:
Clinical demonstration for checking.
(A) Normal consistency, (B) Blanched gingiva
A
B
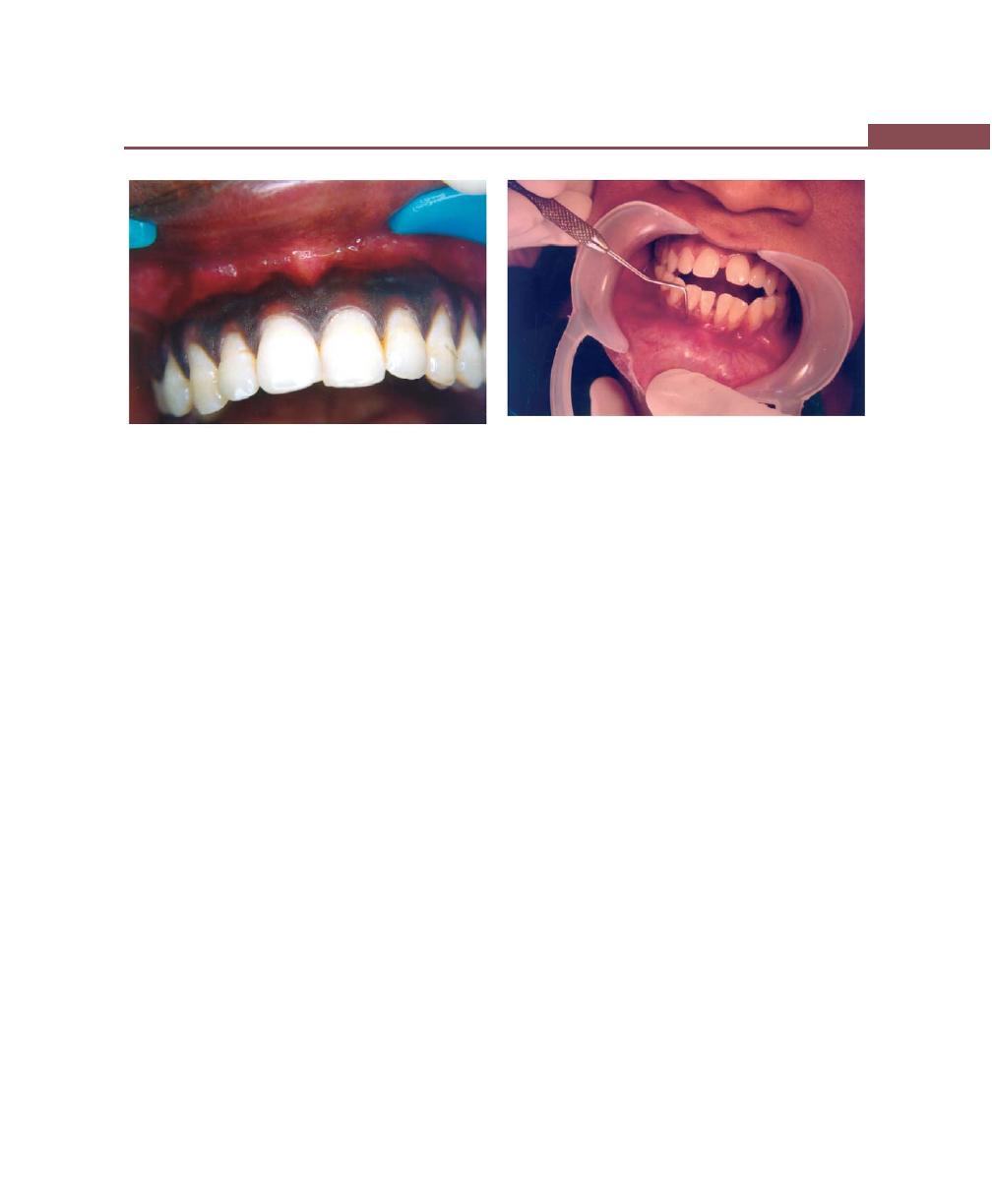
421
Questionnaire for Clinical Case Discussion
covered by stratified squamous epithelium. Stippling is
best viewed by drying the gingiva.
Q16. What is the normal position of the gingiva?
Answer
The normal position of gingiva is 1 millimeter above the
cemento–enamel junction.
Physiologic factors responsible for normal position of
gingiva are:
1. Position of teeth in the arch.
2. Root bone angle
3. Mesiodistal curvature of tooth surface.
Pathologic factors responsible for change in gingival
position are:
1. Tooth brush trauma
2. Gingival inflammation
3. High frenal attachment
4. Tooth malposition
5. Friction from soft tissue
Q17. Define gingival recession and describe the
etiology, classification, clinical significance and
its treatment.
Answer
Gingival recession is defined as apical shift of the gingival
margin to a position apical to the CEJ, with exposure
of root surface to the oral cavity (Fig. 53.10).
Etiology:
Mainly three factors are responsible:
a. Inflammatory: Plaque induced inflammatory perio-
dontal diseases, toothbrush injury, and surgical
treatment of inflammatory periodontal disease.
b. Anatomic factors: Developmental anatomic
abnormalities, (dehiscences, thin bony plates, high
frenum attachments) malocclusion.
c. Iatrogenic factors:
• Deleterious habits (Pressure of foreign objects like
finger nails, pencils, hairpins).
• Clasps and mandibular oral denture bars or
aprons in partial dentures.
• Prolonged orthodontic treatment, improper resto-
rations.
Classification
I. Sullivan and Atkins classified gingival recession as:
a. Shallow narrow
b. Shallow wide
c. Deep narrow
d. Deep wide
II. Millers classification
Class I: Includes marginal tissue recession that does not
extend to the mucogingival junction. There is no loss
of bone or soft tissue in the interdental area. This type
of recession can be narrow or wide (group a and b in
Sullivan and Atkins classification).
Fig. 53.9:
Stippling in normal healthy gingiva
Fig. 53.10:
Gingival recession
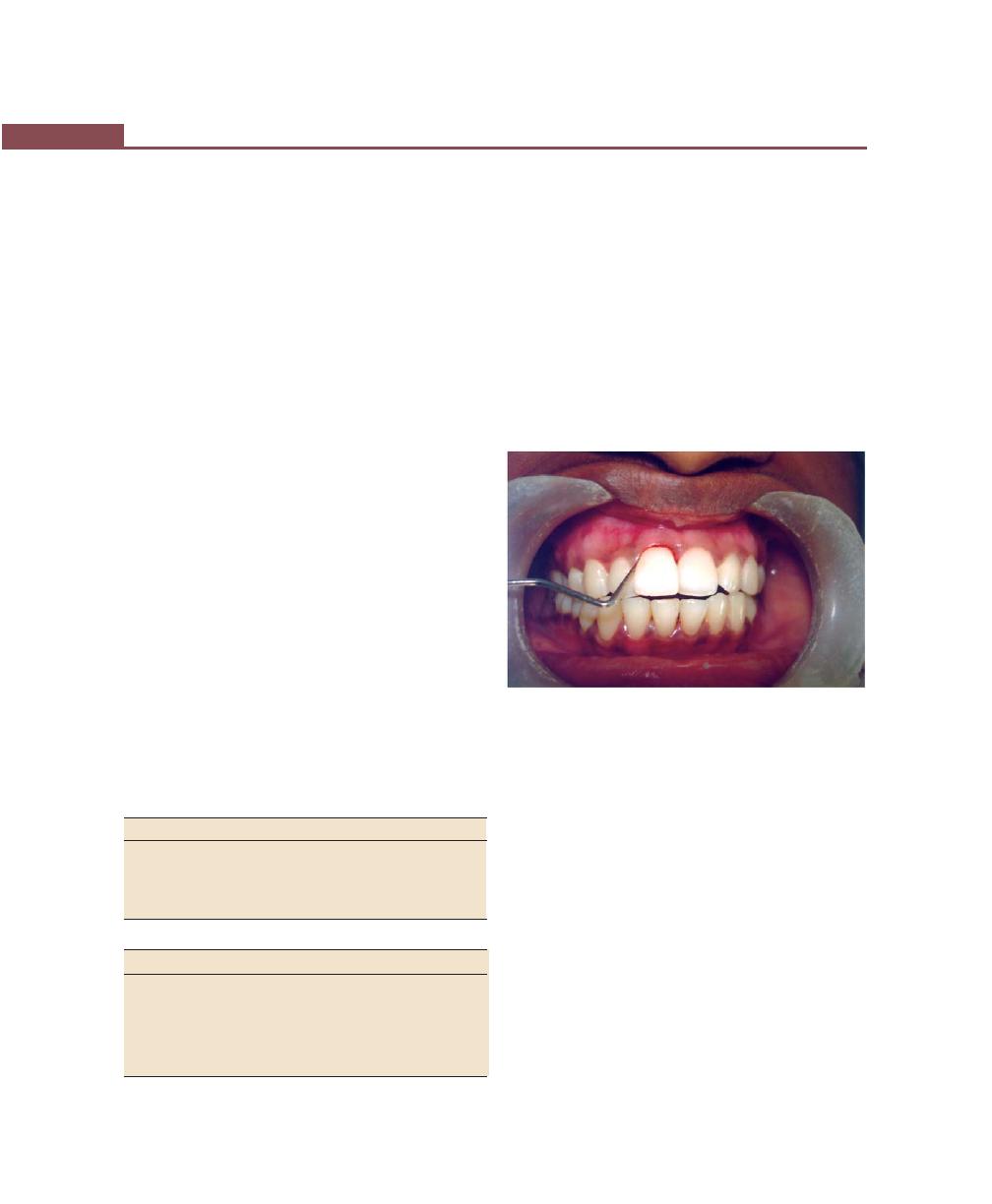
422
Essentials of Clinical Periodontology and Periodontics
Class II: Marginal tissue recession that extends to or
beyond the mucogingival junction. There is no loss of
bone or soft tissue in the interdental area. This type of
recession can be classified into wide and narrow (group
c and d of Sullivan and Atkins classification).
Class III: Marginal tissue recession that extends to or
beyond the mucogingival junction, in addition there is
bone and or soft tissue loss interdentally or malpositioning
of the tooth.
Class IV: Marginal tissue recession that extends to or
beyond the mucogingival junction, with severe bone loss
and soft tissue loss interdentally and or severe tooth-
malpositioning.
Clinical Significance
Exposed root surfaces are susceptible to caries. Wearing
away of the cementum exposed by recession leaves an
underlying dentinal surface that is extremely sensitive.
Hyperemia of the pulp and associated symptoms
may also result from exposure of the root surface.
Interproximal recession creates spaces in which, plaque
and food debris can accumulate.
Treatment
Denuded root surfaces are covered for two purposes:
1. To solve esthetic problem (in anterior teeth)
2. To widen zone of attached gingiva, thereby solving
a possible mucogingival problem
Several procedures are as follows:
I. Treatment based on width of attached gingiva
Adequate
Inadequate
1. Pedicle graft
1. Free soft-tissue autograft.
• Double papillae
• Laterally-displaced
2. Coronally, repositioned
2. Subepithelial connective
flap with semilunar incision
tissue graft.
II. Treatment based on distribution of recession
Localized
Generalized (involving few teeth)
i.Pedicle graft
i. Free soft tissue graft.
• Double papillae
• Laterally-displaced
ii. Coronally repositioned
ii. Subepithelial connective
flap with semilunar incision
tissue graft.
iii. Guided tissue regene ration iii. Coronally-repositioned flap.
Q18. What is the significance of bleeding on
probing? How do you check for gingival bleeding?
Answer
Bleeding on probing is important as it is one of the earliest
signs of gingival inflammation and is also a very sensitive,
objective clinical indicator. However, its relationship
to disease progression is unclear. Histologically,
bleeding on probing results from increased vascularity,
thinning and degeneration of the epithelium and the
proximity of the engorged vessels to the inner surface
(Fig. 53.11).
Fig. 53.11:
Gingival bleeding on probing
It can be checked by using a blunt periodontal probe,
which is carefully introduced to the bottom of pocket
using forces up to 0.75N and gently moved laterally along
the pocket wall. Bleeding may occur spontaneously or
delayed by 30 to 60 seconds.
Q19. What are the etiologic factors responsible
for gingival bleeding?
Local Factors
Chronic bleeding
• Chronic inflammation
Acute bleeding
• Aggressive tooth-brushing
• Sharp pieces of hard food
• Gingival burns
• Acute gingival diseases, e.g. ANUG
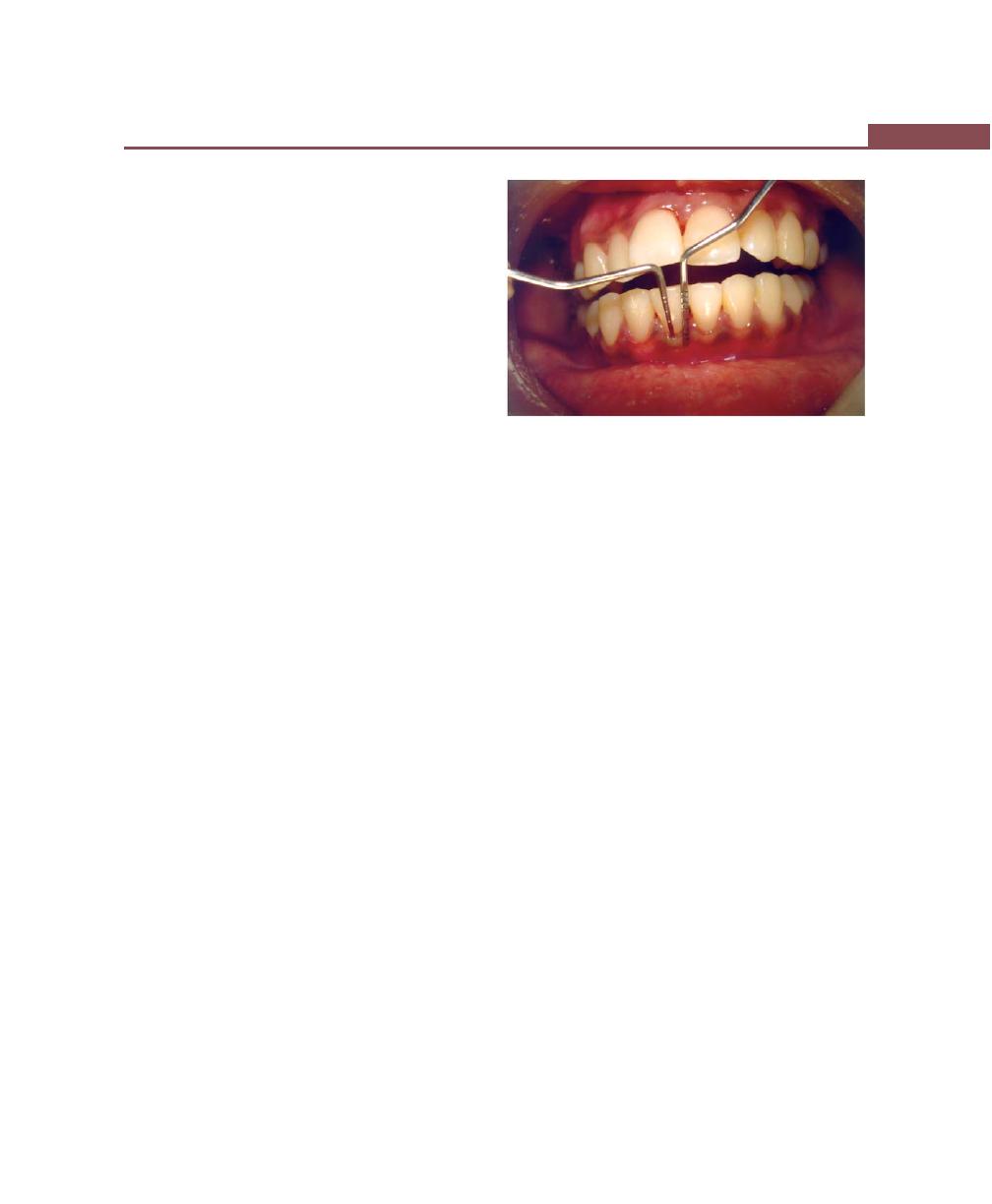
423
Questionnaire for Clinical Case Discussion
Systemic Factors
• Vascular abnormality
– Vitamin C deficiency
– Allergy such as Schonlein-Henoch purpura
• Platelet disorder, e.g. idiopathic thrombocytopenic
purpura.
• Hypoprothrombinemia, e.g. vitamin K deficiency
from liver disease or sprue.
• Coagulation defects, e.g. Hemophilia, Christmas
disease
• Malignancy, e.g. leukemia.
• Deficient platelet thromboplastic factor, e.g. uremia,
multiple myeloma, postrubella purpura.
• Drugs, e.g. salicylates, dicoumarol, heparin.
Q20. What are the microscopic changes asso-
ciated with gingival bleeding?
Answer
Changes in Epithelium
Thinning of epithelium and microulcerations along the
surface of the epithelium
Factors responsible for it are the widening of the
intercellular junctions and destruction of epithelium by
the bacteria and their by- products (e.g. collagenase,
hyaluronidase, protease, chondroitin sulfate or
endotoxin) gaining access into the connective tissue.
Changes in Connective Tissue
Dilation of the capillaries and venules and engorgement
of the blood vessels.
Factors responsible for it are that they are the
manifestations of the gingival inflammation resulting in
diapedesis and emigration of the polymorphonuclear
leukocytes. Because the capillaries are engorged and
closer to the thinned and degenerated epithelium, which
is less protective, any ordinary stimulus causes rupture
of the capillaries and gingival bleeding occurs.
Q21. What is the width of attached gingiva? How
do you measure the width of attached gingiva?
Answer
It is the distance between the mucogingival junction and
the projection on the external surface of the bottom of
the gingival sulcus or the periodontal pocket.
The width of attached gingiva is determined by
subtracting the sulcus or pocket depth from the total
width of the gingiva (Fig. 53.12).
Q22. What are the tests done to identify whether
the width of attached gingiva is adequate or
inadequate?
Answer
There are four different methods used to find the width
of attached gingiva.
1. Measurement approach: In this method first the
pocket depth or the sulcus depth is measured, and
then the total width of gingiva is measured, i.e. from
gingival margin to mucogingival line. Thus by
subtracting these two measurements we get the width
of attached gingiva.
Total gingival width – pocket depth = width of
attached gingiva
2. By using Schiller’s potassium iodide solution
It is similar to measurement approach. Potassium
iodide solution only stains the keratinized epithelium,
i.e. marginal gingiva, attached gingiva and interdental
papilla.
After application of this solution the total width
of gingiva is measured that is from gingival margin
to mucogingival line, and later the sulcus depth or
pocket depth is measured. Then by subtracting the
total gingival width from pocket depth we get the
width of attached gingiva.
Fig. 53.12:
Measurement of width of attached gingiva
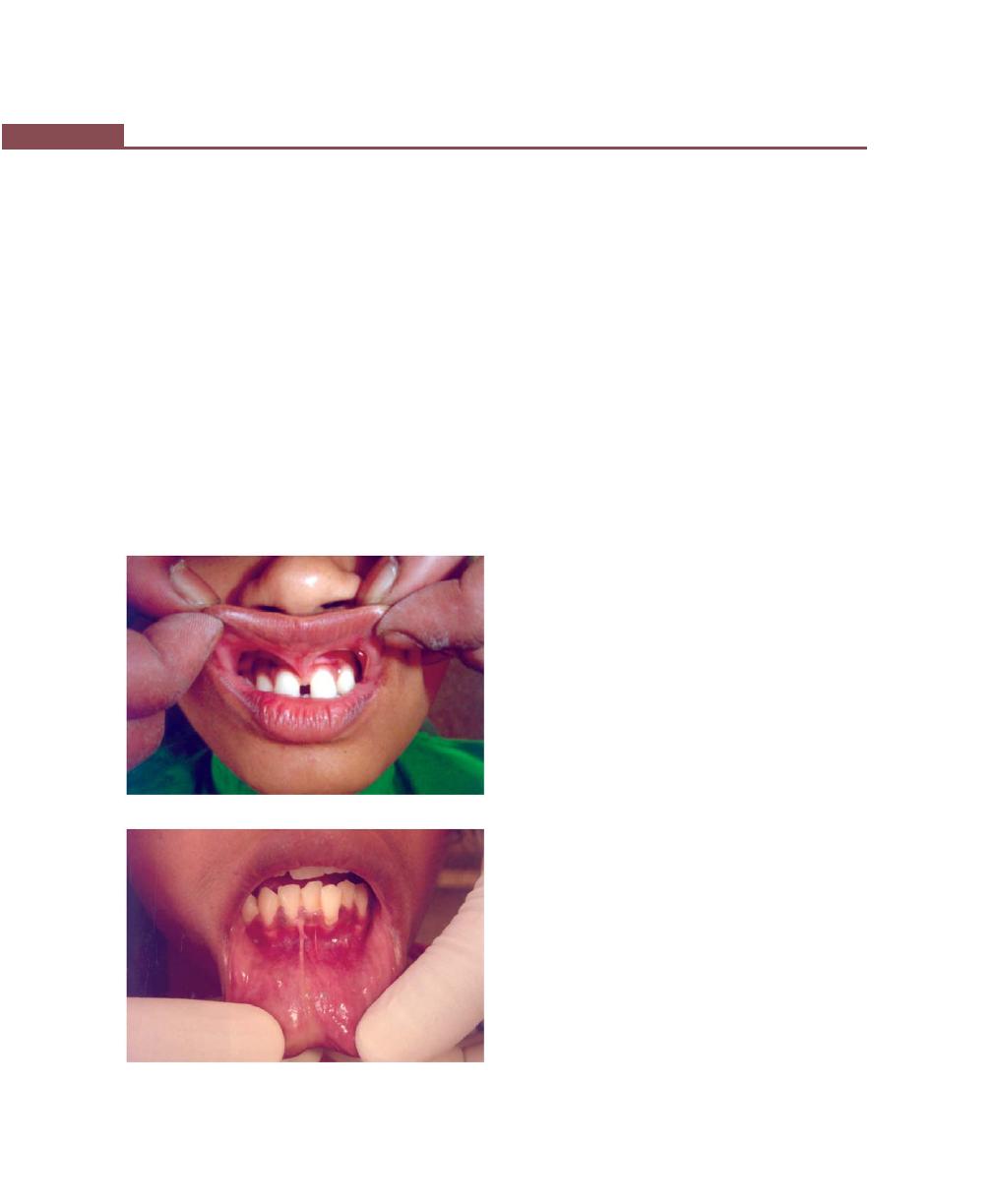
424
Essentials of Clinical Periodontology and Periodontics
Stained total gingival width – pocket depth =
width of attached gingiva.
Here, KI solution is used to know the accurate
width of gingiva, i.e. from free marginal gingiva to
mucogingival line, which is keratinized.
3. Tension test: This is done by stretching the lip or cheek
to demarcate the mucogingival line and to see for
any movement of the free gingival margin. And if
the free gingival margin moves during stretching of
lips then the attached gingiva is considered to be
inadequate (Figs 53.13 and 53.14).
4. Roll test: It is done by pushing the adjacent mucosa
coronally with a dull instrument. If the gingiva moves
with the instrument then the width of attached gingiva
is considered inadequate. In adequate width, the
gingiva does not move because the attached gingiva
is firmly attached to the underlying bone.
Q23. What are the mucogingival problems?
Answer
Mucogingival problems are the disease conditions, which
extends to or beyond the mucogingival junction.
Various mucogingival problems are:
1. Inadequate width of attached gingiva.
2. Gingival recession extending up to mucogingival
junction
3. Decreased vestibular depth
4. Abnormal frenal attachment
5. Pockets extending upto the mucogingival junction
Q24. How do you clinically differentiate between
true, pseudo and combined pockets; suprabony
and infrabony pockets (Figs 53.15 to 53.17)?
Answer
Pseudopockets: It is associated with gingival enlargement
and also while probing, there is increased sulcus depth,
the probe tip does not seem to go beyond the cemento-
enamel junction because junctional epithelium is still
attached at the CEJ.
True pockets: It is associated with apical migration of
junctional epithelium (attachment loss). This is clinically
assessed by probing measurement.
The probing method is as follows, since normally
junctional epithelium is attached at the cemento-enamel
junction.
First: Detect the CEJ by running the probe perpendicular
to the root surface.
Second: Once the CEJ is detected, bring the probe back
to parallel position and observe whether probe can be
probed beyond this point. If it can, then it is understood
that the junctional epithelium is shifted apically, hence
diagnosed as a true pocket.
Combined pocket: It is associated with both gingival
enlargement and attachment loss.
Suprabony pocket: It is a true pocket with base of the
pocket coronal to underlying alveolar bone crest.
Fig. 53.13:
Tension test (for abnormal frenal attachment)
Fig. 53.14:
Tension test (for lack of attached gingiva)
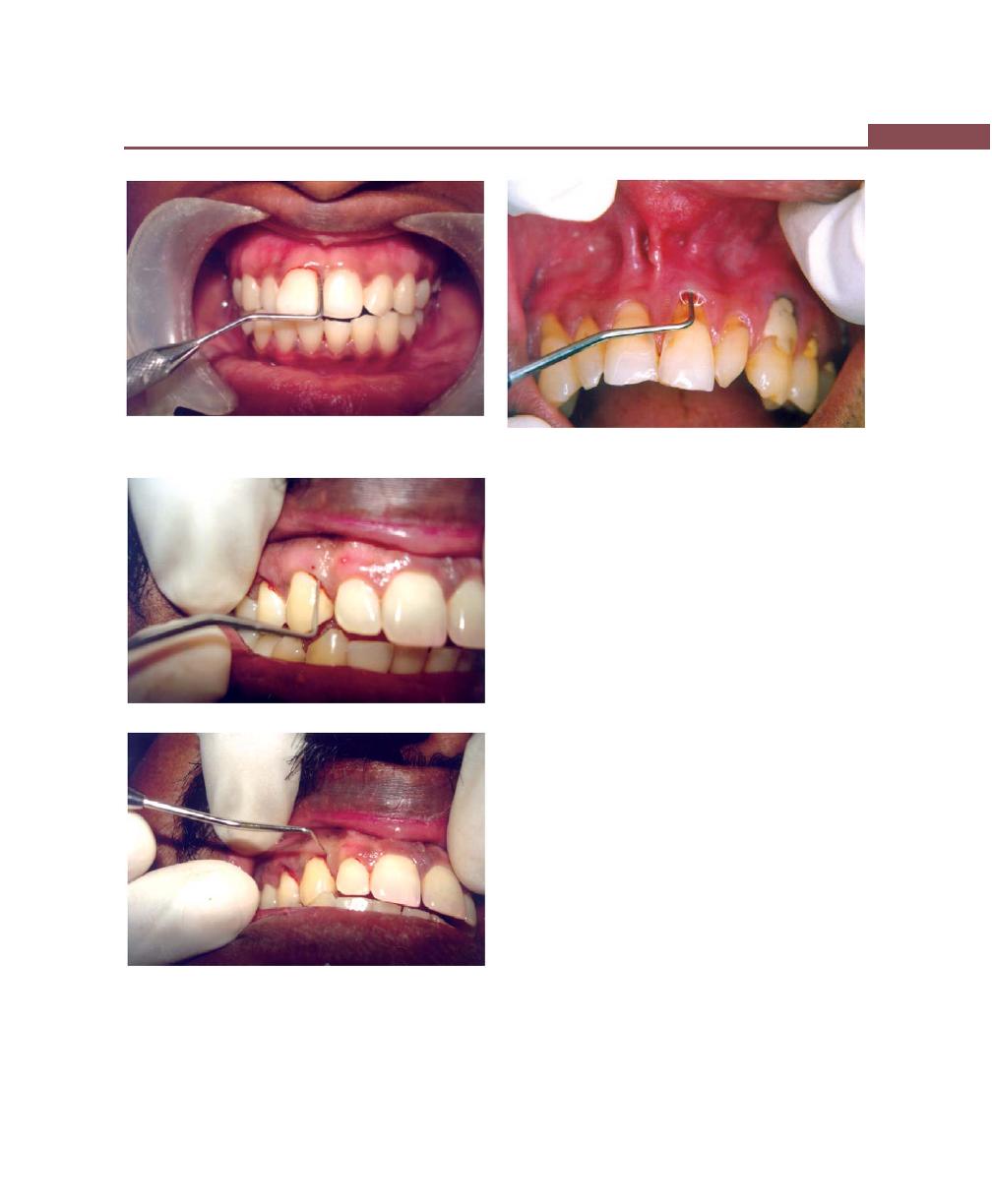
425
Questionnaire for Clinical Case Discussion
Fig. 53.15:
Detection of a pocket (probe placed parallel to
the long axis of the tooth)
Figs 53.16A and B:
True and Pseudopocket. (A) Clinical
detection of true/pseudopocket. (B) Detection of cemento-
enamel junction
Fig. 53.17:
Suprabony/Infrabony pocket (Probe in place
with lateral pressure)
Clinically, it can be assessed by probing measurements.
After the probe is inserted into the gingival suclus and
when lateral pressure is applied, if soft tissue resistance
is felt, then it is diagnosed as suprabony pocket.
Infrabony pocket: It is a true pocket with base of pocket
apical to level of adjacent alveolar bone. Clinically, probe
is inserted into the gingival sulcus and when lateral
pressure is applied, hard tissue resistance is felt.
Q25. How do you treat pockets?
Answer
The various methods of pocket elimination are classified
as follows:
1. New attachment techniques: These offer the ideal
result, because they eliminate pocket depth by
reuniting the gingiva to the tooth at a position coronal
to the bottom of the pre-existing pocket.
2. Removal of the pocket wall is the most common
method. It can be removed by:
a. Retraction or shrinkage by scaling and root
planing.
b. Surgical removal by gingivectomy technique or
by means of an undisplaced flap.
c. Apical displacement with an apically displaced flap.
A
B
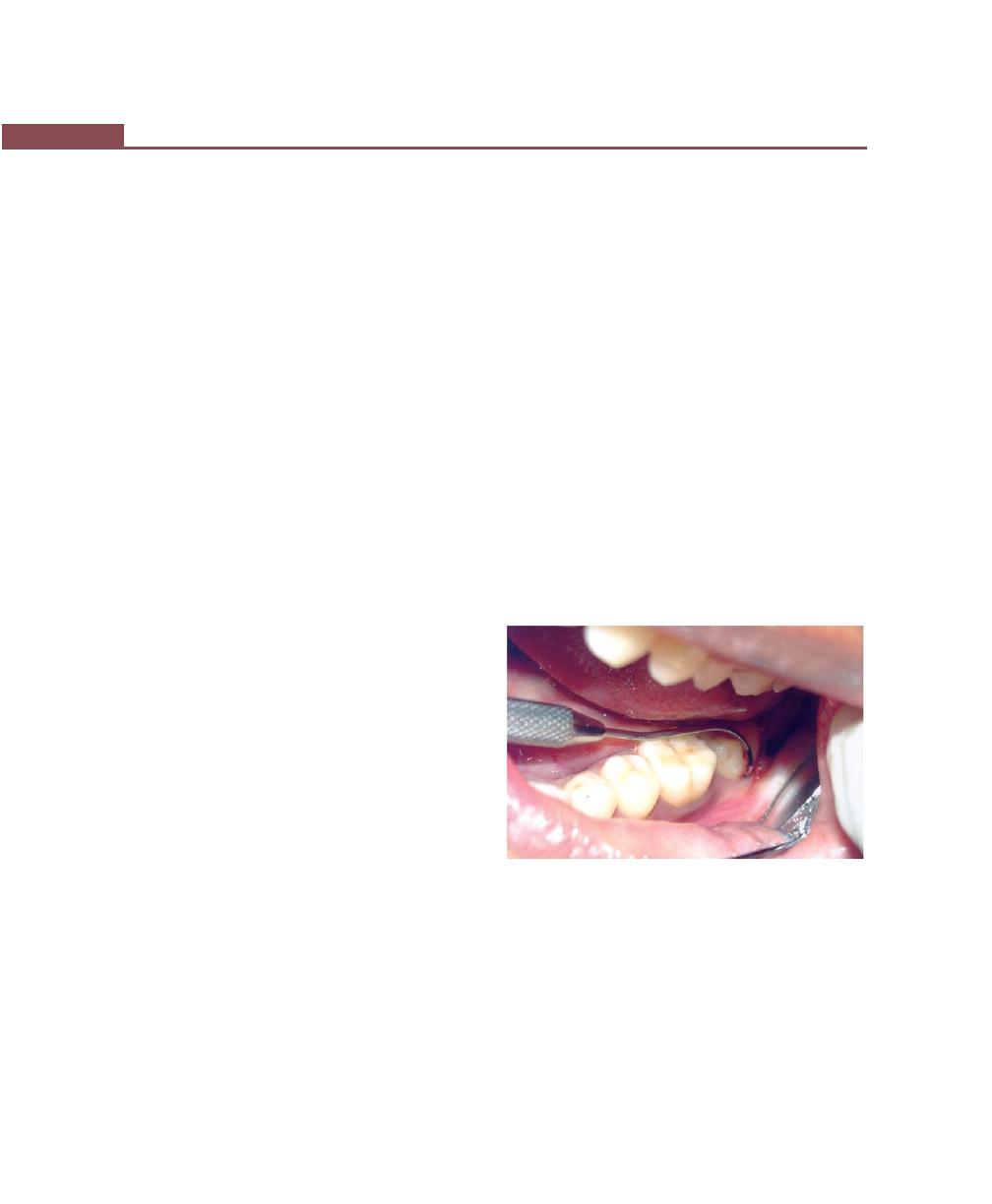
426
Essentials of Clinical Periodontology and Periodontics
3. Removal of the tooth side of the pocket, which
involves either extraction of the tooth or hemisection
of the tooth.
Q26. Describe the sequelae following unreplaced
missing first molars.
Answer
It consists of the following :
i. Second and third molars tilt mesially leading to distal
cusp elevation thereby plunger cusp effect. In the
mesial cusp due to mesial tilt the distance between
the mesial cusp tip and bone is reduced thereby
progression of periodontal disease becomes rapid.
ii. Supra-eruption of opposing first molar may lead to:
↓
Loss of proximal contact
↓
Food impaction
↓
Plaque accumulation
↓
Gingivitis
↓
Periodontitis
iii. Premolars move distally, mandibular incisors tilt
or drift lingually.
Anterior overbite is increased. The mandibular
incisors strike the maxillary incisors near the gingiva
and traumatizes the gingiva.
iv. The maxillary incisors are pushed labially and
laterally.
v. Extrusion of anterior teeth leading to diastema.
Q27. Describe the sequelae following loss of
proximal contact form.
Answer
The tightness of contact should be checked by means
of clinical observation and with a dental floss.
Abnormal contact relation or loss of proximal contact
can cause plaque retention and food impaction →
gingivitis and periodontitis → mobility and loss of tooth.
Q28.How do you diagnose furcation involvement?
Answer
Furcation involvement can be diagnosed by two methods:
1. Clinical examination and
2. Radiographs
A thorough clinical examination is the key to diagnosis
and treatment planning. Careful probing is performed
by using a Naber’s probe. Transgingival probing further
helps to define the anatomy of the furcation defect (Fig.
53.18).
Radiographs can also be used as diagnostic aids in
diagnosing furcation involvement:
Grade I: Radiographic changes are not usually found
Grade II: Radiographs may or may not depict the
furcation involvement
Grade III: Show a radiolucent area in the crotch of the
tooth
Grade IV: Seen as a radiolucent area.
Fig. 53.18:
Diagnosis of furcation involvement using
Naber’s probe
Q29. Describe the etiology, classification and
treatment of furcation involvement.
Answer
Etiology
1. Extension of inflammatory periodontal disease.
2. Trauma from occlusion
3. Cervical enamel projections
427
Questionnaire for Clinical Case Discussion
4. Pulpo-periodontal diseases
5. Iatrogenic co-factors
6. Anatomical factors
Classification of Furcation Involvement
According to Glickman:
Grade I: Pocket formation into the flute of the furcation
but interradiular bone is intact
Grade II: Loss of inter-radicular bone and pocket
formation of various depths into the furcation but not
completely probable to the opposite side of the tooth.
Grade III: Complete loss of interradicular bone with
pocket formation that is completely probable to the
opposite side of the tooth.
Grade IV: Loss of attachment and recession that has made
the entire furcation clinically visible.
According to Goldman and Cohen, furcation
involvement can be:
Grade I: Incipient lesion
Grade II: It is a Cul de sac lesion
Grade III: Through and through furcation involvement
According to Hamp et al.
Degree I: Horizontal loss of periodontal tissue support
less than 3 mm.
Degree II: Horizontal loss of support exceeding 3 mm
but not encompassing the total width of the furcation
area.
Degree III: Horizontal through and through destruction
of the periodontal tissues in the furcation
According to Tarnow and Fletcher
Sub-class A: Vertical destruction to one-third of the total
inter-radicular height (1-3 mm).
Sub-class B: Vertical destruction reaching two-thirds of
the inter-radicular height. (4 to 6 mm).
Sub-class C: Inter-radicular osseous destruction into or
beyond the apical third (more than 7 mm).
Treatment
Traditional procedures
Regenerative
procedures
Grade I
Scaling, root planing,
curettage,gingivectomy,
odontoplasty.
Grade II
Scaling, root planing,
Autogenous and
curettage, odontoplasty,
autologous osseous
osteoplasty with limited
grafting, synthetic
ostectomy, in shallow
grafting, GTR, root
grade II invasions. In
conditioning, coro-
more severe furcation
nally-displaced flap
involvements root resection/
and combination
hemisection is done.
procedures.
Grade III
Tunneling, root sectioning,
GTR and combina-
hemisection, extraction
tion procedures
Grade IV
Maintenance
Q30. What are the causes for tooth mobility? Give
the classification of mobility?
Answer
Causes for mobility can be divided into local and systemic
factors (Fig. 53.19):
Local Factors
a. Bone loss and loss of tooth support—
1. Bone destruction caused by extension of gingival
inflammation, which can be either due to plaque
products or pharmacologically-active substances.
2. Trauma from occlusion, either in absence or
associated with inflammation.
b. Hypofunction
c. Periapical pathology
d. After periodontal therapy
e. Parafunctional habits like bruxism or clenching
f. Pathology of jaws like tumor, cyst etc
g. Traumatic injury to dentoalveolar unit
h. Tooth morphology
i. Overjet and overbite
j. Implant mobility
Systemic Factors
a. Age
b. Sex and Race : Slightly higher incidence seen in
females and Negros
c. Menstrual cycle
d. Oral contraceptives
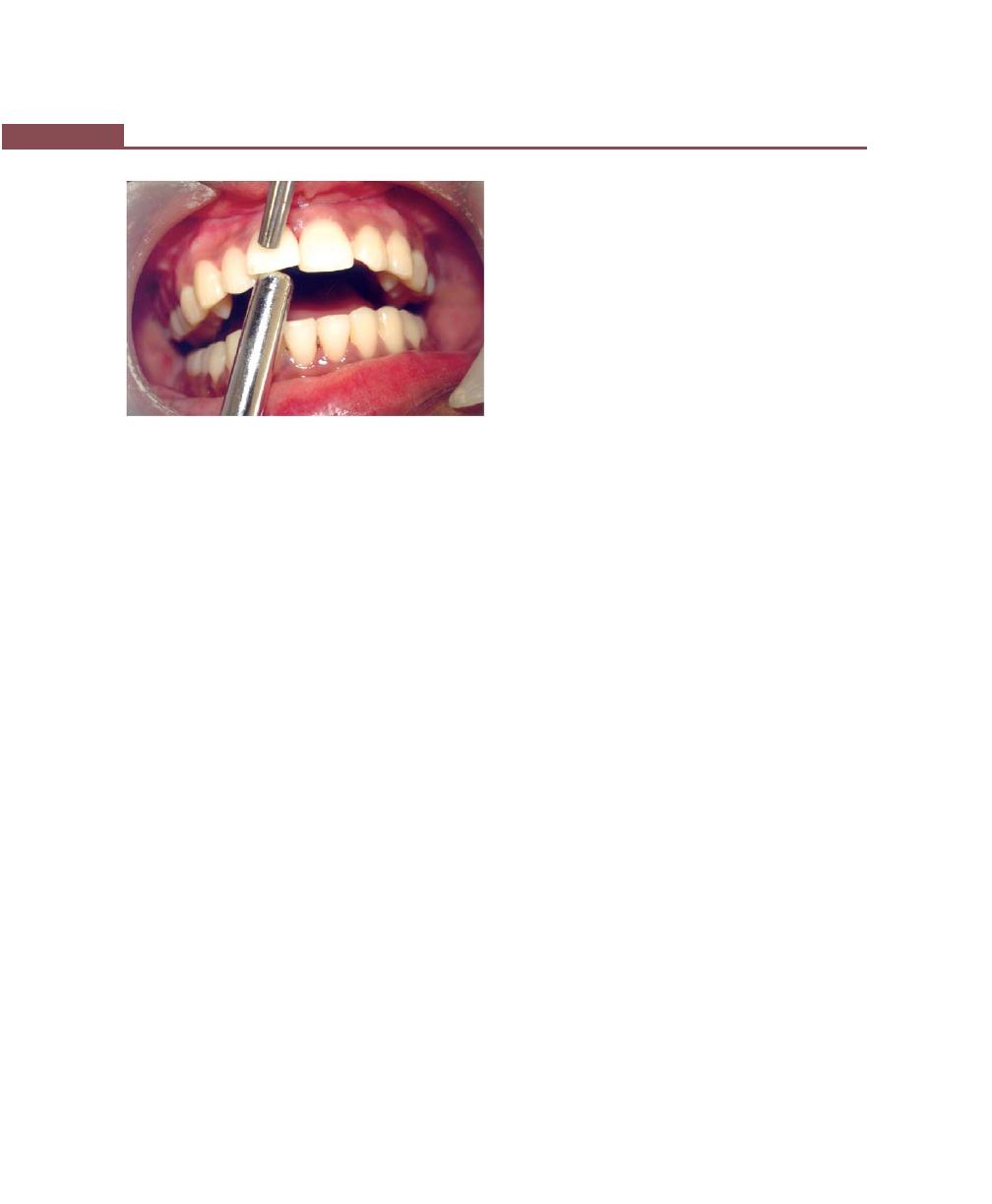
428
Essentials of Clinical Periodontology and Periodontics
e. Pregnancy
f. Systemic diseases such as Papillon-Lefevre syndrome,
Down’s syndrome, neutropenia, Chediak-Higashi
syndrome etc.
Grading of Mobility can be:
Degree I: Mobility of crown of the tooth 0.2-l mm in
horizontal direction
Degree II: Mobility of crown of the tooth exceeding
1 mm in horizontal direction
Degree III: Mobility of crown of the tooth in vertical
direction as well.
Q31. What is pathological migration ? How do you
clinically identify it ?
Answer
Pathologic migration refers to tooth displacement that
results when the balance among the factors that maintain
physiological tooth position is disturbed by periodontal
disease. It is relatively common and maybe an early sign
of disease, or it may occur in association with gingival
inflammation and pocket formation as the disease
progresses.
The characteristic clinical features are as follows:
1. It occurs most frequently in the anterior region
2. It is usually accompanied by mobility rotation and
extrusion of teeth.
3. Drifting of maxillary and mandibular anterior incisors
labially creating diastema between the teeth
4. Should be differentiated from physiological migration
Q32. What are the causes for pathologic migra-
tion?
1. Weakened periodontal support
2. Changes in the forces exerted on the teeth
• Unreplaced missing teeth
• Failure to replace first molars
3. Other causes like:
• Pressure from tongue
• Pressure from granulation tissue of periodontal
Q33. Define attrition, abrasion and erosion.
Answer
Attrition : It is the occlusal wear resulting from functional
contact with opposing teeth or, it is the term used for
dental wear caused by teeth against teeth.
Abrasion : Refers to the loss of tooth substance induced
by mechanical wear other than that of mastication.
Erosion: It is a sharply defined wedge-shaped depression
in the cervical area of the facial tooth surface or wear
to the nonoccluding tooth surface and includes sharply
defined, wedge-shaped depressions in the facial cervical
areas of the tooth.
Q34. When is tooth tender on percussion?
Answer
Various causes for tooth to be tender on percussion are:
A. Periodontal diseases (Tooth is tender on lateral
percussion)
B. Irreversible pulpitis
C. Root fracture
D. Crown fracture
E. Periapical pathology
Q35. What is retrograde periodontitis?
Answer
Normally, periodontitis is caused by extension of
inflammation from the gingiva into deeper periodontal
Fig. 53.19:
Clinical demonstration of mobility
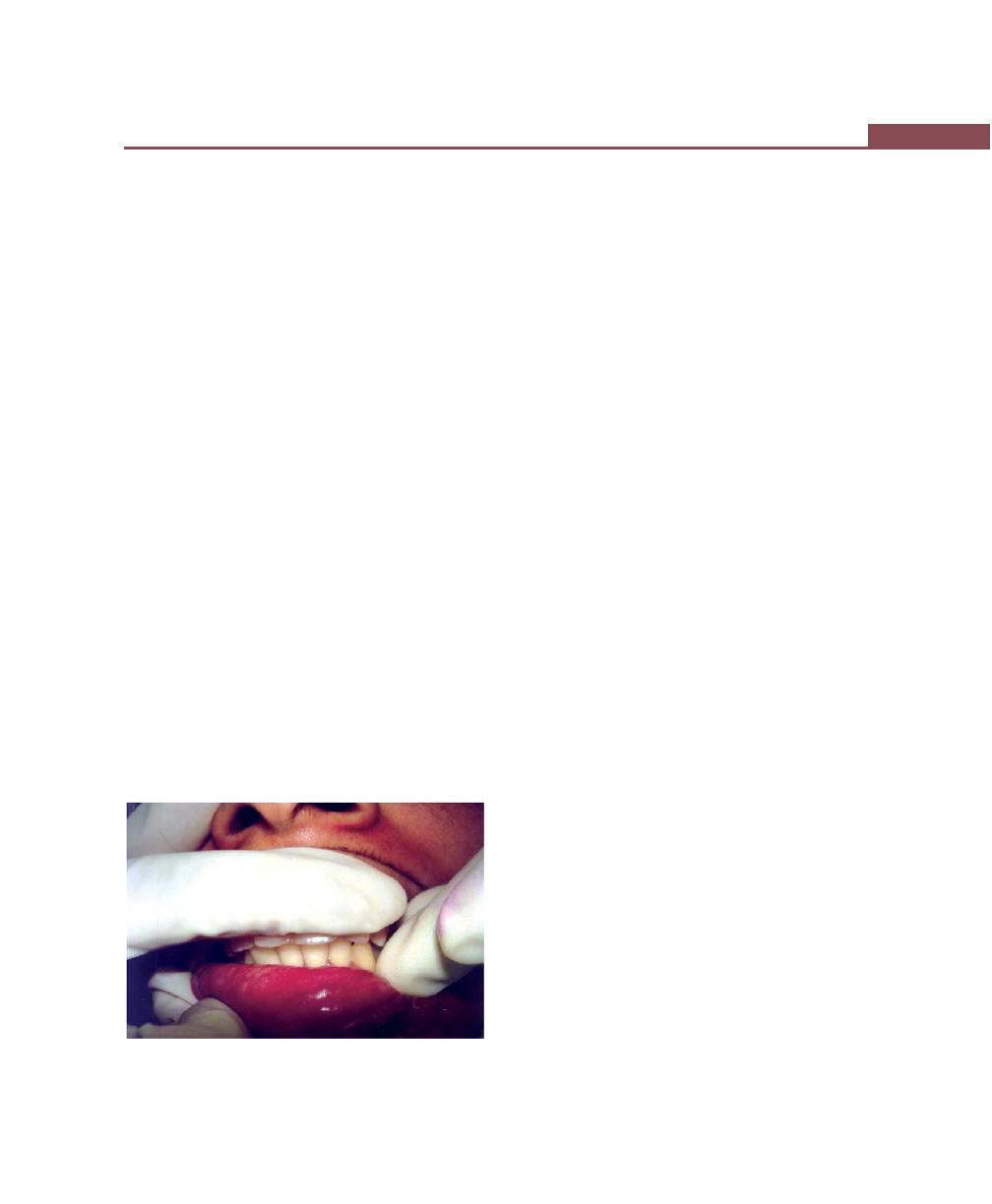
429
Questionnaire for Clinical Case Discussion
tissues. However, periodontitis can also be caused by
pulpal infections that have entered periodontal ligament
either through apical foramen or through the lateral
canal. Such a periodontal lesion is termed as retrograde
periodontitis.
Q36. How do you diagnose a case of trauma from
occlusion?
Answer
a. Fremitus test (Fig. 53.20): It is a measurement of the
vibratory pattern of the teeth when the teeth are
placed in the contacting positions and movements.
To measure fremitus, dampened index finger is
placed along the buccal and labial surfaces of the
maxillary teeth and the patient is asked to tap the
teeth together in the maximum intercuspal position
and then grind systematically in the lateral, protrusive
movements and positions. The teeth that are displaced
by the patient in these positions are identified and
graded. The following classification system is used:
Class I fremitus : Mild vibration or movements
detected.
Class II fremitus : Easily palpable vibration but no
visible movements.
Class III fremitus : Movements visible with naked eye.
b. With help of occlusion registration strips/articulating
paper.
c. Auditory test for trauma from occlusion: In centric
relation, there is a distinct ringing sound during tooth
contact. But in the traumatic occlusion with deflection
present, sound is dull and imperceptible.
Tactile method:
In centric relation and normal excursive movement
response to finger is smooth but with deflection present
as in trauma from occlusion, a roughness can be detected.
Q37. What are the types, signs and symptoms of
trauma from occlusion?
Answer
The various types are:
1. Primary and secondary occlusal trauma.
2. Acute and chronic.
Signs and Symptoms
1. Presence of gingival recession.
2. Presence of Stillman cleft and McCall’s festoons.
3. Presence of traumatic crescent.
4. Presence of infrabony pockets.
5. Food impaction.
6. Excessive wearing of tooth cusps and appearance
of wear facets.
7. Hypersensitivity of the teeth.
8. Increased tooth mobility.
9. Tenderness of TMJ and muscles of mastication.
10. Widening of periodontal ligament and thickening
of lamina dura.
11. Vertical bone loss.
12. Condensation of alveolar bone.
13. Root resorption.
14. Buttressing bone formation seen in an occlusal
radiograph.
15. Smudging of articulating paper.
16. Dull percussion note of an affected tooth.
17. Vibrations are felt on performing Fremitus test.
Q38. What is difference between mobility and
fremitus?
Answer
Mobility : It is a measurement of horizontal and vertical
tooth displacement created by the examiners force
Fig. 53.20:
Fremitus test is performed placing wet finger
on the labial surface of maxillary anterior teeth

430
Essentials of Clinical Periodontology and Periodontics
Fremitus : It is a measurement of the vibratory patterns
of the teeth when the teeth are placed in contacting
positions and movements.
• Fremitus differs from mobility in that fremitus is tooth
displacement created by the patient’s own occlusal
force.
• Therefore the amount of force varies greatly from
patient to patient, unlike mobility, where in the force
with which it is measured tends to be the same for
each examiner.
Q39. What are the various indices used for
assessing oral hygiene?
Answer
1. Oral hygiene index.
2. Simplified oral hygiene index.
3. Patient Hygiene performance index.
4. Plaque component of periodontal disease index.
5. Shick and ash modification of plaque criteria.
6. Turesky- Gilmore- Glickman modification of the
quigley Hein plaque index.
7. Plaque index.
8. Calculus index.
Q40. What are the indices for gingival Bleeding?
Answer
1. Sulcular bleeding index.
2. Papillary bleeding index.
3. Bleeding points index.
4. Interdental bleeding index.
5. Gingival bleeding index.
Q41. Effects of overhanging restorations on perio-
dontium.
Answer
Overhanging restorations causes violation of biologic
width, which may lead to:
1. Ideal locations for the accumulation of plaque
2. Changing the ecologic balance of the gingival sulcus,
i.e. favour the growth of gram-negative anaerobic
species, which altogether leads to the pathogenesis
of chronic inflammatory periodontal disease.
Q42. What is the importance of recording various
lab investigations?
Answer
Complete blood count:
The complete blood count will include:
i. Hemoglobin (Hgb).
ii. Hematocrit (Hct).
iii. Red blood cell count (RBC).
iv. White blood cell count (WBC).
I. Hemoglobin
i. Hgb is the oxygen carrier of the blood. It is dec-
reased in hemorrhage and anemias. It is increased
in hemoconcentration and polycythemia.
ii. The normal range is 14 to 18 g /dl of blood in
men and 12 to 16g /dl of blood in women.
II. Hematocrit : It reflects the relative volume of cells
and plasma in the blood. In anemias and after blood
loss, the hct is lowered. In polycythemia and
dehydration it is raised. The normal Hct range is 40
to 54 percent for men and 37 to 47 percent for
women.
III. RBC count: RBC contains HgB. An increase in RBC’s
may indicate hemoconcentration or polycythemia.
Decrease in number of RBC’s may be indicative
of blood loss or anemia.
IV. WBC count: WBC’s are important in defence against
invading micro-organisms. Normal count is 5000 to
10,000/mm
3
. Increase in WBC is seen in leukemia,
bacterial infection, infectious mononucleosis and
certain parasitic infections. Decrease in WBC’s seen
in aplastic anemia, lupus erythamatosis, acute viral
infections, and drug and chemical toxicity.
a. Neutrophils (50 to 70%)
• Increase in most bacterial infections
• An increase in number of immature neutrophils
is frequently found in acute infections. This is called
shift to the left.
b. Eosinophils (1 to 4%) : Increase in allergic conditions
and parasitic infections.

431
Questionnaire for Clinical Case Discussion
c. Basophils (0 to 1%) : May be increased in blood
dyscrasis.
d. Lymphocyte (25 to 40%) : It increases in measles
and in severe bacterial or chronic infections.
e. Monocyte (4 to 8%) : It may be increased during
recovery from severe infections and Hodgkins
diseases.
V. Blood glucose: Basic tests of blood glucose are:
a. Fasting blood sugar
b. 2 hour postprandial blood sugar test
c. Glucose tolerance test
d. Random blood sugar test
The normal range for blood glucose is 70 to 110
mg/ dl of serum.
VI. Blood urea nitrogen:
• Normal value is 8 to 23 mg /dl of blood.
• Increased value seen in extensive kidney disease,
congestive heart failure, dehydration.
VII. Serology—For screening of syphilis.
• All are nonspecific tests for syphilis and may give
both false positive and false negative results.
Interpretation of these results requires patient
history and clinical findings. Confirmation is by
Fluoroscent treponemal antibody-absorption test
(FTA-abs) or the micro-hemagglutination trepo-
nemal palladium test (MHA-tp).
VIII. Screening test for hemorrhagic disorders:
a. Bleeding time: It is the time required for
hemostasis to occur in a standard wound of the
capillary bed. It varies with vascular and
platelet abnormalities. The normal range is 1 to
7 minutes
b. Platelet count: It is decreased in thrombocytopenic
purpura. In myeloproliferative diseases, platelets
are increased. Normal platelet count is 150,000
to 400,000/mm
3
.
c. Prothrombin time: It is an indirect test of the
clotting ability of the blood. It gives an indication
of prothrombin deficiency arising from liver
disease. Normal range is 12 to 17 sec.
d. Partial thromboplastin time : It is designed to help
the clinician recognize mild to moderate deficiences
of intrinsic clotting factors.
Q43. Effects of improper orthodontic treatment
on periodontium.
Answer
Orthodontic tooth movement is possible because the
periodontal tissues are responsive to externally applied
forces. Orthodontic forces at optimum rate and direction
have no negative impact on the periodontium. Unlikely
when these forces are abnormal, it results in deleterious
effects such as:
• Moderate orthodontic forces ordinarily result in bone
remodelling and repair, whereas excessive forces may
produce necrosis of the periodontal ligament and
adjacent alveolar bone.
• Excessive orthodontic forces also increase the risk of
apical root resorption.
• Use of elastics to close diastema may result in severe
attachment loss with possible tooth loss as the elastics
migrate apically along the root.
• Surgical exposure of impacted teeth and orthodontic
assisted eruption has the potential to compromise
the periodontal attachment on adjacent teeth.
• Dentoalveolar gingival fibers are stretched when teeth
are rotated during orthodontic therapy.
Q44. What are the things you look for in a
radiograph?
Answer
Radiographs help in diagnosis of periodontal disease,
determination of patient’s prognosis and the evaluation
of the outcome of treatment. But radiograph is an adjunct
to the clinical examination, not a substitute for it.
The normal anatomic landmarks to be seen in a
radiograph are (First identify the type of X-ray its side
i.e. left or right and which arch it belongs to):
Maxillary arch
1. Nasopalatine foramen.
2. Median palatal sutures.

432
Essentials of Clinical Periodontology and Periodontics
3. Nasal fossa.
4. Nasal septum.
5. Anterior nasal spine.
6. Maxillary sinus.
7. Maxillary tuberosity.
Mandibular arch
1. Genial tubercles.
2. Lingual foramen.
3. Mental process.
4. Mental foramen.
5. Oblique ridge.
6. Mandibular canal and submandibular fossa.
Lesions to be seen are:
1. Caries
a. Interproximal caries
b. Occlusal caries
c. Root surface caries
2. Periodontal disease i.e. periodontitis—Bone loss.
a. Types of bone loss
i. Vertical or angular
ii. Horizontal bone loss
iii. Crater-like loss
iv. Arc-shaped bone loss.
b. Contour or Architecture of bone
i. Positive
ii. Negative
c. Continuation of lamina dura
d. Trabecular pattern of bone
e. Furcation involvement
f. Amount of bone loss.
3. Tooth fractures
i. Crown fracture
ii. Root fracture
4. Resorption
i. Internal resorption
ii. External resorption
Q45. What are the advantages and disadvantages
of I.O.P.A. radiographs and OPG?
Answer
Advantages of OPG
1. For visualizing the maxilla and mandible in one film.
2. For patient education.
3. To evaluate impacted teeth.
4. In the evaluation of multiple unerupted super-
numerary teeth.
5. To evaluate eruption sequence and pattern,
growth and development.
6. For detecting any pathology involving the jaws.
7. For evaluating traumatic injuries.
8. Radiographic examination of patients with limited
mouth opening.
9. To identify diseases of the temporomandibular joint.
10. Reduces the risk of multiple exposures to radiation.
Disadvantages
1. Images are not as sharp as in an intraoral film.
2. It cannot be used in the diagnosis of caries.
3. It cannot be used in the evaluation of bone loss due
to periodontal disease.
4. It shows super imposition especially in the premolar
region.
5. Structures in the anterior region may not be well
defined.
Advantages of I.O.P.A.
1. Better visibility of periapical region.
2. Helps in the diagnosis of periapical pathology.
3. To study crown and root length and their morphology.
4. Helps in evaluating root apex formation.
5. Evaluation of endodontic treatment.
6. Helps in evaluation of fracture of teeth.
Disadvantages
1. Cannot be taken in case of patients with limited mouth
opening.
2. It cannot detect any pathology involving the jaws.
3. Multiple radiographs have to be taken for the
examination of the entire arch thereby increasing the
risk of exposure to radiation.
Q46. Justify your diagnosis, if diagnosis is gin-
givitis then why? Or if it is periodontitis then why?
Answer
Gingivitis is detected when there is evidence of gingival
inflammation such as swelling, redness, bleeding on

433
Questionnaire for Clinical Case Discussion
probing, often the pocket depth less than or equal to
4 millimeter but by-definition there is no measurable
loss of probing periodontal attachment associated with
periodontal infection. Gingival recession maybe
associated with gingival abrasion or prominent radicular
surface. Plaque is usually present; calculus may or may
not be present. Tooth mobility is rarely present and
radiographic changes do not suggest bone loss.
Periodontitis can be classified as early, moderate,
advanced. According to AAP’s classification, in early
periodontitis there are shallow pockets, minor to
moderate bone loss, satisfactory topography and
generally no tooth mobility. Moderate periodontitis is
characterized by moderate to deep pockets, moderate
to severe bone loss, unsatisfactory topography and slight
tooth mobility. In advanced periodontitis, there are deep
pockets, many areas of severe bone loss, advanced tooth
mobility and often a need for prosthesis to replace missing
teeth or splint the mobile teeth. Periodontitis is by
definition diagnosed when there is measurable
attachment loss (e.g. >2 mm) and often associated with
probing depth more than 4 mm. Plaque and calculus
are usually present. Tooth mobility may also occur.
Radiographic changes seen include alveolar crestal
resorption of varying degree and in advanced cases, loss
of alveolar bone in furcation areas may be seen.
Q47. Define prognosis. What are the factors
responsible for individual and overall tooth
prognosis?
Answer
Prognosis is a prediction of the duration, course and
termination of a disease and its response to treatment
Factors for Individual tooth prognosis are:
• Percentage of bone loss.
• Probing depth.
• Distribution and type of bone loss.
• Presence and severity of furcation involvements.
• Mobility.
• Crown-root ratio.
• Pulpal Involvement.
• Tooth position and occlusal relationship.
• Strategic value.
Factors for overall prognosis are:
• Age
• Medical status
• Individual tooth prognosis (distribution and severity)
• Degree of involvement, duration and history of the
disease
• Patient co-operation
• Economic consideration
• Knowledge and ability of the dentist
• Etiologic factors.
Q48. Discuss the treatment plan in a sequential
order.
Answer
The objectives of treatment are:
a. Elimination of disease.
b. Restoration of efficient function.
c. Production of satisfactory appearance.
Sequence of therapeutic procedures:
a. Preliminary phase/Emergency phase
1. Treatment of emergencies like dental or periapical
and periodontal problems
2. Extraction of hopeless teeth and provisional
replacement, if needed
b. Phase I therapy (Etiotrophic phase).
1. Plaque control
2. Diet control
3. Removal of calculus and root planing
4. Correction of restorative and prosthetic irritational
factors
5. Excavation of caries and restoration
6. Antimicrobial therapy (local/systemic)
7. Occlusal therapy
8. Minor orthodontic movement
9. Provisional splinting.
Evaluation of response to Phase I therapy,
I. Rechecking
• Pocket depth and gingival inflammation
• Plaque and calculus, caries.

434
Essentials of Clinical Periodontology and Periodontics
c. Phase II therapy (Surgical phase)
• Periodontal surgery, including placement of
implants
• Root canal therapy
d. Phase III therapy (Restorative phase).
a. Final restorations
b. Fixed and removable prosthodontics
Evaluation of response to restorative procedures,
periodontal examination
e. Phase IV therapy (Maintenance phase)
Periodic recall visits, re-checking for:
• Plaque and calculus.
• Gingival condition (inflammation, pockets).
• Occlusion, tooth mobility.
• Other pathologic changes.
Q49. Classify periodontal instruments.
Answer
They are classified into surgical and nonsurgical
instruments
I. Nonsurgical Instruments
A. Hand instruments.
B. Mechanical driven instruments.
A. Hand Instruments
1. Diagnostic instruments, examples: explorers and
periodontal probes
2. Scaling and root planing and curettage instru-
ments, examples: sickle scaler, curettes, hoe, chisel
and file
3. Cleaning and polishing instruments, examples:
Dental Tapes, rubber cups and bristlebrushes.
B. Mechanical instruments
i. Scaling and curettage instruments, examples:
ultrasonic, and sonic instruments
ii. Used for other purpose, example: Schwartz
periotrievers – used for retrieval of broken
instruments.
EVA system - correcting over hanging restorations
II. Surgical Instruments
1. Excisional and Incisional Instruments, examples:
• Periodontal knives (Gingivectomy knives)
• Interdental knives No 1-2
• Surgical blades. (Scalpel No 11, 12 and 15)
• Electrosurgery
2. Surgical curettes and Sickles.
Examples:
• Kramer curettes No 1, 2 and 3
• Ball scaler
3. Periosteal elevators, examples: No 24 G elevator
and Goldman fox No: 14
4. Surgical chisels and Hoes, examples: chisel in
Oschsenbein No1-2, Rhodes chisels
5. Surgical files example: Sugarman files
6. Scissors and Nippers, example: Goldman fox No:
16 scissors
7. Needle holders, example: Castroviejo needle
holder.
Q50. Give the characteristics of a scaler and
curette with differences.
Answer
Characteristics of a scaler:
i. Triangular in cross section
ii. Pointed tip not suited for subgingival use
iii. Two cutting edges per working end.
iv. Pointed tip and straight cutting edges do not adapt
well to rounded root surfaces and concavities.
v. Face perpendicular to the lower shank so that
cutting edges are in level with one another
vi. Shank
• Anterior sickles—Simple design
• Posterior sickles—Complex design
vii. Functions: Removal of medium to large-sized
supragingival calculus deposits.
Provides good access to the:
1. Proximal surfaces on anterior crowns
2. Enamel surfaces apical to contact areas of
posterior teeth
viii. Examples: Anterior sickle scaler—OD-1, Jacquette-
30 Jacquette-33, Towner-015, Goldman H6 and
H7, Posterior sickle scalers—Jacquette 34/35,
Jacquette 14/15, Jacquette 31/32, Ball 2/3.
435
Questionnaire for Clinical Case Discussion
Characteristics of Curette
Universal
Area-specific
Cross-section
Semicircular
Semicircular
Back
Rounded
Rounded
Cutting edges
Two cutting edges
One cutting edge
per working end
per-working end
Toe
Rounded
Rounded
Face
Perpendicular to
Tilted at a 60
o
-70
0
to the
the lower shank
lower shank
Application
Used to instrument
limited to use on a
all Tooth surfaces
specific area of specific
in the dentition
tooth surface
Function
Removal of light to
Removal of light
medium-sized
calculus deposits
calculus deposits
Examples
Columbia 2R/2L,
Gracey series
Columbia 13/14,
Kramer-Nevins series,
Rule 3/4,
Turgeon series,
Barnhart 1/2
Barnhart 5/6
After five
Langer 1/2
Mini five
Langer 3/4
Vision curvette series
Langer 5/6
Langer 17/18
Q51. Differences between Universal and Gracey
curette.
Answer
Gracey curette
Universal curette
Area of use
Set of many curettes
One curette designed
designed for specific
for all areas and
areas and surfaces
surfaces
Cutting edge
One cutting edge
Both cutting edges used
use
used; work with
work with either outer
outer edge only
or inner edge
Curvature
Curved in two
Curved in one plane;
planes; Blade curves
Blade curves up, not to
up and to the side
side
Blade angle
Offset blade: face of
Not offset; face of
blade beveled at
blade beveled at 90
0
to
60
0
to shank
shank
Q52. Classify various supragingival and subgin-
gival scalers.
Answer
Supragingival scalers
1. Sickle scaler
2. Surface scaler
3. Cumine scaler
4. Morse scaler
Subgingival scalers
1. Chisel scaler
2. Hoe scaler
3. Periodontal file
4. Islet scaler
Q53. What are the instruments used for root
planing?
Answer
Curette is the instrument of choice for removing deep
subgingival calculus; root planning, altered cementum
and removing the soft tissue lining the periodontal pocket.
These include:
a. Universal curettes
b. Area-specific curettes
In Gracey curettes :
• 1-2, 3-4—Anterior teeth
• 5-6—Anterior teeth and premolars
• 7-8, 9-10—Posterior teeth facial and lingual
• 11-12—Posterior teeth (mesial)
• 13-14—Posterior teeth (distal)
Q54. Define scaling and root planing.
Answer
Scaling is defined as a process by which plaque and
calculus are removed from both supragingival and
subgingival tooth surfaces.
Root planing: It is a process by which residual
embedded calculus and portions of cementum are
removed from the roots to produce a smooth hard, and
clean surface.
OR
It denotes a technique of instrumentation by which the
softened cementum is removed and the root surface is
made hard and smooth.
Q55. What are the principles of instrumentation?
Answer
General principles of Instrumentation are:
1. Accessibility.
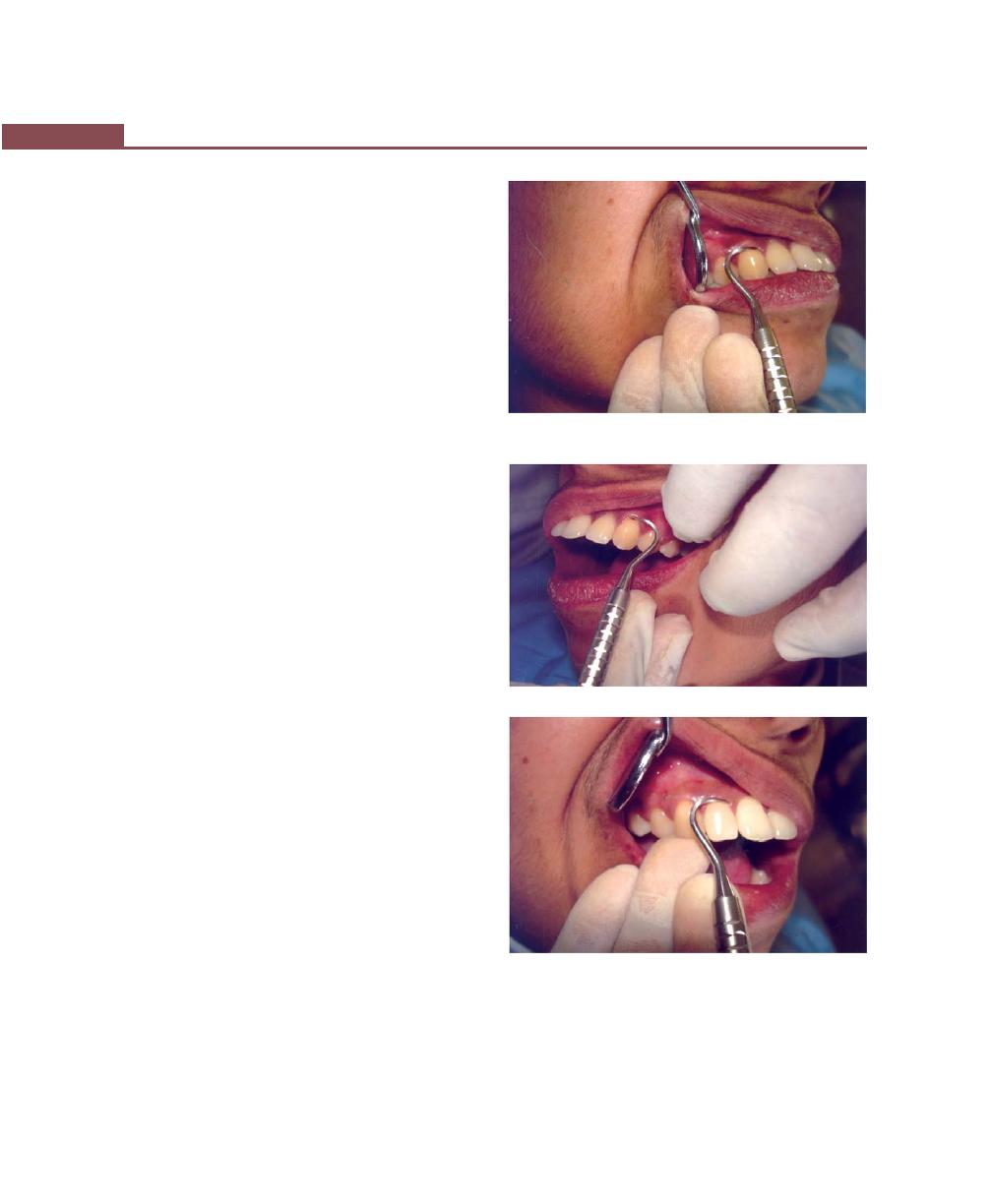
436
Essentials of Clinical Periodontology and Periodontics
2. Visibility, illumination and retraction.
3. Condition of instruments.
4. Maintaining a clean field.
5. Instrument stabilization.
6. Instrument activation.
Q56. What are finger rests and grasps?
Answer
Finger rests serves to stabilize the hand and the
instrument by providing a firm fulcrum as movements
are made to activate the instrument. A good finger rest
prevents injury and laceration of the gingiva and
surrounding tissues by poorly controlled instruments.
Most clinicians for the finger rest prefer the fourth finger.
Maximal control is achieved when the middle finger is
kept between the instrument-shank and fourth finger.
This “built-up fulcrum” is an integral part of the wrist-
forearm action that activates the powerful working stroke
for calculus removal (Figs 53.21 to 53.29).
Finger rest
Extraoral finger rests:
a. Palm up.
b. Palm down.
Intraoral finger rests
a. Conventional
b. Cross-arch
c. Opposite arch
d. Finger on finger
Grasps: A proper grasp is essential for precise control of
movements made during periodontal instrumentation.
The commonly used grasps are:
a. Modified pen grasp: The thumb, index finger, and
middle finger are used to hold the instruments as
pen is held, but the middle finger is positioned so
that the side of the pad next to the finger nail is resting
on the instrument shank. The index finger is bent
at the second joint from the fingertip and is positioned
well above the middle finger on the same side of
the handle. The pad of the thumb is placed midway
between the middle and index fingers on the opposite
side of the hand thereby creating tripod effect.
Fig. 53.21:
Extraoral fulcrums—Palm up
Fig. 53.22:
Extraoral fulcrums—Palm down
Fig. 53.23:
Conventional—Intraoral finger rests
b. Palm and thumb grasp: It is used for stabilizing
instruments during sharpening and for manipulating
air and water syringes, but it is not recommended
for periodontal instrumentation.
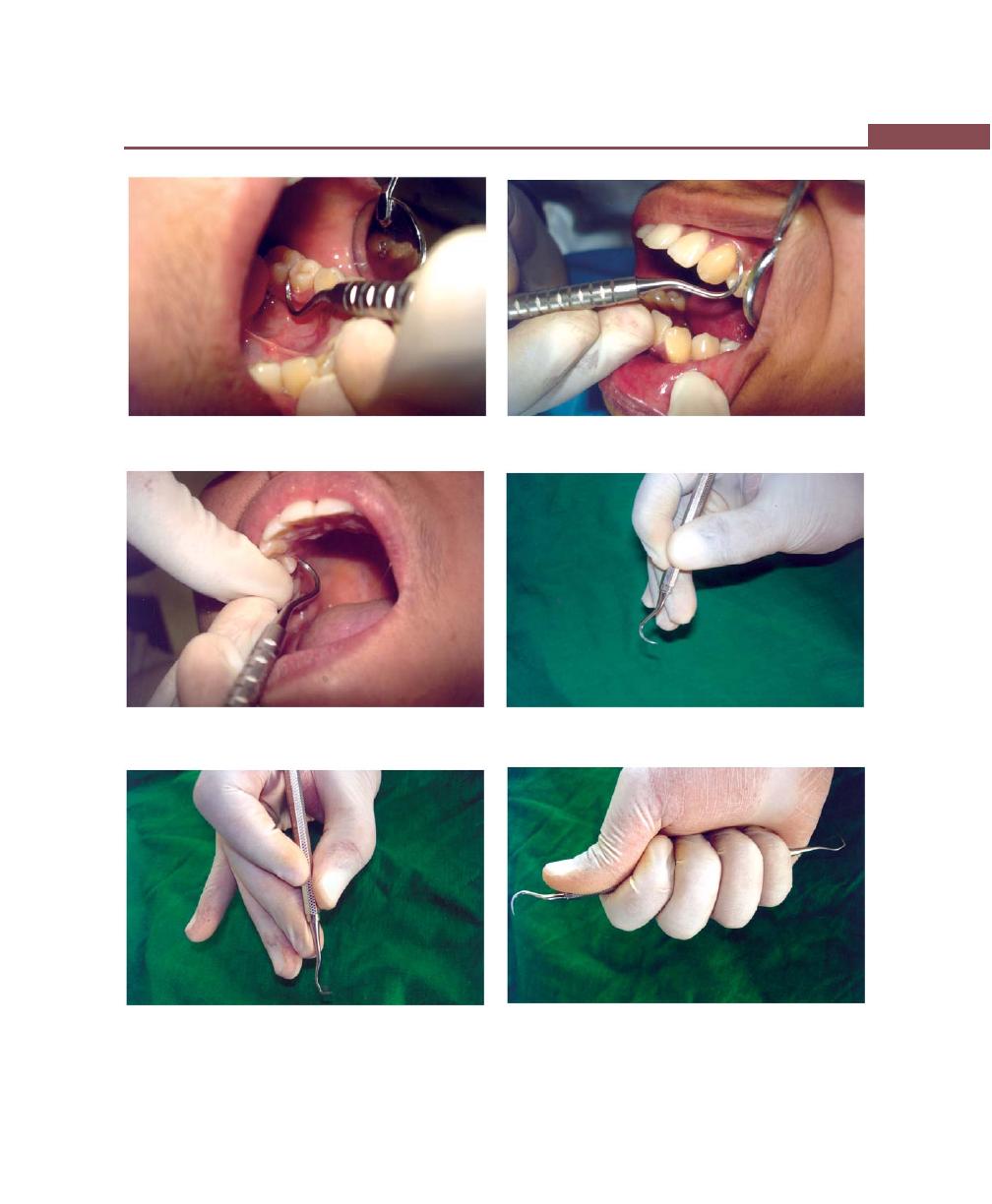
437
Questionnaire for Clinical Case Discussion
Fig. 53.24:
Intraoral finger rests—Cross-arch
Fig. 53.25:
Opposite arch (Intraoral finger rests)
Fig. 53.26:
Finger on finger (Intraoral finger rests)
Fig. 53.27:
Instrument grasps—Pen grasp
Fig. 53.28:
Instrument grasps—Modified pen grasp
Fig. 53.29:
Instrument grasps—Palm and thumb grasp

438
Essentials of Clinical Periodontology and Periodontics
Q57. Different angulations needed for scaling
procedure.
Answer
Angulation refers to the angle between the face of bladed
instrument and tooth surface. It is also called tooth blade
relationship.
a. For subgingival insertion of bladed instruments
angulations should be close to 0 degrees as possible.
b. For scaling and root planing, angulation should be
between 45 to 90°.
c. For gingival curettage angulation>90° is established
so that the cutting edge will engage and remove the
pocket epithelium.
Q58. Recent advances in periodontal instrumen-
tation.
Answer
Advances in Diagnostic Instruments
Probes: They are classified, based on the generation of
development.
Ist
Generation Probes : Conventional probes, e.g.
Williams probe
IInd Generation Probes : Pressure sensitive probes, e.g.
Borodontic probes
IIIrd Generation Probes : Computer aided probes, e.g.
Florida probes
IVth Generation Probes : Records sequential probe
positions along gingival sulcus. They are still under
research.
Vth Generation Probes : Ultrasound probes. They are
still under research.
Dental endoscope : Perioscopy system, which works on
fiber optic system. It is used for subgingival calculus
detection, caries, defective restoration, and fractures.
ADVANCES IN ROOT PLANING INSTRUMENTS
1. Gracey no 15-16: It is a modification of standard
11-12 and has an 11-12 blade with a 13-14 shank.
It is useful for mesial surfaces of posterior teeth.
2. Gracey no 17-18: It is a modification of standard
13-14. It is a 13-14 Gracey curette with a terminal
shank elongated by 3 mm and a more accentuated
angulation of shank.
3. Extended shank curettes (after five curettes) : Here
shank is elongated by 3 mm and is used for deeper
periodontal pockets of 5 mm or more.
4. Mini-bladed curettes (mini five curettes) : They are
modifications of after five curettes with their blade
reduced by half. It is used in furcation area, develop-
mental grooves, tight pockets, deep narrow pockets.
5. Gracey curvettes: Here the blade length is half of
the conventional gracey curette and blade curves
upwards.
6. Langer and mini-langer curettes: It is a combination
of universal and gracey curettes available in 5-6, 11-
12 and 13-14. It has similar shank design to a gracey
curette but blade angle is 90 degrees.

439
Glossary
Abrasion
Refers to the loss of tooth substance induced by mechanical wear other than
that of mastication.
—Carranza.
Abscess
A localized collection of pus.
Acellular Cementum/
Cementum lacking embedded cells.
—Grant/Stern.
Primary Cementum
Acellular Cementum/
Cementum that forms in conjunction with root formation and tooth
Primary Cementum
eruption.
—Lindhe.
Acquired Coatings
It includes those exogenous origins such as saliva, bacteria, calculus and
surface stains.
Acquired Immunodeficiency
It is characterized by profound impairment of the immune system.
Syndrome (AIDS)
Acquired Pellicle
It is a homogenous, membranous, acellular film that covers the tooth surfaces
and frequently forms the interface between the surface of the dental plaque
and calculus.
—Saul Schluger.
Active Eruption
Active Eruption
is the movement of the teeth in the direction of the occlusal
plane.
—Carranza.
Actual Position
It is the level of the epithelial attachment on the tooth.
—Carranza.
Acute Gingivitis
Inflammation of gingiva which is of short duration, sudden onset and can
be painful.
Acute Trauma from Occlusion
It results from abrupt occlusal impact such as that produced by biting on
hard object.
—Carranza.
Adaptation
Refers to the manner in which the working end of the periodontal instrument
is placed against the surface of the tooth.
—Carranza.
Addison’s Disease
It is caused by adrenal dysfunction and produces isolated patches of
discoloration varying from bluish black to brown.
—Carranza
Additive Osseous Surgery
Includes procedures directed at restoring the alveolar bone to its original
level.
Adjuvant
A non-specific stimulant of the immune response which enhances the response
to antigen. Many bacterial cell wall components have this property.
Adult Periodontitis
A form of periodontitis that usually has an onset beyond the age of 35 years.
Bone resorption usually progresses slowly and predominantly in the horizontal
direction.
Glossary

440
Essentials of Clinical Periodontology and Periodontics
Aerobe
A micro-organism which grows in the presence of oxygen.
Ageing of Periodontium
Ageing is a slowing of nature function, a disintegration of the balanced control
and organization that characterize the young adult.
—Carranza.
Aggressive Periodontitis
It is a periodontal destruction that becomes clinically significant around
adolescence or early adulthood.
—Carranza.
Aggressive Periodontitis
It is characterized by the rapid loss of attachment and bone loss occurring
in an otherwise clinically healthy patient with the amount of microbial deposits
inconsistent with disease severity and familial aggregation of diseased
individuals.
Aggressive Periodontitis
Was formerly classified as early onset periodontitis i.e. localized juvenile
periodontitis (LJP). Generalized aggressive periodontitis was previously
classified as Generalized Juvenile Periodontitis (GJP) and Rapidly Progressive
Periodontitis.
Agranulocytosis
Agranulocytosis is characterized by a reduction in the number of circulating
granulocytes and results in severe infection including ulcerative necrotizing
lesions of the oral mucosa, skin, gastrointestinal and genitourinary tracts.
—Carranza.
Alleles
Variations in the nucleotide sequence at a locus is termed as alleles.
Allograft or Homograft
A tissue transfer between individuals of the same species but with non-identical
genes.
Allograft
A graft between genetically dissimilar members of the same species.
—Periodontal Literature Review.
Alloplast Graft
A graft of inert synthetic material, which is sometimes called, implant material.
Alveolar Bone Proper
It is a plate of compact bone, the radiographic image of which is termed
as Lamina dura.
—Genco.
Alveolar Bone Proper
It is the thin lamella of bone surrounding the root, seen as lamina dura
in the radiographs (Opaque line).
—Grant.
Alveolar Mucosa
Mucosa covering the basal part of the alveolar process of continuing without
demarcation into the vestibular fornix and the floor of the mouth. It is loosely
attached to the periosteum and is movable.
—Periodontal Literature Review.
Alveolar Process
Alveolar Process is defined as the parts of the maxilla and mandible that
form and support the sockets of the teeth.
—Lindhe.
Alveolar Process
It is that part of the jawbone which supports the teeth.
Alveolar Process
It is that portion of the maxilla and the mandible that form and support
the sockets of the teeth.
—Carranza.
Alveolar Process
It is the part of the maxilla or mandible that forms and supports the teeth.
—Grant/ Stern /Listgarten.
Anaerobic
Growth in absence of oxygen.
Anemia
It is a deficiency in the quantity or quality of blood as manifested by reduction
in the number of erythrocytes and in the amount of haemoglobin.
—Carranza.

441
Glossary
Angular Chelitis
The commissure appears erythematous with surface crusting and fissuring.
Angular Defects
They are classified on the basis of the number of the osseous walls it may
have one, two or three walls.
Angulation
Refers to the angle between the face of the bladed instrument and the tooth
surface. Also known as tooth-blade relationship.
—Carranza.
Ankylosis
Fusion of the cementum and the alveolar bone with the obliteration of the
periodontal ligament is termed as ankylosis.
—Carranza.
Antibiotics
Are a naturally occurring, semisynthetic or synthetic type of antimicrobial
agent that destroys or inhibits the growth of selective micro-organisms,
generally at low concentrations.
Antibody
A class of serum proteins that are induced following interaction with an
antigen, they bind specifically to the antigen that induced their formation.
Antibody
An immunoglobulin which binds to a specific antigen. Lymphocytes which
have recognized an antigen may differentiate into plasma cells and secrete
antibody against that antigen.
Antigen
Any following material that is specifically bound by antibody.
Antigen
A structure recognized as foreign by the immune system. The word is derived
from antibody generator.
Antigenicity
Capacity of the foreign agent to combine with or be recognized by metabolites
or products of immune response.
Antimicrobial Agent
It is a chemotherapeutic agent that works by reduction in bacterial number.
Antiseptic
Are chemical antimicrobial agents that are applied topically or subgingivally
to mucous membrane wounds, intact dermal surfaces to destroy micro-
organisms and inhibit their metabolism and reproduction.
—Carranza.
Apparent Position
It is the level of the crest of the gingival margin.
Attached Gingiva
Width of the attached gingiva—it is the distance between the mucogingival
junction and the projection on the external surface of the bottom of the
gingival sulcus or the periodontal pocket.
—Carranza.
Attached Gingiva
The attached gingiva is continuous with the marginal gingiva. It is firm, resilient
and tightly bound to the underlying periosteum of the alveolar bone.
—Carranza.
Attrition
Is occlusal wear resulting from functional contacts with opposite teeth.
—Carranza.
Autograft
Tissue transferred from one position to another within the same individual.
Bacterial Succession
The appearance and disappearance of species as mixed flora evolves.
Biofilm
It describes the relatively indefinable microbial community associated with
a tooth surface or any hard non shedding material.
—Wilderer and Charaklis [1989].
Biofilm
It is defined as matrix enclosed bacterial population adherent to each other
and/or to surface or interfaces.
—Costerton.

442
Essentials of Clinical Periodontology and Periodontics
Biofilms
Biofilms are natural communal aggregations of micro-organisms that may
form on wide range of surfaces.
—Lindhe.
Bio-integration
A bonding of the living bone to the surface of an implant which is independent
of any mechanical interlocking mechanism.
—Periodontal Literature Review.
Biologic Depth
It is the distance between the gingival margin and the base of the pocket.
(Coronal end of the junctional epithelium).
—Carranza (it can be measured only in carefully prepared and adequately
oriented histological section).
Biologic Width
It is defined as; the dimension of space that the healthy gingival tissues occupy
above the alveolar bone.
—Carranza.
Bruxism
It is defined as the clenching or grinding of the dentition during non functional
movements of the mastication system.
—Periodontal Literature Review.
Bruxism
It is the clenching /grinding of teeth when individual is not chewing or
swallowing.
Bulbous Bone Contours
Bulbous bone contours are bony enlargements caused by exostosis,
adaptation to function, or buttressing bone formation. Commonly found
in maxilla.
Bullous Pemphigoid
It is a chronic autoimmune subepidermal, bullous disease with tense bullae
that rupture and become flaccid in the skin.
—Carranza.
Bundle Bone
The layer of the alveolar bone into which the principal fibers (Sharpey’s
fibers) are inserted.
—Lindhe.
Buttressing Bone
Bone formation occurring in an attempt to buttress bony trabeculae weakened
Formation/Lipping
by resorption.
Buttressing Bone Formation
If it occurs within jaw, it is termed central buttressing bone formation.
Buttressing Bone Formation
When it occurs on external surface, it is referred to as peripheral buttressing
bone formation.
Bystander damage
Accidental damage to host tissues caused by complements, neutrophils and
macrophages during inflammation.
Calculus
A hard concretion that forms on teeth or dental prosthesis through calcification
of bacterial plaque
—Periodontal Literature Review.
Calculus
Calculus is a calcified plaque.
—Manson and Eley.
Calculus
Calculus is essentially mineralized plaque covered on its external surface by
vital, tightly adherent, non-mineralized plaque.
— Genco.
Calculus
Consists of mineralized bacterial plaque that forms on the surface of the
natural teeth and dental prosthesis.
—James E Hinrichs (Carranza).
Calculus
Is a calcified mass which forms on and adheres to the surface of teeth and
other solid objects in the mouth, e.g. restoration and denture.
Calculus
It is defined as a hard deposit that forms by mineralization of dental plaque
and is generally covered by a layer of unmineralized plaque.
—Carranza.
Calculus
When dental plaque calcifies the resulting deposit is called Dental calculus.
These calcified deposits occur as hard, firmly adhering masses on the clinical
crowns of the teeth.
—Grant.

443
Glossary
Capnophillic
Organisms which require greater concentration of carbon-dioxide.
—Periodontal Literature Review.
Carrier Molecule
Is a part of the antigen recognized by T-Lymphocytes.
—Grant
Cell-mediated Immunity
An immune reaction mediated by T-cells.
Cell-mediated Immunity
An immune reaction mediated by T-Lymphocytes (activated lymphocytes
release biologic response modifiers (lymphokines) on exposure to antigen.
Cellular Cementum/
Cementum that contains cells in lacunae located within the mineralized matrix.
Secondary Cementum
—Grant/ Stern/Listgarten.
Cellular Cementum/
Cementum that forms after tooth eruption and in response to functional
Secondary Cementum
demand.
—Lindhe.
Cementum
Cementum is a calcified connective tissue which covers the root dentine and
into which periodontal fiber bundles are inserted.
—JD Manson & BM Eley.
Cementum
Cementum is a calcified mesenchymal tissue that forms the outer covering
of the anatomic root.
Cementum
Cementum is a hard bone like tissue covering the anatomic roots of the
teeth.
—Genco.
Cementum
Cementum is a hard bone like tissue covering the root surfaces and occasionally
small portions of the crown of the teeth.
—Lindhe.
Cementum
Cementum is mineralized connective tissue that covers the roots of the teeth.
—Grant/ Stern/Listgarten.
Chemotaxis
Locomotion of cells which is directed along an increasing chemical gradient
of a soluble substance.
Chemotaxis
The migration of cells along a concentration gradient of an attractant.
Chemotherapeutic Agents
General term used for a chemical substance that provide chemical therapeutic
benefit.
Chronic Gingivitis
Is slow in onset and is of long duration, and is painless unless complicated
by acute or subacute exacerbations.
—Carranza.
Chronic Periodontitis
An infectious disease resulting in inflammation within the supporting tissues
of the teeth, progressive attachment loss and bone loss.
—Carranza.
Chronic Trauma from
It results from gradual changes in occlusion produced by tooth wear, drifting
Occlusion
movement, extrusion of teeth etc.
—Carranza.
Clenching
It is the closure of the jaws under vertical pressure.
Coatings of Developmental
Those normally formed as part of tooth development (reduced enamel
Origin
epithelium, dental cuticle).
—Carranza.
Co-efficient of Separation
The length of root cones in relation to the length of root complex. —
Lindhe.
Combined Lesion
Combined lesion occurs when pulpal necrosis and periapical lesion occurs
on a tooth that also is periodontally involved.
—Carranza.
Combined Osseous Defect
The number of walls in the apical portion of the defect may be greater than
that in its occlusal portion.

444
Essentials of Clinical Periodontology and Periodontics
Complement
A group of serum proteins involved in the control of inflammation, the
activation of phagocytes and lytic attack on cell membranes. The system
can be activated by interaction with antigen-antibody complexes or bacterial
substances.
—Periodontal Literature Review.
Complement
It is an interacting network of about 30 membrane associated cell receptors
and soluble serum glycoprotein.
—Carranza.
Compromise Osseous
It indicates a bone pattern that cannot be improved without significant osseous
Reshaping
removal that would be detrimental to overall result.
—Carranza.
Contact Inhibition
Inhibition of movement and division caused by physical contact between
cells of the same type.
Contact Inhibition
The process by which the graft material prevents apical proliferation of the
epithelium.
Coronal Cementum
Cementum that is found over enamel.
—Grant/ Stern/Listgarten.
Curettage
It is used in Periodontics for scraping of the gingival walls of the periodontal
pocket to separate the diseased soft tissue.
Curettes
Are manual instruments with cutting edge on both sides of the blade and
rounded toe.
Cuticle
Thin, acellular structure with a homogenous matrix, sometimes enclosed
within clearly demarcated, linear borders.
—Carranza.
Cyst
Cyst is a swelling consisting of fluid in a sac, which is lined by epithelium
or endothelium.
—Bailey and Love.
Cytokine
A small protein messenger released by cells which affects the division,
differentiation and function of other cells, which may be of the same or
different types.
Definitive Osseous Reshaping
Implies that further osseous reshaping would not improve the overall result.
Degree of Separation
It is the angle of separation between two roots.
—Lindhe.
Dehiscence
Alveolar dehiscence is a dipping of the crestal bone margin exposing the
root surface.
—Grant/ Stern /Listgarten.
Dehiscence
Denuded surfaces involving the marginal bone, the defect is called as
dehiscence.
—Carranza.
Dental Calculus
It is a tightly adherent plaque that has undergone mineralization.
Dental Calculus
It is an adherent, calcified or calcifying mass that forms on the surfaces of
teeth and dental appliances.
Dental Epidemiology
Is the study of pattern [distribution] and dynamics of dental diseases in a
human population.
—Carranza.
Dental Implant
Dental implant is perimucosal devices that is biocompatible and bio-functional
and is placed on or within the bone associated with the oral cavity to provide
support for fixed or removable prosthesis.
Dental Plaque
Dental plaque comprises of complex microbial communities that form on
virtually all surfaces of the teeth exposed to the bacteria-laden fluids of the
mouth.
—Robert Wirthilin and Gary C Armitage.

445
Glossary
Dental Plaque
Dental plaque is an adherent, intercellular matrix consisting primarily of
proliferating micro organisms, along with a scattering of epithelial cells
leukocytes and macrophages.
Dental Plaque
Dental plaque is defined as a soft deposit that forms the biofilm adherent
to the tooth surface or other hard surfaces in the oral cavity including
removable and fixed restorations.
—Carranza.
Dental Plaque
It is a host associated biofilm.
Dental Splint
An appliance designed to immobilize and stabilize loose teeth.
Dental Stain
Pigmented deposits on tooth surface.
—Carranza.
Dentifrices
Agent that aids in cleaning and polishing tooth surfaces.
Desmosome
A specialized attachment between epithelial cells which impart strength to
epithelium.
Desquamative Gingivitis
Coined in 1932 by Prinz, to describe a peculiar condition characterized by
intense erythema, desquamation, and ulceration of the free and attached
gingiva.
—Carranza.
Diagnosis
It may be defined as identifying disease from an evaluation of the history,
signs and symptoms, laboratory tests and procedures.
Differentiation
Differentiation involves the process of keratinization, which consists of sequence
of biochemical and morphologic events that occur in the cell as it migrates
from the basal layer.
—Carranza.
Diffuse Gingivitis
It affects gingival margin, the attached gingiva and the interdental papillae.
—Carranza.
Disclosing Agents
They are solutions or wafers capable of staining bacterial deposits on the
surface of teeth, tongue and gingiva.
Disease Atrophy
Non-functional and hypofunction may be expressed as disease atrophy. The
disease of the periodontal tissue is characterized by a rearrangement of fibers
of the periodontal ligament and of the trabeculation of the alveolar bone
without gingival recession.
—Grant.
Disinfectant
An antimicrobial agent that are generally applied to in animate surfaces to
destroy micro-organisms.
Displaced Flap
It is placed apically, coronally or laterally to their original position.
—Carranza.
Disuse or Functional Atrophy
When occlusal forces are reduced, the number and thickness of the trabeculae
are reduced, with atrophy of periodontal ligament appearing thinned, with
reduction in number of fibers and density.
—Carranza.
Divergence
It is the distance between two roots (cones); this distance normally increases
in apical direction.
—Lindhe.
Down’s Syndrome
Is a congenital disease caused by a chromosomal abnormality and
characterized by mental deficiency and growth retardation.
Ecological Niche
The functional position in which bacterial species occupies within a complex
ecosystem such as plaque.
Electrocoagulation
It provides a wide range of coagulation or hemorrhage control obtained
by using electrocoagulation current.

446
Essentials of Clinical Periodontology and Periodontics
Electrosection
Electrotomy or acusection is used for incision, excision and tissue planing.
Electrodesiccation
Uses dehydrating current and is least used.
Electrofulguration
Uses high voltage current.
Electrosurgery (Radiosurgery)
It is currently used to identify surgical techniques performed on soft tissue
using controlled high frequency electrical current in the range of 1.5-7.5
million cycles/sec or MHz.
Electrosurgery
Division of tissue by high frequency electrical current applied locally with
a metal instrument or needle.
Endodontic-periodontal Lesions
Pulpal necrosis precedes periodontal changes. A periapical lesion originating
in pulpal inflammation and necrosis may drain to the oral cavity through
periodontal ligament and adjacent alveolar bone.
—Carranza.
Endotoxin
A complex heat stable toxin which is a structural component of gram-negative
bacterial cell wall. The active component is a lipopolysaccharide.
Epidemiology
The study of the distribution and determinants of health related states or
events in specified populations and the application of this study to control
health problems.
—Carranza.
Epithelial Adaptation
It is the close apposition of the gingival epithelium to the tooth surface without
obliteration of the pocket.
Epulis
It is the generic term to designate all discrete tumor and tumor like mass
of gingiva.
—Carranza.
Erosion (Cuneiform Defect)
It is a sharply defined wedge shaped depression in the cervical area of the
facial tooth surface.
—Carranza.
Etiology
The cause of disease, in the case of gingivitis and periodontal disease, microbial
dental plaque.
Evidence Based Therapy
Current concept of evaluating health care requires a scientific basis for
treatment.
Excisional New Attachment
It is a definitive subgingival curettage performed with a knife.
Procedure
Excursive Movement
Any movement of the mandible away from intercuspal position.
Exostosis
Exostosis is outgrowths of the bone of varied size and shape.
Exploratory Stroke
It is a light “feeling” stroke that is used with probes and explorer to evaluate
the dimensions of the pocket and to detect calculus and irregularities of the
tooth surface.
Exposure
It is defined as a factor that may possibly lead to disease or may be protective
against a disease.
—Mauricio Ronderers.
Extrusion
Pathologic migration in the Occlusal or incisal direction is termed as extrusion.
—Carranza.
Factitious
Pertaining to a state or situation produced by other natural means; self inflicted.
Facultative Anaerobe
Growth in either an aerobic or anaerobic environment.

447
Glossary
F
c
The part of an immunoglobulin molecule which binds to specific receptors
on neutrophils and macrophages, but which does not bind to antigen.
Fenestration
It is a circumscribed hole in the cortical plate over the root and does not
communicate with the crestal margin.
—Grant/ Stern /Listgarten.
Fenestration
An isolated area in which the root is denuded of the bone and the root
surface is covered only by the periosteum and the overlying gingiva are
termed fenestration. Here the margin is intact.
—Grant/ Stern /Listgarten.
Fibroma
Arise from the gingival connective tissue or from the periodontal ligament.
—Carranza.
Fibronectin
A connective tissue protein which binds cells to the extracellular matrix.
Fibro-osseous Integration
In which soft tissue such as fibers and/ or cells, are interposed between the
two surfaces.
Fimbriae
A fine filamentous surface appendage on a bacterium. These are important
for attachment and adhesion and are important antigens on some species.
Finger Rest
The finger rest serves to stabilize the hand instrument by providing a firm
fulcrum as movement is made to activate the instrument.
Flap
A loosened section of the tissue separated from the surrounding tissue except
at its base.
—Periodontal Literature Review.
Flat Architecture
It is reduction of the interdental bone to the same height as radicular
bone.
—Carranza.
Food Impaction
It is the forceful wedging of food into the Periodontium by Occlusal
forces.
—Carranza.
Food Impaction
The forceful wedging of the food into the interproximal space by chewing
pressure or the forcing of food interproximally by tongue or cheek pressure.
Fremitus
A palpable or visible movement of teeth when subjected to occlusal
forces.
Fremitus
It is the measurement of the vibratory patterns of the teeth when teeth are
placed in contacting positions and movements.
—Genco.
Frenectomy
It is complete removal of the frenum, including its attachment to underlying
bone and may be required in correction of an abnormal diastema between
maxillary central incisors.
Frenotomy
It is the incision of frenum.
—Carranza.
Frenum
It is a fold of mucous membrane usually with enclosed muscle fibers that
attaches the lip and cheeks to the alveolar mucosa and / or gingiva and
underlying periosteum.
Full Thickness Flap
All the soft tissue, including the periosteum is reflected to expose the underlying
bone.
Furcational Entrance
It is the transitional area between the divided and undivided part of the
root.
—Lindhe.
Furcational Fornix
The roof of the furcation.

448
Essentials of Clinical Periodontology and Periodontics
Furcation Invasion
Pathologic resorption of bone within the furcation.
—Periodontal Literature Review.
Furcation Involvement
Furcation involvement refers to the invasion of the bifurcation and trifurcation
of multirooted teeth by periodontal disease.
—Carranza.
Furcation
Furcation is defined as the area located between individual root cones.
—Lindhe.
Furcation
It is the anatomic area of a multirooted tooth where the roots diverge.
—Periodontal Literature Review.
Generalized Aggressive
Clinically characterized by “generalized interproximal attachment loss affecting
Periodontitis
at least 3 permanent teeth other than first molars and incisors”.
—Carranza.
Generalized Diffuse Gingivitis
Involves the entire gingiva of the entire mouth.
Generalized Gingivitis
Involves the entire mouth.
Generalized Marginal
Involves the gingival margins in relation to all the teeth.
—Carranza.
Gingivitis
Generalized Periodontitis
It is considered generalized when >30% of the sites assessed in the mouth
demonstrate attachment loss and bone loss.
Genetic Marker
Genetic marker refers to any gene or nucleotide sequence that can be mapped
to a specific location or region on a chromosome.
—Carranza.
Genotype
The generic composition of an organism.
Gingiva
Gingiva is the part of the oral mucosa that covers the alveolar process of
the jaws and surrounds the neck of the teeth.
—Genco.
Gingiva
The fibrous investing tissue covered by keratinized epithelium which
immediately surrounds a tooth and is contiguous with its periodontal ligament
and with the mucosal tissues of the mouth.
—Periodontal Literature Review.
Gingiva
The gingiva is the part of the masticatory mucosa, which covers the alveolar
process and surrounds the cervical portion of the teeth.
—Lindhe.
Gingiva
The gingiva is the part of the oral mucosa that covers the alveolar processes
of the jaws and surrounds the necks of the teeth.
—Carranza.
Gingiva
The gingiva is the part of the oral mucous membrane attached to the teeth
and the alveolar processes of the jaws.
—Grant/ Stern/Listgarten.
Gingiva
The gingiva is that part of the oral mucosa which surrounds the tooth and
covers the alveolar ridge.
—Manson & Eley.
Gingival Abscess
Gingival abscess is a localized, painful, rapidly expanding lesion, which is
usually of sudden onset. It is generally limited to the marginal gingiva or
interdental papilla.
—Carranza.
Gingival Abscesses
Gingival abscesses are those that are localized to the marginal gingiva or
interdental papilla.
—Gary C Armitage.
Gingival Abscesses
Localized in the gingiva, caused by injury to the outer surface of the gingiva
and not involving the supporting structures.
—Carranza.

449
Glossary
Gingival Crevicular Fluid
GCF is an altered serum transudate found in the gingival sulcus.
—Genco.
Gingival Crevicular Fluid
Tissue fluid that seeps through the crevicular epithelium. It is increased in
the presence of inflammation.
—Periodontal Literature Review.
Gingival Curettage
It consists of the removal of inflamed soft tissue lateral to pocket wall.
Gingival Curettage
It means the scraping of the gingival wall of the periodontal pocket to separate
diseased soft tissue.
Gingival Cyst
Found within the gingiva, most commonly in the canine—premolar region.
—Periodontal Literature Review.
Gingival Enlargement
Increase in the size of the gingiva.
Gingival Hyperplasia
Defined as an enlargement of gingiva due to the increase in the number
of cells.
—Periodontal Literature Review.
Gingival Pocket
This type of pocket is formed by gingival enlargement without destruction
(Pseudo Pocket)
of the underlying periodontal tissues.
Gingival Recession
Is the exposure of root surface due to apical shift in the marginal gingiva.
—Grant
Gingival Sulcus
The gingival sulcus is the shallow crevice or space around the tooth bounded
by the surface of the tooth on one side and the epithelia lining the free
margin of the gingiva on the other.
—Carranza.
Gingivectomy
Excision of a portion of the gingiva.
—Periodontal Literature Review.
Gingivectomy
It means excision of gingiva.
—Carranza.
Gingivitis
Inflammation of the gingiva.
Gingivoplasty
Gingivoplasty is “a surgical reshaping of the gingiva”.
Gingivoplasty
It is the reshaping of the gingiva to create physiologic gingival contour, with
the sole purpose of recontouring the gingiva in the absence of periodontal
pocket.
—Carranza.
Glycosaminoglycan
A molecule of repeating sugar subunits which forms a major component
of connective tissue ground substance.
Gnotobiotic Animal
A laboratory animal whose microbial flora is known. Specific micro-organisms
are introduced into germ-free animals to make them gnotobiotic.
Gracey Curettes
Set of many curettes designed for specific area and surfaces.
Graft
Any tissue or organ used for implantation or transplantation.
Graft
It is a viable tissue/ organ that after removal from donor site are implanted
within the host tissue, which is then repaired, restored and remodeled.
Guidance
Pattern of opposing tooth contact during excursive movements of the
mandible. The teeth making such contact cause separation of the other teeth.
Guided Tissue Regeneration
An epithelial exclusionary technique that promotes new connective tissue
attachment without the use of any implant material.
Guided Tissue Regeneration
Procedures attempting to regenerate lost periodontal structures through
differential tissue responses.

450
Essentials of Clinical Periodontology and Periodontics
Habit
An act of repeated performance, almost automatic, such as bruxism or tongue
thrusting.
Halitosis
Also termed as fetor ex ore and oral malodor, is foul or offensive odor,
emnating from oral cavity.
—Carranza.
Hapten
Is a part of antigen recognized by B-Lymphocytes.
—Grant.
Hemidesmosome
Structure which attaches epithelial cells to the basement membrane. It
resembles half a desmosome, but has different structural components.
Hemisection
It is surgical removal of a root with the associated part of crown.
Hemisection
It is the splitting of the two-rooted tooth into two separate portions. The
process has been called Bicuspidisation or Separation.
Hemiseptum
The one wall defect is also called as hemiseptum.
Homeostasis
The production of a stable equilibrium in a body system by means of feedback
mechanisms.
Horizontal Bone Loss
Common pattern of bone loss in which bone is reduced in height, but the
bone margins roughly perpendicular to the tooth surface.
Hydrolytic Enzymes
A class of enzymes which cleaves various types of bond, including peptide,
ester and glycosidic bonds, with the addition of water. Many degradative
and lysosomal enzymes fall into this category.
Hypercementosis
It refers to the prominent thickening of the cementum. It may be localized
or generalized.
—Carranza.
Hyperfunction
Is a functional adaptation in which additional structural elements are formed
on cementum and in bone to withstand the added force expressed clinically
and microscopically as a thickened lamina dura, buttressing bone formation,
osteosclerosis and cemental spurs.
—Grant.
Hypersensitivity
Root surfaces exposed by gingival recession may be hypersensitive to thermal
changes or tactile stimulation.
Iatrogenic Factors
Inadequate dental procedure that contribute to the deterioration of the
periodontal tissues.
—Carranza.
Immune Complex
A complex of antigen and antibody molecules bound to one another and
together.
Immunogenicity
It is the capacity to induce a detectable immune response such as the
production of antibody or the stimulation of cellular immunity.
Immunoglobulin
A glycoprotein composed of heavy and light peptide chains; functions as
antibody in serum and secretions.
—Periodontal Literature Review.
Implant
An alloplastic material or device that is surgically placed into the oral tissue
beneath the mucosal or periosteal layer or within bone for functional,
therapeutic and esthetic purposes.
Implant
To insert a graft or alloplastic device into the oral hard or soft tissue for
replacement of missing or damaged anatomic parts or stabilization of
periodontally compromised tooth or group of teeth.
—Periodontal Literature Review.

451
Glossary
Inadvertent Curettage
Some degree of curettage is done unintentionally while scaling and root
planing is performed.
—Carranza.
Inflammation
A cellular and vascular reaction of tissue to injury.
— Periodontal Literature Review.
Inflammation
A localized protective response elicited by injury or destruction of tissue,
which serves to destroy, dilute or wall off both the injurious agent and injured
tissue.
—Periodontal Literature Review.
Inflammation
Is an observable alteration in tissue associated with changes in vascular
permeability and dilation, often with the infiltration of leucocytes into affected
tissue.
—Periodontal Literature Review.
Intercuspal Position (ICP)
The position of the mandible when there is maximum intercuspation between
the maxillary and mandibular teeth.
—Synonym-Centric occlusion.
Interdental Gingiva
The interdental gingiva occupies the gingival embrasure, which is the
interproximal space beneath the area of tooth contact.
—Carranza.
Interference
Any contact in ICP or excursion that prevents the remaining occlusal surfaces
from achieving stable contact.
—Synonym-Supra contact.
Interleukins
Cytokine which mediate communication between leukocytes.
Intermediate Cementum
It is an ill defined zone near cementodentinal junction of certain teeth that
appear to contain cellular remnants of Hertwig’s sheath embedded in calcified
ground substance.
—Carranza.
Intra Bone/Intra Bony/
Bottom of the pocket is apical to the level of the adjacent alveolar
Supra Crestal Pocket
bone.
—Carranza.
Intrabony Defects
The three-wall vertical defect was originally called Intrabony defect.
Junctional Epithelium
A single or multiple layer of non-keratinizing cells adhering to the tooth surface
at the base of the gingival crevice.
—Periodontal Literature Review.
Junctional Epithelium
It denotes the tissue that joins to the tooth on one side and to the oral
sulcular epithelium and connective tissue on the other.
Juvenile Periodontitis
A disease of Periodontium occurring in an otherwise healthy adolescent which
is characterized by a rapid loss of alveolar bone, about more than one tooth
of the permanent dentition.
Lamina Dura
The compact bone (Alveolar bone proper), which lines the tooth socket,
and in a radiograph appears as a dense opaque line is called as lamina dura.
—Lindhe.
Lateral Pressure
Refer to the pressure created when force is applied against the surface of
a tooth with the cutting edge of a bladed instrument.
—Carranza.
Laterotrusion
Movement of the mandible laterally to the right or left from ICP.
Laterotrusive Movement
The side of either dental arch corresponding to the side of mandible moving
away from the midline.
—Synonym-Working side.
Ledges
Ledges are plateau like bone margins caused by resorption of thickened
bony plates.
—Carranza.

452
Essentials of Clinical Periodontology and Periodontics
Leukemia
It is a malignant neoplasia of WBC precursors.
—Carranza.
Leukoplakia
A white patch or plaque that does not rub off and cannot be diagnosed
as any other disease.
—World Health Organization.
Lichen Planus
It is a relatively common, chronic dermatosis characterized by the presence
of cutaneous, violaceous papules that may coalesce to form plaques.
Lichen Planus
Is defined as unique cutaneous entity consisting of an eruption of pappules
distinct in color and configuration, in patterns and location of appearance
and in microscopic and gross structure.
—Fitz Patrick.
Lipopolysaccharide
The active component of bacterial endotoxins.
Localized Gingivitis
It is confined to the gingiva of a single tooth or group of teeth.
Localized Aggressive
Is characterized as having localized first molar or incisor disease with proximal
Periodontitis
attachment loss on at least 2 permanent teeth, one of which is a first molar.
—Carranza.
Localized Diffuse Gingivitis
It extends from the gingival margin to the mucobuccal fold but is limited
in area.
Localized Marginal Gingivitis
It is confined to one or more areas of the marginal gingiva.
Localized Periodontitis
It is considered localized when <30% of the sites assessed in the mouth
demonstrate attachment loss and bone loss.
Loci
Loci are defined as the specific location on the chromosomes.
Lymphokine
Cytokine secreted by lymphocytes. Cytokine is now preferred term for this
group of substances because they are secreted by other cell types as well.
Malnutrition
Any disorder of nutrition, it may be due to imbalance or insufficient diet
or to defective assimilation or utilization of foods.
—Dorland’s.
Marginal Gingiva
The marginal or attached gingiva, is the terminal edge or border of the gingiva
surrounding the teeth in a collar-like fashion.
—Carranza.
Marginal Gingivitis
Inflammation of the gingival margin and may include a portion of the
contiguous attached gingiva.
Marginal Plaque
Plaque is in direct contact with the gingival margin and also referred to as
marginal plaque.
—Carranza.
Marginal Plaque
It is the supragingival plaque that is in direct contact with the gingival margin.
Masticatory Mucosa
The gingival and the mucosal covering of the hard palate.
Materia Alba
Is a yellowish or whitish, soft loose deposit found in neglected mouths
comprising of a mass of micro-organisms, desquamated epithelial cells, food
debris, leucocytes plus salivary deposits.
—J.D Manson and B.M Eley.
Materia Alba
Is a deposit composed of aggregates of microorganisms, leukocytes,
dead, exfoliated epithelial cells attached to surfaces of the teeth, plaque and
gingiva.
—Saul Schluger.
Materia Alba
It refers to the soft accumulation of bacteria and tissue cells that lack the
organized structure of dental plaque and are easily displaced with a water
spray.
—Carranza.

453
Glossary
McCall’s Festooning
Are life preserver-shaped enlargements of the marginal gingiva that occurs
most frequently in the canine and premolar areas on the facial surface.
Mediotrusive Side
The side of either dental arch corresponding to the side of mandible moving
towards the midline.
—Synonym-Balancing side, non-working side.
Metalloproteinases
Enzyme which degrade protein in the presence of metal ions, usually calcium
or magnesium.
Micro-aerophilic
Organisms which grow best in an atmosphere of reduced oxygen.
Microbial Dental Plaque
Is described as aggregations of bacteria that are tenaciously attached to the
teeth and other surfaces.
—Genco.
Mitogen
A substance that causes DNA synthesis, blast transformation, and mitosis
in lymphocytes.
Mobility
Is a measurement of horizontal and vertical tooth displacement created by
examiner force.
Moderate Periodontitis
Periodontal destruction is generally considered moderate when 3 to 4 mm
of clinical attachment loss has occurred.
Modified Pen Grasp
The thumb, index finger, and middle finger are used to hold the instrument
as a pen is held, but the middle finger is positioned so that the side of the
pad next to the finger nail is resting on the instrument shank. The index
finger is bent at the second joint from the finger tip and is positioned well
above the middle finger on the same side of the handle.
—Carranza.
Modified Widman Flap
A scalloped, replaced, mucoperiosteal flap, accomplished with an internal
bevel incision that provides access for root planing.
— Periodontal Literature Review.
Monocyte
A large mononuclear phagocytic leukocyte, the circulating precursors of
macrophages.
Mucogingival Deformity
It is defined as “a significant departure from the normal shape of gingiva
and alveolar mucosa” and may involve the underlying alveolar bone.
Mucogingival
is defined as “A generic term used to describe the mucogingival junction
and its relationship to the gingiva, alveolar mucosa, frenula, muscle
attachments, vestibular fornices and the floor of the mouth”.
Mucogingival Surgery
Is defined as periodontal surgical procedures designed to correct defects in
the morphology, position, and/or amount of gingiva.
—Carranza.
Mucogingival Surgery
Periodontal surgical procedures used to correct defect in the morphology,
position and or amount of gingiva.
—Periodontal Literature Review.
Mucogingival Surgery
Surgical procedure for the correction of relationships between the gingiva
and oral mucous membrane with reference to three specific problems. Those
associated with attached gingiva, shallow vestibule and a frenum interfering
with the marginal gingiva.
Mucosa
A mucous membrane.
Mucous Membrane
Pemphigoid
Also known as Cicatrical Pemphigoid, is a chronic, vesiculo-bullous auto-
immune disorder of unknown cause that predominantly affects women in
the fifth decade of life.
—Carranza.

454
Essentials of Clinical Periodontology and Periodontics
Multifactorial
Diseases whose etiologies include both genetic and environmental factors.
Muscular Contact Position
The position of the mandible when lifted into contact from resting position.
(MCP)
Necrotizing Ulcerative
Is an extension of necrotizing ulcerative gingivitis (NUG) into the periodontal
Periodontitis
structures, leading to attachment and bone loss.
Necrotizing Ulcerative
It is an inflammatory destructive disease of gingiva, which presents characteristic
Gingivitis
signs and symptoms.
—Carranza.
Necrotizing Ulcerative
It is defined as severe and rapidly progressive disease that has a distinctive
Periodontitis
erythema of the free gingiva, attached gingiva and alveolar mucosa; extensive
soft tissue necrosis; severe loss of periodontal attachment; deep pocket
formation is not evident.
—Periodontal Literature Review.
Negative Architecture
Refers to, if interdental bone is more apical than radicular bone.
Neutrophil
A phagocytic polymorphonuclear leukocyte.
New Attachment
It is the embedding of new periodontal ligament fibers into new cementum
and the attachment of the gingival epithelium to the tooth surface previously
denuded by disease.
—Carranza.
New Attachment
It is the union of the connective tissue or epithelium with a root surface
that has been deprived of its original attachment apparatus.
—Periodontal Literature Review.
Non Displaced Flap
When the flap is returned and sutured in its original position.
Non-thrombocytopenic Purpura
It occurs as a result of either vascular wall fragility or thrombasthenia.
Occlusal Trauma
An injury to the attachment apparatus as a result of excessive occlusal force.
—Lindhe.
Offset Blade
To describe gracey curettes, because they are angled approximately 60-70
degree from the lower shank.
Opsonin
A substance capable of enhancing phagocytosis.
—Periodontal Literature Review.
Opsonin
A substance which facilitates phagocytosis when bound to the surface of
a bacterium or other particle.
Oral Candidiasis
Infection of oral mucous membrane by a fungus of the genus Candida.
Oral Epithelium
Oral epithelium covers the crest and outer surface of the marginal gingiva
and the surface of the attached gingiva.
Oral Epithelium
The oral or outer epithelium covers the crest and outer surface of the marginal
gingiva and the surface of the attached gingiva.
—Carranza.
Oral Mucosa
The tissue lining the oral cavity.
Oral or Outer Epithelium
The outer or oral epithelium covers the crest and outer surface of the marginal
gingiva and the surface of the attached gingiva.
—Carranza.
Osseointegration
A direct contact on the light microscopic level, between living bone tissue
and an implant.

455
Glossary
Osseous Craters
Osseous craters are concavities in the crest of the interdental bone, confined
within the facial and lingual walls.
Osseous Defects
Different types of bone deformities can result from periodontal disease.
Osseous Surgery
It is defined as a procedure by which changes in the alveolar bone can be
accomplished to rid it of deformities induced by the periodontal disease
process or other related factors such as exostosis and tooth supra-eruption.
—Carranza.
Osseous Surgery
Periodontal surgery involving modification of bony support of the teeth.
—Periodontal Literature Review.
Ostectomy
Includes removal of tooth supporting bone.
—Carranza.
Ostectomy
It is defined as the excision of bone or portion of bone in Periodontics;
Ostectomy is done to correct or reduce deformities caused by periodontitis
and includes removal of the supporting bone.
Osteoclast Activating Factor
A mixture of cytokines, including IL-1, TNF, TNF-β
,
and PDGF, which induces
(OAF)
bone resorption and was previously thought to be a distinct cytokine.
Osteoconduction
It is a physical effect by which the matrix of the graft forms scaffold that
favors outside cells to penetrate the graft and form new bone.
Osteoconduction
The graft material acts as passive material, like a trellis or scaffolding for new
bone to cover.
Osteogenesis
Development of the bone; Formation of bone.
Osteogenesis
Refers to formation or development of new bone by cells contained in the
graft.
Osteoinduction
A process by which graft material is capable of promoting cementogenesis,
osteogenesis and new periodontal ligament.
Osteoinduction
It is a chemical process by which molecules contained in the graft (BMPs)
convert the neighbouring cells into osteoblast, which in turn form bone.
Osteoplasty
Thinning of the buccal bone, using diamond burs, included as part of the
surgical technique, results in areas of bone necrosis with reduction in the
bone height, which is later remodeled by new bone.
Osteoplasty
It is defined as the reshaping of the alveolar process to achieve a more
physiologic form without removal of supporting bone.
Osteoplasty
Refers to reshaping the bone without removing tooth supporting bone.
—Carranza.
Osteoplasty
Reshaping the alveolar process to achieve a more physiologic form without
removal of the supporting bone.
—
Periodontal Literature Review.
Papillary Gingivitis
It involves interdental papillae and often extends into the adjacent portion
of the gingival margin.
Papillon-Lefévre Syndrome
Is characterized by hyperkeratotic skin lesions, severe destruction of the
Periodontium and in some cases, calcification of the dura.
Partial Thickness Flap
Includes only the epithelium and a layer of the connective tissue.
—Carranza.

456
Essentials of Clinical Periodontology and Periodontics
Passive Eruption
Passive Eruption is the exposure of the teeth by apical migration of the gingiva.
—Carranza.
Pathogenesis
The mechanism by which a disease develops.
Pathologic Migration
Refers to tooth displacement that results when the balance among the
factors that maintain physiologic tooth position is disturbed by periodontal
disease.
—Carranza.
Pellicle
A thin biofilm.
—Genco.
Pellicle
An organic coating formed when the crown of the teeth emerges and is
exposed to saliva, which is called dental pellicle.
—Grant/ Listgarten.
Pellicle
Pellicle is defined as tooth or mucosal adherent salivary protein.
—Periodontal Literature Review.
Pellicle
The earliest layer of plaque composed of salivary glycoprotein, which is later
colonized by bacteria.
Pemphigoid
Applies to a number of cutaneous, immune-mediated, sub-epithelial bullous
diseases that is characterized by a separation of the basement membrane
zone.
—Carranza.
Pemphigus Vulgaris
Are a group of auto-immune bullous disorders that produce cutaneous and/
or mucous membrane blisters.
—Carranza.
Peri-implant Mucositis
Inflammatory changes confined to the soft tissue surrounding an implant.
—Carranza.
Peri-implantitis
A term used to describe inflammation around a dental implant and/ or its
abutment.
—
Periodontal Literature Review.
Peri-implantitis
Progressive peri-implant bone loss in conjunction with a soft tissue
inflammatory lesion is termed as peri-implantitis.
—Carranza.
Periodontal Abscess
It is a localized accumulation of pus within gingival wall of a periodontal
pocket.
Periodontal Abscess
Localized purulent inflammation in the periodontal tissues.
—Carranza.
Periodontal Abscess
Periodontal abscess are acute lesions that may result in very rapid destruction
of the periodontal tissues.
—Carranza.
Periodontal Abscess
Periodontal abscess are purulent infections localized to the gingival, periodontal
or pericoronal regions.
—Carranza.
Periodontal Cyst
A small cyst of periodontal ligament found most often in the mandibular
canine and premolar area; associated with a vital tooth and postulated to
originate from the rests of mallassez, the rests of the dental lamina or a
supernumarary tooth bud.
Periodontal Disease
Comprises of a group of inflammatory conditions of the supportive tissues
of the teeth that are caused by bacteria.
—Carranza.
Periodontal Dressing/Pack
A protective material applied over the wound created by periodontal surgical
procedures.
—Periodontal Literature Review.
Periodontal Flap
Periodontal flap is a section of the gingiva and\or the mucosa surgically
separated from the underlying tissues to provide visibility and access to the
bone and the root surface.
—Carranza.

457
Glossary
Periodontal Healing/
Restoration of lost Periodontium.
Regeneration
Periodontal Instruments
They are designed for specific purposes, such as removing calculus, planing
root surfaces, curetting the gingiva, removing diseased tissue.
Periodontal Ligament
The periodontal ligament is a dense, fibrous connective tissue attaching the
tooth to the alveolar bone.
—Grant/ Stern /Listgarten.
Periodontal Ligament
The periodontal ligament is the connective tissue that surrounds the root
and connects it to the bone.
—Carranza.
Periodontal Ligament
The periodontal ligament is the soft, richly vascular and cellular connective
tissue which surrounds the roots of the teeth and joins the root cementum
with the lamina dura or the alveolar bone proper, or the socket wall.
—Lindhe.
Periodontal Ligament
The periodontal ligament is the tissue that surrounds the roots of the teeth
and attaches it to the bony alveolus.
Periodontal Plastic Surgery
It is defined as the surgical procedures performed to correct or eliminate
anatomic, developmental or traumatic deformities of the gingiva or alveolar
mucosa.
Periodontal Pocket
Gingival sulcus deepening can occur by coronal movement of gingival margin,
apical displacement of gingival attachment or combination of two processes.
Periodontal Pocket
Periodontal Pocket is defined as “a pathologically deepened gingival sulcus”.
—Carranza.
Periodontal Pocket
Pocket occurred with the destruction of the supporting periodontal tissues.
Periodontal Splint
It is an appliance used for maintaining or stabilizing mobile teeth in their
functional position.
Periodontal Surgery
It is defined as intentional severing or incising of gingival tissue with the purpose
of controlling or eliminating periodontal disease.
Periodontal Trauma
It is a morbid condition produced by repeated mechanical force exerted
on the Periodontium exceeding the physiologic limit of tissue tolerance and
contributing to a breakdown of supporting tissues of tooth.
—Genco.
Periodontal-Endodontic Lesion
The bacterial infection with periodontal pocket associated with loss of
attachment and root exposure may spread through accessory canals to the
pulp, resulting in the pulpal necrosis.
—Carranza.
Periodontics
The clinical science that deals with the Periodontium in health and disease
is called Periodontology, the practice of which is Periodontics.
—Grant/ Stern/ Listgarten.
Periodontitis
Inflammation of the supporting tissues of the teeth. An extension of the
inflammation from gingiva into the adjacent bone and ligament.
—Periodontal Literature Review.
Periodontitis
It is the most common type of periodontal disease and results from extension
of the inflammatory process initiated in the gingiva to the supporting
periodontal tissues.

458
Essentials of Clinical Periodontology and Periodontics
Periodontitis
Periodontitis is defined as “an inflammatory disease of the supporting tissues
of the teeth caused by specific micro-organism, resulting in progressive
destruction of the periodontal ligament and alveolar bone with pocket
formation, recession or both”.
—Carranza.
Periodontitis
Periodontitis is the inflammation combined with loss of attachment.
—Mark U.Thomas, Brain L.Mealey.
Periodontium
Periodontium is the functional unit of the tissues supporting the tooth.
—Grant/ Stern/ Listgarten.
Periodontium
The Periodontium consists of the investing and supporting tissues of the tooth
(gingiva, periodontal ligament, cementum, alveolar bone).
—Micheal G.Newman.
Periodontium
The Periodontium consists of those tissues that surround and anchor the
tooth in the maxillary and mandibular alveolar process.
—Mark I Ryder.
Periodontology
The clinical science that deals with the Periodontium in health and disease
is called Periodontology, the practice of which is Periodontics.
—Grant.
Periodontology
The various diseases of the Periodontium are collectively termed as periodontal
diseases. Their treatment is referred to as periodontal therapy.
Periodontology
The various diseases of the Periodontium are collectively termed as periodontal
disease. The branch of dentistry concerned with prevention and treatment
of periodontal disease is termed Periodontics or Periodontia.
Phagocytosis
Is the process by which cells ingest particles of a size visible by light microscopy.
—Carranza.
Phase I Therapy
To alter or eliminate the microbial etiology and contributing factor for gingival
and periodontal disease.
Phenotype
Collection of traits or characteristics.
Physiologic Mesial Migration
With time and wear the proximal contact areas of the teeth are flattened
and the teeth tend to move mesially. This is referred to as Physiologic mesial
migration.
Physiologic Occlusion
It is one that has demonstrated the ability to survive despite anatomic
obliterations from the hypothetical normal or preconceived ideal form of
occlusion and function.
—Genco.
Plaque Control
It is the removal of dental plaque on a regular basis and prevention of its
accumulation on teeth and adjacent gingival surfaces.
Plaque
An organized mass consisting mainly of micro-organisms that adheres to
teeth, prosthesis and oral surfaces and is found in the gingival crevice and
periodontal pockets.
—Periodontal Literature Review.
Plasma Cell
A mature B lymphocyte which secretes antibody.
Positive Architecture
Refers to radicular bone being apical to interdental bone.
Predictive Value
Refer to the probability of the test results.
Primary Herpetic
It is an infection of the oral cavity caused by the herpes simplex virus
Gingivostomatitis
type-1.

459
Glossary
Primary Trauma from
It is the injury resulting from excessive occlusal forces applied to a tooth
Occlusion
or teeth with normal support.
—Periodontal Literature Review.
Primary Trauma from
It includes a tissue reaction, which is elicited around a tooth with normal
Occlusion
height of the Periodontium.
—Lindhe.
Primary Trauma from
When trauma from occlusion is the result of alteration in the occlusal forces;
Occlusion
it is called primary trauma from occlusion.
—Carranza.
Probing Depth
It is a distance to which an ad hoc instrument (probe) penetrates into pocket.
—Carranza.
Prognosis
Prognosis is a prediction of the probable course, duration and outcome of
the disease based on a general knowledge of the pathogenesis of the disease
and the presence of the risk factors for the disease.
—Carranza.
Proliferation
Proliferation of keratinocytes takes place by mitosis in the basal layer and
less frequently in the suprabasal layers, where a small proportion of cells
remain as a proliferative compartment while a larger number begins to migrate
to the surface.
Proteoglycan
A protein with numerous glycosaminoglycan side chains. A component of
connective tissue ground substance.
Protrusion
Movement of the mandible anteriorly from inter-cuspal position (ICP).
Provisional Prognosis
It allows the clinician to initiate treatment of teeth that have a doubtful outlook
in the hope that a favorable response may tip the balance and allow teeth
to be retained.
Psychosomatic Disorders
Harmful effects that result from psychic influences on the organic control
of the tissues.
—Carranza.
Puberty Gingivitis
A higher prevalence and severity of gingivitis and gingival enlargement is
found in the circumpubertal period.
—Carranza.
Puetz-Jegher’s Syndrome
Produces intestinal polyposis, melanin pigmentation in the oral mucosa &
lips.
—Carranza.
Pyogenic Granuloma
It is a tumor like gingival enlargement that is considered an exaggerated
conditioned response to minor trauma.
—Carranza.
Radicular Cementum
Cementum that covers the root.
Radius of Action
Range of effectiveness of about 1.5–2.5 mm within which bacterial plaque
can induce loss of bone.
—Page and Schroeder.
Reattachment
It is used to refer the repair in areas of the root not previously exposed
to the pocket, such as after surgical detachment of the tissue or the following
traumatic tear in the cementum, tooth fracture or treatment of periapical
lesions.
—Carranza.
Reattachment
To attach again. The reunion of epithelial and connective tissue with root
surfaces and bone such as occurs after an incision or injury.
—Periodontal Literature Review.
Recession
Refers to the location of gingiva, not its condition.
Recurrent Gingivitis
Reappears after having been eliminated by treatment or disappearing
spontaneously.
—Carranza.

460
Essentials of Clinical Periodontology and Periodontics
Redox Potential
A measure of the ease with which oxidation and reduction reactions will
occur.
Refractory Periodontitis
According to American Academy of Periodontology, it has been defined as;
those cases which do not respond to any treatment provided whatever the
thoroughness or frequency.
Refractory Periodontitis
It include patients who are unresponsive to any treatment provided whatever
the thoroughness or frequency, as well as patients with recurrent disease
at single or multiple sites.
—Periodontal Literature Review.
Regeneration
It is the growth and differentiation of new cells and intercellular substances
to form new tissue and parts.
—Carranza.
Regeneration
Reproduction or reconstitution of a lost or injured part.
Repair
Healing of a wound by tissue that does not fully restore the architecture
or function of the part.
—Periodontal Literature Review.
Repair
It simply restores the continuity of the diseased tissue.
—Carranza.
Repair
Simply restores the continuity of the diseased marginal gingiva and re-
establishes a normal gingival sulcus at the same level on the root as the
base of the pre-existing periodontal pocket. Healing by scar formation.
—Carranza.
Reverse Architecture
Reversed architecture defects are produced by loss of interdental bone,
including the facial plates, lingual plates or both, without concomitant loss
of the radicular bone, thereby reversing the normal architecture.
Retrograde Periodontitis
Periodontitis caused by pulpal infection that have entered the periodontal
ligament either through the apical foramen or through the lateral canals.
Retrusion
Movement of the mandible posteriorly from ICP.
Risk Factor
Is defined as an aspect of personal behavior or lifestyle, an environmental
exposure, or an inborn or inherited characteristic, that, on the basis of
epidemiologic evidence, is known to be associated with health conditions
considered important to prevent.
—The Dictionary of Epidemiology.
Risk Factor
May be environmental, behavioral or biologic factor that when present
increase the likelihood that an individual will get the disease.
—Carranza.
Risk Indicator
A probable or putative risk factor that has been associated with the disease
through cross-sectional studies.
Risk Marker
A factor that is associated with increased probability of future disease.
Risk
It is the probability that an individual will get a specific disease in a given
period.
Root Amputation
The removal of a root from mutirooted teeth.
Root Planing Stroke
It is a moderate to light pull stroke that is used for final smoothening and
planing of the root surface.
Root Planing
It is the process by which residual embedded calculus and portion of
cementum are removed from the roots to produce a smooth, hard and
clean surface.
—Carranza.

461
Glossary
Root Resection/Hemisection
It is the splitting of two rooted tooth into two separate portions.
Root Resection
It is the surgical removal of all or a portion of root before or after endodontic
treatment.
Root Resection
Surgical removal of all or portion of a tooth root.
Root Separation/
It is the sectioning of the root complex and maintenance of all roots.
Bicuspidisation
Saccharolytic
Bacteria which break down carbohydrates for energy are saccharolytic.
Saliva
Defined as the fluid secreted by the salivary glands that begins the digestion
of food.
—Genco.
Scaling Stroke
It is short, powerful pull stroke that is used with bladed instruments for the
removal of supragingival and subgingival calculus.
Scaling
Scaling is a process by which plaque and calculus are removed from both
supragingival and subgingival tooth surface.
Secondary Trauma from
Injury resulting from normal occlusal forces applied to tooth or teeth with
Occlusion
inadequate support.
—Periodontal Literature Review.
Secondary Trauma from
Tissue reaction elicited around a tooth by occlusal forces, with reduced height
Occlusion
of Periodontium.
—Lindhe.
Secondary Trauma from
When the trauma result from reduced ability of the tissues to resist the occlusal
Occlusion
forces is known as secondary trauma from occlusion.
—Carranza.
Segregation Analyses
Classic
twin study reared together monozygotic and dizygotic twins are
compared to estimate the effects of shared genes.
—Carranza.
Segregation Analyses
The observed pattern of disease in families is compared with patterns expected
under various models of inheritance.
—
—Carranza.
Sensitivity
Refers to ability of a test or observation to detect the disease whenever it
is present.
Serumal Calculus
Calculus formed apical to gingival margin, often brown or black, hard and
tenacious.
—Periodontal Literature Review.
Severe Periodontitis
Periodontal destruction is considered severe when 5 mm or more of clinical
attachment loss has occurred.
Sickle Scaler
Sickle scalers have a flat surface and two cutting edges that converge in a
sharply pointed tip.
Slight (mild) Periodontitis
Periodontal destruction generally considered slight when no more than 1
to 2 mm of clinical attachment loss has occurred.
Specific Immune Response
The ability of T-cells and B-cells to recognize specific oligomeric structures
on a pathogen and generate progeny that also recognize the structure enables
the immune system to respond more rapidly and effectively when exposed
to that pathogenic agent.
Specificity
Recognition of the structural features of an oligomer by receptors on immune
cells.
—Carranza.
Specificity
Refers to the ability of a test or observation to clearly differentiate one disease
from another.

462
Essentials of Clinical Periodontology and Periodontics
Splint
Any apparatus, appliance, or device employed to prevent motion or
displacement of fractured or movable parts.
Splint
It is an appliance used for immobilization of injured or diseased parts.
Stillman’s Clefts
They are apostrophe shaped indentations extending from and into the gingival
margin for varying distances on the facial surface.
Stippling
Stippling
is a form of adaptive specialization or reinforcement to function.
—Carranza.
Subgingival Calculus
It is located below the crest of the marginal gingiva and not visible in routine
(Serumal Calculus)
clinical examination.
—Carranza.
Subgingival Curettage
Refers to the procedure that is performed apical to the epithelial attachment
severing the connective tissue attachment down to the osseous crest.
—Carranza.
Subgingival Plaque
Can be defined as the community of micro-organisms that develops on tooth
surfaces that are apical to the gingival margin.
—Lindhe.
Subgingival Plaque
Sub-gingival plaque is found below the gingival margin, between the tooth
and gingival sulcular tissue.
—Carranza.
Subacute
Less severe phase of acute condition.
Subclinical Gingivitis
The initial response of the gingiva to bacterial plaque is not apparent.
—Carranza.
Subtraction Radiography
It is a technique that facilitates both qualitative and quantitative visualization
of even minor density changes in bone by removing the unchanged anatomic
structures from the image.
Subtractive Osseous Surgery
Designed to restore the form of the pre-existing alveolar bone to the level
existing at the time of surgery or slightly more apical to this level.
Sulcular Epithelium
The Sulcular epithelium lines the gingival sulcus, it is a thin, non-keratinized
squamous epithelium without rete pegs and extends from the coronal limit
of the junctional epithelium to the crest of the gingival margin.
—Carranza.
Sulcular Epithelium
The soft tissue wall of the gingival sulcus is lined coronally with sulcular
epithelium, extending from the gingival margin to the junctional epithelium.
Suprabony Pocket
In which the bottom of the pocket is coronal to the underlying alveolar
bone.
Supragingival Calculus
Calculus formed coronal to the gingival margin, usually formed more recently
than sub gingival calculus.
—Periodontal Literature Review.
Supragingival Calculus
Supragingival calculus is located coronal to the gingival margin and therefore
visible in the oral cavity. It is usually white or yellowish in color, hard with
clay like consistency and easily detachable from the tooth surface.
—Carranza.
Supragingival Plaque
Supragingival plaque is defined as the community of the micro-organisms
that develop on the tooth surfaces coronal to the gingival margin.
—Lindhe.
Supragingival Plaque
Supragingival plaque is formed at or above the gingival margin.
Surgery
It is the art practice or work of treating diseases, injuries or deformities by
manual operation or instrumental application.

463
Glossary
Susceptibility Genes
Genes involved in complex multifactorial diseases referred to as susceptibility
genes.
—Carranza.
Synergy
An interaction which is cooperative.
Tetralogy of Fallot
It is characterized by pulmonary stenosis, right ventricular enlargement, a
defect in interventricular septum and the malposition of the aorta to the
right. The oral changes include a purple red discoloration of the lip and
gingiva and periodontal destruction.
—Carranza.
Thrombocytopenic Purpura
It is defined as a platelet count < 100000/mm
3
.
Tissue Inhibitor of
An enzyme inhibitor secreted by fibroblasts to control the activity of
Metalloproteinases (TIMP)
metalloproteinases in the turn over of connective tissue ground substance.
Tooth Mobility
The degree of looseness of a tooth beyond physiological movements.
—Periodontal Literature Review.
Trauma from Occlusion
A tooth subjected to excessive occlusal forces but otherwise exhibit health
with intact periodontal support; no loss of gingival connective tissue attachment
and no apical migration of junctional or sulcular epithelium is described as
‘trauma from occlusion’.
—Genco.
Trauma from Occlusion
It is defined as “a condition where injury results to the supporting structures
of the teeth by the act of bringing the jaws into a closed position”.
—Stillman (1917).
Trauma from Occlusion
It is defined as “damage in the Periodontium caused by stress on the teeth
produced directly or indirectly by teeth of the opposing jaws”.
Trauma from Occlusion
It is defined as, “a condition occurring on a tooth having a major loss of
support, which is displaced in the remaining alveolus by a force applied to
it, even the force of the tongue, cheek or chewing soft food”.
—Genco.
Trauma from Occlusion
It is the term used to describe pathologic alteration or adaptive changes
which develop in Periodontium as a result of undue force produced by
masticatory muscles.
—Lindhe.
Trauma from Occlusion
When occlusal forces exceed the adaptive capacity of the tissue, tissue injury
results. The resultant injury is termed as “Trauma from Occlusion”.
—Carranza.
Trauma
Refers to the precise pathologic changes occurring in the tissues.
Traumatic Occlusion
An occlusion which produces such injury is called traumatic occlusion.
—Carranza.
Treatment Plan
It is the blue print for case management. It includes all procedures required
for the establishment and maintenance of oral health.
—Carranza.
Ultrasonic Scaler
An instrument vibrating in the ultrasonic range which, accompanied by the
stream of water, can be used to remove adherent deposits from teeth.
Universal Curette
One curette designed for all area and surfaces.
Vertical or Angular Defects
They occur in an oblique direction, leaving a hollowed-out trough in the
bone alongside the root.
Vertical/Angular Defects
Vertical/ Angular defects are those that occur in an oblique direction, leaving
out a hollowed-out trough in the bone alongside the root; the base of the
defect is located apical to the surrounding bone.

464
Essentials of Clinical Periodontology and Periodontics
Vincent’s Angina
It is a fusospirochetal infection of the oropharynx and throat.
—Carranza.
Wasting Diseases of the Teeth
Wasting is defined as any gradual loss of tooth substance characterized by
the formation of smooth polished surfaces without regard to the possible
mechanism of this loss.
—Carranza.
Wegener’s Granulomatous
It is a rare disease characterized by acute granulomatous necrotizing lesions
of the respiratory tracts, including nasal and oral defects.
—Carranza.
Xenograft or Heterograft
The donor of the graft is from different species from the host.

465
Index
Index
A
A. actinomycetemcomitans 111
A. viscosus 111
Abrasion 428
Acantholysis 189
Acatalasia 100
Accessibility 269
Accordian technique 344
Acoustic streaming 277
Actinobacillus actinomycetemcomitans
31, 244
Actisite 409
Acute trauma from occlusion 88
Adaptation 273
Adaptive capacity 87
Adrenocorticotropic hormone 259
Adult linear IgA dermatosis 185
Adult periodontitis 209
Aerosol 277
After five curettes 266
Agglutination 252
Air-powder abrasive 263
Alendronate a biphosphonate 258
Alkaline phosphatase 221
Allograft 123, 336, 338
Alloplastic graft 337
Alternate pathway 78
Alveolar bone proper 22
Alveolar edges 308
Alveolar process 8
Alveolodental ligament 16
Amelogenin 4
Anaphylactic 113
Anaphylactic reactions 83
Anaphylotoxins 132
Anatomic aberrations 207
Anchoring fibrils 11, 14
Anemia 99
Angioblasts 310
Angiogranuloma 160
Angular cheilitis 225
Angulation 273
Ankylosis 260
Anti-calculus agents 403
Anti-inflammatory drugs 403
Anti-plaque 403
Antibiotics 404
Antimicrobial agents 404
Antimicrobial therapy 256
Antioxidant 97
Antiserum 252
ANUG 111, 245
Apically-displaced flap 316, 320, 328,
346
Aplastic anemia 100
Aplastic cementum 214
Apoptic 188
Arachidonic acid 204
Arteriosclerosis 103
Arthus reactions 84, 113
Asynchronous multiple burst hypothesis
211
Atherosclerosis 115
Atridox 409
Attached gingiva 3, 9
Attrition 428
Atypical gingivitis 158
Atypical ulcers 225
Augmentin 407
Autograft 337
Autoimmune diseases 132
Azathioprine 189
B
B-lymphocytic 203
Bacillary angiomatosis 225
Bacteria 197
Bacterial plaque 95
Bactericidal 288
Bacteroides forsythus 244
BANA test 108
Barnhart curettes 265
Basal lamina 14
Bass method 415
Betamethasone dipropionate 189
Bicuspidization/root 369
Bioactive glass 339
Bioactive integration 383
Biofilms 57
Biologic width 69, 401
Bisbiguanides 288
Bismuth 104
Bleeding on probing 245
Bleeding points index 45, 430
Blunt Naber’s probe 263
Bone blend 337
Bone factor concept 204
Bone swaging 337
Bruxism 74
Bullous pemphigoid 185
Bundle bone 22
Burn-out phenomenon 214
Burtonian line 104, 418
Buttressing bone formation 89, 206
Butyric acid 110
C
Calcium channel blockers 156
Calcium deficiency 27
Calculocementum 68
Calculus 66, 258
Campylobacter 223
Cancrum oris 224
Cannula type 286
Carbon dioxide 312
Cardiac pacemaker 312
Case control or retrospective study 42
Cationic 288
Caustic drugs 305
Cavitation 277
CD
4
82
Cell rests of Malassez 5
Celluloid 281
Cementoblasts 5
Cementoclasts 17
Cementoid 5
Cementopathia 213
Cementum 25
Cervical enamel projections (CEP) 240
Cetrimide 404
Charter’s technique 415
Chédiak-Higashi syndrome 99, 220

466
Essentials of Clinical Periodontology and Periodontics
Cheilitis 161
Chemical curettage 303
Chemiluminescence 108
Chemokines 113
Chemotactic 96
Chemotactic agents 195
Chemotaxis 78, 99, 221
Chemotherapeutic agents 404
Cherubism 166
Chlorhexidine 157, 403
Chronic dermatosis 188
Chronic trauma from occlusion 88
Cicatricial pemphigoid 185
Ciprofloxacin 407
Citric acid 336
Classical pathway 78
Clenching 74
Clobetasol 189
Clotrimazole 225
Co-aggregation 112
Cohort study 42
Col 3
Collagen fibers 17
Collagen splicing 336
Collagenase 134
Combined pocket 424
Community periodontal index of
treatment needs by Ainamo
and associates 52
Complement 77, 112, 132
B-cells 81
B-lymphocytes 81
functions
chemotaxis cellular activation 77
cytolysis 77
opsonization 77
natural killer 81
NK cells 81
T-cells 81
T-lymphocytes 81
Complex pocket 193
Composite splint 379
Contact inhibition 337
Contact stomatitis 191
Continuous paradigm 211
Coronally-positioned flap 353
Coronally-repositioned flap 353
Coronoplasty 379, 395
Cortical plate 22
Corticosteroid 186
CPITN 52
Crevicular domain 131
Crevicular incision 317
Cribriform plate 22
Curettage 153, 303, 304, 306
gingival 306
inadvertent 306
subgingival 306
Curette 434
Curved blade 265
Cyanoacrylates 296
Cyclic neutropenia 243
Cyclooxygenase pathway 204
Cyclophosphamide 189
Cyclosporine 156, 189, 242
Cytokines 110, 113
D
Dapsones 190
Dark-field microscopy 251
Deep violet 289
Defective neutrophil function 220
Dehiscences 205
Delayed healing 225
Delmopinol 404
Dental endoscope 438
Dental implant 381
Dental plaque 57
Dental splinting 378
Dentinal hypersensitivity 308
Dentogingival unit 13
Dentifrices 161
Dermatitis herpetiformis 185
Desmodont 16
Desmosome 11
DFDBA (Demineralized freeze dried bone
allograft) 338
Diabetes 244, 246
Diabetes mellitus 100
Diapedesis 78
Diastema 9, 93, 193
Diet 95
Digitization 250
Discontinuous or continuous incisions
308
Displaced flaps 316
Distal molar surgery 328
Double papilla flap 353
Down’s syndrome 220, 243
Doxycycline 126, 406
Drifting 92
Drug reactions 185
Drug-influenced gingival enlargement
242
E
Edematous pocket 193
Elastin fibers 17
Electrosection 310
Electrocoagulation 310
Electrodesiccation 310
Electrofulguration 310
Elliptical 284
Eluanin 19
Emdogain 336
Emphysema 119
Enamel matrix proteins 336
Enamel pearls 240
Enamel projections 207
Enameloids 5
ENAP 304, 334
Endosteum 17
Endotoxin or lipo-oligosaccharide 111
Endotoxins 111
Envelope flap 317
Envelope technique 358
Enzymes 112
Eosinophils 190
Epitactic concept 69
Epithelial attachment 334
Epithelial barrier 196
Epithelial root sheath of Hertwig 4
Erosion 428
Erythematous candidiasis 225
Erythromycin 190
EVA system 408
Excursive movement 394
Exotoxins 110
Explorers 264
Extraoral fulcrum 276
F
Fanconi’s anemia 100
Faucet 286
Fc-yRII 240
FDBA (Freeze dried bone allograft) 338
Fenestration operation 348
Fenestrations 205
Fibers 14
alveolar gingival 15
circular 15
collagen 14
dentogingival 15
dentoperiosteal 15
elastin 14, 15
intercircular 15
intergingival 15
interpapillary 15
oxytalan 14, 15
periosteogingival 15
reticulin 14
semicircular 15
trans-septal 15
transgingival 15

467
Index
Fibrinolysis 97
Fibro-osseous integration 383
Fibroblast growth factor 336
Fibroblasts 17
Fibroma 165
Fibronectin 14, 19, 336
Fibrotic component 154
Fibrotic pocket 193
Finger pad 272
Finger rests 436
Firm fulcrum 272
Flap surgery 314
Flat architecture 331
Florida probe 248
Flucocinamide 186
Flurbiprofen 258, 407
Food impaction 71, 258
Free gingival autograft 358
Free gingival groove 7
Free soft tissue autograft 342
Fremitus test 429
Functional imbalance 88
Fungicide 296
Funnel-shaped bony defects 204
Furcation areas 263
Furcation involvement 314, 365
G
Gamma beam 249
Gauze strip 417
GBI 43
Gingiva 8
Gingival abscess 152
Gingival bleeding index 430
Gingival curettage 303
Gingival cyst 165
Gingival enlargement 151
Gingival groove 3
Gingival massage 283
Gingival recession 148, 421
Gingival sulcus 9
Gingivectomy 153, 296, 307, 314
Gingivectomy by chemosurgery 308, 312
Gingivectomy by electrosurgery 308
Gingivitis 125, 140, 143
diffuse 143
marginal papillary 143
Gingivitis toxica 73
Gingivomatosis 157
Gingivoplasty 307
Glickman’s concept 90
Glossitis 161
Glossopharyngeal neuralgias 155
Glycoprotiens 19
Glycosaminoglycans 19
Gnawing 193
Golgi apparatus 12
Gomphosis 16
Graft 336
Granulation tissue 310
Granuloma 165
Granuloma pyogenicum 158
Grasps 436
Greene and vermillion 43
GTR 123
Guided tissue regeneration 337
H
Halitosis 106, 224
Handicapped 284
Haversian systems 22
Heel third 274
Hemidesmosomes 11
Hemisection 216, 369
Hemisepta 208
Hemiseptal defect 206
Hemostasis 100
Heparin 18
Herpes zoster 225
Heterogenous nucleation 69
Heterograft 336
High frenal attachment 221
Highly-magnetized 266
Histamine 18
HIV-associated gingivitis (HIV-G) 223
HIV-associated periodontitis (HIV-P)
223
HIV-necrotizing gingivitis (HIV-NG) 223
HIV/acquired immunodeficiency
syndrome 245
HIV/AIDS 246
Homeostasis 76
Homograft 336
Horizontal incisions 316
Host modulation therapy 125
Howship’s lacunae 17, 24
Hyaline layer of Hopewell Smith or
intermediate cementum 5
Hyalinization 398
Hyaluronic acid 19
Hyaluronidase 134
Hydrodynamic mechanism 301
Hydroxyapatite 203, 326
Hydroxyapatite crystals 5
Hypercementosis 26
Hyperkeratosis 188
Hyperparathyroidism 204
Hyperpituitarism 101
Hyperplasia 155, 419
Hyperplastic candidiasis 225
Hyperplastic gingivitis 97
Hypertrophy 419
Hypophosphatasia 220, 243
Hypopituitarism 101
Hypothyroidism 27, 101
Hypoxia 100
I
Ibuprofen 258, 407
Idiopathic gingival enlargement 155
Idiopathic thrombocytopenia 225
IgG2 240
Immunofluorescence 188
Immunosuppressants 156
Impacted tooth 153
Implant surgery 381
Implants 256
Inadvertent curettage 303
Incidence rate 41
Infrabony pockets 308
Infrabony/intrabony/subcrestal/intra-
alveolar pocket 192
Instrumentation zone 276
Insulin-like growth factor 336
Intercuspal position 394
Interdental bleeding 45
Interdental bleeding index 430
Interdental gingiva 3
Interdental incision 317
Interendothelial transmigration 78
Interferon 113
Interleukin 110
Interleukin-1 (IL-1) 240, 245
Interleukin-1 and 6 24
Internal bevel incision 316
Intrasulcular method 282
Iodine 220
J
Junctional epithelium 6, 9, 12, 110
K
Kaposi’s sarcoma 225
Keratinocyte 11
Ketoconazole 225
Ketoprofen 125
Kirkland 308
Klebsiella 223
Knuckle-rest technique 273
L
Lactoferrin 77
Lamina densa 11
Lamina dura 22
Lamina lucida 11, 190
Lamina propria 14
Laminin 14, 19

468
Essentials of Clinical Periodontology and Periodontics
Langer’s technique 358
Langerhans’ cells 11, 188
Laser gingivectomy 308, 312
Lateral pressure 274
Laterally-displaced flap 351
Leukemia 98, 161, 204
Leukocyte adhesion deficiency 220
type I 240
Leukoplakia 165
Leukotriene B4 217
Lichen planus 185
Lining mucosa 3
Lipping 89
Listerine™ 404
Long junctional epithelium 260, 333
Lymphadenopathy 224
Lymphocytes 11, 81
Lysozyme 77
M
Magnification 249
Malodor 106
Mannitol 284
Marginal gingiva 8
Margination 78
Masticatory mucosa 3
Matrix metalloproteinases 126
McCall’s festoons 419
Mediated or delayed hypersensitivity
84
Mefenamic acid 407
Melanocytes 11
Merkel cells 11
Mesenchyme 6
Methacrylic gels 296
Metronidazole 226, 406
Microflora 101
Miniature blade 264
Minocycline 406
Miswak 281
Mitochondria 12
Modified Bass method 415
Modified ridge lap pontic 401
Modified Stillman method 415
Modified Widman flap 316, 320
Mongolism 221
Monotherapy 289
Morse scaler 262
Mouth-breathing 413
Mucogingival line or junction 3
Mucogingival surgery 341
Mucopolysaccharides 31
Multidirectional strokes 278
Myxedema 101
N
N-formyl-methionyl leucylphenylalanine
(FMLP) 217
Naber’s probe 207, 248
Naproxen 407
Nd:YAG lasers 312
Necrotizing stomatitis (NS) 223
Negative architecture 331
Neurosis 72
Neurotic 292
Neutropenia 97, 220
Neutrophil disorders 80
Neutrophils 78, 190
New attachment 259
Niacinamide 190
Nicotinamide 190
Nifedipine 156, 242
Nikolsky’s sign 189
Noma 224
Non-operating hand 271
Non-steroidal anti-inflammatory drugs 258
Nondisplaced flap 316
Nosocomial 120
Nutritional deficiencies 95
Nylon bristles 281
Nystatin 225
O
Occlusal disharmony 88
Occlusal dystrophy 88
Occlusal neurosis 74
Occlusal overload 88
Occlusal trauma 88
Occlusion 394
Ocular mucosa 189
Odontoblasts 4
Odontoplasty 369
Oesophageal reflux 120
Offset blade 265
Opportunistic infections 103
Opsonins 79
Opsonization 79
Oral contraceptive-associated gingivitis
242
Oral epithelium 261
Oral hairy leukoplakia 225
Oral hygiene index 43
Oral hyperpigmentation 225
Oral implantology 381
Oral moniliasis 163
Orban interdental gingivectomy knives
308
Organoleptic method 107
Oropharyngeal or vulvovaginal
candidiasis 224
Oschsenbein chisels 332
Osseointegration 381, 383
Osseous coagulum 337
Osseous surgery 330
Ostectomy 330
Osteoblasts 17, 204
Osteoclasts 17,127
Osteoconduction 337
Osteocytic osteolysis 23
Osteogenin 338
Osteoid 203
Osteoinduction 337
Osteoplasty 330, 369
Osteoporosis 30, 31, 204, 245, 246
Otitis media 221
Overhanging 258
Oviate pontic 402
Oxidized cellulose 300
Oxytalan fibers 19
Oxytalin fibers 17
P
P. gingivalis 111
Paget’s disease 27, 166, 259
Palatal flap 326
Pancytopenia 99
Papillary bleeding index 45, 430
Papilloma 165
Papilloma virus 225
Papillon-Lefévre syndrome 220, 235
Para formaldehyde 312
Paradontosis 213
Parallelism 273
Parallelization 250
Parkinson’s disease 240
PAS stain 11
Pemphigus vulgaris 185
Peri-implant mucositis 387
Peri-implantitis 387
Periapical abscess 153
Pericementum 16
Pericoronal abscess 153
Perio chip 409
Perio-Aid 278
Periodontal abscess 153
Periodontal disease index (PDI) 44
Periodontal diseases 3, 125
Periodontal dressing 308
Periodontal flap 315
Periodontal membrane 16
Periodontal pack 308
Periodontal pocket 314
Periodontal splint 378
Periodontal surgery 314
Periodontal therapy 3

469
Index
Periodontal traumatism 88
Periodontal treatment 44
Periodontal-knives 308
Periodontics 3
Periodontium 3
Periodontology 3
Periosteum 17, 315
cambium layer or cellular layer 17
fibrous layer 17
groups
alveolocrestal group 18
apical group 18
horizontal group 18
inter-radicular fibers 18
oblique group 18
trans-septal group 18
Periotron 134
Peroxidase 76
Petechiae 99
Phagocytic 99
Phagocytosis 18, 79, 101, 131, 196
Phagolysozyme 79
Phenytoin 155, 242
Photodensitometric 250
Plunger cusps 71
Plaque control 414
Plasma cell gingivitis 191
Plasma cells 197
Platelet derived growth factor 336
PMA index 44
Polyvinylchloride 284
Porphyromonas gingivalis 31, 244
Positive architecture 331
Potassium hydroxide 312
Pouch and tunnel technique 360
Povidone 220
Pregnancy 102
Prevalence rate 41
Primary enamel cuticle 6
Primary epithelial attachment 6
Primary occlusal trauma 395
Principal fibers of periodontal ligament 18
Principal group 14
Progenitor cells 203
Prognosis 237, 239, 291, 433
Propionic acids 110
Propylene glycol 284
Prostaglandin 110, 111, 113
Proteins 96
Proteoglycans 19
Proteolytic 101
Protrusion 394
Provisional splinting 256
Pruritus 190
Pseudopockets 307, 424
PSI 43
Psychosomatic 104
Pull stroke 264
Purulent 153
Pyrophosphatase 191
R
Radiating pain 193
Radiation detector 250
Radical treatment 291
Radicular blending 332
Radius of action 204
Random burst theory 211
Reattachment 259
Reciprocal 284
Recurrent periodontitis 219
Reduced enamel epithelium 4
Refractory periodontitis 219
Regeneration 259
Repair 259
Reproducibility 248
Resistance 251
Resistant 287
Resolution 280
Respiratory burst 80
Reticulin fibers 17
Retrograde periodontitis 373, 428
Retrusion 394
Reversal phenomenon 68
Reverse bevel incision 317
Reversed architecture 206
Rickets 221
Ridge lap pontic 401
Risk 237
determinants 246
factors 246
indicators 246
marker 245, 246
Roll technique 415
Roll test 424
Rolling 78
Rongeur forceps 337
Rongeurs 332
Root biomodification 336
Root planing 303, 435
Root resection 369
S
S. sanguis 111
Salivary calculus 66
Salivary peroxidase system 76
Salmonella 233
Sanguinarine 403
Sanitary pontic 401
Sarcoidosis 165
Scaler 434
Scaling 303, 435
Schwartz periotreivers 266
Scrub technique 415
Scurvy 96
Secondary epithelial attachment 7
Secondary group 14
Secondary intention 310
Secondary occlusal trauma 375
Selenium plate 249
Semilunar flap 357
Sequestration 219
Seruminal calculus 67
Sharpey’s fibers 5, 18
Silver bullet 288
Silver halide emulsion 249
Slowly progressive periodontitis 209
Smoker’s palate 73, 414
Sodium lauryl sulphate 285
Specialized mucosa 3
Stability 271
Stellate reticulum 4
Stillman’s clefts 419
Stillman’s technique 283
Stippling 3, 420
Stomatitis medicamentosa 191
Stomatitis venenata 191
Stratum germinativum 11
Stratum intermedium 4
Streptococcus 223
Stress 246
Strip technique 346
Strokes 274
Strontium chloride 301
Subepithelial connective tissue graft 358
Subgingival 262
Subgingival curettage 303
Substantivity 287, 289
Sulcular bleeding index 430
Sulfone 190
Supportive periodontal treatment 390
Supra-alveolar pockets 307
Suprabony pocket 424
Suprabony/supracrestal/supra-alveolar
pocket 192
Supraperiosteal arterioles 16
Surgical curettage 303
Surgical gingivectomy 308
T
T-lymphocyte 203
Tackiness 296
Tenacious 262, 276
Tension test 424
Tensional theory 21
Testosterone 259
Tetracycline 190, 336, 406

470
Essentials of Clinical Periodontology and Periodontics
TGF (transforming growth factor alpha and
beta) 336
Thrush 225
Thyroidectomy 259
TNF 113
Tobacco smoking 246
Toe 281
Tomography 250
Tongue-thrusting 413
Toothpicks 417
Tori 207
Trabeculae 30
Transgingival probing 206
Trauma from occlusion 87
Traumatizing occlusion 88
Triamcinolone 186
Tricalcium phosphate 339
Triclosan 403
Trigeminal ganglion 20
Trisomy 221
True pockets 192, 424
Tumor necrosis factor 113
Tunnel preparation 369
Two-tone dye 289
Tzanck cells 191
U
Ultrasonic curettage 303
Uncontrolled diabetics 240
Undisplaced flap 316, 320
Unitufted brushes 285, 417
V
Vertical grooving 332
Vertical incision 317
Vibratory motion 283
Viscoelastic theory 21
Vitamin C deficiency 259
Vitamins 96
Volkmann’s canals 16
W
Waerhaug’s concept 91
Washed out 301
Water lavage 277
Wedge-shaped 284
Wegener’s granulomatosis 163
William Addis 281
X
Xenograft 336
